COMMELINIDAE Takht.
Takhtajan, Sist. Filog. Cvetk. Rast. [Syst.
Phylog. Magnolioph.]: 514. 4 Feb 1967
[Arecaceae+Dasypogonaceae+[Commelinanae+Cyperales]]
DASYPOGONACEAE Dumort.
Dumortier, Anal. Fam. Plant.: 54, 55. 1829
[’Dasypogoneae’]
Calectasiaceae Endl.
ex Schnizlein, Iconogr. Fam. Regn. Veg. 1: 51******. Jan-Jul 1845
[’Calectasieae’]; Kingiaceae Endl. ex
Schnizlein, Iconogr. Fam. Regn. Veg. 1: 51*****. Jan-Jul 1845;
Baxteriaceae Takht. in Bot. Žurn. 81(2): 85. Mai-Jun
1996; Dasypogonales
Reveal ex Doweld, Tent. Syst. Plant. Vasc.: lxi. 23 Dec 2001
Genera/species 4/16
Distribution Southwestern
Australia; one species of Calectasia in Victoria.
Fossils Unknown.
Habit Bisexual, usually
perennial herbs (in Kingia often with woody trunk and with numerous
adventitious roots, penetrating leaf bases and covering large parts of stem).
More or less xeromorphic. Some species have stilt roots. Baxteria
lacks a supraterranean stem.
Vegetative anatomy Mycorrhiza
absent. Phellogen? Primary vascular tissue in the form of scattered bundles.
Primary lateral growth sometimes considerable (Dasypogon,
Kingia). Secondary lateral growth absent. Vessels present in roots
only. Vessel elements with scalariform (Baxteria, Kingia) or
simple (Calectasia, Dasypogon) perforation plates; lateral
pits? Imperforate tracheary xylem elements tracheids. Wood rays? Axial
parenchyma? Two peripheral phloem strands in foliar bundles (Baxteria
and Kingia with both phloem fibres and thin-walled fibres in outer
bundle envelopes; Baxteria with sclerenchyma girdles formed within
mesophyll reaching from outer bundle envelope cells to epidermis). Sieve tube
plastids P2c type, with cuneate protein crystals, without starch or protein
filaments. Nodes multilacunar with several leaf traces. Calciumoxalate raphides
present in flowers of Calectasia and Dasypogon. Silica bodies
present.
Trichomes Hairs multicellular,
uniseriate or multiseriate, or absent.
Leaves Alternate (spiral),
simple, entire, with ? ptyxis. Stipules absent; leaf sheath well developed.
Leaf bases long persistent. Two peripheral phloem strands present in foliar
vascular bundles. Venation parallelodromous. Stomata paracytic or tetracytic
(Baxteria, Kingia) or of unique type with several subsidiary
cells (Calectasia, Dasypogon). Cuticular wax crystalloids
non-orientated. Epidermal cells usually with silica crystals as druse-shaped
accumulation; in Calectasia and Dasypogon also with silica
sand or amorphous silica crystals. Mesophyll sometimes with calciumoxalate
crystals. Leaf margin usually entire (sometimes finely serrate).
Inflorescence Terminal,
capitate (Dasypogon, Kingia) or flowers solitary
(Baxteria, Calectasia).
Flowers Actinomorphic, in
Baxteria large. Hypogyny. Tepals 3+3, parchment-like to petaloid, dry
or fleshy, persistent, free (Baxteria, Kingia), or entirely
or partially connate (Calectasia, in Dasypogon only outer
tepals largely connate). Septal nectaries in Dasypogon and
Kingia as pits at base of gynoecium. Disc absent.
Androecium Stamens 3+3.
Filaments free, usually adnate to tepals (epitepalous). Anthers usually
basifixed (in Dasypogon dorsifixed), non-versatile?, tetrasporangiate,
introrse, longicidal (usually dehiscing by longitudinal slits; in
Calectasia poricidal, dehiscing by two apical pores or short slits).
Tapetum? Staminodia absent.
Pollen grains
Microsporogenesis successive. Pollen grains monosulcate (in Baxteria
unique pollen type with surface subdivided into separate parts consisting of
repeatedly branched colpus, colpus membrane formed of coarse pieces with
principally similar surface pattern as mesocolpia [intercolpial surfaces]),
shed as monads, ?-cellular at dispersal. Pollen grains without starch. Exine
tectate, with columellate? infratectum, microreticulate or punctate (in
Kingia psilate).
Gynoecium Pistil composed of
three connate carpels. Ovary superior, unilocular (Calectasia) or
trilocular. Style single, simple, long, narrow, with stylar canal. Stigma
trilobate (Baxteria) or capitate, Wet type? Pistillodium absent.
Ovules Placentation usually
axile (in Calectasia basal). Ovules usually one (rarely two) per
carpel, anatropous, ascending, epitropous?, bitegmic, crassinucellar. Micropyle
bistomal, Z-shaped (zig-zag). Outer integument six to eight cell layers thick.
Inner integument two? cell layers thick. Nucellar cap approx. two cell layers
thick. Parietal tissue approx. two cell layers thick. Megagametophyte
monosporous, Polygonum type, usually small. Synergids with a filiform
apparatus? Chalazosperm large, subdermal, starchy, without central axially
orientated cells. Endosperm development ab initio nuclear. Endosperm haustoria?
Embryogenesis?
Fruit Usually nutlike,
indehiscent, enclosed by persistent perianth; in Baxteria a
septifragal explosively dehiscing capsule: each valve split into two halves,
inner wall of each valve forming plate kept under tension and finally springing
upwards dispersing seed.
Seeds Aril absent. Testa light
yellow (phytomelan absent), in Calectasia membranous. Outer epidermis
with pitted thick-walled cells storing fat, aleurone and hemicellulose. Tegmen?
Perisperm not developed. Endosperm copious, without starch
(Dasypogon). Embryo lens-shaped, short, wide, well differentiated,
chlorophyll? Cotyledon one. Cotyledon hyperphyll? Hypocotyl internode absent.
Mesocotyl present in Dasypogon. Coleoptile present? Germination?
Cytology x = 7
(Baxteria, Dasypogon, Kingia), 9
(Calectasia) – In Calectasia the chromosome nine has
mutated, most of the genetic material being inherited as one large supergene,
i.e. permanently conserved heterozygosity.
DNA
Phytochemistry Insufficiently
known. Ferulic acid (in cell walls) and chelidonic acid (in Dasypogon
bromeliifolius) present. Flavonols, ellagic acid, proanthocyanidins, and
cyanogenic compounds not found.
Use Ornamental plants.
Systematics Dasypogonaceae consist of
two main clades (Rudall & Chase 1996), Dasypogoneae and
Kingieae. A weakly supported sister-group relationship between Dasypogonaceae and Arecaceae was identified by
Barrett & al. (2013).
Dasypogoneae Engl. in
Engler et Prantl, Nat. Pflanzenfam. II, 5: 18. 26 Mar 1887
2/14. Dasypogon
(3; D. bromeliifolius, D. hookeri, D. obliquifolius;
southwestern Western Australia), Calectasia
(15; southwestern Western Australia, Victoria). – Southwestern Australia,
Victoria. Vessels with simple perforation plates. Raphides present only in
floral parts. Hairs branched. Foliar vascular bundles without girdles. Stomata
of unique type with several subsidiary cells and aberrant ontogeny of
subsidiary cells. Epidermis with silica. Inflorescence capitate; flowers in
groups or solitary. Tepals more or less connate into a short tube. Stamens
adnate to base of perianth tube. Megasporangium massive, nutrient-storing
(present below megagametophyte). Fruit indehiscent. Tegmen collapsing.
Cotyledon not photosynthesizing. Mesocotyl and coleoptile present. n = 7, 9.
Chelidonic acid present in Dasypogon.
Kingieae Horan., Char.
Ess. Fam.: 39. 17 Jun 1847
2/2. Kingia
(1; K. australis; southwestern Western Australia), Baxteria
(1; B. australis; southwesternmost Western Australia). –
Southwestern Australia. Kingia
with monopodial growth, retarded apical meristem (similar to Arecaceae), and with
epicortical adventitious roots growing downwards inside persistent sheathing
stem-enclosing leaf bases. Vessels with scalariform perforation plates.
Raphides absent. Foliar vascular bundles with girdles formed in mesophyll.
Stomata paracytic or tetracytic. Inflorescence capitate (Kingia)
or one-flowered (Baxteria),
surrounded by bracts. Peduncle (scape) bracteate. Tepals free. Filaments adnate
to tepal bases. Pollen grains extended sulcate-unipantocolpate. Fruit
indehiscent (Kingia)
or explosively septifragal (Baxteria).
n = 7.
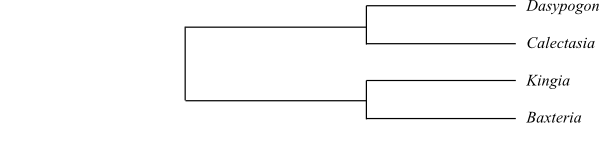 |
Cladogram of Dasypogonaceae
based on DNA sequence data (Rudall & Chase 1996).
|
Literature
Anway JC. 1969. The evolution and taxonomy of
Calectasia cyanea R. Br. (Xanthorrhoeaceae) in
terms of its present-day variation and cytogenetics. – Aust. J. Bot. 17:
147-159.
Barrett RL, Dixon KW. 2001. A revision of the
genus Calectasia (Calectasiaceae) with eight new species described
from South-West Western Australia. – Nuytsia 13: 411-448.
Bedford DJ, Lee AT, Macfarlane TD, Henderson
RJF, George AS. 1986. Xanthorrhoeaceae. – In:
George AS (ed), Flora of Australia 46, Australian Government Publ. Service,
Canberra, pp. 88-171.
Chanda S, Ghosh K. 1976. Pollen morphology
and its evolutionary significance in Xanthorrhoeaceae. – In:
Ferguson JK, Muller J (eds), The evolutionary significance of the exine, Linn.
Soc. Symposium, No. 1, Academic Press, London, New York, pp. 527-559.
Clifford HT, Keighery GJ, Conran JG. 1998. Dasypogonaceae. – In: Kubitzki
K (ed), The families and genera of vascular plants IV. Flowering plants.
Monocotyledons. Alismatanae and Commelinanae (except Gramineae), Springer,
Berlin, Heidelberg, New York, pp. 190-194.
Fahn A. 1954. The anatomical structure of the
Xanthorrhoeaceae
Dumort. – Bot. J. Linn. Soc. 55: 158-184.
Fahn A. 1961. The anatomical structure of the
Xanthorrhoeaceae
Dumort. and its taxonomic position. – In: Recent advances in botany 1,
Toronto, pp. 155-160.
Keighery GJ. 1983. Ballistochory (explosive
seed dispersal) in Baxteria R. Br. (Xanthorrhoeaceae). – W.
Aust. Nat. 15: 163-166.
Keighery GJ. 1984. Chromosome numbers of
Australian Liliaceae. –
Feddes Repert. 95: 523-532.
Krause K. 1930. Liliaceae. – In: Engler A
(ed), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 15a, W. Engelmann,
Leipzig, pp. 227-386.
Neyland R. 2002. A phylogeny inferred from
large-subunit (26S) ribosomal DNA sequences suggests that the family Dasypogonaceae is closely
aligned with the Restionaceae allies. –
Aust. Syst. Bot. 15: 749-754.
Rudall PJ. 1994. The ovule and embryo sac in
Xanthorroeaceae sensu lato. – Flora 189: 335-351.
Rudall PJ, Chase MW. 1996. Systematics of Xanthorrhoeaceae sensu
lato: evidence for polyphyly. – Telopea 6: 185-203.
Shapcott A, Bau B, Katik P. 2008. The
potential implications of rainforest history, hybridization, and climate change
on the phylogenetics of a rare genus of herbs Romnalda (Dasypogonaceae) from New Guinea
and Australia. – Bot. J. Linn. Soc. 157: 455-474.
