[Commelinanae+Cyperales]
CYPERALES Juss. ex Bercht. et J.
Presl
Berchtold et Presl, Přir. Rostlin: 263. Jan-Apr
1820 [‘Cyperaceae’]
Poales Small, Fl.
S.E. U.S.: 48. 22 Jul 1903; Poanae Takht. ex Reveal
et Doweld in Novon 9: 550. 30 Dec 1999
Habit Usually bisexual
(sometimes monoecious, andromonoecious, gynomonocious, polygamomonoecious,
dioecious, androdioecious, or gynodioecious), usually perennial, biennial or
annual herbs (sometimes suffrutices, rarely trees, shrubs, or lianas).
Sometimes hygrophytic or aquatic. Often xerophytes.
Vegetative anatomy Mycorrhiza
often absent. Phellogen absent. Primary vascular tissue two or more cylinders
of vascular bundles or scattered bundles. Secondary lateral growth absent.
Vessels present in roots and/or stem and/or leaves. Vessel elements with
scalariform or simple (rarely reticulate) perforation plates; lateral pits
scalariform or alternating. Imperforate tracheary xylem elements tracheids.
Wood rays absent. Axial parenchyma? Sieve tube plastids usually P2c or P2cf
Type (rarely P2cs type). Nodes? Secretory cavities absent. Idioblasts with
silica bodies (often conical, rounded, saddle-shaped or quadratic) present or
absent. Calciumoxalate raphides present or absent. C4 and CAM
physiology occurring.
Trichomes Hairs unicellular or
multicellular, uniseriate, simple or branched (sometimes stellate or
peltate-lepidote, or T-shaped, and sometimes malpighiaceous, hairs), or absent;
glandular hairs sometimes present; microhairs sometimes present.
Leaves Alternate (distichous
or tristichous, sometimes tetrastichous), simple, entire, usually linear,
sometimes differentiated into pseudopetiole and pseudolamina, sometimes reduced
to leaf sheath, with supervolute/convolute, involute, conduplicate, plicate,
adplicate, revolute, or plicate (rarely circinate) ptyxis. Stipules absent;
leaf sheath usually well developed, open or closed, often with membranous
adaxial ligule at distal end of sheath (ligule sometimes modified into hairs or
absent). Venation usually parallelodromous, often with transverse veins
(sometimes pinnate-parallelodromous). Stomata paracytic or tetracytic (rarely
anomocytic), neighbouring cells with oblique or non-oblique divisions.
Cuticular wax crystalloids usually as longitudinally aggregated rodlets
(Strelitzia type; sometimes scale-like or spherical). Epidermis often
with cell walls containing one or several silica bodies. Mesophyll sometimes
with sclerenchymatous idioblasts. Mucilaginous idioblasts present or absent.
Mesophyll usually without calciumoxalate crystals (sometimes with druses,
styloids or single prismatic crystals). Secretory cavities usually absent.
Cystoliths absent. Leaf margin usually entire (sometimes serrate or
spinose-dentate).
Inflorescence Terminal,
panicle, thyrsoid, corymb, simple or compound, spike-, head-, spadix- or
raceme-like, or spike, raceme or head (flowers sometimes solitary). Floral
prophylls (bracteoles) one, two or absent.
Flowers Usually actinomorphic
(rarely more or less zygomorphic). Hypogyny. Tepals usually three, 3+3
(sometimes 2+2, rarely one, four, 2+4, or more than six) or modified into
scales or bristles (rarely hairs), or absent; outer tepals with contorted or
imbricate (sometimes open, rarely cochlear or conduplicate) aestivation,
sepaloid or petaloid; inner tepals with imbricate or contorted aestivation,
sepaloid or petaloid. Nectary usually absent (septal nectaries sometimes
present). Disc absent.
Androecium Stamens usually
three or 3+3 (sometimes one, two, 2+2, five, or more than six). Filaments
usually free from each other (sometimes more or less connate), usually free
from tepals (sometimes epitepalous). Anthers dorsifixed, subbasifixed or
basifixed, versatile or non-versatile, usually tetrasporangiate (rarely
disporangiate or trisporangiate), introrse, latrorse or extrorse, longicidal
(dehiscing by longitudinal slits) or poricidal (dehiscing by apical pores or
short slits). Tapetum secretory. Staminodia usually absent (female flowers
sometimes with staminodia).
Pollen grains
Microsporogenesis usually successive (rarely simultaneous). Pollen grains
usually monoporate to monoulcerate, diporate to tetraporate (to heptaporate) or
pantotreme (sometimes spiraperturate or trichotomosulcate, rarely disulculate
or inaperturate), often operculate, usually shed as monads (sometimes as
cryptotetrads), usually tricellular (sometimes bicellular) at dispersal. Exine
tectate or semitectate, usually with columellate (sometimes granular)
infratectum, reticulate to punctate, echinulate, echinate, spinulate, finely
scabrate, microverrucate, scrobiculate, or smooth.
Gynoecium Pistil composed of
(one to) three (or four) connate carpels. Ovary superior, usually unilocular or
trilocular (rarely bilocular). Style single, simple, or stylodia two or three,
free or more or less connate. Stigma single, usually bilobate (rarely uni-,
tri- or quadrilobate), or stigmas two or three (to nine), papillate or
non-papillate, Dry type. Pistillodium usually absent (male flowers sometimes
with pistillodium).
Ovules Placentation axile,
basal, apical or parietal. Ovules one to more than 50 per ovary, anatropous,
hemianatropous, amphitropous, orthotropous or campylotropous, ascending,
horizontal or pendulous (often orthotropous pendulous), usually bitegmic
(rarely unitegmic or almost ategmic), crassinucellar or incompletely
tenuinucellar (to pseudocrassinucellar). Micropyle usually endostomal
(sometimes bistomal). Funicular obturator occasionally present. Parietal cell
formed from archesporial cell, or absent. Nucellar cap often present.
Megagametophyte usually monosporous, Polygonum type (rarely disporous,
Allium type, or tetrasporous, close to Drusa type). Synergids
sometimes haustorial. Antipodal cells persistent, sometimes binucleate,
sometimes more than three, sometimes proliferating (rarely absent). Endosperm
development ab initio nuclear or helobial. Endosperm haustoria chalazal and
micropylar, or absent. Embryogenesis usually asterad (sometimes onagrad).
Fruit A loculicidal and/or
septicidal capsule, a nutlet (achene), a follicle, a berry or a nut-like
caryopsis with pericarp usually fused with seed coat in adaxial end (sometimes
a drupe, rarely a nutlet with membranous or gelatinous pericarp free from seed
coat, or a syncarp or coenocarp).
Seeds Aril absent. Caruncular
elaiosome occasionally present. Testa usually dry (sometimes fibrous; sometimes
developing into a sarcotesta; or degenerating). Exotesta sometimes with silica
bodies, sometimes fibrous. Endotesta sometimes persistent, sometimes with
silica bodies. Tegmen sometimes fibrous, sometimes tanniniferous. Exotegmen
sometimes degenerating. Perisperm not or only slightly developed. Endosperm
copious, starchy, usually with proteinaceous tissue (rarely absent), with
aleurone and sometimes oils; starch grains simple or compound. Embryo usually
minute (sometimes large), straight or curved, usually well differentiated
(sometimes rudimentary), with or without chlorophyll. Cotyledon one, not
photosynthesizing. Cotyledon hyperphyll compact (sometimes modified into
haustorium) and not assimilating, or elongate and assimilating. Hypocotyl
internode present or absent. Mesocotyl sometimes present. Coleoptile modified
into substrate-penetrating plumule envelope, with or without lamina, or absent.
Germination phanerocotylar or cryptocotylar.
Cytology x = 2–20
DNA Mitochondrial gene
sdh3 lost.
Phytochemistry Flavonols
(kaempferol, quercetin, myricetin, syringetin), laricitrin, flavonol glycosides
(glycosides of myricetin etc.), flavonol sulfate, flavone sulfates, flavone-5-,
flavone-6- and C-glycosides, catechin, gossypetin, aurones, luteolin,
8-hydroxyluteolin, luteolin-5-methylether, derivatives of 6-hydroxyluteolin and
6-hydroxymyricetin (hydroxyflavonoids), luteolinidin glycoside, tricine
(flavone-3’,5’-dimethyl ether of tricetine), 6-hydroxyflavonoids
(quercetagetin, patuletin), flavanones, cyanidin 3-deoxyanthocyanins,
triterpenes, chalcones, tannins, cinnamic acids, daphnetin, juncosol (phenol),
isoquinoline alkaloids, tryptophane- or tyramine-derived alkaloids (gramine,
hordenine, tyramine, etc.), pyrrolizidine alkaloids as 1-aminopyrrolizidine
derivatives (loline, lolinine, norline, etc.), indole alkaloids, cyanogenic
glycosides (triglochinin), steroidal saponins, tyrosine-derived cyanogenic
compounds, chrysazine (anthraquinone), -sitosterol,
and S-methylcysteine present. Ferulic, diferulic and p-coumaric acids
(esterified) components of non-lignified cell walls. Ellagic acid not found.
Systematics Poales
are sister-group to [Commelinales+Cannales]
(Commelinanae).
Bromeliaceae and Typhaceae are basal branches in
Poales. They were identified as sister-groups in several studies, and
Stevens (2001 onwards) cites the following potential synapomorphies for the
clade [Bromeliaceae+Typhaceae]: stomatal subsidiary
cells with oblique divisions; leaf without distinct sheath; three-nucleotide
deletion in the atpA gene. However, these features may be polarized as
plesiomorphies instead. Oblique divisions of subsidiary cells also occurs
outside Poales (e.g. Iridales). There is a large
variation in leaf base sheathing among monocots in general. Finally, the
three-base deletion may be interpreted as an insertion in the other clades.
Givnish & al. (2010) found Bromeliaceae as sister to the
remaining Poales, whereas Typhaceae are successive
sister-group to the rest.
Rapateaceae are sister to the
remaining Poales except Bromeliaceae and Typhaceae (with various higher or
lower support) in many molecular analyses. The remainder of Poales
form a somewhat different topology depending on the genes and methods used for
the analyses. One possible topology is
[Prionium+[Thurnia+[Juncaceae+Cyperaceae]]]+[[Mayaca+[Xyridaceae+ Eriocaulaceae]]+pooids]].
Another plausible topology may be the following (2012 version of Stevens 2001
onwards): [[[Xyridaceae+Eriocaulaceae]+[Mayaca+[Thurniaceae+[Juncaceae+ Cyperaceae]]]]+pooids], where
Mayacaceae are sister to the
cyperoids. The main clade, representing the vast majority of Poales is
characterized by the features (Stevens 2001 onwards): cellulose fibrils in
outer epidermal cell walls of root elongation zone arranged parallel to root
axis; trichoblast in atrichoblast/trichoblast cell pair adjacent to apical
meristem; absence of septal nectaries; pollen grains tricellular at dispersal;
absence of parietal tissue in ovule; absence (loss) of the mitochondrial gen
sdh4; and presence of (1->3),(1->4)-ß-D-glucans in cell walls.
C4 metabolism is widely distributed here. Moreover, the root hairs
in most of those Poales which have been studied develop exclusively
from special small cells in the epidermal (piliferous) layer. In most vascular
plants the root hairs develop from any cell of the epidermal (piliferous)
layer.
Stevens (2001 onwards) lists the following
potential synapomorphies of the clade [[Eriocaulaceae+Xyridaceae]+[Mayaca+[Prionium+[Thurnia+[Juncaceae+Cyperaceae]]]]]: leaves spirally
arranged; anthers basifixed; outer tepals persistent in fruit; deletions in
ORF2280 region; loss of entire plastid gene accD and mitochondrial
gene sdh4; and presence of flavonoids.
The xyrid clade, comprising Mayacaceae, Eriocaulaceae and Xyridaceae has, e.g., the following
potential synapomorphies (Stevens 2001 onwards): silica bodies absent; spiral
leaves; perianth differentiated into outer sepalous and inner petalous tepals;
basifixed anthers; pollen grains with spinulate exine; style present; micropyle
bistomal; calyx persistent in fruit; seed coat endostomal-tegmic; presence of
operculum (’embryostega’) on the seed; undifferentiated embryo; and
presence of flavonoids. The position of and support of this clade varies
according to analysis methods.
Eriocaulaceae and Xyridaceae (at least Xyris
itself) form a well supported monophyletic group in most analyses,
characterized by the following potential synapomorphies, according to Stevens
(2001 onwards): vessel elements with simple perforation plates; absence of
silica bodies; inflorescence scapose, capitate, and provided with involucral
bracts; clawed inner tepals; filaments adnate to inner tepals, antepetalous;
presence of exothecium; exine provided with spines; seed ridged, with tegmic
operculum; and cuticular layer present between testa and tegmen. The last
character also occurs in Rapateaceae.
The strongly supported cyperoid clade
[Prionium+[Thurnia+[Juncaceae+Cyperaceae]]] has the following
potential synapomorphies (Stevens 2001 onwards): absence of mycorrhiza;
trichoblasts arising from distal cell in a cell pair; culm angular in
cross-section; leaves tristichous, with air canals; leaf sheath usually closed;
perianth small, membranous and undifferentiated, or absent; microsporogenesis
simultaneous; pollen grains porate, shed in tetrads, and tricellular at
dispersal; ovules anatropous; seed coat testal-tegmic; photosynthesizing
unifacial cotyledon hyperphyll (phanomer) usually present; presence of
hypocotyl; seedling collar minute, with rhizoids; chromosomes with diffuse
centromeres; deletions in ORF2280; a 3 bp deletion in the plastid gene
atpA; and presence of amylophilic pteridophyte type starch grains,
3-deoxyanthocyanins and luteolin-5-methylether. The clade [Juncaceae+Cyperaceae] alone has endostomal
micropyle; absence (loss) of plastid gene rpl23; and presence of
luteolin-5’methyl ether.
The clade [[Mayaca+[Eriocaulaceae+Xyridaceae]]+pooids], with fairly
weak support in some molecular analyses, is supported by the characters
tenuinucellate ovules and minute and little differentiated embryo.
The pooid clade is strongly supported by
molecular data and comprises [[Anarthriaceae+ [Centrolepidaceae+Restionaceae]]+[Flagellariaceae+[Joinvilleaceae+Ecdeiocoleaceae+Poaceae]]]]. Stevens (2001 onwards)
cites the following potential synapomorphies: sieve tube plastids with cuneate
and additional loosely packed protein crystals; leaves usually distichous and
sheathing; floral prophylls (bracteoles) often absent; tepals membranous and
undifferentiated; endothecial cell wall thickenings girdle-like; pollen grains
monoporate, annulate/ulcerate and with scrobiculate exine (with minute pores
penetrating tectum and foot layer); stigma plumose, with receptive cells on
multicellular branches; placentation apical; ovule one per carpel,
orthotropous; micropyle bistomal; embryo minute, undifferentiated, broad;
seedling with collar rhizoids; deletions in the ORF2280 region; primary cell
wall also containing (1–3, 1–4)-β-D-glucans; and presence of flavones.
The restioid clade of this monophyletic group
includes Anarthriaceae,
Restionaceae and Centrolepidaceae (the last
group possibly being nested inside Restionaceae) and is supported by
the synapomorphies: usually dioecious; root hairs developing from any epidermal
cell (a reversal); culm with parenchymatous sheath, palisade chlorenchymatous
tissue, and sclerenchymatous cylinder with vascular bundles inside;
chlorenchyma with peg cells; stamens three, antepetalous; anthers dorsifixed;
phanomer (photosynthesizing unifacial cotyledon hyperphyll) present; and loss
of plastid gene rpoC1. Restionaceae and Centrolepidaceae share the
apomorphies: absence of mycorrhiza; absence of hairs; anthers disporangiate and
monothecal; pollen grain pore non-annulate, with irregular margin; cells of
megasporangial epidermis anticlinally elongated; megagametophyte with compound
starch grains (in particular around polar nuclei); and antipodal cells
persistent and proliferating.
The pooids in a strict sense may have the
topology [Flagellariaceae+[[Joinvilleaceae+ Ecdeiocoleaceae]+Poaceae]], which is followed here. On
the other hand, Ecdeiocoleaceae are sometimes
recovered as sister to Poaceae.
In any case, the pooids s.str. clade is characterized by the following
potential synapomorphies (Stevens 2001 onwards): trichoblasts arising from
distal cell of each cell pair; pseudolamina with transverse veins; leaf sheath
with distal ligule; inflorescence a panicle, with adaxial swellings on
branches; presence of nucellar cap; massive suprachalazal zone; indehiscent
fruit; cotyledon non-photosynthesizing; and primary cell walls with
mixed-linkage glucans. Finally, the monophyletic group formed by Joinvilleaceae, Ecdeiocoleaceae and Poaceae may have the synapomorphies:
silica bodies cuboid; epidermis with microhairs; foliar epidermis with long and
silica-containing short cells; guard cells dumbbell-shaped; presence of fusoid
cells (large colourless cells in the central mesophyll); hollow stem; presence
of nucellar cap; first seedling leaf lacking lamina; presence of one 28 kb and
one 6,4 kb inversion in the plastid genome; and loss of the functional plastid
gene ycf2.
ANARTHRIACEAE D. F.
Cutler et Airy Shaw
|
( Back to Cyperales )
|
Cutler et Airy Shaw in Kew Bull. 19: 489. 26 Jul
1965
Hopkinsiaceae B. G.
Briggs et L. A. S. Johnson in Telopea 8: 484. Jul 2000;
Lyginiaceae B. G. Briggs et L. A. S. Johnson in
Telopea 8: 488. Jul 2000
Genera/species 3/11
Distribution Southwestern
Western Australia.
Fossils Unknown.
Habit Usually dioecious
(rarely monoecious), perennial herbs. Graminids. Rarely with stilt roots.
Hygrophytes. Culm branched (Anarthria) or simple (Lyginia),
in cross-section terete or flattened.
Vegetative anatomy Root hairs
lignified, developed from any epidermal cell. Lateral roots developing from a
zone opposite protoxylem poles. Chlorenchymatous layer of peg cells present
inside epidermis; inside this layer a cylinder of vascular bundles.
Anarthria: common parenchymatic and sclerenchymatic envelope not
forming vascular cylinder inside chlorenchyma, instead surrounding each
vascular bundle; palisade tissue absent. Hopkinsia and
Lyginia: subepidermal chlorenchyma separated from cortex by common
sclerenchyma cylinder and parenchyma sheath. Phellogen absent. Secondary
lateral growth absent. Vessels present in stem and leaves (probably absent from
roots). Vessel elements with scalariform perforation plates; lateral pits?
Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial
parenchyma? Sieve tube plastids probably P2c type, with cuneate protein
crystals. Nodes? Silica bodies absent. Calciumoxalate crystals and druses
present in Lyginia.
Trichomes Hairs absent.
Leaves Anarthria:
alternate (distichous), simple, entire, linear, equitant, isobifacial,
laterally flattened, with margin facing culm, with convolute (supervolute)
ptyxis; Hopkinsia and Lyginia: leaves reduced to scale-like
sheaths. Stipules absent; leaf sheath open, with distal ligule. Venation
parallelodromous. Stomata (brachy)paracytic, in furrows, with Poaceae type guard cells. Cuticular
waxes? Epidermis without lines of alternating long and short cells. Mesophyll
with sclerenchymatous idioblasts; mesophyll without calciumoxalate crystals.
Leaf margin entire.
Inflorescence Terminal, few-
to many-flowered, usually panicle (not consisting of spikelets, or spikelets
one-flowered). Each flower subtended by one or two bracts. Floral prophyll
(bracteoles) one, two or absent.
Flowers Actinomorphic, small.
Hypogyny. Tepals 3+3, free, membranous to hard. Nectary absent. Disc absent.
Androecium Stamens three,
antepetalous (outer staminal whorl absent). Filaments filiform, free or connate
(Lyginia), free from tepals. Anthers subbasifixed, versatile,
disporangiate (in Anarthria) or tetrasporangiate (in
Hopkinsia and Lyginia), latrorse-introrse, longicidal
(dehiscing by longitudinal slits). Tapetum secretory? Female flowers often with
staminodia.
Pollen grains
Microsporogenesis successive? Pollen grains graminoid, monoporate to
monoulcerate, operculate? (ulcus with annulus), shed as monads, tricellular at
dispersal. Exine tectate, with columellate infratectum, scrobiculate to smooth
(in Hopkinsia and Lyginia microverrucate).
Gynoecium Pistil usually
composed of three connate antesepalous carpels (in Hopkinsia of one
carpel). Ovary superior, trilocular. Stylodia three, free or connate at base,
with adaxial stigmatic areas. Stigmas three, papillate, Dry type? (sometimes
extremely elongated). Pistillodium absent or rudimentary in male flowers.
Ovules Placentation
apical-axile. Ovule one per carpel, orthotropous, pendulous, bitegmic,
tenuinucellar. Micropyle bistomal? Outer integument ? cell layers thick. Inner
integument ? cell layers thick, tanniniferous. Hypostase present adjacent to
chalazal end. Megagametophyte monosporous, Polygonum type (in
Lyginia with compound starch grains). Antipodal cells? Endosperm
development? Endosperm haustoria? Embryogenesis?
Fruit Usually a loculicidal
capsule (in Hopkinsia a nutlet with fleshy pedicel and persistent
tepals).
Seeds Aril absent. Testa?
Tegmen? Perisperm not developed. Endosperm copious, starchy. Embryo small?,
chlorophyll? Cotyledon one (in Hopkinsia not photosynthesizing).
Cotyledon hyperphyll? Hypocotyl internode? Mesocotyl? Coleoptile absent?
Plumule? Collar hairs? Germination? Phanomer?
Cytology n = 6
(Lyginia); n = 9 (Hopkinsia); n = 11, 22
(Anarthria)
DNA ORF 2280 present. The
plastid gene trnL has a 3 bp deletion and a 5 bp insertion. The
plastid genome lacks the 28 kb inversion often present in Restionaceae, Joinvilleaceae, Ecdeiocoleaceae, and Poaceae.
Phytochemistry Insufficiently
known. Flavonols (kaempferol, quercetin, myricetin) and flavonol glycosides
present. Fructans present in Lyginia. Proanthocyanidins and flavonoid
sulphates not found.
Use Unknown.
Systematics Anarthria
(6; A. gracilis, A. humilis, A. laevis, A.
polyphylla, A. prolifera, A. scabra; southwestern
Western Australia); Hopkinsia (2; H. adscendens, H.
anoectocolea; southwestern Western Australia), Lyginia
(3; L. barbata, L. excelsa, L. imberbis;
southwestern Western Australia).
Anarthriaceae are probably
sister to [Restionaceae+Centrolepidaceae].
Anarthria
is sister to [Hopkinsia+Lyginia]
(Briggs & al. 2010).
 |
Phylogeny of Anarthriaceae based on
DNA sequence data (Briggs & al. 2010).
|
de Jussieu, Gen. Plant.: 49. 4 Aug 1789
[’Bromeliae’], nom. cons.
Bromeliales Link,
Handbuch 1: 207. 4-11 Jul 1829 [’Bromelieae’];
Tillandsiaceae Wilbr., Nat. Pflanzenfam.: 44. Jun-Dec
1834 [’Tillandsieae’]; Bromeliopsida
Brongn., Enum. Plant. Mus. Paris: xv, 23. 12 Aug 1843
[’Bromelioideae’]; Bromelianae R.
Dahlgren ex Reveal in Novon 2: 235. 13 Oct 1992;
Bromeliidae C. Y. Wu in Acta Phytotaxon. Sin. 40:
299. 2002
Genera/species
61–63/3.170–3.200
Distribution Tropical and
subtropical regions of America from Virginia in the United States to Patagonia
in South America; one species of Pitcairnia in tropical West
Africa.
Fossils Uncertain.
Karatophyllum bromelioides, a fossil from Costa Rica of the
mid-Cenozoic, has been attributed to the Bromeliaceae.
Habit Usually bisexual
(sometimes functionally unisexual), usually perennial herbs (rarely trees or
lianas). Many species are epiphytic or epilithic. Some species are succulent.
Two species of Brocchinia and one species of Catopsis are
carnivorous.
Vegatative anatomy Majority of
species with CAM metabolism (evolved multiple times). Intracauline adventitious
roots present in stem cortex in species with a distinct stem. Lateral roots
developing from a zone opposite protoxylem poles. Roots in epiphytes and
epiliths modified into climbing organs; some species lack roots entirely.
Phellogen? Secondary lateral growth absent. Vessels present in roots and often
also in stem and leaves. Vessel elements usually with scalariform (in roots
sometimes simple) perforation plates; lateral pits? Imperforate tracheary xylem
elements tracheids. Wood rays? Axial parenchyma? Sieve tube plastids P2c type,
with cuneate protein crystals, or P2ccl type, with cuneate and
several additional loosely packed protein crystals. Nodes? Stem epidermis with
silica bodies in inner periclinal cell walls. Mucilaginous idioblasts (raphide
sacs) with calciumoxalate raphides.
Trichomes Water absorbing
peltate hairs consisting of a proximal foot cell, a multicellular uniseriate
stalk of living cells and a stellate to discoid peltate upper part consisting
of numerous usually dead cells, often pressed against epidermis; rarely with
multicellular simple uniseriate or stellate hairs or glandular hairs. Water
absorbing stellate or lepidote peltate hairs present especially at leaf
bases.
Leaves Alternate (usually
spiral; in some species of Tillandsia distichous), simple, entire,
often linear (sometimes filiform), usually hard, coriaceous or succulent
(sometimes transformed into spines), with convolute (supervolute), curved or
flat ptyxis. Stipules absent; leaf sheath indistinctly delimited from lamina.
Venation parallelodromous. Stomata tetracytic (sometimes with six subsidiary
cells, sometimes paracytic?), with oblique cell divisions. Cuticular wax
crystalloids usually absent (sometimes as aggregated rodlets, often
longitudinally orientated, Strelitzia type, or as scales). Epidermis
with silica bodies (one per cell, imbedded in inner periclinal cell walls).
Mesophyll with water-storing tissue and mucilaginous idioblasts containing
calciumoxalate raphides. Envelopes of vascular bundles fibrous. Hypodermal
cells often with transparent yellowish tannine drips. Leaf margin serrate
(often spinose-dentate) or entire.
Inflorescence Usually
terminal, spike or raceme (rarely head), often in panicle or thyrse (flowers in
Tillandsia solitary), often with showy colourful bracts (inflorescence
in Deuterocohnia lignified, with cambium-like meristem and anthesis
persisting for several years). Extrafloral nectaries sometimes present.
Flowers Usually actinomorphic
(in Pitcairnia and Tillandsia somewhat zygomorphic). Hypogyny
(probably secondary) to epigyny. Tepals 3+3, free or connate; outer tepals
usually with contorted (sometimes imbricate, rarely cochleate) aestivation,
sepaloid or petaloid, persistent; inner tepals with imbricate or contorted
aestivation, petaloid, often connivent into a tube, often considerably longer
than outer tepals, often with one or two basal adaxial scale-like outgrowths
(nectaries?) and/or longitudinal bulges. Septal nectaries present. Disc
absent.
Androecium Stamens 3+3.
Filaments flattened, more or less fleshy or plicate, usually free (sometimes
connate at base), usually free from tepals (sometimes adnate to inner tepals).
Anthers basifixed or dorsifixed, usually non-versatile?, tetrasporangiate,
introrse to latrorse, longicidal (dehiscing by longitudinal slits); connective
in Androlepis prolonged. Tapetum secretory, with uni- to multinucleate
cells, with ab initio secretory cells subsequently invading. Female flowers
with staminodia.
Pollen grains
Microsporogenesis successive. Pollen grains monosulcate, diporate to
tetraporate or pantotreme (in Aechmea, Canistrum and
Guzmania inaperturate), usually shed as monads (in
Androlepis, Hohenbergiopsis and some Orthophytum as
tetrads), usually bicellular (rarely tricellular) at dispersal. Pollen grains
sometimes without starch. Exine semitectate, with columellate infratectum,
usually reticulate (sometimes scrobiculate or pertectate).
Gynoecium Pistil composed of
three connate carpels; median carpel abaxial. Ovary superior (although
initiated inferior) to inferior, trilocular. Style simple, long, subulate,
straight or curved, trifid at apex (absent in some genera). Stigma usually
trilobate (in Aechmea and Werauhia entire), with straight or
spirally twisted, sometimes foliaceous, branched or irregularly folded lobes,
sometimes cupular, often papillate, Dry or Wet type. Male flowers with
pistillodium; sterile flowers sometimes with pistillodium.
Ovules Placentation axile.
Ovules two to more than 50 per carpel, usually anatropous (rarely
campylotropous or orthotropous), bitegmic, crassinucellar, often with a
chalazal appendage (rarely several). Micropyle endostomal. Outer integument up
to seven cell layers thick. Inner integument two cell layers thick.
Megasporangium one cell layer thick at micropyle; epidermal cells elongate.
Parietal cell formed from archesporial cell. Nucellar cap sometimes two cell
layers thick. Megagametophyte monosporous, Polygonum type. Antipodal
cells sometimes proliferating. Endosperm development ab initio helobial; cell
wall formation in small chalazal space preceding wall formation in large
micropylar spaces. Endosperm haustoria absent? Embryogenesis asterad.
Fruit A usually septicidal
(sometimes septicidal-loculicidal, rarely loculicidal) capsule or a berry with
persistent outer tepals (berries in some species fused with each other and with
inflorescence axis into a syncarp).
Seeds Aril absent. Seed
sometimes winged or plumose (hair tufts in Tillandsioideae formed by
splitting of testa). Operculum sometimes? present. Seed coat testal-tegmic.
Testa dry or developing into a sarcotesta. Exotegmen sometimes thickened.
Endotegmen tanniniferous. Perisperm not developed. Endosperm copious, starchy,
periferal cells with aleurone and lipids; starch grains simple or compound.
Embryo usually small, straight, often lateral, well differentiated, without
chlorophyll, Trillium type. Cotyledon one. Cotyledon hyperphyll
elongate and assimilating, or compact and not assimilating. Hypocotyl internode
present or absent. Coleoptile absent. Radicula absent in
Tillandsioideae. Germination phanerocotylar? or cryptocotylar.
Cytology n = 16, 17, 21, 24,
25, 27, 32, 36, 48 – Chromosomes usually not more than 2.75 μm long.
DNA
Phytochemistry Flavonols
(kaempferol, quercetin), C-glycosylated/6-oxygenated flavones,
derivatives (partially O-methylated) of 6-hydroxyluteolin and
6-hydroxymyricetin, cyaniding?, triterpenes and steroidal saponins present.
Ferulic, diferulic and p-coumaric acids (esterified) components of
non-lignified cellwalls. Ellagic acid and alkaloids not found. Ananas
comosus contains bromelain (a mixture of at least five different
proteolytic enzymes); fruit scent largely due to undecatriene and
undecatetraene.
Use Ornamental plants, fruits
(Ananas comosus, Aechmea, Bromelia,
Greigia), fibres (Ananas lucidus, Aechmea
magdalenae, Neoglaziovia variegata).
Systematics Bromeliaceae have sometimes (with
high support) been identified as sister to the remaining Poales
(Givnish & al. 2010). The subdivision below is according to Givnish &
al. (2006).
Brocchinioideae
Givnish in Aliso 23: 15. Dec 2007
1/21. Brocchinia (21; the
Guayana Highlands in southeastern Colombia, southern Venezuela, Guyana and
northern Brazil). – Stem sometimes erect. Adventitious roots sometimes
intracauline. Stellate chlorenchyma present. Flowers very small. Epigyny.
Septal nectaries present above ovules. Seeds caudate. Basal hair tuft sometimes
present. n = 9?, 23. – Large variation occurs in the mains of nitrogen
absorption. Brocchinia reducta is carnivorous. Brocchinia
acuminata is myrmecophilous.
[Lindmanioideae+[Tillandsioideae+[Hechtioideae+[Navioideae+Pitcairnioideae]+[Puyoideae+
Bromelioideae]]]]
Terminal hair cell dead. Septal
nectaries inserted below ovules.
Lindmanioideae Givnish
in Aliso 23: 15. Dec 2007
1/36. Lindmania (36; the
Guayana Highlands in Venezuela, Guyana and northern Brazil, with their highest
diversity in southern Venezuela). – Dioecious. Chlorenchyma not stellate.
Leaf margin entire or serrate. Outer tepals with contorted aestivation. Stigma
simple, erect. Seeds caudate. Cotyledon hypophyll foliaceous.
[Tillandsioideae+[Hechtioideae+[Navioideae+Pitcairnioideae]+[Puyoideae+Bromelioideae]]]
Inner tepals often with subbasal scales
and/or longitudinally orientated callosities.
Tillandsioideae
Burnett, Outlines Bot.: 442. Feb 1835 [‘Tillandsidae’]
9–10/1.330–1.345. Catopsis
(20; Florida, Mexico, Central America, the West Indies, tropical South
America), Glomeropitcairnia (2; G. ereciflora: Venezuela,
Trinidad; G. penduliflora: the Lesser Antilles), ‘Mezobromelia
hutchisonii’ (Peru), Guzmania (120–125; Florida, Mexico,
Central America, the West Indies, tropical South America), Racinaea
(60–65; southern Mexico, Central America, the Greater Antilles, the
Galápagos Islands, tropical South America), Tillandsia (c 650;
southeastern United States, Mexico, Central America, the West Indies, tropical
South America to central Argentina), Alcantarea (23; eastern Brazil),
Vriesea (c 360; southern Mexico, Central America, tropical South
America), Mezobromelia (8–10; southern Mexico, Central America, the
West Indies, northwestern South America), Werauhia (85–90; tropical
South America). – Tropical and subtropical parts of America from
southern United States to tropical South America. Epiphytic. Roots often
adapted solely for anchoring purpose (sometimes absent, e.g. in adult
individuals of Tillandsia usneoides). Lepidote hairs actinomorphic.
Leaf margin entire. Inflorescence sometimes distichous. Ovules with chalazal
elongated appendage. Outer integument sometimes five cell layers thick. Seeds
caudate due to strongly prolonged outer integument. Apical and/or basal hair
tufts usually developed by longitudinal splitting of outer integument. Radicula
ephemeral or absent. Karyotype bimodal.
[Hechtioideae+[Navioideae+Pitcairnioideae]+Bromelioideae]
Hechtioideae Givnish
in Aliso 23: 16. Dec 2007
1/65–70. Hechtia (65–70;
Texas, Mexico, Central America). – Dioecious. Xeromorphic. Hypodermal
sclerenchymatic tissue present. Internal water storing tissue present.
Chlorenchyma undifferentiated. Hairs arranged in parallel rows. Leaf margin
usually serrate (sometimes entire). Ovary sometimes semi-inferior. Stigma
simple, erect. Seeds usually winged. Cotyledon hypophyll foliaceous. –
Hechtia and Tillandsioideae form a trichotomy together with
the remaining Bromeliaceae
(e.g. Terry & al. 1997). Hechtia is occasionally grouping together
with Tillandsioideae (Crayn & al. 2004).
[Navioideae+Pitcairnioideae]
Navioideae Harms in
Notizbl. Bot. Gart. 10: 575. 30 Mar 1929
5/c 105. Navia (98; northern
tropical South America), Steyerbromelia (6; S. deflexa,
S. diffusa, S. discolor, S. plowmanii, S.
ramosa, S. thomasii; southern Venezuela), Cottendorfia
(1; C. florida; northeastern Brazil), Connellia (6; C.
augustae, C. caricifolia, C. nahoumii, C.
nutans, C. quelchii, C. varadarajanii; the Guayana
Highlands in southern Venezuela and Guyana), Ayensua (1; A.
uaipanensis; the Guayana Shield in southern Venezuela). – The Guayana
Highlands in Venezuela and northern Brazil. Xeromorphic. Peripheral water
storing tissue present. Stellate chlorenchyma present. Leaf margin serrate or
entire. Inner tepals very small. Seeds often winged. – Navioideae
may be sister-group to Pitcairnioideae.
Pitcairnioideae Harms
in Engler et Prantl, Nat. Pflanzenfam., ed. 2, 15a: 99. 1930
6–7/850–855. Pitcairnia (c
395; southern Mexico, Central America, Cuba, tropical South America to
Argentina, with their highest diversity in Colombia to Brazil and Peru, one
species, P. feliciana, in tropical West Africa), Brewcaria
(6; B. brocchinioides, B. duidensis, B. hechtioides,
B. hohenbergioides, B. marahuacae, B. reflexa;
southeastern Colombia, southern Venezuela), Puya (c 220; the
Cordillera in Costa Rica and the Guayana Highlands south to Chile and
Argentina), Fosterella (c 30; southern Mexico, Central America,
tropical South America, with their highest diversity in the Andes),
Encholirium (27; Brazil; in Dyckia?), 'Dyckia'
(155–160; tropical South America; incl. Encholirium?). – Mexico
and Central America southwards to Chile; the occurrence of Pitcairnia
feliciana in West Africa is most probably due to recent long distance
dispersal. Lepidote hairs divided or hairs stellate. Hypogyny to epigyny. Ovule
with chalazal appendage. Outer integument sometimes five cell layers thick.
Parietal tissue sometimes several cell layers thick. Seeds caudate, with body
cells different from ‘tail’; seeds often winged. Embryo often lateral.
Hypocotyl elongated. Cotyledon hypophyll foliaceous. Collar rhizoids present in
Pitcairnia. Karyotype sometimes bimodal. – Puya are usually
xeromorphic, with hypodermal sclerenchymatic tissue and internal water storing
tissue. The chlorenchyma is undifferentiated. The hairs are arranged in
parallel rows. The foliar trichomes have well developed wings. The leaf margins
are serrate. The flowers are zygomorphic. The outer tepals have contorted
aestivation and the inner tepals are clawed, after anthesis densely spirally
twisted. The parietal tissue is several cell layers thick. The seeds are
circumferentially winged and the cotyledon hypophyll is foliaceous.
Bromelioideae Burnett,
Outlines Bot.: 442, 444. Feb 1835 [‘Bromelidae’]
38/765–770. Bromelia (c 55;
southern Mexico, Central America, the West Indies, tropical South America),
Greigia (33; Central America, northwestern South America),
Deinacanthon (1; D. urbanianum; Paraguay, northwestern
Argentina), Ochagavia (4; O. andina, O. carnea,
O. elegans, O. litoralis; southern and central Chile, Juan
Fernandez Islands), Fascicularia (1; F. bicolor; Chile);
Fernseea (2; F. bocainensis, F. itatiaiae;
southeastern Brazil); Acanthostachys (2; A. pitcairnioides,
A. strobilacea; eastern Brazil, Paraguay, northeastern Argentina),
'Quesnelia' (c 20; southeastern Brazil; polyphyletic),
Eduandrea (1; E. sellowiana; southeastern Brazil),
'Wittrockia' (6; W. cyathiformis, W. gigantea,
W. paulistana, W. spiralipetala, W. superba, W.
tenuisepala; eastern Brazil; polyphyletic), 'Neoregelia' (112;
tropical and subtropical South America; polyphyletic), 'Nidularium' (c
45; eastern South America; polyphyletic), 'Canistropsis' (11; tropical
America; polyphyletic), Edmundoa (3; E. ambigua, E.
lindenii, E. perplexa; eastern Brazil), 'Billbergia'
(c 65; southern Mexico, Central America, the West Indies, tropical South
America, with their highest diversity in eastern Brazil; polyphyletic),
'Aechmea' (c 255; Mexico, Central America, the West Indies, tropical
South America; polyphyletic), Ursulaea (2; U. macvaughii,
U. tuitensis; Mexico; in Aechmea?), Hohenbergiopsis
(1; H. guatemalensis; southern Mexico, Guatemala),
'Hohenbergia' (56; southern Mexico, Central America, the West Indies,
northern South America, with their highest diversity in Brazil; polyphyletic),
Hoplocryptanthus (8; Minas Gerais in southeastern Brazil),
Forzzaea (3; F. leopoldo-horstii, F. micra, F.
warasii; Minas Gerais in southeastern Brazil), Lapanthus (2;
L. duartei, L. itambensis; Minas Gerais in southeastern
Brazil), Cryptanthus (c 15; eastern Brazil), Sincoraea (9;
southeastern Brazil), Rokautskyia (14; southeastern Brazil),
Orthophytum (c 40; eastern Brazil), Ronnbergia (11–14;
Central America, northwestern South America), Wittmackia (44;
southeastern Mexico, the West Indies, coastal northeastern South America),
'Lymania' (9; eastern and central Brazil; polyphyletic),
Neoglaziovia (3; N. burle-marxii, N. concolor,
N. variegata; eastern Brazil), Disteganthus (3; D.
basilateralis, D. calatheoides, D. lateralis; French
Guiana, Suriname, Amapá in northern Brazil), Pseudananas (1; P.
sagenarius; tropical South America), Ananas (7; A.
ananassoides, A. bracteatus, A. comosus, A.
erectifolius, A. lucidus, A. macrodontes, A.
parguazensis; Central America, tropical South America),
'Araeococcus' (9; northern South America; polyphyletic),
'Canistrum' (13; eastern Brazil; polyphyletic), Portea (9;
eastern Brazil), Pseudaechmea (1; P. ambigua; Colombia),
Androlepis (1; A. skinneri; southern Mexico to Nicaragua).
– Mexico, the West Indies, Central and South America to Chile. Epiphytic.
Roots often adapted exclusively for anchoring purpose. CAM photosynthesis
frequent. Lepidote hairs irregularly peltate. Leaf margin serrate, often
spinose, or entire. Epigyny or almost epigyny. Perianth tube or hypanthium
sometimes present. Outer tepals sometimes asymmetrical. Inner tepals sometimes
with adaxial subbasal appendages. Pollen grains sometimes porate. Ovary
inferior. Stigma conduplicate, spirally twisted. Ovules with chalazal
(funicular) appendage. Micropyle bistomal or endostomal. Fruit berry-like.
Seeds usually without appendage. Gelatinous sarcotesta frequently present.
Embryo lateral. Cotyledon usually photosynthesizing. Collar rhizoids present.
Radicula prominent. Hypocotyl short. – Greigia,
Deinacanthon, Ochagavia, Fascicularia and perhaps
Bromelia are basal to the remaining Bromeliodeae (Schulte
& al. 2009, Evans & al. 2015).
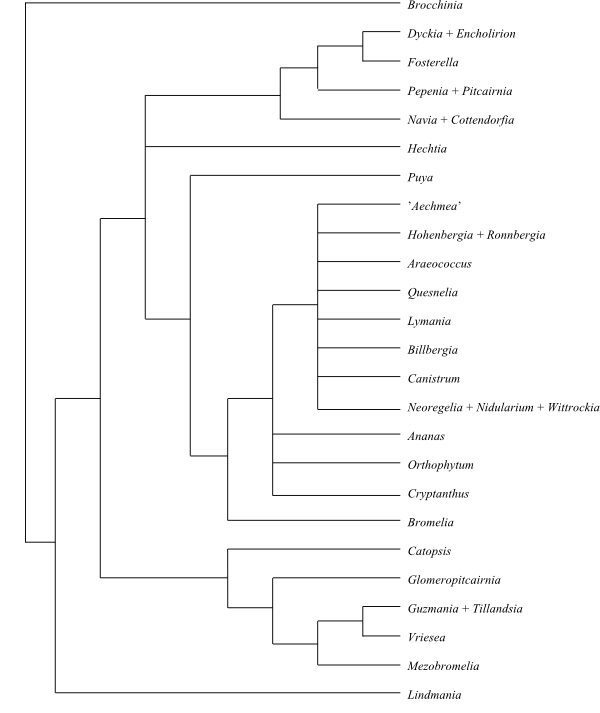 |
Cladogram (simplified) of Bromeliaceae based on DNA
sequence data (Terry & al. 1997; etc.).
|
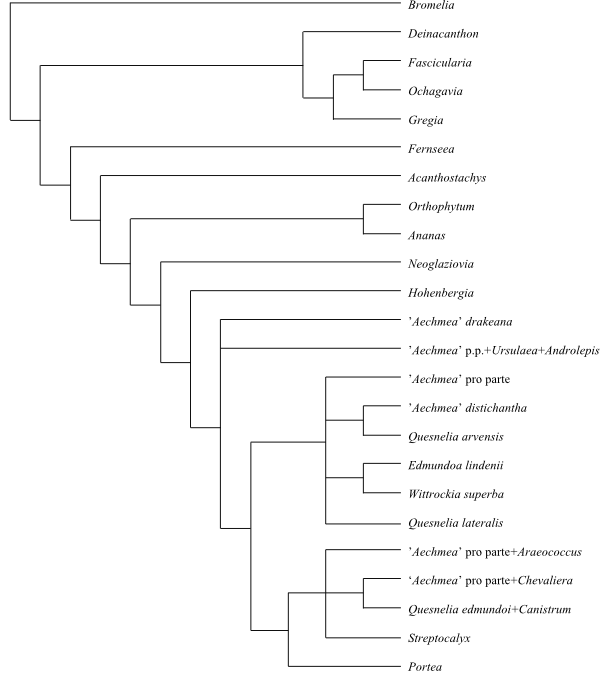 |
Cladogram (simplified) of Bromelioideae based
on DNA sequence data (Schulte & al. 2009).
|
de Jussieu, Gen. Plant.: 26. 4 Aug 1789
[’Cyperoideae’, ’Cyperoïdeae’], nom. cons.
Scirpaceae Batsch ex
Borkh., Bot. Wörterb. 2: 340. 1797 [’Scirpeae’];
Scleriaceae Bercht. et J. Presl, Přir. Rostlin: 263.
Jan-Apr 1820; Caricaceae
Bercht. et J. Presl, Přir. Rostlin: 263. Jan-Apr 1820
[’Caricinae’], nom. illeg. – non Caricaceae
Dumort. 1829; Papyraceae Burnett, Outl. Bot.: 761,
1129. Feb 1835; Caricineae J. Presl in Nowočeská
Bibl. [Wšobecný Rostl.] 7: 1691. 1846; Cyperineae
J. Presl in Nowočeská Bibl. [Wšobecný Rostl.] 7: 1691, 1702. 1846;
Scirpineae J. Presl in Nowočeská Bibl. [Wšobecný
Rostl.] 7: 1691, 1698. 1846; Kobresiaceae Gilly in
Iowa State Coll. J. Sci. 26: 210. Jan 1952;
Mapaniaceae Shipunov in Žurn. Obshchei Biol. 64:
505. Dec 2003
Genera/species
100–105/5.500–5.600
Distribution Cosmopolitan,
with their largest diversity in temperate and alpine regions.
Fossils Fossil pollen and
infructescence of Volkeria messelensis from the mid-Eocene of Germany
were assigned to Mapanioideae. Fossil fruits (Caricoidea,
Cladiocarya, Polycarpella, Scleriocarya, etc. and
also several extant genera) of Cyperaceae (Cyperoideae)
have been recorded from the mid-Paleocene onwards in, above all, Europe, Asia
and North America, and pollen grains are relatively frequent in Cenozoic
layers.
Habit Usually bisexual or
monoecious (rarely andromonoecious, gynomonoecious, dioecious, androdioecious
or gynodioecious; unisexual flowers sometimes in bisexual pseudanthia), usually
perennial (sometimes annual) herbs (rarely shrubs, lianas or epiphytes;
Microdracoides consists of small trees). Graminids. Some species have
bulb-like or tuberous swollen internodes or stem bases. A few species possess
stilt roots. Rhynchospora anomala xeromorphic, with adventitious roots
(with well developed velamen) running down along envelope formed by persistent
leaf bases. Many species are helophytes (sometimes aquatic). Culm usually
medullated (rarely hollow or septate) and terete to sharply triangular in
cross-section (rarely flattened, winged or quadrangular to sexangular in
cross-section).
Vegetative anatomy Mycorrhiza
usually absent (vesicular-arbuscular mycorrhiza present in some species).
Dauciform roots (with dense long root hairs, epidermal cells elongate at right
angles to long axis of root) sometimes present. Lateral roots developing from
zone opposite protophloem or opposite protoxylem poles. Phellogen absent.
Secondary lateral growth absent. Vessels present in roots, stem and leaves.
Vessel elements with scalariform and/or simple perforation plates; lateral
pits? Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial
parenchyma? Sieve tube plastids P2c type, with cuneate protein crystals, or
P2cf, with cuneate protein crystals and peripheral protein filaments. Nodes?
Many species possessing C4 photosynthesis with a green envelope of
Kranz’ cells surrounding vascular bundles (C4 photosynthesis and
Kranz’ anatomy evolved perhaps six times; Kranz’ anatomy present in at
least four types: Chlorocyperus, Fimbristylis,
Rhynchospora, and Eleocharis types; also reversals to
C3 photosynthesis in, e.g., many species of Cyperus), with
or without an envelope of parenchyma cells. Chlorenchyma with lobed cells (peg
cells?) present. Idioblasts with frequent tannins and polyphenols. Silica
bodies conical, inserted on cell walls, usually inner periclinal epidermal cell
walls near veins. Calciumoxalate raphides absent.
Trichomes Hairs usually
unicellular (sometimes multicellular, uniseriate) or absent; often with
unicellular ‘prickle hairs’, with sharp-pointed apex and usually swollen
base.
Leaves Alternate (usually
tristichous, sometimes distichous, tetrastichous or spiral), simple, entire,
usually linear (rarely ensiform or terete), often reduced to a mere leaf
sheath, with conduplicate, plicate, revolute, convolute (supervolute) or
involute ptyxis. Stipules absent; leaf sheath usually closed (in
Coleochloa and Oreobolus open), often with distal ligule
(rarely with distal contraligule). Venation parallelodromous. Stomata usually
paracytic (sometimes tetracytic), with dumbbell-shaped guard cells. Cuticular
wax crystalloids as aggregated rodlets (Strelitzia type) or
non-orientated. Air canals present. Epidermis usually with silica bodies.
Mesophyll without mucilaginous idioblasts and calciumoxalate raphides. Leaf
margin usually entire (sometimes finely serrate-dentate).
Inflorescence Terminal
(sometimes pseudolateral), usually compound (sometimes simple) panicle, corymb,
spike-like, anthela and/or head-like, consisting of axillary one- or
many-flowered spikelets (sometimes a solitary spikelet), each in axil of often
foliaceous bract and with one or more bracts without flowers; bracts with or
without sheath; floral bracts usually small and scale-like, persistent or
caducous; lowermost spikelet scale often prophyll, usually without flower.
Spikelet usually monopodial (indeterminate; sometimes sympodial?). Central axis
of spikelet, rhachilla, persistent or caducous (rarely fragmented into
one-flowered units); rhachilla internodes short or long, sometimes winged or
curved around fruit/fruits. Female spikelet in Carex reduced to pistil
and rhachilla, enclosed by urceolate utriculus, perigynium, formed by basal
prophyll of one-flowered female spikelet.
Flowers Actinomorphic
(zygomorphic through reduction), small. Hypogyny. Tepals usually (one to) three
(or four) or 3+3 (sometimes more than six), usually hair-, bristle- or
scale-like, usually free (sometimes connate), or absent. Nectary absent. Disc
usually absent.
Androecium Stamens (one to)
three (to six; in Chrysithrix numerous [in pseudanthium?]), at least
sometimes antesepalous. Filaments usually free (rarely more or less connate),
usually free from tepals. Anthers basifixed, versatile, tetrasporangiate,
introrse or latrorse, longicidal (dehiscing by longitudinal slits); connective
often comb-shaped and prolonged. Tapetum secretory, with binucleate to
multinucleate cells. Staminodia absent.
Pollen grains
Microsporogenesis simultaneous (Cyperaceae variation). Pollen
grains graminoid, usually monoulcerate or monoporate (in Carex and
other Cyperaceae with
pseudomonads five or six circular or elongate poroids present: one distal and
additional poroids equatorial), shed as pseudomonads (cryptotetrads), three
microspores in each tetrahedral tetrad degenerating and incorporated into wall
of functioning fourth pollen grain, usually tricellular (sometimes bicellular)
at dispersal. Exine tectate, with columellate infratectum, smooth; exine formed
from microsporocyte wall. Intine in Carex sometimes thin below
aperture, thick in interapertural regions.
Gynoecium Pistil composed of
two or three (or four) connate and at least sometimes antesepalous carpels
(median carpel in Carex adaxial, inverted); gynoecium developing from
annular primordium. Ovary superior, unilocular (sometimes on gynophore).
Stylodia two or three (rarely four), simple or branched, free or connate in
lower part; stylar base often enlarged and persistent. Stigmas two or three (to
nine), filiform, adaxially decurrent, papillate or non-papillate, Dry type.
Pistillodium absent.
Ovules Placentation basal.
Ovule one per ovary, anatropous, bitegmic, crassinucellar. Micropyle usually
endostomal (at least in Hypolytrum often bistomal, Z-shaped). Outer
integument two? cell layers thick. Inner integument two? cell layers thick.
Funicular obturator present near micropyle often present. Hypostase present or
absent. Parietal cell formed from archesporial cell. Parietal tissue two to
four cell layers thick. Megasporangium rarely one cell layer thick.
Megagametophyte monosporous, Polygonum type. Endosperm development ab
initio nuclear. Endosperm haustoria chalazal and micropylar, or absent.
Embryogenesis onagrad (Juncus variation).
Fruit Usually a nutlet
(achene), often with persistent tepals as bristles, hairs or scales (achene in
Carex surrounded by utriculus/perigynium at dispersal; in
Cladium, Mapania and Scirpodendron a drupe).
Seeds Aril absent. Seed coat
testal-tegmic. Testa thin, not fused with pericarp. Exotesta with silica
bodies. Remaining testal layers fibrous. Tegmen thin and fibrous. Perisperm not
developed. Endosperm copious, with starch and oils (aleurone?); starch grains
simple. Embryo small, capitate, well or little differentiated, without
chlorophyll, Xyris, Carex, Schoenus or
Scirpus types. Cotyledon one or indistinct, not photosynthesizing.
Cotyledon hypophyll elongate, assimilating. Hypocotyl internode absent.
Mesocotyl sometimes present. Coleoptile with chlorophyll. Plumule? Collar very
small, with rhizoids. Germination cryptocotylar?
Cytology n = 5–>60
(chromosome numbers in Carex extremely variable). – Centromere in
many species diffuse (centric activity present over more or less the entire
chromosomes; not limited to a specific site; spindle fibrils attached at
several sites along chromosomes), easily leading to aberrant chromosome numbers
by chromosome fragmentation, agmatoploidy, detached chromosome segments moving
against poles and remaining functional. Following meiosis three nuclei move
towards one pole and degenerate; finally, they become embedded in the wall of
the surviving microspore.
DNA Deletion encompassing
three base pairs in plastid gene atpA. Plastid gene rpl23
lost. Plastid gene infA lost/defunct (Cyperus). One three
base pairs insertion present in nuclear 5.8S rDNA. Mitochondrial gene
rps14 transferred to nucleus (pseudogene ψrps14 present in
mitochondrial genome).
Phytochemistry Flavonols
(quercetin), flavone-C-glycosides, flavone sulphates, aurones,
flavanones, cyanidin, tricin (tricetin 3’,5’-dimethyl ether; frequent),
6-hydroxyluteolin, luteolin-5’-methyl ether, chalcones, simple indole
alkaloids, and daphnetin present. Carbohydrates stored as kestose and
isokestose oligosaccharides (fructans). Ferulic and p-coumaric acids
(esterified) components of non-lignified cell walls. Ellagic acid not found.
Aluminium accumulation occurring in some species.
Use Ornamental plants,
vegetables (Cyperus esculentus, Eleocharis dulcis), paper
(Cyperus papyrus), thatching (Cladium mariscus etc.), rafts,
canoes, carpets, textiles, basketry, bioenergy, forage plants, etc.
Systematics Cyperaceae are sister-group to
Juncaceae.
Mapanioideae (Hypolytreae)
are sister-group to the remaining Cyperaceae (Cyperoideae).
The subdivision below is based on Muasya & al. (2009) and Hinchliff &
Roalson (2013).
Mapanioideae C. B.
Clarke in W. H. Harvey et O. W. Sonder (ed. W. T. Thiselton-Dyer), Fl. Cap. 7:
150. Dec 1897 [‘Mapanieae’]
11–12/c 170.
Hypolytreae Nees ex Wight et Arn., Contr. Bot. India:
69. Dec 1834. Capitularina (1; C. involucrata; New Guinea),
Exocarya (1; E. sclerioides; Papua New Guinea, Queensland),
Lepironia (1; L. articulata; Madagascar and eastwards to
Polynesia), Chorizandra (5; C. australis, C.
cymbaria, C. enodis, C. multiarticulata, C.
sphaerocephala; Australia, Tasmania, New Caledonia), Chrysitrix
(4; C. capensis, C. dodii, C. junciformis: Western
Cape; C. distigmatosa: southwestern Western Australia),
Diplasia (1; D. karatifolia; Costa Rica to western Brazil),
‘Mapania’ tenuiscapa (Southeast Asia, West Malesia),
Hypolytrum (c 60; tropical and subtropical regions on both
hemispheres), ‘Mapania’ (c 85; pantropical; non-monophyletic),
Paramapania (7; P. flaccida, P. gracillima, P.
longirostris, P. parvibracteata, P. radians, P.
rostrata, P. simplex; Malesia; in Mapania?),
Scirpodendron (2; S. bogneri, S. ghaeri; India and
Sri Lanka to tropical Australia and Polynesia), Principina (1; P.
grandis; Principé in tropical West Africa). – Tropical and subtropical
regions, with their highest diversity in tropical regions in the Old World.
Phytoliths usually absent. Inflorescences possibly consisting of pseudanthia.
Sterile bracts present between stamens and gynoecium. Stamens inserted in axils
of scale-like bracts subtending female flowers.
Cyperoideae Beilschm.
in Flora 16(Beibl. 7): 52. 14 Jun 1833 [‘Cypereae’] (under
construction)
c 98/c 4.300.
Trilepideae Goetgh. in Taxon 34: 629. 29 Nov 1985.
Coleochloa (8; tropical and southern Africa, Madagascar),
Trilepis (5; T. ciliatifolia, T. kanukuensis, T.
lhotzkiana, T. microstachya, T. tenuis; northeastern
South America), Microdracoides (1; M. squamosus; tropical
West and Central Africa). – Cryptangieae Benth. in
J. Linn. Soc. London, Bot. 18: 366. 21feb 1881. Cephalocarpus (4;
C. confertus, C. dracaenula, C. obovoideus, C.
rigidus; tropical South America), Everardia (11; Venezuela,
Guyana), Didymiandrum (1; D. stellatum; tropical South
America), Exochogyne (1; E. amazonica; northern South
America, southeastern Brazil), Lagenocarpus (c 30; Central America,
Cuba, Puerto Rico, tropical South America). –
Sclerieae Wight et Arn., Contr. Bot. India: 71. Dec
1834. Scleria (250–260; tropical and subtropical regions on both
hemispheres). – Bisboeckelereae Pax in H. G. A.
Engler et K. A. E. Prantl, Nat. Pflanzenfam. Nachtr.: 48. 16 Jul 1897
[‘Bisboeckelerieae’]. Calyptrocarya (8; Central America,
the West Indies, tropical South America), Diplacrum (9; pantropical),
Bisboeckelera (4; B. irrigua, B. longifolia, B.
microcephala, B. vinacea; South America), Becquerelia
(7; B. clarkei, B. cymosa, B. discolor, B.
divaricata, B. martii, B. muricata, B.
tuberculata; tropical America). – Koyamaeeae
W. W. Thomas et Davidse in Syst. Bot. 14: 189. 26 Apr 1989
[‘Koyamaeae’]. Koyamaea (1; K. neblinensis;
Venezuela, Brazil). –
'Schoeneae' Dumort., Fl.
Belg.: 145. 1827. Carpha (c 15; Central African mountains, southern
Africa, Madagascar, southern Japan, mountains in New Guinea, Australia, Chile;
incl. Trianoptiles?), Trianoptiles (3; T. capensis,
T. solitaria, T. stipitata; Northern, Western and Eastern
Cape; in Carpha?); Gymnoschoenus (2; G. anceps,
G. sphaerocephalus; southwesternmost and southeastern Australia,
Tasmania), Tricostularia (5; T. compressa, T.
guillauminii, T. neesii, T. pauciflora, T.
undulata; Australia, one species also in Malesia, southern Asia and New
Caledonia), ‘Schoenus’ (100–110; temperate to tropical regions
on both hemispheres, with their highest diversity in Malesia and Australia;
non-monophyletic), Xyroschoenus (1; X. hornei; the
Seychelles), Morelotia (2; M. affinis: New Zealand; M.
gahniiformis: the Hawaiian Islands), Tetrariopsis (1; T.
octandra; southwestern Western Australia), ‘Costularia’
subgenus Lophoschoenus (9; New Caledonia, possibly Borneo and New
Guinea), ‘Tetraria’ pro parte (<50; tropical and southern
Africa, Australia, New Zealand; non-monophyletic; incl. Epischoenus?),
Epischoenus (8; Western and Eastern Cape, KwaZulu-Natal?; in
Tetraria?), Schoenus subgenus Pseudomesomelaena (3;
S. curvifolius, S. grandiflorus, S. turbinatus;
southwestern Western Australia, southeastern Australia, Tasmania),
Machaerina (c 50; tropical and subtropical regions on both
hemispheres, with their highest diversity in Australia), Lepidosperma
(c 65; Malesia to Australia, New Caledonia, New Zealand, with their largest
diversity in Australia), Neesenbeckia (1; N. punctoria;
Western Cape), Cyathochaeta (5; C. avenacea, C.
clandestina, C. diandra, C. equitans, C.
stipoides; southwestern Western Australia, southeastern New South Wales),
‘Gahnia’ (c 40; East Asia, Malesia to Australia, New Caledonia and
islands in the Pacific incl. the Hawaiian Islands; paraphyletic; incl.
Mesomelaena?), Mesomelaena (5; M. graciliceps,
M. preissii, M. pseudostygia, M. stygia, M.
tetragona; southwestern Western Australia; in Gahnia?),
Ptilothrix (1; P. deusta; southeastern Queensland, eastern
New South Wales), Evandra (2; E. aristata, E.
pauciflora; southwestern Western Australia), Caustis (5; C.
blakei, C. dioica, C. flexuosa, C. pentandra,
C. recurvata; western Western Australia, eastern and southeastern
Australia, Tasmania), ‘Cyathocoma’ (3; C. bachmannii,
C. ecklonii, C. hexandra; Western Cape to KwaZulu-Natal,
Mozambique?; non-monophyletic), Capeobolus (1; C.
brevicaulis; Western and Eastern Cape), Chamaedendron (5; C.
kuekenthaliana, C. fragilis, C. neocaledonica, C.
nervosa, C. xyridioides; New Caledonia), Costularia (10;
southeastern Africa, Madagascar, the Mascarene Islands, the Seychelles),
‘Oreobolus’ (17; alpine areas in southeasternmost Australia and
Tasmania, mountains on Pacific islands, southern Andes, subAntarctic islands;
non-monophyletic), ‘Tetraria’ pro parte. –
Rhynchosporeae Wight et Arn., Contr. Bot. India: 71.
Dec 1834. ‘Rhynchospora’
(350–360; almost cosmopolitan, with their highest diversity in tropical and
subtropical South America; paraphyletic; incl. Pleurostachys?),
Pleurostachys (30–35; South America; in Rhynchospora?).
– Cypereae Dumort., Anal. Fam. Plant.: 65. 1829
[‘Cyperineae’]. Androtrichum (1; A. trigynum;
coastal regions in northern Argentina), ‘Androtrichum’
giganteum (northern Argentina), Bolboschoenus (16; temperate
to tropical regions on both hemispheres), Fuirena (c 60;
warm-temperate to tropical regions on both hemispheres), ‘Cyperus’
(c 500; nearly cosmopolitan; non-monophyletic), Kyllingiella (4–5;
K. melanosperma, K. microcephala, K. polyphylla,
K. simpsonii, K. ugandensis; tropical East Africa; in
Cyperus?), Mariscus (c 200; nearly cosmopolitan; in
Cyperus?), ‘Kyllinga’ (c 80; tropical and subtropical
regions on both hemispheres; non-monophyletic; in Cyperus?),
Courtoisina (2; C. assimilis, C. cyperoides;
tropical and southern Africa, Madagascar, India), Sphaerocyperus (1;
S. erinaceus; tropical Africa; in Cyperus?),
‘Pycreus’ (c 120; tropical and subtropical regions on both
hemispheres; paraphyletic; in Cyperus?), Queenslandiella (1;
Q. hyalina; East Africa to eastern Australia; in Cyperus?),
Alinula (4; A. lipocarphioides, A. malawica, A.
paradoxa, A. peteri; tropical and southern Africa, Madagascar;
non-monophyletic?; in Cyperus?), Remirea (1; R.
maritima; pantropical; in Cyperus?), ‘Lipocarpha’ (c
35; tropical and subtropical regions on both hemispheres; paraphyletic; in
Cyperus?), Ascolepis (23; tropical and subtropical regions on
both hemispheres; in Cyperus?), Scirpoides (4; S.
burkei, S. dioeca, S. holoschoenus, S. varius;
the Mediterranean, southern Africa, tropical and subtropical regions in Asia),
Hellmuthia (1; H. membranacea; southern coast of Western
Cape), Ficinia (c 75; tropical and southern Africa, Madagascar, New
Zealand, with their highest diversity in the Cape Provinces),
‘Isolepis’ (75–80; temperate to tropical regions on both
hemispheres, paraphyletic), Erioscirpus (2; E. comosus,
E. microstachyus; northern India, the Himalayas, northern Burma),
Dracoscirpoides (2; D. falsa, D. ficinioides; South
Africa), Afroscirpoides (1; A. dioeca; South Africa),
‘Schoenoplectus’ (50–55; temperate to tropical regions on both
hemispheres; non-monophyletic), Actinoscirpus (1; A. grossus;
tropical and subtropical Asia to northern Australia and islands in the
Pacific), Pseudoschoenus (1; P. inanis; Northern, Western and
Eastern Cape, Free State, Lesotho), Schoenoplectiella (c 30; temperate
to tropical regions on both hemispheres). –
Abildgaardieae Lye in Bot. Not. 126: 328. 1973.
Bulbostylis (210–220; tropical and subtropical regions on both
hemispheres; incl. Nemum?), Nemum (8; tropical Africa; in
Bulbostylis?), Trachystylis (1; T. stradbrokensis;
coastal areas in eastern Queensland and northeastern New South Wales),
Arthrostylis (1; A. aphylla; tropical Australia),
Actinoschoenus (3; A. repens, A. thouarsii, A.
yunnanensis; Madagascar, Sri Lanka, China, northern and northwestern
Australia), Crosslandia (1; C. setifolia; northern
Australia), Fimbristylis (300–310; nearly cosmopolitan). –
Eleocharideae Goetgh. in Taxon 34: 629. 29 Nov 1985.
Eleocharis (280–290; cosmopolitan). –
Cladieae Torr. in Ann. Lyceum Nat. Hist. New York 3:
372. Aug-Dec 1836. Cladium (3; C. costatum: Venezuela,
Guyana; C. mariscoides, C. mariscus: almost cosmopolitan,
especially North America). – Dulichieae W.
Schultze-Motel in Willdenowia 2: 173. 14 Mar 1959. Dulichium (1;
D. arundinaceum; North America), Blysmus (4; B.
compressus, B. mongolicus, B. rufus, B.
sinocompressus; Europe, temperate Asia). –
Scirpeae T. Lestib. in B. C. J. Dumortier, Fl. Belg.:
143. 1827. Khaosokia (1; K. caricoides; peninsular Thailand);
Calliscirpus (2; C. brachythrix, C. crinigerum;
southern Oregon, California, northwestern Mexico); Zameioscirpus (3;
Z. atacamensis, Z. gaimardioides, Z. muticus; the
Andes), Amphiscirpus (1; A. nevadensis; western North
America, Chile, Argentina), Phylloscirpus (4; P. acaulis,
P. andesinus, P. boliviensis, P. deserticola; the
Andes), Rhodoscirpus (1; R. asper; Peru to Argentina);
‘Scirpus’ (c 60; almost cosmopolitan; polyphyletic),
Eriophorum (20; temperate and arctic regions on the Northern
Hemisphere, South Africa); Trichophorum (12; temperate regions on the
Northern Hemisphere, mountains in Southeast Asia, the Andes). –
Sumatroscirpeae Lév.-Bourret et J. R. Starr in Mol.
Phylogen. Evol. 119: 100. 4 Nov 2017. Sumatroscirpus (4; S.
junghuhnii, S. minor, S. paniculatocorymbosus, S.
rupestris; northern Burma, southern China, northern Vietnam, northern
Sumatra). – Cariceae Dumort., Fl. Belg.: 145. 1827.
Carex
(c 1.800; cosmopolitan, with their largest diversity in temperate and alpine
regions). – Unplaced Cyperoideae
Afrotrilepis (2; A. jaegeri, A. pilosa; tropical
West and Central Africa), Nelmesia (1; N. melanostachya;
northern Congo), Neoscirpus (1; N. dioicus; the Korean
Peninsula), Reedia (1; R. spathacea; southwesternmost Western
Australia), Rhynchocladium (1; R. steyermarkii; Venezuela),
Trichoschoenus (1; T. bosseri; Madagascar). – Cosmopolitan,
with their largest diversity in tropical and subtropical regions. Phytoliths
frequent. Tepals as scales, hairs or bristles (sometimes connate), or absent.
Pollen grains obovoid, also with three to six lateral pores/colpi
(pantoporate). Certain species of Rhynchospora are said to be
pollinated by insects (Leppik 1955). – Trilepideae are sister-group
to the remaining Cyperoideae and Cladium
is successive sister to the rest. Larridon & al. (2013) have shown that
most genera recognized in Cypereae (e.g. by Goetghebeur 1998) are
nested inside Cyperus.
An even more comprehensive investigation may reveal that Cyperus
comprises the majority of the Cypereae above. The clade [Blysmus+Dulichium]
is sister-group to
[Khaosokia+Calliscirpus+[Scirpeae+Cariceae]]
in Hinchliff & Roalson (2013). However, the situation in
Cyperoideae at least distal to Rhynchosporeae is chaotic and
every new comprehensive analysis of this part of Cyperaceae presents a new picture
of their phylogeny. Hence, the subdivision above is still provisional.
Sumatroscirpus (Sumatroscirpeae) is sister-group to
Carex (Cariceae) (Léveillé-Bourret & al. 2018).
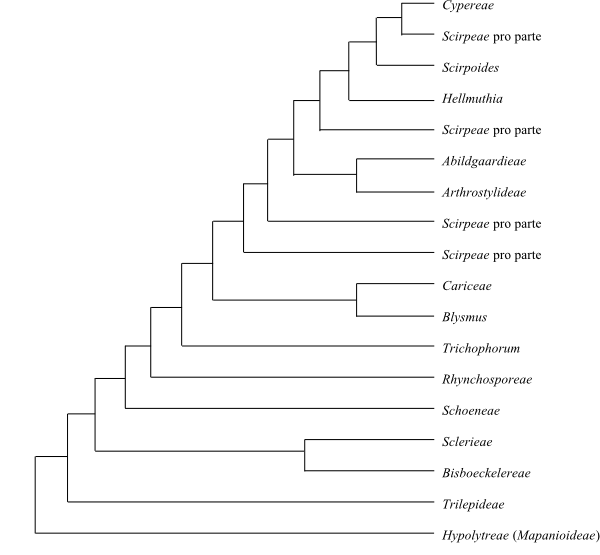 |
Consensus tree (simplified) of Cyperaceae based on successively
weighted DNA sequence data (Muasya & al. 1998, 2000).
|
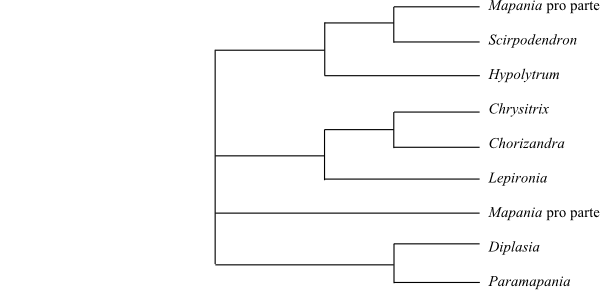 |
Majority rule consensus tree (simplified) of
Mapanioideae (Hinchliff & Roalson 2013).
|
ECDEIOCOLEACEAE D. F.
Cutler et Airy Shaw
|
( Back to Cyperales )
|
Cutler et Airy Shaw in Kew Bull. 19: 495. 26 Jul
1965
Genera/species 2/3
Distribution Western (mainly
coastal) parts of Western Australia.
Fossils Unknown.
Habit Monoecious, perennial
herbs. Graminids. Xeromorphic. Culm terete, branched, furrowed, with swollen
nodes and solid (medullated?) internodes, photosynthesizing.
Vegetative anatomy Culm ridges
sclerenchymatous (sclerenchyma ridges in Georgeantha extending through
chlorenchyma to subepidermal sclerenchymatous layer; not in
Ecdeiocolea). Epidermis in Ecdeiocolea with rows of
alternately long and short cells. Stomata paracytic, in longitudinal furrows
along culm. Phellogen absent. Chlorenchyma with peg cells. Sclerenchyma not
forming a continuous cylinder. Vascular bundles bicollateral, each one
surrounded by sclerenchymatous envelope. Secondary lateral growth absent.
Vessels present in roots, rhizome and culm. Vessel elements with scalariform or
simple perforation plates; lateral pits? Imperforate tracheary xylem elements
tracheids. Wood rays absent. Axial parenchyma? Sieve tube plastids P2c type,
with cuneate protein crystals. Nodes? Secretory cavities absent. Culm
chlorenchyma with cuboid silica bodies as sand. Calciumoxalate crystals absent?
(raphides absent). Special short idioblasts with large single silica bodies
(phytoliths) absent.
Trichomes Hairs absent
(Ecdeiocolea), or multicellular, uniseriate or branched (rhizome, culm
bases and spiklets in Georgeantha); microhairs?
Leaves Alternate (distichous),
on young shoots simple, entire, linear, on older shoots reduced and only
consisting of leaf sheath, with convolute (supervolute) ptyxis. Stipules
absent; leaf sheath closed, auriculate, in Georgeantha caducous;
ligule absent. Venation parallelodromous (also with transverse veins?). Stomata
paracytic, with dumbbell-shaped Poaceae type guard cells. Cuticular
waxes? Mesophyll with fusoid cells? Secretory cavities absent. Mesophyll
without mucilaginous idioblasts or calciumoxalate raphides. Epidermis without
rows of long and short cells. Silica absent from leaf epidermis. Leaf margin
entire.
Inflorescence Terminal,
cymose, usually branched spike-like head, consisting of one
(Ecdeiocolea) or two or three (Georgeantha) racemose
spikelets with male flowers alternating with female flowers; inflorescence
branches not in axils of spatha-like bracts; basal spikelet bracts, glumes,
usually with axillary male or female flowers. Each flower in axil of a bract,
lemma. Floral prophylls (bracteoles) absent.
Flowers Zygomorphic (due to
reduction), dorsiventrally flattened, small. Hypogyny. Tepals 2+2
(Ecdeiocolea) or 3+3 (Georgeantha), bract-like (sepaloid,
glumaceous); two adaxial outer tepals with conduplicate aestivation, keeled,
laterally compressed, ciliated at apex, abaxial outer tepal (in
Georgeantha) and inner tepals flattened, free, surrounded by a stout
scale-like bract. Nectary absent. Disc absent.
Androecium Stamens 2+2
(Ecdeiocolea, possibly corresponding to outer staminal whorl and
adaxial stamen of inner staminal whorl) or 3+3 (Georgeantha).
Filaments filiform, free from each other and from tepals. Anthers basifixed,
versatile, tetrasporangiate, latrorse-introrse, longicidal (dehiscing by
longitudinal slits). Tapetum secretory? Endothecial thicknesses girdle type.
Female flowers with rudimentary staminodia.
Pollen grains Microsporogenes
successive. Pollen grains graminoid, monoporate to monoulcerate, operculate
(with a plug, Poaceae type),
shed as monads, ?-cellular at dispersal. Exine tectate, with columellate
infratectum, smooth (not scrobiculate).
Gynoecium Pistil composed of
two (Ecdeiocolea) or three (Georgeantha) connate carpels.
Ovary superior, unilocular (Ecdeiocolea) or trilocular
(Georgeantha). Stylodia two (Ecdeiocolea) or three
(Georgeantha), free, covered by adaxial stigmatic surfaces. Stigmas
plumose, with papillate hairs, Dry type. Male flowers with rudimentary
pistillodium.
Ovules Placentation
apical-axile. Ovule one per carpel, orthotropous, pendulous, bitegmic,
tenuinucellar. Micropyle bistomal. Outer integument ? cell layers thick. Inner
integument ? cell layers thick. Nucellar cap? Megagametophyte tetrasporous,
16-celled, similar to Drusa type (Ecdeiocolea), or
monosporous, Polygonum type (Ecdeiocolea?). Antipodal cells
absent. Endosperm development? Endosperm haustoria? Embryogenesis?
Fruit A nutlet (achene;
Ecdeiocolea) or a one- or two-seeded loculicidal capsule
(Georgeantha).
Seeds Aril absent. Exotesta
with large cells, in Georgeantha with convex and strongly sinuate
walls; thickness very different in Ecdeiocolea. Endotesta? Tegmen?
Perisperm not developed. Endosperm copious, starchy? Embryo?, chlorophyll?
Cotyledon one, not photosynthesizing. Cotyledon hyperphyll? Hypocotyl
internode? Mesocotyl? Coleoptile? Plumule? Collar hairs? Germination?
Cytology n = c. 24
(Ecdeiocolea); n = 32–33 (Georgeantha)
DNA The plastid genome has an
inversion of 28 kb and an inversion of 6,4 kb (Michelangeli & al. 2003).
Inversion absent from the plastid gene trnT.
Phytochemistry Flavonols
(isorhamnetin, in Georgeantha also quercetin) present. Galactose
present in Georgeantha.
Use Unknown.
Systematics Georgeantha
(1; G. hexandra; westernmost Western Australia), Ecdeiocolea
(2; E. monostachya, E. rigens; western Western Australia).
Ecdeiocoleaceae are probably
sister to Joinvilleaceae
(Marchant & Briggs 2007; Saarela & Graham 2010), although Poaceae have also been identified as
its sister-group (Givnish & al. 2010, etc).
Martinov, Tekhno-Bot. Slovar: 237. 3 Aug 1820
[‘Eriocauleae’], nom. cons.
Eriocaulales Nakai,
Hisi-Shokubutsu: 49. 1930; Eriocaulineae Thorne et
reveal in Bot. Rev. (Lancaster) 73: 85. 29 Jun 2007
Genera/species
10/1.000–1.100
Distribution Tropical and
subtropical regions on the Southern and Northern Hemispheres, with their
largest diversity in the Guayana Highlands and southeastern Brazil; a few
species of Eriocaulon in temperate parts of Europe, East Asia and
North America; Mesanthemum: tropical Africa, Madagascar.
Fossils Uncertain. Subfossil
pollen grains of Eriocaulon septangulare (E. pellucidum) are
known from Pleistocene layers in Ireland and Canada.
Habit Usually monoecious
(rarely dioecious or bisexual), perennial or annual herbs. Many species are
helophytes and some are aquatic.
Vegetative anatomy Mycorrhiza?
Outer part of root cortex in Eriocaulon with specialized aerenchyma
formed by alternating transverse layers of stellate parenchyma cells and
radiate rows of longitudinally widened and flattened cells without contact with
adjacent lateral cells. Root phloem and xylem elements often intermixed in
stele. Lateral roots developing from a zone opposite protophloem or opposite
protoxylem poles. Phellogen absent. Stem endodermal cell walls often U- or
O-shaped in cross-section. Stem vascular bundles alternately on outside and
inside. Secondary lateral growth usually absent (cambium occasionally present,
producing secondary tissue with separate vascular bundles). Vessels usually
present in roots and stem. Vessel elements with scalariform or simple
(sometimes reticulate) perforation plates; lateral pits? Imperforate tracheary
xylem elements tracheids. Wood rays absent. Axial parenchyma? Sieve tube
plastids P2c type, with cuneate protein crystals. Nodes? Silica bodies absent
or nearly so. Calciumoxalate crystals of various types (raphides absent).
Trichomes Hairs multicellular,
uniseriate, with apical cell unbranched, or T-shaped malpighiaceous hairs;
hairs on vegetative parts with foot cell and bulb-like persistent usually dark
basal cell; often also glandular hairs.
Leaves Alternate (usually
spiral, rarely distichous), simple, entire, linear or subulate, with convolute
(supervolute) ptyxis? Stipules absent; leaf sheath indistinct. Vascular bundle
envelope with large cells without chloroplasts. Palisade tissue absent.
Venation parallelodromous. Stomata usually paracytic, with Poaceae type guard cells. Cuticular
wax crystalloids as aggregated rodlets. Epidermis without silica bodies;
subepidermal cells in Paepalanthus with silica bodies. Mesophyll
without mucilaginous idioblasts. Mesophyll with calciumoxalate as druses,
styloids or single prismatic crystals (raphides absent). Leaf margin entire.
Inflorescence Terminal, dense
head-like spike, or compound head- or umbel-like capitulum consisting of up to
1.000 or more partial inflorescences. Pseudanthium surrounded by scale-like
outer bracts and with ten to more than 1.000 flowers in axils of often petaloid
separate inner bracts (absent in Syngonanthus). Peduncle (scape)
narrow, spirally twisted, often with ridges or edges and ebracteate, although
usually with a closed sheathing basal bract; peduncle with central cylinder
consisting of two cylinders of concentric or biconcentric vascular bundles
separated by a sclerenchymatous envelope.
Flowers Actinomorphic or
zygomorphic, very small. Floral receptacle flat or slightly convex. Hypogyny.
Tepals 2+2 or 3+3, with usually open (sometimes valvate) aestivation,
persistent; outer tepals dry, membranous, sepaloid, usually free (sometimes
connate at base; rarely connate into a tube or spathe-like); inner tepals
petaloid, membranous, caducous, usually more or less connate (in, e.g.,
Eriocaulon free), sometimes absent. Tepal nectaries in
Eriocaulon inserted on edges or adaxial side of perianth tube. Septal
nectaries absent. Disc absent.
Androecium Stamens in
Paepalanthoideae two or three (outer staminal whorl absent),
antepetalous; stamens in Eriocaulon and Mesanthemum usually
2+2 or 3+3 (in Syngonanthus amazonicus a single stamen). Stamens
sometimes inserted on androphore. Filaments free; inner filaments in
Eriocaulon and Mesanthemum adnate to inner tepals
(epitepalous). Anthers usually dorsifixed (in Leiothrix basifixed),
non-versatile?, usually tetrasporangiate (in many species of
Paepalanthus disporangiate due to fusion of microsporangia), introrse,
longicidal (dehiscing by longitudinal slits). Tapetum secretory, usually with
uninucleate (rarely binucleate) cells. Female flowers often with (antesepalous)
inconspicuous (often scale-like) staminodia.
Pollen grains
Microsporogenesis usually successive (rarely simultaneous). Pollen grains
usually spiraperturate (one aperture spirally winding over entire pollen grain;
sometimes inaperturate), shed as monads, bicellular or tricellular at
dispersal. Exine tectate, with columellate? infratectum, echinulate to
echinate, often spinulate.
Gynoecium Pistil composed of
two or three connate carpels. Ovary superior, bilocular or trilocular. Stylodia
two or three, free or connate at base, often with stylar appendages. Stigmas
two or three, carinal/dorsal (Eriocaulon, Mesanthemum) or
commissural and non-vascularized (with stylodia-like nectariferous appendages
between stigmas, in Syngonanthus present on same place as vascularized
style in Eriocaulon), simple or bifid, non-papillate, Dry type. Male
flowers often with pistillodium.
Ovules Placentation
axile-apical. Ovule one per carpel, orthotropous, pendulous, bitegmic,
tenuinucellar. Micropyle usually endostomal (in Syngonanthus
bistomal). Outer integument two cell layers thick. Inner integument two cell
layers thick. Hypostase present. Obturator absent. Parietal cell not formed
(parietal tissue absent). Megagametophyte monosporous, Polygonum type.
Unique antipodal cyst, developed by fusion of antipodal cells, present in
megagametophyte. Endosperm development ab initio nuclear, later cellular (or
helobial?). Endosperm haustoria little developed or absent. Embryogenesis
asterad.
Fruit Dorsicidal-loculicidal
capsules with persistent tepals.
Seeds Aril absent. Seed coat
endotestal or tegmic. Exotesta membranous, usually entirely degenerating or
persisting as rows of hooks on seed coat. Endotesta consisting of hexagonal
brownish red to yellow cells with prominent anticlinal walls forming a striate
pattern. Operculum formed from endotegmen. Exotegmen? Endotegmic cells
tanniniferous. Perisperm not developed. Endosperm copious, starchy; starch
grains usually compound. Embryo small, lens- to bell-shaped, rudimentary (lacks
organs but shows differentiation of dermatogen), enclosed by endosperm at
micropylar end, Xyris-Scirpus type, chlorophyll? Cotyledon
one, not photosynthesizing. Cotyledon hyperphyll compact, not assimilating.
Hypocotyl internode absent. Coleoptile absent. Radicula rudimentary or absent.
Collar hairs? Germination?
Cytology n = 8, 9, 15, 20, 25
– Polyploidy occurring.
DNA Significant transfers of
ribosomal protein genes and succinate dehydrogenase genes have taken place from
the mitochondrial genome (to the nuclear genome?) at least in one species of
Paepalanthus (‘Lachnocaulon anceps’). ORF 2280 sometimes
present.
Phytochemistry Flavonols
(kaempferol, myricetin), 6-hydroxyflavonoids quercetagetin and patuletin
(6-methyl ether of quercetagetin) present. Ellagic acid, proanthocyanidins,
alkaloids, and cyanogenic or phenolic compounds not found. Ferulic, diferulic
and p-coumaric acids (esterified) components of non-lignified cell
walls.
Use Ornamental plants.
Systematics The [Eriocaulon+Mesanthemum]
clade is sister to the remaining Eriocaulaceae.
Eriocauloideae
Burnett, Outlines Bot.: 416. Feb 1835 [‘Eriocaulidae’]
2/c 415. Eriocaulon
(c 400; tropical and subtropical regions on both hemispheres, one species,
E. aquaticum, on Ireland and the Hebrides), Mesanthemum (c
15; tropical and subtropical Africa, Madagascar). – Mainly pantropical (few
in temperate regions). Usually aquatic. Roots and leaves usually with
aerenchyma. Inner tepals free, with black (nectar producing?) glandular apex.
Stamens 2+2 or 3+3 stamens, diplostemonous. Inner filaments adnate to inner
tepals (epipetalous). Stigma carinal/dorsal. Testa little developed. Tegmen
tanniniferous.
Paepalanthoideae
Ruhland in H. G. A. Engler, Pflanzenr. 13: 30, 40. 27 Mar 1903
8/580–700. Rondonanthus (6;
R. acopanensis, R. capillaceus, R. caulescens,
R. duidae, R. flabelliformis, R. roraimae; tepuis in
southern Venezuela, the Guayana Highlands and northern Brazil),
Leiothrix (c 65; northern South America to Uruguay, with their highest
diversity in Minas Gerais in northern Brazil), Comanthera (c 40;
northern tropical South America, with their highest diversity in Minas Gerais
and Bahia in northern Brazil), Syngonanthus
(c 140; southeastern United States, Mexico, Central America, Cuba, tropical
South America, tropical Africa, Madagascar, with their highest diversity in
Venezuela and northern Brazil), Actinocephalus (30–35; Brazil, with
their highest diversity in Minas Gerais), Lachnocaulon (7; L.
anceps, L. beyrichianum, L. cubense, L.
digynum, L. ekmanii, L. engleri, L. minus;
southeastern United States, Cuba), Tonina (1; T. fluviatilis;
southern Mexico, Central America, Cuba, northern tropical South America),
Paepalanthus (300–400; tropical America, tropical Africa,
Madagascar, Japan, with their largest diversity in northern tropical South
America). – North and South America, Africa, Madagascar (especially in
tropical South America). Usually terrestrial. Roots and leaves sometimes with
aerenchyma. Secondary lateral growth reported from some species of
Paepalanthus and Syngonanthus.
Inner tepals often connate in middle (at least in male flowers; sometimes
[secondarily?] free; sometimes absent), eglandular. Stamens two or three,
haplostemonous, antepetalous. Anthers sometimes disporangiate and monothecal
(through fusions). Nectariferous carinal stylar appendages usually present.
Male flowers with nectariferous pistillodium. Nectaries consisting of strongly
prolonged epidermal cells. Seed coat endotestal. – Rondonanthus was
sistern to Paepalanthus in a study by de Andrade & al. (2010), but
sister to the remaining Paepalanthoideae in analyses by Trovó &
al. (2013).
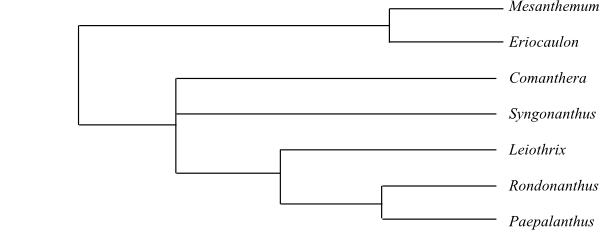 |
Phylogeny of Eriocaulaceae based on
DNA sequence data (de Andrade & al. 2010).
|
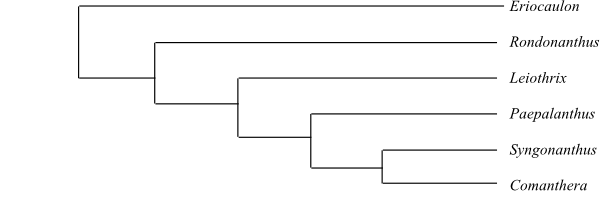 |
Phylogeny of Eriocaulaceae based on
DNA sequence data (Trovó & al. 2013).
|
FLAGELLARIACEAE
Dumort.
|
( Back to Cyperales )
|
Dumortier, Anal. Fam. Plant.: 59, 60. 1829, nom.
cons.
Flagellariales
(Meisn.) Takht. ex Reveal et Doweld in Novon 9: 550. 30 Dec 1999
Genera/species 1/4
Distribution Tropical and
southern Africa, Madagascar, Sri Lanka and southern India, Southeast Asia to
northern Australia and islands in the southwestern Pacific.
Fossils Uncertain. Pollen
grains similar to Flagellaria have been found in Miocene layers on
Borneo.
Habit Usually bisexual
(sometimes unisexual), perennial herbs. Stout, twining and climbing graminids.
Dichotomously branched from distinctly sympodial rhizomes.
Vegetative anatomy Lateral
roots developing from a zone opposite protoxylem poles. Phellogen absent. Culm
solid, with extensive medulla in internodes and compact swollen nodes, without
secretory canals. Vascular bundles embedded in sclerotic tissue between cortex
and central cylinder of aerial stems. Endodermal cells radially elongate.
Chlorenchyma lacking peg cells. Secondary lateral growth absent. Vessels
present in roots, stem and leaves. Vessel elements with scalariform or simple
perforation plates; lateral pits? Imperforate tracheary xylem elements
tracheids. Wood rays absent. Axial parenchyma? Sieve tube plastids
P2ccl type, with cuneate and several additional loosely packed
protein crystals. Nodes? Arm cells and fusoid cells absent. Silica bodies
spherical, present only in association with vascular strands. Cells with
calciumoxalate crystals present.
Trichomes Hairs absent;
multicellular microhairs?
Leaves Alternate (distichous),
simple, entire, linear, with convolute (supervolute) ptyxis below and circinate
ptyxis at apex. Stipules absent; leaf sheath usually closed (rarely open),
auriculate, with one pair of distal lateral lobes (ligule?). Venation
parallelodromous (also with transverse veins). Stomata paracytic, with Poaceae type guard cells; guard cells
not reniform or dumbbell-shaped and without perforations between adjacent guard
cells; adjacent cells with oblique divisions. Cuticular wax crystalloids as
non-orientated platelets (similar to those in Restionaceae), only adjacent to
stomata. Epidermal cells without silica bodies and without phytoliths.
Mesophyll with secretory canals and fibrous cells with granular silica bodies
below and above vascular strands. Mesophyll cells with or without
calciumoxalate crystals. Leaf margin entire. Leaf apex developing into tendril
when in contact with support, due to presence of sensitive adaxial cells.
Extrafloral nectaries present or absent.
Inflorescence Terminal,
compound panicle, with spike- or raceme-like partial inflorescences terminating
branches. Lateral branches often with distinct adaxial swellings at base, and
with first two bracts usually inserted transversely at almost same level.
Floral prophylls (bracteoles) absent.
Flowers Actinomorphic, small.
Hypogyny. Tepals 3+3, pseudouniseriate, sepaloid, membranous, persistent,
connate at base. Nectary absent. Disc absent.
Androecium Stamens usually 3+3
(sometimes fewer, with fertile stamens replaced by staminodia?). Filaments free
from each other and from tepals. Anthers basifixed, versatile, usually
tetrasporangiate (rarely tri-, hexa- or heptasporangiate), latrorse or
introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory, with
binucleate cells. Endothecial thickenings with complete basal plate. Female
flowers with staminodia.
Pollen grains
Microsporogenesis successive. Pollen grains monoporate to monoulcerate, with or
without rudimentary operculum, shed as monads, bicellular at dispersal. Exine
tectate, with columellate infratectum, microperforate, verrucate to
scrobiculate.
Gynoecium Pistil composed of
usually three (sometimes two) connate carpels. Ovary superior, trilocular.
Stylodia three, free or somewhat connate at base. Stigmas three, adaxially
papillate (papillae multicellular), Dry type. Male flowers with
pistillodium.
Ovules Placentation
apical-axile. Ovule one per carpel, almost orthotropous, pendulous, bitegmic,
crassinucellar. Micropyle endostomal; endostoma formed by fast growth of inner
integument. Outer integument apporox. four cell layers thick. Inner integument
two? cell layers thick. Parietal cell formed from non-dividing archesporial
cell. Parietal tissue one cell layer thick. Epidermal cells of megasporangium
dividing periclinally. Megagametophyte at least sometimes disporous,
8-nucleate, Allium type. Endosperm development ab initio nuclear.
Endosperm haustoria? Embryogenesis onagrad or asterad.
Fruit A usually one-seeded
(rarely two-seeded) drupe.
Seeds Aril absent. Seed coat
consisting of exotesta fused with endocarp. Operculum present. Exotesta with
persistent outer periclinal wall. Endotesta? Tegmen? Perisperm not developed.
Endosperm copious, starchy; starch grains simple or compound. Embryo small,
lens-shaped, little differentiated, Xyris-Scirpus type,
chlorophyll? Cotyledon one, not photosynthesizing. Cotyledon hyperphyll?
Hypocotyl internode? Mesocotyl? Coleoptile absent or minute. Plumule? Radicula
with collar hairs. First leaf absent. Germination?
Cytology n = 19
DNA The 6,4 kb and 28 kb
inversions are absent from the plastid genome. ORF2280?
Phytochemistry Flavonols
(kaempferol), alkaloids, cyanogenic glycosides (triglochinin), saponins, and
-sitosterol present. Ellagic acid and
proanthocyanidins not found. Ferulic, diferulic and p-coumaric acids
(esterified) components of non-lignified cell walls.
Use Basketry, medicinal
plants.
Systematics Flagellaria
(5; F. collaris, F. gigantea, F. guineensis, F.
indica, F. neocaledonica; tropical and southern Africa,
Madagascar, the Mascarene Islands, the Seychelles, southern India, Sri Lanka,
Southeast Asia, Malesia, Melanesia, northern Australia, Micronesia and
Polynesia east to Samoa and Niue).
Flagellaria
is sister to a clade comprising Ecdeiocoleaceae, Joinvillea
and Poaceae.
JOINVILLEACEAE Toml. et
A. C. Sm.
|
( Back to Cyperales )
|
Tomlinson et Smith in Taxon 19: 888. 30 Dec
1970
Genera/species 1/4
Distribution West Malesia,
Melanesia on islands in the Pacific to Samoa and the Hawaiian Islands (probably
absent from East Malesia and Australia).
Fossils Uncertain. Ambiguous
fossils from New Zealand have sometimes been assigned to
Joinvillea.
Habit Bisexual, perennial
herbs. Graminids. Culm simple, usually terete, with hollow internodes and
compact swollen nodes.
Vegetative anatomy Phellogen
absent. Secondary lateral growth absent. Vessels present in roots, stem and
leaves. Vessel elements with scalariform or simple (in leaves sometimes also
reticulate) perforation plates; lateral pits? small, numerous. Imperforate
tracheary xylem elements tracheids. Wood rays absent. Axial parenchyma? Sieve
tube plastids P2ccl type, with cuneate and several additional
loosely packed protein crystals. Nodes? Chlorenchyma without peg cells. Arm
cells probably absent. Fusoid cells present. Silica bodies cuboid, especially
frequent in epidermal cell walls and in association with vascular strands.
Special short idioblasts with large single silica bodies (phytoliths)
present.
Trichomes Hairs unicellular or
multicellular, branched or uniseriate, often pointed (‘prickle hairs’);
multicellular microhairs often present.
Leaves Alternate (distichous),
simple, entire, linear, with plicate ptyxis. Stipules absent; leaf sheath open,
auriculate, with distal ligule. Venation parallelodromous to
palmate-parallelodromous; veins anastomosing; main veins connected by
transverse veins. Stomata paracytic, with dumbbell-shaped Poaceae type guard cells. Cuticular
wax crystalloids absent. Mesophyll with fusoid cells? Epidermis with rows of
long cells alternating with short cells containing silica bodies and cuboidal
unlobed phytoliths. Mesophyll without mucilaginous idioblasts and without
calciumoxalate raphides. Mesophyll cells with calciumoxalate crystals, tannins
and silica. Foliar epidermis with microhairs? Leaf margin finely serrate.
Inflorescence Terminal,
compound panicle with spike- or raceme-like partial inflorescences. Lateral
branches with distinct adaxial swellings at base. Prophylls caducous.
Flowers Actinomorphic, small.
Hypogyny. Tepals 3+3, dry, bract-like, sepaloid (outer tepals cucullate),
persistent, free or connate at base. Nectary absent. Disc absent.
Androecium Stamens 3+3.
Filaments free, sometimes adnate at base to tepals. Anthers subbasifixed,
versatile, tetrasporangiate, latrorse, longicidal (dehiscing by longitudinal
slits). Tapetum secretory. Endothecial thickenings girdle type. Staminodia
absent.
Pollen grains
Microsporogenesis successive. Pollen grains graminoid, monoporate to
monoulcerate, with or without rudimentary operculum, shed as monads,
tricellular at dispersal. Exine tectate, with columellate infratectum,
microperforate, scrobiculate.
Gynoecium Pistil composed of
three connate carpels. Ovary superior, trilocular. Stylodia three, free or
somewhat connate at base, sometimes persistent. Stigmas three, with plumose
lobes, and with scattered detached papillate surfaces on multiseriate branches,
Dry? type. Pistillodium absent.
Ovules Placentation
apical-axile. Ovule one per carpel, orthotropous, pendulous, bitegmic,
tenuinucellar? Micropyle bistomal. Outer integument two cell layers thick.
Inner integument two cell layers thick. Parietal cell not formed (parietal
tissue absent). Nucellar cap? Megagametophyte disporous, Allium? type.
Antipodal cells three, binucleate. Endosperm development ab initio nuclear.
Endosperm haustoria? Embryogenesis asterad?
Fruit A one- to three-seeded
drupe with persistent tepals (and sometimes stylar branches).
Seeds Aril absent. Seed coat
tegmic. Testa thin. Exotegmen? Endotegmen tanniniferous. Perisperm not
developed. Endosperm copious, starchy; starch grains compound. Embryo small,
disc-shaped, little differentiated to rudimentary,
Xyris-Scirpus type, chlorophyll? Cotyledon one, not
photosynthesizing. Cotyledon hyperphyll? Hypocotyl internode? Mesocotyl?
Coleoptile? Plumule? Collar hairs? Germination? First seedling leaf only as
leaf sheath.
Cytology n = 18
DNA The plastid genome has an
inversion of 28 kb and one inversion of 6,4 kb. The mitochondrial gene
rps14 is transferred to the nuclear genome, with pseudogene
ψrps14 in mtDNA. The trnT inversion is absent.
Phytochemistry Very
insufficiently known. Ferulic, diferulic and p-coumaric acids
(esterified) components of non-lignified cell walls.
Use Unknown.
Systematics Joinvillea
(4; J. ascendens: the Hawaiian Islands; J. borneensis: West
Malesia, Melanesia and islands in the Pacific to Samoa, the Caroline Islands;
J. bryanii: Samoa; J. plicata: New Caledonia).
Joinvillea
and Poaceae have a similar type
of leaf epidermal structure in the form of alternating long and short cells.
The thickened tepal bases may be homologous to the lodiculae in Poaceae. The stigmas have, as in
Poaceae and some Restionaceae, plumose lobes.
Fusoid cells may be present (Smithson 1957).
de Jussieu, Gen. Plant.: 43. 4 Aug 1789
[’Junci’], nom. cons.
Juncales Bercht. et
J. Presl, Přir. Rostlin: 266. Jan-Apr 1820 [‘Junceae’];
Juncopsida Bartl., Ord. Nat. Plant.: 23, 34. Sep 1830
[’Juncinae’]; Juncanae Takht., Sist.
Filog. Cvetk. Rast. [Syst. Phylog. Magnolioph.]: 510. 4 Feb 1967;
Juncidae Doweld, Tent. Syst. Plant. Vasc.: lxii. 23
Dec 2001
Genera/species 7/430-445
Distribution Cosmopolitan,
with their highest diversity in cold and temperate regions in the Northern and
Southern Hemispheres.
Fossils Fossil seeds have been
found in Late Eocene to Early Oligocene layers in England and from the Miocene
onwards in several places in Europe. Pollen grains, at least of Juncus
and Luzula, are known from the Cenozoic of Russia, Germany and North
America.
Habit Usually bisexual (rarely
monoecious, dioecious or gynodioecious), usually perennial (sometimes annual)
herbs. Graminids. Many species are aquatic or hygrophytic. Culm terete or
flattened in cross-section, smooth or with longitudinal ridges and furrows.
Vegetative anatomy Mycorrhiza
at least usually absent. Dauciform roots (with dense long root hairs, epidermal
cells elongate at right angles to long axis of root) sometimes present. Root
hairs sometimes developing from short cells in root epidermis. Root endodermis
one cell layer thick, with cell wall thickenings U-shaped in cross-section.
Lateral roots developing from a zone opposite protophloem or opposite
protoxylem poles. Central rhizome parenchyma with several scattered vascular
bundles. Culm with one or few peripheral concentric cylinders of vascular
bundles, without endodermis. Phellogen absent. Medulla hollow or consisting of
aerenchyma with stellate cells. Secondary lateral growth absent. Vessels
present in roots, stem and leaves. Vessel elements in Juncus and
Luzula with scalariform and/or simple (in remaining genera usually
entirely scalariform) perforation plates; lateral pits? Imperforate tracheary
xylem elements tracheids. Wood rays absent. Axial parenchyma? Sieve tube
plastids P2c type, with cuneate protein crystals. Nodes? Silica bodies usually
absent (in Juncus rarely as silica sand). Calciumoxalate raphides
absent.
Trichomes Hairs usually absent
(multicellular, uniseriate hairs present on leaf margins in
Luzula).
Leaves Alternate (usually
tristichous, rarely distichous), simple, entire, linear, usually unifacial
(subulate or filiform; rarely laterally flattened and equitant; in some species
consisting of mere leaf sheath), often with convolute (supervolute) ptyxis.
Stipules absent; leaf sheath usually open (in Luzula closed), often
auriculate (sometimes with ligule formed by fusion of auriculae). Venation
parallelodromous. Stomata paracytic (in xeromorphic species Poaceae type paracytic). Cuticular
waxes? Air canals present. Mesophyll without calciumoxalate raphides. Leaf
margin usually entire (in, e.g., Juncus trifidus serrate).
Inflorescence Usually terminal
or pseudolateral, racemose, panicle, corymb, spike- or head-like, in anthela,
drepania or rhipidia (flowers in Marsippospermum and
Rostkovia solitary terminal; in Distichia, Oxychloe
and Patosia solitary lateral). Basal inflorescence bract in some
species of Juncus elongate (appearing as prolongation of culm). Floral
prophylls (bracteoles) one adaxial, two transverse or absent.
Flowers Actinomorphic, usually
small (in Marsippospermum relatively large). Hypogyny. Tepals
(2–)3+(2–)3, sepaloid, caducous or persistent, free. Nectary absent. Disc
absent.
Androecium Stamens usually 3+3
(rarely 2+2, two or three [inner staminal whorl absent]), usually
alternitepalous (in Luzula antetepalous). Filaments filiform or
flattened, free from each other and from tepals. Anthers basifixed,
non-versatile, tetrasporangiate, introrse or latrorse, longicidal (dehiscing by
longitudinal slits); connective sometimes slightly prolonged at apex. Tapetum
secretory, with uninucleate cells. Staminodia absent.
Pollen grains
Microsporogenesis simultaneous (sometimes with ephemeral cell plate following
meiosisI). Pollen grains monoulcerate with indistinct aperture, shed as
tetrahedral tetrads with common exine, tricellular at dispersal. Exine tectate,
thin, with columellate? infratectum, smooth.
Gynoecium Pistil composed of
usually three (rarely two) connate antesepalous carpels; median carpel abaxial.
Ovary superior, usually trilocular (sometimes unilocular and incompletely
septate; rarely bilocular). Stylodia usually three (rarely two), free or
connate below. Stigmas usually three (rarely two), adaxial, papillate (with
unicellular papillae), Dry type, sometimes twisted. Pistillodium absent.
Ovules Placentation usually
axile (when ovary unilocular then parietal placentation; in Luzula
basal). Ovules one (Luzula) or three to c. 50 per carpel, anatropous,
ascending, bitegmic, weakly crassinucellar. Micropyle endostomal or bistomal.
Outer integument two (Juncus), or three or four (Distichia,
Luzula) cell layers thick. Inner integument two cell layers thick.
Funicular obturator (as hair) present. Hypostase present or absent. Parietal
cell formed from archespore. Megagametophyte monosporous, Polygonum
type. Synergids with a filiform apparatus. Antipodal cells persistent.
Endosperm development ab initio helobial. Endosperm haustoria? Embryogenesis
onagrad (Juncus or Luzula variation).
Fruit A usually many-seeded
(in Luzula three-seeded) loculicidal, usually trilocular capsule (in
Distichia, Oxychloe and Patosia more irregularly
dehiscing or sometimes a pyxidium).
Seeds Seeds in many species of
Luzula with a chalazal (apical) or micropylar (basal) fleshy
caruncular elaiosome. Seed coat in Juncus largely
exotestal-endotegmic. Exotesta sometimes mucilaginous; in Luzula
membranous. Endotesta? Exotegmen? Endotegmen in Luzula thickened.
Perisperm not developed. Endosperm copious, starchy, outer layers with aleurone
and oils. Suspensor few-celled, little developed. Embryo small, straight,
without chlorophyll, enclosed by endosperm, Xyris-Scirpus
type. Cotyledon one, usually photosynthesizing. Cotyledon hyperphyll elongate,
assimilating. Hypocotyl rudimentary or absent. Hypocotyl internode present or
absent. Coleoptile absent. Collar very small, with rhizoids. Phanomer
(photosynthesizing unifacial cotyledon hyperphyll) usually present.
Germination?
Cytology n = 9–65
(Juncus); n = 3–42 (Luzula); n = 8 (Oxychloe) –
Centromeres at least in Luzula diffuse (centric activity principally
over the entire chromosomes, not situated at a specific site; spindle fibrils
attaching to several sites along the chromosomes), easily resulting in
anomalous chromosome numbers by chromosome fragmentation, agmatoploidy;
detached chromosome segments move against the poles and remain functional.
DNA A deletion of three base
pairs present in the plastid gene atpA. The plastid gene
rpl23 is absent.
Phytochemistry Flavonols
(quercetin), flavones, flavone sulphates, luteolin-5’-methyl ether,
luteolinidin based glycosides (replacing anthocyanins), cyanidin, tannins,
juncosol (a phytotoxic phenol), 7,8-dihydroxy coumarin, daphnetin including its
8-methyl-ether, and tyrosine-derived cyanogenic compounds present.
Flavone-C-glycosides, tricin, anthocyanins, ellagic acid, alkaloids,
and saponins not found.
Use Ornamental plants,
carpets, baskets, candle wicks (Juncus effusus etc.).
Systematics Luzula
(115–120; cosmopolitan, especially temperate Northern Hemisphere), ‘Juncus’
(300–310; cosmopolitan; non-monophyletic), Marsippospermum (4;
M. gracile: South Island in New Zealand, Campbell Islands, Antipodes
Islands; M. grandiflorum: central and southern Chile, southern
Argentina, Falkland Islands; M. philippii, M. reichei:
southern Chile, southern Argentina), Rostkovia (2; R.
magellanica: South Island in New Zealand, Campbell Islands, Antipodes
Islands, Ecuador, southern Chile, southern Argentina, Falkland Islands, South
Georgia Islands; R. tristanensis: Tristan da Cunha),
‘Oxychloe’ (5; O. andina, O. bisexualis, O.
castellanosii, O. haumaniana, O. mendocina; the Andes
from Peru to northern Patagonia in Argentina; non-monophyletic),
Distichia (3; D. acicularis: the Andes in Ecuador; D.
filamentosa: the Andes in Peru, Bolivia and northern Chile; D.
muscoides: the Andes in Colombia, Ecuador, Peru, Bolivia and northwestern
Argentina), Patosia (1; P. clandestina; Bolivia, Chile,
western Argentina).
Oxychloe has occasionally been
interpreted as sister to Cyperaceae, thus making Juncaceae paraphyletic. It lacks a
groove and additional subepidermal sclerenchyma girdles in the leaf. In, e.g.,
the Plunkett & al. (1995) and Munro & Linder (1998) analyses,
Oxychloe is recovered within Cyperaceae. However, these analyses
were obviously based on mixed samples. The position of Oxychloe in
Juncaceae – near
Distichia and Patosia – has been clarified by later
investigations.
Thurnia (Thurniaceae) and Prionium
(Prioniaceae) are
successive sister-groups to the [Juncaceae+Cyperaceae] clade.
Luzula
elegans is sister to the remaining species of the monophyletic Luzula.
The species is annual and has a cymose inflorescence. The six somatic
chromosomes evolved as a result of chromosome fusion (Nordenskiöld 1951).
‘Juncus’
in the current circumscription is heavily non-monophyletic, according to
Drábková & al. (2003, 2006) and Drábková (2010), with the two species
J. monanthos and J. trifidus forming a sister-group to the
remaining Juncaceae, and
J. capitatus successive sister to the rest. Luzula was
sister to a main clade comprising Juncus pro
parte, Distichia, Marsippospermum, Oxychloe,
Patosia, and Rostkovia. Furthermore, Juncus capensis
and J. lomatophyllus formed a sister-group to Oxychloe,
Distichia and Patosia, where Distichia was
paraphyletic relative to Patosia. Additional molecular analyses,
including even more taxa of Juncus,
are desirable.
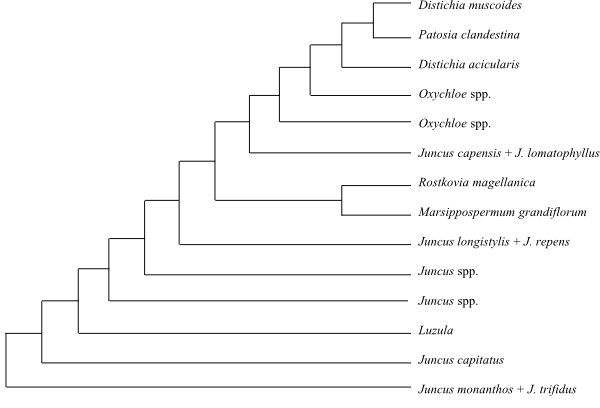 |
Strict consensus tree of Juncaceae based on DNA
sequence data (Drábková 2010). Rostkovia has been added here
as sister to Marsippospermum as revealed in some analyses.
|
Kunth in Abh. Königl. Akad. Wiss. Berlin, Phys.
Abh. 1840: 93. 1842 [’Mayaceae’], nom. cons.
Mayacales Nakai,
Chosakuronbun Mokuroku [Ord. Fam. Trib. Nov]: 214. 20 Jul 1943
Genera/species 1/5
Distribution Central Africa,
southeastern United States, the West Indies, Central and tropical South
America.
Fossils Unknown.
Distribution Bisexual,
perennial herbs. Aquatic or helophytic.
Vegetative anatomy Root hairs
developing from specialized rhizodermal cells. Lateral roots developing from a
zone opposite protophloem poles. Phellogen absent. Cortex aerenchymatous,
consisting of septate air canals. Central stem stele consisting of three to
five collateral vascular bundles in a single cylinder separated by endodermis
from cortex; vascular bundles outside endodermis from that layer. Secondary
lateral growth absent. Vessels present in roots, stem and leaves. Vessel
elements with scalariform (sometimes reticulate?) perforation plates; lateral
pits? Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial
parenchyma? Sieve tube plastids P2c type, with cuneate protein crystals. Nodes?
Uniseriate colleters present. Silica bodies absent. Calciumoxalate raphides
absent.
Trichomes Hairs almost absent
(present in leaf axils); uniseriate glandular hairs present in leaf axils.
Leaves Alternate (spiral),
simple, entire, linear to filiform, with adplicate (flat) ptyxis. Stipules and
leaf sheath absent. Leaf axils with caducous uniseriate glandular hairs
(colleters?). Leaf one-veined. Stomata paracytic, with subsidiary cells with
intersecting oblique cell divisions. Cuticular waxes? Mesophyll without
mucilaginous idioblasts and calciumoxalate crystals. Leaf margin entire. Leaf
apex usually bifid to bidentate.
Inflorescence Flowers axillary
(appearing terminal), solitary, open above water surface. Flowers associated
with a wide adaxial prophyll-like structure (bracteole?).
Flowers Actinomorphic, small.
Hypogyny. Tepals 3+3, free; outer tepals with valvate or subvalvate
aestivation, sepaloid; inner tepals with imbricate aestivation, petaloid,
shortly clawed. Nectary absent. Disc absent.
Androecium Stamens three
(inner staminal whorl absent), antesepalous. Filaments filiform, free from each
other and from tepals. Anthers basifixed, non-versatile, usually
tetrasporangiate (sometimes disporangiate, rarely trisporangiate), extrorse,
poricidal (dehiscing by an apical pore, a short slit or a pore at apex of a
tubular outgrowth). Tapetum secretory, with uninucleate cells. Staminodia
absent.
Pollen grains
Microsporogenesis successive. Pollen grains monosulcate, shed as monads,
bicellular at dispersal. Exine tectate, with columellate infratectum, finely
reticulate, spinulate.
Gynoecium Pistil composed of
three connate antepetalous carpels. Ovary superior, unilocular. Style single,
simple, with stylar canal. Stigma short, capitate or somewhat trilobate, type?
Pistillodium absent.
Ovules Placentation parietal.
Ovules two to c. 30 per ovary, orthotropous, ascending or horizontal, bitegmic,
tenuinucellar to weakly crassinucellar. Micropyle bistomal (endostomal?). Outer
integument ? cell layers thick. Inner integument ? cell layers thick. Hypostase
well developed, tanniniferous. Obturator present. Megasporangial epidermis
basally thickened. Megagametophyte monosporous, Polygonum type.
Endosperm development ab initio nuclear. Endosperm haustoria? Embryogenesis
onagrad.
Fruit A loculicidal
capsule.
Seeds Aril absent. Seeds
operculate. Seed coat tegmic. Testa? Exotegmic cells with U-shaped
lignifications. Operculum (somewhat similar to embryostega in Commelinaceae)
mostly developed from endotegmen. Perisperm not developed. Endosperm copious,
with starch and aleurone, and with a proteinaceous outer layer. Embryo small,
undifferentiated, micropylar, Xyris-Scirpus type. Cotyledon
one, consisting of a closed leaf sheath and an apical haustorium. Radicula
absent. Germination?
Cytology n = 8
DNA
Phytochemistry Very
insufficiently known. Flavonols (quercetin) and phenolic compounds present.
Ellagic acid, proanthocyanidins, and cyanogenic compounds not found.
Use Aquarium plants.
Systematics Mayaca
(5; M. fluviatilis, M. kunthii, M. longipes, M.
sellowiana: southeastern United States, Mexico, Central America, the West
Indies, tropical South America; M. baumii: Central Africa).
Mayaca is sister to [Eriocaulaceae+Xyridaceae]. Mayaca is
sister to Rapateaceae in
Bouchenak-Khelladi & al. (2014). It was sister to [Eriocaulaceae+Xyridaceae] plus the restionoid and
pooid clades in Hochbach & al. (2018).
POACEAE (R. Br.)
Barnhart
|
( Back to Cyperales )
|
Barnhart in Bull. Torrey Bot. Club 22: 7. 15 Jan
1895, nom. cons.
Gramineae Juss., Gen.
Plant.: 28. 4 Aug 1789, nom. cons. et nom. alt.;
Aegilopaceae Martinov, Tekhno-Bot. Slovar: 221. 3 Aug
1820 [‘Egilopeae’, ‘Aegilopeae’];
Agrostidaceae Bercht. et J. Presl, Přir. Rostlin:
264. Jan-Apr 1820 [‘Agrostideae’];
Alopecuraceae Martinov, Tekhno-Bot. Slovar: 19. 3 Aug
1820 [‘Alopecurinae’]; Andropogonaceae
Martinov, Tekhno-Bot. Slovar: 28. 3 Aug 1820 [‘Andropogones’];
Avenaceae Martinov, Tekhno-Bot. Slovar: 60. 3 Aug
1820; Bambusaceae Bercht. et J. Presl, Přir.
Rostlin: 265. Jan-Apr 1820; Bromaceae Bercht. et J.
Presl, Přir. Rostlin: 264. Jan-Apr 1820;
Chloridaceae Bercht. et J. Presl, Přir. Rostlin:
265. Jan-Apr 1820 [‘Chlorideae’];
Hordeaceae Bercht. et J. Presl, Přir. Rostlin: 265.
Jan-Apr 1820; Maydaceae Martinov, Tekhno-Bot Slovar:
387. 1820 [‘Mayseae’], nom. illeg.;
Melicaceae Martinov, Tekhno-Bot. Slovar: 390. 3 Aug
1820 [‘Meliceae’]; Nardaceae Martinov,
Tekhno-Bot. Slovar: 413. 3 Aug 1820 [‘Nardinae’];
Olyraceae Bercht. et J. Presl, Přir. Rostlin: 265.
Jan-Apr 1820 [‘Olyreae’]; Oryzaceae
Bercht. et J. Presl, Přir. Rostlin: 265. Jan-Apr 1820
[‘Oryzeae’]; Panicaceae Bercht. et J.
Presl, Přir. Rostlin: 264. Jan-Apr 1820 [‘Paniceae’];
Saccharaceae Bercht. et J. Presl, Přir. Rostlin:
265. Jan-Apr 1820 [‘Saccharinae’];
Stipaceae Bercht. et J. Presl, Přir. Rostlin: 264.
Jan-Apr 1820; Festucaceae Spreng. in J. Sadler, Fl.
Comit. Pest. 1: 28. 27 Jun 1825; Anthoxanthaceae
Link, Hort. Berol. 1: 271. 1 Oct-27 Nov 1827;
Asperellaceae Link, Hort. Berol. 1: 106, 270. 1
Oct-27 Nov 1827 [’Asperellinae’];
Cenchrinaceae Link, Hort. Berol. 1: 9, 268. 1 Oct-27
Nov 1827 [‘Cenchrinae’]; Chaeturaceae
Link, Hort. Berol. 1: 106, 269. 1 Oct-27 Nov 1827 [‘Chaeturinae’];
Chamagrostidaceae Link, Hort. Berol. 1: 268. 1 Oct-27
Nov 1827 [‘Chamagrostideae’], nom. illeg;
Chondrosaceae Link, Hort. Berol. 1: 269. 1 Oct-27 Nov
1827 [‘Chondrosiaceae’]; Cynodontaceae
Link, Hort. Berol. 1: 51, 269. 1 Oct-27 Nov 1827 [‘Cynodonteae’];
Cynosuraceae Link, Hort. Berol. 1: 271. 1 Oct-27 Nov
1827 [‘Cynosurinae’]; Echinariaceae
Link, Hort. Berol. 1: 271. 1 Oct-27 Nov 1827;
Ehrhartaceae Link, Hort. Berol. 1: 271. 1 Oct-27 Nov
1827 [‘Ehrhartinae’]; Glyceriaceae Link,
Hort. Berol. 1: 271. 1 Oct-27 Nov 1827 [‘Glycerinae’];
Lappaginaceae Link, Hort. Bot. 1: 11, 268. 1 Oct-27
Nov 1827 [‘Lappagineae’], nom. illeg.;
Loliaceae Link, Hort. Berol. 1: 6, 267. 1 Oct-27 Nov
1827; Miliaceae Link, Hort. Berol. 1: 91, 270. 1
Oct-27 Nov 1827; Ophiuraceae Link, Hort. Berol. 1: 3,
268. 1 Oct-27 Nov 1827 [‘Ophiurinae’];
Paspalaceae Link, Hort. Berol. 1: 47, 269. 1 Oct-27
Nov 1827 [‘Paspalinae’, ‘Paspalaceae’];
Phleaceae Link, Hort. Berol. 1: 269. 1 Oct-27 Nov
1827 [‘Phleodeae’]; Spartinaceae Link,
Hort. Berol. 1: 46, 268. 1 Oct-27 Nov 1827;
Triticaceae Link, Hort. Berol. 1: 22, 268. 1 Oct-27
Nov 1827 [’Triticeae’]; Zoysiaceae Link,
Hort. Berol. 1: 8, 268. 1 Oct-27 Nov 1827 [’Zoysinae’];
Holcaceae Link, Hort. Berol. 2: 252. Jul-Dec 1833
[‘Holcoideae’]; Laguraceae Link, Hort.
Berol. 2: 250. Jul-Dec 1833 [‘Lagurinae’];
Phalaridaceae Link, Hort. Berol. 2: 250. Jul-Dec 1833
[‘Phalarideae’]; Tristeginaceae Link,
Hort. Berol. 2: 220. Jul-Dec 1833 [’Tristeginae’], nom. illeg.;
Avenales Bromhead in Edinburgh New Philos. J. 24:
417. Apr 1838; Arundinaceae Döll, Rhein. Fl.: 60.
24-27 Mai 1843; Sesleriaceae Döll, Rhein. Fl.: 60.
24-27 Mai 1843; Coleanthaceae (Link) Pfeiffer,
Nomencl. Bot. 1(2): 818. 3 Oct 1873 [‘Coleanthinae’];
Zeaceae A. Kern., Pflanzenleben 2: 651. 8 Aug 1891;
Arundinellaceae (Stapf) Herter in Revista Sudamer.
Bot. 6: 136. Jun 1940; Eragrostidaceae (Stapf) Herter
in Revista Sudamer. Bot. 6: 145. Jun 1940;
Lepturaceae (Dumort.) Herter in Revista Sudamer. Bot.
6: 147. Jun 1940; Pappophoraceae (Kunth) Herter in
Revista Sudamer. Bot. 6: 145. 1940; Pharaceae (Stapf)
Herter in Revista Sudamer. Bot. 6: 139. Jun 1940;
Anomochloaceae Nakai, Chosakuronbun Mokuroku [Ord.
Fam. Trib. Nov.]: 222. 20 Jul 1943; Parianaceae
Nakai, Chosakuronbun Mokuroku [Ord. Fam. Trib. Nov.]: 222. 20 Jul 1943;
Streptochaetaceae Nakai, Chosakuronbun Mokuroku [Ord.
Fam. Trib. Nov]: 222. 20 Jul 1943
Genera/species
590–596/9.060–9.170
Distribution Cosmopolitan
including polar areas.
Fossils Well preserved fossil
grass phytoliths have been found in a dinosaur coprolith from the Maastrichtian
Deccan Intertrappean Beds of India, and phytoliths (sometimes associated with
epidermal fragments) are known from Cenozoic layers in many places. Pollen
grains resembling those in Poaceae have been found in
Maastrichtian layers, whereas leaves and flowers are known from the Early
Eocene onwards. Paleocene grass pollen have been recorded from Brazil and
tropical West Africa. Inflorescence parts have been detected in Oligocene
layers in Germany and a spikelet was found in the Early Eocene of Tennessee.
Habit Usually bisexual
(sometimes monoecious, andromonoecious, gynomonocious, polygamomonoecious,
dioecious, androdioecious, or gynodioecious), usually perennial, biennial or
annual herbs (sometimes woody, up to c. 40 m tall). Graminids. Sometimes
helophytes, rarely aquatic. Numerous species are xerophytes. Culm terete to
elliptic in cross-section, usually with hollow (fistulose; sometimes medullated
solid) internodes and solid swollen nodes.
Vegetative anatomy
Adventitious roots developing directly from hypocotyl. Endophytic fungi (i.a.
Class I endophytes, Clavicipitaceae) frequent. Vesicular-arbuscular
mycorrhiza often present. Roots producing siderophores that chelate ferric ions
subsequently taken up by plant. Lateral roots developing from zone opposite
protophloem or opposite protoxylem poles. Phellogen absent. Chlorenchyma
without peg cells. Primary vascular tissue two or more cylinders of vascular
bundles or scattered bundles. Secondary lateral growth absent. Vessels present
in roots, stem and leaves. Vessel elements with scalariform or simple
perforation plates; lateral pits? Imperforate tracheary xylem elements
tracheids. Wood rays absent. Axial parenchyma? Sieve tube plastids
P2cc type (with cuneate protein crystals) or P2ccl type
(with cuneate and several additional loosely packed protein crystals); P
proteins absent from sieve elements. Nodes often swollen, with pulvinus;
sclerenchyma usually absent; vascular bundle extending into leaf, with trace
two nodes below leaf and connecting through next node above; nodes containing
diffuse vascular bundles, transverse vascular bundles and expanded vascular
bundles. Secretory cavities absent. Arm cells and fusoid cells present or
absent. Special short idioblasts with large single silica bodies (phytoliths)
of various shape (cuboidal, rounded, quadratic, cross- or saddle-shaped, etc.).
Calciumoxalate absent or scarce. C4 and CAM physiologies frequently
present (at least 50% of species; evolved several times).
Trichomes Hairs unicellular or
multicellular, uniseriate, or absent; glandular hairs sometimes present;
usually bicellular (sometimes multicellular) microhairs present in most Poaceae.
Leaves Alternate (usually
distichous; in Micraira tristichous), simple, entire, usually linear,
with supervolute(-plicate), conduplicate (involute, revolute, convolute, or
plicate) ptyxis (sometimes differentiated into pseudopetiole and pseudolamina).
Stipules absent; leaf sheath well developed, usually open (rarely closed),
long, usually with membranous adaxial distal ligule (sometimes modified into
hairs or absent); sometimes with abaxial contraligule. Venation usually
parallelodromous, often with transverse veins (in Leptaspis and
Pharus pinnate-parallel); midvein usually distinct. Stomata paracytic,
usually with dumbbell-shaped Poaceae type guard cells (not in,
e.g., Neostapfia) and conical to dome-shaped subsidiary cells.
Cuticular wax crystalloids as longitudinally aggregated or non-orientated
rodlets and platelets, chemically dominated by polymeric aldehydes, or as
tubuli, chemically characterized by hydroxy-β-diketones (sometimes
Strelitzia type). Central mesophyll with large uncoloured fusoid
cells. Epidermis usually with rows of long cells alternating with short cells
containing silica (with longitudinal axis parallel to lamina); short ones
usually containing silica crystals. Most Poaceae forming pairs of silica-cork
short cells. Mucilaginous idioblasts absent. Secretory cavities usually absent.
Cystoliths absent. Leaf margin usually entire (sometimes serrate). Epidermis
usually with microhairs. Extrafloral nectaries rarely present.
Inflorescence Terminal,
compound panicle, or simple or branched spike-, head-, spadix- or raceme-like
(sometimes surrounded by spatha), consisting of spikelets subtended by one
basal pair of bracts, glumes (glumae), without axillary flowers; above these
one, several or numerous flowers each subtended and often surrounded by a bract
(or outer tepal?), lemma, and opposite this a two-keeled and usually two-veined
palea (possibly corresponding to a floral prophyll [bracteole] or two connate
outer tepals). Glumes and/or lemmas (rarely paleas) often provided with long
and narrow projections, awns. Lateral branches often bearing distinct adaxial
swellings at base. Spikelet bracts usually suppressed. Some genera have
modified sterile flowers or sterile branchlets serving as dispersal
mechanisms.
Flowers Zygomorphic (due to
reduction), small. Hypogyny. Each flower with usually two (rarely one, three or
more than three) very small scale-like lodiculae, probably corresponding to
reduced tepals of inner whorl (when more than three, then supernumerary
lodiculae possibly corresponding to reduced modified sterile stamens; sometimes
connate; often absent in female flowers). Nectary absent. Disc absent.
Androecium Stamens usually
three (often 3+3, rarely one, two, four, five, or more than six, in
Ochlandra up to c. 120 [to c. 170]). Filaments long, filiform, usually
free from each other, free from lodiculae. Anthers dorsifixed, centrifixed or
subbasifixed, versatile, tetrasporangiate, introrse, latrorse or extrorse,
longicidal (dehiscing by longitudinal slits) or poricidal (dehiscing by apical
pores or short slits). Tapetum secretory, with uni-, bi- or multinucleate
cells. Staminodia absent.
Pollen grains
Microsporogenesis usually successive (in Streptochaeta simultaneous).
Pollen grains graminoid, usually monoporate to monoulcerate, operculate, shed
as monads, usually tricellular at dispersal. Exine tectate, with columellate
infratectum, smooth, echinulate, spinulate, or finely scabrate, with thin
intraexinous channels (not scrobiculate).
Gynoecium Pistil composed of
probably three connate carpels (three vascular bundles reaching stigma), with
abaxial carpel fertile leading to pseudomonomery. Ovary superior, unilocular
(pseudomonomerous). Style single, simple, solid or hollow, or stylodia two
(rarely three; third style often represented by a reduced basal tissue), apical
or lateral, free or more or less connate. Stigma single and usually bilobate
(rarely uni-, tri- or quadrilobate), or stigmas two (rarely three), with often
plumose lobes, papillate or non-papillate, Dry type, usually with scattered
discontinuous receptive surfaces on multiseriate branches (sometimes on
distinct zones) (pollen tubes growing between elongate transfer cells in
multicellular stigmatic lobes). Pistillodium absent.
Ovules Placentation basal to
parietal. Ovule one per ovary, usually hemicampylotropous (sometimes
amphitropous or campylotropous, rarely orthotropous, pendulous), usually
bitegmic (rarely unitegmic or almost ategmic), tenuinucellar or
pseudocrassinucellar. Funicle short. Micropyle usually endostomal (sometimes
bistomal). Outer integument two? cell layers thick, usually degenerating
following fertilization. Inner integument at least two cell layers thick.
Parietal cell not formed (parietal tissue absent). Megasporangium multilayered.
Nucellar cap usually (not in Pooideae) formed through periclinal cell
divisions in megasporangial epidermis. Megagametophyte monosporous,
Polygonum type (Poaceae variation). Synergids
sometimes haustorial (in ’Cortaderia’ and other
Danthonioideae). Antipodal cells binucleate, more than three, often
proliferating (rarely up to c. 300 cells). Endosperm development ab initio
nuclear. Endosperm haustoria? Embryogenesis asterad (special variation).
Polyembryony frequent.
Fruit Usually a nut-like
caryopsis with adaxial end of seed coat usually fused with endocarp (sometimes
an achene with membranous or gelatinous pericarp not adherent to seed coat;
rarely a berry or a drupe).
Seeds Aril absent. Seed-coat
usually tegmic. Testa usually not persistent. Tegmen often crushed. Perisperm
not or only slightly developed. Endosperm copious, starchy, usually with
proteinaceous tissue (rarely absent); peripheral cell layers of mature
endosperm showing meristematic activity, with aleurone and sometimes oils;
starch grains simple or compound. Embryo small to large, straight or curved,
lateral-abaxial (sometimes inserted adjacent to seed coat on one side), well
differentiated, with special and unique shape, usually almost enclosed by
cylindrical coleoptile, without chlorophyll. Cotyledon one, lateral, not
photosynthesizing. Haustorial cotyledon hyperphyll compact, not assimilating,
modified into absorptive scutellum appressed to endosperm. Hypocotyl internode
absent. Collar modified to epiblast, cotyledon ligule, often stout, or absent
(alternatively, epiblast representing projection from first lateral root).
Plumule terminal. Coleoptile first leaf, modified into substrate penetrating
plumular envelope, with or without lamina. Alternatively, coleoptile and
scutellum being different parts of cotyledon. Mesocotyl present as part of axis
between coleoptile and scutellum, or absent. Radicula surrounded by coleorrhiza
and scutellum, or absent. Germination cryptocotylar.
Cytology n = 11, 18
(Anomochlooideae); n = (2) (4) 5–12 (14) (other Poaceae) (n = 2–90) x = 5 or 11? –
Polyploidy and aneuploidy frequently occurring. Agamospermy present in many
genera. – ADP-glucose pyrophosphorylase present in cytosol.
DNA The plastid genome has one
inversion of 28 kb, a second inversion of 6,4 kb, and a third inversion of
0,2–2 kb, and only 17 introns. The plastid gene accD, ORF244 and the
introns 1 and 2 in clpP are often absent (at least in
Bambusa, Oryza and Zea). ORF2280 is absent in many
Poaceae. The intron is absent
from the plastid gene rpoC1 (at least in Oryza and
Zea). An insertion is present in the plastid gene rpoC2. A
trnT inversion is present in the single copy region of the plastid
genome. The IR region of the plastid DNA is expanded. A translocation has taken
place in the plastid genome, thus creating the pseudogene ψrpl23.
Duplication of the nuclear genes
AP1/FUL (FUL1 and FUL2) has taken place in
the crown clade (this duplication may be involved in the evolution of the
spikelet): one copy coding for degradation of starch into fermentable sugars in
endosperm, the second copy with wider expression. Vast gene duplications have
taken place in the API, AG and SEP gene families,
although not in the AP3 evolutionary line. A duplication of the gene
LOFSEP may have taken place in Poaceae (Malcomber & Kellogg
2005), the entire genome being duplicated in a clade comprising at least
Zea, Oryza, Hordeum, and Sorghum. Loss of
nuclear genes ycf1 and ycf2, the c. 200 bp ψycf1
being left in the genome. The mitochondrial gene rps14 has been
transferred to the nuclear genome, the pseudogene ψrps14 being left
in mtDNA.
Phytochemistry Flavonol
sulphates, flavone sulphates, flavone-5- and C-glycosides, luteolin,
tricin (tricetin 3’,5’-dimethyl ether, very frequent), 3-deoxyanthocyanins,
free triterpenes, isoquinoline alkaloids, tryptophane- or tyramine-derived
alkaloids (gramine, hordenine, tyramine, etc.), pyrrolizidine alkaloids
(1-aminopyrrolizidine derivatives; loline, lolinine, norline, etc.), indole
alkaloids, tyrosine-derived cyanogenic compounds, and chelidonic acid present.
Flavonols (kaempferol, quercetin), cyanidin, and saponins rare. Ellagic acid
not found. Primary cell wall with abundant arabinoxylans. Xyloglucans without
fucose. Lignins with p-coumarylic alcohol (lignins acylated with
p-coumarates), and coniferyl and sinapyl monomers.
Hemicellulose/pectin composition different from other seed plants. Aluminium
accumulation occurring in some species.
Use Ornamental plants, starch
sources (cereals), sugar (Saccharum), beer and other alcohol beverages
(Hordeum, Triticum, Saccharum, etc.), vegetables,
spices and perfumes (Cymbopogon), necklaces (Coix), forage
plants, thatching, basketry, textiles, carpets, brushes, bamboo species for
timber, carpentries, tools, ornaments, weapons, musical instruments,
vegetables, paper, etc.
Systematics Poaceae are sister-group either to
Ecdeiocoleaceae or
(perhaps more probable) to the clade [Joinvilleaceae+Ecdeiocoleaceae].
Multicellular microhairs are present in Joinvilleaceae and many Poaceae (also in Ecdeiocoleaceae?). - Poaceae incertae sedis:
Schmidiella (1; S. maxwellii; Laos).
The system below largely follows: Soreng RJ,
Davidse G, Peterson PM, Zuloaga FO, Judziewicz EJ, Filgueiras TS, Morrone O,
Romaschenko. 2012 (and continuously revised). A world-wide phylogenetic
classification of Poaceae
(Gramineae). Published online. Another important source is Peterson
& al. (2011).The potential synapomorphies of the clades are mainly
according to Stevens (2001 onwards).
A possible topology is the following:
[Anomochloa+[Streptochaeta+[Pharoideae+[Puelioideae+[[Aristidoideae+[Panicoideae+Arundinoideae]]]+[Oryzoideae+[Bambusoideae+Pooideae]]]]]]]
Sometimes, the following two alternative
topologies of the “crown clade” are recovered:
[[Pooideae+[Bambusoideae+Oryzoideae]]+[Micrairoideae+[Panicoideae+[[Aristidoideae+Danthonioideae]+[Arundinoideae+Chloridoideae]]]]]
or
[Bambusoideae+[Oryzoideae+[Pooideae+[Panicoideae+[[Aristidoideae+Danthonioideae]+[Arundinoideae+Chloridoideae]]]]]]
Anomochlooideae
Pilg. ex Potzdal in Willdenowia 1: 772. 1 Mar 1957
[‘Anomochloideae’] (paraphyletic?)
2/4. Anomochloa (1; A.
marantoidea; Bahia in southeastern Brazil); Streptochaeta (3;
S. angustifolia [extinct?], S. sodiroana, S.
spicata; Mexico, Central America, the West Indies, tropical South
America). – Central America to tropical South America, forests. Pseudopetiole
with apical (and sometimes basal) pulvinus. Ligule modified into fringe of
hairs. Arm cells absent in Streptochaeta. Inflorescence branches
cymose, with two ’bracts’ along each branch unit and two additional
’bracts’ subtending each flower (Anomochloa), or flowers spirally
arranged along racemose axis, with several spiral ’bracts’ subtending each
flower (Streptochaeta). Tepals 2(–3)+3 or absent.
Anomochloa with four stamens and one carpel. Filaments connate at
base. Anthers subbasifixed or centrifixed (Anomochloa), latrorse.
Anther wall development Reduced type, without endothecium thickenings.
Microsporogenesis simultaneous. Stigma in Streptochaeta not plumose.
Antipodal cells not proliferating (Streptochaeta). Epiblasts present
in Anomochloa, absent in Streptochaeta. First leaf of
seedling without pseudolamina. n = 11, 18. Plastid gene rpoC2
insertion with 21 bp subrepeats. A c. 850 bp pseudogene ψycf1 present
in nuclear genome in Anomochloa. – Streptochaeta and
Anomochloa are sister-groups in some analyses. In others,
Streptochaeta is recovered as successive sister to the remaining
Poaceae (except
Anomochloa). The bicarinate palea in Streptochaeta is
sometimes interpreted as originated from two adaxial outer tepals. Likewise,
the lodiculae have been interpreted as three modified inner tepals. In a way,
morphology indicates that Streptochaeta may be sister to all other
Poaceae (including
Anomochloa).
[Pharoideae+[Puelioideae+[SAPA+EBP]]]
("The spikelet clade")
Leaves with membranous ligules,
sometimes also ciliate. Inflorescence consisting of laterally compressed,
racemose, pedunculate spikelets. Spikelet units with two sterile basal glumes
(spikelet bract + prophyll). Flowers distichous, each with lemma and palea
(possibly equalling one bract and two connate adaxial outer tepals), inverted.
Lodiculae two or three (possibly equalling inner tepal whorl; median lodicula
adaxial). n = 12. 3’ end of the plastid gene matK with 1 bp
deletion. Plastid gene rpoC1 absent (lost). Plastid gene
rpoC2 insertion with 39 bp subrepeats.
Pharoideae L. G. Clark
et Judz. in Taxon 45: 643. 1996
3/13. Leptaspis (3; L.
angustifolia, L. banksia, L. zeylanica; tropical Africa,
Madagascar, the Comoros, tropical Asia from India and Sri Lanka to Queensland
and Melanesia), Scrotochloa (2; S. urceolata, S.
tararaensis; India, Sri Lanka, Burma, Southeast Asia, Malesia to New
Guinea, the Bismarck Archipelago, Solomon Islands, Queensland), Pharus
(7; P. ecuadoricus, P. lappulaceus, P. latifolius,
P. mezii, P. parvifolius, P. virescens, P.
vittatus; Florida, southern Mexico, Central America, the West Indies,
tropical South America). – Pantropical, forests. Microhairs absent. Leaves
resupinate. Lateral veins oblique. Spikelets one-flowered. Stamens 3+3. Anthers
centrifixed, latrorse. Anther wall development in Pharus Reduced type,
without endothecium thickenings. Style hollow (Pharus) or solid.
Micropyle bistomal (Pharus). Antipodal cells not proliferating
(Pharus). Coleoptile with pseudolamina. Scutellus tail absent in
Pharus.
[Puelioideae+[SAPA+IEBP]] ("The
bistigmatic clade")
Phytoliths saddle-shaped. Spikelets
disarticulating above glumes. Anthers usually versatile. Stigmas two, with
stigmatic branching in two orders. Plastid gene ndhF with 15 bp
insertion.
Puelioideae (Soderstr.
et R. P. Ellis) L. G. Clark, M. Kobay., S. Mathews, Spangler et E. A. Kellogg
in Syst. Bot. 25: 184. Apr-Jun 2000
2/11. Guaduella (6; G.
densiflora, G. dichroa, G. humilis, G.
macrostachys, G. marantifolia, G. oblonga; tropical West
and Central Africa), Puelia (5; P. ciliata, P.
coriacea, P. dewevrei, P. olyriformis, P.
schumanniana; tropical West and Central Africa). – Tropical West and
Central Africa. Flowers bisexual or unisexual, polygamous. Perennial with
sympodial rhizome. Kranz’ anatomy absent. Arm cells poorly developed. Fusoid
cells well developed. Microhairs multicellular uniseriate (Guaduella)
or absent (Puelia). Pseudopetiole present. Contraligule present
(Puelia) or absent (Guaduella). Lodiculae three. Stamens 3+3.
Filaments free (Guaduella) or connate (Puelia). Stigmas two
or three. Embryo small. Seedling leaves of unknown shape. n = 12
(Puelia).
[[Aristidoideae+[Panicoideae+[Arundinoideae+[Danthonioideae+Chloridoidae]]]]+[Ehrhartoideae+[Bambusoideae+Pooideae]]]
Arm cells absent. Fusoid cells absent.
No differentiation of leaf into pseudopetiole and pseudolamina. Leaf transverse
veins absent. Lodiculae usually two. Stamens usually three. Stylodia two, not
connate. Antipodal cells usually proliferating. x = 12. Benzoxazinoids (a type
of defense molecules) sometimes present. Genome duplication present. Plastid
gene ndhF with 15 bp insertion. Disease resistance through nuclear
gene Hm1.
[Aristidoideae+[Panicoideae+[Arundinoideae+[Danthonioideae+Chloridoideae]]]]
Phytoliths axial-bilobate or
axial-polylobate.
Aristidoideae Caro in
Dominuezia 4: 16. Feb 1982
3/350–360. Aristida
(c 290; tropical and subtropical regions on both hemispheres),
Sartidia (6; S. angolensis, S. dewinteri, S.
isaloensis, S. jucunda, S. perrieri, S.
vanderystii; Central to southern Africa, Madagascar),
Stipagrostis (c 55; arid and semi-arid subtropical regions in the
Mediterranean, Africa and Asia to China). – Tropical and subtropical regions.
Often with C4 photosynthesis. Ligule lined with hairs. Spikelet
elongated-cylindrical, disarticulating above glume. Lemma awn trifid, or (one
or) three awns, with basal columella. Germination flap present. n = 11, 12.
Insertion of 6 bp absent from 3’ end of plastid gene matK. –
Aristidoideae are possibly sister-group to the clade
[Panicoideae+Arundinoideae+[Danthonioideae+
Chloridoideae]]. However, Aristidoideae are sometimes
revealed as sister-group to Danthonioideae, and the analyses by
Peterson & al. (2011) place Aristideae as sister to the clade [Hakonechloa+[Danthonieae+[Chlorideae+Centropodieae]]].
[Panicoideae+[Arundinoideae+[Danthonioideae+Chloridoideae]]]
(The "PACC clade", the "PACMAD clade", or the "PACCMAD" clade)
C4 photosynthesis present in
approximately 50% of the species. Phytoliths dumbbell-shaped. Ligule often
consisting of hairs. Hilum non-linear. Mesocotyl internode elongated. Epiblast
absent. Plastid gene ndhF extended from short single copy region (SSC)
into inverted repeat (IR). 3’ end of the plastid gene matK usually
with 6 bp insertion.
Panicoideae Link,
Hort. Berol. 1: 202. 1 Oct-27 Nov 1827 [‘Paniceae’]
217/3.075–3.105. Tropical, subtropical
and temperate regions. Culm usually solid. Fusoid cells often absent.
C4 photosynthesis frequent. Elongate microhairs usually present,
Panicoid type (with slender thin-walled cap cells). Stomata with triangular or
dome-shaped subsidiary cells. Spikelet development basipetal. Spikelet dorsally
compressed, without rachilla, two-flowered, lower flower male or sterile,
female floret one. Spikelet shed as one-seeded unit by disarticulation below
glumes. Style sometimes present. Hilum non-linear. Starch grains simple. Embryo
with overlapping leaf margins. Epiblast usually present in ’centothecoids’.
Germination flap present. n = 5, 7, 9, 10 (11–14). Plastid gene
rpl16 intron with 5 bp insertion. Deletion of 4 bp in rpl16
intron in "centothecoids" Pseudogene ψrps14 absent (lost). –
Panicoideae incertae sedis: Alloeochaete (6; A.
andongensis, A. geniculata, A. gracillima, A.
namuliensis, A. oreogena, A. ulugurensis; southern
tropical Africa), Dichaetaria (1; D. wightii; India, Sri Lanka).
Gynerieae Sánchez-Ken
et L. G. Clark in Novon 11: 350. 21 Sep 2001
1/1. Gynerium (1; G.
sagittatum; Central America, South America south to Paraguay). –
Gynerieae may be sister-group to the clade
[[Thysanolaeneae+Centotheceae+Cyperochloeae]+[Chasmanthieae+Zeugiteae]].
According to Morrone & al. (2012), they are instead sister to the clade
[Paniceae+[Paspaleae+[Arundinelleae+Andropogoneae]]].
[[Thysanolaeneae+Centotheceae+Cyperochloeae]+[Chasmanthieae+Zeugiteae]]
[Thysanolaeneae+Centotheceae+Cyperochloeae]
Thysanolaeneae C. E.
Hubb. in J. Hutchinson, Fam. Fl. Plants 2: 222. 20 Jul 1934
1/1. Thysanolaena (1; T.
latifolia; southern and southeastern Asia, southern China). –
Thysanolaena may be sister to the clade
[Centotheceae+Cyperochloeae] or should be included in
Centotheceae.
Centotheceae Ridl.,
Mat. Fl. Malay. Pen. 3: 122. 1907
2/4. Centotheca (3; C.
lappacea, C. philippinensis, C. uniflora; tropical West
and Central Africa, Madagascar, China, Southeast Asia, Malesia to New Guinea,
islands in the Pacific), Megastachya (1; M. mucronata;
tropical and southern Africa, Madagascar). – Tropical regions in the Old
World. – Centotheceae may be sister to Cyperochloeae.
Cyperochloeae L.
Watson et Dallwitz ex Sánchez-Ken et L. G. Clark in Amer. J. Bot. 97: 1744. 1
Oct 2010
2/2. Cyperochloa (1; C.
hirsuta; southwestern Western Australia), Spartochloa (1; S.
scirpoidea; southwestern Western Australia). – Southwestern Western
Australia. – Cyperochloeae should perhaps be included in
Centotheceae.
[Chasmanthieae+Zeugiteae]
Chasmanthieae W. V.
Br. et B. N. Sm. ex Sánchez-Ken et L. G. Clark in Amer. J. Bot. 97: 1744. 1
Oct 2010
1/6. Chasmanthium
(6; C. curvifolium, C. latifolium, C. laxum, C.
nitidum, C. ornithorhynchum: southern Canada, eastern United
States, northern Mexico; C. gossweileri: Tanzania to Angola). –
Eastern and southern tropical Africa, eastern North America. –
Chasmanthieae are sister-group to Zeugiteae.
Zeugiteae Sánchez-Ken
et L. G. Clark in Amer. J. Bot. 97: 1744. 1 Oct 2010
4/18. Chevalierella (1; C.
dewildemanii; Congo), Lophatherum (2; L. gracile, L.
sinense; India, Sri Lanka, the Himalayas, China, the Korean Peninsula,
Japan, Taiwan, Southeast Asia, Malesia to New Guinea and Queensland),
Orthoclada (2; O. africana: southeastern tropical Africa;
O. laxa: southern Mexico, Central America, tropical South America),
Zeugites (13; Mexico, Central America, the West Indies, tropical South
America). – Pantropical.
[[Steyermarkochloeae+Tristachyideae]+[Andropogonodae+Panicodae]]
[Steyermarkochloeae+Tristachyideae]
Steyermarkochloeae
Davidse et R. P. Ellis in Ann. Missouri Bot. Gard. 71: 994. 1985
2/2. Arundoclaytonia (1; A.
dissimilis; Amazonas, Brazil), Steyermarkochloa (1; S.
angustifolia; Colombia, Venezuela). – Tropical South America. –
Steyermarkochloeae are sister to Tristachyideae, according to
Morrone & al. (2012).
Tristachyideae
Sánchez-Ken et L. G. Clark in Amer. J. Bot. 97: 1743. 1 Oct 2010
5/c 70. Danthoniopsis (16;
Africa, the Arabian Peninsula to Pakistan), Gilgiochloa (1; G.
indurata; tropical Africa), Loudetia (c 25; tropical and southern
Africa, Madagascar, tropical South America), Trichopteryx (5; T.
dregeana, T. elegantula, T. fruticulosa, T.
marungensis, T. stolziana; tropical and southern Africa,
Madagascar), Tristachya (22; tropical and southern Africa, Madagascar,
tropical South America). – Africa and Madagascar to Pakistan, tropical South
America. – Tristachyideae may be sister to
Steyermarkochloeae.
[Andropogonodae+Panicodae]
Lecomtelleae Pilg. ex
Potztal in Willdenowia 1: 771. 1957
1/1. Lecomtella (1; L.
madagascariensis; the Andringitra mountain range in southern Madagascar).
– The position of Lecomtella madagascariensis is uncertain. It is
sister-group to [Sacchareae+Paspaleae] or
[Sacchareae+Paniceae] or
[Paniceae+Paspaleae] or
[Paniceae+[Paspaleae+Andropogoneae]] or to
Paniceae (Besnard & al. 2013).
Andropogonodae L. Liu
in Acta Phytotaxon. Sin. 18: 325. Aug 1980 (‘Andropogodae’]
Paspaleae J. Presl in
C. B. Presl, Reliq. Haenk. 1: 208. Jan-Jun 1830 [’Paspalineae’]
38/595–600. Reynaudia (1;
R. filiformis; Cuba, Hispaniola). –
Paspalinae Griseb. in C. F. von Ledebour, Flor. Ross.
4: 466. Jun 1853 [’Paspaleae’]. Aakia (1; A.
tuerckheimii; southern Mexico, Central America), Acostia (1;
A. gracilis; Ecuador), Anthaenantiopsis (5; A.
fiebrigii, A. perforata, A. racemosa, A.
rojasiana, A. trachystachya; tropical and subtropical South
America), Axonopus (c 70; southern United States, Mexico, Central
America, the West Indies, tropical South America, one species, A.
compressus, in Africa, and one species, A. foliacea, in
Mozambique), Gerritea (1; G. pseudopetiolata; Bolivia),
Hopia (1; H. obtusa; southwestern United States, northwestern
Mexico), ‘Ichnanthus‘ pro parte (2), Hildaea (6; H.
breviscrobs, H. nemorosa, H. pallens, H.
parvispiculata, H. ruprechtii, H. tenuis; pantropical),
Streptostachys (6; S. acuminata, S. asperifolia,
S. macrantha, S. ramosa, S. rigidifolia, S.
robusta; Mexico, Central America, the West Indies, tropical South
America), Renvoizea (10; Brazil), Oedochloa (9; tropical
America), Ocellochloa (12; tropical America), Echinolaena (2;
E. gracilis, E. inflexa; southern Mexico, Central America,
tropical South America), Ichnanthus (c 30; Mexico, Central America,
the West Indies, tropical South America), Lecomtella (1; L.
madagascariensis; Madagascar), Osvaldoa (1; O. valida;
Brazil, Uruguay, Argentina), Paspalum (c 330; tropical and subtropical
regions on both hemispheres, with their largest diversity in tropical America).
– Otachyriinae Butzin in Willdenowia 6: 182. 23 Nov
1970. Anthenantia (4; A. lanata, A. rufa, A.
texana, A. villosa; southeastern United States), Rugoloa
(3; R. hylaeica, R. pilosa, R. polygonata; Jamaica,
Brazil), Hymenachne (4; H. amplexicaulis, H. aurita,
H. felliana, H. pseudointerrupta; southern Mexico, Central
America, the West Indies, tropical South America), ’Hymenachne’
pro parte (10; H. assamica, H. donacifolia, H.
grumosa, H. patens, H. pernambucensis, H.
wombaliensis, ’Panicum’ harleyi, P.
hemitomon, P. leptachne, P. stagnatile; tropical Africa,
tropical Asia, Central America, the West Indies, tropical South America),
Plagiantha (1; P. tenella; eastern Brazil),
Otachyrium (15; southern United States to tropical South America). –
Arthropogoninae Butzin in Willdenowia 6: 515. Nov
1972. Apochloa (15; tropical South America), Arthropogon (7;
A. chapadense, A. filifolius, A. piptostachyus,
A. scaber, A. sorengii, A. villosus, A.
xerachne; the Greater Antilles, Colombia, Brazil, Bolivia),
Canastra (2; C. aristella, C. lanceolata; Brazil),
Coleataenia (7; C. anceps, C. longifolia, C.
rigidula, C. scabrida, C. stenodes, C.
stipitata, C. tenera; eastern United States, Mexico, Central
America, tropical South America), Cyphonanthus (1; C.
discrepans; Central America, the West Indies, tropical South America),
Homolepis (5; H. aturensis, H. glutinosa, H.
isocalycia, H. longispicula, H. villaricensis; Mexico,
Central America, the West Indies, tropical South America),
Keratochlaena (1; K. rigidifolia; Guyana, northern Brazil),
Mesosetum (c 25; Mexico, Central America, the West Indies, tropical
South America), Oncorachis (2; O. macrantha, O.
ramosa; Brazil), Oplismenopsis (1; O. najada; Uruguay,
Argentina), Phanopyrum (1; P. gymnocarpon; southeastern
United States), Stephostachys (1; S. mertensii; Mexico,
Central America, tropical South America), Tatianyx (1; T.
arnacites; Brazil), Triscenia (1; T. ovina; Cuba). –
Pantropical, with their largest diversity in tropical America.
[Andropogoneae+Arundinelleae]
Andropogoneae
Dumort., Observ. Gramin. Belg.: 84. Jul-Sep 1824
[‘Andropogineae‘]
82/1.110–1.130. Chrysopogon
(45–50; warmer regions on both hemispheres, especially in tropical region in
the Old World), Thelepogon (1; T. elegans; Ethiopia to
Namibia and east to India). – Arthraxoninae Benth.
in J. Linn. Soc. London, Bot. 19: 67. 1881 [‘Arthraxeae‘].
Arthraxon (27; tropical and subtropical Africa, Madagascar, islands in
the Indan Ocean, India and the Himalayas to southern China and Southeast Asia,
Malesia to New Guinea and northern Australia). –
Tripsacinae Dumort., Anal. Fam. Plant.: 64. 1829
[’Tripsaceae’]. Elionurus (c 15; Macaronesia, tropical
and subtropical Africa, Madagascar, southwestern Asia to Sind in northwestern
India, New Guinea, Solomon Islands, Australia, southern United States, Mexico,
Central America, the West Indies, tropical South America), Oxyrhachis
(1; O. gracillima; tropical East and southern Africa, Madagascar),
Rhytachne (12; tropical and southern Africa, Madagascar, tropical
South America), Tripsacum (16; southern United States, Mexico, Central
America, South America to Paraguay), Urelytrum (7; U.
agropyroides, U. annuum, U. auriculatum, U.
digitatum, U. giganteum, U. henrardii, U.
muricatum; tropical and southern Africa, Madagascar), Vossia (1;
V. cuspidata; tropical Africa to Namibia and northern Botswana,
eastern India to Burma), Zea (6; Z. diploperennis, Z.
luxurians, Z. mays, Z. mexicana, Z.
nicaraguensis, Z. perennis; Mexico, Central America). –
Chionachninae Clayton in Kew Bull. 35: 813. 1981.
Chionachne (12; India to Burma, southern China and Southeast Asia,
Malesia to New Guinea, Solomon Islands and northern Australia; C.
cookei (Trilobachne cookei): the West Indies). –
Coicinae Reichb. ex Clayton et Renvoize in Kew Bull.,
Addit. Ser. 13: 372. 1986. Coix (4; C. aquatica, C.
gasteenii, C. lacryma-jobi, C. puellarum; India to
southern China and Southeast Asia, northern Queensland). –
Rottboelliinae J. Presl in C. Presl, Reliq. Haenk. 1:
329. Jan-Jun 1830 ['Rottboelliaceae']. Chasmopodium (3;
C. afzelii, C. caudatum, C. purpurascens; tropical
West and Central Africa), Eremochloa (14; India, Sri Lanka, Southeast
Asia, Malesia to New Guinea and tropical Australia), Hemarthria (12;
tropical and subtropical regions in the Old World), Ophiuros (4;
O. bombaiensis, O. exaltatus, O. megaphyllus, O.
papillosus; the Horn of Africa, India and eastern Himalayas to Burma,
southern China and Southeast Asia, the Ryukyu Islands, Malesia to New Guinea
and Australia), Mnesithea (6; M. annua, M. formosa,
M. laevis, M. mollicoma, M. pilosa, M.
veldkampii; the Indian Subcontinent, Sri Lanka, China, Japan, Taiwan,
Southeast Asia, Malesia to New Guinea and tropical Australia, islands in the
Pacific), Hackelochloa (2; H. granularis, H.
porifera; sub-Saharan Africa, the Arabian Peninsula, India, eastern
Himalayas to Japan and Southeast Asia, Malesia to New Guinea, Micronesia),
’Phacelurus’ (10; tropical and subtropical Africa, Southwest and
East Asia, tropical Asia to Indochina and Japan, one species, P.
digitatus, on the Balkan Peninsula to Syria; polyphyletic),
Rottboellia (6; R. cochinchinensis, R. coelorachis,
R. goalparensis, R. laevispica, R. paradoxa, R.
purpurascens; tropical Africa, Madagascar, tropical and subtropical Asia
and Australia, Melanesia), Glyphochloa (12; central and southwestern
India), Heteropholis (3; H. sulcata: Central and tropical
East Africa; H. benoistii: Madagascar; H. nigrescens: Sri
Lanka), Lasiurus (1; L. scindicus; Morocco and Mali to the
Middle East and northeastern India), Loxodera (5; L. bovonei,
L. caesitosa, L. ledermannii, L. rhytachnoides,
L. strigosa; tropical Africa), Thaumastochloa (9; New Guinea,
Australia). – Ischaeminae J. Presl in C. Presl,
Reliq. Haenk. 1: 328. Jan-Jun 1830 [’Ischaemeae’].
Andropterum (1; A. stolzii; tropical Africa),
Dimeria (c 60; Madagascar, India and Sri Lanka to China, the Korean
Peninsula, Japan and Southeast Asia, Malesia to New Guinea and tropical
Australia, Micronesia), Ischaemum (c 80; tropical and subtropical
regions on both hemispheres, especially Asia), Kerriochloa (1; K.
siamensis; Southeast Asia), Pogonachne (1; P. racemosa;
the Mumbay area in India), Triplopogon (1; T. ramosissima;
western India). – Germainiinae Clayton in Kew Bull.
27: 465. 1972. Apocopis (16; India and Sri Lanka to southern China,
Southeast Asia and Sulawesi), Germainia (9; Assam, Southeast Asia,
Malesia to Queensland), Lophopogon (2; L. kingii, L.
tridentatus; India), Pogonatherum (4; P. biaristatum,
P. crinitum, P. paniceum, P. rufobarbatum;
Madagascar, the Arabian Peninsula, India to China and Japan, Southeast Asia,
Malesia to New Guinea). – Saccharinae Griseb.,
Spic. Flor. Rumel. 2: 472. Jan 1846 [’Sacchareae’].
Asthenochloa (1; A. tenera; Central Malesia),
Euclasta (2; E. clarkei, E. condylotricha; tropical
Africa, Madagascar, the Comoros, Oman India, Burma, Mexico to tropical South
America), Pseudodichanthium (1; P. serrafalcoides; western
India), Cleistachne (1; C. sorghoides; tropical East and
southeastern Africa, Oman, India), Sorghastrum (20; tropical and
southern Africa, North America, Mexico, Central America, the West Indies,
tropical South America), Sorghum (28; tropical and subtropical regions
in the Old World, India, Sri Lanka, Burma, Southeast Asia Australia, one
species, S. trichocladum, in Mexico and Central America),
Trachypogon (4; T. chevalieri, T. macroglossus,
T. spicatus, T. vestitusa; tropical and southern Africa,
Madagascar, southern United States and Mexico to tropical South America),
’Eulalia’ (34; tropical and subtropical regions in Africa,
Madagascar, Asia and Australia; polyphyletic), Homozeugos (6; H.
conciliatum, H. eylesii, H. fragile, H.
gossweileri, H. huillense, H. katakton; tropical
Africa), Miscanthus (16; tropical and subtropical regions in the Old
World, southern Africa, East Asia), Polytrias (1; P. indica;
Southeast Asia), Pseudosorghum (2; P. fasciculare, P.
zollingeri; the Himalayas, Burma, Yunnan, Southeast Asia, Java, the
Philippines; in Eulalia?), Saccharum (35–40; tropical and
subtropical regions on both hemispheres), Tripidium (>3; T.
bengalense, T. ravennae, T. strictum; southeastern
Europe, the Mediterranean, southwestern and southern Asia), Imperata
(12; tropical and subtropical regions on both hemispheres), Veldkampia
(1; V. sagaingensis; Southeast Asia), Agenium (3; A.
leptocladum, A. majus, A. villosum; Brazil, Uruguay,
Paraguay, Bolivia, Argentina), Eriochrysis (10; tropical and southern
Africa, India, tropical America). – Andropogoninae
J. Presl in C. Presl, Reliq. Haenk. 1: 331. Jan-Jun 1830
[’Andropogoneae’]. Andropogon (c 100; tropical and
subtropical regions on both hemispheres), Bhidea (3; B.
borii, B. burnsiana, B. fischeri; India),
Diheteropogon (4; D. amplectans, D. filifolius,
D. hagerupii, D. microterus; tropical and southern Africa),
Hyparrhenia (c 55; Macaronesia, the Mediterranean, Africa, Madagascar,
southwestern and southern Asia to Pakistan and India, tropical America, with
their highest diversity in Africa), Schizachyrium (c 65; tropical and
subtropical regions on both hemispheres), Anadelphia (21; western to
southeastern tropical Africa), Bothriochloa (c 38; tropical and
subtropical regions on both hemispheres), Capillipedium (18; East
Africa, tropical Asia to Australia and New Caledonia), Clausospicula
(1; C. extensa; Northern Territory), Cymbopogon (55–60;
tropical and subtropical regions in Africa, Madagascar, Asia and Australia),
Dichantium (19–23; tropical and subtropical regions in the Old
World), Elymandra (6; E. androphila, E.
archaelymandra, E. gossweileri, E. grallata, E.
lithophila, E. subulata; tropical and southern Africa, one
species, E. lithophila, also in Brazil), Eremopogon (1;
E. foveolatus; tropical Africa, the Middle East to India and Sri
Lanka), Exotheca (1; E. abyssinica; tropical East Africa,
Vietnam), Heteropogon (5; H. contortus, H.
fischerianus, H. melanocarpus, H. ritchiei, H.
triticeus; tropical and subtropical regions on both hemispheres),
Hyperthelia (7; H. colobantha, H. cornucopiae,
H. dissoluta, H. edulis, H. kottoensis, H.
macrolepis, H. polychaeta; tropical and southern Africa),
Iseilema (c 30; India, Sri Lanka, Southeast Asia, Malesia to New
Guinea and tropical Australia), Monocymbium (3; M.
ceresiiforme, M. deightonii, M. lanceolatum; tropical
and southern Africa), Parahyparrhenia (6; P. annua, P.
bellariensis, P. laegaardii, P. perennis, P.
siamensis, P. tridentata; tropical West and Central Africa,
India, Thailand), Pseudanthistiria (4; P. burmanica, P.
emeinica, P. heteroclita, P. umbellata; India, Thailand,
southern China), Spathia (1; S. neurosa; tropical Australia),
Spodiopogon (16; Turkey, Southwest, Central, East and tropical Asia to
the Russian Far East, Japan, Taiwan and the Malay Peninsula), Themeda
(26; tropical and subtropical regions in Africa, Asia and Australia);
unplaced Andropogoninae: Lakshmia (1; L.
venusta; Sri Lanka, Western Ghats). –
Andropogoneae incertae sedis:
Apluda (1; A. mutica; Madagascar, Oman, Socotra, the
Mascarene Islands, Central Asia, the Indian Subcontinent, Tibet, China, Japan,
the Ryukyu Islands, Taiwan, Southeast Asia, Malesia to New Guinea and
Melanesia), Eulaliopsis (2; E. binata, E. sykesii;
Afghanistan, India, China, Taiwan, the Philippines), Microstegium (c
25; tropical and subtropical Africa and Asia), Sehima (5; S.
galpinii, S. ischaemoides, S. nervosum, S.
notatum, S. sulcatum; the Cape Verde Islands, tropical and
southern Africa, Southwest Asia and India to southern China and Burma,
Southeast Asia to Java, New Guinea). – Coix is sister to
Rottboelliinae (Soreng & al. 2017). – Tropical and subtropical
regions on both hemispheres. Spikelets paired. C4 photosynthetic pathway
(NADP-ME subtype) present.
Arundinelleae Stapf
in W. H. Harvey et O. W. Sonder (ed. W. T. Thiselton-Dyer), Fl. Cap. 7: 314.
Jul 1898
2–4/75–80. Arundinella
(45–50; tropical and subtropical regions on both hemispheres),
Garnotia (c 30; southern and eastern Asia to islands in the Pacific).
– Plausible Arundinelleae: Chandrasekharania (1; C.
keralensis; Kerala in India), Jansenella (1; J.
griffithiana; India, Sri Lanka). – Tropical and subtropical regions,
especially Asia. – Arundinelleae are sister to
Andropogoneae (Sánchez-Ken & Clark 2010). The positions of
Chandrasekharania and Jansenella are uncertain.
Panicodae L. Liu in
Acta Phytotaxon. Sin. 18: 324. Aug 1980
Paniceae R. Br. in M.
Flinders, Voy. Terra Austral. 2: 582. 19 Jul 1814
74/1.190–1.210. Hylebates
(2; H. chlorochloe, H. cordatus; tropical Africa). –
Anthephorinae Benth. in J. Linn. Soc. London, Bot.
19: 30. 24 Dec 1881 ['Anthephoreae']. ’Digitaria‘
(270–280; cosmopolitan; non-monophyletic), Chlorocalymma (1; C.
cryptacanthum; Tanzania), Anthephora (11; tropical and southern
Africa, the Arabian Peninsula, one species, A. hermaphrodita, also in
tropical America), Chaetopoa (2; C. pilosa, C.
taylorii; Tanzania); unplaced Anthephorinae:
Taeniorhachis (1; T. repens; Somalia), Tarigidia (1;
T. aequiglumis; Namibia, North-West, Free State), Thyridachne
(1; T. tisserantii; tropical Africa), Trachys (1; T.
muricata; coastal areas in southern India, Sri Lanka, Bangladesh and
Burma). – Dichantheliinae Zuloaga in Plant Syst.
Evol. 301: 1697. 1 Jun 2015. Adenochloa (14; tropical and subtropical
Africa, Madagascar), Dichanthelium (70–75; North America, Mexico,
Central America, the West Indies, tropical and subtropical South America). –
Boivinellinae Pilg. in H. G. A. Engler et K. A. E.
Prantl, Nat. Pflanzenfam., ed. 2, 14e: 101. 1940. Acroceras (c 25;
tropical and southern Africa, Madagascar, Southeast Asia, Malesia),
Setiacis (1; S. diffusa; Hainan; in Acroceras?),
Alloteropsis (5; A. angusta, A. cimicina, A.
paniculata, A. papillosa, A. semialata; tropical and
subtropical regions in the Old World), Amphicarpum (2; A.
amphicarpon, A. muehlenbergianum; southeastern United States),
Cyphochlaena (2; C. madagascariensis, C.
sclerioides; Madagascar, the Comoros), Cyrtococcum (14; tropical
regions in Africa, Asia and Australia), Echinochloa (c 40;
warm-temperate to tropical regions on both hemispheres), Entolasia (6;
E. imbricata, E. marginata, E. minutifolia, E.
olivacea, E. stricta, E. whiteana; tropical and southern
Africa, tropical Asia to eastern Australia, New Caledonia), Lasiacis
(16; Florida, Mexico, Central America, the West Indies, tropical South
America), Mayariochloa (1; M. amphistemon; Cuba),
Microcalamus (1; M. barbinodis; tropical West Africa),
Morronea (6; M. arundinariae, M. cayoensis, M.
guatemalensis, M. incumbens, M. parviglumis, M.
trichidiachnis; Mexico, Central America, the West Indies, tropical South
America), Oplismenus (7; O. burmannii, O.
compositus, O. flavicomus, O. fujianensis, O.
hirtellus, O. thwaitesii, O. undulatifolius; tropical
and subtropical regions on both hemispheres), Ottochloa (3; O.
gracillima, O. grandiflora, O. nodosa; India, southern
China, Southeast Asia, Malesia to northern Australia),
Parodiophyllochloa (6; P. cordovensis, P. missiona,
P. ovulifera, P. pantricha, P. penicillata, P.
rhizogona; Mexico, Central America, tropical South America),
Poecilostachys (c 20; tropical Africa, Madagascar),
Pseudechinolaena (6; P. camusiana, P.
madagascariensis, P. moratii, P. perrieri, P.
polystachya, P. tenuis; Madagascar, one species, P.
polystachya, pantropical). – Clade.
Sacciolepis (c 25; tropical and subtropical regions on both
hemispheres, with their largest diversity in tropical Africa);
Trichanthecium (38; tropical Africa, tropical America). –
Neurachninae Clayton et Renvoize in Kew Bull., Addit.
Ser. 13: 377. 1986. Cleistochloa (3; C. rigida, C.
sclerachne, C. subjuncea; eastern Queensland, eastern New South
Wales), Neurachne (7; N. alopecuroides, N.
annularis, N. lanigera, N. minor, N. munroi,
N. queenslandica, N. tenuifolia; Australia),
Thyridolepis (3; T. mitchelliana, T. multiculmis,
T. xerophila; arid regions in Australia). – Homopholis (1;
H. belsonii; Australia). – Melinidinae
Stapf in D. Oliver (ed. D. Prain), Flora Trop. Afr. 9: 13. 1 Jul 1917
[’Meliniastrae’]. Thuarea (2; T. perrieri:
Madagascar; T. involuta: Madagascar, tropical and East Asia to
tropical Australia, Melanesia, Micronesia and the Hawaiian Islands),
Rupichloa (2; R. acuminata, R. decidua; Bahia, Minas
Gerais), Moorochloa (3; M. eruciformis, M.
malacodes, M. schoenfelderi; tropical and subtropical America),
Tricholaena (4; T. capensis, T. monachne, T.
teneriffae, T. vestita; the Canary Islands, the Mediterranean,
Africa, Madagascar), Leucophrys (1; L. mesocoma; southern
Namibia, Northern Cape), Melinis (22; tropical and southern Africa,
Madagascar), Urochloa (18; tropical and subtropical regions on both
hemispheres; incl. Chaetium, Eriochloa?), Chaetium
(3; C. bromoides, C. cubanum, C. festucoides;
southern Mexico, Central America, Cuba, Colombia, Venezuela, Brazil; in
Urochloa?), Eriochloa (c 35; tropical and subtropical regions
on both hemispheres; in Urochloa?); unplaced Melinidinae:
Eccoptocarpha (1; E. obconiciventris; southern tropical
Africa), Yvesia (1; Y. madagascariensis; Madagascar). –
Panicinae Fr., Flora Scan.: 195. 1835
['Paniceae']. Louisiella (2; L. fluitans: Sudan,
Congo; L. elephantipes: Mexico, Central America, tropical South
America), ’Panicum’ (c 300; warm-temperate to tropical regions on
both hemispheres; polyphyletic). – Cenchrinae
Dumort., Anal. Fam. Plant.: 64. 1829 ['Cenchreae'].
Acritochaete (1; A. volkensii; mountains in tropical Africa),
Alexfloydia (1; A. repens; Coffs Harbour in New South Wales),
Cenchrus (27; warm-temperate to tropical regions in Africa, India and
America), Chamaeraphis (1; C. hordeacea; northern Australia),
Dissochondrus (1; D. biflorus; the Hawaiian Islands),
Holcolemma (3; H. canaliculatum, H. dispar, H.
inaequale; tropical East Africa, India, Australia), Hygrochloa
(2; H. aquatica, H. cravenii; northern Australia),
Ixophorus (1; I. unisetus; Mexico), Paractaenum (2;
P. novae-hollandiae, P. refractum; Australia),
Paratheria (2; P. glaberrima, P. prostrata; tropical
West and Central Africa, Madagascar, Cuba, Brazil), Pseudochaetochloa
(1; P. australiensis; northern Australia), Pseudoraphis (7;
P. balansae, P. brunoniana, P. jagonis, P.
minuta, P. paradoxa, P. sordida, P. spinescens;
India to China, Japan and Australia), ’Setaria’ (100–105;
warm-temperate to tropical regions on both hemispheres; non-monophyletic?),
Setariopsis (2; S. auriculata, S. latiglumis;
Arizona, Mexico, Central America, Colombia, Venezuela), Spinifex (4;
S. hirsutus, S. littoreus, S. longifolius, S.
sericeus; India to East Asia and islands in the Pacific),
Stenotaphrum (7; S. clavigerum, S. dimidiatum,
S. heiferi, S. micranthum, S. oostachyum, S.
secundatum, S. unilaterale; tropical and subtropical regions on
both hemispheres), Stereochlaena (4; S. annua, S.
caespitosa, S. cameronii, S. tridentata; tropical East
to South Africa), Streptolophus (1; S. sagittifolius;
Angola), Uranthoecium (1; U. truncatum; tropical Australia),
Whiteochloa (6; W. airoides, W. biciliata, W.
capillipes, W. cymbiformis, W. multiciliata, W.
semitonsa; Aru Islands, tropical Australia), Xerochloa (3; X.
barbata, X. imberbis, X. laniflora; Australia),
Zuloagaea (1; Z. bulbosa; Arizona, Mexico, Central America,
northern South America), Zygochloa (1; Z. paradoxa; arid
regions of central Australia). – Unplaced
Paniceae: Hydrothauma (1; H.
manicatum; Zambia), Kellochloa (2; K. brachyantha,
K. verrucosa; eastern and southeastern United States),
Oryzidium (1; O. barnardii; Namibia, northern Botswana to
Zambia), Thedachloa (1; T. nana; northwestern Australia). –
Cosmopolitan, with their highest diversity in tropical and subtropical regions.
– Cenchrinae are highly provisional. Most of their subgroups are
nested inside ’Setaria’ (Silva & al. 2015).
[Arundinoideae+[Danthonioideae+Chloridoideae]]
Arundinoideae
Burmeist., Handb. Naturgesch.: 204. 1837 [‘Arundinaceae’]
20–22/218–220. Ligule hairy. Lemma
awned. Starch grains compound. – The clade
[Arundineae+Molinieae] may be sister to
Micraireae.
[Micrairoideae+[Arundineae+[Crinipedeae+Molinieae]]]
Micrairoideae Pilg. in
H. G. A. Engler et K. A. E. Prantl, Nat. Pflanzenfam., ed. 2, 14d: 167. 16 Feb
1956
9/c 185.
Micraireae Pilg. in Engler et Prantl, Nat.
Pflanzenfam., ed. 2, 14d: 167. 16 Feb 1956. Micraira (16; tropical
Australia). – Eriachneae Eck-Boorsb. in Blumea 26:
2. 128. 1980. Eriachne (c 50; Sri Lanka and southern China to southern
Malesia and Australia). – Isachneae Benth. in J.
Linn. Soc. London, Bot. 19: 30. 24 Dec 1881. Isachne (c 100; tropical
and subtropical regions on both hemispheres, especially southern and eastern
Asia), Coelachne (11; tropical Africa, Madagascar, tropical Asia from
India to New Guinea), Heteranthoecia (1; H. guineensis;
tropical Africa), Sphaerocaryum (1; S. malaccense; India to
China, Taiwan, Southeast Asia and West Malesia), Limnopoa (1; L.
meeboldii; southern India), Hubbardia (2; H. diandra,
H. heptaneuron; western India). – Zenkeria (5; Z.
elegans, Z. jainii, Z. obtusiflora, Z.
sebastinei, Z. stapfii; India, Sri Lanka)? – Pantropical, with
their highest diversity in the Old World tropics. Sometimes xerophytic. C4
photosynthesis present in Eriachne. Leaves tristichous. Stomatal
subsidiary cells dome-shaped. Ligule with hair fringe. Lemma with or without
awn. Embryo small. Starch grains simple. Germination flap present. n = 10. –
Micraireae may be sister-group to
[Arundineae+[Crinipedeae+Molinieae]] (Hardion &
al. 2017). In other analyses they are sister-group to
[Panicoideae+[[Aristidoideae+Danthonioideae]+[Arundinoideae+Chloridoideae]]].
According to Soreng & al. (2017), Micrairoideae are sister to
Arundinoideae.
Arundineae Dumort.,
Observ. Gramin. Belg.: 82. Jul-Sep 1824 [’Arundinaceae’]
4/18. Amphipogon (9;
Australia), Arundo (6; A. collina, A. donax, A.
formosana, A. mediterranea, A. micrantha, A.
plinii; the Mediterranean, Southwest Asia to India, the Ryukyu Islands,
Taiwan, the Philippines), Monachather (1; M. paradoxa;
Australia), Dregeochloa (2; D. calviniensis, D.
pumila; southern Namibia, Northern Cape). – Tropical and subtropical
regions of the Old World.
Crinipedeae Hardion
in Plant Syst. Evol. 303: 1337. Dec 2017
4/8. Crinipes (2; C.
abyssinicus, C. longifolius; Sudan, Ethiopia, Uganda),
Styppeiochloa (3; S. catherineana, S. gynoglossa,
S. hitchcockii; mountains in southern to southeastern tropical Africa,
Madagascar), Elytrophorus (2; E. globularis, E.
spicatus; tropical and southern Africa, the Indian Subcontinent, Yunnan,
Hainan, Indochina, Lesser Sunda Islands, tropical Australia),
Pratochloa (1; P. walteri; Namibia). – Tropical and
subtropical regions of the Old World, with their largest diversity in southern
and eastern Africa.
Molinieae Jirásek in
Preslia 38: 33. Jan-Mar 1966
3–5/7–9. Hakonechloa (1;
H. macra; Japan), Leptagrostis (1; L. schimperiana;
Ethiopia)?, Molinia (2; M. caerulea, M. japonica;
Europe, temperate Asia), Phragmites (4; P. australis, P.
japonicus, P. karka, P. mauritianus; cosmopolitan),
Piptophyllum (1; P. welwitschii; Angola)? – Cosmopolitan,
with their largest diversity in tropical and subtropical regions in the Old
World. Elongate microhairs Panicoid type (with slender thin-walled cap cells).
Hilum short. Epiblasts absent. n = 6, 9, 12.
[Danthonioideae+Chloridoideae]
Hilum short, punctate.
Danthonioideae N. P.
Baker et P. H. Linder in Ann. Missouri Bot. Gard. 88: 421. 17 Sep 2001
19/c 285. Merxmuellera (7;
M. ambalavaoensis, M. davyi, M. drakensbergensis,
M. grandiflora, M. macowanii, M. stereophylla,
M. tsaratananensis; tropical and southern Africa, Madagascar), Capeochloa
(3; C. arundinacea, C. cincta, C. setacea; southern
Africa), Geochloa (3; G. decora, G. lupulina, G.
rufa; tropical and southern Africa), Pentameris
(c 80; tropical and subtropical Africa, Madagascar, southern and eastern Asia),
Chionochloa
(26; 23 spp. in New Zealand; C. antarctica: Auckland and Campbell
Islands; C. howensis: Lord Howe; C. archboldii: New Guinea),
Cortaderia
(20; South America; non-monophyletic?), Chaetobromus (1; C.
involucratus; southern Namibia, Northern and Western Cape),
Pseudopentameris (3; P. brachyphylla, P. caespitosa,
P. macrantha; mountains in Western Cape), Austroderia (5;
A. fulvida, A. richardi, A. splendens, A.
toetoe: New Zealand; A. turbaria: Chatham Islands),
Notochloe (1; N. microdon; Blue Mountains in New South
Wales), Plinthanthesis (3; P. paradoxa, P. rodwayi,
P. urvillei; southeastern Australia), Chimaerochloa (1;
C. archboldii; tropical Asia to New Guinea), Danthonia
(27; Europe, the Mediterranean to Ukraine and the Caucasus, North Africa, the
Russian Far East, Greenland, North, Central and South America, the West
Indies), Tenaxia (8; Africa, temperate to tropical Asia), Schismus
(5; S. arabicus, S. barbatus, S. inermis, S.
scaberrimus, S. schismoides; the Mediterranean, Africa,
southwestern Asia to northwestern India and China, especially southern Africa),
Tribolium
(14; southern Namibia, Northern, Western and Eastern Cape),
Rytidosperma (c 75; New Guinea, Australia, New Zealand, South
America)); unplaced: Phaenanthoecium (1; P. koestlinii;
mountains in northeastern tropical Africa). –
Danthonioideae incertae sedis:
Danthonidium (1; D. gammiei; India). – Cosmopolitan, with
their highest diversity in the Southern Hemisphere, especially in southern and
eastern Africa and Australia (few species in Southeast Asia and Malesia).
Leaves usually symmetrical. Ligules ciliated. Foliar anatomy festucoid.
Prophylls bifid. Spikelets festucoid. Lemma awn trifid or with three awns.
Stylar bases much separated. Synergids haustorial, growing out through
micropyle. n = 6, 7, 9. – In some analyses Danthonioideae are
sister-group to Aristidoideae.
Chloridoideae
Burmeist., Handb. Naturgesch.: 205. 1837 [’Chlorideae’]
125–126/1.405–1.420.
Centropodieae are sister-group to the remaining
Chloridoideae, according to Peterson & al. (2011). –
Unplaced Chloridoideae Gossweilerochloa (1;
G. delicatula; Angola), Indopoa (1; I. paupercula;
India), Lepturopetium (1; L. kuniensis; New Caledonia,
Marshall Islands, Cocos Island), Myriostachya (1; M.
wightiana; southern India, Sri Lanka, Southeast Asia),
Pogonochloa (1; P. greenwayi; southern tropical Africa),
Pseudozoysia (1; P. sessilis; Somalia),
Silentvalleya (1; S. nairii; India). – Tropical,
subtropical and warm-temperate regions, dry habitats (especially in Africa and
Australia). C4 photosynthesis usually present (phosphoenolpyruvate
carboxykinase subtype). Microhairs usually with inflated hemispherical
thick-walled distal cells and elongate secretory basal cell with internal
membranes (’Chloridoid type’; in Neyraudia ‘Panicoid type’).
Leaves usually symmetrical. Spikelets disarticulating above glumes. Hilum
short. Epiblast usually present. Mesocotyl present. Leaf margins of embryo not
overlapping. Plastid gene rpl16 intron with 4 bp insertion. n =
(6–8) 9, 10.
[Centropodieae+[Triraphideae+[Eragrostideae+[Zoysieae+Chlorideae]]]]
Centropodieae P. M.
Peterson, N. P. Barker et H. P. Linder in Taxon 60(4): 1118. Aug 2011
2/6. Centropodia (4; C.
forskalii, C. fragilis, C. glauca, C.
mossamedensis; North Africa, the Middle East to India),
Ellisochloa (2; E. papposa, E. rangei; southern
Africa). – Southern Africa and one species in North Africa eastwards to
India. C3 (Ellisochloa) and C4
(Centropodia) photosynthesis present. Microhairs probably absent.
Haustorial synergids absent. x = 9, 12.
[Triraphideae+[Eragrostideae+[Zoysieae+Chlorideae]]]
Triraphideae P. M.
Peterson in Mol. Phylogen. Evol. 55(2): 591. May 2010
4/14. Habrochloa (1; H.
bullockii; Central Africa), Neyraudia (4; N.
arundinacea, N. curvipes, N. montana, N.
reynaudiana; tropical and subtropical regions in Africa and Asia),
Nematopoa (1; N. longipes; Zambia, Zimbabwe; in
Triraphis?), Triraphis (8; tropical Africa, the Arabian
Peninsula, Australia, one species, T. devia, in central Brazil). –
Tropical and subtropical regions in the Old World, central Brazil.
[Eragrostideae+[Zoysieae+Chlorideae]]
Eragrostideae Stapf
in W. H. Harvey et O. W. Sonder (ed. W. T. Thiselton-Dyer), Fl. Cap. 7: 316.
Jul 1898 [’Eragrosteae’]
13/410–415.
Cotteinae Reeder in Madroño 18: 25. 10 Feb 1965.
Cottea (1; C. pappophorides; southern United States to
central Mexico, Ecuador to Argentina), Enneapogon
(c 25; dry warmer regions on both hemispheres), Kaokochloa (1; K.
nigrirostris; northwestern Namibia), Schmidtia (2; S.
kalahariensis, S. pappophoroides; Africa, Cape Verde Islands,
Pakistan). – Unioliinae Clayton in Kew Bull. 37:
417. 30 Dec 1982. Entoplocamia (1; E. aristulata; Namibia),
Fingerhuthia (2; F. africana, F. sesleriiformis;
southern tropical and southern Africa, the Arabian Peninsula, Afghanistan to
India), Tetrachaete (1; T. elionuroides; Ethiopia, Tanzania,
the Arabian Peninsula), Tetrachne (1; T. dregei; Eastern
Cape, Free State, Lesotho, Pakistan), Uniola (5; U.
condensata, U. paniculata, U. peruviana, U.
pittieri, U. virgata; southern United States, Mexico, Central
America to Ecuador). – Eragrostidinae J. Presl in
C. Presl, Reliq. Haenk. 1: 273. Jan-Jun 1830 [’Eragrostideae’]. Cladoraphis
(2; C. cyperoides, C. spinosa; Namibia, Northern and Western
Cape), Eragrostis
(c 350; tropical to warm-temperate regions on both hemispheres;
non-monophyletic), Richardsiella (1; R. eruciformis; Zambia),
Steirachne (2; S. barbata, S. diandra; northeastern
South America). – Tropical and subtropical (to warm-temperate) regions on
both hemispheres.
[Zoysieae+Chlorideae]
Zoysieae Benth. in J.
Linn. Soc. London, Bot. 19: 29. 24 Dec 1881
4–5/185–190.
Zoysiinae Benth., Flora Austral. 7: 453, 505. 23-30
Mar 1878 [’Zoysieae’]. Zoysia (9; Mauritius to
Polynesia), Urochondra (1; U. setulosa; Sudan and Somalia to
Sind). – Sporobolinae Benth. in J. Linn. Soc.
London, Bot. 19: 30. 24 Dec 1881 [’Sporoboleae’].
Psilolemma (1; P. jaegeri; East Africa), ’Sporobolus’
somalensis (East Africa); Sporobolus
(c 175; warm-temperate to tropical regions on both hemispheres). –
Subcosmopolitan.
Cynodonteae Dumort.,
Observ. Gramin. Belg.: 83. Jul-Sep 1824 [’Cynodoneae’]
95/785–800.
Eleusininae Dumort., Anal. Fam. Plant.: 63. 1829
['Eleusineae']. Dinebra (6; D. haareri, D.
marquisensis, D. perrieri, D. polycarpha, D.
retroflexa, D. somalensis; tropical East and northeastern Africa,
Madagascar, southwestern Asia to India, southern United States),
Eleusine (10; Africa, Madagascar, South America), Diplachne
(5; D. cuspidata, D. fascicularis, D. festuciformis,
D. fusca, D. gigantea; warm-temperate to tropical regions on
both hemispheres), Leptochloa (27; eastern tropical and southern
Africa, Australia, the Marquesas Islands, North and South America),
Astrebla (4; A. elymoides, A. lappacea, A.
pectinata, A. squarrosa; Australia), Disakisperma (4;
D. dubium, D. eleusine, D. obtusiflorum, D.
yemenicum; southern and tropical eastern Africa, southern United States),
Enteropogon (17; tropical and subtropical regions on both
hemispheres), Chloris (60–65; warm-temperate to tropical regions on
both hemispheres), Tetrapogon (11; the Canary Islands, Africa, the
Arabian Peninsula, the Middle East to India and Central Asia),
Lepturus (c 15; coasts in East Africa, South Africa, Sri Lanka,
Australia to Polynesia), Microchloa (6; M. altera, M.
annua, M. caffra, M. ensifolia, M. indica,
M. kunthii; tropical and southern Africa, tropical Asia to tropical
Australia, southwestern United States to Argentina and Chile),
Micrachne (5; M. fulva, M. obtusiflora, M.
patentiflora, M. pilosa, M. simonii; Central and
tropical East Africa), Eustachys (15; United States, Mexico, Central
America, the West Indies, South America, one species, E. paspaloides,
in eastern and southern Africa, Oman and Yemen, one species, E.
tenera, in Southeast Asia to Taiwan and New Guinea), Chrysochloa
(4; C. hindsii, C. hubbardiana, C. orientalis,
C. subaequigluma; tropical Africa), Stapfochloa (6; S.
berroi, S. canterae, S. ciliata, S. elata,
S. lamproparia, S. parvispicula; tropical and subtropical
Africa), Cynodon (10; tropical and subtropical regions on both
hemispheres); unplaced Eleusininae: Afrotrichloris (2; A.
hyaloptera, A. martinii; Somalia; in Schoenefeldia?),
Schoenefeldia (2; S. gracilis, S. transiens;
tropical and southern Africa, Madagascar, India; incl.
Afrotrichloris?), Apochiton (1; A. burttii;
Tanzania), Austrochloris (1; A. dichanthoides; Queensland),
’Coelachyrum’ (1–8; C. brevifolium, C.
lagopoides, C. longiglume, C. piercei, C.
poiflorum; tropical and southern Africa, the Arabian Peninsula, Pakistan;
polyphyletic), Daknopholis (1; D. boivinii; East Africa,
Madagascar, Aldabra), Oxychloris (1; O. scariosa; Australia),
Harpochloa (1; H. falx; southern tropical and southern
Africa), Neostapfiella (3; N. chloridiantha, N.
humbertiana, N. perrieri; Madagascar), Pommereulla (1;
P. cornucopiae; southern India, Sri Lanka), Rheochloa (1;
R. scabiflora; central Brazil), Sclerodactylon (1; S.
macrostachyum; coast of East Africa, islands in the Indian Ocean)? –
Dactylocteniinae P. M. Peterson, Romasch. et Y.
Herrera in Taxon 65(6): 1276. Dec 2016. Acrachne (3; A.
henrardiana, A. perrieri, A. racemosa; tropical and
southern Africa, Madagascar, Southeast Asia, Australia),
Dactyloctenium (13; tropical and subtropical regions on both
hemispheres), Brachychloa (2; B. fragilis, B.
schiemanniana; KwaZulu-Natal, Mozambique), Neobouteloua (2;
N. lophostachya, N. pauciracemosa; Chile, Argentina). –
Triodiinae Benth. in J. Linn. Soc. London, Bot. 19:
30. 24 Dec 1881 ['Triodieae']. Triodia (c 70; Australia). –
Aeluropodinae P. M. Peterson in Mol. Phylogen. Evol.
55: 591. May 2010. Aeluropus (6; A. badghyzii, A.
laciniatus, A. lagopoides, A. littoralis, A.
macrostachyus, A. pilosus; the Mediterranean, southwestern Asia
to India and northern China), Odyssea (2; O. mucronata,
O. paucinervis; coasts of the Red Sea, tropical and southern Africa).
– Orininae P. M. Peterson, Romasch. et Y. Herrera
in Taxon 65(69): 1277. Dec 2016. Cleistogenes (c 13; southern Europe,
Turkey to temperate East Asia), Orinus (6; O. alticulmus,
O. anomala, O. kokonorica, O. longiglumis, O.
thoroldii, O. tibeticus; Central Asia, the Himalayas, Tibet,
western China). – Orcuttiinae P. M Peterson et
Columbus in Aliso 23: 592. Dec 2007. Neostapfia (1; N.
colusana; California), Orcuttia (5; O. californica,
O. inaequalis, O. pilosa, O. tenuis, O.
viscida; California). – Zaqiqahinae P. M.
Peterson, Romaschenko et Herrera Arrieta in Taxon 65(6): 1279. Dec 2016.
Zaqiqah (1; Z. mucronata; Ethiopia, Somalia, Socotra,
southwestern Arabian Peninsula). – Gouiniinae P. M.
Peterson et Columbus in Aliso 23: 592. Dec 2007. Triplasis (2; T.
americana, T. purpurea; central and eastern United States to
Costa Rica), Triplasiella (1; T. eragrostoides; southern
United States, Mexico, the West Indies, Venezuela), Schenckochloa (1;
S. barbata; northern Brazil), Vaseyochloa (1; V.
multinervosa; Texas), Tridentopsis (2; T. eragrostoides,
T. mutica; southwestern United States, northern Mexico, the West
Indies), Gouinia (12; Mexico, Central America, Cuba, Hispaniola,
tropical South America). – Cteniinae P. M.
Peterson, Romaschenko et Herrera Arrieta in Taxon 63(2): 283. Apr 2014.
Ctenium (20; tropical and subtropical Africa, Madagascar, North
America to Bolivia and Paraguay). – Trichoneurinae
P. M. Peterson, Romaschenko et Herrera Arrieta in Taxon 63(2): 284. Apr 2014.
Trichoneura (8; Africa, the Arabian Peninsula, Texas, Mexico, the
Galápagos Islands, Peru, Chile). –
Hubbardochloinae Auquier in Bull. Jard. Bot. Natl.
Belg. 50: 246. 1980. Dignathia (5; D. aristata, D.
ciliata, D. gracilis, D. hirtella, D. villosa;
tropical East Africa, western India), Leptothrium (3; L.
lineare, L. rigidum, L. senegalense; Senegal to
Pakistan, the West Indies), Leptocarydion (1; L. vulpiastrum;
northern Namibia, KwaZulu-Natal, Mpumalanga, Northern Province, Botswana, East
Africa), Bewsia (1; B. biflora; northern Namibia, northern
South Africa, Swaziland), Gymnopogon (14; southern and eastern United
States, Mexico, Central America, the West Indies, tropical South America, one
species, G. delicatulus, in Southeast Asia), Lophacme (2;
L. digitata, L. parva; southern tropical and southern
Africa), Hubbardochloa (1; H. gracilis; mountains in Central
Africa), Decaryella (1; D. madagascariensis; Madagascar). –
Perotidinae P. M. Peterson, Romaschenko et Herrera
Arrieta in Taxon 63(2): 284. Apr 2014. Mosdenia (1; M.
leptostachys; northern South Africa), Perotis (15; tropical and
subtropical Africa, southern Madagascar, temperate to tropical Asia east to
China, New Guinea and northern Australia), Trigonochloa (2; T.
rupstris, T. uniflora; tropical, southern and eastern Africa, the
Arabian Peninsula to India and Sri Lanka). –
Farragininae P. M. Peterson, Romaschenko et Herrera
Arrieta in Taxon 63(2): 283. Apr 2014. Farrago (1; F.
racemosa; Tanzania), Craspedorhachis (9; southern tropical and
southern Africa). – Tripogoninae Stapf in D. Oliver
(ed. D. Prain), Flora Trop. Afr. 9: 22. 1 Jul 1917
['Schizachyriastrae']. Desmostachya (1; D.
bipinnata; North Africa, southwestern Asia to India and Southeast Asia),
Melanocenchris (3; M. abyssinica, M. jacquemontii,
M. monoica; Chad and tropical northeastern Africa to India and Sri
Lanka), Halopyrum (1; H. mucronatum; coasts along the Indian
Ocean), Eragrostiella (6; E. bifaria, E.
brachyphylla, E. collettii, E. leioptera, E.
lolioides, E. nardoides; East Africa, Sri Lanka to northern
Australia), Tripogonella (3; T. minima: tropical and southern
Africa, Madagascar; T. loliiformis: Australia; T. spicata:
North America, Central America, the West Indies, South America),
Oropetium (6; O. aristatum, O. capense, O.
minimum, O. roxburghianum, O. thomaeum, O.
villosulum; arid and semiarid regions in tropicl West Africa to India and
Southeast Asia), Tripogon (c 50; tropical and southern Africa,
Madagascar, India to Australia). – Pappophorinae
Dumort., Anal. Fam. Plant.: 63. 1829 ['Pappophoreae'].
Neesiochloa (1; N. barbata; northeastern Brazil),
Pappophorum (7; P. bicolor, P. caespitosum, P.
hassleri, P. krapovickasii, P. mucronulatum, P.
pappiferum, P. philippianum; southern United States, Mexico,
Central America, the West Indies, tropical South America), Tridens
(15; southern Canada, eastern and southern United States, Mexico, Central
America, Cuba, tropical South America). – Traginae
P. M. Peterson et Columbus in Aliso 23: 592. Dec 2007. Polevansia (1;
P. rigida; eastern Eastern Cape, Lesotho), Pogononeura (1;
P. biflora; tropical East Africa), Monelytrum (1; M.
luederitzianum; southern Angola, Namibia), Tragus (8; tropical to
warm-temperate regions in the Old World east to China, Australia and New
Caledonia, Argentina), Orthacanthus (1; O. pedunculatus;
southern Africa), Willkommia (4; W. annua, W.
newtonii, W. sarmentosa, W. texana; southern tropical
and southern Africa, Texas). – Muhlenbergiinae
Pilg. in H. G. A. Engler et K. A. E. Prantl, Nat. Pflanzenfam., ed. 2, 14d:
168. 16 Feb 1956. Muhlenbergia (155–160; Canada, United States,
Mexico, Central America, tropical and subtropical South America, few species in
Central to Northeast Asia, the Himalayas and Southeast Asia to New Guinea). –
Sohnsiinae P. M. Peterson, Romasch. et Y. Herrera in
Phytoneuron 2017-44: 6. 18 Jul 2017. Sohnsia (1; S.
filifolia; Mexico). – Scleropogoninae Pilg. in
H. G. A. Engler et K. A. E. Prantl, Nat. Pflanzenfam., ed. 2, 14d: 167. 16 Feb
1956. Scleropogon (1; S. brevifolius; southwestern United
States, Mexico, Chile, Argentina), Swallenia (1; S.
alexandrae; Eureka Valley Sand Dunes in Inyo County in California),
Blepharidachne (4; B. benthamiana, B. bigelovii,
B. hitchcockii, B. kingii; western United States, Argentina),
Erioneuron (2; E. avenaceum, E. pilosum; southern
United States, Mexico, Peru, Bolivia, Argentina), Munroa (5; M.
andina, M. argentina, M. decumbens, M.
mendocina, M. squarrosa; western and southwestern United States,
northen Mexico, Chile, Argentina). – Jouveinae P.
M. Peterson, Romasch. et Y. Herrera in Phytoneuron 2017-44: 5. 18 Jul 2017.
Jouvea (2; J. pilosa, J. straminea; Baja California
to Panamá). – Allolepiinae P. M. Peterson,
Romasch. et Y. Herrera in Phytoneuron 2017-44: 4. 18 Jul 2017.
Allolepis (1; A. texana; southern United States, Mexico). –
Hilariinae P. M. Peterson et Columbus in Aliso 23:
592. Dec 2007. Hilaria (10; southern United States, Mexico,
Guatemala). – Kaliniinae P. M. Peterson, Romasch.
et Y. Herrera in Phytoneuron 2017-44: 5. 18 Jul 2017. Kalinia (1;
K. obtusiflora; southwestern United States to central Mexico). –
Monanthochloinae Pilg. ex Potztal in Willdenowia 5:
472. Nov 1969. Distichlis (11; Canada, United States, Mexico, the West
Indies, South America, one species, D. distichophylla, in Australia).
– Boutelouinae Stapf in D. Oliver (ed. D. Prain),
Flora Trop. Afr. 9: 22. 1 Jul 1917. Bouteloua (c 40; southern Canada,
United States, Mexico, Central America, the West Indies, tropical South
America). – Cynodonteae incertae sedis:
Kampochloa (1; K. brachyphylla; Zambia, Angola; possibly
close to Ctenium?), Lepturidium (1; L. insulare;
Isla de la Juventud West of Cuba), Vietnamochloa (1; V.
aurea; Vietnam). – Tropical and subtropical (to warm-temperate) regions
on both hemispheres.
[Oryzoideae+[Bambusoideae+Pooideae]]
("The BEP clade")
Phytoliths in Oryzoideae and
Bambusoideae transverse-bilobate, in Brachyelytrum and
Pooideae axial-bilobate or axial-polylobate. x = 12. Endosperm
softness gene present. – Transverse-bilobates were possibly lost in the
common ancestor of Brachyelytrum and Pooideae. Alternatively,
this feature is a synapomorphy of [Oryzoideae+Bambusoideae]
(Rudall & al. 2014).
Oryzoideae Kunth ex
Beilschm., Flora 16(2): 52, 109. 1833
19/115. Widely distributed, especially
on the Southern Hemisphere. Microhairs usually present. Fusoid cells absent.
Longitudinal walls of epidermal cells sometimes straight. Spikelet developing
basipetally. Glumes minute. Stamens (one to) six. Stylodia almost entirely
separate. n = (10, 15).
Streptogyneae C. E.
Hubb. ex Calderón et Soderstr. in Smithsonian Contr. Bot. 44: 18. 13 Feb
1980
1/2. Streptogyna (2; S.
crinita: tropical West and Central Africa; S. americana: southern
Mexico, Central America, tropical South America). – Epidermal papillae
absent. Spikelet several-flowered. Lemma with awns. Lodiculae three.
Multicellular microhairs present on lodiculae in S. crinita. Stamens
two. Stigmas two or three. Hilum linear extending along entire fruit. Starch
grains aggregated or simple. Embryo without mesocotyl internode. Epiblast
prominent. First seedling leaf with strongly overlapping margins. n = 12. –
Streptogyna may be sister to the clade
[Panicoideae+Arundinoideae] (Peterson & al. 2011),
although Soreng & al. (2012) assigned Streptogyna
(Streptogyneae) to Ehrhartoideae.
Ehrharteae Nevski in
Trudy Bot. Inst. Akad. Nauk S.S.S.R., ser. 1, Fl. Sist. Vyssh. Rast. 4: 227.
1937
1/c 35. Ehrharta
(c 35; South Africa to Ethiopia, the Mascarenes, Malesia, the Philippines, Java
to Australia and islands in the Pacific, mountains in New Zealand, Western and
Eastern Cape). – Tropical and subtropical regions in the Old World. Arm cells
absent. Epiblasts absent at least in Ehrharta.
Roots in Ehrharta
at scutellar node.
Oryzeae Dumort.,
Observ. Gramin. Belg.: 83. Jul-Sep 1824
13–14/72–73.
Oryzinae Griseb. in C. F. von Ledebour, Flora Ross.
4: 465. Jun 1853 [‘Oryzeae‘]. Leersia
(18; warm-temperate to tropical regions on both hemispheres),
Maltebrunia (4; M. leersioides, M. letestui, M.
maroana, M. schliebenii; tropical to southern Africa,
Madagascar), Oryza (19;
tropical and subtropical regions on both hemispheres; incl.
Porteresia?), Prosphytochloa (1; P. prehensilis;
Eastern Cape, KwaZulu-Natal, Mpumalanga, Northern Province). –
Zizaniinae Benth. in J. Linn. Soc. London, Bot. 19:
54. 24 Dec 1881. Chikusichloa (3; C. aquatica, C.
brachyathera, C. mutica; China, Japan, the Ryukyu Islands,
Sumatra), Hygroryza (1; H. aristata; southern and Southeast
Asia to China), Luziola (12; southern United States, Mexico, Central
America, the West Indies, tropical South America), Potamophila (1;
P. parviflora; northern New South Wales), Rhynchoryza (1;
R. subulata; Paraguay, Argentina), Zizania (4; Z.
aquatica, Z. latifolia, Z. palustris, Z.
texana; eastern India to East Asia, North America), Zizaniopsis
(5; Z. bonariensis, Z. killipii, Z. microstachya,
Z. miliacea, Z. villanensis; southern United States, Mexico,
tropical South America to Argentina). – Phyllorachideae
C. E. Hubb.in Hooker’s Icon. Plant. 34: t. 3386, p. 5.
Mar 19. Humbertochloa (2; H. bambusiuscula, H.
greenwayi; tropical East Africa, Madagascar), Phyllorachis (1;
P. sagittata; southern tropical Africa). – Subcosmopolitan. Arm
cells present. First seedling leaf only sheath. – Suddia (1; S.
sagittifolia; Sudan) may belong in Phyllorachideae.
[Bambusoideae+Pooideae]
Epiblast present. First seedling leaf
with overlapping margins.
Bambusoideae Luerss.,
Grundz. Bot., ed. 5: 451. Jun 1893 [‘Bambusaceae’]
118/1.555–1.580. Tropical, subtropical
and temperate regions, often forests. Woody, sometimes tree-like. Mesophyll
differentiated into palisade and spongy tissues. Fusoid cells and strongly
asymmetrically invaginated arm cells present. Microhairs elongate, with slender
thin-walled cap cells (Panicoid type). Leaves with pseudopetiole, often with
inner and outer distal ligules. Culm leaves often very different from other
leaves (heterophylly). Lodiculae two or three. Stamens (two to) six (to c.
140). Filaments sometimes connate at base. Stigmas (one or) two or three. Fruit
sometimes a berry. First seedling leaf only sheath. n = 7, 9–12. – Woody
and some herbaceous species have a synchronized anthesis, and many are
monocarpic (hapaxanthic); this may be a plesiomorphy in Bambusoideae.
The woody temperate species perhaps form a sister-group to the remainder.
Arundinarieae Hackel
in Engler et Prantl, Nat. Pflanzenfam. II, 2: 92, 93. Nov 1887
30/c 550. Ampelocalamus (13;
the Himalayas to China), Bergbambos (1; B. tessellata;
southern and eastern South Africa), Thamnocalamus (4; T.
chigar, T. spathiflorus, T. tesellatus, T.
unispiculatus; the Himalayas), ’Indocalamus’ (c 35; China,
Japan; polyphyletic), Fargesia (c 80; eastern Himalayas, Tibet,
China), Phyllostachys (c 50; the Himalayas, China to Japan),
Gaoligongshania (1; G. megalothyrsa; southern Yunnan),
Oldeania (1; O. alpina; tropical East Africa, Cameroon),
Chimonocalamus (16; eastern Himalayas to Yunnan), Kuruna (7;
K. debilis, K. densifolia, K. floribunda, K.
scandens, K. serrulata, K. walkeriana, K.
wightiana; southern India, Sri Lanka), Ferrocalamus (2; F.
fibrillosus, F. strictus; southern Yunnan), Shibataea
(7; S. chiangshanensis, S. chinensis, S. hispida,
S. kumasasa, S. lancifolia, S. nanpingensis, S.
strigosa; southeastern China, southwestern Japan), Acidosasa (11;
southern China, Vietnam), Pleioblastus (23; China, the Korean
Peninsula, Japan, Vietnam), Sasa (c 60; temperate East Asia, with
their highest diversity in Japan), Arundinaria (3; A.
appalachiana, A. gigantea, A. tecta; eastern United
States), Bashania (4; B. abietina, B. fansipanensis,
B. fargesii, B. qingchengshanensis; China; in
Arundinaria?); unplaced Arundinarieae:
Chimonobambusa (42; the Himalayas, China, Japan),
Drepanostachyum (13; the Himalayas to southwestern China; incl.
Himalayacalamus?), Himalayacalamus (9; the Himalayas to
southwestern China; in Drepanostachyum?), Gelidocalamus (13;
southeastern China), Indosasa (20; southern China, Vietnam),
Oligostachyum (17; China), Pseudosasa (21; China, the Korean
Peninsula, Japan), Sarocalamus (3; S. faberi, S.
racemosus, S. spanostachyus; southern and eastern Himalayas to
southwestern China), Sasaella (13; Japan), Semiarundinaria
(7; S. densiflora, S. fastuosa, S. fortis, S.
kagamiana, S. shapoensis, S. sinica, S.
yashadake; China, Japan; hybrid origin), Sinobambusa (13;
southwestern and southern China, northern Vietnam), Vietnamocalamus
(1; V. catbaensis; Vietnam), ’Yushania’ (c 70; southern
Himalayas to southern China and Peninsular Thailand, Taiwan, Indochina, the
Philippines, northern Borneo; Madagascar; polyphyletic). – The Himalayas to
East Asia, North America, with their largest diversity in China. – The
Malagasy species of ’Yushania’ should probably be separated from
the rest. Ampelocalamus may be sister to the remaining
Arundinarieae. Arundinarieae aresister-group
to[Bambuseae+Olyreae].
Bambuseae Kunth ex
Dumort., Anal. Fam. Plant.: 63. 1829 [‘Bambusaceae’]
67/890–915.
Melocanninae Benth. in J. Linn. Soc. London, Bot. 19:
31. 24 Dec 1881 ['Melocanneae']. ’Cephalostachyum’ (14;
Madagascar, eastern Himalayas, Tibet, Yunnan, Burma, Southeast Asia, the
Philippines; polyphyletic), Melocanna (2; M. arundina, M.
baccifera; India, Southeast Asia, Malesia), Schizostachyum (c 65;
southeastern and northeastern India, Sri Lanka, the Himalayas, China, Southeast
Asia), Annamocalamus (1; A. kontumensis; Vietnam). –
Hickeliinae A. Camus in H. Lecomte, Compt. Rend.
Hebd. Seances Acad. Sci. 179: 480. 1 Sep 1924. Hickelia (4; H.
africana, H. alaotrensis, H. madagascariensis, H.
perrieri; Tanzania, Madagascar), Nastus (c 15; Madagascar,
Réunion, Southeast Asia, Malesia to New Guinea), Sirochloa (1; S.
parvifolia; Madagascar, the Comoros), Cathariostachys (2; C.
capitata, C. madagascariensis; Madagascar), Decaryochloa
(1; D. diadelpha; Madagascar), Hitchcockella (1; H.
baronii; Madagascar), Perrierbambus (2; P.
madagascariensis, P. tsarasaotrensis; Madagascar),
Sokinochloa (7; S. australis, S. bosseri, S.
brachyclada, S. chapelieri, S. chiataniae, S.
perrieri, S. viguieri; Madagascar), Valiha (2; V.
diffusa, V. perrieri; Madagascar). –
Bambusinae J. Presl in C. Presl, Reliq. Haenk. 1:
256. Jan-Jun 1830 ['Bambusaceae']. Bonia (5; B.
amplexicaulis, B. laevigata, B. parvifloscula, B.
saxatilis, B. tonkinensis; southern China, Vietnam),
Neomicrocalamus (4; N. andropogonifolius, N.
dongvanensis, N. prainii, N. yunnanensis; East Asia),
Soejatmia (1; S. ridleyi; the Malay Peninsula),
Pseudoxytenanthera (4; P. bourdillonii, P.
monadelpha, P. ritcheyi, P. stocksii; southern India,
Sri Lanka), Maclurochloa (1; M. montana; the Malay
Peninsula), ’Melocalamus’ (13; India, southern China, Southeast
Asia; polyphyletic), ’Dendrocalamus’ (55–60; India, Sri Lanka,
Southeast Asia to China and the Philippines; polyphyletic),
‘Gigantochloa‘ (c 70; India, Sri Lanka, Southeast Asia, Malesia to
New Guinea; non-monophyletic), ’Bambusa’ (145–150; tropical and
subtropical regions in Asia, Australia and America; polyphyletic; incl.
Fimbribambusa?), Fimbribambusa (2; F. horsfieldii,
F. microcephala; Central and East Malesia; in Bambusa?),
Oreobambos (1; O. buchwaldii; tropical East Africa),
Oxytenanthera (1; O. abyssinica; tropical Africa),
Vietnamosasa (3; V. ciliata, V. darlacensis, V.
pusilla; southern Vietnam), Thyrsostachys (2; T.
oliveri, T. siamensis; Burma and Thailand to China),
Cochinchinochloa (1; C. braiana; southern Vietnam),
Yersinochloa (1; Y. dalatensis; southern Vietnam),
Temochloa (1; T. liliana; southern Thailand). –
Racemobambosinae Stapleton in Edinburgh J. Bot. 51:
323. Oesterr. Bot. 1994. Racemobambos (19; India, East and Southeast
Asia, Malesia to New Guinea), Chloothamnus (10; Southeast Asia,
Malesia to New Guinea), Widjajachloa (2; New Guinea). –
Dinochloinae K. M. Wong et W. L. Goh, Sandakania 22.
2016. Mullerochloa (1; M. moreheadiana; northern Queensland),
Dinochloa (c 35; Burma to the Philippines), Neololeba (3;
N. atra, N. glabra, N. hirsuta; Central Malesia to
New Guinea and tropical Australia), Parabambusa (2; P. kaini,
P. marginata; New Guinea), Sphaerobambos (3; S.
hirsuta, S. philippinensis, S. subtilis; Malesia),
Cyrtochloa (7; C. fenixii, C. hirsuta, C.
luzonica, C. major, C. mindoroensis, C. puser,
C. toppingii; the Philippines). –
Greslaniinae K. M. Wong et W. L. Goh, Sandakania 22.
2016. Greslania (4; G. circinnata, G. montana,
G. multiflora, G. rivularis; New Caledonia). –
Holttumochloinae K. M. Wong et W. L. Goh, Sandakania
22. 2016. Kinabaluchloa (1; K. wrayi; Thailand, Vietnam, the
Malay Peninsula), Holttumochloa (3; H. korbuensis, H.
magica, H. pubescens; the Malay Peninsula, Borneo),
Nianhochloa (1; N. bidoupensis; Mount Bidoup in southern
Vietnam). – Temburongiinae K. M. Wong, Sandakania
22. 2016. Temburongia (1; T. simplex; Temburong River in
Brunei). – Chusqueinae Soderstr. et R. P. Ellis in
T. R. Soderstrom et al. (eds.), Grass Syst. Evol.: 238. Mar 1988.
Chusquea (155–160; Mexico, Central America, tropical South America
including the Andes, Chile, western Argentina). –
Guaduinae Soderstr. et R. P. Ellis in T. R.
Soderstrom et al. (eds.), Grass Syst. Evol.: 238. Mar 1988. Tibisia
(3; T. angustifolia, T. farcta, T. pinifolia; the
West Indies), Apoclada (1; A. simplex; Brazil),
Eremocaulon (4; E. amazonicum, E. asymmetricum,
E. aureofimbriatum, E. capitatum; Brazil), Guadua
(26; Mexico, Central America, tropical South America), Olmeca (5;
O. clarkiae, O. fulgor, O. recta, O.
reflexa, O. zapotecorum; Mexico), Otatea (10; Mexico,
Central America, northern Colombia). –
Arthrostylidiinae Soderstr. et R. P. Ellis in T. R.
Soderstrom et al. (eds.), Grass Syst. Evol.: 238. Mar 1988.
Glaziophyton (1; G. mirabile; mountains in eastern Brazil),
Cambajuva (1; C. ulei; southern Brazil),
Actinocladum (1; A. verticillatum; Brazil),
Athroostachys (2; A. capitata, A. shepherdiana;
eastern Brazil), Merostachys (c 45; Central America, tropical South
America), Filgueirasia (2; F. arenicola, F.
cannavieira; Brazil), Alvimia (3; A. auriculata, A.
gracilis, A. lancifolia; Brazil), Atractantha (5; A.
amazonica, A. aureolanata, A. cardinalis, A.
falcata, A. radiata, A. shepherdiana; eastern Brazil),
Colanthelia (7; C. burchellii, C. cingulata, C.
distans, C. intermedia, C. lanciflora, C.
macrostachya, C. rhizantha; southern Brazil, northeastern
Argentina), Aulonemia (c 45; Central America, tropical South America),
’Arthrostylidium’ (30–35; southern Mexico, Central America, the
West Indies, tropical South America; non-monophyletic; incl.
Rhipidocladum?), Rhipidocladum (16; southern Mexico, Central
America, tropical South America; in Arthrostylidium?),
Elytrostachys (2; E. clavigera, E. typica; Central
America, Colombia, Venezuela), Didymogonyx (2; D. geminatum,
D. longispiculatum; Colombia, Venezuela); unplaced
Arthrostylidiinae: Myriocladus (12; the Roraimas in
Venezuela). – Unplaced Bambuseae:
Ruhooglandia (1; New Guinea). – Pantropical, the Himalayas, East
Asia, New Caledonia, with their largest diversity in tropical Asia and tropical
South America. – Bambuseae are sister-group to Olyreae. The
American clade
[Chusqueinae+[Guaduinae+Arthrostylidiinae]] is
sister-group the the remaining Bambuseae (the Paleotropical clade;
Zhou & al. 2017).
Olyreae Kunth ex
Spenn. Fl. Friburg. 1: 172. 1825
21/c 115.
Parianinae Hack. in H. G. A. Engler et K. A. E.
Prantl, Nat. Pflanzenfam. II, 2: 88. Nov 1887 ['Parianeae'].
’Pariana’ (c 30; Costa Rica and Panamá to Amazonian and
southeastern Brazil and Bolivia; paraphyletic; incl. Eremitis?),
Eremitis (1; E. parviflora; Atlantic Brazil; in
Pariana?), Parianella (2; P. carvalhoi, P.
lanceolata; southern Bahia in Brazil). –
Buergersiochloinae L. G. Clark et Judz. in Aliso 23:
311. Dec 2007. Buergersiochloa (1; B. bambusioides; New
Guinea). – Olyrinae Kromb., Flore Luxembourg: 496.
1875. Agnesia (1; A. lancifolia; Amazonas),
Cryptochloa (8; southern Mexico, Central America, tropical South
America), ’Olyra’ (24; Mexico, Central America, tropical South
America, one species, O. latifolia, also in tropical Africa,
Madagascar, the Comoros and the West Indies; paraphyletic), Arberella
(7; A. bahiensis, A. costaricensis, A. dressleri,
A. flaccida, A. grayumii, A. lancifolia, A.
venezuelae; Costa Rica to Brazil; in Olyra?), Lithachne
(4; L. horizontalis, L. humilis, L. pauciflora,
L. pinetii; southern Mexico, Central America, Cuba, tropical South
America; in Olyra?), Reitzia (1; R. smithii;
Venezuela and Trinidad to southern Brazil), Diandrolyra (3; D.
bicolor, D. pygmaea, D. tatianae; southeastern Brazil),
Ekmanochloa (2; E. aristata, E. subaphylla; eastern
Cuba), Froesiochloa (1; F. boutelouoides; tropical South
America), Maclurolyra (1; M. tecta; Panamá),
Mniochloa (1; M. pulchella; Cuba), ’Parodiolyra’
(6; P. aratityopensis, P. colombiensis, P.
lateralis, P. luetzelburgii, P. micrantha, P.
ramosissima; Central America, tropical South America; paraphyletic),
Piresiella (1; P. strephioides; Cuba), Raddiella (8;
R. esenbeckii, R. kaieteurana, R. lunata, R.
malmeana, R. minima, R. molliculma, R.
potaroensis, R. vanessiae; Central America, tropical South
America), ’Sucrea’ (3; S. maculata, S.
monophylla, S. sampaiana; Brazil; paraphyletic), Raddia
(9; Guyana, eastern Brazil), Rehia (1; R. nervata; tropical
South America). – Tropical America, one species in New Guinea. –
Olyreae are sister-group to Bambuseae. Species of
Olyra and Pariana are said to be pollinated by insects
(Soderstrom & Calderón 1971).
Pooideae Benth., Fl.
Hongk.: 407. Feb 1861 [’Poaeaceae’]
186–188/3.630–3.680. Mainly
temperate regions in the Northern Hemisphere. Epichloe endophytes
pervasive. C3 photosynthesis present. C4 photosynthesis
probably absent. Asymmetric division of root epidermal cells forming
trichoblast-atrichoblast pair. Longitudinal walls of epidermal cells straight.
Microhairs absent. Stomatal subsidiary cells with parallel margins. Primary
inflorescence branches distichous. Lemma usually with five veins. Lodiculae
slightly or not vascularized. Styles almost entirely separate (connate only at
base). Hilum often short. Embryo small. Epiblast present at least in most
Pooideae (absent in Bromus and
in some Brachypodium
and Hordeeae). Scutellum not peltate. Scutellum tail absent (also from
some Brachyelytrum). n = (2, 4–)7(–13). Duplication of β-amylase
gene. Fructose oligosaccharides present in stem. – Epichloe
(Clavicipitaceae), an endophytic group of ascomycetes, is only known
in Pooideae. A similar group is Neotyphodium. At least 30% of
the species in Pooideae are associated with this kind of symbioses and
vertical as well as horizontal transfers of these fungi take place. Numerous
alkaloids (e.g. ergotamins) are produced as a result of these symbioses.
Brachyelytreae Ohwi in
Bot. Mag. (Tokyo) 55: 361. 1941
1/3. Brachyelytrum (3; B.
aristosum, B. erectum, B. japonicum; Japan, southeastern
China, eastern United States). – Stomatal subsidiary cells with parallel
sides. n = 11. – Brachyelytrum is probably sister to the remaining
Pooideae. On the other hand, it is sometimes placed as part of a
polytomy including also Bambusoideae and Ehrhartoideae (see,
e.g., Blaner & al. 2014).
Nardeae W. D. J. Koch,
Syn. Fl. Germ. Helv.: 830. Jan-Oct 1837
1/1. Nardus (1;
N. stricta; Europe except the Mediterranean, western Asia). –
Europe, western Asia, the Mediterranean. Microhairs bicellular. Lodiculae
absent. Style and stigma single. n = 10, 13. – Nardeae are
successive sister-group to the remaining Pooideae except
Brachyelytrum, but see Soreng & al. (2017).
Lygeeae J. Presl,
Wsobecny Rostl. 2: 1708, 1753. 1846
1/1. Lygeum (1; L.
spartum; the Mediterranean, North Africa). Microhairs bicellular.
Lodiculae absent. Inflorescence spatheate. Spikelet very modified. Style and
stigma single. – Nardeae, Lygeeae, Duthieeae and
Phaenospermateae are joined in the supertribe Nardodae by
Soreng & al. (2017).
Duthieeae Röser et
Jul. Schneider in Syst. Biodivers. 9: 41. Mar 2011
7/11. Stephanachne (2; S.
nigrescens, S. pappophorea; Central Asia, western China),
Sinochasea (1; S. trigyna; western China),
Danthoniastrum (1; D. compactum; the Balkan Peninsula, the
Caucasus), Pseudodanthonia (1; P. himalaica; mountains in
northern India), Metcalfia (1; M. mexicana; Mexico),
Anisopogon (1; A. avenaceus; eastern New South Wales, eastern
Victoria), Duthiea (4; D. brachpodium, D. bromoides,
D. oligostachya, D. nepalensis; Afghanistan to China). –
Temperate and subtropical regions in the Old World, Mexico.
Phaenospermateae
Renvoize et Clayton in Kew Bull. 40: 478. 4 Jul 1985
1/1. Phaenosperma (1; P.
globosa; Assam, southeastern China, the Korean Peninsula, Japan, Taiwan).
– Northeastern India to East Asia including Taiwan.
Meliceae Link ex
Endl., Fl. Poson.: 116. Mai 1830 [’Melicaceae’]
8/133.
Brylkiniinae Ohwi in Acta Phytotax. Geobot. 10: 107.
1 Jun 1941. Brylkinia (1; B. caudata; eastern Russia, China,
Japan). – Melicinae Fr., Flora Scan.: 204, 208.
1835 [’Meliceae’]. Glyceria
(38; temperate regions on the Northern Hemisphere, with their largest diversity
in North America), Lycochloa (1; L. avenacea; Lebanon),
Schizachne (1; S. purpurascens; arctic and temperate Russia,
Siberia, Central Asia, Mongolia, China, the Korean Peninsula, temperate and
arctic North America, mountains in southwestern United States), Melica (78;
temperate regions on both hemispheres except Australia), Pleuropogon
(6; P. californicus, P. davyi, P. hooverianus,
P. oregonus, P. refractus, P. sabinei; western
United States, one species, P. sabinei, arctic circumpolar),
Triniochloa (6; T. andina, T. gracilis, T.
laxa, T. micrantha, T. stipoides, T. talpensis;
Mexico, Central America, northwestern South America), Koordersiochloa
(2; K. longiarista, K. sanjappae; tropical and southern
Africa, Réunion, southern India, mountains in Indonesia and the Philippines).
– Temperate regions on the Northern Hemisphere, few species in tropical
regions and Chile. – Meliceae may be sister-group to the remaining
Pooideae. Brylkinia and Koordersiochloa have a
cylindrical to lanceoloid style and a punctate hilum. Some species of
Melica have seeds possessing elaiosome formed by clavate lemmas or
sterile florets.
Ampelodesmeae (Conert)
Tutin in Bot. J. Linn. Soc. 76(4): 369. Jun 1978
1/1. Ampelodesmos (1; A.
mauritanicus; the Mediterranean). – Ampelodesmos probably
originated from ancient hybridization between members of Duthieeae and
Stipeae (Soreng & al. 2017). It is often recovered as sister to
Stipeae (e.g. Hochbach & al. 2015).
Stipeae Dumort.,
Observ. Gramin. Belg.: 83. Jul-Sep 1824 [’Stipaceae’]
23–25?/545–550. Macrochloa
(1; M. tenacissima; the Mediterranean), ’Stipa’ (c
150; Europe, North Africa, temperate and drier subtropical regions in Asia,
with their highest diversity in southwestern and southern Asia; paraphyletic),
Ortachne (3; O. breviseta, O. erectifolia, O.
rariflora; Costa Rica to Peru, Chile and Argentina), Psammochloa
(1; P. villosa; the Gobi Desert), Trikeraia (4; T.
hookeri, T. oreophila, T. pappiformis, T.
tianshanica; Pakistan to China), ’Ptilagrostis’ (13; Russia
to China; polyphyletic), Piptatheropsis (5; P. canadensis,
P. exigua, P. micrantha, P. pungens, P.
shoshoneana; Canada, United States), Hesperostipa (5; H.
comata, H. curtiseta, H. neomexicana, H.
saxicola, H. spartea; Canada, United States, northern Mexico),
Piptochaetium (35; semi-arid grasslands in the United States and
Mexico to Argentina), Aciachne (3; A. acicularis, A.
flagellifera, A. pulvinata; the High Andes in Costa Rica to
northern Argentina), Anatherostipa (10; the Andes in Costa Rica till
Chile), Patis (3; P. coreana, P. obtusa, P.
racemosa; temperate regions on the Northern Hemisphere), Oryzopsis
(1; O. asperifolia; North America), ’Achnatherum’
(c 40; Europe, North Africa, temperate regions in Asia east to Japan),
Eriocoma (>3?; North America)?, Celtica (1; C.
gigantea; western Mediterranean), Anemanthele (1; A.
lessoniana; New Zealand), Austrostipa
(c 60; Australia, one species also in New Zealand), Nassella
(115–120; western and southwestern United States, Mexico, Central America,
the West Indies, tropical and subtropical South America, with their largest
diversity in the Andes), Jarava (c
55; South America), Oloptum (1; O. miliaceum; the
Mediterranean, southwestern Asia), Orthoraphium (1; O.
roylei; the Himalayas), Pappostipa (23; the United States and
Mexico to Chile and Argentina), Stipellula (5; S. capensis,
S. nitens, S. parviflora, S. staintonii, S.
tigrensis; warm-temperate and subtropical regions in the Old World),
Timouria (4?; Pakistan and Central Asia to Mongolia and China; in
Psammochloa?)? – Subcosmopolitan, with their highest diversity in
temperate and alpine regions. – The clade
[Stipeae+Ampelodesmos] may be sister-group to all the
Pooideae below but see Soreng & al. (2017). The taxonomy within
Stipeae is still unclear. However, Macrochloa is a potential
sister to the remaining Stipeae.
Diarrheneae (Ohwi)
Tateoka ex C. S. Campb. in J. Arnold Arbor. 66: 188. 8 Apr 1985
1/5. Diarrhena (5; D.
americana, D. fauriei, D. japonica, D.
mandshurica, D. obovata; East Asia, North America). – Embryo of
Bambusoid type.
Brachypodieae Harz in
Linnaea 43: 15. 1880
1/22. Brachypodium
(22; Europe, the Mediterranean, temperate regions in Asia, southern Africa,
tropical mountains in Mexico to Bolivia). – Brachypodium may be
sister to the rest of Pooideae, although Soreng & al. (2017)
joined Ampelodesmeae, Stipeae and Diarrheneae
together with Brachypodieae in the supertribe Stipodae L.
Liu.
Triticeae Dumort.,
Observ. Gramin. Belg.: 82. Jul-Sep 1824
20/510–520.
Littledaleinae Röser in J. Schneider et al., Taxon
58: 420. 28 Mai 2009. Littledalea (4; L. alaica, L.
przevalskyi, L. racemosa, L. tibetica; Central Asia and
Tibet to western China). – Brominae Dumort., Anal.
Fam. Plant.: 63. 1829 ['Bromeae']. Bromus (c 165; temperate
regions on the Northern Hemisphere, the Mediterranean, southern Africa,
tropical mountains in South America). – Triticinae
Fr., Flora Scan.: 210. 1835 ['Triticeae']. Aegilops (c 25;
the Canary Islands, the Mediterranean to Pakistan and Central Asia; in
Triticum?), Triticum (17; the Mediterranean and southwestern
Asia to China; incl. Aegilops?), Dasypyrum (2; D.
hordeaceum, D. villosum; the Mediterranean), Thinopyrum
(11; coastal regions in Europe, the Mediterranean, southwestern Asia to Iran).
– Hordeinae Dumort., Anal. Fam. Plant.: 63. 1829
['Hordeaceae']. Agropyron (13; temperate regions in the Old
World), Australopyrum (5; A. calcis, A. pectinatum,
A. retrofractum, A. uncinatum, A. velutinum; New
Guinea, eastern Australia, Tasmania, New Zealand), Crithopsis (1;
C. delileana; Crete and Libya to Iran), Elymus (150–155;
temperate regions on the Northern Hemisphere, one species, Connorochloa
tenuis, in New Zealand), Eremopyrum (4; E. bonaepartis,
E. distans, E. orientale, E. triticeum; southern
Europe, the Mediterranean, Morocco to western China), Henrardia (2;
H. persica, H. pubescens; Turkey and Iran to Central Asia),
Heteranthelium (1; H. piliferum; Turkey to Pakistan),
Hordeum (c 35; temperate regions on both hemispheres),
Hordelymus (1; H. europaeus; Europe and North Africa to the
Caucasus; in Leymus?), Leymus (c 55; temperate regions on the
Northern Hemisphere, one species, L. erianthus, also in Chile and
Argentina), Peridictyon (1; P. sanctum; the Balkan
Peninsula), Psathyrostachys (10; eastern Mediterranean to Central
Asia), Secale (9; eastern Europe and eastwards to Central Asia, the
Mediterranean, the Middle East, Roggeveld in Western Cape),
Taeniatherum (1; T. caput-medusae; the Iberian Peninsula, the
Mediterranean to Pakistan and Central Asia). – Temperate and subtropical
regions on both hemispheres, with their highest diversity in temperate Eurasia.
– Triticeae are sister-group to Poeae. Littledalea
is sister to the remaining Triticeae. Bromus is successive
sister to the rest.
Poeae R. Br. in M.
Flinders, Voy. Terra Austral. 2: 582. 19 Jul 1814 [’Poaceae’]
121/2.395–2.430.
Torreyochloinae Soreng et J. I. Davis in Contr. U. S.
Natl. Herb. 48: 721. Oct 2003. Amphibromus (12; Australia, New
Zealand, temperate South America), Torreyochloa (5; T.
erecta, T. fernaldii, T. natans, T. pallida,
T. pauciflora; northeast Asia, Canada, United States). –
Phalaridinae Fr., Flora Scan.: 195. 1835
['Phalarideae']. Phalaris (17; temperate regions on the
Northern Hemisphere, the Mediterranean, Andean and southern South America). –
Aveninae J. Presl in C. Presl, Reliq. Haenk. 1: 246.
Jan-Jun 1830 ['Avenaceae']. Arrhenatherum (7; A.
album, A. calderae, A. elatius, A. kotschyii,
A. longifolium, A. palaestinum, A. pallens; Europe,
the Mediterranean, northern and western Asia), Avena (22; Europe, the
Mediterranean, North Africa to Ethiopia, southwestern Asia),
Helictotrichon (60; western United States, Eurasia, the Mediterranean,
East and Southeast Asia, tropical mountains). –
Koeleriinae Asch. et Graebn., Syn. Mitteleur. Fl.
2(1): 342. 22 Mai 1900. Sphenopholis (7; S. filiformis,
S. intermedia, S. interrupta, S. longiflora, S.
nitida, S. obtusata, S. pensylvanica; Canada, United
States, Mexico, Hispaniola, the Hawaiian Islands), Cinnagrostis
(80–90; Central America, South America), Peyritschia (7; P.
conferta, P. deyeuxioides, P. erythraea, P.
humilis, P. koelerioides, P. pringlei, P.
roederi; Mexico, Central America, northwestern tropical South America),
Trisetopsis (20–25; tropical to southern Africa, Madagascar, Yemen,
the Himalayas, central China, southern India and Sri Lanka, Sumatra, Java),
Trisetaria (16; the Mediterranean to western Himalayas),
Koeleria (c 45; temperate regions on both hemispheres, tropical and
subtropical African mountains), Rostraria (12; Europe, the
Mediterranean, southern Africa), Gaudinia (4; G. coarctata,
G. fragilis, G. hispanica, G. maroccana; the Azores,
the Mediterranean), Graphephorum (2; G. melicoides, G.
wolfii; North America, Mexico, Central America), Trisetum (c 75;
temperate regions on the Northern Hemisphere), Tzveleviochloa (3;
T. burmanica, T. parviflora, T. potaninii; Assam,
northern Burma, central China). – Lagurus (1; L. ovatus;
the Mediterranean), Tricholemma (2; T. breviaristatum, T.
jahandiezii; Morocco, Algeria). –
Anthoxanthinae A. Gray, Man. Bot., ed. 2: 538. 1 Sep
1856 ['Anthoxantheae']. Anthoxanthum (c 50; temperate and
alpine regions on the Northern Hemisphere, tropical mountains in Africa and
Asia). – Brizinae Tzvelev in Bot. Zhurn. (Moscow et
Leningrad) 53: 310. 5 Mar 1968. Airopsis (1; A. tenella;
southern Europe, northwestern Africa), Briza (5; B. humilis,
B. marcowiczii, B. maxima, B. media, B.
minor; Europe, temperate Asia, the Mediterranean, South America). –
Echinopogoninae Soreng in J. Syst. Evol. 55(4): 262.
2017. Ancistragrostis (1; A. uncinioides; New Guinea,
tropical Australia)?, Bromidium (2; B. ramboi, B.
trisetoides; South America)?, Dichelachne (9; East Malesia to New
Guinea and Australia, New Zealand), Echinopogon (7; E.
caespitosus, E. cheelii, E. intermedius, E.
mckiei, E. nutans, E. ovatus, E. phleoides; New
Guinea, Australia, New Zealand), Pentapogon (1; P.
quadrifidus; southeastern South Australia, southeastern New South Wales,
Victoria, Tasmania), Relchela (1; R. panicoides; Chile,
Argentina). – Calothecinae Soreng 2015.
Chascolytrum (23; Mexico, Central America, South America). –
Agrostidinae Fr., Flora Scan.: 196. 1835
['Agrostideae']. Agrostis (190–200; temperate regions on
both hemispheres, tropical mountains, Macquarie Island, subAntarctic South
America, Falkland Islands, Crozet Islands, Kerguélen, Prince Edward Islands),
’Calamagrostis’ sensu stricto (c 260; temperate regions on both
hemispheres; non-monophyletic), Gastridium (2; G. phleoides,
G. ventricosum; western Europe, Macaronesia, the Mediterranean to the
Caucasus and Iran), Hypseochloa (2; H. cameroonensis, H.
matengoensis; Mount Cameroon, mountains in Tanzania), Limnodea
(1; L. arkansana; southern United States), Polypogon (21;
warm-temperate regions on both hemispheres, tropical mountains),
Triplachne (1; T. nitens; the Mediterranean). –
Scolochloinae Tzvelev in Komarov. Chten. 37: 33.
1987. Dryopoa (1; D. dives; southeastern New South Wales,
Victoria, Tasmania), Scolochloa (1; S. festucacea; temperate
regions on the Northern Hemisphere). – Sesleriinae
Parl., Flora Palerm.: 127. Mar-Dec 1845 ['Seslerieae'].
Sesleriella (1; S. sphaerocephala; central and eastern Alps),
Mibora (2; M. maroccana, M. minima; western Europe,
northwestern Africa), Oreochloa (5; O. blanka, O.
confusa, O. disticha, O. elegans, O.
seslerioides; the Pyrenees, the Alps, the Carpathians), Echinaria
(1; E. capitata; southern and southeastern Europe, the Mediterranean,
southwestern Asia to the Caucasus), Psilathera (1; P. ovata;
central and eastern Alps), Sesleria (c 30; Europe, Morocco, Libya,
western Asia to the Caucasus and Iran). – Airinae
Fr., Flora Scan.: 196, 198. 1835 ['Aireae']. Avenella (1;
A. flexuosa; temperate regions on the Northern Hemisphere),
Aira (8; Europe, the Mediterranean to Iran), Antinoria (2;
A. agrostidea, A. insularis; the Mediterranean),
Corynephorus (5; C. canescens, C. deschampsioides,
C. divaricatus, C. fasciculatus, C. macrantherus;
Europe, the Mediterranean to Iran), Helictochloa (22; central and
southern Europe, the Mediterranean, the Canary Islands, North Africa, the
Balkan Peninsula, eastern Europe to Crimea and the Caucasus, the Middle East,
one species, H. hookeri, in Russia, Ukraine, Central Asia, Mongolia,
China and North America), Periballia (3; P. involucrata,
P. laevis, P. minuta; the Mediterranean). –
Holcinae Dumort. in Bull. Soc. Roy. Bot. Belgique 7:
68. 1868 ['Holceae']. Holcus (10; Europe, the Mediterranean,
North and southern Africa, southwestern Asia), Vahlodea (1; V.
atropurpurea; cold-temperate and arctic-alpine regions on the Northern
Hemisphere, subAntarctic South America). –
Aristaveninae F. Albers et Butzin in Willdenowia 8:
82. 25 Aug 1977. Deschampsia (c 40; temperate and polar regions on
both hemispheres, the Andes from 34°10’ south latitude to Tierra del Fuego,
Falkland Islands, South Georgia Island, South Shetland Islands, the Antarctic
Peninsula and adjacent islands, South Orkney Islands, South Sandwich Islands,
Prince Edward Islands, Crozet islands, Kerguelen Islands, Heard Island). –
Loliinae Dumort., Anal. Fam. Plant.: 63. 1829
['Loliaceae']. Castellia (1; C. tuberculosa;
Macaronesia, the Mediterranean, North Africa to Pakistan), Festuca
(470–480?; temperate, polar and alpine regions on both hemispheres, Tierra
del Fuego, Falkland Islands, South Georgia Islands, Kerguelen Islands,
Macquarie Island, tropical mountains), Leucopoa (10?; temperate Asia,
Canada, United States), Drymochloa (5; D. donax, D.
drymeja, D. grandis, D. lasto, D. sylvatica;
Europe, western Mediterranean, North Africa; in Leucopoa?),
Lolium (12; temperate regions on the Northern Hemisphere, the Canary
Islands, the Mediterranean), Megalachne (3; M. berteroniana,
M. robinsoniana, M. masafuerana; Juan Fernandez Islands),
Patzkea (1–5; P. paniculata; Europe, the Mediterranean),
Podophorus (1; P. bromoides; Juan Fernandez Islands). –
Pseudobromus (6; P. africanus, P. ambilobensis,
P. breviligulatus, P. engleri, P. humbertianus,
P. tenuifolius; tropical and subtropical Africa)? –
Dactylidinae Stapf in W. H. Harvey et O. W. Sonder
(ed. W. T. Thiselton-Dyer), Flora Cap. 7: 317. Jul 1898
['Dactylideae']. Dactylis (2; D. glomerata: Europe,
temperate Asia; D. smithii: Macaronesia), Lamarckia (1;
L. aurea; the Mediterranean, the Middle East to Pakistan). –
Cynosurinae Fr., Flora Scan.: 204. 1835
['Cynosureae']. Cynosurus (9; Europe, the Mediterranean,
southwestern Asia). – Ammochloinae Tzvelev, Zlaki
SSSR: 535. 1976. Ammochloa (3; A. involucrata, A.
palaestina, A. pungens; the Mediterranean, southwestern Asia).
– Parapholiinae Caro in Dominguezia 4: 41. 1982.
Agropyropsis (1; A. lolium; Algeria), Cutandia (6;
C. dichotoma, C. divaricata, C. maritima, C.
memphitica, C. rigescens, C. stenostachya; the
Mediterranean, Macaronesia, southwestern Asia), Desmazeria (4; D.
lorentii, D. philistaea, D. pignattii, D.
sicula; Europe, the Mediterranean, North Africa, southwestern Asia to
Iran), Hainardia (1; H. cylindrica; the Mediterranean),
Parapholis (6; P. filiformis, P. gracilis, P.
incurva, P. marginata, P. pycnantha, P.
strigosa; Europe, the Mediterranean, southern Asia), Sphenopus
(2; S. divaricatus, S. ehrenbergii; the Mediterranean,
southwestern Asia to Iran), Vulpiella (1; V. tenuis; western
Mediterranean). – Avenula (1; A. pubescens; temperate
Eurasia). – Coleanthinae Rouy, Flore France 14: 28,
55. Apr 1913. Catabrosa (3; C. aquatica: temperate regions on
the Northern Hemisphere, southern South America; C. drakensbergensis:
Lesotho, KwaZulu-Natal; C. werdermannii: the Andes in Bolivia, Chile
and Argentina), Coleanthus (1; C. subtilis; temperate regions
on the Northern Hemisphere), Phippsia (3; P. algida, P.
concinna, P. wilczekii; arctic regions on the Northern
Hemisphere), Puccinellia (c 125; temperate and arctic regions on the
Northern Hemisphere, southern Africa, one species, P. stricta, in
Australia), Sclerochloa (2; S. dura, S. woronowii;
the Mediterranean, North Africa and the Middle East to Central Asia and China).
– Poinae Dumort., Anal. Fam. Plant.: 63. 1829
['Poeae']. Poa (>500; temperate, alpine and polar regions
on both hemispheres, tropical mountains). –
Miliinae Dumort., Anal. Fam. Plant.: 64. 1829
[’Miliaceae’]. Milium (6; M. atropatanum, M.
effusum, M. pedicellare, M. schmidtianum, M.
transcaucasicum, M. vernale; Europe, temperate Asia, eastern
North America). – Phleinae Dumort. in Bull. Soc.
Roy. Bot. Belgique 7: 69. 1868 [‘Phleae‘]. Phleum (16;
temperate regions on the Northern Hemisphere, temperate South America). –
Beckmanniinae Nevski in Trudy Bot. Inst. Akad. Nauk
S.S.S.R., ser. 1, Fl. Sist. Vyssh. Rast. 4: 228. 1937. Beckmannia (2;
B. eruciformis, B. syzigachne; temperate regions on the
Northern Hemisphere), Pholiurus (1; P. pannonicus;
southeastern Europe to Central Asia), Pseudophleum (2; P.
anatolicum, P. gibbum; Turkey to Iran), Rhizocephalus
(1; R. orientalis; the Mediterranean to Iran). –
Cinninae Caruel, Epit. Flor. Europ. 1: 69. Jan 1892
[’Cinneae’]. Cinna (4; C. arundinacea, C.
bolanderi, C. latifolia, C. poiformis; temperate regions
on the Northern Hemisphere), Aniselytron (2; A. agrostoides,
A. treutleri; northern India to Japan), Cyathopus (1; C.
sikkimensis; the Himalayas), Simplicia (2; S.
buchananii, S. laxa; New Zealand), Agrostopoa (3; A.
barclayae, A. wallisii, A. woodii; Colombia)? –
Alopecurinae Dumort., Anal. Fam. Plant.: 64. 1829
[’Alopecureae’]. Alopecurus (c 40; temperate regions on
the Northern Hemisphere, temperate South America), Cornucopiae (2;
C. alopecuroides, C. cucullatum; eastern Mediterranean to
Iraq), Limnas (3; L. malyschevii, L. stelleri,
L. veresczagini; Central Asia to northeastern Siberia). –
Ventenatinae Holub ex L. J. Gillespie, Cabi et Soreng
in J. Syst. Evol. 55(4): 264. 2017. Ventenata (8; southern Europe, the
Mediterranean to the Caspian Sea), Bellardiochloa (5; B.
argaea, B. carica, B. polychroa, B. variegata,
B. violacea; central and southern Europe, the Mediterranean,
southwestern Asia), Nephelochloa (1; N. orientalis; Turkey),
Apera (5; A. baytopiana, A. intermedia, A.
interrupta, A. spica-venti, A. triaristata; Europe,
southwestern Asia to Afghanistan), Parvotrisetum (1; P.
myrianthum; northern Italy, southern and western Balkan Peninsula). –
Cosmopolitan, with their largest diversity in temperate regions on the Northern
Hemisphere. – Poeae are sister-group to Triticeae. –
Poeae incertae sedis: Brizochloa (1; B.
humilis; eastern Europe to the Caucasus and western Asia),
Dupontia (2; D. fisheri, D. fulva; arctic regions),
Dupontiopsis (1; D. hayachinensis; mountains in northern
Japan), Arctagrostis (1; A. latifolia; arctic regions),
Hookerochloa (1; H. hookeriana; eastern New South Wales,
Victoria, Tasmania), Nicoraepoa (6; N. andina, N.
chonotica, N. erinacea, N. pugionifolia, N.
robusta, N. subenervis; the Andes in South America),
Saxipoa (1; S. saxicola; southeastern New South Wales,
eastern Victoria, Tasmania), Sylvipoa (1; S. queenslandica;
southeastern Queensland, eastern New South Wales).
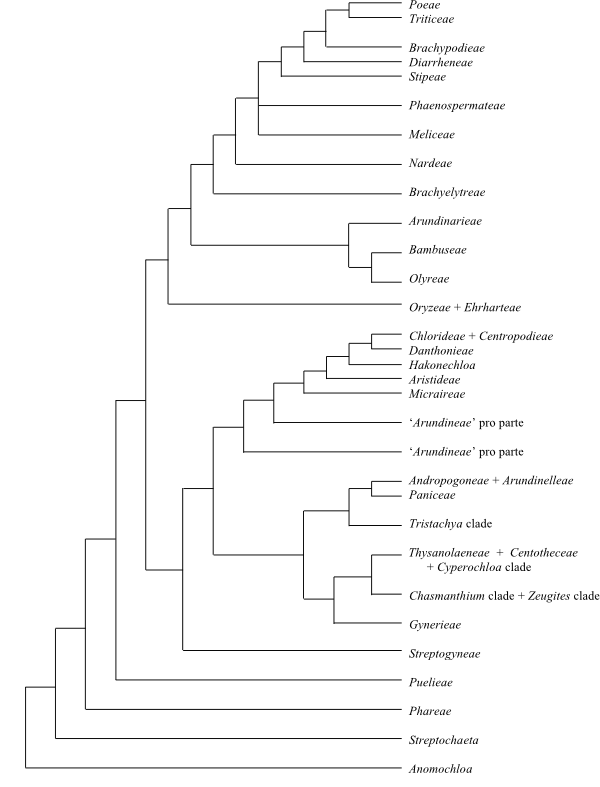 |
Cladogram (simplified and slightly modified)
of Poaceae based on DNA
sequence data (Bouchenak-Khelladi & al. 2008; Schneider & al.
2009). Anomochloa and Streptochaeta are sister-groups
in some analyses. Isachne is sister toEriachne
inMicrairoideae
(=Micraireae)in, e.g., Peterson &
al. (2011).
|
PRIONIACEAE S. L. Munro
et H. P. Linder
|
( Back to Cyperales )
|
S. L. Munro et H. P. Linder in Syst. Bot. 23: 51.
4 Mai 1998
Genera/species 1/1
Distribution Southern South
Africa.
Fossils Unknown.
Habit Bisexual, perennial
herbs or, in older individuals, with lignified up to one metre tall (sometimes
taller) aerial stem. Graminids. Helophyte. Culm angular in cross-section,
erect.
Vegetative anatomy Mycorrhiza
absent? Aerial stem with thin-walled epidermis, cortex with loosely packed
parenchyma cells and scattered vascular bundles, and central cylinder with
scattered amphivasal bundles. Phellogen absent. Secondary lateral growth
absent. Vessels present in roots, stem and leaves. Vessel elements usually with
scalariform (in stems sometimes simple) perforation plates; lateral pits?
Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial
parenchyma? Sieve tube plastids P2c type, with cuneate protein crystals. Nodes?
Silica bodies spherical, in epidermal cells; silica bodies absent from
parenchyma cells. Tanniniferous cells scattered. Calciumoxalate crystals
absent.
TrichomesHairs absent, except
for small unicellular hairs on pedicels.
Leaves Alternate
(tristichous), simple, entire, linear, flat or V-shaped in cross-section (with
spinose-serrate keel), unifacial, with convolute (supervolute) ptyxis. Stipules
absent; leaf sheath closed, without ligule? Venation parallelodromous. Stomata
paracytic. Cuticular wax crystalloids as longitudinally aggregated rodlets,
Strelitziatype. Air canals with chlorenchyma. Epidermis with silica
bodies? Mesophyll without mucilaginous idioblasts or calciumoxalate crystals.
Leaf margin spinose-serrate.
Inflorescence Terminal, richly
branched, panicle. Floral prophyll (bracteole) absent.
Flowers Actinomorphic, small.
Hypogyny. Tepals 3+3, sepaloid, membranous, persistent, free. Nectary absent.
Disc absent.
Androecium Stamens 3+3.
Filaments filiform, free from each other and from tepals. Anthers basifixed,
non-versatile?, tetrasporangiate, latrorse?, longicidal (dehiscing by
longitudinal slits). Tapetum secretory, with uninucleate cells. Staminodia
absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains with indistinct aperture
(ulcerate), shed as tetrahedral and tetragonal tetrads, tricellular at
dispersal. Exine tectate, with granular? infratectum, scabrate or ulcerate.
Gynoecium Pistil composed of
three carpels, connate only at base. Ovary superior, trilocular, narrowing
towards apex. Stylodia three, free or slightly connate at base. Stigmas three,
papillate, Dry? type, Pistillodium absent.
Ovules Placentation axile.
Ovules one to seven per carpel, anatropous, ascending, apotropous, bitegmic,
crassinucellar. Micropyle endostomal. Outer integument one or two cell layers
thick. Inner integument two cell layers thick. Parietal cell formed from
archesporial cell. Megagametophyte monosporous, Polygonumtype.
Synergids with a filiform apparatus. Antipodal cells persistent. Endosperm
development ab initio helobial. Endosperm haustoria? Embryogenesis onagrad
(Juncusvariation).
Fruit A loculicidal
capsule.
Seeds Aril absent. Seed coat
testal-tegmic. Exotesta consisting of one to three layers of sclerenchymatous
fibres. Endotesta consisting of one or two parenchymatous layers. Tegmen
consisting of elongate tightly packed tanniniferous cells. Perisperm not
developed. Endosperm copious, starchy. Embryo small, straight, well
differentiated, Xyris-Scirpustype, with chlorophyll.
Cotyledon one, photosynthesizing. Cotyledon hyperphyll? Hypocotyl absent.
Mesocotyl? Coleoptile absent. Plumule terminal. Collar very small, with
rhizoids. Germination phanerocotylar.
Cytology n = ? – Chromosomes
with diffuse centromeres.
DNA Deletion of three base
pairs in plastid gene atpA.
Phytochemistry Very
insufficiently known. Flavone-C-glycosides present.
Luteolin-5-methylether? Cyanogenic compounds not found.
Use Fibre plant.
Systematics Prionium
(1; P. serratum; Western and Eastern Cape, southern Kwazulu-Natal).
Prionium
is sister to [Thurnia+[Juncaceae+Cyperaceae]], according to Munro
& Linder (1998).
Dumortier, Anal. Fam. Plant., 60, 62. 1829, nom.
cons.
Rapateales (Meisn.)
Colella ex Reveal et Doweld in Novon 9: 551. 30 Dec 1999;
Rapateanae Doweld, Tent. Syst. Plant. Vasc.: lxii. 23
Dec 2001
Genera/species 16/90–95
Distribution Tropical Central
and South America, tropical West Africa, with their highest diversity in the
Guayana and Venezuelan Highlands.
Fossils Unknown.
Habit Bisexual, perennial
herbs. Usually hygrophytic (a few species are epiphytic).
Vegetative anatomy Phellogen
absent. Stems with wide outer cortex consisting of parenchyma cells with
starch; inner cortex consisting of several sclereid layers. Central stem
cylinder consisting of sclerified parenchyma and collateral and amphivasal
vascular bundles. Secondary lateral growth absent. Vessels present in roots and
stem (rarely in leaves). Vessel elements with simple and/or scalariform
perforation plates; lateral pits scalariform. Imperforate tracheary xylem
elements tracheids. Wood rays absent. Axial parenchyma? Sieve tube plastids P2c
type, with cuneate protein crystals. Nodes? Mucilage canals present in some
species. Tanniniferous cells often frequent (at least in hypodermis). Silica
bodies (druses) and/or silica sand usually present. Calciumoxalate probably
absent.
Trichomes Axillary,
multicellular, uniseriate, mucilage secreting eglandular hairs often abundant,
especially on young shoots.
Leaves Alternate (usually
distichous, sometimes spiral), simple, entire, often linear, unifacial to
bifacial, usually V-shaped, often twisted 90°, with conduplicate ptyxis,
sometimes with spines. Stipules absent; leaf sheath open (rarely with distal
ligule), symmetrical or asymmetrical. Colleters uniseriate, secreting mucilage.
Venation parallelodromous. Stomata paracytic, with dumbbell-shaped Poaceae type guard cells; subsidiary
cells oily. Cuticular wax crystalloids usually absent (sometimes amorphous and
spherical, non-orientated). Epidermis with silica druses. Mesophyll without
mucilaginous cells and calciumoxalate crystals. Leaf margin entire.
Inflorescence Terminal or
axillary, simple or compound spherical or flattened thyrses or thyrsoid
inflorescences with one to more than 70 many-flowered cymose partial
inflorescences as spike-like bostryces with terminal flower and coriaceous
bracts, often with two or more, sometimes more or less connate, spathe-like
bracts.
Flowers Actinomorphic or
somewhat zygomorphic, large. Hypogyny. Tepals 3+3; outer tepals with imbricate
aestivation, sepaloid, free or connate at base; inner tepals usually with
imbricate aestivation, petaloid, caducous, connate at base. Septal nectaries
present at least in Monotrematoideae. Disc absent. Colleters present
on floral parts.
Androecium Stamens 3+3.
Filament bases usually connate and/or adnate to inner tepals (in one species of
Rapatea hairy). Anthers basifixed to subbasifixed, non-versatile,
usually tetrasporangiate (sometimes disporangiate), extrorse to introrse,
poricidal (dehiscing by usually one or two, in Schoenocephalium four,
apical to adaxial pores or short slits); connective sometimes with apical
appendages. Tapetum secretory, with mostly binucleate cells. Staminodia
absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains monosulcate or trichotomosulcate
(sometimes zonisulcate or zonisulculate, rarely disulculate), shed as monads,
bicellular at dispersal. Exine tectate or semitectate, with columellate
infratectum, reticulate, foveolate or scrobiculate.
Gynoecium Pistil composed of
three connate carpels. Ovary superior, trilocular or (due to reduction of two
carpels) unilocular; septa often incomplete. Style single, simple. Stigma
capitate to punctate, often finely hairy, type? Pistillodium absent.
Ovules Placentation usually
axile (sometimes basal). Ovules usually one to eight (sometimes up to c. 50)
per carpel, anatropous, ascending, apotropous, bitegmic, crassinucellar.
Micropyle usually bistomal (sometimes endostomal), directed downwards. Outer
integument three to ten cell layers thick. Inner integument ? cell layers
thick. Funicular obturator present in some representatives. Parietal cell
formed from archesporial cell. Epidermal megasporangium cells often radially
prolonged. Megagametophyte monosporous, Polygonum type. Antipodal
cells sometimes more than three. Endosperm development ab initio nuclear.
Endosperm haustoria? Embryogenesis asterad.
Fruit A septicidal capsule,
often with only one or two locules fertile.
Seeds Aril absent. Sometimes
with a conical or cap-like spongy caruncle or elaiosome at chalazal end. Seed
coat testal-tegmic, sometimes winged. Exotesta thin, with silica bodies.
Endotesta sometimes with silica bodies; endotestal cells thick-walled (in
Spathanthus with U-shaped wall thickenings). Cuticular layer present
between testa and tegmen. Tegmic cells tanniniferous. Perisperm not developed.
Endosperm copious, starchy, oily and proteinaceous; starch grains simple.
Embryo small, little differentiated, micropylar,
Xyris-Scirpus type. Cotyledon one. Coleoptile absent. Collar
absent. Rhizoids absent. Radicula little developed. Germination?
Cytology n = 11
(Maschalocephalus); n = 26 (Cephalostemon,
Spathanthus)
DNA
Phytochemistry Virtually
unknown. Aluminium accumulation occurring in many species.
Use Unknown.
Systematics Rapateaceae are sister to all
other Poales except Bromeliaceae and Typhaceae. In some analyses, Rapateaceae are recovered as
sister to the remaining Poales (incl. Bromeliaceae and Typhaceae), although the support is
fairly low.
Spathanthus
1/2. Spathanthus (2; S.
bicolor, S. unilateralis; northern South America). – Ovule one
per carpel. Seeds ovoid-oblongoid. Endotestal cells with U-shaped wall
thickenings. – Spathanthus is sister to all other Rapateaceae (Givnish & al.
2004).
[Rapateoideae+[Monotrematoideae+Saxofridericioideae]]
Rapateoideae Meisn.,
Plant. Vasc. Gen.: Tab. Diagn. 405. 17-20 Aug 1842 [‘Rapateaceae’]
2/31. Rapatea (22; Panama,
tropical South America), Cephalostemon (9; C. affinis, C.
angustatus, C. cyperaceoideus, C. flavus, C.
gracilis, C. junciformis, C. microglochin, C.
riedelianus, C. squarrosus; southeastern Colombia, southern
Venezuela, northern Brazil). – The Guayana Highlands and south to Matto
Grosso (Brazil) and Bolivia. Ovule one per carpel. Seeds ovoid-oblongoid,
sometimes with a papillate apical appendage. – Rapateoideae are
sister-group to [Monotrematoideae+Saxofridericioideae].
[Monotrematoideae+Saxofridericioideae]
Monotrematoideae
Givnish et P. E. Berry in T. J. Givnish et al. in Intern. J. Plant Sci.
165(Suppl. 4): S45. 27 Sep 2004 [’Monotremoideae’]
4/7. Monotrema (4; M.
aemulans, M. arthrophyllum, M. bracteatum, M.
xyridoides; Colombia, Venezuela, Guyana, Brazil), Potarophytum
(1; P. riparium; the Kaieteur Nat. Park in Guyana),
Windsorina (1; W. guianensis; the Kaieteur Nat. Park in
Guyana), Maschalocephalus (1; M. dinklagei; Guinea, Sierra
Leone, Liberia, Ivory Coast; most probably due to late long distance dispersal;
Givnish & al. 2004). – Guyana, the upper Rio Negro in Colombia and
Venezuela, tropical West Africa. Septal nectaries present. Ovule one per
carpel. Seeds ovoid-oblongoid, white-granulate (muriculate), with a flattened
apical appendage. – Monotrematoideae are sister to
Saxofridericioideae.
Saxofridericioideae
Maguire in B. Maguire et al. in Mem. New York Bot. Gard. 10: 21. 1 Jul 1958
9/50–55.
Stegolepideae Givnish et P. E. Berry in T. J. Givnish
et al., Intern. J. Plant Sci. 165(Suppl. 4): S45. 27 Sep 2004.
Epidryos (3; E. allenii, E. guayanensis, E.
micrantherus; Panamá, Colombia, southern Venezuela, northern Ecuador),
Phelpsiella (1; P. ptericaulis; Cerro Parú in southern
Venezuela), Marahuacaea (1; M. schomburgkii; Cerro Marahuaca
in southern Venezuela), Amphiphyllum (1; A. rigidum; Cerro
Duida in southern Venezuela), Stegolepis (30–35; southern Venezuela,
Guyana, northern Brazil, with their highest diversity on mountains in
southeastern Venezuela). – Saxofridericieae Maguire
in B. Maguire et al., Mem. New York Bot. Gard. 10: 21. 1 Jul 1958.
Saxofridericia (8; S. aculeata, S. compressa, S.
duidae, S. grandis, S. inermis, S. petiolata,
S. regalis, S. spongiosa; northern South America, with their
highest diversity in southern Venezuela), Kunhardtia (2; K.
radiata, K. rhodantha; southern Venezuela), Guacamaya
(1; G. superba; along Río Guainía in eastern Colombia and western
Venezuela), Schoenocephalium (4; S. cucullatum, S.
martianum, S. schultesii, S. teretifolium; southeastern
Colombia, southern Venezuela, northwestern Brazil). – Panamá, northern South
America, with their largest diversity in the Guayana Highlands (the Guayana
Shield). Seeds prismatic, pyramidal, lens-shaped or crescent-shaped. –
Stegolepis has auriculate leaf sheath. Saxofridericieae have
leaves often differentiated into pseudopetiole and pseudolamina.
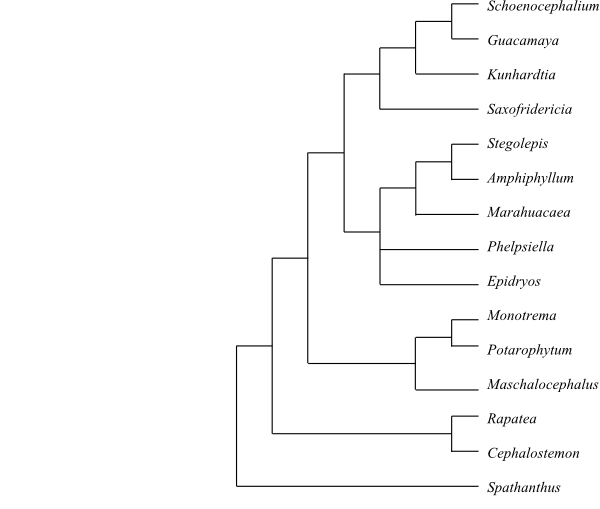 |
Cladogram of Rapateaceae based on DNA
sequence data (Givnish & al. 2000, 2004).
|
Brown, Prodr. Fl. Nov.-Holl.: 243. 27 Mar 1810
[’Restiaceae’], nom. cons.
Restionales R. Br. ex
Bercht. et J. Presl, Přir. Rostlin: 266. Jan-Apr 1820
[’Restiaceae’]; Restionineae Link,
Handbuch 1: 134. 4-11 Jul 1829 [‘Restiaceae’];
Devauxiaceae Dumort., Anal. Fam. Plant.: 62, 63.
1829, nom. illeg.; Centrolepidaceae Endlicher, Gen.
Plant.: 119. Dec 1836 [’Centrolepideae’], nom. cons.;
Elegiaceae Raf., Fl. Tellur. 4: 32, 33. med 1838
[’Elegides’]; Centrolepidales R.
Dahlgren ex Takht., Divers. Classif. Fl. Pl.: 553. 24 Apr 1997
Genera/species c
46/500–505
Distribution Tropical and
southern Africa, Madagascar, southern China, Southeast Asia and Malesia to New
Guinea, Australia, Tasmania, New Zealand, Chile, with their largest diversity
in southwestern Australia and the Western Cape Province in South Africa.
Fossils Restiocarpus
comprises fossil fruits from the Eocene/Oligocene border of Queensland and may
be remnants of Restionaceae or some allied
group. Pollen grains with the formal name of Milfordia are known from
Eocene strata in Queensland, Europe, Asia and North America. They have often
been assigned to Restionaceae, although they may
emanate from other graminoid groups. Plausible Restionaceae pollen have been
described from the Neogene at several places in the Southern Hemisphere, from
the earliest Paleocene onwards.
Habit Usually dioecious (in
Coleocarya and Lepyrodia monoecious; in, e.g., some species
of Lepyrodia sometimes bisexual; in Centrolepidoideae
monoecious, andromonoecious or polygamomonoecious), perennial (sometimes
annual) herbs. Graminids. Often xeromorphic. Culm photosynthesizing, simple or
branched (branches sometimes verticillate), usually terete (rarely quadrangular
or flattened in cross-section), smooth, verrucose, striate, furrowed or pitted,
medullated or with a hollow centre, often with compact swollen nodes.
Vegetative anatomy Mycorrhiza
absent. Root hairs developing from any epidermal cell, usually persistent,
lignified, very long and densely spaced. Root in Centrolepidoideae
without pericycle. Lateral roots developing from a zone usually opposite
protoxylem poles (in Centrolepidoideae from a zone opposite
protophloem poles). Rhizome with endodermis-like envelope. Phellogen absent.
Rhizome with endodermoid sheath. One or several subepidermal palisade-like
chlorenchyma layers with peg cells (in Centrolepidoideae usually
without peg cells); inside these layers a parenchymatic cylinder and inside
this a sclerenchymatous cylinder; medulla with scattered collateral vascular
bundles. Culm with protective lignified chlorenchymatous cells lining
substomatal cavities. Secondary lateral growth absent. Vessels present in roots
and stem (rarely in leaves). Vessel elements with scalariform or simple
(sometimes reticulate) perforation plates; lateral pits scalariform or
alternating. Imperforate tracheary xylem elements tracheids. Wood rays absent.
Axial parenchyma? Sieve tube plastids P2cc type (with cuneate
protein crystals), P2ccl type (with cuneate and several additional
loosely packed protein crystals) or P2cf (with cuneate protein crystals and
peripheral protein filaments). Nodes? Silica as spheroidal-nodular bodies or
granular crystal sand (in Centrolepidoideae silica bodies and crystals
usually absent?); parenchyma and sclerenchyma cells often filled with silica
bodies, usually in association with vascular strands (especially in parenchyma
sheath between chlorenchyma and sclerenchyma cylinders, or in outer
sclerenchymatous layer). Calciumoxalate crystals absent.
Trichomes Hairs unicellular or
multicellular, usually uniseriate (rarely T-shaped, in Gaimardia often
branched), multiseriate-stellate, stalked (sometimes peltate) or absent;
multicellular microhairs rarely? present.
Leaves Alternate (spiral or
distichous to spirodistichous), simple, entire, linear (rarely subulate),
unifacial, often with convolute (supervolute) ptyxis, usually reduced to mere
sheaths or bristle-like. Stipules absent; leaf sheath well developed, usually
open (rarely closed), usually persistent (sometimes caducous), often with one
pair of membranous lobes or a membranous distal edge (ligule?), usually without
ligule (in Gaimardia with distal ligule). Venation parallelodromous.
Stomata (brachy)paracytic; often Poaceae type guard cells, sometimes
sunken. Cuticular wax crystalloids as non-orientated platelets, only associated
with stomata. Epidermis with or without rows of alternately long and short
cells; epidermal cells with or without silica bodies. Mesophyll without
mucilaginous idioblasts and usually without calciumoxalate crystals (raphides
absent). Leaf margin entire. Epidermis with or without hairs and papillae.
Inflorescence Terminal,
compound, simple or branched panicle, corymb, spike- or head-like with one- or
many-flowered partial inflorescences as spikelets (inflorescence sometimes
consisting of a single spikelet); inflorescence branches in axils of often
spatha-like bracts; lower spikelet bracts often without flowers; flowers
usually with a single bract (rarely two bracts). Inflorescences often with
large sexual dimorphism. Floral prophylls (bracteoles) often absent (sometimes
two). Inflorescence in Centrolepidoideae terminal, few- to
many-flowered usually bisexual pseudanthia, each pseudanthial unit being a
spikelet with usually two (sometimes one, three or no) hyaline membranous
secondary bracts (floral prophylls?); pseudanthia concentrated in capitate or
distichous spike-like inflorescences, each subtended by two (or more)
scale-like or green primary bracts.
Flowers Actinomorphic to
somewhat zygomorphic, small. Hypogyny. Tepals usually 3+3 (rarely two, three,
2+2, 3+2, or absent), dry, membranous to hard (in male flowers rarely reduced),
often persistent, usually free (sometimes connate at base); outer lateral tepal
often with a hairy (sometimes winged) keel. Nectary absent. Disc absent.
Androecium Stamens usually
three (rarely – such as in Centrolepidoideae – one or two),
antepetalous (outer staminal whorl absent). Filaments filiform, free from each
other and from tepals. Anthers dorsifixed, sometimes versatile, usually
disporangiate and monothecal (rarely tetrasporangiate and dithecal), usually
introrse (rarely latrorse), longicidal (dehiscing by longitudinal slits);
connective sometimes slightly prolonged. Tapetum secretory, with uninucleate to
quadrinucleate cells; anther walls highly tanniniferous. Female flowers often
with staminodia.
Pollen grains
Microsporogenesis successive. Pollen grains graminoid, monoporate or
monoulcerate (aperture with various shape; most African representatives with a
very wide pore), with or without operculum or annulus, shed as monads, usually
tricellular (sometimes bicellular) at dispersal. Exine tectate, with
columellate (sometimes granular) infratectum, perforate, rugulate or
scrobiculate, usually microverrucate or smooth (rarely verrucate or
spinulate).
Gynoecium Pistil composed of
one to three ascidiate connate antesepalous carpels (sometimes only one out of
three carpels fertile leading to pseudomonomery); pistil in
Centrolepidoideae composed of a single carpel or, alternatively, one
to 30 (rarely up to 45) free or (seemingly) connate carpels. Ovary superior,
unilocular to trilocular, sometimes shortly stipitate; ovary walls richly
tanniniferous. Stylodia usually three (rarely one or two), usually free (rarely
connate at base), with adaxial stigmatic surfaces; style in
Centrolepidoideae single, simple, filiform, persistent, with adaxial
pollen receptive surface. Stigmas usually three (rarely one or two, in
Centrolepidoideae one), much branched, with papillate branches, Dry
type. Male flowers often with pistillodium (at least rudimentary).
Ovules Placentation usually
axile to apical (apical or marginal, when ovary monomerous then unilocular).
Ovule one per carpel, orthotropous, pendulous, bitegmic, usually tenuinucellar
(in at least Alexgeorgea crassinucellar). Micropyle usually bistomal
(in Willdenowia and some species of Leptocarpus endostomal).
Outer integument two? cell layers thick. Inner integument two? cell layers
thick; inner epidermis of inner integument strongly tanniniferous. Parietal
cell usually not formed (parietal tissue one cell layer thick present in at
least, e.g., Alexgeorgea). Hypostase usually present (no widening of
micropylar part of integument; in Centrolepidoideae absent).
Megasporangial epidermis with anticlinally elongate cells (radial elongation of
megasporangial epidermis). Megagametophyte monosporous, Polygonum type
(sometimes Poaceae variation),
with large compound starch grains (polar nuclei surrounded by large starch
bodies). Synergids sometimes with a filiform apparatus. Antipodal cells usually
binucleate?, in Restioneae at least sometimes proliferating. Endosperm
development ab initio nuclear. Endosperm haustoria? Embryogenesis onagrad.
Fruit A loculicidal usually
three-seeded capsule or a one-seeded unilocular nutlet; fruit stalk sometimes
fleshy and elaiosome-like (spikelet axis in Hypolaena fleshy); nutlet
sometimes with persistent tepals with wing-shaped keel; fruit in
Centrolepidoideae a one-seeded, abaxially dehiscing follicle with
membranous pericarp (in Aphelia indehiscent), collateral ovaries often
fused into a capsule-like coenocarp. Fruit in Alexgeorgea hypogeal.
Seeds Aril absent. Elaiosome
present in some African genera. Seed coat endotegmic. Exotesta persistent
(testa and exotegmen in at least Centrolepidoideae degenerating).
Endotesta? Tegmen? (endotegmen in at least Centrolepidoideae
membranous, tanniniferous, persistent) Cuticle between integument well
developed. Perisperm not or only slightly developed. Endosperm copious,
starchy; starch grains in at least Centrolepidoideae compound. Embryo
small, often lens-shaped or conical, little differentiated, rudimentary,
Xyris-Scirpus type, chlorophyll? Cotyledon one, usually
photosynthesizing. Cotyledon hyperphyll prolonged, assimilating. Hypocotyl and
collar rudimentary. Hypocotyl internode absent. Coleoptile absent. Collar
rhizoids present. Germination phanerocotylar. First leaf of seedling unifacial,
with sheath-like or (in Centrolepidoideae) disc-shaped part; with
isodiametric or palisade chlorenchyma cells. Photosynthesizing unifacial
cotyledon hyperphyll (phanomer) usually present in at least
Centrolepidoideae.
Cytology n = 6, 7, 9, 10
(Centrolepidoideae), 11, 12 (Australian genera); n = 16, 20 (African
genera) – Agamospermy probably occurs in some genera. Some species are
parthenocarpic and known as female plants only.
DNA The plastid genome has an
inversion of 28 kb in some species (present at least in Askidiosperma,
Baloskion, Leptocarpus, Rhodocoma, and Elegia
fenestrata; absent from Desmocladus, Elegia cuspidata,
and Centrolepis).
Phytochemistry Flavonols
(kaempferin, quercetin), flavones, flavone-C-glycosides and flavone
sulphates sometimes present (flavones and glycoflavones present in some African
clades). Proanthocyanidins present in almost all African species, whereas
remaining species (including a few African species) possess 8-hydroxyflavonoids
gossypetin and hypolaetin (8-hydroxyluteolin). Glycosides of myricetin,
laricitrin and syringetin present in ’Elegia’ and
Chondropetalum. S-methylcysteine (an amino acid) present in
Lepyrodia. Tricin, ellagic acid, alkaloids, saponins, and cyanogenic
compounds not found. Aluminium accumulation occurring in
Centrolepis.
Use Ornamental plants
(Chondropetalum tectorum, Elegia capensis etc.), thatching
(Thamnochortus insignis etc.), forage plants.
Systematics Restionaceae are sister-group to
Anarthriaceae.
The diversification in the Cape Region (c. 350
species) may have started during the Late Eocene or the Early Oligocene (Hardy
& al. 2004), and trans-oceanic dispersal – rather than vicariance – may
be the explanation for the presence of Restionaceae in both South Africa
and Australasia. South African Restionaceae have protecting
cells in the form of lignified chlorenchyma cells surrounding subepidermal
cavities, flavonols (sometimes myricetin), proanthocyanidins, and fewer
flavones. Australian Restionaceae have usually terete
leaves (not in Anthochortus); pollen grains with large pores without
annulus; non-photosynthesizing cotyledon; elongate internodes on seedling culm;
and chlorenchymatous palisade cells; sulphated flavonoids; few flavonols
(quercetin more frequent) and rarely proanthocyanidins.
Parsimony analysis of trnK and
trnL-F identified Centrolepidaceae as sister-group to Restionaceae (Briggs & Linder
2009). On the other hand, Bayesian-inference analysis of the trn sequences and
data from rbcL analyses recovered the clade (on a very long branch) as embedded
within Restionaceae
(Briggs & Linder 2009; Briggs & al. 2014). This is also found in some
maximum-likelihood and maximum-parsimony analyses (e.g., Linder & al. 2000;
Briggs & al. 2010, 2014). In these cases, the following topology was:
[Restionoideae+[Sporadanthoideae+[Leptocarpoideae+Centrolepidoideae]]].
The leaves in Centrolepidoideae
resemble the seedling leaves of Restionaceae, and
Centrolepidoideae may have evolved from neotenic Restionaceae. The apertures of
the pollen grains in some Australian Leptocarpoideae are similar to
those in Centrolepidoideae, having a granular infratectum instead of
the columellate infratectum seen in the majority of Restionaceae. Endexine is absent
from or very poorly developed in Centrolepidoideae.
The interpretation of the complex inflorescence
of Centrolepidoideae (particularly in Aphelia) has been
extremely difficult. The reproductive units in Centrolepis were
interpreted by Sokoloff & al. (2009, 2010) as distichously arranged in
lateral double spikelets each subtended by a bract. Usually, each reproductive
unit (flower?) is rarely subtended by a bract (one important exception is
Centrolepis racemosa) and consists of a single stamen and one to c. 30
(to 45) carpels. Gaimardia, on the other hand, has simple terminal
spikelets. An alternative explanation was presented by Cooke (1998), who
interpreted the inflorescence as being a bisexual pseudanthium consisting of a
varying number of unisexual atepalous flowers, each comprising one or two
stamens and a single carpel, respectively.
Restionoideae Arn.,
Botany: 135. 9 Mar 1832 [‘Restieae’]
c 17/350–355. Africa south of Sahara,
Madagascar, Australia. Substomatal protective cells often present – modified
cells of the chlorenchyma with slightly to moderately thickened lignified walls
surrounding a substomatal cavity forming a tube extending all or part the way
through chlorenchyma. Pillar cells absent. ‘False pillar cells’ –
lignified cells of the chlorenchyma that extend outwards from ridges of the
sclerenchyma – sometimes present. Sclerenchyma ribs sometimes present,
alternating with outer vascular bundles. Pollen type ‘African restionoid’,
i.e. tectum raised around a relatively small (4–10 µm) aperture, some
species with thickened foot layer. Flavonols (usually glycoside derivatives of
myricetin and its methyl ethers larycitin and syringetin), non-hydrolyzable
tannins, and proanthocyanidins present. n = 16, 20.
Restioneae Bartl.,
Ord. Nat. Plant.: 36. Sep 1830
c 9/290–295. Soroveta (1;
S. ambigua; Western Cape), Platycaulos (12; western and
southwestern Eastern Cape), Staberoha (9; Western Cape),
'Restio subg. Pendulostemon' (2; ‘Restio’
egregius, ‘Restio’ micans; Western Cape),
Rhodocoma (8; Western and Eastern Cape, KwaZulu-Natal),
Thamnochortus (c 35; Western and Eastern Cape), Askidiosperma
(12; Western Cape), Elegia (c 50; Northern, Western and Eastern Cape),
Restio
(160–165; tropical and southern Africa, Madagascar, Australia). – Tropical
and southern Africa, Madagascar, Australia. Sclerenchyma ribs absent. ’False
pillar cells’ absent. Chlorenchyma cells radially elongated. Silica bodies
often present in parenchyma sheath (absent from sclerenchyma cylinder). Style
one to three. Antipodal cells at least sometimes proliferating. Fruit a capsule
or a soft-walled nut. Immature seed coat tanniniferous. – Soroveta
is sister to the remainder (Linder & Hardy 2010).
Willdenowieae Masters
in A. L. P. P. de Candolle, Monogr. Phan. 1: 314. Jun 1878
[‘Willdenovieae’]
8/c 62. Nevillea
(3; N. obtusissimus, N. singularis, N. vlokii;
Western Cape), ’Willdenowia’
(12; Northern and Western Cape; non-monophyletic), Mastersiella (3;
M. digitata, M. purpurea, M. spathulata; Western
Cape), Anthochortus (7; A. capensis, A. crinalis,
A. ecklonii, A. graminifolius, A. insignis, A.
laxiflorus, A. singularis; Western Cape), Hydrophilus
(1; H. rattrayi; Western and Eastern Cape), ’Hypodiscus’
(c 15; Northern, Western and Eastern Cape; non-monophyletic), Cannomois
(13; Western and Eastern Cape), Ceratocaryum (8; Western Cape). –
Southwestern South Africa. ‘False pillar cells’ present in some species.
Sclerenchyma ridges often alternate with outer vascular bundles, extending from
sclerenchyma sheath all or part-way through chlorenchyma. Chlorenchyma cells
often radially short and flattened. Silica bodies usually present only in
sclerenchyma cylinder. Anterior carpel absent, with displacement of remaining
carpels. Styles two (rarely connate at base. Antipodal cells proliferating?
Fruit a nut, usually with heavily lignified pericarp. Pedicel in fruit often
fleshy and modified into elaiosome-like structure (fruits often
myrmecochorous). Immature seed coat not tanniniferous. –
Willdenowieae was unresolved in Eldenäs & Linder (2000).
Possibly, Nevillea
is sister to the remainder in one of the main clades.
[Sporadanthoideae+[Leptocarpoideae+Centrolepidoideae]]
Chlorenchyma cells palisade. Pollen type
‘Australian restionoid’, i.e. tectum not raised around a large (8–25 µm)
and usually irregular aperture, distinct annulus and thickened foot layer
absent. Cotyledon photosynthesizing or non-photosynthesizing. Culm internodes
of seedling elongated. First leaves of seedling terete. Flavonols rarely
present (except quercetin). Flavones very diverse. Sulphated flavonoids
present. Non-hydrolyzable tannins rare. n = 6, 7, 9, 11, 12.
Sporadanthoideae B. G.
Briggs et H. P. Linder in Telopea 12: 338. Oct 2009
3/22. Lepyrodia
(13; Australia except the central areas, Tasmania), Calorophus (2;
C. elongatus, C. erostris; Victoria, western Tasmania),
Sporadanthus (7; S. caudatus, S. ferrugineus, S.
gracilis, S. interruptus, S. strictus, S.
tasmanicus, S. traversii; southwestern Western Australia,
southeastern Queensland, New South Wales, Tasmania, North Island of New
Zealand, Chatham Islands). – Australia, Tasmania, New Zealand, Chatham
Islands. Some species of Lepyrodia
monoecious or bisexual. Substomatal protective cells present (modified
chlorenchyma cells with slightly to moderately thickened lignified walls
surrounding a substomatal cavity forming a tube extending all or part way
through chlorenchyma). Pillar cells absent. ‘False pillar cells’ absent.
Sclerenchyma girdles or ridges absent. Spikelets often absent (spikelets
reduced in Lepyrodia
and Sporadanthus), flowers then solitary and bracteolate. Capsule
usually trilocular. Myricetin and quercetin often present (myricetin absent in
Calorophus). Flavones and proanthocyanidins usually absent. – Lepyrodia
may be sister to [Calorophus+Sporadanthus].
Leptocarpoideae B. G.
Briggs et H. P. Linder in Telopea 12: 339. Oct 2009 (under construction)
23/c 90. Eurychorda (1; E.
complanata; southeastern Queensland, eastern New South Wales, Victoria,
Tasmania); Winifredia (1; W. sola; southwestern Tasmania),
Taraxis (1; T. grossa; southwestern Western Australia), Empodisma
(2; E. gracillimum, E minus; Australia, Tasmania, New
Zealand), Alexgeorgea (3; A. ganopoda, A. nitens,
A. subterranea; southwestern Western Australia), Chaetanthus
(3; C. aristatus, C. leptocarpoides, C. tenellus;
southwestern Western Australia), Dapsilanthus (4; D.
disjunctus, D. elatior, D. ramosus, D.
spathaceus; Hainan, Thailand, Indochina, the Malay Peninsula, Aru Islands,
New Guinea, northernmost Northern Territory, northern Queensland),
Leptocarpus (2; L. laxus, L. tenax; southwestern
Western Australia, New South Wales, Tasmania), Loxocarya (5; L.
albipes, L. cinerea, L. gigas, L. magna, L.
striata; southwestern Western Australia), Tremulina (2; T.
cracens, T. tremula; southwestern Western Australia),
Chordifex (c 20; southwestern Western Australia, New South Wales
[‘Saropsis’], Tasmania [‘Acion’]), Baloskion
(8; southeastern Queensland, eastern New South Wales, Victoria, southern South
Australia, Tasmania), Dielsia (1; D. stenostachya;
southwestern Western Australia), Melanostachya (1; M.
ustulata; southwestern Western Australia), Tyrbastes (1; T.
glaucescens; southwestern Western Australia), Coleocarya (1;
C. gracilis; southeastern Queensland, northeastern New South Wales),
Lepidobolus
(6; L. basiflorus, L. chaetocephalus, L. deserti,
L. drapetocoleus, L. preissianus, L. spiralis;
southern Australia, especially southwestern Western Australia), Desmocladus
(c 15; southwestern Western Australia). – Unplaced
Leptocarpoideae Apodasmia (3; A. brownii,
A. chilensis, A. similis; South Australia, Tasmania, New
Zealand, Chile), Catacolea (1; C. enodis; southwestern
Western Australia), Cytogonidium (1; C. leptocarpoides;
southwestern Western Australia), Hypolaena (7; H. caespitosa,
H. exsulca, H. fastigiata, H. humilis, H.
pubescens, H. robusta, H. viridis; Australia, with their
highest diversity in southwestern Western Australia), Platychorda (2;
P. applanata, P. rivalis; southwestern Western Australia).
– Southeast Asia to New Guinea, Australia, New Zealand, Chile. Substomatal
protective cells absent; substomatal cavity in members of Desmocladus
group and Alexgeorgea protected by elongated and thick-walled
epidermal cells. Pillar cells (elongate palisade-like cells of parenchyma
sheath, usually with moderately thickened lignified walls, radiating from
sclerenchyma sheath to epidermis, dividing chlorenchyma into longitudinal
bands) present in Eurychorda, Alexgeorgia, western Australian
Chordifex, Dielsia, Loxocarya, Taraxis and
the Leptocarpus group. Girdles (sclerenchyma ridges) sometimes present
opposite outer vascular bundles, extending from sclerenchyma sheath all or
part-way through chlorenchyma. Flavones (usually luteolin and hypolaetin) and
sulphated flavonoids and flavonoid derivatives present (e.g. gossypetin).
Flavonols and proanthocyanidins usually absent. – Eurychorda is
sister to the remaining Leptocarpoideae.
Centrolepidoideae
Burnett, Outlines Bot.: 416. Feb 1835 [‘Centrolepidae’]
3/36. Gaimardia (4; G.
amblyphylla, G. fitzgeraldii: Tasmania; G. setacea: New
Guinea, Tasmania, South Island in New Zealand; G. australis: Tierra
del Fuego, the Falkland Islands), Aphelia (6; A. brizula,
A. cyperoides, A. drummondii, A. gracilis, A.
nutans, A. pumilio; Australia), Centrolepis (26; Hainan,
Indochina, Malesia to Australia).
Gaimardia is sister to
[Aphelia+Centrolepis] (Briggs & al. 2010). One
synapomorphy common to Aphelia and Centrolepis may be their
absence of a distal foliar ligule.
 |
Phylogeny of Centrolepidoideae (Centrolepidaceae)
based on DNA sequence data (Briggs & al. 2010).
|
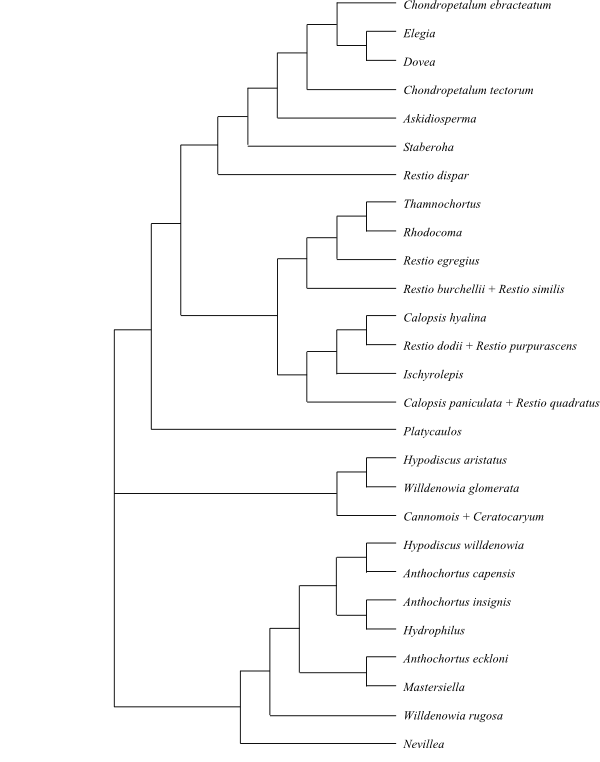 |
Cladogram of Restionaceae in South
Africa based on morphology and DNA sequence data (Eldenäs & Linder
2000). Chondropetalum and Dovea are nested in Elegia.
|
 |
Phylogeny of Restionaceae in Australia
based on DNA sequence data (Briggs 2000; Briggs & al. 2010).
Acion, Guringalia and Soropsis are nested in
Chordifex. In the Briggs (2000) analysis the basal clade of
Calorophus, Lepyrodia
and Sporadanthus collapsed into a trichotomy.
|
Engler, Syllabus, ed. 5: 94. Jul 1907, nom.
cons.
Genera/species 1/3
Distribution Northeastern
South America.
Fossils Unknown.
Habit Bisexual, perennial
herbs. Graminids. Often helophytic or aquatic. Culm angular in cross-section,
erect.
Vegetative anatomy Mycorrhiza
absent? Phellogen absent. Secondary lateral growth absent. Vessels present in
roots, stem and leaves. Vessel elements usually with scalariform (in stems
sometimes simple) perforation plates; lateral pits? Imperforate tracheary xylem
elements tracheids. Wood rays absent. Axial parenchyma? Sieve tube plastids P2c
type, with cuneate protein crystals. Nodes? Silica bodies spherical, in
epidermal cells, and silica sand in parenchyma cells. Tanniniferous cells
scattered. Calciumoxalate crystals absent.
Trichomes Hairs absent, except
for small unicellular hairs on pedicels.
Leaves Alternate (tristichous
or tetrastichous), simple, entire, linear, flat or V-shaped in transverse
section, unifacial, with convolute (supervolute) ptyxis. Stipules absent; leaf
sheath closed?, with ligule? Venation parallelodromous; vascular bundles in
vertical pairs (superposed) with a larger adaxial non-inverted (with phloem on
lower side) and a smaller abaxial inverted bundle (with phloem on upper side),
the two phloems facing each other. Stomata usually paracytic (sometimes
tetracytic). Cuticular wax crystalloids as aggregated rodlets. Epidermis with
silica bodies. Mesophyll without mucilaginous idioblasts or calciumoxalate
crystals. Leaf margin entire to hispid.
Inflorescence Flowers in one
or several terminal dense racemose heads subtended by narrowly elongate bracts.
Peduncle (culm) triangular or quadrangular in transverse section.
Flowers Actinomorphic, small.
Hypogyny. Tepals 3+3, sepaloid, membranous, persistent, free. Nectary absent.
Disc absent.
Androecium Stamens 3+3.
Filaments filiform, free from each other, somewhat adnate at base to tepals.
Anthers basifixed, non-versatile?, tetrasporangiate, introrse, longicidal
(dehiscing by longitudinal slits). Tapetum secretory, with uninucleate? cells.
Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains with indistinct aperture
(ulcerate), shed as tetrahedral tetrads, tricellular at dispersal. Exine
tectate, with granular? infratectum, scabrate or ulcerate.
Gynoecium Pistil composed of
three connate carpels. Ovary superior, trilocular, narrowing towards apex.
Style single, trilobate. Stigmas three, papillate, Dry type. Pistillodium
absent.
Ovules Placentation axile to
axile-basal or lateral. Ovules one to seven per carpel, anatropous, ascending,
apotropous, bitegmic, crassinucellar. Micropyle bistomal, Z-shaped (zig-zag).
Outer integument one or two cell layers thick. Inner integument two cell layers
thick. Parietal cell formed from archesporial cell. Megagametophyte
monosporous, Polygonum type. Synergids with a filiform apparatus.
Endosperm development ab initio nuclear. Endosperm haustoria? Embryogenesis?
Fruit A loculicidal
capsule.
Seeds Aril present. Seed coat
testal-tegmic, with one subulate outgrowth at each end. Testa puberoulous.
Exotesta consisting of one to three layers of sclerenchymatous fibres.
Endotesta consisting of one or two parenchymatous layers. Tegmen consisting of
elongate tightly packed tanniniferous cells. Perisperm not developed. Endosperm
copious, starchy. Embryo small, straight, well differentiated,
Xyris-Scirpus type, chlorophyll? Cotyledon one,
photosynthesizing. Cotyledon hyperphyll? Hypocotyl absent. Mesocotyl?
Coleoptile absent. Plumule terminal. Collar very small, with rhizoids.
Germination phanerocotylar.
Cytology n = ? – Chromosomes
with diffuse centromeres.
DNA Deletion of three base
pairs in the plastid gene atpA.
Phytochemistry Virtually
unknown. Flavone-C-glycosides? Luteolin-5-methylether? Cyanogenic
compounds not found.
Use Unknown.
Systematics Thurnia
(3; T. jenmanii, T. polycephala, T. sphaerocephala;
mountains from southeastern Colombia and southern Venezuela to northern Brazil,
the Guayana Highlands).
Thurnia is sister to [Juncaceae+Cyperaceae], according to Munro
& Linder (1998).
de Jussieu, Gen. Plant.: 25. 4 Aug 1789
[’Typhae’] , nom. cons.
Sparganiaceae Hanin,
Cours Bot.: 400. 16-23 Apr 1811 [’Spargania’], nom. cons.;
Typhales Juss. ex Bercht. et J. Presl, Přir.
Rostlin: 263. Jan-Apr 1820 [‘Typhaceae’];
Typhanae Thorne ex Reveal in Novon 2: 237. 13 Oct
1992
Genera/species 2/c 52
Distribution Cosmopolitan.
Fossils Fruits assigned to
Typha have been found in the Maastrichtian of Central Europe and
Sparganium fruits were reported from Paleocene and younger layers. The
oldest fruits are quinquelocular. Sparganium and Typha have
been recorded more frequently from the Eocene onwards. Typhaspermum,
representing seeds similar to Typha, is known from Late Eocene to
Oligocene layers in Queensland.
Habit Monoecious, perennial
herbs. Aquatic or helophytic. Rhizome rich in starch.
Vegetative anatomy Mycorrhiza
probably absent. Lateral roots developing from a zone opposite protoxylem
poles. Phellogen absent. Secondary lateral growth absent. Vessels present in
roots, stem and leaves. Vessel elements with scalariform perforation plates;
lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays absent.
Axial parenchyma? Sieve tube plastids P2cs type, with cuneate protein crystals
and starch grains. Nodes? Silica bodies present or absent. Calciumoxalate as
raphides, druses or single prismatic or rod-shaped crystals. Calciumoxalate
styloids present in rows of cells above sclerenchyma bundles in Typha.
Starch grains simple.
Trichomes Hairs absent from
vegetative organs.
Leaves Alternate (distichous),
simple, entire, aerial leaves linear, in Sparganium sometimes keeled
and laterally flattened, with ? ptyxis. Stipules absent; leaf sheath well
developed, in Typha with adaxial mucilaginous cells. Venation
parallelodromous. Stomata paracytic (subsidiary cells obliquely divided).
Cuticular wax crystalloids as longitudinally aggregated rodlets
(Strelitzia type), chemically dominated by wax esters. Epidermis with
or without silica bodies; epidermis with tanniniferous myriophyllin cells.
Mesophyll with or without mucilaginous idioblasts and sacs containing
calciumoxalate raphides. Leaf margin entire.
Inflorescence Terminal (in
Sparganium often axillary), complex multiple compound racemose, with
male inflorescence(s) in upper part and female inflorescence(s) in lower part;
in Typha spike-like (stachyoid) or spadix-like (spadicioid); in
Sparganium spherical and capitate (cephalioid); inflorescences compact
and consisting of reduced one- or few-flowered branched partial inflorescences.
Male and female inflorescences in Typha at least initially separated
by a bract.
Flowers Actinomorphic, small
to minute. Hypogyny. Tepals one to c. 20, in male flowers usually three, in
female flowers one to four, filiform (Typha), or three or 3+3 sepaloid
and scale-like and with quincuncial aestivation (Sparganium),
persistent, free or connate at base. Nectary absent. Disc absent.
Androecium Stamens in
Typha usually three (sometimes one, two or up to eight); in
Sparganium (one to) three or 3+3, on androphore. Filaments linear to
filiform, free from each other or connate at base, free from tepals. Anthers
basifixed, non-versatile, tetrasporangiate, extrorse to latrorse, longicidal
(dehiscing by longitudinal slits); connective in Typha somewhat
prolonged. Tapetum intermediate between secretory and amoeboid-periplasmodial,
in Sparganium with 8-nucleate cells (Typha type: tapetal
nuclei dividing, each cell becoming ab initio binucleate, subsequently dividing
twice). Staminodia absent.
Pollen grains
Microsporogenesis successive. Pollen grains monoulcerate with indistinct
pore-like aperture, usually shed as monads (in Typha occasionally as
tetrads), usually bicellular at dispersal. Exine tectate, with columellate
infratectum, finely reticulate, echinulate or smooth.
Gynoecium Pistil in
Typha composed of one stipitate carpel; pistil in Sparganium
composed of two or three connate carpels (usually only one carpel fertile,
pseudomonomerous). Ovary superior, unilocular, sometimes on gynophore. Style
(stylodium) single, simple or bifid or trifid in upper part. Stigma two or
three, decurrent, fairly elongate, Dry type, in Sparganium papillate.
Pistillodium absent.
Ovules Placentation apical or
subapical. Ovule one per carpel, anatropous, pendulous, apotropous, bitegmic,
crassinucellar. Micropyle bistomal (in Typha endostomal?). Outer
integument ? cell layers thick. Inner integument ? cell layers thick. Obturator
present. Parietal cell formed from archesporial cell and further developing
into parietal tissue. Nucellar cap approx. two cell layers thick.
Megagametophyte monosporous, Polygonum type. Antipodal cells in
Sparganium often proliferating (to c. 150 cells). Endosperm
development ab initio helobial of aberrant type: small chalazal endosperm
chamber with rapid cell divisions (free nuclear divisions up to 16-nucleate
stage or cell wall formation earlier), large micropylar chamber developing into
nutrient tissue. Endosperm haustoria absent. Embryogenesis asterad
(Typha) or onagrad (Sparganium).
Fruit In Typha a
hairy follicular nutlet with strongly reduced endocarp and mesocarp; in
Sparganium a drupe with usually spongy exocarp, decaying during
maturation, and hard endocarp.
Seeds Aril absent. Operculum
present. Seed coat in Sparganium testal-tegmic, with testa and tegmen
membranous except at micropylar end, thick-walled cells here forming a conical
double operculate structure; testa in Typha compressed, with
thin-walled cells, tegmen with thick-walled cells. Perisperm thin, weakly
developed. Endosperm copious, with micropylar chamber containing aleurone,
starch (starch grains pteridophyte type, amylophilic), proteins (in
Sparganium), and oils (in Typha); chalazal chamber with very
small amounts of nutrients. Embryo long, straight, slender, without
chlorophyll, Trillium type. Cotyledon one, terminal. Cotyledon
hyperphyll elongate, assimilating. Hypocotyl present. Hypocotyl internode
absent (Typha) or short (Sparganium). Mesocotyl absent.
Coleoptile absent (Typha) or present (Sparganium). Plumule
lateral. Collar rhizoids present. Germination?
Cytology x = 15
DNA Deletion in ORF2280
Phytochemistry Flavonols (in
Typha kaempferol, quercetin, cinnamic acids; in Sparganium
quercetin, myricetin), catechin, and cyanidin (in Sparganium also
delphinidin?) present. Alkaloids and cyanogenic compounds rare. Ellagic acid,
chelidonic acid, and saponins not found. Ferulic acid (ferulate, esterified)
component of non-lignified cell walls.
Use Ornamental plants,
Typha also for matting, basketry, vegetables (starchy rhizome),
etc.
Systematics Typha
(8–13), Sparganium
(15).
Sparganium
1/22. Temperate and arctic regions, one
or two species in Southeast Asia, Malesia, New Guinea, Australia, the Southern
Hemisphere south to New Zealand. Aquatic. Leaves linear. Leaf sheath
indistinct. Stomatal subsidiary cells with intersecting oblique divisions.
Inflorescence spherical, cephalioid. Tepals one to six; when three, then median
tepal adaxial. Stamens usually three or 3+3. Anthers extrorse-latrorse. Pistil
composed of two or three carpels. Ovary sometimes with three fertile locules;
some flowers with a second sterile ovary locule (some fossil fruits with up to
seven locules). Embryogenesis onagrad. Fruit a spongy drupe with persistent
tepals and micropylar plug. Testa membranous. Endosperm also proteinaceous.
Cotyledon non-photosynthesizing, with envelope. Hypocotyl internode short.
Coleoptile present.
Typha
1/c 30. Cosmopolitan except arctic
regions. Styloids in cell rows above sclerenchyma bundles. Cuticular wax
crystalloids as aggregated rodlets. Leaf sheath distinct. Inflorescence dense
stachyoid or spadicioid. Pedicel with long hairs. Tepals absent. Stamens (one
to) three (to eight). Filaments connate. Tapetum with octonucleate? cells.
Pollen grains occasionally shed in tetrads. Pistil composed on a single carpel.
Embryogenesis asterad. Fruit an achene with small operculum. Endosperm also
oily. Hypocotyl internode absent. Coleoptile absent.
Agardh, Aphor. Bot.: 158. 23 Mai 1823
[’Xyrideae’], nom. cons.
Xyridales Lindl.,
Veg. Kingd.: lvii, 185. 14-28 Mar 1846; Abolbodaceae
Nakai, Chosakuronbun Mokuroku [Ord. Fam. Trib. Nov.]: 221. 20 Jul 1943;
Xyridineae thorne et Reveal in Bot. Rev. (Lancaster)
73: 85. 29 Jun 2007
Genera/species 5/275–290
Distribution Tropical and
subtropical regions on the Southern and Northern Hemispheres, with their
largest diversity in northern South America; few species in temperate areas.
Fossils Fossilized seeds
attributed to Xyris have been found in the mid- and Late Miocene of
Germany (Mai 2000).
Habit Bisexual, usually
perennial (sometimes annual) herbs. Often helophytic (rarely aquatic); some
species are xerophytic.
Vegetative anatomy Mycorrhiza
absent. Roots fibrous. Root endodermis uni- or multilayered, consisting of
large evenly thick cells with specific orientation. Root stele relatively
narrow. Root phloem and xylem elements usually irregularly scattered in
parenchyma. Lateral roots developing from a zone opposite protophloem poles.
Phellogen absent. Stem vascular bundles amphivasal. Secondary lateral growth
absent. Vessels present in roots and, usually, in stem and leaves. Vessel
elements usually with simple (sometimes scalariform) perforation plates;
lateral pits scalariform. Imperforate tracheary xylem elements tracheids. Wood
rays absent. Axial parenchyma? Sieve tube plastids P2ccof type, with
cuneate and orthogonal protein crystals and peripheral protein filaments.
Nodes? Silica bodies absent. Calciumoxalate as single crystals; raphides
absent.
Trichomes Hairs unicellular or
multicellular (few-celled), uniseriate, usually simple; in Xyris
uniseriate hairs with bulb-like terminal cell producing mucilage present in
leaf axils.
Leaves Alternate (usually
distichous, sometimes spiral), simple, entire, linear, usually equitant,
laterally flattened, sometimes subulate, with convolute (supervolute) ptyxis?
Stipules absent; leaf sheath distinct, open, some species of Xyris
with distal ligule (or auriculae). Venation parallelodromous. Stomata paracytic
or anomocytic (in Abolboda sometimes tetracytic). Cuticular waxes
absent. Cuticle with insoluble secretions. Outer epidermal cell walls often
with papillae, tubercles or other types of outgrowths, or thickened. Mesophyll
without mucilaginous cells, often with calciumoxalate as druses or solitary
prismatic crystals (raphides absent). Leaf margin entire.
Inflorescence Terminal or
lateral, dense, spike- or head-like condensed panicle (usually stachyoid; in
Achlyphila open) consisting of one- or sometimes two- or
three-flowered cymose partial inflorescences (in some species spikes in
panicles; in Aratitiyopea racemose; in Achlyphila solitary
flowers, each of which enclosed by an involucral bract), on long peduncle
bracteate in lower part. Each flower usually subtended by one or several pairs
of bracts. Floral prophyll (bracteole) absent.
Flowers Actinomorphic or
zygomorphic (in Orectanthe bilabiate). Hypogyny. Tepals usually 3+3
(in Abolboda and Orectanthe sometimes 2+3); outer tepals
usually three (sometimes two, carinate), sepaloid, free or two lateral connate
at base; median tepal in Xyris membranous, caducous, or all outer
tepals persistent (median outer tepal in Abolboda strongly reduced or
absent); inner tepals with imbricate aestivation, petaloid, clawed, early
caducous, usually free (sometimes large and often connate at base into a tube).
Nectary absent. Disc absent.
Androecium Stamens usually
three (outer staminal whorl absent), antepetalous (sometimes also one to three
staminodia; one species of Xyris with six fertile stamens). Filaments
often filiform, with flattened base, usually connate, usually adnate to inner
tepals (epipetalous; in Achlyphila free). Anthers basifixed,
non-versatile, tetrasporangiate (microsporangia sometimes connate), usually
latrorse or extrorse (rarely introrse), longicidal (dehiscing by longitudinal
slits). Tapetum usually secretory (in Abolbodoideae
amoeboid-periplasmodial), with binucleate cells. Exothecium present. Staminodia
(in Xyris and sometimes in Abolboda) usually three (sometimes
one or two), alternipetalous, extrastaminal, free from tepals, simple, bifid or
quadrifid, usually provided with long (often plumose) hairs (secondary pollen
presentation).
Pollen grains
Microsporogenesis successive. Pollen grains monosulcate (in Xyris;
sometimes disulcate) or inaperturate (in Abolbodoideae), shed as
monads, usually bicellular (sometimes tricellular) at dispersal. Exine tectate,
with columellate? infratectum, spinulate or smooth.
Gynoecium Pistil composed of
three connate carpels. Ovary superior, unilocular to entirely or partially (at
least basally) trilocular. Style single, filiform, terete or triangular in
cross-section in lower part, usually trilobate (in Achlyphila simple),
in Abolboda, Aratitiyopea and Orectanthe trifid in
upper part and with three lateral appendages. Stigmas three, lobate or
U-shaped, often infundibuliform to subcapitate, usually fimbriate or papillate,
type? Pistillodium absent.
Ovules Placentation in
Xyris marginal, parietal (sometimes intrusively so), basal,
basal-axile, free central or axile, in Abolbodoideae parietal. Ovules
usually numerous (sometimes one) per carpel, anatropous (Achlyphila,
Abolboda; in Orectanthe anatropous to slightly
campylotropous)ororthotropous(Xyris),
bitegmic, tenuinucellar (Xyris, Abolboda) to more or less
crassinucellar (Achlyphila). Micropyle bistomal. Outer integument two
cell layers thick. Inner integument two cell layers thick. Hypostase absent (in
Xyris) or present (in Abolbodoideae). Obturator absent.
Parietal cell not formed (parietal tissue absent). Megasporangial epidermis in
Xyris sometimes with periclinal cell divisions. Megagametophyte
monosporous, Polygonum type, or disporous, Allium type.
Endosperm development ab initio helobial (or possibly sometimes nuclear?).
Endosperm haustoria absent. Embryogenesis asterad.
Fruit Usually a loculicidal,
many-seeded (rarely one-seeded) capsule (in Xyris rarely a pyxidium)
with persistent tepals.
Seeds Aril absent. Seed coat
testal-endotegmic, sometimes winged. Exotestal cells thin-walled. Endotestal
cells thick-walled. Seeds sometimes operculate; operculum micropylar, formed
from exotegmen or (in Xyris) both integuments. Tegmen mechanical, rich
in resins and tannins. Perisperm not developed. Endosperm copious, starchy and
proteinaceous (and sometimes oily); starch grains compound (Xyris) or
simple (Abolboda). Embryo small (Xyris) or large, straight or
curved (in Abolbodoideae large and curved), little differentiated or
rudimentary, with chlorophyll, Xyris-Scirpus type. Cotyledon
one. Cotyledon hyperphyll prolonged, assimilating. Hypocotyl internode short,
with rhizoids. Coleoptile absent. Collar rhizoids usually present.
Germination?
Cytology n = 8, 9, 13, 16, 17
– Polyploidy frequently occurring.
DNA Deletions present in
ORF2280.
Phytochemistry Flavonols
(kaempferol, quercetin), flavone glycosides, cyanidin, saponins, and chrysazine
(an anthraquinone) present. Esterified ferulic acid (diferulic acid?,
p-coumaric acid?) component of non-lignified cell walls. Phenolic
compounds present or absent. Ellagic acid and alkaloids not found. Aluminium
accumulated.
Use Aquarium plants, medicinal
plants.
Systematics Xyridaceae are sister to Eriocaulaceae with high support.
Xyris
is sister to the remaining Xyridaceae. Comprehensive molecular
phylogenetic analyses of Xyridaceae are needed.
Xyris
1/250–260. Tropical to warm-temperate
regions on both hemispheres, with their highest diversity, c 150 species, in
Brazil. Stem vascular bundles forming a single cylinder. Leaves distichous,
equitant, isobifacial, ligulate. Mucilaginous hairs present in leaf axils.
Peduncle sometimes spirally twisted. Median tepal membranous, caducous. Stamens
sometimes 3+3. Tapetum secretory, with binucleate cells. Pollen grains usually
monosulcate. Exine not spiny. Staminodia usually three, branched and with
moniliform hairs on branch apices. Placentation marginal, parietal (sometimes
intrusively), basal, basal-axile, free central or axile. Ovule orthotropous.
Hypostase absent. Endosperm development usually nuclear. Endotestal cells
thickened. Starch grains compound. Cotyledon bifacial. Cotyledon hypophyll
bifacial, photosynthesizing. Hypocotyl present. Collar rhizoids present. n =
8?, 9, 13, 14, 16, 17 or more. Polyploidy frequently occurring.
Abolbodoideae Suess.
et Beyerle in Bot. Jahrb. Syst. 67: ?. 1935 (Abolbodaceae Nakai,
Chosakuronbun Mokuroku [Ord. Fam. Trib. Nov.]: 221. 20 Jul 1943)
4/25–30. Achlyphila (1;
A. disticha; Serranía de la Neblina Nat. Park in Amazonas in southern
Venezuela), Aratitiyopea (1; A. lopezii; Venezuela, Colombia,
Peru, northwestern Brazil), Orectanthe (2; O. ptaritepuiana,
O. sceptrum; tepuis in Venezuela, Guyana and northwestern Brazil),
Abolboda (20–25; northern South America, with their highest
diversity in the Venezuelan highlands). – Northern South America, with their
largest diversity in the Venezuelan and the Guayana Highlands. Stem vascular
bundles present alternately on inside and outside of thickened sclerified
cylinder, also scattered in centre. Leaves usually spiral (rarely distichous),
rarely equitant (in Achlyphila isobifacial). Inflorescence sometimes
branched, in Achlyphila and some species of Abolboda open.
Peduncle in Achlyphila and Abolboda with one or more pairs of
opposite bracts. Outer tepals in Abolboda two or three; median outer
tepal strongly reduced or absent. Anthers sometimes introrse. Tapetum
amoeboid-periplasmodial (Abolboda). Staminodia usually absent (in
Abolboda sometimes filamentous). Pollen grains spheroidal,
inaperturate (in Orectanthe up to c. 185 μm in diameter). Style often
solid (not hollow), usually trifid in upper part and with three vascularized
nectariferous lateral carinal (non-commissural) appendages (absent in
Achlyphila). Placentation axile. Ovule usually anatropous, in
Abolboda sometimes crassinucellar. Hypostase present. Endosperm
development usually helobial. Exotesta in Orectanthe mechanical,
thick-walled. Endotestal cells in Abolboda large, alternating with
projecting exotegmic cells. Exotegmen thick-walled. Starch grains simple. n =
8–10, 13, 17.
Literature
Acedo C. 1995. Revisión taxonómica del
género Bromus L. (Poaceae) en
la Península Ibérica. – Memoria de Doctorado, Universidad de León,
Spain.
Acedo C, Llamas F. 1997. Two new
brome-grasses (Bromus, Poaceae)
from the Iberian Peninsula. – Willdenowia 27: 47-55.
Acedo C, Llamas F. 1999. The genus
Bromus L. (Poaceae) in the
Iberian Peninsula. – Phanerog. Monogr. 22.
Acedo C, Llamas F. 2003. A new species of
Tridens (Poaceae) from Brazil.
– Syst. Bot. 28: 313-316.
Acosta J, Scataglini M, Reinheimer R, Zuloaga
F. 2014. A phylogenetic study of subtribe Otachyriinae (Poaceae, Panicoideae, Paspaleae). – Plant
Syst. Evol. 300: 2155-2166. – Erratum: idem p. 2167.
Adams KL, Palmer JD. 2003. Evolution of
mitochondrial gene content: gene loss and transfer to the nucleus. – Mol.
Phylogen. Evol. 29: 380-395.
Adamson MA. 1935. A revision of the South
African species of Juncus. – Bot. J. Linn. Soc. 50: 1-38.
Adati S. 1958. Cytogenetics of Japanese wild
forage Miscanthus species. – Proceedings of the Tenth International
Congress of Genetics 2: 1-2.
Agafonov AV, Salomon B. 2002. Genepools in
SH-genomic Elymus species in Boreal Eurasia. – In: Hernández P
(ed), Proceedings of the 4th International Triticeae Symposium,
September 10-12, 2001, Córdoba, Spain, Sevilla, pp. 37-41.
Aguirre-Santoro J. 2017. Taxonomy of the
Ronnbergia Alliance (Bromeliaceae: Bromelioideae): new
combinations, synopsis, and new circumscriptions of Ronnbergia and the
resurrected genus Wittmackia. – Plant Syst. Evol. 303: 615-640.
Aguirre-Santoro J, Betancur J, Holst BK.
2015. Three new additions to the Guayana Shield-endemic Steyerbromelia
(Bromeliaceae) for Colombia with
comments on the problematic generic delimitation within the subfamily
Navioideae. – Syst. Bot. 40: 737-745.
Aguirre-Santoro J, Michelangeli FA, Stevenson
DW. 2016. Molecular phylogenetics of the Ronnbergia Alliance (Bromeliaceae, Bromelioideae) and
insights into their morphological evolution. – Mol. Phylogen. Evol. 100:
1-20.
Ahmad NM, Martin PM, Vella JM. 2009. Floral
morphogenesis and proliferation in Poa labillardieri (Poaceae). – Aust. J. Bot. 57: 602-618.
Ahsan SMN, Vahidy AA, Ali SI. 1994.
Chromosome numbers and incidence of polyploidy in Panicoideae (Poaceae) from Pakistan. – Ann. Missouri
Bot. Gard. 81: 775-783.
Ahumada O, Vegetti AC. 2009. Inflorescence
structure in species of Scleria subgenus Hypoporum and
subgenus Scleria (Sclerieae-Cyperaceae). – Plant Syst. Evol. 281:
115-135.
Aiken SG, Consaul LL. 1995. Leaf cross
sections and phytogeography: a potent combination for identifying members of
Festuca subg. Festuca and Leucopoa (Poaceae), occurring in North America. –
Amer. J. Bot. 82: 1287-1299.
Aiken SG, Darbyshire SJ. 1990. Fescue grasses
of Canada. – Biosystematics Research Institute, Agriculture Canada,
Ottawa.
Aiken SG, Lefkovitch LP. 1990.
Arctagrostis (Poaceae), tribe
Pooideae in North America and Greenland. – Can. J. Bot. 68: 2422-2432.
Aiken SG, Lefkovitch LP. 1993. On the
separation of two species of Festuca subg. Obtusae (Poaceae). – Taxon 42: 323-337.
Aiken SG, Consaul LL, Lefkovitch LP. 1995.
Festuca edlundiae (Poaceae), a
high Arctic, new species compared enzymatically and morphologically with
similar Festuca species. – Syst. Bot. 20: 374-392.
Ainouche ML, Bayer RJ. 1997. On the origins
of the tetraploid Bromus species (section Bromus, Poaceae): insights from internal
transcribed spacer sequences of nuclear ribosomal DNA. – Genome 40:
730-743.
Ainouche ML, Baumel A, Salmon A, Yannic G.
2003. Hybridization, polyploidy and speciation in Spartina (Poaceae). – New Phytol. 161: 165-172.
Ainscought MM, Barker CM, Stace CA. 1986.
Natural hybridizations between Festuca and species of Vulpia.
– Watsonia 16: 143-151.
Aitken K, Li J, Wang L, Qing C, Fan YH,
Jackson P. 2007. Characterization of intergeneric hybrids of Erianthus
rockii and Saccharum using molecular markers. – Gen. Res. Crop
Evol. 54: 1395-1405.
Albert B, Matamoro-Vidal A, Raquin C, Nadot
S. 2010. Formation and function of a new pollen aperture pattern in
angiosperms: the proximal sulcus of Tillandsia leiboldiana (Bromeliaceae). – Amer. J. Bot. 97:
365-368.
Albert B, Ressayre A, Nadot S. 2011.
Correlation between pollen aperture pattern and callose deposition in late
tetrad stage in three species producing atypic pollen grains. – Amer. J. Bot.
98: 189-196.
Albert B, Toghranegar Z, Nadot S. 2014.
Diversity and evolution of microsporogenesis in Bromeliaceae. – Bot. J. Linn. Soc.
176: 36-45.
Al-Beyroutiová M, Sabo M, Sleziak P,
Dušinský, Birčák E, Hauptvogel P, Kilian A, Švec M. 2016. Evolutionary
relationships in the genus Secale revealed by DArTseq DNA
polymorphism. – Plant Syst. Evol. 302: 1083-1091.
Alexeev E. 1980. Genus Colpodium
Trin. s. str. – Novsti Sist. Vysš. Rast. 17: 4-10. [In Russian]
Alexeev E. 1981. Genus Paracolpodium
(Tzvel.) Tzvel. (Poaceae). – Novsti
Sist. Vysš. Rast. 18: 86-95. [In Russian]
Alexeev E. 1985. Festuca L. (Poaceae) in Alaska and Canada. – Novsti
Sist. Vysš. Rast. 22: 5-35. [In Russian]
Alexeev E. 1986. New narrow-leaved
Festuca (Poaceae) members from
Tropical and South Africa. – Bot. Žurn. 71: 1109-1117. [In Russian]
Alexeev E. 1987. Fescues of the Festuca
abyssinica s.l. (Poaceae) group.
– Bot. Žurn. 72: 1260-1268. [In Russian]
Aliscioni SS. 2000. Anatomía ecológica de
algunas especies del género Paspalum (Poaceae, Panicoideae, Paniceae). –
Darwiniana 38: 187-207.
Aliscioni SS. 2002. Contribución a la
filogenia del género Paspalum (Poaceae: Panicoideae: Paniceae). – Ann.
Missouri Bot. Gard. 89: 504-523.
Aliscioni SS, Giussani LM, Zuoaga FO, Kellogg
EA. 2003. A molecular phylogeny of Panicum (Poaceae: Paniceae). Tests of monophyly and
phylogenetic placement within the Panicoideae. – Amer. J. Bot. 90:
796-821.
Aliscioni SS, Ospina JC, Gomiz NE. 2016.
Morphology and leaf anatomy of Setaria s.l. (Poaceae: Panicoideae: Paniceae) and its
taxonomic significance. – Plant Syst. Evol. 302: 173-185.
Allred KW. 1982. Describing the grass
inflorescences. – Range Managem. 35: 672-695.
Allred KW, Gould FW. 1983. Systematics of the
Bothriochloa saccharoides complex (Poaceae: Andropogoneae). – Syst. Bot. 8:
168-184.
Almeida VR, Ferreira da Costa A, Mantovani A,
Gonçalves-Esteves V, de Oliveira R do C, Forzza RC. 2009. Morphological
phylogenetics of Quesnelia (Bromeliaceae, Bromelioideae). –
Syst. Bot. 34: 660-672.
Alves MV, Thomas WW. 2002. Four new species
of Hypolytrum Rich. (Cyperaceae) from Costa Rica and Brazil.
– Feddes Repert. 113: 261-270.
Alves MV, Thomas WW, Das Gracas Lapa
Wanderley M. 2002. New species of Hypolytrum Rich. (Cyperaceae) from the Neotropics. –
Brittonia 54: 124-135.
Alves MV, Estelita MEM, Wanderley MGL, Thomas
WW. 2002. Aplicações taxonômicas da anatomia foliardas espécies brasileiras
de Hypolytrum Rich. (Cyperaceae). – Rev. Brasileira Bot.
25: 1-9.
Alves MV, Araújo AC, Prata AP, Vitta F,
Hefler S, Trevisan R, Gil AB, Martins S, Thomas WW. 2010. Documenting Cyperaceae in diverse tropical
countries: the example of Brazil. – In: Seberg O, Petersen G, Barfod AS,
Davis JI (eds), Diversity, phylogeny, and evolution in the monocotyledons,
Aarhus University Press, Århus, pp. 417-424.
Alves PGM, Scatena VL, Trovó M. 2013.
Anatomy of scapes, bracts, and leaves of Paepalanthus sect.
Diphyomene (Eriocaulaceae, Poales) and its
taxonomic implications. – Brittonia 65: 262-272.
Amalraj VA, Balasundaram N. 2006. On the
taxonomy of the members of ‘Saccharum complex’. – Gen. Res. Crop
Evol. 53: 35-41.
Amarilla LD, Chiapella JO, Nagahama N, Antón
AM. 2013. Inclusion of Dasyochloa in the amphitropical genus
Munroa (Poaceae, Chloridoideae)
based on morphologicdal evidence. – Darwiniana, n.s., 1: 241-252.
Amarilla LD, Chiapella JO, Sosa V, Moreno NC,
Antón AM. 2015. A tale of North and South America: time and mode of dispersal
of the amphitropical genus Munroa (Poaceae, Chloridoideae). – Bot. J. Linn.
Soc. 179: 110-125.
Amarasinghe V, Watson L. 1988. Comparative
ultrastructure of microhairs in grasses. – Bot. J. Linn. Soc. 98: 303-319.
Amarasinghe V, Watson L. 1990. Taxonomic
significance of microhair morphology in the genus Eragrostis Beauv.
(Poaceae). – Taxon 39: 59-65.
Ammann K. 1981. Bestimmungsschwierigkeiten
bei europäischen Bromus-Arten. – Bot. Jahrb. Syst. 102: 459-469.
Amorim AM, Leme EMC. 2009. Two new species of
Quesnelia (Bromeliaceae:
Bromelioideae) from the Atlantic rainforest of Bahia, Brazil. – Brittonia 61:
14-21.
Amsler AI, Vegetti AC. 1999. Tiplogía de la
inflorescencia en Rhynchoryza (Oryzeae-Poaceae). – Candollea 54: 54-65.
Amsler AI, Perreta MG, Tivano JC, Vegetti AC.
2004. Anatomía caulinar de especies de Oryzeae. – Candollea 59: 135-155.
Anamthawat-Jonsson K. 2014. Molecular
cytogenetics of Leymus: mapping the Ns genome-specific repetitive
sequences. – J. Syst. Evol. 52: 716-721.
Anderson DE. 1974. Taxonomy of the genus
Chloris (Gramineae). – Brigham Young Univ. Sci. Bull., Biol. Ser.
29: 1-133.
Andrade MJG de. 2007. Filogenia e taxonomia
em Eriocaulaceae neotropicais. –
Ph.D. diss., Universidade Estadual de Feira de Santana, Feira de Santana,
Brazil.
Andrade MJG de, Giulietti AM, Rapini A,
Queiroz LP de, Conceição A de Souza, Almeida PRM de, Berg C van den. 2010. A
comprehensive phylogenetic analysis of Eriocaulaceae: evidence from nuclear
(ITS) and plastid (psbA-trnH and trnL-F) DNA
sequences. – Taxon 59: 379-388.
Andrés JM. 1941. Numero de cromosomas en las
especies del género Hordeum espontáneas en los alrededores de Buenos
Aires. – Rev. Fac. Agron. 2: 100-107.
Angelov GB. 2003. Relationships among
Agropyron, Dasypyrum and Lophopyrum (Triticeae: Poaceae) viewed from isoenzyme variation of
esterase, peroxidase and acid phosphatase. – Acta Bot. Croat. 62: 11-19.
Ansari R, Balakrishnan NP. 1994. The family
Eriocaulaceae in India. – Bishen
Singh Mahendra Pal Singh, Dehra Dun, India.
Antón AM. 1977. Notas criticas sobre
gramíneas de Argentina II. – Kurtziana 10: 51-67.
Antón AM. 1981. The genus Tragus
(Gramineae). – Kew Bull. 36: 55-61.
Antón AM, Cocucci AE. 1984. The grass
megagametophyte and its possible phylogenetic implications. – Plant Syst.
Evol. 146: 117-121.
Antón AM, Connor HE. 1995. Floral biology
and reproduction in Poa (Poeae: Gramineae). – Aust. J. Bot. 43:
577-599.
Appel O, Bayer C. 1998. Flagellariaceae. – In: Kubitzki K
(ed), The families and genera of vascular plants IV. Flowering plants.
Monocotyledons. Alismatanae and Commelinanae (except Gramineae), Springer,
Berlin, Heidelberg, New York, pp. 208-211.
Appels R, Dvořák J. 1982. Relative rates of
divergence of spacer and gene sequences within the rDNA region of species in
the Triticeae: implications for the maintenance of homogeneity of a repeated
gene family. – Theor. Appl. Gen. 63: 361-365.
Araújo AC, Fischer EA, Sazima M. 1994.
Floração sequencial e polinização de três espécies de Vriesea
(Bromeliaceae) na região de
Juréia, sudeste do Brasil. – Rev. Bras. Bot. 17: 113-118.
Araújo AC, Longhi-Wagner HM, Thomas WW.
2003. New unicapitate species of Rhynchospora (Cyperaceae) from South America. –
Brittonia 55: 30-36.
Araújo AC, Longhi-Wagner HM, Thomas WW.
2012. A synopsis of Rhynchospora sect. Pluriflorae (Cyperaceae). – Brittonia 64:
381-393.
Arber A. 1923. Leaves of the Gramineae. –
Bot. Gaz. 76: 374-388.
Arber A. 1928. Studies in the Gramineae V. 1.
On Luziola and Dactylis; 2. On Lygeum and
Nardus. – Ann. Bot. 52: 291-407.
Arber A. 1929. Studies in the Gramineae VI.
1. Streptochaeta. 2. Anomochloa. 3. Ichnanthus. –
Ann. Bot. 43: 35-53.
Arber A. 1934. The Gramineae: a study of
cereal, bamboo, and grass. – Cambridge University Press, Cambridge.
Arekal GD, Ramaswamy SN. 1980. Embryology of
Eriocaulon hookerianum Stapf and the systematic position of Eriocaulaceae. – Bot. Not. 133:
295-309.
Arenas P, Arroyo SC. 1988. Las espécies
comestibles del género Bromelia (Bromeliaceae) del Gran Chaco. –
Candollea 43: 645-660.
Armstrong KC. 1973. Chromosome pairing in
hexaploid hybrids from Bromus erectus x B. inermis (2n=56).
– Can. J. Gen. Cytol. 15: 427-436.
Armstrong KC. 1977. Hybrids of the annual
Bromus arvensis with perennial B. inermis and B.
erectus. – Can. J. Genet. Cytol. 21: 65-71.
Armstrong KC. 1981. The evolution of
Bromus inermis and related species of Bromus sect.
Pnigma. – Bot. Jahrb. Syst. 102: 427-443.
Armstrong KC. 1982. Hybrids between the
tetraploids of Bromus inermis and Bromus pumpellianus. –
Can. J. Bot. 60: 476-482.
Armstrong KC. 1983. The relationship between
some Eurasian and American species of Bromus section Pnigma
as determined by the karyotypes of some F1 hybrids. – Can. J. Bot.
61: 700-707.
Armstrong KC. 1984a. The genomic relationship
of the diploid Bromus variegatus to Bromus inermis. – Can.
J. Genet. Cytol. 62: 469-474.
Armstrong KC. 1984b. Chromosome pairing
affinities between Old and New World species of Bromus section
Pnigma. – Can. J. Bot. 62: 581-585.
Armstrong KC. 1985. Chromosome pairing
failure in an intersectional amphiploid of Bromus altissimus x B.
arvensis. – Can. J. Genet. Cytol. 27: 705-709.
Armstrong KC. 1991. Chromosome evolution in
Bromus. – In: Tsuchiya T, Gupta PK (eds), Chromosome engineering in
plants: genetics, breeding, evolution, part B, Elsevier, Amsterdam, pp.
366-377.
Arnow LA. 1981. Poa secunda Presl
versus P. sandbergii Vasey (Poaceae). – Syst. Bot. 6: 412-421.
Arrais M das Graças Medina. 1989. Aspectos
anatômicos de espécies de Bromeliaceae da Serra do Cipö-MG, com
especial referência à vascularização floral. – Ph.D. diss., São Paulo,
Brazil.
Arriaga MO. 1990. Desarrollo de la estructura
Kranz en tallo de especies de Eriochloa (Paniceae-Poaceae). – Bol. Soc. Argent. Bot. 26:
177-195.
Arriaga MO. 1992. Salt glands in flowering
culms of Eriochloa species (Poaceae). – Bothalia 22: 111-117.
Arriaga MO, Barkworth ME. 2006.
Amelichloa: a new genus in the Stipeae (Poaceae). – Sida 22: 145-149.
Arthan W, Traiperm P, Gale SW, Norsaengsri M,
Kethirun L. 2016. Re-evaluation of the taxonomic status of
Hackelochloa (Poaceae) based on
anatomical and phonetic analyses. – Bot. J. Linn. Soc. 181: 224-245.
Arthan W, McKain MR, Traipem P, Welker CAD,
Teisher JK, Kellogg EA. 2017. Phylogenomics of Andropogoneae (Panicoideae: Poaceae) of Mainland Southeast Asia. –
Syst. Bot. 42: 418-431.
Asano T, Tsudzuki T, Takahashi S, Shimada H,
Kadowaki K. 2004. Complete nucleotide sequence of the sugarcane (Saccharum
officinarum) chloroplast genome: a comparative analysis of four monocot
chloroplast genomes. – DNA Research 11: 93-99.
Ashan SMN, Vahidi AA, Ali SI. 1994.
Chromosome numbers and incidence of polyploidy in Panicoideae (Poaceae) from Pakistan. – Ann. Missouri
Bot. Gard. 81: 775-783.
Asplund I. 1968. Embryological studies in the
genera Sparganium and Typha. A preliminary report. – Svensk
Bot. Tidskr. 62: 410-412.
Asplund I. 1972. Embryological studies in the
genus Typha. – Svensk Bot. Tidskr. 66: 1-17.
Asplund I. 1973. Embryological studies in the
genus Sparganium. – Svensk Bot.Tidskr. 67: 177-200.
Assadi M. 1996. A taxonomic revision of
Elymus sect. Caespitosae and sect. Elytrigia (Poaceae, Triticeae) in Iran. –
Willdenowia 26: 251-271.
Assadi M, Runemark 1995. Hybridisation,
genomic constitution and generic delimitation in Elymus s.l. (Poaceae: Triticeae). – Plant Syst. Evol.
194: 189-205.
Astegiano MA. 1973. Sobre la presencia del
genero Aciachne (Gramineae) en la Argentina. – Kurtziana 7:
43-47.
Aston HI. 1987. Sparganiaceae. – In: George
AS (ed), Flora of Australia 45, Australian Government Publ. Service, Canberra,
pp. 6-8.
Atkins RJ, Barkworth ME, Dewey DR. 1984. A
taxonomic study of Leymus ambiguus and L. salinus (Poaceae: Triticeae). – Syst. Bot. 9:
279-294.
Attigala L, Triplett JK, Kathriarachchi HS,
Clark LG. 2014. A new genus and a major temperate bamboo lineage of the
Arundinarieae (Poaceae: Bambusoideae)
from Sri Lanka based on a multi-locus plastid phylogeny. – Phytotaxa 174:
187-205.
Attigala L, Wysocki WP, Duvall MR, Clark LG.
2016. Phylogenetic estimation and morphological evolution of Arundinarieae
(Bambusoideae: Poaceae) based on
plastome phylogenomic analysis. – Mol. Phylogen. Evol. 101: 111-121.
Attigala L, Kathriarachchi H-S, Clark LG.
2016. Taxonomic revision of the temperate woody bamboo genus Kuruna
(Poaceae: Bambusoideae: Arundinarieae).
– Syst. Bot. 41: 174-196.
Auquier P. 1980. Hubbardochloa, a
new genus of grasses from Rwanda and Burundi. – Bull. Jard. Bot. Natl. Belg.
50: 241-247.
Auquier P, Somers Y. 1967. Recherches
histotaxonomiques sur le chaume des Poaceae. – Bull. Soc. Roy. Bot. Belg.
100: 95-140.
Avdulov NP. 1931. Karyo-systematische
Untersuchungen der Familie Gramineen. – Bull. Appl. Bot. Genet. Plant Breed.
[Suppl.] 44: 1-428. [In Russian with German summary]
Bačič T, Jogan N, Koce JD. 2007.
Luzula sect. Luzula in the south-eastern Alps – karyology
and genome size. – Taxon 56: 129-136.
Backer CA. 1951a. Juncaceae. – In: Steenis CGGJ van (ed),
Flora Malesiana I, 4(3), Noordhoff-Kolff N. V., Batavia, pp. 210-215.
Backer CA. 1951. Flagellariaceae. – In: Steenis
CGGJ van (ed), Flora Malesiana I, 4(3), Noordhoff-Kolff, N. V., Batavia, pp.
245-250.
Baden C. 1991. A taxonomic revision of
Psathyrostachys Nevski (Poaceae). – Nord. J. Bot. 11: 3-26.
Baden C, Bothmer R von. 1994. A taxonomic
revision of Hordeum sect. Critesion. – Nord. J. Bot. 14:
117-136.
Baeza PCM. 1996. Los géneros
Danthonia D.C. y Rytidosperma Steud. (Poaceae) en América – una revision. –
Sendtnera 3: 11-93.
Baeza PCM, Stuessy TF, Marticorena C. 2002.
Notes on the Poaceae of the Robinson
Crusoe (Juan Fernandez) Islands, Chile. – Brittonia 54: 154-163.
Baeza PCM, Finot VL, Matther O. 2006.
Micromorfología de la epidermis de la lemma de Trisetum y géneros
afines (Poaceae, Pooideae). –
Darwiniana 44: 32-57.
Bahadur KN, Jain SS. 1981. Rare bamboos of
India. – Indian J. For. 4: 280-286.
Bailey LH. 1886. A preliminary synopsis of
North American carices. – Proc. Amer. Acad. Arts Sci. 3: 59-157.
Baillon MH. 1892. Une Graminée uniflore. –
Bull. Mens. Soc. Linn. Paris 130: 1034-1036.
Baillon MH. 1893. Nouvelle note sur
l’Aciachne. – Bull. Mens. Soc. Linn. Paris 135: 1073.
Baker JG. 1889. Handbook of the Bromeliaceae. – George Bell &
Sons, London.
Bakker K. 1958. Restionaceae. – In: Steenis CGGJ van
(ed), Flora Malesiana I, 5(4), Noordhoff-Kolff N. V., Djakarta, pp. 416-420.
Baldini RM. 1995. Revision of the genus
Phalaris L. (Gramineae). – Webbia 49: 265-329.
Balke M, Gómez-Zurita J, Ribera I, Viloria
A, Zillikens A, Steiner J, García M, Hendrich L, Vogler AP. 2008. Ancient
associations of aquatic beetles and tank bromeliads in the neotropical forest
canopy. – Proc. Natl. Acad. Sci. U.S.A. 105: 6356-6361.
Ball PW. 1990. Some aspects of the
phytogeography of Carex. – Can. J. Bot. 68: 1462-1472.
Ballard F. 1932. The genus
Mariscopsis. – Kew Bull. 1932: 457-458.
Ballard F. 1933. Queenslandiella
hyalina (Vahl) Ballard. – Hooker’s Icones Plantarum ser. 5, tab.
3208.
Balslev H. 1979. 208. Juncaceae. – In: Harling G, Sparre B
(eds), Flora of Ecuador 11, Swedish Natural Science Research Council,
Stockholm, pp. 1-44.
Balslev H. 1982. A systematic monograph of
the neotropical Juncaceae. – Ph.D.
diss., City University of New York, New York.
Balslev H. 1996. Flora Neotropica Monograph
68. Juncaceae. – The New York
Botanical Garden, Bronx, New York.
Balslev H. 1998. Juncaceae. – In: Kubitzki K (ed), The
families and genera of vascular plants IV. Flowering plants. Monocotyledons.
Alismatanae and Commelinanae (except Gramineae), Springer, Berlin, Heidelberg,
New York, pp. 252-260.
Baltisberger M, Leuchtmann A. 1991.
Investigations on some Gramineae from Albania and Greece. – Ber. Geobot.
Inst. ETH, Stiftung Rübel, Zürich 57: 182-192.
Bamboo Phylogeny Group. 2006. The Bamboo
Phylogeny Project. – BAMBOO, Mag. Amer. Bamboo Soc. 27: 11-14.
Bamboo Phylogeny Group. 2012. An updated
tribal and subtribal classification for the Bambusoideae (Poaceae). Bamboo Science & Culture. –
J. Amer. Bamboo Soc. 25: 3-27.
Banerjee U. 1967. Ultrastructure of the
tapetal membranes in grasses. – Grana Palynol. 7: 2-3.
Banfi E, Bracchi G, Galasso G, Romani E.
2005. Agrostologia placentina. – Mem. Soc. Ital. Sci. Nat. Mus. Civico Storia
Milano 33: 1-80.
Bao Y, Ge S. 2004. Origin and phylogeny of
Oryza species with the CD genome based on multiple-gene sequence data.
– Plant Syst. Evol. 249: 55-66.
Baquar SR. 1978. Cytological studies of some
Southern Nigerian Cyperaceae. – La
Kromosomo II, 9: 263-270.
Baranova MA. 1975. Stomatographical studies
of the family Flagellariaceae.
– Bot. Žurn. 60: 1690-1697. [In Russian]
Barber JC, Hames KA, Cialdella AM, Giussani
LM, Morrone O. 2009. Phylogenetic relationships of Piptochaetium Presl
(Poaceae: Stipeae) and related genera
reconstructed from nuclear and chloroplast sequence datasets. – Taxon 58:
375-380.
Baresch A, Smith JAC, Winter K, Valerio AL,
Jaramillo C. 2011. Karatophyllum bromelioides L. D. Gómez revisited:
a probable fossil CAM bromeliad. – Amer. J. Bot. 98: 1905-1908.
Barfuss MHJ. 2012. Molecular studies in Bromeliaceae: implications of plastid
and nuclear DNA markers for phylogeny, biogeography, and character evolution
with emphasis on a new classification of Tillandsioideae. – Ph.D. diss.,
University of Vienna, Austria.
Barfuss MHJ, Samuel MM, Till W. 2004.
Molecular phylogeny in subfamily Tillandsioideae (Bromeliaceae) based on six cpDNA
markers: an update. – J. Brom. Soc. 54: 9-17.
Barfuss MHJ, Samuel MR, Till W, Stuessy TF.
2005. Phylogenetic relationships in subfamily Tillandsioideae (Bromeliaceae) based on DNA sequence
data from seven plastid regions. – Amer. J. Bot. 92: 337-351.
Barfuss MHJ, Till W, Leme EMC, Pinzón JP,
Manzanares JM, Halbritter H, Samuel R, Brown GK. 2016. Taxonomic revision of Bromeliaceae subfam. Tillandsioideae
based on a multi-locus DNA sequence phylogeny and morphology. – Phytotaxa
279: 1-97.
Barker NP. 1993. A biosystematic study of
Pentameris (Arundineae, Poaceae). – Bothalia 23: 25-47.
Barker NP. 1994. External fruit morphology of
southern African Arundineae (Arundinoideae: Poaceae). – Bothalia 24: 55-66.
Barker NP. 1995. A molecular phylogeny of the
subfamily Arundinoideae (Poaceae). –
Ph.D. diss., University of Cape Town, Republic of South Africa.
Barker NP. 1997. The relationships of
Amphipogon, Elytrophorus and Cyperochloa (Poaceae) as suggested by rbcL
sequence data. – Telopea 7: 205-213.
Barker NP, Linder HP, Harley EH. 1995.
Polyphyly of the Arundinoideae (Poaceae): evidence from rbcL
sequence data. – Syst. Bot. 20: 423-435.
Barker NP, Linder HP, Harley EH. 1998.
Sequences of the grass-specific insert in the chloroplast rpoC2 gene
elucidate generic relationships of the Arundinoideae (Poaceae). – Syst. Bot. 23: 327-350.
Barker NP, Clark LG, Davis JI, Duvall MR,
Guala GF, Hsiao C, Kellogg EA, Linder HP, Mason-Gamer R, Mathews S, Soreng R,
Spangler R. 2000. A phylogeny of the grass family (Poaceae), as inferred from eight character
sets. – In: Jacobs SWL, Everett J (eds), Grasses: systematics and evolution,
Proceedings of the 2nd International Conference on the Comparative
Biology of Monocotyledons, Sydney, Australia, Vol. 2, CSIRO Publ., Melbourne,
pp. 3-7
Barker NP, Morton CM, Linder HP. 2000. The
Danthonieae: generic composition and relationships. – In: Jacobs SWL, Everett
J (eds), Grasses: systematics and evolution, Proceedings of the 2nd
International Conference on the Comparative Biology of Monocotyledons, Sydney,
Australia, Vol. 2, CSIRO Publ., Melbourne, pp. 221-230.
Barker NP, Clark L, Davis JI, Duvall MR,
Guala G, Hsiao C, Kellogg EA, Linder HP, Mason-Gamer R, Mathews S, Simmons M,
Soreng R, Spangler R. 2001. Phylogeny and subfamilial classification of the
grasses (Poaceae). – Ann. Missouri
Bot. Gard. 8: 373-457..
Barker NP, Linder HP, Morton CM, Lyle M.
2003. The paraphyly of Cortaderia (Danthonioideae; Poaceae): evidence from morphology,
chloroplast and nuclear DNA sequence data. – Ann. Missouri Bot. Gard. 90:
1-24.
Barker NP, Galley C, Verboom GA, Mafa P,
Gilbert M, Linder HP. 2007. The phylogeny of the austral grass subfamily
Danthonioideae: evidence from multiple data sets. – Plant Syst. Evol. 264:
135-156.
Barkworth ME. 1981. Foliar epidermis and
taxonomy of North American Stipeae (Gramineae). – Syst. Bot. 6: 136-152.
Barkworth ME. 1982. Embryological characters
and the taxonomy of the Stipeae (Gramineae). – Taxon 31: 233-243.
Barkworth ME. 1983. Ptilagrostis in
North America and its relationship to other Stipeae (Gramineae). – Syst. Bot.
8: 395-419.
Barkworth ME. 1988. New taxa in
Piptochaetium (Stipeae, Gramineae) from Mexico. – Syst. Bot. 13:
196-201.
Barkworth ME. 1990. Nassella
(Gramineae, Stipeae): revised interpretation and nomenclatural changes. –
Taxon 39: 597-614.
Barkworth ME. 1993. North American Stipeae
(Gramineae): taxonomic changes and other comments. – Phytologia 74: 1-25.
Barkworth ME. 2001. Distribution and
diagnostic characters of Nassella (Poaceae: Stipeae). – Taxon 50:
439-468.
Barkworth ME. 2006. Manual of grasses for
North America North of Mexico. – University of Utah, Logan.
Barkworth ME, Everett J. 1987. Evolution in
the Stipeae: identification and relationships of its monophyletic taxa. – In:
Soderstrom TR, Hilu KW, Campbell CS, Barkworth ME (eds), Grass systematics and
evolution, Smithsonian Institution Press, Washington, D.C., pp. 251-264.
Barkworth ME, Torres MA. 2001. Distribution
and diagnostic characters of Nassella (Poaceae: Stipeae). – Taxon 50:
439-468.
Barkworth ME, Arriaga MO, Smith JF, Jacobs
SWL, Valdés-Reyna J, Bushman BS. 2008. Molecules and morphology in South
American Stipeae (Poaceae). – Syst.
Bot. 33: 719-731.
Barkworth ME, Jacobs SWL, Zhang H-Q. 2009.
Connorochloa: a new genus in Triticeae. – Breeding Sci. 59:
685-686.
Barkworth ME, Cutler DR, Rollo JS, Jacobs
SWL, Rashid A. 2009. Morphological identification of genomic genera in the
Triticeae. – Breeding Sci. 59: 561-570.
Barnard C. 1957a. Floral histogenesis in the
monocotyledons I. The Gramineae. – Aust. J. Bot. 5: 1-20.
Barnard C. 1957b. Floral histogenesis in the
monocotyledons II. The Cyperaceae.
– Aust. J. Bot. 5: 115-128.
Barnard C. 1958. Floral histogenesis in
monocotyledons III. The Juncaceae. –
Aust. J. Bot. 6: 285-298.
Barreto L, Echternacht L, Garcia Q. 2013.
Seed coat sculpture in Comanthera (Eriocaulaceae) and its implications
on taxonomy and phylogenetics. – Plant Syst. Evol. 299: 1461-1469.
Barrett RL, Wilson KL. 2012. A review of the
genus Lepidosperma Labill. (Cyperaceae: Schoeneae). – Aust. Syst.
Bot. 25: 225-294.
Barros M. 1928. Ciperáceas Argentinas I.
Heleocharis. – An. Mus. Argent. Ci. Nat. “Bernardino Rivadavia”
34: 425-496.
Barros M. 1935. Ciperáceas Argentinas II.
Géneros Kyllingia, Scirpus, Carex. – An Mus. Nac.
Hist. Nat. Buenos Aires 37: 133-263.
Barros M. 1960. Las Ciperáceas del estado de
Santa Catalina. – Sellowia 12: 181-450.
Barrow J, Lucero M, Reyes-Vera I, Havstad K.
2007. Endosymbiotic fungi structurally integrated with leaves reveals a
lichenous condition of C4 grasses. – In Vitro Cell. Devel. Biol.
Plant 43: 65-70.
Battaglia E. 1954. Assenza si centromero
localizzato in Heleocharis uniglumis (Link) Schult. – Caryologia 6:
319-332.
Battaglia E. 1955. A consideration of a new
type of meiosis (mis-meiosis) in Juncaceae (Luzula) and
Hemiptera. – Bull. Torrey Bot. Club 82: 383-396.
Battaglia E, Boyes JW. 1955. Post-reductional
meiosis: its mechanism and causes. – Caryologia 8: 87-133.
Baum BR. 1968. Delimitation of the genus
Avena (Gramineae). – Can. J. Bot. 46: 121-132.
Baum BR. 1969. The use of lodicule type in
assessing the origin of Avena fatuoids. – Can. J. Bot. 47:
931-944.
Baum BR. 1973. The genus
Danthoniastrum, about its circumscription, past and present status,
and some taxonomic principles. – Österr. Bot. Zeitschr. 122: 51-57.
Baum BR. 1975. Cladistic analysis of the
diploid and hexaploid oats (Avena, Poaceae) using numerical techniques. –
Can. J. Bot. 53: 2115-2127.
Baum BR. 1977. Oats: wild and cultivated. A
monograph of the genus Avena L. (Poaceae). – Biosyst. Res. Inst., Ottawa,
Canada, Monogr. 14.
Baum BR. 1987. Numerical taxonomic analyses
of the Poaceae. – In: Soderstrom TR,
Hilu KH, Campbell CS, Barkworth ME (eds), Grass systematics and evolution,
Smithsonian Institution Press, Washington, D.C., pp. 334-342.
Baum BR, Appels R. 1992. Evolutionary change
at the 5S DNA loci of species in the Triticeae. – Plant Syst. Evol. 183:
195-208.
Baum BR, Bailey LG. 1990. Key and synopsis of
North American Hordeum species. – Can. J. Bot. 68: 2433-2442.
Baum BR, Estabrook GF. 1996. Impact of
outgroup inclusion on estimates by parsimony of undirected branching of ingroup
phylogenetic lines. – Taxon 45: 243-257.
Baum BR, Tulloch AP, Bailey LG. 1980. A
survey of epicuticular waxes among genera of Triticeae I. Ultrastructure of
glumes and some leaves as observed with the scanning electron microscope. –
Can. J. Bot. 58: 2467-2480.
Baum BR, Estes JR, Pushpendra K, Gupta PK.
1987. Assessment of the genomic system of classification in the Triticeae. –
Amer. J. Bot. 74: 1388-1395.
Baum BR, Tulloch AP, Bailey LG. 1989.
Epicuticular waxes of the genus Hordeum: a survey of their chemical
composition and ultrastructure. – Can. J. Bot. 67: 3219-3226.
Baum BR, Edwards T, Johnson DA. 2009.
Phylogenetic relationships among diploid Aegilops species inferred
from 5S rDNA units. – Mol. Phylogen. Evol. 53: 34-44.
Baumel A, Ainouche ML, Bayer RJ, Ainouche AK,
Misset MT. 2002. Molecular phylogeny of hybridizing species from the genus
Spartina Schreb. (Poaceae). –
Mol. Phylogen. Evol. 22: 303-314.
Bauters K, Larridon I, Reynders M, Asselman
P, Vrijdaghs A, Muasya AM, Simpson DA, Goetghebeur P. 2014. A new
classification for Lipocarpha and Volkiella as infrageneric
taxa of Cyperus s.l. (Cypereae, Cyperoideae, Cyperaceae): insights from species tree
reconstruction supplemented with morphological and floral developmental data.
– Phytotaxa 166: 1-32.
Bauters K, Asselman P, Simpson DA, Muasya AM,
Goetghebeur P, Larridon I. 2016. Phylogenetics, ancestral state reconstruction,
and a new infrageneric classification of Scleria (Cyperaceae) based on three DNA markers.
– Taxon 65: 444-466.
Bayer C, Appel O. 1998. Joinvilleaceae. – In: Kubitzki K
(ed), The families and genera of vascular plants IV. Flowering plants.
Monocotyledons. Alismatanae and Commelinanae (except Gramineae), Springer,
Berlin, Heidelberg, New York, pp. 249-251.
Beaman RS. 1989. Systematics of
Tillandsia subgenus Pseudalcantarea (Bromeliaceae) and cladistic
relationships of the Bromelioideae, Pitcairnioideae, and Tillandsioideae. –
M.Sc. thesis, University of Florida, Gainesville, Florida.
Beaman RS, Judd SW. 1996. A systematics of
Tillandsia subgenus Pseudalcantarea (Bromeliaceae). – Brittonia 48:
1-19.
Beetle AA. 1943. The North American
variations of Distichlis spicata. – Bull. Torrey Bot. Club 70:
638-650.
Beetle AA. 1945. Distichlis spicata
in Australia. – Rhodora 47: 148.
Beetle AA. 1946. Studies in the genus
Scirpus L. VIII. Notes on its taxonomy, phylogeny and distribution.
– Amer. J. Bot. 33: 660-666.
Beetle AA. 1955a. The grass genus
Distichlis. – Rev. Argentina Agron. 22: 86-94.
Beetle AA. 1955b. The four subfamilies of the
Gramineae. – Bull. Torrey Bot. Club 82: 196-197.
Beetle AA, Manrique Forceck E, Jaramillo
Luque V, Guerreo Sánchez P, Miranda Sánchez A, Núñez Tancredi I, Chimal
Hernández A. 1987. Las gramíneas de México 2. – Secretaría de Agricultura
y Recursos Hidráulicos, México.
Begum M. 1966. Sporogenesis and the
development of gametophtes in Eriocaulon quinquangulare Linn. –
Curr. Sci. 35: 262-263.
Begum M. 1968. Embryological studies in
Eriocaulon quinquangulare L. – Proc. Indian Acad. Sci., Sect. B, 67:
148-156.
Bell HL. 2007. Phylogenetic relationships
within Chloridoideae (Poaceae) with
emphasis on subtribe Monanthochloinae. – Ph.D. diss., Claremont Graduate
University, Claremont, California.
Bell HL, Columbus JT. 2008. Proposal for an
expanded Distichlis (Poaceae,
Chloridoideae): support from molecular, morphological, and anatomical
characters. – Syst. Bot. 33: 536-551.
Bell HL, Columbus JT, Ingram AL. 2013.
Kalinia, a new North American genus for a species long misplaced in
Eragrostis (Poaceae,
Chloridoideae). – Aliso 30: 85-95.
Bell TL, Pate JS. 1993. Morphotypic
differentiation in the SW Australian restiad Lyginia barbata R. Br.
(Restionaceae). – Aust. J. Bot.
41: 91-104.
Benko-Iseppson AM, Wanderley MGL. 2002.
Cytogenetic studies on Brazilian Xyris species (Xyridaceae). – Bot. J. Linn. Soc. 138:
245-252.
Benner U, Schnepf E. 1975. Die Morphologie
der Nektarausscheidung bei Bromeliaceen: Beteiligung des Golgi-Apparates. –
Protoplasma 85: 337-349.
Bennetzen JL. 2007. Patterns in grass genome
evolution. – Curr. Opinion Plant Biol. 10: 176-181.
Bentham G. 1881. Notes on Gramineae. – Bot.
J. Linn. Soc. 19: 14-134.
Benzing DH. 1976. Bromeliad trichomes:
structure, function, and ecological significance. – Selbyana 1: 330-348.
Benzing DH. 1980. The biology of the
Bromeliads. – Mad River Press, Eureka, California.
Benzing DH. 1990. Vascular epiphytes. –
Cambridge University Press, Cambridge.
Benzing DH. 1994. How much is known about Bromeliaceae in 1994? – Selbyana 15:
1-7.
Benzing DH. 2000. Bromeliaceae – Profile of an
adaptive radiation. – Cambridge University Press, Cambridge.
Benzing DH, Renfrow A, 1974. The mineral
nutrition of Bromeliaceae. – Bot.
Gaz. 135: 281-288.
Benzing DH, Henderson K, Kessel B, Sulak J.
1976. The absorptive capacities of bromeliad trichomes. – Amer. J. Bot. 63:
1009-1014.
Benzing DH, Seemann J, Renfrow A. 1978. The
foliar epidermis in Tillandsioideae (Bromeliaceae) and its role in habitat
selection. – Amer. J. Bot. 65: 359-365.
Benzing DH, Givnish TJ, Bermudes D. 1985.
Absorptive trichomes in Brocchinia reducta (Bromeliaceae) and their evolutionary
and systematic significance. – Syst. Bot. 10: 81-91.
Berg RY. 1985. Spiklet structure in
Panicum australiense (Poaceae):
taxonomic and ecological implications. – Aust. J. Bot. 33: 579-583.
Berger CA. 1946. The cytology of
Luzula. – Amer. J. Bot. 36: 794.
Bergner I, Jensen U. 1989. Phytoserological
contribution to the systematic placement of the Typhales. – Nord. J. Bot. 8:
447-456.
Bernardello LM, Galetto L, Juliani HR. 1991.
Floral nectar, nectary structure and pollinators in some Argentinian Bromeliaceae. – Ann. Bot., N. S.,
67: 401-411.
Bernardello LM, Galetto L, Jaramillo J,
Grijalba E. 1994. Floral nectar chemical composition of some species from
reserva Río Guajalito, Ecuador. – Biotropica 26: 113-116.
Berry PE. 1999. 212B. Rapateaceae. – In: Harling G,
Andersson L (eds), Flora of Ecuador 63, Nord. J. Bot., Copenhagen, pp.
39-45.
Besnard G, Muasya AM, Russier F, Roalson EH,
Salamin N, Christin P-A. 2009. Phylogenomics of C4 photosynthesis in
sedges (Cyperaceae): multiple
appearances and genetic convergence. – Mol. Biol. Evol. 26: 1909-1919.
Besnard G, Christin P-A, Malé P-J G, Coissac
E, Ralimanana H, Vorontsova MS. 2013. Phylogenomics and taxonomy of
Lecomtelleae (Poaceae), an isolated
panicoid lineage from Madagascar. – Ann. Bot. 112: 1057-1066.
Bess EC, Doust AN, Kellogg EA. 2005. A naked
gass in the ‘bristle clade’: a phylogenetic and developmental study of
Panicum section Bulbosa (Paniceae: Poaceae). – Intern. J. Plant Sci. 166:
371-381.
Bess EC, Doust AN, Davidse G, Kellogg EA.
2006. Zuloagaea, a new genus of neotropical grass within the
“Bristle Clade” (Poaceae: Paniceae).
– Syst. Bot. 31: 656-670.
Besse P, Mcintyre CL, Berding N. 1997.
Characterization of Erianthus sect. Ripidium and
Saccharum germplasm (Andropogoneae-Saccharinae) using RFLP markers.
– Euphytica 93: 283-292.
Bessey EA. 1917. The phylogeny of the
grasses. – Rep. Michigan Acad. Sci. 19: 239-245.
Betancur J, Miranda-Esquivel DR. 1999. Existe
Sodiroa? – Rev. Acad. Colomb. Cienc. 23: 189-194.
Betancur J, Salinas NR. 2006. El ocaso de
Pseudaechmea (Bromeliaceae: Bromelioideae): the
Pseudaechmea (Bromeliaceae: Bromelioideae) twilight.
– Caldasia 28: 157-164.
Bews JW. 1929. The world’s grasses: their
differentiation, distribution, economics, and ecology. – Longmans, Green
& Co., London.
Bhanwra RK. 1988. Embryology in relation to
systematics of Gramineae. – Ann. Bot., N. S., 62: 215-233.
Bhanwra RK, Kaur N, Garg A. 1991.
Embryological studies in some grasses and their taxonomic significance. –
Bot. J. Linn. Soc. 107: 405-417.
Bhanwra RK, Sharma ML, Vij SP. 2001.
Comparative embryology of Bambusa tulda Roxb. and Thyrsostachys
siamensis Gamble (Poaceae:
Bambuseae). – Bot. J. Linn. Soc. 135: 113-124.
Bieniek W, Mizianty M, Szklarczyk M. 2015.
Sequence variation at the three chloroplast loci (matK, rbcL,
trnH-psbA) in the Triticeae tribe (Poaceae): comments on the relationships and
utility in DNA barcoding of selected species. – Plant Syst. Evol. 301:
1275-1286.
Bin J, Peterson PM, Qing L. 2011. Caryopsis
micromorphology of Eleusine Gaertn. (Poaceae) and its systematic implications.
– J. Trop. Subtrop. Bot. 19: 195-204.
Bir SS, Sahni M. 1985. Cytological
investigations on some grasses from Punjab Plain, North India. – Proc. Indian
Natl. Sci. Acad., Part B, Biol. Sci. 5: 609-626.
Bir SS, Sidhu M, Kamra S. 1982. Cytological
studies on certain sedges from Punjab, India. – Cell Chromosome Res. 5:
25-28.
Bir SS, Sidhu M, Kamra S. 1986. Karyotypic
studies on certain members of Cyperaceae from Punjab, North West
India. – Cytologia 51: 95-106.
Bir SS, Cheema P, Kumari S, Sidhu M. 1993.
Occurrence of B-chromosomes and karyotypic analysis in sedge genus
Eleocharis R. Br. – Curr. Sci. 64: 322-325.
Birch JL, Cantrill DJ, Walsh NG, Murphy DJ.
2014. Phylogenetic investigation and divergence dating of Poa (Poaceae, tribe Poeae) in the Australasian
region. – Bot. J. Linn. Soc. 175: 523-552.
Birch JL, Berwick FM, Walsh NG, Cantrill DJ,
Murphy DJ. 2015. Distribution of morphological diversity within widespread
Australian species of Poa (Poaceae, tribe Poeae) and implications for
taxonomy of the genus. – Aust. Syst. Bot. 27: 333-354.
Birch WR. 1963. Epiblast in Gramineae. –
Nature 198(4877): 304.
Birge WI. 1911. The anatomy and some
biological aspects of the ball moss, Tillandsia recurvata L. – Bull.
Univ. Texas 194: 1-24.
Bisht MS, Mukai Y. 2002. Genome organization
and polyploid evolution in the genus Eleusine (Poaceae). – Plant Syst. Evol. 233:
243-258.
Björkman SO. 1956. Zingeria
biebersteiniana (Claus) P. Smirn. – one more grass species with the
chromosome number 2n = 8. – Svensk Bot. Tidskr. 50: 513-515.
Björkman SO. 1960. Studies in
Agrostis and related genera. – Symb. Bot. Upsal. 17(1): 1-12.
Blake ST. 1939. A monograph of the genus
Eleocharis in Australia and New Zealand. – Proc. Roy. Soc.
Queensland 50: 88-132.
Blake ST. 1940. Notes on Australian Cyperaceae 3. – Proc. Roy. Soc.
Queensland 51: 32-50.
Blake ST. 1972a. Neurachne and its
allies (Gramineae). – Contr. Queensland Herb. 13: 1-53.
Blake ST. 1972b. Plinthanthesis and
Danthonia and a review of the Australian species of
Leptochloa (Gramineae). – Contr. Queensland Herbarium 14: 1-19.
Blakey CA, Goldman SL, Dewald CL. 2001.
Apomixis in Tripsacum: comparative mapping of a multigene phenomenon.
– Genome 44: 222-230.
Blaner A, Schneider J, Röser M. 2014.
Phylogenetic relationships in the grass family (Poaceae) based on the nuclear single copy
locus topoisomerase 6 compared with chloroplast DNA. – Syst. Biodivers. 1:
111-124.
Blaser NW. 1941a. Studies in the morphology
of the Cyperaceae I. Morphology of
flowers: A. Scirpoid genera. – Amer. J. Bot. 28: 542-551.
Blaser NW. 1941b. Studies in the morphology
of the Cyperaceae I. Morphology of
flowers: B. Rhynchosporoid genera. – Amer. J. Bot. 28: 832-838.
Blaser NW. 1944. Studies in the morphology of
the Cyperaceae II. The prophyll. –
Amer. J. Bot. 31: 53-64.
Blatter E, McDann C. 1935. The Bombay
grasses. – Sci. Monogr. No. 5, Imper. Council Agricult. Res., New Delhi 21:
324.
Blattner FR. 2004. Phylogenetic analysis of
Hordeum (Poaceae) as inferred
by nuclear rDNA ITS sequences. – Mol. Phylogen. Evol. 33: 289-299.
Böcher TW. 1972. Leaf anatomy in
Sporobolus rigens (Tr.) Desv. (Gramineae). – Bot. Not. 125:
344-360.
Bodard M. 1962. Notes préliminaires à la
révision de genre Bulbostylis (Cypéracées). – Bull. Soc. Bot.
France 108: 307-310.
Bodard M. 1963. Première contribution à la
revision du genre Bulbostylis (Cyperaceae) en Afrique. – Ann. Fac.
Sci. Univ. Dakar 9: 51-80.
Boechat SC. 2005. O gênero
Ichnanthus (Poaceae-Panicoideae-Paniceae) no Brasil.
– Iheringia, Sér. Bot. l60: 189-248.
Boeckeler JO. 1888. Beiträge zur Kenntniss
der Cyperaceen 1. Cyperaceae novae.
– Breitschädel & Vogt, Varel.
Böhling N, Scholz H. 2003. The Gramineae (Poaceae) flora of the Southern Aegean
islands (Greece). Checklist, new records, internal distribution. – Ber. Inst.
Landschafts-Pflanzenökologie Univ. Hohenheim, Beih. 16.
Böhme S. 1988. Bromelienstudien III.
Vergleichende Untersuchungen zu Bau, Lage und systematischer Verwertbarkeit der
Septalnektarien von Bromeliaceen. – Trop. Subtrop. Pflanzenwelt 62:
125-274.
Bonde SD. 1986. A new gramineous stem from
the Deccan Intertrappean beds of Nawargaon in Wardha District, Majharashtra,
India. – Biovigyanam 12: 39-43.
Bonnefille R, Hamilton AC, Linder HP, Riollet
G. 1990. 30,000-Year-old fossil Restionaceae pollen from central
equatorial Africa and its biogeographical significance. – J. Biogeogr. 17:
307-314.
Booth TA, Richards AJ. 1976. Studies in the
Hordeum murinum aggregate I. Morphology. – Bot. J. Linn. Soc. 72:
149-159.
Booth TA, Richards AJ. 1978. Studies in the
Hordeum murinum L. aggregate: disc electrophoresis of seed proteins.
– Bot. J. Linn. Soc. 76: 115-125.
Bor NL. 1951 [1952]. Notes on some Indian
grasses. – Kew Bull. 6: 445-453.
Bor NL. 1952. Notes on Dimeria. –
Kew Bull. 6: 455-459.
Bor NL. 1952 [1953]. Notes on Asiatic grasses
XI. The genus Dimeria R. Br. in India & Burma. – Kew Bull. 7:
553-592.
Bor NL. 1954. Notes on Asiatic grasses XXII.
Trikeraia Bor, a new genus of Stipeae. – Kew Bull. 9: 555-557.
Bor NL. 1960. The grasses of Burma, Ceylon,
India and Pakistan (excluding Bambuseae). – Series of Pure and Applied Biol.,
Bot. Div. 1: 136-145.
Borges RLM de, Santos F de A dos, Giuletti
AM. 2009. Comparative pollen morphology and taxonomic considerations in Eriocaulaceae. – Rev. Palaeobot.
Palynol. 154: 91-105.
Borre A van den, Watson L. 1994. The
infrageneric classification of Eragrostis (Poaceae). – Taxon 43: 383-422.
Borre A van den, Watson L. 1997. On the
classification of the Chloridoideae (Poaceae). – Aust. Syst. Bot. 10:
491-531.
Borre A van den, Watson L. 2000. On the
classification of the Chloridoideae: results from morphological and leaf
anatomical data analyses. – In: Jacobs SWL, Everett J (eds), Grasses:
systematics and evolution, Proceedings of the 2nd International
Conference on the Comparative Biology of Monocotyledons, 1998, Sydney,
Australia, Vol. 2, CSIRO Publ., Melbourne, Victoria, pp. 180-183.
Borrill M. 1962. The experimental taxonomy of
Anthoxanthum species. – Proc. Linn. Soc. London 1960: 106-109.
Borrill M. 1963. Experimental studies of
evolution in Anthoxanthum (Gramineae). – Genetica 34: 183-210.
Bortiri E, Coleman-Derr D, Lazo G, Anderson
O, Gu Y. 2008. The complete chloroplast genome sequence of Brachypodium
distachyon: sequence comparison and phylogenetic analysis of eight grass
plastomes. – BMC Research Notes 1: 61-68.
Borwein B, Goetsee ML, Krupko S. 1949.
Development of the embryo sac of Restio dodii and Elegia
racemosa. – J. South Afr. Bot. 15: 1-11.
Bosser J. 1969. Graminées des paturages et
des cultures à Madagascar. – Mem. ORSTOM 35: 1440.
Botha DJ, Schijff HP van der. 1976. The
structure and ontogeny of the chlorenchyma in the stems of Elegia L.
(Restionaceae). – J. South Afr.
Bot. 42: 57-62.
Botha DJ, Schijff HP van der, Tonder EMA van.
1972. The position, structure and ontogeny of the stomata in Elegia
vaginulata. – Tydskr. Natuurwet. 13: 193-199.
Bothmer R von. 1979. Revision of the Asiatic
taxa of Hordeum sect. Stenostachys. – Bot. Tidsskr. 74:
117-147.
Bothmer R von, Jacobsen N. 1979. A taxonomic
revision of Hordeum secalinum and H. capense. – Bot.
Tidsskr. 74: 223-235.
Bothmer R von, Jacobsen N. 1980. Wild species
of Hordeum (barley) in Argentina and Chile. – Geogr. Tidsskr. 80:
3-4, 18-21, 27.
Bothmer R von, Jacobsen N, Nicora E. 1980.
Revision of Hordeum sect. Anisolepis Nevski. – Bot. Not.
130: 539-554.
Bothmer R von, Jacobsen N, Bagger Jørgensen
R, Nicora E. 1982. Revision of the Hordeum pusillum group. – Nord.
J. Bot. 2: 307-321.
Bothmer R von, Salomon B, Enomoto T, Watanabe
O. 2005. Distribution, habitat and status for perennial Triticeae species in
Japan. – Bot. Jahrb. Syst. 126: 317-346.
Boubier A-M. 1895. Remarques sur l’anatomie
systématique des Rapateacées et des familles voisines. – Bull. Herb. Boiss.
3: 115-120.
Bouchenak-Khelladi Y. 2007. Grass evolution
and diversificiation: a phylogenetic approach. – Ph.D. diss., Trinity
College, Dublin.
Bouchenak-Khelladi Y, Salamin N, Savolainen
V, Forest F, Bank M van den, Chase MW, Hodkinson TR. 2008. Large multi-gene
phylogenetic trees of the grasses (Poaceae): progress towards complete tribal
and generic level sampling. – Mol. Phylogen. Evol. 47: 488-505.
Bouchenak-Khelladi Y, Verboom GA, Hodkinson
TR, Salamin N, François O, Ní Chonghaile G, Savolainen V. 2009. The origins
and diversification of C4 grasses and savanna-adapted ungulates. –
Global Change Biol. 15: 2397-2417.
Bouchenak-Khelladi Y, Savolainen V, Hodkinson
TR. 2010. Diversification of the grasses (Poaceae): a phylogenetic approach to reveal
macro-evolutionary patterns. – In: Seberg O, Petersen G, Barfod AS, Davis JI
(eds), Diversity, phylogeny, and evolution in the monocotyledons, Aarhus
University Press, Århus, pp. 451-475.
Bouchenak-Khelladi Y, Verboom GA, Savolainen
V, Hodkinson TR. 2010. Biogeography of the grasses (Poaceae): a phylogenetic approach to reveal
evolutionary history in geographical space and geological time. – Biol. J.
Linn. Soc. 162: 543-557.
Bouchenak-Khelladi Y, Muasya AM, Linder HP.
2014. A revised evolutionary history of Poales: origins and diversification.
– Bot. J. Linn. Soc. 175: 4-16.
Bouchenak-Khelladi Y, Slingsby JA, Verboom
GA, Bond WJ. 2014. Diversification of C4 grasses (Poaceae) does not coincide with their
ecological dominance. – Amer. J. Bot. 101: 300-307.
Boudko E. 2014. Phylogenetic analysis of
subtribe Alopecurinae sensu lato (Poaceae). – M.Sc. thesis, University of
Ottawa, Ottawa.
Bouton JH, Brown RH, Bolton JK, Campagnoli
RP. 1981. Photosynthesis of grass species differing in carbon dioxide fixation
pathways. – Plant Physiol. 67: 433-437.
Bowden BN. 1971. Studies on Andropogon
gayanus Kunth VI. The leaf nectaries of Andropogon gayanus var.
bisquamulatus (Hochst.) Hack. (Gramineae). – Bot. J. Linn. Soc. 64:
77-80.
Bowden WM. 1959. Chromosome numbers and
taxonomic notes on northern grasses I. Tribe Triticeae. – Can. J. Bot. 37:
1143-1151.
Bowden WM. 1960a. Chromosome numbers and
taxonomic notes on northern grasses II. Tribe Festuceae. – Can. J. Bot. 38:
117-131.
Bowden WM. 1960b. Chromosome numbers and
taxonomic notes on northern grasses III. Twenty-five genera. – Can. J. Bot.
38: 541-557.
Bowden WM. 1961. Chromosome numbers and
taxonomic notes on northern grasses IV. Tribe Festuceae: Poa and
Puccinellia. – Can. J. Bot. 39: 123-138.
Bowden WM. 1962. Cytotaxonomy of the native
and adventive species of Hordeum, Eremopyrum,
Secale, Sitanion and Triticum in Canada. – Can. J.
Bot. 40: 1675-1711.
Bowden WM. 1965. Cytotaxonomy of the
Eurasiatic and South American species of the barley genus, Hordeum L.
– Can. J. Genet. Cytol. 7: 394-399.
Bowden WM. 1966. Chromosome numbers in seven
genera of the tribe Triticeae. – Can. J. Genet. Cytol. 8: 130-136.
Bowman CM, Bonnard G, Dyer TA. 1983.
Chloroplast DNA variation between species of Triticum and
Aegilops. Location of the variation in the chloroplast genome and its
relevance to the inheritance and classification of the cytoplasm. – Theor.
Appl. Gen. 65: 247-262.
Boykin LM, Pockman WT, Lowrey TK. 2008. Leaf
anatomy of Orcuttieae (Poaceae:
Chloridoideae): more evidence of C4 photosynthesis without Kranz
anatomy. – Madroño 55: 143-150.
Bozek M, Leitch AR, Leitch IJ, Záveská
Drábková L, Kuta E. 2012. Chromosome and genome size variation in
Luzula (Juncaceae), a genus
with holocentric chromosomes. – Bot. J. Linn. Soc. 170: 529-541.
Brain CK. 1934. A key to the sedges (Cyperaceae) of Southern Rhodesia. –
Proc. Rhodesia Sci. Assoc. 33: 51-96.
Brandenburg DM, Estes JR, Collins SL. 1991. A
revision of Diarrhena (Poaceae)
in the United States. – Bull. Torrey Bot. Club 118: 128-136.
Braselton JP. 1971. The ultrastructure of the
non-localized kinetochores of Luzula and Cyperus. –
Chromosoma 36: 89-99.
Braselton JP. 1981. The ultrastructure of the
meiotic kinetochores of Luzula. – Chromosoma 82: 143-151.
Brassac J, Blattner FR. 2015. Species-level
phylogeny and polyploid relationships in Hordeum (Poaceae) inferred by next-generation
sequencing and in silico cloning of multiple nuclear loci. – Syst. Biol. 64:
792-808.
Brassac J, Jakob SS, Blattner FR. 2012.
Progenitor-derivative relationships of Hordeum polyploids (Poaceae, Triticeae) inferred from sequences
of Topo6, a nuclear low-copy gene region. – PloS ONE 7(3): e33808.
Bremer K. 2002. Gondwanan evolution of the
grass alliance of families (Poales). – Evolution 56: 1374-1387.
Brichambaut GP de, Sauvage C. 1954. Notes
agrostologiques. –Bull. Soc. Sci. Nat. Maroc 34: 235-254.
Briggs BG. 1963. Chromosome numbers in
Lepyrodia and Restio in Australia. – Contr. New South Wales
Natl. Herb. 3: 223-232.
Briggs BG. 1966. Chromosome numbers of some
Australian monocotyledons. – Contr. New South Wales Natl. Herb. 4: 24-34.
Briggs BG. 1987. Typhaceae. – In: George AS (ed), Flora
of Australia 45, Australian Government Publ. Service, Canberra, pp. 8-10.
Briggs BG. 2000. What is significant – the
wollemi pine or the southern rushes? – Ann. Missouri Bot. Gard. 87: 72-80.
Briggs BG. 2001. Proposal to conserve the
name Leptocarpus (Restionaceae) with a conserved type.
– Taxon 50: 919-921.
Briggs BG. 2004. Restionaceae (Poales) in the footsteps
of Robert Brown. – Telopea 10: 499-503.
Briggs BG. 2014a. Leptocarpus (Restionaceae) enlarged to include
Meeboldina and Stenotalis, with new subgenera and Western
Australian species. – Telopea 16: 19-41.
Briggs BG. 2014b. Desmocladus (Restionaceae) enlarged to include the
Western Australian genera Harperia, Kulinia and
Onychosepalum. – Telopea 17: 29-33.
Briggs BG, Johnson LAS. 1998a.
Georgeantha hexandra, a new genus and species of Ecdeiocoleaceae (Poales) from
Western Australia. – Telopea 7: 307-312.
Briggs BG, Johnson LAS. 1998b. A guide to a
new classification of Restionaceae
and allied families. – In: Meney KA, Pate JS (eds) Australian rushes –
biology, identification and conservation of Restionaceae and allied families,
University of Western Australia Press, Nedlands, Western Australia, pp.
25-56.
Briggs BG, Johnson LAS. 1998c. New genera and
species of Australian Restionaceae
(Poales). – Telopea 7: 345-373.
Briggs BG, Johnson LAS. 1998d. New
combinations arising from a new classification of non-African Restionaceae. – Telopea 8: 21-33.
Briggs BG, Johnson LAS. 1999. A guide to a
new classification of Restionaceae
and allied families. – In: Meney KA, Pate JS (eds), Australian rushes.
Biology, identification and conservation of Restionaceae and allied families,
University of Western Australia Press, Nedlands, Western Australia, pp.
25-56.
Briggs BG, Johnson LAS. 2000. Hopkinsiaceae
and Lyginiaceae, two new families of Poales in Western Australia, with
revisions of Hopkinsia and Lyginia. – Telopea 8:
477-502.
Briggs BG, Johnson LAS. 2001a. The genus
Desmocladus (Restionaceae)
and new species from the south of Western Australia and South Australia. –
Telopea 9: 227-245.
Briggs BG, Johnson LAS. 2001b. New species of
Harperia, Loxocarya, Onychosepalum,
Platychorda and Tremulina (Restionaceae) in Western Australia.
– Telopea 9: 247-257.
Briggs BG, Johnson LAS. 2004a. New Western
Australian species of Hypolaena (Restionaceae) and a new section. –
Telopea 10: 573-580.
Briggs BG, Johnson LAS. 2004b. New
combinations in Chordifex (Restionaceae) from eastern Australia
and new species from Western Australia. – Telopea 10: 683-700.
Briggs BG, Johnson LAS. 2012. New species of
Sporadanthus and Lepyrodia (Restionaceae) from eastern and western
Australia. – Telopea 14: 11-28.
Briggs BG, Linder HP. 2009. A new subfamilial
and tribal classification of Restionaceae (Poales). – Telopea 12:
333-345.
Briggs BG, Johnson LAS, Krauss SL. 1990. The
species of Alexgeorgia, a Western Australian genus of the Restionaceae. – Aust. Syst. Bot. 3:
751-758.
Briggs BG, Marchant AD, Gilmore S, Porter CL.
2000. A molecular phylogeny of Restionaceae and allies. – In:
Wilson KL, Morrison DA (eds), Monocots: systematics and evolution, Proceedings
of the 2nd International Conference on the Comparative Biology of
Monocotyledons, Sydney, Australia, Vol. 2, CSIRO Publ., Melbourne, pp.
661-671.
Briggs BG, Marchant AD, Perkins AJ. 2010.
Phylogeny and features in Restionaceae, Centrolepidaceae and Anarthriaceae (restiid clade of
Poales). – In: Seberg O, Petersen G, Barfod AS, Davis JI (eds), Diversity,
phylogeny, and evolution in the monocotyledons, Aarhus University Press,
Århus, pp. 357-388.
Briggs BG, Marchant AD, Perkins AJ. 2014.
Phylogeny of the restiid clade (Poales) and implications for the classification
of Anarthriaceae, Centrolepidaceae and Australian Restionaceae. – Taxon 63: 24-46.
Brink D, Wet JMJ de. 1983. Supraspecific
groups in Tripsacum (Gramineae). – Syst. Bot. 8: 243-249.
Britton NL. 1907. The sedges of Jamaica. –
Bull. Dept. Agric. Jamaica 5: 10.
Brix K. 1974. Sexual reproduction in
Eragrostis curvula (Schrad.) Nees. – Zeitschr. Pflanzenzüchtung 71:
25-32.
Brown GK, Gilmartin AJ. 1984. Stigma
structure and variation in Bromeliaceae – neglected taxonomic
characters. – Brittonia 36: 364-374.
Brown GK, Gilmartin AJ. 1986. Chromosomes of
the Bromeliaceae. – Selbyana 9:
88-93.
Brown GK, Gilmartin AJ. 1988. Comparative
ontogeny of bromeliaceous stigmas. – In: Leins P, Tucker SC, Endress PK
(eds), Aspects of floral development, J. Cramer, Berlin, Stuttgart, pp.
191-204.
Brown GK, Gilmartin AJ. 1989a. Stigma types
in Bromeliaceae – a systematic
survey. – Syst. Bot. 14: 110-132.
Brown GK, Gilmartin AJ. 1989b. Chromosome
numbers in Bromeliaceae. – Amer.
J. Bot. 76: 657-665.
Brown GK, Leme EMC. 2000. Cladistic analysis
in the nidularioid complex. – In: Leme EMC(ed), Nidularium –
bromeliads of the Atlantic forest, Sexante Artes, Rio de Janeiro, pp.
240-247.
Brown GK, Leme EMC. 2005. The
re-establishment of Andrea (Bromeliaceae: Bromelioideae), a
monotypic genus from southeastern Brazil threatened with extinction. – Taxon
54: 63-70.
Brown GK, Terry RG. 1992. Petal appendages in
Bromeliaceae. – Amer. J. Bot. 79:
1051-1071.
Brown SW. 1950. Spurious secondary
associations and asymmetric spindles in a Luzula. – Cytologia 15:
259-268.
Brown SW. 1954. Mitosis and meiosis in
Luzula campestris DC. – Univ. Calif. Publ. Bot. 27: 231-278.
Brown WV. 1948. A cytological study in the
Gramineae. – Amer. J. Bot. 35: 382-395.
Brown WV. 1950. A cytological study of some
Texas grasses. – Bull. Torrey Bot. Club 77: 63-76.
Brown WV. 1951. Chromosome numbers of some
Texas grasses. – Bull. Torrey Bot. Club 78: 292-299.
Brown WV. 1958a. Leaf anatomy in grass
systematics. – Bot. Gaz. 119: 170-178.
Brown WV. 1958b. Apomixis as related to
geographical distribution in the panicoid grass tribes. – J. South Afr. Bot.
24: 191-200.
Brown WV. 1959. The epiblast and coleoptile
of the grass embryo. – Bull. Torrey Bot. Club 86: 13-16.
Brown WV. 1960. The morphology of the grass
embryo. – Phytomorphology 10: 215-323.
Brown WV. 1965. The grass embryo: a rebuttal.
– Phytomorphology 15: 274-284.
Brown WV. 1977. The Kranz syndrome and its
subtypes in grass systematics. – Mem. Torrey Bot. Club 23: 1-97.
Brown WV, Emery WHP. 1957. Some South African
apomictic grasses. – J. South Afr. Bot. 23: 123-125.
Brown WV, Emery WHP. 1958. Apomixis in the
Gramineae: Panicoideae. – Amer. J. Bot. 45: 253-263.
Brown WV, Johnson SC. 1962. The fine
structure of the grass guard cell. – Amer. J. Bot. 49: 110-115.
Brown WV, Harris WE, Graham JD. 1959. Grass
morphology and systematics I. The internode. – Southw. Natur. 4: 115-125.
Browning J. 1992. Hypogynous bristles and
scales in basal florets in amphicarpous Schoenoplectus species (Cyperaceae). – Nord. J. Bot. 12:
171-175.
Browning J. 1994. Floret position in
Costularia (Cyperaceae): a
new interpretation. – Nord. J. Bot. 14: 653-655.
Browning J, Gordon-Gray KD. 1993. Notes on
tropical Africa Cyperaceae. – Nord.
J. Bot. 13: 507-510.
Browning J, Gordon-Gray KD. 1995. Studies in
Cyperaceae in southern Africa 24.
Three species of Scirpoides. – South Afr. J. Bot. 60: 315-320.
Browning J, Gordon-Gray KD. 1999. The
inflorescence in southern Africa species of Bolboschoenus (Cyperaceae). – Ann. Bot. Fenn. 36:
81-97.
Browning J, Gordon-Gray KD. 2000. Patterns of
fruit morphology in Bolboschoenus (Cyperaceae) and their global
distribution. – South Afr. J. Bot. 66: 63-71.
Browning J, Gordon-Gray KD, Smith SG. 1995.
Achene structure and taxonomy of North American Bolboschoenus (Cyperaceae). – Brittonia 47:
433-445.
Browning J, Gordon-Gray KD, Smith SG. 1997.
Achene morphology and pericarp anatomy of the type specimens of the Australian
and New Zealand species of Bolboschoenus (Cyperaceae). – Aust. Syst. Bot. 10:
49-58.
Bruhl JJ. 1990. Taxonomic relationships and
photosynthetic pathways in the Cyperaceae. – Ph.D. diss., Australian
National University, Canberra.
Bruhl JJ. 1991. Comparative development of
some taxonomically critical floral/inflorescence features in Cyperaceae. – Aust. J. Bot. 39:
119-127.
Bruhl JJ. 1994. Amphicarpy in the Cyperaceae, with novel variation in the
wetland sedge Eleocharis caespitosissima Baker. – Aust. J. Bot. 42:
441-448.
Bruhl JJ. 1995. Sedge genera of the world:
relationships and a new classification of the Cyperaceae. – Aust. Syst. Bot. 8:
125-305.
Bruhl JJ, Whalley RDB (Wal). 2011.
Walwhalleya jacobsiana (Poaceae, Paniceae), a new, rare species of
grass from South Australia. – Telopea 13: 77-92.
Bruhl JJ, Wilson KL. 2007 [2008]. Towards a
comprehensive survey of C3 and C4 photosynthetic pathways
in Cyperaceae. – In: Columbus JT,
Friar EA, Porter JM, Prince LM, Simpon MG (eds), Monocots: comparative biology
and evolution. Poales, Rancho Santa Ana Botanical Garden, Claremont,
California, pp. 99-148.
Bruhl JJ, Watson L, Dallwitz MJ. 1992. Genera
of Cyperaceae: interactive
identification and information retrieval. – Taxon 41: 225-234.
Bruhl JJ, Wilson PG, Wills KE. 2006. Grass
not fungus: Walwhalleya nom. nov. (Poaceae, Paniceae). – Aust. Syst. Bot.
19: 327-328.
Bryson JJ, Wilson KL. 2008. The significance
of Cyperaceae as weeds. – In: Naczi
RFC, Ford BA (eds), Sedges: uses, diversity and systematics of the Cyperaceae, Missouri Botanical Garden
Press, St. Louis, Missouri, pp. 30-53.
Brysting AK, Aiken SG, Lefkovitch LP, Boles
RL. 2003. Dupontia (Poaceae) in
North America. – Can. J. Bot. 81: 769-779.
Brysting AK, Fay MF, Leitch IJ, Aiken SG.
2004. One or more species in the Arctic grass genus Dupontia? – A
contribution to the Panarctic flora project. – Taxon 53: 365-382.
Buchenau F. 1865. Der Blüthenstand der
Juncaceen. – Jahrb. Wiss. Bot. 4: 385-440.
Buchenau F. 1875. Monographie der Juncaceen
vom Cap. – Abh. Naturwiss. Ver. Bremen 4: 393-512.
Buchenau F. 1885. Kritische Zusammenstellung
der europäischen Juncaceen. – Engl. Bot. Jahrb. Syst. 7: 153-176.
Buchenau F. 1888. Juncaceae. – In: Engler A, Prantl K
(eds), Die natürlichen Pflanzenfamilien II(5), W. Engelmann, Leipzig, pp.
1-7.
Buchenau F. 1890a. Monographia Juncacearum.
– Engl. Bot. Jahrb. Syst. 12: 1-495.
Buchenau F. 1890b. Monographia Juncacearum.
Supplement. – Engl. Bot. Jahrb. Syst. 12: 71-74.
Buchenau F. 1898. Luzula campestris
und verwandte Arten. – Österr. Bot. Zeitschr. 48: 161-167, 209-220, 243-246,
284-297.
Buckler ES, Holtsford TP. 1996a. Zea
systematics: ribosomal ITS evidence. – Mol. Biol. Evol. 13: 612-622.
Buckler ES, Holtsford TP. 1996b. Zea
ribosomal repeat evolution and substitution patterns. – Mol. Biol. Evol. 13:
623-632.
Budnowski A. 1922. The septal glands of the
Bromeliaceae. – Bot. Arch. 1:
47-80.
Buell CR. 2009. Poaceae genomes: going from unattainable to
becoming a model clade for comparative plant genomics. – Plant Physiol. 149:
111-116.
Bugg C, Smith C, Blackstock N, Simpson D,
Ashton PA. 2013. Consistent and variable leaf anatomical characters in
Carex (Cyperaceae). – Bot.
J. Linn. Soc. 172: 371-384.
Bulinska-Rodomska Z, Lester RN. 1988.
Intergeneric relationships of Lolium, Festuca and
Vulpia (Poaceae) and their
phylogeny. – Plant Syst. Evol. 159: 217-227.
Burkart A. 1975. Evolution of grasses and
grasslands in South America. – Taxon 24: 53-66.
Burke SV, Grennan CP, Duvall MR. 2012.
Plastome sequences of two new world bamboos Arundinaria gigantea and
Cryptochloa strictiflora (Poaceae) extended phylogenomic
understanding of Bambusoideae. – Amer. J. Bot. 99: 1951-1961.
Burke SV, Clark LG, Triplett JK, Grennan CP,
Duvall MR. 2014. Biogeography and phylogenomics of New World Bambusoideae (Poaceae), revisited. – Amer. J. Bot. 101:
886-891.
Burman AG. 1980. A new species of
Paspalum (Gramineae) from Brazil. – Kew Bull. 35: 297-298.
Burman AG. 1985 [1987]. The genus
Thrasya H.B.K. (Gramineae). – Acta Bot. Venezuelica 14: 7-93.
Burson BL. 1975. Cytology of some apomictic
Paspalum species. – Crop Sci. 15: 229-232.
Burson BL. 1978. Genome relations between
Paspalum conspersum and two diploid Paspalum species. –
Can. J. Genet. Cytol. 20: 365-372.
Burton GW. 1942. A cytological study of some
species in the tribe Paniceae. – Amer. J. Bot. 29: 355-359.
Burt-Utley K, Utley JF. 1988. New and
noteworthy species of Hechtia (Bromeliaceae) from Guerrero, Mexico.
– Syst. Bot. 13: 276-282.
Butcher D. 2009. Names and synonyms. – Bromeliaceae 43: 4-5.
Butzin F. 1965. Neue Untersuchungen über die
Blüte der Gramineae. – Ph.D. diss., Freie Universität Berlin, Germany.
Butzin F. 1970. Die systematische Gliederung
der Paniceae. – Willdenowia 6: 179-192.
Butzin F. 1973. Die Namen der
supragenerischen Einheiten der Gramineae (Poaceae). – Willdenowia 7: 113-168.
Butzin F. 1977. Evolution der Infloreszenzen
in der Borstehhirsen-Verwandtschaft. – Willdenowia 8: 67-79.
Caceres MR. 1969. La anatomía foliar de
Monanthochloë. – Rev. Fac. Ci. Agr. Univ. Nac. Cuyo 15: 39-45.
Cáceres ME, Mazzucato A. 1995. Cytological
and embryological studies in Setaria cordobensis Herrmann and
Setaria leiantha Hackel (Poaceae). – Caryologia Collation 48:
255-263.
Caetano-Anollés G. 2005. Evolution of genome
size in the grasses. – Crop Sci. 45: 1809-1816.
Cahoon AB, Sharpe RM, Mysayphonh C, Thompson
EJ, Ward AD, Lin A. 2010. The complete chloroplast genome of tall fescue
(Lolium arundinaceum; Poaceae)
and comparison of whole plastomes from the family Poaceae. – Amer. J. Bot. 97: 49-58.
Cai L-B, Wu Z-L. 1997. Studies on the
biosystematicf relationships and geographic distribution of Trikeraia
Bor and Stephanachne Keng. – Bull. Bot. Res (Zhiwuyanjiu: jikan) 17:
380-388.
Calderón CE, Soderstrom TR. 1973.
Morphological and anatomical considerations of the grass subfamily Bambusoideae
based on the new genus Maclurolyra. – Smithsonian Contr. Bot. 11:
1-55.
Calderón CE, Soderstrom TR. 1980. The genera
of Bambusoideae (Poaceae) of the
American continent: keys and comments. – Smithsonian Contr. Bot. 44: 1-27.
Cámara Hernández J, Gambino S. 1990.
Ontogeny and morphology of Zea diploperennis inflorescences and the
origin of maize (Zea mays ssp. mays). – Maydica 35:
113-124.
Cámara Hernández J, Gambino S. 1993. The
synflorescence of Tripsacum dactyloides (Poaceae). – Beitr. Biol. Pflanzen 66:
295-303.
Cámara Hernández J, Miante Alzogaray AM.
1995. Polytely: a general character in Poaceae. – Beitr. Biol. Pflanzen 68:
249-261.
Cámara Hernández J, Rua G. 1992. The
synflorescence of Poaceae. – Beitr.
Biol. Pflanzen 66: 297-311.
Cambecèdes J, Vaillancourt RE, Potts BM.
1999. Morphological and genetic variation in Centrolepis paludicola
and C. monogyna (Centrolepidaceae). – Aust. Syst.
Bot. 12: 679-688.
Camelbeke K, Goetghebeur P. 1999. The ligule,
a new diagnostic character in Scleria (Cyperaceae). – Syst. Geogr. Plants 68:
73-84.
Camelbeke K, Zijlstra G, Goetghebeur P. 2001.
Nomenclature of genera and subdivisions of genera within Scleria P.
J.Bergius (Cyperaceae). – Taxon 50:
479-486.
Campbell CS. 1986. Phylogenetic
reconstructions and two new varieties in the Andropogon virginicus
complex (Poaceae: Andropogoneae). –
Syst. Bot. 11: 280-292.
Campbell CS, Kellogg EA. 1987. Sister group
relationships of the Poaceae. – In:
Soderstrom TR, Hilu KW, Campbell CS, Barkworth ME (eds), Grass systematics and
evolution, Smithsonian Institution Press, Washington, D.C., pp. 217-224.
Campbell CS, Garwood PE, Specht LP. 1986.
Bambusoid affinities of the North temperate genus Brachyelytrum
(Gramineae). – Bull. Torrey Bot. Club 113: 135-141.
Campbell DH. 1899a. Studies on the flower and
embryo of Sparganium. – Proc. Calif. Acad. Sci., Ser. III, Bot. 1:
293-328.
Campbell DH. 1899b. Notes on the structure of
the embryo-sac in Sparganium and Lysichiton. – Bot. Gaz.
27: 153-166.
Campbell LM. 2004. Anatomy and systematics of
Xyridaceae, with special reference to
Aratitiyopea Steyerm. & P. E. Berry. – Ph.D. diss, The City
University of New York, New York.
Campbell LM. 2005. Contributions towards a
monograph of Xyridaceae: a revised
nomenclature for Abolboda (Xyridaceae). – Harvard Pap. Bot. 10:
137-146.
Campbell LM. 2012. Pollen morphology of Xyridaceae systematic (Poales) and its
potential. – Bot. Rev. 78: 428-439.
Campbell LM, Stevenson DW. 2005. Vegetative
anatomy of Aratitiyopea lopezii (Xyridaceae). – Acta Bot. Venez. 28:
395-407.
Campbell LM, Stevenson DW. 2007 [2008].
Inflorescence architecture and floral morphology in Aratitiyopea
lopezii (Xyridaceae). – In:
Columbus JT, Friar EA, Porter JM, Prince LM, Simpson MG (eds), Monocots:
comparative biology and evolution. Poales, Rancho Santa Ana Botanical Garden,
Claremont, California. – Aliso 23: 227-233.
Camus AA. 1925. Lecomtella, genre
nouveau de graminées malgaches. – Compt. Rend. Hedb. Sé. Acad. Sci. Paris
181: 567-568.
Camus AA. 1943. Le genre Vulpia dans
la flore française. – Not. Syst. 11: 124-131.
Camus AA. 1948. Chasechloa A. Camus
(Graminées), genre nouveau de Madagascar et de Nossi-Bé. – Bull. Soc. Bot.
France 95: 329-331.
Camus AA. 1954. Chasechloa A. Camus,
genre de Graminées Malgaches. – Mém. Inst. Sci. Madagascar, Sér. B, Biol.
Vég. 5: 201-204.
Camus EG. 1913. Les Bambusées. – Paul
Lechevalier, Paris.
Canela MBF, Paz NPL, Wendt T. 2003. Revision
of the Aechmea multiflora complex (Bromeliaceae). – Bot. J. Linn. Soc.
143: 189-196.
Carle R. 1992. Ananas. – In: Hänsel R,
Keller K, Rimpler H, Schneider G (eds), Hagers Handbuch der pharmazeutischen
Praxis, 5th ed., Springer, Berlin, Heidelberg, New York, pp.
272-276.
Carlquist SJ. 1960. Anatomy of Guayana Xyridaceae: Abolboda,
Orectanthe, and Achlyphila. – Mem. New York Bot. Gard. 10:
65-117.
Carlquist SJ. 1961. Pollen morphology of Rapateaceae. – Aliso 5: 39-66.
Carlquist SJ. 1966. Anatomy of Rapateaceae. Roots and stems. –
Phytomorphology 16: 17-38.
Carlquist SJ. 1969. Rapateaceae. – In: Metcalfe CE (ed),
Anatomy of the monocotyledons 3, Oxford, pp. 128-145.
Carlquist SJ. 1976. Alexgeorgea, a
bizarre new genus of Restionaceae
from Western Australia. – Aust. J. Bot. 24: 281-295.
Carniel K. 1962. Beiträge zur
Entwicklungsgeschichte des sporogenen Gewebes der Gramineen und Cyperaceen II.
Cyperaceae. – Österr. Bot.
Zeitschr. 109: 81-95.
Carniel K. 1972. Elektronenmikroskopische
Analyse der Pollenentwicklung von Heleocharis palustris. – Österr.
Bot. Zeitschr. 120: 223-234.
Caro JA. 1966. Las especies de Stipa
de la region central Argentina. – Kurtziana 3: 7-119.
Caro JA. 1981. Antonella Caro, un
nuevo genero de Gramíneas. – Dominguezia 2: 18-20.
Caro JA. 1982. Sinopsis taxonómica de las
gramíneas Argentinas. – Dominguezia 4: 1-51.
Caro JA, Sánchez E. 1971a. La identidad de
Stipa brachychaeta Godron, S. caudata Trinius y S.
bertrandii Philippi. – Darwiniana 16: 637-653.
Caro JA, Sánchez E. 1971b. Contribuciones al
mejor conocimiento de las Chlorideae (Gramineae) argentinas. – Kurtziana 6:
219-232.
Caro JA, Sánchez E. 1973. Las especies de
Stipa (Gramineae) del subgénero Jarava. – Kurtziana 7:
61-116.
Carolin RC, Jacobs SWL,Vesk M. 1977.The
ultrastructure of Kranz cells in the family Cyperaceae. – Bot. Gaz. 138:
413-419.
Carter S. 1966. Juncaceae. – In: Milne-Redhead E,
Polhill RM (eds), Flora of tropical East Africa, Crown Agents for Oversea
Governments and Administrations, London, pp. 1-11.
Caruel F. 1867. Observations organogéniques
sur la fleur femelle des Carex. – Ann. Sci. Nat., sér. 5, Bot., 7:
104-111.
Carvalho MLS de, Nakamura AT, Sajo M das G.
2009. Floral anatomy of neotropical species of Mayacaceae. – Flora 204: 220-227.
Castro NM. 1986. Estudos morphologicos dos
órgãos vegetativos de especies de Paepalanthus Kunth (Eriocaulaceae). – Ph.D. diss.,
Instituto de Biociências, Universidad de São Paulo, Brazil.
Castroviejo S. 1995. Rhynchospora
modesti-lucennoi, sp. nov. (Cyperaceae), from the western
Mediterranean, Madagascar and Africa. – Nord. J. Bot. 15: 577-570.
Catalán P, Olmstead RG. 2000. Phylogenetic
reconstruction of the genus Brachypodium P. Beauv. (Poaceae) from combined sequences of
chloroplast ndhF gene and nuclear ITS. – Plant Syst. Evol. 220:
1-19.
Catalán P, Shi Y, Armstrong L, Draper J,
Stace CA. 1995. Molecular phylogeny of the grass genus Brachypodium P.
Beauv. based on RFLP and RAPD analysis. – Bot. J. Linn. Soc. 117: 263-280.
Catalán P, Kellogg EA, Olmstead RG. 1997.
Phylogeny of Poaceae subfamily Pooideae
based on chloroplast ndhF gene sequences. – Mol. Phylogen. Evol. 8:
150-166.
Catalán P, Torrecilla P, Rodriguez JAL,
Olmstead RG. 2004. Phylogeny of the festucoid grasses of subtribe Loliinae and
allies (Poeae, Pooideae) inferred from ITS and trnL-F sequences. –
Mol. Phylogen. Evol. 31: 517-541.
Catalán P, Soreng RJ, Peterson PM. 2009.
Festuca aloha and F. molokaiensis (Poaceae: Loliinae), two new species from
Hawai’i. – J. Bot. Res. Inst. Texas 3: 51-58.
Catling PM, Reznicek AA, Crins WJ. 1993.
Carex juniperorum (Cyperaceae), a new species from
northeastern North America, with a key to Carex sect.
Phyllostachys. – Syst. Bot. 18: 496-501.
Cayouette J, Catling PM. 1992. Hybridization
in the genus Carex with special reference to North America. – Bot.
Rev. 58: 351-440.
Cayouette J, Morisset P. 1985. Chromosome
studies on natural hybrids between maritime species of Carex (sections
Phacocystis and Cryptocarpae) in northeastern North America
and their taxonomic implication. – Can. J. Bot. 63: 1957-1985.
Cebolla Lozano C, Rivas Ponce MA. 2003.
Catálogo del género Festuca L. (Poaceae) en la Península Ibérica. –
Candollea 58: 189-213.
Čelakovský L. 1885. Über die Infloreszenz
von Typha. – Flora 68: 617-630.
Čelakovský L. 1887. Über die
ährchenartigen Partialinfloreszenzen der Rhynchosporen. – Ber. Deutsch. Bot.
Ges. 5: 148-152.
Čelakovský L. 1889a. Über den Ährchenbau
der brasilianischen Grasgattung Streptochaeta Schrad. – Sitzungsber.
Königl. Böhm. Ges. Wiss. Prag, Math.-Naturwiss. Kl., 3: 14-42.
Čelakovský L. 1889b. Über die
Blütenstände der Cariceen. – Sitzungsber. Königl. Böhm. Ges. Wiss. Prag,
Math.-Naturwiss. Kl., 3: 91-113.
Čelakovský L. 1891. Über die
Verwandtschaft von Typha und Sparganium. – Österr. Bot.
Zeitschr. 41: 117-121, 154-160, 195-199, 224-228, 266-272.
Čelakovský L. 1897. Über die Homologien
des Grasembryos. – Bot. Zeitschr. 55: 141-174.
Celarier RP. 1959. Cytotaxonomy of the
Andropogoneae III. Subtribe Sorgheae, genus Sorghum. – Cytologia 23:
395-418.
Cerros-Tlatilpa R, Columbus JT, Barker NP.
2011. Phylogenetic relationships of Aristida and relatives (Poaceae, Aristidoideae) based on noncoding
chloroplast (trnL-F, rpl16) and nuclear (ITS) DNA sequences.
– Amer. J. Bot. 98: 1868-1886.
Chacón J, Madriñán S, Chase MW, Bruhl JJ.
2006. Molecular phylogenetics of Oreobolus (Cyperaceae) and the origin and
diversification of the American species. – Taxon 55: 359-366.
Chanda S. 1965. On the pollen morphology of
the Flagellariaceae with
reference to taxonomy. – Trans. Bose Res. Inst. Calcutta 8: 53-55.
Chanda S. 1966. On the pollen morphology of
the Centrolepidaceae, Restionaceae and Flagellariaceae, with special
reference to taxonomy. – Grana Palynol. 6: 355-415.
Chanda S, Ferguson IK. 1978. Pollen
morphology of Calorophus and Empodisma (Restionaceae) and its taxonomic
significance. – Kew Bull. 33: 411-415.
Chanda S, Rowley S. 1967. Apertural types in
pollen of the Restionaceae and Flagellariaceae. – Grana Palynol.
7: 16-36.
Chandra N. 1963. Morphological studies in the
Gramineae III. On the nature of the gynoecium in the Gramineae. – J. Indian
Bot. Soc. 42: 252-259.
Chao C-S. 1989. A revision of the species
described under Arundinaria (Gramineae) in Southeast Asia and Africa.
– Kew Bull. 44: 349-367.
Chao C-S, Renvoize SA. 1988a. Two new bamboos
from the eastern Himalaya and southern Burma. – Kew Bull. 43: 409-413.
Chao C-S, Renvoize SA. 1988b. Notes on some
species of Phyllostachys (Gramineae: Bambusoideae). – Kew Bull. 43:
415-422.
Chao C-Y. 1964. Megasporogenesis,
megagametogenesis and embryogeny in Paspalum orbiculare. – New Asia
College Acad. Ann. 6: 15-25.
Chao C-Y. 1974. Megasporogenesis and
megagametogenesis in Paspalum commersonii and P. longifolium
at two polyploid levels. – Bot. Not. 127: 267-275.
Chapman GP. 1990. The widening perspective:
reproductive biology of bamboos, some dryland grasses and cereals. – In:
Champman GP (ed), Reproductive versatility in the grasses, Cambridge University
Press, Cambridge, pp. 240-257.
Chapman GP (ed). 1992. Grass evolution and
domestication. – Cambridge University Press, London.
Chapman GP. 1996. The biology of grasses. –
CAB International, Wallingford, England.
Charmet G, Balfourier F. 1994. Isozyme
variation and species relationships in the genus Lolium L. (rye
grasses, Gramineae). – Theor. Appl. Gen. 87: 641-649.
Chase A. 1906. Notes on genera of Paniceae I.
– Proc. Biol. Soc. Washington 19: 184-192.
Chase A. 1908a. Notes on genera of Paniceae
II. – Proc. Biol. Soc. Washington 21: 1-10.
Chase A. 1908b. Notes on genera of Paniceae
III. – Proc. Biol. Soc. Washington 21: 175-188.
Chase A. 1911. Notes on genera of Paniceae
IV. – Proc. Biol. Soc. Washington 24: 103-160.
Chase A. 1924. Aciachne, a
cleistogamous grass of the high Andes. – J. Washington Acad. Sci. 14:
364-366.
Chase A. 1929. The North American species of
Paspalum. – Contr. U.S. Natl. Herb. 28: 1-310.
Chase A. 1939. Papuan grasses collected by L.
J. Brass II. – J. Arnold Arbor. 20: 304-316.
Chase A. 1943. Papuan grasses collected by L.
J. Brass III. – J. Arnold Arbor. 24: 77-89.
Chase A. 1964. First book of grasses. The
structure of grasses explained for beginners. – Smithsonian Institution,
Washington, D.C.
Chaudhary SA. 1989. Grasses of Saudi Arabia.
– Riyadh.
Chaudhary SA, Cope TA. 1983. Studies in the
flora of Arabia VI. A checklist of grasses of Saud Arabia. – Arab Gulf J.
Sci. Res. 1: 313-354.
Cheadle VI. 1955a. Conducting elements in the
xylem of the Bromeliaceae. –
Bull. Bromeliad Soc. 5: 3-7.
Cheadle VI. 1955b. The taxonomic use of
specialization of vessels in the metaxylem of Gramineae, Cyperaceae, Juncaceae, and Restionaceae. – J. Arnold Arbor. 36:
141-157.
Cheadle VI, Kosakai H. 1972. Vessels in the
Cyperaceae. – Bot. Gaz. 133:
214-223.
Cheadle VI, Kosakai H. 1974. Vessels in
Juncales I. Juncaceae and Thurniaceae. – Phytomorphology 23:
80-87.
Cheadle VI, Kosakai H. 1975. Vessels in
Juncales II. Centrolepidaceae
and Restionaceae. – Amer. J. Bot.
62: 1017-1026.
Cheadle VI, Kosakai H. 1982. Occurrence and
specialization of vessels in Xyridales. – Nord. J. Bot. 2: 97-109.
Chemisquy MA, Giussani LM, Scataglini MA,
Kellogg EA, Morrone O. 2010. Phylogenetic studies favour the unification of
Pennisetum, Cenchrus and Odontelytrum (Poaceae): a combined nuclear, plastid and
morphological analysis, and nomenclatural combinations in Cenchrus.
– Ann. Bot. 106: 107-130.
Chen C-H, Veldkamp J-F, Kuoh C-S. 2012.
Taxonomic revision of Microstegium s.str. (Andropogoneae, Poaceae). – Blumea 57: 160-189.
Chen R, Li X, Song W, Liang G, Zhang P, Lin
R, Zong W, Chen C, Fung H. 2004. Chromosome atlas of major economic plant
genome in China (Tomus IV). Chromosome atlas of various bamboo species. –
Science Press, Beijing.
Chennaveeraiah MS. 1960. Karyomorphologic and
cytotaxonomic studies in Aegilops. – Acta Horti Gothob. 23:
85-178.
Chermezon H. 1919a. Mariscus
(Cypéracées) nouveaux de Madagascar. – Bull. Mus. Natl. Hist. Nat. Paris
25: 300-304, 405-410.
Chermezon H. 1919b [1920]. Cyperus
nouveaux de Madagascar. – Bull. Soc. Bot. France 66: 338-353.
Chermezon H. 1920 [1921]. Diagnoses de
Pycreus et Cyperus nouveaux de Madagascar. – Bull. Soc.
Bot. France 67: 326-330.
Chermezon H. 1921. Scirpées nouvelles de
Madagascar. – Bull. Soc. Bot. France 68: 417-426.
Chermezon H. 1922a. Sur la position
systématique du genre Remirea. – Bull. Soc. Bot. France 69:
809-814.
Chermezon H. 1922b. Révision des
Cypéracées de Madagascar 2. – Ann. Mus. Col. Marseille 30: 1-62.
Chermezon H. 1925a. Sur la dissémination de
quelques Cypéracées. – Bull. Soc. Bot. France 71: 849-861.
Chermezon H. 1925b. Diagnoses des
Cypéracées nouvelles de Madagascar. – Bull. Soc. Bot. France 72: 18-22.
Chermezon H. 1925c. Observations sur quelques
Cypéracées de Madagascar. – Bull. Soc. Bot. France 72: 168-174.
Chermezon H. 1929. Les Cypéracées à
feuille ensiformes. – Arch. Bot. Bull. Mens. 3: 73-101.
Chermezon H. 1931. Les Cypéracées du
Haut-Oubangui. – Arch. Bot. (Caen) 4, Mém. 7: 1-25.
Chermezon H. 1934. Cypéracées nouvelles du
Congo Belge. – Rev. Zool. Bot. Afr. 24: 294-299.
Chermezon H. 1936. Contribution à la Flore
Cypérologique du Sénégal. Cypéracées récoltées par M. Trochain. –
Arch. Bot. (Caen) 7, Mém. 4: 1-32.
Chermezon H. 1937. Cypéracées. – In:
Humbert H (ed), Flore de Madagascar, Tananarive.
Chevalier A. 1933. Deux cypéracées
arbustiformes remarquables de l’ouest africain. – Reprint from “La Terre
et la Vie” No de Mars 1933.
Chia L-C, Fung H-L, But P P-H. 1983. Notes on
Gramineae: Bambusoideae in Hong Kong. – Kew Bull. 37: 591-595.
Chiapella J. 2000. The Deschampsia
cespitosa complex in central and northern Europe: a morphological
analysis. – Bot. J. Linn. Soc. 134: 495-512.
Chiapella J. 2007. A molecular phylogenetic
study of Deschampsia (Poaceae:
Aveneae) inferred from nuclear ITS and plastid trnL sequence data:
support for the recognition of Avenella and Vahlodea. –
Taxon 56: 55-64.
Chiapella J. 2008. On Jarava, or
putting the cart before the horse. – Taxon 57: 695-697.
Chiapella J, Probatova NS. 2003. The
Deschampsia cespitosa complex (Poaceae: Aveneae) with special reference to
Russia. – Bot. J. Linn. Soc. 142: 213-228.
Chiapella J, Zuloaga FO. 2010. A revision of
Deschampsia, Avenella, and Vahlodea (Poaceae, Poeae, Airinae) in South America.
– Ann. Missouri Bot. Gard. 97: 141-162.
Chin TC. 1941. The cytology of some wild
species of Hordeum. – Ann. Bot., N. S., 5: 535-545.
Chippindall LKA. 1959. A guide to the
identification of grasses in South Africa. – In: Meredit (ed), The grasses
and pastures of South Africa, Parow, C. P., pp. 1-527.
Chokthaweepanich H. 2014. Phylogenetics and
evolution of the Paleotropical woody bamboos (Poaceae: Bambusoideae: Bambuseae). –
Ph.D. diss., Iowa State University, Ames, Iowa.
Choo MK, Soreng RJ, Davis JI. 1994.
Phylogenetic relationships among Puccinellia and allied genera of Poaceae as inferred from chloroplast DNA
restriction site variation. – Amer. J. Bot. 81: 119-126.
Chopanov P, Yurtsev VN. 1976. Chromosome
numbers of some grasses of Turkmenia II. – Bot. Žurn. 61: 1240-1244.
Christensen J, Horner H, Lersten N. 1972.
Pollen wall and tapetal orbicular wall development in Sorghum bicolor
(Gramineae). – Amer. J. Bot. 59: 43-58.
Christin P-A, Besnard G. 2009. Two
independent C4 origins in Aristidoideae (Poaceae) revealed by the recruitment of
distinct phosphoenolpyruvate carboxylase genes. – Amer. J. Bot. 96:
2234-2239.
Christin P-A, Salamin N, Savolainen V,
Besnard G. 2007. A phylogenetic study of the phosphoenolpyruvate
carboxylase multigene family in Poaceae:
understanding the molecular changes linked to C4 photosynthesis
evolution. – Kew Bull. 62: 455-462.
Christin P-A, Salamin N, Savolainen V, Duvall
M, Besnard G. 2007. C4 photosynthesis evolved in grasses via
parallel adaptive genetic changes. – Curr. Biol. 17: 1241-1247.
Christin P-A, Besnard G, Samaritani E, Duvall
MR, Hodkinson TR, Savolainen V, Salamin N. 2008. Oligocene CO2
decline promoted C4 photosynthesis in grasses. – Curr. Biol. 18:
37-43.
Christin P-A, Petitpierre B, Salamin N,
Büchi L, Besnard G. 2009. Evolution of C4
phosphoenolpyruvate carboxykinase in grasses, from genotype to
phenotype. – Mol. Biol. Evol. 26: 357-365.
Christin P-A, Salamin N, Kellogg EA,
Vicentini A, Besnard G. 2009. Integrating phylogeny into studies of
C4 variation in the grasses. – Plant Physiol. 149: 82-87.
Christin P-A, Samaritani E, Petitpierre B,
Salamin N, Besnard G. 2009. Evolutionary insights on C4 photosynthetic subtypes
in grasses from genomics and phylogenetics. – Genome Biol. Evol. 1:
221-230.
Christin P-A, Edwards EJ, Besnard G, Boxall
SF, Gregory R, Kellogg EA, Hartwell J, Osborne CP. 2012. Adaptive evolution of
C4 photosynthesis through recurrent lateral gene transfer. – Curr.
Biol. 22: 445-449.
Christin P-A, Wallace MJ, Clayton H, Edwards
EJ, Furbank RT, Hattersley PW, Sage RF, et al. 2012. Multiple photosynthetic
transitions, polyploidy, and lateral gene transfer in the grass subtribe
Neurachninae. – J. Exper. Bot. 63: 6297-6308.
Christin P-A, Osborne CP, Chatelet DS,
Columbus JT, Besnard G, Hodkinson TR, Garrison LM, Vorontsova MS, Edwards EJ.
2013. Anatomical enablers and the evolution of C4 photosynthesis in
grasses. – Proc. Natl. Acad. Sci. U.S.A. 110: 1381-1386.
Christin P-A, Spriggs E, Osborne CP,
Strömberg CAE, Salamin N, Edwards EJ. 2014. Molecular dating, evolutionary
rates, and the age of the grasses. – Syst. Biol. 63: 153-165.
Christopher J, Mini LS, Pillai TN. 1989.
Karyomorphological studies of Coix aquatica Roxb. – Cytologia 54:
169-172.
Christophersen E. 1931. Notes on
Joinvillea. – Bernice P. Bishop Mus. Bull. 9: 2-7.
Chrtek J. 1965. Bemerkungen zur Gliederung
der Gattung Trisetum Pers. – Bot. Not. 118: 210-224.
Chrtek J. 1966. Beitrag zur Kenntnis einiger
Arten der Gattung Trisetum der Türkei. – Bot. Not. 119: 486-490.
Chu C-D, Chao C-S. 1979. Acidosasa
– a new genus of Chinese Bambusoideae. – J. Nanjing Coll. Forest Prod. Ind.
1-2: 142-145.
Church GL. 1949. A cytotaxonomic study of
Glyceria and Puccinellia. – Amer. J. Bot. 36: 155-165.
Church GL. 1952. The genus
Torreyochloa. – Rhodora 54: 197-200.
Ciaffi W, Paolacci AR, Tanzarella OA,
Porceddu E. 2011. Molecular aspects of flower development in grasses. – Sex
Plant Repr. 24: 247-282.
Cialdella AM, Giussani LM. 2002. Phylogenetic
relationships of the genus Piptochaetium (Poaceae, Pooideae, Stipeae): evidence from
morphological data. – Ann. Missouri bot. Gard. 89: 305-336.
Cialdella AM, Morrone O, Zuloaga FO. 2006.
Revisión de las especies de Axonopus (Poaceae, Panicoideae, Paniceae), serie
Suffulti. – Ann. Missouri Bot. Gard. 93: 592-633.
Cialdella AM, Giussani LM, Aagesen L, Zuloaga
FO, Morrone O. 2007. A phylogeny of Piptochaetium (Poaceae: Pooideae: Stipeae) and related
genera based on a combined analysis including trnL-F, rpl16,
and morphology. – Syst. Bot. 32: 545-559.
Cialdella AM, Salariato DL, Aagesen L,
Giussani LM, Zuoaga FO, Morrone O. 2010. Phylogeny of New World Stipeae (Poaceae): an evaluation of the monophyly of
Aciachne and Amelichloa. – Cladistics 26: 563-578.
Cialdella AM, Sede SM, Romaschenko K,
Peterson PM, Soreng RJ, Zuloaga FO, Morrone O. 2014. Phylogeny of
Nassella (Stipeae, Pooideae, Poaceae) based on analyses of chloroplast
and nuclear ribosomal DNA and morphology. – Syst. Bot. 39: 814-828.
Clark LG. 1986. Systematics of
Chusquea Section Chusquea, Section Swallonochloa,
Section Verticillatae, Section Serpentes, and Section
Longifoliae (Poaceae:
Bambusoideae). – Ph.D. diss., Iowa State University, Ames, Iowa.
Clark LG. 1989. Systematics of
Chusquea section Swallenochloa, section
Verticillatae, section Serpentes, and section
Longifoliae (Poaceae:
Bambusoideae). – Syst. Bot. Monogr. 27: 1-127.
Clark LG. 1990. Chusquea sect.
Longiprophyllae (Poaceae:
Bambusoideae): a new Andean section and new species. – Syst. Bot. 15:
617-634.
Clark LG. 1992. Chusquea sect.
Swallenochloa (Poaceae:
Bambusoideae) and allies in Brazil. – Brittonia 44: 387-422.
Clark LG. 1995. Diversity and distribution of
the Andean woody bamboos (Poaceae:
Bambusoideae). – In: Churchill SP, Balslev H, Forero E, Luteyn JL (eds),
Biodiversity and conservation of neotropical montane forests, New York
Botanical Garden, Bronx, New York, pp. 501-512.
Clark LG. 1996. Four new species of
Chusquea (Poaceae:
Bambusoideae) from Brazil and Ecuador. – Brittonia 48: 250-262.
Clark LG. 1997a. Diversity, biogeography, and
evolution in Chusquea (Poaceae:
Bambusoideae). – In: Chapman GP (ed), The bamboos, Academic Press, London,
pp. 33-44.
Clark LG. 1997b. Bamboos: the centrepiece of
the grass family. – In: Chapman GP (ed), The Bamboos, Academic Press, London,
pp. 237-248.
Clark LG. 2004. The grasses (Poaceae): Robert Brown and now. – Telopea
10: 505-514.
Clark LG, Cortés G. 2004. A new species of
Otatea from Chiapas, Mexico. – Bamboo Sci. Cult. 18: 1-6.
Clark LG, Judziewicz EJ. 1996. The grass
subfamilies Anomochlooideae and Pharoideae (Poaceae). – Taxon 45: 641-645.
Clark LG, Londoño X. 1991a. Miscellaneous
new taxa of bamboo (Poaceae: Bambuseae)
from Colombia, Ecuador and Mexico. – Nord. J. Bot. 11: 323-331.
Clark LG, Londoño X. 1991b. A new species
and new sections of Rhipidocladum (Poaceae: Bambusoideae). – Amer. J. Bot.
78: 1260-1279.
Clark LG, Davidse G, Ellis RP. 1989. Natural
hybridization in bamboos: evidence from Chusquea sect.
Swallenochloa (Poaceae:
Bambusoideae). – Natl. Geogr. Res. 5: 459-476.
Clark LG, Zhang W-P, Wendel JF. 1995. A
phylogeny of the grass family (Poaceae)
based on ndhF sequence data. – Syst. Bot. 20: 436-460.
Clark LG, Cortes R G, Chazaro B M. 1997. An
unusual new species of Chusquea (Poaceae: Bambusoideae) from Mexico. –
Syst. Bot. 22: 219-228.
Clark LG, Kobayashi M, Mathews S, Spangler
RE, Kellogg EA. 2000. The Puelioideae, a new subfamily of Poaceae. – Syst. Bot. 25: 181-187.
Clark LG, Dransfield S, Triplett J,
Sánchez-Ken JG. 2007 [2008]. Phylogenetic relationships among the
one-flowered, determinate genera of Bambuseae (Poaceae: Bambusoideae). – In: Columbus
JT, Friar EA, Porter JM, Prince LM, Simspon MG (eds), Monocots: comparative
biology and evolution. Poales, Rancho Santa Ana Botanical Garden, Claremont,
California, [Aliso 23] pp. 315-332.
Clark LG, Londoño X, Ruiz-Sanchez E. 2015.
Bamboo taxonomy and habitat. – In: Liese W, Köhl M (eds), Bamboos. The plant
and its uses, Springer Internat. Publ., Switzerland, pp. 1-30.
Clarke CB. 1883. On Hemicarex
Benth., and its allies. – Bot. J. Linn. Soc. 20: 374-403.
Clarke CB. 1884. On the Indian species of
Cyperus. – Bot. J. Linn. Soc. 21: 1-202.
Clarke CB. 1896. New East African Cyperaceae. – J. Bot. 34: 224-226.
Clarke CB. 1908. New genera and species of Cyperaceae. – Kew Bull., Add. Ser., 8:
1-196.
Clarke CB. 1909. Illustrations of Cyperaceae. – London.
Clarkson RB. 1961. Fraser’s sedge,
Cymophyllus fraseri (Andrews) Mackenzie. – Castanea 26: 129-136.
Clayton WD. 1966. Studies in the Gramineae
IX. Andropogoneae. – Kew Bull. 20: 257-274.
Clayton WD. 1967a. Studies in the Gramineae
XIII. – Kew Bull. 21: 99-10.
Clayton WD. 1967b. Studies in the Gramineae
XVI. A remarkable new genus from Tanzania. – Kew Bull. 21: 125-127.
Clayton WD. 1969. A revision of the genus
Hyparrhenia. – Kew Bull., Add. Ser., 2: 1-169.
Clayton WD. 1970a. Studies in the Gramineae
XXI. Coelorhachis and Rhytachne: a study in numerical
taxonomy. – Kew Bull. 24: 309-314.
Clayton WD. 1970b. Gramineae (Part 1). –
In: Milne-Redhead, Polhill RM (eds), Flora of tropical East Africa, Crown
Agents for Oversea Governments and Administrations, London, pp. 1-176.
Clayton WD. 1972a. Studies in the Gramineae:
XXVI. – Kew Bull. 27: 151-153.
Clayton WD. 1972b. Studies in the Gramineae
XXXI. The awned genera of Andropogoneae. – Kew Bull. 27: 457-474.
Clayton WD. 1972 [1973]. Studies in the
Gramineae XXXIII. The awnless genera of Andropogoneae. – Kew Bull. 28:
49-58.
Clayton WD. 1974. Gramineae (Part 2). - In:
Polhill RM (ed), Flora of tropical East Africa, Crown Agents for Oversea
Governments and Administrations, London, pp. 177-449.
Clayton WD. 1975. Chorology of the genera of
Gramineae. – Kew Bull. 30: 111-132.
Clayton WD. 1976. Studies in the Gramineae
XLI. Some discriminant functions for Hyparrhenia. – Kew Bull. 30:
511-520.
Clayton WD. 1980. Notes on the tribe
Andropogoneae (Gramineae). – Kew Bull. 35: 813-818.
Clayton WD. 1981a. Evolution and distribution
of grasses. – Ann. Missouri Bot. Gard. 68: 5-14.
Clayton WD. 1981b. New grasses from Tanzania.
– Kew Bull. 36: 234.
Clayton WD. 1981c. Early sources of tribal
names in Gramineae. – Kew Bull. 36: 483-485.
Clayton WD. 1982. Notes on subfamily
Chloridoideae (Gramineae). – Kew Bull. 37: 417-420.
Clayton WD. 1985. Miscellaneous notes on
pooid grasses. – Kew Bull. 40: 727-729.
Clayton WD. 1987a. Andropogoneae. – In:
Soderstrom TR, Hilu KH, Campbell CS, Barkworth ME (eds), Grass systematics and
evolution, Smithsonian Institution Press, Washington, D.C., pp. 334-342.
Clayton WD. 1987b. Miscellaneous notes on
panicoid grasses. – Kew Bull. 42: 401-403.
Clayton WD. 1990. The spikelet. – In:
Chapman GP (ed), Reproductive versatiliby in the grasses, Cambridge University
Press, Cambridge, pp. 32-51.
Clayton WD, Renvoize SA. 1982. Gramineae
(Part 3). – In: Polhill RM (ed), Flora of tropical East Africa, A. A.
Balkema, Rotterdam, pp. 451-898.
Clayton WD, Renvoize SA. 1986. Genera
Graminum. Grasses of the world. – Kew Bull., Add. Ser., 13: 1-389.
Clayton WD, Richardson FR. 1973. Studies in
the Gramineae XXXII. The tribe Zoysieae Miq. – Kew Bull. 28: 37-48.
Clayton WD, Snow N. 2010. A key to Pacific
grasses. – Kew Publishing, Kew, United Kingdom.
Clayton WD, Vorontsova MS, Harman KT,
Williamson H. 2012. GrassBase – The online World grass flora. – The Board
of Trustees, Royal Botanic Gardens, Kew.
http://www.kew.org/data/grasses-db/index.htm
Clegg MT, Rawson JRY, Thomas K. 1984.
Chloroplast DNA variation in pearl millet and related species. – Genetics
106: 449-461.
Clifford HT. 1961. Floral evolution in the
family Gramineae. – Evolution 15: 455-460.
Clifford HT. 1965. The classification of Poaceae: a statistical study. – Papers,
Dept. of Biology, University of Queensland 4: 243-253.
Clifford HT. 1967. A contribution to the
leaf-anatomy of Hubbardia heptaneuron Bor (Gramineae). – Kew Bull.
21: 169-174.
Clifford HT. 1970. Monocotyledon
classification with special reference to the origin of grasses (Poaceae). – In: Robson NKB, Cutler DF,
Gregory M (eds), New research in plant anatomy, Academic Press, London, pp.
25-34.
Clifford HT. 1987. Spikelet and floral
morphology. – In: Soderstrom TR, Hilu KW, Campbell CS, Barkworth ME (eds),
Grass systematics and evolution, Smithsonian Institution Press, Washington,
D.C., pp. 21-30.
Clifford HT. 1988. The taxonomic significance
of the ability of grass ‘seed’ to germinate under waterlogged conditions.
– Kew Bull. 43: 327-328.
Clifford HT, Watson L. 1977. Identifying
grasses: data, methods and identification. – University of Queensland Press,
St. Lucia.
Clifford HT, Williams WT, Lance GN. 1969. A
further numerical contribution to the classification of Poaceae. – Aust. J. Bot. 17: 119-131.
Coan AI, Scatena VL. 2004. Embryology and
seed development of Blastocaulon scirpeum and Paepalanthus
scleranthus (Eriocaulaceae).
– Flora 199: 47-57.
Coan AI, Alves MV, Scatena VL. 2008.
Comparative study of ovule and fruit development in species of
Hypolytrum and Rhynchospora (Cyperaceae, Poales). – Plant Syst.
Evol. 272: 181-195.
Coan AI, Alves MV, Scatena VL. 2010. Evidence
of pseudomonad pollen formation in Hypolytrum (Mapanioideae, Cyperaceae). – Aust. J. Bot. 58:
663-672.
Coan AI, Stützel T, Scatena VL. 2010.
Comparative embryology and taxonomic considerations in Eriocaulaceae (Poales). – Feddes
Repert. 121: 268-284.
Cocks PS, Boyce KG, Kloot PM. 1976. The
Hordeum murinum complex in Australia. – Aust. J. Bot. 24:
651-662.
Cocucci AE, Anton AM. 1988. The grass flower:
suggestions on its origin and evolution. – Flora 181: 353-362.
Coffani-Nunes JV, Versieux LM, Wanderley MGL,
Pirani JR. 2010. Flora da Serra do Cipó, Minas Gerais: Bromeliaceae – Tillandsioideae. –
Bol. Bot. Univ. São Paulo 28: 35-54.
Columbus JT. 1996. Lemma micromorphology,
leaf blade anatomy, and phylogenetics of Bouteloua, Hilaria,
and relatives (Gramineae: Chloridoideae: Boutelouinae). – Ph.D. diss.,
University of California, Berkeley, California.
Columbus JT. 1999a. An expanded
circumscription of Bouteloua (Gramineae: Chloridoideae): new
combinations and names. – Aliso 18: 61-65.
Columbus JT. 1999b. Morphology and leaf blade
anatomy suggest a close relationship between Bouteloua aristidoides
and B. (Chondrosium) eriopoda (Gramineae:
Chloridoideae). – Syst. Bot. 23: 467-478.
Columbus JT, Smith JP Jr. 2010. Nomenclatural
changes for some grasses in California and the Muhlenbergia clade (Poaceae). – Aliso 28: 65-67.
Columbus JT, Kinney MS, Pant R, Delgado MES.
1998. Cladistic parsimony analysis of internal transcribed spacer region
(nrDNA) sequences of Bouteloua and relatives (Gramineae:
Chloridoideae). – Aliso 17: 99-130.
Columbus JT, Kinney MS, Delgado MES, Porter
JM. 2000. Phylogenetics of Bouteloua and relatives (Gramineae:
Chloridoideae): cladistic parsimony analysis of internal transcribed spacer
(nrDNA) and trnL-F (cpDNA) sequences. – In: Jacobs SWL, Everett J
(eds), Grasses: systematics and evolution, Proceedings of the 2nd
International Conference on the Comparative Biology of Monocotyledons, Sydney,
Australia, Vol. 2, CSIRO Publ., Melbourne, pp. 189-194.
Columbus JT, Cerros-Tlatilpa R, Kinney MS,
Siqueiros-Delgado ME, Bell HL, Griffith MP, Refulio-Rodriguez NF. 2007 [2008].
Phylogenetics of Chloridoideae (Gramineae): a preliminary study based on
nuclear ribosomal internal transcribed spacer and chloroplast trnL-F
sequences. – In: Columbus JT, Friar EA, Porter JM, Prince LM, Simpson MG
(eds), Monocots: comparative biology and evolution. Poales, Rancho Santa Ana
Botanical Garden, Claremont, California. – Aliso 23: 565-579
Columbus JT, Peterson PM, Rodríguez NFR,
Tlatilpa RC, Kinney MS. 2010. Phylogenetics of Muhlenbergiinae (Poaceae: Chloridoideae, Cynodonteae) based
on ITS and trnL-F DNA sequences. – In: Seberg O, Petersen G, Barfod
AS, Davis JI (eds), Diversity, phylogeny, and evolution in the monocotyledons,
Aarhus University Press, Århus, pp. 477-495.
Comparot-Moss S, Denyer K. 2009. The
evolution of the starch biosynthetic pathway in cereals and other grasses. –
J. Experim. Bot. 60: 2481-2492.
Conceição S, De Toni KLG, Costa CG. 2007.
Particularidades do nucelo de Dyckia pseudococcinea L. B. Smith (Bromeliaceae). – Rev. Bras. Bioci.
5: 846-848.
Conert HJ. 1959. Über die Stellung der
Gattung Phaenosperma im System der Gramineae. – Bot. Jahrb. Syst.
78: 195-207.
Conert HJ. 1961. Die Systematik und Anatomie
der Arundineae. – Weinheim.
Conert HJ. 1970. Merxmuellera, eine
neue Gattung der Gramineen. – Schenckenberg. Biol. 51: 129-133.
Conert HJ. 1971. The genus Danthonia
in Africa. – Mitt. Bot. Staatssamml. München 10: 299-308.
Conert HJ. 1987. Current concepts in the
systematics of the Arundinoideae. – In: Soderstrom TR, Hilu KW, Campbell CS,
Barkworth ME (eds), Grass systematics and evolution, Smithsonian Institution
Press, Washington, D.C., pp. 239-250.
Conert HJ, Lobin W. 1984. Revision der
kapverdischen Sporobolus-Arten (Poaceae). – Garcia de Orta, Sér. Bot. 6:
51-68.
Conn BJ, Doust ANL. 1997. Xyris L.
Section Pomatoxyris Endl. (Xyridaceae) in Australia. – Aust.
Syst. Bot. 10: 189-248.
Connor HE. 1970. Gynodioecism in
Danthonia archboldii. – Aust. J. Bot. 18: 233-236.
Connor HE. 1979. Breeding systems in the
grasses: a survey. – New Zealand J. Bot. 17: 547-574.
Connor HE. 1981. Evolution of reproductive
systems in the Gramineae. – Ann. Missouri Bot. Gard. 68: 48-74.
Connor HE. 1987. Reproductive biology in
grasses. – In: Soderstrom TR, Hilu KW, Campbell CS, Barkworth ME (eds), Grass
systematics and evolution, Smithsonian Institution Press, Washington, D.C., pp.
117-132.
Connor HE. 2004. Flora of New Zealand –
Gramineae Supplement I: Danthonioideae. – New Zealand J. Bot. 42: 771-795.
Connor HE. 2008. Floral biology of Australian
species of Hierochloë (Gramineae). – Aust. J. Bot. 50: 166-176.
Connor HE. 2012. Flower and floral biology of
the holy grasses (Hierochloë and Anthoxanthum: Aveneae,
Gramineae). – Flora 207: 323-333.
Connor HE, Edgar E. 1974. Names and types in
Cortaderia Stapf (Gramineae). – Taxon 23: 595-605.
Connor HE, Jacobs SWL. 1991. Sex ratios in
dioecious Australian grasses: a preliminary assessment. – Cunninghamia 2:
385-390.
Consaul LL, Gillespie LJ, Waterway MJ. 2008.
Systematics of North American arctic diploid Puccinellia (Poaceae): morphology, DNA content, and AFLP
markers. – Syst. Bot. 33: 251-261.
Consaul LL, Gillespie LJ, Waterway MJ. 2010a.
Evolution and polyploidy origins in North American Arctic Puccinellia
(Poaceae) based on nuclear ribosomal
spacer and chloroplast DNA sequences. – Amer. J. Bot. 97: 324-336.
Consaul LL, Gillespie LJ, Waterway MJ. 2010b.
Polyploid speciation and evolution in Arctic Puccinellia (Poaceae: Puccinelliinae) – a review. –
In: Seberg O, Petersen G, Barfod AS, Davis JI (eds), Diversity, phylogeny, and
evolution in the monocotyledons, Aarhus University Press, Århus, pp.
645-662.
Coode MJE. 1978. Flore des Mascareignes 187:
Flagellariacées. – In: Bosser J, Cadet T, Julien HR, Marais W (eds), Flore
des Mascareignes: La Réunion, Maurice, Rodrigues, The Sugar Research
Institute, Mauritius.
Cook CDK, Nicholls MS. 1986. A monographic
study of the genus Sparganium (Sparganiaceae) 1. Subgenus
Xanthosparganium Holmberg. – Bot. Helvetica 96: 213-267.
Cook CDK, Nicholls MS. 1987. A monographic
study of the genus Sparganium (Sparganiaceae) 2. Subgenus
Sparganium. – Bot. Helvetica 97: 1-44.
Cooke DA. 1980. Studies in Australian
Centrolepidaceae I: the scapeless species of Centrolepis Labill. –
Muelleria 4: 265-272.
Cooke DA. 1992. A taxonomic revision of
Centrolepis (Centrolepidaceae) in Australia. – J. Adelaide Bot.
Gard. 15: 1-63.
Cooke DA. 1995. A taxonomic revision of
Aphelia (Centrolepidaceae). – J. Adelaide Bot. Gard. 16: 95-109.
Cooke DA. 1998. Centrolepidaceae. – In:
Kubitzki K (ed), The families and genera of vascular plants IV. Flowering
plants. Monocotyledons. Alismatanae and Commelinanae (except Gramineae),
Springer, Berlin, Heidelberg, New York, pp. 106-109.
Cope TA. 1983. Centropodia: an
earlier name for Asthenatherum (Gramineae). – Kew Bull. 37:
657-659.
Cope TA. 1984. Some new Arabian grasses. –
Kew Bull. 39: 833-836.
Cope TA. 1985. Studies in the flora of Arabia
XX. A key to the grasses of the Arabian Peninsula. – Arab Gulf J. Sci. Res.,
Spec. Publ. 1: 1-82.
Cope TA. 1987. Euthryptochloa: a new
genus of Gramioneae from China. – Kew Bull. 42: 707-709.
Cope TA. 1992a. Some new Somali grasses. –
Kew Bull. 47: 277-282.
Cope TA. 1992b. Some new Arabian grasses II.
– Kew Bull. 47: 655-664.
Cope TA. 1993. Taeniorhachis: a new
genus of Gramineae from Somalia. – Kew Bull. 48: 403-405.
Cope TA. 1995a. Some new Somali grasses II.
– Kew Bull. 50: 109-117.
Cope TA. 1995b. Two new species of
Perotis Aiton (Gramineae) from tropical Africa. – Kew Bull. 50:
611-614.
Cope TA. 1998a. A synopsis of
Eragrostis Wolf (Poaceae) in
the Flora Zambesiaca area. – Kew Bull. 53: 129-164.
Cope TA. 1998b. A synopsis of
Sporobolus R. Br. (Poaceae) in
the Flora Zambesiaca area. – Kew Bull. 53: 165-172.
Cope TA. 2006. Three new Arabian grasses. –
Kew Bull. 61: 243-244.
Cope TA, Hosni HA. 1991. A key to Egyptian
grasses. – Kew.
Costa AF da, Rodrigues PJFP, Wanderley MDGL.
2009. Morphometric analysis and taxonomic revision of the Vriesea
paraibica complex (Bromeliaceae). – Bot. J. Linn. Soc.
159: 163-181.
Costa FN. 2006. Three new species of
Actinocephalus Sano (Eriocaulaceae) from Minas Gerais,
Brazil. – Novon 16: 212-215.
Costa FN, Sano PT. 2013. New circumscription
of the endemic Brazilian genus Actinocephalus (Eriocaulaceae). – Novon 22:
281-287.
Costa Pereira S, Barreto IL. 1985. O gênero
Chloris Swartz (Gramineae) no Rio Grande do Sul. – Rodriguésia 62:
9-20.
Cotton JL, Wysocki WP, Clark LG, Kelchner SA,
Pires JC, Edger PP, Mayfield-Jones D, Duvall MR. 2015. Resolving deep
relationships of PACMAD grasses: a phylogenomic approach. – BMC Plant Biol.
15: 178.
Cotton R. 1974. Cytotaxonomy of the genus
Vulpia. – Ph.D. diss., University of Manchester, England.
Cotton R, Stace CA. 1976. Taxonomy of the
genus Vulpia (Gramineae) I. Chromosome numbers and geographical
distribution of the Old World species. – Genetica 46: 235-255.
Cotton R, Stace CA. 1977. Morphological and
anatomical variation of Vulpia (Gramineae). – Bot. Not. 130:
173-187.
Covas G. 1949. Taxonomic observations on the
North American species of Hordeum. – Madroño 10: 1-21.
Covas G. 1951. Nuevo Hordeum
hexaploide indigena en la Patagonia. – Rev. Argentina Agron. 18: 74-77.
Covas G. 1952. Nota taxonómica sobre
especies sudamericanas de “Hordeum”. – Rev. Argentina Agron. 19:
140-142.
Covas G. 1981. Las especies pampeanas de
Chloris (gramineae). – Apuntes Fl. Pampa 1: 262-264.
Crawford FC. 1910. Anatomy of the British
Carices. – Oliver & Boyd, Edinburgh.
Crayn DM, Terry RG, Smith JAC, Winter K.
2000. Molecular systematic investigations in Pitcairnioideae (Bromeliaceae) as a basis for
under-standing the evolution of crassulacean acid metabolism (CAM). – In:
Wilson KL, Morrison DA (eds.), Monocots: systematics and evolution, Proceedings
of the 2nd International Conference on the Comparative Biology of
Monocotyledons, Sydney, Australia, Vol. 2, CSIRO Publ., Melbourne, pp.
569-579.
Crayn DM, Winter K, Smith JAC. 2004. Multiple
origins of crassulacean acid metabolism and the epiphytic habit in the
neotropical family Bromeliaceae.
– Proc. Natl. Acad. Sci. U.S.A. 101: 3703-3708.
Crayn DM, Winter K, Schulte K, Smith JAC.
2015. Photosynthetic pathways in Bromeliaceae: phylogenetic and
ecological significance of CAM and C3 based on carbon isotope ratios for 1893
species. – Bot. J. Linn. Soc. 178: 169-221.
Crepet WL, Feldman GD. 1991. The earliest
remains of grasses in the fossil record. – Amer. J. Bot. 78: 1010-1014.
Crepet WL, Friis EM. 1987. The evolution of
insect pollination in angiosperms. – In: Friis EM, Chaloner WG, Crane PR
(eds), The origin of angiosperms and their biological consequences, Cambridge
University Press, Cambridge, pp. 181-201.
Crins WJG. 1985. The taxonomy of
Carex section Ceratocystis in North America and Northern
Eurasia. – Ph.D. diss., Dept. of Botany, University of Toronto, Canada.
Crins WJG. 1990. Phylogenetic considerations
below the sectional level in Carex. – Can. J. Bot. 68: 1433-1440.
Crins WJG, Ball PW. 1988. Sectional limits
and phylogenetic considerations in Carex section Ceratocystis
(Cyperaceae) in North America. –
Brittonia 40: 38-47.
Crins WJG, Ball PW. 1989a. Taxonomy of the
Carex flava complex (Cyperaceae) in North America and
northern Eurasia I. Numerical taxonomy and character analysis. – Can. J. Bot.
67: 1032-1047.
Crins WJG, Ball PW. 1989b. Taxonomy of the
Carex flava complex (Cyperaceae) in North America and
northern Eurasia II. Taxonomic treatment. – Can. J. Bot. 67: 1048-1065.
Cross RA. 1980. Distribution of sub-families
of Gramineae in the Old World. – Kew Bull. 35: 279-289.
Cruz GAS, Zizka G, Silvestro D, Leme EMC,
Schulte K, Benko-Iseppon AM. 2017. Molecular phylogeny, character evolution and
historical biogeography of Cryptanthus Otto & A. Dietr. (Bromeliaceae). – Mol. Phylogen.
Evol. 106: 152-165.
Cummings MP, King LM, Kellogg EA. 1994.
Slipped strand mispairing in a plastid gene: rpoC2 in grasses (Poaceae). – Mol. Biol. Evol. 11: 1-8.
Curtis WM. 1984. New species of Tasmanian
Monocotyledones in the families Juncaceae, Centrolepidaceae and Cyperaceae. – Brunonia 7: 297-304.
Cusset F, Tran TTH. 1965. La ligule de la
feuille végétative des Carex. – Bull. Soc. Bot. France 112:
42-54.
Cutler DF. 1965. Vegetative anatomy of Thurniaceae. – Kew Bull. 19:
431-441.
Cutler DF. 1969. Juncales. – In: Metcalfe
CR (ed), Anatomy of the monocotyledons IV, Clarendon Press, Oxford, pp.
1-358.
Cutler DF. 1972. Vicarious species of Restionaceae in Africa, Australia and
South America. – In: Valentine DH (ed), Taxonomy, phytogeography and
evolution, Academic Press, London, pp. 73-83.
Cutler DF, Airy Shaw HK. 1965. Anarthriaceae and Ecdeiocoleaceae: two new
monocotyledonous families, separated from the Restionaceae. – Kew Bull. 19:
489-499.
Cvelev NN. 1972. On the taxonomy and
phylogeny of genus Festuca of the USSR II. Evolution of subgenus
Festuca. – Bot. Žurn. 57: 161-172.
Dahlgren O. 1917. Die jüngeren
Entwicklungsstadien der Samenanlagen von Typha latifolia L. – Svensk
Bot. Tidskr. 12: 207-211.
Dandin SB, Chennaveeraiah MS. 1977.
Chromosome number and cytology of some species of Paspalum. – Proc.
Indian Sci. Congress Assoc. 64: 146.
Dandin SB, Chennaveeraiah MS. 1983.
Chromosome number and meiotic behaviour in interpretation of basic chromosome
number in the genus Paspalum. – J. Cytol. Genet. 18: 26-33.
Dandin SB, Chennaveeraiah MS. 1988.
Cytological evidence for apomixis in some species of Paspalum 1. –
J. Cytol. Genet. 23: 61-67.
Danin A. 2004. Arundo (Gramineae) in
the Mediterranean reconsidered. – Willdenowia 34: 361-369.
Danton P. 2002. Bromeliaceae et Orchidaceae de l’archipel
Juan Fernández (Chili). – Richardiana 2: 93-110.
Darbyshire SJ. 1993. Realignment of
Festuca subgenus Schedonorus with the genus Lolium
(Poaceae). – Novon 3: 239-243.
Darbyshire SJ, Warwick SI. 1992. Phylogeny of
North American Festuca (Poaceae) and related genera using
chloroplast DNA restriction site variation. – Can. J. Bot. 70: 2415-2429.
David R. 1976. Nomenclature of the British
taxa of the Carex muricata L. aggregate. – Watsonia 11: 59-65.
David R, Chater AO. 1977. Carex
polyphylla Kar. & Kir. and Carex leersiana Rauschert. –
Watsonia 11: 253-254.
David R, Kelcey JG. 1975. Carex
muricata L. sensu Nelmes and Carex bullockiana Nelmes. –
Watsonia 10: 412-414.
Davidse G. 1987. Fruit dispersal in the Poaceae. – In: Soderstrom TR, Hilu KW,
Campbell CS, Barkworth ME (eds), Grass systematics and evolution, Smithsonian
Institution Press, Washington, D.C., pp. 143-155.
Davidse G, Ellis RP. 1984.
Steyermarkochloa, a new genus from Venezuela and Colombia (Poaceae; Arundinoidese;
Steyermarkochloeae). – Ann. Missouri Bot. Gard. 71: 994-1012.
Davidse G, Ellis RP. 1987.
Arundoclaytonia, a new genus of the Steyermarkochloeae (Poaceae: Arundinoideae) from Brazil. –
Ann. Missouri Bot. Gard. 74: 479-490.
Davidse G, Filgueiras TS. 1993. Paspalum
longiaristatum (Poaceae: Paniceae),
a new serpentine endemic from Goiás, and the first awned species in the genus.
– Novon 3: 129-132.
Davidse G, Kral R. 1988. Two new species of
Calyptrocarya (Cyperaceae:
Sclerieae) from Venezuela and observations on the inflorescence morphology of
the genus. – Ann. Missouri Bot. Gard. 75: 853-861.
Davidse G, Pohl RW. 1971. Chromosome numbers
of Costa Rica grasses. – Brittonia 23: 293-324.
Davidse G, Pohl RW. 1972. Chromosome numbers
and notes on some Central American grasses. – Can. J. Bot. 50: 273-283.
Davidse G, Pohl RW. 1974. Chromosome numbers,
meiotic behaviour, and notes on tropical American grasses (Gramineae). – Can.
J. Bot. 52: 317-328.
Davidse G, Pohl RW. 1978. Chromosome numbers
of tropical American grasses (Gramineae). – Ann. Missouri Bot. Gard. 65:
637-649.
Davidse G, Pohl WP. 1992. New taxa and
nomenclatural combinations of Mesoamerican grasses (Poaceae). – Novon 2: 81-110.
Davidse G, Hoshino T, Simon BK. 1986.
Chromosome counts of Zimbabwean grasses (Poaceae) and an analysis of polyploidy in
the grass flora of Zimbabwe. – South Afr. J. Bot. 52: 521-527.
Davidse G, Soderstrom TR, Ellis RP. 1986.
Pohlidium petiolatum (Poaceae:
Centotheceae), a new genus and species from Panama. – Syst. Bot. 11:
131-144.
Davidse G, Morrone O, Zuloaga FO. 2001. Two
new species of Paspalum (Poaceae: Panicoideae) from Brazil. –
Novon 11: 389-394.
Davidse G, Soreng RJ, Peterson PM. 2009.
Agrostopoa (Poaceae, Pooideae,
Poeae, Poinae), a new genus with three species from Colombia. – Novon 19:
32-40.
Davies EW. 1956a. Cytology, evolution, and
origin of the aneuploid series in the genus Carex. – Hereditas 42:
349-365.
Davies EW. 1956b. Some new chromosome numbers
in the Cyperaceae. – Watsonia 3:
242-243.
Davies J, Briarty LG, Rieley JO. 1973.
Observations on the swollen lateral roots of the Cyperaceae. – New Phytol. 72:
167-174.
Davies RJ-P, Craigie AI, Mackay DA, Whalen
MA, Cheong JP-E, Leach GJ. 2007. Resolution of the taxonomy of
Eriocaulon (Eriocaulaceae) taxa endemic to
Australian mound springs, using morphometrics and AFLP markers. – Aust. Syst.
Bot. 20: 428-447.
Davis JI. 1983. Phenotypic plasticity and the
selection of taxonomic characters in Puccinellia (Poaceae). – Syst. Bot. 8: 341-353.
Davis JI. 1988. Genetic and environmental
contributions to multivariate morphological pattern in Puccinellia (Poaceae). – Can. J. Bot. 66:
2436-2444.
Davis JI, Manos PS. 1991. Isozyme variation
and species delimitation in the Puccinellia nuttalliana complex (Poaceae): an application of the
phylogenetic species concept. – Syst. Bot. 16: 431-445.
Davis JI, Soreng RJ. 1993. Phylogenetic
structure in the grass family (Poaceae)
as inferred from chloroplast DNA restriction site variation. – Amer. J. Bot.
80: 1444-1454.
Davis JI, Soreng RJ. 2007. A phylogenetic
analysis of Poaceae tribe Poeae sensu
lato based on morphological characters and sequence data from three
plastid-encoded genes: evidence for reticulation, and a new classification for
the tribe. – Kew Bull. 62: 425-454.
Davis JI, Soreng RJ. 2007 [2008]. A
preliminary phylogenetic analysis of the grass subfamily Pooideae (Poaceae), with attention to structural
features of the plastid and nuclear genomes, including an intron loss in GBSSI.
– In: Columbus JT, Friar EA, Porter JM, Prince LM, Simpson MG (eds),
Monocots: comparative biology and evolution. Poales, Rancho Santa Ana Botanical
Garden, Claremont, California. – Aliso 23: 335-348.
Davis JI, Soreng RJ. 2010. Migration of
endpoints of two genes relative to boundaries between regions of the plastid
genome in the grass family (Poaceae).
– Amer. J. Bot. 97: 874-892.
Dean M, Ashton PA. 2008. Leaf surfaces as a
taxonomic tool: the case of Carex section Phacocystis (Cyperaceae) in the British Isles. –
Plant Syst. Evol. 273: 97-105.
De Bustos A, Jouve N. 2002. Phylogenetic
relationships of the genus Secale based on the characterisation of
rDNA ITS sequences. – Plant Syst. Evol. 235: 147-154.
Decker HF. 1964a. An anatomic-systematic
study of the classical tribe Festuceae (Gramineae). – Amer. J. Bot. 51:
453-463.
Decker HF. 1964b. Affinities of the grass
genus Ampelodesmos. – Brittonia 16: 76-79.
deKoning R, Sosef MSM, Veldkamp JF. 1983. A
revision of Heteropholis and Thaumastochloa (Gramineae). –
Gard. Bull. Straits Settlem. (Singapore) 36: 137-162.
De Langhe JE. 1944. Sur le groupe du
Carex muricata L. en Belgique. – Bull. Soc. Roy. Bot. Belg. 76:
39-50.
Dellaferrera IM, Vegetti A. 2015.
Inflorescence structure in Koyamaeae and its relationship with Sclerieae,
Bisboeckelereae, Cryptagieae and Trilepideae tribes (Cyperoideae-Cyperaceae). – Bot. Rev. 81:
179-188.
De Moraes Fernandes MI, Barreto IL, Salzano
FM, Sacchet MOF. 1974. Cytological and evolutionary relationships in Brazilian
forms of Paspalum (Gramineae). – Caryologia 27:455-465.
Dengler NG, Dengler RE, Hattersley PW. 1986.
Comparative bundle sheath and mesophyll differentiation in the leaves of the
C4 grasses Panicum effusum and P. bulbosum. –
Amer. J. Bot. 73: 1431-1442.
Denham SS. 2005. Revisión sistemática del
subgénero Harpostachys de Paspalum (Poaceae, Panicoideae, Paniceae). – Ann.
Missouri Bot. Gard. 92: 463-532.
Denham SS, Zuloaga FO. 2007 [2008].
Phylogenetic relationships of the Decumbentes group of
Paspalum, Thrasya, and Thrasyopsis (Poaceae: Panicoideae: Paniceae). – In:
Columbus JT, Friar EA, Porter JM, Prince LM, Simpson MG (eds), Monocots:
comparative biology and evolution. Poales, Rancho Santa Ana Botanical Garden,
Claremont, California. – Aliso 23: 545-562.
Denham SS, Zuloaga FO, Morrone O. 2002.
Systematic revision and phylogeny of Paspalum subgenus
Ceresia (Poaceae: Panicoideae:
Paniceae). – Ann. Missouri Bot. Gard. 89: 337-399.
Denton MF. 1983. Anatomical studies of the
Luzulae group of Cyperus (Cyperaceae). – Syst. Bot. 8:
250-262.
De Oliveira L, de Sousa F, Wendt T, Brown GK,
Tuthill DE, Evans TM. 2007. Monophyly an phylogenetic relationships in
Lymania (Bromeliaceae:
Bromelioideae) based on morphology and chloroplast DNA sequences. – Syst.
Bot. 32: 264-270.
De Oliveira L, de Sousa F, Wendt T. 2008.
Taxonomy and conservation of the genus Lymania (Bromeliaceae) in the southern Bahian
Atlantic Forest of Brazil. – Bot. J. Linn. Soc. 157: 47-66.
De Oliveira L, De Aquino AJG, Guedès MLS,
Cotias de Oliveira ALP. 2008. Cytogenetics of Brazilian species of Bromeliaceae. – Bot. J. Linn. Soc.
158: 189-193.
De Paula LFA, Forzza RC, Neri AV, Bueno ML,
Porembski S. 2016. Sugar Loaf Land in south-eastern Brazil: a centre of
diversity for mat-forming bromeliads on inselbergs. – Bot. J. Linn. Soc. 181:
459-476.
Derieg NJ, Sangaumphai A, Bruederle LP. 2008.
Genetic diversity and endemism in North American Carex section
Ceratocystis (Cyperaceae).
– Amer. J. Bot. 95: 1287-1296.
De Sousa GM, Wanderley MGL, Cruz-Barros MAV.
1997. Morfologia polínica de espécies de Aechmea Ruiz & Pav. (Bromeliaceae) de Pernambuco, Brazil.
– Bol. Bot. Univ. São Paulo 16: 21-30.
De Sousa GM, Lapa Wanderly MDG, Alves M.
2009. Inflorescence architecture in Brazilian species of Aechmea
subgenus Chevaliera (Bromeliaceae-Bromelioideae) dagger.
– Bot. J. Linn. Soc. 158: 584-592.
Dettmann ME, Clifford HT. 2000. Fossil
Typha in Australia. – Mem. Queensland Mus. Nat. 45: 234.
Devos KM. 2010. Grass genome organization and
evolution. – Curr. Opin. Plant Biol. 13: 139-145.
Dewey DR. 1982. Genomic and phylogenetic
relationships among North American perennial Triticeae grasses. – In: Estes
JE et al. (eds), Grasses and grassland, University of Oklahoma Press, Norman,
pp. 51-80.
Dewey DR, Hsiao C. 1983. A cytogenetic basis
for transferring Russian wildrye from Elymus to
Psathyrostachys. – Crop Sci. 23: 123-126.
Dhooge S, Goetghebeur P, Muasya AM. 2003.
Zameioscirpus, a new genus of Cyperaceae from South America. – Plant
Syst. Evol. 243: 73-84.
Diao X, Freeling M, Lisch D. 2006. Horizontal
transfer of a plant transposon. – PLOS Biol. 4: e5.
doi:10.1371/journal.pbio.0040005
Díaz O, Salomon B, Bothmer R von. 1998.
Description of isozyme polymorphisms in Elymus species by using starch
gel electrophoresis. – In: Jaradat AA (ed), Triticeae III, Enfield, New
Hampshire, pp. 199-208.
Díaz-Pérez AJ, Sharifi-Tehrani M, Inda LA,
Catalán P. 2014. Polyphyly, gene-duplication and extensive allopolyploidy
framed the evolution of the ephemeral Vulpia grasses and other
fine-leaved Loliinae (Poaceae). –
Molec. Phylogen. Evol. 79: 92-105.
Didrichsen A. 1894. Om cyperaceens kim I. –
Bot. Tidsskr. 19: 1-6.
Didrichsen A. 1897. Om cyperaceens kim II.
– Bot. Tidsskr. 21: 1-10.
Diekmann K, Hodkinson TR, Wolfe KH, Bekerom R
van den, Dix PJ, Barth S. 2009. Complete chloroplast genome sequence of a major
allogamous forage species, perennial ryegrass (Lolium perenne L.). –
DNA Research 16: 165-176.
Dillon SL, Lawrence PK, Henry RJ. 2001. the
use of ribosomal ITS to determine phylogenetic relationships within
Sorghum. – Plant Syst. Evol. 230: 97-110.
Dillon SL, Lawrence PK, Henry RJ, Ross L,
Price HJ, Johnston JS. 2004. Sorghum laxiflorum and S.
macrospermum, the Australian native species most closely related to the
cultivated S. bicolor based on ITS1 and ndhF sequence
analysis of 25 Sorghum species. – Plant Syst. Evol. 249: 233-246.
Dillon SL, Lawrence PK, Henry RJ, Price HJ.
2007. Sorghum resolved as a distinct genus based on combined ITS1,
ndhF and Adh1 analyses. – Plant Syst. Evol. 268: 29-43.
Dillon SL, Shapter FM, Henry RJ, Cordeiro G,
Izquierdo L, Lee LS. 20007. Domestication to crop improvement: genetic
resources for Sorghum and Saccharum (Andropogoneae). – Ann.
Bot. 100: 975-989.
Ding Hou. 1958. Centrolepidaceae. – In: Steenis
CGGJ van (ed), Flora Malesiana I, 5(4), Noordhoff-Kolff N. V., Djakarta, pp.
421-428.
Doebley JF. 1989. Molecular evidence for a
missing wild relative of maize and the introgression of its chloroplast genome
into Zea perennis. – Evolution 43: 1555-1559.
Doebley JF, Goodman MM, Stuber CW. 1984.
Isoenzymatic variation in Zea (Gramineae). – Syst. Bot. 9:
203-218.
Doebley JF, Renfroe W, Blanton A. 1987.
Restriction site variation in the Zea chloroplast genome. – Genetics
117: 139-147.
Doebley JF, Durbin M, Golenberg E, Clegg M,
Ma D. 1990. Evolutionary analysis of the large subunit of carboxylase
(rbcL) nucleotide sequence among the grasses (Gramineae). –
Evolution 44: 1097-1108.
Doebley JF, Bothmer R von, Larson S. 1992.
Chloroplast DNA variation and the phylogeny of Hordeum (Poaceae). – Amer. J. Bot. 79: 576-584.
Doğan M. 1988. A scanning electron
microscope survey of the lemma in Phleum, Pseudophleum and
Rhizocephalus (Gramineae). – Notes Roy. Bot. Gard. Edinb. 45:
117-124.
Doğan M. 1991. A taxonomical revision of the
genus Phleum L. (Gramineae). – Karaca Arb. Mag. 1: 53-70.
Doğan M, Us J. 1996. Infrageneric
classification of the genus Phleum L. (Gramineae) estimated by
numerical taxonomy. – In: Öztürk M, Seçmen Ö, Görk G (eds), Plant life
in Southwest and Central Asia, Izmir.
Doğan M, Behçet L, Sinan A. 2015.
Pseudophleum anatolicum, a new endemic species of
Pseudophleum (Poaceae) from
East Anatolia, Turkey. – Syst. Bot. 40: 454-460.
Dokkedall AL, Salatino A. 1992. Flavonoids of
Brazilian Leiothrix Ruhland (Eriocaulaceae). – Biochem. Syst.
Ecol. 20: 31-32.
Domin K. 1907. Monographie der Gattung
Koeleria. – Bibl. Bot. 65.
Donadío S, Giussani LM, Kellogg EA, Zuloaga
FO, Morrone O. 2009. A preliminary molecular phylogeny of Pennisetum
and Cenchrus (Poaceae-Paniceae)
based on the trnL-F, rpl16 chloroplast markers. – Taxon 58:
392-404.
Donadío S, Pozner R, Giussani L. 2015.
Phylogenetic relationships within Tillandsia subgenus
Diaphoranthema (Bromeliaceae, Tillandsioideae) based
on a comprehensive morphological dataset. – Plant Syst. Evol. 301:
387-410.
Döring E. 2009. Molekulare Phylogenie der
Hafer-Gräser (Poaceae: Pooideae:
Aveneae). – Ph.D. diss., Martin-Luther-Universität Halle-Wittenberg,
Halle/Saale, Germany.
Döring E, Schneider J, Hilu KW, Röser M.
2007. Phylogenetic relationships in the Aveneae/Poeae complex (Pooideae, Poaceae). – Kew Bull. 62: 407-424.
Dörr H, Leist N. 1984. Morphologie der
Lodiculae in der Gattung Avena L. und ihre systematische
Verwertbarkeit. – Landw. Forsch. 3, Kongreßband 1984: 556-569.
Doust AN, Conn BJ. 1994. Xyris L.
section Xyris (Xyridaceae)
in Australia. – Aust. Syst. Bot. 7: 455-484.
Doust AN, Kellogg EA. 2002a. Integrating
phylogeny, developmental morphology and genetics: a case study of inflorescence
evolution in the ‘bristle grass’ clade (Panicoideae: Poaceae). – In: Cronk QCB, Bateman RM,
Hawkins JA (eds), Developmental genetics and plant evolution, Taylor and
Francis, London, pp. 298-314.
Doust AN, Kellogg EA. 2002b. Inflorescence
diversification in the panicoid “bristle grass” clade (Paniceae, Poaceae): evidence from molecular
phylogenies and developmental morphology. – Amer. J. Bot. 89: 1203-1222.
Doust AN, Penly AM, Jacobs SWL, Kellogg EA.
2007. Congruence, conflict, and polyploidization shown by nuclear and
chloroplast markers in the monophyletic “bristle clade” (Paniceae,
Panicoideae, Poaceae). – Syst. Bot.
32: 531-544.
Doyle JJ, Davis JI, Soreng RJ, Garvin D,
Anderson MJ. 1992. Chloroplast DNA inversions and the origin of the grass
family (Gramineae). – Proc. Natl. Acad. Sci. U.S.A. 89: 7722-7726.
Drábková LZ. 2010. Phylogenetic
relationships within Juncaceae:
evidence from five regions of plastid, mitochondrial and nuclear ribosomal DNA,
with notes on morphology. – In: Seberg O, Petersen G, Barfod AS, Davis JI
(eds), Diversity, phylogeny, and evolution in the monocotyledons, Aarhus
University Press, Århus, pp. 389-416.
Drábková LZ, Vlaček C. 2007. The
phylogenetic position of Oxychloë (Juncaceae): evidence from morphology,
nuclear and plastid DNA regions. – Taxon 56: 95-102.
Drábková LZ, Vlaček Č. 2009. DNA
variation within Juncaceae: comparison
of impact of organelle regions on phylogeny. – Plant Syst. Evol. 278:
169-186.
Drábková LZ, Kirschner J, Seberg O,
Petersen G, Vlaček C. 2003. Phylogeny of the Juncaceae based on rbcL
sequences, with special emphasis on Luzula DC. and Juncus L.
– Plant Syst. Evol. 240: 133-147.
Drábková LZ, Kirschner J, Vlček Č, Pačes
V. 2004. TrnL-trnF intergenic spacer and trnL intron
define clades within Luzula and Juncus (Juncaceae). – J. Mol. Evol. 59:
1-10.
Drábková LZ, Kirschner J, Vlček Č. 2006.
Phylogenetic relationships within Luzula DC. and Juncus L.
(Juncaceae): a comparison of
phylogenetic signals of trnL-trnF intergenic spacer,
trnL intron and rbcL plastome sequence data. – Cladistics
22: 132-143.
Dragon JA, Barrington DS. 2009. Systematics
of the Carex aquatilis and C. lenticularis lineages:
geographically and ecologically divergent sister clades of Carex
section Phacocystis (Cyperaceae). – Amer. J. Bot. 96:
1896-1906.
Dransfield S. 1981. The genus
Dinochloa (Gramineae-Bambusoideae) in Sabah. – Kew Bull. 36:
613-633.
Dransfield S. 1983a. The genus
Racemobambos (Gramineae: Bambusoideae). – Kew Bull. 37: 661-679.
Dransfield S. 1983b. Notes on
Schizostachyum (Gramineae-Bambusoideae) from Borneo and Sumatra. –
Kew Bull. 38: 321-332.
Dransfield S. 1989. Sphaerobambos, a
new genus of bamboo (Gramineae-Bambusoideae) from Malesia. – Kew Bull. 44:
425-434.
Dransfield S. 1992. The bamboos of Sabah. –
Forest Records 14, Forest Research Centre, Forestry Department, Sabah,
Malaysia.
Dransfield S. 1994. The genus
Hickelia (Gramineae: Bambusoideae). – Kew Bull. 49: 429-443.
Dransfield S. 1996. New species of
Dinochloa (Gramineae-Bambusoideae) in Malesia and notes on the genus.
– Kew Bull. 51: 103-117.
Dransfield S. 1997. Notes on the genus
Decaryochloa (Gramineae-Bambusoideae) from Madagascar. – Kew Bull.
52: 593-600.
Dransfield S. 1998a. Valiha and
Cathariostachys, two new bamboo genera (Gramineae-Bambusoideae) from
Madagascar. – Kew Bull. 53: 375-397.
Dransfield S. 1998b. Cyrtochloa, a
new genus of bamboo (Gramineae-Bambusoideae) from the Philippines. – Kew
Bull. 53: 857-873.
Dransfield S. 2002. Sirochloa, a new
bamboo genus from Madagascar (Poaceae-Bambusoideae). – Kew Bull. 57:
963-970.
Dransfield S. 2003. A new species and a new
combination of Cyrtochloa (Poaceae-Bambusoideae) from the Philippines.
– Kew Bull. 58: 981-985.
Dransfield S. 2016. Sokinochloa, a
new bamboo genus (Poaceae-Bambusoideae)
from Madagascar. – Kew Bull. 71: 40 DOI 10.1007/S12225-016-9650-9
Dransfield S, Widjaja EA. 1995. Plant
resources of south-east Asia. No. 7: Bamboos. – Backhuys, Leiden.
Dransfield S, Wong K-M. 1996.
Temburongia, a new genus of bamboo (Gramineae: Bambusoideae) from
Brunei. – Sandakania 7: 49-58.
Druyts-Voets E. 1970. Types van stengel- en
bladstrukturen in het genus Cyperus L. – Natuurwet. Tijdschr. (Gent)
52: 28-49.
Dubcovsky J, Zuloaga FO. 1991. Números
cromosómicos de especies sudamericanas de Panicum (Poaceae: Paniceae). – Bol. Soc. Argentina
Bot. 27: 201-206.
Dugas DP, Ratallack GJ. 1993. Middle Miocene
fossil grasses from Fort Ternan, Kenya. – J. Paleontology 67: 113-128.
Duhamel G. 1994. Flore pratique illustrée
des Carex de France. – Boubée, Paris.
Dunbar A. 1973. Pollen development in the
Eleocharis palustris group (Cyperaceae) I. Ultrastructure and
ontogeny. – Bot. Not. 126: 197-254.
Dunn RE, Thien-Y T Le, Strömberg CAE. 2015.
Light environment and epidermal cell morphology in grasses. – Intern. J.
Plant Sci. 176: 832-847.
Du Plessis H, Spies JJ. 1992. Chromosome
numbers in the genus Pentaschistis (Poaceae, Danthonieae). – Taxon 41:
709-720.
Duvall MR. 1987. A systematic evaluation of
the genus Zizania (Poaceae).
– Ph.D. diss., University of Minnesota, St. Paul, Minnesota.
Duvall MR, Biesboer D. 1988. Anatomical
distinctions between the pistillate spikelets of the species of wild-rice
(Zizania, Poaceae). – Amer.
J. Bot. 75: 157-159.
Duvall MR, Doebley JF. 1990. Restriction site
variation in the chloroplast genome of Sorghum (Poaceae). – Syst. Bot. 15: 472-480.
Duvall MR, Morton BR. 1996. Molecular
phylogenetics of Poaceae: an expanded
analysis of rbcL sequence data. – Mol. Phylogen. Evol. 5:
352-358.
Duvall MR, Peterson PM, Terrell EE,
Christensen AH. 1993. Phylogeny of North American oryzoid grasses as construed
from maps of plastid DNA restriction sites. – Amer. J. Bot. 80: 83-88.
Duvall MR, Peterson PM, Christensen AH. 1994.
Alliances of Muhlenbergia (Poaceae) within New World Eragrostideae are
identified by phylogenetic analysis of mapped restriction sites form plastid
DNAs. – Amer. J. Bot. 81: 622-629.
Duvall MR, Noll JD, Minn AH. 2001.
Phylogenetics of Paniceae (Poaceae). –
Amer. J. Bot. 88: 1988-1992.
Duvall MR, Saar DE, Grayburn WS, Holbrook GP.
2003. Complex transitions between C3 and C4
photosynthesis during the evolution of Paniceae: a phylogenetic case study
emphasizing the position of Steinchisma hians (Poaceae), a C3-C4
intermediate. – Intern. J. Plant Sci. 164: 949-958.
Duvall MR, Davis JI, Clark LG, Noll JD,
Goldman DH, Sánchez-Ken G. 2007 [2008]. Phylogeny of the grasses (Poaceae) revisited. – In: Columbus JT,
Friar EA, Porter JM, Prince LM, Simpson MG (eds), Monocots: comparative biology
and evolution. Poales, Rancho Santa Ana Botanical Garden, Claremont,
California. – Aliso 23: 237-247.
Duvall MR, Leseberg CH, Grennan CP, Morris
LM. 2010. Molecular evolution and phylogenetics of complete chloroplast genomes
in Poaceae. – In: Seberg O, Petersen
G, Barfod AS, Davis JI (eds), Diversity, phylogeny, and evolution in the
monocotyledons, Aarhus University Press, Århus, pp. 437-451.
Duvall MR, Fisher AE, Columbus JT, Ingram AL,
Wysocki WP, Burke SV, Clark LG, Kelchner SA. 2016. Phylogenomics and plastome
evolution of the chloridoid grasses (Chloridoideae: Poaceae). – Intern. J. Plant Sci. 177:
235-246.
Duvall MR, Yadav SR, Burke SV, Wysocki WP.
2017. Grass plastomes reveal unexpected paraphyly with endemic species of
Micrairoideae from India and new haplotype markers in Arundinoideae. – Amer.
J. Bot. 104: 286-295.
Ebinger JE. 1963. A new subgenus in
Luzula (Juncaceae). –
Brittonia 15: 169-174.
Ebinger JE, Carlen JL. 1975. Culm morphology
and grass systematics. – Trans. Illinois State Acad. Sci. 68: 87-101.
Echternacht L, Sano PT, Bonillo C, Cruaud C,
Couloux A, Dubuisson J-Y. 2014. Phylogeny and taxonomy of Syngonanthus
and Comanthera (Eriocaulaceae): evidence from
expanded sampling. – Taxon 63: 47-63.
Echternacht L, Sano PT, Dubuisson J-Y. 2015.
Taxonomic study of Comanthera subg. Thysanocephalus (Eriocaulaceae). – Syst. Bot. 40:
136-150.
Edgar E. 1966. Luzula in New
Zealand. – New Zealand J. Bot. 4: 159-184.
Edgar E. 1970. Centrolepidaceae. – In:
Moore LB, Edgar E (eds), Flora of New Zealand 2, Wellington.
Edgar E, Connor HE. 1998. Zotovia
and Microlaena: New Zealand ehrhartoid Gramineae. – New Zealand J.
Bot. 36: 565-586.
Edgar E, Forde MB. 1991. Agrostis in
New Zealand. – New Zealand J. Bot. 29: 139-161.
Edwards EJ, Smith SA. 2010. Phylogenetic
analyses reveal the shady history of C4 grasses. – Proc. Natl.
Acad. Sci. U.S.A. 107: 2532-2537.
Edwards EJ, Still CJ. 2008. Climate,
phylogeny and the ecological distribution of C4 grasses. – Ecol. Lett. 11:
266-276.
Egorova TV. 1980. Rod Eleocharis R. Br. vo
flore dal’nego vostoka SSSR. – Nov. Sist. Vysš. Rast. 17: 65-81.
Egorova TV. 1981. Sistema i konspekt roda
Eleocharis R. Br. (Cyperaceae) flory SSSR. – Nov. Sist.
Vysš. Rast. 18: 95-124.
Egorova TV. 1999. The sedges (Carex
L.) of Russia and adjacent states (within the limits of the former USSR).
–State Chemical-Pharmaceutical Academy, St. Petersburg, and Missouri
Botanical Garden Press, St. Louis.
Egorova TV, Khoi NK. 1980. Konspekt rodov
Scirpus L., Eriophorum L., Fuirena Rottb. i
Eleocharis R. Br. flory V’etnama. – Nov. Sist. Vysš. Rast. 17:
54-63.
Ehler N. 1977. Neue Untersuchungen zur
Entwicklung, Struktur und Funktion der Bromelien-Trichome. – Bromelienstudien
II: 473-508.
Ehler N, Schill R. 1973. Die
Pollenmorphologie der Bromeliaceae.
– Pollen Spores 15: 13-45.
Eig A. 1929. Monographisch-kritische
Übersicht der Gattung Aegilops. – Feddes Repert. 55.
Eiten LT. 1964. Egleria, a new genus
of Cyperaceae from Brazil. –
Phytologia 9: 481-487.
Eiten LT. 1970. Notes on Brazilian Cyperaceae II. – Phytologia 20:
273-276.
Eiten LT. 1976a. Inflorescence units in the
Cyperaceae. – Ann. Missouri Bot.
Gard. 63: 81-112.
Eiten LT. 1976b. The morphology of some
critical Brazilian species of Cyperaceae. – Ann. Missouri Bot. Gard.
63: 113-199.
Eldenäs PK, Linder HP. 2000. Congruence and
complementarity of morphological and trnL-trnF sequence data
and the phylogeny of the African Restionaceae. – Syst. Bot. 25:
692-707.
Elias MK. 1942. Tertiary prairie grasses and
other herbs from the high plains. – Geol. Soc. America Spec. Pap. 41:
1-176.
Elliott TL, Muasya AM. 2017. Taxonomic
realignment in the southern African Tetraria (Cyperaceae, tribe Schoeneae; Schoenus
clade). – South Afr. J. Bot. 112: 354-360.
Ellis RP. 1976. A procedure for standardizing
comparative leaf anatomy in the Poaceae
I. The leaf-blade as viewed in transverse section. – Bothalia 12: 65-109.
Ellis RP. 1977. Distribution of the Kranz
syndrome in the Southern African Eragrostoideae and Panicoideae according to
bundle sheath anatomy and cytology. – Agroplantae 9: 73-110.
Ellis RP. 1979. A procedure for standardizing
comparative leaf anatomy in the Poaceae
II. The epidermis as seen in surface view. – Bothalia 12: 641-671.
Ellis RP. 1982. Leaf anatomy of South African
Danthonieae (Poaceae) VII.
Merxmuellera dura and M. rangei. – Bothalia 14: 95-99.
Ellis RP. 1984a. Leaf anatomy of the South
African Danthonieae (Poaceae) IX.
Asthenatherum glaucum. – Bothalia 15: 153-159.
Ellis RP. 1984b. Eragrostis walteri
– a first record of non-Kranz leaf anatomy in the subfamily Chloridoideae (Poaceae). – South Afr. J. Bot. 3:
380-386.
Ellis RP. 1987a. A review of comparative leaf
blade anatomy in the systematics of the Poaceae: the past twenty-five years. –
In: Soderstrom TR, Hilu KW, Campbell CS, Barkworth ME (eds), Grass systematics
and evolution, Smithsonian Institution Press, Washington, D.C., pp. 3-10.
Ellis RP. 1987b. Leaf anatomy of the genus
Ehrharta (Poaceae) in southern
Africa: the Setacea group. – Bothalia 17: 75-89.
Ellis RP. 1987c. Leaf anatomy of the genus
Ehrharta (Poaceae) in southern
Africa: the Villosa group. – Bothalia 17: 195-204.
Ellis RP. 1988. Leaf anatomy and systematics
of Panicum (Poaceae:
Panicoideae) in southern Africa. – In: Goldblatt P, Lowry PP II (eds), Modern
systematic studies in African botany, Monogr. Syst. Bot. Missouri Bot. Gard.
25: 129-156.
Emery WHP. 1957. A cyto-taxonomical study of
Setaria macrostachya (Gramineae) and its relatives in the Southwestern
United States and Mexico. – Bull. Torrey bot. Club 84: 94-104.
Endo TR, Gill BS. 1984. The heterochromatin
distribution and genome evolution in diploid species of Elymus and
Agropyron. – Can. J. Genet. Cytol. 26: 669-678.
Engelmann G. 1866-1868. A revision of the
North American species of the genus Juncus, with a description of new
and imperfectly known species. – Trans. Acad. Sci. St. Louis 2: 424-499.
Engler A. 1886. Über die Familie Typhaceae. – Bot. Centralbl. 25:
127.
Engler A. 1888a. Flagellariaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien II(4), W. Engelmann, Leipzig,
pp. 1-3.
Engler A. 1888b. Mayacaceae. – In: Engler A, Prantl K
(eds), Die natürlichen Pflanzenfamilien II(4), W. Engelmann, Leipzig, pp.
16-18.
Engler A. 1888c. Xyridaceae. – In: Engler A, Prantl K
(eds), Die natürlichen Pflanzenfamilien II(4), W. Engelmann, Leipzig, pp.
18-20.
Engler A. 1888d. Rapateaceae. – In: Engler A, Prantl K
(eds), Die natürlichen Pflanzenfamilien II(4), W. Engelmann, Leipzig, pp.
28-31.
Engler A. 1889a. Typhaceae. – In: Engler A, Prantl K
(eds), Die natürlichen Pflanzenfamilien II(1), W. Engelmann, Leipzig, pp.
183-186.
Engler A. 1889b. Sparganiaceae. – In:
Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien II(1), W.
Engelmann, Leipzig, pp. 192-193.
Engler A. 1930. Flagellariaceae. – In: Engler A
(ed), Die natürlichen Pflanzenfamilien, 2. Augl., Bd. 15a, W. Engelmann,
Leipzig, pp. 6-8.
Engler A, Krause K. 1911. Über den
anatomischen Bau der baumartigen Cyperaceae Schoenodendron
bücheri Engl. aus Kamerun. – Abh. Kön. Preuß. Akad. Wissensch.
1911.
Erdtman G, Praglowski J. 1974. A note on
pollen morphology. – In: Smith LB, Downs RJ (eds), Pitcairnioideae (Bromeliaceae). Flora Neotropica 14,
Hafner Press, New York, pp. 28-33.
Ertter B. 1986. The Juncus triformis
complex. – Mem. New York Bot. Gard. 39: 1-90.
Escobedo-Sarti J, Ramírez I, Leopardi C,
Carnevali G, Magallón S, Duno R, Mondragón D. 2013. A phylogeny of Bromeliaceae (Poales,
Monocotyledoneae) derived from an evaluation of nine supertree methods. – J.
Syst. Evol. 51: 743-757.
Escudero M, Luceño M. 2009. Systematics and
evolution of Carex sects. Spirostachyae and Elatae
(Cyperaceae). – Plant Syst. Evol.
279: 163-189.
Escudero M, Luceño M. 2011. Taxonomic
revision of the tropical African group of Carex subsect.
Elatae (sect. Spirostachyae, Cyperaceae). – An. Jard. Bot. Madrid
68: 225-247.
Escudero M, Valcárcel V, Vargas P, Luceño
M. 2007. Evolution in Carex L. sect. Spirostachyae (Cyperaceae): a molecular and cytogenetic
approach. – Organisms Divers. Evol. 7: 271-291.
Escudero M, Valcárcel V, Vargas P, Luceño
M. 2010. Bipolar disjunctions in Carex: long-distance dispersal,
vicariance, or parallel evolution? – Flora 205: 118-127.
Escudero M, Hipp AL, Waterway MJ, Valente LM.
2012. Diversification rates and chromosome evolution in the most diverse
angiosperm genus of the temperate zone (Carex, Cyperaceae). – Mol. Phylogen. Evol.
63: 650-655.
Esen A, Hilu KW. 1989. Immunological
affnities among subfamilies of the Poaceae. – Amer. J. Bot. 76: 196-203.
Esen A, Hilu KW. 1991. Electrophoretic and
immunological studies of prolamins in the Poaceae II. Phylogenetic affinities of the
Aristideae. – Taxon 40: 5-17.
Espejo-Serna A. 2002. Viridantha, un
género nuevo de Bromeliaceae
(Tillandsioideae) endémico de México. – Acta Bot. Mexicana 60: 25.
Essi L, Longhi-Wagner HM, Souza-Chies TT de.
2008. Phylogenetic analysis of the Briza complex (Poaceae). – Mol. Phylogen. Evol. 47:
1018-1029.
Essi L, Souza Chies TT, Longhi Wagner HM.
2010. Three new taxa of Chascolytrum (Poaceae, Pooideae, Poeae) from South
America. – Novon 20: 149-156.
Essi L, Longhi-Wagner HM, Souza-Chies TT de.
2011. New combinations within the Briza complex (Poaceae, Pooideae, Poeae). – Novon 21:
326-330.
Essi L, Longhi-Wagner HM, de Souza Chies TT.
2017. A synopsis of Briza, Brizochloa, and
Chascolytrum (Poaceae,
Pooideae, Poeae). – Ann. Missouri Bot. Gard. 102: 466-519.
Estep MC, Diaz DV, Zhong J, Kellogg EA. 2012.
Eleven diverse nuclear-encoded phylogenetic markers for the subfamily
Panicoideae (Poaceae). – Amer. J. Bot.
99: e443-e446.
Estes JR, Tyrl RJ. 1982. The generic concept
and generic circumscription in the Triticeae: an end paper. – In: Estes JR,
Tyrl RJ, Brunken JN (eds), Grasses and grasslands, systematics and ecology,
University of Oklahoma Press, Norman, pp. 145-164.
Evans TM, Brown GK. 1989. Plicate staminal
filaments in Tillandsia subgenus Anoplophytum. – Amer. J.
Bot. 76: 1478-1485.
Evans TM, Jabaily RS, Gelli de Faria AP, de
Sousa L de OF, Wendt T, Brown GK. 2015. Phylogenetic relationships in Bromeliaceae subfamily Bromeliodeae
based on chloroplast DNA sequence data. – Syst. Bot. 50: 116-128.
Everett J. 1991. Systematic relationships of
the Australian Stipeae (Poaceae). –
M.Sc. thesis, University of Sydney, New South Wales, Australia.
Everett J, Jacobs SWL. 1983. Studies in
Australian Stipa (Poaceae). –
Telopea 2: 391-400.
Everett J, Jacobs SWL. 1990. Notes on
Stipa (Poaceae) in Australia
and Easter Island. – Telopea 4: 7-11.
Fagundes NF, Mariath JE de A. 2010.
Morphoanatomy and ontogeny of fruit in Bromeliaceae species. – Acta Bot.
Brasilica 24: 765-779.
Fagundes NF, Mariath JEA. 2014. Ovule
ontogeny in Billbergia nutans in the evolutionary context of Bromeliaceae (Poales). – Plant Syst.
Evol. 300: 1323-1336.
Fahmy AG. 2008. Diversity of lobate
phytoliths in grass leaves from the Sahel region, West Tropical Africa: tribe
Paniceae. – Plant Syst. Evol. 270: 1-23.
Fan X, Zhang HQ, Sha LN, Zhang L, Yang RW,
Ding CB, Zhou YH. 2007. Phylogenetic analysis among Hystrix,
Leymus and its affinitive genera (Poaceae: Triticeae) based on the sequences
of a gene encoding plastid acetyl-CoA carboxylase. – Plant Sci. 172:
701-707
Fan X, Sha LN, Yang RW, Zhang HQ, Kang HY,
Ding CB, Zhang L, Zheng YL, Zhou YH. 2009. Phylogeny and evolutionary history
of Leymus (Triticeae; Poaceae)
based on a single-copy nuclear gene encoding plastid acetyl-CoA carboxylase.
– BMC Evol. Biol. 9: 247.
Fan X, Liu J, Sha L-N, Sun G-L, Hu Z-Q, Zeng
J, Kang H-Y, Zhang H-Q, Wang Y, Wang X-L, Zhang L, Ding C-B, Yang R-W, Zheng
Y-L, Zhou Y-H. 2014. Evolutionary pattern of rDNA following polyploidy in
Leymus (Triticeae: Poaceae).
– Molec. Phylogen. Evol. 77: 296-306.
Fang W, Wu J-R, Sheng Y-C. 1991. Chromosome
numbers of ten scattered bamboos. – J. Zhejiang Forest College 8: 127-129.
Faria APG, Wendt T, Brown GK. 2004. Cladistic
relationships of Aechmea (Bromeliaceae, Bromelioideae) and
allied genera. – Ann. Missouri Bot. Gard. 91: 303-319.
Fassett NC. 1925. Notes on
Distichlis. – Rhodora 27: 67-72.
Faulkner JS. 1972. Chromosome studies on
Carex section Acutae in North-west Europe. – Bot. J. Linn.
Soc. 65: 271-302.
Felber F. 1986. Distribution des cytodèmes
d’Anthoxanthum odoratum L. s. lat. en Suisse. Les relations
Alpes-Jura. Index des nombres chromosomiques des Spermatophytes de la Suisse
III. Poaceae, genre
Anthoxanthum. – Bot. Helv. 96: 145-158.
Felber F. 1988. Distribution des cytodèmes
d’Anthoxanthum odoratum L. s. l. en France et dans les regions
limitrophes. – Bull. Soc. Bot. France 135: 281-293.
Fernandes A, Queiros M. 1969. Contribution à
la connaissance cytotaxinomique des Spermatophyta du Portugal I. Gramineae. –
Bol. Soc. Brot., ser. II, 43: 20-140.
Fernández Souto DP, Catalano SA, Tosto D,
Bernasconi P, Sala A, Wagner M, Corach D. 2006. Phylogenetic relationships of
Deschampsia antarctica (Poaceae): insights from nuclear ribosomal
ITS. – Plant Syst. Evol. 261: 1-9.
Ferrari RC, Oriani A. 2017. Floral anatomy
and development of Saxofridericia aculeata (Rapateaceae) and its taxonomic and
phylogenetic significance. – Plant Syst. Evol. 303: 187-201.
Ferrari RC, Scatena V, Oriani A. 2014. Leaf
and inflorescence peduncle anatomy: a contribution to the taxonomy of Rapateaceae. – Plant Syst. Evol. 300:
1579-1590.
Ferreira FM, Berg C van den, Hollowell VC,
Oliveira RP. 2013. Parianella (Poaceae, Bambusoideae): morphological and
biogeographical information reveals a new genus of herbaceous bamboos from
Brazil. – Phytotaxa 77: 27-32.
Ferris C, King RA, Gray AJ. 1997. Molecular
evidence for the maternal parentage in the hybrid origin of Spartina
anglica C. E. Hubbard. – Mol. Ecol. 6: 185-187.
Fijten F. 1975. A taxonomic revision of
Buergersiochloa Pilg. (Gramineae). – Blumea 22: 415-418.
Filgueiras TS. 1982. Taxonomia e
distribuição de Arthropogon Nees (Gramineae). – Bradea 3:
303-322.
Filgueiras TS. 1990. Revisão de
Mesosetum Steudel (Gramineae: Paniceae). – Acta Amazonica 14:
47-114.
Filgueiras TS. 1993. Nomenclatural and
critical notes on some Brazilian species of Paspalum (Poaceae: Paniceae). – Acta Amazonica 23:
147-161.
Filgueiras TS. 1994. A new species of
Echinolaena (Poaceae: Paniceae)
from Ecuador and a key to the New World species of the genus. – Nord. J. Bot.
14: 379-381.
Filgueiras TS. 1995. Paspalum
niquelandiae (Poaceae: Paniceae), a
new species from serpentine outcrops of central Brazil. – Novon 5: 30-33.
Filgueiras TS. 1996. Arthropogon
rupestris (Poaceae: Arthropogoneae)
a new species from the Brazilian cerrado vegetation and a revised key for the
genus. – Nord. J. Bot. 16: 69-72.
Filgueiras TS, Davidse G. 1994. Paspalum
biaristatum (Poaceae: Paniceae), a
new serpentine endemic from Goiás, Brazil, and the second awned species in the
genus. – Novon 4: 18-22.
Filgueiras TS, Davidse G. 1995. Two new
species of Paspalum (Poaceae:
Paniceae) from Brazil. – Novon 5: 146-151.
Filgueiras TS, Zuloaga FO. 1999. A new
Triraphis (Poaceae:
Eragrostoideae) from Brazil: first record of a native species in the New World.
– Novon 9: 36-41.
Filgueiras TS, Davidse G, Zuloaga FO. 1993.
Ophiochloa, a new endemic serpentine grass genus (Poaceae: Paniceae) from the Brazilian
cerrado vegetation. – Novon 3: 360-366.
Filgueiras TS, Peterson PM, Herrera-Arrieta
Y. 1999. Rheochloa (Poaceae:
Chloridoideae), a new genus from Central Brazil. – Syst. Bot. 24: 123-127.
Filgueiras TS, Morrone O, Zuloaga FO. 2001.
Paspalum burmanii (Poaceae:
Paniceae), a new species from central Brazil. – Novon 11: 36-39.
Filgueiras TS, Davidse G, Zuloaga FO, Morrone
O. 2001. The establishment of the new genus Altoparadisium and a
reevaluation of the genus Arthropogon (Poaceae, Paniceae). – Ann. Missouri Bot.
Gard. 88: 351-372.
Filho PJS, Trevisan R, Boldrini II. 2017.
Revision of Rhynchospora (Cyperaceae) sect. Luzuliformes.
– Syst. Bot. 42: 175-184.
Fincher GB. 2009. Revolutionary times in our
understanding of cell wall biosynthesis and remodelling in the grasses. –
Plant Physiol. 149: 27-37.
Finlayson CM, Roberts J, Chick AJ, Sale PJM.
1983. The biology of Australian weeds II. Typha dominguensis Pers. and
Typha orientalis Presl. – J. Aust. Inst. Agric. Sci. 49: 3-10.
Finot VL, Peterson PM, Zuloaga FO, Soreng RJ,
Matthei O. 2005. A revision of Trisetum (Poaceae: Pooideae: Aveninae) in South
America. – Ann. Missouri Bot. Gard. 92: 533-568.
Finot VL, Peterson PM, Soreng RJ, Zuloaga FO.
2005. A revision of Trisetum and Graphephorum (Poaceae: Pooideae: Aveninae) in North
America north of México. – Sida 1419-1453.
Finot VL, Baeza CM, Matthei O. 2006.
Micromorfología de la epidermis de la lemma de Trisetum y géneros
afines (Poaceae, Pooideae). –
Darwiniana 44: 32-57.
Finot VL, Barrera JA, Marticorena C, Rojas G.
2011. Systematic diversity of the family Poaceae (Gramineae) in Chile. – In:
Grillo O (ed.), The dynamical processes of biodiversity – case studies of
evolution and spatial distribution, publ. by InTech, Rijeka & Shanghai, pp.
71-108.
Finot VL, Barrera JA, Marticorena C, Rojas G.
2011. Systematic diversity of the family Poaceae (Gramineae) in Chile. – In:
Grillo O (ed.), The dynamical processes of biodiversity – case studies of
evolution and spatial distribution, publ. by InTech, Rijeka & Shanghai, pp.
71-108.
Fisher AE, Triplett JK, H C-S, Schiller AD,
Oltrogge KA, Schroder ES, Kelchner SA, Clark LG. 2009. Paraphyly in the bamboo
subtribe Chusqueinae (Poaceae:
Bambusoideae) and a revised infrageneric classification for Chusquea.
– Syst. Bot. 34: 673-683.
Fisher AE, Clark LG, Kelchner SA. 2014.
Molecular phylogeny estimation of the bamboo genus Chusquea (Poaceae: Bambusoideae: Bambuseae) and
description of two new subgenera. – Syst. Bot. 39: 829-844.
Fisher AE, Hasenstab KM, Bell HL, Blaine E,
Ingram AL, Columbus JT. 2016. Evolutionary history of chloridoid grasses
estimated from 122 nuclear loci. – Mol. Phylogen. Evol. 105: 1-14.
Fisher JB. 1971. Inverted vascular bundles in
the leaf of Cladium (Cyperaceae). – Bot. J. Linn. Soc. 64:
277-293.
Flovik K. 1938. Cytological studies on arctic
grasses. – Hereditas 24: 265-376.
Foggi B, Rossi G. 1996. A survey of the genus
Festuca L. (Poaceae) in Italy
I. The species of the summit flora in the Tuscan-Emilian Apennines and Apuan
Alps. – Willdenowia 26: 183-215.
Foggi B, Scholz H, Valdés B. 2005. The
Euro+Med treatment of Festuca (Gramineae) – new names and new
combinations in Festuca and allied genera. – Willdenowia 35:
241-244.
Foggi B, Parolo G, Šmarda P, Coppi A,
Lastrucci L, Lakušić D, Eastwood R, Rossi G. 2012. Revision of the
Festuca alpina group (Festuca section Festuca, Poaceae) in Europe. – Bot. J. Linn. Soc.
170: 618-639.
Fonseca MA. 2003. Systematic studies on the
genus Glyphochloa. – Ph.D. thesis, University of Goa, Goa, India.
Fontana A. 1963. Micorriza ectotrofiche in
una ciperacea: Kobresia bellardii. – Degl. Giorn. Bot. Ital. 70:
639-641.
Ford BA, Ball PW. 1992. The taxonomy of the
circumpolar short-beaked taxa of Carex sect. Vesicariae (Cyperaceae). – Syst. Bot. 17:
620-639.
Ford BA, Ball PW, Ritland K. 1991. Allozyme
diversity and genetic relationships among North American members of the
short-beaked taxa of Carex sect. Vesicariae (Cyperaceae). – Syst. Bot. 16:
116-131.
Ford BA, Iranpour M, Naczi RFC, Starr JR,
Jerome CA. 2006. Phylogeny of Carex subg. Vignea (Cyperaceae) based on non-coding nrDNA
sequence data. – Syst. Bot. 31: 70-82.
Ford BA, Ghazvini H, Naczi RFC, Starr JR.
2012. Phylogeny of Carex subg. Vigna (Cyperaceae) based on amplified fragment
length polymorphism and nrDNA data. – Syst. Bot. 37: 913-925.
Fortune PM, Schierenbeck KA, Ainouche AK,
Jacquemin J, Wendel JF, Ainouche ML. 2007. Evolutionary dynamics of Waxy and
the origin of hexaploid Spartina species (Poaceae). – Mol. Phylogen. Evol. 43:
1040-1055.
Fortune PM, Pourtau N, Viron N, Ainouche ML.
2008. Molecular phylogeny and reticulate origins of the polyploid
Bromus species from section Genea (Poaceae). – Amer. J. Bot. 95: 454-464.
Forzza RC. 2001. Filogenia da tribo Puyeae
Wittm. e revisão taxonômica do gênero Encholirium Mart. ex Schult.
& Schult. f. (Pitcairnioideae-Bromeliaceae). – Ph.D. diss.,
University of São Paulo, Brazil.
Forzza RC. 2005. Revisão taxonômica de
Encholirium Mart. ex. Schult. & Schult. f. (Pitcairnioideae – Bromeliaceae). – Bol. Bot. Inst.
Bioci. Univ. São Paulo 23: 1-49.
Forzza RC, Zappi D. 2011. Side by side: two
remarkable new species of Encholirium Mart. ex Schult. & Schult.
f. (Bromeliaceae) found in the
Cadeia do Espinhaço, Minas Gerais, Brazil. – Kew bull. 66: 281-287.
Forzza RC, Christianini AV, Wanderley MGL,
Buzato S. 2003. Encholirium (Pitcairnioideae – Bromeliaceae): conhecimento atual e
sugestões para conservação. – Vidalia 1: 7-20.
Fox DL, Koch PL. 2004. Carbon and oxygen
isotopic variability in Neogene paleosol carbonates: constraints on the
evolution of C4-grasslands of the Great Plains, USA. –
Palaeogeogr. Palaeoclim. Palaeoecol. 207: 305-329.
Francis A. 1990. The Tripsacinae: an
interdisciplinary review of maize (Zea mays) and its relatives. –
Acta Bot. Fenn. 140: 1-51.
Franklin DC. 2003. Morphology and taxonomy of
the Top End Bamboo Bambusa arnhemica F. Muell., a little-known bamboo
from northern Australia. – Bamboo Sci. Cult. 17: 44-54.
Franklin DC. 2008. Taxonomic interpretations
of Australian native bamboos (Poaceae:
Bambuseae) and their biogeographic implications. – Telopea 12: 179-191.
Franklin EF. 1983. Two new species of
Scleria (Cyperaceae) from
South Africa. – Kew Bull. 38: 33-36.
Freckmann RW, Lelong MG. 2003. Nomenclatural
changes and innovations in Panicum and Dichanthelium (Poaceae: Paniceae). – Sida 20:161-174.
Frederiksen S. 1982. Festuca
brachyphylla, F. saximontana and related species in North
America. – Nord. J. Bot. 2: 525-536.
Frederiksen S. 1986. Revision of
Taeniatherum (Poaceae). –
Nord. J. Bot. 6: 389-397.
Frederiksen S. 1991. Taxonomic studies in
Dasypyrum (Poaceae). – Nord.
J. Bot. 11: 135-142.
Frederiksen S. 1991. Taxonomic studies in
Eremopyrum (Poaceae). – Nord.
J. Bot. 11: 271-285.
Frederiksen S. 1993. Taxonomic studies in
some annual genera of the Triticeae (Poaceae). – Nord. J. Bot. 13: 481-493.
Frederiksen S, Bothmer R von. 1986.
Relationships in Taeniatherum (Poaceae). – Can. J. Bot. 64:
2343-2347.
Frederiksen S, Seberg O. 1992. Phylogenetic
analysis of the Triticeae (Poaceae). –
Hereditas 116: 15-19.
Freitag H. 1975. The genus
Piptatherum (Gramineae) in Southwest and South Asia. – Notes Roy.
Bot. Gard. Edinb. 33: 341-408.
Freitag H. 1985. The genus Stipa
(Gramineae) in Southwest and South Asia. – Notes Roy. Bot. Gard. Edinb. 42:
355-489.
Freitag H. 1989. Piptatherum and
Stipa (Gramineae) in the Arabian Peninsula and tropical East Africa.
– In: Tan K (ed), The Davis and Hedge festschrift, Edinburgh University
Press, Edinburgh, pp. 115-132.
Freter LE, Brown WV. 1955. A cytotaxonomic
study of Bouteloua curtipendula and B. uniflora. – Bull.
Torrey Bot. Club 82: 121-130.
Friar E, Kochert G. 1991. Bamboo germplasm
screening with nuclear restriction fragment length polymorphisms. – Theor.
Appl. Gen. 82: 697-703.
Friedland S. 1941. The American species of
Hemicarpha. – Amer. J. Bot. 28: 855-861.
Friedman J, Harder LD. 2004. Inflorescence
architecture and wind pollination in six grass species. – Funct. Ecol. 18:
851-860.
Fuente García V de la, Ortuñez E. 1998.
Biosistemática de la sección Festuca del género Festuca L.
(Poaceae) en la Península Ibérica. –
Madrid.
Fuente García V de la, Sanchez Mata D. 1986.
Datos taxonómicas sobre el género Festuca L. (Gramineae) en la
Península Ibérica. – Candollea 41: 441-448.
Fuente García V de la, Ferrero LM, Ortuñez
Rubio E. 2001. Chromosome counts in the genus Festuca L. section
Festuca (Poaceae) in the
Iberian Peninsula. – Bot. J. Linn. Soc. 137: 385-398.
Gabel ML, Bich H. 1988. A range extension for
the Tertiary fossil Eleofimbris (Cyperaceae). – The Southw. Natur. 33:
110-112.
Gabel ML, Backlund DC, Haffner J. 1992. Sedge
(Cyperaceae) achenes from the Late
Barstovian of Nebraska. – J. Paleontology 66: 525-529.
Galbreath EC. 1974. Stipoid grass “seeds”
from the Oligocene and Miocene deposits of northeastern Colorado. – Trans.
Illinois State Acad. Sci. 67: 366-368.
Gale MD, Devos KM. 1998. Comparative genetics
in the grasses. – Proc. Natl. Acad. Sci. U.S.A. 95: 1971-1974.
Galetto L, Bernardello LM. 1992. Extrafloral
nectaries that attract ants in Bromeliaceae: structure and nectar
composition. – Can. J. Bot. 70: 1101-1106.
Galley C, Linder HP. 2007. The phylogeny of
the Pentaschistis clade (Danthonioideae, Poaceae) based on chloroplast DNA, and the
evolution and loss of complex characters. – Evolution 61: 864-884.
Gamble JS. 1923. Neohouzeaua, a new
genus of bamboos. – Bull. Misc. Inform. – Kew 1923: 89-93.
Gandhi KN, Barkworth ME. 2003. Proposal to
conserve the name Munroa (Gramineae) with that spelling. – Taxon 52:
137-138.
Gao Q, Yang Z-L. 2010. Ectomycorrhizal fungi
associated with two species of Kobresia in an alpine meadow in the
eastern Himalaya. – Mycorrhiza 20: 281-287.
Garbari F. 1972. Il genere Paspalum
L. (Gramineae) in Italia. – Atti Soc. Tosc. Sci. Nat. Pisa Mem., Ser. B, 79:
52-65.
Garber ED. 1950. Cytotaxonomic studies in the
genus Sorghum. – Univ. Calif. Publ. Bot. 23: 283-361.
García-Madrid AS, Muasya AM, Álvarez I,
Cantó P, Molina JA. 2015. Towards resolving phylogenetic relationships in the
Ficinia clade and description of the new genus Afroscirpoides
(Cyperaceae: Cypereae). – Taxon 64:
688-702.
Gardner CS. 1982. A systematic study of
Tillandsia subgenus Tillandsia. – Ph.D. diss., Texas A.
& M. University, Corpus Cristi, Texas.
Gardner CS. 1986a. Inferences about
pollination in Tillandsia (Bromeliaceae). – Selbyana 9:
76-87.
Gardner CS. 1986b. Preliminary classification
of Tillandsia based on floral characters. – Selbyana 9: 130-146.
Gaut BS, Clegg MT. 1991. Molecular evolution
of alcohol dehydrogenase 1 in members of the grass family. – Proc. Natl.
Acad. Sci., U.S.A. 90: 2060-2064.
Gaut BS, Morton BR, McCaig BC, Clegg MT.
1996. Substitution rate comparisons between grasses and palms: synonymous rate
differences at the nuclear gene Adh parallel rate differences at the
plastid gene rbcL. – Proc. Natl. Acad. Sci. U.S.A. 93:
10274-10279.
Gaut BS, Clark LG, Wendel JF, Muse SV. 1997.
Comparison of the molecular evolutionary process at rbcL and
ndhF in the grass family (Poaceae). – Mol. Biol. Evol. 14:
769-777.
Gebauer S, Starr JR, Hoffman MH. 2014.
Parallel and convergent diversification in two northern hemispheric
species-rich Carex lineages (Cyperaceae). – Organ. Div. Evol. 14:
247-258.
Gebauer S, Röser M, Hoffman MH. 2015.
Molecular phylogeny of the species-rich Carex sect. Racemosae
(Cyperaceae) based on four nuclear
and chloroplast markers. – Syst. Bot. 40: 433-447.
Gehrke BS. 2011. Synopsis of Carex
(Cyperaceae) from sub-Saharan Africa
and Madagascar. – Bot. J. Linn. Soc. 166: 51-99.
Gehrke BS, Martín-Bravo S, Muasya M, Luceño
M. 2010. Monophyly, phylogenetic position and the role of hybridization in
Schoenoxiphium Nees (Cariceae, Cyperaceae). – Mol. Phylogen. Evol.
56: 380-392.
Gehrke BS, Vrijdaghs A, Smets E, Muasya AM.
2012. Unisexual flowers as a robust synapomorphy in Cariceae (Cyperaceae)? Evidence for bisexual
flowers in Schoenoxiphium. – South Afr. J. Bot. 78: 150-158.
Geng B-J, Yi T-P. 1982. Bashania, a
new bamboo genus from western China. – J. Nanjing Univ. (Nat. Sci.) 3:
722-732.
Gervais C. 1968. Sur un critère anatomique
nouveau utilisable dans la taxinomie chez les avoines vivaces. – Ber.
Schweiz. Bot. Ges. 78: 369-372.
Gervais C. 1973. Contribution à l’étude
cytologique et taxinomique des avoines vivaces (G. Helictotrichon
Bess. et Avenochloa Holub). – Denkschr. Schweiz. naturf. Ges. 88.
Ghamkhar K, Marchant AD, Wilson KL, Bruhl JJ.
2007 [2008]. Phylogeny of Abildgaardieae (Cyperaceae) inferred from ITS and
trnL-F data. – In: Columbus JT, Friar EA, Porter JM, Prince LM,
Simpson MG (eds), Monocots: comparative biology and evolution. Poales, Rancho
Santa Ana Botanical Garden, Claremont, California. – Aliso 23: 149-164.
Ghasemkhani M, Akhani H, Sahebi J, Scholz H.
2008. The genera Aristida and Stipagrostis (Poaceae) in Iran. – Willdenowia 38:
135-148.
Gibbs-Russell GE. 1986. Significance of
different centers of diversity in subfamilies of Poaceae in southern Africa. – Palaeoecol.
Africa 17: 183-192.
Gibbs-Russell GE. 1987. Taxonomy of the genus
Ehrharta (Poaceae) in southern
Africa: the Setacea group. – Bothalia 17: 67-73.
Gibbs-Russell GE, Ellis RP. 1987. Species
groups in the genus Ehrharta (Poaceae) in southern Africa. – Bothalia
17: 51-65.
Gibbs-Russell GE, Ellis RP. 1988. Taxonomy
and leaf anatomy of the genus Ehrharta (Poaceae) in southern Africa: the
Dura group. – Bothalia 18: 165-171.
Gibbs-Russell GE, Watson L, Koekemoer M,
Smook L, Barker NP, Anderson HM, Dallwitz MJ. 1990. Grasses of southern Africa.
– Mem. Bot. Surv. South Africa 58: 1-437.
Giles BE, Lefkovitch LP. 1986. A taxonomic
investigation of the Hordeum murinum complex (Poaceae). – Plant Syst. Evol. 153:
181-187.
Gilg E. 1891. Beiträge zur vergleichenden
Anatomie der xerophilen Familie der Restionaceae. – Engl. Bot. Jahrb.
Syst. 13: 541-606.
Gilg-Benedict C. 1930a. Restionaceae. – In: Engler A (ed),
Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 15a, W. Engelmann, Leipzig,
pp. 8-27.
Gilg-Benedict C. 1930b. Centrolepidaceae. – In: Engler A
(ed), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 15a, W. Engelmann,
Leipzig, pp. 27-33.
Gillespie LJ, Soreng RJ. 2005. A phylogenetic
analysis of the bluegrass genus Poa based on cpDNA restriction site
data. – Syst. Bot. 30: 84-105.
Gillespie LJ, Archambault A, Soreng RJ. 2007
[2008]. Phylogeny of Poa (Poaceae) based on
trnT-trnF sequence data: major clades and basal
realtionships. – In: Columbus JT, Friar EA, Porter JM, Prince LM, Simpson MG
(eds), Monocots: comparative biology and evolution. Poales, Rancho Santa Ana
Botanical Garden, Claremont, California. – Aliso 23: 420-434.
Gillespie LJ, Soreng RJ, Bull, RD, Jacobs
SWL, Refulio-Rodriguez NF. 2008. Phylogenetic relationships in subtribe Poinae
(Poaceae, Poeae) based on nuclear ITS
and plastid trnT-trnF sequences. – Botany 86: 938-967.
Gillespie LJ, Soreng RJ, Jacobs SWL. 2009.
Phylogenetic relationships of Australian Poa (Poaceae: Poinae), including molecular
evidence for two new genera, Saxipoa and Sylvipoa. – Aust.
Syst. Bot. 22: 413-436.
Gillespie LJ, Soreng RJ, Paradis M, Bull RD.
2010. Phylogeny and reticulation in subtribe Poinae and related subtribes (Poaceae) based on nrITS, ETS, and
trnTLF data. – In: Seberg O, Petersen G, Barfod AS, Davis JI (eds),
Diversity, phylogeny, and evolution in the monocotyledons, Aarhus University
Press, Århus, pp. 589-617.
Gilliland HB. 1971. Grasses of Malaya. –
In: Burkill HM (ed), A revised flora of Malaya 3, Government Printing Office,
Singapore.
Gilly CL. 1941a. The genus
Everardia. – Bull. Torrey Bot. Club 68: 20-31.
Gilly CL. 1941b. A new cyperaceous genus from
Northern South America. – Bull. Torrey Bot. Club 68: 330-332.
Gilly CL. 1942. The genus
Cephalocarpus Nees (Cyperaceae). – Bull. Torrey Bot. Club
69: 290-297.
Gilly CL. 1943. An Afro-South-American
cyperaceous complex. – Brittonia 5: 1-20.
Gilly CL. 1952. Phylogenetic development of
the inflorescence and generic relationships in the Kobresiaceae. – Iowa State
College J. Sci. 26: 210-212.
Gilmartin AJ. 1968. Taxonomic notes on
Ecuadorian Bromeliaceae. –
Phytologia 16: 153-167.
Gilmartin AJ. 1972. Bromeliaceae of Ecuador. – Monogr.
Phanerog. 4, J. Cramer, Lehre.
Gilmartin AJ. 1981. Morphological variation
within five angiosperm families: Asclepiadaceae, Bromeliaceae, Melastomataceae,
Piperaceae, and Rubiaceae. – Syst.
Bot. 6: 331-345.
Gilmartin AJ. 1983. Evolution of mesic and
xeric habits in Tillandsia ad Vriesea (Bromeliaceae). – Syst. Bot. 8:
233-242.
Gilmartin AJ, Brown GK. 1986a. Cladistic
tests of hypotheses concerning the evolution of xerophytes and mesophytes in
Tillandsia subg. Phytarrhiza (Bromeliaceae). – Amer. J. Bot. 73:
387-397.
Gilmartin AJ, Brown GK. 1986b.
Glomeropitcairnia, an enigmatic tillandsioid genus. – J. Bromeliad
Soc. 36: 104-106.
Gilmartin AJ, Brown GK. 1987. Bromeliales,
related monocots, and resolution of relationships among Bromeliaceae subfamilies. – Syst.
Bot. 12: 493-500.
Gilmartin AJ, Brown GK, Varadarajan GS,
Neighbours M. 1989. Status of Glomeropitcairnia within evolutionary
history of Bromeliaceae. – Syst.
Bot. 14: 339-348.
Gilmour CN, Starr JR, Naczi RFC. 2013.
Calliscirpus, a new genus for two narrow endemics of the California
Floristic Province, C. criniger and C. brachythrix sp. nov.
(Cyperaceae). – Kew Bull. 68:
85-105.
Giraldo-Cañas D. 2005. Las especies
colombianas del género Digitaria (Poaceae: Panicoideae: Paniceae). –
Caldasia 27: 25-87.
Gitaí J, Horres R, Benko-Iseppon AM. 2005.
Chromosomal features and evolution of Bromeliaceae. – Plant Syst. Evol.
253: 65-80.
Giulietti AM. 1978. Modificações
taxonômicos no gênero Eriocaulon L. – Bol. Bot. Univ. São Paulo
6: 39-47.
Giulietti AM. 1984. Estudos taxonômicos no
gênero Leiothrix Ruhl. – These de Livre docencia, Universidad de
São Paulo, Brazil.
Giulietti AM, Hensold NC. 1990. Padrões de
distribuição geográfica dos gêneros de Eriocaulaceae. – Acta Bot.
Brasilica 4: 133-158.
Giulietti AM, Hensold NC. 1991a.
Nomenclatural changes and range extension in Leiothrix flavescens
(Bong.) Ruhl. (Eriocaulaceae). –
Novon 1: 45-49.
Giulietti AM, Hensold NC. 1991b.
Synonymization of the genera Comanthera and Carptotepala with
Syngonanthus (Eriocaulaceae). – Ann. Missouri
Bot. Gard. 78: 460-464.
Giulietti AM, Meikle RD. 1982. A problematic
transatlantic disjunction in Paepalanthus (Eriocaulaceae). – Kew Bull. 37:
291-293.
Giulietti AM, Monteiro WR, Mayo SJ, Stephens
J. 1984. A preliminary survey of testa sculpture in Eriocaulaceae. – Beitr. Biol.
Pflanzen 62: 189-209.
Giulietti AM, Amaral MCE, Bittrich V. 1995.
Phylogenetic analysis of inter- and infrageneric relationships of
Leiothrix Ruhland (Eriocaulaceae). – Kew Bull. 50:
55-71.
Giulietti AM, Scatena VL, Sano PT, Parra LR,
de Queiroz LP, Harley RM, Menezes NL, Ysepon AMB., Salatino A, Satino ML,
Vilegas W, Santos LC, Ricci CV, Bonfim MCP, Miranda EB. 2000. Multidisciplinary
studies on neotropical Eriocaulaceae. – In: Wilson KL,
Morrison DA (eds), Monocots: systematics and evolution, Proceedings of the
2nd International Conference on the Comparative Biology of
Monocotyledons, Sydney, Australia, Vol. 2, CSIRO Publ., Melbourne, pp.
580-589.
Giulietti AM, Hensold N, Parra LR, Gomes de
Andrade MJ, Berg C van den, Harley RM. 2012. The synonymization of
Philodice with Syngonanthus (Eriocaulaceae). – Phytotaxa 60:
50-56.
Giussani LM, Cota-Sánchez JH, Zuloaga FO,
Kellogg EA. 2001. A molecular phylogeny of the grass subfamily Panicoideae (Poaceae) shows multiple origins of
C4 photosynthesis. – Amer. J. Bot. 88: 1993-2012.
Giussani LM, Zuloaga FO, Quarín CL,
Cota-Sánchez JG, Ubayasena K, Morrone O. 2009. Phylogenetic relationships in
the genus Paspalum (Poaceae:
Panicoideae: Paniceae): an assessment of the Quadrifaria and
Virgata informal groups. – Syst. Bot. 34: 32-43.
Givnish TJ, Burkhardt EL, Happel R, Weintraub
J. 1984. Carnivory in the bromeliad Brocchinia reducta, with a
cost/benefit model for the general restriction of carnivorous plants to sunny,
moist, nutrient-poor habitats. – Amer. Natur. 124: 479-497.
Givnish TJ, Sytsma KJ, Smith JF, Hahn WJ,
Benzing DH, Burkhardt EM. 1997. Molecular evidence and adaptive radiation in
Brocchinia (Bromeliaceae:
Pitcairnioideae) atop tepuis of the Guayana Shield. – In: Givnish TJ, Sytsma
KJ (eds), Molecular evolution and adaptive radiation, Cambridge University
Press, New York, pp. 259-311.
Givnish TJ, Evans TM, Zjhra ML, Patterson TB,
Berry PE, Sytsma KJ. 2000. Molecular evolution, adaptive radiation, and
geographic diversification in the amphiatlantic family Rapateaceae: evidence from
ndhF and morphology. – Evolution 54: 1915-1937.
Givnish TJ, Milliam KC, Evans TM, Hall JC,
Pires JC, Berry PE, Sytsma KJ. 2004. Ancient vicariance or recent long-distance
dispersal? Inferences about phylogeny and South American-African disjunctions
in Rapateaceae and Bromeliaceae based on ndhF
sequence data. – Intern. J. Plant Sci. 165(Suppl.): S35-S54.
Givnsh TJ, Milliam KC, Berry PE, Sytsma KJ.
2007. Phylogeny, adaptive radiation, and historical biogeography of Bromeliaceae inferred from
ndhF sequence data. – Aliso 23: 3-26.
Givnish TJ, Pires JC, Graham SW, McPherson
MA, Prince LM, Patterson TB. 2007 [2008]. Phylogeny, biogeography, and
ecological evolution in Bromeliaceae: insights from
ndhF sequences. – In: Columbus JT, Friar EA, Porter JM, Prince LM,
Simpson MG (eds), Monocots: comparative biology and evolution. Poales, Rancho
Santa Ana Botanical Garden, Claremont, California. – Aliso 23: 3-26.
Givnish TJ, Leebens-Mack JH, Ames Sevillano
M, McNeal JR, Steele PR, Davis JI, Ané C, Soltis DE, Soltis PS. 2010.
Assembling the tree of the monocotyledons: plastome sequence phylogeny and
evolution of Poales. – Ann. Missouri Bot. Gard. 97: 584-616.
Givnish TJ, Barfuss MHJ, Van Ee B, Riina R,
Schulte K, Horres R, Gonsiska PA, Jabaily RS, Crayn DM, Smith JAC, Winter K,
Brown GK, Evans TM, Holst BK, Luther H, Till W, Zizka G, Berry PE, Sytsma KJ.
2011. Phylogeny, adaptive radiation, and historical biogeography in Bromeliaceae: insights from an
eight-locus plastid phylogeny. – Amer. J. Bot. 98: 872-895.
Givnish TJ, Barfuss MHJ, Ee BV, Riina R,
Schulte K, Horres R, Gonsiska PA, Jabaily RS, Crayn DM, Smith JAC, Winter K,
Brown GK, Evans TM, Holst BK, Luther H, Till W, Zizka G, Berry PE, Sytsma KJ.
2014. Adaptive radiation, correlated and contingent evolution, and net species
diversification in Bromeliaceae.
– Molec. Phylogen. Evol. 71: 55-78.
Glémin S, Bataillon T. 2009. A comparative
review of the evolution of grasses under domestication. – New Phytol. 183:
273-290.
Global Carex Group. 2015. Making
Carex monophyletic (Cyperaceae, tribe Cariceae): a new
broader circumscription. – Bot. J. Linn. Soc. 179: 1-42.
Global Carex Group. 2016. Megaphylogenetic
specimen-level approaches to the Carex (Cyperaceae) phylogeny using ITS, ETS,
and matK sequences: implications for classification. – Syst. Bot. 41:
500-518.
Glon HE, Shiels DR, Linton E, Starr JR,
Shorkey AL, Fleming S, Lichtenwald SK, Schick ER, Pozo D, Monfils AK. 2017. A
five gene phylogenetic study of Fuireneae (Cyperaceae) with a revision of
Isolepis humillima. – Syst. Bot. 42: 26-36.
Goebel K. 1895. Ein Beitrag zur Morphologie
der Gräser. – Flora 81: 17-29.
Goetghebeur P. 1977. Studies in Cyperaceae 1. Taxonomic notes on
Ascolepis and Marisculus, a new genus of the tribe Cypereae.
– Bull. Jard. Bot. Nat. Belgique 47: 435-447.
Goetghebeur P. 1980. Studies in Cyperaceae 2. Contribution towards a
revision of the mainly African genus Ascolepis Nees ex Steudel. –
Adansonia sér. 2, 19: 269-305.
Goetghebeur P. 1985. Studies in Cyperaceae 6. Nomenclature of the
suprageneric taxa in the Cyperaceae.
– Taxon 34: 617-632.
Goetghebeur P. 1986. Genera Cyperacearum. Een
bijdrage tot de kennis van de morfologie, systematiek en fylogenese van de Cyperaceae-genera. – Ph.D. diss.,
Rijksuniversiteit Gent, Belgium.
Goetghebeur P. 1989. Studies in Cyperaceae 9. Problems in the
lectotypification and infrageneric taxonomy of Cyperus L. – Bull.
Soc. Roy. Bot. Belg. 122: 103-114.
Goetghebeur P. 1998. Cyperaceae. – In: Kubitzki K (ed), The
families and genera of vascular plants IV. Flowering plants. Monocotyledons.
Alismatanae and Commelinanae (except Gramineae), Springer, Berlin, Heidelberg,
New York, pp. 141-190.
Goetghebeur P, Borre A van den. 1989. Studies
in Cyperaceae 8. A revision of
Lipocarpha, including Hemicarpha and Rikliella. –
Wageningen Agric. Univ. Papers 89-1: 1-87.
Goetghebeur P, Coudijzer J. 1984. Studies in
Cyperaceae 3. Fimbristylis
and Abildgaardia in Central Africa. – Bull. Jard. Bot. Natl. Belg.
54: 65-89.
Goetghebeur P, Coudijzer J. 1985. Studies in
Cyperaceae 5. The genus
Bulbostylis in Central Africa. – Bull. Jard. Bot. Natl. Belg. 55:
236-238.
Goetghebeur P, Simpson DA. 1991. Critical
notes on Actinoscirpus, Bolboschoenus, Isolepis,
Phylloscirpus and Amphiscirpus (Cyperaceae). – Kew Bull. 46:
169-178.
Goetghebeur P, Vorster P. 1988. Studies in Cyperaceae 7. The genus Alinula
J. Raynal: a reappraisal. – Bull. Jard. Bot. Nat. Belgique 58: 457-465.
Goetze M, Schulte K, Palma-Silva C, Zanella
CM, Büttow MV, Capra F, Bered F. 2016. Diversification of Bromelioideae (Bromeliaceae) in the Brazilian
Atlantic rainforest: a case study in Aechmea subgenus
Ortgiesia. – Mol. Phylogen. Evol. 98: 346-357.
Goh WL, Chandran S, Lin R-S, Xia N-H, Wong
KM. 2010. Phylogenetic relationships among Southeast Asian climbing bamboos (Poaceae: Bambusoideae) and the
Bambusa complex. – Mol. Phylogen. Evol. 38: 764-773.
Goh WL, Chandran S, Franklin DC, Isagi Y,
Koshy KC, Sungkaew S, Yang HQ, Xia NH, Wong KM. 2013. Multigene region
phylogenetic analyses suggest reticulate evolution and a clade of Australian
origin among paleotropical woody bamboos (Poaceae: Bambusoideae: Bambuseae). –
Plant Syst. Evol. 299: 239-257.
Golovnina KA, Glushkov SA, Blinov AG, Mayorov
VI, Adkison LR, Goncharov NP. 2007. Molecular phylogeny of the genus
Triticum L. – Plant Syst. Evol. 264: 195-216.
Gómez P LD. 1972. Karatophyllum
bromelioides L. D. Gómez (Bromeliaceae), nov. gen. et sp., del
terciário medio de Costa Rica. – Rev. Biol. Trop. 20: 221-229.
Gomes-da-Silva J, da Costa AF. 2013. An
updated overview of taxonomy and phylogenetic history of Tillandsioideae genera
(Bromeliaceae: Poales). – Global
J. Bot. Sci. 1: 1-8.
Gomes-da-Silva J, Souza-Chies TT. 2018. What
actually is Vriesea? A total evidence approach in a polyphyletic genus
of Tillandsioideae (Bromeliaceae,
Poales). – Cladistics 34: 181-199.
Gomes-da-Silva J, da Costa Vargens FA, de
Oliveira Arruda R do C, da Costa AF. 2012. A morphological cladistic analysis
of the Vriesea corcovadensis group (Bromeliaceae: Tillandsioideae), with
anatomical descriptions: new evidence of the non-monophyly of the genus. –
Syst. Bot. 37: 641-654.
Gómez-Martínez R. 1998. A systematic study
of the grass tribe Paniceae with special emphasis on the genus
Axonopus. – Ph.D. diss., University of Reading, England.
Gómez-Martínez R, Culham A. 2000. Phylogeny
of the subfamily Panicoideae with emphasis on the tribe Paniceae: evidence from
the trnL-F cpDNA region. – In: Jacobs SWL, Everett J (eds), Grasses:
systematics and evolution, Proceedings of the 2nd International
Conference on the Comparative Biology of Monocotyledons, Sydney, Australia,
Vol. 2, CSIRO Publ., Melbourne, Australia, pp. 136-140.
Gómez-Sánchez M, Koch SD. 1998. Estudio
anatómico comparativo de la lámina foliar de Eragrostis (Poaceae: Chlorioideae) de México. – Acta
Bot. Mexicana 43: 33-56.
Gómez-Sánchez M, Dávila-Aranda P,
Valdés-Reyna J. 2001. Estudio anatómico de Swallenia (Poaceae: Eragrostideae: Monanthochlinae),
un género monotípico de Norte América. – Madroño 48: 152-161.
Goncharov NP. 2011. The genus
Triticum L. taxonomy: the present and the future. – Plant Syst.
Evol. 295: 1-11.
González-Elizondo MS, Peterson PM. 1997. A
classification of and key to the supraspecific taxa in Eleocharis (Cyperaceae). – Taxon 46: 433-449.
González-Elizondo MS, Reznicek AA. 1996. New
Eleocharis (Cyperaceae) from
Venezuela. – Novon 6: 356-365.
González-Elizondo MS, Tena-Flores JA,
Alarcón-Herrera MT, Flores-Tavizón E, Barajas-Acosta N. 2005. An
arsenic-tolerant new species of Eleocharis (Cyperaceae) from Chihuahua, Mexico. –
Brittonia 57: 150-154.
Gonzalo R, Aedo C, Nickrent DL, García MA.
2012. A numerical taxonomic investigation of Stipa Sect.
Smirnovia and S. Sect. Subsmirnovia (Poaceae). – Syst. Bot. 37: 655-670.
Gonzalo R, Aedo C, García MA. 2013.
Taxonomic revision of the Eurasian Stipa Subsections Stipa
and Tirsae (Poaceae). – Syst.
Bot. 38: 344-378.
Goossens AP. 1938. A study of the South
African species of Sporobolus R. Br. with special reference to leaf
anatomy. – Trans. Roy. Soc. South Afr. 26: 173-223.
Gordon-Gray KD. 1968. Studies in Cyperaceae in Southern Africa V. – J.
South Afr. Bot. 34: 371-396.
Gordon-Gray KD. 1971. Fimbristylis
and Bulbostylis: generic limits as seen by a student of Southern
African species. – Mitt. Bot. Staatssamml. München 10: 549-574.
Gordon-Gray KD. 1995. Cyperaceae in Natal. – Strelitzia 2:
1-218.
Gorenflot R. 1976. Le complexe polyploïde du
Phragmites australis (Cav.) Trin. ex. Steud. (P. communis
Trin.). – Bull. Soc. Bot. France 123: 261-267.
Gosavi KVC, Yadav SR, Karanth KP, Surveswaran
S. 2016. Molecular phylogeny of Glyphochloa (Poaceae, Panicoideae), an endemic grass
genus fom the Western Ghats, India. – J. Syst. Evol. 54: 162-174.
Gould FW. 1960. Chromosome numbers in
southwestern grasses. – Amer. J. Bot. 47: 873-877.
Gould FW. 1968a. Chromosome numbers of Texas
grasses. – Can. J. Bot. 46: 1315-1325.
Gould FW. 1968b. Grass systematics. – New
York.
Gould FW. 1975. The grasses of Texas. –
Texas A & M University Press, College Station, Texas.
Gould FW. 1979. The genus Bouteloua
(Poaceae). – Ann. Missouri Bot. Gard.
66: 348-416.
Gould FW, Shaw RB. 1983. Grass systematics.
2nd ed. – Texas A & M University Press, College Station,
Texas.
Gould FW, Soderstrom TR. 1967. Chromosome
numbers of tropical American grasses. – Amer. J. Bot. 54: 676-683.
Govaerts R. 2006. World checklist of Eriocaulaceae. – The Board of
Trustees of the Royal Botanic Gardens, Kew. http://www.kew.org/wcsp/, accessed
16 October 2009.
Govaerts R, Simpson DA, Goetghebeur P, Wilson
KL, Egorova T, Bruhl J. 2007. World checklist of Cyperaceae. – The Board of Trustees of
the Royal Botanic Gardens, Kew. http://www.kew.org/wcsp/, accessed 16 October
2009.
Govaerts R, Simpson DA, Goetghebeur P, Wilson
KL, Egorova T, Bruhl J. 2012. World Checklist of Cyperaceae. – The Board of Trustees of
the Royal Botanic Gardens, Kew. http://www.kew.org/wcsp/monocots/, accessed 1
March 2012.
Govaerts R, Jiménez-Mejías P, Koopman J,
Simpson D, Goetghebeur P, Wilson K, Egorova T, Bruhl J. 2015. World Checklist
of Cyperaceae. – The Board of
Trustees of the Royal Botanic Gardens, Kew. http://apps.kew.org/wcsp/,
retrieved Dec 2015.
Govil GM, Lavania S. 1982. Floral vasculature
of Eriocaulon L. – J. Indian Bot. Soc. 61: 371-376.
Govindappa DA. 1953. The embryo sac of
Xyris pauciflora Willd. – Curr. Sci. 22: 386.
Govindappa DA. 1955. Embryological studies in
Xyris pauciflora Willd. – Proc. Indian Acad. Sci., Sect. B, 42:
47-57.
Govindappa DA (Arekal GD), Ramaswamy SN.
1980. Embryology of Eriocaulon hookerianum Stapf and the systematic
position of Eriocaulaceae. –
Bot. Not. 133: 295-309.
Govindarajalu E. 1975. Studies in Cyperaceae 14. Endomorphic evidences for
placing Cyperus hyalinus under the new subgenus
Queenslandiella. – Reinwardtia 9: 187-195.
Graef PE. 1955. Ovule and embryo sac
development in Typha latifolia. – Amer. J. Bot. 42: 806-809.
Gram K. 1961. The inflorescence of the
grasses. – Bot. Tidsskr. 56: 293-313.
Grande Allende JR. 2014. Novitates
agrostologicae, IV. Additional segregates from Panicum incertae sedis.
– Phytoneuron 2014-22: 1-6.
Grant JR. 1993a. New combinations in
Mezobromelia and Racinaea (Bromeliaceae: Tillandsioideae). –
Phytologia 74: 428-430.
Grant JR. 1993b. True tillandsias misplaced
in Vriesea (Bromeliaceae:
Tillandsioideae). – Phytologia 75: 170-175.
Grant JR. 1994a. The reduction of
Platyaechmea under Hoplophytum, and a new name in
Tillandsia (Bromeliaceae).
– Phytologia 77: 99-101.
Grant JR. 1994b. Three new species of
Racinaea (Tillandsioideae: Bromeliaceae) from Ecuador and Peru.
– Phytologia 76: 284-289.
Grant JR. 1995a. Bromelienstudien. Addendum
to ‘the resurrection of Alcantarea and Werauhia, a new
genus’. – Phytologia 78: 119-123.
Grant JR. 1995b. New combinations and new
taxa in the Bromeliaceae. –
Phytologia 79: 254-256.
Grant JR, Till W. 2005. Proposal to reject
the name Dendropogon (Bromeliaceae: Tillandsioideae). –
Taxon 54: 549.
Grant JR, Zijlstra G. 1998. An annotated
catalogue of the generic names of the Bromeliaceae. – Selbyana 19:
91-121.
Grass Phylogeny Working Group (GPWG). 2000. A
phylogeny of the grass family (Poaceae),
as inferred from eight character sets. – In: Jacobs SWL, Everett J (eds),
Grasses: systematics and evolution, Proceedings of the 2nd
International Conference on the Comparative Biology of Monocotyledons, Sydney,
Australia, Vol. 2, CSIRO Publ., Melbourne, Australia, pp. 3-7.
Grass Phylogeny Working Group (GPWG). 2001.
Phylogeny and subfamilial classification of the grasses (Poaceae). – Ann. Missouri Bot. Gard. 88:
373-457.
Grass Phylogeny Working Group (GPWG). 2011.
New grass phylogeny resolves deep evolutionary relationships and discovers
C4 origins. – New Phytol. 193: 304-312.
Grassl CO. 1972. Taxonomy of
Saccharum relatives: Sclerostachya, Narenga, and
Erianthus. – Proceedings of 14th Congress, International Society of
Sugar Cane Technologists (ISSCT): 240-248.
Grebenstein B, Grebenstein O, Sauer W,
Hemleben V. 1996. Distribution and complex organization of satellite DNA
sequences in Aveneae species. – Genome 39: 1045-1050.
Grebenstein B, Röser M, Sauer W, Hemleben V.
1998. Molecular phylogenetic relationships in Aveneae (Poaceae) species and other grasses as
inferred from ITS1 and ITS2 rDNA sequences. – Plant Syst. Evol. 213:
233-250.
Greuning JV van, Schijff HP van der. 1973.
Chlorenchyma and sclerenchyma in the culms of Willdenowia Thunb. and
Hypodiscus Nees. – Ber. Deutsch. Bot. Ges. 86: 537-550.
Greuning JV van, Schijff HP van der. 1974.
External morphology of the genera Hypodiscus Nees and
Willdenowia Thunb. and key to genera and species based on external
morphological properties. – Kirkia 9: 331-347.
Griffin PC, Robin C, Hoffmann AA. 2011. A
next-generation sequencing method for overcoming the multiple gene copy problem
in polyploidy phylogenetics, applied to Poa grasses. – BMC Biology
9: 19.
Grob A. 1896. Beiträge zur Anatomie der
Epidermis der Gramineenblätter. – Bibl. Bot. 36: 1-122.
Groß E. 1988a. Bromelienstudien IV. Zur
Morphologie der Bromeliaceen-Samen unter Berücksichtigung
systematisch-taxonomischer Aspekte. – Trop. Subtrop. Pflanzenwelt 64:
415-625.
Groß E. 1988b. Über die Keimung der
Bromeliaceen. – Beitr. Biol. Pflanzen 63: 101-113.
Guaglianone ER. 1970. Un nuevo carácter,
útil en la distinción genérica entre Fimbristylis Vahl y
Bulbostylis Kunth (Cyperaceae). – Darwiniana 16:
40-48.
Guaglianone ER. 1979. Sobre Rhynchospora
rugosa (Vahl) Gale (Cyperaceae)
y algunas especies afines. – Darwiniana 22: 155-311.
Guaglianone ER, Ueno O. 1991. A disjunct
species in Eleocharis (Cyperaceae). – Darwiniana 30:
223-229.
Guala II GF. 1986. The relation of space and
geography to cladogenic events in Agenium and Homozeugos (Poaceae: Andropogoneae) in South America
and Africa. – In: Soderstrom TR, Hilu KW, Campbell CS, Barkworth ME (eds),
Grass systematics and evolution, Smithsonian Institution Press, Washington,
D.C., pp. 159-166.
Guala II GF. 1995a. Andropogon
crispifolius (Poaceae:
Andropogoneae): a new species from the cerrado of central Brazil. – Nord. J.
Bot. 15: 59-62.
Guala II GF. 1995b. A cladistic analysis and
revision of the genus Apoclada (Poaceae: Bambusoideae: Bambusodae). –
Syst. Bot. 20: 207-223.
Guala II GF. 2003. A new genus of bamboo from
Cerrados of Brazil (Poaceae:
Bambusoideae). – Bamboo Sci. Cult. 17: 1-3.
Guala II GF, Bogler D, Sadle J,
Francisco-Ortega J. 2000. Molecular evidence for polyphyly in the genus
Apoclada (Poaceae:
Bambusoideae). – Bamboo Sci. Cult. 14: 15-20.
Guarise NJ, Vegetti AC. 2008. The
inflorescences structure of Cyperus L. section Luzuloidei
Kunth (Cyperaceae). – Plant Syst.
Evol. 271: 41-63.
Guarise NJ, Vegetti AC, Pozner R. 2012.
Multiple origins of congested inflorescences in Cyperus s.s. (Cyperaceae): developmental and
structural evidence. – Amer. J. Bot. 99: 1276-1288.
Guédès M. 1967. Stipules médianes et
stipules ligulaires chez quelques Liliacées, Joncacées et Cypéracées. –
Beitr. Biol. Pflanzen 43: 59-103.
Guerreiro C, Rodríguez MF, Rúgolo de
Agrasar ZE. 2013. Culm anatomy: a contribution to the identification of
vegetative Andean woody bamboos in southernmost America. – Kew Bull. 68:
209-218.
Guglieri A, Longhi-Wagner HM, Zuloaga FO.
2006. Panicum complanatum (Poaceae: Panicoideae: Paniceae), a new
species for southeastern Brazil. – Syst. Bot. 31: 506-511.
Guignard JL. 1961. Recherches sur
l’embryogénie des Graminées: rapports des Graminées avec les autres
Monocotylédones. – Ann. Sci. Nat. Bot., 12th ser., 2: 491-610.
Guisinger MM, Chumley TW, Kuehl JV, Boore JL,
Jansen RK. 2010. Implications of the plastid genome sequence of Typha
(Typhaceae, Poales) for understanding
genome evolution in Poaceae. – J. Mol.
Evol. 70: 149-166.
Guldahl AS, Borgen L, Nordal I. 2001.
Variation in the Festuca brachyphylla (Poaceae) complex in Svalbard, elucidated by
chromosome numbers and isozymes. – Bot. J. Linn. Soc. 137: 107-126.
Guo J-H, Skinner DZ, Liang G-H. 1996.
Phylogenetic relationships of sorghum taxa inferred from mitochondrial DNA
restriction fragment analysis. – Genome 39: 1027-1034.
Guo Y-L, Ge S. 2005. Molecular phylogeny of
Oryzeae (Poaceae) based on DNA sequences
from chloroplast, mitochondrial, and nuclear genomes. – Amer. J. Bot. 92:
1548-1558.
Guo Z-H, Li D-Z. 2004. Phylogenetics of the
Thamnocalamus group and its allies (Gramineae: Bambusoideae):
inference from the sequences of GBSSI gene and ITS spacer. – Mol.
Phylogen. Evol. 30: 1-12.
Guo Z-H, Chen Y-Y, Li D-Z, Yang J-B. 2001.
Genetic variation and evolution of the alpine bamboos (Poaceae: Bambusoideae) using DNA sequence
data. – J. Plant Res. 114: 315-322.
Guo Z-H, Chen Y-Y, Li D-Z. 2002. Phylogenetic
studies on Thamnocalamus group and its allies (Bambusoideae: Poaceae) based on ITS sequence data. –
Mol. Phylogen. Evol. 22: 20-30.
Gupta PK. 1963. Meiotic studies in some
members of the tribe Paniceae. – Curr. Sci. 32: 180-181.
Gupta PK, Singh RV. 1977. Variations in
chromosomes and flavonoids in Setaria Beauv. – Nucleus 20:
167-171.
Guterrez M, Edwards GE, Brown WV. 1976. PEP
carboxykinase containing species in the Brachiaria group of the
subfamily Panicoideae. – Biochem. Syst. Ecol. 4: 47-49.
Guzmán R, Anaya MC, Santana M. 1984. El
género Otatea (Bambusoideae), en México y Centroamérica. – Bol.
Inst. Bot. 5: 2-20.
Hackel E. 1882. Monographia Festucarum
Europaearum. – Kassel, Berlin.
Hackel E. 1887. Gramineae (echte Gräser).
– In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien II(2), W.
Engelmann, Leipzig, pp. 1-97; Nachträge zu II(2), pp. 39-47.
Hackel E. 1899. Enumeratio Graminum Japoniae.
Verzeichnis der Gräser Japans. – Bull. Herb. Boissier 7: 701-726.
Hackel E. 1901. Neue Gräser. – Österr.
Bot. Zeitschr. 51: 372-373.
Hadač E. 1961. The family Cyperaceae in Iraq. – Bull. Coll. Sci.
6: 1-27.
Haines RW. 1967. Prophylls and branching in
Cyperaceae. – J. East Afr. Nat.
Hist. Soc. 26: 51-70.
Haines RW. 1971. Amphicarpy in East African
Cyperaceae. – Mitt. Bot.
Staatssamml. München 10: 534-538.
Haines RW. 1983. The sedges and rushes of
East Africa. Appendix 3A. – Nairobi.
Haines RW, Lye KA. 1971. Studies in African
Cyperaceae IV. Lipocarpha R.
Br., Hemicarpha Nees and Isolepis R. Br. – Bot. Not. 124:
473-482.
Haines RW, Lye KA. 1972. Studies in African
Cyperaceae VII. Panicle morphology
and possible relationships in Sclerieae and Cariceae. – Bot. Not. 125:
331-343.
Haines RW, Lye KA. 1973. Studies in African
Cyperaceae IX. The morphology of
Coleochloa Gilly and Afrotrilepis J. Rayn. – Bot. Not. 126:
330-339.
Haines RW, Lye KA. 1976. Studies in African
Cyperaceae XIV. The genus
Hellmuthia Steud. – Bot. Not. 129: 61-67.
Haines RW, Lye KA. 1977. Studies in African
Cyperaceae XV. Amphicarpy and
spikelet structure in Trianoptiles solitaria. – Bot. Not. 130:
235-240.
Haines RW, Lye KA. 1978. Studies in African
Cyperaceae XVII.
Kyllingiella R. Haines and K. Lye, gen. nov. – Bot. Not. 131:
175-177.
Haines RW, Lye KA. 1983. The sedges and
rushes of East Africa. – East African Natural History Society, Nairobi.
Håkansson A. 1954. Meiosis and pollen
mitosis in X-rayed and untreated spikelets of Eleocharis palustris.
– Hereditas 40: 325-345.
Håkansson A. 1958. Holocentric chromosomes
in Eleocharis. – Hereditas 44: 531-540.
Halbritter H. 1988. Bromeliaceae: Pollenmorphologie und
Systematik. Die Entwicklung des Pollens von Tillandsia sinuosa L. B.
Smith. – Ph.D. diss., Universität Wien, Austria.
Halbritter H. 1992. Morphologie und
systematische Bedeutung des Pollens der Bromeliaceae. – Grana 31:
197-212.
Halbritter H, Till W. 1998. Pollen morphology
of Nidularium and related genera. – In: Leme EMC (ed), Canistropis
– bromeliads of the Atlantic forest, Salamandra Rio de Janeiro, pp.
112-121.
Halbritter H, Weber M, Hesse M. 2010. Unique
aperture stratification Carex (Cyperaceae) pollen. – Grana 49:
1-11.
Hall BM. 1955. Genetic analysis of
interspecific hybrids in the genus Bromus, section
Ceratochloa. – Genetics 40: 175-192.
Hall JB, Morton AJ, Hooper SS. 1976.
Application of principal component analyses with constant character number in a
study of the Bulbostylis/Fimbristylis (Cyperaceae) complex in Nigeria. – Bot.
J. Linn. Soc. 73: 333-354.
Hamann U. 1962. Beitrag zur Embryologie der
Centrolepidaceen mit Bemerkungen über den Bau der Blüten und Blütenstände
und die systematische Stellung der Familie. – Ber. Deutsch. Bot. Ges. 75:
153-171.
Hamann U. 1963. Über die Entwicklung und den
Bau des Spaltöffnungsapparates der Centrolepidaceen. – Bot. Jahrb. Syst. 82:
316-320.
Hamann U. 1975. Neue Untersuchungen zur
Embryologie und Systematik der Centrolepidaceen. – Bot. Jahrb. Syst. 96:
154-191.
Hamasha HR, Hagen KB von, Röser M. 2012.
Stipa (Poaceae) and allies in
the Old World: molecular phylogenetics realigns genus circumscription and gives
evidence on the origin of American and Australian lineages. – Plant Syst.
Evol. 298: 351-367.
Hamby RK, Zimmer EA. 1988. Ribosomal RNA
sequences for inferring phylogeny within the grass family (Poaceae). – Plant Syst. Evol. 160:
29-37.
Hamlin BG. 1959. A revision of the genus
Uncinia (Cyperaceae-Caricoideae) in New Zealand.
– Dominion Mus. Bull. (Wellington) 19.
Hammel BE, Reeder JR. 1979. The genus
Crypsis (Gramineae) in the United States. – Syst. Bot. 4:
267-280.
Hammer K. 1980. Zur Taxonomie und Nomenklatur
der Gattung Aegilops L. – Feddes Repert. 91: 225-258.
Hamoud MA, Haroun SA, Macleod RD, Richards
AJ. 1994. Cytological relationships of selected species of Panicum L. –
Biologia Plantarum 36: 37-45.
Hand ML, Cogan NO, Stewart AV, Forster JW.
2010. Evolutionary history of tall fescue morphotypes inferred from molecular
phylogenetics of the Lolium-Festuca species complex. – BMC Evol.
Biol. 10: 303.
Hansen DJ, Dayanandan P, Kaufman PB,
Brotherson JD. 1976. Ecological adaptations of salt marsh grass, Distichlis
spicata (Gramineae), and environmental factors affecting its growth and
distribution. – Amer. J. Bot. 63: 635-650.
Hansen I, Potztal E. 1954. Beiträge zur
Anatomie und Systematik der Leptureae. – Bot. Jahrb. Syst. 76: 251-270.
Harada I. 1947. Chromosome numbers in
Pandanus, Sparganium and Typha. – Cytologia 14:
214-218.
Harborne JB. 1979. Correlations between
flavonoid chemistry, anatomy and geography in the Restionaceae. – Phytochemistry 18:
1323-1327.
Harborne JB, Clifford HT. 1969. Flavonoid
patterns of the Restionaceae.
Gossypetin in Restio and a new flavone in Hypolaena. –
Phytochemistry 8: 2071-2075.
Harborne JB, Boardley M, Linder HP. 1985.
Variations in flavonoid patterns within the genus Chondropetalum (Restionaceae). – Phytochemistry 24:
273-278.
Harborne JB, Williams CA, Briggs BG, Johnson
LAS. 2000. Flavonoid patterns and the phylogeny of the Restionaceae. – In: Wilson KL,
Morrison DA (eds), Monocots: systematics and evolution, Proceedings of the
2nd International Conference on the Comparative Biology of
Monocotyledons, Sydney, Australia, Vol. 2, CSIRO Publ., Melbourne, pp.
672-675.
Hardion L, Verlaque R, Baumel A, Juin M, Vila
B. 2012. Revised systematics of Mediterranean Arundo (Poaceae) based on AFLP fingerprints and
morphology. – Taxon 61: 1217-1226.
Hardion L, Verlaque R, Haan-Archipoff G,
Cahen D, Hoff M, Vila B. 2017. Cleaning up the grasses dustbin: systematics of
the Arundinoideae subfamily (Poaceae).
– Plant Syst. Evol. 303: 1331-1339.
Hardion L, Peterson PM, Soreng RJ. 2018.
Validation of Pratochloa (Poaceae: Arundinoideae) and a new
combination, Pratochloa walteri, based on Eragrostis walteri.
– Phytoneuron 2018-31: 1-3.
Hardy CR, Linder HP. 2007 [2008]. Phylogeny
and historical ecology of Rhodocoma (Restionaceae) from the Cape floristic
region. – In: Columbus JT, Friar EA, Porter JM, Prince LM, Simpon MG (eds),
Monocots: comparative biology and evolution. Poales, Rancho Santa Ana Botanical
Garden, Claremont, California. – Aliso 23: 213-226.
Hardy CR, Moline P, Linder HP. 2008. A
phylogeny for the African Restionaceae and new perspectives on
morphology’s role in generating complete species phylogenies for large
clades. – Intern. J. Plant Sci. 169: 377-390.
Harlan JR, Brooks MH, Borgaonkar DS, Wet JMJ
de. 1964. Nature and inheritance of apomixis in Bothriochloa and
Dichanthium. – Bot. Gaz. 125: 41-46.
Harms H. 1930. Bromeliaceae. – In: Engler A (ed),
Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 15a, W. Engelmann, Leipzig,
pp. 65-159.
Harris PJ, Hartley RD. 1976. Detection of
bound ferulic acid in cell-walls of Gramineae by ultraviolet fluorescence
microscopy. – Nature 259: 508-510.
Hartley W. 1950. The global distribution of
tribes of the Gramineae in relation to historical and environmental factors.
– Aust. J. Agric. Res. 1: 355-373.
Hartley W. 1958. Studies on the origin,
evolution, and distribution of the Gramineae II. The tribe Paniceae. – Aust.
J. Bot. 6: 343-357.
Hartley W. 1964. The distribution of the
grasses. – In: Bernard C (ed), Grasses and grasslands, MacMillan, London, pp.
29-46.
Hartley W. 1973. Studies on the origin,
evolution, and distribution of the Gramineae V. The subfamily Festucoideae. –
Aust. J. Bot. 21: 201-234.
Hartley W, Slater C. 1960. Studies on the
origin, evolution, and distribution of the Gramineae III. The tribes of the
subfamily Eragrostoideae. – Aust. J. Bot. 8: 256-276.
Hartvig P. 1986. Chromosome numbers in Nordic
populations of the Carex muricata group (Cyperaceae). –Symb. Bot. Ups. 27(2)
127-138.
Harz CO. 1880-1882. Beiträge zur Systematik
der Gramineen. – Linnaea 43: 1-30.
Hatch SL, Haile KC. 2012. Checklist of Texas
grass species and a key to the genera. – Phytoneuron 2012-57: 1-60.
Hatfield RD, Marita JM, Frost K, Grabber J,
Ralph J, Lu F, Kim H. 2009. Grass lignin acylation:p-coumaroyl
transferase activity and cell wall characteristics of C3 and C4 grasses. –
Planta 229: 1253-1267.
Hattersley PW. 1984. Characterization of
C4 type leaf anatomy in grasses (Poaceae). Mesophyll: bundle sheath area
ratios. – Ann. Bot., N. S., 53: 163-179.
Hattersley PW. 1987. Variations in
photosynthetic pathway. – In: Soderstrom TR, Hilu KW, Campbell CS, Barkworth
ME (eds), Grass systematics and evolution, Smithsonian Institution Press,
Washington, D.C., pp. 49-64.
Hattersley PW, Browning AJ. 1981. Occurrence
of the suberized lamella in leaves of grasses of different photosynthetic types
I. In parenchymatous bundle sheaths and PCR (“Kranz”) sheaths. –
Protoplasma 109: 371-401.
Hattersley PW, Esen A. 1988. Prolamin size
diversity in the Poaceae. – Biochem.
Syst. Ecol. 16: 457-465.
Hattersley PW, Johnson JL. 1991. Chloroplast
DNA reassociation and grass phylogeny. – Plant Syst. Evol. 176: 21-31.
Hattersley PW, Watson L. 1976. C4
grasses: an anatomical criterion for distinguishing between NADP-malic enzyme
species and PCK or NAD-malic enzyme species. – Aust. J. Bot. 24: 297-308.
Hattersley PW, Watson L. 1992.
Diversification of photosynthesis. – In: Chapman GP (ed), Grass evolution and
domestication, Cambridge University Press, Cambridge, pp. 38-116.
Hattersley PW, Wright K. 1982. Systematics of
Gramineae: a cluster analysis study. – Taxon 31: 9-36.
Hattersley PW, Watson L, Johnston CR. 1982.
Remarkable leaf anatomical variations in Neurachne and its allies (Poaceae) in relation to C3 and C4
photosynthesis. – Bot. J. Linn. Soc. 84: 265-272.
Hawkins JS, Ramachandran D, Henderson A,
Freeman J, Carlise M, Harris A, Willison-Headley Z. 2015. Phylogenetic
reconstruction using four low-copy nuclear loci strongly supports a
polyphyletic origin of the genus Sorghum. – Ann. Bot. 116:
291-299.
Hayasaka E. 2012. Delineation of
Schoenoplectiella Lye (Cyperaceae), a genus newly segregated
from Schoenoplectus (Rchb.) Palla. – Fukui Bot. Gard. 186:
169-186.
Hayek A. 1925. Zur Systematik der Gramineen.
– Österr. Bot. Zeitschr. 74: 249-255.
Hedberg I. 1976. A cytotaxonomic
reconnaissance of tropical African Anthoxanthum L. (Gramineae). –
Bot. Not. 129: 85-90.
Hedberg O. 1952. Cytological studies in East
African mountain grasses. – Hereditas 38: 256-266.
Hedberg O. 1967. Chromosome numbers of
vascular plants from arctic and sub-arctic North America. – Ark. f. Bot.,
Ser. II, 6(6): 309-326.
Hedberg O, Hedberg I. 1994. The genus
Colpodium (Gramineae) in Africa. – Nord. J. Bot. 14: 601-607.
Heilborn O. 1918. Zur Embryologie und
Zytologie einiger Carex-Arten. – Svensk Bot. Tidskr. 12: 212-219.
Heilborn O. 1922. Die Chromosomenzahlen der
Gattung Carex. – Svensk Bot. Tidskr. 16: 271-274.
Heilborn O. 1924. Chromosome numbers, species
formation, and phylogeny in Carex. – Hereditas 5: 129-216.
Heilborn O. 1928. Chromosome studies in Cyperaceae. – Hereditas 11:
182-192.
Heilborn O. 1939. Chromosome studies in Cyperaceae III-IV. – Hereditas 25:
224-240.
Helfgott DM, Mason-Gamer RJ. 2004. The
evolution of North American Elymus (Triticeae, Poaceae) allotetraploids: evidence from
phosphoenolpyruvate carboxylase gene sequences. – Syst. Bot. 29: 850-861.
Heller S, Leme EMC, Schulte K, Benko-Iseppon
A, Zizka G. 2015. Elucidating phylogenetic relationships in the
Aechmea alliance: AFLP analysis of Portea and the
Gravisia complex (Bromeliaceae, Bromelioideae). –
Syst. Bot. 40: 716-725.
Henderson S, Schäfer H. 2003. Synopsis of
the genus Rostraria (Poaceae)
in the Azores. – Bot. J. Linn. Soc. 141: 125-131.
Hendrichs M, Oberwinkler F, Begerow D, Bauer
R. 2004. Carex, subgenus Carex (Cyperaceae) – a phylogenetic approach
using ITS sequence. – Plant Syst. Evol. 246: 89-107.
Hendrichs M, Michalski S, Begerow D,
Oberwinkler F, Hellwig FH. 2004. Phylogenetic relationships in Carex,
subgenus Vignea (Cyperaceae), based on ITS sequences. –
Plant Syst. Evol. 246: 109-125.
Henrard JT. 1926. A critical revision of the
genus Aristida I. – Meded. Rijks Herb. Leiden 54.
Henrard JT. 1927. A critical revision of the
genus Aristida II. – Meded. Rijks Herb. Leiden 54A.
Henrard JT. 1928. A critical revision of the
genus Aristida III. – Meded. Rijks Herb. Leiden 54B.
Henrard JT. 1929. A monograph of the genus
Aristida I. – Meded. Rijks Herb. Leiden 58.
Henrard JT. 1932a. A monograph of the genus
Aristida II. – Meded. Rijks Herb. Leiden 58A.
Henrard JT. 1932b. A monograph of the genus
Aristida III. – Meded. Rijks Herb. Leiden 58B.
Henrard JT. 1936. Chloothamnus, a
neglected genus of Bambusaceae. – Blumea 2: 60-73.
Henrard JT. 1940. Notes on the nomenclature
of some grasses. – Blumea 3: 411-480.
Henrard JT. 1950. Monograph of the genus
Digitaria. – Universitaire Pers Leuven, Leiden, Netherlands.
Hensold NC. 1988. Morphology and systematics
of Paepalanthus subgenus Xeractis (Eriocaulaceae). – Syst. Bot.
Monogr. 23: 1-150.
Hensold NC. 1991. Revisionary studies in Eriocaulaceae of Venezuela. – Ann.
Bissouri Bot. Gard. 78: 424-440.
Hensold NC. 2016. The Andean Paepalanthus
pilosus complex (Eriocaulaceae): a revision with three
new taxa. – PhytoKeys 64: 1-57.
Hensold NC, Giulietti AM. 1991. Revision and
redefinition of the genus Rondonanthus Herzog. – Ann. Missouri Bot.
Gard. 78: 441-459.
Henzen FA, Vegetti AC. 1994. Typology of the
inflorescences in Cyperus corymbosus var. subnodosus and
C. rotundus (Cyperaceae).
– Beitr. Biol. Pflanzen 68: 263-273.
Hermann FJ. 1970. Manual of the carices of
the Rocky Mountains and Colorado Basin. – U.S. Forest Service, Washington.
Hermann FJ. 1974. Manual of the genus
Carex in Mexico and Central America. – U. S. Department of
Agriculture. Agriculture Handbook 467: 1-219.
Herrera-Arrieta Y. 1998. A revision of the
Muhlenbergia montana (Nutt.) Hitchc. complex (Poaceae: Chloridoideae). – Brittonia 50:
23-50.
Herrera-Arrieta Y, Peterson PM, Cerda Lemus
M. 2004. Revisión de Bouteloua Lag. (Poaceae). – Instituto Politécnico
Nacional, Centro Interdisciplinario de Investigación para el Desarrollo
Integral Regional, Unidad Durango, Oaxaca, Mexico.
Hess H. 1955. Zur Kenntnis der Eriocaulaceae von Angola und dem
unteren Belgischen Kongo. – Ber. Schweiz. Bot. Ges. 65: 115-204.
Hieronymus G. 1873. Beiträge zur Kenntnis
der Centrolepidaceen. – Abhandl. Naturf. Ges. Halle 12: 115-222.
Hieronymus G. 1886. Über Blüte und
Blütenstand der Centrolepidaceen. – Engl. Bot. Jahrb. Syst. 7: 319-330.
Hieronymus G. 1888a. Restionaceae. – In: Engler A, Prantl
K (eds), Die natürlichen Pflanzenfamilien II(4), W. Engelmann, Leipzig, pp.
3-10.
Hieronymus G. 1888b. Centrolepidaceae. –
In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien II(4), W.
Engelmann, Leipzig, pp. 11-16.
Hieronymus G. 1888c. Eriocaulaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien II(4), W. Engelmann, Leipzig,
pp. 21-27.
Hilu KW. 1984. Leaf epidermes of
Andropogon sect. Leptopogon (Poaceae) in North America. – Syst. Bot.
9: 247-257.
Hilu KW. 1985. Trends of variation and
systematics of Poaceae. – Taxon 34:
102-114.
Hilu KW. 1986. Chloroplast DNA in the
systematics and evolution of the Poaceae. – In: Soderstrom TR, Hilu KW,
Campbell CS, Barkworth ME (eds), Grass systematics and evolution, Smithsonian
Institution Press, Washington, D.C., pp. 65-72.
Hilu KW. 1994. Evidence from RAPD markers in
the evolution of Echinochloa Millets (Poaceae). – Plant Syst. Evol. 189:
247-257.
Hilu KW. 2004. Phylogenetics and chromosomal
evolution in the Poaceae (grasses). –
Aust. J. Bot. 52: 13-22.
Hilu KW. 2007a. Skewed distribution of
species number in grass genera: is it a taxonomic artefact? – In: Hodkinson
TR, Parnell JAN (eds), Reconstructing the Tree of Life: taxonomy and
systematics of species rich taxa, Syst. Assoc. Spec. Vol. Ser. 72, CRC Press,
Boca Raton, Florida.
Hilu KW. 2007b. A century of progress in
grass systematics. – Kew Bull. 62: 355-373.
Hilu KW, Alice LA. 1999. Evolutionary
implications of matK indels in Poaceae. – Amer. J. Bot. 86:
1735-1741.
Hilu KW, Alice LA. 2000. Phylogenetic
relationships in subfamily Chloridoideae (Poaceae) based on matK sequences:
a preliminary assessment. – In: Jacobs SWL, Everett J (eds), Grasses:
systematics and Evolution, Proceedings of the 2nd International
Conference on the Comparative Biology of Monocotyledons, 1998, Sydney,
Australia, Vol. 2, CSIRO Publ., Collingwood, Victoria, pp. 173-179.
Hilu KW, Alice LA. 2001. A phylogeny of
Chloridoideae (Poaceae) based on
matK sequences. – Syst. Bot. 26: 386-405.
Hilu KW, Esen A. 1988. Prolamin size
diversity in the Poaceae. – Biochem.
Syst. Ecol. 16: 457-465.
Hilu KW, Esen A. 1990. Prolamins in
immunological Studies in the Poaceae I.
Subfamily Arundinoideae. – Plant Syst. Evol. 173: 57-70.
Hilu KW, Esen A. 1993. Prolamin and
immunological studies in the Poaceae
III. Subfamily Chloridoideae. – Amer. J. Bot. 80: 104-113.
Hilu KW, Johnson JL. 1991. Chloroplast DNA
reassociation and grass phylogeny. – Plant Syst. Evol. 176: 21-31.
Hilu KW, Randall JL. 1984. Convenient method
for studying grass leaf epidermis. – Taxon 33: 413-415.
Hilu KW, Wright K. 1982. Systematics of
Gramineae: a cluster analysis study. – Taxon 31: 9-36.
Hilu KW, Alice LA, Liang H. 1999. Phylogeny
of Poaceae inferred from matK
sequences. – Ann. Missouri Bot. Gard. 86: 835-851.
Hinchliff CE, Roalson EH. 2009. Stem
architecture in Eleocharis subgenus Limnochloa (Cyperaceae): evidence of dynamic
morphological evolution in a group of pantropical sedges. – Amer. J. Bot. 96:
1487-1499.
Hinchliff CE, Roalson EH. 2013. Using
supermatrices for phylogenetic inquiry: an example using the sedges. – Syst.
Biol. 62: 205-219.
Hinchliff CE, Lliully A, AE, Carey T, Roalson
EH. 2010. The origins of Eleocharis (Cyperaceae) and the status of
Websteria, Egleria, and Chillania. – Taxon 59:
709-719.
Hipp AL. 2007. Nonuniform processes of
chromosome evolution in sedges (Carex: Cyperaceae). – Evolution 61:
2175-2194.
Hipp AL, Rothrock PE, Roalson EH. 2009. The
evolution of chromosome arrangements in Carex (Cyperaceae). – Bot. Rev. 75:
96-109.
Hipp AL, Reznicek AA, Rothrock PE, Weber JA.
2006. Phylogeny and classification of Carex section Ovales
(Cyperaceae). – Intern. J. Plant
Sci. 167: 1029-1048.
Hirahara T, Katsuyama T, Hoshino T. 2007.
Suprageneric phylogeny of Japanese Cyperaceae based on DNA sequences from
chloroplast ndhF and 5.8 S nuclear ribosomal DNA. – Acta Phytotaxon.
Geobot. 58: 57-68.
Hiremath SC, Salimath SS. 1991. Quantitative
nuclear DNA changes in Eleusine (Gramineae). – Plant Syst. Evol.
178: 225-233.
Hitchcock AS. 1920. The genera of grasses of
the United States with special reference to the economic species. – Bull.
U.S.D.A. 772: 1-307.
Hitchcock AS. 1925a. The North American
species of Stipa. – Contr. U.S. Natl. Herb. 24: 215-262.
Hitchcock AS. 1925b. Synopsis of the South
American species of Stipa. – Contr. U.S. Natl. Herb. 24: 263-289.
Hitchcock AS. 1927. The grasses of Ecuador,
Peru and Bolivia. – Contr. U.S. Natl. Herb. 24: 291-556.
Hitchcock AS. 1935. Manual of the grasses of
the United States. – U.S.D.A. Misc. Publ. 200, Washington, D.C.
Hitchcock AS. 1936. Manual of the grasses of
the West Indies. – United States Government Printing Office, Washington,
D.C.
Hitchcock AS. 1951. Manual of the grasses of
the United States. 2nd ed. rev. by A. Chase. – U.S.D.A. Misc.
Publ. 200, Washington, D.C.
Hitchcock AS, Chase A. 1910. The North
American species of Panicum. – Contr. U.S. Natl. Herb. 15: 1-396.
Hitchcock AS, Chase A. 1915. Tropical North
American species of Panicum. – Contr. U.S. Natl. Herb. 17:
459-539.
Hitchcock AS, Chase A. 1950. Manual of the
grasses of the United States. 2nd ed. (rev. by A. Chase). –
U.S.D.A. Misc. Publ. 200, Washington, D.C.
Hochbach A, Schneider J, Röser M. 2015. A
multi-locus analysis of phylogenetic relationships within grass subfamily
Pooideae (Poaceae) inferred from
sequences of nuclear single copy gene regions compared with plastid DNA. –
Mol. Phylogen. Evol. 87: 14-27.
Hochbach A, Linder HP, Röser M. 2018.
Nuclear genes, matK and the phylogeny of the Poales. – Taxon 67:
521-536.
Hochuli PA. 1979. Ursprung und Verbreitung
der Restionaceen. – Vierteljahrsschr. Naturf. Ges. Zürich 124: 109-131.
Hodgon J, Bruhl JJ, Wilson KL. 2006.
Systematic studies in Lepidosperma (Cyperaceae: Schoeneae) with particular
reference to L. laterale. – Aust. Syst. Bot. 19: 273-288.
Hodkinson TR, Renvoize SA, Chonghaile GN,
Stapleton CMA, Chase MW. 2000. A comparison of ITS nuclear rDNA sequence data
and AFLP markers for phylogenetic studies in Phyllostachys
(Bambusoideae, Poaceae). – J. Plant
Res. 113: 259-269.
Hodkinson TR, Chase MW, Lledo MD, Salamin N,
Renvoize SA. 2002. Phylogenetics of Miscanthus, Saccharum and
related genera (Saccharinae, Andropogoneae, Poaceae) based on DNA sequences from ITS
nuclear ribosomal DNA and plastid trnL intron and trnL-F
intergenic spaces. – J. Plant Res. 115: 381-392.
Hodkinson TR, Savolainen V, Jacobs SWL,
Bouchenak-Khelladi Y, Kinney MS, Salamin N. 2007. Supersizing: progress in
documenting and understanding grass species richness. – In: Hodkinson TR,
Parnell JAN (eds), Reconstructing the Tree of Life: taxonomy and systematics of
species rich taxa, Syst. Assoc. Spec. Vol. Ser. 72, CRC Press, Boca Raton,
Florida.
Hodkinson TR, Salamin N, Chase MW,
Bouchenak-Khelladi Y, Renvoize SA, Savolainen V. 2007 [2008]. Large trees,
supertrees, and diversification of the grass family. – In: Columbus JT, Friar
EA, Porter JM, Prince LM, Simpson MG (eds), Monocots: comparative biology and
evolution. Poales, Rancho Santa Ana Botanical Garden, Claremont, California.
– Aliso 23: 248-258.
Hodkinson TR, Ni Chonghaile G, Sungkaew S,
Chase MW, Salamin N, Stapleton CMA. 2010. Phylogenetic analyses of plastid and
nuclear DNA sequences indicate a rapid late Miocene radiation of the temperate
bamboo tribe Arundinarieae (Poaceae,
Bambusoideae). – Plant Ecol. Divers. 3: 109-120.
Hoffmann MH, Gebauer S. 2016. Quantitative
morphological and molecular divergence in replicated and parallel radiations in
Carex (Cyperaceae) using
symbolic data analysis. – Syst. Bot. 41: 552-557.
Hoffmann MH, Schneider J, Hase P, Röser M.
2013. Rapid and recent world-wide diversification of bluegrasses (Poa,
Poaceae) and related genera. – PLoS
ONE 8:e60061. doi: 10.1371/journal.pone.0060061
Hohendorff U. 1981. Embryologische
Untersuchungen an Eriocaulaceen. – Thesis, Universität Bochum, Germany.
Hojsgaard D, Honfi AI, Rua G, Daviña J.
2009. Chromosome numbers and ploidy levels of Paspalum species from
subtropical South America (Poaceae). –
Genet. Res. Crop Evol. 56: 533-545.
Hollowell VC. 1987. Systematics of the
subtribe Parianinae (Poaceae:
Bambusoideae: Olyreae). – Ph.D. diss., University of South Carolina,
Columbia, South Carolina.
Hollowell VC. 1997. Systematic relationships
of Pariana and associated Neotropical taxa. – In: Chapman GP (ed),
The bamboos, Academic Press, London, pp. 45-60.
Holm T. 1891. A study of some anatomical
characters of North American Gramineae III. – Bot. Gaz. (Chicago) 16:
275-281.
Holm T. 1896a. Studies in the Cyperaceae I. On the monopodial
ramification in certain North American species of Carex. – Amer. J.
Sci. 1: 348-350.
Holm T. 1896b. Studies upon the Cyperaceae II. The clado- and
anthoprophyllon in the genus Carex. – Amer. J. Sci. 2: 214-220.
Holm T. 1899. Studies in the Cyperaceae IX. The genus
Lipocarpha. – Amer. J. Sci. 7: 171-183.
Holm T. 1900. Studies in the Cyperaceae XIII. Carex
willdenowii and its allies. – Amer. J. Sci. 10: 33-47.
Holm T. 1901. Some new anatomical characters
for certain Gramineae. – Beih. Bot. Centralbl. 11: 101-133.
Holm T. 1903. Studies in the Cyperaceae XX. “Greges Caricum”. –
Amer. J. Sci. 16: 445-464.
Holmen K. 1964. Cytotaxonomical studies in
the arctic Alaskan flora. The genus Festuca. – Bot. Not. 117:
109-118.
Holst BK. 1997. Bromeliaceae. – In: Steyermark JA,
Berry PE, Holst BK (eds), Flora of the Venezuelan Guayana 3, Timber Press,
Portland, Oregon, pp. 548-676.
Holttum RE. 1948. The spikelet in Cyperaceae. – Bot. Rev. 14:
525-541.
Holttum RE. 1955. The bamboo-genera
Nastus and Chloothamnus. – Kew Bull. 10: 591-594.
Holttum RE. 1956. The classification of the
bamboos. – Phytomorphology 6: 73-90.
Holttum RE. 1958. The bamboos of the Malay
Peninsula. – Gard. Bull. Singapore 16: 1-135.
Holub J. 1958. Bemerkungen zur Taxonomie der
Gattung Helictotrichon Bess. – In: Nemec B & al. (ed), Philip
Maximilian Opiz und seine Bedeutung für die Pflanzentaxonomie, Verlag der
Tschekoslowakischen Akademien der Wissenschaften, Praha, pp. 101-133.
Holub J. 1962. Ein Beitrag zur Abgenzung der
Gattungen in der Tribus Aveneae: die Gattung Avenochloa Holub. –
Acta Horti Bot. Prag. 1962: 75-86.
Holub J. 1976. A newly adopted restriction of
illegitimity in generic names and its consequence for Avenochloa Holub
1962. – Folia Geobot. Phytotaxon. 11: 281-300.
Holub J. 1977. Notes on some species of
Avenula and Helictotrichon. – Preslia 49: 203-221.
Honfi AI, Quarín CL, Valls JF. 1990 [1991].
Estudios cariológicos en gramíneas sudamericanas. – Darwiniana 30:
87-94.
Hooper SS. 1972. New taxa, names and
combinations in Cyperaceae for the
“Flora of West Tropical Africa”. – Kew Bull. 26: 577-583.
Hooper SS. 1983. Remirea or
Mariscus? – New support for a monotypic genus in Cyperaceae. – Kew Bull. 38:
479-480.
Hooper SS. 1984. Two montane forest species
of Carex in Yemen and Northeast Africa: new distributional records and
a new variety. – Kew Bull. 39: 747-751.
Hooper SS. 1985. Notes on tropical African
sedges: Mariscus, Schoenoplectus & Pycreus. –
Kew Bull. 40: 781-784.
Hooper SS. 1986a. A concept of the cyperoid
spikelet and its terminology, illustrated by new species of Lipocarpha
(Cyperaceae). – Kew Bull. 41:
423-428.
Hooper SS. 1986b. Volkiella (Cyperaceae), a specialised cyperoid
genus re-examined. – Kew Bull. 41: 945-948.
Hooper SS, Raynal J. 1969. New species and
names in African Pycreus P. Beauv. (Cyperaceae). – Kew Bull. 23:
313-314.
Hooper SS, Simpson DA. 1997. A new species of
Scirpodendron (Cyperaceae)
with a discussion of the inflorescence in the genus. – Kew Bull. 52:
683-687.
Hoover RF. 1941. The genus Orcuttia.
– Bull. Torrey Bot. Club 68: 149-156.
Horn SP, Clark LG. 1992. Pollen viability in
Chusquea subtessellata (Poaceae: Bambusoideae). – Biotropica 24:
577-579.
Horn af Rantzien H. 1946. Notes on the Mayacaceae of the Regnellian Herbarium
in the Riksmuseum, Stockholm. – Svensk Bot. Tidskr. 40: 405-424.
Hornung-Leoni CT, Sosa V. 2005. Morphological
variation in Puya (Bromeliaceae): an allometric study.
– Plant Syst. Evol. 256: 35-53.
Hornung-Leoni CT, Sosa V. 2008. Morphological
phylogenetics of Puya subgenus Puya (Bromeliaceae). – Bot. J. Linn. Soc.
156: 93-110.
Horres R. 2003. Untersuchungen zur Systematik
und Phylogenie der Bromeliaceae
unter besonderer Berücksichtigung molekularer Merkmale. – Ph.D. diss.,
Johann Wolfgang von Goethe-Universitet, Frankfurt am Main, Germany.
Horres R, Zizka G. 1995. Untersuchungen zur
Blattsukkulenz bei Bromeliaceae.
– Beitr. Biol. Pflanzen 69: 43-76.
Horres R, Zizka G, Kahl G, Weising K. 2000.
Molecular systematics of Bromeliaceae: evidence from
trnL(UAA) intron sequences of the chloroplast genome. – Plant Biol.
2: 306-315.
Horres R, Schulte K, Weising K, Zizka G. 2007
[2008]. Systematics of Bromelioideae (Bromeliaceae): evidence from molecular
and anatomical studies. – In: Columbus JT, Friar EA, Porter JM, prince LM,
Simpson MG (eds), Monocots: comparative biology and evolution. Poales, Rancho
Santa Ana Botanical Garden, Claremont, California. – Aliso 23: 27-43.
Hoshikawa K. 1969. Underground organs of the
seedlings and the systematics of Gramineae. – Bot. Gaz. 130: 192-203.
Hoshino T. 1981. Karyomorphological and
cytogenetical studies on aneuploidy in Carex. – J. Sci. Hiroshima
Univ., Ser. B., Div. II, Bot. 17: 155-238.
Hoshino T, Davidse G. 1988. Chromosome
numbers of grasses (Poaceae) from
southern Africa I. – Ann. Missouri Bot. Gard. 75: 866-873.
Hoshino T, Masaki T, Nishimoto M. 2011.
Illustrated sedges of Japan. – Heibonsha Ltd., Tokyo.
Hosoyama Y, Hoshida K, Takeoka S, Miyata S.
2002. On the inter-generic hybrid Sasaella ramosa. – Proc. Inst.
Natl. Sci. Nihon Univ. 37: 209-216.
Hsiao C, Wang RR-C, Dewey DR. 1986. Karyotype
analysis and genome relationships of 22 diploid species in the tribe Triticeae.
– Can. J. Genet. Cytol. 28: 109-120.
Hsiao C, Chatterton NJ, Asay KH, Jensen KB.
1994. Phylogenetic relationships of 10 grass species: an assessment of
phylogenetic utility of the internal transcribed spacer region in nuclear
ribosomal DNA in monocots. – Genome 37: 112-120.
Hsiao C, Chatterton NJ, Asay KH, Jensen KB.
1995a. Molecular phylogeny of the Pooideae (Poaceae) based on nuclear rDNA (ITS)
sequences. – Theor. Appl. Gen. 90: 389-398.
Hsiao C, Chatterton NJ, Asay KH, Jensen KB.
1995b. Phylogenetic relationships of the monogenomic species of the wheat
tribe, Triticeae (Poaceae), inferred
from nuclear rDNA (internal transcribed spacer) sequences. – Genome 38:
211-223.
Hsiao C, Jacobs SWL, Barker NP, Chatterton
NJ. 1998. A molecular phylogeny of the subfamily Arundinoideae (Poaceaee) based on sequences of rDNA. –
Aust. Syst. Bot. 11: 41-52.
Hsiao C, Jacobs SWL, Chatterton NJ, Asay KH.
1999. A molecular phylogeny of the grass family (Poaceae) based on the sequences of nuclear
ribosomal DNA (ITS). – Aust. Syst. Bot. 11: 667-688.
Hsu CC. 1965. The classification of
Panicum (Gramineae) and its allies, with special reference to the
characters of lodicule, style-base and lemma. – J. Fac. Sci. Univ. Tokyo,
sect. III (Botany), 9: 43-150.
Hsueh C-J, Yi T-P. 1980. Two new genera of
Bambusoideae from S.W. China 2. Qiongzhuea Hsueh et Yi. – Acta Bot.
Yunn. 2: 91-99.
Hsueh C-J, Yi T-P, Li D-Z. 1996. Validation
of Qiongzhuea and correlated species names (Gramineae, Bambusoideae).
– Taxon 45: 217-221.
Huang S, Sirikhachornkit A, Faris JD, Su X,
Gill BS, Haselkorn R, Gornicki P. 2002. Phylogenetic analysis of the acetyl-CoA
carboxylase and 3-phosphoglycerate kinase loci in wheat and other grasses. –
Plant Mol. Biol. 48: 805-820.
Hubbard CE. 1936. The species of
Helictotrichon in tropical Africa. – Bull. Misc. Inform. Kew 1936:
330-335.
Hubbard CE. 1946. Henrardia, a new
genus of the Gramineae. – Blumea, Suppl. 3: 10-21.
Hubbard CE. 1954. Grasses. – London.
Hughes DK. 1923. The genus Panicum
of the Flora Australiensis. – Kew Bull. 9: 305-332.
Humphreys AM, Antonelli A, Pirie MD, Linder
HP. 2010. Ecology and evolution of the diaspore “burial syndrome”. –
Evolution 65: 1163-1180.
Humphreys AM, Pirie MD, Linder HP. 2010. A
plastid tree can bring order to the chaotic generic taxonomy of
Rytidosperma Steud. s.l. (Poaceae). – Mol. Phylogen. Evol. 55:
911-928.
Hunt HV, Badakshi F, Romanova O, Howe CJ,
Jones MK, Heslop-Harrison JSP. 2014. Reticulate evolution in Panicum
(Poaceae): the origin of tetraploid
broomcorn millet, P. miliaceum. – J. Exper. Bot. 65: 3165-3175.
Hunter AM, Orlovich DA, Lloyd KM, Lee WG,
Murphy DJ. 2004. The generic position of Austrofestuca littoralis and
the reinstatement of Hookerochloa and Festucella (Poaceae) based on evidence from nuclear
(ITS) and chloroplast (trnL-trnF) DNA sequences. – New
Zealand J. Bot. 42: 253-262.
Hunter AWS. 1934. A karyosystematic
investigation in the Gramineae. – Can. J. Res. 11: 213-241.
Hunziker JH. 1989. Chromosome studies on
Anomochloa and other Bambusoideae (Gramineae). – Darwiniana 29:
41-45.
Hunziker JH, Stebbis GL. 1987. Chromosomal
evolution in the Gramineae. – In: Soderstrom TR, Hilu KH, Campbell CS,
Barkworth ME (eds), Grass systematics and evolution, Smithsonian Institution
Press, Washington, D.C., pp. 179-187.
Hunziker JH, Wulff AF, Soderstrom TR. 1982.
Chromosome studies on the Bambusoideae (Gramineae). – Brittonia 34: 30-35.
Hunziker JH, Wulff AF, Soderstrom TR. 1989.
Chromosome studies on Anomochloa and other Bambusoideae (Gramineae).
– Darwiniana 29: 41-45.
Hunziker JH, Zuloaga GO, Morrone O, Escobar
A. 1998. Estudios cromosómicos en Paniceas sudamericanas (Poaceae: Panicoideae). – Darwiniana 35:
29-36.
Hurcombe R. 1946. Chromosome studies in
Cynodon. – South Afr. J. Sci. 42: 144-146.
Hurcombe R. 1947. A cytological and
morphological study of cultivated Cynodon species. – J. South Afr.
Bot. 13: 107-117.
Hutton EM. 1961. Inter-variety variation in
Rhodes grass (Chloris gayana Kunth). – J. Br. Grassl. Soc. 16:
23-29.
Huygh W, Larridon I, Reynders M, Muasya AM,
Govaerts R, Simpson DA, Goetghebeur P. 2010. Nomenclature and typification of
names of genera and subdivisions of genera in Cypereae (Cyperaceae) 1. Names of genera in the
Cyperus clade. – Taxon 59: 1883-1890.
Ibrahim DG, Burke T, Ripley BS, Osborne CP.
2009. A molecular phylogeny of the genus Alloteropsis (Panicoideae, Poaceae) suggests an evolutionary reversion
from C4 to C3 photosynthesis. – Ann. Bot. 103: 127-136.
Idrobo JM. 1954. Xiridáceas de Colombia. –
Caldasia 6: 184-260.
Inda LA, Segarra-Moragues JG, Müller J,
Peterson PM, Catalán P. 2008. Dated historical biogeography of the temperate
Loliinae (Poaceae, Pooideae) grasses in
the northern and southern hemispheres. – Mol. Phylogen. Evol. 46: 932-957.
Inglis C. 1994. Checklist of economically
important Cyperaceae. – Cyperaceae Newsl. 13: 13-15.
Ingram AL, Doyle JJ. 2003. The origin and
evolution of Eragrostis tef (Poaceae) and related polyploids: evidence
from nuclear waxy and plastid rps16. – Amer. J. Bot. 90:
116-122.
Ingram AL, Doyle JJ. 2004. Is
Eragrostis (Poaceae)
monophyletic? Insights from nuclear and plastid sequence data. – Syst. Bot.
29: 545-552.
Ingram AL, Doyle JJ. 2007 [2008].
Eragrostis (Poaceae): monophyly
and infrageneric classification. – In: Columbus JT, Friar EA, Porter JM,
prince LM, Simpson MG (eds), Monocots: comparative biology and evolution.
Poales, Rancho Santa Ana Botanical Garden, Claremont, California. – Aliso 23:
595-604.
Ingram AL, Christin PA, Osborne CP. 2011.
Molecular phylogenies disprove a hypothesized C4 reversion in
Eragrostis walteri (Poaceae).
– Ann. Bot. 107: 321-325.
Ishii A, Miyata S, Hosoyama Y. 2003. The
maternal species of hannouzasa (Sasaella ramosa). – Bamboo J. 20:
12-18.
Ito Y, Tanaka N, Kim C, Kaul RB, Albach DC.
2016. Phylogeny of Sparganium (Typhaceae) revisited: non-monophyletic
nature of S. emersum sensu lato and resurrection of S. acaule. –
Plant Syst. Evol. 302: 129-135.
Ito Y, Viljoen J-A, Tanaka N, Yano O, Muasya
AM. 2016. Phylogeny of Isolepis (Cyperaceae) revisited: non-monophyletic
nature of I. fluitans sensu lato and resurrection of I.
lenticularis. – Plant Syst. Evol. 302: 231-238.
Ivanova NA. 1939. The genus Kobresia
Willd., its morphology and systematics. – Bot. Žurn. 24: 455-503. [In
Russian with English summary]
Jabaily RS, Sytsma KJ. 2010. Phylogenetics of
Puya (Bromeliaceae):
placement, major lineages, and evolution of Chilean species. – Amer. J. Bot.
97: 337-356.
Jabaily RS, Sytsma KJ. 2013. Historical
biogeography and life-history evolution of Andean Puya (Bromeliaceae). – Bot. J. Linn. Soc.
171: 201-224.
Jacobs BF, Kingston JD, Jacobs BL. 1999. The
origin of grass-dominated ecosystems. – Ann. Missouri Bot. Gard. 86:
590-643.
Jacobs SWL. 1985. A new grass genus from
Australia. – Kew Bull. 40: 659-661.
Jacobs SWL. 1987. Systematics of the
chloridoid grasses. – In: Soderstrom TR, Hilu KW, Campbell CS, Barkworth ME
(eds), Grass systematics and evolution, Smithsonian Institution Press,
Washington, D.C., pp. 277-286.
Jacobs SWL. 1990. Notes on Australian grasses
(Poaceae). – Telopea 3: 601-603.
Jacobs SWL. 1992. New taxa and a new
combination in Triodia (Poaceae). – Telopea 4: 653-659.
Jacobs SWL. 2001a. The genus
Lachnagrostis (Gramineae) in Australia. – Telopea 9: 439-448.
Jacobs SWL. 2001b. Four new species of
Agrostis (Gramineae) from Australia. – Telopea 9: 679-683.
Jacobs SWL. 2002. Corrections to and a new
combination in Lachnagrostis (Gramineae). – Telopea 9: 837-838.
Jacobs SWL. 2004a. Thedachloa, a new
grass genus (Gramineae: Paniceae) from the northern Kimberley, Western
Australia. – Telopea 10: 635-637.
Jacobs SWL. 2004b. The tribe Triodieae
(Chloridoideae: Gramineae). – Telopea 10: 701-704.
Jacobs SWL, Everett J. 1996.
Austrostipa, a new genus, and new names for the Australasian species
formerly included in Stipa (Gramineae). – Telopea 6: 579-595.
Jacobs SWL, Everett J. 1997. Jarava
plumosa (Gramineae), a new combination for the species formerly known as
Stipa papposa. – Telopea 7: 301-302.
Jacobs SWL, Highet J. 1988. Re-evaluation of
the characters used to distinguish Enteropogon from Chloris
(Poaceae). – Telopea 3: 217-221.
Jacobs SWL, Lapinpuro L. 1986. The Australian
species of Amphibromus (Poaceae). – Telopea 2: 715-729.
Jacobs SWL, McClay KL, Simon BK. 1993. Review
of Dichelachne (Gramineae) in Australia. – Telopea 5: 325-328.
Jacobs SWL, Everett J, Barkworth ME. 1995.
Clarification of morphological terms used in the Stipeae (Gramineae), and a
reassessment of Nassella in Australia. – Taxon 44: 33-41.
Jacobs SWL, Everett J, Barkworth ME, Hsiao C.
2000. Relationships within the stipoid grasses (Gramineae). – In: Jacobs SWL,
Everett J (eds), Grasses: systematics and evolution, Proceedings of the
2nd International Conference on the Comparative Biology of
Monocotyledons, Sydney, Australia, 1998, Vol. 2, CSIRO Publ., Melbourne, pp.
75-82.
Jacobs SWL, Bayer R, Everett J, Arriaga MO,
Barkworth ME, Sabin-Badereau A, Torres MA, Vazquez FM, Bagnall N. 2007 [2008].
Systematics of the tribe Stipeae (Gramineae) using molecular data. – In:
Columbus JT, Friar EA, Porter JM, Prince LM, Simpson MG (eds), Monocots:
comparative biology and evolution. Poales, Rancho Santa Ana Botanical Garden,
Claremont, California. – Aliso 23: 349-361.
Jacobs SWL, Gillespie LJ, Soreng RJ. 2008.
New combinations in Hookerochloa and Poa (Gramineae). –
Telopea 12: 273-278.
Jacobsen N, Bothmer R von. 1992.
Supraspecific groups in the genus Hordeum. – Hereditas 116:
21-24.
Jacobsen N, Bothmer R von. 1995. Taxonomy in
the Hordeum murinum complex (Poaceae). – Nord. J. Bot. 15: 449-458.
Jacques-Félix H. 1961. Observations sur la
variabilité morphologique de Coix lacrima-jobi. – J. Agric. Trop.
Bot. Appl. 8: 44-56.
Jacques-Félix H. 1962. Les graminées
d’Afrique tropicale. – Institut de Recherches Agronomiques Tropicales et
des Culture Vivrières, Paris.
Jain SK. 1970. The genus Manisuris
L. (Poaceae) in India. – Bull. Bot.
Surv. India 12: 6-17.
Jakob SS, Blattner FR. 2006. A chloroplast
genealogy of Hordeum (Poaceae):
long-term persisting haplotypes, incomplete lineage sorting, regional
extinction, and the consequences for phylogenetic inference. – Mol. Biol.
Evol. 23: 1602-1612.
Jakob SS, Blattner FR. 2010. Two extinct
diploid progenitors were involved in allopolyploid formation in the Hordeum
murinum (Poaceae: Triticeae) taxon
complex. – Mol. Phylogen. Evol. 55: 650-659.
Jakob SS, Meister A, Blattner FR. 2004. The
considerable genome size variation of Hordeum species (Poaceae) is linked to phylogeny, life form,
ecology, and speciation rates. – Mol. Biol. Evol. 21: 860-869.
Janetzky W, Vareschi E. 1993. Phytotelmata in
bromeliads as microhabitats for limnetic organisms. – In: Barthlott W,
Naumann CM, Schmidt-Loske K, Schuchmann K-L (eds), Animal-plant interactions in
tropical environments, Zool. Forschungsinst. Mus. A. Koenig, Bonn, pp.
199-209.
Jansen P. 1953. Notes on Malaysia grasses I.
– Reinwardtia 2: 265-267.
Janzen DH. 1976. Why bamboos wait so long to
flower. – Ann. Rev. Ecol. Syst. 7: 347-391.
Jarvie JK. 1992. Taxonomy of
Elytrigia sect. Caespitosae and sect. Junceae
(Gramineae: Triticeae). – Nord. J. Bot. 12: 155-169.
Jarvie JK, Barkworth ME. 1990. Isozyme
similarity in Thinopyrum and its relatives (Triticeae: Gramineae). –
Genome 33: 885-891.
Jenkins TJ. 1933. Interspecific and
intergeneric hybrids in herbage grasses. Initial crosses. – J. Genet. 28:
205-264.
Jermy AC, Charter AO, David RW. 1982. Sedges
of the British Isles (a new edition of British sedges). 2nd ed. –
Botanical Society of the British Isles 1: 1-268.
Jermy AC, Simpson DA, Foley MJY, Porter MS.
2007. Sedges of the British Isles, ed. 3. – Botanical Society of the British
Isles, London.
Jesus-Costa C de, Clark LG, Santos-Gonçalves
AP. 2018. Molecular phylogeny of Atractantha, and the phylogenetic
position and circumscription of Athroostachys (Poaceae: Bambusoideae: Bambuseae:
Arthrostylidiinae). – Syst. Bot. 43: 656-663.
Jiang Z-H. 2007. Bamboo and rattan in the
world. – China Forestry Publ. Hous, Beijing.
Jiménez-Mejías P, Martinetto E. 2013.
Toward an accurate taxonomic interpretation of Carex fossil fruits (Cyperaceae): a case study in section
Phacocystis in the Western Palearctic. – Amer. J. Bot. 100:
1580-1603.
Jiménez-Mejías P, Escudero M,
Guerra-Cárdenas S, Lye KA, Luceño M. 2011. Taxonomic delimitation and drivers
of speciation in the Ibero-North African Carex sect.
Phacocystis river-shore group (Cyperaceae). – Amer. J. Bot. 98:
1855-1867.
Jiménez-Mejías P, Martín-Bravo S, Luceño
M. 2012. Systematics and taxonomy of Carex sect. Ceratocystis
(Cyperaceae) in Europe: a molecular
and cytogenetic approach. – Syst. Bot. 37: 382-398.
Jiménez-Mejías P, Martinetto E. 2013.
Toward an accurate taxonomic interpretation of Carex fossil fruits (Cyperaceae): a case study in section
Phacocystis in the Western Palearctic. – Amer. J. Bot. 100:
1580-1603.
Jiménez-Mejías P, Rodríguez-Palacios GE,
Amini-Rad M, Martín-Bravo S. 2015. Taxonomic notes on some problematic
Carex (Cyperaceae) names
from SW Asia. – Phytotaxa 219: 183-189.
Jiménez-Mejías P, Luceño M, Wilson KL,
Waterway MJ, Roalson EH. 2016. Clarification of the use of the terms perigynium
and utricle in Carex L. (Cyperaceae). – Syst. Bot. 41:
519-528.
Jiménez-Mejías P, Martinetto E, Momohara A,
Popova S, Smith SY, Roalson EH. 2016. A commented synopsis of the
pre-Pleistocene fossil record of Carex (Cyperaceae). – Bot. Rev.
doi:10.1007/s12229-016-9169-7
Jiménez-Mejías P, Strong M, Gebauer S,
Hilpold A, Martín-Bravo S, Reznicek AA. 2018. Taxonomic, nomenclatural and
chorological reports on Carex (Cyperaceae) in the Neotropics. –
Willdenowia 48: 117-124.
Jin X-F, Zhou YY, Hipp A, Hin S-H, Oda J,
Ikeda H, Yano O, Nagamasu H. 2014. Nutlet micromorphology of Carex
section Rhomboidales sensu Kükenthal (Cyperaceae) and its systematic
implications. – Bot. J. Linn. Soc. 175: 123-143.
Jirasek V. 1969. Morphologie der Schüppchen
(Lodiculae) von Gräsern und ihre Terminologie. Ein weiterer Beitrag zur
Kenntnis des Baues der Lodiculae. – Acta Univ. Carol. Biol. 1968: 321-344.
Jirasek V. 1970. Beitrag zur Kenntnis
zweizelliger Haare bei Gräsern mit Benützung von Pfeifengräsen –
Molinia caerulea (L.) Moench s.l. – Acta Univ. Carol. Biol.
1969: 383-402.
Jirasek V, Jozifova M. 1968. Morphology of
lodicules, their variability and importance in the taxonomy of the Poaceae family. – Bol. Soc. Argentina
Bot. 12: 324-349.
Johnson BL. 1945. Cytotaxonomic studies in
Oryzopsis. – Bot. Gaz. 107: 1-32.
Johnson BL. 1972. Polyploidy as a factor in
the evolution and distribution of grasses. – In: Younger VB, McKell CM (eds),
The biology and utilization of grasses, Academic Press, New York, pp. 18-35.
Johnson LAS. 1993. New species of
Juncus (Juncaceae) in eastern
Australia. – Telopea 5: 309-318.
Johnson LAS, Briggs BG. 1981. Three old
southern families: Myrtaceae, Proteaceae, and Restionaceae. – In: Keast A (ed),
Ecological biogeography of Australia, Junk, Utrecht, pp. 427-469.
Johnson LAS, Briggs BG. 1986a. A new species
and a new genus of Restionaceae
from Tasmania. – Telopea 2: 737-740.
Johnson LAS, Briggs BG. 1986b.
Alexgeorgea nitens, a new combination in Restionaceae. – Telopea 2:
781-782.
Johnson LAS, Cutler DF. 1973.
Empodisma: a new genus of Australasian Restionaceae. – Kew Bull. 28:
381-385.
Johnston CR, Watson L. 1976. Microhairs: a
universal characteristic of non-festucoid grass genera? – Phytomorphology 26:
297-301.
Johnston CR, Watson L. 1981. Germination
flaps in grass lemmas. – Phytomorphology 31: 78-85.
Jones BMG, Ponti J, Tavasolli A, Dixon PA.
1978. Relationships of the Ethiopian cereal t’ef (Eragrostis tef
(Zucc.) Trotter): evidence from morphology and chromosome number. – Ann.
Bot., N. S., 42: 1369-1373.
Jones E, Hodkinson T, Parnell J, Chase MW.
2007 [2008]. The Juncaceae-Cyperaceae interface: a combined plastid
gene analysis. – In: Columbus JT, Friar EA, Porter JM, Prince LM, Simpson MG
(eds), Monocots: comparative biology and evolution. Poales, Rancho Santa Ana
botanical Garden, Claremont, California, pp. 3-26.
Jones S, Burke S, Duvall M. 2014.
Phylogenomics, molecular evolution, and estimated ages of lineages from the
deep phylogeny of Poaceae. – Plant
Syst. Evol. 300: 1421-1436.
Jones SD. 1994. A new species of
Carex (Cyperaceae:
Phaestoglochin) from Oklahoma and Texas: typification of section
Phaestoglochin, and notes in sections Bracteosae and
Phaestoglochin. – Sida 16: 341-353.
Jørgensen RB, 1986. Relationships in the
barley genus (Hordeum): an electrophoretic examination of proteins.
– Hereditas 104: 273-291.
Judziewicz EJ. 1984. Scrotochloa –
a new genus of paleotropical pharoid grasses. – Phytologia 56: 299-304.
Judziewicz EJ. 1987. Taxonomy and morphology
of the tribe Phareae (Poaceae:
Bambusoideae). – Ph.D. diss. University of Wisconsin, Madison, Wisconsin.
Judziewicz EJ. 1990. A new South American
species of Sacciolepis (Poaceae: Panicoideae: Paniceae), with a
summary of the genus in the New World. – Syst. Bot. 15: 415-420.
Judziewicz EJ. 1992. A revision of
Atractantha (Poaceae:
Bambusoideae: Bambuseae). – Ann. Missouri Bot. Gard. 79: 160-183.
Judziewicz EJ. 1998. A revision of
Myriocladus (Poaceae:
Bambusoideae: Bambuseae). – Brittonia 50: 430-446.
Judziewicz EJ. 2009. Toliara (Poaceae, Chloridoideae, Cynodonteae), a new
grass genus endemic to southern Madagascar. – Adansonia, sér. III, 31:
273-277.
Judziewicz EJ, Clark LG. 1993. The South
American species of Arthrostylidium (Poaceae: Bambusoideae: Bambuseae). –
Syst. Bot. 18: 80-99.
Judziewicz EJ, Clark LG. 2007 [2008].
Classification and biogeography of New World grasses: Anomochlooideae,
Pharoideae, Ehrhartoideae, and Bambusoideae. – In: Columbus JT, Friar EA,
Porter JM, Prince LM, Simpson MG (eds), Monocots: comparative biology and
evolution. Poales, Rancho Santa Ana Botanical Garden, Claremont, California.
– Aliso 23: 303-314.
Judziewicz EJ, Soderstrom TR. 1989.
Morphological, anatomical, and taxonomic studies in Anomochloa and
Streptochaeta (Poaceae:
Bambusoideae). – Smithsonian Contr. Bot. 68: 1-52.
Judziewicz EJ, Tyrrell CD. 2007.
Aulonemia yanachagensis (Poaceae: Bambusoideae: Bambuseae): a new
species from central Peru. – Brittonia 59: 83-87.
Judziewicz EJ, Clark LG, Londoño X, Stern
MJ. 1999. American bamboos. – Smithsonian Institution Press, Washington,
D.C.
Judziewicz EJ, Soreng RJ, Davidse G, Peterson
PM, Filgueiras TS, Zuloaga FO. 2000. Catalogue of New World grasses (Poaceae) I. Subfamilies Anomochlooideae,
Bambusoideae, Ehrhartoideae, and Pharoideae. – Contr. U.S. Natl. Herb. 39:
1-128.
Jung J, Choi H-K. 2010. Systematic
rearrangement of Korean Scirpus L. s.l. (Cyperaceae) as inferred from nuclear ITS
and chloroplast rbcL sequences. – J. Plant Biol. 53: 222-232.
Jung J, Choi H-K. 2013. Recognition of two
major clades and early diverged groups within the subfamily Cyperoideae (Cyperaceae) including Korean sedges. –
J. Plant Res. 126: 335-349.
Kabeer KAA, Nair VJ. 2007. Polypogon
nilgiricus – a new species of Poaceae from India. – Nord. J. Bot. 25:
9-11.
Kabeer KAA, Nair VJ. 2009. Flora of Tamil
Nadu. Grasses. – Botanical Survey of India, Calcutta.
Kabuye CHS, Renvoize SA. 1975. The genus
Alloeochaete, Tribe Danthonieae (Gramineae). – Kew Bull. 30:
569-577.
Kabuye CHS, Wood D. 1968. A first record of
multicellular glandular hairs in the Gramineae. – Bot. J. Linn. Soc. 62:
69-70.
Kaczmarek RM. 1914. Priority of
Hierochloa. – Amer. Midl. Natur. 3: 198.
Kahler AL, Krzakowa M, Allard RW. 1981.
Isozyme phenotypes in five species of Bromus sect. Genea. –
Bot. Jahrb. Syst. 102: 401-409.
Kakudidi EKZ, Lazarides M, Carnahan JA. 1988.
A revision of Enneapogon (Poaceae, Pappophoreae) in Australia. –
Aust. Syst. Bot. 1: 325-353.
Kalliola R, Renvoize SA. 1994. One or more
species of Gynerium (Poaceae)?
– Kew Bull. 49: 305-320.
Kam YK, Maze J. 1974. Studies on the
relationships and evolution of supraspecific taxa utilizing developmental data
II. Relationships and evolution of Oryzopsis hymenoides, O.
virescens, O. kingii, O. micrantha, and O.
asperifolia. – Bot. Gaz. 135: 227-247.
Kamra S, Sidhu M, Cheema PC. 1988.
Cytomorphological studies on some members of Cyperaceae from North India. – J.
Cytol. Genet. 23: 14-37.
Kanno A, Hirai A. 1992. Comparative studies
of the structure of chloroplast DNA from four species of Oryza:
cloning and physical maps. – Theor. Appl. Gen. 83: 791-798.
Katayama H, Ogihara Y. 1993. Structural
alterations of the chloroplast genome found in grasses are not common in
monocots. – Curr. Genet. 23: 160-165.
Katayama H, Ogihara Y. 1996. Phylogenetic
affinities of the grasses to other monocots as revealed by molecular analysis
of chloroplast DNA. – Curr. Genet. 29: 572-581.
Kaul RB. 1971. Diaphragms and aerenchyma in
Scirpus validus. – Amer. J. Bot. 58: 808-816.
Kaul RB. 1972. Adaptive leaf architecture in
emergent and floating Sparganium. – Amer. J. Bot. 59: 270-278.
Kawano S, Tatewaki M. 1961. A new species of
Poa from Hokkaido, Japan. – J. Jap. Bot. 36: 337-341.
Kelchner SA, Clark LG. 1997. Molecular
evolution and phylogenetic utility of the chloroplast rpl16 intron in
Chusquea and the Bambusoideae (Poaceae). – Mol. Phylogen. Evol. 8:
385-397.
Kelchner SA, Bamboo Phylogeny Group. 2013.
Higher level phylogenetic relationships within the bamboos (Poaceae: Bambusoideae) based on five
plastid markers. – Mol. Phylogen. Evol. 67: 404-413.
Kellogg EA. 1989. Comments on genomic genera
in the Triticeae. – Amer. J. Bot. 76: 796-805.
Kellogg EA. 1990a. Ontogenetic studies of
florets in Poa (Gramineae): allometry and heterochrony. – Evolution
44: 1978-1989.
Kellogg EA. 1990b. Variation and species
limits in agamospermous grasses. – Syst. Bot. 15: 112-123.
Kellogg EA. 1992a. Tools for studying the
chloroplast genome in the Triticeae (Gramineae): an EcoRI map, a
diagnostic deletion, and support for Bromus as an outgroup. – Amer.
J. Bot. 79: 186-197.
Kellogg EA. 1992b. Restriction site variation
in the chloroplast genomes of the monogenomic Triticeae. – Hereditas 116:
43-47.
Kellogg EA. 1998. Relationships of cereal
crops and other grasses. – Proc. Natl. Acad. Sci. U.S.A. 95: 2005-2010.
Kellogg EA. 2000a. Molecular and
morphological evolution in the Andropogoneae. – In: Jacobs SWL, Everett J
(eds), Grasses: systematics and evolution, Proceedings of the 2nd
International Conference on the Comparative Biology of Monocotyledons, Sydney,
Australia, Vol. 2, CSIRO Publ., Melbourne, pp. 149-158.
Kellogg EA. 2000b. The grasses: a case study
in macro-evolution. – Ann. Rev. Ecol. Syst. 31: 217-238.
Kellogg EA. 2001. Evolutionary history of the
grasses. – Plant Physiol. 125: 1198-1205.
Kellogg EA. 2006. Beyond taxonomy: prospects
for understanding morphological diversity in the grasses (Poaceae). – Darwiniana 44: 7-17.
Kellogg EA. 2007. Floral displays: genetic
control of grass inflorescences. – Curr. Opinion Plant Biol. 10: 26-31.
Kellogg EA. 2009. The evolutionary history of
Ehrhartoideae, Oryzeae, and Oryza. – Rice 2: 1-14.
Kellogg EA. 2015a. The families and genera of
vascular plants XIII. Flowering plants – eudicots – Poaceae. – Springer,
416 pp.
Kellogg EA. 2015b. Poaceae – General
information. – In: Kellogg EA (ed), The families and genera of vascular
plants XIII. Flowering plants – eudicots – Poaceae. Springer, pp.
3–130.
Kellogg EA, Appels R. 1995. Intraspecific and
interspecific variation in 5S RNA genes are decoupled in diploid wheat
relatives. – Genetics 140: 325-343.
Kellogg EA, Birchler JA. 1993. Linking
phylogeny and genetics: Zea mays as a tool for phylogenetic studies.
– Syst. Biol. 4: 415-439.
Kellogg EA, Campbell CS. 1987. Phylogenetic
analyses of the Gramineae. – In: Soderstrom TR, Hilu KW, Campbell CS,
Barkworth ME (eds), Grass systematics and evolution, Smithsonian Institution
Press, Washington, D.C., pp. 310-322.
Kellogg EA, Linder HP. 1995. Phylogeny of
Poales. – In: Rudall PJ, Cribb PJ, Cutler DF, Humphries CJ (eds),
Monocotyledons: systematics and evolution, Royal Botanic Gardens, Kew, pp.
511-542.
Kellogg EA, Watson L. 1993. Phylogenetic
studies of a large data set I. Bambusoideae, Andropogonoideae, and Pooideae
(Gramineae). – Bot. Rev. 59: 273-343.
Kellogg EA, Appels R, Mason-Gamer RA. 1996.
When genes tell different stories: the diploid genera of Triticeae (Gramineae).
– Syst. Bot. 21: 321-347.
Kellogg EA, Hiser KM, Doust AN. 2004.
Taxonomy, phylogeny, and inflorescence development of the genus
Ixophorus (Panicoideae: Poaceae). – Intern. J. Plant Sci. 165:
1089-1105.
Kellogg EA, Aliscioni S, Morrone O, Pensiero
J, Zuloaga F. 2009. A phylogeny of Setaria (Poaceae, Panicoideae, Paniceae) and related
genera based on the chloroplast gene ndhF. – Intern. J. Plant Sci.
17: 117-131.
Keng PC. 1982a. A revision of genera of
bamboos from the world I. – J. Bamboo Res. 1: 1-19.
Keng PC. 1982b. A revision of genera of
bamboos from the world II. – J. Bamboo Res. 1: 31-46.
Keng PC. 1983a. A revision of genera of
bamboos from the world III. – J. Bamboo Res. 2: 11-27.
Keng PC. 1983b. A revision of genera of
bamboos from the world IV. – J. Bamboo Res. 2: 1-17.
Keng Y-L, Chen S-L. 1963. A revision of the
genus Roegneria C. Koch of China. – J. Nanjing Univ. (Nat. Sci.) 3:
1-92.
Kennedy PB. 1899. The structure of the
caryopsis of grasses with reference to their morphology and classification. –
U.S.D.A. Div. Agrostol. Bull. 19: 1-44.
Kerby K, Kuspira J. 1987. The phylogeny of
polyploid wheats Triticum aestivum (bread wheat) and Triticum
turgidum (macaroni wheat). – Genome 29: 722-737.
Kerguélen M. 1975. Les Gramineae (Poaceae) de la flore française. Essai de
mise au point taxonomique et nomenclaturale. – Lejeunia 75.
Kerguélen M, Plonka F. 1988. Le genre
Festuca dans la flore française. Taxons nouveaux, observations
nomenclaturales et taxinomiques. – Bull. Soc. Bot. Centre-Ouest, sér. II,
19: 15-30.
Kerguélen M, Plonka F. 1989. Les
Festuca de la flore de France (Corse comprise). – Bull. Soc. Bot.
Centre-Ouest, sér. II, Num. Spéc. 10.
Kern JH. 1955. Florae Malesianae precursores
X. Notes on Malaysian and some S. E. Asian Cyperaceae III. – Blumea 8:
110-169.
Kern JH. 1958. Florae Malesianae precursores
XXI. Notes on Malaysian and some S. E. Asian Cyperaceae VII. Acta Bot. Neerl. 7:
786-800.
Kern JH. 1961. Florae Malesianae precursores
XXX. The genus Scleria in Malaysia. – Blumea 11: 140-218.
Kern JH. 1962. New look at some Cyperaceae mainly from the tropical
standpoint. – Advanc. Sci. 19: 141-148.
Kern JH. 1974. Cyperaceae I. – In: Steenis CGGJ van
(ed), Flora Malesiana I, 7(3), Junk, Den Haag, pp. 435-753.
Kern JH (†), Nooteboom HP. 1979. Cyperaceae II. – In: Steenis CGGJ van
(ed), Flora Malesiana I, 9(1), Sijthoff & Noordhoff International Publ.,
Alphen aan den Rijn, The Netherlands, pp. 107-187.
Kern VG, Guarise NJ, Vegetti AC. 2008.
Inflorescence structure in species of Spartina Schreb. (Poaceae: Chloridoideae: Cynodonteae). –
Plant Syst. Evol. 273: 51-61.
Kerstens S, Verbelen J-P. 2002. Cellulose
orientation in the outer epidermal wall of angiosperm roots: implications for
biosystematics. – Ann. Bot. 90: 669-676.
Killeen TJ. 1990. The grasses of
Chiquitanía, Santa Cruz, Bolivia. – Ann. Missouri Bot. Gard. 77: 125-201.
Killeen TJ, Agrasar ZE R de. 1992. Taxonomy
and reproductive biology of Digitaria dioica and D. neesiana
(Gramineae: Paniceae). – Syst. Bot. 17: 594-606.
Kim C, Choi H-K. 2011. Molecular systematic
and character evolution of Typha (Typhaceae) inferred from nuclear and
plastid DNA sequence data. – Taxon 60: 1417-1428.
Kim C, Tang H, Paterson AH. 2009. Duplication
and divergence of grass genomes: integrating the chloridoids. – Trop. Plant
Biol. 2: 51-62.
Kim C, Wang X, Lee TH, Jakob K, Lee GJ,
Paterson AH. 2014. Comparative analysis of Miscanthus and
Saccharum reveals a shared whole-genome duplication but different
evolutionary fates. – The Plant Cell 26: 2420-2429.
Kim CM, Dolan L. 2011. Root hair development
involves asymmetric cell division in Brachypodium distachyon and
symmetric division in Oryza sativa. – New Phytol. 192: 601-610.
Kim ES, Bolsheva NL, Samatadze TE, Nosov NN,
Nosova IV, Zelenin AV, Punina EO, Muravenko OV, Rodionov AV. 2009. The unique
genome of two-chromosome grasses, Zingeria and Colpodium, its
origin, and evolution. – Russian J. Gen. 45: 1329-1337.
Kim S, Kim C-S, Le J, Lee I-Y, Chung Y-J, Cho
M-S, Kim S-C. 2015. Phylogenetic relationships among species of
Setaria (Paniceae; Panicoideae; Poaceae) in Korea: insights from nuclear
(ITS and kn1) and chloroplast DNA sequence data. – Plant Syst. Evol.
301: 725-736.
Kimpouni V. 1992. Trois espèces nouvelles du
genre Syngonanthus (Eriocaulaceae) pour la Flore
d’Afrique centrale (Zaire, Rwanda, Burundi). – Fragm. Flor. Geobot. 37:
147-155.
King MG, Roalson EH. 2008. Exploring
evolutionary dynamics of nrDNA in Carex subgenus Vignea (Cyperaceae). – Syst. Bot. 33:
514-524.
Kinges H. 1961. Merkmale des
Gramineenembryos, ein Beitrag zur Systematik der Gräser. – Bot. Jahrb. Syst.
81: 50-93.
Kinney MS, Columbus JT, Friar EA. 2003.
Molecular evolution of the maize sex-determining gene TASSELSEED2 in
Bouteloua (Poaceae). – Mol.
Phylogen. Evol. 29: 519-528.
Kinney MS, Columbus JT, Friar EA. 2008.
Unisexual flower, spikelet, and inflorescence development in
monoecious/dioecious Bouteloua dimorpha (Poaceae, Chloridoideae). – Amer. J. Bot.
95: 123-132.
Kiran Raj MS. 2008. Taxonomic revision of the
subtribe Dimeriinae Hack. of Andropogoneae (Panicoideae-Poaceae) in Peninsular India. – Ph.D.
thesis, University of Calicut, India.
Kiran Raj MS, Sivadasan M, Veldkamp JF,
Alfarhan AH, Thomas J. 2013. Nanooravia gen. nov., subtribe Dimeriinae
(Poaceae-Panicoideae-Andropogoneae) from
India. – Nord. J. Bot. 31: 161-165.
Kiran Raj MS, Sivadasan M, Veldkamp JF,
Alfarhan AH, Amal Tamimi ASM. 2015. A revised infrageneric classification of
Dimeria R. Br. (Poaceae-Andropogoneae). – Bangladesh J.
Plant Taxon. 22: 47-54.
Kircher E. 1977. Embryologische
Untersuchungen an Xyris capensis Thunb. – Thesis, Universität
Bochum, Germany.
Kircher P. 1986. Untersuchungen zur Blüten-
und Infloreszenzmorphologie, Embryologie und Systematik der Restionaceen im
Vergleich mit Gramineen und verwandten Familien. – Diss. Bot. 94: 1-219.
Kirpes CC. 1993. Arrangement of pollen in the
anthers of grasses (Poaceae) and related
families. – M.Sc. thesis, Iowa State University, Ames, Iowa.
Kirpes CC, Clark LG, Lersten NR. 1996.
Systematic significance of pollen arrangement in microsporangia of Poaceae and Cyperaceae: review and observations on
representative taxa. – Amer. J. Bot. 83: 1609-1622.
Kirschner J. 1993. Taxonomic survey of
Luzula sect. Luzula (Juncaceae) in Europe. – Folia Geobot.
Phytotaxon. 28: 141-182.
Kirschner J. 2001. Taxonomic and
nomenclatural notes on Luzula and Juncus (Juncaceae). – Taxon 50: 1107-1113.
Kirschner J et al. 2003. Species Plantarum,
Flora of the World 6. Juncaceae.
Rostkovia to Luzula; 7. Juncus subgen.
Juncus; 8. Juncus subgen. Agathyron. – Australian
Biological Resources Studies, Canberra.
Kjellqvist E, Löve Á. 1963. Chromosome
numbers of some Carex species from Spain. – Bot. Not. 116:
241-248.
Klaus H. 1966. Ontogenetische und
histogenetische Untersuchungen an der Gerste (Hordeum distichon L.).
– Bot. Jahrb. Syst. 85. 45-79.
Klokov MV, Ossycznjuk V. 1976. Stipeae
Ucrainicae. – Novosti Sist. Vyssh. Nizsh. Rast. 1975: 7-92.
Klopper KC, Spies JJ, Visser B. 1998.
Cytogenetic studies in the genus Pentaschistis (Poaceae: Arundinoideae). – Bothalia 28:
231-238.
Knapp WM, Naczi RFC. 2008. Taxonomy,
morphology, and geographic distribution of Juncus Longii (Juncaceae). – Syst. Bot. 33:
685-694.
Knobloch IW. 1968. A checklist of crosses in
the Gramineae. – East Lansing, Michigan.
Knowles PF. 1944. Interspecific hybridization
of Bromus. – Genetics 29: 128-140.
Knox RB. 1967. Apomixis: seasonal and
population differences in a grass. – Science 157: 325-326.
Kobayashi M. 1997. Phylogeny of world bamboos
analysed by restriction fragment length polymorphisms of chloroplast DNA. –
In: Chapman GP (ed), The bamboos, Academic Press, London, pp. 227-234.
Köbele CP, Tillich H-J. 2001. Die
Infloreszensen der Juncaceae. –
Sendtnera 7: 137-161.
Köbele CP, Tillich H-J. 2002. The aberrant
inflorescence of Luzula elegans Lowe (Juncaceae) compared to other
Luzula species. – Sendtnera 8: 77-84.
Koch SD, Sánchez Vega I. 1985.
Eragrostis mexicana, E. neomexicana, E. orcuttiana,
and E. virescens: the resolution of a taxonomic problem. –
Phytologia 58: 377-381.
Körnicke F. 1867. Eriocaulaceae. – Ann. Mus. Bot.
Lugd.-Bat. 3: 162-164, 238-241.
Körnicke F. 1873. Monographie der
Rapateaceen. – Linnaea 37: 417-494.
Kowal T. 1958. A study on the morphology of
fruits of Europaean genera from the subfamilies Scirpoideae Pax,
Rhynchosporoideae Aschers. et Graebner and som genera of Caricoideae Pax. –
Monogr. Bot. 6: 97-141.
Koyama T. 1954. New carices from Nepal
collected by Sasuke Nakao. – Acta Phytotaxon. Geobot. 15: 111-114.
Koyama T. 1957. Taxonomic studies of Cyperaceae 7: the systematic position of
Carex sect. Decorae with a taxonomic treatment of the
Japanese species. – Bot. Mag. (Tokyo) 70: 347-357.
Koyama T. 1958. Taxonomic study of the genus
Scirpus Linné. – J. Fac. Sci. Univ. Tokyo, sect. III, Bot. 7:
271-366.
Koyama T. 1959. Taxonomic study of Cyperaceae 9. – Bull. Arts Sci. Div.,
Univ. Ryukyus (Math. Nat. Sci.) 3: 65-76.
Koyama T. 1961. Classification of the family
Cyperaceae 1. – J. Fac. Sci. Univ.
Tokyo, Sect. 3, Bot. 8: 37-148.
Koyama T. 1962a. Classification of the family
Cyperaceae 2. – J. Fac. Sci. Univ.
Tokyo, Sect. 3, Bot. 8: 149-278.
Koyama T. 1962b. The genus Scirpus
Linn. Some North American aphylloid species. – Can. J. Bot. 40: 913-937.
Koyama T. 1965. Interrelationships between
the tribes Lagenocarpeae and Sclerieae (Cyperaceae). – Bull. Torrey Bot. Club
92: 250-265.
Koyama T. 1967. Cyperaceae-Mapanioideae. – In: Maguire
B (ed), The botany of the Guayana Highland 7, Mem. New York Bot. Gard. 17:
23-79.
Koyama T. 1969. Delimitation and
classification of the Cyperaceae-Mapanioideae. – In: Gunckel
JE (ed), Current topics in plant science, New York, London, pp. 201-228.
Koyama T. 1970. The American species of the
genus ‘Hypolytrum’ (Cyperaceae). – Darwiniana 16:
49-92.
Koyama T. 1971. Systematic interrelationships
among Sclerieae, Lagenocarpeae and Mapanieae (Cyperaceae). – Mitt. Bot. Staatssamml.
München 10: 604-617.
Koyama T. 1973. New Cyperaceae from Nepal and adjoining
Tibet. – Bot. Mag. (Tokyo) 86: 275-283.
Koyama T. 1976. Nomenclatural remarks on some
Cyperaceae from southeastern Asia.
– J. Jap. Bot. 51: 316-317.
Koyama T. 1980. The genus
Bolboschoenus Palla in Japan. – Acta Phytotaxon. Geobot. 31:
139-148.
Koyama T. 1982. The genus Lipocarpha
R. Brown, its morphology and systematic position in the family Cyperaceae. – Acta Phytotaxon. Geobot.
33: 218-226.
Koyama T. 1987. Grasses of Japan and its
neighboring regions. – Kodansha, Tokyo.
Koyama T, Maguire B. 1965. Cyperaceae-Lagenocarpeae. – In:
Maguire B (ed), The botany of the Guayana Highland 6, Mem. New York Bot. Gard.
12: 8-54.
Kožuharov SI, Petrova AV. 1981. Caryological
studies on Bulgarian Poaceae. – Bol.
Soc. Brot., ser. II, 53: 1161-1175.
Kožuharov SI, Petrova AV, Ehrendorfer F.
1981. Evolutionary patterns in some bromegrass species (Bromus,
Gramineae) of the Balkan Peninsula. – Bot. Jahrb. Syst. 102: 381-391.
Kral R. 1966. Xyris (Xyridaceae) of the continental United
States and Canada. – Sida 2: 177-260.
Kral R. 1971. A treatment of
Abildgaardia, Bulbostylis and Fimbristylis (Cyperaceae) for North America. – Sida
4: 57-227.
Kral R. 1988. The genus Xyris (Xyridaceae) in Venezuela and contiguous
northern South America. – Ann. Missouri Bot. Gard. 75: 522-722.
Kral R. 1992. A treatment of American Xyridaceae exclusive of Xyris.
– Ann. Missouri Bot. Gard. 79: 819-885.
Kral R. 1998. Xyridaceae. – In: Kubitzki K (ed), The
families and genera of vascular plants IV. Flowering plants. Monocotyledons.
Alismatanae and Commelinanae (except Gramineae), Springer, Berlin, Heidelberg,
New York, pp. 461-469.
Kral R. 1999. 212A. Xyridaceae. – In: Harling G, Andersson
L (eds), Flora of Ecuador 63, Nord. J. Bot., Copenhagen, pp. 15-36.
Kral R, Wanderley MGL. 1988. Ten novelties in
Xyris (Xyridaceae) from the
Planalto of Brazil. – Ann. Missouri Bot. Gard. 75: 352-372.
Kral R, Wanderley MGL. 1993. Five new taxa of
Xyris (Xyridaceae) from
Brazil. – Kew Bull. 48: 577-588.
Krapp F. 2013. Phylogenie und Evolution der
Gattung Dyckia (Bromeliaceae). – Ph.D. diss.,
Universität von Kassel, Germany.
Krapp F, Barros Pinangé D, Benko-Iseppon A,
Leme E, Weising K. 2014. Phylogeny and evolution of Dyckia (Bromeliaceae) inferred from
chloroplast and nuclear sequences. – Plant Syst. Evol. 300: 1591-1614.
Krassilov VA, Dobruskina IA. 1998. A
graminoid plant from the Cretaceous of the Middle East. – Paleontol. J. 32:
429-434.
Krattinger K. 1978. Biosystematische
Untersuchungen innerhalb der Gattung Typha L. – Mitt. Bot. Mus.
Univ. Zürich 298: 1-270.
Kraus JE, Sajo MG, Dias-Leme CL, Wanderley
MGL. 1994. Aspectos morphológicos do desenvolvimento pós-seminal em species
de Xyris L. (Xyridaceae).
– Hoehnea 21: 29-38.
Krauss RW. 1950. A taxonomic revision of the
Hawaiian species of the genus Carex. – Pac. Sci. 4: 249-282.
Kreczetovicz VI. 1936. Are the sedges of
subgenus Primocarex Kük. primitive? – Bot. Žurn. 21: 395-425. [In
Russian with English summary]
Kreczetovicz VI. 1937. Cyperacearum
novitiates. – Not. Syst. 7: 27-37.
Krishnan S, Samson NP, Ravichandran P,
Narasimhan D, Dayanandan P. 2000. Phytoliths of Indian grasses and their
potential use in identification. – Bot. J. Linn. Soc. 132: 241-252.
Kristiansen KA, Cilieborg M, Drábková L,
Jørgensen T, Petersen G, Seberg O. 2005. DNA taxonomy – the riddle of
Oxychloë (Juncaceae). –
Syst. Bot. 30: 284-289.
Kronfeld M. 1887. Über Raphiden bei
Typha. – Bot. Centralbl. 29: 154-156.
Kronfeld M. 1889. Monographie der Gattung
Typha Tourn. (Typhinae Agdh., Typhaceae Schur-Engl.). – Verh.
Zool.-Bot. Ges. Wien 39: 89-190.
Krügel P. 1993. Biologie und Ökologie der
Bromelienfauna von Guzmania weberbaueri im amazonischen Peru ergänzt
durch eine umfassende Boibliographie der Bromelien-Phytotelmata. – Biosyst.
Ecol. Ser. 2: 1-93.
Krupko S. 1962. Embryological and cytological
investigations in Hypodiscus aristatus Nees (Restionaceae). – J. South Afr. Bot.
28: 21-44.
Krupko S. 1963. Macrosporogenesis and embryo
sac development in Chondropetalum hookerianum (Mast.) Pillans (Restionaceae). – Acta Soc. Bot.
Polon. 32: 17-190.
Krupko S. 1966. Some loose embryological and
cytological observations on members of the Restionaceae family. – Bull. Soc.
Amis Sci. Poznan, Ser. D, 7: 59-67.
Kubitzki K. 1966. Untersuchungen über den
Blütenbau von Oreobolus R. Br. – Bot. Jahrb. Syst. 85: 80-87.
Kubitzki K. 1998a. Thurniaceae. – In: Kubitzki K (ed),
The families and genera of vascular plants IV. Flowering plants.
Monocotyledons. Alismatanae and Commelinanae (except Gramineae), Springer,
Berlin, Heidelberg, New York, pp. 455-457.
Kubitzki K. 1998b. Typhaceae. – In: Kubitzki K (ed), The
families and genera of vascular plants IV. Flowering plants. Monocotyledons.
Alismatanae and Commelinanae (except Gramineae), Springer, Berlin, Heidelberg,
New York, pp. 457-461.
Kuhn SA, Nogueira FM, Fagundes NF, Mariath
JEA. 2016. Morphoanatomy of the ovary and ovule in Bromeliaceae subfamily Tillandsioideae
and its systematic relevance. – Bot. J. Linn. Soc. 181: 343-361.
Kükenthal G. 1913. Cyperaceae novae III. – Feddes Repert.
12: 91-95.
Kükenthal G. 1935-1936. Cyperaceae-Scirpoideae-Cypereae. – In:
Engler A (ed), Die natürlichen Pflanzenfamilien, Wilhelm Engelmann, Leipzig,
IV, 20, pp. 1-160, 161-671.
Kükenthal G. 1940. Neue Cyperaceen aus dem
Malayischen und Papuanischen Gebiet I. – Bull. Jard. Bot. Buitenzorg, sér.
III, 16: 300-323.
Kükenthal G. 1943. Zur Kenntnis der Gattung
Rhynchospora. – Boissiera 7: 100-104.
Kükenthal G. 1944. Vorarbeiten zu einer
Monographie der Rhynchosporoideae 14. – Feddes Repert. Spec. Nov. Regni
Veget. 53: 187-219.
Kükenthal G, Peter A. 1936-1937. Plantarum
novarum Africae orientalis descriptiones (Cyperaceae). – Feddes Repert. 40,
Anhang: 123-142.
Kukkonen I. 1964. Facts and speculations
about the factors affecting the distribution of Anthracoidea scirpi as
a parasite of Trichophorum cespitosum. – Ann. Univ. Turku A 2:
140-148.
Kukkonen I. 1967a. Gedanken und Probleme zur
Systematik der Familie Cyperaceae.
Eine Zusammenfassung. – Aquilo, Ser. Bot. 6: 18-42.
Kukkonen I. 1967b. Vegetative anatomy of
Uncinia (Cyperaceae). –
Ann. Bot., N. S., 31: 523-544.
Kukkonen I. 1967c. Spikelet morphology and
anatomy of Uncinia Pers. (Cyperaceae). – Kew Bull. 21: 93-97.
Kukkonen I. 1978. Two new species of
Schoenoxiphium (Cyperaceae).
– Bot. Not. 131: 263-267.
Kukkonen I. 1983. The genus
Schoenoxiphium (Cyperaceae).
A preliminary account. – Bothalia 14: 819-823.
Kukkonen I. 1984a. On the inflorescence
structure in the family Cyperaceae.
– Ann. Bot. Fenn. 21: 257-264.
Kukkonen I. 1984b. New infraspecific taxa and
nomenclatural combinations in Carex (Cyperaceae) in the Flora Iranica area.
– Ann. Bot. Fenn. 21: 383-389.
Kukkonen I. 1986a. Special features of the
inflorescence structure in the family Cyperaceae. – Ann. Bot. Fenn. 23:
107-119.
Kukkonen I. 1986b. Schoenoxiphium.
– In: Hilliard OM, Burtt BL (eds), Notes of some plants of Africa chiefly
from Natal XIII, Notes Roy. Bot. Gard. Edinb. 43: 345-405.
Kukkonen I. 1990. On the genus
Eleocharis (Cyperaceae) in
the Flora Iranica area, with revised infrageneric classification and
nomenclature. – Ann. Bot. Fenn. 27: 109-117.
Kukkonen I. 1994. Definition of descriptive
terms for the Cyperaceae. – Ann.
Bot. Fenn. 31: 37-43.
Kukkonen I, Timonen T. 1979. Species of
Ustilaginales, especially of the genus Anthracoidea, as tools in plant
taxonomy. – Symb. Bot. Ups. 22: 166-176.
Kukkonen I, Toivonen H. 1988. Taxonomy of
wetland carices. – Aquatic Bot. 30: 5-22.
Kupicha FK, Cope TA. 1985. Three new grasses
from the ‘Flora Zambesiaca’ area. – Kew Bull. 40: 89-91.
Kuwabara Y. 1961. On the shape and direction
of leaves of the grass seedling. – J. Jap. Bot. 36: 368-373.
Kuzmanović N, Lakušić D. Frajman B, Alegro
A, Schönswetter P. 2017. Phylogenetic relationships in Seslerieae (Poaceae) including resurrection of
Psilathera and Sesleriella, two monotypic genera endemic to
the Alps. – Taxon 66: 1349-1370.
La Cour LF. 1952. The Luzula system
analyzed by x-rays. – Heredity 6(Suppl.): 77-81.
Ladd PG. 1977. Pollen morphology of some
members of the Restionaceae and
related families, with notes on the fossil record. – Grana 16: 1-14.
Ladizinsky G, Zohary D. 1971. Notes on
species delimitation, species relationships and polyploidy in Avena L.
– Euphytica 20: 380-395.
Laessle AM. 1961. A micro-limnological study
of Jamaican bromeliads. – Ecology 42: 499-517.
Lægaard S. 1987. The genus Aciachne
(Poaceae). – Nord. J. Bot. 7:
667-672.
Lægaard S. 1990. Three new species of
Muhlenbergia and Uniola (Poaceae) from Northern Peru. – Nord. J.
Bot. 10: 437-441.
Lægaard S. 1997. 214(1). Gramineae (part 1).
– In: Harling G, Andersson L (eds), Flora of Ecuador 57, Nord. J. Bot.,
Copenhagen, pp. 1-54.
Lægaard S, Peterson PM. 2001. 214(2).
Gramineae (part 2). Subfam. Chloridoideae. – In: Harling G, Andersson L
(eds), Flora of Ecuador 68, Botanical Institute, Göteborg University, pp.
1-129.
Lakshmanan KK. 1967. Embryological studies in
the Bromeliaceae I. Lindmannia
penduliflora (C. H. Wright) Stapf. – Proc. Indian Acad. Sci., Sect. B,
65: 49-55.
Lambertini C, Gustafsson MHG, Frydenberg J,
Lissner J, Speranza M, Brix H. 2006. A phylogeographic study of the
cosmopolitan genus Phragmites (Poaceae) based on AFLPs. – Plant Syst.
Evol. 258: 161-182.
Lamont BB. 1974. The biology of dauciform
roots in the sedge Cyathochaete avenacea. – New Phytol. 73:
985-996.
Lamont BB. 1978. The root system of sedges.
– Aust. Plants 9: 258-261.
Lange D. 1995. Untersuchungen zur Systematik
und Taxonomie der Gattung Helictotrichon Besser ex J. A. Schultes
& J. H. Schultes (Poaceae) in
Südosteuropa und Vorderasien. – Bibl. Bot. 144.
Lange P de, Smissen RD, Rolfe JR, Ogle CC.
2016. Systematics of Simplicia Kirk (Poaceae, Agrostidinae) – an endemic,
threatened New Zealand grass genus. – PhytoKeys 75: 119-144.
Lanning FC. 1972. Ash and silica in
Juncus. – Bull. Torrey Bot. Club 99: 196-198.
Lanning FC, Eleuterius LN. 1989. Silica
deposition in some C3 and C4 species of grasses, sedges
and composites in the USA. – Ann. Bot., N. S., 63: 395-410.
Laren L van, Gordon-Gray KD, Browning J.
1989. Studies in Cyperaceae in
southern Africa 15. A review of Rhynchospora brownii (Cyperaceae) and its relationships with
the African R. angolensis and the South American R.
barrosiana. – South Afr. J. Bot. 55: 498-508.
Larridon I, Reynders M, Huygh W, Bauters K,
Vrijdaghs A, Leroux O, Muasya AM, Simpson DA, Goetghebeur P. 2011. Taxonomic
changes in C3 Cyperus (Cyperaceae) supported by molecular data,
morphology, embryography, ontogeny and anatomy. – Plant Ecol. Evol. 144:
327-356.
Larridon I, Reynders M, Huygh W, Bauters K,
Putte K van de, Muasya AM, Boeckx P, Simpson DA, Vrijdaghs A, Goetghebeur P.
2011. Affinities in C3 Cyperus lineages (Cyperaceae) revealed using molecular
phylogenetic data and carbon isotope analysis. – Bot. J. Linn. Soc. 167:
19-46.
Larridon I, Huygh W, Reynders M, Muasya AM,
Govaerts R, Simpson DA, Goetghebeur P. 2011. Nomenclature and typification of
names of genera and subdivisions of genera in Cypereae (Cyperaceae) 2. Names of subdivisions of
Cyperus. – Taxon 60: 868-884.
Larridon I, Bauters K, Reynders M, Huygh W,
Muasya AM, Simpson DA, Goetghebeur P. 2013. Towards a new classification of the
giant paraphyletic genus Cyperus (Cyperaceae): phylogenetic relationships
and generic delimitation in C4 Cyperus. – Bot. J. Linn.
Soc. 172: 106-126.
Larridon I, Bauters K, Reynders M, Juygh W,
Goetghebeur P. 2014. Taxonomic changes in C4 Cyperus (Cypereae,
Cyperoideae, Cyperaceae): combining
the sedge genera Ascolepis, Kyllinga and Pycreus
into Cyperus s.l. – Phytotaxa 166: 33-48.
Larridon I, Bauters K, Semmouri I, Viljoen
J-A, Prychid CJ, Muasya AM, Bruhl JJ, Wilson KL, Senterre B, Goetghebeur P.
2018. Molecular phylogenetics of the genus Costularia (Schoeneae, Cyperaceae) reveals multiple distinct
evolutionary lineages. – Mol. Phylogen. Evol. 126: 196-209.
Larsen K. 1972. Flagellariaceae, Hanguanaceae. –
Flora of Thailand 2(2): 162-166.
Larson S. 1990. Genetic diversity in the
Tripsacum and Zea chloroplast genome and nuclear ribosomal
DNA: phylogeny and rates of evolution. – M.Sc. thesis, University of
Minnesota, Minneapolis, Minnesota.
Larson S, Doebley J. 1994. Restriction site
variation in the chloroplast genome of Tripsacum (Poaceae): phylogeny and rates of sequence
evolution. – Syst. Bot. 19: 21-34.
Launert E. 1971. 200. Gramineae
(Bambuseae-Pappophoreae). – In: Fernandes A, Launert E, Wild H (eds), Flora
Zambesiaca 10 (Part 1), Crown Agents for Oversea Governments and
Administrations, London.
Laurent M. 1904. Recherches sur le
développement des Joncées. – Ann. Sci. Nat. Bot. 19: 97-194.
Lavania UC. 1987. Chromosomal instability in
lemon grass, Cymbopogon flexuosus (Steudel) Wats. – Genetica 72:
211-215.
Lazarević M, Kuzmanović N, Lakušić D,
Alegro A, Schönswetter P, Frajman B. 2015. Patterns of cytotype distribution
and genome size variation in the genus Sesleria Scop. (Poaceae). – Bot. J. Linn. Soc. 179:
126-143.
Lazarides M. 1961. The genus
Ottochloa Dandy (Gramineae) in Australia and its relationship to
Ichnanthus oblongus Hughes. – Aust. J. Bot. 9: 209-215.
Lazarides M. 1972. A revision of Australian
Chlorideae (Gramineae). – Aust. J. Bot., Suppl. Ser. 5.
Lazarides M. 1976. The genus
Eragrostiella Bor (Poaceae,
Eragrostideae). – Contr. Herb. Austral. 22: 1-7.
Lazarides M. 1977. The genus
Whiteochloa C. E. Hubbard (Poaceae, Paniceae). – Brunonia 1:
69-93.
Lazarides M. 1979a. Micraira Fuell.
(Poaceae, Micrairoideae). – Brunonia
2: 67-84.
Lazarides M. 1979b. Hygrochloa, a
new genus of aquatic grasses from the Northern Territory. – Brunonia 2:
85-91.
Lazarides M. 1980a. The genus
Leptochloa Beauv. (Poaceae,
Eragrostideae) in Australia and Papua New Guinea. – Brunonia 3: 247-269.
Lazarides M. 1980b. Aristida L. (Poaceae, Aristideae) in Australia. –
Brunonia 3: 271-333.
Lazarides M. 1980c. The tropical grasses of
Southeast Asia. – Phanerogamarum monographiae 12.
Lazarides M. 1984. New taxa of tropical
Australian grasses (Poaceae). –
Nuytsia 5: 273-303.
Lazarides M. 1995. The genus
Eriachne (Eriachneae, Poaceae).
– Aust. Syst. Bot. 8: 355-452.
Lazarides M. 1997a. A revision of
Eragrostis (Eragrostideae, Eleusininae, Poaceae) in Australia. – Aust. Syst.
Biol. 10: 77-187.
Lazarides M. 1997b. A revision of
Triodia including Plectrachne (Poaceae, Eragrostideae, Triodiinae). –
Aust. Syst. Biol. 10: 381-489.
Lazarides M, Watson L. 1986.
Cyperochloa, a new genus in the Arundinoideae Dumortier (Poaceae). – Brunonia 9: 215-221.
Lazarides M, Webster RD. 1984.
Yakirra (Paniceae, Poaceae), a
new genus for Australia. – Brunonia 7: 289-296.
Lazarides M, Lenz J, Watson L. 1991.
Clausospicula, a new Australian genus of grasses (Poaceae, Andropogoneae). – Aust. Syst.
Bot. 4: 391-405.
Lazarides M, Hacker JB, Andrew MH. 1991.
Taxonomy, cytology and ecology of indigenous Australian sorghums
(Sorghum Moench: Andropogoneae: Poaceae). – Aust. Syst. Bot. 4:
591-635.
Leach GJ. 2000. Notes and new species of
Eriocaulon (Eriocaulaceae) from Australia. –
Aust. Syst. Bot. 13: 755-772.
Leandro TD, Scremin-Dias E, Oliveira Arruda R
do C de. 2016. Micromorphology and anatomy of the leaf blade: a contribution to
the taxonomy of Luziola (Poaceae, Oryzoidee) from the Pantanal,
Brazil. – Plant Syst. Evol. 302: 265-273.
Lebgue T, Valerio A. 1986. Manual para
identificar las gramíneas de Chihuahua. – Talleres Graficos del Gobierno de
Estado Chihuahua, Mexico.
Le Cohu M-C. 1967. Recherches taxinomiques
sur les Carex du Massif Armoricain. – Bot. Rhedonica, sér. A,
no 3: 1-213.
Lee DW, Fairbrothers DE. 1972. Taxonomic
placement of the Typhales within the monocotyledons: preliminary serological
investigation. – Taxon 21: 39-44.
Lee DW, Pin YK, Yew LF. 1975. Serological
evidence on the distinctness of the monocotyledonous families Flagellariaceae, Hanguanaceae, and Joinvilleaceae. – Bot. J. Linn.
Soc. 70: 77-81.
Lehnebach CA. 2012. Re-evaluating species
limits in Uncinia angustifolia, U. caespitosa s.str., U.
rupestris, U. viridis and U. zotovii (Cyperaceae). – Aust. Syst. Bot. 24:
405-420.
Lehväslaiho H, Saura A, Lokki J. 1987.
Chloroplast DNA variation in the grass tribe Festuceae. – Theor. Appl. Gen.
74: 298-302.
Lei Y-X, Liu J, Fan X, Sha L-N, Wang Y, Kang
H-Y, Zhou Y-H, Zhang H-Q. 2018. Phylogeny and molecular evolution of the DMC1
gene in the polyploid genus Roegneria and its affinitive genera (Poaceae: Triticeae). – Bot. J. Linn. Soc.
186: 129-142.
Lei Y-X, Liu J, Fan X, Sha L-N, Wang Y, Kang
H-Y, Zhou Y-H, Zhang H-Q. 2018. Phylogeny and maternal donor of
Roegneria and its affinitive genera (Poaceae: Triticeae) based on sequence data
for two chloroplast DNA regions (ndhF and
trnH-psbA). – J. Syst. Evol. 56: 105-119.
Leistner OA (ed), Gibbs-Russell GE, Watson L,
Koekemoer M, Smook L, Barker NP, Anderson HM, Dallwitz MJ. 1990. Grasses of
southern Africa. – Mem. Bot. Surv. South Africa 58: 1-437.
Leme EMC. 1996. Revision of the lithophytic
Vriesea species from Minas Gerais State, Brazil I. – J. Bromeliad
Soc. 46: 244-246.
Leme EMC. 1997. Canistrum.
Bromélias da Mata Atlântica. – Salamandra Consultoria Editorial Ltda, Rio
de Janeiro.
Leme EMC. 2003. Nominal extinction and the
taxonomist’s responsibility: the example of Bromeliaceae in Brazil. – Taxon 52:
299-302.
Leme EMC. 1998. Canistropsis.
Bromélias da Mata Atlântica. – GMT Editores Ltda, Rio de Janeiro.
Leme EMC. 2000. Nidularium.
Bromélias da Mata Atlântica. – Sexante Arts, Rio de Janeiro.
Leme EMC. 2003. Nominal extinction and the
taxonomist’s responsibility: the example of Bromeliaceae in Brazil. – Taxon 52:
299-302.
Leme EMC. 2004. Studies on
Orthophytum, an endemic genus of Brazil I. – J. Bromeliad Soc. 54:
36-43.
Leme EMC. 2010. Miscellaneous new species in
the Brazilian Bromeliaceae. –
Selbyana 30: 129-133.
Leme EMC, Costa A. 1994. Vriesea
saundersii e V. botafogensis, duas espécies distintas. –
Bromélia 1: 11-18.
Leme EMC, Kollmann LJC. 2013. Miscellaneous
new species of Brazilian Bromeliaceae. – Phytotaxa 108:
1-40.
Leme EMC, Marigo LC. 1993. Bromeliads in the
Brazilian wilderness. – Marigo Comunicação Visual, Rio de Janeiro.
Leme EMC, Rezende B. 2002. On the
revalidation of Aechmea cariocae L. B. Sm. – J. Bromeliad Soc. 52:
262-268.
Leme EMC, Siqueira Filho JA. 2007a. Bromeliad
taxonomy in Atlantic Forest fragments of Pernambuco and Alagoas. – In: Leme
EMC, Siqueira Filho JA (eds), Fragments of the Atlantic Forest of Northeast
Brazil, Andrea Jakobsson Estúdio, Rio de Janeiro, Brazil, pp. 191-378.
Leme EMC, Siqueira Filho JA. 2007b. Taxonomic
considerations correlated with the Bromeliaceae of Pernambuco and
Alagoas. – In: Leme EMC, Siqueira Filho JA (eds), Fragments of the Atlantic
Forest of Northeast Brazil, Andrea Jakobsson Estúdio, Rio de Janeiro, Brazil,
pp. 383-407.
Leme EMC, Heller S, Zizka G, Halbritter H.
2017. New circumscription of Cryptanthus and new Cryptanthoid genera
and subgenera (Bromeliaceae:
Bromelioideae) based on neglected morphological traits and molecular phylogeny.
– Phytotaxa 318(1): 1-88.
Leppik EE. 1955. Dichromena ciliata,
a noteworthy entomophilous plant among Cyperaceae. – Amer. J. Bot. 42:
455-458.
Lerman JC, Raynal J. 1972. La teneur en
isotopes stables du carbone chez les Cypéracées: sa valeur taxonomique. –
Compt. Rend. Acad. Sci. Paris, sér. D, 275: 1391-1394.
Le Roux LG, Kellogg EA. 1999. Floral
development and the formation of unisexual spikelets in the Andropogoneae (Poaceae). – Amer. J. Bot. 86: 354-366.
Leseberg CH, Duvall MR. 2009. The complete
chloroplast genome of Coix lacryma-jobi and a comparative molecular
evolutionary analysis of plastomes in cereals. – J. Mol. Evol. 69:
311-318.
Léveillé-Bourret É, Gilmour CN, Starr JR,
Naczi RFC, Spalink D, Sytsma KJ. 2014. Searching for the sister to sedges
(Carex): resolving relationships in the Cariceae-Dulichieae-Scirpeae
clade (Cyperaceae). – Bot. J. Linn.
Soc. 176: 1-21.
Léveillé-Bourret É, Donadío S, Gilmour
CN, Starr JR. 2015. Rhodoscirpus (Cyperaceae: Scirpeae), a new South
American sedge genus supported by molecular, morphological, anatomical and
embryological data. – Taxon 64: 931-944.
Léveillé-Bourret É, Starr JR, Ford BA.
2018. Why are there so many sedges? Sumatroscirpeae, a missing piece in the
evolutionary puzzle of the giant genus Carex (Cyperaceae). – Mol. Phylogen. Evol.
119: 93-104.
Léveillé-Bourret É, Starr JR, Ford BA.
2018. A revision of Sumatroscirpus (Sumatroscirpeae, Cyperaceae) with discussions on
Southeast Asian biogeography, general collecting, and homologues with
Carex (Cariceae, Cyperaceae). – Syst. Bot. 43:
510-531.
Levering CA, Thomson WW. 1971. The
ultrastructure of the salt gland of Spartina foliosa. – Planta 97:
183-196.
Levy AA, Feldman M. 2002. The impact of
polyploidy on grass genome evolution. – Plant Physiol. 130: 1587-1593.
Levyns MR. 1943. A revision of
Trianoptiles Fenzl. – J. South Afr. Bot. 9: 21-26.
Levyns MR. 1944. Notes on Scirpus
and descriptions of three new species. – J. South Afr. Bot. 10: 25-32.
Levyns MR. 1945. A comparative study of the
inflorescence in four species of Schoenoxiphium and its significance
in relations to Carex and its allies. – J. South Afr. Bot. 11:
79-89.
Lewton-Brain L. 1904. On the anatomy of the
leaves of British grasses. – Trans. Linn. Soc. London, Bot., Ser. II, 6:
315-359.
Li D-Z. 1996a. Proposal to conserve the name
Sinarundinaria Nakai (Gramineae) with a conserved type. – Taxon 45:
321-322.
Li D-Z. 1996b. Proposal to conserve the name
Sasa (Gramineae) with a conserved type. – Taxon 45: 543-544.
Li D-Z. 1997. The Flora of China Bambusoideae
project – problems and current understanding of bamboo taxonomy in China. –
In: Chapman GP (ed), The bamboos, Academic Press, London, pp. 61-81.
Li D-Z. 1998. Taxonomy and biogeography of
the Bambuseae (Gramineae: Bambusoideae). – In: Rao AN, Ramantha Rao V (eds),
Bamboo – Conservation, diversity, ecogeography, germplasm resource
utilization and taxonomy: Proceedings of training course cum workshop, 10–17
May 1998, Kunming and Xishuanbanna, Yunnan, China. Serdang, Malaysia:
IPGRI-APO, pp. 235-247.
Li D-Z, Stapleton CMA, Xia N-H. 2005. New
combinations for Chinese bamboos (Poaceae, Bambuseae). – Novon 15:
599-601.
Li M-R, Jones MB. 1994. Kranzkette, a unique
C4 anatomy occurring in Cyperus japonicus leaves. –
Photosynthetica 30: 117-131.
Li M-R, Wedin DA, Tieszen LL. 1999.
C3 and C4 photosynthesis in Cyperus (Cyperaceae) in temperate eastern North
America. – Can. J. Bot. 77: 18-209.
Li X-L, Liu S, Spong W-Q, Chen R-Y, Wang Y-Z.
1999. Chromosome number of forty species of scattered bamboos. – Acta
Phytotaxon. Sin. 37: 541-544.
Li X-L, Lin R-S, Fung H-L, Qi Z-X, Song W-Q,
Chen R-Y. 2001. Chromosome numbers of some caespitose bamboos native in or
introduced to China. – Acta Phytotaxon. Sin. 39: 433-442.
Li Y-H, Lubke RA, Phipps JB. 1966. Studies in
Arundinelleae (Gramineae) IV. Chromosome numbers of 23 species. – Can. J.
Bot. 44: 387-393.
Liang H, Hilu KW. 1996. Application of the
matK gene sequences to grass systematics. – Can. J. Bot. 74:
125-134.
Lin S-Y, Hao J-J, Xin H, Ding Y-L. 2009. The
megasporogenesis, microsporogenesis and the development of their female and
male gametophyte in Menstruocalamus sichuanensis. – J. Nanjing
Forest Univ. (Nat. Sci. Ed.) 33: 9-12.
Linde-Laursen I, Bothmer R von, Jacobsen N.
1980. Giemsa C-banding in Asiatic taxa of Hordeum section
Stenostachys with notes on chromosome morphology. – Hereditas 93:
235-254.
Linde-Laursen I, Bothmer R von, Jacobsen N.
1986. Giemsa C-banded karyotypes of Hordeum taxa from North America.
– Can. J. Genet. Cytol. 28: 42-62.
Linde-Laursen I, Bothmer R von, Jacobsen N.
1992. Relationships in the genus Hordeum: Giemsa C-banded karyotypes.
– Hereditas 116: 111-116.
Linder HP. 1984. A phylogenetic
classification of the genera of the African Restionaceae. – Bothalia 15:
11-76.
Linder HP. 1985. Conspectus of the African
species of Restionaceae. –
Bothalia 15: 387-503.
Linder HP. 1986. A review of the tropical
African and Malagasy Restionaceae.
– Kew Bull. 41: 99-106.
Linder HP. 1987. The evolutionary history of
the Poales/Restionales: a hypothesis. – Kew Bull. 42: 297-318.
Linder HP. 1991a. A review of the southern
African Restionaceae. – Contr.
Bolus Herb. 14: 209-264.
Linder HP. 1991b. Confidence limits in
phylogenies: an example from the African Restionaceae. – Taxon 40:
253-266.
Linder HP. 1992a. The structure and evolution
of the female flower of the African Restionaceae. – Bot. J. Linn. Soc.
109: 401-425.
Linder HP. 1992b. The gynoecia of Australian
Restionaceae: morphology, anatomy
and systematic implications. – Aust. Syst. Bot. 5: 227-245.
Linder HP. 1995a. Ceratocaryum
pulchrum, a new restioid from the Bredasdorp plains. – South Afr. J.
Bot. 61: 222-225.
Linder HP. 1995b. Restio
mlanjiensis, a new species of Restionaceae from south-central
Africa. – Kew Bull. 50: 623-625.
Linder HP. 1999. Rytidosperma
vickeryae – a new danthonioid grass from Kosciuszko (New South Wales,
Australia): morphology, phylogeny and biogeography. – Aust. Syst. Bot. 12:
743-755.
Linder HP. 2000. Vicariance, climate change,
anatomy and phylogeny of Restionaceae. – Bot. J. Linn. Soc.
134: 159-177.
Linder HP. 2001a. On areas of endemism, with
an example from the African Restionaceae. – Syst. Biol. 50:
892-912.
Linder HP. 2001b. Two new species of
Ceratocaryum (Restionaceae). – Kew Bull. 56:
465-477.
LLinder HP. 2005. Evolutionary history of
Poales. – Ann. Rev. Ecol. Syst. 36: 107-124.
Linder HP, Barker NP. 2000. Biogeography of
the Danthonieae. – In: Jacobs SWL, Everett J (eds), Grasses: systematics and
evolution, Proceedings of the 2nd International Conference on the
Comparative Biology of Monocotyledons, Sydney, Australia, Vol. 2, CSIRO Publ.,
Melbourne, pp. 231-238.
Linder HP, Barker NP. 2005. From Nees to now
– changing questions in the systematics of the grass subfamily
Danthonioideae. – Nova Acta Leop. 92, 342: 29-44
Linder HP, Caddick LR. 2001. Restionaceae seedlings: morphology,
anatomy and systematic implications. – Feddes Repert. 112: 59-80.
Linder HP, Ellis RP. 1990a. A revision of
Pentaschistis (Arundineae: Poaceae). – Contr. Bolus Herb. 12:
1-124.
Linder HP, Ellis RP. 1990b. Vegetative
morphology and interfire survival strategies in the Cape fynbos grasses. –
Bothalia 20: 91-103.
Linder HP, Ferguson IK. 1985. On the pollen
morphology and phylogeny of the Restionales and Poales. – Grana 24: 65-76.
Linder HP, Hardy CR. 2010. A generic
classification of the Restioneae (Restionaceae), southern Africa. –
Bothalia 40: 1-35.
Linder HP, Mann DM. 1998. The phylogeny and
biogeography of Thamnochortus (Restionaceae). – Bot. J. Linn. Soc.
128: 319-357.
Linder HP, Rudall PJ. 1993. The
megagametophyte in Anarthria (Anarthriaceae, Poales) and its
implications for the phylogeny of the Poales. – Amer. J. Bot. 80:
1455-1464.
Linder HP, Rudall PJ. 2005. Evolutionary
history of Poales. – Ann. Rev. Ecol. Syst. 36: 107-124.
Linder HP, Verboom GA. 1996. Generic limits
in the Rytidosperma (Danthonieae, Poaceae) complex. – Telopea 6:
597-627.
Linder HP, Vlok JH. 1991. The morphology,
taxonomy and evolution of Rhodocoma (Restionaceae). – Plant Syst. Evol.
175: 139-160.
Linder HP, Thompson JF, Ellis RP, Perold SM.
1990. The occurrence, anatomy and systematic implications of the glands in
Pentaschistis and Prionanthium (Poaceae, Arundinoideae, Arundineae). –
Bot. Gaz. (Crawfordsville) 151: 221-233.
Linder HP, Verboom GA, Barker NP. 1997.
Phylogeny and evolution in the Crinipes group of grasses
(Arundinoideae: Poaceae). – Kew Bull.
52: 91-110.
Linder HP, Briggs BG, Johnson LAS. 1998a. Anarthriaceae. – In: Kubitzki K
(ed), The families and genera of vascular plants IV. Flowering plants.
Monocotyledons. Alismatanae and Commelinanae (except Gramineae), Springer,
Berlin, Heidelberg, New York, pp. 19-21.
Linder HP, Briggs BG, Johnson LAS. 1998b. Ecdeiocoleaceae. – In: Kubitzki K
(ed), The families and genera of vascular plants IV. Flowering plants.
Monocotyledons. Alismatanae and Commelinanae (except Gramineae), Springer,
Berlin, Heidelberg, New York, pp. 195-197.
Linder HP, Briggs BG, Johnson LAS. 1998c. Restionaceae. – In: Kubitzki K (ed),
The families and genera of vascular plants IV. Flowering plants.
Monocotyledons. Alismatanae and Commelinanae (except Gramineae), Springer,
Berlin, Heidelberg, New York, pp. 425-445.
Linder HP, Briggs BG, Johnson LAS. 2000. Restionaceae: a morphological
phylogeny. – In: Wilson KL, Morrison DA (eds), Monocots: systematics and
evolution, Proceedings of the 2nd International Conference on the
Comparative Biology of Monocotyledons, Sydney, Australia, Vol. 1, CSIRO Publ.,
Melbourne, pp. 653-660.
Linder HP, Eldenäs P, Briggs BG. 2003.
Contrasting patterns of radiation in African and Australian Restionaceae. – Evolution 57:
2688-2702.
Linder HP, Hardy CR, Rutschmann F. 2005.
Taxon sampling effects in molecular clock dating: an example from the African
Restionaceae. – Mol. Phylogen.
Evol. 35: 569-582.
Linder HP, Baeza M, Barker NP, Galley C,
Humphreys AM, Lloyd KM, Orlovich DA, Pirie MD, Simon BK, Walsh N, Verboom GA.
2010. A generic classification of the Danthonioideae (Poaceae). – Ann. Missouri Bot. Gard. 97:
306-364.
Lindquist B. 1932. Taxonomical remarks on
Juncus alpinus Vill. and some related species. – Bot. Not. 85:
313-372.
Liphschitz N, Waisel Y. 1974. Existence of
salt glands in various genera of the Gramineae. – New Phytol. 73: 507-512.
Lipkin R. 1983. Systematics of the arctic
grass genus Dupontia R. Br. – M.Sc. thesis, University of Alaska,
Fairbanks, Alaska.
Lisowski S. 1999. Xyris nouveaux (Xyridaceae) du Haut-Katanga
(Congo-Kinshasa). – Syst. Geogr. Plants 69: 205-214.
Litardière R de. 1945. Contribution à
l’étude du genre Festuca. – Candollea 10: 103-146.
Litke R. 1968. Über den Nachweis tertiärer
Gramineen. – Monatsber. Deutsch. Akad. Wiss. Berlin 19: 462-471.
Liu H, Hu XY, Liu YX, Liu Q. 2015. Caryopsis
micromorphologicdal survey of Sorghum (Poaceae) – Taxonomic implications. –
South Afr. J. Bot. 99: 1-11.
Liu Q, Zhao N-X, Hao G, Hu X-Y, Liu Y-X.
2005. Caryopsis morphology of the Chloridoideae (Gramineae) and its systematic
implications. – Bot. J. Linn. Soc. 148: 57-72.
Liu Q, Zhao N-X, Hao G. 2005a. The phylogeny
of the Chloridoideae (Gramineae): a cladistic analysis. – J. Trop. Subtrop.
Bot. 13: 432-442.
Liu Q, Zhao N-X, Hao G. 2005b. Inflorescence
structures and evolution in subfamily Chloridoideae (Gramineae). – Plant
Syst. Evol. 251: 183-198.
Liu Q, Ge S, Zhang X, Zhu G, Lu B-R. 2006.
Phylogenetic relationships in Elymus (Poaceae: Triticeae) based on the nuclear
ribosomal internal transcribed spacer and chloroplast trnL-F
sequences. – New Phytol. 170: 411-420.
Liu Q, Zhang D-X, Peterson PM. 2010. Lemma
micromorphological characters in the Chloridoideae (Poaceae) optimized on a molecular
phylogeny. – South Afr. J. Bot. 76: 196-209.
Liu Q, Triplett JK, Wen J, Peterson PM. 2011.
Allotetraploid origin and divergence in Eleusine (Chloridoideae, Poaceae): evidence from low-copy nuclear
gene phylogenies and a plastid gene chronogram. – Ann. Bot. 108:
1287-1298.
Liu Q, Jiang B, Wen J, Peterson PM. 2014.
Low-copy nuclear gene McGISH resolves polyploid history of Eleusine
coracana and morphological character evolution in Eleusine. –
Turkish J. Bot. 38: 1-12.
Liu Q, Liu H, Wen J, Peterson PM. 2014.
Infrageneric phylogeny and temporal divergence of Sorghum
(Andropogoneae, Poaceae) based on
low-copy nuclear and plastid sequences. – PLoS ONE 9: e104933.
Liu Z, Chen Z, Pan J, Li X, Su M, Wang L, Li
H, Liu G. 2008. Phylogenetic relationships in Leymus (Poaceae: Triticeae) revealed by the nuclear
ribosomal internal transcribed spacer and chloroplast trnL-F
sequences. – Mol. Phylogen. Evol. 46: 278-289.
Lizarazu MA, Nicola MV, Salariato DL. 2014.
Taxonomic re-evaluation of Panicum sections Tuerckheimiana
and Valida (Poaceae:
Panicoideae) using morphological and molecular data. – Taxon 63: 265-274.
Llauradó M. 1984. El género
Paspalum L. a Catalunya. – Bull. Inst. Catalana Hist. Nat., Secc.
Bot. 51: 101-108.
Lobin W. 1982. Beitrag zur Kenntnis der Cyperaceae (Phanerogamae,
Monocotyledoneae) der Kapverdischen Inseln. – Courier Forschungsinst.
Senckenberg 52: 265-276.
Lock JM. 1998. Notes on the genus
Xyris (Xyridaceae) in East
Africa. – Kew Bull. 53: 883-895.
Lock JM. 1999a. Xyridaceae. – In: Beentje HJ,
Whitehouse CM (eds), Flora of tropical East Africa, A. A. Balkema, Rotterdam,
pp. 1-24.
Lock JM. 1999b. A synopsis of Xyris
(Xyridaceae) in South-Central Africa.
– Kew Bull. 54: 301-326.
Lock JM. 2001 [2002]. Monographs and
revisions of African genera. Short communications: Xyris (Xyridaceae) in Africa: a progress
report. – Syst. Geogr. Plants 71: 443-445.
Lock JM. 2002. New species of Xyris
(Xyridaceae) from Tanzania. – Kew
Bull. 57: 445-450.
Lohauss K. 1905. Der anatomische Bau der
Laubblätter der Festucaceen und dessen Bedeutung für die Systematik. –
Bibl. Bot. 13: 1-114.
Lonard RI, Gould FW. 1974. The North American
species of Vulpia (Gramineae). – Madroño 22: 217-230.
Londoño X, Clark LG. 1998. Eight new taxa
and two new reports of Bambuseae (Poaceae: Bambuseae) from Colombia. –
Novon 8: 408-428.
Londoño X, Clark LG. 2002a. A revision of
the Brazilian bamboo genus Eremocaulon (Poaceae: Bambuseae: Guaduinae). – Syst.
Bot. 27: 703-721.
Londoño X, Clark LG. 2002b. Three new taxa
of Guadua (Poaceae:
Bambusoideae) from South America. – Novon 12: 64-76.
Londoño X, Peterson PM. 1991. Guadua
sarcocarpa (Poaceae: Bambuseae), a
new species of Amazonian bamboo with fleshy fruits. – Syst. Bot. 16:
630-638.
Londoño X, Peterson PM. 1992. Guadua
chacoensis (Poaceae: Bambuseae),
its taxonomic identity, morphology, and affinities. – Novon 2: 41-47.
Longhi-Wagner HM. 1994. Aristidia
(Poaceae): two new species from Brazil.
– Kew Bull. 49: 817-821.
Longhi-Wagner HM. 2005. New neotropical taxa
in the genus Ctenium (Poaceae-Chloridoideae-Cynodonteae). – Kew
Bull. 60: 123-127.
Longhi-Wagner HM, Cope TA. 2014. The genus
Ctenium (Poaceae:
Chloridoideae: Chlorideae) in Africa. – Kew Bull. 69: 9541.
Longhi-Wagner HM, Renvoize SA. 2004. The
genus Ctenium (Poaceae-Cynodonteae) in Bolivia. – Kew
Bull. 59: 305-309.
Loos GH. 1996. Zur Identität von Carex
leersiana Rauschert, C. chaberti F. W. Schultz, C.
polyphylla Kar. und Kir. und C. guestphalica (Boenn. ex Rchb.)
Boenn. ex O. F. Lang. – Feddes Repert. 107: 61-74.
López A, Morrone O. 2012. Phylogenetic
studies in Axonopus (Poaceae,
Panicoideae, Paniceae) and related genera: morphology and molecular (nuclear
and plastid) combined analyses. – Syst. Bot. 37: 671-676.
López J, Devesa JA. 1991. Contribución al
conocimiento de la anatomía foliar de las Aveneae (Poaceae, Pooideae) del Centro-Oeste de
España. – Anales Jard. Bot. Madrid 48: 171-187.
López MG, Prata AP, Thomas WW. 2007. New
synonymy and new distributional records in Bulbostylis (Cyperaceae) from South America. –
Brittonia 59: 88-96.
Lorch J. 1962. A revision of Crypsis
Ait. s.l. (Gramineae). – Bull. Res. Counc. Israel 11D: 91-116.
Lorougnon G. 1973. Le vecteur pollinique chez
les Mapania et les Hypolytrum, cypéracées du sous-bois des
forêts tropicales ombrophiles. – Bull. Jard. Bot. Natl. Belg. 45:
181-184.
Lourteig A. 1999. 211. Mayacaceae. – In: Harling G, Andersson
L (eds), Flora of Ecuador 63, Nord. J. Bot., Copenhagen, pp. 7-11.
Louis-Marie F. 1928. The genus
Trisetum in America. – Rhodora 30: 209-223, 237-245.
Loureiro J, Kopecký D, Castro S, Santos C,
Silveira P. 2007. Flow cytometric and cytogenetic analyses of Iberian Peninsula
Festuca spp. – Plant Syst. Evol. 269: 89-105.
Lourteig A. 1952. Mayacaceae. – Notulae Syst. (Paris)
14: 234-248.
Lourteig A. 1968. Étude sur Uncinia
compacta R. Br. (Cyperaceae).
– Bull. Comité Natl. Français Rech. Antarctique – Biol. 23: 25-31.
Louzada RB. 2008. Taxonomia e citogenética
das espécies de inflorescência séssil do gênero Orthophytum Beer
(Bromeliaceae). – Instituto de
Botânica, São Paulo, pp. 1-102.
Louzada RB, Versieux LM. 2010.
Lapanthus (Bromeliaceae,
Bromelioideae): a new genus from the southern Espinhaço Range, Brazil. –
Syst. Bot. 35: 497-503.
Louzada RB, Wanderley M das GL. 2010.
Revision of Orthophytum (Bromeliaceae): the species with
sessile inflorescences. – Phytotaxa 13: 1-26.
Louzada RB, Schulte K, Graças L. Wanderley M
das, Silvestro D, Zizka G, Barfuss MHJ, Palma-Silva C. 2014. Molecular
phylogeny of the Brazilian endemic genus Orthophytum (Bromelioideae,
Bromeliaceae) and its implications
on morphological character evolution. – Molec. Phylogen. Evol. 77: 54-64.
Löve Á. 1984. Conspectus of the Triticeae.
– Feddes Repert. 95: 425-521.
Lu B-R. 1993. Biosystematic investigations of
Asiatic wheatgrasses – Elymus L. (Triticeae, Poaceae). – Svalöv.
Lu B-R. 1995. Taxonomy and morphology of the
Elymus parviglumis group (Triticeae: Poaceae). – Nord. J. Bot. 15: 3-37.
Lu B-R, Salomon B. 1993. Two new Tibetan
species of Elymus (Poaceae:
Triticeae) and their genomic relationships. – Nord. J. Bot. 13: 353-367.
Lucas H, Jahier J. 1988. Phylogenetic
relationships in some diploid species of Triticinae: cytogenetic analysis of
interspecific hybrids. – Theor. Appl. Gen. 75: 498-502.
Luceño M. 1992. Cytotaxonomic studies in
Iberian and Macaronesian species of Carex (Cyperaceae). – Willdenowia 22:
149-165.
Luceño M. 1994. Monografía del género
Carex L. en la Península Ibérica e Islas Baleares. – Ruizia 14:
1-139.
Lucero LE, Vegetti AC, Reinheimer R. 2014.
Evolution and development of the spikelet and flower of Rhynchospora
(Cyperaceae). – Intern. J. Plant
Sci. 175: 186-201.
Luo MC, Deal KR, Akhunov ED, Akhunova AR,
Anderson OD, Anderson JA, Blake N, Clegg MT, Coleman-Derr D, Conley EE,
Crossman CC, Dubcovsky J, Gill BS, Gu Y-Q, Hadam J, Heo H, Huo N, Lazo GR,
Lundy KE, Ma Y, Matthews DE, Mcguire PE, Morrell PL, Nicolet CM, Qualset CO,
Renfro J, Tabano D, Talbert LE, Tian A, Toleno DM, Warburton ML, You F-M, Zhang
W-J, Dvorak J. 2009. Genome comparisons reveal a dominant mechanism of
chromosome number reduction in grasses and accelerated genome evolution in
Triticeae. – Proc. Natl. Acad. Sci. U.S.A. 106: 15780-15785.
Luo S, Peng J, Li K, Wang M, Kuang H. 2011.
Contrasting evolutionary patterns of the Rp1 resistance gene family in
different species of Poaceae. – Mol.
Biol. Evol. 28: 313-325.
Luther HE. 1989. A provisional checklist of
the Bromeliaceae of Ecuador. –
Phytologia 67: 312-330.
Luther HE. 1992. Three new species of Bromeliaceae from Ecuador. – Nord.
J. Bot. 12: 219-221.
Luther HE. 1994. A new species and two new
combinations of Ecuadorian Bromeliaceae (Tillandsioideae). –
Nord. J. Bot. 14: 327-329.
Luther HE. 2002. An alphabetical list of
bromeliad binomials. – The Bromeliad Society International, Sarasota,
Florida.
Luther HE. 2003. Miscellaneous new taxa of Bromeliaceae XVI. – Brittonia 54:
279-285. – Erratum: 57 (2005): 202.
Luther HE. 2006. An alphabetical list of
bromeliad binomials. – The Bromeliad Society International, Sarasota,
Florida.
Luther HE. 2010. An alphabetical list of
bromeliad binomials. 12th ed. – Sarasota Bromeliad Society and Marie Selby
Botanical Gardens, Sarasota, Florida.
Luther HE. 2012. An alphabetical list of
bromeliad binomials. 13th ed. – Marie Selby Botanical Gardens and The
Bromeliad Society International, Sarasota, Florida.
Luther HE, Norton KF. 2008. Epiphytism in Bromeliaceae: a synopsis. – Selbyana
29: 215-216.
Luther HE, Sieff E. 1991. An alphabetical
list of bromeliad binomials. – The Bromeliad Society, Inc., Orlando,
Florida.
Lye KA. 1971a. Studies in African Cyperaceae II. The genus
Oxycaryum Nees. – Bot. Not. 124: 280-286.
Lye KA. 1971b. Studies in African Cyperaceae III. A new species of
Schoenoplectus and some new combinations. – Bot. Not. 124:
287-291.
Lye KA. 1971c. The generic concept of
Bulbostylis Kunth ex C. B. Cl. – Mitt. Bot. Staatssamml. München
10: 539-547.
Lye KA. 1971d. Moderne oppfatning av slekta
Scirpus L. – Blyttia 29: 141-147.
Lye KA. 1972a. Studies in African Cyperaceae V. Sphaerocyperus K.
Lye, gen. nov. – Bot. Not. 125: 212-216.
Lye KA. 1972b. Studies in African Cyperaceae VI. New species and
combinations in Kyllinga – Bot. Not. 125: 217-219.
Lye KA. 1973. Studies in African Cyperaceae VIII. The taxonomic position
of Abildgaardia Vahl and Nemum Hamilton. – Bot. Not. 126:
325-329.
Lye KA. 1974a. Studies in African Cyperaceae X. New taxa and combinations
in Fuirena Rottb. – Bot. Not. 127: 109-112.
Lye KA. 1974b. Studies in African Cyperaceae XI. New taxa and combinations
in Abildgaardia Vahl. – Bot. Not. 127: 493-497.
Lye KA. 1974c. Studies in African Cyperaceae XII. New taxa and
combinations in Fimbristylis Vahl. – Bot. Not. 127: 498-499.
Lye KA. 1981a. Studies in African Cyperaceae 18. Two new subgenera of
Cyperus. – Nord. J. Bot. 1: 57-61.
Lye KA. 1981b. Studies in African Cyperaceae 19. The genera
Anosporum Nees and Sorostachys Steudel. – Nord. J. Bot. 1:
186-191.
Lye KA. 1981c. Studies in African Cyperaceae 20. New taxa and combinations
in Pycreus Beauv. – Nord. J. Bot. 1: 617-622.
Lye KA. 1981d. Studies in African Cyperaceae 21. New taxa and combinations
in Kyllinga Rottb. – Nord. J. Bot. 1: 741-747.
Lye KA. 1981e. Studies in African Cyperaceae 22. New taxa and combinations
in Abildgaardia Vahl II. – Nord. J. Bot. 1: 749-758.
Lye KA. 1982a. Studies in African Cyperaceae 23. New taxa and combinations
in Fimbristylis Vahl II. – Nord. J. Bot. 2: 333-335.
Lye KA. 1982b. Studies in African Cyperaceae 24. New taxa and combinations
in Ascolepis and Isolepis. – Nord. J. Bot. 2: 561-566.
Lye KA. 1983a. Studies in African Cyperaceae 25. New taxa and combinations
in Cyperus L. – Nord. J. Bot. 3: 213-232.
Lye KA. 1983b. Studies in African Cyperaceae 26. New taxa and combinations
in Abildgaardia Vahl III. – Nord. J. Bot. 3: 233-239.
Lye KA. 1984b. Studies in African Cyperaceae 27. Miscellaneous new taxa
and combinations. – Nord. J. Bot. 3: 241-244.
Lye KA. 1987. Studies in African Cyperaceae 28. New taxa and combinations
in Abildgaardia Vahl IV. – Nord. J. Bot. 7: 39-50.
Lye KA. 1988. Two new yellow species of
Cyperus L. from Africa. – Candollea 43: 505-511.
Lye KA. 1989. A new species of Nemum
(Cyperaceae) from West Africa. –
Lidia 2: 33-36.
Lye KA. 1992. The history of the genus
Mariscus (Cyperaceae). –
Lidia 3: 37-72.
Lye KA. 1996a. A new species of
Eriocaulon (Eriocaulaceae) from Ethiopia. –
Nord. J. Bot. 16: 63-68.
Lye KA. 1996b. Eight new species of
Cyperus (Cyperaceae) from
Somalia. – Nord. J. Bot. 16: 367-377.
Lye KA. 2003. Schoenoplectiella Lye,
gen. nov. (Cyperaceae). – Lidia 6:
20-29.
Lye KA. 2016. Chemistry of fruit wall and
seed in Cyperaceae tribe Trilepideae.
– South Afr. J. Bot. 106: 104-109.
Lye KA, Haines RW. 1970. Studies in African
Cyperaceae I. A new species of
Scirpus (Cyperaceae) from
Mt. Elgon. – Bot. Not. 123: 430-432.
Lye KA, Haines RW. 1974. Studies in African
Cyperaceae XIII. New taxa and
combinations in Isolepis R. Br. – Bot. Not. 127: 522-526.
Lye KA, Haines RW. 1977. Studies in African
Cyperaceae XVI. New taxa of
Isolepis R. Br. – Bot. Not. 130: 311-313.
Ma P-F, Guo Z-H, Li D-Z. 2012. Rapid
sequencing of the bamboo mitochondrial genome using Illumina technology and
parallel episodic evolution of organelle genomes in grasses. – PloS One 7:
e30297.
Ma P-F, Zhang Y-X, Zeng C-X, Guo Z-H, Li D-Z.
2014. Chloroplast phylogenomic analyses resolve deep-level relationships of an
intractable bamboo tribe Arundinarieae (Poaceae). – Syst. Biol. 63: 933-950.
McClintock D. 1983. New combinations in some
temperate bamboos, and a new variety. – Kew Bull. 38: 485-486.
McClure FA. 1940. New genera and species of
Bambusaceae from eastern Asia. – Sci. Bull. Lingnan Univ. 9: 19-20.
McClure FA. 1966. The bamboos. A fresh
perspective. – Harvard University Press, Cambridge, Massachusetts.
McClure FA. 1973. Genera of bamboos native to
the New World. – Smithsonian Contr. Bot. 9: 1-148.
Macfarlane TD. 1987. Poaceae subfamily Pooideae. – In:
Soderstrom TR, Hilu KW, Campbell CS, Barkworth ME (eds), Grass systematics and
evolution, Smithsonian Institution Press, Washington, D.C., pp. 265-276.
Macfarlane TD, Watson L. 1980. The
circumscription of Poaceae subfamily
Pooideae, with notes on some controversial genera. – Taxon 29: 645-666.
Macfarlane TD, Watson L. 1982. The
classification of Poaceae subfamily
Pooideae. – Taxon 31: 178-203.
McInerney FA, Strömberg CAE, White JWC.
2011. The Neogene transition from C3 to C4 grasslands in
North America: stable carbon isotope ratios of fossil phytoliths. –
Paleobiology 37: 23-49.
McNeill J. 1979. Diplachne and
Leptochloa (Poaceae) in North
America. – Brittonia 31: 399-404.
McNeill J, Wiersema JH, Gandhi K. 2010. The
complex nomenclature of Panicum sect. or subg. Agrostoidea
and of P. agrostoides. – Taxon 59: 1581-1584.
McWilliams EL. 1968. Natural and cultivated
bromeliads in southeastern Brazil. – Bromeliad Soc. Bull. 18: 123-137.
McWilliams EL. 1974. Chromosome number and
evolution. – In: Smith LB, Downs RJ (eds), Bromeliaceae. Flora Neotropica 14,
Hafner Press, New York, pp. 33-40.
Magalhães R, Mariath J. 2012. Seed
morphoanatomy and its systematic relevance to Tillandsioideae (Bromeliaceae). – Plant Syst. Evol.
298: 1881-1895.
Maguilla E, Escudero M, Waterway MJ, Hipp AL,
Luceño M. 2015. Phylogeny, systematics, and trait evolution of Carex
section Glareosae. – Amer. J. Bot. 102: 1128-1144.
Maguire B. 1958a. Xyridaceae. – In: Maguire B, Wurdack
JJ et al. (eds), The botany of the Guayana Highland III, Mem. New York Bot.
Gard. 10: 1-19.
Maguire B. 1958b. Rapateaceae. – In: Maguire B, Wurdack
JJ et al. (eds), The botany of the Guayana Highland III, Mem. New York Bot.
Gard. 10: 19-49.
Maguire B. 1965. Rapateaceae. – In: Maguire B, Wurdack
JJ et al. (eds), The botany of the Guayana Highland VI, Mem. New York Bot.
Gard. 12: 69-102.
Maguire B. 1979. Additions to the Rapateaceae. – Acta Amazonica 9:
267-269.
Maguire B, Smith LB. 1963. Xyridales. – In:
Maguire B, Wurdack JJ et al. (eds), The botany of the Guyana Highland V, Mem.
New York Bot. Gard. 10: 7-37.
Maguire B, Wurdack JJ. 1960. Xyridaceae. – In Maguire B, Wurdack JJ
et al. (eds), The botany of the Guyana Highland IV, Mem. New York Bot. Gard.
10: 11-15.
Maguire B, Wurdack JJ. 1965. The botany of
the Guyana Highland VI. – Mem. New York Bot. Gard. 12: 69-102.
Mahelka V, Kopecky D. 2010. Gene capture from
across the grass family in the allohexaploid Elymus repens (L.) Gould
(Poaceae, Triticeae) as evidenced by
ITS, GBSSI, and molecular cytogenetics. – Mol. Biol. Evol. 27: 1370-1390.
Mai DH. 2000. Die mittelmiozänen und
obermiozänen Floren aus der Meuroer und Raunoer Folge in der Lausitz. Teil 1.
Farnpflanzen, Koniferen und Monocotyledonen. – Palaeontographica, Ser. B,
256: 1-68.
Maier R, Neckermann K, Igloi G, Kossel H.
1995. Complete sequence of the maize chloroplast genome: gene content, hotspots
of divergence and fine tuning of genetic information by transcript editing. –
J. Mol. Biol. 251: 614-628.
Makde KH. 1982. Pollen development in the Cyperaceae. – J. Indian Bot. Soc. 61:
242-249.
Makde KH, Untawale AG. 1989. Contribution to
the embryology of Fimbristylis Vahl with a brief discussion on its
systematic position. – Beitr. Biol. Pflanzen 64: 231-242.
Malato-Beliz J. 1970. Gramíneas da Ilha de
Maio (Arquipélago de Cabo Verde). – Bot. Soc. Brot., ser. II, 44:
251-277.
Malcev AI. 1930. Ovsjugi i ovsy Sectio
Euavena Griseb. – Trudy Prikl. Bot. Suppl. 38.
Malcomber ST, Kellogg EA. 2005.
SEPALLATA gene diversification: brave new whorls. – Trends Plant
Sci. 10: 427-435.
Malcomber ST, Preston JC, Reinheimer R,
Kossuth J, Kellogg EA. 2006. Developmental gene evolution and the origin of
grass inflorescence diversity. – Adv. Bot. Res. 44: 423-479.
Males J. 2016. Think tank: water relations of
Bromeliaceae in their evolutionary
context. – Bot. J. Linn. Soc. 181: 415-440.
Malheiros N, Gardé A. 1947. Contribuiçoes
para o estudo citológico do género Luzula Link. – Agron. Lusit. 9:
75-79.
Malheiros N, Castro D, Câmara A. 1947.
Cromosomas sem centrómero localizado; o casa da Luzula purpurea Link.
– Agron. Lusitana 9: 51-74.
Malik CP, Thomas PT. 1966. Karyotypic studies
in some Lolium and Festuca species. – Caryologia 19:
167-196.
Malmanche LA. 1919. Contribution à
l’étude anatomique des eriocaulonacées et des familles voisines:
restiacées, centrolépidacées, xyridacées, philydracées, mayacacées. –
Thèse Fac. Sci., Paris, Imprimeries Girault, St. Cloud, Paris.
Malme GOA. 1925. Xyridologische Beiträge.
– Ark. f. Bot. 19(13): 1-8.
Malme GOA. 1930. Xyridaceae. – In: Engler A (ed), Die
natürlichen Pflanzenfamilien, 2. Aufl., Bd. 15a, W. Engelmann, Leipzig, pp.
35-38.
Malme GOA. 1933. Beiträge zur Kenntnis der
südamerikanischen Xyridazeen. – Ark. f. Bot. 25(12): 1-18.
Manning JC, Linder HP. 1990. A cladistic
analysis of patterns of endothecial thickenings in the Poales/Restionales. –
Amer. J. Bot. 77: 196-210.
Mant JG, Bayer RJ, Crisp MD, Trueman JWH.
2000. A phylogeny of Triodieae (Poaceae:
Chloridoideae) based on the ITS region of nrDNA: Testing conflict between
anatomical and inflorescence characters. – In: Jacobs SWL, Everett J (eds),
Grasses: systematics and evolution, Proceedings of the 2nd
International Conference on the Comparative Biology of Monocotyledons, 1998,
Sydney, Australia, Vol. 2, CSIRO Publ., Melbourne, pp. 213-217.
Mantovani A, Venda A, Almeida V, Costa A,
Forzza R. 2012. Leaf anatomy of Quesnelia (Bromeliaceae): implications for the
systematics of core bromelioids. – Plant Syst. Evol. 298: 787-800.
Marchant AD, Briggs BG. 2007. Ecdeiocoleaceae and Joinvilleaceae, sisters to Poaceae (Poales): evidence from
rbcL and matK data. – Telopea 11: 437-450.
Marchant CJ. 1967. Chromosome evolution in
the Bromeliaceae. – Kew Bull. 21:
161-168.
Marek S. 1958. A study of the anatomy of
fruits of Europaean genera in the subfamilies Scirpoideae Pax,
Rhynchosporoideae Aschers. et Graebner and some genera of Caricoideae Pax. –
Monogr. Bot. 6: 151-177.
Marhold K, Hroudová Z, Ducháček M
Zákravský P. 2004. The Bolboschoenus maritimus group (Cyperaceae), in Central Europe,
including B. laticarpus, spec. nova. – Phyton 44: 1-21.
Maria GA, López MG. 2010. Development and
morphology of the gynoecium and nutlet in two South-American
Bulbostylis (Cyperaceae)
species. – Flora 205: 211-220.
Markgraf-Dannenberg I. 1976. Die Gattung
Festuca in Griechenland. – Veröff. Geobot. Inst. ETH Stiftung
Rübel Zürich 56: 92-182.
Markgraf-Dannenberg I. 1981. The genus
Festuca (Gramineae) in Turkey: new taxa and new names. – Willdenowia
11: 201-210.
Martel E, Poncet V, Lamy F, Siljak-Yakovlev
S, Lejeune B, Sarr A. 2004. Chromosome evolution of Pennisetum species
(Poaceae): implications of ITS
phylogeny. – Plant Syst. Evol. 249: 139-149.
Martin CE. 1994. Physiological ecology of the
Bromeliaceae. – Bot. Rev. 60:
1-82.
Marín-Bravo S, Escudero M, Míguez M,
Jiménez-Mejías P, Luceño M. 2013. Molecular and morphological evidence for a
new species from South Africa: Carex rainbowii (Cyperaceae). – South Afr. J. Bot. 87:
85-91.
Martinelli G. 1994. Reproductive biology of
Bromeliaceae in the Atlantic
rainforest of southeastern Brazil. – Ph.D. diss., University of St. Andrews,
England.
Martinetto E, Bouvet D, Vassio E,
Jiménez-Mejías P. 2014. A new protocol for the collection and cataloguing of
reference material for the study of fossil Cyperaceae fruits: the modern
carpological collection. – Rev. Palaeobot. Palyn. 201: 56-74.
Martínez AMS, Salazar GA, Aranda PD. 2007.
Phylogenetic relationships of Zeugites (Poaceae: Centothecoideae) inferred from
plastid and nuclear DNA sequences and morphology. – Syst. Bot. 32:
722-730.
Martínez-y-Pérez JL, Sosa V, Mejia-Saules
T. 2006. Species delimitation in the Luziola peruviana (Poaceae) complex. – Brittonia 58:
362-375.
Martínez-y-Pérez JL, Mejía-Saulés T, Sosa
V. 2008. A taxonomic revision of Luziola (Poaceae: Oryzeae). – Syst. Bot. 33:
702-718.
Martinovský JO. 1967. Neue submediterrane
Stipa-Arten und die taxonomische Einteilung der Federgrassippen der
Serie Pulcherrimae Martinovský. – Preslia 39: 260-275.
Martinovský JO. 1972. Beitrag zur Kenntnis
der Gattung Stipa XXIV. Studien über einige submediterrane
Federgrassippen. – Preslia 44: 7-23.
Martinovský JO. 1976. Neue
Stipa-Sippen und einige Ergänzungen der früher beschriebenen
Stipa-Taxa. – Preslia 48: 186-188.
Martins S, Alves M. 2009. Anatomical features
of species of Cyperaceae from
northeastern Brazil. – Brittonia 61: 189-200.
Martins S, Scatena VR. 2011. Bundle sheath
ontogeny in Kranz and non-Kranz species of Cyperaceae (Poales). – Aust. J. Bot.
59: 554-562.
Maruyama I, Okamura H, Murata G. 1979. On a
new hybrid genus Hibanobambusa. – Acta Phytotaxon. Geobot. 30:
148-152. [In Japanese]
Mary Z, Pattan Shetty JK, Yoganarasimhan SN.
1985. Pharmacognostical studies on Flagellaria indica L. (Flagellariaceae). – J. Econ.
Taxon. Bot. 6: 1-8.
Mashau AC. 2016. A synopsis of
Anthoxanthum (Poaceae:
Pooideae: Poeae) in southern Africa and description of a new subspecies. –
Kew Bull. 71: 18 DOI 10.1007/S12225-016-9629-6
Mashau AC, Fish L, Wyk AE van. 2010. Two new
species of Helictotrichon (Pooideae: Aveneae) from South Africa. –
Bothalia 40: 179-183.
Mason-Gamer RJ. 2001. Origin of North
American Elymus (Poaceae:
Triticeae) allotetraploids based on granule-bound starch synthase gene
sequences. – Syst. Bot. 26: 757-768.
Mason-Gamer RJ. 2004. Reticulate evolution,
introgression, and intertribal gene capture in an allohexploid grass. – Syst.
Biol. 53: 25-37.
Mason-Gamer RJ. 2005. The β-amylase genes of
grasses and a phylogenetic analysis of the Triticeae (Poaceae). – Amer. J. Bot. 92:
1045-1058.
Mason-Gamer RJ. 2008. Allohexaploidy,
introgression, and the complex phylogenetic history of Elymus repens.
– Mol. Phylogen. Evol. 47: 598-611.
Mason-Gamer RJ. 2013. Phylogeny of a
genomically diverse group of Elymus (Poaceae) allopolyploids reveals multiple
levels of reticulation. – PLoS ONE 8: e78449.
Mason-Gamer RJ, Kellogg EA. 1996a.
Chloroplast DNA analysis of the monogenomic Triticeae: phylogenetic
implications and genome-specific markers. – In: Jauhaur P (ed), Methods of
genome analysis in plants: their merits and pitfalls, CRC Press, Boca Raton,
Florida, pp. 301-325.
Mason-Gamer RJ, Kellogg EA. 1996b. Testing
for phylogenetic conflict among molecular data sets in the tribe Triticeae
(Gramineae). – Syst. Biol. 45: 524-545.
Mason-Gamer RJ, Kellogg EA. 2000.
Phylogenetic analysis of the Triticeae using the starch synthase gene, and a
preliminary analysis of some North American Elymus species. – In:
Jacobs SWL, Everett J (eds), Grasses: systematics and evolution, Proceedings of
the 2nd International Conference on the Comparative Biology of
Monocotyledons, 1998, Sydney, Australia, Vol. 2, CSIRO Publ., Melbourne, pp.
102-109.
Mason-Gamer RJ, Burns MM, Naum M. 2010a.
Phylogenetic relationships and reticulation among Asian Elymus (Poaceae) allotetraploids: analyses of three
nuclear gene trees. – Mol. Phylogen. Evol. 54: 10-22.
Mason-Gamer RJ, Burns MM, Naum BM. 2010b.
Reticulate evolutionary history of a complex group of grasses: phylogeny of
Elymus StStHH allotetraploids based on three nuclear genes. – PloS
One 5: e10989.
Mathews S, Sharrock RA. 1996. The phytochrome
gene family in grasses (Poaceae): a
phylogeny and evidence that grasses have a subset of the loci found in dicot
angiosperms. – Mol. Biol. Evol. 13: 1141-1150.
Mathews S, Tsai RC, Kellogg EA. 2000.
Phylogenetic structure in the grass family (Poaceae): evidence from the nuclear gene
phytochrome B. – Amer. J. Bot. 87: 96-107.
Mathews S, Spangler RE, Mason-Gamer RJ,
Kellogg EA. 2002. Phylogeny of Andropogoneae inferred from phytochrome B,
GBSSI, and ndhF. – Intern. J. Plant Sci. 163: 441-450.
Mattei G, Tropea C. 1908. Graminacee proviste
di nettarii estranuziali. – Boll. R. Orto. Bot. (Palermo) 7: 113-117.
Mattfeld J. 1936. Zur Morphologie und
Systematik der Cyperaceae. – Proc.
Zesde Intern. Bot. Congres 1: 330-332.
Matthei OR. 1965. Estudio crítico de las
gramíneas del género Stipa en Chile. – Gayana, Bot. 13: 1-137.
Matthei OR. 1975. Der Briza-Komplex
in Südamerika: Briza, Calotheca, Chascolytrum,
Poidium (Gramineae). – Willdenowia 8: 1-168.
Matthei OR. 1986. El género Bromus
L. (Poaceae) en Chile. – Gayana, Bot.
43: 47-110.
Matthei O, Marticorena C, Rodríguez R, Kalin
Arroyo M, Muñoz M, Squeo FA, Arancio G. 1998. Nuevas citas y nuevas
combinaciones en Poaceae para la flora
de Chile. – Gayana, Bot. 54: 189-192.
Matuszak-Renger S, Paule J, Heller S, Leme
EMC, Stinbeisser GM, Barfuss MHJ, Zizka G. 2018. Phylogenetic relationships
among Ananas and related taxa (Bromelioideae, Bromeliaceae) based on nuclear,
plastid and AFLP data. – Plant Syst. Evol. 304: 841-851.
Mavrodiev EV. 2000. A new species of cat-tail
(Typha L.) from sect. Engleria (Leonova) N. Tzvel. – Feddes
Repert. 111: 571-575.
Mavrodiev EV. 2002. Two new species of
Typha L. (Typhaceae Juss.)
from the Far East of Russia and from Mongolia. – Feddes Repert. 113:
281-288.
Mayorga NC. 1999. La tribu chlorideae
(Gramineae) para Colombia. – Tesis Facultad de Ciencias, Dpto. de Biología,
Universidad Nacional de Colombia, Bogotá, Colombia.
Maze J. 1972. Notes on the awn anatomy of
Stipa and Oryzopsis (Gramineae). – Syesis 5: 169-171.
Maze J, Lin S-C. 1975. A study of the mature
megagametophyte of Stipa elmeri. – Can. J. Bot. 53: 2958-2977.
Medico JML, Tosto DS, Rua GH, Agrasar ZER de,
Scataglini MA, Vega AS. 2017. Phylogeny of Digitaria Sections
Trichachne and Trichophorae (Poaceae, Panicoideae, Paniceae): a
morphological and molecular analysis. New circumscription and synopsis. –
Syst. Bot. 42: 37-53.
Medina E. 1974. Dark CO2 fixation,
habitat preference and evolution within the Bromeliaceae. – Evolution 28:
677-686.
Meert M, Goetghebeur P. 1979. Comparative
floral morphology of Bisboeckelereae and Cariceae (Cyperaceae) on the basis of the anthoid
concept. – Bull. Soc. Roy. Bot. Belg. 112: 128-143.
Meeuse ADJ. 1975. Interpretative floral
morphology of the Cyperaceae on the
basis of the anthoid concept. – Acta Bot. Neerl. 24: 291-304.
Mehra PN, Chaudhary JD. 1981. Male meiosis in
some grasses of the tribe Paniceae from northeastern India I. Genus
Paspalum. – Cytologia 46: 265-278.
Mehra PN, Sachdeva SK. 1975. Cytology of some
W. Himalayan Cyperaceae. –
Cytologia 40: 497-515.
Mehra PN, Sharma ML. 1975. Cytological
studies in some central and eastern Himalayan grasses II. The Paniceae. –
Cytologia 40: 75-89.
Mehra PN, Khosla PK, Kohli BL, Koonar JS.
1968. Cytological studies in the North Indian grasses I. – Res. Bull. Panjab
Univ. 19: 157-230.
Meikle RD. 1948. Tropical African plants XX.
Eriocaulon adamesii Meikle, sp. nov. – Kew Bull. 3: 472-473.
Meikle RD. 1950. Eriocaulon
nigericum Meikle, sp. nov. – In: Brenan JPM (ed), More new plants from
the Idanre Hills, Nigeria, Kew Bull. 5: 231-232.
Meikle RD. 1954. Revision of the Flora of
West Tropical Africa VI (ed. R. W. J. Keay), Eriocaulaceae. – Kew Bull. 9:
275.
Meikle RD. 1968. Notes on Eriocaulaceae of West Tropical
Africa. – Kew bull. 22: 141-144.
Mejia-Saulés T, Bisby FA. 2000. Preliminary
views on the tribe Meliceae (Gramineae: Pooideae). – In: Jacobs SWL, Everett
J (eds), Grasses: systematics and evolution, Proceedings of the 2nd
International Conference on the Comparative Biology of Monocotyledons, 1998,
Sydney, Australia, Vol. 2, CSIRO Publ., Melbourne, pp. 83-88.
Mejia-Saulés T, Bisby FA. 2003. Silica
bodies and hooked papillae in lemmas of Melica species (Gramineae:
Pooideae). – Bot. J. Linn. Soc. 141: 447-463.
Melderis A. 1956. New taxa of afroalpine
grasses. – Svensk Bot. Tidskr. 50: 535-547.
Melderis A. 1978. Taxonomic notes on the
tribe Triticeae (Gramineae), with special reference to the genera
Elymus L. s.l., and Agropyron Gaertn. s.l. – Bot. J. Linn.
Soc. 76: 369-384.
Mendes SP. 2008. Endospermogênese e
embriogênese de Dyckia pseudococcinea L. B. Smith (Bromeliaceae). – Ph.D. diss.,
Federal University of Rio de Janeiro, Brazil.
Mendes SP, Costa CG, De Toni KLG. 2012.
Androecium development in the bromeliad Dyckia pseudococcinea L. B.
Sm. (Pitcairnioideae-Bromeliaceae),
an endangered species endemic to Brazil: implications for conservation. –
Flora 207: 622-627.
Meney KA, Pate JS (eds). 1999. Australian
rushes. Biology, identification and conservation of Restionaceae and allied families. –
University of Western Australia Press, Nedlands, Western Australia.
Meney KA, Pate JS, Dixon KW. 1990.
Comparative morphology, anatomy, phenology and reproductive biology of
Alexgeorgea spp. (Restionaceae) from south-western
Western Australia. – Aust. J. Bot. 38: 523-541.
Meney KA, Dixon KW, Scheltema M, Pate JS.
1993. Occurrence of vesicular mycorrhizal fungi in dryland species of Restionaceae and Cyperaceae from SW Western Australia.
– Aust. J. Bot. 41: 733-737.
Meney KA, Pate JS, Dixon KW. 1996. New
species of Restionaceae from
Western Australia. – Telopea 6: 649-666.
Meney KA, Pate JS, Hickman EJ. 1999.
Morphological and anatomical descriptions of Restionaceae and allied families and
their distribution. – In: Meney KA, Pate JS (eds), Australian rushes.
Biology, identification and conservation of Restionaceae and allied families,
University of Western Australia Press, Nedlands, Western Australia, pp.
161-461.
Menezes de Sequeira M, Castroviejo S. 2007.
Holcus azoricus M. Seq. & Castrov. (Poaceae), a new species from the Azores
Islands. – Bot. J. Linn. Soc. 154: 259-267.
Merxmüller H, Czech G. 1953. Eine neue
Gattung der Cyperaceae. – Mitt.
Bot. Staatssamml. München I(8): 317-323.
Messing J, Bennetzen JL. 2008. Grass genome
structure and evolution. – In: Volff JN (ed), Genome dynamics Vol. 4. Plant
Genomes, Karger, Basel, pp. 41-56.
Metcalfe CR. 1960. Anatomy of the
monocotyledons I. Gramineae. – Clarendon Press, Oxford.
Metcalfe CR. 1969. Anatomy as an aid to
classifying the Cyperaceae. – Amer.
J. Bot. 56: 782-790.
Meyer FJ. 1933. Beiträge zur vergleichenden
Anatomie der Typhaceae (Gattung
Typha). – Beitr. Bot. Centralbl. 51: 335-376.
Meyer NR, Yaroshevskaya AS. 1976. The
phylogenetic significance of the development of pollen grain walls in Liliaceae, Juncaceae and Cyperaceae. – In: Ferguson IK, Muller
J (eds), The evolutionary significance of the exine, Linnaean Society Symposium
Series No. 1, pp. 91-100.
Mez C. 1904. Physiologische
Bromeliaceen-Studien I. Die Wasser-Ökonomie der extreme atmosphärischen
Tillandsien. – Jahrb. Wissensch. Bot. 40: 157-229.
Michael PW, Vickery JW. 1975. Two new species
and a new combination in Echinochloa. – Telopea 1: 44-48.
Michael PW, Vickery JW. 1980. Three new
annual species of Echinochloa from Northern Australia. – Telopea 2:
25-29.
Michalska A. 1953. Cytological investigations
on Luzula. – Acta Soc. Bot. Polon. 22: 169-186.
Michelangeli FA, Davis JI, Stevenson DW.
2003. Phylogenetic relationships among Poaceae and related families as inferred
from morphology, inversions in the plastid genome, and sequence data from the
mitochondrial and plastid genomes. – Amer. J. Bot. 90: 93-106.
Minaya M, Hackel J, Namaganda M, Brochmann C,
Vorontsova MS, Besnard G, Catalán P. 2017. Contrasting dispersal histories of
broad- and fine-leaved temperate Loliinae grasses: range expansion, founder
events, and the roles of distance and barriers. – J. Biogeogr. 44: 1-14.
Mirjalili SA, Bennett SJ, Poorazizi E. 2008.
A phenetic analysis on the genus Lolium (Poaceae) in Iran. – Plant Syst. Evol.
274: 203-208.
Mitchell WM. 1967. Taxonomic synopsis of
Bromus section Bromopsis (Gramineae) in Alaska. – Can. J.
Bot. 45: 1309-1313.
Mlada J. 1977. The histological structure of
the grass embryo and its significance for the taxonomy of the family Poaceae. – Acta Univ. Carol. Biol. 1974:
51-156.
Mlodzianowski F. 1964. The structure and the
later stages of development in the embryo sac of Thamnochortus
fruticosus Berg (Restionaceae). – Bull. Soc. Amis
Sci. Lett. Poznan, Ser. D, 4: 3-11.
Moffett AA. 1944. Note on the cytology of
Rhodes grass. – Rhod. Agr. J. 41: 11-13.
Moffett AA, Hurcombe RE. 1949. Chromosome
numbers in South African grasses. – Heredity 3: 369-373.
Mohammed AH, Gould FW. 1966. Biosystematic
studies in the Bouteloua curtipendula complex V. Megasporogenesis and
embryo sac development. – Amer. J. Bot. 53: 166-169.
Mohlenbrock RH, Drapalik DJ. 1960.
Eleocharis, subseries Palustres, in Illinois. – Amer. Midl.
Natur. 64: 224.
Moinuddin M, Vahidy AA, Ali SI. 1994.
Chromosome counts in Arundinoideae, Chloridoideae, and Pooideae (Poaceae) from Pakistan. – Ann. Missouri
Bot. Gard. 81: 784-791.
Molina AM. 1981. El género
Erianthus (Gramineae) en la Argentina y países limítrofes. –
Darwiniana 323: 559-585.
Molina AM. 1996. Revisión taxonómica del
género Eustachys Desv. (Poaceae: Chloridoideae, Cynodonteae) de
Sudamérica. – Candollea 51: 225-272.
Molina AM, Rúgolo de Agrasar ZE. 2004.
Revisión taxonómica de las especies del género Chloris (Poaceae: Chloridoideae) en Sudamérica. –
Candollea 59: 347-428.
Molina A, Acedo C, Llamas F. 2006a.
Typification of some Hudson plant names in Carex. – Taxon 55:
1009-1013.
Molina A, Acedo C, Llamas F. 2006b.
Delimitación taxonómica de Carex grupo muricata (Cyperaceae) en Europa. Resultados
preliminares. – Bull. Soc. Hist. Nat. Toulouse 141: 57-61.
Molina A, Acedo C, Llamas F. 2008. Taxonomy
and new taxa in Eurasian Carex (Section Phaestoglochin, Cyperaceae). – Syst. Bot. 33:
237-250.
Molina A, Acedo C, Llamas F. 2012. A
comparative study of the inflorescence in the genus Carex (Cyperaceae). – Syst. Bot. 37:
365-381.
Molina A, Chung K-S, Hipp AL. 2015. Molecular
and morphological perspectives on the circumscription of Carex section
Heleoglochin (Cyperaceae).
– Plant Syst. Evol. 301: 2419-2439.
Moline PM, Linder HP. 2005. Molecular
phylogeny and generic delimitation in the Elegia group (Restionaceae, South Africa) based on a
complete taxon sampling and four chloroplast DNA regions. – Syst. Bot. 30:
759-772.
Molloy BPJ, Edgar E, Heenan PB, De Lange PJ.
1999. New species of Poa (Gramineae) and Ischnocarpus (Brassicaceae)
from limestone, North Otago, South Island, New Zealand. – New Zealand J. Bot.
37: 41-50.
Monoyer A. 1934. Contribution à l’anatomie
du genre Scirpus. – Bot. Arch. Univ. Liège11: 1-185.
Monteiro-Scannavacca WR, Mazzoni SC. 1978.
Embryological studies in Leiothrix fluitans (Mart.) Ruhl. (Eriocaulaceae). – Rev. Brasil. Bot.
1: 59-64.
Monteiro WR, Giulietti AM, Moraes Castro M
de. 1984. Aspects of leaf structure of some species of Eriocaulon (Eriocaulaceae) from Serra do Cipó
(Minas Gerais, Brasil). – Rev. Brasil. Bot. 7: 137-147.
Monteiro WR, Moraes Castro M de, Giulietti
AM. 1985. Aspects of leaf structure of some species of Leiothrix Ruhl.
(Eriocaulaceae) from Serra do
Cipó (Minas Gerais, Brasil). – Rev. Brasil. Bot. 8: 109-125.
Moore G, Guaglianone ER, Zartman C. 2003.
Rhynchospora pseudomacrostachya, a new Brazilian species of Cyperaceae. – Brittonia 54:
340-343.
Moore RJ, Calder JA. 1964. Some chromosome
numbers of Carex species of Canada and Alaska. – Can. J. Bot. 42:
1387-1391.
Morakinyo JA, Olorode O. 1988. Cytogenetic
studies in Sorghum bicolor (L.) Moench. – Cytologia 53: 653-658.
Moraldo B. 1986. Il genere Stipa L.
(Gramineae) in Italia. – Webbia 40: 203-278.
Mora-Osejo LE. 1960. Beiträge zur
Entwicklungsgeschichte und vergleichenden Morphologie der Cyperaceen. –
Beitr. Biol. Pflanzen 35: 293-341.
Mora-Osejo LE. 1966. Las inflorescencias
parciales de ultimo orden de Uncinia Pers. y la agrupación
sistemática de las Caricoideae Kükenthal. – Caldasia, Bot. 9: 277-293.
Mora-Osejo LE. 1982. Consideraciones sobre la
morfoligía, anatómica y posición sistemática de Vesicarex
Steyermark (Cyperaceae). – Acta
Biol. Colombiana 1: 31-41.
Mora-Osejo LE. 1989. La bioforma de
Bulbostylis leucostachya Kunth (Cyperaceae) y de otras monocotiledoneas
arboriformes tropicales. – Rev. Acad. Colomb. Ci. 17: 215-230.
Morden CW. 1985. A biosystematic study of the
Muhlenbergia repens complex (Poaceae). – Ph.D. diss., Texas A & M
University, College Station, Texas.
Morikawa T, Leggett JM. 1990. Isozyme
polymorphism in natural populations of Avena canariensis from the
Canary Islands. – Heredity 64: 403-411.
Morillo IMR. 1996. Systematics, phylogeny,
and chromosome number evolution of Cryptanthus (Bromeliaceae). – Ph.D. diss.,
University of Missouri, St. Louis, Missouri.
Morong T. 1888. Studies in the Typhaceae. – Bull. Torrey Bot. Club 15:
1-8.
Morong T. 1891. Notes on the North American
species of Eriocauleae. – Bull. Torrey Bot. Club 28: 351-362.
Morris LM, Duvall MR. 2010. The chloroplast
genome of Anomochloa marantoidea (Anomochlooideae, Poaceae) comprises a mixture of grass-like
and unique features. – Amer. J. Bot. 97: 620-627.
Morrison JW. 1959. Cytogenetic studies in the
genus Hordeum I. Chromosome morphology. – Can. J. Bot. 37:
527-538.
Morrison JW, Rajhathy T. 1959. Cytogenetic
studies in the genus Hordeum III. Pairing in some interspecific and
intergeneric hybrids. – Can. J. Genet. Cytol. 1: 65-77.
Morrone O, Zuloaga FO. 1991. Revisión del
género Streptostachys (Poaceae: Panicoideae: Paniceae), su
posición sistemática dentro de la tribu Paniceae. – Ann. Missouri Bot.
Gard. 78: 359-376.
Morrone O, Zuloaga FO. 1992. Revisión de las
especies sudamericanas natives e introducidas de los géneros
Brachiaria y Urochloa (Poaceae: Panicoideae: Paniceae). –
Darwiniana 31: 43-109.
Morrone O, Zuloaga FO. 1993. Sinopsis del
género Urochloa (Poaceae:
Panicoideae: Paniceae) para México y América Central. – Darwiniana 32:
59-75.
Morrone O, Zuloaga FO. 2003. New species of
Paspalum (Poaceae: Panicoideae:
Paniceae) from Brazil. – Syst. Bot. 28: 307-312.
Morrone O, Zuloaga FO. 2009.
Keratochlaena, el nombre correcto para Sclerochlamys (Poaceae, Paniceae). – Darwiniana 47:
231.
Morrone O, Filgueiras TS, Zuloaga FO,
Dubcovsky J. 1993. Revision of Anthaenantiopsis (Poaceae: Panicoideae: Paniceae). – Syst.
Bot. 18: 434-453.
Morrone O, Hunziker JH, Zuloaga FO, Escobar
A. 1995. Números cromosómicos en Paniceae sudamericanas (Poaceae: Panicoideae). – Darwiniana 33:
53-60.
Morrone O. Zuloaga FO, Carbonó E. 1995.
Revisión del grupo Racemosa del género Paspalum (Poaceae: Panicoideae: Paniceae). – Ann.
Missouri Bot. Gard. 82: 82-116.
Morrone O, Zuloaga FO, Arriaga MO, Pozner R,
Aliscioni SS. 1998. Revisión sistemática y análisis cladístico del género
Chaetium (Poaceae: Panicoideae:
Paniceae). – Ann. Missouri Bot. Gard. 85: 404-424.
Morrone O, Zuloaga FO, Davidse G, Filgueiras
TS. 2001. Canastra, a new genus of Paniceae (Poaceae, Panicoideae) segregated from
Arthropogon. – Novon 11: 429-436.
Morrone O, Escobar A, Zuloaga FO. 2006.
Chromosome studies in American Panicoideae (Poaceae). – Ann. Missouri Bot. Gard. 93:
647-657.
Morrone O, Scataglini A, Zuloaga FO. 2007.
Cyphonanthus, a new genus segregated from Panicum (Poaceae: Panicoideae: Paniceae) based on
morphological, anatomical and molecular data. – Taxon 56: 521-532.
Morrone O, Denham SS, Aliscioni S, Zuloaga
FO. 2008. Parodiophyllochloa, a new genus segregated from
Panicum (Paniceae, Poaceae)
based on morphological and molecular data. – Syst. Bot. 33: 66-76.
Morrone O, Aagesen L, Scataglini MA,
Salariato DL, Denham SS, Chemisquy MA, Sede SM, Giussani LM, Kellogg EA,
Zuloaga FO. 2012. Phylogeny of the Paniceae (Poaceae: Panicoideae) integrating plastid
DNA sequences and morphology into a new classification. – Cladistics 28:
333-356.
Morton BR, Clegg MT. 1993. A chloroplast DNA
mutational hotspot and gene conversion in a noncoding region near rbcL
in the grass family (Poaceae). – Curr.
Genet. 24: 357-365.
Morton BR, Gaut BS, Clegg MT. 1996. Evolution
of alcohol dehydrogenase genes in the palm and grass families. – Proc. Natl.
Acad. Sci. U.S.A. 93: 11735-11739.
M’Ribu K, Hilu KW. 1996. Application of
random amplified polymorphic DNA to study genetic diversity in Paspalum
scrobiculatum L. (Kodo millet, Poaceae). – Gen. Res. Crop Evol. 43:
203-210.
Mtotomwema K. 1990. New species of
Kyllinga (Cyperaceae) from
tropical Africa. – Nord. J. Bot. 9: 637-641.
Muasya AM. 1998. A synopsis of
Fuirena (Cyperaceae) for the
Flora of tropical East Africa. – Kew Bull. 53: 187-202.
Muasya AM, Baquar SR. 1995. Taxonomic studies
in the Fuirena pubescens complex (Cyperaceae) in Kenya. – Nord. J. Bot.
15: 407-410.
Muasya AM, Lange PJ de. 2010. Ficinia
spiralis (Cyperaceae), a new
genus and combination for Desmoschoenus spiralis. – New Zealand J.
Bot. 48: 31-39.
Muasya AM, Simpson DA. 2002. A monograph of
the genus Isolepis R. Br. (Cyperaceae). – Kew Bull. 57:
257-362.
Muasya AM, Simpson DA, Chase MW, Culham A.
1998. An assessment of suprageneric phylogeny in Cyperaceae using rbcL DNA
sequences. – Plant Syst. Evol. 211: 257-271.
Muasya AM, Bruhl JJ, Simpson DA, Culham A,
Chase MW. 2000. Suprageneric phylogeny of Cyperaceae: a combined analysis. – In:
Wilson KL, Morrison DA (eds), Monocots: systematics and evolution, Proceedings
of the 2nd International Conference on the Comparative Biology of
Monocotyledons, 1998, Sydney, Australia, Vol. 1, CSIRO Publ., Melbourne, pp.
593-601.
Muasya AM, Simpson DA, Chase MW, Culham A.
2000. Phylogenetic relationships within the heterogeneous Scirpus s.
lat. (Cyperaceae) inferred from
rbcL and trnL-F sequence data. – In: Wilson KL, Morrison DA
(eds), Monocots: systematics and evolution, Proceedings of the 2nd
International Conference on the Comparative Biology of Monocotyledons, 1998,
Sydney, Australia, Vol. 1, CSIRO Publ., Melbourne, pp. 610-614.
Muasya AM, Simpson DA, Chase MW, Culham A.
2001. A phylogeny of Isolepis (Cyperaceae) inferred using plastid
rbcL and trnL-F sequence data. – Syst. Bot. 26: 342-353.
Muasya AM, Simpson DA, Chase MW. 2001 [2002].
Generic relationships and character evolution in Cyperus s.l. (Cyperaceae). – Syst. Geogr. Plants 71:
539-544.
Muasya AM, Simpson DA, Chase MW. 2002.
Phylogenetic relationships in Cyperus L. s.l. (Cyperaceae) inferred from plastid DNA
sequence data. – Bot. J. Linn. Soc. 138: 145-153.
Muasya AM, Simpson DA, Verboom GA,
Goetghebeur P, Naczi RFC, Chase MW, Smets E. 2009. Phylogeny of Cyperaceae based on DNA sequence data:
current progress and future prospects. – Bot. Rev. 75: 2-21.
Muasya AM, Vrijdaghs A, Simpson DA, Chase MW,
Goetghebeur P, Smets E. 2009. What is a genus in Cypereae: phylogeny, character
homology assessment and generic circumscription in Cypereae. – Bot. Rev. 75:
52-66.
Muasya AM, Reynders M, Goetghebeur P, Simpson
DA, Vrijdaghs A. 2012. Dracoscirpoides (Cyperaceae) – a new genus from
Southern Africa, its taxonomy and floral ontogeny. – South Afr. J. Bot. 72:
104-115.
Muasya AM, Viljoen JA, Dludlu MN, Demissew S.
2014. Phylogenetic position of Cyperus cladestinus (Cypereae, Cyperaceae) clarified by morphological
and molecular evidence. – Nord J. Bot. 32: 106-114.
Mühlberg H. 1970. Wuchsformen der Gattung
Brachypodium (Poaceae). –
Feddes Repert. 81: 119-130.
Mukherjee SK. 1958. Revision of the genus
Erianthus Michx. (Gramineae). – Lloydia 21: 157-188.
Müller C. 1983. Untersuchungen an
südamerikanischen Gramineen I. 2 neue Varietäten südamerikanischer
Chloris-Arten. – Feddes Repert. 94: 625-630.
Müller C. 1985. Untersuchungen an
südamerikanischen Gramineen II. Bestimmungsschlüssel der
Chloris-Arten Südamerikas. – Feddes Repert. 96: 269-277.
Müller J. 2006. Novelties in
Festuca (Gramineae-Poeae) from southern Bolivia. – Brittonia 58:
42-45.
Müller J, Catalán P. 2006. Notes on the
infrageneric classification of Festuca L. (Gramineae). – Taxon 55:
139-144.
Müller-Doblies U. 1969a [1970]. Über die
Blütenstände und Blüten sowie zur Embryologie von Sparganium. –
Bot. Jahrb. Syst. 89: 359-450.
Müller-Doblies D. 1969b [1970]. Über die
Verwandtschaft von Typha und Sparganium in Infloreszenz- und
Blütenbau. – Bot. Jahrb. Syst. 89: 451-562.
Munro SL, Linder HP. 1997. The embryology and
systematic relationships of Prionium serratum (Juncaceae: Juncales). – Amer. J. Bot.
84: 850-860.
Munro SL, Linder HP. 1998. The phylogenetic
position of Prionium (Juncaceae) within the order Juncales
based on morphological and rbcL sequence data. – Syst. Bot. 23:
43-55.
Munro SL, Kirschner J, Linder HP. 2001.
Species plantarum. Flora of the world 5. Prioniaceae. – Australian Biological
Resources, Canberra.
Müntzing A. 1933. Apomictic and sexual seed
formation in Poa. – Hereditas 17: 131-154.
Müntzing A. 1940. Further studies on
apomixis and sexuality in Poa. – Hereditas 26: 115-190.
Murphy MA. 2003. Relationships among taxa of
Elymus (Poaceae: Triticeae) in
Australia: reproductive biology. – Aust. Syst. Bot. 16: 633-642.
Murphy MA, Jones CE. 1999. Observations on
the genus Elymus (Poaceae:
Triticeae) in Australia. – Aust. Syst. Bot. 12: 593-604.
Murray BG, Lange PJ de, Ferguson AR. 2005.
Nuclear DNA variation, chromosome numbers and polyploidy in the endemic and
indigenous grass flora of New Zealand. – Ann. Bot. 96: 1293-1305.
Murty YS, Kumar V. 1972. Flowering impulse
and floral histogenesis in Fimbristylis diphylla Vahl. – J. Ind.
Bot. Soc. 51: 185-195.
Musili PM, Gibbs AK, Wilson KL, Bruhl JJ.
2016. Schoenus (Cyperaceae)
is not monophyletic based on ITS nrDNA sequence data. – Aust. Syst. Bot. 29:
265-283.
Naczi RFC. 1992. Systematics of
Carex section Griseae (Cyperaceae). – Ph.D. diss., University
of Michigan, Ann Arbor, Michigan.
Naczi RFC. 2009. Insights on using
morphologic data for phylogenetic analysis in sedges (Cyperaceae). – Bot. Rev. 75: 67-95.
Naczi RFC, Ford BA. 2001. Systematics of the
Carex jamesii complex (Cyperaceae: sect.
Phyllostachyae). – Sida 19: 853-884.
Naczi RFC, Ford BA (eds). 2008. Sedges: uses,
diversity, and systematics of the Cyperaceae. – Missouri Botanical
Garden Press, St. Louis, Missouri.
Nadot S, Bajon R, Lejeune B. 1994. The
chloroplast gene rps4 as a tool for the study of Poaceae phylogeny. – Plant Syst. Evol.
191: 27-38.
Nagahama N, Vegetti AC, Anton AM, Norrmann
GA. 2013. Synflorescence analysis in South American species of
Andropogon section Leptopogon (Andropogoneae, Poaceae): a tool to identify different
ploidy levels. – Phytotaxa 82: 45-63.
Nagels A, Muasya AM, Huysmans S, Vrijdaghs A,
Smets E, Vinckier S. 2008. Pollen diversity in Cyperaceae. – South Afr. J. Bot. 74:
374.
Nagels A, Muasya AM, Huysmans S, Vrijdaghs A,
Smets E, Vinckier S. 2009. Palynological diversity and major evolutionary
trends in Cyperaceae. – Plant Syst.
Evol. 277: 117-142.
Nair NV, Selvi A, Sreenivasan TV, Pushpalatha
KN, Mary S. 2005. Molecular diversity among Saccharum,
Erianthus, Sorghum, Zea and their hybrids. – Sugar
Tech 7: 55-59.
Nakai T. 1925. Two new genera of Bambusaceae,
with special remarks on the related genera growing in eastern Asia. – J.
Arnold Arbor. 6: 145-153.
Nakamatte E, Lye KA. 2007. AFLP-based
differentiation in north Atlantic species of Carex sect.
Phacocystis. – Nord. J. Bot. 25: 318-328.
Namaganda M, Lye KA. 2007. The species
distinction of the narrow-leaved Festuca from East Africa based on
AFLP fingerprinting and morphology. – Nord. J. Bot. 25: 85-95.
Namaganda M, Lye KA. 2008. A taxonomic
comparison between tropical African and related European broad-leaved species
of Festuca L. (Poaceae). –
South Afr. J. Bot. 74: 295-305.
Nannfeldt JA. 1977. The species of
Anthracoidea (Ustilaginales) on Carex subgen. Vignea
with special regard to the Nordic species. – Bot. Not. 130: 351-375.
Napper DM. 1963a. Cyperaceae of East Africa I. – J. East
Afr. Nat. Hist. Soc. 24: 1-18.
Napper DM. 1963b. Notes on East African
grasses. – Kirkia 3: 112-130.
Napper DM. 1964. Cyperaceae of East Africa II. – J.
East Afr. Nat. Hist. Soc. 24: 23-46.
Napper DM. 1965. Cyperaceae of East Africa III. – J.
East Afr. Nat. Hist. Soc. 25: 1-27.
Napper DM. 1971a. Flagellariaceae. – In:
Milne-Redhead E, Polhill RM (eds), Flora of tropical East Africa, Crown Agents
for Oversea Governments and Administrations, London, pp. 1-3.
Napper DM. 1971b. Typhaceae. – In: Milne-Redhead E,
Polhill RM (eds), Flora of tropical East Africa, Crown Agents for Oversea
Governments and Administrations, London, pp. 1-6.
Napper DM. 1971c. Cyperaceae of East Africa V. – J. East
Afr. Nat. Hist. Soc. Nat. Mus. 28, no. 124.
Negby M, Koller D. 1962. Homologies in the
grass embryo: a reevaluation. – Phytomorphology 12: 289-296.
Negby M, Sargent JA. 1986. The scutellum of
Avena: a structure to maximize exploitation of endosperm reserves. –
Bot. J. Linn. Soc. 93: 247-258.
Negritto MA, Anton AM. 2006. Three new
species of Poa from the Andes of Colombia and Peru. – Syst. Bot. 31:
83-88.
Nelmes E. 1946. A key to the carices of
Malaysia and Polynesia. – Kew Bull. 1946: 5-29.
Nelmes E. 1947. Two critical groups of
British sedges. – Rep. Bot. Exchange Club, Brit. Isles 13: 99-105.
Nelmes E. 1949. Notes on Cyperaceae XX. The genus
Uncinia in Malaysia. – Kew Bull. 1949: 140-145.
Nelmes E. 1951a. Facts and speculations on
phylogeny in the tribe Cariceae of the Cyperaceae. – Kew Bull. 6: 427-436.
Nelmes E. 1951b. The genus Carex in
Malaysia. – Reinwardtia 1: 221-450.
Nelmes E. 1952. Facts and speculations on
phylogeny in the tribe Cariceae of the Cyperaceae. – Kew Bull. 1951:
427-436.
Nelmes E. 1953. Notes on Cyperaceae XXXI. The African genus
Coleochloa. – Kew Bull. 8: 373-381.
Nelmes E. 1955a. Notes on Cyperaceae XXXIII. The African species
of Hypolytrum. – Kew Bull. 10: 63-82.
Nelmes E. 1955b. Notes on Cyperaceae XXXIV. Allies of Carex
flava L. in the southern hemisphere. – Kew Bull. 10: 83-88.
Nelmes E. 1955c. Notes on Cyperaceae XXXVIII. Scleria
Berg., Sect. Hypoporum (Nees) Endl. in Africa. – Kew Bull. 13:
415-453.
Nemirovich-Danchenko EN. 1983. The structure
of the seed-coat in the representatives of the Bromeliaceae. – Bot. Žurn. 68:
1094-1101. [In Russian]
Neves SS, Swire-Clark G, Hilu KH, Baird WV.
2005. Phylogeny of Eleusine (Poaceae: Chloridoideae) based on nuclear
ITS and plastid trnT-trnF sequences. – Mol. Phylogen. Evol.
35: 395-419.
Nevski SA. 1933. Agrostologische Studie über
das System der Tribe Horeae Benth. – Trudy Bot. Inst. Akad. Nauk SSSR, Ser.
I, Fl. Sist. Vysš. Rast. 1: 9-32.
Nevski SA. 1936. Conspectus Loliearum,
Nardearum, Leptuearum hordecarumque florae Unionis Rerum Publicarum
Sovieticarum Socialisticarum. – Trudy Bot. Inst. Akad. Nauk SSR, ser. I, 2:
33-90.
Newell TK. 1969. A study of the genus
Joinvillea (Flagellariaceae). – J. Arnold
Arbor. 50: 527-555.
Newell TK, Stone BC. 1967.
Flagellaria (Chortodes) plicata Hooker fil. is a
Joinvillea. – Taxon 16: 192-194.
Newmaster SG, Balasubramaniam V, Murugesan M,
Ragupathy S. 2008. Tripogon cope (Poaceae: Chloridoideae), a new species
supported by morphometric analysis and a synopsis of Tripogon in
India. – Syst. Bot. 33: 695-701.
Newton RJ, Bond WJ, Farrant JM. 2002. Seed
development, morphology and quality testing in selected species of nut-fruited
Restionaceae. – South Afr. J.
Bot. 68: 226-230.
Neyland R. 2002. A phylogeny inferred from
large-subunit (26S) ribosomal DNA sequences suggests that the family Dasypogonaceae is
closely aligned with the Restionaceae allies. – Aust. Syst.
Bot. 15: 749-754.
Ng’uni D, Geleta M, Fatih M, Bryngelsson T.
2010. Phylogenetic analysis of Sorghum based on combined sequence data
from cpDNA regions and ITS generate well-supported trees with two major
lineages. – Ann. Bot. 105: 471-480.
Nguyen HN, Tran VT. 2012.
Nianhochloa gen. nov. (Poaceae,
Bambusoideae), a new bamboo genus endemic to Bidoup Mountain, southern Vietnam.
– Adansonia 34: 257-264.
Nguyen HN, Tran VT. 2013.
Cochinchinochloa (Gramineae: Bambusoideae-Bambusineae), a new bamboo
genus endemic to Braian mountain, southern Vietnam. – Blumea 58: 28-32.
Nguyen HN, Tran VT. 2016.
Yersinochloa gen. nov. (Gramineae: Bambusoideae-Bambusineae) endemic
to the Lam Vien Plateau, southern Vietnam. – Nord. J. Bot. 34: 400-404.
Nicholls MS, Cook CDK. 1986. The function of
pollen tetrads in Typha (Typhaceae). – Veröff. Geobot. Inst.
ETH, Stiftung Rübel, Zürich 94: 112-119.
Ní Chonghaile G. 2002. Systematics of the
woody bamboos (tribe Bambuseae). – Ph.D. diss., University of Dublin, Trinity
College, Dublin.
Nicola MV, Lizarazu MA, Scataglini MA. 2015.
Phylogenetic analysis and taxonomic position of section Verrucosa of
Panicum and its relationship with taxa of the
Sacciolepis-Trichanthecium clade (Poaceae: Panicoideae: Paniceae). –
Plant Syst. Evol. 301: 2247-2260.
Nicora EG. 1941. Contribucion al estudio
histologico de las glandulas epidermicas de algunas especies de
Eragrostis. – Darwiniana 5: 316-321.
Nicora EG. 1973. Novedades agrostologicas
patagonicas. – Darwiniana 18: 80-106..
Nicora EG. 1995. Los generos
Diplachne y Leptochloa (Gramineae: Eragrosteae) de la
Argentina y paises limitrofes. – Darwiniana 33: 233-256.
Nicora EG, Rúgolo de Agrasar Z. 1987. Los
géneros de gramíneas de América Austral. – Hemisferio Sur, Buenos
Aires.
Nielson EL. 1939. Grass studies III.
Additional somatic chromosome complements. – Amer. J. Bot. 26: 366-371.
Nijalingappa BH-M. 1975. Cytological studies
in Fimbristylis (Cyperaceae). – Cytologia 40:
177-183.
Niño SM, Clark LG, Dorr LJ. 2006. Una nueva
especie de Chusquea (Poaceae:
Bambusoideae) de la Cordillera de Mérida, Venezuela. – Brittonia 58:
46-51.
Nogueira FM, Fagundes NF, Kuhn SA, Fregonezi
JN, Mariath JEA. 2015. Ovary and ovule anatomy in the nidularioid complex and
its taxonomic utility (Bromelioideae: Bromeliaceae). – Bot. J. Linn. Soc.
177: 66-77
Noltie HJ. 1999. Notes relating to the flora
of Bhutan XXXXVIII. Gramineae I, Tribe Stipeae. – Edinb. J. Bot. 56:
285-291.
Nordenskiöld H. 1951. Cyto-taxonomical
studies in the genus Luzula I. Somatic chromosomes and chromosome
numbers. – Hereditas 37: 325-355.
Nordenskiöld H. 1956. Cyto-taxonomical
studies in the genus Luzula II. – Hereditas 42: 7-73.
Nordenskiöld H. 1961. Tetrad analysis and
the course of meiosis in three hybrids of Luzula campestris. –
Hereditas 47: 203-238.
Nordenskiöld H. 1964. The effect of
X-irradiation on diploid and polyploidy Luzula. – Hereditas 51:
344-374.
Nordenskiöld H. 1969. The genus
Luzula in Australia. – Bot. Not. 122: 69-89.
Norlindh T. 1972. Notes on the variation and
taxonomy in the Scirpus maritimus complex. – Bot. Not. 125:
397-405.
Norrmann GA, Quarín CL, Killeen TJ. 1994.
Chromosome numbers in Bolivian grasses (Gramineae). – Ann. Missouri Bot.
Gard. 81: 768-774.
Norstog K. 1963. Apomixis and polyembyony in
Hierochloë odorata. – Amer. J. Bot. 50: 815-821.
Novara LJ. 1976. Contribución al
conocimiento de las inflorescencias de Juncus y su significación
taxonómica. – Kurtziana 9: 41-61.
Novikov VS. 1990a. Conspectus of the system
of the genus Juncus L. (Juncaceae). – Bjull. Moskovsk. Obšč.
Isp. Prir., Odt. Biol. 95(5): 111-125. [In Russian]
Novikov VS. 1990b. The synopsis of the genus
Luzula DC. system (Juncaceae). – Bjull. Moskovsk. Obšč.
Isp. Prir., Odt. Biol. 95(6): 63-70. [In Russian]
Núñez O. 1952. Investigaciones
cariosistemáticas en las gramíneas Argentinas de la tribu Paniceae. – Rev.
Fac. Agron. Univ. Nac. La Plata 28: 229-255.
Núñez O. 1968. El problema de la pálea de
Oryza L. – Bol. Soc.Argent. Bol. 12: 57-97.
Nygren A. 1946. The genesis of some
Scandinavian species of Calamagrostis. – Hereditas 32: 131-262.
Nygren A. 1949a. Studies on vivipary in the
genus Deschampsia. – Hereditas 35: 27-32.
Nygren A. 1949b. Apomictic and sexual
reproduction in Calamagrostis purpurea. – Hereditas 35: 285-300.
Nygren A. 1950. Cytological and embryological
studies in arctic Poae. – Symb. Bot. Ups. 10(4).
Nygren A. 1954. Investigations on North
American Calamagrostis. – Hereditas 40: 377-397.
Nygren A. 1962. Artificial and natural
hybridization in European Calamagrostis. – Symb. Bot. Upsal. 10(4):
1-64.
Ogden EL. 1897. Leaf structure of
Jouvea and of Eragrostis obtusiflora. – In: Studies on
American grasses, U. S. Department of Agriculture, Division of Agrostology,
Bull. No. 8, pp. 12-20.
Ogihara Y, Tsunewaki K. 1988. Diversity and
evolution of the chloroplast DNA in Triticum and Aegilops as
revealed by restriction fragment analysis. – Theor. Appl. Gen. 76:
321-332.
Ogihara Y, Isono K, Kojima T, Endo A, Hanaoka
M, Shiina T, Terachi T et al. 2002. Structural features of a wheat plastome as
revealed by complete sequencing of chloroplast DNA. – Mol. Genet. Gen. 266:
740-746.
Ohrnberger D. 1999. The bamboos of the world:
annotated nomenclature and literature of the species and the higher and lower
taxa. – Elsevier, Amsterdam.
Ohwi J. 1936. Cyperaceae Japonicae I. A synopsis of
the Caricoideae of Japan, including Saghalien, Kuriles, Iorea and Formosa. –
Mem. Coll. Sci. Kyoto Imp. Univ., Ser. B, Biol. 11: 229-530.
Ohwi J. 1941. Stipeae (Gramineae) of Japan,
Manchuria, and Northern China. – J. Jap. Bot. 17: 399-405.
Ohwi J. 1942. Gramina Japonica. – Acta
Phytotaxon. Geobot. 11: 27-56.
Oja T. 1998. Isoenzyme diversity and
phylogenetic affinities in the section Bromus of the genus
Bromus (Poaceae). – Biochem.
Syst. Ecol. 28: 403-413.
Oliveira JA, Arbones E, Bregu R. 1995.
Diversidad genetica en poblaciones naturales de Lolium canariense. –
In: Proceedings of 35th Reunion Cientifica de la Sociedad Española
para el Estudio de los Pastos, Centro de Investigación y Technología Agrarias
& Universidad de la Laguna, Tenerife, Spain, pp. 21-24.
Oliveira RC. 2004. Estudo taxonômico das
espécies de Paspalum L., grupo Plicatula (Poaceae-Panicoideae-Paniceae) no Brasil.
– Ph.D. diss., University of Campinas, Campinas, São Paulo, Brazil.
Oliveira RC, Rua GH. 2005. A new species of
Paspalum (Poaceae, Paniceae)
from Central Brazil. – Syst. Bot. 30: 530-532.
Oliveira RC, Valls JFM. 2002. Taxonomia de
Paspalum L., grupo Linearia (Gramineae-Paniceae) do Brasil.
– Rev. Brasileira Bot. 25: 371-389.
Oliveira RP de. 2001. A tribo Olyreae (Poaceae: Bambusoideae) no estado da Bahia,
Brasil. – Master’s thesis, Universidade Estadual de Feira de Santan,
Brazil.
Oliveira RP de, Longhi-Wagner HM, Hollowell
VC. 2004. A new species of Pariana (Poaceae: Bambusoideae: Olyreae) endemic to
the Atlantic moist forest in the State of Bahia, Brazil. – Novon 14:
208-211.
Oliveira RP de, Longhi-Wagner HM, Leite KRB.
2008. A contribuição da anatomia foliar para a taxonomia de Raddia
Bertol. (Poaceae: Bambusoideae). –
Acta Bot. Brasilica 22: 1-19.
Oliveira RP de, Borba EL, Longhi-Wagner HM.
2008. Morphometrics of herbaceous bamboos of the Raddia brasiliensis
complex (Poaceae-Bambusoideae):
implications for the taxonomy of the genus and new species from Brazil. –
Plant Syst. Evol. 270: 159-182.
Oliveira RP de, Longhi-Wagner HM, Leite KRB,
Hollowell VC. 2008. Pariana multiflora (Poaceae, Bambusoideae, Olyreae), a new
species from eastern Brazil, with notes on the leaf anatomy of the genus. –
Syst. Bot. 33: 262-266.
Oliveira RP de, Borba EL, Longhi-Wagner HM,
Pereira ACS, Lambert SM. 2008. Genetic and morphological variability in the
Raddia brasiliensis complex (Poaceae: Bambusoideae). – Plant Syst.
Evol. 274: 25-35.
Oliveira RP de, Clark LG, Schnadelbach AS,
Monteiro SHN, Borba EL, Longhi-Wagner HM, Berg C van den. 2014. A molecular
phylogeny of Raddia and its allies within the tribe Olyreae (Poaceae, Bambusoideae) based on noncoding
plastid and nuclear spacers. – Molec. Phylogen. Evol. 78: 105-117.
Oliveira Furtado de Sousa L de, Wendt T,
Brown GK, Tuthill DE, Evans TM. 2007. Monophyly and phylogenetic relationships
in Lymania (Bromeliaceae:
Bromelioideae) based on morphology and chloroplast DNA sequences. – Syst.
Bot. 32: 264-270.
Oliveira Nardi K de, Scatena VL, Oriani A.
2015. Development of ovule, fruit and seed of Xyris (Xyridaceae, Poales) and taxonomic
considerations. – Bot. J. Linn. Soc. 177: 619-628.
Olivier J, Otto T, Roddaz M, Antoine P-O,
Londoño X, Clark LG. 2009. First macrofossil evidence of a pre-Holocene thorny
bamboo cf. Guadua (Poaceae:
Bambusoideae: Bambuseae: Guaduinae) in south-western Amazonia (Madre de Dios
– Peru). – Rev. Palaeobot. Palynol. 153: 1-7.
Ong HC, Palmer JD. 2006. Pervasive survival
of expressed mitochondrial rps14 pseudogenes in grasses and their
relatives for 80 million years following three functional transfers to the
nucleus. – BMC Evol. Biol. 6: 55.
http://www.biomedcentral.com/1471-2148/6/55
Oostermeijer JGB. 1989. Myrmecochory in
Polygala vulgaris L., Luzula campestris (L.) DC., and
Viola curtisii Forster in a Dutch dune area. – Oecologia 78:
302-311.
Oriani A, Scatena VL. 2007. Intracellular
papillae of Actinocephalus (Eriocaulaceae-Poales) roots and their
interaction with fungi: a light and transmission electron microscopy study. –
Micron 38: 611-617.
Oriani A, Scatena VL. 2011. Reproductive
biology of Abolboda pulchella and A. poarchon (Xyridaceae: Poales). – Ann. Bot. 107:
611-619.
Oriani A, Scatena VL, Sano PT. 2008.
Morphological architecture of Actinocephalus (Koern.) Sano (Eriocaulaceae-Poales). – Flora 203:
341-349.
Oriani A, Sano PT, Scatena VL. 2009.
Pollination biology of Syngonanthus elegans (Eriocaulaceae-Poales). – Aust. J.
Bot. 57: 94-105.
Oriani A, Scatena VL. 2012. Floral anatomy of
xyrids (Poales): contributions to their reproductive biology, taxonomy, and
phylogeny. – Intern. J. Plant Sci. 173: 767-779.
Oriani A, Scatena VL. 2013. The taxonomic
value of floral characters in Rapateaceae (Poales-Monocotyledons).
– Plant Syst. Evol. 299: 291-303.
Oriani A, Scatena VL. 2014. Ovule, fruit and
seed development in Abolboda (Xyridaceae, Poales): implications for
taxonomy and phylogeny. – Bot. J. Linn. Soc. 175: 144-154.
Oriani A, Scatena VL. 2015. Anther wall
development, microsporogenesis, and microgametogenesis in Abolboda and
Orectanthe: contributions to the embryology of Xyridaceae (Poales). – Intern. J.
Plant Sci. 176: 324-332.
Oriani A, Scatena VL, Sano PT. 2008.
Morphological architecture of Actinocephalus (Koern.) Sano (Eriocaulaceae-Poales). – Flora 203:
341-349.
Oriani A, Sano PT, Scatena VL. 2009.
Pollination biology of Syngonanthus elegans (Eriocaulaceae-Poales). – Aust. J.
Bot. 57: 94-105.
Oross JW, Thomson WW. 1982. The
ultrastructure of the salt glands of Cynodon and Distichlis
(Poaceae). – Amer. J. Bot. 69:
939-949.
Orr AR, Sundberg MD. 2004. Inflorescence
development in a new teosinte: Zea nicaraguensis (Poaceae). – Amer. J. Bot. 91: 165-173.
Ortíz JJ. 1991. Schenckochloa (Poaceae, Chloridoideae, Eragrostideae), un
género nuevo del noreste de Brasil. – Candollea 46: 241-249.
Ortíz-Diaz JJ. 1993. Studio sistematico del
género Gouinia (Gramineae, Chloridoideae, Eragrostideae). – Acta
Bot. Mex. 23: 1-33.
Ortíz-Diaz J-J., Culham A. 2000.
Phylogenetic relationships of the genus Sporobolus (Poaceae: Eragrostideae) based on nuclear
ribosomal DNA ITS sequences. – In: Jacobs SWL, Everett J (eds), Grasses:
systematics and evolution, CSIRO, Collingwood, Australia, pp. 184-188.
Ortlieb U, Winkler S. 1977. Ökologische
Differenzierungsmuster in der Evolution der Bromeliaceen. – Bot. Jahrb. Syst.
97: 586-602.
Osada T. 1993. Illustrated grasses of Japan.
Enlarged edition. – Heibonsha Ltd., Tokyo.
Osborne CP. 2008. Atmosphere, ecology and
evolution: what drove the Miocene expansion of C4 grasslands? – J.
Ecol. 96: 35-45.
Osborne CP, Salomaa A, Kluyver TA, Visser V,
Kellogg EA, Morrone O, Vorontsova MS, Clayton WD, Simpson DA. 2014. A global
database of C4 photosynthesis in grasses. – New Phytol. doi:
10.1111/nph.12942
Oteng-Yeboah AA. 1974. Four new genera in Cyperaceae-Cyperoideae. – Notes Roy
Bot. Gard. Edinb. 33: 307-310.
Oteng-Yeboah AA. 1975. Morphology, anatomy
and taxonomy of the genus Remirea Aublet (Cyperaceae). – Boissiera 24A:
197-205.
Ovadiahu-Yavin Z. 1969. Cytotaxonomy of the
genus Bromus of Palestine. – Israel J. Bot. 18: 195-216.
Owen TP, Thomson WW. 1991. Structure and
function of a specialized cell wall in the trichomes of the carnivorous
bromeliad Brocchinia reducta. – Can. J. Bot. 69: 1700-1706.
Owen TP, Benzing DH, Thomson WW. 1988.
Apoplastic and ultrastructural characterizations of the trichomes from the
carnivorous bromeliad Brocchinia reducta. – Can. J. Bot. 66:
941-948.
Padhye MD, Makde KH. 1982. Pollen morphology
of Cyperaceae. – J. Palynol. 16:
71-81.
Page JS. 1978. A scanning electron microscope
survey of grass pollen. – Kew Bull. 32: 313-319.
Page VM. 1947. Leaf anatomy of
Streptochaeta and the relation of this genus to the bamboos. – Bull.
Torrey Bot. Club 74: 232-239.
Page VM. 1951. Morphology of the spikelet of
Streptochaeta. – Bull. Torrey Bot. Club 78: 22-37.
Paisooksantivatana Y, Pohl RW. 1992.
Morphology, anatomy and cytology of the genus Lithachne (Poaceae: Bambusoideae). – Rev. Biol.
Trop. 40: 47-72.
Palaci CA, Brown GK, Tuthill DE. 2004. The
seeds of Catopsis (Bromeliaceae: Tillandsioideae). –
Syst. Bot. 29: 518-527.
Palla E. 1888a. Über die Gattung
Scirpus. – Verhandl. Zool.-Bot. Ges. Wien 38, Sitzungsberichte, p.
49.
Palla E. 1888b. Zur Kenntnis der Gattung
Scirpus. – Engl. Bot. Jahrb. Syst. 10: 293-301.
Palla E. 1900. Die Gattungen der
mitteleuropäischen Scirpoideen. – Allg. Bot. Zeitschr. 6: 199-201,
213-217.
Palla E. 1905. Über den morphologischen Wert
der Blüte der Gattungen Lipocarpha und Platylepis. – Ber.
Deutsch. Bot. Ges. 23: 316-323.
Palla E. 1908. Über Hemicarpha. –
Österr. Bot. Zeitschr. 58: 417-422.
Palma-Silva C, Leal BSS, Chaves CJN, Fay MF.
2016. Advances in and perspectives on evolution in Bromeliaceae. – Bot. J. Linn. Soc.
181: 305-322.
Palmer PG, Tucker AE. 1981. A scanning
electron microscopy survey of the epidermis of East African grasses I. –
Smithsonian Contr. Bot. 49: 1-84.
Panajiotidis S, Athanasiadis N, Symeonidis L,
Karataglis S. 2000. Pollen morphology in relation to the taxonomy and phylogeny
of some native Greek Aegilops species. – Grana 39: 126-132.
Papini A, Mosti S, Milocani E, Tani G, Di
Falco P, Brighigna L. 2011. Megasporogenesis and programmed cell death in
Tillandsia (Bromeliaceae).
– Protoplasma 248: 651-662.
Parihar SK, Tripathi SN. 1989. Karyotypes of
Pennisetum species. – J. Indian Bot. Soc. 68: 295-299.
Parker PF. 1972. Studies in Dactylis
II. natural variation, distribution and systematics of the Dactylis
smithii Link complex in Madeira and other Atlantic islands. – New
Phytol. 71: 371-378.
Parmelee J, Savile BDO. 1954. Life history
and relationship of rusts of Sparganium and Acorus. –
Mycologia 46: 823-836.
Parodi LR. 1919. Las Chlorídeas de la
República Argentina. – Rev. Fac. Agron. Veterin. 2: 233-335.
Parodi LR. 1925. Notas sobre gramíneas de la
Flora Argentina. – Physis (Buenos Aires) 8: 59-81.
Parodi LR. 1935. Notas sobre gramíneas
Argentinas. – Physis (Buenos Aires) 11: 497-498.
Parodi LR. 1943. Gramíneas austroamericanas
nuevas o críticas II. – Not. Mus. La Plata, Bot. 8(40): 75-100.
Parodi LR. 1944. Revisión de las gramíneas
australes americanas del género Piptochaetium. – Rev. Mus. La
Plata, Secc. Bot. 6: 213-310.
Parodi LR. 1945. Una nueva especie de
Gramínea del género Chloris y sus relaciones con
Gymnopogon. – Rev. Argent. Agron. 12: 45-50.
Parodi LR. 1946. The Andean species of the
genus Stipa allied to Stipa obtusa. – Blumea 3: 63-70.
Parodi LR. 1947. Las especies de gramineas
del género Nassella de la Argentina y Chile. – Darwiniana 7:
369-395.
Parodi LR. 1953. Gramíneas argentinas nuevas
o críticas II. – Rev. Argent. Agron. 20: 11-30.
Parodi LR. 1960. Las especies de
Stipa del subgénero Pappostipa de la Argentina y Chile. –
Rev. Argent. Agron. 27: 65-106.
Parodi LR. 1961. Gramíneas argentinas nuevas
o críticas III. – Rev. Argent. Agron. 28: 100-125.
Parodi LR, Nicora EG. 1977. Novedades en el
genero Hordeum (Gramineae). – Hickenia 1: 55-62.
Parolly G, Scholz H. 2004. Oreopoa
gen. novum, two other new grasses and further remarkable records from Turkey.
– Willdenowia 34: 145-158.
Parra LR. 2000. Redelimitação e revisão de
Syngonanthus sect. Eulepis (Bong. ex Körn.) Ruhland –
Ericaulaceae. – Ph.D. diss., Universidade de São Paulo, São Paulo.
Parra LR, Giulietti AM. 1997. Nomenclatural
and taxonomic changes in Brazilian Syngonanthus (Eriocaulaceae). – Willdenowia 27:
227-233.
Parra LR, Giulietti AM, Andrade MJG de, Berg
C van den. 2010. Reestablishment and new circumscription of Comanthera
(Eriocaulaceae). – Taxon 59:
1135-1146.
Pate JS, Delfs JC. 1999. Anatomical features
of Restionaceae and allied
families. – In: Meney KA, Pate JS (eds), Australian rushes. Biology,
identification and conservation of Restionaceae and allied families,
University of Western Australia Press, Nedlands, Western Australia, pp.
57-70.
Pate JS, Meney KA, Dixon KW. 1991.
Contrasting growth and morphological characteristics of fire-sensitive
(obligate seeder) and fire-resistant (resprouter) species of Restionaceae (S. Hemisphere restiads)
from south-western Australia. – Aust. J. Bot. 39: 505-525.
Patel CM, Patel DS. 1964. The morphological
and embryological studies in Eriocaulon cinereum R. Br. – J. Gujarat
Univ. 7: 58-70.
Patel CM, Shah CK. 1960. The embryology of
Kyllingia triceps Rottb. – Vidya. J. Gujarat Univ. 3: 61-74.
Paterson A, Bowers JE, Chapman BA. 2004.
Ancient polyploidization predating divergence of the cereals, and its
consequences for comparative genomics. – Proc. Natl. Acad. Sci. U.S.A. 101:
9903-9908.
Paunero E. 1953. Las especies españolas del
género Anthoxanthum L. – An. Inst. Bot. AJ Cavanilles 12:
401-442.
Paunero E. 1963. El género
Ctenopsis De Not. en la flora española. – An. Inst. Bot. Cavanilles
21: 357-386.
Paunero E. 1964. Notos sobre gramíneas 2.
Consideraciones acerca de las especies españolas del genera Vulpia
Gmel. – An. Inst. Bot. Cavanilles 22: 83-114.
Pavlick LE. 1995. Bromus L. of North
America. – Victoria.
Pax F. 1886. Beiträge zur Morphologie und
Systematik der Cyperaceen. – Engl. Bot. Jahrb. Syst. 7: 287-318.
Pax F. 1887. Cyperaceae (Riedgräser). – In: Engler
A, Prantl K (eds), Die natürlichen Pflanzenfamilien II(2), W. Engelmann,
Leipzig, pp. 98-126.
Pée-Laby E. 1898. Étude anatomique de la
feuille des Graminées de la France. – Ann. Sci. Nat., Bot., sér. 8, 8:
227-346.
Peichoto MC, Arbo MM, Rúgolo de Agrasar ZE,
Neffa VS. 2007. Identity of Schizachyrium sulcatum and S.
brevifolium (Poaceae:
Andropogoneae). – Brittonia 59: 65-78.
Peichoto MC, Mazza SM, Neffa VGS. 2008.
Morphometric analysis of Schizachyrium condensatum (Poaceae) and related species. – Plant
Syst. Evol. 276: 177-189.
Peñailillo P. 1996. Anatherostipa,
un Nuevo género de Poaceae (Stipeae).
– Gayana, Bot. 53: 277-284.
Peñailillo P. 2002. El género
Jarava Ruiz et Pav. (Stipeae-Poaceae): delimitacion y nuevas
combinaciones. – Gayana Bot. 59: 27-34.
Peñailillo P. 2005. Los géneros nativos de
la tribu Stipeae (Poaceae, Pooideae) en
Chile. – Teoria 14: 125-140.
Peng S, Yang H-Q, Li D-Z. 2008. Highly
heterogeneous generic delimitation within the temperate bamboo clade (Poaceae: Bambusoideae): evidence from
GBSSI and ITS sequences. – Taxon 57: 799-810.
Peng Y-Y, Wei Y-M, Baum BR, Jiang Q-T, Lan
X-J, Dai S-F, Zheng Y-L. 2010. Phylogenetic investigation of Avena
diploid species and the maternal genome donor of Avena polyploids. –
Taxon 59: 1472-1482.
Pensiero JF. 1999. Las especies sudamericanas
del género Setaria (Poaceae,
Paniceae). – Darwiniana 37: 37-151.
Pénzes A. 1936. Notes on Bromus.
– Bot. Közlem. 33: 98-138.
Perak JT. 1943. Numero de cromosomas de
algunas especies de Hordeum espontáneas en Argentina. – An. Inst.
Fitotec. S. Catalina 3: 7-11.
Pereira E, Leme EMC. 1986. Contribuição ao
estudo do gênero Nidularium (Bromeliaceae) I. Subgênero
Canistropsis. – Bradea 4: 219-254.
Pereira MP, Pérez GE, Balbuena ES. 2007.
European sweet vernal grasses (Anthoxanthum: Poaceae, Pooideae, Aveneae): a morphometric
taxonomical approach. – Syst. Bot. 32: 43-59.
Perreta MG, Vegetti AC. 2002. The
inflorescence of Cyperus giganteus Vahl (Cyperaceae). – Feddes Repert. 113:
256-260.
Perreta MG, Vegetti AC. 2006. Structure and
development of the branching system in Melica sarmentosa Nees (Poaceae). – Feddes Repert. 117:
264-271.
Perreta MG, Tivano JC, Vegetti AC. 2000.
Forma de crecimiento en Leptochloa chloridiformis (Poaceae). – Darwiniana 38: 219-226.
Perreta MG, Ramos JC, Vegetti AC. 2009.
Development and structure of the grass inflorescence. – Bot. Rev. 75:
377-396.
Peters J. 2009. Revision of the genus
Fosterella L. B. Sm. (Bromeliaceae). – Ph.D. diss.,
Universität von Kassel, Germany.
Peters J, Vásquez R, Osinaga A, Leme E,
Weising K, Ibisch PL. 2008. Towards a taxonomic revision of the genus
Fosterella (Bromeliaceae).
– Selbyana 29: 182-194.
Petersen G, Seberg O. 1997. Phylogenetic
analysis of the Triticeae (Poaceae)
based on rpoA sequence data. – Mol. Phylogen. Evol. 7: 217-230.
Petersen G, Seberg O. 2003. Phylogenetic
analyses of the diploid species of Hordeum (Poaceae) and a revised classification of
the genus. – Syst. Bot. 28: 293-306.
Petersen G, Seberg O, Baden C. 2004. A
phylogenetic analysis of the genus Psathyrostachys (Poaceae) based on one nuclear gene, three
plastid genes, and morphology. – Plant Syst. Evol. 249: 99-110.
Petersen G, Seberg O, Aagesen L, Frederiksen
S. 2004. An empirical test of the treatment of indels during optimization
alignment based on the phylogeny of the genus Secale (Poaceae). – Mol. Phylogen. Evol. 30:
733-742.
Petersen G, Seberg O, Hyde M, Berthelsen K.
2006. Phylogenetic relationships of Triticum and Aegilops and
evidence for the origin of the A, B, and D genomes of common wheat
(Triticum aestivum). – Mol. Phylogen. Evol. 39: 70-82.
Petersen G, Aagesen L, Seberg O, Larsen ID.
2011. When is enough, enough in phylogenetics? A case in point from
Hordeum (Poaceae). –
Cladistics 27: 428-446.
Petersen G, Seberg O, Salomon B. 2011. The
origin of the H, St, W, and Y genomes in allotetraploid species of
Elymus L. and Stenostachys Turcz. (Poaceae: Triticeae). – Plant Syst. Evol.
291: 197-210.
Peterson PM. 1989. Lemma micromorphology in
the annual Muhlenbergia (Poaceae). – Southw. Natur. 34: 61-71.
Peterson PM. 1997. A classification of and
key to the supraspecific taxa in Eleocharis (Cyperaceae). – Taxon 46: 433-449.
Peterson PM. 2000. Systematics of the
Muhlenbergiinae (Chloridoideae: Eragrostideae). – In: Jacobs SWL, Everett J
(eds), Grasses: systematics and evolution, Proceedings of the 2nd
International Conference on the Comparative Biology of Monocotyledons, 1998,
Sydney, Australia, Vol. 2, CSIRO Publ., Melbourne, pp. 195-212.
Peterson PM, Annable CR. 1990. A revision of
Blepharoneuron (Poaceae:
Eragrostideae). – Syst. Bot. 15: 515-525.
Peterson PM, Rieseberg LH. 1987. Flavonoids
of the annual Muhlenbergia (Poaceae). – Biochem. Syst. Ecol. 15:
647-652.
Peterson PM, Soreng R. 2016. A revision of
Poa subsection Aphanelytrum (Poaceae, Pooideae, Poeae, Poinae); and a
new species, Poa auriculata. – PhytoKeys 63: 107-125.
Peterson PM, Valdès-Reyna J. 2005.
Eragrostis (Poaceae:
Chloridoideae: Eragrostideae: Eragrostidinae) from northeastern México. –
Sida 21: 1363-1418.
Peterson PM, Vega IS. 2007.
Eragrostis (Poaceae:
Chloridoideae: Eragrostideae: Eragrostidinae) of Peru. – Ann. Missouri Bot.
Gard. 94: 745-790.
Peterson PM, Annable CR, Franceschi VR. 1989.
Comparative leaf anatomy of the annual Muhlenbergia (Poaceae). – Nord. J. Bot. 8: 575-583.
Peterson PM, Webster RD, Valdés-Reyna J.
1995. Subtribal classification of the New World Eragrostideae (Poaceae: Chloridoideae). – Sida 16:
529-544.
Peterson PM, Webster RD, Valdés-Reyna J.
1997. Genera of New World Eragrostideae (Poaceae; Chloridoideae). – Smithsonian
Contr. Bot. 87: 1-50.
Peterson PM, Soreng RJ, Davidse G. 1998.
Proposal to conserve the name Elionurus (Poaceae, Andropogoneae) with that spelling.
– Taxon 47: 737-738.
Peterson PM, Soreng RJ, Davidse G, Filgueiras
TS, Zuloaga FO, Judziewicz EJ. 2001. Catalogue of New World grasses (Poaceae) II. Subfamily Chloridoideae. –
Contr. U. S. Natl. Herb. 41: 1-255.
Peterson PM, Columbus JT, Pennington SJ. 2007
[2008]. Classification and biogeography of New World grasses: Chloridoideae.
– In: Columbus JT, Friar EA, Porter JM, Prince LM, Simpson MG (eds),
Monocots: comparative biology and evolution. Poales, Rancho Santa Ana Botanical
Garden, Claremont, California. – Aliso 23: 580-594.
Peterson PM, Romaschenko K, Johnson G. 2010a.
A phylogeny and classification of the Muhlenbergiinae (Poaceae: Chloridoideae: Cynodonteae) based
on plastid and nuclear DNA sequences. – Amer. J. Bot. 97: 1532-1554.
Peterson PM, Romaschenko K, Johnson G. 2010b.
A classification of the Chloridoideae (Poaceae) based on multi-gene phylogenetics
trees. – Mol. Phylogen. Evol. 55: 580-598.
Peterson PM, Romaschenko K, Barker NP, Linder
HP. 2011. Centropodieae and Ellisochloa, a new tribe and genus in
Chloridoideae (Poaceae). – Taxon 60:
1113-1122.
Peterson PM, Romaschenko K, Snow N, Johnson
G. 2012. A molecular phylogeny and classification of Leptochloa (Poaceae: Chloridoideae: Chlorideae)
sensu lato and related genera. – Ann. Bot. 109: 1317-1330.
Peterson PM, Romaschenko K, Arrieta YH. 2014.
A molecular phylogeny and classification of the Cteniinae, Farragininae,
Gouiniinae, Gymnopogoninae, Perotidinae, and Trichoneurinae (Poaceae: Chloridoideae: Cynodonteae). –
Taxon 63: 275-286.
Peterson PM, Romaschenko K, Soreng RJ. 2014.
A laboratory guide for generating DNA barcodes in grasses: a case study of
Leptochloa s.l. (Poaceae:
Chloridoideae). – Webbia 69: 1-12.
Peterson PM, Romaschenko K, Arrieta YH,
Saarela JM. 2015. A molecular phylogeny and new subgeneric classification of
Sporobolus (Poaceae:
Chloridoideae: Sporobolinae). – Taxon 63: 1212-1243.
PPeterson PM, Romaschenko K, Arrieta YH.
2015. A molecular phylogeny and classification of the Eleusininae with a new
genus, Micrachne (Poaceae:
Chloridoideae: Cynodonteae). – Taxon 64: 445-467.
Peterson PM, Romaschenko K, Herrera Arrieta
Y. 2015. Phylogeny and subgeneric classification of Bouteloua with a
new species, B. herrera-arrietae (Poaceae: Chloridoideae: Cynodonteae:
Boutelouinae). – J. Syst. Evol. 53: 351-366.
Peterson PM, Romaschenko K, Herrera Arrieta
Y. 2016. A molecular phylogeny and classification of the Cynodonteae (Poaceae: Chloridoideae) with four new
genera: Orthacanthus, Triplasiella, Tripogonella,
and Zaqiqah; three new subtribes: Dactylocteniinae, Orininae, and
Zaqiqahinae; and a subgeneric classification of Distichlis. – Taxon
65: 1263-1287.
Peterson PM, Romaschenko K, Herrera Arrieta
Y. 2017a. A molecular phylogeny of the subtribe Sporobolinae and an
classification of the subfamily Chloridoideae (Poaceae). – Mem. New York Bot. Gard. 118:
127-151.
Peterson PM, Romaschenko K, Herrera Arrieta
Y. 2017b. Four new subtribes: Allolepiinae, Jouveinae, Kaliniinae, and
Sohnsiinae in the Cynodonteae (Poaceae:
Chloridoideae). – Phytoneuron 44: 1-9.
Petrova LR, Tsvelev NN. 1974. On the
evolution of inflorescence in Poaceae:
on the nature and functions of lodicules. – Bot. Žurn. 59: 1713-1720. [In
Russian with English summary]
Petrova OA. 1975. On the main chromosome
numbers in the genus Milium. – Bot. Žurn. 60: 393-394. [In
Russian]
Pfeiffer HH. 1920. Über die Stellung der
Gattung Caustis R. Br. im natürlichen System 2. – Ber. Deutsche
Bot. Ges. 38: 207-216.
Pfeiffer HH. 1921a. Revision der Gattung
Ficinia Schrad. – Bremen.
Pfeiffer HH. 1921b. Beiträge zur Morphologie
und Systematik der Gattungen Lagenocarpus und Cryptangium.
– Ber. Deutsch. Bot. Ges. 39: 125-134.
Pfeiffer HH. 1921c. Conspectus Cyperacearum
in America meridionali nascentium I. Genus Heleocharis R. Br. –
Clavis analytica. – Herbarium 57: 65-68.
Pfeiffer HH. 1922. De novis et criticis
speciebus generum saepe ignotorum Scleriearum. – Feddes Repert. 18:
375-385.
Pfeiffer HH. 1925. Vorarbeiten zur
systematischen Monographie der Cyperaceae-Mapanieae. – Bot. Arch. 12:
446-472.
Pfeiffer L. 1927. Untersuchungen zur
vergleichenden Anatomie der Cyperaceen 1. Anatomie der Blätter. – Beih. Bot.
Centralbl. 44, Abt. 1: 90-176.
Pfeiffer HH. 1930. Decas Cyperacearum
criticarum vel emendatarum III. – Feddes Repert. 29: 171-186.
Pfeiffer HH. 1942.
Polarisationsmikroskopische Befunde an den Pollentetraden einiger
Cyperaceen-Arten. – Veröff. Deutsch. Kolon. Ûbersee-Mus. 3: 238-243.
Philcox D. 1992a. Notes on South American Bromeliaceae. – Kew Bull. 47:
261-276.
Philcox D. 1992b. Notes on South American Bromeliaceae: corrections. – Kew
Bull. 47: 544.
Philipson MN. 1977. Haustorial synergids in
Cortaderia (Gramineae). – New Zealand J. Bot. 15: 777-778.
Philipson MN, Connor HE. 1984. Haustorial
synergids in danthonioid grasses. – Bot. Gaz. 145: 78-82.
Philipson WR. 1985. Is the grass gynoecium
monocarpellary? – Amer. J. Bot. 72: 1954-1961.
Phillips SM. 1973. The genus Dinebra
Jacq. (Gramineae). – Kew Bull. 28: 411-418.
Phillips SM. 1974. Studies in the Gramineae
XXV. – Kew Bull. 29: 267-270.
Phillips SM. 1975. A review of the genus
Oropetium (Gramineae). – Kew Bull. 30: 467-470.
Phillips SM. 1982. A numerical analysis of
the Eragrostideae (Gramineae). – Kew Bull. 37: 133-162.
Phillips SM. 1986a. Agrostis
kilimandscharica (Gramineae) and its allies in eastern Africa. – Kew
Bull. 41: 131-140.
Phillips SM. 1986b. Four new grasses from
North East tropical Africa. – Kew Bull. 41: 1027-1030.
Phillips SM. 1987. Notes on
Eragrostis (Gramineae) from northeast tropical Africa. – Kew Bull.
42: 929-931.
Phillips SM. 1989. The genus Poa
(Gramineae) in Ethiopia. – Kew Bull. 44: 127-137.
Phillips SM. 1991a. Four new species of
Eragrostis (Gramineae) from northeast tropical Africa. – Kew Bull.
46: 111-117.
Phillips SM. 1991b. Some new species of
Gramineae from Ethiopia. – Kew Bull. 46: 535-537.
Phillips SM. 1994a. Notes on some
Eriocaulon species from Ceylon. – Kew Bull. 49: 287-303.
Phillips SM. 1994b. Two new species of
Hyparrhenia (Gramineae) from Ethiopia. – Kew Bull. 49: 537-542.
Phillips SM. 1995. A new species of
Eriocaulon (Eriocaulaceae) from Ceylon, with
notes on some other Ceylonese species. – Kew Bull. 50: 733-738.
Phillips SM. 1996a. Eriocaulon
schimperi (Eriocaulaceae) and
some related species in eastern Africa. – Kew Bull. 51: 333-342.
Phillips SM. 1996b. Some new African taxa of
Eriocaulon, with notes on their systematic position. – Kew Bull. 51:
625-647.
Phillips SM. 1997a. Eriocaulaceae. – In: Polhill RM
(ed), Flora of tropical East Africa, A. A. Balkema, Rotterdam, pp. 1-41.
Phillips SM. 1997b. The Eriocaulon
transvaalicum complex and some other species of Eriocaulon from
Africa. – Kew Bull. 52: 51-72.
Phillips SM. 1997c. The genus
Syngonanthus (Eriocaulaceae) in Eastern and
Southern Africa. – Kew Bull. 52: 73-89.
Phillips SM. 1998. Two new species of
Eriocaulon from West Africa. – Kew Bull. 53: 943-948.
Phillips SM. 2000. Two more new species of
Eriocaulon from West Africa. – Kew Bull. 55: 195-202.
Phillips SM. 2002. The genus
Tripogon (Poaceae) in China.
– Kew Bull. 57: 911-924.
Phillips SM, Chen SL. 2002. The genus
Tripogon (Poaceae) in China.
– Kew Bull. 57: 911-924.
Phillips SM, Launert E. 1971. A revision of
the African species of Tripogon Roem. & Schult. – Kew Bull. 25:
301-322.
Phillips SM, Mesterházy A. 2015. Revision of
small ephemeral species of Eriocaulon (Eriocaulaceae) in West Africa with
long involucral bracts. – Kew Bull. 70: 5 DOI 10.1007/S12225-014-9557-2
Phipps JB. 1967a. Studies in the
Arundinelleae (Gramineae) VI. Development of generic concepts. – Bol. Soc.
Broter., Ser. II, 41: 27-55.
Phipps JB. 1967b. Studies in the
Arundinelleae (Gramineae) VIII. The phylogeny – a hypothesis. – Kirkia 15:
477-517.
Piech K. 1924. Zur Entwicklung der
Pollenkörner bei Scirpus lacustris L. – Bull. Acad. Polon. Sci.
Lettr., Sér. B, 1924: 113-123.
Piech K. 1928a. Über die Entstehung der
generativen Zelle bei Scirpus uniglumis Link durch “freie
Zellbildung”. – Planta 6: 96-117.
Piech K. 1928b. Zytologische Studien an der
Gattung Scirpus. – Bull. Intern. Acad. Polon. Sci. Lettres 1928:
1-43.
Pienaar R de V. 1953. Cytological studies in
some South African species of the genus Eragrostis Host. – Ph.D.
diss., University of Witwatersrand, Johannesburg, Republic of South Africa.
Pienaar R de V, Littlejohn GM, Sears ER.
1988. Genomic relationships in Thinopyrum. – South Afr. J. Bot. 54:
541-550.
Piérart P. 1951. Les espèces du genre
Scleria Berg. du Congo Belge et Ruanda Urundi. – Lejeunea Mém. 13:
1-70.
Pierce S. 2007 [2008]. The jewelled armor of
Tillandsia – multifaceted or elongated trichomes provide
photoprotection. – In: Columbus JT, Friar EA, Porter JM, Prince LM, Simpson
MG (eds), Monocots: comparative biology and evolution. Poales, Rancho Santa Ana
Botanical Garden, Claremont, California, pp. 44-52.
Pierce S, Maxwell K, Griffiths H, Winter K.
2001. Hydrophobic trichome layers and epicuticular wax powders in Bromeliaceae. – Amer. J. Bot. 88:
1371-1389.
Pignotti L, Mariotti LM. 2004.
Micromorphology of Scirpus (Cyperaceae) and related genera in
South-West Europe. – Bot. J. Linn. Soc. 145: 45-58.
Pilger R. 1906. Gramineae andinae II. –
Engl. Bot. Jahrb.Syst. 37: 373-381.
Pilger R. 1920. Gramineae austro-americanae
imprimis Weberbauerianae V. – Engl. Bot. Jahrb. Syst. 56: 23-30.
Pilger R. 1930a. Mayacaceae. – In: Engler A (ed), Die
natürlichen Pflanzenfamilien, 2. Augl., Bd. 15a, W. Engelmann, Leipzig, pp.
33-35.
Pilger R. 1930b. Thurniaceae. – In: Engler A (ed), Die
natürlichen Pflanzenfamilien, 2. Aufl., Bd. 15a, W. Engelmann, Leipzig, pp.
58-59.
Pilger R. 1930c. Rapateaceae. – In: Engler A (ed), Die
natürlichen Pflanzenfamilien, 2. Aufl., Bd. 15a, W. Engelmann, Leipzig, pp.
59-65.
Pilger R. 1940. Gramineae III. – In: Engler
A (†), Harms H, Mattfeld J (eds), Die natürlichen Pflanzenfamilien, 2.
Aufl., Bd. 14e, W. Engelmann, Leipzig.
Pilger R (†), Potztal E. 1954a. Beiträge
zur Flora von Südwestafrika I. Gramineae. – Willdenowia 1: 199-274.
Pilger R (†), Potztal E. 1954b. Das System
der Gramineae unter Ausschluß der Bambusoideae. – Bot. Jahrb. Syst. 76:
281-384.
Pilger R (†), Potztal E. 1956. Gramineae
II. Nachtrag zu Band 14e, Gramineae III. – In: Engler A (†), Harms H (†),
Mattfeld J (†), Melchior H, Werdermann E (eds), Die natürlichen
Pflanzenfamilien, 2. Aufl., Bd. 14d, Duncker & Humblot, Berlin.
Pilger R. 1954. Das System der Gramineae. –
Bot. Jahrb. Syst. 76: 281-384.
Pillans NS. 1928. The African genera and
species of Restionaceae. – Trans.
Roy. Soc. South Africa 16: 207-439.
Pillay M. 1993. Chloroplast genome
organization of bromegrass, Bromus inermis Leyss. – Theor. Appl.
Gen. 86: 281-287.
Pillay M, Hilu KW. 1990. Chloroplast DNA
variation in diploid and polyploidy species of Bromus (Poaceae) subgenera Festucaria and
Ceratochloa. – Theor. Appl. Gen. 80: 326-332.
Pillay M, Hilu KW. 1995. Chloroplast-DNA
restriction site analysis in the genus Bromus (Poaceae). – Amer. J. Bot. 82: 239-249.
Pimentel M, Estévez G, Sahuquillo E. 2007.
European sweet vernal grasses (Anthoxanthum, Poaceae; Pooideae; Aveneae): a morphometric
taxonomical approach. – Syst. Bot. 32: 43-59.
Pimentel M, Sahuquillo E, Catalán P. 2007.
Genetic diversity and spatial correlation patterns unravel the biogeographic
history of the European sweet vernal grasses (Anthoxanthum L., Poaceae). – Mol. Phylogen. Evol. 44:
667-684.
Pimentel M, Sahuquillo E, Torrecilla Z, Popp
M, Catalán P, Brochmann C. 2013. Hybridization and long-distance colonization
at different time scales: towards resolution of long-term controversies in the
sweet vernal grasses (Anthoxanthum). – Ann. Bot. 112: 1015-1030.
Pinangé Diego SB, Krapp F, Zizka G,
Silvestro D, Leme EMC, Weising K, Benko-Iseppon AM. 2017. Molecular
phylogenetics, historical biogeography and character evolution in
Dyckia (Bromeliaceae,
Pitcairnioideae). – Bot. J. Linn. Soc. 183: 39-56.
Piper CV. 1906. North American species of
Festuca. – Contr. U.S. Natl. Herb. 10: 1-48.
Piperno D, Pearsall DM. 1998. The silica
bodies of tropical American grasses: morphology, taxonomy, and implications for
grass systematics and fossil phytolith identification. – Smithsonian Contr.
Bot. 85: 1-40.
Piperno D, Sues H-D. 2005. Dinosaurs dined on
grass. – Science 310: 1126-1128.
Pirie MD, Humphreys AM, Galley C, Barker NP,
Verboom GA, Orlovich D, Draffin SJ, Lloyd K, Baeza CM, Negritto M, Ruiz E,
Sanchez JHC, Reimer E, Linder HP. 2008. A novel supermatrix approach improves
resolution of phylogenetic relationships in a comprehensive sample of
danthonioid grasses. – Mol. Phylogen. Evol. 48: 1106-1119.
Pirie MD, Humphreys AM, Barker NP, Linder HP.
2009. Reticulation, data combination, and inferring evolutionary history: an
example from Danthonioideae (Poaceae).
– Syst. Biol. 58: 612-628.
Pirie MD, Lloyd KM, Lee WG, Linder HP. 2010.
Diversification of Chionochloa (Poaceae) and biogeography of the New
Zealand Southern Alps. – J. Biogeogr. 37: 379-392.
Pirie MD, Humphreys AM, Antonelli A, Galley
C, Linder HP. 2012. Model uncertainty in ancestral area reconstruction: a
parsimonious solution? – Taxon 61: 652-664.
Pittendrigh CS. 1948. The
bromeliad-Anopheles-malaria complex in Trinidad I. The bromeliad
flora. – Evolution 2: 58-89.
Pleines T, Blattner FR. 2008. Phylogeographic
implications of an AFLP phylogeny of the American diploid Hordeum
species (Poaceae: Triticeae). – Taxon
57: 875-881.
Plowman AB. 1906. The comparative anatomy and
phylogeny of the Cyperaceae. – Ann.
Bot. 20: 1-30.
Plunkett GM. 1994. A molecular-phylogenetic
approach to the “family-pair dilemma” in Apiales and Cyperales. – Ph.D. diss., Washington
State University, Pullman, Washington.
Plunkett GM, Soltis DE, Soltis PS, Brooks RE.
1995. Phylogenetic relationships between Juncaceae and Cyperaceae: insights from rbcL
sequence data. – Amer. J. Bot. 82: 520-525.
Poddubnaya-Arnoldi VA. 1978.
Cytoembryological characteristics of the Poaceae. – Bull. Main Bot. Gard. 109:
57-60. [In Russian]
Pohl RW. 1969. Muhlenbergia,
subgenus Muhlenbergia (Gramineae) in North America. – Amer. Midl.
Natur. 82: 512-542.
Pohl RW. 1972. New taxa of
Hierochloë, Pariana and Triplasis from Costa Rica.
– Iowa State J. Res. 47: 71-78.
Pohl RW. 1980a. On the flowering of bamboos
in Central America. – Brenesia 19: 465-475.
Pohl RW. 1980b. Gramineae. – In: Burger W
(ed), Flora Costaricensis, Fieldiana Bot. N, ser. 4: 1-608.
Pohl RW, Clark LG. 1992. New chromosome
counts for Chusquea and Aulonemia (Poaceae: bambusoideae). – Amer. J. Bot.
79: 478-480.
Pohl RW, Davidse G. 1971. Chromosome numbers
of Costa Rican grasses. – Brittonia 23: 293-324.
Pohl RW, Lersten NR. 1975. Stem aerenchyma as
a character separating Hymenachne and Sacciolepis (Gramineae:
Panicoideae). – Brittonia 27: 223-227.
Poilecot P. 1999. Les Poaceae du Niger. Description,
illustration, écologie, utilisations. – Boissiera 56.
Poinar GO Jr. 2004. Programinis
burmitis gen. et sp. nov., and P. laminatus sp. nov., early
Cretaceous grass-like monocots in Burmese amber. – Aust. Syst. Bot. 17:
497-504.
Poinar GO Jr. 2011. Silica bodies in the
Early Cretaceous Programinis laminatus (Angiospermae: Poales). –
Palaeodivers. 4: 1-6.
Poinar GO Jr, Columbus JT. 1992. Adhesive
grass spikelet with mammalian hair in Dominican amber: first fossil evidence of
epizoochory. – Experientia 48: 906-908.
Pollock CJ, Cairns AJ. 1991. Fructan
metabolism in grasses and cereals. – Ann. Rev. Plant Physiol. Plant Mol.
Biol. 42: 77-101.
Porembski S, Barthlott W. 1995. On the
occurrence of a velamen radicum in Cyperaceae and Velloziaceae. – Nord.
J. Bot. 15: 625-629.
Portal R. 1995. Bromus de France.
– Vals-près-Le Puy.
Portal R. 2004. Quelques Bromus sur
la sellette. – Bull. Assoc. Bot. Digitalis 3: 18-30.
Potztal E. 1951. Anatomisch-systematische
Untersuchungen an den Gattungen Arrhenatherum und
Helictotrichon. – Bot. Jahrb. Syst. 75: 321-332.
Potztal E. 1952. Über die Blattanatomie der
Isachneae. – Bot. Jahrb. Syst. 75: 551-569.
Potztal E. 1953. Über die Anatomie von
Micraira subulifolia F. Muell. – Bot. Jahrb. Syst. 76: 134-138.
Potztal E. 1954. Die Anatomie der Gräser und
ihre Bedeutung für die Systematik. – Ber. Deutsch. Bot. Ges. 66:
(19)-(21).
Potztal E. 1957. Beschreibung einiger
systematischer Gruppen der Gräser. – Willdenowia 1: 771-772.
Potztal E. 1959. Zwei neue Gräser aus dem
südlichen Chile. – Willdenowia 2: 166-169.
Poulsen VA. 1888. Anatomiske Studier over
Eriocaulaceerne. – Vidensk. Medd. Danske Naturhist. Foren. 1888: 221-385.
Pozzobon MT, Machado ACC, Vaio M, Valls JFM,
Peñaloza APS, Santos S dos, Côrtes AL, Rua GH. 2008. Cytogenetic studies in
Paspalum (Gramineae) reveal new diploid species and accessions. –
Ciência Rural 38: 1292-1299.
Prakash N. 1969. The floral development and
embryology of Centrolepis fascicularis. – Phytomorphology 19:
285-291.
Prasad V, Strömberg CAE, Alimohammadian H,
Sahni H. 2005. Dinosaur coprolites and the early evolution of grasses and
grazers. – Science 310: 1177-1180.
Prat H. 1932. L’épiderme des Graminées:
étude anatomique et systématique. – Ann. Sci. Nat. Bot., sér. 10, 14:
117-324.
Prat H. 1936. La systématique des
Graminées. – Ann. Sci. Nat., Bot., sér. 10, 18: 165-258.
Prat H. 1960. Vers une classification
naturelle des Graminées. – Bull. Soc. Bot. France 107: 32-79.
Prendergast HDV, Hattersley PW. 1987.
Australian C4 grasses (Poaceae): leaf blade anatomical features in
relation to C4 acid decarboxylation types. – Aust. J. Bot. 35:
355-382.
Prendergast HDV, Hattersley PW, Stone NE.
1987. New structural/biochemical associations in leaf blades of C4
grasses (Poaceae). – Aust. J. Plant
Phys. 14: 403-420.
Preston JC, Kellogg EA. 2006. Reconstructing
the evolutionary history of paralogous APETALA1/FRUITFULL-like genes
in grasses (Poaceae). – Genetics 174:
421-437.
Preston JC, Kellogg EA. 2008. Discrete
developmental roles for temperate cereal grass
VERNALIZATION1/FRUITFULL-like genes in flowering competency and the
transition to flowering. – Plant Physiol. 146: 265-276.
Preston JC, Christensen A, Malcomber ST,
Kellogg EA. 2009. MADS-box gene expression and implications for developmental
origins of the grass spikelet. – Amer. J. Bot. 96: 1419-1429.
Price HJ, Dillon SL, Hodnett G, Rooney WL,
Ross L, Johnston S. 2005. Genome evolution in the genus Sorghum (Poaceae). – Ann. Bot. 95: 219-227.
Pritchard AJ. 1970. Meiosis and embryo sac
development in Urochloa mosambicensis and three Paspalum
species. – Aust. J. Agric. Res. 21: 649-652.
Probatova NS. 1973. Poacearum species novae
et rariores ex Oriente extreme. – Nov. Syst. Plant. Vasc. 10: 68-79. [In
Russian]
Probatova NS. 1974. De genere novo
Arctopoa (Griseb.) Probat. (Poaceae). – Novost. Sist. Vyssh. Rast.
11: 44-54. [In Russian]
Probatova NS. 2003. The genus
Arctopoa (Griseb.) Probat. (Poaceae): taxonomy, chromosome numbers,
biogeography and differentiation. – Komar. Čtenija (Vladivostok) 49:
89-130.
Probatova NS, Sokolovskaya AP. 1980. A
karyotaxonomic study of the grasses of the Altai Mts. – Bot. Žurn. 65:
509-520. [In Russian]
Probatova NS, Yurtzev BA. 1984. New taxa of
the family Poaceae from the north-east
of the USSR. – Bot. Žurn. 69: 688-692. [In Russian]
Proctor MCF, Bradshaw ME. 2014. Scanning
electron micrographs of leaves of British Carex species 2. Subgenus
Vignea. Carices with several hermaphrodite spikes. – New J. Bot. 4:
47-60.
Proctor MCF, Bradshaw ME. 2015. Scanning
electron micrographs of leaves of British Carex species 4. Subgenus
Carex: sections Paludosae, Porocystis,
Atratae, Paniceae, Pachystylae, Glaucae,
Ceratocystis and Strigosae. – New J. Bot. 5: 45-58.
Proença SL, Sajo M das G. 2008. Rhizome and
root anatomy of 14 species of Bromeliaceae. – Rodriguésia 59:
113-128.
Prychid CJ, Bruhl JJ. 2013. Floral ontogeny
and gene protein localization rules out euanthial interpretation of
reproductive units in Lepironia (Cyperaceae, Mapanioideae,
Chrysitricheae). – Ann. Bot. 112: 161-177.
Punt W. 1975. The Northwest European pollen
flora 5. Sparganiaceae and Typhaceae.
– Rev. Palaeobot. Palynol. 19: 75-88.
Quarín CL. 1977. Recuentos cromosómicos en
gramíneas de Argentina subtropical. – Hickenia 1: 73-78.
Quarín CL, Burson BL. 1983. Cytogenetic
relations among Paspalum notatum var. saurae, P.
pumilum, P. indecorum, and P. vaginatum. – Bot. Gaz.
144: 433-438.
Quezada IM, Gianoli E. 2011. Crassulacean
acid metabolism photosynthesis in Bromeliaceae: an evolutionary key
innovation. – Bot. J. Linn. Soc. 104: 480-486.
Quintanar A, Catalán P, Castroviejo S. 2006.
Adscription of Parafestuca albida (Lowe) E. B. Alexeev to
Koeleria Pers. – Taxon 55: 664-670.
Quintanar A, Castroviejo S, Catalán P. 2007.
Phylogeny of the tribe Aveneae (Pooideae, Poaceae) inferred from plastid
trnT-F and nuclear ITS sequences. – Amer. J. Bot. 94: 1554-1569.
Quintanar A, Catalán P, Castroviejo S. 2010.
A review of the systematics and phylogenetics of the Koeleriinae (Poaceae: Poeae). – In: Seberg O, Petersen
G, Barfod AS, Davis JI (eds), Diversity, phylogeny, and evolution in the
monocotyledons, Aarhus University Press, Århus, pp. 539-556.
Raab TK, Lipson DA, Monson RK. 1999. Soil
amino acid utilization among species of Cyperaceae: plant and soil processes.
– Ecology 80: 2408-2419.
Radhakrishnaiah M, Nageshwar G, Narayana LL.
1984. Chemotaxonomy of Pandanus and Typha. – Curr. Sci. 53:
759-760.
Ragonese AM, Guaglianone ER, Dizeo de
Strittmatter C. 1984. Desarrollo del pericarpio con cuerpos de silice de dos
especies de Rhynchospora Vahl (Cyperaceae). – Darwiniana 25:
27-41.
Rajbhandari KR. 1991. A revision of the genus
Poa L. (Gramineae) in the Himalaya. – Bull. Univ. Mus. Univ. Tokyo
34: 169-249.
Rajhathy T, Morrison JM. 1961. Cytogenetic
studies in the genus Hordeum V. H. jubatum and the new world
species. – Can. J. Genet. Cytol. 3: 378-390.
Ramadan T. 2001. Dynamics of salt secretion
by Sporobolus spicatus (Vahl) Kunth from sites of differing salinity.
– Ann. Bot. 87: 259-266.
Ramaswamy SN. 1975. Embryology and systematic
position of Eriocaulaceae. –
Ph.D. diss., University of Mysore, India.
Ramaswamy SN, Arekal GD. 1980. Embryology of
Eriocaulon setaceum (Eriocaulaceae). – Plant Syst. Evol.
138: 175-188.
Ramaswamy SN, Arekal GD. 1982. Embryology of
Eriocaulon xeranthemum Mart. (Eriocaulaceae). – Acta Bot. Neerl.
31: 41-54.
Ramaswamy SN, Raju MVS. 1982. The embryo sac
of Xyris schoenoides Mart. (Xyridaceae). – Bull. Torrey Bot. Club
109: 325-329.
Ramaswamy SN, Arekal GD, Gaju MVS. 1983.
Developmental anatomy of seed coat and pericarp in two species of
Eriocaulon L. (Eriocaulaceae). – Bull. Torrey Bot.
Club 110: 287-291.
Ramírez I. 1991. Systematic revision of
Neoregelia subgenus Hylaeaicum (Bromeliaceae). – M.Sc. thesis,
University of Missouri, St. Louis, Missouri.
Ramírez IM, Brown GK. 2001. The origin of
the low chromosome number in Cryptanthus (Bromeliaceae). – Syst. Bot. 26:
722-726.
Ramos COC, Borba EL, Funch LS. 2005.
Pollination in Brazilian Syngonanthus (Eriocaulaceae) species: evidence for
entomophily instead of anemophily. – Ann. Bot. 96: 387-397.
Randall JL, Hilu KW. 1986. Biosystematic
studies of North American Trisetum spicatum (Poaceae). – Syst. Bot. 11: 567-578.
Ranker TA, Soltis DE, Soltis PS, Gilmartin
AJ. 1990. Subfamilial phylogenetic relationships of the Bromeliaceae: evidence from
chloroplast DNA restriction site variation. – Syst. Bot. 15: 425-434.
Rao AN, Wee YC. 1979. Embryology of the
pineapple, Ananas comosus (L.) Merr. – New Phytol. 83: 485-497.
Rao PN, Nirmala A. 1990. Cytogenetic
variation and fertility in Coix aquatica (Maydeae). – Proc. Indian
Sci. Congress Assoc. 77: 135.
Rao TA, Naidu TRB. 1981. On the epidermal
fibrelike sclereids in the two sibling genera of the Poaceae. – Curr. Sci. 50: 958-959.
Raole VM, Desai RJ. 2008. Desmostachya
pingalaiae sp. nov. from Gujarat, India. – Nord. J. Bot. 26: 196-198.
Rath SP, Patnaik SN. 1974. A note on the
cytotaxonomy of East Indian species of the genus Fuirena Rottb. –
Bot. Mag. (Tokyo) 87: 333-338.
Rath SP, Patnaik SN. 1978. Cytological
studies in Cyperaceae with special
reference to its taxonomy II. – Cytologia 43: 643-653.
Rath SP, Mahaptra J, Patnaik SN. 1973.
Cytotaxonomic studies in the genus Cyperus L. (s.l.). – Proc. Indian
Sci. Congr. Assoc. 60: 314-316.
Rauh W. 1979. Bromeliads for home, garden and
greenhouse. – Blandford Press, Poole, Great Britain.
Rauh W. 1981. Bromelien. 2. Aufl. – Eugen
Ulmer, Stuttgart.
Rauh W. 1990. Bromelien. Tillandsien und
andere kulturwürdige Bromelien. 3. Aufl. – Eugen Ulmer, Stuttgart.
Ravi N, Mohanan N, Shaju T, Kiran Raj MS,
Rajesh R. 1998. Three new species of Ischaemum L. (Poaceae) from Kerala, India. – Rheedea 8:
149-158.
Ravi N, Mohanan N, Kiran Raj MS, Shaju T,
Rajesh R. 2000. Two new species of Poaceae from Kerala, India. Rheedea 10:
91-98.
Ravi N, Mohanan N, Shaju T, Kiran Raj MS,
Rajesh R. 2001. Three more new species of Ischaemum L. (Poaceae) from Kerala, India. – Bot. Bull.
Acad. Sin. 42: 223-230.
Raymond M. 1949. Notes sur le genre
Carex II. La valeur taxonomique de C. arctogena. – Contr.
Inst. Bot. Univ. Montréal 64: 37-41.
Raymond M. 1959. Carices Indo-chinenses nec
non Siamenses. – Mém. Jard. Bot. Montréal 53: 1-125.
Raymond M. 1966. Studies in the flora of
Thailand 39. Cyperaceae. – Dansk
Bot. Ark. 23: 311-374.
Raynal J. 1963. Notes cypérologiques 1.
Afrotrilepis, nouveau genre africain. – Adansonia, sér. II, 3:
250-265.
Raynal J. 1964. Notes cypérologiques 2. Deux
nouveaux Scleria ouest-africains. – Adansonia, sér. II, 4:
148-155.
Raynal J. 1967a. Notes cypérologiques 7. Sur
quelques Lipocarpha africains. – Adansonia, sér. II, 7: 81-87.
Raynal J. 1967b. Notes cypérologiques 8. Le
genre Actinoschoenus Benth. – Adansonia, sér. II, 7: 89-95.
Raynal J. 1968a. Notes cypérologiques 11.
Sur quelques Scirpus et Ascolepis de l’ancien monde. –
Adansonia, sér. II, 8: 85-104.
Raynal J. 1968b. Notes cypérologiques 14.
Mapania rhynchocarpa, nouvelle espèce ouest-africaine. – Adansonia,
sér. II, 8: 417-422.
Raynal j. 1968c. Notes cypérologiques 15.
Les Hypolytrum ‘mapanioides’ d’Afrique équatoriale.
– Adansonia, sér. II, 8: 423-430.
Raynal J. 1971a. Répartition géographique
des Rhynchospora africains et malgaches. – Mitt. Bot. Staatssamml.
München 10: 135-148.
Raynal J. 1971b. Quelques notes
morphologiques sur les Cyperacées. – Mitt. Bot. Staatssamml. München 10:
589-603.
Raynal J. 1972. Répartition et évolution
des modes de photosynthèse chez les Cypéracées. – Compt. Rend. Acad. Sci.
Paris, sér. D, 275: 2231-2234.
Raynal J. 1973. Notes cypérologiques 19.
Contribution à la classification de la sous-famille des Cyperoideae. –
Adansonia, sér. II, 13: 145-171.
Raynal J. 1974. Notes cypérologiques 22. Les
Costularia de Nouvelle-Calédonie. – Adansonia, sér. II, 14:
337-377.
Raynal J. 1976a. Notes cypérologiques 25. Le
genre Schoenoplectus I. Sur quelques espèces sud-africaines. –
Adansonia, sér. II, 15: 537-542.
Raynal J. 1976b. Notes cypérologiques 26. Le
genre Schoenoplectus II. L’amphicarpie et la sect. Supini.
– Adansonia, sér. II, 16: 119-155.
Raynal J. 1977. Notes cypérologiques 31.
Mélanges nomenclaturaux (Cyperoideae). – Adansonia, sér. II, 17: 43-47.
Raynal J. 1978. Notes cypérologiques 33.
Mélanges nomenclaturaux (2). – Adansonia, sér. II, 17: 273-280.
Read RW. 1968. A new combination in
Vriesea (Bromeliaceae).
– Phytologia 16: 457-458.
Read RW, Baensch HU. 1994. Ursulaea;
a new genus of Mexican bromeliads. – J. Bromeliad Soc. 44: 205-211.
Read RW, Luther HE. 1991. The
Aechmea/Gravisia complex (Bromeliaceae). – Selbyana 12:
54-67.
Reeder JR. 1943. The status of Distichlis
dentata. – Bull. Torrey Bot. Club 70: 53-57.
Reeder JR. 1953. The embryo of
Streptochaeta and its bearing on the homology of the coleoptile. –
Amer. J. Bot. 40: 77-80.
Reeder JR. 1957. The embryo in grass
systematics. – Amer. J. Bot. 44: 756-768.
Reeder JR. 1960. The systematic position of
the grass genus Anthephora. – Trans. Amer. Microsc. Soc. 79:
211-218.
Reeder JR. 1962. The bambusoid embryo: a
reappraisal. – Amer. J. Bot. 49: 639-641.
Reeder JR. 1965. The tribe Orcuttieae and the
subtribes of the Pappophoreae (Gramineae). – Madroño 18: 18-28.
Reeder JR. 1967. Notes on Mexican grasses VI.
Miscellaneous chromosome numbers. – Bull. Torrey Bot. Club 94: 1-17.
Reeder JR. 1971. Notes on Mexican grasses IX.
Miscellaneous chromosome numbers 3. – Brittonia 23: 105-117.
Reeder JR. 1977. Chromosome numbers in
western grasses. – Amer. J. Bot. 64: 102-110.
Reeder JR. 1982. Systematics of the tribe
Orcuttieae (Gramineae) and the description of new segregate genus,
Tuctoria. – Amer. J. Bot. 69: 1082-1095.
Reeder JR, Reeder CG. 1968.
Parodiella, a new genus of grasses from the high Andes. – Bol. Soc.
Argent. Bot. 12: 268-283.
Reeder JR, Reeder CG. 1980. Systematics of
Bouteloua breviseta and B. ramosa (Gramineae). – Syst. Bot.
5: 312-321.
Reeder JR, Singh DN. 1968. Chromosome numbers
in the tribe Pappophoreae (Gramineae). – Madroño 19: 183-187.
Reeder JR, Toolin LJ. 1989. Notes on
Pappophorum (Gramineae: Pappophoreae). – Syst. Bot. 14: 349-358.
Refulio-Rodriguez NF, Columbus JT, Gillespie
LJ, Peterson PM, Soreng RJ. 2012. Molecular phylogeny of Dissanthelium
(Poaceae: Pooideae) and its taxonomic
implications. – Syst. Bot. 37: 122-133.
Reid CS, Carter R, Urbatsch LE. 2014.
Phylogenetic insights into New World Cyperus (Cyperaceae) using nuclear ITS sequences.
– Brittonia 66: 292-305.
Reihmann D. 1977. Vergleichende Embryologie
und systematische Stellung der Juncaceae mit neuen Beiträgen zur
Samenentwicklung von Juncus und Luzula. – Thesis,
Universität Bochum, Germany.
Reimer E, Cota-Sánchez JH. 2007. An SEM
survey of the leaf epidermis in danthonioid grasses (Poaceae: Danthonioideae). – Syst. Bot.
32: 60-70.
Reinert F, Russo CAM, Salles LO. 2003. The
evolution of CAM in the subfamily Pitcairnioideae (Bromeliaceae). – Bot. J. Linn. Soc.
80: 261-268.
Reinheimer R, Kellogg EA. 2009. Evolution of
AGL6-like MADS box genes in grasses (Poaceae): ovule expression is ancient and
palea expression is new. – Plant Cell 21: 2591-2605.
Reinheimer R, Vegetti AC. 2008. Inflorescence
diversity and evolution in the PCK clade (Poaceae: Panicoideae: Paniceae). – Plant
Syst. Evol. 275: 133-167.
Reinheimer R, Pozner R, Vegetti AC. 2005.
Inflorescence, spikelet, and floral development in Panicum maximum and
Urochloa plantaginea (Poaceae).
– Amer. J. Bot. 92: 565-575.
Reinheimer R, Zuloaga FO, Vegetti AC, Pozner
R. 2010. Changes in floret development patterns that may correlate with sex
determination in the PCK clade (Poaceae). – Intern. J. Plant Sci. 171:
24-33.
Reinheimer R, Vegetti AC, Rua GH. 2013.
Macroevolution of panicoid inflorescences: a history of contingency and order
of trait acquisition. – Ann. Bot. 112: 1613-1628.
Renata R, Zuloaga FO, Vegetti AC, Pozner R.
2009. Diversification of inflorescence development in the PCK clade (Poaceae: Panicoideae: Paniceae). – Amer.
J. Bot. 96:549-564.
Renvoize SA. 1968. Studies in the Gramineae
XVIII. – Kew Bull. 22: 481-488.
Renvoize SA. 1978. Studies in the Gramineae
XLIII. The genus Panicum group Lorea (Gramineae). – Kew
Bull. 32: 419-428.
Renvoize SA. 1979. A new genus in the tribe
Arundineae (Gramineae). – Kew Bull. 33: 525-527.
Renvoize SA. 1979 [1980]. New species of
Panicum (Poaceae) from tropical
Africa. – Kew Bull. 34: 551-555.
Renvoize SA. 1981. The subfamily
Arundinoideae and its position in relation to a general classification of the
Gramineae. – Kew Bull. 36: 85-102.
Renvoize SA. 1982a. A survey of leaf-blade
anatomy in grasses I. Andropogoneae. – Kew Bull. 37: 315-321.
Renvoize SA. 1982b. A new genus and several
new species of grasses from Bahia (Brazil). – Kew Bull. 37: 323-333.
Renvoize SA. 1982c. A survey of leaf-blade
anatomy in grasses II. Arundinelleae. – Kew Bull. 37: 489-495.
Renvoize SA. 1982d. A survey of leaf-blade
anatomy in grasses III. Garnotieae. – Kew Bull. 37: 497-500.
Renvoize SA. 1983. A survey of leaf-blade
anatomy in grasses IV: Eragrostideae. – Kew Bull. 38: 469-478.
Renvoize SA. 1984a. New grasses from Bahia.
– Kew Bull. 39: 179-183.
Renvoize SA. 1984b. New combinations of
grasses from Bahia. – Kew Bull. 39: 184.
Renvoize SA. 1985a. A note on
Jansenella (Gramineae). – Kew Bull. 40: 470.
Renvoize SA. 1985b. A survey of leaf blade
anatomy in grasses V: the bamboo allies. – Kew Bull. 40: 509-535.
Renvoize SA. 1985c. A survey of leaf-blade
anatomy in grasses VI: Stipeae. – Kew Bull. 40: 731-736.
Renvoize SA. 1985d. A survey of leaf-blade
anatomy in grasses VII: Pommereulleae, Orcuttieae & Pappophoreae. – Kew
Bull. 40: 737-744.
Renvoize SA. 1985e. A review of
Tribolium (Gramineae). – Kew Bull. 40: 795-799.
Renvoize SA. 1986a. A survey of leaf-blade
anatomy in grasses VIII: Arundinoideae. – Kew Bull. 41: 323-338.
Renvoize SA. 1986b. A survey of leaf blade
anatomy in grasses IX: Centothecoideae. – Kew Bull. 41: 339-342.
Renvoize SA. 1987a. A survey of leaf-blade
anatomy in grasses X: Bambuseae. – Kew Bull. 42: 201-207.
Renvoize SA. 1987b. A survey of leaf-blade
anatomy in grasses XI: Paniceae. – Kew Bull. 42: 739-768.
Renvoize SA. 1987c. New grasses from Paraná,
Brazil. – Kew Bull. 42: 921-925.
Renvoize SA. 1988. Hatschbachs Paraná
Grasses. – Royal Botanic Gardens, Kew.
Renvoize SA. 1989. New species of
Panicum (Gramineae) from southern tropical Africa. – Kew Bull. 44:
543-546.
Renvoize SA. 1994. Notes on
Sporobolus & Bromus (Gramineae) from the Andes. – Kew
Bull. 49: 543-546.
Renvoize SA. 1995a. Three new species of
Panicum group Lorea (Gramineae) from the Pico das Almas,
Bahia, Brazil. – Kew Bull. 50: 161-164.
Renvoize SA. 1995b. Two new species of
Eriochloa (Gramineae) from South America. – Kew Bull. 50:
343-347.
Renvoize SA. 1998. Gramíneas de Bolivia. –
Royal Botanic Gardens, Kew.
Renvoize SA, Clayton WD. 1985. Two new tribal
names in Gramineae. – Kew Bull. 40: 478.
Renvoize SA, Clayton WD. 1992. Classification
and evolution of grasses. – In: Chapman GP (ed), Grass evolution and
domestication, Cambridge University Press, Cambridge, London, pp. 3-37.
Renvoize SA, Zuloaga FO. 1984. The genus
Panicum group Lorea (Gramineae). – Kew Bull. 39:
185-202.
Renvoize SA, Zuloaga FO. 1995. Three new
species of Panicum group Lorea (Gramineae) from the Pico das
Almas, Bahia, Brazil. – Kew Bull. 50: 161-164.
Renvoize SA, Lock JM, Denny P. 1984. A
remarkable new grass genus from the southern Sudan. – Kew Bull. 39:
455-461.
Renvoize S, Vega AS, Rúgolo de Agrasar ZE.
2006. 214(3). Gramineae (part 3). Subfam. Panicoideae. – In: Harling G,
Persson C (eds), Flora of Ecuador 78, Department of Plant and Environmental
Sciences, Göteborg University, pp. 1-216.
Repka R. 2003. The Carex muricata
aggregate in the Czech Republic: multivariate analysis of quantitative
morphological characters. – Preslia (Praha) 75: 233-248.
Repka R, Danihelka J. 2005. Typification of
the name Carex muricata var. lamprocarpa Wallr. and its
nomenclatural consequences. – Preslia (Praha) 77: 129-136.
Retallack GJ, Dugas DP, Bestland EA. 1990.
Fossil soils and grasses of a middle Miocene East African grassland. –
Science 247: 1325-1328.
Reutemann AG, Guarise NJ, López MG, Vegetti
AC. 2009. Structure of the inflorescences of selected South American species of
Abildaardia Vahl, Bulbostylis Kunth, and
Fimbristylis Vahl (Abildgaardieae-Cyperoideae-Cyperaceae). – Plant Syst. Evol. 283:
93-110.
Reutemann AG, Pilatti V, Guarise N, Vegetti
AC. 2014. Typical cyperoid reproductive structures in Lipocarpha
humboldtiana and Ascolepis brasiliensis (Cypereae-Cyperoideae-Cyperaceae): new evidence from a
developmental perspective. – Flora 200: 15-22.
Rex M, Patzolt K, Schulte K, Zizka G,
Vásquez R, Ibisch PL, Weising K. 2007. AFLP analysis of genetic relationships
in the genus Fosterella L. B. Smith (Pitcarnioideae, Bromeliaceae). – Genome 50:
90-105.
Rex M, Schulte K, Zizka G, Peters J, Vasquez
R, Ibisch PL, Weising K. 2009. Phylogenetic analysis of Fosterella L.
B. Sm. (Pitcairnioideae, Bromeliaceae) based on four
chloroplast DNA regions. – Mol. Phylogen. Evol. 51: 472-485.
Reynders M, Goetghebeur P. 2010.
Reestablishment of Pycreus section Tuberculati (Cyperaceae). – Blumea 55: 226-230.
Reynders M, Huygh W, Larridon I, Muasya AM,
Govaerts R, Simpson DA, Goetghebeur P. 2011. Nomenclature and typification of
names of genera and subdivisions of genera in the Cypereae (Cyperaceae) 3. Names in segregate genera
of Cyperus. – Taxon 60: 885-895.
Reynders M, Vrijdaghs A, Muasya AM, Larridon
I, Goetghebeur P, Smets E. 2012. Evolution of the gynoecium in Cyperoideae (Cyperaceae): congenital fusion of
carpels facilitates pistil modifications. Combining evidence from floral
ontogeny and anatomy. – Plant Ecol. Evol. 145: 96-125.
Reznicek AA. 1982. Two new species of
Carex (Cyperaceae) from
southern Mexico. – Syst. Bot. 7: 340-344.
Reznicek AA. 1986. The taxonomy of
Carex sect. Hymenochlaenae (Cyperaceae) in Mexico and Central
America. – Syst. Bot. 11: 56-87.
Reznicek AA. 1990. Evolution in sedges
(Carex, Cyperaceae). –
Can. J. Bot. 68: 1409-1432.
Reznicek AA. 2001. Sectional names in
Carex (Cyperaceae) for the
Flora of North America. – Novon 11: 454-459.
Reznicek AA, Catling PM. 1986. Vegetative
shoots in the taxonomy of sedges (Carex, Cyperaceae). – Taxon 35: 495-501.
Reznicek AA, Murray DF. 2013. A re-evaluation
of Carex specuicola and the Carex parrayana complex (Cyperaceae). – J. Bot. Res. Inst.
Texas 7: 37-51.
Ricci CV, Patricio MCB, Salatino MLF, Antonio
A, Giulietti AM. 1996. Flavonoids of Syngonanthus Ruhland (Eriocaulaceae): taxonomic
implications. – Biochem. Syst. Ecol. 24: 577-583.
Richards JH, Bruhl JJ, Wilson KW. 2006.
Morphology and development of reproductive structures in Exocarya (Cyperaceae, Mapanioideae,
Chrysitricheae). – Amer. J. Bot. 93: 1081-1090.
Ridley NN. 1894. The Cyperaceae of the West Coast of Africa
in the Welwitsch Herbarium. – Trans. Linn. Soc., Ser. II, Bot. 2: 121-171.
Rikli M. 1895. Beiträge zur vergleichenden
Anatomie der Cyperaceen mit besonderer Berücksichtigung der inneren
Parenchymscheide. – Jahrb. Wiss. Bot. 27: 485-580.
Ripley B, Frole K, Gilbert M. 2010.
Difference in drought sensitivities and photosynthetic limitations between
co-occurring C3 and C4 (NADP-ME) panicoid grasses. –
Ann. Bot. 105: 493-503.
Roalson EH. 2005. Phylogenetic relationships
in the Juncaceae inferred from nuclear
ribosomal DNA internal transcribed spacer sequence data. – Intern. J. Plant
Sci. 166: 397-413.
Roalson EH, Columbus JT. 1999. Glume absence
in the Orcuttieae (Gramineae: Chloridoideae) and a hypothesis of intratribal
relationships. – Aliso 18: 67-70.
Roalson EH, Friar EA. 2000. Infrageneric
classification of Eleocharis (Cyperaceae) revisited: evidence from the
internal transcribed spacer (ITS) region of nuclear ribosomal DNA. – Syst.
Bot. 25: 323-336.
Roalson EH, Friar EA. 2004a. Phylogenetic
relationships and biogeographic patterns in North American members of
Carex section Acrocystis (Cyperaceae) using nrDNA ITS and ETS
sequence data. – Plant Syst. Evol. 243: 175-187.
Roalson EH, Friar EA. 2004b. Phylogenetic
analysis of the nuclear alcohol dehydrogenase (Adh) gene family in
Carex section Acrocystis (Cyperaceae) and combined analyses of
Adh and nuclear ribosomal ITS and ETS sequences for inferring species
relationships. – Mol. Phylogen. Evol. 33: 671-686.
Roalson EH, Columbus JT, Friar EA. 2001.
Phylogenetic relationships in Cariceae (Cyperaceae) based on ITS (nrDNA) and
trnT-L-F (cpDNA) region sequences: assessment of subgeneric and
sectional relationships in Carex with emphasis on section
Acrocystis. – Syst. Bot. 26: 318-341.
Roalson EH, McCubbin AG, Whitkus R. 2007
[2008]. Chromosome evolution in Cyperales. – In: Columbus JT, Friar EA,
Porter JM, Rince LM, Simpson MG (eds), Monocots: comparative biology and
evolution. Poales, Rancho Santa Ana Botanical Garden, Claremont, California.
– Aliso 23: 62-71.
Roalson EH, Hinchcliff CE, Treviswan R, Silva
CRM da. 2010. Phylogenetic relationships in Eleocharis (Cyperaceae): C4
photosynthesis origins and patterns of diversification in the spikerushes. –
Syst. Bot. 35: 257-271.
Robertson A. 1979. History of the
classification of the genus Carex. – Taxon 28: 535-548.
Robertson A. 1984. Carex of
Newfoundland. – Newfoundland Forest Research Centre, St. John.
Robertson IH. 1981. Chromosome numbers in
Brachypodium Beauv. (Gramineae). – Genetica 56: 55-60.
Robinson H. 1969. A monograph on foliar
anatomy of the genera Connellia, Cottendorfia and
Navia (Bromeliaceae). –
Smithsonian Contr. Bot. 2: 1-41.
Roberty G. 1960. Monographie systématique
des Andropogonées du globe. – Boissiera 9: 1-455.
Robinson H, Taylor DC. 1999. The status of
the pitcairnioid genera of the Bromeliaceae. – Harvard Papers Bot.
4: 195-202.
Rodionov AV, Kim ES, Punina EO, Machs EM,
Tyupa NB, Nosov NN. 2007. Evolution of chromosome numbers in the tribes Aveneae
and Poeae inferred from comparative analysis of the internal transcribed
spacers ITS1 and ITS2 of nuclear 45S rRNA genes. – Bot. Žurn. 92: 57-71.
Roig FA. 1964. Las Gramíneas Mendocinas del
género Stipa I. Taxonomía. – Rev. Fac. Ci. Agrar. Univ. Nac. Cuyo
11: 3-110.
Rojas PF. 1997. New species and new
combinations for the tribe Stipeae (Poaceae) in Bolivia. – Gayana, Bot. 54:
163-182.
Romaschenko K, Garcia-Jacas N, Peterson PM,
Soreng RJ, Futorna O, Susanna A. 2008. Molecular phylogenetic analysis of the
American Stipeae (Poaceae) resolves
Jarava sensu lato polyphyletic: evidence for a new genus,
Pappostipa. – J. Bot. Res. Inst. Texas 2: 165-192.
Romaschenko K, Peterson PM, Soreng RJ,
Garcia-Jacas N, Susanna A. 2010. Phylogenetics of Stipeae (Poaceae: Pooideae) based on plastid and
nuclear DNA sequences. – In: Seberg O, Petersen G, Barfod AS, Davis JI (eds),
Diversity, phylogeny, and evolution in the monocotyledons, Aarhus University
Press, Århus, pp. 511-537.
Romaschenko K, Peterson PM, Soreng RJ,
Futorna O, Susanna A. 2011. Phylogenetics of Piptatherum s.l. (Poaceae: Stipeae): evidence for a new
genus, Piptatheropsis, and resurrection of Patis. – Taxon
60: 1703-1716.
Romaschenko K, Peterson PM, Soreng RJ,
Garcia-Jacas N, Futorna O, Susanna A. 2012. Systematics and evolution of the
needle grasses (Poaceae: Pooideae:
Stipeae) based on analysis of multiple chloroplast loci, ITS, and lemma
micromorphology. – Taxon 61: 18-44.
Romaschenko K, Garcia-Jacas N, Peterson PM,
Soreng RJ, Vilatersana R, Susanna A. 2014. Miocene-Pliocene speciation,
introgression, and migration of Patis and Ptilagrostis (Poaceae: Stipeae). – Molec. Phylogen.
Evol. 70: 244-259.
Romero-Zarco C. 1984a. Revisión taxonómica
del género Avenula (Dumort.) Dumort. en la Península Ibérica e
Islas Baleares. – Lagascalia 13: 39-146.
Romero-Zarco C. 1984b. Revisión del género
Helictotrichon Besser ex Schultes & Schultes fil. (Gramineae) en
la Península Ibérica I. Estudio taxonómico. – Anales Jard. Bot. Madrid 41:
98-124.
Romero-Zarco C. 1985a. Revisión del género
Arrhenatherum Beauv. (Gramineae) en la peninsula Ibérica. – Acta
Bot. Malac. 10: 123-154.
Romero-Zarco C. 1985b. Estudio taxonónomico
del género Pseudarrhenatherum Rouy (Gramineae) en la Península
Ibérica. – Lagascalia 13: 255-273.
Romero-Zarco C. 1985c. Revisión del género
Helictotrichon Besser ex Schultes & Schultes fil. (Gramineae) en
la Península Ibérica II. Estudio experimental. – Anales Jard. Bot. Madrid
42: 133-154.
Romero-Zarco C. 1993. Observaciones sobre las
Avenula del grupo marginata en Andalucia. – Acta Bot. Malac. 18:
147-151.
Romero-Zarco C. 1996. Sinópsis del género
Avena L. (Poaceae, Aveneae) en
España peninsular y Baleares. – Lagascalia 18: 171-198.
Romero-Zarco C. 2007. Helictotrichon
devesae, a new endemic grass species from Castilla-La Mancha (Central
Spain). – Anales Jard. Bot. Madrid 64: 205-211.
Romero-Zarco C. 2011. Helictochloa
Romero Zarco (Poaceae), a new genus of
oat grass. – Candollea 66: 87-103.
Rondeau R, Rouch C, Besnard G. 2005.
NADP-malate dehydrogenase gene evolution in Andropogoneae (Poaceae): gene duplication followed by
sub-functionalization. – Ann. Bot., N. S., 96: 1307-1314.
Ronse De Craene L-P, Smets E, Clinckemaillie
D. 2001. Floral ontogenetic evidence in support of the Willdenowia
clade of South African Restionaceae. – J. Plant Res. 114:
329-342.
Ronse De Craene L-P, Linder HP, Smets EF.
2002. Ontogeny and evolution of the flowers of South African Restionaceae with special emphasis on
the gynoecium. – Plant Syst. Evol. 231: 225-258.
Roodt R, Spies JJ, 2003a. Chromosome studies
in the grass subfamily Chloridoideae I. Basic chromosome numbers. – Taxon 52:
557-566.
Roodt R, Spies JJ. 2003b. Chromosome studies
in the grass subfamily Chloridoideae II. An analysis of polyploidy. – Taxon
52: 736-746.
Roodt-Wilding R, Spies JJ. 2006. Phylogenetic
relationships in southern African chloridoid grasses (Poaceae) based on nuclear and chloroplast
sequence data. – Syst. Biodiv. 4: 401-415.
Rosa MM, Scatena VL. 2003. Floral anatomy of
Eriocaulon elichrysoides and Syngonanthus caulescens (Eriocaulaceae). – Flora 198:
188-199.
Rosa MM, Scatena VL. 2007. Floral anatomy of
Paepalanthoideae (Eriocaulaceae,
Poales) and their nectariferous structures. – Ann. Bot. 99: 131-139.
Roscoe MV. 1927. Cytological studies in the
genus Typha. – Bot. Gaz. 84: 392-406.
Rosen DJ, Hatch SL. 2007. A new species of
Eleocharis subgen. Limnochloa (Cyperaceae) from Bolivia. – Brittonia
59: 377-379.
Rosengurtt B, Izaguirre de Artucio P. 1968.
Nuevas especies de Chloris de Uruguay y Paraguay. – Bot. Soc.
Argent. Bot. 12: 117-131.
Rosengurtt B, Arrillaga de Maffei BR,
Izaguirre de Artucio P. 1970. Gramíneas uruguayas. – Universidad de la
República, Montevideo.
Rosengurtt B, Laguardia A, Arrillaga de
Maffei BR. 1972. El character lipido del endosperma central en especies de
Gramineas. – Bol. Fac. Agron. Univ. Montevideo 124: 1-43.
Röser M. 1989. Karyologische, systematische
und chorologische Untersuchungen an der Gattung Helictotrichon Besser
ex Schultes & Schultes im westlichen Mittelmeergebiet. – Diss. Bot. 145:
1-250.
Röser M. 1996. Ecogeography of the grass
genus Helictotrichon (Poaceae:
Aveneae) in the Mediterranean and adjacent regions. – Plant Syst. Evol. 203:
181-281.
Röser M. 1997. Patterns of diversification
in Mediterranean oat grasses (Poaceae:
Aveneae). – Lagascalia 19: 101-120.
Röser M. 1998. Character evolution of the
genus Helictotrichon (Poaceae:
Aveneae) reconsidered in view of recent results in Ibero-Mauritanian and
Eurasian species. – Flora 193: 425-447.
Röser M. 2012. Stipellula, a new
genus, and new combinations in feather grasses (Poaceae tribe Stipeae). – Schlechtendalia
24: 91-93.
Röser M, Winterfeld G, Grebenstein B,
Hemleben V. 2001. Molecular diversity and physical mapping of 5S rDNA in wild
and cultivated oat grasses (Poaceae:
Aveneae). – Mol. Phylogen. Evol. 21: 198-217.
Röser M, Döring E, Winterfeld G, Schneider
J. 2009. Generic realignments in the grass tribe Aveneae (Poaceae). – Schlechtendalia 19: 27-38.
Röser M, Winterfeld G, Grebenstein B,
Hemleben V. 2001. Molecular diversity and physical mapping of 5S rDNA in wild
and cultivated oat grasses (Poaceae:
Aveneae). – Mol. Phylogen. Evol. 21: 198-217.
Roshevits R Yu. 1951. De genere
Piptatherum P. B. notae criticae. – Bot. Mater. Gerb. Bot. Inst.
Komarova Akad. Nauk S.S.S.R. 14: 78-129.
Roth I. 1955. Zur morphologischen Deutung des
Grasembryos und verwandter Embryotypen. – Flora 142: 564-600.
Rothrock PE, Reznicek AA, Hipp AL. 2009.
Taxonomic study of the Carex tenera group (Cyperaceae). – Syst. Bot. 34:
297-311.
Rourke JP. 1974. On restios and roofs. –
Veld Flora 4: 57-59.
Rousseau H, Rousseau-Gueutin M, Dauvergne X,
Boutte J, Simon G, Marnet N, Bouchereau A, Guiheneuf S, Bazureau J-P, Morice J,
Ravanel S, Cabello-Hurtado F, Ainouche A, Salmon A, Wendel JF, Ainouche ML.
2017. Evolution of DMSP (dimethylsulfoniopropionate) biosynthesis pathway:
origin and phylogenetic distribution in polyploid Spartina (Poaceae, Chloridoideae). – Mol. Phylogen.
Evol. 114: 401-414.
Rouy G. 1921. Le Thorea longifolia
deviant le Pseudarrhenatherum longifolium Rouy. – Bull. Soc. Bot.
France 68: 401-402.
Row HC, Reeder JR. 1957. Root-hair
development as evidence of relationships among genera in the Gramineae. –
Amer. J. Bot. 44: 596-601.
Rowlatt U. 1992. Architecture of the leaf of
the greater reed mace, Typha latifolia L. – Bot. J. Linn. Soc. 110:
161-170.
Royen P van. 1953. Xyridaceae. – In: Steenis CGGJ van
(ed), Flora Malesiana I, 4(4), Noordhoff-Kolff N. V., Batavia, pp. 366-376.
Rua GH. 1993. The synflorescence of
Paspalidium rarum (Poaceae) and
the alternative hypothesis about the evolution of some poaceous inflorescences.
– Aust. Syst. Bot. 6: 261-267.
Rua GH. 1996. The inflorescences of
Paspalum L. (Poaceae,
Paniceae): the Quadrifaria group and the evolutionary pathway towards
the fully homogenized, truncated common type. – Plant Syst. Evol. 201:
199-209.
Rua GH, Aliscioni SS. 2002. A
morphology-based cladistic analysis of Paspalum sect.
Pectinata (Poaceae). – Syst.
Bot. 27: 489-501.
Rua GH, Boccaloni KB. 1996. The
inflorescences of Digitaria phaeotrix: morphological and developmental
aspects. – Flora 191: 117-119.
Rua GH, Gróttola MC. 1997. Growth form
models within the genus Paspalum L. (Poaceae, Paniceae). – Flora 192:
65-80.
Rua GH, Speranza PR, Vaio M, Arakaki M. 2010.
A phylogenetic analysis of the genus Paspalum (Poaceae) based on cpDNA and morphology. –
Plant Syst. Evol. 288: 227-243.
Rua GH, Valls JFM. 2012. On the taxonomic
status of the genus Thrasyopsis (Poaceae, Panicoideae, Paspaleae): new
combinations in Paspalum. – Phytotaxa 73: 60-66.
Rua GH, Weberling F. 1995. Growth form and
inflorescence structure of Paspalum L. (Poaceae, Paniceae): a comparative
morphological approach. – Beitr. Biol. Pflanzen 69: 363-431.
Rua GH, Oliveira RC, Valls JFM. 2006.
Ophiochloa bryoides (Poaceae,
Paniceae), a new grass species from Central Brazil. – Syst. Bot. 31:
493-496.
Rua GH, Valls JFM, Graciano-Ribeiro D,
Oliveira RC. 2008. Four new species of Paspalum (Poaceae, Paniceae) from Central Brazil, and
resurrection of an old one. – Syst. Bot. 33: 267-276.
Rua GH, Speranza PR, Vaio M, Arakaki M. 2010.
A phylogenetic analysis of the genus Paspalum (Poaceae) based on cpDNA and morphology. –
Plant Syst. Evol. 288: 227-243.
Rudall PJ. 1990. Development of the ovule and
megagametophyte in Ecdeiocolea monostachya. – Aust. Syst. Bot. 3:
265-274.
Rudall PJ, Linder HP. 1988. Megagametophyte
and nucellus in Restionaceae and Flagellariaceae. – Amer. J. Bot.
75: 1777-1786.
Rudall PJ, Sajo MG. 1999. Systematic position
of Xyris: flower and seed anatomy. – Intern. J. Plant Sci. 160:
795-808.
Rudall PJ, Stuppy W, Cunniff J, Kellogg EA,
Briggs BG. 2005. Evolution of reproductive structures in grasses (Poaceae) inferred by sister-group
comparison with their putative closest living relatives, Ecdeiocoleaceae. – Amer. J. Bot.
92: 1432-1443.
Rudall PJ, Prychid CJ, Gregory T. 2014.
Epidermal patterning and silica phytoliths in grasses: an evolutionary history.
– Bot. Rev. 80: 59-71.
Rúgolo de Agrasar ZE. 1974. Las especies del
género Digitaria (Gramineae) de la Argentina. – Darwiniana 19:
65-166.
Rúgolo de Agrasar ZE. 2006. Las especies del
género Deyeuxia (Poaceae,
Pooideae) de la Argentina y notas nomenclaturales. – Darwiniana 44:
131-293.
Rúgolo de Agrasar ZE, Rodriguez MF. 2002.
Cauline anatomy of native woody bamboos in Argentina and neighbouring areas:
epidermis. – Bot. J. Linn. Soc. 138: 45-55.
Rúgolo de Agrasar ZE, Sulekic AA. 2006. Una
nueva especie de Gymnopogon (Poaceae, Cynodonteae) pará la Argentina.
– Darwiniana 44: 504-507.
Rúgolo de Agrasar ZE, Vega AS. 2004.
Tripogon nicorae, a new species and synopsis of Tripogon (Poaceae: Chloridoideae) in America. –
Syst. Bot. 29: 874-882.
Rúgolo de Agrasar ZE, Vega AS. 2007.
Novedades taxonómicas y synopsis del género Digitaria (Poaceae, Panicoideae, Paniceae) en América
Central. – Darwiniana 45: 92-119.
Ruhland W. 1900. Kritische Revision der
afrikanischen Arten der Gattung Eriocaulon L. – Engl. Bot. Jahrb.
Syst. 27: 65-85.
Ruhland W. 1930. Eriocaulaceae. – In: Engler A (ed),
Die natürlichen Pflanzenfamilien, 2. Augl., Bd. 15a, W. Engelmann, Leipzig,
pp. 39-57.
Ruiz-Sanchez E, Sosa V. 2010. Delimiting
species boundaries within the Neotropical bamboo Otatea (Poaceae: Bambusoideae) using molecular,
morphological and ecological data. – Mol. Phylogen. Evol. 54: 344-356.
Ruiz-Sanchez E, Sosa V. 2015. Origin and
evolution of fleshy fruit in woody bamboos. – Mol. Phylogen. Evol. 91:
123-134.
Ruiz-Sanchez E, Sosa V, Mejía-Saules MT.
2008. Phylogenetics of Otatea inferred from morphology and chloroplast
DNA sequence data, and recircumscription of Guaduinae (Poaceae: Bambusoideae). – Syst. Bot. 33:
277-283.
Ruiz-Sanchez E, Sosa V, Mejía-Saules MT.
2011. Molecular phylogenetics of the Mesoamerican bamboo Olmeca (Poaceae, Bambuseae): implications for
taxonomy. – Taxon 60: 89-98.
Runemark H. 1962. A revision of
Parapholis and Monerma in the Mediterranean. – Bot. Not.
115: 1-17.
Runemark H, Heneen WK. 1968. Elymus
and Agropyron, a problem of generic delimitation. – Bot. Not. 121:
51-79.
Rychlewski J. 1962. Cyto-embryological
studies in the apomictic species Nardus stricta L. – Acta Biol.
Cracov., Ser. Bot., 4: 1-23.
Saarela JM, Ford BA. 2001. Taxonomy of the
Carex backii complex (Section Phyllostachyae, Cyperaceae). – Syst. Bot. 26:
704-721.
Saarela JM, Graham SW. 2010. Inference of
phylogenetic relationships among the subfamilies of grasses (Poaceae: Poales) using meso-scale
exemplar-based sampling of the plastid genome. – Botany 88: 65-84.
Saarela JM, Peterson PM, Soreng RJ, Chapman
RE. 2003. A taxonomic revision of the eastern North American and eastern Asian
disjunct genus Brachyelytrum (Poaceae): evidence from morphology,
phytogeography and AFLPs. – Syst. Bot. 28: 674-692.
Saarela JM, Peterson PM, Keane RM, Cayouette
J, Graham SW. 2007 [2008]. Molecular phylogenetics of Bromus (Poaceae: Pooideae) based on chloroplast and
nuclear DNA sequence data. – In: Columbus JT, Friar EA, Porter JM, Rince LM,
Simpson MG (eds), Monocots: comparative biology and evolution. Poales, Rancho
Santa Ana Botanical Garden, Claremont, California. – Aliso 23: 450-467.
Saarela JM, Liu Q, Peterson PM, Soreng RJ,
Paszko B. 2010. Phylogenetics of the grass ’Aveneae-Type Plastid DNA Clade’
(Poaceae: Pooideae, Poeae) based on
plastid and nuclear ribosomal DNA sequence data. – In: Seberg O, Petersen G,
Barfod AS, Davis JI (eds), Diversity, phylogeny, and evolution in the
monocotyledons, Aarhus University Press, Århus, pp. 557-587.
Saarela JM, Peterson PM, Valdés-Reyna J.
2014. A taxonomic revision of Bromus (Poaceae: Pooideae: Bromeae) in Mexico and
Central America. – Phytotaxa 185: 1-147.
Saarela JM, Wysocki WP, Barrett CF, Soreng
RJ, Davis JI), Clark LG, Kelchner SA, Pires JC, Edger PP, Mayfield DR, Duvall
MR. 2015. Plastid phylogenomics of the cool-season grass subfamily:
clarification of relationships among early-diverging tribes. – AoB PLANTS 7:
plv046.
Saarela JM, Bull RD, Paradis MJ, Ebata SN,
Peterson PM, Soreng RJ, Paszko B. 2017. Molecular phylogenetics of cool-season
grasses in the subtribes Agrostidinae, Anthoxanthinae, Aveninae, Brizinae,
Calothecinae, Koeleriinae and Phalaridinae (Poaceae, Pooideae, Poeae, Poeae chloroplast
group I). – PhytoKeys 87: 1-139.
Saarela JM, Burke SV, Wysocki WP, Barrett MD,
Clark LG, Craine JM, Peterson PM, Soreng RJ, Vorontsova MS, Duvall MR. 2018. A
250 plastome phylogeny of the grass family (Poaceae): topological support under
different data partitions. – PeerJ. 2018; 6: e4299
Sabelli PA, Larkins BA. 2009. The development
of endosperm in grasses. – Plant Physiol. 149: 14-26.
Sack FD. 1994. Structure of the stomatal
complex of the monocot Flagellaria indica. – Amer. J. Bot. 81:
339-344.
Saez L, Rossello JA. 2000. A new species of
Agrostis (Gramineae) in the A. alpina complex. – Bot. J.
Linn. Soc. 133: 359-370.
Sahni M. 1989. Cytological variability in
genus Setaria P. Beauv. from Punjab Plains. – Aspects of Plant
Sciences 11: 467-473.
Sahni M, Bir SS. 1985. SOCGI plant chromosome
number reports – III. – J. Cytol. Genet. 20: 205-206.
Sahuquillo E, Lumaret R. 1995. Variation in
the subtropical group of Dactylis glomerata L. I. Evidence from
allozyme polymorphism. – Biochem. Syst. Ecol. 23: 407-418.
Saint-Yves A. 1922. Les Festuca
(subgen. Eu-Festuca) de l’Afrique du Nord et des Îles Atlantiques.
– Candollea 1: 1-63.
Saint-Yves A. 1925. Contribution à
l’étude des Festuca (subgen. Eu-Festuca) de l’Amérique
du Nord et du Méxique. – Candollea 2: 229-316.
Saint-Yves A. 1928. Contribution à
l’étude des Festuca (subgen. Eu-Festuca) de l’Orient,
Asie et région méditerranéenne voisine. – Candollea 3: 321-466.
Saint-Yves A. 1931. Contribution à
l’étude des Avena sect. Avenastrum (Eurasie et region
méditerranéenne). – Candollea 4: 353-504.
Saint-Yves A. 1934. Contribution à
l’étude des Brachypodium (Europe et Région méditerranéenne). –
Candollea 5: 427-493.
Sajo MG. 1992. Estudos morfoanatômicos em
órgãos foliares de Xyris L. (Xyridaceae). – Bol. Bot. Univ. São
Paulo 13: 67-86.
Sajo MG, Rudall PJ. 1999. Systematic
vegetative anatomy and ensiform leaf development in Xyris (Xyridaceae). – Bot. J. Linn. Soc. 130:
171-182.
Sajo MG, Rudall PJ. 2012. Morphological
evolution in the graminid clade: comparative floral anatomy of the grass
relatives Flagellariaceae and Joinvilleaceae. – Bot. J. Linn.
Soc. 170: 393-404.
Sajo MG, Wanderley MGL. 1998. Estrutura
foliar e a taxonomia de 5 taxons de Xyris L. (Xyridaceae). – Bol. Bot. Univ. São
Paulo 17: 31-38.
Sajo MG, Wanderley MGL, Carvalho LM. 1995.
Caracterização anatômica foliar para 14 espécies de Xyris L. (Xyridaceae) da Serra do Cipó, M.G. –
Acta Bot. Bras. 9: 101-114.
Sajo MG, Prychid CJ, Rudall PJ. 2004.
Structure and development of the ovule in Bromeliaceae. – Kew Bull. 59:
261-267.
Sajo MG, Rudall PJ, Prychid CJ. 2004. Floral
anatomy of Bromeliaceae, with
particular reference to the evolution of epigyny and septal nectaries in
commelinid monocots. – Plant Syst. Evol. 247: 215-231.
Sajo MG, Furness CA, Prychid CJ, Rudall PJ.
2005. Microsporogenesis and anther development in Bromeliaceae. – Grana 44: 65-74.
Sajo MG, Longhi-Wagner HM, Rudall PJ. 2007.
Floral development and embryology in the early-divergent grass Pharus.
– Intern. J. Plant Sci. 168: 181-191.
Sajo MG, Longhi-Wagner HM, Rudall PJ. 2008.
Reproductive morphology of the early-divergent grass Streptochaeta and
its bearing on the homologies of the grass spikelet. – Plant Syst. Evol. 275:
245-255.
Sajo MG, Furness CA, Rudall PJ. 2009.
Microsporogenesis is simultaneous in the early-divergent grass
Streptochaeta, but successive in the closest grass relative,
Ecdeiocolea. – Grana 48: 27-37.
Sajo MG, Pabón-Mora N, Jardim J, Stevenson
DW, Rudall PJ. 2012. Homologies of the flower and inflorescence in the
early-divergent grass Anomochloa (Poaceae). – Amer. J. Bot. 99: 614-628.
Sajo MG, Moreira FF, Oliveira RP, Rudall PJ.
2015. Developmental morphology of a dimorphic grass inflorescence: the
Brazilian bamboo Eremitis (Poaceae). – Intern. J. Plant Sci. 176:
544-553.
Sajo MG, Oriani A, Scatena VL, Rudall PJ.
2017. Floral ontogeny and vasculature in Xyridaceae, with particular reference to
staminodes and stylar appendages. – Plant Syst. Evol. 303: 1293-1310.
Sakamoto S. 1967. Genome analysis of the
genus Eremopyrum. – Wheat Inform. Serv. 23-24: 21-22.
Sakamoto S. 1973. Patterns of phylogenetic
differentiation in the tribe Triticeae. – Rep. Kihara Inst. Biol. 24:
11-31.
Sakamoto S. 1974. Intergeneric hybridization
among three species of Heteranthelium, Eremopyrum and
Hordeum, and its significance for the genetic relationships within the
tribe Triticeae. – New Phytol. 73: 341-350.
Sakamoto S. 1979. Generic relationships among
four species of the genus Eremopyrum in the tribe Triticeae.
Gramineae. – Mem. Coll. Agron. Kyoto Univ. 114: 1-27.
Sakamoto S, Muramatsu M. 1965. Morphological
and cytological studies on various species of Gramineae collected in Pakistan,
Afghanistan, and Iran. – Result of the Kyoto University Scientific expedition
to the Karakorane and Hindukush 1955, 1: 119-140.
Sakamoto S, Muramatsu M. 1966. Cytogenetic
studies in the tribe Triticeae II. Tetraploid and hexaploid hybrids of
Agropyron. – Jap. J. Genet. 41: 155-168.
Salamin N, Hodkinson TR, Savolainen V. 2002.
Building supertrees: an empirical assessment using the grass family (Poaceae). – Syst. Bot. 51: 136-150.
Salariato DL, Giussani LM, Morrone O, Zuloaga
FO. 2009. Rupichloa, a new genus segregated from Urochloa (Poaceae) based on morphological and
molecular data. – Taxon 58: 381-391.
Salariato DL, Zuloaga FO, Giussani LM,
Morrone O. 2010. Molecular phylogeny of the subtribe Melinidinae (Poaceae: Panicoideae: Paniceae) and
evolutionary trends in the homogenization of inflorescences. – Mol. Phylogen.
Evol. 56: 355-369.
Salariato DL, Morrone O, Zuloaga FO. 2012.
Mayariochloa, a new monotypic genus segregated from Scutachne
(Poaceae, Panicoideae, Paniceae). –
Syst. Bot. 37: 105-116.
Salatino A, Faria Salatino M, Santos D dos,
Patricio M. 2000. Distribution and evolution of secondary metabolites in Eriocaulaceae, Lythraceae and Velloziaceae from
“campos rupestres”. – Gen. Mol. Biol. 23: 931-940.
Sales F. 1993. Taxonomy and nomenclature of
Bromus sect. Genea. – Edinburgh J. Bot. 50: 1-31.
Salgado-Labouriau ML, Rinaldi M. 1990.
Palynology of Gramineae of the Venezuelan mountains. – Grana 29: 119-128.
Salgado-Labouriau ML, Nilsson S, Rinaldi M.
1992. Exine sculpture in Pariana pollen (Gramineae). – Grana 32:
243-249.
Salomon B. 1994. Taxonomy and morphology of
the Elymus semicostatus group (Poaceae). – Nord. J. Bot. 14: 7-21.
Salomon B, Lu B-R. 1992. Genomic groups,
morphology, and sectional delimitation in Eurasian Elymus (Poaceae, Triticeae). – Plant Syst. Evol.
180: 1-13.
Salomon B, Bothmer R von, Yang J-L, Lu B-R.
1988. Notes on the perennial Triticeae species in northern Pakistan. – Bot.
Jahrb. Syst. 110: 7-15.
Salse J, Piegu B, Cooke R, Delseny M. 2004.
New in silico insight into the synteny between rice (Oryza sativa L.)
and maize (Zea mays L.) highlights reshuffling and identifies new
duplications in the rice genome. – Plant J. 38: 396-409.
Salse J, Bolot S, Throude M, Jouffe V, Piegu
B, Quraishi UM, CalcagnoT, Cooke R, Delseny M, Feuillet C. 2008. Identification
and characterisation of shared duplications between rice and wheat provide new
insight into grass genome evolution. – Plant Cell 20: 11-24.
Salse J, Abrouk M, Murat F, Quraishi UM,
Feuillet C. 2009. Improved criteria and comparative genomics tool provide new
insights into grass paleogenomics. – Brief Bioinform. 10: 619-630.
Sánchez E. 1971. Anatomía foliar de las
Chlorideae (Gramineae) argentinas. – Kurtziana 6: 103-218.
Sanchez E. 1979. Estructura Kranz en tallos
de Gramineae (Eragrosteae). – Kurtziana 12-13: 113-118.
Sanchez E. 1981a. Desarrollo de la estructura
Kranz en tallos de Gramineae. – Lilloa 35: 37-40.
Sanchez E. 1981b. Variación del a estructura
Kranz en el tallo de Diandrochloa glomerata (Walter) Burkart. –
Lilloa 35: 41-46.
Sanchez E. 1984. Estudios anatómicos en el
género Munroa (Poaceae,
Chloridoideae, Eragrostideae). – Darwiniana 25: 43-57.
Sanchez E, Arriaga M, Ellis R. 1989. Kranz
distinctive cells in the culm of Arundinella (Arundinelleae;
Panicoideae; Poaceae). – Bothalia 19:
45-52.
Sánchez-Ken JG, Davila Aranda P. 1995.
Numeros cromosomicos de algunas especies Mexicanas de Aristida (Poaceae: Aristideae). – Ann. Missouri
Bot. Gard. 82: 593-595.
Sánchez-Ken JG, Clark LG. 2007 [2008].
Phylogenetic relationships within the Centothecoideae + Panicoideae clade (Poaceae) based on ndhF and
rpl16 intron sequences and structural data. – In: Columbus JT, Friar
EA, Porter JM, Prince LM, Simpson MG (eds), Monocots: comparative biology and
evolution. Poales, Rancho Santa Ana Botanical Garden, Claremont, California.
– Aliso 23: 487-502.
Sánchez-Ken JG, Clark LG. 2010. Phylogeny
and a new tribal classification of the Panicoideae s.l. (Poaceae) based on plastid and nuclear
sequence data and structural data. – Amer. J. Bot. 97: 1732-1748.
Sánchez-Ken JG, Clark LG, Kellogg EA, Kay
EE. 2007. Reinstatement and emendation of subfamily Micrairoideae (Poaceae). – Syst. Bot. 32: 71-80.
Sano PT. 2004. Actinocephalus
(Körn.) Sano (Paepalanthus sect. Actinocephalus), a new
genus of Eriocaulaceae, and other
taxonomic and nomenclatural changes involving Paepalanthus Mart. –
Taxon 53: 99-107.
Santos-Gonçalves AP. 2005. Taxonomic and
morpho-anatomical studies in Colanthelia (Poaceae: Bambusoideae: Bambuseae). –
Ph.D. diss., Universidade Estadual de Campinas, Instituto de Biologia,
Brazil.
Santos-Silva F, Venda AKL, Hallbritter HM,
Leme EMC, Mantovani A, Forzza RC. 2017. Nested in chaos: insights on the
relations of the ’Nidularioid Complex’ and the evolutionary history of
Neoregelia (Bromelioideae-Bromeliaceae). – Brittonia 69:
133-147.
Sanyal B. 1972. Cytological studies in Indian
Cyperaceae II. Tribe Cypereae. –
Cytologia 37: 33-42.
Sanyal B, Sharma A. 1972. Cytological studies
in Indian Cyperaceae I. Tribe
Scirpeae. – Cytologia 37: 13-32.
Saraiva DP, Mantovani A, Forzza RC. 2015.
Insights into the evolution of Pitcairnia (Pitcairnioideae-Bromeliaceae), based on morphological
evidence. – Syst. Bot. 40: 726-736.
Sarkar P. 1955. A karyomorphological study on
the genus Eremopyrum. – Wheat Inform. Serv. 2: 17-19.
Sarkar P. 1958. Cytotaxonomic studies on
Eremopyrum. – Can. J. Bot. 36: 539-546.
Sartori JS. 2008. Desenvolvimento floral em
Vriesea carinata Wawra (Tillandsioideae-Bromeliaceae). – Ph.D. diss.,
Federal University of Rio Grande do Sul, Brazil.
Sasan R;, Bidochka MJ. 2012. The
insect-pathogenic fungus Metarhizium roberstii (Clavicipitaceae) is
also an endophyte that stimulates plant root development. – Amer. J. Bot. 99:
101-107.
Saski C, Lee SB, Fjellheim S, Guda C, Jansen
RK, Luo H, Tomkins J, Rognli OA, Daniell H, Clarke JL. 2007. Complete
chloroplast genome sequences of Hordeum vulgare, Sorghum
bicolor and Agrostis stolonifera, and comparative analyses with
other grass genomes. – Theor. Appl. Gen. 115: 571-590.
Sass C, Specht CD. 2010. Phylogenetic
estimation of the core bromelioids with an emphasis on the genus
Aechmea (Bromeliaceae).
– Mol. Phylogen. Evol. 55: 559-571.
Satake Y. 1933. Systematic and anatomical
studies on some Japanese plants II. Juncaceae. – J. Fac. Sci. Univ. Tokyo,
sect. III, 4: 131-224.
Sauer W. 1984. Die Gattung Avenula
(Dumort.) Dumort. auf der Balkanhalbinsel. – Acta Bot. Croat. 43: 315-328.
Sauer W, Chmelitschek H. 1976. Beiträge zur
Kenntnis ausdauernder Wildhafer: die Gattung Avenula (Dumort.) Dumort.
in den Ostalpen. – Mitt. Bot. Staatssamml. München 12: 513-608.
Saura F. 1941. Cariologia de algunas especies
del género Paspalum. – Publ. Inst. Gen. Univ. Buenos Aires 2:
41-48.
Savile DBO. 1990. Relationships of Poaceae, Cyperaceae, and Juncaceae reflected by their fungal
parasites. – Can. J. Bot. 68: 731-734.
Savile DBO, Calder JA. 1953. Phylogeny of
Carex in the light of parasitism by the smut fungi. – Can. J. Bot.
31: 164-174.
Sazima I, Vogel S, Sazima M. 1989. Bat
pollination of Encholirium glaziovii, a terrestrial bromeliad. –
Plant Syst. Evol. 168: 167-179.
Scataglini MA, Giussani LM, Denham SS,
Zuloaga FO, Morrone O. 2007. Una aproximación a la filogenia de
Paspalum (Poaceae, Panicoideae,
Paniceae) utilizando tres marcadores de ADN de cloroplasto. – Darwiniana 45:
124-125.
Scataglini MA, Zuloaga FO. 2013.
Morronea, a new genus segregated from Panicum (Paniceae, Poaceae) based on morphological and
molecular data. – Syst. Bot. 38: 1076-1086.
Scataglini MA, Zuloaga FO, Giussani L, Denham
S, Morrone O. 2014. Phylogeny of New World Paspalum (Poaceae, Panicoideae, Paspaleae) based on
plastid and nuclear markers. – Plant Syst. Evol. 300: 1051-1070.
Scataglini MA, Lizarazu MA, Zuloaga FO. 2014.
A peculiar amphitropical genus of Paniceae (Poaceae, Panicoideae). – Syst. Bot. 39:
1108-1119.
Scatena VL, Bouman F. 2001. Embryology and
seed development of Paepalanthus sect. Actinocephalus
(Koern.) Ruhland (Eriocaulaceae).
– Plant Biol. 3: 341-350.
Scatena VL, Giulietti AM, Borba EL, Berg C
van den. 2005. Anatomy of Brazilian Eriocaulaceae: correlation with
taxonomy and habitat using multivariate analyses. – Plant Syst. Evol. 253:
1-22.
Schaffner JH. 1897. The development of the
stamens and carpels of Typha latifolia. – Bot. Gaz. 24: 93-102.
Schardl CL. 2010. The Epichloeae, symbionts
of the grass subfamily Pooideae. – Ann. Missouri Bot. Gard. 97: 646-665.
Schardl CL, Leuchtmann A, Chung KR, Penny D,
Siegel MR. 1997. Coevolution by common descent of fungal symbionts
(Epichloë spp.) and grass hosts. – Mol. Biol. Evol. 14: 133-143.
Schardl CL, Leuchtmann A, Spiering MJ. 2004.
Symbioses of grasses with seedborne fungal endophytes. – Ann. Rev. Plant
Biol. 55: 315-340.
Schill R, Dannenbaum C, Jentzsch E-M. 1988.
Untersuchungen an Bromeliennarben. – Beitr. Biol. Pflanzen 63: 221-252.
Schippmann U. 1991. Revision der
europäischen Arten der Gattung Brachypodium Palisot de Beauvois (Poaceae). – Boissiera 45: 1-249.
Schmid B. 1983. Notes on the nomenclature and
taxonomy of the Carex flava group in Europe. – Watsonia 14:
309-319.
Schmidt W. 2003. Iron solutions: acquisition
strategies and signalling pathways in plants. – Trends Plant Sci. 8:
188-193.
Schmidt Jabaily R, Sytsma K. 2010.
Phylogenetics of Puya (Bromeliaceae): placement, major
lineages, and evolution of Chilean species. – Amer. J. Bot. 97: 337-356.
Schneider J, Döring E, Hilu KW, Röser M.
2009. Phylogenetic structure of the grass subfamily Pooideae based on
comparison of plastid matK gene-3’trnK exon and nuclear ITS
sequences. – Taxon 58: 405-424.
Schneider J, Winterfeld G, Hoffmann MH.
Röser M. 2011. Duthieeae, a new tribe of grasses (Poaceae) identified among the early
diverging lineages of subfamily Pooideae: molecular phylogenetics,
morphological delineation, cytogenetics and biogeography. – Syst. Biodiv. 9:
27-44.
Schneider J, Winterfeld G, Röser M. 2012.
Polyphyly of the grass tribe Hainardieae (Poaceae: Pooideae): identification of its
different lineages based on molecular phylogenetics, including morphological
and cytogenetic characteristics. – Organ. Div. Ecol. 12: 113-132.
Schneider M. 1932. Untersuchungen über die
Embryobildung und -entwicklung der Cyperaceen. – Beih. Bot. Centralbl. 49:
649-674.
Schneider M del P, Vegetti AC. 1993. The
synflorescence in species of Sorghinae (Andropogoneae-Poaceae). – Beitr. Biol. Pflanzen 67:
225-239.
Schnepf E, Benner U. 1978. Die Morphologie
der Nektarausscheidung bei Bromeliaceen II. Experimentelle und quantitatve
Untersuchungen bei Billbergia nutans. – Biochem. Physiol. Pflanzen
173: 23-36.
Schoenebeck B. 1924. Die Antipodenvermehrung
der Typhaceen. – Ber. Deutsch. Bot. Ges. 42: 296-299.
Scholz H. 1968. Systematik und Verbreitung
einiger Taxa der Gattung Poa, Sektion Ochlopoa, im
Mittelmeergebiet. – Ber. Deutsch. Bot. Ges. 81: 17-21.
Scholz H. 1969. Bemerkungen zu einigen
Stipagrostis-Arten (Gramineae) aus Afrika und Arabien. – Österr.
Bot. Zeitschr. 117: 284-292.
Scholz H. 1971a. Zur Systematik der Gattung
Bromus L. Subgenus Bromus (Gramineae). – Willdenowia 6:
139-159.
Scholz H. 1971b. Zwei neue Gramineen-Arten
aus Libyen und einige nomenklatorische Änderungen. – Willdenowia 6:
291-296.
Scholz H. 1972.Der Stipagrostis
plumosa-Komplex (Gramineae) in Nord-Afrika. – Willdenowia 6: 519-552.
Scholz H. 1981. Der
Bromus-pectinatus-Komplex (Gramineae) im Nahen und Mittleren Osten.
– Bot. Jahrb. Syst. 102: 471-495.
Scholz H. 1982. Über Mikro- und Makrohaare
einiger Piptatherum- und Stipa-Arten (Stipeae, Gramineae).
– Willdenowia 12: 235-240.
Scholz H. 1986. Poa studies 5. The
genus Poa (Gramineae) in Greece: annotated check-list and key to the
species. – Willdenowia 15: 393-400.
Scholz H. 1988. Ene neue
Stipagrostis (Gramineae) aus den Sand-Wüsten Irans und Afghanistan.
– Willdenowia 17: 107-109.
Scholz H. 1989. Floristisches und
taxonomisches über einige Gramineae von Rhodos und Symi (Ost-Ägäis,
Griechenland). – Willdenowia 19: 105-110.
Scholz H. 1990. Neue und wenig bekannte
Gramineen-Taxa. – Willdenowia 19: 405-412.
Scholz H. 1991a. Stipa tunetana,
eine neue Art aus Tunesien, und das St. lagascae-Aggregat (Gramineae).
– Willdenowia 20: 77-80.
Scholz H. 1991b. Notizen zur Gramineenflora
von Kos und Nisyros (Ost-Ägäis, Griechenland). – Willdenowia 21:
131-141.
Scholz H. 1995a. Bromus regnii
(Gramineae), a new endemic serpentine annual brome grass from Cyprus. –
Willdenowia 25: 235-238.
Scholz H. 1995b. Monerma P. Beauv.
(Poaceae) not an illegitimate name. –
Feddes Repert. 106: 169-171.
Scholz H. 1996. Two new Eragrostis
taxa (Gramineae). – Willdenowia 26: 229-232.
Scholz H. 1998. Notes on Bromus
danthoniae and relatives (Gramineae). – Willdenowia 28: 143-150.
Scholz H. 1999. Short notes on
Phleum sect. Achnodon (Gramineae). – Willdenowia 29:
45-49.
Scholz H. 2000a. Old and new taxa of the
genus Catapodium (Gramineae). – Bot. Chron. 13: 95-104.
Scholz H. 2000b. New Aristida and
Stipagrostis taxa (Gramineae). – Willdenowia 30: 273-277.
Scholz H. 2002. Panicum riparium H.
Scholz – eine neue indigene Art der Flora Mitteleuropas. – Feddes Repert.
113: 273-280.
Scholz H. 2006. Kikuyuochloa, genus
novum (Poaceae: Paniceae). – Feddes
Repert. 117: 512-518.
Scholz H. 2007. Notes on Aegilops
(Poaceae). – Willdenowia 37:
431-434.
Scholz H. 2008. Some comments on the genus
Bromus (Poaceae) and three new
species. – Willdenowia 38: 411-422.
Scholz H, Böcker R. 1996. Ergänzungen und
Anmerkungen zur Grasflora (Poaceae) der
Kanaren. – Willdenowia 25: 571-582.
Scholz H, Müller J. 2004.
Stapfochloa (Poaceae:
Cynodonteae, Chloridinae), a new genus from Africa. – Willdenowia 34:
129-133.
Scholz H, Scholz U. 1983. Flore descriptive
des Cypéracées et Graminées du Togo. – J. Cramer, Vaduz.
Scholz H, Stierstorfer C, Gaisberg MV. 2000.
Lolium edwardii sp. nova (Gramineae) and its relationship with
Schedonorus sect. Plantynia Dumort. – Feddes Repert. 111:
561-565.
Schönland S. 1922. South African Cyperaceae. – Mem. Bot. Survey South
Afr. (Pretoria) 3.
Schouten Y, Veldkamp JF. 1985. A revision of
Anthoxanthum including Hierochloë (Gramineae) in Malesia and
Thailand. – Blumea 30: 319-351.
Schulte K, Zizka G. 2008. Multi locus plastid
phylogeny of Bromelioideae (Bromeliaceae) and the taxonomic
utility of petal appendages and pollen characters. – Candollea 63:
209-225.
Schulte K, Horres R, Zizka G. 2005. Molecular
phylogeny of Bromelioideae and its implications on biogeography and the
evolution of CAM in the family. – Senckenbergiana Biol. 85: 113-125.
Schulte K, Barfuss MHJ, Zizka G. 2009.
Phylogeny of Bromelioideae (Bromeliaceae) inferred from nuclear
and plastid DNA loci reveals the evolution of the tank habit within the
subfamily. – Mol. Phylogen. Evol. 51: 327-339.
Schultze-Motel W. 1959a.
Entwicklungsgeschichtliche und vergleichend-morphologische Untersuchungen im
Blütenbereich der Cyperaceae. –
Bot. Jahrb. Syst. 78: 129-170.
Schultze-Motel W. 1959b. Dulichieae, eine
neue Tribus der Cyperaceae-Scirpoideae. – Willdenowia
2: 170-175.
Schulz A. 1887. Zur Morphologie der Cariceae.
– Ber. Deutsch. Bot. Ges. 5: 27-43.
Schulz-Schaeffer J. 1960. Cytological
investigations in the genus Bromus III. The cytotaxonomic significance
of the satellite chromosomes. – J. Heredity 51: 269-277.
Schulz-Schaeffer J, Jurasits P. 1962.
Biosystematic investigations in the genus Agropyron I. Cytological
studies of species karyotypes. – Amer. J. Bot. 49: 940-953.
Schumann K. 1892. Morphologische Studien IV.
Sproßaufbau und Blüthenentwicklung in Scirpus setaceus L. –
Leipzig.
Schürhoff PN. 1920. Die Antipodenvermehrung
der Sparganiaceae. – Ber. Deutsch. Bot. Ges. 38: 346-349.
Schuster J. 1910. Über die Morphologie der
Grasblüte. – Flora (Jena) 100: 213-266.
Schütz N. 2013. Systematics, morphology and
taxonomy of the genus Deuterocohnia L. B. Sm. (Bromeliaceae). – Ph.D. diss.,
Universität von Kassel, Germany.
Schütz N, Krapp F, Wagner N, Weising K.
2016. Phylogenetics of Pitcairnioideae s.s. (Bromeliaceae): evidence from nuclear
and plastid DNA sequence data. – Bot. J. Linn. Soc. 181: 323-342.
Schuyler AE. 1971a. Two new species of
Scirpus (Cyperaceae) in
Southern Africa. – Notul. Natl. Acad. Nat. Sci. Philadelphia 438.
Schuyler AE. 1971b. Scanning electron
microcscopy of achene epidermis in species of Scirpus. – Proc. Acad. Nat.
Sci. Philadelphia 123: 29-52.
Schwab CA. 1972. Floral structure and
embryology of Diarrhena (Gramineae). – Diss. Abstr. Intern. B. The
Sciences and Engineering 32: 3812-3813.
Schwabe H. 1948. Contribución a la anatomía
foliar de algunas Agrostideas. Las especies Argentinas de los generos
Muhlenbergia y Lycurus, sus relaciones con especies
Americanas y las relaciones intragenericas de Muhlenbergia,
Lycurus, Sporobolus y Epicampes. – Lilloa 16:
141-160.
Schwabe H. 1949. Contribucion al studio
anatómico de las especies argentinas del género Sporobolus y sus
relaciones con los géneros afines. – Bol. Soc. Argent. Bot. 2: 253-270.
Schweickerdt H. 1937. A revision of the South
African species of Helictotrichon Bess. ex Schultes. – Bothalia 3:
185-203.
Sclovich SE, Giussani LM, Cialdella AM, Sede
SM. 2015. Phylogenetic analysis of Jarava (Poaceae, Pooideae, Stipeae) and related
genera: testing the value of the awn indumentum in the circumscription of
Jarava. – Plant Syst. Evol. 301: 1625-1641.
Scogin R. 1985. Floral anthocyanins in the
genus Puya. – Biochem. Syst. Ecol. 13: 387-389.
Scrivanti LR, Anton AM, Zygadlo JA. 2009.
Essential oil composition of Bothriochloa Kuntze (Poaceae) from South America and their
chemotaxonomy. – Biochem. Syst. Ecol. 37: 206-213.
Seberg O. 1986. Schoenoides, a new
genus of Cyperaceae from Tasmania.
– Willdenowia 16: 181-186.
Seberg O. 1988. Taxonomy, phylogeny and
biogeography of the genus Oreobolus R. Br. (Cyperaceae), with comments on the
biogeography of the South Pacific continents. – Bot. J. Linn. Soc. 96:
119-195.
Seberg O, Frederiksen S. 2001. A phylogenetic
analysis of the monogenomic Triticeae (Poaceae) based on morphology. – Bot. J.
Linn. Soc. 136: 75-97.
Seberg O, Linde-Laursen I. 1996.
Eremium, a new genus of the Triticeae (Poaceae) from Argentina. – Syst. Bot. 21:
3-15.
Seberg O, Petersen G. 2007 [2008]. Phylogeny
of Triticeae (Poaceae) based on three
organelle genes, two single-copy nuclear genes, and morphology. – In:
Columbus JT, Friar EA, Porter JM, Rince LM, Simpson MG (eds), Monocots:
comparative biology and evolution. Poales, Rancho Santa Ana Botanical Garden,
Claremont, California. – Aliso 23: 362-371.
Seberg O, Frederiksen S, Baden C,
Linde-Laursen I. 1991. Peridictyon, a new genus from the Balkan
Peninsula, and its relationship with Festucopsis (Poaceae). – Willdenowia 21: 87-104.
Sede SM, Morrone O, Giussani LM, Zuloaga FO.
2008. Phylogenetic studies in Paniceae (Poaceae): a realignment of section
Lorea of Panicum. – Syst. Bot. 33: 284-300.
Sede SM, Zuloaga FO, Morrone O. 2009.
Phylogenetic studies in the Paniceae (Poaceae-Panicoideae): Ocellochloa,
a new genus from the New World. – Syst. Bot. 34: 684-692.
Sede SM, Morrone O, Aliscioni SS, Giussani
LM, Zuloaga FO. 2009. Oncorachis and Sclerochlamys, two new
segregated genera from Streptostachys (Poaceae, Panicoideae, Paniceae): a revision
based on molecular, morphological and anatomical characters. – Taxon 58:
365-374.
Sede SM, Escobar A, Morrone O, Zuloaga FO.
2010. Chromosome studies in American Paniceae (Poaceae, Panicoideae). – Ann. Missouri
Bot. Gard. 97: 128-138.
Sendulsky T. 2001. Merostachys
Spreng. (Poaceae, Bambusoideae,
Bambuseae): a new species from Brazil and critical notes on “Group
Speciosa”. – Kew Bull. 56: 627-638.
Sendulsky T, Soderstrom TR. 1984. Revision of
the South American genus Otachyrium (Poaceae: Panicoideae). – Smithsonian
Contr. Bot. 57: 1-24.
Sendulsky T, Filguerias TS, Burman AG. 1987.
Fruits, embryos and seedlings. – In: Soderstrom TR, Hilu KW, Campbell CS,
Barkworth ME (eds), Grass systematics and evolution, Smithsonian Institute
Press, Washington, D.C., pp. 31-36.
Sequeira M, Diaz-Pérez A, Santos-Guerra A,
Catalán P. 2009. Karyological analysis of the five native Macaronesian
Festuca (Gramineae) grasses supports a distinct diploid origin of two
schizoendemic groups. – An. Jard. Bot. Madrid 66: 55-63.
Serebryakova TY. 1969. Branching and
tillering in the Poaceae family. – J.
Bot. Soc. U.S.S.R. 54: 858-871. [In Russian]
Sevenster JG, Veldkamp JF. 1983. A revision
of Helictotrichon (Gramineae) in Malesia. – Blumea 28: 329-342.
Sha L-N, Fan X, Yang R-W, Kang H-Y, Ding C-B,
Zhang L, Zheng Y, Zhou Y-H. 2010. Phylogenetic relationship between
Hystrix and its closely related genera (Triticeae; Poaceae) based on nuclear Acc1, DMC1 and
chloroplast trnL-F sequences. – Mol. Phylogen. Evol. 54: 327-335.
Sha L-N, Fan X, Zhang H-Q, Kang H-Y, Wang Y,
Wang X-L, Zhang L, Ding C-B, Yang R-W, Zhou Y-H. 2014. Phylogenetic
relationships in Leymus (Triticeae; Poaceae): evidence from chloroplast
trnH-psbA and mitochondrial coxII intron sequences.
– J. Syst. Evol. 52: 722-734.
Sha L-N, Fan X, Zhang H-Q, kang H-Y, Wang Y,
Wang X-L, Yu X-F, Zhou Y-H. 2016. Phylogeny and molecular evolution of the
DMC1 gene in the polyploid genus Leymus (Triticeae: Poaceae) and its diploid relatives. – J.
Syst. Evol. 54: 250-263.
Sha L-N, Fan X, Li J, Lao J-Q, Zeng J, Wang
Y, Kang H-Y, Zhang H-Q, Zheng Y-L Zhou Y-H. 2017. Contrasting evolutionary
patterns of multiple loci uncover new aspects in the genome origin and
evolutionary history of Leymus (Triticeae; Poaceae). – Mol. Phylogen. Evol. 114:
175-188.
Shah CK. 1962. Pollen development in some
members of the Cyperaceae. – Plant
embryology, a symposium, 1960, CSIR, New Delhi, pp. 81-93.
Shah CK. 1963. The life history of Juncus
bufonius. – J. Indian Bot. Soc. 42: 238-251.
Shah CK. 1965. Embryogeny in some Cyperaceae. – Phytomorphology 15:
1-9.
Shah CK. 1967. A taxonomic evaluation of the
families Cyperaceae and Juncaceae. – Bull. Indian Natl. Inst.
Sci., Sect. B, 34: 248-256.
Shah CK. 1968. Development of pericarp and
seed coat in the Cyperaceae. –
Naturaliste Canad. 95: 39-48.
Shah CK. 1974. Morphology and embryology of
the family Cyperaceae. – Adv. Plant
Morph. 1972: 102-112.
Shah CK, Neelakandan N. 1971. Embryogeny in
some Cyperaceae II. – Beitr. Biol.
Pflanzen 47: 215-227.
Shahid-Masood M, Nishikawa T, Fukuoka S,
Njenga P, Tsudzuki T, Kadowaki K. 2004. The complete nucleotide sequence of
wild rice (Oryza nivara) chloroplast genome: first genome wide
comparative sequence analysis of wild and cultivated rice. – Gene 340:
133-139.
Shama Kumari K. 1960. Cytogenetic
investigations in Paniceae: occurrence of apospory in a diploid species of
Panicum – P. antidotale Retz. – Curr. Sci. 29: 191.
Shane MW, Dixon KW, Lambers H. 2005. The
occurrence of dauciform roots amongst Western Australian reeds, rushes and
sedges, and the impact of phosphorus supply on dauciform-root development in
Schoenus unispiculatus (Cyperaceae). – New Phytol. 165:
887-898.
Shapter FM, Henry RJ, Lee LS. 2008. Endosperm
and starch grain morphology in wild cereal relatives. – Plant Gen. Res. 6:
85-97.
Sharma A, Bal AK. 1956. A cytological
investigation of some members of Cyperaceae. – Phyton 6: 7-22.
Sharma AK, De DN. 1956. Cytology of some of
the millets. – Caryologia 8: 294-308.
Sharma AK, Ghosh I. 1971. Cytotaxonomy of the
family Bromeliaceae. – Cytologia
36:237-247.
Sharma M. 1965. Pollen morphological studies
of Eriocaulon Linn. from India. – Palyn. Bull. 1: 45-48.
Sharma ML. 1979. Some considerations on the
phylogeny and chromosomal evolution in grasses. – Cytologia 44: 679-685.
Sharp D, Simon BK. 2002. AusGrasses. Grasses
of Australia. CD ROM. – CSIRO Publ., Collingwood, Australia.
Shaw RB, Webster RD. 1983. Characteristics of
the upper anthecia of Ichnanthus (Poaceae: Paniceae). – Bot. Gaz. 144:
363-370.
Shekhovtsov SV, Shekhovtsova IN, Peltek SE.
2012. Phylogeny of Siberian species of Carex sect. Vesicariae
based on nuclear and plastid markers. – Nord. J. Bot. 30: 343-351.
Shen D, Wang S, Chen H, Zhu Q-H, Helliwell C,
Fan L. 2009. Molecular phylogeny of miR390-guided trans-acting siRNA genes
(TAS3) in the grass family. – Plant Syst. Evol. 283: 125-132.
Shetty BV, Subramanyam K. 1964. Cytology of
Flagellaria indica Linn. – Curr. Sci. 33: 279-280.
Shiels DR, Monfils AK. 2012. New combinations
in North American Schoenoplectiella (Cyperaceae). – Novon 22: 87-90.
Shiels DR, Hurlbut DL, Lichtenwald SK,
Monfils AK. 2014. Monophyly and phylogeny of Schoenoplectus and
Schoenoplectiella (Cyperaceae): evidence from chloroplast
and nuclear DNA sequences. – Syst. Bot. 39: 132-144.
Shreekumar PV, Nair VJ. 1991. Flora of
Kerala. Grasses. – Botanical Survey of India, Calcutta.
Shrestha S, Adkins SW, Graham GC, Loch DS.
2003. Phylogeny of the Sporobolus indicus complex, based on internal
transcribed spacer (ITS) sequences. – Aust. Syst. Bot. 16: 165-176.
Siqueiros-Delgado ME, Ainouche M, Columbus
JT, Ainouche A. 2013. Phylogeny of the Bouteloua curtipendula complex
(Poaceae: Chloridoideae) based on
nuclear ribosomal and plastid DNA sequences from diploid taxa. – Syst. Bot.
38: 379-389.
Signorini MA, Foggi B, Nardi E. 2003.
Taxonomic and nomenclatural notes on Festuca (Poaceae): the Festuca vizzavonae
and ”F. cyrnea” problem. – Taxon 52: 591-594.
Silva C, Silva L, Andrade CJ, Trevisan R,
González-Elizondo M, Vanzela A. 2012. Ornamentation of achene silica walls and
its contribution to the systematics of Eleocharis (Cyperaceae). – Plant Syst. Evol. 298:
391-398.
Silva C, Snak C, Schnadelbach AS, Berg C van
den, Oliveira RP. 2015. Phylogenetic relationships of Echinolaena and
Ichnanthus within Panicoideae (Poaceae) reveal two new genera of tropical
grasses. – Mol. Phylogen. Evol. 93: 212-233.
Silva Filho PJS da, Trevisan R, Boldrini II.
2013. Taxonomic novelties in Rhynchospora (Cyperaceae). – Phytotaxa 149: 1-11.
Silvestro D, Zizka G, Schulte K. 2014.
Disentagling the effects of key innovations on diversification of Bromelioideae
(Bromeliaceae). – Evolution 68:
163-175.
Simon BK. 1986. Studies in Australian grasses
2. – Austrobaileya 2: 238-242.
Simon BK. 1990. A key to Australian grasses.
– Queensland Department of Primary Industries, Brisbane.
Simon BK. 1992a. A revision of the genus
Aristida (Poaceae) in
Australia. – Aust. Syst. Bot. 5: 129-226.
Simon BK. 1992b. Studies in Australian
grasses 5. New species of and new combinations for Queensland panicoid grasses.
– Austrobaileya 3: 585-607.
Simon BK. 1992c. Studies in Australian
grasses 6. Alexfloydia, Cliffordiochloa and
Dallwatsonia, three new panicoid grass genera from Eastern Australia.
– Austrobaileya 3: 669-681.
Simon BK. 1999. Steinchisma hians
(Elliot) Nash, the correct name for Fasciculochloa sparshottiorum B.
K. Simon & C. M. Weiller. – Austrobaileya 5: 583-584.
Simon BK. 2003. Steinchisma laxa
(Sw.) Zuloaga, the correct name for Cliffordiochloa parvispiculata B.
K. Simon. – Austrobaileya 6: 561-562.
Simon BK. 2007 [2008]. Grass phylogeny and
classification: conflict of morphology and molecules. – In: Columbus JT,
Friar EA, Porter JM, Prince LM, Simpson MG (eds), Monocots: comparative biology
and evolution. Poales, Rancho Santa Ana Botanical Garden, Claremont,
California. – Aliso 23: 487-502.
Simon BK. 2010. New taxa, nomenclatural
changes and notes on Australian grasses in the tribe Paniceae (Poaceae: Panicoideae). – Austrobaileya 8:
187-219.
Simon BK, Jacobs SWL. 1990. Gondwanan grasses
in the Australian flora. – Austrobaileya 3: 239-260.
Simon BK, Jacobs SWL. 1999. Revision of the
genus Sporobolus (Poaceae,
Chloridoideae) in Australia. – Aust. Syst. Bot. 12: 375-448.
Simon BK, Jacobs SWL. 2003.
Megathyrsus, a new generic name for Panicum subgenus
Megathyrsus. – Austrobaileya 6: 571-574.
Simon BK, Weiller CM. 1995.
Fasciculochloa, a new grass genus (Poaceae: Paniceae) from south-eastern
Queensland. – Austrobaileya 4: 369-379.
Simpson DA. 1987. Notes on Brazilian Cyperaceae II. New species of
Cyperus and Eleocharis from Bahia. – Kew Bull. 42:
897-901.
Simpson DA. 1988. Notes on Brazilian Cyperaceae III. Some problems in
Eleocharis. – Kew Bull. 43: 127-134.
Simpson DA. 1989a. Some interesting
geographical disjunctions in Mapania sect. Mapania (Cyperaceae). – Kew Bull. 44:
139-142.
Simpson DA. 1989b. Notes on Brazilian Cyperaceae IV. Taxonomic changes and new
taxa in Cyperus, Pycreus and Mariscus. – Kew Bull.
44: 279-287.
Simpson DA. 1990. A revision of
Cyperus sect. Leucocephali. – Kew Bull. 45: 485-501.
Simpson DA. 1992. A revision of the genus
Mapania. – The Royal Botanic Gardens, Kew.
Simpson DA. 1993a. Notulae cyperologicae 10.
Genera of Cyperaceae recognised at
Kew. – Cyperaceae Newsl. 12:
8-12.
Simpson DA. 1993b. Notes on Brazilian Cyperaceae VI. New species and a new
combination in Cyperaceae from
Brazil. – Kew Bull. 48: 699-713.
Simpson DA. 1995. Relationships within Cyperales. – In: Rudall PJ, Cribb PJ,
Cutler DF, Humphries CJ (eds), Monocotyledons: systematics and evolution, Royal
Botanic Gardens, Kew, pp. 497-509.
Simpson DA. 1996. New taxa and combinations
in Mapania (Cyperaceae) from
South America. – Kew Bull. 51: 729-740.
Simpson DA. 2008. Frosted curls to tiger
nuts: ethnobotany of Cyperaceae. –
Monogr. Syst. Bot. Missouri Bot. Gard. 108: 1-14.
Simpson DA, Muasya AM. 2004. Three new
species of Cyperus (Cyperaceae) from Eastern and Southern
Africa. – Kew Bull. 59: 593-598.
Simpson DA, Noltie HJ. 1995. The status of
the genus Microschoenus. – Kew Bull. 50: 169-170.
Simpson DA, Furness CA, Hodkinson TR, Muasya
AM, Chase MW. 2003. Phylogenetic relationships in Cyperaceae subfamily Mapanioideae
inferred from pollen and plastid DNA sequence data. – Amer. J. Bot. 90:
1071-1086.
Simpson DA, Muasya AM, Chayamarit K, Parnell
JAN, Suddee S, Wilde BDE, Jones MB, Bruhl JJ, Pooma R. 2005. Khaosokia
caricoides, a new genus an species of Cyperaceae from Thailand. – Bot. J.
Linn. Soc. 149: 357-364.
Simpson DA, Muasya AM, Alves MV, Bruhl JJ,
Dhooge S, Chase MW, Furness CA, Ghamkhar K, Goetghebeur P, Hodkinson TR,
Marchant AD, Reznicek AA, Nieuwborg R, Roalson EH, Smets E, Starr JR, Thomas
WW, Wilson KL, Zhang X. 2007 [2008]. Phylogeny of Cyperaceae based on DNA sequence data
– a new rbcL analysis. – In: Columbus JT, Friar EA, Porter JM,
Prince LM, Simpson MG (eds), Monocots: comparative biology and evolution.
Poales, Rancho Santa Ana Botanical Garden, Claremont, California. – Aliso 23:
72-83.
Simpson MG. 1988. A critique of
‘Bromeliales, related monocots, and resolution of relationships among Bromeliaceae subfamilies’. – Syst.
Bot. 13: 610-614.
Sindhu A, Chintamanani S, Brandt AS, Zanis M,
Scofield SR, Johal GS. 2008. A guardian of grasses: specific origin and
conservation of a unique disease-resistance gene in the grass lineage. –
Proc. Natl. Acad. Sci. U.S.A. 105: 1762-1767.
Singh DN, Goodward MBE. 1960. Cytological
studies in the Gramineae. – Heredity 15: 193-197.
Singh RJ, Röbbelen G. 1975. Comparison of
somatic Giemsa banding pattern in several species of rye. – Zeitschr.
Pflanzenzüchtung 74: 270-285.
Sinha N, Kellogg E. 1996. Parallelism and
diversity in multiple origins of C4 photosynthesis in the grass
family. – Amer. J. Bot. 83: 1458-1470.
Sinha RRP, Bhardwaj AK, Singh RK. 1990. SOCGI
plant chromosome numbers reports IX. – J. Cytol. Gen. 25: 140-143.
Siqueira Filho JA, Leme EMC. 2002. An
addition to the genus Canistrum: a new combination for the old species
from Pernambuco and new species from Alagoas, Brazil. – J. Bromeliad Soc. 52:
105-121.
Siqueiros-Delgado ME. Ainouche M, Columbus
JT, Ainouche A. 2013. Phylogeny of the Bouteloua curtipendula complex
(Poaceae: Chloridoideae) based on
nuclear ribosomal and plastid DNA sequences from diploid taxa. – Syst. Bot.
38: 379-389.
Skendzic EM, Columbus JT, Cerros-Tlatilpa R.
2007 [2008]. Phylogenetics of Andropogoneae (Poaceae: Panicoideae) based on nuclear
ribosomal internal transcribed spacer and chloroplast trnL-F
sequences. – In: Columbus JT, Friar EA, Porter JM, Prince LM, Simpson MG
(eds), Monocots: comparative biology and evolution. Poales, Rancho Santa Ana
Botanical Garden, Claremont, California. – Aliso 23: 530-544.
Skvarla JJ, Larson DA. 1963. Nature of
cohesion within pollen tetrads of Typha latifolia. – Science 140:
173-175.
Skvortsov AK. 1977. Once more on the
morphological nature of the parts of embryo and seedling. – Bull. Moscow Soc.
Natur. 82: 96-111. [In Russian with English summary]
Skvortsov AK. 2003. Prophylls in grasses:
three storeys of homology. – Bull. Moscow Soc. Natur. Biol. Ser. 108:
32-37.
Slageren MW van. 1994. Wild wheats: a
monograph of Aegilops L. and Amblypyrum (Jaub. & Spach)
Eig (Poaceae). – Papers Wageningen
Agric. Univ. 94(7).
Slingsby JA, Verboom GA. 2006. Phylogenetic
relatedness limits co-occurrence at fine spatial scales: evidence from the
schoenoid sedges (Cyperaceae:
Schoeneae) of the Cape Floristic Region, South Africa. – Amer. Nat. 168:
14-27.
Slingsby JA, Britton MN, Verboom GA. 2014.
Ecology limits the diversity of the Cape flora: phylogenetics and
diversification of the genus Tetraria. – Molec. Phylogen. Evol. 72:
61-70.
Small JK. 1901. Juncoides in the
South Eastern States. – Torreya 1: 73-75.
Šmarda P, Bures P, Horova L, Foggi B, Rossi
G. 2008. Genome size and GC content evolution of Festuca: ancestral
expansion and subsequent reduction. – Ann. Bot. 101: 421-433.
Šmarda P, Šmerda J, Knoll A, Bureš P,
Danihelka J. 2007. Revision of Central European taxa of Festuca ser.
Psammophilae Pawlus: morphometrical, karyological and AFLP analysis.
– Plant Syst. Evol. 266: 197-232.
Smirnov PA. 1953. Morphological studies on
grasses. – Bull. Moscow Soc. Natur. Biol. Ser. 58: 71-75. [In Russian]
Smissen RD, Lange PJ de, Thorsen MJ, Ogle CC.
2011. Species delimitation and genetic variation in the rare New Zealand
endemic grass Simplicia. – New Zealand J. Bot. 49: 187-199.
Smith AC. 1978. Flagellaria. –
Allertonia 1: 341-344.
Smith BG, Harris PJ. 1999. The polysaccharide
composition of Poales cell walls: Poaceae cell walls are not unique. –
Biochem. Syst. Ecol. 27: 33-53.
Smith BS, Brown WV. 1973. The Kranz syndrome
in the Gramineae as indicated by carbon isotopic ratios. – Amer. J. Bot. 60:
505-513.
Smith DL. 1966. Development of the
inflorescence in Carex. – Ann. Bot., N. S., 30: 475-486.
Smith DL. 1967. The experimental control of
inflorescence development in Carex. – Ann. Bot., N. S., 31:
19-30.
Smith DL, Faulkner JS. 1976. The
inflorescence of Carex and related genera. – Bot. Rev. 42: 53-81.
Smith JAC. 1989. Epiphytic bromeliads. –
In: Lüttge U (ed), Vascular plants as epiphytes, evolution and ecophysiology,
Springer, Berlin, pp. 108-138.
Smith JAC, Griffiths H, Luttge H. 1986.
Comparative ecophysiology of CAM and C3 bromeliads I. The ecology of
the Bromeliaceae in Trinidad. –
Plant Cell Envir. 9: 359-376.
Smith JP Jr. 1971. Taxonomic revision of the
genus Gymnopogon (Gramineae). – Iowa State J. Sci. 45: 319-385.
Smith LB. 1930-1954. Studies in the Bromeliaceae I-XVII. – Contr.
Gray/Reed Herb.
Smith LB. 1934. Geographical evidence on the
lines of evolution in the Bromeliaceae. – Bot. Jahrb. Syst.
66: 446-468.
Smith LB. 1941. Studies in the Bromeliaceae I. – Contr. Gray Herb.
Harv. Univ. 89: 5-89.
Smith LB. 1955. The Bromeliaceae of Brazil. –
Smithsonian Misc. Coll. 126: 1-290.
Smith LB. 1960. Notes on the Bromeliaceae XIV. – Phytologia 7:
169-172.
Smith LB. 1966. The great bromeliad hoax. –
Bromeliad Soc. Bull. 16: 4-5.
Smith LB. 1967. Notes on Bromeliaceae XXV. – Phytologia 14:
457-465.
Smith LB. 1970. Notes on Bromeliaceae. – Phytologia 19:
281-282.
Smith LB. 1984. Bromeliaceae. – In: Steyermark J,
Maguire B et al. (eds), Nuevos taxa de la Guayana Venezolana, Acta Bot. Venez.
4: 1-45.
Smith LB. 1986. Revision of the Guayana
Highland Bromeliaceae. – Ann.
Missouri Bot. Garden 73: 689-721.
Smith LB. 1988. New key to the genera of the
Bromeliaceae. – Beitr. Biol.
Pflanzen 63: 403-411.
Smith LB, Downs RJ. 1974. Flora Neotropica.
Monograph 14:1. Bromeliaceae 1.
Pitcairnioideae. – New York Botanical Garden, Hafner Press, New York, pp.
1-660.
Smith LB, Downs RJ. 1977. Flora Neotropica.
Monograph 14:2. Bromeliaceae 2.
Tillandsioideae. – New York Botanical Garden, Hafner Press, New York, pp.
661-1492.
Smith LB, Downs RJ. 1979. Flora Neotropica.
Monograph 14:3. Bromeliaceae 3.
Bromelioideae. – New York Botanical Garden, Hafner Press, New York, pp.
1493-2142.
Smith LB, Kress WJ. 1989. New or restored
genera of Bromeliaceae. –
Phytologia 66: 70-79.
Smith LB, Kress WJ. 1990. New genera of Bromeliaceae. – Phytologia 69:
271-274.
Smith LB, Looser G. 1934. Notas sobre las
Bromeliáceas Chilenas. – Rev. Univ. (Santiago) 18: 1075-1081.
Smith LB, Read RW. 1976. Notes on Bromeliaceae XXXVIII. – Phytologia
33: 429-443.
Smith LB, Spencer MA. 1992. Reduction of
Streptocalyx (Bromeliaceae: Bromelioideae). –
Phytologia 72: 96-98.
Smith LB, Spencer MA. 1993.
Racinaea, a new genus of Bromeliaceae (Tillandsioideae). –
Phytologia 74: 151-160.
Smith LB, Till W. 1998. Bromeliaceae. – In: Kubitzki K (ed),
The families and genera of vascular plants IV. Flowering plants.
Monocotyledons. Alismatanae and Commelinanae (except Gramineae), Springer,
Berlin, Heidelberg, New York, pp. 74-99.
Smith PM. 1970. Taxonomy and nomenclature of
the brome-grasses (Bromus L. s.l.). – Notes Roy. Bot. Gard. Edinb.
3: 361-375.
Smith PM. 1972. Serology and species
relationships in annual bromes (Bromus L. sect. Bromus). –
Ann. Bot., N. S., 36: 1-30.
Smith PM. 1985. Observations on Turkish
brome-grasses I. Some new taxa, new combinations and notes on typification. –
Notes Roy. Bot. Gard. Edinb. 42: 491-501.
Smith PM, Sales F. 1993. Bromus L.
sect. Bromus: taxonomy and relationship of some species with small
spikelets. – Edinburgh J. Bot. 50: 149-171.
Smith RW. 1910. The floral development and
embryogeny of Eriocaulon septangulare. – Bot. Gaz. 49: 281-289.
Smith SG. 1987. Typha: its taxonomy
and the ecological significance of hybrids. – Arch. Hydrobiol. 27:
129-138.
Smith SG. 1995. New combinations in North
American Schoenoplectus, Bolboschoenus, Isolepis,
and Trichophorum (Cyperaceae). – Novon 5: 97-102.
Smith SG, Hayasaka E. 2002. New combinations
within North American Schoenoplectus smithii and S.
purshianus and comparison with eastern Asian allies. – Novon 12:
106-111.
Smith SY, Collinson ME, Simpson DA, Rudall
PJ, Marone F, Stampanoni M. 2009. Elucidating the affinities and habitat of
ancient, widespread Cyperaceae:
Volkeria messelensis gen. et sp. nov., a fossil mapanioid sedge from
the Eocene of Europe. – Amer. J. Bot. 96: 1506-1518.
Smith SY, Collinson ME, Rudall PJ, Simpson
DA. 2010. Cretaceous and Paleogene fossil record of Poales: review and current
research. – In: Seberg O, Petersen G, Barfod AS, Davis JI (eds), Diversity,
phylogeny, and evolution in the monocotyledons, Aarhus University Press,
Århus, pp. 333-356.
Smith TW, Waterway MJ. 2008. Evaluating the
taxonomic status of the globally rare Carex roanensis and allied
species using morphology and amplified fragment length polymorphisms. – Syst.
Bot. 33: 525-535.
Smithson E. 1957. Comparative anatomy of the
Flagellariaceae. – Kew Bull.
11(1956): 491-501.
Snell RS. 1936. Anatomy of the spikelets and
flowers of Carex, Kobresia, and Uncinia. – Bull.
Torrey Bot. Club 63: 277-295.
Snogerup S. 1958. Studies in the genus
Juncus. Some cytological observations. – Bot. Not. 111: 249-250.
Snogerup S. 1963. Studies in the genus
Juncus III. Observations on the diversity of chromosome numbers. –
Bot. Not. 116: 142-156.
Snogerup S. 1978. A revision of the
Juncus atratus group. – Bot. Not. 131: 189-196.
Snogerup S. 1993. A revision of
Juncus subgen. Juncus (Juncaceae). – Willdenowia 23: 23-73.
Snow N. 1996. The phylogenetic utility of
lemmatal micromorphology in Leptochloa s.l. and related genera in
subtribe Eleusininae (Poaceae,
Chloridoideae, Eragrostideae). – Ann. Missouri Bot. Gard. 83: 504-529.
Snow N. 1997. Phylogeny and systematics of
Leptochloa P. Beauv. sensu lato (Poaceae, Chloridoideae). – Ph.D. diss. ,
Washington University, St. Louis, Missouri.
Snow N. 1998a. A new species of
Leptochloa (Poaceae,
Chloridoideae) from Sri Lanka. – Novon 8: 183-186.
Snow N. 1998b. Caryopsis morphology of
Leptochloa sensu lato (Poaceae,
Chloridoideae). – Sida 18: 271-282.
Snow N. 2000. A new Leptochloa (Poaceae, Chloridoideae) from Papua New
Guinea and the Torres Strait Islands of Australia. – Novon 10: 238-241.
Snow N, Peterson PM. 2012a. Systematics of
Trigonochloa (Poaceae,
Chloridoideae, Chlorideae). – PhytoKeys 13: 25-38.
Snow N, Peterson PM. 2012b. Nomenclatural
notes on Dinebra, Diplachne, Disakisperma and
Leptochloa (Poaceae:
Chloridoideae). – Phytoneuron 2012-71: 1-2.
Snow N, Peterson PM, Romaschenko K. 2013.
Systematics of Disakisperma (Poaceae, Chloridoideae, Chlorideae). –
PhytoKeys 26: 21-70.
Snow N, Simon BK. 1997. Leptochloa
southwoodii (Poaceae:
Chloridoideae), a new species from south-east Queensland. – Austrobaileya 5:
137-143.
Snow N, Peterson PM, Romaschenko K, Simon BK.
2018. Monograph of Diplachne (Poaceae, Chloridoideae, Cynodonteae). –
PhytoKeys 93: 1-102.
Snyder LA. 1957. Apomixis in Paspalum
secans. – Amer. J. Bot. 44: 318-324.
Soderstrom TR. 1967. Taxonomic study of
subgenus Podosemum and section Epicampes of
Muhlenbergia (Gramineae). – Contr. U.S. Natl. Herb. 34: 75-189.
Soderstrom TR. 1980. A new species of
Lithachne (Poaceae:
Bambusoideae). – Brittonia 32: 495-501.
Soderstrom TR. 1981a. Some evolutionary
trends in the Bambusoideae (Poaceae).
– Ann. Missouri Bot. Gard. 68: 15-47.
Soderstrom TR. 1981b. The grass subfamily
Centothecoideae. – Taxon 30: 614-616.
Soderstrom TR. 1998. Aulonemia
fulgor (Poaceae: Bambusoideae), a
new species from Mexico. – Brittonia 40: 22-31.
Soderstrom TR, Calderón CE. 1971. Insect
pollination in tropical rain forest grasses. – Biotropica 3: 1-16.
Soderstrom TR, Calderón CE. 1974. Primitive
forest grasses and evolution of the Bambusoideae. – Biotropica 6: 141-153.
Soderstrom TR, Calderón CE. 1978a. The
species of Chusquea (Poaceae:
Bambusoideae) with verticillate buds. – Brittonia 30: 154-164.
Soderstrom TR, Calderón CE. 1978b.
Chusquea and Swallenochloa (Poaceae: Bambusoideae): generic
relationships and new species. – Brittonia 30: 297-312.
Soderstrom TR, Calderón CE. 1979. A
commentary on the bamboos (Poaceae:
Bambusoideae). – Biotropica 11: 161-172.
Soderstrom TR, Decker HF. 1964.
Reederochloa, a new genus of dioecious grasses from Mexico. –
Brittonia 16: 334-339.
Soderstrom TR, Decker HF. 1965.
Allolepis: a new segregate of Distichlis (Gramineae). –
Madroño 18: 33-39.
Soderstrom TR, Decker HF. 1973.
Calderonella, a new genus of grasses, and its relationships to the
centostecoid genera. – Ann. Missouri Bot. Gard. 60: 427-441.
Soderstrom TR, Ellis RP. 1987. The position
of the bamboo genera and allies in a system of grass classification. – In:
Soderstrom TR, Hilu KW, Campbell CS, Barkworth ME (eds), Grass systematics and
evolution, Smithsonian Institution Press, Washington, D.C., pp. 225-238.
Soderstrom TR, Ellis RP. 1988. The woody
bamboos (Poaceae: Bambuseae) of Sri
Lanka: a morphological-anatomical study. – Smithsonian Contr. Bot. 72:
1-75.
Soderstrom TR, Judziewicz EJ. 1987.
Systematics of the amphi-atlantic bambusoid genus Streptogyna (Poaceae). – Ann. Missouri Bot. Gard. 74:
871-888.
Soderstrom TR, Londoño X. 1987. Two new
genera of Brazilian bamboos related to Guadua (Poaceae: Bambusoideae: Bambuseae). –
Amer. J. Bot. 74: 27-39.
Soderstrom TR, Londoño X. 1988. A
morphological study of Alvimia (Poaceae: Bambuseae), a new Brazilian bamboo
with fleshy fruit. – Amer. J. Bot. 75: 819-839.
Soderstrom TR, Zuloaga FO. 1989. A revision
of the genus Olyra and the new segregate genus Parodiolyra
(Poaceae: Bambusoideae: Olyreae). –
Smithsonian Contr. Bot. 69: i-iv, 1-79.
Soderstrom TR, Ellis RP, Judziewicz EJ. 1987.
The Phareae and Streptogyneae (Poaceae)
of Sri Lanka: a morphological-anatomical study. – Smithsonian Contr. Bot 65:
1-26.
Soderstrom TR, Hilu KH, Campbell CS,
Barkworth ME (eds). 1987. Grass systematics and evolution. – Smithsonian
Institution Press, Washington, D.C.
Soderstrom TR, Judziewicz EJ, Clark LG. 1988.
Distribution patterns in neotropical bamboos. – In: Vanzolini PE, Heyer WR
(eds), Proceedings of the Neotropical biotic distribution pattern workshop, Rio
de Janeiro, 1987, Academia Brasileira de Ciências, Rio de Janeiro, pp.
120-156.
Søgaard B, Wettstein-Knowles P von. 1987.
Barley: genes and chromosomes. – Carlsberg Res. Commun. 52: 123-196.
Sohns ER. 1955. Cenchrus and
Pennisetum: fascicle morphology. – J. Washington Acad. Sci. 45:
135-143.
Sokoloff DD, Remizova MV, Linder HP, Rudall
PJ. 2009. Morphology and development of the gynoecium in Centrolepidaceae: the most
remarkable range of variation in Poales. – Amer. J. Bot. 96: 1925-1940.
Sokoloff DD, Remizowa MV, Rudall PJ. 2009. A
new species of Centrolepis (Centrolepidaceae: Poales) from
Northern Australia, with remarkable inflorescence architecture. – Bot. Žurn.
94: 92-100.
Sokoloff DD, Remizowa MV, Linder HP,
Macfarlane T, Rudall PJ. 2010. Arrangement of reproductive units in
Centrolepis (Poales: Centrolepidaceae): cincinnus or
spikelet? – In: Seberg O, Petersen G, Barfod AS, Davis JI (eds), Diversity,
phylogeny, and evolution in the monocotyledons, Aarhus University Press,
Århus, pp. 425-436.
Sokoloff DD, Remizowa MV, Barrett MD, Conran
JG, Rudall PJ. 2015. Morphological diversity and evolution of Centrolepidaceae
(Poales), a species-poor clade with diverse body plans and developmental
patterns. – Amer. J. Bot. 102: 1219-1249.
Sokolovskaya AP, Probatova NS. 1979.
Chromosome numbers of some grasses (Poaceae) of the U.S.S.R. flora III. –
Bot. Žurn. 64: 1245-1258.
Soltis DE, Gilmartin AJ, Rieseberg L, Gardner
S. 1987. Genetic variation in the epiphytes Tillandsia ionantha and
T. recurvata (Bromeliaceae). – Amer. J. Bot. 74:
531-537.
Soreng RJ. 1990. Chloroplast-DNA
phylogenetics and biogeography in a reticulating group: study in Poa
(Poaceae). – Amer. J. Bot. 77:
1383-1400.
Soreng RJ. 1991. Systematics of the
‘Epiles’ group of Poa (Poaceae). – Syst. Bot. 16: 507-528.
Soreng RJ. 1998. An infrageneric
classification for Poa in North America, and other notes on sections,
species, and subspecies of Poa, Puccinellia, and
Dissanthelium (Poaceae). –
Novon 8: 187-202.
Soreng RJ (ed). 2003. Catalogue of New World
grasses (Poaceae) IV. Subfamily
Pooideae. – Contr. U.S. Natl. Herb. 48.
Soreng RJ. 2010. Coleataenia Griseb.
(1879): the correct name for Sorengia Zuloaga & Morrone (2010) (Poaceae: Paniceae). – J. Bot. Res. Inst.
Texas 4: 691-692.
Soreng RJ, Davis JI. 1998. Phylogenetics and
character evolution in the grass family (Poaceae): simultaneous analysis of
morphological and chloroplast DNA restriction site character sets. – Bot.
Rev. (Lancaster) 64: 1-85.
Soreng RJ, Davis JI. 2000. Phylogenetic
structure in Poaceae subfamily Pooideae
as inferred from molecular and morphological characters: misclassification
versus reticulation. – In: Jacobs SWL, Everett J (eds), Grasses: systematics
and evolution, Proceedings of the 2nd International Conference on
the Comparative Biology of Monocotyledons, 1998, Sydney, Australia, Vol. 2,
CSIRO Publ., Melbourne, pp. 61-74.
Soreng RJ, Gillespie LJ. 2007.
Nicoraepoa (Poaceae, Poeae), a
new South American genus based on Poa subg. Andinae, and
emendation of Poa sect. Parodiochloa of the Sub-Antarctic
Islands. – Ann. Missouri Bot. Gard. 94: 821-849.
Soreng RJ, Peterson PM. 2012. Revision of
Poa L. (Poaceae, Pooideae,
Poeae, Poinae) in Mexico: new records, re-evaluation of P. ruprechtii,
and two new species, P. palmeri and P. wendtii. – PhytoKeys
15: 1-104.
Soreng RJ, Davis JI, Doyle JJ. 1990. A
phylogenetic analysis of chloroplast DNA restriction site variation in Poaceae subfam. Pooideae. – Plant Syst.
Evol. 172: 83-97.
Soreng RJ, Peterson PM, Davidse G, Judziewicz
EJ, Zuloaga FO, Filgueiras TS, Morrone O. 2003. Catalogue of New World grasses
(Poaceae) IV. Subfamily Pooideae. –
Contr. U.S. Natl. Herb. 48: 1-730.
Soreng RJ, Davidse G, Peterson PM, Zuloaga
FO, Judziewicz EJ, Filgueiras TS, Morrone O. 2003 and onwards. On-line
taxonomic novelties and updates, distributional additions and corrections, and
editorial changes since the four published volumes of the Catalogue of New
World Grasses (Poaceae) published in
Contributions from the United States National Herbarium vols. 39, 41, 46, and
48 [on-line]. Available from www.tropicos.org/Project/CNWG/
Soreng RJ, Davis JI, Voionmaa MA. 2007. A
phylogenetic analysis of Poaceae tribe
Poeae sensu lato based on morphological characters and sequence data from three
plastid-encoded genes: evidence for reticulation, and a new classification of
the tribe. – Kew Bull. 62: 425-454.
Soreng RJ, Gillespie LJ, Jacobs SWL. 2009.
Saxipoa and Sylvipoa – two new genera and a new
classification for Australian Poa (Poaceae: Poinae). – Aust. Syst. Bot. 22:
401-412.
Soreng RJ, Bull RD, Gillespie LJ. 2010.
Phylogeny and reticulation in Poa based on plastid trnTLF and
nrITS sequences with attention to diploids. – In: Seberg O, Petersen G,
Barfod AS, Davis JI (eds), Diversity, phylogeny, and evolution in the
monocotyledons, Aarhus University Press, Århus, pp. 619-643.
Soreng RJ, Gillespie LJ, Boudko K, Bull RD,
Koba H, Smissen R, Lange P de. 2011. CD. The New Zealand genus
Simplicia and a new genus endemic to Japan: two new members of
subtribe Poinae (Poaceae). – Intern.
Bot. Congress: 17-29.
Soreng RJ, Peterson PM, Romaschenko K,
Davidse G, Zuloaga FO, Judziewicz EJ, Filgueiras, Davis JI, Morrone O. 2015. A
worldwide phylogenetic classification of the Poaceae (Gramineae). – J. Syst. Evol. 53:
117-137.
Soreng RJ, Gillespie LJ, Koba H, Boudko E,
Bull RD. 2015. Molecular and morphological evidence for a new grass genus,
Dupontiopsis (Poaceae tribe
Poeae subtribe Poinae s.l.), endemic to alpine Japan, and implications for the
reticulate origin of Dupontia and Arctophila within Poinae
s.l. – J. Syst. Evol. 53: 138-162.
Soreng RJ, Peterson PM, Romaschenko K,
Davidse G, Teisher JK, Clark LG, Barbera P, Gillespie LJ, Zuloaga FO. 2017. A
worldwide phylogenetic classification of the Poaceae (Gramineae) II: an update and a
comparison of two 2015 classifications. – J. Syst. Evol. 55: 259-290.
Sørensen T. 1953. A revision of the
Greenland species of Puccinellia Parl. with contributions to our
knowledge of the Arctic Puccinellia flora in general. – Meddel.
Grønland 136(3): 1-179.
Sørensen T. 1954. New species of
Hierochloë, Calamagrostis and Braya. – Meddel.
Grønland 136(8): 1-24.
Soros CL, Bruhl JJ. 2000. Multiple
evolutionary origins of C4 photosynthesis in the Cyperaceae. – In: Wilson KL, Morrison
DA (eds), Monocots: systematics and evolution, Proceedings of the
2nd International Conference on the Comparative Biology of
Monocotyledons, Sydney, Australia, Vol. 2, CSIRO Publ., Melbourne, pp.
629-636.
Sousa LOF de, Wendt T, Brown GK, Tuthill DE,
Evans TM. 2007. Monophyly and phylogenetic relationships in Lymania
(Bromeliaceae: Bromelioideae) based
on morphology and chloroplast DNA sequences. – Syst. Bot. 32: 264-270.
Spalink D. 2015. Space, time, and form:
patterns of diversification at the family, genus, species, and floristic level
over 90 million years of sedge (Cyperaceae) evolution. – Ph.D. diss.,
University of Wisconsin, Madison, Wisconsin.
Spalton LM. 2001. Brome-grasses with small
lemmas. – Bot. Soc. Brit. Isles News 87: 21-23.
Spangler RE. 1986. Andropogoneae systematics
and generic limits in Sorghum. – In: Soderstrom TR, Hilu KW,
Campbell CS, Barkworth ME (eds), Grass systematics and evolution, Smithsonian
Institution Press, Washington, D.C., pp. 167-170.
Spangler RE. 2003. Taxonomy of
Sarga, Sorghum and Vacoparis (Poaceae: Andropogoneae). – Aust. Syst.
Bot. 16: 279-299.
Spangler RE, Zaitchik B, Russo E, Kellogg EA.
1999. Andropogoneae evolution and generic limits in Sorghum (Poaceae) using ndhF sequences. –
Syst. Bot. 24: 267-281.
Spat C. 2012. Embriologia de Tillandsia
aeranthos (Loisel.) L. B. Sm. (Tillandsioideae-Bromeliaceae). – Ph.D. diss.,
Federal University of Santa Maria, Rio Grande de Sul, Brazil.
Spatafora JW, Sung GH, Sung JM, Hywel-Jones
NL, White JF Jr. 2007. Phylogenetic evidence for an animal pathogen origin of
ergot and the grass endophytes. – Mol. Ecol. 16: 1701-1711.
Spegazzini C. 1901. Stipeae platenses. –
An. Mus. Nac. Montevideo 4: 1-173.
Spegazzini C. 1925. Stipeae platenses. Novae
v. criticae. – Rev. Argent. Bot. 1: 9-51.
Spencer MA, Smith LB. 1992. A revision of the
genus Deuterocohnia Mez (Bromeliaceae, Pitcairnioideae). –
Bradea 4: 141-146.
Spencer MA, Smith LB. 1993.
Racinaea, a new genus of Bromeliaceae (Tillandsioideae). –
Phytologia 74: 151-160.
Speranza PR. 2009. Evolutionary patterns in
the Dilatata group (Paspalum, Poaceae). – Plant Syst. Evol. 282:
43-56.
Spies JJ. 1982. Stomatal area as an
anatomical criterion for the determination of chromosome number in the
Eragrostis curvula complex. – Bothalia 14: 119-122.
Spies JJ, Du Plessis H, Barker NP, Wyk SMC
van. 1990. Cytogenetic studies in the genus Chaetobromus. – Genome
33: 646-658.
Spies JJ, Merwe E van der, Du Plessis H,
Saayman EJL. 1991. Basic chromosome numbers and polyploid levels in some South
African and Australian grasses (Poaceae). – Bothalia 21: 163-170.
Spies JJ, Davidse G, Du Plessis H. 1992.
Cytogenetic studies in the genus Tribolium (Poaceae: Arundineae). – Amer. J. Bot. 79:
689-700.
Spies JJ, Spies SK, Wyk SMC van, Malan AF,
Liebenberg EJL. 1996. Cytogenetic studies of the subfamily Pooideae (Poaceae) in South Africa 1. The tribe
Aveneae, subtribe Aveninae. – Bothalia 26: 53-61.
Spies JJ, Wyk SMC van, Nieman IC, Liebenberg
EJL. 1997. Cytogenetic studies in some representatives of the subfamily
Pooideae (Poaceae) in South Africa 3.
The tribe Poeae. – Bothalia 27: 75-82.
Spriggs EL, Christin P-A, Edwards EJ. 2014.
C4 photosynthesis promoted species diversification during the Miocene grassland
expansion. – PLoS ONE 9: e97722.
Srivastava SK. 2011. The occurrence of the
fossil genus Graminidites in the Maastrichtian Scollard Formation,
Alberta, Canada, and its palaeoecological and palaeogeographical significance.
– Bot. J. Linn. Soc. 167: 235-248.
Stančik D. 2004. Festuca dinirica
and F. guaramacalana (Poaceae,
Loliinae), two new species from the Venezuelan Andes. – Novon 14: 341-344.
Standley LA. 1987. Anatomical and chromosomal
studies of Carex section Phacocystis in eastern North
America. – Bot. Gaz. 148: 507-518.
Stapf O. 1927. Lecomtella
madagascariensis, A. Camus. – Hookers’s Icones plantarum, Tabula
3123.
Stapleton CMA. 1994a. The bamboos of Nepal
and Bhutan II: Arundinaria, Thamnocalamus, Borinda,
and Yushania (Gramineae: Poaceae, Bambusoideae). – Edinb. J. Bot.
51: 275-295.
Stapleton CMA. 1994b. The bamboos of Nepal
and Bhutan III: Drepanostachyum, Himalayacalamus,
Ampelocalamus, Neomicrocalamus and Chimonobambusa
(Gramineae: Poaceae, Bambusoideae). –
Edinburgh J. Bot. 51: 301-330.
Stapleton CMA. 1997. The morphology of woody
bamboos. – In: Chapman GP (ed), The bamboos, Linnean Society of London
Symposium Series, Academic Press, London, pp. 251-267.
Stapleton CMA. 2013. Bergbambos and
Oldeania, new genera of African bamboos (Poaceae, Bambusoideae). – PhytoKeys 25:
87-103.
Stapleton CMA, Xia N-H. 2004.
Qiongzhuea and Dendrocalamopsis (Poaceae-Bambusoideae): publication by
descriptio generico-specifico and typification. – Taxon 53: 526-528.
Stapleton CMA, Chonghaile GN, Hodkinson TR.
2004. Sarocalamus, a new Sino-Himalayan bamboo genus (Poaceae: Bambusoideae). – Novon 14:
345-349.
Starr JR, Ford BA. 2001. The taxonomic and
phylogenetic utility of vegetative anatomy and fruit epidermal silica bodies in
Carex section Phyllostachys (Cyperaceae). – Can. J. Bot. 79:
362-379.
Starr JR, Ford BA. 2009. Phylogeny and
evolution in Cariceae (Cyperaceae):
current knowledge and future directions. – Bot. Rev. 75: 110-137.
Starr JR, Bayer RJ, Ford BA. 1999. The
phylogenetic position of Carex section Phyllostachys and its
implications for phylogeny and subgeneric circumscription in Carex (Cyperaceae). – Amer. J. Bot. 86:
563-577.
Starr JR, Harris SA, Simpson DA. 2003.
Potential of the 5’ and 3’ ends of the intergenic spacer (IGS) of rDNA in
the Cyperaceae: new sequences for
lower level phylogenies in sedges with an example from Uncinia. –
Intern. J. Plant Sci. 164: 213-227.
Starr JR, Harris SA, Simpson DA. 2004.
Phylogeny of the unispicate taxa in Cyperaceae tribe Cariceae I: generic
relationships and evolutionary scenarios. – Syst. Bot. 29: 528-544.
Starr JR, Gravel G, Bruneau A, Muasya AM.
2007 [2008]. Phylogenetic implications of a unique 5.8S nrDNA insertion in Cyperaceae. – In: Columbus JT, Friar
EA, Porter JM, Prince LM, Simpson MG (eds), Monocots: comparative biology and
evolution. Poales, Rancho Santa Ana Botanical Garden, Claremont, California.
– Aliso 23: 84-98.
Starr JR, Harris SA, Simpson DA. 2008.
Phylogeny of the unispicate taxa in Cyperaceae tribe Cariceae II: the limits
of Uncinia. – In: Naczi RFC, Ford BA (eds), Sedges: uses, diversity
and systematics of the Cyperaceae, Monographs in Systematic Botany from the
Missouri Botanical Garden 108, pp. 243-267.
Starr JR, Naczi RFC, Chouinard BN. 2009.
Plant DNA barcodes and species resolution in sedges (Carex, Cyperaceae). – Mol. Ecol. Res.
9(Suppl. 1): 151-163.
Starr JR, Janzen FH, Ford BA. 2015. Three
new, early diverging Carex (Cariceae, Cyperaceae) lineages from East and
Southeast Asia with important evolutionary and biogeographic implications. –
Mol. Phylogen. Evol. 88: 105-120.
Stebbins GL. 1947. The origin of the complex
of Bromus carinatus and its phytogeographic implications. – Contr.
Gray Herb. Harvard Univ. 165: 42-55.
Stebbins GL. 1956. Cytogenetics and evolution
of the grass family. – Amer. J. Bot. 43: 890-905.
Stebbins GL. 1972. The evolution of the grass
family. – In: Younger VB, McKell CM (eds), The biology and utilization of
grasses, Academic Press, New York, pp. 1-17.
Stebbins GL. 1975. The role of polyploid
complexes in the evolution of North American grasslands. – Taxon 24:
91-106.
Stebbins GL. 1981a. Coevolution of grasses
and herbivores. – Ann. Missouri Bot. Gard. 68: 75-86.
Stebbins GL. 1981b. Chromosomes and evolution
in the genus Bromus (Gramineae). – Bot. Jahrb. Syst. 102:
359-379.
Stebbins GL. 1982. Major trends of evolution
in the Poaceae and their possible
significance. – In: Estes JR, Tyrl RJ, Brunken JN (eds), Grasses and
grasslands, University of Oklahoma Press, Norman, Oklahoma, pp. 3-36.
Stebbins GL. 1983. Cytogenetics and phylogeny
in the family Poaceae. – Amer. J. Bot.
70: 96-97.
Stebbins GL. 1987. Grass systematics and
evolution: past, present and future. – In: Soderstrom TR, Hilu KW, Campbell
CS, Barkworth ME (eds), Grass systematics and evolution, Smithsonian
Institution Press, Washington, D.C., pp. 359-367.
Stebbins GL, Crampton B. 1961. A suggested
revision of the grass genera of temperate North America. – In: Recent
advances in botany, from lectures and symposia presented to the IX
International Botanical Congress, Montreal 1959, vol. 1, University of Toronto
Press, Toronto, pp. 133-145.
Stebbins GL, Love RM. 1941. A cytological
study of California forage grasses. – Amer. J. Bot. 28: 371-382.
Stebbins GL, Tobgy HA. 1944. The cytogenetics
of hybrids in Bromus 1. Hybrids within the section
“Ceratochloa”. – Amer. J. Bot. 31: 1-11.
Stebbins GL, Zohary D. 1959. Cytogenetic and
evolutionary studies in the genus Dactylis I. Morphology,
distribution, and interrelationship of the diploid subspecies. – Univ. Calif.
Publ. Bot. 31(1).
Steele PR, Hertweck KL, Mayfield D, McKain
MR, Leebens-Mack J, Pires JC. 2012. Quality and quantity of data recovered from
massively parallel sequencing: examples in Asparagales and Poaceae. – Amer. J. Bot. 99: 330-348.
Stephenson SN. 1972. A putative
Distichlis x Monanthocloë (Poaceae) hybrid from Baja California,
Mexico. – Madroño 21: 125-127.
Stevenson DW. 1983. Systematic implications
of the floral morphology of the Mayacaceae. – Amer. J. Bot. 70(5, pt.
2): 32.
Stevenson DW. 1998. Mayacaceae. – In: Kubitzki K (ed), The
families and genera of vascular plants IV. Flowering plants. Monocotyledons.
Alismatanae and Commelinanae (except Gramineae), Springer, Berlin, Heidelberg,
New York, pp. 294-296.
Stevenson DW, Colella M, Boom B. 1998. Rapateaceae. – In: Kubitzki K (ed),
The families and genera of vascular plants IV. Flowering plants.
Monocotyledons. Alismatanae and Commelinanae (except Gramineae), Springer,
Berlin, Heidelberg, New York, pp. 415-424.
Steyermark JA. 1984. Flora of the Venezuelan
Guyana 1. – Ann. Missouri Bot. Gard. 71: 297-340.
Stieber MT. 1982. Revision of
Ichnanthus sect. Ichnanthus (Gramineae, Panicoideae). –
Syst. Bot. 7: 85-115.
Stieber MT. 1987. Revision of
Ichnanthus sect. Foveolatus (Gramineae, Panicoideae). –
Syst. Bot. 12: 187-216.
St. John H. 1941. Teratological
Typha. – Rhodora 43: 85-91.
St. John H. 1978. Revision of
Joinvillea (Joinvilleaceae). Pacific plant
studies 37. – Phytologia 40: 369-374.
St. John H. 1984. Pacific plant studies 41.
Machaerina and Uncinia (Cyperaceae) on Rapa, Austral Islands.
– Nord. J. Bot. 4: 57-60.
Stock WD, Chuba DK, Verboom GA. 2004.
Distribution of South African C3 and C4 species of Cyperaceae in relation to climate and
phylogeny. – Aust. Ecol. 29: 313-319.
Stoeva M, Popova E. 1997. A taxonomic study
of Carex sect. Phaestoglochin and sect. Stellulatae
(Cyperaceae) in Bulgaria. –
Bocconea 5: 787-796.
Stoll M, Begerow D, Piepenbring M,
Oberwinkler F. 2003. Co-evolution of “smut fungi” (Ustilaginales) and
grasses (Poaceae). – Palm. Hortus
Francofurt. 7: 135.
Stone BC. 1981. Nomenclature of
Joinvillea (Joinvilleaceae). – Gard. Bull.
(Singapore) III, 34: 223-225.
Stover EL. 1934. Development and
differentiation of tissue in the stem tips of grasses. – Ohio J. Sci. 34:
150-159.
Strandhede S-O. 1965a. Chromosome studies in
Eleocharis, subser. Palustres III. Observations on Western
European taxa. – Opera Bot. 9(2): 1-86.
Strandhede S-O. 1965b. Chromosome studies in
Eleocharis, subser. Palustres IV. A possible case of an
extra, reductional division giving rise to hemi-haploid pollen nuclei. – Bot.
Not. 118: 243-253.
Strandhede S-O. 1966. Morphologic variation
and taxonomy in European Eleocharis, subser. Palustres. –
Opera Bot. 10(2): 1-187.
Strandhede S-O. 1967. Eleocharis,
Subser. Eleocharis in North America. Taxonomical comments and
chromosome numbers. – Bot. Not. 120: 355-368.
Strandhede S-O. 1973. Pollen development in
the Eleocharis palustris group (Cyperaceae) II. Cytokinesis and
microspore degeneration. – Bot. Not. 126: 255-265.
Streetman LJ. 1963a. Reproduction of the
lovegrasses, the genus Eragrostis I. E. chloromelas Steud.,
E. curvula (Schrad.) Nees, E. lehmaniana Nees and E.
superba Peyr. – Wrightia 3: 41-51.
Streetman LJ. 1963b. Reproduction of the
lovegrasses, the genus Eragrostis II. E. bicolor Nees, E.
plana Nees, E. intermedia Hitchc. and E. obtusa Munro.
– Wrightia 3: 52-60.
Strehl T, Winkler S. 1982. Vergleichende
Untersuchungen über die Trichome der Bromeliaceen. – Beitr. Biol. Pflanzen
56: 415-438.
Strehl T, Winkler S. 1983. Vergleichende
Studien zum Bau der Bromeliaceenwurzeln. – Beitr. Biol. Pflanzen 58:
219-235.
Strömberg CAE. 2005. Decoupled taxonomic
radiation and ecological expansion of open-habitat grasses in the Cenozoic of
North America. – Proc. Natl. Acad. Sci. U.S.A. 102: 11980-11984.
Strömberg CAE, McInerney FA. 2011. The
Neogene transition from C3 to C4 grasslands in North
America: assemblage analysis of fossil phytoliths. – Paleobiology 37:
50-71.
Strömberg CAE, Werdelin L, Friis EM, Saraç
Gl. 2007. The spread of grass-dominated habitats in the Eastern Mediterranean
during the Cainozoic: phytolith evidence. – Palaeogeogr. Palaeoclim.
Palaeoecol. 250: 18-49.
Strydom A, Spies JJ. 1994. A cytotaxonomic
study of some representatives of the tribe Cynodonteae (Chloridoideae, Poaceae). – Bothalia 24: 92-96.
Stuckert T. 1904. Contribución al
conocimiento de las gramináceas argentinas. – An. Mus. Nac. Hist. Nat.
Buenos Aires, ser. III, 4: 43-161.
Stuckert T. 1906. Segunda contribución al
conocimiento de las gramináceas argentinas. – An. Mus. Nac. Hist. Nat.
Buenos Aires, ser. III, 6: 409-555.
Stuckert T. 1911. Tercera contribución al
concimiento de las gramináceas argentinas. – An. Mus. Nac. Hist. Nat. Buenos
Aires, ser. III, 14: 1-214.
Stützel T. 1984. Blüten- und
infloreszenzmorphologische Untersuchungen zur Systematik der Eriocaulaceen. –
Diss. Bot. 71: 1-108.
Stützel T. 1985a. Die epipetalen Drüsen der
Gattung Eriocaulon (Eriocaulaceae). – Beitr. Biol.
Pflanzen 60: 271-276.
Stützel T. 1985b. Die Bedeutung
monothecat-bisporangiater Antheren als systematisches Merkmal zur Gliederung
der Eriocaulaceae. – Bot. Jahrb.
Syst. 105: 433-438.
Stützel T. 1987. On the morphological and
systematic position of the genus Moldenkeanthus (Eriocaulaceae). – Plant Syst. Evol.
156: 133-141.
Stützel T. 1988. Untersuchungen zur
Wurzelanatomie der Eriocaulaceen. – Flora 180: 223-239.
Stützel T. 1990. “Appendices” am
Gynoeceum der Xyridaceen. – Beitr. Biol. Pflanzen 65: 275-299.
Stützel T. 1998. Eriocaulaceae. – In: Kubitzki K
(ed), The families and genera of vascular plants IV. Flowering plants.
Monocotyledons. Alismatanae and Commelinanae (except Gramineae), Springer,
Berlin, Heidelberg, New York, pp. 197-207.
Stützel T, Gansser N. 1995. Floral
morphology of North American Eriocaulaceae and its taxonomic
implications. – Feddes Repert. 106: 495-502.
Stützel T, Trovó M. 2013. Inflorescences
and taxonomy in Eriocaulaceae. –
Ann. Bot. 112: 1505-1522.
Stützel T, Weberling F. 1982. Untersuchungen
über Verzweigung und Infloreszenzaufbau von Eriocaulaceen. – Flora 172:
105-112.
Su X, Liu Y-P, Wu G-L, Luo W-C, Liu J-Q.
2017. A taxonomic revision of Orinus (Poaceae) with a new species, O.
intermedius, from the Qinghai-Tibet Plateau. – Novon 25: 206-213.
Subils R. 1973. Poliembrionía en especies
Argentinas de Tillandsia (Bromeliaceae). – Kurtziana 7:
266-267.
Subramanyam K, Narayana HS. 1972. Some
aspects of the floral morphology and embryology of Flagellaria indica
L. – In: Murty YS, Johri BM, Mohan Ram HY, Varghese TM (eds), Advances in
plant morphology, Trakashan, Neerut, India, pp. 211-217.
Suessenguth K. 1943. Einige neue Gattungen
und Arten der Cyperaceae aus
Südamerika. – Bot. Jahrb. Syst. 73: 113-125.
Suessenguth K, Beyerle R. 1935. Über die
Xyridaceengattung Abolboda Humb. et Bonpl. – Bot. Jahrb. Syst. 67:
132-142.
Sujatha DM, Manga V, Rao MVS, Murty JSR.
1989. Meiotic studies in some species of Pennisetum (L.) Rich. (Poaceae). – Cytologia 54: 641-652.
Sulekic AA. 2006. Una nueva especie de
Paspalum (Poaceae, Paniceae)
para la Argentina. – Darwiniana 44: 127-130.
Sulekic AA, Zapater MA. 2001. El género
Tragus (Poaceae, Zoisieae) en
la Argentina. – Darwiniana 39: 247-254.
Sulman JD, Drew BT, Drummond C, Hayasaka E,
Sytsma KJ. 2013. Systematics, biogeography, and character evolution of
Sparganium (Typhaceae):
diversification of a widespread, aquatic lineage. – Amer. J. Bot. 100:
2023-2039.
Sun G. 2014. Molecular phylogeny revealed
complex evolutionary process in Elymus species. – J. Syst. Evol. 52:
706-711.
Sun G, Komatsuda T. 2010. Origin of the
Y genome in Elymus and its relationship to other
genomes in Triticeae based on evidence from elongation factor G (EF-G)
gene sequences. – Mol. Phylogen. Evol. 56: 727-733.
Sun Y, Skinner DZ, Liang G-H, Hulbert SH,
1994. Phylogenetic analysis of Sorghum and related taxa using internal
transcribed spacers of nuclear ribosomal DNA. – Theor. Appl. Gen. 89:
26-32.
Sun Y, Xia N, Lin R. 2005. Phylogenetic
analysis of Bambusa (Poaceae:
Bambusoideae) based on internal transcribed spacer sequences of nuclear
ribosomal DNA. – Biochem. Genet. 43: 603-612.
Sun Y, Xia N, Stapleton CMA. 2006.
Relationships between Bambusa species (Poaceae, Bambusoideae) revealed by random
amplified polymorphic DNA. – Biochem. Syst. Ecol. 34: 417-423.
Sundberg MD, Orr AR. 1986. Early
inflorescence and floral development in Zea diploperennis,
diploperennial teosinte. – Amer. J. Bot. 73: 1699-1712.
Sungkaew S. 2008. Taxonomy and systematics of
Dendrocalamus (Bambuseae; Poaceae). – Ph.D. diss., University of
Dublin, Trinity College, Dublin, Ireland.
Sungkaew S, Teerawatananon A, Parnell JAN,
Stapleton CMA, Hodkinson TR. 2008. Phuphanochloa, a new bamboo genus
(Poaceae: Bambusoideae) from Thailand.
– Kew Bull. 63: 669-673.
Sungkaew S, Stapleton CM, Salamin N,
Hodkinson TR. 2009. Non-monophyly of the woody bamboos (Bambuseae; Poaceae): a multi-gene region phylogenetic
analysis of Bambusoideae s.s. – J. Plant Res. 122: 95-108.
Sungkaew S, Stapleton CMA, Hodkinson TR.
2010. Phylogenetics of Dendrocalamus (Poaceae: Bambusoideae, Bambuseae,
Bambusionae) and its related genera based on combined multi-gene analyses. –
In: Seberg O, Petersen G, Barfod AS, Davis JI (eds), Diversity, phylogeny, and
evolution in the monocotyledons, Aarhus University Press, Århus, pp.
497-510.
Sur PR. 2001. A revision of the genus
Ischaemum Linn. (Poaceae) in
India. – J. Econ. Taxon. Bot. 25: 407-438.
Süssenguth K. 1943. Einige neue Gattungen
der Cyperaceae aus Südamerika. –
Bot. Jahrb. Syst. 73: 113-125.
Suzuki S. 1978. Index to Japanese
Bambusaceae. – Gakken Co. Ltd., Tokyo.
Svenson HK. 1929. Monographic studies in the
genus Eleocharis. – Rhodora 31: 121-135.
Svenson HK. 1934. Monographic studies in the
genus Eleocharis. – Rhodora 36: 377-389.
Svenson HK. 1929. Monographic studies in the
genus Eleocharis. – Rhodora 39: 271-272.
Svenson HK. 1939. Monographic studies in the
genus Eleocharis. – Rhodora 41: 13-19; 43-77; 95-104.
Swallen JR. 1931. Five new grasses from
Colombia. – J. Washington Acad. Sci. 21: 14-16.
Swallen JR. 1935. The grass genus
Gouinia. – Amer. J. Bot. 22: 31-41.
Swallen JR. 1965. The grass genus
Luziola. – Ann. Missouri Bot. Gard. 52: 472-475.
Swallen JR. 1966. Notes on grasses. –
Phytologia 14: 65-98.
Swallen JR. 1967. New species of
Paspalum. – Phytologia 14: 358-389.
Swallen JR. 1968. Acostia, a new
genus of grasses from Ecuador. – Bol. Soc. Argent. Bot. 12: 109-110.
Swami UBS. 1963. The chromosome numbers in
some of the grasses of Andra Pradesh, India. – Curr. Sci. 32: 267-268.
Sykes GR, Christensen AH, Peterson PM. 1997.
A chloroplast DNA analysis of Chaboissaea (Poaceae: Eragrostideae). – Syst. Bot. 22:
291-302.
Sylvester SP, Soreng RJ, Peterson PM.
Sylvester MDPV. 2016. An updated checklist and key to the open-panicled species
of Poa L. (Poaceae) in Peru
including three new species, Poa ramoniana, Poa
tayacajaensis, and Poa urubambensis. – PhytoKeys 65: 57-90.
Syme AE. 2012. Diversification rates in the
Australasian endemic grass Austrostipa: 15 million years of constant
evolution. – Plant Syst. Evol. 298: 221-227.
Syme AE, Murphy DJ, Holmes GD, Gardner S,
Fowler R, Cantrill DJ. 2012. An expanded phylogenetic analysis of
Austrostipa (Poaceae: Stipeae)
to test infrageneric relationships. – Aust. Syst. Bot. 25: 1-10.
Szidat L. 1922. Die Samen der Bromeliaceen in
ihrer Anpassung an den Epiphytismus. – Bot. Archiv 1: 29-46.
Takahashi H, Sohma K. 1984. Development of
pollen tetrads in Typha latifolia L. – Pollen Spores 26: 5-18.
Takahashi K, Watano Y, Shimizu T. 1994.
Allozyme evidence for intersectional and intergeneric hybridization in the
genus Sasa and its related genera (Poaceae: Bambusoideae). – J. Phytogeogr.
Taxon. 42: 49-60.
Talavera S. 1978. Aportación al studio
cariologico de las gramíneas españolas. – Lagascalia 7: 133-142.
Tanaka N. 1939a. Chromosome studies in Cyperaceae IV. Chromosome numbers of
Carex species. – Cytologia 10: 51-58.
Tanaka N. 1939b. Chromosome studies in Cyperaceae V. Pollen development of
Carex grallatoria Maxim. var. heteroclita Kückenth. ex
Matsum. – Jap. J. Genet. 15: 153-157.
Tanaka N. 1941. Chromosome studies in Cyperaceae XII. Pollen development in
five genera, with special reference to Rhynchospora. – Bot. Mag.
Tokyo 55: 55-65.
Tanaka N. 1942a. Chromosome studies in Cyperaceae XIX. Chromosome numbers of
Carex (Vignea I). Med. & Biol. 2: 215-219.
Tanaka N. 1942b. Chromosome studies in Cyperaceae XX. Chromosome numbers of
Carex (Vignea II). – Med. & Biol. 2: 220-224.
Tanaka N. 1942c. Chromosome studies in Cyperaceae XV. Chromosome numbers of
Eucarex species (5). – Med. & Biol. 2: 421-424.
Tanaka N. 1942d. Chromosome studies in Cyperaceae XVI. Chromosome numbers of
Eucarex species (6). – Med. & Biol. 2: 425-428.
Tanaka N. 1948. Chromosome studies in Cyperaceae, with special reference to
the problem of aneuploidy. – Biol. Contr. Japan 4: 1-327.
Tang L, Zou X-H, Achoundong G, Potgieter C,
Second G Zhang D-Y, Ge S. 2010. Phylogeny and biogeography of the rice tribe
(Oryzeae): evidence from combined analysis of 20 chloroplast fragments. –
Mol. Phylogen. Evol. 54: 266-277.
Tang L, Zou X-H, Zhang L-B, Ge S. 2015.
Multilocus species tree analyses resolve the ancient radiation of the subtribe
Zizaniinae (Poaceae). – Mol. Phylogen.
Evol. 84: 232-239.
Tanji S. 1925. Chromosome numbers of wild
barleys. – Bot. Mag. (Tokyo) 39: 55-57.
Tatanov IV. 2004. Vnutrirodovaja sistema roda
Bolboschenus (Aschers.) Palla (Cyperaceae). – Novosti Sist. Vysš.
Rast. 36: 80-95.
Tateoka T. 1954a. Karyotaxonomic studies in
Poaceae I. – Ann. Rep. Natl. Inst.
Genet., Misima 4: 45-47.
Tateoka T. 1954b. Karyotaxonomy in Poaceae II. Somatic chromosomes of some
species. – Cytologia 19: 317-328.
Tateoka T. 1955. Karyotaxonomic studies in Poaceae III. – Ann. Rep. Natl. Inst.
Genet., Misima 6: 73-74.
Tateoka T. 1957a. Notes on some grasses III.
5. Affinities of the genus Brylkinia; 6. Systematic position of the
genus Diarrhena. – Bot. mag. (Tokyo) 70: 8-12.
Tateoka T. 1957a. Notes on some grasses IV.
Systematic position of the genus Brachyelytrum. – J. Jap. Bot. 32:
111-114.
Tateoka T. 1957b. Miscellaneous papers on the
phylogeny of Poaceae X. Proposition of a
new phylogenetic system of Poaceae. –
J. Jap. Bot. 32: 275-287.
Tateoka T. 1960. Cytology in grass
systematics: a critical review. – Nucleus 3: 81-110.
Tateoka T. 1962a. Starch grains of endosperm
in grass systematics. – Bot. Mag. (Tokyo) 75: 336-343.
Tateoka T. 1962b. A cytological study of some
Mexican grasses. – Bull. Torrey Bot. Club 89: 77-81.
Tateoka T. 1963. Notes on some grasses XIII.
Relationship between Oryzeae and Ehrharteae, with special reference to leaf
anatomy and histology. – Bot. Gaz. (Chicago) 124: 264-270.
Tateoka T. 1964. Notes on some grasses XVI.
Embryo structure of the genus Oryza in relation to systematics. –
Amer. J. Bot. 51: 539-543.
Tateoka T. 1965a. Chromosome numbers of some
grasses from Madagascar. – Bot. Mag. (Tokyo) 78: 306-311.
Tateoka T. 1965b. Chromosome numbers of some
East African grasses. – Amer. J. Bot. 52: 864-869.
Tateoka T. 1985. Chromosome numbers and their
taxonomic implications in the genus Poa of Japan. – Bot. Mag.
(Tokyo) 98: 413-437.
Tateoka T. 1986. Chromosome numbers in the
tribe Stipeae (Poaceae) in Japan. –
Bull. Natl. Sci. Mus. Tokyo, Ser. B, 12: 151-154.
Tateoka T, Inoue S, Kawano S. 1959. Notes on
some grasses IX. Systematic significance of bicellular microhairs of leaf
epidermis. – Bot. Gaz. (Chicago) 121: 80-91.
Taylor DC, Robinson H, Robinson HE. 1999. A
rejection of Pepinia (Bromeliaceae: Pitcairnioideae) and
taxonomic revisions. – Harv. Pap. Bot. 4(1): 203-218.
Teerawatananon A, Jacobs SWL, Hodkinson TR.
2011. Phylogenetics of Panicoideae (Poaceae) based on chloroplast and nuclear
DNA sequences. – Telopea 13: 115-142.
Teerawatananon A, Boontia V, Chantarasuwan B,
Hodkinson TR, Sungkaew S. 2014. A taxonomic revision of the genus
Dimeria (Poaceae: Panicoideae)
in Thailand. – Phylotaxa 186: 137-147.
Teisher JK. 2016. Systematics and evolution
of the Arundinoideae and Micrairoideae (Poaceae). – Washington University, St.
Louis, Missouri.
Teisher JK, McKain MR, Schaal BA, Kellogg EA.
2017. Polyphyly of Arundinoideae (Poaceae) and evolution of the twisted
geniculate lemma awn. – Ann. Bot. 120: 725-738.
Tejavathi DH. 1987. Seed development in some
members of Cyperaceae. – Beitr.
Biol. Pflanzen 62: 43-55.
Teppner H. 2002. Poaceae in the greenhouses of the Botanic
Garden of the Institute of Botany in Graz (Austria, Europe). – Fritschiana
(Graz) 31: 1-42.
Terauchi T, Ogihara Y, Tsunewaki K. 1987. The
molecular basis of genetic diversity among cytoplasms of Triticum and
Aegilops VI. Complete nucleotide sequences of the rbcL genes
encoding H- and L-type rubisco large subunits in common wheat and Aegilops
crassa 4x. – Jap. J. Genet. 62: 375-388.
Termeh F. 1975. Contribution à l’étude de
quelques graminées nouvelles pour la flore de l’Iran. – Publ. Dep. Bot.
Plant Pest Diseases Res. Inst. 5.
Terrell EE. 1968. A taxonomic revision of the
genus Lolium. – Techn. Bull. U.S. Dept. Agric. 1392.
Terrell EE. 1971. Survey of occurrences of
liquid or soft endosperm in grass genera. – Bull. Torrey Bot. Club 98:
264-268.
Terrell EE, Peterson PM. 1993. Caryopsis
morphology and classification in the Triticeae (Pooideae: Poaceae). – Smithsonian Contr. Bot. 83:
1-25.
Terrell EE, Robinson H. 1974. Luziolinae, a
new subtribe of oryzoid grasses. – Bull. Torrey Bot. Club 101: 235-245.
Terry RG, Brown GK. 1996. A study of
evolutionary relationships in Bromeliaceae based on comparison of
DNA sequences from the chloroplast gene ndhF. – J. Bromeliad Soc.
46: 107-112, 123.
Terry RG, Brown GK, Olmstead RG. 1997a.
Examination of subfamilial phylogeny in Bromeliaceae using comparative
sequencing of the plastid locus ndhF. – Amer. J. Bot. 84:
664-670.
Terry RG, Brown GK, Olmstead RG. 1997b.
Phylogenetic relationships in subfamily Tillandsioideae (Bromeliaceae) using ndhF
sequences. – Syst. Bot. 22: 333-345.
Testoni D, Linder HP. 2017. Synoptic taxonomy
of Cortaderia Stapf (Danthonioideae, Poaceae). – PhytoKeys 76: 39-69.
Thanikaimoni G. 1965. Contribution to the
pollen morphology of Eriocaulaceae. – Pollen Spores 7:
181-191.
Theile HL, Clifford HT, Rogers RW. 1996.
Diversity in the grass pistil and its taxonomic significance. – Aust. Syst.
Bot. 9: 903-912.
The International Brachypodium
Initiative. 2010. Genome sequencing and analysis of the model grass
Brachypodium distachyon. – Nature 463: 763-768.
Thimm U. 1985. Zur Embryologie, Blüten- und
Fruchtanatomie der isolierten Juncales-Gattungen Prionia und
Thurnia. – Diploma Thesis, Universität Bochum, Germany.
Thomas WW. 1984a. The systematics of
Rhynchospora section Dichromena. – Mem. New York Bot. Gard.
37: 1-116.
Thomas WW. 1984b. Insect pollination of
Cymophyllus fraseri (Andrews) Mackenzie. – Castanea 49: 94-95.
Thomas WW, Davidse G. 1989. Koyamaea
neblinensis, a new genus and species of Cyperaceae (Sclerioideae) from Cerro de
la Neblina, Venezuela and Brazil. – Syst. Bot. 14: 189-196.
Thomas WW, Araújo AC, Alves MV. 2009. A
preliminary molecular phylogeny of the Rhynchosporeae (Cyperaceae). – Bot. Rev. 75: 22-29.
Thomasson JR. 1978. Epidermal patterns of the
lemma in some fossil and living grasses and their phylogenetic significance.
– Science 199: 975-977.
Thomasson JR. 1980. Paleoeriocoma
(Gramineae, Stipeae) from the Miocene of Nebraska: taxonomic and phylogenetic
significance. – Syst. Bot. 5: 233-240.
Thomasson JR. 1981. Micromorphology of the
lemma in Stipa robusta and Stipa viridula (Gramineae:
Stipeae): taxonomic significance. – S. W. Natur. 26: 211-214.
Thomasson JR. 1982. Fossil grass anthoecia
and other plant fossils from arthropod burrows in the Miocene of Western
Nebraska. – J. Paleontology 56: 1011-1017.
Thomasson JR. 1985. Miocene fossil grasses:
possible adaptations in reproductive bracts (lemma and palea). – Ann.
Missouri Bot. Gard. 72: 843-851.
Thomasson JR. 1986a. Fossil grasses:
1820-1986 and beyond. – In: Soderstrom TR, Hilu KW, Campbell CS, Barkworth ME
(eds), Grass systematics and evolution, Smithsonian Institution Press,
Washington, D.C., pp. 159-167.
Thomasson JR. 1986b. Lemma epidermal features
in the North American species of Melica and selected species of
Briza, Catabrosa, Glyceria, Neostapfia,
Pleuropogon, and Schizachne (Gramineae). – Syst. Bot. 11:
253-262.
Thomasson JR. 1987. Fossil grasses: 1820-1986
and beyond. – In: Soderstrom TR, Hilu KW, Campbell CS, Barkworth ME (eds),
Grass systematics and evolution, Smithsonian Institution Press, Washington,
D.C., pp. 159-167.
Thomasson JR. 2005. Berriochloa
gabelii and Berriochloa hulettii (Gramineae: Stipeae), two new
grass species from the late Miocene Ash Hollow Formation of Nebrfaska and
Kansas. – J. Paleontol. 79: 185-199.
Thomasson JR, Nelson ME, Zarkzewski RJ. 1986.
A fossil grass (Gramineae: Chloridoideae) from the Miocene with Kranz anatomy.
– Science 233: 876-878.
Thompson BE, Bartling L, Whipple C, Hall DH,
Sakai H, Schmidt R, Hake S. 2009. bearded ear encodes a MADS box
transcription factor critical for maize floral development. – Plant Cell 21:
2578-2590.
Tieghem P van. 1897. Morphologie de
l’embryon et de la plantule chez les Graminées et les Cyperacées. – Ann.
Sci. Nat. Bot. Biol. Vég. 8: 259-309.
Tiemann A. 1985. Untersuchungen zur
Embryologie, Blütenmorphologie und Systematik der Rapateaceen und der
Xyridaceen-Gattung Abolboda (Monocotyledoneae). – Diss. Bot. 82:
1-202.
Tietze M. 1906. Physiologiche
Bromeliaceen-Studien II. Die Entwicklung der Wasseraufnehmenden
Bromeliaceen-Trichome. – Zeitschr. Naturwiss. 78: 1-49.
Till W. 1984. Sippendifferenzierung innerhalb
von Tillandsia subgenus Diaphoranthema in Südamerika mit
besonderer Berücksichtigung des Andenostrandes und der angrenzenden Gebiet.
– Ph.D. diss., Botantisches Institut, Universität Wien, Austria.
Till W. 1992. Systematics and evolution of
the tropical-subtropical Tillandsia subgenus Diaphoranthema
(Bromeliaceae). – Selbyana 13:
88-94.
Tillich H-J. 1996. Seeds and seedlings in Hanguanaceae and Flagellariaceae (monocotyledons).
– Sendtnera 3: 187-197.
Tillich H-J. 2007. Seedling diversity and
homologies of seedling organs in the order Poales (monocotyledons). – Ann.
Bot. 100: 1413-1429.
Tillich H-J, Sill E. 1999. Morphological and
anatomical systematics of Hanguana Blume (Hanguanaceae) and
Flagellaria L. (Flagellariaceae) with a description
of a new species, Hanguana bogneri spec. nov. – Sendtnera 6:
215-238.
Timonen T. 1985. Synflorescence morphology
and anatomy in Kobresia laxa (Cyperaceae). – Ann. Bot. Fenn. 22:
153-171.
Timonen T. 1989. Synflorescence structure of
Schoenoxiphium lanceum (Cyperaceae). – Ann. Bot. Fenn. 26:
319-342.
Timonen T. 1993. Synflorescence structure of
some hetero-, homo- and monostachyae sedges (Carex, Cyperaceae). – Ann. Bot. Fenn. 30:
21-42.
Timonen T. 1998. Inflorescence structure in
the sedge tribe Cariceae (Cyperaceae). – Publ. Bot. Univ.
Helsinki 26: 1-35.
Tinney FW. 1940. Cytology of parthenogenesis
in Poa pratensis. – J. Agric. Res. 60: 351-360.
Toderash LG. 1979. Chromosome numbers of four
species of the genus Carex L. – Izv. Akad. Nauk. Moldavsk. SSR, Ser.
Biol. Khim. (Kishinev) 4: 82-87. [In Russian]
Toivonen H. 1981. Spontaneous Carex
hybrids of Heleonastes and related sections in Fennoscandia. – Acta
Bot. Fenn. 116: 1-51.
Tomlinson PB. 1969. Commelinales-Zingiberales.
– In: Metcalfe CR (ed), Anatomy of the monocotyledons 3, Oxford.
Tomlinson PB. 1970. Dichotomous branching in
Flagellaria indica (monocotyledons). – Bot. J. Linn. Soc. 63
[Suppl.] 1: 1-14.
Tomlinson PB, Posluszny U. 1977. Features of
dichotomizing apices in Flagellaria indica (Monocotyledones). –
Amer. J. Bot. 64: 1057-1065.
Tomlinson PB, Smith AC. 1970. Joinvilleaceae, a new family of
monocotyledons. – Taxon 19: 887-889.
Toolin L, Reeder JR. 2000. The status of
Setaria macrostachya and its relationship to S. vulpiseta
(Gramineae). – Syst. Bot. 25: 26-32.
Toon A, Crisp MD, Gamage H, Mant JG, Morris
DC, Schmidt S, Cook LG. 2015. Key innovation or adaptive change? A test of leaf
traits using Triodiinae in Australia. – Sci. Rep. 5: 12398.
Torrecilla P, Catalán P. 2002. Phylogeny of
broad-leaved and fine-leaved Festuca lineages (Poaceae) based on nuclear ITS sequences.
– Syst. Bot. 27: 241-251.
Torrecilla P, López-Rodríguez JA, Stancik
D, Catalán P. 2003. Systematics of Festuca sects. Eskia
Willk., Pseudatropis Kriv., Amphigenes (Janka) Tzvel.,
Pseudoscariosa Kriv. and Scariosae Hack. based on analysis of
morphological characters and DNA sequences. – Plant Syst. Evol. 239:
113-139.
Torres AM. 1993. Revisión del género
Stipa (Poaceae) en la provincia
de Buenos Aires. – Monogr. Comis. Invest. Ci. [ Buenos Aires], Comisión de
Investigaciones Científicas, La Plata, 12: 3-62.
Torres AM. 1997a. Nassella
(Gramineae) del noroeste de la Argentina. – Monogr. Comis. Invest. Ci.
[Buenos Aires], Comisión de Investigaciones Científicas, La Plata, 13:
5-45.
Torres AM. 1997b. Stipa (Gramineae)
del noroeste de la Argentina. – Monogr. Comis. Invest. Ci. [Buenos Aires],
Comisión de Investigaciones Científicas, La Plata, 13: 46-67.
Torres AM. 1997c. Nicoraella
(Gramineae) un nuevo género para America del Sur. – Monogr. Comis. Invest.
Ci. [Buenos Aires], Comisión de Investigaciones Científicas, La Plata, 13:
69-77.
Torres Gonzalez AM, Morton CM. 2005.
Molecular and morphological phylogenetic analysis of Brachiaria and
Urochloa (Poaceae). – Mol.
Phylogen. Evol. 37: 36-44.
Tournay R. 1961. La nomenclature des sections
du genre Bromus L. (Gramineae). – Bull. Jard. Bot. État 31:
289-299.
Tovar O. 1993. Las gramíneas (Poaceae) del Perú. – Ruizia 13:
9-480.
Trabut ML. 1889. Révision des caractères
des Stipa gigantea Lag., Lagascae R. et Sch.,
Letourneuxii sp. nov., Fontanesii Parlat.; Cleistogamie chez
les Stipa. – Bull. Soc. Bot. France 36: 404-407.
Traiperm P, Boonkerd T, Chantaranothai P,
Simpson DA. 2012. Notes on the genus Ischaemum (Poaceae). – Kew Bull. 67: 75-79.
Tran VT, Nguyen HN, Xia N-H. 2013.
Annamocalamus H. N. Nguyen, N.-H. Xia & V. T. Tran, a new genus of
bamboo (Poaceae) from Vietnam. –
Candollea 68: 159-165.
Trethewey JAK, Campbell LM, Harris PJ. 2005.
(1->3),(1->4)-ß-D-glucans in the cell walls of the Poales (sensu lato):
an immunogold labelling study using a monoclonal antibody. – Amer. J. Bot.
92: 1660-1674.
Trigas P, Constantinidis T, Touloumenidou T.
2009. A new hexaploid species of Centaurea section Acrolophus
(Asteraceae)
from Evvia Island, Greece. – Bot. J. Linn. Soc. 158: 762-774.
Triplett JK. 2008. Phylogenetic relationships
among the temperate bamboos (Poaceae:
Bambusoideae) with an emphasis on Arundinaria and allies. – Iowa
State University, Amers, Iowa.
Triplett JK, Clark LG. 2010. Phylogeny of the
temperate bamboos (Poaceae:
Bambusoideae: Bambuseae) with an emphasis on Arundinaria and allies.
– Syst. Bot. 35: 102-120.
Triplett JK, Oltrogge KA, Clark LG. 2010.
Phylogenetic relationships and natural hybridization among the North American
woody bamboos (Poaceae: Bambusoideae:
Arundinaria). – Amer. J. Bot. 97: 471-492.
Triplett JK, Wang Y, Zhong J, Kellogg EA.
2012. Five nuclear loci resolve the polyploid history of switchgrass
(Panicum virgatum L.) and relatives. – PLoS ONE 7: e38702.
Triplett JK, Clark LG, Fisher AE, Wen J.
2014. Independent allopolyploidiztion events preceded speciation in the
temperate and tropical woody bamboos. – New Phytol. 204: 66-73.
Trofimovskaja AJ, Kobylianskij VD. 1964.
Classification of wild barleys for breeding purposes. – Bull. Appl. Bot.
Genet. Plant Breeding 36: 53-88.
Trovó M, Sano PT. 2010. Taxonomic survey of
Paepalanthus section Diphyomene (Eriocaulaceae). – Phytotaxa 14:
49-55.
Trovó M, Stützel T. 2013. On the
morphological position of Paepalanthus subgenus Psilandra (Eriocaulaceae). – Plant Syst. Evol.
299: 115-121.
Trovó M, Andrade MJG, Sano PT, Ribeiro PL,
Berg C. 2013. Molecular phylogenetics and biogeography of Neotropical
Paepalanthoideae with emphasis on Brazilian Paepalanthus (Eriocaulaceae). – Bot. J. Linn.
Soc. 171: 225-243.
Tucker GC. 1982. A new species of
Cyperus (Cyperaceae) from
Costa Rica and Mexico. – Syst. Bot. 7: 345-347.
Tucker GC. 1983. The taxonomy of
Cyperus (Cyperaceae) in
Costa Rica and Panama. – Syst. Bot. Monogr. 2.
Tucker GC, Miller NG. 1990. Achene
microstructure in Eriophorum (Cyperaceae): taxonomic implications and
paleobotanical applications. – Bull. Torrey Bot. Club 117: 266-283.
Tulloch AP. 1973. Composition of leaf surface
waxes of Triticum species: variation with age and tissue. –
Phytochemistry 12: 2225-2232.
Tulloch AP. 1981. Composition of epicuticular
waxes from 28 genera of Gramineae: differences between subfamilies. – Can. J.
Bot. 59: 1213-1221.
Tulloch AP, Baum BR, Hoffman LL. 1980. A
survey of epicuticular waxes among genera of Triticeae 2. Chemistry. – Can.
J. Bot. 58: 2602-2615.
Türpe AM. 1984. Revision of the South
American species of Schizachyrium (Gramineae). – Kew Bull. 39:
169-178.
Tutin G. 1936. A revision of the genus
Pariana (Gramineae). – Bot. J. Linn. Soc. 50: 337-362.
Tyrrell CD, Santos-Gonçalves AP, Londoño X,
Clark LG. 2012. Molecular phylogeny of the arthrostylidioid bamboos (Poaceae: Bambusoideae: Bambuseae:
Arthrostylidiinae) and new genus Didymogonyx. – Mol. Phylogen. Evol.
65: 136-148.
Tyrrell CD, Londoño X, Prieto RO, Attigala
L, McDonald K, Clark LG. 2018. Molecular phylogeny and cryptic morphology
reveal a new genus of West Indian woody bamboo (Poaceae: Bambusoideae: Bambuseae) hidden by
convergent character evolution. – Taxon 67: 916-930.
Tzvelev NN. 1964. De genere
Colpodium Trin. – Nov. Sist. Vysh. Rast. 1964: 5-19. [In Russian]
Tzvelev NN. 1970. De generibus
Trisetum Pers. et Koeleria Pers. in URSS notulae
systematicae. – Nov. Sist. Vyssh. Rast. 7: 59-73. [In Russian]
Tzvelev NN. 1973a. Conspectus specierum
tribus Triticeae Dum. familiae Poaceae
in Flora URSS. – Nov. Syst. Plant. Vasc., 10: 19-59. [In Russian]
Tzvelev NN. 1973b. Notae de graminis florae
URSS 7. – Nov. Syst. Plant Vasc. 10: 79-98. [In Russian]
Tzvelev NN. 1974. Notulae de tribu Stipeae
Dum. (Fam. Poaceae) in URSS. – Novosti
Sist. Vysš. Rast. 11: 4-21. [In Russian]
Tzvelev NN. 1976. Grasses of the Soviet
Union. – Nauka Publ., Leningrad. [In Russian]
Tzvelev NN. 1977. On the origin and evolution
of feathergrasses (Stipa L.). – In: Lebedev DV, Karamysheva ZV
(eds), Problemy ekologii, geobotaniki, botanicheskoi geografii i floristiki,
Academiya Nauk SSSR, Leningrad, pp. 139-150. [In Russian]
Tzvelev NN. 1983. Grasses of the Soviet
Union. Transl. from Russian by B. R.Sharma, Smithsonian Institution. –
Amerind Publ. Co., New Delhi.
Tzvelev NN. 1989. The system of grasses (Poaceae) and their evolution. – Bot. Rev.
55: 141-203.
Tzvelev NN. 2005. O proishozhdenii i
evolyutzii kovyley (Stipa L.) [On the origin and evolution of
feathergrasses (Stipa L.)]. – In: Geltman DV (ed), Problemy
teoreticheskiy morfologii i evolyutzii vysšikh rasteniy, Tovarischestvo
nauchnykh izdaniy KMK, Moskwa & St.-Petersburg, pp. 332-349.
Tzvelev NN, Bolchovskieh V. 1965. On the
genus Zingeria P. Smirn. and related genera in the family Gramineae
– a karyosystematic study. – Bot. Žurn. 50: 1317-1320. [In Russian]
Überfeld M. 1925. Beiträge zur Kenntniss
des sexuellen Dimorphismus der Restionaceae. – Engl. Bot. Jahrb.
Syst. 60: 176-206.
Uchikawa L. 1935. Karyological studies in
Japanese bamboo II. Further studies on chromosome numbers. – Jap. J. Gen. 11:
308-313.
Ueno O, Koyama T. 1987. Distribution and
evolution of C4 syndrome in Rhynchospora (Rhynchosporeae-Cyperaceae). – Bot. Mag. (Tokyo) 100:
63-85.
Ueno O, Samejima M. 1989. Structural features
of NAD-malic enzyme type C4 Eleocharis: an additional
report of C4 acid decarboxylation types of the Cyperaceae. – Bot. Mag. (Tokyo) 102:
393-402.
Ueno O, Takeda T, Maeda E. 1988. Leaf
ultrastructure of C4 species possessing different Kranz anatomical
types in the Cyperaceae. – Bot.
Mag. (Tokyo) 101: 141-152.
Ueno O, Samejima M, Koyama T. 1989.
Distribution and evolution of C4 syndrome in Eleocharis, a
sedge group inhabiting wet and aquatic environments, based on culm anatomy and
carbon isotope ratios. – Ann. Bot., N. S., 64: 425-438.
Ueno O, Kawano Y, Wakayama M, Takeda T. 2006.
Leaf vascular systems in C3 and C4 grasses: a two-dimensional analysis. –
Ann. Bot. 97: 611-621.
Uitten H. 1935. Principia, genus
novum Cyperacearum Africanum. – Recl. Trav. Bot. Néerland. 32: 282-285.
Unwin MM. 2004. Molecular systematics of the
Eriocaulaceae Martinov. – Ph.D.
diss., Miami University, Oxford, Ohio.
Uphof JCT. 1924. The physiological anatomy of
Mayaca fluviatilis. – Ann. Bot. 38: 389-393.
Utley JF. 1978. A revision of the Costa Rican
thecophylloid Vrieseas (Bromeliaceae). – Ph.D. diss., Duke
University, Durham, North Carolina.
Utley JF. 1983. A revision of the Middle
American thecophylloid Vrieseas (Bromeliaceae). – Tulane Stud. Zool.
Bot. 24: 1-81.
Utley JF, Luther HE. 1991. Studies in middle
American Bromeliaceae II. – Ann.
Missouri Bot. Gard. 78: 270.
Valdés B. 1973. Revisión de lase species
anuales del género Anthoxanthum (Gramineae). – Lagascalia 3:
99-141.
Valdés B, Scholz H. 2006. The Euro+Med
treatment of Gramineae – a generic synopsis and some new names. –
Willdenowia 36: 657-669.
Valdés-Reyna J, Hatch SL. 1991. Lemma
micromorphology in the Eragrostideae (Poaceae). – Sida 14: 531-549.
Valdés-Reyna J, Hatch SL. 1997. A revision
of Erioneuron and Dasyochloa (Poaceae: Eragrostideae). – Sida 17:
645-666.
Valdés-Reyna J, Morden CW, Hatch SL. 1986.
Gouldochloa, a new genus of centothecoid grasses from Tamaulipas,
Mexico. – Syst. Bot. 11: 112-119.
Vallenback P, Jaarola M, Ghatnekar L,
Bengtsson BO. 2008. Origin and timing of the horizontal transfer of a
PgiC gene from Poa to Festuca ovina. – Mol.
Phylogen. Evol. 46: 890-896.
Valls JFM. 1978. A biosystematics study of
Leptochloa with special emphasis on Leptochloa dubia
(Gramineae: Chloridoideae). – PhD. diss., Texas A & M University, College
Station, Texas.
Vanky K. 1995. Taxonomical studies on
Ustilaginales 12. – Mycotaxon 54: 215-238.
Vanky K. 1996. New Ustilaginales on Cyperaceae from Australia. – Mycotaxon
58: 167-183.
Vanzela ALL, Luceno M, Guerra M. 2000.
Karyotype evolution and cytotaxonomy in Brazilian species of
Rhynchospora Vahl (Cyperaceae). – Bot. J. Linn. Soc. 134:
557-566.
Varadarajan GS. 1986a. Taxonomy and evolution
of the subfamily Pitcairnioideae (Bromeliaceae). – Ph.D. diss.,
Washington State University, Pullman, Washington.
Varadarajan GS. 1986b. Habitats of
Brocchinia, a descriptive account. – J. Bromeliad Soc. 36:
209-216.
Varadarajan GS. 1987a. Explorations for
Pitcairnioideae in South America 1. – J. Bromeliad Soc. 37: 16-25.
Varadarajan GS. 1987b. Explorations for
Pitcairnioideae in South America 2. – J. Bromeliad Soc. 37: 62-66.
Varadarajan GS, Brown GK. 1988. Morphological
variation of some floral features of the subfamily Pitcairnioideae (Bromeliaceae) and their significance
in pollination biology. – Bot. Gaz. 149: 82-91.
Varadarajan GS, Forero E. 1987. Genus
Pitcairnia (Bromeliaceae):
new taxa from Colombia, nomenclatural changes, and species complexes. – Syst.
Bot. 12: 410-415.
Varadarajan GS, Gilmartin AJ. 1983. Phenetic
and cladistic analyses of North American Chloris (Poaceae). – Taxon 32: 380-386.
Varadarajan GS, Gilmartin AJ. 1987. Foliar
scales in the subfamily Pitcairnioideae (Bromeliaceae). – Syst. Bot. 12:
562-571.
Varadarajan GS, Gilmartin AJ. 1988a. Seed
morphology of the subfamily Pitcairnioideae (Bromeliaceae) and its systematic
implications. – Amer. J. Bot. 75: 808-818.
Varadarajan GS, Gilmartin AJ. 1988b.
Phylogenetic relationships of groups of genera within the subfamily
Pitcairnioideae (Bromeliaceae). –
Syst. Bot. 13: 283-293.
Varadarajan GS, Gilmartin AJ. 1988c.
Taxonomic realignments within the subfamily Pitcairnioideae (Bromeliaceae). – Syst. Bot. 13:
294-299.
Vasey G. 1893. Descriptions of new grasses
from Mexico. – Contr. U.S. Natl. Herb. 1: 281-285.
Vazques Pardo FM, Devesa JA. 1996. Revisión
del género Stipa L. y Nassella Desv. (Poaceae) en la Península Ibérica e Islas
Baleares. – Acta Bot. Malac. 21: 125-189.
Vázquez FM, Barkworth ME. 2004. Resurrection
and emendation of Macrochloa (Gramineae: Stipeae). – Bot. J. Linn.
Soc. 144: 483-495.
Vázquez FM, Ramos S. 2007. Two new taxa and
a new combination for Stipa (Gramineae: Stipeae) in Tunisia. – Bot.
J. Linn. Soc. 153: 439-444.
Vedel F, Lebacq P, Quetier F. 1980.
Cytoplasmic DNA variation and relationships in cereal genomes. – Theor. Appl.
Gen. 58: 219-224.
Vega AS, Rugolo de Agrasar ZE. 2002.
Digitaria killeenii (Poaceae:
Panicoideae: Paniceae), a new species from Bolivia. – Syst. Bot. 27:
252-256.
Vega AS, Rua GH, Fabbri LT, Rúgolo de
Agrasar ZE. 2009. A morphology-based cladistic analysis of Digitaria
(Poaceae, Panicoideae, Paniceae). –
Syst. Bot. 34: 312-323.
Vega AS, Rúgolo de Agrasar ZE, Axelrod FS.
2010. A new species of Tarigidia (Poaceae, Panicoideae, Paniceae) from Puerto
Rico and additional evidence for a hybrid origin of the genus. – Syst. Bot.
35: 96-101.
Vegetti AC. 1991. Sobre politelia en las
inflorescencias de Poaceae. –
Kurtziana 21: 267-278.
Vegetti AC. 1992a. Typology of the
inflorescence in species of Schoenoplectus (Cyperaceae) of Austral America. –
Beitr. Biol. Pflanzen 67: 241-249.
Vegetti AC. 1992b. La sinflorescencia en
Schizachyrium tenerum y S. salzmannii. – Darwiniana 31:
341-344.
Vegetti AC. 1993a. Contribution to the study
of the synflorescence in Themeda Forssk. (Andropogoneae-Poaceae). – Beitr. Biol. Pflanzen 67:
251-258.
Vegetti AC. 1993b. Tipología de la
sinflorescencia en Hemarthria altissima. – Parodiana 8: 69-75.
Vegetti AC. 1994a. Typology of the
inflorescence in species of Isolepis. – Beitr. Biol. Pflanzen 68:
21-26.
Vegetti AC. 1994b. Tipología de la
sinflorescencia en Andropogoneae (Poaceae). – Ph.D. diss., Universidad
Nacional de Córdoba, Argentina.
Vegetti AC. 1997a. Sobre la estructura de la
inflorescencia en especies de Anthistiriinae (Poaceae-Andropogoneae). – Candollea 52:
87-103.
Vegetti AC. 1997b. Sobre la estructura de la
inflorescencia en especies de Rottboelliinae (Poaceae-Andropogoneae). – Candollea 52:
475-495.
Vegetti AC. 1997c. The structure of the
paracladial zone in Luziolinae (Oryzeae-Poaceae). – Beitr. Biol. Pflanzen 70:
101-106.
Vegetti AC. 1998. Sobre la estructura de la
inflorescencia en especies de Saccharinae, Germainiinae, Dimerliinae e
Ischaeminae (Poaceae-Andropogoneae). –
Candollea 53: 51-70.
Vegetti AC. 1999. Typology of the
synflorescence of Andropogoneae (Poaceae), additional comments. – Feddes
Repert. 110: 111-126.
Vegetti AC. 2000. Typology of synflorescences
in Oryzeae (Poaceae). – Phyton (Horn)
40: 71-88.
Vegetti AC. 2002a. Caracterización de los
sistemas de ramificación en especies de Oryzeae (Poaceae). – Candollea 57: 251-260.
Vegetti AC. 2002b. Typological
reinterpretation of the inflorescences in Cariceae (Cyperaceae). – Phyton 42: 159-168.
Vegetti AC, Anton AM. 1995. Some evolution
trends in the inflorescence of Poaceae.
– Flora 190: 225-228.
Vegetti AC, Anton AM. 1996. The
synflorescence in Poaceae. – Flora
191: 231-234.
Vegetti AC, Anton AM. 2000. The grass
inflorescence. – In: Jacobs SWL, Everett J (eds), Grasses: systematics and
evolution, Proceedings of the 2nd International Conference on the
Comparative Biology of Monocotyledons, 1998, Sydney, Australia, Vol. 2, CSIRO
Publ., Melbourne, pp. 29-31.
Vegetti AC, Tivano JC. 1991a. Inflorescence
typology in Schoenoplectus californicus (Cyperaceae). – Beitr. Biol. Pflanzen
66: 323-345.
Vegetti AC, Tivano JC. 1991b. La
inflorescencia en Aciachne (Poaceae). – Bol. Soc. Argent. Bot. 27:
91-96.
Vegetti AC, Tivano JC. 1992. Synflorescence
in Schizachyrium microstachyum (Poaceae). – Beitr. Biol. Pflanzen 66:
165-178.
Vegetti AC, Weberling F. 1996. The structure
of the paracladial zone in Poaceae. –
Taxon 45: 453-460.
Veken P van der. 1955. Trois taxa nouveaux de
Cypéracées du Congo Belge. – Bull. Jard. Bot. État, Bruxelles 25:
143-147.
Veken P van der. 1965. Contribution à
l’embryographie systématique des Cyperaceae-Cyperoideae. – Bull. Jard.
Bot. État, Bruxelles 35: 285-354.
Veldkamp JF. 1973. Notes on Malesian grasses
VI. A revision of Digitaria Haller (Gramineae) in Malesia. – Blumea
21: 1-80.
Veldkamp JF. 1985. Anemanthele
Veldk. (Gramineae: Stipeae), a new genus from New Zealand. – Acta Bot. Neerl.
34: 105-109.
Veldkamp JF. 1994. Poa L.
(Gramineae) in Malesia. – Blumea 38: 409-457.
Veldkamp JF. 1996a. Proposal to conserve the
name Brachiaria (Trin.) Griseb. (Gramineae) with a conserved type. –
Taxon 45: 319-320.
Veldkamp JF. 1996b. Name changes in
Agrostis, Arundinella, Deyeuxia,
Helictotrichon, Tripogon (Gramineae). – Blumea 41:
407-411.
Veldkamp JF. 2015. Arundinella
(Gramineae) in Malesia with notes on other taxa and on aluminium accumulation.
– Blumea 59: 167-179.
Veldkamp JF. 2016a. A revision of
Themeda (Gramineae) in Malesia with a new species from Laos. –
Blumea 61: 29-40.
Veldkamp JF. 2016b. A revision of
Dimeria (Gramineae-Dimeriinae) in Malesia with a note on Cymbachne.
– Blumea 61: 207-214.
Veldkamp JF. 2018. Schmidiella
Veldk., gen. nov., an enigmatic new genus of Gramineae from Laos. –
Adansonia, sér. 3, 40: 55-58.
Veldkamp JF, Nowack R. 1994.
Vietnamochloa aurea (Gramineae: Eragrostideae), a new genus and
species from Vietnam. – Bull. Mus. Natl. Hist. Nat., Section B, Adansonia 16:
213-218.
Veldkamp JF, deKoning R, Sosef MSM. 1986.
Generic delimitation of Rottboellia and related genera (Gramineae).
– Blumea 31: 281-307.
Veldkamp JF, Eriks M, Smit SS. 1991.
Bromus (Gramineae) in Malesia. – Blumea 35: 483-497.
Veldkamp JF, Teerawatananon A, Sungkaew S.
2015. A revision of Garnotia (Gramineae) in Malesia and Thailand. –
Blumea 59: 229-237.
Venturelli M, Bouman F. 1986. Embryology and
seed development in Mayaca fluviatilis (Mayacaceae). – Acta Bot. Neerl. 35:
497-516.
Venturelli M, Bouman F. 1988. Development of
ovule and seed in Rapateaceae. –
Bot. J. Linn. Soc. 97: 267-294.
Verbelen JP. 1970. Systematische embryografie
van de Cyperaceae-Rhynchosporineae.
– Biol. Jaarb. Dodonaea 38: 151-166.
Verboom GA. 2006. A phylogeny of the
schoenoid sedges (Cyperaceae:
Schoeneae) based on plastid DNA sequences, with special reference to the genera
found in Africa. – Mol. Phylogen. Evol. 38: 79-89.
Verboom GA, Linder HP, Barker NP. 1994.
Haustorial synergids: an important character in the systematics of danthonioid
grasses (Arundinoideae: Poaceae). –
Amer. J. Bot. 81: 1601-1610.
Verboom GA, Linder HP, Stock WD. 2003.
Phylogenetics of the grass genus Ehrharta: evidence for radiation in
the summer-arid zone of the South African Cape. – Evolution 57: 1008-1021.
Verboom GA, Linder HP, Stock WD. 2004.
Testing the adaptive nature of radiation: growth form and life history
divergence in the African grass genus Ehrharta (Poaceae: Ehrhartoideae). – Amer. J. Bot.
91: 1364-1370.
Verboom GA, Ntsohi R, Barker NP. 2006.
Molecular phylogeny of African Rytidosperma-affiliated danthonioid
grasses reveals generic polyphyly and convergent evolution in spikelet
morphology. – Taxon 55: 337-348.
Verloove F. 2001. A revision of the genus
Panicum (Poaceae, Paniceae) in
Belgium. – Syst. Geogr. Plants 71: 53-72.
Verloove F. 2005. A synopsis of
Jarava Ruiz & Pav. and Nassella E. Desv. (Stipa
L. s.l.) (Poaceae: Stipeae) in
southwestern Europe. – Candollea 60: 97-117.
Verloove F. 2008. Studies within the genus
Digitaria Haller (Poaceae,
Panicoideae) in southwestern Europe. – Candollea 63: 227-233.
Verloove F, Gullón ES. 2012. A taxonomic
revision of non-native Cenchrus s.str. (Paniceae, Poaceae) in the Mediterranean area. –
Willdenowia 42: 67-75.
Verloove F, Reynders M. 2007. Studies in the
genus Paspalum (Paniceae, Poaceae) in Europe 2. The
Quadrifaria group. – Willdenowia 37: 423-430.
Versieux LM. 2009. Checklist and one new
species of Bromeliaceae from Pico
do Itambé, Minas Gerais, Brazil. – Bot. J. Linn. Soc. 158: 709-715.
Versieux LM, Wanderley M das Graças Lapa.
2007. Two new species of Alcantarea (Bromeliaceae, Tillandsioideae) from
Brazil. – Brittonia 59: 57-64.
Versieux LM, Wendt T, Louzada RB, Wanderley
MGL. 2008. Bromeliaceae da Cadeia
do Espinhaço. – Megadiversidade 4: 98-110.
Versieux LM, Louzada RB, Viana PL, Mota N,
Wanderley M das GL. 2010. An illustrated checklist of Bromeliaceae from Parque Estadual do
Rio Preto, Minas Gerais, Brazil, with notes on phytogeography and one new
species of Cryptanthus. – Phytotaxa 10: 1-16.
Versieux LM, Barbará T, Wanderley MGL,
Calvente A, Fay MF, Lexer C. 2012. Molecular phylogenetics of the Brazilian
giant bromeliads (Alcantarea, Bromeliaceae): implications for
morphological evolution and biogeography. – Mol. Phylogen. Evol 64:
177-189.
Viana PL, Filgueiras TS, Clark LG. 2013.
Cambajuva (Poaceae:
Bambusoideae: Bambuseae: Arthrostylidiinae), a new woody bamboo genus from
southern Brazil. – Syst. Bot. 38: 97-103.
Vicentini A, Barber JC, Alscioni SS, Giussani
LM, Kellogg EA. 2008. The age of the grasses and clusters of origins of
C4 photosyntesis. – Global Change Biol. 14: 2963-2977.
Vicioso C. 1959. Estudio monográfico sobre
el género Carex en España. – Bol. Inst. Forest. Invest. Exp. 79:
1-205.
Vickery JW. 1975. Contributions to the
taxonomy of Australian grasses III. – Telopea 1: 40-43.
Vickery JW. 1980. Four new species of
Stipa (Poaceae). – Telopea 2:
11-15.
Vickery JW, Jacobs SWL. 1980.
Nassella and Oryzopsis in New South Wales. – Telopea 2:
17-23.
Vickery JW, Jacobs SWL, Everett J. 1986.
Taxonomic studies in Stipa (Poaceae) in Australia. – Telopea 3:
1-132.
Vieira CM. 1999. Quesnelia Gaudich.
(Bromelioideae: Bromeliaceae) do
estado do Rio de Janeiro. – M.Sc. thesis, University of Rio de Janeiro,
Brazil.
Vieira RC, Gomes DMS, Sarahyba LS, Arruda
RCO. 2002. Leaf anatomy of three herbaceous bamboo species. – Brazilian J.
Biol. 62(4B): 907-922.
Vierhapper F. 1906. Zur Systematik der
Gattung Avena. – Verh. Zool.-Bot. Ges. Wien 56: 369-370.
Vierhapper F. 1930. Juncaceae. – In: Engler A (ed), Die
natürlichen Pflanzenfamilien, 2. Augl., Bd. 15a, W. Engelmann, Leipzig, pp.
192-224.
Vignal C. 1984. Étude phytodermologique de
la sous-famille des Chloridoideae (Gramineae). – Bull. Mus. Natl. Hist. Nat.
Paris, sér. IV, sect. B, Adansonia: Botanique Phytochimie 6: 279-295.
Villamil CB. 1969. El género
Monanthochloë (Gramineae). Estudios morfológicos y taxonómicos con
especial referencia a la especie Argentina. – Kurtziana 5: 369-391.
Villaverde T, Maguilla E, Escudero M,
Márquez-Corro JI, Jiménez-Mejías P, Gehrke B, Martín-Bravo S, Luceño M.
2017. New insights intothe systematics of the Schoenoxiphium clade
(Carex, Cyperaceae). –
Intern. J. Plant Sci. 178: 320-329.
Visser NC, Spies JJ, Venter HJT. 1998.
Meiotic chromosome behavior in Cenchrus ciliaris (Poaceae: Panicoideae). – Bothalia 28:
83-90.
Vitta FA. 2002. Trilepis tenuis (Cyperaceae: Trilepideae), a new species
from Rio de Janeiro, southeastern Brazil. – Brittonia 54: 120-123.
Voight PW. 1971. Discovery of sexuality in
Eragrostis curvula (Schrad.) Nees. – Crop Sci. 11: 424-425.
Voight PW, Bashaw EC. 1972. Apomixis and
sexuality in Eragrostis curvula (Schrad.) Nees. – Crop Sci. 12:
843-847.
Voight PW, Bashaw EC. 1976. Facultative
apomixes in Eragrostis curvula. – Crop Sci. 16: 803-806.
Voight PW, Burson BL, Sherman RA. 1992. Mode
of reproduction in cytotypes of Lehmann lovegrass. – Crop Sci. 32:
118-121.
Vollbrecht E, Springer PS, Goh L, Buckler ES,
Martienssen R. 2005. Architecture of floral branch systems in maize and related
grasses. – Nature 436: 1119-1126.
Vollmann F. 1903. Der Formenkreis der
Carex muricata und seine Verbreitung in Bayern. – Denkschr. K.
Bayer. Bot. Ges. Regensburg 2(8): 55-90.
Vorontsova MS, Simon BK. 2012. Updating
classifications to reflect monophyly: 10 to 20 percent of species names change
in Poaceae. – Taxon 61: 735-746.
Vorontsova MS, Ratovonirina G,
Randriamboavonjy T. 2013. Revision of Andropogon and
Diectomis (Poaceae: Sacchareae)
in Madagascar and the new Andropogon itremoensis from the Itremo
Massif. – Kew Bull. 68: 193-207.
Vorontsova MS, Haevermans T, Haevermans A,
Razanatsoa J, Lundgren MR, Besnard G. 2015. The genus Sartidia (Poaceae: Aristidoideae) in Madagascar. –
Syst. Bot. 40: 448-453.
Vorster TB, Liebenberg H. 1977. Cytogenetic
studies in the Eragrostis curvula complex. – Bothalia 12:
215-221.
Vorster TB, Liebenberg H. 1984.
Classification of embryo sacs in the Eragrostis curvula complex. –
Bothalia 15: 167-174.
Voshell SM, Baldini RM, Kumar R, Tatalovich
N, Hilu KW. 2011. Canary grasses (Phalaris, Poaceae): molecular phylogenetics,
polyploidy and floret evolution. – Taxon 60: 1306-1316.
Voshell SM, Baldini RM, Hilu KW. 2016.
Infrageneric treatment of Phalaris (Canary grasses, Poaceae) based on molecular phylogenetics
and floret structure. – Aust. Syst. Bot. 28: 355-367.
Voster P. 1990. Anatomy of the South African
species of Mariscus (Cyperaceae), and its relation to
environmental conditions. – Mitt. Inst. Allg. Bot. Hamburg 23: 367-386.
Vrijdaghs A. 2006. A floral ontogenetic
approach to homology questions in non-mapanioid Cyperaceae. – PhD diss., K. U. Leuven,
Leuven, Belgium.
Vrijdaghs A, Goetghebeur P, Muasya AM, Smets
E, Caris P. 2004. The nature of the perianth in Fuirena (Cyperaceae). – South Afr. J. Bot. 70:
587-594.
Vrijdaghs A, Caris P, Goetghebeur P, Smets E.
2005. Floral ontogeny in Scirpus, Eriophorum and
Dulichium (Cyperaceae), with
special reference to the perianth. – Ann. Bot. 95: 1199-1209.
Vrijdaghs A, Goetghebeur P, Muasya AM, Caris
P, Smets E. 2005. Floral ontogeny in Ficinia and Isolepis (Cyperaceae), with focus on the nature
and origin of the gynophore. – Ann. Bot. 96: 1247-1264.
Vrijdaghs A, Goetghebeur P, Smets E, Muasya
AM. 2006. The floral scales in Hellmuthia (Cyperaceae, Cyperoideae) and
Paramapania (Cyperaceae,
Mapanioideae): an ontogenetic study. – Ann. Bot. 98: 619-630.
Vrijdaghs A, Goetghebeur P, Smets E, Caris P.
2007 [2008]. The Schoenus spikelet: a rhipidium? A floral ontogenetic
answer. – In: Columbus JT, Friar EA, Porter JM, Prince LM, Simpson MG (eds),
Monocots: comparative biology and evolution. Poales, Rancho Santa Ana Botanical
Garden, Claremont, California, [Aliso 23] pp. 204-209.
Vrijdaghs A, Muasya AM, Goetghebeur P, Caris
P, Nagels A, Smets E. 2009. A floral ontogenetic approach to questions of
homology within the Cyperoideae (Cyperaceae). – Bot. Rev. 75: 30-51.
Vrijdaghs A, Reynders M, Larridon I, Muasya
AM, Smets E, Goetghebeur P. 2010. Spikelet structure and development in
Cyperoideae (Cyperoideae): a monopodial general model based on ontogenetic
evidence. – Ann. Bot. 105: 555-571.
Vrijdaghs A, Reynders M, Muasya AM, Larridon
I, Goetghebeur P, Smets E. 2011. Morphology and development of spikelets and
flowers in Cyperus and Pycreus (Cyperaceae). – Plant Ecol. Evol. 144:
44-63.
Wagner ND, Wöhrmann T, Öder V, Burmeister
MMA, Weising K. 2015. Reproduction biology and chloroplast inheritance: a case
study in Fosterella. – Plant Syst. Evol. 301: 2231-2246.
Wagnon KH. 1952. A revision of the genus
Bromus, section Bromopsis, of North America. – Brittonia 7:
415-480.
Wagstaff SJ, Clarkson BR. 2012. Systematics
and ecology of the Australasian genus Empodisma (Restionaceae) and description of a new
species from peatlands in northern New Zealand. – PhytoKeys 13: 39-79.
Wahl HA. 1940. Chromosome numbers and meiosis
in the genus Carex. – Amer. J. Bot. 27: 458-470.
Wallace EC. 1975. Carex L. – In:
Stace CA (ed), Hybridization and the flora of the British Isles, Academic
Press, London, pp. 513-540.
Waller FR, Morden CW. 1983. Panicum
tamaulipense (Poacecae: Paniceae): a new species from Mexico. – Syst.
Bot. 8: 221-222.
Walters MS. 1952. Spontaneous chromosome
breakage and atypical chromosome movement in meiosis of the hybrid Bromus
arginatus x B. pseudolaevipes. – Genetics 37: 8-25.
Wanderley MGL. 1990. Diversidade e
distribuição geográfica das espécies de Orthophytum (Bromeliaceae). – Acta Bot. Bras. 4:
169-175.
Wanderley MGL. 1992. Estudos taxonômicos no
gênero Xyris L. (Xyridaceae) da Serra do Cipó, Minas
Gerais, Brasil. – Ph.D. diss., Instituto de Biociências, São Paulo.
Wanderley MGL. 2011. Flora da Serra do Cipó,
Minas Gerais: Xyridaceae. – Bol.
Bot. Univ. São Paulo 29: 69-134.
Wang RRC, Lu B-R. 2014. Biosystematic and
evolutionary relationships of perennial Triticeae species revealed by genomic
analyses. – J. Syst. Evol. 56: 697-705.
Wang S, Henwood MJ. 1999. The taxonomic
utility of micromorphological characters in Australian and New Zealand
Elymus species (Poaceae). –
Telopea 8: 351-362.
Wang Z-P, Ye G-H. 1980. Taxonomic problems in
Chinese scattered bamboos. – Acta Phytotaxon. Sin. 18: 283-290.
Wang Z-P, Ye G-H. 1982.
Oligostachyum – a new genus of Chinese Bambusoideae. – J. Nanjing
Univ. (Nat. Sci.) 1: 95-101.
Wang Z-Y, Tanksley G, Tanksley SD. 1992.
Polymorphism and phylogenetic relationships among species in the genus
Oryza as determined by analysis of nuclear RFLPs. – Theor. Appl.
Gen. 83: 565-581.
Warwick SI, Aiken SG. 1986. Electrophoretic
evidence for the recognition of two species in annual wild rice
(Zizania, Poaceae). – Syst.
Bot. 11: 464-473.
Washburn JD, Schnable JC, Davidse G, Pires
JC. 2015. Phylogeny and photosynthesis of the grass tribe Paniceae. – Amer.
J. Bot. 102: 1493-1505.
Watanabe M, Ito M, Kurita S. 1994.
Chloroplast DNA phylogeny of Asian bamboos (Bambusoideae, Poaceae) and its systematic implication.
– J. Plant Res. 107: 253-261.
Waterway MJ, Starr JR. 2007 [2008].
Phylogenetic relationships in tribe Cariceae (Cyperaceae) based on nested analyses of
four molecular data sets. – In: Columbus JT, Friar EA, Porter JM, Prince LM,
Simpson MG (eds), Monocots: comparative biology and evolution. Poales, Rancho
Santa Ana Botanical Garden, Claremont, California. – Aliso 23: 165-192.
Waterway MJ, Hoshino T, Masaki T. 2009.
Phylogeny, species richness, and ecological specialization in Cyperaceae tribe Cariceae. – Bot. Rev.
75: 138-159.
Watson L. 1990. The grass family, Poaceae. – In: Chapman GP (ed),
Reproductive versatility in the grasses, Cambridge University Press, Cambridge,
pp. 1-31.
Watson L, Clifford HT. 1976. The major groups
of Australian grasses: a guide to sampling. – Aust. J. Bot. 24:489-507.
Watson L, Dallwitz MJ. 1992. The grass genera
of the world. – C. A. B. International, Wallingford, Oxon, United Kingdom.
Watson L, Clifford HT, Dallwitz MJ. 1985. The
classification of the Poaceae:
subfamilies and supertribes. – Aust. J. Bot. 33: 433-484.
Watson L, Barker NP, Jacobs SWL. 1998.
Amphipogon (Gramineae) does have microhairs. – Telopea 8: 155.
Webber JM, Ball PW. 1984. The taxonomy of the
Carex rosea group (section Phaestoglochin) in Canada. –
Can. J. Bot. 62: 2058-2073.
Weber H. 1963. Über die Wuchsform von
Bulbostylis paradoxa (Sprengl.) Lindm. (Cyperaceae). – Abh. Math. Nat. Kl.
Akad. Wiss. Mainz 5: 267-284.
Webster RD. 1983. A revision of the genus
Digitaria Haller (Paniceae: Poaceae) in Australia. – Brunonia 6:
131-216.
Webster RD. 1987. The Australian Paniceae (Poaceae). – J. Cramer, Berlin,
Stuttgart.
Webster RD. 1988. Genera of the North
American Paniceae (Poaceae:
Panicoideae). – Syst. Bot. 13: 576-609.
Webster RD. 1992. Character significance and
generic similarities in the Paniceae (Poaceae: Panicoideae). – Sida 15:
185-213.
Webster RD, Valdes-Reyna J. 1988. Genera of
Mesoamerican Paniceae (Poaceae:
Panicoideae). – Sida 13: 187-221.
Webster RD, Kirkbride JH, Reyna JV. 1989. New
World genera of the Paniceae (Poaceae:
Panicoideae). – Sida 13: 393-417.
Wee YC, Rao AN. 1974. Gametophytes and seed
development in pineapple. – Curr. Sci. 43: 171-173.
Wei F, Coe E, Nelson W, Bharti AK, Engler F,
Butler E, Kim H, Goicoechea JL, Chen M, Lee S, Fuks G, Sanchez-Villeda H,
Schroeder S, Fang Z, McMullen M, Dvis G, Bowers JE, Paterson AH, Schaeffer M,
Gardiner J, Cone K, Messing J, Soderlund C, Wing RA. 2007. Physical and genetic
structure of the maize genome reflects its complex evolutionary history. –
PloS Genet. 3(7): e123.
Weimarck G. 1971a. Variation and taxonomy of
Hierochloë (Gramineae) in the Northern Hemisphere. – Bot. Not. 124:
129-175.
Weimarck G. 1971b. Studies of
Hierochloë (Gramineae) in the Northern hemisphere. – Ph.D. diss.,
University of Lund, Sweden.
Weimarck G. 1973. Male meiosis in some
amphimictic and apomictic Hierochloë (Gramineae). – Bot. Not. 126:
7-36.
Weimarck H. 1946. Studies in Juncaceae, with special reference to the
species in Ethiopia and the Cape. – Svensk Bot. Tidskr. 40: 141-176.
Weinzieher S. 1914. Beiträge zur
Entwicklungsgeschichte von Xyris indica L. – Flora 106: 393-432.
Welker CAD, Longhi-Wagner HM. 2012. The
genera Eriochrysis P. Beauv., Imperata Cirillo and
Saccharum L. (Poaceae-Andropogoneae-Saccharinae) in the
state of Rio Grande do Sul, Brazil. – Brazil. J. Bot. 35: 87-105.
Welker CAD, Longhi-Wagner HM, Souza-Chies TT
de. 2012. New record for Eriochrysis (Poaceae: Andropogoneae) in the State of Rio
Grande do Sul, Brazil, and a key to the species of Eriochrysis in
Brazil. – Phytotaxa 71: 1-4.
Welker CAD, Kellogg EA, Prado J. 2014.
Andropogoneae versus Sacchareae (Poaceae: Panicoideae): the end of a great
controversy. – Taxon 63: 643-646.
Welker CAD, Souza-Chies TT, Longhi-Wagner HM,
Peichoto MC, McKain MR, Kellogg EA. 2015. Phylogenetic analysis of
Saccharum s.l. (Poaceae;
Andropogoneae), with emphasis on the circumscription of the South American
species. – Amer. J. Bot. 102: 248-263.
Welker CAD, Souza-Chies TT, Longhi-Wagner HM,
Peichoto MC, McKain MR, Kellogg EA. 2016. Multilocus phylogeny and
phylogenomics of Eriochrysis P. Beauv. (Poaceae-Andropogoneae): taxonomic
implications and evidence of interspecific hybridization. – Mol. Phylogen.
Evol. 99: 155-167.
Wen T-H. 1985. Some ideaws about the origin
of bamboos. – J. Amer. Bamboo Soc. 6: 104-111.
Wen T-H. 1987. New taxa of
Sinobambusa and others. – J. Bamboo Res. 6: 29-34.
Wen T-H. 1989. Some new bamboos from southern
Yangtze river. – J. Bamboo Res. 8: 13-24.
Wen T-H. 1991. Some ideas on the taxonomy of
several Bambusoideae taxon. – J. Bamboo Res. 10: 11-25.
Wendt T. 1997. A review of the subgenus
Pothuava (Baker) Baker of Aechmea Ruiz & Pav. (Bromeliaceae) in Brazil. – Bot. J.
Linn. Soc. 125: 245-271.
Wendt T, Ferreira Canela MB, Morrey-Jones JE,
Henriques AB, Iglesias Rios R. 2000. Recognition of Pitcairnia
corcovadensis (Bromeliaceae)
at the species level. – Syst. Bot. 25: 389-398.
Wendt T, Coser TS, Matallana G, Guilherme
FAG. 2008. An apparent lack of prezygotic reproductive isolation among 42
sympatric species of Bromeliaceae
in southeastern Brazil. – Plant Syst. Evol. 275: 31-41.
Weng D-M, Zhang H-W, Xu S-F, Jin X-F. 2009.
Carex qingliangensis sp. nov. (Cyperaceae) from Zhejiang, eastern
China. – Nord. J. Bot. 27: 7-10.
Wepfer PH, Linder HP. 2014. The taxonomy of
Flagellaria (Flagellariaceae). – Aust. Syst.
Bot. 27: 159-179.
West JG, McIntyre CL, Appels R. 1988.
Evolution and systematic relationships in the Triticeae (Poaceae). – Plant Syst. Evol. 160:
1-28.
Wet JMJ de. 1954a. The genus
Danthonia in grass phylogeny. – Amer. J. Bot. 41: 204-211.
Wet JMJ de. 1954b. Chromosome numbers of a
few South African grasses. – Cytologia 19: 97-103.
Wet JMJ de. 1958. Additional chromosome
numbers in Transvaal grasses. – Cytologia 23: 113-118.
Wet JMJ de. 1960a. Chromosome numbers and
some morphological attributes of various South African grasses. – Amer. J.
Bot. 47: 44-49.
Wet JMJ de. 1960b. Culm anatomy in relation
to taxonomy. – Bothalia 7: 311-316.
Wet JMJ de. 1987. Hybridization and
polyploidy in the Poaceae. – In:
Soderstrom TR, Hilu KW, Campbell CS, Barkworth ME (eds), Grass systematics and
evolution, Smithsonian Institution Press, Washington, D.C., pp. 188-194.
Wet JMJ de, Anderson LJ. 1956. Chromosome
numbers in Transvaal grasses. – Cytologia 21: 1-10.
Wet JMJ de, Harlan JR. 1970a. Apomixis,
polyploidy, and speciation in Dichanthium. – Evolution 24:
270-277.
Wet JMJ de, Harlan JR. 1970b. Biosystematics
of Cynodon L. C. Rich. – Taxon 19: 565-569.
Wheeler DJB, Jacobs SWL, Norton BE. 1984.
Grasses of the New South Wales. 2nd ed. – University of New
England, Armidale, Australia.
Wheeler GA. 1987. The taxonomy of
Carex sect. Abditispicae sect. nov. (Cyperaceae) from austral South America.
– Syst. Bot. 12: 572-585.
Wheeler GA. 1988. A new species of
Carex sect. Junciformes (Cyperaceae) from austral South America
and notes on the sectional placement of C. sorianoi. – Syst. Bot.
13: 202-206.
Wheeler GA. 1989a. A new species of
Carex sect. Abditispicae (Cyperaceae) from northern Argentina and
the status of Vesicarex collumanthus in South America. – Syst. Bot.
14: 37-42.
Wheeler GA. 1989b. The taxonomy of
Carex sect. Aciculares (Cyperiaceae) in South America. –
Syst. Bot. 14: 168-188.
Wheeler GA. 1990. Taxonomy of the Carex
atropicta complex (Cyperaceae)
in South America. – Syst. Bot. 15: 643-659.
Wheeler GA. 2007. Carex and
Uncinia (Cyperaceae,
Cariceae) from the Juan Fernandez Archipelago, Chile. – Darwiniana 45:
120-142.
Whipple CJ, Schmidt RJ. 2006. Genetics of
grass flower development. – Adv. Bot. Res. 44: 385-424.
Whipple CJ, Zanis MJ, Kellogg EA, Schmidt RJ.
2007. Conservation of B class gene expression in the second whorl of a basal
grass and outgroups links the origin of lodicules and petals. – Proc. Natl.
Acad. Sci. U.S.A. 104: 1081-1086.
Whipple CJ, Hall DH, DeBlasio S,
Taguchi-Shiobara F, Schmidt RJ, Jackson DP. 2010. A conserved mechanism of
bract suppression in the grass family. – Plant Cell 22: 565-578.
Whiting DA. 1985. Lignans and neolignans. –
Nat. Prod. Rep. 2: 191-211.
Whitkus R. 1992. Allozyme variation within
the Carex pachystachya complex (Cyperaceae). – Syst. Bot. 17:
16-24.
Wichelen J van, Camelbeke K, Chaerle P,
Goetghebeur P, Huysmans S. 1999. Comparison of different treatments for LM and
SEM studies and systematic value of pollen grains in Cyperaceae. – Grana 38: 50-58.
Widjaja EA. 1997. New taxa in Indonesian
bamboos. – Reinwardtia 11: 57-152.
Widmer Y, Clark LG. 1991. New species of
Chusquea (Poaceae:
Bambusoideae) from Costa Rica. – Ann. Missouri Bot. Gard. 78: 164-171.
Wilczek E. 1892. Beiträge zur Kenntnis des
Baues der Frucht und des Samens der Cyperaceen. – Bot. Centralbl. 51:
129-138, 193-201, 225-233, 257-265.
Will B, Zizka G. 1999. The genus
Greigia Regel in Chile. – Harvard Pap. Bot. 4: 225-240.
Wille F. 1926. Beiträge zur anatomie des
Cyperaceenrhizoms. – Beih. Bot. Zeitschr. 43: 267-309.
Wille N. 1882. Om Pollenkornens Udvikling hos
Juncaceer og Cyperaceer. – Christiania Videnskaps Selsk. Forh. 1882(16).
Williams CA. 1978. The systematic
implications of the complexity of leaf flavonoids in the Bromeliaceae. – Phytochemistry 17:
729-734.
Williams CA, Harborne JB. 1971. Flavonoid
patterns in the monocotyledons. Flavonols and flavons in some families
associated with the Poaceae. –
Phytochemistry 10: 1059-1063.
Williams CA, Harborne JB. 1975. Luteolin and
daphnetin derivatives in the Juncaceae
and their systematic significance. – Biochem. Syst. Ecol. 3: 181-190.
Williams CA, Harborne JB, Greenham J, Briggs
BG, Johnson LAS. 1997. Flavonoid evidence and the classification of the Anarthriaceae within the Poales. –
Phytochemistry 45: 1189-1196.
Williams CA, Harborne JB, Greenham J, Briggs
BG, Johnson LAS. 1998. Flavonoid patterns and the revised classification of
Australian Restionaceae. –
Phytochemistry 49: 529-552.
Wills KE, Whalley RDB, Bruhl JJ. 2000.
Systematic studies in Paniceae (Poaceae): Homopholis and
Whalleya gen. et sp. nov. – Aust. Syst. Bot. 13: 437-468.
Wilson KL. 1980. Notes on some Australian
species of Cyperaceae. – Telopea 1:
457-467.
Wilson KL. 1981a. A synopsis of the genus
Scirpus sens. lat. (Cyperaceae) in Australia. – Telopea 2:
153-172.
Wilson KL. 1981b. Revision of the genus
Mesomelaena (Cyperaceae).
– Telopea 2: 181-195.
Wilson KL. 1983. Cyperaceae. – In: Morley BD, Toelken
HR (eds), Flowering plants in Australia, Rigby, Adelaide, pp. 364-368.
Wilson KL. 1991. Systematic studies in
Cyperus section Pinnati (Cyperaceae). – Telopea 4: 361-496.
Wilson KL. 1994. New taxa and combinations in
the family Cyperaceae in eastern
Australia. – Telopea 5: 589-625.
Wilson KL, Johnson LAS. 2001. The genus
Juncus (Juncaceae) in Malesia
and allied septate-leaved species in adjoining regions. – Telopea 9:
357-397.
Wingfield R. 1977. A note on Fuirena
ciliaris (Cyperaceae) and
related species. – Bot. Not. 130: 319-320.
Winkler S. 1990. Zur Evolution der Gattung
Tillandsia L. – Bot. Jahrb. Syst. 122: 43-77.
Winter B de. 1965. The South African Stipeae
and Aristideae (Gramineae). An anatomical, cytological and taxonomic study. –
Bothalia 8: 201-404.
Winterfeld G. 2006. Molekular-cytogenetische
Untersuchungen an Hafergräsern (Aveneae) und anderer Poaceae. – Stapfia 86: 1-170.
Winterfeld G, Röser M. 2007a. Disposal of
ribosomal DNAs in the chromosomes of perennial oats (Poaceae: Aveneae). – Bot. J. Linn. Soc.
155: 193-210.
Winterfeld G, Röser M. 2007b. Chromosomal
localization and evolution of satellite DNAs and heterochromatin in grasses (Poaceae), especially tribe Aveneae. –
Plant Syst. Evol. 264: 75-100.
Winterfeld G, Döring E, Röser M. 2009.
Chromosome evolution in wild oat grasses (Aveneae) revealed by molecular
phylogeny. – Genome 52: 361-380.
Winterfeld G, Schneider J, Röser M. 2009.
Allopolyploid origin of Mediterranean species in Helictotrichon (Poaceae) and its consequences for karyotype
repatterning and homogenisation of rDNA repeat units. – Syst. Biodivers. 7:
1-18.
Wipff JK. Hatch SL. 1994. A systematic study
of Digitaria sect. Pennatae (Poaceae: Paniceae) in the New World. –
Syst. Bot. 613-627.
Wittmack L. 1888. Bromeliaceae. – In: Engler A, Prantl
K (eds), Die natürlichen Pflanzenfamilien II(4), W. Engelmann, Leipzig, pp.
32-59; Wittmack L. 1897. Nachträge zu II(4), pp. 61-69.
Wölk A, Röser M. 2013. The new genus
Trisetopsis and new combinations in oat-like grasses (Poaceae). – Schlechtendalia 25: 57-61.
Wölk A, Röser M. 2014. Polyploid evolution,
intercontinental biogeographical relationships and morphology of the recently
described African oat genus Trisetopsis (Poaceae). – Taxon 63: 773-788.
Wölk A, Röser M. 2017. Hybridization and
long-distance colonization in oat-like grasses of South and East Asia,
including an amended circumscription of Helictotrichon and the
description of the new genus Tzveleviochloa (Poaceae). – Taxon 66: 20-43.
Wölk A, Winterfeld G, Röser M. 2015. Genome
evolution in a Mediterranean species complex: phylogeny and cytogenetics of
Helictotrichon (Poaceae)
allopolyploids based on nuclear DNA sequences (rDNA, topoisomerase gene) and
FISH. – Syst. Biodivers. 13: 326-345.
Wong K-M. 1986. The growth habits of Malayan
bamboos. – Kew Bull. 41: 703-720.
Wong K-M. 1993. Four new genera of bamboos
(Gramineae: Bambusoideae) from Malesia. – Kew Bull. 48: 517-532.
Wong K-M. 1995. The morphology, anatomy,
biology and classification of Peninsular Malaysian bamboos. – University of
Malaya botanical monographs 1, University of Malaya, Kuala Lumpur.
Wong K-M. 2005. Mullerochloa, a new
genus of bamboo (Poaceae: Bambusoideae)
from north-east Australia and notes on the circumscription of Bambusa.
– Blumea 50: 425-441.
Wong K-M, Goh W-L, Chokthaweepanich H, Clark
LG, Sungkaew S, Widjaja EA, Xia N-H. 2016. A subtribal classification of
Malesian and South Pacific woody bamboos (Poaceae: Bambusoideae: Bambuseae) informed
by morphological and molecular studies. – Sandakania 22: 11-36.
Wu C-C, Diggle PK, Friedman WE. 2011. Female
gametophyte development and double fertilization in Balsas teosinte, Zea
mays subsp. parviglumis (Poaceae). – Sex. Plant Repr. 24:
219-229.
Wu F-H, Kan D-P, Lee S-B, Daniell H, Lee Y-W,
Lin C-C, Lin C-S. 2009. Complete nucleotide sequence of Dendrocalamus
latiflorus and Bambusa oldhamii chloroplast genomes. – Tree
Physiol. 29: 847-856.
Wu M, Lan S, Cai B, Chen S, Chen H, Zhou S.
2015. The complete chloroplast genome of Guadua angustifolia and
comparative analyses of Neotropical-Paleotropical bamboos. – PLoS ONE 10:
e0143792.
Wu Z-Q, Ge S. 2012. The phylogeny of the BEP
clade in grasses revisited: evidence from the whole-genome sequences of
chloroplasts. – Mol. Phylogen. Evol. 62: 573-578.
Wulff HD. 1939. Die Pollenentwicklung der
Juncaceen nebst einer Auswertung der embryologischen Befunde hinsichtlich einer
Verwandtschaft zwischen den Juncaceen und Cyperaceen. – Jahrb. Wiss. Bot. 87:
533-556.
Wysocki WP, Clark LG, Kelchner SA, Burke SV,
Pires JC, Edger PP, Mayfield DR, Triplett JK, Columbus JT, Ingram AL, Duvall
MR. 2014. A multi-step comparison of short-read full plastome sequence assembly
methods in grasses. – Taxon 63: 899-910.
Wysocki WP, Clark LG, Attigala L,
Ruiz-Sanchez E, Duvall MR. 2015. Evolution of the bamboos (Bambusoideae; Poaceae): a full plastome phylogenomic
analysis. – BMC Evol. Biol. 15: 50.
Xia N-H. 1996. A study of the genus
Bonia (Gramineae: Bambusoideae). – Kew Bull. 51: 565-569.
Xu G, Kong H. 2007. Duplication and
divergence of floral MADS-Box genes in grasses: evidence for the generation and
modification of novel regulator. – J. Integr. Plant Biol. 49: 927-939.
Xu J-H, Messing J. 2008. Organization of the
prolamin gene family provides insight into the evolution of the maize genome
and gene duplications in grass species. – Proc. Natl. Acad. Sci. U.S.A. 105:
14330-14335.
Yabuno T. 1966. Biosystematic study of the
genus Echinochloa. – Jap. J. Bot. 19: 277-323.
Yadav SR. 210. Know your grass genera through
hand lens. – Shivaji University Press, Kolhapur.
Yakovlev MS. 1950. Endosperm and embryo
structure of grasses as a taxonomic feature. – Trudy Komarov Bot. Inst. Acad.
Sci. USSR, ser. 7, Morfol. Anat. Rast. 1: 121-218. [In Russian]
Yamaguchi T, Tsukaya H. 2010. Evolutionary
and developmental studies of unifacial leaves in monocots: Juncus as a
model system. – J. Plant Res. 123: 35-41.
Yamaguchi T, Yano S, Tsukaya H. 2010. Genetic
framework for flattened leaf blade formation in unifacial leaves of Juncus
prismatocarpus. – Plant Cell 22: 2141-2155.
Yaneshita M, Sasakuma T, Ogihara Y. 1993.
Phylogenetic relationships of turfgrasses as revealed by restriction fragment
analysis of chloroplast DNA. – Theor. Appl. Gen. 87: 129-135.
Yang G-Y, Chao C-S. 1994. A revision of the
genus Arundinaria Michaux in China. – J. Bamboo Res. 13: 1-23.
Yang HM, Zhang YX, Yang JB, Li D-Z. 2013. The
monophyly of Chimonocalamus and conflicting gene trees in
Arundinarieae (Poaceae: Bambusoideae)
inferred from four plastid and two nuclear markers. – Mol. Phylogen. Evol.
68: 340-356.
Yang H-Q, Peng S, Li D-Z. 2007. Generic
delimitations of Schizostachyum and its allies (Gramineae:
Bambusoideae) inferred from GBSSI and trnL-F sequence phylogenies. –
Taxon 56: 45-54.
Yang H-Q, Wang H, Li D-Z. 2008. Comparative
morphology of the foliage leaf epidermis, with emphasis on papillae characters,
in key taxa of woody bamboos of the Asian tropics (Poaceae: Bambusoideae). – Bot. J. Linn.
Soc. 156: 411-423.
Yang H-Q, Yang J-B, Peng Z-H, Gao J, Yang
Y-M, Peng S, Li D-Z. 2008. A molecular phylogenetic and fruit evolutionary
analysis of the major groups of the paleotropical woody bamboos (Gramineae:
Bambusoideae) based on nuclear ITS, GBSSI gene and plastid trnL-F DNA
sequences. – Mol. Phylogen. Evol. 48: 809-824.
Yang J-B, Yang H-Q, Li D-Z, Wong K-M; Yang
Y-M. 2010. Phylogeny of Bambusa and its allies (Poaceae: Bambusoideae) inferred from
nuclear GBSSI gene and plastid psbA-trnH,
rpl32-trnL and rps16 intron DNA sequences. – Taxon
59: 1102-1110.
Yano O, Hoshino T. 2005. Molecular phylogeny
and choromosomal evolution of Japanese Schoenoplectus (Cyperaceae), based on ITS and ETS 1f
sequences. – Acta Phytotaxon. Geobot. 56: 177-189.
Yano O, Katsuyama T, Tsubota H, Hoshino T.
2004. Molecular phylogeny of Japanese Eleocharis (Cyperaceae) based on ITS sequence data,
and chromosomal evolution. – J. Plant Res. 117: 409-419.
Yano O, Ikeda H, Watson MF, Rajbhandari KR,
Jin X-F, Hoshino T, Muasya AM, Ohba H. 2012. Phylogenetic position of the
Himalayan genus Erioscirpus (Cyperaceae) inferred from DNA sequence
data. – Bot. J. Linn. Soc. 170: 1-11.
Yano O, Ikeda H, Jin X-F, Hoshino T. 2014.
Phylogeny and chromosomal variations in East Asian Carex,
Siderostictae group (Cyperaceae), based on DNA sequences and
cytological data. – J. Plant Res. 127: 99-107.
Yates HO. 1966a. Revision of grasses
traditionally referred to Uniola I. Uniola and
Leptochloöpsis. – Southw. Natur. 11: 372-394.
Yates HO. 1966b. Morphology and cytology of
Uniola (Gramineae). – Southw. Natur. 11: 145-189.
Yavin ZO. 1969. Cytotaxonomy of the genus
Bromus of Palestine. – Israel J. Bot. 18: 195-216.
Yen AC, Olmstead RG. 2000a. Phylogenetic
analysis of Carex (Cyperaceae): generic and subgeneric
relationships based on chloroplast DNA. – In: Wilson KL, Morrison DA (eds),
Monocots: systematics and evolution, Proceedings of the 2nd
International Conference on the Comparative Biology of Monocotyledons, Sydney,
Australia, Vol. 1, CSIRO Publ., Melbourne, pp. 602-609.
Yen AC, Olmstead RG. 2000b. Molecular
systematics of Cyperaceae tribe
Cariceae based on two chloroplast DNA regions: ndhF and trnL
intron-intergenic spacer. – Syst. Bot. 25: 479-494.
Yen C, Yang J-L. 1990. Kengyilia
gobicola, a new taxon from west China. – Can. J. Bot. 68: 1894-1897.
Yen C, Yang J-L, Baum BR. 2005.
Douglasdeweya: a new genus, with a new species and a new combination
(Triticeae: Poaceae). – Can. J. Bot.
83: 413-419.
Yeo RR. 1964. Life history of the common
cattail. – Weeds 12: 284-288.
Yeoh H-H, Watson L. 1986. Systematic
variation in amino acid compositions of grass caryopses. – Phytochemistry 20:
1041-1051.
Yeoh H-H, Watson L. 1987. Taxonomic patterns
in protein amino acid profiles of grass leaves and caryopses. – In:
Soderstrom TR, Hilu KW, Campbell CS, Barkworth ME (eds), Grass systematics and
evolution, Smithsonian Institution Press, Washington, D.C., pp. 88-96.
Yi T-P. 1982. New taxa of bamboos from
Sichuan. – Bull. Bot. Res. 2: 99-111.
Yi T-P. 1985a. Classification and
distribution of the food bamboos of the giant panda I. – J. Bamboo Res. 4:
11-27.
Yi T-P. 1985b. Classification and
distribution of the food bamboos of the giant panda II. – J. Bamboo Res. 4:
28-45.
Yi T-P. 1992. A new genus of Chinese bamboo
– Menstruocalamus. – J. Bamboo Res. 11: 38-41.
You R, Jensen WA. 1985. Ultrastructural
observations of the mature megagametophyte and fertilization in wheat
(Triticum aestivum). – Can. J. Bot. 63: 163-178.
Yuan Z, Gao S, Xue D-W, Luo D, Li L-T, Ding
S-Y, Yao X, Wilson ZA, Qian Q, Zhang D-B. 2009. RETARDED PALEA1
controls palea development and floral zygomorphy in rice. – Plant Physiol.
149: 235-244.
Zaitchik BF, Leroux LG, Kellogg EA. 2000.
Development of male flowers in Zizania aquatica (North America
wild-rice; Gramineae). – Intern. J. Plant Sci. 161: 345-351.
Zanis MJ. 2007. Grass spikelet genetics and
duplicate gene comparisons. – Intern. J. Plant Sci. 168: 93-110.
Zeng C-X, Zhang Y-X, Triplett JK, Yang J-B,
Li D-Z. 2010. Large multi-locus plastid phylogeny of the tribe Arundinarieae
(Poaceae: Bambusoideae) reveals ten
major lineages and low rate of molecular divergence. – Mol. Phylogen. Evol.
56: 821-839.
Zhang C, Fan X, Yu H-Q, Zhang H-Q, Wang X-L,
Zhou Y-H. 2009. Phylogenetic analysis of questionable tetraploid species in
Roegneria and Pseudoroegneria (Poaceae: Triticeae) inferred from a gene
encoding plastid acetyl-CoA carboxylase. – Biochem. Syst. Ecol. 37:
412-420.
Zhang D-X, Nagabhyru P, Schardl CL. 2009.
Regulation of a chemical defense against herbivory produced by symbiotic fungi
in grass plants. – Plant Physiol. 150: 1072-1082.
Zhang G-Z. 1985. Studies on chromosome number
of some sympodial bamboos. – Bamboo Res. 2: 1-7.
Zhang G-Z. 1987. Studies on the chromosome
numbers of some bamboo species with clump rhizomes. – In: Rao AN, Dhanarajan
G, Sastry CB (eds), Recent research on bamboos, the Chinese Academy of
Forestry, People’s Republic of China and International Development Research
Centre, Canada, pp. 175-178.
Zhang H-Q, Yang R-W, Yang C-R, Huang Y, Fan
X, Sha L-N, Kang H-Y, Wang Y, Zhou Y-H. 2014. What became of Hystrix?
– J. Syst. Evol. 52: 712-715.
Zhang L-L, Lin R-S, Xia N-H. 2008.
Schizostachyum diaoluoshanense sp. nov. (Poaceae-Bambusoideae) from Hainan, China.
– Nord. J. Bot. 26: 21-24.
Zhang P, Friebe B, Gill BS. 2002. Variation
in the distribution of a genome-specific DNA sequence on chromosomes reveals
evolutionary relationships in the Triticum and Aegilops
complex. – Plant Syst. Evol. 235: 169-179.
Zhang S-H, Second G. 1989. Phylogenetic
analysis of the tribe Oryzeae. Total chloroplast DNA restriction fragment
analysis. A preliminary report. – Rice Genet. Newsl. 6: 76-80.
Zhang S-R. 2001. A preliminary revision of
the supraspecific classification of Kobresia Willd. (Cyperaceae). – Bot. J. Linn. Soc. 135:
289-294.
Zhang W-P. 1992. The classification of
Bambusoideae (Poaceae) in China. – J.
Amer. Bamboo Soc. 9: 25-42.
Zhang W-P. 1996. Phylogeny and classification
of the bamboos (Poaceae: Bambusoideae)
based on molecular and morphological data. – Ph.D. diss., Iowa State
University, Ames, Iowa.
Zhang W-P. 2000. Phylogeny of the grass
family (Poaceae) from rpl16
intron sequence data. – Mol. Phylogen. Evol. 15: 135-146.
Zhang W-P, Clark LG. 2000. Phylogeny and
classification of the Bambusoideae (Poaceae). – In: Jacobs SWL, Everett J
(eds), Grasses: systematics and evolution, Proceedings of the 2nd
International Conference on the Comparative Biology of Monocotyledons, 1998,
Sydney, Australia, Vol. 2, CSIRO Publ., Melbourne, pp. 35-42.
Zhang W, Qu LJ, Gu H, Gao W, Liu M, Chen J,
Chen Z. 2002. Studies on the origin and evolution of tetraploid wheats based on
the internal transcribed spacer (ITS) sequences of nuclear ribosomal DNA. –
Theor. Appl. Gen. 104: 1099-1106.
Zhang X, Wilson KL, Bruhl JJ. 2004. Sympodial
structure of spikelets in the tribe Schoeneae (Cyperaceae). – Amer. J. Bot. 91:
24-36.
Zhang X, Marchant A, Wilson KL, Bruhl JJ.
2004. Phylogenetic relationships of Carpha and its relatives
(Schoeneae, Cyperaceae) inferred from
chloroplast trnL intron and trnL-trnF intergenic
spacer sequences. – Mol. Phylogen. Evol. 31: 647-657.
Zhang X, Wilson KL, Bruhl JJ. 2006. Species
limits in Carpha (Schoeneae, Cyperaceae) based on phenetic analyses.
– Aust. Syst. Bot. 19: 437-465.
Zhang X, Bruhl JJ, Wilson KL, Marchant A.
2007. Phylogeny of Carpha and related genera (Schoeneae, Cyperaceae) inferred from morphological
and molecular data. – Aust. Syst. Bot. 20: 93-106.
Zhang X-Z, Zeng C-X, Ma P-F, Haevermans T,
Zhang Y-X, Zhang L-N, Guo Z-H, Li D-Z. 2016. Multi-locus plastid phylogenetic
biogeography supports the Asian hypothesis of the temperate woody bamboos (Poaceae: Bambusoideae). – Mol. Phylogen.
Evol. 96: 118-129.
Zhang Y-J, Ma P-F, Li D-Z. 2011.
High-throughput sequencing of six bamboo chloroplast genomes: phylogenetic
implications for temperate woody bamboos (Poaceae: Bambusoideae). – PloS One 6:
e20596.
Zhang Y-X, Zeng C-X, Li D-Z. 2012. Complex
evolution in Arundinarieae (Poaceae:
Bambusoideae): incongruence betwen plastid and nuclear GBSSI gene phylogenies.
– Mol. Phylogen. Evol. 63: 777-797.
Zhang Y-X, Ma P-F, Havermans T, Vorontsova
MS, Zhang T, Nanjarisoa OP, Li D-Z. 2017. In search of the phylogenetic
affinity of the temperate woody bamboos from Madagascar, with description of a
new species (Bambusoideae, Poaceae). –
J. Syst. Evol. 55: 453-465.
Zhao L, Zhang N, Ma PF, Liu Q, Li DQ, Guo ZH.
2013. Phylogenomic analyses of nuclear genes reveal the evolutionary
relationships within the BEP clade and the evidence of positive selection in Poaceae. – PLoS ONE 8: e64642.
Zhou M-B, Lu J-J, Zhong H, Liu X-M, Tang D-Q.
2010. Distribution and diversity of PIF-like transposable elements in the
Bambusoideae subfamily. – Plant Sci. 179: 257-266.
Zhou M-Y, Zhang Y-X, Haevermans T, Li D-Z.
2017. Towards a complete generic-level plastid phylogeny of the paleotropical
woody bamboos (Poaceae: Bambusoideae).
– Taxon 66: 539-553.
Zhou Y-Y, Jin X-F. 2014. Notes on
Carex (Cyperaceae) from
China: three new species. – Phytotaxa 164: 133-140.
Zhu T, Xu P-Z, Liu J-P, Peng S, Mo X-C, Gao
L-Z. 2014. Phylogenetic relationships and genome divergence among the AA-genome
species of the genus Oryza as revealed by 53 nuclear genes and 16
intergenic regions. – Molec. Phylogen. Evol. 70: 348-361.
Zhuge Q, Diong Y-L, Xu C, Zou H-Y, Huang M-R,
Wang M-X. 2004. Phylogeny of the genus Arundinaria based on nucleotide
sequences. – Acta Gen. Sin. 31: 349-356.
Žukovsky PM. 1928. A critical-systematic
survey of the species of the genus Aegilops L. – Bull. Appl. Bot.
Plant Breed. 18: 417-609.
Zimmermann MH, Tomlinson PB. 1968. Vascular
construction and development in the aerial stem of Prionium (Juncaceae). – Amer. J. Bot. 55:
1100-1109.
Zimmermann T, Bocksberger G, Brüggemann W,
Berberich T. 2013. Phylogenetic relationship and molecular taxonomy of African
grasses of the genus Panicum inferred from four chloroplast
DNA-barcodes and nuclear gene sequences. – J. Plant Res. 126: 363-371.
Zizka G, Horres R, Nelson CE, Weising K.
1999. Revision of the genus Fascicularia Mez (Bromeliaceae). – Bot. J. Linn. Soc.
129: 315-332.
Zizka G, Trumpler K, Zöllner O. 2002.
Revision of the genus Ochagavia (Bromeliaceae, Bromelioideae). –
Willdenowia 32: 331-350.
Zöllner O, Oyanedel E. 1991. A Chilean
bromeliad genus of the temperate zone. – J. Bromeliad Soc. 41: 169-171.
Zona S. 2001. Starchy pollen in commelinoid
monocots. – Ann. Bot. 87: 109-116.
Zotov VD. 1971. Simplicia T. Kirk
(Gramineae). – New Zealand J. Bot. 9: 539-544.
Zou X-H, Zhang F-M, Zhang J-G, Zang L-L, Tang
L, Wang J, Sang T, Ge S. 2008. Analysis of 142 genes resolves the rapid
diversification of the rice genus. – Genome Biol. 9: R49.
http://genomebiology.com/2008/9/3/R49
Zuloaga FO. 1987a. Systematics of New World
species of Panicum (Poaceae:
Paniceae). – In: Soderstrom T, Hilu K, Campbell C, Barkworth M (eds), Grass
systematics and evolution, Smithsonian Institution Press, Washington, D.C., pp.
287-306.
Zuloaga FO. 1987b. A revision of
Panicum subgenus Panicum section Rudgeana (Poaceae: Paniceae). – Ann. Missouri Bot.
Gard. 74: 463-478.
Zuloaga FO. 1989. El género Panicum en la
República Argentina III. – Darwiniana 29: 289-370.
Zuloaga FO, Morrone O. 1996. Revisión de las
especies americanas de Panicum subgénero Panicum sección
Panicum (Poaceae; Panicoideae:
Paniceae). – Ann. Missouri Bot. Gard. 83: 200-280.
Zuloaga FO, Morrone O. 2005. Revisión de las
especies de Paspalum para América del Sur austral (Argentina,
Bolívia, sur Del Brasil, Chile, Paraguay y Uruguay). – Monogr. Syst. Bot.
Missouri Bot. Gard. 102: 1-297.
Zuloaga FO, Sendulsky T. 1988. A revision of
Panicum Subg. Phanopyrum Sect. Stolonifera (Poaceae: Paniceae). – Ann. Missouri Bot.
Gard. 75: 420-455.
Zuloaga FO, Soderstrom TR. 1985.
Classification of the outlying species of Panicum (Poaceae: Paniceae). – Smithsonian Contr.
Bot. 59: 1-63.
Zuloaga FO, Saénz AA, Morrone O. 1986. El
género Panicum (Poaceae:
Paniceae) sect. Cordovensia. – Darwiniana 27: 403-429.
Zuloaga FO, Morrone O, Saenz AA. 1987.
Estudio exomorfológico e histofoliar de las especies americanas del género
Acroceras (Poaceae: Paniceae).
– Darwiniana 28: 191-217.
Zuloaga FO, Morrone O, Dubcovsky J. 1989.
Exomorphological, anatomical, and cytological studies in Panicum
validum (Poaceae: panicoideae:
Paniceae): its systematic position within the genus. – Syst. Bot. 14:
220-230.
Zuloaga FO, Ellis RP, Morrone O. 1992. A
revision of Panicum subgenus Phanopyrum section Laxa
(Poaceae: Panicoideae: Paniceae). –
Ann. Missouri Bot. Gard. 79: 770-818.
Zuloaga FO, Ellis RP, Morrone O. 1993. A
revision of Panicum subg. Dichanthelium sect.
Dichanthelium (Poaceae:
Panicoideae: Paniceae) in Mesoamerica, the West Indies, and South America. –
Ann. Missouri Bot. Gard. 80: 119-190.
Zuloaga FO, Dubcovsky J, Morrone O. 1993.
Infrageneric phenetic relations in new world Panicum (Poaceae: Panicoideae: Paniceae): a
numerical analysis. – Can. J. Bot. 71: 1312-1327.
Zuloaga FO, Morrone O, Judziewicz EJ. 1993.
Endemic herbaceous bamboo genera of Cuba (Poaceae: Bambusoideae: Olyreae). – Ann.
Missouri Bot. Gard. 80: 846-861.
Zuloaga FO, Morrone O, Killeen T. 1993.
Gerritea, a new genus of Paniceae (Poaceae: Panicoideae) from South America.
– Novon 3: 213-219.
Zuloaga FO, Nicora EG, Rúgolo de Agrasar ZE,
Morrone O, Pensiero J, Cialdella AM. 1994. Catálogo de la familia Poaceae en la República Argentina. –
Monogr. Syst. Bot. Missouri Bot. Gard. 47: 1-178.
Zuloaga FO, Morrone O, Vega AS, Giussani LM.
1998. Revisión y análisis cladístico de Stenchisma (Poaceae: Panicoideae: Paniceae). – Ann.
Missouri Bot. Gard. 85: 631-656.
Zuloaga FO, Morrone O, Giussani LM. 2000. A
cladistic analysis of the Paniceae: a preliminary approach. – In: Jacobs SWL,
Everett J (eds), Grasses: systematics and evolution, Proceedings of the
2nd International Conference on the Comparative Biology of
Monocotyledons, 1998, Sydney, Australia, Vol. 2, CSIRO Publ., Melbourne, pp.
123-135.
Zuloaga FO, Morrone O, Davidse G, Filgueiras
TS, Peterson PM, Soreng RJ, Judziewicz EJ. 2003. Catalogue of New World grasses
(Poaceae) III. Subfamilies Panicoideae,
Aristidoideae, Arundinoideae, and Danthonioideae. – Contr. U.S. Natl. Herb.
46: 1-662.
Zuloaga FO, Pensiero J, Morrone O. 2004.
Systematics of Paspalum group Notata (Poaceae-Panicoideae-Paniceae). – Syst.
Bot. Monogr. 71: 1-75.
Zuloaga FO, Giussani LM, Morrone O. 2006. On
the taxonomic position of Panicum aristellum (Poaceae: Panicoideae: Paniceae). – Syst.
Bot. 31: 497-505.
Zuloaga FO, Giussani LM, Morrone O. 2007.
Hopia, a new genus segregated from Panicum (Poaceae: Panicoideae: Paniceae). – Taxon
56: 145-156.
Zuloaga FO, Morrone O, Davidse G, Pennington
SJ. 2007 [2008]. Classification and biogeography of Panicoideae (Poaceae) in the New World. – In: Columbus
JT, Friar EA, Hamilton CW, Porter JM, Prince LM, Simpson MG (eds), Moncots:
comparative biology and evolution – Poales, Rancho Santa Ana Botanic Garden,
Claremont, California. – Aliso 23: 503-529.
Zuloaga FO, Scataglini MA, Morrone O. 2010. A
phylogenetic evaluation of Panicum sects. Agrostoidea,
Megista, Prionitia and Tenera (Panicoideae, Poaceae): two new genera,
Stephostachys and Sorengia. – Taxon 59: 1535-1546.
Zuloaga FO, Morrone O, Scataglini MA. 2011.
Monograph of Trichanthecium, a new genus segregated from
Panicum (Poaceae, Paniceae)
based on morphological and molecular data. – Syst. Bot. Mongr. 94: 1-101.
Zuloaga FO, Salomón L, Scataglini MA. 2015.
Phylogeny of sections Clavelligerae and Pectinatae of
Panicum (Poaceae, Panicoideae,
Paniceae): establishment of the new subtribe Dichantheliinae and the genus
Adenochloa. – Plant Syst. Evol. 301: 1693-1711.
Zwickl DJ, Stein JC, Wing RA, Ware D,
Sanderson MJ. 2014. Disentangling methodological and biological sources of gene
tree discordance on Oryza (Poaceae) chromosome 3. – Syst. Biol. 63:
645-659.













