
Phylogeny (maximum likelihood bootstrap consensus tree) of Iridales based on DNA sequence data (Wurdack & Dorr 2009).
[Iridales+Commelinidae]
Iridanae Doweld, Tent. Syst. Plant. Vasc.: lvi. 23 Dec 2001
Fossils Paleoallium billgenseli from the latest Early Eocene of Washington State possesses both sessile bulbils, pedicellate flowers and a spathe and is provided with a bulb with roots. It may be a member of either Alliaceae or Amaryllidaceae or was at least closely allied to those lineages.
Habit Usually bisexual (rarely monoecious, andromonoecious, gynomonoecious, polygamomonoecious, dioecious, gynodioecious, or polygamodioecious), usually perennial herbs (sometimes biennial herbs, or evergreen or deciduous trees, shrubs, suffrutices or lianas). Often with bulb or corm rich in polysaccharides. Often xerophytic.
Vegetative anatomy Usually Arum type arbuscular mycorrhiza. Single- or multi-layered velamen often present. Phellogen absent. Secondary lateral growth usually absent (sometimes anomalous). Vessels present only in roots. Vessel elements with scalariform or simple perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial parenchyma? Sieve tube plastids P2c or P2cf types (rarely P2cs type). Nodes usually multilacunar with several leaf traces. Laticifers sometimes present. Silica bodies sometimes present. Mucilage cells and mucilage sacs frequent, with calciumoxalate as raphides, pseudo-raphides, styloids, cubes, crystal sand, or rhomboidal or acicular crystals. Epidermal cells often with crystals.
Trichomes Hairs usually absent (sometimes unicellular or multicellular, uniseriate or multiseriate, stellate or lepidote, rarely dendritic).
Leaves Alternate (spiral or distichous), simple, entire, often linear, usually bifacial (sometimes equitant), with revolute, supervolute, involute, conduplicate, plicate, flat or curved (sometimes explicative) ptyxis, sometimes differentiated into pseudopetiole and pseudolamina. Stipules absent; leaf sheath open or closed, often well developed (sometimes absent). Venation parallelodromous or pinnate-parallelodromous (sometimes acrodromous). Stomata anomocytic, paracytic, tricytic or tetracytic; neighbouring cells usually with oblique or non-oblique divisions. Cuticular wax crystalloids as parallel platelets (Convallaria type, resembling ‘electromagnetic field lines’) or unordered platelets, rodlets or filiform reticulate processes. Dimorphic hypodermal cells sometimes present. Mesophyll with mucilaginous idioblasts containing calciumoxalate raphides. Leaf margin usually entire (sometimes serrate).
Inflorescence Terminal, umbel-, raceme-, spike- or head-like, panicle, fascicle, corymb, or thyrsoid, often compound and consisting of bostrychoid or helicoid monochasial partial inflorescences, or racemes or spikes, sometimes subtended by membranous spathae; or flowers solitary. Floral prophylls (bracteoles) sometimes lateral, pairwise or absent.
Flowers Actinomorphic or zygomorphic. Hypogyny or epigyny (rarely half epigyny). Tepals (2–)3(–5)+(2–)3(–5) (rarely 7+7), usually petaloid (sometimes sepaloid), usually more or less connate into infundibuliform or tubular perianth (rarely free). Septal nectaries present, usually infralocular, or absent. Disc usually absent (sometimes nectar-secreting).
Androecium Stamens (2–)3(–6)+(2–)3(–6) (sometimes one or three, rarely two, five, nine to more than 18). Filaments free or more or less connate, often more or less adnate to tepals (rarely to style). Anthers usually dorsifixed or basifixed (sometimes centrifixed), versatile or non-versatile, tetrasporangiate, usually introrse (sometimes latrorse, rarely extrorse), usually longicidal (dehiscing by longitudinal slits; rarely poricidal, dehiscing by apical pore). Tapetum usually secretory (rarely amoeboid-periplasmodial), with binucleate to quadrinucleate cells. Staminodia three or absent (female flowers often with staminodia). Pollinaria present in most Orchidaceae, consisting of pollinia and translator.
Pollen grains Microsporogenesis usually successive (sometimes simultaneous). Pollen grains usually monosulcate (sometimes monosulcoidate or inaperturate, rarely disulcate, disulculate, trisulcate, trichotomosulcate, zonosulcate, zonosulculate, dizonosulculate, spiraperturate, mono- to tetraporate, ulcerate, or foraminate), usually shed as monads (rarely tetrads), bicellular at dispersal. Exine tectate or semitectate (rarely intectate), with columellate infratectum, reticulate, rugulate, foveolate, echinate, verrucate, spinulate, or smooth (sometimes absent).
Gynoecium Pistil composed of (two or) three (to seven) connate carpels. Ovary superior or inferior (rarely semi-inferior), (unilocular to) trilocular (to quinquelocular). Style single, simple, with stylar canal (rarely trilobate). Stigma capitate, punctate or slightly to deeply trilobate, papillate or non-papillate, Dry or Wet type. Pistillodium usually absent (male flowers often with pistillodium).
Ovules Placentation usually axile (sometimes parietal, rarely basal or apical). Ovules one to more than 50 per carpel (sometimes hundreds to several thousands, rarely to four million per ovary), anatropous or campylotropous (sometimes semicampylotropous, hemianatropous or orthotropous), ascending or horizontal, apotropous or epitropous, usually bitegmic (sometimes unitegmic, rarely ategmic), usually crassinucellar (sometimes tenuinucellar, sometimes pseudocrassinucellar or pseudotenuinucellar). Micropyle endostomal or bistomal (rarely exostomal). Funicular obturator sometimes present. Parietal cell formed from archesporial cell or absent. Periclinal cell divisions often occurring in megasporangial epidermis. Nucellar cap sometimes present. Megagametophyte usually monosporous, Polygonum type (sometimes disporous, Allium type, rarely tetrasporous, Scilla, Adoxa, Fritillaria, or Drusa type). Synergids sometimes with a filiform apparatus. Antipodal cells usually persistent, sometimes proliferating. Endosperm development usually ab initio helobial (sometimes nuclear). Endosperm haustoria chalazal or absent. Embryogenesis asterad or onagrad (sometimes caryophyllad, chenopodiad or solanad).
Fruit Usually a loculicidal capsule (rarely septicidal, irregularly dehiscent or indehiscent) or a berry (sometimes a nut, rarely a drupe, a samara or a schizocarp).
Seeds Aril usually absent. Strophiolus rarely present. Caruncular, chalazal or raphal elaiosome occasionally present. Seed coat usually exotestal, sometimes fleshy or winged, sometimes with caruncular elaiosome at chalazal end. Exotesta usually with thin phytomelan layer on epidermal cell walls. Mesotesta and endotesta often collapsed. Tegmen usually collapsed, sometimes well developed. Perisperm usually not developed (sometimes well developed, with lipids and proteins). Endosperm copious, with oils, aleurone or hemicellulose (sometimes rich in water and/or starch), sometimes thin-walled. Embryo straight or slightly curved, little differentiated, with or without chlorophyll. Cotyledon one, sometimes photosynthesizing. Cotyledon hyperphyll elongate, dorsiventrally flattened and assimilating, or compact and not assimilating. Hypocotyl internode short or absent. Coleoptile absent. Radicula well developed, contractile. Germination phanerocotylar or cryptocotylar.
Cytology n = 2–23
DNA Mitochondrial genes rpl2 and sdh3 lost.
Phytochemistry Flavonols (kaempferol, quercetin, isorhamnetin), flavone-C-glycosides, lanaroflavone (biflavonoid), isoflavones, amentoflavone (biflavone), homoisoflavanones, cyanidin, chalcones, 6-hydroxyapigenin methyl ethers, tannins, phenols, norbelladine alkaloids (toxic tyrosine derivatives, e.g. crinine, belladine, galanthanine, haemanthanine, homolyrine, lycorenine, lycorine, pancratiostatine, and tazettine), polyhydroxyalkaloids, cholestane glycosides (cardiotoxic bufodienolides, cardenolide glycosides and spirostanol glycosides), phenolic glycosides, steroidal saponins and sapogenins (e.g. agapanthagenin, yuccagenin, diosgenin, hecogenin, neotigogenin, sarsasapogenin, smilagenin, tigogenin, yamogenin, aigogenin, markogenin diglycoside, timosaponins, anemarsaponins, gitogenin, aspidirin), cyanogenic compounds (e.g. cyanogenic glycosides), anthrones (e.g. anthrone-C-glycoside [aloin, barbaloin] in leaves), tetrahydroanthracenones (chrysophanol, 10,7’-bichrysophanol, knipholone, asphodeline and other lipophilic anthranoid aglucones), naphthoquinones (imbricatonol, plumbagin, stypandrone), quinonoid pigments, polyacetate-derived arthroquinones, nepodin, dianellidin, stypandrol, dianellidone, magniferin (glycosylic xanthone), chromones, phenylpyrones, phenolic amines, acetidine carbonic acid, chelidonic acid, ascorbic acid, tuliposides, allyl sulfides, allyl disulfides, propyl sulfides, vinyl disulfides, alliin, propionaldehyde, propionthiol, hydroxycinnamic acid, eicosanyl arachidate, crocein, phytosterols, non-protein amino acids (tricine etc.), meta-carboxysubstituted aromatic amino- and γ-glutamic peptides, acaroid resins, and stem fructans present. Ellagic acid not found.
Systematics Iridales are sister-group to Commelinidae. Orchidaceae seem to be sister to the remaining Iridales. Stevens (2001 onwards) has suggested potential synapomorphies for some of the main clades in Iridales.
The clade [[Boryaceae-Hypoxidaceae]+[Ixioliriaceae+Tecophilaeaceae]+[Doryanthaceae-Ruscaceae]] is characterized by, e.g.: cuticular wax crystalloids as parallel platelets; tepals often connate; stamens often inserted on a perigonal tube; seed with well developed exotesta and phytomelan layer; and presence of stem fructans.
The first of these main clades is [Boryaceae+[Blandfordiaceae+[Lanariaceae+[Asteliaceae+ Hypoxidaceae]]]], which has the following potential synapomorphies: septal nectaries external; hypostase often present in ovule; megagametophyte often with chalazal constriction; and antipodal cells usually persistent. The clade [Blandfordiaceae+[Lanariaceae+[Asteliaceae+Hypoxidaceae]]] is characterized by ovule usually with parietal cell and nucellar cap. The [Lanariaceae+ [Asteliaceae+Hypoxidaceae]] has: multicellular and often branched hairs; paracytic stomata; pseudolamina with distinct midrib; often internal septal nectaries; and bistomal micropyle. Finally, potential synapomorphies of the clade [Asteliaceae+Hypoxidaceae] include presence of mucilage canals; thin-walled endosperm; cotyledon not photosynthesizing; an elongate ligule; and presence of flavonols (Stevens 2001 onwards).
Ixioliriaceae and Tecophilaeaceae have the following characteristics in common (Stevens 2001 onwards): cormose stem; spiral leaves; outer tepals mucronate to aristate; short perigonal tube; and stamens arising from the mouth of the perianth tube.
The second main clade is [Iridaceae+[Xeronemataceae-Ruscaceae]], and this has the potential synapomorphies (Stevens 2001 onwards): often absence (loss) of Arabidopsis type telomeres; human-type telomere ([TTAGGG]n type telomere) dominating; often glucomannans as reserve carbohydrates; and often secondary growth (from meristem producing tissue inwards; separate vascular bundles in this tissue surrounded by ground parenchyma). The clade [Xeronemataceae+[Hemerocallidaceae-Ruscaceae]] has lost the mitochondrial gene rpl2. Characteristic features of the clade [[Hemerocallidaceae+[Xanthorrhoeaceae+Asphodelaceae]]+ [Alliaceae-Ruscaceae]] include: pedicels articulated; septal nectaries infralocular; hypogyny; microsporogenesis successive; and parietal tissue two or three cell layers thick. The clade [Hemerocallidaceae+[Xanthorrhoeaceae+Asphodelaceae]] has: styloids; cymose inflorescence branches; outer integument more than three cell layers thick; hypostase; non-photosynthesizing cotyledon; and anthraquinones. [Xanthorrhoeaceae+Asphodelaceae] is characterized by: secondary thickening and lateral growth sometimes present; stamens not adnate to tepals; and angular seeds.
The monophyletic group [[Agapanthaceae+[Amaryllidaceae+Alliaceae]]+[Aphyllanthaceae-Ruscaceae]] has the potential synapomorphy of successive microsporogenesis. The [Agapanthaceae+[Amaryllidaceae+Alliaceae]] clade has the common features (Stevens 2001 onwards): presence of laticifers and mucilage cells; leaves distichous; inflorescence scapose, umbel-like cymose, with scarious spatha, with two (or more, external) inflorescence bracts; pedicels not articulated; stigma usually Dry type; parietal tissue absent; presence of flavonols and saponins; and lectins binding mannose (in Agapanthus?).
The clade [[[Aphyllanthaceae+[Themidaceae+Hyacinthaceae]+[Anemarrhenaceae-Behniaceae]]+[Laxmanniaceae+[Asparagaceae+Ruscaceae]]] has racemose inflorescence. Themidaceae and Hyacinthaceae share the features: spiral leaves; inflorescence scapose, with bracteate pedicels; presence of raphides in carpel wall; anatropous ovules; non-photosyntesizing cotyledon; and presence of steroidal saponins. In [Agavaceae+[Behniaceae+[Anthericaceae+ Herreriaceae]]] are nucellar cap and hypostase present, whereas articulated pedicels, capsular fruit, a thick-walled, pitted and hemicellulosic helobial endosperm, and presence of steroidal saponins are characteristic features of the clade [Laxmanniaceae+[Asparagaceae+Ruscaceae]].

|
Phylogeny (maximum likelihood bootstrap consensus tree) of Iridales based on DNA sequence data (Wurdack & Dorr 2009). |
AGAPANTHACEAE F. Voigt |
( Back to Iridales ) |
Genera/species 1/c 7
Distribution Southern and southeastern South Africa.
Fossils Unknown.
Habit Bisexual, perennial herbs. Rhizome tuberous.
Vegetative anatomy Roots fleshy, contractile, with multiple velamen. Phellogen absent. Secondary lateral growth absent. Vessels present in roots. Vessel elements usually with scalariform (sometimes simple) perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial parenchyma? Sieve tube plastids P2c type, with cuneate protein crystals. Nodes? Laticifers absent? Calciumoxalate raphides present.
Trichomes Hairs absent.
Leaves Alternate (distichous), simple, entire, linear, with flat ptyxis. Stipules absent; leaf sheath well developed. Venation parallelodromous. Stomata anomocytic. Cuticular wax crystalloids? Mesophyll with calciumoxalate raphides. Leaf margin entire.
Inflorescence Terminal, umbel-like, consisting of condensed helicoid monochasia, subtended by two membranous caducous bracts (spathae) connate on one side, which enclosed floral buds. Peduncle (scape) long and stout, terete or somewhat compressed. Floral bracts and prophylls filiform, persistent.
Flowers Zygomorphic, large. Pedicel not articulated. Hypogyny. Tepals 3+3, petaloid, fleshy, with abaxial median ridge and adaxial median furrow, more or less connate at base. Septal nectaries infralocular. Disc absent.
Androecium Stamens 3+3. Filaments free from each other, adnate at base to tepals. Anthers dorsifixed, versatile, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory, with binucleate cells. Staminodia absent.
Pollen grains Microsporogenesis successive. Pollen grains monosulcate, shed as monads, bicellular at dispersal. Exine semitectate, with columellate? infratectum, reticulate.
Gynoecium Pistil composed of three connate carpels. Ovary superior, trilocular. Style single, simple, narrow, curved, with stylar canal. Stigma small, Dry type. Pistillodium absent.
Ovules Placentation axile. Ovules numerous per carpel, semi-campylotropous, apotropous, bitegmic, crassinucellar. Micropyle ?-stomal. Outer integument ? cell layers thick. Inner integument ? cell layers thick. Parietal cell formed from archesporial cell. Megagametophyte monosporous, Polygonum type. Endosperm development ab initio nuclear. Endosperm haustoria? Embryogenesis onagrad.
Fruit A loculicidal capsule with persistent style.
Seeds Aril absent. Testa winged at funicular end. Seed coat exotestal. Exotesta with thin phytomelan layer on epidermal cell walls. Mesotesta, endotesta and tegmen collapsed. Perisperm not developed. Endosperm copious, with oil, aleuronic, starch and hemicellulose. Embryo large, well developed, without chlorophyll. Cotyledon one. Germination cryptocotylar.
Cytology n = (14) 15 (16) – Chromosomes 4–9 µm long.
DNA The mitochondrial gene rpl2 is absent.
Phytochemistry Insufficiently known. Steroidal saponins (e.g. agapanthagenin and yuccagenin) present. Flavonols, allyl sulphides, alliin etc. not found.
Use Ornamental plants.
Systematics Agapanthus (c 7; A. africanus, A. campanulatus, A. caulescens, A. coddii, A. inapertus, A. praecox, A. walshii; southern and southeastern South Africa from Western Cape northwards to just south of Limpopo River, southern Mozambique).
Agapanthus is sister to [Alliaceae+Amaryllidaceae].
AGAVACEAE Dumort. |
( Back to Iridales ) |
Funkiaceae Horan., Prim. Lin. Syst. Nat.: 52. 2 Nov 1834 [’Funkiaceae s. Hemerocallideae’], nom. illeg.; Yuccaceae J. Agardh, Theoria Syst. Plant.: 8. Apr-Sep 1858; Agavales Hutch., Fam. Fl. Pl., Monocot. 2: 14, 149. 20 Jul 1934; Hesperocallidaceae Traub in Plant Life 28: 131. 22 Feb 1972 [’Hesperocallaceae’]; Hostaceae B. Mathew in Kew Bull. 43: 302. Jun 1988; Chlorogalaceae (Baker) Doweld et Reveal in Reveal in Bot. Rev. (Lancaster) 71: 53. 20 Mai 2005
Genera/species 12/305–320
Distribution East Asia, Central and southwestern North America and southwards to tropical and subtropical South America and the West Indies, with their largest diversity in Mexico.
Fossils Protoyucca shadishii, resembling extant Yucca, is represented by fossilized stems from the mid-Miocene of Nevada.
Habit Usually bisexual (rarely andromonoecious, gynomonoecious or dioecious), usually perennial herbs, sometimes giant herbs (Agave, Furcraea, etc.; some species of Yucca are arborescent). Rhizome often tuberous; in Hesperocallis a bulb with tunica and onion-like smell. Many species are xerophytic.
Vegetative anatomy Crassulacean acid metabolism (CAM) frequent (evolved three times [Hesperaloe, Yucca, Agave] from C3 ancestors). Roots fibrous or fleshy, in Agave with multilayered velamen; root endodermis in Yucca thick. Phellogen? Primary vascular tissue as scattered bundles (atactostele). Secondary lateral growth usually absent (Agave, Furcraea and Yucca have anomalous secondary lateral growth). Vessels present in roots. Vessel elements with scalariform or simple perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays? Axial parenchyma? Sieve tube plastids P2c type, with cuneate protein crystals. Nodes multilacunar with ? leaf traces. Mucilage cells abundant. Silica bodies absent. Calciumoxalate as pseudo-raphides (in Agave hexagonal in cross-section), raphides, styloids, and prismatic or acicular crystals.
Trichomes Hairs usually absent (sometimes unicellular or multicellular, uniseriate).
Leaves Alternate (spiral), simple, entire, often linear, sometimes succulent (in Hosta sometimes differentiated into pseudopetiole and pseudolamina), with ? ptyxis. Stipules absent; leaf sheath well developed. Adaxial vascular bundles inverted. Venation parallelodromous (in Hosta sometimes pinnate-parallelodromous); primary veins in Hosta usually distinct, connected by transversal secondary veins. Stomata anomocytic, paracytic, tricytic or tetracytic, sunken, in Agave, Beschorneria, Furcraea, ‘Prochnyanthes’, and Yucca surrounded by complex spaces. Cuticle in Agave, Beschorneria, Furcraea, ’Prochnyanthes’, and Yucca thick. Cuticular wax crystalloids usually as parallel platelets (Convallaria type; in Hosta as non-orientated tubuli, chemically dominated by nonacosan-10-ol). Mesophyll with calciumoxalate as raphides or single prismatic crystals, without mucilaginous idioblasts. Bundles of strongly lignified fibres present near leaf surface; particularly long and strong fibres present in species of Agave, Hesperaloe and Yucca. Vascular bundles in Agave and Yucca with acicular calciumoxalate crystals (sometimes with styloids or suberized cells containing pseudo-raphides). Leaf margin usually entire (sometimes serrate, with tiny translucent teeth, in Agave and Furcraea with large hard teeth), in some species filiferous and separated by layers of lignified cells and abscission cells. Leaf apex often acute (in Agave, Hesperaloe and Yucca with a hard sclerenchymatous terminal spine).
Inflorescence Terminal or axillary, panicle, raceme- or spike-like, with one- to many-flowered partial inflorescences. Bracts caducous or persistent.
Flowers Actinomorphic or zygomorphic, usually large. Pedicel usually not articulated and without pericladium (in Chlorogalum, Hesperocallis and Schoenolirion articulated). Hypogyny to epigyny. Tepals 3+3 (median outer tepal adaxial), petaloid, caducous or marcescent, usually connate into a tube, often connate at base (sometimes free). Septal nectaries present or absent. Disc absent.
Androecium Stamens 3+3 (sometimes three fertile and three staminodial). Filaments narrow to wide and thick, free from each other, usually adnate to tepals (epitepalous) (filaments in some species of Hosta inserted on ovary; filaments in some species of Yucca hairy). Anthers dorsifixed, versatile, tetrasporangiate, usually introrse, longicidal (dehiscing by longitudinal slits); connective in Hosta cucullate and widening around filament apex (filaments inserted in a pit on connective). Tapetum secretory, with binucleate to quadrinucleate cells. Staminodia three or absent.
Pollen grains Microsporogenesis successive. Pollen grains usually monosulcate (rarely disulculate), usually shed as monads (rarely tetrads), bicellular at dispersal. Exine tectate or semitectate, with ? infratectum, usually reticulate (in Hosta usually rugulate or verrucate).
Gynoecium Pistil composed of three connate carpels. Ovary superior to inferior, trilocular. Style usually single, simple (stylodia in most species of Yucca three, free), usually narrow (in Beschorneria, Furcraea and Yucca thick; in Hesperocallis persistent; in Camassia with three canals). Stigma capitate, punctate or trilobate (stigmas in most species of Yucca three), papillate, Dry or Wet type. Pistillodium absent.
Ovules Placentation axile. Ovules six to more than 50 per carpel, anatropous, bitegmic, usually crassinucellar (sometimes tenuinucellar). Micropyle ?-stomal. Outer integument (four to) nine to 14 cell layers thick. Inner integument two cell layers thick. Obturator present in Beschorneria. Parietal cell formed from archesporial cell (also in Hosta and Hesperocallis). Hypostase present (also in Hosta). Parietal tissue one or two cell layers thick or absent. Nucellar cap present, sometimes two cell layers thick. Megagametophyte monosporous, Polygonum type (micropylar megaspore cell dividing in Furcraea). Synergids sometimes with a filiform apparatus. Antipodal cells persistent. Chalazal part of megagametophyte possibly with haustorial function. Endosperm development usually helobial (also in Hesperocallis and Hosta; in Camassia and Furcraea nuclear). Endosperm haustoria absent. Embryogenesis onagrad or caryophyllad (Yucca).
Fruit Usually a loculicidal capsule (in Yucca a septicidal and/or loculicidal capsule, a dry indehiscent fruit or a berry).
Seeds Aril absent. Seeds usually flat, sometimes winged. Seed coat testal. Exotesta with a thin layer of phytomelan on epidermal cell walls. Mesotesta and endotesta present in, e.g., Agave. Tegmen collapsed. Perisperm in Agave and Yucca conspicuous, with proteins and lipids (starch absent). Endosperm usually copious (in Yucca ruminate), with oil, aleurone, protein and hemicellulose (starch absent); endosperm with usually thick pitted cell walls (in Hosta with thin cell walls). Embryo large, straight or slightly curved, without chlorophyll. Cotyledon one, photosynthesizing (in Hosta little differentiated, not photosynthesizing). Cotyledon hyperphyll usually elongate, assimilating (in Hosta compact, not photosynthesizing). Hypocotyl internode up to 4 mm long. Coleoptile absent. Radicula persistent (Agave, Yucca) or ephemeral, often branched. Collar rhizoids usually present. Germination in Hosta cryptocotylar.
Cytology n = 30; n = 24 (Hesperocallis) – Polyploidy and aneuploidy occurring in, e.g., Agave and Hosta. Chromosomes 0,4–10 µm long. Karyotype bimodal, with four (Hosta), five (Agave, Yucca), or five or six (Hesperocallis) long, and c. 25 short chromosomes (in Hesperocallis 18 or 19 short chromosomes; in Hosta also two medium-sized chromosomes). Adventitious polyembryony present in Hosta (at least in H. ventricosa).
DNA The mitochondrial gene rpl2 is absent (lost).
Phytochemistry Flavonols and other flavonoids (kaempferol xyloside, kaempferol-3-glycoside, kaempferol-3-rutinoside, etc.), steroidal saponins (diosgenin, hecogenin, neotigogenin, sarsasapogenin, smilagenin, tigogenin, yamogenin and nearly 30 other sapogenins, especially in seeds), chelidonic and ascorbic acid, non-protein amino acids, and fructans present. Homoisoflavanones present in Chlorogaleae. Ellagic acid, tannins, proanthocyanidins, alkaloids, and cyanogenic compounds not found.
Use Ornamental plants, textile plants (fibres from Agave sisalana and A. fourcroydes), soap (Yucca), medicinal plants, stimulants and beverages (Agave tequilana).
Systematics Hesperocallis (1; H. undulata; southern California, western Arizona); Hosta (23; China, the Korean Peninsula, Japan, the Russian Far East); Hesperoyucca (2; H. newberryi: northwestern Arizona; H. whipplei: southern California, Baja California), Schoenolirion (3; S. albiflorum, S. croceum, S. wrightii; Texas, southeastern United States), Hesperaloe (8; southwestern United States, northwestern Mexico); Hastingsia (4; H. alba, H. atropurpurea, H. bracteosa, H. serpentinicola; mainly on serpentine in northern California and southwestern Oregon), Camassia (6; C. angusta, C. cusickii, C. howellii, C. leichtlinii, C. quamash, C. scilloides; southern Canada, the United States), Chlorogalum (5; C. angustifolium, C. grandiflorum, C. parviflorum, C. pomeridianum, C. purpureum; California, Baja California); Yucca (c 50; central and western United States, California, Mexico, Central America, the West Indies), Furcraea (23; southern Mexico, Central America, the West Indies, tropical South America), Beschorneria (8; central and southern Mexico, northern Central America), Agave (255–265; southern United States, Mexico, Central America, the West Indies, Venezuela).
Agavaceae are sister-group to the clade [Anthericaceae+[Behniaceae+Herreriaceae]].
Hosta is sister to the remaining Agavaceae in Bogler & Simpson (1996). On the other hand, Hesperocallis is sister to Agavaceae in analyses by Seberg & al. (2012). Hesperocallis undulata and Hosta plantaginea have pollen grains with similar unibaculate muri.
Chlorogaleae Baker in Bot. J. Linn. Soc. London 13: 215. 4 Dec 1872 – comprising Camassia, Chlorogalum, Hastingsia and Schoenolirion – are successive sister-group to Agavaceae minus Hesperocallis and Hosta. They have rhexigenetic lacunae like Scilloideae (Hyacinthaceae) and at least Camassia has tepals with a simple leaf trace. In a Bayesian analysis of molecular data (Kim & al. 2010), Camassia was recovered with very high support as sister to the clade [Agavaceae+[Anthericaceae+[Behniaceae+Herreriaceae]]]. Unfortunately, Chlorogalum, Hastingsia or Schoenolirion were not included in that study. Awaiting analyses of all four genera, they will be kept in Agavaceae.
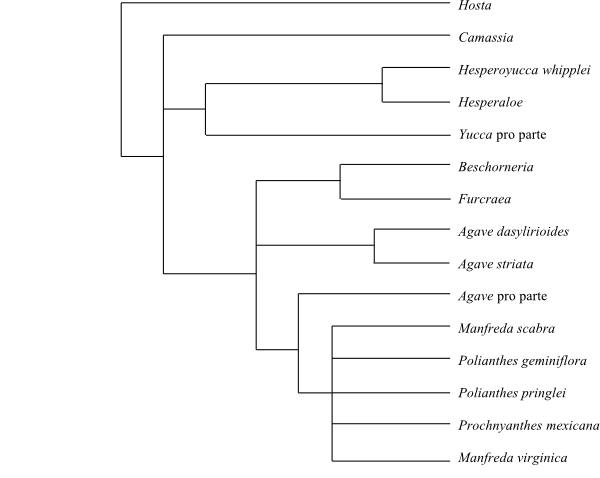 |
Cladogram of Agavaceae based on DNA sequence data (Bogler & Simpson 1996). Hesperocallis is sister to the remaining Agavaceae in analyses by Seberg & al. (2012). |
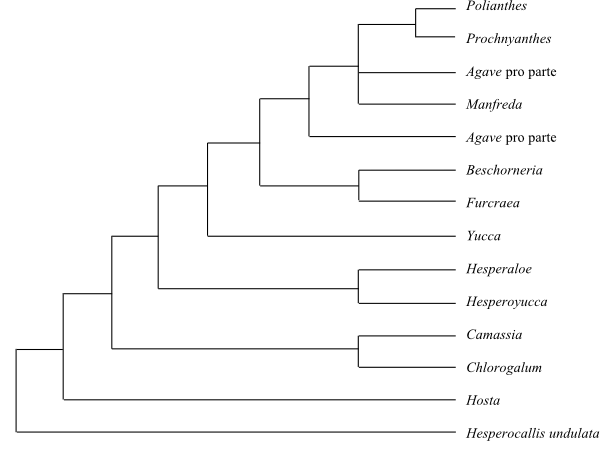 |
Strict consensus tree (simplified) of Agavaceae based on DNA sequence data (Bogler & al. 2006). |
 |
Strict consensus tree (simplified) of Agavaceae based on DNA sequence data (Archibald & al. 2015). |
ALLIACEAE Batsch ex Borkh. |
( Back to Iridales ) |
Alliales Bercht. et J. Presl, Přir. Rostlin: 267. Jan-Apr 1820 [’Alliaceae’]; Gilliesiaceae Lindl. in Bot. Reg. 12: ad t. 992. 1 Jul 1826 [’Gilliesieae’]; Alliineae Link, Handbuch 1: 152, 820. 4-11 Jul 1829 [‘Alliaceae’]; Gilliesiales Lindl. in C. F. P. von Martius, Consp. Regn. Veg.: 7. Sep-Oct 1835 [‘Gilliesieae’]; Cepaceae Salisb., Gen. Plant.: 88. 15-31 Mai 1866 [’Cepaeeae’]; Tulbaghiaceae Salisb., Gen. Plant.: 87. 15-31 Mai 1866 [’Tulbagheae’]; Milulaceae Traub in Plant Life 28: 131. 22 Feb 1972
Genera/species 14/855–900?
Distribution Temperate and subtropical regions in the Northern Hemisphere, South Africa, Sri Lanka, Mexico.
Fossils Unknown.
Habit Bisexual, perennial or biennial herbs. Usually with a bulb surrounded by membranous scales (rarely a bulb-like corm; in Tulbaghia a tuberous rhizome). Often with a strong characteristic odour (‘onion smell’). New bulb developing in axile of uppermost leaf.
Vegetative anatomy Roots of young bulb often contractile. Phellogen absent. Secondary lateral growth absent. Vessels present in roots. Vessel elements with scalariform and/or simple perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial parenchyma? Sieve tube plastids P2c type, with cuneate protein crystals. Nodes? Laticifers present in at least Allium, Nothoscordum, Tristama, and Tulbaghia. Mucilage cells abundant. Calciumoxalate raphides present in some genera, absent from at least Allium and Tulbaghia; calciumoxalate as single crystals, styloids or other configurations present in Allium and Nothoscordum.
Trichomes Hairs unicellular or absent.
Leaves Alternate (spiral or distichous), simple, entire, often linear or filiform, often fleshy, often angular in transverse section or terete, with ? ptyxis. Stipules absent; leaf sheath closed, long, in Allium etc. with short ligule. Venation parallelodromous; vascular bundles in many species of Allium (especially with cylindrical leaves) arranged in a cylinder with phloem adjacent to epidermis. Stomata anomocytic. Cuticular wax crystalloids as platelets without particular orientation (in several species of Allium), as tubuli (chemically dominated by nonacosan-10-ol) or as filiform reticulate processes (in Ipheion uniflorum and Nothoscordum bivalve). Mesophyll often with calciumoxalate raphides. Leaf margin entire.
Inflorescence Usually terminal (rarely lateral), umbel-like (in one species of Allium spike-like), consisting of one or several condensed helicoid monochasia (rarely a single flower). Inflorescence subtended by usually two (sometimes one or more than two) membranous, usually free (sometimes connate) bracts (spathae), enclosing floral buds and in some species caducous prior to anthesis. Peduncle (scape) usually long and terete (occasionally angular or somewhat compressed in transverse section). Floral bracts and prophylls usually absent. Flowers in some species of Allium often replaced by bulbils.
Flowers Usually actinomorphic (in Gilliesia, Miersia and Solaria zygomorphic). Usually hypogyny (in Allium siculum and A. tripedale almost half epigyny). Tepals usually 3+3 (rarely three or 3+2), petaloid, free or more or less connate; usually with appendages of various shape between tepals or stamens, often as corona. Septal nectaries infralocular (in Allium with orifice close to ovary base; in Nothoscordum with orifice near style). Disc absent.
Androecium Stamens usually 3+3 (rarely two or three stamens, or two fertile stamens and three staminodia, or three fertile stamens and two staminodia). Filaments free or connate, often adnate to tepals (in some species with apical, dorsal or lateral appendages). Anthers basifixed, versatile, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory, with uninucleate (Allium) or binucleate to quadrinucleate (Tulbaghia) cells. Staminodia usually absent (rarely two or three).
Pollen grains Microsporogenesis successive. Pollen grains monosulcate, shed as monads, bicellular at dispersal. Exine semitectate, with columellate infratectum, reticulate.
Gynoecium Pistil composed of three connate carpels. Ovary usually superior (rarely semi-inferior), trilocular. Style single, simple, terminal or (in Allium) gynobasic, without stylar canal (style solid). Stigma capitate or trilobate, papillate (with unicellular papillae), usually Dry (in Allium) or Wet (in Leucocoryne) type. Pistillodium absent.
Ovules Placentation axile. Ovules two to numerous per carpel, anatropous or campylotropous, apotropous?, bitegmic, crassinucellar or tenuinucellar. Micropyle usually endostomal? (sometimes bistomal). Outer integument ? cell layers thick. Inner integument usually two or three cell layers thick. Obturator present (funicular?). Parietal cell not formed (parietal tissue absent). Hypostase present. Nucellar cap usually formed by periclinal cell divisions of megasporangial epidermis. Megagametophyte disporous, Allium type (Allium, Leucocoryne), or monosporous, Polygonum type (Nothoscordum). Endosperm development cellular or nuclear (Allium), or helobial (Nothoscordum, Tulbaghia). Endosperm haustoria? Embryogenesis onagrad or asterad.
Fruit A loculicidal capsule.
Seeds Aril absent. Seed coat exotestal. Exotesta with often coarse phytomelan layer on epidermal cell walls. Mesotesta, endotesta and tegmen usually compressed or collapsed. Perisperm not developed. Endosperm copious, with oil and aleurone (hemicellulose?, starch absent); endosperm cells usually with pitted walls. Embryo short or long, usually curved (in Tulbaghia straight), without chlorophyll. Cotyledon one, usually elongate, usually photosynthesizing. Hypocotyl internode absent. Coleoptile absent. Germination cryptocotylar.
Cytology n = 4–15 – Chromosomes 2–20 µm long. Polyploidy and adventitious embryony (seed formed from megasporangium) occurring (rarely apospory).Minisatellite telomeres are absent in Allium.
DNA Plastid gene infA lost/defunct (Allium). Mitochondrial gene rpl2 lost. Mitochondrial ribosomal protein genes and succinate dehydrogenase genes in Allium transferred to nuclear genome.
Phytochemistry Flavonoids (quercetin etc., especially in bulb scales), steroidal saponins (with, e.g., aigogenin as sapogenin), chelidonic acid, cysteine derived sulphur compounds (strongly smelling onion oils) in the form of, e.g., allyl disulfides, propyl sulfides, vinyl disulfides, propionaldehyde, and propionthiol present in Allium, Milula, Tulbaghia, Gilliesia, Ipheion, Leucocoryne, and Tristagma; in wounded tissues alliin, an S-substituted cystein derived amino acid, converted into water-soluble allicin, pyruvic acid and ammonia, a reaction catalyzed by alliinase. The strongly smelling compound propantial-S-oxide (in, e.g., Allium cepa, although nearly absent from Allium ampeloprasum and A. sativum) is formed from propenylic sulphuric acid and liberated from crushed cells. Ellagic acid, proanthocyanidins and alkaloids not found. Carbohydrates stored in bulb scales as galactan, raffinose, and fructans in, e.g., Allium; in Nothoscordum, Ipheion, and Tulbaghia as starch. Lectines binding mannose.
Use Ornamental plants, vegetables and spices (Allium spp.).
Systematics Alliaceae are sister-group to Amaryllidaceae.
The topology [Allieae+[Tulbaghieae+[Gilliesieae+Leucocoryneae]]] is according to Fay & al. (2006) and Li & al. (2016). Souza & al. (2016) recovered Tulbaghia as sister to Leucocoryneae.
Allieae Dumort., Fl. Belg.: 139. 1827
1/750–780? Allium (750–780?; temperate regions on the Northern Hemisphere, northern, northeastern and southern Africa, southern Asia southwards to Sri Lanka, Mexico, with their highest diversity in the Mediterranean area, Central Asia and southwestern North America). – Bulbs without starch. Root vessel elements often with simple perforation plates. Leaves sometimes unifacial. Seemingly bifacial leaves in at least some species of Allium with inverted vascular bundles along adaxial surface and bundles with normal orientation along abaxial surface. Tepals usually connate at base. Median outer tepal adaxial. Corona absent. Filaments often winged, connate at base, adnate to inner tepals. Tapetum with uninucleate cells. Ovary sometimes semi-inferior, sometimes with paired projections. Style entire, gynobasic. Ovules two, epitropous, to 14 per carpel. Endosperm development often? cellular. Caruncle sometimes present. Embryo long, curved. n = (7) 8 (9). Numerous transfers of ribosomal protein and succinate dehydrogenase genes from mitochondrial to nuclear genome. Minisatellite telomeres lost. – Allium is sister to [Tulbaghioideae+ Gilliesioideae] (Fay & Chase 1996).
[Tulbaghieae+[Gilliesieae+Leucocoryneae]]
Bulbs with starch. Endosperm development helobial.
Tulbaghieae Endl. ex Meisn., Pl. Vasc. Gen.: Tab. Diagn. 397, 399, Comm. 302. 17–20 Dec 1842
1/20. Tulbaghia (20; southern tropical and southern Africa). – Often with rhizome. Leaf sheath short. Floral bracts present. Inner tepals largely connate, often with connate lobes. Corona well developed. Filaments adnate to tepals and/or corona lobes. Ovules two or more per carpel. Seeds flattened. n = 6.
[Gilliesieae+Leucocoryneae]
Gilliesieae Baker in J. Linn. Soc. London, Bot. 14: 509. 24 Apr 1875
6/25. Speea (2; S. humilis, S. triloba; Chile), Solaria (6; S. atropurpurea, S. attenuata, S. brevicoalita, S. curacavina, S. cuspidata, S. miersioides; southern Chile, southern Argentina), Schickendantziella (1; S. trichosepala; Bolivia, Argentina), Trichlora (4; T. huascarana, T. lactea, T. peruviana, T. sandwithii; Peru), Miersia (5; M. chilensis, M. leporina, M. myodes, M. rusbyi, M. tenuiseta; Bolivia, Chile), Gilliesia (7; G. curicana, G. dimera, G. graminea, G. isopetala, G. monophylla, G. montana, G. nahuelbutae; Chile, western Argentina). – Flowers in Gilliesia and Miersia very strongly zygomorphic. Tepals in Schickendantziella and Trichlora three, caudate. Stamens in Gilliesia two.
Leucocoryneae Ravenna in Onira 5(11): 43. 17 Jan 2001
6/60–75. Nothoscordum (20–25; southern and eastern United States to South America; paraphyletic?), Beauverdia (4; B. dialystemon, B. hirtella, B. sellowiana, B. vittata; southern Brazil, Uruguay, Argentina; in Nothoscordum?), Zoellnerallium (2; Z. andinum, Z. serenense; the Andes in central Chile and Argentina), Leucocoryne (15–20; Chile), Ipheion (3; I. sessile, I. tweedieanum, I. uniflorum; Mexico to Chile and Argentina), Tristagma (17–20; Chile, southern Argentina). – Southern and eastern United States, Mexico, Central America, South America. Corona present or absent. Stamens sometimes two or three. Filaments sometimes connate and adnate to tepals. Anthers sometimes extrorse. Staminodia sometimes present. Style in Nothoscordum entire. Ovules two to numerous per carpel. Embryo short. n = 4 or more. – Zoellnerallium was sister to Nothoscordum and Beauverdia was sister-group to those two, according to Sassone & al. (2014). On the other hand, Nothoscordum (including Beauverdia) was sister to the remaining Leucocoryneae in Souza & al. (2016).
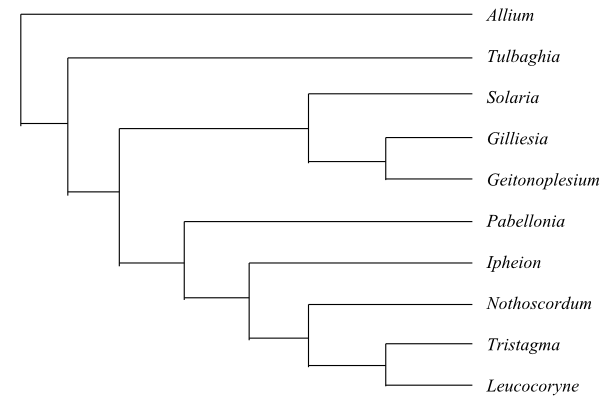 |
Phylogeny of Alliaceae based on DNA sequence data (Li & al. 2016). Pabellonia and Stemmatium are usually treated as congeneric with Leucocoryne (Fay & al. 2006). |
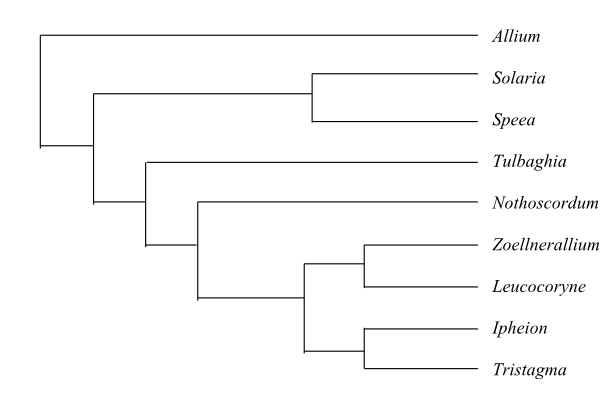 |
Phylogeny of Alliaceae based on DNA sequence data (Souza & al. 2016). Beauverdia was nested in Nothoscordum. Only Solaria and Speea from Gilliesieae were included in their analysis. |
AMARYLLIDACEAE J. St.-Hil. |
( Back to Iridales ) |
Narcissaceae Juss., Gen. Plant.: 54. 4 Aug 1789 [’Narcissi’]; Leucojaceae Batsch ex Borkh., Bot. Wörterb. 1: 372. 1797; Crinaceae Vest, Anleit. Stud. Bot.: 267, 283. 1818 [‘Crinoideae’]; Amaryllidales Link, Handbuch 1: 193. 4-11 Jul 1829 [’Amarillydeae’]; Narcissales Dumort., Anal. Fam. Plant.: 57. 1829 [‘Narcissarieae’]; Brunsvigiaceae Horan., Prim. Lin. Syst. Nat.: 51. 2 Nov 1834 [‘Brunswigiaceae’]; Crinopsida Horan., Prim. Lin. Syst. Nat.: 51. 2 Nov 1834 [’Crinoideae’]; Galanthaceae G. Mey., Chloris Han.: 510, 560. Jul-Aug 1836 [‘Galantheae’]; Gethyllidaceae Raf., Fl. Tellur. 4: 19. med 1838 [‘Gethylides’]; Amaryllidineae J. Presl in Nowočeská Bibl. [Wšobecný Rostl.] 7: 1528. 1846 [‘Amarylleae’]; Narcissineae J. Presl in Nowočeská Bibl. [Wšobecný Rostl.] 7: 1528, 1533. 1846 [‘Narcisseae’]; Pancratiaceae Horan., Char. Ess. Fam.: 50. 30 Jun 1847 [‘Pancratiaceae. nob. (Amaryllideae)’]; Cyrtanthaceae Salisb., Gen. Plant.: 138. 15-31 Mai 1866 [‘Cyrtantheae’]; Haemanthaceae Salisb., Gen. Plant.: 129. 15-31 Mai 1866 [‘Haemantheae’]; Oporanthaceae Salisb., Gen. Plant.: 97. 15-31 Mai 1866 [‘Oporantheae’]; Strumariaceae Salisb., Gen. Plant.: 126. 15-31 Mai 1866 [’Strumareae’]; Zephyranthaceae Salisb., Gen. Plant.: 133. 15-31 Mai 1866 [’Zephyrantheae’]
Genera/species c 80/870–900
Distribution Tropical and subtropical regions on both hemispheres northwards to Western Europe and eastwards to East Asia, with their largest diversity in the Mediterranean, southern Africa and South America.
Fossils Unknown.
Habit Bisexual, perennial herbs. Usually with a bulb rich in polysaccharides (in Clivia, Cryptostephanus and Scadoxus corm or rhizome). Rarely epiphytic or aquatic.
Vegetative anatomy Roots contractile, perennial or fibrous, ephemeral; root velamen two- to four-layered; root exodermis one-layered, with alternating long and short cells. Bulb with tunica. Phellogen absent. Lacunae sometimes formed by degradation of parenchyma. Secondary lateral growth absent. Vessels present in roots. Vessel elements with scalariform perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial parenchyma? Sieve tube plastids P2cc type (with cuneate protein crystals), P2cco type (with one to three orthogonal and many cuneate protein crystals) (in Worsleya P2ccf type, with cuneate protein crystals and peripheral protein filaments; in Rauhia P2ccof type, with one to three orthogonal and many cuneate protein crystals and peripheral protein filaments). Nodes? Mucilage cells and mucilage sacs frequent, with calciumoxalate raphides and rhomboedric crystals. Epidermal cells often with calcium oxalate crystals.
Trichomes Hairs usually absent (rarely unicellular or multicellular, uniseriate or multiseriate, or aggregated).
Leaves Alternate (usually distichous), simple, entire, often linear, usually bifacial (rarely unifacial), with revolute, involute or flat (sometimes explicative) ptyxis, sometimes differentiated into pseudopetiole and pseudolamina. Stipules absent; leaf sheath sometimes well developed. Venation parallelodromous, sometimes with inverted vascular bundles. Stomata anomocytic, sometimes sunken. Cuticular wax crystalloids as non-orientated rodlets or platelets (similar to those in Asphodelaceae and Hyacinthaceae). Mesophyll with mucilaginous idioblasts containing calciumoxalate raphides. Leaf margin entire.
Inflorescence Terminal, umbel- or head-like, consisting of reduced helicoid partial inflorescences (flowers rarely solitary), usually surrounded by (one or) two (to eight) often membranous free or connate equitant or obvolute bracts (spathae), usually marcescent (absent in Leptochiton). Peduncle (scape) often long, sometimes subterranean, in Amaryllideae with a cylinder of sclerenchyma between cortex and central vascular tissue.
Flowers Zygomorphic or (secondarily) actinomorphic, large (some species of Phaedranassa with split zygomorphy, androecial members appearing through an abaxial slit in perianth tube). Pedicel not articulated. Epigyny. Tepals 3+3, petaloid, with median outer tepal adaxial, usually connate in lower part (rarely free) and infundibuliform to tubular. In some clades a corona (paraperigone) present as non-vascularized outgrowths from filaments (in, e.g., Hymenocallis) or vascularized appendages from tepals and forming a tube (e.g. Narcissus), sometimes small and consisting of a narrow border or a ring of scales or fringes in perianth orifice. Septal nectaries usually infralocular (in, e.g., Galanthus tepal nectaries present on distal part of inner tepals; nectariferous glands, associated with septal nectaries, present on filament bases in some species of Eucrosia and Hessea). Disc usually absent (in Galanthus and Leucojum present, nectar-secreting).
Androecium Stamens usually 3+3 (in some species of Griffinia five; in Gethyllis 9–18 or more). Filaments free or more or less connate at base, often adnate to perianth tube (in Carpolyza and Strumaria adnate to style), often with prolonged lobed outgrowths forming a corona (e.g. in Hymenocallis). Anthers usually dorsifixed (sometimes centrifixed or basifixed), usually versatile, tetrasporangiate, usually introrse (rarely latrorse), usually longicidal (dehiscing by longitudinal slits; in Galanthus and Leucojum poricidal, dehiscing by an apical pore). Tapetum usually secretory (rarely amoeboid-periplasmodial), with usually binucleate (sometimes uninucleate) cells. Staminodia absent.
Pollen grains Microsporogenesis successive. Pollen grains usually monosulcate (rarely disulculate), shed as monads, bicellular at dispersal. Exine usually semitectate (rarely tectate, fossulate or intectate), with columellate infratectum, usually reticulate (sometimes spinulate or absent, rarely perforate).
Gynoecium Pistil composed of three connate carpels. Ovary inferior, usually trilocular (rarely unilocular). Style single, simple, filiform, with stylar canal (rarely trilobate). Stigma capitate, punctate or slightly trifid to deeply trilobate, usually papillate (with unicellular or multicellular papillae), usually Dry (sometimes Wet) type. Pistillodium absent.
Ovules Placentation usually axile (rarely basal). Ovules approx. twelve to more than 50 per carpel, anatropous, usually bitegmic (in Amaryllideae unitegmic; in some species of Crinum probably ategmic), usually crassinucellar (in Zephyranthes tenuinucellar). Micropyle bistomal (sometimes Z-shaped, zig-zag). Outer integument usually two (sometimes three or more) cell layers thick. Inner integument two cell layers thick. Parietal cell formed from archesporial cell or absent. Parietal tissue absent. Hypostase usually absent. Periclinal cell divisions often occurring in megasporangial epidermis (parietal cell not formed). Megagametophyte usually monosporous, Polygonum type (rarely disporous, Allium type). Synergids with a filiform apparatus. Endosperm development usually helobial (sometimes nuclear; in Crinum cellular). Endosperm haustoria? Embryogenesis asterad or onagrad. Polyembryony occurring in at least Crinum and Hymenocallis.
Fruit Usually a loculicidal capsule (in, e.g., Boophone and Cybistetes irregularly dehiscent; in Calostemmateae indehiscent; in, e.g., Gethyllideae a berry).
Seeds Aril absent. Seed coat testal, sometimes fleshy or winged, sometimes (in, e.g., Galanthus and Leucojum) with caruncular elaiosome at chalazal end. Exotesta usually with thin phytomelan layer on epidermal cell walls (absent in water-rich seeds of Amaryllideae, Calostemmateae, Haemantheae, and Hymenocallideae, having fleshy seed coat and starchy endosperm). Endotesta? Tegmen collapsed. Perisperm not developed. Endosperm copious, with oils, aleuronic, starch or hemicellulose (sometimes rich in water), sometimes thin-walled, in Amaryllideae chlorophyllous. Embryo straight, small, little differentiated, with or without chlorophyll. Cotyledon one, bifacial, usually photosynthesizing. Cotyledon hyperphyll elongate, dorsiventrally flattened and assimilating, or compact and not assimilating. Hypocotyl internode absent. Coleoptile absent. Radicula well developed, contractile. Germination phanerocotylar.
Cytology n = 5–13, 20–23 or more (n = 52–55 in Ismene narcissiflora, n = 69 in Eucharis caucana, n = 59–89 in Sprekelia formosissima); x = 11 – Polyploidy frequently occurring. Chromosomes (1,5–)3–28 µm long. Agamospermy occurring in Crinum, Habranthus and Zephyranthes.
DNA The mitochondrial gene rpl2 is absent (lost).
Phytochemistry Flavonols (kaempferol, quercetin), norbelladine alkaloids (toxic tyrosine derivatives, more than 200 structures known at present, at least 79 of which from Narcissus; e.g. crinine, belladine, narwedine, galanthamine, haemanthamine, homolyrine, lycorenine, lycorine, homolycorine, pancratiostatine, and tazettine), benzylisoquinoline alkaloids, cyanogenic compounds, chelidonic acid and non-protein amino acids present. Mannans present as storage carbohydrates. Ellagic acid, proanthocyanidins, steroidal saponins, and allyl disulfides not found. Lectins binding mannose.
Use Ornamental plants, medicinal plants.
Systematics Amaryllidaceae are sister-group to Alliaceae.
Amaryllideae are sister to the remaining Amaryllidaceae, according to Meerow & al. (1999).
The variation in sieve tube plastids is large.
Amaryllideae Dumort., Anal. Fam. Plant.: 58. 1829
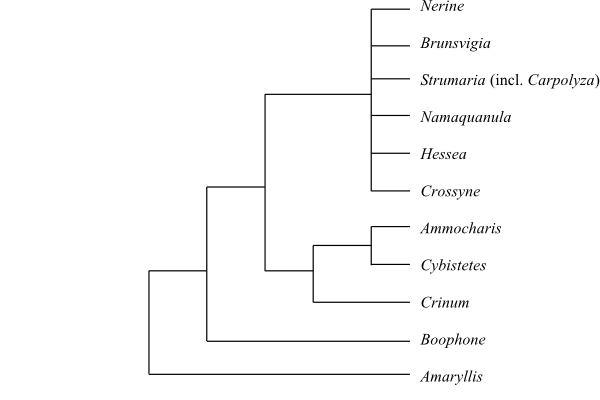
9–10/c 165. Amaryllis (2; A. belladonna, A. paradisicola; Northern and Western Cape), Nerine (c 23; southern Africa), Brunsvigia (c 20; southern Africa), Crossyne (2; C. flava, C. guttata; Northern and Western Cape), Hessea (14; Namibia, Northern and Western Cape; incl. Namaquanula?), Namaquanula (2; N. bruce-bayeri, N. bruynsii; southwestern Namibia, northernmost Northern Cape; in Hessea?), Strumaria (28; southern Africa, especially Namibia and southwesternmost South Africa), Boophone (2; B. disticha, B. haemanthoides; East to southern Africa), Crinum (c 65; pantropical, south to South Africa), Ammocharis (7; A. angolensis, A. baumii, A. coranica, A. deserticola, A. longifolia, A. nerinoides, A. tinneana; tropical and southern Africa). – Pantropical, with their highest diversity in South Africa. Bulb scales forming strongly elastic cotton-like fibres, when injured (due to presence of foliar fibres). Leaves with extensive fibres (with spiral cell wall thickenings); leaves developed prior to flowers (leaves in many Crininae perennial). Stomata paracytic; subsidiary cells with oblique divisions. Filaments usually connate at base (in Crininae free), sometimes with small processes (filament bases in Strumaria and Carpolyza adnate to style). Pollen grains usually disulcate. Exine intectate, columellate, often gemmate, with scattered spinulae. Style sometimes laterally inserted. Ovules unitegmic (sometimes ategmic?). Megagametophyte Allium type. Seeds watery, without resting period. Testa up to 25 cell layers thick, usually with chlorophyll and anomocytic stomata (absent in Amaryllis) or more or less collapsing, without phytomelan, or absent (e.g. in Crinum). Endosperm with starch and sometimes (e.g. in Crinum) with a suberine chlorophyllous layer. Embryo with chlorophyll. Very long tubular cotyledon envelope sometimes formed during germination. n = 10, 12, 15. Chromosomes 5,3–20,5 µm long. – Amaryllis and Boophone are successive sister-groups to the remaining Amaryllideae (Meerow & al. 1999).
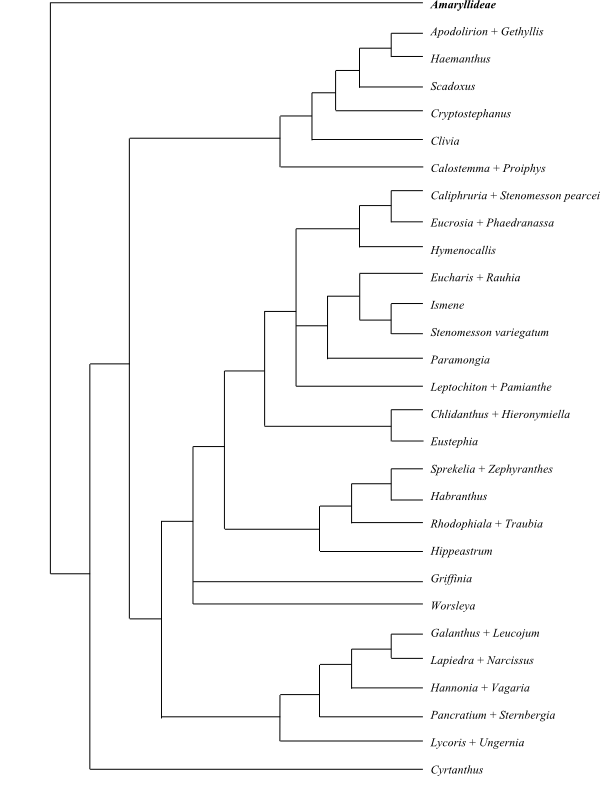
[Cyrtantheae+[[Calostemmateae+Haemantheae]+[[Lycorideae+[Pancratieae+Narcisseae]]+ [Griffinia+ Worsleya+[Hippeastreae+[Eustephieae+[Hymenocallideae]]]]]]]
Vascular bundles with parenchymatic envelope cells.
Cyrtantheae Traub in Herbertia 5: 111. Nov 1938
1/c 50. Cyrtanthus (c 50; tropical and southern Africa, especially South Africa). – Peduncle without sclerenchyma cylinder. Collenchyma subepidermal. Rhizodermis one-layered. Velamen absent. Seeds flat, horizontally packed. Phytomelan present. n = (7) 8 (11).
[[Calostemmateae+Gethyllideae]+[[Lycorideae+[Pancratieae+Narcisseae]]+[Griffinia+ Worsleya+[Hippeastreae+[Eustephieae+[Hymenocallideae]]]]]]
[Calostemmateae+Gethyllideae]
Fruit indehiscent.
Calostemmateae D. Müll.-Doblies et U. Müll.-Doblies in Feddes Repert. 107: 7. Dec 1996
2/7. Calostemma (3; C. abdicatum: South Australia; C. luteum: South Australia to Queensland; C. purpureum: south central and southeastern Australia), Proiphys (4; P. alba, P. amboinensis, P. cunninghamii, P. infundibularis; Peninsular Thailand, Malesia to New Guinea, tropical and eastern Australia). – Malesia, tropical and eastern Australia. Bulbils as dispersal units. Ovules two or three per carpel. Embryo slowly germinating and producing a bulbil. Phytomelan absent. n = 10.
Gethyllideae Dumort., Anal. Fam. Plant.: 58. 1829
6/c 84. Clivia (6; C. caulescens, C. gardenii, C. miniata, C. mirabilis, C. nobilis, C. robusta; South Africa, Swaziland), Cryptostephanus (3; C. densiflorus, C. haemanthoides, C. vansonii; tropical East Africa, Mozambique to Angola and Namibia), Scadoxus (9; tropical and southern Africa), Haemanthus (22; southern Africa, with their highest diversity in Namaqualand and Western Cape), Apodolirion (6; A. amyanum, A. bolusii, A. buchananii, A. cedarbergense, A. lanceolatum, A. macowanii; South Africa, Swaziland), Gethyllis (c 38; Namibia, Northern, Western and Eastern Cape, Free State, North-West). – Tropical and southern Africa (with their largest diversity in South Africa). Sometimes with rhizome. Rhizoderm one-layered. Velamen absent. Peduncle without sclerenchyma cylinder. Subepidermal collenchyma present. Inflorescence bracts usually connate. Gethyllis has up to 18 stamens. Fruit a berry. Seeds angular. Phytomelan usually absent. n = 6, 8, 9, 11, 12, 14. Alkaloids absent in Gethyllis.
[[Lycorideae+[Pancratieae+Narcisseae]]+[Griffinia+Worsleya+[Hippeastreae+[Eustephieae+ [Hymenocallideae]]]]]
[Lycorideae+[Pancratieae+Narcisseae]]
Seeds almost spherical, turgid.
Lycorideae Nakai, Chosakuronbun Mokuroku [Ord. Fam. Trib. Nov.]: 235. 20 Jul 1943
2/25–32. Lycoris (15–22; China, the Korean Peninsula, Japan, Burma), Ungernia (10; Central Asia to Japan). – Temperate to subtropical East Asia to Iran, Central Asia and Burma. Seeds in Ungernia irregularly discoid. n = 11 or higher.
[Pancratieae+Narcisseae]
Pancratieae Dumort., Anal. Fam. Plant.: 58. 1829
2/28–29. Pancratium (20–21, the Canary Islands, the Mediterranean, West Africa, Namibia, southern Asia), Sternbergia (8; southeastern Europe, the Caucasus, southwestern Asia to Kashmir). – Southeastern Europe, the Canary Islands, the Mediterranean, West Africa, Namibia, southwestern and southern Asia. Staminal tube dentate. n = 11.
Narcisseae Lam. et DC., Syn. Plant. Fl. Gall.: 165. 30 Jun 1806 [’Narcissi’]
7/c 72. Narcissus (c 37; Europe, the Mediterranean), Lapiedra (1; L. martinezii; the Iberian Peninsula, western Mediterranean), Acis (9; the Mediterranean), Galanthus (c 20; Europe to Iran), Leucojum (2; L. aestivum, L. vernum; Europe to the Caucasus and Iran), Hannonia (1; H. hesperidium; Morocco), Vagaria (2; V. olivieri: Morocco; V. parviflora: Turkey to Syria and Palestine). – Europe, the Mediterranean, northwestern Africa, Crimea, the Caucasus, western Asia to Iran. Peduncular bracts connate at base or along one side. Corona usually present in Narcissus. Elaiosome usually present. n = 7–9, 11, 12 (13) or higher. – Especially Galanthus have the inner tepal whorl very different from the outer one.
[Griffinia+Worsleya+[Hippeastreae+[Eustephieae+[Hymenocallideae]]]]
Rhizoderm one-layered. Velamen absent. Peduncle without sclerenchyma cylinder. Subepidermal collenchyma present. Bracts obvolute. Phytomelan frequently present.
Hippeastreae Herb. ex Sweet, Brit. Fl. Gard., ser. 2, 1: ad t. 14. 1 Sep 1829 [’Hippeastriformes’]
c 14/265–280. Griffiniinae D. Müll.-Doblies et U. Müll.-Doblies in Feddes Repert. 107(Short commun.): 6. Dec 1996. Griffinia (15–20; Brazil). – Traubiinae D. Müll.-Doblies et U. Müll.-Doblies in Feddes Repert. 107(Short commun.): 6. Dec 1996. Traubia (1; T. modesta; Chile), ’Rhodolirium’ (6; R. andicola, R. chilense, R. fulgens, R. laetum, R. montanum, R. speciosum; Chile, western Argentina; non-monophyletic), ’Phycella’ (c 8–10; P. australis, P. brevituba, P. cyrtanthoides, P. herbertiana, P. scarlatina; Venezuela, Chile, Argentina; paraphyletic; incl. Placea?), ’Placea’ (6; P. amoena, P. arzae, P. davidii, P. germainii, P. lutea, P. ornata; Chile; polyphyletic; in Phycella?). – Hippeastrinae Walp., Ann. Bot. Syst. 3: 616. 28–29 Sep 1852 [’Hippeastreae’]. Cearanthes (1; C. fuscoviolacea; northeastern Brazil), Tocantinia (1; T. mira; Paraná in Brazil), Hippeastrum (c 90; Mexico, Central America, the West Indies, South America to Bolivia and Argentina; incl. Worsleya?), Worsleya (1; W. procera; Organ Mountains in Brazil; in Hippeastrum?), ’Zephyranthes’ (70–75; southeastern United States, Central America, the West Indies, South America to Argentina; paraphyletic; incl. Habranthus and Rhodophiala pro parte?), ’Habranthus’ (35–40; Chile, Argentina; polyphyletic; in Zephyranthes?), Eithea (1; E. blumenavia; southern Brazil),’Rhodophiala’ (c 30; Brazil, Uruguay, Bolivia, Chile, Argentina; polyphyletic), Sprekelia (2; S. formosissima, S. howardii; Mexico, Guatemala). – Southeastern United States, Mexico, Central America, the West Indies, South America. Peduncular bracts often connate at base (sometimes only along one side). Flowers zygomorphic. Corona sometimes present. Stamens declinate, of various lengths. Seeds flat, winged or D-shaped. n = 8–13, 17 or more. Chromosomes 3–16.7 µm long. – Griffinia and Worsleya were each identified by Meerow & al. (1999) as an isolated clade in a basal trichotomy outside of Hippeastreae, Eustephieae and Hymenocallideae.
[Eustephieae+Hymenocallideae]
Mesophyll without palisade. Flowers actinomorphic. Tetraploidy often occurring.
Eustephieae Hutch., Fam. Fl. Plants 2: 130. 20 Jul 1934
3/24. Chlidanthus (10; southern Peru, Bolivia, northwestern Argentina), Eustephia (6; E. armifera, E. coccinea, E. darwinii, E. hugoei, E. kawidei, E. longibracteata; southern Peru, Bolivia), Hieronymiella (8; Bolivia, Argentina). – The Central Andes in Peru, Bolivia and Argentina. Stamens of two different lengths (dimorphic). Seeds flattened, winged.
Hymenocallideae Small, Man. S.E. Fl.: 315. 30 Nov 1933 [’Hymenocalleae’]
13/150–160. Hymenocallis (60–65; southeastern United States, the West Indies, northeastern South America), Leptochiton (2; L. helianthus, L. quitoensis; the Andes in Ecuador and Peru), Stenomesson (16; the Andes, especially in Peru), Clinanthus (24; the Andes in Ecuador to northwestern Argentina), Paramongaia (1; P. weberbaueri; Peru), Pamianthe (3; P. cardenasii, P. parviflora, P. peruviana; the Andes in Ecuador, Peru and Bolivia), Phaedranassa (9; the Cordillera in Costa Rica, Colombia and Ecuador), Rauhia (5; R. decora, R. multiflora, R. occidentalis, R. sagasteguiana, R. staminosa; Peru), Eucrosia (8; the Andes in Eucador and Peru), Eucharis (15–20; Central America, tropical South America; incl. Caliphruria?), Caliphruria (4; C. hartwegiana, C. korsakoffii, C. subedentata, C. tenera; western Colombia, Peru; in Eucharis?), Plagiolirion (1; P. horsmannii; Cauca Valley in Colombia), Urceolina (7; U. ayacucensis, U. cuzcoensis, U. fulva, U. latifolia, U. microcrater, U. robledoana, U. urceolata; the Andes in south central Perusouthern central Peruvian Andes). – Southeastern United States, the West Indies, Central America, the Andes, tropical South America. Velamen present in Pamianthe. Leaves often petiolate, lorate. Flowers usually actinomorphic (sometimes zygomorphic). Androecial cup usually present. Pollen grains auriculate (the two ends tapering and with different sculpturing). Seeds flattened and obliquely winged, or spherical, turgid, and with glossy seed coat. Testa thick, spongy, vascularized, with chlorophyll. Phytomelan usually absent (present in Leptochiton). Embryo starchy. n = sometimes 19, 20, 22. Chromosomes 2.3–11.8 µm long.
 |
Cladogram of Amaryllideae based on morphology and DNA sequence data (Meerow & Snijman 2001). |
 |
Cladogram (one of 5.000 successively weighted trees) of Amaryllidaceae based on DNA sequence data (Meerow & al. 1999). |
ANEMARRHENACEAE Conran, M. W. Chase et Rudall |
( Back to Iridales ) |
Genera/species 1/1
Distribution Northern China, the Korean Peninsula.
Fossils Unknown.
Habit Bisexual, perennial herb. Rhizome short, thick. Roots thick.
Vegetative anatomy Phellogen absent. Secondary lateral growth absent. Vessels present in roots? Vessel elements with ? perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial parenchyma? Sieve tube plastids P2c type, with cuneate protein crystals. Nodes? Idioblasts with calciumoxalate raphides sometimes present in foliar mesophyll.
Trichomes Hairs absent?
Leaves Alternate (spiral), simple, entire, linear, with ? ptyxis. Stipules absent; leaf sheath relatively well developed. Venation parallelodromous. Stomata anomocytic. Cuticular wax crystalloids? Leaf surface papillate. Leaf margin entire, papillate.
Inflorescence Terminal, spike-like panicle, with few-flowered partial inflorescences.
Flowers Actinomorphic, small. Pedicel articulated. Pericladium present. Hypogyny. Tepals 3+3, petaloid, more or less clawed, marcescent, usually free (sometimes partially connate at base). Septal nectaries with openings at stylar base. Disc absent.
Androecium Stamens three, antepetalous. Filaments flattened, free, adnate at middle of inner tepals. Anthers basifixed, non-versatile, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits). Tapetum probably secretory (pre-Ubisch-bodies present, resulting in Pollenkitt), with multinucleate cells. Staminodia absent.
Pollen grains Microsporogenesis successive. Pollen grains monosulcate, shed as monads, bicellular at dispersal. Exine semitectate, with columellate infratectum, reticulate.
Gynoecium Pistil composed of three connate carpels. Ovary superior, trilocular. Style short, subulate. Stigma small, capitate, type? Pistillodium absent.
Ovules Placentation axile. Ovules two per carpel, anatropous, apotropous, bitegmic, crassinucellar. Micropyle ?-stomal. Outer integument four to six cell layers thick. Inner integument two or three cell layers thick. Parietal cell formed from archesporial cell. Nucellar cap absent. Hypostase present. Megagametophyte monosporous, Polygonum type. Antipodal cells persistent. Megagametophyte haustoria present. Endosperm development helobial. Endosperm haustoria? Embryogenesis?
Fruit A loculicidal capsule.
Seeds Aril absent. Seed coat exotestal. Exotesta with phytomelan layer on epidermal cell walls. Mesotesta, endotesta and tegmen collapsed? Perisperm not developed. Endosperm copious, fleshy, with pitted cell walls, with starch (with oil, aleurone, and hemicellulose?). Embryo relatively large, curved, chlorophyll? Cotyledon one (occasionally two?), well developed; cotyledon apex a haustorium hidden beyond seed coat. Cotyledon hyperphyll elongate. Hypocotyl internode absent. Coleoptile absent. Germination?
Cytology n = 11
DNA The mitochondrial gene rpl2 is absent (lost).
Phytochemistry Steroidal saponins (sarsasaponigene, markogenin diglycoside, timosaponins A-III and B-II and Zhi-mu-, hinokiresinol-, smilageninoside- and anemarsaponin A1, A2 and B), glucanes (anemarin A, B, C and D), xanthone-C-glycoside and mangiferin present in rhizome.
Use Ornamental plant, medicinal plant.
Systematics Anemarrhena (1; A. asphodeloides; northern China, the Korean Peninsula).
Anemarrhena is sister to [Agavaceae+[Anthericaceae+[Behniaceae+Herreriaceae]]].
ANTHERICACEAE J. Agardh |
( Back to Iridales ) |
Genera/species 9/340–355
Distribution Europe, northern and eastern Africa, Madagascar, South and East Asia, northern and eastern Australia, southwestern North America to South America.
Fossils Unknown.
Habit Bisexual, perennial herbs (stem in Chlorophytum suffruticosum more or less lignified).
Vegetative anatomy Roots fibrous or thick and fleshy (sometimes with root nodules); velamen present at least in Anthericum and Chlorophytum; root exodermis sometimes thickened. Phellogen absent. Secondary lateral growth absent. Vessels present at least in roots (sometimes in stem). Vessel elements usually with simple (in stem also scalariform) perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays absent? Axial parenchyma? Sieve tube plastids P2c type, with cuneate protein crystals. Nodes? Styloids present in Chlorophytum. Mucilage cells and mucilage canals present at least in Echeandia. Tanniniferous cells absent. Silica bodies and laticifers absent. Calciumoxalate raphides usually abundant.
Trichomes Hairs?, usually absent.
Leaves Alternate (spiral or distichous), simple, entire, usually linear, with ? ptyxis. Stipules absent; leaf sheath well developed. Venation parallelodromous. Stomata anomocytic. Cuticular wax crystalloids as platelets, parallel to stomata (Convallaria type). Mesophyll with calciumoxalate raphides. Leaf margin entire.
Inflorescence Terminal, thyrsoid, panicle or raceme-like (rarely umbel-like), with bostrychoid partial inflorescences, or raceme.
Flowers Usually actinomorphic (rarely zygomorphic). Pedicel usually articulated. Pericladium usually present. Hypogyny. Tepals 3+3, petaloid, often marcescent, usually free (in Diora and Leucocrinum connate at base into a tube). Septal nectaries present. Disc absent.
Androecium Stamens 3+3. Filaments usually free (in Echeandia connate), usually adnate at base (in Leucocrinum for most of their length) to tepals. Anthers basifixed or dorsifixed, often versatile, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory, usually with binucleate cells. Staminodia absent.
Pollen grains Microsporogenesis successive. Pollen grains monosulcate, shed as monads, bicellular at dispersal. Exine semitectate, with columellate infratectum, reticulate.
Gynoecium Pistil composed of three connate carpels. Ovary superior, trilocular (in Chlorophytum stipitate, on gynophore). Style single, simple, filiform. Stigma capitate or trilobate, usually papillate (with unicellular papillae), Dry type. Pistillodium absent.
Ovules Placentation axile. Ovules two to numerous per carpel, anatropous or campylotropous, bitegmic, crassinucellar. Micropyle ?-stomal. Outer integument approx. four cell layers thick. Inner integument two cell layers thick. Hypostase present. Parietal cell formed from archesporial cell. Nucellar cap in Leucocrinum formed by periclinal cell divisions in apical part of megasporangial epidermis. Megagametophyte monosporous, Polygonum type. Synergids with a filiform apparatus (Chlorophytum). Antipodal cells often persistent. Megagametophyte haustoria frequently present. Endosperm development helobial. Endosperm haustoria? Embryogenesis?
Frui A loculicidal capsule (rarely a schizocarp or a nut) often with persistent tepals.
Seeds Aril absent. Seed coat testal. Exotesta with phytomelan layer on epidermal cell walls. Outer testal layers sometimes with thickened periclinal cell walls. Endotesta? Tegmen? Perisperm not developed. Endosperm copious, fleshy, with lipids and starch; endosperm cell walls with pits. Embryo cylindrical, incurved, without chlorophyll. Cotyledon one, short, with apex transformed into haustorium, not photosynthesizing, in Chlorophytum with a tubular elongation of cotyledon sheath. Cotyledon hyperphyll? Hypocotyl internode? Mesocotyl absent. Coleoptile present in Chlorophytum. Germination cryptocotylar.
Cytology n = 7, 8, 10, 11, 13–15 or more – Polyploidy frequently occurring. Chromosomes 2–10 (to 13.8) µm long.
DNA The mitochondrial gene rpl2 is absent (lost). A genome duplication has taken place in Chlorophytum.
Phytochemistry Steroidal saponins, phenylalanine-derived cyanogenic glycosides (in Chlorophytum), and chelidonic acid present. Flavonols, ellagic acid, alkaloids, and anthraquinones not found.
Use Ornamental plants.
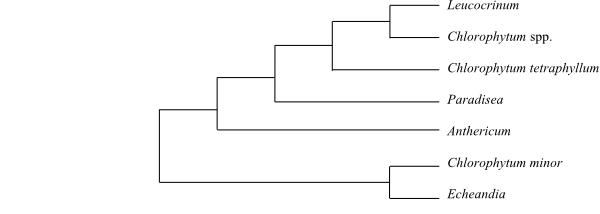
Systematics Echeandia (75–80; southwestern United States, Mexico, Central America, northern South America to Peru), Anthericum (c 65; Europe, North and East Africa to Tanzania, southwestern Asia), Paradisea (2; P. liliastrum: mountains in southern Europe; P. lusitanica: the Iberian Peninsula), ‘Chlorophytum’ (190–200; Africa, Madagascar, India to northern and eastern Australia; polyphyletic), Leucocrinum (1; L. montanum; southwestern United States). – Unplaced Anthericaceae: Diamena (1; D. stenantha; upper Cerro de las Cabras near Trujillo in Peru, possibly extinct), Diora (1; D. cajamarcaensis; the Andes in Peru), Eremocrinum (1; E. albomarginatum; Utah, northern Arizona), Hagenbachia (6; H. brasiliensis, H. columbiana, H. ecuadorensis, H. hassleriana, H. matogrossensis, H. panamensis; Costa Rica, Panamá, tropical South America).
Anthericaceae are sister-group to [Behnia+[Herreriaceae]].
The Echeandia clade is sister to the remaining Anthericaceae (Kim & al. 2010).
 |
Cladogram (simplified) of Anthericaceae based on DNA sequence data (Kim & al. 2010). |
APHYLLANTHACEAE Burnett |
( Back to Iridales ) |
Genera/species 1/1
Distribution Western Mediterranean from southern France to northern Morocco.
Fossils Unknown.
Habit Bisexual, perennial herbs. Xeromorphic, with photosynthesizing stems. Tufts consisting of long-scapose inflorescences.
Vegetative anatomy Roots fibrous; mature roots without cortex; all root tissues except phloem lignified. Rhizome stele differentiated into inner, primary, irregularly oriented traces and outer, secondary, serial vascular traces. Phellogen absent. Secondary lateral growth (in rhizome) anomalous. Vessels present in roots. Vessel elements with simple or scalariform perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial parenchyma? Sieve tube plastids P2c type (in roots), with cuneate protein crystals. Nodes? Mucilage cells abundant. Idioblasts with calciumoxalate raphides frequent in rhizome cortex. Cuticular wax crystalloids on stems as non-orientated platelets.
Trichomes Hairs absent.
Leaves Alternate (distichous), simple, entire, scale-like, reduced to non-photosynthesizing leaf sheath; uppermost leaf with rudimentary pseudolamina and with short ligule, with supervolute subinvolute ptyxis. Stipules absent; leaf sheath well developed. Venation parallelodromous. Stomata absent from leaves. Mesophyll often with calciumoxalate raphides. Epidermal cells of abaxial side elongate, with thickened and lignified walls. Leaf margin entire.
Inflorescence Terminal, unbranched, one- or few-flowered capitate. Peduncle (scape) long and narrow, terete, photosynthesizing, with thickened epidermis and rows of deeply sunken anomocytic stomata in upper part; medullary parenchyma often collapsing and replaced by an air canal; cuticle thick; cuticular wax crystalloids as parallel rodlets (Convallaria type); calciumoxalate as raphides. Bracts dry, scale-like, membranous, one or two free and five connate.
Flowers Actinomorphic. Pedicel not articulated, very short, or absent. Hypogyny. Tepals 3+3, petaloid, clawed, marcescent, free. Septal nectaries infralocular. Disc absent.
Androecium Stamens 3+3. Filaments free from each other, adnate to base of tepals (epitepalous). Anthers dorsifixed, versatile?, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory. Staminodia absent.
Pollen grains Microsporogenesis successive. Pollen grains spiraperturate, shed as monads, bicellular at dispersal. Exine tectate, with columellate? infratectum, echinate.
Gynoecium Pistil composed of three connate carpels. Ovary superior, trilocular. Style single, simple, with stylar canal. Stigma trilobate with short filiform lobes, papillate, Dry type. Pistillodium absent.
Ovules Placentation axile. Ovule one per carpel, anatropous or somewhat campylotropous-amphitropous, bitegmic, crassinucellar. Micropyle ?-stomal. Outer integument three or four cell layers thick. Inner integument two cell layers thick. Parietal cell formed from archesporial cell. Megagametophyte monosporous, Polygonum type. Endosperm development helobial. Endosperm haustoria? Embryogenesis?
Fruit A loculicidal capsule with persistent style.
Seeds Aril absent. Seed coat exotestal. Exotesta with thin phytomelan layer on epidermal cell walls. Mesotesta, endotesta and tegmen more or less collapsed. Perisperm not developed. Endosperm copious, with lipids and aleurone (starch absent). Embryo large, straight, well differentiated, with chlorophyll. Cotyledon one, well developed; cotyledon leaf sheath with ligule. Cotyledon hyperphyll elongate, terete, assimilating. Hypocotyl internode short. Coleoptile absent. Germination cryptocotylar?
Cytology n = 16
DNA The mitochondrial gene rpl2 is absent (lost).
Phytochemistry Flavonols, steroidal saponins (genin, 25D- and 25L-saponogene), and wax alcohols present. Chelidonic acid? Ellagic acid, proanthocyanidins, and alkaloids not found.
Use Unknown.
Systematics Aphyllanthes (1; A. monspeliensis; southern France, the Iberian Peninsula, northern Morocco).
In the analysis by Wurdack & Dorr (2009), Aphyllanthes is recovered as part of a trichotomy in the same clade as Hyacinthaceae, Themidaceae, Anemarhenaceae and the Agavaceae group.
ASPARAGACEAE Juss. |
( Back to Iridales ) |
Asparagales Link, Handbuch 1: 272. 4-11 Jul 1829 [’Asparaginae’]; Asparagineae J. Presl in Nowočeská Bibl. [Wšobecný Rostl.]7: 1556. 1846 [‘Asparageae’]; Hemiphylacaceae Doweld, New Syllabus Plant Fam.: 921. Apr 2007
Genera/species 2/165–195(–300?)
Distribution Eurasia, Africa, Australia, Mexico.
Fossils Unknown.
Habit Usually bisexual (sometimes monoecious, andromonoecious, dioecious, or gynodioecious), evergreen or deciduous shrubs or suffrutices, or perennial herbs, often twining and climbing. Tuberous stem present in Asparagus ovatus and A. undulatus. Branches usually green and assimilating. Branch ends in Asparagus transformed into foliaceous or terete phyllocladia. Spines consisting of transformed branches or leaf bases present in some species.
Vegetative anatomy Lateral roots sometimes tuberous; sometimes with membranous multiple velamen; roots in Hemiphylacus contractile. Phellogen? Secondary lateral growth absent. Vessels present in roots and stem. Vessel elements with scalariform (in roots often also simple) perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays?, Axial parenchyma? Sieve tube plastids P2c type, with cuneate protein crystals. Nodes? Idioblasts (raphide cells) with calciumoxalate raphides.
Trichomes Hairs usually absent (rarely unicellular?).
Leaves Alternate (spiral), simple, entire, needle- or scale-like (in Hemiphylacus linear), with basal spur (sometimes transformed into spine), with ? ptyxis. Stipules and leaf sheath absent. Venation parallelodromous. Stomata anomocytic. Cuticular wax crystalloids as parallel platelets (Convallaria type) or absent. Mesophyll usually with mucilaginous idioblasts containing calciumoxalate raphides. Leaf margin entire. Extrafloral nectaries present in some species.
Inflorescence Axillary, fasciculate, panicle or umbel-like, or flowers solitary (in Hemiphylacus raceme or thyrse). Floral prophylls (bracteoles?) in Hemiphylacus lateral.
Flowers Actinomorphic, usually small. Pedicel articulated; pericladium separated from pedicel by a swelling. Hypogyny. Tepals 3+3, petaloid, free or connate in lower part or at base into a tube (in Hemiphylacus connate half-way up, twisted following anthesis). Septal nectaries present. Disc absent.
Androecium Stamens usually 3+3 (in Hemiphylacus three fertile stamens and three staminodia). Filaments filiform or flattened, free from each other, adnate at base to tepals; in Hemiphylacus three inner antepetalous fertile stamens adnate to inner tepals, and three outer staminodia adnate to outer tepals. Anthers usually basifixed (in Hemiphylacus dorsifixed), versatile, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory. Female flowers with staminodia. In Hemiphylacus flowers bisexual, with three staminodia.
Pollen grains Microsporogenesis successive. Pollen grains monosulcate, starchy, shed as monads, bicellular at dispersal. Exine tectate or semitectate, with columellate? infratectum, psilate-microperforate or somewhat reticulate.
Gynoecium Pistil composed of three connate carpels; carpels in Hemiphylacus antepetalous. Ovary superior, trilocular (sometimes slightly stipitate). Style single, simple, with stylar canal. Stigma capitate to trilobate, Dry or Wet type. Male flowers with pistillodium.
Ovules Placentation axile. Ovules two to twelve (in Hemiphylacus three to six) per carpel, hemianatropous or almost orthotropous (in Hemiphylacus campylotropous), bitegmic, crassinucellar. Micropyle at least in Hemiphylacus endostomal. Outer integument five to seven cell layers thick. Inner integument two cell layers thick. Obturator present near micropyle in Hemiphylacus. Hypostase present in Hemiphylacus. Parietal cell in Hemiphylacus formed from archesporial cell. Micropylar megasporangial epidermal cells enlarged and forming a uniseriate layer, crushed in mature seed (also in Hemiphylacus). Nucellar cap absent. Megagametophyte monosporous, Polygonum type. Megagametophyte curved and asymmetrical (also in Hemiphylacus). Endosperm development usually nuclear (in Hemiphylacus helobial). Endosperm haustoria? Embryogenesis?
Fruit Usually a one- or several-seeded berry (rarely a nut; in Hemiphylacus a loculicidal capsule).
Seeds Aril absent. Pseudo-operculum sometimes present. Seed coat exotestal. Testa multiplicative. Exotesta massive, with phytomelan layer on epidermal cell walls (always?). Mesotesta and endotesta collapsed. Tegmen inconspicuous, collapsed. Perisperm not developed. Endosperm copious, with oil, aleurone and hemicellulose (starch absent); endosperm cells thick, with pitted walls. Embryo large, straight to curved, well differentiated, without chlorophyll. Cotyledon one. Cotyledon hyperphyll? Hypocotyl internode short. Coleoptile absent. Radicula persistent. Germination?
Cytology n = 10 (Asparagus), n = 56 (Hemiphylacus) – Polyploidy frequently occurring in Asparagus. Sex chromosomes present in, e.g., Asparagus officinalis. Chromosomes in Hemiphylacus 1–3 µm long.
DNA The mitochondrial gene rpl2 is absent (lost).
Phytochemistry Flavonols (kaempferol, quercetin), steroidal saponins, acetidine carbonic acid, and chelidonic acid present. Carbohydrates stored as inulin-like fructans and mannans. Ellagic acid, proanthocyanidins, alkaloids, and cyanogenic compounds not found.
Use Ornamental plants, vegetables (Asparagus officinalis, A. albus etc.).
Systematics Asparagus (160–190[–300?]; mainly arid and subarid regions in Europe, Africa, Madagascar, the Mascarene Islands, Asia, and one species in Australia), Hemiphylacus (5; H. alatostylus, H. hintoniorum, H. latifolius, H. mahindae, H. novogalicianus; semiarid regions in central Mexico).
Asparagaceae are sister to Ruscaceae.
ASPHODELACEAE Juss. |
( Back to Iridales ) |
Aloaceae Batsch, Tab. Affin. Regni Veg.: 138. 2 Mai 1802 [‘Alooideae’]; Asphodelales Doweld, Tent. Syst. Plant. Vasc.: lvii. 23 Dec 2001; Asphodelineae Thorne et Reveal in Bot. Rev. (Lancaster) 73: 80. 29 Jun 2007
Genera/species 18–19/790–830
Distribution Southern and eastern Europe, the Mediterranean, Africa, Madagascar, Socotra, the Arabian Peninsula, the Mascarene Islands, West and Central Asia, Australia, New Zealand, with their largest diversity in South Africa.
Fossils Uncertain.
Habit Bisexual, usually perennial herbs (some species of Aloe and Kniphofia are pachycaul shrubs or trees). Rarely with bulb. Many representatives are leaf succulents. Roots often somewhat succulent, sometimes contractile, sometimes swollen.
Vegetative anatomy Root velamen present in some species. Stem usually fibrous. Phellogen? Secondary lateral growth anomalous (present especially in Aloe, but also in Bulbine, Kniphofia and Trachyandra) or absent. Vessels present at least in roots (in some species also in stem). Vessel elements in roots usually with simple perforation plates, in stem with scalariform perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays? Axial parenchyma? Sieve tube plastids P2c type, with cuneate protein crystals, or (in Alooideae) P2cf type, with cuneate protein crystals and peripheral protein filaments. Nodes? Mucilage cells with calciumoxalate as raphides or prismatic crystals.
Trichomes Hairs absent?
Leaves Alternate (spiral or distichous), simple, entire, often linear or subulate, in many species succulent (some species have ‘window leaves’ with transparent tissue in distal part), with ? ptyxis. Stipules absent; leaf sheath usually closed (sometimes absent). Venation parallelodromous; veins often indistinct; vascular bundles often inverted; large group of thin-walled parenchymatous (secretory?) aloin cells present adjacent to phloem in envelope of inner vascular bundle (in, e.g., Kniphofia these cells with lignified walls). Stomata usually anomocytic (rarely paracytic; in Alooideae tetracytic). Cuticular wax crystalloids as non-orientated rodlets or platelets. Mesophyll with mucilaginous idioblasts containing calciumoxalate as raphides or single prismatic crystals. Leaf margin serrate or entire. Leaf apex often pointed.
Inflorescence Terminal, panicle, raceme- or spike-like, often branched.
Flowers Usually actinomorphic (in Haworthia and few species of Aloe weakly zygomorphic, bilabiate, in Gasteria curved). Pedicel in Asphodelus, Asphodeline and Bulbine articulated. Hypogyny. Tepals 3+3, petaloid, usually free (in Kniphofia and Aloeae connate into a tubular or campanulate perianth). Septal nectaries well developed, infralocular. Disc absent.
Androecium Stamens 3+3. Filaments subulate to filiform, free from each other and from tepals (in Bulbine with numerous long hairs). Anthers usually dorsifixed (in Eremurus basifixed; filaments often? inserted in a connective pit), versatile?, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory (tapetal material slightly invading anther locule at tetrad stage in Aloe). Staminodia absent.
Pollen grains Microsporogenesis simultaneous. Pollen grains monosulcate or trichotomosulcate, shed as monads, bicellular at dispersal. Exine tectate or semitectate, with columellate infratectum, perforate or reticulate.
Gynoecium Pistil composed of three connate carpels. Ovary superior, trilocular. Style single, simple. Stigma punctate, usually Dry (sometimes Wet) type. Pistillodium absent.
Ovules Placentation axile. Ovules one to more than 40 per carpel, anatropous, orthotropous (Asphodeleae), hemitropous or campylotropous, bitegmic, crassinucellar. Micropyle usually endostomal? (in Kniphofia bistomal). Outer integument three or four cell layers thick. Inner integument two cell layers thick. Parietal cell formed from archesporial cell, forming parietal tissue between megasporocyte and megasporangial epidermis. Hypostase present. Megasporangial endothelium present in Kniphofia. Megagametophyte monosporous, Polygonum type. Synergids in Asphodelus with a filiform apparatus. Endosperm development ab initio helobial. Endosperm haustoria? Embryogenesis onagrad, asterad or chenopodiad.
Fruit A loculicidal capsule (in some species of Aloe a fleshy berry-like capsule).
Seeds Seeds sometimes winged. Arilloid structure formed as annular invagination at distal part of funicle (seemingly from an extra ‘integument’), two or several cell layers thick. Seed coat exotestal. Exotesta usually with phytomelan layer on epidermal cell walls. Endotesta and tegmen collapsed. Perisperm usually absent (sometimes sparsely developed). Endosperm copious, thick-walled, with lipids and aleuronic (sometimes with hemicellulose?). Embryo long, straight, well differentiated, without chlorophyll. Cotyledon one. Cotyledon hyperphyll compact, not assimilating. Hypocotyl internode present or absent. Mesocotyl absent. Coleoptile sometimes present. Germination cryptocotylar?
Cytology n = 2–7, 12–16, 20, 40, 44, 49; x = 7 (Aloeae) – Karyotype in Aloeae bimodal, in Bulbine etc. with four long and three short chromosomes. Chromosomes 1,5–20 µm long.
DNA The mitochondrial gene rpl2 is absent. Loss of the 3’-rps12-intron (Bulbine?).
Phytochemistry Flavonoids, alkaloids, cyanogenic substances, anthraquinones (1-methyl-8-hydroxyanthraquinones in roots), anthrones (e.g. anthrone-C-glycoside [aloin, barbaloin] in leaves), tetrahydroanthracenones (chrysophanol, 10,7’-bichrysophanol, knipholone, asphodeline and other lipophilic anthranoid aglucones), chromones, phenylpyrones, polyacetate derived arthroquinones, phenolic amines, chelidonic acid, bitter substances, and resins present. Mannans present as storage carbohydrates. Flavonols, ellagic acid, proanthocyanidins, and steroidal saponins not found.
Use Ornamental plants, medicinal plants and cosmetics (Aloe vera, A. ferox etc.).
Systematics Asphodelaceae are probably sister-group to [Hemerocallidaceae+Xanthorrhoeaceae] (or, alternatively, to Xanthorrhoea).
A probable topology is [Asphodeleae+[Kniphofieae+Aloeae]] (Treutlein & al. 2003).
Asphodeleae Lam. et DC., Syn. Plant. Fl. Gall.: 160. 30 Jun 1806 [‘Asphodeli’]
2/29. Asphodelus (12; the Mediterranean, southwestern Asia), Asphodeline (17; the Mediterranean to Turkey, the Caucasus and Iran). – The Mediterranen, southwestern Asia. – Asphodeleae are sister to all other Asphodelaceae.
[Kniphofieae+Aloeae]
Kniphofieae Hutch., Fam. Fl. Plants 2: 83, 90. 20 Jul 1934
4/195–210. Eremurus (55–60; eastern Europe to Russia, temperate and alpine regions in West and Central Asia, China), Trachyandra (50–55; tropical and southern Africa, Madagascar, with their highest diversity in Western Cape); Bulbinella (c 23; Northern, Western and Eastern Cape, New Zealand), Kniphofia (65–70; tropical and southern Africa, Madagascar, the Arabian Peninsula). – Eastern Europe to Central Asia, tropical and southern Africa, Madagascar, the Arabian Peninsula, New Zealand. Aloin cells are absent and replace by a well developed sclerenchymatic cap (n.b. also in some Aloeae). Aloin cells have also been reported from Dianella (Hemerocallidaceae). Bulbinella and Kniphofia have knipholone.
Aloeae A. Rich. in C. H. D. d’Orbigny, Dict. Univ. Hist. Nat. 1: 291. 18 Dec 1841 [‘Aloineae’]
12–13/565–590. Bulbine (c 50; tropical and southern Africa, Australia, with their highest diversity in Western Cape; incl. Jodrellia?), Jodrellia (2; J. fistulosa, J. migiurtina; Central to northeastern Africa; in Bulbine?); Aloidendron (7; A. barberae, A. dichotomum, A. eminens, A. pillansii, A. ramosissimum, A. sabaeum, A. tongaense; southern Africa, Somalia), Kumara (2; K. haemantifolia, K. plicatilis; Western Cape), Haworthia (c 40; Namibia, South Africa, with their highest diversity in Western and Eastern Cape), Aloiampelos (7; A. ciliaris, A. commixta, A. decumbens, A. gracilis, A. juddii, A. striatula, A. tenuior; southern and eastern South Africa, especially Western and Eastern Cape, Swaziland), Aloe (400–420; tropical, eastern and southern Africa, Madagascar, the Arabian Peninsula, Socotra), Gasteria (25–30; Namibia, South Africa, Mozambique), Haworthiopsis (18; Western Cape), Astroloba (8; Western and Eastern Cape), Aristaloe (1; A. aristata; mountains in southeastern South Africa), Gonialoe (3; G. dinteri, G. sladeniana, G. variegata; southern Angola, Namibia, arid regions in South Africa), Tulista (4; T. kingiana, T. marginata, T. minima, T. pumila; Western Cape). – Africa, Madagascar, the Arabian Peninsula, Socotra, Australia, with their largest diversity in South Africa. Sometimes shrub-like. Sieve tube plastids P2cf type, with cuneate protein crystals and peripheral protein filaments. Stomata usually tetracytic. Foliar vascular bundles forming a cylinder; globuli present in outer vascular bundle envelope (also present in Kniphofia). Karyotype bimodal. 1-methyl-8-hydroxy anthraquinones (e.g. chrysophanol) present in roots and anthrone-C-glycosides in leaves. – Bulbine (including Jodrellia?) is sister to the remaining Aloeae. Bulbine has knipholone and a bimodal karyotype based on n = 7: four long and three short chromosomes.
 |
Phylogeny (simplified) of Asphodelaceae based on DNA sequence data (Treutlein & al. 2003; Daru & al. 2012; Grace & al. 2013). The following changes have been made in accordance with Manning & al. (2014): Haworthia only includes Haworthia Subgenus Haworthia; the species in ‘Haworthia’ Subgenus Robustipedunculares are included in Tulista; likewise, ‘Haworthia’ Subgenus Hexangulares II comprises Haworthiopsis; ‘Aloe’ aristata is now Aristaloe aristata; finally, ‘Aloe’ Section Serrulatae comprises Gonialoe. |
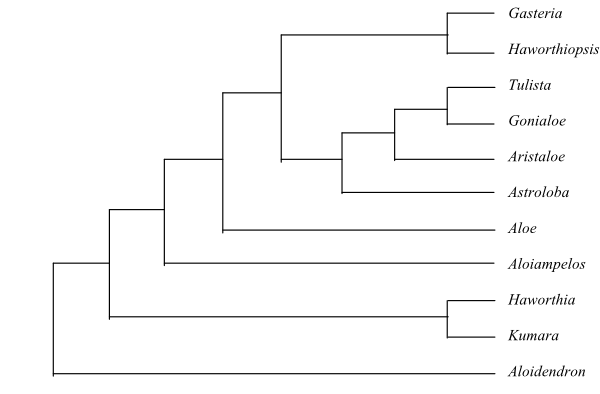 |
Phylogeny of Asphodelaceae based on Manning & al. (2014). |
ASTELIACEAE Dumort. |
( Back to Iridales ) |
Asteliales Dumort., Anal. Fam. Plant.: 59. 1829 [‘Astelarieae’]
Genera/species 3/36
Distribution The Mascarene Islands, New Guinea, New Caledonia, Fiji, Samoa, the Society Islands, the Marquesas Islands, the Hawaiian Islands, temperate regions in southeastern Australia, Tasmania, New Zealand, Auckland, Campbell and Chatham Islands, southern Chile and Argentina, the Falkland Islands.
Fossils Pollen of ‘Astelia’ is known from the Late Eocene onwards in New Zealand, and macrofossils from the Miocene of New Zealand.
Habit Bisexual (Milligania), dioecious or gynodioecious, perennial herbs. Rarely epiphytic.
Vegetative anatomy Phellogen absent. Secondary lateral growth absent. Vessels present in roots. Vessel elements with scalariform perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial parenchyma? Sieve tube plastids P2c type, with cuneate protein crystals, or P2cs type, with cuneate protein crystals and starch grains. Nodes? Idioblasts with calciumoxalate raphides frequent. Crystals of various shape present in Astelia and Collospermum.
Trichomes Hairs unicellular or multicellular, uniseriate or multiseriate, stellate or lepidote, often long and with multicellular base, marginal cells often caducous.
Leaves Alternate (spiral), simple, entire, often linear, with ? ptyxis. Stipules absent; leaf sheath open or closed (sometimes absent). Venation parallelodromous; often with two distinct lateral veins (often larger than midvein) and, in connection with these, a well developed sclerenchyma. Stomata paracytic. Cuticular wax crystalloids as parallel platelets (Convallaria type). At least young leaves densely pubescent. Adaxial hypodermis present. Mesophyll with calciumoxalate as raphides and single prismatic crystals. Some species of ‘Astelia’ and Collospermum with secretory cavities (mucilaginous canals). Leaf margin entire.
Inflorescence Terminal, branched system of racemes or spikes. Bracts large. Floral prophylls (bracteoles) absent.
Flowers Actinomorphic. Usually hypogyny (in Milligania half epigyny). Tepals usually 3+3 (in Neoastelia spectabilis sometimes 5+5 or 7+7), sepaloid, petaloid or bract-like, membranous or fleshy, usually connate at base (sometimes free). Septal nectaries branched, in furrows on ovary, sometimes external, on abaxial side of ovary (in Milligania short, in upper part of ovary). Disc absent.
Androecium Stamens 3+3. Filaments usually free (in Milligania adnate at base to tepals). Anthers basifixed to dorsifixed, versatile?, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory, with binucleate cells. Female flowers with staminodia.
Pollen grains Microsporogenesis usually successive (in Milligania simultaneous). Pollen grains usually monosulcate (sometimes trichotomosulcate), shed as monads, bicellular at dispersal. Exine tectate, with columellate? infratectum, foveolate, echinate, spinulate, or smooth.
Gynoecium Pistil composed of three (to seven) connate carpels. Ovary usually superior (in Milligania semi-inferior), usually trilocular (in some species of ‘Astelia’ unilocular), in many species of ‘Astelia’ and Collospermum with mucilage hairs on inner side (from funicles and placentae). Stylodia usually absent (in Milligania short). Stigma usually trilobate, decurrent (in some species of ‘Astelia’ connate into a wide stigmatic surface), papillate, Dry type. Male flowers with pistillodium (sometimes with fertile ovules).
Ovules Placentation usually axile (in some species of ‘Astelia’ parietal). Ovules four to c. 15? per carpel, anatropous, bitegmic, pseudocrassinucellar. Funicle hairy in many species (not in Milligania). Micropyle bistomal, also Z-shaped (zig-zag). Outer integument two to five cell layers thick. Inner integument two cell layers thick. Parietal cell formed from archesporial cell, degenerating (parietal tissue absent). Hypostase present. Nucellar cap absent. Megagametophyte monosporous, Polygonum type, with chalazal constriction. Antipodal cells persistent. Endosperm development helobial. Endosperm haustoria? Embryogenesis?
Fruit Usually a berry (in Milligania a loculicidal capsule dehiscing from apex).
Seeds Aril absent. Seeds in Collospermum surrounded by mucilage hairs. Seed coat exotestal. Testa hard. Exotesta with phytomelan layer on epidermal cell walls. Endotesta collapsed? Tegmen in ‘Astelia’ reduced to a layer of empty cells. Perisperm not developed. Endosperm copious, with oil and aleurone (starch and hemicellulose absent). Embryo straight or somewhat curved, well differentiated, chlorophyll? Cotyledon one. Cotyledon hyperphyll elongate to compact, often assimilating. Hypocotyl internode short. Coleoptile absent. Radicula well developed, ephemeral. Germination?
Cytology n = 8, 30, 35, 105 (x = 8) – Polyploidy occurring. Chromosomes 4–6 μm long.
DNA
Phytochemistry Flavonols (kaempferol, quercetin, isorhamnetin), steroidal saponins, and chlorogenic acid present. Ellagic acid and proanthocyanidins not found.
Use Unknown.
Systematics Astelia (30; the Mascarene Islands, New Guinea, New Caledonia, temperate parts of southeastern Australia, Tasmania, New Zealand, Auckland, Campbell and Chatham Islands, Vanuatu, Fiji, Samoa, the Society Islands, the Marquesas Islands, the Hawaiian Islands, southern Chile and Argentina, the Falkland Islands), Neoastelia (1; N. spectabilis; northeastern New South Wales), Milligania (5; M. densiflora, M. johnstonii, M. lindoniana, M. longifolia, M. stylosa; Tasmania).
Collospermum is nested within Astelia. Milligania and Neoastelia are sister-groups.
In a study by Kocyan & al. (2011), Asteliaceae form a paraphyletic grade basal to Blandfordia (Blandfordiaceae), Lanaria (Lanariaceae) and Hypoxidaceae.
 |
Bayesian inference 50% consensus tree (simplified) of Asteliaceae based on plastid and nuclear DNA (Birch & al. 2012). |
BEHNIACEAE Conran, M. W. Chase et Rudall |
( Back to Iridales ) |
Genera/species 1/1
Distribution Southeastern Africa.
Fossils Unknown.
Habit Dioecious (cryptodioecious), perennial herb with more or less lignified stems, occasionally somewhat twining or climbing. Xeromorphic. Aerial roots absent.
Vegetative anatomy Roots fibrous; velamen one-layered; root exodermis uniseriate. Phellogen? Stem cortex with a cylinder of fibre bundles and small collateral vascular bundles; centre of stem with scattered bundles. Rhizome with lignified secondary xylem, parenchymatous secondary phloem and anomalous secondary lateral growth. Vessels present in roots and stem. Vessel elements with simple or scalariform perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial parenchyma? Sieve tube plastids P2c type, with cuneate protein crystals. Nodes? Mucilage cells and laticifers absent. Tanniniferous cells absent. Silica bodies absent. Calciumoxalate raphides abundant; druses sometimes present.
Trichomes Hairs absent?
Leaves Alternate (distichous), simple, entire, linear, with supervolute? ptyxis, differentiated into pseudopetiole and pseudolamina. Stipules and leaf sheath absent. Venation pinnate-parallelodromous, acrodromous; midvein distinct, longitudinal main veins connected with parallel transverse secondary veins. Stomata paracytic; subsidiary cells with oblique division. Cuticular wax crystalloids? Mesophyll with calciumoxalate raphides. Leaf margin entire.
Inflorescence Axillary, bostryx, or flowers solitary.
Flowers Actinomorphic, small. Pedicel articulated. Pericladium short. Hypogyny. Tepals 3+3, petaloid, marcescent, connate into a tube. Septal nectaries present (also in male flowers). Disc absent.
Androecium Stamens 3+3. Filaments filiform, free from each other, adnate in lower part to tepals (epitepalous). Anthers basifixed, non-versatile, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory, with binucleate cells. Female flowers with staminodia.
Pollen grains Microsporogenesis successive. Pollen grains monosulcate, shed as monads, bicellular at dispersal. Exine semitectate, with columellate infratectum, reticulate, spinulate.
Gynoecium Pistil composed of three connate carpels. Ovary superior, trilocular, on gynophore. Style single, simple. Stigma trilobate, papillate, Wet type. Male flowers with pistillodium.
Ovules Placentation axile. Ovules two or three per carpel, anatropous, bitegmic, tenuinucellar (crassinucellar?). Micropyle endostomal. Outer integument three or four cell layers thick. Inner integument two cell layers thick. Hypostase present. Parietal cell formed from archesporial cell. Parietal tissue absent. Nucellar cap present. Megagametophyte monosporous, Polygonum type. Antipodal cells persistent. Endosperm development helobial. Endosperm haustoria? Embryogenesis?
Fruit A few- to many-seeded berry.
Seeds Aril absent. Testal and tegmic cells thin-walled. Phytomelan layer probably absent. Exotesta collapsed, sometimes caducous. Endotesta parenchymatous, partially collapsed. Tegmen crushed. Perisperm not developed. Endosperm copious, with oil and aleurone (starch and hemicellulose absent); endosperm cells walls not pitted. Embryo short, straight, chlorophyll? Cotyledon one, little differentiated (ligule absent). Cotyledon hyperphyll? Hypocotyl internode? Coleoptile absent? Germination?
Cytology n = ?
DNA The mitochondrial gene rpl2 is absent (lost).
Phytochemistry Very insufficiently known. Steroidal saponins present.
Use Unknown.
Systematics Behnia (1; B. reticulata; Zimbabwe, South Africa, Swaziland).
Behnia is sister to Herreriaceae.
BLANDFORDIACEAE R. Dahlgren et Clifford |
( Back to Iridales ) |
Genera/species 1/4
Distribution Eastern Australia, Tasmania.
Fossils Unknown.
Habit Bisexual, perennial herbs. Rhizome tuberous.
Vegetative anatomy Roots fibrous, with uniseriate velamen. Root cortex starchy. Phellogen absent. Stem cortex collenchymatous, reduced, with a thick cylinder of lignified fibre bundles; inner cortex with small collateral bundles; centre of stem hollow. Secondary lateral growth absent. Vessels present in roots. Vessel elements with scalariform or simple perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial parenchyma? Sieve tube plastids P2c, with cuneate protein crystals. Nodes? Calciumoxalate druses present. Raphides and styloids absent.
Trichomes Hairs absent.
Leaves Alternate (distichous), simple, entire, linear, with flat, curved or somewhat concave ptyxis. Stipules absent; leaf sheath well developed. Venation parallelodromous; midvein prominent. Stomata anomocytic. Cuticular wax crystalloids? Secretory cavities absent. Mesophyll often with cells containing calciumoxalate druses (raphides absent). Leaf margin entire.
Inflorescence Usually terminal, simple raceme; flowers sometimes solitary axillary. Floral prophylls (bracteoles) two.
Flowers Actinomorphic, large. Pedicel articulated. Pericladium long, pedicel-like. Hypogyny. Tepals 3+3, petaloid, persistent, connate into a long tube. Septal nectaries external, as deep furrows on abaxial side of ovary and gynophore. Disc absent.
Androecium Stamens 3+3. Filaments filiform, free from each other, adnate in lower part to perianth tube (epitepalous). Anthers centrifixed (seemingly basifixed), versatile, tetrasporangiate, latrorse, longicidal (dehiscing by longitudinal slits); connective hollow in lower part and sheathing filament. Tapetum secretory, with binucleate cells. Staminodia absent.
Pollen grains Microsporogenesis simultaneous. Pollen grains trichotomosulcate, shed as monads, bicellular at dispersal. Exine tectate? with ? infratectum, perforate?, verrucate?
Gynoecium Pistil composed of three connate carpels. Ovary superior, trilocular, stipitate (on gynophore). Style single, simple, short. Stigma entire, somewhat trilobate, punctate, with three furrows, papillate?, Dry type. Pistillodium absent.
Ovules Placentation axile. Ovules c. 40 to c. 50 per carpel, anatropous, bitegmic, pseudotenuinucellar (functionally tenuinucellar). Micropyle endostomal. Outer integument four or five cell layers thick, somewhat shorter than inner integument. Inner integument two cell layers thick. Parietal cell formed from archesporial cell, early degenerating (parietal tissue absent). Hypostase present. Nucellar cap approx. two cell layers thick, formed by periclinal divisions of epidermal cells of megasporangium. Megagametophyte monosporous, Polygonum type, with chalazal constriction. Synergids with a filiform apparatus. Antipodal cells persistent, sometimes becoming binucleate. Endosperm development helobial. Endosperm haustoria? Embryogenesis?
Fruit A septicidal capsule with accrescent gynophore.
Seeds Aril absent. Seed coat exotestal. Exotesta with papillar hairs, without phytomelan. Endotesta? Tegmen? Perisperm not developed. Endosperm copious, with oil and aleurone (without starch). Embryo short, somewhat curved, well differentiated, chlorophyll? Cotyledon one, as a photosynthesizing hyperphyll without leaf sheath. Hypocotyl internode? Coleoptile absent? Germination phanerocotylar.
Cytology n = 17, 27
DNA
Phytochemistry Flavonols (kaempferol, quercetin), flavone-C-glycosides, cyanidin, and chelidonic acid present. Steroidal saponins? Ellagic acid and cyanogenic compounds not found.
Use Ornamental plants.
Systematics Blandfordia (4; B. cunninghamii, B. grandiflora, B. nobilis, B. punicea; eastern Australia from southeastern Queensland to Tasmania).
A probable topology is [Blandfordia+[Asteliaceae+[Hypoxidaceae+Lanaria]]].
BORYACEAE (Baker) M. W. Chase, Rudall et Conran |
( Back to Iridales ) |
Genera/species: 2/12
Distribution: Australia.
Fossils: Unknown.
Habit: Bisexual, perennial herbs. Xerophytes. Roots in Borya tuberculate and slightly lignified (with Rhizoctonia or other fungi?), sometimes stilt roots.
Vegetative anatomy: Roots fibrous, with mycorrhiza, Root hypodermis lignified; endodermis strongly thickened; inner cortex sclerenchymatous; medulla lignified. Phellogen absent. Stem endodermis in Borya strongly lignified; vascular strands in peripheral cylinder inside endodermis; medulla lignified, with fibres (in Alania parenchymatous). Secondary lateral growth usually absent (older stems in Borya with anomalous secondary lateral growth). Vessels present in roots. Vessel elements with scalariform perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial parenchyma? Sieve tube plastids P2c type, with cuneate protein crystals. Nodes? Calciumoxalate as raphides.
Trichomes: Hairs absent?
Leaves: Alternate (spiral), simple, entire, linear to filiform, with ? ptyxis. Stipules absent; leaf sheath well developed. Venation parallelodromous. Vascular bundles containing lateral phloem. Stomata anomocytic. Cuticular wax crystalloids? Hypodermis with lignified fibres. Inner epidermal cell walls lignified. Leaf margin finely serrate or entire.
Inflorescence: Terminal, raceme- or spike-like. Peduncle (scape) long. Inflorescence surrounded by an involucre; involucral bracts in Alania dry, in Borya differentiated into subulate foliaceous outer and scale-like inner bracts. Pedicel in Alania with several floral prophylls (bracteoles), in Borya with a single floral prophyll.
Flowers: Actinomorphic. Hypogyny. Tepals 3+3, petaloid, persistent, free (Alania), or connate in lower part into a short tube (Borya). Septal nectaries external-basal, as deep furrows in ovary wall. Disc absent.
Androecium: Stamens 3+3. Filaments filiform, free from each other, in Borya adnate to perianth tube (epitepalous). Anthers almost as wide as long, sometimes glandular, usually basifixed (sometimes centrifixed), non-versatile?, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory, with binucleate cells. Staminodia absent.
Pollen grains: Microsporogenesis simultaneous. Pollen grains monosulcate, shed as monads, bicellular at dispersal. Exine semitectate, with columellate infratectum, reticulate.
Gynoecium: Pistil composed of three connate carpels. Ovary superior, trilocular. Style single, simple, filiform (in Alania geniculate). Stigma capitate, type? Pistillodium absent.
Ovules: Placentation axile. Ovules numerous per carpel, anatropous, bitegmic, pseudotenuinucellar (functionally tenuinucellar, developmentally crassinucellar). Micropyle bistomal. Outer integument two cell layers thick. Inner integument two cell layers thick. Parietal cell formed from archesporial cell, early degenerating (parietal tissue absent). Hypostase present. Megasporangial tissue one cell layer thick. Nucellar cap absent. Megagametophyte monosporous, Polygonum type, with chalazal constriction. Synergids without filiform apparatus. Antipodal cells persistent. Endosperm development ab initio helobial. Endosperm haustoria absent. Embryogenesis?
Fruit: A loculicidal capsule with persistent perianth.
Seeds: Aril absent. Seeds in Borya papillate. Seed coat exotestal. Exotesta with phytomelan layer on epidermal cell walls. Exotestal cells with thickened walls. Endotesta? Tegmen degenerating. Hypostase somewhat lignified. Perisperm not developed. Endosperm copious, with oil and aleurone (starch absent). Embryo small, ovoid, chlorophyll? Cotyledon one. Cotyledon hyperphyll? Hypocotyl internode? Coleoptile? Germination?
Cytology: n = 11 (Alania); n = 14, 28 (Borya)
DNA:
Phytochemistry: Very insufficiently known. Chelidonic acid present.
Use: Unknown.
Systematics: Borya (11; Western Australia, Queensland, Victoria), Alania (1; A. endlicheri; mountains west of Sydney in New South Wales).
Boryaceae are sister to the clade [Blandfordia+[Asteliaceae+[Lanaria+Hypoxidaceae]]]. Sometimes, Boryaceae have been recovered as sister-group to Orchidaceae.
DORYANTHACEAE R. Dahlgren et Clifford |
( Back to Iridales ) |
Genera/species 1/2
Distribution Coastal eastern Australia.
Fossils Unknown.
Habit Bisexual, perennial giant herbs. Bulb consisting of large leaf bases rich in starch. Secondary bulbs developing from lower axillary shoot primordia following anthesis.
Vegetative anatomy Phellogen absent. Secondary lateral growth absent. Vascular bundles enclosed by fibres. Vessel elements? Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial parenchyma? Sieve tube plastids P2c type, with cuneate protein crystals. Nodes? Idioblasts with calciumoxalate as single, double or triple crystals. Styloids present. Calciumoxalate raphides absent from vegetative organs (present in tepals).
Trichomes Hairs?
Leaves Alternate (spiral), simple, entire, long, often linear, with ? ptyxis. Stipules absent; leaf sheath well developed, enclosing stem. Venation parallelodromous. Stomata paracytic; subsidiary cells with oblique divisions. Cuticular wax crystalloids as parallel platelets (Convallaria type). Mesophyll with lignified parenchyma between vascular bundles, with scattered tanniniferous cells and with calciumoxalate as single prismatic crystals. Leaf margin entire. Epidermis at apex of older leaves caducous, leaving a tuft of dry threads (sometimes dissolved into fibres).
Inflorescence Terminal, panicle or capitate thyrse. Peduncle (scape) very long. Flowers sometimes replaced by bulbils.
Flowers Actinomorphic to somewhat zygomorphic, large. Epigyny. Tepals 3+3, petaloid, partially caducous, connate at base. Septal nectaries well developed, opening around stylar base. Disc absent.
Androecium Stamens 3+3. Filaments linear or widened at base, free from each other, often adnate in lower part to tepals (epitepalous). Anthers peltate, pseudo-basifixed (centrifixed), non-versatile, tetrasporangiate, latrorse (to introrse?), longicidal (dehiscing by longitudinal slits); filament apex enclosed by tube formed by massive connective; anthers sometimes adnate to tepal bases. Tapetum secretory, outer layer with uninucleate cells, inner layer with multinucleate cells. Staminodia absent.
Pollen grains Microsporogenesis simultaneous. Pollen grains trichotomosulcate, shed as monads, bicellular at dispersal. Exine semitectate, with columellate infratectum, reticulate.
Gynoecium Pistil composed of three connate carpels. Ovary inferior, trilocular. Style single, simple, narrow, with stylar canal. Stigma triangular to punctate, papillate?, Dry type. Pistillodium absent.
Ovules Placentation axile. Ovules five to more than 50 per carpel, anatropous, bitegmic, crassinucellar. Micropyle ?-stomal. Outer integument five to seven cell layers thick. Inner integument two cell layers thick. Parietal cell formed from archesporial cell. Parietal tissue approx. five cell layers thick. Hypostase present. Nucellar cap approx. two cell layers thick, formed by periclinal cell divisions in megasporangial epidermis. Megagametophyte monosporous, Polygonum type. Antipodal cells persistent, proliferating (up to five cells). Endosperm development ab initio helobial. Endosperm haustoria? Embryogenesis?
Fruit A lignified loculicidal capsule.
Seeds Aril absent. Seeds often winged. Seed coat testal-tegmic. Testa multilayered, multiplicative, with phlobaphene and degradation products of this substance. Phytomelan absent. Tegmen thick, with distinct cuticle. Perisperm not developed. Endosperm copious, with thin-walled cells rich in oils and aleurone (starch absent). Embryo straight, flattened, chlorophyll? Cotyledon one, wide, obtriangular. Cotyledon hyperphyll? Hypocotyl internode? Coleoptile present. Germination? Seedling with laterally compressed haustorium.
Cytology n = 17, 18, 22, 24. – Karyotype bimodal.
DNA
Phytochemistry Cyanidin and steroidal saponins present. Flavonols, ellagic acid, and cyanogenic compounds not found.
Use Ornamental plants.
Systematics Doryanthes (2; D. excelsa, D. palmeri; coastal areas in southeastern Queensland and eastern New South Wales). Doryanthaceae are sometimes recovered as sister-group to Iridaceae (e.g. Rudall 2003).
HEMEROCALLIDACEAE R. Br. |
( Back to Iridales ) |
Phormiaceae J. Agardh, Theoria Syst. Plant.: 7. Apr-Sep 1858; Dianellaceae Salisb., Gen. Plant.: 66. 15-30 Mai 1866 [’Dianelleae’]; Johnsoniaceae J. P. Lotsy, Vortr. Bot. Stammesgesch. 3: 731. 3-20 Sep 1911; Geitonoplesiaceae R. Dahlgren ex Conran in Telopea 6: 39. Nov 1994; Eccremidaceae Doweld, New Syllabus Plant Fam.: 942. Apr 2007
Genera/species 19/110–115
Distribution Europe, tropical East Africa, southern and southeastern Africa, Madagascar, the Mascarene Islands, the Seychelles, West and Central Asia eastwards to China and Japan, Southwest, South and Southeast Asia, Malesia, New Guinea, Australia, New Caledonia, New Zealand (including the Chatham Islands), Norfolk Island, Fiji, Polynesia including the Hawaiian Islands, the Andes.
Fossils Pollen and leaf fossils assigned to Phormium are described from the Oligocene and the Miocene of New Zealand. Fossil pollen is known from the Eocene onwards in Australia and the Miocene in Antarctica.
Habit Dioecious, perennial herbs or shrubs (in Corynotheca deciduous; in Geitonoplesium climbing). Phyllocladia present in Hensmania, Hodgsoniola, Johnsonia, and Stawellia. Roots often swollen, contractile, sometimes tuberous (sometimes fibrous; rarely stilt roots).
Vegetative anatomy Root endodermis with stout U-formed cell wall thickenings (not in Arnocrinum). Phellogen? Secondary lateral growth absent. Vessels present in roots (in Caesia, Geitonoplesium, Johnsonia, and Tricoryne also in stem). Vessel elements with scalariform or simple perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial parenchyma? Sieve tube plastids P2c type, with cuneate protein crystals. Nodes? Mucilage cells and mucilage canals absent. Tanniniferous cells present in at least some species. Calciumoxalate as cubes or crystal sand; raphides absent.
Trichomes Hairs absent.
Leaves Alternate (usually distichous, sometimes spiral), simple, entire, usually linear (sometimes semi-equitant, unifacial), with conduplicate to conduplicate-flat ptyxis, often unifacially keeled. Stipules absent; leaf sheath closed. Venation parallelodromous. Stomata anomocytic. Cuticular wax crystalloids as parallel platelets (Convallaria type) or non-orientated platelets or rodlets. Mesophyll often with calciumoxalate as druses or single prismatic crystals. Leaf margin serrate or entire.
Inflorescence Terminal, usually panicle, sometimes raceme- or spike-like, possibly consisting of one or two double helicoid cymes (in Tricoryne with umbel-like partial inflorescences; in Hensmania, Hodgsoniola, Johnsonia, and Stawellia simple raceme or spike; flowers in Herpolirion solitary). Floral prophylls (bracteoles) sometimes (in, e.g., Dianella and Hemerocallis) lateral.
Flowers Usually actinomorphic (in Hemerocallis and Phormium zygomorphic), sometimes large. Pedicel usually articulated. Usually (secondary) hypogyny (in Pasithea half epigyny). Tepals 3+3 (median outer tepal in Hemerocallis adaxial), petaloid, usually marcescent (often twisted), free or connate in lower part into a tube (in Arnocrinum and Hemerocallis connate in lower half). Septal nectaries infralocular (in Johnsonia and Tricoryne external at ovary base; absent in Hemerocallis and Phormium). Androecial nectaries present in Dianella. Disc absent.
Androecium Stamens usually 3+3 (in Hodgsonia three). Filaments often ornamented, usually free from each other (in Eccremis slightly connate at base), often adnate to tepal bases (in Eccremis three stamens hypogynous and three stamens slightly adnate to tepals [epitepalous]); if stamens three then filaments adnate at base to inner tepals, somewhat upcurved (in Simethis and Stypandra more or less hairy; filaments in, e.g., Dianella, Rhuacophila and Stypandra with swelling, struma). Anthers usually basifixed (sometimes dorsifixed to centrifixed or peltate), sometimes versatile, tetrasporangiate, latrorse or introrse (in Corynotheca also extrorse), usually longicidal (dehiscing by longitudinal slits; in Dianella etc. pseudoporicidal, dehiscing by apical pores and subsequently longicidal; anthers in Geitonoplesium and Stypandra coiled after anthesis; anthers in Arnocrinum connate and forming a tube around style), often twisted. Tapetum secretory, with binucleate cells (remains of degenerating protoplasts sometimes invading anther locule in Dianella). Staminodia usually absent (in Hodgsonia three).
Pollen grains Microsporogenesis usually simultaneous (in Hemerocallis successive). Pollen grains usually trichotomosulcate (in Hemerocallis monosulcate), shed as monads, bicellular at dispersal. Exine tectate (sometimes semitectate) or intectate, with columellate infratectum, usually perforate or microreticulate (in Hemerocallis and Phormium reticulate), tuberculate.
Gynoecium Pistil composed of three connate carpels (in Tricoryne almost free). Ovary usually (secondarily) superior (rarely semi-inferior), trilocular or unilocular. Style single, simple, filiform, usually terminal (in Tricoryne gynobasic). Stigma small, usually capitate or punctate (in Hemerocallis as a short hair tuft; in Pasithea trilobate), papillate?, Dry or Wet type. Pistillodium absent.
Ovules Placentation axile (ovary trilocular; sometimes basal-axile or apical-axile) or parietal (ovary unilocular). Ovules one to c. 30 (to more than 50) per carpel, anatropous or campylotropous, ascending to pendulous, bitegmic, tenuinucellar (to weakly crassinucellar). Micropyle endostomal. Outer integument six or seven cell layers thick. Inner integument two to four cell layers thick. Parietal cell not formed (parietal tissue absent). Nucellar cap approx. two cell layers thick, sometimes formed by periclinal cell divisions starting in epidermal layer of megasporangium (absent in Hemerocallis). Hypostase absent. Megagametophyte monosporous, Polygonum type. Chalazal nucleus well developed. Synergids with a filiform apparatus (at least in some genera). Antipodal cells large, persistent. Endosperm development usually helobial (in Hemerocallis nuclear). Endosperm haustoria? Embryogenesis Graminad.
Fruit Usually a dry loculicidal capsule (in Eccremis fleshy, with loculicidal exocarp and septicidal endocarp; sometimes a berry; in Corynotheca a nut; in Tricoryne a schizocarp).
Seeds Seed often with strophiole or aril (from hilum; in Phormium winged). Elaiosome absent. Seed coat exotestal. Exotesta thick, with phytomelan layer on epidermal cell walls. Endotesta compressed. Tegmen collapsed. Perisperm not developed. Endosperm copious, with oil and aleurone (with usually little or no hemicellulose or starch). Embryo long or short, usually straight (in Arnocrinum curved), well differentiated, without chlorophyll. Cotyledon one, unifacial, with a prolonged closed and tubular sheath (in Caesia, Dianella and Tricoryne epigeal hyperphyll but not cotyledon photosynthesizing). Cotyledon hyperphyll elongate, assimilating, or compact, not assimilating. Epicotyl often longer. Hypocotyl internode short (sometimes absent). Coleoptile absent. Collar sometimes present. Radicula well developed, often branched, persistent. Germination?
Cytology n = 4, 8, 9, 11, 12, 14, 16, 22, 24, 38, 40, 42 – Chromosomes 0,8–17 µm long.
DNA The mitochondrial gene rpl2 is absent (lost). Loss of the 3’-rps12 intron is a synapomorphy of the clade [Hemerocallidoideae+Johnsonioideae].
Phytochemistry Flavonols (kaempferol, quercetin, in Hemerocallis), phenolic glycosides, steroidal saponins (at least in Pasithea), naphthoquinones (e.g. imbricatonol), chelidonic acid, and polyacetate derived arthroquinones present. Eicosanyl arachidate, chrysophanol, and nepodin present in roots. Dianellidin, dianellidone, imbricatonol, stypandrone, and stypandrol (hemerocallin, a toxic binaphthalene tetrol) present in, e.g., Dianella, Hemerocallis and Stypandra. Cyanidin present in Dianella. Ellagic acid not found.
Use Ornamental plants, medicinal plants, textile plants (Phormium tenax).
Systematics Hemerocallidaceae are probably sister to Xanthorrhoeaceae. However, Asphodelaceae have sometimes been recovered as sister to Hemerocallidaceae (e.g. McPherson & al. 2004; Chase & al. 2006). The loss of the 3’-rps12 intron is a character present in both Hemerocallidoideae and Johnsonioideae.
Phormioideae (J. Agardh) Thorne et Reveal in Bot. Rev. (Lancaster) 73: 80. 29 Jun 2007
10/50–55. Pasithea (1; P. caerulea; Chile); Phormium (2; P. colensoi, P. tenax; New Zealand incl. Stewart Island, Norfolk Island, Chatham Islands), Agrostocrinum (1; A. scabrum; southwestern Western Australia), Geitonoplesium (1; G. cymosum; the Philippines, Central and East Malesia to New Guinea, eastern Queensland, eastern New South Wales, eastern Victoria, Norfolk Island, Lord Howe, New Caledonia, Fiji), Thelionema (3; T. caespitosum, T. grande, T. umbellatum; southeasternmost Queensland, eastern New South Wales, Victoria, Tasmania), Rhuacophila (1; R. javanica; Malesia, New Caledonia, Fiji), Herpolirion (1; H. novae-zelandiae; southeastern New South Wales, eastern Victoria, Tasmania, New Zealand), Stypandra (1; S. glauca; southwestern Western Australia, southern South Australia, southeastern Queensland to Victoria, New Caledonia), Dianella (c 40; East Africa, Madagascar, East Asia to Japan, tropical Asia from India to Malesia, Australia, New Zealand, islands in southwestern Pacific, the Hawaiian Islands, with their highest diversity in Australia), Eccremis (1; E. coarctata; Costa Rica, the Andes from Venezuela to Bolivia, Suriname, Brazil). – East Africa, Madagascar, East and tropical Asia, Australia, Melanesia, New Zealand, Polynesia, Central and South America, with their largest diversity in southwestern and eastern Australia. Leaves often bifacial basally and distally, being semi-ensiform and unifacial in middle just after diverging from sheath (Wurdack & Dorr 2009, etc.). – Pasithea, with entirely bifacial leaves, is sister to the remaining Phormioideae. The Andean Eccremis coarctata (sister to Dianella) has articulated pedicels, filaments connate at base, three filaments adnate to tepals, and a septicidal endocarp. Aloin cells (see Asphodelaceae) have been reported from Dianella.
[Hemerocallidoideae+Johnsonioideae]
Hemerocallidoideae (Bartl.) Lindl., Veg. Kingd.: 201, 205. Jan-Mai 1846 [‘Hemerocallideae’]
2/20. Hemerocallis (19; Central Europe, temperate Asia to China, the Korean Peninsula and Japan), Simethis (1; S. planifolia; southwestern Europe to southwestern Ireland and Italy, western Mediterranean). – Eurasia. – Hemerocallidoideae are sister-group to Johnsonioideae, according to the analyses by Wurdack & Dorr (2009).
Johnsonioideae Nakai, Chosakuronbun Mokuroku [Ord. Fam. Trib. Nov.]: 233. 20 Jul 1943
7/c 40. Tricoryne (9; New Guinea, Australia), Corynotheca (6; C. asperata, C. flexuosissima, C. lateriflora, C. licrota, C. micrantha, C. pungens; Australia), Caesia (c 12; Northern, Western and Eastern Cape, Madagascar, New Guinea, Australia), Arnocrinum (3; A. drummondii, A. gracillimum, A. preissii; southwestern Western Australia), Stawellia (2; S. dimorphantha, S. gymnocephala; southwestern Western Australia), Hensmania (3; H. chapmanii, H. stoniella, H. turbinata; southwestern Western Australia), Johnsonia (5; J. acaulis, J. inconspicua, J. lupulina, J. pubescens, J. teretifolia; southwestern Western Australia). – South Africa, Madagascar, New Guinea, Australia, with their highest diversity in southwestern Western Australia. Aril present. – Tricoryne is sister to the remaining Johnsonioideae. Hodgsoniola (1; H. junciformis; southwestern Western Australia), sometimes included in Anthericaceae and possessing phylloclades, is possibly closely allied to the clade [Stawellia+[Johnsonia+Hensmania]].
 |
Maximum likelihood bootstrap consensus tree of Hemerocallidaceae based on DNA sequence data (Wurdack & Dorr 2009). |
HERRERIACEAE Kunth |
( Back to Iridales ) |
Genera/species 2/9
Distribution Madagascar, South America.
Fossils Unknown.
Habit Bisexual, perennial herbs or suffrutices, usually climbing or twining. Rhizome often tuberous. Climbing roots absent. Aerial stem spiny or absent.
Vegetative anatomy Roots fibrous; root exodermis uniseriate. Phellogen absent? Stem cortex with a cylinder of fibre bundles and small collateral vascular bundles; centre of stem with scattered bundles. Rhizome in Herreria with lignified secondary xylem and parenchymatous secondary phloem. Aerial stem at least in Herreria montevidensis with secondary lateral growth. Vessels present in roots and in Herreria also in stem. Vessel elements with scalariform perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial parenchyma? Sieve tube plastids P2c type, with cuneate protein crystals. Nodes? Mucilage cells and laticifers absent. Tanniniferous cells present. Calciumoxalate raphides frequent.
Trichomes Hairs absent?
Leaves Alternate (spiral), simple, entire, often linear, cladode-like, with supervolute (Herreria) ptyxis. Stipules and leaf sheath absent. Venation parallelodromous. Stomata anomocytic, in parallel rows on adaxial side (Herreria). Cuticular wax crystalloids as parallel platelets (Convallaria type). Mesophyll with cells containing calciumoxalate raphides. Leaf margin entire.
Inflorescence Terminal or axillary, panicle or raceme.
Flowers Actinomorphic. Pedicel not articulated. Hypogyny. Tepals 3+3, petaloid, persistent or caducous, free. Septal nectaries present in Herreria. Outer tepals in Herreriopsis with saccate tepal nectaries at base. Disc absent.
Androecium Stamens 3+3. Filaments free from each other and from tepals. Anthers basifixed, versatile, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory (tapetal material slightly invading anther locule at dyad stage). Staminodia absent.
Pollen grains Microsporogenesis successive. Pollen grains monosulcate, shed as monads, bicellular at dispersal. Exine semitectate, with columellate infratectum, perforate, microreticulate or reticulate.
Gynoecium Pistil composed of three connate carpels. Ovary superior, trilocular. Style single, simple, narrow. Stigma capitate or trilobate, type? Pistillodium absent.
Ovules Placentation intrusively axile. Ovules one to more than 50 per carpel, anatropous, bitegmic, crassinucellar. Micropyle ?-stomal. Outer integument four cell layers thick. Inner integument two cell layers thick. Hypostase present. Parietal tissue? Nucellar cap present? Megagametophyte monosporous, Polygonum type. Endosperm development ab initio helobial. Endosperm haustoria? Embryogenesis?
Fruit A septicidal capsule with partially persistent tepals.
Seeds Aril absent. Seeds flattened, spirally winged. Exotesta with phytomelan layer on outer epidermal cell walls, collapsed. Endotesta? Tegmen? Perisperm not developed. Endosperm copious, with oils and aleurone (starch absent); endosperm cell walls without pits. Embryo small, straight, chlorophyll? Cotyledon one, without sheath. Cotyledon hyperphyll long, photosynthesizing. Hypocotyl internode? Coleoptile? Germination cryptocotylar?
Cytology n = 27 (Herreria) – Chromosomes 0.7–3.7 µm long, dimorphic (bimodal, with one large chromosome).
DNA The mitochondrial gene rpl2 absent.
Phytochemistry Flavonols, tannins, phenols, steroidal saponins (e.g. gitogenin in roots), and water-soluble steroids present. Chelidonic acid? Ellagic acid not found.
Use Unknown.
Systematics Herreria (8; H. bonplandii, H. cipoana, H. glaziovii, H. grandiflora, H. latifolia, H. montevidensis, H. salsaparilha, H. stellata; Brazil to Chile and Argentina), Herreriopsis (1; H. elegans; Madagascar).
Herreriaceae is sister to Behnia (Behniaceae).
HYACINTHACEAE Batsch ex Borkh. |
( Back to Iridales ) |
Scillaceae Vest, Anleit. Stud. Bot.: 267, 284. 1818 [’Scilloideae’]; Hyacinthineae Link, Handbuch 1: 160. 4-11 Jul 1829 [’Hyacinthinae’]; Eucomidaceae Salisb., Gen. Plant.: 16. 15-31 Mai 1866 [’Eucomeae’]; Lachenaliaceae Salisb., Gen. Plant.: 20. 15-31 Mai 1866 [’Lachenaleae’]; Ornithogalaceae Salisb., Gen. Plant.: 33. 15-31 Mai 1866 [’Ornithogaleae’]
Genera/species 38/805–845
Distribution Europe, Africa, southwestern Asia to Burma and Sri Lanka, southwestern South America (Chile and the Andes), with their largest diversity in South Africa, the Mediterranean and southwestern Asia.
Fossils Unambiguous fossils not known.
Habit Usually bisexual (rarely polygamomonoecious), perennial herbs. Usually bulbous.
Vegetative anatomy Bulb usually with a membranous tunica; bulb scales free or more or less coalescent. Roots usually contractile; carbohydrate storing roots present in Ledebouria. Phellogen absent. Stem and leaves with large rhexigenetic lacunae. Secondary lateral growth absent. Vessels present in roots. Vessel elements with scalariform and/or simple perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial parenchyma? Sieve tube plastids P2c type, with cuneate protein crystals. Nodes? Mucilage cells abundant, often with calciumoxalate raphides.
Trichomes Hairs absent.
Leaves Alternate (usually spiral, sometimes distichous), simple, entire, often linear or filiform, with ? ptyxis. Stipules absent; leaf sheath well developed, bulb scale with closed or open sheath. Venation parallelodromous. Stomata anomocytic. Cuticular wax crystalloids usually as non-orientated platelets (sometimes parallel platelets, Convallaria type). Mesophyll with mucilaginous idioblasts containing calciumoxalate raphides. Leaf margin entire.
Inflorescence Terminal, simple, raceme (rarely spike, head or branched raceme; flowers rarely solitary) on elongated peduncle (scape). Floral prophylls (bracteoles) usually absent.
Flowers Usually actinomorphic (in Lachenalia and Daubneya aurea zygomorphic with median outer tepal adaxial; inner tepals rarely strongly reduced). Pedicel usually not articulated (in Schizobasis articulated). Usually hypogyny (in Bowiea half epigyny). Tepals 3+3, petaloid, free or connate into a tubular, campanulate or urceolate perianth. Corona sometimes present. Septal nectaries usually present, of simple shape (rarely basally connate; absent in Autonoe; in some species of Ornithogalum also a stylar nectary). Disc absent.
Androecium Stamens usually 3+3 (in, e.g., some species of Ornithogalum three fertile stamens and three staminodia). Filaments filiform or broad and flat (sometimes with apical or basal appendages), usually free (sometimes connate at base; in Puschkinia forming a paracorolla), often adnate at base to tepals. Anthers usually dorsifixed, versatile?, tetrasporangiate, introrse, usually longicidal (dehiscing by longitudinal slits; rarely poricidal, dehiscing by apical pores). Tapetum secretory, with binucleate cells. Staminodia usually absent (sometimes three, extrastaminal).
Pollen grains Microsporogenesis successive. Pollen grains monosulcate, shed as monads, bicellular at dispersal. Exine semitectate, with columellate? infratectum, reticulate.
Gynoecium Pistil composed of three connate carpels (sometimes syncarpously connate in lower part and paracarpously connate in upper part). Ovary usually superior (rarely semi-inferior), trilocular. Style single, simple (sometimes very short), with a stylar canal. Stigma punctate to somewhat trilobate, papillate, Dry or Wet type. Pistillodium absent.
Ovules Placentation axile. Ovules two to c. 50 (in Barnardia one) per carpel, anatropous, bitegmic, crassinucellar. Micropyle ?-stomal. Outer integument three or more cell layers thick. Inner integument two cell layers thick. Obturator often present. Parietal cell formed from archesporial cell. Parietal tissue usually two to four cell layers thick. Nucellar cap often present. Megasporangial epidermis sometimes radially elongate. Megagametophyte usually monosporous, Polygonum type (sometimes disporous, Allium type, or tetrasporous, Scilla type [Hyacinthoides type, Endymion type], Adoxa type, DrusaI type, or Fritillaria type). Synergids with a filiform apparatus. Endosperm development usually helobial (sometimes nuclear). Endosperm haustoria? Embryogenesis asterad, caryophyllad or chenopodiad.
Fruit A loculicidal capsule, dry or fleshy.
Seeds Aril absent. Caruncular elaiosome sometimes present. Seed coat exotestal. Exotesta usually with phytomelan layer on epidermal cell walls (absent in some species of Hyacintheae). Mesotesta, endotesta and tegmen usually collapsed. Perisperm not developed. Endosperm copious, with oils, aleurone and hemicellulose (starch usually absent); endosperm cells with pitted walls. Embryo large, usually straight (sometimes curved), without chlorophyll. Cotyledon one. Cotyledon hyperphyll elongate and assimilating, or compact and non-assimilating. Hypocotyl internode absent. Coleoptile absent. Collar rhizoids sometimes present. Germination cryptocotylar? or phanerocotylar? Seedling foliage distichous.
Cytology x = 2–12 – Polyploidy and aneuploidy frequently occurring (even deca- and dodecaploidy). Chromosomes 1.2–18 µm long; chromosomes sometimes bimodal or trimodal.
DNA The mitochondrial gene rpl2 is absent (lost).
Phytochemistry Flavonols (kaempferol, quercetin), flavones, flavone-C-glycosides, flavonoid sulphates, homoisoflavanones, polyhydroxyalkaloids, cholestane glycosides (cardiotoxic bufodienolides [e.g. scilliroside] and cardenolides), steroidal saponins, cyanogenic compounds, salicylic acid, and chelidonic acid present. Tricine rare. Carbohydrates in bulb mainly stored as fructans (sometimes also as starch). Ellagic acid, proanthocyanidins and alkaloids not found.
Use Ornamental plants, medicinal plants, vegetables (Muscari).
Systematics Hyacinthaceae are sister to Themidaceae.
A plausible topology is [Oziroeoideae+[Urgineoideae+[Ornithogaloideae+Hyacinthoideae]]].
Oziroeoideae Speta in Phyton (Horn) 38: 51. 14 Aug 1998
1/5. Oziroe (5; O. acaulis, O. argentinensis, O. arida, O. biflora, O. pomensis; Peru, Bolivia, Paraguay, Chile, Argentina). – Filaments at base connate and adnate to inner tepals. Seeds rounded. Embryo as long as seed. n = 15, 17. – Oziroe is sister to the remaining Hyacinthaceae.
[Urgineoideae+[Ornithogaloideae+Hyacinthoideae]]
Rhexigenetic lacunae present. Styloids present.
Urgineoideae Speta in Phyton (Horn) 38: 51. 14 Aug 1998
2/c 60. Bowiea (1; B. volubilis; Uganda and Kenya to South Africa); Drimia (c 60; the Mediterranean, Africa, Madagascar, India). – The Mediterranean, Africa, Madagascar, the Arabian Peninsula to Sri Lanka and Burma. Bracts with spurs (in Bowiea as small leaves). Seeds flattened and/or winged. Testa fragile, not tightly adherent to endosperm. n = 6, 7, 10 or more. Bufadienolides present. – Urgineoideae are sister-group to [Ornithogalum+Hyacinthoideae]. Bowiea is sister to the remaining Urgineoideae.
[Ornithogaloideae+Hyacinthoideae]
Ornithogaloideae Speta in Phyton (Horn) 38: 51. 14 Aug 1998
1/270–300. Ornithogalum (270–300; Europe, the Mediterranean to the Caucasus, subtropical and southern Africa, Madagascar, southern Arabian Peninsula, Socotra, southwestern Asia to India and Sri Lanka, with their largest diversity in South Africa). – Stamens sometimes three. Filaments sometimes flat, provided with appendages. Seeds flattened and/or short. Cell nuclei containing protein crystalloids. Cotyledon often photosynthesizing. n = 2–10 or more. Cardenolides present.
Hyacinthoideae Speta in Phyton (Horn) 38: 51. 14 Aug 1998
34/470–480. Leaves sometimes with pustulae or coloured spots. Megagametophyte varying. Seeds often rounded. Elaiosome sometimes present. Homoisoflavanones present.
Pseudoprospereae J. C. Manning et Goldblatt in Edinburgh J. Bot. 60: 557. 14 April 2004
1/1. Pseudoprospero (1; P. firmifolium; southern and eastern South Africa). – Prophylls present. Ovules two per carpel. One seed per locule. Cotyledon non-photosynthesizing. n = 9. – Pseudoprospero is probable sister to [Massonieae+Hyacintheae].
[Massonieae+Hyacintheae]
Massonieae Baker in Bot. J. Linn. Soc. London 11: 355. 17 Sep 1870
12/230–235. Merwilla (3; M. dracomontana, M. lazulina, M. plumbea; southern Africa to Zimbabwe); Eucomis (c 10; South Africa to Zimbabwe and Malawi); Spetaea (1; S. lachenaliiflora; Western Cape), Daubenya (8; Northern and Western Cape); Veltheimia (2; V. bracteata, V. capensis; Northern, Western and Eastern Cape), Massonia (c 10; Namibia, South Africa, Lesotho), Lachenalia (c 120; Namibia, Northern, Western and Eastern Cape, Free State), Namophila (1; N. urotepala; southern Namibia); Schizocarphus (1; S. nervosus; tropical East to southern Africa), Ledebouria (55–60; tropical and southern Africa, Madagascar, the Arabian Peninsula, southern India, Sri Lanka), Drimiopsis (14; tropical and southern Africa), Resnova (5; R. humifusa, R. lachenalioides, R. maxima, R. minor, R. pilosa; southern Africa). – Tropical and southern Africa, Madagascar, the Arabian Peninsula, southern India, Sri Lanka. Floral prophylls (bracteoles) sometimes present. Flowers sometimes zygomorphic. Filament tube present in some species of Daubenya. Ovary and style usually sulcate. Style with three canals. Ovules two to numerous per carpel. Elaiosome sometimes present. Cotyledon usually non-photosynthesizing. n = 5–10 or more. – Merwilla seems to be sister to the remaining Massonieae.
Hyacintheae Dumort., Fl. Belg.: 141. 1827
21/240–245. Barnardia (1; B. japonica: eastern China, the Korean Peninsula, Japan, the Russian Far East); Tractema (4; T. liliohyacinthus, T. monophyllos, T. ramburei, T. verna; western Europe, western Mediterranean, northwesternmost Africa), Oncostema (5–10; O. caerulea, O. cupanii, O. dimartinoi, O. hughii, O. peruviana, O. sicula; the Iberian Peninsula, western Mediterranean, northwesternmost Africa), Autonoe (2; A. haemorrhoidalis, A. maderensis; Macaronesia), Hyacinthoides (11; western Europe to North Africa); Brimeura (3; B. amethystina: the Pyrenees; B. duvigneaudii: Mallorca; B. fastigiata: Mallorca, Menorca, Corsica, Sardinia), Hyacinthella (18; southeastern Europe, southwestern Asia), Alrawia (2; A. bellii: western Iran; A. nutans: northeastern Iraq), Prospero (c 15; western, southern and southeastern Europe, the Mediterranean, northernmost Africa, Turkey, the Caucasus), Puschkinia (3; P. bilgineri, P. peshmenii, P. scilloides; Turkey, Lebanon, Syria, the Caucasus, northern Iran), Othocallis (c 20; Europe, the Mediterranean, western Asia, the Middle East to Iran), Fessia (10; Iran, Pakistan, Central Asia), Pfosseria (1; P. bithynica; eastern Balkan Peninsula, western Turkey), Zagrosia (1; Z. persica; southestern Turkey, Iraq, western Iran), Hyacinthus (1; H. orientalis; western and Central Asia); Chouardia (2; C. lakusicii, C. litardierei; the Balkan Peninsula), Nectaroscilla (1; N. hyacinthoides; eastern Mediterranean), Schnarfia (2; S. albanica, S. messeniaca; southern Balkan Peninsula), Muscari (42; Europe, the Mediterranean, western and southwestern Asia), Bellevalia (c 65; the Mediterranean to Iran, northern Afghanistan and Pakistan), Scilla (c 30; Europe, Macaronesia, the Mediterranean, North Africa, West Asia). – Europe to southwestern Asia, North Africa, East Asia. Bract sometimes absent. Prophylls fairly frequently present. Style with a single papillate canal. Ovules two to eight (to numerous) per carpel. Outer integument four or five cell layers thick. Parietal tissue two or three cell layers thick. Antipodal cells large. Elaiosome sometimes present. Phytomelan sometimes absent. Cotyledon often photosynthesizing. n = 4–8 or more. – Barnardia japonica seems to be sister to all other Hyacintheae. ‘Barnardia’numidica (the Balearic Islands, Algeria, Tunisia, Libya) is only distantly related to Barnardia japonica (Ali & al. 2012).
 |
Cladogram (simplified) of Hyacinthaceae based on DNA sequence data (Pfosser & Speta 1999; with the addition of Pseudoprospereae). |
HYPOXIDACEAE R. Br. |
( Back to Iridales ) |
Hypoxidales R. Br. in C. F. P. von Martius, Consp. Regn. Veg.: 9. Sep-Oct 1835 [‘Hypoxideae’]
Genera/species c 6/140–180?
Distribution Africa, the Mascarene Islands, the Seychelles, tropical and subtropical regions in Asia to eastern Himalaya, southwestern China and Japan, New Guinea, Australia, Tasmania, New Zealand, eastern USA to the West Indies and South America to Uruguay, with the largest diversity in South Africa.
Fossils Unknown.
Habit Usually bisexual (in Curculigo rarely unisexual), perennial herbs. Tuberous rhizome or corm.
Vegetative anatomy Velamen present or absent. Roots often contractile. Dimorphic root hypodermis absent. Phellogen absent. Rhizome and stem nodules with inner parenchymatous zone containing irregularly running vascular bundles and fibrous tissue, surrounded by starchy parenchyma usually with secretory cavities (mucilage canals). Secondary lateral growth absent. Vessels present in roots. Vessel elements with scalariform perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial parenchyma? Sieve tube plastids P2c type, with cuneate protein crystals. Silica bodies absent. Calciumoxalate raphides usually present.
Trichomes Hairs unicellular or multicellular, uniseriate or multiseriate, often dichotomously branched and with multicellular base, sometimes stellate or lepidote.
Leaves Alternate (usually tristichous), simple, entire, often linear, sometimes plicate or unifacial and subulate in transverse section (in Curculigo, Hypoxidia and Molineria differentiated into pseudopetiole and pseudolamina), with plicate to conduplicate-plicate (sometimes conduplicate-flat) ptyxis. Stipules absent; leaf sheath open or closed, persistent. Venation parallelodromous (sometimes pinnate-parallel), with distinct main veins. Stomata usually paracytic (rarely tetracytic), with oblique or parallel cell divisions. Cuticular waxes absent. Secretory cavities (mucilage canals) present above vascular bundles in some genera (in Empodium in mesophyll). Mesophyll with calciumoxalate raphides. Leaf margin entire.
Inflorescence Axillary, often on long peduncle (scape), corymbose, spike- or umbel-like cymose (flowers sometimes solitary).
Flowers Actinomorphic. Epigyny. Tepals usually 3+3 (in Hypoxis and Spiloxene rarely 2+2), petaloid, often with valvate aestivation, persistent, usually free (in Hypoxidia, Pauridia and Saniellia connate into a tube). Septal nectaries absent. Disc absent.
Androecium Stamens usually 3+3 (rarely 2+2; in Pauridia three antepetalous stamens and three staminodia adnate to style into a gynostemium). Filaments short, filiform or subulate, free, often adnate to lower part of tepals (epitepalous). Anthers basifixed, centrifixed or slightly dorsifixed, occasionally somewhat versatile, tetrasporangiate, usually introrse or latrorse (sometimes extrorse), longicidal (dehiscing by longitudinal slits); connective rarely somewhat prolonged at apex. Tapetum secretory or amoeboid-periplasmodial, with uni- to multinucleate cells. Staminodia usually absent (in Pauridia three intrastaminal staminodia).
Pollen grains Microsporogenesis successive. Pollen grains usually monosulcate (sometimes trisulcate or inaperturate, in Pauridia disulcate), shed as monads, bicellular at disperal. Exine tectate or semitectate, with columellate infratectum, perforate or finely reticulate.
Gynoecium Pistil composed of three connate carpels. Ovary inferior, usually trilocular (in Empodium unilocular; in Hypoxidia and some species of Spiloxene trilocular in lower part and unilocular in upper part); ovary often with rostrum (small apical elongation). Style single, simple, stout, or stylodia three, more or less connate; with a stylar canal. Stigma trilobate or consisting of three vertical furrows along upper part of style, papillate, Dry or Wet type. Pistillodium absent.
Ovules Placentation usually axile (in Empodium parietal). Ovules few to numerous per carpel, usually anatropous (in Empodium and Pauridia possibly hemianatropous to campylotropous), apotropous, bitegmic, pseudocrassinucellar. Micropyle bistomal (sometimes Z-shaped, zig-zag), often prolonged. Outer integument two cell layers thick. Inner integument two cell layers thick. Parietal cell sometimes formed (from archesporial cell), degenerating (parietal tissue absent). Hypostase present. Nucellar cap formed by periclinal cell divisions in megasporangial epidermis, two or three cell layers thick, or absent. Megagametophyte usually monosporous, Polygonum type (in Empodium sometimes disporous, Allium type), with chalazal constriction. Synergids sometimes with a filiform apparatus. Antipodal cells ephemeral or persistent. Endosperm development usually helobial (in Pauridia and sometimes in Spiloxene nuclear). Endosperm haustorium chalazal or absent. Embryogenesis onagrad, solanad or asterad.
Fruit Usually a loculicidal capsule or a pyxidium (capsule sometimes denticidal, poricidal or irregularly dehiscing – with ovary beak – or baccate), usually with persistent parts of tepals.
Seeds Raphe prominent. Strophiole present in Curculigo and Empodium in association with hilum. Seed coat exotestal. Exotesta with phytomelan layer on epidermal cell walls, often palisade. Endotesta? Exotegmen? Endotegmen sometimes persistent. Perisperm not developed. Endosperm copious, with oils and aleurone (starch absent). Embryo small, straight, often little differentiated, chlorophyll? Cotyledon one, non-photosynthesizing, with reduced open leaf sheath and with apex transformed into haustorium. Cotyledon hyperphyll compact, non-assimilating. Hypocotyl internode and mesocotyl absent. Coleoptile present. Germination?
Cytology n = 6–9, 11, 16, 18, 19, 27, 36, 48 or more – Polyploidy occurring at least in Hypoxis. Chromosomes 2–5 µm long. Agamospermy present inHypoxis.
DNA
Phytochemistry Flavonols (quercetin) and chelidonic acid present. Ellagic acid, proanthocyanidins, alkaloids, steroidal saponins, and cyanogenic compounds not found. Ferulic acid absent from cell walls.
Use Ornamental plants.
Systematics Hypoxis (agamospermy present, with perhaps 50–100 species; tropical and southern Africa, East and Southeast Asia, tropical to temperate America, with their highest diversity in South Africa), Empodium (9–10; South Africa, Swaziland, Lesotho), Pauridia (c 55; Namibia, Northern, Western and Eastern Cape, KwaZulu-Natal, Lesotho, Australia, Tasmania, New Zealand), ‘Curculigo’ (c 20; tropical regions on both hemispheres; polyphyletic), ‘Molineria’ (7; M. capitulata, M. crassifolia, M. gracilis, M. latifolia, M. oligantha, M. prainiana, M. trichocarpa; tropical Asia; paraphyletic; incl. Curculigo pro parte), Hypoxidia (2; H. maheensis, H. rhizophylla; the Seychelles).
Hypoxidaceae are probably sister-group to Lanaria (Lanariaceae) (or, possibly, to Asteliaceae).
Some analyses based on morphology indicate a close relationship between Hypoxidaceae and Orchidaceae.
 |
Cladogram of Hypoxidaceae based on DNA sequence data (Kocyan & al. 2011). |
IRIDACEAE Juss. |
( Back to Iridales ) |
Crocaceae Vest, Anleit. Stud. Bot.: 266, 283. 1818 [’Crocoideae’]; Ixiaceae Horan., Prim. Lin. Syst. Nat.: 51. 2 Nov 1834 [’Jxiaceae. (Jrideae)’]; Ixiales Lindl., Key Bot.: 70. 15-30 Sep 1835; Galaxiaceae Raf., New Fl. N. Amer. 1: 72. Dec 1836 [’Galaxidia’]; Gladiolaceae Raf., Fl. Tellur. 4: 34. med 1838 [’Gladiolina’]; Geosiridaceae Jonker in Receuil Trav. Bot. Néerl. 36 [Meded. Bot. Mus. Herb. Rijksuniv. Utrecht 60]: 477. seors. impr. 18 Mai 1939, nom. cons.; Hewardiaceae Nakai, Chosakuronbun Mokuroku [Ord. Fam. Trib. Nov.]: 234. 20 Jul 1943, nom. illeg.; Iridineae Engl., Syllabus, ed. 2: 94. Mai 1898; Isophysidaceae (Hutch.) F. A. Barkley in Revista Fac. Nac. Agron. Medellin Univ. Antioquia 8: 152. Sep 1948
Genera/species 67/2.370–2.465
Distribution Nearly cosmopolitan, with their largest diversity in southern Africa, eastern Mediterranean, southwestern Asia and western South America.
Fossils Uncertain. Fossil pollen grains, Liliacidites goldblattii, resembling those in Isophysis (and Doryanthes), were described from the latest Campanian to earliest Maastrichtian Vilui Basin sediments in Siberia.
Habit Bisexual, usually perennial (some species of Sisyrinchium annual) herbs (Klattia, Nivenia and Witsenia are suffruticose with a more or less lignified stem base). Usually with a rhizome (often tuberous) or a tunicated corm (with basal innovation; rarely bulb). Geosiris aphylla in Madagascar is an achlorophyllous mycotrophic holoparasite with scale-like membranous leaves.
Vegetative anatomy Roots with mycorrhiza; often contractile; root stele central, surrounded by endodermis often with lignified cell walls; root hairs absent; root hypodermis dimorphic; root nodules or fleshy roots present in some genera. Rhizome sometimes with lignified endodermis-like layer. Phellogen? Stem parenchyma often starchy. Secondary lateral growth anomalous (Klattia, Nivenia, Witsenia) or absent. Vessels present in roots (in some genera also in stem and leaves). Vessel elements with scalariform or simple perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays? Axial parenchyma? Sieve tube plastids usually P2c type, with cuneate protein crystals (rarely P2ccl type, with cuneate and several additional loosely packed protein crystals). Secretory cavities containing mucilage present. Tanniniferous cells present in many genera. Idioblasts with calciumoxalate as styloids or single prismatic crystals usually frequent (raphides absent).
Trichomes Hairs usually absent (sometimes unicellular or multicellular, simple).
Leaves Alternate (distichous), simple, entire, often linear or equitant, sometimes terete and filiform, unifacial, isobifacial or heterobifacial, with ad-abaxially plicate or conduplicate (sometimes conduplicate-plicate) ptyxis. Stipules absent; leaf sheath open or closed (ligule present in Geissorhiza). Venation parallelodromous; midvein often distinct (sometimes thickened). Stomata usually anomocytic (subsidiary cells in Diplarrhena and some species of Moraea formed by oblique cell divisions). Cuticular wax crystalloids as parallel platelets, Convallaria type. Secretory cavities with mucilage. Mesophyll with calciumoxalate as single prismatic crystals. Leaf margin entire.
Inflorescence Terminal, either thyrse, panicle or cyme composed of one or more umbel-like, corymbose or reduced rhipidia, or spike-like with often unilaterally rotated flowers (flowers often solitary). Bracts of various shape, often spathe-like. Extrafloral nectaries rarely present on bracts.
Flowers Actinomorphic or zygomorphic, often large. Some species with hypanthium. Usually epigyny (in Isophysis hypogyny). Tepals 3+3, petaloid, often aristate, free (Isophysis, Aristea, Geosiris, most Iridoideae), or connate into an infundibuliform or tubular perianth; perianth in zygomorphic flowers often bilabiate, with adaxial tepal usually largest (often dome-shaped) and three abaxial tepals smallest and patterned with nectar guides. Septal nectaries usually present, with antepetalous orifices at stylar base (in Crocoideae), or tepal nectaries inserted at tepal bases (in most Iridoideae and Aristea spiralis); tepal nectaries present as nectariferous or oil-secreting glandular hairs, elaiophores, usually unicellular and inserted at inner tepals; androecial (on filaments) or gynoecial nectaries often present in, e.g., Iris (nectaries absent in Isophysis, Geosiris and most species of Aristea). Disc absent.
Androecium Stamens usually three (in Diplarrhena two: outer abaxial stamen absent), antesepalous (in some genera asymmetrically placed), representing outer staminal whorl (inner whorl absent). Filaments coarse to filiform, usually free (sometimes more or less connate), often adnate to tepal bases or to perianth tube when present. Anthers usually basifixed or subbasifixed (sometimes centrifixed), sometimes versatile, tetrasporangiate, usually extrorse (rarely latrorse, with wide connective, or introrse), usually longicidal (dehiscing by longitudinal slits; in Cobana poricidal, dehiscing with apical pores). Tapetum secretory, with usually binucleate (sometimes multinucleate) cells. Staminodia absent.
Pollen grains Microsporogenesis usually simultaneous (in at least Geosiris successive). Pollen grains usually monosulcate (rarely disulcate, trisulcate, trichotomosulcate, zonosulculate, dizonosulculate, spiraperturate, or inaperturate), sometimes operculate, shed as monads, usually bicellular at dispersal. Exine usually tectate or semitectate (in Diplarrhena and Patersonia intectate), with columellate infratectum, usually reticulate or microreticulate (sometimes perforate to microscabrate; rarely areolate or rugulate).
Gynoecium Pistil composed of three connate carpels; median carpel abaxial. Ovary usually inferior (in Isophysis superior), usually trilocular (in some species of Iris unilocular). Style single, filiform, usually trilobate, often further branched or petaloid (in Zygotritonia entire), with thin to wide (rarely hairy or fimbriate) branches, with stylar canal; stylar branches often widened at apex, in Iridoideae longitudinally folded, often flattened or thickened and with paired apical appendages, in Iris, Moraea and some other Irideae tangentially flattened, wide, petaloid, with two apical appendages and an apical stigma. Stigma papillate (with unicellular bifid papillae), Dry type; stigmatic branches adaxial on stylar lobes, inserted at apex, along margins or on surface of stylar branches. Pistillodium absent.
Ovules Placentation usually axile (in some species of Iris parietal). Ovules one to c. 50 per carpel, anatropous or campylotropous, bitegmic, crassinucellar or tenuinucellar. Micropyle endostomal or exostomal. Outer integument usually four to six cell layers thick (in Aristea, Klattia, Nivenia and Witsenia two or three cell layers thick). Inner integument two cell layers thick. Parietal cell formed from archesporial cell. Parietal tissue one or two cell layers thick. Hypostase formed in Ixioideae from megasporangium at chalazal end of megagametophyte and surrounding antipodal cells. Megagametophyte monosporous, Polygonum type. Synergids sometimes with a filiform apparatus. Antipodal cells in some genera proliferating. Endosperm development usually nuclear (in Geosiris and Isophysis helobial). Endosperm haustoria? Embryogenesis asterad or caryophyllad.
Fruit Usually a loculicidal capsule (rarely indehiscent), hard (sometimes lignified), usually xerochastic (rarely hygrochastic).
Seeds Aril usually absent (present in some species of Iris). Elaiosome (arising from chalaza) present in Patersonia and some species of Iris (in Patersonia also from raphe). Seed coat testal-tegmic (rarely winged). Exotesta usually with phlobaphene (absent in Klattia, Nivenia, and Witsenia), without phytomelan. Endotesta often with lipids. Tegmen often partially collapsed. Exotegmen and endotegmen usually pigmented. Perisperm not developed. Endosperm copious, hard, thick-walled, with oils, proteins and aleurone (rarely starch), with hemicellulose in pitted cell walls. Embryo small to fairly large, straight, usually without chlorophyll. Cotyledon one, terminal, coleoptile-like, usually not photosynthesizing (in, e.g., Sisyrinchium photosynthesizing; in, e.g., Tigridia with ligule or coleoptile). Cotyledon hyperphyll elongate or compact, often assimilating. Hypocotyl internode short or absent. Mesocotyl absent. Plumule lateral. Germination phanerocotylar.
Cytology n = (3–)5–17, 19, 20 – Polyploidy and aneuploidy occurring (in, e.g., Crocus, Iris, Moraea, and Sisyrinchium).
DNA
Phytochemistry Flavonols (kaempferol, quercetin etc.), flavone-C-glycosides, isoflavones (irigenin, tectorigenin, irisolon, and numerous others), amentoflavone (a biflavone present in Isophysis and Patersonia), cyanidin, tannins, alkaloids (e.g. homeridin), steroidal saponins, naphthoquinones (plumbagin), anthraquinones, polyacetate derived arthroquinones (in Gladiolus and Libertia), quinonoid pigments, mangiferin (a glycosylic xanthone), crocein (a yellow carotenoid in Crocus), hydroxycinnamic acid, chelidonic acid, and meta-carboxysubstituted aromatic amino- and γ-glutamylic peptides present. Tricine rare. Ellagic acid not found. Carbohydrates (e.g. saccharose, fructan and starch) stored in corms and rhizomes.
Use Ornamental plants, flavours (styles and stigmas from Crocus sativus, containing β- and γ-carotene, lycopin, zeaxanthin, crocin, safranal, etc.), perfumes (rhizome of Iris germanica), medicinal plants.
Systematics Iridaceae are sister-group to the remaining Iridales, comprising Xeronemataceae to Ruscaceae. Isophysis (Tasmania) is sister to all other Iridaceae.
Isophysidoideae (Hutch.) Takht. ex Thorne et Reveal in Bot. Rev. (Lancaster) 73: 79. 29 Jun 2007
1/1. Isophysis (1; I. tasmanica; Tasmania). – Rhizomatous herb. Root vessel elements with scalariform perforation plates. Flower solitary, spathaceous, with a pair of ’bracts’ subtending the single flower (perhaps corresponding to a reduced rhipidium). Hypogyny. Tepals free. Septal nectaries absent. Pistil composed of three connate carpels. Style shortly trifid, branches alternating with anthers. Stigmatic surfaces adaxial. Seedling unknown. n = ? Amentoflavone (a biflavonoid) present. – Isophysis tasmanica is sister to all other Iridaceae.
[Iridoideae+[Patersonioideae+[Geosiridoideae+[Aristeoideae+[Nivenioideae+Crocoideae]]]]]
Root vessel elements with usually simple perforation plates. Inflorescence usually rhipidium or spike, with cymose partial inflorescences with flowers successively arising from axils of prophylls. Epigyny. Septal nectaries, tepal nectaries or oil glands often present. Pollen grains sometimes operculate and sulcus often with two exine bands. Endosperm development usually nuclear. Xanthone (mangiferin) present.
Iridoideae Eaton, Bot. Dict., ed. 4: 28. Apr-Mai 1836 [‘Irideae’]
29/1.010–1.050. Diplarrheneae Goldblatt in P. J. Rudall et P. Goldblatt, Ann. Bot. (Italy), n.s., 1(2): 65. 2001. Diplarrhena (2; D. latifolia, D. moraea; southeastern New South Wales, southern Victoria, Tasmania). – Irideae Kitt. in A. Richard, Nouv. Elém. Bot., ed. 3, Germ. transl.: 736. 1840 [‘Irideae verae’]. Bobartia (15; Western and Eastern Cape), Iris (270–280; temperate regions on the Northern Hemisphere), Ferraria (18; southern tropical Africa to South Africa), Moraea (220–230; the Mediterranean, Africa, southwestern Asia, with their highest diversity in Western Cape). – Sisyrinchieae Klatt in C. F. P. von Martius, Fl. Bras. 3(1): 511, 529. 1 Jul 1871. Libertia (16; New Guinea, eastern New South Wales, Victoria, Tasmania, New Zealand, Chatham Islands, the Andes), Orthrosanthus (10; southwestern Western Australia, South Australia, Victoria, southern Mexico, Central America, western South America), Tapeinia (1; T. pumila; southern Chile, southern Argentina), Solenomelus (2; S. pedunculatus, S. segethi; Chile, southern Argentina), Sisyrinchium (180–200; Canada, United States, Mexico, Central America, South America to Chile and Argentina), Olsynium (17; southwestern Canada, western United States, southern Chile, southern Argentina).– Trimezieae Ravenna in Wrightia 7: 12. 1 Jun 1981. Neomarica (31; Central America, tropical South America, with their highest diversity in Brazil), Pseudiris (1; P. speciosa; Chapada Diamantina in Brazil), Trimezia (16; Central America, tropical South America), Pseudiris (1; P. speciosa; Chapada Diamantina in Brazil); Cypella (c 20; Mexico and southwards to Argentina), Deluciris (2; D. rupestris, D. violacea; Brazil), Pseudotrimezia (24; tropical South America, with their highest diversity in Minas Gerais in Brazil). – Tigridieae Kitt. in A. Richard, Nouv. Elém. Bot., ed. 3, Germ. transl.: 736. 1840 [‘Tigrideae’]. Cypella (38; Mexico to northern Argentina), Hesperoxiphion (5; H. herrerae, H. huilense, H. niveum, H. pardalis, H. peruvianum; the Andes in Colombia, Peru and Bolivia), Cipura (8; Mexico, Central America, the West Indies, tropical South America), Calydorea (c 20; Mexico, South America), Eleutherine (4; E. angusta, E. bulbosa, E. citriodora, E. latifolia; Mexico, Central America, the West Indies, tropical South America), Gelasine (7; G. caldensis, G. coerulea, G. elongata, G. gigantea, G. paranaensis, G. rigida, G. uruguaiensis; southern Brazil, Paraguay, Uruguay, northern Argentina), Ennealophus (5; E. boliviensis, E. euryandrus, E. fimbriatus, E. foliosus, E. simplex; Ecuador, Peru, Brazil, Bolivia, northwestern Argentina), Herbertia (9; South America from Colombia to central Chile and northern Argentina, one species, H. lahue, north to southern United States), Mastigostyla (27; western South America), Nemastylis (6; N. floridana, N. geminiflora, N. nuttallii, N. selidandra, N. tenuis, N. tuitensis; southern United States, Mexico, northern Central America), Alophia (5; A. drummondii, A. intermedia, A. medusa, A. silvestris, A. veracruzana; southern United States, Mexico, Central America, tropical South America), Tigridia (c 50; Mexico, Central America, South America), Cobana (1; C. guatemalensis; Guatemala, Honduras). – Temperate regions on the Northern Hemisphere, Africa, Australia, Tasmania, New Zealand, North America to southern South America, with their largest diversity in South Africa and South America. Rhipidia simple. Habitus varying. Tepal nectaries usually present (in Diplarrhena septal nectaries). Oil glands or oil hairs often present. Anther endothecium with spiral thickenings. Stylar branches long and tubular. γ-glutamyl peptides and metacarboxy aminoacids present. – Five well supported main clades have been recognized in Iridoideae (Reeves & al. 2001): Diplarrhena (Australia), the sister-group to the remaining Iridoideae; Irideae; Sisyrinchieae; Tigridieae; and Mariceae.
[Patersonioideae+[Geosiridoideae+[Aristeoideae+[Nivenioideae+Crocoideae]]]]
Rhipidia two fused, each unit with two to numerous flowers; tepals connate; anther endothecium with base-plate or U-shaped thickenings; extra codon often present in gene rps4.
Patersonioideae Goldblatt in P. Goldblatt et J. C. Manning, Iris Fam.: 93. 12 Nov 2008
1/25. Patersonia (25; Sumatra, the Philippines, northern Borneo, New Guinea, Australia, Tasmania, New Caledonia). – Stem rhizomatous and often lignified. Secondary lateral growth present. Root vessel elements often with scalariform perforation plates. Inner tepals sometimes reduced to scales or absent. Septal nectaries absent. Filaments connate. Pollen grains spheroidal, inaperturate. Exine intectate. Embryo small. n = 11, 21. Two extra codons present in gene rps4. Amentoflavone (a biflavonoid) present.
[Geosiridoideae+[Aristeoideae+[Nivenioideae+Crocoideae]]]
Geosiridoideae Goldblatt et J. C. Manning, Iris Fam.: 95. 12 Nov 2008
1/3. Geosiris (3; G. aphylla, G. albiflora; Madagascar, Mayotte in the Comoros; G. australiensis: northeastern Queensland). –Achlorophyllous root mycoparasitic herb. Leaves heterobifacial, reduced to scales. Flowers sessile. Tepals connate only at base. Septal nectaries absent. Microsporogenesis successive. Endosperm development helobial. Seeds extremely small. Endosperm with starch. n = ?
[Aristeoideae+[Nivenioideae+Crocoideae]]
Aristeoideae Vines, Stud. Text-book Bot. 2: 566. Mar 1895 [‘Aristinae’]
1/c 55. Aristea (c 55; South Africa to Senegal and Ethiopia, Madagascar, with their highest diversity in the Cape Provinces). – Root vessel elements with scalariform or simple perforation plates. Tepals connate only at base. Septal nectaries absent. Pollen morphology highly varying. Embryo small. n = 16. Plumbagin (a naphthoquinone) present.
[Nivenioideae+Crocoideae]
Flowers persistent, long-lived.
Nivenioideae Schulze ex Goldblatt in Ann. Missouri Bot. Gard. 77: 621. 16 Nov 1990
3/15. Nivenia (11; Western Cape), Klattia (3; K. flava, K. partita, K. stokoei; Western Cape), Witsenia (1; W. maura; Western Cape). – Western Cape. Stem woody, with pronounced secondary lateral growth. Inflorescence biseriate, consisting of two terminal monochasia each with one or two flowers and a single bract. Tepals connate into a long tube. Pollen grains sometimes with encircling sulcus. Heterostyly present in Nivenia. Seeds peltate (tangentially flattened). Testa two or three cell layers thick. Exotesta transparent, not thickened. Exotegmen well developed. n = 16.
Crocoideae Burnett, Outlines Bot.: 451. Feb 1835 [’Crocidae’]
31/1.260–1.305. Watsonia (50–55; southern Africa, with their largest diversity in Western Cape), Thereianthus (11; Western Cape), Micranthus (7; M. alopecuroides, M. cruciatus, M. filifolius, M. plantagineus, M. simplex, M. thereianthoides, M. tubulosus; Western Cape), Codonorhiza (6–7; C. azurea, C. corymbosa, C. elandsmontana, C. falcata, C. fastigiata, C. micrantha, C. neglecta; western Western Cape), Schizorhiza (1; S. neglecta; mountains in western Western Cape), Lapeirousia (27; South Africa to Nigeria and Ethiopia, with their highest diversity in Northern and Western Cape), Afrosolen (16; Nigeria, Ethiopia to Zimbabwe, Mozambique and Angola, southern Africa), Savannosiphon (1; S. euryphyllus; Congo and Tanzania to Zambia, Malawi and northern Mozambique), Cyanixia (1; C. socotrana; Socotra), Zygotritonia (6; Z. benishangula, Z. bongensis, Z. nyassana, Z. praecox, Z. hysterantha, Z. teretifolia; tropical West Africa to Ethiopia, Tanzania, Zambia and Socotra); Tritoniopsis (24; Western and Eastern Cape), Babiana (90–95; southern Africa, southern Zimbabwe, with their largest diversity in Northern and Western Cape), Gladiolus (250–260; Europe, the Mediterranean, mountains in tropical Africa, Madagascar, southwestern Asia, with their highest diversity in Western Cape and the Drakensberg), Radinosiphon (2; R. leptostachya, R. lomatensis; northeastern South Africa to southern Tanzania), Xenoscapa (2; X. fistulosa, X. uliginosa; southern Namibia, Northern, Western and Eastern Cape), Geissorhiza (90–100; Northern, Western and Eastern Cape), Hesperantha (80–85; South Africa to Ethiopia, with their largest diversity in Western Cape and the Drakensberg), Melasphaerula (1; M. ramosa; southern Namibia, Northern and Western Cape), Romulea (90–95; the Canary Islands, the Mediterranean, Africa, Socotra, with their highest diversity in the Cape Provinces), Syringodea (8; southern Africa, especially Northern and Western Cape), Crocus (c 90; central and southern Europe, the Mediterranean, North Africa, southwestern Asia and eastwards to Central Asia and western China), Freesia (16; South Africa to Sudan, with their largest diversity in Western and Eastern Cape), Dierama (44; South Africa to Ethiopia), Ixia (85–90; Western and Eastern Cape), Sparaxis (16; Northern and Western Cape), Tritonia (c 30; South Africa to Tanzania), Duthiastrum (1; D. linifolium; northern Karroo in Northern Cape), Crocosmia (9; tropical and southern Africa, Madagascar), Devia (1; D. xeromorpha; Roggeveld Escarpment in Northern and Western Cape), Chasmanthe (3; C. aethiopica, C. bicolor, C. floribunda; Western and Eastern Cape). – Europe, the Mediterranean, Africa, Madagascar, Socotra, southwestern Asia to western China. Corm usually present. Root vessel elements with simple perforation plates. Leaves usually with pseudo-midvein (absent in Pillansia). Leaf sheath closed. Inflorescence spike-like or consisting of paired rhipidia usually with a single flower. Flowers actinomorphic or zygomorphic, often sessile. Tepals usually connate. Septal nectaries present or absent. Anther endothecium with spiral thickenings. Pollen grains with aperture having one or two longitudinal bands forming an operculum, sometimes zonaperturate. Exine tectate, perforate-scabrate. Ovules campylotropous. Chalazal hypostase prominent. Exotesta sometimes exfoliating. n = 9–17.
 |
Cladogram (simplified) of Iridaceae based on DNA sequence data (Reeves & al. 2001). |
IXIOLIRIACEAE (Pax) Nakai |
( Back to Iridales ) |
Genera/species 1/4
Distribution Egypt, Israel, eastern Turkey to Central Asia and Pakistan.
Fossils Unknown.
Habit Bisexual, perennial herbs. Rhizome a corm surrounded by a tunica.
Vegetative anatomy Root exodermis not dimorphic. Phellogen absent. Secondary lateral growth absent. Vessels present in roots. Vessel elements with scalariform perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial parenchyma? Sieve tube plastids P2c type, with cuneate protein crystals. Nodes? Mucilage cells abundant. Calciumoxalate raphides frequent.
Trichomes Hairs bicellular to sexacellular, uniseriate.
Leaves Alternate (spiral), simple, entire, linear, with ? ptyxis. Stipules absent; leaf sheath well developed. Venation parallelodromous, with unequally sized vascular bundles. Stomata anomocytic. Cuticular wax crystalloids? Mesophyll with calciumoxalate raphides. Leaf margin entire. Leaf apex short, subulate.
Inflorescence Terminal, leafy thyrse or almost umbel-like, sometimes botryoid or corymb with helicoid partial inflorescences (bostryces). Peduncle with sclerenchymatous cylinder; some vascular bundles of inflorescence axis arranged in a cylinder and enclosing remaining scattered bundles. Floral prophyll (bracteole) often lateral.
Flowers Actinomorphic. Epigyny. Tepals 3+3, petaloid, thin, connate at base; outer tepals with somewhat prolonged apex arising on abaxial side at a point immediately below apex. Septal nectaries absent. Disc absent.
Androecium Stamens 3+3. Filaments free from each other, adnate in lower part (at base?) to tepals (epitepalous). Anthers basifixed to centrifixed, non-versatile, tetrasporangiate, introrse to latrorse, longicidal (dehiscing by longitudinal slits). Tapetum secretory, with uninucleate cells. Staminodia absent.
Pollen grains Microsporogenesis simultaneous. Pollen grains monosulcate, shed as monads, bicellular at dispersal. Exine semitectate, with columellate infratectum, reticulate.
Gynoecium Pistil composed of three connate carpels. Ovary inferior, trilocular. Style single, simple, narrow. Stigma trilobate, papillate?, Dry type. Pistillodium absent.
Ovules Placentation axile. Ovules c. 15 to more than 50 per carpel, anatropous, ascending, bitegmic, crassinucellar. Micropyle ?-stomal. Outer integument ? cell layers thick. Inner integument ? cell layers thick. Parietal cell formed from archesporial cell. Hypostase? Megagametophyte monosporous, Polygonum type. Endosperm development helobial. Endosperm haustoria? Embryogenesis?
Fruit A loculicidal capsule, apically dehiscing.
Seeds Aril absent. Seed coat testal. Exotesta with phytomelan layer on epidermal cell walls. Several testal layers with thin and reddish-brown cell walls. Mesotesta and endotesta with flat, empty cells. Tegmen crushed, membranous. Perisperm not developed. Endosperm copious, with thin and pitted cell walls, with oils and aleurone (starch almost absent, present in cells adjacent to embryo). Embryo large, straight, well differentiated, without chlorophyll. Cells surrounding embryo starchy. Cotyledon one. Cotyledon hyperphyll? Hypocotyl internode? Coleoptile? Germination?
Cytology n = 12
DNA
Phytochemistry Very insufficiently known. Chelidonic acid present. Steroidal saponins? Alkaloids not found.
Use Ornamental plants.
Systematics Ixiolirion (4; I. ferganum: Kyrgyzstan; I. karateginum: Pakistan, Tajikistan; I. songaricum: Xinjiang; I. tataricum: northeastern Egypt, Israel, eastern Turkey to Kashmir and Central Asia).
Ixiolirion is sister to Tecophilaeaceae.
LANARIACEAE H. Huber ex R. Dahlgren et A. E. van Wyk |
( Back to Iridales ) |
Genera/species 1/1
Distribution The Cape Provinces in South Africa.
Fossils Unknown.
Habit Bisexual, perennial herbs. Rhizome vertical.
Vegetative anatomy Roots fibrous. Phellogen absent. Secondary lateral growth absent. Vessels present in roots? Vessel elements with scalariform? perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial parenchyma? Sieve tube plastids P2ccps type, with a single large loosely-packed polygonal protein crystal and many cuneate ones, and with starch grains. Nodes? Calciumoxalate raphides absent; cuboidal styloids sometimes present.
Trichomes Hairs unicellular, usually dendritic, long, especially on inflorescences and abaxial side of flowers.
Leaves Alternate (spiral or distichous), simple, entire, linear, with ? ptyxis. Stipules absent; leaf sheath closed? Venation parallelodromous. Stomata paracytic. Cuticular wax crystalloids? Epidermal cells associated with vascular strands and leaf margins often with lignified walls. Leaf margin entire.
Inflorescence Terminal, corymbose panicle with short branches. Inflorescence covered with dendritic hairs.
Flowers Actinomorphic. Epigyny to slightly half epigyny. Tepals 3+3, petaloid, connate in lower half into a tube. Abaxial side of tepals covered with dendritic hairs. Adaxial side of tepals with simple unicellular hairs and papillae. Septal nectaries external, present along upper abaxial side of ovary. Disc absent.
Androecium Stamens 3+3, inner stamens somewhat shorter than outer stamens. Filaments free from each other, adnate to mouth of perianth tube. Anthers dorsifixed, versatile, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory, with binucleate to multinucleate cells. Staminodia absent.
Pollen grains Microsporogenesis simultaneous. Pollen grains monosulcate, shed as monads, bicellular at dispersal. Exine tectate, with columellate infratectum, perforate or microreticulate, granulate.
Gynoecium Pistil composed of three more or less connate carpels. Ovary inferior to slightly semi-inferior, trilocular, with a central canal. Style single, simple, filiform, long. Stigma punctate, type? Pistillodium absent.
Ovules Placentation axile to subbasal. Ovules two per carpel, anatropous, apotropous, bitegmic, crassinucellar to pseudocrassinucellar. Micropyle bistomal, Z-shaped (zig-zag). Outer integument five to seven cell layers thick. Inner integument two cell layers thick. Obturator present (funicular?). Parietal cell formed from archesporial cell. Parietal tissue approx. three cell layers thick. Hypostase present. Nucellar cap two or three cell layers thick. Chalazal part of megasporangium relatively well developed. Megagametophyte monosporous, Polygonum type; with chalazal constriction. Antipodal cells ephemeral. Endosperm development helobial. Endosperm haustoria? Embryogenesis?
Fruit A one-seeded loculicidal capsule.
Seeds Aril absent. Seed coat exotestal. Exotesta with phytomelan layers on epidermal cell walls, palisade, with thin cell walls, remaining cells rounded. Mesotesta? Endotesta? Tegmen developed at micropyle, persistent. Perisperm not developed. Endosperm copious, ab initio with starch. Embryo?, chlorophyll? Cotyledon one? Cotyledon hyperphyll? Hypocotyl internode? Coleoptile? Germination?
Cytology n = 18
DNA
Phytochemistry Very insufficiently known. Lanaroflavone (a biflavone) and chelidonic acid present.
Use Unknown.
Systematics Lanaria (1; L. lanata; mountains in the Hottentots Holland in Western Cape to near Port Elizabeth in Eastern Cape).
Lanaria is sister-group to Hypoxidaceae.
The sieve tube plastids are of a unique type.
LAXMANNIACEAE Bubani |
( Back to Iridales ) |
Xerotaceae (Endl.) Hassk., Cat. Hort. Bot. Bogor.: 27. Oct 1844 [’Xerotideae’], nom. illeg.; Lomandraceae Lotsy, Vortr. Bot. Stammesgesch. 3: 761. 3-20 Sep 1911; Eustrephaceae Chupov in Bot. Žurn. 79(3): 7. Mar-Sep 1994
Genera/species 14/190–200
Distribution Tropical Asia, New Guinea, Australia, Tasmania, New Caledonia, New Zealand, South America, with their largest diversity in Australia.
Fossils Pollen assigned to Cordyline are known from the Early Miocene onwards. Fossil leaves from the mid-Eocene of South Australia (Paracordyline) are very similar to Australasiatic species of Cordyline, and leaf fossils from the Oligocene of Kerguelen resemble extant species of the genus from New Zealand.
Habit Bisexual or dioecious, usually perennial herbs (in Cordyline often fruticose or arborescent). Some species are xerophytic.
Vegetative anatomy Roots fibrous or fleshy (sometimes tuberous or stilt roots), in some species with a sclerenchymatous cortical zone outside root endodermis. Phellogen? Secondary lateral growth usually absent (anomalous secondary lateral growth present in stems of some species of Cordyline and Lomandra). Vessels usually present only in roots. Vessel elements with scalariform or simple perforation plates (in Acanthocarpus and Arthropodium in leaves also, then with scalariform perforation plates; in Eustrephus also in stem and leaves, with scalariform perforation plates); lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays? Axial parenchyma? Sieve tube plastids P2c type, with cuneate protein crystals, or P2cf type, with cuneate protein crystals and peripheral protein filaments (in Acanthocarpus P2ccl type, with cuneate and several additional loosely packed protein crystals). Nodes? Tanniniferous cells probably absent. Silica bodies absent. Idioblasts (raphide cells) with calciumoxalate raphides.
Trichomes Hairs unicellular or multicellular, uniseriate?, or absent.
Leaves Alternate (spiral or distichous), simple, entire, usually linear, sometimes terete or triangular in cross-section (in Cordyline sometimes differentiated into pseudopetiole and pseudolamina), with supervolute, conduplicate or flat to curved ptyxis. Stipules absent; leaf sheath well developed (in Laxmannia and Sowerbaea with ligule at sheath apex). Venation parallelodromous. Stomata usually anomocytic (sometimes paracytic or tetracytic). Cuticular wax crystalloids? Mesophyll with calciumoxalate raphides. Epidermal cells in Lomandra with calciumoxalate as styloid-like crystals (rhomboidal or polyhedral crystals present in leaf cells in some species of Lomandra and Romnalda). Leaf margin entire or spinulate-dentate.
Inflorescence Terminal, panicle, raceme-, umbel- or spike-like, sometimes with cymose partial inflorescences.
Flowers Actinomorphic, often small. Pedicel usually articulated, with pericladium. Hypogyny. Tepals 3+3, petaloid (in Thysanotus with inner tepals fimbriate), dry or fleshy, marcescent, usually free (sometimes connate at base). Septal nectaries infralocular (absent in some species). Disc absent.
Androecium Stamens 3+3, or three inner stamens (three outer stamens sometimes staminodial or absent). Filaments free from each other and usually from tepals (all or only inner stamens sometimes adnate to tepals; filaments in Arthropodium hairy). Anthers basifixed, centrifixed or dorsifixed, versatile?, tetrasporangiate, introrse or extrorse, usually longicidal (dehiscing by longitudinal slits; in Eustrephus and some species of Arthropodium and Thysanotus poricidal, dehiscing with apical pores). Tapetum usually secretory (in Eustrephus possibly amoeboid-periplasmodial), with binucleate cells. Staminodia sometimes three (in Arthropodium and Trichopetalum with staminodial appendage).
Pollen grains Microsporogenesis successive. Pollen grains usually monosulcate (in Acanthocarpus and Chamaexeros zonosulcate, with a single sulcus surrounding entire pollen grain and dividing it into two halves; in Lomandra sometimes spiraperturate), shed as monads, bicellular at dispersal. Exine semitectate, with columellate infratectum, reticulate, in Acanthocarpus negatively reticulate, in Lomandra spinulate, echinate or negatively reticulate.
Gynoecium Pistil composed of three connate carpels. Ovary superior, trilocular. Style single, simple. Stigmas one to three, Dry or Wet type. Pistillodium?
Ovules Placentation axile. Ovules one, several or numerous per carpel, anatropous to campylotropous, bitegmic, crassinucellar. Micropyle ?-stomal. Outer integument ? cell layers thick. Inner integument ? cell layers thick. Parietal cell formed from archesporial cell (at least in Lomandra). Megasporangium with enlarged multilayered basal zone (proximal zone) with central ’Zuleitungsbahn’ consisting of axially elongated cells. Dermal chalazal cells enlarged. Nucellar cap present or absent. Megagametophyte monosporous, Polygonum typ. Nuclei of egg cell, synergids and antipodal cells enlarged. Synergids in some species with a filiform apparatus. Antipodal cells enlarged. Lateral megagametophyte haustoria present in at least Arthropodium. Endosperm development helobial. Endosperm haustoria? Embryogenesis?
Fruit Usually a loculicidal capsule (in Lomandra spicata a drupe; in Eustrephus a berry-like capsule) with persistent tepals.
Seeds Aril (formed from hilum) present in Murchisonia and Thysanotus. Strophiole (juicy, sweet) present in Eustrephus. Testa thin. Exotesta with or without phytomelan layer on epidermal cell walls. Mesotesta and endotesta often compressed or collapsed. Tegmen collapsed. Perisperm not developed. Endosperm copious, with lipids, aleurone and hemicellulose (starch absent); endosperm in Lomandra group and Eustrephus with thick, pitted cell walls. Embryo usually small, straight, curved or spirally twisted, chlorophyll? Cotyledon one, with apex sometimes transformed into haustorium, usually not photosynthesizing. Cotyledon hyperphyll? Hypocotyl internode in Cordyline long. Mesocotyl absent. Coleoptile usually absent (present in Sowerbaea). Radicula in Cordyline persistent, branched. Germination cryptocotylar?
Cytology n = 4 (Laxmannia, Sowerbaea); n = 6 (Chamaescilla); n = 7 (Chamaexeros, Lomandra); n = 8 (Acanthocarpus, Laxmannia, Lomandra); n = 10 (Eustrephus); n = 11 (Arthropodium, Chamaescilla, Murchisonia, Thysanotus); n = 19 (Cordyline) – Polyploidy frequently occurring. Chromosomes in Laxmannia, Sowerbaea and the Lomandra Group 2–7 µm long, or in Arthropodium and the Cordyline Group 0.5–2 µm long. Karyotype in Cordyline bimodal.
DNA The mitochondrial gene rpl2 is absent (lost).
Phytochemistry Steroidal saponins, naphthoquinones (in Lomandra), polyacetate derived arthroquinones (in Lomandra), phytosterols (in Cordyline), and chelidonic acid present. Polysaccharides in some genera stored as fructans. Seeds in Cordyline rich in linolic and oil acid based lipids. Flavonols, ellagic acid and alkaloids not found.
Use Ornamental plants, textile plants (Cordyline, Lomandra).
Systematics Laxmanniaceae are sister to [Asparagaceae+Ruscaceae].
The leaves in Xanthorrhoeaceae, some Lomandreae and in Dasypogonaceae are superficially similar. The leaves have sclerenchyma ribs extending from the inner envelopes of the vascular bundles. However, in Xanthorrhoea they arise from the mesophyll, whereas Dasypogonaceae lack such an envelope.
The subdivision below is according to Stevens (2001 onwards). A detailed phylogenetic analysis is in demand.
Lomandreae Engl. in Engler et Prantl, Nat. Pflanzenfam. II, 5: 18. 26 Mar 1887
5/65–70. Lomandra (50–55; New Guinea, Australia, New Caledonia), Romnalda (4; R. grallata, R. ophiopogonoides, R. papuana, R. strobilacea; Papua New Guinea, New Britain, eastern Queensland), Chamaexeros (4; C. fimbriata, C. longicaulis, C. macranthera, C. serra; southwestern Western Australia), Acanthocarpus (7; A. canaliculatus, A. humilis, A. parviflorus, A. preissii, A. robustus, A. rupestris, A. verticillatus; coastal regions in Western Australia); Xerolirion (1; X. divaricata; southwestern Western Australia). – New Guinea, Australia, New Caledonia. Tubers absent. Leaves distichous, flat or curved. Sclerenchyma ribs of leaf extending from inner envelope of vascular bundle to surface; outer envelope with enlarged cells. Leaf margin sometimes serrate. Pedicel usually articulated. Pollen grains in Lomandra sometimes spiraperturate. Stigma Wet type. Ovules one or two per carpel. Nucellar cap present. Megasporangium with axially arranged central vascular passage. Tegmen brown, collapsed. Phytomelan absent. Endosperm with hemicellulose. n = 7, 8. Chromosomes 2–7 μm long.
Xerolirion divaricata has solitary terminal female flowers, male flowers in cymes, non-articulated pedicels, one ovule per carpel, lacks silica bodies, and has cell walls containing ferulates. Its systematic position is obscur.
Laxmannioideae Thorne et Reveal in Bot. Rev. 73(2): 82. 29 Jun 2007 [‘Laxmannieae’ Engl. 1887]
8/92. Arthropodium (14; Madagascar, Australia, New Caledonia, New Zealand), Murchisonia (2; M. fragrans, M. volubilis; southern, northern and western Australia), Thysanotus (50; southern China to New Guinea, Australia), Trichopetalum (2; T. chosmalensis: western Argentina; T. plumosum: Chile), Eustrephus (1; E. latifolius; southern and eastern New Guinea, eastern Queensland, eastern New South Wales, eastern Victoria, New Caledonia), Laxmannia (14; Australia), Sowerbaea (5; S. alliacea, S. juncea, S. laxiflora, S. multicaulis, S. subtilis; southwestern and eastern Australia), Chamaescilla (4; C. corymbosa, C. gibsonii, C. maculata, C. spiralis; southwestern Western Australia, southern South Australia, Victoria, Tasmania). – Southeast Asia to Australia, New Caledonia, New Zealand, Chile. Vesicular-arbuscular mycorrhiza present. Storage roots present. Mucilage cells present. Leaves spiral, with supervolute or conduplicate ptyxis. Leaf sheath in Laxmannia and Sowerbaea with apical ligule. Flowers fasciculate or solitary. Pedicel often articulated. Anthers sometimes poricidal. Stigma Wet type. Megasporangium with axially oriented vascular tissue. Aril present in Murchisonia and Thysanotus. Exotesta often papillate, remaining testal layers cellular. Phytomelan present. Tegmen thin. Endosperm thin-walled. n = 4, 11. Chromosomes 0.5–2 μm long.
Cordylineae Nakai, Chosakuronbun Mokuroku [Ord. Fam. Trib. Nov.]: 227. 20 Jul 1943
1/15–20. Cordyline (15–20; the Mascarene Islands, India to New Guinea, tropical Australia, New Zealand, islands in the Pacific; one species in tropical America from southern Brazil to Bolivia and northern Argentina). – Sometimes arborescent. Storage roots present. Mucilage cells present. Leaves spiral, in some species differentiated into pseudopetiole and pseudolamina, with supervolute or conduplicate ptyxis. Vascular bundles complex, amphivasal. Stomata paracytic; subsidiary cells with oblique divisions. Leaf sheath sometimes with apical ligule. Flowers solitary. Pedicel not articulated. Testa with phytomelan layer. n = 3, 6, 19. Chromosomes 0.5–2.4 μm long. Karyotype bimodal.
ORCHIDACEAE Juss. |
( Back to Iridales ) |
Orchidales Raf., Anal. Nat.: 197. Apr-Jul 1815 [’Orchidia’]; Orchidopsida Bartl., Ord. Nat. Plant.: 24, 54. Sep 1830 [’Orchideae’]; Apostasiaceae Lindl., Nix. Plant.: 22. 17 Sep 1833 [’Apostasieae’], nom. cons.; Cypripediaceae Lindl., Nix. Plant.: 22. 17 Sep 1833 [’Cypripedieae’]; Neottiaceae Horan., Prim. Lin. Syst. Nat.: 50. 2 Nov 1834 [’Neottiaceae (Orchideae)’]; Apostasiales Blume in C. F. P. von Martius, Consp. Regn. Veg.: 10. Sep-Oct 1835 [’Apostasieae’]; Vanillaceae Lindl., Key Bot.: 73. 15-30 Sep 1835; Limodoraceae Horan., Char. Ess. Fam.: 44. 30 Jun 1847 [’Limodoraceae s. Orchideae’]; Cohniaceae Pfeiffer, Nomencl. Bot. 1(2): 815. ante 24 Jan 1873, nom. illeg.; Liparidaceae Vines, Stud. Text-book Bot. 2: 567. Mar 1895 [’Liparidinae’]; Ophrydaceae Vines, Stud. Text-book. Bot. 2: 566. Mar 1895 [‘Ophrydinae’]; Orchididae Heintze, Cormofyt. Fylog.: 10. 1927 [‘Orchidiformes’]; Neuwiediaceae (Burns-Bal. et V. A. Funk) R. Dahlgren ex Reveal et Hoogland in Bull. Mus. Natl. Hist. Nat., sér. IV, sect. B, Adansonia, 13: 91. 4 Oct 1991; Orchidanae Doweld, Tent. Syst. Plant. Vasc.: lviii. 23 Dec 2001
Genera/species 776/27.410–27.530
Distribution Cosmopolitan except polar regions, with the highest diversity in tropical and subtropical Asia and tropical Central and South America.
Fossils Meliorchis caribea is represented by a pollinium attached to a bee preserved in Oligocene to Miocene amber from the Dominican Republic.
Habit Usually bisexual (rarely monoecious or dioecious), perennial herbs. Many species have rhizome, root tubers or tuberous stem. Numerous species (c. 70%) are epiphytes, often with internodes modified into swollen water-storing pseudobulbs, often with contractile aerial roots covered with a thick layer of dead water-absorbing tissue, velamen, formed from epidermis; other species are climbing. Some genera consist of achlorophyllous holoendoparasites on fungi (intracellular modified ectomycorrhiza with mostly basidiomycetes). Several genera (especially within Vandeae) lack photosynthesizing leaves, which are replaced by photosynthesizing roots.
Vegetative anatomy CAM physiology often present (especially in Epidendroideae). Roots fibrous or tuberous, with mycorrhiza and usually with water-absorbing velamen (especially in epiphytic representatives). Plants as young (protocorms; sometimes also as mature) mycotrophic parasites on basidiomycetes (Russulaceae, Ceratobasidiaceae, Sebacinaceae, Thelephoraceae, Tulasnellaceae, sometimes Atractiellomycetes; sometimes ascomycetes, i.a. Tuber), with modified ectomycorrhiza as hyphal coils, pelotones, in cortical cells; also arbuscular mycorrhiza with Glomales. Phellogen absent. Primary vascular tissue as scattered bundles, centrifugal. Secondary lateral growth absent. Vessels present in roots (sometimes also in stem, rarely in leaves). Vessel elements usually with simple perforation plates (sometimes scalariform; in leaves only scalariform); lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial parenchyma? Sieve tube plastids P2c type, with cuneate prostein crystals. Nodes? Envelopes of vascular bundles with idioblasts, stegmata, containing silica bodies; envelopes with fibres. Mucilage cells usually present. Calciumoxalate as raphides.
Trichomes Absent or multicellular, uniseriate, eglandular or glandular.
Leaves Alternate (spiral or distichous), simple, entire, often linear (sometimes equitant), often succulent, sometimes scale-like, with convolute (supervolute), conduplicate or (conduplicate-)plicate ptyxis. Stipules absent; leaf sheath usually closed. Colleters sometimes present. Venation parallelodromous. Fibre bundles sometimes present. Stomata usually paracytic (sometimes anomocytic or tetracytic). Cuticular wax crystalloids usually absent (rarely as non-orientated rodlets or scales). Epidermis often with stegmata containing silica bodies. Mesophyll often with mucilaginous idioblasts containing calciumoxalate raphides. Leaf margin usually entire. Extrafloral nectaries sometimes present.
Inflorescence Terminal or axillary, spike, raceme, or panicle (sometimes umbel or head; flowers sometimes solitary). Extrafloral nectaries sometimes present on bracts or abaxial side of outer tepals.
Flowers Usually zygomorphic (in Apostasioideae almost actinomorphic; in Neuwiedia resupinate; in Apostasia non-resupinate). Flower usually resupinate (usually by 180°; in some species re-resupinate, in other species non-resupinate; female flowers in Catasetum non-resupinate, male flowers resupinate). Epigyny. Tepals 3+3, usually with imbricate aestivation, caducous, usually free (sometimes connate at base); outer tepals sepaloid or petaloid; upper (median) tepal in outer whorl abaxial (usually seemingly adaxial due to resupination); inner tepals usually petaloid; upper (median) tepal in inner whorl usually modified into adaxial labellum (usually seemingly abaxial due to resupination; in Cypripedioideae slipper-shaped; in Orchidoideae and Epidendroideae often spurred), often larger and formed in different way than remaining tepals and often with spur with or without nectar. Tepal nectaries usually present. Septal nectaries absent. Oil secretion sometimes present (sometimes with elaiophores). Osmophores often present. Disc absent.
Androecium Stamens one, two or three; one median in outer whorl and two laterals in inner whorl; in Apostasioideae median outer stamen fertile or staminodial and lateral inner stamens fertile; in Cypripedioideae median outer stamen staminodial and lateral inner stamens fertile; in Vanilloideae, Orchidoideae and Epidendroideae median outer stamen fertile and lateral inner stamens staminodial. When filaments two or three then connate at base, free from tepals and partially adnate to style; when filament one then entirely or partially (at least at base) and congenitally adnate to style into a gynostemium and sometimes adnate to tepals (epitepalous). Anthers basifixed or dorsifixed, non-versatile, tetrasporangiate, latrorse or introrse, longicidal (dehiscing by longitudinal slits; sometimes with appendage); thecae usually transformed into pollinia. Placentoid often present. Tapetum secretory, with usually uninucleate or binucleate (rarely multinucleate) cells. Staminodia two intrastaminal or one extrastaminal or absent.
Pollen grains Microsporogenesis simultaneous. Pollen grains usually inaperturate (sometimes monosulcate, disulcate, monoporate, diporate, triporate, tetraporate, ulcerate, sulculate, or foraminate), usually shed as tetrads and coherent into massulae or in two (to eight) pollinia with single pollen grains or tetrads adhered to each other through strands of sterile sporogenous material (in some genera shed as monads), bicellular at dispersal. Exine tectate or semitectate, with usually columellate (sometimes granular) infratectum, reticulate to perforate, rugulate, scabrate, verrucate, psilate or smooth (exine rarely absent).
Gynoecium Pistil composed of three connate carpels; median carpel abaxial. Ovary inferior, usually unilocular (in Apostasioideae and some Cypripedioideae trilocular). Style single, simple, solid or with a stylar canal. Stigma capitate, usually trilobate (rarely bilobate), papillate, Wet type; median stigmatic lobe usually transformed into a sterile rostellum with viscidium. Pistillodium absent.
Ovules Placentation usually parietal-marginal (when ovary unilocular; in Apostasioideae and some Cypripedioideae axile); placentae branched. Ovules usually c. 30 to more than 1.500 per carpel (in one species almost four million per ovary), anatropous, usually bitegmic (rarely unitegmic), usually tenuinucellar (sometimes crassinucellar). Funicle non-vascularized. Micropyle endostomal. Outer integument one or two cell layers thick. Inner integument one or two cell layers thick. Parietal cell not formed (parietal tissue absent). Hypostase present. Nucellar cap absent. Megagametophyte usually monosporous, Polygonum type (rarely disporous, Allium type, or tetrasporous, ? type), with development usually delayed until after pollination. Antipodal cells persistent. Endosperm development ab initio nuclear; endosperm usually not developing, or development very early arrested. Endosperm haustoria absent. Embryogenesis asterad or onagrad (sometimes possibly piperad). Polyembryony occurs in some genera.
Fruit Usually a loculicidal or septicidal capsule (sometimes indehiscent; rarely a berry).
Seeds Aril absent. Seeds usually extremely small (often less than 0,1 µm). Seed coat membranous, mainly exotestal. Testa hyaline. Phytomelan absent. Tegmen sometimes thick. Perisperm not developed. Endosperm little developed or absent. Embryo minute, usually undifferentiated (devoid of organs), usually with chlorophyll. Suspensor haustoria usually well developed, with various structures (often branched). Cotyledon one, rarely developed. Coleoptile absent. Germination taking place together with fungal hyphae; embryo developing into a tuberous protocorm with basal rhizoids. Radicula usually absent (rarely present and then ephemeral).
Cytology n = 9, 10, 12, 14–16, 18, 19, 24, 29 or more
DNA
Phytochemistry Flavonols, flavone-C-glycosides, flavonoid sulphates, pyrrolizidine alkaloids in the form of esters of arylic and aralkylic acids (above all in Kerosphaereae) and other alkaloids, and 6-hydroxyapigenin methyl ethers, and aliphatic monocarboxylic esters present. Tricine rare. Mannans present as storage carbohydrates. Chelidonic acid? Ellagic acid, proanthocyanidins and cyanogenic compounds not found. Aluminium accumulation reported from Spathoglottis.
Use Ornamental plants, flavours and perfumes (Vanilla), medicinal plants.
SystematicsOrchidaceae are sister-group to all other Iridales.
Apostasioideae are sister to the clade [Vanilloideae+[Cypripedioideae+[Orchidoideae+Epidendroideae]]].
The taxonomy below is according to Chase & al. (2015).
Apostasioideae Horan., Char. Ess. Fam.: 46. 17Jun 1847 [‘Apostasieae‘]
2/16. Neuwiedia (8; N. borneensis, N. elongata, N. griffithii, N. inae, N. malipoensis, N. siamensis, N. veratrifolia, N. zollingeri; Yunnan to Malesia and Melanesia), Apostasia (8; A. fogangica, A. latifolia, A. nuda, A. odorata, A. parvula, A. ramifera, A. shenzhenica, A. wallichii; Sri Lanka, northeastern India, Japan, Southeast Asia, Malesia, northeastern Queensland, Melanesia). – Sri Lanka, northeastern India, Japan, Southeast Asia, Malesia, northeastern Queensland, Melanesia. Vessels present in roots and sometimes in stem. Vessel elements with simple or scalariform perforation plates. Leaves spiral, with plicate ptyxis. Stomata tetracytic. Flowers almost actinomorphic; in Neuwiedia resupinate; in Apostasia non-resupinate, without labellum. Simultaneous initiation of inner tepals. Tepals apiculate, carinate. Stamens in Neuwiedia three fertile (staminodium absent); in Apostasia two lateral inner fertile stamens and one median outer staminodium. Pollen grains sometimes operculate. Ovary trilocular. Style entire. Placentation axile. Micropyle bistomal. Megagametophyte in Neuwiedia disporous, Allium type. Seed coat endotestal. Exotegmen in Neuwiedia sclerified. Outer periclinal cell walls collapsing. n = 24. Chromosomes small. Plastid gene matK possibly transition between functional gene and pseudogene.
[Vanilloideae+[Cypripedioideae+[Orchidoideae+Epidendroideae]]]
Velamen often present. Tilosomes (‘fibrous bodies‘, ‘rod bodies‘; lignified appendages from cell walls of innermost cell layer of root velamen adjacent to exodermis) often present. Vessel elements usually with scalariform perforation plates. Leaves spiral or distichous. Flowers strongly zygomorphic, with well developed labellum. Tepal nectaries sometimes present. Style and filaments almost entirely congenitally fused into gynostemium. Pollen grains viscid. Stigma asymmetric. Placentation parietal. Ovules not entirely developed at pollination (fertilization often delayed up to several months). Seeds dispersed prior to embryo maturation. Radicula absent or ephemeral.
Vanilloideae (Lindl.) Szlach. in Fragm. Forist. Geobot., Suppl. 3: 48. 1995
14/248–250. Pogonieae Pfetzer ex Garay et dunsterv., Venez. Orchids Ill. 2: 28. 1961. Duckeella (4; D. adolphii, D. alticola, D. humboldtii, D. pauciflora; Colombia, Venezuela, Brazil), Cleistes (64; tropical America to Florida), Cleistesiopsis (3; C. bifaria, C. divaricata, C. oricamporum; eastern and southeastern United States), Isotria (2; I. medeoloides, I. verticillata; eastern and central United States), Pogonia (5; P. japonica, P. minor, P. yunnanensis: China, the Korean Peninsula, Japan, Taiwan; P. trinervia: the Moluccas Islands; P. ophioglossoides: eastern North America). – Vanilleae Blume in Rumphia 1: 196. Dec 1837. Epistephium (21; northern South America), Lecanorchis (c 20; tropical Asia, Japan), Clematepistephium (1; C. smilacifolium; New Caledonia), Eriaxis (1; E. rigida; New Caledonia); Vanilla (106; tropical and subtropical regions on both hemispheres), Erythrorchis (2; E. altissima: Southeast Asia, Malesia, Taiwan, the Ryukyu Islands; E. cassythoides: Queensland, New South Wales), Pseudovanilla (≥8; Malesia to tropical Australia and islands in the Pacific), Cyrtosia (5; C. integra, C. javanica, C. nana, C. plurialata, C. septentrionalis; India, southern China, Southeast Asia, Japan, Ryukyu Islands), Galeola (6; G. cathcarthii, G. faberi, G. falconeri, G. humblotii, G. lindleyana, G. nudifolia; India and the Himalayas to China and Southeast Asia, Taiwan, Malesia to New Guinea, one species, G. humblotii, on Madagascar and the Comoros). – Pantropical, with their largest diversity in tropical Asia, few species in eastern North America. CAM physiology sometimes present. Silica bodies absent. Stomata usually tetracytic. Simultaneous initiation of inner tepals? Tepals often carinate, sometimes persistent. Median outer stamen fertile and lateral inner stamens staminodial. Anther incumbent due to strong extension of apical connective. Pseudopollinia soft (pollinia absent). Pollen grains sometimes polyporate, often shed as tetrads. Viscidium absent. Rostellum (part of median stigmatic lobe) present. Placentation usually parietal (sometimes axile). Seeds fusiform, crustose. Seed coat exotestal, with outer parietal wall well developed. Tegmen persistent. Endosperm up to 16-nucleate. n = 9, 10, 12, 14–16, 18 and more. Chromosomes comparatively large. Plastid gene matK pseudogene. – Vanilloideae are sister-group to the remaining Orchidaceae.
[Cypripedioideae+[Orchidoideae+Epidendroideae]]
Cypripedioideae Lindl. ex Endl., Gen. Plant.: 220. Jun 1837 [’Cypripedieae’]
5/160–165. Cypripedium (51; temperate regions on the Northern Hemisphere), Paphiopedilum (80–85; India to southern China and Southeast Asia, Malesia to New Guinea, the Bismarck Archipelago and Solomon Islands), Selenipedium (6; S. aequinoctiale, S. chica, S. isabelianum, S. palmifolium, S. steyermarkii, S. vanillocarpum; tropical South America), Phragmipedium (23; Mexico, Central America tropical South America), Mexipedium (1; M. xerophyticum; Mexico). – Tropical, subtropical and temperate regions on the Northern Hemisphere to East Malesia, southern India, southwestern Pacific and southwards to central South America. Leaves with conduplicate or plicate ptyxis. Stomata often paracytic. Simultaneous initiation of inner tepals. Tepals sometimes caducous. Two abaxial outer tepals connate. Labellum strongly saccate. Two lateral inner stamens fertile. Median outer stamen staminodial. Tapetum with binucleate cells. Microsporogenesis successive? Pollen grains operculate, sometimes shed as tetrads. Ovary sometimes trilocular. Median stigmatic lobe larger than lateral lobes. Placentation usually parietal (sometimes axile). Micropyle sometimes bistomal. Megagametophyte sometimes disporous, octocellular, Allium type. n = 9 or more. Chromosomes comparatively large. Plastid gene matK pseudogene. – Cypripedioideae are sometimes sister-group to Vanilloideae or to the clade [Vanilloideae+[Orchidoideae+Epidendroideae]]. Selenipedium, in particular, have a number of plesiomorphies, e.g. pollen grains shed as monads, trilocular ovary, fleshy fruit, and hard dark testa, suggesting a position of Cypripedioideae at least as basal as Vanilloideae. Yet, I have followed the most commonly recovered topology here.
[Orchidoideae+Epidendroideae]
Floral primordium transversely elliptic-oval. Tepals not caducous. Median outer stamen fertile. Two lateral inner stamens usually absent (sometimes staminodial). Pollinia adnate to viscidium (part of median stigmatic lobe). Pollinarian stipe formed from caudicula (part of anther), tegula (epidermis on rostellum), or hamulus (apex of rostellum). Microsporogenesis simultaneous, with tetraedric tetrads. Pollen grains porate or ulcerate, usually shed in tetrads. Median carpel developed prior to lateral carpels. Rostellum (part of median stigmatic lobe) present. Endosperm not developing. Tegmen not persistent. n = 9 or more (often 19).
Orchidoideae Eaton, Bot. Dict., ed. 4: 29. Apr-Mai 1836 [‘Orchideae’]
194/4.925–5.000. Codonorchideae P. J. Cribb in P. J. Cribb et P. J. Kores, Lindleyana 15: 169. 29 Sep 2000. Codonorchis (2; C. canisioi, C. lessonii; Brazil to southern South America, the Falkland Islands). – Orchideae Small, Man. S.E. Fl.: 363. 30 Nov 1933. Brownleeinae H. P. Linder et H. Kurzweil in Ann. Missouri Bot. Gard. 81: 710. 1994. Brownleea (8; tropical and southern Africa, Madagascar), Disperis (78; Africa, Madagascar, the Mascarene Islands, India, Sri Lanka, Thailand, China, Japan, the Ryukyu Islands, Taiwan, the Philippines, Java, the Lesser Sunda Islands, New Guinea, the Caroline Islands). – Coryciinae Benth. in J. Linn. Soc. London, Bot. 18: 288. 21 Feb 1881 [‘Corycieae’]. Ceratandra (6; C. atrata, C. bicolor, C. globosa, C. grandiflora, C. harveyana, C. venosa; Western and Eastern Cape), Evotella (2; E. carnosa, E. rubiginosa; Western Cape), Pterygodium (32; southern Africa to Malawi and southern Tanzania, with their highest diversity in Western Cape and the Drakensberg in KwaZulu-Natal). – Pachites clade: Pachites (2; P. appressus, P. bodkinii; Western Cape). – Disinae Benth. in J. Linn. Soc. London, Bot. 18: 288. 21 Feb 1881 [‘Diseae’]. Disa (170–180; Africa, Madagascar, the Mascarene Islands, the Arabian Peninsula), Huttonaea (5; H. fimbriata, H. grandiflora, H. oreophila, H. pulchra, H. woodii; Eastern Cape, KwaZulu-Natal, Mpumalanga, Free State).– Orchidinae Reveal in Phytoneuron 2012-33: 2. 9 Apr 2012. Satyrium (85–90; tropical and southern Africa, Madagascar, the Arabian Peninsula, India, Sri Lanka, Burma, China), Peristylus (c 100; China to islands in the Pacific), Pecteilis (8; tropical and East Asia; in Habenaria?), Hsenhsua (1; H. chrysea; eastern Himalayas, Bhutan, Yunnan, Xizang), Herminium (c 50; Europe, temperate Asia), Androcorys (10; the Himalayas, China, Honshu in Japan), Porolabium (1; P. biporosum; China), Gennaria (1; G. diphylla; Macaronesia, western Mediterranean; in Habenaria?), Nujiangia (1; N. griffithii; the Himalayas),‘Habenaria’ (c 840; tropical and subtropical regions on both hemispheres; paraphyletic), Bonatea (13; eastern Africa to Western Cape, the Arabian Peninsula; in Habenaria?), Sirindhornia (3; S. mirabilis, S. monophylla, S. pulchella; Burma, Yunnan, Thailand), 'Ponerorchis' (c 55; the Himalayas, China, Japan; paraphyletic), Tsaiorchis (1; T. neottianthoides; southeastern Yunnan, Guangxi), Hemipilia (13; the Himalayas, southeastern Tibet, East Asia, Thailand), Ophrys (10–55; Europe, the Mediterranean, North Africa, western Asia), Serapias (13; the Azores, the Mediterranean), Orchis (21; temperate regions on the Northern Hemisphere to southwestern China and northern India), Gymnadenia (23; Europe, temperate Asia, eastern North America), Coeloglossum (1; C. viride; temperate regions on the Northern Hemisphere), Dactylorhiza(c 40; Europe, Macaronesia, the Mediterranean, temperate Asia, Alaska), Pseudorchis (1–2; P. albida, P. straminea; Europe, northern Asia to Kamchatka, eastern North America, Greenland), Galearis (10; the Himalayas, Tibet, Burma, China, the Korean Peninsula, Japan, the Russian Far East, Kamtchatka, Canada, temperate United States, western Greenland), ’Platanthera’ (c 135; temperate to tropical regions on the Northern Hemisphere south to mountains in Malesia; polyphyletic), Anacamptis (11; Europe, the Mediterranean to Iran), Bartholina (2; B. burmanniana, B. etheliae; southern Namibia, Northern, Western and Eastern Cape), Benthamia (c 30; Madagascar, the Mascarene Islands?), Bhutanthera (6; B. albomarginata, B. albosanguinea, B. albovirens, B. alpina, B. fimbriata, B. himalayana; Nepal, eastern Himalayas to southwestern China), Brachycorythis (c 35; tropical and southern Africa, tropical Asia), Centrostigma (3; C. clavatum, C. occultans, C. papillosum; southern tropical and southern Africa), Chamorchis (1; C. alpina; northern Scandinavia, the Carpathians, the Alps), Cynorkis (c 170; eastern Africa, Madagascar, the Mascarene Islands, with their highest diversity in Madagascar), Diplomeris (3; D. hirsuta, D. josephi, D. pulchella; the Himalayas and Tibet to southern China and Southeast Asia), Dracomonticola (1; D. virginea; the Drakensberg in KwaZulu-Natal and Lesotho), Frigidorchis (1; F. humidicola; Qinghai in southern China), Himantoglossum (11; Europe, the Canary Islands, the Mediterranean to western Iran, North Africa), Holothrix (c 45; tropical and southern Africa, the Arabian Peninsula), Megalorchis (1; M. regalis; eastern Madagascar), Neobolusia (3; N. ciliata, N. stolzii, N. tysonii; Tanzania to Zimbabwe and South Africa), Neotinea (4; N. lactea, N. maculata, N. tridentata, N. ustulata; Europe, Macaronesia, the Mediterranean), Odisha (1; O. cleistantha; Orissa in India; in Habenaria?), Oligophyton (1; O. drummondii; Zimbabwe), Physoceras (12; Madagascar), Platycoryne (c 20; tropical Africa, Madagascar), Roeperocharis (5; R. alcicornis, R. bennettiana, R. maleveziana, R. urbaniana, R. wentzeliana; tropical East Africa), Schizochilus (11; eastern and southeastern Africa), Silvorchis (4; S. aurea, S. colorata, S. furcata, S. vietnamica; Vietnam, Java), Stenoglottis (8; eastern South Africa, Swaziland, one species in eastern Africa to Tanzania), Steveniella (1; S. satyroides; Crimea to western Asia), Thulinia (1; T. albolutea; Nguru Mountains in Tanzania), Traunsteinera (2; T. globosa, T. sphaerica; Europe, the Mediterranean to Turkey, Ukraine and the Caucasus), Tylostigma (8; Madagascar), Veyretella (2; V. flabellata, V. hetaerioides; Gabon). – Cranichideae Pfeiff., Nomencl. Bot. 1(2): 901. ante 3 Oct 1873 (polyphyletic). Chloraeinae Pfitzer, Entwurf. Anordn. Orch.: 98. Jan-Apr 1887 [’Chloraeeae’]. Bipinnula (10–11; Brazil, Uruguay, Argentina, southern Chile), ‘Chloraea‘ (52; southern Chile, southern Argentina), Gavilea (16; southern Chile, southern Argentina). – Pterostylidinae Pfitzer, Entwurf. Anordn. Orch.: 97. Jan-Apr 1887 [’Pterostylideae’]. Achlydosa (1; A. glandulosa; New Caledonia), Pterostylis (c 210; East Malesia, Queensland to South Australia, Tasmania, New Caledonia, New Zealand). – Goodyerinae Ridl., Mat. Flora Malay Penins. 1: 12. 1907. Aenhenrya (1; A. rotundifolia; southern India), Anoectochilus (43; tropical Asia to the Hawaiian Islands), Aspidogyne (c 60; southern Mexico, Central America, tropical South America), Chamaegastrodia (3; C. inverta, C. shikokiana, C. vaginata; the Himalayas, East Asia), Cheirostylis (53; tropical and subtropical regions of the Old World), Cystorchis (c 20; Thailand and eastwards to Micronesia), Danhatchia (1; D. australis; New Zealand), Dossinia (1; D. marmorata; Borneo), Erythrodes (26; tropical Asia, New Caledonia to Samoa and Tonga), Eurycentrum (7; E. amblyoceras, E. atroviride, E. fragrans, E. goodyeroides, E. monticola, E. obscurum, E. salomonense; New Guinea, Solomon Islands, Vanuatu), Gonatostylis (2; G. bougainvillei, G. vieillardii; New Caledonia), Goodyera (100–105; temperate regions on the Northern Hemisphere, Mozambique, Madagascar, tropical Asia), Halleorchis (1; H. aspidogynoides; Cameroon, Gabon), Herpysma (1; H. longicaulis; southern and southeastern Asia to Sumatra), Hetaeria (c 30; Sri Lanka, Southeast Asia, Malesia to Tahiti), Hylophila (7; H. cheangii, H. gracilis, H. lanceolata, H. mollis, H. nipponica, H. orientalis, H. rubra; Thailand to Solomon Islands), Kreodanthus (14; Mexico, Central America, Cuba, northern tropical South America), Kuhlhasseltia (10; the Korean Peninsula, Japan to New Guinea), Lepidogyne (1; L. longifolia; Malesia), Ludisia (1; L. discolor; Southeast Asia, West Malesia), Macodes (11; Japan, Vietnam, Malesia to Vanuatu), Microchilus (c 75; Mexico, Central America, the West Indies, tropical South America), Myrmechis (17; the Himalayas, Tibet, Southeast Asia, Malesia to New Guinea, Taiwan, the Korean Islands, Japan, the Kuril Islands), Odontochilus (c 25; the Himalayas, tropical Asia, the Hawaiian Islands), Orchipedum (3; O. echinatum, O. plantaginifolium, O. wenzelii; Southeast Asia, Java, the Philippines), Pachyplectron (2; P. arifolium, P. neocaledonicum; New Caledonia), Papuaea (1; P. reticulata; New Guinea), Platylepis (17; tropical and southern Africa, Madagascar, the Mascarene Islands, the Seychelles, the Moluccas, Tahiti), Rhamphorhynchus (1; R. mendoncae; Brazil), Rhomboda (22; the Himalayas, southern China, Southeast Asia, Malesia to New Guinea, the Bismarck Archipelago, Solomon Islands, New Caledonia, Queensland, Taiwan, Japan, the Ryukyu Islands), Schuitemania (1; S. merrillii; the Philippines), Stephanothelys (5; S. colombiana, S. rariflora, S. siberiana, S. sororia, S. xystophylloides; the Andes), Vrydagzynea (43; southeastern China, Southeast Asia, the Nicobar Islands, Malesia to New Guinea, Solomon Islands, Vanuatu, Queensland, Taiwan, Fiji, Samoa, Tonga), Zeuxine (c 75; tropical and subtropical regions in the Old World), Zeuxinella (1; Z. vietnamica; Vietnam). – Galeottiellinae Salazar et M. W. Chase in Lindleyana 17: 172. 9 Oct 2002. Galeottiella (2; G. orchioides, G. sarcoglossa; Mexico, Guatemala). – Manniellinae Schltr. in Notizbl. Bot. Gart. Berlin-Dahlem 9: 572, 585. 22 Jul 1926 [’Mannielleae’]. Manniella (2; M. cypripedioides, M. gustavi; tropical West Africa). – Spiranthinae Lindl. ex Meisn., Plant. Vasc. Gen.: Tab. Diagn. 385, Comm. 288. 17-20 Aug 1842 [’Spiranthideae’]. Cotylolabium (1; C. lutzii; Espírito Santo and Minas Gerais in southeastern Brazil); Coccineorchis (7; C. bracteosa, C. cernua, C. cristata, C. dressleri, C. navarrensis, C. standleyi, C. warszewicziana; Mexico, Central America, northwestern South America), Cyclopogon (c 85; Florida, Mexico, Central America, the West Indies, tropical South America), Sarcoglottis (45–50; Mexico, Central America, Grenada, tropical South America), Sauroglossum (11; South America), Pelexia (77; Florida, Mexico, Central America, the West Indies, tropical South America), Odontorrhynchus (6; O. alticola, O. castillonii, O. domeykoanus, O. erosus, O. monstrosus, O. variabilis; the Andes), Brachystele (21; Mexico, Central America, tropical South America), Stenorrhynchos (5; S. albidomaculatum, S. austrocompactum, S. glicensteinii, S. speciosum, S. vaginatum; Mexico, Central America, the West Indies, tropical South America), Eltroplectris (13; Florida, the West Indies, tropical South America), Nothostele (2; N. acianthiformis, N. brasiliaensis; Brazil), Mesadenella (7; M. angustisegmenta, M. atroviridis, M. cuspidata, M. meeae, M. peruviana, M. tunduzii, M. variegata; Mexico, Central America, tropical South America), Pteroglossa (11; South America), Lyroglossa (2; L. grisebachii, L. pubicaulis; southern Mexico, Central America, Brazil), Skeptrostachys (13; tropical South America), Sacoila (8; Florida, Mexico, Central America, the West Indies, tropical South America), Quechua (1; Q. glabrescens; Ecuador, Peru), Lankesterella (11; Costa Rica, the Greater Antilles, tropical South America), Eurystyles (20; southern Mexico, Central America, the West Indies, tropical South America), Hapalorchis (10; Central America, the West Indies, tropical South America), Funkiella (27; Texas, Mexico, Guatemala, Costa Rica), Sotoa (1; S. confusa; western Texas, northern and central Mexico), Svenkoeltzia (4; S. congestiflora, S. luzmariana, S. pamelae, S. patriciae; Mexico), Beloglottis (6; B. boliviensis, B. costaricensis, B. ecallosa, B. hameri, B. mexicana, B. subpandurata; southeastern United States to Brazil), Aulosepalum (8; Mexico to Costa Rica), Spiranthes (42–45; temperate regions on the Northern Hemisphere, Malesia to eastern Australia, islands in the Pacific, Canada to Central America), Physogyne (3; P. garayana, P. gonzalezii, P. sparsiflora; Mexico), Pseudogoodyera (1; P. wrightii; Central America, Cuba), Mesadenus (7; M. affinis, M. chiangii, M. glaziovii, M. lucayanus, M. polyanthus, M. rhombiglossus, M. tenuissimus; Florida, Central America, the West Indies, southeastern Brazil), Greenwoodiella (3; G. deserticola, G. micrantha, G. wercklei; southern Texas, Mexico, Central America, the West Indies), Kionophyton (4; K. pyramidalis, K. riodelayensis, K. sawyeri, K. seminuda, Mexico, Guatemala), Schiedeella (22; southwestern United States, Mexico, Central America, the West Indies), Dichromanthus (4; D. aurantiacus, D. cinnabarinus, D. michuacanus, D. yucundaa; Texas, Arizona, Mexico, Central America), Deiregyne (18; Mexico, Guatemala). Unplaced Spiranthinae: Aracamunia (1; A. liesneri; southern Venezuela), Buchtienia (4; B. boliviensis, B. claudiae, B. nitida, B. rosea; tropical South America), Cybebus (1; C. grandis; Colombia), Degranvillea (1; D. dermatoptera; French Guiana), Helonoma (4; H. americana, H. bifida, H. chiropterae, H. peruviana; the Guayana Highlands, the Andes in Peru), Stalkya (1; S. muscicola; the Andes in Venezuela), Thelyschista (1; T. ghillanyi; eastern Brazil). – Discyphinae Salazar et Van den Berg in Phytotaxa 173(2): §136. 25 Jun 2014. Discyphus (1; D. scopulariae; Brazil). – Cranichidinae Lindl. ex Meisn., Plant. Vasc. Gen.: Tab. Diagn. 384, Comm. 288. 17-20 Aug 1842 [’Cranichideae’].Aa (c 25; the Andes), Altensteinia (7; A. boliviensis, A. citrina, A. elliptica, A. fimbriata, A. longispicata, A. marginata, A. virescens; the Andes), Baskervilla (10; the Andes, southern Brazil), Cranichis (53; Florida, Mexico, Central America, the West Indies, tropical South America), Fuertesiella (1; F. pterichoides; eastern Cuba, Hispaniola), Galeoglossum (3; G. cactorum, G. thysanochilum, G. tubulosum; Mexico), Prescottia (26; Florida, Mexico, Central America, the West Indies, tropical South America), Gomphichis (24; Costa Rica, Brazil, the Andes), Myrosmodes (12; the Andes), Ponthieva (c 65; southeastern United States, Mexico, Central America, the West Indies, tropical South America), Porphyrostachys (2; P. parviflora, P. pilifera; the Andes in Ecuador and Peru), Pseudocentrum (7; P. bursarium, P. guadalupense, P. hoffmannii, P. macrostachyum, P. minus, P. purdii, P. sylvicola; Central America, the West Indies, tropical South America), Pterichis (c 20; Costa Rica, Jamaica, the Andes), Solenocentrum (4; S. asplundii, S. costaricense, S. lueri, S. maasii; Costa Rica, Ecuador), Stenoptera (7; S. acuta, S. ciliaris, S. ecuadorana, S. huilaensis, S. laxiflora, S. montana, S. peruviana; the Andes). – Diurideae Endl. ex Butzin in Willdenowia 6: 311. 22 Oct 1971. Rhizanthellinae R. S. Rogers in J. Roy. Soc. W. Australia 15: 4. 17 Oct 1928. Rhizanthella (3; R. gardneri, R. omissa, R. slateri; southwestern Western Australia, eastern Australia). – Acianthinae Schltr. in Bot. Jahrb. Syst. 45: 381. 21 Feb 1922.Acianthus (20; New Guinea, eastern Queensland to southeastern South Australia, Tasmania, New Caledonia, New Zealand), Corybas (c 130; Japan, tropical Asia, eastern Queensland to southeastern South Australia, Tasmania, New Zealand, Macquarie Island, Polynesia), Cyrtostylis (5; C. oblonga, C. reniformis, C. robusta, C. rotundifolia, C. tenuissima; southwestern Western Australia, southeastern Queensland to southeastern South Australia, Tasmania, New Zealand), Stigmatodactylus (10; the Himalayas, China, Japan, Malesia to New Guinea, Solomon Islands), Townsonia (2; T. deflexa, T. viridis; Tasmania, New Zealand). – Caladeniinae Pfitzer, Entwurf. Anordn. Orch.: 97. Jan-Apr 1887 [’Caladenieae’]. Adenochilus (2; A. gracilis, A. nortonii; eastern New South Wales, New Zealand), Aporostylis (1; A. bifolia; New Zealand incl. Stewart Island, Chatham Islands, Auckland Islands, Campbell Island, the Antipode Islands), Caladenia (c 300; Australia, one species, C. catenata, also in Malesia, New Caledonia, New Zealand, Antipode Islands and Chatham Islands), Eriochilus (c 9; southwestern Western Australia, southeastern Australia, Tasmania), Leptoceras (1; L. menziesii; southwestern Western Australia, southeastern South Australia, Victoria, Tasmania), Praecoxanthus (1; P. aphyllus; southwestern Western Australia). – Prasophyllinae Schltr. in Bot. Jahrb. Syst. 45: 381. 21 Feb 1911. Genoplesium (47; eastern Queensland to southern Western Australia, Tasmania, New Caledonia, New Zealand), Microtis (19; China, Japan to southwestern Western Australia, New Zealand), Prasophyllum (c 130; southeastern Queensland to southwestern Western Australia, Tasmania, New Zealand). – Diuridinae Lindl. ex Meisn., Plat. Vasc. Gen.: Tab. Diagn. 387, Comm. 289. 17-20 Aug 1842 [‘Diurideae‘]. Diuris (c 70; Timor, Australia), Orthoceras (2; O. novae-zeelandiae, O. strictum; southeastern Queensland to southeastern South Australia, Tasmania, New Caledonia, New Zealand). – Cryptostylidinae Schltr. in Notizbl. Bot. Gart. Berlin-Dahlem 9: 570, 583. 22 Jul 1926 [‘Cryptostylideae‘]. Coilochilus (1; C. neocaledonicus; New Caledonia), Cryptostylis (c 25; Taiwan, tropical Asia to Australia, New Caledonia, Vanuatu, New Zealand and Samoa). – Drakaeinae Schltr. in Bot. Jahrb. Syst. 45: 381. 21 Feb 1911. Arthrochilus (c 15; New Guinea, tropical Australia to northeastern New South Wales), Caleana (1; C. major; southwestern Western Australia, southeastern Queensland to South Australia, Tasmania), Chiloglottis (c 25; southeastern Queensland to southern Victoria, Tasmania, New Zealand), Drakaea (10; southwestern Western Australia), Paracaleana (13; eastern, southern and western Australia, Tasmania, North Island in New Zealand, with their highest diversity in southwestern Western Australia), Spiculaea (1; S. ciliata; southwestern Western Australia, southeastern New South Wales, southern Victoria). – Megastylidinae Schltr. in Notizbl. Bot. Gart. Berlin-Dahlem 9: 572, 585. 22 Jul 1926 [‘Megastylideae‘].Burnettia (1; B. cuneata; southeastern New South Wales, southern Victoria, Tasmania), Leporella (1; L. fimbriata; southwestern Western Australia, southeastern South Australia, Victoria), Lyperanthus (2; L. serratus, L. suaveolens; southwestern Western Australia, southeastern Australia, Tasmania, New Zealand), Megastylis (6; M. gigas, M. latilabris, M. latissima, M. montana, M. paradoxa, M. rara; New Caledonia, Vanuatu; polyphyletic), Pyrorchis (2; P. forrestii, P. nigricans; southwestern Western Australia, southeastern South Australia, eastern New South Wales, Victoria, Tasmania), Rimacola (1; R. elliptica; eastern New South Wales), Waireia (1; W. stenopetala; New Zealand). – Thelymitrinae Lindl. ex Meisn., Plant. Vasc. Gen.: Tab. Diagn. 387, Comm. 289. 17-20 Aug 1842 [’Thelymitrideae’]. Calochilus (27; New Guinea, southeastern Queensland to southeastern South Australia, southwestern Western Australia, Tasmania, New Caledonia, New Zealand), Epiblema (1; E. grandiflorum; southwestern Western Australia), Thelymitra (c 110; Malesia to New Guinea, eastern Queensland to southeastern South Australia, southwestern Western Australia, Tasmania, New Caledonia, New Zealand). – Cosmopolitan, with their highest diversity in temperate regions. Mycoheterotrophy absent. Stem or root tuber frequent. Stem and leaf sclerenchyma rare (in leaves as fibre bundles or associated with vascular bundles). Stegmata with silica bodies absent. Leaves spiral or distichous. Stomata usually anomocytic. Anther usually erect (sometimes incumbent). Pollinia present, soft, sectile. Staminodia reduced. Hamulus (pollinium stalk developed from modified rostellum apex) sometimes present. n = 12–24. Chromosomes small. Plastid gene matK pseudogene.
Epidendroideae Lindl. ex Endl., Gen. Plant.: 193. Jun 1837 [‘Epidendreae’]
561/22.060–22.115. Neottieae Lindl., Orchid. Select.: 7, 9. Jan 1826. Palmorchis (21; Central America, tropical South America); Cephalanthera (19; temperate regions on the Northern Hemisphere), Neottia (c 65; Europe, temperate Asia, North America), Aphyllorchis (22; Sri Lanka to Japan and eastern Queensland), Limodorum (3; L. abortivum, L. rubriflorum, L. trabuianum; the Mediterranean to Iran), Epipactis (70–75; Europe, tropical Africa, temperate Asia, the Himalayas, Southeast Asia). – Sobralieae Pfitzer, Entwurf. Anordn. Orch.: 99. Jan-Apr 1887 [’Sobraliinae’]. Elleanthus (c 110; Mexico, Central America, the West Indies, tropical South America; incl. Sertifera?), Sertifera (9; the Andes; in Elleanthus?), Sobralia (c 150; Mexico, Central America, tropical South America), Brasolia (23; northern and northwestern South America). – Tropidieae Dressler in Telopia 2: 422. 13 Oct 1983. Corymborkis (8; tropical Africa to South Africa, Madagascar, the Mascarene Islands, India to southern China and Southeast Asia, Malesia to New Guinea, Queensland and islands in the Pacific, Mexico, Central America, the West Indies, tropical South America), Kalimantanorchis (1; K. nagamasui; western Borneo), Tropidia (c 30; India, Sri Lanka and the Himalayas, Tibet and China to Southeast Asia, the Andamans, Malesia to New Guinea, tropical Australia to Melanesia and Norfolk Island, Taiwan, Japan and islands in western Pacific, Florida, Central America, the West Indies, northwestern South America).– Diceratostele clade. Diceratostele (1; D. gabonensis; western and central tropical Africa; position uncertain). – Nervilieae Dressler in Lindleyana 5: 124. 29 Jun 1990. Nervilia (c 80; tropical and southern Africa, Madagascar, the Arabian Peninsula, tropical Asia to New Guinea and northern Australia, islands in the Indian and Pacific oceans); Epipogium (3; E. aphyllum: Europe, temperate Asia to the Himalayas and Kamchatka; E. japonicum: Sichuan, Honshu in Japan, Taiwan; E. roseum: tropical Africa, India, China, Japan, Southeast Asia, Malesia to New Guinea, eastern Queensland and northeastern New South Wales, western Pacific islands), Stereosandra (1; S. javanica; Southeast Asia, West Malesia). – Wullschlaegelieae Dressler in Telopea 2: 422. 13 Oct 1983.Wullschlaegelia (2; W. aphylla, W. calcarata; Mexico, Central America, the West Indies, tropical South America). – Triphoreae Dressler in Selbyana 5: 204. 27 Dec 1979. Monophyllorchis (1; M. microstyloides; Colombia, Ecuador), Pogoniopsis (2; P. nidus-avis, P. schenkii; Brazil), Psilochilus (8; Mexico, Central America, the West Indies, tropical South America), Triphora (19; Florida, Mexico, Central America, the West Indies, tropical South America, one species, T. trianthophora, in eastern North America to Mexico). – Xerorchideae P. J. Cribb in Kew Bull. 60: 143. Jul 2005. Xerorchis (2; X. amazonica, X. trichorhiza; tropical South America). – Gastrodieae Lindl., Orchid. Select.: 7, 11. Jan 1826. Auxopus (4; A. kamerunensis, A. letouzeyi, A. macranthus, A. madagascariensis; tropical Africa, Madagascar), Didymoplexiella (8; Southeast Asia, West Malesia to Japan and the Ryukyu Islands), Didymoplexiopsis (1; D. khiriwongensis; Thailand, Laos, Vietnam, Hainan; in Didymoplexiella?), Didymoplexis (17; tropical and southeastern Africa, Madagascar, the Himalayas, Southeast Asia, China, Malesia to New Guinea, northern Australia, Melanesia, Taiwan and islands in western Pacific), Gastrodia (c 65; tropical Africa, East Asia to Japan, the Ryukyu Islands and Taiwan, the Himalayas and Southeast Asia to New Guinea, Melanesia, Australia, Tasmania and New Zealand), Uleiorchis (4; U. liesneri, U. longipedicellata, U. prataensis, U. ulei; Central America, tropical South America). – Thaieae X. H. Jin et X. G. Xiang in Taxon 61: 52. 21 Feb 2012.Thaia (1; T. saprophytica; Thailand). – Arethuseae Lindl., Orchid. Select.: 7, 10. Jan 1826. Arethusinae Benth. in J. Linn. Soc. London, Bot. 18: 288. 21 Feb 1881 [‘Arethuseae’]. Arundina (1; A. graminifolia; the Himalayas to islands in the Pacific), Arethusa (1; A. bulbosa; southern Canada, eastern United States), Eleorchis (1; E. japonica; Japan); Anthogonium (1; A. gracile; eastern Himalayas to Southeast Asia), Calopogon (5; C. barbatus, C. multiflorus, C. oklahomensis, C. pallidus, C. tuberosus; eastern United States, the West Indies). – Coelogyninae Benth. in J. Linn. Soc. London, Bot. 18: 287. 21 Feb 1881 [’Coelogyneae’].Aglossorhyncha (13; East Malesia to New Guinea), Bletilla (5; B. chartacea, B. foliosa, B. formosana, B. ochracea, B. striata; temperate East Asia), Bracisepalum (2; B. densiflorum, B. selebicum; Sulawesi), Bulleyia (1; B. yunnanensis; eastern Himalayas, southwestern China), Chelonistele (13; West Malesia, with their highest diversity on Borneo), Coelogyne (>200; India, southern China, Southeast Asia, Malesia to New Guinea and islands in western Pacific), Dendrochilum (c 280; Southeast Asia, Malesia to New Guinea), Dickasonia (1; D. vernicosa; northeastern India, Burma), Dilochia (8; Malesia), Entomophobia (1; E. kinabaluensis; northern Borneo), Geesinkorchis (4; G. alaticallosa, G. breviunguiculata, G. phaiostele, G. quadricarinata; West Malesia), Glomera (>130; Laos, Malesia to New Guinea and Melanesia), Gynoglottis (1; G. cymbidioides; Sumatra), Ischnogyne (1; I. mandarinorum; China), Nabaluia (3; N. angustifolia, N. clemensii, N. exaltata; northern Borneo), Otochilus (5; O. albus, O. fuscus, O. lancilabius, O. porrectus, O. pseudoporrectus; eastern Himalayas to Southeast Asia), Panisea (13; northeastern India to Southeast Asia), Pholidota (31; India to southern China and Southeast Asia, Malesia to New Guinea, tropical and eastern Australia, islands in the Pacific), Pleione (16–22; Nepal to Taiwan and Thailand), Thunia (5; T. alba, T. bensoniae, T. brymeriana, T. candidissima, T. pulchra; the Himalayas to Burma). – Malaxideae Lindl., Collect. Bot. 8: 7. Jan 1826. Dendrobiinae G. Don in R. Sweet, Hort. Brit., ed. 3: 638. late 1839 [’Dendrobieae’]. Bulbophyllum (c 1.870; tropical and subtropical regions on both hemispheres), Dendrobium (c 1.510; tropical and subtropical regions in Asia, tropical and eastern Australia, Tasmania and the Pacific), Genyorchis (10; tropical West and Central Africa; in Dendrobium?). – Malaxidinae Benth. et Hook. f., Gen. Plant. 3: 463, 465. 14 Apr 1883. Alatiliparis (5; A. angustiflora, A. filicornes, A. lepanthes, A. otochilus, A. speculifera; Sumatra, Java), Crepidium (c 260; islands in the Indian Ocean, India, China, Southeast Asia, Malesia to New Guinea, northern Australia, islands in the Pacific), Crossoglossa (26; Central America, tropical South America), Crossoliparis (1; C. wendlandii; Central America), Dienia (2; D. ophrydis, D. seidenfadeniana; India, southern China, Southeast Asia, Malesia to northern Australia and Melanesia, Japan), Hammarbya (1; H. paludosa; temperate and subarctic regions on the Northern Hemisphere), Hippeophyllum (c 10; Malesia), ’Liparis’ (c 425; nearly cosmopolitan; non-monophyletic),’Malaxis’ (c 180; nearly cosmopolitan; non-monophyletic), Oberonia (c 325; tropical and southern Africa, Madagascar, the Mascarene Islands, India, Southeast Asia, Malesia to New Guinea and northern Australia), Oberonioides (2; O. oberoniiflora, O. pusillus; China, Thailand), Orestias (4; O. elegans, O. foliosa, O. micrantha, O. stelidostachya; tropical Africa), Stichorkis (8; islands in the Indian Ocean, India, Southeast Asia, Malesia to New Guinea, islands in the Pacific), Tamayorkis (4; T. ehrenbergii, T. hintonii, T. porphyrea, T. wendtii; Mexico, Central America). – Cymbidieae Pfitzer, Entwurf. Anordn. Orch.: 105. Jan-Apr 1887 [’Cymbidiinae’]. Cymbidiinae Benth. in J. Linn. Soc. London, Bot. 18: 287. 21 Feb 1881 [’Cymbidieae’]. Acriopsis (10; Southeast Asia to Solomon Islands), Ansellia (1; A. africana; tropical and southern Africa), Claderia (2; C. papuana, C. viridiflora; Thailand, Malesia to New Guinea), Cymbidium (c 70; northwestern India to Japan and Australia), Dipodium (c 25; Malesia to tropical Australia and New Caledonia), Grammatophyllum (13; Malesia to Polynesia), Graphorkis (4; G. concolor, G. ecalcarata, G. lurida, G. medemiae; tropical Africa, Madagascar, the Mascarene Islands), Imerinaea (1; I. madagascarica; northern Madagascar)?, Porphyroglottis (1; P. maxwelliae; Malaysia, Sumatra, Borneo), Thecopus (2; T. maingayi, T. secunda; Southeast Asia, Borneo), Thecostele (1; T. alata; Burma to West Malesia). – Cyrtopodiinae Benth. in J. Linn. Soc. London, Bot. 18: 288. 21 Feb 1881 [’Cyrtopodieae’]. Cyrtopodium (47; Florida, Mexico, Central America, the West Indies, tropical South America).– Eulophiinae Benth. in J. Linn. Soc. London, Bot. 18: 287. 21 Feb 1881 [’Eulophieae’]. Grammangis (2; G. ellisii, G. spectabilis; Madagascar), Eulophiella (5; E. capuroniana, E. elisabethae, E. ericophila, E. galbana, E. roempleriana; Madagascar), Cymbidiella (3; C. falcigera, C. flabellata, C. pardalina; Madagascar), Orthochilus (c 35; tropical Africa, Madagascar, tropical and subtropical America), Paralophia (2; P. epiphytica, P. palmicola; Madagascar), Oeceoclades (31; tropical Africa, Madagascar, Indian Ocean islands), Acrolophia (7; A. barbata, A. bolusii, A. capensis, A. cochlearis, A. lamellata, A. micrantha, A. ustulata; Western Cape to KwaZulu-Natal), Eulophia (c 215; tropical and subtropical regions on both hemispheres). – Catasetinae Schltr. in Notizbl. Bot. Gart. Berlin-Dahlem 9: 577, 588. 22 Jul 1926 [’Cataseteae’]. Catasetum (c 175; Mexico, Central America, the West Indies, tropical South America), Clowesia (7; C. amazonica, C. dodsoniana, C. glaucoglossa, C. rosea, C. russelliana, C. thylaciochila, C. warczewitzii; Central America, tropical South America), Cyanaeorchis (3; C. arundinae, C. minor, C. praetermissa; Brazil), Cycnoches (c 35; southern Mexico, Central America, tropical South America), Dressleria (12; Central America), Galeandra (38; Central America, tropical South America), Grobya (5; G. amherstiae, G. cipoensis, G. fascifera, G. galeata, G. guieselii; Brazil), Mormodes (c 80; Mexico, Central America, tropical South America). – Oncidiinae Benth. in J. Linn. Soc. London, Bot. 18: 288. 21 Feb 1881 [’Oncidieae’]. Ada (17; Central America, the Andes in Colombia to Bolivia)?, Amparoa (1; A. beloglossa; southern Mexico, Central America; possibly in Rhynchostele)?, Aspasia (7; A. epidendroides, A. lunata, A. omissa, A. principissa, A. psittacina, A. silvana, A. variegata; southern Mexico, Central America, tropical South America), Brachtia (8; northwestern South America)?, Brassia (c 65; Florida, Mexico, Central America, the West Indies, tropical South America), Caluera (3; C. surinamensis, C. tavaresii, C. vulpina; northern South America), Capanemia (9; tropical South America), Caucaea (9; northwestern South America), Centroglossa (5; C. castellensis, C. greeniana, C. macroceras, C. nunes-limae, C. tripollinica; Brazil, Peru, Paraguay), Chytroglossa (3; C. aurata, C. marileoniae, C. paulensis; southeastern Brazil), Cischweinfia (11; Central America, northwestern tropical South America), Comparettia (c 80; Mexico, Central America, the West Indies, tropical South America), Cuitlauzina (7; C. candida, C. convallarioides, C. dubia, C. egertonii, C. pendula, C. pulchella, C. pygmaea; Mexico, Central America), Cypholoron (2; C. convexum, C. frigidum; Venezuela, Ecuador), Cyrtochiloides (3; C. ochmatochila, C. panduriformis, C. riopalenqueana; Central America, tropical South America), Cyrtochilum (c 140; Venezuela and Colombia to Peru), Dunstervillea (1; D. mirabilis; Venezuela, Ecuador, Brazil), Eloyella (10; Panamá, northern South America), Erycina (7; E. crista-galli, E. echinata, E. glossomystax, E. hyalinobulbon, E. pumilio, E. pusilla, E. zamorensis; Mexico, Central America, Trinidad, tropical South America), Fernandezia (c 50; southern Mexico, Central America, tropical South America), Gomesa (c 120; Central America, South America), Grandiphyllum (7; G. auricula, G. divaricatum, G. edwallii, G. hians, G. micranthum, G. pohlianum, G. schunkeanum; Brazil, Argentina), Hintonella (1; H. mexicana; Mexico), Hofmeisterella (2; H. biglobulosa, H. eumicroscopica; Ecuador), Ionopsis (6; I. burchellii, I. minutiflora, I. papillosa, I. satyrioides, I. utricularioides, I. zebrina; Florida, Mexico, Central America, the West Indies, tropical South America), Leochilus (12; Florida, Mexico, Central America, the West Indies, tropical South America), Lockhartia (c 30; Mexico, Central America, tropical South America), Macradenia (11, Florida, Central America, tropical South America), Macroclinium (42; Florida, southern Mexico, Central America, tropical South America), Mesospinidium (8; western tropical South America)?, Miltonia (12; central and southern Brazil to northern Argentina), Miltoniopsis (5; M. bismarckii, M. phalaenopsis, M. roezlii, M. vexillaria, M. warszewiczii; Central America, northwestern South America), Nohawilliamsia (1; N. pirarensis; Venezuela, Guyana, northern Brazil)?, Notylia (c 55; Central America, the West Indies, tropical South America), Notyliopsis (1; N. beatricis; Colombia), Oliveriana (6; O. brevilabia, O. ecuadorensis, O. egregia, O. lehmannii, O. ortizii, O. simulans; northern tropical South America), Oncidium (c 330; Florida, Mexico, Central America, the West Indies, tropical South America to Chile and Argentina), Ornithocephalus (c 55; southern Mexico, Central America, tropical South America), Otoglossum (13; Central America, northern South America), Phymatidium (10; Brazil, Paraguay, Argentina), Platyrhiza (1; P. quadricolor; Brazil), Plectrophora (10; Trinidad, northeastern South America), Polyotidium (1; P. huebneri; Colombia, Venezuela, Brazil), Psychopsiella (1; P. limminghei; tropical America; in Psychopsis?), Psychopsis (4; P. krameriana, P. papilio, P. sanderae, P. versteegiana; tropical America; incl. Psychopsiella?), Pterostemma (3; P. antioquiense, P. benzingii, P. escobarii; Colombia, Ecuador), Quekettia (5; Q. microscopica, Q. papillosa, Q. peruviana, Q. pygmaea, Q. vermeuleniana; Trinidad, northern tropical South America), Rauhiella (3; R. brasiliensis, R. seehaweri, R. silvana; Brazil), Rhynchostele (17; Mexico, Central America, Venezuela), Rodriguezia (c 50; southern Mexico, Central America, tropical South America), Rossioglossum (9; Mexico, Central America, tropical South America), Sanderella (2; S. discolor, S. riograndensis; Brazil, Bolivia, Argentina), Saundersia (2; S. mirabilis, S. paniculata; Brazil), Schunkea (1; S. vierlingii; Peru), Seegeriella (2; S. crothersii, S. pinifolia; Bolivia), Solenidium (3; S. lunatum, S. portillae, S. racemosum; northern South America), Suarezia (1; S. ecuadorana; Ecuador), Sutrina (2; S. bicolor, S. garayi; Peru, Bolivia), Systeloglossum (5; S. acuminatum, S. bennettii, S. costaricense, S. ecuadorense, S. panamense; Costa Rica, Panamá, Ecuador, Peru), Telipogon (c 205; Central America, tropical South America), Thysanoglossa (3; T. jordanensis, T. organensis, T.spiritu-sanctensis; southeastern Brazil), Tolumnia (27; Florida, Mexico, Central America, the West Indies, northern South America), Trichocentrum (c 70; Florida, Mexico to Argentina), Trichoceros (10; northern South America), Trichopilia (c 45; Mexico, Central America, the West Indies, tropical South America), Trizeuxis (1; T. falcata; southern Central America, Trinidad, tropical South America), Vitekorchis (4; V. aurifera, V. excavaga, V. lucasiana, V. vasquezii; Central America, tropical South America), Warmingia (4; W. buchtienii, W. eugenii, W. holopetala, W. zamorana; Costa Rica, Ecuador, Brazil, Paraguay, Argentina), Zelenkoa (1; Z. venusta; Panamá, Colombia to Peru), Zygostates (22; tropical South America). – Zygopetalinae Schltr. in Notizbl. Bot. Gart. Berlin-Dahlem 9: 577, 588. 22 Jul 1926 [’Zygopetaleae’]. Aetheorhyncha (1; A. andreettae; Ecuador), Aganisia (3; A. cyanea, A. fimbriata, A. pulchella; tropical South America), Batemannia (5; B. armillata, B. colleyi, B. leferenzii, B. lepida, B. wolteriana; tropical South America), Benzingia (9; Costa Rica to Peru), Chaubardia (3; C. heteroclita, C. klugii, C. surinamensis; tropical South America), Chaubardiella (8; Costa Rica, tropical South America), Cheiradenia (1; C. cuspidata; northeastern South America), Chondrorhyncha (7; C. hirtzii, C. inedita, C. macronyx, C. panguensis, C. rosea, C. suarezii, C. velastiguii; Colombia, Venezuela, Ecuador), Chondroscaphe (14; southern Central America, northwestern tropical South America), Cochleanthes (4; C. aromatica, C. flabelliformis, C. lueddemanniana, C. trinitatis; Mexico, Central America, the West Indies, tropical South America), Cryptarrhena (3; C. guatemalensis, C. kegelii, C. lunata; Central America, the West Indies, tropical South America), Daiotyla (4; D. albicans, D. crassa, D. maculata, D. xanthina; Costa Rica to Colombia), Dichaea (c 120; Mexico, Central America, the West Indies, tropical South America), Echinorhyncha (5; E. antonii, E. ecuadorensis, E. litensis, E. manzurii, E. vollesii; Colombia, Ecuador), Euryblema (2; E. anatonum, E. andreae; Panamá, Colombia), Galeottia (12; southern Mexico, Central America, tropical South America), Hoehneella (2; H. gehrtiana, H. heloisae; Brazil), Huntleya (14; Central America, tropical South America), Ixyophora (5; I. aurantiaca, I. carinata, I. fosterae, I. luerorum, I. viridisepala; tropical South America), Kefersteinia (c 70; Central America, tropical South America, with their highest diversity in the Andes of Colombia and Ecuador), Koellensteinia (17; Central America, Puerto Rico, Trinidad, tropical South America), Neogardneria (1; N. murrayana; Brazil), Otostylis (4; O. alba, O. brachystalix, O. lepida, O. paludosa; Trinidad, tropical South America), Pabstia (5; P. jugosa, P. modestior, P. placanthera, P. schunkiana, P. viridis; Brazil), Paradisanthus (4; P. bahiensis, P. micranthus, P. mosenii, P. neglectus; Brazil), Pescatoria (23; Costa Rica, Panama, tropical South America), Promenaea (18; Brazil), Stenia (22; Trinidad, northern South America), Stenotyla (9; southern Mexico, Central America), Vargasiella (2; V. peruviana: Peru; V. venezuelana: Venezuela), Warczewiczella (11; Central America, Cuba, tropical South America), Warrea (3; W. costaricensis, W hookeriana, W. warreana; southern Mexico, Central America, tropical South America), Warreella (2; W. cyanea, W. patula; Colombia), Warreopsis (4; W. colorata, W. pardina, W. parviflora, W. purpurea; southern Central America, northwestern South America), Zygopetalum (14; Peru, Bolivia, Brazil, northern Argentina), Zygosepalum (8; tropical South America). – Eriopsidinae Szlach. in Fragm. Florist. Geobot., Suppl. 3: 97. 1995.Eriopsis (5; E. amazonica, E. biloba, E. mesae, E. rutidobulbon, E. sceptrum; Central America, tropical South America). – Maxillariinae Benth. in J. Linn. Soc. London, Bot. 18: 288. 21 Feb 1881 [’Maxillarieae’].Anguloa (9; tropical South America), Bifrenaria (21; Panamá, Trinidad, tropical South America), Camaridium (c 140; Florida, Mexico, Central America, the West Indies, tropical South America southwards to Bolivia)?, Christensonella (14; Mexico, Central America, tropical South America)?, Cryptocentrum (22; Central America, tropical South America)?, Cyrtidiorchis (5; C. alata, C. frontinoensis, C. gerardi, C. rhomboglossa, C. stumpfiei; Colombia, Venezuela, Ecuador, Peru)?, Guanchezia (1; G. maguirei; Venezuela), Heterotaxis (15; Florida, Mexico, Central America, the West Indies, tropical South America)?, Horvatia (1; H. andicola; Peru), Hylaeorchis (1; H. petiolaris; northwestern and northernmost South America)?, Inti (2; I. bicallosa, I. chartacifolia; Central America, northern South America)?, Lycaste (32; Mexico, Central America, the West Indies, tropical South America), Mapinguari (4; M. auyantepuiensis, M. desvauxianus, M. foldatsianus, M. longipetiolatus; Central America, northern tropical South America)?, Maxillaria (c 660; Mexico, Central America, the West Indies, tropical South America), Maxillariella (c 45; Mexico, Central America, the West Indies, tropical South America)?, Mormolyca (c 25; Mexico, Central America, the West Indies, northern South America)?, Neomoorea (1; N. wallisii; Panamá to Ecuador), Nitidobulbon (3; N. cymbidioides, N. nasutum, N. proboscideum; Mexico, Central America, northwestern South America)?, Ornithidium (c 20; Mexico, Central America, the West Indies, tropical South America)?, Pityphyllum (7; P. amesianum, P. antioquiense, P. hirtzii, P. huancabambae, P. laricinum, P. pinoides, P. saragurense; northwestern South America)?, Rhetinantha (14; Mexico, Central America, tropical South America)?, Rudolfiella (6; R. aurantiaca, R. bicornaria, R. floribunda, R. lindmaniana, R. peruviana, R. picta; Panama, Trinidad, tropical South America), Sauvetrea (11; Central America, northern South America; in Maxillaria?), Scuticaria (11; tropical South America), Sudamerlycaste (c 40; the West Indies, tropical South America), Teuscheria (7; T. cornucopia, T. dodsonii, T. elegans, T. horichiana, T. integrilabia, T. pickiana, T. wageneri; southern Mexico, Central America, northern tropical South America), Trigonidium (c 25; southern Mexico, Central America, tropical South America)?, Xylobium (c 30; Mexico, Central America, tropical South America). – Coeliopsidinae Szlach. in Fragm. Florist. Geobot., Suppl. 3: 97. 1995. Coeliopsis (1; C. hyacinthosma; Central America), Lycomormium (5; L. ecuadorense, L. elatum, L. fiskei, L. schmidtii, L. squalidum; tropical South America), Peristeria (13; Costa Rica, Panamá, tropical South America). – Stanhopeinae Benth. in J. Linn. Soc. London, Bot. 18: 288. 21 Feb 1881 [’Stanhopieae’]. Acineta (17; Mexico, Central America, tropical South America), Archivea (1; A. kewensis; Brazil)?, Braemia (1; B. vittata; northern South America), Cirrhaea (7; C. dependens, C. fuscolutea, C. loddigesii, C. longiracemosa, C. nasuta, C. seidelii, C. silvana; Brazil), Coryanthes (c 60; Mexico, Central America, tropical South America), Embreea (2; E. herrenhusana, E. rodigasiana; Colombia, Ecuador), Gongora (c 75; Central America, tropical South America), Horichia (1; H. dressleri; Panamá), Houlletia (9; Mexico, Central America, tropical South America), Kegeliella (4; K. atropilosa, K. houtteana, K. kupperi, K. orientalis; Central America, tropical South America), Lacaena (2; L. bicolor, L. spectabilis; Central America), Lueckelia (1; L. breviloba; Brazil), Lueddemannia (3; L. dalessandroi, L. pescatorei, L. striata; western tropical South America), Paphinia (16; Costa Rica, Panama, tropical South America), Polycycnis (17; Central America, tropical South America), Schlimmia (7; S. alpina, S. condorana, S. garayana, S. jasminodora, S. jennyana, S. pandurata, S. stevensonii; northern Andes), Sievekingia (20; Central America, tropical South America), Soterosanthus (1; S. shepheardii; Colombia, Ecuador), Stanhopea (c 60; Central America, tropical South America), Trevoria (5; T. chloris, T. escobariana, T. glumacea, T. lehmannii, T. zahlbruckneriana; Colombia, Ecuador), Vasqueziella (1; V. boliviana; Bolivia). – Epidendreae Lindl., Collect. Bot.: 7, 16. Jan 1826. Agrostophyllinae Szlach. in Fragm. Florist. Geobot., Suppl. 3: 66. 1995.Agrostophyllum (c 100; the Seychelles to islands in western Pacific), Earina (7; E. aestivalis, E. autumnalis, E. deplanchei, E. floripecten, E. mucronata, E. sigmoidea, E. valida; New Caledonia, Vanuatu, Fiji, Samoa, New Zealand, Chatham Islands). – Calypsoinae Camus, Monogr. Orchid.: 376. 1908. Coelia (5; C. bella, C. densiflora, C. guatemalensis, C. macrostachya, C. triptera; southern Mexico, Central America, the West Indies), Dactylostalix (1; D. ringens; Japan), Ephippianthus (2; E. sachalinensis, E. sawadanus; the Korean Peninsula, Japan, Sakhalin), Changnienia (1; C. amoena; central and eastern China), Tipularia (7; T. cunninghamii, T. discolor, T. harae, T. japonica, T. josephi, T. odorata, T. szechuanica; Assam, the Himalayas, Burma, Tibet, China, the Korean Peninsula, Japan, Taiwan, eastern United States), Calypso (1; C. bulbosa; temperate regions on the Northern Hemisphere), Yoania (4; Y. amagiensis, Y. flava, Y. japonica, Y. prainii; northeastern India, the Himalayas, China, Vietnam, Japan, Taiwan), Govenia (c 25; Florida, Mexico, Central America, the West Indies, tropical South America), Aplectrum (1; A. hyemale; Japan, North America), Cremastra (5; C. aphylla, C. appendiculata, C. guizhouensis, C. malipoensis, C. unguiculata; the Himalayas, Tibet, China, the Korean Peninsula, Japan, Taiwan, the Russian Far East), Danxiaorchis (1; D. singchiana; Danxiashan in Guangdong), Oreorchis (16; western Himalayas to Japan and Taiwan), Corallorhiza (11; temperate regions on the Northern Hemisphere, with their highest diversity in North America).– Bletiinae Benth. in J. Linn. Soc. London, Bot. 18: 287. 21 Feb 1881 [’Bletieae’]. Basiphyllaea (7; B. carabiaiana, B. corallicola, B. hamiltoniana, B. hoffmannii, B. sarcophylla, B. volubilis, B. wrightii; Florida, the West Indies), Bletia (33; Florida, Mexico, Central America, the West Indies, tropical South America), Chysis (10; Mexico, Central America, tropical South America), Hexalectris (10; southern United States, Mexico).– Ponerinae Pfitzer, Entwurf. Anordn. Orch.: 101. Jan-Apr 1887 [’Ponereae’]. Helleriella (2; H. guerrerensis, H. nicaraguensis; Central America), Isochilus (13; Mexico, Central America, Cuba, tropical South America), Nemaconia (5; N. dressleriana, N. glomerata, N. graminifolia, N. pellita, N. striata; southern Mexico, Central America, tropical South America), Ponera (2; P. exilis, P. juncifolia; Mexico, Central America). – Laeliinae Benth. in J. Linn. Soc. London, Bot. 18: 287. 21 Feb 1881 [’Laelieae’]. Acrorchis (1; A. roseola; Central America), Adamantinia (1; A. miltonioides; Serra do Sincorá in Brazil), Alamania (1; A. punicea; Mexico), Amoana (2; southern central Mexico)?, Arpophyllum (3; A. giganteum, A. laxiflorum, A. spicatum; Mexico, Central America, Jamaica, Colombia, Venezuela), Artorima (1; A. erubescens; Mexico), Barkeria (17; Mexico, Central America), Brassavola (22; Mexico, Central America, the West Indies, tropical South America), Broughtonia (6; B. cubensis, B. domingensis, B. lindenii, B. negrilensis, B. ortgiesiana, B. sanguinea; the Greater Antilles, the Bahamas), Cattleya (c 115; Central America, tropical South America), Caularthron (4; C. amazonicum, C. bicornutum, C. bilamellatum, C. kaenzlinianum; Central America, tropical South America), Constantia (6; C. australis, C. cipoensis, C. cristinae, C. gutfreundiana, C. microscopica, C. rupestris; Brazil), Dimerandra (8; Mexico, Central America, Jamaica, tropical South America), Dinema (1; D. polybulbon; southern Mexico, Central America, Cuba, Jamaica), Domingoa (4; D. gemma, D. haematochila, D. nodosa, D. purpurea; Central America, Cuba, Hispaniola, Venezuela), Encyclia (c 165; Florida, Mexico, Central America, the West Indies, tropical South America), Epidendrum (1.410–1.415; southeastern United States, Mexico, Central America, the West Indies, tropical South America; incl. Prosthechea?), Guarianthe (4; G. aurantiaca, G. bowringiana, G. patinii, G. skinneri; southern Mexico, Central America, Trinidad, Colombia, Venezuela), Hagsatera (2; H. brachycolumna, H. rosilloi; Mexico), Homalopetalum (8; Mexico, Central America, the Greater Antilles, tropical South America), Isabelia (3; I. pulchella, I. violacea, I. virginalis; Brazil), Jacquiniella (12; Mexico, Central America, the West Indies, tropical South America), Laelia (23; 25; Mexico, Central America, tropical South America; incl. Schomburgkia?), Schomburgkia (22–24; tropical South America; in Laelia?), Leptotes (9; tropical South America), Loefgrenianthus (1; L. blanche-amesii; Brazil), Meiracyllium (2; M. gemma, M. trinasutum; southern Mexico, Central America), Microepidendrum (1; M. subulatifolium; Mexico), Myrmecophila (10; southern Mexico, Central America, the West Indies, Venezuela), Nidema (2; N. bothii, N. ottonis; Mexico, Central America, the West Indies, tropical South America), Oestlundia (4; O. cyanocolumna, O. distantiflora, O. ligulata, O. luteorosea; Mexico, Central America, Colombia, Venezuela), Orleanesia (9; tropical South America), Prosthechea (c 120; Florida, Mexico, Central America, the West Indies, tropical South America; in Epidendrum?), Pseudolaelia (19; Brazil), Psychilis (14; the West Indies), Pygmaeorchis (2; P. brasiliensis, P. seidelii; Brazil), Quisqueya (4; Q. ekmanii, Q. holdridgei, Q. karstii, Q. rosea; the West Indies), Rhyncholaelia (2; R. digbyana, R. glauca; Mexico, Central America), Scaphyglottis (c 70; Mexico, Central America, the West Indies, tropical South America), Tetramicra (13; the West Indies). – Pleurothallidinae Lindl. ex G. Don in R. Sweet, Hort. Brit., ed. 3: 636. late 1839 [’Pleurothalleae’]. Acianthera (c 120; Mexico, Central America, the West Indies, South America to Chile), Anathallis (c 150; Mexico, Central America, the West Indies, tropical and subtropical South America), Andinia (13; tropical South America), Barbosella (19; Mexico, Central America, the West Indies, tropical South America), Brachionidium (c 75; Central America, the West Indies, tropical South America), Chamelophyton (1; C. kegelii; Venezuela, Guyana), Dilomilis (5; D. bissei, D. elata, D. montana, D. oligophylla, D. scirpoidea; Cuba, Hispaniola, Jamaica, Puerto Rico), Diodonopsis (5; D. anachaeta, D. erinacea, D. hoeijeri, D. pterygiophora, D. pygmaea; mountain areas in tropical America; in Masdevallia?), Draconanthes (2; D. aberrans, D. bufonis; the Andes, Ecuador), Dracula (125–130; Mexico, Central America, Colombia, Ecuador, Peru), Dresslerella (13; Central America, northwestern South America), Dryadella (c 55; southern Mexico, Central America, tropical South America), Echinosepala (11; Central America, tropical South America), Frondaria (1; F. caulescens; central Colombia to central Bolivia), Jostia (1; J. teaguei; Colombia, Ecuador, Peru)?, Kraenzlinella (9; southern Mexico, Central America, tropical South America), Lepanthes (c 1.085; Mexico, Central America, the West Indies, tropical South America), Lepanthopsis (c 45; Florida, southern Mexico, Central America, the West Indies, tropical South America), Masdevallia (c 590; Mexico, Central America, the West Indies and tropical South America, especially in mountain areas), Myoxanthus (c 50; Central America, tropical South America), Neocogniauxia (2; N. hexaptera, N. monophylla; the West Indies), Octomeria (c 160; Mexico, Central America, the West Indies, tropical South America), Pabstiella (c 30; southern Mexico, Central America, tropical South America), Phloeophila (11; Mexico, Central America, tropical South America), Platystele (c 100; Mexico, Central America, the West Indies, tropical South America), Pleurothallis (c 550; Mexico, Central America, the West Indies, tropical South America), Pleurothallopsis (18; Central America, tropical South America), Porroglossum (43; the Andes), Restrepia (53; southern Mexico, Central America, the Andes of northwestern South America), Restrepiella (3; R. guatemalensis, R. lueri, R. ophiocephala; Central America), Scaphosepalum (c 45; southern Mexico, Central America, tropical South America), Specklinia (c 135; Mexico, Central America, the West Indies, tropical South America), Stelis (c 880; Florida, Mexico, Central America, the West Indies, tropical South America), Teagueia (13; the Andes), Tomzanonia (1; T. filicina; Massif de la Hotte in Haiti), Trichosalpinx (c 110; Mexico, Central America, the West Indies, tropical South America), Trisetella (23; Central America, tropical South America), Zootrophion (23; Central America, Jamaica, northern tropical South America). – Collabieae Pfitzer, Entwurf. Anordn. Orch.: 100. Jan-Apr 1887 [’Collabiinae’]. Acanthephippium (13; India and Southeast Asia to China, Japan, Taiwan, Malesia to New Guinea and islands in southwestern Pacific to Tonga), Ancistrochilus (2; A. rothschildianus, A. thomsonianus; tropical Africa), Ania (11; India, southern China, Southeast Asia, Malesia to New Guinea, the Solomon Islands, Queensland), Calanthe (c 215; tropical and subtropical regions in Madagascar, India and Southeast Asia to China, the Korean Peninsula, Japan, Malesia to New Guinea, Australia and Tahiti, one species in tropical and southeastern Africa, one species in Central America and the West Indies), Cephalantheropsis (4; C. halconensis, C. laciniata, C. longipes, C. obcordata; eastern Himalayas, Tibet, southern China, Japan, the Ryukyu Islands, Southeast Asia, West Malesia, Taiwan; in Calanthe?), Chrysoglossum (4; C. assamicum, C. ensigerum, C. ornatum, C. reticulatum; the Himalayas, Tibet, Taiwan, Southeast Asia, Malesia to New Guinea and east to Fiji and Samoa), Collabium (14; the Himalayas, southern China, Southeast Asia, Malesia to New Guinea and Melanesia), Devogelia (1; D. intonsa; the Moluccas, New Guinea)?, Diglyphosa (3; D. celebica, D. elmeri, D. latifolia; eastern Himalayas, Southeast Asia, Malesia to New Guinea), Eriodes (1; E. barbata; northeastern India to Vietnam), Gastrorchis (8; Madagascar; in Calanthe?), Hancockia (1; H. uniflora; Yunnan, Vietnam, Japan, the Ryukyu Islands, Taiwan), Ipsea (3; I. malabarica, I. speciosa, I. thailandica; India, Sri Lanka, Thailand), Nephelaphyllum (11; the Himalayas, southern China, Southeast Asia, West Malesia to Borneo and the Philippines), Pachystoma (2; P. nutans, P. pubescens; the Himalayas, southern China, Southeast Asia, the Andaman and Nicobar Islands, Taiwan and Malesia to New Guinea, New Caledonia and tropical Australia), Paraphaius (3; P. columnaris, P. flavus, P. takeoi; subtropical regions in East and Southeast Asia), Phaius (c 45; tropical Africa, tropical Asia to southern China and eastern Queensland and northeastern New South Wales; in Calanthe?), Pilophyllum (1; P. villosum; Peninsular Thailand, Malesia to New Guinea and Solomon Islands), Plocoglottis (41; Southeast Asia, Malesia to New Guinea, the Bismarck Archipelago and Solomon Islands), Risleya (1; R. atropurpurea; the Himalayas, western China), Spathoglottis (c 50; India, Southeast Asia, Malesia to New Guinea, Solomon Islands, northeastern Queensland, New Caledonia, Samoa, Niue), Tainia (23; India and Sri Lanka to China and Japan, Southeast Asia, Malesia to New Guinea and northeastern Queensland). – Podochileae Pfitzer, Entwurf. Anordn. Orch.: 101. Jan-Apr 1887 [’Podochilinae’]. Appendicula (c 130; India, southern China, Southeast Asia, Malesia to New Guinea, New Caledonia to Tonga), Ascidieria (8; southern Thailand, West Malesia to the Philippines), Bryobium (8; Sri Lanka, southern China, Southeast Asia, Malesia to New Guinea, eastern Queensland, Melanesia), Callostylis (5; C. bambusifolia, C. carnosissima, C. cyrtosepala, C. pulchella, C. rigida; Assam, southern China, Southeast Asia, Java), Campanulorchis (5; C. globifera, C. leiophylla, C. pellipes, C. pseudoleiophylla, C. thao; Hainan, Southeast Asia, Malesia to New Guinea), Ceratostylis (c 150; India, southern China, Southeast Asia, Malesia to New Guinea and Melanesia), Conchidium (10; India, southern China, Indochina, the Malay Peninsula), Cryptochilus (5; C. ctenostachyus, C. luteus, C. petelotii, C. roseus, C. sanguineus; Assam, the Himalayas, Burma, Yunnan, southeastern China, Vietnam), Dilochiopsis (1; D. scortechinii; the Malay Peninsula), Epiblastus (22; East Malesia and eastwards to Polynesia), 'Eria' (>500; India, the Himalayas, southern China, Southeast Asia, Malesia to New Guinea, Melanesia and islands in western Pacific; polyphyletic), Mediocalcar (17; Sulawesi to Samoa, with their largest diversity on New Guinea), Mycaranthes (36; the Himalayas, southern China, Southeast Asia, Malesia to New Guinea), Notheria (1; N. diaphana; Sulawesi), Octarrhena (c 50; Sri Lanka, Malesia to New Caledonia and Polynesia), Oxystophyllum (36; southern China, Southeast Asia, Malesia to New Guinea, the Solomon Islands), Phreatia (c 210; northeastern India, Southeast Asia, Malesia to New Guinea and tropical Australia, Taiwan, islands in the Pacific), Pinalia (105; the Himalayas, northeastern India to Burma, southern China, Thailand, Indochina, Malesia to New Guinea, Queensland, islands in the Pacific), Poaephyllum (7; P. fimbriatum, P. grandiflorum, P. pauciflorum, P. podochiloides, P. selebicum, P. tenuipes, P. trilobum; Southeast Asia, Malesia), Podochilus (60–65; tropical Asia to southwestern China and islands in western Pacific), Porpax (14; India, Yunnan, Southeast Asia, West Malesia), Pseuderia (c 20; East Malesia, New Guinea to Fiji, Samoa and Micronesia), Ridleyella (1; R. paniculata; New Guinea), Sarcostoma (5; S. borneense, S. brevipes, S. celebicum, S. javanicum, S. subulatum; the Malay Peninsula, Java, Sulawesi), Stolzia (15; tropical Africa), Thelasis (27; the Himalayas, southern China, Southeast Asia, the Andaman and Nicobar Islands, Malesia to New Guinea, Solomon Islands, Queensland, Melanesia, Micronesia), Trichotosia (c 50; the Himalayas, southern China, Southeast Asia, Malesia to New Guinea, Solomon Islands and Vanuatu). – Vandeae Lindl., Orchid. Select.: 7, 14. Jan 1826. Polystachyinae Ridl., Mat. Flora Malay Penins. 1: 10. 1907 [’Polystachyae’]. Hederorkis (2; H. scandens: the Mascarene Islands; H. seychellensis: the Seychelles), Imerinaea (1; I. madagascarica; Madagascar; in Polystachya?), Polystachya (c 235; tropical to southern Africa, Madagascar, tropical Asia to New Guinea and tropical Australia, Mexico, Central America, the West Indies, tropical South America). – Adrorhizinae Schltr. in Notizbl. Bot. Gart. Berlin-Dahlem 9: 573, 586. 22 Jul 1926 [’Adrorhizeae’]. Adrorhizon (1; A. purpurascens; Sri Lanka), Bromheadia (c 30; Sri Lanka and Burma to eastern Queensland), Sirhookera (2; S. lanceolata, S. latifolia; southern India, Sri Lanka). – Aeridinae Pfitzer, Entwurf. Anordn. Orch.: 108. Jan-Apr 1887 [’Aerideae’]. Abdominea (1; A. minimiflora; peninsular Thailand, the Malay Peninsula, Java, the Philippines; in Tuberolabium?)?, Acampe (8; tropical and southern Africa, tropical Asia to southern China and the Philippines, the Andaman Islands), Adenoncos (17; Thailand, Vietnam, Malesia to New Guinea), Aerides (c 20; East Asia, tropical Asia to New Guinea), Amesiella (1; A. philippinensis; the Philippines), Arachnis (13; Southeast Asia , Malesia to the Solomon Islands), Armodorum (4; A. calcarata, A. senapatianum, A. siamense, A. sulingi; northeastern India, Burma, Southeast Asia, West Malesia), Ascochilopsis (2; A. lobata, A. myosurus; Thailand to Borneo), Ascochilus (6; A. emarginatus, A. fasciculatus, A. leytensis, A. mindanaensis, A. nitidus, A. siamensis; Thailand to Borneo and the Philippines), Biermannia (12; eastern Himalayas, southern China, Southeast Asia, Sumatra, Bali), Bogoria (4; B. merrillii, B. papuana, B. raciborskii, B. taeniorhiza; Java, New Guinea), Brachypeza (7; B. archytas, B. indusiata, B. koeteiensis, B. laotica, B. minimipes, B. stenoglottis, B. zamboangensis; Malesia to New Guinea, Christmas Island), Calymmanthera (5; C. filiformis, C. major, C. montana, C. paniculata, C. tenuis; New Guinea), Ceratocentron (1; C. fesselii; the Philippines), Chamaeanthus (2; C. brachystachys, C. wenzelii; Thailand, Indochina, Malesia to Borneo), Chiloschista (c 20; India, southern China, Southeast Asia, Malesia to New Guinea, northern Australia, Fiji, Micronesia), Chroniochilus (5; C. ecalcaratus, C. minimus, C. sinicus, C. thrixspermoides, C. virescens; Thailand to Borneo), Cleisocentron (6; C. abasii, C. gokusingii, C. kinabaluense, C. klossii, C. merrillianum, C. pallens; eastern Himalayas to Vietnam and Borneo), Cleisomeria (2; C. lanatum, C. pilosulum; Southeast Asia, Borneo), Cleisostoma (c 90; India and the Himalayas, southern China, Southeast Asia, Malesia to New Guinea, New Caledonia and Fiji), Cleisostomopsis (2; C. eberhardtii, C. elytrigera; southern China, Indochina), Cordiglottis (7; C. breviscapa, C. filiformis, C. fulgens, C. major, C. multicolor, C. pulverulenta, C. westenenkii; southern Thailand, West Malesia), Cottonia (1; C. macrostachya; southern India, Sri Lanka), Cryptopylos (1; C. clausus; Southeast Asia, Sumatra), Deceptor (1; D. bidoupensis; Vietnam), Dimorphorchis (5; D. graciliscapa, D. lowii, D. rohaniana, D. rossii, D. tenomensis; Borneo), Diplocentrum (2; D. congestum, D. recurvum; India), Diploprora (2; D. championii, D. truncata; India, Sri Lanka, the Himalayas, Burma, southern China, Southeast Asia, Taiwan), Dryadorchis (5; D. barbellata, D. dasystele, D. huliorum, D. minor, D. singularis; New Guinea), Drymoanthus (4; D. adversus, D. flavus, D. minimus, D. minutus; northeastern Queensland, New Zealand), Dyakia (1; D. hendersoniana; Borneo), Eclecticus (1; E. chungii; Thailand), Esmeralda (3; E. bella, E. cathcartii, E. clarkei; the Himalayas, Assam, Tibet, southern China, northern Burma, northern Thailand, Hainan, Vietnam), Gastrochilus (c 55; the Himalayas and China to Japan and Taiwan), Grosourdya (11; Southeast Asia, West Malesia), Gunnarella (9; New Caledonia), Holcoglossum (14; Southeast Asia to southern Japan and Taiwan; in Vanda?), Hygrochilus (2; H. parishii, H. tsii; Assam, Bhutan, Yunnan, Burma, Southeast Asia; in Phalaenopsis?), Hymenorchis (12; the Philippines to New Guinea, tropical Australia and New Caledonia), Jejewoodia (6; J. crockerensis, J. jiewhoei, J. jongirii, J. linusii, J. longicalcarata, J. rimauensis; Borneo), Luisia (c 40; India and China to Japan, Southeast Asia, Malesia to New Guinea, Melanesia and Micronesia), Macropodanthus (8; Malesia), Malleola (c 30; southern China, Southeast Asia, Malesia to New Guinea and Melanesia), Megalotus (1; M. bifidus; the Philippines; in Saccolabium?), Micropera (21; the Himalayas, Burma, Hainan, Southeast Asia, Malesia to New Guinea, Queensland, Melanesia, Micronesia), Microsaccus (12; Burma, Southeast Asia, West Malesia), Microtatorchis (50–55; Southeast Asia, Taiwan, Malesia to New Guinea, tropical Australia, islands in western Pacific), Mobilabium (1; M. hamatum; northeastern Queensland), Omoea (2; O. micrantha: Java; O. philippinensis: the Philippines), Ophioglossella (1; O. chrysostoma; New Guinea), Ornithochilus (4; O. cacharensis, O. difformis, O. moretonii, O. yingjiangensis; the Himalayas, Burma, southern China, Southeast Asia, West Malesia; in Phalaenopsis?), Papilionanthe (11; India, Sri Lanka, the Himalayas, Burma, southern China, Southeast Asia, Malesia to Timor, Taiwan), Papillilabium (1; P. beckleri; southeastern Queensland, northeastern New South Wales), Paraphalaenopsis (4; P. denevei, P. labukensis, P. laycockii, P. serpentilingua; western Borneo), Pelatantheria (8; the Himalayas, Burma, China, the Korean Peninsula, Japan, Taiwan, Southeast Asia, the Andaman Islands, West Malesia), Pennilabium (15; eastern Himalayas, southern China, Southeast Asia, West Malesia), Peristeranthus (1; P. hillii; eastern Queensland, northeastern New South Wales), Phalaenopsis (c 70; the Himalayas, southern China, Southeast Asia, Malesia to New Guinea, the Bismarck Archipelago, Queensland, Taiwan), Phragmorchis (1; P. teretifolia; the Philippines), Plectorrhiza (3; P. brevilabris, P. erecta, P. tridentata; eastern Queensland, eastern New South Wales, estern Victoria, Lord Howe), Pomatocalpa (c 25; India, Sri Lanka, eastern Himalayas, southern China, Southeast Asia, the Andaman and Nicobar Islands, Malesia to New Guinea, Solomon Island, Queensland, Vanuatu, Taiwan), Porrorhachis (2; P. galbina, P. macrosepala; Malesia), Pteroceras (27; India, Yunnan, Southeast Asia, the Andaman and Nicobar Islands, West and Central Malesia), Renanthera (c 25; the Himalayas, southern China, Southeast Asia, Malesia to New Guinea and Solomon Islands), Rhinerrhiza (1; R. divitiflora; New Guinea, eastern Queensland, northeastern New South Wales, the Solomon Islands), Rhinerrhizopsis (3; R. matutina, R. moorei, R. ramuana; New Guinea, Solomon Islands, northern Queensland), Rhynchogyna (3; R. fallax, R. luisifolia, R. saccata; Thailand, Vietnam, the Malay Peninsula), Rhynchostylis (4; R. coelestis, R. gigantea, R. retusa, R. rieferi; India, Sri Lanka, eastern Himalayas, Burma, southern China, Southeast Asia, the Andaman and Nicobar Islands, West Malesia), Robiquetia (c 45; India, Sri Lanka, Burma, southern China, Southeast Asia, the Andaman and Nicobar Islands, Malesia to New Guinea, Queensland, Melanesia to Samoa and Tonga), Saccolabiopsis (14; India, eastern Himalayas, Burma, southern China, Southeast Asia, the Andaman Islands, Malesia to New Guinea, eastern Queensland and Fiji, Taiwan), Saccolabium (5; S. congestum, S. longicaule, S. pusillum, S. rantii, S. sigmoideum; India, Sumatra, Java), Sedirea (2; S. japonica, S. subparishii; China, the Korean Peninsula, Japan; in Aerides?), Santotomasia (1; S. wardiana; Luzon in the Philippines), Sarcanthopsis (5; S. hansemannii, S. nagarensis, S. quaifei, S. warocqueana, S. woodfordii; New Guinea, the Bismarck Archipelago, Melanesia), Sarcochilus (25; estern Queensland to eastern Victoria, Tasmania, New Caledonia), Sarcoglyphis (12; eastern Himalayas, Burma, Yunnan, Southeast Asia, West Malesia), Sarcophyton (3; S. crassifolium, S. pachyphyllum, S. taiwanianum; the Philippines, Taiwan), Schistotylus (1; S. purpuratus; eastern Queensland, eastern New South Wales), Schoenorchis (c 25; India, Sri Lanka, the Himalayas, Tibet, Burma, southern China, Southeast Asia, Malesia to New Guinea, Queensland, Melanesia to Samoa), Seidenfadenia (1; S. mitrata; Burma, Thailand), Seidenfadeniella (2; S. filiformis, S. rosea; India, Sri Lanka), Singchia (1; S. malipoensis; southeastern Yunnan), Smithsonia (3; S. maculata, S. straminea, S. viridiflora; India), Smitinandia (3; S. helferi, S. micrantha, S. selebensis; Yunnan, Southeast Asia, the Andaman Islands, Borneo, Sulawesi), Spongiola (1; S. lohokii; Borneo), Stereochilus (7; S. brevirachis, S. dalatensis, S. erinaceus, S. hirtus, S. laxus, S. pachyphyllus, S. ringens; eastern Himalayas, Yunnan, Burma, Thailand, Vietnam, the Malay Peninsula, the Philippines), Taeniophyllum (185–200; tropical Africa, islands in the Indian Ocean, India, China, Japan, Southeast Asia, Malesia, Australia, islands in the Pacific east to Tahiti), Thrixspermum (c 160; India to Southeast Asia, Malesia to New Guinea, tropical Australia and islands in western Pacific, Taiwan), Trachoma (14; Indochina, Malesia to New Guinea, Melanesia, northeastern Queensland and islands in southwestern Pacific), Trichoglottis (c 70; Taiwan, tropical Asia and east to Polynesia), Tuberolabium (11; Assam, Burma, Southeast Asia, Malesia to the Moluccas, Queensland, Taiwan, Cook Islands, the Mariana Islands), Uncifera (6; U. acuminata, U. dalatensis, U. lancifolia, U. obtusifolia, U. thailandica, U. verrucosa; Assam, the Himalayas, Burma, southern China, Southeast Asia, West Malesia), Vanda (c 75; India, Sri Lanka, the Himalayas, China, the Korean Peninsula, Japan, Taiwan, Burma, Southeast Asia, Melanesia to New Guinea, northern Queensland), Vandopsis (4; V. gigantea, V. lissochiloides, V. shanica, V. undulata; southern China, Southeast Asia, the Philippines, New Guinea). – Angraecinae Summerh. in Kew Bull. 20: 188. 9 Sep 1966. Aeranthes (43; Zimbabwe, Madagascar, the Mascarene Islands), Jumellea (c 60; eastern tropical Africa, Madagascar, the Mascarene Islands, with their highest diversity in Madagascar), ’Angraecum’ (c 200; tropical and southern Africa, Madagascar, the Mascarene Islands, Sri Lanka; polyphyletic), Solenangis (8; tropical and southeastern Africa, Madagascar, the Mascarene Islands), Calyptrochilum (2; C. christyanum, C. emarginatum; tropical Africa, Brazil), Ancistrorhynchus (17; tropical Africa), Bolusiella (4; B. iridifolia, B. maudiae, B. talbotii, B. zenkeri; tropical and southern Africa), Microcoelia (c 30; tropical and southern Africa, Madagascar), Angraecoides (3–5; A. chermezoni, A. clavigerum, A. elliotii, A. moandense, A. sedifolium; Madagascar, the Mascarene Islands), ’Angraecoides’ pro parte (c 10?; tropical continental Africa), Campylocentrum (c 65; Florida, Mexico, Central America, the West Indies, tropical South America), Dendrophylax (14; Florida, the West Indies), Afropectinariella (5; A. atlantica, A. doratophylla, A. gabonensis, A. pungens, A. subulata; tropical West and Central Africa, São Tomé), Pectinariella (7; P. dasycarpa, P. edmundi, P. hermannii, P. humblotiana, P. pectinata, P. pterophylla, P. scroticalcar; Madagascar, the Comoros, the Mascarene Islands), Dolabrifolia (5; D. aporoides, D. bancoensis, D. disticha, D. podochiloides; tropical West and Central Africa), Rhipidoglossum (40–45; tropical and subtropical Africa to the Cape Provinces), Mystacidium (c 10?; southern and southeastern Africa to Tanzania), Angraecopsis (10–13; tropical Africa, Madagascar, the Mascarene Islands), Sphyrarhynchus (6?; S. amaniensis, S. brevilobus, S. longicaudatus, S. muscicola, S. schliebenii, S. trilobatus; tropical and southern Africa), Diaphananthe (c 30; tropical West and Central Africa), Aerangis (c 60; tropical and southern Africa, Madagascar, the Mascarene Islands, Sri Lanka), Eurychone (2; E. galeandrae, E. rothschildiana; tropical Africa), Nephrangis (2; N. bertauxiana, N. filiformis; tropical Africa), Cyrtorchis (18; tropical and southern Africa), Podangis (1; P. dactyloceras; tropical Africa), Ypsilopus (5; Y. erectus, Y. leedalii, Y. liae, Y. longifolius, Y. viridiflorus; tropical East to southern Africa), Tridactyle (c 50; tropical and southern Africa), Listrostachys (1; L. pertusa; tropical West and Central Africa), Plectrelminthus (1; P. caudatus; tropical West Africa); unplaced: Ambrella (1; A. longituba; Madagascar), Beclardia (2; B. grandiflora, B. macrostachya; the Mascarene Islands), Erasanthe (1; E. henrici; Madagascar), Lemurella (4; L. culicifera, L. pallidiflora, L. papillosa, L. virescens; Madagascar), Lemurorchis (1; L. madagascariensis; Madagascar), Neobathiea (6; N. comet-halei, N. grandidierana, N. hirtula, N. keraudrenae, N. perrieri, N. spatulata; Madagascar), Oeonia (5; O. brauniana, O. curvata, O. madagascariensis, O. rosea, O. volucris; Madagascar, the Mascarene Islands), Oeoniella (2; O. aphrodite, O. polystachys; Madagascar, the Comoros, the Mascarene Islands, the Seychelles), Sobennikoffia (4; S. fournieriana, S. humbertiana, S. poissoniana, S. robusta; the Mascarene Islands); Cardiochilos (1; C. williamsonii; southern Tanzania, Malawi), Chauliodon (1; C. deflexicalcaratum; tropical West Africa), Cryptopus (4; C. brachiatus, C. dissectus, C. elatus, C. paniculatus; Madagascar, the Mascarene Islands), Dinklageella (4; D. liberica, D. minor, D. scandens, D. villiersii; tropical West and Central Africa), Distylodon (1; D. comptum; Cameroon, Uganda), ’Mystacidium’ tanganyikense (tropical East Africa), Summerhayesia (2; S. laurentii, S. zambesiaca; tropical Africa), Taeniorrhiza (1; T. gabonensis; Gabon), Triceratorhynchus (1; T. viridiflorus; tropical East Africa). – Mainly tropical epiphytes; few species in temperate regions. Sometimes mycoheterotrophic holoparasites without leaves. CAM physiology often present. Roots sometimes with pneumathodes. Velamen usually present. Stegmata with silica bodies sometimes absent. Tilosomes often present. Leaves usually distichous, with conduplicate(-plicate) ptyxis, usually articulated, simetimes caducous or absent. Stomata often paracytic or tetracytic. Median outer stamen fertile and lateral inner stamens staminodial. Anther incumbent (by elongation of columella or by very early bending of anther; sometimes strongly convex), operculate and with ‘beak’. Pollinia usually clavate and hard (sometimes sectile); pollinium stipe developed from apical part of median stigmatic lobe. Pollen grains often inaperturate. Cotyledon usually very little developed. n = 5 and more. Plastid gene matK pseudogene. – Palmorchis may be sister to Neottieae or these two successive sister-groups to the remaining Epidendroideae. The position of Devogelia is unknown due to lack of fresh material.
 |
Cladogram (simplified) of Orchidaceae based on DNA sequence data (Cameron 2004). |
 |
Cladogram of Apostasioideae, Cypripedioideae, and Vanilloideae based on DNA sequence data (Cameron 2004, 2009). |
 |
Cladogram of Orchidoideae, based on DNA sequence data (Cameron 2004). |
 |
Cladogram of a part of Epidendroideae, based on DNA sequence data (Cameron 2004). |
 |
Cladogram of a part of Epidendroideae, based on DNA sequence data (Cameron 2004). |
RUSCACEAE M. Roem. |
( Back to Iridales ) |
Convallariaceae Horan., Prim. Lin. Syst. Nat.: 53. 2 Nov 1834 [’Convallariaceae s. Smilaceae’]; Ophiopogonaceae (Endl.) Meisn., Plant. Vasc. Gen., Tab. Diagn. 397, Comm. 308. 12-14 Feb 1842 [’Ophiopogoneae’]; Aspidistraceae (Endl.) Hassk., Cat. Hort. Bot. Bogor.: 32. Oct 1844 [’Aspidistreae’]; Eriospermaceae (Endl.) Lem. in V. V. D. d’Orbigny, Dict. Univ. Hist. Nat. 5: 402. 29 Mar 1845 [’Eriospermeae’]; Tupistraceae Schnizl., Iconogr. Fam. Regni Veg. 1: 72. Jan-Apr 1846 [’Tupistreae’]; Dracaenaceae Salisb., Gen. Plant.: 73. 15-31 Mai 1866 [’Dracaeneae’], nom. cons.; Peliosanthaceae Salisb., Gen. Plant.: 63. 15-31 Mai 1866 [’Peliosantheae’]; Platymetraceae Salisb., Gen. Plant.: 9. 15-31 Mai 1866 [‘Platymetreae’], nom. illeg.; Polygonataceae Salisb., Gen. Plant.: 63. 15-31 Mai 1866 [’Polygonateae’]; Sansevieriaceae Nakai in J. Jap. Bot. 12: 779. 1936; Nolinaceae Nakai, Chosakuronbun Mokuroku [Ord. Fam. Trib. Nov.]: 226. 20 Jul 1943
Genera/species 24/740–765
Distribution Temperate to tropical regions on the Northern Hemisphere south to West Malesia and Central America, with their largest diversity in East and Southeast Asia, North America, Mexico and South Africa.
Fossils Maianthemophyllum was described from Paleocene layers in Alberta, and Soleredera from the mid-Eocene of British Columbia.
Habit Usually bisexual (sometimes monoecious, polygamomonoecious, dioecious, or polygamodioecious), usually perennial herbs (sometimes suffrutices; rarely epiphytes; in Dracaena, Beaucarnea and Nolina frutescent or arborescent). Danae, Ruscus and Semele have green assimilating branches with ends modified into phyllocladia. Often xerophytic. Many species of Eriospermum and Comospermum have a hypocotyledonary tuber. Some species have lignotuber.
Vegetative anatomy Roots fibrous or fleshy (in some species of Ophiopogon swollen and carnose), often tuberous (tuber in Comospermum and Eriospermum developed from hypocotyl); velamen often present (sometimes multilayered). Danae, Ruscus and Semele have their phyllocladia and prophyll lateral or entirely fused with axillary shoot, together forming a compound phyllocladium. Phellogen? Secondary lateral growth usually absent (Dracaena, Beaucarnea, Nolana etc. with anomalous secondary lateral growth). Vessels present in roots and, in some species, in stem (at least rhizome) or leaves. Vessel elements in roots with scalariform or simple perforation plates (in stem only scalariform or absent); lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays? Axial parenchyma? Vascular bundles sometimes amphivasal. Sieve tube plastids P2c type, with cuneate protein crystals. Nodes? Tanniniferous cells present in some genera. Dracaena secreting a blackish-red substance, hard and vitreous when oxidized and dry. Silica bodies absent. Mucilage cells with calciumoxalate raphides and styloids. Suberized cells with pseudo-raphides present in some genera.
Trichomes Hairs usually absent; leaf hairs in Eriospermum simple or compound; exotestal hairs in Eriospermum (and Comospermum?) unicellular, long, finally air-filled.
Leaves Usually alternate (spiral or distichous; rarely verticillate; in Polygonatum oppositifolium opposite), simple, entire, sometimes linear (in Danae, Ruscus and Semele reduced, scale-like, not photosynthesizing), often caducous (sometimes differentiated into pseudopetiole and pseudolamina), with conduplicate, supervolute or flat to curved ptyxis. Stipules absent (Peliosanthes sometimes with stipule-like outgrowths above leaf sheath); leaf sheath usually more or less well developed (sometimes absent). Leaf base in Eriospermum with filiform, bottle brush or feather duster like (sometimes bushy) appendages. Venation parallelodromous, sometimes acrodromous; midvein sometimes prominent; primary and secondary veins parallel. Stomata usually anomocytic (rarely paracytic or tetracytic), in Beaucarnea, Dasylirion and Nolina with oily guard cells. Cuticular wax crystalloids as parallel rodlets or platelets (Convallaria type) or non-orientated. Mesophyll often with mucilaginous idioblasts or cavities containing calciumoxalate raphides and/or single prismatic crystals. Leaf margin usually entire (in Dasylirion serrate-dentate).
Inflorescence Terminal or axillary, panicle, spike-, raceme- or head-like, or raceme or spike (flowers rarely solitary). In Semele and Ruscus one group (rarely two groups) of flowers on margin (umbel-like inflorescence) and on surface (raceme-like inflorescence) of a phyllocladium. Abaxial side of floral bracts sometimes with small nectaries.
Flowers Usually actinomorphic (sometimes slightly zygomorphic), usually small. Pedicel articulated, with pericladium. Hypogyny or half epigyny. Tepals (2–)3(–5)+(2–)3(–5), petaloid, caducous or marcescent, free or connate into a campanulate, urceolate or tubular perianth (in Aspidistra up to 13-lobate). Septal nectaries (infralocular?) usually present (sometimes absent; Convallaria without septal nectaries but with osmophores as tepaline oil glands; Tupistra with gynoecial nectaries). Extrafloral nectaries usually present at pedicel bases in Dracaena. Disc absent.
Androecium Stamens (2–)3(–6)+(2–)3(–6), usually as many (rarely twice as many) as tepals (outer staminal whorl sometimes staminodial or absent?). Filaments filiform to wide and flat, free or connate into a tube, free from or adnate to tepals or corona. Anthers basifixed or dorsifixed, in Dracaena and Eriospermum versatile, tetrasporangiate, usually introrse (sometimes latrorse or extrorse), longicidal (dehiscing by longitudinal slits). Tapetum secretory, usually with binucleate cells. Female flowers often with staminodia (bisexual flowers and male flowers sometimes with three staminodia).
Pollen grains Microsporogenesis successive. Pollen grains monosulcate, monosulcoidate or inaperturate, shed as monads, usually bicellular (sometimes tricellular) at dispersal. Exine tectate or semitectate, with columellate infratectum, perforate, reticulate, or foveolate.
Gynoecium Pistil composed of (two or) three (to five) connate carpels. Ovary superior to semi-inferior, (unilocular to) trilocular (to quinquelocular). Style single, simple, filiform to stout, often with a stylar canal, or absent. Stigma capitate or trilobate (in ‘Aspidistra’ peltately widened), papillate or non-papillate, usually Wet type. Male flowers often with pistillodium.
Ovules Placentation usually axile (sometimes intrusively axile; sometimes parietal, basal, or apical). Ovules usually one to six (sometimes numerous) per carpel, anatropous, hemianatropous?, campylotropous or orthotropous, apotropous or epitropous, bitegmic, usually crassinucellar (sometimes tenuinucellar). Micropyle usually bistomal (in Danae, Ruscus, Semele, and Eriospermum endostomal). Outer integument two to eight cell layers thick. Inner integument two cell layers thick. Funicular obturator or ovary wall obturator often present (e.g. in Eriospermum). Parietal cell often formed from archesporial cell (absent in, e.g., Nolana, Beaucarnea and Dasylirion), and sometimes further dividing by periclinal divisions. Parietal tissue one to three (or four) cell layers thick. Nucellar cap usually absent (in, e.g., Dracaena, formed by periclinal cell divisions in megasporangial epidermis; no parietal cell formed). Megagametophyte usually monosporous, Polygonum type, or disporous, 8-nucleate, Allium type (in Maianthemum tetrasporous, 16-nucleate, Scilla or Drusa type). Chalazal end of megagametophyte in Ruscus contracted around antipodal cells and surrounded by a hypostase, a sclerotized lignified sheath. Synergids sometimes with a filiform apparatus. Antipodal cells usually not persistent (sometimes proliferating to five cells). Megagametophyte haustorium present in Dasylirion. Endosperm development usually helobial (sometimes nuclear). Endosperm haustoria? Embryogenesis in Eriospermum solanad, with megasporangial tissue enclosing chalazal parts of large embryo.
Fruit Usually a one- or several-seeded berry (in Tricalistra a drupe; in Comospermum, Eriospermum and Gonioscypha usually a loculicidal capsule; in Nolina, Beaucarnea, Calibanus and Dasylirion a samara; fruit in Liriope, Ophiopogon and Peliosanthes usually an irregularly dehiscing capsule).
Seeds Aril absent. Testa thin, degenerating (Liriope, Ophiopogon and Peliosanthes usually with sarcotesta; testa absent in Dracaena; testa in Eriospermum with long hairs). Exotesta without phytomelan layer, often with phlobaphene. Tegmen persistent, with thick cell walls, or collapsed. Perisperm usually not developed (in Eriospermum and possibly Comospermum thin, with oils and aleurone). Endosperm usually copious, with oils, aleurone and hemicellulose (starch absent); endosperm cell walls with pits or pores (endosperm absent from ripe seeds in Eriospermum). Embryo small to large (in Comospermum with coma), straight or curved, usually well differentiated, without chlorophyll. Cotyledon one, not photosynthesizing. Cotyledon hyperphyll compact, not assimilating. Hypocotyl internode short. Mesocotyl absent. Coleoptile present in Liriope, Ophiopogon and Peliosanthes. Radicula well developed, simple or branched, ephemeral or persistent. Germination? Seedling without ligule.
Cytology n = 5–7, 9, 10, 18–21 – Polyploidy occurring. Chromosomes 0.5–19 µm long. Karyotype bimodal (six large and 14 small chromosomes) in Danae, Ruscus, Semele and certain other species of Eriospermum. Adventitious polyembryony present (in, e.g., Maianthemum stellatum).
DNA The mitochondrial gene rpl2 is absent.
Phytochemistry Flavonols (e.g. 3-O-methyl-8-C-methylquercetin in, e.g., Nolina and Dasylirion, kaempferol in, e.g., Danae and Dracaena), cardenolide glycosides (Convallaria, Polygonatum, Speirantha), spirostanol glycosides (in Peliosanthes), steroidal saponins and sapogenins (e.g. diosgenin in Nolina, and aspidirin, a diosgenin-3-O-β-lycotetraoside, in Aspidistra), chelidonic acid, and azetidin-2-carboxylic acid (a non-protein amino acid) present. Fructans, polyacetate derived arthroquinones and chrysophanol present in roots of Ruscus. Ellagic acid, proanthocyanidins, alkaloids, and cyanogenic compounds not found.
Use Ornamental plants, medicinal plants, alcohol (sotol), textiles, basketry etc. (Nolina, Beaucarnea, Dasylirion, etc.).
Systematics Ruscaceae are sister-group to Asparagaceae.
Eriospermeae are sister to the remaining Ruscaceae.
Eriospermeae Endl. ex Meisn., Plant. Vasc. Gen.: Tab. Diagn. 397, 400, Comm. 304. 17-20 Aug 1842
1/100–105. Eriospermum (100–105; tropical and southern Africa, with their highest diversity in Western Cape). – Tuber of Eriospermum develop from hypocotyls. Leaves sometimes with various branched, peltate or stellate-haired etc. enations on adaxial surface. Pedicels non-articulated. Fruit a loculicidal capsule. Seeds often exposed early during development. Testa hairy. Embryo massive. Perisperm present. Endosperm absent. Embryo massive. Cotyledon unifacial, photosynthesizing. n = 20.
Rusceae Dumort., Anal. Fam. Plant.: 60. 1829 [‘Ruscineae’]
23/640–660. Disporopsis (7; D. aspersa, D. fuscopicta, D. jinfushanensis, D. longifolia, D. luzoniensis, D. pernyi, D. undulata; southern China, Southeast Asia, the Philippines), Polygonatum (66; temperate regions on the Northern Hemisphere, with their highest diversity in southwestern China), Heteropolygonatum (6; H. ginfushanicum, H. ogisui, H. pendulum, H. roseolum, H. urceolatum, H. xui; southern China, Vietnam), Maianthemum (c 40; temperate regions on the Northern Hemisphere south to the Himalayas and Central America); Theropogon (1; T. pallidus; the Himalayas, Tibet, Yunnan), Comospermum (1; C. yedonense; Japan), Chrysodracon (6; C. aurea, C. auwahiensis, C. fernaldii, C. forbesii, C. halapepe, C. hawaiiensis; the Hawaiian Islands), Dracaena (190–200; the Canary Islands, warm-temperate to tropical regions in the Old World, one species, D. americana, in Central America, one species, D. marginata, on Cuba), Danae (1; D. racemosa; southern Turkey, northwestern Syria, the Caspian area), Semele (3; S. androgyna: the Canary Islands, Madeira; S. gayae: Gran Canaria; S. menezesii: Madeira), Ruscus (7; R. aculeatus, R. colchicus, R. hypoglossum, R. hypophyllum, R. hyrcanus, R. microglossus, R. streptophyllus; western Europe, Macaronesia, the Mediterranean to the Caucasus and Iran); Peliosanthes (23; eastern Himalayas, southern China, Southeast Asia, Taiwan, West Malesia), Liriope (6; L. graminifolia, L. kansuensis, L. longipedicellata, L. minor, L. muscari, L. spicata; China, the Korean Peninsula, Japan, the Ryukyu Islands, Vietnam, the Philippines), Ophiopogon (65–70; the Himalayas and Tibet to the Korean Peninsula, Japan and Southeast Asia, the Philippines); Dasylirion (22; southwestern United States, Mexico), Nolina (28; southern United States, Mexico, Central America), Beaucarnea (10; Mexico, Central America); Speirantha (1; S. gardenii; eastern China), Convallaria (1–3; C. majalis: Europe, the Caucasus; C. keiskei: eastern China and the Korean Peninsula to the Russian Far East, Sakhalin and Japan; C. montana: southeastern United States), ’Aspidistra’ (100–105; India to China, the Korean Peninsula, Japan, Taiwan and Southeast Asia; paraphyletic), Reineckea (1; R. carnea; China, Japan), Rohdea (17; northern India, southern Himalayas, China, Japan, Indochina, Taiwan), Tupistra (c 20; northern India, southern Himalayas, southwestern and southern China, Southeast Asia, West Malesia, Ambon). – Temperate to tropical regions on the Northern Hemisphere southwards to West Malesia and Central America. – The clade [Peliosanthes+[Liriope+Ophiopogon]] has irregularly dehiscing capsule, sarcotesta and coleoptiles. Homoisoflavanones are present in Ophiopogon. Comospermum has two ovules per carpel, apotropous, tenuinucellate and the embryo has a coma (hair tuft).
 |
Bayesian inference tree of Ruscaceae based on DNA sequence data (Kim & al. 2010). |
TECOPHILAEACEAE Leyb. |
( Back to Iridales ) |
Androsynaceae Salisb., Gen. Plant.: 61. 15-31 Mai 1866 [‘Androsyneae’]; Cyanellaceae Salisb., Gen. Plant.: 46. 15-31 Mai 1866 [‘Cyanelleae’]; Conantheraceae (Endl. ex Meisn.) Endl. in L. K. G. Pfeiffer, Nomencl. Bot. 1: 839. 21 Feb 1873 [’Conanthereae’]; Cyanastraceae Engl. in Bot. Jahrb. Syst. 28: 357. 22 Mai 1900; Tecophilaeales Traub ex Reveal in Phytologia 74: 176. 25 Mar 1993; Walleriaceae (R. Dahlgren) H. Huber ex Takht. in Bot. Žurn. 79(12): 65. 7-28 Feb 1995, nom. cons.
Genera/species 8/26
Distribution Tropical and southern Africa, Madagascar, California, Chile.
Fossils Unknown.
Habit Bisexual, perennial herbs. Rhizome developed into a tuber or a corm (with basal innovation).
Vegetative anatomy Roots fibrous. Tuberous rhizome in most tunicate genera consisting of fibrous to membranous scale-like leaves, leaf sheaths, or vascular and fibre bundles (absent from Cyanastrum, Kabuyea and Walleria). Vascular bundles in one row, with or without sclerenchyma. Phellogen absent. Secondary lateral growth absent. Vessels present in roots. Vessel elements with scalariform perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial parenchyma? Sieve tube plastids P2c, with cuneate protein crystals. Nodes? Calciumoxalate raphides usually present (absent in Cyanastrum and Kabuyea). Anthocyanin cells present in Cyanastrum and Kabuyea.
Trichomes Hairs usually absent (in Cyanella unicellular or short and multicellular, multiseriate; in Walleria scabrate).
Leaves Alternate (usually spiral, sometimes distichous), simple, entire, often linear (in Cyanastrum and Kabuyea differentiated into pseudopetiole and pseudolamina), with ? ptyxis. Stipules absent; leaf sheath open? (sometimes absent). Venation parallelodromous (in Cyanastrum and Kabuyea pinnate-parallel or palmate-parallel), with distinct midvein. Stomata usually anomocytic (in, e.g., Cyanastrum indistinctly paracytic), adjacent cells with parallel divisions. Cuticular wax crystalloids absent (Cyanella). Mesophyll usually with cells containing calciumoxalate raphides. Mesophyll in Cyanastrum and Kabuyea with schizogenous secretory cavities (oil canals). Leaf margin entire.
Inflorescence Usually terminal, panicle or thyrsoid, often with raceme-like branches (flowers in Tecophilaea one or few, in Walleria solitary axillary, in Cyanastrum and Kabuyea in a simple raceme or pairwise). Some species of Cyanella have either monomorphic or dimorphic enantiostyly.
Flowers Actinomorphic or zygomorphic. Usually half epigyny (in Walleria almost hypogyny). Tepals 3+3, with imbricate aestivation, petaloid, free or connate at base into a tube; outer median tepal abaxial; outer tepals pointed. Coronae (enations), consisting of six small interstaminal appendages, present in Odontospermum and Zephyra. Septal nectaries usually situated over entire ovary (absent from or reduced to short septal slits in ovary apex in Cyanella, Tecophilaea and Walleria). Disc absent.
Androecium Stamens usually 3+3, all stamens fertile, or two (Zephyra) or three stamens (usually upper ones) staminodial; stamens in Cyanella usually dimorphic (heteranthery), with three small posterior and three large anterior stamens, or with five small posterior staminodial stamens and one large anterior fertile stamen (rarely all six stamens identical). Filaments subulate, usually free (rarely connate at base), adnate to tepals (epitepalous). Anthers usually free (in Conanthera connate), basifixed or subbasifixed (in Tecophilaea and Zephyra with basal caudate appendage), non-versatile, tetrasporangiate, poricidal (dehiscing by an apical or subapical pore), or introrse and dehiscing by short slits; connective sometimes apically prolonged. Tapetum secretory, with usually uni- or binucleate (in Cyanastrum multinucleate) cells. Staminodia sometimes two or three (rarely five). Enantiostyly occurring in some species of Cyanella.
Pollen grains Microsporogenesis simultaneous. Pollen grains monosulcate (sometimes trichotomosulcate), heteropolar, usually operculate (operculum absent in Cyanastrum and Kabuyea), shed as monads, bicellular at dispersal. Exine tectate, with columellate infratectum, usually foveolate, rugulate, granulate, in Kabuyea psilate or perforate.
Gynoecium Pistil composed of three, usually connate (in Cyanastrum free), antesepalous carpels; median carpel abaxial. Ovary usually semi-inferior (in Walleria almost superior), trilocular. Style single, simple, subulate, straight or curved (in Cyanastrum, Kabuyea and Walleria sunken and thus lateral on each carpel, gynobasic), with stylar canal. Stigma punctate or more or less trilobate, papillate, type? Pistillodium absent.
Ovules Placentation axile (in Cyanastrum and Kabuyea basal-axile?). Ovules usually four to c. 50 (in Cyanastrum, Kabuyea and Odontostomum two) per carpel, anatropous or campylotropous, bitegmic, crassinucellar. Micropyle endostomal or bistomal. Outer integument several cell layers thick. Inner integument two? cell layers thick. Funicular obturator usually present. Parietal cell formed from archesporial cell. Chalazal end of megasporangium usually well developed. Megagametophyte monosporous, Polygonum type. Endosperm development in Odontostomum and Cyanastrum probably helobial, in Cyanella nuclear. Endosperm haustorium in Cyanella chalazal. Embryogenesis?
Fruit A loculicidal capsule (in Walleria somewhat baccate; in Cyanastrum and Kabuyea? a schizocarp with three mericarps?).
Seeds Aril absent. Seed coat testal. Testa multilayered, thick-walled. Exotesta usually with phytomelan layer on epidermal cell walls (absent in Cyanastrum, Kabuyea? and Walleria), sometimes palisade, with thick cell walls. Endotesta? Tegmen? Perisperm not developed. Endosperm usually copious, thick-walled, with or without pits on surface, starchy? Endosperm absent in Cyanastrum, replaced by a swollen zone, chalazosperm, filled with starch grains, formed by cell divisions in chalazal tissue and consisting of loosely packed thin-walled cells (a similar starchy chalazal tissue present in Odontostomum). Embryo long or short, well differentiated, chlorophyll? Cotyledon one, not photosynthesizing. Cotyledon hyperphyll in Cyanella and Walleria with apex as an haustorium, a short tubular leaf sheath and a coleoptile. Hypocotyl absent in Cyanastrum. Radicula usually long (absent in Cyanastrum). Germination? Polyembryony present in Cyanella.
Cytology n = 8, 10–12, 14, 24 – Polyploidy and aneuploidy occurring. Chromosomes 2–4 µm long. Adventitious embryony present at least in Cyanella.
DNA
Phytochemistry Chelidonic acid present in Conanthera, and tuliposides in Tecophilaea. Steroidal saponins present in Conanthera? Flavonols, ellagic acid, proanthocyanidins, and cyanogenic compounds not found.
Use Ornamental plants.
Systematics Tecophilaea (2; T. cyanocrocus: central Chile; T. violiflora: Peru, central Chile); Cyanastrum (3; C. cordifolium, C. goetzeanum, C. johnstonii; Central and tropical East Africa), Kabuyea (1; K. hostifolia; Kenya, Tanzania); Odontostomum (1; O. hartwegii; California), Cyanella (7–8; Namibia, South Africa, with their highest diversity in Western Cape), Walleria (3; W. gracilis, W. mackenziei, W. nutans; tropical and southern Africa, Madagascar), Zephyra (2; Z. compacta, Z. elegans; northern and central Chile), Conanthera (5; C. bifolia, C. campanulata, C. parvula, C. trimaculata, C. urceolata; Chile).
Tecophilaeaceae are sister to Ixiolirion (Ixioliriaceae).
Tecophilaea in Chile is sister to the remaining Tecophilaeaceae and the African clade [Cyanastrum+Kabuyea] is successive sister-group to the rest. Odontostomum in California is sister to the African clade [Cyanella+Walleria] (Jesson & Barrett 2003).
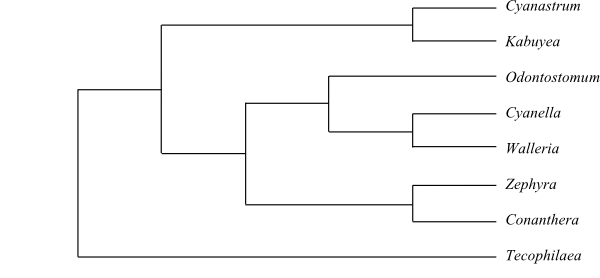 |
Cladogram of Tecophilaeaceae based on DNA sequence data (Jesson & Barrett 2003). |
THEMIDACEAE Salisb. |
( Back to Iridales ) |
Genera/species 12/65–70
Distribution Western North America to British Columbia, Mexico and Guatemala, with their largest diversity in southwestern United States and Mexico.
Fossils Unknown.
Habit Bisexual, perennial herbs. Corm fibrous, rich in starch, and with basal innovation. Bulb absent.
Vegetative anatomy Young corm surrounded by dry leaf sheaths, cataphylls, of previous year, forming a membranous or fibrous tunica. Phellogen absent. Secondary lateral growth absent. Vessels present in roots. Vessel elements usually with scalariform or simple? perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial parenchyma? Sieve tube plastids P2c type, with cuneate protein crystals. Nodes? Laticifers present at least in ‘Brodiaea’ and ‘Milla’. Mucilage cells? Calciumoxalate raphides present at least in Brodiaea.
Trichomes Hairs absent?
Leaves Alternate, simple, entire, linear, usually bifacial (in ‘Brodiaea’ unifacial), often fleshy, flattened, triangular or rounded in cross-section, with ? ptyxis. Stipules absent; leaf sheath closed. Venation parallelodromous. Stomata anomocytic. Cuticular wax crystalloids? Mesophyll with or without cells containing calciumoxalate. Leaf margin entire.
Inflorescence Terminal, umbel-like, consisting of condensed helicoid monochasia (flowers rarely solitary), subtended by four or more, dry, membranous, early caducous bracts (spathae), not enclosing floral buds. Peduncle (scape) often long.
Flowers Actinomorphic. Pedicel often articulated. Hypogyny. Tepals 3+3, petaloid, usually connate in lower part into a tube (rarely free). Appendages, scales or corona often present between tepals and stamens (extrastaminal). Septal nectaries? Disc absent.
Androecium Stamens usually 3+3 (sometimes three fertile stamens, or three fertile stamens and three staminodia). Filaments usually subulate (sometimes flattened), often with basal, lateral, dorsal or apical appendages, free or connate (sometimes tubular), usually adnate to tepal (epitepalous); basal filament appendages in some species forming a nectary. Anthers basifixed or dorsifixed, usually versatile (not in ‘Brodiaea’ and ‘Dichelostemma’), tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory. Staminodia three or absent.
Pollen grains Microsporogenesis successive. Pollen grains monosulcate, shed as monads, bicellular at dispersal. Exine semitectate, with columellate? infratectum, reticulate.
Gynoecium Pistil composed of three connate carpels. Ovary superior, trilocular, often stipitate (with gynophore), in Bessera, ‘Milla’ and Petronymphe adnate to tepals by antesepalous flanges. Style single, simple, with stylar canal. Stigma capitate or trilobate (in ‘Brodiaea’ more or less squarrose), usually Dry (in Bloomeria Wet) type. Pistillodium absent.
Ovules Placentation axile. Ovules two or more per carpel, usually anatropous (rarely campylotropous), bitegmic, crassinucellar. Micropyle ?-stomal. Outer integument three or four cell layers thick. Inner integument usually two or three (sometimes more than three, in Dichelostemma five to seven) cell layers thick; inner integument and megasporangial base sometimes massive. Parietal tissue three or four cell layers thick. Megasporangial epidermal cells enlarged. Nucellar cap usually absent (sometimes present). Megagametophyte monosporous, Polygonum type (Brodiaea, Muilla). Endosperm development ab initio nuclear (‘Brodiaea’) or helobial (‘Muilla’, Triteleia). Endosperm haustoria? Embryogenesis?
Fruit A loculicidal capsule.
Seeds Aril absent. Seed coat exotestal. Exotesta with thick phytomelan layer on epidermal cell walls. Mesotesta and endotesta compressed or collapsed. Tegmic cells usually strongly enlarged (not so in, e.g., Triteleia). Perisperm not developed. Endosperm copious, with oils and aleurone (starch absent). Embryo short, straight, without chlorophyll? Cotyledon one, not photosynthesizing. Cotyledon hyperphyll? Hypocotyl internode? Coleoptile absent. Radicula persistent. Germination?
Cytology n = 5–12, 14–16, 18, 21, 24, 25, 27, 36, 42 – Polyploidy and aneuploidy frequently occurring.
DNA The mitochondrial gene rpl2 is absent (lost).
Phytochemistry Insufficiently known. Saponins present. Chlorogenic acid present at least in Brodiaea. Alkaloids and onion oils not found.
Use Ornamental plants.
Systematics ‘Milla’ (c 10; one species, M. biflora, in southwestern United States to Guatemala and Honduras; non-monophyletic), Bessera (3; B. elegans: central and southern Mexico; B. tenuiflora; southern Baja California; B. tuitensis: southwestern Mexico), Petronymphe (1; P. decora; southern Mexico), Jaimehintonia (1; J. gypsophila; northeastern Mexico), Dandya (4; D. balsensis, D. hannibalii, D. purpusii, D. thadhowardii; Mexico); Androstephium (2; A. breviflorum: western and southwestern United States, northern Mexico; A. coeruleum: Kansas, Oklahoma, Texas), ‘Muilla’ (3; M. coronata, M. maritima, M. transmontana; southwestern United States, northern Baja California; non-monophyletic), Bloomeria (3; B. clevelandii, B. crocea, B. humilis; southern California, Baja California), Triteleia (15–20; southwestern Canada, western United States); Triteleiopsis (1; T. palmeri; Arizona, Baja California), ‘Brodiaea’ (17; southwestern Canada, western United States, northern Baja California; non-monophyletic; in Dichelostemma?), ‘Dichelostemma’ (5; D. capitatum, D. congestum, D. ida-maia, D. multiflorum, D. volubile; southwestern Canada, western United States, northwestern Mexico; non-monophyletic; incl. Brodiaea?).
Themidaceae are sister to Hyacinthaceae.
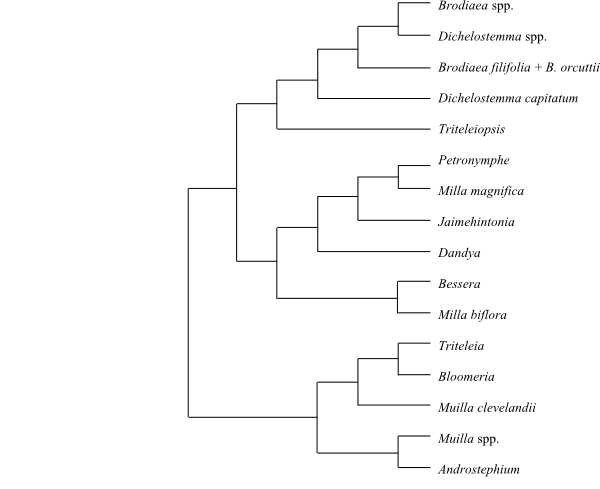 |
Cladogram (simplified) of Themidaceae based on DNA sequence data (Pires & Sytsma 2002). |
XANTHORRHOEACEAE Dumort. |
( Back to Iridales ) |
Xanthorrhoeales Takht. ex Reveal et Doweld in Novon 9: 552. 30 Dec 1999
Genera/species 1/c 30
Distribution Australia, Tasmania.
Fossils Unknown.
Habit Bisexual, evergreen, arborescent with aerial stem, or fruticose with subterranean stem. Stem thick, often tuberous, with leaves concentrated at apex.
Vegetative anatomy Roots contractile. Root cortex consisting of an ephemeral fleshy outer zone and a thickened inner zone; endodermal cells with U-shaped wall thickenings. Phellogen? Primary vascular stem tissue as scattered vascular bundles. Secondary lateral growth anomalous. Secondary thickening meristem dracaenoid. Vessels present at least in roots and leaves (sometimes in stem). Root vessel elements with simple perforation plates; lateral pits?; foliar and stem vessel elements with scalariform perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays? Axial parenchyma? Sieve tube plastids P2c type, with cuneate protein crystals. Nodes? Secretory ducts frequent, with yellow, brown or red acaroid resins containing essential oils. Silica bodies absent. Calciumoxalate raphides absent.
Trichomes Hairs?
Leaves Alternate (spiral), simple, entire, linear, unifacial, with ? ptyxis. Stipules absent; leaf sheath in young stems open (absent in older stems). Leaf bases thick and resiniferous, persistent as a dry coat around stem. Venation parallelodromous. Stomata paracytic, present in furrows. Cuticular wax crystalloids non-orientated and irregular. Mesophyll with cells containing calciumoxalate as single prismatic crystals. Secretory cavities and ducts with resin. Sclerenchymatic layers present inside epidermis. Leaf centre consisting of parenchyma with vascular bundles, some of them inverted. Epidermal cells with calciumoxalate as styloid-like crystals. Leaf margin entire.
Inflorescence Terminal, dense spike-like thyrse with alternate reduced cymes as partial inflorescences. Peduncle lignified. Each flower subtended by several bracts.
Flowers Actinomorphic. Pedicel not articulated. (Secondary) hypogyny. Tepals 3+3, sepaloid to bracteoid, persistent, free; outer tepals hard, dry; inner tepals membranous, almost petaloid. Septal nectaries well developed, infralocular. Disc absent.
Androecium Stamens 3+3. Filaments flattened, free from each other and from tepals. Anthers dorsifixed, versatile?, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory? Staminodia absent.
Pollen grains Microsporogenesis successive. Pollen grains monosulcate with widened sulcus, shed as monads, bicellular at dispersal. Exine semitectate, with columellate infratectum, reticulate.
Gynoecium Pistil composed of three connate carpels. Ovary (secondarily) superior, trilocular. Style single, simple, subulate, narrowing at apex, with stylar canal. Stigma capitate, punctate or trilobate, probably Wet type. Pistillodium absent.
Ovules Placentation axile. Ovules three to eight per carpel, anatropous, ascending or horizontal, bitegmic, crassinucellar. Micropyle endostomal. Outer integument approx. three cell layers thick. Inner integument two cell layers thick. Hypostase present. Megasporangium acute at apex. Megasporangial epidermal cells and parietal cells often dividing periclinally forming tissue up to six cell layers thick. Megagametophyte monosporous, Polygonum type. Endosperm development helobial? Endosperm haustoria? Embryogenesis?
Fruit A lignified to cartilaginous loculicidal capsule.
Seeds Seeds flattened. Aril absent. Seed coat exotestal. Exotesta with phytomelan layer on epidermal cell walls. Endotesta? Tegmen collapsed, with inner cuticle of endotegmen present. Perisperm not developed. Endosperm copious, with oils and aleurone (starch absent), with relatively thick-walled cells containing small amounts of hemicellulose. Embryo straight to more or less curved, usually transversely oriented relative to seed axis, chlorophyll? Cotyledon one, not photosynthesizing. Cotyledon hyperphyll elongate. Hypocotyl internode short. Coleoptile absent. Germination phanerocotylar. First leaves distichous.
Cytology n = 11 – Karyotype bimodal.
DNA The mitochondrial gene rpl2 is absent (lost).
Phytochemistry Flavonols (kaempferol, quercetin), flavans (e.g. 4’,5,7-trimethoxyflavan), chalcones, anthraquinones, polyacetate derived arthroquinones, and acaroid resins present (with, e.g., 2-hydroxy-4-methoxy acetophenone, 2-hydroxy-4,6-dimethoxy acetophenone, p-oxybenzoic aldehyde, xanthorrhein, citronellol, paeonol, cinnamoyl alcohol derivatives, p-coumaric acid and chrysophanic acid; in yellow resin also polymerized derivatives of cinnamic acids). Chelidonic acid? Ellagic acid, proanthocyanidins and cyanogenic compounds not found. Ferulic acid absent from cell walls.
Use Wooden vessels, varnish (‘Grass Tree Gum’ and ‘Red Acaroid Gum’).
Systematics Xanthorrhoea (c 30; Australia, Tasmania).
Xanthorrhoea is sister-group to Hemerocallidaceae or, alternatively, to Asphodelaceae.
XERONEMATACEAE M. W. Chase, Rudall et M. F. Fay |
( Back to Iridales ) |
Genera/species 1/2
Distribution New Caledonia, northernmost New Zealand.
Fossils Leaf fossils provisionally assigned to Xeronema are known from the Oligocene of New Zealand.
Habit Bisexual, perennial, rhizomatous herbs.
Vegetative anatomy Phellogen absent. Secondary lateral growth absent. Vessels present in roots? Vessel elements with scalariform? perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial parenchyma? Sieve tube plastids P2c type, with cuneate protein crystals. Nodes? Idioblasts with calciumoxalate raphides. Styloids absent.
Trichomes Hairs absent.
Leaves Alternate (distichous), simple, entire, linear, equitant, unifacial, isobilaterally flattened, with ? ptyxis. Stipules absent; leaf sheath well developed. Venation parallelodromous. Stomata anomocytic? Cuticular wax crystalloids? Leaf margin entire.
Inflorescence Terminal, spike-like, unilateral.
Flowers Actinomorphic, directed upwards (secund). Hypogyny. Tepals 3+3, petaloid, free. Septal nectaries well developed, branched. Disc absent?
Androecium Stamens 3+3. Filaments thick, subulate, free from each other and from tepals, long and strongly exserted. Anthers dorsifixed to subbasifixed, non-versatile, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory. Staminodia absent.
Pollen grains Microsporogenesis simultaneous? Pollen grains monosulcate, boat-shaped, shed as monads, bicellular at dispersal. Exine semitectate, with columellate infratectum, finely to coarsely reticulate.
Gynoecium Pistil composed of three connate carpels. Ovary superior, stipitate, trilocular. Style single, simple, solid. Stigma capitate, type? Pistillodium absent.
Ovules Placentation axile. Ovules several per carpel, anatropous, bitegmic, crassinucellar. Micropyle endostomal? Outer integument ? cell layers thick. Inner integument ? cell layers thick. Parietal cell? Parietal tissue? Nucellar cap formed? Megagametophyte monosporous, Polygonum type. Antipodal cells? Endosperm development helobial. Endosperm haustoria? Embryogenesis?
Fruit A loculicidal capsule.
Seeds Aril absent. Seeds echinate or papillate. Seed coat testal. Exotesta with phytomelan layer on epidermal cell walls. Endotesta? Tegmen collapsed. Perisperm not developed. Endosperm copious, without starch. Embryo straight?, chlorophyll? Cotyledon one. Cotyledon hyperphyll? Hypocotyl internode? Coleoptile absent. Germination?
Cytology n = 17, 18
DNA The mitochondrial gene rpl2 is absent (lost).
Phytochemistry Unknown.
Use Ornamental plants (X. callistemon).
Systematics Xeronema (2; X. moorei: New Caledonia; X. callistemon: Taranga and Poor Knights Island off northeastern New Zealand).
Xeronema is sister to the remaining Iridales (Hemerocallidaceae to Ruscaceae).
Literature
Abe K. 1971. Contributions to the embryology of the family Orchidaceae IV. Development of the embryo sac in Bletilla striata. – Sci. Rep. Tôhoku Imp. Univ., ser. IV (Biol.) 35: 213-218.
Abe K. 1972a. Contributions to the embryology of the family Orchidaceae VI. Development of the embryo sac in 15 species of orchids. – Sci. Rep. Tôhoku Imp. Univ., ser. IV (Biol.) 36: 135-178.
Abe K. 1972b. Contributions to the embryology of the family Orchidaceae VII. A comparative study of the orchid embryo sac. – Sci. Rep. Tôhoku Imp. Univ., ser. IV (Biol.) 36: 179-201.
Abele D, Rudolph B, Thiede J, Schirarend C. 2005. Phylogeny of the genus Masdevallia Ruiz & Pav. based on morphological and molecular data. – In: Raynal-Roques A, Roguenant A, Prat D (eds), Proceedings of the 18th World Orchid Conference, Naturalia Publ., Turriers, France, pp. 111-116.
Aceto S, Caputo P, Cozzolino S, Gaudio L, Moretti A. 1999. Phylogeny and evolution or Orchis and allied genera based on ITS DNA variation: morphological gaps and molecular continuity. – Mol. Phylogen. Evol. 13: 67-76.
Ackermann JD. 1983. On the evidence for a primitively epiphytic habit in orchids. – Syst. Bot. 8: 474-477.
Ackermann JD. 2014. Orchid flora of the Greater Antilles. – Mem. New York Bot. Gard. 109.
Ackermann JD, Williams NH. 1980. Pollenmorphology of the tribe Neottieae and its impact on the classification of the Orchidaceae. – Grana 19: 7-18.
Ackermann JD, Williams NH. 1981. Pollen morphology of the Chloraeinae (Orchidaceae: Diurideae) and related subtribes. – Amer. J. Bot. 68: 1392-1402.
Adams PB. 2011. Systematics of Dendrobiinae (Orchidaceae) with special reference to Australian taxa. – Bot. J. Linn. Soc. 166: 105-126.
Adams PB, Burke JM, Lawson SD. 2006a. Systematic analysis of Dendrobium Swartz section Dendrocoryne in the Australian region. – Plant Syst. Evol. 260: 65-80.
Adams PB, Burke JM, Lawson SD. 2006b. Dendrobium speciosum (Dendrocoryne: Orchidaceae) complex in north Queensland. – Aust. Syst. Bot. 19: 259-271.
Adams PB, Burke JM, Lawson SD. 2006c. A review of the taxonomy and relationships of the Dendrobium speciosum complex (Orchidaceae), and recognition of two new taxa. – Telopea 11: 195-232.
Adams SP, Leitch IJ, Bennett MD, Chase MW, Leitch AR. 2000. Ribosomal DNA evolution and phylogeny in Aloe (Asphodelaceae). – Amer. J. Bot. 84: 1578-1583.
Adamson RS. 1926. On the phylogeny of some shrubby Iridaceae. – Trans. Roy. Soc. South Afr. 13: 373-378.
Adamson RS. 1942. Some Peninsula species of Urginea. – J. South Afr. Bot. 8: 237-242.
Aden P. 1988. The Hosta book. – Timber Press, Portland, Oregon.
Agababian MV, Oganesian ME. 2000. Allium sect. Acanthoprason (Alliaceae) in southern Transcaucasia: a survey, with the description of two new species. – Willdenowia 30: 93-104.
Agapova ND. 1974. Comparative karyological characteristics of three species of the genus Ornithogalum L. – Bot. Žurn. 57: 406-414.
Agapova ND. 1977. Novyj priznak v sistematike roda Ornithogalum L. (Liliaceae). – Bot. Žurn. 62: 883-885.
Agapova ND. 1980. Cytosystematic study of the European representatives of the genus Ornithogalum (Liliaceae) of the Flora of the USSR II. Subgenus Ornithogalum. – Bot. Žurn. 65: 783-794.
Agapova ND. 1997. Ornithogalum gabrielianae (Hyacinthaceae), a new endemic species from Armenia. – Willdenowia 27: 199-206.
Ahmad NM, Martin PM, Vella JM. 2008. Floral structure and development in the dioecious Australian endemic Lomandra longifolia (Lomandraceae). – Aust. J. Bot. 56: 666-683.
Akhani H. 2002. Notes on the flora of Iran: 1. Asparagus (Asparagaceae) and Nitraria (Zygophyllaceae). – Edinburgh J. Bot. 59: 295-302.
Aksenova LM, Sedova ED. 1981. Bulb structure and morphogenesis of some representatives of the family Amaryllidaceae. – Ukr. J. Bot. 4: 41-45. [In Russian]
Albert VA. 1994. Cladistic relationships of the slipper orchids (Cypripedioideae: Orchidaceae) from congruent morphological and molecular data. – Lindleyana 9: 115-132.
Albert VA, Chase MW. 1992. Mexipedium: a new genus of slipper orchid (Cypripedioideae: Orchidaceae). – Lindleyana 7: 172-176.
Albert VA, Pettersson B. 1994. Expansion of genus Paphiopedilum to include all conduplicate-leaved slipper orchids (Cypripedioideae: Orchidaceae). – Lindleyana 9: 133-139.
Ali SS, Yu Y, Pfosser M, Wetschnig W. 2012. Inferences of biogeographical histories within subfamily Hyacinthoideae using S-DIVA and Bayesian binary MCMC analysis implemented in RASP (Reconstruct Ancestral State in Phylogenies). – Ann. Bot. 109: 95-107.
Alrich P, Higgins W. 2008. Illustrated dictionary of orchid genera. – Comstock Publ. Assoc., Cornell University Press, Ithaca.
Alvarez A. 1990. El complejo estomático en la familia Agavaceae II. Epidermis adulta. – Feddes Repert. 101: 113-134.
Alvarez A, Cameron KM. 2009. Molecular phylogenetics of Precottiinae s.l. and their close allies (Orchidaceae, Cranichideae) inferred from plastid and nuclear ribosomal DNA sequences. – Amer. J. Bot. 96: 1020-1040.
Alvarez A, Köhler E. 1987. Morfología del polen de las Agavaceae y algunos généros afines. – Grana 26: 25-46.
Alvarez de Zayas A. 1990. El compleja estomático en la família Agavaceae 2. Epidermis adultsa. – Feddes Repert. 101: 113-134.
Àlvarez-Molina A, Cameron KM. 2009. Molecular phylogenetics of Prescottiinae s. l. and their close allies (Orchidaceae, Granichideae) inferred from plastid and nuclear ribosomal DNA sequences. – Amer. J. Bot. 96: 1020-1040.
Ameele RJ. 1982. The transmitting tract in Gladiolus I. The stigma and the pollen-stigma interaction. – Amer. J. Bot. 69: 389-401.
Ames O. 1913. Notes on Philippine orchids with description of new species 6. – Philipp. J. Sci., Sect. C, Bot. 8, 6: 407-440.
Ames O. 1915. The genera and species of Philippine orchids. – Orchidaceae, fasc. 5, Boston.
Ames O. 1922a. A discussion of Pogonia and its allies in the northeastern United States. – Orchidaceae, vol. 7, Merrymount Press, Boston, Massachusetts, pp. 3-39.
Ames O. 1922b. New or noteworthy orchids from different parts of the world. – Orchidaceae 7: 88-140.
Ames O. 1924. Additions to the orchid flora of tropical America. – Sched. Orch. 7: 1-36.
Ames O. 1925. Enumeration of Philippine Apostasiaceae and Orchidaceae. – In: Merrill ED (ed), Enumeration of Philippine flowering plants 1, Bur. Sci. Publ. 18, Manila, 1924, pp. 252-458.
Ames O. 1946. The evolution of the orchid flower. – Amer. Orchid. Soc. Bull. 14: 355-360.
Ames O. 1948. Orchids in retrospect: a collection of essay on the Orchidaceae. – Cambridge, Massachusetts.
Ames O. 1992. A discussion of Pogonia and its allies in the northeastern United States with reference to extra-limital genera and species. – Orchidaceae 7: 3-38.
Ames O, Correll DS. 1952-1953. Orchids of Guatemala. – Fieldiana Bot. 26: 1-727.
Andersen TF, Johansen B, Lund I, Rasmussen FN, Rasmussen H, Sørensen I. 1988. Vegetative architecture of Eria. – Lindleyana 3: 117-132.
Anderson CE. 1940. Some studies on the floral anatomy of the Liliales. – Ph.D. diss., Cornell University, Ithaca, New York.
Anderson E. 1931. The chromosome complements of Allium stellatum and Nothoscordum bivalve. – Ann. Missouri Bot. Gard. 18: 465-467.
Andriananjamanantsoa HN, Engberg S, Louis Jr EE, Brouillet L. 2016. Diversification of Angraecum (Orchidaceae, Vandeae) in Madagascar: revised phylogeny reveals species accumulation through time rather than rapid radiation. – PloS One 11, e0163194.
Angold R. 1967. The ontogeny and fine structure of the pollen grain of Endymion non-scriptus. – Rev. Palaeobot. Palynol. 3: 205-212.
Angold R. 1968. The formation of the generative cell in the pollen grain of Endymion non-scriptus (L.). – J. Cell Sci. 3: 573-578.
Ansari R, Balakrishnan R, Wood J. 1991. A revision of the Indian species of Oberonia (Orchidaceae). – Orchid Monogr. 4, Rijksherbarium/Hortus Botanicus, Leiden.
Ansari R, Nair NC, Nair VJ. 1982. An analysis of the lip of Oberonias in Andhra Pradesh, Kerala and Tamilnadu. – J. Econ. Taxon. Bot. 3: 113-119.
Arber A. 1920. On the leaf structure of certain Liliaceae, considered in relation to the phyllode theory. – Ann. Bot. 34: 447-465.
Arber A. 1921. The leaf structure of the Iridaceae, considered in relation to the phyllode theory. – Ann. Bot. 35: 301-336.
Arber A. 1924a. Danae, Ruscus, and Semele: a morphological study. – Ann. Bot. 38: 229-260.
Arber A. 1924b. Myrsiphyllum and Asparagus. – Ann. Bot. 38: 635-659.
Arber A. 1935. The “needles” of Asparagus with special reference to A. sprengeri Regel. – Ann. Bot. 49: 337-344.
Arber A. 1937. Studies in flower structure III. On the ‘corona’ and androecium in certain Amaryllidaceae. – Ann. Bot., N. S., 1: 293-304.
Archer RH, Snijman DA, Brummitt RK. 2001. Proposal to conserve the name Boophone Herbert with that spelling (Amaryllidaceae). – Taxon 50: 569-571.
Archibald JK, Kephart SR, Theiss KE, Petrosky AL, Culley TM. 2015. Multilocus phylogenetic inference in subfamily Chlorogaloideae and related genera of Agavaceae – informing questions in taxonomy an multiple ranks. – Mol. Phylogen. Evol. 84: 266-283.
Arditti J. 1969. Floral anthocyanins in species and hybrids of Broughtonia, Brassavola, and Cattleyopsis (Orchidaceae). – Amer. J. Bot. 56: 59-68.
Arditti J (ed). 1984. Orchid biology: reviews and perspectives III. – Cornell University Press, Ithaca, New York.
Arditti J (ed). 1987. Orchid biology: reviews and perspectives IV. – Cornell University Press, Ithaca, New York.
Arditti J. 1992. Fundamentals of orchid biology. – John Wiley and Sons, New York.
Arekal GC. 1967. Contribution to the embryology of Hypoxis aurea Lour. – J. Indian Bot. Soc. 46: 193-198.
Arends JC, Laan FM van der. 1986. Cytotaxonomy of Vandeae. – Lindleyana 1: 33-41.
Arnold ML, Bennett BD, Zimmer EA. 1990. Natural hybridization between Iris fulva and Iris hexagona: pattern of ribosomal DNA variation. – Evolution 44: 1512-1521.
Arnold ML, Buckner CM, Robinson JJ. 1991. Pollen mediated introgression and hybrid speciation in Louisiana irises. – Proc. Natl. Acad. U.S.A. 88: 1398-1402.
Arnott HJ. 1962. The seed, germination and seedling of Yucca. – Univ. Calif. Publ. Bot. 35: 1-144.
Arroyo SC. 1981. Systematic anatomical studies on Amaryllidaceae including morphological, ecological, cytological and phytogeographical considerations. – Ph.D. diss., University of Reading, England.
Arroyo SC. 1982a. Anatomia vegetativa de Ixiolirion Fisch. ex Herb. (Liliales) y su significado taxonomico. – Parodiana 1: 271- 286.
Arroyo SC. 1982b. The chromosomes of Hippeastrum, Amaryllis and Phycella (Amaryllidaceae). – Kew Bull. 37: 211-216.
Arroyo SC. 1984. Contribución al conocimiento de los bulbos de Amaryllidaceae. – Kurtziana 17: 55-70.
Arroyo SC. 1986. Leaf anatomy in the Tecophilaeaceae. – Bot. J. Linn. Soc. 93: 323-328.
Arroyo SC, Cutler DF. 1984. Evolutionary and taxonomic aspects of the internal morphology in Amaryllidaceae from South America and southern Africa. – Kew Bull. 39: 467-498.
Arroyo-Leuenberger S, Leuenberger BE. 1992. Notes on Rhodophiala rhodolirion (Amaryllidaceae) in Mendoza, Argentina. – Herbertia 47: 80-87.
Arroyo-Leuenberger S, Leuenberger BE. 2009. Revision of Zephyranthes andina (Amaryllidaceae) including five new synonyms. – Willdenowia 39: 145-159.
Artyushenko ZT. 1974. Galanthus L. (Amaryllidaceae) in Greece. – Ann. Mus. Coulandris 2: 9-21.
Artyushenko ZT. 1989. Aspects of research on Amaryllidaceae Jaume. – Herbertia 45: 131-137.
Ashurmetov OA, Yengalycheva SS, Fritsch RM. 2001. Morphological and embryological characters of three middle Asian Allium L. species (Alliaceae). – Bot. J. Linn. Soc. 137: 51-64.
Atwood JT Jr. 1984. The relationships of the slipper orchids (subfamily Cypripedioideae, Orchidaceae). – Selbyana 7: 129-247.
Atwood JT Jr. 1986. The size of the Orchidaceae and the systematic distribution of epiphytic orchids. – Selbyana 9: 171-186.
Atwood JT Jr. 2003. Miscellaneous new species of Maxillaria (Orchidaceae). – Selbyana 24: 30-43.
Atwood JT Jr, Mora de Retana DE. 1999. Orchidaceae: tribe Maxillarieae: subtribes Maxillariinae and Oncidiinae. – Fieldiana, Bot. 40: 1-182.
Averyanov LV. 1988. New and rare species of orchids in the Vietnamese flora. – Bot. Žurn. 73: 720-729.
Averyanov LV. 1990. New and rare species of the Orchidaceae family in the Vietnamese flora. – Bot. Žurn. 75: 721-723.
Averyanov LV, Ponert J, Nguyen PT, Duy NV, Khang NS, Nguyen VC. 2016. A survey of Dendrobium Sw. sect. Formosae (Benth. & Hook. f.) Hook. f. in Cambodia, Laos and Vietnam. – Adansonia 38: 199-217.
Aybeke M. 2012. Comparative anatomy of selected rhizomatous and tuberous taxa of subfamilies Orchidoideae and Epidendroideae (Orchidaceae) as an aid to identification. – Plant Syst. Evol. 298: 1643-1658.
Ayensu ES, Williams NH. 1972. Leaf anatomy of Palumbina and Odontoglossum subgenus Osmoglossum. – Bull. Amer. Orchid Soc. 41: 687-696.
Bacchetta G, Brullo S, D’Emerico S, Pontecorvo C, Salmeri C. 2012. Charybdis glaucophylla (Asparagaceae), a new species from Sardinia. – Phytotaxa 69: 16-26.
Backhouse GN, Jeanes J. 1995. The orchids of Victoria. – Meigunyah Press, Carlton, Victoria.
Badawi AA, Elwan Z. 1986. A taxonomic study of Liliaceae sensu lato 1 & 2. – Phytologia 60: 201-221.
Baijnath H. 1978. Jodrellia, a new genus of Liliaceae from tropical Africa. – Kew Bull. 32: 571-578.
Baijnath H. 1980. A contribution to the study of leaf anatomy of the genus Kniphofia Moench (Liliaceae). – In: Brickell CD, Cutler DF, Gregory M (eds), Petaloid monocotyledons. Horticultural and botanical research, Linn. Soc. Symp. Ser. 8, Academic Press, London, pp. 89-103.
Baijnath H, Cutler DF. 1993. A contribution to the anatomy and surface details of leaves in the genus Bulbine (Asphodelaceae) in Africa. – South Afr. J. Bot. 59: 109-115.
Baijnath H, Perry P. 1980. Preliminary observations of leaf surface structures in Eriospermum Jacq. – Proc. Electr. Microsc. Soc. South Africa 10: 39-40.
Baker HG. 1986. Yuccas and yucca moths – a historical commentary. – Ann. Missouri Bot. Gard. 73: 556-564.
Baker JG. 1870. Monograph of Scilla, Ledebouria and Drimiopsis. – Saunder’s Ref. Bot. 3, App.: 1-18.
Baker JG. 1871. A revision of the genera and species of herbaceous capsular gamophyllous Liliaceae. – Bot. J. Linn. Soc. 11: 349-436.
Baker JG. 1873. Revision of the genera and species of Scilleae and Chlorogaleae. – Bot. J. Linn. Soc. 13: 209-293.
Baker JG. 1875a. Albuca glandulosa. – Gard. Chron. 1: 814.
Baker JG. 1875b. Revision of the species and genera of the Asparagaceae. – Bot. J. Linn. Soc. 14: 508-632.
Baker JG. 1876. Revision of the genera and species of Anthericeae and Eriospermeae. – Bot. J. Linn. Soc. 15: 253-363.
Baker JG. 1877 [1878]. Systema Iridacearum. – Bot. J. Linn. Soc. 16: 61-180.
Baker JG. 1878a. A synopsis of Hypoxidaceae. – Bot. J. Linn. Soc. 17: 93-126.
Baker JG 1878b. Descriptions of new and little known Liliaceae. – J. Bot. 16: 321-326.
Baker JG. 1880. A synopsis of Aloineae and Yuccoideae. – Bot. J. Linn. Soc. 18: 148-241.
Baker JG. 1881a. A synopsis of the known species of Crinum L. – Gard. Chron. 15: 763, 786.
Baker JG. 1881b. A synopsis of the known species of Crinum L. – Gard. Chron. 16: 39, 72, 180, 398, 495, 588, 760, 785.
Baker JG. 1888. Handbook of the Amaryllideae including the Alstroemerieae and Agaveae. – George Bell & Sons, London.
Baker JG. 1892. Handbook of the Irideae. – George Bell & Sons, London.
Baker JG. 1896. The genus Brodiaea and its allies. – Gard. Chron., Ser. III, 20: 213-214.
Baker RK. 1972. Foliar anatomy of the Laeliinae (Orchidaceae). – Ph.D. diss., University of Washington.
Balakrishnan NP, Chowdhury S. 1967. Notes on orchids of Bhutan II: some new or imperfectly known species. – Bull. Bot. Surv. India 9: 88-94.
Balicka-Iwanowska G. 1893. Contribution à l’étude anatomique et systématique du genre Iris et des genres voisines. – Lab. Bot. Univ. Genève, sér. I, 6: 67-120.
Balogh P. 1982. Generic redefinition in subtribe Spiranthinae (Orchidaceae). – Amer. J. Bot. 69: 1119-1132.
Banerji ML, Thapa BB. 1969. Orchids of Nepal 2. – J. Bombay Nat. Hist. Soc. 66: 577-583.
Baranow P, Mytnik-Ejsmont J. 2009. Two new species of Polystachya Hook. (Orchidaceae) from Africa. – Plant Syst. Evol. 281: 11-16.
Baranow P, Szlachetko D. 2016. The taxonomic revision of Sobralia Ruiz & Pav. (Orchidaceae) in the Guyanas (Guyana, Suriname, French Guiana). – Plant Syst. Evol. 302: 333-355.
Baranow P, Dudek M, Szlachetko DL. 2017. Brasolia, a new genus highlighted from Sobralia (Orchidaceae). – Plant Syst. Evol. 303: 853-871.
Bareka P. 2001. A karyosystematic study of the genus Leucojum L. (Amaryllidaceae) in Greece. – M.Sc. thesis, University of Patras, Greece. [In Greece with English summary]
Bareka P, Kamari G, Phitos D. 2003. Cytogeographic study of the genus Leucojum L. (Amaryllidaceae) in Greece. – Bocconea 16: 529-536.
Bareka P, Phitos D, Kamari G. 2008. A karyosystematic study of the genus Bellevalia Lapeyr. (Hyacinthaceae) in Greece. – Bot. J. Linn. Soc. 157: 723-739.
Barigozzi C. 1979. First data on the chromosome number of Curculigo seychellensis Boj (Amaryllidaceae). – Atti Accad. Naz. Lincei, Rend., Sci. Fis. Mat. Nat. 66: 453-454.
Barkman TJ, Simpson BB. 2001. Origin of high-elevation Dendrochilum species (Orchidaceae) endemic to Mount Kinabalu, Sabah, Malaysia. – Syst. Bot. 26: 658-669.
Barone Lumaga MR, Cozzolino S, Kocyan A. 2006. Exine micromorphology of Orchidinae (Orchidoideae, Orchidaceae): phylogenetic constraints or ecological influences? – Ann. Bot. 98: 237-244.
Barrett CF, Freudenstein JV. 2008. Molecular evolution of rbcL in the mycoheterotrophic coralroot orchids (Corallorhiza Gagnebin, Orchidaceae). – Mol. Phylogen. Evol. 47: 665-679.
Barrett SCH, Lloyd DG, Arroyo J. 1993. Stylar polymorphisms and the evolution of heterostyly in Narcissus. – In: Lloyd DG, Barrett SCH (eds), Floral biology. Studies on floral evolution in animal-pollinated plants, Chapman & Hall, New York, pp. 339-376.
Barringer K. 1987. Two new species of Elleanthus (Orchidaceae) from Ecuador and Peru. – Syst. Bot. 12: 162-166.
Barros Neves J de. 1952. Estudos caaryologicos no genero Ornithogalum L. – Bol. Soc. Brot. 26, ser. 2A: 5-192.
Barthet MM, Moukarzel K, Smith KN, Patel J, Hilu KW. 2015. Alternative translation initiation codons for the plastid maturase matK: unravelling the pseudogene misconception in the Orchidaceae. – BMC Evol. Biol. 15: 210.
Barthlott W. 1976a. Struktur und Funktion des Velamen radicum der Orchideen. – In: Senghas K (ed), Proceedings of the Eighth World Orchid Conference, Deutsche Orchideen-Gesellschaft, Frankfurt, pp. 438-443.
Barthlott W. 1976b. Morphologie der Samen von Orchideen im Hinblick auf taxonomische und funktionelle Aspekte. – In: Senghas K (ed), Proceedings of the Eighth World Orchid Conference, Deutsche Orchideen-Gesellschaft, Frankfurt, pp. 444-452.
Barthlott W, Capesius I. 1975. Mikromorphologische und funktionelle Untersuchungen am Velamen radicum der Orchideen. – Ber. Deutsch. Bot. Ges. 88: 379-390.
Barthlott W, Ziegler B. 1981. Mikromorphologie der Samenschalen als systematisches Merkmal bei Orchideen. – Ber. Deutsch. bot. Ges. 94: 267-273.
Bartolo G, Brullo S, Pavone P, Terrasi MC. 1984. Cytotaxonomical notes on some Liliaceae of N Cyrenaica. – Webbia 38: 601-622.
Barykina RP, Gulenkova MA. 1985. Ontomorphogenesis, anatomy and the nature of leaf-like organs in Asparagus sprengeri (Asparagaceae). – Bot. Žurn. 70: 322-331. [In Russian]
Bastide J, Viladomat F. 2002. Alkaloids of Narcissus. – In: Hanks GR (ed), Narcissus and daffodil, Taylor & Francis, London, pp. 141-214.
Bateman AJ. 1968. The role of heterostyly in Narcissus and Mirabilis. – Evolution 22: 645-646.
Bateman RM, Pridgeon AM, Chase MW. 1997. Phylogenetics of subtribe Orchidinae (Orchidoideae, Orchidaceae) based on nuclear ITS sequences 2. Infrageneric relationships and reclassification to achieve monophyly of Orchis sensu stricto. – Lindleyana 12: 113-141.
Bateman RM, Hollingsworth PM, Preston J, Luo Y-B, Pridgeon AM, Chase MW. 2003. Molecular phylogenetics and evolution of Orchidinae and selected Habenariinae (Orchidaceae). – Bot. J. Linn. Soc. 142: 1-40.
Bateman RM, James KE, Luo Y-B, Lauri RK, Fulcher T, Cribb PJ, Chase MW. 2009. Molecular phylogenetics and morphological reappraisal of the Platanthera clade (Orchidaceae: Orchidinae) prompts expansion of the generic limits of Galearis and Platanthera. – Ann. Bot. 104: 431-445.
Bateman RM, Bradshaw E, Devey DS, Glover BJ, Malmgren S, Sramkó G, Thomas MM, Rudall PJ. 2011. Species arguments: clarifying competing concepts of species delimitation in the pseudo-copulatory orchid genus Ophrys. – Bot. J. Linn. Soc. 165: 336-347.
Bates RJ, Weber JZ. 1990. Orchids of South Australia. – The Flora and Fauna of South Australia Handbooks Committee, Adelaide.
Batista JAN, Bianchetti L de B. 2004. Three new taxa in Cyrtopodium (Orchidaceae) from central and southeastern Brazil. – Brittonia 56: 260-274.
Batista JAN, Bianchetti L de B, Miranda Z de JG. 2006. A revision of Habenaria section Macroceratitae (Orchidaceae) in Brazil. – Brittonia 58: 10-41.
Batista JAN, Bianchetti L de B, Miranda Z de JG. 2008. Two new species of Habenaria (Orchidaceae) from the Brazilian cerrado and campo rupestre. – Kew Bull. 63: 449-456.
Batista JAN, Carvalho BM, Ramalho AJ, Bianchetti LB. 2010. Three new species of Habenaria (Orchidaceae) from Serra da Canastra, Minas Gerais, Brazil. – Phytotaxa 13: 27-39.
Batista JAN, Meneguzzo TEC, Salazar GA, Bianchetti L de B, Ramalho AJ. 2011. Phylogenetic placement, taxonomic revision. and a new species of Nothostele (Orchidaceae), an enigmatic genus endemic to the cerrado of central Brazil. – Bot. J. Linn. Soc. 165: 348-363.
Batista JAN, Borges KS, Faria MWF de, Proite K, Ramalho AJ, Salazar GA, Berg C van den. 2013. Molecular phylogenetics of the species-rich genus Habenaria (Orchidaceae) in the New World based on nuclear and plastid DNA sequences. – Mol. Phylogen. Evol. 67: 95-109.
Batista JAN, Mota ACM, Proite K, Bem Bianchetti L de, Romero-González GA, Huerta H, Salazar GA. 2014. Molecular phylogenetics of Neotropical Cyanaeorchis (Cymbidieae, Epidendroideae, Orchidaceae): geographical rather than morphologidcal similarities plus a new species. – Phytotaxa 156: 251-272.
Baumann B, Lorenz R. 2005. Beiträge zur Taxonomie europäischer und mediterraner Orchideen. – J. Eur. Orch. 37: 705-743.
Bauml JA. 1979. A study of the genus Hymenocallis (Amaryllidaceae) in Mexico. – M.Sc. thesis, Cornell University, Ithaca, New York.
Bayer MB. 1971. Changes in the genus Haworthia Duval. – Cact. Succ. J. (Los Angeles) 43: 157-162.
Bayer MB. 1972. reinstatement of the genera Astroloba and Poellnitzia Uitew. (Liliaceae-Aloineae). – Natl. Cact. Succ. J. 27: 77-79.
Bayer MB. 1973. Natural variation and species delimitation in Haworthia Duval 3. Haworthia reinwardtii Haw. Haworthia coarctata Haw. – Natl. Cact. Succ. J. 28: 80-86.
Bayer MB. 1976. Haworthia handbook. – National Botanic Gardens of South Africa, Kirstenbosch.
Bayer MB. 1980. Gasteria. – Excelsa 9: 29-33.
Bayer MB. 1982. The new Haworthia handbook. – National Botanic Gardens of South Africa, Kirstenbosch, Cape Town.
Bayer MB. 1999. Haworthia revisited: a revision of the genus. – Ed. by François Steffens, Umdaus Press, Hatfield, Republic of South Africa.
Bayer MB. 2002. Haworthia update: essays on Haworthia, vol. 1. – Umdaus Press, Hatfield, Republic of South Africa.
Bayer MB. 2009. Haworthia supplement. – The Haworthia Society, London.
Bayer C, Appel O, Rudall PJ. 1998. Asteliaceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 141-145.
Bay-Smidt MGK, Jäger AK, Krydsfeldt K, Meerow AW, Stafford GI, Staden J van, Rønsted N. 2011. Phylogenetic selection of target species in Amaryllidaceae tribe Haemantheae for acetylcholinesterase inhibition and affinity to serotonin reuptake transport protein. – South Afr. J. Bot. 77: 175-183.
Baytop T, Mathew B, Brighton C. 1975. Four new taxa in Turkish Crocus (Iridaceae). – Kew Bull. 30: 241-246.
Beardsell D, Beardsell C. 1992. A rare new Caladenia species from central Victoria, and its relationship with other recentrly described taxa in south-eastern Australia. – Aust. Syst. Bot. 5: 513-519.
Beaumont J, Cutler DF, Reynolds T, Vaughan JG. 1985. The secretory tissue of aloes and their allies. – Israel J. Bot. 34: 265-282.
Bedford DJ, Lee AT, Macfarlane TD, Henderson RJF, George AS. 1986. Xanthorrhoeaceae. – In: George AS (ed), Flora of Australia 46, Australian Government Publ. Service, Canberra, pp. 88-171, 221-230.
Beever RE. 1981. Self-incompatibility in Cordyline kaspar (Agavaceae). – New Zealand J. Bot. 19: 13-16.
Beever RE, Parkes SL. 1996. Self-incompatibility in Cordyline australis (Asteliaceae). – New Zealand J. Bot. 34: 135-137.
Bell AK, Roberts DL, Hawkins JA, Rudall PJ, Box MS, Bateman RM. 2009. Comparative micromorphology of nectariferous and nectarless labellar spurs in selected clades of subtribe Orchidinae (Orchidaceae). – Bot. J. Linn. Soc. 160: 369-387.
Bell WD. 1977. More potentials in Amaryllis breeding. – Plant Life 33: 65-69.
Bellstedt DU, Linder HP, Harley E. 2001. Phylogenetic relationships in Disa based on non-coding trnL-trnF chloroplast sequences: evidence of numerous repeat regions. – Amer. J. Bot. 88: 2088-2100.
Bellusci F, Pellegrino G, Palermo AM, Musacchio A. 2008. Phylogenetic relationships in the orchid genus Serapias L. based on noncoding regions of the chloroplast genome. – Mol. Phylogen. Evol. 47: 986-991.
Bennett BC. 1983. Primitive habit in Orchidaceae. – Syst. Bot. 8: 472-474.
Bennett DE. 1998. New species of Peruvian Orchidaceae IV. – Brittonia 50: 186-191.
Bennett DE, Christenson EA. 1993. Icones orchidacearum peruviarum. – Pastorelli de Bennett, Lima-Sarasota.
Bennett DE Jr, Christenson EA. 1998. Icones orchidacearum peruviarum. – Pastorelli de Bennett, Lima-Sarasota.
Bentham G. 1881. Notes on Orchideae. – J. Linn. Soc. 18: 281-360.
Bentzer B. 1969. Chromosome morphology in Aegean populations of Leopoldia Parl. (Liliaceae). – Bot. Not. 122: 457-480.
Bentzer B. 1973. Taxonomy, variation and evolution in representatives of Leopoldia Parl. (Liliaceae) in the Southern and Central Aegean. – Bot. Not. 126: 69-132.
Bentzer B. 1974. Karyotypes and meiosis in Leopoldia Parl. (Liliaceae) from the Southern and Central Aegean (Greece). – Bot. Not. 127: 69-86.
Bentzer B, Bothmer R von, Wendelbo P. 1972. Chromosome morphology in Afghanian Bellevalias (Liliaceae). – Bot. Not. 125: 153-156.
Bentzer B, Bothmer R von, Wendelbo P. 1974. Cytology and morphology of the genus Hyacinthus L. s. str. (Liliaceae). – Bot. Not. 127: 297-301.
Benzing DH. 1987. Major patterns and processes in orchid evolution: a critical synthesis. – In: Arditt J (ed), Orchid Biology IV: 34-77.
Benzing DH, Atwood JT Jr. 1984. Orchidaceae: ancestral habitats and current status in forest canopies. – Syst. Bot. 9: 155-165.
Berg C van den, Higgins WE, Dressler RL, Whitten WM, Soto Arenas A, Culham A, Chase MW. 2000. A phylogenetic analysis of Laeliinae (Orchidaceae) based on sequence data from internal transcribed spacers (ITS) of nuclear ribosomal DNA. – Lindleyana 15: 96-114.
Berg C van den, Goldman DH, Freudenstein JV, Pridgeon AM, Cameron KM, Chase MW. 2005. An overview of the phylogenetic relationships within Epidendroideae inferred from multiple DNA regions and recircumscription of Epidendreae and Arethuseae (Orchidaceae). – Amer. J. Bot. 92: 613-624.
Berg C van den, Higgins WE, Dressler RL, Whitten WM, Soto-Arenas MA, Chase MW. 2009. A phylogenetic study of Laeliinae (Orchidaceae) based on combined nuclear and plastid DNA sequences. – Ann. Bot. 104: 417-430.
Berg RY. 1978. Development of ovule, embryo sac, and endosperm in Brodiaea (Liliales). – Norw. J. Bot. 25: 1-7.
Berg RY. 1996. Development of ovule, embryo sac, and endosperm in Dipterostemon and Dichelostemma (Alliaceae) relative to taxonomy. – Amer. J. Bot. 83:790-801.
Berg RY. 2003. Development of ovule, embryo sac, and endosperm in Triteleia (Themidaceae) relative to taxonomy. – Amer. J. Bot. 90: 937-948.
Berg RY, Maze JR. 1966. Contribution to the embryology of Muilla, with a remark on the taxonomic position of the genus. – Madroño 18: 143-151.
Berger A. 1905. Über die systematische Gliederung der Gattung Aloë. – Engl. Bot. Jahrb. Syst. 36: 42-68.
Bernardos S, Amich F, Gallego F. 2003. Karyological and taxonomic notes on Ophrys (Orchidoideae, Orchidaceae) from the Iberian Peninsula. – Bot. J. Linn. Soc. 142: 395-406.
Bernardos S, Tyteca D, Revuelta JL, Amich F. 2004. A new endemic species of Epipactis (Orchidaceae) from North-East Portugal. – Bot. J. Linn. Soc. 145: 239-249.
Bernardos S, Crespi A, Rey F del, Amich F. 2005. The section Pseudophrys in the Iberian Peninsula, a morphometric and molecular analysis. – Bot. J. Linn. Soc. 148: 359-375.
Bernhardt P. 1995. The floral ecology of Dianella caerulea var. assera (Phormiaceae). – Cunninghamia 4: 9-20.
Bernhardt P, Burns-Baloch P. 1986. Floral mimesis in Thelymitra nuda (Orchidaceae). – Plant Syst. Evol. 151: 187-202.
Bernhardt P, Goldblatt P. 2006. The role of phylogenetic constraints in the evolution of pollination mechanisms in Iridaceae of sub-Saharan Africa. – In: Columbus JT, Friar EA, Porter JM, Prince LM, Simpson MG (eds), Monocots: comparative biology and evolution. Excluding Poales, Rancho Santa Ana Botanical Garden, Claremont, California. – Aliso 22: 434-444.
Bernhardt P, Montalvo EA. 1977. The reproductive phenology of Echeandia macrocarpa Greenm. (Liliaceae) with a re-examination of the floral morphology. – Bull. Torrey Bot. Club 104: 320-323.
Bidartondo MI, Read DJ. 2008. Fungal specificity bottlenecks during orchid germination and development. – Mol. Ecol. 17: 3707-3716.
Bidartondo MI, Burghardt B, Gebauer G, Bruns TD, Read DJ. 2004. Changing partners in the dark: isotopic and molecular evidence of ectomycorrhizal liaisons between forest orchids and trees. – Proc. Roy. Soc., Ser. B, 271: 1799-1806.
Birch JL. 2015. A revision of infrageneric classification in Astelia Banks & Sol. ex R. Br. (Asteliaceae). – PhytoKeys 52: 105-132. – Corrigenda: PhytoKeys 56: 127-128.
Birch, J. L. & Keeley, S. C. 2013: Dispersal pathways across the Pacific: the historical biogeography of Astelia s.l. (Asteliaceae, Asparagales). – J. Biogeogr. 40: 1914–1927.
Birch JL, Keeley SC, Morden CW. 2012. Molecular phylogeny and dating of Asteliaceae (Asparagales): Astelia s.l. evolution provides insight into the Oligocene history of New Zealand. – Mol. Phylogen. Evol. 65: 102-115.
Bjorå CS, Hoell G, Kativu S, Nordal I. 2008. New taxa of Chlorophytum (Anthericaceae) from southern tropical Africa with notes on their sister group relationships. – Bot. J. Linn. Soc. 157: 223-238.
Bjorå CS, Hemp A, Hoell Gry, Nordal I. 2008. A taxonomic and ecological analysis of two forest Chlorophytum taxa (Anthericaceae) on Mount Kilimanjaro, Tanzania. – Plant Syst. Evol. 274: 243-253.
Bjorå CS, Kwembeya EG, Bogner J, Nordal I. 2009. Geophytes diverging in rivers – a study on the genus Crinum, with two new rheophytic taxa from Cameroon. – Taxon 58: 561-571.
Bjørnstad IN. 1973. A revision of the genus Pancratium L. (Amaryllidaceae) in Africa south of the Sahara. – Norw. J. Bot. 20: 281-291.
Bjørnstad IN, Friis I. 1972a. Studies on the genus Haemanthus (Amaryllidaceae) I. The infrageneric taxonomy. – Norw. J. Bot. 19: 187-206.
Bjørnstad IN, Friis I. 1972b. Studies on the genus Haemanthus (Amaryllidaceae) II. A revision of the section Demeusea (De Wild. & Th. Dur.) Pax & Hoffm. emend. I. Bjørnstad & I. Friis. – Norw. J. Bot. 19: 207-222.
Bjørnstad IN, Friis I. 1974. Studies on the genus Haemanthus L. (Amaryllidaceae) III. A revision of the sections Gyaxis and Nerissa. – Norw. J. Bot. 21: 243-275.
Bjørnstad J. 1970. Comparative embryology of Asparagoideae-Polygonateae, Liliaceae. – Nytt Mag. Bot. 17: 169-207.
Blackman SJ, Yeung EC. 1983. Structural development of the caudicel of an orchid (Epidendrum). – Amer. J. Bot. 70: 97-105.
Blackmore S. 1981a. A new species of Kniphofia (Liliaceae) from Malawi. – Kew Bull. 35: 793-795.
Blackmore S. 1981b. Kniphofia monticola (Liliaceae), a new species from Malawi. – Nord. J. Bot. 1: 481-483.
Blanco MA, Barboza G. 2005. Pseudocopulatory pollination in Lepanthes (Orchidaceae: Pleurothallidinae) by fungus gnats. – Ann. Bot. 95: 763-772.
Blanco MA, Carnevali G, Whitten WM, Singer RB, Koehler S, Williams NH, Ojeda I, Neubig KM, Endara L. 2007. Generic realignments in Maxillariinae (Orchidaceae). – Lankesteria 7: 515-537.
Blunden G, Binns WW. 1970. The leaf anatomy of Yucca glauca Nutt. – Bot. J. Linn. Soc. 63: 133-141.
Blunden G, Yi Y, Jewers K. 1973. The comparative leaf anatomy of Agave, Beschorneria, Doryanthes, and Furcraea species (Agavaceae: Agaveae). – Bot. J. Linn. Soc. 66: 157-179.
Blunden G, Yi Y, Jewers K. 1978. Steroidal sapogenins from leaves of Agaveae species. – Phytochemistry 17: 1923-1925.
Bockemühl L. 1984. Die Gattung Odontoglossum H.B.K. Studien zu einer natürlichen Gliederung (8. Fortsetzung). – Die Orchidee 35: 213-218.
Bockemühl L. 1989. Odontoglossum, a monograph and iconograph. – Bruecke Verlag, Hildesheim.
Bogdanovic S, Brullo S, Mitic B, Salmeri C. 2008. A new species of Allium (Alliaceae) from Dalmatia, Croatia. – Bot. J. Linn. Soc. 158: 106-114.
Bogler DJ. 1994. Taxonomy and phylogeny of Dasylirion (Nolinaceae). – Ph.D. diss., University of Texas, Austin, Texas.
Bogler DJ. 1995. Systematics of Dasylirion: taxonomy and molecular phylogeny. – Bol. Soc. Bot. Méx. 56: 69-767.
Bogler DJ. 1998. Nolinaceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 392-397.
Bogler DJ, Simpson BB. 1995. A chloroplast DNA study of the Agavaceae. – Syst. Bot. 20: 191-205.
Bogler DJ, Simpson BB. 1996. Phylogeny of Agavaceae based on ITS rDNA sequence variation. – Amer. J. Bot. 83: 1225-1235.
Bogler DJ, Neff JL, Simpson BB. 1995. Multiple origins of the yucca-yucca moth association. – Proc. Natl. Acad. Sci. U.S.A. 92: 6864-6867.
Bogler DJ, Pires JC, Francisco-Ortega J. 2006. Phylogeny of Agavaceae based on ndhF, rbcL, and ITS sequences: implications of molecular data for classification. – In: Columbus JT,Friar EA, Porter JM, Prince LM, Simpson MG (eds), Monocots: comparative biology and evolution. Excluding Poales, Rancho Santa Ana Botanical Garden, Claremont, California. – Aliso 22: 313-328.
Bonde SD. 2005. Eriospermocormus indicus gen. et sp. nov. (Liliales: Eriospermaceae): first record of a monocotyledonous corm from the Deccan Intertrappean beds of India. – Cretaceous Res. 26: 197-205.
Bone RE, Sanz E, Buerki S. 2014. Notes on the flora of Madagascar, 38. The transfer of Eulophia beravensis Rchb. f. to Oeceoclades Lindl., a genus with its centre of diversity in Madagascar (Eulophiinae, Orchidaceae). – Candollea 69: 189-193.
Bone RE, Cribb PJ, Buerki S. 2015. Phylogenetics of Eulophiinae (Orchidaceae: Epidendroideae): evolutionary patterns and implications for generic delimitation. – Bot. J. Linn. Soc. 179: 43-56.
Borba EL, Salazar GA, Mazzoni-Viveiros S,
Batista JAN. 2014. Phylogenetic position and floral morphology of the Brazilian
endemic, monospecific genus Cotylolabium: a sister group for the
remaining Spiranthinae (Orchidaceae).
– Bot. J. Linn. Soc. 175: 29-46.
Borg-Karlson AK. 1990. Chemical and ethological studies of pollination in the genus Ophrys (Orchidaceae). – Phytochemistry 29: 1359-1387.
Bos JJ. 1980. Dracaena surculosa Lindl. – Misc. Pap. Landbouwhoogesch. Wageningen 19: 71-79.
Bos JJ. 1984. Dracaena in West Africa. – Agric. Univ. Wagen. Pap. 84(1): 1-126.
Bos JJ. 1998. Dracaenaceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 238-241.
Bos JJ, Cullen J. 1986. Dracaena. – In: Walters SM, Brady A, Brickell CD, Cullen J, Green PS, Lewis J, Matthews VA, Webb DA, Yeo PF, Alexander JCM (eds), European garden flora 1, Cambridge University Press, Cambridge, pp. 285-287.
Bos JJ, Obermeyer AA. 1992. Dracaenaceae. – In: Leistner OA (ed), Flora of Southern Africa 5, 3, Nat. Bot. Inst., Pretoria, pp. 1-9.
Bos JJ, Graven P, Hetterscheid WLA, Wege JJ van de. 1993. Wild and cultivated Dracaena fragrans. – Edinburgh J. Bot. 49: 311-331.
Bose S, Flory WS. 1963. A study of phylogeny and of karyotype evolution in Lycoris. – Nucleus 6: 141-156.
Bosser J. 1976. Le genre Hederorkis Thou. (Orchidaceae) aux Mascareignes et aux Seychelles. – Adansonia, sér. II, 16: 225-228.
Bosser J. 2000. Contribution à l’étude des Orchidaceae de Madagascar et des Mascareignes XXIX. Révision de la section Kainochilus du genre Bulbophyllum. – Adansonia, sér. III, 22: 167-182.
Bosser J. 2002. Contribution à l’étude des Orchidaceae de Madagascar, des Comores et des Mascareignes XXXII. Un Cynorkis nouveau des Comores et un Eulophia nouveau de La Réunion. – Adansonia, sér. III, 24: 21-25.
Bosser J. 2004. Contribution à l’étude des Orchidaceae de Madagascar, des Comores et des Mascareignes XXXIII. – Adansonia, sér. III, 26: 53-61.
Bosser J. 2006. Contribution à l’étude des Orchidaceae de Madagascar, des Comores et des Mascareignes XXXV. Description d’un Oeceoclades nouveau de Madagascar, et notes sur trois genres nouveaux pour les Mascareignes. – Adansonia, sér. III, 28: 45-54.
Bosser J. 2007. Contribution à l’étude des Orchidaceae de Madagascar, des Comores et des Mascareignes XXXVI. Description d’un Cynorkis nouveau de la Réunion et d’un Angraecum nouveau de Madagascar. – Adansonia, sér. III, 29: 13-17.
Bosser J, Cribb PJ. 2001. Trois nouvelles espèces de Bulbophyllum (Orchidaceae) de Madagascar. – Adansonia, sér. III, 23: 129-135.
Bosser J, Cribb PJ. 2003. Contribution à l’étude des Orchidaceae de Madagascar, des Comores et des Mascareignes XXXIV. Bathiorchis, nouveau genre monotypique de Madagascar. – Adansonia, sér. III, 25: 229-231.
Bosser J, Morat P. 2001. Contribution à l’étude des Orchidaceae de Madagascar et des Mascareignes XXXI. Espèces et combinaisons nouvelles dans les genres Oeceoclades, Eulophia et Eulophiella. – Adansonia, sér. III, 23: 7-22.
Bosser J, la Croix I, Cribb PJ. 2002. The genus Disperis (Orchidaceae) in Madagascar, the Comores, the Mascarenes and the Seychelles. – Adansonia, sér. III, 24: 55-87.
Botha CEJ, Evert RF, Cross RHM, Marshall DM. 1982. The suberin lamella, a possible barrier to water movement from the veins to the mesophyll of Themeda triandra Forsk. – Protoplasma 112: 1-8.
Bothmer R von. 1970. Cytological studies in Allium 1. Chromosome numbers and morphology in Allium Sect. Allium from Greece. – Bot. Not. 123: 518-550.
Bothmer R von. 1972. Four species of Allium sect. Allium in Greece. – Bot. Not. 125: 62-76.
Bothmer R von. 1974. Studies in the Aegean flora XXI. Biosystematic studies in the Allium ampeloprasum complex. – Opera Bot. 34: 1-104.
Bothmer R von, Wendelbo P. 1981. Cytological and morphological variation in Bellevalia. – Nord. J. Bot. 1: 4-11.
Bouck A, Peeler R, Arnold ML, Wessler SR. 2005. Genetic mapping of species boundaries in Louisiana irises using IRRE retrotransposon display markers. – Genetics 171: 1289-1303.
Bouetard A, Lefeuvre P, Gigant R, Bory S, Pignal M, Besse P, Grisoni M. 2010. Evidence of transoceanic dispersion of the genus Vanilla based on plastid DNA phylogenetic analysis. – Mol. Phylogen. Evol. 55: 621-630.
Bouvier W. 1915. Beiträge zur vergleichenden Anatomie der Asphodeloideae (Tribus: Asphodeleae und Hemerocallideae). – Akad. Wiss. Wien, Math.-Naturwiss. Kl., Denkschr. 91: 539-577.
Bower CC. 1992. The use of pollinators in the taxonomy of sexually deceptive orchids in the subtribe Caladeniinae (Orchidaceae). – Orchadian 10: 331-338.
Box MS, Bateman RM, Glover BJ, Rudall PJ. 2008. Floral ontogenetic evidence of repeated speciation via paedomorphosis in subtribe Orchidinae (Orchidaceae). – Bot. J. Linn. Soc. 157: 429-454.
Braas LA, Braem GJ, Mohr H. 1982. Beiträge zur Subtribus Pleurothallidinae I. – Orchidee (Hamburg) 33: 147-156.
Brackett A. 1923. Contributions from the Gray Herbarium of Harvard University I. Revision of the American species of Hypoxis. – Rhodora 69: 120-147.
Braem GJ. 1988. Paphiopedilum: eine Monographie aller Frauenschuh-Orchideen der asiatischen Tropen und Subtropen. – Schmersow, Hildesheim.
Brandham PE. 1969. Chromosome behaviour in the Aloineae. The nature and significance of E-type bridges. – Chromosoma 26: 270-286.
Brandham PE. 1971. The chromosomes of the Liliaceae II. Polyploidy and karyotype variation in the Aloïneae. – Kew Bull. 25: 381-399.
Brandham PE, Bhandol PS. 1997. Chromosomal relationships between the genera Amaryllis and Hippeastrum (Amaryllidaceae). – Kew Bull. 52: 973-980.
Brandham PE, Carter S. 1990. A revision of the Aloe tidmarshii/A. ciliaris complex in South Africa. – Kew Bull. 45: 637-645.
Brandham PE, Cutler DF. 1981. Polyploidy, chromosome interchange and leaf surface anatomy as indicators of relationships within Haworthia section Coarctatae Baker (Liliaceae-Aloineae). – J. South Afr. Bot. 47: 507-546.
Brandham PE, Johnson MAT. 1977. Population cytology of structural and numerical chromosome variants in the Aloïneae (Liliaceae). – Plant Syst. Evol. 128: 105-122.
Brandham PE, Johnson MAT. 1982. Polyploidy and chromosome interchange in Aloe (Liliaceae) from Somalia. – Kew Bull. 37: 389-395.
Brandham PE, Kirton PR. 1987. The chromosomes of species, hybrids and cultivars of Narcissus L. (Amaryllidaceae). – Kew Bull. 42: 65-102.
Brandham PE, Carter S, Reynolds T. 1994. A multidisciplinary study of relationships among the cremnophilous aloes of northeastern Africa. – Kew Bull. 49: 415-428.
Breitenbach W. 1878. Über Asparagus officinalis, eine triözische Pflanze. – Bot. Zeitung 36: 163-167.
Brieger FG. 1977. On the Maxillariinae (Orchidaceae) with sepaline spur. – Bot. Jahrb. Syst. 97: 548-574.
Brieger FG, Maatsch R, Senghas K (eds). 1970-1995. Rudolph Schlechter: Die Orchideen. 3rd ed. – Paul Parey, Berlin, Hamburg.
Briggs BG. 1986. Chromosome numbers in Lomandra (Dasypogonaceae). – Telopea 2: 741-744.
Briggs LH, Briggs LR, King AW. 1975. New Zealand phytochemical survey 14. Constituents of Dianella nigra Col. – New Zealand J. Bot. 18: 559-563.
Brighton CA. 1976. Cytology of Crocus olivieri and its allies. – Kew Bull. 31: 209-219.
Brighton CA, Mathew B, Marchant CJ. 1973. Chromosome counts in the genus Crocus. – Kew Bull. 28: 451-464.
Brittan NH. 1981. Revision of the genus Thysanotus R. Br. (Liliaceae). – Brunonia 4: 67-181.
Brown NE. 1914: Notes on the genera Cordyline, Dracaena, Pleomele, Sansevieria and Taetsia. – Kew Bull. 1914: 273-279.
Brown NE. 1915. Sansevieria: a monograph of all the known species. – Kew Bull. 1915: 185-261.
Brown WH. 1909. the embryo sac of Habenaria. – Bot. Gaz. 48: 241-250.
Brown WV. 1951. Apomixis in Zephyranthes texana Herb. – Amer. J. Bot. 38: 697-702.
Broyles SB, Wyatt R. 1991. The breeding system of Zephyranthes atamasco (Amaryllidaceae). – Bull. Torrey Bot. Club 118: 137-140.
Brullo S, Pavone P, Salmeri C. 1991. Allium kollmannianum, a new species from Israel. – Flora Medit. 1: 15-20.
Brullo S, Pavone P, Salmeri C. 1993. Three new species of Allium (Alliaceae) from Cyprus. – Candollea 48: 279-290.
Brullo S, Pavone P, Salmeri C. 1996. Allium daninianum (Alliaceae), a new species from the Middle East. – Willdenowia 26: 237-244.
Brullo S, Guglielmo A, Pavone P, Salmeri C, Terrasi MC. 2003. Three new species of Allium sect. Codonoprasum from Greece. – Plant Biosystems 137: 131-140.
Brullo S, Giusso del Galdo G, Terrasi MC. 2006. A new species of Oncostema (Hyacinthaceae) from Tunisia. – Bocconea 19: 169-175.
Brullo S, Guglielmo A, Pavone P, Salmeri C. 2008. Taxonomic study on Allium dentiferum Webb & Berthel. (Alliaceae) and its relations with allied species from the Mediterranean. – Taxon 57: 243-253.
Brummitt RK, Rudall PJ, Banks H, Johnson MAT, Docherty KA, Jones K, Chase MW. 1998. Taxonomy of the Cyanastroideae (Tecophilaeaceae): a multidisciplinary approach. – Kew Bull. 53: 769-803.
Buchner L. 1948. Vergleichende embryologische Studien an Scilloideae. – Österr. Bot. Zeitschr. 95: 428-450.
Buerki S, Callmander MW, Schüpfer F, Ravokatra M, Küpfer P, Alvarez N. 2009. Malagasy Dracaena Vand. ex L. (Ruscaceae): an investigation of discrepancies between morphological features and spatial genetic structure at a small evolutionary scale. – Plant Syst. Evol. 280: 15-28.
Buerki S, Manning JC, Forest F. 2013. Spatio-temporal history of the disjunct family Tecophilaeaceae: a tale involving the colonization of three Mediterranean-type ecosystems. – Ann. Bot. 111: 361-373.
Bugnon F. 1960. Contributions à l’étude de quelques problèmes d’anatomie végétale V. Vascularisation de l’éperon foliar chez l’Asparagus sprengeri Regel. – Bull. Soc. Bourgogne 20: 65-71.
Buijsen JRM. 1993. Alliaceae. – In: Kalkman C et al. (eds), Flora Malesiana I, 11(2), Rijksherbarium/Hortus Botanicus, Leiden, pp. 375-384.
Bundrant LH. 1985. Polianthes tuberosa and its hybrids. – Herbertia 41: 55-60.
Büneker HM, Bastian RE, Soares KP, Costa CM. 2016. The genus Tocantinia (Amaryllidaceae, Amaryllidoideae) and two new species from Brazil. – Balduinia 53: 1-14.
Burbank MP. 1941. Cytological and taxonomic studies in the genus Brodiaea. – Bot. Gaz. 103: 247-265.
Burbank MP. 1944. Cytological and taxonomic studies in the genus Brodiaea II. – Bot. Gaz. 105: 339-345.
Burbidge RB. 1978. A revision of the genus Tulbaghia (Liliaceae). – Notes Roy. Bot. Gard. Edinburgh 35: 77-103.
Burke JM, Adams PB. 2002. Variation in the Dendrobium speciosum (Orchidaceae) complex: a numerical approach to the species problem. – Aust. Syst. Bot. 15: 63-80.
Burke JM, Bayly MJ, Adams PB, Ladiges PY. 2008. Molecular phylogenetic analysis of Dendrobium (Orchidaceae), with emphasis on the Australian section Dendrocoryne, and implications for generic classification. – Aust. Syst. Bot. 21: 1-14.
Burns-Balogh P. 1983. A theory on the evolution of the exine in Orchidaceae. – Amer. J. Bot. 70: 1304-1312.
Burns-Balogh P. 1984. Classification of the tribe Diurideae (Orchidaceae). – Selbyana 7: 318-327.
Burns-Balogh P, Bernhardt P. 1985. Evolutionary trends in the androecium of the Orchidaceae. – Plant Syst. Evol. 149: 119-134.
Burns-Balogh P, Bernhardt P. 1988. Floral evolution and phylogeny in the tribe Thelymitreae (Orchidaceae: Neottioideae). – Plant Syst. Evol. 159: 19-47.
Burns-Balogh P, Funk VA. 1986. A phylogenetic analysis of the Orchidaceae. – Smithsonian Contr. Bot. 61: 1-79.
Burns-Balogh P, Hesse M. 1988. Pollen morphology of the cypripedioid orchids – Plant Syst. Evol. 158: 165-182.
Burns-Balogh P, Robinson H. 1983. Evolution and phylogeny of the Pelexia alliance (Orchidaceae: Spiranthoideae: Spiranthinae). – Syst. Bot. 8: 263-268.
Burns-Balogh P, Szlachetko DL, Dafni A. 1987. Evolution, pollination, and systematics of the tribe Neottieae (Orchidaceae). – Plant Syst. Evol. 156: 91-115.
Burtt BL. 2000. Saniella and its relation to other South African genera of Hypoxidaceae. – Edinb. J. Bot. 57: 63-70.
Buzatto CR, Davies KL, Singer RB, Pires dos Santos R, Berg C van den. 2012. A comparative survey of floral characters in Capanemia Barb. Rodr. (Orchidaceae: Oncidiinae). – Ann. Bot. 109: 135-144.
Byrne LT, Colegate SM, Dorling PR, Huxtable CR. 1987. Imbricatonol, a naphthol-naphthoquinone dimer isolated from Stypandra imbricata and Dianella revoluta. – Aust. J. Chem. 40: 1315-1320.
Bytebier B, Bellstedt DU, Linder HP. 2007. A molecular phylogeny for the large African orchid genus Disa. – Mol. Phylogen. Evol. 43: 75-90.
Bytebier B, Oliver EGH, Liltved WR. 2007. Disa linderiana (Orchidaceae), a new orchid from the Western Cape of South Africa. – South Afr. J. Bot. 73: 558-562.
Bytebier B, Bellstedt DU, Linder PH. 2008. A new phylogeny-based sectional classification for the large African orchid genus Disa. – Taxon 57: 1233-1251.
Bytebier B, Niet T van der, Bellstedt DU, Linder HP. 2008. The phylogenetic position of the enigmatic orchid genus Pachites. – South Afr. J. Bot. 74: 306-312.
Cadavid I, Gato A, Callej MJ. 1985. Chrysophanol and nepodin constituents of Simethis bicolor. – Intern. J. Crude Drug Res. 23: 13-15.
Cai J, Liu X, Vanneste K, Proost S, Tsai W-C, Liu K-W, Chen L-J, He Y, Xu Q, Bian C, Zheng Z, Sun F, Liu W, Hsiao Y-Y, Pan Z-J, Hsu C-C, Yang Y-P, Hsu Y-C, Chuang Y-C, Dievart A, Dufayard J-F, Xu X, Wang J-Y, Wang J, Xiao X-J, Zhao X-M, Du R, Zhang G-Q, Wang M, Su Y-Y, Xie G-C, Liu G-H, Li L-Q, Huang L-Q, Luo Y-B, chen H-H. Van de Peer Y, Liu Z-J. 2015. The genome sequence of the orchid Phalaenopsis equestris. – Nat. Genet. 47: 65-72.
Cameron KM. 2004. Utility of plastid psaB gene sequences for investigating intrafamilial relationships within Orchidaceae. – Mol. Phylogen. Evol. 31: 1157-1180.
Cameron KM. 2005a. Leave it to the leaves: a molecular phylogenetic study of Malaxideae (Epidendroideae, Orchidaceae). – Amer. J. Bot. 92: 1025-1032.
Cameron KM. 2005b. Molecular systematics of Orchidaceae: a literature review and an example using five plastid genes. – In: Nair H, Arditti J (eds), Proceedings of the Seventeenth World Orchid Conference: “Sustaining orchids for the future”, Kota Kinabalu, Natural History Publ., Borneo, pp. 80-96.
Cameron KM. 2006. A comparison and combination of plastid atpB and rbcL gene sequences for inferring phylogenetic relationships within Orchidaceae. – In: Columbus JT, Friar EA, Porter JM, Prince LM, Simpson MG (eds), Monocots: comparative biology and evolution. Excluding Poales, Rancho Santa Ana Botanical Garden, Claremont, California. – Aliso 22: 447-464.
Cameron KM. 2007. Molecular phylogenetics of Orchidaceae: first decade of DNA sequencing. – In: Cameron KM, Arditti J, Kull T (eds), Orchid biology. Reviews and perspectives, IX, New York Botanical Garden, New York, pp. 163-200.
Cameron KM. 2009. On the value of nuclear and mitochondrial gene sequences for reconstructing the phylogeny of vanilloid orchids. – Ann. Bot. 104: 377-385.
Cameron KM, Chase MW. 1998. Seed morphology of the vanilloid orchids. – Lindleyana 13: 148-169.
Cameron KM, Chase MW. 1999. Phylogenetic relationships of Pogoniinae (Vanilloideae, Orchidaceae): an herbaceous example of the eastern North American-eastern Asia phytogeographic disjunction. – J. Plant Res. 112: 317-329.
Cameron KM, Chase MW. 2000. Nuclear 18S rDNA sequences of Orchidaceae confirm the subfamilial status and circumscription of Vanilloideae. – In: Wilson KL, Morrison DA (eds), Monocots: systematics and evolution, CSIRO Publ., Melbourne, pp. 457-464.
Cameron KM, Dickison WC. 1998. Foliar architecture of vanilloid orchids: insights into the evolution of reticulate leaf venation in monocots. – Bot. J. Linn. Soc. 128: 45-70.
Cameron KM, Molina MC. 2006. Photosystem II gene sequences of psbB and psbC clarify the phylogenetic position of Vanilla (Orchidaceae). – Cladistics 22: 239-248.
Cameron KM, Chase MW, Whitten WM, Kores PJ, Jarrell DC, Albert VA, Yukawa,T, Hills HG, Goldman DH. 1999. A phylogenetic analysis of the Orchidaceae: evidence from rbcL nucleotide sequences. – Amer. J. Bot. 86: 208-224.
Campos-Rocha A, Meerow AW, Lopes EFM, Semir J, Mayer JLS, Dutilh JHA. 2017. Eithea lagopaivae, a new critically endangered species in the previously monotypic genus Eithea Ravenna (Amaryllidaceae). – PhytoKeys 85: 45-58.
Capeder E. 1898. Beiträge zur Entwicklungsgeschichte einiger Orchideen. – Flora 85: 368-423.
Caporali E, Marziani G, Servettaz O, Spada A, Grünanger P. 2001. Molecular (RAPD) analysis of some taxa of the Ophrys bertolonii aggregate (Orchidaceae). – Israel J. Plant Sci. 49: 85-89.
Cárdenas M. 1973. The genus Haylockia in Bolivia. – Plant Life (Stanford) 29: 41-45.
Cardoso-Gustavson P, Davis AR, Bona C, Campbell LM, De Barros F. 2017. The rostellum, stigma, style and ovarian transmitting tissue in Pleurothallidinae (Orchidaceae: Epidendroideae). – Bot. J. Linn. Soc. 185: 393-412.
Carlquist SJ, Schneider EL. 2006. Origins and nature of vessels in monocotyledons 8. Orchidaceae. – Amer. J. Bot. 93: 963-971.
Carlquist SJ, Schneider EL. 2007. Origin and nature of vessels in monocotyledons 9. Sansevieria. – South Afr. J. Bot. 73: 196-203.
Carlsward BS, Stern WL. 2009. Vegetative anatomy and systematics of Triphorinae (Orchidaceae). – Bot. J. Linn. Soc. 159: 203-210.
Carlsward BS, Whitten WM, Williams NH. 2003. Molecular phylogenetics of leafless Angraecinae (Orchidaceae): reevaluation of generic concepts. – Intern. J. Plant Sci. 164: 43-51.
Carlsward BS, Whitten WM, Williams NH, Bytebier B. 2006. Molecular phylogenetics of Vandeae (Orchidaceae) and the evolution of leaflessness. – Amer. J. Bot. 93: 770-786.
Carlsward BS, Stern WL, Bytebier B. 2006. Comparative vegetative anatomy and systematics of the angraecoids (Vandeae, Orchidaceae) with an emphasis on the leafless habit. – Bot. J. Linn. Soc. 151: 165-218.
Carnevali G, Ramírez de Carnevali I. 1993. New or noteworthy orchids for the Venezuelan Flora IX: new taxa, new records, and nomenclatural changes, mainly from the Guayana Shield and northern Amazonas. – Novon 3: 102-125.
Carnevali G, Ramírez de Carnevali I. 1998. Notes on the orchid flora of the Cruz Carrillo National Park (Guaramacal), Venezuela. – Harvard Pap. Bot. 3: 239-252.
Carnevali G, Cetzal-Ix W, Balam R, Leopardi C, Romero-Gonalez A. 2013. A combined evidence phylogenetic re-circumscription and a taxonomic revision of Lophiarella (Orchidaceae: Oncidiinae). – Syst. Bot. 38: 46-63.
Carr CE. 1929. Some Malayan orchids. – Gard. Bull. Straits Settlem. (Singapore) 5(1-2): 1-41.
Carr CE. 1932a. The genus Taeniophyllum in the Malay Peninsula. – Gard. Bull. Straits Settlem. (Singapore) 7: 61-86.
Carr CE. 1932b. Some Malayan orchids 3. – Gard. Bull. Straits Settlem. (Singapore) 8: 1-55.
Carr GW. 2006. Dianella tenuissima (Hemerocallidaceae), a remarkable new species from the Blue Mountains, New South Wales, Australia. – Telopea 11: 300-306.
Carr GW. 2007. Taxonomy of Dianella and implications for conservation. – Australasian Plant Cons. 16: 11-13.
Carrillo-Reyes P, Avina RV, Ramirez-Delgadillo R. 2003. Agave rzedowskiana, a new species in subgenus Littaea (Agavaceae) from western Mexico. – Brittonia 55: 240-244.
Carter S. 1962. Revision of Walleria and Cyanastrum (Tecophilaeaceae). – Kew Bull. 16: 185-195.
Carter S. 1966. Tecophilaeaceae. – In: Milne-Redhead E, Polhill RM (eds), Flora of tropical East Africa, Crown Agents for Oversea Governments and Administrations, London, pp. 1-7.
Carter S. 1996. New Aloe taxa in the Flora Zambesiaca area. – Kew Bull. 51: 777-785.
Carter S, Cutler DF, Reynold T, Brandham PE. 1984. A multidisciplinary approach to a revision of the Aloe somaliensis complex (Liliaceae). – Kew Bull. 39: 611-633.
Carter S, Lavranos JJ, Newton LE, Walker CC. 2011. Aloes: the definitive guide. – Royal Botanic Gardens, Kew; University of Chicago Press, Chicago.
Castetter EF, Bell WH, Grove AR. 1938. The early utilization and distribution of agave in the American southwest. – Bull. Univ. New Mexico, Biol. Ser. pp. 1-92.
Catalano G. 1931. Sulla morfologia delle infiorescenze di Agave. – R. Inst. Bot. Palermo, Lavori 2: 99-107.
Cave MS. 1939. Macrosporogenesis in Leucocoryne ixioides Lindl. – Cytologia 9: 407-411.
Cave MS. 1948. Sporogenesis and embryo sac development of Hesperocallis and Leucocoryne in relation to their systematic position. – Amer. J. Bot. 35: 343-349.
Cave MS. 1952. Sporogenesis and gametogenesis in Odontostomum hartwegii. – Phytomorphology 2: 210-214.
Cave MS. 1955. Sporogenesis and the female gametophyte of Phormium tenax. – Phytomorphology 5: 247-253.
Cave MS. 1964. Cytological observations on some genera of the Agavaceae. – Madroño 17: 163-170.
Cave MS. 1970. Chromosomes of California Liliaceae. – Univ. Calif. Publ. Bot. 57: 1-48.
Cave MS. 1975. Embryological studies in Stypandra (Liliaceae). – Phytomorphology 25: 95-99.
Celep F, Koyuncu M, Fritsch RM, Kahraman A, Doğan M. 2012. Taxonomic importance of seed morphology in Allium (Amaryllidaceae). – Syst. Bot. 37: 893-912.
Cetzal-Ix W, Carnevali G, Noguera-Savelli E, Romero-González GA. 2013. What is Cohniella cebolleta? Recircumscription and new and reinstated species and combinations (Orchidaceae). – Syst. Bot. 38: 606-623.
Chakroun S, Hébant C. 1983. Developmental anatomy of Aphyllanthes monspeliensis, a herbaceous monocotyledon with secondary growth. – Plant Syst. Evol. 141: 231-241.
Chanda S, Ghosh K. 1976. Pollen morphology and its evolutionary significance in Xanthorrhoeaceae. – In: Ferguson JK, Muller J (eds), The evolutionary significance of the exine, Linn. Soc. Symposium, No. 1, Academic Press, London, New York, pp. 527-559.
Chang C-C, Lin H-C, Lin I-P, Chow T-Y, Chen H-H, Chen W-H, Cheng C-H, Lin C-Y, Liu S-M, Chang C-C, Chaw S-M. 2006. The chloroplast genome of Phalaenopsis aphrodite (Orchidaceae): comparative analysis of evolutionary rate with that of grasses and its phylogenetic implications. – Mol. Biol. Evol. 23: 279-291.
Chang H-J, Hsu C-C. 1974. A cytotaxonomical study on some Formosan Liliaceae. – Taiwania 19: 58-71.
Charanasri U, Kamemoto H. 1975. Additional chromosome numbers in Oncidium and allied genera. – Amer. Orchid Soc. Bull. 44: 686-691.
Chardard R. 1958. L’ultrastructure des grains de pollen d’Orchidacées. – Rev. Cytol. Biol. Vég. 19: 223-235.
Chardard R. 1962. Recherches sur les cellules-mères des microspores des Orchidées. – Rev. Cytol. Biol. Vég. 19: 223-235.
Chardard R. 1963. Contribution à l’étude cytotaxonomique des Orchidées. – Rev. Cytol. Biol. Vég. 26: 1-58.
Chardard R. 1969. Aspects infrastructuraux de la maturation des grains de pollen de quelques Orchidacées. – Rev. Cytol. Biol. Vég. 32: 67-100.
Chase MW. 1986a. Two new species in the genus Leochilus (Orchidaceae). – Syst. Bot. 11: 242-246.
Chase MW. 1986b. A reappraisal of the oncidioid orchids. – Syst. Bot. 11: 477-491.
Chase MW. 1986c. A monograph of Leochilus (Orchidaceae). – Syst. Bot. Monogr. 14.
Chase MW. 1987a. Systematic implications of pollinarium morphology in Oncidium Sw., Odontoglossum Kunth, and allied genera (Orchidaceae). – Lindleyana 2: 8-28.
Chase MW. 1987b. Obligate twig epiphytes in the Oncidiinae and other neotropical orchids. – Selbyana 10: 24-30.
Chase MW. 2003. The origin and biogeography of Orchidaceae. – In: Pridgeon AM, Cribb PJ, Chase MW, Rasmussen FN (eds), Genera orchidacearum 3. Orchidoideae (part 2), Vanilloideae, Oxford University Press, Oxford, pp. 1-5.
Chase MW. 2009. Subtribe Oncidiinae. – In: Pridgeon AM, Chase MW, Cribb PJ, Rasmussen FN (eds), Genera orchidacearum 5. Epidendroideae (part 2), Oxford University Press, Oxford, pp. 211-394.
Chase MW, Hills HG. 1992. Orchid phylogeny, flower sexuality, and fragrance-seeking bees. – Bioscience 42: 43-49.
Chase MW, Olmstead RG. 1988. Isozyme number in subtribe Oncidiinae (Orchidaceae): an evaluation of polyploidy. – Amer. J. Bot. 75: 1080-1085.
Chase MW, Palmer JD. 1988. Chloroplast DNA variation, geographical distribution, and morphological parallelism in subtribe Oncidiinae (Orchidaceae). – Amer. J. Bot. 75: 163-164.
Chase MW, Palmer JD. 1989. Chloroplast DNA systematics of lilioid monocots: resources, feasibility, and an example from the Orchidaceae. – Amer. J. Bot. 76: 1720-1730.
Chase MW, Palmer JD. 1992. Floral morphology and chromosome number in subtribe Oncidiinae (Orchidaceae): evolutionary insights from a phylogenetic analysis of chloroplast DNA restriction site variation. – In: Soltis PD, Soltis DE, Doyle JJ (eds.), Molecular systematics of plants, Chapman & Hall, pp. 324-334.
Chase MW, Palmer JD. 1997. Leapfrog radiation in floral and vegetative traits among twig epiphytes in the orchid subtribe Onchidiinae. – In: Givnish TJ, Sytsma KJ (eds), Molecular evolution and adaptive radiation, Cambridge University Press, Cambridge, pp. 331-352.
Chase MW, Pippen JS. 1988. Seed morphology in the Oncidiinae and related subtribes (Orchidaceae). – Syst. Bot. 13: 313-323.
Chase MW, Pippen JS. 1990. Seed morphology and phylogeny in subtribe Catasetinae (Orchidaceae). – Lindleyana 5: 126-133.
Chase MW, Whitten WM. 2011. Further taxonomic transfers in Oncidiinae (Orchidaceae). – Phytotaxa 20: 26-32.
Chase MW, Cameron KM, Hills HG, Jarrell D. 1994. DNA sequences and phylogenetics of the Orchidaceae and other lilioid monocots. – In: Pridgeon A (ed), Proceedings of the Fourteenth World Orchid Conference, Her Majesty’s Stationary Office, Glasgow, pp. 61-73.
Chase MW, Rudall PJ, Conran JG. 1996. New circumscriptions and a new family of asparagoid lilies: genera formerly included in the Anthericaceae and associated taxa. – Kew Bull. 51: 667-680.
Chase MW, Rudall PJ, Conran JG. 1997. Validation of the family name Boryaceae. – Kew Bull. 52: 416.
Chase MW, Bruijn AY de, Cox AV, Reeves G, Rudall PJ, Johnson MAJ, Eguiarte LE. 2000. Phylogenetics of Asphodelaceae (Asparagales): an analysis of plastid rbcL and trnL-F sequences. – Ann. Bot. 86: 935-956.
Chase MW, Rudall PJ, Fay MF, Stobart KL. 2000. Xeronemataceae, a new family of asparagoid lilies from New Caledonia and New Zealand. – Kew Bull. 55: 865-870.
Chase MW, Freudenstein JF, Cameron KM. 2003. DNA data and Orchidaceae systematics: a new phylogenetic classification. – In: Dixon KW, Pell SP, Barrett RL, Cribb PJ (eds), Orchid Conservation, Natural History Publ., Kota Kinabalu, Sabah, Malaysia, pp. 69-89.
Chase MW, Hanson L, Albert VA, Whitten WM, Williams NH. 2005. Life history evolution and genome size in subtribe Oncidiinae (Orchidaceae). – Ann. Bot. (Oxford) 95: 191-199.
Chase MW, Williams NH, Neubig KM, Whitten WM. 2008. Taxonomic transfers in Oncidiinae to accord with Genera Orchidacearum, vol. 5. – Lindleyana (in Orchids) 21: 20-31.
Chase MW, Williams NH, Faria AD de, Neubig KM, Amaral M do CE, Whitten WM. 2009. Floral convergence in Oncidiinae (Cymbidieae; Orchidaceae): an expanded conept of Gomesa and a new genus Nohawilliamsia. – Ann. Bot. 104: 387-402.
Chase MW, Reveal JW, Fay MF. 2009. A subfamilial classification for the expanded asparagalean families Amaryllidaceae, Asparagaceae and Xanthorrhoeaceae. – Bot. J. Linn. Soc. 161: 132-136.
Chase MW, Cameron KM, Freudenstein JV,
Pridgeon AM, Salazar G, Berg C van den Schuiteman A. 2015. An updated
classification of Orchidaceae. –
Bot. J. Linn. Soc. 177: 151-174.
Chaudhary SA. 1972a. A new species of Iris subgenus Oncocyclus. – Bot. Not. 125: 259-260.
Chaudhary SA. 1972b. Three new taxa of Iris subgenus Oncocyclus from Lebanon and Syria. – Bot. Not. 125: 497-500.
Chaudhary SA, Kirkwood G, Weymouth C. 1975. Iris subgenus Susiana in Lebanon and Syria. – Bot. Not. 128: 380-407.
Chaudhary SA, Chaudhary GA, Akram M. 1977. Karyotypes of some Iris taxa. – Bot. Not. 130: 263-267.
Chauhan KPS, Brandham PE. 1985. The significance of robertsonian fusion and monosomy in Cardiocrinum (Liliaceae). – Kew Bull. 40: 567-571.
Chauveau O, Eggers L, Raquin C, Silvério A, Brown S, Couloux A, Cruaud C, Kaltchuk-Santos E, Yockteng R, Souza-Chies TT, Nadot S. 2011. Evolution of oil-producing trichomes in Sisyrinchium (Iridaceae): insights from the first comprehensive phylogenetic analysis of the genus. – Ann. Bot. 107: 1287-1312.
Chauveau O, Eggers L, Souza-Chies TT, Nadot S. 2012. Oil-producing flowers within the Iridoideae (Iridaceae): evolutionary trends in the flowers of the New World genera. – Ann. Bot. 110: 713-729.
Cheadle VI. 1963. Vessels in Iridaceae. – Phytomorphology 13: 245-248.
Cheadle VI. 1969. Vessels in Amaryllidaceae and Tecophilaeaceae. – Phytomorphology 19: 8-16.
Cheadle VI. 1970. Vessels in Pontederiaceae, Ruscaceae, Smilacaceae, and Trilliaceae. – In: Robson NKB, Cutler DF, Gregory M (eds), New research in plant anatomy, Bot. J. Linn. Soc. 63 [Suppl. 1]: 45-50.
Cheadle VI, Kosakai H. 1971. Vessels in Liliaceae. – Phytomorphology 21: 320-333.
Cheadle VI, Kosakai H. 1982. The occurrence and kinds of vessels in the Orchidaceae. – Phyta (India), Studies on living and fossil plants, Plant Commemoration Vol., 1982: 45-57.
Chemisquy MA, Morrone O. 2012. Molecular phylogeny of Gavilea (Chloraeinae: Orchidaceae) using plastid and nuclear markers. – Mol. Phylogen. Evol. 62: 889-897.
Chen S-C. 1965. A primitive new orchid genus Tangtsinia and its meaning in phylogeny. – Acta Phytotaxon. Sin. 10: 193-206.
Chen S-C. 1982. The origin and early differentiation of the Orchidaceae. – Acta Phytotaxon. Sin. 20: 1-22.
Cheadle VI. 1970. Vessels in Pontederiaceae, Ruscaceae, Smilacaceae, and Trilliaceae. – In: Robson NKB, Cutler DF, Gregory M (eds), New research in plant anatomy, Bot. J. Linn. Soc. 63 [Suppl. 1]: 45-50.
Chen S-C. 1979. On Diplandrorchis, a very primitive and phylogenetically significant new genus of Orchidaceae. – Acta Phytotaxon. Sin. 17: 1-6.
Chen S-C. 1980. The orchid genus Panisea Lindl. in China. – Acta Bot. Yunnan. 2: 300-305.
Chen S-C. 1982. The origin and early differentiation of the Orchidaceae. – Acta Phytotaxon. Sin. 20: 1-22.
Chen S-C, Tang T. 1982. A general review of the orchid flora of China. – In: Arditti J (ed), Orchid biology reviews and perspectives II, Cornell University Press, Ithaca, New York, pp. 39-81.
Chen S-C, Tsi Z-H. 1984. On Paphiopedilum malipoense sp. nov. – an intermediate form between Paphiopedilum and Cypripedium. – Acta Phytotaxon. Sin. 22: 119-124.
Chen S-C, Liu Z-J, Zhu G-H, Lang K-Y, Li Z-H, Luo Y-B,Jin X-H, Cribb PH, Wood JJ, Gale SW, Ormerod P, Vermeulen JJ, Wood HP, Clayton D, Bell A. 2009. Orchidaceae. – In: Wu Z-Y, Raven PH, Hong DY (eds), Flora of China 25, Science Press, Beijing; Missouri Botanical Garden Press, St. Louis, Missouri, pp. 174-195.
Chen S-C, Kim D-K, Chase MW, Kim JH. 2013. Networkds in a large-scale phylogenetic analysis. reconstructing evolutionary history of Asparagales (Lilianae) based on four plastid genes. – PLoS ONE 8: 1-18.
Chen W, Shui Y. 2005. A new species of Thrixspermum (Orchidaceae) from China. – Brittonia 57: 55-58.
Chen Z-K, Wang F-H, Zhou F. 1988a. On the origin, development and ultrastructure of the orbicules and pollenkitt in the tapetum of Anemarrhena asphodeloides (Liliaceae). – Grana 27: 273-282.
Chen Z-K, Wang F-H, Zhou F. 1988b. The ultrastructural aspect of tapetum and Ubisch bodies in Anemarrhena asphodeloides. – Acta Bot. Sin. 30: 1-15. [In Chinese]
Chen Z-K, Zhou F, Wang F-X, Wang F-H. 1988. Investigation on the development of male gametophyte in Anemarrhena asphodeloides. – Acta Bot. Sin. 30: 569-573. [In Chinese]
Chen Z-K, Wang F-H, Li Z-H. 1990. Investigation on embryology of Anemarrhena asphodeloides. – Acta Phytotaxon. Sin. 28: 223-227. [In Chinese]
Chichiricco G. 1999. Developmental stages of the pollen wall and tapetum in some Crocus species. – Grana 38: 31-41.
Chiron GR. 2007a. Phylogenetic study of the genus Baptistonia (Orchidaceae, Oncidiinae) sensu lato, based on morphological characters. – J. Bot. Res. Inst. Texas 1: 913-931.
Chiron GR, Castro Neto VP. 2004. Contribution à la connaissance des orchidées du Brésil III. Rétablissement du genre Baptistonia Barbosa Rodrigues. – Richardiana 4: 109-120.
Chiron GR, Guiard J. 2008. Anatomie foliaire du genre Baptistonia Barb. Rodr. (Orchidaceae, Oncidiinae). – Candollea 63: 101-113.
Chiron GR, Oliveira RP, Santos TM, Bellvert F, Bertrand C, Berg C. 2009. Phylogeny and evolution of Baptistonia (Orchidaceae, Oncidiinae) based on molecular analyses, morphology and floral oil evidences. – Plant Syst. Evol. 281: 35-49.
Chiron GR, Guiard J, Berg C van den. 2012. Phylogenetic relationships in Brazilian Pleurothallis sensu lato (Pleurothallidinae, Orchidaceae): evidence from nuclear ITS rDNA sequences. – Phytotaxa 46: 34-58.
Chochai A, Leitch IJ, Ingrouille MJ, Fay MF. 2012. Molecular phylogenetics of Paphiopedilum (Cypripedioideae; Orchidaceae) based on nuclear ribosomal ITS and plastid sequences. – Bot. J. Linn. Soc. 170: 176-196.
Chodat R, Balicka-Iwanowska G. 1892. La feuille des Iridées, essai d’anatomie systematique. – J. Bot. 6: 220-232, 253-267.
Choi HJ, Oh BU. 2011. A partial revision of Allium (Amaryllidaceae) in Korea and north-eastern China. – Bot. J. Linn. Soc. 167: 153-211.
Choi HJ, Davis AR, Cota-Sánchez JH. 2011. Comparative floral structure of four New World Allium (Amaryllidaceae) species. – Syst. Bot. 36: 870-882.
Choi HJ, Giussani LM, Jang CG, Oh BU, Cota-Sánchez JH. 2012. Systematics of disjunct northeastern Asian and northern North American Allium (Amaryllidacee). – Botany 90: 491-508.
Choob VV. 1999. Phantom leaves: a new look to the old problem of branching in Galanthus (Amaryllidaceae). – Syst. Geogr. Plants 68: 67-72.
Choob VV. 2001. Patterns of flower and inflorescence architecture in Crocus L. (Iridaceae). – Ann. Bot. (Roma), n. s., 1: 91-104.
Chouard P. 1926. Révision de quelques genres et sous-genres de Liliacées bulbeuses d’après de développement de l’appareil végétatif (Scilla, Endymion, Hyacinthus). – Bull. Mus. Natl. Hist. Nat. Paris, sér. II, 2: 698-706.
Chouard P. 1931. Types de développement de l’appareil végétatif chez les Scillées. – Ann. Sci. Nat. Bot. X, 13: 131-323.
Christenson EA. 1986. An historical review of Saccolabium (Orchidaceae) and excluded species. – Kew Bull. 41: 833-853.
Christenson EA. 1999. Proposal to conserve the name Anneliesia against Gynizodon (Orchidaceae). – Taxon 48: 589.
Christenson EA. 2006. Brevilongium, un nouveau genre neotropical (Oncidiinae). – Richardiana 6: 45-49.
Chukr NS, Giulietti AM. 2003. Revisão de Pseudotrimezia Foster (Iridaceae). – Sitientibus Ci. Biol. 3: 44-80.
Chukr NS, Giulietti AM. 2008. Revisão de Trimezia Salisb. ex Herb. (Iridaceae) para o Brasil. – Sitientibus Ci. Biol. 8: 15-58.
Chung M-G, Jones SB Jr. 1989. Pollen morphology of Hosta Tratt. (Funkiaceae) and related genera. – Bull. Torrey Bot. Club 116: 31-44.
Chupov VS, Kutiavina NG. 1980. Phylogeny of some groups of Liliales based on the data of serological analysis. – In: Zhilin SG (ed), Systematics and evolution of higher plants, Nauka, Leningrad, pp. 101-110. [In Russian]
Chupov VS, Kutiavina NG. 1981a. Serological studies in the order Liliales. – Bot. Žurn. 66: 75-81. [In Russian]
Chupov VS, Kutiavina NG. 1981b. Serological studies in the order Liliales II. – Bot. Žurn. 66: 408-416. [In Russian]
Ciesacutelicka D. 2006. The problem of infrageneric classification of Eulophia R. Br. ex Lindl. (Orchidaceae, Cymbidiinae). – Biodivers. Res. Conserv. 3-4: 210-212.
Cingel NA van der. 1995. An atlas of orchid pollination: European orchids. – A. A. Balkema, Rotterdam.
Cingel NA van der. 2001. An atlas of orchid pollination: America, Africa, Asia and Australia. – A. A. Balkema, Rotterdam.
Cisternas MA, Araneda L, García N, Baeza CM. 2010. Karyotypic studies in the Chilean genus Placea (Amaryllidaceae). – Gayana. Bot. 67: 198-205.
Cisternas MA, Salazar GA, Verdugo G, Novoa P, Calderón X, Negritto MA. 2012. Phylogenetic analysis of Chloraeinae (Orchidaceae) based on plastid and nuclear DNA sequences. – Bot. J. Linn. Soc. 168: 258-277.
Clary KH. 2001. The genus Hesperoyucca (Agavaceae) in the western United States and Mexico: new nomenclatural combinations. – Sida 19: 839-847.
Clary KH, Simpson BB. 1995. Systematics and character evolution in the genus Yucca (Agavaceae): evidence from morphology and molecular analyses. – Bol. Soc. Bot. Méx. 56: 77-88.
Clausen RT. 1940. A review of Cyanastraceae. – Gentes Herb. 4: 293-304.
Clements MA. 1988. Orchid mycorrhizal associations. – Lindleyana 3: 73-86.
Clements MA. 1989. Catalogue of Australian Orchidaceae. – Aust. Orchid Res. 1: 1-160.
Clements MA. 1995. Reproductive biology in relation to phylogeny of the Orchidaceae, especially the tribe Diurideae. – Ph.D. diss., Australian National University, Canberra.
Clements MA. 1999. Embryology. – In: Pridgeon AM, Cribb PJ, Chase MW, Rasmussen FN (eds), Genera Orchidacearum 1, Oxford University Press, Oxford.
Clements MA. 2003. Molecular phylogenetic systematics in the Dendrobiinae (Orchidaceae), with emphasis on Dendrobium section Pedilonum. – Telopea 10: 247-298.
Clements MA. 2006. Molecular phylogenetic systematics in Dendrobieae (Orchidaceae). – In: Columbus JT, Friar EA, Porter JM, Prince LM, Simpson MG (eds), Monocots: comparative biology and evolution. Excluding Poales, Rancho Santa Ana Botanical Garden, Claremont, California. – Aliso 22: 465-480.
Clements MA, Jones DL. 1997. Cannaeorchis, a new genus of Dendrobiinae (Orchidaceae) for the taxon previously known as Dendrobium Sw. sect. Macrocladium Schltr. – Lasianthera 1: 132-147.
Clements MA, Jones DL, Sharma IK, Nightingale ME, Garratt MJ, Fitzgerald KJ, Mackenzie KM, Molloy BPJ. 2002. Phylogenetics of Diurideae (Orchidaceae) based on the internal transcribed spacer (ITS) regions of nuclear ribosomal DNA. – Lindleyana 17: 135-171.
Clements MA, Otero JT, Miller JT. 2011. Phylogenetic relationships in Pterostylidinae (Cranichideae: Orchidaceae): combined evidence from nuclear ribosomal and plastid DNA sequences. – Aust. J. Bot. 59: 99-117.
Clements MA, Howard CG, Miller JT. 2015. Caladenia revisited: results of molecular phylogenetic analyses of Caladeniinae plastid and nuclear loci. – Amer. J. Bot. 102: 581-597.
Clifford HT. 1998a. Doryanthaceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 236-238.
Clifford HT. 1998b. Xanthorrhoeaceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 467-470.
Clifford HT, Conran JG. 1998a. Blandfordiaceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 148-150.
Clifford HT, Conran JG. 1998b. Johnsoniaceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 336-340.
Clifford HT, Lavarack PS. 1974. The role of vegetative and reproductive attributes in the classification of the Orchidaceae. – Bot. J. Linn. Soc. 6: 97-110.
Clifford HT, Conran JG, Henderson RJF, Hewson HJ, Keighery GJ, Williams JB et al. 1987. Liliaceae. – In: George AS (ed), Flora of Australia 45, Australian Government Publ. Service, Canberra, pp. 148-419, 466-496.
Clifford HT, Henderson RJF, Conran JG. 1998. Hemerocallidaceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 245-253.
Coates F, Walsh NG, James EA. 2002. Threats to the survival of the Grampians pincushion lily (Borya mirabilis, Liliaceae) – a short-range endemic from western Victoria. – Aust. Syst. Bot. 15: 477-485.
Cocucci AA, Jensen WA. 1969. Orchid embryology: pollen tetrads of Epidendrum scutella in the anther and on the stigma. – Planta 84: 215-229.
Cocucci AA, Vogel S. 2003. Oil-producing flowers of Sisyrinchium species (Iridaceae) and their pollinators in southern South America. – Flora 196: 26-46.
Cocucci AE. 1969. El género Camassia Lindl. (Liliaceae) en Sudamérica. – Kurtziana 5: 181-190.
Cocucci AE. 1973. Orchid embryology: the membrane systems and the pollen-tube growth. – Caryologia 25 [Suppl.]: 201-206.
Codd LE. 1967. The status of the genus Notosceptrum Benth. (Liliaceae). – Bot. Not. 120: 41-45.
Codd LE. 1968. The South African species of Kniphofia. – Bothalia 9: 363-513.
Coetzee J, Schijff HP van der. 1969. Secondary thickening in geophytic Liliaceae. – J. South Afr. Bot. 35: 99-108.
Colasante M, Rudall PJ (eds). 2002. Irises and Iridaceae: biodiversity and systematics. – Ann. Bot. (Roma), n. s., 1: 1-209.
Colegate SM, Dorling PR, Huxtable CR, Skelton BW, White AH. 1985. Stypandrol, a toxic binaphthalenetetrol isolated from Stypandra imbricata. – Aust. J. Chem. 38: 1233-1241.
Colegate SM, Dorling PR, Huxtable CR. 1987. Stypandrone: a toxic naphthalene-1,4-quinone from Stypandra imbricata and Dianella revoluta. – Phytochemistry 26: 979-982.
Comber JB. 1984. Bulbophyllum Section Cirrhopetalum in Java. – Orch. Dig. 48: 77-82.
Comber JB. 1990. Orchids of Java. – Bentham-Moxon Trust, Royal Botanic Gardens, Kew.
Conner AJ, Conner LN. 1988. Germination and dormancy of Arthropodium cirratum seeds. – New Zealand J. Bot. 15: 3-10.
Conover MH. 1983. The vegetative anatomy of the reticulate-veined Liliiflorae. – Telopea 2: 401-412.
Conover MH. 1991. Epidermal patterns of the reticulate-veined Liliiflorae and their parallel-veined allies. – Bot. J. Linn. Soc. 107: 295-312.
Conran JG. 1987. Variation in Eustrephus R. Br. ex Ker Gawler and Geitonoplesium Cunn. ex. R. Br. (Asparagales: Luzuriagaceae). – Muelleria 6: 363-369.
Conran JG. 1989. Cladistic analyses of some net-veined Liliiflorae. – Plant Syst. Evol. 168: 123-141.
Conran JG. 1994. The Geitonoplesiaceae Dahlgren ex Conran (Liliiflorae: Asparagales): a new family of monocotyledons. – Telopea 6: 39-41.
Conran JG. 1995. Family distributions in the Liliiflorae and their biogeographical implications. – J. Biogeogr. 22: 1023-1034.
Conran JG. 1997a. Paracordyline kerguelensis, an Oligocene monocotyledon macrofossil from the Kerguélen Islands. – Alcheringa 21: 129-140.
Conran JG. 1997b. A preliminary investigation of leaf venation and cuticle features to characterise taxa within Cordyline Comm. ex R. Br.. (Agavaceae s.l.). – In: Dransfield J, Coode MJE, Simpson DA (eds), Plant diversity in Malesia III, Royal Botanic Gardens, Kew, pp. 71-89.
Conran JG. 1998a. Anthericaceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 114-121.
Conran JG. 1998b. Aphyllanthaceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 122-124.
Conran JG. 1998c. Behniaceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 146-148.
Conran JG. 1998d. Boryaceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 151-154.
Conran JG. 1998e. Herreriaceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 253-255.
Conran JG. 1998f. Lomandraceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 354-365.
Conran JG. 1999. Anatomy and morphology of Behnia (Behniaceae) and its relationships within Lilianae: Asparagales. – Bot. J. Linn. Soc. 131: 115-129.
Conran JG, Christophel DC. 1998. Paracordyline aureonemoralis: an Eocene monocotyledon macrofossil from Adelaide, South Australia. – Alcheringa 22: 351-359.
Conran JG, Clifford HT. 1986b. Smilacaceae. – In: George AS (ed), Flora of Australia 46, Australian Government Publ. Service, Canberra, pp. 180-196.
Conran JG, Rudall PJ. 1998. Anemarrhenaceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 111-114.
Conran JG, Tamura MN. 1998. Convallariaceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 186-198.
Conran JG, Temby A. 2000. Embryology and affinities of the Boryaceae (Asparagales). – In: Wilson KL, Morrison DA (eds), Monocots: systematics and evolution, CSIRO Publ., Melbourne, pp. 401-406.
Conran JG, Christophel DC, Scriven LJ. 1994. Petermanniopsis angleseaënsis: an Australian fossil net-veined monocotyledon from Eocene Victoria. – Intern. J. Plant Sci. 155: 816-827.
Conran JG., Chase MW, Rudall PJ. 1997. Two new monocotyledon families: Anemarrhenaceae and Behniaceae (Lilianae: Asparagales). – Kew Bull. 52: 995-999.
Conran JG, Christophel DC, Cunningham LK. 2003. An Eocene fossil monocotyledon from Nelly Creek, Central Australia with affinities to Hemerocallidaceae (Lilianae: Asparagales). – Alcheringa 27: 107-116.
Conran JG, Forster PI, Donnon M. 2008. Romnalda ophiopogonoides (Asparagales: Laxmanniaceae), a new and endangered species from the wet tropics bioregion of north-east Queensland. – Telopea 12: 167-178.
Conran JG, Bannister JM, Lee DE. 2009. Earliest orchid macrofossils: Early Miocene Dendrobium and Earina (Orchidaceae: Epidendroideae) from New Zealand. – Amer. J. Bot. 96: 466-474.
Cooke DA. 1986. Iridaceae. – In: George AS (ed), Flora of Australia 46, Australian Government Publ. Service, Canberra, pp. 1-66.
Cooney-Sovetts C, Sattler R. 1986. Phylloclade development in the Asparagaceae: an example of homoeosis. – Bot. J. Linn. Soc. 94: 327-371.
Couderc H, Gorenflot R, Moret J, Siami A. 1984. Variation chromosomique et biosystématique chez plusieurs espèces d’Ornithogalum L. – Webbia 38: 671-679.
Cowley J, Özhatay N, Mathew B. 1994. New species of Alliaceae & Hyacinthaceae from Turkey. – Kew Bull. 49: 481-489.
Cox AV, Pridgeon AM, Albert VA, Chase MW. 1997. Phylogenetics of the slipper orchids (Cypripedioideae, Orchidaceae): nuclear rDNA ITS sequences. – Plant Syst. Evol. 208: 197-223.
Cozzolini S, Widmer A. 2005. Orchid diversity: an evolutionary consequence of deception? – Trends Ecol. Evol. 20: 487-495.
Craig JL. 1989. Seed set in Phormium: interactive effects of pollinator behaviour, pollen carryover and pollen source. – Oecologia 81: 1-5.
Craig JL, Stewart AM. 1988. Reproductive biology of Phormium tenax: a honeyeater-pollinated species. – New Zealand J. Bot. 26: 453-464.
Cranwell LM. 1933. A new locality for Xeronema callistemon. – New Zealand J. Sci. Technol. 34: 234-236.
Cribb PJ. 1977. New orchids from South Central Africa. – Kew Bull. 32: 137-187.
Cribb PJ. 1980. New or little-known orchids from East Africa. – Kew Bull. 34: 321-340.
Cribb PJ. 1983a. Dendrobium sect. Ceratobium (Orchidaceae) in the Pacific Islands. – Kew Bull. 37: 577-590.
Cribb PJ. 1983b. A revision of Dendrobium sect. Latouria (Orchidaceae). – Kew Bull. 38: 229-306.
Cribb PJ. 1984. Orchidaceae (Part 2). – In: Polhill RM (ed), Flora of tropical East Africa, A. A. Balkema, Rotterdam, pp. 1-411.
Cribb PJ. 1986. A revision of Dendrobium sect. Spatulata (Orchidaceae). – Kew Bull. 41: 615-692.
Cribb PJ. 1987a. The genus Paphiopedilum. – Collingridge, London.
Cribb PJ. 1987b. The genus Eulophia in Africa. The world orchid Hiroshima symposium. – Excutive Committee of the World Orchid Hiroshima Symposium, Hiroshima, pp. 97-103.
Cribb PJ. 1989. Orchidaceae (Part 3). – In: Polhill RM (ed), Flora of tropical East Africa, A. A. Balkema, Rotterdam, pp. 1-651.
Cribb PJ. 1995. New species of Dendrobium and Bulbophyllum (Orchidaceae) from the Pacific Islands. – Kew Bull. 50: 785-789.
Cribb PJ. 1996. New species and records of Orchidaceae from West Africa. – Kew Bull. 51: 353-363.
Cribb PJ. 1997. The genus Cypripedium. – Timber Press, Portland, Oregon.
Cribb PJ. 2004. Five new taxa of Tridactyle (Orchidaceae) from West Central Africa. – Kew Bull. 59: 195-205.
Cribb PJ. 2005. Xerorchideae, a new tribe in Orchidaceae subfamily Epidendroideae. – Kew Bull. 60: 143-144.
Cribb PJ, Chase MA. 2001. Proposal to conserve the name Dactylorhiza Necker ex Nevski over Coeloglossum Hartm. (Orchidaceae). – Taxon 50: 581-582.
Cribb PJ, Fay JM. 1987. Orchids of the Central African Republic – a provisional check-list. – Kew Bull. 42: 711-737.
Cribb PJ, Hermans J. 2009. Field guide to the orchids of Madagascar. – Kew Publ., London.
Cribb PJ, Kores PJ. 2000. The systematic position of Codonorchis (Orchidaceae: Orchidoideae). – Lindleyana 15: 169-170.
Cribb PJ, Kurzweil H. 1994. The genus Corycium (Orchidaceae) newly reported for Tanzania. – Kew Bull. 49: 555-557.
Cribb PJ, la Croix I. 1996. New Polystachya species (Orchidaceae) from tropical West and South-Central Africa. – Kew Bull. 51: 571-577.
Cribb PJ, la Croix I. 1997a. A synopsis of the genus Cribbia Senghas (Orchidaceae), with two new species from São Tomé. – Kew Bull. 52: 743-748.
Cribb PJ, la Croix I. 1997b. Two new orchids from the Flora Zambesiaca area. – Kew Bull. 52: 1001-1004.
Cribb PJ, Mathew B. 1998. The genus Paphiopedilum. 2nd ed. A Botanical Magazine Monograph. – Natural History Publ., Borneo, The Royal Botanic Gardens, Kew.
Cribb PJ, Ormerod P. 2000. Notes on Orchidaceae from the Pacific Islands. – Kew Bull. 55: 231-236.
Cribb PJ, Pollard BJ. 2002. New orchid discoveries in Western Cameroon. – Kew Bull. 57: 653-659.
Cribb PJ, Stewart J. 1985. Additions to the orchid flora of tropical Africa. – Kew Bull. 40: 399-419.
Cribb PJ, Tang CZ. 1982. Spathoglottis (Orchidaceae) in Australia and athe Pacific Islands. – Kew Bull. 36: 721-729.
Cribb PJ, Whistler WA. 1996. Orchids of Samoa. – The Royal Botanic Gardens, Kew.
Cribb PJ, Whistler WA. 2011. The orchids of Tonga, Niue, and the Cook islands. – Lankesteriana 11: 96-177.
Cribb PJ, Laan FM van der, Arends JC. 1989. Two new species of Orchidaceae from West Africa. – Kew Bull. 44: 479-483.
Cribb PJ, Hermans J, Roberts DL. 2007. Erasanthe (Orchidaceae, Epidendroideae, Vandeae, Aerangidinae), a new endemic orchid genus from Madagascar. – Adansonia, sér. III, 29: 27-30.
Crosa O. 1972. Estudios cariología en el género Nothoscordum (Liliaceae). – Bol. Fac. Agron. Uruguay 122: 3-8.
Crosa O. 1975a. Zoellnerallium, un género nuevo para la tribu Allieae (Liliaceae). – Darwiniana 19: 331-334.
Crosa O. 1975b. Las especies unifloras del género Nothoscordum Kunth y el género Ipheion Rafinesque de la tribu Allieae (Liliaceae). – Darwiniana 19: 335-344.
Crosa O. 1981. Los chromosomas de cinco especies del género Tristagma (Liliaceae). – Darwiniana 23: 361-366.
Crosa O. 1988. Los cromosomas de nueve especies del genero chileno Leucocoryne Lindley (Allieae-Alliaceae). – Bol. Fac. Agron. Uruguay 17: 1-12.
Crosa O. 2004. Segunda especie y justificación del género Zoellnerallium (Alliaceae). – Darwiniana 42: 165-168.
Crosa O. 2005. Una nueva especie uniflora de Nothoscordum secc. Nothoscordum (Alliaceae). – Hickenia 3: 253-256.
Crosa O. 2006. Nothoscordum izaguirreae, nueva especie uniflora de Alliaceae de Uruguay. – Hickenia 1: 271-275.
Cruden RW. 1968. Three new species of Tigridia (Iridaceae) from México. – Brittonia 20: 314-320.
Cruden RW. 1971. The systematics of Rigidella (Iridaceae). – Brittonia 23: 217-225.
Cruden RW. 1975. New Tigridieae (Iridaceae) from Mexico. – Brittonia 27: 103-109.
Cruden RW. 1981. New Echeandia (Liliaceae) from Mexico. – Sida 9: 139-146.
Cruden RW. 1987. Hagenbachia, a misplaced genus of New World Liliaceae. – Nord. J. Bot. 7: 255-260.
Cruden RW. 2009. A synopsis of South American Echeandia (Anthericaceae). – Ann. Missouri Bot. Gard. 96: 251-267.
Cruden RW, Maas PJM, Maas-van de Kamer H. 1991. Hagenbachia (Liliaceae): reexamination of its familial placement, its authors, the lectotype, and other collections. – Taxon 40: 445-452.
Cruz C, Castillo L, Robert M, Ondarza RN (eds). 1985. Biología y aprovechamiento integral del henequen y otros agaves. – Centro de Investigación Científica de Yucatán, A. C.
Cullen J, Ratter JA. 1967. Taxonomic and cytological notes on Turkish Ornithogalum. – Notes Roy. Bot. Gard. Edinb. 27: 293-339.
Čupov VS. 1987. On the genera Geitonoplesium and Simethis: their position in the system. – Bot. Žurn. 72: 904-908. [In Russian]
Čupov VS. 1994. Phylogeny and systematics of the Liliales and Asparagales. – Bot. Žurn. 79: 1-12. [In Russian with English summary]
Čupov VS. 1995. Kompensirujuscij vozvrat priznakov v primitivnoe sostojanie. – Bot. Žurn. 80: 1-7.
Čupov VS, Kutjavina NG. 1978. The comparative immunoelectrophoretic investigation of seed proteins of Liliaceae. – Bot. Žurn. 63: 473-493. [In Russian]
Čupov VS, Kutjavina NG. 1980. Phylogeny of some groups of Liliales based on the data of serological analysis. – In: Žilin SG (ed), Systematics and evolution of higher plants, Nauka, Leningrad, pp. 101-110. [In Russian]
Čupov VS, Kutjavina NG. 1981a. Serological studies in the order Liliales I. – Bot. Žurn. 66: 75-81. [In Russian]
Čupov VS, Kutjavina NG. 1981b. Serological studies in the order Liliales II. – Bot. Žurn. 66: 408-416. [In Russian]
Currah RS, Zelmer CD, Hambleton S, Richardson KA. 1997. Fungi from orchid mycorrhizas. – In: Arditti J, Pridgeon AM (eds), Orchid biology: reviews and perspectives VII, Kluwer, Dordrecht, pp. 117-170.
Cutler DF. 1972. Leaf anatomy of certain Aloë and Gasteria species and their hybrids. – In: Ghouse AKM, Yunus M (eds), Research trends in plant anatomy – A. K. Chowdhury Commemoration Volume, Tata McGraw-Hill, New Delhi, pp. 103-122.
Cutler DF. 1979. Leaf surface studies in Aloë and Haworthia species (Liliaceae): taxonomic implications. – Trop. Subtrop. Pflanzenwelt 28: 447-471.
Cutler DF. 1982. Cuticular structure and habitat in certain Aloë species (Liliaceae) from southern Africa. – In: Cutler DF, Alvin KL, Price CE (eds), The plant cuticle, Academic Press, London, pp. 425-444.
Cutler DF. 1985. Haworthia spp. (Liliaceae) with window leaves: SEM studies of leaf surface adaptations. – Parodiana 3: 203-223.
Cutler DF. 1992. Vegetative anatomy of Ophiopogoneae (Convallariaceae). – Bot. J. Linn. Soc. 110: 385-419.
Cutler DF, Gregory M. 1983. Current anatomical research in Liliaceae, Amaryllidaceae and Iridaceae. – Telopea 2: 425-452.
Cutler DF, Furness CA, Chase MW, Fay MF. 1997. Microsporogenesis and pollen sulcus type in Asparagales (Lilianae). – Can. J. Bot. 75: 408-430.
Dafni A, Bernhardt P. 1990. Pollination of terrestrial orchids of southern Australia and the Mediterranean region. – In: Hecht MK, Wallace B, Macyntire RJ (eds), Evol. Biol. 24: 193-252.
Dafni A, Werker E. 1982. Pollination ecology of Sternbergia clusiana (Ker-Gawler) Spreng. (Amaryllidaceae). – New Phytol. 91: 571-577.
Dafni A, Ivri Y, Brantjes NBM. 1981. Pollination of Serapias vomeracea Briq. (Orchidaceae) by imitation of holes for sleeping solitary male bees (Hymenoptera). – Acta Bot. Neerl. 30: 60-73.
Dagne E, Yenesew A. 1994. Anthraquinones and the chemotaxonomy of the Asphodelaceae. – Pure Appl. Chem. 66: 2395-2398.
Dagne E, Yenesew A, Asmellash S, Sebsebe D, Mavi S. 1994. The distribution of some anthraquinones, preanthraquinones and isoleutherol in the roots of some Aloe species. – Phytochemistry 35: 401-406.
Dagne E, Wyk B-E van, Mueller M, Steglich W. 1996. Three dihydroanthracenones from Gasteria bicolor. – Phytochemistry 41: 795-800.
Dagne E, Bisrat D, Viljoen A, Wyk B-E van. 2000. Chemistry of Aloe species. – Curr. Org. Chem. 4: 1055-1078.
Dahlgren R, Wyk AE van. 1988. Structures and relationships of families endemic to or centered in southern Africa. – Monogr. Syst. Bot. Missouri Bot. Gard. 25: 1-94.
Dalström S. 1999. The genus Solenidiopsis Senghas (Orchidaceae: Oncidiinae), a discussion and revision. – Selbyana 20: 1-9.
Dalström S. 2001. New species and combinations in the Oncidiinae (Orchidaceae) and a synopsis of the Cochlioda clade (Oncidiinae). – Selbyana 22: 135-145.
Dalström S. 2012. New combinations in Odontoglossum (Orchidaceae: Oncidiinae) and a solution to a taxonomic conundrum. – Lankesteriana 12: 53-60.
D’Amato F. 1949. Studio citologico e embriologico di Bowiea volubilis Harv. – Caryologia 2: 60-70.
Damboldt J, Phitos D. 1975. Die Karyosystematik der Gattung Leucojum L. (Amaryllidaceae) in Giechenland. – Plant Syst. Evol. 123: 119-131.
Damme EJM van, Goldstein IJ, Peumans WJ. 1991. A comparative study of mannose-binding lectins from the Amaryllidaceae and Alliaceae. – Phytochemistry 30: 509-514.
Dammer U. 1905. Liliaceae africanae. – Engl. Bot. Jahrb. Syst. 38: 62-66.
Dammer U. 1912. Liliaceae africanae IV. – Engl. Bot. Jahrb. Syst. 48: 360-366.
Dannenbaum C, Wolter M, Schill R. 1989. Stigma morphology of the orchids. – Bot. Jahrb. Syst. 110: 441-460.
Danton P. 2002. Bromeliaceae et Orchidaceae de l’archipel Juan Fernández (Chili). – Richardiana 2: 93-110.
Daru BH, Manning JC, Boatwright JS, Maurin O, Maclean N, Schaefer H, Kuzmina M, Bank M van der. 2013. Molecular and morphological analysis of subfamily Alooideae (Asphodelaceae) and the inclusion of Chortolirion in Aloe. – Taxon 62: 62-76.
Darwin C. 1862. On the various contrivances by which British and foreign orchids are fertilized by insects. – John Murray, London.
Dathe S, Dietrich H. 2006. Comparative molecular and morphological studies in selected Maxillariinae orchids. – Willdenovia 36 (Spec. issue): 89-102.
Dauncey EA, Cribb PJ. 1993. Dendrobium (Sect. Pedilonum) purpureum Roxb. (Orchidaceae) and its allies. – Kew Bull. 48: 545-576.
David JC. 2012. The genus Lachenalia. – Bot. Mag. Monogr., Kew Publ. Royal Botanic Gardens, Kew.
Davies KL, Stpiczynska M. 2008. Labellar micromophology of two euglossine-pollinated orchid genera; Scuticaria Lindl. and Dichaea Lindl. – Ann. Bot. 102: 805-824.
Davies KL, Turner MP. 2004a. Pseudopollen in Dendrobium unicum Seidenf. (Orchidaceae): reward or deception? – Ann. Bot. 94: 129-132.
Davies KL, Turner MP. 2004b. Pseudopollen in Eria Lindl. Section Mycaranthes Rchb. f. (Orchidaceae). – Ann. Bot. 94: 707-715.
Davies KL, Winters C. 1998. Ultrastructure of the labellar epidermis in selected Maxillaria species (Orchidaceae). – Bot. J. Linn. Soc. 126: 349-361.
Davies KL, Winters C, Turner MP. 2000. Pseudopollen: its structure and development in Maxillaria (Orchidaceae). – Ann. Bot. 85: 887-895.
Davies KL, Roberts DL, Turner MP. 2002. Pseudopollen and food-hair diversity in Polystachya Hook. (Orchidaceae). – Ann. Bot. 90: 477-484.
Davies KL, Turner MP, Gregg A. 2003a. Atypical pseudopollen-forming hairs in Maxillaria (Orchidaceae). – Bot. J. Linn. Soc. 143: 151-158.
Davies KL, Turner MP, Gregg A. 2003b. Lipoidal labellar secretions in Maxillaria Ruiz & Pav. (Orchidaceae). – Ann. Bot. 91: 439-446.
Davies KL, Turner MP. 2004. Morphology of floral papillae in Maxillaria Ruiz & Pav. (Orchidaceae). – Ann. Bot. 93: 75-86.
Davies TJ, Savolainen V, Chase MW, Goldblatt P, Barraclough TG. 2005. Environment, area and diversification in the species rich flowering plant family Iridaceae. – Amer. Natur. 186: 418-425.
Daviña JR. 2001. Estudios citogenéticos en algunos géneros argentinos de Amaryllidaceae. – Thesis, Universidad Nactional de Córdoba, Argentina.
Davis AP, Barnett JR. 1997. The leaf anatomy of the genus Galanthus L. (Amaryllidaceae J. St.-Hil.). – Bot. J. Linn. Soc. 123: 333-352.
Davis AP, Ozhatay N. 2001. Galanthus trojanus: a new species of Galanthus (Amaryllidaceae) from north-western Turkey. – Bot. J. Linn. Soc. 137: 409-412.
Davis PH, Stuart DC. 1967. Three new species of Muscari. – The Lily Year Book 30: 123-126.
De Barros F, De Azevedo Lourenco R. 2004. Synopsis of the Brazilian orchid genus Grobya, with the description of two new species. – Bot. J. Linn. Soc. 145: 119-127.
De Castro O, Brullo S, Colombo P, Jury S, De Luca P, Di Maio A. 2012. Phylogenetic and biogeographical inferences for Pancratium (Amaryllidaceae), with an emphasis on the Mediterranean species based on plastid sequence data. – Bot. J. Linn. Soc. 170: 12-28.
Delannoy E, Fujii S, Colas des Francs-Small C, Brudrett M, Small I. 2011. Rampant gene loss in the underground orchid Rhizanthella gardneri highlights evolutionary constraints on plastid genomes. – Mol. Biol. Evol. 28: 2077-2086.
Delaunay LN. 1915. Étude comparée caryologique de quelques espèces du genre Muscari Mill. – Mém. Soc. Nat. Kiew. 25: 33-62.
Delaunay LN. 1922. Vergleichende Untersuchungen einiger Muscari Mill. und Bellevalia Lapeyr. Arten. – Vestn. Tiflisk. Bot. Sada 2(1): 24-55.
Delaunay LN. 1923. Vergleichende Untersuchungen einiger Muscari Mill. und Bellevalia Lapeyr.-Arten. – Vestnik Tiflis Bot. Sada II, 1: 24-55.
Delgadillo RR. 1992. Una nueva especie de Bessera (Liliaceae) del Occidente de Jalisco, México. – Bol. Inst. Bot. Univ. Guadalajara 1: 131-136.
D’Emerico S, Cozzolino A, Pellegrino I. Pignone O, Scrugli A. 2002. Karyotype structure, supernumerary chromosomes and heterochromatin distribution suggest a pathway of karyotype evolution in Dactylorhiza (Orchidaceae). – Bot. J. Linn. Soc. 138: 85-91.
D’Emerico S, Pignone D, Bartolo G, Pulvirenti S, Terrasi C, Stuto S, Scrugli A. 2005. Karyomorphology, heterochromatin patterns and evolution in the genus Ophrys (Orchidaceae). – Bot. J. Linn. Soc. 148: 87-99.
Demirelma H, Uysal T. 2007. Allium ertugrulii sp. nov. (Alliaceae) from southern Turkey. – Nord. J. Bot. 25: 315-317.
Demissew S. 2008. Four new species of Asparagus (Asparagaceae) from the Flora Zambesiaca area. – Kew Bull. 63: 269-275.
Deng H, Zhang L-S, Zhang G-Q, Zheng B-Q, Liu Z-J, Wang Y. 2016. Evolutionary history of PEPC genes in green plants: implications for the evolution of CAM in orchids. – Mol. Phylogen. Evol. 94: 559-564.
Denk T, Gåuner HT, Grimm GW. 2014. From mesic to arid: leaf epidermal features suggest preadaptation in Miocene dragon trees (Dracaena). – Rev. Palaeobot. Palynol. 200: 211-228.
Deori NC, Das GC. 1976. Notes on rare orchids from north-eastern India 2. – Bull. Bot. Surv. India 18: 233-235.
De Tullio L, Roitman G, Bernardello G. 2008. Tamia (Iridaceae), a synonym of Calydorea: cytological and morphological evidence. – Syst. Bot. 33: 509-513.
Devey DS, Leitch I, Rudall PJ, Pires JC, Pillon Y, Chase MW. 2006. Systematics of Xanthorrhoeaceae sensu lato, with an emphasis on Bulbine. – In: Columbus JT, Friar EA, Porter JM, Prince LM, Simpson MG (eds), Monocots: comparative biology and evolution. Excluding Poales, Rancho Santa Ana Botanical Garden, Claremont, California. – Aliso 22: 345-351.
Devey DS, Bateman RM, Fay MF, Hawkins JA. 2008. Friends or relatives? Phylogenetics and species delimitation in the controversial European orchid genus Ophrys. – Ann. Bot. 101: 385-402.
Devillers P, Devillers-Terschuren J. 1994. Essai d’analyse systematique du genre Ophrys. – Naturalistes belges 75: 273-400.
De Vos MP. 1948. The development of the ovule and seed in the Hypoxideae I. Ianthe Salisb. – J. South Afr. Bot. 14: 159-169.
De Vos MP. 1949. The development of the ovule and seed in the Hypoxideae II. The genera Pauridia Harv. and Forbesia Ecklon. – J. South Afr. Bot. 15: 13-22.
De Vos MP. 1950. Die Ontwikkeling van die Saadknop en Saad by Cyanella capensis: ‘n Gefal von Polyembryonie. – South Afr. J. Sci. 46: 220-226.
De Vos MP. 1963. Studies on the embryology and relationships of South African genera of the Haemodoraceae: Lanaria Ait. – South Afr. J. Bot. 29: 79-90.
De Vos MP. 1970. Bydrae tot die morphologie en anatomie van Romulea II. Die blare. – J. South Afr. Bot. 36: 271-286.
De Vos MP. 1972. The genus Romulea in South Africa. – J. South Afr. Bot. [Suppl.] 9: 1-307.
De Vos MP. 1974. Die Suid-Afrikaanse genus Syringodea. – J. South Afr. Bot. 40: 201-254.
De Vos MP. 1977. Knol ontwikkeling by sommige genera van die Iridaceae en die systematise posisie. – Tydskr. Natuurwet. 17: 9-19.
Devos N, Raspe O, Jacquemart A-L, Tyteca D. 2006. On the monophyly of Dactylorhiza Necker ex Nevski (Orchidaceae): is Coeloglossum viride (L.) Hartman a Dactylorhiza? – Bot. J. Linn. Soc. 152: 261-269.
Devos N, Raspe O, Oh SH, Tyteca D, Jacquemart AL. 2006. The evolution of Dactylorhiza (Orchidaceae) allotetraploid complex: insights from nrDNA sequences and cpDNA PCR-RFLP data. – Mol. Phylogen. Evol. 38: 767-778.
De Wilde-Duyfjes BEE. 1976. A revision of the genus Allium L. (Liliaceae) in Africa. – Meded. Landbouwh. Wageningen 76: 1-238.
De Wildemann E. 1921. Hypoxidaceae. – Plant. Bequaert. 1: 48.
Diannelidis T. 1951. Cytologische Studien an einigen Allium-Arten aus Nord-Griechenland. – Port. Acta Biol. 3: 151-170.
Díaz Lifante Z. 1992. Karyological studies in Asphodelaceae and Anthericaceae I. Chromosome numbers in Asphodelus and Anthericum from N Africa. – Willdenowia 22: 143-148.
Díaz Lifante Z, Valdés B. 1996. Revisión del género Asphodelus L. (Asphodelaceae) en el Mediterráneo Occidental. – Boissiera 52: 5-189.
Díaz Lifante Z, Díez MJ, Fernández I. 1990. Morfología polínica de las subfamilies Melanthioideae y Asphodeloideae (Liliaceae) en la Península Ibérica y su importantcía taxonómica. – Lagascalia 16: 211-225.
Dice JC. 1988. Systematic studies in the Nolina bigelovii-N. parryi (Nolinaceae) complex. – M.A. thesis, San Diego State University, San Diego, California.
Dickson A. 1885. On the occurrence of foliage leaves in Ruscus (Semele) androgynus; with some structural and morphological considerations. – Trans. Proc. Bot. Soc. Edinb. 16: 130-149.
Diels L. 1930. Iridaceae. – In: Engler A (ed), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd 15a, W. Engelmann, Leipzig, pp. 463-505.
Dietrich H. 1984. Vorläufiges Gattungs- und Artenverzeichnis cubanischer Orchideen. – Wiss. Zeitschr. Friedrich-Schiller-Univ., Naturwiss. Reihe 33: 707-721.
Diez MJ, Pastor J. 1985. Contribución al studio del polen y semillas de la tribu Scilleae (Liliaceae) en Andalucia occidental. – An. Jard. Bot. Madrid 41: 351-360.
Di Fulvio E. 1973a. Sobre el gineceo de Allium y Nothoscordum. – Kurtziana 7: 241-253.
Di Fulvio E. 1973b. Contribución al conocimiento cariológico de Amaryllidaceae. Estudio cromosómico en Hieronymiella y otras généros afines. – Kurtziana 7: 117-131.
Di Fulvio TE, Cave MS. 1964. Embryology of Blandfordia nobilis Smith (Liliaceae), with special reference to its taxonomic position. – Phytomorphology 14: 487-499.
Diggle PK, DeMason DA. 1983. The relationship between the primary thickening meristem and the secondary thickening meristem in Yucca whipplei Torr. I. Histology of the mature stem; II. Ontogenetic relationship within the vegetative stem. – Amer. J. Bot. 70: 1195-1204, 1205-1216.
Dixon KW. 1985. The underground orchids of Australia – an appraisal. – Orchidarian 8: 75-79.
Dixon KW, Hopper SD. 2009. An introduction to Caladenia R. Br. – Australasia’s jewel among terrestrial orchids. – Aust. J. Bot. 57: ii-vii.
Docha-Neto A, Baptista DH, Campacci MA. 2006. Novos generos baseados em Oncidium. – Colet Orquídeas Brasil 3: 65-96.
Dockrill AW. 1967. Australasian Sarcanthinae. – Surrey, Australia.
Dodd RJ, Linhart YB. 1994. Reproductive consequences of interactions between Yucca glauca (Agavaceae) and Tegiticula yuccasella (Lepidoptera) in Colorado. – Amer. J. Bot. 81: 815-825.
Dodson CH. 1962. The importance of pollination in the evolution of the orchids of tropical America. – Amer. Orchid Soc. Bull. 31: 525-534, 641-649, 731-735.
Dodson CH. 1998. Miscellaneous new orchid genera and species. – Orquideologia 21: 61-67.
Dodson CH, Luer CA. 2005. 225(2). Orchidaceae. Genera Aa–Cyrtidiorchis. – In: Harling G, Andersson L (†) (eds), Flora of Ecuador 76, Botanical Institute, Göteborg University, pp. 1-345.
Dodson CH, Luer CA. 2009. 225(9). Orchidaceae. Masdevallia and affiliates. – In: Harling G, Persson C (eds), Flora of Ecuador 84, Department of Plant and Environmental Sciences, Göteborg University, pp. 1-393.
Donato R, Leach C, Conran JG. 2000. Relationships of Dietes (Iridaceae) inferred from ITS2 sequences. – In: Wilson KL, Morrison DA (eds), Monocots: systematics and evolution, CSIRO Publ., Melbourne, pp. 407-413.
Dong J-X, Han G-Y. 1992. Studies on the active constituents of Anemarrhena asphodeloides Bunge. – Acta Pharm. Sin. 27: 26-32.
Dönmez EO, Isik S. 2008. Pollen morphology of Turkish Amaryllidaceae, Ixioliriaceae and Iridaceae. – Grana 47: 15-38.
Dora G, Edwards JM. 1991. Taxonomic status of Lanaria lanata and isolation of a novel biflavone. – J. Nat. Prod. 54: 796-801.
Douzery JP, Pridgeon AM, Kores P, Kurzweil H, Linder P, Chase MW. 1999. Molecular phylogenetics of Diseae (Orchidaceae): a contribution from nuclear ribosomal ITS sequences. – Amer. J. Bot. 86: 887-899.
Dransfield J, Comber JB, Smith G. 1986. A synopsis of Corybas (Orchidaceae) in West Malesia and Asia. – Kew Bull. 41: 575-613.
Dressler RL. 1961. The structure of the orchid flower. – Missouri Bot. Gard. Bull. 49: 60-69.
Dressler RL. 1974. Classification of the orchid family. – In: Ospina M (ed), Proceedings of the Seventh World Orchid Conference, Medellin, Colombia, pp. 259-278.
Dressler RL. 1979. The subfamilies of the Orchidaceae. – Selbyana 5: 197-206.
Dressler RL. 1981. The orchids. Natural history and classification. – Harvard University Press, Cambridge, Massachusetts.
Dressler RL. 1983. Classification of the Orchidaceae and their probable origin. – Telopea 2: 413-424.
Dressler RL. 1986a. Recent advances in orchid phylogeny. – Lindleyana 1: 5-20.
Dressler RL. 1986b. Features of pollinaria and orchid classification. – Lindleyana 1: 125-130.
Dressler RL. 1987. Cladistic analysis of the Orchidaceae: a commentary. – Lindleyana 2: 66-71.
Dressler RL. 1989. The vandoid orchids: a polyphyletic grade? – Lindleyana 4: 89-93.
Dressler RL. 1990a. The Neottieae in orchid classification. – Lindleyana 5: 101-109.
Dressler RL. 1990b. The Spiranthoideae: grade or subfamily? – Lindleyana 5: 110-116.
Dressler RL. 1990c. The major clades of the Orchidaceae-Epidendroideae. – Lindleyana 5: 117-125.
Dressler RL. 1993. Phylogeny and classification of the orchid family. – Dioscorides Press, Portland, Oregon.
Dressler RL, Chase MW. 1995. Whence the orchids? – In: Rudall PJ, Cribb PJ, Cutler DF, Humphries CJ (eds), Monocotyledons: systematics and evolution, Royal Botanic Gardens, Kew, pp. 217-226.
Dressler RL, Cook SL. 1988. Conical silica bodies in Eria javanica. – Lindleyana 3: 224-225.
Dressler RL, Dodson CH. 1960. Classification and phylogeny in the Orchidaceae. – Ann. Missouri Bot. Gard. 47: 25-68.
Dressler RL, Dodson CH. 1993. Phylogeny and classification of the orchid family. – Dioscorides Press, Portland, Oregon.
Dressler RL, Salazar GA. 1991. Viscarium, a term for the glue-bearing area of the rostellum. – Orchid Research Newsletter 17: 11-12.
Dressler RL, Whitten WM, Williams NH. 2004. Phylogenetic relationships of Scaphyglottis and related genera (Laeliinae: Orchidaceae) based on nrDNA ITS sequence data. – Brittonia 56: 58-66.
Drewes SE, Khan F. 2004. The African potato (Hypoxis hemerocallidea): a chemical-historical perspective. – South Afr. J. Sci. 100: 425-430.
Driguez A, Sytsma KJ. 2006. Phylogenetics of the “tiger-flower” group (Tigridieae: Iridaceae): molecular and morphological evidence. – In: Columbus JT, Friar EA, Porter JM, Prince LM, Simpson MG (eds), Monocots: comparative biology and evolution. Excluding Poales, Rancho Santa Ana Botanical Garden, Claremont, California. – Aliso 22: 412-424.
Droissart V, Simo M, Sonké B, Cawoy V, Stévart T. 2009. Le genre Stolzia (Orchidaceae) en Afrique central avec deux nouveaux taxons. – Adansonia, sér. III, 31: 25-40.
Droissart V, Sonké B, Nguembou K C, Djuikouo K M-N, Parmentier I, Stévart T. 2009. Synopsis of the genus Chamaeangis (Orchidaceae), including two new taxa. – Syst. Bot. 34: 285-296.
Droissart V, Cribb PJ, Simo-Droissar M,
Stévart T. 2014. Taxonomy of Atlantic Central African orchids 2. A second
species of the rare genus Distylodon (Orchidaceae, Angraecinae) collected in
Cameroon. – PhytoKeys 36: 27-34.
Druselmann S. 1992. Vergleichende Untersuchungen an Vertretern der Alliaceae Agardh 1. Morphologie der Keimpflanzen der Gattung Allium L. – Flora 186: 37-52.
Dubouzet JG, Shinoda K. 1998. Phylogeny of Allium L. subg. Melanocrommyum (Webb et Berth.) Rouy based on DNA sequence analyses of the internal transcribed spacer region of nrDNA. – Theor. Appl. Genet. 97: 541-549.
Dubouzet JG, Shinoda K. 1999. Relationships among old and new world Allium according to ITS DNA sequence analysis. – Theor. Appl. Genet. 98: 422-433.
Duchartre P. 1892. Observations sur les feuilles ensiforme des Iridées. – J. Soc. Nat. Hort. France, sér. III, 14: 556-579.
Dulberger R, Ornduff R. 1978. The breeding system in dimorphic Cyanella alba and C. lutea (Tecophilaeaceae). – Bot. Soc. 27: 31-32.
Dulberger R, Ornduff R. 1980. Floral morphology and reproductive biology of four species of Cyanella (Tecophilaeaceae). – New Phytol. 86: 45-56.
DuPuy DJ, Ford-Lloyd BV, Cribb PJ. 1985. A numerical taxonomic analysis of Cymbidium, Section Iridorchis (Orchidaceae). – Kew Bull. 40: 421-434.
Duthie AV. 1940. Contribution to our knowledge of the genus Eriospermum. – Ann. Univ. Stellenbosch, Reeks A, Wiss.-Natuurk. 18: 1-64.
Duthie JF. 1906. The orchids of North Western Himalaya. – Ann. Roy. Bot. Gard. Calcutta 9, Calcutta.
Dutt BSM. 1962. A contribution to the life history of Crinum defixum. – In: Plant embryology – a symposium, Council of Scientific and Industrial Research, New Delhi, pp. 37-48.
Dutt BSM. 1970a. Comparative embryology of angiosperms: Cyanastraceae. – Bull. Indian Natl. Sci. Acad. 41: 362-364.
Dutt BSM. 1970b. Comparative embryology of angiosperms: Amaryllidaceae. – Bull. Indian Natl. Sci. Acad. 41: 365-367.
Dutt BSM. 1970c. Comparative embryology of angiosperms: Hypoxidaceae. – Bull. Indian Natl. Sci. Acad. 41: 368-372.
Dyer WT. 1874. The tree aloes of South Africa. – Gard. Chron., n. s. 1: 566-569.
Dykes WR. 1913. The genus Iris. – Cambridge.
Ebert I. 1993. Systematische Karyologie und Embryologie von Prospero Salisb. und Barnardia Lindl. (Hyacinthaceae). – Ph.D. diss., Universität Wien, Austria.
Ebert I, Greilhuber J, Svoma E, Speta F. 1983. Taxonomic significance of embryology in Scilla. – Acta Bot. Neerl. 32: 356-357.
Efimov PG. 2004. Rod Epipactis Zinn (Orchidaceae) na territorii Rossii. – Turczaninowia 7: 8-42.
Efimov PG. 2008. Notes on Epipactis condensata, E. rechingeri and E. purpurata (Orchidaceae) in the Caucasus and Crimea. – Willdenowia 38: 71-80.
Efimov PG, Lauri R, Bateman R. 2009. Neolindleya Kraenzl. (Orchidaceae), an enigmatic and largely overlooked autogamous genus from temperate East Asia. – Kew Bull. 64: 661-671.
Eguiarte LE. 1995. Hutchinson (Agavales) vs. Huber y Dahlgren (Asparagales): análisis moleculares sobre la filogenia y evolucíon de la familia Agavaceae sensu Hutchinson dentro de las monocotiledóneas. – Bull. Soc. Bot. México 56: 45-56.
Eguiarte LE, Búrquez A. 1987. Reproductive ecology of Manfreda brachystachya, an iteroparous species of Agavaceae. – Southwest Natur. 32: 169-178.
Eguiarte LE, Duvall MR, Learn GH Jr, Clegg MT. 1994. The systematic status of the Agavaceae and Nolinaceae and related Asparagales in the monocotyledons: an analysis based on the rbcL gene sequence. – Bol. Soc. Bot. México 54: 35-56.
Eguiarte LE, Souza V, Silva-Montellano A. 2000. Evolución de la familia Agavaceae: filogenia, biología, reproductiva y genética de poblaciones. – Bol. Soc. Bot. México 66: 131-150.
Ehrhardt W. 1992. Hemerocallis: Day lilies. – Batsford, London.
Eid SE. 1963. Cytological studies in section Molium of the genus Allium. – Genet. Today 1: 134.
Ekberg L. 1969. Studies in the genus Allium II. A new subgenus and new sections from Asia. – Bot. Not. 122: 57-68.
Ekberg L. 1970. Studies in the genus Allium III. Wind dispersal of Allium bulbs. – Bot. Not. 123: 115-118.
Ekberg L. 1972a. Studies in the genus Allium IV. Vegetative reproduction in Allium unifolium and some other American species. – Bot. Not. 125: 82-86.
Ekberg L. 1972b. Studies in the genus Allium V. Bulb structure in the Section Anguinum. – Bot. Not. 125: 87-92.
Ekberg L. 1972c. Studies in the genus Allium VI. Bulb structure in the Subgenus Melanocrommyum. – Bot. Not. 125: 93-101.
Eker I, Koyuncu M. 2008. Muscari babachii sp. nov. (Hyacinthaceae) from south Anatolia. – Nord. J. Bot. 26: 49-52.
El-Hamidi A. 1952. Vergleichend-morphologische Untersuchungen am Gynoeceum der Unterfamilien Melanthioideae und Asphodelioideae der Liliaceae. – Arb. Inst. Allg. Bot., Univ. Zürich, Ser. A, 4: 1-50.
El-Moghazy AM, Ali AA, Ross SA, El-Shanaway MA. 1980. Phytochemical studies on Polianthes tuberosa L. – Fitoterapia 51: 179-181.
Ely F, Luque Arias R. 2006. Estudio morfoanatómico comparado de Eccremis coarctata (Ruiz & Pav.) Baker (Phormiaceae) en diferentes altitudes de la Cordillera de Mérida. – Plantula 4: 23-37.
Emons RW. 1945. A revision of the Central American species of Smilacina. – Ann. Missouri Bot. Gard. 32: 395-411.
Engelmann G. 1873. Notes on the genus Yucca. – Trans. Acad. Sci. St. Louis 3: 17-54.
Engler A. 1888. Liliaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien II(5), W. Engelmann, Leipzig, pp. 10-91; Engler A. 1897. Nachträge zu II(5), pp. 71-77.
Engler A. 1930. Cyanastraceae. – In: Engler A (ed), Die natürlichen Pflanzenfamilien, 2. Augl., Bd. 15a, W. Engelmann, Leipzig, pp. 188-190.
Erol O, Küçüker O. 2003. Morpho-anatomical observations on three Romulea (Iridaceae) taxa of Turkey. – Bocconea 16: 607-613.
Erol O, Küçüker O, Uzen E. 2008. Corm tunic morphology of Turkish Crocoideae (Iridaceae) and their systematic significance. – Nord. J. Bot. 26: 66-73.
Escobar I, Ruiz E, Baeza C. 2012. Estudios cariotípicos em espécies de Gilliesieae Lindl. (Gilliesioideae-Alliaceae) de Chile central. – Gayana Botánica 69: 240-250.
Espejo E, López-Ferrari AR. 1997. Las monoctiledóneas mexicanas: una sinopsis florística 7: Orchidaceae 1. – Consejo Nacional de la Flora de México, Mexico City.
Espejo E, López-Ferrari AR. 1998. Las monoctiledóneas mexicanas: una sinopsis florística 8: Orchidaceae 2. – Consejo Nacional de la Flora de México, Mexico City.
Eum SM, Yukawa T, Luo Y, Freudenstein JV, Lee NS. 2008. Reappraisal of Diplolabellum coreanum (Orchidaceae) as inferred from molecular data. – J. Plant Biol. 51: 20-24.
Eunus AM. 1952. Contributions to the embryology of Liliaceae III. Embryogeny and development of the seed of Asphodelus tenuifolius Cav. – Lloydia 15: 149-155.
Fahn A. 1954. The anatomical structure of the Xanthorrhoeaceae Dumort. – Bot. J. Linn. Soc. 55: 158-184.
Fahn A. 1961. The anatomical structure of the Xanthorrhoeaceae Dumort. and its taxonomic position. – In: Recent advances in botany 1, Toronto, pp. 155-160.
Fan J, Qin H-N, Li D-Z, Jin X-H. 2009. Molecular phylogeny and biogeography of Holcoglossum (Orchidaceae: Aeridinae) based on nuclear ITS, and chloroplast trnL-F and matK. – Taxon 58: 849-861.
Fangan BM, Nordal I. 1993. A comparative analysis of morphology, chloroplast-DNA and distribution within the genus Crinum (Amaryllidaceae). – J. Biogeogr. 20: 55-61.
Faria A. 2004. Sistematica filogenetica e delimitação dos generos da subtribo Oncidiinae. – Ph.D. diss., Université d’État de Campinas, Brésil.
Faria E. 2016. Diversité du genre Corybas Salisb. (Orchidaceae, Diurideae) en Nouvelle-Calédonie. – Adansonia 38: 175-198.
Farminhão J, Meerts P, Descourvières P, Droissart V, Simo-Droissart M, Stévart T. 2018. A revised concept of Rhipidoglossum (Angraecinae, Orchidaceae). – Phytotaxa 349(3). DOI: 10.11646/phytotaxa 349.3.5
Fay MF, Chase MW. 1996. Resurrection of Themidaceae for the Brodiaea alliance, and recircumscription of Alliaceae, Amaryllidaceae and Agapanthoideae. – Taxon 45: 441-451.
Fay MF, Rudall PJ, Sullivan S, Stobart KL, Bruijn AY de, Reeves G, Qamaruz-Zaman F, Hong W-P, Joseph J, Hahn WJ, Conran JG, Chase MW. 2000. Phylogenetic studies of Asparagales based on four plastid DNA regions. – In: Wilson KL, Morrison DA (eds), Monocots: systematics and evolution, CSIRO Publ., Melbourne, pp. 360-371.
Fay MF, Rudall PJ, Chase MW. 2006. Molecular studies of subfamily Gilliesioideae (Alliaceae). – In: Columbus JT, Friar EA, Porter JM, Prince LM, Simpson MG (eds), Monocots: comparative biology and evolution. Excluding Poales, Rancho Santa Ana Botanical Garden, Claremont, California [Aliso 22], pp. 367-371.
Feinbrun N. 1938-1940. A monographic study on the genus Bellevalia Lapeyr. (caryology, taxonomy, geography). – Palest. J. Bot., Jerusalem Ser., 1: 42-54, 131-142, 336-409.
Feinbrun N. 1943. Allium section Porrum of Palestine and the neighbouring countries. – Palest. J. Bot. 3: 1-21.
Feinbrun N. 1950. Chromosome counts in Palestinian Allium species. – Palest. J. Bot. 5: 13-16.
Feinbrun N. 1961. Revision of the genus Hyacinthella Schur. – Bull. Res. Counc. Israel 10D: 324-347.
Felix LP, Guerra M. 2005. Basic chromosome numbers of terrestrial orchids. – Plant Syst. Evol. 254: 131-148.
Felix LP, Guerra M. 2010. Variation in chromosome number and the basic chromosome number of subfamily Epidendroideae (Orchidaceae). – Bot. J. Linn. Soc. 163: 234-278.
Felix WJP, Felix LP, Melo NF, Oliveira MBM, Dutilh JHA, Carvalho R. 2011. Karyotype variability in species of the genus Zephyranthes Herb. (Amaryllidaceae-Hippeastreae). – Plant Syst. Evol. 294: 263-271.
Fernandes A. 1935. Remarque sur l’hétérostylie de Narcissus triandrus L. et de N. reflexus Brot. – Bol. Soc. Broteriana, sér. II, 10: 278-288.
Fernandes A. 1942. Summary of work on cytology of Narcissus L. – Herbertia 9: 126-133.
Fernandes A. 1943. Sur la caryo-systematique de la section Autumnales Gay du genre Narcissus L. – Bol. Soc. Brot., sér. II, 17: 5-54.
Fernandes A. 1966. Nouvelles études caryologiques sur la section Jonquilla DC. du genre Narcissus L. – Bol. Soc. Brot., sér. II, 40: 207-248.
Fernandes A. 1967. Contribution à la connaissance de la biosystématique de quelques espèces du genre Narcissus L. – Portug. Acta Biol. 9: 1-44.
Fernandes A. 1968. Improvements in the classification of the genus Narcissus L. – Plant Life 24: 51-57.
Fernandes A. 1969. Contribution to the knowledge of the biosystematics of some species of genus Narcissus L. – Publ. Univ. Sevilla (V Simposio de Flora Europaea): 245-284.
Fernandez A, Daviña JR. 1991. Heterochromatin and genome size in Fortunatia and Camassia (Hyacinthaceae). – Kew Bull. 46: 307-316.
Fernandez-Alonso JL, Groenendijk JP. 2004. A new species of Zephyranthes Herb. s.l. (Amaryllidaceae, Hippeastreae), with notes on the genus in Colombia. – Rev. Acad. Colomb. Ci. Exact. 28: 177-186.
Fernández-Concha GC, Muñoz JLT, Gómez-Juárez M. 2001. A synopsis of the Maxillaria rufescens complex in Mexico, Central America, and the Greater Antilles. – Brittonia 53: 454-465.
Ficker T. 1951. Chromosomes of two narrow-leaved Amaryllis species, and the generic type species of Amaryllis belladonna L. – Plant Life 7: 68.
Figueiredo E, Smith GF. 2010. What’s in a name: epithets in Aloe L. (Asphodelaceae) and what to call the next new species. – Bradleya 28: 79-102.
Figueroa C, Salazar GA, Zavaleta HA, Engleman EM. 2008. Root character evolution and systematics in Cranichidinae, Prescottiinae and Spiranthinae (Orchidaceae, Cranichideae). – Ann. Bot. 101: 509-520.
Finet MEA. 1896. Note sur deux espèces nouvelles d’Oreorchis. – Bull. Soc. Bot. France 43: 697-698.
Finet MEA. 1897. Sur le genre Oreorchis Lindley. – Bull. Soc. Bot. France 44: 69-74.
Fischer GA, Gravendeel B, Sieder A, Andriantiana J, Heiselmayer P, Cribb PJ, Smidt E de C, Samuel R, Kiehn M. 2007. Evolution of resupination in Malagasy species of Bulbophyllum (Orchidaceae). – Mol. Phylogen. Evol. 45: 358-376.
Fischer GA, Sieder A, Cribb PJ, Kiehn M. 2007. Description of two new species and one new section of Bulbophyllum (Orchidaceae) from Madagascar. – Adansonia, sér. III, 29: 19-25.
Fishbein M, Kephart SR, Wilder M, Halpin KM, Datwyler SL. 2010. Phylogeny of Camassia (Agavaceae) inferred from plastid rpl16 intron and trnD-trnY-trnE-trnT intergenic spacer DNA sequences: implications for species delimitation. – Syst. Bot. 35: 77-85.
Fisher JB, Tomlinson PB. 1971. Morphological studies in Cordyline (Agavaceae) I. Introduction and general morphology. – J. Arnold Arbor. 52: 459-478.
Flory WS. 1939. Parthenogenesis in Zephyrantheae. – Herbertia 6: 196-202.
Flory WS. 1940. Cytology of Zephyranthes. – Ann. Rep. Texas Agric. Exp. Sta. 53: 29.
Flory WS. 1967. Sprekelia chromosomes. – Plant Life 23: 54-57.
Flory WS. 1968. Chromosome diversity in species, and in hybrids, of tribe Zephyrantheae. – Nucleus 11: 79-95.
Flory WS. 1976. Distribution, chromosome number and types of various species and taxa of Hymenocallis. – Nucleus 19: 204-227.
Flory WS. 1977. Overview of chromosomal evolution in the Amaryllidaceae. – Nucleus 20: 70-88.
Flory WS, Coulthard Jr RF. 1981. New chromosome counts, numbers, and types in genus Amaryllis. – Plant Life 37: 43-56.
Flory WS, Flagg RO. 1958. A cytological study of the genus Habranthus. – The Nucleus 1: 267-280.
Flory WS, Schmidhauser TF. 1957. Mitotic chromosome numbers in Hymenocallis with a consideration of factors possibly affecting numbers and karyotypes. – Genetics 42: 369-370.
Fluellen BL. 1974. Studies in the genus Albuca L. – Ph.D. diss., University of Bristol, England.
Forster PI, Thompson EJ. 1997. Borya inopinata (Anthericaceae), a new species of resurrection plant from North Queensland. – Austrobaileya 4: 597-600.
Forster W, Souza VC. 2007. Epidendrum caparaoense (Orchidaceae), a new species from Minas Gerais, Brazil. – Bot. J. Linn. Soc. 155: 157-159.
Fosberg FR. 1985. Cordyline fruticosa (L.) Chevalier (Agavaceae). – Baileya 22: 180-181.
Foster RC. 1937. A cyto-taxonomic survey of the North American species of Iris. – Contr. Gray Herb. Harvard Univ. 119: 1-82.
Foster RC. 1941. Studies in Iridaceae II. A revision of Geissorhiza Ker-Gawl. – Contr. Gray Herb. Harvard Univ. 135: 1-78.
Foster RC. 1945. Studies in the Iridaceae III. – Contr. Gray Herb. Harvard Univ. 155: 3-54.
Foster RC. 1950. Studies in the Iridaceae IV. – Contr. Gray Herb. Harvard Univ. 165: 106-111.
Fowden L. 1959. Nitrogenous compounds and nitrogen metabolism in the Liliaceae 6. Changes in the nitrogenous composition during the growth of Convallaria and Polygonatum. – Biochem. J. 71: 643-648.
Fowden L, Steward FC. 1957. Nitrogenous compounds and nitrogen metabolism in the Liliaceae 1. The occurrence of soluble nitrogen compounds. – Ann. Bot., N. S., 21: 53-67.
Francisco A, Porto M, Ascensão L. 2015. Morphological phylogenetic analysis of Ophrys (Orchidaceae): insights from morpho-anatomical floral traits into the interspecific relationships in an unresolved clade. – Bot. J. Linn. Soc. 179: 454-476.
François E. 1928. Deux orchidées nouvelles de Madagascar. – Rev. Hort., n. s., 21: 304-306.
Fredrikson M, Carlsson K, Franksson O. 1988. Confocal scanning laser microscopy, a new technique used in an embryological study of Dactylorhiza maculata (Orchidaceae). – Nord. J. Bot. 8: 369-374.
Freitag H, Wendelbo P. 1970. Studies in the flora of Afghanistan. The genus Bellevalia in Afghanistan. – Israel J. Bot. 19: 220-224.
Frello S, Ørgaard M, Jacobsen N, Heslop-Harrison JS. 2004. The genomic organization and evolutionary distribution of a tandemly repeated DNA sequence in the genus Crocus (Iridaceae). – Hereditas 141: 81-88.
Freudenstein JV. 1991. A systematic study of endothecial thickenings in the Orchidaceae. – Amer. J. Bot. 78: 766-781.
Freudenstein JV. 1994. Gynostemium structure and relationships of the Corallorhizinae (Orchidaceae: Epidendroideae). – Plant Syst. Evol. 193: 1-19.
Freudenstein JV. 1996. Proposal to conserve the name Corallorhiza Gagnebin (Orchidaceae) with a conserved spelling. – Taxon 45: 695-696.
Freudenstein JV, Barrett CF. 2010. Mycoheterotrophy and diversity in Orchidaceae. – In: Seberg O, Petersen G, Barfod AS, Davis JI (eds), Diversity, phylogeny, and evolution in the monocotyledons, Aarhus University Press, Århus, pp. 25-37.
Freudenstein JV, Chase MW. 2001. Analysis of mitochondrial nad1b-c intron sequences in Orchidaceae: utility and coding of length-change characters. – Syst. Bot. 26: 643-657.
Freudenstein JV, Chase MW. 2015. Phylogenetic relationships in Epidendroideae (Orchidaceae), one of the great flowering plant radiations: progressive specialization and diversification. – Ann. Bot. 115: 665-681.
Freudenstein JV, Doyle JJ. 1994a. Character transformation and relationships in Corallorhiza (Orchidaceae: Epidendroideae) I. Plastid DNA. – Amer. J. Bot. 81: 1449-1457.
Freudenstein JV, Doyle JJ. 1994b. Plastid DNA, morphological variation, and the phylogenetic species concept: the Corallorhiza maculata (Orchidaceae) complex. – Syst. Bot. 19: 273-290.
Freudenstein JV, Rasmussen FN. 1996. Pollinium development and number in the Orchidaceae. – Amer. J. bot. 83: 813-824.
Freudenstein JV, Rasmussen FN. 1997. Sectile pollen and relationships in Orchidaceae. – Plant Syst. Evol. 205: 125-146.
Freudenstein JV, Rasmussen FN. 1999. What does morphology tell us about orchid relationships? A cladistic analysis. – Amer. J. Bot. 86: 225-248.
Freudenstein JV, Senyo DM. 2008. Relationships and evolution of matK in a group of leafless orchids (Corallorhiza and Corallorhizinae; Orchidaceae: Epidendroideae). – Amer. J. Bot. 95: 498-505.
Freudenstein JV, Barrett CF. 2010. Mycoheterotrophy and diversity in Orchidaceae. – In: Seberg O, Petersen G, Barfod AS, Davis JI (eds), Diversity, phylogeny, and evolution in the monocotyledons, Aarhus University Press, Århus, pp. 25-37.
Freudenstein JV, Senyo DM, Chase MW. 2000. Mitochondrial DNA and relationships in the Orchidaceae. – In: Wilson KL, Morrison DA (eds), Monocots: systematics and evolution, CSIRO, Melbourne, pp. 421-429.
Freudenstein JV, Harris EM, Rasmussen FN. 2002. The evolution of anther morphology in orchids: incumbent anthers, superposed pollinia, and the vandoid complex. – Amer. J. Bot. 89: 1747-1755.
Freudenstein JV, van den Berg C, Goldman DH, Kores PJ, Molvray M, Chase MW. 2004. An expanded plastid DNA phylogeny of Orchidaceae and analysis of jackknife branch support strategy. – Amer. J. Bot. 91: 149-157.
Freudenstein JV, Yukawa T, Luo Y. 2017. A reanalysis of relationships among Calypsoinae (Orchidaceae: Epidendroideae): floral and vegetative evolution and the placement of Yoania. – Syst. Bot. 42: 17-25.
Friedmann F. 1984. Note sur les Hypoxidaceae des Seychelles et description du genre nouveau Hypoxidia. – Bull. Mus. Natl. Hist. Paris, sér. III, sect. B, Adansonia, 4: 453-460.
Fries TCE. 1919. Der Samenbau bei Cyanastrum. – Svensk Bot. Tidskr. 13: 295-304.
Friesen N. 1988. Lukovye Sibiri. Sistematika, kariologija, chorologija. – Nauka, Novosibirsk.
Friesen N, Blattner FR. 2000. RAPD analysis reveals geographic differentiations within Allium schoenoprasum L. (Alliaceae). – Plant Biol. 2: 297-305.
Friesen N, Fritsch RM, Bachmann K. 1997. Hybrid origin of some ornamentals of Allium subgenus Melanocrommyum verified with GISH and RAPD. – Theor. Appl. Genet. 95: 1229-1238.
Friesen N, Fritsch RM, Pollner S, Blattner FR. 2000. Molecular and morphological evidence for an origin of the aberrant genus Milula within Himalayan species of Allium (Alliaceae). – Mol. Phylogen. Evol. 17: 209-218.
Friesen N, Fritsch RM, Blattner FR. 2006. Phylogeny and new infrageneric classification of Allium (Alliaceae) based on nuclear ribosomal DNA ITS sequences. – In: Columbus JT, Friar EA, Porter JM, Prince LM, Simpson MG (eds), Monocots: comparative biology and evolution. Excluding Poales, Rancho Santa Ana Botanical Garden, Claremont, California. – Aliso 22: 372-395.
Friis I, Bjørnstad IN. 1971. A new species of Haemanthus (Amaryllidaceae) from southwest Ethiopia. – Norw. J. Bot. 18: 227-233.
Friis I, Nordal I. 1976. Studies on the genus Haemanthus (Amaryllidaceae) IV. Division of the genus into Haemanthus s. str. and Scadoxus with notes on Haemanthus s. str. – Norw. J. Bot. 23: 63-77.
Friis I, Rasmussen FN. 1974. The two alternative systems of nomenclature proposed and used for orchids by Du Petit-Thouars, with special regard to Corymborkis Thouars. – Taxon 24: 307-318.
Frisch B, Nagl W. 1979. Patterns of endopolyploidy and 2 C nuclear DNA content (Feulgen) in Scilla bifolia (Liliaceae). – Plant Syst. Evol. 131: 261-276.
Fritsch RM. 1988. Anatomical investigations of the leaf blade of Allium L. (Alliaceae) I. Species having one row of vascular bundles. – Flora 181: 83-100.
Fritsch RM. 1992a. Septal nectaries in the genus Allium shape, position and excretory canals. – In: Hanelt P, Hammer K, Knüpffer H (eds), The genus Allium – taxonomic problems and genetic resources, Proceedings of an International Symposium in Gatersleben, June 11-13, 1991, Gatersleben, pp. 77-85.
Fritsch RM. 1992b. Infra-subgeneric grouping in subgenus Melanocrommyum (Webb et Berth.) Rouy. – In: Hanelt P, Hammer K, Knüpffer H (eds), The genus Allium – taxonomic problems and genetic resources, Proceedings of an International Symposium in Gatersleben, June 11-13, 1991, Gatersleben, pp. 77-85.
Fritsch RM. 1992c. Zur Wurzelanatomie in der Gatttung Allium L. (Alliaceae). – Beitr. Biol. Pflanzen 67: 129-160.
Fritsch RM. 1993. Taxonomic and nomenclatural remarks on Allium L. subgen. Melanocrommyum (Webb & Berth.) Rouy sect. Megaloprason Wendelbo. – Candollea 48: 417-430.
Fritsch RM. 1996. The Iranian species of Allium subg. Melanocrommyum sect. Megaloprason (Alliaceae). – Nord. J. Bot. 16: 9-17.
Fritsch RM. 2001. Contribution from IPK Gatersleben: taxonomy of the genus Allium. – Herbertia 56: 19-50.
Fritsch RM. 2009. New Allium (Alliaceae) species from Tajikistan, Kyrgyzstan, and Uzbekistan. – Bot. Jahrb. Syst. 127: 459-471.
Fritsch RM, Astanova SB. 1998. Uniform karyotypes in different sections of Allium L. subgen. Melanocrommyum (Webb & Berth.) Rouy from Central Asia. – Feddes Repert. 109: 539-549.
Fritsch RM, Friesen N. 2002. Evolution, domestication, and taxonomy. – In: Rabinowitch HD, Currah L (eds), Allium crop science. Recent advances, CABI Publ, Wallingford, England, pp. 5-30.
Fritsch RM, Friesen N. 2009. Allium oreotadzhikorum and Allium vallivanchense, two new species of Allium subg. Polyprason (Alliaceae) from the Central Asian republic Tajikistan. – Feddes Repert. 120: 221-231.
Fritsch RM, Keusgen M. 2006. Occurrence and taxonomic significance of cystein sulphoxides in the genus Allium L. (Alliaceae). – Phytochemistry 67: 1127-1135.
Fritsch RM, Khassanov FO. 2008. New taxa of Allium L. subg. Allium (Alliaceae) from Tajikistan and Uzbekistan. – Feddes Repert. 119: 625-633.
Fritsch RM, Kruse J, Adler K, Rutten T. 2006. Testa sculptures in Allium L. subg. Melanocrommyum (Webb & Berth.) Rouy (Alliaceae). – Feddes Repert. 117: 250-263.
Fritz F. 1935. Über die Kutikula von Aloe- und Gasteriaarten. – Jahrb. Wissensch. Bot. 81: 718-746.
Fritz GPJ. 2012. Revie of the three species accepted in Chortolirion A. Berger (Xanthorrhoeaceae: Asphodeloideae). – Aloe 49: 4-10.
Fuchs A, Ziegenspeck H. 1927a. Die Dactylorchisgruppe der Ophrydineen. – Bot. Archiv 19: 163-274.
Fuchs A, Ziegenspeck H. 1927b. Entwicklung, Axen und Blätter einheimischer Orchideen IV. – Bot. Archiv 20: 275-422.
Fuchs A, Ziegenspeck H. 1927c. Entwicklungsgeschichte der Axen der einheimischen Orchideen und ihre Physiologie und Biologie III. – Bot. Archiv 18: 378-475.
Fuchsig H. 1911. Vergleichende Anatomie der Vegetationsorgane der Lilioideen. – Sitzungsber. K. Akad. Wiss. Wien, Math.-Naturw. Kl., 120: 1-43.
Fujishima A. 1975. Karyotypes of some species in the genus Crinum. – La Kromosoma 99: 3063-3071.
Fujita N. 1976. The genus Hosta in Japan. – Acta Phytotaxon. Geobot. 27: 66-96. [In Japanese with English summary]
Fukuda T, Ashizawa H, Suzuki R, Ochiai T, Nakamuru T, Kanno A, Kameya T, Yokoyama J. 2005. Molecular phylogeny of the genus Asparagus inferred from plastid petB intron and petD-rpoA intergenic spacer sequences. – Plant Species Biol. 20: 121-132.
Furness CA, Conran JG, Gregory T, Rudall PJ. 2014. The trichotomosulcate asparagoids: pollen morphology of Hemerocallidaceae in relation to systematics and pollination biology. – Aust. Syst. Bot. 26: 393-407.
Gagnepain MF. 1934a. Quelques liliacées nouvelle d’Indo-Chine. – Bull. Soc. Bot. France 81: 286-289.
Gagnepain MF. 1934b. Les aspidistrées d’Indo-Chine. – Bull. Mus. Natl. Hist. Nat. Paris 2: 189-192.
Gagnepain MF. 1951. Deux collections précieuse d’orchidées d’Indochine. – Notul. Syst. Paris 14, 2: 114-132.
Galli MG, Bracale M, Falavigna A, Raffaldi F, Savini C, Vigo A. 1993. Different kinds of male flowers in the dioecious plant Asparagus officinalis L. – Sex. Plant Reprod. 6: 16-21.
Gamarra R, Dorda E, Scrugli A, Galán P, Ortúñez E. 2007. Seed micromorphology in the genus Neotinea Rchb. f. (Orchidaceae, Orchidinae). – Bot. J. Linn. Soc. 153: 133-140.
Gamarra R, Galán P, Herrera I, Ortunez E. 2008. Seed micromorphology supports the splitting of Limnorchis from Platanthera (Orchidaceae). – Nord. J. Bot. 26: 61-65.
Gamarra R, Ortúñez E, Cela PG, Guadaño V. 2012. Anacamptis versus Orchis (Orchidaceae): seed micromorphology and its taxonomic significance. – Plant Syst. Evol. 298: 597-607.
Gamisans J, Fridlender A, Moret J, Jeanmonod D. 1994. Les espèces du genre Romulea en Corse. – Candollea 49: 509-526.
Gándara E, Specht CD, Sosa V. 2014. Origin
and diversification of the Milla clade (Brodiaeoideae, Asparagoaceae): a
neotropical group of six geophytic genera. – Molec. Phylogen. Evol. 75:
118-125.
Garay LA. 1956. Studies in American orchids I. – Can. J. Bot. 34: 241-260.
Garay LA. 1960. On the origins of the Orchidaceae. – Bot. Mus. Leafl. Harvard Univ. 19: 57-96.
Garay LA. 1963. Oliveriana and its position in the Oncidieae. – Amer. Orchid Soc. Bull. 32: 19-24.
Garay LA. 1967. The genus Restrepiella G. & D. – Orchid Digest 31: 38-40.
Garay LA. 1968. Studies in American orchids VII. – Caldasia 10: 231-238.
Garay LA. 1969. Orquideas colombianas nuevas o críticas I. – Orquideologia 4: 14-21, 32-33.
Garay LA. 1972a. On the origins of the Orchidaceae II. – J. Arnold Arbor. 53: 202-215.
Garay LA. 1972b. On the systematics of the monopodial orchids I. – Bot. Mus. Leafl. Harvard Univ. 23: 149-212.
Garay LA. 1972c. Orquideas colombianas nuevas o críticas VIII. – Orquideologia 7: 197-202, 230-232.
Garay LA. 1973. Systematics of the genus Angraecum (Orchidaceae). – Kew Bull. 28: 495-516.
Garay LA. 1974. Orquideas colombianas nuevas o críticas XII. – Orquideologia 9: 133-141.
Garay LA. 1975. Orquideas colombianas nuevas o críticas XIII. – Orquideologia 10: 63-71.
Garay LA. 1977. Systematics of the Physurinae (Orchidaceae) in the New World. – Bradea 2: 191-208.
Garay LA. 1978. 225(1). Orchidaceae (Cypripedioideae, Orchidoideae and Neottioideae). – In: Flora of Ecuador 9, Swedish Natural Science Research Council, Stockholm, pp. 1-304.
Garay LA. 1979. The genus Phragmipedium. – Orchid Digest 43: 133-148.
Garay LA. 1982. A generic revision of the Spiranthinae. – Bot. Mus. Leafl. Harvard Univ. 28: 277-425.
Garay LA. 1986. Olim Vanillaceae. – Bot. Mus. Leafl. Harvard Univ. 30: 223-237.
Garay LA, Stacy JE. 1974. Synopsis of the genus Oncidium. – Bradea 1: 393-428.
Garay LA, Sweet HR. 1974. Orchids of southern Ryukyu Islands. – Cambridge, Massachusetts.
Garay LA, Wirth M. 1959. On the genera Mormolyca Fenzl and Cyrtoglottis Schltr. – Can. J. Bot. 37: 479-490.
Garay LA, Hamer F, Siegerist ES. 1994. The genus Cirrhopetalum and the genera of the Bulbophyllum alliance. – Nord. J. Bot. 14: 609-646.
Garbari F. 1968a. Sul rango tassonomico di Leopoldia Parl., Muscarimia Kostel., Muscari Mill. – Giorn. Bot. Ital. 101: 300-301.
Garbari F. 1968b. Il genere Muscari (Liliaceae): contributo alla revisione citotassonomica. – Giorn. Bot. Ital. 102: 87-105.
Garbari F. 1968c. Iconografia cromosomica di alcune Liliaceae. – Atti Soc. Tosc. Sci. Nat. Mem., Ser. B., 75: 163-178.
Garbari F. 1969. Nuove osservazioni citologiche sui generi Muscari e Leopoldia. – Giorn. Bot. Ital. 103: 1-9.
Garbari F, Greuter W. 1970. On the taxonomy and typification of Muscari Miller (Liliaceae) and allied genera, and on the typification of generic names. – Taxon 19: 329-335.
Garbari F, Senatori E. 1975. Il genere Allium L. in Italia VI. Contributo alla citosistematica di alcune specie. – Atti Soc. Tosc. Sci. Nat., Mem., Ser. B, 82: 1-23.
Garbari F, Greuter W, Miceli P. 1979. The Allium cupanii group: a preliminary taxonomic, caryological and leaf anatomical study. – Webbia 34: 459-480.
García N, Meerow AW, Soltis DE, Soltis PS.
2014. Testing deep reticulate evolution in Amaryllidaceae tribe Hippeastreae
(Asparagales) with ITS and chloroplast sequence data. – Syst. Bot. 39:
75-89.
García N, Folk RA, Meerow AW, Chamala S, Gitzendanner MA, Oliveira RS de, Soltis DE, Soltis PS. 2017. Deep reticulation and incomplete lineage sorting obscure the diploid phylogeny of rain-lilies and allies (Amaryllidaceae tribe Hippestreae). – Mol. Phylogen. Evol. 111: 231-247.
Garcia-Cruz J, Sosa V. 2005. Phylogenetic relationships and character evolution in Govenia (Orchidaceae). – Can. J. Bot. 83: 1329-1339.
Garcia-Cruz J, Sosa V. 2006. A new species of Govenia (Orchidaceae) from Chiapas, Mexico. – Brittonia 58: 259-263.
García-Mendoza A. 1987. Monografía del género Beschorneria Kunth, Agavaceae. – M.Sc. thesis, Universidad Nacional Autonoma de México, México, DF.
García-Mendoza A. 1998. Con sabor a maguey: guía de la colección nacional de Agavaceas y Nolináceas del Jardín Botánico del Instituto de Biología-UNAM. – Instituto de Biología, Universidad Nacional Autónoma de México, México DF.
García-Mendoza A. 2000. Revisión taxonómica de las species arborescentes de Furcraea (Agavaceae) en México y Guatemala. – Bol. Soc. Bot. México 66: 113-129.
García-Mendoza A. 2002. Distribution of Agave (Agavaceae) in Mexico. – Cact. Succ. J. (Los Angeles) 74: 177-187.
García-Mendoza A, Galván VR. 1995. Riquezas de las familias Agavaceae y Nolinaceae en México. – Bull. Soc. Bot. México 56: 7-24.
Gardiner LM, Kocyan A, Motes M, Roberts DL, Emerson BC. 2013. Molecular phylogenetics of Vanda and related genera (Orchidaceae). – Bot. J. Linn. Soc. 173: 549-572.
Garside S. 1936. The South African species of Spiloxene Salisb. – J. Bot. 74: 267-269.
Geerinck DJL. 1968. Considérations taxonomique au sujet des Haemodoraceae et des Hypoxidaceae (Monocotylédones). – Bull. Soc. Bot. Belg. 101: 265-278.
Geerinck DJL. 1969. Genera des Haemodoraceae et des Hypoxidaceae. – Bull. Jard. Bot. Nat. Belg. 39: 47-82.
Geerinck DJL. 1971. Hypoxidaceae in Flore du Congo, du Rwanda et du Burundi. – Jardin Botanique National de Belgique, Bruxelles.
Geerinck DJL. 1974. Revision of Australian Iridaceae. – Bull. Jard. Bot. Natl. Belg. 44: 29-60.
Geerinck DJL. 1977. Iridaceae. – In: Steenis CGGJ van (ed), Flora Malesiana I, 8(2), Sijthoff & Noordhoff International Publ., Alphen aan den Rijn, The Netherlands, pp. 77-84.
Geerinck DJL. 1984. Orchidaceae 1. – In: Flore d’Afrique centrale, Jardin botanique national de Belgique, Bruxelles.
Geerinck DJL. 1993. Amaryllidaceae (including Hypoxidaceae). – In: Kalkman C et al. (eds), Flora Malesiana I, 11(2), Rijksherbarium/Hortus Botanicus, Leiden, pp. 353-373.
Geerinck DJL. 2000. Quatre nouveaux taxons d’Orchidaceae des genres Liparis, Bulbophyllum, Polystachya et Calanthe découverts à São Tomé et Príncipe. – Syst. Geogr. Plants 70: 141-148.
Geerinck D, Parmentier I, Lejoly J. 2003. A new species of Polystachya sect. Polychaete (Orchidaceae) from Central Africa. – Syst. Geogr. Plants 73: 281-285.
Geitler L. 1941. Embryosäcke aus Pollenkörnern bei Ornithogalum. – Ber. Deutsch. Bot. Ges. 59: 419-423.
Gentry HS. 1982. Agaves of continental North America. – University of Arizona Press, Tucson, Arizona.
Gentry HS, Sauck JR. 1978. The stomatal complex in Agave: groups Deserticolae, Campaniflorae, Umbelliflorae. – Proc. Calif. Acad. Sci. 41: 371-387.
George A, Gonzales C, Stauss MS, Arditti J. 1973. Chemotaxonomic and ecological implications of anthocyanins in Elythranthera. – Biochem. Syst. 1: 45-49.
George AS. 1963. Notes on Western Australian Orchidaceae III. Elythranthera, a new genus for the enamel orchids. – Western Aust. Natur. 9: 3-9.
George AS. 1986. Acanthocarpus. – In: George AS (ed) Flora of Australia 46, Australian Government Publ. Service, Canberra, pp. 92-98.
Gereau RE, Meerow AW, Brako L. 1993. New combinations in Hippeastrum, Ismene, and Leptochiton (Amaryllidaceae) for the Flora of Peru. – Novon 3: 28-30.
Ghazanfar SA. 1996. The genus Dipcadi (Hyacinthaceae) in the Arabian Peninsula. – Kew Bull. 51: 803-807.
Gheshmedziev I. 1975. Cytotaxonomic studies of several species of onion from section Codonoprasum Reichenb. – Compr. Rend. Acad. Bulg. Sci. 28: 795-798.
Gheshmedziev I, Terzijski D. 1997. A scanning electron microscopic study of the spermoderm in Allium subgen. Codonoprasum (Allium
Ghosh S, Shivanna KR. 1984. Structure and cytochemistry of the stigma and pollen-pistil interactions in Zephyranthes. – Ann. Bot., N. S., 53: 91-105.
Gil ASB, Chukr NS, Giulietti AM, Amaral MCE. 2008. Pseudiris speciosa, a new genus and species of Trimezieae (Iridoideae, Iridaceae) from Chapada Diamantina, Brazil. – Proc. California Acad. Sci. Ser. 4, 59: 723-729.
Gilbert MG, Demissew S. 1992. Notes on the genus Aloe in Ethiopia: misinterpreted taxa. – Kew Bull. 47: 647-653.
Gilbert MG, Demissew S. 1997. Further notes on the genus Aloe in Ethiopia and Eritrea. – Kew Bull. 52: 139-152.
Giménez-Martín G. 1959a. Numero chromosomica en especies de Scilla. – Genet. Iber. 11: 97.
Giménez-Martín G. 1959b. Cariología de Scilla II. – Phyton 13: 145-152.
Gledhill D, Oyewole SO. 1972. The taxonomy of Albuca in West Africa. – Bol. Soc. Brot. 46: 93-106.
Godfrey MJ. 1928. Classification of the genus Ophrys. – J. Bot. London 66: 33-36.
Gohil RN, Koul AK. 1972. Desynapsis in some diploid and polyploid alliums. – Can. J. Genet. Cytol. 13: 723-728.
Gohil RN, Koul AK. 1973. Some adaptive genetic-evolutionary processes accompanying polyploidy in the Indian alliums. – Bot. Not. 126: 426-432.
Goldblatt P. 1971. Cytological and morphological studies in the southern African Iridaceae. – J. South Afr. Bot. 37: 317-460.
Goldblatt P. 1973. Contributions to the knowledge of Moraea (Iridaceae) in the summer rainfall region of South Africa. – Ann. Missouri Bot. Gard. 60: 204-249.
Goldblatt P. 1975. Revision of the bulbous Iridaceae of North America. – Brittonia 27: 373-385.
Goldblatt P. 1976a. Evolution, cytology and subgeneric classification in Moraea (Iridaceae). – Ann. Missouri Bot. Gard. 63: 1-23.
Goldblatt P. 1976b. Barnardiella: a new genus of the Iridaceae and its relationship to Gynandriris and Moraea. – Ann. Missouri Bot. Gard. 63: 309-313.
Goldblatt P. 1976c. Chromosome cytology of Hessea, Strumaria, and Carpolyza (Amaryllidaceae). – Ann. Missouri Bot. Gard. 63: 314-320.
Goldblatt P. 1976d. A revision of Moraea (Iridaceae) in the winter rainfall region of southern Africa. – Ann. Missouri Bot. Gard. 63: 657-786.
Goldblatt P. 1977. Systematics of Moraea (Iridaceae) in tropical Africa. – Ann. Missouri Bot. Gard. 64: 243-295.
Goldblatt P. 1979a. Preliminary cytology of Australasian Iridaceae. – Ann. Missouri Bot. Gard. 66: 851-855.
Goldblatt P. 1979b. Biology and systematics of Galaxia (Iridaceae). – J. South Afr. Bot. 45: 385-423.
Goldblatt P. 1980a. Redifinition of Homeria and Moraea (Iridaceae) in the light of biosystematic data, with Rheome gen. nov. – Bot. Not. 133: 85-95.
Goldblatt P. 1980b. Systematics of Gynandriris (Iridaceae), a Mediterranean-southern African disjunct. – Bot. Not. 133: 239-260.
Goldblatt P. 1981. Systematics, phylogeny, and evolution in Dietes (Iridaceae). – Ann. Missouri Bot. Gard. 68: 131-152.
Goldblatt P. 1982a. Chromosome cytology in relation to suprageneric systematics of Neotropical Iridaceae. – Syst. Bot. 7: 186-198.
Goldblatt P. 1982b. Corm morphology in Hesperantha (Iridaceae, Ixioideae) and a proposed infrageneric taxonomy. – Ann. Missouri Bot. Gard. 69: 370-378.
Goldblatt P. 1982c. Systematics of Freesia Klatt (Iridaceae). – J. South Afr. Bot. 48: 39-91.
Goldblatt P. 1982d. Notes on Geissorhiza (Iridaceae): the species in Madagascar. – Ann. Missouri Bot. Gard. 69: 379-381.
Goldblatt P. 1985. Systematics of the southern African genus Geissorhiza (Iridaceae-Ixioideae). – Ann. Missouri Bot. Gard. 72: 277-447.
Goldblatt P. 1989. The genus Watsonia. – Ann. Kirstenbosch Bot. Gard. 19: 1-148.
Goldblatt P. 1989b. Revision of the tropical African genus Zygotritonia (Iridaceae). – Bull. Mus. Hist. Nat. sér. 4, B, Adansonia 11: 199-212.
Goldblatt P. 1990a. Phylogeny and classification of the Iridaceae. – Ann. Missouri Bot. Gard. 77: 607-627.
Goldblatt P. 1990b. Status of the southern African Anapalina and Antholyza (Iridaceae), genera based solely on characters for bird pollination, and a new species of Tritoniopsis. – South Afr. J. Bot. 56: 577-582.
Goldblatt P. 1991. An overview of the systematics, phylogeny and biology of the southern African Iridaceae. – Contr. Bolus Herb. 13: 1-74.
Goldblatt P. 1992. Phylogenetic analysis of the South African genus Sparaxis (including Synnotia) (Iridaceae: Ixioideae), with two new species and a review of the genus. – Ann. Missouri Bot. Gard. 79: 143-159.
Goldblatt P. 1993. The woody Iridaceae: Nivenia, Klattia, and Witsenia. Systematic biology and evolution. – Timber Press, Portland, Oregon.
Goldblatt P. 1996a. Gladiolus in tropical Africa. – Timber Press, Portland, Oregon.
Goldblatt P. 1996b. Iridaceae. – In: Polhill RM (ed), Flora of tropical East Africa, A. A. Balkema, Rotterdam, pp. 1-89.
Goldblatt P. 1998. Reduction of Barnardiella, Galaxia, Gynandriris, Hexaglottis and Homeria in Moraea (Iridaceae: Irideae). – Novon 8: 371-377.
Goldblatt P. 2001. Phylogeny and classification of the Iridaceae and the relationships of Iris. – In: Colasante MA, Rudall PJ (eds), Irises and Iridaceae: biodiversity and systematics, Ann. Bot. (Roma), n. s., 1: 13-28.
Goldblatt P. 2006. Radiation of pollination systems in the Iridaceae of sub-Saharan Africa. – Ann. Bot. 97: 317-344.
Goldblatt P. 2012. Systematics of Patersonia (Iridaceae, Patersonioideae) in the Malesian Archipelago. – Ann. Missouri Bot. Gard. 98: 514-523.
Goldblatt P. 2013. Aristea (Iridaceae, Aristeoideae), a subgeneric classification. – Novon 22: 415-417.
Goldblatt P, Bernhardt P. 1990. Pollination biology of Nivenia (Iridaceae) and the presence of heterostylous self-compatibility. – Israel J. Bot. 39: 93-111.
Goldblatt P, Bernhardt P. 1999. Pollination mechanics of Moraea species (Iridaceae) with a staminal column. – Ann. Missouri Bot. Gard. 86: 47-56.
Goldblatt P, Henrich JE. 1987. Notes on Cipura (Iridaceae) in South and Central America, an new species from Venezuela. – Ann. Missouri Bot. Gard. 74: 333-340.
Goldblatt P, Henrich JE. 1991. Calydorea Herbert (Iridoideae-Tigridieae): notes on this New World genus and reduction to synonymy of Catila, Cardiostigma, Itysa, and Salpingostylis. – Ann. Missouri Bot. Gard. 78: 504-511.
Goldblatt P, Le Thomas A. 1997. Palynology, phylogenetic reconstruction and the classification of the Afro-Madagascan genus Aristea Aiton (Iridaceae). – Ann. Missouri Bot. Gard. 84: 263-284.
Goldblatt P, Mabberley DJ. 2005. Belamcanda Adanson included in Iris Linnaeus, and the new combination, I. domestica (Linnaeus) Goldblatt & Mabberley (Iridaceae: Irideae). – Novon 15: 128-132.
Goldblatt P, Manning JC. 1989. Chromosome number in Walleria (Tecophilaeaceae). – Ann. Missouri Bot. Gard. 76: 925-926.
Goldblatt P, Manning JC. 1990. Devia xeromorpha, a new genus and species of Iridaceae-Ixioideae from the Cape Province, South Africa. – Ann. Missouri Bot. Gard. 77: 359-364.
Goldblatt P, Manning JC. 1995. Phylogeny of the African genera Anomatheca and Freesia (Iridaceae: Ixioideae), and a new genus Xenoscapa. – Syst. Bot. 20: 161-178.
Goldblatt P, Manning JC. 1996. Aristeas and beetle pollination. – Veld Flora 82: 17-19.
Goldblatt P, Manning JC. 1998. Gladiolus in southern Africa. Systematics, biology, and evolution. – Fernwood Press, Cape Town.
Goldblatt P, Manning JC. 2006. Radiation of pollination systems in the Iridaceae of sub-Saharan Africa. – Ann. Bot. 97: 317-344.
Goldblatt P, Manning JC. 2007. Floral biology of Babiana (Iridaceae: Crocoideae): adaptive floral radiation and pollination. – Ann. Missouri Bot. Gard. 94: 709-733.
Goldblatt P, Manning JC. 2008. The Iris family: natural history and classification. – Timber Press, Portland, Oregon.
Goldblatt P, Manning JC. 2010. Geosiris albiflora (Geosiridoideae), a new species from the Comoro Archipelago. – Bothalia 40: 169-171.
Goldblatt P, Manning JC. 2014. Taxonomy of the Moraea saxicola complex (Iridaceae: Iridoideae) of arid, western southern Africa, with the new species, M. acocksii, M. geminifolia, M. quartzicola and M. teretifolia. – South Afr. J. Bot. 91: 75-83.
Goldblatt P, Manning JC. 2015. Systematics and biology of Lapeirousia, Codonorhiza, Psilosiphon & Schizorhiza in Southern Africa. – Strelitzia 35: 1-146.
Goldblatt P, Manning JC. 2016. Systematics of the southern African genus Ixia L. (Iridaceae): 5. Synopsis of section Ixia, including five new species. – South Afr. J. Bot. 104: 175-198.
Goldblatt P, Marais W. 1979. Savannosiphon gen. nov., a segregate of Lapeirousia (Iridaceae-Ixioideae). – Ann. Missouri Bot. Gard. 66: 845-850.
Goldblatt P, Rudall PJ. 1992. Relationships of Bobartia. – South Afr. J. Bot. 58: 304-309.
Goldblatt P, Takei M. 1993. Chromosome cytology of the African genus Lapeirousia (Iridaceae-Ixioideae). – Ann. Missouri Bot. Gard. 80: 961-973.
Goldblatt P, Takei M. 1997. Chromosome cytology of Iridaceae – patterns of variation, determination of base number, and modes of karyotype change. – Ann. Missouri Bot. Gard. 84: 285-304.
Goldblatt P, Vos MP de. 1989. The reduction of Oenostachys, Homoglossum and Anomalesia, putative sunbird pollinated genera, in Gladiolus L. (Iridaceae: Ixioideae). – Bull. Mus. Natl. Hist. Nat. Paris, sér. IV, sect. B, Adansonia 11: 417-428.
Goldblatt P, Henrich JE, Rudall PJ. 1984. Occurrence of crystals in Iridaceae and allied families and their phylogenetic significance. – Ann. Missouri Bot. Gard. 71: 1013-1020.
Goldblatt P, Rudall PJ, Cheadle VL, Dorr LJ, Williams CA. 1987. Affinities of the Madagascan endemic Geosiris, Iridaceae or Geosiridaceae. – Bull. Mus. Natl. Hist. Nat. Paris, sér. IV, sect. B, Adansonia 9: 239-248.
Goldblatt P, Rudall PJ, Henrich JE. 1990. The genera of the Sisyrinchium alliance (Iridaceae: Iridoideae): phylogeny and relationships. – Syst. Bot. 15: 497-510.
Goldblatt P, Manning JC, Bari A. 1991. Sulcus and operculum structure in the pollen grains of Iridaceae subfamily Ixioideae. – Ann. Missouri Bot. Gard. 78: 950-961.
Goldblatt P, Takei M, Razzaq ZA. 1993. Chromosome cytology in tropical African Gladiolus (Iridaceae). – Ann. Missouri Bot. Gard. 80: 461-470.
Goldblatt P, Manning JC, Bernhardt P. 1995. Pollination biology of Lapeirousia subgenus Lapeirousia (Iridaceae) in southern Africa: floral divergence and adaptation for longtongued fly pollination. – Ann. Missouri Bot. Gard. 82:517-534.
Goldblatt P, Manning JC, Rudall PJ. 1998. Iridaceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 295-333.
Goldblatt P, Manning JC, Bernhardt P. 2000. Adaptive radiation of pollination mechanisms in Sparaxis (Iridaceae: Ixioideae). – Adansonia, sér. III, 22: 57-70.
Goldblatt P, Bernhardt P, Manning JC. 2002. Floral biology of Romulea (Iridaceae: Crocoideae): a progression from a generalist to a specialist pollination system. – Adansonia, sér. III, 24: 243-262.
Goldblatt P, Savolainen V, Porteous O, Sostaric I, Powell M, Reeves G, Manning JC, Barraclough TG, Chase MW. 2002. Radiation in the Cape flora and the phylogeny of the peacock irises Moraea (Iridaceae) based on four plastid DNA regions. – Mol. Phylogen. Evol. 25: 341-360.
Goldblatt P, Manning JC, Davies TJ, Savolainen V, Rezai S. 2004. Cyanixia, a new genus for the Socotran endemic Babiana socotrana (Iridaceae-Crocoideae). – Edinburgh J. Bot. 60: 517-532.
Goldblatt P, Manning JC, Dunlop G. 2004. Crocosmia and Chasmanthe: biology, classification and cultivation. – Timber Press, Portland, Oregon.
Goldblatt P, Le Thomas A, Suarez-Cervera M. 2004. Phylogeny of the Afro-Madagascan Aristea (Iridaceae) revisited in the light of new data on pollen morphology. – Bot. J. Linn. Soc. 144: 41-68.
Goldblatt P, Bernhardt P, Manning JC. 2005. Pollination mechanisms in the African genus Moraea (Iridaceae, Iridoideae): floral divergence and adaptation for pollinators. – Adansonia, sér. III, 27: 21-46.
Goldblatt P, Davies TJ, Manning JC, Bank M van der, Savolainen V. 2006. Phylogeny of Iridaceae subfamily Crocoideae based on a combined multigene plastid DNA analysis. – In: Columbus JT, Friar EA, Porter JM, Prince LM, Simpson MG (eds), Monocots: comparative biology and evolution. Excluding Poales, Rancho Santa Ana Botanical Garden, Claremont, California. – Aliso 22: 399-411.
Goldblatt P, Rodriguez A, Powell MP, Davies TJ, Manning JC, Bank M van der, Savolainen V. 2008. Iridaceae ‘out of Australasia’? Phylogeny, biogeography, and divergence time based on plastid DNA sequences. – Syst. Bot. 33: 495-508.
Goldblatt P, Bernhardt P, Manning JC. 2009. Adaptive radiation of the putrid perianth: Ferraria (Iridaceae; Irideae) and its unusual pollinators. – Plant Syst. Evol. 278: 53-65.
Goldblatt P, Phillipson PB, Manning JC. 2013. The new species Aristea farafangana Goldblatt & Phillipson, sp. nov. (Iridaceae) from Madagascar, biogeographic notes on Aristea Aiton in Madagascar and a revised key to the genus. – Adansonia 35: 47-53.
Goldblatt P, Manning JC, Schnitzler J. 2013. A revised infrageneric classification and synopsis of the Afro-Eurasian genus Moraea (Iridaceae: Irideae). – Bothalia 43: 29-42.
Goldblatt P, Manning JC, Demissew SS. 2015. Two new species of Zygotritonia Mildbr. (Iridaceae: Crocoideae) from eastern tropical Africa with notes on the morphology of the genus. – South Afr. J. Bot. 96: 37-41.
Goldblatt P, Manning JC, Helme NA. 2015. New species and taxonomic notes in Ixia (Iridaceae: Crocoideae) from western South Africa. – South Afr. J. Bot. 98: 108-113.
Goldblatt P, Manning JC, Wyk PCV van 2015. New species, cominations and range extensions in Hesperantha Ker Gawl. (Iridaceae: Crocoideae) from western South Africa. – South Afr. J. Bot. 98: 114-121.
Goldblatt P, Manning JC, Roux A le. 2015. New taxa in Moraea subgenera Moraea and Vieusseuxia (Iridaceae: Irideae) from Western Cape, South Africa. – South Afr. J. Bot. 99: 69-74.
Goldblatt P, Manning JC, Demissew S, Malakasi P, Forest F. 2016. Relationships of the sub-Saharan African genus Zygotritonia Mildbr. (Iridaceae: Crocoideae) inferred from molecular analysis. – South Afr. J. Bot. 106: 5-7.
Goldman DH, Freudenstein JV, Kores PJ, Molvray M, Jarrell DC, Whitten WM, Cameron KM, Jansen RK, Chase MW. 2001. Phylogenetics of Arethuseae (Orchidaceae) based on plastid matK and rbcL sequences. – Syst. Bot. 26: 670-695.
Goldman DH, van den Berg C, Griffith MP. 2004. Morphometric circumscription of species and infraspecific taxa in Calopogon R. Br. (Orchidaceae). – Plant Syst. Evol. 247: 37-60.
Gómez-Pompa A, Villalobos-Pietrini R, Chimal A. 1971. Studies in the Agavaceae I. Chromosome morphology and number of seven species. – Madroño 21: 208-221.
Gomiz NE, Torretta JP, Aliscioni SS. 2017. New evidence of floral elaiophores and characterization of the oil flowers in the subtribe Oncidiinae (Orchidaceae). – Plant Syst. Evol. 303: 433-449.
González R. 1992 [1993]. Schiedeella garayana (Orchidaceae), una nueva especie del estado de Jalisco. – Bol. IBUG 1: 42-48.
Good-Avila SV, Souza V, Gaut BS, Eguiarte LE. 2006. Timing and rate of speciation in Agave (Agavaceae). – Proc. Natl. Acad. Sci. U.S.A. 103: 9124-9129.
Goodspeed TH. 1940. Amaryllidaceae from the University of California botanical expedition to the Andes. – Herbertia 7: 17-31.
Gorham A. 1953. The question of fertilization in Smilacina racemosa. – Phytomorphology 3: 44-50.
Górniak M, Mytnik-Ejsmont J, Rutkowski P, Tukallo P, Minasiewicz J, Szlachetko DL. 2006. Phylogenetic relationships within the subtribe Spiranthinae s.l. (Orchidaceae) inferred from the nuclear ITS region. – Biodivers. Res. Conserv. 1-2: 18-24.
Górniak M, Paun O, Chase MW. 2010. Phylogenetic relationships within Orchidaceae based on a low-copy nuclear coding gene, Xdh: congruence with organellar and nuclear ribosomal DNA results. – Mol. Phylogen. Evol. 56: 784-795.
Gottlieb-Tannenhain P von. 1904. Studien über die Formen der Gattung Galanthus. – Abh. Zool.-Bot. Ges. Wien 2(4).
Gou D, Li S, Chi Q, Sun W-G, Sha Z-F. 1991. Isolation and structure determination of a new saponin of Anemarrhena asphodeloides. – Acta Pharm. Sin. 26: 619-621. [In Chinese]
Gould FW. 1942. A systematic treatment of the genus Camassia Lindl. – Amer. Midl. Natur. 28: 712-742.
Gouws JB. 1949. Karyology of some South African Amaryllidaceae. – Plant Life 5: 54-81.
Govaerts R, Hjertson M. 2005. Proposal to conserve the name Zygopetalum (Orchidaceae) with that spelling. – Taxon 54: 835.
Govaerts R, Cribb P, Wood J. 2003. Monocot checklist – provisional checklist of Orchidaceae. – Royal Botanic Gardens, Kew.
Govaerts R & al. 2014. World Checklist of Orchidaceae. – The Royal Botanic Gardens, Kew. http://apps.kew.org/wcsp/
Govindappa DA. 1967. Contribution to the embryology of Hypoxis aurea Lour. – J. Indian Bot. Soc. 46: 193-198.
Govindappa DA, Shamakumari K. 1957. Development of embryo in Hypoxis aurea Lour. – J. Indian Bot. Soc. 36: 324-327.
Grace OM, Rønsted N. 2012. Unlocking the secrets of DNA in the classification of the aloes. – Aloe 49: 4-5.
Grace OM, Simmonds MSJ, Smith GF, Wyk A van. 2010. Chemosystematic evaluation of Aloe section Pictae (Asphodelaceae). – Biochem. Syst. Ecol. 38: 57-62.
Grace OM, Klopper RR, Figueiredo E, Smith GF. 2011. The aloe names book. – Strelitzia 28: 1-232.
Grace OM, Klopper RR, Smith GF, Crouch NR, Figueiredo E, Rønsted N, Wyk AE van. 2013. A revised generic classification for Aloe (Xanthorrhoeaceae subfam. Asphodeloideae). – Phytotaxa 76: 7-14.
Graham SW, Barrett S. 2004. Phylogenetic reconstruction of the evolution of stylar polymorphisms in Narcissus (Amaryllidaceae). – Amer. J. Bot. 91: 1007-1021.
Granick EB. 1944. A karyosystematic study of the genus Agave. – Amer. J. Bot. 31: 283-298.
Grant B. 1895. The orchids of Burma (including the Andaman Islands) described. – Hanthawaddy Press, Rangoon.
Grau J, Bayer E. 1991. Zur systematischen Stellung der Gattung Traubia Moldenke (Amaryllidaceae). – Mitt. Bot. Staatssamml. München 30: 479-484.
Graven P, Wittich PE, Bouman F, Nachtegal G, Kentgens APM. 1998. Characterization of phytomelan in the seed coat of Gasteria verrucosa (Mill.) H. Duval. – In: Wittich PE (ed), Seed development and carbohydrates, Landbouwuniversiteit Wageningen, Wageningen.
Gravendeel B, Chase MW, Vogel EF De, Roos MC, Mes THM, Bacmann K. 2001. Molecular phylogeny of Coelogyne (Epidendroideae, Orchidaceae) based on plastid RFLPs, matK and nuclear ribosomal ITS sequences: evidence for polyphyly. – Amer. J. Bot. 88: 1915-1927.
Gravendeel B, Smithson A, Slik FJW, Schuiteman A. 2004. Epiphytism and pollinator specialization: drivers for orchid diversity? – Phil. Trans. Roy. Soc. London, Ser. B, 359: 1523-1536.
Gravendeel B, Eurlings MCM, Berg C van den, Cribb PJ. 2004. Phylogeny of Pleione (Orchidaceae) and parentage analysis of its wild hybrids based on plastid and nuclear ribosomal ITS sequences and morphological data. – Syst. Bot. 29: 50-63.
Gravendeel B, Schuiteman A, de Vogel EF. 2005. Molecular dating and vicariance analysis of Coelogyninae (Orchidaceae). – In: Bakker FT, Chatrou LW, Gravendeel B, Pelser PB (eds), Plant species-level systematics: new perspectives on pattern and process, Regnum Vegetabile vol. 143, A. R. G. Gantner, Ruggel, Liechtenstein, pp. 131-148.
Gray B, Low YW. 2017. First record of Geosiris (Iridaceae: Geosiridoideae) from Australasia: a new record and a new species from the Wet Tropics of Queensland, Australia. – Candollea 72: 249-255.
Greene EL. 1886. Studies in the botany of California and parts adjacent I. Some genera which have been confused with the name Brodiaea. – Bull. Calif. Acad. Sci. 2: 125-144.
Greenwood EW. 1982. Viscidium types in Spiranthinae. – Orquídea (Mexico City), n.s. 8: 283-290.
Gregory M, Fritsch RM, Friesen NW, Khassanov FO, McNeal DW. 1998. Nomenclator alliorum: Allium names and synonyms – a world guide. – Royal Botanic Gardens, Kew.
Greilhuber J. 1982. Trends in der Chromosomenevolution von Scilla (Liliaceae). – Stapfia 10: 11-51.
Greilhuber J, Speta F. 1976. C-banded karyotypes in the Scilla hohenackeri group, S. persica, and Puschkinia (Liliaceae). – Plant Syst. Evol. 126: 149-188.
Greilhuber J, Speta F. 1978. Quantitative analyses of C-banded karyotypes, and systematics in the cultivated species of the Scilla siberica-group (Liliaceae). – Plant Syst. Evol. 129: 63-109.
Greilhuber J, Speta F. 1989. A Giemsa C-banding and DNA content study in Scilla cilicica and S. morrisii, two little known sibling species of the S. siberica alliance (Hyacinthaceae). – Plant Syst. Evol. 165: 71-83.
Greilhuber J, Deumling B, Speta F. 1981. Evolutionary aspects of chromosome banding, heterochromatin, satellite DNA, and genome size in Scilla (Liliaceae). – Ber. Deutsch. Bot. Ges. 94: 249-266.
Greizerstein EJ, Naranjo CA. 1987. Estudios cromosómicos en especies de Zephyranthes (Amaryllidaceae). – Darwiniana 28: 169-186.
Grindlay D, Reynolds T. 1986. The Aloe vera phenomenon: a review of the properties and modern uses of the leaf parenchyma gel. – J. Ethnopharm. 16: 117-151.
Groenewald BH. 1941. Die Aalwyne van Suid-Afrika, Suidwes-Afrika, Portugees Oos-Afrika, Swaziland, Basoetolanden ‘n Spesiale Ondersoek van die Klassifikasie, Chromosome en Areale van die Aloe maculatae. – Nasionale Pers, Bloemfontein, South Africa.
Grosvenor RK. 1976. A list of orchids indigenous in Rhodesia. – Excelsa 6: 77-86.
Grove AR. 1941. Morphological study of Agave lechuguilla. – Bot. Gaz. 103: 354-365.
Grundmann M, Rumsey FJ, Ansell SW, Russell SJ, Darwin SC, Vogel JC, Spencer M, Squirrell J, Hollingsworth PM, Ortiz S, Schneider H. 2010. Phylogeny and taxonomy of the bluebell genus Hyacinthoides, Asparagaceae [Hyacinthaceae]. – Taxon 59: 68-82.
Guaglianone ER. 1972. Sinopsis de las especies de Ipheion Raf. y Nothoscordum Kunth (Liliáceas) de Entre Rios y regions vecinas. – Darwiniana 17: 159-242.
Guaglianone ER. 1973. Nothoscordum andinum, espècie de Liliaceae nueva para la flora Argentina. – Darwiniana 18: 31-36.
Guaglianone ER, Arroyo-Leuenberger S. 2002. The South American genus Oziroë (Hyacinthaceae-Oziroëoideae). – Darwiniana 40: 61-76.
Guignard J-L, Mestre J-C. 1969. L’origine du cotyledon chez l’Agapanthus umbellatus L’Hér. – Compt. Rend. Hebd. Séances Acad. Sci. Paris 268: 3068-3070.
Guillaumin A. 1958. Plantes nouvelles, rares ou critiques des Serres du Muséum. (Notules sur quelques Orchidées d’Indo-Chine 19). – Bull. Mus. Natl. Hist. Nat. Paris, sér. II, 30(5): 458-463.
Guillaumin A. 1961. Plantes nouvelles, rares ou critiques des Serres du Muséum. (Notules sur quelques Orchidées d’Indo-Chine 26). – Bull. Mus. Natl. Hist. Nat. Paris, sér. II, 33(3): 332-335.
Guo J. 2015. Comparative micromorphology and anatomy of crested sepals in Iris (Iridaceae). – Intern. J. Plant Sci. 176: 627-642.
Guo Y-Y, Luo Y-B, Liu Z-J, Wang X-Q. 2012. Evolution and biogeography of the slipper orchids: Eocene vicariance of the conduplicate genera in the Old and New World tropics. – PLoS ONE 7(6), e38788.
Gurushidze M, Mashayekhi S, Blattner FR, Friesen N, Fritsch RM. 2007. Phylogenetic relationships of wild and cultivated species of Allium section Cepa inferred by nuclear rDNA ITS sequence analysis. – Plant Syst. Evol. 269: 259-269.
Gurushidze M, Fritsch RM, Blattner FR. 2008. Phylogenetic analysis of Allium subg. Melanocrommyum infers cryptic species and demands a new sectional classification. – Mol. Phylogen. Evol. 49: 997-1007.
Gurushidze M, Fritsch RM, Blattner FR. 2010. Species-level phylogeny of Allium subg. Melanocrommyum: incomplete lineage sorting, hybridization and trnF gene duplication. – Taxon 59: 829-840.
Gustafsson ALS, Verola CF, Antonelli A. 2010. Reassessing the temporal evolution of orchids with new fossils and a Bayesian relaxed clock, with implications for the diversification of the rare South American genus Hoffmannseggella (Orchidaceae: Epidendroideae). – BMC Evol. Biol. 10: 177. doi:10.1185/1471-2148-10-177.
Gustafsson M, Wendelbo P. 1975. Karyotype analysis and taxonomic comments on irises from SW and C Asia. – Bot. Not. 128: 208-226.
Gutiérrez J, Terrazas T, Luna-Vega I, Salazar GA. 2017. Phylogenetic analyses of the Milla complex (Brodiaeoideae: Asparagaceae), with an emphasis on Milla. – Bot. J. Linn. Soc. 185: 445-462.
Haeckel I. 1930. Über Iridaceen. – Flora 25: 1-82.
Hagerup O. 1952. The morphology and biology of some primitive orchid flowers. – Phytomorphology 2: 134-138.
Hágsater E, Soto MA, Salazar GA, Jiménez-Machorro R, López MA, Dressler RL. 2005. Orchids of Mexico. Instituto Chinoin, Mexico City.
Håkansson A. 1951. Parthenogenesis in Allium. – Bot. Not. 103: 143-176.
Hall AV. 1965. Studies of the South African species of Eulophia. – J. South Afr. Bot. 5(Suppl.): 1-248.
Hall AV. 1982. A revision of the southern African species of Satyrium. – Contr. Bolus Herb. 10: 1-142.
Hall IR. 1976. Vesicular mycorrhiza infection in the orchid Corybas macranthus. – Trans. Brit. Mycol. Soc. 66: 160.
Hallé N. 1977. Orchidacées. – In: Aubréville A, Leroy JF (eds), Flore de la Nouvelle-Calédonie et Dependances, Muséum National d’Histoire naturelle, Paris, 8, pp. 1-565.
Hallé N. 1986. Les élatères des Sarcanthinae et additions aux Orchidaceae de la Nouvelle-Calédonie. – Bull. Mus. Natl. Hist. Nat. Paris, sér. IV, sect. B, Adansonia: Botanique Phytochimie 3: 215-239.
Hallé N, Toilliez J. 1971. Le genre Nervilia (Orchidaceae) en Côte d’Ivoire. – Adansonia, sér. II, 11: 443-461.
Hallier H. 1897. Über Paphiopedilum amabile und die Hochgebirgsflora des Berges K’Lamm in West-Borneo nebst einer Übersicht über die Gattung Paphiopedilum. – Ann. Jard. Bot. Buitenzorg 14: 18-52.
Halpin KM. 2011. A chloroplast phylogeny of Agavaceae subfamily Chlorogaloideae with a focus on species relationships in Hastingsia. – Botany, Oklahoma State University, Stillwater, Oklahoma.
Halpin KM, Fishbein M. 2013. A chloroplast phylogeny of Agavaceae subfamily Chlorogaloideae: implications for the tempo of evolution on serpentine soils. – Syst. Bot. 38: 996-1011.
Handa K, Tsuji S, Tamura MN. 2001. Pollen morphology of Japanese Asparagales and Liliales (Lilianae). – Jap. J. Hist. Bot. 9: 85-125.
Hanelt P. 1990. Taxonomy, evolution and history. – In: Rabinowitch H, Brewster JL (eds), Onions and allied crops, vol. 1, Boca Raton, Florida, pp. 1-26.
Hanelt P, Fritsch R. 1994. Notes on some infrageneric taxa in Allium L. – Kew Bull. 49: 559-564.
Hanelt P, Fritsch R, Kruse J, Maaß H, Ohle H, Pistrick K. 1989. Allium L. sect. Porphyroprason Ekberg – Merkmale und systematische Stellung. – Flora 182: 69-86.
Hanelt P, Hammer K, Knüpffer H (eds). 1992. The genus Allium – taxonomic problems and genetic resources. Proceedings of an International Symposium held at Gatersleben, June 11-13, 1991, Institut für Pflanzengenetik und Kulturpflanzenforschung, Gatersleben.
Hanelt P, Schultze-Motel J, Fritsch R, Kruse J, Maaß HI, Ohle H, Pistrick K. 1992. Infrageneric grouping of Allium – the Gatersleben approach. – In: Hanelt P, Hammer K, Knüpffer H (eds), The genus Allium – Taxonomic problems and genetic resources, Institut für Pflanzengenetik und Kulturpflanzenforschung, Gatersleben, pp. 107-123.
Hankey AJ, Edwards TJ. 2008. Ledebouria mokobulanensis A. J. Hankey and T. J. Edwards (Hyacinthaceae) a new species from the high altitude grasslands of Mpumalanga. – South Afr. J. Bot. 74: 214-217.
Hannibal LS. 1967. A primitive amaryllid – Hannonia hesperidium. – Plant Life 23: 143-144.
Hanson MA. 1993. Dispersed unidirectional introgression from Yucca schidigera into Y. baccata (Agavaceae). – Ph.D. diss., Claremont Graduate School, Claremont, California.
Hapeman J, Inoeu K. 1997. Plant-pollinator interactions and floral radiation in Platanthera (Orchidaceae. – In: Givnish TJ, Sytsma KJ (eds), Molecular evolution and adaptive radiation, Cambridge University Press, Cambridge, pp. 433-454.
Harborne JB, Williams CA. 2001. The phytochemical richness of the Iridaceae and its systematic significance. – Ann. Bot. (Roma), n. s., 1: 43-50.
Harpke D, Meng S, Rutten T, Kerndorff H, Blattner FR. 2013. Phylogeny of Crocus (Iridaceae) based on one chloroplast and two nucler loci: ancient hybridization and chromosome number evolution. – Mol. Phylogen. Evol. 66: 617-627.
Harris W, Mann JD. 1994. Preliminary investigation of the suitability of Cordyline australis (Asphodelaceae) as a crop for fructose production. – New Zealand J. Crop Hort. Sci. 22: 439-451.
Hartl D, Severin I. 1981. Verwachsungen in Umfeld des Griffels bei Allium, Cyanastrum und Heliconia und den Monocotylen allgemein. – Beitr. Biol. Pflanzen 55: 235-260.
Hashimoto T. 1990. A taxonomic review of the Japanese Lecanorchis (Orchidaceae). – Ann. Tsukuba Bot. Gard. 9: 1-40.
Hashimoto T. 1991. Japanese indigenous orchids in colour. 2nd ed. – Tenchikari Assoc., Tokyo.
Havey MJ. 1991. Phylogenetic relationships among cultivated Allium species from restriction enzyme analysis of the chloroplast genome. – Theor. Appl. Genet. 81: 752-757.
Havey MJ. 1992a. Restriction enzyme analysis of the nuclear 45S ribosomal DNA of six cultivated alliums (Alliaceae). – Plant Syst. Evol. 181: 45-55.
Havey MJ. 1992b. Restriction enzyme analysis of the chloroplast and nuclear 45S ribosomal DNA of Allium sections Cepa and Phyllodolon (Alliaceae). – Plant Syst. Evol. 183: 17-31.
Hawkes AD. 1965. Encyclopedia of cultivated orchids. – Faber & Faber, London.
He C-X. 1994. Effects of extract from Hemerocallis citrina barroni (EHCB) and epidermal growth factor (EGF) on human dermal fibroblast proliferation. – Zhonghua Pifuke Zazhi 27: 218-220, 269. [In Chinese]
Heberle R. 1982. Caladenia in Western Australia and natural hybridization. – Orchadian 7: 78-83.
Hedrén M. 2001. Systematics of the Dactylorhiza euxina/incarnata/maculata polyploid complex (Orchidaceae) in Turkey: evidence from allozyme data. – Plant Syst. Evol. 229: 23-44.
Hedrén M, Sofie Nordström, Persson Hovmalm HA, Pedersen HAe, Hansson S. 2007. Patterns of polyploid evolution in Greek marsh orchids (Dactylorhiza; Orchidaceae) as revealed by allozymes, AFLPs, and plastid DNA data. – Amer. J. Bot. 94: 1205-1218.
Heenan PB, Lange PJ de. 2007. Two new species of Dianella (Hemerocallidaceae) from New Zealand. – New Zealand J. Bot. 45: 269-285.
Heenan PB, Mitchell AD, Lange PJ de. 2004. Arthropodium bifurcatum (Lomandraceae), a new species from northern New Zealand. – New Zealand J. Bot. 42: 233-246.
Hegde SN. 1984. Orchids of Arunachal Pradesh. – Forest Dept. Arunachal Pradesh, Itanagar.
Heideman ME. 1979. Taxonomic studies in the genus Hypoxis L. (Hypoxidaceae) on Witwatersrand. – M.Sc. thesis, University of Witwatersrand, Republic of South Africa.
Heideman ME. 1983. Studies of diagnostic features in the genus Hypoxis L. (Hypoxidaceae R. Br.) on the Witwatersrand. – Bothalia 14: 889-893.
Heldreich T von. 1878. Über die Liliaceen-Gattung Leopoldia und ihre Arten. – Bull. Soc. Imp. Nat. Moscou 53: 56-75.
Henderson RJF. 1982. Romnalda grallata, a new species of the Xanthorrhoeaceae from Queensland. – Kew Bull. 37: 229-235.
Henderson RJF. 1985. Thelionema, a new genus of the Phormiaceae from Australia. – Austrobaileya 2: 109-111.
Henderson RFJ. 1987a. Dianella, Stypandra, Thelionema. – In: George AS (ed), Flora of Australia 45, Australian Government Printing Service, Canberra, pp. 193-230.
Henderson RFJ. 1987b. Chamaescilla. – In: George AS (ed), Flora of Australia 45, Australian Government Printing Service, Canberra, pp. 288-292.
Henderson RFJ. 1987c. Corynotheca. – In: George AS (ed), Flora of Australia 45, Australian Government Printing Service, Canberra, pp. 299-306.
Henderson RFJ. 1987d. Chlorophytum. – In: George AS (ed), Flora of Australia 45, Australian Government Printing Service, Canberra, pp. 349-350.
Henderson RFJ, Clifford HT. 1984. A recircumscription of the Phormiaceae Agardh. – Taxon 33: 423-427.
Hepper FN. 1968. Notes on tropical African monocotyledons. – Kew Bull. 21: 491-498.
Herden T, Hanelt P, Friesen N. 2016. Phylogeny of Allium L. subgenus Anguinum (G. Don ex W. D. J. Koch) N. Friesen (Amaryllidaceae). – Mol. Phylogen. Evol. 95: 79-93.
Hernández S LG. 1993. Cladistic analysis of the American genera of Asparagales and the systematic study of Beaucarnea (Nolinaceae) and Hemiphylacus (Hyacinthaceae). – Ph.D. diss., University of Texas, Austin, Texas.
Hernández S LG. 1995a. Análisis cladístico de la familia Agavaceae. – Bol. Soc. Bot. Méx. 56: 57-68.
Hernández S LG. 1995b. Taxonomic study of the Mexican genus Hemiphylacus (Hyacinthaceae). – Syst. Bot. 20: 546-554.
Hernández S LG, Zamudio S. 2003. Two new remarkable Nolinaceae from Central Mexico. – Brittonia 55: 226-232.
Herter WG. 1943. Beauverdia genus
novum Liliacearum. – Boissiera 7: 505-512.
Heslop-Harrison Y. 1977. The pollen-stigma interaction: pollen-tube penetration in Crocus. – Ann. Bot., N. S., 41: 913-922.
Hesse M, Burns-Balogh P, Wolff M. 1989. Pollen morphology of the “primitive” epidendroid orchids. – Grana 28: 261-278.
Hesse M, Halbritter H, Weber M. 2009. Beschorneria yuccoides and Asimina triloba (L.) Dun.: examples for proximal polar germinating pollen in angiosperms. – Grana 48: 151-159.
Heusser K. 1915. Die Entwicklung der generativen Organe von Himantoglossum hircinum Spr. – Beih. Bot. Centralbl. 32: 218-277.
Heyduk K, McKain MR, Lalani F, Leebens-Mack J. 2016. Evolution of a CAM anatomy predates the origins of Crassulacean acid metabolism in the Agavoideae (Asparagaceae). – Mol. Phylogen. Evol. 105: 102-113.
Hidayat T, Yukawa T, Ito M. 2005. Molecular phylogenetics of subtribe Aeridinae (Orchidaceae): insights from plastid matK and nuclear ribosomal ITS sequences. – J. Plant Res. 118: 271-284.
Hilliard OM, Burtt BL. 1978. Notes on some plants of southern Africa, chiefly form Natal VII (Hypoxidaceae). – Notes Roy Bot. Gard. Edinb. 36: 43-76.
Hilliard OM, Burtt BL. 1988. A revision of Galtonia (Liliaceae). – Notes Roy. Bot. Gard. Edinb. 45: 95-104.
Hillman EM, Warren A. 1973. Survey of Ruscus aculeatus on Bookham Common: the first two years. – Lond. Natur. 52: 93-103.
Hilton-Taylor C, Smith GF. 1994. The conservation status of Aloaceae in southern Africa. – In: Huntley BJ (ed), Botanical diversity in southern Africa, Strelitzia 1, National Botanical Institute, Pretoria, pp. 287-303.
Hirmer M. 1920. Beiträge zur Organographie der Orchideenblüte. – Flora 113: 213-310.
Hirsch A. 1977. A developmental study of the phylloclades of Ruscus aculeatus L. – Bot. J. Linn. Soc. 74: 355-365.
Hirschegger P, Jakse J, Trontelj P, Bohanec B. 2010. Origins of Allium ampeloprasum horticultural groups and a molecular phylogeny of the section Allium (Allium: Alliaceae). – Mol. Phylogen. Evol. 54: 488-497.
Ho PH. 1960. Orchidaceae. – Cây-Co Miên-Nam Vietnam, ed. 1, Saigon, pp. 596-630.
Ho PH. 1972. Orchidaceae. – Cây-Co Miên-Nam Vietnam 2, ed. 2, Saigon, pp. 998-1101.
Hochstätter F. 2009. The genus Hesperaloe (Agavaceae). – Cactus World 27: 97-106.
Hoffman MT. 1988. The pollination ecology of Aloe ferox Mill. – South Afr. J. Bot. 54:345-350.
Hoffman N, Brown A. 1992. Orchids of South-West Australia. 2nd ed. – University of Western Australia Press, Perth, Western Australia.
Hoffman N, Brown A. 1998. Orchids of South-West Australia. 2nd ed. with supplement. – University of Western Australia Press, Perth, Western Australia.
Hofmann C-C, Zetter R. 2010. Upper Cretaceous sulcate pollen from the Timerdyakh formation, Vilui Basin (Siberia). – Grana 49: 170-193.
Holford P, Croft JH, Newbury HJ. 1991. Differences between, and possible origins of the cytoplasms found in fertile and male-sterile onions (Allium cepa L.). – Theor. Appl. Genet. 82: 737-744.
Holland PG. 1978. An evolutionary biogeography of the genus Aloe. – J. Biogeogr. 5: 213-226.
Holmber EL. 1905. Amaryllidáceas argentinas, indígenas y exóticas cultivadas. – An. Mus. Nac. Buenos Aires, ser. III, 5: 75-192.
Holttum RE. 1957. Orchids of Malaya. – In: Holttum RE (ed), Flora of Malaya 1, 2nd ed., Government Printing Office, Singapore.
Holttum RE. 1959. Evolutionary trends in the Sarcanthine orchids. – Bull. Amer. Orchid Soc. 28: 747-754.
Holttum RE. 1964. Orchids of Malaya. 3rd ed. – In: Holttum RE (ed), Flora of Malaya 1, 4th ed., Government Printing Office, Singapore.
Holtzmeier MA, Stern WL, Judd WS. 1998. Comparative anatomy and systematics of Senghas’s cushion species of Maxillaria (Orchidaceae). – Bot. J. Linn. Soc. 127: 43-82.
Hong D-Y, Zhu X-Y. 1990. Report on karyotypes of 6 species in 4 genera of Poligonateae from China. – Acta Phytotaxon. Sin. 28: 185-198. [In Chinese with English summary]
Hong D-Y, Ma LM, Chen T. 1987. A discussion on the karyotype and evolution of the tribe Convallarieae s.l. (Liliaceae). – In: Hong DY (ed), Plant chromosome research, Nishiki Print Co., Hiroshima.
Hooker JD. 1892. Pasithea caerulea. – Bot. Mag. 118: t. 7249.
Hoover RF. 1939a. A revision of the genus Brodiaea. – Amer. Midl. Natur. 22: 551-574.
Hoover RF. 1939b. A definition of the genus Brodiaea. – Bull. Torrey Bot. Club 66: 161-166.
Hoover RF. 1940a. A monograph of the genus Chlorogalum. – Madroño 5: 137-147.
Hoover RF. 1940b. The genus Dichelostemma. – Amer. Midl. Natur. 24: 463-476.
Hoover RF. 1941. A systematic study of Triteleia. – Amer. Midl. Natur. 25: 73-100.
Hoover RF. 1955. Further observations on Brodiaea and some related genera. – Plant Life 11: 13-22.
Hopper SD. 2009. Taxonomic turmoil down-under: recent developments in Australian orchid systematics. – Ann. Bot. 104: 447-455.
Hopper SD, Brown AP. 2000. New genera, subgenera, combinations, and species in the Caladenia alliance (Orchidaceae: Diurideae). – Lindleyana 15: 120-126.
Hopper SD, Brown AP. 2001. Contributions to Western Australian orchidology 2. New taxa and circumscriptions in Caladenia (spider, fairy and dragon orchids of Western Australia). – Nuytsia 14:27-314.
Hopper SD, Brown AP. 2004a. Robert Brown’s Caladenia revisited, including a revision of its sister genera Cyanicula, Ericksonella and Pheladenia (Caladeniinae: Orchidaceae). – Aust. Syst. Bot. 17: 171-240.
Hopper SD, Brown AP. 2004b. Robert Brown’s Caladenia and Pterostylis revisited. – Orchadian 14: 366-371.
Hopper SD, Brown AP. 2006. Australia’s wasp-pollinated flying duck orchids revised (Paracaleana: Orchidaceae). – Aust. Syst. Bot. 19: 211-244.
Hopper SD, Brown AP. 2007. A revision of Australia’s hammer orchids (Drakaea: Orchidaceae), with some field data on species-specific sexually deceived wasp pollinators. – Aust. Syst. Bot. 20: 252-285.
Horowitz A. 1901-1902. Ueber den anatomischen Bau und das Aufspringen der Orchideen-früchte. – Beih. Bot. Centralbl. 11: 486-521.
Hosseinzadeh NH, Saeidi MS, Zarre S, Fritsch R. 2009. Pollen morphology of selected species of Allium (Alliaceae) distributed in Iran. – Nord. J. Bot. 27: 54-60.
Hou M-F, Liu Y, Kono Y, Peng C-I. 2009. Aspidistra daxinensis (Ruscaceae) a new species from limestone areas in Guangxi, China. – Bot. Stud. 50: 371-378.
Howard TH. 1970. Some bulbous and cormous plants of Mexico and Guatemala. – Plant Life 26: 14-32.
Howard TM. 1999. Three new Milla species from Mexico. – Herbertia 54: 232-237.
Howell DJ, Roth S. 1981. Sexual reproduction in agaves: the benefits of bats; the cost of semelparous advertising. – Ecology 62: 1-7.
Howell G, Prakash N. 1990. Embryology and reproductive ecology of the Darling lily, Crinum flaccidum Herbert. – Aust. J. Bot. 38: 433-444.
Hu G-W, Long C-L, Jin X-H. 2008. Dendrobium wangliangii (Orchidaceae), a new species belonging to section Dendrobium from Yunnan, China. – Bot. J. Linn. Soc. 157: 217-221.
Hu S-Y. 1972. The Orchidaceae of China III. – Quart. J. Taiwan Mus. 25: 41-67
Hu S-Y. 1974a. The Orchidaceae of China VII. – Quart. J. Taiwan Mus. 27: 156-190.
Hu S-Y. 1974b. The Orchidaceae of China VIII. – Quart. J. Taiwan Mus. 27: 419-467.
Hu S-Y, Barretto G. 1976. New species and varieties of Orchidaceae from Hong Kong. – Chung Chi J. 13: 1-34.
Hu S-Y, Yang J. 1989. The structure of pollen wall and its relation to pollen aggregation in Cymbidium goeringii (Rchb. f.) Rchb.f. – Acta Bot. Sin. 31: 414-421.
Huang D-Q, Yang J-T, Zhou C-J, Zhou S-D, He
X-J. 2014. Phylogenetic reappraisal of Allium subgenus
Cyathophora (Amaryllidaceae) and related taxa,
with a proposal of two new sections. – J. Plant Res. 127: 275-286.
Huang J, Li H. 1990. Study on the taxonomic system of the genus Tupistra. – Acta Bot.Yunnan. [Suppl.] 3: 49-61. [In Chinese with English summary]
Huang S-M, Sterling C. 1970. Laticifers in bulb scales of Allium. – Amer. J. Bot. 57: 1000-1002.
Hume HH. 1938. The genus Haylockia. – Proc. Florida Acad. Sci. 2: 91.
Hume HH. 1961. Ophiopogon-Liriope complex. – Baileya 9: 134-158.
Hunt PF. 1968. African orchids XXXII. – Kew Bull. 22: 489-492.
Hunziker AT. 1969. Estudios sobre Amaryllidaceae III. Sinopsis provisional de Hieronymiella, y novedades Argentinas sobre Zephyranthes. – Kurtziana 5: 343-367.
Hunziker AT, Di Fulvio ET. 1973. Una nueva especie de Habranthus (Amaryllidaceae) de la provincia de Buenos Aires. – Kurtziana 7: 255-259.
Hutchinson J. 1939. The tribe Gilliesieae of Amaryllidaceae. – Herbertia 6: 136-145.
Hutchison PC. 1951. Studies in the genus Haworthia Duval 1. Vegetative variation in the section Fenestratae Poellnitz. – Cact. Succ. J. (Los Angeles) 23: 99-102.
Huynh DL. 1977. Quelques phenomènes de polarité du pollen des Orchidaceae. – Ber. Schweiz. Bot. Ges. 86: 115-128.
Immelman K. 1979. A revision of the genus Holothrix. – Thesis, University of Cape Town, Republic of South Africa.
Inácio CD, Chauveau O, Souza-Chies TT, Sauquet H, Eggers L. 2017. An updated phylogeny and infrageneric classification of the genus Sisyrinchium (Iridaceae): challenges of molecular and morphological evidence. – Taxon 66: 1317-1348.
Inariyama S. 1931. Cytological studies in the genus Lycoris. – Bot. Mag. (Tokyo) 45: 11-26.
Inariyama S. 1933. Phylogeny of Lycoris from the karyological point of view. – Rep. Jap. Sci. Congr. 8.
Inariyama S. 1937. Karyotype studies in Amaryllidaceae I. – Sci. Rep. Tokyo Bunrika Daigaku, Sect. B, 3(52): 95-113.
Inariyama S. 1953. Cytological studies in Lycoris. – Seiken Ziho 6: 5-10.
Inda LA, Pimental M, Chase MW. 2010. Contribution of mitochondrial cox1 intron sequences to the phylogenetics of the tribe Orchideae (Orchidaceae): Do the distribution and sequence of this intron in orchids also tell us something about its evolution? – Taxon 59: 1053-1064.
Inda LA, Pimentel M, Chase MW. 2012. Phylogenetics of tribe Orchideae (Orchidaceae: Orchidoideae) based on combined DNA matrices: inferences regarding timing of diversification and evolution of pollination syndromes. – Ann. Bot. 110: 71-90.
Indsto JO, Weston PH, Clements MA. 2009. A molecular phylogenetic analysis of Diuris (Orchidaceae) based on AFLP and ITS reveals three major clades and a basal species. – Aust. Syst. Bot. 22: 1-15.
Ingram JA. 1953. A monograph of the genera Bloomeria and Muilla (Liliaceae). – Madroño 12: 19-27.
Ising G. 1969. Cytogenetic studies in Cyrtanthus III. Aneuploidy and structural chromosome changes. – Hereditas 63: 213-256.
Ising G. 1970. Evolution of karyotypes in Cyrtanthus. – Hereditas 65: 1-28.
Ito M, Kawamoto A, Kita Y, Yukawa T, Kurita S. 1999. Phylogeny of Amaryllidaceae based on matK sequence data. – Jap. J. Plant Res. 112: 207-216.
Jaarsveld EJ van. 1989. A revision of the genus Gasteria (Asphodelaceae). – M.Sc. thesis, University of Natal, Pietermaritzburg, Republic of South Africa.
Jaarsveld EJ van. 1992. A synopsis of the genus Gasteria. – Aloe 29: 5-33.
Jaarsveld EJ van. 1994. Gasterias of South Africa: a new revision of a major succulent group. – Fernwood Press and the National Botanical Institute, Cape Town, Republic of South Africa.
Jaarsveld EJ van. 2007. The genus Gasteria: a synoptic review (new taxa and combinations). – Aloe 44: 84-104.
Jaarsveld EJ van. 2010. Aloe tongaensis, a new species from Tongaland, KwaZulu-Natal (South Africa), and a new sectional arrangement of the tree aloes. – Aloe 47: 64-71.
Jaarsveld EJ van, Smith GF, Wyk B-E van. 1994. A cladistic analysis of Gasteria (Aloaceae). – South Afr. J. Sci. 90: 467-470.
Jaarsveld EJ van, Wyk AE van, Blake T. 2007. Ornithogalum cremnophilum. – Flow. Plants Africa 60: 8-13.
Jain SK, Mehrotra A. 1984. A preliminary inventory of Orchidaceae of India. – Botanical Survey of India, Department of Environment, Calcutta.
Jakubska A. 2007. Analysis of the exine micromorphology of Epipactis helleborine (L.) Crantz and Epipactis albensis Nováková et Rydlo (Orchidaceae: Neottieae) and its application to genus taxonomy. – Genus Suppl. 14: 47-49.
Jalal JS, Jayanthi J, Schuteman A. 2014.
Xenikophyton Garay (Orchidaceae – Aeridinae), a new
synonym of Schoenorchis Reinw. ex Blume. – Kew Bull. 69: 9508.
Janes JK, Duretto MF. 2010. A new classification for subtribe Pterostylidinae (Orchidaceae) reaffirming Pterostylis in the broad sense. – Aust. Syst. Bot. 23: 260-269.
Jang CG, Pfosser M. 2002. Phylogenetics of Ruscaceae sensu lato based on plastid rbcL and trnL-F DNA sequences. – Stapfia 80: 333-348.
Jara-Arancio P, Arroyo MTK, Guerrero PC, Hinojosa LF, Arancio G, Méndez MA. 2013. Phylogenetic perspectives on biome shifts in Leucocoryne (Alliaceae) in relation to climatic niche evolution in western South America. – J. Biogeogr. 41: 328-338.
Jauzein P, Tison J-M. 1999. Hypothèses sur les liens entre Allium oleraceum L., Allium oporinanthum Brullo, Pavone & Salmeri et Allium savii Parl. – J. Bot. Soc. Bot. France 11: 55-58.
Jauzein P, Tison J-M. 2001. Étude analytique du genre Allium L., sous-genre Codonoprasum (Reichenb.) Zahar., section Codonoprasum Reichenb., en France. – J. Bot. Soc. Bot. France 15: 29-49.
Jayeola AA, Thorpe JR. 2000. A scanning electron microscope study of the genus Calyptrochilum Kraenzl. (Orchidaceae) in West Africa. – Feddes Repert. 111: 315-320.
Jeffrey C. 1980. The genus Polygonatum (Liliaceae) in eastern Asia. – Kew Bull. 34: 435-471.
Jeffrey C. 1982. Further note on eastern Asian Polygonatum (Liliaceae). – Kew Bull. 34: 335-339.
Jensen BS, Christensen SB, Jäger AC, Rønsted N. 2011. Amaryllidaceae alkaloids from the Australian tribe Calostemmateae with acetylcholinesterase inhibitory activity. – Biochem. Syst. Ecol. 39: 153-155.
Jeppe B. 1969. South African Aloes. – Purnell, Cape Town, South Africa.
Jernstedt JA. 1980. Anthesis and floral senescence in Chlorogalum pomeridianum (Liliaceae). – Amer. J. Bot. 67: 824-832.
Jersáková J, Johnson SD, Kindlmann P. 2006. Mechanisms and evolution of deceptive pollination in orchids. – Biol. Rev. 81: 219-235.
Jesson LK, Barrett SCH. 2003. The comparative biology of mirror-image flowers. – Intern. J. Plant Sci. 164(Suppl.): S237-S249.
Jessop JP. 1966. The genus Asparagus in South Africa. – Bothalia 9: 31-96.
Jessop JP. 1970. Studies in the bulbous Liliaceae in South Africa 1. Scilla, Schizocarphus and Ledebouria. – J. South Afr. Bot. 36: 223-266.
Jessop JP. 1972a. Studies in the bulbous Liliaceae in South Africa 2. Drimiopsis and Resnova. – J. South Afr. Bot. 38: 151-162.
Jessop JP. 1972b. Studies in the bulbous Liliaceae in South Africa 3. The meiotic chromosomes of Ledebouria. – J. South Afr. Bot. 38: 249-259.
Jessop JP. 1975. Studies in the bulbous Liliaceae in South Africa 5. Seed surface characters and generic groupings. – J. South Afr. Bot. 41: 67-85.
Jessop JP. 1976. A revision of Peliosanthes (Liliaceae). – Blumea 23: 141-159.
Jessop JP. 1977. Studies in the bulbous Liliaceae in South Africa 7. The taxonomy of Drimia and certain allied genera. – J. South Afr. Bot. 43: 265-319.
Jessop JP. 1979. Liliaceae I. – In: Steenis CGGJ van (ed), Flora Malesiana I, 9(1), Sijthoff & Noordhoff International Publ., Alphen aan den Rijn, The Netherlands, pp. 189-235.
Jeyanayaghy S, Rao AN. 1966. Flower and seed development in Bromheadia finlaysoniana. – Bull. Torrey Bot. Club. 93: 97-103.
Jin W-T, Jin X-H, Schuiteman A, Li D-Z, Xiang
X-G, Huang W-C, Li J-W, Huang L-Q. 2014. Molecular systematics of subtribe
Orchidinae and Asian taxa of Habernariinae (Orchideae, Orchidaceae) based on plastid
matK, rbcL and nuclear ITS. – Mol. Phylogen. Evol. 77:
41-53.
Jin X. 1986. The chromosomes of Hemerocallis (Liliaceae). – Kew Bull. 41: 379-391.
Jin X-H. 2005a. Generic delimitation and a new infrageneric system in the genus Holcoglossum (Orchidaceae: Aeridinae). – Bot. J. Linn. Soc. 149: 465-468.
Jin X-H. 2005b. Bulbophyllum wuzhishanensis (Orchidaceae), a new species from Hainan, China. – Brittonia 57: 255-257.
Jin X-H, Li H. 2007a. A new species of Calanthe (Orchidaceae) from Yunnan, China. – Nord. J. Bot. 25: 20-22.
Jin X-H, Li H. 2007b. Listera fugongensis (Orchidaceae), a new species from Yunnan, China. – Brittonia 59: 243-244.
Jin X-H, Chen S-C, Li D-Z. 2007. Holcoglossum nujiangense (Orchidaceae: Aeridinae) – a new species and its pollination system. – Nord. J. Bot. 25: 125-128.
Jin X-H, Zhang T, Gu Z-J, Li D-Z. 2007. Cytological studies on the genus Holcoglossum (Orchidaceae). – Bot. J. Linn. Soc. 154: 283-288.
Jin X-H, Li D-Z, Xiang X-G, Lai Y-J, Shi X-C. 2012. Nujiangia (Orchidaceae: Orchideae): a new genus from the Himalayas. – J. Syst. Evol. 50: 64-71.
Johansen DA. 1932. The chromosomes of the Californian Liliaceae I. – Amer. J. Bot. 19: 779-783.
Johansen LB, Frederiksen S. 2002. Orchid flowers: development and evolution. – In: Cronk QCB, Bateman RM, Hawkins JA (eds), Developmental genetics and plant evolution, Taylor and Francis, London, pp. 206-219.
Johnson IM. 1938. The species of Sisyrinchium in Uruguay, Paraguay and Brazil. – J. Arnold Arbor. 19: 376-401.
Johnson KA, Morrison DA, Goldsack G. 1994. Post-fire flowering patterns in Blandfordia nobilis (Liliaceae). – Aust. J. Bot. 42: 49-60.
Johnson M, Garbari F, Mathew B. 1991. A cytotaxonomical approach to species delimitation of a group of early flowering Turkish Ornithogalum species (Hyacinthaceae). – Bot. Hron. (Patras) 10: 827-839.
Johnson MAT. 1994. Cytology of three new geophytes from Turkey. – Kew Bull. 49: 491-498.
Johnson MAT. 1999. Two new chromosome numbers in an unusual Albuca (Hyacinthaceae) from Saudi Arabia. – Kew Bull. 54: 437-444.
Johnson MAT. 2003. Polyploidy and karyotype variation in Turkish Bellevalia (Hyacinthaceae). – Bot. J. Linn. Soc. 143: 87-98.
Johnson MAT, Gale RM. 1983. Observations on the leaf anatomy, pollen, cytology and propagation of Calibanus Hookeri (Lem.) Trel. – Bradleya 1: 25-32.
Johnson MAT, Guner A. 2002. Iris stenophylla Hausskn. & Siehe ex Baker from Turkey and its cytology. – Bot. J. Linn. Soc. 140: 115-127.
Johnson SD, Edwards TJ. 2000. The structure and function of orchid pollinaria. – Plant Syst Evol. 222: 243-269.
Johnson SD, Linder HP, Steiner KE. 1998. Phylogeny and radiation of pollination systems in Disa (Orchidaceae). – Amer. J. Bot. 85: 402-411.
Johri MM. 1966. The style, stigma, and pollen tube III. Some taxa of the Amaryllidaceae. – Phytomorphology 16: 142-157.
Jones AG. 1979. A study of wild leek, and the recognition of Allium burdickii (Liliaceae). – Syst. Bot. 4: 29-43.
Jones DL. 1970. The pollination of Corybas diemenicus H. M. R. Rupp and W. H. Nicholls ex H. M. R. Rupp. – Victorian Natur. 87: 372-374.
Jones DL. 1974a. The pollination of Calochilus holtzei F. Muell. – Amer. Orchid Soc. Bull. 43: 604-606.
Jones DL. 1974b. The pollination of Acianthus caudatus R. Br. – Victorian Natur. 91: 272-274.
Jones DL. 1981. The pollination of selected Australian orchids. – In: Lawler L, Kerr RD (eds), Proceedings of the orchid symposium, 13th International Botanical Congress, Sydney, Australia, 1981, Orchid Society of New South Wales, Sydney, pp. 40-43.
Jones DL. 1988. Native orchids of Australia. – Reed Books, Frenchs Forest, Australia.
Jones DL. 2006a. A complete guide to native orchids of Australia including the island territories. – Reed New Holland, Sydney, New South Wales.
Jones DL. 2006b. Miscellaneous new species of Australian Orchidaceae. – Aust. Orchid Res. 5: 45-111.
Jones DL, Clements MA. 2002a. A review of Pterostylis R. Br. (Orchidaceae). – Aust. Orchid Res. 4: 1-168.
Jones DL, Clements MA. 2002b. Nomenclatural notes arising from studies in the tribe Diurideae (Orchidaceae): Calonemorchis. – Orchadian 14: 33-42.
Jones DL, Clements MA. 2003. Nomenclatural notes arising from studies in the tribe Diurideae (Orchidaceae): Jonesiopsis. – Orchadian 154: 179-183.
Jones DL, Wapstra H, Tonelli P, Harris S. 1999. The orchids of Tasmania. – Melbourne University Press, Carlton South, Australia.
Jones DL, Clements MA, Sharma IK, Mackenzie AM. 2001. A new classification of Caladenia R. Br. (Orchidaceae). – Orchadian 13: 389-419.
Jones DL, Clements MA, Sharma IK, Mackenzie AM, Molloy BPJ. 2002. Nomenclatural notes arising from studies into the tribe Diurideae (Orchidaceae). – Orchadian 13: 437-468.
Jones K, Smith JB. 1967a. Chromosome evolution in the genus Crinum. – Caryologia 20: 163-179.
Jones K, Smith JB. 1967b. The chromosomes of the Liliaceae I. The karyotypes of twenty-five tropical species. – Kew Bull. 21: 31-38.
Jones K, Lim KY, Cribb PJ. 1982. The chromosomes of orchids VII. Dendrobium. – Kew Bull. 37: 221-227.
Jonker FP. 1939. Les Geosiridacées: une nouvelle famille de Madagascar. – Rec. Trav. Bot. Néerl. 36: 473-479.
Jonsson L. 1979. The African member of Taeniophyllum (Orchidaceae). – Bot. Not. 132: 511-519.
Jonsson L. 1981. A monograph of the genus Microcoelia (Orchidaceae). – Symb. Bot. Ups. 23(4): 1-151.
Joseph J. 1982. Orchids of Nilgiris. – Rec. Bot. Surv. India 22: 1-144.
Joshi AC. 1937. Megasporogenesis in Aloe vera Linn. – J. Indian Bot.Soc. 15: 297-300.
Joshi AC, Pantulu JV. 1941. A morphological and cytological study of Polianthes tuberosa Linn. – J. Indian Bot. Soc. 20: 37-71.
Joyeux L. 1928. Valeur morphologique du cladode chez les Ruscées. – Mém. Acad. Roy. Belg., Bruxelles 9: 1-94.
Judd WS, Stern WL, Cheadle VI. 1993. Phylogenetic position of Apostasia and Neuwiedia (Orchidaceae). – Bot. J. Linn. Soc. 113: 87-94.
Julou T, Burghardt B, Gebauer G, Berveiller D, Damesin C, Selosse MA. 2005. Mixotrophy in orchids: insights from a comparative study of green individuals and nonphotosynthetic individuals of Cephalanthera damasonium. – New Phytol. 166: 639-653.
Jumelle H, Perrier de la Bâthie H. 1912. Les Nervilia et les Bulbophyllum du Nord-Ouest de Madagascar. – Ann. Fac. Sci. Marseille 21: 187-216.
Kamari G. 1982. A biosystematic study of the genus Galanthus (Amaryllidaceae) in Greece I. Taxonomy. – Bot. Jahrb. Syst. 103: 107-135.
Karasawa K. 1979. Karyomorphological studies in Paphiopedilum, Orchidaceae. – Bull. Hiroshima Bot. Gard. 2: 1-149.
Karasawa K. 1980. Karyomorphological studies in Phragmipedium, Orchidaceae. – Bull. Hiroshima Bot. Gard. 3: 1-49.
Karasawa K. 1986. Karyomorphological studies on nine taxa of Paphiopedilum. – Bull. Hiroshima Bot. Gard. 8: 23-42.
Karasawa K, Aoyama M. 1986. Karyomorphological studies on Cypripedium in Japan and Formosa. – Bull. Hiroshima Bot. Gard. 31: 1-22.
Karasawa K, Aoyama M. 1988. Karyomorphological studies on two species of Paphiopedilum. – Bull. Hiroshima Bot. Gard. 10: 1-6.
Karasawa K, Saito K. 1982. A revision of the genus Paphiopedilum (Orchidaceae). – Bull. Hiroshima Bot. Gard. 5: 1-69.
Karasawa K, Tanaka R. 1980. C-banding study on centric fission in the chromosomes of Paphiopedilum. – Cytologia 45: 97-102.
Karasawa K, Tanaka R. 1981. A revision of chromosome numbers in some hybrids of Paphiopedilum. – Bull. Hiroshima Bot. Gard. 4: 1-8.
Karavokyrou E, Tzanoudakis D. 1991. The genus Allium in Greece II. A cytogeographical study of the E Aegean species. – Bot. Chron. 10: 777-784.
Karlén T. 1985. Karyotypes and chromosome numbers of five species of Muscari (Liliaceae). – Willdenowia 14: 313-320.
Karremans A, Bakker F, Pupulin F, Solano-Gómez R, Smulders M. 2013. Phylogenetics of Stelis and closely related genera (Orchidaceae: Pleurothallidinae). – Plant Syst. Evol. 299: 151-176.
Karst L, Wilson CA. 2012. Phylogeny of the New World genus Sisyrinchium (Iridaceae) based on analyses of plastid and nuclear DNA sequence data. – Syst. Bot. 37: 87-95.
Katayama Y. 1936. Chromosome studies in some Alliums. – J. Coll. Agric. Tokyo Imp. Univ. 13: 431-441.
Kativu S. 1993. Four new species of Chlorophytum (Anthericaceae) from tropical Africa. – Nord. J. Bot. 13: 501-505.
Kativu S. 1994. Anthericaceae in Zimbabwe: a study on cytology and reproduction. – In: Seyani JH, Chikuni AC (eds), Proceedings of the XIIIth Plenary Meeting AETFAT, Malawi, pp. 524-534.
Kativu S, Nordal I. 1993. New combinations of African species in the genus Chlorophytum (Anthericaceae). – Nord. J. Bot. 13: 59-65.
Kato M. 1995. The aspidistra and the amphipod. – Nature 377: 293.
Kauff F, Rudall PJ, Conran JG. 2000. Systematic root anatomy of Asparagales and other monocotyledons. – Plant Syst. Evol. 223: 139-154.
Kawano S, Ihara M, Suzuki M, Iltis HH. 1967. Biosystematic studies on Maianthemum (Liliaceae- Polygonateae) I. Somatic chromosome number and morphology. – Bot. Mag. (Tokyo) 80: 345-352.
Kawano S, Ihara M, Suzuki M. 1968. Biosystematic studies in Maianthemum (Liliaceae-Polygonateae) II. Geography and ecological life history. – Jap. J. Bot. 20: 35-65.
Kawano S Suzuki M, Kojima S. 1971. Biosystematic studies in Maianthemum (Liliaceae-Polygonateae) V. Variation in gross morphology, karyology and ecology of North American populations of M. dilatatum sensu lato. – Bot. Mag. (Tokyo) 84: 299-318.
Kay QON, Davies EW, Rich TCG. 2001. Taxonomy of the western European endemic Asparagus prostratus (A. officinalis subsp. prostratus) (Asparagaceae). – Bot. J. Linn. Soc. 137: 127-137.
Keator G. 1967. Ecological and taxonomic studies of the genus Dichelostemma. – Ph.D. diss., University of California, Berkeley, California.
Keator G. 1989. The brodiaeas. – Four Seasons 8: 4-11.
Keator G. 1992. Blud dicks Brodiaea (Dichelostemma capitatum): a common but problematic species. – Four Seasons 9: 24-39.
Keighery GJ. 1984a. Chromosome numbers of Australian Liliaceae. – Feddes Repert. 95: 523-532.
Keighery GJ. 1984b. The Johnsonieae (Liliaceae): biology and classification. – Flora 175: 103-108.
Keighery GJ. 1987. Laxmannia. – In: George AS (ed), Flora of Australia 45, Australian Government Publ. Service, Canberra, pp. 254-264.
Keighery GJ. 2001. Taxonomic notes on the genus Johnsonia (Anthericaceae). – Nuytsia 13: 479-481.
Kenton A, Heywood CA. 1984. Cytological studies in South American Iridaceae. – Plant Syst. Evol. 146: 87-104.
Kenton A, Rudall P. 1987. An unusual case of complex heterozygosity in Gelasine azurea (Iridaceae), and its implications for reproductive biology. – Evol. Trends Plants 1: 95-103.
Kenton A, Dickie JB, Langton DH, Bennett MD. 1990. Nuclear DNA amount and karyotype symmetry in Cypella and Hesperoxiphion (Tigridieae; Iridaceae). – Evol. Trends Plants 4: 59-69.
Kereszty Z, Zilágyi L, Borhidi A. 1986. Biosystematic studies of the Scilla bifolia complex in Hungary. – Symb. Bot. Ups. 27: 107-112.
Kerr AFG. 1933. A collection of orchids from Laos. – J. Siam Soc., Nat. Hist. [Suppl. 9], 2: 225-243.
Khaleel TF, Boraiah G. 1974. Cytotaxonomic studies in three species of Zephyranthes. – Bot. Not. 127: 508-512.
Khassanov FO. 1992. A revision of the genus Allium L. in the flora och Uzbekistan. – In: Hanelt P, Hammer K, Knüpffer H (eds), The genus Allium – taxonomic problems and genetic resources, Proceedings of an International Symposium in Gatersleben, June 11-13, 1991, Gatersleben, pp. 153-159.
Khassanov FO, Fritsch R. 1994. New taxa in Allium L. subg. Melanocrommyum (Webb et Berth.) Rouy from Central Asia. – Linzer Biol. Beitr. 26: 965-990.
Khoshoo TN, Raina SN. 1968. Cytogenetics of tropical bulbous ornamentals I. Heterozygosity in Crinum latifolium. – Cytologia 33: 209-219.
Kidd KK. 1963. An analysis of beard-hair morphology in the section Iris. – Aril Soc. Yearb. (1963), pp. 20-23.
Kim C, Cameron KM, Kim J-H. 2017. Molecular systematics and historical biogeography of Maianthemum s.s. – Amer. J. Bot. 104: 939-952.
Kim D-K, Kim JS, Kim J-H. 2012. The phylogenetic relationships of Asparagales in Korea based on five plastid DNA regions. – J. Plant Biol. 55: 325-345.
Kim J-H, Kim D-K, Forest F, Fay MF, Chase MW. 2010. Molecular phylogenetics of Ruscaceae sensu lato and related families (Asparagales) based on plastid and nuclear DNA sequences. – Ann. Bot. 106: 775-790.
Kim S-C, Lee NS. 2007. Generic delimitation and biogeography of Maianthemum and Smilacina (Ruscaceae sensu lato): preliminary results based on partial 3’matK and trnK 3’ intron sequences of cpDNA. – Plant Syst. Evol. 265: 1-12.
King G, Pantling R. 1896. Some new orchids from Sikkim. – J. Asiatic Soc. Bengal 65: 17-34.
King G, Pantling R. 1898. The orchids of Sikkim Himalaya. – Ann. Roy. Bot. Gard. Calcutta 8: 1-342.
Kizu H, Yamamoto M, Shimana H, Tomimori T. 1994. Seasonal variation of the contents of timosaponin B-II and mangiferin in the rhizome of Anemarrhena asphodeloides Bunge. – Shoyakugaku Zasshi 47: 426-428. [In Japanese]
Klatt FW. 1882. Ergänzungen und Berichtigungen zu Baker’s Systema Iridacearum. – Abhandl. Naturforsch. Ges. Halle 15: 335-404.
Klein E. 2005. Versuch einer Gliederung der Gattung Epipactis Zinn (Orchidaceae-Neottieae). – J. Eur. Orch. 37: 121-130.
Klercker JEF. 1883. Recherches sur la structure anatomique de l’Aphyllanthes monspeliensis Lin. – Bih. Kungl. Sv. Vetensk.-Akad. Handl. 8(6): 1-23.
Klopper RR, Smith GF. 2007. The genus Aloe L. (Asphodelaceae: Alooideae) in Namaqualand, South Africa. – Haseltonia 13: 1-13.
Klopper RR, Wyk B-E van, Smith GF. 2010. Phylogenetic relationships in the family Asphodelaceae (Asparagales). – Schumannia 6: 9-36.
Knudtzon SH, Stedje B. 1986. Taxonomy and cytology of the genus Albuca (Hyacinthaceae) in East Africa. – Nord. J. Bot. 6: 773-786.
Ko SC, Kim YO, Kim YS. 1985. A cytotaxonomical study of the tribe Ophiopogoneae in Korea. – Korean J. Plant Taxon. 15: 111-125.
Kocyan A. 2007. The discovery of polyandry in Curculigo (Hypoxidaceae): implications for androecium evolution of asparagoid monocotyledons. – Ann. Bot. 100: 241-248.
Kocyan A. 2010. Apostasioideae – the least known orchid subfamily. – Malesian Orchid J. 5: 125-138.
Kocyan A, Endress PK. 2001a. Floral structure and development of Apostasia and Neuwiedia (Apostasioideae) and their relationships to other Orchidaceae. – Intern. J. Plant Sci. 162: 847-867.
Kocyan A, Endress PK. 2001b. Floral structure and development and systematic aspects of some “lower” Asparagales. – Plant Syst. Evol. 229: 187-216.
Kocyan A, Qiu Y-L, Endress PK, Conti E. 2004. A phylogenetic analysis of Apostasioideae (Orchidaceae) based on ITS, trnL-F and matK sequences. – Plant Syst. Evol. 247: 203-213.
Kocyan A, Vogel EF de, Conti E, Gravendeel B. 2008. Molecular phylogeny of Aerides (Orchidaceae) based on one nuclear and two plastid markers: a step forward in understanding the evolution of the Aeridinae. – Mol. Phylogen. Evol. 48: 422-443.
Kocyan A, Snijman DA, Forest F, Devey DS, Freudenstein JV, Wiland-Szymańska J, Chase MW, Rudall PJ. 2011. Molecular phylogenetics of Hypoxidaceae – evidence from plastid DNA data and inferences on morphology and biogeography. – Mol. Phylogen. Evol. 60: 122-136.
Koehler S. 2004. A taxonomic study of the South American genus Bifrenaria Lindl. (Orchidaceae). – Brittonia 56: 314-345.
Koehler S, Williams NH, Whitten WM, do Amaral U, do Carmo E. 2002. Phylogeny of the Bifrenaria (Orchidaceae) complex based on morphology and sequence data from nuclear rDNA internal transcribed spacers (ITS) and chloroplast trnL-trnF region. – Intern. J. Plant Sci. 163: 1055-1066.
Koehler S, Cabral JS, Whitten WM, Williams NH, Singer RB, Neubig KM, Guerra M, Souza AP, Amaral M do CE. 2008. Molecular phylogeny of the neotropical genus Christensonella (Orchidaceae, Maxillariinae): species delimitation and insights into chromosome evolution. – Ann. Bot. 102: 491-507.
Koehler S, Singer RB, Amaral MCE. 2012. Taxonomic revision of the neotropical genus Christensonella (Maxillariinae, Orchidaceae). – Bot. J. Linn. Soc. 168: 449-474.
Koketsu M, Kim M, Yamamoto T. 1996. Antifungal activity against food-borne fungi of Aspidistra elatior Blume. – Phytochemistry 44: 301-303.
Kolanowska M, Szlachetko DL. 2014. Notes on Erycina-complex with descriptions of new Colombian species. – Plant Syst. Evol. 300: 527-534.
Kolanowska M, Szlachetko DL. 2015a. Notes on
Pachyphyllinae (Vandoideae, Orchidaceae) with a description of a new
genus. – Plant Syst. Evol. 301: 95-111.
Kolanowska M, Szlachetko DL. 2015b. Overview
of Cranichis (Orchidaceae,
Cranichidinae) and allied genera with notes on their Colombian representatives.
– Plant Syst. Evol. 301: 709-724.
Kolanowska M, Szlachetko DL, 2016. Problems
with generic delimitation in the Odontoglossum complex (Orchidaceae, Oncidiinae) and an attempt
for a solution. – Plant Syst. Evol. 302: 203-217.
Kolanowska M, Szlachetko DL. 2017. Synopsis of the genus Pterichis (Orchidacee) in Colombia. – Ann. Missouri Bot. Gard. 102: 87-124.
Kolanowska M, Szlachetko DL, Kras M. 2014.
Synopsis of the genus Psilochilus (Orchidaceae) in Colombia. – Syst. Bot.
39: 750-758.
Kollmann F. 1969. Cytotaxonomic polymorphism in the Allium erdelii group. – Israel J. Bot., Jer. Ser., 18: 61-75.
Kollmann F. 1985. The genus Allium in Israel. – Rotem 15.
Komar GA. 1976. The ultrastructure of seed appendages (elaiosomes) in Scilla sibirica, Scilla mischtschenkoana, and Chionodoxa gigantea (Liliaceae). – Bot. Žurn. 61: 332-341. [In Russian with English summary]
Komar GA. 1978. Arils and aril-like formations in some Liliales. – Bot. Žurn. 63: 937-955.
Königer W. 1995. New species of the genera Cyrtochilum, Masdevallia, Oncidium and Sigmatostalix. – Arcula 3: 58-89.
Königer W. 1996. Portillia – einie neue Gattung in der Subtribus Pleurothallidinae, neue Arten der Gattungen Portillia, Odontoglossum, Oncidium, Sigmatostalix, Trichocentrum und Trigonochilum. – Arcula 6: 154-185.
Konta F, Hayakawa E. 1982. Preliminary study of the pollen tetrads in the Orchidaceae. – Rep. Fac. Sci. Shizuoka Univ. 16: 103-108.
Konta F, Tsuji M. 1982. The types of pollen tetrads and their formation in some species of Orchidaceae in Japan. – Acta Phytotaxon. Geobot. 33: 206-217. [In Japanese]
Kores PJ. 1995. A systematic study of the genus Acianthus (Orchidaceae: Diurideae). – Allertonia 7: 1-220.
Kores PJ, Molvray M, Darwin SP. 1993. Morphometric variation in three species of Cyrtostylis (Orchidaceae). – Syst. Bot. 18: 274-282.
Kores PJ, Cameron KM, Molvray M, Chase MW. 1997. The phylogenetic relationships of Orchidoideae and Spiranthoideae (Orchidaceae) as inferred from rbcL plastid sequences. – Lindleyana 12: 1-11.
Kores PJ, Weston PH, Molvray M, Chase MW. 2000. Phylogenetic relationships within the Diurideae (Orchidaceae): inferences from plastid matK DNA sequences. – In: Wilson KL, Morrison DA (eds), Monocots: systematics and evolution, CSIRO, Melbourne, pp. 449-456.
Kores PJ, Molvray M, Weston PH, Hopper SD, Brown AP, Cameron KM, Chase MW. 2001. A phylogenetic analysis of Diurideae (Orchidaceae) based on plastid DNA sequence data. – Amer. J. Bot. 88: 1903-1914.
Kosenko VN. 1994. Pollen morphology of the families Phormiaceae, Blandfordiaceae, and Doryanthaceae. – Bot. Žurn. 79: 1-12. [In Russian with English summary]
Kosenko VN, Sventorzhetskaya O. 1999. Pollen morphology in the family Asphodelaceae (Asphodeleae, Kniphofieae). – Grana 38: 218-227.
Koshimizu T. 1930. Carpobiological studies of Crinum asiaticum L. var. japonicum Bak. – Mem. Coll. Sci. Kyoto Imp. Univ., Ser. B, Biology 5: 183-227.
Kottke I, Suárez JP, Herrera P, Cruz D, Bauer R, Haug I, Garnica S. 2010. Atractiellomycetes belonging to the ’rust’ lineage (Pucciniomycotina) form mycorrhizae with terrestrial and epiphytic neotropical orchids. – Proc. Roy. Soc., Ser. B, 277: 1289-1298.
Kozhevnikova AD, Vinogradova TN. 1999. Pseudobulb structure in some boreal terrestrial orchids. – Syst. Geogr. Plants 68: 59-65.
Kränzlin F. 1897. Orchidacearum et species plantarum. – Mayer & Müller, Berlin.
Kränzlin F. 1922. Orchidaceae-Monandrae. Tribus Oncidiinae – Odontoglosseae (Pars II). – Engelmann, Leipzig.
Krause K. 1930. Liliaceae. – In: Engler A (ed), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 15a, W. Engelmann, Leipzig, pp. 227-386.
Krenn L. 1990. Über die Bufadienolide des Urginea maritima Aggregates. – Ph.D. diss., Universität Wien, Austria.
Krenn L. 1994. Urginea. – In: Hagers Handbuch der pharmazeutischen Praxis, 5. Aufl., Bd. 6, F. v. Bruchhausen, pp. 1030-1050.
Krenn L, Kopp B, Speta F, Kubelka W. 2001. Chemotaxonomische Untersuchung der Gattung Charybdis Speta (Urgineoideae, Hyacinthaceae). – Stapfia 75: 101-120.
Kristiansen KA, Rasmussen FN, Rasmussen HN. 2001. Seedlings of Neuwiedia have typical orchidaceous mycotrophic protocorms. – Amer. J. Bot. 85: 956-959.
Kristiansen KA, Freudenstein JV, Rasmussen FN, Rasmussen HN. 2004. Molecular identification of mycorrhizal fungi in Neuwiedia veratrifolia (Orchidaceae). – Mol. Phylogen. Evol. 33: 251-258.
Krupko S, Israelstam GF, Martinovic B. 1954. Embryosac development and chromosome number in Vanilla roscheri from Inhaca Island. – South Afr. J. Sci. 51: 115-117.
Kubitzki K. 1998a. Agapanthaceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 58-60.
Kubitzki K. 1998b. Hostaceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 256-260.
Kubitzki K. 1998c. Ixioliriaceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 334-335.
Kubitzki K, Rudall PJ. 1998. Asparagaceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 125-129.
Kubo S, Mimaki Y, Terao M, Sashida Y, Nikaido T, Ohmoto T. 1992. Acylated cholestane glycosides from the bulbs of Ornithogalum saundersiae. – Phytochemistry 31: 3969-3973.
Kubo S, Mimaki Y, Sashida Y, Nikaido T, Ohmoto T. 1992. New cholestane bisdesmosides from the bulbs of Ornithogalum thyrsoides. – Bull. Chem. Soc. Japan 65: 1120-1124.
Kulak M, Górniak M, Romowicz A. 2006. Tribal and subtribal relationships of Epidendroideae Lindl. (Orchidaceae) with emphasis on Epidendreae Humb., Bonpl. & Kunth based on matK gene. – Biodivers. Res. Conserv. 3-4: 205-209.
Kumar M, Manilal KS. 1988. Floral anatomy of Apostasia odorata and the taxonomic status of apostasioids (Orchidaceae). – Phytomorphology 38: 159-162.
Kumar M, Sequiera S, Wood J. 2002. A new species of the hitherto monospecific genus Xenikophyton Garay (Orchidaceae) from India. – Kew Bull. 57: 227-230.
Kurita M. 1956. Karyotypes of some species in Allium. – Mem. Ehime Univ. Sect. II (Sci.) B. Biol. 2: 239-245.
Kurita S. 1986. Variation and evolution in the karyotype of Lycoris, Amaryllidaceae I. General karyomorphological characteristics of the genus. – Cytologia 51: 803-815.
Kurita S. 1987a. Variation and evolution on the karyotype of Lycoris, Amaryllidaceae II. Karyotype analysis of ten taxa, among which seven are native in China. – Cytologia 52: 19-40.
Kurita S. 1987b. Variation and evolution in the karyotype of Lycoris, Amaryllidaceae III. Intraspecific variation in the karyotype of L. traubii Hayward. – Cytologia 52: 117-128.
Kurita S. 1987c. Variation and evolution in the karyotype of Lycoris, Amaryllidaceae IV. – Cytologia 52: 137-149.
Kurita S. 1989. Variation and evolution of the karyotype of Lycoris, Amaryllidaceae V. Chromosomal variation in L. sanguinea Maxim. – Plant Spec. Biol. 4: 47-60.
Kurzweil H. 1985. Entwicklungsgeschichtliche Untersuchungen an Orchideenblüten unter besonderer Berücksichtigung des Gynostemiums. – Ph.D. diss., Universität Wien, Austria.
Kurzweil H. 1987a. Developmental studies in orchid flowers I: epidendroid and vandoid species. – Nord. J. Bot. 7: 427-442.
Kurzweil H. 1987b. Developmental studies in orchid flowers II: orchidoid species. – Nord. J. Bot. 7: 443-451.
Kurzweil H. 1988. Developmental studies in orchid flowers III: neottioid species. – Nord. J. Bot. 8: 271-282.
Kurzweil H. 1989. An investigation of the floral morphogenesis in Bonatea speciosa (L. f.) Willd. (Orchidaceae). – South Afr. J. Bot. 55: 433-437.
Kurzweil H. 1990. Floral morphology and ontogeny in the orchid subtribe Disinae. – Bot. J. Linn. Soc. 102: 61-83.
Kurzweil H. 1991a. Floral morphology of southern African Orchideae I: Orchidinae. – Nord. J. Bot. 11: 155-178.
Kurzweil H. 1991b. The unusual structure of the gynostemium in the Orchidaceae-Coryciinae. – Bot. Jahrb. Syst. 112: 273-293.
Kurzweil H. 1993a. Developmental studies in orchid flowers IV: cypripedioid species. – Nord. J. Bot. 13: 423-430.
Kurzweil H. 1993b. Seed morphology in Southern African Orchidoideae (Orchidaceae). – Plant Syst. Evol. 185: 229-247.
Kurzweil H. 1998. Floral ontogeny of orchids: a review. – Beitr. Biol. Pflanzen 71: 45-100.
Kurzweil H. 2000. The value of early floral ontogeny in the systematics of Orchidaceae. – In: Wilson KL, Morrison DA (eds), Monocots: systematics and evolution, CSIRO Publ., Collingwood, pp. 436-440.
Kurzweil H, Kocyan A. 2002. Ontogeny of orchid flowers. – In: Kull T, Arditti J (eds), Orchid biology: reviews and perspectives VIII, Kluwer, Dordrecht, pp. 83-130.
Kurzweil H, Manning JC. 2005. A synopsis of the genus Disperis Sw. (Orchidaceae). – Adansonia, sér. III, 27: 155-207.
Kurzweil H, Weber A. 1991. Floral morphology of southern African Orchideae I. Orchidinae. – Nord. J. Bot. 11: 155-178.
Kurzweil H, Weber A. 1992. Floral morphology of southern African Orchideae II. Habenariinae. – Nord. J. Bot. 12: 39-61.
Kurzweil H, Linder HP, Chesselet P. 1991. The phylogeny and evolution of the Pterygodium-Corycium complex (Coryciinae, Orchidaceae). – Plant Syst. Evol. 175: 161-223.
Kurzweil H, Linder HP, Stern WL, Pridgeon AM. 1995. Comparative vegetative anatomy and classification of Diseae (Orchidaceae). – Bot. J. Linn. Soc. 117: 171-220.
Kurzweil H, Weston PH, Perkins AJ. 2005. Morphological and ontogenetic studies on the gynostemium of some Australian members of Diurideae and Cranichideae (Orchidaceae). – Telopea 11: 11-33.
Kushnir U, Galil J, Feldman M. 1977. Cytology and distribution of Ornithogalum in Israel I. Section Heliocharmos Bak. – Israel J. Bot. 26: 63-82.
Kwembeya EG, Stedje B. 2007. A numerical taxonomy of the pan species of Crinum based on morphological characters. – Nord. J. Bot. 25: 137-144.
Kwembeya EG, Bjorå CS, Stedje B, Nordal I. 2007. Phylogenetic relationships of the genus Crinum (Amaryllidaceae) with emphasis on tropical African species: evidence from trnL-F and nuclear ITS DNA sequence data. – Taxon 56: 801-810.
Laan FM van der, Cribb PJ. 1986. Ossiculum (Orchidaceae), a new genus from Cameroun. – Kew Bull. 41: 823-826.
la Croix IF. 1993. Two new species of Habenaria sect. Podandria (Orchidaceae) from West Africa. – Kew Bull. 48: 369-373.
la Croix IF. 2004. Two new species of Sansevieria thumb. (Dracaenaceae) from the Flora Zambesiaca area. – Kew Bull. 59: 617-620.
la Croix IF, Cribb PJ. 1993. New taxa on Habenaria, Satyrium & Schizochilus (Orchidaceae) from the Flora Zambesiaca area. – Kew Bull. 48: 357-367.
Laferrière JE. 1995. Nomenclature and type speciemens in Eustrephus and Geitonoplesium (Geitonoplesiaceae). – Austrobaileya 4: 391-399.
Laferrière JE. 1996. Geitonoplesiaceae. – In: Kalkman C et al. (eds), Flora Malesiana I, 12(2), Flora Malesiana Foundation, Rijksberbarium/Hortus Botanicus, Leiden, pp. 731-736.
LaFrankie JV Jr. 1985a. Morphological and systematic studies in the genus Smilacina (Liliaceae). – Ph.D. diss., Harvard University, Cambridge, Massachusetts.
LaFrankie JV. 1985b. A note on seedling morphology and establishment growth in the genus Smilacina (Liliaceae). – Bull. Torrey Bot. Club 112: 313-317.
LaFrankie JV Jr. 1986a. Morphology and taxonomy of the New World species of Maianthemum (Liliaceae). – J. Arnold Arbor. 67: 371-439.
LaFrankie JV Jr. 1986b. Transfer of species of Smilacina to Maianthemum (Liliaceae). – Taxon 35: 584-589.
LaFrankie JV Jr. 1986c. A new species of Maianthemum (Liliaceae) from Costa Rica with an upright and aerial rhizome. – Amer. J. Bot. 73: 1258-1260.
Lakshmanian KK, Phillip VJ. 1971. A contribution to the embryology of Iridaceae. – Proc. Indian Acad. Sci. 73: 110-116.
Lakshmi N. 1978. Cytological studies in two allopolyploid species of the genus Hymenocallis. – Cytologia 43: 555-563.
Lakshmi N. 1979. Cytology, cytotaxonomy, interchange heterozygosity and position effect studies in Curculigo crassifolia Hook. f. – Cytol. Genet. 14: 173-178.
Lamont BB, Wittkuhn R, Korczynskyj D. 2004. Ecology and ecophysiology of grasstrees. – Aust. J. Bot. 52: 561-582.
Landström T. 1989. The species of Ornithogalum L. subg. Ornithogalum (Hyacinthaceae) in Greece. – Ph.D. diss., University of Lund, Sweden.
Lang K-Y, Tsi Z-H. 1976. Orchidaceae. Iconographia cormophytorum sinicorum 5. – Science Press, Peking, pp. 603-1146.
Larsen K. 1966. Two new Liliaceae from the Khao Yai National Park. – Bot. Not. 119: 196-200.
Larsen MM, Adsersen A, Davis AP, Lledó MD, Jäger AK, Rønsted N. 2010. Using a phylogenetic approach to selection of target plants in drug discovery of acetylcholinesterase inhibiting alkaloids in Amaryllidaceae tribe Galantheae. – Biochem. Syst. Ecol. 38: 1026-1034.
Larsen PO, Sorensen FT, Wieczorkowska E, Goldblatt P. 1981. Meta-carboxy-substituted aromatic amino acids and γ-glutamyl peptides: chemical characters for classification in the Iridaceae. – Biochem. Syst. Ecol. 9: 313-323.
Larsen PO, Sundahl M, Sorensen FT, Wieczorkowska E, Goldblatt P. 1987. Relationship between subfamilies, tribes and genera in Iridaceae inferred from chemical characteristics. – Biochem. Syst. Ecol. 18: 575-579.
Lavarack PS. 1971. The taxonomic affinities of the Neottioideae. – Ph.D. diss., University of Queensland, Saint Lucia, Queensland.
Lavarack PS. 1976. The taxonomic affinities of the Australian Neottioideae. – Taxon 25: 289-296.
Lavarack PS, Gray B. 1985. Tropical orchids of Australia. – Nelson, Melbourne.
Lavranos JJ. 1969. The genus Aloe in the Socotra Archipelago, Indian Ocean: a revision. – Cact. Succ. J. (Los Angeles) 41: 202-207.
Lawler LJ. 1979. Ethnobotany of the Orchidaceae. – In: Arditti J (ed), Orchid biology. Reviews and perspectives III, Comstock Publ. Assoc., Ithaca & London, pp. 27-149.
Lawrence GHM. 1953. A reclassification of the genus Iris. – Gentes Herb. 8: 346-371.
Lazarte JE, Palser BF. 1979. Morphology, vascular anatomy, and embryology of pistillate and staminate flowers of Asparagus officinalis. – Amer. J. Bot. 66: 753-764.
Leavitt RG. 1901. Notes on the embryology of some New England orchids. – Rhodora 3: 202-205.
Leavitt RG. 1909. The genus Eria in the Philippine Islands. – Phil. J. Sci., Sect. C, Bot. 4: 201-245.
Lebatha P, Buys MH. 2006. The taxonomic significance of the floral morphology in the Ledebouriinae (Hyacinthaceae). – In: Ghazanfar SA, Beentje H (eds), Taxonomy and ecology of African plants, their conservation and sustainable use, Royal Botanic Gardens, Kew, pp. 85-95.
Lebatha P, Buys MH, Stedje B. 2006. Ledebouria, Resnova and Drimiopsis: a tale of three genera. – Taxon 55: 643-652.
Lee AT, Macfarlane TD. 1986. Lomandra. – In: George AS (ed), Flora of Australia 46, Australian Government Publ. Service, Canberra, pp. 100-141.
Lee N-S, Kim M, Lee B-S, Park K-R. 2001. Isozyme evidence for the allotriploid origin of Lycoris flavescens (Amaryllidaceae). – Plant Syst. Evol. 227: 227-234.
Lehnebach CA. 2003. Preliminary checklist of the orchids of Chile. – Bot. J. Linn. Soc. 143: 449-451.
Leighton FM. 1944. A revision of the South African species of Ornithogalum L. I. – J. South Afr. Bot. 10: 83-122.
Leighton FM. 1945. A revision of the South African species of Ornithogalum L. II. – J. South Afr. Bot. 11: 135-189.
Leighton FM. 1965. The genus Agapanthus L’Heritier. – South Afr. J. Bot. [Suppl.] 4: 1-50.
Leinfellner W. 1963. Das Perigon der Liliaceen ist staminaler Herkunft. – Österr. Bot. Zeitschr. 110: 448-467.
Leitch IJ, Kahanadawala I, Suda J, Hanson L, Ingrouille MJ, Chase MW, Fay MF. 2009. Genome size diversity in Orchidaceae: consequences and evolution. – Ann. Bot. 104: 469-481.
Lenski I. 1958. Über die Brutzwiebelbildung auf den blättern von Drimiopsis kirkii Bak. – Planta 50: 579-621.
Lenz LW. 1958. A revision of the Pacific coast irises. – Aliso 4: 1-72.
Lenz LW. 1975a. A biosystematic study of Triteleia (Liliaceae) I. Revision of the species of section Calliprora. – Aliso 8: 221-258.
Lenz LW. 1975b. The chromosomes of Bloomeria and Muilla (Liliaceae) and range extensions for Muilla coronata and Muilla transmontana. – Aliso 8: 259-262.
Lenz LW. 1976. The nature of the floral appendages in four species of Dichelostemma (Liliaceae). – Aliso 8: 379-381.
Leopardi C, Carnevali G, Romero-González GA. 2012. Amoana (Orchidaceae, Laeliinae), a new genus and species from Mexico. – Phytotaxa 65: 23-35.
Leopardi-Verde CL, Carnevali G, Romero-González GA. 2017. A phylogeny of the genus Encyclia (Orchidaceae: Laeliinae), with emphasis on the species of the Northern Hemisphere. – J. Syst. Evol. 55: 110-123.
Le Thomas A, Suárez-Cervera M, Goldblatt P. 1996. Deux types polliniques originaux dans le genre Aristea (Iridaceae-Nivenioideae): implications phylogéniques. – Grana 35: 87-96.
Le Thomas A, Suárez-Cervera M, Goldblatt P. 2001a. Pollen of Nivenioideae and its phylogenetic implications. – Ann. Bot. (Roma), n. s., 1: 67-72.
Le Thomas A, Suárez-Cervera M, Goldblatt P. 2001b. Ontogeny of the exine in pollen of Aristea (Iridaceae). – Grana 40: 35-44.
Levan A. 1932. Cytological studies in Allium II. Chromosome morphological contributions. – Hereditas 16: 257-294.
Levan A. 1935. Cytological studies in Allium VI. The chromosome morphology of some diploid species of Allium. – Hereditas 20: 289-330.
Levan A. 1944. Notes on the cytology of Dipcadi and Bellevalia. – Hereditas 30: 217-224.
Lewis BA. 1992. Additions to the orchid flora of Vanuatu in the South-West Pacific. – Kew Bull. 47: 685-691.
Lewis BA, Cribb PJ. 1989. Orchids of Vanuatu. – The Royal Botanic Gardens, Kew.
Lewis GJ. 1941. Iridaceae. New genera and species and miscellaneous notes. – J. South Afr. Bot. 7: 19-59.
Lewis GJ. 1954. Some aspects of the morphology, phylogeny, and taxonomy of the South African Iridaceae. – Ann. South Afr. Mus. 40: 15-113.
Lewis GJ. 1959. The genus Babiana. – J. South Afr. Bot. Suppl. 3.
Lewis GJ. 1962. South African Iridaceae. The genus Ixia. – J. South Afr. Bot. 28: 45-195.
Lewis GJ, Obermeyer AA, Barnard TT. 1972. A revision of the South African species of Gladiolus. – J. South Afr. Bot. [Suppl.] 10: 1-316.
Lewis WH, Oliver RL. 1961. Meiotic chromosomes in six Texas and Mexican Nemastylis and Sisyrinchium (Iridaceae). – The Southw. Natur. 6: 45-46.
Lewitsky GA, Tron EJ. 1930. Zur Frage der karyotypischen Evolution der Gattung Muscari Mill. – Planta 9: 760-775.
Li G-Z (ed). 2004. The genus Aspidistra. – Guangxi Science and Technology Publ. House, Guangxi.
Li H. 1995. The phylogenetic place and origination of the genus Diuranthera. – Acta Bot. Yunnan. 17:268-276. [In Chinese]
Li J-H, Liu Z-J, Salazar GA, Bernhardt P, Perner H, Tomohisa Y, Jin X-H, Chung S-W, Luo Y-B. 2011. Molecular phylogeny of Cypripedium (Orchidaceae: Cypripedioideae) inferred from multiple nuclear and chloroplast regions. – Mol. Phylogen. Evol. 61: 308-320.
Li L, Yan H-F, 2013. A remarkable new species of Liparis (Orchidaceae) from China and its phylogenetic implications. – PLoS One 8: e78112.
Li L, Yan H-F, Niu M, Tu T-Y, Li S-J, Xing
F-W. 2014. Re-establishment of the genus Ania Lindl. (Orchidaceae). – PloS One. 2014; 9(7):
e103129. doi: 10.1371/journal.pone.0103129
Li L-C, Hsu P-S. 1983. Karyotype analysis of Anemarrhena asphodeloides Bunge (Liliaceae). – Acta Phytotaxon. Sin. 21: 445-448.
Li M-H, Zhang G-Q, Lan S-R, Liu Z-J, China Phylogeny Consortium. 2016. A molecular phylogeny of Chinese orchids. – J. Syst. Evol. 54: 349-362.
Li Q-Q, Zhou S-D, He X-J, Yu Y, Zhang Y-C, Wei X-Q. 2010. Phylogeny and biogeography of Allium (Amaryllidaceae: Allieae) based on nuclear ribosomal internal transcribed spacer and chloroplast rps16 sequences, focusing on the inclusion of species endemic to China. – Ann. Bot. 106: 709-733.
Li Q-Q, Zhou S-D, Huang D-Q, He X-J, Wei X-Q. 2016. Molecular phylogeny, divergence time estimates and historical biogeography within one of the world’s largest monocot genera. – AoB PLANTS 8: plw041;doi:10.1093/aobpla/plw041
Li RJ, Shang ZY, Cui TC, Xu JM. 1996. Studies on karyotypes and phylogenetic relationship of Allium sect. Caloscordum (Liliaceae) from China. – Acta Phytotax. Sin. 34: 288-295. [In Chinese]
Lifante ZD. 1992. Karyological studies in Asphodelaceae and Anthericaceae I. Chromosome numbers in Asphodelus and Anthericum from N Africa. – Willdenowia 22: 143-148.
Lim KY, Jones K. 1982. The chromosomes of orchids VI. Bulbophyllum. – Kew Bull. 37: 217-219.
Linder HP. 1980. An annotated revision of the genus Schizochilus Sond. (Orchidaceae). – J. South Afr. Bot. 46: 379-434.
Linder HP. 1981a. Taxonomic studies on the Disinae I. A revision of the genus Brownleea Lindl. – J. South Afr. Bot. 47: 13-48.
Linder HP. 1981b. Taxonomic studies on the Disinae II. A revision of the genus Schizodium Lindl. – J. South Afr. Bot. 47: 339-371.
Linder HP. 1981c. Taxonomic studies on the Disinae III. A revision of Disa Berg. excluding sect. Micranthae. – Contr. Bolus Herb. 9: 1-370.
Linder HP. 1981d. Taxonomic studies in the Disinae IV. A revision of Disa Berg. sect. Micranthae Lindl. – Bull. Jard. Bot. Natl. Belg. 51: 255-346.
Linder HP. 1981e. Taxonomic studies in the Disinae V. A revision of the genus Monadenia. – Bothalia 13: 399-363.
Linder HP. 1981f. Taxonomic studies in the Disinae VI. A revision of the genus Herschelia. – Bothalia 13: 365-388.
Linder HP. 1985. Notes on the orchids of southern tropical Africa I. Brownleea and Herschelia. – Kew Bull. 40: 125-129.
Linder HP. 1986. Notes on the phylogeny of the Orchidoideae, with particular reference to the Diseae. – Lindeyana 1: 51-64.
Linder HP. 1989. Notes on southern African angraecoid orchids. – Kew Bull. 44: 317-319.
Linder HP. 1995. Setting conservation priorities: the importance of endemism and phylogeny in the Southern African orchid genus Herschelia. – Conserv. Biol. 9: 585-595.
Linder HP, Hitchcock AN. 2006. Disa remota, a remarkable new orchid species from the Western Cape. – South Afr. J. Bot. 72: 627-629.
Linder HP, Kurzweil H. 1990. Floral morphology and phylogeny of the Disinae (Orchidaceae). – Bot. J. Linn. Soc. 102: 287-302.
Linder HP, Kurzweil H. 1994. The phylogeny and classification of the Diseae (Orchidoideae: Orchidaceae). – Ann. Missouri Bot. Gard. 81: 687-713.
Linder HP, Kurzweil H. 1995. Taxonomic notes on the African Orchidoideae (Orchidaceae): a new genus and combination. – Willdenowia 25: 229-234.
Linder HP, Kurzweil H. 1996. Ontogeny and phylogeny of Brownleea (Orchidoideae: Orchidaceae). – Nord. J. Bot. 16: 345-357.
Linder HP, Williamson G. 1986. Notes on the orchids of southern tropical Africa II. Oligophyton drummondii, gen. et sp. nov. – Kew Bull. 41: 313-317.
Linne von Berg G, Samoylov A, Klaas M, Hanelt P. 1996. Chloroplast DNA restriction analysis and the infrageneric grouping of Allium (Alliaceae). – Plant Syst. Evol. 200: 253-261.
Lisowski S, Wiland-Szymańska J. 2002. Espèce nouvelle du genre Kniphofia du Haut-Katanga (R. D. Congo). – Syst. Geogr. Plants 72: 235-238.
Liu T, Su H. 1971. Three new species of Cirrhopetalum. – Quart. J. Taiwan. Mus. 24: 173-180.
Liu Z-J, Chen L-J. 2009. Singchia and Gunnaria, two new genera of Orchidaceae. – J. Syst. Evol. 47: 599-604.
Lledó MD, Davis AP, Crespo MB, Chase MW, Fay MF. 2004. Phylogenetic analysis of Leucojum and Galanthus (Amaryllidaceae) based on plastid matK and nuclear ribosomal spacer (ITS) DNA sequences and morphology. – Plant Syst. Evol. 246: 223-243.
Lloyd DG, Webb CJ, Dulberger R. 1990. Heterostyly in species of Narcissus (Amaryllidaceae), Hugonia (Linaceae) and other disputed cases. – Plant Syst. Evol. 172: 215-227.
Lock GW. 1969. Sisal. 2nd ed. – Longmans, Green, London.
Logacheva MD, Schelkunov MI, Penin AA. 2011. Sequencing and analysis of plastid genome in mycoheterotrophic orchid Neottia nidus-avis. – Genome Biol. Evol. 3: 1296-1303.
Løjtnant B. 1977a. Observations on the Elleanthus linifolius alliance (Orchidaceae) in S America. – Bot. Not. 129: 445-453.
Løjtnant B. 1977b. New and noteworthy species of Neottioideae (Orchidaceae) from Ecuador. – Bot. Not. 130: 163-172.
Løjtnant B. 1977c. Notes on the genus Epidendrum (Orchidaceae) in Ecuador. – Bot. Not. 130: 321-328.
Lonay H. 1902. Recherches anatomiques sur les feuilles de l’Ornithogalum caudatum AIT. – Mém. Soc. Roy. Sci. Liège III, 4: 1-82.
Lopes RC. 2003 Herreriaceae Endlicher: revisão taxonômica dos gê neros neotropicais Herreria Ruiz & Pavon e Clara Kunth. – Ph.D. diss., UFRJ/Museu Nacional, Rio de Janeiro.
Lopes RC, Andreata RHP. 2003. Clara Kunth (Herreriaceae): novo poicionamento taxonômico para o gênero. – Bradea 9: 417-420.
Lopes RC, Andreata RHP. 2005. Clara Kunth (Herreriaceae): novos nomes. – Bradea 11: 17-10.
Lopes RC, Andreata RHP. 2006. A new species of Clara (Herreriaceae) from Brazil. – Syst. Bot. 31: 298-301.
Lopes RC, Andreata RHP, Cartaxo-Pinto S, Trovó M, Gonçalves-Esteves V. 2013. Pollen morphology and wall structure of Neotropical species of Herreria and Clara (Asparagaceae-Agavoideae) and its taxonomic implications. – Plant Syst. Evol. 299: 25-34.
Lopez-Ferrari AR, Espejo A. 1992. Una nueva especie de Dandya (Alliaceae) de la Cuenca del Rio Balsas, México. – Acta Bot. Mexicana 18: 11-15.
Loptien H. 1979. Identification of the sex chromosome pair in asparagus (Asparagus officinalis L.). – Zeitschr. Pflanzenzücht. 82: 162-173.
Lovo J, Winkworth RC, Mello-Silva R. 2012. New insights into Trimezieae (Iridaceae) phylogeny: what do molecular data tell us? – Ann. Bot. 110: 689-702.
Lovo J, Winkworth RC, Santos Bragança Gil A dos, Carmo E. Amaral M do, Bittrich V, Mello-Silva R. 2018. A revised genus-level taxonomy for Trimezieae (Iridaceae) based on expanded molecular and morphological analyses. – Taxon 67: 503-520.
Lu A-M. 1985. Embryology and probable relationships of Eriospermum (Eriospermaceae). – Nord. J. Bot. 5: 229-240.
Lu P-L, Morden C. 2010. Phylogenetics of the plant genera Dracaena and Pleomele (Asparagaceae). – J. Plant Sci. 7: 64-72.
Lu P-L, Morden CW. 2014. Phylogenetic
relationships among dracaenoid genera (Asparagaceae: Nolinoideae) inferred
from chloroplast DNA loci. – Syst. Bot. 39: 90-104.
Lungeanu I. 1972. Contributions to the caryologic study of the genus Ornithogalum. – Acta Bot. Horti Bucurestiensis 1970-71: 147-151.
Luer CA. 1975. The native orchids of the United States and Canada. – New York Botanical Gardens, Bronx, New York.
Luer CA. 1990. Icones Pleurothallidinarum VI. Systematics of Pleurothallis subgenus Ancipitia, subgenus Scopula and Trisetella. – Syst. Bot. Monogr., Missouri Botanical Garden, St. Louis.
Luer CA. 1990. Icones Pleurothallidinarum VII. Systematics of Platystele (Orchidaceae). – Syst. Bot. Monogr., Missouri Botanical Garden, St. Louis.
Luer CA. 1993. Icones Pleurothallidinarum X. Systematics of Dracula. – Syst. Bot. Monogr., Missouri Botanical Garden, St. Louis.
Luer CA. 1995. Icones Pleurothallidinarum XI. Systematics of Lepanthes subgenus Brachycladium and Pleurothallis subgenus Aenigma, subgenus Elongata, subgenus Kraenzlinella. – Syst. Bot. Monogr., Missouri Botanical Garden, St. Louis.
Luer CA. 1996. Icones Pleurothallidinarum XIV. Systematics of Draconanthes, Lepanthes subgenus Marsipanthes and subgenus Lepanthes of Ecuador. – Syst. Bot. Monogr., Missouri Botanical Garden, St. Louis.
Luer CA. 1998. New species of orchids from Cuba. – Lindleyana 13: 138-147.
Luer CA. 1999. New species of pleurothallids from Cuba and Hispaniola. – Lindleyana 14: 106-121.
Luer CA. 2006. Icones Pleurothallidinarum XXVIII. Reconsideration of Masdevallia, and the systematics of Specklinia and vegetatively similar genera. – Monogr. Syst. Bot. 105, Missouri Botanical Garden, St. Louis, Missouri, St. Louis.
Lund ID. 1987. The genus Panisea (Orchidaceae), a taxonomic revision. – Nord. J. Bot. 7: 511-527.
Lund ID. 1988. The genus Cremastra (Orchidaceae), a taxonomic revision. – Nord. J. Bot. 8: 197-203.
Lungeanu I. 1972. Contributions to the caryologic study of the genus Ornithogalum. – Acta Bot. Horti Bucurestiensis 1970-1971: 147-151.
Lüning B. 1974. Alkaloids of the Orchidaceae. – In: Withner CL (ed), The orchids, New York, pp. 349-382.
Lüning B, Leander K. 1965. Studies on Orchidaceae alkaloids III. The alkaloids in Dendrobium primulinum Lindl. and Dendrobium chrysanthum Wall. – Acta Chem. Scand. 19: 1607-1611.
Luo Y-B. 2004. Cytological studies on some representative species of the tribe Orchideae (Orchidaceae) from China. – Bot. J. Linn. Soc. 145: 231-238.
Luo Y-B, Chen S-C. 2000. The floral morphology and ontogeny of some Chinese representatives of orchid subtribe Orchidinae. – Bot. J. Linn. Soc. 134: 529-548.
Lynch AH, Rudall PJ, Cutler DF. 2001. Leaf anatomy and systematics of Hyacinthaceae. – Kew Bull. 61: 145-159.
Ma X-H, Qin R-L, Xing W-B. 1985. Chromosome observation of twenty species of drug plants in Xinjiang. – Acta Phytotaxon. Sin. 22: 243-249.
Maad J, Nilsson LA. 2004. On the mechanism of floral shifts in speciation: gained pollination efficiency from tongue- to eye-attachment of pollinia in Platanthera (Orchidaceae). – Biol. J. Linn. Soc. 83: 481-495.
McAllister F. 1914. The development of the embryo sac in the Convallariaceae. – Bot. Gaz. 58: 137-153.
MacBride JF. 1918. Further new or otherwise interesting Liliaceae. – Contr. Gray Herb. 56: 1-20.
McCabe S. 1995. Pollination of Aloe polyphylla. – Cact. Succ. J. (U.S.) 67: 166-168.
McCarthy EM. 1928. The structure and development of Astelia nervosa var. sylvestris. – Trans. Proc. New Zealand Inst. 59: 343-360.
McCook L. 1989. Systematics of Phragmipedium (Cypripedioideae: Orchidaceae). – Ph.D. diss., Cornell University, Ithaca, New York.
MacFarlane TD. 1994. Chamaexeros longicaulis (Dasypogonaceae), a new species from Walpole, southwestern Australia. – Nuytsia 9: 375-382.
McGee P. 1986. Growth response to and morphology of mycorrhizas of Thysanotus (Anthericaceae: Monocotyledonae). – New Phytol. 109: 459-464.
Maciunas E, Conran JG, Bannister JM, Paull R, Lee DE. 2011. Miocene Astelia (Asparagales: Asteliaceae) macrofossils from southern New Zealand. – Aust. Syst Bot. 24: 19-31.
McKain MR, Wickett N, Zhang Y, Ayyampalayam S, McCombie WR, Chase MW, Pires JC., dePamphilis CW, Leebens-Mack J. 2012. Phylogenomic analysis of transcriptome data elucidates co-occurrence of a paleopolyploid event and the origin of bimodal karyotypes in Agavoideae (Asparagaceae). – Amer. J. Bot. 99: 397-406.
McKain MR, NcNeal JR, Kellar PR, Eguiarte LE, Pires JC, Leebens-Mack J. 2016. Timing of rapid diversification and convergent origins of active pollination within Agavoideae (Asparagaceae). – Amer. J. Bot. 103: 1717-1729.
McKelvey SD. 1938. Yuccas of the Southwestern United States. Part One. – Arnold Arboretum of Harvard University, Jamaica Plain, Massachusetts.
McKelvey SD. 1947. Yuccas of the Southwestern United States. Part Two. – Arnold Arboretum of Harvard University, Jamaica Plain, Massachusetts.
McKelvey SD, Sax K. 1933. Taxonomic and cytological relationships of Yucca and Agave. – J. Arnold Arbor. 14: 76-81.
McLaughlin SP, Schuck SM. 1991. Fiber properties of several species of Agavaceae from the southwestern United States and northern Mexico. – Econ. Bot. 45: 480-486.
McMurtry D, Edwards TJ, Bytebier B. 2006. A new species of Disa (Orchidaceae) from Mpumalanga, South Africa. – South Afr. J. Bot. 72: 551-554.
McNaughton JE, Robertson BL. 1974. Megasporogenesis and megagametogenesis in Aloe africana Mill. – J. South Afr. Bot. 40: 75-79.
McPherson MA, Fay MF, Chase MW, Graham SW. 2004. Parallel loss of a slowly evolving intron from two closely related families in Asparagales. – Syst. Bot. 29: 296-307.
Maciunas E, Conran JG, Paull R, Lee DE, Bannister JM. 2009. Phormium and Asteliaceae macrofossils from New Zealand: using leaf cuticular details to determine phylogenetic affinities. – In: Barrell DJA, Tulloch A (eds), Joint Geological and Geophysical Societies Conference, 2009: programme and abstracts. Geol. Soc. New Zealand Misc. Publ. 128A, Geological Society of New Zealand, Oamaru, p. 129.
Maciunas E, Conran JG, Bannister JM, Paull R, Lee DE. 2011. Miocene Astelia (Asparagales: Asteliaceae) macrofossils from southern New Zealand. – Aust. Syst. Bot. 24: 19-31.
Maheshwari P. 1934. Contributions to the morphology of some Indian Liliaceae I. – Proc. Indian Acad. Sci., Sect. B, 1: 197-204.
Malcomber ST, Demissew S. 1993. The status of Protasparagus and Myrsiphyllum in the Asparagaceae. – Kew Bull. 48: 63-78.
Malguth R. 1901. Biologische Eigenthümlichkeiten der Früchte epiphytischer Orchideen. – Ph.D. diss., Breslau, Poland.
Maekawa F. 1935. Dua genera nova orchidacearum japonensium. – Bot. Mag. (Tokyo) 49: 596-599.
Maekawa F. 1971. The wild orchids of Japan in colour. – Tokyo.
Maekawa F, Kaneko K. 1968. Evolution of karyotype in Hosta (Liliaceae). – J. Jap. Bot. 43: 132-140. [In Japanese with English summary]
Mann LK. 1959. The Allium inflorescence: some species of the section Molium. – Amer. J. Bot. 46: 730-739.
Mann LK. 1960. Bulb organisation in Allium: some species of section Molium. – Amer. J. Bot. 47: 765-771.
Manning JC, Goldblatt P. 1990. Endothecium in Iridaceae and its systematic implications. – Amer. J. Bot. 77: 527-532.
Manning JC, Goldblatt P. 1991. Seed coat structure in the shrubby Cape Iridaceae, Nivenia, Klattia and Witsenia. – Bot. J. Linn. Soc. 107: 387-404.
Manning JC, Goldblatt P. 2001. A synoptic review of Romulea (Iridaceae: Crocoideae) in sub-Saharan Africa, the Arabian Peninsula and Socotra including new species, biological notes, and a new infrageneric classification. – Adansonia, sér. III, 23: 59-108.
Manning JC, Goldblatt P. 2016. Nomenclatural adjustments in African plants 2. – Bothalia 46(1). doi: 10.4102/abc.v46i1.2024
Manning JC, Linder HP. 1992. Pollinators and evolution in Disperis (Orchidaceae), or why are there so many species? – South Afr. J. Sci. 88: 38-49.
Manning JC, Smith GF. 2000. Asphodelaceae: Alooideae. The genus Poellnitzia included in Astroloba. – Bothalia 30: 53.
Manning JC, Goldblatt P, Fay MF. 2004. A revised generic synopsis of Hyacinthaceae in sub-Saharan Africa, based on molecular evidence, including new combinations and the new tribe Pseudoprospereae. – Edinburgh J. Bot. 60: 533-568.
Manning JC, Forest F, Devey DS, Fay MF, Goldblatt P. 2009. A molecular phylogeny and a revised classification of Ornithogaloideae (Hyacinthaceae) based on an analysis of four plastid DNA regions. – Taxon 58: 77-107.
Manning JC, Deacon J, Goldblatt P. 2014a. A
review of the Litanthus group of Drimia Jacq. (Hyacinthaceae: Urgineoideae) with the
description of a second species, Drimia stenocarpa, from Western Cape.
– South Afr. J. Bot. 90: 96-100.
Manning JC, Deacon J, Goldblatt P. 2014b. A
review of the Schizobasis group of Drimia Jacq. (Hyacinthaceae: Urgineoideae), and the
new species D. sigmoidea from Western Cape, South Africa. – South
Afr. J. Bot. 94: 263-269.
Manning JC, Boatwright JS, Daru BH, Maurin O,
Bank M van der. 2014. A molecular phylogeny and generic classification of Asphodelaceae subfamily Alooideae: a
final resolution of the prickly issue of polyphyly in the alooids? – Syst.
Bot. 39: 55-74.
Mansfeld R. 1934. Orchideologische Mitteilungen III. – Feddes Repert. 38: 199-205.
Mansfeld R. 1937a. Über das System der Orchidaceae. – Blumea [Suppl.] 1: 25-37.
Mansfeld R. 1937b. Über das System der Orchidaceae-Monandrae. – Notizbl. Königl. Bot. Gart. Mus. Berlin-Dahlem 13: 666-676.
Mansfeld R. 1954. Über die Verteilung der Merkmale innerhalb der Orchidaceae-Monandrae. – Flora 142: 65-80.
Mant JG, Schiestl FP, Peakall R, Weston PH. 2002. A phylogenetic study of pollinator conservatism among sexually deceptive orchids. – Evolution 56: 888-898.
Marais W. 1973. A revision of the tropical species of Kniphofia (Liliaceae). – Kew Bull. 28: 465-483.
Marais W, Reilly J. 1978. Chlorophytum and its related genera (Liliaceae). – Kew Bull. 32: 653-663.
Markötter EI. 1936. Die lewensgeskiedenis van sekere geslagte van die Amaryllidaceae. – Ann. Univ. Stellenbosch XIV.A.2: 1-84.
Markova M, Popova M, Radenkova J, Ivanova P. 1974. Karyologischen Untersuchungen der in Bulgarien wildwachsenden Vertreter der Gattung Ornithogalum. – Bull. Inst. Bot. 25: 63-92.
Marloth R. 1929. Eriospermums. – South Afr. Gard. Country Life 19: 249-261, 325-327.
Marrero A, Almeida RS, González-Martín M. 1998. A new species of the wild dragon tree, Dracaena (Dracaenaceae), from Gran Canaria and its taxonomic and biogeographic implications. – Bot. J. Linn. Soc. 128: 291-314.
Martín J, Raymúndez M, Vallès J, Garnatje T, Raimúndez E. 2012. Palynological study of the Venezuelan species of the genus Hymenocallis (Amaryllidaceae). – Plant Syst. Evol. 298: 695-701.
Martin SF. 1987. The Amaryllidaceae alkaloids. – In: Brossi A (ed), The alkaloids, vol. 30, Academic Press, New York, pp. 252-376.
Martínez A. 1985. The chromosomes of orchids VIII. Spiranthinae and Cranichidinae. – Kew Bull. 40: 139-147.
Martínez-Azorin M. 2008. Sistemática del género Ornithogalum L. (Hyacinthaceae) en el Mediterráneo Occidental: implicaciones taxonómicas, filogenéticas y biogeográficas. – Ph.D. diss., Universidad de Alicante, Spain.
Martínez-Azorín M, Crespo MB, Spencer M. 2006. Typification of names and taxa in Ornithogalum L. subg. Cathissa (Salisb.) Baker (Hyacinthaceae). – Taxon 5: 1014-1018.
Martínez-Azorín M, Crespo MB, Juan A, Fay MF. 2011. Molecular phylogenetics of subfamily Ornithogaloideae (Hyacinthaceae) based on nuclear and plastid DNA regions, including a new taxonomic arrangement. – Ann. Bot. 107: 1-37.
Martínez-Azorín M, Crespo MB, Dold AP, Wetschnig W, Pinter M, Pfosser M, Jaarsveld E van. 2013. Sagittanthera (Hyacinthaceae, Urgineoideae), a new buzz pollinated genus from the Eastern Cape Province of South Africa. – Phytotaxa 98: 43-54.
Martos F, Johnson SD, Peter CI, Bytebier B.
2014. A molecular phylogeny reveals paraphyly of the large genus
Eulophia (Orchidaceae): a
case for the reinstatement of Orthochilus. – Taxon 63: 9-23.
Martos F, Le Péchon T, Johnson SD, Bytebier B. 2018. A reassessment of Angraecopsis, Mystacidium and Sphyrarhynchus (Orchidaceae: Vandeae) based on molecular and morphological evidence. – Bot. J. Linn. Soc. 186: 1-17.
Mashayekhi S, Zarre S, Fritsch RM, Attar F. 2005. A new species of Allium subgen. Melanocrommyum sect. Compactoprason (Alliaceae) from Iran. – Feddes Repert. 116: 191-194.
Masters MT. 1887. On the floral conformation of the genus Cypripedium. – Bot. J. Linn. Soc. 22: 402-422.
Mather K. 1932. Chromosome variation in Crocus I. – J. Genet. 26: 129-142.
Mathew B. 1976. Crocus olivieri and its allies (Iridaceae). – Kew Bull. 31: 201-214.
Mathew B. 1982. The Crocus. A revision of the genus Crocus (Iridaceae). – Batsford, London.
Mathew B. 1983. The Greek species of Crocus (Iridaceae), a taxonomic survey. – Ann. Mus. Goulandris 6: 63-86.
Mathew B. 1988. Hostaceae, a new name for the invalid Funkiaceae. – Kew Bull. 43: 302.
Mathew B. 1989. Notes on tropical African Asparagaceae. – Kew Bull. 44: 181-182.
Mathew B. 1996. A review of Allium section Allium. – The Royal Botanic Gardens, Kew.
Mathew B, Brighton C. 1977. Crocus tournefortii and its allies (Iridaceae). – Kew Bull. 31: 775-786.
Mathew B, Petersen G, Seberg O. 2009. A reassessment of Crocus based on molecular analysis. – The Plantsman, N.S., 8: 50-57.
Matine F. 1976. Contribution à l’étude de la famille Alliaceae en Iran. – Inst. Rech. Ent. Phytopath. d’Evine, Sect. Bot. 6, Tehran.
Matuszkiewicz A, Tukallo P. 2006. Infrageneric reclassification and phylogenetic inference in the genus Masdevallia Ruiz & Pav. (Orchidaceae, Pleurothallidinae) – preliminary results of nrDNA based analysis. – Biodivers. Res. Conserv. 3-4: 213-219.
Mayer JLS, Cardoso-Gustavson P, Appezzato-da-Gloria B. 2011. Colleters in monocots: new record for Orchidaceae. – Flora 206: 185-190.
Meerow AW. 1984. Karyotype evolution in the Amaryllidaceae. – Herbertia 40: 139-154.
Meerow AW. 1987a. A monograph of Eucrosia (Amaryllidaceae). – Syst. Bot. 12: 460-492.
Meerow AW. 1987b. Chromosome cytology of Eucharis, Caliphruria, and Urceolina. – Amer. J. Bot. 74: 1559-1575.
Meerow AW. 1987c. The identities and systematic affinities of Mathieua Klotzsch and Plagiolirion Baker (Amaryllidaceae). – Taxon 36: 566-572.
Meerow AW. 1987d. Biosystematics of tetraploid Eucharis (Amaryllidaceae). – Ann. Missouri Bot. Gard. 74: 291-309.
Meerow AW. 1989a. A monograph of the Amazon lilies, Eucharis and Caliphruria (Amaryllidaceae). – Ann. Missouri Bot. Gard. 76: 136-220.
Meerow AW. 1989b. Systematics and evolution of the Stenomesseae (Amaryllidaceae). – Herbertia 45: 138-151.
Meerow AW. 1990. 202. Amaryllidaceae. – In: Harling G, Andersson L (eds), Flora of Ecuador 41, Nord. J. Bot., Copenhagen, pp. 1-52.
Meerow AW. 1995. Towards a phylogeny of the Amaryllidaceae. – In: Rudall PJ, Cribb PJ, Cutler DF, Humphries CJ (eds), Monocotyledons: systematics and evolution, Royal Botanic Gardens, Kew, pp. 169-179.
Meerow AW. 2010. Convergence or reticulation? Mosaic evolution in the canalized American Amaryllidaceae. – In: Seberg O, Petersen G, Barfod AS, Davis JI (eds), Diversity, phylogeny, and evolution in the monocotyledons, Aarhus University Press, Århus, pp. 145-168.
Meerow AW, Clayton JR. 2004. Generic relationships among the baccate-fruited Amaryllidaceae (tribe Haemantheae) inferred from plastid and nuclear non-coding DNA sequences. – Plant Syst. Evol. 244: 141-155.
Meerow AW, Dehgan B. 1985a. The auriculate pollen grain of Hymenocallis quitoensis Herb. (Amaryllidaceae) and its systematic implications. – Amer. J. Bot. 72: 540-547.
Meerow AW, Dehgan B. 1985b. A new species and a new combination in the genus Eucrosia (Amaryllidaceae). – Brittonia 37: 47-55.
Meerow AW, Dehgan B. 1988. Pollen morphology of the Eucharideae (Amaryllidaceae). – Amer. J. Bot. 75: 1857-1870.
Meerow AW, Snijman DA. 1998. Amaryllidaceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 83-110.
Meerow AW, Snijman DA. 2001. Phylogeny of Amaryllidaceae tribe Amaryllideae based on nrDNA ITS sequences and morphology. – Amer. J. Bot. 88: 2321-2330.
Meerow AW, Snijman DA. 2006. The never-ending story: multigene approaches to the phylogeny of Amaryllidaceae. – In: Columbus JT, Friar EA, Porter JM, Prince LM, Simpson MG (eds), Monocots: comparative biology and evolution. Excluding Poales, Rancho Santa Ana Botanical Garden, Claremont, California. – Aliso 22: 355-366.
Meerow AW, Werff H van der. 2004. Pucara (Amaryllidaceae) reduced to synonymy with Stenomesson on the basis of nuclear and plastid DNA spacer sequences, and a new related species of Stenomesson. – Syst. Bot. 29: 511-517.
Meerow AW, Dehgan NB, Dehgan B. 1986. Pollen tetrads in Stenomesson elwesii (Amaryllidaceae). – Amer. J. Bot. 73: 1642-1644.
Meerow AW, Scheepen J van, Dutilh JHA. 1997. Transfers from Amaryllis to Hippeastrum (Amaryllidaceae). – Taxon 46: 15-19.
Meerow AW, Fay MF, Guy CL, Li Q-B, Zaman FQ, Chase MW. 1999. Systematics of Amaryllidaceae based on cladistic analysis of plastid rbcL and trnL-F sequence data. – Amer. J. Bot. 86: 1325-1345.
Meerow AW, Fay MF, Chase MW, Guy CL, Li Q-B. 1999. The new phylogeny of the Amaryllidaceae. – Herbertia 54: 180-203.
Meerow AW, Fay MF, Chase MW, Guy CL, Li Q-B, Snijman D, Yang S-L. 2000. Phylogeny of Amaryllidaceae: molecules and morphology. – In: Wilson KL, Morrison DA (eds), Monocots: systematics and evolution, CSIRO Publ., Melbourne, pp. 372-386.
Meerow AW, Guy CL, Li Q-B, Yang S-L. 2000. Phylogeny of the American Amaryllidaceae based on nrDNA ITS sequences. – Syst. Bot. 25: 708-726.
Meerow AW, Guy CL, Li Q-B, Clayton JR. 2002. Phylogeny of the tribe Hymenocallideae (Amaryllidaceae) based on morphology and molecular characters. – Ann. Missouri Bot. Gard. 89: 400-413.
Meerow AW, Preuss KD, Tombolato FC. 2002. Griffinia (Amaryllidaceae), a critically endangered Brazilian geophyte with horticultural potential. – In: Littlejohn G (ed), Acta Horticulturae (ISHS), pp. 57-64.
Meerow AW, Lehmiller DJ, Clayton JR. 2003. Phylogeny and biogeography of Crinum L. (Amaryllidaceae) inferred from nuclear and limited plastid non-coding DNA sequences. – Bot. J. Linn. Soc. 141: 349-363.
Meerow AW, Francisco-Ortega J, Kuhn DN, Schnell RJ. 2006. Phylogenetic relationships and biogeography within the Eurasian clade of Amaryllidaceae based on plastid ndhF and nrDNA ITS sequences: lineage sorting in a reticulate area? – Syst. Bot. 31: 42-60.
Meerow AW, Reveal JL, Snijman DA, Dutilh JH. 2007. Proposal to conserve the name Amaryllidaceae against Alliaceae, a “superconservation” proposal. – Taxon 56: 1299-1300.
Meinecke EP. 1894. Beiträge zur Anatomie der Luftwurzeln der Orchideen. – Flora 78: 133-203.
Meney KA, Pate JS, Dixon KW. 1996. New species of Restionaceae from Western Australia. – Telopea 6: 649-666.
Meng Q-W, Luo Y-B. 2009. A new species of Galearis (Orchidinae: Orchidaceae) from Sichuan, China. – Bot. J. Linn. Soc. 158: 689-695.
Meng Q-W, Song X-Q, Luo Y-B. 2007. A new species of Gastrodia (Orchidaceae) from Hainan Island, China, and its conservation status. – Nord. J. Bot. 25: 23-26.
Meng Y, Wen J, Nie Z-L, Sun H, Yang Y-P. 2008. Phylogeny and biogeographic diversification of Maianthemum (Ruscaceae: Polygonatae). – Mol. Phylogen. Evol. 49: 424-434.
Meng Y, Nie Z-L, Deng T, Wen J, Yang Y-P.
2014. Phylogenetics and evolution of phyllotaxy in the Solomon’s seal genus
Polygonatum (Asparagaceae:
Polygonateae). – Bot. J. Linn. Soc. 176: 435-451.
Mensinkai SW. 1939. Cytogenetic studies in the genus Allium. – J. Genet. 39: 1-45.
Menz G. 1922. Osservazioni sull’anatomia degli organi vegetativi delle specie italiane del genere Allium (Tourn.) L. – Bull. Istit. Bot. Univ. Sass. 1, 5: 1-27.
Mes THM, Friesen N, Fritsch RM, Klaas M, Bachmann K. 1997. Criteria for sampling in Allium based on chloroplast DNA PCR-RFLP’s. – Syst. Bot. 22: 701-712.
Mes THM, Fritsch RM, Pollner S, Bachmann K. 1999. Evolution of the chloroplast genome and polymorphic ITS regions in Allium subg. Melanocrommyum. – Genome 42: 237-247.
Metcalf LF. 1972. Some characteristic New Zealand Liliaceae. – Lilies 1973: 72-93.
Meyer NR, Yaroshevskaya AS. 1976. The phylogenetic significance of the development of pollen grain walls in Liliaceae, Juncaceae and Cyperaceae. – In: Ferguson IK, Muller J (eds), The evolutionary significance of the exine, Linnaean Society Symposium Series No. 1, pp. 91-100.
Miceli P, Garbari F. 1979. Chromosomi ed anatomia fogliare de quattro Allium diploidi di Grecia. – Atti Soc. Toscana Sci. Nat., Mem., Ser. B, 876: 37-51.
Micheneau C. Carlsward BS, Fay MF, Bytebier B, Pailler T, Chase MW. 2008. Phylogenetics and biogeography of Mascarene angraecoid orchids (Vandeae, Orchidaceae). – Mol. Phylogen. Evol. 46: 908-922.
Migliorato E. 1910. Sull’impollinazione di Rohdea japonica per mezzo delle formiche. – Ann. Bot. 8: 241-242.
Mildbraed J. 1923. Iridaceae Africanae. – Bot. Jahrb. Syst. 58: 230-233.
Miller JT, Clements MA. 2014. Molecular
phylogenetic analyses of Drakaeinae: Diurideae (Orchidaceae) based on DNA sequences of
the internal transcribed spacer region. – Aust. Syst. Bot. 27: 3-22.
Mimaki Y,Sashida Y, Kawashima K. 1992. New steroidal constituents of the bulbs of Camassia cusickii Wats. – Chem. Pharm. Bull. 40: 148-152.
Misra S. 1980. Additions to the orchidaceous flora of Orissa. – Bull. Bot. Surv. India 22: 147-156.
Misra S. 1982. Additions to the orchidaceous flora of Orissa 2. – J. Orissa Bot. Soc. 4: 23-32.
Misra S. 1983. Orchidaceous flora of the Mahendragiri and Singaraj Hills of Orissa. – Ind. J. For. 6: 309-313.
Mitra A. 1966. Karyological studies in Curculigo orchioides Gaertn. – Sci. Cult. 32: 201-202.
Mitra J. 1956. Karyotype analysis of bearded irises. – Bot. Gaz. 117: 265-293.
Mitra J, Randolph LF. 1959. Karyotype analysis of bulbous Iris. – Bot. Gaz. 120: 125-131.
Mogensen HL. 1968. Floral ontogeny and interpretation of the inferior ovary in Agave parryi. – Can. J. Bot. 47: 23-26.
Mogensen HL. 1970. Megagametophyte development in Agave parryi. – Phytomorphology 20: 16-22.
Møller J, Rasmussen H. 1984. Stegmata in Orchidales: character state distribution and polarity. – Bot. J. Linn. Soc. 89: 53-76.
Molseed E. 1968. Fosteria, a new genus of Mexican Iridaceae. – Brittonia 20: 232-234.
Molseed E. 1970. The genus Tigridia (Iridaceae) of Mexico and Central America. – Univ. Calif. Publ. Bot. 54: 1-113.
Molseed E, Cruden RW. 1969. Sessilanthera, a new genus of American Iridaceae. – Brittonia 21: 191-193.
Molvray M, Kores PJ. 1995. Character analysis of the seed coat in Spiranthoideae and Orchidoideae, with special reference to the Diurideae (Orchidaceae). – Amer. J. Bot. 82: 1443-1454.
Molvray M, Kores PJ, Chase MW. 2000. Polyphyly of mycoheterotrophic orchids and functional influences on floral and molecular characters. – In: Wilson KL, Morrison DA (eds), Monocots: systematics and evolution, CSIRO, Melbourne, pp. 441-448.
Mondragón-Palomino M, Theißen G. 2008. MADS about the evolution of orchid flowers. – Trends Plant Sci. 13: 51-59.
Mondragón-Palomino M, Theißen G. 2009. Why are orchid flowers so diverse? Reduction of evolutionary constraints by paralogues of class B floral homeotic genes. – Ann. Bot. 104: 583-594.
Moore DM. 1971. Tapeinia Comm. ex Juss. (Iridaceae) and the rediscovery of Galaxia obscura Cav. – Bot. Not. 124: 82-86.
Moore HE Jr. 1951. Petronymphe, a new genus of Amaryllidaceae. – Gentes Herb. 8: 258-260.
Moore HE Jr. 1953. The genus Milla (Amaryllidaceae-Allieae) and its allies. – Gentes Herb. 8: 262-294.
Moore HE Jr. 1954-1955. The cultivated Allium. – Baileya 2: 103-113, 117-124; 3: 137-149, 156-167.
Moore LB. 1957. The species of Xeronema. – Pacific Sci. 11: 355-362.
Moore LB. 1966. Australasian asteliads (Liliaceae) with special reference to New Zealand species of Astelia subgenus Tricella. – New Zealand J. Bot. 4:201-240.
Moore LB. 1975. Hybridism in Cordyline (Agavaceae). – New Zealand J. Bot. 13: 305-316.
Moore LB. 1980. Hybrid asteliads (Liliaceae). – New Zealand J. Bot. 18: 37-42.
Mootmura H, Yukawa T, Ueno O, Kagawa A. 2008. The occurrence of crassulacean acid metabolism in Cymbidium (Orchidaceae) and its ecological and evolutionary implications. – J. Plant Res. 121: 163-177.
Morães AP, Leitch IJ, Leitch AR. 2012. Chromosome studies in Orchidaceae: karyotype divergence in Neotropical genera in subtribe Maxillariinae. – Bot. J. Linn. Soc. 170: 29-39.
Morães AP, Souza-Chies TT, Stiehl-Alves EM,
Burchardt P, Eggers L, Siljak-Yakovlev S, Brown SC, Chauveau O, Nadot S, Bourge
M, Viccini LF, Kaltchuk-Santos E. 2015. Evolutionary trends in Iridaceae: new cytogenetic findings from
the New World. – Bot. J. Linn. Soc. 177: 27-49.
Mordak EV. 1971. Scilla of the Soviet Union II. Taxonomy and geography. – Bot. Žurn. 56: 1444-1458.
Moret J. 1987. Étude cytogénétique des taxons des sous-genres Beryllis et Cathissa du genre Ornithogalum L. au Maroc: discussion des resultats dans le cadre d’une systématique évolutive du genre. – Webbia 41: 143-153.
Moret J. 1988. Taxonomie numérique et classification supraspécifique des Ornithogales d’Afrique du Nord. – Rev. Cytol. Biol. Végét., Bot. 11: 3-11.
Moret J, Couderc H. 1986. Contribution of caryology to the systematic knowledge of the Ornithogalum genus in North Africa: the Heliocharmos Baker sub-genus. – Caryologia 39: 259-272.
Moret J, Couderc H, Hubac J-M, Gorenflot R. 1986. Contributions of numerical taxonomy to Ornithogalum subgen. Beryllis (Hyacinthaceae) in Morocco. – Plant Syst. Evol. 154: 103-110.
Moret J, Couderc H, Gorenflot R, Hubac J. 1988. La variabilité morphologique des taxons marocains du genre Ornithogalum L., sous-genre Heliocharmos: une étude biométrique. – Can. J. Bot. 66: 2178-2186.
Moret J, Couderc H, Bari A, Delarue Y. 1989. Micromorphology of seeds of Ornithogalum (Hyacinthaceae) in North Africa. – Nord. J. Bot. 9: 461-468.
Moret J, Guern M, Baudoin R, Baudière A. 2000. Étude phénétique du genre Romulea (Iridaceae) en France. – Monde Plant. 468: 24-30.
Morice IM. 1962. Seed fats in New Zealand Agavaceae. – J. Sci. Food Agric. 13: 666-669.
Morice IM. 1967. Seed fats of Astelia and Collospermum, family Liliaceae. – J. Sci. Food Agric. 18: 343-346.
Morice IM. 1975. Seed fats of further species of Astelia. – Phytochemistry 14: 1315-1318.
Morris MW, Stern WL, Judd WS. 1996. Vegetative anatomy and systematics of subtribe Dendrobiinae (Orchidaceae). – Bot. J. Linn. Soc. 120: 89-144.
Morton JK. 1965. The experimental taxonomy of the West African species of Pancratium L. (Amaryllidaceae). – Kew Bull. 19: 337-347.
Motte J. 1939. Sur quelques aspects considérés comme tératologique du cladode de Ruscus. – Bull. Soc. Bot. France 86: 73-80.
Mulay BN, Deshpande BD. 1959. Velamen in terrestrial monocots – I. Ontogeny and morphology in the Liliaceae. – J. Indian Bot. Soc. 38: 383-390.
Müller-Doblies D. 1977. Über den geometrischen Zusammenhang der monochasialen Verzweigungen am Beispiel einiger Liliifloren. – Ber. Deutsch. Bot. Ges. 90: 351-362.
Müller-Doblies D, Müller-Doblies U. 1972. Galanthus ist doch sympodial gebaut. – Ber. Deutsch. Bot. Ges. 84: 665-682.
Müller-Doblies D, Müller-Doblies U. 1975. Studies on the morphology, cytology, and distribution of Leucojum subgenus Ruminia. – Plant Syst. Evol. 123: 117-118.
Müller-Doblies D, Müller-Doblies U. 1978a. Bulbs and morphology: Ungernia. – Lagascalia 8: 13-23.
Müller-Doblies D, Müller-Doblies U. 1978b. Studies on tribal systematics of Amaryllidaceae 1. The systematic position of Lapiedra Lag. – Lagascalia 8: 13-23.
Müller-Doblies D, Müller-Doblies U. 1985. De liliifloris notulae 2: de taxonomia subtribus Strumariinae (Amaryllidaceae). – Bot. Jahrb. Syst. 107: 17-47.
Müller-Doblies D, Müller-Doblies U. 1994. De liliifloris notulae 5: some new taxa and combinations in the Amaryllidaceae tribe Amaryllideae from arid South Africa. – Feddes Repert. 105: 331-363.
Müller-Doblies D, Müller-Doblies U. 1996. Tribes and subtribes and some species combinations in Amaryllidaceae J. St.-Hil. emend. R. Dahlgren et al. 1985. – Feddes Repert. 107: S.c. 1-9.
Müller-Doblies U. 1994. Enumeratio Albucarum (Hyacithaceae) austro-africanarum adhuc cognitarum 1. Subgenus Albuca. – Feddes Repert. 105: 365-368.
Müller-Doblies U. 1995. Enumeratio Albucarum (Hyacithaceae) austro-africanarum adhuc cognitarum 2. Subgenus Falconera (Salisb.) Baker emend. U. M-D. 1987. – Feddes Repert. 106: 353-370.
Müller-Doblies U. 2006. Enumeratio Albucarum (Hyacinthaceae) austro-africanarum adhuc cognitarum 3. Additions and additional notes to Albuca subgenus Falconera and A. subgenus Albuca. – Feddes Repert. 117: 96-138.
Müller-Doblies U, Müller-Doblies D. 1975. De Liliifloris notulae I. Zum Merkmalsbestand von Haemanthus (Amaryllidaceae). – Bot. Jahrb. Syst. 96: 324-327.
Müller-Doblies U, Müller-Doblies D. 1978. Zum Bauplan von Ungernia, der einzigen endemischen Amaryllidaceen-Gattung Zentralasiens. – Bot. Jahrb. Syst. 99: 249-263.
Müller-Doblies U, Müller-Doblies D. 1996. Revisionula incompleta Ornithogalorum austro-africanorum (Hyacinthaceae). – Feddes Repert. 107: 361-548.
Müller-Doblies U, Müller-Doblies D. 1997. A partial revision of the tribe Massonieae (Hyacinthaceae) 1. Survey, including three novelties from Namibia: a new genus, a second species in the monotypic Whiteheadia, and a new combination in Massonia. – Feddes Repert. 108: 49-96.
Munguía-Lino G, Vargas-Amado G, Vázquez-García LM, Rodríguez A. 2015. Riqueza de especies y distribución geográfica de la tribu Tigridieae (Iridaceae) en Norteamérica. – Rev. Mexicana Biodivers. 86: 80-98.
Munguía-Lino G, Escalante T, Morrone JJ,
Rodríguez A. 2016. Areas of endemism of the North American species of
Tigridieae (Iridaceae). – Aust. Syst.
Bot. 29: 142-156.
Muñoz M, Riegel R, Seemann P, Peñalillo P,
Schiappacasse F, Núñez J. 2011. Relaciones filogenéticas de Rhodolirium
montanum Phil. y especies afines, basadas en secuencias nucleotídicas de
la region ITS y análisis cariotípico. – Gayana Bot. 68: 40-48.
Murray BG, Ran Y, De Lange PJ, Hammett KRW, Truter JT, Swanevelder ZH. 2004. A new species of Clivia (Amaryllidaceae) endemic to the Pondoland centre of endemism, South Africa. – Bot. J. Linn. Soc. 146: 369-374.
Mytnik-Ejsmont J, Rutkowski P. 2006. Taxonomic position of Zhukowskia Szlach., R. Gonzalez T. & Rutk. (Cyclopogoninae, Spirantheae, Orchidaceae). – Biodiv. Res. Conserv. 1-2: 7-10.
Nackejima C. 1973. Preliminary notes on the noteworthy Orchidaceae from Formosa, Ryukyus, Bonin Islands and Southern Japan. – Biol. Mag. Okinawa 10: 26-36.
Nadot S, Penet L, Dreyer LD, Forchioni A, Ressayre A. 2006. Aperture pattern and microsporogenesis in Asparagales. – In: Columbus JT, Friar EA, Porter JM, Prince LM, Simpson MG (eds), Monocots: comparative biology and evolution. Excluding Poales, Rancho Santa Ana Botanical Garden, Claremont, California. – Aliso 22: 197-203.
Nakai T. 1936. Subdivision of Convallariaceae Link. – Jap. J. Bot. 12: 145-150.
Naranjo CA. 1969. Cariotipos de nueve especies Argentinas de Rhodophiala, Hippeastrum, Zephyranthes y Habranthus (Amaryllidaceae). – Kurtziana 5: 67-87.
Naranjo CA. 1974. Karyotypes of four Argentine species of Habranthus and Zephyranthes (Amaryllidaceae). – Phyton (Buenos Aires) 32: 61-71.
Naranjo CA. 1975. Chromosomal studies in Hypoxis L. Karyotype of H. decumbens L. – Phyton (Buenos Aires) 33: 45-49.
Naranjo CA, Andrada AB. 1975. El cariótipo fundamental en el genéro Hippeastrum Herb. (Amaryllidaceae). – Darwiniana 19: 566-582.
Naranjo CA, Poggio L. 1988. A comparison of karyotype, Ag-NOR bands and DNA content in Amaryllis and Hippeastrum (Amaryllidaceae). – Kew Bull. 43: 317-325.
Naranjo CA, Poggio L. 2000. Karyotypes of five Rhodophiala species (Amaryllidaceae). – Bol. Soc. Argentina Bot. 35: 335-343.
Nash RJ, Beaumont J, Veitch NC, Reynolds T, Benner J, Hughes CNG, Dring JV, Bennet RN, Dellar JE. 1992. Phenylethylamine and pyperidine alkaloids in Aloe species. – Planta Medica 58: 84-87.
Negritto MA, Ruiz E, Beck S, Escobar I, Baeza CM. 2010. Schickendantziella trichosepala (Alliaceae), new record from Bolivia. – Gayana Bot. 67: 135-137.
Nel GC. 1914. Studien über die Amaryllidaceae-Hypoxidaea, under besonderer Berücksichtigung der afrikanischen Arten. – In: Engler A (ed), Beiträge zur Flora von Afrika 43, Engl. Bot. Jahrb. Syst. 51: 234-338.
Nelson E. 1965. Zur organophyletischen Natur des Orchideenlabellums. – Bot. Jahrb. Syst. 84: 175-214.
Nelson E. 1976. Monographie und Ikonographie der Orchidaceen-Gattung Dactylorhiza. – Zürich.
Neshati F, Fritsch RM. 2009. Seed characters and testa sculptures of some Iranian Allium L. species (Alliaceae). – Feddes Repert. 120: 322-332.
Neshati F, Fritsch RM, Zarre S. 2009. Pollen morphology of some Allium L. species (Alliaceae) from Iran. – Bot. Jahrb. Syst. 127: 433-451.
Neto E de M. 1948. Chromosome numbers in Amaryllis Linn. – Herbertia 15: 25-29.
Neubig KM, Whitten WM, Carlsward BS, Blanco MA, Endara L, Williams NH, Moore M. 2009. Phylogenetic utility of ycf1 in orchids: a plastid gene more variable than matK. – Plant Syst. Evol. 277: 75-84.
Neubig KM, Whitten WM, Williams NH, Blanco MA, Endara L, Burleigh JG, Silvera K, Cushman JC, Chase MW. 2012. Generic recircumscriptions of Oncidiinae (Orchidaceae: Cymbidieae) based on maximum likelihood analysis of combined DNA datasets. – Bot. J. Linn. Soc. 168: 117-146.
Neves J de B. 1952. Estudios cariologicos no genero Ornithogalum L. – Bol. Soc. Brot., ser. IIa, 26: 1-193.
Neves J de B. 1962. Dados cariológicos sobre algumas espécies Africanas de Ornithogalum L. – Bol. Soc. Brot., ser. IIa, 36: 151-173.
Neves J de B. 1973. Contribution à la connaissance cytotaxonomique des Spermatophyta du Portugal VIII. Liliaceae. – Bol. Soc. Brot., ser. IIa, 47: 157-212.
Newman IV. 1928. The life history of Doryanthes excelsa I. Some ecological and vegetative features on spore production. – Proc. Linn. Soc. New South Wales 53: 499-558.
Newman IV. 1929. The life history of Doryanthes excelsa II. The gametophytes, seed production, chromosome number, and general conclusion. – Proc. Linn. Soc. New South Wales 54: 411-435.
Newton GD, Williams NH. 1978. Pollen morphology of the Cypripedioideae and the Apostasioideae (Orchidaceae). – Selbyana 2: 169-182.
Neyland R, Urbatsch LE. 1993. Anatomy and morphology of the articulation between ovary and pedicel in Pleurothallidinae (Orchidaceae). – Lindleyana 8: 189-192.
Neyland R, Urbatsch LE. 1995. A terrestrial origin for the Orchidaceae suggested by a phylogeny inferred from ndhF chloroplast gene sequences. – Lindleyana 10: 244-251.
Neyland R, Urbatsch LE. 1996a. Evolution in the number and position of fertile anthers in Orchidaceae inferred from ndhF chloroplast gene sequences. – Lindleyana 11: 47-53.
Neyland R, Urbatsch LE. 1996b. Phylogeny of subfamily Epidendroideae (Orchidaceae) inferred from ndhF chloroplast gene sequences. – Amer. J. Bot. 83: 1195-1206.
Neyland R, Urbatsch LE, Pridgeon AM. 1995. A phylogenetic analysis of subtribe Pleurothallidinae. – Bot. J. Linn. Soc. 117: 13-28.
Ng YP, Schuiteman A, Pedersen HÆ, Petersen G, Watthana S, Seberg O, Pridgeon AM, Cribb PJ, Chase MW. 2018. Phylogenetics and systematics of Eria and related genera (Orchidaceae: Podochileae). – Bot. J. Linn. Soc. 186: 179-201.
Nguyen NH, Driscoll HE, Specht CD. 2008. A molecular phylogeny of the wild onions (Allium; Alliaceae) with a focus on the western North American center of diversity. – Mol. Phylogen. Evol. 47: 1157-1172.
Niehaus TF. 1968. A biosystematic study of the genus Brodiaea (Amaryllidaceae). – Ph.D. diss., University of California, Berkeley, California.
Niehaus TF. 1971. A biosystematic study of the genus Brodiaea (Amaryllidaceae). – Univ. Calif. Publ. Bot. 60: 1-66.
Niehaus TF. 1980. The Brodiaea complex. – Four Seasons 6: 11-21.
Niet T van der, Linder HP. 2008. Dealing with incongruence in the quest for the species tree: a case study from the orchid genus Satyrium. – Mol. Phylogen. Evol. 47: 154-174.
Niet T van der, Linder HP, Bytebier B, Bellstedt DU. 2005. Molecular markers reject monophyly of the subgenera of Satyrium (Orchidaceae). – Syst. Bot. 30: 263-274.
Niet T van der, Liltved WR, Oliver EGH. 2009. Satyrium situsanguinum (Orchidaceae): a new species from the Western Cape, South Africa. – South Afr. J. Bot. 75: 22-26.
Nietsch H. 1941. Zur systematischen Stellung von Cyanastrum. – Österr. Bot. Zeitschr. 90: 31-52.
Nilsson LA, Jonsson L, Ralison L, Randrianjohany E. 1987. Angraecoid orchids and hawkmoths in central Madagascar: specialized pollination systems and generalist foragers. – Biotropica 19: 310-318.
Nishimura G, Tamura M. 1993. Seed coat formation in Apostasia nipponica. – J. Jap. Bot. 68: 219-223.
Nobel PS. 1988. Environmental biology of agaves and cacti. – Cambridge University Press, Cambridge.
Noguera-Savelli EJ, Carnevali F-CG, Romero-González GA. 2008. Description of a new species and notes on Crossoglossa (Orchidaceae: Epidendroideae: Malaxideae) from the eastern Andes in Colombia and Venezuela. – Brittonia 60: 240-244.
Nordal I. 1977. Revision of the East African taxa of the genus Crinum (Amaryllidaceae). – Norw. J. Bot. 24: 179-194.
Nordal I. 1982. Amaryllidaceae. – In: Polhill RM (ed), Flora of tropical East Africa, A. A. Balkema, Rotterdam, pp. 1-30.
Nordal I. 1998. Hypoxidaceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 286-295.
Nordal I, Duncan T. 1984. A cladistic analysis of Haemanthus and Scadoxus. – Nord. J. Bot. 4: 145-153.
Nordal I, Iversen I. 1987. Hypoxidaceae. – Flore du Cameroun 30: 33-47.
Nordal I, Kwembeya EG. 2004. Crinum binghamii sp. nov.: with a key to Crinum species with radially symmetrical flowers in mainland Africa. – Kew Bull. 59: 599-603.
Nordal I, Stedje B. 1993. The Ornithogalum tenuifolium complex (Hyacinthaceae) in Zimbabwe. – Kirkia 14: 12-18.
Nordal I, Thulin M. 1993. Synopsis of Anthericum and Chlorophytum (Anthericaceae) in the Horn of Africa, including the description of nine new species. – Nord. J. Bot. 13: 257-280.
Nordal I, Wahlström R. 1980. A study of the genus Crinum (Amaryllidaceae) in Cameroun. – Adansonia, sér. II, 20: 179-198.
Nordal I, Wahlström R. 1982. Studies in the Crinum zeylanicum complex in East Africa. – Nord. J. Bot. 2: 465-473.
Nordal I, Rorslett B, Laane MM. 1977. Species delimitation within the Crinum ornatum group (Amaryllidaceae) in East Africa. – Norw. J. Bot. 24: 195-212.
Nordal I, Laane MM, Holt E, Staubo I. 1985. Taxonomic studies of the genus Hypoxis in East Africa. – Nord. J. Bot. 5: 15-30.
Nordal I, Eriksen TE, Fosby M. 1990. Studies in the generic delimitation of Anthericaceae. – In: Proceedings of the 12th plenary meeting of AETFAT, Mitt. Staatsinst. Allg. Bot. Hamburg 23b: 535-559.
Nordal I, Kativu S, Poulsen AD. 1997. Anthericaceae. – In: Polhill RM (ed), Flora of tropical East Africa, A. A. Balkema, Rotterdam, pp. 1-67.
Nordenstam B. 1964. Studies in South African Liliaceae II. Two small species of Bulbine. – Bot. Not. 117: 183-187.
Nordenstam B. 1970a. Studies in South African Liliaceae III. The genus Rhadamanthus. – Bot. Not. 123: 155-182.
Nordenstam B. 1970b. Notes on South African Iridaceae: Lapeirousia and Babiana. – Bot. Not. 123: 433-443.
Nordström S, Hedrén M. 2008. Genetic differentiation and postglacial migration of the Dactylorhiza majalis ssp. traunsteineri/lapponica complex into Fennoscandia. – Plant Syst. Evol. 276: 73-87.
Norup MF, Petersen G, Burrows S, Bouchenak-Khelladi Y, Leebens-Mack J, Pires JC, Linder HP, Seberg O. 2015. Evolution of Asparagus L. (Asparagaceae): out-of-South-Africa and multiple origins of sexual dimorphism. – Mol. Phylogen. Evol. 92: 25-44.
Nowak S, Szlachetko D, Mytnik-Ejsmont J,
Cleef A. 2015. Eight new species of Gomphichis (Orchidaceae, Spiranthoideae) from
Colombia. – Plant Syst. Evol. 301: 61-76.
Nozeran R. 1946. L’inflorescence d’Aphyllanthes monspeliensis L. – Ann. Sci. Nat., sér. 11, Bot. Boil. Veg., 7: 147-151.
Nuñez O, Fraissinet N, Rodríguez RH, Jones K. 1974. Cytogenetic studies in the genus Nothoscordum Kunth I. The N. inodorum polyploidy complex. – Caryologia 27: 403-441.
Obermeyer AA. 1962. A revision of the South African species of Anthericum, Chlorophytum, and Trachyandra. – Bothalia 7: 669-767.
Obermeyer AA. 1973. Liliaceae: Aloe, Chamaealoe, Haworthia, Astroloba, Poellnitzia and Chortolirion. – Bothalia 11: 119.
Obermeyer AA. 1978. Ornithogalum: a revision of the southern African species. – Bothalia 12: 323-376.
Obermeyer AA. 1980. The genus Sypharissa (Liliaceae). – Bothalia 13: 111-114.
Ocampo López A, Camacho ES, Casas HS. 2000. Morfología de semillas, germinación y desarrollo postemergente de tres species del género Polianthes L. (Agavaceae). – Bol. Soc. Bot. México 66: 55-65.
Oganezova GG. 1981. Anatomical and morphological study in Ixiolirion tataricum ssp. montanum. – Bot. Žurn. 66: 702-713. [In Russian]
Oganezova GG. 1982. On the anatomical structure of fruit and seed of some Liliaceae in relation to systematics of the family: 2. Scilloideae. – Bot. Žurn. 67: 729-742. [In Russian]
Oganezova GG. 1986. Morphological and anatomical characters of seed and fruit in some members of the subfamily Allioideae (Liliaceae) in relation to their systematics and phylogeny. – Bot. Žurn. 71: 300-310. [In Russian with English summary]
Oganezova GG. 1987a. Characteristics of seed and fruit anatomical structure in some representatives of subfamily Asphodeloideae (Liliaceae) in connection with their systematics and phylogeny. – Bot. Žurn. 72: 436-447. [In Russian]
Oganezova GG. 1987b. Systematic position of some disputable genera for Asphodeloideae (Liliaceae) based on the anatomical structure of their fruits and seeds. – Bot. Žurn. 72: 1009-1020. [In Russian]
Oganezova GG. 1990. Seed and fruit anatomy of some Amaryllidaceae in connection with their systematics and phylogeny. – Bot. Žurn. 75: 615-630. [In Russian with English summary]
Oganezova GG. 1995. On the systematical position of the families Haemodoraceae, Hypoxodaceae and Taccaceae. – Bot. Žurn. 80: 12-25. [In Russian]
Oganezova GG. 2000a. Fruit and seed structure of some Asparagaceae s.l. in connection with the volume and phylogeny of the family. – Bot. Žurn. 85: 14-32.[In Russian]
Oganezova GG. 2000b. Systematic position of the Trilliaceae, Smilacaceae, Dioscoreaceae, Herreriaceae, Tecophilaeaceae families and the volume and phylogeny of the Asparagales (based on seed structure). – Bot. Žurn. 85: 9-25. [In Russian]
Ogura Y. 1953. Anatomy and morphology of the subterranean organs in some Orchidaceae. – J. Fac. Sci. Univ. Tokyo, Sect. III, Botany 6: 135-157.
Ogura-Tsujita Y, Gebauer G, Hashimoto T, Umata H, Yukawa T. 2009. Evidence for novel and specialized mycorrhizal parasitism: the orchid Gastrodia confusa gains carbohydrate from saprotrophic Mycena. – Proc. Roy. Soc. London, Ser. B, 276: 761-767.
Ohri D, Fritsch RM, Hanelt P. 1998. Evolution of genome size in Allium (Alliaceae). – Plant Syst. Evol. 210: 57-86.
Ojeda I, Carnevali G, Williams NH, Whitten WM. 2003. Phylogeny of the Heterotaxis Lindley complex (Maxillariinae): evolution of the vegetative architecture and pollination syndromes. – Lankesteriana 7: 45-47.
Ojeda L, Ludlow-Wiechers B. 1995. Palinología de Agavaceae, una contribución biosistemática. – Bol. Soc. Bot. México 56: 25-43.
Ojeda L, Ludlow-Wichers B, Orellana R. 1984. Palinología de la familia Agavaceae pará la península de Yucatán. – Biotica 9: 379-398.
Okada H. 1988. Karyomorphological observations of Apostasia nuda and Neuwiedia veratrifolia (Apostasioideae, Orchidaceae). – J. Jap. Bot. 63: 344-350.
Olatunji OA, Nengim RO. 1980. Occurrence and distribution of tracheoidal elements in the Orchidaceae. – Bot. J. Linn. Soc. 80: 357-370.
Oliveira IG de, Moraes AP, Almeida EM de, Assis FNM de, Cabral JS, Barros F de, Felix LP. 2015. Chromosomal evolution in Pleurothallidinae (Orchidaceae: Epidendroideae) with an emphasis on the genus Acianthera: chromosome numbers and heterochromatin. – Bot. J. Linn. Soc. 178: 102-120.
Oliveira RS de. 2012. O gênero Hippeastrum Herb. (Amaryllidaceae) no Brasil: evidência de evolução reticulada e análise de caracteres florais. – Universidade Estadual de Campinas, Brazil.
Oliveira RS de, Semir J, Dutilh JHA. 2013. Four new endemic species of Hippeastrum (Amaryllidaceae) from Serra da Canastra, Minas Gerais State, Brazil. – Phytotaxa 145: 38-46.
Oliver EGH, Liltved WR, Pauw A. 2008. Pterygodium vermiferum (Coryciinae), a new, autonomously self-pollinating, oil-secreting orchid from the Western Cape of South Africa. – South Afr. J. Bot. 74: 617-622.
Ono R, Hashimoto A. 1956. Chromosome studies in Orchidaceae I. Chromosome numbers in four species of Orchidaceae. – Bot. Mag. (Tokyo) 69: 286-288.
Ono T. 1928. Endosperm formation in the Liliaceae. – Bot. Mag. (Tokyo) 42: 445-449.
Ornduff R. 1979a. Chromosome numbers and relationships of certain African and American genera of Haemodoraceae. – Ann. Missouri Bot. Gard. 66: 577-580.
Ornduff R. 1979b. Chromosome numbers in Cyanella (Tecophilaeaceae). – Ann. Missouri Bot. Gard. 66: 581-583.
Ornduff R. 1983. Studies on the reproductive system of Nivenia corymbosa an apparently androdioecious species – Ann. Missouri Bot. Gard. 70: 146-148.
Ortiz S, Rodríguez-Oubiña J. 1996. Taxonomic characterization of populations of Hyacinthoides sect. Somera (Hyacinthaceae) in the northwestern Iberian Peninsula. – Plant Syst. Evol. 202: 111-119.
Ortiz Valdivieso P. 1997. New orchids from Colombia. – Orquideologia 20: 314-327.
Otero JT, Flanagan NS. 2006. Orchid diversity – beyond deception. – Trends Ecol. Evol. 21: 64-65.
Otero JT, Ackerman JD, Bayman P. 2002. Diversity and host specificity of endophytic Rhizoctonia-like fungi from tropical orchids. – Amer. J. Bot. 89: 1852-1858.
Otero JT, Thrall PH, Clements M, Burdon JJ, Miller JT. 2011. Codiversification of orchids (Pterostylidinae) and their associated mycorrhizal fungus. – Aust. J. Bot. 59: 480-497.
Owenby BP. 1944. The liliaceous genus Polygonatum in North America. – Ann. Missouri Bot. Gard. 31: 373-413.
Ownbey M, Aase HC. 1955. Cytotaxonomic studies in Allium I. The Allium canadense alliance. – Res. Stud. State Coll. Wash. 23, Monogr. Suppl. 1: 1-106.
Oyewole SO. 1971. Biosystematic studies in the genus Albuca L. with particular reference to those species occurring in Nigeria. – Ph.D. diss., University of Ibadan, Nigeria.
Oyewole SO. 1972. Cytological and cytogenetic studies in the genus Albuca L. in West Africa. – Bol. Soc. Brot., ser. IIa, 46: 149-170.
Oyewole SO. 1975. Cytotaxonomic studies in the genus Urginea in West Africa 1. Karyotype analysis in Urginea altissima, Urginea gigantea and Urginea viridula. – Bol. Soc. Brot., ser. IIa, 49: 213-223.
Oyewole SO. 1984. Pachyten analyses and the karyotype of Drimiopsis barteri. – Cytologia 49: 81-86.
Özhatay N. 1978. The chromosomes of Milula spicata (Liliaceae). – Kew Bull. 32: 453-454.
Özhatay N. 1986. Allium species grown in northern Anatolia and their chromosome number. – Doga TU Bio. D. 10: 452-458.
Özhatay N, Mathew B. 1995. New taxa and notes on the genus Allium (Alliaceae) in Turkey and Arabia. – Kew Bull. 50: 723-731.
Pabón-Mora N, González F. 2008. Floral ontogeny of Telipogon spp. (Orchidaceae) and insights on the perianth symmetry in the family. – Intern. J. Plant Sci. 169: 1159-1173.
Pace L. 1909. The gametophytes of Calopogon. – Bot. Gaz. 48: 126-137.
Pacek A, Stpiczyńska M, Davies KL, Szymczak G. 2012. Floral elaiophore structure in four representatives of the Ornithocephalus clade (Orchidaceae: Oncidiinae). – Ann. Bot. 110: 809-820.
Pacini E. 2008. Pollination biology: orchid pollen dispersal units and reproductive structures. – In: Kull T, Arditti J, Wong SM (eds), Orchid biology: reviews and perspecitives X, Springer, pp. 187-218.
Pacini E, Hesse M. 2002. Types of pollen dispersal units in orchids, and their consequences for germination and fertilization. – Ann. Bot. (London) 89: 653-664.
Palestina RA, Sosa V. 2002. Morphological variation in populations of Bletia purpurea (Orchidaceae) and description of the new species B. riparia. – Brittonia 54: 99-111.
Palomino G, Martinez J. 1994. Cytotypes and meiotic behaviour in Mexican populations of three species of Echeandia (Liliaceae). – Cytologia 59: 295-304.
Pandolfi T, Pacini E, Calder DM. 1993. Ontogenesis of monad pollen in Pterostylis plumosa (Orchidaceae, Neottioideae). – Plant Syst. Evol. 186: 175-185.
Pansarin ER, Barros F de. 2008. Taxonomic notes on Pogonieae (Orchidaceae): Cleistesiopsis, a new genus segregated from Cleistes, and description of two new South American species, Cleistes batistana and C. elongata. – Kew Bull. 63: 441-448.
Pansarin ER, Salatino A, Salatino MLF. 2008. Phylogeny of South American Pogonieae (Orchidaceae, Vanilloideae) based on sequences of nuclear ribosomal (ITS) and chloroplast (psaB, rbcL, rps16, and trnL-F) DNA, with emphasis on Cleistes and discussion of biogeographic implications. – Organisms Divers. Evol. 8: 171-181.
Pansarin LM, Pansarin ER, Sazim M. 2014.
Osmophore structure and phylogeny of Cirrhaea (Orchidaceae, Stanhopeinae). – Bot. J.
Linn. Soc. 176: 369-383.
Papanicolaou K, Zacharof E. 1980. Crocus in Greece: new taxa and chromosome numbers. – Bot. Not. 133: 155-163.
Papanicolaou K, Zacharof E. 1983. Cytological notes and taxonomic comments of four Galanthus L. taxa from Greece. – Israel J. Bot. 32: 22-32.
Parlatore P. 1855. Note sur l’Aphyllanthes monspeliensis et la nouvelle famille des Aphyllanthacées. – Bull. Soc. Bot. France 2: 529-532.
Pascher A. 1959. Zur Blütenbiologie einer aasblumigen Liliacee und zur Verbreitungsbiologie abfallender geflügelter Kapseln. – Flora 148: 153-178.
Pastor J. 1982. Karyology of Allium species from the Iberian Peninsula. – Phyton (Horn) 22: 171-200.
Pastor J, Valdés B. 1983. Revision del género Allium (Liliaceae) en la Peninsula Iberica e Islas Baleares. – Sevilla.
Pathak GN. 1940. Studies in the cytology of Crocus. – Ann. Bot., N. S., 4: 227-256.
Pauli GF. 1995. The cardenolids of Speirantha convallarioides. – Planta Medica 61: 162-166.
Paulus H, Gack C. 1994. Signalfälschung als Bestäubungsstrategie in der mediterranen Orchideengattung Ophrys – Probleme der Artbildung un der Artabgrenzung. Proc. Int. Symp. European Orchids, Utrecht/Haarlem.
Paun O, Luna JA, Fay MF, Bateman RM, Chase MW. 2010. Genomic responses drive adaptation in allotetraploid species of Dactylorhiza (Orchidaceae; Orchidinae). – In: Seberg O, Petersen G, Barfod AS, Davis JI (eds), Diversity, phylogeny, and evolution in the monocotyledons, Aarhus University Press, Århus, pp. 169-192.
Pauw A. 2006. Floral syndromes accurately predict pollination by a specialized oil-collecting bee (Rediviva peringueyi, Mellitidae) in a guild of South African orchids (Coryciinae). – Amer. J. Bot. 93: 917-926.
Pavillard G. 1919. Sur la structure femelle des Ruscus. – Compt. Rend. Acad. Sci. Paris 168: 113-115.
Pax F. 1888a. Amaryllidaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien II(5), W. Engelmann, Leipzig, pp. 97-124; Pax F. 1897. Nachträge zu II(5), pp. 77-80.
Pax F. 1888b. Iridaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien II(5), W. Engelmann, Leipzig, pp. 137-157.
Pax F. 1890. Beiträge zur Kenntnis der Amaryllidaceae. – Englers Bot. Jahrb. Syst. 11: 318-337.
Pax F, Hoffmann K. 1930. Amaryllidaceae. – In: Engler A (ed), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd 15a, W. Engelmann, Leipzig, pp. 391-430.
Peakall R. 2007. Speciation in the Orchidaceae: confronting the challenge. – Mol. Ecol. 16: 2834-2837.
Peakall R, Beattie AJ. 1989. Pollination of the orchid Microtis parviflora R. Br. by flightless worker ants. – Funct. Ecol. 3: 515-522.
Pedersen HÆ. 1992. Notes on the genus Pteroceras (Orchidaceae). – Nord. J. Bot. 12: 387-388.
Pedersen HÆ. 1993. The genus Pteroceras (Orchidaceae) – a taxonomic revision. – Opera Bot. 117: 1-64.
Pedersen HÆ. 1995. Thirteen new species of Dendrochilum (Orchidaceae), a new record from Burma, and a checklist of the genus in East Malesia. – Nord. J. Bot. 15: 381-402.
Pedersen HÆ. 1997. The genus Dendrochilum (Orchidaceae) in the Philippines – a taxonomic revision. – Opera Bot. 131: 1-205.
Pedersen HÆ. 2010. Inadequate morphometric analyses have contributed to oversplitting in European orchids: a case study in Dactylorhiza (Orchidaceae). – In: Seberg O, Petersen G, Barfod AS, Davis JI (eds), Diversity, phylogeny, and evolution in the monocotyledons, Aarhus University Press, Århus, pp. 193-212.
Pedersen HÆ, Wood JJ, Comber JB. 1997. A revised subdivision and bibliographical survey of Dendrochilum (Orchidaceae). – Opera Bot. 130: 1-85.
Pedersen K, Wendelbo P. 1966. Chromosome numbers of some SW Asian Allium species. – Blyttia 24: 307-313.
Pedley L. 1986. Cordyline. – In: George AS (ed), Flora of Australia 46, Australian Government Publ. Service, Canberra, pp. 81-86.
Pedley L, Forster PI. 1986. Agavaceae. – In: George AS (ed), Flora of Australia 46, Australian Government Publ. Service, Canberra, pp. 71-88.
Pedron M, Buzatto CR, Ramalho AJ, Carvalho,
BM, Radins JA, Singer RB, Batista JAN. 2014. Molecular phylogenetics and
taxonomic revision of Habenaria section Pentadactylae (Orchidaceae, Orchidinae). – Bot. J.
Linn. Soc. 175: 47-73.
Pellmyr O. 2003. Yuccas, yucca moths, and coevolution: a review. – Ann. Missouri Bot. Gard. 90: 35-55.
Pellmyr O, Leebens-Mack J. 1999. Forty million years of mutualism: evidence for Eocene origin of the yucca-yucca moth association. – Proc. Natl. Acad. Sci. U.S.A. 96: 9178-9183.
Pellmyr O, Thompson JN, Brown JM, Harrison RG. 1996. Evolution of pollination and mutualism in the yucca moth lineage. – Amer. Natur. 148: 827-847.
Pellmyr O, Segraves KA, Althoff DM, Balcázar-Lara M, Leebens-Mack J. 2007. The phylogeny of yuccas. – Mol. Phylogen. Evol. 43: 493-501.
Penet L, Nadot S, Ressayre A, Forchioni A, Dreyer L, Gouyon PH. 2005. Multiple developmental pathways leading to a single morph: monosulcate pollen (examples from the Asparagales). – Ann. Bot. 95: 331-343.
Penet L, Laurin M, Gouyon PH, Nadot S. 2007. Constraints and selection: insights from microsporogenesis in Asparagales. – Evol. Developm. 9: 460-471.
Peraza-Flores LN, Carnevali G, van den Berg C. 2016. A molecular phylogeny of the Laelia alliance (Orchidaceae) and a reassessment of Laelia and Schomburgkia. – Taxon 65: 1249-1262.
Pérez-Hérnandez H, Damon A, Valle-Mora J, Sánchez-Guillen D. 2011. Orchid pollination: specialization in chance? – Bot. J. Linn. Soc. 165: 251-266.
Perrier de la Bâthie H. 1934. Deux liliacées nouvelles de Madagascar. – Bull. Soc. Bot. France 81: 819-823.
Perrier de la Bâthie H. 1939. 49. Orchidées 1. – In: Humbert N (ed), Flore de Madagascar et des Comores, Imprimerie officielle, Tananarive.
Perrier de la Bâthie H. 1941. 49. Orchidées 2. – In: Humbert N (ed), Flore de Madagascar et des Comores, Imprimerie officielle, Tananarive.
Perry P. 1987. A synoptic review of the genus Bulbinella (Asphodelaceae) in South Africa. – South Afr. J. Bot. 53: 431-444.
Perry PL. 1994. A revision of the genus Eriospermum (Eriospermaceae). – Contr. Bolus Herb. 17: 1-320.
Perry PL, Rudall PJ. 1998. Eriospermaceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 241-244.
Persson K. 2006. One new and one emended species of Bellevalia (Hyacinthaceae) from Turkey. – Bot. J. Linn. Soc. 150: 253-260.
Persson K, Persson J. 1992. A new species and additional chromosome counts of Hyacinthella in Turkey. – Nord. J. Bot. 12: 615-620.
Persson K, Wendelbo P. 1979a. Bellevalia hyacinthoides, a new name for Strangweja spicata. – Bot. Not. 132: 65-70.
Persson K, Wendelbo P. 1979b. The taxonomic position of Nectaroscordum koelzii (Liliaceae). – Bot. Not. 132: 191-196.
Persson K, Wendelbo P. 1979c. Alrawia, a new genus of Liliaceae-Scilloideae. – Bot. Not. 132: 201-206.
Persson K, Wendelbo P. 1981. Taxonomy and cytology of the genus Hyacinthella (Liliaceae-Scilloideae) with special reference to the species in S. W. Asia I. – Candollea 36: 513-541.
Persson K, Wendelbo P. 1982. Taxonomy and cytology of the genus Hyacinthella (Liliaceae-Scilloideae) with special reference to the species in S. W. Asia II. – Candollea 37: 157-175.
Pessoa E, Alves M. 2016. Taxonomic revision of Campylocentrum (Orchidaceae-Vandeae-Angraecinae): species with terete leaves. – Syst. Bot. 41: 700-713.
Pessoa EM, Viruel J, Alves M,Bogarín D, Whitten WM, Chase MW. 2018. Evolutionary history and systematics of Campylocentrum (Orchidaceae: Vandeae: Angraecinae): a phylogenetic and biogeographical approach. – Bot. J. Linn. Soc. 186: 158-178.
Petersen G, Seberg O, Thorsøe S, Jørgensen T, Mathew B. 2008. The phylogeny of the genus Crocus (Iridaceae) based on sequence data from five plastid regions. – Taxon 57: 487-499.
Peterson PM, Annable CR, Rieseberg LH. 1988. Systematic relationships and nomenclatural changes in the Allium douglasii complex (Alliaceae). – Syst. Bot. 13: 207-214.
Pettersson B. 1990. Studies in the genus Nervilia (Orchidaceae) in Africa. – Nord. J. Bot. 9: 487-497.
Pettersson B. 1991. The genus Nervilia (Orchidaceae) in Africa and the Arabian Peninsula. – Orchid. Monogr. 5.
Pettit GR, Pettit GR III, Backhaus RA, Boyd MR, Meerow AW. 1993. Antineoplastic agents 256. Cell growth-inhibitory isocarbostyrils from Hymenocallis. – J. Nat. Prod. 56: 1682-1687.
Pettit GR, Pettit GR III, Groszek G, Backhaus RA, Doubek DL, Meerow AW. 1995. Antineoplastic agents 301. An investigation of the genus Hymenocallis Salisbury (Amaryllidaceae). – J. Nat. Prod. 58: 756-759.
Pfitzer EHH. 1882. Grundzüge einer vergleichenden Morphologie der Orchideen. – Carl Winters Universitätsbuchhandlung, Heidelberg.
Pfitzer EHH. 1886. Morphologische Studien über die Orchideenblüthe. – Carl Winters Universitätsbuchhandlung, Heidelberg.
Pfitzer EHH. 1887. Entwurf einer natürlichen Anordnung der Orchideen. – Carl Winters Universitätsbuchhandlung, Heidelberg.
Pfitzer EHH. 1888. Untersuchungen über Bau und Entwicklung der Orchideenblüte I: Cypripediinae, Ophrydinae, Neottiinae. – Pringsheim Jahrb. Wissensch. Bot. 19: 155-177.
Pfitzer EHH. 1889. Orchidaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien II(6), W. Engelmann, Leipzig, pp. 52-218; Pfitzer EHH. 1897. Nachträge zu II(6), pp. 97-113.
Pfitzer EHH. 1894. Beiträge zur Systematik der Orchideen. – Engl. Bot. Jahrb. Syst. 19: 1-42.
Pfitzer EHH. 1905. Über den morphologischen Aufbau der Coelogyninae. – Engl. Bot. Jahrb. Syst. 34: 55-59.
Pfosser MF, Speta F. 1999. Phylogenetics of Hyacinthaceae based on plastid DNA sequences. – Ann. Missouri Bot. Gard. 86: 852-875.
Pfosser MF, Speta F. 2001. Bufadienolide und DNA-Sequenzen: über Zusammenhalt und Aufteilung der Urgineoideae (Hyacinthaceae). – Stapfia 75: 177-242.
Pfosser MF, Speta F. 2004. From Scilla to Charybdis – is our voyage safer now? – Plant Syst. Evol. 246: 245-263.
Pfosser M, Wetschnig W, Ungar S, Prenner G. 2003. Phylogenetic relationships among genera of Massonieae (Hyacinthaceae) inferred from plastid DNA and seed morphology. – J. Plant Res. 116: 115-132.
Pfosser M, Wetschnig W, Speta F. 2006. Drimia cryptopoda, a new combination in Hyacinthaceae from Madagascar. – Linzer Biol. Beitr. 38: 1731-1739.
Phillips RD, Backhouse G, Brown AP, Hopper SD. 2009. Biogeography of Caladenia (Orchidaceae), with special reference to the South-west Australian Floristic Region. – Aust. J. Bot. 57: 259-275.
Pienaar R de V. 1963. Sitogenetiese studies in die genus Ornithogalum L. – J. South Afr. Bot. 29: 111-130.
Pijl L van der, Dodson CH. 1966. Orchid flowers, their pollination and evolution. – Fairchild Tropical Garden and University of Miami Press, Coral Gables, Florida.
Pillon Y, Fay MF, Hedrén M, Bateman RM, Devey DS, Shipunov AB, Bank M van der, Chase MW. 2007. Evolution and temporal diversification of wetstern European polyploid species complexes in Dactylorhiza (Orchidaceae). – Taxon 56: 1185-1208.
Pinheiro F, Koehler S, Corrêa AM, Salatino MLF, Salatino A, Barros F. 2009. Phylogenetic relationships and infrageneric classification of Epidendrum subgenus Amphiglottium (Laeliinae, Orchidaceae). – Plant Syst. Evol. 283: 165-177.
Piper JK. 1986. Effects of habitat and size of fruit display on removal of Smilacina stellata (Liliaceae) fruits. – Can. J. Bot. 64: 1050-1054.
Piper JK. 1989. Light, flowering, and fruiting within patches of Smilacina racemosa and Smilacina stellata (Liliaceae). – Bull. Torrey Bot. Club 116: 247-257.
Pires JC. 2000. Biosystematics and molecular phylogenetics of Brodiaea (Themidaceae) and lilioid relatives. – Ph.D. diss., University of Wisconsin, Madison, Wisconsin.
Pires JC, Sytsma KJ. 2002. A phylogenetic evaluation of a biosystematic framework: Brodiaea and related petaloid monocots (Themidaceae). – Amer. J. Bot. 89: 1342-1359.
Pires JC, Fay MF, Davis WS, Hufford L, Rova J, Chase MW, Sytsma KJ. 2001. Molecular and morphological phylogenetic analyses of Themidaceae (Asparagales). – Kew Bull. 56: 601-626.
Pires JC, Maureira IJ, Rebman JP, Salazar GA, Cabrera LI, Fay MF, Chase MW. 2004. Molecular data confirm the phylogenetic placement of the enigmatic Hesperocallis (Hesperocallidaceae) with Agave. – Madroño 51: 307-311.
Pires JC, Maureira IJ, Givnish TJ, Sytsma KJ, Seberg O, Petersen G, Davis JI, Stevenson DW, Rudall PJ, Fay MF, Chase MW. 2006. Phylogeny, genome size, and chromosome evolution in Asparagales. – In: Columbus JT, Friar EA, Porter JM, Prince LM, Simpson MG (eds), Monocots: comparative Biology and Evolution. Excluding Poales, Rancho Santa Ana Botanical Garden, Claremont, California. – Aliso 22: 287-304.
Pires M de Fatima de Oliveira, Semir J, Pinna GF de A Melo de, Felix LP. 2003. Taxonomic separation of the genera Prosthechea and Encyclia (Laeliinae: Orchidaceae) using leaf and root anatomical features. – Bot. J. Linn. Soc. 143:293-303.
Pizzolato TD. 2009. Procambial initiation for the vascular system in the rhizome of Ophiopogon (Asparagales). – Intern. J. Plant Sci. 170: 701-723.
Plummer JA, Crawford AD, Taylor SK. 1995. Germination of Lomandra sonderi (Dasypogonaceae) promoted by pericarp removal and chemical stimulation of the embryo. – Aust. J. Bot. 43: 223-230.
Poddubnaya-Arnoldi VA. 1967. Comparative embryology of the Orchidaceae. – Phytomorphology 17: 312-320.
Poellnitz K von. 1941. Zwei neue Anthericum-Arten aus Ost-Afrika. – Feddes Repert. 50: 322-323.
Poellnitz K von. 1942. Die Anthericum-Arten Deutsch-Ost-Afrikas. – Feddes Repert. 51: 17-32, 66-80, 113-139.
Poellnitz K von. 1943a. Neue Anthericum-Arten aus Südamerika. – Rev. Sudamericana Bot. 7: 99-104.
Poellnitz K von. 1943b. Die Anthericum-Arten Angolas. – Bol. Soc. Brot. 17: 56-91.
Poellnitz K von. 1943c. Die Anthericum-Arten Kameruns. – Mitt. Thuring. Bot. Ver., N. F., 50: 179-190.
Poellnitz K von. 1943d. Zur Kenntnis der Gattung Chlorophytum. – Ber. Deutsch. Bot. Ges. 61: 126-131.
Poellnitz K von. 1944. Neue Anthericum-Arten. – Feddes Repert. 53: 126-137.
Poellnitz K von. 1945. Neue Chlorophytum-Arten Afrikas. – Portug. Acta Biol., ser. B, 1: 221-233.
Poellnitz K von. 1946. Die Chlorophytum-Arten Tanganyikas. – Portug. Acta Biol., ser. B, 1: 255-383.
Poggio L, Naranjo CA, Jones K. 1986. The chromosomes of orchids IX. Eulophia. – Kew Bull. 41: 45-49.
Poggio L, Gonzalez G, Naranjo CA. 2007. Chromosome studies in Hippeastrum (Amaryllidaceae): variation in genome size. – Bot. J. Linn. Soc. 155: 171-178.
Pogosian A. 1983. Hromosomnye čisla nekotoryh vidov roda Allium (Alliaceae), rasprostrannennyh na territorii Armenii i Irana. – Bot. Žurn. 68: 652-660.
Polhill R. 1962. Common perennial lilies of Kenya with ephemeral flowering shoots. – J. East Afr. Nat. Hist. Soc. 24: 1-32.
Ponsie ME, Mitchell A, Edwards TJ, Johnson SD. 2007. Phylogeny of Bonatea (Orchidaceae: Habenariinae) based on molecular and morphological data. – Plant Syst. Evol. 263: 253-268.
Ponsie ME, Edwards TJ, Johnson SD. 2007. A taxonomic revision of Bonatea Willd. (Orchidaceae: Orchidoideae: Habenariinae). – South Afr. J. Bot. 73: 1-21.
Porembski S, Barthlott W. 1988. Velamen radicum micromorphology and classification of Orchidaceae. – Nord. J. Bot. 8: 117-137.
Porter CL, Morrison DA, Johnson KA. 1992. Morphological variation within the genus Blandfordia (Liliaceae) in relation to its environment. – Aust. Syst. Bot. 5: 373-385.
Poulsen AD, Nordal I. 1999. Two new species of Chlorophytum from Central Africa. – Kew Bull. 54: 941-949.
Poulsen AD, Nordal I. 2005. A phenetic analysis and revision of Guineo-Congolean rain forest taxa of Chlorophytum (Anthericaceae). – Bot. J. Linn. Soc. 148: 1-20.
Powell JA, Mackie RA. 1966. Biological interrelationships of moths and Yucca whipplei. – Univ. Calif. Publ. Entomol. 42: 1-46.
Pradhan GM. 1979. Cirrhopetalums of North India. – Orch. Dig. 43: 230-236.
Pradhan UC. 1979. Indian orchids. – Paridabad.
Prain D. 1895. On Milula, a new genus of Liliaceae from eastern Himalaya. – Sci. Mem. Med. Offic. Army India 9: 25-27.
Prakash N, Ramsey M. 2000. Embryological development in Blandfordia and Neoastelia with comments on their systematic position. – In: Wilson KL, Morrison DA (eds), Monocots: systematics and evolution, CSIRO Publ., Collingwood, pp. 214-217.
Pridgeon AM. 1981. Absorbing trichomes in the Pleurothallidinae (Orchidaceae). – Amer. J. Bot. 68: 64-71.
Pridgeon AM. 1982. Diagnostic anatomical characters in the Pleurothallidinae (Orchidaceae). – Amer. J. Bot. 69: 921-938.
Pridgeon AM. 1987. The velamen and exodermis of orchid roots. – In: Arditti J (ed), Orchid biology, reviews and perspectives IV, Comstock, Cornell University Press, Ithaca, New York, pp. 139-192.
Pridgeon AM. 1993. Systematic leaf anatomy of Caladenia (Orchidaceae). – Kew Bull. 48: 533-543.
Pridgeon AM. 1994. Multicellular trichomes in tribe Diurideae (Orchidaceae): systematic and biological significance. – Kew Bull. 49: 569-579.
Pridgeon AM, Chase MW. 1995. Subterranean axes in tribe Diurideae (Orchidaceae): morphology, anatomy, and systematic significance. – Amer. J. Bot. 82: 1473-1495.
Pridgeon AM, Chase MW. 2001. A phylogenetic reclassification of Pleurothallidinae (Orchidaceae). – Lindleyana 16: 235-271.
Pridgeon AM, Stern WL, Benzing DH. 1983. Tilosomes in roots of Orchidaceae: morphology and systematic occurrence. – Amer. J. Bot. 70: 1365-1377.
Pridgeon AM, Bateman RM, Cox AV, Hapeman JR, Chase MW. 1997. Phylogenetics of subtribe Orchidinae (Orchidoideae; Orchidaceae) based on nuclear ITS sequences 1. Intergeneric relationships and polyphyly of Orchis sensu lato. – Lindleyana 12: 89-109.
Pridgeon AM, Cribb PJ, Chase MW (eds). 1999. Genera orchidacearum 1. General Introduction, Apostasioideae, Cypripedioideae. – Oxford University Press, Oxford.
Pridgeon AM, Cribb PJ, Chase MW, Rasmussen FN (eds). 2001. Genera orchidacearum 2. Orchidoideae (Part 1). – Oxford University Press, Oxford.
Pridgeon AM, Solano R, Chase MW. 2001. Phylogenetic relationships in Pleurothallidinae (Orchidaceae): combined evidence from nuclear and plastid DNA sequences. – Amer. J. Bot. 88: 2286-2308.
Pridgeon AM, Cribb PJ, Chase MW, Rasmussen FN (eds). 2003. Genera orchidacearum 3. Orchidoideae (Part 2), Vanilloideae. – Oxford University Press, Oxford.
Pridgeon AM, Cribb PJ, Chase MW, Rasmussen FN (eds). 2005. Genera orchidacearum 4. Epidendroideae (Part 1). – Oxford University Press, Oxford.
Pridgeon AM, Cribb PJ, Chase MW, Rasmussen FN (eds). 2009. Genera orchidacearum 5. Epidendroideae (Part 2). – Oxford University Press, Oxford.
Pridgeon AM, Cribb, PJ, Chase MW, Rasmussen FN (eds). 2014. Genera orchidacearum 6. Epidendroideae (Part 3). – Oxford Unviersity Press, Oxford.
Prutsch J, Schill R. 2000a. Die Ontogenese der Narbe bei den Orchideen. – Bibl. Bot. 151: 1-82.
Prutsch J, Schill R. 2000b. The stigma of Cephalanthera (Orchidaceae) provides a link between primitive and derived Epidendroideae. – Nord. J. Bot. 20: 599-604.
Pupulin F. 2001. Contributions toward a reassessment of Costa Rican Zygopetalinae (Orchidaceae). The genus Kefersteinia Rchb. f. – Ann. Naturhist. Mus. Wien 103B: 525-555.
Pupulin F, Bogarín D. 2004. Two new species of Lepanthes (Orchidaceae: Pleurothallidinae) from Costa Rica. – Kew Bull. 59: 559-563.
Pupulin F, Bogarín D. 2012. A taxonomic revision of Encyclia (Orchidaceae: Laeliinae) in Costa Rica. – Bot. J. Linn. Soc. 168: 395-448.
Pupulin F, Dressler RL. 2006. A new species of Ornithocephalus (Orchidaceae) from Panama. – Brittonia 58: 314-317.
Pupulin F, Merino G. 2008. Two new species of Kefersteinia (Orchidaceae: Zygopetalinae). – Willdenowia 38: 187-193.
Pupulin F, Merino G. 2010. Comparettia sotoana (Orchidaceae: Oncidiinae), a new Ecuadorian species. – Lankesteriana 9: 399-402.
Pyke GH, Day LP, Wale KA. 1988. Pollination ecology of Christmas bells (Blandfordia nobilis Sm.): effects of adding artificial nectar on pollen removal and seed set. – Aust. J. Ecol. 13: 279-284.
Raamsdonck LWD van. 1986. Biosystematic studies on the umbellatum-angustifolium complex of the genus Ornithogalum (Liliaceae) II. Genome characterization and evolution. – Nord. J. Bot. 6: 525-544.
Raamsdonck LWJ van, Skiech MP, Sandbrink JM. 1997. Introgression explains incongruence between nuclear and chloroplast DNA-based phylogenies in Allium section Cepa. – Bot. J. Linn. Soc. 123: 91-108.
Radulescu D. 1973. Recherches morpho-palynologiques sur la famille Liliaceae. – Acta Horti Bot. Bucur. 1972-1973: 133-248.
Rahn K. 1998a. Alliaceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 70-78.
Rahn K. 1998b. Themidaceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 436-441.
Raina SN. 1978. Genetic mechanisms underlying evolution in Crinum. – Cytologia 43: 575-580.
Raina SN, Khoshoo TN. 1971. Cytogenetics of tropical bulbous ornamentals II. Variation in mitotic complement in Crinum. – The Nucleus 14: 23-39.
Raina SN, Khoshoo TN. 1972. Cytogenetics of tropical bulbous ornamentals IX. Breeding systems in Zephyranthes. – Euphytica 21: 317-323.
Raju MUS. 1957. Some aspects of the embryology of Dianella nemorosa. – J. Indian Bot. Soc. 36: 223-226.
Rakotoarivelo FP, Razafimandimbison SG, Mallet B, Faliniaina L, Pailler T. 2012. Molecular systematics and evolutionary trends and relationships in the genus Jumellea (Orchidaceae): implications for its species limits. – Taxon 61: 534-544.
Rakotoarivelo FP, Pailler T, Faliniaina L. 2013. Revision of the genus Jumellea Schltr. (Orchidaceae) from the Comoros Archipelago. – Adansonia 35: 33-46.
Ramdhani S, Barker NP, Baijnath H. 2009. Rampant non-monophyly of species in Kniphofia Moench (Asphodelaceae) suggests a recent Afromontane radiation. – Taxon 58: 1141-1152.
Ramdhani S, Barker NP, Cowling RM. 2011. Revisiting monophyly in Haworthia Duval (Asphodelaceae): incongruence, hybridization and contemporary speciation. – Taxon 60: 1001-1014.
Ramírez SR, Gravendeel B, Singer RB, Marshall CR, Pierce NE. 2007. Dating the origin of the Orchidaceae from a fossil orchid with its pollinator. – Nature 448: 1042-1045.
Ramsey M, Prakash N, Cairns S. 1993. Breeding systems of disjunct populations of Christmas bells (Blandfordia grandiflora R. Br., Liliaceae): variation in self-fertility and an ovular mechanism regulating self-fertilisation. – Aust. J. Bot. 41: 35-47.
Ramstad E. 1953. Über das Vorkommen und die Verbreitung von Chelidonsäure in einigen Pflanzenfamilien. – Pharm. Acta Helv. 28: 45-57.
Randolph LF. 1934. Chromosome numbers in native American and introduced species and cultivated varieties of Iris. – Bull. Amer. Iris Soc. 52: 61-66.
Randolph LF, Mitra J. 1956. Chromosome numbers of Iris species. – Bull. Amer. Iris Soc. 140: 1-12.
Randolph LF, Mitra J. 1960. Chromosomes of aril and aril-bred irises. – Bull. Amer. Iris Soc. 137: 41-60.
Randolph LF, Mitra J. 1961. Karyotypes of Iris species indigenous to the USSR. – Amer. J. Bot. 48: 862-870.
Rangaswami K, Ramarethinam S. 1965. Floral anatomy of Zephyranthes carinata Herb. with special reference to gynoecium. – Bot. Not. 118: 166-170.
Ranker TA, Schnabel AF. 1986. Allozymic and morphological evidence for a progenitor-derivative species pair in Camassia (Liliaceae). – Syst. Bot. 11: 433-445.
Rao AN. 1967. Flower and seed development in Arundina graminifolia. – Phytomorphology 17: 291-300.
Rao AN. 1986. Orchids of Arunachal Pradesh – a conspectus. – In: Vij SP (ed), Biology, conservation and culture of orchids, East-West Press Private Ltd., New Delhi, pp. 323-349.
Rao AN. 1988. Two new species of Cheirostylis (Orchidaceae) from Arunachal Pradesh, India. – Nord. J. Bot. 8: 339-340.
Rao PRM, Sood SK. 1979. Embryology of Habenaria densa (Orchidaceae). – Bot. Not. 132: 145-148.
Rao RP, Kaur A. 1979a. Embryology and systematic position of Ophiopogon intermedius. – Proc. Indian Natl. Sci. Acad., Sect. B, 45: 175-187.
Rao RP, Kaur A. 1979b. Sporogenesis and gametophytes of Polygonatum cirrhifolium. – Phytomorphology 29: 93-97.
Rao VS. 1969. The floral anatomy and relationships of the rare Apostasia. – J. Indian Bot. Soc. 68: 374-385.
Rao VS. 1974. The relationships of the Apostasiaceae on the basis of floral anatomy. – Bot. J. Linn. Soc. 68: 319-327.
Raskoti BB, Jin W-T, Xiang X-G, Schuiteman A, Li D-Z, Li J-W, Huang W-C, Jin X-H, Huang L-Q. 2016. A phylogenetic analysis of molecular and morphological characters of Herminium (Orchidaceae, Orchideae): evolutionary relationships, taxonomy, and patterns of character evolution. – Cladistics 32: 198-210.
Raskoti BB, Schuiteman A, Jin W-T, Jin X-H. 2017. A taxonomic revision of Herminium L. (Orchidoideae, Orchidaceae). – PhytoKeys 79: 1-74.
Rasmussen FN. 1977. The genus Corymborkis Thou. (Orchidaceae). – Bot. Tidsskr. 71: 161-192.
Rasmussen FN. 1982. The gynostemium of the neottioid orchids. – Opera Bot. 65: 1-96.
Rasmussen FN. 1985. The gynostemium of Bulbophyllum ecornutum (J. J. Smith) J. J. Smith (Orchdiaceae). – Bot. J. Linn. Soc. 91: 447-456.
Rasmussen FN. 1986a. On the various contrivances by which pollinia are attached to viscidia. – Lindleyana 1: 21-32.
Rasmussen FN. 1986b. Ontogeny and phylogeny in the Orchidaceae. – Lindleyana 2: 114-124.
Rasmussen FN. 1995a. Relationships of Burmanniales and Orchidales. – In: Rudall PJ, Cribb PJ, Cutler DF, Humphries CJ (eds), Monocotyledons: systematics and evolution, Royal Botanic Gardens, Kew, pp. 227-241.
Rasmussen FN. 1995b. Terrestrial orchids: from seed to mycotrophic plant. – Cambridge University Press, Cambridge.
Rasmussen FN. 2000. Ins and outs of orchid phylogeny. – In: Wilson KL, Morrison DA (eds), Monocots: systematics and evolution, CSIRO Publ., Melbourne, pp. 430-435.
Rasmussen FN, Rasmussen H. 1979. Notes on the morphology and taxonomy of Diceratostele gabonensis (Orchidaceae). – Bull. Jard. Bot. Natl. Belg. 49: 139-148.
Rasmussen FN, Frederiksen S, Johansen B, Jørgensen LB, Petersen G, Seberg O. 2006. Fleshy fruits in liliiflorous monocots. – In: Columbus JT, Friar EA, Porter JM, Prince LM, Simpson MG (eds), Monocots: comparative biology and evolution. Excluding Poales, Rancho Santa Ana Botanical Garden, Claremont, California. – Aliso 22: 135-147.
Rasmussen H. 1981. The diversity of stomatal development within Orchidaceae subfamily Orchidoideae. – Bot. J. Linn. Soc. 82: 381-393.
Rasmussen H. 1982. Branching pattern and inflorescence bud displacement in Flickingeria (Orchidaceae). – Nord. J. Bot. 2: 235-248.
Rasmussen H. 1983. Stomatal development in families of Liliales. – Bot. Jahrb. Syst. 104: 261-287.
Rasmussen H. 1986a. Epidermal cell differentiation during leaf development in Anemarrhena asphodeloides. – Can. J. Bot. 64: 1277-1285.
Rasmussen H. 1986b. Pattern formation and cell interactions in epidermal development of Anemarrhena asphodeloides (Liliaceae). – Nord. J. Bot. 6: 467-477.
Rasmussen H. 1987. Orchid stomata – structure, differentiation, function and phylogeny. – In: Arditti J (ed), Orchid biology, reviews and perspectives IV, Comstock, Cornell University Press, Ithaca, New York, pp. 105-138.
Ratsirarson J. 1995. Pollination ecology of Aloe divaricata Berger (Liliaceae): an endemic plant species of Southwest Madagascar. – South. Afr. J. Bot. 61: 249-252.
Rauh W. 1974. Window-leaved succulents. – Cact. Succ. J. (Los Angeles) 46: 12-25.
Rauschert E. 1983. Beitrag zur Nomenclatur der Orchidaceae. – Feddes Repert. 94: 433-471.
Ravenna P. 1964. Notas sobre Iridaceae I. – Rev. Inst. Municipal Bot., Jard. Bot. ‘Carlos Thays’, 2: 51-60.
Ravenna P. 1965. Notas sobre Iridaceae II. – Bol. Soc. Arg. Bot. 10: 311-322.
Ravenna P. 1967. Estudios sobre Liliáceas. El género Solaria, su presencia en la flora argentina. – Bol. Soc. Argent. Bot. 11: 157-164.
Ravenna P. 1968. Notas sobre Iridaceae III. – Bonplandia 2: 273-291.
Ravenna P. 1969. Contribution to South American Amaryllidacae II. – Plant Life 25: 55-76.
Ravenna P. 1970. Rhodophiala cipoana Rav., sp. nov. – Plant Life 26: 86-87.
Ravenna P. 1971. Contribution to South American Amaryllidaceae IV. – Plant Life 27: 61-89.
Ravenna P. 1974a. Contribution to South American Amaryllidaceae VI. – Plant Life 30: 29-80.
Ravenna P. 1974b. Studies in the genus Griffinia. – Plant Life 30: 64-70.
Ravenna P. 1974c. Cobana, a new genus of Central American Iridaceae. – Bot. Not. 127: 104-108.
Ravenna P. 1976. Neotropical species threatened and endangered by human activity in the Iridaceae, Amaryllidaceae and allied bulbous families. – In: Prance GT, Elias TS (eds), Extinction is forever, New York Botanical Garden, Bronx, New York, pp. 257-265.
Ravenna P. 1977a. Notas sobre Iridaceae V. – Notic. Mens. Mus. Nac. Hist. Nat. Santiago 249: 7-9.
Ravenna P. 1977b. Correct name and new synonyms for some Amaryllidaceae of the northern hemisphere. – Plant Life 33: 36-38.
Ravenna P. 1978a. Contribution to South American Amaryllidaceae VII. – Plant Life 34: 69-91.
Ravenna P. 1978b. Studies in Allieae II. – Plant Life 34: 130-151.
Ravenna P. 1979a. New species of Iridaceae from Galápagos and Colombia. – Bot. Not. 132: 463-466.
Ravenna P. 1979b. Ainea, a new genus of Iridaceae from Mexico. – Bot. Not. 132: 467-469.
Ravenna P. 1980. A new yellow-flowered Hymenocallis (Amaryllidaceae) from North Peru. – Bot. Not. 133: 97-98.
Ravenna P. 1981a. The tribe Trimezieae of the Iridaceae. – Wrightia 7: 12.
Ravenna P. 1981b. A submerged new species of Cypella (Iridaceae), and a new section for the genus (s. str.). – Nord. J. Bot. 1: 489-492.
Ravenna P. 1981c. Sympa, a new genus of Iridaceae from Rio Grande do Sul, Brazil. – Wrightia 7: 10-11.
Ravenna P. 1981d. Kelissa, a new genus of Iridaceae from South Brazil. – Bull. Mus. Natl. Hist. Nat., sér. B, Adansonia 3: 105-110.
Ravenna P. 1981e. Eight new species and two new subspecies of Cypella (Iridaceae). – Wrightia 7: 13-22.
Ravenna P. 1981f. Contribution to South American Amaryllidaceae VIII. – Plant Life 37: 57-83.
Ravenna P. 1982. Zephyranthes briquetii transferred to Mastigostyla (Iridaceae). – Plant Life 38: 116-117.
Ravenna P. 1983. Catila and Onira, two new genera of South American Iridaceae. – Nord. J. Bot. 3: 197-205.
Ravenna P. 1984. The delimitation of Gelasine (Iridaceae), and G. uruguaiensis sp. nov. from Uruguay. – Nord. J. Bot. 4: 347-350.
Ravenna P. 1985. New or critical Liliaceae. – Phytologia 57: 327-328.
Ravenna P. 1986. Itysa and Lethia, two new genera of neotropical Iridaceae. – Nord. J. Bot. 6: 581-588.
Ravenna P. 1987. Diamena and Diora, two new genera of Anthericaceae from Peru. – Opera Bot. 92: 185-193.
Ravenna P. 1988a. Six new species of Anthericum (Anthericaceae) from Bolivia and Peru. – Onira Bot. Leafl. 1: 24-30.
Ravenna P. 1988b. Studies in the genus Stenomesson (Amaryllidaceae). – Onira, Bot. Leafl. 1: 17-21.
Ravenna P. 1999. New species of Zephyranthes and Habranthus (Amaryllidaceae) I. – Onira, Bot. Leafl. 3: 52-61.
Ravenna P. 2000a. Nothoscordum subgen. Latace and the illegitimacy of Zoellnerallium (Alliaceae). – Onira, Bot. Leafl. 4: 15-18.
Ravenna P. 2000b. Tocantinia and Cearanthes, two new genera, and Tocantinieae new tribe, of Brazilian Amaryllidaceae. – Onira, Bot. Leafl. 5: 9-12.
Ravenna P. 2000c. The family Gilliesiaceae. – Onira, Bot. Leafl. 4: 11-14.
Ravenna P. 2000d. Five new species in the genus Griffinia (Amaryllidaceae). – Onira, Bot. Leafl. 4: 19-22.
Ravenna P. 2000e. New or noteworthy Leucocoryne species (Alliaceae). – Onira, Bot. Leafl. 4: 3-10.
Ravenna P. 2000f. New or noteworthy Leucocoryne species (Alliaceae) 2. – Onira, Bot. Leafl. 5: 24-30.
Ravenna P. 2001a. The Iridaceae of the Cuyo region, Argentina. – Onira, Bot. Leafl. 6: 1-18.
Ravenna P. 2001b New or noteworthy Leucocoryne species (Alliaceae) 3. – Onira, Bot. Leafl. 5: 42-43.
Ravenna P. 2001c. New species of Zephyranthes and Habranthus (Amaryllidaceae) III. – Onira, Bot. Leafl. 6: 38-43.
Ravenna P. 2001d. New or critical
Tristagma species (Alliaceae).
– Onira, Bot. Leafl. 6: 24-33.
Ravenna P. 2002a. New Rauhia species from northern Peru (Amaryllidaceae). – Onira, Bot. Leafl. 7(4): 11.
Ravenna P. 2002b. New or noteworthy Leucocoryne species (Alliaceae) 4. – Onira, Bot. Leafl. 7: 1-3.
Ravenna P. 2003a. Eidema, a new genus of neotropical Amaryllidaceae. – Onira, Bot. Leafl. 8: 1-4.
Ravenna P. 2003b. Heliacme, a new genus of New World Hypoxidaceae. – Onira, Bot. Leafl. 8: 5-9.
Ravenna P. 2003c. A new neotropical species of Zephyranthes (Amaryllidaceae), the infrageneric divisions of the genus, and other notes. – Onira, Bot. Leafl. 8: 41-45.
Ravenna P. 2003d. Elucidation and systematics of the Chilean genera of Amaryllidaceae. – Bot. Australis 2: 1-21.
Ravenna P. 2004. New or noteworthy Leucocoryne species (Alliaceae) 5. – Onira, Bot. Leafl. 9: 35-42.
Ravenna P. 2009. A survey in the genus Cypella and its allies (Iridaceae). – Onira, Bot. Leafl. 12: 1-11.
Reeves G, Goldblatt P, Rudall P, Chase MW. 2001. Molecular systematics of Iridaceae: a combined analysis of four plastid DNA sequence matrices. – Ann. Bot. (Roma), n. s., 1: 29-42.
Reeves G, Chase MW, Goldblatt P, Rudall PJ, Fay MF, Cox AV, Lejeune B, Souza-Chies T. 2001. Molecular systematics of Iridaceae: evidence from four plastid DNA regions. – Amer. J. Bot. 88: 2074-2087.
Regel E. 1875. Alliorum adhuc cognitorum monographia. – Acta Horti Petropol., III, 2: 1-266.
Regel E. 1880. Ixiolirion tataricum (Amaryllis) Pall. γ Ledebouri. – Gartenflora 1880: 193-195, T. 1014.
Reiche K. 1893. Beiträge zur Kenntnis der Liliaceae-Gilliesieae. – Engl. Bot. Jahrb. Syst. 16: 262-277.
Reichenbach HG. 1877. Morphologische Mitteilungen in Bezug auf die Orchideenblüte. – Bot. Zeitung 35: 38-43.
Reichenbach HG. 1884. Über das System der Orchideen. – Bull. Congres Intern. Bot. Horticult. St.-Petersbourg 1884: 39-58.
Reid C, Dyer RA. 1984. A review of the Southern African species of Cyrtanthus. – American Plant Life Society, La Jolla, California.
Reinhammar L-G. 1995. Evidence for two distinctive species of Pseudorchis (Orchidaceae) in Scandinavia. – Nord. J. Bot. 15: 469-481.
Reinke J. 1898. Die Assimilationsorgane der Asparageen. – Jahrb. Wiss. Bot. 31: 207-272.
Rendle AB. 1901. The bulbiform seeds of certain Amaryllidaceae. – J. Roy. Hortic. Soc. 26: 89-96.
Renny AT. 1972. Nervilia. A dwarf species from the Northern Transvaal. – South Afr. Orch. J., Jun.-Jul.
Renz J. 1948. Beiträge zur Kenntnis der süd- und zentralamerikanischen Orchideen I. Orchideae-Cranichidinae. – Candollea 11: 243-276.
Repetur CP, Welzen PC van, De Vogel EF. 1997. Phylogeny and historical biogeography of the genus Bromheadia (Orchidaceae). – Syst. Bot. 22: 465-477.
Reveal JL. 1998. Proposals to conserve the names Tricyrtidaceae and Walleriaceae (Magnoliophyta). – Taxon 47: 173-174.
Reynolds GW. 1947. Genus Leptoaloe Stapf, restoration to Aloe Linn.- J. South Afr. Bot. 13: 99-150.
Reynolds GW. 1950. The aloes of South Africa. – Aloes of South Africa Book Fund, Johannesburg, South Africa.
Reynolds GW. 1965. Two new aloes from Madagascar. – J. South Afr. Bot. 31: 279-284.
Reynolds GW. 1966. The aloes of tropical Africa and Madagascar. – The Trustees, The Aloe Book Fund, Mbabane, Swaziland.
Reynolds GW. 1969. The aloes of South Africa. – A. A. Balkema, Cape Town.
Reynolds GW, Bally PRO. 1958. Notes on the aloes of Somaliland Protectorate. – J. South Afr. Bot. 24: 169-190.
Reynolds T. 1985a. The compounds in Aloë leaf exudates: a review. – Bot. J. Linn. Soc. 90: 157-177.
Reynolds T. 1985b. Observations on the phytochemistry of the Aloë leaf exudate compounds. – Bot. J. Linn. Soc. 90: 179-199.
Reynolds T. 1994. A chromatographic examination of some old samples of drug aloes. – Pharmazie 49: 524-529.
Reynolds T (ed). 2004. Aloes, the genus Aloe. – CRC Press, Boca Raton, Florida.
Rheede van Oudtshoorn MCB. 1964. Chemotaxonomic investigations in Asphodeleae and Aloineae (Liliaceae). – Phytochemistry 3: 383-390.
Ribeiro PL, Borba EL, Camargo Smidt E, Lambert SM, Schnadelbach AS, Berg C. 2008. Genetic and morphological variation in the Bulbophyllum exaltatum (Orchidaceae) complex occurring in the Brazilian ‘campos rupestres’: implications for taxonomy and biogeography. – Plant Syst. Evol. 270: 109-137.
Richter KS, Weis AE. 1995. Differential abortion in the yucca. – Nature 376: 557-558.
Ridley HN. 1885. The orchids of Madagascar. – Bot. J. Linn. Soc. 21: 456-522.
Ridley HN. 1886. A monograph of the genus Liparis. – Bot. J. Linn. Soc. 22: 244-297.
Ridley HN. 1896a. An enumeration of all Orchideae hitherto recorded from Borneo. – Bot. J. Linn. Soc. 31: 261-306.
Ridley HN. 1896b. The Orchideae and Apostasiaceae of the Malay Peninsula. – Bot. J. Linn. Soc. 32: 213-416.
Ridley HN. 1921. Orchidaceae. – J. Nat. Hist. Soc. Siam 4, 3: 114-121.
Riley HP. 1959a. Polyploidy in Gasteria and Haworthia. – Bull. Torrey Bot. Club 86: 81-87.
Riley HP. 1959b. Polyploidy in South African species of Aloë. – Amer. J. Bot. 46: 126-129.
Riley HP. 1959c. Chromosome studies in Gasteria species and hybrids. – Cytologia 24: 438-446.
Riley HP, Majumdar SK. 1979. The Aloineae: a biosystematic survey. – University Press of Kentucky, Lexington, Kentucky.
Ríos-Ruiz S, Rivera-Nuñez DR, Alcaraz-Ariza F, Obon-de Castro C. 1999. Three new species of Narcissus L. subgenus Ajax Spach (Amaryllidaceae), restricted to the meadows and forests of south-eastern Spain. – Bot. J. Linn. Soc. 131: 153-165.
Rita J. 1989-1990. El género Romulea Maratti (Iridáceas) en las Islas Baleares. – Bol. Soc. Hist. Nat. Baleares 33: 263-268.
Robatsch K. 1995. Beiträge zur Kenntnis der europäischen Epipactis-Arten (Orchidaceae) und zur Evolution der Autogamie bei europäischen und asiatischen Gattungen der Neottioideae. – J. Eur. Orch. 27: 125-177.
Robbins S. 1992. Additional records of Orchidaceae for the flora of Arabia. – Kew Bull. 47: 721-724.
Robbins WW, Borthwick HA. 1925. Development of the seeds of Asparagus officinalis. – Bot. Gaz. 80: 426-438.
Robert P. 1964. Contribution à l’étude ontogénique de Ruscus aculeatus L. Développement du rhizome et formation des cladodes. – Ann. Sci. Nat. Bot. Biol. Veg. 5: 739-752.
Roberts Reinecke P. 1965. The genus Astroloba Uitewaal (Liliaceae). – M.Sc. thesis, University of Cape Town, South Africa.
Robinson H, Burns-Balogh P. 1982. Evidence for a primitively epiphytic habit in Orchidaceae. – Syst. Bot. 7: 353-358.
Rocha M, Good-Ávila SV, Molina-Freaner F, Arita HT, Castillo A, García-Mendoza A, Silva-Montellano A, Gaut BS, Souza V, Equiarte LE. 2006. Pollination biology and adaptive radiation in Agavaceae, with special emphasis on the genus Agave. – In: Columbus JT, Friar EA, Porter JM, Prince LM, Simpson MG (eds), Monocots: comparative biology and evolution. Excluding Poales, Rancho Santa Ana Botanical Garden, Claremont, California. – Aliso 22: 329-344.
Roche SA, Carter RJ, Peakall R, Smith LM, Whitehead MR, Lind CC. 2010. A narrow group of Tulasnella (Tulasnellaceae) symbiont lineages are associated with multiple species of Chiloglottis (Orchidaceae): implications for orchid diversity. – Amer. J. Bot. 97: 1313-1327.
Rodolphe G, Séverine B, Michel G, Pascale B. 2011. Biodiversity and evolution in the Vanilla genus. – In: Grillo O (ed.), The dynamical processes of biodiversity – case studies of evolution and spatial distribution, publ. by InTech, Rijeka & Shanghai, pp. 1-26.
Rodionenko GJ. 1961. Rod iris – Iris L. – Isdatelstwo ANSSSR, Moskow. [In Russian]
Rodolphe G, Séverine B, Michel G, Pascale B.
2011. Biodiversity and evolution in the Vanilla genus. – In: Grillo
O (ed.), The dynamical processes of biodiversity – case studies of evolution
and spatial distribution, publ. by InTech, Rijeka & Shanghai, pp. 1-26.
Rodrigues Souza LG, Crosa O, Guerra M. 2010.
Karyological circumscription of Ipheion Raf. (Gilliesioideae, Alliaceae). – Plant Syst. Evol. 28:
119-127.
Rodriguez A. 1999. Molecular and morphological systematics of the “tiger flower” group (tribe Tigridieae: Iridaceae), biogeography and evidence for the adaptive radiation of the subtribe Tigridiinae. – Ph.D. diss., University of Wisconsin, Madison, Wisconsin.
Rodriguez A, Garcia-Mendoxa A. 2004. Tigridia amatlanensis (Tigredieae: Iridaceae), a new species from Oaxaca, Mexico. – Brittonia 56: 128-131.
Rodríguez A, Ortiz-Catedral L. 2001. La tribu Tigridieae (Iridaceae) en México. – Scientia-CUCBA 32: 123-136.
Rodríguez A, Ortiz-Catedral L. 2003. Colima (Tigridieae: Iridaceae), new genus from western Mexico and a new species: Colima tuitensis from Jalisco. – Acta Bot. Mexicana 65: 51-60.
Rodríguez A, Sytsma K. 2006. Phylogeny of the ‘tiger-flower’ group (Tigridieae: Iridaceae): molecular and morphological evidence. – In: Columbus JT, Friar EA, Porter JM, Prince LM, Simpson MG (eds), Monocots: comparative biology and evolution. Excluding Poales, Rancho Santa Ana Botanical Garden, Claremont, California. – Aliso 22: 412-424.
Rodríguez A, Vargas O, Villegas E, Sytsma KJ. 1996. Nuevos informes de iridáceas (Tigridieae) en Jalisco. – Bol. Inst. Bot. 4: 39-47.
Rogers GK. 2000. A taxonomic revision of the genus Agave (Agavaceae) in the Lesser Antilles, with an ethnobotanical hypothesis. – Brittonia 52: 218-233.
Rohrbach P. 1866. Über den Blütenbau und die Befruchtung von Epipogium gmelini. – Göttinger Preisschrift.
Roitman GG, Castillo A. 2004. A new species Herbertia crosae (Iridaceae), from Uruguay. – Brittonia 56: 361-364.
Roitman GG, Castillo A. 2005. Calydorea alba (Iridaceae-Tigridieae), a new species from Uruguay. – Bol. Soc. Argentina Bot. 40: 311-312.
Rojas Leal A. 1993. Anatomia foliar camparada de Lemboglossum (Orchidaceae: Oncidiinae) y géneros relacionados. – Ph.D. diss., Université nationale autonome de Mexico.
Rojas-Piña V, Olson ME, Alvarado-Cárdenas
LO, Eguiarte LE. 2014. Molecular phylogenetics and morphology of
Beaucarnea (Ruscaceae) as
distinct from Nolina, and the submersion of Calibanus into
Beaucarnea. – Taxon 63: 1193-1211.
Rolfe RA. 1896. The Cypripedium group. – Orchid Rev. 4: 327-334, 363-367.
Rolfe RA. 1901. New orchids. Decade 25. – Kew Bull. 1901: 146-150.
Rolfe RA. 1909, 1910. The evolution of the Orchidaceae. – Orchid Rev. 17: 129-132, 193-196, 289-292, 353-356 (1909); 18: 33-36, 87-99, 129-132, 162-166, 289-294, 321-325 (1910).
Romero-González GA. 1990. Phylogenetic relationships in subtribe Catasetinae (Orchidaceae). – Lindleyana 5: 160-181.
Romero-González GA, Dodson CH. 2010. Ala tercera se gana: the validation of Benzingia (Orchidaceae: Zygopetalinae). – Lankesteriana 9: 526-528.
Romowicz A, Szlachetko DL. 2006. Genera et
species orchidalium. 12. Oncidieae. – Polish Bot. J. 51: 43-47.
Rønsted N, Savolainen V, Mølgaard P, Jäger AK. 2008. Phylogenetic selection of Narcissus species for drug discovery. – Biochem. Syst. Ecol. 36: 417-422.
Rose JN. 1899. A proposed rearrangement of the suborder Agaveae. – Contr. U. S. Natl. Herb. 5: 151-157.
Rose JN. 1903. Amaryllidaceae in studies of Mexican and central American plants. – Contr. U. S. Natl. Herb. 8: 8-23.
Rose JN. 1906. Liliaceae in studies of Mexican and central American plants. – Contr. U. S. Natl. Herb. 10: 79-93.
Rose SL. 1959. Vesicular-arbuscular endomycorrhizal associations of some desert plants of Baja-California, Mexico. – Can. J. Bot. 59: 1056-1061.
Rosso SW. 1966. The vegetative anatomy of the Cypripedioideae (Orchidaceae). – Bot. J. Linn. Soc. 59: 309-341.
Rowley GD. 1967a. Zygomorphy in Bulbine. – Natl. Cact. Succ. J. 22: 10.
Rowley GD. 1967b. A numerical survey of the genera of Aloineae. – Natl. Cact. Succ. J. 22: 71-75.
Rowley GD. 1976a. Generic concepts in the Aloineae 1. The genus – to split or to lump? – Natl. Cact. Succ. J. 31: 26-31.
Rowley GD. 1976b. Generic concepts in the Aloineae 2. Generic names in the Aloineae. – Natl. Cact. Succ. J. 31: 54-56.
Rowley GD. 1996. The berried aloes: Aloe section Lomatophyllum. – Excelsa 17: 59-62.
Rübsamen T. 1986. Morphologische, embryologische und systematische Untersuchungen an Burmanniaceae und Corsiaceae (mit Ausblick auf die Orchidaceae-Apostasioideae). – Diss. Bot. 92: 1-310, J. Cramer, Berlin, Stuttgart.
Rübsamen-Weustenfeld T, Mukielka V, Hamann U. 1994. Zur Embryologie, Morphologie und systematischen Stellung von Geosiris aphylla Baillon (Monocotyledoneae-Geosiridaceae/Iridaceae) mit einigen embryologischen Daten zur Samenanlage von Isophysis tasmanica (Hook.) T. Moore (Iridaceae). – Bot. Jahrb. Syst. 115: 475-545.
Rudall PJ. 1983. Leaf anatomy and relationships of Dietes (Iridaceae). – Nord. J. Bot. 3: 471-478.
Rudall PJ. 1984. Taxonomic and evolutionary implications of rhizome structure and secondary thickening in Iridaceae. – Bot. Gaz. 145: 524-534.
Rudall PJ. 1986. Taxonomic significance of leaf anatomy in Australasian Iridaceae. – Nord. J. Bot. 6: 277-289.
Rudall PJ. 1989. Stem thickening growth in bulbous Iridaceae. – Bot. Gaz. (Crawfordsville) 150: 132-138.
Rudall PJ. 1991. Leaf anatomy of Tigridieae (Iridaceae). – Plant Syst. Evol. 175: 1-10.
Rudall PJ. 1993. Leaf anatomy and systematics of Mariceae (Iridaceae). – Kew. Bull. 48: 151-160.
Rudall PJ. 1994a. Leaf anatomy and systematics of the Iridaceae. – Bot. J. Linn. Soc. 114: 1-21.
Rudall PJ. 1994b. The ovule and embryo sac in Xanthorroeaceae sensu lato. – Flora 189: 335-351.
Rudall PJ. 1995. Iridaceae. – In: Cutler DF, Gregory M (eds), Anatomy of the monocotyledons VIII, Clarendon Press, Oxford.
Rudall PJ. 1998. Lanariaceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 340-342.
Rudall PJ. 1999. Flower anatomy and systematics of Comospermum (Asparagales). – Syst. Geogr. Plants 68: 195-202.
Rudall PJ. 2003. Unique floral structures and iterative evolutionary themes in Asparagales: insights from a morphological cladistic analysis. – Bot. Rev. 68: 488-509.
Rudall PJ, Bateman RM. 2002. Roles of synorganisation, zygomorphy and heterotopy in floral evolution: the gynostemium and labellum of orchids and other lilioid monocots. – Biol. Rev. 77: 403-441.
Rudall PJ, Burns P. 1989. Leaf anatomy of the woody South African Iridaceae. – Kew Bull. 44: 525-532.
Rudall PJ, Campbell G. 1999. Flower and pollen structure of Ruscaceae in relation to Aspidistreae and other Convallariaceae. – Flora 194: 201-214.
Rudall PJ, Chase MW. 1996. Systematics of the Xanthorrhoeaceae sensu lato: evidence for polyphyly. – Telopea 6: 629-647.
Rudall PJ, Cutler DF. 1995. Asparagales: a reappraisal. – In: Rudall PJ, Cribb PJ, Cutler DF, Humphries CJ (eds), Monocotyledons: systematics and evolution, Royal Botanic Gardens, Kew, pp. 157-168.
Rudall PJ, Goldblatt P. 1991. Leaf anatomy and phylogeny of Ixioideae (Iridaceae). – Bot. J. Linn. Soc. 106: 329-345.
Rudall PJ, Goldblatt P. 1992. Relationships of the southern African genus Bobartia (Iridaceae). – South Afr. J. Bot. 58: 304-309.
Rudall PJ, Goldblatt P. 1993. Leaf anatomy and systematics of Homeriinae (Iridaceae). – Bot. J. Linn. Soc. 111: 379-397.
Rudall PJ, Mathew B. 1990. Leaf anatomy in Crocus (Iridaceae). – Kew Bull. 45: 535-544.
Rudall PJ, Wheeler A. 1988. Pollen morphology in Tigridieae (Iridaceae). – Kew Bull. 43: 693-701.
Rudall PJ, Owens SJ, Kenton AY. 1984. Embryology and breeding systems in Crocus (Iridaceae) – a study in causes of chromosome variation. – Plant Syst. Evol. 148: 119-134.
Rudall PJ, Kenton AY, Lawrence J. 1986. An anatomical and chromosomal investigation of Sisyrinchium and allied genera. – Bot. Gaz. 147: 466-477.
Rudall PJ, Chase MW, Conran JG. 1996. New circumscriptions and a new family of asparagoid lilies: genera formerly included in Anthericaceae. – Kew Bull. 51: 667-680.
Rudall PJ, Furness CA, Chase MW, Fay MF. 1997. Microsporogenesis and pollen sulcus type in Asparagales (Lilianae). – Can. J. Bot. 75: 408-430.
Rudall PJ, Chase MW, Cutler DF, Rusby J, Bruijn AY de. 1998. Anatomical and molecular systematics of Asteliaceae and Hypoxidaceae. – Bot. J. Linn. Soc. 127: 1-42.
Rudall PJ, Engelman EM, Hanson L, Chase MW. 1998. Systematics of Hemiphylacus, Asparagus and Anemarrhena (Asparagales). – Plant Syst. Evol. 211: 181-199.
Rudall PJ, Conran JG, Chase MW. 2000. Systematics of Ruscaceae/Convallariaceae: a combined morphological and molecular investigation. – Bot. J. Linn. Soc. 134: 73-92.
Rudall PJ, Bateman RM, Fay MF, Eastman A. 2002. Floral anatomy and systematics of Alliaceae with particular reference to Gilliesia, a presumed insect mimic with strongly zygomorphic flowers. – Amer. J. Bot. 89: 1867-1883.
Rudall PJ, Manning JC, Goldblatt P. 2003. Evolution of floral nectaries in Iridaceae. – Ann. Missouri Bot. Gard. 90: 613-631.
Ruiz Rejón M. 1978. Estudios cariologicos en espècies españolas del orden Liliales III. Familia Liliaceae. – An. Inst. Bot. Antonio José Cavanilles 34: 733-759.
Ruiz Rejón M, Oliver JL. 1980. Cytogenetic and electrophoretic evidence for a polyploid origin of the basic chromosome number x=14 in the Asphodelus (Liliaceae). – Plant Syst. Evol. 136: 259-265.
Russell A, Samuel R, Rupp B, Barfuss MHJ, Šafran M, Besendorfer V, Chase MW. 2010. Phylogenetics and cytology of a pantropical orchid genus Polystachya (Polystachyinae, Vandeae, Orchidaceae): evidence from plastid DNA sequence data. – Taxon 59: 389-404.
Sachs T. 1974. The developmental origin of stomata pattern in Crinum. – Bot. Gaz. 135: 314-318.
Saha PS, Sengupta M, Jha S. 2017. Ribosomal DNA ITS1, 5.8S and ITS2 secondary structure, nuclear DNA content and phytochemical analyses reveal distinctive characteristics of four subclades of Protasparagus. – J. Syst. Evol. 55: 54-70.
Saito K. 1975. Studies on the occurrence of polyploidy and its contributions to the flower plant breeding XII. On the nature and sterility in the spontaneous triploid cultivars of Rhodohypoxis baurii Nil. – Jap. J. Breed. 25: 355-362.
Salazar GA. 2005. A new species of Ponthieva (Orchidaceae, Cranichidinae) from Veracruz, Mexico. – Brittonia 57: 252-254.
Salazar GA, Ballesteros-Barrera C. 2010. Sotoa, a new genus of Spiranthinae (Orchidaceae) from Mexico and the southern United States. – Lankesteriana 9: 491-504.
Salazar GA, De Santiago R. 2007. A new species of Malaxis (Orchidaceae) from Guerrero, Mexico. – Brittonia 59: 238-242.
Salazar GA, Dressler RL. 2011. The leaves got it right again: DNA phylogenetics supports a sister-group relationship between Eurystyles and Lankesterella (Orchidaceae, Spiranthinae). – Lankesteriana 11: 337-347.
Salazar GA, Jost L. 2012. Quechua, a new monotypic genus of Andean Spiranthinae (Orchidaceae). – Syst. Bot. 37: 78-86.
Salazar GA, Chase MW, Soto Arenas MA, Ingrouille M. 2003. Phylogenetics of Cranichideae with emphais on Spiranthinae (Orchidaceae, Orchidoideae): evidence from plastid and nuclear DNA sequences. – Amer. J. Bot. 90: 777-795.
Salazar GA, Cabrera LI, Madriñán S, Chase MW. 2009. Phylogenetic relationships of Cranichidinae and Prescottiinae (Orchidaceae, Cranichideae) inferred from plastid and nuclear DNA sequences. – Ann. Bot. 104: 403-416.
Salazar GA, Cabrera LI, Figueroa C. 2011. Molecular phylogenetics, floral convergence and systematics of Dichromanthus and Stenorrhynchos (Orchidaceae: Spiranthinae). – Bot. J. Linn. Soc. 167: 1-18.
Salazar GA, Berg C van den, Popovkin A. 2014. Phylogenetic relationships of Discyphus scopulariae (Orchidaceae, Cranichideae) inferred from plastid and nuclear DNA sequences: evidence supporting recognition of a new subtribe, Discyphinae. – Phytotaxa 173: 127-139.
Salazar GA, Hernández-López TJ, Sharma J, Jiménez-Machorro R, Cabrera LI, Treviño-Carreón J. 2016. Greenwoodiella, a new genus of Spiranthinae (Orchidaceae) from North and Central America and the Greater Antilles, with a new species from the Chihuahuan Desert. – Syst. Bot. 41: 823-838.
Salazar GA, Batista JAN, Cabrera LI, van den Berg C, Whitten WM, Smidt EC, Buzatto CR, Singer RB, Gerlach G, Jiménez-Machorro R, Radins JA, Insaurralde IS, Guimarães LRS, De Barros F, Tobar F, Linares JL, Mújica E, Dressler RL, Blanco MA, Hágsater E. 2018. Phylogenetic systematics of subtribe Spiranthinae (Orchidaceae: Orchidoideae: Cranichideae) based on nuclear and plastid DNA sequences of a nearly complete generic sample. – Bot. J. Linn. Soc. 186: 273-303.
Salmeri C. 1998. Allium brulloi (Alliaceae), a new species from Astypalea (Aegean Islands, Greece). – Willdenowia 28: 69-75.
Samson MAH, Karstens WKH. 1971. Bulbs and bulbils of Ornithogalum caudatum Ait. – Acta Bot. Neerl. 20: 600-610.
Samyolov A, Klaas M, Hanelt P. 1995. Use of chloroplast DNA polymorphisms for the phylogenetic study of the subgenera Amerallium and Bromatorrhiza (genus Allium). – Feddes Repert. 106: 161-167.
Sandoval LH. 1995. Análisis cladístico de la familia Agavaceae. – Bull. Soc. Bot. México 56: 57-68.
Sandoval-Zapotitla E. 1993. Anatomia foliar de Cuitlauzina pendula. – Orquidea (Mexico City) 13: 181-190.
Sandoval-Zapotitla E, Terrazas T. 2001. Leaf anatomy of 16 taxa of the Trichocentrum clade (Orchidaceae, Oncidiinae). – Lindleyana 16: 81-93.
Sanford WW, Adanlawo I. 1973. Velamen and exodermis characters of West African epiphytic orchids in relation to taxonomic grouping and habitat tolerance. – Bot. J. Linn. Soc. 66: 307-321.
Santos-Gally R, Vargas P, Arroyo J. 2012. Insights into Neogene Mediterranean biogeography based on phylogenetic relationships of mountain and lowland lineages of Narcissus (Amaryllidaceae). – J. Biogeogr. 39: 782-798.
Sapir Y, Shmida A, Fragman O, Comes HP. 2002. Morphological variation of the Oncocyclus irises (Iris: Iridaceae) in the southern Levant. – Bot. J. Linn. Soc. 139: 369-382.
Sargent C. 1976. The occurrence of a secondary cuticle in Libertia elegans (Iridaceae). – Ann. Bot., N. S., 40: 355-359.
Sarkar AK, Datta N, Chatterjee U. 1981 [1982]. Cytology of Peliosanthes Andr. (Liliaceae) as an aid to taxonomy. – Caryologia 34: 467-472.
Sassone AB, Giussani LM, Guaglianone ER.
2013. Multivariate studies of Ipheion (Amaryllidaceae, Allioideae) and
related genera. – Plant Syst. Evol. 299: 1561-1575.
Sassone AB, Giussani LM, Guaglianone ER.
2014. Beauverdia, a resurrected genus of Amaryllidaceae (Allioideae,
Gilliesieae). – Syst. Bot. 39: 767-776.
Sassone AB, Arroyo-Leuenberger SC, Giussani LM. 2014. Nueva circumscripción de la tribu Leucocoryneae (Amaryllidaceae, Allioideae). – Darwinia, n.s. 2: 197-206.
Sathekge NR, Kritzinger Q, Prinsloo G. 2008. Chemical analysis of five Hypoxis species. – South Afr. J. Bot. 74: 389.
Satô D. 1935a. Analysis of the karyotypes in Yucca, Agave, and related genera with special reference to the phylogenetic significance. – Jap. J. Genet. 11: 272-278.
Satô D. 1935b. Chromosome studies in Scilla I. Analysis of karyotypes in Scilla with special reference to the origin of aneuploids. – Bot. Mag. (Tokyo) 49: 298-305.
Satô D. 1938. Karyotype alteration and phylogeny IV. Karyotypes in Amaryllidaceae with special reference to SAT-chromosomes. – Cytologia 9: 203-242.
Satô D. 1942. Karyotype alteration and phylogeny of Liliaceae and allied families. – Jap. J. Bot. 12: 58-161.
Sato S, Nagase S, Ichinose K. 1994. New steroidal saponins from the rhizomes of Anemarrhena asphodeloides Bunge (Liliaceae). – Chem. Pharm. Bull. 42: 2342-2345.
Savile DBO. 1962. Taxonomic disposition of Allium. – Nature 196: 792.
Scepánková I, Hudák J. 2004. Leaf and tepal anatomy, plastid ultrastructure and chlorophyll content in Galanthus nivalis L. and Leucojum aestivum L. – Plant Syst. Evol. 243: 211-219.
Schaffer WM, Schaffer MV. 1977. The reproductive biology of the Agavaceae I. Pollen and nectar production in four Arizona agaves. – Southw. Natur. 22: 157-168.
Scharf W. 1892. Beiträge zur Anatomie der Hypoxideen und einiger verwandter Pflanzen. – Beih. Bot. Centralbl. 52: 145-153, 177-184, 209-217, 241-249, 289-296, 321-327.
Schelpe EACLE. 1958. Gasteria – a problem genus of South African succulent plants. – J. Bot. Soc. South Afr. 44: 17-20.
Schelpe EACLE. 1966a. Taxonomic notes on some South African orchids. – The Orchid Review 74: 394.
Schelpe EACLE. 1966b. An introduction to South African orchids. – Purnell, Cape Town.
Schelpe EACLE. 1976a. A provisional check-list of the Orchidaceae of Angola. – J. South Afr.. Bot. 42: 383-388.
Schelpe EACLE. 1976b. A provisional check-list of the Orchidaceae of Moçambique. – J. South Afr. Bot. 42: 389-393.
Schelpe EACLE. 1978. Aspects of the phytogeography of the South African Orchidaceae. – Bot. Jahrb. Syst. 99: 146-151.
Schiestl FP 2012. Animal pollination and speciation in plants: general mechanisms and examples from the orchids. – In: Patiny S (ed), Evolution of plant-pollinator relationships, Cambridge University Press, Cambridge, pp. 263-278.
Schiestl FP, Cozzolino S. 2008. Evolution of sexual mimicry in the orchid subtribe Orchidinae: the role of preadaptations in the attraction of male bees as pollinators. – BMC Evol. Biol. 8: 27.
Schill R. 1973. Studien zur systematischen Stellung der Gattung Lomatophyllum Willd. – Beitr. Biol. Pflanzen 49: 272-289.
Schill R. 1978. Palynologische Untersuchungen zur systematischen Stellung der Apostasiaceae. – Bot. Jahrb. Syst. 99: 353-362.
Schill R, Pfeiffer W. 1977. Untersuchungen an Orchideenpollinien unter besonderer Berücksichtigung ihrer Feinskulptur. – Pollen Spores 19: 5-118.
Schill R, Wolter M. 1986. On the presence of elastoviscin in all subfamilies of the Orchidaceae and the homology to pollenkitt. – Nord. J. Bot. 6: 321-324.
Schill R, Wyk AE van. 1992. Systematic leaf anatomy of selected genera of the Alooideae (Asphodelaceae). – South Afr. J. Bot. 58: 349-357, 534.
Schlag M, Hesse M. 1993. Morphogenesis of the sporoderm in Polystachia pubescens (Orchidaceae). – Grana 32: 22-28.
Schlechter R. 1893. Beiträge zur Kenntnis der Orchidaceen und Asclepiadaceen Süd-Afrikas. – Verh. Bot. Ver. Prov. Brandenbrg 35: 44-54.
Schlechter R. 1898. Monographie der Disperideae. – Bull. Herb. Boissier, sér I, 6: 800-821.
Schlechter R. 1902. Monographie der Diseae. – Engl. Bot. Jahrb. Syst. 31: 134-313.
Schlechter R. 1906a. Neue Orchidaceen der Flora des Monsun-Gebietes. – Bull. Herb. Boissier, sér. II, 6: 295-310, 453-472.
Schlechter R. 1906b. Orchidaceae africanae IV. – Engl. Bot. Jahrb. Syst. 38: 144-165.
Schlechter R. 1910. Revision der Orchidaceen von Deutsch-Samoa. – Feddes Repert. 9: 82-96.
Schlechter R. 1911a. Zur Kenntnis der Orchidaceen von Celebes. – Feddes Repert. 10: 66-96.
Schlechter R. 1911b. Beiträge zur Kenntnis der Orchidaceen-Flora von Sumatra. – Engl. Bot. Jahrb. Syst. 45, Beibl. 104, 3: 1-61.
Schlechter R. 1911c. Die Polychondreae (Neottiinae Pfitz.) und ihre systematische Einteilung. – Engl. Bot. Jahrb. Syst. 45: 375-410.
Schlechter R. 1911-1914. Die Orchideen von Deutsch Neu-Guinea. – Feddes Repert. Beih. 1: 1-1079.
Schlechter R. 1915a. Die Orchideen, ihre Beschreibung, Kultur und Züchtung. – Paul Parey, Berlin.
Schlechter R. 1915b. Orchidaceae Stolzianae. Ein Beitrag zur Orchideenkunde des Nyassa-Landes. – Engl. Bot. Jahrb. Syst. 53: 477-605.
Schlechter R. 1915c. Kritische Aufzählung der bisher von Madagscar, den Maskarenen, Komoren und Seychellen bekanntgewordenen Orchidaceen. – Beih. Bot. Centralbl. 33: 390-440.
Schlechter R. 1918a. Versuch einer natürlichen Neuordnung der afrikanischen angraekoiden Orchidaceen. – Beih. Bot. Centralbl. 36: 62-181.
Schlechter R. 1918b. Kritische Aufzählung der bisher aus Zentral-Amerika bekanntgewordenen Orchidaceen. – Beih. Bot. Centralbl. 36: 321-520.
Schlechter R. 1919a. Orchideologiae Sino-Japonicae prodromus. – Feddes Repert. Beih. 4: 1-319.
Schlechter R. 1919b. Die Orchideenfloren der südamerikanischen Kordillerenstaaten I. Venezuela. – Feddes Rep. Beih. 6.
Schlechter R. 1920a. Orchidaceae novae et criticae. – Feddes Repert. 16: 353-358.
Schlechter R. 1920b. Versuch einer systematischen Neuordnung der Spiranthinae. – Beih. Bot. Centralbl. 37: 317-454.
Schlechter R. 1921a. Revision von Schizochilus Sond. and Brachycorythis Lindl. – Beih. Bot. Centralbl. 38: 80-131.
Schlechter R. 1921b. Die Orchideenfloren der südamerikanischen Kordillerenstaaten III. Ecuador. – Feddes Rep. Beih. 8.
Schlechter R. 1921c. Die Orchidaceen von Mikronesien. – Engl. Bot. Jahrb. Syst. 56: 434-501.
Schlechter R. 1924a. Orchidaceae novae et criticae LXXVII. Die Gattungen Cymbidium Sw. und Cyperorchis Bl. – Feddes Repert. 20: 372-383.
Schlechter R. 1924b. Contributions to South African orchideology. – Ann. Transv. Mus. 10: 233-252.
Schlechter R. 1925. Orchidaceae Perrierianae. – Feddes Repert. Beih. 33:
Schlechter R. 1926. Das System der Orchideen. – Notizbl. Bot. Gart. Berlin-Dahlem 9: 563-591.
Schlechter R. 1930. Blütenanalysen neuer Orchideen I. Südamerikanische Orchideen. – Feddes Rep. Beih. 58. [Herausg. R. Mansfeld]
Schlechter R. 1932(†). Blütenanalysen neuer Orchideen III. Afrikanische und madagassische Orchideen. – Feddes Repert. Beih. 68.
Schlechter R. 1934(†). Blütenanalysen neuer Orchideen IV. – Feddes Repert. Beih. 74.
Schlechter R (Brieger FG, Butzin F, Senghas K [eds]). 1992. Die Orchideen. 3. Aufl., Bd. 1A. – Paul Parey, Berlin.
Schlechter R (Brieger FG, Butzin F, Senghas K [eds]). 1995. Die Orchideen. 3. Aufl., Bd. 1B. – Blackwell, Berlin.
Schlechter R (Brieger FG, Butzin F, Senghas K [eds]). 1996. Die Orchideen. Dritte Aufl., Bd 1C. – Blackwell, Berlin.
Schlechter R (Senghas K [ed]). 2003. Die Orchideen. 3. Aufl., Literaturverzeichnis und Register zu Bd. I/A, B und C. – Parey, Berlin.
Schlimbach H. 1924. Beiträge zur Kenntnis der Samenanlagen und Samen der Amaryllidaceen mit Berücksichtigung des Wassergehaltes der Samen. – Flora 117: 41-54.
Schlittler J. 1940. Monographie der Liliaceengattung Dianella Lam. – Mitt. Bot. Mus. Univ. Zürich 163: 5-283.
Schlittler J. 1943. Die Blütenabgliederung und die Perikladien bei den Vertretern des Anthericumtypus sowie ihre Bedeutung für die Systematik. – Mitt. Bot. Mus. Univ. Zürich 164: 491-507.
Schlitter J. 1945. Untersuchungen über den Bau der Blütenstände im Bereich des Anthericumtypus (Asphodelinae-Anthericinae-Dianellinae). – Mitt. Bot. Mus. Univ. Zürich 174: 200-239.
Schlittler J. 1951. Die Gattung Eustrephus R. Br. ex Sims und Geitonoplesium (R. Br.) A. Cunn.: morphologische-anatomische Studie mit Berücksichtigung der systematischen, nomenklatorischen und arealgeographischen Verhältnisse. – Ber. Schweiz. Bot. Ges. 61: 175-239.
Schlittler J. 1953. Die Blütenartikulation und die Phyllokladien der Liliaceen organphylogenetisch betrachtet I-II. – Feddes Repert. 55: 154-258.
Schlittler J. 1954. Die Liliaceengattung Dianella Lam. in Neukaledonien und auf den benachbarten Inseln. – Schweiz. Bot. Ges. Ber. 64: 185-198.
Schlittler J. 1957. Die Verbreitung der Liliaceengattung Dianella Lam. im Zusammenhang mit der Organdifferenzierung und der Arealbildung. – Mitt. Bot. Mus. Univ. Zürich 207: 1-38.
Schlittler J. 1960. Die Asparageenphyllokladien erweisen sich auch ontogenetisch als Blätter. – Bot. Jahrb. Syst. 79:428-446.
Schmid M. 1938. Vergleichende Untersuchungen der leitenden Elemente im Gynoeceum einiger Liliaceae. – Ph.D. diss., Universität Wien, Austria.
Schmid R, Schmid MJ. 1973. Fossils attributed to the Orchidaceae. – Amer. Orchid Soc. Bull. 42: 201-205.
Schmid R, Schmid MJ. 1974. On Massalongo’s fossils: Protorchis and Palaeorchis. – Amer. Orchid Soc. Bull. 43: 213-216.
Schmid R, Schmid MJ. 1977. Fossil history of the Orchidaceae. – In: Arditti J (ed), Orchid biology – reviews and perspectives I, Cornell University Press, Ithaca, New York, pp. 25-45.
Schmid WG. 1991. The genus Hosta. – Timber Press, Portland, Oregon.
Schmidt C. 1891. Über die Blätter einiger xerophiler Liliifloren. – Beih. Bot. Centralbl. 47: 1-6, 33-42, 97-107, 164-170.
Schnarf K. 1929. Die Embryologie der Liliaceae und ihre systematische Bedeutung. – Sitzungsber. Akad. Wiss. Wien, Math.-Naturwiss. Kl., Abt. I, 138: 69-92.
Schnarf K. 1948. Der Umfang der Lilioideae im natürlichen System. – Österr. Bot. Zeitschr. 95: 257-269.
Schnarf K, Wunderlich R. 1939. Zur vergleichenden Embryologie der Liliaceae-Asphodeloideae. – Flora 133: 297-327.
Schnepf E, Pross E. 1976. Differentiation and redifferentiation of a transfer cell: development of septal nectaries of Aloe and Gasteria. – Protplasma 89: 105-115.
Schönland S. 1903. On some South African species of Aloe with special reference to those represented in the Herbarium of the Albany Museum. – Records of the Albany Museum 1: 32-47.
Schuiteman A. 2004. Devogelia (Orchidaceae), a new genus from the Moluccas and New Guinea. – Blumea 49: 361-366.
Schuiteman A, Adams PB. 2011. New combinations in Dendrobium (Orchidaceae). – Muelleria 29: 62-68.
Schuiteman A, Ormerod P. 1998. Ophioglossella (Orchidaceae-Aeridinae), a new genus from Papua New Guinea. – Kew Bull. 741-745.
Schuiteman A, Kruizinga J, Scheindelen HJ van, Vogel EF de, Thomas SA, Burgh W van der. 1997. Revisions of the orchid genera Agrostophyllum (section Appendiculopsis), Mediocalcar-Bromheadia-Acanthephippium-Chrysoglossum, Collabium, Diglyphosa, Pilophyllum. – Orchid Monographs 8: 1-272.
Schulze R. 1893. Beiträge zur vergleichenden Anatomie der Liliaceen, Haemodoraceen, Hypoxidaceen und Velloziaceen. – Engl. Bot. Jahrb. Syst. 17: 295-334.
Schulze W. 1971. Beiträge zur Pollenmorphologie der Iridaceae und ihre Bedeutung für die Taxonomie. – Feddes Repert. 82: 101-124.
Schulze W. 1975. Beiträge zur Taxonomie der Liliifloren I. Asphodelaceae. – Wiss. Zeitschr. Friedrich-Schiller-Univ. Jena, Math.-Naturwiss. Reihe, 24: 403-415.
Schulze W. 1980a. Beiträge zur Taxonomie der Liliifloren V. Alliaceae. – Wiss. Zeitschr. Friedrich-Schiller-Univ. Jena, Math.-Naturwiss. Reihe, 29: 595-606.
Schulze W. 1980b. Beiträge zur Taxonomie der Liliifloren VI. Der Umfang der Liliaceae. – Wiss. Zeitschr. Friedrich-Schiller-Univ. Jena, Math.-Naturwiss. Reihe, 29: 607-636.
Schulze W. 1981. Beiträge zur Taxonomie der Liliifloren VII. Philesiaceae. – Wiss. Zeitschr. Friedrich-Schiller-Univ. Jena, Math.-Naturwiss. Reihe, 31: 277-283.
Schulze W. 1982a. Beiträge zur Taxonomie der Liliifloren IX. Anthericaceae. – Wiss. Zeitschr. Friedrich-Schiller-Univ. Jena, Math.-Naturwiss. Reihe, 31: 291-307.
Schulze W. 1982b. Beiträge zur Taxonomie der Liliifloren X. Asparagaceae. – Wiss. Zeitschr. Friedrich-Schiller-Univ. Jena, Math.-Naturwiss. Reihe, 31: 309-330.
Schulze W. 1983a. Beiräge zur Taxonomie der Liliiflorae XI. Tecophilaeaceae und Cyanastraceae. – Wiss. Zeitschr. Friedrich-Schiller-Univ. Jena, Math.-Naturwiss. Reihe, 32: 957-964.
Schulze W. 1983b. Beiträge zur Taxonomie der Liliifloren XII. Der Umfang der Agavaceen. – Wiss. Zeitschr. Friedrich-Schiller-Univ. Jena, Math.-Naturwiss. Reihe, 32: 965-979.
Schulze W. 1983c[1984]. Beiträge zur Taxonomie der Liliifloren XIV. Der Umfang der Amaryllidaceae. – Wiss. Zeitschr. Friedrich-Schiller-Univ. Jena, Math.-Naturwiss. Reihe, 32: 985-1003.
Schweinfurth C. 1940. Notes on American orchids, including new species and nomenclatorial changes. – Bot. Mus. Leafl. 8: 33-64.
Schweinfurth C. 1944. Notes on tropical American orchids. – Bot. Mus. Leafl. 11: 173-200.
Schweinfurth C. 1956. Notes on Peruvian orchids. – Bot. Mus. Leafl. 17: 211-215.
Schweinfurth C. 1958. Orchids of Peru I. – Fieldiana Bot. 30.
Schweinfurth C. 1959. Orchids of Peru II. – Fieldiana Bot. 30.
Schweinfurth C. 1970. First supplement to the flora of the orchids of Peru. – Fieldiana Bot. 33.
Scopece G, Cozzolino S, Bateman RM. 2010. Just what is a genus? Comparing levels of postzygotic isolation to test alternative taxonomic hypotheses in Orchidaceae subtribe Orchidinae. – Taxon 59: 1754-1764.
Scotland RW. 2013. Some observations on the homology of the daffodil corona. – In: Wilkin P, Mayo SJ (eds.), Early events in monocot evolution, Systematics Association Spec. Vol. Series, Cambridge University Press, Cambridge. doi:10.1017/CBO9781139002950.013
Scott CL. 1985. The genus Haworthia: a taxonomic revision. – Aloe Books, Johannesburg.
Scott G. 1991. A revision of Cyanella (Tecophilaeaceae) excluding C. amboensis. – South Afr. J. Bot. 57: 34-54.
Sealy JR. 1937. Lepidochiton quitoensis. – Curtis’s Bot. Mag.: t. 9491.
Sealy JR. 1954. Review of the genus Hymenocallis. – Kew Bull. 2: 201-240.
Seberg O, Petersen G, Davis JI, Pires JC, Stevenson DW, Chase MW, Fay MF, Devey DS, Jørgensen T, Sytsma KJ, Pillon Y. 2012. Phylogeny of the Asparagales based on three plastid and two mitochondrial genes. – Amer. J. Bot. 99: 875-889.
Seidenfaden G. 1968. The genus Oberonia in Mainland Asia. – Dansk Bot. Ark. 25(3): 1-125.
Seidenfaden G. 1969. Contributions to the orchid flora of Thailand. – Bot. Tidsskr. 65: 100-162.
Seidenfaden G. 1972. Contributions to the orchid flora of Thailand 4. – Bot. Tidsskr. 67: 76-127.
Seidenfaden G. 1973a. Notes on Cirrhopetalum Lindl. – Dansk Bot. Ark. 29(1): 1-260.
Seidenfaden G. 1973b. Contributions to the orchid flora of Thailand 5. – Bot. Tidsskr. 68: 41-95.
Seidenfaden G. 1974. Notes on Cirrhopetalum Lindl. – Dansk bot. Ark. 29(1): 1-260.
Seidenfaden G. 1975a. Contributions to a revision of the orchid flora of Cambodia, Laos, and Vietnam 1. – Fredensborg.
Seidenfaden G. 1975b. Orchid genera in Thailand I. Calanthe R. Br. – Dansk Bot. Ark. 29(2): 1-50.
Seidenfaden G. 1975c. Contributions to the orchid flora of Thailand 6. – Bot.tidsskr. 70: 64-97.
Seidenfaden G. 1976a. Contributions to the orchid flora of Thailand 7. – Bot. Tidsskr. 71: 1-30.
Seidenfaden G. 1976b. Orchid genera in Thailand IV. Liparis L. C. Rich. – Dansk Bot. Ark. 31(1): 1-105.
Seidenfaden G. 1977a. Orchid genera in Thailand V. Orchidoideae. – Dansk Bot. Arkiv 31(2): 1-149.
Seidenfaden G. 1977b. Contributions to the orchid flora of Thailand VII. – Bot. Tidsskr. 71: 1-14.
Seidenfaden G. 1978a. Orchid genera in Thailand VI. Neottioideae Lindl. – Dansk Bot. Arkiv 32(2): 1-195.
Seidenfaden G. 1978b. Orchid genera in Thailand VII. Oberonia Lindl. & Malaxis Sol. ex Sw. – Dansk Bot. Ark. 33(1): 1-94.
Seidenfaden G. 1979. Orchid genera in Thailand VIII. Bulbophyllum Thou. – Dansk Bot. Ark. 33(3): 1-228.
Seidenfaden G. 1980. Orchid genera in Thailand IX. Flickingeria Hawkes and Epigeneium Gagnep. – Dansk Bot. Ark. 34(1): 1-104.
Seidenfaden G. 1981. Contributions to the orchid flora of Thailand IX. – Nord. J. Bot. 1: 192-217.
Seidenfaden G. 1982. Orchid genera in Thailand X. Trichotosia Bl. & Eria Lindl. – Opera Bot. 62: 1-157.
Seidenfaden G. 1984a. A note on the section Cymboglossum Schltr. of Eria (Orchidaceae). – Nord. J. Bot. 4: 39-45.
Seidenfaden G. 1984b. Orchid genera in Thailand XI. Cymbidieae Pfitz. – Opera Bot. 72: 1-123.
Seidenfaden G. 1985a. Contributions to the orchid flora of Thailand XI. – Nord. J. Bot. 5: 157-167.
Seidenfaden G. 1985b. Orchid genera in Thailand XII. Dendrobium Sw. – Opera Bot. 83: 1-295.
Seidenfaden G. 1986a. A collection of orchids from Malaya. – Nord. J. Bot. 6: 157-181.
Seidenfaden G. 1986b. Orchid genera in Thailand XIII. Thirty-three epidendroid genera. – Opera Bot. 89: 1-216.
Seidenfaden G. 1988. Orchid genera in Thailand XIV. Fifty-nine vandoid genera. – Opera Bot. 95: 1-398.
Seidenfaden G. 1992. The orchids of Indochina. – Opera Bot. 114: 1-502.
Seidenfaden G. 1994. The genus Chamaegastrodia (Orchidaceae). – Nord. J. Bot. 14: 293-301.
Seidenfaden G. 1995. Contributions to the orchid flora of Thailand XII. – Opera Bot. 124: 1-90.
Seidenfaden G. 1996. A remarkable new Bulbophyllum, sect. Sestochilus (Orchidaceae) from Thailand. – Nord. J. Bot. 16: 287-289.
Seidenfaden G, Smitinand T. 1959-1965. The orchids of Thailand, a preliminary list. – Science Society, Bangkok.
Seidenfaden G, Wood JJ. 1992. The orchids of Peninsular Malaysia and Singapore. A revision of R. E. Holttum: Orchids of Malaya. – Olsen & Olsen, Fredensborg.
Seisums AG. 1994. Podrod Melanocrommyum (Webb et Berth.) Rouy roda Allium L. (Monograficheskij obzor). – Avtoreferat dissertatsii doktora biol. nauk. Riga.
Selosse MA, Faccio A, Scappaticci G, Bonfante P. 2004. Chlorophyllous and achlorophyllous specimens of Epipactis microphylla (Neottieae, Orchidaceae) are associated with ectomycorrhizal septomycetes, including truffles. – Microbiol. Ecol. 47: 416-426.
Sen S. 1973. Polysomaty and its significance in Liliales. – Cytologia 38: 737-751.
Sen S. 1974. Chromosome evolution in Polygonateae. – Bull. Bot. Soc. Bengal 28: 103-111.
Sen S. 1975. Cytotaxonomy of Liliales. – Feddes Repert. 86: 255-305.
Senghas K. 1964a. Sur quelques orchidées nouvelles ou critiques de Madagascar. – Adansonia, sér. II, 4: 301-314.
Senghas K. 1964b. Die Gattung Graphorkis (=Eulophiopsis). – Die Orchidee 15: 61-66.
Senghas K. 1968. Taxonomische Übersicht der Gattung Dactylorhiza Necker ex Nevski. – Jahresb. Nat. Wiss. Ver. Wuppertal 21-22: 32-67.
Senghas K. 1972. Leucorchis versus Pseudorchis. – Orchidee 23: 203-205.
Senghas K. 1978. Hapalochilus, eine alte, neue Orchideengattung aus Neu-Guinea. – Die Orchideen 29: 245-248.
Senghas K. 1994. Pterostemma, eine von der Evolution vergessene Orchideengattung? Erstnachweis der Gattung Cypholoron aus der Kultur. – Die Orchidee (Hamburg) 45: 146-154.
Senghas K, Garay LA. 1996. Sarmenticola, eine neue Orchideengattung (Orchidaceae, Oncidieae, Notyliinae). – Willdenowia 25: 655-658.
Sera T. 1990. Karyomorphological studies on Goodyera and its allied genera in Orchidaceae. – Bull. Hiroshima Bot. Gard. 12: 71-144.
Seth CJ. 1982. Cymbidium aloifolium (Orchidaceae) and its allies. – Kew Bull. 37: 397-402.
Shah GL, Gopal BV. 1970. Structure and development of stomata on the vegetative and floral organs of some Amaryllidaceae. – Ann. Bot., N. S., 34: 737-749.
Sharma AK, Chaudhuri M. 1964. Cytological studies as an aid in assessing the status of Sansevieria, Ophiopogon, and Curculigo. – Nucleus 7: 43-58.
Sharma AK, Datta PC. 1960. Chromosome studies in species of Dracaena with special reference to their means of speciation. – J. Genet. 57: 43-76.
Sharma AK, Ghosh C. 1954. Further investigations on the cytology of the family Amaryllidaceae and its bearing on the interpretation of its phylogeny. – Genet. Iber. 6: 71-100.
Sharma AK, Ghosh I. 1968. Cytotaxonomy of Dracaena. – J. Biol. Sci. 11: 45-55.
Sharma AK, Mallick R. 1966. Interrelationships and evolution of the tribe Aloineae as reflected in its cytology. – J. Genet. 59: 20-47.
Sharma AK, Talukdar C. 1959. Cytotaxonomical studies on some members of the Iridaceae with special reference to the structural heterozygosity of Cipura paludosa Aubl. – Nucleus 2: 63-84.
Sherman HL. 1969. A systematic study of the genus Schoenolirion (Liliaceae). – General Biology, Vanderbilt University, Nashville, Tennessee.
Sherman HL, Becking RW. 1991. The generic distinctness of Schoenolirion and Hastingsia. – Madroño 38: 130-138.
Shevock J. 1984. Redescription and distribution of Muilla coronata (Liliaceae). – Aliso 10: 621-627.
Shinners LH. 1966. Texas Polianthes, including Manfreda (Agave subgenus Manfreda) and Runyonia (Agavaceae). – Sida 2: 333-338.
Shinwari ZK. 2000. Chloroplast DNA variation in Polygonatae sensu lato (Liliaceae). – Pakistan J. Bot. 32: 7-14.
Shinwari ZK, Kato H, Terauchi R, Kawano S. 1994. Phylogenetic relationships among genera in the Liliaceae-Asparagoideae-Polygonatae sensu lato inferred from rbcL gene sequence data. – Plant Syst. Evol. 192: 263-277.
Shneyer VS. 1983. The relationships between the Iridaceae and Liliaceae s.l. as revealed by the serological analysis of seed proteins. – Bot. Žurn. 68: 49-54. [In Russian]
Shu P, Qin M-J, Shen W-J, Wu G. 2009. A new coumaronochromone and phenolic constituents from the leaves of Iris bungei Maxim. – Biochem. Syst. Ecol. 37: 20-23.
Shukla AK. 1984. A clarification on the use of the term viscin thread in Orchidaceae. – Grana 23: 127.
Sigler A, Rauwald HW. 1994a. Tetrahydroanthracenes as markers for the subterranean anthranoid metabolism in Aloe species. – J. Plant Physiol. 143: 596-600.
Sigler A, Rauwald HW. 1994b. Aloe plants accumulate anthrone-type anthranoids in inflorescence and leaves, and tetrahydroanthracenes in roots. – Zeitschr. Naturforsch. 49c: 286-292.
Silvera K, Santiago LS, Cushman JC, Winter K. 2009. Crassulacean acid metabolism and epiphytism linked to adaptive radiations in the Orchidaceae. – Plant Physiology 149: 1838-1847.
Silvera K, Santiago LS, Cushman JC, Winter K. 2010. The incidence of crassulacean acid metabolism in Orchidaceae derived from carbon isotope ratios: a checklist of the flora of Panama and Costa Rica. – Bot. J. Linn. Soc. 163: 194-222.
Silvera K, Winter K, Rodriguez BL, Albion RL, Cushman JC. 2014. Multiple isoforms of phosphoenolpyruvate carboxylase in the Orchidaceae (subtribe Oncidiinae): implications for the evolution of crassulacean acid metabolism. – J. Exp. bot. 65: 3623-3636.
Simo-Droissart M, Sonké B, Droissart V,
Geerinck D, Micheneau C, Lowry II PP, Plunkett GM, Hardy OJ, Stévart T. 2014.
Taxonomic revision of the continental African species of Angraecum
Section Pectinaria (Orchidaceae). – Syst. Bot. 39:
725-739.
Simo-Droissart M, Sonké B, Droissart V, Micheneau C, Lowry II PP, Hardy OJ, Plunkett GM, Stévart T. 2016. Morphometrics and molecular phylogenetics of Angraecum section Dolabrifolia (Orchidaceae, Angraecinae). – Plant Syst. Evol. 302: 1027-1045.
Simo-Droissart M, Plunkett GM, Droissart V, Edwards MB, Farminhão JNM, Ječmenica V, D’haijère T, Lowry PP II, Sonké B, Micheneau C, Carlsward BS, Azandi L, Verlynde S, Hardy OJ, Martos F, Bytebier B, Fischer E, Stévart T. 2018. New phylogenetic insights toward developing a natural generic classification of African angraecoid orchids (Vandeae, Orchidaceae). – Mol. Phylogen. Evol. 126: 241-249.
Simo-Droissart M, Stévart T, Sonké B, Mayogo S, Kamdem N, Droissart V. 2018. New taxonomic and conservation status of Ossiculum (Vandeae, Orchidaceae), a highly threatened and narrow-endemic angraecoid orchid from Central Africa. – PhytoKeys 98: 85-97.
Simo-Droissart M, Sonké B, Droissart V, Stévart T. 2018. Afropectinariella (Vandeae, Orchidaceae), a new genus of the Angraecum alliance. – PhytoKeys 96: 79-86.
Simonet M. 1928. Le nombre des chromosomes dans le genre Iris. – Compt. Rend. Soc. Biol. Paris 99: 1314-1316.
Simonet M. 1932. Recherches cytologiques et genetique chez les Iris. – Bull. Biol. France Belg. 105: 255-444.
Simpson MG. 1983. Pollen ultrastructure of the Haemodoraceae and its taxonomic significance. – Grana 22: 79-103.
Simpson MG. 1985. Pollen ultrastructure of the Tecophilaeaceae. – Grana 24: 77-92.
Simpson MG. 1990. Phylogeny and classification of the Haemodoraceae. – Ann. Missouri Bot. Gard. 77: 722-784.
Simpson MG, Rudall PJ. 1998. Tecophilaeaceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 429-436.
Simpson P. 2000. Dancing leaves: the story of New Zealand’s cabbage tree, Ti-Ko-uka. – Canterbury University Press, Christchurch, New Zealand.
Singer RB, Koehler S. 2003. Toward a phylogeny of Maxillariinae orchids: multidisciplinary studies with emphasis on Brazilian species. – Lankesteriana 7: 57-60.
Singer RB, Koehler S. 2004. Pollinarium morphology and floral rewards in Brazilian Maxillariinae (Orchidaceae). – Ann. Bot. 93: 39-51.
Singh H. 1981. Development and organisation of stomata in Orchidaceae. – Acta Bot. Indica 9: 94-100.
Singh V. 1972. Floral morphology of the Amaryllidaceae I. Subfamily Amaryllidoideae. – Can. J. Bot. 50: 1555-1565.
Singh Y. 2006. Hypoxis (Hypoxidaceae) in Africa: list of species and infraspecific names. – Bothalia 36: 13-23.
Singh Y. 2007. Hypoxis (Hypoxidaceae) in southern Africa: taxonomic notes. – South Afr. J. Bot. 73: 360-365.
Sinitsyna TA, Herden T, Friesen N. 2016. Dated phylogeny and biogeography of the Eurasian Allium section Rhizirideum (Amaryllidaceae). – Plant Syst. Evol. 302: 1311-1328.
Sirisena UM. 2010. Systematic studies on Thysanotus R. Br. (Asparagales: Laxmanniaceae). – Ph.D. diss., University of Adelaide, South Australia.
Sitko M, Takallo P, Gorniak M. 2006. Introduction to the phylogenetic analysis of Maxillaria Ruiz & Pav. (Maxillariinae, Orchidaceae). – Biodivers. Res. Cons. 3-4: 200-204.
Skottsberg C. 1934a. Studies in the genus Astelia Banks et Solander. – Kungl. Sv. Vetensk.-Akad. Handl. III(14): 1-106.
Skottsberg C. 1934b. Astelia and Pipturus of Hawaii. – Bernice P. Bishop Mus. Bull. 117: 3-39.
Skottsberg C. 1960. Astelia on Mauritius. – Svensk Bot. Tidskr. 54: 477-482.
Skrzypczak L. 1977. Zwiazki flawonoidowe w chemicznej taksonomii rodziny Liliaceae. – Herba Polon. 22: 336-349.
Slob A. 1973. Tulip allergens in Alstroemeria and some other Liliiflorae. – Phytochemistry 12: 811-815.
Smidt EC, Borba EL, Gravendeel B, Fischer GA, Berg C van den. 2011. Molecular phylogeny of the Neotropical sections of Bulbophyllum (Orchidaceae) using nuclear and plastid spacers. – Taxon 60: 1050-1064.
Smith CI, Pellmyr O, Althoff DM, Balcázar-Lara M, Leebens-Mack J, Seagraves KA. 2008. Pattern and timing of diversification in Yucca (Agavaceae): specialized pollination does not escalate rates of diversification. – Proc. Roy. Soc. London, Ser. B, 275: 249-258.
Smith CM, Smith GA. 1970. An electrophoretic comparison of six species of Yucca and of Hesperaloe. – Bot. Gaz. 131: 201-205.
Smith FH. 1930. The corm and contractile roots of Brodiaea lactea. – Amer. J. Bot. 17: 916-927.
Smith GF. 1985. Die taksonomiese status van die monotipiese genusse Chortolirion Berger en Poellnitzia Uitewaal in die tribus Aloineae (familie Asphodelaceae). – B.Sc.(Hons.) Scription, University of Pretoria, Republic of South Africa.
Smith GF. 1988. A scanning electron microscopic investigation of the pollen morphology of Chortolirion Berger (Aloineae; Liliaceae). – South Afr. J. Sci. 84: 428-430.
Smith GF. 1990. Nomenclatural notes on the subsection Bowieae in Aloe (Asphodelaceae: Alooideae). – South Afr. J. Bot. 56: 303-308.
Smith GF. 1991a. Studies on the reproductive biology and palynology of Chortolirion Berger (Asphodelaceae: Aloideae) in southern Africa. – Taxon 40: 61-73.
Smith GF. 1991b. The chromosomes of Chortolirion and Poellnitzia (Asphodelaceae: alooideae). – Bothalia 21: 171-175.
Smith GF. 1991c. Historical review of the taxonomy of Chortolirion Berger (Asphodelaceae: Alooideae). – Aloe 28: 90-95.
Smith GF. 1995a. FSA contributions 2: Asphodelaceae/Aloaceae, 1029010 Chortolirion. – Bothalia 25: 31-33.
Smith GF. 1995b. FSA contributions 3: Asphodelaceae/Aloaceae, 1028010 Poellnitzia. – Bothalia 25: 35-36.
Smith GF, Steyn EMA. 2004. Taxonomy of Aloaceae. – In: Reynolds T (ed), Aloes, the genus Aloe, CRC Press, Boca Raton, Florida, pp. 15-36.
Smith GF, Wyk AE van. 1993. The generic status of Chortolirion (Aloaceae): evidence from leaf anatomy. – Kew Bull. 48: 105-113.
Smith GF, Wyk B-E van. 1991. Generic relationships in the Alooideae (Asphodelaceae). – Taxon 40: 557-581.
Smith GF, Wyk B-E van. 1992. Systematic leaf anatomy of selected genera of southern African Alooideae (Asphodelaceae). – South Afr. J. Bot. 58: 349-357, 543.
Smith GF, Wyk B-E van. 1993. The generic status of Chortolirion (Aloaceae): evidence from leaf anatomy. – Kew Bull. 48: 105-113.
Smith GF, Wyk B-E van. 1998. Asphodelaceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 130-140.
Smith GF, Theron PD, Loots GC. 1992. Notes on the microfaunal complement and pollination mechanisms of Poellnitzia rubriflora (Asphodelaceae: Alooideae): an example of mite-flower domatia association. – Taxon 41: 437-450.
Smith GF, Wyk B-E van, Mössmer M, Viljoen A. 1995. The taxonomy of Aloinella, Guillauminia and Lemeea (Aloaceae). – Taxon 44: 513-517.
Smith GF, Wyk B-E van, Steyn EMA, Breuer I. 2001 [2002]. Infrageneric classification of Haworthia (Aloaceae): perspectives from nectar sugar analysis. – Syst. Geogr. Plants 71: 391-397.
Smith GL, Flory WS. 1990. Studies on Hymenocallis henryae (Amaryllidaceae). – Brittonia 42: 212-220.
Smith GL, Flory WS. 2001. Hymenocallis (Amaryllidaceae) in Texas, with a new varietal combination. – Novon 11: 229-232.
Smith GL, Garland MA. 2003. Nomenclature of Hymenocallis taxa (Amaryllidaceae) in southeastern United States. – Taxon 52: 805-817.
Smith GL, Anderson LC, Flory WS. 2001. A new species of Hymenocallis (Amaryllidaceae) in the Lower Central Florida Panhandle. – Novon 11: 233-240.
Smith JJ. 1904. Neue Orchideen. – Rec. Trav. Bot. Neerl. 1: 146-159.
Smith JJ. 1905. Die Orchideen von Java. – Flora von Buitenzorg 6: 1-672.
Smith JJ. 1912. Bulbophyllum Thou. Sect. Cirrhopetalum. – Bull. Buitenz. sér. II, 8: 19-29.
Smith JJ. 1918. Die Orchideen von Java. Fünfter Nachtrag. – Bull. Buitenz. sér. II, 26: 1-135.
Smith JJ. 1925. Die Orchideen der zweiten Frankfurter Sunda-Expedition 1909-1910. – Meded. Rijksherb. Leiden 53: 1-17.
Smith JJ. 1926. Ephemeral orchids. – Ann. Jard. Bot. Buitenzorg 35: 55-70.
Smith JJ. 1928. Orchidaceae Buruenses. – Bull. Jard. Bot. Buitenzorg III, 9(3-4): 439-481.
Smith JJ. 1930. Icones orchidacearum Malayensium. – Bull. Buitenz. [Suppl.] 2: T. 1-50.
Smith JJ. 1932. Drei neue Orchideen. – Feddes Repert. 31: 76-82.
Smith JJ. 1933a. Orchidaceae selbenses Kjellbergiano. – Bot. Jahrb. Syst. 65: 449-508.
Smith JJ. 1933b. Enumeration of the Orchidaceae of Sumatra and neighbouring islands. – Feddes Repert. 32: 129-386.
Smith S, Stansbie J. 2003. Alliaceae. – In: Beentje HJ, Ghazanfar SA (eds), Flora of tropical East Africa, A. A. Balkema Publ., Lisse, pp. 1-8.
Smith SD, Cowan RS, Gregg KB, Chase MW, Maxted N, Fay MF. 2004. Genetic discontinuities among populations of Cleistes (Orchidaceae, Vanilloideae) in North America. – Bot. J. Linn. Soc. 145: 87-95.
Smitinand T. 1958. The genus Eria Ldl. in Thailand. – Nat. Hist. Bull. Siam Soc. 19: 7-43.
Snijman DA. 1984. A revision of the genus Haemanthus L. (Amaryllidaceae). – South Afr. J. Bot., Suppl. Vol. 12.
Snijman DA. 1991. Kamiesbergia, a new monotypic genus of the Amaryllideae-Strumariinae (Amaryllidaceae) from the north-western Cape. – Bothalia 21: 125-128.
Snijman DA. 1992. Systematic studies in the tribe Amaryllideae (Amaryllidaceae). – Ph.D. diss., University of Cape Town, Republic of South Africa.
Snijman DA. 1994. Systematics of Hessea, Strumaria and Carpolyza (Amaryllideae: Amaryllidaceae). – Contr. Bolus Herb. 16: 1-162.
Snijman DA. 2006. Two new species of Spiloxene (Hypoxidaceae) from the northwestern Cape, South Africa. – Bothalia 36: 133-138.
Snijman DA, Kocyan A. 2013. The genus Pauridia (Hypoxidaceae) amplified to include Hypoxis sect. Ianthe, Saniella and Spiloxene, with revised nomenclature and typification. – Phytotaxa 116: 19-33.
Snijman DA, Linder HP. 1996. Phylogenetic relationships, seed characters, and dispersal systems evolution in Amaryllideae (Amaryllidaceae). – Ann. Missouri Bot. Gard. 83: 362-386.
Snijman DA, Meerow AW. 2010. Floral and macroecological evolution within Cyrtanthus (Amaryllidaceae): inferences from combined analyses of plastid ndhF and nrDNA ITS sequences. – South Afr. J. Bot. 76: 217-238.
Snijman DA, Williamson G. 1994. A taxonomic reassessment of Ammocharis herrei and Cybistetes longifolia (Amaryllidaceae). – Bothalia 24: 127-132.
Solano-Gómez R, Salazar GA, Jiménez-Machorro R. 2011. New combinations in Orchidaceae of Mexico. – Acta Bot. Mex. 97: 49-56.
Soliva M, Kocyan A, Widmer A. 2001. Molecular phylogenetics of the sexually deceptive orchid genus Ophrys (Orchidaceae) based on nuclear and chloroplast DNA sequences. – Mol. Phylogen. Evol. 20: 78-88.
Soó R. 1960. Synopsis generis Dactylorhiza (Dactylorchis). – Ann. Univ. Sci. Budapest Rolando Eötvös (sect. biol.) 3: 335-357.
Sood SK, Mohana Rao PR. 1988.Studies in the embryology of the diandrous orchid Cypripedium cordigerum (Cypripedieae, Orchidaceae). – Plant Syst. Evol. 160: 159-168.
Šopova M. 1972. Cytology of ten Crocus species from Macedonia. – God. Zborn. 24: 73-85.
Sosa V. 2007. A molecular and morphological phylogenetic study of subtribe Bletiinae (Epidendreae, Orchidaceae). – Syst. Bot. 32: 34-42.
Sosa V, Chase MW, Salazar GE, Whitten WM, Williams NH. 2001. Phylogenetic position of Dignathe (Orchidaceae: Oncidiinae): evidence from nuclear ITS ribosomal DNA sequence. – Lindleyana 16: 94-101.
Soto MA. 1988. Listado actualizado de las orquídeas de México. – Orquídeas (Mexico City) 11: 233-277.
Soto MA, Salazar GA, Hagsáter E. 1990. Phragmipedium xerophyticum, una nueva especie del sureste de México. – Orquídea (México) 12: 1-10.
Soto MA, Hágsater E, Jiménez-Machorro R, Salazar GA, Solano R, Flores R, Ruiz I. 2007. The orchids of Mexico: digital catalogue. – Instituto Chinoin, Mexico City.
Soto-Arenas MA, Cribb P. 2010. A new infrageneric classification and synopsis of the genus Vanilla Plum. ex Mill. (Orchidaceae: Vanillinae). – Lankesteria 9: 355-398.
Souza LGR, Crosa O, Guerra M. 2010. Karyological circumscription of Ipheion Rafinesque (Gilliesioideae, Alliaceae). – Plant Syst. Evol. 287: 119-127.
Souza LGR, Crosa O, Speranza P, Guerra M. 2012. Cytogenetic and molecular evidence suggest multiple origins and geographical parthenogenesis in Nothoscordum gracile (Alliaceae). – Ann. Bot. 109: 5987-5999.
Souza LGR, Crosa O, Guerra M. 2015. Karyological, morphological, and phylogenetic diversification in Leucocoryne Lindl. (Allioideae, Amaryllidaceae). – Plant Syst. Evol. 301: 2013-2023.
Souza LGR, Crosa O, Speranza P, Guerra M. 2016. Phylogenetic relations in tribe Leucocoryneae (Amaryllidaceae, Allioideae) and the validation of Zoellnerallium based on DNA sequences and cytomolecular data. – Bot. J. Linn. Soc. 182: 811-824.
Souza-Chies TT, Bittar G, Nadot S, Carter L, Besin E, Lejeune B. 1997. Phylogenetic analysis of Iridaceae with parsimony and distance methods using the plastid gene rps4. – Plant Syst. Evol. 204: 109-123.
Speta F. 1971. Beitrag zur Systematik von Scilla L. subgen. Scilla (inklusive Chionodoxa Boiss.). – Österr. Bot. Zeitschr. 119: 6-18.
Speta F. 1974a. Scilla messeniaca Boiss. (Liliaceae) und ihre verwandtschaftlichen Beziehungen. – Ann. Mus. Goulandris 2: 59-67.
Speta F. 1974b. Cytotaxonomische und arealkundliche Untersuchungen an der Scilla bifolia-Gruppe in Oberösterreich, Niederösterreich und Wien. – Naturk. Jahrb. Stadt Linz 19: 9-54.
Speta F. 1976a. Über Chionodoxa Boiss., ihre Gliederung und Zugehörigkeit zu Scilla L. – Naturk. Jahrb. Stadt Linz 21: 9-79.
Speta F. 1976b. Cytotaxonomischer Beitrag zur Kenntnis der Scilla nivalis-Gruppe. – Linzer Biol. Beitr. 8: 293-322.
Speta F. 1979. Karyological investigations in Scilla in regard to their importance for taxonomy. – Webbia 34: 419-431.
Speta F. 1980. Karyosystematik, Kultur und Verwendung der Meerzwiebel (Urginea Steinh., Liliaceae s.l.). – Linzer Biol. Beitr. 12: 193-238.
Speta F. 1981. Die frühjahrsblühenden Scilla-Arten des östlichen Mittelmeerraumes. – Naturk. Jahrb. Stadt Linz 25: 19-198.
Speta F. 1982a. Über die Abgrenzung und Gliederung der Gattung Muscari und über ihre Beziehungen zu anderen Vertretern der Hyacinthaceae. – Bot. Jahrb. Syst. 103: 247-291.
Speta F. 1982b. Die Gattungen Scilla L. s. str. und Prospero Salisb. im pannonischen Raum. – Veröff. Intern. Arbeitsgem. Clusius-Forschung Güssing 5: 1-19.
Speta F. 1985. Nomenklatorische, morphologische und karyologische Bemerkungen über Lindneria clavata (Masters) Speta (Hyacinthaceae). – Bot. Jahrb. Syst. 106: 123-134.
Speta F. 1986. Über die herbstblühenden Scilla-Arten des Mittelmeerraumes. – Linzer Biol. Beitr. 18: 399-416.
Speta F. 1987. Die verwandtschaftlichen Beziehungen von Brimeura Salisb.: ein Vergleich mit den Gattungen Oncostema Rafin., Hyacinthoides Medic. und Camassia Lindl. (Hyacinthaceae). – Phyton (Horn) 26: 698-706.
Speta F. 1989. Muscari (subg. Leopoldia) mirum Speta spec. nova, im Kreise seiner nächsten Verwandten. – Phyton (Horn) 29: 105-117.
Speta F. 1991. Zwei neue Scilla-Arten (Hyacinthaceae) aus der S-Türkei. – Willdenowia 21: 157-166.
Speta F. 1993. The autumn-flowering squills of the Mediterranean region. – Proc. 5th OPTIMA meeting, Istanbul 8-15 Sep 1986, pp. 109-124.
Speta F. 1998a. Systematische Analyse der Gattung Scilla L. s.l. (Hyacinthaceae). – Phyton (Horn) 38: 1-141.
Speta F. 1998b. Hyacinthaceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 261-285.
Speta F. 2000. Beitrag zur Kenntnis der Gattung Prospero Salisb. (Hyacinthaceae) auf der griechischen Insel Kreta. – Linzer Biol. Beitr. 32: 1323-1326.
Speta F. 2001. Die echte und die falsche Meerzwiebel: Charybdis Speta und Stellarioides Medicus (Hyacinthaceae), mit Neubeschreibungen und Neukombination im Anhang. – Stapfia 75: 139-176.
Spies JJ, hardy DS. 1983. A karyotypic and anatomical study of an unidentified liliaceous plant. – Bothalia 14: 215-217.
Sprague TA. 1928. Marica and Neomarica. – Kew Bull. 1928: 278-281.
Sprumont G. 1928. Chromosomes et satellites dans quelques espèces d’Ornithogalum. – La Cellule 38: 271-292.
Sramkó G, Attila MV, Hawkins JA, Bateman RM.
2014. Molecular phylogeny and evolutionary history of the Eurasiatic orchid
genus Himantoglossum s.l. (Orchidaceae). – Ann. Bot. 114:
1609-1626.
Stafford GI, Wikkelsø MJ, Nancke L, Jäger AK, Möller M, Rønsted N. 2016. The first phylogenetic hypothesis for the southern African endemic genus Tulbaghia (Amaryllidaceae, Allioideae) based on plastid and nuclear DNA sequences. – Bot. J. Linn. Soc. 181: 156-170.
Ståhlberg D, Hedrén M. 2008. Systematics and phylogeography of the Dactylorhiza maculata complex (Orchidaceae) in Scandinavia: insights from cytological, morphological and molecular data. – Plant Syst. Evol. 273: 107-132.
Stapf O. 1933. Pamianthe peruviana. – Curtis’s Bot. Mag.: t. 9315.
Starr G. 1995. Hesperaloe: aloes of the west. – Desert Plants 11: 3-8.
Starr G. 1997. A revision of the genus Hesperaloe. – Madroño 44: 282-296.
Stearn WT. 1944 [1946]. Notes on the genus Allium in the Old World. – Herbertia 11: 11-34.
Stearn WT. 1960. Allium and Milula in the central and eastern Himalaya. – Bull. Brit. Mus. (Nat. Hist.) Bot. 2: 159-191.
Stearn WT. 1978a. European species of Allium and allied genera of Alliaceae: a synonymic enumeration. – Ann. Mus. Goulandris 4: 83-198.
Stearn WT. 1978b. Mediterranean and Indian species of Drimia (Liliaceae): a nomenclatural survey with special reference to the medical squill, D. maritima (syn. Urginea maritima). – Ann. Mus. Goulandris 4: 199-210.
Stearn WT. 1983. The Linnean species of Ornithogalum (Liliaceae). – Ann. Mus. Goulandris 6: 139-170.
Stedje B. 1987. A revision of the genus Drimia (Hyacinthaceae) in East Africa. – Nord. J. Bot. 7: 655-666.
Stedje B. 1994. A revision of the genus Drimiopsis (Hyacinthaceae) in East Africa. – Nord. J. Bot. 14: 45-50.
Stedje B. 1996a. Hyacinthaceae. – In: Polhill RM (ed), Flora of tropical East Africa, A. A. Balkema, Rotterdam, pp. 1-32.
Stedje B. 1996b. Karyotypes of some species of Hyacinthaceae from Ethiopia and Kenya. – Nord. J. Bot. 16: 121-126.
Stedje B. 1998. Phylogenetic relationships and generic delimitation of sub-Saharan Scilla (Hyacinthaceae) and allied African genera as inferred from morphological and DNA sequence data. – Plant Syst.Evol. 211: 1-11.
Stedje B. 2000. The evolutionary relationships of the genera Drimia, Thuranthos, Bowiea and Schizobasis discussed in the light of morphology and chloroplast DNA sequence data. – In: Wilson KL, Morrison DA (eds), Monocots: systematics and evolution, CSIRO Publ., Melbourne, pp. 414-417.
Stedje B. 2001. The generic delimitation within Hyacinthaceae, a comment on works by F. Speta. – Bothalia 31: 192-195.
Stedje B. 2001 [2002]. Generic delimitation of Hyacinthaceae, with special emphasis on sub-Saharan genera. – Syst. Geogr. Plants 71: 449-454.
Stedje B, Nordal I. 1984. Taxonomy and cytology of the genus Ornithogalum (Liliaceae) in East Africa. – Nord. J. Bot. 4: 749-759.
Stedje B, Nordal I. 1987. Cytogeographical studies of Hyacinthaceae in Africa south of the Sahara. – Nord. J. Bot. 7: 53-65.
Stedje B, Nordal I. 1994. A contribution to the discussion of the family delimitation of Anthericaceae versus Asphodelaceae. – In: Seyani JH, Chikuni AC (eds), Proceedings of the XIIIth Plenary Meeting AETFAT, Malawi, pp. 513-524.
Stedje B, Thulin M. 1995. Synopsis of Hyacinthaceae in tropical East and North-East Africa. – Nord. J. Bot. 15: 591-601.
Steele PR, Hertweck KL, Mayfield D, McKain MR, Leebens-Mack J, Pires JC. 2012. Quality and quantity of data recovered from massively parallel sequencing: examples in Asparagales and Poaceae. – Amer. J. Bot. 99: 330-348.
Steiner KE. 2010. Twin oil sacs facilitate the evolution of a novel type of pollination unit (meranthium) in a South African orchid. – Amer. J. Bot. 97: 311-323.
Steiner KE, Kaiser R, Döterl S. 2011. Strong phylogenetic effects of floral scent variation of oil-secreting orchids in South Africa. – Amer. J. Bot. 98: 1663-1679.
Stenar H. 1925. Embryologische Studien II. Die Embryologie der Amaryllidaceen. – Ph.D. diss., University of Uppsala, Sweden.
Stenar H. 1928a. Zur Embryologie der Veratrum- und Anthericum-Gruppe. – Bot. Not. 1928: 357-378.
Stenar H. 1928b. Zur Embryologie der Asphodeline-Gruppe. Ein Beitrag zur systematischen Stellung der Gattungen Bulbine und Paradisia. – Svensk Bot. Tidskr. 22: 145-159.
Stenar H. 1933. Zur Embryologie der Agapanthus-Gruppe. – Bot. Not. 1933: 520-530.
Stenar H. 1949. Zur Kenntnis der Embryologie und der Raphiden-Zellen bei Bowiea volubilis Harvey und anderen Liliaceen. – Acta Horti Berg. 15: 45-63.
Stenar H. 1950. Studien über das Endospermbei Galtonia candicans (Bak.) Decne und anderen Scilloideen. – Acta Horti Berg. 15: 169-184.
Stenar H. 1951. Zur Embryologie von Haemanthus Katherinae Bak. Erörterungen über das helobiale Endosperm in den Amaryllidaceae und Liliaceae. – Acta Horti Berg. 16: 57-72.
Stenar H. 1953. The embryo sac type in Smilacina, Polygonatum and Theropogon. – Phytomorphology 3: 326-338.
Stenzel H. 2000. Pollen morphology of the subtribe Pleurothallidinae Lindl. (Orchidaceae). – Grana 39: 108-125.
Stenzel H. 2001. New species of Orchidaceae subtribe Pleurothallidinae from Cuba. – Lindleyana 16: 26-30.
Stenzel H. 2002. New species of Platystele and Pleurothallis (Orchidaceae) from Cuba. – Willdenowia 32: 99-104.
Sterling C. 1974. Comparative morphology of the carpel in the Liliaceae: Baeometra, Burchardia, and Walleria. – Bot. J. Linn. Soc. 68: 115-125.
Sterling C, Huang S-M. 1972. Notes on the laticifers of Allium, Caloscordum, Nothoscordum, Tristagma, and Tulbaghia. – Plant Life 28: 43-46.
Stern FC. 1956. Snowdrops and snowflakes. A study of the genera Galanthus and Leucojum. – London.
Stern WL. 1993. Comparative vegetative anatomy and systematics of Spiranthoideae (Orchidaceae). – Bot. J. Linn. Soc. 113: 161-197.
Stern WL. 1997a. Vegetative anatomy of subtribe Orchidinae (Orchidaceae). – Bot. J. Linn. Soc. 124: 121-136.
Stern WL. 1997b. Vegetative anatomy of subtribe Habenariinae (Orchidaceae). – Bot. J. Linn. Soc. 124: 211-227.
Stern WL, Carlsward BS. 2006. Comparative vegetative anatomy and systematics of the Oncidiinae (Maxillarieae, Orchidaceae). – Bot. J. Linn. Soc. 152: 91-107.
Stern WL, Carlsward BS. 2009. Comparative vegetative anatomy and systematics of Laeliinae (Orchidaceae). – Bot. J. Linn. Soc. 160: 21-41.
Stern WL, Judd WS. 1999. Comparative vegetative anatomy and systematics of Vanilla (Orchidaceae). – Bot. J. Linn. Soc. 131: 353-382.
Stern WL, Judd WS. 2000. Comparative anatomy and systematics of the orchid tribe Vanilleae excluding Vanilla. – Bot. J. Linn. Soc. 134: 179-202.
Stern WL, Judd WS. 2001. Comparative anatomy and systematics of Catasetinae (Orchidaceae). – Bot. J. Linn. Soc. 136: 153-178.
Stern WL, Judd WS. 2002. Systematic and comparative anatomy of Cymbidieae (Orchidaceae). – Bot. J. Linn. Soc. 139: 1-27.
Stern WL, Warcup JH. 1994. Root tubercles in apostasioid orchids. – Amer. J. Bot. 81: 1571-1575.
Stern WL, Whitten WM. 1999. Comparative vegetative anatomy of Stanhopeinae (Orchidaceae). – Bot. J. Linn. Soc. 129: 87-103.
Stern WL, Cheadle VA, Thorsch J. 1993. Apostasiads, systematic anatomy, and the origins of Orchidaceae. – Bot. J. Linn. Soc. 111: 411-455.
Stern WL, Morris MW, Judd WS, Pridgeon AM, Dressler RL. 1993. Comparative vegetative anatomy and systematics of Spiranthoideae (Orchidaceae). – Bot. J. Linn. Soc. 113: 161-197.
Stern WL, Aldrich HC, McDowell LM, Morris MW, Pridgeon AM. 1993. Amyloplasts from cortical root cells of Spiranthoideae (Orchidaceae). – Protoplasma 172: 49-55.
Stern WL, Judd WS, Carlsward BS. 2004. Systematic and comparative anatomy of Maxillarieae (Orchidaceae). – Bot. J. Linn. Soc. 144: 251-274.
Stévart T, Cribb PJ. 2004. New species and records of Orchidaceae from São Tomé and Príncipe. – Kew Bull. 59: 77-86.
Stévart T, Nguema N. 2004. Trois espèces et trois combinaisons nouvelles de Polystachya (Orchidaceae) du Cameroun, de Guinée Équatoriale et du Gabon. – Adansonia, sér. III, 26: 217-233.
Stévart T, Parmentier I, Droissart V. 2007. Deux espèces nouvelles de Polystachya (Orchidaceae) de Guinée Équatoriale. – Adansonia, sér. III, 29: 31-38.
Stevens PF. 1978. Generic limits in the Xeroteae (Liliaceae sensu lato). – J. Arnold Arbor. 59: 129-155.
Stevenson DW. 1973. Phyllode theory in relation to leaf ontogeny in Sansevieria trifasciata. – Amer. J. Bot. 60: 387-395.
Steward AN. 1930. The Polygoneae of Eastern Asia. – Contr. Gray Herb. Harvard Univ. 5: 1-129.
Stewart DA, Barlow BA. 1976. Genomic differentiation and polyploidy in Sowerbaea (Liliaceae). – Aust. J. Bot. 24: 349-367.
Stewart J, la Croix IF. 1987. Notes on the orchids of southern tropical Africa III: Aerangis. – Kew Bull. 42: 215-219.
Steyn EMA, Smith GF. 1998. Ovule orientation, curvature and internal structure in the Aloaceae. – South Afr. J. Bot. 64: 192-197.
Steyn EMA, Smith GF. 2001. Are ovules and seeds in Lomatophyllum Willd. (Aloe Sect. Lomatophyllum sensu auct.) anatropous and exarillate? – Bothalia 31: 237-240.
Steyn EMA, Smith GF, Nilsson S, Grafström E. 1998. Pollen morphology in Aloe (Aloaceae). – Grana 37: 23-27.
Stirton CH. 1976. Thuranthos: notes on generic status, morphology, phenology and pollination biology. – Bothalia 12: 161-165.
Stockhouse RE, Wells H. 1978. Pollination ecology of Chlorogalum pomeridianum (DC.) Kunth (Liliaceae). – Bull. South. Calif. Acad. Sci. 77: 124-129.
Stpiczyńska M, Davies KL. 2008. Elaiophore structure and oil secretion in flowers of Oncidium trulliferum Lindl. and Ornithophora radicans (Rchb. f.) Garay & Pabst (Oncidiinae: Orchidaceae). – Ann. Bot. 101: 375-384.
Stpiczyńska M, Davies KL, Gregg A. 2007. Elaiophore diversity in three contrasting members of Oncidiinae (Orchidaceae). – Bot. J. Linn. Soc. 155: 135-148.
Stpiczyńska M, Davies KL, Pacek-Bieniek A, Kamińska M. 2013. Comparative anatomy of the floral elaiophore in representatives of the newly re-circumscribed Gomesa and Oncidium clades (Orchidaceae: Oncidiinae). – Ann. Bot. 112: 839-854.
Strid AK. 1974. A taxonomic revision of Bobartia L. (Iridaceae). – Opera Bot. 37: 1-45.
Stringer S, Conran JG. 1991. Stamen and seed cuticle morphology in some Arthropodium and Dichopogon species (Anthericaceae). – Aust. J. Bot. 39: 129-135.
Stuart DC. 1965. Muscari and allied genera. A lily group discussion. – The Lily Year Book 29: 125-138.
Stuart DC. 1970. Chromosome numbers in the genus Muscari Mill. – Notes Roy. Bot. Gard. Edinb. 30: 189-196.
Su H-J. 2002. The systematic position of genus Didiciea (Orchidaceae) and its occurrence in Taiwan. – Taiwania 47: 232-238.
Suárez-Santiago VN, Salinas MJ, Romero-García AT, Garrido-Ramos MA, Herrán R de la, Ruiz-Rejón C, Ruiz-Rejón M, Blanca G. 2007. Polyploidy, the major speciation mechanism in Muscari subgenus Botryanthus in the Iberian Peninsula. – Taxon 56: 1171-1184.
Subramanian SS, Nair AGR. 1970. Chlorogenin and kaempferol glycosides from the flowers of Agave americana. – Phytochemistry 9: 2582.
Summerhayes VS. 1927. African orchids I. – Kew Bull. 1927: 415-419.
Summerhayes VS. 1936. African orchids VIII. – Kew Bull. 1936: 221-233.
Summerhayes VS. 1937. African orchids IX. – Kew Bull. 1937: 457-476.
Summerhayes VS. 1943. African orchids XIII. – Bot. Mus. Leafl. Harvard Univ. 11: 137-170.
Summerhayes VS. 1945. African orchids XV. – Bot. Mus. Leafl. Harvard Univ. 11: 249-260.
Summerhayes VS. 1953. African orchids XXI. – Kew Bull. 1953: 129-162.
Summerhayes VS. 1955. A revision of the genus Brachycorythis. – Kew Bull. 1955: 221-264.
Summerhayes VS. 1956. African orchids XXIII. – Kew Bull. 11: 217-236.
Summerhayes VS. 1957. African orchids XXIV. – Kew Bull. 12: 107-126.
Summerhayes VS. 1958a. African orchids XXV. – Kew Bull. 13: 57-87.
Summerhayes VS. 1958b. African orchids XXVI. – Kew Bull. 13: 257-281.
Summerhayes VS. 1960. African orchids XXVII. – Kew Bull. 14: 126-159.
Summerhayes VS. 1966. African orchids XXX. – Kew Bull. 20: 165-199.
Summerhayes VS. 1968. Orchidaceae (Part 1). – In: Milne-Redhead E, Polhill RM (eds), Flora of tropical East Africa, Crown Agents for Oversea Governments and Administrations, London, pp. 1-235.
Sundermann H. 1977. Neue Beiträge zur Zytotaxonomie der Erdorchideen II (Ophrys, Orchis, Neotinea, Steveniella und Gennaria). – Orchidee 28: 146-147.
Suomalainen E. 1947. On the cytology of the genus Polygonatum group Alternifolia. – Ann. Acad. Sci. Fenn., Ser. A, 13: 1-65.
Supaibulwatana K, Mii M. 1997. Organogenesis and somatic embryogenesis from young flower buds of Agapanthus africanus Hoffmanns. – Plant Biotechn. 14: 23-28.
Svoma E. 1981. Zur systematischen Embryologie der Gattung Scilla L. (Liliaceae). – Stapfia 9: 1-124.
Svoma E, Greilhuber J. 1988. Studies on systematic embryology in Scilla (Hyacinthaceae). – Plant Syst. Evol. 161: 169-181.
Svoma E, Greilhuber J. 1989. Systematic embryology of the Scilla siberica alliance (Hyacinthaceae). – Nord. J. Bot. 8: 585-600.
Swamy BGL. 1946. Embryology of Habenaria. – Proc. Indian Natl. Inst. Sci., Sect. B, 12: 413-426.
Swamy BGL. 1947. On the life history of Vanilla planifolia. – Bot. Gaz. 108: 449-459.
Swamy BGL. 1948. Vascular anatomy of orchid flowers. – Bot. Mus. Leafl. Harvard Univ. 13: 61-95.
Swamy BGL. 1949. Embryological studies in the Orchidaceae II. Embryogeny. – Amer. Midl. Natur. 41: 202-232.
Sweet HR. 1970. Orquideas andinas poco conocidas III. Xerorchis Schltr. – Orchideologia 5: 89-95.
Sýkorová E, Fajkus J, Mezniková M, Lim KY, Neplechová K, Blattner FR, Chase MW, Leitch AR. 2006. Minisatellite telomeres occur in the family Alliaceae but are lost in Allium. – Amer. J. Bot. 93: 814-823.
Sýkorová E, Lim KY, Kunicka Z, Chase MW, Bennett MD, Fajkus J, Leitch AR. 2003. Telomere variability in the monocotyledonous plant order Asparagales. – Proc. Roy. Soc. London, Ser. B, 270: 1893-1904.
Szelubsky R. 1950. Caryology and morphology of some Palestinian species of Allium. – Palest. J. Bot., Jer. Ser., 5: 1-12.
Szlachetko DL. 1991. Thelymitroideae, a new subfamily within Orchidaceae. – Fragm. Florist. Geobot. 36: 33-49.
Szlachetko DL. 1992a. Notes on Eurystyles (Orchidaceae), with a description of a new species from Mesoamerica. – Fragm. Flor. Geobot. 37: 13-19.
Szlachetko DL. 1992b. Genera and species of the subtribe Spiranthinae (Orchidaceae) 2. A revision of Schiedeella. – Fragm. Flor. Geobot. 37: 157-204.
Szlachetko DL. 1993. Schiedeella romeroana (Orchidaceae, Spiranthinae), a new and interesting species from Mexico. – Rhodora 95: 1-5.
Szlachetko DL. 1995a. Systema orchidalium. – Fragm. Florist. Geobot. [Suppl.] 3: 1-152.
Szlachetko DL. 1995b. Two new species of the genus Pelexia (Orchidaceae, Spiranthinae) from South America. – Nord. J. Bot. 15: 173-175.
Szlachetko DL. 2001a. Genera et species orchidalium I. – Polish Bot. J. 46: 11-26.
Szlachetko DL. 2001b. Nomenclatural adjustments in the Thelymitroideae (Orchidaceae). – Polish Bot. J. 46: 137-144.
Szlachetko DL. 2003a. Senghasia, eine neue Gattung der Zygopetaleae. – J. Orchideenfreund 10: 332-344.
Szlachetko DL. 2003b. Genera et species Orchidalium. 7. Vandeae. – Ann. Bot. Fennici 40: 67-70.
Szlachetko DL. 2003c. Nomenclatural adjustments in Caladeniinae (Orchidaceae, Thelymitroideae). – Ann. Bot. Fenn. 40: 143-145.
Szlachetko DL, Górniak M. 2006. New taxa in the subtribe Oncidiinae. – Biodivers. Res. Conservation 1-2: 11-13.
Szlachetko DL, Kolanowska M. 2015. Reconsideration of Heteranthocidium (Oncidiinae, Orchidaceae): new species and taxonomic transfers. – Plant Syst. Evol. 301: 1793-1805.
Szlachetko DL, Kolanowska M. 2016. Notes on the genus Selenipedium (Orchidaceae, Cypripedioideae) with descriptions of new taxa. – Syst. Bot. 41: 142-159.
Szlachetko DL, Kolanowska M. 2017. Synopsis of the genus Ocampoa (Orchidaceae). – Ann. Missouri Bot. Gard. 102: 710-729.
Szlachetko DL, Margonska HB. 2002. Gynostemia orchidalium II. Orchidaceae (Epidendroideae). – Acta Bot. Fenn. 173: 1-275.
Szlachetko DL, Mytnik-Ejsmont J. 2009. Gynostemia orchidalium IV. – Acta Bot. Fenn. 180: 1-313.
Szlachetko DL, Romowicz A. 2006. Notes sur le genre Senghasia Szlachetko (Orchidaceae, Huntleyinae). Richardiana 6: 180-182.
Szlachetko DL, Romowicz A. 2007. Dolabrifolia, un nouveau genre d’orchidées de l’alliance Angraecum. – Richardiana 7: 53-54.
Szlachetko DL, Rutkowski P. 2000. Gynostemia orchidalium I. Apostasiaceae, Cypripediaceae, Orchidaceae (Thelymitroideae, Orchidoideae, Tropidioideae, Spiranthoideae, Neottioideae, Vanilloideae). – Acta Bot. Fenn. 169: 1-380.
Szlachetko DL, Margonska HB. 2002. Gynostemia orchidalium II. Orchidaceae (Epidendroideae). – Acta Bot. Fenn. 173: 1-275.
Szlachetko DL, Rutkowski P. 2003. Quelques note additionelles sur la sous-famille Thelymitroideae (Orchidaceae). – Richardiana 3: 90-100.
Szlachetko DL, Rutkowski P, Mytnik J. 2005. Contributions to the taxonomic revision of the subtribes Spiranthinae, Stenorrhynchidinae and Cyclopogoninae (Orchidaceae) in Mesoamerica and the Antilles. – Polish Bot. Stud. 20: 3-387.
Szlachetko DL, González Tamayo R, Rutkowski P. 2000. Zhukowskia, a new orchid genus from Mesoamerica. – Adansonia, sér. III, 22: 235-238.
Szlachetko DL, Mytnik-Ejsmont J, Romowicz A.
2006. Genera et species orchidalium 14. Oncidieae. – Polish Bot. J. 51:
53-55.
Szlachetko DL, Mytnik-Ejsmont J, Nowak S,
Kolanowska M. 2014. Revision of the genus Myrosmodes (Orchidaceae, Spiranthoideae) in
Colombia. – Syst. Bot. 39: 740-749.
Szlachetko DL, Baranow P, Dudek M. 2018. Materials towards taxonomic revision of the genus Palmorchis (Orchidaceae). – Syst. Bot. 43: 130-152.
Takahashi H, Niwa S, Ukai A. 1988. Germination characteristics of Hosta sieboldiana (Lodd.) Engler, H. albomarginata (Hook.) Ohwi and H. longissima Honda. – Sci. Rep. Fac. Educ., Gifu Univ. (Nat. Sci.) 12: 3-10.
Takahashi H, Goto Y, Kanematsu S, Niwa S, Mori K, Nozaki K. 1994. Pollination biology of Hosta sieboldiana (Lodd.) Engler and H. sieboldii (Paxton) J. Ingram (Liliaceae). – Plant Species Biol. 9: 23-30.
Takahashi M, Konno C, Hikino H. 1985. Isolation and hypoglycaemic activity of anemarans A, B, C and D, glycans of Anemarrhena asphodeloides rhizomes. – Planta Med. 1985: 100-102.
Tamanyan KG. 1988. Pollen morphology of Asparagus L. – Flora rastitel “nost” i rastitel’nye resursy (Armenskoj SSR) 11: 96-102. [In Russian]
Tamayo RG, Szlachetko DL. 1998. A new
definition of the genus Tamayorkis. – Ann. Bot. Fennici 35:
21-27.
Tamura MN. 1990. Biosystematic studies on the genus Polygonatum (Liliaceae) I. Karyotype analysis of species indigenous to Japan and its adjacent regions. – Cytologia 55: 443-466.
Tamura MN. 1991. Biosystematic studies on the genus Polygonatum (Liliaceae) II. Morphology of staminal filaments of species indigenous to Japan and its adjacent regions. – Acta Phytotaxon. Geobot. 42: 1-18.
Tamura MN. 1993. Biosystematic studies on the genus Polygonatum (Liliaceae) III. Morphology of staminal filaments and karyology of eleven Eurasian species. – Bot. Jahrb. Syst. 115: 1-26.
Tamura MN. 1995. A karyological review of the orders Asparagales and Liliales (Monocotyledonae). – Feddes Repert. 106: 83-111.
Tamura MN, Ogisu M, Xu J-M. 1997. Heteropolygonatum, a new genus of the tribe Polygonateae (Convallariaceae) from West China. – Kew Bull. 52: 949-956.
Tamura MN, Schwarzbach AE, Kruse S, Reski R. 1997. Biosystematic studies on the genus Polygonatum (Convallariaceae) IV. Molecular phylogenetic analysis based on restriction site mapping of the chloroplast gene trnK. – Feddes Repert. 108: 159-168.
Tan K. 1969. The systematic status of the genus Bletilla (Orchidaceae). – Brittonia 21: 202-214.
Tanaka N. 2003. Inclusion of Tricalistra and Gonioscypha muricata in Tupistra (Convallariaceae). – Novon 13: 334-336.
Tanaka R. 1965. Chromosome numbers of some species of Orchidaceae from Japan and its neighbouring areas. – J. Jap. Bot. 40: 65-77.
Tanaka R. 1971. Types of resting nuclei in Orchidaceae. – Bot. Mag. (Tokyo) 84: 118-122.
Tanaka R. 1976. Cytological studies on the wide-crossing in Orchidaceae. – Rec. Adv. Breedings 12: 95-112. [In Japanese]
Tang T, Wang F-T. 1951a. Contributions to the knowledge of eastern Asiatic orchids 2. – Acta Phytotaxon. Sin. 1: 24-102. [In Chinese]
Tang T, Wang F-T. 1951b. On the identity of eight Gagnepain’s orchidaceous genera from Indo-China. – Acta Scient. Sin. 2: 312-322.
Tang T, Wang F-T, Lang K-L. 1980. Materiae ad genus orchidem L. sinicam. – Acta Phytotaxon. Sin. 18: 408-419.
Taylor WR. 1925. Cytological studies on Gasteria II. A comparison of the chromosomes of Gasteria, Aloë, and Haworthia. – Amer. J. Bot. 12: 219-223.
Tedersoo L, Pellet P, Kõljalg U, Selosse M-A. 2007. Parallel evolutionary paths to mycoheterotrophy in understorey Ericaceae and Orchidaceae: ecological evidence for mixotrophy in Pyroleae. – Oecologia 151: 206-217.
Teixeira de Souza-Chies T, Bittar G, Nadot S, Carter L, Besin E, Lejeune B. 1997. Phylogenetic analysis of Iridaceae with parsimony and distance methods using the plastid gene rps2. – Plant Syst. Evol. 204: 109-123.
Telepova-Texier M. 2009. Acampe hulae Telepova (Orchidaceae), une nouvelle espèce du Cambodge et du Laos. – Adansonia, sér. III, 31: 267-272.
Terekhin ES, Kamelina OP. 1969. Endosperm of the Orchidaceae. – Bot. Žurn. 54: 657-666. [In Russian]
Therman E. 1951. Somatic and secondary pairing in Ornithogalum. – Heredity 5: 253-269.
Therman E. 1953. Chromosomal evolution in the genus Polygonatum. – Hereditas 39: 277-288.
Therman E. 1956. Cytotaxonomy of the tribe Polygonateae. – Amer. J. Bot. 43: 134-142.
Therman-Suomalainen E. 1949. Investigations on secondary constrictions in Polygonatum. – Hereditas 35: 86-108.
Thiv M. 1994. Beitrag zur Kenntnis der Gattung Macroclinium Barb. Rodr. – J. Orchideenfreund 2: 22-27.
Thomas S, Cribb PJ. 1996. New species of Habenaria (Orchidaceae) from Ethiopia. – Kew Bull. 51: 145-154.
Thompson MF. 1976. Studies in the Hypoxidaceae I. Vegetative morphology and anatomy. – Bothalia 12: 111-117.
Thompson MF. 1978. Studies in the Hypoxidaceae II. Floral morphology and anatomy. – Bothalia 12: 429-435.
Thompson MF. 1979. Studies in the Hypoxidaceae III. The genus Pauridia. – Bothalia 12: 621-625.
Thongpukdee A. 1989. The taxonomic position of the genus Tricoryne R. Br. – Ph.D. diss., University of Queensland, St. Lucia.
Thorne FM. 1965. The taxonomy of the genus Nolina Michx. (Agavaceae) in the southeastern United States. – Ph.D. diss., University of Georgia, Athens, Georgia.
Thorsch J, Stern WL. 1997. Tracheary studies and the terrestrial ancestry of Orchidaceae. – Intern. J. Plant Sci. 158: 222-227.
Thulin M. 1995. Trachyandra triquetra (Asphodelaceae), a new species from the Horn of Africa. – Nord. J. Bot. 15: 571-573.
Tidwell WD, Parker LR. 1990. Protoyucca shadishii gen. et spec. nov., an arborescent monocotyledon with secondary growth from the Middle Miocene of northwestern Nevada, USA. – Rev. Palaeobot. Palynol. 62: 79-95.
Tillich H-J. 1995. Früchte, Samen und Keimpflanzen bei den Cyanastraceae Engler 1900 und einigen vermuteten Verwandten. – Feddes Repert. 106: 483-493.
Tillich H-J. 2003. Seedling morphology in Iridaceae: indications for relationships within the family and to related families. – Flora 198: 220-242.
Tillich H-J. 2005. A key for Aspidistra (Ruscaceae), including fifteen new species from Vietnam. – Feddes Repert. 116: 313-338.
Tillich H-J. 2006. Four new species in Aspidistra Ker-Gawl. (Ruscaceae) from China, Vietnam and Japan. – Feddes Repert. 117: 139-145.
Tillich H-J. 2008. An updated and improved determination key for Aspidistra Ker-Gawl. (Ruscaceae, Monocotyledons). – Feddes Repert. 119: 449-462.
Tillich H-J, Averyanov LV. 2008. Two new species and one new subspecies of Aspidistra Ker-Gawl. (Ruscaceae) from Vietnam. – Feddes Repert. 119: 37-41.
Tillie N, Chase MW, Hall T. 2001. Molecular studies in the genus Iris: a preliminary study. – In: Colasante MA, Rudall PJ (eds), Irises and Iridaceae: biodiversity and systematics, Ann. Bot. (Roma), N. S., 1: 105-112.
Tilton VR. 1980a. The nucellar epidermis and micropyle of Ornithogalum caudatum (Liliaceae) with a review of these structures in other taxa. – Can. J. Bot. 58: 1872-1884.
Tilton VR. 1980b. Hypostase development in Ornithogalum caudatum (Liliaceae) and notes on other types of modifications in the chalaza of angiosperm ovules. – Can. J. Bot. 58: 2059-2066.
Tilton VR. 1981. Ovule development in Ornithogalum caudatum (Liliaceae) with a review of selected papers on angiosperm reproduction IV. Egg apparatus structure and function. – New Phytol. 88: 505-531.
Tilton VR, Horner HT Jr. 1980. Stigma, style, and obturator of Ornithogalum caudatum (Liliaceae) and their function in the reproductive process. – Amer. J. Bot. 67: 1113-1131.
Tilton VR, Horner HT Jr. 1983. Carpel development, anatomy, and function in the reproductive process in Ornthogalum caudatum (Liliaceae). – Flora 173: 1-31.
Titova GE. 2003. Algorithms of embryo morphogenesis in Agapanthus praecox Willd. (Alliaceae) in monocotyly, dicotyly and transitional forms. – Acta Biol. Cracov., Ser. Bot. 45: 161-165.
Tohda H. 1983. Seed morphology in Orchidaceae I. Dactylorchis, Orchis, Ponerorchis, Chondradenia and Galeorchis. – Sci. Rep. Tôhoku Imp. Univ., Ser. IV, Biology 38: 253-268.
Tohda H. 1985. Seed morphology in Orchidaceae II. Tribe Cranichideae. – Sci. Rep. Tôhoku Imp. Univ., Ser. IV, Biology 39: 21-43.
Tohda H. 1986. Seed morphology in Orchidaceae III. Tribe Neottieae. – Sci Rep. Tôhoku Imp. Univ., Ser. IV, Biology 39: 103-119.
Tomita K. 1931. Über die Entwicklung des nackten Embryos von Crinum latifolium L. – Sci. Rep. Tôhoku Imp. Univ., ser. IV, Biology, 6: 163-169.
Tomlinson PB. 1965. Notes on the anatomy of Aphyllanthes (Liliaceae) and comparison with Eriocaulaceae. – Bot. J. Linn. Soc. 59: 163-173.
Tomlinson PB, Fisher JB. 1971. Morphological studies in Cordyline (Agavaceae) I. Introduction and general morphology. – J. Arnold Arbor. 52: 459-478.
Topik H, Yukawa T, Ito M. 2005. Molecular phylogenetics of subtribe Aeridinae (Orchidaceae): insights from plastid matK and nuclear ribosomal ITS sequences. – J. Plant Res. 118: 271-284.
Tören J. 1968. Mégasporogénèse et la formation du sac embryonnaire chez Scilla amoena L. – Istanbul Univ. Fen Fak. Mec., ser. B, 33: 1-10.
Tornadore N, Garbari F. 1979. Il genere Ornithogalum L. (Liliaceae) in Italia 3. Contributo alla revisione citotassonomica. – Webbia 33: 379-423.
Tornadore N, Marcucci R, Garbari F. 2003. Ornithogalum umbratile (Hyacinthaceae), a new species from Gargano’s promontory, southeastern Italy. – Taxon 52: 577-582.
Toscano de Brito AL. 1992a. Two new species of Habenaria (Orchidaceae) from Bahia, Brazil. – Kew Bull. 47: 731-735.
Toscano de Brito AL. 1992b. Notes on Brazilian Orchidaceae I. – Kew Bull. 47: 774.
Toscano de Brito AL, Luer CA. 1993. Notes on Brazilian Orchidaceae II. – Kew Bull. 48: 326.
Trapnell DW, Hamrick JL, Giannasi DE. 2004. Genetic variation and species boundaries in Calopogon (Orchidaceae). – Syst. Bot. 29: 308-315.
Traub HP. 1943. The Ixiolirion tribe. – Herbertia 9: 53-59.
Traub HP. 1951. Amaryllid notes. – Plant Life 7: 40-43.
Traub HP. 1952. Biosystematic experiments involving Zephyranthes, Habranthus and Amaryllis. – Taxon 8: 121-123.
Traub HP. 1953.The genera Rhodophiala Presl, Phycella Lindl. and Amaryllis L. – Plant Life 9: 59-63.
Traub HP. 1957a. Genus Rauhia and R. peruviana gen. & sp. nov. – Plant Life 13: 73-75.
Traub HP. 1957b. Classification of the Amaryllidaceae – subfamilies, tribes, and genera. – Plant Life 13: 76-83.
Traub HP. 1962. Key to the subgenera, alliances and species of Hymenocallis. – Plant Life 18: 55-72.
Traub HP. 1963a. The genera of Amaryllidaceae. – The American Plant Life Society, La Jolla, California.
Traub HP. 1963b. Tristagma Poepp. – Plant Life 19: 60-61.
Traub HP. 1965. Addenda to Traub’s “The genera of the Amaryllidaceae (1963)”. – Plant Life 21: 88-89.
Traub HP. 1966. Polyembryony in Hymenocallis mexicana. – Plant Life 22: 49.
Traub HP. 1968a. New Guatemalan and Mexican alliums – Plant Life 24: 127-142.
Traub HP. 1968b. The subgenera, sections and subsections of Allium L. – Plant Life 24: 147-163.
Traub HP. 1970. An introduction to Herbert’s “Amaryllidaceae, etc.” 1837 and related works. Reprint. – J. Cramer, Lehre, Germany.
Traub HP. 1972a. The order Alliales. – Plant Life 28: 129-132.
Traub HP. 1972b. Genus Allium L. – subgenera, sections and subsections. – Plant Life 28: 132-137.
Traub HP. 1976. Tribe Gilliesieae, family Alliaceae, order Alliales. – Plant Life 32: 124-126.
Traub HP. 1980. Sections and alliances, genus Hymenocallis Salisb. – Plant Life 36: 46-49.
Traub HP. 1982. Order Alliales. – Plant Life 38: 119-132.
Traub HP, Moldenke HN. 1949. Amaryllidaceae: tribe Amarylleae. – American Plant Life Society, La Jolla, California.
Traub HP, Moldenke HN. 1955. The genus Ipheion, diagnosis, key to species and synonymy. – Plant Life 11: 125-130.
Trelease W. 1893. Further studies of yuccas and their pollination. – Ann. Rep. Missouri Bot. Gard. 4: 181-226.
Trelease W. 1902. The Yucceae. – Ann. Rep. Missouri Bot. Gard. 13: 27-133.
Trelease W. 1910. Observations on Furcraea. – Extr. Ann. Jard. Bot. Buitenzorg, ser. II, Suppl. III.
Trelease W. 1911. The desert group Nolineae. – Proc. Amer. Philos. Soc. 50: 404-443.
Tremblay RL, Ackerman JD, Zimmerman JK, Calvo RN. 2005. Variation in sexual reproduction in orchids and its evolutionary consequences: a spasmodic journey to diversification. – Biol. J. Linn. Soc. 84: 1-54.
Treub M. 1879. Notes sur l’embryogénie de quelques Orchidées. – Verh. Kon. Ned. Akad. Wet. Afd. Natuurk. 19: 1-50.
Treutlein J, Smith GF, Wyk B-E van, Wink M. 2003. Phylogenetic relationships in Asphodelaceae (subfamily Alooideae) inferred from chloroplast DNA sequences (rbcL, matK) and from genomic finger-printing (ISSR). – Taxon 52: 193-207.
Treutlein J, Smith GF, Wyk B-E van, Wink M. 2003. Evidence for the polyphyly of Haworthia (Asphodelaceae subfamily Alooideae; Asparagales) inferred from nucleotide sequences of rbcL, matK, ITS1 and genomic fingerprinting with ISSR-PCR. – Plant Biol. 5: 513-521.
Trueblood EWE. 1973. Omixochitl – the tuberose (Polianthes tuberosa). – Econ. Bot. 27: 157-173.
Tsai C-C, Huang S-C, Chou C-H. 2005. Molecular phylogeny of Phalaenopsis Blume (Orchidaceae) based on the internal transcribed spacer of the nuclear ribosomal DNA. – Plant Syst. Evol. 256: 1-16.
Tsai C-C, Huang S-C, Chou C-H. 2009. Phylogenetics, biogeography, and evolutionary trends of the Phalaenopsis sumatrana complex inferred from nuclear and chloroplast DNA. – Biochem. Syst. Ecol. 37: 633-639.
Tscholokaschvili N. 1975. Ad cognitionem systematis generis Allium. – Zam. Sist. Geogr. Rast. (Tbilisi) 31: 36-54.
Tso CL. 1933. Notes on the orchid flora of Kwangtung. – Sunyatsenia 1: 131-156.
Tsutsumi C, Yukawa T, Lee NS, Lee CS, Kato M. 2007. Phylogeny and comparative seed morphology of epiphytic and terrestrial species of Liparis (Orchidaceae) in Japan. – J. Plant Res. 120: 405-412.
Tullio Luisina de, Roitman Germán, Bernardello G. 2008. Tamia (Iridaceae), a synonym of Calydorea: cytological and morphological evidence. – Syst. Bot. 33: 509-513.
Turner BL. 1993. Jaimehintonia (Amaryllidaceae: Allieae), a new genus from northeastern Mexico. – Novon 3: 86-88.
Tuzlaci E. 1987. Revision of the genus Asphodeline (Liliaceae) – a new infrageneric classification. – Candollea 42: 559-576.
Tyteca D, Dufrene M. 1994. Biostatistical studies of Western European allogamous populations of the Epipactis helleborine (L.) Crantz species group (Orchidaceae). – Syst. Bot. 424-442.
Tyteca D, Ceinos M, Gathoye J-L, Brys R, Jacquemyn H. 2012. On the morphological, biological and genetic heterogeneity of the genus Orchis (Orchidaceae, Orchidinae). – Phytotaxa 75: 19-32.
Tzagolova VG. 1977. K sistematike sektsii Molium G. Don Kazakhstanskikh lukov. – Botg. Mat. Gerb. Inst. Bot. Akad. Nauk Kaz. SSR 10: 13-14.
Tzanoudakis D. 1983a. Karyotypes of ten taxa of Allium sect. Scorodon from Greece. – Caryologia 36: 259-284.
Tzanoudakis D. 1983b. New taxa of Allium from Greece. – Candollea 38: 317-323.
Tzanoudakis D. 1992. Karyotype variation and evolution in the Greek Allium. – In: Hanelt P, Hammer K, Knüpffer H (eds), The genus Allium – taxonomic problems and genetic resources. Proceedings of an International Symposium held at Gatersleben, Germany, June 11-13, 1991, pp. 305-320.
Tzanoudakis D. 2000. The genus Allium in Greece, species and identification keys. – In: Kamari G, Psaras G, Konstantinidis T (eds), Proceedings of the 8th scientific congress of the Greek Botanical Society, Patras, pp. 405-418.
Tzanoudakis D. 2001. The genus Allium in Greece: a genus representative of the floristic richness and diversity of the area. – In: Özhatay N (ed), Plants of the Balkan Peninsula: into the next millennium 1, Istanbul, pp. 353-358.
Tzanoudakis D, Kypriotakis Z. 2008. Allium brussalisii (Alliaceae), a new species from Greece. – Bot. J. Linn. Soc. 158: 140-146.
Tzanoudakis D, Vosa CG. 1988. The cytogeographical distribution pattern of Allium (Alliaceae) in the Greek Peninsula and Islands. – Plant Syst. Evol. 159: 193-215.
Tzanoudakis D, Iatrou G, Kypriotakis Z, Christodoulakis D. 1991. Cytogeographical studies in some Aegean Liliaceae. – Bot. Chron. 10: 761-775.
Uitewaal AJA. 1940. Een nieuw geslacht der Aloineae. – Succulenta (Leeuwarden) 22: 61-64.
Uitewaal AJA. 1947. A first attempt to subdivide the genus Haworthia, based on floral characters. – Desert Plant Life 19: 133-136.
Utech FH. 1979. Floral vascular anatomy of the Himalayan Theropogon pallidus Maxim. (Liliaceae-Convallarieae). – Ann. Carnegie Mus. 48: 25-41.
Uysal T, Ertugrul K, Dural H. 2005. A new species of Ornithogalum (Liliaceae) from South Anatolia, Turkey. – Bot. J. Linn. Soc. 148: 501-504.
Uysal T, Ertugrul K, Dural H, Kucukoduk M. 2007. Muscari turcicum (Liliaceae/Hyacinthaceae), a new species from South Anatolia, Turkey. – Bot. J. Linn. Soc. 154: 233-236.
Vaikos NP, Pai RM. 1982. The floral anatomy of Kniphofia uvaria Hook. (Liliaceae: Kniphofieae). – Proc. Indian Acad. Sci., Plant Sci., 91: 351-356.
Vaikos NP, Pai RM. 1986. The floral anatomy of Bowiea volubilis Harv. – J. Indian Bot. Soc. 65: 516-518.
Vaikos NP, Markandeya SK, Pai RM. 1978. The floral anatomy of the Liliaceae: the tribe Aloineae. – Indian J. Bot. 1: 61-68.
Vaikos NP, Markandeya SK, Pai RM. 1981. The floral anatomy of the Liliaceae: the tribe Hemerocallideae. – J. Indian Bot. Soc. 60: 222-231.
Vaikos NP, Markandaya SK, Pai RM. 1989. The floral anatomy of the Liliaceae: the tribe Convallarieae. – Proc. Indian Acad. Sci., Plant Sci., 99: 91-95.
Vakhtina LJ. 1964. The chromosome numbers of some Allium species distributed in USSR. – Bot. Žurn. 49: 870-875. [In Russian]
Vakhtina LJ. 1965. A comparative caryological investigation of some species of Allium belonging to the section Rhizirideum Don. – Bot. Žurn. 50: 387-394. [In Russian]
Vakhtina LJ. 1969. A comparative caryological investigation of some species of Allium belonging to the section Molium Don. – Bot. Žurn. 54: 143-150. [In Russian]
Valente LM, Savolainen V, Manning JC, Goldblatt P, Vargas P. 2011. Explaining disparities in species richness between Mediterranean floristic regions: a case study in Gladiolus (Iridaceae). – Global Ecol. Biogeogr. 20: 881-892.
Vargas C. 1975. Contribution to Peruvian Amaryllidaceae. – Plant Life 31: 27-32.
Vásquez R, Ibisch PL, Gerkmann B. 2003. Diversity of Bolivian Orchidaceae – a challenge for taxonomic, floristic and conservation research. – Organisms Divers. Evol. 3: 93-102.
Ved Brat S. 1965. Genetic systems in Allium I. Chromosome variation. – Chromosoma 16: 486-499.
Ved Brat S. 1966. Genetic systems in Allium II. Sex differences in meiosis. – Chromosomes today 1: 31-40.
Ved Brat S. 1967. Genetic systems in Allium IV. Balance in hybrids. – Heredity 22: 387-396.
Véla E. 2000. Les Ophrys du complexe sphegodes (Orchidaceae). – Bull. Soc. Linn. Provence 51: 51-70.
Velarde MO. 1949. Pseudostenomesson, un nuevo genero de Amarilidáceas. – Rev. Cienc. (Lima) 51: 47-51.
Velenovský J. 1903. Zur Deutung der Phyllokladien der Asparageen. – Beih. Bot. Centralbl. 15: 257-268.
Venhuis C, Venhuis P, Oostermeijer JGB, Tienderen PH. 2007. Morphological systematics of Serapias L. (Orchidaceae) in Southwest Europe. – Plant Syst. Evol. 265: 165-177.
Venkataswarlu J, Raju CSK. 1958. Male and female gametophytes in four species of Asparagus. – J. Indian Bot. Soc. 37: 290-299.
Venter S. 2008. Synopsis of the genus Ledebouria Roth (Hyacinthaceae) in South Africa. – Herbertia 62: 85-155.
Verdcourt B. 1986. A key to the East African species of Disperis (Orchidaceae) with two new species. – Kew Bull. 41: 51-57.
Verhoek S. 1978a. Floral biology of Manfreda virginica (L.) Rose (Agavaceae). – Bot. Soc. Amer., Misc. Ser., Publ. 156: 82.
Verhoek S. 1978b. Huaco and amole: a survey of the uses of Manfreda and Prochnyanthes (Agavaceae). – Econ. Bot. 32: 124-130.
Verhoek S. 1998. Agavaceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 60-70.
Verhoek-Williams SE. 1975. A study of the tribe Poliantheae (including Manfreda) and revisions of Manfreda and Prochnyanthes (Agavaceae). – Ph.D. diss., Cornell University, Ithaca, New York.
Verlynde S, Dubuisson J-Y, Stévart T, Simo-Droissart M, Geerinck D, Sonké B, Cawoy V, Descourvières P, Droissart V. 2013. Taxonomic revision of the genus Bolusiella (Orchidaceae, Angraecinae) with a new species from Cameroon, Burundi and Rwanda. – Phytotaxa 114: 1-22.
Verlynde S, Ramandimbisoa B, Bosser J, Stévart T. 2016. Contribution to the study of Orchidaceae from Madagascar. XXXVIII. Two new species and one new combination for the genus Pectinariella Szlach., Mytnik & Grochocka in Madagascar. – Adansonia 38: 219-232.
Verlynde S, D’Haese CA, Plunkett GM, Simo-Droissart M, Edwards M, Droissart V, Stévart T. 2018. Molecular phylogeny of the genus Bolusiella (Orchidaceae, Angraecinae). – Plant Syst. Evol. 304: 269-279.
Vermeulen JJ. 1987. A taxonomic revision of the continental African Bulbophyllinae. – Orch. Monogr. 2: 1-300.
Vermeulen JJ. 1991. Orchids of Borneo II. Bulbophyllum. – Roy. Bot. Gard., Kew, and Tihaan Publ. Comp., Kinabalu, Kuala Lumpur.
Vermeulen JJ. 1992. Additions to the taxonomic revision of the continental African Bulbophyllinae (Orchidaceae). – Kew Bull. 47: 137-140.
Vermeulen JJ. 1993a. New species of Bulbophyllum, sections Macrobulbon, Sestochilus and Vesicisepalum (Orchidaceae). – Blumea 38: 145-155.
Vermeulen JJ. 1993b. A taxonomic revision of Bulbophyllum, Sections Adelopetalum, Lepantanthe, Macrouris, Pelma, Peltopus and Uncifera (Orchidaceae). – Orch. Monogr. 7: 1-324.
Vermeulen JJ. 1996. Some taxonomic corrections in the subtribe Bulbophyllinae (Orchidaceae). – Nord. J. Bot. 16: 379-381.
Vermeulen JJ. 2008. New species of Bulbophyllum from eastern Malesia (Orchidaceae). – Nord. J. Bot. 26: 129-195.
Vermeulen P. 1938. Chromosomes in Orchis. – Chron. Bot. 4: 107-108.
Vermeulen P. 1947. Studies on dactylorchids. – Utrecht.
Vermeulen P. 1955. The rostellum of the Orchideae. – Bull. Amer. Orchid Soc. 24: 239-245.
Vermeulen P. 1959. The different structure of the rostellum in Ophrydeae and Neottieae. – Acta Bot. Neerl. 8: 338-355.
Vermeulen P. 1965. The place of Epipogium in the system of the Orchidales. – Acta Bot. Neerl. 14: 230-241.
Vermeulen P. 1966. The system of the Orchidales. – Acta Bot. Neerl. 15: 224-253.
Vermeulen P. 1976. Die Säulchenstruktur von Gymnadenia, Platanthera, Habenaria und verwandten Genera. – Sonderh. Zeitschr. ’Die Orchidee’, Kurt Schersow, Hannover, pp. 144-152.
Vermeulen P. 1987. Two new species of Bulbophyllum (Orchidaceae) in Africa. – Kew Bull. 42: 263-266.
Veyret Y. 1965. Embryogénie comparée et blastogénie chez les Orchidaceae-Monandrae. – Mém. Office Rech. Sci. Tech. Outre-Mer 12: 1-106.
Vijayaraghavan MR, Shukla AK. 1980. Viscin threads in Zeuxine strateumatica (Orchidaceae). – Grana 19: 173-175.
Vijayavalli B, Mathew PM. 1990. Cytotaxonomy of the Liliaceae and allied families. – Kerala.
Viljoen AM. 1999. A chemotaxonomic study of phenolic leaf compounds in the genus Aloe. – Ph.D. diss., Rand Afrikaans University, Johannesburg, Republic of South Africa.
Viljoen AM, Wyk B-E van. 1999. The chemotaxonomic value of two cinnamoyl chromones, aloeresin E and F, in Aloe (Aloaceae). – Taxon 48: 747-754.
Viljoen AM, Wyk B-E van, Dagne E. 1996. The chemotaxonomic value of 10-hydroxyaloin B and its derivatives in Aloe Series Asperifoliae Berger. – Kew Bull. 51: 159-168.
Viljoen AM, Wyk B-E van, Bank H van der, Smith GF, Bank M van der. 1996. A chemotaxonomic and biochemical evaluation of the identity of Aloe candelabrum (Aloaceae). – Taxon 45: 461-471.
Viljoen AM, Wyk B-E van, Heerden FR van. 1998. Distribution and chemotaxonomic significance of flavonoids in Aloe (Asphodelaceae). – Plant Syst. Evol. 211: 31-42.
Viljoen AM, Wyk B-E van, Newton LE. 1999. Plicatiloside in Aloe – a chemotaxonomic appraisal. – Biochem. Syst. Ecol. 27: 507-517.
Vogel EF de. 1969. Monograph of the tribe Apostasieae (Orchidaceae). – Blumea 17: 313-350.
Vogel S. 1959. Organographie der kapländischen Ophrydeen, mit Bemerkungen zum Koaptations-Problem I-II. – Abh. Akad. Wiss. Lit. (Mainz) Math.-Naturwiss. Kl. 6-7, F. Steiner, Wiesbaden, pp. 265-532.
Vogel S. 1966a. Pollination neotropischer Orchideen durch duftstoff-höselnde Prachtbienen-Männchen. – Österr. Bot. Zeitschr. 113: 181-182.
Vogel S. 1966b. Parfümsammelnde Bienen als Bestäuber von Orchideen und Gloxinia. – Österr. Bot. Zeitschr. 113: 302-361.
Vogel S. 1969. Über synorganisierte Blütensporne bei einigen Orchideen. – Österr. Bot. Zeitschr. 116: 244-262.
Vos MP de. 1950. Die ontwikkeling van die saadknop en saad by Cyanella capensis. – South Afr. J. Sci. 46: 220.
Vos MP de. 1963. Studies on the embryology and relationships of South African genera of the Haemodoraceae: Lanaria Ait. – J. South Afr. Bot. 29: 79-90.
Vosa CG. 1975. The cytotaxonomy of the genus Tulbaghia. – Ann. Bot. (Roma) 34: 47-121.
Vosa CG. 1980. Chromosome analysis and heterochromatin recognition in the Southern African species of Ornithogalum 1. Ornithogalum seineri (Engl. & Kr.) Oberm. – J. South Afr. Bot. 46: 445-450.
Vosa CG. 1986. Chromosome studies in the genus Gethyllis (Amaryllidaceae). – Caryologia 39: 251-257.
Vosa CG. 2000. A revised cytotaxonomy of the genus Tulbaghia (Alliaceae). – Caryologia 53: 83-112.
Vosa CG, Marchi PD. 1980. Chromosome analysis of Haemanthus and Scadoxus (Amaryllidaceae). – Plant Syst. Evol. 135: 119-126.
Vosa CG, Snijman DA. 1984. The cytology of the genus Haemanthus L. (Amaryllidaceae). – J. South Afr. Bot. 50: 237-259.
Wahlström R, Laane MM. 1979. Chromosome analyses in African Crinum species (Amaryllidaceae). – Hereditas 91: 183-206.
Wang G-Y, Meng Y, Huang J-L, Yang Y-P. 2014.
Molecular phylogeny of Ophiopogon (Asparagaceae) inferred from nuclear and
plastid DNA sequences. – Syst. Bot. 39: 776-784.
Wang J-H, Humphreys DJ, Stodulski GBJ, Middleton DJ, Barlow RM, Lee JB. 1989. Structure and distribution of a neurotoxic principle, hemerocallin. – Phytochemistry 28: 1825-1826.
Warcup JH. 1981. The mycorrhizal relationships of Australian orchids. – New Phytol. 87: 371-381.
Waterhouse JT. 1987. The phylogenetic significance of Dracaena-type growth. – Proc. Linn. Soc. New South Wales 109: 131-138.
Waterman RJ, Pauw A, Barraclough TG, Savolainen V. 2009. Pollinators underestimated: a molecular phylogeny reveals widespread floral convergence in oil-secreting orchids (sub-tribe Coryciinae) of the Cape of South Africa. – Mol. Phylogen. Evol. 51: 100-110.
Waterman RJ, Bidartondo MI, Stofberg J, Combs JK, Genauer G, Savolainen V, Barraclough TG, Pauw A. 2011. The effects of above- and belowground mutualisms on orchid speciation and coexistence. – Amer. Nat. 177: E54-E68.
Watson EM. 1986a. Cytoevolutionary studies in the genus Bulbine Wolf (Liliaceae) I. The Australian perennial taxa (B. bulbosa s.l.). – Aust. J. Bot. 34: 481-504.
Watson EM. 1986b. Cytoevolutionary studies in the genus Bulbine Wolf (Liliaceae) II. The Australian annual taxa (B. semibarbata s.l.). – Aust. J. Bot. 34: 505-522.
Watson S. 1879. Contributions to American botany I. Revision of the North American Liliaceae. – Proc. Amer. Acad. Arts. Sci. 14: 213-288, 301.
Wattendorf J. 1976. A third type of raphide crystal in the plant kingdom; six-sided raphides with laminated sheaths in Agave americana L. – Planta 130: 303-311.
Webber HJ. 1895. Studies in the dissemination and leaf reflexion of Yucca aloifolia and other species. – Ann. Rep. Missouri Bot. Gard. 6: 91-112.
Webber JM. 1953. Yuccas of the Southwestern United States. – Agric. Monogr. U.S.D.A. 17: 1-97.
Weber D. 1973. Deux orchidacées nouvelles pour la flore des Îles Galapagos. – Bull. Soc. Neuchâtel. Sci. Nat. 96: 17-30.
Weber E. 1929. Entwicklungsgeschichtliche Untersuchungen über die Gattung Allium. – Bot. Arch. 25: 1-44.
Weichhardt-Kulessa K, Börner T, Schmitz J, Müller-Doblies U, Müller-Doblies D. 2000. Controversial taxonomy of Strumariinae (Amaryllidaceae) investigated by nuclear rDNA (ITS) sequences. – Plant Syst. Evol. 223: 1-13.
Weimarck H. 1939. Types of inflorescences in Aristea and some allied genera. – Bot. Not. 40: 616-626.
Weimarck H. 1940. Monograph of the genus Aristea. – Lunds Univ. Årsskr., N. F., Avd. II, 36(1): 1-140.
Wenck S. 1935. Entwicklungsgeschichtliche Untersuchungen über die Assimilationsorgane von Semele, Ruscus, Danae und Myrsiphyllum. – Beih. Bot. Centralbl. 53A: 1-25.
Wendelbo P. 1963. Studies in oriental Liliiflorae 3-4. – Nytt Mag. Bot. 10: 81-84.
Wendelbo P. 1964. On the genus Eremurus (Liliaceae) in Southwest Asia. – Acta Univ. Bergensis, Ser. Math.-Nat. 5: 1-45.
Wendelbo P. 1966. New taxa and synonyms in Allium and Nectaroscordum of SW Asia. – Acta Horti Gothoburg. 28: 15-55.
Wendelbo P. 1967a. New species of Bellevalia, Ornithogalum and Tulipa from Iran. – Nytt Mag. Bot. 14: 96-100.
Wendelbo P. 1967b. New species of Allium from W. Pakistan. – Nytt Mag. Bot. 4: 101-104.
Wendelbo P. 1968. Studies in the Flora of Afghanistan 9. Some new species of Allium (Liliaceae) from Afghanistan. – Bot. Not. 121: 269-277.
Wendelbo P. 1969a. New subgenera, sections and species of Allium. – Bot. Not. 122: 25-37.
Wendelbo P. 1969b. Studies in the flora of Afghanistan 10. Two new species of Iris from Afghanistan. – Bot. Not. 122: 204-206.
Wendelbo P. 1973. Contributions to the flora of Iraq 12. New species and new combinations in the Liliaceae. – Kew Bull. 28: 29-35.
Werker E, Fahn A. 1975. Seed anatomy of Pancratium species from three different habitats. – Bot. Gaz. 136: 396-403.
Westphalen G, Conran JG. 1994. Chromosome numbers in the Arthropodium-Dichopogon complex (Asparagales: Anthericaceae). – Taxon 43: 377-381.
Wet JMC de 1957. Chromosome numbers in the Scilleae. – Cytologia 22: 145-159.
Wet JMC de. 1960. Chromosome morphology in Kniphofia. – Bothalia 7: 295-297.
Wetschnig W, Pfosser M. 2003. The Scilla plumbea puzzle – present status of the genus Scilla sensu lato in southern Africa and descriptions of Spetaea lachenaliiflora, a new genus and species of Massonieae (Hyacinthaceae). – Taxon 52: 75-91.
Wetschnig W, Pfosser M, Prenner G. 2002. Zur Samenmorphologie der Massonieae Baker 1871 (Hyacinthaceae) im Lichte phylogenetisch interpretierter molekularer Befunde. – Stapfia 80: 349-379.
Wetschnig W, Knirsch W, Ali SS, Pfosser M. 2007. Systematic position of three little known and frequently misplaced species of Hyacinthaceae from Madagascar. – Phyton (Horn) 47: 321-337.
Weymouth CG, Chaudhary SA. 1974. Karyotypes of Iris subgenus Susiana Spach in Lebanon and Syria. – Bot. Not. 127: 513-521.
Wheeler EJ, Mashayekhi S, McNeal DW, Columbus JT, Pires JC. 2013. Molecular systematics of Allium subgenus Amerallium (Amaryllidaceae) in North America. – Amer. J. Bot. 100: 701-711.
Wheeler JM. 1966. Cytotaxonomy of the large asteliads (Liliaceae) of the North Island of New Zealand. – New Zealand J. Bot. 4: 95-113.
Whitaker TW. 1934. Chromosome constitution of certain monocotyledons. – J. Arnold Arbor. 15: 135-143.
White JH. 1907. On polystely in roots of Orchidaceae. – Univ Toronto Stud., Biol. Ser. 6: 1-20.
Whitehead MR, Brown CA. 1940. The seeds of the spider lily Hymenocallis occidentalis. – Amer. J. Bot. 27: 199-203.
Whitehouse C. 1996. Eriospermaceae. – In: Polhill RM (ed), Flora of tropical East Africa, A. A. Balkema, Rotterdam, pp. 1-7.
Whitehouse C. 2002. Asphodelaceae. – In: Beentje HJ, Smith SAL (eds), Flora of tropical East Africa, A. A. Balkema, Rotterdam, pp. 1-19.
Whitten WM, Williams NH, Chase MW. 2000. Subtribal and generic relationships of Maxillarieae (Orchidaceae) with emphasis on Stanhopeinae: combined molecular evidence. – Amer. J. Bot. 87: 1842-1856.
Whitten WM, Williams NH, Dressler RL, Gerlach G, Pupulin F. 2005. Generic relationships of Zygopetalinae (Orchidaceae: Cymbidieae): combined molecular evidence. – Lankesteriana 5: 87-107.
Whitten WM, Blanco MA, Williams NH, Koehler S, Carnevali G, Singer RB, Endara L, Neubig K. 2007. Molecular phylogenetics of Maxillaria and related genera (Orchidaceae: Cymbidieae) based on combined molecular data sets. – Amer. J. Bot. 94: 1860-1889.
Whitten WM, Neubig KM, Williams NH. 2014. Generic and subtribal relationships in Neotropical Cymbidieae (Orchidaceae) based on matK/ycf1 plastid data. – Lankesteriana 13: 375-392.
Wiland J. 1997. The genus Curculigo (Hypoxidaceae) in Central Africa (Zaire, Rwanda, Burundi). – Fragm. Flor. Geobot. 42: 9-24.
Wiland-Szymańska J. 2001. The genus Hypoxis (Hypoxidaceae) in Central Africa. – Ann. Missouri Bot. Gard. 88: 302-350.
Wiland-Szymańska J. 2009. The genus Hypoxis L. (Hypoxidaceae) in the East Tropical Africa: variability, distribution and conservation status. – Biodiv. Res. Conserv. 14: 1-129.
Wiland-Szymańska J, Nordal I. 2006. Hypoxidaceae. – Flora of tropical East Africa, the Royal Botanic Gardens, Kew.
Wild H. 1953. Dianella ensifolia: a new generic record for continental Africa. – Kew Bull. 8: 251-252.
Wildman WC. 1968. The Amaryllidaceae alkaloids. – Alkaloids 11: 307-405.
Willemse MTM, Franssen-Verheijen MAW. 1986. Stylar development in the open flower of Gasteria verrucosa (Mill.) H. Duval. – Acta Bot. Neerl. 35: 297-309.
Willemse MTM, Kapil RN. 1981. Antipodals of Fasteria verrucosa (Liliaceae). An ultrastructural study. – Acta Bot. Neerl. 30: 25-32.
Williams CA. 1975. Biosystematics of the Monocotyledoneae – flavonoid patterns in leaves of the Liliaceae. – Biochem. Syst. Ecol. 3: 229-244.
Williams CA, Harborne JB, Goldblatt P. 1986. Correlations between phenolic patterns and tribal classification in the family Iridaceae. – Phytochemistry 25: 2135-2154.
Williams CA, Harborne JB, Mathew B. 1988. A chemical appraisal via leaf flavonoids of Dahlgren’s Liliiflorae. – Phytochemistry 27: 2609-2629.
Williams LO. 1941. Orchidaceae austro-americanae. – Lilloa 6: 241-246.
Williams LO. 1951. The Orchidaceae of Mexico. – Ceiba 2: 1-321.
Williams LO. 1952. Encyclia, a segregate from Epidendrum? – Ceiba 3: 154-156.
Williams MD. 1981. Chromosome count for Paramongaia weberbaueri Velarde. – Plant Life 37: 83-89.
Williams M, Dudley TR. 1984. Chromosome count for Hippeastrum iguazuanum. – Taxon 33: 271-275.
Williams NH. 1970. Some observations on pollinaria in the Oncidiinae I & II. – Bull. Amer. Orchid Soc. 39: 32-43, 207-220.
Williams NH. 1972. Additional studies on pollinaria in the Oncidiinae. – Bull. Amer. Orchid Soc. 41: 222-230.
Williams NH. 1975. Stomatal development in Ludisia discolor (Orchidaceae): mesoperigenous subsidiary cells in the monocotyledons. – Taxon 24: 281-288.
Williams NH. 1979. Subsidiary cells in the Orchidaceae: their general distribution with special reference to development in the Oncidieae. – Bot. J. Linn. Soc. 78: 41-66.
Williams NH. 1982. The biology of orchids and euglossine bees. – In: Arditti J (ed), Orchid biology: reviews and perspectives, Vol. 2, Cornell University, Ithaca, New York, pp. 119-171.
Williams NH, Broome CR. 1976. Scanning electron microscope studies of orchid pollen. – Bull. Amer. Orchid Soc. 45: 699-707.
Williams NH, Whitten WM. 1983. Orchid floral fragrances and male euglossine bees: methods and advances in the last sesquidecade. – Biol. Bull. 164: 355-395.
Williams NH, Whitten WM. 2003. Molecular phylogenetics and generic concepts in the Maxillarieae (Orchidaceae). – Lankesteriana 7: 61-62.
Williams NH, Chase MW, Fulcher T, Whitten WM. 2001. Molecular systematics of the Oncidiinae based on evidence from four DNA sequence regions: expanded circumscriptions of Cyrtochilum, Erycina, Otoglossum, and Trichocentrum and a new genus (Orchidaceae). – Lindleyana 16: 113-139.
Williams NH, Chase MW, Whitten WM. 2001. Phylogenetic positions of Miltoniopsis, Caucaea, a new genus, Cyrtochiloides, and Oncidium phymatochilum (Orchidaceae: Oncidiinae) based on nuclear and plastid DNA sequence data. – Lindleyana 16: 272-285.
Williams NH, Whitten WM, Dressler RL. 2005. Molecular systematics of Telipogon (Orchidaceae: Oncidiinae) and its allies: nuclear and plastid DNA sequence data. – Lankesteriana 5: 163-184.
Williamson G. 1977. The orchids of south central Africa. – J. M. Dent & Sons Ltd., London.
Williamson G. 1980. Contribution to the orchid flora of South Central Africa. – Plant Syst. Evol. 134: 53-77.
Wilsenach R. 1965. On the caryology and phylogeny of some genera of Amaryllidaceae. – Plant Life 21: 82-83.
Wilsenach R. 1967. Cytological observations on Hypoxis 1. Somatic chromosomes and meiosis in some Hypoxis species. – J. South Afr. Bot. 33: 75-84.
Wilsenach R, Papenfuss JN. 1967. Cytological observations on Hypoxis II. Pollen germination, pollen tube growth and haploid chromosome numbers in some Hypoxis species. – J. South Afr. Bot. 33: 111-116.
Wilsenach R, Warren JL. 1967. Cytological observations on Hypoxis. Embryo-sac development in H. rooperi and H. filiformis. – J. South Afr. Bot. 33: 133-140.
Wilson CA. 1998. A cladistic analysis of Iris Series Californicae based on morphological data. – Syst. Bot. 23: 73-88.
Wilson CA. 2003. Phylogenetic relationships in Iris series Californicae based on ITS sequences of nuclear ribosomal DNA. – Syst. Bot. 28: 39-46.
Wilson CA. 2004. Phylogeny of Iris based on chloroplast matK gene and trnK intron sequence data. – Mol. Phylogen. Evol. 33: 402-412.
Wilson CA. 2006. Patterns of evolution in characters that define Iris subgenera and sections. – In: Columbus JT, Friar EA, Porter JM, Prince LM, Simpson MG (eds), Monocots: comparative biology and evolution. Excluding Poales, Rancho Santa Ana Botanical Garden, Claremont, California. – Aliso 22: 425-433.
Wilson CA. 2009. Phylogenetic relationships among the recognized Series in Iris Section Limniris. – Syst. Bot. 34: 285-296.
Wilson CA. 2011. Subgeneric classification in Iris re-examined using chloroplast sequence data. – Taxon 60: 27-35.
Wilson M, Belle C, Dang A, hannan P, Kellogg L, Kenyon C, Low H, Mochizuki A, Nguyen A, Sheade N, Shan L, Shum A, Stayton T, Volz C, Vosburgh B, Wellman H, Woolley M. 2013. A preliminary phylogenetic analysis of Pleurothallis sensu lato based upon nuclear and plastid sequences. – Lankesteriana 13: 139.
Wimber DE. 1993. The chromosome evolution for slipper orchids. – In: Pridgeon AM (ed), Proceedings of the 14th World Orchid Conference, Her Majesty’s Stationery Office, Edinburgh, pp. 228-232.
Winge H. 1959. Studies on cytotaxonomy and polymorphism of the genus Alophia (Iridaceae). – Rev. Brasileira Biol. 19: 195-201.
Winkler H. 1906. Über den Blütendimorphismus von Renanthera lowii. – Ann. Jard. Bot. Buitenzorg 20: 1-12.
Wirth M. 1964. Supraspecific variation and classification in the Oncidiinae (Orchidaceae). – Ph.D. diss., Washington University, St. Louis, Missouri.
Wirth M, Withner CL. 1959. Embryology and development in the Orchidaceae. – In: Withner CL (ed), The orchids: a scientific survey, John Wiley & Sons, New York, pp. 155-188.
Withner CL (ed). 1959. The orchids: scientific studies. – Chron. Bot. 32, Ronald Press, New York.
Withner CL (ed). 1974. The orchids: a scientific survey. – John Wiley & Sons, New York.
Withner CL. 1996. The cattleyas and their relatives 4. The Bahamian and Caribbean species. – Portland.
Wittmann H. 1985. Beitrag zur Systematik der Ornithogalum. Arten mit verlängert-traubiger Infloreszenz. – Stapfia 13: 1-117.
Wolf FT. 1940. Macrosporogenesis and the development of the embryo sac in Yucca aloifolia. – Bull. Torrey Bot. Club 67: 755-671.
Wolf T. 1865. Beiträge zur Entwicklungsgeschichte der Orchideenblüte. – Pringsheim Jahrb. Wissensch. Bot. 4: 261-384.
Wolter M, Schill R. 1986. Ontogenie von Pollen, Massulae und Pollinien bei den Orchideen. – Trop. Subtrop. Pflanzenwelt 56: 1-93.
Wolter M, Seuffert C, Schill R. 1988. The ontogeny of pollinia and elastoviscin in the anther of Doritis pulcherrima (Orchidaceae). – Nord. J. Bot. 8: 77-88.
Wood JJ. 1983a. Notes on Pacific Bulbophyllum (Orchidaceae). – Kew Bull. 38: 71-74.
Wood JJ. 1983b. Notes on Himantoglossum (Orchidaceae). – Kew Bull. 38: 75-78.
Wood JJ. 1984. New orchids from Gunung Mulu National Park, Sarawak. – Kew Bull. 39: 73-98.
Wood JJ. 1986. Notes on Asiatic and New Guinea Orchidaceae. – Kew Bull. 41: 811-822.
Wood JJ. 1988. Muluorchis reduced to Tropidia (Orchidaceae). – Kew Bull. 43: 663-665.
Wood JJ. 1990. Notes on Trachoma, Tuberolabium and Parapteroceras (Orchidaceae). – Nord. J. Bot. 10: 481-486.
Wood JJ, Comber JB. 1986. New orchids from Java and Bali. – Kew Bull. 41: 697-702.
Wood JRI. 1983. The aloes of the Yemen Arab Republic. – Kew Bull. 38: 13-31.
Wood SE. 1976. A contribution to the knowledge of the genus Hypoxis L. (Hypoxidaceae) in Natal, South Africa. – M.Sc. thesis, University of Pietermaritsburg, Republic of South Africa.
Wu S, Yang J, Rao G. 2001. Systematic position of Polygonatum simizui (Convallariaceae) based on morphological, cytological and chloroplast DNA sequence data. – Bot. J. Linn. Soc. 137: 291-296.
Wunderlich R. 1936. Vergleichende Untersuchungen von Pollenkörnern einiger Liliaceen und Amaryllidaceen. – Österr. Bot. Zeitschr. 85: 30-55.
Wunderlich R. 1937. Zur vergleichenden Embryologie der Liliaceae-Scilloideae. – Flora 132: 48-90.
Wunderlich R. 1950. Die Agavaceen Hutchinson’s im Lichte ihrer Embryologie, ihres Gynözeum-, Staubblatt- und Blattbaues. – Österr. Bot. Zeitschr. 97: 437-502.
Wurdack KJ, Dorr LJ. 2009. The South American genera of Hemerocallidaceae (Eccremis and Pasithea): two introductions to the New World. – Taxon 58: 1122-1132.
Wyk B-E van, Smith GF. 2003. Guide to aloes of South Africa. – Briza Publ., Pretoria, Republic of South Africa.
Wyk B-E van, Whitehead CS, Glen HF, Hardy DS, Jaarsveld EJ van, Smith GF. 1993. Nectar sugar composition in the subfamily Alooideae (Asphodelaceae). – Biochem. Syst. Ecol. 21: 249-253.
Wyk B-E van, Viljoen AM, Dagne E. 1995. Chemotaxonomic studies in African Aloaceae and Asphodelaceae. – In: Dagne E (ed), Proceedings of the 6th NAPRECA Symposium on Natural Products, Natural Products Research Network for Eastern and Central Africa (NAPRECA), Addis Ababa Univesity, Addis Ababa, pp. 15-18.
Wyk B-E van, Yenesew A, Dagne E. 1995a. Chemotaxonomic survey of anthraquinones and pre-anthraquinones in roots of Aloe species. – Biochem. Syst. Ecol. 23: 267-275.
Wyk B-E van, Yenesew A, Dagne E. 1995b. Chemotaxonomic significance of anthraquinones in the roots of Asphodeloideae (Asphodelaceae). – Biochem. Syst. Ecol. 23: 277-281.
Wyk B-E van, Yenesew A, Dagne E. 1995c. The chemotaxonomic significance of anthraquinones and pre-anthraquinones in the genus Lomatophyllum (Asphodelaceae). – Biochem. Syst. Ecol. 23: 805-808.
Wyk B-E van, Rheede van Oudtshoorn MCB van, Smith GF. 1995. Geographical variation in the major compounds of Aloe ferox leaf exudate. – Planta Medica 61: 250-253.
Xiang X-G, Li D-Z, Jin W-T, Zhou H-L, Li J-W, Jin X-H. 2012. Phylogenetic placement of the enigmatic orchid genera Thaia and Tangtsinia: evidence from molecular and morphological characters. – Taxon 61: 45-54.
Xiong Z-T, Chen S, Hong D, Luo Y. 1998. Pollen morphology and its evolutionary significance in Hemerocallis (Liliaceae). – Nord. J. Bot. 18: 183-189.
Xu S, Schlüter PM, Schiestl FP. 2012. Pollinator-driven speciation in sexually deceptive orchids. – Intern. J. Ecol. ID 285081. doi: 10.1155/2012/285081
Yakouchi Y. 1964. Chromosome studies on Zephyranthes 11. Karyotype of Z. carinata. – La Kromosoma 57-52: 1902-1909.
Yam TW, Arditti J, Cameron KM. 2009. “The orchids have been a splendid sport” – an alternative look at Charles Darwin’s contribution to orchid biology. – Amer. J. Bot. 96: 2128-2154.
Yamamoto Y. 1924. Genus novum Orchidacearum ex Formosa. – Bot. Mag. (Tokyo) 38: 209-212.
Yamamura Y. 1984. Matter production processes of Reineckea carnea Kunth, an evergreen forest floor herb in the warm-temperate region of Japan. – Bot. Mag. (Tokyo) 97: 179-191.
Yamashita J, Tamura MN. 2000. Molecular phylogeny of the Convallariaceae (Asparagales). – In: Wilson KL, Morrison DA (eds), Monocots: systematics and evolution, CSIRO Publ., Melbourne, pp. 387-400.
Yamashita J, Tamura MN. 2004. Phylogenetic analysis and chromosome evolution in Convallarieae (Ruscaceae sensu lato), with some taxonomic treatments. – J. Plant Res. 117: 363-370.
Yan D, Wang L-J, Zhao C-H, Zhao Y-Y, Liu J-X. 2017. Embryology of Hemerocallis L. and its systematic significance. – Plant Syst. Evol. 303: 663-673.
Yang D-Q, Zhu K-F. 1990. Studies on karyotypes of 5 species of Rohdea and Tupistra. – Acta Phytotaxon. Sin. 28: 199-206. [In Chinese with English summary]
Yang Q-E, Hong D-Y. 1993. Karyotype study of the genus Theropogon. – Acta Bot. Yunnan. 15: 263-268. [In Chinese]
Yang S-H. 1982. Studies on the development of the flower in Gastrodia elata Bl. – Acta Bot. Sin. 24: 21-27.
Yang Y, Li H, Liu X, Katsuhiko K. 1990. Karyotype study on the genus Ophiopogon in Yunnan. – Acta Bot. Yunnan. [Suppl.] 3: 94-102.
Yasui K, Sawada N. 1940. On the spore and embryo sac formation with special reference to the sterility of Iris japonica. – Bot. Mag. (Tokyo) 54: 96-102.
Yeo PF. 1968. A contribution to the taxonomy of the genus Ruscus. – Notes Roy. Bot. Gard. Edinb. 28: 237-264.
Yeo PF. 1998. Ruscaceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 412-416.
Yeung EC. 1987a. Development of pollen and accessory structures in orchids. – In: Arditti J (ed), Orchid biology, reviews and perspectives IV, Comstock, Cornell University Press, Ithaca, New York, pp. 193-226.
Yeung EC. 1987b. Mechanisms of pollen aggregation into pollinia in Epidendrum ibaguense (Orchidaceae). – Grana 26: 47-52.
Yeung EC. 2005. The structural organisation of orchid embryos: a functional interpretation. – In: Nair H, Arditti J (eds), Proceedings of the Seventeenth World Orchid Conference: “Sustaining orchids for the future”, Kota Kinabalu, Natural History Publ., Borneo, pp. 123-130.
Yoneda K. 1982. Studies on flowering of orchids II: on the floral initiation, development and flowering in Epidendrum radicans Pavon. – Nihon Daigaku Nojuigakuba Gakujutsu Kenkyu Hokoku 39: 35-46.
Young DP. 1970. Bestimmung und Verbreitung der autogamen Epipactis-Arten. – Jahresber. Naturwiss. Ver. Wuppertal 23: 43-52.
Yukawa T. 2016. Taxonomic notes on the Orchidaceae of Japan and adjacent regions. – Bull. Natl. Mus. Nat. Sci, Ser. B (Bot.) 42: 103-111.
Yukawa T, Stern WL. 2002. Comparative vegetative anatomy and systematics of Cymbidium (Cymbidieae: Orchidaceae). – Bot. J. Linn. Soc. 138: 383-419.
Yukawa T, Uehara K. 1996. Vegetative diversification and radiation in subtribe Dendrobiinae (Orchidaceae): evidence from chloroplast DNA and morphological characters. – Plant Syst. Evol. 201: 1-14.
Yukawa T, Kurita S, Nisida M, Hasebe M. 1993. Phylogenetic implications of chloroplast DNA restriction site variation in subtribe Dendrobiinae (Orchidaceae). – Lindleyana 8: 211-221.
Yukawa T, Ohba H, Kurita S, Cameron KM, Chase MW. 1996. Chloroplast DNA phylogeny of subtribe Dendrobiinae (Orchidaceae): insights from a combined analysis based on rbcL sequences and restriction site variation. – J. Plant Res. 109: 169-176.
Yukawa T, Kita K, Handa T. 2000. DNA phylogeny and morphological diversification of Australian Dendrobium (Orchidaceae). – In: Wilson KM, Morrison DA (eds), Monocots: systematics and evolution, CSIRO Publ., Collingwood, pp. 465-471.
Yukawa T, Ogura-Tsujita Y, Shefferson RP, Yokoyama J. 2009. Mycorrhizal diversity in Apostasia (Orchidaceae) indicates the origin and evolution of orchid mycorrhiza. – Amer. J. Bot. 96: 1997-2009.
Zahariadi C. 1962. Caractères morphologiques, anatomiques et biologiques dans la taxonomie du genre Ornithogalum. – Rev. Biol. 7: 5-41.
Zahariadi C. 1965. Sous-genres et sectiones mésogéens du genre Ornithogalum et la valeur comparative de leurs caractères différentiels. – Rev. Roum. Biol. Bot. 10: 271-291.
Zahariadi C. 1975. Le sous-genre Codonoprasum (genre Allium L. fam. Alliaceae Agardh, 1858) en Grèce et en Roumanie 2. – Biologia Gallo-Hellen. 6: 27-64.
Zahariadi C. 1977a. Notes on the intrageneric classification of the genus Ornithogalum L. (Liliaceae). – Bot. Žurn. 62: 1624-1639. [In Russian]
Zahariadi C. 1977b. Cinq espèces nouvelles du genre Ornithogalum (Liliaceae) trouvées en Grèce. – Ann. Mus. Goulandris 3: 51-75.
Zahariadi C. 1981. Nouvelles espèces du genre Ornithogalum L. du Proche Orient et de la Grèce. – Bull. Soc. Bot. France 128: 303-314.
Zakhariyeva OI, Makushenko LM. 1969. Chromosome numbers of monocotyledons belonging to the families Liliaceae, Iridaceae, Amaryllidaceae and Araceae. – Bot. Žurn. 54: 1213-1227. [In Russian]
Zaman MA, Chakraborty BN. 1974. Cytogenetics of Amaryllidaceae I. Karyomorphology and meiotic behaviour of inversion heterozygote Eurycles sylvestris Salisb. – Bangladesh J. Bot. 3: 51-58.
Zavada MS. 1990. A contribution to the study of pollen wall ultrastructure of orchid pollinia. – Ann. Missouri Bot. Gard. 77: 785-801.
Zavada MS, Scott G. 1993. Pollen morphology of Cyanella species (Tecophilaeaceae). – Grana 32: 189-192.
Zhai J-W, Zhang G-Q, Chen L-J, Xiao X-J, Liu
K-W, Tsai W-C, Hsiao Y-Y, Tian H-Z, Zhu J-Q, Wang M-N, Wang F-G, Xing F-W, Liu
Z-J. 2013. A new orchid genus, Danxiaorchis, and phylogenetic analysis
of the tribe Calypsoeae. – PLoS One. 2013 Apr 4; 8(4):e60371. doi:
10.1371/journal.pone.0060371
Zhai J-W, Zhang G-Q, Li L, Wang Meina Chen
L-J, Chung S-W, Rodríguez FJ, Francisco-Orgega J, Lan S-R, Xing F-W, Liu Z-J.
2014. A new phylogenetic analysis sheds new light on the relationships in the
Calanthe alliance (Orchidaceae) in China. – Molec.
Phylogen. Evol. 77: 216-222.
Zhang D, Zhuo L-H, Shen X-H. 2010. Sporogenesis and gametogenesis in Agapanthus praecox Willd. ssp. orientalis (Leighton) Leighton and their systematic implications. – Plant Syst. Evol. 288: 1-11.
Zhang D, Ren L, Shen X-H, Zhuo L-H. 2011. Fertilization and embryogeny in Agapanthus praecox ssp. orientalis (Leighton) Leighton. – Plant Syst. Evol. 295: 25-30.
Zhang G-Q, Li M-H, Su Y-Y, Chen L-J, Lan S-R, Liu Z-J. 2015. A new myco-heterotropic genus, Yunorchis, and the molecular phylogenetic relationships of the tribe Calypsoeae (Epidendroideae, Orchidaceae) inferred from plastid and nuclear DNA sequences. – PLoS ONE 10(4):e0123382.
Zhou T, Jin X-H. 2018. Molecular systematics and the evolution of mycoheterotrophy of tribe Neottieae (Orchidaceae, Epidendroideae). – PhytoKeys 94: 39-49.
Ziegler B. 1981. Mikromorphologie der Orchidaceen-samen unter Berücksichtigung taxonomischer Aspekte. – Ph.D. diss., Ruprecht-Karls Universität, Heidelberg, Germany.
Zimmer K, Hynson NA, Gebauer G, Allen EB, Allen MF, Read DJ. 2007. Wide geographic and ecological distribution of nitrogen and carbon gains from fungi in pyroloids and monotropoids (Ericaceae) and in orchids. – New Phytol. 175: 166-175.
Zimmerman M, Pyke GH. 1988a. Pollination ecology of Christmas Bells (Blandfordia nobilis): effects of pollen quantity and source on seed set. – Aust. J. Ecol. 13: 93-100.
Zimmerman M, Pyke GH. 1988b. Pollination ecology of Christmas Bells (Blandfordia nobilis): patterns of standing crop nectar. – Aust. J. Ecol. 13: 301-310.
Zimmermann MH, Tomlinson PB. 1969. The vascular system in the axis of Dracaena fragrans (Agavaceae) 1. Distribution and development of primary strands. – J. Arnold Arbor. 50: 370-383.
Zimmermann MH, Tomlinson PB. 1970. The vascular system in the axis of Dracaena fragrans (Agavaceae) 2. Distribution and development of secondary vascular tissue. – J. Arnold Arbor. 51: 478-491.
Zimudzi C. 1994a. Studies of the family Hypoxidaceae in South Central Africa with emphasis on variation patterns in the genus Hypoxis. – Ph.D. diss., Dept. of Biological Sciences, University of Zimbabwe, Harare, Zimbabwe.
Zimudzi C. 1994b. Revision of the genus Curculigo (Hypoxidaceae) in the Flora Zambesiaca area. – Nord. J. Bot. 14: 311-314.
Zimudzi C. 1995. The cytology and reproduction of the genus Hypoxis. – In: Seyani JH, Chikuni AC (eds), Proc. XIIIth Plenary Meeting AETFAT, Malawi, Vol. 1, pp. 535-543.
Zimudzi C. 1996. A synopsis of the Hypoxidaceae in the Flora Zambesiaca area. – Kirkia 16: 11-19.
Zöllner O, Arriagada L. 1998. The tribe Gilliesieae (Alliaceae) in Chile. – Herbertia 53: 104-107.
Zomlefer WB. 1999. Advances in angiosperm systematics: examples from the Liliales and Asparagales. – J. Torrey Bot. Soc. 126: 58-62.
Zonneveld BJM. 2007. Nuclear DNA content of ploidy chimeras of Hosta Tratt. (Hostaceae) demonstrate three apical layers in all organs, but not in the adventitious root. – Plant Syst. Evol. 269: 29-38.
Zonneveld BJM. 2008. The systematic value of nuclear DNA content for all species of Narcissus L. (Amaryllidaceae). – Plant Syst. Evol. 275: 109-132.
Zonneveld BJM. 2015. Nuclear genome sizes of 343 accessions of wild collected Haworthia and Astroloba (Asphodelaceae, Alooideae), compared with the genome sizes of Chortolirion, Gasteria and 83 Aloe species. – Plant Syst. Evol. 301: 931-953.
Zonneveld BJM, Duncan GD. 2006. Genome size for the species of Nerine Herb. (Amaryllidaceae) and its evident correlation with growth cycle, leaf width and other morphological characters. – Plant Syst. Evol. 257: 251-260.
Zonneveld BJM, Fritz GPJ. 2010. Three species accepted in Chortolirion Berger (Xanthorrhoeaceae: Asphodeloideae). – Bradleya 28: 27-36.
Zonneveld BJM, Jaarsveld EJ van. 2005. Taxonomic implications of genome size for all species of the genus Gasteria Duval (Aloaceae). – Plant Syst. Evol. 251: 217-227.
Zou L-H, Huang J-X, Zhang G-Q, Liu Z-J, Zhuang X-Y. 2015. A molecular phylogeny of Aeridinae (Orchidaceae: Epidendroideae) inferred from multiple nuclear and chloroplast regions. – Mol. Phylogen. Evol. 85: 247-254.
Zweigelt F. 1913. Vergleichende Anatomie der Asparagoideae, Ophiopogonoideae, Aletroideae, Luzuriagoideae und Smilacoideae nebst Bemerkungen über die Beziehungen Ophiopogonoideae und Dracaenoideae. – Denkschr. Akad. Wiss. Wien 88: 397-476.