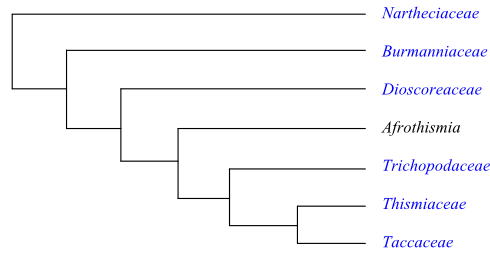
Bayesian inference tree of Taccales based on DNA sequence data (Merckx & Bidartondo 2008; Merckx & al. 2009, 2010). Stenomeris is nested within Dioscorea as sister to Dioscorea althaeoides.
Dioscoreales R. Br. in C. F. P. von Martius, Consp. Regn. Veg.: 9. Sep-Oct 1835 [‘Dioscoreae’]; Dioscoreanae (R. Br.) Takht. ex Reveal et Doweld in Novon 9: 550. 30 Dec 1999
Habit Bisexual, monoecious or dioecious (rarely polygamous), perennial or annual herbs. Rhizome often tuberous, starchy. Often mycoheterotrophic (then usually achlorophyllous).
Vegetative anatomy Stem tuber developed from epicotyl, hypocotyl, etc. Phellogen subepidermal. Stele often eusteloid. Secondary lateral growth usually absent (sometimes anomalous, from an annular cambium). Vascular bundles usually arranged in two rings. Vessels present in root, and often in stem and pseudopetiole; tracheids often present in pseudolamina, rhizome and stem tuber. Vessel elements with scalariform perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial parenchyma? Sieve tube plastids P2c or P2cs types, or absent. Nodes trilacunar to multilacunar with several leaf traces. Secretory cells with resins or tannins and mucilaginous idioblasts with raphides present. Starch grains of several different types. Calciumoxalate as druses, raphides or prismatic crystals.
Trichomes Hairs unicellular or multicellular, uniseriate or multiseriate, simple, T-shaped, stellate or dendritic, or absent; glandular hairs, consisting of unicellular stalk and multicellular or bicellular head, often present.
Leaves Usually alternate (spiral or distichous; rarely opposite), palmately compound, or simple and entire or palmately lobed, differentiated into pseudopetiole and pseudolamina (sometimes ensiform, equitant), with conduplicate or plicate ptyxis (sometimes scale-like or absent). Stipule-like structures caducous or absent; leaf sheath short, well developed or absent. Venation parallelodromous, palmate, acrodromous, actinodromous, or camptodromous. Stomata anomocytic (adjacent cells with irregular divisions) or absent. Cuticular wax crystalloids as parallel or non-oriented scales or platelets, or absent. Mesophyll usually with mucilaginous idioblasts containing calciumoxalate raphides. Leaf margin entire.
Inflorescence Axillary, panicle, spike- or raceme-like (rarely umbel-like), spike, raceme or consisting of dichotomously branched cincinni (flowers sometimes solitary). Floral prophyll (bracteole) usually single (rarely two lateral prophylls).
Flowers Usually actinomorphic (rarely slightly zygomorphic). Usually epigyny (sometimes hypogyny or half epigyny). Tepals usually 3+3 (rarely three or 4+4), with valvate or induplicate (rarely contorted) aestivation, sepaloid or petaloid, usually connate at base (rarely connate into urceolate, tubular or infundibuliform perianth). Septal nectaries or tepal nectaries sometimes present. Disc usually absent (sometimes aberrant).
Androecium Stamens usually 3+3, or three outer fertile stamens and three inner staminodia, or absent (sometimes three stamens and no staminodia; rarely one stamen). Filaments usually free (rarely connate into tube), usually adnate to tepals (epitepalous). Anthers basifixed or dorsifixed, non-versatile, tetrasporangiate, usually introrse (sometimes extrorse or latrorse), longicidal (dehiscing by longitudinal slits) or dehiscing by transverse slits; connective often extended at apex. Tapetum secretory. Staminodia six, three intrastaminal or absent.
Pollen grains Microsporogenesis simultaneous or successive. Pollen grains usually monosulcate, monoporate or diporate (rarely triporate, disulcate, trisulcate, quadri- or quinqueforaminate, or inaperturate), usually shed as monads (rarely dyads or tetrads), bicellular or tricellular at dispersal. Exine tectate or semitectate, with columellate infratectum, perforate, reticulate, striate, rugulate or foveolate, verrucate, undulate, plicate, gemmate or psilate.
Gynoecium Pistil composed of three more or less connate carpels; carpel sometimes congenitally fused and partially ascidiate, partially plicate. Ovary usually inferior (sometimes superior or semi-inferior), unilocular to trilocular. Style single, simple, often with stylar canal (hollow), or absent. Stigma bilobate or trilobate, usually non-papillate, Dry or Wet type. Male flowers often with pistillodium.
Ovules Placentation axile (when ovary trilocular), basal or parietal (when ovary unilocular). Ovules one to more than 50 per carpel, anatropous or campylotropous, pendulous, usually bitegmic (rarely unitegmic), crassinucellar or incompletely tenuinucellar. Micropyle usually bistomal (sometimes endostomal). Parietal cell usually formed from archesporial cell. Megagametophyte usually monosporous, Polygonum type (rarely disporous, Allium type). Antipodal cells persistent, not proliferating (sometimes absent). Endosperm development ab initio nuclear or helobial (rarely cellular?). Endosperm haustoria chalazal or absent. Embryogenesis asterad or onagrad.
Fruit Usually a loculicidal or septicidal capsule (sometimes a pyxidium or a berry, rarely a samara) usually with persistent tepals, often with ridges or wings.
Seeds Aril usually absent (rarely present). Seed coat testal-tegmic. Testa often with phlobaphene, often winged or ridged, sometimes collapsed. Endotestal cells elongate, thick-walled, usually with calciumoxalate crystal and phlobaphene. Exotegmen usually with sculptured cell walls (sometimes compressed). Endotegmic cells thin-walled (sometimes compressed), tanniniferous. Perisperm not developed. Endosperm sparse or copious, starchy or oily, proteinaceous and often with hemicelluloses, often with thick cell walls (rarely ruminate). Embryo small, straight, well differentiated or undifferentiated, without chlorophyll. Cotyledon one, wide, usually lateral, or two (one of which degenerating). Sometimes with a terminal plumule. Hypocotyl internode present or absent (sometimes modified into nutrient-storing organ). Cotyledon hyperphyll elongate or compact, not assimilating. Coleoptile absent. Germination phanerocotylar? or cryptocotylar.
Cytology n = 6–15, 18, 21, 22, 26, 27
DNA
Phytochemistry Flavonols (kaempferol, quercetin), flavonoid glycosides (taccalin etc.), cyanidin, tannins, tropane alkaloids (dioscorine), steroidal saponins, lactone, chelidonic acid, -sitosterol, ceryl alcohol and other alcohols present. Ellagic acid and cyanogenic compounds not found.
Systematics Taccales are sister to Pandanales in most molecular analyses and Nartheciaceae are sister-group to the remaining Taccales. Steven (2001 onwards) lists the following features as potential synapomorphies of Taccales minus Nartheciaceae: stem containing endodermis; a wide perianth tube; an inferior ovary (epigyny); bilobate stigmatic lobes; a winged fruit; and tanniniferous exotesta and endotesta.
Burmanniaceae are successive sister to the clade [Dioscorea+[Afrothismia+[Trichopus+[Thismiaceae+Tacca]]]], according to Merckx & al. (2010). ‘Burmannia’ as traditionally circumscribed is paraphyletic, with Burmannia congesta sister to Campylosiphon. Stenomeris is nested within Dioscorea, as sister to D. althaeoides. Afrothismia, sister to the [Trichopus+[Tacca+ Thismiaceae]] clade, has gained its mycoheterotrophic habit in parallel with Thismiaceae s.str. and many species of Burmanniaceae. Merckx & al. (2010) concluded that six or more independent origins of a mycoheterotrophic habit (several within Burmanniaceae) are most probable.
The clade [Thismiaceae+Tacca] is sister to Trichopus, and characterized by the potential synapomorphies: stamens reflexed at anthesis; absence of septal nectaries; and a parietal placenta (Steven 2001 onwards).

|
Bayesian inference tree of Taccales based on DNA sequence data (Merckx & Bidartondo 2008; Merckx & al. 2009, 2010). Stenomeris is nested within Dioscorea as sister to Dioscorea althaeoides. |
BURMANNIACEAE Blume |
( Back to Taccales ) |
Tripterellaceae Dumort., Anal. Fam. Plant.: 54, 55. 1829 [’Tripterelleae’], nom. illeg.; Burmanniales Blume in C. F. P. von Martius, Consp. Regn. Veg.: 9. Sep-Oct 1835 [‘Burmanniaceae’]; Burmanniidae Heintze, Cormofyt. Fylog.: 10. 1927 [’Burmanniaeformes’]
Genera/species 8/90–95
Distribution Tropical and subtropical regions on both hemispheres, with their largest diversity in tropical South America.
Fossils Unknown.
Habit Bisexual, perennial or annual herbs. Green autotrophs? or hemiparasites (Burmannia) or achlorophyllous holoparasites (mycoheterotrophic) on fungi. Roots often tuberous, sometimes coral-like.
Vegetative anatomy Arbuscular mycorrhiza present. Roots with or without velamen. Root stele diarch to pentarch; medulla sometimes absent; central root vascular cylinder surrounded by parenchyma (not endodermis). Phellogen absent. Stem with endodermis? Secondary lateral growth absent. Vessels usually present in root only (in photosynthesizing species of Burmannia also in stem and rarely in leaves). Vessel elements with scalariform perforation plates; lateral pores? Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial parenchyma? Sieve tube plastids P2c type, with cuneate protein crystals. Nodes? Silica absent. Calciumoxalate raphides usually absent.
Trichomes Hairs absent?
Leaves Alternate (usually spiral, sometimes distichous), simple, entire, in autotrophic species green, in holoparasitic species scale-like, with ? ptyxis. Stipules absent; leaf sheath short. Venation parallelodromous. Stomata anomocytic or absent. Cuticular wax crystalloids as transversely arranged parallel platelets (Convallaria type, resembling ‘electromagnetic field lines’). Mesophyll sometimes with calciumoxalate raphides. Leaf margin entire.
Inflorescence Terminal, usually dichotomously branched cincinni (double rhipidia; in Apteria a simple cincinnus), or flowers solitary. Floral prophylls (bracteoles) in Burmannia lateral.
Flowers Usually actinomorphic (sometimes slightly zygomorphic), often small. Epigyny. Tepals usually 3+3 (occasionally 4+4, in Marthella three with outer tepals present), with valvate or induplicate aestivation, petaloid, sometimes persistent, connate at base into a tubular or infundibuliform perianth; perianth tube entirely or partially persistent; tepals sometimes (in, e.g., many species of Burmannia) with three longitudinal wings or ridges, sometimes with ornamented annulus around perianth mouth; outer tepals usually larger and enclosing inner ones. Paired nectariferous glands at apex of ovary or gynoecial-septal nectaries on upper part of ovary septa or in furrows on placentae. Disc aberrant.
Androecium Stamens three, antepetalous. Filaments usually free, adnate to perianth tube (epitepalous; sometimes alternating with interstaminal lobes), often very short, sometimes absent. Anthers basifixed to subbasifixed, non-versatile, tetrasporangiate, latrorse, dehiscing by transversal slits; thecae separated by connective; connective usually expanded, usually with appendage. Placentoid present. Tapetum secretory, with binucleate cells. Staminodia absent.
Pollen grains Microsporogenesis successive. Pollen grains usually monoporate or diporate (sometimes inaperturate), usually shed as monads (rarely dyads or tetrads), usually tricellular (sometimes bicellular) at dispersal. Exine tectate or semitectate, with columellate infratectum, psilate or undulate to plicate.
Gynoecium Pistil composed of three more or less connate antesepalous carpels. Ovary inferior, usually entirely or partially unilocular (in Burmannia trilocular), sometimes (in, e.g., many species of Burmannia) with three longitudinal wings or ridges. Style single, simple, long, cylindrical. Stigma usually trilobate, infundibuliform (in Cymbocarpa and some species of Gymnosiphon with glanduliferous appendages), type? Pistillodium absent.
Ovules Placentation axile (when ovary trilocular; Burmannia, Campylosiphon) or parietal (when ovary unilocular with three free central placentae). Placentae with paired glands (modified septal nectaries) at apex. Ovules ten to more than 50 per carpel, anatropous, bitegmic, tenuinucellar. Micropyle bistomal. Outer integument ? cell layers thick. Inner integument ? cell layers thick. Hypostase present. Megasporangium crushed (except chalazal part) during megagametophyte development. Parietal cell not formed (parietal tissue absent). Megagametophyte usually monosporous, Polygonum type (in at least some species of Burmannia disporous, Allium type). Antipodal cells in Gymnosiphon persistent. Endosperm development helobial. Endosperm haustoria chalazal. Embryogenesis onagrad or asterad?
Fruit Usually a loculicidal or septicidal winged capsule (occasionally a pyxidium), longitudinally, transversally or irregularly dehiscing, sometimes with entire or part of perianth persistent (in some species of Gymnosiphon indehiscent).
Seeds Aril absent. Seeds often winged. Seed coat testal. Testal cells spiral-shaped. Exotestal epidermis with elevated anticlinal cell walls and collapsing thin outer periclinal cell walls. Endotesta? Tegmic cells often compressed, sometimes tanniniferous. Perisperm not developed. Endosperm sparse or copious, with thick-walled cells, ab initio with starch, later with mainly proteins and lipids. Cell divisions starting in chalazal endosperm chamber. Embryo very small, often four- to ten-celled, undifferentiated, starchy and with lipids, chlorophyll? Cotyledon one. Germination?
Cytology n = 6–8, 11–13, 68, 87–99, etc. – Polyploidy frequent. Agamospermy occurring at least in Burmannia.
DNA
Phytochemistry Unknown. Saponins?
Use Unknown.
Systematics Campylosiphon (1 or 2; C. purpurascens; tropical South America; incl. Burmannia congesta?), Dictyostega (1; D. orobanchoides; Mexico to Bolivia and southeastern Brazil), Hexapterella (2; H. gentianoides: northern South America; H. steyermarkii: Cerro Aracamuni in southern Venezuela), Gymnosiphon (c 25; tropical Africa, Madagascar, tropical Asia, Central America, the Greater Antilles, tropical South America), Apteria (1; A. aphylla; southern United States and the West Indies to central South America), Burmannia (60–65; tropical and southeastern Africa, Madagascar, East and Southeast Asia, Malesia to southeastern Australia, North to South America), Miersiella (1; M. umbellata; tropical South America), Marthella (1; M. trinitalis; Mount Tucuche on Trinidad).
Some species of Burmannia are chlorophyllous and photosynthesizing (autotrophic?; Merckx & al. 2010). According to analyses of DNA data (Merckx & al. 2008), photosynthesis has been lost at least eight times in Burmanniaceae from the Late Cretaceous to the Oligocene.
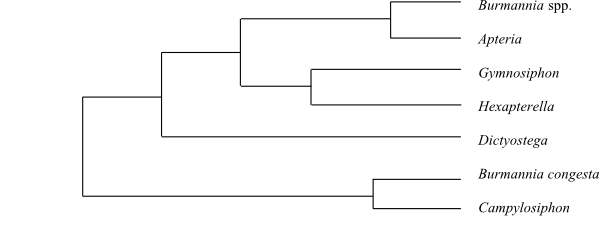 |
Cladogram (simplified) of Burmanniaceae based on DNA sequence data (Merckx & al. 2008). |
DIOSCOREACEAE R. Br. |
( Back to Taccales ) |
Tamaceae Bercht. et J. Presl, Přir. Rostlin: 267. Jan-Apr 1820 [’Tameae’]; Tamnaceae J. Kickx f., Descr. Plant. Louvan.: 307. 1827 [’Dioscoreae (sive Tamneae)’]; Tamales Dumort., Anal. Fam. Plant.: 57. 1829 [’Tamarieae’]; Stenomeridaceae J. Agardh, Theoria Syst. Plant.: 66. Apr-Sep 1858 [’Stenomerideae’], nom. cons.
Genera/species 1/600–800
Distribution Pantropical, with some species in warm-temperate regions.
Fossils There are no known
unambiguous Cretaceous Dioscoreaceae. Leaves from the
Late Cretaceous of North America have been described as Dioscorites
cretaceus, although their relationship to Dioscoreaceae is doubtful. Pan
& al. (2014) reported Dioscorea from Late Oligocene (c 27 Ma)
layers in northwestern Ethiopia.
Habit Bisexual (the ‘Stenomeris clade’), or monoecious or dioecious, usually perennial herbs (often twining, creeping or climbing). Rhizome usually tuberous, starchy. Roots sometimes transformed into spines.
Vegetative anatomy Roots usually with mycorrhiza. Roots sometimes with velamen (formed from epidermis). Stem tuber developed from epicotyl, hypocotyl, etc. Phellogen subepidermal. Endodermis aberrant. Eusteloid stele. Secondary lateral growth usually absent (in rhizome or tuber of some species anomalous, from an annular cambium). Vascular bundles usually in two cylinders. Two main categories of characteristic vascular bundles alternating in stem: (1) small ’common bundles’, often V-shaped in cross-section, and (2) larger ‘cauline bundles’, elliptic in cross-section and receding inwards. Both ’common bundles’ and ’cauline bundles’ containing at least two separated phloem strands. ’Cauline bundles’ at nodes interrupted by ’glomeruli’, where vessels in successive internodes do not fuse but are replaced by numerous short tracheids; sieve tubes replaced in a corresponding way by ’phloem glomeruli’ consisting of thin-walled cells without sieve pores but with small sieve areas. Vessels present in roots, aerial stem and pseudopetiole (absent from pseudolamina); tracheids usually present in pseudolamina, rhizome and stem tuber. Vessel elements with scalariform perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial parenchyma? Larger ’cauline bundles’ with one or two phloem strands inside metaxylem. Sieve tube plastids P2c or P2cs types, with cuneate protein crystalloids and often starch grains. Nodes 3:3?, trilacunar with three? leaf traces. Secretory cells with resins or tannins. Mucilage idioblasts with raphides. Tanniniferous cells usually absent (sometimes present). Starch grains of several different types. Calciumoxalate druses?
Trichomes Hairs unicellular or multicellular, uniseriate, T-shaped, stellate or dendritic; glandular hairs consisting of unicellular stalk and usually multicellular (in the ‘Stenomeris clade’ bicellular) head.
Leaves Usually alternate (distichous; rarely opposite), simple or palmately compound (due to localized activity of marginal blastozone), entire or palmately lobed, differentiated into pseudopetiole and pseudolamina, with flat to curved or conduplicate ptyxis. Stipule-like paired basal processes without vascular bundles, caducous, or absent; leaf sheath absent. Pseudopetiole vascular bundle transection usually annular (in, e.g., Dioscorea hemicrypta and the ‘Stenomeris clade’ arcuate); pseudopetiole usually pulvinate proximally and distally. Venation usually palmate, acrodromous, actinodromous, or camptodromous; midvein and reticulate fine venation or sometimes with several parallel longitudinal veins connected to transversal secondary veins; vein endings sometimes free. Stomata usually anomocytic, with irregular ontogeny; guard cells often penetrated by a protruding border. Cuticular wax crystalloids non-orientated or absent. Mesophyll usually with calciumoxalate raphides. Leaf margin entire. Extrafloral nectaries present in some species on pseudopetiole or abaxial side of pseudolamina (sometimes sunken into parenchyma).
Inflorescence Axillary, panicle, spike- or raceme-like, two or more together. Floral prophyll (bracteole) usually single (sometimes two lateral prophylls).
Flowers Actinomorphic, usually small. Usually epigyny (rarely half epigyny). Tepals 3+3, with imbricate aestivation, usually sepaloid, usually connate at base (in some species connate into an urceolate or tubular perianth; sometimes free); median outer tepal adaxial. Septal nectaries present in some species. Disc absent.
Androecium Stamens 3+3 or three outer fertile stamens and three intrastaminal staminodia, usually erect (not in the ‘Stenomeris clade’), or absent (rarely a single stamen). Filaments usually free (rarely connate into a tube), usually partially or completely adnate to tepals. Anthers basifixed or dorsifixed, non-versatile, tetrasporangiate, usually introrse (sometimes extrorse), longicidal (dehiscing by longitudinal slits); connective sometimes wide (connective in the ‘Stenomeris clade’ strongly prolonged, inflexed and connivent above stigma). Tapetum secretory. Staminodia six, three intrastaminal or absent.
Pollen grains Microsporogenesis simultaneous. Microsporocytes in the ‘Stenomeris clade’ distinctly elongate. Pollen grains usually monosulcate (sometimes disulcate or trisulcate), shed as monads, bicellular at dispersal. Exine semitectate, with columellate infratectum, usually reticulate (sometimes striate).
Gynoecium Pistil composed of three connate plicate carpels; odd carpel posterior (adaxial). Compitum present. Ovary usually inferior (rarely semi-inferior), usually trilocular (in ‘Stenomeris’ filled with secretions). Style single, simple, bifid or usually trifid, with secretory stylar canal, or absent. Stigma usually trilobate (sometimes bilobate), usually non-papillate, usually Wet type. Male flowers often with pistillodium.
Ovules Placentation usually axile. Ovules usually one or few (in the ‘Stenomeris clade’ numerous) per carpel, anatropous, pendulous, bitegmic, crassinucellar. Micropyle usually bistomal (sometimes endostomal). Outer integument three to five cell layers thick. Inner integument two? cell layers thick. Hypostase present. Parietal cell formed from archesporial cell. Parietal tissue often six to eight cell layers thick. Megagametophyte monosporous, Polygonum type. Endosperm development ab initio nuclear. Endosperm haustoria? Embryogenesis asterad.
Fruit Usually a loculicidal capsule (in some species a samara, samaroid or a berry; rarely with two sterile locules and a third locule one-seeded) with persistent tepals, often with ridges or wings.
Seeds Aril? Testa with phlobaphene, often winged. Exotesta? Endotestal cells elongate, thick-walled, usually with a calciumoxalate crystal (absent in the ‘Stenomeris clade’) and phlobaphene. Exotegmen sclerotic, usually with sculptured cell walls (absent in the ‘Stenomeris clade’). Endotegmic cells thin-walled, usually tanniniferous (tegmen in the ’Stenomeris clade’ collapsed). Perisperm not developed. Endosperm copious, horny, oily, proteinaceous, and with cellulose and aleurone (starch usually absent), with hemicellulose, usually thickened (in the ‘Stenomeris clade’ thin, without hemicellulose), with cell walls usually thick or very thick. Embryo small, wide, well differentiated, without chlorophyll. Cotyledon one, wide, flattened, lateral, or two (one of which degenerating), often photosynthesizing. Cotyledon hyperphyll elongate or compact, not assimilating. Hypocotyl internode present or absent (sometimes transformed into nutrient-storing organs). Sometimes with a terminal plumule. Coleoptile absent. Germination?
Cytology n = (8) 9, 10, 12 (rarely 18, 27 or up to c. 200) – Polyploidy and aneuploidy occurring.
DNA
Phytochemistry Flavonols (kaempferol, quercetin), tannins, cyanidin, tropane alkaloids (dioscorine in some species), steroidal saponins, chelidonic acid, and alcohols present. Ellagic acid and cyanogenic compounds not found. Tubers containing large amounts of steroidal saponins. Mannans often storage polysaccharides.
Use Ornamental plants, medicinal plants, starch sources, arrow poison.
Systematics Dioscorea (600–800; tropical and subtropical regions on both hemispheres; some species in warm-temperate regions; the ‘Stenomeris clade‘ in West Malesia).
Dioscoreaceae are sister to [Afrothismia+[Trichopodaceae+[Thismiaceae+Taccaceae]]] (Merckx & Bidartondo 2008). Caddick & al. (2002) identified Dioscorea as sister to Tacca.
NARTHECIACEAE E. M. Fries ex J. Bjurzon |
( Back to Taccales ) |
Abaminaceae J. Agardh, Theoria Syst. Plant.: 3. Apr-Sep 1858 [’Abamineae’], nom. illeg.; Lophiolaceae Nakai, Chosakuronbun Mokuroku [Ord. Fam. Trib. Nov.]: 225. 20 Jul 1943; Nartheciales Reveal et Zomlefer in Novon 8: 176. 15 Jul 1998
Genera/species 5/30–35
Distribution Western Europe, eastern Himalayas, East Asia to Japan, West Malesia, western and eastern United States, northeastern South America.
Fossils Unknown.
Habit Usually bisexual (rarely polygamous), perennial herbs. In one species of Aletris corm; in Lophiola stolons.
Vegetative anatomy Root fibrous; root cortex with central air chamber. Phellogen absent. Secondary lateral growth absent. Vessels present in root. Vessel elements with scalariform (to almost reticulate) perforation plates; lateral pits? Imperforate tracheary xylem element tracheids. Wood rays absent. Axial parenchyma? Phloem with alternating sieve cells and fibres. Sieve tube plastids P2cc type, with only starch and many cuneate protein crystals, or P2ccl (in Narthecium), with starch, many cuneate and additional loosely-packed square, rhomboidal etc. protein crystals. Nodes? Calciumoxalate druses usually present. Vascular bundle envelopes in Narthecium and Aletris with prismatic crystals. Calciumoxalate raphides present in leaf cells, usually absent from stem (present in stem in some species of Aletris).
Trichomes Hairs absent from vegetative parts?
Leaves Alternate (usually distichous; in Aletris spiral), simple, entire, often equitant (ensiform), usually isobifacial (in Aletris bifacial), with ? ptyxis. Stipules absent; leaf sheath well developed. Venation parallelodromous. Stomata anomocytic. Cuticular wax crystalloids as parallel or non-oriented platelets. Calciumoxalate raphides present. Leaf margin entire.
Inflorescence Terminal, usually spike, simple or compound raceme (in Lophiola and Nietneria cymose), in Lophiola woolly haired. Floral prophyll (bracteole) usually absent (in Metanarthecium abaxial?).
Flowers Actinomorphic, usually small. Hypogyny to half epigyny. Tepals 3+3, petaloid (in Aletris often recurved), persistent, free or more or less connate at base. Nectaries present at tepal bases. Septal nectaries often absent. Disc absent.
Androecium Stamens 3+3. Filaments thin, usually glabrous (in Narthecium densely tomentose), free from each other, adnate to tepal bases. Anthers basifixed to dorsifixed, non-versatile?, tetrasporangiate, extrorse or introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory. Staminodia absent.
Pollen grains Microsporogenesis successive? Pollen grains monosulcate, shed as monads, bicellular at dispersal. Exine tectate or semitectate, with columellate infratectum, reticulate, microreticulate, rugulate, perforate-foveolate or gemmate (Aletris).
Gynoecium Pistil composed of three entirely or largely connate carpels; carpel congenitally or postgenitally fused, plicate and ascidiate; compitum often present. Ovary superior to semi-inferior, trilocular. Style single, usually simple (in Aletris trilobate). Stigmas punctate or stigma trilobate, type? Pistillodium?
Ovules Placentation basal to axile. Ovules numerous per carpel, anatropous or (ana)campylotropous, bitegmic (in Narthecium unitegmic?), crassinucellar. Micropyle ?-stomal. Outer integument ? cell layers thick. Inner integument ? cell layers thick. Integumentary obturator present in Aletris aurea. Parietal cell? Megagametophyte monosporous, Polygonum type. Antipodal cells uninucleate. Endosperm development helobial. Endosperm haustoria? Embryogenesis?
Fruit A loculicidal capsule with persistent tepals.
Seeds Seeds in Narthecium with terminal filifom appendages at both ends. Aril absent. Testa collapsed. Tegmen usually thin, more or less collapsed (in Narthecium flattened, persistent). Exotegmen in Lophiola with thickened outer anticlinal cell walls. Perisperm not developed. Endosperm copious, in Aletris rich in starch. Embryo?, chlorophyll? Cotyledon one, sometimes bifacial. Cotyledon hyperphyll? Hypocotyl internode? Radicula weakly developed. Collar rhizoids? Germination?
Cytology n = (12) 13 (22) (26); n = 21 (Lophiola) – Polyploidy rare. Chromosomes 0,7–1,4 µm long.
DNA
Phytochemistry Unsufficiently known. Steroidal saponins and chelidonic acid present (Narthecium). Flavones and alkaloids not found. Aluminium accumulation occurring in Aletris.
Use Medicinal plants.
Systematics Aletris (20–25; eastern Himalayas, East Asia, West Malesia, Canada, United States), Metanarthecium (1; M. luteoviride; Japan), Lophiola (1; L. aurea; southeastern United States), Narthecium (7; N. americanum: eastern United States; N. californicum: western United States; N. asiaticum: Japan; N. balansae: Turkey to the Caucasus; N. ossifragum: western and northern Europe; N. reverchonii: Corsica; N. scardicum: the Balkan Peninsula), Nietneria (2; N. corymbosa, N. paniculata; Venezuela, Guyana, northern Brazil).
Nartheciaceae are sister to the remaining Taccales. The position of Nietneria is unknown.
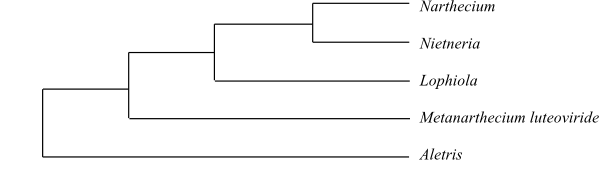 |
Simplified cladogram of Nartheciaceae based on DNA sequence data (Zhao & al. 2012). Metanarthecium has in previous studies often been nested inside Aletris. |
TACCACEAE Dumort. |
( Back to Taccales ) |
Genera/species 1/10–15
Distribution Tropical regions in the Old World east to Southwest Pacific islands.
Fossils Fossil leaves of Tacca were found in an Early Miocene (c 22 Ma) layer in northwestern Ethiopia (Pan & al. 2014).
Habit Bisexual, perennial herbs. Rhizome usually tuberous, starchy. Leaves basal.
Vegetative anatomy Root usually with mycorrhiza. Root with (one-layered) or without velamen. Stem tuber developed from epicotyl or hypocotyl, etc. Phellogen absent. Endodermis aberrant. Secondary lateral growth (in rhizome) anomalous (from meristematic ring surrounding primary vascular bundles). ’Vessels’ (vessel-like tracheids) present in root (usually tracheids in pseudolamina, rhizome and stem tuber). ‘Vessel’ elements with scalariform perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids with ? pits. Wood rays absent. Axial parenchyma? Sieve tube plastids P2c type, with cuneate protein crystals. Nodes? Starch grains of several different types. Calciumoxalate raphides present.
Trichomes Hairs (on young leaves) with multicellular short stalk, multicellular head and above this an additional line of cells.
Leaves Alternate (spiral), simple or palmately (sometimes pinnately) compound, entire or palmately lobed, often bipinnate, differentiated into pseudopetiole and pseudolamina, with plicate ptyxis. Stipule-like structures caducous or absent; leaves somewhat sheathing at base. Pseudopetiole usually pulvinate at both ends. Pseudopetiole vascular bundle transection annular. Venation palmate or pinnate, acrodromous, actinodromous or camptodromous, with midvein and reticulate secondary and tertiary venation. Stomata anomocytic or surrounded by a single cell (’axillocytic’). Cuticular wax crystalloids as platelets without distinct orientation or absent. Mesophyll usually with mucilaginous idioblasts containing calciumoxalate raphides. Extrafloral nectaries glandular (sometimes sunken into parenchyma). Leaf margin or leaflet margin entire.
Inflorescence Terminal (axillary?), umbel-like, consisting of groups of cincinni, surrounded by (two to) four (to twelve) foliaceous bracts and usually with numerous long filiform bracts subtending flowers. Floral prophyll (bracteole) usually single (rarely two lateral prophylls).
Flowers Actinomorphic. Epigyny. Tepals 3+3, petaloid, usually connate basally to a short tube or forming a campanulate perianth. Septal nectaries probably absent (or nectar secretion from stylar base?). Disc present (in, e.g., Tacca leontopetaloides) or absent.
Androecium Stamens 3+3, or three outer fertile stamens and three inner staminodia?, recurved at anthesis? Filaments flat, somewhat petaloid, connate at base, adnate to lower part of tepals (epitepalous), together with connective forming a tepaloid apical hood-shaped structure above each anther. Anthers basifixed or dorsifixed, non-versatile, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits); connective wide, together with filament forming a tepaloid apical hood-shaped structure above each reflexed anther, not prolonged. Anther wall formation Dicotyledonous type, outer secondary wall layer forming endothecium and mid-layer, and inner wall layer forming only tapetum. Tapetum at least usually secretory (in some species possibly amoeboid-periplasmodial), with binucleate cells. Staminodia six, three intrastaminal ones or absent.
Pollen grains Microsporogenesis simultaneous. Pollen grains usually monosulcate (rarely disulcate or trisulcate), shed as monads, bicellular at dispersal. Exine tectate or semitectate, with columellate infratectum, perforate, striate or verrucate.
Gynoecium Pistil composed of three connate plicate antepetalous carpels; odd carpel posterior (adaxial). Ovary inferior, unilocular, with six longitudinal ridges on abaxial side. Style single, simple, with stylar canal (secreting), three-winged. Stigma trilobate (stigmatic lobes often petaloid), usually non-papillate, usually Dry (sometimes Wet) type. Compitum present. Male flowers often with pistillodium.
Ovules Placentation intrusively parietal. Ovules numerous per carpel, anatropous, pendulous, apotropous, bitegmic, crassinucellar. Micropyle endostomal. Outer integument ? cell layers thick. Inner integument ? (several) cell layers thick. Hypostase present. Parietal cell formed from archesporial cell. Nucellar cap absent. Megasporocytes several. Megagametophyte monosporous, Polygonum type. Polar nuclei fused prior to fertilization. Antipodal cells three, not proliferating, ephemeral. Endosperm development ab initio nuclear, later cellular. Endosperm haustoria? Embryogenesis?
Fruit A berry or a loculicidal capsule with fleshy pericarp and persistent tepals, often with six ridges or wings.
Seeds Aril and fleshy raphe present in at least Tacca leontopetaloides. Testa strongly ridged or winged. Endotestal cells very elongate, thick-walled, usually with a calciumoxalate crystal and phlobaphene. Exotegmen usually with sculptured thick and elongate cell walls. Endotegmic cells thin-walled. Perisperm not developed. Endosperm copious, horny, oily, fatty, proteinaceous and with aleurone (starch absent); endosperm cell walls weak, without hemicellulose. Embryo short to very small, well differentiated, without chlorophyll. Cotyledon one, wide, laterally inserted, bifacial? Cotyledon hyperphyll elongate or compact, not assimilating. Hypocotyl internode present. Plumule terminal. Coleoptile absent. Germination?
Cytology n = 15
DNA
Phytochemistry Flavonoid glycosides (taccalin, quercetin-3-arabinoside), tropane alkaloids (dioscorine), steroidal saponins with diosgenin as aglucone, and lactone present. Cyanidin? Tannins? Flavonols, ellagic acid and cyanogenic compounds not found. Tubers containing betulinic acid, castanogenin (a sapogenin), steroidal saponins, -sitosterol, ceryl alcohol etc.
Use Starch sources (arrowroot from Tacca leontopetaloides), fibres.
Systematics Tacca (10–15; tropical Africa, Southeast Asia, Malesia to New Guinea, tropical Australia, Solomon Islands, Fiji, Samoa, Micronesia, with their largest diversity in Malesia).
Tacca has sometimes been placed as an ingroup within an extended Dioscoreaceae. Other DNA data indicate that Tacca is sister to Thismiaceae.
The anther wall development according to the ‘Dicotyledon type’ is otherwise known only in Acorus among the monocotyledons.
THISMIACEAE J. Agardh |
( Back to Taccales ) |
Arachnitideae Kuntze in T. E. von Post et C. E. O. Kuntze, Lex. Gen. Phan.: 629. 20-30 Nov 1903
Genera/species 5/50–55
Distribution Tropical West Africa, Sri Lanka, Southeast Asia, Malesia, southeastern Australia, New Zealand, locally in eastern North America, tropical Central and South America to Chile, with their largest diversity in Brazil.
Fossils Unknown.
Habit Bisexual, perennial or annual herbs. Achlorophyllous mycoheterotrophic holoparasites.
Vegetative anatomy Root with mycorrhiza, often tuberous, sometimes coral-shaped; root with or without velamen. Root stele diarch to pentarch, sometimes without medulla (central root vascular cylinder surrounded by parenchyma, not by endodermis). Phellogen absent. Stem with endodermis? Secondary lateral growth absent. Vessels present in root only. Vessel elements with scalariform perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial parenchyma? Sieve tube plastids not found. Silica bodies absent. Calciumoxalate raphides abundant in Thismia.
Trichomes Hairs absent?
Leaves Alternate (spiral), simple, entire, scaly (reduced). Stipules absent; leaf sheath short. Venation parallelodromous. Stomata usually absent (or anomocytic). Cuticular wax crystalloids usually as parallel platelets. Mesophyll (sometimes?) with calciumoxalate raphides. Leaf margin entire.
Inflorescence Usually (pseudo)terminal, raceme-like, or flowers solitary terminal or lateral.
Flowers Usually actinomorphic (sometimes zygomorphic), often small. Epigyny. Tepals 3+3, inner tepals often longer and narrower than outer tepals, usually with valvate or induplicate (in Thismia contorted) aestivation, petaloid, sometimes persistent, connate in lower part into a tube; perianth tube entirely or partially persistent; tepals sometimes with an ornamented annulus or a diaphragm around perianth mouth; inner tepals in Thismia larger than outer tepals (outer tepals sometimes absent) and sometimes connate into a mitre-shaped structure or provided with long outgrowths. Septal nectaries absent; at least in Thismia twelve swollen appendages (nectariferous glands?) present at perianth base. Disc absent.
Androecium Stamens usually 3+3 (in Oxygyne three, antepetalous), recurved at anthesis. Filaments usually free (all filaments in Sarcosiphon, Scaphiophora and Thismia connate into a collar), adnate to perianth tube (sometimes alternating with interstaminal lobes). Anthers basifixed, non-versatile, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits); connective often somewhat enlarged at apex, in some species of Thismia connate and tubular (sometimes with nectariferous glands?), sometimes connate to stigma. Tapetum secretory, with uninucleate cells. Staminodia absent.
Pollen grains Microsporogenesis successive. Pollen grains usually monoporate or diporate (rarely sulcate, polyforaminate, triporate or inaperturate), shed as monads, usually bicellular (sometimes tricellular) at dispersal. Exine tectate or semitectate, with columellate infratectum, reticulate or psilate.
Gynoecium Pistil composed of three more or less connate antesepalous carpels. Ovary inferior, usually entirely or partially unilocular. Style usually single, long, cylindrical, usually trifid at apex (in Thismia simple; stylodia in Arachnitis three, free); stylar parts of gynoecium fused early during ontogeny. Stigmas three, capitate (in Arachnitis papillate), type? Pistillodium absent.
Ovules Placentation parietal (when ovary unilocular), sometimes with three free central placental columellae. Ovules numerous per carpel, anatropous, bitegmic, crassinucellar. Micropyle bistomal. Outer integument ? cell layers thick. Inner integument ? cell layers thick. Hypostase present? Megasporangium crushed (except chalazally) during embryogenesis. Parietal cell not formed (parietal tissue absent). Megagametophyte usually monosporous, Polygonum type (at least in some species of Thismia disporous, Allium type). Antipodal cells not developed (nuclei early degenerating). Endosperm development usually helobial (in some species of Thismia cellular). Endosperm haustoria chalazal? Embryogenesis onagrad or asterad?
Fruit Usually a loculicidal or septicidal capsule, apically dehiscent, sometimes with perianth tube entirely or partially persistent and urceolate or campanulate (fruit in Thismia fleshy).
Seeds Aril absent. Seeds often winged. Testal cells spiral. Exotestal epidermis with raised anticlinal cell walls and collapsing thin outer periclinal cell walls. Endotesta? Tegmic cells often compressed, tanniniferous. Perisperm not developed. Endosperm sparse or copious, with thick-walled cells, ab initio with starch, later mainly with proteins and lipids. Embryo very small, often few-celled, undifferentiated (in Thismia globular with three-celled suspensor), with starch and lipids. Cotyledon one. Germination cryptocotylar.
Cytology n = 6–8, 11–13 – Polyploidy? Agamospermy occurring at least in Thismia.
DNA
Phytochemistry Unknown. Saponins?
Use Unknown.
Systematics Afrothismia (12; Central and tropical East Africa); Oxygyne (4; O. hyodoi, O. shinzatoi, O. yamashitae: Japan; O. triandra: Cameroon), Haplothismia (1; H. exannulata; Western Ghats in southern India), Thismia (c 35; tropical Asia, Japan, Taiwan, southeastern Australia, New Zealand, near Chicago in Illinois, tropical America; paraphyletic or polyphyletic?), Tiputinia (1; T. foetida; Amazonian Ecuador); Arachnitis (2; Bolivia, southern Chile, the Falkland Islands)?
Thismiaceae are sister to Taccaceae, according to Merckx & al. 2010.
Afrothismia and Haplothismia are nested inside a paraphyletic Thismia, according to Merckx & al. (2006). It is possible, however, that Afrothismia is sister to [Trichopus+[Tacca+Thismiaceae s.str.] (Merckx & al. 2009, 2010). The tropical Central and East African Afrothismia has many peculiar features different from Thismiaceae s.str. On its rhizome, Afrothismia has bulbils, formed by roots. The at least seemingly zygomorphic flowers are borne in a cymose inflorescence, and the perianth tube is often partitioned by a ring-like constriction into two chambers. The perianth lobes have often reflexed basal appendages, and the anther connectives are adnate to the stigma. The fruit dehisces circumscissilely at its apex.
TRICHOPODACEAE Hutch. |
( Back to Taccales ) |
Avetraceae Takht., Divers. Classif. Fl. Pl: 524. 24 Apr 1997
Genera/species 1/2
Distribution Madagascar, India, Sri Lanka, the Malay Peninsula.
Fossils Unknown.
Habit Bisexual, perennial, climbing herbs. A rhizome rich in starch produces several aerial shoots, each one with a single terminal leaf subtending one to three flowers.
Vegetative anatomy Roots usually with mycorrhiza. Roots sometimes with velamen (formed from epidermis). Stem tuber developed from epicotyl, hypocotyl, etc. Phellogen subepidermal? Endodermis fibrous. Eusteloid stele. Secondary lateral growth absent. Vascular bundles in single cylinder; without distinct vascular bundles, or with two main types of vascular bundles alternating in stem: (1) small ’common bundles’, often V-shaped in cross-section, and (2) larger ‘cauline bundles’, elliptic in cross-section and receding inwards. Both ’common bundles’ and ’cauline bundles’ containing at least two separated phloem strands. ’Cauline bundles’ at nodes interrupted by ’glomeruli’, where vessels in successive internodes do not fuse but are replaced by numerous short tracheids; sieve tube replaced in a corresponding way by ’phloem glomeruli’ consisting of thin-walled cells without sieve pores but with small sieve areas. Vessels present in roots, aerial stem and pseudopetiole (absent from pseudolamina); tracheids usually present in pseudolamina, rhizome and stem tuber. Vessel elements with scalariform perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial parenchyma? Larger ’cauline bundles’ with one or two phloem strands inside metaxylem. Sieve tube plastids P2c type, with cuneate protein crystals, without starch. Nodes 3:3?, trilacunar with three? leaf traces. Secretory cells with resins or tannins. Mucilage idioblasts with raphides. Tanniniferous cells absent. Starch grains of several different kinds. Crystals?
Trichomes Hairs unicellular or multicellular, uniseriate, T-shaped, stellate or dendritic; glandular heads with numerous transversely elongate cells in series.
Leaves Alternate (spiral), simple, entire, differentiated into pseudopetiole and pseudolamina, with conduplicate ptyxis. Stipule-like structures caducous or absent; leaf sheath absent. Pseudopetiole vascular bundle transection arcuate; pseudopetiole with basal pulvinus (absent in one species). Venation pinnate or palmate, acrodromous, actinodromous, or camptodromous; midvein and reticulate fine venation or sometimes parallelodromous, with three to five parallel longitudinal primary veins connected by transverse secondary veins. Stomata anomocytic, with irregular ontogeny; guard cells often penetrated by a protruding border. Cuticular wax crystalloids without regular orientation or absent. Mesophyll usually with calciumoxalate raphides. Leaf margin entire. Extrafloral nectaries absent.
Inflorescence Axillary (at base of pseudopetiole), reduced umbel-like panicle? (two or three flowers arising in axil) or flower solitary. Floral prophyll (bracteole) single.
Flowers Actinomorphic, small. Epigyny. Tepals 3+3, with imbricate aestivation, petaloid, connate in lower part into a tube, persistent. Nectary absent. Disc absent.
Androecium Stamens 3+3, diplostemonous. Filaments free, adnate to tepals. Anthers basifixed, non-versatile, tetrasporangiate, laterally connivent, introrse, longicidal (dehiscing by longitudinal slits); connective strongly prolonged, inflexed and connivent above stigma. Tapetum secretory. Staminodia absent.
Pollen grains Microsporogenesis successive. Microsporocytes distinctly elongate. Pollen grains monosulcate or quadri- or quinquepantoporate, shed as monads, bicellular at dispersal. Exine semitectate, with columellate infratectum, reticulate, spinulate.
Gynoecium Pistil composed of three connate plicate carpels; odd carpel posterior (adaxial). Compitum present. Ovary inferior, trilocular (filled with secretions?). Style single, simple, bifid or usually trifid, with stylar canal, or absent. Stigma trilobate, stigmatic lobes somewhat bifid, usually non-papillate, type? Pistillodium absent.
Ovules Placentation axile. Ovules one or two per carpel, anatropous, pendulous, apotropous, bitegmic, tenuinucellar. Micropyle bistomal, Z-shaped (zig-zag). Outer integument two cell layers thick. Inner integument two? cell layers thick. Hypostase present. Obturator present. Nucellar cap absent. Parietal cell not formed (parietal tissue absent). Lateral megasporangial cells present. Megagametophyte monosporous, Polygonum type. Synergids with hook. Antipodal cells ephemeral. Endosperm development ab initio nuclear. Endosperm haustoria? Embryogenesis possibly solanad.
Fruit A samara or a dry berry with persistent tepals three longitudinal wings, and thick pericarp.
Seeds Aril absent. Testa with phlobaphene, not winged. Endotestal cells elongate, not thickened, with phlobaphene; calciumoxalate crystal absent? Exotegmic cells elongate, with reticulate wall thickenings. Endotegmic cells thin-walled. Perisperm not developed. Endosperm copious, horny, oily, proteinaceous, and with cellulose and aleuronic (starch usually absent), with hemicelluloses, ruminate, with cell walls usually thick or very thick. Embryo small, straight, well differentiated, without chlorophyll. Cotyledon one, wide, lateral, with subterminal plumule. Cotyledon hyperphyll elongate or compact, not assimilating. Hypocotyl internode? Coleoptile absent. Germination?
Cytology n = 14.
DNA
Phytochemistry Unsufficiently known. Flavonoid glycosides, flavones, glycolipids and steroidal saponins present. Tannins? Tropane alkaloids? Ceryl alcohol?
Use Ornamental plants, medicinal plants, arrow poison.
Systematics Trichopus (2; T. sempervirens: eastern Madagascar; T. zeylanicus: southern and central India, Sri Lanka, peninsular Thailand, the Malay Peninsula).
Trichopodaceae are sister to [Thismiaceae+Taccaceae], according to Merckx & Bidartondo (2008), and embedded in Dioscoreaceae (sister to Dioscorea) in a study by Caddick & al. (2002).
Literature
Airy-Shaw HK. 1952. A new genus and species of Burmanniaceae from South India. – Kew Bull. 1952: 277-279.
Akasawa Y. 1950. A new species of Glaziocharis (Burmanniaceae) found in Japan. – J. Jap. Bot. 25: 193-196.
Ambrose JD. 1985. Lophiola, familial affinity with the Liliaceae. – Taxon 34: 149-150.
Archibald EEA. 1967. The genus Dioscorea in the Cape Province west of East London. – J. South Afr. Bot. 33: 1-46.
Ayensu ES. 1966. Taxonomic status of Trichopus: anatomical evidence. – Bot. J. Linn. Soc. 59: 425-430.
Ayensu ES. 1969. Aspects of the complex nodal anatomy of the Dioscoreaceae. – J. Arnold Arbor. 50: 124-137.
Ayensu ES. 1970. Analysis of the complex vascularity in stems of Dioscorea composita. – J. Arnold Arbor. 51: 228-240.
Ayensu ES. 1972. Dioscoreales. – In: Metcalfe CR (ed), Anatomy of the monocotyledons, Vol. 6, Clarendon Press, Oxford.
Beccari O. 1870. Nota sull embryone della Dioscoreacee. – Nuov. Giorn. Bot. Ital. 2: 149-155.
Beccari O. 1890. Le Triuridaceae della Malesia. – Malesia 3: 318-344.
Behnke H-D. 1965a. Über das Phloem der Dioscoreaceen unter besonderer Berücksichtigung ihrer Phloembecken I. Mitt.: lichtoptische Untersuchungen zur Struktur der Phloembecken und ihrer Einordnung in das Sproßleitsystem. – Zeitschr. Pflanzenphysiol. 53: 97-125.
Behnke H-D. 1965b. Über das Phloem der Dioscoreaceen unter besonderer Berücksichtigung ihrer Phloembecken II. Mitt.: elektronenoptische Untersuchungen zur Feinstruktur des Phloembeckens. – Zeitschr. Pflanzenphysiol. 53: 214-244.
Behnke H-D. 1967. Über den Aufbau der Siebelement-Plastiden einiger Dioscoreaceen. – Zeitschr. Pflanzenphysiol. 57: 243-254.
Behnke H-D. 1990. Sieve elements in internodal and nodal anastomoses of the monocotyledon liana Dioscorea. – In: Behnke H-D, Sjolund RD (eds), Sieve elements: comparative structure, induction, and development, Springer, Berlin, pp. 161-178.
Bouman F. 1995. Seed structure and systematics in Dioscoreales. – In: Rudall PJ, Cribb PJ, Cutler DF, Humphries CJ (eds), Monocotyledons: systematics and evolution, Royal Botanic Gardens, Kew, pp. 139-156.
Braun HJ. 1957. Die Leitbündelbecken in den Nodien der Dioscoreaceae, mit besonderer Berücksichtigung eines neuartigen Typs assimilate-leitender Zellen. – Ber. Deutsch. Bot. Ges. 70: 305-322.
Browne ET Jr. 1961. Morphological studies in Aletris I. Development of the ovule, megaspores and megagametophyte of A. aurea and their connection with the systematics of the genus. – Amer. J. Bot. 48: 143-147.
Bruni A, Tosi B, Modenesi P. 1987. Morphology and secretion of glandular trichomes in Tamus communis. – Nord. J. Bot. 7: 79-84.
Bucherer E. 1889. Beiträge zur Morphologie und Anatomie der Dioscoreaceen. – Bibl. Bot. 3(16): 1-34.
Burkill IH. 1937. The life cycle of Tamus communis L. – J. Bot. (London) 75: 1-12, 33-34, 65-74.
Burkill IH. 1939. Notes on the genus Dioscorea in the Belgian Congo. – Bull. Jard Bot. Natl. Belg. 15: 345-392.
Burkill IH. 1946. Flied of the family Empididae and other insect-visitors to the flowers of Tamus communis. – Proc. Linn. Soc. London 157: 99-102.
Burkill IH. 1947. A plea for a description from life of the African Dioscorea minutiflora Engl. – Proc. Linn. Soc. London 159: 77-81.
Burkill IH. 1951. Dioscoreaceae. – In: Steenis CGGJ van (ed), Flora Malesiana I, 4(3), Noordhoff N. V., Batavia, pp. 293-335.
Burkill IH. 1960. The organography and evolution of Dioscoreaceae, the family of the yams. – Bot. J. Linn. Soc. 56: 319-412.
Burkill IH, Perrier de la Bâthie H. 1950. 44e Famille. Dioscoréacées. – In: Humbert H (ed), Flore de Madagascar et des Comores, Muséum National d’Histoire Naturelle, Paris, pp. 1-78.
Caddick E. 1999. Systematics of Dioscoreaceae. – Ph.D. diss., University of Reading, England.
Caddick LR, Wilkin P. 1998. A revision of the genus Stenomeris (Dioscoreaceae). – Kew Bull. 53: 703-712.
Caddick LR, Furness CA, Stobart KL, Rudall PA. 1998. Microsporogenesis and pollen morphology in Dioscoreales and allied taxa. – Grana 37: 321-336.
Caddick LR, Rudall PJ, Wilkin P. 2000. Floral morphology and development in Dioscoreales. – Feddes Repert. 111: 189-230.
Caddick LR, Rudall PJ, Wilkin P, Chase MW. 2000. Yams and their allies: systematics of Dioscoreales. – In: Wilson KL, Morrison DA (eds), Monocots: systematics and evolution, CSIRO Publ., Melbourne, pp. 475-487.
Caddick LR, Rudall PJ, Wilkin P, Hedderson TAJ, Chase MW. 2002. Phylogenetics of Dioscoreales based on combined analyses of morphological and molecular data. – Bot. J. Linn. Soc. 138: 123-144.
Caddick LR, Wilkin P, Rudall PJ, Hedderson TAJ, Chase MW. 2002. Yams reclassified: a recircumscription of Dioscoreaceae and Dioscoreales. – Taxon 51: 103-114.
Carter S. 1962. Taccaceae. – In: Hubbard CE, Milne-Redhead E (eds), Flora of tropical East Africa, Crown Agents for Oversea governments and Administrations, London, pp. 1-4.
Castillon L. 1927. Las dioscoreáceas Argentinas. – Bol. Mus. Hist. Nat. Univ. Nac.Tucumán 11: 1-41.
Chakrapani P, Raj B. 1971. Pollen morphological studies in the Burmanniaceae. – Grana 11: 164-179.
Cheadle VI, Kosakai H. 1976. Vessels in Dioscoreales. – Phyta 1: 41-53.
Cheek M. 2003. A new species of Afrothismia (Burmanniaceae) from Kenya. – Kew Bull. 58: 951-955.
Cheek M, Jannerup P. 2005. A new species of Afrothismia (Burmanniaceae) from Tanzania. – Kew Bull. 60: 593-596.
Cheek M, Williams S, Brown A. 2008. Gymnosiphon marieae sp. nov. (Burmanniaceae) from Madagascar, a species with tepal-mediated stigmatic extension. – Nord. J. Bot. 26: 230-234.
Chin H-C, Chang M-C, Ling P-P, Ting C-T, Dou F-P. 1985. A cytotaxonomic study on Chinese Dioscorea L. – Acta Phytotaxon. Sin. 23: 11-18.
Colloza A. 1910. Contributo allo studio anatomico delle Burmanniaceae. – Boll. Soc. Ital. 1910: 106-115.
Coursey DG. 1976. The origins and domestication of yams in Africa. – In: Harlan JR, De Wit JMJ, Stemler ABL (eds), Origins of African plant domestication, Mouton Publ., The Hague, Paris, pp. 383-408.
Cowley EJ. 1988. Burmanniaceae. – In: Polhill RM (ed), Flora of tropical East Africa, A. A. Balkema, Rotterdam, The Netherlands, pp. 1-9.
Cribb PJ, Wilken P, Clements M. 1995. Corsiaceae: a new family for the Falkland Islands. – Kew Bull. 50: 171-172.
Dauby G, Parmentier I, Stévart T. 2007. Afrothismia gabonensis sp. nov. (Burmanniaceae) from Gabon. – Nord. J. Bot. 25: 268-271.
De Wildeman E. 1938. Dioscorea alimentaires et toxique (morphologie et biologie). – Inst. Roy. Col. Belge, Sect. Sci. Nat. Méd., Mém., coll. in 8o, 7: 1-262.
Dimitri JM. 1972. Una nueva especie del género Arachnitis Phil. (Corsiaceae). – Rev. Fac. Agron. Univ. Nac. La Plata 48: 37-45.
Dominguez LS, Sersic A. 2006. “Prepackaged symbioses”: propagules on roots of the mycoheterotrophic plant Arachnitis uniflora. – New Phytol. 169: 191-198.
Drenth E. 1972. A revision of the family Taccaceae. – Blumea 20: 367-406.
Drenth E. 1976. Taccaceae. – In: Steenis CGGJ van (ed), Flora Malesiana I, 7, Noordhoff, Leyden, pp. 806-819.
Edeoga HO, Ikem CI. 2001. Midrib anatomy and systematics in Dioscorea L. (Dioscoreaceae). – In: Maheshwari JK, Jain AP (eds), Recent researches in plant anatomy and morphology, Scientific Publ., Jodhpur, pp. 191-195.
Engler A. 1889. Burmanniaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien II(6), W. Engelmann, Leipzig, pp. 44-51.
Engler A. 1905. Thismia winkleri Engl., eine neue afrikanische Burmanniaceae. – Engl. Bot. Jahrb. Syst. 38: 89-91.
Ernst A, Bernard C. 1910. Beiträge zur Kenntnis der Saprophyten Javas III. – Ann. Jard. Bot. Buitenzorg 23: 48-61.
Franke T. 2004. Afrothismia saingei (Burmanniaceae), a new myco-heterotrophic plant from Cameroon. – Syst. Geogr. Plants 74: 27-33.
Franke T, Sainge MN, Agerer R. 2004. A new species of Afrothismia (Burmanniaceae; tribe Thismieae) from the western foothills of Mt. Cameroon. – Blumea 49: 451-456.
Fuse S, Tamura MN. 2000. A phylogenetic analysis of the plastid matK gene with emphasis on Melanthiaceae sensu lato. – Plant Biol. 2: 415-427.
Fuse S, Lee N, Tamura M. 2012. Biosystematic studies on the family Nartheciaceae (Dioscoreales) I. Phylogenetic relationships, character evolution and taxonomic re-examination. – Plant Syst. Evol. 298: 1575-1584.
Groom P. 1895. On Thismia aseroe (Beccari) and its mycorhiza. – Ann. Bot. 9: 327-361.
Hansen DR, Dastidar SG, Cai Z, Penaflor C, Kuehl JV, Boore JL, Ransen RK. 2007. Phylogenetic and evolutionary implications of complete chloroplast genome sequences of four early-diverging angiosperms: Buxus (Buxaceae), Chloranthus (Chloranthaceae), Dioscorea (Dioscoreaceae), and Illicium (Schisandraceae). – Mol. Phylogen. Evol. 45: 547-563.
Hara H. 1967. The status of the genus Metanarthecium Maxim. – J. Jap. Bot. 42: 312-316.
Hatusima S. 1976. Two new species of Burmanniaceae from Japan. – J. Geobot. 24: 2-10.
Hewson HJ. 1986. Taccaceae. – In: George AS (ed), Flora of Australia 46, Australian Government Publ. Service, Canberra, pp. 174-176.
Ho GWC, Mar SS, Saunders RMK. 2009. Thismia tentaculata (Burmanniaceae tribe Thismieae) from Hong Kong: first record of the genus and tribe from continental China. – J. Syst. Evol. 47: 605-607.
Hsu K-m, Tsai J-L, Chen M-y, Ku H-M, Liu S-C. 2013. Molecular phylogeny of Dioscorea (Dioscoreaceae) in East and Southeast Asia. – blumea 58: 21-27.
Huber H. 1998a. Dioscoreaceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 216-235.
Huber H. 1998b. Trichopodaceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 441-444.
Ibisch P:, Neinhuis C, Rojas NP. 1996. On the biology, biogeography, and taxonomy of Arachnitis Phil. nom. cons. (Corsiaceae) in respect to a new record from Bolivia. – Willdenowia 26: 321-332.
Imhof S. 1999. Anatomy and mycotrophy of the achlorophyllous Afrothismia winkleri. – New Phytol. 144: 533-540.
Imhof S, Sainge MN. 2008. Ontogeny of the mycoheterotrophic species Afrothismia hydra (Burmanniaceae). – Bot. J. Linn. Soc. 157: 31-36.
Jessop JP. 1979. Liliaceae I. – In: Steenis CGGJ van (ed), Flora Malesiana I, 9(1), Sijthoff & Noordhoff International Publ., Alphen aan den Rijn, The Netherlands, pp. 189-235.
Jonker FP. 1938. A monograph of the Burmanniaceae. – Meded. Bot. Mus. Herb. Rijks Univ. Utrecht 51: 1-279.
Kale NN, Pai AM. 1979. The floral anatomy of Trichopus zeylanicus Gaertn. – Proc. Indian Acad. Sci., Sect. B, 88: 63-67.
Karnick CR. 1970. New aspects regarding the ‘nodal plexus’ of some Dioscorea species of India. – Pharmaceut. Biol. 10: 1607-1625.
Kitagawa I, Nakanishi T. 1981. Saponin and sapogenol XXX. Furostanol glycosides from Metanarthecium luteo-viride MAXIM.: bisdesmosides of furometagenin and furometanarthogenin. – Chem. Pharm. Bull. 29: 1299-1311.
Kitagawa M. 1983. Aletris luteoviridis (Maxim.) Franch. – In: Ohwi J, Kitagawa M (eds), New flora of Japan, Shibundo, Tokyo, p. 436. [In Japanese]
Knuth R. 1930. Dioscoreaceae. – In: Engler A (ed), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd 15a, W. Engelmann, Leipzig, pp. 438-462.
Kosenko VN. 1987. Pollen morphology of Tofieldieae, Narthecieae, Xerophylleae, and Melanthieae (Melanthiaceae). – Bot. Žurn. 72: 1318-1330. [In Russian]
Krause K. 1930. Liliaceae. – In: Engler A (ed), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 15a, W. Engelmann, Leipzig, pp. 227-386.
Kubitzki K. 1998. Taccaceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 425-428.
Larsen K, Averyanov LV. 2007. Thismia annamensis and Thismia tentaculata, two new species of Thismiaceae from central Vietnam. – Rheedea 17: 13-19.
Ling P-P. 1981. Stomatal studies in Chinese Taccaceae with a discussion of its taxonomical significance. – Bull. Nanjing Bot. Gard., Mem. Sun Yat Sen, 1981: 20-24.
Maas PJM, Maas-van der Kamer H, Benthem J van, Snelders HCM, Rübsamen T. 1986. Flora Neotropica. Monograph 42. Burmanniaceae. – New York Botanical Garden, Bronx, New York.
Maas-van de Kamer H. 1998. Burmanniaceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 154-164.
Maas-van de Kamer H. 2003. Afrothismia gesnerioides, another new species of Afrothismia (Burmanniaceae) from tropical Africa. – Blumea 48: 475-478.
Malme GOA. 1896. Die Burmannien der ersten Regnell’schen Expedition. – Bih. Kungl. Sv. Vetensk.-Akad. Handl. 22: 1-32.
Merckx V, Bidartondo MI. 2008. Breakdown and cospeciation in the arbuscular mycorrhizal mutualism. – Proc. Roy. Soc., Sect. B, 275: 1029-1035.
Merckx V, Schols V, Maas-van de Kamer H, Maas P, Huysmans S, Smets EF. 2006. Phylogeny and evolution of Burmanniaceae (Dioscoreales) based on nuclear and mitochondrial data. – Amer. J. Bot. 93: 1684-1698.
Merckx V, Schols P, Geuten K, Huysmans S, Smets E. 2008. Phylogenetic relationships in Nartheciaceae (Dioscoreales), with focus on pollen and orbicule morphology. – Belg. J. Bot. 141: 64-77.
Merckx V, Chatrou LW, Lemaire B, Sainge MN, Huysmans S, Smets EF. 2008. Diversification of myco-heterotrophic angiosperms: evidence from Burmanniaceae. – BMC Evol. Biol. 8: 178. http://www.biomedcentral.com/1471-2148/8/178.
Merckx V, Bakker F, Huysmans S, Smets E. 2009. Bias and conflict in phylogenetic inference of myco-heterotrophic plants: a case study in Thismiaceae. – Cladistics 25: 64-77.
Merckx V, Huysmans S, Smets E. 2010. Cretaceous origins of myco-heterotrophic lineages in Dioscoreales. – In: Seberg O, Petersen G, Barfod AS, Davis JI (eds), Diversity, phylogeny, and evolution in the monocotyledons, Aarhus University Press, Århus, pp. 39-53.
Miers J. 1866. On Myostoma, a new genus of the Burmanniaceae. – Trans. Linn. Soc. London 25: 461-476.
Milne-Redhead E. 1975. Dioscoreaceae. – In: Milne-Redhead E, Polhill RM (eds), Flora of tropical East Africa, Crown Agents for Oversea Governments and Administrations, London, pp. 1-26.
Minoletti ML. 1986. Arachnitis uniflora Phil. una curiosa monocotiledónea de la flora chilena. – Bol. Soc. Biol. Concepción (Chile) 57: 7-20.
Nagarjuna Rao A. 1955. Embryology of Trichopus zeylanicus Gaertn. – J. Indian Bot. Soc. 34: 213-221.
Neyland R. 2002. A phylogeny inferred from large-subunit (26S) ribosomal DNA sequences suggests that Burmanniales are polyphyletic. – Aust. Syst. Bot. 15: 19-28.
Neyland R, Hennigan M. 2003. A phylogeny inferred from large-subunit (26S) ribosome DNA sequences suggests that the Corsiaceae are polyphyletic. – New Zealand J. Bot. 41: 1-11.
Oganezova GG. 1995. On the systematical position of the families Haemodoraceae, Hypoxodaceae and Taccaceae. – Bot. Žurn. 80: 12-25. [In Russian]
Oganezova GH. 2000. Systematic position of the Trilliaceae, Smilacaceae, Herreriaceae, Tecophilaeaceae, Dioscoreaceae families and the volume and phylogeny of the Asparagales (based on seed structure). – Bot. Žurn. 85: 9-25. [In Russian]
Ogura-Tsujita Y, Umata H, Yukawa T. 2013. High mycorrhizal specificity in the mycoheterotrophic Burmannia nepalensis and B. itoana (Burmanniaceae). – Mycoscience 54: 444-448.
Orr MY. 1923. The leaf glands of Dioscorea macroura Harms. – Notes Roy. Bot. Gard. Edinb. 14: 57-72.
Orr MY 1926. On the secretory organs of the Dioscoreaceae. – Notes Roy. Bot. Gard. Edinb. 15: 133-146.
Paetow W. 1931. Embryologische Untersuchungen an Taccaceen, Meliaceen und Dilleniaceen. – Planta 14: 441-470.
Pai RM. 1966. Studies in the floral morphology and anatomy of the Burmanniaceae: I. Vascular anatomy of the flower of Burmannia pusilla (Wall. ex Miers) Thw. – Proc. Indian Acad. Sci., Sect. B, 63: 301-308.
Pan AD, Jacobs BF, Currano ED. 2014. Dioscoreaceae fossils from the late
Oligocene and early Miocene of Ethiopia. – Bot. J. Linn. Soc. 175: 17-28.
Pax F. 1888a. Taccaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien II(5), W. Engelmann, Leipzig, pp. 127-130.
Pax F. 1888b. Dioscoreaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien II(5), W. Engelmann, Leipzig, pp. 130-137; Uline EB. 1897. Nachträge zu II(5), pp. 80-87.
Pax F. 1930. Taccaceae. – In: Engler A (ed), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd 15a, W. Engelmann, Leipzig, pp. 434-437.
Perrier de la Bâthie H. 1924. Un nouveau genre de Dioscoréacées. – Bull. Soc. Bot. France 71: 25-28.
Perrier de la Bâthie H. 1950. Famille 44e bis: Trichopodacées. – In: Humbert H (ed), Flore de Madagascar, Mus. Natl. Hist. Nat., Paris.
Pfeiffer NE. 1914. Morphology of Thismia americana. – Bot. Gaz. 57: 122-135.
Pfeiffer NE. 1918. The sporangia of Thismia americana. – Bot. Gaz. 66: 354-363.
Pushpangadan P, Rajasekharan S, Ratheesh Kumar PK, Jawahar CR, Velayudhan Nair V, Lakshmi N, Sarada Amma L. 1988. “Arogyapacha” (Trichopus zeylonicus Gaertn). The Ginzeng of Kani tribes of Agasthyar Hills (Kerala) for evergreen health and vitality. – Ancient Sci. Life 8: 13-16.
Ramachandran K. 1968. Cytological studies in the Dioscoreaceae. – Cytologia 33: 401-410.
Rao NA. 1953. Embryology of Dioscorea oppositifolia L. – Phytomorphology 3(2): 121-126.
Rao VS. 1969a. Certain salient features in the floral anatomy of Burmannia, Gymnosiphon, and Thismia. – J. Indian Bot. Soc. 48(1-2): 22-29.
Rao VS. 1969b. The vascular anatomy of Tacca pinnatifida. – J. Univ. Bombay 38, 65: 18-24.
Rao VS. 1970. Certain salient features in the floral anatomy of Burmannia, Gymnosiphon and Thismia. – J. Indian Bot. Soc. 48: 22-29.
Rasmussen FN. 1995. Relationships of Burmanniales and Orchidales. – In: Rudall PJ, Cribb PJ, Cutler DF, Humphries CJ (eds), Monocotyledons: systematics and evolution, Royal Botanic Gardens, Kew, pp. 227-241.
Remizowa M, Sokoloff D, Rudall PJ. 2006a. Evolution of the monocot gynoecium: evidence from comparative morphology and development in Tofieldia, Japonolirion, Petrosavia and Narthecium. – Plant Syst. Evol. 258: 183-209.
Remizowa M, Sokoloff D, Rudall PJ. 2006b. Comparative patterns of floral orientation, bracts and bracteoles in Tofieldia, Japonolirion, and Narthecium. – Aliso 24: 157-169.
Remizowa M, Sokoloff D, Rudall PJ. 2006c. Patterns of floral structure and orientation in Japonolirion, Narthecium and Tofieldia. – In: Columbus JT, Friar EA, Porter JM, Prince LM, Simpson MG (eds), Monocots: comparative biology and evolution. Excluding Poales, Rancho Santa Ana Botanical Garden, Claremont, California. – Aliso 22: 159-171.
Remizowa M, Sokoloff D, Kondo K. 2008. Floral evolution in the monocot family Nartheciaceae (Dioscoreales): evidence from anaomy and development in Metanarthecium luteo-viride Maxim. – Bot. J. Linn. Soc. 158: 1-18.
Remizowa M, Sokoloff DD, Kondo K. 2010. Early flower and inflorescence development in Dioscorea tokoro (Dioscoreales): shoot chirality, handedness of cincinni and common tepal-stamen primordia. – Wulfenia 17: 77-97.
Rübsamen T. 1983. Nectaries of the Burmanniaceae (Burmannieae). – Acta Bot. Neerl. 32: 351.
Rübsamen T. 1986. Morphologische, embryologische und systematische Untersuchungen an Burmanniaceae und Corsiaceae (mit Ausblick auf die Orchidaceae-Apostasioideae). – Diss. Bot. 92: 1-310.
Rudall P, Morley S. 1992. Embryosac and early post-fertilisation development in Thismia (Burmanniaceae). – Kew Bull. 47: 625-632.
Sainge MN, Franke T. 2005. A new species of Afrothismia (Burmanniaceae) from Cameroon. – Nord. J. Bot. 23: 299-303.
Sasidharan N, Sujanapal P. 2000. Rediscovery of Haplothismia exannulata Airy Shaw (Burmanniaceae) from its type locality. – Rheedea 10: 131-134.
Satô D. 1942. Karyotype alteration and phylogeny in Liliaceae and allied families. – Jap. J. Bot. 12: 57-161.
Sato Y, Kirito E. 1988. Formation of embryo-sac and callose deposition during its development in Aletris luteoviridis. – Sci. Rep. Yokohama Natl. Univ. (Sect. II) 35: 47-56.
Saw LG. 1993. Tacca: flowering and fruiting behaviour. – Nat. Malaysiana 18: 3-6.
Schlechter R. 1906. Burmanniaceae africanae. – Engl. Bot. Jahrb. Syst. 38: 137-143.
Schlechter R. 1921. Die Thismieae. – Notizbl. Bot. Gart. Mus. Berlin-Dahlem 8: 31-45.
Schols P. 2004. Contributions to the palynology and phylogeny of Dioscorea (Dioscoreaceae). – PhD diss., Katholieke Universiteit Leuven, Leuven, Belgium
Schols P, Furness CA, Wilkin P, Huysmans S, Smets E. 2001. Morphology of pollen and orbicules in some Dioscorea species and its systematic implications. – Bot. J. Linn. Soc. 136: 295-311.
Schols P, Furness CA, Wilkin F, Smets E, Cielen V, Huysmans S. 2003. Pollen morphology of Dioscorea (Dioscoreaceae) and its relation to systematics. – Bot. J. Linn. Soc. 143: 375-390.
Schols P, Furness CA, Merckx V, Wilkin P, Smets E. 2005. Comparative pollen development in Dioscoreales. – Intern. J. Plant Sci. 166: 909-924.
Schols P, Wilkin P, Furness CA, Huysmans S, Smets E. 2005. Pollen evolution in yams (Dioscorea: Dioscoreaceae). – Syst. Bot. 30: 750-758.
Shah GL, Gopal BV. 1972. Some observations on the diversity of stomata and trichomes in six species of Dioscorea. – Ann. Bot., N. S., 36: 997-1004.
Sharma OP. 1974. Anatomy, origin and development of tuber of Dioscorea glabra. – Phytomorphology 24: 297-305.
Shin T. 1974. Two species of the genus Glaziocharis (Burmanniaceae) from southern Kyushu. – J. Jap. Bot. 49: 3-6. [In Japanese]
Simpson MG. 1981. Embryological development of Lachnanthes caroliniana (Lam.) Dandy and Lophiola aurea Ker-Gawler (Haemodoraceae) and its taxonomic significance. – Bot. Soc. Amer., Misc. Ser. 160: 78.
Simpson MG, Dickison WC. 1981. Comparative anatomy of Lachnanthes and Lophiola (Haemodoraceae). – Flora 171: 95-113.
Sivarajan VV, Pushpangadan P, Ratheesh Kumar PK. 1990. A revision of Trichopus. – Kew Bull. 45: 353-360.
Smith J. 1909. Burmanniaceae, Corsiaceae. – Nova Guinea 8: 193-197.
Sochor M, Hroneš M, Dančák M. 2018. New insights into variation, evolution and taxonomy of fairy lanterns (Thismia, Thismiaceae) with four new species from Borneo. – Plant Syst. Evol. 304: 699-721.
Stenar H. 1931. Die Art der Pollenbildung bei Narthecium ossifragum Huds. – Bot. Not. 1931: 51-54.
Stone BC. 1980. Rediscovery of Thismia clavigera (Becc.) F. v. M. (Burmanniaceae). – Blumea 26: 419-425.
Sujanapal P, Robi AJ, Dantas KJ, Sumod M, MerckxVSFT. 2017. Thismia (Thismiaceae): the first record of the mycoheterotrophic genus to the Flora of India with a new species revealing the phytogeographical significance of Western Ghats. – Blumea 62: 97-102.
Susan Chacko, Sethuraman MG, George V, Pushpangadan P. 2002. Phytochemical constituents of Trichopus zeylanicus ssp. travancoricus. – J. Med. Arom. Plant Sci. 24: 703-706.
Takahashi M, Kawano S. 1989. Pollen morphology of the Melanthiaceae and its systematic implications. – Ann. Missouri Bot. Gard. 76: 863-876.
Takeda K. 1961. The structure of luvigenin, a new sapogenin from Metanarthecium luteo-viride Maxim. – Tetrahedron 15: 183-186.
Tamura MN. 1998. Nartheciaceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 381-392.
Tamura MN, Fus S, Azuma H, Hasebe M. 2004. Biosystematic studies on the family Tofieldiaceae I. Phylogeny and circumscription of the family inferred from DNA sequences of matK and rbcL. – Plant Biol. 6: 562-567.
Teichman und Logischen I von, Robbertse PJ, Schijff HP van der. 1977. The subterranean intermediary organs of Dioscorea cotinifolia Kunth 1. The germination, development, morphology and vegetative reproduction of the tuberous swollen and cylindrical intermediary organs. – J. South Afr. Bot. 43: 41-56.
Telford IRH. 1986. Dioscoreaceae. – In: George AS (ed), Flora of Australia 46, Australian Government Publ. Service, Canberra, pp. 196-202.
Tenorio V, Couto RS, Albuquerque ESB de, Medeiros AML, Oliveira Ferreira R de, Braga JMA, Vieira RC. 2017. Stem anatomy of neotropical Dioscorea L. (Dioscoreaceae) and its importance to the systematics of the genus. – Plant Syst. Evol. 303: 775-786.
Thiele KR, Jordan P. 2002. Thismia clavarioides (Thismiaceae), a new species of fairy lantern from New South Wales. – Telopea 9: 765-771.
Torshilova AA, Titova GE. 2010. Seed formation and morphogenetic correlations in structure development in Dioscorea nipponica (Dioscoreaceae). – Bot. Žurn. 95: 298-325. [in Russian]
Torshilova AA, Titova GE, Batygina TB. 2003. Female reproductive structures and seed development in Dioscorea nipponica Makino (Dioscoreaceae). – Acta Biol. Cracov. 435: 149-154.
Tsukaya H, Yokota M, Okada H. 2007. Chromosomal characters of Oxygyne shinzatoi (Burmanniaceae) and its phylogenetic significance. – Acta Phytotaxon. Geobot. 58: 100-106.
Téllez-Valdés O, Geeta R. 2007. Dioscorea howardiana, a new species in Dioscorea section Trigonobasis (Dioscoreaceae). – Brittonia 59: 370-373.
Ugarte E, Arriagada J. 1983. Presencia de Arachnitis uniflora Phil. (Corsiaceae) en la peninsula de Hualpén, Concepción, Chile. – Bol. Soc. Biol. Concepción 54: 167-170.
Utech FH. 1978. Floral vascular anatomy of monotypic Japanese Metanarthecium luteoviride Maxim. (Liliaceae-Melanthioideae). – Ann. Carnegie Mus. 47: 455-477.
Varitchak B. 1940. Le développement du sac embryonnaire et le nombre de chromosomes chez la plante Narthecium scadicum Kosanin. – Bull. Acad. Sci. (Beograd), Ser. B, 6: 97-105.
Viruel J, Forest F, Paun O, Chase MW, Devey D, Couto RS, Segarra-Moragues JG, Catalán P, Wilkin P. 2018. A nuclear Xdh phylogenetic analysis of yams (Dioscorea: Dioscoreaceae) congruent with plastid trees reveals a new Neotropical lineage. – Bot. J. Linn. Soc. 187: 232-246.
Watson EV. 1936. A study of the anatomy of Trichopus zeylanicus Gaertn. – Notes Roy. Bot. Gard. Edinb. 19: 135-156.
Wilkin P. 1999. A revision of the compound-leaved yams (Dioscorea, Dioscoreaceae) of Africa. – Kew Bull. 54: 19-39.
Wilkin P. 2001. Dioscoreaceae of South-Central Africa. – Kew Bull. 56: 361-404.
Wilkin P, Caddick L, Foster C, Schols P. 2000. A new species of Dioscorea (Dioscoreaceae) from eastern Madagascar and its pollen morphology. – Kew Bull. 55: 427-434.
Wilkin P, Rakotonasolo F, Schols P, Furness CA. 2002. A new species of Dioscorea (Dioscoreaceae) from Western Madagascar and its pollen morphology. – Kew Bull. 57: 901-909.
Wilkin P, Schols P, Chase MW, Chayamarit K, Furness CA, Huysmans S, Rakotonasolo F, Smets E, Thapyai C. 2005. A plastid gene phylogeny of the yam genus, Dioscorea: roots, fruits and Madagascar. – Syst. Bot. 30: 736-749.
Wilkin P, Hladik A, Labat J-N, Barthelat F. 2007. A new edible yam (Dioscorea L.) species endemic to Mayotte, new data on D. comorensis R. Knuth and a key to the yams of the Comoro Archipelago. – Adansonia, sér. III, 29: 215-228.
Wilkin P, Peterson Andrianantenaina W, Jeannoda V, Hladik A. 2008. The species of Dioscorea L. (Dioscoreaceae) from Madagascar with campanulate tori, including a new species from Eastern Madagascar. – Kew Bull. 63: 583-600.
Wilkin P, Hladik A, Jeannoda V, Weber O. 2009. The threatened edible yams of the Dioscorea sambiranensis R. Knuth species complex (Dioscoreaceae): a new species and subspecies. – Adansonia, sér. III, 31: 249-266.
Wilkin P, Muasya AM, Banks H, Furness CA, Vollesen K, Weber O, Demissew S. 2009. A new species of yam from Kenya, Dioscorea kituiensis. Pollen morphology, conservation status, and speciation. – Syst. Bot. 34: 652-659.
Woodward CL, Berry PE, Maas-van de Kamer H, Swing K. 2007. Tiputinia foetida, a new mycoheterotrophic genus of Thismiaceae from Amazonian Ecuador, and a likely case of deceit pollination. – Taxon 56: 157-162.
Yang S, Saunders RMK, Hsu C. 2002. Thismia taiwanensis sp. nov. (Burmanniaceae tribe Thismieae): first record of the tribe in China. – Syst. Bot. 27: 485-488.
Yokoyama J, Koizumi Y, Yokota M, Tsukaya H. 2008. Phylogenetic position of Oxygyne shinzatoi (Burmanniaceae) inferred from 18S rDNA sequences. – J. Plant Res. 121: 27-32.
Zavada M, Xu X, Edwards JM. 1983. On the taxonomic status of Lophiola aurea Ker-Gawler. – Rhodora 85: 73-81.
Zhang D, Saunders RMK. 2000. Reproductive biology of a mycoheterotrophic species, Burmannia wallichii (Burmanniaceae). – Bot. J. Linn. Soc. 132: 359-367.
Zhao Y-M, Wang W, Zhang S-R. 2012. Delimitation and phylogeny of Aletris (Nartheciaceae) with implications for perianth evolution. – J. Syst. Evol. 50: 135-145.