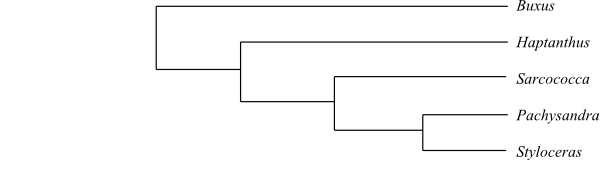
Cladogram of Buxaceae based on DNA sequence data (Shipunov & Shipunova 2011). The position of Haptanthus is somewhat uncertain, since it may be sister to Buxus, according to some gene data.
[Didymelales+Gunneridae]
Fossils The Late Albian to Early Cenomanian Spanomera from Maryland is represented by monoecious decussate inflorescences with units bearing a terminal female flower and a pair of lateral male flowers. The pollen grains are tricolpate, the two carpels basally slightly connate and the fruits follicular. Lusistemon striatus is male flowers with up to six antetepalous stamens from the Late Aptian to the Early Albian of Portugal. The pollen grains are tricolpate with a striate exine, and the fruits – developed from two connate carpels – have been described as Lusicarpus planatus. Aguacarpus hirsutus and Valecarpus pedicellatus comprise two tricarpellate syncarpous gynoecia with reflexed styles from the Late Aptian to the Early Albian of Portugal.
Habit Usually monoecious (rarely dioecious, polygamodioecious or bisexual), evergreen trees or shrubs (sometimes perennial herbs).
Vegetative anatomy Phellogen ab initio subepidermal or pericyclic. Vessel elements with scalariform perforation plates; lateral pits usually opposite or alternate (rarely intermediary between scalariform and opposite), bordered pits. Imperforate tracheary xylem elements usually vessel-like tracheids (rarely fibre tracheids) with usually bordered (sometimes simple) pits, non-septate (also vasicentric tracheids). Wood rays uniseriate or multiseriate, homocellular or heterocellular. Axial parenchyma usually apotracheal diffuse or diffuse-in-aggregates, or paratracheal scanty (sometimes unilateral; occasionally absent). Compression wood present. Parenchyma often with secretory cells and sclereids. Sieve tube plastids Pc or Pcs type, with a central globular protein crystal. Nodes 1:1, unilocular with one leaf trace. Druses sometimes present. Chains of small irregularly thickened sclereids with calciumoxalate crystals, surrounding a larger fibre, sometimes present.
Trichomes Hairs usually unicellular (sometimes multicellular), simple, or absent.
Leaves Alternate (spiral) or opposite, simple, entire, usually coriaceous, with ? ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle transection arcuate. Venation usually pinnate, usually brochidodromous (rarely craspedodromous, eucamptodromous or palmate). Stomata usually laterocytic (sometimes cyclocytic or anomocytic). Cuticular wax crystalloids as curved rods or irregular platelets. Mesophyll often with sclerenchymatous idioblasts. Lamina usually with secretory canals. Idioblasts with ethereal oils absent. Calciumoxalate as druses or single prismatic crystals. Leaf margin usually entire (rarely serrate, with chloranthoid teeth). Peltate hairs sometimes present.
Inflorescence Usually terminal or axillary (or supra-axillary) racemes, spikes, heads or panicles (female flowers sometimes solitary, axillary).
Flowers Actinomorphic, small. Hypogyny. Tepals in male flowers (two to) four (or five), sepaloid or bracteate, with decussate or imbricate aestivation, connate at base (sometimes absent); tepals in female flowers (one to) four to six (rarely up to c. 20 or absent), some of them usually spiral. Nectaries often present between stylodia (sometimes absent). Disc absent.
Androecium Stamens (two or) four (or six; rarely up to c. 45), antetepalous (when six stamens, then two pairs opposite inner tepals). Filaments usually free from each other (sometimes connate at base) and from tepals (sometimes absent). Anthers basifixed or dorsifixed, non-versatile, tetrasporangiate, usually introrse (sometimes extrorse), longicidal (dehiscing by longitudinal slits). Tapetum secretory. Female flowers often with staminodia?
Pollen grains Microsporogenesis simultaneous. Pollen grains 3–7-zonocolporate, 5–12-pantocolporate (with two or more ora per colpus), 5–12-pantocolpate or 12–40-pantoporate (sometimes colpodiorate), with circular ectoapertures, shed as monads, bicellular at dispersal. Exine semitectate, with columellate infratectum, reticulate, often with supratectal spinules or pilate-verrucate elements (tectum rarely crotonoid, with pores and lumina surrounded by upraised structures consisting of discoid or triangular elements).
Gynoecium Pistil composed of one to four almost free carpels; carpel plicate, postgenitally completely fused, without canal. Ovary superior, uni-, bi- or trilocular (sometimes quadri- or sexalocular due to secondary septum). Stylodium one or absent, or stylodia two to four, marginal, usually free (rarely connate at base), with two crests. Stigmas usually decurrent (stylodia often stigmatic their entire length; rarely truncate), often with a median furrow (rarely somewhat connate at base), papillate, Dry or Wet type. Male flowers usually with pistillodium.
Ovules Placentation axile to apical. Ovules (one or) two per carpel, anatropous, hemianatropous or campylotropous, pendulous, apotropous or epitropous, bitegmic, crassinucellar. Micropyle usually endostomal (sometimes bistomal or exostomal). Placental obturator usually present. Nucellar cap usually well developed. Megagametophyte monosporous, Polygonum type. Synergids usually with a filiform apparatus. Antipodal cells sometimes proliferating. Endosperm development usually cellular (rarely nuclear). Endosperm haustoria? Embryogenesis onagrad.
Fruit Usually a loculicidal capsule with persistent style (sometimes a berry or a drupe).
Seeds Aril rudimentary or absent. Seeds often with caruncle formed from outer integument. Seed coat exotestal-mesotestal. Testa sometimes multiplicative. Exotesta palisade, with lignified cell walls. Mesotesta sometimes sclerotic. Endotesta often palisade. Tegmen unspecialized? Perisperm usually not developed. Endosperm copious, oily. Embryo short to long, usually straight (rarely curved), well differentated, without chlorophyll. Cotyledons two. Germination phanerocotylar.
Cytology n = 10, 12–14, 24, 27.
DNA Mitochondrial genes rps2 and rps11 absent (lost). Nuclear gene paleoAP3?
Phytochemistry Flavonols (kaempferol, quercetin), iridoids, proanthocyanidins, steroidal pregnane pseudoalkaloids (aminopregnanes, formed from triterpenoids), dhurrin (a cyanogenic glycoside), pinitol, simmondsin-like compounds, and arbutin present. Ellagic acid, tannins, benzylisoquinoline alkaloids, and saponins not found.
Systematics Buxaceae are sister to Didymeles (Didymelaceae).
BUXACEAE Dumort. |
Pachysandraceae J. Agardh, Theoria Syst. Plant.: 358. Apr-Sep 1858 [‘Pachysandreae’]; Stylocerataceae (Pax) Takht. ex Reveal et Hoogland in Bull. Mus. Natl. Hist. Nat., sér. 4, sect. B, Adansonia, 12: 206. 24 Nov 1990; Buxales Takht. ex Reveal in Novon 2: 238. 13 Oct 1992; Buxanae (Müll. Arg.) Takht. ex Reveal et Doweld in Novon 9: 549. 30 Dec 1999; Haptanthaceae C. Nelson in Ceiba 42: 33. Mai 2001
Genera/species 5/90–100
Distribution Tropical and southern Africa, Madagascar, Socotra, northeastern Africa, northern Macaronesia, western and central Mediterranean, eastern Turkey, the Caucasus and northern Iran, Afghanistan, the Himalayas, southern India, Sri Lanka, East Asia to the Korean Peninsula and Japan, Southeast Asia, West and Central Malesia, southern North America, Central America, northwestern South America (the Andes).
Fossils Polyporate pollen grains similar to those in some extant Buxaceae have been found in Campanian to Maastrichtian and Cenozoic layers in Europe, Siberia and North America, and described as Erdtmanipollis. Paleocene and younger pollen grains assigned to Buxapollis and similar to Buxus are known from several sites in the Northern Hemisphere.
Habit Usually monoecious (rarely dioecious, polygamodioecious or bisexual), evergreen trees or shrubs; Pachysandra consists of perennial herbs with a rhizome.
Vegetative anatomy Phellogen ab initio subepidermal or pericyclic. Primary cortex with long fibres and large stone cells. Vessel elements with scalariform perforation plates; lateral pits usually opposite or alternate (in, e.g., Styloceras intermediary between scalariform and opposite), bordered pits. Imperforate tracheary xylem elements usually vessel-like tracheids (in Styloceras fibre tracheids) with usually bordered pits (sometimes simple pits), non-septate (also vasicentric tracheids). Wood rays uniseriate or multiseriate, homocellular or heterocellular. Axial parenchyma usually apotracheal diffuse or diffuse-in-aggregates, or paratracheal scanty (sometimes unilateral; occasionally absent). Parenchyma with secretory cells, single or in branched rows. Sieve tube plastids Pc or Pcs type, with a central globular protein crystal, with or without starch grains. Nodes 1:1, unilocular with one leaf trace, or 3:3, trilocular with one trace (in Sarcococca 1:3, unilocular with three traces). Heartwood sometimes with resinous substances. Chains of small irregularly thickened sclereids with calciumoxalate crystals, surrounding a larger fibre, present in Sarcococca.
Trichomes Hairs usually unicellular (sometimes multicellular, uniseriate), or absent.
Leaves Alternate (spiral; Pachysandra, Sarcococca, Styloceras) or opposite (Buxus, Haptanthus), simple, entire, usually coriaceous, with curved or flat ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle transection arcuate, three bundles present in upper part of petiole. Venation usually pinnate, usually brochidodromous (in Pachysandra craspedodromous, in Styloceras eucamptodromous; rarely almost palmate). Stomata usually laterocytic (sometimes cyclocytic or anomocytic). Cuticular wax crystalloids as twisted rodlets or irregular platelets or as transitional types between twisted rodlets and tubuli, chemically characterized by presence of β-diketones. Lamina with secretory cells and secretory canals. Idioblasts with ethereal oils absent. Calciumoxalate as druses or single prismatic crystals. Lamina usually entire (in Pachysandra serrate, with chloranthoid teeth).
Inflorescence Usually terminal or axillary raceme or botryoid, spike- or head-like (female flowers sometimes solitary, axillary); in Buxus terminal flower of cyme female and lateral flowers male; in Pachysandra lowermost lateral flower of cyme female and remaining flowers male; in Haptanthus terminal stipitate female flower subtended by raceme-like cymose male partial inflorescence. Female flowers in Buxus subtended by bract-like paired scales.
Flowers Actinomorphic, small. Hypogyny. Tepals in male flowers (two to) four (or five), sepaloid or bracteate (in Haptanthus four, very small, or absent), decussate, with imbricate aestivation, connate at base (absent in Styloceras); tepals in female flowers usually four to six (rarely up to c. 20; in Haptanthus very small or absent), spiral (at least some), free? Nectaries in Buxus inserted between stylodia (not in Pachysandra, Sarcococca and Styloceras). Disc absent.
Androecium Stamens usually four (sometimes three or six; in Styloceras six to c. 45; in Haptanthus two), antetepalous (when six stamens, then two pairs opposite inner tepals); stamens in Pachysandra, Sarcococca and Styloceras showy. Filaments often wide, strongly exserted, free from each other and from tepals (in Haptanthus adnate to an elliptic structure [filament?] with an arcuate vascular bundle), in Buxus very short or absent. Anthers basifixed or dorsifixed, non-versatile, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits); endothecium in Haptanthus significantly developed, massive. Tapetum secretory, with binucleate cells. Staminodia absent.
Pollen grains Microsporogenesis simultaneous. Pollen grains 3–7-zonocolporate, 5–12-pantocolporate (with two or more ora in each colpus), 5–12-pantocolpate or 12–40-pantoporate (in Haptanthus tricolp[oroid]ate), shed as monads, bicellular at dispersal. Exine semitectate, with columellate infratectum, reticulate, often with spinules or supratectal pilate-verrucate elements (Sarcococca with crotonoid tectum, pores and lumina surrounded by upraised structures consisting of discoid and triangular elements).
Gynoecium Pistil in Buxus and Haptanthus composed of usually three (sometimes four), in Pachysandra, Sarcococca and Styloceras usually two, almost free carpels; carpel plicate, postgenitally completely fused, without canal. Ovary superior, usually bi- or trilocular (in Pachysandra and Styloceras quadri- or sexalocular due to secondary septum; in Haptanthus unilocular). Styles (stylodia) usually three (sometimes two or four; in Haptanthus absent), marginal, usually free (rarely connate at base), often persistent. Stigmas bilobate, decurrent, wide, often with a median furrow (rarely somewhat connate at base; in Haptanthus trilobate with recurved lobes, stigmatic their entire length), papillate, Dry or slightly Wet type. Male flowers usually with pistillodium (absent in, e.g., Haptanthus).
Ovules Placentation usually axile to apical (parietal in Haptanthus). Ovules usually two (in Haptanthus eight to 15) per carpel, anatropous or campylotropous, pendulous, apotropous, bitegmic, crassinucellar. Micropyle in Buxus endostomal, in Pachysandra, Sarcococca and Styloceras bistomal. Outer integument two to ten cell layers thick. Inner integument two or three cell layers thick. Obturator, formed from placenta, usually present (absent in Styloceras?). Parietal tissue four to more than 15 cell layers thick. Nucellar cap present, often weakly developed. Megagametophyte monosporous, Polygonum type. Synergids with a filiform apparatus. Antipodal cells sometimes proliferating. Endosperm development usually ab initio cellular (in Sarcococca nuclear). Endosperm haustoria? Embryogenesis onagrad.
Fruit A loculicidal capsule (Buxus, Pachysandra), with persistent style, or baccate to drupaceous (Sarcococca, Styloceras); in Haptanthus unknown.
Seeds Seeds often with caruncle (in Buxus rudimentary), formed from outer integument. Seed coat exotestal-mesotestal. Testa in Pachysandra and Sarcococca multiplicative. Exotestal cells with lignified walls, palisade. Mesotesta sometimes sclerotized. Endotesta often palisade. Tegmen unspecialized? Hypodermal cell walls often lignified. Perisperm usually not developed (present in Sarcococca). Endosperm copious, oily. Embryo short to elongate, usually straight (rarely curved), well differentated, without chlorophyll. Cotyledons two, thin, flattened. Germination phanerocotylar.
Cytology n = 10, 12–14, 24, 27 – Agamospermy occurring in some species of Sarcococca.
DNA Mitochondrial genes rps2 and rps11 absent (lost). Nuclear gene paleoAP3?
Phytochemistry Flavonols (kaempferol, quercetin), steroidal pregnane alkaloids (aminopregnanes), triterpene alkaloids (e.g. cycloprotobuxine-C and buxamine-G), proanthocyanidins, dhurrin (a cyanogenic glycoside), arbutin, pinitol, and simmondsin-like compounds present. Iridoids? Ellagic acid, tannins, benzylisoquinoline alkaloids, and saponins not found. Raffinose and stachyose often present in phloem exudate. Nickel accumulated in some species of Buxus.
Use Ornamental plants (Buxus sempervirens, Pachysandra terminalis etc.), timber, carpentry, engravings (Buxus).
Systematics Buxaceae are sister to Didymeles (Didymelaceae).
Buxus is probably sister to the remaining Buxaceae.
Buxeae Dumort., Anal. Fam. Plant.: 45. 1829 [‘Buxineae’]
1/70–80. Buxus (70–80; western and southern Europe, northern Africa to South Africa, Madagascar, southwestern, southern and eastern Asia, Central America and the West Indies, especially Cuba). – Cortical vascular bundles sometimes present. Female inflorescences with opposite basal bracteoid scale-like leaves. Hypogyny. Nectaries usually present between stylodia (sometimes absent). Pollen grains tri- to polycolpate (colpus with two to four endoapertures) or polyporate. Carpels three, median carpel adaxial. Micropyle endostomal. Outer integument five to ten cell layers thick. Capsule loculicidal, explosively dehiscent. Seeds with rudimentary caruncle. Embryo curved. Radicula elongated. n = 14, 20. – The pollen grains in Buxus have often several ora per colpus and hence are similar to those in Didymeles (Didymelaceae).
Stylocerateae Pax in Engler et Prantl, Nat. Pflanzenfam. III, 5: 132, 134. Jun 1892 [‘Stylocereae’]
4/27? Haptanthus (1; H. hazlettii; Matarras in Honduras); Sarcococca (c 15?; tropical and subtropical Asia from Afghanistan and the Himalayas to China, Southeast Asia and Malesia including the Philippines, Guatemala?), Pachysandra (5; P. axillaris, P. procumbens, P. coriacea, P. stylosa, P. terminalis; India, Nepal, Burma, China, Japan, Taiwan, southeastern United States), Styloceras (6; S. brokawii, S. columnare, S. connatum, S. kunthianum, S. laurifolium; the Andes in South America). – East Asia to West Malesia, eastern North America, northern South America. Fibres in Haptanthus storied. Nodes 1:3 (Sarcococca). Chains of small irregularly thickened sclereids, containing calciumoxalate crystals, surrounding a larger fibre. Leaves usually spiral. Female flowers with numerous spiral bracts. Floral prophylls (bracteoles) absent in Haptanthus. Male flowers in Styloceras and Haptanthus without perianth. Nectary absent. Stamens usually four (in Styloceras six to c. 45; in Haptanthus two, with wide filaments and anthers attached their entire length). Pollen grains pantoporate, with crotonoid exine surface elements. Carpels usually two (sometimes with divided loculi; in Haptanthus three, stipitate). Male flowers usually with pistillodium (pistillodium absent in Haptanthus). Placentation in Haptanthus parietal. Ovules eight to 15 per carpel. Micropyle bistomal. Outer integument usually two or three cell layers thick. Endosperm development in Sarcococca nuclear. Testa often multiplicative. Endotegmic cell walls at least in Sarcococca lignified. Perisperm present in Sarcococca. Embryo with short radicula. n = 12–14. – Haptanthus may be sister to the remaining three genera in Stylocerateae, according to maximum parsimony analyses (Shipunov & Shipunova 2011).
 |
Cladogram of Buxaceae based on DNA sequence data (Shipunov & Shipunova 2011). The position of Haptanthus is somewhat uncertain, since it may be sister to Buxus, according to some gene data. |
DIDYMELACEAE Leandri |
Genera/species 1/2
Distribution Eastern and northern Madagascar, the Comoros.
Fossils Uncertain. Pollen grains which have been found in Palaeogene layers in the Ninetyeast Ridge in the Indian Ocean, in Australia and in New Zealand have been described as Schizocolpus marlinensis and may be assigned to Didymelaceae.
Habit Dioecious, evergreen trees.
Vegatative anatomy Phellogen? Cork tissue late and/or weakly developed. Medulla wide, chambered. Non-xylem tissues in bark, stem and leaves with numerous corse, thick fibres. Vessel elements with scalariform perforation plates; lateral pits opposite or alternate, bordered pits? Imperforate tracheary xylem elements tracheids with small bordered pits, non-septate? Pericyclic fibres absent. Wood rays multiseriate?, heterocellular, ten to c. 30 cells tall, with wings of erect cells. Axial parenchyma absent, replaced by lignified cells. Sieve tube plastids ? type. Nodes? Secretory canals absent. Pericyclic and cortical sclereids present. Druses present.
Trichomes Hairs small, peltate (on leaves).
Leaves Alternate (spiral), simple, entire, often coriaceous, with ? ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle transection arcuate with one central and two inverted lateral vascular bundles. Venation pinnate, brochidodromous. Stomata cyclocytic. Cuticular waxes? Mesophyll with sclerenchymatous idioblasts. Calciumoxalate druses present. Leaf margin entire.
Inflorescence Axillary (or supra-axillary), raceme-like (with female flowers solitary or three together) or short panicle (reduced thyrso-paniculate?; with male flowers).
Flowers Actinomorphic, small. Hypogyny? Male flowers without tepals, often with one or two scales (floral prophylls?). Female flowers (in axil of bract) with one to three scale-like tepals (floral prophylls and bract?) or without tepals. Nectary absent. Disc absent.
Androecium Stamens two. Filaments short, connate at base. Anthers basifixed to somewhat dorsifixed, non-versatile, tetrasporangiate, extrorse, longicidal (dehiscing with longitudinal slits). Tapetum? Staminodia absent.
Pollen grains Microsporogenesis? Pollen grains tricolpodiorate (with two ora per colpus) with linear operculum in colpus, shed as monads, ?-cellular at dispersal. Exine semitectate, with columellate simplibaculate infratectum, reticulate.
Gynoecium Pistil composed of one carpel with adaxial suture; carpel plicate? Ovary superior, unilocular (monocarpellate). Scale-like structure present between bract and ovule. Stylodium very short or absent. Stigma truncate or obliquely decurrent, bifid, papillate, type? Pistillodium absent.
Ovules Placentation apical or subapical. Ovule usually one (rarely two) per carpel, hemianatropous, pendulous, epitropous, bitegmic, crassinucellar. Micropyle exostomal. Integuments prolonged to an elongate annular collar into stylar canal. Outer integument ? cell layers thick, fimbriate at apex. Inner integument ? cell layers thick. Chalaza conically prolonged. Megagametophyte monosporous, Polygonum type? Endosperm development cellular? Endosperm haustoria? Embryogenesis?
Fruit A one-seeded drupe with lateral furrow and persistent stigma.
Seeds Aril rudimentary or absent. Seed coat testal? Testal cell walls thickened (lignified?). Tegmen? Perisperm not developed? Endosperm absent. Embryo large, straight, well differentiated, chlorophyll? Cotyledons two, thick. Germination?
Cytology n = ?
DNA Mitochondrial genes rps2 and rps11 absent? Nuclear gene paleoAP3?
Phytochemistry: Insufficiently known. Steroid pregnane pseudoalkaloids (aminopregnanes) present. Iridoids? Aluminium accumulated.
Use Unknown.
Systematics Didymeles (2; D. integrifolia: eastern coast of Madagascar, Moheli in the Comoros; D. perrieri: Tsaratanana, Montagne d’Ambre and Andohahela in Madagascar).
Literature
Ahond A, Debray MM, Picot F, Poupat C, Sánchez V, Potier P. 1980. Alkaloïdes stéroïdique de Didymeles cf. madagascariensis. – Planta Med. 39: 204.
Balthazar M von, Endress PK. 2002a. Development of inflorescences and flowers in Buxaceae and the problem of perianth interpretation. – Intern. J. Plant Sci. 163: 847-876.
Balthazar M von, Endress PK. 2002b. Reproductive structures and systematics of Buxaceae. – Bot. J. Linn. Soc. 140: 193-228.
Balthazar M von, Endress PK, Qiu Y-L. 2000. Phylogenetic relationships in Buxaceae based on nuclear internal transcribed spacers and plastid ndhF sequences. – Intern. J. Plant Sci. 161: 785-792.
Balthazar M von, Schatz GE, Endress PK. 2003. Female flowers and inflorescences of Didymelaceae. – Plant Syst. Evol. 237: 199-208.
Barabé D, Bergeron Y, Vincent GA. 1987. La répartition des caractères dans la classification des Hamamelididae (Angiospermae). – Can. J. Bot. 65: 1756-1767.
Baranova MA. 1980. Comparative stomatographic studies in the families Buxaceae and Simmondsiaceae. – In: Žilin SG (ed), Systematics and evolution of higher plants, Nauka, Leningrad, pp. 68-75. [In Russian]
Batdorf LR. 1995. Boxwood handbook. – American Boxwood Society, Boyce.
Behnke H-D. 1982. Sieve-element plastids, exine sculpturing, and the systematic affinities of the Buxaceae. – Plant Syst. Evol. 139: 257-266.
Bessedik M. 1983. Le genre Buxus L. (Nagyipollis Kedves 1962) au Tertiaire en Europe occidentale: évolution et implications paléogéographiques. – Pollen Spores 25: 461-486.
Boltenhagen E. 1967. Spores et pollen de Crétacé supérieure du Gabon. – Pollen Spores 9: 335-355.
Brückner P. 1985. Blattnervatur und Pollenmorphologie eurasiatischer Arten der Gattung Buxus L. (Buxaceae Dumort.) und ihre Bedeutung für die Systematik. – Ph.D. diss., Universität Berlin, Germany.
Brückner P. 1993. Pollen morphology and taxonomy of Eurasiatic species of the genus Buxus (Buxaceae). – Grana 32: 65-78.
Carlquist SJ. 1982. Wood anatomy of Buxaceae: correlations with ecology and phylogeny. – Flora 172: 463-491.
Cerny V, Sorm F. 1967. Steroid alkaloids: alkaloids of Apocynaceae and Buxaceae. – In: Manske RHF (ed), The alkaloids 9: 305-426.
Dang V-L. 1959. Embryogénie des Buxacées: développement de l’embryon chez le Buxus sempervirens L. – Compt. Rend. Acad. Sci. Paris 248: 1844-1847.
Daumann E. 1974. Zur Frage nach dem Vorkommen eines Septalnektariums bei Dicotyledonen, zugleich ein Beitrag zur Blütenmorphologie und Bestäubungsökologie von Buxus L. und Cneorum L. – Preslia 46: 97-109.
Dierickx PJ. 1973. New β-diketones from Buxus sempervirens. – Phytochemistry 12: 1498-1499.
Doust AN, Stevens PF. 2005. A reinterpretation of the staminate flowers of Haptanthus. – Syst. Bot. 30: 779-785.
Drinnan AN, Crane PR, Friis EM, Pedersen KR. 1991. Angiosperm flowers and tricolpate pollen of buxaceous affinity from the Potomac group (mid-Cretaceous) of eastern North America. – Amer. J. Bot. 78: 153-176.
Friis I. 1989. A synopsis of the Buxaceae in Africa South of the Sahara. – Kew Bull. 44: 293-299.
Gentry AH, Foster R. 1981. A new Peruvian Styloceras (Buxaceae): discovery of a phytogeographical missing link. – Ann. Missouri Bot. Gard. 68: 122-124.
Goldberg A, Alden HA. 2005. Taxonomy of Haptanthus Goldberg & C. Nelson. – Syst. Bot. 30: 773-778.
Goldberg A, Nelson CS. 1989. Haptanthus, a new dicotyledonous genus from Honduras. – Syst. Bot. 14: 16-19.
Gray J, Sohma K. 1964. Fossil Pachysandra from Western America with a comparative study of pollen in Pachysandra and Sarcococca. – Amer. J. Sci. 262: 1159-1197.
Haas K. 1989. Chemical composition and structure of epicuticular wax in box leaves. – Biol. Chem. Hoppe-Seyler 370: 796.
Hans AS. 1973. Chromosomal conspectus of the Euphorbiaceae. – Taxon 22: 591-636.
Hansen DR, Dastidar SG, Cai Z, Penaflor C, Kuehl JV, Boore JL, Ransen RK. 2007. Phylogenetic and evolutionary implications of complete chloroplast genome sequences of four early-diverging angiosperms: Buxus (Buxaceae), Chloranthus (Chloranthaceae), Dioscorea (Dioscoreaceae), and Illicium (Schisandraceae). – Mol. Phylogen. Evol. 45: 547-563.
Hjelmqvist H. 1948. Studies on the floral morphology and phylogeny of the Amentiferae. – Bot. Not. Suppl. 2(1): 1-171.
Jarvis CE. 1989. A review of the family Buxaceae Dumortier. – In: Crane PR, Blackmore S (eds), Evolution, systematics, and fossil history of the Hamamelidae 1. Introduction and ‘lower’ Hamamelidae, Syst. Assoc. Special Vol. 40A, Clarendon Press, Oxford, pp. 273-278.
Kedves M. 1962. Nagyipollis, a new pollen fgen. from the Hungarian Lower Eocene. – Acta Biol. (Szeged) 8: 83-84.
Köhler E. 1979. Ergebnisse palynotaxonomischer Untersuchungen an cubanischen Buxus-Arten. – Wiss. Zeitschr. Friedrich-Schiller-Univ. Jena, Math.-Naturwiss. Reihe 28: 683-689.
Köhler E. 1980. Zur Pollenmorphologie und systematischen Stellung der Didymelaceae Leandri. – Feddes Repert. 91: 581-591.
Köhler E. 1981. Pollen morphology of the West Indian-Central American species of the genus Buxus L. (Buxaceae) with reference to taxonomy. – Pollen Spores 23: 37-91.
Köhler E. 1982. Drei neue Buxus-Arten für die Flora von Cuba. – Wiss. Zeitschr. Friedrich-Schiller-Univ. Jena, Math.-Naturwiss. Reihe 31: 239-250.
Köhler E. 1984. Zur Blattnervatur der neotropischen Buxus-Arten und ihre Bedeutung für die Systematik (Buxaceae). – Flora 175: 345-374.
Köhler E. 1985. Vorstellungen zur Evolution und Chorogenese der neotropischen Buxus-Arten. – Feddes Repert. 96: 663-675.
Köhler E. 1987. Taxonomical and ecological aspects of scanning electron microscopic (SEM) studies of leaf surfaces of the Cuban species of the genus Buxus L. (Buxaceae). – In: Resumenes IV Conferencia sobre la Flora de Cuba, La Habana, p. 13.
Köhler E. 1990. Zur Blattnervatur der afrikanischen Buxus- und Notobuxus-Arten. – Feddes Repert. 101: 243-255.
Köhler E. 1993. Blattnervatur-Muster der Buxaceae Dumortier und Simmondsiaceae van Tieghem. – Feddes Repert. 104: 145-167.
Köhler E. 1994. Parallel evolution of pollen characters in the genus Buxus L. (Buxaceae). – Acta Bot. Gallica 141: 223-232.
Köhler E. 1998. Weitere neue Buxus-Arten der Flora von Cuba. – Feddes Repert. 109: 351-363.
Köhler E. 2006a. Buxaceae. – In: Kubitzki K (ed), The families and genera of vascular plants IX. Flowering plants. Eudicots. Berberidopsidales, Buxales, Crossosomatales, Fabales p. p., Geraniales, Gunnerales, Myrtales p. p., Proteales, Saxifragales, Vitales, Zygophyllales, Clusiaceae Alliance, Passifloraceae Alliance, Dilleniaceae, Huaceae, Picramniaceae, Sabiaceae, Springer, Berlin, Heidelberg, New York, pp. 40-47.
Köhler E. 2006b. Didymelaceae. – In: Kubitzki K (ed), The families and genera of vascular plants IX. Flowering plants. Eudicots. Berberidopsidales, Buxales, Crossosomatales, Fabales p. p., Geraniales, Gunnerales, Myrtales p. p., Proteales, Saxifragales, Vitales, Zygophyllales, Clusiaceae Alliance, Passifloraceae Alliance, Dilleniaceae, Huaceae, Picramniaceae, Sabiaceae, Springer, Berlin, Heidelberg, New York, pp. 129-131.
Köhler E. 2006c. Three new Buxus species (Buxaceae) from eastern Cuba. – Willdenowia 36 (Spec. issue): 479-489.
Köhler E, Brückner P. 1982. Die Pollenmorphologie der afrikanischen Buxus-Arten und Notobuxus-Arten (Buxaceae) und ihre systematische Bedeutung. – Grana 21: 71-82.
Köhler E, Brückner P. 1989. The genus Buxus (Buxaceae): aspects of its differentiation in space and time. – Plant Syst. Evol. 162: 267-283.
Köhler E, Brückner P. 1990. Considerations on the evolution and chorogenesis of the genus Buxus (Buxaceae). – Mem. New York Bot. Gard. 55: 153-168.
Köhler E, Schirarend C. 1989. Zur Blattanatomie der neotropischen Buxus-Arten und ihre Bedeutung für die Systematik. – Flora 183: 1-38.
Köhler E, Fernández R, Zamudio S. 1993. Buxus moctezmae Köhler Fernández et Zamudio (Buxaceae) una especie nova de Estado de Querétaro, México. – Feddes Repert. 104: 295-305.
Krutzsch W. 2008. Buxus – (Buxaceen) Pollenformen aus alttertiären Ablagerungen Mitteleuropas und ihre paläochorologische Bedeutung. – Feddes Repert. 119: 207-216.
Kvacek Z, Buzek C, Holý F. 1982. Review of Buxus-fossils and a new large-leaved species from the Miocene of Central Europe. – Rev. Palaeobot. Palynol. 37: 361-394.
Leandri J. 1937. Sur l’aire et la position systématique du genre malgache Didymeles Thouars. – Ann. Sci. Nat. Bot., sér. X, 19: 304-318.
Lowry II PP, Schatz GE. 2006. A new restricted-range species of Buxus L. (Buxaceae) from central Madagascar. – Adansonia, sér. III, 28: 67-70.
Lu A-M, Li J-Q, Xu K-X. 1991. A phylogenetic analysis of families in the Hamamelidae. – Acta Phytotaxon. Sin. 29: 481-493. [In Chinese with English summary]
Martin-Sans E. 1930. Généralité de la présence d’alkaloides chez les Buxacées. – Compt. Rend. Acad. Sci. Paris 191: 625-626.
Martin-Sans E, Ponchet J. 1930. Sur l’appareil sécréteur des Buxus. – Bull. Soc. Hist. Nat. Toulouse 60: 231-232.
Mathou T. 1939. Recherches sur la famille des Buxacées: étude anatomique, microchimique, et systématique. – Douladoure, Toulouse.
Mathou T. 1940. Recherches sur la famille des Buxacées. – Trav. Lab. Bot. Fac. Méd. & Pharmacol. Univ. Toulouse 1940: 130-136.
Melikian AP. 1968. On the position of the families Buxaceae and Simmondsiaceae in the system. – Bot. Žurn. 53: 1043-1047. [In Russian with English summary]
Melikian AP. 1973. Seed-coat types of Hamamelidaceae and allied families in relation to their systematics. – Bot. Žurn. 58: 350-359. [In Russian]
Naumova TN. 1980. Nucellar polyembryony in the genus Sarcococca (Buxaceae). – Bot. Žurn. 65: 230-240. [In Russian]
Nelson CS. 2001. Plantas descritas originalmente de Honduras y sus nomenclaturas equivalentes actuales. – Ceiba 42: 1-71.
Outer RW den. 1985. Wood anatomy of Buxus madagascarica Baill. – Acta Bot. Neerl. 34: 111-113.
Pax F. 1896. Buxaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(5), W. Engelmann, Leipzig, pp. 130-135.
Radcliffe-Smith A. 1981. A remarkable new species of Notobuxus (Buxaceae) from Tanzania. – Kew Bull. 36: 39-41.
Radcliffe-Smith A. 1985. A further note on Notobuxus cordata (Buxaceae). – Kew Bull. 40: 88.
Robbins HC. 1962. A monographic study of the genus Pachysandra (Buxaceae). – Ph.D. diss., Vanderbilt University, Nashville, Tennessee.
Robbins HC. 1968. The genus Pachysandra (Buxaceae). – Sida 3: 211-248.
Sánchez V, Ahond A, Debray M-M, Picot F, Poupat C. 1984. Alcaloïdes des écorces de tronc de Didymeles cf. madagascariensis (Didymélacées). – Bull. Soc. Chim. France 2: 71-76.
Schatz GE, Lowry II PP. 2002. A synoptic revision of the genus Buxus L. (Buxaceae) in Madagascar and the Comoro Islands. – Adansonia, sér. III, 24: 179-196.
Schatz GE, Lowry II PP. 2003. Buxus rabenantoandroi G. E. Schatz & Lowry, a new name for a Malagasy Buxaceae. – Adansonia, sér. III, 25: 129-130.
Sealy JR. 1986. A revision of the genus Sarcococca (Buxaceae). – Bot. J. Linn. Soc. 92: 117-159.
Shipunov A, Shipunova E. 2011. Haptanthus story: rediscovery of enigmatic flowering plant from Honduras. – Amer. J. Bot. 98: 761-763.
Srivastava SK. 1972. Pollen genus Erdtmanipollis Krutzsch 1962. – Pollen Spores 14: 309-322.
Straka H. 1966. Palynologia Madagassica et Mascarenica: Didymelaceae. – Pollen Spores 8: 242-243, 246-247.
Sutton DA. 1989. The Didymelales: a systematic review. – In: Crane PR, Blackmore S (eds), Evolution, systematics, and fossil history of the Hamamelidae 1, Introduction and ‘lower’ Hamamelidae, Syst. Assoc. Spec. Vol. 40A, Clarendon Press, Oxford, pp. 279-284.
Takhtajan AL, Shilkina IA, Yatsenko-Khmelevsky AA. 1986. Wood anatomy of Didymeles madagascariensis in the connection with the systematic status of the family Didymelaceae. – Bot. Žurn. 71: 1203-1206. [In Russian]
Tieghem P van. 1897. Sur les Buxacées. – Ann. Sci. Nat. Bot., sér. VIII, 5: 289-338.
Tomko J, Votický Z. 1973. Steroid alkaloids: the Veratrum and Buxus groups. – In: Manske RHF (ed), The alkaloids XIV, Academic Press, New York, pp. 5-82.
Uemura K. 1979. Leaf compressions of Buxus from the upper Miocene of Japan. – Bull. Nat. Sci. Mus. Tokyo, C, 5: 1-8.
Verdcourt B. 1962. Buxaceae. – In: Hubbard CE, Milne-Redhead E (eds), Flora of tropical East Africa, Crown Agents for Oversea Governments and Administrations, London, pp. 1-3.
Vogel S. 1998. Remarkable nectaries: structure, ecology, organophyletic perspectives IV. Miscellaneous cases. – Flora 193: 225-248.
Webster GL. 1987. The saga of the spurges: a review of classification and relationships in the Euphorbiales. – Bot. J. Linn. Soc. 94: 3-46.
Wiger J. 1935. Embryological studies on the families Buxaceae, Meliaceae, Simarubaceae, and Burseraceae. – Ph.D. diss., University of Lund, Sweden.