SUPERROSIDAE W. S. Judd, D. E.
Soltis et P. S. Soltis
Judd, Soltis et Soltis in Amer. J. Bot. 98:
E21-E22. Apr 2011
[Saxifragales+Rosidae]
SAXIFRAGALES Bercht. et J.
Presl
Berchtold et Presl, Přir. Rostlin: 259. Jan-Apr
1820 [‘Saxifrageae’]
Hamamelidanae Takht.,
Sist. Filog. Cvetk. Rast. [Syst. Phylog. Magnolioph.]: 113. 4 Feb 1967;
Hamamelididae Takht., Sist. Filog. Cvetk. Rast.
[Syst. Phylog. Magnolioph.]: 461. 4 Feb 1967, pro parte;
Saxifraganae Reveal in Phytologia 76: 4. 2 Mai
1994
Fossils Archamamelis
bivalvis is represented by fossil flowers from the Late Santonian to the
Early Campanian of Sweden. The hexamerous or heptamerous flowers have a
bicyclic perianth, one series of stamens, one whorl of staminodia, and a
gynoecium of three connate carpels. Divisestylus from the Turonian of
New Jersey comprises fossil pentamerous flowers with alternipetalous stamens
containing striate pollen, an intrastaminal nectariferous disc and two carpels
connate at stigmatic and ovary regions; the capsules were apically dehisced and
the stomata anomocytic. The Coniacian Lindacarpa pubescens from
eastern Siberia is a spherical female inflorescence, the flowers of which
having connate sepals and a semi-inferior gynoecium of two connate carpels.
Microaltingia apocarpela, from the Turonian of New Jersey, consists of
stalked spherical female inflorescences with two basally connate semi-inferior
ovaries per flower.
Habit Bisexual, monoecious,
andromonoecious, polygamomonoecious, dioecious, androdioecious or
polygamodioecious, evergreen or deciduous trees, shrubs or suffrutices (rarely
lianas), perennial, biennial or annual herbs. Many representatives are
succulent and/or xerophytic with CAM-physiology. Sometimes aquatic.
Vegetative anatomy Roots often
diarch. Phellogen ab initio subepidermal, pericyclic or outer-cortical.
Secondary lateral growth normal or absent. Tension wood sometimes present.
Vessel elements usually with scalariform (sometimes simple, rarely reticulate)
perforation plates; lateral pits scalariform, opposite or alternate (rarely
reticulate), simple or bordered pits, non-septate. Vestured pits sometimes
present. Imperforate tracheary elements fibre tracheids or libriform fibres
(sometimes tracheids) with simple or bordered pits, usually non-septate, or
absent (sometimes also vasicentric tracheids). Wood rays uniseriate or
multiseriate, homocellular or heterocellular, or absent. Axial parenchyma
apotracheal diffuse or diffuse-in-aggregates, or paratracheal scanty,
vasicentric, unilateral or banded, or absent. Sieve tube plastids Ss or
S0 type. Nodes usually 3:3, trilacunar with three leaf traces
(sometimes 1:1–3 or ≥5:≥5, unilacunar or multilacunar with one or several
traces). Cystoliths sometimes numerous. Secretory cells sometimes with
proanthocyanidins and/or ellagitannins. Idioblasts with cyanogenic compounds or
druses unusual. Schizolysigenous resinous canals with balsam sometimes
abundant. Tanniniferous cells sometimes frequent. Prismatic calciumoxalate
crystals, druses or crystal sand often present (rarely elongate crystals or
styloids).
Trichomes Hairs unicellular or
multicellular, uniseriate or multiseriate, often branched (furcate, stellate,
sometimes rarely arachnoid), stalked or unstalked, or absent (sometimes tufted,
peltate, clavate or vesicular; often with multicellular apical gland);
glandular hairs sometimes frequent, sometimes peltate or lepidote; prickles
sometimes abundant on branch epidermis.
Leaves Usually alternate
(spiral or distichous; sometimes opposite or verticillate), simple or pinnately
or palmately compound, entire or lobed, with usually conduplicate or involute
(sometimes flat or curved, rarely plicate, supervolute or convolute) ptyxis
(leaves sometimes succulent). Stipules usually absent (sometimes intrapetiolar,
rarely pairwise); leaf base usually absent (sometimes sheathing). Colleters
sometimes present. Petiole vascular bundle transection arcuate or annular,
sometimes with medullary or adaxial bundles. Venation pinnate or palmate,
actinodromous, eucamptodromous, brochidodromous or craspedodromous; lateral
veins often running out into (glandular) leaf teeth. Stomata usually anomocytic
or paracytic (sometimes anisocytic or diacytic, rarely helicocytic), often only
on adaxial side of lamina. Cuticular wax crystalloids usually as tubuli (often
clustered tubuli [Berberis type, at least in some woody Saxifragales], sometimes as
rodlets, threads or platelets), chemically often dominated by nonacosan-10-ol.
Domatia as pockets or hair tufts, or absent. Mesophyll and petiolar cortex
sometimes with calciumoxalate as druses or single prismatic crystals. Leaf
margin usually serrate (sometimes entire, crenate or biserrate); leaf teeth
usually rosoid, sometimes platanoid (glandular with cavity, lateral veins
almost reaching teeth), fothergilloid (with transparent glandular apex, lateral
veins almost reaching teeth) or chloranthoid (with persistent transparent
swollen structure, into which high order lateral veins proceed).
Inflorescence Terminal or
axillary, racemes, spikes or heads, or raceme-, spike- or head-like, pleio-,
di- or monochasia, thyrsoids, fasciculate, panicles, corymbs, racemes or
cincinnate (flowers rarely solitary). Floral prophylls (bracteoles) sometimes
absent.
Flowers Usually actinomorphic
(rarely zygomorphic). Hypanthium sometimes present. Epigyny or half epigyny
(sometimes hypogyny). Sepals (two to) four or five (sometimes eight or ten),
with imbricate or valvate aestivation, free (sometimes connate at base;
sometimes spiral or absent). Petals (two to) four or five (to 13), with
imbricate or valvate aestivation, free (sometimes spiral or absent). Nectary
and disc absent, or nectariferous disc intrastaminal.
Androecium Stamens (one to)
four or five (to more than 100), usually whorled, alternisepalous, or
(4–)5+(4–)5, diplostemonous or obdiplostemonous (sometimes spiral).
Filaments usually free (rarely connate at base, usually free from tepals
(rarely epipetalous). Anthers usually basifixed (usually with filament attached
at basal pit; rarely dorsifixed), usually non-versatile, usually
tetrasporangiate (rarely disporangiate), usually latrorse (sometimes introrse
or extrorse), usually longicidal (dehiscing by longitudinal slits; rarely
valvate or with a basal pore or short apical slits). Tapetum secretory.
Staminodia usually absent (sometimes antepetalous; female flowers sometimes
with staminodia).
Pollen grains
Microsporogenesis simultaneous. Pollen grains 2–3-colpate,
2–3-colpor(oid)ate or polypantoporate (sometimes diporate, rately triporate),
shed as monads, bicellular at dispersal. Exine tectate or semitectate, with
usually columellate (rarely granular) infratectum, perforate, reticulate,
rugulate or striate, verrucate, echinulate, spinulate, scabrate or psilate.
Gynoecium Pistil composed of
one to 32, usually connate (sometimes free, at least in distal part) carpels,
sometimes unsealed distally-ventrally (sometimes spiral); carpel plicate. Ovary
inferior or semi-inferior (sometimes superior), unilocular (apocarpy) to
quadrilocular. Stylodia two to numerous, free, or absent. Stigmas usually
capitate (sometimes decurrent), papillate, Dry or Wet type. Pistillodium
usually absent (male flowers sometimes with pistillodium/pistillodia).
Ovules Placentation axile or
parietal (sometimes apical or marginal to laminar). Ovules one to more than 100
per carpel, anatropous (sometimes hemianatropous), pendulous, horizontal or
ascending, apotropous or epitropous, usually bitegmic (rarely unitegmic),
usually crassinucellar (sometimes tenuinucellar). Micropyle usually bistomal,
often Z-shaped (zig-zag; rarely exostomal). Nucellar cap sometimes present.
Megagametophyte usually monosporous, Polygonum type (rarely disporous,
Allium type). Synergids often with a filiform apparatus, rarely
haustorial. Endosperm development usually cellular (rarely helobial or
nuclear). Endosperm haustorium chalazal or absent. Embryogenesis solanad,
caryophyllad or chenopodiad.
Fruit Usually a follicle, a
septicidal and/or loculicidal capsule or a nut (sometimes a drupe, a berry, or
an assemblage of follicles or nutlets, or a syncarp consisting of follicles or
septicidal capsules, rarely a schizocarp with nut-like mericarps).
Seeds Aril usually absent
(sometimes funicular aril enclosing seed entirely or partially). Seed coat
testal or exotestal (sometimes mesotestal or endotegmic). Exotestal cells often
with outer wall (sometimes radial walls) thickened (sometimes partially
crushed). Inner pigmented layer often present. Tegmen usually crushed (inner
exotegmic layer sometimes pigmented). Endotegmen sometimes with thick
sclerotic, cutinized, tanniniferous and/or crystalliferous cell walls.
Perisperm usually not developed (sometimes more or less developed). Endosperm
copious or sparse, oily, proteinaceous (rarely starchy). Embryo large or small,
straight or curved, without chlorophyll. Cotyledons two. Germination
phanerocotylar.
Cytology n = 5–37
DNA Insertion present in 18S
rDNA. I copy of nuclear gene RPB2 lost. Mitochondrial intron
coxII.i3 sometimes lost.
Phytochemistry Flavonols
(kaempferol, quercetin, myricetin, apigenin, luteolin, etc.), afzelechin (a
flavan-3-ol), methylated and non-methylated flavones, acylated flavonol
glycosides, pelargonidin, chalcones, dihydrochalcones, cyanidin, delphinidin,
mono- and sesquiterpenes, oleanolic acid derivatives, toxic bufadienolides,
Group I carbocyclic iridoids (e.g. daphylloside, monotropein, asperuloside,
daphniphylloside), ellagic and gallic acids, bergeniin (C-glycoside of
gallic acid), ellagic or gallic acid based hydrolyzable tannins (tellimagrandin
I and II etc.), condensed tannins (from procyanidin or prodelphinidin),
proanthocyanidins (prodelphinidins), myriophyllin, pyridine alkaloids,
glycosides (e.g. paeonol, paeonolide, paeonoside, paeoniflorin,
oxypaeoniflorin, benzoylpaeoniflorin and alliflorin), isoleucine-, tyrosine- or
valine-derived cyanogenic compounds, coumaric acid, arbutin, acetophenones,
galloylic esters, and germacrane-like compounds present. Squalene alkaloids of
the daphniphyllin group rare. Saponins not found.
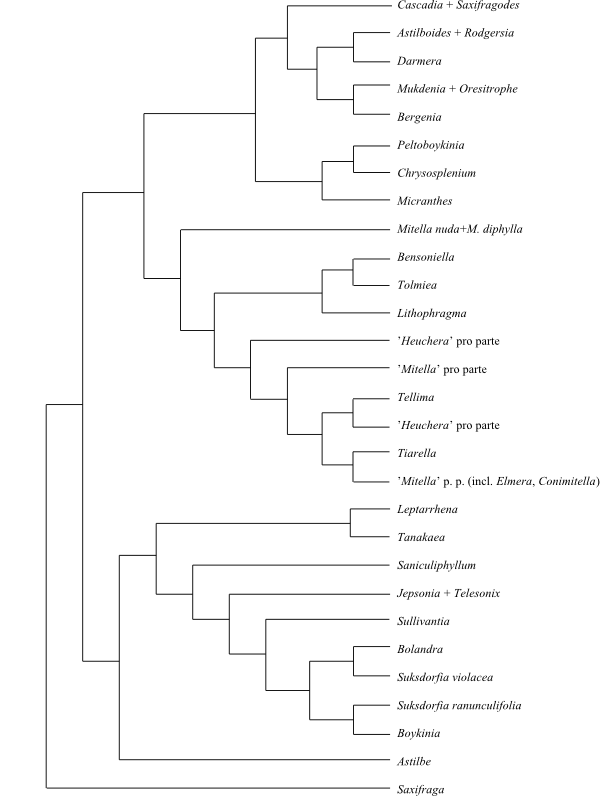
Systematics Saxifragales are sister to
Rosidae.
[Peridiscaceae+Medusandra]
are sister-group to the remaining Saxifragales.
The remaining Saxifragales have the
following more or less strongly supported topology: [[Paeoniaceae+[Altingiaceae+[Hamamelidaceae+[Cercidiphyllaceae+Daphniphyllaceae]]]]+
[[Crassulaceae+[Aphanopetalaceae+[Tetracarpaeaceae+[Haloragaceae+Penthoraceae]]]]+ [[Iteaceae+Pterostemonaceae]+[Grossulariaceae+Saxifragaceae]]]]. It is
supported by the potential synapomorphies (Stevens 2001 onwards): floral apex
flat to concave early in development; and ovary often inferior to
semi-inferior.
The first main clade, with the topology [Paeoniaceae+[Altingiaceae+[Hamamelidaceae+ [Cercidiphyllaceae+Daphniphyllaceae]]]], has
the synapomorphies leaves with basically palmate venation; and absence of
mitochondral intron coxII.i3. The clade [Altingiaceae+ [Hamamelidaceae+[Cercidiphyllaceae+Daphniphyllaceae]]] is
characterized by cuticular wax crystalloids as tubuli, dominated by
nonacosan-10-ol; usually racemose inflorescence; absence of pedicel; valvicidal
anther with apically protruding connective; and an elongate embryo. Cercidiphyllaceae and
Daphniphyllaceae
share the potential synapomorphies dioecy; small flowers; absence of petals;
and hypogyny.
The second main clade [[Crassulaceae+[Aphanopetalaceae+[Tetracarpaeaceae+[Haloragaceae+Penthoraceae]]]]+[[Iteaceae+Pterostemonaceae]+[Grossulariaceae+Saxifragaceae]]] has the
following potential synapomorphies, according to Stevens (2001 onwards):
superficial phellogen; vessel elements with simple perforation plates; petiole
bundle transection arcuate; cuticular wax crystalloids not as tubuli; ovules
apotropous; and calyx persisting as withered. The clade [Crassulaceae+[Aphanopetalaceae+[Tetracarpaeaceae+[Haloragaceae+Penthoraceae]]]] has the
synapomorphies: endodermis usually present in stem; nodes 1:1; absence of
stipules; and tricolporate pollen grains. The [[Iteaceae+Pterostemonaceae]+[Grossulariaceae+Saxifragaceae]] clade has
spiral leaves; presence of hypanthium; antesepalous stamens as many as sepals;
ovules numerous per carpel; and a septicidal capsule. Iteaceae and Pterostemonaceae share the
features: presence of stipules; one style; axile placentation; sparse
endosperm; and presence of flavone-C-glycosides. Likewise, Grossulariaceae and Saxifragaceae share the
characters two or three connate carpels; and sometimes helobial endosperm
development.

|
Bayesian consensus tree of Saxifragales based on
DNA sequence data (Jian & al. 2008). [Peridiscaceae+Medusandra]
are sister group to the remaining Saxifragales with a
high bootstrap support. The bootstrap support for Paeonia
being sister to the woody Saxifragales was only
59% in analyses by Soltis & al. (2011), whereas the remaining
clades are very strongly supported. Paeoniaceae were recovered
as sister to the herbaceous groups
Crassulaceae–Saxifragaceae by Dong & al. (2018). There
is significant support for placing Cynomorium
(Cynomoriaceae) either within Saxifragales or as
sister to this clade (Su & al. 2015).
|
Horaninov, Osnov. Bot.: 271. 1841, nom. cons.
Altingiales Doweld,
Ann. Bot. (London) 82: 435. Oct 1998
Genera/species 1/13–15
Distribution Eastern
Mediterranean to eastern Himalayas, India, China, Taiwan, Indochina, West
Malesia, eastern and northeastern North America, Mexico, Central America.
Fossils Altingiaceae are richly
represented by Cenozoic fossils, particularly from the Miocene and the
Pliocene. Infructescences of Steinhauera from Eocene layers in Europe
resemble those in Liquidambar.
Habit Monoecious, usually
deciduous (sometimes evergreen) trees.
Vegetative anatomy Phellogen
ab initio superficial. Vessel elements with scalariform perforation plates;
lateral pits scalariform or opposite, simple pits. Imperforate tracheary xylem
elements ? with bordered pits, non-septate. Wood rays uniseriate or
multiseriate, homocellular. Axial parenchyma apotracheal diffuse, or
paratracheal scanty, or absent. Sieve tube plastids Ss type. Nodes trilacunar?
Schizolysigenous resinous canals with balsam abundant in most tissues.
Calciumoxalate crystals?
Trichomes Hairs multicellular,
uniseriate or stellate, or absent.
Leaves Alternate (spiral),
simple, usually palmately lobed (rarely entire), with flat-conduplicate ptyxis.
Stipules (intra?)petiolar, small, usually caducous; leaf sheath absent. Petiole
vascular bundle transection annular; petiole with medullary bundles. Venation
palmate, usually actinodromous (sometimes camptodromous). Stomata paracytic.
Cuticular wax crystalloids as tubuli? Domatia as pockets or hair tufts. Leaf
margin usually serrate (rarely entire); leaf teeth platanoid (glandular with
cavity).
Inflorescence Terminal, cymose
(condensed panicles); male inflorescences capitula in spike- or raceme-like
partial inflorescences; female inflorescences globular, with intercarpellary
outgrowths corresponding to sterile flowers.
Flowers Actinomorphic, small.
Epigyny? Tepals absent (or as tiny rudimentary scales?). Nectary absent. Disc
absent.
Androecium Stamens four to
ten. Filaments free. Anthers basifixed, non-versatile, tetrasporangiate,
latrorse, longicidal (dehiscing by longitudinal valves or slits). Tapetum
secretory. Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains triporate to polypantoporate,
shed as monads, bicellular at dispersal. Exine semitectate, with columellate
infratectum, finely reticulate.
Gynoecium Pistil composed of
two connate carpels; carpels with various orientation (sometimes transversely
orientated), unsealed, only lower carpels fertile. Ovary inferior?, bilocular.
Style absent. Stigmas much decurrent, with multicellular appendages (sometimes
unicellular papillae?), Dry type. Pistillodium absent.
Ovules Placentation axile.
Ovules c. 20 to 47 (only lower ovules fertile) per carpel, anatropous?,
horizontal, apotropous, bitegmic, crassinucellar. Micropyle usually bistomal?
(sometimes endostomal). Outer integument approx. two cell layers thick. Inner
integument approx. five cell layers thick. Megagametophyte monosporous,
Polygonum type. Endosperm development ab initio nuclear. Endosperm
haustoria? Embryogenesis?
Fruit Septicidal (and
occasionally loculicidal) lignified capsules (sometimes more or less connate).
Endocarp cells thickened, transversely elongate relative to longitudinal axis
of fruit.
Seeds Aril absent. Seed winged
(wings developed from elongation of integuments surrounding micropyle). Seed
coat testal (or exotegmic?). Exotestal cell walls often lignified. Mesotestal
cells more or less sclerotized. Endotestal cells oblong, with lignified walls.
Exotegmen well developed? Endotegmen? Perisperm not developed. Endosperm poorly
developed. Embryo long, straight, chlorophyll? Cotyledons two, flattened.
Germination phanerocotylar.
Cytology n = 15, 16 –
Polyploidy occurring.
DNA Mitochondrial intron
coxII.i3 lost.
Phytochemistry Flavonols
(quercetin, myricetin), cyanidin, Group I carbocyclic iridoids (daphylloside,
monotropein), ellagic acid, tannins, and proanthocyanidins (prodelphinidins)
present. Cyanogenic compounds not found.
Use Ornamental plants, timber,
carpentry, medicinal plants (gum from Liquidambar orientalis).
Systematics Liquidambar
(13–15; eastern Mediterranean [the Aegean Islands, Rhodos, Cyprus, southern
Turkey], India, Assam, the Himalayas, China, Taiwan, Indochina, West Malesia,
Taiwan, southeastern Canada, eastern United States, eastern and southern Mexico
to Costa Rica).
A basal branching of Liquidambar
comprises one East Asiatic clade and one European-American clade (Ickert-Bond
& Wen 2006).
The Cretaceous fossils Lindacarpa,
Microaltingia and Vilyungia do not belong in Altingiaceae, according to
Friis & al. (2011). They are instead considered “unassigned Saxifragales”.
Doweld, Tent. Syst. Plant. Vasc.: xxvii. 23 Dec
2001
Genera/species 1/2
Distribution Western and
southeastern Australia.
Fossils Unknown.
Habit Bisexual, evergreen
climbing shrub or liana. Lenticels elevated and prominent.
Vegetative anatomy Phellogen
ab initio superficial. Endodermis distinctly delimited, as innermost cortical
layer. Pericyclic fibres absent. Vessel elements with scalariform perforation
plates; lateral pits?, bordered pits. Imperforate tracheary xylem elements
fibre tracheids with bordered pits?, non-septate. Wood rays uniseriate
homocellular or multiseriate heterocellular. Axial parenchyma apotracheal
diffuse, or paratracheal scanty. Ray parenchyma cells starchy. Sieve tube
plastids Ss type? Nodes 1:1(–2), unilacunar with one bifid leaf trace.
Crystals?
Trichomes Hairs absent.
Leaves Usually opposite
(rarely verticillate), simple, entire, with ? ptyxis. Stipules? present on both
sides of nodes, modified into tooth-like colleters; leaf sheath absent. Petiole
bundle transection arcuate?; petiole with usually three (rarely one) vascular
bundles. Venation pinnate. Stomata anomocytic. Cuticular waxes? Leaf margin
entire or serrate; leaf teeth one-veined, salicoid, without glands.
Inflorescence Axillary,
paniculate cymose, or flowers solitary.
Flowers Actinomorphic.
Hypanthium present, short. Half epigyny. Sepals four (or five), with imbricate
aestivation, persistent, largely free. Petals four (or five), rudimentary and
visible only as young, free, or absent. Tepals connate at base into tube.
Nectary? Disc absent.
Androecium Stamens usually
eight (sometimes ten). Filaments connate at base into a tube, adnate to tetals
(epitepalous). Anthers elongate, almost basifixed, non-versatile,
tetrasporangiate, latrorse-introrse, longicidal (dehiscing by longitudinal
slits); connective prolonged. Tapetum secretory? Staminodia absent.
Pollen grains
Microsporogenesis simultaneous? Pollen grains tricolporate, shed as monads,
bicellular at dispersal. Exine tectate, with columellate? infratectum,
rugulate-stellate.
Gynoecium Pistil composed of
four connate antepetalous carpels. Ovary semi-inferior, quadrilocular. Style
single, quadrilobate, with four stylar canals. Stigma papillate, type?
Pistillodium?
Ovules Placentation
apical-axile. Ovule usually one (rarely two) per carpel, anatropous, pendulous,
epitropous, bitegmic, crassinucellar. Funicle long, thick. Micropyle bistomal.
Outer integument ? cell layers thick. Inner integument ? cell layers thick.
Parietal tissue? Megagametophyte monosporous, Polygonum type.
Endosperm development? Endosperm haustoria? Embryogenesis?
Fruit A one-seeded nut with
persistent and accrescent calyx.
Seeds Aril absent. Seed
reniform or hippocrepomorphic, often winged or with long hairs. Testa? Tegmen?
Perisperm not developed. Endosperm copious?, fleshy. Embryo curved,
chlorophyll? Cotyledons two. Germination?
Cytology n = ?
DNA
Phytochemistry Unknown.
Use Ornamental plants
(Aphanopetalum resinosum).
Systematics
Aphanopetalum (2; A. clematideum: westernmost Western
Australia; A. resinosum: warm-temperate parts of southern Queensland
and New South Wales).
Aphanopetalum is sister to the clade
[Tetracarpaeaceae+[Haloragaceae+Penthoraceae]].
Engler, Syllabus, ed. 5: 126. Jul 1907, nom.
cons.
Cercidiphyllales Hu
ex Reveal in Phytologia 74: 174. 25 Mar 1993
Genera/species 1/1–2
Distribution: China, Japan.
Fossils Reports of Cercidiphyllaceae from
the Cretaceous, based on leaves and fruits, are ambiguous. On the other hand,
the Paleocene Joffrea speirsiae from Alberta (Canada) has an elongated
receptacle and a bicarpellate pistil with the adaxial sutures directed against
one another, resembling extant Cercidiphyllaceae.
Similar fossils are known from the Maastrichtian of western North America.
Fossilized leaves and inflorescences (with flowers possessing sometimes
pentamerous perianth) indicate that Cercidiphyllum was widely
distributed in the Northern Hemisphere during the Cenozoic.
Habit Usually dioecious
(rarely monoecious), deciduous trees with very distinct difference between long
and short shoots.
Vegetative anatomy Phellogen
ab initio outer-cortical. Primary medullary rays narrow. Primary stem with
continuous vascular cylinder. Vessel elements with scalariform perforation
plates; lateral pits opposite to scalariform, simple and/or bordered pits.
Imperforate tracheary xylem elements fibre tracheids with bordered pits,
non-septate. Wood rays biseriate with uniseriate ends, heterocellular. Axial
parenchyma apotracheal diffuse?, or paratracheal scanty or banded, or absent.
Tyloses abundant. Sieve tube plastids Ss type. Nodes on short shoots unilacunar
(with one leaf trace?), on long shoots trilacunar (with three traces?).
Parenchyma with prismatic calciumoxalate crystals.
Trichomes Hairs usually
absent.
Leaves Usually opposite
(rarely verticillate) on long shoots, a few alternate on short shoots, simple,
entire, with involute ptyxis. Stipules adaxial-petiolar (intrapetiolar), small,
caducous; leaf sheath absent. Petiole vascular bundles? Leaf bases on long
shoots cuneate, on short shoots cordate. Venation palmate brochidodromous.
Stomata anomocytic. Cuticular wax crystalloids as clustered tubuli
(Berberis type), chemically dominated by nonacosan-10-ol. Mesophyll
and petiolar cortex with calciumoxalate as druses or single prismatic crystals.
Leaf margin on long shoots entire to finely serrate, on short shoots crenate;
leaf teeth chloranthoid (with persistent transparent swollen structure, into
which higher order lateral veins proceed).
Inflorescence Terminal, dense,
few-flowered on sympodial short shoots; female inflorescences capitate
pseudanthia, with two to eight flowers, often surrounded by involucre of
sepaloid bracts; male inflorescences raceme-like, short, with (lower flowers)
or without (upper flowers) bracts. Prophyll adaxial.
Flowers At least male flowers
zygomorphic (with stamens on abaxial side). Hypogyny? Tepals absent. Nectary
absent. Disc absent.
Androecium Stamens 16–35, in
fascicles (flowers?) each with one to 13 stamens. Each staminal fascicle
(flower?) subtended by a single bract. Filaments long, thin, free. Anthers
basifixed, non-versatile, tetrasporangiate, latrorse, longicidal (dehiscing by
longitudinal slits); connective somewhat prolonged at apex. Tapetum secretory,
with binucleate cells. Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains tricolpate, shed as monads,
bicellular at dispersal. Exine tectate, with columellate infratectum, perforate
or finely reticulate, beset with supratectal verrucae.
Gynoecium Pistil composed of
one carpel (monocarpellate); carpel plicate, somewhat stipitate, with abaxial
ventral suture. Ovary superior?, unilocular (apocarpy). Stylodium long. Stigma
decurrent its entire length, papillate, Dry type. Pistillodium absent.
Ovules Placentation
laminal-lateral (marginal). Ovules c. 15 to c. 30 per carpel, anatropous,
pendulous, apotropous, bitegmic, crassinucellar. Micropyle bistomal. Outer
integument four or five cell layers thick. Inner integument two or three cell
layers thick. Chalazal end elongating into a wing-like structure during
maturation. Megagametophyte monosporous, Polygonum type. Endosperm
development ab initio cellular. Endosperm haustoria? Embryogenesis
caryophyllad.
Fruit A follicle. Groups of
adjacent follicles forming a syncarp (multifolliculus).
Seeds Aril absent. Seed small,
with a chalazal wing-like appendage with hairpin-like vascular bundle. Seed
coat testal. Testa indistinct. Exotestal cells enlarged, somewhat thickened.
Tegmen tanniniferous, degenerating. Perisperm not developed. Endosperm poorly
developed, oily, with one large suspensor cell. Embryo large, straight, well
differentiated, without chlorophyll. Cotyledons two. Germination
phanerocotylar.
Cytology n = 19
DNA Mitochondrial intron
coxII.i3 lost.
Phytochemistry Flavonols
(kaempferol, quercetin), cyanidin, chalcones, dihydrochalcones, ellagic acid,
and tannins present. Cyanogenic compounds and saponins not found.
Use Ornamental plants,
carpentry.
Saint-Hilaire, Expos. Fam. Nat. 2: 123. Feb-Apr
1805 [‘Crassuleae’], nom. cons.
Sempervivaceae Juss.,
Gen. Plant.: 207. 4 Aug 1789 [‘Sempervivae’];
Sedaceae Roussel, Fl. Calvados, ed. 2: 291. 1806
[’Sedoideae’]; Cotyledonaceae Martinov,
Tekhno-Bot. Slovar: 168. 3 Aug 1820 [‘Cotyledones’];
Rhodiolaceae Martinov, Tekhno-Bot. Slovar: 546. 3 Aug
1820 [‘Rhodioleae’]; Sempervivales Juss.
ex Bercht. et J. Presl, Přir. Rostlin: 238. Jan-Apr 1820
[‘Semperviveae’]; Tillaeaceae Martinov,
Tekhno-Bot. Slovar: 635. 3 Aug 1820; Sedales Reichb.,
Bot. Damen: 462. 1828 [’Sediflorae’];
Crassulales Link, Handbuch 2: 18. 4-11 Jul 1829
[’Crassulaceae’];
Sedineae Rchb., Deutsch. Bot. Herb.-Buch: lxiii. Jul
1841 [‘Sediflorae’]; Crassulopsida
Brongn., Enum. Plant. Mus. Paris: xxviii, 106. 12 Aug 1843
[’Crassulineae’]
Genera/species
30?/1.270–1.325
Distribution Temperate,
subtropical and alpine regions on both hemispheres, with their largest
diversity in Mexico and southern Africa.
Fossils Uncertain.
Habit Usually bisexual (rarely
unisexual), usually perennial (rarely annual or biennial) herbs, suffrutices or
evergreen shrubs (rarely trees or epiphytes). Leaf succulents. Usually
xerophytes. Rarely aquatic (i.a. Crassula aquatica). Many species
produce adventitious roots from broken leaves; some species form bulbils along
leaf margins or in inflorescences.
Vegetative anatomy Mycorrhiza
usually absent. Roots usually fibrous. CAM physiology frequently present; acid
metabolism often Crassulaceae type (via
isocitrate). Mycorrhiza absent. Phellogen ab initio usually subepidermal
(sometimes cortical). Young stem with separate vascular bundles. Cortical
and/or medullary vascular bundles present or absent. Endodermis present or
absent. Secondary lateral growth usually normal (rarely anomalous). Vessel
elements (usually short) usually with simple (in Sedum rarely
reticulate) perforation plates; lateral pits scalariform(-reticulate) or
alternate, simple pits? Imperforate tracheary xylem elements libriform fibres
with simple pits, non-septate, or absent. Wood rays absent. Axial parenchyma
usually paratracheal scanty vasicentric. Wood elements often storied. Phloem
little developed. Sieve tube plastids S0 type, without starch or
protein inclusions. Nodes 1:1, 1:2, 1:3, unilacunar with one to three leaf
traces, or ≥3:≥3, trilacunar to multilacunar with several traces. Cork
tissue usually without resin. Prismatic crystals or crystal sand present or
absent.
Trichomes Hairs unicellular or
multicellular, usually uniseriate or biseriate (sometimes furcate, stellate,
rarely arachnoid), vesicular hairs frequent; glandular hairs often frequent.
Leaves Usually alternate
(spiral) or opposite (in some species of Sedum verticillate), usually
simple (rarely pinnately or palmately compound), usually entire (rarely lobed),
often round in cross-section, usually succulent, with curved to flat ptyxis.
Stipules and leaf sheath absent. Petiole vascular bundle transection arcuate.
Venation pinnate or palmate, camptodromous or reticulate. Stomata usually
anisocytic (rarely helicocytic with second ring of subsidiary cells outside
anisocytic cell configuration), often on both laminal surfaces (amphistomatic).
Cuticular wax crystalloids as platelets, rodlets (sometimes as elongated
rosettes, sometimes longitudinally furrowed), threads or lobate to smooth
platelets, chemically characterized by high amounts of triterpenoids.
Hydathodes abundant. Epidermis often with mucilage cells. Lamina usually
without palisade tissue. Mesophyll in succulent leaves often with outer
chlorenchyma grading into inner chlorophyll-poor water storage parenchyma.
Tanniniferous cells abundant. Crystals and druses frequent (sometimes crystal
sand). Leaf margin serrate, crenate or entire.
Inflorescence Usually terminal
(rarely axillary), thyrsoid, pleio-, di- or monochasium (cincinnus or corymb;
rarely panicle, raceme- or spike-like). Extrafloral nectaries rarely
(Adromischus) present.
Flowers Usually actinomorphic
(rarely zygomorphic). Hypanthium present. Hypogyny to half epigyny. Sepals
(three to) five (to 32), with imbricate aestivation, free or connate at base.
Petals (three to) five (to 32), with quincuncial, cochlear, contorted,
imbricate or valvate aestivation, free or entirely or partially connate into a
tube. Nectaries as abaxial usually scale-like or elongate (in
’Monanthes’ petaloid) outgrowths from carpel bases. Disc
absent.
Androecium Stamens usually
twice as many (sometimes as many) as petals, usually in two whorls,
obdiplostemonous (sometimes a single whorl, antesepalous). Filaments usually
free (sometimes connate at base), free from or (when sympetalous) adnate to
corolla tube (antepetalous stamens inserted somewhat higher up than
antesepalous stamens). Anthers basifixed, non-versatile, tetrasporangiate,
usually latrorse (rarely somewhat introrse), longicidal (dehiscing by
longitudinal slits). Tapetum secretory, with uninucleate cells. Staminodia
usually absent (female flowers often with staminodia).
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually tricolporate, shed as
monads, bicellular at dispersal. Exine tectate or semitectate, with columellate
infratectum, reticulate or rugulate, usually with striae.
Gynoecium Carpels (three to)
five to ten (to 32), as many as petals, antepetalous, usually free (sometimes
connate at base; in Crassula pageae entirely connate), sometimes
stipitate. Ovary superior to semi-inferior, unilocular (apocarpy). Stylodia
usually short (style rarely single, branched), usually with abaxial
nectariferous scale at base (secreting nectar through stomata). Stigmas small,
usually apical, capitate to punctate, papillate, usually Dry (sometimes Wet)
type. Pistillodia usually absent (male flowers often with pistillodia).
Ovules Placentation usually
marginal to laminar (rarely parietal). Ovule one to usually numerous per
carpel, anatropous, pendulous to horizontal, bitegmic, crassinucellar or (in
Crassuloideae) tenuinucellar. Micropyle usually bistomal (sometimes
exostomal or endostomal). Outer integument approx. two cell layers thick. Inner
integument two or three cell layers thick. Nucellar cap approx. four cell
layers thick. Hyponucellus (part of megasporangium present below developing
megagametophyte) strongly enlarged during development (Sedum and
Crassula types of megasporangium). Megasporangial apex sometimes
extending beyond outer integument; apical cell seemingly subpalisade.
Megasporocyte usually single (archespore sometimes multicellular). Megaspore
haustoria present in some species. Megagametophyte usually monosporous,
Polygonum type (in Hylotelephium disporous, Allium
type). Synergids sometimes haustorial (synergidal haustoria present). Antipodal
cells sometimes proliferating and haustorial. Micropylar suspensor haustorium
often well developed. Endosperm development usually cellular (rarely helobial
or nuclear; endosperm in e.g. Crassula aquatica reduced with chalazal
chamber of bicellular stage not dividing but becoming haustorial). Endosperm
haustorium chalazal. Embryogenesis caryophyllad.
Fruit Usually an assemblage of
follicles (rarely nutlets or a capsule; in Diamorpha secondarily fused
into a syncarp).
Seeds Aril absent. Testa
usually with papillae or longitudinal ridges. Outer wall of exotestal cells
thickened. Two layers crushed. Inner exotegmic layer pigmented. Perisperm not
developed. Endosperm sparse (thin layer surrounding hypocotyl), oily, or
absent. Suspensor uniseriate; basal suspensor cell with hypha-like haustorial
branches. Embryo small, straight, elongate, without chlorophyll. Plumule
absent. Cotyledons two, fleshy. Germination phanerocotylar.
Cytology n = 4-≥22 –
Karyologically very variable; even the base number varying at least between x =
5 and x = 37 (especially in Sedum; S. suaveolens has the
highest haploid chromosome number among angiosperms: n = 320, 40x). Polyploidy
and aneuploidy frequently occurring.
DNA
Phytochemistry Flavonols
(kaempferol, quercetin, myricetin), methylated and non-methylated flavones,
acylated flavonol glycosides, cyanidin, red anthocyanins (frequent, also in
roots), toxic bufadienolides and similar substances (in Tylecodon,
Cotyledon and Kalanchoe), gallic acid, proanthocyanidins
(prodelphinidins), non-hydrolyzable tannins, pyrridine alkaloids and other
alkaloids, cyanogenic compounds, arbutin, acetophenones, galloylic esters and
isocitrate present. Sedoheptulose main reserve sugar. Aluminium accumulated in
some species. Ellagic acid and hydrolyzable tannins not found. Cyanogenesis via
isoleucine or valine.
Use Ornamental plants,
medicinal plants.
Systematics Crassulaceae are sister-group
to the clade [Aphanopetalaceae+[Tetracarpaeaceae+[Haloragaceae+Penthoraceae]]].
A possible topology is
[Crassuloideae+[Kalanchoideae+Sempervivoideae]].
Crassuloideae Burnett,
Outline Bot.: 736, 1092, 1131. Feb 1835 [’Crassulidae’]
2/200–210. Crassula
(200–210; almost cosmopolitan), Hypagophytum (1; H.
abyssinicum; Ethiopia). – Subcosmopolitan. The Tillaea clade of
Crassulais
nearly cosmopolitan and the only indigenous clade of Crassulaceae in Australia.
Leaves opposite. Leaf margin with hydathodes. Sepals four (or five). Petals
four (or five. Stamens four (or five). Anthers somewhat introrse. Carpels three
to nine. Median carpel in Tillaea clade abaxial. Ovules tenuinucellar.
Parietal tissue absent. Archespore sometimes multicellular. Megasporangial
epidermal cells often large. First division of micropylar endosperm cell
horizontal. Follicles dehiscing by apical pore. Testal cells often with a
single papilla and sinuous anticlinal walls. Tegmen absent. x = 7, 8.
[Kalanchoideae+Sempervivoideae]
Leaf margin usually with only one
(sub)apical hydathode. Anthers introrse only in early bud. Placentae sometimes
lobed. Parietal tissue present, one to four cell layers thick. First division
of micropylar endosperm cell vertical. Seeds costate.
Kalanchoideae A.
Berger in Engler et Prantl, Nat. Pflanzenfam., ed. 2, 18a: 383. 3 Mai 1930
4/185–190. Adromischus
(c 26; southern Africa), Kalanchoe (c
125; eastern and southern Africa, Madagascar), Tylecodon (c
27; southern Africa), Cotyledon (9;
eastern and southern Africa, the Arabian Peninsula). – Africa, Madagascar,
the Arabian Peninsula. Shrubs or suffrutices (sometimes small trees).
Calciumoxalate present as crystal sand. Petals connate. Anther connective with
spherical prolongation. Megasporangial epidermal cells sometimes large. Seeds
with four to six costae and micropylar corona. x = 9, 17 (18). Toxic
bufadienolides (cardiac glycosides) present.
Sempervivoideae Arn.,
Botany: 112. 9 Mar 1832 [‘Sempervivae’]
24?/985–1.025. Usually perennial
(sometimes annual) herbs, shrubs, or suffrutices. Hylotelephium
clade Sinocrassula (7; S. ambigua, S.
densirosulata, S. diversifolia, S. indica, S.
longistyla, S. techinensis, S. yunnanensis; the
Himalayas, southwestern China; incl. Kungia?), Kungia (2;
K. aliciae, K. schoenlandii; southern China; in
Sinocrassula?), Meterostachys (1; M. sikokianus;
southern Korean Peninsula, southern Japan), Orostachys
(14; Europe, Central Asia, southern Siberia, Mongolia, China, the Korean
Peninsula, Japan), Hylotelephium
(c 33; temperate regions on the Northern Hemisphere), Perrierosedum
(1; P. madagascariense; Madagascar). –
Umbiliceae Meisn., Plant. Vasc. Gen.: Tab. Diagn.
134, Comm. 98. 8-14 Apr 1838. Umbilicus (c
10; Europe, the Mediterranean to Iran, North and East African mountains),
Pseudosedum (7; P. acutisepalum, P. affine, P.
condensatum, P. koelzii, P. lievenii, P.
longidentatum, P. multicaule; Central Asia), Rhodiola
(60–65; alpine and mountain areas on the Northern Hemisphere, with their
highest diversity in the Himalayas, Tibet and western China), Phedimus (18;
Europe, temperate Asia). – Semperviveae Dumort.,
Fl. Belg.: 85. 1827. Sempervivum
(35–40; Europe, the Mediterranean, Morocco, western Asia). –
Sedeae Fr., Fl. Scan.: 97. 1835. Aeonium
(60–65; Macaronesia, the Mediterranean to Tanzania, the Arabian Peninsula),
Pistorinia (4; P. attenuata, P. brachyantha, P.
breviflora, P. hispanica; the Iberian Peninsula, Morocco,
Algeria), Rosularia (c
30; Europe, the Mediterranean, North Africa, Turkey to Central Asia and the
Himalayas), Prometheum (9; Europe, temperate Asia), Sedella
(4; S. congdonii, S. leiocarpa, S. pentandra, S.
pumila; southern Oregon, California), Dudleya (c 45;
southwestern United States, northwestern Mexico), ’Sedum’
(390–400; Europe, Africa, Asia, North to South America; non-monophyletic),
Villadia (c 45; Texas, Mexico, Central America, northwestern South
America to Peru), Lenophyllum (7; L. acutifolium, L.
guttatum, L. latum, L. obtusum, L. reflexum,
L. texanum, L. weinbergii; Texas, northeastern Mexico), Graptopetalum
(18; Arizona, Mexico), Thompsonella (7; T. colliculosa,
T. garcia-mendozae, T. minutiflora, T. mixtecana,
T. platyphylla, T. spathulata, T. xochipalensis;
Mexico), Echeveria
(150–170; southern United States, Mexico, Central America, the Andes, with
their highest diversity in Mexico), Pachyphytum
(17; Mexico). – Temperate, subtropical and alpine regions on the Northern
Hemisphere and southwards to Madagascar and Peru. Leaves usually alternate
(spiral; sometimes opposite). Flowers tetramerous to 32-merous. Petals usually
free (rarely connate). Carpels three to 32. Megasporangium prolonged, with
central vascular bundle. Follicle in Sedum rarely with
abaxial dehiscence. Seeds with more than six costae. Micropylar suspensor cell
vertically dividing. Suspensor often with compound plasmodesmata. x = 5 or
more. Pyrrolidine and piperidine alkaloids often present. Nonhydrolyzable
tannins not found. – All species of Echeveria
appear to be interfertile. The highly polyphyletic ‘Sedum’ occurs
in five of the seven main clades of Sempervivoideae.
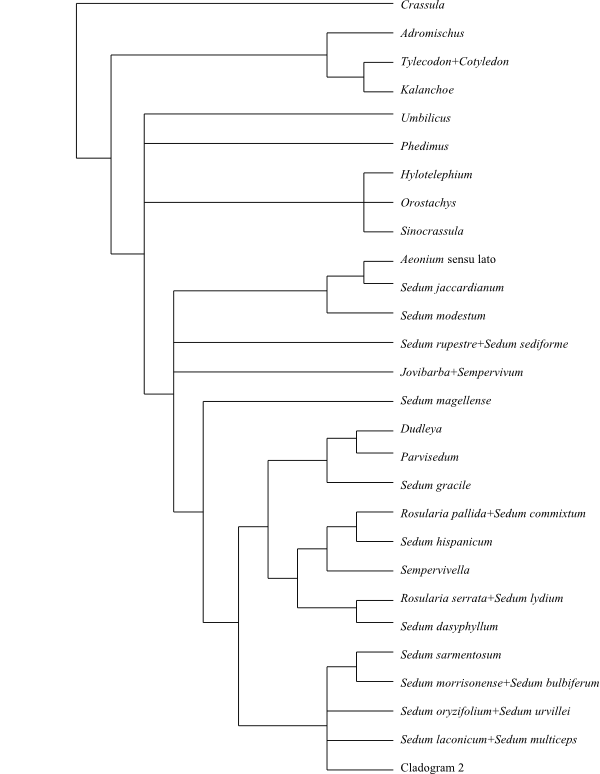 |
Cladogram 1 of Crassulaceae based on
DNA sequence (matK) data (Mort & al. 2001).
|
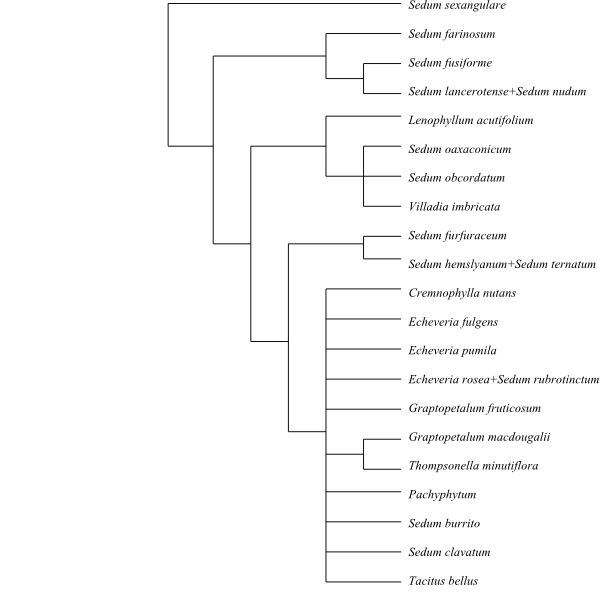 |
Cladogram 2 of Crassulaceae pro parte
based on DNA (matK) data (Mort & al. 2001).
|
Lindley, Nix. Plant.: 23. 17 Sep 1833
[‘Cynomorieae’], nom. cons.
Cynomoriales Burnett,
Outl. Bot.: 1100. Feb 1835
Genera/species 1/1
Distribution The
Mediterranean, Turkey, the Arabian Peninsula to Central Asia and Mongolia.
Fossils Unknown.
Habit Usually monoecious (or
polygamomonoecious?; rarely bisexual), perennial herbs. Achlorophyllous root
holoendoparasites on Nitraria, different Cistaceae and Amaranthaceae,
Tamarix, and other halophytes.
Vegetative anatomy Mycorrhiza
absent. Root modified into fleshy tuberous haustorium, often with host tissue
incorporated; small lateral roots with root hairs abundant, arising from
rhizome. Phellogen absent. Vascular tissue much reduced. Secondary lateral
growth absent. Vessel elements with simple perforation plates; lateral pits?
Imperforate tracheary xylem elements? Wood rays absent. Axial parenchyma? Sieve
tube plastids Ss type? Calciumoxalate crystals abundant.
Trichomes Epidermal hairs
absent.
Leaves Alternate (spiral),
simple, entire, scale-like. Stipules and leaf sheath absent. Venation absent?
Stomata anomocytic, rudimentary. Cuticular waxes? Leaf margin entire.
Inflorescence Terminal,
clavate, on thick, fleshy inflorescence axis.
Flowers Actinomorphic, very
small. Epigyny. Tepals usually (one to) four or five (to eight), sepaloid, free
or connate at base (somewhat more differentiated in male flowers than in female
flowers), with ? aestivation. Pistillodial nectary present. Disc absent.
Androecium Stamen single.
Filament adnate at base to tepals. Anther dorsifixed, versatile,
tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits).
Tapetum secretory. Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains tricolporate to tricolporoidate,
shed as monads, bicellular at dispersal. Exine semitectate, with columellate
infratectum, reticulate.
Gynoecium Pistil probably
composed of one carpel (possibly two carpels). Ovary inferior, unilocular
(possibly pseudomonomerous). Style single, simple (with two vascular bundles),
long, with longitudinal furrow and with conduplicate distal part. Stigma
bilobate, type? Male flowers usually with one (sometimes two) yellow hypogynous
or epigynous pistillodium (stylodium) modified into nectary.
Ovules Placentation apical.
Ovule one per carpel/ovary, orthotropous, pendulous, ategmic to unitegmic,
almost crassinucellar. Integument five to seven cell layers thick, or absent.
Nucellar cap possibly present? Megagametophyte monosporous, Polygonum
type? Endosperm development cellular. Endosperm haustoria? Embryogenesis?
Fruit An achene.
Seeds Aril absent. Seed coat
testal. Testa approx. seven cell layers thick, persistent, with little
thickened cells. Tegmen absent. Perisperm not developed. Endosperm copious,
thick-walled. Embryo small, poorly differentiated, chlorophyll? Cotyledons two.
Germination?
Cytology n = 12 – Karyotype
highly bimodal.
DNA
Phytochemistry Virtually
unknown. Tannins present. Triterpenes with HIV-inhibiting properties (Ma &
al. 1999, Nakamura 2004).
Use Medicinal plants, food.
Systematics
Cynomorium (1; C. coccineum; the Mediterranean from coastal
areas of southern Iberian Peninsula and northwestern Africa, Sahara to Egypt,
Turkey, the Arabian Peninsula to Central Asia and Mongolia).
Cynomorium was sister to
Rosaceae (Rosales) with weak support, according to Zhang
& al. (2009) and Moore & al. (2011) using sequences of the plastid
inverted repeat. The mitochondrial genes atp1 and coxI
indicate an inclusion into Sapindales (Barkman
& al. 2007), whereas Cynomorium belongs in Santalales,
according to Jian & al (2008). However, analyses of 18S rDNA and the
mitochondrial gene matR suggest placement of Cynomorium
within Saxifragales
(Nickrent 2002, Nickrent & al. 2005).
Cynomorium may have acquired genes
from different hosts via horizontal gene transfers (Nickrent 2002, Nickrent
& al. 2005, Barkman & al. 2007, Jian & al. 2008, Zhang & al.
2009), and this makes interpretations of its systematic position fairly
difficult.
Müller Argau in A.-P. de Candolle et A. L. P. P.
de Candolle, Prodr. 16(1): 1. med Nov 1869, nom. cons.
Daphniphyllales Pulle
ex Cronquist, Integr. Syst. Class. Fl. Pl.: 178. 10 Aug 1981;
Daphniphyllanae Takht., Divers. Classif. Fl. Pl.:
140. 24 Apr 1997
Genera/species 1/c 30
Distribution Southwestern
India, Sri Lanka, the Himalayas, southern Tibet, Assam, East Asia to Taiwan,
the Korean Peninsula and Japan, Southeast Asia, Malesia to New Guinea, Solomon
Islands, tropical Australia, with their largest diversity in Yunnan.
Fossils Unknown.
Habit Usually dioecious
(rarely polygamodioecious), evergreen trees or shrubs. Branches provided with
numerous lenticels.
Vegetative anatomy Phellogen?
Medulla often septated by diaphragms. Pericyclic fibres absent. Vessel elements
with scalariform perforation plates; lateral pits opposite to scalariform,
simple and/or bordered pits, non-septate. Imperforate tracheary xylem elements
tracheids with bordered pits, non-septate? Wood rays uniseriate or
multiseriate, heterocellular. Axial parenchyma apotracheal diffuse or banded.
Tyloses common. Sieve tube plastids Ss type, with approx. five globular starch
grains. Nodes? Chambered calciumoxalate crystals present in some species.
Trichomes Hairs absent.
LeavesUsually alternate
(spiral; rarely opposite), simple, entire, with flat ptyxis. Stipules and leaf
sheath absent. Petiole vascular bundles? Venation pinnate. Stomata usually
paracytic (sometimes anomocytic or laterocytic). Cuticular wax crystalloids as
clustered tubuli (Berberis type), chemically dominated by
nonacosan-10-ol. Mesophyll with calciumoxalate druses. Leaf margin usually
entire.
Inflorescence Axillary,
usually raceme.
Flowers Actinomorphic, small.
Hypogyny. Sepals two to six, with imbricate aestivation, very small, or absent.
Petals absent. Nectary absent. Disc absent.
Androecium Stamens five to 12
(to 24), usually as many as or twice the number of sepals. Filaments short,
free from each other and from tepals. Anthers basifixed, non-versatile,
tetrasporangiate, latrorse, longicidal (dehiscing by longitudinal valves);
connective often somewhat prolonged. Tapetum secretory. Female and male flowers
sometimes with staminodia.
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually tricolpate (rarely
tricolporoidate), shed as monads, bicellular at dispersal. Exine tectate, with
columellate infratectum, microperforate, psilate or verrucate.
Gynoecium Pistil composed of
usually two (rarely three or four) connate carpels. Ovary superior,
incompletely bilocular (rarely trilocular or quadrilocular). Stylodia usually
two (rarely three or four), short, recurved or circinate, connate at base.
Stigmas relatively massive, decurrent, with multicellular appendages,
non-papillate, Dry type. Male flowers sometimes with pistillodium.
Ovules Placentation apical to
axile or parietal. Ovules (one or) two per carpel, anatropous, pendulous,
epitropous, bitegmic, crassinucellar. Micropyle exostomal, Z-shaped (zig-zag)?
Outer integument three to six cell layers thick. Inner integument four or five
cell layers thick. Hypostase present. Megagametophyte monosporous,
Polygonum type. Synergids with a filiform apparatus. Endosperm
development cellular. Endosperm haustoria? Embryogenesis? Polyembryony
sometimes occurring.
Fruit A usually one-seeded
(rarely two-seeded) drupe, often with persistent stylodia and staminodia.
Seeds Aril absent. Testa
persistent, thin-walled, crushed. Exotegmen? Endotegmen with tannins, and
thickened and often sclerotized and cutinized cell walls. Perisperm thin, with
protein crystals. Endosperm copious, oily and proteinaceous. Embryo small,
straight, apical, well differentiated, chlorophyll? Cotyledons two, as wide as
radicula. Germination phanerocotylar.
Cytology n = 16
DNA Mitochondrial intron
coxII.i3 lost?
Phytochemistry Flavonols
(quercetin, apigenin, luteolin, etc.), Group I carbocyclic iridoids (e.g.
asperuloside, daphniphylloside), hydrolyzable tannins, and unique triterpene
(squalene) alkaloids of the daphniphylline group (daphniphylline,
codaphniphylline etc.) present. Myricetin? Ellagic acid, proanthocyanidins,
condensed tannins, and cyanogenic compounds not found. Aluminium
accumulated.
Use Ornamental plants.
Systematics
Daphniphyllum (c 30; the Western Ghats in southwestern India, Sri
Lanka, Missuri to Bhutan in southern Himalayas, southern Tibet, Khasia Hills in
Assam, China, the Korean Peninsula and Japan, Taiwan, Southeast Asia, Malesia
to New Guinea, Solomon Islands, tropical Australia, with their largest
diversity in Yunnan).
Daphniphyllum is probably sister to Cercidiphyllum
(Cercidiphyllaceae).
de Candolle in de Lamarck et A. P. de Candolle,
Fl. Franç., ed. 3, 4(2): 405. 17 Sep 1805 [’Grossulariae’], nom.
cons.
Grossulariales DC. ex
Bercht. et J. Presl, Přir. Rostlin: 236. Jan-Apr 1820
[‘Grossulariae’]; Ribesiaceae Marquis,
Esq. Règne Vég.: 66. 15-22 Jul 1820 [’Ribesioïdeae’]
Genera/species 1/180–200
Distribution Temperate parts
of Eurasia, the Mediterranean, northeastern Africa, North America to southern
Chile.
Fossils Leaves similar to
Ribes are known from Eocene and younger sediments in North America.
Habit Usually bisexual
(sometimes dioecious), usually deciduous (rarely evergreen) shrubs, upright,
creeping (often with subterranean stems) or occasionally somewhat climbing;
branches differentiated into long shoots and short shoots. Leaves sometimes
modified into spines.
Vegetative anatomy Phellogen
ab initio outer-cortical to pericyclic. Subterranean stems with well developed
endodermis. Pericyclic fibres absent. Vessel elements usually with scalariform
(rarely simple) perforation plates; lateral pits usually alternate (sometimes
scalariform), simple or bordered pits. Imperforate tracheary xylem elements
fibre tracheids with simple or bordered pits, septate or non-septate (also
vasicentric tracheids). Wood rays uniseriate or multiseriate, homocellular or
heterocellular. Axial parenchyma usually absent (rarely apotracheal diffuse or
paratracheal scanty or banded). Sieve tube plastids Ss type. Nodes 1:1,
unilacunar with one leaf trace, or 3:3, trilacunar with three traces.
Tanniniferous cells abundant. Non-lignified stem tissues with numerous
cystoliths. Calciumoxalate druses present.
Trichomes Hairs unicellular or
multicellular, stalked or unstalked, uniseriate or branched, with or without
glandular apex, often peltate-lepidote glandular hairs; epidermal prickles
frequent at nodes and internodes.
Leaves Alternate (spiral),
simple or almost palmately compound, usually lobed (rarely entire), usually
with conduplicate-plicate (rarely convolute) ptyxis. Stipules intrapetiolar,
membranous, or absent; leaf sheath absent. Petiole vascular bundle transection
arcuate?; petiole bundles connate. Venation palmate. Stomata anomocytic
(paracytic?). Cuticular waxes? Domatia abaxial, as pockets. Druses present in
parenchyma and collenchyma. Tanniniferous cells numerous. Leaf margin usually
coarsely serrate; leaf teeth with hydathodes.
Inflorescence Terminal,
raceme, usually pendent (sometimes erect).
Flowers Actinomorphic, small.
Pedicel articulated. Hypanthium present, with basal nectaries. Epigyny or
almost epigyny. Sepals usually five (rarely four), with imbricate or subvalvate
aestivation, often petaloid, persistent, usually recurved (sometimes connivent
into a tube), free. Petals (in reality staminodia?) usually five (rarely four),
with open or imbricate aestivation, scale-like to subulate, persistent, free
(rarely absent). Nectariferous disc often quinquelobate.
Androecium Stamens usually
five (rarely four), antesepalous, alternipetalous. Filaments filiform, inserted
on top of hypanthium, free, adnate to sepals (free from petals?). Anthers
usually basifixed (sometimes dorsifixed), non-versatile, tetrasporangiate,
introrse, longicidal (dehiscing by longitudinal slits); connective sometimes
with apical nectary. Tapetum secretory. Female flowers with staminodia.
Pollen grains
Microsporogenesis simultaneous. Pollen grains (5–)8–14-pantoporate or
zonocolporate (rarely pentacolpodiorate), shed as monads, bicellular at
dispersal. Ectoapertures present (rugulate surfaces surrounding endoapertures).
Exine pertectate, with columellate? infratectum, rugulate, punctate or
spinulate.
Gynoecium Pistil usually
composed of two paracarpous, connate and usually median (sometimes transverse)
carpels. Ovary inferior or almost inferior, unilocular. Style single, simple or
bifid, or stylodia two, free. Stigmas two, capitate, non-papillate, Wet type.
Male flowers with pistillodium.
Ovules Placentation parietal
(with two somewhat intruding placentae). Ovules four to more than 100 per
ovary, anatropous, bitegmic, crassinucellar. Micropyle exostomal. Outer
integument three to five cell layers thick, with tanniniferous outer epidermal
cells. Inner integument two or three cell layers thick. Funicular obturator
present. Parietal tissue three or four cell layers thick. Hypostase present.
Megagametophyte monosporous, Polygonum type. Endosperm development
usually cellular (sometimes helobial or nuclear?). Endosperm haustorium
chalazal. Embryogenesis irregular.
Fruit A many-seeded berry-like
fruit with persistent perianth and staminal remnants.
Seeds Funicular aril
surrounding entire or parts of seed. Seed coat endotestal. Exotesta
mucilaginous (myxotesta); exotestal cells palisade, large with thin walls;
epidermal cells large and pulpy. Endotesta hard; endotestal cells small,
cuboid, with calciumoxalate crystals, and with radial and inner walls
lignified. Tegmic cells elongate, tanniniferous. Perisperm not developed.
Endosperm copious, oily, proteinaceous and with hemicellulose, with somewhat
thickened walls. Embryo small, straight, well differentiated, without
chlorophyll. Cotyledons two, with main vein ending in hydathodal tooth.
Germination phanerocotylar.
Cytology n = 8 (16)
DNA
Phytochemistry Flavonols
(kaempferol, quercetin, myricetin), cyanidin, delphinidin, mono- and
sesquiterpenes, ellagic acid, ellagitannins, and tyrosine-derived cyanogenic
compounds present. Iridoids not found. Aluminium accumulated in some
species.
Use Ornamental plants, fruits,
tea (leaves from Ribes nigrum), medicinal plants.
Systematics Ribes
(180–200; Europe, the Mediterranean, northeastern Africa, temperate Asia,
North and Central America, the Pacific coast of South America, the Andes south
to Tierra del Fuego).
Ribes is
sister to Saxifragaceae.
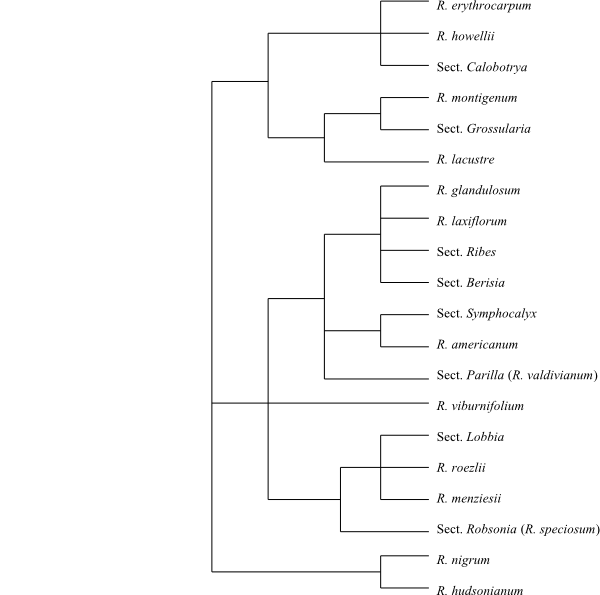 |
50% majority rule consensus tree of Ribes
based on DNA sequence data (Messinger & al. 1999).
|
Brown in M. Flinders, Voy. Terra Austral. 2: 549.
19 Jul 1814 [’Halorageae’], nom. cons.
Cercodiaceae Juss. in
F. Cuvier, Dict. Sci. Nat. 7: 441. 24 Mai 1817 [‘Cercodianae’];
Haloragales Link, Handbuch 2: 52. 4-11 Jul 1829
[’Halorageae’]; Myriophyllaceae Schultz
Sch., Nat. Syst. Pflanzenr.: 324. 30 Jan-10 Feb 1832
[’Myriophylleae’]; Haloragineae Engl.,
Syllabus, ed. 2: 162. Mai 1898 [‘Halorrhagidineae’]
Genera/species 8–9/c 120
Distribution Largest diversity
on the Southern Hemisphere, particularly in Australia; Myriophyllum
cosmopolitan; Proserpinaca in North America and the West Indies.
Fossils Obispocaulis
myriophylloides and Tarahumara sophiae from the Campanian to the
Maastrichtian of Mexico have both been assigned to either Haloragaceae or very close to
that clade. Tarahumara had unisexual epigynous flowers with four
basally connate uniovulate carpels, and a drupaceous fruit.
Obispocaulis is represented by fossil stems containing aerenchyma and
bearing adpressed leaves. Cenozoic pollen and fruits of Haloragaceae are frequent in
both the Northern and Southern Hemispheres. Fossils of Proserpinaca
are known from pre-Pliocene layers in Europe.
Habit Usually monoecious or
polygamomonoecious (rarely bisexual or dioecious), perennial or annual herbs,
suffrutices or evergreen small shrubs (‘Haloragodendron’ consists
of shrubs and small trees). Numerous species are aquatic, other representatives
are amphibious, hygrophytes or terrestrial mesophytes. Myriophyllum in
the Northern Hemisphere produces turions (condensed reproductive and
hibernating shoots). The main root in aquatic and amphibious species is
replaced by adventitious roots (without root hairs), anchoring the plant to the
substrate (adventitious roots in Haloragis inserted between the
leaves).
Vegetative anatomy Phellogen?
Primary cortex with numerous aerial cavities (especially in aquatic species).
Primary vascular tissue strongly reduced in aquatic species. Endodermis
significant especially in aquatic species. Secondary lateral growth normal or
absent. Vessel elements with simple perforation plates; lateral pits?
Imperforate tracheary xylem elements ?, with simple pits
(Glischrocaryon). Wood rays uniseriate or multiseriate, homocellular
or heterocellular. Axial parenchyma? Sieve tube plastids Ss type. Nodes 1:1,
unilacunar with one leaf trace. Calciumoxalate crystals often present in
hair-shaped cortical cells.
Trichomes Hairs unicellular or
multicellular, uniseriate, often with silica, or absent.
Leaves Usually opposite or
verticillate (sometimes alternate, spiral), simple or compound (often pinnately
compound), entire or often finely lobed, with conduplicate-flat ptyxis (in
Myriophyllum and Proserpinaca heterophylly). Stipules very
small or absent; leaf sheath absent. Colleters present? Petiole vascular bundle
transection arcuate? Venation pinnate (or leaves one-veined). Stomata usually
anomocytic (often absent). Cuticular wax crystalloids usually absent (rarely as
parallel grouped platelets, Hypericum type). Leaf margin serrate or
entire, eglandular.
Inflorescence Terminal or
axillary, thyrso-paniculate, thyrsoid, fasciculate etc., or raceme- or
spike-like, or flowers solitary, axillary. Floral prophylls (bracteoles)
present or absent.
Flowers Actinomorphic, small.
Epigyny. Sepals usually four (in Proserpinaca three, rarely two), with
valvate aestivation, persistent (absent in female flowers of
Myriophyllum). Petals usually four (in Proserpinaca three,
rarely two), with imbricate aestivation, caducous (usually absent in
Proserpinaca and female flowers in Myriophyllum and
Laurembergia). Nectary absent. Disc absent.
Androecium Stamens usually
(two to) four or 4+4, as many as or twice the number of sepals (when four then
antesepalous). Filaments short, narrow, free from each other and from tepals.
Anthers basifixed, non-versatile, tetrasporangiate, introrse, longicidal
(dehiscing by longitudinal slits); connective sometimes slightly prolonged.
Tapetum secretory. Antesepalous staminodia present in some species of
Gonocarpus.
Pollen grains
Microsporogenesis simultaneous. Pollen grains 4–6(–20)-colpate or
4–6(–20)-porate, shed as monads, usually tricellular (rarely bicellular) at
dispersal. Exine tectate, with columellate infratectum, microperforate and
beset with small supratectal processes.
Gynoecium Pistil composed of
usually four (rarely two or three) connate carpels; carpels antepetalous or
median carpel adaxial. Ovary inferior, (unilocular to) quadrilocular (septa
sometimes poorly developed, absent in Laurembergia and most species of
Glischrocaryon). Stylodia separate, clavate, somewhat swollen at base
(sometimes absent). Stigmas capitate or penicillate, papillate, Dry type.
Pistillodium?
Ovules Placentation apical.
Ovules one or two (one aborting) per carpel, anatropous or hemianatropous,
pendulous, usually apotropous (sometimes epitropous), bitegmic, crassinucellar
(or tenuinucellar?). Micropyle endostomal (Myriophyllum). Outer
integument two or three cell layers thick. Inner integument approx. two cell
layers thick. Parietal tissue two or three cell layers thick. Archesporial cell
hypodermal (Myriophyllum). Funicular obturator poorly developed or
absent. Hypostase often present. Nucellar cap often present. Megagametophyte
monosporous, Polygonum type. Synergids with a filiform apparatus.
Antipodal cells persistent. Endosperm development usually cellular (in
Laurembergia and some species of Myriophyllum nuclear).
Haustorial suspensor present. Endosperm haustoria? Embryogenesis
caryophyllad.
Fruit A one- to four-seeded
nut (in Proserpinaca three-seeded), a drupe with one- to four-seeded
pyrene (in Meziella) or a schizocarp with two to four nut-like
mericarps (in Myriophyllum).
Seeds Aril absent. Seed coat
exotestal. Exotesta (sometimes also hypodermal layer) persistent, with
thin-walled cells; other parts of testa and tegmen degrading and crushed.
Perisperm not developed. Endosperm usually copious, starchy, oily. Embryo
usually large, straight, without chlorophyll. Cotyledons two. Germination
phanerocotylar.
Cytology n = (6) 7 (8) 14, 28,
42, 56 – Polyploidy occurring.
DNA Mitochondrial intron
coxII.i3 lost (Haloragis).
Phytochemistry Flavones,
pelargonidin (in leaves), cyanidin, ellagic and gallic acid, tannins,
myriophyllin (in hair glands of Myriophyllum), cyanogenic compounds,
saponins, and coumaric acid present. Flavonols (kaempferol, quercetin)?
Use Ornamental plants,
aquarium plants (Myriophyllum).
Systematics Glischrocaryon
(5; G. angustifolium, G. aureum, G. behrii, G.
flavescens, G. roei; southern Western Australia, southern South
Australia, New South Wales, Victoria; incl. Haloragodendron?),
‘Haloragodendron’ (5–6; H. baeuerlenii, H.
gibsonii, H. glandulosum, H. lucasii, H.
monospermum, H. racemosum; southwestern Western Australia,
southeastern New South Wales, eastern Victoria, Tasmania; paraphyletic; in Glischrocaryon?);
Proserpinaca (4; P. amblygona, P. intermedia, P.
palustris, P. pectinata; southeastern United States, the West
Indies), Meionectes (2; M. brownii, M. preissii;
southwestern Western Australia, southeastern South Australia, southern
Victoria, Tasmania), Trihaloragis (1; T. hexandra;
southwestern Western Australia), Haloragis (17?; Australia, New
Caledonia, New Zealand, Rapa Island, Juan Fernández), Gonocarpus (c
40; Southeast Asia and Malesia to Japan, Australia and New Zealand),
Laurembergia (4; L. minor, L. repens, L.
tetrandra, L. veronicifolia; tropical and subtropical regions on
both hemispheres), Myriophyllum
(c 70; cosmopolitan, with their highest diversity in Australia).
Haloragaceae are sister-group
to Penthorum (Penthoraceae).
The Haloragodendron clade (probably
including Glischrocaryon)
is sister to the remaining Haloragaceae. The crown clade
is not completely resolved, since Proserpinaca and Meionectes
are parts of a basal trichotomy together with the remainder.
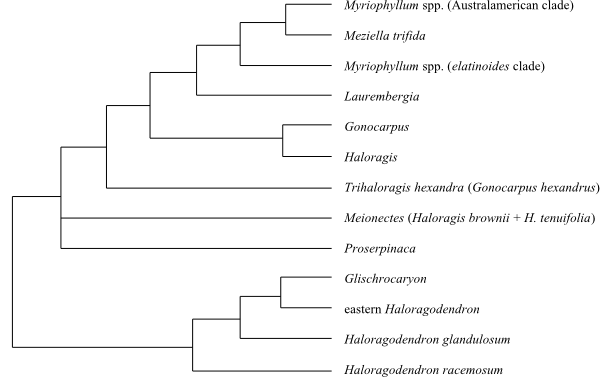 |
Majority rule consensus tree (simplified) from
Bayesian analysis of Haloragaceae based on
DNA sequence data (Moody & Les 2007).
|
Brown in C. Abel, Narr. Journey China: 374. 15
Aug 1818 [‘Hamamelideae’], nom. cons.
Fothergillaceae
Nutt., Gen. N. Amer. Pl. 1: 108. 14 Jul 1818 [‘Fothergilleae’];
Fothergillales Link, Handbuch 2: 445. 4-11 Jul 1829
[’Fothergilleae’]; Hamamelidales Link,
Handbuch 2: 4. 4-11 Jul 1829 [’Hamamelideae’];
Parrotiaceae Horan., Prim. Lin. Syst. Nat.: 79. 2 Nov
1834 [‘Parrotiaceae (Hamamelideae)’];
Hamamelidopsida Brongn., Enum. Plant. Mus. Paris:
xxix, 109. 12 Aug 1843 [’Hamamelineae’];
Bucklandiaceae J. Agardh, Theoria Syst. Plant.: 155.
Apr-Sep 1858 [’Bucklandieae’], nom. illeg.;
Disanthaceae Nakai, Chosakuronbun Mokuroku [Ord. Fam.
Trib. Nov.]: 246. 20 Jul 1943; Rhodoleiaceae Nakai,
Chosakuronbun Mokuroku [Ord. Fam. Trib. Nov.]: 246. 20 Jul 1943;
Exbucklandiaceae Reveal et Doweld in Novon 9: 552. 30
Dec 1999; Hamamelidineae Thorne et Reveal in Bot.
Rev. (Lancaster) 73: 91. 29 Jun 2007
Genera/species 26/110–111
Distribution Eastern and
southern Africa, Madagascar, southern Turkey, southeastern Trans-Caucasus,
northern Iran, western and eastern Himalayas, Assam, Manipure, East and
Southeast Asia to the Korean Peninsula and Japan, Malesia to New Guinea,
northeastern Australia (Queensland), eastern North America, Central America,
northeastern South America.
Fossils Allonia
decandra from the Late Santonian of Georgia (the United States) is a
pentamerous male flower with connate sepals and two staminal whorls, tricolpate
pollen having reticulate exine and columellate infratectum, and with an adaxial
lobate (nectariferous?) disc. Allonia seems to be sister-group to
Maingaya. Androdecidua endressii is another pentamerous
staminate flower from the Late Santonian of Georgia. Numerous fossils
(especially leaves and seeds) of Hamamelidaceae are known
from the Cenozoic. Seeds and bilocular fruits of Rhodoleia have been
found in Maastrichtian to Miocene layers in Europe.
Habit Usually bisexual
(sometimes monoecious, andromonoecious or polygamomonoecious), evergreen or
deciduous trees or shrubs.
Vegetative anatomy Phellogen
ab initio superficial. Primary vascular tissues as a cylinder, without separate
bundles. Vessel elements with usually scalariform (sometimes reticulate)
perforation plates; lateral pits usually opposite to scalariform (rarely
alternate), simple pits. Vestured pits sometimes present. Imperforate tracheary
xylem elements tracheids or fibre tracheids with bordered pits, non-septate.
Wood rays uniseriate or multiseriate, homocellular or heterocellular. Axial
parenchyma apotracheal diffuse or diffuse-in-aggregates, or paratracheal scanty
(or unilateral), or absent. Tyloses sometimes abundant. Sieve tube plastids Ss
type, with five (to ten) starch grains. Nodes usually 3:3, trilacunar with
three leaf traces (in Chunia and Mytilaria 5:5, pentalacunar
with five traces). Medulla in Mytilaria with resiniferous secretory
canals. Branched sclereids frequent. Some species possess secretory canals.
Parenchyma usually with prismatic calciumoxalate crystals.
Trichomes Hairs usually
multicellular (sometimes unicellular), often early sclerified, usually stellate
or tufted (sometimes uniseriate).
Leaves Usually alternate
(usually distichous, sometimes spiral; rarely opposite), simple, entire, with
conduplicate-flat or conduplicate-plicate ptyxis. Stipules usually caducous
(sometimes cauline); leaf sheath absent. Petiole vascular bundle transection
usually annular (rarely arcuate; petiole sometimes with adaxial bundle).
Venation usually palmate or with stout veins at base (rarely pinnate),
eucamptodromous or actinodromous and brochidodromous or craspedodromous.
Stomata usually paracytic (sometimes laterocytic), often only on adaxial side
of lamina. Cuticular wax crystalloids usually absent (rarely as irregular
platelets, or as clustered tubuli of Berberis type, chemically
dominated by nonacosan-10-ol). Domatia rarely present. Epidermis with or
without mucilage cells. Mesophyll with calciumoxalate crystals and usually
sclerenchymatous idioblasts with sclereids of different appearance
(brachysclereids, columnar, etc.). Leaf margin usually serrate (rarely entire);
leaf teeth often fothergilloid (with transparent glandular apex; gland often
absent).
Inflorescence Terminal or
axillary, thyrse, botryoid, panicle, spike-, catkin- or head-like (rarely
raceme; in Rhodoleia pendant capitulate pseudanthium with involucre
consisting of bracts).
Flowers Usually actinomorphic.
Hypanthium present or absent. Usually epigyny (rarely hypogyny). Sepals (two
to) four or five (to seven), usually with imbricate aestivation, usually free
(rarely connate), often persistent (rarely absent). Petals (two to) four or
five, with open or valvate (or imbricate) aestivation, adaxially circinate,
usually band-like, often spirally twisted, sometimes stipitate, caducous, free
(rarely absent). Nectaries as an intrastaminal annular nectariferous disc or
staminodial nectaries (in Hamamelis), at petal bases (in
Disanthus) or as nectariferous glands around filament (in
Rhodoleia).
Androecium Stamens (one to)
four or five (to 24), in one or two whorls, often as many as sepals,
antesepalous. Filaments free from each other and from tepals. Anthers usually
basifixed, non-versatile, usually tetrasporangiate (rarely disporangiate),
latrorse or introrse, longicidal (usually dehiscing by one or two valves or
slits; rarely by basal pore); connective usually more or less prolonged.
Tapetum secretory, with uni-, bi- or multinucleate cells. Staminodia
antepetalous, sometimes alternating with fertile stamens, or absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually tricolpate (rarely
tetracolpate, pantocolpate, hexarugate,polyrugate or polyforate), shed as
monads, bicellular at dispersal. Exine semitectate, with columellate
infratectum, reticulate.
Gynoecium Pistil composed of
usually two (rarely three) partially or entirely connate carpels. Ovary usually
inferior (rarely superior), usually bilocular (rarely trilocular). Stylodia
usually two (rarely three), long, free or connate below. Stigmas terminal or
decurrent, with multicellular appendages, non-papillate, Dry type. Male flowers
often with pistillodium.
Ovules Placentation axile to
apical. Ovule usually one or several (rarely numerous) per carpel, anatropous,
usually pendulous (along carpellary edges when numerous), apotropous to
epitropous (half epitropous, half apotropous), bitegmic, crassinucellar.
Micropyle endostomal or bistomal, sometimes Z-shaped (zig-zag). Outer
integument usually six to twelve (rarely two) cell layers thick. Inner
integument two or three cell layers thick. Hypostase present. Parietal tissue
usually eight to ten cell layers thick. Nucellar cap sometimes two cell layers
thick. Archespore usually unicellular (rarely multicellular). Megagametophyte
monosporous, Polygonum type. Endosperm development usually nuclear (at
least in Parrotiopsis cellular). Endosperm haustoria? Embryogenesis
chenopodiad (Distylium, Hamamelis) or solanad
(Parrotiopsis).
Fruit A loculicidal and/or
septicidal double-walled capsule with coriaceous or lignified exocarp and
lignified or corneous endocarp, often with persistent calyx (in
Rhodoleia a syncarp with capsular unities).
Seeds Aril absent. Hilum
large. Seed coat (meso)testal. Testa usually thick, hard, multiplicative,
shining and often two-coloured, winged or unwinged. Exotestal cells usually
thickened. Mesotesta massive, usually consisting of sclerotized fibrous cells.
Tegmen not included in mature seed coat; tegmic cells tanniniferus. Perisperm
more or less developed. Endosperm usually sparse, oily. Embryo straight,
usually small, well differentiated. Cotyledons two. Germination phanerocotylar.
Polyembryony sometimes occurring.
Cytology n = 8, 12 –
Polyploidy occurring in some species.
DNA Mitochondrial intron
coxII.i3 lost. Plastid gene rpl22 absent.
Phytochemistry Flavonols
(kaempferol, quercetin, myricetin), flavone-C-glycosides, cyanidin,
delphinidin, ellagic acid, and tannins present. Chalcones, alkaloids, and
cyanogenic compounds not found.
Use Ornamental plants,
medicinal plants (also healing substances etc. from Hamamelis
virginiana), timber.
Systematics Hamamelidaceae are
sister-group to the clade [Cercidiphyllaceae+Daphniphyllaceae].
Exbucklandioideae are sister to the
remainder.
Exbucklandioideae (A.
Reinsch) H. T. Chang in Acta Sci. Nat. Univ. Sunyatseni 1973(1): 54. Nov
1973
3/10. Exbucklandia (3; E.
longipetala, E. populnea, E. tonkinensis; Assam, eastern
Himalayas, southern China, the Malay Peninsula, Sumatra), Rhodoleia
(6; R. championii, R. forrestii, R. henryi, R.
macrocarpa, R. parvipetala, R. stenopetala; southern
China, Southeast Asia to Sumatra), Mytilaria (1; M.
laosensis; Guangxi, Laos). – Assam to Southeast Asia and southern China,
West Malesia. Inflorescence usually capitate. Epigyny or half epigyny. Petals
sometimes absent. Micropyle in Exbucklandia with partially prolonged
outer integument. Outer integument in Exbucklandia two cell layers
thick. Seed coat in Exbucklandia and Rhodoleia winged. Only
exotestal cells thickened. n = 8, 12.
[Disanthoideae+Hamamelidoideae]
Disanthoideae Harms in
Engler et Prantl, Nat. Pflanzenfam., ed. 2, 18a: 314. 3 Mai 1930
1/1. Disanthus (1; D.
cercidifolius; Japan). – Hypogyny. Nectaries present at petal bases.
Anthers dehiscing with longitudinal slits. n = 8.
Hamamelidoideae
Burnett, Outlines Bot.: 733, 1132. Feb 1835 [‘Hamamelidae’]
22/99–100. Chunia (1; C.
bucklandioides; Hainan), Corylopsis
(c 25; eastern Himalayas, China, the Korean Peninsula, Japan),
Dicoryphe (13; Madagascar, the Comoros), Distyliopsis (6;
D. dunnii, D. lanata, D. laurifolia, D.
salicifolia, D. tutcheri, D. yunnanensis; Burma,
Thailand, Indochina, Malesia, Taiwan), Distylium (c 15; Southeast
Asia, Malesia), Embolanthera (2; E. glabrescens, E.
spicata; Southeast Asia, the Philippines), Eustigma (3; E.
balansae, E. lenticellatum, E. oblongifolium; southern
China, Southeast Asia), Fortunearia (1; F. sinensis; central
and eastern China), Fothergilla
(2–3; F. gardenia, F. major, F. monticola;
southeastern United States), Hamamelis
(6; H. mexicana, H. ovalis, H. vernalis, H.
virginiana: southeastern Canada, eastern United States, Mexico; H.
mollis: China; H. japonica: Japan), Loropetalum
(3; L. chinense, L. lanceum, L. subcordatum;
northern and northeastern India to China, Japan), Maingaya (1; M.
malayana; Penang and Perak on the Malay Peninsula), Matudaea (2;
M. colombiana, M. trinervia; central Mexico to Honduras),
Molinadendron (3; M. guatemalense, M. hondurense,
M. sinaloense; Mexico, Central America), Neostrearia (1;
N. fleckeri; northeastern Queensland), Noahdendron (1; N.
nicholasii; northeastern Queensland), Ostrearia (1; O.
australiana; northeastern Queensland), Parrotia (2;
P. persica: southwestern Caspian area; P. subaequalis:
eastern China), Parrotiopsis
(2; P. involucrata, P. jacquemontiana; the Himalayas), Sinowilsonia
(1; S. henryi; western and central China), Sycopsis (2;
S. sinensis, S. triplinervia; Assam, China, Taiwan),
Trichocladus (6; T. crinitus, T. dentatus, T.
ecklonianus, T. ellipticus, T. goetzei, T.
grandiflorus; tropical and southern Africa). – Distribution as for
Hamamelidaceae.
Leaves with craspedodromous venation. Sepals usually absent (sometimes five to
nine). Petals usually absent. Stamens four to 24. Anthers dehiscing by valves.
Ovule one (in reality three, two of which sterile) per carpel, usually more or
less epitropous (sometimes apotropous). Fruit with ballistic seed dispersal. n
= 12. – Corylopsideae Harms in H. G. A. Engler et K. A. E. Prantl,
Nat. Pflanzenfam., ed. 2, 18a: 325. 3 Mai 1930 are sister to a clade consisting
of Eustigmateae Harms in H. G. A. Engler et K. A. E. Prantl, Nat.
Pflanzenfam., ed. 2, 18a: 314. 3 Mai 1930 and Hamamelideae DC., Prodr.
4: 268. late Sep 1830 [‘Hamameleae’] (Li & al. 1999).
 |
Cladogram of Hamamelidaceae based
on DNA (nuclear ITS) sequence data (Li & al. 1999).
|
Agardh, Theoria Syst. Plant.: 151. Apr-Sep 1858,
nom. cons.
Iteales Doweld, Tent.
Syst. Plant. Vasc.: xxviii. 23 Dec 2001
Genera/species 1/c 20
Distribution Eastern and
southern Africa, eastern Himalayas to the Korean Peninsula, Japan, Taiwan,
Southeast Asia, West Malesia, southeastern United States.
Fossils Iteaceae fossils – mostly
diporate pollen grains, but also leaves, fruits and seeds – have been
described from the Eocene to the Pliocene of Europe. Ademanthemum
iteoides, a flower fossilized in Baltic amber, may be assigned to Iteaceae. Fossil pollen grains of
’Choristylis’ (Itea) are known from Europe and leaves
similar to those in Itea have been found in Eocene and younger layers
in western North America.
Habit Bisexual or
polygamomonoecious, evergreen or deciduous trees or shrubs (rarely
climbing).
Vegetative anatomy Phellogen
superficial. Young stem with separate vascular bundles. Medulla septated (by
diaphragms?). Vessel elements with scalariform perforation plates; lateral pits
opposite to scalariform, bordered pits. Imperforate tracheary xylem elements ?
with bordered pits, non-septate. Wood rays uniseriate or multiseriate,
heterocellular. Axial parenchyma apotracheal diffuse or diffuse-in-aggregates
(sometimes absent). Sieve tube plastids Ss type. Nodes 3:3, trilacunar with
three leaf traces. Calciumoxalate druses present in cortex and medulla.
Trichomes Hairs
unicellular.
Leaves Alternate (spiral),
simple, entire, sometimes coriaceous, with conduplicate ptyxis. Stipules small,
subulate, inserted at petiole base or stem, or absent; leaf sheath absent.
Petiole vascular bundle transection arcuate? Venation pinnate. Stomata
anomocytic (paracytic?). Cuticular wax crystalloids? Leaf margin usually
glandular-serrate (rarely entire; in Itea ilicifolia
spinose-dentate).
Inflorescence Terminal or
axillary, racemose or panicle.
Flowers Actinomorphic, small.
Hypanthium present. Hypogyny to epigyny. Sepals five, with open or valvate
aestivation, persistent, connate at base into tube. Petals five, with valvate
aestivation, persistent, free. Nectariferous disc intrastaminal, annular, on
hypanthium rim.
Androecium Stamens five,
antesepalous, alternipetalous. Filaments subulate, inserted at margin of
nectariferous disc, free from each other and from tepals. Anthers dorsifixed,
non-versatile, tetrasporangiate, introrse, longicidal (dehiscing by
longitudinal slits); connective slightly prolonged. Tapetum secretory.
Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains bilateral/heteropolar, usually
diporate (rarely triporate), shed as monads, bicellular at dispersal. Exine
tectate, with granular infratectum, psilate to scabrate.
Gynoecium Pistil composed of
two ab initio free, finally connate carpels. Ovary superior to almost inferior,
bilocular. Style single, simple or bifid, at anthesis with connivent stylodia.
Stigma capitate or lobate, at anthesis with connivent (often connate) and in
fruit separate lobes, non-papillate?, Wet type. Pistillodium absent.
Ovules Placentation axile.
Ovules four to c. 50 per carpel, usually bitegmic (in
’Choristylis’ unitegmic), crassinucellar. Micropyle bistomal?, not
Z-shaped. Outer integument ? cell layers thick. Inner integument ? cell layers
thick. Parietal tissue three (to seven) cell layers thick. Megagametophyte
monosporous, Polygonum type. Endosperm development? Endosperm
haustoria? Embryogenesis?
Fruit A septicidal capsule
with persistent perianth (valves often connate in stigmatic region).
Seeds Aril absent. Seed coat
exotestal. Exotestal cells tanniniferous, with thickened outer walls. Mesotesta
and endotesta crushed. Tegmen? Perisperm not developed. Endosperm sparse, with
lipids and aleuron. Embryo large, curved, well differentiated, chlorophyll?
Cotyledons two. Germination?
Cytology n = 11
DNA
Phytochemistry
Flavone-C-glycosides, proanthocyanidins (prodelphinidins),
tyrosine-derived cyanogenic compounds, and allitol present. Flavonols and
ellagic acid not found.
Use Ornamental plants,
medicinal plants.
Systematics Itea (c 15; eastern
and southern Africa [I. rhamnoides], eastern Himalayas, China, the
Korean Peninsula, Japan, Taiwan, Southeast Asia, West Malesia, southeastern
United States).
Itea is sister to
Pterostemon (Pterostemonaceae).
Brenan in Kew Bull. 7: 228. 25 Jul 1952, nom.
cons.
Medusandrales F.
Novák ex Takht., Divers. Classif. Fl. Pl.: 353. 24 Apr 1997
Genera/species 1/2
Distribution Central
Africa.
Fossils Unknown.
Habit Bisexual, evergreen
trees.
Vegetative anatomy Phellogen
ab initio superficial. Vessel elements very long, with scalariform perforation
plates (with numerous transverse ridges); lateral pits scalariform or opposite,
bordered pits. Imperforate tracheary xylem elements fibre tracheids with
bordered pits, non-septate? Wood rays usually uniseriate, heterocellular. Axial
parenchyma usually paratracheal scanty, or diffuse. Sieve element plastids S
type. Nodes? Laticifers present in outer part of medulla, with lemon-coloured
latex. Some phloem cells with aggregated crystals.
Trichomes Hairs unicellular,
with lignified walls and swollen base.
Leaves Alternate (spiral),
simple (unifoliolate?), entire, with ? ptyxis. Stipules cauline, small, early
caducous; leaf sheath absent. Petiole apex with pulvinus having complex
anatomy. Petiole vascular bundle transection annular; petiole with medullary
annular bundle. Venation palmate, with two sides of base converging in apical
part of distally swollen petiole (veins arising from base or near base);
secondary veins numerous, parallel. Stomata anomocytic. Cuticular wax
crystalloids? Leaf margin crenate to weakly serrate.
Inflorescence Axillary, single
or pairwise, catkin-like.
Flowers Actinomorphic, small.
Hypogyny. Sepals five, with apert, open (valvate?) aestivation, persistent,
accrescent, free or connate at base. Petals five, with imbricate aestivation,
free. Nectary absent. Disc absent.
Androecium Stamens five,
antepetalous. Filaments free, glabrous, free from petals or adnate
(epipetalous) at least near base. Anthers dorsifixed, non-versatile,
tetrasporangiate, latrorse, valvate (dehiscing by longitudinal recurved
valves). Tapetum? Staminodia five, antesepalous, very long (filaments much
longer than petals), densely pubescent with clavate hairs, apically with
aborted anther reduced to quadrilobate outgrowth.
Pollen grains
Microsporogenesis simultaneous? Pollen grains (2–)3-colpor(oid)ate, shed as
monads, ?-celled at dispersal. Exine semitectate, infratectum columellate,
reticulate.
Gynoecium Pistil composed of
usually three (sometimes four) connate carpels. Ovary superior, unilocular,
with central column free except at base and apex. Stylodia usually three
(sometimes four), separate. Stigma punctate, non-papillate, type? Pistillodium
absent.
Ovules Placentation free
central-apical. Ovules two per carpel, anatropous, pendulous, epitropous,
bitegmic?, crassinucellar? Micropyle ?-stomal. Outer integument ? cell layers
thick. Inner integument ? cell layers thick. Megagametophyte monosporous?,
Polygonum type? Endosperm development? Endosperm haustoria?
Embryogenesis?
Fruit A single-seeded leathery
capsule with persistent, accrescent and strongly recurved sepals.
Seeds Aril absent. Seed coat
testal. Testal cells thin-walled. Exotestal cells? Endotesta? Tegmen crushed?
Perisperm not developed. Endosperm copious, somewhat ruminate. Embryo minute,
straight, chlorophyll? Cotyledons two. Germination?
Cytology n = ?
DNA Insertion of a single base
(adenosine) in 18S rDNA (synapomorphy of Saxifragales).
Phytochemistry Unknown.
Aluminium accumulation?
Use Unknown.
Systematics
Medusandra (2; M. richardsiana, M. mpomiana;
Cameroon).
Medusandra is sister to Peridiscaceae and assigned to
these by Wurdack & Davis (2009). Medusandra and Peridiscaceae share
synapomorphies in both morphology and DNA.
Rafinesque, Anal. Nat.: 176. Apr-Jul 1815
[‘Peonidia’], nom. cons.
Paeoniineae Mart.,
Consp. Regn. Veg.: 40. Sep-Oct 1835 [‘Paeoniaceae’];
Paeoniales Heintze, Cormofyt. Fylog.: 12, 97. 1927;
Paeonianae Doweld, Tent. Syst. Plant. Vasc.: xxvii.
23 Dec 2001; Paeoniidae C. Y. Wu in Acta Phytotaxon.
Sin. 40: 302. 2002
Genera/species 1/33–35
Distribution South and
Southeast Europe, the Mediterranean, subtropical, temperate and subarctic parts
of Asia, western North America.
Fossils Uncertain.
Paeoniaecarpum hungaricum are fossil fruits from the Miocene of
Hungary, which have sometimes been assigned to Paeoniaceae.
Habit Bisexual, perennial
herbs, often with root or rhizome tubers, or suffrutices (rarely deciduous
shrubs).
Vegetative anatomy Phellogen
ab initio inner-cortical. Cortical vascular bundles present. Vessel elements
usually with scalariform (sometimes reticulate or simple) perforation plates;
lateral pits alternate or transitional (rarely scalariform). Imperforate
tracheary xylem elements tracheids with bordered pits, non-septate? Wood rays
uniseriate or multiseriate, homocellular or heterocellular (with lignified cell
walls). Axial parenchyma apotracheal (some cells among fibres) or paratracheal
scanty. Sieve tube plastids Ss type. Nodes 3:3, trilacunar with three leaf
traces, or 5:5, pentalacunar with five traces. Calciumoxalate crystals (druses
etc.) present.
Trichomes Hairs usually absent
(rarely unicellular).
Leaves Alternate (spiral),
pinnately compound (sometimes twice or several timespinnately compound) or
palmately compound to ternate or simple, lobed, with ? ptyxis. Stipules and
leaf sheath absent. Petiole vascular bundle transection annular. Venation
palmate. Stomata anomocytic. Cuticular wax crystalloids as transversely ridged
rodlets (Aristolochia type), chemically dominated by palmitone
(hentriacontan-16-one). Mesophyll with calciumoxalate druses. Leaf margin
serrate.
Inflorescence Flowers
terminal, usually solitary (sometimes few together in cymose inflorescences).
Extrafloral nectaries frequent on bracts enclosing floral buds.
Flowers Actinomorphic, large.
Hypogyny. Sepals (three to) five (to seven), with imbricate aestivation,
spiral, coriaceous, persistent, free, intergrading into petals. Petals five to
eight (to 13), with imbricate aestivation, spiral, caducous, free.
Nectariferous disc intrastaminal, annular or lobate, fleshy (developed from
receptacle or stamens).
Androecium Stamens c. 50 to
more than 150, spiral, initiated from five primordia, centrifugally developing
and continuing spiral corolla phyllotaxis. Filaments narrow, more or less
connivent in five fascicles (staminal vascular strands five, branched). Anthers
basifixed, non-versatile, tetrasporangiate, extrorse, longicidal (dehiscing by
longitudinal slits). Tapetum secretory. Staminodia modified into intrastaminal
glands or absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains tricolp(oroid)ate, shed as
monads, bicellular at dispersal. Exine tectate to semitectate, with columellate
infratectum, perforate reticulate, punctate, rugulate, foveolate, or
irregularly tuberculate-foveolate.
Gynoecium Carpels (two or)
three to eight (to 15), spiral (secondarily), free (secondarily), arcuately
diverging. Ovary superior, unilocular (apocarpy). Style very short or absent.
Stigma expanded, oblique, papillate, Wet type. Pistillodium absent.
Ovules Placentation marginal
(ovules biseriate, inserted on placental outgrowths, and richly vascularized).
Ovules approx. ten to more than 100 per carpel, anatropous, bitegmic,
crassinucellar (megasporangium degenerating after fertilization or prior to
anthesis). Micropyle exostomal to bistomal. Outer integument c. 10 to c. 20
cell layers thick, two-layered. Inner integument three or four cell layers
thick. Hypostase present. Parietal tissue approx. five cell layers thick.
Nucellar cap massive, approx. twelve cell layers thick. Megasporangium usually
absorbed prior to anthesis. Archespore often multicellular (often several
germinating). Megagametophyte monosporous, Polygonum type. Synergids
with a filiform apparatus. Endosperm development ab initio nuclear. Endosperm
haustorium chalazal. Embryogenesis unique among angiosperms, including
asymmetrical first division of zygote, degeneration of smaller daughter cell,
and free nuclear divisions in larger cell; this resulting in nuclear stage
(coenocytic proembryo), from which one or usually several embryos are formed
through budding; cell walls produced six or seven (sometimes eight) free
nuclear divisions later; only a single embryo maturing.
Fruit An assemblage of
sometimes fleshy follicles with persistent calyx.
Seeds Seed large, usually with
small fleshy funicular aril. Seed coat mesotestal. Testa a fleshy sarcotesta,
vascularized, ab initio red, later black, glossy; in some species developed
black fertilized seeds as well as undeveloped showy red unfertilized seeds
present. Exotestal cells palisade, thickened. Hypodermis palisade, lignified.
Mesotesta sometimes thickened. Endotesta crushed. Tegmen absent. Perisperm not
developed. Endosperm copious, oily, with amyloid (xyloglucans). Embryo very
small, well differentiated, without chlorophyll. Cotyledons two. Germination
phanerocotylar or cryptocotylar.
Cytology n = 5, 10. –
Chromosomes 10–15 µm long.
DNA Plastid gene infA
defunct (pseudogene present). Mitochondrial intron coxII.i3 lost.
Phytochemistry Flavonols
(kaempferol, quercetin etc.), flavones, Group I carbocyclic iridoids, oleanolic
acid derivatives, phenolic substances and glycosides (e.g. paeonol, paeonolide,
paeonoside, paeoniflorin, oxypaeoniflorin, benzoylpaeoniflorin, and
alliflorin), acetophenones, amids and ethereal oils present. Alkaloids? Ellagic
acid, proanthocyanidins, tannins, saponins, and cyanogenic compounds not
found.
Use Ornamental plants,
medicinal plants.
Britton, Man. Fl. N. States: 475. Oct 1901, nom.
cons.
Genera/species 1/2
Distribution Northeastern
Asia, China, the Korean Peninsula, Japan, northern Vietnam, eastern United
States.
Fossils Unknown.
Habit Bisexual, perennial
herbs. Helophytes. Roots fibrous.
Vegetative anatomy Phellogen
(in root) ab inition superficial. Cortex consisting of outer exodermis and
inner aerenchyma. Endodermis? Pericyclic fibres absent. Pseudosiphonostele
present in young stems. Secondary lateral growth normal or absent. Vessel
elements with scalariform perforation plates; lateral pits?, bordered pits.
Imperforate tracheary xylem elements fibre tracheids and tracheids with
bordered pits, non-septate (also vasicentric tracheids). Wood rays usually
uniseriate or biseriate, homocellular. Axial parenchyma absent. Sieve tube
plastids Ss type, with starch, but without protein inclusions. Nodes 1:1,
unilacunar with one leaf trace. Crystals?
Trichomes Hairs (in
Penthorum sedoides) multicellular, triseriate or quadriseriate,
glandular, or absent (in P. chinense).
Leaves Alternate (spiral),
simple, entire, with supervolute ptyxis. Stipules and leaf sheath absent.
Colleters present. Petiole vascular bundle transection arcuate. Venation
pinnate, brochidodromous, with distinct mid-vein. Stomata anomocytic. Cuticular
wax crystalloids? Leaf margin glandular serrate; teeth often with apical
hydathode.
Inflorescence Terminal or
axillary, usually scorpioid monochasium. Bracts lateral, perpendicular to
pedicel.
Flowers Actinomorphic. Ab
initio epigyny (half epigyny with ovary somewhat sunken into receptacle), later
hypogyny. Hypanthium present. Sepals five (to eight), with valvate aestivation,
unequal, persistent, connate at base. Petals usually absent (sometimes one to
eight, small, free, usually shorter than sepals). Nectary absent. Disc
absent.
Androecium Stamens 5+5 (to
8+8), twice as many as sepals. Filaments filiform, free from each other and
from tepals. Anthers basifixed, non-versatile, disporangiate, latrorse,
longicidal (dehiscing by longitudinal slits). Tapetum secretory. Staminodia
five (to eight).
Pollen grains
Microsporogenesis simultaneous. Pollen grains tricolporate(or tricolporoidate),
shed as monads, ?-cellular at dispersal. Exine tectate, with columellate?
infratectum, perforate or microreticulate.
Gynoecium Pistil composed of
five (to eight) antesepalous carpels, free or connate at base. Ovary ab initio
inferior (semi-inferior) and later superior, unilocular (apocarpy) or
multilocular in proximal part. Stylodia five (to eight), short, submarginal.
Stigmas small, capitate, type? Pistillodium absent.
Ovules Placentation
apical-axile, later intrusive. Ovules numerous per carpel, anatropous,
pendulous, bitegmic, crassinucellar. Funicle long. Micropyle ?-stomal. Outer
integument ? cell layers thick. Inner integument ? cell layers thick. Parietal
tissue? Megasporangium multicellular in chalazal part only. Megagametophyte
monosporous, Polygonum-type. Endosperm development cellular. Basal
suspensor cell giving way to micropylar endosperm haustorium. Embryogenesis?
Fruit An assemblage of
follicles (with persistent and recurved sepals); free part of carpel
circumscissilely dehiscing at base and falling off like a lid.
Seeds Aril absent. Seed coat
exotestal. Exotestal cells prominent, papillate, tanniniferous, with thickened
outer walls. Other testal cell layers absent. Tegmen crushed, consisting of
endostomal micropylar operculum (formed from inner integument). Perisperm not
developed. Endosperm sparse. Embryo large, straight; suspensor with strongly
enlarged not dividing micropylar cell, chlorophyll? Cotyledons two.
Germination? First two pairs of seedling leaves opposite.
Cytology n = 8, 9
DNA
Phytochemistry Mono- and
diglycosides of flavonols (kaempferol, quercetin) present. Myricetin, flavones,
non-hydrolyzable tannins, and cyanogenic compounds not found.
Use Medicinal plants.
Systematics Penthorum
(2; P. chinense: the Russian Far East, China, the Korean Peninsula,
Japan, northern Vietnam; P. sedoides: eastern United States from Maine
to Ontario and Minnesota, and south to Florida and Texas).
Penthorum is sister to Haloragaceae.
Kuhlmann in Arq. Serv. Florest. 3: 4. 1950, nom.
cons.
Peridiscales Doweld,
New Syllabus Pl. Fam.: 574. Apr 2007
Genera/species 3/9–11
Distribution Tropical West
Africa, tropical South America.
Fossils Unknown.
Habit Bisexual, evergreen
trees or shrubs.
Vegetative anatomy Phellogen?
Stem cortex with secretory cells and few fibres. Medullary bundles usually
absent (present in Peridiscus). Wide primary medullary strands
alternating with narrow ones. Vessel elements with scalariform perforation
plates; lateral pits scalariform to opposite, bordered pits. Imperforate
tracheary xylem elements fibre tracheids and long libriform fibres with
bordered pits, usually non-septate. Wood rays uniseriate and homocellular or
heterocellular (Soyauxia), or multiseriate heterocellular
(Peridiscus, Whittonia). Axial parenchyma apotracheal
diffuse. Sieve tube plastids S (Ss?) type. Nodes? Acicular and elongate
crystals, crystal sand, styloids, etc. present in parenchyma cells in
Soyauxia.
Trichomes Hairs unicellular or
absent.
Leaves Alternate (distichous),
simple, entire, coriaceous, with ? ptyxis. Stipules single, adaxial,
intrapetiolar or pairwise lateral, almost perfoliate, early caducous; leaf
sheath absent. Petiole in Peridiscus with pulvinus. Petiole vascular
bundle transection annular; petiole with wing bundles (Soyauxia), an
adaxial plate (Peridiscus) or adaxial annular bundle
(Whittonia). Venation usually pinnate (rarely palmate). Stomata
anomocytic or paracytic. Cuticular wax crystalloids as platelets in rosettes.
Domatia in vein axils on abaxial side of lamina (Soyauxia?).
Idioblasts with crystals. Leaf margin entire.
Inflorescence Axillary,
fasciculate, or raceme or spike. Floral prophylls (bracteoles) large,
persistent.
Flowers Actinomorphic.
Hypogyny to half epigyny. Sepals four to seven, with imbricate? aestivation,
caducous, free. Petals five with tubular corona (Soyauxia), free, or
absent. Nectariferous disc intrastaminal, cupular or annular, sometimes
hairy.
Androecium Stamens c. 30 to
more than 100; outer much longer than inner. Filaments free or somewhat connate
at base into fascicles (Whittonia), free from tepals. Anthers
basifixed or subdorsifixed, non-versatile, disporangiate (Peridiscus,
Whittonia) or tetrasporangiate (Soyauxia), introrse,
longicidal (dehiscing by longitudinal slits, Peridiscus,
Whittonia) or by lateral flaps (Soyauxia). Tapetum secretory.
Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains tricolpor(oid)ate, shed as
monads, ?-cellular at dispersal. Exine semitectate (Soyauxia), with
columellate infratectum, reticulate (Soyauxia).
Gynoecium Pistil composed of
usually three or four (rarely five) carpels, connate in lower parts. Ovary
superior to semi-inferior, unilocular (in Soyauxia with central
columella). Stylodia three or four, separate, short. Stigmas punctate,
non-papillate, type? Pistillodium absent.
Ovules Placentation usually
apical (sometimes free central). Ovules usually six to eight per ovary,
orthotropous?, pendulous, bitegmic?, crassinucellar? Micropyle ?-stomal. Outer
integument ? cell layers thick. Inner integument ? cell layers thick.
Megagametophyte monosporous, Polygonum type? Endosperm development
nuclear? Endosperm haustoria? Embryogenesis?
Fruit A single-seeded
loculicidal capsule (Soyauxia) or a one-seeded drupe
(Peridiscus, Whittonia).
Seeds: Aril absent. Seed coat
testal. Testal cells tanniniferous (sometimes dark-staining), thin-walled, more
or less collapsed. Tegmen? Perisperm not developed. Endosperm copious,
cartilagineous, with thick cell walls (or absent?), probably with
hemicellulose. Embryo minute, straight? (curved?), well differentiated,
chlorophyll? Cotyledons two, foliaceous. Germination?
Cytology n = ?
DNA
Phytochemistry Virtually
unknown. Aluminium accumulated.
Use Unknown.
Systematics Soyauxia
(7–9; tropical West and Central Africa), Peridiscus (1; P.
lucidus; Venezuela, Amazonian Brazil), Whittonia (1; W.
guianensis; known only from the type material from below Kaieteur Falls in
Guyana and possibly extinct).
The clade [Peridiscaceae+Medusandra]
is sister-group to all other Saxifragales.
Soyauxia is probably sister to
[Peridiscus+Whittonia]. The position of Whittonia
within Peridiscaceae
is uncertain due to lack of material, but it is most probably sister to
Peridiscus since they share several potential morphological
synapomorphies.
Small in Britton, N. Amer. Fl. 22(1): 2. 22 Mai
1905, nom. cons.
Genera/species 1/3
Distribution Mexico.
Fossils Unknown.
Habit Bisexual, evergreen
shrubs.
Vegetative anatomy Phellogen
ab initio superficial. Pericyclic fibres weakly developed. Vessel elements
(some of them fibre-like) with scalariform or simple perforation plates;
lateral pits? Imperforate tracheary xylem elements tracheids (rare), grading
into fibre tracheids and libriform fibres. Wood rays uniseriate or
multiseriate, heterocellular? Axial parenchyma apotracheal diffuse, or
paratracheal scanty. Xylem diffusely porose, non-storied. Sieve tube plastids
Ss type. Nodes 3:3, trilacunar with three leaf traces. Secretory cavities
absent. Crystals?
Trichomes Hairs unicellular or
multicellular, uniseriate, peltate or clavate; glandular hairs cone-shaped to
peltate or spherical, multicellular, resin-secreting.
Leaves Alternate (spiral),
simple, entire, membranaceous to coriaceous, sticky and resinous on adaxial
side, with ? ptyxis. Stipules cauline, very small, early caducous; leaf sheath
absent. Petiole vascular bundle transection arcuate? Venation pinnate. Stomata
anomocytic. Cuticular waxes? Domatia as pockets in axils of lateral veins on
abaxial side of lamina. Secretory cavities absent. Mesophyll with
calciumoxalate, usually as druses (usually in association with vascular
strands). Leaf margin serrate; leaf teeth with hydathodes.
Inflorescence Terminal or
subterminal, few-flowered corymbose cyme.
Flowers Actinomorphic.
Hypanthium present. Epigyny. Sepals five, with valvate aestivation, persistent,
free. Petals five, with imbricate aestivation, persistent, free. Nectary
absent. Disc absent.
Androecium Stamens five,
antesepalous, alternipetalous. Filaments flattened, dentate at apex, winged,
free from each other and from tepals. Anthers basifixed to dorsifixed,
versatile, tetrasporangiate, introrse, poricidal (dehiscing by basal pores).
Tapetum secretory. Staminodia five, intrastaminal, antepetalous, as thin
filaments without anthers; each staminodium with one lateral tooth.
Pollen grains
Microsporogenesis simultaneous. Pollen grains tricolporoidate or tricolporate,
shed as monads, bicellular or tricellular at dispersal. Exine tectate, with
columellate? infratectum, perforate or suprarugulate.
Gynoecium Pistil composed of
five connate carpels. Ovary inferior, usually quinquelocular (sometimes
sexalocular). Styles five, connate in lower part, short. Stigmas five,
quinquelobate, arising more or less radially, type? Pistillodium absent.
Ovules Placentation axile.
Ovules four to eight per carpel, anatropous, ascending, apotropous, bitegmic,
crassinucellar. Micropyle ?-stomal. Outer integument ? cell layers thick. Inner
integument ? cell layers thick. Parietal tissue approx. seven cell layers
thick. Megagametophyte monosporous, Polygonum type? Endosperm
development? Endosperm haustoria? Embryogenesis?
Fruit A lignified septicidal
usually single-seeded capsule, with persistent upright sepals and recurved
petals.
Seeds: Aril absent? Seed coat
testal. Testa more or less cartilaginous. Tegmen? Perisperm not developed.
Endosperm sparse or absent? Embryo large, well differentiated, chlorophyll?
Cotyledons two. Germination?
Cytology n = ?
DNA
Phytochemistry Very
insufficiently known. Quercetin-3-O-glycosides and
flavone-C-glycosides present. Cyanogenic compounds? Ellagic acid?
Use Unknown.
Systematics
Pterostemon (3; P. bravoanus, P. mexicanus, P.
rotundifolius; high mountains in arid and semiarid regions of central and
southern Mexico).
Pterostemon is sister to Itea (Iteaceae).
Pterostemon and Ribes (Grossulariaceae) have
similar peltate glandular hairs.
de Jussieu, Gen. Plant.: 308. 4 Aug 1789
[’Saxifragae’], nom. cons.
Chrysospleniaceae
Bercht. et J. Presl, Přir. Rostlin: 259. Jan-Apr 1820
[’Chrysospleniae’]; Pectiantiaceae Raf.,
Fl. Tellur. 2: 73. Jan-Mar 1837 [’Pectantidia’];
Saxifragopsida Brongn., Enum. Plant. Mus. Paris:
xxviii, 107. 12 Aug 1843 [’Saxifragineae’];
Brachycaulaceae Panigrahi et Dikshit in Panigrahi,
Proc. 90th Indian Sci. Congr. Part III (Advance Abstr.): 54. Jan
2003
Genera/species c
35/720–735
Distribution Mainly in
northern temperate and polar areas; a few species in southern temperate
regions, and on tropical mountains (in the Andes).
Fossils Uncertain.
Habit Usually bisexual (rarely
androdioecious), usually perennial herbs (sometimes annual or biennial, rarely
somewhat lignified). Some species are succulent and/or xerophytic.
Vegetative anatomy Phellogen
ab initio subepidermal or pericyclic. Cortical and medullary vascular bundles
present or absent. Young stem with vascular tissue in separate bundles
(pseudosiphonostele). Secondary lateral growth usually absent (sometimes
present, normal?). Vessel elements usually with simple (rarely scalariform)
perforation plates; lateral pits? Imperforate tracheary elements ? with
bordered pits, non-septate?, or absent. Wood rays indistinct. Axial parenchyma?
Sieve tube plastids Ss type. Nodes usually 3:3, trilacunar with three leaf
traces (sometimes 1:1 [Chrysosplenium], 1:2 [Micranthes],
unilacunar with one or two traces, or >3:>3 [Astilbe, etc.],
multilacunar with several traces; in some species more than 50 vascular bundles
proceeding into petiolar base). Secretory cells with proanthocyanidins and/or
ellagitannins. Idioblasts with cyanogenic compounds or druses unusual.
Calciumoxalate as druses or acicular crystals present in several genera.
Trichomes Hairs unicellular or
multicellular, uniseriate or multiseriate; apex often with multicellular gland;
chalk glands present in some species?
Leaves Usually alternate
(spiral; usually in basal rosette; rarely opposite in inflorescences), simple
or pinnately or palmately compound, entire or lobate, with various types of
ptyxis. Often with stipules? in inflorescences; leaves sometimes with
persistent stipule-like outgrowths (stipules?) at or near base or on petiole
(in Astilbe cauline); leaf base often sheathing. Colleters often
present. Petiole vascular bundle transection arcuate or annular; petiole
sometimes with medullary or adaxial bundles. Venation palmate or pinnate.
Stomata usually anomocytic (sometimes anisocytic or diacytic), often present
only on adaxial side of lamina. Cuticular wax crystalloids? Hydathodes
frequent. Leaf margin usually serrate, often glandular-serrate (rarely
entire).
Inflorescence Terminal raceme,
spike or head, or raceme-, spike- or head-like, or fasciculate or panicle
(flowers rarely solitary).
Flowers Usually actinomorphic
(rarely obliquely zygomorphic). Hypanthium usually present. Epigyny or half
epigyny (sometimes pseudohypogyny; variation also between different morphs of
heterostylous species). Sepals (three to) five (to ten), with imbricate or
valvate aestivation, median sepal adaxial, free. Petals (four or) five (to
ten), with imbricate or valvate aestivation, free (sometimes absent).
Nectariferous disc intrastaminal (sometimes absent).
Androecium Stamens (three to)
five, alternisepalous (sometimes obhaplostemonous), or (4–)5+(4–)5,
obdiplostemonous (rarely up to 15). Filaments free from each other and from
tepals. Anthers usually basifixed (rarely somewhat dorsifixed), usually
non-versatile, usually tetrasporangiate (in Leptarrhena and
Tanakaea disporangiate), introrse or latrorse (in Tolmiea
median anthers extrorse and lateral anthers introrse), usually longicidal
(dehiscing by longitudinal slits; in Leptarrhena and Tanakaea
apically). Tapetum secretory. Staminodia absent?
Pollen grains
Microsporogenesis simultaneous. Pollen grains 2–3-colporoidate,
2–3-colpate, 2–3-colporate or 6–9-porate, shed as monads, bicellular at
dispersal. Exine tectate or semitectate, with columellate infratectum,
perforate, reticulate, or striate, echinulate or spinulate.
Gynoecium Pistil composed of
two (to five) usually connate carpels (connate at least at base; in
Darmera and certain species of Astilbe free) with various
orientation, numerous species with median carpels (some species with oblique
carpels), sometimes more or less open ventrally in free distal part. Ovary
inferior or semi-inferior, unilocular (apocarpy) or bilocular (rarely
trilocular). Stylodia usually two (rarely three), free. Stigmas usually
capitate (rarely decurrent), papillate, Dry or Wet type. Pistillodium
absent.
Ovules Placentation axile to
parietal. Ovules nine to more than 30 per carpel, anatropous, pendulous or
ascending, usually bitegmic (in Darmera and Micranthes
unitegmic), crassinucellar. Micropyle bistomal, often Z-shaped (zig-zag). Outer
integument two cell layers thick. Inner integument two cell layers thick
(integument in Darmera four to six cell layers thick). Parietal tissue
three to five cell layers thick. Nucellar cap present. Megagametophyte usually
monosporous, Polygonum type (rarely disporous, Allium type).
Synergids usually with a filiform apparatus. Endosperm development ab initio
cellular, helobial or nuclear. Endosperm haustoria of various types present in
some species. Embryogenesis solanad.
Fruit Usually a septicidal
capsule (sometimes an assemblage of follicles).
Seeds Aril absent? Seed coat
exotestal (and sometimes endotegmic), usually with various sculpturing (in
Leptarrhena and Sullivantia with wings). Exotestal cells
often with outer wall (sometimes radial walls) thickened. Inner pigmented layer
often present. Tegmen usually crushed. Endotegmen in Heuchera and
Tolmiea thick-walled, crystalliferous. Perisperm not developed.
Endosperm usually copious, oily. Embryo usually small (sometimes large),
straight, without chlorophyll. Cotyledons two. Germination phanerocotylar.
Cytology x = 7 (most Saxifragaceae), x = 9–14
(many species of Saxifraga), x = 11 (Chrysosplenium,
Peltoboykinia), x = 12 (Chrysosplenium), x = 15
(Rodgersia), x = 17 (Astilboides, Bergenia,
Darmera, Mukdenia), x = 18 (Rodgersia) –
Polyploidy (also autopolyploidy) and aneuploidy often occurring; in
Saxifraga the diploid chromosome number varies widely (2n = 12 to c.
220).
DNA Intron absent from plastid
gene rpl2. Different combinations of plastid and nuclear genomes
present (plastid genome in Tellima also present in
Mitella).
Phytochemistry Flavonols
(kaempferol, quercetin, myricetin), afzelechin (a flavan-3-ol), cyanidin,
oleanolic acid derivatives, bergeniin (C-glycoside of gallic acid),
proanthocyanidins (prodelphinidins), hydrolyzable ellagic acid or gallic acid
based tannins (tellimagrandin I, tellimagrandin II), condensed tannins (from
procyanidin or prodelphinidin), cyanogenic compounds, germacrane-like
compounds, and arbutin present. Cyanogenesis via isoleucine or valine. Iridoids
not found. Aluminium accumulated in some species.
Use Ornamental plants,
medicinal plants.
Systematics Saxifraga (c
390; Europe, arctic and temperate Asia, North Africa, temperate and alpine
North America, temperate and alpine South America); Astilbe
(18–23; the Himalayas, China, Japan, Southeast Asia, Malesia to New Guinea,
southeastern Canada, eastern United States), Leptarrhena (1; L.
pyrolifolia; western Canada, northwestern United States incl. Alaska),
Tanakaea (1; T. radicans; China, Japan),
Saniculiphyllum (1; S. guangxiense; northwestern Guangxi,
southeastern Yunnan), Jepsonia (3; J. heterandra, J.
malvifolia, J. parryi; southern California, northwestern Mexico),
Telesonix (2; T. heucheriformis, T. jamesii; Rocky
Mountains in the United States), Sullivantia (3; S. oregana,
S. renifolia, S. sullivantii; the United States),
Bolandra (2; B. californica, B. oregana; western
United States), Suksdorfia (1; S. violacea; southwestern
Canada, western United States), Boykinia (8
incl. ‘Suksdorfia’ ranunculifolia; Japan, western Canada,
western United States incl. Alaska, northwestern Mexico), Hieronymusia
(1; H. alchemilloides; Bolivia, Argentina)?;
Mitella (2; M. diphylla, M. nuda; southern
Canada, United States), Bensoniella (1; B. oregona; western
United States), Tolmiea (2; T. diplomenziesii, T.
menziesii; western Canada, western United States incl. Alaska),
Lithophragma (9; western United States),
‘Heuchera’ (55–60; Canada, United States incl. southern Alaska,
Mexico; polyphyletic), Pectiantia (c 13; P. breweri, P.
ovalis, ‘Mitella’ furusei, P. pentandra;
‘Mitella’ kiusiana, M. yoshinagae; M.
acerina, M. amamiana, M. doiana, M. formosana,
M. japonica, M. koshiensis, M. pauciflora, M.
stylosa; Japan, the Ryukyu Islands, Taiwan, western Canada, western United
States incl. Alaska), Tellima (10; western Canada, western United
States incl. southern Alaska), Tiarella (3; T. cordifolia,
T. polyphylla, T. trifoliata; eastern Himalayas, China,
Japan, western and southeastern Canada, western and eastern United States),
‘Mitella’ integripetala (Japan), Elmera (1;
E. racemosa; southwestern British Columbia, Washington, Oregon),
Mitellastra (1; M. caulescens; southwestern Canada, western
United States), Ozomelis (4; O. diversifolia, O.
stauropetala, O. trifida, Conimitella williamsii;
southwestern Canada, western United States incl. southern Alaska),
Cascadia (1; C. nuttallii; Oregon, Washington),
Saxifragodes (1; S. albowiana; 51°S in Chile to Tierra del
Fuego); Darmera (1;
D. peltata; southwestern Oregon, northern California),
Astilboides (1; A. tabularis; northern China), Rodgersia
(5; R. aesculifolia, R. henrici, R. pinnata, R.
podophylla, R. sambucifolia; Nepal, the Himalayas, China, the
Korean Peninsula, Japan), Bergenia (10;
temperate and subtropical regions of western China to Japan), Mukdenia (2;
M. acanthifolia, M. rossii; northern China, Manchuria, the
Korean Peninsula), Oresitrophe (1; O. rupifraga; northeastern
China); Peltoboykinia (1; P. tellimoides; Japan),
Chrysosplenium (70–75; Europe, temperate and arctic Asia, North
America, southern South America); Micranthes
(c 45; Europe, arctic and temperate Asia, arctic and mountain regions in North
America, alpine and mountain regions in Mexico and South America).
Saxifragaceae are
sister-group to Ribes (Grossulariaceae).
According to the study by Soltis, Morgan,
Grable & al. (1993), Astilbe
(Astilbeae Small, Man. S.E. Fl.: 590. 30 Nov 1933), Chrysosplenium
(Chrysosplenieae Dumort., Fl. Belg.: 84. 1827) and
Peltoboykinia form three basal clades, Astilbe being
sister to Saxifragoideae Beilschm. in Flora 16(Beibl. 7): 94. 14 Jun
1833 [‘Saxifrageae’] which comprise all other Saxifragaceae. On the other
hand, Deng & al. (2015) reveals Saxifraga as
sister to the remaining Saxifragaceae. Two main
clades may be identified: the saxifragoids (Saxifrageae Dumort., Fl.
Belg.: 84. 1827), the astilboids and the heucheroids (Heuchereae
Bartl., Ord. Nat. Plant.: 312. Sep 1830 [‘Heucherea’]).
 |
Cladogram of Saxifragaceae based
on DNA restriction site analysis data (Soltis, Morgan, Grable & al.
1993).
|
Nakai, Chosakuronbun Mokuroku [Ord. Fam. Trib.
Nov.]: 244. 20 Jul 1943
Genera/species 1/1
Distribution Tasmania.
Fossils Unknown.
Habit Bisexual, evergreen
shrub.
Vegetative anatomy Phellogen?
Stem without endodermis. Vessel elements with scalariform perforation plates;
lateral pits scalariform to opposite. Imperforate tracheary xylem elements very
short thick-walled tracheids. Wood rays uniseriate and homocellular, or
uniseriate or biseriate and heterocellular. Axial parenchyma apotracheal
diffuse or diffuse-in-aggregates, or paratracheal scanty. Sieve tube plastids
Ss type. Nodes 1:1, unilacunar with one leaf trace. Crystals?
Trichomes Hairs absent.
Leaves Alternate (spiral),
simple, entire, coriaceous, with ? ptyxis. Stipules and leaf sheath absent.
Petiole vascular bundle transection arcuate? Venation pinnate. Stomata
anomocytic. Cuticular wax crystalloids? Cuticle thick. Leaf margin biserrate;
leaf teeth eglandular, possibly with hydathodes (water pores absent).
Inflorescence Terminal,
raceme.
Flowers Actinomorphic, small.
Hypanthium present? Hypogyny. Sepals four (or five), with imbricate
aestivation, persistent, connate at base. Petals four (or five), with imbricate
aestivation, stipitate, caducous, free. Nectary absent. Disc absent.
Androecium Stamens four or
eight, in one whorl (or two whorls?), antepetalous and alternipetalous.
Filaments filiform, connate at base, free from tepals. Anthers basifixed,
non-versatile, tetrasporangiate, latrorse, longicidal (dehiscing by
longitudinal slits). Tapetum secretory. Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains tricolporate, shed as monads,
?-cellular at dispersal. Exine semitectate,with columellate? infratectum,
reticulate to rugulate.
Gynoecium Pistil composed of
four (or five), stipitate, antepetalous carpels, connate only at base. Ovary
superior, unilocular (apocarpy) or usually quadrilocular (sometimes
quinquelocular in proximal part?). Stylodia very short. Stigmas small, type?
Pistillodium absent.
Ovules Placentation
(sub)marginal (ventral, with branched placentae). Ovules c. 15 to more than 100
per carpel, anatropous, bitegmic, crassinucellar. Micropyle exostomal. Outer
integument ? cell layers thick. Inner integument ? cell layers thick. Parietal
tissue approx. four cell layers thick. Megagametophyte monosporous,
Polygonum type. Endosperm development ab initio cellular. Endosperm
haustoria? Embryogenesis?
Fruit An assemblage of stalked
follicles.
Seeds Aril absent. Seed very
small. Seed coat exotestal. Testa thin, with ridges and one or two narrow
longitudinal wings, extended at both ends. Exotestal cells longitudinally
elongate, with thickened outer walls, without mechanical layers. Inner testal
cell layers often crushed. Tegmen? Perisperm not developed. Endosperm copious,
fleshy. Embryo very small, chlorophyll? Cotyledons two? Germination?
Cytology n = ?
DNA
Phytochemistry Unknown.
Use Unknown.
Systematics Tetracarpaea (1;
T. tasmannica; mountains of Tasmania).
Tetracarpaea is sister to [Haloragaceae+Penthoraceae].
Literature
Abe K. 1982. Embryological studies in the
family Saxifragaceae (s.l.) I.
Development of the ovule and embryo sac in Saxifraga fortunei var.
partita (Makino) Nakai. – Amer. J. Bot. 69: 416-420.
Acevedo-Rosas R, Cameron K, Sosa S, Pell S.
2004. A molecular phylogenetic study of Graptopetalum (Crassulaceae) based on ETS, ITS,
rpl16 and trnL-F nucleotide sequences. – Amer. J. Bot. 91:
1099-1104.
Acevedo-Rosas R, Sosa V, Lorea FG. 2004.
Phylogenetic relationships and morphological patterns in Graptopetalum
(Crassulaceae). – Brittonia
56: 185-194.
Agababian VS. 1960. On the palynosystematics
of the family Iteaceae. – Izvest.
Akad. Nauk Armajanskoj S.S.R. (Bull. Armenian Acad. Sci., Biol.) 13: 99-102.
[In Russian.]
Agababian VS. 1961. Materials toward the
palynosystematic study of the family Saxifragaceae s.l. – Izvest.
Biol. Nauki 14: 45-61. [In Russian]
Agababian VS. 1963. Pollen morphology of the
genus Ribes. – Izvest. Akad. Nauk Armajanskoj S.S.R. 16: 93-98.
Agababian VS. 1964. Evolution of pollen in
the order Cunoniales and Saxifragales in relation to some
questions of their systematics and phylogeny. – Izvest. Akad. Nauk
Armajanskoj S.S.R. 17: 59-72.
Aiken SG. 1979. North American species of
Myriophyllum (Haloragaceae). – Ph.D. diss.,
University of Minnesota, Minneapolis, Minnesota.
Akiyama S, Ohba H, Wu S-K. 2001. A new
variety of Sinocrassula paoshingensis (S. H. Fu) H. Ohba et al. (Crassulaceae). – J. Jap. Bot. 76:
222-226.
Alexander EJ. 1942. A new Mexican
Sedum. – Cact. Succ. J. 14: 76-78.
Alm T. 2004. Ethnobotany of Rhodiola
rosea (Crassulaceae) in
Norway. – Sida 21: 321-344.
Al-Shammary KI. 1991. Systematic studies of
the Saxifragaceae, chiefly from
the Southern Hemisphere. – Ph.D. diss., University of Leicester, England.
Al-Shammary KI, Gornall RJ. 1994. Trichome
anatomy of the Saxifragaceae
s.l. from the Southern Hemisphere. – Bot. J. Linn. Soc. 114: 99-131.
Babalola KA, Victoria AA. 2009. Foliar
epidermal morphology of two West African genera of Haloragaceae R. Br. (Saxifragales). – J. Sci. Res.
Dev. 11: 84-91.
Baehni C. 1937. Villadia et
Altamiranoa. Étude sur la fusion de deux genres de Crassulacées. –
Candollea 7: 283-286.
Bahadur B, Ramaswamy N, Srikanth R. 1986.
Studies on the floral biology and nectar secretion in some Kalanchoe
species (Crassulaceae). – In:
Kapil RP (ed), Pollination biology – an analysis, Inter-India Publ., New
Delhi, pp. 251-259.
Baldwin JT Jr. 1935. Somatic chromosome
numbers in the genus Sedum. – Bot. Gaz. 96: 558-564.
Baldwin JT Jr. 1937. The cyto-taxonomy of the
Telephium sections of Sedum. – Amer. J. Bot. 24:
126-132.
Baldwin JT Jr. 1938. Kalanchoe: the
genus and its chromosomes. – Amer. J. Bot. 25: 572-579.
Baldwin JT Jr. 1939. Certain cytophyletic
relations in the Crassulaceae.
– Chronica Bot. 5: 415-417.
Baldwin JT Jr. 1940. Cytophyletic analysis of
certain annual and biennial Crassulaceae. – Madroño 5:
184-192.
Baldwin JT Jr, Speese BM. 1951.
Penthorum: its chromosomes. – Rhodora 53: 89-91.
Bañares Baudet Á. 1990. Híbridos de la
familia Crassulaceae en las
Islas Canarias. Novedades y datos corológicos II. – Vieraea 18: 65-85.
Bañares Baudet Á. 1992. Aeonium
pseudourbicum sp. nov. (Crassulaceae), nuevo endemismo de
Tenerife (Islas Canarias). – An. Jard. Bot. Madrid 50: 175-182.
Bañares Baudet Á. 1999. Notes on the
taxonomy of Aeonium urbicum and A. appendiculatum sp. nov.
(Crassulaceae). – Willdenowia
29: 95-103.
Bañares Baudet Á. 2002. On some poorly
known taxa of Aichryson sect. Aichryson and A.
bituminosum sp. nov. (Crassulaceae). – Willdenowia 32:
221-230.
Bañares Baudet Á, Scholz S. 1990.
Monanthes wildpretii sp. nov. (Crassulaceae), nuevo endemismo de
Tenerife (Islas Canarias). – Stud. Bot. 9: 129-138.
Bañares Baudet Á, Gómez MVM, Scholz S.
2008. Taxonomic and nomenclatural notes on Crassulaceae of the Canary Islands,
Spain. – Willdenowia 38: 475-489.
Barabé D. 1984. Application du cladisme à
la systématique des angiosperms: cas des Hamamélidales. – Candollea 39:
51-70.
Barber HN. 1941. Evoluton in genus
Paeonia. – Nature 148(3747): 227-228.
Barkman TJ, McNeal JR, Lim S-H, Coat G, Croom
HB, Young ND, dePamphilis CW. 2007. Mitochondrial DNA suggests at least 11
origins of parasitism in angiosperms and reveals genomic chimerism in parasitic
plants. – BMC Evol. Biol. 7: 248.
http://dx.doi.org/10.1186/1471-2148-4-248
Baskin JM, Baskin CC. 1972. Germination
characteristics of Diamorpha cymosa seeds and an ecological
interpretation. – Oecologia 10: 17-28.
Baskin JM, Baskin CC. 1977. Germination
ecology of Sedum pulchellum Michx. (Crassulaceae). – Amer. J. Bot.
64: 1242-1247.
Bates JC. 1933. Comparative anatomical
research within the genus Ribes. – Univ. Kansas Sci. Bull. 21:
369-398.
Bate-Smith EC. 1976. Chemistry and taxonomy
of Ribes. – Biochem. Syst. Ecol. 4: 13-23.
Bawa SB. 1969a. Embryological studies on the
Haloragidaceae II. Laurembergia brevipes Schindl. and a discussion of
systematic considerations. – Proc. Natl. Inst. Sci. India, Sect. B, 35:
273-290.
Bawa SB. 1969b. Embryological studies on the
Haloragidaceae III. Myriophyllum intermedium DC. – Beitr. Biol.
Pflanzen 45: 447-464.
Bawa SB. 1970. Haloragaceae. – Bull. Natl. Sci.
Acad. India 41: 226-229.
Bayer C. 2006. Peridiscaceae. – In: Kubitzki K
(ed), The families and genera of vascular plants IX. Flowering plants.
Eudicots. Berberidopsidales,
Buxales, Crossosomatales, Fabales p. p., Geraniales, Gunnerales, Myrtales p. p., Proteales, Saxifragales, Vitales, Zygophyllales,
Clusiaceae
Alliance, Passifloraceae
Alliance, Dilleniaceae, Huaceae, Picramniaceae,
Sabiaceae, Springer, Berlin,
Heidelberg, New York, pp. 297-300.
Beamish KI, Lin SC. 1965. Fertilization and
seed development in Saxifraga integrifolia Hook. – Can. J. Bot. 43:
861-865.
Behnke H-D. 1986. Contributions to the
knowledge of sieve-element plastids in Gunneraceae and allied
families. – Plant Syst. Evol. 151: 215-222.
Benedict JC, Pigg KB, DeVore ML. 2008.
Hamawilsonia boglei gen. et sp. nov. (Hamamelidaceae) from the Late
Paleocene Almont flora of central North Dakota. – Intern. J. Plant Sci. 169:
687-700.
Bensel CR, Palser BF. 1975. Floral anatomy in
the Saxifragaceae sensu lato
II. Saxifragoideae and Iteoideae. – Amer. J. Bot. 62: 661-675.
Berbeek-Reuvers AAML. 1980. Grossulariaceae. – In: Punt W,
Clarke GCS (eds), The Northwest European pollen flora, Elsevier, New York, pp.
107-116.
Berger A. 1924. A taxonomic review of
currants and gooseberries. – New York Agric. Exp. Sta. Techn. Bull. 109:
3-118.
Berger A. 1930. Crassulaceae. – In: Engler A,
Harms H (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 18a, W.
Engelmann, Leipzig, pp. 352-483.
Bhatnagar AK, Garg M. 1977. Affinities of
Daphniphyllum – palynological approach. – Phytomorphology 27:
92-97.
Bhatnagar AK, Kapil RN. 1982. Seed
development in Daphniphyllum himalayense with a discussion on
taxonomic position of Daphniphyllaceae. –
Phytomorphology 32: 66-81.
Bhatnagar AK, Garg M. 1977. Affinities of
Daphniphyllum – palynological approach. – Phytomorphology 27:
92-97.
Bogle AL. 1968. Floral vascular anatomy and
the nature of the hamamelidaceous flowers. – Ph.D. diss., University of
Minnesota, Minneapolis, Minnesota.
Bogle AL. 1970. Floral morphology and
vascular anatomy of the Hamamelidaceae: the apetalous
genera of Hamamelidoideae. – J. Arnold Arbor. 51: 310-366.
Bogle AL. 1984. Floral morphology and
vascular anatomy of Maingaya Oliv. (Hamamelidaceae, Hamamelidoideae,
Hamamelideae). – Amer. J. Bot. 71: 19.
Bogle AL. 1986. The floral morphology and
vascular structure of the Hamamelidaceae subfamily
Liquidambaroideae. – Ann. Missouri Bot. Gard. 73: 325-347.
Bogle AL. 1987. Inflorescence and flower
ontogeny in the pseudanthium of Rhodoleia (Hamamelidaceae). – Amer. J.
Bot. 74: 607-608.
Bogle AL. 1989. The floral morphology,
vascular anatomy, and ontogeny of the Rhodoleioideae (Hamamelidaceae) and their
significance in relation to the ’lower’ hamamelids. – In: Crane PR,
Blackmore S (eds), Evolution, systematics, and fossil history of the
Hamamelidae 1: Introduction and ‘lower’ Hamamelidae, Syst. Assoc. Spec.
Vol. 40A, Clarendon Press, Oxford, pp. 201-226
Bogle AL. 1990. Multilacunar nodal anatomy in
Mytilaria (Hamamelidaceae). – J. Arnold
Arbor. 71: 111-118.
Bogle AL, Philbrick CT. 1980. A generic atlas
of hamamelidaceous pollens. – Contr. Gray Herb. 210: 29-103.
Bohm BA. 1979. Flavonoids of Tolmiea
menziesii. – Phytochemistry 18: 1079-1080.
Bohm BA. 1993. External and vacuolar
flavonoids of Ribes viscosissimum. – Biochem. Syst. Evol. 21:
745.
Bohm BA, Bhat UG. 1985. Flavonoids of
Astilbe and Rodgersia compared to Aruncus. –
Biochem. Syst. Ecol. 13: 437-440.
Bohm BA, Collins FW. 1979. Flavonoids of some
species of Chrysosplenium. – Biochem. Syst. Ecol. 7: 195-201.
Bohm BA, Ornduff R. 1978. Chemotaxonomic
studies in the Saxifragaceae
s.l. 9. Flavonoids of Jepsonia. – Madroño 52: 39-43.
Bohm BA, Wilkins CK. 1976. Flavonoids and
gallic acid derivatives from Peltiphyllum peltatum. – Phytochemistry
15: 2012-2013.
Bohm BA, Wilkins CK. 1978a. The flavonoids of
Heuchera cylindrica. – Can. J. Bot. 56: 1174-1176.
Bohm BA, Wilkins CK. 1978b. Chemosystematic
studies in the Saxifragaceae
s.l. 8. The flavonoids of Elmera racemosa (Watson) Rydberg. –
Brittonia 30: 327-333.
Bohm BA, Collins FW, Bose R. 1977a.
Flavonoids of Bergenia, Francoa, and Parnassia. –
Biochem. Syst. Ecol. 13: 221-233.
Bohm BA, Collins FW, Bose R. 1977b.
Flavonoids of Chrysosplenium tetrandrum. – Phytochemistry 16:
1205-1209.
Bohm BA, Donevan LS, Bhat UG. 1986.
Flavonoids of some species of Bergenia, Francoa,
Parnassia and Lepuropetalon. – Biochem. Syst. Ecol. 14:
75-77.
Bohm BA, Chalmers G, Bhat UG. 1988.
Flavonoids and the relationship of Itea to the Saxifragaceae. – Phytochemistry
27: 2651-2653.
Bohm BA, Yang JY, Page JE, Soltis DS. 1999.
Flavonoids, DNA and relationships of Itea and Pterostemon.
– Biochem. Syst. Ecol. 27: 79-83.
Boiteau P, Allorge-Boiteau L. 1995.
Kalanchoe (Crassulacées) de Madagascar. Systématique,
écophysiologie et phytochimie. – Karthala, Paris.
Borissova AG. 1969. Konspekt sistemy sem. Crassulaceae DC. flory SSSR
(dobavlenija is ismenenija). – Novosti Sist. Vyšs. Rast. 6: 112-121.
Böttcher W, Jäger EJ. 1984. Zur
Interpretation der Verbreitung der Gattung Sedum L. s.l. (Crassulaceae) und ihrer
Wuchsformtypen. – Wissensch. Zeitschr. Univ. Halle 33: 127-141.
Boutique R, Verdcourt B. 1973. Haloragaceae. – In: Milne-Redhead
E, Polhill RM (eds), Flora of tropical East Africa, Balkema, London, Rotterdam,
pp. 1-4.
Bowman RN. 1983. Intraspecific variability of
leaf cuticle alkanes in Sedum lanceolatum along an elevational
gradient. – Biochem. Syst. Ecol. 11: 195-198.
Bramwell D. 1968. Notes on the taxonomy and
nomenclature of the genus Aichryson. – Bol. Inst. Nac. Invest.
Agron. (Madrid) 28: 203-213.
Bramwell D. 1970. Generic delimitation of the
Sempervivum group. – Natl. Cact. Succ. J. 25: 50-51.
Brenan JPM. 1952. Plants of the Cambridge
Expedition, 1947-1948: II. A new order of flowering plants from the British
Cameroons. – Kew Bull. 7: 227-236.
Brenan JPM. 1954. Soyauxia, a new
genus of Medusandraceae. –
Kew Bull. 9: 507-511.
Brennan RM. 1992. Currants and gooseberries
(Ribes). – In: Moore JN, Ballington JR (eds), Genetic resources of
temperate fruit and nut crops, International Society of Horticultural Sciences,
Wageningen, pp. 457-488.
Breuer B, Stuhlfauth T, Fock H, Huber H.
1987. Fatty acids of some Cornaceae, Hydrangeaceae, Aquifoliaceae,
Hamamelidaceae and Styracaceae. –
Phytochemistry 26: 1441-1445.
Britton EG. 1887. Elongation of the
inflorescence of Liquidambar. – Bull. Torrey Bot. Club 14: 95-96.
Britton NL, Rose JN. 1903. New or noteworthy
North American Crassulaceae. –
Bull. New York Bot. Gard. 3: 1-45.
Britton NL, Rose JN. 1904.
Lenophyllum, a new genus of Crassulaceae. – Smithsonian Misc.
Coll. 47: 159-162.
Britton NL, Rose JN. 1909.
Thompsonella, a new genus of Crassulaceae from Mexico. –
Contr. U.S. Natl. Herb. 12: 391-392.
Brochmann C, Xiang Q-Y, Brunsfeld SJ, Soltis
DE, Soltis PS. 1998. Molecular evidence for polyploid origins in
Saxifraga (Saxifragaceae): the narrow arctic
endemic S. svalbardensis and its widespread allies. – Amer. J. Bot.
85: 135-143.
Brown RW. 1939. Fossil leaves, fruits, and
seeds of Cercidiphyllum. – J. Palaeontol. 13: 485-499.
Byalt VV. 1997. Meterostachys
sikokianus (Crassulaceae),
a new species and genus for the flora of China. – Bot. Žurn. 82: 128-130.
[In Russian with English summary]
Byalt VV. 1998. Orostachys paradoxa,
a rare species from the Russian Far East. – Cact. Succ. J. 70: 262-263.
Byalt VV, Sokolova IV. 1999. Ohbaea
Byalt & I. V. Sokolova, a replacement name for Balfouria (H. Ohba)
H. Ohba (Crassulaceae). – Kew
Bull. 54: 476.
Bywater M. 1980. Observations on seeds of
Crassula sect. Rosulares. – Kew Bull. 35: 401-402.
Bywater M, Wickens GE. 1983. New World
species of the genus Crassula. – Kew Bull. 39: 699-728.
Caballero A, Jiménez MS. 1977. Contribución
al estudio anatómico foliar de las crassuláceas canarias. – Vieraea 7:
115-132.
Calder JA, Savile DBO. 1960. Studies in Saxifragaceae III. Saxifraga
odontolma and lyalli and North American subspecies of S.
punctata. – Can. J. Bot. 38: 409-435.
Calie PJ. 1981. Systematic studies in
Sedum section Ternata (Crassulaceae). – Brittonia 33:
498-507.
Camp WH, Hubbard MM. 1963. Vascular supply
and structure of the ovule and aril in peony and of the aril in nutmeg. –
Amer. J. Bot. 50: 174-178.
Carlquist SJ. 1982. Wood anatomy of Daphniphyllaceae: ecological
and phylogenetic considerations, review of pittosporalean families. –
Brittonia 34: 252-266.
Carlström A. 1985. Two new species of
Sedum (Crassulaceae)
from S Greece and SW Turkey. – Willdenowia 15: 107-113.
Carlsward BS, Judd WS, Soltis DE, Manchester
S, Soltis PS. 2011. Putative morphological synapomorphies of Saxifragales and their major
subclades. – J. Bot. Res. Inst. Texas 5: 179-196.
Carniel K. 1967. Über die Embryobildung in
der Gattung Paeonia. – Österr. Bot. Zeitschr. 114: 4-19.
Carrillo-Reyes P, Lomelí-Sención JA. 2008.
Sedum chazaroi (Crassulaceae), an endemic new
species from southern Jalisco, Mexico. – Bol. Soc. Bot. Mexico 83: 77-80.
Carrillo-Reyes P, Sosa V, Mort ME. 2008.
Thompsonella and the “Echeveria group“ (Crassulaceae): phylogenetic
relationships based on molecular and morphological characters. – Taxon 57:
863-874.
Carrillo-Reyes P, Sosa V, Mort ME. 2009.
Molecular phylogeny of the Acre clade (Crassulaceae): dealing with the
lack of definitions for Echeveria and Sedum. – Mol.
Phylogen. Evol. 53: 267-276.
Castroviejo S, Calvo R. 1981. Datos
citotaxonomicos en Sedum series Rupestria Berger. – Anal.
Jard. Bot. Madrid 38: 37-50.
Catling PM, Dumouchel L, Brownell VR. 1998.
Pollination of the Miccosukee Gooseberry (Ribes echinellum). –
Castanea 63: 402-407.
Cave MS, Arnott HJ, Cook SA. 1961. Embryogeny
in the California paeonies with reference to their taxonomic position. –
Amer. J. Bot. 48: 397-404.
Ceska O. 1977. Studies in aquatic macrophytes
XVII: phytochemical differentiation of Myriophyllum taxa collected in
British Columbia. – Water Investigation Branch, Ministry of Environment
Victoria, British Columbia.
Chang CT. 1959. The pollen morphology of
Liquidambar L. and Altingia Nor. – Bot. Žurn. 44:
1375-1380. [In Russian with English summary]
Chang H-T. 1948. Additions to the
hamamelidaceous flora of China. – Sunyatsenia 7: 63-74.
Chang H-T. 1962. Semiliquidambar,
novum Hamamelidacearum genus Sinicum. – Sunyatsen Univ. Bull. Nat. Sci. 1:
34-44. [In Chinese]
Chang H-T. 1973. A revision of the
hamamelidaceous flora of China. – Sunyatsen Univ. Bull. Nat. Sci. 1: 54-71.
[In Chinese]
Chapman M. 1933. The ovule and embryo sac of
Saxifraga virginiensis. – Amer. J. Bot. 20: 151-158.
Chen LY, Zhao SY, Mao KS, Les DH, Wang QF,
Moody ML. 2014. Historical biogeography of Haloragaceae: an out-of-Australia
hypothesis with multiple intercontinental dispersals. – Mol. Phylogen. Evol.
78: 87-95.
Clausen RT. 1940. Studies in the Crassulaceae: Villadia,
Altamiranoa, and Thompsonella. – Bull. Torrey Bot. Club 67:
195-198.
Clausen RT. 1943. The section
Sedastrum of Sedum. – Bull. Torrey Bot. Club 70:
289-296.
Clausen RT. 1959. Sedum of the
Trans-Mexican Volcanic Belt: an exposition of taxonomic methods. – Cornell
University Press, Ithaca, New York.
Clausen RT. 1975. Sedum of North
America North of the Mexican Plateau. – Cornell University Press, Ithaca, New
York.
Clausen RT. 1977. Biennial species of
Sedum of the Sierra Madre Occidental and the Mexican Plateau. –
Bull. Torrey Bot. Club 104: 209-217.
Clausen RT. 1979. Sedum in six areas
of the Mexican Cordilleran Plateau. – Bull. Torrey Bot. Club 106: 205-216.
Collins FW, Bohm BA. 1974. Chemotaxonomic
studies in the Saxifragaceae
s.l. 1. The flavonoids of Tellima grandiflora – Can. J. Bot. 52:
307-312.
Collins FW, Bohm BA, Wilkins CK. 1975.
Flavonol glycoside gallates from Tellima grandiflora. –
Phytochemistry 14: 1099-1102.
Conradi R. 1960. Astilbe. – Norsk
Hagetid. 76: 192-193.
Conti E, Soltis DE, Hardig TM, Schneider J.
1999. Phylogenetic relationships of the silver saxifrages (Saxifraga,
sect. Ligulatae Haworth): implications for the evolution of substrate
specificity, life histories, and biogeography. – Mol. Phylogen. Evol. 13:
536-555.
Crane PR. 1984. A reevaluation of
Cercidiphyllum-like plant fossils from the British early Tertiary. –
Bot. J. Linn. Soc. 89: 199-230.
Crane PR, Stockey RA. 1985. Growth and
reproductive biology of Joffrea speirsii gen. et sp. nov., a
Cercidiphyllum-like plant from the Late Paleocene of Alberta, Canada.
– Can. J. Bot. 63: 340-364.
Crane PR, Stockey RA. 1986. Morphology and
development of pistillate inflorescences in extant and fossil Cercidiphyllaceae. – Ann.
Missouri Bot. Gard. 73: 382-393.
Crepet WL, Nixon KC, Friis EM, Freudenstein
JV. 1992. Oldest fossil flowers of hamamelidaceous affinity, from the Late
Cretaceous of New Jersey. – Proc. Natl. Acad. Sci. U.S.A. 89: 8986-8989.
Croizat L. 1941. On the systematic position
of Daphniphyllum and its allies. – Lingnan Sci. J. 20: 79-103.
Dahlgren KVO. 1930. Zur Embryologie der
Saxifragoideen. – Svensk Bot. Tidskr. 24: 429-448.
Dandy JE. 1927. The genera of Saxifragaceae. – In: Hutchinson
J (ed), Contributions towards a phylogenetic classification of flowering
plants, Kew Bull. 6: 100-118.
Dark SOS. 1936. Meiosis in diploid and
tetraploid Paeonia species. – J. Genet. 32: 353-372.
Darlington CD. 1929. A comparative study of
the chromosome complement in Ribes. – Genetica 11: 267-272.
Davezac T. 1957. La place systématique du
genre Paeonia et forme de jeunesse de P. lusitanica Mill. –
Bull. Soc. Hist. Natur. Toulouse 92: 197-201.
Davis CC, Chase MW. 2004. Elatinaceae are
sister to Malpighiaceae;
Peridiscaceae belong to Saxifragales. – Amer. J. Bot. 91:
262-273.
Deng J-B, Drew BT, Mavrodiev EV, Gitzendanner
MA, Soltis PS, Soltis DE. 2015. Phylogeny, divergence times, and historical
biogeography of the angiosperm family Saxifragaceae.– Mol. Phylogen.
Evol. 83: 86-98.
Denton MF. 1979. Cytological and reproductive
differentiation in Sedum section Gormania (Crassulaceae). – Brittonia 31:
197-211.
Denton MF. 1982. Revision of Sedum
section Gormania (Crassulaceae). – Brittonia 34:
48-77.
Denton MF, Kerwin JL. 1980. Survey of
vegetative flavonoids of Sedum section Gormania (Crassulaceae). – Can. J. Bot. 58:
902-905.
Deschatres R. 1954. Recherches sur la
phyllotaxie du genre Sedum. – Rev. Gén. Bot. 61: 501-570.
Descoings B. 2006. Le genre
Kalanchoe (Crassulaceae): structure et
définition. – J. Bot. Soc. France 33: 3-28.
Dickison WC. 1975. Studies on the floral
anatomy of the Cunoniaceae. –
Amer. J. Bot. 62: 433-447.
Dickison WC. 1980a. Diverse nodal anatomy of
the Cunoniaceae.
– Amer. J. Bot. 67: 975-981.
Dickison WC. 1980b. Comparative wood anatomy
and evolution of the Cunoniaceae. –
Allertonia 2: 281-321.
Dickison WC, Hils HM, Lugansky TW, Stern WL.
1994. Comparative anatomy and systematics of woody Saxifragaceae.
Aphanopetalum Endl. – Bot. J. Linn. Soc. 114: 167-182.
Dong W, Xu C, Cheng T, Lin K, Zhou S. 2013.
Sequencing angiosperm plastid genomes made easy: a complete set of universal
primers and a case study on the phylogeny of Saxifragales.– Genome Biol. Evol.
2013: 5: 989-997.
Dong W, Xu C, Wu P, Chen T, Yu J, Zhou S,
Hong D-Y. 2018. Resolving the systematic positions of enigmatic taxa:
manipulating the chloroplast genome data of Saxifragales. – Mol. Phylogen.
Evol. 126: 321-330.
Donoghue MJ, Doyle JA. 1989. Phylogenetic
analysis of angiosperms and the relationships of the Hamamelidae. – In: Crane
PR, Blackmore S (eds), Evolution, systematics, and fossil history of the
Hamamelidae 1: Introduction and ”lower” Hamamelidae, Syst. Assoc. Spec.
Vol. 40A, Clarendon Press, Oxford, pp. 17-45.
Dorofeev PI. 1976. K sistematike neogenovych
Proserpinaca Belorusii. – Doklady Akademia Nauk SSSR 20:
1036-1038.
Ebel F, Hagen A, Kümmel F. 1991a.
Beobachtungen zur Wuchsrhythmik von Orostachys spinosus (L.) Sweet (Crassulaceae). – Wissensch.
Zeitschr. Univ. Halle 40: 47-68.
Ebel F, Hagen A, Kümmel F. 1991b.
Beobachtungen zur Wuchsrhythmik und “Knospenbildung” einiger
Greenovia- und Aeonium-Arten (Crassulaceae). – Flora 85:
187-200.
Egger K, Reznik H. 1961. Die
Flavonolglykoside der Hamamelidaceen. – Planta 57: 239-249.
Eggli U. 1988. A monographic study of the
genus Rosularia (Crassulaceae). – Bradleya
[Suppl.] 6: 1-119.
Eggli U (ed). 2003. Illustrated handbook of
succulent plants VI. Crassulaceae. – Springer,
Berlin.
Eggli U, ‘t Hart H, Nyffeler R. 1995.
Towards a consensus classification of the Crassulaceae. – In: ‘t Hart H,
Eggli U (eds), Evolution and systematics of the Crassulaceae, Backhuys Publ.
Leiden, pp. 173-192.
Elst P van der. 1909. Beiträge zur Kenntnis
der Samenauflage der Saxifragaceae. – Ph.D. diss.,
University of Utrecht, The Netherlands.
Elvander PE. 1984. The taxonomy of
Saxifraga (Saxifragaceae) section
Boraphila subsection Integrifoliae in western North America.
– Syst. Bot. Monogr. 3: 1-44.
Endress PK. 1967. Systematische Studie über
die verwandtschaftlichen Beziehungen zwischen den Hamamelidaceen und
Betulaceen. – Bot. Jahrb. Syst. 87: 431-525.
Endress PK. 1968. Zur systematischen Stellung
von Parrotia C. A. Mey. und Sycopsis Oliv. und zum neuen
Bastard xSycoparrotia semidecidua P. Endress et J. Anliker. –
Schweiz. Beitr. Dendrol. 16-18: 11-28.
Endress PK. 1969. Molinadendron,
eine neue Hamamelidaceen-Gattung aus Zentralamerika. – Bot. Jahrb. Syst. 89:
354-359.
Endress PK. 1970. Die Infloreszenzen der
apetalen Hamamelidaceen, ihre grundsätzliche morphologische und systematische
Bedeutung. – Bot. Jahrb. Syst. 90: 1-54.
Endress PK. 1971. Blütenstände und
morphologische Interpretation der Blüten bei apetalen Hamamelidaceen. – Ber.
Deutsch. Bot. Ges. 84: 183-185.
Endress PK. 1976. Die Androeciumanlage bei
polyandrischen Hamamelidaceen und ihre systematische Bedeutung. – Bot. Jahrb.
Syst. 97: 436-457.
Endress PK. 1977. Evolutionary trends in the
Hamamelidales-Fagales group. – In: Kubitzki K (ed), Flowering plants:
evolution and classification of higher categories, Plant Syst. Evol. [Suppl.]
1: 321-347.
Endress PK. 1978a. Stipules in
Rhodoleia (Hamamelidaceae). – Plant Syst.
Evol. 130: 157-160.
Endress PK. 1978b. Blütenontogenese,
Blütenabgrenzung und systematische Stellung der perianthlosen Hamamelidoideae.
– Bot. Jahrb. Syst. 100: 249-317.
Endress PK. 1989a. Aspects of evolutionary
differentiation of the Hamamelidaceae and the Lower
Hamamelididae. – Plant Syst. Evol. 162: 193-211.
Endress PK. 1989b. A suprageneric taxonomic
classification of the Hamamelidaceae. – Taxon 38:
371-376.
Endress PK. 1989c. Phylogenetic relationships
in the Hamamelidoideae. – In: Crane PR, Blackmore S (eds), Evolution,
systematics, and fossil history of the Hamamelidae 1: Introduction and
‘lower’ Hamamelidae, Syst. Assoc. Spec. Vol. 40A, Clarendon Press, Oxford,
pp. 227-240.
Endress PK. 1993a. Cercidiphyllaceae. – In:
Kubitzki K, Rohwer JG, Bittrich V (eds), The families and genera of vascular
plants II. Flowering plants. Dicotyledons. Magnoliid, hamamelid and
caryophyllid families, Springer, Berlin, Heidelberg, New York, pp. 250-252.
Endress PK. 1993b. Hamamelidaceae. – In: Kubitzki
K, Rohwer JG, Bittrich V (eds), The families and genera of vascular plants II.
Flowering plants. Dicotyledons. Magnoliid, hamamelid and caryophyllid families,
Springer, Berlin, Heidelberg, New York, pp. 322-331.
Endress PK, Anliker J. 1968.
xSycoparrotia semidecidua hybr. nov. (= Parrotia persica C.
A. Mey. x Sycopsis sinensis Oliv.). – Schweiz. Beitr. Dendrol.
16-18: 6-28.
Endress PK, Friis EM. 1991.
Archamamelis, hamamelidalean flowers from the Upper Cretaceous of
Sweden. – Plant Syst. Evol. 175: 101-114.
Endress PK, Hyland BPM, Tracey JG. 1985.
Noahdendron, a new Australian genus of the Hamamelidaceae. – Bot. Jahrb.
Syst. 107: 369-378.
Engelmann W. 1960. Endogene Rhythmik und
photoperiodische Blühinduktion bei Kalanchoe. – Planta 55:
496-511.
Engler A. 1891. Saxifragaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien III(2a), W. Engelmann,
Leipzig, pp. 41-93; Engler A. 1897. Nachträge zu III(2a), p. 180.
Engler A. 1930. Saxifragaceae. – In: Engler A,
Harms H (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 18a, W.
Engelmann, Leipzig, pp. 74-226.
Fægri K. 1982. The Myriophyllum
spicatum group in North Europe. – Taxon 31: 467-471.
Fairfield KN, Mort ME, Santos-Guerra A. 2004.
Phylogenetics and evolution of the Macaronesian members of the genus
Aichryson (Crassulaceae) inferred from nuclear
and chloroplast sequence data. – Plant Syst. Evol. 248: 71-83.
Favarger C. 1957. Sur deux critères nouveaux
utilisables dans la taxinomie des Saxifragacées. – Rev. Cytol. Biol. Vég.
18: 125-137.
Fehrenbach S, Barthlott W. 1988.
Mikromorphologie der Epicuticular-Wachse der Rosales s.l. und deren
systematische Bedeutung. – Bot. Jahrb. Syst. 109: 407-428.
Feng G-P, Li C-S, Zhilin SG, Wang Y-F,
Gabrielyan IG. 2000. Nyssidium jiayinense sp. nov. (Cercidiphyllaceae) of the
Early Tertiary from North-East China. – Bot. J. Linn. Soc. 134: 471-484.
Feng Y-X, Chen Z-D, Wang X-Q, Pan K-Y, Hong
D-Y. 1999. A taxonomic revision of the
Loropetalum-Tetrathyrium complex and its systematic position
in the Hamamelidoideae, based on morphology and ITS sequence data. – Taxon
48: 689-700.
Ferguson DK. 1989. A survey of the
Liquidambaroideae (Hamamelidaceae) with a view to
elucidating its fossil record. – In: Crane PR, Blackmore S (eds), Evolution,
systematics, and fossil history of the Hamamelidae 1: Introduction and
‘lower’ Hamamelidae, Syst. Assoc. Spec. Vol. 40A, Clarendon Press, Oxford,
pp. 249-272.
Ferguson IK, Webb DA. 1970. Pollen morphology
in the genus Saxifraga and its taxonomic significance. – Bot. J.
Linn. Soc. 63: 295-311.
Fernald ML. 1919. Two new Myriophyllums and a
species new to the United States. – Rhodora 21: 120-124.
Fernandes RB. 1978. Crassulaceae africanae novae vel
minus cognitae. – Botl. Soc. Brot., Sér. II, 52: 165-220.
Fernandes RB. 1983. 67. Crassulaceae. – In: Launert E
(ed), Flora Zambesiaca 7 (Part 1), Flora Zambesiaca Managing Committee, London,
pp. 3-74.
Fétré J, Lebègue A. 1964. Embryogénie des
Crassulacées. – Compt. Rend. Acad. Sci. Paris 258: 5035-5038.
Fishbein M, Soltis DE. 2004. Further
resolution of the rapid radiation of Saxifragales (angiosperms,
eudicots) supported by mixed-model Bayesian analysis. – Syst. Bot. 29:
883-891.
Fishbein M, Hibsch-Jetter C, Soltis DE,
Hufford L. 2001. Phylogeny of Saxifragales (angiosperms,
eudicots): analysis of a rapid, ancient radiation. – Syst. Biol. 50:
817-847.
Folk RA, Freudenstein JV. 2014a. Phylogenetic
relationships and character evolution in Heuchera (Saxifragaceae) on the basis of
multiple nuclear loci. – Amer. J. Bot. 101: 1532-1550.
Folk RA, Freudenstein JV. 2014b. Revision of
Heuchera Section Rhodoheuchera Subsections
Hemsleyanae and Rosendahliae Subsectio Nova (Saxifragaceae). – Syst. Bot. 39:
850-8974.
Franchet AR. 1890. Monographie du genre
Chrysosplenium Tournefort. – Nouv. Arch. Mus. Natl. Hist. Nat.
Paris, sér. III, 2: 87-114.
Freire-Fierro A. 2002. A new species of
Ribes (Grossulariaceae), along with
notes and a key to the Ecuadorian species. – Syst. Bot. 27: 14-18.
Freire-Fierro A, Romoleroux K. 2004. 73. Crassulaceae, 74. Saxifragaceae, 75B. Grossulariaceae. – In: Harling
G, Andersson L (eds), Flora of Ecuador 73, Botanical Institute, Göteborg
University, pp. 3-24, 41-66.
Friedrich H-C. 1973. Zur Cytotaxonomie der
Gattung Crassula. – Garcia de Orta, Sér. Bot., 1: 49-65.
Friedrich H-C. 1979. Vorarbeiten zu einer
Monographie der Gattung Crassula L. 3. Die hydrophylen Sippen in Süd-
und Ostafrika. – Mitt. Bot. München 15: 577-598.
Fröderström H. 1930. The genus
Sedum L. I. – Acta Horti Gothoburg. 5, Appendix: 1-75.
Fröderström H. 1932a. The genus
Sedum L. II. – Acta Horti Gothoburg. 6, Appendix: 1-111.
Fröderström H. 1932b. The genus
Sedum L. III. – Acta Horti Gothoburg. 7, Appendix: 1-126.
Fröderström H. 1936. The genus
Sedum L. IV. – Acta Horti Gothoburg. 11, Appendix: 1-262.
Fu K-T. 1965. Species et combinationes novae
Crassulacearum Sinicarum. – Acta Phytotaxon. Sin. 10: 111-128.
Fu K-T. 1974. Revision of the section
Oreades of Chinese Sedum. – Acta Phytotaxon. Sin. 12:
51-77. [In Chinese].
Gandolfo MA, Nixon KC, Crepet WL. 1998.
Tylerianthus crossmanensis gen. et sp. nov. (aff. Hydrangeaceae) from
the Upper Cretaceous of New Jersey. – Amer. J. Bot. 85: 376-386.
Garcês HMP, Champagne CEM, Townsley BT, Park
S, Malhó R, Pedroso MC, Harada JJ, Sinha NR. 2007. Evolution of asexual
reproduction in leaves of the genus Kalanchoë. – Proc. Natl. Acad.
Sci. U.S.A. 104: 15578-15583.
García MA, Nicholson EH, Nickrent DL. 2004.
Extensive intraindividual variation in plastid rDNA sequences from the
holoparasite Cynomorium coccineum (Cynomoriaceae). – J. Mol. Evol.
58: 322-332.
Gäumann E. 1919. Studien über die
Entwicklungsgeschichte einiger Saxifragales. – Rec. Trav. Bot.
Néerl. 16: 285-322.
Ge L-P, Lu A-M, Pan K-Y. 2002. Floral
ontogeny in Itea yunnanensis (Iteaceae). – Acta Bot. Sin. 44:
1261-1267.
Gehrig H, Gaußmann O, Max H, Schwarzott D,
Kluge M. 2001. Molecular phylogeny of the genus Kalanchoe (Crassulaceae) inferred from
nucleotide sequences of the ITS-1 and ITS-2 regions. – Plant Sci. 160:
827-835.
Gelius L. 1967. Studien zur
Entwicklungsgeschichte an Blüten der Saxifragales sensu lato mit
besonderer Berücksichtigung des Androeceums. – Bot. Jahrb. Syst. 87:
253-303.
George G, Candela C, Quinet M, Fellous R.
1974. Identification of the diterpenic acid of Ribes nigrum buds. –
Helv. Chim. Acta 57: 1247-1249.
Gess S, Gess F, Gess R. 1998. Birds, wasps
and Tylecodon. Pollination strategies of two members of the genus
Tylecodon in Namaqualand. – Veld and Flora 84: 56-57.
Gilbert MG. 1985. The genus Sedum in
Ethiopia. – Bradleya 3: 48-52.
Gluchoff-Fiasson K, Fenet B, Leclerc J-C,
Reynaud J, Lussignol M, Jay M. 2001. Three new flavonol malonylrhamnosides from
Ribes alpinum. – Chem. Pharmaceut. Bull. 49: 768-770.
Goldblatt P, Endress PK. 1977. Cytology and
evolution in Hamamelidaceae.
– J. Arnold Arbor. 58: 67-71.
Goldschmidt E. 1964. Cytological species and
interspecific hybrids of the genus Ribes. – Hereditas 52:
139-150.
Gontcharova SB. 1999. Ornamentation of the
testa of some Eastern Asian Sedoideae (Crassulaceae). – Bull. Natl. Sci.
Mus., Tokyo, ser. B, 25: 131-141.
Gontcharova SB, Artiukova EV, Gontcharov AA.
2006. Phylogenetic relationships among members of the subfamily Sedoideae (Crassulaceae) inferred from the ITS
region sequences of nuclear rDNA. – Genetika 42: 803-811.
Gorenflot R, Moreau F. 1970. Types
stomatiques et phylogénie des Saxifraginées (Saxifragacées). – Compt.
Rend. Acad. Sci. Paris 270: 2802-2805.
Gornall RJ. 1980. Generic limits and
systematics of Boykinia and allies (Saxifragaceae). – Ph.D. diss.,
University of British Columbia, Vancouver, British Columbia.
Gornall RJ. 1986. Trichome anatomy and the
taxonomy of Saxifraga (Saxifragaceae). – Nord. J. Bot.
6: 257-275.
Gornall RJ. 1987a. An outline of a revised
classification of Saxifraga L. – Bot. J. Linn. Soc. 95: 273-292.
Gornall RJ. 1987b. Foliar crystals in
Saxifraga and segregate genera (Saxifragaceae). – Nord. J. Bot.
7: 233-238.
Gornall RJ. 1989. Anatomical evidence and the
taxonomic position of Darmera (Saxifragaceae). – Bot. J. Linn.
Soc. 100: 173-182.
Gornall RJ, Bohm BA. 1980. The use of
flavonoids in the taxonomy of Boykinia and allies (Saxifragaceae). – Can. J. Bot.
58: 1768-1779.
Gornall RJ, Bohm BA. 1984. Breeding systems
and relationships among species of Boykinia and related genera (Saxifragaceae). – Can. J. Bot.
62: 33-37.
Gornall RJ, Bohm BA. 1985. A monograph of
Boykinia, Peltoboykinia, Bolandra, and
Suksdorfia (Saxifragaceae). – Bot. J. Linn.
Soc. 90: 1-71.
Gornall RJ, Bohm BA. Taylor RL. 1983.
Chromosome numbers in Boykinia and allies (Saxifragaceae). – Can. J. Bot.
61: 1979-1982.
Gruas-Cavagnetto C, Praglowski J. 1977.
Pollen d’Haloragacées dans le Thanétien et le Cuisien du basin de Paris.
– Pollen Spores 19: 299-308.
Gruhlich V. 1984. Generic division of
Sedoideae in Europe and adjacent regions. – Preslia 56: 29-54.
Grund C, Jensen U. 1981. Systematic
relationships of the Saxifragales revealed by
serological characteristics of seed proteins. – Plant Syst. Evol. 137:
1-22.
Günthart A. 1902. Beiträge zur
Blütenbiologie der Cruciferen, Crassulaceen und der Gattung
Saxifraga. – Bibl. Bot. 58: 1-97.
Guzmán JM, Gordillo MM, Durán RC, Ramírez
JJ, Pérez-Amador MC. 2013. A synopsis of Pterostemon (Iteaceae), a group endemic to Mexico.
– Amer. J. Plant Sci. 4: 1-9.
Hallier H. 1904. Über die Gattung
Daphniphyllum, ein Übergangsglied von den Magnoliaceen und
Hamamelidaceen zu den Kätzchenblütlern. – Bot. Mag. (Tokyo) 18: 55-69.
Ham RCHJ van. 1994. Phylogenetic implications
of chloroplast DNA variation in the Crassulaceae. – Ph.D. diss.,
Universiteit Utrecht, The Netherlands.
Ham RCHJ van. 1995. Phylogenetic
relationships in the Crassulaceae inferred from
chloroplast DNA variation. – In: Hart H ‘t, Eggli U (eds), Evolution and
Systematics of the Crassulaceae,
Backhuys, Leiden, pp. 17-29.
Ham RCHJ van, Hart H ’t. 1994. Evolution of
Sedum series Rupestria (Crassulaceae): evidence from
chloroplast DNA and biosystematic data. – Plant Syst. Evol. 190: 1-20.
Ham RCHJ van, ‘t Hart H. 1998. Phylogenetic
relationships in the Crassulaceae inferred from
chloroplast DNA restriction-site variation. – Amer. J. Bot. 85: 123-134.
Ham RCHJ van, ‘t Hart H, Mes THM, Sandbrink
JM. 1994. Molecular evolution of noncoding regions of the chloroplast genome in
the Crassulaceae and related
species. – Curr. Genet. 25: 558-566.
Hamel J. 1953. Contribution à l’étude
cytotaxinomique des Saxifragacées. – Rev. Cytol. Biol. Vég. 14: 113-311.
Hamet R. 1929. Contribution à l’étude
phytogéographique du genre Sedum. – Candollea 4: 1-52.
Hara H. 1957. Synopsis of the genus
Chrysosplenium L. (Saxifragaceae). – J. Fac. Sci.
Univ. Tokyo, Sect. III, Bot. 7: 1-90.
Harms H. 1916. Über die Blütenverhältnisse
und die systematische Stellung der Gattung Cercidiphyllum Sieb. et
Zucc. – Ber. Deutsch. Bot. Ges. 34: 272-283.
Harms H. 1930. Hamamelidaceae. – In: Engler A,
Harms H (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 18a, W.
Engelmann, Leipzig, pp. 303-345, 487.
Harmsen L. 1939. Studies in the embryology
and cytology of Saxifraga. – Medd. Grønl. 125: 1-15.
Hart H ‘t. 1972. Chromosome numbers in the
series Rupestria Berger of the genus Sedum L. – Acta Bot.
Neerl. 21: 428-435.
Hart H ‘t. 1975. The pollen morphology of
24 European species of the genus Sedum L. – Pollen Spores 16:
373-387.
Hart H ‘t. 1978. Biosystematic studies in
the Acre group and the series Rupestria Berger of the genus
Sedum L. (Crassulaceae). – Ph.D. diss.,
Universiteit Utrecht, The Netherlands.
Hart H ‘t. 1982a. The systematic position
of Sedum tuberosum Coss. & Let. (Crassulaceae). – Proc. Kon.
Nederl. Akad. Wet., Ser. C, 85: 497-508.
Hart H ‘t. 1982b. The white-flowered
European Sedum species 1. Principles of a phylogenetic classification
of the Sedoideae (Crassulaceae)
and the position of the white-flowered Sedum species. – Proc. Kon. Nederl.
Akad. Wet., Ser. C, 85: 663-675.
Hart H ‘t. 1985a. Sexual reproduction and
hybridisation in Sedum telephium (Crassulaceae). – Acta Bot. Neerl.
34: 1-4.
Hart H ‘t. 1985b. The vascular pattern of
the flowers of Sedum anacampseros (Crassulaceae). – Acta Bot. Neerl.
34: 119-121.
Hart H ‘t. 1985c. Chromosome numbers in
Sedum (Crassulaceae)
from Greece. – Willdenowia 15: 115-135.
Hart H ‘t. 1987a. Natural hybrids in
Sedum (Crassulaceae) 1.
Two new hybrids of S. series Rupestria and a new locality of
S. x brevierei. – Bot. Jahrb. Syst. 109: 1-16.
Hart H ‘t. 1987b. Evolution of the
Eurasiatic Sedum flora. – I.O.S. Bull. 4: 209-210.
Hart H ‘t. 1990. Variation in the structure
of the flowers of Sedum. – Sedum Soc. Newslett. 13: 11-17.
Hart H ‘t. 1991. Evolution and
classification of the European Sedum species (Crassulaceae). – Flora Mediterr.
1, OPTIMA, Madrid, pp. 31-61.
Hart H ‘t. 1992. The archetype of the Crassulaceae. – Acta Bot. Neerl.
41: 107-108.
Hart H ‘t. 1994a. The evolution of
life-forms, growth-forms and secondary growth in Eurasian Sedoideae (Crassulaceae). – Bradleya 12:
37-56.
Hart H ‘t. 1994b. The unilacunar two-trace
nodal structure of the caudex of Rhodiola rosea L. (Crassulaceae). – Bot. J. Linn.
Soc. 116: 235-241.
Hart H ‘t. 1995. Infrafamilial and generic
classification of the Crassulaceae. – In: Hart H ‘t,
Eggli U (eds), Evolution and systematics of the Crassulaceae, Backhuys, Leiden, pp.
159-172.
Hart H ‘t. 1997. Diversity within
Mediterranean Crassulaceae. –
Lagascalia 19: 93-100.
Hart H ‘t, Alpinar K. 1991. Biosystematic
studies in Sedum (Crassulaceae) of Turkey 1. Notes on
four hitherto little known species collected in the western part of Anatolia.
– Willdenowia 21: 143-156.
Hart H ‘t, Alpinar K. 1999. Sedum
ince (Crassulaceae), a new
species from southern Anatolia. – Edinburgh J. Bot. 56: 181-194.
Hart H ‘t, Arkel J van. 1985. Quantitative
aspects of the influence of day-length and temperature on Sedum
telephium (Crassulaceae).
– Acta Bot. Neerl. 34: 115-118.
Hart H ’t, Berendsen W. 1980. Ornamentation
of the testa in Sedum (Crassulaceae). – Plant Syst.
Evol. 135: 107-117.
Hart H ‘t, Berg AJJ van den 1982. The
white-flowered European Sedum species 2. Cytotaxonomic notes on S.
album L. and S. gypsicolum Boiss. & Reuter. – Proc. Kon.
Nederl. Akad. Wet., Ser. C, 85: 677-691.
Hart H ‘t, Bleij B. 1999. Nieuwe namen in
Sempervivum Sect. Jovibarba (Crassulaceae). – Succulenta 78:
35-42.
Hart H ‘t, Eggli U. 1995a. Introduction:
evolution of Crassulaceae
systematics. – In: Hart H ‘t, Eggli U (eds), Evolution and systematics of
the Crassulaceae, Backhuys
Publ., Leiden, The Netherlands, pp. 7-15.
Hart H ‘t, Eggli U (eds). 1995b. Evolution
and systematics of the Crassulaceae. – Backhuys Publ.,
Leiden, The Netherlands.
Hart H ‘t, Eggli U. 1998. Cytotaxonomic
studies in Rosularia (Crassulaceae). – Bot. Helvetica
98: 223-234.
Hart H ‘t, Koek-Noorman J. 1989. The origin
of the woody Sedoideae (Crassulaceae). – Taxon 38:
535-544.
Hart H ’t, Sandbrink JM, Csikos I, Ooyen A,
Brederode J van. 1993. Natural hybrids in Sedum (Crassulaceae) 4. The allopolyploid
origin of Sedum rupestre subsp. rupestre (Crassulaceae). – Plant Syst.
Evol. 184: 195-206.
Hart H ’t, Ham RDHJ van, Stevens JF, Elema
ET, Klis H van, Gadella TWJ. 1999. Biosystematic, molecular and phytochemical
evidence for the multiple origin of sympetaly in Eurasian Sedoideae (Crassulaceae). – Biochem. Syst.
Ecol. 27: 407-426.
Haskins ML, Hayden WJ. 1987. Anatomy and
affinities of Penthorum. – Amer. J. Bot. 74: 164-177.
Hayden WJ, Lewandowski JD. 1997. Gynoecium
structure in Penthorum. – Amer. J. Bot. 84: 201.
Hébert LP. 1975. Contribution à la
cytotaxonomie du genre Sedum L. – Bull. Soc. Neuchât., Sci. Nat.
98: 59-70.
Hébert LP. 1976. Nouvelle contribution à la
cytotaxonomie du genre Sedum L. – Bull. Soc. Neuchât., Sci. Nat.
99: 97-107.
Hébert LP. 1983. Analyse d’un complexe
chromosomique en Mediterranée: Sedum ser. Rupestria Berger
emend. – Rev. Cytol. Biol. Végét. Bot. 6: 179-224.
Heel WA van. 1986. A deviating female flower
of Cercidiphyllum japonicum (Cercidiphyllaceae). – Blumea
31: 273-276.
Heel WA van. 1987. Note on the morphology of
the male inflorescences in Cercidiphyllum (Cercidiphyllaceae). – Blumea
32: 303-309.
Henry JK. 1919. Ribes divaricatum x
Ribes lobbii. – Can. Field-Natur. 19: 94.
Hermsen EJ, Gandolfo MA, Nixon KC, Crepet WL.
2003. Divisestylus gen. nov. (aff. Iteaceae), a fossil saxifrage from the
late Cretaceous of New Jersey, USA. – Amer. J. Bot. 90: 1373-1383.
Hermsen EJ, Nixon KC, Crepet WL. 2006. The
impact of extinct taxa on understanding the early evolution of angiosperm
clades: an example incorporating fossil reproductive structures of Saxifragales. – Plant Syst. Evol.
260: 141-169.
Hernández E, Bañares A. 1996. Aeonium
volkeri sp. nov., nuevo endemiso de la isla de Tenerife, islas Canárias
(Crassulaceae). – Vieraea 25:
159-168.
Hernández-Castillo GR, Cevallos-Ferriz SRS.
1999. Reproductive and vegetative organs with affinities to Haloragaceae from the Upper
Cretaceous Huepac Chert locality of Sonora, Mexico. – Amer. J. Bot. 86:
1717-1734.
Herr JM. 1954. The development of the ovule
and female gametophyte in Tiarella cordifolia. – Amer. J. Bot. 41:
333-338.
Hewson HJ. 1989. Hamamelidaceae. – In: George AS
(ed), Flora of Australia 3, Australian Government Publ. Service, Canberra, pp.
1-4.
Hideux M. 1981. Le pollen données nouvelles
de la microscopie électronique et de l’informatique. Structure du sporoderme
de Rosidae-Saxifragales, étude
comparative et dynamique. – Agence de Coopération Culturelle et Technique,
Paris.
Hideux M, Abadie M. 1985. Cytologie
ultrastructurale de l’anthère de Saxifraga I. Période
d’initiation des précurseurs des sporopollénines au niveau des principaux
types exiniques. – Can. J. Bot. 63: 97-112.
Hideux M, Ferguson IK. 1976. The
stereostructure of the exine and its evolutionary significance in Saxifragaceae sensu lato. – In:
Ferguson IK, Muller J (eds), The evolutionary significance of the exine, Linn.
Soc. Symposium, No. 1, Academic Press, London and New York, pp. 327-377.
Hiepko P. 1965. Das zentrifugale Androecium
der Paeoniaceae. – Ber.
Deutsch. Bot. Ges. 77: 427-435.
Hiepko P. 1966. Zur Morphologie, Anatomie und
Funktion des Diskus der Paeoniaceae. – Ber. Deutsch. Bot.
Ges. 79: 233-245.
Hils MH. 1985. Comparative anatomy and
systematics of twelve woody Australasian genera of the Saxifragaceae. – Ph.D. diss.,
University of Florida, Gainesville, Florida.
Hils MH, Dickison WC, Lucansky TW, Stern WL.
1988. Comparative anatomy and systematics of woody Saxifragaceae:
Tetracarpaea. – Amer. J. Bot. 75: 1687-1700.
Hoey MT, Parks CR. 1991. Isozyme divergence
between eastern Asian, North American, and Turkish species of
Liquidambar (Hamamelidaceae). – Amer. J.
Bot. 78: 938-947.
Hoey MT, Parks CR. 1994. Genetic divergence
in Liquidambar styraciflua, L. formosana, and L.
acalycina (Hamamelidaceae). – Syst. Bot.
19: 308-316.
Holderegger R. 1996. Reproduction of the rare
monocarpic species Saxifraga mutata L. – Bot. J. Linn. Soc. 122:
301-313.
Holderegger R, Abbott RJ. 2003.
Phylogeography of the arctic-alpine Saxifraga oppositifolia (Saxifragaceae) and some related
taxa based on cpDNA and ITS sequence variation. – Amer. J. Bot. 90:
931-936.
Holle G. 1893. Beiträge zur Anatomie der
Saxifragaceen und deren systematische Verwerthung. – Bot. Centralbl. 53: 1-9,
33-41, 65-70, 97-102, 129-136, 161-169, 209-222.
Hong D-Y. 1989. Studies on the genus
Paeonia 2. The characters of leaf epidermis and their systematic
significance. – Chinese J. Bot. 1: 145-154.
Hong D-Y, Zhou S-L. 2003. Paeonia
(Paeoniaceae) in the Caucasus.
– Bot. J. Linn. Soc. 143: 135-150.
Hong D-Y, Zhang Z-X, Zhu X-Y. 1988. Studies
on the genus Paeonia 1. Report of karyotypes of some wild species in
China. – Acta Phytotaxon. Sin. 26: 33-43. [In Chinese]
Hong D-Y, Pan K-Y, Rao G-Y. 2001.
Cytogeography and taxonomy of the Paeonia obovata polyploid complex
(Paeoniaceae). – Plant Syst.
Evol. 227: 123-136.
Hong D-Y, Wang X-Q, Zhang D-M. 2004.
Paeonia saueri (Paeoniaceae), a new species from the
Balkans. – Taxon 53: 83-90.
Hong D-Y, Zhang D-M, Wang X-Q, Koruklu ST,
Tzanoudakis D. 2008. Relationships and taxonomy of Paeonia arietina G.
Anderson complex (Paeoniaceae)
and its allies. – Taxon 57: 922-932.
Horne AL. 1914. A contribution to the study
of the evolution of the flower, with special reference to the Hamamelidaceae, Caprifoliaceae,
and Cornaceae. –
Trans. Linn. Soc. London, Bot., 2nd ser., 8: 239-309.
Huang G-L. 1986. Comparative anatomical
studies on the woods of Hamamelidaceae in China. – Acta
Sci. Nat. Univ. Sunyatsenia 1986, 1: 22-28. [In Chinese with English
summary]
Huang G-L, Lee C-L. 1982. Anatomical studies
of Chunia wood. – Acta Bot. Sin. 24: 506-511. [In Chinese]
Huang T-C. 1965. Monograph of
Daphniphyllum, I. – Taiwania 11: 57-98.
Huang T-C. 1966. Monograph of
Daphniphyllum, II. – Taiwania 12: 137-234.
Huang T-C. 1996. Notes on taxonomy and pollen
of Malesian Daphniphyllum (Daphniphyllaceae). – Blumea
41: 231-244.
Huang T-C. 1997. Daphniphyllaceae. – In:
Kalkman C et al. (eds), Flora Malesiana, I, 13, Flora Malesiana Foundation,
Rijksherbarium/Hortus Botanicus, Leiden, pp. 145-168.
Hufford L, Crane PR. 1989. A preliminary
phylogenetic analysis of the ‘lower’ Hamamelidae. – In: Crane PR,
Blackmore S (eds), Evolution, systematics and fossil history of the Hamamelidae
1: Introduction and ‘lower’ Hamamelidae, Syst. Assoc. Spec. Vol. 40A,
Clarendon Press, Oxford, pp. 175-192.
Ickert-Bond SM, Wen J. 2006. Phylogeny and
biogeography of Altingiaceae:
evidence from combined analysis of five non-coding chloroplast regions. –
Mol. Phylogen. Evol. 39: 512-528.
Ickert-Bond SM, Pigg KB, Wen J. 2005.
Comparative infructescence morphology in Liquidambar (Altingiaceae) and its evolutionary
significance. – Amer. J. Bot. 92: 1234-1255.
Ickert-Bond SM, Pigg KB, Wen J. 2007.
Comparative infructescence morphology in Altingia (Altingiaceae) and discordance
between morphological and molecular phylogenies. – Amer. J. Bot. 94:
1094-1115.
Ignat’eva IP. 1995. Ontogenetic
morphogenesis in vegetative organs of peony (Paeonia anomala L.). –
Izvestiya TCXA 4: 108-134. [In Russian]
Ikeda H, Itoh K. 2001. Germination and water
dispersal of seeds from a threatened plant species, Penthorum
chinense. – Ecol. Res. 16: 99-106.
Jaarsveld EJ van. 1994. The distribution of
Tylecodon and Cotyledon (Crassulaceae) in South Africa and
Namibia. – In: Seyani JH, Chikuni AC (eds), Proc. XIII Plenary Meeting
AETFAT, Malawi, pp. 1157-1163.
Jacobsen V. 1954. Handbuch der sukkulenten
Pflanzen 2. – Gustav Fischer, Jena.
Jäger-Zúrn I. 1989. Zur Kenntnis von
Crassula pageae Tölken (syn. Pagella archeri). –
Trop.-Subtrop. Pflanzenwelt 70: 1-71
Jähnichen H, Mai DH, Walther H. 1980.
Blätter und Früchte von Cercidiphyllum Siebold et Zuccarini im
mitteleuropäischen Tertiär. – Schriftenr. Geol. Wiss. 16: 357-399.
Janczewski E de. 1906a. Species generis
Ribes L. II. Subgenera Ribesia et Coreosma. –
Bull. Int. Acad. Polon. Sci., Cl. Sci. Math., Sér. B, Sci. Nat. 1906: 1-13.
Janczewski E de. 1906b. Species generis
Ribes L. III. Subgenera Grossularioides,
Grossularia, et Berisia. – Bull. Int. Acad. Polon. Sci.,
Cl. Sci. Math., Sér. B, Sci. Nat. 1906: 280-293.
Janczewski E de. 1907. Monographie des
groseilliers, Ribes L. – Mém. Soc. Phys. Genève 35: 199-517.
Jay M. 1967. Recherches chimiotaxinomiques
sur les plantes vasculaires 1. Distribution des flavonoides chez les
Saxifragacées. – Compt. Rend. Acad. Sci. Paris 264: 1754-1756.
Jay M. 1968. Distribution des flavonoides
chez les Hamamelidacées et familles affines. – Taxon 17: 136-147.
Jay M. 1969. Contribution biochimique à la
connaissance taxonomique et phylogénetique des Saxifragacées et familles
affinés. – Ph.D. diss., l’Université de Lyon, France.
Jay M. 1971. Quelques problèmes taxinomiques
et phylogénétiques des Saxifragacées vus à la lumière de la biochimie
flavonique. – Bull. Mus. Natl. Hist. Nat. Paris, sér. II, 42: 754-775.
Jay M, Voirin B. 1976. Les flavonoides de
deux espèces du genre Chrysosplenium. – Phytochemistry 15:
517-519.
Jensen LCW. 1966. Comparative anatomical
studies in three subfamilies of the Crassulaceae. – Ph.D. diss.,
University of Minnesota, Minneapolis, Minnesota.
Jensen LCW. 1968. Primary stem vascular
patterns in three subfamilies of the Crassulaceae. – Amer. J. Bot. 55:
553-563.
Jha UN. 1978. Chemotaxonomy of the Hamamelidaceae. – J. Indian
Bot. Soc. 56: 44-48.
Jian S, Soltis PS, Gitzendanner MA, Moore MJ,
Li R, Hendry TA, Qiu Y-L, Dhingra A, Bell C, Soltis DE. 2008. Resolving an
ancient, rapid radiation in Saxifragales. – Syst. Biol. 57:
38-57.
Johnson AM. 1923. A revision of the North
American species of the section Boraphila Engler of the genus
Saxifraga (Tourn.) L. – Univ. Minnesota Stud. Biol. Sci. 4:
1-109.
Johnson AM. 1927. The status of Saxifraga
nuttallii. – Amer. J. Bot. 14: 38-43.
Johnson LA, Soltis DE. 1994. matK
DNA sequences and phylogenetic reconstruction in Saxifragaceae s. str. – Syst.
Bot. 19: 143-156.
Johnson LA, Soltis DE. 1995. Phylogenetic
inference in Saxifragaceae
sensu stricto and Gilia (Polemoniaceae)
using matK sequences. – Ann. Missouri Bot. Gard. 82: 149-175.
Johnson SD, Ellis A, Carrick P, Swift P,
Horner N, Janse van Rensburg S, Bond WJ. 1993. Moth pollination and rhythms of
advertisement and reward in Crassula fascicularis (Crassulaceae). – South Afr. J.
Bot. 59: 511-513.
Jørgensen MH, Elven R, Tribsch A,
Gabrielsen, Stedje B, Brochmann C. 2006. Taxonomy and evolutionary
relationships in the Saxifraga rivularis complex. – Syst. Bot. 31:
702-729.
Jørgensen TH, Frydenberg J. 1999.
Diversification in insular plants; inferring the phylogenetic relationship in
Aeonium (Crassulaceae)
using ITS sequences of nuclear ribosomal DNA. – Nord. J. Bot. 19: 613-621.
Jørgensen TH, Olesen JM. 2000. Growth rules
based on the modularity of the Canarian Aeonium (Crassulaceae) and their
phylogenetic value. – Bot. J. Linn. Soc. 132: 223-240.
Jørgensen TH, Olesen JM. 2001. Adaptive
radiation of island plants: evidence from Aeonium (Crassulaceae) of the Canary
Islands. – Perspect. Plant Ecol. Evol. Syst. 4: 29-42.
Judd WS. Soltis DE, Soltis PS, Ionta G. 2007.
Tolmiea diplomenziesii: a new species from the Pacific Northwest and
the diploid sister taxon of the autotetraploid T. menziesii (Saxifragaceae). – Brittonia 59:
217-225.
Juel HO. 1902. Entwicklungsgeschichte des
Samens von Cynomorium. – Beih. Bot. Centralbl. 13: 194-202.
Juel HO. 1907. Studien über die
Entwicklungsgeschichte von Saxifraga granulata. – Nova Acta Reg.
Soc. Sci. Upsal. 4(1): 1-41.
Juel HO. 1910. Cynomorium und
Hippuris. – Svensk Bot. Tidskr. 4: 151-159.
Jürgens N. 1995. Contributions to the
phytogeography of Crassula. – In: Hart H ‘t, Eggli U (eds),
Evolution and systematics of the Crassulaceae, Backhuys, Leiden, pp.
136-150.
Kapil RN, Bawa SB. 1968. Embryological
studies on the Haloragidaceae I. Haloragis colensoi Skottsb. – Bot.
Not. 121: 11-28.
Kapil RN, Kaul U. 1974. Embryologically
little known taxon – Parrotiopsis jacquemontiana. –
Phytomorphology 22: 234-245.
Kaplan K. 1981. Embryologische, pollen- und
samenmorphologische Untersuchungen zur Systematik von Saxifraga (Saxifragaceae). – Bibl. Bot.
134: 1-54.
Kaplan K, Strohschneider M. 1984.
Mikromorphologische Untersuchungen an Samenoberflächen in der Gattung
Chrysosplenium (Saxifragaceae). – Bot. Jahrb.
Syst. 104: 469-482.
Kaul U, Kapil RN. 1974. Exbucklandia
populnea: from flower to fruit. – Phytomorphology 24: 217-228.
Keefe JM, Moseley MF Jr. 1978. Wood anatomy
and phylogeny of Paeonia section Moutan. – J. Arnold Arbor.
59: 274-297.
Keep E. 1962. Interspecific hybridization in
Ribes. – Genetica 33: 1-23.
Keep E. 1975. Currants and gooseberries. –
In: Janick J, Moore JN (eds), Advances in fruit breeding, Purdue University
Press, West Lafayette Indian, pp. 197-268.
Kern P. 1966. The genus Tiarella in
western North America. – Madroño 18: 152-160.
Kim J-H. 1994. Pollen morphology of genus
Sedum in Korea. – J. Plant Biol. 37: 245-252.
Kim J-H, Hart H ‘t, Mes THM. 1996. The
phylogenetic position of East Asian Sedum species (Crassulaceae) based on chloroplast
DNA trnL (UAA) – trnF (GAA) intergenic spacer sequence
variation. – Acta Bot. Neerl. 45: 309-321.
Kimnach M. 1978. Sedum suaveolens, a
remarkable new species from Durango, Mexico. – Cact. Succ. J. 50: 3-7.
Klopfer K. 1968. Beiträge zur floralen
Morphogenese und Histogenese der Saxifragaceae 1. Die
Infloreszenz-Entwicklung von Tellima grandiflora. – Flora 157:
461-476.
Klopfer K. 1968. Beiträge zur floralen
Morphogenese und Histogenese der Saxifragaceae 2. Die
Blütenentwicklung von Tellima grandiflora. – Flora 158: 1-21.
Klopfer K. 1970. Beiträge zur floralen
Morphogenese und Histogenese der Saxifragaceae 4. Die
Blütenentwicklung einiger Saxifraga-Arten. – Flora 159: 347-365.
Klopfer K. 1973. Florale Morphogenese und
Taxonomie der Saxifragaceae
sensu lato. – Feddes Repert. 84: 475-516.
Kluge M, Brulfert J. 1996. Crassulacean acid
metabolism in the genus Kalanchoë: ecological, physiological and
biochemical aspects. – In: Winter K, Smith AP, Smith JAC (eds), Crassulacean
acid metabolism. Biochemistry, ecophysiology and evolution, Springer, Berlin,
Heidelberg, New York, pp. 324-335.
Knaben G. 1954. Saxifraga osloensis
n. sp., a tetraploid species of the Tridactylites section. – Nytt
Mag. Bot. 3: 117-138.
Knapp U. 1994. Skulptur der Samenschale und
Gliederung der Crassulaceae. –
Bot. Jahrb. Syst. 116: 157-187.
Knapp U. 1997. Samenoberfläche und
Systematik der Saxifragaceae
und Crassulaceae. – Ph.D.
diss., Universität Kaiserslautern, Germany.
Köhlein F. 1980. Saxifragen und andere
Steinbrechgewächse. – Eugen Ulmer GmbH, Stuttgart.
Kozieradzka-Kiszkurno M, Plachno BJ,
Bohdanowicz J. 2011. Are unusual plasmodesmata in the embryo-suspensor
restricted to species from the genus Sedum among Crassulaceae? – Flora 206:
684-690.
Kozyrenko MM, Gontcharova SB, Gontcharov AA.
2013. Phylogenetic relationships among Orostachys subsection
Orostachys species (Crassulaceae) based on nuclear and
chloroplast DNA data. – J. Syst. Evol. 51: 578-589.
Krach JE. 1976. Samenanatomie der Rosifloren
I. Die Samen der Saxifragaceae.
– Bot. Jahrb. Syst. 97: 1-60.
Krach JE. 1977. Seed characters in and
affinities among the Saxifragineae. – In: Kubitzki K (ed), Flowering plants
– Evolution and classification at higher categories, Plant Syst. Evol.
[Suppl.] 1: 141-153.
Kubitzki K. 2006a. Aphanopetalaceae. – In:
Kubitzki K (ed), The families and genera of vascular plants IX. Flowering
plants. Eudicots. Berberidopsidales,
Buxales, Crossosomatales, Fabales p. p., Geraniales, Gunnerales, Myrtales p. p., Proteales, Saxifragales, Vitales, Zygophyllales,
Clusiaceae
Alliance, Passifloraceae
Alliance, Dilleniaceae, Huaceae, Picramniaceae,
Sabiaceae, Springer, Berlin,
Heidelberg, New York, pp. 29-30.
Kubitzki K. 2006b. Daphniphyllaceae. – In:
Kubitzki K (ed), The families and genera of vascular plants IX. Flowering
plants. Eudicots. Berberidopsidales,
Buxales, Crossosomatales, Fabales p. p., Geraniales, Gunnerales, Myrtales p. p., Proteales, Saxifragales, Vitales, Zygophyllales,
Clusiaceae
Alliance, Passifloraceae
Alliance, Dilleniaceae, Huaceae, Picramniaceae,
Sabiaceae, Springer, Berlin,
Heidelberg, New York, pp. 127-128.
Kubitzki K. 2006c. Haloragaceae. – In: Kubitzki K
(ed), The families and genera of vascular plants IX. Flowering plants.
Eudicots. Berberidopsidales,
Buxales, Crossosomatales, Fabales p. p., Geraniales, Gunnerales, Myrtales p. p., Proteales, Saxifragales, Vitales, Zygophyllales,
Clusiaceae
Alliance, Passifloraceae
Alliance, Dilleniaceae, Huaceae, Picramniaceae,
Sabiaceae, Springer, Berlin,
Heidelberg, New York, pp. 184-190.
Kubitzki K. 2006d. Iteaceae. – In: Kubitzki K (ed), The
families and genera of vascular plants IX. Flowering plants. Eudicots. Berberidopsidales,
Buxales, Crossosomatales, Fabales p. p., Geraniales, Gunnerales, Myrtales p. p., Proteales, Saxifragales, Vitales, Zygophyllales,
Clusiaceae
Alliance, Passifloraceae
Alliance, Dilleniaceae, Huaceae, Picramniaceae,
Sabiaceae, Springer, Berlin,
Heidelberg, New York, pp. 202-204.
Kubitzki K. 2006e. Pterostemonaceae. – In:
Kubitzki K (ed), The families and genera of vascular plants IX. Flowering
plants. Eudicots. Berberidopsidales,
Buxales, Crossosomatales, Fabales p. p., Geraniales, Gunnerales, Myrtales p. p., Proteales, Saxifragales, Vitales, Zygophyllales,
Clusiaceae
Alliance, Passifloraceae
Alliance, Dilleniaceae, Huaceae, Picramniaceae,
Sabiaceae, Springer, Berlin,
Heidelberg, New York, pp. 405-406.
Kubitzki K. 2006f. Tetracarpaeaceae. – In:
Kubitzki K (ed), The families and genera of vascular plants IX. Flowering
plants. Eudicots. Berberidopsidales,
Buxales, Crossosomatales, Fabales p. p., Geraniales, Gunnerales, Myrtales p. p., Proteales, Saxifragales, Vitales, Zygophyllales,
Clusiaceae
Alliance, Passifloraceae
Alliance, Dilleniaceae, Huaceae, Picramniaceae,
Sabiaceae, Springer, Berlin,
Heidelberg, New York, pp. 456-457.
Kuhlmann JG. 1947. Peridiscaceae (Kuhlmann). – Arq.
Serv. Florestal 3: 3-5.
Kumazawa M. 1935. The structure and
affinities of Paeonia. – Bot. Mag. (Tokyo) 49: 306-315. [In
Japanese]
Küpfer P, Rais JR. 1983. Index des nombres
chromosomiques de spermatophytes de la Suisse I. Saxifragaceae. – Bot. Helvetica
93: 11-25.
Kurita M. 1959. Chromosome studies in Ranunculaceae XII.
Satellites on the Paeonia chromosomes. – Rep. Biol. Inst. Ehime
Univ. 8: 7-11.
Kurkin VA, Zapesochnaya GG. 1986. The
chemical composition and pharmacological properties of Rhodiola
plants. – Khim-farm. Žurn. 20: 1231-1244. [In Russian]
Kuzoff RK, Soltis DE, Hufford L, Soltis PS.
2000. Phylogenetic relationships within Lithophragma (Saxifragaceae): hybridization,
allopolyploidy, and ovary diversification. – Syst. Bot. 24: 598-615.
Kuzoff RK, Hufford L, Soltis DE. 2001.
Structural homology and developmental transformations associated with ovary
diversification in Lithophragma (Saxifragaceae). – Amer. J. Bot.
88: 196-205.
Lakela O. 1937. A monograph of the genus
Tiarella L. in North America. – Amer. J. Bot. 24: 344-351.
Leinfellner W. 1954. Beiträge zur
Kronblattmorphologie III. Die Kronblätter der Gattung Pachyphytum.
– Österr. Bot. Zeitschr. 101: 586-591.
Le Lous J, Majoie B, Morinière JL, Wulfert
E. 1975. Study of the flavonoids of Ribes nigrum. – Ann. Pharmaceut.
Franç. 33: 393-399.
Lems K. 1960. Botanical notes on the Canary
Islands II. The evolution of plant forms in the islands: Aeonium. –
Ecology 41: 1-17.
Lercker G, Cocchi M, Turchetto E. 1988.
Ribes nigrum seed oil. – Riv. Ital. Sost. Grasse 65(1): 1-6.
Leroy JE. 1980. Développement et
organogenèse chez le Cercidiphyllum japonicum: un cas semblant unique
chez les Angiospermes. – Compt. Rend. Acad. Sci. Paris., sér. D, 290:
679-682.
Levin GA, Mulroy TW. 1985. Floral morphology,
nectar production, and breeding systems in Dudleya subgenus
Dudleya (Crassulaceae).
– Trans. San Diego Soc. Nat. Hist. 21: 57-70.
Li H-M, Hickey LJ. 1988. Leaf architecture
and systematics of the Hamamelidaceae sensu lato. –
Acta Phytotaxon. Sin. 26: 96-110. [In Chinese]
Li J-H. 1997. Systematics of the Hamamelidaceae based on
morphological and molecular evidence. – Ph.D. diss., University of New
Hampshire, Durham, New Hampshire.
Li J-H, Bogle AL. 2001. A new suprageneric
classification system of Hamamelidoideae based on morphology and sequences of
nuclear and chloroplast DNA. – Harvard Pap. Bot. 5: 499-515.
Li J-H, Donoghue MJ. 1999. More molecular
evidence for interspecific relationships in Liquidambar (Hamamelidaceae). – Rhodora 101:
87-91.
Li J-H, Bogle AL, Klein AS, Pan KY. 1997.
Close relationship between Shaniodendron and Parrotia (Hamamelidaceae), evidence from
ITS sequences of nuclear ribosomal DNA. – Acta Phytotaxon. Sin. 35:
481-493.
Li J-H, Bogle AL, Klein AS. 1998.
Phylogenetic relationships in the Corylopsis complex (Hamamelidaceae) based on
sequences of the internal transcribed spacers of nuclear ribosomal DNA and
morphology. – Rhodora 99: 302-318.
Li J-H, Bogle AL, Klein AS, Donoghue MJ.
2000. Phylogeny and biogeography of Hamamelis (Hamamelidaceae). – Harvard Pap.
Bot. 5: 171-178.
Li J-M, Hickey LJ. 1988. Leaf architecture
and systematics of the Hamamelidaceae sensu lato. –
Acta Phytotaxon. Sin. 27: 96-110.
Li J, Bogle AL. 2001. A new suprageneric
classification system of Hamamelidoideae based on morphology and sequences of
nuclear and chloroplast DNA. – Harvard Pap. Bot. 5: 499-515.
Li J, Bogle AL, Klein AS. 1999a. Phylogenetic
relationships of the Hamamelidaceae inferred from
sequences of internal transcribed spacers (ITS) of nuclear ribosomal DNA. –
Amer. J. Bot. 86: 1027-1037.
Li J, Bogle AL, Klein AS. 1999b. Phylogenetic
relationships of the Hamamelidaceae: evidence from the
nucleotide sequences of the plastid gene matK. – Plant Syst. Evol.
218: 205-219.
Liu H-Y. 1989. Systematics of
Aeonium (Crassulaceae).
– National Museum of Natural Science, Taichung, Taiwan, Spec. Publ. No. 3:
1-102.
Lösch R. 1990. Funktionelle Voraussetzungen
der adaptiven Nischenbesetzung in der Evolution der makaronesischen
Semperviven. – Diss. Bot. 146: 1-482.
Lozano-Contreras G. 1996. Hallazgo de la
familia Hamamelidaceae en
suramérica y descripción de una nueva especie de Matudaea de
Colombia. – Rev. Acad. Colombiana Ci. Exact. Físic. Natur. 20: 443-445.
Lu Y-R, Foo L-Y, Wong H. 2002.
Nigrumin-5-p-coumarate and nigrumin-5-ferulate, two unusial nitrile-containing
metabolites from black currant (Ribes nigrum) seed. – Phytochemistry
59: 465-468.
Luizet D. 1913. Classification naturelle des
saxifrages de la section Dactyloides Tausch. – Rev. Gén. Bot. 25:
273-284.
Magallón SA. 2007. From fossils to
molecules: phylogeny and the core eudicot floral groundplan in Hamamelidoideae
(Hamamelidaceae, Saxifragales). – Syst. Bot. 32:
317-347.
Magallón SA, Crane PR, Herendeen PS. 1999.
Phylogenetic pattern, diversity, and diversification of eudicots. – Ann.
Missouri Bot.Gard. 86: 297-372.
Magallón SA, Herendeen PS, Crane PR. 2001.
Androdecidua endressii gen. et sp. nov. from the Late Cretaceous of
Georgia (United States): further floral diversity in Hamamelidoideae (Hamamelidaceae). – Intern. J.
Plant Sci. 162: 963-983.
Magallón-Puebla S, Herendeen PS, Endress PK.
1996. Allonia decandra: flora remains of the tribe Hamamelideae (Hamamelidaceae) from Campanian
strata of Southeastern USA. – Plant Syst. Evol. 202: 177-198.
Mai DH. 1985. Beiträge zur Geschichte
einiger holziger Saxifragales-Gattungen. –
Gleditschia 13: 75-88.
Mai DH. 2001. The fossils of
Rhodoleia champion (Hamamelidaceae) in Europe. –
Acta Palaeobot. 41: 161-175.
Manheim BS Jr, Mulroy TW, Hogness DK, Kerwin
JL. 1979. Interspecific variation in leaf wax of Dudleya. – Biochem.
Syst. Ecol. 7: 17-19.
Manos PS, Steele KP. 1997. Phylogenetic
analyses of ”higher” Hamamelididae based on plastid sequence data. –
Amer. J. Bot. 84: 1407-1419.
Manos PS, Nixon KC, Doyle JJ. 1993. Cladistic
analysis of restriction site variation within the chloroplast DNA inverted
repeat region of selected Hamamelididae. – Syst. Bot. 18: 551-562.
Marchant TA, Alarcon R, Simonsen JA,
Koopowitz H. 1998. Population ecology of Dudleya multicaulis (Crassulaceae): a rare narrow
endemic. – Madroño 45: 215-220.
Martin CE, Willert DJ von. 2000. Leaf
epidermal hydathodes and the ecophysiological consequences of foliar water
uptake in species of Crassula from the Namib desert in Southern
Africa. – Plant Biol. 2: 229-242.
Maslova NP. 2003. Extinct and extant Platanaceae and Hamamelidaceae: morphology,
systematics, and phylogeny. – Paleontol. J. 37(Suppl.) 5: S467-S590.
Maslova NP, Golovneva LB. 2000a. A hamamelid
inflorescence with in situ pollen grains from the Cenomanian of
eastern Siberia. – Paleontol. J. 34(Suppl.) 1: S40-S49.
Maslova NP, Golovneva LB. 2000b.
Lindacarpa gen. nov., a new hamamelid fructification from the Upper
Cretaceous of eastern Siberia. – Paleontol. J. 34: 462-468.
Maslova NP, Herman A. 2004. New finds of
fossil hamamelids and data on the phylogenetic relationships between the Platanaceae and Hamamelidaceae. – Paleontol. J.
38: 563-575.
Maslova NP, Golovneva LB, Tekleva MV. 2005.
Infructescences of Kasicarpa gen. nov. (Hamamelidales) from the Late
Cretaceous (Turonian) of the Chulym-Enisey Depression, Western Siberia, Russia.
– Acta Palaeobot. 45: 121-137.
Matthew CJ. 1980. Embryological studies in Hamamelidaceae: development of
female gametophyte and embryogeny in Hamamelis virginiana. –
Phytomorphology 30: 172-180.
Matthiessen A. 1962. A contribution to the
embryology of Paeonia. – Acta Horti Berg. 20: 57-61.
Mauritzon J. 1930. Beitrag zur Embryologie
der Crassulaceen. – Bot. Not. 1930: 233-250.
Mauritzon J. 1933. Studien über Embryologie
der Familien Crassulaceae und Saxifragaceae. – Ph.D. diss,
University of Lund, Sweden.
Mayuzumi S, Ohba H. 2004. The phylogenetic
position of eastern Asian Sedoideae (Crassulaceae) inferred from
chloroplast and nuclear DNA sequences. – Syst. Bot. 29. 587-598.
Meijden R van der, Caspers N. 1971. Haloragaceae. – In: Steenis CGGJ
van (ed), Flora Malesiana I, 7, Wolters-Noordhoff, Groningen, pp. 239-263.
Melikian AP. 1973. Seed-coat types of Hamamelidaceae and allied
families in relation to their systematics. – Bot. Žurn. 58: 350-359. [In
Russian]
Melville R. 1983. The affinity of
Paeonia and a second genus of Paeoniaceae. – Kew Bull. 38:
87-105.
Mendes EJ. 1978. 71. Haloragaceae. – In: Launert E
(ed), Flora Zambesiaca 4, Flora Zambesiaca Managing Committee, London, pp.
74-81.
Mendes EJ, Vidigal MP. 1978. 70. Hamamelidaceae. – In: Launert E
(ed), Flora Zambesiaca 4, Flora Zambesiaca Managing Committee, London, pp.
71-73.
Merxmüller H, Friedrich H-C, Grau J. 1971.
Cytotaxonomische Untersuchungen zur Gattungsstruktur von Crassula. –
Ann. Naturh. Mus. Wien 75: 111-119.
Mes THM. 1995. Phylogenetic and systematic
implications of chloroplast and nuclear spacer sequence variation in the
Macaronesian Sempervivoideae and related Sedoideae (Crassulaceae). – In: Hart H ‘t,
Eggli U (eds), Evolution and systematics of the Crassulaceae, Backhuys Publ.,
Leiden, The Netherlands, pp. 30-44.
Mes THM. 1996. Origin and evolution of the
Macaronesian Sempervivoideae (Crassulaceae). – Ph.D. diss.,
Universeit Utrecht, The Netherlands.
Mes THM, Hart H ‘t. 1994. Sedum
surculosum and S. jaccardianum (Crassulaceae) share a unique 70 bp
deletion in the chloroplast DNA trnL(UAA)-trnF(GAA)
intergenic spacer. – Plant Syst. Evol. 193: 213-221.
Mes THM, Hart H ‘t. 1996. The evolution of
growth-forms in the Macaronesian genus Aeonium (Crassulaceae) inferred from
chloroplast RFLPs and morphology. – Mol. Ecol. 5: 351-363.
Mes THM, Brederode J van, Hart H ’t. 1996.
Origin of the woody Macaronesian Sempervivoideae and the phylogenetic position
of the East African species of Aeonium. – Bot. Acta 109: 477-491.
Mes THM, Wiejers GJ, Hart H ‘t. 1997.
Phylogenetic relationships in Monanthes (Crassulaceae) based on
morphological, chloroplast and nuclear DNA variation. – J. Evol. Biol. 10:
193-216.
Mesler MR, Cole JC, Wilson P. 1991. Natural
hybridization in western gooseberries (Ribes subg.
Grossularia, Grossulariaceae). – Madroño
38: 115-129.
Messinger W, Liston A, Hummer K. 1993.
Restriction site mapping of Ribes nuclear ribosomal DNA. – Acta
Hort. 352: 175-184.
Messinger W, Hummer K, Liston A. 1999.
Ribes (Grossulariaceae) phylogeny as
indicated by restriction-site polymorphisms of PCR-amplified chloroplast DNA.
– Plant Syst. Evol. 217: 185-195.
Metcalfe CR. 1952. Medusandra
richardsiana Brenan: anatomy of the leaf, stem, and wood. – Kew Bull. 7:
237-244.
Metcalfe CR. 1962. Notes on the systematic
anatomy of Whittonia and Peridiscus. – Kew Bull. 15:
472-475.
Meurman O. 1928. Cytological studies in the
genus Ribes. – Hereditas 11: 289-356.
Meyrán J, López L. 2003. Las Crasuláceas
de México. – Soc. Mexic. Cactol., A.C. México, D.F., pp. 1-234.
Mildbraed J. 1954. Die Schausamen von
Paeonia corallina Retz. – Ber. Deutsch. Bot. Ges. 67: 73-74.
Miller JM, Bohm BA. 1979. Flavonoids of
Leptarrhena pyrolifolia. – Phytochemistry 18: 1412-1413.
Miller JM, Bohm BA. 1980. Flavonoid variation
in some North American Saxifraga species. – Biochem. Syst. Ecol. 8:
279-289.
Miller RB. 1975. Systematic anatomy of the
xylem and comments on the relationships of Flacourtiaceae. – J. Arnold Arbor.
56: 20-102.
Mione T, Bogle AL. 1987. Comparative ontogeny
of the flowers of Hamamelis virginiana and Loropetalum
chinense (Hamamelidaceae). – Amer. J.
Bot. 74: 620-621.
Mione T, Bogle AL. 1990. Comparative ontogeny
of the inflorescence and flower of Hamamelis virginiana and
Loropetalum chinense (Hamamelidaceae). – Amer. J.
Bot. 77: 77-91.
Mizushima M. 1968. On the flower of
Disanthus cercidifolius Maxim. – J. Jap. Bot. 43: 522-544.
Mohana Rao PR. 1974. Seed anatomy in some Hamamelidaceae and phylogeny. –
Phytomorphology 24: 113-139.
Mohana Rao PR. 1986. Seed and fruit anatomy
in Cercidiphyllum japonicum with a discussion on the affinities of Cercidiphyllaceae. – Flora
178: 243-249.
Moody ML. 2004. Systematics of the angiosperm
family Haloragaceae R. Br.
emphasizing the aquatic genus Myriophyllum: phylogeny, hybridization
and character evolution. – Ph.D. diss., University of Connecticut, Storrs,
Connecticut.
Moody ML, Les DH. 2007. Phylogenetic
systematics and character evolution in the angiosperm family Haloragaceae. – Amer. J. Bot. 94:
2005-2025.
Moody ML, Les DH. 2010. Systematics of the
aquatic angiosperm genus Myriophyllum (Haloragaceae). – Syst. Bot. 35:
121-139.
Moore DM. 1969. Saxifragodes, a new
genus of Saxifragaceae from
Tierra del Fuego. – Bot. Not. 122: 322-329.
Moran RV. 1942a. Delimitation of genera and
subfamilies in the Crassulaceae.
– Desert Plant Life 14: 125-128.
Moran RV. 1942b. The status of
Dudleya and Stylophylum. – Desert Plant Life 14:
149-157.
Moran RV. 1949. Graptopetalum
bartramii in Chihuahua. – Desert Plant Life 21: 53-56.
Moran RV. 1966. The section
Centripetalia of Sedum. – Cact. Succ. J. 38: 75-81.
Moran RV. 1967. Echeveria procera, a
new species from Oaxaca, Mexico. – Cact. Succ. J. 39: 182-185.
Moran RV. 1978. Resurrection of
Cremnophila. – Cact. Succ. J. 50: 139-146.
Moran RV. 1992. Thompsonella Britton
& Rose (Crassulaceae) with
T. colliculosa, a new species. – Cact. Succ. J. 64: 37-44.
Moran RV. 1994. The genus
Lenophyllum. – Haseltonia 2: 1-19.
Moran RV. 1996. Altamiranoa into
Sedum (Crassulaceae).
– Haseltonia 4: 46.
Moran RV. 2000. Circumscission in
Cotyledon: with thoughts on what is Cotyledon, and on how A.
P. de Candolle was right all along and they should have listened. – Cact.
Succ. J. 72: 306-308.
Moran RV, Meyrán J. 1974. Tacitus
bellus, un nuevo género y especie de Crassulaceae de Chihuahua, México.
– Cact. Succ. Mex. 19: 75-84.
Moreau F. 1971. Apport des caractères
stomatiques à la taxinomie et à la phylogénie des Saxifragées. – Bull.
Soc. Bot. France 118: 381-427.
Moreau F. 1976. Types stomatiques et trichome
foliaire des Saxifragoïdées (Saxifragacées). – Rev. Cytol. Biol. Vég. 39:
329-341.
Moreau F. 1984. Contribution
phytodermatologique à la systématique des Saxifragacées sensu stricto et des
Crassulacées. – Rev. Cytol. Biol. Vég. 7: 31-92.
Morf E. 1950. Vergleichend-morphologische
Untersuchungen am Gynoeceum der Saxifragaceen. – Ber. Schweiz. Bot. Ges. 60:
516-590.
Morgan DR, Soltis DE. 1993. Phylogenetic
relationships among members of Saxifragaceae sensu lato based on
rbcL sequence data. – Ann. Missouri Bot. Gard. 80: 631-660.
Mort ME, Mori SA. 2004. Crassulaceae. – In: Smith N, Mori
SA, Henderson A, Stevenson DW, Heald SV (eds), Flowering plants of the
Neotropics, Princeton University Press, New York, pp. 118-120.
Mort ME, Soltis DE. 1999. Phylogenetic
relationships and the evolution of ovary position in Saxifraga section
Micranthes. – Syst. Bot. 24: 139-147.
Mort ME, Soltis DE, Soltis PS,
Francisco-Ortega J, Santos-Guerra A. 2001. Phylogenetic relationships and
evolution of Crassulaceae
inferred from matK sequence data. – Amer. J. Bot. 88: 76-91.
Mort ME, Soltis DE, Soltis PS,
Francisco-Ortega J, Santos-Guerra A. 2002. Phylogenetics and evolution of the
Macaronesian clade of Crassulaceae inferred from nuclear
and chloroplast sequence data. – Syst. Bot. 27: 271-288.
Mort ME, Levsen N, Randle CP, Jaarsveld E
van, Palmer A. 2005. Phylogenetics and diversification of Cotyledon
(Crassulaceae) inferred from
nuclear and chloroplast DNA sequence data. – Amer. J. Bot. 92: 1170-1176.
Mort ME, Soltis DE, Soltis PS, Santos-Guerra
A, Francisco-Ortega J. 2007. Physiological evolution and association between
physiology and growth form in Aeonium (Crassulaceae). – Taxon 56:
453-464.
Mort ME, Randle CP, Burgoyne P, Smith G,
Jaarsveld E, Hopper SD. 2009. Analyses of cpDNA matK sequence data
place Tillaea (Crassulaceae) within
Crassula. – Plant Syst. Evol. 283: 211-217.
Mort ME, O’Leary TR, Carillo-Reyes P,
Nowell T, Archibald JK, Randle CP. 2010. Phylogeny and evolution of Crassulaceae: past, present and
future. – Schumannia 6: 69-86.
Moskov IV. 1964. On the development of the
embryo in some species of Paeonia L. – Bot. Žurn. 49: 887-894. [In
Russian]
Moteetee A, Nagendran CR. 1997. Comparative
anatomical studies in five southern African species of Crassula I.
Structure of the stem and the root. – South Afr. J. Bot. 63: 90-94.
Mu X-J, Wang F-X. 1985. The early development
of embryo and endosperm of Paeonia lactiflora. – Acta Bot. Sin. 87:
7-12. [In Chinese]
Murgai P. 1959. The development of the embryo
in Paeonia – a reinvestigation. – Phytomorphology 9: 275-277.
Nagaraj M, Nijalingappa BHM. 1967.
Embryological studies in Myriophyllum intermedium DC. – Proc. Indian
Acad. Sci., Sect. B, 65: 210-220.
Nagaraj M, Nijalingappa BHM. 1974.
Embryological studies in Laurembergia hirsuta. – Bot. Gaz. 135:
19-28.
Nakazawa M, Wakabayashi M, Ono M, Murata J.
1997. Molecular phylogenetic analysis of Chrysosplenium (Saxifragaceae) in Japan. – J.
Plant Res. 110: 265-274.
Nelson EA, Sage TL, Sage RF. 2005. Functional
leaf anatomy of plants with crassulacean acid metabolism. – Funct. Plant
Biol. 32: 409-419.
Nemirovich-Danchenko EN. 1994. Seed structure
in Penthorum sedoides and P. chinense (Penthoraceae). – Bot. Žurn. 79:
64-69. [In Russian]
Nicholls KW, Bohm BA. 1984. The flavonoids of
Lithophragma (Saxifragaceae). – Can. J. Bot.
62: 1636-1639.
Nicholls KW, Bohm BA, Wells EF. 1986. The
flavonoids of Mitella, Bensoniella, and Conimitella
(Saxifragaceae). – Can. J.
Bot. 64: 525-530.
Nickrent DL, Der JP, Anderson FE. 2005.
Discovery of the photosynthetic relatives of the ‘Maltese mushroom’
Cynomorium. – BMC Evol. Biol. 5: 38.
http://dx.doi.org/10.1186/1471-2148-5-38
Niedenzu F. 1891. Hamamelidaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien III(2a), W. Engelmann,
Leipzig, pp. 115-130.
Nikulin AY, Nikulin VY, Gontcharova SB,
Gontcharov AA. 2015. ITS rDNA sequence comparisons resolve phylogenetic
relationships in Orostachys subsection Appendiculatae (Crassulaceae). – Plant Syst.
Evol. 301: 1441-1453.
Nowicke JW, Bittner JL, Skvarla J. 1986.
Paeonia: exine substructure and plasma ashing. – In: Blackmore S,
Ferguson IK (eds.), Pollen and spores: form and function, Linnean Society,
London, pp. 81-95.
Nyffeler R. 1992. A taxonomic revision of the
genus Monanthes Haworth (Crassulaceae). – Bradleya 10:
49-82.
Nyffeler R. 1995. Hybridization in
Monanthes (Crassulaceae). – In: Hart H ’t,
Eggli U (eds), Evolution and systematics of the Crassulaceae, Backhuys Publ.,
Leiden.
Oginuma K, Tobe H. 1991. Karyomorphology and
evolution in some Hamamelidaceae and Platanaceae (Hamamelididae:
Hamamelidales). – Bot. Mag. (Tokyo) 104: 115-135.
Ohba H. 1977. The taxonomic status of
Sedum telephium and its allied species (Crassulaceae). – Bot. Mag.
(Tokyo) 90: 41-56.
Ohba H. 1978. Generic and infrageneric
classification of the Old World Sedoideae (Crassulaceae). – J. Fac. Sci.
Univ. Tokyo, Sect. III, 12: 139-198.
Ohba H. 1989. Biogeography of the genus
Rhodiola (Crassulaceae), with special
reference to the floristic interaction between the Himalaya and the Arctic
Region. – In: Ohba H, Hayami I, Mochizuki K (eds), Current aspects of
biogeography in West Pacific and East Asian regions, University of Tokyo, pp.
115-133.
Ohba H. 1995. Systematic problems of Asian
Sedoideae. – In: Hart H ‘t, Eggli U (eds), Evolution and systematics of the
Crassulaceae, Backhuys, Leiden,
pp. 151-158.
Okada H, Tamura M. 1979. Karyomorphology and
relationship of the Ranunculaceae. –
J. Jap. Bot. 54: 65-77.
Okuyama Y, Kato M, Murakami N. 2004.
Pollination by fungus gnats in four species of the genus Mitella (Saxifragaceae). – Bot. J. Linn.
Soc. 144: 449-460.
Okuyama Y, Fujii N, Wakabayashi M, Kawakita
A, Ito M, Watanabe M, Murakami N, Kato M. 2005. Nonuniform concerted evolution
and chloroplast capture: heterogeneity of observed introgression patterns in
three molecular data partition phylogenies of Asian Mitella (Saxifragaceae). – Mol. Biol.
Evol. 22: 285-296.
Okuyama Y, Pellmyr O, Kato M. 2008. Parallel
floral adaptations to pollination by fungus gnats within the genus
Mitella (Saxifragaceae). – Mol. Phylogen.
Evol. 46: 560-575.
Olfelt JP, Furnier GP, Luby JL. 1998.
Reproduction and development of the endangered Sedum integrifolium
ssp. leedyi (Crassulaceae). – Amer. J. Bot.
85: 346-351.
Olfelt JP, Furnier GP, Luby JL. 2001. What
data determine whether a plant’s taxon is distinct enough to merit legal
protection? A case study of Sedum integrifolium (Crassulaceae). – Amer. J. Bot.
88: 401-410.
Oliver D. 1896. Peridiscus lucidus,
Benth. – Hooker’s Ic. Pl. IV, 25: pl. 2441.
Orchard AE. 1975. Taxonomic revisions in the
family Haloragaceae I. The
genera Haloragis, Haloragodendron, Glischrocaryon,
Meziella, and Gonocarpus. – Bull. Auckland Inst. Mus. 10:
1-293.
Orchard AE. 1979. Myriophyllum (Haloragaceae) in Australasia 1. New
Zealand: a revision of the genus and a synopsis of the family. – Brunonia 2:
247-287.
Orchard AE. 1981. A revision of South
American Myriophyllum (Haloragaceae), and its
repercussions on some Australian and North American species. – Brunonia 4:
27-65.
Orchard AE. 1985. Myriophyllum (Haloragaceae) in Australasia 2. The
Australian species. – Brunonia 8: 173-291.
Orchard AE. 1990. Haloragaceae. – In: George AS
(ed), Flora of Australia 18, Australian Government Publ. Service, Canberra, pp.
5-85.
Orchard AE, Keighery GJ. 1993. The status,
ecology and relationships of Meziella (Haloragaceae). – Nuytsia 9:
111-117.
Orchard AE, Lepschi BJ, Hislop M. 2005. New
taxa, a new record and a rediscovery in Western Australian Haloragis
(Haloragaceae). – Nuytsia 15:
431-443.
Ornduff RO. 1969. Ecology, morphology, and
systematics of Jepsonia (Saxifragaceae). – Brittonia 21:
286-298.
Ornduff RO. 1971. The reproductive system of
Jepsonia heterandra. – Evolution 25: 300-311.
Oskolski A, Kodrul T, Jin J. 2012.
Altingioxylon hainanensis sp. nov.: earliest fossil wood record of the
family Altingiaceae in Eastern
Asia and its implications for historical biogeography. – Plant Syst. Evol.
298: 661-669.
Ozdilek A, Gengel B, Kandemir G, Tayanc Y,
Velioglu E, Kaya Z. 2012. Molecular phylogeny of relict-endemic Liquidambar
orientalis Mill. based on sequence diversity of the chloroplast-encoded
matK gene. – Plant Syst. Evol. 298: 337-349.
Packer JG. 1963. The taxonomy of some North
American species of Chrysosplenium L., section Alternifolia
Franchet. – Can. J. Bot. 41: 85-103.
Pan JT. 1978. The genus Saxifraga in
Qing-Zang Plateau. – Acta Phytotaxon. Sin. 16: 11-35.
Pan K-Y, Yang Q-E. 1994. Karyotypes of
Disanthus and Mytilaria (Hamamelidaceae). – Acta
Phytotaxon. Sin. 32: 235-239.
Pan K-Y, Lu A-M, Wen J. 1990. Characters of
leaf epidermis in Hamamelidaceae (s.l.). – Acta
Phytotaxon. Sin. 28: 1-26. [In Chinese with English summary]
Pan K-Y, Lu A-M, Wen J. 1991. A systematic
study on the genus Disanthus Maxim. (Hamamelidaceae). – Cathaya 3:
1-28. [In Chinese]
Parnell J. 1991. Pollen morphology of
Jovibarba Opiz and Sempervivum L. (Crassulaceae). – Kew Bull. 46:
733-738.
Parra V, Vargas CF, Eguiarte LE. 1993.
Reproductive biology, pollen and seed dispersal, and neighborhood size in the
hummingbird-pollinated Echeveria gibbiflora (Crassulaceae). – Amer. J. Bot.
80: 153-159.
Parra V, Vargas CF, Eguiarte LE. 1998. Is
Echeveria gibbiflora (Crassulaceae) fecundity limited by
pollen availability? An experimental study. – Funct. Ecol. 12: 591-595.
Pastre A, Pons A. 1973. Quelques aspects de
la systématique des Saxifragacées à la lumière des données de la
palynologie. – Pollen Spores 15: 117-133.
Pérez-Calix E. 1998. Sedum
mocinianum (Crassulaceae)
una especie nueva del centro de México. – Acta Bot. Mex. 45: 49-54.
Petersen OG. 1893. Halorrhagidaceae. – In:
Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(7), W.
Engelmann, Leipzig, pp. 226-237.
Pigg KB, Ickert-Bond SM, Wen J. 2004.
Anatomically preserved Liquidambar (Altingiaceae) from the middle
Miocene of Yakima Canyon, Washington State, USA, and its biogeographic
implications. – Amer. J. Bot. 91: 499-509.
Pilon-Smits EAH. 1992. Variation and
evolution of crassulacean acid metabolism in Sedum and
Aeonium (Crassulaceae).
– Ph.D. diss., Universiteit Utrecht, The Netherlands.
Pilon-Smits EAH, Hart H ‘t, Maas JW,
Meesterburrie JAN, Kreuler R van, Brederode J. 1992. The evolution of
Crassulacean Acid Metabolism in Aeonium inferred from carbon isotope
composition and enzyme activities. – Oecologia 91: 548-553.
Pino G. 2006. Little-known Crassulaceae of Central Peru. –
Haseltonia 12: 55-66.
Pinto P, Ruiz PM (eds). 1984. Haloragaceae. – Instituto de
Ciencias Naturales, Bogotá, Colombia.
Plouvier V. 1965. Études chimiotaxinomiques
sur les Saxifragacées. – Bull. Soc. Bot. France, Mém., 112: 150-161.
Praeger RL. 1921. An account of the genus
Sedum as found in cultivation. – J. Roy. Horticult. Soc. 46:
1-314.
Praeger LR. 1928a. On some doubtful species
of the African section of the Sempervivum group. – Proc. Roy. Irish
Acad., sect. B, 38: 1-24.
Praeger LR. 1928b. The Canarian
Sempervivum-flora: its distribution and origin. – J. Bot. 66:
218-229.
Praeger LR. 1929. Semperviva of the
Canary Islands Area. – Proc. Roy. Irish Acad., Sect. B, 38: 454-499.
Praeger RL. 1932. An account of the
Sempervivum group. – Royal Horticultural Society, London.
Praglowski J. 1970. The pollen morphology of
the Haloragaceae with reference
to taxonomy. – Grana 10: 159-239.
Praglowski J. 1974. Pollen morphology of the
Trochodendraceae,
Tetracentraceae,
Cercidiphyllaceae, and Eupteleaceae with
reference to taxonomy. – Pollen Spores 16: 449-467.
Prantl K. 1889. Beiträge zur Morphologie und
Systematik der Ranunculaceen. – Engl. Bot. Jahrb. Syst. 9: 225-273.
Prantl K. 1891. Trochodendraceae.
– In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(2), W.
Engelmann, Leipzig, pp. 21-23.
Qi XS, Chen C, Comes HP, Sakaguchi S, Liu YH,
Tanaka N, Sakio H, Qiu YX. 2012. Molecular data and ecological niche modelling
reveal a highly dynamic evolutionary history of the East Asian Tertiary relict
Cercidiphyllum (Cercidiphyllaceae). – New
Phytol. 196: 617-630.
Qiu Y-L, Chase MW, Hoot SB, Conti E, Crane
PR, Sytsma KJ, Parks CR. 1998. Phylogenetics of the Hamamelidae and their
allies: parsimony analyses of nucleotide sequences of the plastid gene
rbcL. – Intern. J. Plant Sci. 159: 891-905.
Quimby MW. 1971. The floral morphology of the
Crassulaceae. – Ph.D. diss.,
Cornell University, Ithaca, New York.
Raadts E. 1972. Zwei neue Kalanchoë
aus Arabien und Somaliland. – Bot. Jahrb. Syst. 91: 478-482.
Raadts E. 1977. The genus Kalanchoë
(Crassulaceae) in tropical East
Africa. – Willdenowia 8: 101-157.
Raadts E. 1979.
Rasterelektronenmikroskopische und anatomische Untersuchungen an
Konnektivdrüsen von Kalanchoe (Crassulaceae). – Willdenowia 9:
169-175.
Raadts E. 1981. Über zwei arabische
Kalanchoë-Arten (Crassulaceae). – Willdenowia 11:
327-331.
Raadts E. 1983. Cytotaxonomische
Untersuchungen an Kalanchoë (Crassulaceae) 1. Kalanchoë
marmorata Baker und 2 neue Kalanchoë-Arten aus Ostafrika. –
Willdenowia 13: 373-385.
Raadts E. 1985. Cytotaxonomische
Untersuchungen an Kalanchoë (Crassulaceae) 2. Chromosomenzahlen
intermediärer Formen. – Willdenowia 15: 157-166.
Raadts E. 1989. Cytotaxonomische
Untersuchungen an Kalanchoë (Crassulaceae) 3. Chromosomenzahlen
ostafrikanischer Kalanchoë-Sippen. – Willdenowia 19: 169-174.
Raadts E. 1995. Über zwei
Kalanchoë-Arten (Crassulaceae) und eine neue
Varietät aus dem Jemen. – Willdenowia 25: 253-259.
Rabe AJ, Soltis DE. 1999. Pollen tube growth
and self-incompatibility in Heuchera micrantha var.
diversifolia (Saxifragaceae). – Intern. J.
Plant Sci. 160: 1157-1162.
Radtke MG, Pigg KB, Wehr W. 2005. Fossil
Corylopsis and Fothergilla leaves (Hamamelidaceae) from the lower
Eocene flora of Republic, Washington, USA, and their evolutionary and
biogeographic significance. – Intern. J. Plant Sci. 166: 347-356.
Rakotobe EA. 1996. Le genre endemique
malgache Dicoryphe Du Petit-Thouars (Hamamélidacées): repartition et
phytogéographie. – Biogéographie de Madagascar: 177-182.
Ramamonjiarisoa BA. 1980. Comparative anatomy
and systematics of African and Malagasy woody Saxifragaceae sensu lato. –
Ph.D. diss., University of Massachusetts, Amherst, Massachusetts.
Rao PRM. 1974. Seed anatomy in some Hamamelidaceae and phylogeny. –
Phytomorphology 24: 113-139.
Rao TA, Bhupal OP. 1974. Typology of foliar
sclereids in various taxa of Hamamelidaceae. – Proc. Indian
Acad. Sci., Sect. B, 79: 127-138.
Record SJ. 1941. American woods of the family
Flacourtiaceae. – Trop. Woods 68: 40-57.
Reinsch A. 1889 [1890]. Über die
anatomischen Verhältnisse der Hamamelidaceae mit Rücksicht auf
ihre systematische Gruppierung. – Engl. Bot. Jahrb. Syst. 11: 347-395.
Rich SM, Ludwig M, Pedersen O, Colmer TD.
2010. Aquatic adventitious roots of the wetland plant Meionectes
brownii can photosynthesize: implications for root function during
flooding. – New Phytol. 190: 311-319.
Rieseberg LH, Soltis DE. 1987. Allozyme
differentiation between Tolmiea menziesii and Tellima
grandiflora (Saxifragaceae). – Syst. Bot. 12:
154-161.
Rocén T. 1928. Beitrag zur Embryologie der
Crassulaceen. – Svensk Bot. Tidskr. 22: 368-376.
Rodríguez ACB, Caballero-Ruano A, Parrondo
MSJ. 1979. Estudio anatómico-fisiológico del leño de las Crassulaceas en
relación con el habitat. – An. Edafol. Agrobiol. 38: 2169-2179.
Rombach S. 1911. Die Entwicklung der
Samenknospe bei den Crassulaceen. – Rec. Trav. Bot. Néerl. 8: 189-200.
Ronse De Craene L-P, Roels P, Smets E,
Backlund A. 1998. The floral development and floral anatomy of
Chrysosplenium alternifolium, an unusual member of the Saxifragaceae. – J. Plant Res.
111: 573-580.
Rosendahl CO. 1914. A revision of the genus
Mitella with a discussion of geographical distribution and
relationships. – Engl. Bot. Jahrb. Syst. [Suppl.] 50: 375-397.
Rosendahl CO, Butters FK, Lakela O. 1936. A
monograph on the genus Heuchera. – Minnesota Studies in Plant
Science 2, University of Minnesota Press, Minneapolis, Minnesota, pp. 1-180.
Rosenthal K. 1931. Daphniphyllaceae. – In:
Engler A (†), Harms H, Pax F (eds), Die natürlichen Pflanzenfamilien, 2.
Aufl., Bd. 19c, W. Engelmann, Leipzig, pp. 233-235.
Rowley JR. 1992. Pollen of
Cercidiphyllum (Cercidiphyllaceae). – Bot.
Žurn. 77: 1-3.
Rudramuniyappa CK, Annigeri BG. 1984. A
histochemical study of meiocytes, microspores, pollen and the tapetum in
Kalanchoë. – Nord. J. Bot. 4: 661-667.
Rüffle L. 1980. Wachstums-Modus und
Blattmorphologie bei altertümlichen Fagales und Hamamelidales der Kreide und
der Gegenwart. – In: 100 Jahre Arboretum Berlin, Akademie-Verlag, Berlin, pp.
329-341.
Rünger W, Wehr B. 1969. Über den Einfluss
der Tageslänge und der Temperatur auf die Blütenbildung einiger
Echeveria-Arten. – Gartenbauwissenschaft 34: 111-143.
Rylova TB. 1989. Morphological features of
pollen in some fossil and extant species of Itea (Iteaceae). – Bot. Žurn. 74: 694-699.
[In Russian]
Said C. 1982. Les nectaires floreaux des
Crassulacées. Étude morphologique, histologique et anatomique. – Bull. Soc.
Bot. France Lett. Bot. 129: 231-240.
Sandwith NY. 1962. Contribution to the flora
of tropical America LXIX. A new genus of Peridiscaceae. – Kew Bull. 15:
467-471.
Sang T, Zhang D. 1999. Reconstructing hybrid
speciation using sequences of low copy nuclear genes: hybrid origins of five
Paeonia species based on Adh gene phylogenies. – Syst. Bot.
24: 148-163.
Sang T, Crawford DJ, Stuessy TF. 1995.
Documentation of reticulate evolution in peonies (Paeonia) using
internal spacer sequences of nuclear ribosomal DNA: implications for
biogeography and concerted evolution. – Proc. Natl. Acad. Sci. U.S.A. 92:
6813-6817.
Sang T, Crawford DJ, Stuessy TF. 1997.
Chloroplast DNA phylogeny, reticulate evolution, and biogeography of
Paeonia (Paeoniaceae).
– Amer. J. Bot. 84: 1120-1136.
Sang T, Donoghue MJ, Zhang D. 1997. Evolution
of alcohol dehydrogenase genes in peonies (Paeonia): phylogenetic
relationships of putative nonhybrid species. – Mol. Biol. Evol. 14:
994-1007.
Sato Y. 1972. Development of the embryo sac
of Daphniphyllum macropodum var. humile (Maxim.) Rosenth. –
Sci. Rep. Tohoku Imp. Univ., Ser. IV (Biol.), 36: 129-133.
Savile DBO. 1953. Splash-cup dispersal
mechanism in Chrysosplenium and Mitella. – Science 117:
250-251.
Savile DBO. 1954. Taxonomy, phylogeny, host
relationship and phytogeography of the microcyclic rusts of Saxifragaceae. – Can. J. Bot.
32: 400-425.
Savile DBO. 1975. Evolution and biogeography
of Saxifragaceae with guidance
from their rust parasites. – Ann. Missouri Bot. Gard. 62: 354-361.
Savile DBO. 1976. Conimitella (Saxifragaceae) and its rust. –
Can. J. Bot. 54: 1977-1978.
Sawada M. 1971. Floral vascularization of
Paeonia japonica with some consideration of systematic position of the
Paeoniaceae. – Bot. Mag.
(Tokyo) 84: 51-60.
Sax K. 1931. Chromosome numbers in the
ligneous Saxifragaceae. – J.
Arnold Arbor. 12: 198-205.
Saxena NP. 1964. Studies in the family Saxifragaceae I. A contribution to
the morphology and embryology of Saxifraga diversifolia Wall. –
Proc. Indian Acad. Sci., Sect. B, 60: 38-51.
Saxena NP. 1972. Studies in the family Saxifragaceae IX. Anatomy of the
flower of some members of Saxifragineae. – J. Indian Bot. Soc. 52:
251-266.
Scherwin PA, Wilbur RL. 1971. The
contributions of floral anatomy to the generic placement of Diamorpha
smallii and Sedum pusillum. – J. Elisha Mitchell Sci. Soc. 87:
103-114.
Schindler AK. 1904. Die Abtrenning der
Hippuridaceen von den Halorrhagaceen. – Engl. Bot Jahrb. Syst. 34, Beibl. 77:
1-77.
Schoenagel E. 1931. Chromosomenzahl und
Phylogenie der Saxifragaceen. – Bot. Jahrb. Syst. 64: 266-308.
Schöffel K. 1932. Untersuchungen über den
Blütenbau der Ranunculaceen. – Planta 17: 315-371.
Schönland S. 1891. Crassulaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien III(2a), W. Engelmann,
Leipzig, pp. 23-38.
Schultheis LM, Donoghue MJ. 2004. Molecular
phylogeny and biogeography of Ribes (Grossulariaceae), with an
emphasis on gooseberries (subg. Grossularia). – Syst. Bot. 29:
77-96.
Schwaighofer KF. 1908. Ist
Zahlbrucknera als eigene Gattung beizubehalten oder wieder mit
Saxifraga zu vereinigen? – Sitzungsber. Kaiserl. Akad. Wiss.
Math.-Naturwiss. Kl., Wien 117: 25-52.
Segraves KA, Thompson JN. 1999. Plant
polyploidy and pollination: floral traits and insect visits to diploid and
tetraploid Heuchera grossulariifolia. – Evolution 53: 1114-1121.
Senters AE, Soltis DE. 2003. Phylogenetic
relationships in Ribes (Grossulariaceae) inferred from
ITS sequence data. – Taxon 52: 51-66.
Shamrov II. 1997. Ovule and seed development
in Paeonia lacitiflora (Paeoniaceae). – Bot. Žurn.
82: 24-46. [In Russian]
Shamrov II. 1998. Formirovanie gipostazy,
podiuma i postamenta v semyazachatke Nuphar lutea (Nymphaeaceae) i
Ribes aureum (Grossulariaceae). – Bot.
Žurn. 83: 3-14.
Sharma AK, Gosh S. 1967. Cytotaxonomy of Crassulaceae. – Biol. Zentralbl.
Suppl. 86: 313-336.
Shi S, Chang HT, Chen Y, Qu L, Wen J. 1998.
Phylogeny of the Hamamelidaceae based on the ITS
sequences of nuclear ribosomal DNA. – Biochem. Syst. Ecol. 26: 55-69.
Shi S, Huang Y-L, Zhong Y, Du Y, Zhang Q,
Chang H, Boufford DE. 2001. Phylogeny of the Altingiaceae based on cpDNA
matK, PY-IGS and nrDNA ITS sequences. – Plant Syst. Evol.
230: 13-24.
Sin J-H, Yoo Y-G, Park K-R. 2002. A
palynotaxonomic study of the Korean Crassulaceae. – Korean J.
Electron Microscopy 32: 345-360.
Sinnott QP. 1985. A revision of
Ribes L. subg. Grossularia (Mill.) Pers. sect.
Grossularia (Mill.) Nutt. (Grossulariaceae) in North
America. – Rhodora 87: 189-286.
Siplivinsky V. 1983. Sections of the genus
Saxifraga L. described by A. Haworth. – Taxon 31: 548-549.
Skvortsova NT. 1960. The structure of
epidermis in the members of the family Hamamelidaceae. – Bot. Žurn.
45: 712-717.
Small JK. 1896. Two new genera of Saxifragaceae. – Bull. Torrey
Bot. Club 23: 18-20.
Smith H. 1958. Saxifraga of the
Himalaya 1. Section Kabschia. – Bull. Brit. Mus. Nat. Hist. (Bot.)
2: 85-129.
Smith H. 1960. Saxifraga of the
Himalaya 2. Some new species. – Bull. Brit. Mus. Nat. Hist. (Bot.) 2:
229-265.
Snezhkova SA. 1990. Structure of woods of
some representatives of Hydrangeaceae and
Grossulariaceae. – Byull.
Glav. Bot. Sada 158: 78-80.
Snow R. 1969. Permanent translocation
heterozygosity associated with an inversion system in Paeonia brownii.
– Heredity 60: 103-106.
Solereder H. 1899. Zur Morphologie und
Systematik der Gattung Cercidiphyllum Sieb. et Zucc. mit
Berücksichtigung der Gattung Eucommia Oliv. – Ber. Deutsch. Bot.
Ges. 17: 387-406.
Soltis DE. 1980a. Karyotypic relationships
among species of Boykinia, Heuchera, Mitella,
Sullivantia, Tiarella, and Tolmiea (Saxifragaceae). – Syst. Bot. 5:
17-29.
Soltis DE. 1980b. Flavonoids of
Sullivantia: taxonomic implications at the genetic level within the
Saxifraginae. – Biochem. Syst. Ecol. 8: 149-151.
Soltis DE. 1981. Heterochromatin banding in
Boykinia, Heuchera, Mitella, Sullivantia,
Tiarella and Tolmiea (Saxifragaceae). – Amer. J. Bot.
69: 108-115.
Soltis DE. 1982. Allozymic variability in
Sullivantia (Saxifragaceae). – Syst. Bot. 7:
26-34.
Soltis DE. 1984a. Karyotypic relationships
among Elmera, Heuchera, and Tellima (Saxifragaceae). – Syst. Bot. 9:
6-11.
Soltis DE. 1984b. Karyotypes and
relationships of species of Jepsonia (Saxifragaceae). – Syst. Bot. 9:
137-141.
Soltis DE. 1984c. Karyotypes of
Leptarrhena and Tanakaea (Saxifragaceae). – Can. J. Bot.
62: 671-673.
Soltis DE. 1985. Allozymic differentiation
among Heuchera americana, H. parviflora, H.
pubescens, and H. villosa (Saxifragaceae). – Syst. Bot. 10:
193-198.
Soltis DE. 1986. Karyotypic relationships
among Astilboides, Bergenia, Darmera, and
Mukdenia and their implications for subtribal boundaries in
Saxifrageae (Saxifragaceae).
– Can. J. Bot. 64: 586-588.
Soltis DE. 1987. Karyotypes and relationships
among Bolandra, Boykinia, Peltoboykinia, and
Suksdorfia (Saxifragaceae: Saxifrageae). –
Syst. Bot. 12: 14-20.
Soltis DE. 1988. Karyotypes of
Bensoniella, Conimitella, Lithophragma, and
Mitella, and relationships in Saxifrageae (Saxifragaceae). – Syst. Bot. 13:
64-72.
Soltis DE. 1991. A revision of
Sullivantia (Saxifragaceae). – Brittonia 43:
27-53.
Soltis DE. 2006. Saxifragaceae. – In: Kubitzki K
(ed), The families and genera of vascular plants IX. Flowering plants.
Eudicots. Berberidopsidales,
Buxales, Crossosomatales, Fabales p. p., Geraniales, Gunnerales, Myrtales p. p., Proteales, Saxifragales, Vitales, Zygophyllales,
Clusiaceae
Alliance, Passifloraceae
Alliance, Dilleniaceae, Huaceae, Picramniaceae,
Sabiaceae, Springer, Berlin,
Heidelberg, New York, pp. 418-435.
Soltis DE, Bohm BA. 1982. Flavonoids of
Penthorum sedoides. – Biochem. Syst. Ecol. 10: 221-224.
Soltis DE, Bohm BA. 1984. Karyology and
flavonoid chemistry of the disjunct species of Tiarella (Saxifragaceae). – Syst. Bot. 9:
441-447.
Soltis DE, Bohm BA. 1986. Flavonoid chemistry
of diploid and tetraploid cytotypes of Tolmiea menziesii (Saxifragaceae). – Syst. Bot. 11:
20-25.
Soltis DE, Hufford L. 2002. Ovary position
diversity in Saxifragaceae:
clarifying the homology of epigyny. – Intern. J. Plant Sci. 163: 277-293.
Soltis DE, Kuzoff RK. 1995. Discordance
between nuclear and chloroplast phylogenies in the Heuchera group (Saxifragaceae). – Evolution 49:
727-742.
Soltis DE, Soltis PS. 1986. Intergeneric
hybridization between Conimitella williamsii and Mitella
stauropetala (Saxifragaceae). – Syst. Bot. 11:
293-297.
Soltis DE, Soltis PS. 1993. Molecular data
and the dynamic nature of polyploidy. – Crit. Rev. Plant Sci. 12: 243-273.
Soltis DE, Soltis PS. 1997. Phylogenetic
relationships in Saxifragaceae
sensu lato: a comparison of topologies based on 18S rDNA and rbcL
sequences. – Amer. J. Bot. 84: 504-522.
Soltis DE, Soltis PS, Ness BD. 1989.
Chloroplast-DNA variation and multiple origins of autopolyploidy in
Heuchera micrantha (Saxifragaceae). – Evolution 43:
650-656.
Soltis DE, Soltis PS, Bothel KD. 1990.
Chloroplast DNA evidence for the origins of the monotypic Bensoniella
and Conimitella (Saxifragaceae). – Syst. Bot. 15:
349-362.
Soltis DE, Soltis PS, Clegg MT, Durbin M.
1990. rbcL sequence divergence and phylogenetic relationships in Saxifragaceae sensu lato. –
Proc. Natl. Acad. Sci. U.S.A. 87: 4640-4644.
Soltis DE, Soltis PS, Collier TG, Edgerton
ML. 1991. Chloroplast DNA variation within and among genera of the
Heuchera group (Saxifragaceae): evidence for
chloroplast transfer and paraphyly. – Amer. J. Bot. 78: 1091-1112.
Soltis DE, Mayer MS, Soltis PS, Edgerton M.
1991. Chloroplast-DNA variation in Tellima grandiflora (Saxifragaceae). – Amer. J. Bot.
78: 1379-1390.
Soltis DE, Soltis PS, Thompson JN, Pellmyr O.
1992. Chloroplast DNA variation in Lithophragma (Saxifragaceae). – Syst. Bot. 17:
607-619.
Soltis DE, Morgan DR, Grable A, Soltis PS,
Kuzoff R. 1993. Molecular systematics of Saxifragaceae sensu stricto. –
Amer. J. Bot. 80: 1056-1081.
Soltis DE, Johnson LA, Looney C. 1996.
Discordance between ITS and chloroplast topologies in the Boykinia
group (Saxifragaceae). –
Syst. Bot. 21: 169-185.
Soltis DE, Kuzoff RK, Conti E, Gornall R,
Ferguson K. 1996. matK and rbcL gene sequence data indicate
that Saxifraga (Saxifragaceae) is polyphyletic.
– Amer. J. Bot. 83: 371-382.
Soltis, DE, Kuzoff RK, Mort ME, Zanis M,
Fishbein M, Hufford L, Koontz J, Arroyo MK. 2001. Elucidating deep-level
phylogenetic relationships in Saxifragaceae using sequences for
six chloroplast and nuclear DNA regions. – Ann. Missouri Bot. Gard. 88:
669-693.
Soltis DE, Tago-Nakazawa M, Xiang Q-Y, Kawano
S, Murat J, Wakabayashi M. 2001. Phylogenetic relationships and evolution in
Chrysosplenium (Saxifragaceae) based on
matK sequence data. – Amer. J. Bot. 88: 883-893.
Soltis DE, Clayton JW, Davis CC, Gitzendanner
MA, Cheek M, Savolainen V, Amorim AM, Soltis PS. 2007. Monophyly and
relationships of the enigmatic family Peridiscaceae. – Taxon 56:
65-73.
Soltis DE, Mort ME, Latvis M, Mavrodiev EV,
O’Meara BC, Soltis PS, Burleigh JG, Casas RR de. 2013. Phylogenetic
relationships and character evolution analysis of Saxifragales using a supermatrix
approach. – Amer. J. Bot. 100: 916-929.
Soltis PS, Soltis DE. 1986. Anthocyanin
content in diploid and tetraploid cytotypes of Tolmiea menziesii (Saxifragaceae). – Syst. Bot. 11:
32-34.
Sopova M. 1971. The cytological study of two
Paeonia species from Macedonia. – Fragm. Balc. Mus. Macedon. Sci.
Nat. 8: 137-142.
Souèges R. 1936. Les rélations
embryogéniques des Crassulacées, Saxifragacées et Hypéricacées. – Bull.
Soc. Bot. France 83: 317-329.
Spongberg SA. 1979. Cercidiphyllaceae hardy in
temperate North America. – J. Arnold Arbor. 60: 367-376.
Stearn WT. 1946. A study of the genus
Paeonia. – The Royal Horticultural Society, London.
Stebbins GL. 1938. Cytogenetic studies in
Paeonia II. The cytology of the diploid species and hybrids. –
Genetics 23: 83-110.
Stebbins GL. 1939. Notes on some systematic
relationships in the genus Paeonia. – Univ. Calif. Publ. Bot. 19:
245-266.
Steen SW, Gielly L, Taberlet P, Brochmann C.
2000. Same parental species, but different taxa: molecular evidence for hybrid
origins of the rare endemics Saxifraga opdalensis and S.
svalbardensis (Saxifragaceae). – Bot. J. Linn.
Soc. 132: 153-164.
Steindl F. 1945. Beitrag zur Pollen- und
Embryobildung bei Cynomorium L. – Arch. Julius Klaus-stiftung
Vererbungsf. 20, Suppl.: 342-355.
Stephenson R. 1994. Sedum,
cultivated stonecrops. – Timber Press, Portland, Oregon.
Stern FC. 1944. Geographical distribution of
the genus Paeonia. – Proc. Linn. Soc. London 155: 76-80.
Stern FC. 1946. A study of the genus
Paeonia. – The Royal Horticultural Society, London.
Stern WL, Sweitzer EM, Phipps RE. 1970.
Comparative anatomy and systematics of woody Saxifragaceae: Ribes. –
In: Robson NKB, Cutler DF, Gregory M (eds), New research in plant anatomy, Bot.
J. Linn. Soc. 63 [Suppl.], London and New York, pp. 215-237.
Stevens JF. 1995a. The systematic and
evolutionary significance of phytochemical variation in the Eurasian Sedoideae
and Sempervivoideae (Crassulaceae). – Ph.D. diss.,
Rijks Universiteit, Groningen, The Netherlands.
Stevens JF. 1995b. Chemotaxonomy of the
Eurasian Sedoideae and Sempervivoideae. – In: Hart H ‘t, Eggli U (eds),
Evolution and systematics of the Crassulaceae, Backhuys, Leiden, pp.
30-44.
Stevens JF, Hart H ‘t, Hendriks H,
Malingré TM. 1992. Alkaloids of some European and Macaronesian Sedoideae and
Sempervivoideae (Crassulaceae).
– Phytochemistry 31: 3917-3924.
Stevens JF, Hart H ‘t, Hendricks H,
Malingré TM. 1993. Alkaloids of the Sedum acre-group (Crassulaceae). – Plant Syst.
Evol. 185: 207-217.
Stevens JF, Hart H ‘t, Bolck A, Zwaving JH,
Malingré TM. 1994. Epicuticular wax composition of some European
Sedum species. – Phytochemistry 35: 389-399.
Stevens JF, Hart H ‘t, Ham RCHJ van, Elema
ET, Ent MMVX van den, Wildeboer M, Zwaving JH. 1995. Distribution of alkaloids
and tannins in the Crassulaceae.
– Biochem. Syst. Ecol. 23: 157-165.
Stevens JF, Hart H ’t, Wollenweber E. 1995.
The systematic and evolutionary significance of exudate flavonoids in
Aeonium. – Phytochemistry 39: 805-813.
Stevens JF, Hart H ’t, Elema ET, Bolck A.
1996. Flavonoid variation in Eurasian Sedum and Sempervivum.
– Phytochemistry 41: 503-512.
Stockey RA, Crane PR. 1983. In situ
Cercidiphyllum-like seedlings from the Paleocene of Alberta, Canada.
– Amer. J. Bot. 70: 1564-1568.
Stolt KAH. 1928. Die Embryologie von
Myriophyllum alterniflorum DC. – Svensk Bot. Tidskr. 22: 305-319.
Stopp K. 1957. Aberrante Dehiszenzformen bei
Früchten einiger Crassula-Arten. – Beitr. Biol. Pflanzen 34:
165-175.
Stubbs RL, Folk RA, Xiang C-L, Soltis DE,
Cellinese N. 2018. Pseudo-parallel patterns of disjunctions in an Arctic-alpine
plant lineage. – Mol. Phylogen. Evol. 123: 88-100.
Su HJ, Hu JM, Anderson FE, Der JP, Nickrent
DL. 2015. Phylogenetic relationships of Santalales with insights into the
origins of holoparasitic Balanophoraceae. Appendix S1.
Justification for why Cynomorium was excluded from this study. –
Taxon 64(3): S1-S2.
Subramanyam K. 1962. Embryology in relation
to systematic botany, with particular reference to the Crassulaceae. – In: Plant
embryology. A symposium, New Delhi: C.S.I.R., pp. 94-112.
Subramanyam K. 1970. Comparative embryology
of angiosperms: Crassulaceae, Campanulaceae,
Sphenocleaceae,
Pentaphragmataceae,
Stylidiaceae.
– Bull. Natl. Sci. Acad. India 41: 84-89, 306-312, 313-316, 317-320,
321-324.
Supratman U, Fujita T, Akiyama K, Hayashi H.
2001. Insecticidal compounds from Kalanchoe daigremontiana x
tubiflora. – Phytochemistry 58: 311-314.
Sutton DA. 1989. The Daphniphyllaceae: a systematic
review. – In: Crane PR, Blackmore S (eds), Evolution, systematics, and fossil
history of the Hamamelidae 1: Introduction and ”lower” Hamamelidae, Syst.
Assoc. Spec. Vol. 40A, Clarendon Press, Oxford, pp. 285-291.
Swamy BGL, Bailey IW. 1949. The morphology
and relationships of Cercidiphyllum. – J. Arnold Arbor. 30:
187-210.
Takahashi A. 1985. Wood anatomical studies of
Polycarpicae I. Magnoliales. – Sci.
Rep. Osaka Univ. 34: 29-83.
Tamura M. 2006. Paeoniaceae. – In: Kubitzki K
(ed), The families and genera of vascular plants IX. Flowering plants.
Eudicots. Berberidopsidales,
Buxales, Crossosomatales, Fabales p. p., Geraniales, Gunnerales, Myrtales p. p., Proteales, Saxifragales, Vitales, Zygophyllales,
Clusiaceae
Alliance, Passifloraceae
Alliance, Dilleniaceae, Huaceae, Picramniaceae,
Sabiaceae, Springer, Berlin,
Heidelberg, New York, pp. 265-269.
Taneyama M, Yoshida S. 1978. Studies on
C-glycosides in higher plants I. Occurrence of bergenin in Saxifragaceae. – Bot. Mag.
(Tokyo) 91: 109-112.
Tang M-S, Yang Y-P, Sheue C-R. 2009.
Comparative morphology of the leaves of Daphniphyllum (Daphniphyllaceae). – Blumea
54: 63-68.
Tang Y. 1943. Systematic anatomy of the woods
of the Hamamelidaceae. –
Bull. Fan Mem. Inst. Biol., N. S., 1: 8-63.
Tank DC, Sang T. 2001. Phylogenetic utility
of the glycerol-3-phosphate acyltransferase gene: evolution and implications in
Paeonia (Paeoniaceae).
– Mol. Phylogen. Evol. 19: 421-429.
Tattje DHE, Bos R, Bruins AP. 1980.
Constituents of essential oil from leaves of Liquidambar styraciflua
L. – Planta Medica 38: 79-85.
Taylor RL. 1965. The genus
Lithophragma (Saxifragaceae). – Univ. Calif.
Publ. Bot. 37: 1-122.
Teeri JA, Overton J. 1981. Chloroplast
ultrastructure in two Crassulacean species and an F1 hybrid with
differing biomass delta 13C values. – Plant Cell Environm. 4:
427-431.
Teeri JA, Stowe LG, Murawski DA. 1978. The
climatology of two succulent plant families: Cactaceae and Crassulaceae. – Can. J. Bot. 56:
1750-1758.
Teryokhin ES, Nikiticheva ZI, Yakovlev MS.
1975. Development of seed, embryo, and endosperm in Cynomorium
songaricum Rupr. (Cynomoriaceae). – Bot. Žurn.
60: 1603-1613. [In Russian with English summary]
Thiede J. 1995. Quantitative phytogeography,
species richness, and evolution of American Crassulaceae. – In: Hart H ‘t,
Eggli U (eds), Evolution and systematics of the Crassulaceae, Backhuys, Leiden, pp.
89-123.
Thiede J. 2004. The genus Dudleya
Britton & Rose (Crassulaceae): its systematics and
biology. – Cact. Succ. J. 76: 4-11.
Thiede J. 2006. Penthoraceae. – In: Kubitzki K
(ed), The families and genera of vascular plants IX. Flowering plants.
Eudicots. Berberidopsidales,
Buxales, Crossosomatales, Fabales p. p., Geraniales, Gunnerales, Myrtales p. p., Proteales, Saxifragales, Vitales, Zygophyllales,
Clusiaceae
Alliance, Passifloraceae
Alliance, Dilleniaceae, Huaceae, Picramniaceae,
Sabiaceae, Springer, Berlin,
Heidelberg, New York, pp. 292-296.
Thiede J, Eggli U. 2006. Crassulaceae. – In: Kubitzki K
(ed), The families and genera of vascular plants IX. Flowering plants.
Eudicots. Berberidopsidales,
Buxales, Crossosomatales, Fabales p. p., Geraniales, Gunnerales, Myrtales p. p., Proteales, Saxifragales, Vitales, Zygophyllales,
Clusiaceae
Alliance, Passifloraceae
Alliance, Dilleniaceae, Huaceae, Picramniaceae,
Sabiaceae, Springer, Berlin,
Heidelberg, New York, pp. 83-118.
Thiede J, Hart H ‘t. 1999. Transfer of four
Peruvian Altamiranoa species to Sedum (Crassulaceae). – Novon 9:
124-125.
Thouvenin M. 1890. Recherches sur la
structure des Saxifragacées. – Ann. Sci. Nat., sér. VII (Bot.), 12:
1-174.
Thulin M. 1993. Crassulaceae. – In: Lock JM (ed),
Flora of Somalia, Royal botanic Gardens, Kew, pp. 87-93.
Tiaghi YD. 1970. Comparative embryology of
angiosperms: Paeoniaceae. –
Bull. Natl. Sci. Acad. India 41: 53-58.
Tieghem P van. 1898. Sur le genre Penthore
considéré comme type d’une famille nouvelle, les Penthoracées. – J. Bot.
(Morot) 12: 150-154.
Tillson AH. 1940.The floral anatomy of the
Kalanchoideae. – Amer. J. Bot. 27: 595-600.
Tkach N, Röser M, Miehe G, Muellner-Riehl
AN, Ebersbach J, Favre A, Hoffmann MH. 2015. Molecular phylogenetics,
morphology and a revised classification of the complex genus Saxifraga
(Saxifragaceae). – Taxon 64:
1159-1187.
Tkach N, Röser M, Hoffmann MH. 2015.
Molecular phylogenetics, character evolution and systematics of the genus
Micranthes (Saxifragaceae). – Bot. J. Linn.
Soc. 178: 47-66.
Toelken HR. 1977. A revision of the genus
Crassula in southern Africa 1-2. – Contr. Bolus Herb. 8: 1-331,
332-595.
Tong K. 1930. Studien über die Familie der
Hamamelidaceae, mit besonderer
Berücksichtigung der Systematik und Entwicklungsgeschichte von
Corylopsis. – Bull. Dept. Biol., Coll. Sci., Sun Yatsen Univ. 2:
1-72.
Tzanoudakis D. 1977. Cytotaxonomic study of
the genus Paeonia in Greece. – Ph.D. diss., Botanical Inst.,
University of Patras, Greece.
Tzanoudakis D. 1983. Karyotypes of four wild
Paeonia species from Greece. – Nord. J. Bot. 3: 307-318.
Uhl CH. 1948. Cytotaxonomic studies in the
subfamilies Crassuloideae, Kalanchoideae, and Cotyledonoideae of the Crassulaceae. – Amer. J. Bot. 35:
695-706.
Uhl CH. 1956. The Crassulaceae and cytotaxonomy. –
Cact. Succ. J. 48: 225-229.
Uhl CH. 1961a. Some cytotaxonomic problems in
the Crassulaceae. – Evolution
15: 375-383.
Uhl CH. 1961b. The chromosomes of the
Sempervivoideae (Crassulaceae).
– Amer. J. Bot. 48: 114-123.
Uhl CH. 1963. Chromosomes and phylogeny of
the Crassulaceae. – Cact.
Succ. J. 35: 3-7.
Uhl CH. 1970. Chromosomes of
Graptopetalum and Thompsonella (Crassulaceae). – Amer. J. Bot.
57: 1115-1121.
Uhl CH. 1976a. Chromosomes, hybrids and
ploidy of Sedum cremnophila and Echeveria linguifolia (Crassulaceae). – Amer. J. Bot.
63: 806-820.
Uhl CH. 1976b. Chromosomes of Mexican
Sedum I. Annual and biennial species. – Rhodora 78: 629-640.
Uhl CH. 1976-1992. Chromosomes of Mexican
Sedum I-VI. – Rhodora 79: 629-640; 80: 491-512; 82: 377-402; 85:
243-252; 87: 381-423; 94: 362-370.
Uhl CH. 1978. Chromosomes of Mexican
Sedum II. Section Pachysedum. – Rhodora 80: 491-512.
Uhl CH. 1980. Chromosomes of Mexican
Sedum III. Section Centripetalia, Fruticisedum and
other woody species. – Rhodora 82: 377-402.
Uhl CH. 1989. The hybrid origin of
Echeveria x Sayulensis. – Cact. Succ. J. 61: 279-284.
Uhl CH. 1992a. Polyploidy, dysploidy, and
chromosome pairing in Echeveria (Crassulaceae) and its hybrids. –
Amer. J. Bot. 79: 556-566.
Uhl C. 1992b. Chromosomes of Mexican
Sedum VI. Section Sedastrum. – Rhodora 94: 362-370.
Uhl CH. 1993-1995. Intergeneric hybrids in
the Mexican Crassulaceae I-V.
– Cact. Succ. J. 65: 271-273; 66: 74-80, 175-179, 214-217; 67: 144-147.
Uhl CH. 1993. Tacitus and
polyploidy. – Haseltonia 1: 29-34.
Uhl CH. 1994-2005. Chromosomes and hybrids of
Echeveria (Crassulaceae) I-IX. – Haseltonia
2: 79-88; 3: 25-33; 3: 34-48; 4: 66-88; 5: 21-36; 6: 63-90; 8: 71-82; 9:
121-145; 11: 138-149.
Uhl CH. 1996. Chromosomes and polyploidy in
Lenophyllum (Crassulaceae). – Amer. J. Bot.
83: 216-220.
Uhl CH, Moran R. 1972. Chromosomes of Crassulaceae from Japan and South
Korea. – Cytologia 37: 59-81.
Uhl CH, Moran R. 1973. The chromosomes of
Pachyphytum (Crassulaceae). – Amer. J. Bot.
60: 648-656.
Uhl CH, Moran R. 1999. Chromosomes of
Villadia and Altamiranoa (Crassulaceae). – Amer. J. Bot.
86: 387-397.
Umemoto K. 1974. Chemical composition and
form of crystalline inorganic components in Japanese saxifragaceous plants. –
Yakugaku Zasshi 94: 1627-1633.
Vandeputte E. 1992. Bloemontogenetische
observaties en systematische verwantschappen binnen de Saxifragaceae sensu lato. –
Thesis, Katholieke Universiteit Leuven, Belgium.
Vargas P. 1991. On the vegetative and floral
development in Saxifraga ser. Ceratophyllae and ser.
Pentadactylis (Saxifragaceae). – Taxon 40:
41-43.
Vargas P, Morton CM, Jury SL. 1999.
Biogeographic patterns in Mediterranean and Macaronesian species of
Saxifraga (Saxifragaceae) inferred from
phylogenetic analyses of ITS sequences. – Amer. J. Bot. 86: 724-734.
Velajos M, Carrasco MA, Monge C. 1990. Dos Crassulaceae de Ciudad Real
(España). – An. Jard. Bot. Madrid 47: 53-58.
Verdcourt B. 1971. Hamamelidaceae. – In:
Milne-Redhead E, Polhill RM (eds), Flora of tropical East Africa, Crown Agents
for Oversea Governments and Administrations, London, pp. 1-6.
Verdcourt B. 1973a. Haloragaceae. – In: Milne-Redhead
E, Polhill RM (eds), Flora of tropical East Africa, Crown Agents for Oversea
Governments and Administrations, London, pp. 1-10.
Verdcourt B. 1973. Escalloniaceae.
– In: Polhill RM (ed), Flora of tropical East Africa, Crown Agents for
Oversea Governments and Administration, London, pp. 1-4.
Verdcourt B. 1983. 64a. Escalloniaceae.
– In: Launert E (ed), Flora Zambesiaca 7 (Part 1), Flora Zambesiaca Managing
Committee, London, pp. 1-3.
Vink W. 1957. Hamamelidaceae. – In: Steenis
CGGJ van (ed), Flora Malesiana I, 5(3), Noordhoff-Kolff N. V., Djakarta, pp.
363-379.
Wadhwa BM. 1983. Two new species of
Saxifraga from Burma. – Kew Bull. 38: 487-490.
Wakabayashi M. 1970. On the affinity in Saxifragaceae s. lato with special
reference to the pollen morphology. – Acta Phytotaxon. Geobot. 24: 128-145.
[In Japanese with English summary]
Wakabayashi M. 1973. On Saxifraga
Sect. Diptera of Japan, with description of a new species. – Acta
Phytotaxon. Geobot. 25: 154-169.
Wakabayashi M, Ohba H. 1999. Chromosome
numbers of seven species of Sedum and Sinocrassula indica (Crassulaceae) in East Himalaya. –
J. Jap. Bot. 74: 228-235.
Walters JL. 1952. Heteromorphic chromosome
pairs in Paeonia californica. – Amer. J. Bot. 39: 145-151.
Walters JL. 1962. Megasporogenesis and
gametophyte selection in Paeonia californica. – Amer. J. Bot. 49:
787-794.
Walther E. 1936. Phylogeny of
Echeveria. – Cact. Succ. J. 8: 82-88.
Walther E. 1938. Notes on Crassulaceae. – Cact. Succ. J.
10: 22-24.
Walther E. 1972. Echeveria. –
California Academy of Sciences, San Francisco, California.
Wang X-Z. 1992. Palaeopalynological evidence
of phylogeny in Hamamelidaceae. – Acta
Phytotaxon. Sin. 30: 137-145. [In Chinese with English summary]
Warburg O. 1894. Flacourtiaceae. – In:
Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(6a), W.
Engelmann, Leipzig, pp. 1-56.
Warming E. 1909. The structure and biology of
arctic flowering plants IV. Saxifragaceae 1. Morphology and
biology. – Medd. Grønl. 36: 169-236.
Wassmer A. 1955. Vergleichend-morphologische
Untersuchungen an den Blüten der Crassulaceen. – Ph.D. diss., Universität
Zürich, Switzerland.
Watari S. 1939. Anatomical studies on the
leaves of some saxifragaceous plants, with special reference to the vascular
system. – J. Fac. Sci. Imp. Univ. Tokyo, Sect. III, Bot. 5: 195-316.
Watari S. 1941. Notes on the crystals in
leaves of saxifragaceous plants 1. – Bot. & Zool. (Tokyo) 9: 479-488.
Webb DA, Gornall RJ. 1989a. Saxifrages of
Europe with notes on African, American and some Asiatic species. –
Christopher Helm, Bromley, London.
Webb DA, Gornall RJ. 1989b. A manual of
saxifrages and their cultivation. – Timber Press, Portland, Oregon.
Webb DA, Press JR. 1987. The genus
Saxifraga L. in the Madeiran Archipelago. – Bocagiana 105: 1-4.
Weberling F. 1976. Weitere Untersuchungen zur
Morphologie des Unterblattes bei den Dikotylen IX. Saxifragaceae s.l., Brunelliaceae,
und Bruniaceae.
– Beitr. Biol. Pflanzen 52: 163-181.
Weiblen GD, Brehm BG. 1996. Reproductive
strategies and barriers to hybridization between Tellima grandiflora
and Tolmiea menziesii (Saxifragaceae). – Amer. J. Bot.
83: 910-918.
Weigend M. 2006. Grossulariaceae. – In:
Kubitzki K (ed), The families and genera of vascular plants IX. Flowering
plants. Eudicots. Berberidopsidales,
Buxales, Crossosomatales, Fabales p. p., Geraniales, Gunnerales, Myrtales p. p., Proteales, Saxifragales, Vitales, Zygophyllales,
Clusiaceae
Alliance, Passifloraceae
Alliance, Dilleniaceae, Huaceae, Picramniaceae,
Sabiaceae, Springer, Berlin,
Heidelberg, New York, pp. 168-176.
Weigend M, Binder M. 2001a. A revision of the
genus Ribes (Grossulariaceae) in Bolivia. –
Bot. Jahrb. Syst. 123: 111-134.
Weigend M, Binder M. 2001b. Three new species
of Ribes L. (Grossulariaceae) from Central
and South America. – Syst. Bot. 26: 727-732.
Weigend M, Mohr O, Motley TJ. 2002. Phylogeny
and classification of the genus Ribes (Grossulariaceae) based on 5S-NTS
sequences and morphological and anatomical data. – Bot. Jahrb. Syst. 124:
163-182.
Weigend M, Gottschling M, Hoot S, Ackerman M.
2004. A preliminary phylogeny of Loasaceae subfam.
Loasoideae (Angiospermae: Cornales) based on trn(UAA)
sequence data, with consequences for systematics and historical biogeography.
– Organisms Divers. Evol. 4: 73-90.
Wells EF. 1984. A revision of the genus
Heuchera (Saxifragaceae) in eastern North
America. – Syst. Bot. Monogr. 3: 45-121.
Wells EF, Bohm BA. 1980. Chemotaxonomic
studies in the Saxifragaceae
s.l. 15. The flavonoids of subsection Villosae section
Heuchera in the genus Heuchera. – Can. J. Bot. 58:
1459-1463.
Wen J, Shi S. 1999. A phylogenetic and
biogeographic study of Hamamelis (Hamamelidaceae), an eastern Asian
and eastern North American disjunct genus. – Biochem. Syst. Ecol. 27:
55-66.
Wetzstein HY, Sommer HE. 1982. Leaf anatomy
of tissue-cultured Liquidambar styraciflua (Hamamelidaceae) during
acclimatization. – Amer. J. Bot. 69: 1579-1586.
Wickens GE. 1987. Crassulaceae. – In: Polhill RM
(ed), Flora of tropical East Africa, A. A. Balkema, Rotterdam, pp. 1-66.
Wickens GE. 1982. Studies in the Crassulaceae for the ‘Flora of
tropical East Africa’ III. Miscellaneous notes on Crassula,
Bryophyllum and Kalanchoe. – Kew Bull. 36: 665-674.
Wickens GE, Bywater M. 1980. Seed studies in
Crassula subgen. Disporocarpa. – Kew Bull. 34: 629-637.
Wiggins IL. 1959. Development of the ovule
and megagametophyte in Saxifraga hieracifolia. – Amer. J. Bot. 46:
692-697.
Wilkins CK, Bohm BA. 1976. Chemotaxonomic
studies in the Saxifragaceae
s.l. 4. The flavonoids of Heuchera micrantha var.
diversifolia. – Can. J. Bot. 54: 2133-2140.
Wilkinson HP. 1994. Leaf and stem anatomy of
the Pterostemonaceae (Engl.)
Small: ecological and systematic features. – Bot. J. Linn. Soc. 115:
115-131.
Wilson PG, Moody ML. 2006.
Haloragodendron gibsonii (Haloragaceae), a new species from
the Blue Mountains, New South Wales. – Telopea 11: 141-146.
Wisniewski M, Bogle AL. 1982. The ontogeny of
the inflorescence and flower of Liquidambar styraciflua L. (Hamamelidaceae). – Amer. J.
Bot. 69: 1612-1624.
Worsdell WC. 1908. The affinities of
Paeonia. – J. Bot. 46: 114-116.
Wu C-Y, Ku T-C. 1992. A new tribe with a new
monotypic genus of Saxifragaceae (s.l.) from China.
– Acta Phytotaxon. Sin. 30: 193-196. [In Chinese]
Wu W, Zhou R, Huang Y, Boufford DE, Shi S.
2010. Molecular evidence for the natural intergeneric hybridization between
Liquidambar and Altingia. – J. Plant Res. 123: 231-239.
Wyatt R, Stoneburger A. 1981. Patterns of
ant-mediated pollen dispersal in Diamorpha smallii (Crassulaceae). – Syst. Bot. 6:
1-7.
Xi Y-Z. 1984. The pollen morphology and exine
ultrastructure of Paeonia in China. – Acta Bot. Sin. 26: 241-246.
[In Chinese]
Xie L, Yi T-S, Li R, Li D-Z, Wen J. 2010.
Evolution and biogeographic diversification of the witch-hazel genus
(Hamamelis L., Hamamelidaceae) in the northern
hemisphere. – Mol. Phylogen. Evol. 56: 675-689.
Xu J-F, Liu C-B, Han A-M, Feng P-S, Su Z-G.
1998. Strategies for the improvement of salidroside production in cell
suspension cultures of Rhodiola sachalinensis. – Plant Cell Rep. 17:
288-293.
Yakovlev MS. 1961. Further studies on the new
type of embryogenesis in angiosperms. – Bot. Žurn. 46: 1402-1421. [In
Russian]
Yakovlev MS, Yoffe MD. 1957. On some peculiar
features in the embryogeny of Paeonia L. – Phytomorphology 7:
74-87.
Yamagishi T, Haruna M, Yan X-Z, Chang J-J,
Lee K-H. 1989. Antitumor agents, 110. 1,2 bryophyllin B, a novel potent
cytotoxic bufadienolide from Bryophyllum pinnatum. – J. Nat. Prod.
52: 1071-1079.
Yan X-L, Ren Y, Tian X-H, Zhang X-H. 2007.
Morphogenesis of pistillate flowers of Cercidiphyllum japonicum (Cercidiphyllaceae). – J.
Integr. Plant Biol. 49: 1400-1408.
Yen TK. 1936. Floral development and vascular
anatomy of the fruit of Ribes aureum. – Bot. Gaz. 98: 105-120.
Yeo PF. 1966. A revision of the genus
Bergenia Moench (Saxifragaceae). – Kew Bull. 26:
113-148.
Yoshikawa M, Shimada H, Shimoda H, Murakami
N, Yamahara J, Matsuda H. 1996. Bioactive constituents of Chinese natural
medicines II. Rhodiolae Radix (1): chemical structures and antiallergic
activity of rhodiocyanosides A and B from the underground part of Rhodiola
sachalinensis (Pall.) Fisch. et Mey. (Crassulaceae). – Chem.
Pharmaceut. Bull. (Tokyo) 44: 2086-2091.
Yu J, Xiao P-G. 1987. A preliminary study of
the chemistry and systematics of Paeoniaceae. – Acta Bot. Sin. 25:
172-179. [In Chinese]
Zavada MS, Dilcher DL. 1986. Comparative
pollen morphology and its relationship to phylogeny of pollen in the Hamamelidaceae. – Ann. Missouri
Bot. Gard. 73: 348-381.
Zhang J-Q, Meng S-Y, Wen J, Rao G-Y. 2014.
Phylogenetic relationships and character evolution of Rhodiola (Crassulaceae) based on nuclear
ribosomal ITS and plastid trnL-F and psbA-trnH
sequences. – Syst. Bot. 39: 441-451.
Zhang J-Q, Meng S-Y, Allen GA, Wen J, Rao
G-Y. 2014. Rapid radiation and dispersal out of the Qinghai-Tibetan Plateau of
an alpine plant lineage Rhodiola (Crassulaceae). – Mol. Phylogen.
Evol. 77: 147-158.
Zhang J-T, Zhang D-W. 1991. Studies on pollen
morphology of the genus Corylopsis. – Acta Phytotaxon. Sin. 29:
347-351. [In Chinese]
Zhang Z-H, Li C-Q, Li J. 2009. Phylogenetic
placement of Cynomorium in Rosales inferred from sequences of the
inverted repeat region of the chloroplast genome. – J. Syst. Evol. 47:
297-304.
Zhang Z-Y, Lu A-M. 1989. Phylogenetic
relationship of the Daphniphyllaceae. – Acta
Phytotaxon. Sin. 27: 17-26. [In Chinese with English summary]
Zhang Z-Y, Lu A-M. 1995. Hamamelidaceae: geographic
distribution, fossil history and origin. – Acta Phytotaxon. Sin. 33:
313-339.
Zhao L-C, Li D-Y. 2008. Anatomically
preserved seeds of Corylopsis (Hamamelidaceae) from the Miocene
of Yunnan, China, and their phytogeographic implications. – Intern. J. Plant
Sci. 169: 483-494.
Zhou Z-K, Crepet WL, Nixon KC. 2001. The
earliest fossil evidence of the Hamamelidaceae: Late Cretaceous
(Turonian) inflorescences and fruits of Altingioideae. – Amer. J. Bot. 88:
753-766.
Zhu W-D, Nie Z-L, Wen J, Sun H. 2013.
Molecular phylogeny and biogeography of Astilbe (Saxifragaceae) in Asia and eastern
North America. – Bot. J. Linn. Soc. 171: 377-394.
Zielinski QB. 1953. Chromosome numbers and
meiotic studies in Ribes. – Bot. Gaz. 114: 265-274.