
Cladogram of Malvales based on DNA sequence data (Stevens 2001 onwards, May 2012 version).
Habit Usually bisexual (rarely monoecious, andromonoecious, trimonoecious, polygamomonoecious, dioecious, androdioecious, or gynodioecious), evergreen or deciduous trees, shrubs or suffrutices (rarely lianas), perennial, biennial or annual herbs. Some species are xerophytic.
Vegetative anatomy Ectomycorrhiza frequent. Phellogen ab initio epidermal, subepidermal or outer-cortical. Vessel elements usually with simple (sometimes scalariform) perforation plates; lateral pits usually alternate (sometimes scalariform or opposite), simple or bordered pits. Vestured pits sometimes present. Imperforate tracheary xylem elements tracheids, fibre tracheids or libriform fibres with simple or bordered pits, usually non-septate (also vasicentric tracheids). Wood rays uniseriate or multiseriate, homocellular or heterocellular. Axial parenchyma apotracheal diffuse or diffuse-in-aggregates, or paratracheal, aliform, lozenge-aliform, winged-aliform, confluent, reticulate, unilateral, vasicentric, or banded (sometimes scanty or scalariform), or absent. Tile cells often present. Secondary phloem often stratified into soft parenchymatous and harder fibrous layers; phloem rays cuneate. Sieve tube plastids usually S0 or Ss type (sometimes Pcs type). Nodes usually 3:≥3, trilacunar with three or more leaf traces (sometimes 1:1, unilacunar with one trace, rarely ≥5:≥5, penta- or multilacunar with five or more traces). Lysigenic or schizogenic mucilage ducts, cavities and cells often present (especially in epidermis, cortex and medulla). Heartwood sometimes with gum-like deposits. Silica bodies present in some species. Cortex with or without cristarque cells. Prismatic or acicular calciumoxalate crystals frequent (usually in groups), sometimes druses, styloids, or crystal sand.
Trichomes Hairs unicellular or multicellular, uniseriate or multiseriate, simple or branched, fasciculate, lanate, bristle-like, furcate, multi-armed, stellate, peltate, lepidote; glandular hairs (also peltate) often abundant.
Leaves Usually alternate (spiral or distichous; sometimes opposite, rarely verticillate), simple or palmately compound (rarely unifoliolate), entire or palmately lobed, with conduplicate to plicate, supervolute, involute or curved ptyxis. Stipules often intrapetiolar, usually well developed, sometimes large and foliaceous, often early caducous (rarely reduced); leaf sheath absent. Petiole vascular bundle transection arcuate or annular; petiole sometimes also with inner ring of bundles (sometimes with medullary bundles), or petiole anatomy simple. Venation pinnate or palmate, brochidodromous, eucamptodromous, actinodromous or acrodromous (sometimes parallelodromous); one vein proceeding into non-glandular apex. Stomata usually anomocytic (sometimes anisocytic, paracytic, tetracytic, cyclocytic or helicocytic). Cuticular wax crystalloids as rosettes of platelets (Fabales type) or absent. Domatia as pockets or hair tufts (rarely pits) or absent. Epidermis and mesophyll with or without mucilaginous idioblasts. Mesophyll often with sclerenchymatous idioblasts. Parenchyma sometimes with schizogenous secretory ducts. Cystoliths sometimes present. Leaf margin serrate (sometimes glandular-serrate), crenate, sinuate or entire; leaf teeth often malvoid.
Inflorescence Terminal or axillary, cymose of various shape, thyrsoid, panicle, raceme-like (sometimes raceme, head, or racemose panicle), or flowers solitary. Inflorescence often composed of cymose modified ’bicolor’ units, consisting of one terminal flower with three bracts, two of which having one cymose partial inflorescence each, with normal number of floral prophylls (bracteoles), and lowermost bract without axillary partial inflorescence. Floral prophylls (bracteoles) sometimes absent.
Flowers Usually actinomorphic (rarely zygomorphic or asymmetrical). Epicalyx, usually consisting of three bracts, sometimes present. Usually hypogyny (rarely epigyny or half epigyny). Sepals (three to) five (or six), usually with valvate (sometimes imbricate or contorted) aestivation, persistent or caducous, sometimes petaloid, free or more or less connate in lower part. Petals (three to) five (to nine), usually with contorted, valvate or convolute (sometimes imbricate or open) aestivation, sometimes clawed, usually free (rarely connate at base; sometimes absent). Nectaries often as groups of glandular hairs, usually adaxial on sepal bases, or absent. Disc extrastaminal to intrastaminal, annular, or absent.
Androecium Stamens usually (secondarily) numerous (rarely five, 5+5 or up to more than 1.000), usually in five alternisepalous or antesepalous fascicles (rarely in several whorls), centrifugally developing. Filaments simple or branched, free or connate at base in groups, or connate in two whorls into tube, outer usually antepetalous, usually with fertile stamens, inner usually consisting of staminodia; filaments free from or adnate to petal bases. Anthers basifixed or dorsifixed, versatile or non-versatile, usually tetrasporangiate (sometimes di-, hexa-, or polysporangiate; sometimes septate), usually introrse (sometimes extrorse or latrorse), usually longicidal (dehiscing by longitudinal slits; rarely poricidal, dehiscing by apical or lateral pores or short slits). Tapetum usually secretory (rarely amoeboid-periplasmodial). Antesepalous extrastaminal staminodia often present, usually adnate to fertile stamens.
Pollen grains Microsporogenesis simultaneous. Pollen grains 3(–4)-colpate, (2–)3(–9)-colpor(oid)ate, 3(–9)-porate, spiraperturate or polypantoporate, usually shed as monads (rarely tetrads), usually bicellular (sometimes tricellular) at dispersal. Exine tectate or semitectate (rarely intectate), with columellate or acolumellate infratectum, perforate, microperforate, reticulate, microreticulate, striate, rugulate, foveolate, verrucate, spinulate, psilate or smooth. Pollen grains often germinating with several pollen tubes.
Gynoecium Pistil composed of (one to) five (to numerous) usually connate (rarely free, secondarily apocarpous) and usually antepetalous (sometimes antesepalous) carpels; when three carpels, then median carpel abaxial or adaxial. Ovary usually superior (rarely semi-inferior), (unilocular to) quinquelocular (to multilocular), sometimes on gynophore or androgynophore. Style single, simple, or stylodia (two to) five (to numerous), free or more or less connate in lower part. Stigma capitate or lobate, or stigmas capitate or punctate, papillate or non-papillate, usually Dry (rarely Wet) type. Pistillodium?
Ovules Placentation usually axile (sometimes parietal, apical, basal, lateral or free central). Ovules two or several (sometimes numerous; rarely one) per carpel, anatropous or hemianatropous to campylotropous (sometimes orthotropous), ascending, horizontal or pendulous, epitropous, bitegmic, crassinucellar (rarely pseudocrassinucellar). Micropyle usually bistomal (sometimes exostomal or endostomal). Funicular, placental or stylar obturator sometimes present. Archespore sometimes multicellular. Nucellar cap sometimes present. Megagametophyte usually monosporous, Polygonum type. Synergids sometimes with a filiform apparatus. Antipodal cells often long persistent, sometimes proliferating (up to c. 20 cells). Endosperm development usually nuclear. Endosperm haustorium chalazal. Embryogenesis usually asterad (sometimes caryophyllad or solanad, rarely onagrad or irregular).
Fruit Usually a loculicidal (sometimes septicidal, rarely denticidal or poricidal) capsule, often a schizocarp, regma, with few to numerous usually nutlike (rarely samaroid, baccate, drupaceous or follicular) mericarps (rarely a berry, drupe, nut, nutlet or multifolliculus). Endocarp or centre of fruit sometimes hairy or fleshy.
Seeds Aril or strophiole sometimes present. Seed coat exotegmic or endotestal-exotegmic. Exotesta sometimes wooly or winged. Endotestal cells often with calciumoxalate crystals. Exotegmen usually palisade, often consisting of malpighiacean cells with thickened lignified walls. Exotegmen sometimes with palisade layer invaginating chalazal region, into which hypostase plug, with core and annulus, fits (outer hypostase forming core, ’bixoid chalazal region’). Endotegmic cell walls sometimes thickened. Perisperm not developed. Endosperm usually copious, oily or starchy (sometimes sparse or absent). Embryo straight or curved, usually with chlorophyll. Cotyledons two, often thin. Radicula often short. Germination phanerocotylar or cryptocotylar.
Cytology x = 5–11(–13)
DNA Plastid gene infA lost/defunct. Mitochondrial intron coxII.i3 lost.
Phytochemistry Flavonols (kaempferol, quercetin, myricetin, herbacetin, gossypetin, hibiscetin, etc.) and their glycosides, flavones, afzelechin, flavonoid sulfates, biflavonoids, cyanidin, delphinidin, apigenin, monoterpenes (e.g. borneol), triterpenes, (e.g. dryobalanone), toxic diterpene esters (e.g. phorbol ester diterpenes), oleanolic acid derivatives, dammaranes, dammarane triterpenoids (dipterocarpol), cucurbitacins, arjunolic acid derivatives, quinoid and/or phenolic sesquiterpenes (gossypol, ishwarane etc.), sesquiterpene lactones, ellagic acid, methylated ellagic and gallic acids, hydrolyzable and non-hydrolyzable tannins, proanthocyanidins (prodelphinidins), caffeic acid, p-coumaric acid, indole alkaloids (e.g. theophylline, caffeine and theobromine), saponins, cyanogenic glycosides and other cyanogenic compounds, naphthoquinones, anthraquinones, polyacetate-derived arthroquinones, acetophenones, coumarins (daphnin, daphnetin), quebrachitol, lignans (syringaresinol, pinoresinol), pinitol, chelidonic acid, ferulic acid, sinapic acid, lipids of cyclopropane and cyclopropenoid fatty acids (sterculiic acid, malvic acid, etc.) and their derivatives, and sterols present. Mucilage as heteropolysaccharides of galacturonic and glucuronic acids with galactose, rhamnose, glucose, and arabinose.
Systematics Malvales are sister-group to Capparales.
Neuradaceae are sister to the remaining Malvales and Thymelaeaceae successive sister to the rest.
The clade [Thymelaeaceae+[Sphaerosepalaceae+[Bixaceae+Cochlospermaceae]+[Cistaceae+ [Sarcolaenaceae+Dipterocarpaceae]]+[Muntingiaceae+Cytinaceae]+Malvaceae]] is characterized by the following potential synapomorphies, according to Stevens (2001 onwards): presence of vestured pits; stratified phloem; cuneate (wedge-shaped) phloem rays; and much thickened and lignified palisade exotegmen.
The clade [Sphaerosepalaceae+[Bixaceae+Cochlospermaceae]+[Cistaceae+[Sarcolaenaceae+ Dipterocarpaceae]]+[Muntingiaceae+Cytinaceae]+Malvaceae] has the potential synapomorphies: hairs often stellate; stipules often well developed; stamens numerous, developing centrifugally, with five or ten (or 15) vascular bundles (when five, then often antepetalous); ovules six or more per carpel; and micropyle bistomal.
The clade [[Bixaceae+Cochlospermaceae]+[Cistaceae+[Sarcolaenaceae+Dipterocarpaceae]]] has the following characteristics in common (Stevens 2001 onwards): presence of secretory ducts; sepals with imbricate aestivation; unique differentiation of ovule chalazal part: plug of hypostase tissue fitting into curved dome-shaped structure formed by exotegmic palisade layer, plug containing core and annulus (bixoid chalaza; Nandi 1998). The unique bixoid chalazal plug forms an opening through the often very hard seed coat of these plants; hence, water may enter the seed and facilitate germination.
Bixaceae, Cochlospermaceae and Cistaceae share: foliar teeth with one vein proceeding into transparent caducous tooth apex; and elongate embryo with thin curved or plicate cotyledons and short stout radicula. However, molecular data recover Cistaceae as sister to [Dipterocarpaceae+Sarcolaenaceae] or at least as member of the trichotomy [Cistaceae+Sarco-laenaceae+Dipterocarpaceae].
Bixaceae and Cochlospermaceae share the characters (Stevens 2001 onwards): secretory ducts present; resiniferous cells present outside veins; hairs glandular, not tufted or stellate; leaf margin serrate; terminal inflorescence; large flowers; sepals with imbricate aestivation; anthers poricidal; stigma slightly lobate to capitate; ovules numerous per carpel; long funicle; micropyle Z-shaped (zig-zag); outer hypostase forming core.
The clade [Cistaceae+[Sarcolaenaceae+Dipterocarpaceae]] has the following potential synapomorphies (Stevens 2001 onwards): ectomycorrhizae present; presence of tracheids; presence of secretory canals; sepals with imbricate quincuncial aestivation; two outer sepals often different from remaining sepals; filaments not articulated; ovules both anatropous and orthotropous; exotegmen curved inwards in chalazal region; hypostase plug with core and annulus; endosperm starchy; and presence of ellagic acid.
Shape of indumentum, hollow style, morphology of stigma, number of carpels, and several other features unite Cistaceae and Sarcolaenaceae. In both Fumana (Cistaceae) and Dipterocarpoideae (Dipterocarpaceae) the outer integument is prolonged and ectomycorrhiza is frequent.
Sarcolaenaceae and Dipterocarpaceae share the characters: usually well developed stipules and complex petiolar anatomy.
Muntingiaceae and Cytinaceae both have many-seeded berry and ellagitannins.

|
Cladogram of Malvales based on DNA sequence data (Stevens 2001 onwards, May 2012 version). |
BIXACEAE Kunth |
( Back to Malvales ) |
Bixales Link, Handbuch 2: 371. 4-11 Jul 1829 [’Bixinae’]; Diegodendraceae Capuron in Adansonia, sér. 2, 3: 392. Oct-Dec 1964
Genera/species 2/6
Distribution Tropical South America, Madagascar.
Fossils Unknown.
Habit Bisexual, evergreen shrubs or small trees. Leaves in Diegodendron fragrant (like camphor). Juice coloured (orange to red).
Vegetative anatomy Phellogen ab initio superficial. Vessel elements with simple perforation plates; lateral pits alternate, bordered pits. Vestured pits? Imperforate tracheary xylem elements fibre tracheids or libriform fibres with bordered (Bixa) or simple (Diegodendron) pits, non-septate (radial tracheids present in Bixa arborea). Wood rays in Bixa uniseriate, homocellular; in Diegodendron biseriate, homocellular. Axial parenchyma in Bixa apotracheal diffuse; in Diegodendron apotracheal diffuse or diffuse-in-aggregates. Wood elements storied (Bixa). Secondary phloem stratified into hard fibrous and soft parenchymatous layers. Sieve tube plastids S type. Nodes 3:?, trilacunar with ? leaf traces (Bixa), with extrafloral nectariferous glands. Mucilage cells? Medullary parenchyma with secretory canals (with bixine?). Exudate in Bixa red to orange. Idioblasts with calciumoxalate raphides.
Trichomes Hairs in Bixa multicellular, stellate or peltate-lepidote, reddish-brown; glandular hairs peltate.
Leaves Alternate (in Bixa spiral; in Diegodendron distichous), simple or palmately compound, entire or palmately lobed, with conduplicate ptyxis. Stipules in Bixa enclosing bud, in Diegodendron intrapetiolar, unequal in size, sheathing and surrounding stem/branch, caducous; leaf sheath absent. Petiole vascular bundle transection annular; petiole with medullary bundles. Lamina in Bixa densely beset with reddish-brown hairs, in Diegodendron densely gland-dotted, glabrous. Venation in Bixa palmate, brochido-actinodromous, in Diegodendron pinnate. Stomata anomocytic, paracytic or anisocytic (in Diegodendron sometimes cyclocytic). Cuticular wax crystalloids? Parenchyma with schizogenous secretory canals. Resinous secretory cells present outside veins. Hairs stellate or peltate and brown (Bixa), or absent (Diegodendron). Leaf margin serrate or entire. Leaf teeth with one vein running into transparent caducous tooth apex.
Inflorescence Terminal, thyrsoid (in Diegodendron usually panicle), or flowers solitary.
Flowers Usually actinomorphic (in Bixa rarely somewhat zygomorphic), large. Pedicel in Bixa with five large extrafloral nectariferous glands. Hypogyny. Sepals five (or six), with imbricate quincuncial or contorted? (Bixa) aestivation, caducous (Bixa) or persistent (Diegodendron), in Bixa with distinct abaxial glands at base, outer sepals in Diegodendron smaller, free. Petals five (or six), usually with imbricate (in Diegodendron rarely contorted) aestivation, caducous (Diegodendron), free. Nectary absent. Disc in Bixa absent, in Diegodendron indistinct or absent.
Androecium Stamens numerous (in Diegodendron sometimes more than 400), centrifugally developing. Filaments filiform (Diegodendron), free from each other and from tepals. Anthers basifixed (Diegodendron), in Bixa hippocrepomorphic and horizontally folded, non-versatile (Diegodendron), tetrasporangiate, latrorse (Diegodendron), in Bixa poricidal (dehiscing by short lateral, seemingly apical, slits or pores), in Diegodendron longicidal (dehiscing by longitudinal slits); connective in Bixa curved into loop. Tapetum secretory. Staminodia absent.
Pollen grains Microsporogenesis simultaneous. Pollen grains tricolporate, shed as monads, bicellular at dispersal. Exine tectate or semitectate, with columellate infratectum, rugulate, microperforate (Bixa) or verrucate to insulate (Diegodendron).
Gynoecium Pistil composed of (one or) two (to four) more or less connate antesepalous, alternipetalous carpels. Ovary superior, unilocular in Bixa (carpels connate), in Diegodendron usually bilocular (sometimes uni-, tri- or quadrilocular); carpels free below (apocarpy). Style single, simple, in Diegodendron gynobasic, persistent, in Bixa apical. Stigma capitate or somewhat lobate (in Diegodendron punctate), type? Pistillodium absent.
Ovules Placentation parietal (Bixa) or basal (Diegodendron). Ovules usually two (Diegodendron) or numerous (Bixa) per carpel, anatropous, ascending and epitropous (Diegodendron), bitegmic, pseudocrassinucellar (Bixa) or crassinucellar (Diegodendron). Funicle long (Bixa). Micropyle exostomal to bistomal, somewhat Z-shaped (zig-zag; Bixa). Outer integument ? cell layers thin. Inner integument in Bixa four or five cell layers thick, in Diegodendron ? cell layers thick. Hypostase present (Bixa). Megagametophyte monosporous, Polygonum type. Endosperm development ab initio nuclear. Endosperm haustoria? Embryogenesis irregular (Bixa).
Fruit A loculicidal capsule, usually with long prickles, dehiscing by two valves or remaining indehiscent (Bixa), or a schizocarp with glands and one (to four) indehiscent mericarps (Diegodendron).
Seeds Funicular aril present in Bixa (absent in Diegodendron). Testa in Bixa with red sarcotesta (with bixine, a carotenoid), in Diegodendron thin with viscid outer layer. Exotegmen in Bixa with palisade layer (absent in Diegodendron) invaginating chalazal region, into which hypostase plug, with core and annulus, fits (outer hypostase forming core, ’bixoid chalazal region’). Endotegmic cell walls in Bixa more or less thickened. Hypodermal layer consisting of clepsydromorphous (hour-glass-shaped) cells. Perisperm not developed. Endosperm starchy (Bixa) or absent (Diegodendron). Embryo long, straight, without chlorophyll (Bixa). Cotyledons two, in Bixa spatulate, thin, curved or folded, in Diegodendron thick. Radicula short, stout. Germination phanerocotylar.
Cytology n = 7 (Bixa)
DNA
Phytochemistry Flavones, flavonoid sulphates, cyanidin, arjunolic acid derivatives, sesquiterpenes (e.g. ishwarane), ellagic acid, tannins, and proanthocyanidins (prodelphinidins) present (in Bixa). Flavonols (kaempferol, quercetin, myricetin), alkaloids, saponins, and cyanogenic compounds not found.
Use Dyeing substances (bixine for staining of margarine and other food stuffs, cosmetics; Bixa orellana: annatto, arnotto, roucou).
Systematics Bixa (5; B. arborea, B. excelsa, B. orellana, B. platycarpa, B. urucurana; Mexico, Central America, the West Indies, tropical South America), Diegodendron (1; D. humbertii; Madagascar).
Bixaceae are sister to Cochlospermaceae or, possibly, to Sphaerosepalaceae (Johnson-Fulton & Watson 2017).
CISTACEAE Juss. |
( Back to Malvales ) |
Cistales DC. ex Bercht. et J. Presl, Přir. Rostlin: 220. Jan-Apr 1820 [’Cisti’]; Cistopsida Bartl., Ord. Nat. Plant.: 221, 277. Sep 1830 [’Cistiflorae’]; Helianthemaceae G. Mey., Chloris Han.: 9, 185. Jul-Aug 1836 [’Helianthemeae’]; Cistineae Rchb., Deutsch. Bot. Herb.-Buch: lxxvi. Jul 1841
Genera/species 8/205–210
Distribution Temperate and subtropical regions on the Northern Hemisphere east to Central Asia, with their largest diversity in the Mediterranean area, eastern North Africa and the eastern United States; few species in the West Indies and southern South America.
Fossils Uncertain. Fossil fruits and wood assigned to Cistaceae from the Late Eocene have been reported from European strata.
Habit Bisexual, evergreen shrubs or suffrutices, or annual herbs. Numerous species are xerophytes.
Vegetative anatomy Root hairs (rhizoids) absent at least in seedlings. Ectomycorrhiza present in at least Fumana and Helianthemum; endomycorrhiza at least sometimes present. Phellogen ab initio usually superficial (rarely deeply seated). Endodermis sometimes prominent. Vessel elements with simple perforation plates; lateral pits alternate, bordered pits. Vestured pits present. Imperforate tracheary xylem elements fibre tracheids or tracheids with bordered pits, non-septate. Wood rays usually uniseriate (sometimes multiseriate, in Pakaraimaea biseriate), heterocellular. Axial parenchyma poorly developed (apotracheal diffuse or diffuse-in-aggregates, paratracheal scanty vasicentric) or absent. Wood elements not storied. Phloem not stratified. Intraxylary phloem present in Pakaraimaea. Sieve tube plastids Ss type. Nodes 1:1, unilacunar with one leaf trace. Secretory canals? Heartwood vessels with gum-like substances. Mucilage cells absent? Prismatic calciumoxalate crystals (sometimes druses) present or absent.
Trichomes Hairs unicellular or multicellular, uniseriate or multiseriate, simple, fasciculate or stellate or peltate (characteristic, seemingly bicellular, each cell with basal inner compartment); peltate-lepidote glandular hairs sometimes present (in Cistus with resinous balsam and ethereal oils).
Leaves Usually opposite (sometimes verticillate, rarely spiral), simple, entire, often coriaceous, sometimes scale-like or ericoid, with conduplicate to curved ptyxis. Stipules probably absent (or intrapetiolar?); leaf sheath absent. Petiole vascular bundle transection arcuate. Leaf bases sometimes connate. Venation pinnate or palmate (lamina often single-veined or with at least three parallel veins). Stomata anomocytic. Cuticular wax crystalloids usually absent (sometimes as platelets or annular rodlets). Epidermis with or without mucilaginous idioblasts. Cystoliths often present. Leaf margin serrate or entire.
Inflorescence Terminal or axillary, cymes of various shape, or flowers solitary.
Flowers Actinomorphic. Receptacle often prolonged into androphore or gynophore? Hypogyny. Sepals (three or) five, with imbricate quincuncial (inner sepals sometimes with contorted) aestivation, when five then two outer much smaller than three inner sepals, usually persistent, free or more or less connate. Petals usually five (in Lechea three small), with usually contorted aestivation (in opposite direction to sepals; in Lechea and Pakaraimaea imbricate quincuncial aestivation), wrinkled in bud, caducous, free (rarely absent); petal epidermis with approx. ten micropapillae per cell. Androgynophore present in Pakaraimaea. Nectary usually absent (annular nectariferous disc rarely present?). Disc extrastaminal to intrastaminal, annular.
Androecium Stamens (three to) numerous (androecium with ten vascular bundles), centrifugally developing, often sensitive. Filaments free from each other and from tepals. Anthers basifixed, non-versatile (basifixed-versatile in Pakaraimaea), tetrasporangiate, introrse, longicidal (dehiscing by usually longitudinal slits); anthers in some cleistogamous flowers adnate to stigma and apically dehiscing. Tapetum secretory. Staminodia usually absent (in Fumana extrastaminal staminodia present).
Pollen grains Microsporogenesis simultaneous. Pollen grains (2–)3(–5)-colpor(oid)ate, often starchy, usually shed as monads (rarely tetrads), bicellular at dispersal. Exine tectate or semitectate, with columellate infratectum, reticulate, microreticulate, rugulate, striate or striate-reticulate.
Gynoecium Pistil composed of (two or) three to five (to twelve) connate carpels; carpels alternisepalous antepetalous, or median carpel abaxial. Ovary superior, unilocular or incompletely multilocular due to intruding and often connate parietal placentae. Style single, simple, hollow, or absent. Stigma usually one (stigmas rarely three), large, capitate or discoid, often lobate (rarely fringed), papillate (with multicellular multiseriate papillae), Dry type. Pistillodium absent.
Ovules Placentation parietal (often deeply intrusive). Ovules two to numerous (rarely one) per carpel, usually orthotropous (in Fumana anatropous or hemianatropous), ascending, bitegmic, crassinucellar. Funicle usually long (in Fumana short). Micropyle usually bistomal (in Fumana exostomal). Outer integument approx. two cell layers thick. Inner integument two to four cell layers thick. Parietal tissue approx. two cell layers thick. Nucellar cap approx. two cell layers thick. Hypostase present. Archespore sometimes multicellular. Megagametophyte monosporous, Polygonum type. Synergids with a filiform apparatus. Endosperm development ab initio nuclear. Endosperm haustoria? Embryogenesis solanad.
Fruit Usually loculicidal (septifragal?) capsule (in Pakaraimaea many-seeded nut), often surrounded by persistent sepals.
Seeds Aril absent. Testa thin, starchy, as wet sometimes gelatinous and mucilaginous. Tegmen very hard. Exotegmen palisade, with lignified malphighiacean cells; exotegmen invaginating chalazal region, into which hypostase plug, with core and annulus, fits (outer hypostase forming core, ’bixoid chalazal region’). Endotegmic cells with thickened radial and/or tangential walls. Perisperm not developed. Endosperm copious, starchy. Embryo usually often strongly curved (in Lechea almost straight), long, well developed, with or without chlorophyll. Cotyledons two, thin, curved or plicate. Radicula short, stout. Germination phanerocotylar.
Cytology n = 5, 7, 9–12, 16 (Fumana), 18, 20, 24 – Polyploidy occurring.
DNA
Phytochemistry Flavonols (kaempferol, quercetin, myricetin, etc.), flavonoid sulphates, cyanidin, delphinidin, dammaranes, ellagic and gallic acids, hydrolyzable and condensed tannins, proanthocyanidins (prodelphinidins), saponins, acetophenones, and pinitol present. Alkaloids and cyanogenic compounds not found. Certain species of Cistus secrete aromatic balsam (ladanum, labdanum), a mixture of ethereal oils and resin (diterpene and triterpene esters).
Use Ornamental plants, perfumes (Cistus), medicinal plants.
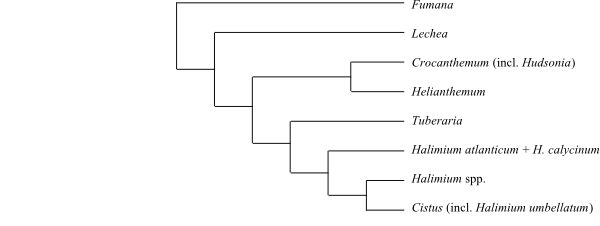
Systematics Pakaraimaeoideae Maguire, P. S. Ashton et de Zeeuw in B. Maguire et P. S. Ashton in Taxon 26: 353. 23 Sep 1977. Pakaraimaea (1; P. dipterocarpacea; the Guayana and Venezuelan Highlands in northern South America). – Cistoideae Eaton, Bot. Dict., ed. 4: 42. Apr–Mai 1836 [‘Cistineae’]. Fumana (15–18; Europe, the Mediterranean, North Africa); Lechea (17–18; North America, Mexico, Belize, Cuba); Crocanthemum (21; southern Canada, United States, Mexico, Central America, Hispaniola, South America), Helianthemum (c 110; Europe, the Canary Islands, the Mediterranean, North Africa to Central Asia), Tuberaria (c 12; West and Central Europe, the Mediterranean), Halimium (12; the Mediterranean, North Africa, Turkey), Cistus (19–20; the Canary Islands, the Mediterranean, Turkey, the Caucasus).
Cistaceae are possibly sister-group to [Dipterocarpaceae+Sarcolaenaceae].
Fumana (with several plesiomorphies) is sister to all other Cistaceae except Pakaraimaea, and Lechea successive sister-group to the remainder.
Pakaraimaea dipterocarpacea. Evergreen tree or shrub. Ectomycorrhiza present. Wood rays usually biseriate. Intraxylary phloem present. Hairs fasciculate. Stipules elongate, caducous. Petiole non-geniculate. Inflorescence panicle. Hypogyny. Petals with imbricate aestivation, shorter than sepals. Stamens c. 40 to c. 50, on short androgynophore. Anthers basifixed-versatile. Pollen grains tricolporate. Exine semitectate, infratectum columellate, reticulate. Pistil composed of five connate carpels. Ovary (quadrilocular or) quinquelocular. Ovules (two to) four per carpel. Fruit a many-seeded nut, probably with five accrescent almost equally sized sepals. Endosperm absent. n = ?
 |
Bayesian inference analysis tree of Cistaceae-Cistoideae based on DNA sequence data (Guzmán & Vargas 2009). |
COCHLOSPERMACEAE Planch. |
( Back to Malvales ) |
Cochlospermineae Engl., Syllabus, ed.2: 154. Mai 1898
Genera/species 2/17
Distribution Tropical Africa, India, Sri Lanka, southern Himalayas, northern Burma, northern Thailand, northern Laos, eastern New Guinea, northern Australia, southern United States, Mexico, Central America, the West Indies, tropical South America.
Fossils Unknown.
Habit Bisexual, deciduous trees or shrubs (Cochlospermum), or perennial herbs with woody subterranean stem (Amoreuxia). Some species are xerophytes. Juice coloured (yellow), resinous.
Vegetative anatomy Phellogen? Young stem with vascular tissue forming continuous cylinder. Vessel elements with simple perforation plates; lateral pits alternate, scalariform or opposite, simple or bordered pits. Imperforate tracheary xylem elements fibre tracheids with simple or bordered pits (Amoreuxia without imperforate tracheary elements), non-septate. Wood rays multiseriate, homocellular or heterocellular. Axial parenchyma apotracheal diffuse, paratracheal scanty vasicentric, unilateral, or banded. Wood elements often partially storied. Secondary phloem stratified into hard fibrous and soft parenchymatous layers. Sieve tube plastids Pcs type, with protein crystals and starch. Nodes 3:3?, trilacunar with three? leaf traces. Parenchyma in Cochlospermum with branched unicellular resinous idioblasts. Cortex and medulla with mucilage cells and lysigenic mucilage canals. Crystals?
Trichomes Hairs unicellular, simple, or on young shoots multicellular, stalked; sometimes gland-like and peltate-lepidote.
Leaves Alternate (spiral), palmately compound or simple and palmately lobed, with conduplicate ptyxis. Stipules usually small, narrow, caducous; leaf sheath absent. Petiole vascular bundles three, with transection annular; petiole without medullary bundles. Venation palmate. Stomata anomocytic. Cuticular wax crystalloids? Epidermis often with mucilage cells. Resinous secretory cells present outside veins. Leaf margin serrate or entire. Leaf teeth with one vein proceeding into transparent caducous apex.
Inflorescence Usually terminal (rarely axillary), thyrsoid, panicle or raceme-like. Floral prophylls (bracteoles) absent.
Flowers Slightly zygomorphic (Amoreuxia, buds in Cochlospermum) or actinomorphic (open flowers in Cochlospermum), large. Hypogyny. Sepals (four or) five, with imbricate or contorted aestivation (sepals unequal in size, or three sepals and two floral prophylls?; median sepal abaxial), caducous, free. Petals (four or) five, with contorted or imbricate aestivation, free. Nectary probably absent. Disc indistinct or absent.
Androecium Stamens numerous, often in two fascicles (Amoreuxia; upper stamens in Amoreuxia much shorter than lower stamens) or in five fascicles (Cochlospermum), centrifugally developing. Filaments free from each other and from tepals. Anthers basifixed, non-versatile, tetrasporangiate, introrse, poricidal (dehiscing by one or two apical, sometimes also two basal, pores or pore-like slits). Tapetum secretory. Staminodia absent.
Pollen grains Microsporogenesis simultaneous. Pollen grains tricolpate, tricolporate or tricolporoidate, shed as monads, bicellular at dispersal. Exine intectate to semitectate, with columellate infratectum, reticulate or psilate.
Gynoecium Pistil composed of three to five connate carpels; antepetalous or odd carpel adaxial. Ovary superior, unilocular or incompletely trilocular. Style single, simple. Stigma small, entire or somewhat lobate, type? Pistillodium absent.
Ovules Placentation entirely parietal (when ovary unilocular), or axial at base and apex and parietal in central part (when ovary multilocular). Ovules numerous per carpel, campyloanatropous, bitegmic, crassinucellar. Funicle long. Micropyle exostomal to bistomal, somewhat Z-shaped (zig-zag). Outer integument three cell layers thick. Inner integument three or four cell layers thick. Megagametophyte monosporous, Polygonum type. Endosperm development ab initio nuclear. Endosperm haustoria? Embryogenesis?
Fruit A capsule with loculicidal lignified exocarp/mesocarp separating from septicidal membranous endocarp.
Seeds Aril absent. Testa lanate or glabrous, thin. Tegmen thick, hard, with enlarged outer hypodermal cells. Exotegmen invaginating chalazal region, into which hypostase plug, with core and annulus, fits; chalaza closed by hypostase plug (outer hypostase forming core, ’bixoid chalazal region’). Endotegmen? Perisperm not developed. Endosperm thick, oily, without starch. Embryo elongate, usually curved (sometimes straight), chlorophyll? Cotyledons two, spatulate, thin, curved or plicate. Radicula short, stout. Germination phanerocotylar.
Cytology n = 6, 9, 12, 18 – Polyploidy frequently occurring.
DNA
Phytochemistry Flavonols (kaempferol, quercetin, myricetin, etc.), cyanidin, afzelechin, ellagic acid (Cochlospermum), gum substances (’coutira gum’) with frequently branched acetylated polysaccharides consisting of galactose, rhamnose, glucuronic acid and galacturonic acid (Cochlospermum), and saponins present.
Use Ornamental plants (Cochlospermum), medicinal plants, timber.
Systematics Amoreuxia (4; A. gonzalezii, A. malvifolia, A. palmatifida, A. wrightii; southern Arizona, Mexico, Central America, Colombia, Peru), Cochlospermum (13; western and central tropical Africa, southern and eastern India, Sri Lanka, southern Himalayas, northern Burma, northern Thailand, northern Laos, eastern New Guinea, northern and Western Australia, southern United States, Mexico, Central America, the West Indies, tropical South America to southeastern Brazil and northern Argentina).
Cochlospermaceae are sister to Bixaceae.
CYTINACEAE Brongn. ex A. Rich. |
( Back to Malvales ) |
Cytinales Dumort., Anal. Fam. Plant.: 13. 1829 [‘Cytinarieae’]
Genera/species 2/13
Distribution The Canary Islands, the Mediterranean, Turkey, southern and western Caucasus, southern Africa, Madagascar, southern Mexico, Central America.
Fossils Unknown.
Habit Monoecious or dioecious, achlorophyllous perennial herbaceous endophytic root holoparasites without rhizome or normal roots. Cytinus subgenus Cytinus on species of Cistaceae; Cytinus subgenus Hypolepis on species of at least five different clades of angiosperms; Bdallophytum mostly on Bursera (Burseraceae).
Vegetative anatomy Mycorrhiza absent. Hypha-like cell threads invading host plant and forming endophytic system in roots. Phellogen absent. Secondary lateral growth absent. Xylem elements present in at least Cytinus. Vessels absent. Imperforate tracheary xylem elements? Wood rays absent. Axial parenchyma? Phloem elements present in at least Cytinus. Sieve tube plastids S0 type, without starch or protein inclusions. Nodes? Crystals?
Trichomes Indumentum absent on vegetative parts.
Leaves Alternate (spiral), reduced, scale-like. Stipules and leaf sheath absent. Venation? Stomata poorly differentiated (Cytinus). Cuticular waxes absent? Leaf margin entire.
Inflorescence Terminal, in Bdallophytum spicate, in Cytinus raceme-like or sometimes capitular. Flowers and inflorescence branches pressing themselves outwards through host cortex.
Flowers Actinomorphic. Epigyny. Tepals four to six (Cytinus) or five to nine (Bdallophytum), in one whorl, connate at base; tepals rarely adnate by dissepiment to stamens and stylodium. Thin tissue, diaphragm, present on top of perigonal tube and special outgrowths, ramenta, inserted inside perigonal tube in Cytinus. Nectariferous tissue in Cytinus as hippocrepomorphic glands at adaxial side of perigonal base (absent in Bdallophytum?). Disc absent.
Androecium Stamens six to ten (to more than 20), whorled. Filaments absent. Anthers connate into synandrium (in Cytinus with nectariferous pits between staminal bases), dorsifixed, non-versatile, disporangiate, monothecal, extrorse, longicidal (dehiscing by longitudinal slits); connective thick, usually with terminal and often branched appendage. Tapetum secretory? Female flowers without staminodia.
Pollen grains Microsporogenesis simultaneous? Pollen grains di- to tetraporate or indistinctly di- to tetracolpate, usually shed as monads (in some species of Cytinus as tetrads), bicellular at dispersal. Exine tectate to semitectate, with columellate infratectum, perforate or microreticulate.
Gynoecium Pistil composed of five to eight (to 14) connate carpels. Ovary inferior, ab initio septate, multilocular, later unilocular. Style single, simple, long, enlarged and discoid at apex, with nectariferous cavities near base. Stigma commissural, as annular radiate capitate structure on abaxial edge or lower side of discoid apex of central column, in Cytinus with six to eight lobes, in Bdallophytum discoid, type? Male flowers with pistillodium.
Ovules Placentation intrusively parietal, with branched placentae. Ovules five to 14 per ovary, orthotropous, unitegmic or bitegmic (then outer integument higly reduced), tenuinucellar. Micropyle endostomal. Outer integument two or three cell layers thick, poorly developed. Inner integument approx. two cell layers thick. Megasporangial epidermis persistent. Megagametophyte monosporous, Polygonum type. Antipodal cells persistent. Endosperm development ab initio nuclear. Endosperm haustoria? Embryogenesis caryophyllad or solanad?
Fruit A many-seeded berry (Cytinus) or a syncarp with berry-like solitary fruits (Bdallophytum).
Seeds Aril absent. Seeds in Cytinus with rudimentary exotesta. Seed coat exotegmic. Exotesta in Bdallophytum? Endotesta hard. Exotegmic cells thickened around. Endotegmen? Perisperm not developed. Endosperm present. Embryo rudimentary, undifferentiated (few-celled in few tiers), chlorophyll? Cotyledons two. Germination phanerocotylar?
Cytology n = 12, 16 (Cytinus hypocistis)
DNA
Phytochemistry Very insufficiently known. Ellagitannins (isoterchebin) present.
Use Unknown.
Systematics Cytinus (8; subgenus Cytinus in the Mediterranean, the Canary Islands, Turkey, southern and western Caucasia; subgenus Hypolepis in southern Africa and Madagascar); Bdallophytum (5; B. americanum, B. andrieuxii, B. bambusarum, B. ceratantherum, B. oxylepis; southern Mexico, Central America)
The position of Cytinaceae in Malvales is not clarified. They sometimes appear as sister to Muntingiaceae, although with fairly weak support (Nickrent 2007). Both Cytinaceae and other Malvales have exotegmic seeds and the perianth morphology in Cytinaceae resembles that in Malvaceae. The position of Bdallophytum is uncertain. It is tentatively placed as sister to Cytinus.
DIPTEROCARPACEAE Blume |
( Back to Malvales ) |
Monotaceae (Gilg) Kosterm. in Taxon 38: 123. 27 Feb 1989
Genera/species 14/530–535
Distribution Tropical Africa, Madagascar, the Seychelles, tropical Asia from Sri Lanka and India to New Guinea, Colombian Amazonas, the Guayana and the Venezuelan Highlands, with their highest diversity in lowland rainforests of West Malesia.
Fossils Fossil wood assigned to Dipterocarpaceae has been found on many places. Woburnia porosa was described from England and is probably of Early Cenozoic age. Other fossil woods possibly attributable to Dipterocarpaceae are Dipterocarpoxylon, Dryobalanoxylon, Grewioxylon and Shoreoxylon from tropical Asia and eastern Africa. Pollen grains of Oligocene age have been recorded from Borneo.
Habit Bisexual, evergreen trees or shrubs. Often with large plank buttresses.
Vegetative anatomy Ectomycorrhiza present. Root hairs (rhizoids) sometimes absent. Phellogen ab initio superficial to outer-cortical. Cortical vascular bundles present or absent. Vessel elements usually with simple (rarely scalariform) perforation plates; lateral pits usually alternate (rarely opposite), simple pits. Vestured pits present. Imperforate tracheary xylem elements fibre tracheids with simple and/or bordered pits, non-septate (also vasicentric tracheids). Wood rays in Dipterocarpoideae multiseriate, in Monotoideae usually uniseriate, homocellular or heterocellular. Axial parenchyma apotracheal diffuse or diffuse-in-aggregates, or banded, or paratracheal scanty vasicentric, aliform, lozenge-aliform, winged-aliform, confluent, unilateral, or banded. Cambium and wood elements sometimes storied. Tyloses frequent (sometimes crystalliferous). Secondary phloem stratified into hard fibrous and soft parenchymatous layers. Sieve tube plastids S type. Nodes 3:3, trilacunar with three leaf traces, or 5:5, pentalacunar with five traces. Cortex, wood and medulla with branched vertical system of intercellular resinous ducts. Medulla often with secretory mucilage cavities. Silica bodies present in many species. Prismatic calciumoxalate crystals often frequent. Styloids, acicular crystals and/or crystal sand present in some species.
Trichomes Hairs unicellular or multicellular, uniseriate or multiseriate, simple or branched, fasciculate, stellate or peltate; glandular hairs unicellular or multicellular, often peltate-lepidote.
Leaves Alternate (spiral or distichous), simple, entire, coriaceous, usually with conduplicate (in Dipterocarpus and Parashorea conduplicate-plicate) ptyxis. Stipules intrapetiolar, caducous (in Stemonoporus early caducous) or persistent; leaf sheath absent. Petiole usually geniculate. Petiole vascular bundle transection annular; bundle surrounding central vascular tissue (complex, often with siphonostele with medullary bundles?). Stipules and leaf bases rarely with nectariferous glands (extrafloral nectaries). Venation pinnate; secondary veins parallel; tertiary veins usually scalariform. Stomata usually anomocytic or cyclocytic (rarely paracytic), sometimes on adaxial side only. Cuticular wax crystalloids? Domatia present as pits along mid-vein. Mesophyll with or without mucilaginous idioblasts and resinous and mucilage canals. Leaf margin entire or sinuate. Adaxial or abaxial surface of young leaves usually with peltate extrafloral nectaries.
Inflorescence Terminal or axillary, usually panicle or raceme-like, often branched and more or less monochasial (flowers rarely solitary).
Flowers Actinomorphic. Usually hypogyny (in Anisoptera half epigyny). Sepals two to five, with imbricate or valvate aestivation, usually persistent and strongly accrescent, usually free (rarely connate at base). Petals five, usually with contorted aestivation, usually free (in many Dipterocarpoideae connate at base). Androgynophore present in Monotoideae. Nectary probably absent. Disc absent.
Androecium Stamens five, 5+5, or 5+5+5 (rarely up to c. 110, irregularly inserted; androecial bundles five, or five alternipetalous and five alternisepalous), centrifugally developing. Filaments flattened or filiform, free or connate below, free from or adnate at base to petals (epipetalous). Anthers basifixed (Dipterocarpoideae) or basifixed-versatile (Monotoideae), usually tetrasporangiate (rarely disporangiate), usually latrorse, longicidal (usually dehiscing by longitudinal slits; rarely poricidal, dehiscing by two apical pores); connectives usually prolonged at apex. Tapetum secretory, with binucleate cells. Staminodia absent.
Pollen grains Microsporogenesis simultaneous. Pollen grains usually tricolpate (in Monotoideae tricolporate), shed as monads, bicellular at dispersal. Exine semitectate, with columellate infratectum, reticulate.
Gynoecium Pistil composed of (two or) three (to five) connate carpels; median carpel abaxial. Ovary usually superior (rarely semi-inferior), usually trilocular (rarely bilocular, quadrilocular, or quinquelocular). Style single, simple, or stylodia three, free or connate below, often widened at base into stylopodium. Stigmas usually three (stigma rarely single, entire to sexalobate), type? Pistillodium absent.
Ovules Placentation usually axile or lateral (sometimes apical). Ovules (one or) two (to four) per carpel, usually anatropous (when placenta lateral) or pendulous (when placenta axile), epitropous, bitegmic, crassinucellar. Micropyle endostomal or exostomal (Dipterocarpoideae). Outer integument two to ten cell layers thick. Inner integument two to nine cell layers thick, in Dipterocarpus vascularized. Megagametophyte monosporous, Polygonum type. Endosperm development ab initio nuclear. Endosperm haustoria? Embryogenesis asterad or irregular.
Fruit Usually a single-seeded nut (in Monotoideae and Upuna a capsule) with one to five wings formed by persistent and strongly (and often non-uniform) accrescent calyx (absent in some representatives).
Seeds Aril usually absent (present in Upuna). Testa vascularized. Exotesta? Endotesta? Exotegmic cells usually palisade, with sclereids; exotegmen invaginating chalazal region, into which hypostase plug, with core and annulus, fits (outer hypostase forming core, ’bixoid chalazal region’)? Endotegmen? Perisperm not developed. Endosperm usually absent (present in Monotoideae and Dipterocarpus). Embryo curved, with chlorophyll. Cotyledons two, often plicate, usually unequal in size, entire or lobed, enclosing radicula, starchy or with lipids. Germination phanerocotylar or cryptocotylar.
Cytology n = 6, 7, 10–13 (Dipterocarpoideae) – Polyploidy occurring.
DNA Mitochondrial coxI intron present (Shorea).
Phytochemistry Flavonols (kaempferol, quercetin, myricetin), afzelechin, cyanidin, monoterpenes (e.g. borneol in ‘borneo-camphor’), sesquiterpenes and triterpenes (dryobalanone etc.) in balsam, dipterocarpol (hydroxydammaradienone-II, a triterpenoid), oleo-resins, sesquiterpenol-resins, non-methylated and methylated ellagic acids, gallic acid, bergenin (gallic acid derivative) tannins, proanthocyanidins (prodelphinidins), ursolic acid, oleanolic acid derivatives, dammaranes, anthraquinones, polyacetate-derived arthroquinones, and stilbenoids (e.g. resveratrol; hydroxylated derivatives of stilbene) present. Alkaloids, saponins, and cyanogenic compounds not found. Aluminium accumulated in some species.
Use Timber (hardwood), resins (dammar), camphor (Dryobalanops aromatica), butter fats from fruits.
Systematics
Dipterocarpaceae are sister to Sarcolaenaceae (Heckenhauer & al. 2017).
Monotoideae Thonner, Bl.-pfl. Afr.: 386. Oct 1908
3/c 34. Marquesia (3; M. acuminata, M. excelsa, M. macroura; tropical Africa), Monotes (c 30; tropical Africa, Madagascar), Pseudomonotes (1; P. tropenbosii; Amazonian Colombia). – Tropical Africa, Madagascar, Amazonian Colombia. Ectomycorrhiza? Wood rays usually uniseriate. Leaf base with adaxial gland. Hypogyny. Androgynophore present. Anthers basifixed-versatile. Pollen grains tricolporate. Exine reticulate, with columellate infratectum. Pistil composed of three (to five) connate carpels. Ovules (one or) two per carpel, sometimes orthotropous. Fruit a capsule. Exostome prolonged. Endosperm present, without starch. n = ?
Dipterocarpoideae Burnett, Outlines Bot.: 824, 1094, 1120. Feb 1835 [’Dipterocarpidae’]
11/495–500. Vatica (c 65; tropical Asia), Dipterocarpus (c 70; tropical Asia, with their highest diversity in West Malesia), Dryobalanops (7; D. aromatica, D. beccarii, D. fusca, D. keithii, D. lanceolata, D. oblongifolia, D. rappa; the Malay Peninsula, Sumatra, Borneo), 'Shorea' (c 300; Sri Lanka, northern India, southern China, Southeast Asia, Malesia to the Moluccas and the Lesser Sunda Islands, with their largest diversity in West Malesia to the Philippines; paraphyletic), Parashorea (14; southern China, Southeast Asia, Malesia; in Shorea?), Anisoptera (10; Southeast Asia, Malesia to New Guinea), Cotylelobium (5; C. burckii, C. lanceolatum, C. lewisianum, C. melanoxylon, C. scabriusculum; Sri Lanka, peninsular Thailand, the Malay Peninsula, Sumatra, Borneo), Upuna (1; U. borneensis; Borneo), Vateria (4; V. acuminata, V. copallifera, V. indica, V. macrocarpa; southern India, Sri Lanka), Vateriopsis (1; V. seychellarum; the Seychelles), Stemonoporus (c 20; Sri Lanka). – The Seychelles, Sri Lanka, India, Southeast Asia, Malesia to New Guinea, with their largest diversity in West Malesia. Ectomycorrhiza present. Wood rays usually multiseriate. Intercellular resinous canals abundant in wood, vascular tissue and internode pits. At least lateral leaf traces leaving central cylinder well below node, before entering petiole. Usually hypogyny (rarely epigyny). Sepals with valvate or imbricate aestivation. Androgynophore absent. Stamens five antesepalous, ten to c. 110. Anthers basifixed; connectives usually prolonged. Pollen grains tricolpate. Pistil composed of (two or) three connate carpels. Ovary usually superior (rarely semi-inferior). Ovules two (or three) per carpel. Micropyle endostomal. Outer integument two to five (in Dipterocarpus up to ten) cell layers thick. Inner integument two to nine cell layers thick. Fruit usually a single-seeded nut (in Upuna a loculicidal capsule). Endocarp hairy. Sepals persistent, non-uniformly accrescent and thickening. Thin aril present in Upuna. Exotegmen usually palisade. Endosperm usually absent (present in Dipterocarpus, without starch). n = (6–)7, (10–)11(–13).
Neobalanocarpus heimii – here included in Shorea – is sometimes interpreted as a hybrid between species of Shorea and Hopea, the latter being nested in Shorea (together with the sections Anthoshorea and Doona, according to Dayanandan & al. 1999).
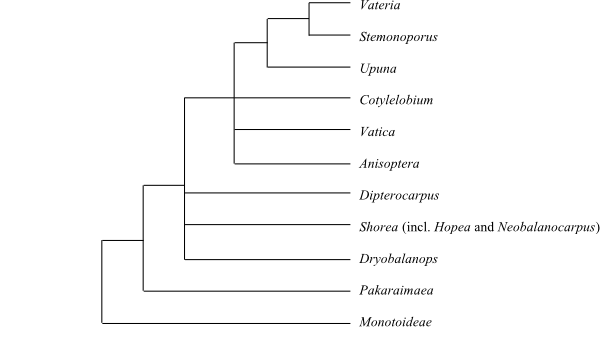 |
Cladogram of Dipterocarpaceae based on DNA sequence data (Dayanandan & al. 1999). |
MALVACEAE Juss. |
( Back to Malvales ) |
Tiliaceae Juss., Gen. Plant.: 289. 4 Aug 1789, nom. cons.; Sterculiaceae Vent. in R. Salisbury, Parad. Lond. 2: ad t. 69. 1 Mai 1807, nom. cons.; Byttneriaceae R. Br. in M. Flinders, Voy. Terra Austr. 2: 540. 19 Jul 1814 [’Buttneriaceae’], nom. cons.; Malvopsida R. Br. in Tuckey, Narr. Exped. Zaire: 429. 5 Mar 1818 [’Malvaceae’]; Fugosiaceae Martinov, Tekhno-Bot. Slovar: 273. 3 Aug 1820 [‘Fugosiae’], nom. illeg.; Hermanniaceae Marquis, Esq. Règne Vég.: 52. 15-22 Jul 1820 [‘Hermannieae’]; Pentapetaceae Bercht. et J. Presl, Přir. Rostlin: 222. Jan-Apr 1820 [‘Pentapeticae’]; Sidaceae Bercht. et J. Presl, Přir. Rostlin: 222. Jan-Apr 1820 [‘Sideae’]; Sterculiales Vent. ex Bercht. et J. Presl, Přir. Rostlin: 222. Jan-Apr 1820 [‘Sterculiae’]; Tiliales Juss. ex Bercht. et J. Presl, Přir. Rostlin: 222. Jan-Apr 1820 [‘Tiliaceae’]; Bombacaceae Kunth, Malvac., Büttner., Tiliac.: 5. 20 Apr 1822 [’Bombaceae’], nom. cons.; Lasiopetalaceae Reichb. in Mag. Aesth. Bot.: ad t. 37. 1823 [’Lasiopetaleae’]; Byttneriales Link, Handbuch 2: 350. 4-11 Jul 1829 [’Buettneriaceae’]; Dombeyaceae Kunth in Desfontaines, Tabl. École Bot., ed. 3: 252. 15-21 Mar 1829 [’Dombeyeae’], nom. cons. prop.; Triplobaceae Raf., Sylva Tellur.: 110. Oct-Dec 1838 [’Triplobides’], nom. illeg.; Malvineae Rchb. Deutsch. Bot. Herb.-Buch: lxxxi. Jul 1841; Tiliineae Rchb., Detusch. Bot. Herb.-Buch: lxxxv. Jul 1841; Philippodendraceae (Endl.) A. Juss. in V. V. D. d’Orbigny, Dict. Univ. Hist. Nat. 9: 735. 1847 [’Philippodendreae’]; Fremontiaceae J. Agardh, Theoria Syst. Plant.: 264. Apr-Sep 1858 [‘Fremontieae’], nom. illeg.; Helicteraceae J. Agardh, Theoria Syst. Plant.: 264. Apr-Sep 1858 [’Helictereae’]; Hibiscaceae J. Agardh, Theoria Syst. Plant.: 275. Apr-Sep 1858 [’Hibisceae’]; Melochiaceae J. Agardh, Theoria Syst. Plant.: 271. Apr-Sep 1858 [’Melochieae’]; Plagianthaceae J. Agardh, Theoria Syst. Plant.: 201. Apr-Sep 1858 [’Plagiantheae’]; Sparmanniaceae J. Agardh, Theoria Syst. Plant.: 260. Apr-Sep 1858; Theobromataceae J. Agardh, Theoria Syst. Plant.: 264. Apr-Sep 1858 [‘Theobromeae’]; Chiranthodendraceae A. Gray in Proc. Amer. Acad. Arts 22: 303. 4 Mar 1887 [’Cheiranthodendreae’]; Cacaoaceae Augier ex T. Post et Kuntze, Lex. Gen. Phan.: 667, 710. 20-30 Nov 1903, nom. illeg.; Berryaceae Doweld, Tent. Syst. Plant. Vasc.: xxxvii. 23 Dec 2001; Grewiaceae (Dippel) Doweld et Reveal in Reveal in Bot. Rev. (Lancaster) 71: 100. 20 Mai 2005; Durionaceae Cheek, Kew Bull. 61: 443. 8 Dec 2006
Genera/species 242/4.355–4.875
Distribution Cosmopolitan except polar areas, with their largest diversity in tropical forests.
Fossils From Paleocene and younger strata, pollen grains and macrofossils (mainly wood) of Grewioideae, Sterculioideae, Malvoideae, and perhaps Dobeyoideae are known. Fossils attributed to Byttnerioideae have been found in the Miocene of Mexico and the Čech Republic. Fossil pollen grains and wood of Bombacoideae are known from Paleocene layers in South America and Africa, and from younger strata in Asia, Antarctica, Australia, and New Zealand. Fossil pollen grains of Helicteroideae have been found in Cenozoic layers in Europe and Asia and Tilia is known from numerous Cenozoic fossils. Craigia – with extant occurrences only in China – was, according to records from the Eocene onwards, distributed in North America, Europe, Spitsbergen and Asia during the Cenozoic.
Habit Usually bisexual (rarely monoecious, polygamomonoecious or dioecious), evergreen or deciduous trees, shrubs or suffrutices (rarely lianas), perennial, biennial or annual herbs. Some species are xerophytes. Some genera with tough fibres in bark and stem.
Vegetative anatomy Roots often with phloem fibres. Phellogen ab initio epidermal or outer cortical. Vessel elements usually with simple perforation plates; lateral pits usually alternate (rarely scalariform), simple and/or bordered pits. Vestured pits sometimes present? Imperforate tracheary xylem elements usually fibre tracheids or (sometimes very long) libriform fibres (sometimes tracheids) with simple pits, usually non-septate (also vasicentric tracheids). Wood rays uniseriate or multiseriate, usually heterocellular, usually dilated. Axial parenchyma apotracheal diffuse or diffuse-in-aggregates, or paratracheal aliform, lozenge-aliform, winged-aliform, confluent (in concentric bands), reticulate, vasicentric, or banded (rarely scanty or scalariform). Wood elements often storied. Wood often fluorescent. Tyloses sometimes frequent (sometimes crystalliferous). Several types of tile cells (almost unique to Malvaceae) present in many species. Secondary phloem in young stems and branches often tangentially stratified into hard fibrous and soft (non-fibrous) parenchymatous layers. Phloem rays cuneate, dilated. Sieve tube plastids S type; sieve tubes with non-dispersive protein bodies. Nodes usually 3:3?, trilacunar with three? leaf traces (rarely penta- or multilacunar with five or more? traces). Lysigenic or schizogenic mucilaginous canals, cavities and cells often present (especially in epidermis, cortex and medulla). Heartwood sometimes with gum-like deposits. Silica bodies present in some species. Cortex with or without cristarque cells. Calciumoxalate crystals (usually in groups) sometimes as druses, styloids, crystal sand or acicular crystals. Prismatic crystals abundant, especially in some wood ray and axial parenchyma cells.
Trichomes Hairs unicellular or multicellular, simple or branched, fasciculate (as hair tufts), multi-armed, stellate, peltate, lepidote; glandular hairs often frequent (also peltate; rarely prickles).
Leaves Usually alternate (spiral or distichous, rarely opposite), simple or palmately compound (rarely unifoliolate), entire or palmately lobed, usually with conduplicate(-plicate) ptyxis. Stipules usually well developed, sometimes large and foliaceous, often early caducous (rarely reduced); leaf sheath absent. Petiole usually pulvinate proximally and distally. Petiole vascular bundle transection usually arcuate or annular; petiole sometimes also with inner cylinder of bundles. Adaxial hypodermis sometimes present. Venation usually palmate (sometimes pinnate); one vein proceeding into non-glandular tooth apex. Stomata usually anomocytic (rarely paracytic, tetracytic, or helicocytic). Cuticular wax crystalloids as rosettes of rodlets, chemically characterized by presence of special triterpenoids, or sometimes as membranous crystalloids often with filiform extensions. Domatia as pockets or hair tufts (rarely pits) or absent. Epidermis and mesophyll with or without mucilaginous idioblasts. Mesophyll with or without sclerenchymatous idioblasts. Leaf margin serrate (often with malvoid teeth; sometimes glandular-serrate), crenate or entire. Stipules, petiole and abaxial side of lamina often with extrafloral nectaries.
Inflorescence Terminal or axillary, cymose of various shape, or flowers solitary (in Sterculioideae panicle; in Tilioideae sometimes supra-axillary: floral or cymular bract simultaneously subtending vegetative bud). Inflorescence usually composed of cymose, often modified, ’bicolor units’, consisting of one terminal flower with three bracts, two of which usually subtending one cymose partial inflorescence each, with normal number of floral prophylls (bracteoles), and lowermost bract not subtending any partial inflorescence. Extrafloral nectaries sometimes present on bracts or pedicels.
Flowers Usually actinomorphic (rarely zygomorphic or asymmetrical), often large. Epicalyx (present in some genera) usually consisting of three (rarely more than three) bracts and together with remaining floral parts probably representing reduced ‘bicolor unit’. Receptacle often elongated into androgynophore, androphore or gynophore. Hypogyny (rarely epigyny?). Sepals (three to) five, usually with valvate (sometimes imbricate) aestivation, persistent or caducous, sometimes petaloid, free or more or less connate in lower part. Petals (three to) five, usually with contorted or valvate (sometimes imbricate or open?) aestivation, sometimes clawed, usually free (rarely connate at base; sometimes absent). Nectaries usually as groups of densely packed multicellular glandular hairs, usually adaxial on sepal bases (rarely on petals or androgynophore). Disc absent.
Androecium Stamens usually numerous (in Adansonia up to more than 1.000; rarely five or 5+5), usually in five alternisepalous or antesepalous fascicles, fundamentally obdiplostemonous, centrifugally developing. Filaments often branched, free or connate at base in fascicles or connate usually in two whorls into tube, outer whorl usually antepetalous, usually with fertile stamens, inner whorl usually staminodial; filaments free from or adnate to petal bases (epipetalous). Anthers basifixed or dorsifixed, often versatile, usually tetrasporangiate (sometimes di-, hexa-, or polysporangiate; sometimes septate), introrse or extrorse, usually longicidal (dehiscing by longitudinal slits; rarely poricidal, dehiscing by apical pores or short slits); connective sometimes slightly prolonged at apex. Tapetum usually secretory (rarely amoeboid-periplasmodial). Usually antesepalous and extrastaminal staminodia often present, usually adnate to fertile stamens.
Pollen grains Microsporogenesis simultaneous. Pollen grains 3(–4)-colpate, 3(–9)-colporate, 3(–9)-porate, spiraperturate or polypantoporate, usually shed as monads (rarely tetrads), usually bicellular (rarely tricellular) at dispersal. Mature pollen grains sometimes starchy. Exine tectate or semitectate, with columellate or acolumellate infratectum, perforate to reticulate, rugulate, verrucate, spinulate, covered with spines, or smooth. Pollen grains at germination often with several pollen tubes.
Gynoecium Pistil composed of (two to) five (to numerous) usually connate (sometimes secondarily free, apocarpous) and usually antepetalous (sometimes antesepalous) carpels; when three carpels, then median carpel abaxial or adaxial; carpels sometimes (in Sterculioideae) opening as seed develops. Ovary superior, (unilocular to) quinquelocular (to multilocular; locules in Malopeae horizontally divided by secondary septa), stipitate (on gynophore) or on androgynophore. Stylodia (two to) five (to numerous), free or more or less connate in lower part. Stigmas capitate or lobate, papillate or non-papillate, usually Dry (rarely Wet) type (large variation in Malvoideae). Pistillodium absent.
Ovules Placentation usually axile (rarely parietal or free central). Ovules two or several (sometimes numerous; rarely one) per carpel, anatropous or hemitropous to campylotropous (rarely orthotropous), ascending, horizontal or pendulous, bitegmic, crassinucellar. Micropyle usually bistomal, Z-shaped (zig-zag; sometimes exostomal, rarely endostomal). Outer integument two to eight cell layers thick. Inner integument three to ten (to 15) cell layers thick. Funicular, placental or stylar obturator sometimes present. Hypostase sometimes present. Archespore sometimes multicellular. Parietal tissue two to seven cell layers thick. Nucellar cap sometimes formed (through divisions of epidermal megasporangial cells). Megagametophyte usually monosporous, Polygonum type. Synergids sometimes with a filiform apparatus. Antipodal cells often long persistent and sometimes proliferating (up to c. 20 cells). Endosperm development usually nuclear. Endosperm haustorium chalazal. Embryogenesis usually asterad (sometimes caryophyllad, rarely onagrad). Polyembryony sometimes occurring. Sporophytic self-incompatibility often present.
Fruit Usually a loculicidal (sometimes septicidal, rarely denticidal or poricidal) capsule, often a schizocarp, regma, with few to numerous usually nutlike (rarely samaroid, baccate, drupaceous or follicular) mericarps (rarely berry, drupe, nutlet or assemblage of follicles), sometimes entirely or partially lignified, coriaceous, carnose or membranous. Endocarp or centre of fruit sometimes hairy or fleshy.
Seeds Aril or strophiole sometimes present. Seed coat usually endotestal-exotegmic. Exotesta sometimes lanate or winged. Endotestal cells with calciumoxalate crystals. Exotegmen palisade, often consisting of malpighiacean cells with lignified walls (in Leptonychia with short fibres). Endotegmen? Perisperm not developed. Endosperm usually copious, oily or starchy (sometimes sparse or absent). Embryo straight or curved, with or without chlorophyll. Cotyledons two, thin or fleshy, sometimes folded or inrolled. Germination phanerocotylar or cryptocotylar.
Cytology n = 7–10 (Grewioideae); n = (5–7) 10(–13) (Byttnerioideae); n = 9, 14, 20, 25 or higher (Helicteroideae); n = 19, 20, 30 or higher (Dombeyoideae); n = 41, c. 80, 82 (Tilioideae); n = 10, 20 (Brownlowioideae); n = (15, 16, 18) 20 (21 or higher) (Sterculioideae); n = (20, 36–)43–46 (up to at least 138) (Bombacoideae); n = 5–28 (to more than 50) (Malvoideae) – Polyploidy occurring.
DNA Plastid gene infA lost (Glycine; present in nuclear genome).
Phytochemistry Flavonols (kaempferol, quercetin, myricetin, herbacetin, gossypetin, hibiscetin, etc.) and their glycosides, flavones, cyanidin, delphinidin, quinoid and/or phenolic sesquiterpenes (e.g. gossypol), dammaranes, cucurbitacins, ellagic and gallic acids, sesquiterpene lactones, caffeic acid, indole alkaloids (e.g. theophylline, caffeine and theobromine), cyanogenic compounds, p-coumaric acid, naphthoquinones, acetophenones, quebrachitol, lignans (syringaresinol), ferulic acid, sinapic acid, and lipids of cyclopropenoid fatty acids (sterculic acid, malvic acid, etc.) and their derivatives present. Saponins? Iridoids? Mucilage as heteropolysaccharides of galacturonic and glucuronic acids with galactose, rhamnose, glucose, and arabinose.
Use Ornamental plants, textile plants (seed hairs from Gossypium; endocarp hairs, kapok, from Ceiba pentandra, Bombax etc.; phloem fibres from Corchorus capsularis, Abroma augusta, Hibiscus cannabinus, H. sabdariffa, etc.), fruits (aril from Durio zibethinus, etc.), vegetables (Hibiscus esculentus etc.), spices, beverages and stimulants (Theobroma cacao, T. grandiflorum, Cola spp., etc.), medicinal plants (Althaea officinalis etc.), seed oils (Gossypium), timber, carpentries (balsa from Ochroma pyramidale etc.), forage plants (Hermannia spp.).
Systematics The sister-group relationships of Malvaceae as well as among their main clades are largely unresolved. Grewioideae and Byttnerioideae (Byttneriina) form a basal clade sister to the remainder (=Malvadendrina), and Helicteroideae may be sister to the rest, yet with fairly low support. Dombeyoideae, Tilioideae, Brownlowioidae, Sterculioideae, and [Bombacoideae+Malvoideae] (Malvatheca) form a polytomy.
[Grewioideae+Byttnerioideae] (Byttneriina)
Grewioideae Dippel, Handb. Laubholzk. 3: 56. Oct-Nov 1893 [‘Grewieae’]
25/680–695. Grewieae Endl., Gen. Plant.: 1006. 1-14 Feb 1840. Luehea (c 25; southern Mexico, Central America, the West Indies, tropical South America), Lueheopsis (11; tropical South America), Colona (35–40; southern China, Southeast Asia, Malesia), Goethalsia (1; G. meiantha; Central America, Colombia), Desplatsia (5; D. chrysochlamys, D. dewevrei, D. floribunda, D. mildbraedii, D. subericarpa; tropical West and Central Africa), Duboscia (2; D. macrocarpa, D. viridiflora; tropical West and Central Africa), Trichospermum (35–40; Malesia to Solomon Islands, the Santa Cruz Islands, Fiji, Samoa, tropical America), Mollia (17–18; tropical South America), Microcos (13–15; tropical Asia to Fiji), Grewia (c 320; tropical and subtropical regions of the Old World), Eleutherostylis (1; E. renistipulata; the Moluccas, New Guinea), Hydrogaster (1; H. trinervis; eastern Brazil), Tetralix (6; T. brachypetalus, T. cristalensis, T. eriophora, T. jaucoensis, T. moanensis, T. nipensis; Cuba), Vasivaea (2; V. alchorneoides, V. podocarpa; Brazil, Peru). – Apeibeae Benth. in J. Proc. Linn. Soc., Bot. 5(Suppl. 2): 55. 1861. Apeiba (10; tropical South America), Ancistrocarpus (3; A. bequaertii, A. comperei, A. densispinosus; tropical Africa), Sparrmannia (4; S. africana, S. discolor, S. ricinocarpa, S. subpalmata; tropical Africa, Madagascar), Entelea (1; E. arborescens; New Zealand, Three Kings Islands), Clappertonia (3; C. ficifolia, C. minor, C. polyandra; tropical West and Central Africa), Glyphaea (2; G. brevis, G. tomentosa; tropical Africa), Corchorus (75–80; tropical and subtropical regions on both hemispheres), Pseudocorchorus (5; P. alatus, P. cornutus, P. greveanus, P. mamillatus, P. pusillus; Madagascar), Triumfetta (c 100; tropical regions on both hemispheres), Heliocarpus (1; H. americanus; Mexico, Central America), Erinocarpus (1; E. nimmonii; southwestern India). – Tropical and subtropical regions. Sepals free, without nectaries. Petals with various adaxial epidermal modifications (in Grewia and Luehea with nectariferous hairs at base). Androgynophore usually present (in Triumfetta nectariferous). First five stamens antesepalous (sometimes absent), additional stamens centrifugally developing. Filaments usually free. Staminodia absent. Pollen grains prolate. Pistil composed of two to ten connate carpels. Outer integument two or three cell layers thick. Inner integument three to seven cell layers thick. Micropyle exostomal or endostomal. n = 7–9 (10).
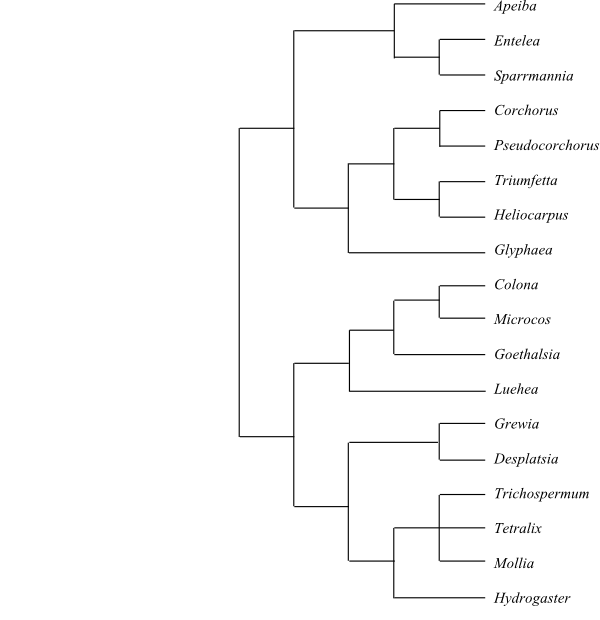 |
Bayesian majority rule consensus tree of Grewioideae based on DNA sequence data (Brunken & Muellner 2012; Pseudocorchorus added). |
Byttnerioideae Burnett, Outlines Bot.: 821, 1119. Feb 1835 [‘Buttneridae’]
25/700–725. Pantropical. Durio type tile cells present. Leaves alternate (often distichous), usually simple and entire (in Herrania palmately lobed). Petiole vascular bundle transection incurved-arcuate. Sepals usually connate. Petals wide at base (with margins inflexed), later clawed, often spatulate, linear or bilobate (rarely absent). Stamens five (to c. 30), only in antepetalous fascicles. Filaments adnate to petals (epipetalous). Tapetum seemingly amoeboid-periplasmodial (cell content resorbed). Antesepalous petaloid staminodia usually present. Staminal fascicles and staminodia together forming tube. Nectar usually secreted through nectarostomata. Style branched at apex. Parietal placentation present in Leptonychia. Outer integument two to four cell layers thick. Inner integument three to ten cell layers thick. n = (5–7) 10(–13). – The subdivision below follows Whitlock & al. (2001).
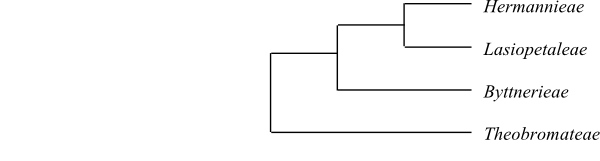 |
Cladogram (simplified) of Byttnerioideae based on DNA sequence data (Whitlock & al. 2001). |
[Theobromateae+[Byttnerieae+[Lasiopetaleae+Hermannieae]]]
Theobromateae A. Stahl, Estud. Fl. Puerto-Rico 2: 103. 1884 [‘Theobromeae’]
4/43. Guazuma (3; G. crinita, G. longipedicellata, G. ulmifolia; southern Mexico, Central America, the West Indies, tropical South America), Glossostemon (1; G. bruguieri; the Arabian Peninsula, Iraq, Iran), ’Theobroma’ (22; Central America, tropical South America; paraphyletic; incl. Herrania?), Herrania (17; tropical South America; in Theobroma?). – Southwest Asia, tropical America.
[Byttnerieae+[Lasiopetaleae+Hermannieae]]
Byttnerieae DC., Prodr. 1: 484. Jan (med.) 1824 [‘Büttneriaceae verae’]
8/260–265. Scaphopetalum (12; tropical Africa), Leptonychia (c 25; tropical regions in the Old World), Abroma (1; A. augusta; tropical Asia, northeastern Queensland), Kleinhovia (1; K. hospita; tropical Asia, northeastern Queensland), Rayleya (1; R. bahiensis; Bahia in Brazil), ’Byttneria’ (c 140; tropical regions on both hemispheres; paraphyletic; incl. Ayenia?), Ayenia (c 80; southern United States to Argentina; in Byttneria), Megatritheca (2; M. devredii, M. grossedenticulata; Gabon, Congo). – Tropical regions on both hemispheres.
[Lasiopetaleae+Hermannieae]
Lasiopetaleae DC., Prodr. 1: 488. Jan (med.) 1824
9/150–155. Seringia (>20; Madagascar, New Guinea, Australia), Commersonia (c 30; Southeast Asia, Malesia to New Guinea, Australia, New Caledonia), Androcalva (14?; Australia), Maxwellia (1; M. lepidota; New Caledonia), Hannafordia (4; H. bissillii, H. kessellii, H. quadrivalvis, H. shanesii; central Australia), Guichenotia (17; southwestern Western Australia), Lysiosepalum (3; L. abollatum, L. aromaticum, L. rugosum; southwestern Western Australia), Lasiopetalum (30–35; southwestern Western Australia, southeastern Australia, Tasmania), Thomasia (c 30; southwestern Western Australia, southeastern South Australia, Victoria). – Madagascar, New Guinea, Australia, Tasmania, New Caledonia.
Hermannieae DC., Prodr. 1: 490. Jan (med.) 1824
4/250–265. ’Melochia’ (55–60; tropical and subtropical regions on both hemispheres, with their highest diversity in tropical America; non-monophyletic), Hermannia (c 120; tropical and subtropical regions on both hemispheres, with their largest diversity in the Cape Provinces in South Africa), Dicarpidium (1; D. monoicum; northwestern Australia), Waltheria (45–50; tropical Africa, Madagascar, the Malay Peninsula, Taiwan, tropical America). – Tropical and subtropical regions on both hemispheres.
[Helicteroideae+Dombeyoideae+Tilioideae+Brownlowioideae+Sterculioideae+[Bombacoideae+Malvoideae]] (Malvadendrina)
Deletion of 21 bp present in plastid gene ndhF.
Helicteroideae Meisn., Plant. Vasc. Gen.: Tab. Diagn. 29, Comm. 25. 26 Mar-1 Apr 1837 [‘Helictereae’]
12/128–133. Tropical regions, with their highest diversity in Southeast Asia and West Malesia. Durio type tile cells present. Hairs sometimes lepidote. Leaf venation in Durioneae pinnate. Sepals connate. Petals often with lateral constrictions. Androgynophore present. Stamens usually in fascicles and/or forming short tube. Pollen grains columellate or microverrucate to suprareticulate. Outer integument two cell layers thick. Inner integument two cell layers thick. Aril usually absent (sometimes present). Testa sometimes multiplicative. n = 9, 14, 20, 25 or higher. – Helicteroideae may be sister-group to the remaining Malvadendrina (Baum & al. 1998; Alverson & al. 1999).
Helictereae Schott et Endl., Melet. Bot.: 30. 1831
6/75–80. Reevesia (c 15; tropical Asia, Central America), Ungeria (1; U. floribunda; Norfolk Island), Helicteres (55–60; tropical Asia, tropical America), Neoregnellia (1; N. cubensis; Cuba, Hispaniola), Mansonia (2; M. altissima, M. diatomanthera; tropical Africa, Assam, Burma), Triplochiton (2; T. scleroxylon, T. zambesiacus; tropical Africa). – Pantropical.
Durioneae Becc. in Malesia 3: 206. Sep 1889
6/c 53. Neesia (8; West Malesia), Coelostegia (6; C. borneensis, C. chartacea, C. griffithii, C. kostermansii, C. montana, C. neesiocarpa; West Malesia), Kostermansia (1; K. malayana; the Malay Peninsula), Cullenia (2; C. exarillata, C. rosayroana; India, Sri Lanka), Boschia (6; B. acutifolia, B. excelsa, B. grandiflora, B. griffithii, B. mansonii, B. oblongifolia; Burma, Malesia), Durio (c 30; West Malesia). – India, Sri Lanka, Burma, Malesia. Hairs lepidote. Epicalyx ab initio connate. Anthers often multilocular. n = 14.
Dombeyoideae (Lindl.) Beilschm. in Flora 16(Beibl. 7): 86, 106. 14 Jun 1833 [‘Dombeyaceae s. Wallichieae’]
21/370–375. Nesogordonia (c 20; tropical Africa, Madagascar), Pterospermum (25–30?; tropical Asia), Schoutenia (c 10; Thailand to Central Malesia, northern Australia), Sicrea (1; S. godefroyana; Cambodia), Burretiodendron (7; B. brilletii, B. esquirolii, B. hsienmu, B. kydiifolium, B. obconicum, B. siamense, B. yunnanense; southwestern China, Southeast Asia), Melhania (c 45; tropical regions in the Old World), Paramelhania (1; P. decaryana; southeastern Madagascar), Harmsia (1; H. lepidota; northeastern Africa), Astiria (1; A. rosea; Mauritius, extinct), Cheirolaena (1; C. linearis; Antsiranana in Madagascar), Corchoropsis (1; C. tomentosa; East Asia to Japan), Paradombeya (1; P. sinensis; Burma, Yunnan), Pentapetes (1; P. phoenicea; tropical Asia), Trochetiopsis (3; T. ebenus, T. erythroxylon, T. melanoxylon; St. Helena), ‘Dombeya’ (>220; tropical Africa, Madagascar, Mauritius; paraphyletic), Trochetia (6; T. blackburniana, T. boutoniana, T. parviflora, T. triflora, T. uniflora: Mauritius; T. granulata: Réunion), Ruizia (1; R. cordata; Réunion), Andringitra (5; A. leiomacrantha, A. leucomacrantha, A. macrantha, A. moratii, A. muscosa; Madagascar), Eriolaena (c 10; the Himalayas, southern China, Southeast Asia), Helmiopsiella (2; H. leandrii, H. madagascariensis; Madagascar), Helmiopsis (9; Madagascar). – Tropical regions in the Old World, St. Helena, with their largest diversity in Madagascar. Leaves alternate (spiral). Epicalyx usually present. Sepals free or connate at base, with basal nectaries. Stamens (five or) ten (to c. 30). Filaments usually connate (rarely free). Staminodia antesepalous, elongate, forming short tube (sometimes absent). Tapetum usually secretory (sometimes amoeboid-periplasmodial). Secondary pollen display sometimes occurring. Pollen grains often porate, with spinulate exine. Pistil composed of (two to) five (or ten) connate carpels. Micropyle bistomal. Outer integument three or four cell layers thick. Inner integument four or five cell layers thick. Archespore sometimes multicellular. Nucellar cap sometimes present. Endocarp often pubescent. Seed with umbonate sarcotestal outgrowths. Cotyledons bilobate. n = 19, 20, 30 or higher. – Dombeyoideae may be sister to the remaining Malvadendrina (Nyffeler & al. 2005) or to Tilioideae (with low support; Alverson & al. 1999).
Tilioideae Arn., Botany: 100. 9 Mar 1832 [‘Tilieae’]
3/c 62. Tilia (c 45; temperate regions on the Northern Hemisphere), Craigia (2; C. kwangsiensis, C. yunnanensis; China, northern Vietnam), Mortoniodendron (c 15; Mexico, Central America, northern Colombia). – Temperate regions on the Northern Hemisphere south to China and northern Vietnam, Mexico, Central America. Ectomycorrhiza present. Leaves alternate (usually distichous), usually with horizontally conduplicate ptyxis. Petiole vascular bundle transection annular; petiole with medullary phloem strands and inverted bundles. Sepals free. Stamens and staminodia antepetalous (antesepalous sectors empty). Filaments free. Carpels antesepalous. Outer integument three or four cell layers thick. Inner integument four or five cell layers thick. Aril usually absent (sometimes present). Cotyledons plicate. n = 41, c. 80, 82. Stachyose and raffinose present (in phloem exudate in Tilia).
Brownlowioideae Burett in Notizbl. Bot. Gart. Berlin-Dahlem 9: 599, 605. 22 Jul 1926
9/c 90. Diplodiscus (12; Sri Lanka, West Malesia to the Philippines), Indagator (1; I. fordii; northern Queensland), Brownlowia (c 30; Southeast Asia, Malesia to Solomon Islands), Pentace (c 25; Burma, Southeast Asia, West Malesia), Pityranthe (2; P. trichosperma, P. verrucosa; Sri Lanka, southern China, Taiwan), Jarandersonia (4; J. paludosa, J. parvifolia, J. rinoreoides, J. spinulosa; Borneo), Christiana (5; C. africana, C. eburnea, C. macrodon, C. mennegae, C. vescoana; tropical Africa, Tahiti [extinct], tropical South America), Berrya (6; B. cordifolia, B. javanica, B. mollis, B. pacifica, B. papuana, B. rotundifolia; India, Sri Lanka, Southeast Asia, Malesia), Carpodiptera (5; C. africana, C. cubensis, C. hexaptera, C. mirabilis, C. simonis; tropical East Africa, the Comoros, southern Mexico, the West Indies, Trinidad). – Tropical regions in the Old World eastwards to Taiwan and the Solomon Islands, tropical America. Hairs often lepidote. Inflorescences axillary. Sepals splitting irregularly into two or three lobes, connate into campanulate calyx, persistent. Stamens c. 30, in antepetalous fascicles. Anthers with sagittate thecae. Staminodia petaloid, antesepalous (sometimes absent). Style one or stylodia several. Ovules approx. two per carpel. Fruit with persistent sepals. n = 10, 20.
Sterculioideae (Lindl.) Beilschm. in Flora 16(Beibl. 7): 86. 14 Jun 1833 [‘Sterculieae’]
13/365–370. Sterculia (c 90; tropical regions on both hemispheres), Brachychiton (31; New Guinea, Australia), Cola (c 120; tropical Africa), Octolobus (3; O. grandis, O. heteromerus, O. spectabilis; tropical Africa), Acropogon (c 25; New Caledonia), Pterygota (11–15; tropical regions on both hemispheres), Franciscodendron (1; F. laurifolium; northeastern Queensland), Argyrodendron (4; A. actinophyllum, A. peralatum, A. polyandrum, A. trifoliolatum; eastern Queensland, northeastern New South Wales), Firmiana (16; tropical regions in the Old World from tropical East Africa and eastwards), Hildegardia (7; H. ankaranensis, H. barteri, H. cubensis, H. erythrosiphon, H. merrittii, H. migeodii, H. perrieri; tropical Africa, Madagascar, tropical Asia, northern Northern Territory, Cuba), Scaphium (8; tropical Asia), Pterocymbium (c 15; Southeast Asia, Malesia to Fiji), Heritiera (c 35; tropical Africa, tropical Asia, eastern Queensland, northeastern New South Wales, New Caledonia). – Pantropical. Flowers unisexual (monoecy). Leaves alternate (spiral), often palmately compound. Petiole vascular bundle transection annular; petiole with medullary bundle. Inflorescence axillary, panicle, without distinct ‘bicolor units’ (possibly strongly modified). Epicalyx absent. Epicalyx absent. Sepals petaloid. Petals absent. Androgynophore present, sometimes with nectariferous hairs at base. Filaments very short, connate, or absent. Staminodia absent. Carpels largely (secondarily) free (in Firmiana early opening, exposing unripe seeds on carpellary margins); compitum developing after postgenital fusion of apical stylar parts. Stylodia connate at apex. Stigma single. Micropyle endostomal. Outer integument three to five cell layers thick. Inner integument three to six cell layers thick. Integument in Sterculia lobate. Fruit usually a follicle (sometimes coriaceous; sometimes a nut). Endocarp sometimes pubescent. n = (15, 16, 18) 20 (21 or higher). – Sterculioideae may be sister to the clade [Bombacoideae+Malvoideae].
[Bombacoideae+Malvoideae] (Malvatheca)
Leaves alternate (spiral). Floral development starting with ring of primordials; subsequently five alternisepalous androecial sections develop, each with two rows of centrifugally developing secondary androecial primordia. Sepals connate, at base provided with adaxial multicellular clavate nectariferous hairs. Each staminal unit hypothesized as consisting of (usually sterile) antesepalous primordium with its own vascular bundle, on either side flanked by one primordium developed from separate antepetalous primordia, each giving rise to one theca (half anther) each of which supplied by branch from antepetalous bundle. Stamens in contorted fascicles and/or forming tube. Filaments adnate at base to petals (epipetalous). Anthers usually disporangiate (monothecal; occasionally locellate); thecae with simple antesepalous vascular bundle.
Bombacoideae Burnett, Outlines Bot.: 816, 818, 1094, 1119. Feb 1835 [‘Bombacidae’]
18/160–175. Bernoullieae Carv.-Sobr. in Mol. Phylogen. Evol 101: 68. 2016. Bernoullia (3; B. flammea, B. jaliscana, B. uribeana; Mexico, Central America, Colombia), Gyranthera (2; G. caribensis, G. darienensis; Panamá, Venezuela), Huberodendron (3; H. allenii, H. patinoi, H. swietenioides; tropical South America). – Adansonieae Horan., Char. Ess. Fam.: 192. 17 Jun 1847 [‘Adansoniae’]. Adansonia (8; tropical Africa, Madagascar, northwestern Australia), Cavanillesia (5; C. arborea, C. chicamochae, C. hylogeiton, C. platanifolia, C. umbellata; Panamá, tropical South America), Aguiaria (1; A. excelsa; Amazonian Brazil), Catostemma (c 15; northern South America), Scleronema (3; S. guianense, S. micranthum, S. praecox; tropical South America). – Bombaceae Kunth, Syn. Plant. 3: 258. 28 Feb 1824. Bombax (9; tropical regions in the Old World), Rhodognaphalon (2; R. brevicuspe, R. lukayense; tropical Africa), Pachira (45–50; tropical Africa, Central America, tropical South America), Pochota (1; P. squamigera; Central America, northern tropical South America), Eriotheca (20–25; tropical America), Spirotheca (5; S. awadendron, S. mahechae, S. michaeli, S. rivieri, S. rosea; Central America, tropical South America), Neobuchia (1; N. paulinae; Haiti), Ceiba (c 17; Mexico, Central America, the West Indies, tropical South America, one species, C. pentandra, also in tropical West Africa), Pseudobombax (20–25; southern Mexico, Central America, tropical South America), Septotheca (1; S. tessmannii; northwestern tropical South America). – Pantropical, with their highest diversity in tropical America. Stem often stout, sometimes with parenchymatous water-storage tissue and with thin and often green bark occasionally with large prickles. Leaves usually palmately compound or simple and palmately lobed. Inflorescence axillary, consisting of one or two flowers. Stamens usually numerous, in fascicles (in, e.g., Ceiba five antepetalous). Anthers sometimes disporangiate, monothecal. Staminodia usually absent. Pollen grains flattened, triangular in polar view. Outer integument five to eight cell layers thick. Inner integument six to nine cell layers thick. Nucellar cap present. Endocarp pubescent. Testa in Adansonia six to eight cell layers thick, vascularized. Embryo curved. Cotyledons plicate. n = (20, 36–)43–46.
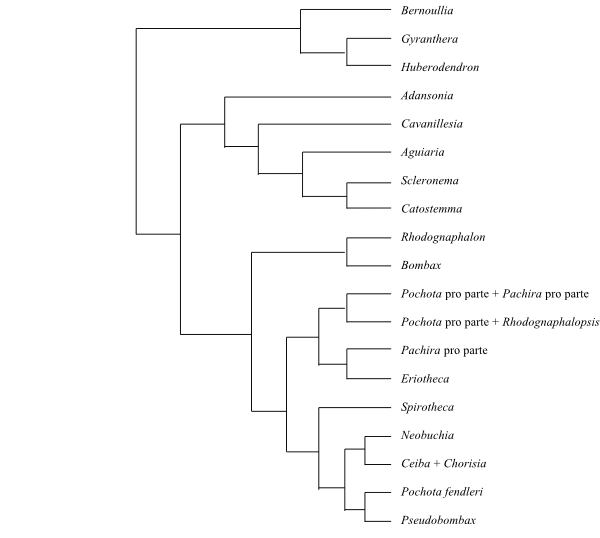 |
Cladogram (simplified) of Bombacoideae based on DNA sequence data (Carvalho-Sobrinho & al. 2016) |
Malvoideae Burnett, Outlines Bot.: 816, 1094, 1118. Feb 1835 [’Malvidae’]
116/1.800–2.250. Temperate to tropical regions, mainly in the Northern Hemisphere. Durio type tile cells present. Glands with gossypol present in Gossypieae. Petiole vascular bundle transection annular. Epiphyllous inflorescence present in Nototriche. Epicalyx sometimes present. Hypogyny. Median sepal often abaxial. Sepals in Ochroma with imbricate aestivation. Petals free or connate at base (absent in Fremontodendron). Stamens from five antepetalous primordia (sometimes split into two), centrifugally developing. Filaments usually connate into tube. Staminal tube often with five apical teeth. Anthers sometimes disporangiate, monothecal. Thecae usually with simple synlateral (antesepalous) vascular bundle. Tapetum amoeboid-periplasmodial. Staminodia in fascicles. Pollen grains often polyporate (with seven or more pores); exine often spinulate. Pistil composed of one carpel or (two or) three to numerous connate carpels. Style often branched at apex or with separate stylodia. Stigma decurrent to capitate, hairy. Ovules one to numerous per carpel, usually campylotropous (sometimes anatropous). Outer integument two to six cell layers thick. Inner integument four to eight (to 15) cell layers thick. Parietal tissue two to seven cell layers thick. Nucellar cap sometimes approx. two cell layers thick. Fruit usually a loculicidal capsule (sometimes a schizocarp, regma). Seeds sometimes hairy. Embryo usually curved. Cotyledons plicate. n = 5–28 (to more than 50). Deletion of 6 bp absent from conserved part of plastid gene matK in Fremontodendron and Chiranthodendron (present in all other investigated species of Malvatheca). – Unplaced Malvoideae Fremontodendron (3; F. californicum, F. decumbens, F. mexicanum; southwestern United States, northwestern Mexico), Chiranthodendron (1; C. pentadactylon; Mexico, Guatemala), Patinoa (4; P. almirajo, P. ichthyotoxica, P. paraensis, P. sphaerocarpa; tropical South America), Ochroma (1; O. pyramidale; southern Mexico to southern Brazil and Bolivia), Phragmotheca (5; P. ecuadorensis, P. fuchsia, P. leucoflora, P. mammosa, P. siderosa; Panamá to Peru), Pentaplaris (3; P. davidsmithii, P. doroteae, P. huaoranica; Costa Rica, Ecuador, Peru, Bolivia), Matisia (c 25; tropical South America), Quararibea (85–90; southern Mexico, Central America, the West Indies, tropical South America). – Howittia (1; H. trilocularis; southeastern Queensland, eastern New South Wales, Victoria), Uladendron (1; U. codesuri; Venezuela), Camptostemon (3; C. aruense, C. philippinense: coasts in Central Malesia; C. schultzii: coasts in New Guinea and northern Australia), Lagunaria (1; L. patersonia; Queensland, Norfolk Island, Lord Howe). – Fremontodendron, Chiranthodendron, Patinoa, Ochroma, Phragmotheca, Pentaplaris, Matisia, and Quararibea may be basal in Malvoideae (Matisieae K. Schum. in Engl. et Prantl, Nat. Pflanzenfam. III, 6: 58, 63. Sep 1890).
Hibisceae Rchb., Fl. Germ. Excurs. 2(2): 770, 774. 1832
23/325–760. Radyera (2; R. urens: arid regions in southern Nambia and central South Africa; R. farragei: arid regions in Australia), ‘Hibiscus’ (240–675; tropical to warm-termperate regions on both hemispheres; paraphyletic), Peltaea (18; Central America, tropical South America; in Hibiscus?), Hibiscadelphus (6; H. bombycinus, H. crucibracteatus, H. distans, H. giffardianus, H. hualalaiensis, H. wilderianus; the Hawaiian Islands; in Hibiscus?), Wercklea (11; Central America, the West Indies, tropical South America), Symphyochlamys (1; S. erlangeri; northeastern tropical Africa), Megistostegium (3; M. microphyllum, M. nodulosum, M. perrieri; Madagascar), Perrierophytum (6–9; P. glomeratum, P. humbertii, P. paniculatum, P. rubrum, P. viridiflorum, P. viscosum; Madagascar), Humbertiella (5; H. decaryi, H. foliosa, H. henricii, H. quararibeoides, H. tormeyi; southwestern Madagascar), Helicteropsis (1; H. microsiphon; northwestern Madagascar), Humbertianthus (1; H. cardiostegius; Madagascar), Cenocentrum (1; C. tonkinensis; southern China, Southeast Asia), Urena (c 10; tropical regions on both hemispheres), Malachra (8; tropical and subtropical regions on both hemispheres), Phragmocarpidium (1; P. heringeri; central Brazil), Rojasimalva (1; R. tetrahedralis; Venezuela), Anotea (1; A. flavida; Mexico), Jumelleanthus (1; J. perrieri; Madagascar), Julostylis (3; J. ampumalensis, J. angustifolia, J. polyandra; southwestern India, Sri Lanka), Dicellostyles (2; D. axillaris, D. jujubifolia; Sri Lanka, the Himalayas, Thailand), Nayariophyton (1; N. zizyphifolium; eastern Himalayas, Yunnan), Kydia (2; K. calycina, K. glabrescens; southern Himalayas, Yunnan, Southeast Asia). – ‘Hibiscus’ as hitherto recognized is highly paraphyletic and comprises most if not all of Hibisceae.
Gossypieae Alef. in Bot. Zeitung (Berlin) 19: 301. 11 Oct 1861 [‘Gossypiidae’]
9/125–130. Cephalohibiscus (1; C. peekelii; New Guinea), Cienfuegosia (c 30; tropical Africa, tropical and subtropical America), Lebronnecia (1; L. kokioides; the Marquesas Islands), Hampea (20–25; Mexico, Central America, Colombia), Thespesia (11; tropical regions on both hemispheres), Thepparatia (1; T. thailandica; Thailand), Gossypioides (2; G. brevilanatum, G. kirkii; tropical Africa, Madagascar), Kokia (3; K. cookei, K. drynarioides, K. kauaiensis; the Hawaiian Islands), Gossypium (c 50; warm-temperate to tropical regions on both hemispheres). – Tropical regions on both hemispheres.
Malveae J. Presl, Fl. Sicula: 173. 1826 [‘Malvaceae’]
71/1.210–1.220. Neobaclea (1; N. spirostegia; southern Argentina), Herissantia (≥6; tropical America), Akrosida (2; A. floribunda, A. macrophylla; Brazil), Rhynchosida (2; R. kearneyi, R. physocalyx; southern Texas, northern Mexico, Bolivia, Argentina), Krapovickasia (4; K. flavescens, K. macrodon, K. physaloides, K. urticifolia; Central America, tropical South America), Cristaria (c 75; Peru, Chile, Andean Argentina), Corynabutilon (8; C. bicolor, C. ceratocarpum, C. crassa, C. hirsutum, C. ochsenii, C. salicifolium, C. viride, C. vitifolium; temperate Chile and Argentina), Malvella (4; M. lepidota, M. leprosa, M. sagittifolia, M. sherardiana; the Mediterranean, southwestern United States, northwestern Mexico), Meximalva (2; M. filipes, M. venusta; Mexico), Neobrittonia (1; N. acerifolia; Mexico to Panamá), Tetrasida (4; T. chachapoyensis, T. serrulata, T. tulla, T. weberbaueri; Peru), Allosidastrum (4; A. dolichophyllum, A. hilarianum, A. interruptum, A. pyramidatum; tropical South America), ‘Sida’ (c 155; tropical and subtropical regions on both hemispheres, with their highest diversity in tropical America; polyphyletic), Dendrosida (6; D. batesii, D. breedlovei, D. cuatrecasasii, D. oxypetala, D. parviflora, D. sharpiana; Mexico, Colombia, Venezuela; in Sida?), Bordasia (1; B. bicornis; Paraguay), Robinsonella (16; Central America), Sidastrum (8; islands in the Pacific, Texas, Mexico, Central America, the West Indies, tropical South America), Dirhamphis (2; D. mexicana: western Mexico; D. balansae: Bolivia, Paraguay), Batesimalva (5; B. killipii, B. lobata, B. pulchella, B. stipulata, B. violacea; northeastern Mexico, Venezuela), Pseudabutilon (19; southern United States, Mexico, Central America, the West Indies, tropical South America), Hochreutinera (2; H. amplexifolia, H. hassleriana; Mexico, temperate Chile and Argentina), Briquetia (5; B. brasiliensis, B. denudata, B. inermis, B. sonorae, B. spicata; southern Mexico, Central America, Cuba, tropical South America), Fryxellia (1; F. pygmaea; southwestern United States, northwestern Mexico), Herissantia (5; H. crispa, H. dressleri, H. intermedia, H. nemoralis, H. tiubae; Mexico, Central America, the West Indies, tropical South America), Gaya (39; Central America, tropical South America), Callianthe (c 40; southern Mexico, Central America, tropical South America to northern Argentina), ‘Abutilon’ (c 215; warm-temperate to tropical regions on both hemispheres; non-monophyletic; incl. Bastardia and Bastardiopsis?), Bastardia (7; Texas, Mexico, Central America, the West Indies, tropical South America; in Abutilon?), Bastardiopsis (6; B. densiflora, B. eggersii, B. grewiifolia, B. myrianthus, B. turumiquirensis, B. yaracuyensis; tropical South America; in Abutilon?), Spirabutilon (1; S. citrinum; Espirito Santo in Brazil), Billieturnera (1; B. helleri; southern Texas, northeastern Mexico), Allowissadula (10; Texas, Mexico), Wissadula (c 35; tropical regions on both hemispheres, with their highest diversity in tropical America), Bastardiastrum (8; Mexico), Horsfordia (4; H. alata, H. exalata, H. newberryi, H. rotundifolia; southwestern United States, northwestern Mexico), Anoda (23; southern United States, Mexico, Central America, Colombia to Bolivia, Chile and Argentina), Periptera (5; P. ctenotricha, P. lobelioides, P. macrostelis, P. punicea, P. trichostemon; western Mexico, Guatemala), Bakeridesia (26; Mexico, Central America, tropical South America), Phymosia (8; southern Mexico, Central America, the West Indies), Malacothamnus (16; California, northern Baja California in northwestern Mexico), Iliamna (8; southwestern Canada, United States), Palaua (16; the Andes), Kearnemalvastrum (2; K. lacteum, K. subtriflorum; Mexico, Central America, Colombia), Malvastrum (27; tropical and subtropical regions on both hemispheres), Andeimalva (4; A. chilensis, A. machupicchensis, A. mandonii, A. spiciformis; the Andes), Sphaeralcea (50–55; arid and semiarid regions in America), Tarasa (27; Mexico, the Andes), Fuertesimalva (14; Mexico, the Andes), Calyculogygas (1; C. uruguayensis; Uruguay), Monteiroa (10; southern and southeastern Brazil), Calyptraemalva (1; C. catharinensis; Brazil), Napaea (1; N. dioica; eastern to central United States), Eremalche (3; E. exilis, E. parryi, E. rotundifolia; California, northern Baja California in northwestern Mexico), Sidasodes (1; S. jamesonii; Colombia, Ecuador, Peru), Acaulimalva (21; the Andes), Nototriche (c 95; South America), Cristaria (25–30; Peru, Chile, Andean Argentina), Lecanophora (5; L. ameghinoi, L. chubutensis, L. ecristata, L. heterophylla, L. jarae; temperate Argentina), Modiola (1; M. caroliniana; America), Modiolastrum (5; M. australe, M. gilliesii, M. lateritum, M. malvifolium, M. palustre; South America), Kitaibela (1; K. vitifolia; Lower Danube in western Balkan Peninsula), Malope (2; M. malacoides, M. trifida; the Mediterranean), Anisodontea (20; South Africa, Lesotho), Alcea (75–80; the Mediterranean to Central Asia), Althaea (17; Europe, the Mediterranean, temperate Asia to northeastern Siberia), ‘Malva’ (c 30; Europe, Macaronesia, the Mediterranean, tropical African mountains, temperate and Central Asia, northwestern Himalayas, southern Australia, Tasmania, America; polyphyletic), Callirhoe (9; southern Canada, United States, Mexico), Sidalcea (c 30; southwestern Canada, western United States, northwestern Mexico), Hoheria (6; H. angustifolia, H. glabrata, H. lyallii, H. ovata, H. populnea, H. sexstylosa; New Zealand), Lawrencia (5?; L. buchananensis, L. chrysoderma, L. cinerea, L. helmsii, L. viridigrisea; Australia, Tasmania), Plagianthus (3; P. divaricatus, P. regius, P. squamatus; New Zealand), Gynatrix (2; G. macrophylla, G. pulchella; southeastern New South Wales, Victoria, Tasmania), Asterotrichion (1; A. discolor; Tasmania). – Subcosmopolitan, with their highest diversity in America.
Alyogyne clade
1/5. Alyogyne (5; A. cravenii, A. cuneiformis, A. hakeifolia, A. huegelii, A. pinoniana; Western Australia, South Australia, southern Northern Territory).
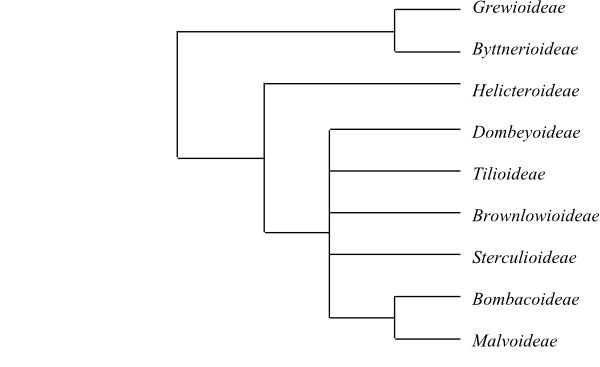 |
Cladogram (simplified) of Malvaceae based on DNA sequence data (Baum & al. 1998; Alverson & al. 1999). |
MUNTINGIACEAE C. Bayer, M. W. Chase et M. F. Fay |
( Back to Malvales ) |
Genera/species 3/3
Distribution Florida, Mexico, Central America, the West Indies, tropical South America southwards to northern Argentina.
Fossils Unknown.
Habit Usually bisexual (rarely unisexual), evergreen trees or shrubs.
Vegetative anatomy Phellogen ab initio superficial? Vessels single. Vessel elements with simple perforation plates; lateral pits alternate, simple pits. Vestured pits absent. Imperforate tracheary xylem elements tracheids with simple or bordered pits, usually non-septate (in Dicraspidia sometimes septate?). Wood rays usually multiseriate (rarely uniseriate), high, homocellular (Muntingia), in young secondary wood often much widened. Axial parenchyma apotracheal diffuse or diffuse-in-aggregates, or paratracheal? scanty vasicentric, scalariform, or banded. Wood elements and parenchyma sometimes partially storied. Pericyclic fibres absent. Secondary phloem as young stratified into hard fibrous and soft parenchymatous layers. Sieve tube plastids S type. Nodes 3?:3?, trilacunar? with three? leaf traces. At least plagiotropic branches with filiform, foliaceous, peltate or unilaterally reduced stipule-like dimorphic appendages. Secretory mucilage canals and cavities absent. Yellowish deposits common in vessel elements (Muntingia). Dark-staining deposits often present in wood elements. Rhomboidal calciumoxalate crystals sometimes present in ray and parenchyma cells (Muntingia). Druses sometimes present in phloem rays and cortex (Muntingia).
Trichomes Hairs unicellular or multicellular, uniseriate or multiseriate, simple or branched, bristle-like, lanate, stellate, or fasciculate (in Muntingia hair tufts and erect uniseriate hairs); glandular hairs sometimes present?
Leaves Alternate (distichous), simple, entire, often distinctly asymmetrical, with conduplicate-subplicate ptyxis (Muntingia). Stipules absent; leaf sheath absent. Prophylls basal on axillary shoots, stipule-like (in Dicraspidia heteromorphic, on adaxial side of branch orbicular, foliaceous, persistent, on abaxial side of branch linear, thin, caducous; prophyll in Muntingia one, adaxial, narrow). Petiole vascular bundle transection annular; pericyclic fibres absent. Leaf base asymmetrical. Venation pinnate to palmate. Stomata? Cuticular wax crystalloids? Mesophyll without mucilaginous idioblasts. Hair bases on abaxial side of lamina with calciumoxalate crystals. Leaf margin serrate.
Inflorescence Supra-axillary (extra-axillary; floral bract or cymule bract simultaneously supporting vegetative bud) few-flowered fascicles, or flowers solitary.
Flowers Actinomorphic. Hypogyny (Muntingia), epigyny (Neotessmannia) or half epigyny (Dicraspidia). Sepals (four or) five (to seven), with valvate aestivation, caducous (Muntingia) or persistent (Dicraspidia), connate at base. Petals (four or) five (to seven), with imbricate aestivation, shortly clawed, caducous, free, crumpled in bud. Nectaries intrastaminal, present on adaxial side of wide disc-like annular structure (Muntingia).
Androecium Stamens numerous, inserted on massive, almost discoid adaxially hairy androphore, centrifugally? developing. Filaments filiform, usually free from each other, free from tepals. Anthers basifixed or subbasifixed (to dorsifixed?), sometimes versatile, tetrasporangiate, introrse, usually longicidal (dehiscing by longitudinal slits; sometimes poricidal, with apical pore-like slits). Tapetum secretory, with binucleate cells. Staminodia?
Pollen grains Microsporogenesis simultaneous. Pollen grains tricolpor(oid)ate, shed as loose tetrads (Neotessmannia) or monads (Muntingia, Dicraspidia), bicellular at dispersal. Exine semitectate, with columellate infratectum, finely reticulate to almost scabrate.
Gynoecium Pistil composed of five (to seven) connate antepetalous carpels with numerous septa. Ovary superior, inferior or semi-inferior, quinquelocular (Dicraspia), or multilocular in lower part and unilocular in upper part (Neotessmannia); often multilocular due to secondary septa. Style single, simple, short, stout. Stigma conical, with five (to seven) ridges, or capitate, type? Pistillodium?
Ovules Placentation axile-laminar (Dicraspidia, Neotessmannia), or placentae bilobate axile-pendulous (Muntingia). Ovules numerous per carpel, anatropous, pendulous, epitropous, bitegmic, crassinucellar. Funicle long (Muntingia). Micropyle exostomal, Z-shaped (zig-zag). Outer integument approx. two cell layers thick. Inner integument approx. three cell layers thick. Funicle long (Muntingia). Hypostase present. Parietal tissue ? cell layers thick. Archespore multicellular. Megagametophyte monosporous, quadrinucleate, with micropylar megaspore (Muntingia). Endosperm development ab initio nuclear. Endosperm haustoria? Embryogenesis onagrad.
Fruit A many-seeded berry, with persistent (Dicraspidia, Neotessmannia?) or caducous (Muntingia) sepals.
Seeds Aril absent. Funicle mucilaginous. Exotesta mucilaginous. Endotestal cells crystalliferous. Exotegmic cells somewhat elongate, fibre-like. Endotegmen? Perisperm not developed. Endosperm diploid, starchy (Muntingia). Embryo straight, chlorophyll? Cotyledons two. Germination?
Cytology n = (14) 15 (Muntingia)
DNA
Phytochemistry Insufficiently known. Hydrolyzable tannins (ellagitannins), and ellagic and gallic acids present.
Use Ornamental plants, fruits, fibres, timber (Muntingia).
Systematics Muntingia (1; M. calabura; Florida, Mexico, Central America, the West Indies, tropical South America to Argentina), Dicraspidia (1; D. donnell-smithii; Central America, Colombia), Neotessmannia (1; N. uniflora; eastern Peru).
Muntingiaceae are sometimes recovered as sister to Cytinaceae (Nickrent 2007).
There is no available phylogeny of Muntingiaceae. Neotessmannia, in particular, is very insufficiently known.
NEURADACEAE Kostel. |
( Back to Malvales ) |
Grielaceae Martinov, Tekhno-Bot. Slovar: 294. 3 Aug 1820 [’Grielinae’]; Neuradales DC. in C. F. P. von Martius, Consp. Regn. Veg.: 64. Sep-Oct 1835 [‘Neuradeae’]
Genera/species 3/7
Distribution Arid regions of northern Africa, the Arabian Peninsula, Syria, Iraq, northern Iran, Afghanistan and Pakistan to India, southern and southeastern Africa.
Fossils Unknown.
Habit Bisexual, usually annual herbs (rarely perennial herbs, occasionally woody at base), usually procumbent and creeping.
Vegetative anatomy Phellogen absent? Secondary lateral growth normal or absent. Cambium storied? Vascular bundles surrounded by mucilaginous envelope. Wood with wide xylem bundles. Vessel elements with simple perforation plates; lateral pits usually opposite (sometimes almost alternate), simple pits. Vestured pits present. Imperforate tracheary xylem elements ? with simple pits. Wood rays wide. Axial parenchyma? Sieve tube plastids Pcs type, with protein crystalloids and starch. Nodes usually 3:3, trilacunar with three leaf traces. Medulla with lysigenous mucilaginous canal. Calciumoxalate crystals present in groups in stem and near foliar vascular strands. Raphides absent.
Trichomes Hairs unicellular, simple, lanate.
Leaves Alternate (spiral), pinnately compound, or simple and entire or pinnately lobed, with ? ptyxis. Stipules? and leaf sheath absent. Prophylls stipule-like, developing later than leaves. Petiole anatomy simple; petiole vascular bundle transection? Venation almost palmate. Stomata anomocytic. Cuticular waxes absent. Epidermal cells with tannins. Leaf margin serrate or sinuate.
Inflorescence Terminal (seemingly axillary), modified (reduced) cymose of various shape, or flowers solitary terminal.
Flowers Actinomorphic (Neurada, Neuradopsis) or somewhat obliquely zygomorphic (Grielum). Epicalyx-like structure consisting of five (in Neurada and Neuradopsis spiny) bracteole-like appendages, persistent in fruit, or absent. Hypanthium short. Receptacle accrescent. Epigyny or partial epigyny. Sepals five, with valvate aestivation, free. Petals five, usually with contorted (sometimes imbricate) aestivation, free. Nectary? Disc absent.
Androecium Five outer longer stamens and five inner shorter stamens, diplostemonous. Filaments with wide base and narrowing at apex, free from each other and from tepals, adnate to hypanthium. Anthers dorsifixed, versatile, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory, usually with binucleate cells. Staminodia absent.
Pollen grains Microsporogenesis simultaneous. Pollen grains oblate, bipolar, syncolpate and di- to tetraporate, with usually trifid (sometimes quadrifid) aperture (pore) at each pole; each aperture branch (semicolpus) with one pore (os), shed as monads, tricellular at dispersal. Exine semitectate, with columellate infratectum, reticulate (Neurada) or foveolate to finely reticulate (Grielum).
Gynoecium Pistil composed of usually ten connate antesepalous carpels, often with two to four adaxial carpels reduced or at least sterile; carpels dorsally adnate to hypanthium, ventrally free; carpels as young ascidiate. Ovary inferior or semi-inferior, multilocular (two to four locules reduced), often with persistent spines. Stylodia usually ten, free, usually persistent. Stigmas capitate, type? Gynoecium gradually asymmetrical due to non-uniform development. Pistillodium absent.
Ovules Placentation apical-axile. Ovule one (primarily two, one of which degenerating) per carpel, anatropous, pendulous, apotropous, bitegmic, crassinucellar. Micropyle bistomal. Outer integument approx. four cell layers thick. Inner integument approx. four cell layers thick. Parietal tissue approx. two cell layers thick. Megagametophyte monosporous, Polygonum type. Endosperm development ab initio nuclear. Endosperm haustoria? Embryogenesis?
Fruit A dry, indehiscent, often spiny nutlike fruit (sometimes ventrally dehiscent?) with accrescent calyx and persistent often spiny stylodia.
Seeds Aril absent. Seeds exotegmic. Testal cells collapsing. Endotestal cells small. Tegmen multiplicative. Exotegmen fibrous, with cells longitudinally and tangentially expanded, crystalliferous. Mesotegmic and endotegmic cells persistent. Perisperm not developed. Endosperm absent. Embryo curved, well differentiated, proteinaceous and oily, chlorophyll? Cotyledons two. Germination cryptocotylar (seeds germinating inside fruit).
Cytology n = 7
DNA
Phytochemistry Insufficiently known. Tannins and cyclopropenoid fatty acids (in seeds of at least Neurada) present. Ellagic acid?
Use Ornamental plants.
Systematics Neurada (1; N. procumbens; the Mediterranean, North Africa, the Arabian Peninsula, Southwest Asia to northwestern India), Grielum (4; G. cuneifolium, G. grandiflorum, G. humifusum, G. sinuatum; Namibia, Botswana, southwestern South Africa), Neuradopsis (2; N. austroafricana, N. bechuanensis; Namibia, Botswana, Northern Cape).
Neuradaceae are sister to the remaining Malvales.
There is no available phylogeny of Neuradaceae.
SARCOLAENACEAE Caruel |
( Back to Malvales ) |
Schizolaenaceae Barnhart in Bull. Torrey Bot. Club 22: 17. 15 Jan 1895 [‘Schizochlaenaceae’]; Rhodolaenaceae Bullock in Kew Bull. 12: 410. 1958
Genera/species 9/59
Distribution Madagascar, with their largest diversity in the eastern and central parts.
Fossils Uncertain. Fossil pollen tetrads similar to those in Sarcolaenaceae are reported from Miocene layers in South Africa.
Habit Bisexual, usually evergreen (sometimes deciduous) trees or shrubs.
Vegetative anatomy Ectomycorrhiza? Phellogen? Primary medullary rays narrow, uniseriate. Vessel elements with simple perforation plates; lateral pits alternate?, simple and/or bordered pits. Vestured pits present. Imperforate tracheary xylem elements tracheids, fibre tracheids or libriform fibres with bordered pits, non-septate. Wood rays usually uniseriate (rarely biseriate), homocellular. Axial parenchyma apotracheal diffuse, diffuse-in-aggregates, or banded. Wood elements not storied. Tyloses frequent. Secondary phloem stratified into hard fibrous and soft parenchymatous layers. Sieve tube plastids S type. Nodes 3:3, trilacunar with three leaf traces. Medullary and cortical cells often with sclereids. Resinous or mucilaginous canals and mucilage cells frequent. Cystoliths sometimes present. Wood ray cells often with silica bodies. Primary cortex and medulla with calciumoxalate crystals.
Trichomes Hairs unicellular or multicellular, simple or branched, furcate, stellate, peltate, lepidote, fasciculate; glandular hairs unicellular or multicellular, often peltate-lepidote.
Leaves Alternate (distichous), simple, entire, usually coriaceous, with involute ptyxis. Stipules of various shape, intrapetiolar, usually caducous, in Sarcolaena and Xerochlamys large and connate in pairs; leaf sheath absent. Petiole vascular bundle transection annular; petiole with cylinder of bundles surrounding central complex vascular tissue (often with siphonostele with medullary bundles?). Venation pinnate (secondary veins sometimes parallel). Stomata anomocytic. Cuticular wax crystalloids? Mesophyll with calciumoxalate druses; mesophyll with mucilaginous idioblasts. Leaf margin entire.
Inflorescence Usually terminal panicle (sometimes umbel-like; in Pentachlaena and Rhodolaena bifloral axillary). One or two flowers surrounded at base by large cupular, foliaceous, persistent and in fruit accrescent involucral outgrowth (consisting of free or connate bracts or bracteoles?) of pedicel, with dentate or lobate margin. Floral buds in some species enclosed by bracts, each consisting of rudimentary lamina surrounded by two connate stipules.
Flowers Actinomorphic. Usually hypogyny. Sepals three (to five; when five, then two outer sepals smaller than three inner sepals; when four then one or three outer sepals smaller than inner sepals), with imbricate-contorted aestivation, usually persistent, connate at base. Petals five (or six), with imbricate-contorted aestivation (in opposite direction of sepals), free or slightly connate at base. Nectariferous disc extrastaminal, annular to cupular or quinquelobate (staminodial?).
Androecium Stamens usually numerous (in Leptolaena five?, or ten diplostemonous), usually persistent. Filaments usually free (rarely connate at base into five or ten fascicles), free from tepals. Anthers basifixed or dorsifixed, non-versatile, tetrasporangiate, introrse or extrorse, longicidal (dehiscing by longitudinal slits). Tapetum secretory. Staminodia probably absent (alternatively five or ten staminodia forming nectariferous disc?).
Pollen grains Microsporogenesis simultaneous. Pollen grains 3(–6)-colporate (parasyncolpate), shed as tetrads with common apertures (apertures meeting in six confluent pairs), bicellular at dispersal. Exine tectate or semitectate, with columellate infratectum, perforate, microreticulate or reticulate, pilate or spinulate to smooth, with columellae often concentrated on ridges encircling each pollen grain and running in parallel along apertures.
Gynoecium Pistil composed of (one to) three (to five) densely hairy connate carpels. Ovary superior, (unilocular to) trilocular (to quinquelocular). Style single, simple, hollow, usually persistent. Stigma capitate to lobate, with multicellular papillae, type? Pistillodium absent.
Ovules Placentation apical, axile or basal. Ovules (one or) two to six (to more than 30) per carpel, anatropous, pendulous to ascending, bitegmic, crassinucellar. Micropyle exostomal to somewhat bistomal and Z-shaped (zig-zag). Outer integument ? cell layers thick. Inner integument ? cell layers thick. Megagametophyte monosporous, Polygonum type. Endosperm development ab initio nuclear. Endosperm haustoria? Embryogenesis?
Fruit Usually a loculicidal capsule (sometimes more or less indehiscent, nutlike), surrounded by persistent and often accrescent, thin, fleshy or woody involucre, bracts or cupule. Endocarp hairy.
Seeds Seeds hairy or glabrous. Aril absent. Exotesta? Endotesta? Exotegmen usually palisade, lignified; at least in Leptolaena with palisade layer invaginating chalazal region, into which hypostase plug, with core and annulus, fits (outer hypostase forming core, ’bixoid chalazal region’). Endotegmen? Perisperm not developed. Endosperm usually copious (rarely sparse or absent), often starchy (sometimes ruminate). Embryo straight, well differentiated, often starchy, chlorophyll? Cotyledons two, thin in species with copious endosperm, or thick in species without or with little endosperm, cordate. Germination?
Cytology n = 11
DNA
Phytochemistry Flavonols (myricetin etc.) and their glycosides, gallic acid, hydrolyzable and condensed tannins, saponins, and cyclopropenoid and cyclopropanoid fatty acids and their lipids present.
Use Ornamental plants, timber.
Systematics Sarcolaena (8; S. codonochlamys, S. delphinensis, S. eriophora, S. grandiflora, S. humbertiana, S. isaloensis, S. multiflora, S. oblongifolia; Madagascar), Leptolaena (8; L. abrahamii, L. cuspidata, L. delphinensis, L. gautieri, L. masoalensis, L. multiflora, L. pauciflora, L. raymondii; Madagascar), Xyloolaena (5; X. humbertii, X. perrieri, X. richardii, X. sambiranensis, X. speciosa; Madagascar), Perrierodendron (5; P. boinense, P. capuronii, P. occidentale, P. quartzitorum, P. rodoense; Madagascar), Eremolaena (2; E. humblotiana, E. rotundifolia; eastern Madagascar), Schizolaena (c 20; Madagascar), Rhodolaena (7; R. acutifolia, R. altivola, R. bakeriana, R. coriacea, R. humblotii, R. leroyana, R. macrocarpa; Madagascar), Pentachlaena (3; P. betamponensis, P. latifolia, P. orientalis; Madagascar), Mediusella (1; M. bernieri; Madagascar).
Sarcolaenaceae are sister to Dipterocarpaceae.
 |
Phylogeny (simplified) of Sarcolaenaceae based on DNA sequence data (Aubriot & al. 2016) |
SPHAEROSEPALACEAE (Warb.) Tiegh. ex Bullock |
( Back to Malvales ) |
Rhopalocarpaceae Hemsl. ex Takht., Sist. Magnoliof. [Systema Magnoliophytorum]: 130. 24 Jun 1987
Genera/species 2/20
Distribution Madagascar, with their highest diversity in the northeastern parts.
Fossils Unknown.
Habit Bisexual, deciduous trees or shrubs.
Vegetative anatomy Phellogen ab initio subepidermal. Medulla with parenchyma (rarely resinous). Vessel elements with simple perforation plates; lateral pits alternate, bordered pits. Vestured pits absent. Imperforate tracheary xylem elements tracheids or fibre tracheids, surrounding libriform fibres, with simple or bordered pits, non-septate (also vasicentric tracheids). Wood rays uniseriate to multiseriate, heterocellular. Axial parenchyma paratracheal, scalariform vasicentric or banded. Wood elements partially storied (tall wood rays not storied). Secondary phloem in young stems tangentially stratified into hard fibrous and soft parenchymatous layers. Sieve tube plastids S type. Nodes 3:3, trilacunar with three leaf traces. Cortex (and sometimes medulla) with secretory cavities (containing mucilage?). Pith and/or cortex in some species with vertical lysigenous secretory cavities. Prismatic calciumoxalate crystals frequent.
Trichomes Hairs unicellular, simple.
Leaves Alternate (spiral or distichous), simple, entire, often coriaceous, with conduplicate ptyxis. Stipules intrapetiolar, wide, more or less sheathing and connate, caducous; leaf sheath absent. Petiole distally pulvinate. Petiole vascular bundle transection arcuate to annular (rarely with adaxial plate); medullary vascular bundles sometimes present. Venation pinnate or palmate, brochidodromous, eucamptodromous, actinodromous, or acrodromous. Stomata usually anomocytic or anisocytic (sometimes cyclocytic). Cuticular wax crystalloids? Petiole with lysigenous secretory cavities containing mucilage. Mesophyll with mucilaginous idioblasts, often with calciumoxalate druses. Resiniferous cells present outside veins. Some species with branched transverse sclereids. Leaf margin entire.
Inflorescence Terminal or axillary, panicle or thyrse (with subumbelliform cymules, panicle, or with cymose partial inflorescences). Floral bracts several, caducous. Floral prophylls (bracteoles) usually small, caducous.
Flowers Actinomorphic. Receptacle somewhat dome-shaped, usually with short gynophore-like structure. Hypogyny to partial epigyny. Sepals usually 2+2 (rarely 3+3), with imbricate aestivation, coriaceous, unequal in size (outer sepals smaller, median, inner sepals larger), usually caducous (sometimes persistent), free. Petals usually four antesepalous (sometimes three, rarely up to nine), usually with imbricate aestivation, somewhat clawed, unequal in size, with numerous resinous lines, caducous, free. Nectary at apex of gynophore-like structure. Disc intrastaminal, annular, large, cupular, dentate.
Androecium Stamens usually c. 50 to c. 80 (rarely c. 25 to c. 30, or more than 200), in two to four whorls. Filaments filiform, usually inflexed in bud, with resiniferous ducts, usually connate at base in fascicles, free from tepals. Anthers dorsifixed, versatile, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits); connective broad, glandular. Tapetum secretory. Staminodia usually absent (in Rhopalocarpus longipetiolatus one to four petaloid staminodia and one smaller non-petaloid staminodium).
Pollen grains Microsporogenesis simultaneous. Pollen grains 3–5(–7)-colpor(oid)ate, with endoapertures colpiform, larger than and encompassing ectoapertures, shed as monads, ?-cellular at dispersal. Exine semitectate to almost intectate, with columellate infratectum, reticulate or microreticulate, scabrate, verrucate or spinulate.
Gynoecium Pistil composed of usually two or four (sometimes three, rarely one or five) antesepalous (Rhopalocarpus) or usually four (sometimes five; Dialyceras) connate carpels. Ovary superior to semi-inferior, in Dialyceras consisting of usually four (sometimes five) distinct (except at base) ovarioles surrounding central gynobasic style; ovary in Rhopalocarpus (unilocular or) bilocular (to quinquelocular) and narrowing towards apex into style. Style single, simple, gynobasic (Dialyceras) or terminal (Rhopalocarpus). Stigma capitate or punctate to discoid or somewhat infundibuliform, papillate, type? Carpels secreting exudate when injured. Pistillodium absent.
Ovules Placentation basal-axile (Rhopalocarpus) or basal-marginal (Dialyceras). Ovules two to nine per carpel, anatropous, ascending, epitropous, bitegmic, crassinucellar. Micropyle endostomal. Outer integument four or five cell layers thick. Inner integument three or four cell layers thick. Hypostase present. Megagametophyte monosporous, Polygonum type. Endosperm development ab initio nuclear. Endosperm haustoria? Embryogenesis?
Fruit A prickly and deeply lobed nutlike schizocarp with separate carpels developing into two to five single-seeded mericarps (Dialyceras), or a prickly nutlike capsule with ovary undeveloped on one side (Rhopalocarpus).
Seeds Seed usually with (in Rhopalocarpus similis without) partially lignified funicular aril. Testa often surrounded by viscid substance. Exotesta mucilaginous. Endotesta c. 15 to c. 20 cells thick, red. Exotegmen palisade, often distinctly invaginating either side of hypostase. Endotegmen? Operculum present. Perisperm not developed. Endosperm copious, often ruminate (due to invaginating exotegmen), oily and with numerous elliptic starch grains. Embryo well differentiated, straight, embedded in endosperm, chlorophyll? Cotyledons two, thin, cordate, bilobate at apex, with sinuate to highly dissected margins. Radicula straight. Germination?
Cytology n = 19
DNA
Phytochemistry Insufficiently known. Ellagic and gallic acids, hydrolyzable and condensed tannins (Rhopalocarpus), and saponins present.
Use Fibres for rope.
Systematics Dialyceras (3; D. coriaceum, D. discolor, D. parvifolium; northeastern Madagascar), Rhopalocarpus (17; Madagascar).
The sister-group relationships of Sphaerosepalaceae are unresolved, although they may be sister-group to Bixaceae (Johnson-Fulton & Watson 2017).
THYMELAEACEAE Juss. |
( Back to Malvales ) |
Daphnaceae Vent., Tabl. Règne Vég. 2: 235. 5 Mai 1799 [’Daphnoideae’]; Thymelaeales R. Br. ex Bercht. et J. Presl, Přir. Rostlin: 234. Jan-Apr 1820 [‘Thymeleae’]; Gnidiaceae Bercht. et J. Presl, Přir. Rostlin 1(116*-120): 1, 2. 1823; Aquilariaceae R. Br. ex DC., Prodr. 2: 59. Nov 1825 [’Aquilarineae’]; Aquilariales Link, Handbuch 2: 123. 4-11 Jul 1829 [’Aquilarinae’]; Daphnales Lindl., Nix. Plant.: 15. 17 Sep 1833; Thymelaeopsida Endl., Gen. Plant.: 313. Oct 1837 [’Thymelaeae’]; Daphnopsida Meisn., Plant. Vasc. Gen.: Comm.: 235. 18-24 Jul 1841 [’Daphnoideae’]; Phaleriaceae Meisn., Plant. Vasc. Gen.: Tab. Diagn. 323, 329, Comm. 241. 18-24 Jul 1841 [’Phalerieae’]; Gonystylaceae Tiegh. in Bot. Jahresber. (Just) 21(2): 389, 390. Jul-Dec 1896 [’Gonystyleae’], nom. cons.; Thymelaeineae Engl., Syllabus, ed. 2: 158. Mai 1898; Tepuianthaceae Maguire et Steyerm. in Mem. New York Bot. Gard. 32: 8. 20 Mai 1981
Genera/species 45/915–940
Distribution Cosmopolitan except polar areas, although mainly in tropical and southern Africa, the Mediterranean, tropical Asia and Australia.
Fossils Uncertain. Fossil pollen grains assigned to Octolepidoideae are known from the Eocene of India, and from the Oligocene and the Miocene of Borneo. Pollen attributed to Thymelaeoideae have been found in Miocene and younger layers.
Habit Usually bisexual (sometimes andromonoecious, trimonoecious, polygamomonoecious, dioecious, androdioecious, or gynodioecious), evergreen or deciduous trees, shrubs or suffrutices (rarely lianas or perennial herbs), usually poisonous. Many species are xerophytes. Bark strongly fibrous.
Vegetative anatomy Phellogen ab initio superficial. Primary medullary rays narrow? Secondary lateral growth usually normal (sometimes anomalous, from several concentric cambia or from one cylindrical cambium). Vessel elements with simple perforation plates; lateral pairs usually alternate, bordered pits. Vestured pits present. Imperforate tracheary xylem elements tracheids, fibre tracheids or libriform fibres, usually with bordered (sometimes simple) pits, non-septate (also vasicentric tracheids). Wood rays uniseriate or multiseriate, homocellular or heterocellular. Axial parenchyma usually paratracheal, scanty vasicentric, aliform, lozenge-aliform, winged-aliform, confluent, unilateral, or banded (rarely apotracheal diffuse or diffuse-in-aggregates or banded, or absent). Wood often fluorescent. Wood elements sometimes partially storied. Intraxylary diffuse phloem (often with fibres) usually present in young stems and branches of Thymelaeoideae (absent in Arnheimia and Drapetes; in, e.g., Aquilaria interxylary phloem, developing inwards from cambium). Secondary phloem fibres non-septate, sometimes lignified. Tough pericyclic fibres often present (absent in, e.g., Edgeworthia, Tepuianthus). Sieve tube plastids Ss type. Nodes 1:1, unilacunar with one leaf trace. Secretory cavities or cells, mucilage cells and cells with crystal sand or aggregations of crystals present. Calciumoxalate as styloids or prismatic crystals.
Trichomes Hairs usually unicellular, simple (sometimes furcate).
Leaves Alternate (usually spiral) or opposite, simple, entire, often coriaceous, in some genera ericoid, usually with supervolute (sometimes conduplicate) ptyxis. Stipules minute or absent; leaf sheath absent. Petiole vascular bundle transection usually arcuate. Venation usually pinnate, often brochidodromous or parallelodromous. Stomata usually anomocytic (sometimes cyclocytic). Cuticular wax crystalloids as rosettes of rodlets. Epidermis often with mucilage cells. Mesophyll with or without sclerenchymatous idioblasts; mesophyll cells sometimes with calciumoxalate druses. Leaf margin entire.
Inflorescence Terminal or axillary, usually racemose, capitate, fascicular or paniculate (rarely cymose). Involucre consisting of bracts present in many species. Inflorescence bracts rarely (e.g. in Phaleria) with extrafloral nectaries.
Flowers Usually actinomorphic (rarely zygomorphic), often small. Pedicel often articulated. Calyx tube (“hypanthium”) usually present (in Octolepidoideae short, in Thymelaeoideae long), persistent or caducous. Hypogyny. Sepals (three or) four or five (or six), usually with imbricate (rarely valvate) aestivation, often petaloid, free (possibly connate into tubular hypanthium-like structure?). Petals (three or) four or five (or six), with imbricate aestivation, as many as or twice as many as (sometimes more than twice as many as) sepals, often as scales, fringes, glands or hairs, usually free (sometimes entirely or only at base connate), or absent. Nectariferous disc of various shape (annular or of separate parts) often present in Thymelaeoideae (absent in Octolepidoideae).
Androecium Stamens usually 4+4 or 5+5 (rarely four, five, or numerous; in Pimelea two), usually diplostemonous or haplostemonous (antesepalous or alternisepalous; rarely polystemonous). Filaments usually free (in Synandrodaphne connate into tube), free from tepals, inserted at hypanthium. Anthers straight or curved (in some genera hippocrepomorphic), usually basifixed (in, e.g., Synandrodaphne dorsifixed), non-versatile, usually tetrasporangiate, usually introrse (rarely extrorse), longicidal (dehiscing by longitudinal slits). Tapetum secretory, with binucleate to sexanucleate cells. Staminodia absent (or three to twelve petaloid, scale-like?).
Pollen grains Microsporogenesis simultaneous. Pollen grains usually polyporate or forate (in Octolepis triporate or tetraporate; in Tepuianthus 3–6-colporate), shed as monads, tricellular at dispersal. Exine tectate or semitectate, with columellate infratectum, perforate, reticulate or microreticulate, microechinate.
Gynoecium Pistil composed of two (Thymelaeoideae, with abaxial carpel usually sterile and degenerated), or three to twelve (Octolepidoideae) connate carpels. Ovary superior, in Octolepidoideae trilocular to duodecemlocular; in Thymelaeoideae unilocular (pseudomonomerous) or bilocular. Style single, simple, usually terminal (lateral in species with one fertile carpel; rarely absent). Stigma capitate to peltate, usually papillate, Dry type. Male flowers in Tepuianthus with pistillodium.
Ovules Placentation axile to apical (when ovary multilocular), or parietal to apical (when ovary unilocular). Ovule one per carpel/ovary, anatropous or hemianatropous, pendulous, epitropous, bitegmic, crassinucellar. Micropyle usually endostomal (rarely Z-shaped). Outer integument three to six cell layers thick. Inner integument three to ten cell layers thick. Obturator usually present from near stylar canal base to micropyle. Hypostase usually present. Parietal tissue approx. seven cell layers thick. Nucellar cap approx. two cell layers thick. Megagametophyte monosporous, Polygonum type. Synergids sometimes with a filiform apparatus. Antipodal cells usually persistent and proliferating (forming up to c. 30 or more cells). Endosperm development ab initio nuclear. Endosperm haustoria? Embryogenesis asterad.
Fruit A loculicidal capsule (often with coriaceous to carnose pericarp), a drupe or berry (sometimes nutlike).
Seeds Seed with or without arilloid lignified appendage (caruncle?) from chalaza and/or raphe (funicular aril absent). Seed coat tegmic. Testa usually thin (sometimes carnose). Exotegmen palisade, with lignified cell walls. Endotegmen thickened and often lignified. Perisperm not developed. Endosperm usually sparse or absent. Embryo well differentiated, straight, without chlorophyll. Cotyledons two, thick or thin. Germination phanerocotylar or cryptocotylar.
Cytology n = (7–)9(–10) (Thymelaeoideae) – Polyploidy frequently occurring.
DNA
Phytochemistry Flavonols (kaempferol, quercetin), biflavonoids, apigenin etc., cyanidin, toxic diterpene esters, alkaloids, cucurbitacins, cyclopropenoid fatty acids and their lipids, cyanogenic glycosides, coumarins (daphnin, daphnetin), lignans (syringaresinol, pinoresinol), sterols, and chelidonic acid present. Ellagic acid not found.
Use Ornamental, textile and paper plants (bast fibres of Edgeworthia chrysantha etc., Daphne, Lagetta, Thymelaea, Wikstroemia), incense (Wikstroemia) and perfumes (sesquiterpene alcohols in fungal infected wood), medicinal plants, cosmetics (Aquilaria etc.), timber.
Systematics Thymelaeaceae are sister to the remaining Malvales except Neuradaceae.
Tepuianthus is sister to all other Thymelaeaceae. A probable topology of Thymelaeaceae is the following [Tepuianthus+[Synandrodaphne+[Octolepidoideae+Thymelaeoideae]]].
Tepuianthus
1/6. Tepuianthus (6; T. aracensis, T. auyantepuiensis, T. colombanus, T. sarisarinamensis, T. savannensis, T. yapacanensis; lowland savannah and Roraima sandstone in Colombia, Venezuela, the Guyanas and northern Brazil). – Bisexual or unisexual (functional dioecy or androdioecy), evergreen trees or shrubs with bitter-tasting bark and resin. Pericyclic fibres absent. Wood rays uniseriate. Secondary phloem stratified into sclerenchymatic and non-sclerenchymatic layers. Phloem rays narrow. Nodes 1:1, unilacunar with one leaf trace. Resiniferous cells present. Hairs unicellular, simple, depressed. Leaves alternate or opposite, simple, entire, often coriaceous, subpeltate. Stipules and leaf sheath absent. Venation pinnate. Lamina gland-dotted. Inflorescence terminal or axillary cyme. Flowers actinomorphic, small. Hypanthium absent. Hypogyny. Sepals five, imbricate, free, with adaxial colleters? at base. Petals five, imbricate, clawed, free. Nectariferous disc extrastaminal, consisting of five to ten scale-like glands (staminodia?). Stamens five antesepalous or twelve to 16(–22) in antepetalous? fascicles of (one to) three or four each, in one to three whorls. Filaments free from each other and from petals. Anthers dorsifixed, versatile, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits); connective sometimes prolonged. Staminodia absent. Pollen grains 3–6-colp(or)ate, ?-cellular at dispersal. Exine reticulate. Pistil composed of (two or) three connate carpels. Ovary (bilocular or) trilocular. Stylodia (two or) three, deeply bilobate. Male flowers with pistillodium. Placentation apical-axile. Ovule one per carpel, anatropous, pendulous. Fruit a loculicidal capsule. Seed non-arillate. Raphe perpendicular. Testa approx. six cell layers thick, unlignified. Exotegmen palisade, with lignified cell walls. Endotegmic cells low, with lignified walls. Endosperm copious. Embryo small, poorly differentiated. Cotyledons two. n = ?
[Synandrodaphne+[Octolepidoideae+Thymelaeoideae]]
Petals absent. Perianth tubular, with apical appendages. Pollen grains oligoporate to polyporate. Exine echinate. Style single. Stigma capitate, Dry type. Endotegmen with stripes on inner surface.
Synandrodaphne
1/1. Synandrodaphne (1; S. paradoxa; Central Africa). – Bisexual tree. Sepals four or five. Calyx tube (“hypanthium”) absent. Petals absent. Disc absent. Stamens four or five. Anthers introrse. Pistil composed of two connate carpels. Fruit a capsule. n = ? – Synandrodaphne is sometimes recovered as sister to Thymelaeoideae.
[Octolepidoideae+Thymelaeoideae]
Octolepidoideae Engl. et Gilg in H. G. A. Engler, Syllabus, ed. 7: 275. Oct 1912-Mar 1913
8/57–62. Octolepis (7; O. aymoniniana, O. casearia, O. decalepis, O. dioica, O. ibityensis, O. oblanceolata, O. ratovosonii; tropical Africa, Madagascar), Deltaria (1; D. brachyblastophora; New Caledonia), Lethedon (14; northeastern Queensland, New Caledonia, Vanuatu), Solmsia (2; S. calophylla, S. chrysophylla; New Caledonia), Arnhemia (1; A. cryptantha; Northern Territory); Gonystylus (30–35; Malesia to New Guinea, the Solomon Islands, Fiji), Amyxa (1; A. pluricornis; Borneo), Aetoxylon (1; A. sympetalum; Borneo). – Tropical Africa, Madagascar, Malesia to New Guinea, northeastern Queensland, Melanesia. Trees, shrubs or lianas. Intraxylary phloem absent. Secretory cavities present (lamina punctate). Stomata in Octolepis anomocytic. Hypanthium short or absent. Hypogyny. Sepals (three to) five (or six), with valvate or imbricate aestivation. Glandular scales four to c. 40. Stamens eight to c. 80. Filaments not adnate to calyx tube, sometimes connate. Anthers usually reflexed and hippocrateromorphic; connective well developed; basal layer with pendulous internal outgrowths. Pistil composed of (two or) three to five (to eight) connate carpels. Clavate or subglobose parastyles sometimes present. Stigma capitate to punctate. Raphal aril present, or seed angular at raphe, or funicle swollen. Chalazal fold only on ventral side. Nucellar tracheids present. Tegmen sometimes multiplicative. Endosperm usually copious. n = ? Cyclopentenoid cyanogenic glycosides present in Lethedon.
Thymelaeoideae Burnett, Outlines Bot.: 570, 1091, 1145. Feb 1835 [‘Thymaelidae’]
35/850–870. Aquilaria (22; northeastern India, Southeast Asia, Malesia to New Guinea), Gyrinops (9; Sri Lanka, Laos, East Malesia to New Guinea); Linostoma (3; L. decandrum, L. pauciflorum, L. persimile; tropical Asia, tropical Australia), Jedda (1; J. multicaulis; northern Queensland), Lophostoma (4; L. amoenum, L. calophylloides, L. dinizii, L. ovatum; northern tropical South America), Enkleia (3; E. malaccensis, E. paniculata, E. thorelii; the Andaman Islands, Southeast Asia, Malesia), Dicranolepis (c 20; tropical Africa), Synaptolepis (5; S. alternifolia, S. kirkii, S. oliveriana, S. perrieri, S. retusa; tropical and southern Africa, Madagascar), Craterosiphon (11; tropical Africa), Linodendron (3; L. aroniifolium, L. cubanum, L. venosum; Cuba), Stephanodaphne (9; Madagascar, the Comoros), Phaleria (c 25; Sri Lanka, Malesia to New Guinea, Northern Territory, eastern Queensland, eastern New South Wales, the Caroline Islands), Peddiea (14; tropical and southeastern Africa, Madagascar), Daphnopsis (c 75; southern Mexico, Central America, the West Indies, tropical South America), Goodallia (1; G. guianensis; Guyana), Funifera (4; F. brasiliensis, F. ericiflora, F. grandifolia, F. insulae; Brazil), Schoenobiblus (10; Central America, tropical South America), Ovidia (3; O. andina, O. pillopillo, O. sericea; Bolivia, central Chile, the Andes in Argentina), Dirca (4; D. decipiens, D. mexicana, D. occidentalis, D. palustris; southern Canada, United States, northern Mexico), Lagetta (3; L. lagetto, L. valenzuelana, L. wrightiana; the West Indies), Daphne (90–95; Europe, the Mediterranean, temperate Asia), Wikstroemia (c 90; Southeast Asia to islands in the Pacific incl. the Hawaiian Islands), Rhamnoneuron (1; R. balansae; Southeast Asia), Edgeworthia (4; E. eriosolenoides, E. gardneri, E. longipes, E. tomentosa; China, Japan), Thymelaea (c 35; southern Europe, the Mediterranean, temperate Asia, the Himalayas, Tibet), Diarthron (c 15; Europe, western Asia), Stellera (1; S. chamaejasme; Iran, Central Asia, the Himalayas, Tibet, China), Dais (2; D. cotinifolia: south tropical and southern Africa; D. glaucescens: Madagascar), Struthiola (35–40; tropical and southern Africa, with their highest diversity in the Cape Provinces in South Africa), Lachnaea (35–40; Northern, Western and Eastern Cape), Passerina (c 20; Central to southern Africa, with their highest diversity in the Cape Provinces in South Africa), Drapetes (1; D. muscosus; Tierra del Fuego, the Falkland Islands), Kelleria (11; Gunong Kinabalu in Sabah, New Guinea, eastern Victoria, Tasmania, New Zealand), Gnidia (150–155; tropical and southern Africa, Madagascar, the Arabian Peninsula, western India, Sri Lanka, with their largest diversity in the Cape Provinces in South Africa), Pimelea (c 130; the Philippines to Australia, Tasmania, Lord Howe, New Zealand), Lasiadenia (2; L. ottohuberi, L. rupestris; tropical South America). – Cosmopolitan, with their largest diversity in South Africa and Australia. Trees, shrubs, lianas or herbs. Intraxylary phloem usually present. Vascular bundles bicollateral. Lignified pit membrane with torus present in numerous species. Leaves often opposite. Stomata sometimes anomocytic. Inflorescence often capitate. Calyx tube (“hypanthium”) usually long. Hypogyny. Sepals usually four or five. Petaloid appendages up to twice as many as sepals or absent. Nectariferous disc present (sometimes elongate and tubular) or absent. Stamens two to five, usually antesepalous (sometimes alternisepalous), or ten. Filaments usually inserted in upper part of (= adnate to) calyx tube. Pollen grains crotonoid. Pistil composed of two connate carpels. Ovary in principle bilocular (one locule often undeveloped). Micropyle in Gnidia Z-shaped (zig-zag). Fruit a drupe or an achene. Seed sometimes with chalazal fold and/or carunculus. Nucellar tracheids sometimes present. Testal cells sometimes enlarged. Tegmen often multiplicative. Exotegmen usually palisade. Endosperm usually absent (sometimes copious). n = (7–)9(–10); polyploidy frequently occurring. Myricetin, phorbol ester diterpenes (mostly orthoesters and 1-alkyldaphnane derivatives), chelidonic acid, and cyclopropenoid fatty acids present. Tannins not found. – Aquilaria and Gyrinops – Aquilarioideae (R. Br. ex DC.) Meisn. in DC., Prodr. 14: 495, 601. mid Oct 1856 – form a sister-group to the remaining Thymelaeoideae (Bank & al. 2002).
 |
One of 27 most-parsimonious cladograms (successively weighted) of Thymelaeaceae based on DNA sequence data (Bank & al. 2002). The position of Synandrodaphne is fairly weakly supported. |
Literature
Adolf W, Seip EH, Hecker E. 1988. Irritant principles of the mezereon family (Thymelaeaceae) V. New skin irritants and tumor promoters of the daphnane and 1α-alkyldaphnane type from Synaptolepis kirkii and Synaptolepis retusa. – J. Nat. Prod. (Lloydia) 51: 662-674.
Aguilar JF, Fryxell PA, Jansen RK. 2003. Phylogenetic relationships and classification of the Sida generic alliance (Malvaceae) based on nrDNA ITS evidence. – Syst. Bot. 28: 352-364.
Airy Shaw HK. 1953. Thymelaeaceae-Gonystyloideae. – In: Steenis CGGJ van (ed), Flora Malesiana I, 4(4), Noordhoff-Kolff N. V., Batavia, pp. 349-365.
Airy Shaw HK. 1979. Three interesting plants from the Northern Territory of Australia (Thymelaeaceae, Flacourtiaceae and Hanguanaceae). – Kew Bull. 33: 1-5.
Alvarado-Cárdenas LO. 2009. Systemática del género Bdallophytum (Cytinaceae). – Acta Bot. Mexicana 87: 1-21.
Álvarez I, Cronn R, Wendel JF. 2005. Phylogeny of the New World diploid cottons (Gossypium L., Malvaceae) based on sequences of three low-copy nuclear genes. – Plant Syst. Evol. 252: 199-214.
Alverson WS. 1986. Quararibea Aubl. s.l. (Bombacaceae) in Mexico, Central America and the Antilles: a taxonomic study. – Ph.D. diss., University of Wisconsin, Madison, Wisconsin.
Alverson WS. 1989a. Quararibea (Bombacaceae): five new species from moist and wet forests of Costa Rica and Panama. – Brittonia 41: 61-74.
Alverson WS. 1989b. Matisia and Quararibea (Bombacaceae) should be retained as separate genera. – Taxon 38: 377-388.
Alverson WS. 1991. A synopsis of Phragmotheca (Bombacaceae), with two new species and a new subspecies. – Brittonia 43: 73-87.
Alverson WS. 1994. New species and combinations of Catostemma and Pachira (Bombacaceae) from the Venezuelan Guayana. – Novon 4: 3-8.
Alverson WS, Duarte MC. 2015. Hello again Pochota, farewell Bombacopsis. – Novon 24: 115-119.
Alverson WS, Steyermark JA. 1997. Bombacaceae. – In: Steyermark JA, Berry PE, Holst BK (eds), Flora of the Venezuelan Guayana 3. Araliaceae-Cactaceae, Missouri Botanical Garden, St. Louis, Missouri, pp. 496-527.
Alverson WS, Karol KG, Baum DA, Chase MW, Swensen SM, McCourt R, Sytsma KJ. 1998. Circumscription of the Malvales and relationships to other Rosidae: evidence from rbcL sequence data. – Amer. J. Bot. 85: 876-887.
Alverson WS, Whitlock BA, Nyffeler R, Bayer C, Baum DA. 1999. Phylogeny of the core Malvales: evidence from ndhF sequence data. – Amer. J. Bot. 86: 1474-1486.
Anderson GJ. 1976. The pollination biology of Tilia. – Amer. J. Bot. 63: 1203-1212.
Andreasen K. 2012. Phylogeny, hybridization, and evolution of habit and breeding system in Sidalcea and Eremalche (Malvaceae). – Intern. J. Plant Sci. 173: 532-548.
Andreasen K, Baldwin BG. 2001. Unequal evolutionary rates between annual and perennial lineages of checker mallows Sidalcea (Malvaceae): evidence from 18S-26S rDNA internal and external transcribed spacers. – Mol. Biol. Evol. 18: 936-944.
Andreasen K, Baldwin BG. 2003. Nuclear ribosomal DNA sequence polymorphism and hybridization in checker mallows (Sidalcea, Malvaceae). – Mol. Phylogen. Evol. 29: 563-581.
Andriamihajarivo T, Lowry PP II, Schatz GE. 2016. Endemic families of Madagascar XIV. A new restricted range species of Pentachlaena (Sarcolaenaceae) from central Madagascar. – Candollea 71: 167-172.
Anjaneyulu ASR, Raju SN. 1984. Stercurensin, a new C-methylchalcone from leaves of Sterculia urens. – Indian J. Chem. 23B: 1010-1011.
Appanah S. 1987. Insect pollination and the diversity of dipterocarps. – In: Kostermans AJGH (ed), Proc. 3rd Round Table Conf. Dipterocarps, UNESCO, Jakarta, pp. 277-291.
Appanah S. 1998. Root symbiosis and nutrition. – In: Appanah S, Turnbull JM (eds), A review of dipterocarps: taxonomy, ecology and silviculture, CIFOR, pp. 99-114.
Appanah S, Chan HT. 1981. Thrips: the pollinators of some dipterocarps. – Malaysian For. 44: 234-252.
Applequist WL. 2009. A revision of the Malagasy endemic Helmiopsis (Malvaceae s.l.). – Ann. Missouri Bot. Gard. 96: 521-540.
Applequist WL. 2014. A taxonomic revision of
Dombeya Sect. Decastemon (Malvaceae). – Ann. Missouri Bot. Gard.
99: 553-619.
Arbo MM. 1972. Estructura y ontogenia de los nectarios foliares del género Byttneria (Sterculiaceae). – Darwiniana 17: 104-158.
Arbo MM. 1973. Los nectarios foliares de Megatritheca (Sterculiacae). – Darwiniana 18: 272-276.
Archangelsky DB. 1966. Pollen grains of Thymelaeaceae and Gonystylaceae. – Bot. Žurn. 51: 484-494. [In Russian]
Archangelsky DB. 1971. The palynotaxonomy of Thymelaeaceae s.l. – In: Kuprianova LA, Jakovlev MS (eds), Pollen morphology, Nauka, Leningrad, pp. 104-234. [In Russian]
Areces-Berazain F, Ackerman JD. 2016. Phylogenetics, delimitation and historical biogeography of the pantropical tree genus Thespesia (Malvaceae, Gossypieae). – Bot. J. Linn. Soc. 181: 171-198.
Argout X, Salse J, Aury JM, Guiltinan MJ, Droc G, Gouzy J, Allegre M, Chaparro C, Legavre T, Maximova SN, Abrouk M, Murat F, Fouet O, Poulain J, Ruiz M, Roguet Y, Rodier-Goud M, Barbosa-Neto JF, Sabot F, Kudrna D, Ammiraju JSS, Schuster SC, Carlson JE, Sallet E, Schiex T, Dievart A, Kramer M, Gelley L, Shi Z, Bérard A, Viot C, Boccara M, Risterucci AM, Guignon V, Sabau X., Axtell M, Ma Z, Zhang Y, Brown S, Bourge, M., Golser, W., Song, X., Clement, D., Rivalan, R., Tahi, M., Akaza, J. M., Pitollat, B., Gramacho, K., D’Hont, A., Brunel, D., Infante, D., Kebe, I., Costet, P., Wing, R., McCombie, W. R., Guiderdoni, E., Quetier F, Panaud O, Wincker P, Sidibe-Bocs S, Lanaud C. 2010. The genome of Theobroma cacao. – Nature Genetics 43: 101-110.
Arrington JM. 2004. Systematics of the Cistaceae. – Ph.D. diss., Department of Biology, Duke University, North Carolina.
Arrington JM, Kubitzki K. 2002. Cistaceae. – In: Kubitzki K, Bayer C (eds), The families and genera of vascular plants V. Flowering plants. Dicotyledons. Malvales, Capparales and non-betalain Caryophyllales, Springer, Berlin, Heidelberg, New York, pp. 62-70.
Ashton PS. 1962. The taxonomy and ecology of the Dipterocarpaceae of Brunei State. – Ph.D. diss., Cambridge University, Cambridge, England.
Ashton PS. 1963. Taxonomic notes on Bornean Dipterocarpaceae. – Gard. Bull. (Singapore) 20: 229-284.
Ashton PS. 1972. Precursor to a taxonomic revision of Ceylon Dipterocarpaceae. – Blumea 20: 357-366.
Ashton PS. 1979. Phylogenetic speculations on Dipterocarpaceae. – In: Maury-Lechon G (ed), Diptérocarpacées: taxonomie-phylogénie-écologie, Mem. Mus. Natl. Hist. Nat., sér. B, Botanique 26, Éditions du Muséum, Paris, pp. 145-149.
Ashton PS. 1982. Dipterocarpaceae. – In: Steenis CGGJ van (ed), Flora Malesiana I, 9(2), Martinus Nijhoff, Junk Publ., The Hague, Boston, London, pp. 237-552.
Ashton PS. 1989. Dipterocarp reproductive biology. – In: Lieth H, Werger MJA (eds), Ecosystems of the world 14B. Tropical rain forest ecosystems, Elsevier, Amsterdam.
Ashton PS. 2002. Dipterocarpaceae. – In: Kubitzki K, Bayer C (eds), The families and genera of vascular plants V. Flowering plants. Dicotyledons. Malvales, Capparales and non-betalain Caryophyllales, Springer, Berlin, Heidelberg, New York, pp. 182-197.
Ashton PS, Givnish TJ, Appanah S. 1988. Staggered flowering in the Dipterocarpaceae: new insights into floral induction and the evolution of mast fruiting in the aseasonal tropics. – Amer. Natur. 132: 44-66.
Aspinall GO, Fraser RN, Sanderson GR. 1965. Plant gums of the genus Sterculia III. Sterculia setigera and Cochlospermum gossypium gums. – J. Chem. Soc. London 1965: 4325-4329.
Aubréville A. 1975. Essais de géophylétique des Bombacacées. – Adansonia, sér. II, 15: 57-64.
Aubréville A. 1976. Essai d’interprétation nouvelle de la distribution des Diptérocarpacées. – Adansonia, sér. II, 16: 205-210.
Aubriot X, Soulebeau A, Haevermans T, Schatz GE, Cruaud C, Lowry II PP. 2016. Molecular phylogenetics of Sarcolaenaceae (Malvales), Madagascar’s largest endemic plant family. – Bot. J. Linn. Soc. 182: 729-743.
Awasthi N. 1969. Revision of some dipterocarpaceous woods previously described from the Tertiary of South India. – Palaeobotanist 18: 229.
Baas P, Werker E. 1981. A new record of vestured pits in Cistaceae. – IAWA Bull., N. S., 2: 41-42.
Baer DF. 1976. Systematics of the genus Bixa and geography of the cultivated annatto tree. – Ph.D. diss., University of California, Los Angeles, California.
Bahadur B, Srikanth R. 1984. Pollination biology and the species problem in the Waltheria indica complex. – Phytomorphology 33: 96-107.
Baillon H. 1870. Traité du développement de la fleur et du fruit: Buettnériées. – Adansonia 9: 336-351.
Baillon H. 1872. Sur le fruit d’une nouvelle Chlaenacée. – Adansonia 10: 234-239.
Baillon H. 1883. Les Ropalocarpées, à propos d’un nouveau Ropalocarpus. – Bull. Mens. Soc. Linn. Paris 1: 393-394.
Baillon H. 1884. Les Xylolaena et la valeur de la famille des Chlénacées. – Bull. Mens. Soc. Linn. Paris 1: 410-414.
Baillon H. 1886. Nouvelles observations sur les Chlénacées. – Bull. Mens. Soc. Linn. Paris 1: 570-572.
Baker HG. 1965. The evolution of the cultivated kapok tree: a probably West African product. – In: Brokensha D (ed), Research Studies no. 9. Ecology and economic development in Africa, Inst. Intern. Stud., University of California, Berkeley, California, pp. 185-216.
Baker HG. 1983. Ceiba pentandra (Ceyba, Ceiba, Kapok Tree). – In: Janzen D (ed), Costa Rican natural history, University of Chicago Press, Chicago, ILL, pp. 212-215.
Baker HG, Baker I. 1968. Chromosome numbers in the Bombacaceae. – Bot. Gaz. (London) 129: 294-296.
Bakhuizen van der Brink RC. 1924. Revisio Bombacacearum. – Bull. Jard. Bot. Buitenzorg, sér. III, 6: 161-240.
Balasubramanian A, Sekar T, Devadoss C. 1995. The vascular system of stem-node-leaf in Bixa orellana L. – Phytomorphology 45: 219-227.
Balthazar M von, Nyffeler R. 2002. The peculiar androecium of Cullenia (Durioneae, Malvaceae s.l.). – In: Schönenberger, Balthazar M von, Matthews M (eds), Flowers: diversity, development & evolution, Institute of Systematic Botany, Universität Zürich, Switzerland.
Balthazar M von, Alverson WS, Schönenberger J, Baum DA. 2004. Comparative floral development and androecium structure in Malvoideae (Malvaceae s..). – Intern. J. Plant Sci. 165: 445-473.
Balthazar M von, Schönenberger J, Alverson WS, Janka H, Bayer C, Baum DA. 2006. Structure and evolution of the androecium in the Malvatheca clade (Malvaceae s.l.) and implications for Malvaceae and Malvales. – Plant Syst. Evol. 260: 171-197.
Bancroft H. 1935a. The taxonomy, history and geographical distribution of Monotoideae. – Amer. J. Bot. 22: 505-519.
Bancroft H. 1935b. The wood anatomy of representative members of the Monotoideae. – Amer. J. Bot. 22: 717-739.
Banerji I. 1941. A note on the development of the female gametophyte in Abroma angusta L. and Pentapetes phoenicea L. – Curr. Sci. 10: 30.
Bank M van der, Fay MF, Chase MW. 2002. Molecular phylogenetics of Thymelaeaceae with particular reference to African and Australian genera. – Taxon 51: 329-339.
Barnett LC. 1987. Two new species of Nesogordonia (Sterculiaceae) from Madagascar. – Bull. Mus. Hist. Nat. Paris, sér. IV, 9, sect. B, Adansonia 1: 95-100.
Barnett LC. 1988. Systematics of Nesogordonia Baillon (Sterculiaceae). – Ph.D. diss., University of Texas, Austin, Texas.
Barth OM, Barbosa AF. 1973. Catálogo sistemático das pólens das plantas arbóreas do Brasil meridional XVII. Elaeocarpaceae e Tiliaceae. – Mem. Inst. Oswaldo Cruz 71: 203-217.
Bates DM. 1965. Notes on cultivated Malvaceae 1. Hibiscus. – Baileya 13: 56-130.
Bates DM. 1968. Generic relationships in the Malvaceae, tribe Malveae. – Gentes Herb. 10: 117-135.
Bates DM. 1969. Systematics of the South African genus Anisodontea Presl (Malvaceae). – Gentes Herb. 10: 215-383.
Bates DM. 1973. A revision of Bakeridesia Hochreutiner subgenus Bakeridesia (Malvaceae). – Gentes Herb. 10: 425-484.
Bates DM. 1976. Chromosome numbers in the Malvales III. Miscellaneous counts from the Byttneriaceae and Malvaceae. – Gentes Herb. 11: 143-150.
Bates DM. 1992. Gynodioecy, endangerment, and status of Eremalche kerensis (Malvaceae). – Phytologia 72: 48-54.
Bates DM, Blanchard OJ Jr. 1970. Chromosome numbers in the Malvales II. New or otherwise noteworthy counts relevant to classification in the Malvaceae, tribe Malveae. – Amer. J. Bot. 57: 927-934.
Bates DM, Dorr LJ, Blanchard OJ Jr. 1989. Chromosome numbers in Callirhoe (Malvaceae). – Brittonia 41: 143-151.
Bate-Smith EC, Whitmore TC. 1959. Chemistry and taxonomy in Dipterocarpaceae. – Nature 184: 795-796.
Baum DA. 1995a. The comparative pollination and floral biology of baobabs (Adansonia-Bombacaceae). – Ann. Missouri Bot. Gard. 82: 322-348.
Baum DA. 1995b. A systematic revision of Adansonia (Bombacaceae). – Ann. Missouri Bot. Gard. 82: 440-471.
Baum DA, Oginuma K. 1994. A review of chromosome numbers in Bombacaceae with new counts for Adansonia. – Taxon 43: 11-20.
Baum DA, Alverson WS, Nyffeler R. 1998. A durian by any other name: taxonomy and nomenclature of the core Malvales. – Harvard Pap. Bot. 3: 317-332.
Baum DA, Small RL, Wendel JF. 1998. Biogeography and floral evolution of baobabs (Adansonia, Bombacaceae) as inferred from multiple data sets. – Syst. Biol. 47: 181-207.
Baum DA, Smith SD, Yen A, Alverson WS, Nyffeler R, Whitlock BA, Oldham RL. 2004. Phylogenetic relationships of Malvatheca (Bombacoideae and Malvoideae; Malvaceae sensu lato) as inferred from plastid DNA sequences. – Amer. J. Bot. 91: 1863-1871.
Baumann K. 1994. Der Zistrosenwurger, Cytinus hypocistis (L.) L. – Palmengarten 58: 154-157.
Bawa KS, Frankie GW. 1983. Cochlospermum vitifolium. – In: Janzen DH (ed), Costa Rican natural history, The University of Chicago Press, Chicago, pp. 215-216.
Bawa KS, Webb CJ. 1983. Floral variation and sexual differentiation in Muntingia calabura (Elaeocarpaceae), a species with hermaphrodite flowers. – Evolution 37: 1271-1282.
Bayer C. 1994. Zur Infloreszenzmorphologie der Malvales. – Diss. Bot. 212: 1-280.
Bayer C. 1995. Zur Verzweigung der vegetativen und blühenden Achsen einiger Bombacaceen. – Feddes Repert. 106: 407-413.
Bayer C. 1998. Synflorescences of Malvaceae. – Nord. J. Bot. 18: 335-338.
Bayer C. 1999. The bicolor unit – homology and transformation of an inflorescence structure unique to core Malvales. – Plant Syst. Evol. 214: 187-198.
Bayer C. 2000. On the identity of Cotylonychia Stapf (Sterculiaceae). – Kew Bull. 55: 499-500.
Bayer C. 2002a. Diegodendraceae. – In: Kubitzki K, Bayer C (eds), The families and genera of vascular plants V. Flowering plants. Dicotyledons. Malvales, Capparales and non-betalain Caryophyllales, Springer, Berlin, Heidelberg, New York, pp. 175-177.
Bayer C. 2002b. Muntingiaceae. – In: Kubitzki K, Bayer C (eds), The families and genera of vascular plants V. Flowering plants. Dicotyledons. Malvales, Capparales and non-betalain Caryophyllales, Springer, Berlin, Heidelberg, New York, pp. 315-319.
Bayer C. 2002c. Neuradaceae. – In: Kubitzki K, Bayer C (eds), The families and genera of vascular plants V. Flowering plants. Dicotyledons. Malvales, Capparales and non-betalain Caryophyllales, Springer, Berlin, Heidelberg, New York, pp. 325-328.
Bayer C. 2002d. Sarcolaenaceae. – In: Kubitzki K, Bayer C (eds), The families and genera of vascular plants V. Flowering plants. Dicotyledons. Malvales, Capparales and non-betalain Caryophyllales, Springer, Berlin, Heidelberg, New York, pp. 345-352.
Bayer C. 2002. Sphaerosepalaceae. – In: Kubitzki K, Bayer C (eds), The families and genera of vascular plants V. Flowering plants. Dicotyledons. Malvales, Capparales and non-betalain Caryophyllales, Springer, Berlin, Heidelberg, New York, pp. 359-362.
Bayer C, Dorr LJ. 1999. A synopsis of the neotropical genus Pentaplaris, with remarks on its systematic position within core Malvales. – Brittonia 51: 134-148.
Bayer C, Hoppe JR. 1990. Die Blütenentwicklung von Theobroma cacao L. – Beitr. Biol. Pflanzen 65: 301-312.
Bayer C, Kubitzki K. 1996. Inflorescence morphology of some Australian Lasiopetaleae (Sterculiaceae). – Telopea 6: 721-728.
Bayer C, Kubitzki K. 2002. Malvaceae. – In: Kubitzki K, Bayer C (eds), The families and genera of vascular plants V. Flowering plants. Dicotyledons. Malvales, Capparales and non-betalain Caryophyllales, Springer, Berlin, Heidelberg, New York, pp. 225-311.
Bayer C, Chase MW, Fay MF. 1998. Muntingiaceae, a new family of dicotyledons with malvalean affinities. – Taxon 47: 37-42.
Bayer C, Fay MF, De Bruijn AY, Savolainen V, Morton CM, Kubitzki K, Alverson WS, Chase MW. 1999. Support for an expanded family concept of Malvaceae within a recircumscribed order Malvales: a combined analysis of plastid atpB and rbcL DNA sequences. – Bot. J. Linn. Soc. 129: 267-303.
Beaumont AJ, Edwards TJ, Smith FR. 2001a [2002]. Leaf and bract diversity in Gnidia (Thymelaeaceae): patterns and taxonomic value. – Syst. Geogr. Plants 71: 399-418.
Beaumont AJ, Edwards TJ, Smith FR. 2001b [2002]. Patterns of diversity among involucral bracts, inflorescences and flowers in Gnidia (Thymelaeaceae). – Syst. Geogr. Plants 71: 419-431.
Beaumont AJ, Edwards TJ, Manning J, Maurin O, Rautenbach M, Motsi MC, Fay MF, Chase MW, Bank M van der. 2009. Gnidia (Thymelaeaceae) is not monophyletic: taxonomic implications for Thymelaeoideae and a partial new generic taxonomy for Gnidia. – Bot. J. Linn. Soc. 160: 402-417.
Bechi N, Corsi G, Pagni AM. 1994. On the glandular hairs of Cistus laurifolius L. (Cistaceae). – Giorn. Bot. Ital. 128: 741-749.
Beentje H. 1989. Bombacaceae. – In: Polhill RM (ed), Flora of tropical East Africa, A. A. Balkema, Rotterdam, pp. 1-9.
Behnke H-D. 1984. Ultrastructure of sieve-element plastids of Myrtales and allied groups. – Ann. Missouri Bot. Gard. 71: 824-831.
Bell SAJ, Copeland LM. 2004. Commersonia rosea (Malvaceae s.l.: Lasiopetaleae): a new, rare fire-ephemeral species from the upper Hunter Valley of New South Wales. – Telopea 10: 582-586.
Benitez, Vieyra S, Hempel de Ibarra N, Wertien AM, Cocucci AA. 2007. How to look like a mallow: evidence of floral mimicry between Turneraceae and Malvaceae. – Proc. Roy. Soc. London, B, 274: 2239-2248.
Bentley BL. 1977. The protective function of ants visiting the extrafloral nectaries of Bixa orellana (Bixaceae). – J. Ecol. 65: 27-38.
Bentley BL. 1983. Bixa orellana (Achiote, Annatto). – In: Janzen DH (ed), Costa Rican natural history, Chicago University Press, Chicago, pp. 193-194.
Bentley KW. 1998. The isoquinoline alkaloids. – Harwood, Australia.
Berazaín FA. 2006. New records of Malvaceae from Cuba. – Willdenowia 36: 881-884.
Beyers JBP, Marais EM. 1998. Palynological studies of the Thymelaeaceae of the Cape flora. – Grana 37: 193-202.
Beyers JBP, Walt JJA van der. 1995. The generic delimitation of Lachnaea and Cryptadenia (Thymelaeaceae). – Bothalia 25: 65-85.
Bhat RB. 1996. Leaf architecture in Adansonia, Bombax and Ceiba (Bombacaceae). – Aust. Syst. Bot. 9: 255-260.
Bondeson WE. 1979. Ovarian anatomy of Quararibea guianensis och Q. cordata. – Bot. Not. 132: 491-509.
Borrone JW, Meerow AW, Kuhn DN, Whitlock BA, Schnell RJ. 2007. The potential of the WRKY gene family for phylogenetic reconstruction: an example from the Malvaceae. – Mol. Phylogen. Evol. 44: 1141-1154.
Borssum Waalkes J van. 1966. Malesian Malvaceae revised. – Blumea 14: 1-251.
Bosch J. 1992. Floral biology and pollinators of three co-occurring Cistus species (Cistaceae). – Bot. J. Linn. Soc. 109: 39-55.
Bosser J. 1973. Sur trois Rhopalocarpus de Madagascar. – Adansonia, sér. II, 13: 55-62.
Bouman F, Meijer W. 1994. Comparative structure of ovules and seeds in Rafflesiaceae. – Plant Syst. Evol. 193: 187-212.
Boureau E. 1958. Contribution à l’étude anatomique des espèces actuelles de R[h]opalocarpaceae. – Bull. Mus. Natl. Hist. Nat. (Paris), sér. II, 30: 213-221.
Boureau E, Tardieu-Blôt ML. 1955. Répartition géographique des Diptérocarpacées vivantes et fossiles. – Compt. Rend. Som. Séances Soc. Biogéogr.: 107-114.
Boursnell JG. 1950. The symbiotic seed-borne fungus in the Cistaceae I. – Ann. Bot., N. S., 14: 217-243.
Boyd RS. 1994. Pollination biology of the rare shrub Fremontodendron decumbens. – Madroño 41: 277-289.
Boyd RS. 1996. Ant-mediated seed dispersal of the rare chaparral shrub Fremontodendron decumbens (Sterculiaceae). – Madroño 43: 299-315.
Brandis D. 1895. An enumeration of the Dipterocarpaceae, based chiefly upon the specimens preserved at the Royal Herbarium and Museum, Kew, and the British Museum; and remarks on the genera and species. – Biol. J. Linn. Soc. 31: 1-148.
Brandis D, Gilg E. 1895. Dipterocarpaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(6), W. Engelmann, Leipzig, pp. 243-273.
Brandt U, Gottsberger G. 1988. Flower phenology, pollinating insects and breeding systems in Cistus, Halimium and Tuberaria species in Portugal. – Lagascalia 15 (Extra): 625-634.
Brazier JD. 1979. Classifying the Dipterocarpaceae: the wood technologist’s view. – In: Maury-Lechon G (ed), Diptérocarpacées: taxonomie-phylogénie-écologie, Mem. Mus. Natl. Hist. Nat., sér. B, Botanique 26, Éditions du Muséum, Paris, pp. 76-80.
Bredenkamp CL, Wyk AE van. 1996. Palynology of the genus Passerina (Thymelaeaceae): form and function. – Grana 35: 335-346.
Bredenkamp CL, Wyk AE van. 1999. Structure of mucilaginous epidermal cell walls in Passerina (Thymelaeaceae). – Bot. J. Linn. Soc. 129: 223-238.
Bridson DM. 1975. Bixaceae. – In: Polhill RM (ed), Flora of tropical East Africa, Crown Agents for Oversea Governments and Administrations, London, pp. 1-3.
Brodie S, Cheek M, Staniforth M. 1998. Trochetiopsis ebenus. Sterculiaceae. – Curtis’s Bot. Mag. 15: 27-36.
Brundrett MC. 2011. Commentary on the de Vega et al. (2010) paper on hyphae in the parasitic plant Cytinus: mycorrhizal fungi growing within plants are not always mycorrhizal. – Amer. J. Bot. 98: 595-596.
Brunken U. 2003. Androecial structure and ontogeny of Grewioideae (Malvaceae s.l.). – Palm. Hortus Francofurtensis 7: 135.
Brunken U. 2007. Blütenstruktur und Phylogenie der Malvaceae-Grewioideae. – Ph.D. diss., Universität Frankfurt/Main, Germany.
Brunken U, Muellner AN. 2012. A new tribal classification of Grewioideae (Malvaceae) based on morphological and molecular phylogenetic evidence. – Syst. Bot. 37: 699-711.
Buerki S, Devey DS, Callmander MW, Phillipson PB, Forest F. 2013. Spatio-temporal history of the endemic genera of Madagascar. – Bot. J. Linn. Soc. 171: 304-329.
Bunninger L. 1972. Untersuchungen über die morphologische Natur des Hypanthiums bei Myrtales- und Thymelaeales-Familien II. Myrtaceae; III. Vergleich mit den Thymelaeaceae. – Beitr. Biol. Pflanzen 48: 79-156.
Burandt Jr CL. 1992. A monograph of Sida Sect. Oligandrae (Malvaceae). – Syst. Bot. 17: 164-179.
Burandt Jr CL, Fryxell PA. 1990. A reappraisal of Abutilon reflexum (Malvaceae) and its allies. – Syst. Bot. 15: 49-56.
Burgoyne PM. 2006. A new species of Cytinus (Cytinaceae) from South Africa and Swaziland, with a key to the southern African species. – Novon 16: 315-319.
Burret M. 1924. Neotessmannia, eine neue Tiliaceen-Gattung. – Notizbl. Bot. Gart. Mus. Berlin-Dahlem 9: 125-127.
Burret M. 1926. Beiträge zur Kenntnis der Tiliaceen. – Notizbl. Bot. Gart. Mus. Berlin-Dahlem 9: 592-880.
Burret M. 1940. Palmen und Tiliaceen von der Südsee aus der Sammlung des Bernice P. Bishop Museums, Honolulu, Hawaii. – Notizbl. Bot. Gart. Berlin-Dahlem 15: 85-96.
Burrows CJ. 1960. Studies in Pimelea I – the breeding system. – Trans. Roy. Soc. New Zealand (Bot.) 88: 29-45.
Burrows CJ. 1962. Studies in Pimelea II – taxonomy of some mountain species. – Trans. Roy. Soc. New Zealand (Bot.) 1: 217-223.
Burrows CJ. 2008. The genus Pimelea (Thymelaeaceae) in New Zealand 1. Taxonomic treatment of seven endemic glabrous-leaved species. – New Zealand J. Bot. 46: 127-176.
Burrows CJ. 2009a. The genus Pimelea (Thymelaeaceae) in New Zealand 2. The P. prostrata and P. urvilliana species complexes. – New Zealand J. Bot. 47: 163-229.
Burrows CJ. 2009b. The genus Pimelea (Thymelaeaceae) in New Zealand 3. The taxonomic treatment of six endemic hairy-leaved species. – New Zealand J. Bot. 47: 325-354.
Burrows CJ. 2011a. The genus Pimelea (Thymelaeaceae) in New Zealand 4. The taxonomic treatment of 10 endemic hairy-leaved species. – New Zealand j. Bot. 49: 41-106.
Burrows CJ. 2011b. The genus Pimelea (Thymelaeaceae) in New Zealand 5. The taxonomic treatment of five endemic species with both adaxial and abaxial leaf hair. – New Zealand J. Bot. 49: 367-412.
Buttrose MS, Grant WJR, Lott JNA. 1977. Reversible curvature of style branches of Hibiscus trionum L., a pollination mechanism. – Aust. J. Bot. 25: 567-570.
Buzato S, Sazima M, Sazima I. 1994. Pollination of three species of Abutilon (Malvaceae) intermediate between bat and hummingbird flower syndromes. – Flora 189: 327-334.
Cabi E, Baser B, Uzunhisarcikli ME, Yavru A. 2009. Pollen morphology of Alcea L. and Althaea L. genera (Malvaceae) in Turkey. – Feddes Repert. 120: 405-418.
Callmander MW, Phillipson PB, Schatz GE, Andriambololonera S, Rabarimanarivo M, Rakotonirina N, Raharimampionona J, Chatelain C, Gautier L, Lowry PP II. 2011. The endemic and non-endemic vascular flora of Madagascar updated. – Plant Ecol. Evol. 144: 121-125.
Capuron R. 1952. 127ème Famille – Rhopalocarpacées. – In: Humbert H (ed), Flore de Madagascar et des Comores, Muséum National d’Histoire Naturelle, Paris.
Capuron R. 1962. Révision des Rhopalocarpacées. – Adansonia, sér. II, 2: 228-267.
Capuron R. 1963a. Révision des Tiliacées de Madagascar et des Comores. – Adansonia, sér. II, 3: 91-129.
Capuron R. 1963b. Contributions à l’étude de la flore de Madagascar XV. Diegodendron R. Capuron gen. nov., type de la nouvelle famille des Diegodendraceae (Ochnales sensu Hutchinson). – Adansonia, sér. II, 3: 385-392.
Capuron R. 1963c. Contributions à l’étude de la flore de Madagascar XVI. Deux nouveaux Schizolaena Dupetit-Thouars (Sarcolaenacées). – Adansonia, sér. II, 3: 392-400.
Capuron R. 1965. Description des fruits du Diegodendron Humbertii R. Capuron (Diegodendracées). – Adansonia, sér. II, 5: 503-505.
Capuron R. 1970. Observations sur les Sarcolaenacées. – Adansonia, sér. II, 10: 247-265.
Capuron R. 1973. Un Pentachlaena (Sarcolaenacées) nouveau. – Adansonia, sér. II, 13: 289-293.
Carlquist SJ. 1964. Pollen morphology and evolution of Sarcolaenaceae (Chlaenaceae). – Brittonia 16: 231-254.
Carlquist SJ. 2005. Wood and bark anatomy of Muntingiaceae: a phylogenetic comparison within Malvales s.l. – Brittonia 57: 59-67.
Carvalho MR, Herrera FA, Jaramillo CA, Wing SL, Callejas R. 2011. Paleocene Malvaceae from northern South America and their biogeographical implications. – Amer. J. Bot. 98: 1337-1355.
Carvalho-Sobrinho JG, Queiroz LP. 2011. Morphological cladistics analysis of Pseudobombax Dugand (Malvaceae, Bombacoideae) and allied genera. – Rev. Bras. Bot. 34: 197-209.
Carvalho-Sobrinho JG, Santos FAR, Queiroz LP. 2009. Morfologia dos tricomas das pétalas de espécies de Pseudobombax Dugand (Malvaceae, Bombacoideae) e seu significado taxonômico. – Acta Bot. Bras. 23: 929-934.
Carvalho-Sobrinho JG, Queiroz LP, Dorr LJ. 2013. Does Pseudobombax have prickles? Assessing the enigmatic species Pseudobombax endecaphyllum (Malvaceae: Bombacoideae). – Taxon 62: 814-818.
Carvalho-Sobrinho JG, Alverson WS, Alcantara S, Queiroz LP, Mota AC, Baum DA. 2016. Revisiting the phylogeny of Bombacoideae (Malvaceae): novel relationships, morphologically cohesive clades, and a new tribal classification based on multilocus phylogenetic analyses. – Mol. Phylogen. Evol. 101: 56-74.
Carvalho MR, Herrera FA, Jaramillo CA, Wing SL, Callejas R. 2011. Paleocene Malvaceae from northern South America and their biogeographical implications. – Amer. J. Bot. 98: 1337-1355.
Cascante-Marin A. 1997. La familia Bombacacee (Malvales) en Costa Rica. – Brenesia 47: 17-36.
Catalina Londoño A, Alvarez E, Forero E, Morton CM. 1995. A new genus and species of Dipterocarpaceae from the Neotropics I. Introduction, taxonomy, ecology, and distribution. – Brittonia 47: 237-247.
Catarino L, Martins ES, Abreu JA, Figueira R. 2012. Revision of the family Dipterocarpaceae in Angola. – Blumea 57: 263-274.
Cavaco A. 1951. Remarques sur les genres Leptolaena et Xerochlamys (Chlaenacées). Un nouveau genre de Chlaenaceae. – Bull. Mus. Natl. Hist. Nat. Paris, sér. II, 23: 133-139.
Cavaco A. 1952a. Recherches sur les Chlaenacées, famille endémique de Madagascar. – Mém. Inst. Sci. Madagascar, Sér. B, Biol. Vég. 4: 52-92.
Cavaco A. 1952b. 126ème Famille – Chlaenacées. – Flore de Madagascar et des Comores, Muséum National d’Histoire Naturelle, Paris.
Chambers T, Godwin H. 1961. The fine structure of the pollen wall of Tilia platyphyllos. – New Phytol. 60: 393-399.
Chattaway MM. 1932. The wood of Sterculiaceae I. Specialization of the vertical wood parenchyma within the subfamily Sterculieae. – New Phytol. 31: 119-132.
Chattaway MM. 1933a. Tile-cells in the rays of the Malvales. – New Phytol. 32: 261-273.
Chattaway MM. 1933b. Ray development in the Sterculiaceae. – Forestry 7: 93-108.
Chattaway MM. 1934. Anatomical evidence that Grewia and Microcos are distinct. – Trop. Woods 38: 9-11.
Chattaway MM. 1937. The wood anatomy of the Sterculiaceae. – Phil. Trans. Roy. Soc. London 228: 313-365.
Chattaway MM. 1956. Crystals in woody tissues II. – Trop. Woods 104: 100-124.
Chauhan JS, Kumar S, Chaturvedi R. 1984. A new flavanonol glycoside from Adansonia digitata roots. – Planta Medica 50: 113.
Cheek M, Frimodt-Møller C. 1998. The genus Octolobus (Sterculiaceae) new to East Africa. – Kew Bull. 53: 682.
Cheek M, Leach G. 1991. Hildegardia (Sterculiaceae) new to Australia. – Kew Bull. 46: 72.
Cheeseman EE. 1927. Fertilisation and embryogeny in Theobroma cacao L. – Ann. Bot. 41: 107-126.
Chen Z, Grover CE, Li P, Wang Y, Nie H, Zhao Y, Wang M, Liu F, Zhou Z, Wang X, Cai X, Wang K, Wendel JF, Hua J. 2017. Molecular evolution of the plastid genome during diversification of the cotton genus. – Mol. Phylogen. Evol. 112: 268-276.
Chiarugi A. 1925. Embriologia delle Cistaceae. – Nuovo Giorn. Bot. Ital., n. s., 32: 223-314.
Choong CY, Wickneswari R, Norwati M, Abbott RJ. 2008. Phylogeny of Hopea (Dipterocarpaceae) inferred from chloroplast DNA and nuclear PgiC sequences. – Mol. Phylogen. Evol. 48: 1238-1243.
Chopra RN, Kaur H. 1965. Embryology of Bixa orellana Linn. – Phytomorphology 15: 211-214.
Christensen PB. 1986a. Pollen morphological studies in the Malvaceae. – Grana 25: 95-117.
Christensen PB. 1986b. Evolutionary trends in the pollen morphology in Malvaceae. – In: Blackmore S, Ferguson IK (eds), Pollen and spores: form and function, Academic Press, London, pp. 425-427.
Chung RCK. 2003. New taxa and new combinations of Microcos (Tiliaceae) from Peninsular Malaysia and Borneo. – Kew Bull. 58: 329-349.
Chung RCK, Soepadmo E. 2011. Taxonomic revision of the genus Microcos (Malvaceae-Grewioideae) in Peninsular Malaysia and Singapore. – Blumea 56: 273-299.
Civeyrel L, Leclercq J, Demoly JP, Agnan Y, Quèbre N, Pélissier C, Otto T. 2011. Molecular systematics, character evolution, and pollen morphology of Cistus and Halimium (Cistaceae). – Plant Syst. Evol. 295: 23-54.
Clarkson JR. 1986. Jedda, a new genus of Thymelaeaceae (subtribe Linostomatinae) from Australia. – Austrobaileya 2: 203-210.
Clarkson JR, Clifford HT. 1987. Germination of Jedda multicaulis (Thymelaeaceae). An example of cryptogeal germination in the Australian Flora. – Aust. J. Bot. 35: 715-720.
Classen R. 1988. Beiträge zur Kenntnis der Gattung Lasiopetalum (Sterculiaceae). – Bot. Jahrb. Syst. 109: 501-527.
Coetzee JA, Muller J. 1984. The phytogeographic significance of some extinct Gondwana pollen types from the Tertiary of the southwestern Cape (South Africa). – Ann. Missouri Bot. Gard. 71: 1088-1099.
Cok OF. 1916. Branching and flowering habits of cacao and patashte. – Contr. U.S. Natl. Herb. 17: 609-625.
Cope FW. 1958. Incompatibility in Theobroma cacao. – Nature 181: 279.
Cope FW. 1962. The mechanisms of pollen imcompatibility in Theobroma cacao. – Heredity 17: 157-182.
Corner EJH. 1949. The durian theory or the origin of the modern tree. – Ann. Bot., N. S., 13: 367-414.
Corner EJH. 1953. The durian theory extended I. – Phytomorphology 3: 465-476.
Costa L, Oliveira Á, Carvalho-Sobrinho J, Souza G. 2017. Comparative cytomolecular analyses reveal karyotype variability related to biogeographic and species richness patterns in Bombacoideae (Malvaceae). – Plant Syst. Evol. 303: 1131-1144.
Costantin JN, Poisson HL. 1908. Macrocalyx tomentosa. – Compt. Rend. Acad. Sci. Paris 147: 637.
Craven LA, Fryxell PA. 1989. Two new species of Decaschistia (Malvaceae) from Australia. – Aust. Syst. Bot. 2: 461-468.
Craven LA, Pfeil BE. 2004. Australian representatives of Macrostelia transferred to Hibiscus (Malvaceae) with the description of a new species. – Adansonia, sér. III, 26: 235-240.
Craven LA, Wilson FD, Fryxell PA. 2003. A taxonomic review of Hibiscus sect. Furcaria (Malvaceae) in Western Australia and the Northern Territory. – Aust. Syst. Bot. 16: 185-218.
Cristóbal CL. 1960. Revisión del género ’Ayenia’ (Sterculiaceae). – Opera Lilloana (Tucuman) 4: 1-230.
Cristóbal CL. 1965. Megatritheca (Sterculiaceae), género nuevo de Africa tropical. – Adansonia, sér. II, 5: 365-373.
Cristóbal CL. 1967. Cromosomas de Malvales. – Kurtziana 4: 139-142.
Cristóbal CL. 1968. Estudio morfológico de los granos de pollen de Byttneria (Sterculiaceae). – Pollen Spores 10: 57-72.
Cristóbal CL. 1976. Estudio taxonómico del género Byttneria Loefling (Sterculiaceae). – Bonplandia 4: 1-428.
Cristóbal CL. 1981. Rayleya, nueva Sterculiaceae de Bahia-Brasil. – Bonplandia 5: 43-50.
Cristóbal CL. 1989. Comentarios acerca de Guazuma ulmifolia (Sterculiaceae). – Bonplandia 6: 183-196.
Cristóbal CL. 2001. Taxonomía del género Helicteres (Sterculiaceae). Revisión de las espécies americanas. – Bonplandia 11: 1-206.
Cronk QCB. 1990. The history of the endemic flora of St Helena: Late Miocene ‘Trochetiopsis-like’ pollen from St Helena and the origin of Trochetiopsis. – New Phytol. 114: 159-165.
Cronk QCB. 1995. A new species and hybrid in the St Helena endemic genus Trochetiopsis. – Edinburgh J. Bot. 52: 205-213.
Cronn RC, Zhao X, Paterson AH, Wendel JF. 1996. Polymorphism and concerted evolution in a tandemly repeated gene family: 5S ribosomal DNA in diploid and allopolyploid cottons. – J. Mol. Evol. 42: 685-705.
Cruickshank RH. 1953. Chromosome numbers in the genus Pimelea. – Pap. Proc. Roy. Soc. Tasmania 87: 13-16.
Cuatrecasas J. 1953. Un nouveau genre de Bombacées, Patinoa. – Rev. Int. Bot. Appl. Agric. Trop. 33: 306-313.
Cuatrecasas J. 1954a. Novelties in the Bombacaceae. – Phytologia 4: 465-480.
Cuatrecasas J. 1954b. Disertaciones sobre Bombacáceas. – Rev. Acad. Colomb. Ci. Exact. 9: 164-177.
Cuatrecasas J. 1964. Cacao and its allies – a taxonomic revision of the genus Theobroma. – Contr. U.S. Natl. Herb. 35: 379-614.
Curran LM. 1994. The ecology and evolution of mast-fruiting in Bornean Dipterocarpaceae: a general ectomycorrhizal theory. – Ph.D. diss, Princeton University, Princeton, New Jersey.
Curran LM, Leighton M. 2000. Vertebrate responses to spatiotemporal variation in seed production of mast-fruiting Dipterocarpaceae. – Ecol. Monogr. 70: 101-128.
Curtis WF. 1976. Chromosome counts in Grielum and Cercis. – Ann. Missouri Bot. Gard. 63: 379-380.
Danguy P. 1915. Observations sur le genre Eremolaena. – Bull. Mus. Natl. Hist. Nat. Paris 21: 210-203.
Dansereau PM. 1939. Definizione del genere Halimiocistus. – Nuovo Giorn. Bot. Ital. II, 46: 357-360.
Daoud HS, Wilbur RL. 1965. A revision of the North American species of Helianthemum (Cistaceae). – Rhodora 67: 63-82, 201-216, 255-312.
Das AB, Mukherjee AK, Das P. 2001. Molecular phylogeny of Heritiera Aiton (Sterculiaceae), a tree mangrove: variations in RAPD markers and nuclear DNA content. – Bot. J. Linn. Soc. 136: 221-229.
Dass HC, Randhawa GS. 1962. Vascular anatomy of the flower of Grewia asiatica L. – Phyton (Buenos Aires) 19: 185-193.
Dathan ASR, Singh D. 1972. Development of embryo sac and seed in Bixa L. and Cochlospermum Kunth. – J. Indian Bot. Soc. 51: 254-266.
Davie JH. 1933. Cytological studies in the Malvaceae and certain related families. – J. Genet. 28: 33-67.
Dayanandan S. 1996. Phylogeny of the tropical tree family Dipterocarpaceae based on nucleotide sequences of the chloroplast rbcL gene and morphology. – Ph.D. diss., Boston University, Boston, Massachusetts.
Dayanandan S, Attygalle DNC, Abeygunasekera L, Gunatilleke CVS, Gunatilleke IAUN. 1990. Phenology and floral morphology in relation to pollination of Sri Lankan dipterocarps. – In: Bawa KS, Hadley M (eds), Reproductive ecology of tropical forest plants, UNESCO, Paris and Parthenon Publ. Carnforth.
Dayanandan S, Ashton PS, Williams SM, Primack RB. 1999. Phylogeny of the tropical tree family Dipterocarpaceae based on nucleotide sequences of the chloroplast rbcL gene. – Amer. J. Bot. 86: 1182-1190.
Dehay C. 1941. L’appareil libéro-ligneux foliaire des Sterculiacées. – Ann. Sci. Nat., XI, Bot., 2: 45-128.
Dehay C. 1942. Remarques sur l’appareil libéro-ligneux foliaire des Sterculiacées. – Bull. Soc. Bot. France 89: 76-78.
Dehay C. 1944. L’appareil libéro-ligneux foliaire des Tiliaceés. – Bull. Soc. Bot. France 91: 27-29.
Dehay C. 1957. Anatomie comparée de la feuille des Chlénacées. – Mém. Inst. Sci. Madagascar, sér. B, Biol. Vég. 8: 145-203.
Dehay C. 1958. Anatomie comparée de la famille des Chlénacées. – Mém. Inst. Sci. Madagascar, sér. B, Biol. Vég. 8: 145-203.
DeJoode DR, Wendel JF. 1992. Genetic diversity and origin of the Hawaiian islands cotton, Gossypium tomentosum. – Amer. J. Bot. 79: 1311-1319.
Dempsey RE, Garwood NC. 1994. A study of Bixa (Bixaceae), with particular reference to the leaf undersurface indumentum as a diagnostic character. – Bull. Brit. Mus. (Nat. Hist.) Bot. 24: 173-179.
Den Outer RW, Schütz PR. 1981. Wood anatomy of some Sarcolaenaceae and Rhopalocarpaceae and their systematic position. – Meded. Landbouwh. Wageningen 81(8): 1-25.
Den Outer RW, Vooren AP. 1980. Bark anatomy of some Sarcolaenaceae and Rhopalocarpaceae and their systematic position. – Meded. Landbouwh. Wageningen 80(6): 1-15.
Détienne P, Loureiro AA, Jacquet P. 1983. Estudo anatômico do lenho da familia Bombacaceae da América. – Acta Amazon. 13: 831-867.
De Vega C, Ortiz PL, Arista M, Talavera S. 2007. The endophytic system of Mediterranean Cytinus (Cytinaceae) developing on five host Cistaceae species. – Ann. Bot. 100: 1209-1217.
De Vega C, Berjano R, Arista M, Ortiz PL, Talavera S, Stuessy TF. 2008. Genetic races associated with the genera and sections of host species in the holoparasitic plant Cytinus (Cytinaceae) in the western Mediterranean basin. – New Phytol. 178: 875-887.
De Vega C, Arista M, Ortiz PL, Herrera CM, Talavera S. 2009. The ant-pollination system of Cytinus hypocistis (Cytinaceae), a Mediterranean root holoparasite. – Ann. Bot. 103: 1065-1075.
Dicke IA, Guza RC, Krazewski SE, Reich PB. 2004. Shared ectomycorrhizal fungi between a herbaceous perennial (Helianthemum bicknellii) and oak (Quercus) seedlings. – New Phytol. 164: 375-382.
Dickison WC. 1988. Xylem anatomy of Diegodendron humbertii. – IAWA Bull., N. S., 9: 332-336.
Ding Hou. 1960. Thymelaeaceae. – In: Steenis CGGJ van (ed), Flora Malesiana I, 6, Wolters-Noordhoff, Groningen, pp. 1-48.
Ditsch F, Barthlott W. 1994. Mikromorphologie der Epicuticularwachse und die Systematik der Dilleniales, Lecythidales, Malvales und Theales. – Trop. Subtrop. Pflanzenwelt 88: 1-74.
Domke W. 1934a. Untersuchungen über die systematische und geographische Gliederung der Thymelaeaceen nebst einer Neubeschreibung ihrer Gattungen. – Bibl. Bot. 27, 111: 1-151.
Domke W. 1934b. Zur Kenntnis einiger Thymelaeaceae. – Notizbl. Bot. Gart. Berlin-Dahlem 9(1930-1934): 348-363.
Donnell AA, Ballard HE Jr, Cantino PD. 2012. Callianthe (Malvaceae): a new genus of Neotropical Malveae. – Syst. Bot. 37: 712-722.
Dorr LJ. 1990a. An expansion and revision of the Malagasy genus Humbertiella (Malvaceae). – Bull. Mus. Natl. Hist. Nat. (Paris), sér. IV, sect. B, Adansonia 12: 7-27.
Dorr LJ. 1990b. A revision of the North American genus Callirhoe (Malvaceae). – Mem. New York Bot. Gard. 56: 1-76.
Dorr LJ. 1996. Ayenia saligna (Sterculiaceae), a new species from Colombia. – Brittonia 48: 213-216.
Dorr LJ. 2004. A remarkable new species of Sterculia (Sterculioideae, Malvaceae) from Madagascar. – Adansonia, sér. III, 26: 161-165.
Dorr LJ, Barnett LC. 1989. A revision of Melochia section Physodium (Sterculiaceae) from Mexico. – Brittonia 41: 404-423.
Doweld A, Reveal JL. 2007. Proposal to conserve the name Dombeyaceae. – Taxon 56: 265-266.
Drake del Castillo E. 1903. Hibiscus nodulosus sp. nov. – Bull. Mus. Natl. Hist. Nat. Paris 9: 38.
Duarte MC, Esteves GL, Salatino MLF, Walsh KC, Baum DA. 2011. Phylogenetic analyses of Eriotheca and related genera (Bombacoideae, Malvaceae). – Syst. Bot. 36: 690-701.
Ducke A. 1935. Aguiaria, novo gênero de Bombacáceas, a árvore maior do Alto Rio Negro. – Anais Acad. Bras. Ci. 7: 329-332.
Ducousso M, Béna G, Bourgeois C, Buyck B, Eyssartier G, Vincelette M, Rabevohitra R, Randrihasipara L, Dreyfus B, Prin Y. 2004. The last common ancestor of Sarcolaenaceae and Asian dipterocarp trees was ectomycorrhizal before the India-Madagascar separation, about 88 million years ago. – Mol. Ecol. 13: 231-236.
Dugand A. 1943. Revalidacion de Bombax Ceiba L. como especie tipica del genero Bombax L. y descripcion de Pseudobombax gen. nov. – Caldasia 2: 47-68.
Dumont A. 1887. Recherches sur l’anatomie comparée des Malvacées, Bombacacées, Tiliacées, Sterculiacées. – Ann. Sci. Nat. Bot., sér. 7, 6: 129-242.
Duncan EJ. 1970. Ovule and embryo ontogenesis in Bombacopsis glabra (Pasq.) A. Robyns. – Ann. Bot., N. S., 34: 677-683.
Dute R, Jandrlich MD, Thornton S, Callahan N, Jansen CJ. 2011. Tori in species of Diarthron, Stellera and Thymelaea (Thymelaeaceae). – IAWA J. 32: 54-66.
Dutta S, Tripathi SM, Mallick M, Mathews RP, Greenwood PF, Rao MR, Summons RE. 2011. Eocene out-of-India dispersal of Asian dipterocarps. – Rev. Palaeobot. Palyn. 166: 63-68.
Duvigneaud P. 1961. 27. Dipterocarpaceae. – In: Exell AW, Wild H (eds), Flora Zambesiaca 1 (Part 2), Crown Agents for Oversea Governments and Administrations, London, pp. 407-420.
Edlin HL. 1935. A critical revision of certain taxonomic groups of the Malvales 1-2. – New Phytol. 34: 1-20, 122-143.
Edmonds JM. 1990. Herbarium survey of African Corchorus L. species. Systematic and ecogeographic studies on crop genepools 4. – International Board for Plant Genetic Resources, Rome.
Eguiarte L, Rio CM del, Arita H. 1987. El nectar y el polen como recursos: el papel ecologico de los visitantes a las flores de Pseudobombax ellipticum (HBK) Dugand. – Biotropica 19: 74-82.
Eichler AW. 1872. Abermals ein neues Balanophoreen-Geschlecht. – Bot. Zeit. (Berlin) 30: 709-715.
Eisenhut G. 1959. Beiträge zur Kenntnis der Blütenbildung und Fruchtentwicklung in der Gattung Tilia. – Flora 147: 43-75.
Endress PK, Jenny M, Fallen ME. 1983. Convergent elaboration of apocarpous gynoecia in higher advanced dicotyledons (Sapindales, Malvales, Gentianales). – Nord. J. Bot. 3: 293-300.
Erickson BJ, Young AM, Strand MA, Erickson EH Jr. 1987. Pollination biology of Theobroma and Herrania (Sterculiaceae) II. Analysis of floral oils. – Insect Sci. Appl. 8: 301-310.
Escobar García P, Schönswetter P, Fuertes Aguilar J, Nieto Feliner G, Schneeweiss GM. 2009. Five molecular markers reveal extensive morphological homoplasy and reticulate evolution in the Malva alliance (Malvaceae). – Mol. Phylogen. Evol. 50: 226-239.
Escobar García P, Pakravan M, Schönswetter P, Fuertes Aguilar J, Schneeweiss GM. 2012. Phylogenetic relationships in the species-rich Irano-Turanian genus Alcea (Malvaceae). – Taxon 61: 324-332.
Esser H-J, Taylor SE. 1983. Pro-inflammatory, tumour-promoting and anti-tumour diterpenes of the families Euphorbiaceae and Thymelaeaceae. – Prog. Chem. Org. Nat. Prod. 44: 1-99.
Esteves GL. 2000. Taxonomic characters of the staminal tube and epicalyx in Brazilian Pavonia (Malvaceae). – Brittonia 52: 256-264.
Esteves GL, Krapovickas A. 2002. New species of Abutilon (Malvaceae) from São Paulo State, Brazil. – Kew Bull. 57: 479-482.
Eurlings MCM, Gravendeel B. 2005. trnL-trnF sequence data imply paraphyly of Aquilaria and Gyrinops (Thymelaeaceae) and provide new perspectives for agarwood identification. – Plant Syst. Evol. 254: 1-12.
Evans FJ, Taylor SE. 1983. Pro-inflammatory, tumour-promoting and anti-tumour diterpenes of the families Euphorbiaceae and Thymelaeaceae. – Progr. Chem. Org. Nat. Prod. 44: 1-99.
Exell AW. 1939. Some new species of Dombeya, Grewia and Combretum from tropical Africa. – J. Bot. 77: 172-173.
Exell AW. 1961. 28. Malvaceae. – In: Exell AW, Wild H (eds), Flora Zambesiaca 1 (Part 2), Crown Agents for Oversea Governments and Administrations, London, pp. 420-511.
Eyken PAAF. 1906. Sur l’essence du bois de Gonystylus miquelianus, T. & B. – Rec. Trav. Chim. Pay-Bas (et Belge): 44-47.
Fagerlind F. 1940. Zytologie und Gametophytenbildung in der Gattung Wikstroemia. – Hereditas 26: 23-50.
Fay MF, Bayer C, Alverson WS, Bruijn AY de, Chase MW. 1998. Plastid rbcL sequence data indicate a close affinity between Diegodendron and Bixa. – Taxon 47: 43-50.
Fedalto LC. 1983. Estudo anatomico do lenho de Bixa arborea Huber. – Acta Amazonica 12: 389-399.
Fehrenbach S, Barthlott W. 1988. Mikromorphologie der Epicuticular-Wachse der Rosales s.l. und deren systematische Gliederung. – Bot. Jahrb. Syst. 109: 407-428.
Fernald ML. 1917. Helianthemum dumosum on the mainland of New England. – Rhodora 19: 58-60.
Fernald ML. 1941. Another century of additions to the flora of Virginia. Crocanthemum; has it really stable generic characters? – Rhodora 43: 609-616.
Fernández A. 1974. Recuentos cromosómicos en Malváceas. – Bol. Soc. Argent. Bot. 15: 403-410.
Fernández A. 1981. Recuentos cromosómicos en Malvales. – Bonplandia 5: 63-71.
Fernández A, Krapovickas A, Lavia G, Seijo G. 2003. Cromosomas de Malváceas. – Bonplandia 12: 141-145.
Fernández-Alonso JL. 1998. Novedades taxonómicas, corológicas y nomenclaturales en el género Pachira Aubl. (Bombacaceae). – An. Jard. Bot. Madrid 52: 305-314.
Fernández-Alonso JL. 2001. Bombacaceae neotropicae novae vel minus cognitae V. Novedades en Pseudobombax Dugand y synopsis de las especies Colombianas. – Rev. Acad. Colomb. Ci. Exact. 25: 467-476.
Fernández-Alonso JL. 2001. Bombacaceae neotropicae novae vel minus cognitae VI. Novedades en los géneros Cavanillesia, Eriotheca, Matisia y Pachira. – Rev. Acad. Colomb. Ci. Exact. 27: 25-37.
Fischer EA. 1997. The role of plumes in Eriotheca pentaphylla (Bombacaceae) seed survival in south-eastern Brazil. – J. Trop. Ecol. 13: 133-138.
Fleming TH, Williams CF, Bonaccorso FJ, Herbst LH. 1985. Phenology, seed dispersal and colonization in Muntingia calabura, a neotropical pioneer tree. – Amer. J. Bot. 72: 383-391.
Forsaith CC. 1915. Some features in the anatomy of the Malvales. – Amer. J. Bot. 2: 238-246.
Forstmeier L, Weberling F, Weber HC. 1983. Zum Parasitismus von Cytinus hypocistis L. (Rafflesiaceae). – Beitr. Biol. Pflanzen 58: 299-311.
Foster CSP, Cantrill DJ, James EA, Syme AE, Jordan R, Douglas R, Ho SYW, Henwood MJ. 2016. Molecular phylogenetics provides new insights into the systematics of Pimelea and Thecanthes (Thymelaeaceae). – Aust. Syst. Bot. 29: 185-196.
Foster CSP, Henwood MJ, Ho SYW. 2018. Plastome sequences and exploration of tree-space help to resolve the phylogeny of riceflowers (Thymelaeaceae: Pimelea). – Mol. Phylogen. Evol. 127: 156-167.
Freeman PW, Murphy ST, Nemorin JE, Taylor WC. 1981. The constituents of Australian Pimelea species II. The isolation of unusual flavones from P. simplex and P. decora. – Aust J. Chem. 34: 1779-1784.
Freytag GF. 1951. A revision of the genus Guazuma. – Ceiba 1: 193-225.
Friedel J. 1933. Sur l’anatomie de l’Oceanopapaver neo-caledonicum Guillaumin: importance de cette espèce au point de vue systématique. – Bull. Soc. Bot. France 80: 33-35.
Friedrich-Holzhammer M. 1968. Neuradaceae. – In: Merxmüller H (ed), Prodromus einer Flora von Südwestafrika 56, Cramer, Lehre.
Fries RE. 1908. Entwurf einer Monographie der Gattungen Wissadula und Pseudabutilon. – Kungl. Sv. Vetensk.-Akad. Handl. 43(4): 1-114.
Fries RE. 1914. Die Gattung Marquesia und ihre systematische Stellung. – Engl. Bot. Jahrb. Syst. 51: 349.
Fries RE. 1947. Zur Kenntnis der Süd- und Zentralamerikanischen Malvaceenflora. – Kungl Sv. Vetensk.-Akad. Handl., Ser. III, 24: 1-37.
Fryxell PA. 1968a. A redefinition of the tribe Gossypieae. – Bot. Gaz. 129: 296-308.
Fryxell PA. 1968b. Taxonomic notes on Australian Malvaceae. – Proc. Linn. Soc. New South Wales 92: 262-265.
Fryxell PA. 1974a. New species of Gossypium, Decaschistia, and Macrostelia (Malvaceae) from Australia. – Aust. J. Bot. 22: 183-193.
Fryxell PA. 1974b. Cienfuegosia Cav. extended to Madagascar. – Ann. Missouri Bot. Gard. 61: 491-493.
Fryxell PA. 1975. Generic relationships of Decaschistia (Malvaceae) and the description of a new tribe, Decaschistieae. – Amer. J. Bot. 62: 172-175.
Fryxell PA. 1976a. New species and new combinations in Briquetia and Hochreutinera, and a discussion of the Briquetia generic alliance (Malvaceae). – Brittonia 28: 318-325.
Fryxell PA. 1976b. Mexican species of Abutilon sect. Armata, including descriptions of three new species. – Madroño 23: 320-334.
Fryxell PA. 1979a. The natural history of the cotton tribe. – Texas A & M University Press, College Station, Texas.
Fryxell PA. 1979b. Taxonomic notes on Chiapas Malvaceae. – Syst. Bot. 4: 253-256.
Fryxell PA. 1980. A new species of Hampea (Malvaceae) from El Salvador. – Syst. Bot. 5: 442-444.
Fryxell PA. 1983. A revision of Abutilon sect. Oligocarpae (Malvaceae) including a new species from Mexico. – Madroño 30: 84-92.
Fryxell PA. 1984. Four new species of Malvaceae from Mexico. – Syst. Bot. 9: 415-422.
Fryxell PA. 1985a. Sidus Sidarum V. The North and Central American species of Sida. – Sida 11: 62-91.
Fryxell PA. 1985b. Additional novelties in Mexican Malvaceae. – Syst. Bot. 10: 268-281.
Fryxell PA. 1987. Three new species (from Australia and Venezuela) and three new names (of Mexican plants) in the Malvaceae. – Syst. Bot. 274-280.
Fryxell PA. 1988. Malvaceae of Mexico. – Syst. Bot. Monogr. 25: 1-522.
Fryxell PA. 1992a. A revised taxonomic interpretation of Gossypium L. (Malvaceae). – Rheedea 2: 108-165.
Fryxell PA. 1992b. 118. Malvaceae. – In: Harling G, Andersson L (eds), Flora of Ecuador 44, Nord. J. Bot., Copenhagen, pp. 1-141.
Fryxell PA. 1997a. The American genera of Malvaceae II. – Brittonia 49: 204-269.
Fryxell PA. 1997b. A revision and redefinition of Pseudabutilon (Malvaceae). – Contr. Univ. Michigan Herb. 21: 175-195.
Fryxell PA. 2002. An Abutilon nomenclator (Malvaceae). – Lundellia 5: 79-118.
Fryxell PA, Clase GT. 2007. Akrosida floribunda (malvaceae), a new arborescent mallow from the Dominican Republic. – Brittonia 59: 385-388.
Fryxell PA, Craven LA. 1989. Urena (Malvaceae) in Australia. – Aust. Syst. Bot. 2: 455-460.
Fryxell PA, Fuertes J. 1992. A re-evaluation of the Abutilothamnus complex (Malvaceae) I. Two new species and two new genera, Sidasodes and Akrosida. – Brittonia 44: 436-447.
Fryxell PA, Olivera MDG. 2001. New Mexican species of Byttneria (Sterculiaceae), Bakeridesia (Malvaceae), and Triumfetta (Tiliaceae). – Brittonia 53: 59-65.
Fryxell PA, Stelly DM. 1993. Additional chromosome counts in the Malvaceae. – Sida 15: 639-647.
Fryxell PA, Craven LA, Stewart J McD. 1992. A revision of Gossypium Sect. Grandicalyx (Malvaceae), including the description of six new species. – Syst. Bot. 17: 91-114.
Fryxell PA, Guadarram Olivera M de los Angeles. 2001. New Mexican species of Byttneria (Sterculiaceae), Bakeridesia (Malvaceae), and Triumfetta (Tiliaceae). – Brittonia 53: 59-65.
Fuchs A. 1938. Beiträge zur Embryologie der Thymelaeaceae. – Österr. Bot. Zeitschr. 87: 1-41.
Fuchs HP. 1967. Pollen morphology of the family Bombacaceae. – Rev. Palaeobot. Palynol. 3: 119-132.
Fuertes-Aguilar J, Ray MF, Francisco-Ortega J, Santos-Guerra A, Jansen RK. 2002. Molecular evidence from chloroplast and nuclear markers for multiple colonizations of Lavatera (Malvaceae) in the Canary Islands. – Syst. Bot. 27: 74-83.
Gaafar K. 2001. The stipules of Dicraspidia donnell-smithii (Muntingiaceae): a surprise. – Abstr. 15th Intern. Symp. Biodiversität Evolutionsbiologie, Bochum.
Gadrinab LU, Belin M. 1981. Biology of the green spots in leaves of some dipterocarps. – Malaysian For. 44: 203-266.
Galati BG, Monacci F, Gotelli MM, Rosenfeldt S. 2007. Pollen, tapetum and orbicule development in Modiolastrum malvifolium (Malvaceae). – Ann. Bot. 99: 755-763.
Galicia-Herbada D. 2006. Origin and diversification of Thymelaea (Thymelaeaceae): inferences from a phylogenetic study based on ITS (rDNA) sequences. – Plant Syst. Evol. 257: 159-187.
Gallego MJ, Aparicio A. 1993. Karyological studies in the genus Tuberaria Sect. Scorpioides (Cistaceae): taxonomic and evolutionary inferences. – Plant Syst. Evol. 184: 11-25.
Gandoger M. 1924. Le genre Sida (Malvacées). – Bull. Soc. Bot. France 71: 627-633.
Garcia PE, Schönswetter P, Aguilar JF, Feliner GN, Schneeweiss GM. 2009. Five molecular markers reveal extensive morphological homoplasy and reticulate evolution in the Malva alliance (Malvaceae). – Mol. Phylogen. Evol. 50: 226-239.
García-Barriga H. 1952. Contribución al studio de las Bombacaceae de Colombia. – Mutisia 2: 1-5.
García-Franco JG. 1996. Distribution and host specificity in the holoparasite Bdallophyton bambusarum (Rafflesiaceae) in a tropical deciduous forest in Veracruz, Mexico. – Biotropica 28: 759-762.
García-Franco JG, Rico-Gray V. 1997a. Reproductive biology of the holoparasitic endophyte Bdallophyton bambusarum (Rafflesiaceae). – Bot. J. Linn. Soc. 123: 237-247.
García-Franco JG, Rico-Gray V. 1997b. Dispersión, viabilidad, germinación y banco de semillas de Bdallophyton bambusarum (Rafflesiaceae) en la costa de Veracruz, México. – Rev. Biol. Trop. 44-45: 87-94.
García-Franco J, Sousa V, Eguiarte L, Rico-Gray V. 1998. Genetic variation, genetic structure and effective population size in the tropical holoparasitic endophyte Bdallophyton bambusarum (Rafflesiaceae). – Plant Syst. Evol. 210: 271-288.
García-Franco JG, López-Portillo J, Ángeles G. 2006. The holoparasitic endophyte Bdallophyton americanum affects root water conductivity of the tree Bursera simaruba. – Trees – Structure and Function 21: 215-220.
Garwood NC. 1994. Morphology and ecology of seedlings, fruits andseeds of Panama: Bixaceae and Cochlospermaceae. – Bull. Brit. Mus. (Nat. Hist.) Bot. 24: 161-171.
Gasson P. 1996. Wood anatomy of the Elaeocarpaceae. – In: Donaldson LA (ed), Recent advances in wood anatomy, New Zealand Forestry Research Institute, pp. 44-71.
Gaume R. 1912. Germination, développement et structure anatomique de quelques Cistinées. – Rev. Gén. Bot. 24: 273-295.
Gaydou EM, Ramanoelina ARP. 1983. A survey of the Sarcolaenaceae for cyclopropene fatty acids. – Phytochemistry 22: 1725-1728.
Gazet du Chatelier G. 1940a. Recherches sur les Sterculiacées. – Rev. Gén. Bot. 52: 174-191, 211-233, 257-284, 305-332.
Gazet du Chatelier G. 1940b. La structure florale des Sterculiacées. – Compt. Rend. Acad. Sci. Paris 210: 57-59.
George AS. 1982a. Bixaceae. – In: George AS (ed), Flora of Australia 8, Australian Government Publ. Service, Canberra, pp. 84-88.
Gérard F. 1915. Contribution à l’étude des genres Sarcochlaena et Xerochlamys, Chlaenacées de Madagascar. – Compt. Rend. Assoc. Fr. Avancem. Sci., session 43: 404-410.
Gérard F. 1919. Étude systématique, morphologique et anatomique des Chlaenacées. – Thèse, Fac. Sci. Paris; Ann. Mus. Col. Marseille III, 7.1: 1-135.
Giannasi DE, Niklas KJ. 1977. – In: Maguire B, Ashton PS (eds), Pakaraimoideae, Dipterocarpaceae of the western hemisphere II. Systematic and phyletic consideration. – Taxon 26: 380-385.
Gibbs PE, Alverson WS. 2006. How many species of Spirotheca (Malvaceae s.l., Bombacoideae)? – Brittonia 58: 245-258.
Gibbs PE, Bianchi M. 1993. Post-pollination events of Chorisia (Bombacaceae) and Tabebuia (Bignoniaceae) with late-acting self-incompatibility. – Bot. Acta 106: 64-71.
Gibbs PE, Semir J. 2003. A taxonomic revision of the genus Ceiba Mill. (Bombacaceae). – An. Jard. Bot. Madrid 60: 259-300.
Gibbs PE, Semir J, da Cruz ND. 1988. A proposal to unite the genera Chorisia Kunth and Ceiba Miller (Bombacaceae). – Notes Roy. Bot. Gard. Edinb. 45: 125-136.
Gilg E. 1894a. Thymelaeaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(6a), W. Engelmann, Leipzig, pp. 216-245.
Gilg E. 1894b. Studien über die Verwandtschaftsverhältnisse der Thymelaeales und über die anatomische Methode. – Engl. Bot. Jahrb. Syst. 18: 489-574.
Gilg E. 1897. Gonystylaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien, Nachträge zu III(6), W. Engelmann, Leipzig, pp. 231-232.
Gilg E. 1901a. Über der systematische Stellung der Gattung Monotes und deren Arten. – Engl. Bot. Jahrb. Syst. 28: 127.
Gilg E. 1901b. Über die Gattung Octolepis und ihre Zugehörigkeit zu den Thymelaeaceae. – Engl. Bot. Jahrb. Syst. 28: 139-147.
Gilg E. 1915. Eine neue interessante Gattung der Thymelaeaceae aus dem tropischen Afrika. – Engl. Bot. Jahrb. Syst. 53: 362-365.
Gilg E. 1921. Über die Phylogenese der Thymelaeaceae. – Ber. Freien Verein. Pflanzengeogr. 1919: 60-68.
Gilg E. 1925. Dipterocarpaceae. – In: Engler A, Gilg E (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 21, W. Engelmann, Leipzig, pp. 237-269.
Gillett J. 1954. The genus Helianthemum in Somaliland. – Kew Bull. 1954: 493-494.
Goldberg A. 1967. The genus Melochia L. (Sterculiaceae). – Contr. U.S. Natl. Herb. 34: 191-363.
Goldblatt P, Dorr LJ. 1986. Chromosome number in Sarcolaenaceae. – Ann. Missouri Bot. Gard. 73: 828-829.
Gomez LD. 1983. Rafflesiaceae. – In: Burger W (ed), Flora Costaricensis, Fieldiana Bot. II, 13: 89-93.
Gore UR. 1935. Morphogenetic studies on the inflorescence of cotton. – Bot. Gaz. 97: 118-138.
Gottsberger G. 1967. Blütenbiologische Beobachtungen an brasilianischen Malvaceen. – Österr. Bot. Zeitschr. 114: 349-378.
Gottsberger G. 1972. Blütenmorphologische Beobachtungen an brasilianischen Malvaceen II. – Österr. Bot. Zeitschr. 120: 439-509.
Gottwald H, Parameswaran N. 1966. Das sekundäre Xylem der Familie Dipterocarpaceae. Anatomische Untersuchungen zur Taxonomie und Phylogenie. – Bot. Jahrb. Syst. 85: 410-508.
Graham A. 1979. Mortoniodendron (Tiliaceae) and Sphaeropteris/Trichipteris (Cyatheaceae) in cenozoic deposits of the Gulf-Caribbean region. – Ann. Missouri Bot. Gard. 66: 572-576.
Greene EL. 1906. Certain malvaceous types. – Leafl. Bot. Observ. Crit. 1: 205-209.
Gribel R. 1988. Visits of Caluromys lanatus (Didelphidae) to flowers of Pseudobombax tomentosum (Bombacaceae): a problem case of pollination by marsupials in Central Brazil. – Biotropica 20: 344-347.
Grover CE, Grupp K, Wanzek R, Wendel J. 2012. Assessing the monophyly of polyploidy Gossypium species. – Plant Syst. Evol. 298: 1177-1183.
Grover CE, Gallagher JP, Jareczek JJ, Page JT, Udall JA, Gore MA, Wendel JF. 2015. Re-evaluating the phylogeny of allopolyploid Gossypium L. – Mol. Phylogen. Evol. 92: 45-52.
Güemes J. 1999. A new species of Fumana (Cistaceae) from Rif, Morocco. – Folia Geobot. Phytotaxon. 34: 363-372.
Guérin P. 1916. Reliquiae treubianae I. Recherches sur la structure anatomique de l’ovule et de la graine des Thyméléacées. – Ann. Jard. Bot. Buitenzorg, sér. 2, 14: 3-35.
Guillaumin A. 1932. Matériaux pour la flore de la Nouvelle-Calédonie XXVIII. Papavéracées. – Bull. Soc. Bot. France 79: 225-226.
Gülz PG, Müller E, Moog B. 1988. Epicuticular leaf waxes of Tilia tomentosa Moench and Tilia x europaea L., Tiliaceae. – Zeitschr. Naturforsch. 43: 516-526.
Günther K-F. 1986. Amoreuxia wrightii A. Gray (Cochlospermaceae) neu für Cuba. – Feddes Repert. 97: 73-78.
Guymer GP. 1988. A taxonomic revision of Brachychiton (Sterculiaceae). – Aust. Syst. Bot. 1: 199-323.
Guymer GP. 2005. New species of Commersonia J. R. Forst. & G. Forst. (Sterculiaceae) from Eastern Australia and Vanuatu. – Austrobaileya 7: 231-250.
Guymer GP. 2006. New species of Commersonia J. R. Forst. & G. Forst. (Sterculiaceae) from Queensland. – Austrobaileya 7: 365-372.
Guzmán B, Vargas P. 2005. Systematics, character evolution, and biogeography of Cistus L. (Cistaceae) based on ITS, trnL-trnF, and matK sequences. – Mol. Phylogen. Evol. 37: 644-660.
Guzmán B, Vargas P. 2009. Historical biogeography and character evolution of Cistaceae (Malvales) based on analysis of plastid rbcL and trnL-trnF sequences. – Organisms Divers. Evol. 9: 83-99.
Guzmán B, Lledo MD, Vargas P. 2009. Adaptive radiation in Mediterranean Cistus (Cistaceae). – PLoS ONE 4: e6362.
Guzowska I. 1964. Reinvestigation of embryo sac development, fertilization, and early embryogeny in Cytinus hypocistis. – Acta Soc. Bot. Polon. 33: 157-166.
Guzowska I. 1966. Microsporogenesis and chromosome number in Cytinus hypocistis L. – Acta Soc. Bot. Polon. 35: 445-454.
Gwynne-Evans DC, Hedderson TAJ. 2007. Preliminary cladistics of the genus Hermannia and intriguing morphological adaptations. – South Afr. J. Bot. 73: 290.
Haber WA, Frankie, GW. 1982. Pollination of Luehea (Tiliaceae) in Costa Rican deciduous forest. – Ecology 63: 1740-1750.
Haevermans T. 1999. Phylogénie des genres de Sarcolaenaceae, une famille endémique de Madagascar. – Mémoire de DEA, Muséum National d’Histoire Naturelle, Paris.
Halda JJ. 2001. The genus Daphne. – Sen, Dobré.
Hall JW, Swain AM. 1971. Pedunculate bracts of Tilia from the Tertiary of western United States. – Bull. Torrey Bot. Club 98: 95-100.
Hallé F, Ng FSP. 1981. Crown construction in mature dipterocarp trees. – Mal. For. 44: 222.
Hallier H. 1922. Beiträge zur Kenntnis der Thymelaeaceen und ihrer natürlichen Umgrenzung. – Meded. Rijks-Herb. 44: 1-31.
Hamaya T. 1955. A dendrological monograph of the Thymelaeaceae of Japan. – Bull. Tokyo Univ. For. 50: 45-96.
Hansen B, Straka H. 1978. Palynologia Madagassica et Mascarenica, Fam. 61-64. – Pollen Spores 20: 157-166.
Harborne JB. 1975. Flavonoid bisulphates and their co-occurrences with ellagic acid in the Bixaceae, Frankeniaceae, and related families. – Phytochemistry 14: 1331-1337.
Harms H. 1935. Rafflesiaceae. – In: Engler A (†), Harms H (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 16b, W. Engelmann, Leipzig, pp. 243-281.
Hassan NM. 2011. Pollen morphology of the family Cistaceae in Egypt and its systematic significance. – J. Syst. Evol. 49: 362-371.
Hayek A von. 1912. Über die Blütenbiologie von Cytinus hypocistis L. – Österr. Bot. Zeitschr. 62: 238-240.
Heads MJ. 1990. A revision of the genera Kelleria and Drapetes (Thymelaeaceae). – Aust. Syst. Bot. 3: 595-652.
Heads MJ. 1994. Biogeography and biodiversity in New Zealand Pimelea (Thymelaeaceae). – Candollea 49: 37-53.
Heckenhauer J, Samuel R, Ashton PS, Turner B, Barfuss MHJ, Jang T-S, Temsch EM, McCann J, Abu Salim K, Attanayake AMAS, Chase MW. 2017. Phylogenetic analyses of plastid DNA suggest a different interpretation of morphological evolution than those used as the basis for previous classifications of Dipterocarpaceae (Malvales). – Bot. J. Linn. Soc. 185: 1-26.
Heckenhauer J, Samuel R, Ashton PS, Salim KA, Paun O. 2018. Phylogenomics resolves evolutionary relationships and provides insights into floral evolution in the tribe Shoreeae (Dipterocarpaceae). – Mol. Phylogen. Evol. 127: 1-13.
Hecker E. 1977. New toxic, irritant and cocarcinogenic diterpene esters from Euphorbiaceae and Thymelaeaceae. – Pure Appl. Chem. 49: 1423-1431.
Heel WA van. 1966. Morphology of the androecium in Malvales. – Blumea 13: 177-394.
Heel WA van. 1978. Morphology of the pistil in Malvaceae-Ureneae. – Blumea 24: 123-127.
Heel WA van. 1995. Morphology of the gynoecium of Kitaibelia vitifolia Willd. and Malope trifida L. (Malvaceae-Malopeae). – Bot. Jahrb. Syst. 117: 485-493.
Heim F. 1892. Recherches sur les Dipterocarpacées. – Ph.D. diss., La Faculté des Sciences, Université de Paris, Paris, France.
Heinig KH. 1951. Studies in the floral morphology of the Thymelaeaceae. – Amer. J. Bot. 38: 113-132.
Heinricher E. 1917. Zur Kenntnis der Blüte von Cytinus hypocistis. – Ber. Deutsch. Bot. Ges. 35: 513-517.
Heinricher E. 1934. Zur Frage der Artbildung bei Cytinus hypocistis nebst anderen Bemerkungen. – Ber. Deutsch. Bot. Ges. 52: 48-53.
Hemsley WB. 1882. Cytinaceae. – In: Godman FD, Salvin O (eds), Biologia Centrali Americana. Botany III, R. H. Porter Ltd, London, pp. 40-41.
Herber BE. 2002a. Pollen morphology of Thymelaeaceae in relation to its taxonomy. – Plant Syst. Evol. 232: 107-121.
Herber BE. 2002b. Thymelaeaceae. – In: Kubitzki K, Bayer C (eds), The families and genera of vascular plants V. Flowering plants. Dicotyledons. Malvales, Capparales and non-betalain Caryophyllales, Springer, Berlin, Heidelberg, New York, pp. 373-396.
Herrera J. 1992. Flower variation and breeding system in the Cistaceae. – Plant Syst. Evol. 179: 245-255.
Hesse M. 1978. Entwicklungsgeschichte und Ultrastruktur des Pollenkitts bei Tilia (Tiliaceae). – Plant Syst. Evol. 129: 13-30.
Heydacker F. 1963. Les types polliniques dans la famille des Cistacées. – Pollen Spores 5: 41-49.
Hislop M, Thiele KR, Brassington D. 2013.
Cochlospermum macnamarae (Bixaceae), a rare, new endemic from the
Pilbara bioregion of Western Australia. – Nuytsia 23: 89-94.
Hitzemann C. 1886. Beiträge zur vergleichende Anatomie der Terstroemiaceen, Dilleniaceen, Dipterocarpaceen und Chlaenaceen. – von Giebel & Oehlschlägel, Osterode.
Hochreutiner BPG. 1913. Bakeridesia, un nouveau genre de Malvacées. – Ann. Cons. Jard. Bot. Genève 15-16: 297-303.
Hochreutiner BPG. 1914. Notes sur les Tiliacées avec descriptions d’espèces, de sections et de sous-familles nouvelles ou peu connues. – Ann. Cons. Jard. Bot. Genève 18: 68-128.
Hochreutiner BPG. 1915. Malvacées de Madagascar: Perrierophytum, Perrieranthus et Megistostegium. – Ann. Cons. Jard. Bot. Genève 18: 215-237.
Hochreutiner BPG. 1920a. Organes carpiques nouveaux ou méconnus chez les Malvacées. – Ann. Cons. Jard. Bot. Genève 21: 347-387.
Hochreutiner BPG. 1920b. Notes sur les genres Cristaria, Bakeridesia, Malvastrum. – Ann. Cons. Jard. Bot. Genève 21: 405-428.
Hochreutiner BPG. 1924. Genres nouveaux et genres discutés de la famille des Malvacées; Jumelleanthus. – Candollea 2: 79-83.
Hochreutiner BPG. 1925a. Réforme et extension du genre Perrierophytum Hochr. – Candollea 2: 145-154.
Hochreutiner BPG. 1925b. Encore un genre nouveau de Malvacées de Madagascar. – Candollea 2: 155-158.
Hochreutiner BPG. 1926. Humbertiella, un genre nouveau de Malvacées de Madagascar. – Candollea 3: 1-4.
Hochreutiner BPG. 1932. Extension et affinités du genre Humbertiella Hochr. – Candollea 5: 1-4.
Hochreutiner BPG. 1937. La valeur relative de groupes systematique. – Boissiera 2: 1-7.
Hochreutiner BPG. 1940. Neohumbertiella, nouveau genre de Malvacées. – Candollea 8: 27-34.
Hochreutiner BPG. 1952. Macrostelia, un nouveau genre extraordinaire de Malvacées de Madagascar. – Not. Syst. (Paris) 14: 229-234.
Hochreutiner BPG. 1955. Malvacées. – In: Humbert H (ed), Flore de Madagascar et des Comores 129, Muéum National d’Histoire Naturelle, Paris, pp. 1-170.
Hong-Wa C. 2008. Multivariate analyses of morphological characters of Leptolaena Thouars s.l. subgenera Mediusella and Xerochlamys (Sarcolaenaceae). – Bot. J. Linn. Soc. 157: 559-574.
Hong-Wa C. 2009. Endemic families of Madagascar XII. Resurrection and taxonomic revision of the genera Mediusella (Cavaco) Hutchinson and Xerochlamys Baker (Sarcolaenaceae). – Adansonia, sér. III, 31: 311-339.
Horn JW. 2004. The morphology and relationships of Sphaerosepalaceae (Malvales). – Bot. J. Linn. Soc. 144: 1-40.
Hosamani KN. 1994. Ricinoleic and cyclopropen acids in Trichodesma zeylanicum seed oils. – Phytochemistry 37: 1621-1624.
Huard J. 1965a. Anatomie des Rhopalocarpacées. – Adansonia, sér. II, 5: 103-123.
Huard J. 1965b. Palynologia Madagassica et Mascarenica: Fam. 127. Rhopalocarpaceae. – Pollen Spores 7: 303-312.
Huard J. 1965c. Remarques sur la position systématique des Rhopalocarpacées d’après leur anatomie et leur morphologie pollinique. – Bull. Soc. Bot. France 112: 252-254.
Huber H. 1993. Neurada, eine Gattung der Malvales. – Sendtnera 1: 7-10.
Huertas ML, Schneider JV, Zizka G. 2007. Phylogenetic analysis of Palaua (Malveae, Malvaceae) based on plastid and nuclear sequences. – Syst. Bot. 32: 157-165.
Humeau L, Pailler T, Thompson JD. 1999a. Cryptic dioecy and leaky dioecy in endemic species of Dombeya (Sterculiaceae) on La Réunion. – Amer. J. Bot. 86: 1437-1447.
Humeau L, Pailler T, Thompson JD. 1999b. Variation in the breeding system of two sympatric Dombeya species on La Réunion island. – Plant Syst. Evol. 218: 77-87.
Hutchinson JB. 1947. Notes on the classification and distribution of genera related to Gossypium. – New Phytol. 46: 123-141.
Iconomidis J. 1958. Les principaux stades du développement proembryonnaire chez le Cistus incanus Rehb. (Cistus villosus var. incanus Freyn.), Cistacées. – Bull. Soc. Bot. France 105: 128-131.
Inamdar JA, Chohan AJ. 1969. Epidermal structure and stomatal development in some Malvaceae and Bombacaceae. – Ann. Bot., N. S., 33: 865-878.
Inamdar JA, Balakrishna Bhat R, Ramana Rao TV. 1983. Structure, ontogeny, classification, and taxonomic significance of trichomes in Malvales. – Korean J. Bot. 26: 151-160.
Ingram JS, Francis BJ. 1969. The annatto tree (Bixa orellana L.) – a guide to its occurrence, cultivation, preparation and uses. – Trop. Sci. 9: 97-102.
Jabeen F, Prabhakar M, Leelavathi P. 1995. Crystalliferous cells in leaf epidermis of Malvales in relation to taxonomy. – Geophytology 24: 213-217.
Jain A, Bhartiya HP, Vishwakarma AN. 1982. A chalcone glycoside from the heartwood of Shorea robusta. – Phytochemistry 21: 957.
Jain TC, Bhattacharyya SC. 1959. Structure, stereochemistry and absolute configuration of agarol, a new sesquiterpene alcohol from agarwood oil. – Tetrahedron Lett. 9: 13-17.
Jain TC, Maheswari ML, Bhattacharyya SC. 1962. The composition of oil from uninfected agarwood. – Parfumery Essential Oil Rec. 53: 294-298.
Janchen E. 1907. Helianthemum canum (L.) Baumg. und seine nächsten Verwandten. – Abhandl. Zool.-Bot. Ges. Wien 4(1).
Janchen E. 1909. Die Cistaceen Östterreich-Ungarns. – Mitt. Naturwiss. Ver. Univ. Wien 7: 1-124.
Janchen E. 1925. Cistaceae. – In: Engler A, Gilg E (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 21, W. Engelmann, Leipzig, pp. 289-313.
Janda C. 1935. Die extranuptialen Nektarien der Malvaceen. – Österr. Bot. Zeitschr. 86: 81-130.
Janka H. 2003. Floral structure of Malvaceae-Malvoideae. – Palm. Hortus Francofurtensis 7: 135.
Janka H, Balthazar M, Alverson WS, Baum DA, Semir J, Bayer C. 2008. Structure, development and evolution of the androecium in Adansonieae (core Bombacoideae, Malvaceae s.l.). – Plant Syst. Evol. 275: 69-91.
Jansen S, Baas P, Smets E. 2000. Vestured pits in Malvales s.l.: a character with taxonomic significance hidden in the secondary xylem. – Taxon 49: 169-182.
Janzen DH. 1974. Tropical blackwater rivers, animals and mast fruiting by the Dipterocarpaceae. – Biotropica 6: 69-103.
Janzen DH.1982. Natural history of guacimo fruits (Sterculiaceae: Guazuma ulmifolia) with respect to consumption by large mammals. – Amer. J. Bot. 69: 1240-1250.
Jean M-T, Pons A. 1963. Contribution à l’étude palynologique des Cistacées de la flore de France. – Ann. Sci. Nat. Bot., sér. 12, 4: 159-204.
Jenny M. 1985. Struktur, Funktion und systematische Bedeutung des Gynoeciums bei Sterculiaceen. – Ph.D. diss., Universität Zürich, Switzerland.
Jenny M. 1988. Different gynoecium types in Sterculiaceae: ontogeny and functional aspects. – In: Leins P, Tucker SC, Endress PK (eds), Aspects of floral development, J. Cramer, Berlin, Stuttgart, pp. 225-236.
Jenny M, Bayer C, Dorr LJ. 1999. Aethiocarpa reduced to Harmsia (Malvaceae, Dombeyoideae). – Taxon 48: 3-6.
Jiménez-Reyes N. 2003. Morfología de los granos de polen de la familia Malvaceae de Jalisco, México II: Anoda, Bakeridesia, Bastardia, Bastardiastrum, Briquetia, Gaya y Gossypium. – Scientia-CUCBA 5: 1-30.
Johnson SD, Burgoyne PM, Harder LD, Dötterl S. 2011. Mammal pollinators lured by the scent of a parasitic plant. – Proc. Roy. Soc., Sect. B, 278: 2303-2310.
Johnson-Fulton SB. 2014. Systematics, biogeography, and ethnobotany of the pantropical family Cochlospermaceae (Malvales). – Ph.D. diss., Miami University, Miami, Florida.
Johnson-Fulton SB, Watson LB. 2017. Phylogenetic systematics of Cochlospermaceae (Malvales) based on molecular and morphological evidence. – Syst. Bot. 42: 271-282.
Jong K. 1976. Cytology of the Dipterocarpaceae. – In: Burley J, Styles BT (eds), Tropical trees, variation, breeding and conservation, Academic Press, London, pp. 79-84.
Jong K, Kaur A. 1979. A cytotaxonomic view of Dipterocarpaceae with some comments on polyploidy and apomixes. – In: Maury-Lechon G (ed), Diptérocarpacées: taxonomie-phylogénie-écologie, Mem. Mus. Natl. Hist. Nat., sér. B, Botanique 26, Éditions du Muséum, Paris, pp. 41-49.
Jong K, Lethbridge A. 1967. Cytological studies in the Dipterocarpaceae 1. Chromosome numbers of certain Malaysian genera. – Notes Roy. Bot. Gard. Edinb. 27: 175-184.
Joshi AC. 1936. Anatomy of the flowers of Stellera chamaejasme. – J. Indian Bot. Soc. 15: 77-85.
Joshi K. 2003. Leaf flavonoid patterns in Dipterocarpus and Hopea (Dipterocarpaceae). – Bot. J. Linn. Soc. 143: 43-46.
Joshi PC, Wadhwani AM, Johri BM. 1967. Morphological and embryological studies of Gossypium L. – Proc. Indian Natl. Inst. Sci., Sect. B, 33: 37-93.
Judd WS, Manchester SR. 1997. Circumscription of Malvaceae (Malvales) as determined by a preliminary cladistic analysis of morphological, anatomical, palynological, and chemical characters. – Brittonia 49: 384-405.
Kajita T, Kamiya K, Nakamura K, Tachida H, Wickneswari R, Tsumura Y, Yoshimaru H, Yamazaki T. 1998. Molecular phylogeny of Dipterocarpaceae in Southeast Asia based on nucleotide sequences of matK, trnL intron, and trnL-trnF intergenic spacer region in chloroplast DNA. – Mol. Phylogen. Evol. 10: 202-209.
Kania W. 1973. Entwicklungsgeschichtliche Untersuchungen an Rosaceeenblüten. – Bot. Jahrb. Syst. 93: 175-246.
Kamiya K, Harada K, Ogino K, Kayita T, Yamazaki T, Lee HS, Ashton PS. 1998. Molecular phylogeny of dipterocarp species using nucleotide sequences of two non-coding regions in chloroplast DNA. – Tropics 7: 195-207.
Kamiya K, Harada K, Tachida H, Ashton PS. 2005. Phylogeny of pgiC gene in Shorea and its closely related genera (Dipterocarpaceae), the dominant trees in Southeast Asian rain forests. – Amer. J. Bot. 92: 775-788.
Kapil RN, Maheshwari R. 1964. Embryology of Helianthemum vulgare Gaertn. – Phytomorphology 14: 547-557.
Kaur A, Jong K, Sands VE, Soepadmo E. 1986. Cytoembryology of some Malaysian dipterocarps, with some evidence of apomixes. – Bot. J. Linn. Soc. 92: 75-88.
Kaur H. 1969. Embryological investigations on Bixa orellana Linn. – Proc. Indian Natl. Inst. Sci., Sect. B, 35: 487-506.
Kausik SB. 1940. Structure and development of the ovule and embryo sac of Lasiosiphon eriocephalus Decne. – Proc. Indian Natl. Inst. Sci., Sect. B, 6: 117-132.
Kearney TH. 1935. The North American species of Sphaeralcea subgenus Eusphaeralcea. – Univ. Calif. Publ. Bot. 19: 1-128.
Kearney TH. 1949. Malvaceae: a new subtribe and genus and new combinations. – Leafl. West. Bot. 5: 189-191.
Kearney TH. 1951. The American genera of Malvaceae. – Amer. Midl. Natur. 46: 93-131.
Kearney TH. 1952a. Notes on Malvaceae II. – Leafl. West. Bot. 6: 165-172.
Kearney TH. 1952b. Notes on Malvaceae III. Abutilon and Pseudabutilon in the Galapagos Islands. – Madroño 11: 285-289.
Kearney TH. 1955a. Malvastrum A. Gray. A re-definition of the genus. – Leafl. West. Bot. 7: 238-241.
Kearney TH. 1955b. A tentative key to the North American species of Abutilon Miller. – Leafl. West. Bot. 7: 241-254.
Kearney TH. 1958. A tentative key to the South American species of Abutilon Miller. – Leafl. West. Bot. 8: 201-216.
Keating RC. 1968. Comparative morphology of Cochlospermaceae I. Synopsis of family and wood anatomy. – Phytomorphology 18: 379-392.
Keating RC. 1970. Comparative morphology of Cochlospermaceae II. Anatomy of the young vegetative shoot. – Amer. J. Bot. 57: 889-898.
Keating RC. 1972. Comparative morphology of Cochlospermaceae III. The flower and pollen. – Ann. Missouri Bot. Gard. 59: 282-296.
Keating RC. 1975 [1976]. Trends of specialization in pollen of Flacourtiaceae with comparative observations of Cochlospermaceae and Bixaceae. – Grana 15: 29-49.
Keighery GJ. 1975. Parallel evolution of floral structures in Darwinia (Myrtaceae) and Pimelea (Thymelaeaceae). – West. Aust. Natur. 13: 46-50.
Kelman WM. 1991. A revision of Fremontodendron (Sterculiaceae). – Syst. Bot. 16: 3-20.
Kidway P. 1974. Epidermal structure and stomatal development in Bombax ceiba L. (Bombacaceae). – Bot. J. Linn. Soc. 68: 227-234.
Knoll F. 1914. Zur Ökologie und Reizphysiologie des Andrözeums von Cistus salvifolius L. – Jahrb. Wiss. Bot. 54: 498-527.
Koechlin J. 1972. L’appareil floral des Sarcolaenacées et la notion de l’angiocarpie. – Candollea 27: 171-179.
Koernicke M. 1918. Über die extrafloralen Nectarien einiger Hibisceen. – Flora 111: 526-540.
Köhler E. 1973. Über einen bemerkenswerten Pollendimorphismus in der Gattung Waltheria L. – Grana 13: 57-64.
Köhler E. 1976. Pollen dimorphism and heterostyly in the genus Waltheria L. (Sterculiaceae). – In: Ferguson IK, Muller J (eds), The evolutionary significance of the exine, Academic Press, London, pp. 147-161.
Koopman MM, Baum DA. 2008. Phylogeny and biogeography of tribe Hibisceae (Malvaceae) on Madagascar. – Syst. Bot. 33: 364-374.
Kostermans AJGH. 1960a. Miscellaneous botanical notes 1. – Reinwardtia 5: 233-254.
Kostermans AJGH. 1960b. Jarandersonia, a new Bornean genus of Tiliaceae-Brownlowieae. – Reinwardtia 5: 319-331.
Kostermans AJGH. 1961. A monograph of the genus Brownlowia Roxb. (Tiliaceae). – Comm. For. Res. Inst. Bogor 41: 401-430.
Kostermans AJGH. 1964. A monograph of the genus Pentace Hassk. (Tiliaceae). – Comm. For. Res. Inst. Bogor 87: 1-78.
Kostermans AJGH. 1970. Jarandersonia (Tiliaceae). – Reinwardtia 8: 117-130.
Kostermans AJGH. 1972. A synopsis of the Old World species of Trichospermum Bl. (Tiliaceae). – Trans. Bot. Soc. Edinb. 41: 401-430.
Kostermans AJGH. 1978. Pakaraimaea dipterocarpacea Maguire & Ashton belongs to Tiliaceae and not to Dipterocarpaceae. – Taxon 27: 357-359.
Kostermans AJGH. 1981a. The Ceylonese species of Balanocarpus Bedd. (Dipterocarpaceae). – Bull. Mus. Natl. Hist. Nat. Paris, sér. IV, 3, B, Adansonia: 173-177.
Kostermans AJGH. 1981b. Stemonoporus Thw. (Dipterocarpaceae). A monograph 1-2. – Bull. Mus. Natl. Hist. Nat. Paris, sér. IV, 3, sect. B, Adansonia 4: 321-358, 373-405.
Kostermans AJGH. 1985. Family status for the Monotoideae Gilg and the Pakaraimoideae Ashton, Maguire and de Zeeuw (Dipterocarpaceae). – Taxon 34: 426-435.
Kostermans AJGH. 1987. The genera Sunaptea (Griff.) and Cotylelobium (Pierre) (Dipterocarpaceae). – In: Kostermans AJGH (ed), Proceedings of the 3rd Round Table Conference on Dipterocarps, UNESCO, Paris, pp. 603-627.
Kostermans AJGH. 1989. Monotaceae, a new family allied to Tiliaceae. – Taxon 38: 123-124.
Kostermans AJGH. 1992. A handbook of the Dipterocarpaceae of Sri Lanka. – Wildlife Heritage Trust of Sri Lanka, Colombo.
Krapovickas A. 1949. Las espécies de Sphaeralcea de Argentina y Uruguay. – Lilloa 17: 179-222.
Krapovickas A. 1954. Estudio de las espècies de ‘Anurum’, nueva sección del género Urocarpidium Ulbr. (Malvaceae). – Darwiniana 10: 606-636.
Krapovickas A. 1957. Números cromosómicos de Malváceas americanas de la tribu Malveae. – Rev. Agron. Noroeste Argent. 2: 245-260.
Krapovickas A. 1960. Poliploidia y area en el género Tarasa (Malvaceae). – Lilloa 30: 233-249.
Krapovickas A. 1965. Notas sobre Malvaceae III. – Kurtziana 2: 113-126.
Krapovickas A. 1967. Notas citotaxonómicas sobre Malveae. – Kurtziana 4: 29-37.
Krapovickas A. 1969a. Notas citotaxonómicas sobre Malváceas. – Bonplandia 3: 9-24.
Krapovickas A. 1969b. Notas sobre el género Abutilon Mill. (Malvaceae) I. La sección Tetrasida (Ulbr.) Krapov. – Bonplandia 3: 25-47.
Krapovickas A. 1971. Evolución del género Tarasa (Malvaceae). – In: Mejia RH, Moguilevsky JA (eds), Recientes adelantos en biología, Bona, Buenos Aires, pp. 232-241.
Krapovickas A. 2006. Las species Argentinas y de países vecinos de Sida Secc. Nelavaga (Malvaceae, Malveae). – Bonplandia 15: 5-45.
Krapovickas A, Cristóbal CL. 1965. Revisión del género Peltaea (Malvaceae). – Kurtziana 2: 135-216.
Krapovickas A, Fryxell PA, Bates DM. 1988. Allosidastrum, un nuevo género de Malvaceae de los neotrópicos. – Bol. Soc. Bot. México 48: 23-34.
Krebs G. 1990. Zur Monophylie der Tribus Malopeae Reichenb. – Catalogus Herbarii Lipsiensis, Plantae Neotropicae 3, Universität/Botanischer Garten, Leipzig, pp. 45-51.
Krebs G. 1994a. Taxonomische Untersuchungen in der Subtribus Malvinae. – Feddes Repert. 105: 7-18.
Krebs G. 1994b. Taxonomische Untersuchungen an der Subtribus Malvinae II. Dinacrusa. – Feddes Repert. 105: 299-315.
Krishnan N. 1977. Cytotaxonomical studies on Bixaceae and Samydadeae from South India with collected evidences from palynology, anatomy and biochemistry. – Ph.D. diss., Annamalai University, India.
Krutzsch W. 1970a. Reevesiapollis, ein neues Pollengenus der Sterculiaceen aus dem mitteleuropäischen Tertiär. – Feddes Repert. 81: 371-384.
Krutzsch W. 1970b. Einige neue Pollenformen aus den Familien der Tiliaceen, Bombacaceen und Sterculiaceen aus dem mitteleuropäischen Alttertiär. – Jahrb. Geol. Bot. 3: 275-307.
Krutzsch W. 1989. Paleogeography and historical phytogeography (paleochorology) in the Neophyticum. – Plant Syst. Evol. 162: 5-61.
Kubitzki K. 1995. Asterophorum and Tahitia congeneric with Christiana (Tiliaceae). – Bot. Jahrb. Syst. 116: 537-542.
Kubitzki K. 2002. Conspectus of the families of Malvales. – In: Kubitzki K, Bayer C (eds), The families and genera of vascular plants V. Flowering plants. Dicotyledons. Malvales, Capparales and non-betalain Caryophyllales, Springer, Berlin, Heidelberg, New York, pp. 17-18.
Kubitzki K. 2002. Tepuianthaceae. – In: Kubitzki K, Bayer C (eds), The families and genera of vascular plants V. Flowering plants. Dicotyledons. Malvales, Capparales and non-betalain Caryophyllales, Springer, Berlin, Heidelberg, New York, pp. 371-372.
Kubitzki K, Chase MW. 2002. Introduction to Malvales. – In: Kubitzki K, Bayer C (eds), The families and genera of vascular plants V. Flowering plants. Dicotyledons. Malvales, Capparales and non-betalain Caryophyllales, Springer, Berlin, Heidelberg, New York, pp. 12-16.
Kuhlmann JG. 1935. Novas especies botanicas da Hyléa: Hydrogaster Kuhlmann g. nov. – Arch. Inst. Biol. Veg. 2: 86-87.
Kukachka BF. 1962. Wood anatomy of Petenaea cordata Lundell (Elaeocarpaceae). – Wrightia 3: 36-40.
Kukachka BF, Rees LW. 1943. Systematic anatomy of the woods of the Tiliaceae. – Bull. Univ. Minnesota Agric. Exp. Stn. Techn. 158: 1-70.
Kuntze G. 1891. Beiträge zur vergleichenden Anatomie der Malvaceen 1-6. – Bot. Centralbl. 45: 161-168, 197-202, 229-234, 261-268, 293-299, 325-329.
Kvaček Z, Buzek C, Manchester SR. 1991. Fossil fruits of Pteleaecarpum Weyland – tiliaceous, not sapindaceous. – Bot Gaz. 152: 522-523.
LaDuke JC, Doebley J. 1995. A chloroplast DNA based phylogeny of the Malvaceae. – Syst. Bot. 20: 259-271.
Leandri J. 1930. Recherches anatomiques sur les Thyméléacées. – Ann. Sci. Nat. Bot., sér. 10, 12: 125-237.
Lee SS. 1998. Root symbiosis and nutrition. – In: Appanah S, Turnbull JM (eds), A review of dipterocarps: taxonomy, ecology and silviculture, CIFOR, Bogor, Indonesia, pp. 99-114.
Leinfellner W. 1960. Zur Entwicklungsgeschichte der Kronblätter der Sterculiaceae-Buettnerieae. – Österr. Bot. Zeitschr. 107: 153-176.
Leitão CAE, Meira RMSA, Azevedo AA, Araújo JM de, Silva KLF, Collevatti RG. 2005. Anatomy of the floral, bract, and foliar nectaries of Triumfetta semitriloba (Tiliaceae). – Can. J. Bot. 83: 279-286.
Leitão MT, Alves MC. 1976. Contribuição para o conhecimento citotaxonomico das Spermatophyta de Portugal 14. Cistaceae. – Bol. Soc. Brot. 50: 247-263.
Le Péchon TL, Cao N, Dubuisson J-Y, Gigord LDB. 2009. Systematics of Dombeyoideae (Malvaceae) in the Mascarene archipelago (Indian Ocean) inferred from morphology. – Taxon 58: 519-531.
Le Péchon TL, Dubuisson J-Y, Haevermans T, Cruaud C, Couloux A, Gigord LDB. 2010. Multiple colonizations from Madagascar and converged acquisition of dioecy in the Mascarene Dombeyoideae (Malvaceae) as inferred from chloroplast and nuclear DNA sequence analyses. – Ann. Bot. 106: 343-357.
Le Péchon T, Dai Q, Zhang L-B, Gao X-F, Sauquet H. 2015. Diversification of Dombeyoideae (Malvaceae) in the Mascarenes: old taxa on young islands? – Intern. J. Plant Sci. 176: 211-221.
Leroy J-F. 1973. Recherches sur la speciation et l’endémisme dans la flore malgache III. Note sur le genre Dialyceras R. Cap. (Sphaerosépalacées). – Adansonia, sér. II, 13: 37-53.
Leroy J-F. 1975. Espèces et speciation. Remarques à propos du genre Schizolaena (Sarcolaenaceae). – Boissiera 24: 339-344.
Li Q-M, He T-H, Xu Z-F. 2004. Generic relationships of Parashorea chinensis Wang Hsie (Dipterocarpaceae) based on cpDNA sequences. – Taxon 53: 461-466.
Litchfield WH. 1966. The pollen morphology of Australian Sterculiaceae. – Pollen Spores 8: 439-453.
Lloyd RM. 1965. A new species of Fremontodendron (Sterculiaceae) from the Sierra Nevada foothills, California. – Brittonia 17: 382-384.
Londoño C, Alvarez E, Forero E, Morton C. 1995. A new genus and species of Dipterocarpaceae from the Neotropics I. Introduction, taxonomy, ecology, and distribution. – Brittonia 47: 225-236.
Long H, He L-K, Hsue H-H. 1985. Pollen morphology of Craigia with reference to its systematic position. – Acta Phytotaxon. Sin. 23: 179-184.
Lowry PP II, Rabehevitra D. 2006. Endemic families of Madagascar IX. A new littoral forest species of Schizolaena (Sarcolaenaceae). – Adansonia, sér. III, 28: 149-153.
Lowry PP II, Wolf A-E. 2000. Endemic families of Madagascar VI. A synoptic revision of Rhodolaena (Sarcolaenaceae). – Adansonia, sér. III, 22: 239-252.
Lowry PP II, Schatz GE, Leroy J-F, Wolf A-E. 1999. Endemic families of Madagascar III. A synoptic revision of Schizolaena (Sarcolaenaceae). – Adansonia, sér. III, 21: 183-212.
Lowry PP II, Haevermans T, Labat J-N, Schatz GE, Leroy J-F, Wolf A-E. 2000. Endemic families of Madagascar V. A synoptic revision of Eremolaena, Pentachlaena and Perrierodendron (Sarcolaenaceae). – Adansonia, sér. III, 22: 11-31.
Lowry PP II, Schatz GE, Wolf A-E. 2002. Endemic families of Madagascar VIII. A synoptic revision of Xyloolaena Baill. (Sarcolaenaceae). – Adansonia, sér. III, 24: 7-19.
Lowry PP II, Nusbaumer L, Randrianasolo A, Schatz GE, Hong-Wa C. 2014. Endemic families of Madagascar XIII. New, restricted range species of Eremolaena Baill. and Schizolaena Thouars (Sarcolaenaceae). – Candollea 69: 183-193.
Lundell CL. 1962. Plantae Mayanae – V. Petenaea cordata, a new genus and species in the Elaeocarpaceae, and other taxonomic notes. – Wrightia 3: 21-25.
Lutz ML. 1899. Observations sur l’ovaire du Cytinus hypocistis. – Bull. Soc. Bot. France 46: 299-301.
MacFarlane AT, Mori SA, Purzycki K. 2003. Notes on Eriotheca longitubulosa (Bombacaceae), a rare, putatively hawkmoth-ollinated species new to the Guianas. – Brittonia 55: 305-316.
Maguire B, Ashton PS. 1980. Pakaraimaea dipterocarpacea II. – Taxon 29: 225-231.
Maguire B, Steyermark JA. 1981a. Tepuianthaceae, Sapindales. – In: Maguire B et al. (eds), The botany of the Guayana Highland XI, Mem. New York Bot. Gard. 32: 4-21.
Maguire B, Steyermark JA. 1981b. Pakaraimaea dipterocarpacea III. – In: Maguire B et al. (eds), The botany of the Guayana Highland XI, Mem. New York Bot. Gard. 32: 306-309.
Maguire B, Ashton PS, Zeeuw C de, Giannasi DE, Niklas KJ. 1977. Pakaraimoideae, Dipterocarpaceae of the Western Hemisphere I-IV. – Taxon 26: 341-385.
Mai DH. 1961. Über eine fossile Tiliaceen-Blüte und tilioiden Pollen aus dem deutschen Tertiär. – Geologie 10, Beih. 32: 54-93.
Manchester SR. 1979. Triplochitoxylon (Sterculiaceae): a new genus of wood from the Eocene of Oregon and its bearing on xylem evolution in the extant genus Triplochiton. – Amer. J. Bot. 66: 699-708.
Manchester SR. 1980. Chattawaya (Sterculiaceae): a new genus of wood from the Eocene of Oregon and its implications for xylem evolution of the extant genus Pterospermum. – Amer. J. Bot. 67: 59-67.
Manchester SR. 1992. Flowers, fruits, and pollen of Florissantia, an extinct Malvalean genus from the Eocene and Oligocene of Western North America. – Amer. J. Bot. 79: 996-1008.
Manchester SR. 1994. Inflorescence bracts of fossil and extant Tilia in North America, Europe, and Asia: patterns of morphological divergence and biogeographic history. – Amer. J. Bot. 81: 1176-1185.
Manchester SR, Miller RB. 1978. Tile cells and their occurrence in malvalean fossil woods. – IAWA Bull. 1978: 23-28.
Manske RH. 1963. The genus Oceanopapaver. – Nature 200: 1123.
Marais W. 1981. Trochetiopsis (Sterculiaceae), a new genus from St Helena. – Kew Bull. 36: 645-646.
Marais W. 1983. Notes on Mascarene Malvaceae. – Kew Bull. 38: 41-46.
Marcano-Berti L. 1971. Uladendron, nuevo género de las Malvaceae. – Pittieria 3: 9-17.
Marinho RC, Mendes-Rodrigues C, Balao F,
Ortiz PL, Yamagishi-Costa J, Bonetti AM, Oliveira PE. 2014. Do chromosome
numbers reflect phylogeny? New counts for Bombacoideae and a review of Malvaceae s.l. – Amer. J. Bot. 101:
1456-1465.
Markova M. 1975. Karyosystematische Untersuchungen an den Cistaceae Bulgariens. – Plant Syst. Evol. 123: 283-315.
Marrero Á. 1992. Notas taxonómicas del género Helianthemum Miller en Lanzarote. – Bot. Macaronés. 19-20: 65-78.
Marrero Á, Mesa R. 2003. El género Helianthemum Mill. en la isla de La Gomera, Islas Canarias. – Candollea 58: 149-162.
Marrero Á, González-Martín M, González-Artiles F. 1995. Descripción de una nueva especie de Helianthemum Miller para Gran Canaria, islas Canarias. – Bot. Macaronés. 22: 3-11.
Martin FW. 1967. Distyly, self-incompatibility, and evolution in Melochia. – Evolution 21: 493-499.
Martin PG, Dowd JM. 1984. The study of plant phylogeny using amino acid sequences of ribulose-1,5-bisphosphate carboxylase III. Addition of Malvaceae and Ranunculaceae to the phylogenetic tree. – Aust. J. Bot. 32: 283-290.
Martinez-Hernández E, Fernández P, Lozano S. 1978. Pollen of tropical trees I. Tiliaceae. – J. Arnold Arbor. 59: 299-309.
Martinson VA. 1972a. Polyembryony in Theobroma cacao L. – Ann. Bot., N. S., 36: 947-951.
Martinson VA. 1972b. Embryological studies on hybridization between Theobroma cacao and Theobroma grandiflora. – Can. J. Bot. 50: 2117-2124.
Massicotte HB, Peterson RL, Melville LH, Tackaberry LE. 2010. Hudsonia ericoides and Hudsonia tomentosa: anatomy of the mycorrhizas of two members in the Cistaceae from eastern Canada. – Botany 88: 607-616.
Masters MT. 1869. On some points in the morphology of the Malvales, together with a description of a new genus of Buettnerieae. – Bot. J. Linn. Soc. 10: 18-30.
Masters MT. 1875. Monographic sketch of the Durioneae. – Bot. J. Linn. Soc. 14: 495-506.
Maury G. 1978. Dipterocarpacées: du fruit à la plantule. – Ph.D. diss., l’Université Paul Sabatier, Toulouse, France.
Maury G. 1979. Interprétation phylogénique des caractères des pollens, fruits-germinations, embryons et plantules des Diptérocarpacées. – In: Maury-Lechon G (ed), Diptérocarpacées: taxonomie-phylogénie-écologie, Mem. Mus. Natl. Hist. Nat., sér. B, Botanique 26, Éditions du Muséum, Paris, pp. 139-144.
Maury-Léchon G. 1979. Conséquences taxonomiques de l’étude des caractères des fruits – germinations, embryons et plantules de Diptérocarpacées. – Mém. Mus. Natl. Hist. Nat. Paris, B, Botanique 26: 81-106.
Maury-Léchon G, Curtet L. 1998. Biogeography and evolutionary systematics of Dipterocarpaceae. – In: Appanah S, Turnbull JM (eds), A review of dipterocarps: taxonomy, ecology and silviculture, CIFOR, Bogor, Indonesia, pp. 5-44.
Maury-Léchon G, Muller J, Lugardon B. 1975. Notes on the morphology and fine structure of the exine of some pollen types in Dipterocarpaceae. – Rev. Palaeobot. Palynol. 19: 241-289.
Mayer SS. 1990. The origin of dioecy in Hawaiian Wikstroemia (Thymelaeaceae). – Mem. New York Bot. Gard. 55: 76-82.
Mayer SS. 1991. Morphological variation in Hawaiian Wikstroemia (Thymelaeaceae). – Syst. Bot. 16: 693-704.
Meher-Homji VM. 1979. Distribution of Dipterocarpaceae: some phytogeographic considerations on India. – Phytocoenologia 6: 85-93.
Meijer W. 1993. Rafflesiaceae. – In: Kubitzki K, Rohwer JG, Bittrich V (eds), The families and genera of vascular plants II. Flowering plants. Dicotyledons. Magnoliid, hamamelid and caryophyllid families, Springer, Berlin, Heidelberg, New York, pp. 557-563.
Merrill ED. 1923. Distribution of the Dipterocarpaceae. – Philipp. J. Sci. 23: 1-32.
Meyer T, Barkley FA. 1973. Revisión del género Schinopsis. – Lilloa 33: 207-258.
Mildbraed J. 1921. Zur Kenntnis der afrikanischen Sterculiaceae-Mansonieae. – Notizbl. Bot. Gart. Mus. Berlin-Dahlem 7: 486-490.
Mohana Rao PR. 1976. Seed and fruit anatomy of Pterospermum acerifolium (Sterculiaceae). – Phytomorphology 26: 363-369.
Molau U. 1983. 127. Bixaceae, 128. Cochlospermaceae. – In: Harling G, Sparre B (eds), Flora of Ecuador 20, Swedish Natural Science Research Council, Stockholm, pp. 3-15.
Molby EE. 1931. The preliminary study of the epidermal appendages of the mallow family. – Trans. Ill. Acad. Sci. 23: 169-173.
Molero BJ, Montserrat MJM. 2007. A new species of Lavatera Sect. Olbia (Medik.) DC. (Malvaceae) from North-East Morocco. – Bot. J. Linn. Soc. 153: 445-454.
Möller-Lindenhof Y, Richter HG, Trockenbrodt M. 1999. The genus Mansonia (Sterculiaceae): wood structure and affinities within the order Malvales. – Mus. Roy. Afr. Centr., Tervuren, Belg., Ann. Sci. Econ., Wood to Survive 25: 43-57.
Monteiro Filho H. 1955. Malvaceae brasilienses novae vel criticae I. – Bol. Soc. Portuguesa Ci. Nat. 5: 119-140.
Monteiro Filho H. 1973. Malvaceae brasilienses novae vel criticae IV. – An. Soc. Bot. Brasil 23: 115-135.
Morat P, Chalopin M. 2003. Quatre nouvelles espèces d’Acropogon (Malvaceae: Sterculieae) endémiques de la Nouvelle-Calédonie. – Adansonia, sér. III, 25: 191-203.
Morat P, Chalopin M. 2005. Quatre autres nouvelles espèces d’Acropogon Schltr. (Malvaceae, Sterculieae) endémiques de Nouvelle-Calédonie. – Adansonia, sér. III, 27: 255-266.
Morat P, Chalopin M. 2007. Contribution à l’étude des Malvaceae, Sterculieae de la Nouvelle-Calédonie: nouvelles espèces dans le genre Acropogon Schltr. – Adansonia, sér. III, 29: 93-104.
Morawetz W. 1981. Zur systematischen Stellung der Gattung Prockia: Karyologie und Epidermisstruktur im Vergleich zu Flacourtia (Flacourtiaceae), Grewia (Tiliaceae) und verwandten Gattungen. – Plant Syst. Evol. 139: 57-76.
Morawetz W. 1986. Remarks on karyological differentiation patterns in tropical woody plants. – Plant Syst. Evol. 152: 49-100.
Morgan DR, Soltis DE, Robertson KR. 1994. Systematic and evolutionary implications of rbcL sequence variation in Rosaceae. – Amer. J. Bot. 81: 890-903.
Morse LE. 1979. Systematics and ecological biogeography of the genus Hudsonia (Cistaceae), the sand heather. – Ph.D. diss, Harvard University, Cambridge, Massachusetts.
Morton CM. 1995. A new genus and species of Dipterocarpaceae from the Neotropics II. Stem anatomy. – Brittonia 47: 237-247.
Morton CM. 2009. Phylogenetic relationships of the Aurantioideae (Rutaceae) based on the nuclear ribosomal DNA ITS region and three noncoding chloroplast DNA regions, atpB-rbcL spacer, rps16, and trnL-trnF. – Organisms Divers. Evol. 9: 52-68.
Morton CM, Dayanandan S, Dissanayake D. 1999. Phylogeny and biosystematics of Pseudomonotes (Dipterocarpaceae) based on molecular and morphological data. – Plant Syst. Evol. 216: 197-205.
Motsi MC, Rye B, Bank M van der. 2008. Molecular phylogenetics of the genera Pimelea and Thecanthes (Thymelaeaceae). – South Afr. J. Bot. 74: 373.
Motsi MC, Moteetee AN, Beaumont AJ, Rye BL, Powell MP, Savolainen V, Bank M van der. 2010. A phylogenetic study of Pimelea and Thecanthes (Thymelaeaceae): evidence from plastid and nuclear ribosomal DNA sequence data. – Aust. Syst. Bot. 23: 270-284.
Moyersoen B. 2006. Pakaraimaea dipterocarpacea is ectomycorrhizal, indicating an ancient Gondwanaland origin for the ectomycorrhizal habit in Dipterocarpaceae. – New Phytol. 172: 753-762.
Müller-Stoll WR, Müller-Stoll H. 1949. Sterculioxylon rhenanum nov. spec. aus dem Alttertiär Südwest-deutschlands. – Palaeontographica, Abt. B, 89: 204-218.
Murbeck S. 1916. Über die Organisation, Biologie, und verwandtschaftlichen Beziehungen der Neuradoideen. – Lunds Univ. Årsskr. (Acta Univ. Lund.), N. F., Avd. II, 12(6): 1-29.
Myoland BPM, Steenis CGGJ van. 1987. Franciscodendron (Sterculiaceae), a new tree genus from Queensland. – Brunonia 10: 211-214.
Naggar SM el. 2004. Pollen morphology of Egyptian Malvaceae: an assessment of taxonomic value. – Turkish J. Bot. 28: 227-240.
Nandi OI. 1998a. Ovule and seed anatomy of Cistaceae and related Malvanae. – Plant Syst. Evol. 209: 239-264.
Nandi OI. 1998b. Floral development and systematics of Cistaceae. – Plant Syst. Evol. 212: 107-134.
Neubig KM,Blanchard jr OJ, Whitten WM, McDaniel SF. 2015. Moecular phylogenetics of Kosteletzkya (Malvaceae, Hibisceae) reveals multiple independent and successive polyploid speciation events. – Bot. J. Linn. Soc. 179: 421-435.
Nevling LI. 1959. A revision of the genus Daphnopsis. – Ann. Missouri Bot. Gard. 46: 257-353.
Nevling LI. 1961a. A revision of the Asiatic genus Linostoma (Thymelaeaceae). – J. Arnold Arbor. 42: 295-320.
Nevling LI. 1961b. A revision of the Asiatic genus Enkleia. – J. Arnold Arbor. 42: 373-396.
Nevling LI. 1963. A revision of the genus Lophostoma (Thymelaeaceae). – J. Arnold Arbor. 44: 143-164.
Nickrent DL. 2007. Cytinaceae are sister to Muntingiaceae (Malvales). – Taxon 56: 1129-1135.
Nilsson S, Randrianasolo A. 1999. Morphology and functional aspects of pollen in the Sarcolaenaceae. – Palaeoecology of Africa 26: 191-200.
Nilsson S, Robyns A. 1974. Pollen morphology and taxonomy of the genus Quararibea s.l. (Bombacaceae). – Bull. Jard. Bot. Natl. Belg. 44: 77-99.
Nilsson S, Robyns A. 1986. Bombacaceae Kunth. – In: Nilsson S (ed), World Pollen and Spore Flora 14: 1-59.
Nilsson S, Coetzee J, Grafström E. 1996. On the origin of the Sarcolaenaceae with reference to pollen morphological evidence. – Grana 35: 321-334.
Nowicke JW, Patel V, Skvarla J. 1985. Pollen morphology and relationships of Aëtoxylon, Amyxa, and Gonystylus to the Thymelaeaceae. – Amer. J. Bot. 72: 1106-1113.
Nyffeler R, Baum DA. 2000. Phylogenetic relationships of the durians (Bombacaceae-Durioneae or Malvaceae/Helicteroideae/Durioneae) based on chloroplast and nuclear ribosomal DNA sequences. – Plant Syst. Evol. 224: 55-82.
Nyffeler R, Baum DA. 2001. Systematics and character evolution in Durio s. lat. (Malvaceae/Helicteroideae/Durioneae or Bombacaceae-Durioneae). – Organisms Divers. Evol. 1: 165-178.
Nyffeler R, Bayer C, Alverson WS, Yen A, Whitlock BA, Chase MW, Baum DA. 2005. Phylogenetic analysis of the Malvadendrina clade (Malvaceae s.l.) based on plastid DNA sequences. – Organisms Divers. Evol. 5: 109-123.
Oginuma K, Fujita K. 1997. Karyomorphology of Neurada procumbens L. (Neuradaceae). – Acta Phytotaxon. Geobot. 48: 69-71.
Oliveira PE, Gibbs PE, Barbosa AA, Talavera S. 1992. Contrasting breeding systems in two Eriotheca (Bombacaceae) species of the Brazilian cerrados. – Plant Syst. Evol. 179: 207-219.
Ourisson G. 1979. Chimie-taxonomie des Dipterocarpacées. – In: Maury-Lechon G (ed), Diptérocarpacées: taxonomie-phylogénie-écologie, Mem. Mus. Natl. Hist. Nat., sér. B, Botanique 26, Éditions du Muséum, Paris, pp. 57-67.
Outer RW den, Schütz PR. 1981. Wood anatomy of some Sarcolaenaceae and Rhopalocarpaceae and their systematic position. – Meded. Landbouwh. Wageningen 81(8): 1-25.
Outer RW den, Vooren AP. 1980. Bark anatomy of some Sarcolaenaceae and Rhopalocarpaceae and their systematic position. – Meded. Landbouwh. Wageningen 80(6): 3-15.
Pan AD, Jacobs BF. 2009. The earliest record of the genus Cola (Malvaceae sensu lato: Sterculioideae) from the Late Oligocene (28-27 Ma) of Ethiopia and leaf characteristics within the genus. – Plant Syst. Evol. 283: 247-262.
Parameswaran N, Gottwald H. 1979. Problematic taxa in the Dipterocarpaceae. Their anatomy and taxonomy. – In: Maury-Lechon G (ed), Diptérocarpacées: taxonomie-phylogénie-écologie, Mem. Mus. Natl. Hist. Nat., sér. B, Botanique 26, Éditions du Muséum, Paris, pp. 69-75.
Paris RR, Jacquemin H, Linard A. 1975. Plantes de Madagascar XV. Sur quelques Chlaenacées malgaches: Leptolaena pauciflora Baker, L. diospyroidea Cavaco var. tampoketsensis et Sarcolaena multiflora Dup. Thou.; presence d’hétérosides du myricétol. – Plant Méd. Phytothér. 9: 230-237.
Paula JEP. 1969. Estudos sobre Bombacaceae I. Contribuiçäo para o conhecimento dos gêneros Catostemma Benth. e Scleronema Benth. da Amazônia Brasileira. – Ci. Cult. 21: 697-705.
Paula JEP. 1975. Estudos sobre Bombacaceae V. Investigação anatômica das madeiras de Catostemma commune Sandwith, Catostemma sclerophyllum Ducke e Scleronema micranthum (Ducke) Ducke, com vistas à polpa, papel e taxinomia. – Acta Amazon. 6: 155-161.
Paula VF, Barbosa LCA, Demuner AJ, Piló-Veloso D. 1997. A química da família Bombacaceae. – Quím. Nova 20: 627-630.
Perrier de la Bâthie H. 1925. Nouvelles remarques sur les Chlaenacées. – Bull. Soc. Bot. France 72: 307-313.
Perrier de la Bâthie H. 1931. Remarques sur les Chlaenacées 2. – Bull. Soc. Bot. France 78: 46-65.
Perrier de la Bâthie H. 1955. Notes concernant l’homme et les plantes utiles à Madagascar. – J. Agric. Trop. Got. Appl. 2: 298-329.
Perveen A, Qaiser M. 1997. Pollen flora of Pakistan VII. Neuradaceae. – Pak. J. Bot. 29: 39-42.
Perveen A, Qaiser M. 2007. Pollen flora of Pakistan LII. Malvaceae-Grewioideae. – Pak. J. Bot. 39: 1-7.
Perveen A, Grafström E, El-Ghazaly G. 2004. World Pollen and Spore Flora 23. Malvaceae Adams. p.p. subfamilies: Grewioideae, Tilioideae, Brownlowioideae. – Grana 43: 129-155.
Peterson B. 1959. Some interesting species of Gnidia. – Bot. Notiser 112: 465-480.
Peterson B. 1978. Thymelaeaceae. – In: Polhill RM (ed), Flora of tropical East Africa, Crown Agents for Oversea Governments and Administrations, London, pp. 1-37.
Pettigrew FRS, Jack D, Bell KL, Bhagwandin A, Grinan E, Jillani N, Meyer J, Wabuyele E, Vickers CE. 2012. Morphology, ploidy and molecular phylogenetics reveal a new diploid species from Africa in the baobab genus Adansonia (Malvaceae: Bombacoideae). – Taxon 61: 1240-1250.
Pfeil BE, Craven LA. 2002. New taxa in Glycine (Fabaceae: Phaseoleae) from north-western Australia. – Aust. Syst. Bot. 15: 565-573.
Pfeil BE, Crisp MD. 2005. What to do with Hibiscus? A proposed nomenclatural resolution for a large and well known genus of Malvaceae and comments on paraphyly. – Aust. Syst. Bot. 18: 49-60.
Pfeil BE, Brubaker CL, Craven LA, Crisp MD. 2002. Phylogeny of Hibiscus and the tribe Hibisceae (Malvaceae) using chloroplast DNA sequences of ndhF and the rpl16 intron. – Syst. Bot. 27: 333-350.
Pfeil BE, Brubaker CL, Craven LA, Crisp MD. 2004. Paralogy and orthology in the Malvaceae rpb2 gene family: investigation of gene duplication in Hibiscus. – Mol. Biol. Evol. 21: 1428-1437.
Phillips EP. 1944. Notes of some genera of the Thymelaeaceae. – South Afr. J. Bot. 10: 61.
Phuphathanaphong L, Puangpen S, Nuvongsri G. 2006. Thepparatia (Malvaceae), a new genus from Thailand. – Thai For. Bull. (Bot.) 34: 195-200.
Piccioli L. 1901. Il legno e la corteccia delle Cistacee. – Nuovo Giorn. Bot. Ital., n. s., 8: 473-504.
Pilger R. 1925a. Bixaceae. – In: Engler A, Gilg E (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 21, W. Engelmann, Leipzig, pp. 313-315.
Pilger R. 1925b. Cochlospermaceae. – In: Engler A, Gilg E (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 21, W. Engelmann, Leipzig, pp. 316-320.
Pires SM, Cristóbal CL. 2001. El pollen de Helicteres (Sterculiaceae) y su comparación con géneros vecinos. – Bonplandia 11: 207-230.
Pittier H. 1914. Gyranthera Pittier, gen. nov. Bombacacearum. – Repert. Spec. Nov. Regni Veg. 13: 318-319.
Pittier H. 1921. Contribuciones para la flora de Venezuela II. Acerca del genero Gyranthera Pittier. – Typografia Americana, Caracas, Venezuela.
Plowman T, Nevling LI. 1986. A new species of Lasiadenia (Thymelaeaceae) from Venezuela. – Brittonia 38: 114-118.
Pobedimova EG. 1941. Restella Pob., a new genus and its origin. – Bot. Žurn. 26: 36-43. [In Russian]
Poisson HL. 1912. Macrocalyx tomentosa Cost. et Poisson. – Flore Mérid. Madagascar, sér. A, 605: 26-27.
Polevova S, Tekleva M, Neumann FH, Scott L, Stager JC. 2010. Pollen morphology, ultrastructure and taphonomy of the Neuradaceae with special reference to Neurada procumbens L. and Grielum humifusum E. Mey. ex Harv. et Sond. – Rev. Palaeobot. Palynol. 160: 163-171.
Ponzi R, Pizzolongo P. 1976. Cytinus hypocistis L. embryogenesis: ultrastructural aspect of megasporogenesis and megagametogenesis. – J. Submicroscop. Cytol. 8: 327-336.
Ponzi R, Pizzolongo P. 1982. Cytinus hypocistis L. embryogenesis: some biological and ultrastructural aspects of fertilization and embryo development. – Plant Biosystems 116: 149-166.
Poppendieck H-H. 1980. A monograph of the Cochlospermaceae. – Bot. Jahrb. Syst. 101: 191-265.
Poppendieck H-H. 1981. Flora Neotropica. Monograph 27. Cochlospermaceae. – New York Botanical Garden, Bronx, New York.
Poppendieck H-H. 2002a. Bixaceae. – In: Kubitzki K, Bayer C (eds), The families and genera of vascular plants V. Flowering plants. Dicotyledons. Malvales, Capparales and non-betalain Caryophyllales, Springer, Berlin, Heidelberg, New York, pp. 33-35.
Poppendieck H-H. 2002b. Cochlospermaceae. – In: Kubitzki K, Bayer C (eds), The families and genera of vascular plants V. Flowering plants. Dicotyledons. Malvales, Capparales and non-betalain Caryophyllales, Springer, Berlin, Heidelberg, New York, pp. 71-74.
Powell RG, Weisleder D, Smith CR Jr. 1985. Daphnane diterpenes from Diarthron vesiculosum: vesiculosin and isovesiculosin. – J. Nat. Prod. (Lloydia) 48: 102-107.
Pradeep AK, Sivarajan VV. 1993. A revision of the genus Julostylis Thwaites (Malvaceae) with a new species from India. – Bot. Bull. Acad. Sin. 34: 277-286.
Prain D. 1905. Mansonieae, a new tribe of the natural order Sterculiaceae. – Bot. J. Linn. Soc. 37: 250-263.
Presting D, Straka H, Friedrich B. 1983. Palynologia Madagassica et Mascarenica, Familien 128 bis 146. – Akad. Wiss. Lit. Mainz, Trop. Subtrop. Pflanzenwelt 44: 1-93.
Proctor MCF. 1955. Some chromosome counts in the European Cistaceae. – Watsonia 3: 154-159.
Purohit KM, Panigrahi G. 1983. The Neuradaceae J. G. Agardh (Rosales) in India. – J. Econ. Taxon. Bot. 4: 1033-1037.
Rabehevitra D, Lowry II PP. 2009. Endemic families of Madagascar XI. A new critically endangered species of Schizolaena (Sarcolaenaceae) from Tapia woodland in south-central Madagascar. – Adansonia, sér. III, 31: 149-155.
Raffauf RF, Zennie TM. 1983. The phytochemistry of Quararibea funebris. – Bot. Mus. Leafl. 29: 151-158.
Raffauf RF, Zennie TM, Onan KD, LeQuesne P. 1984. Funebrine, a structurally novel pyrrole alkaloid, and other gamma-hydroxyisoleucine-related metabolites of Quararibea funebris (Llave) Vischer (Bombacaceae). – J. Org. Chem. 49: 2714-2718.
Ramadan AA, El-Keblawy A, Shaltout KH, Lovett-Doust J. 1994. Sexual polymorphism, growth and reproductive effort in Egyptian Thymelaea hirsuta (Thymelaeaceae). – Amer. J. Bot. 81: 847-857.
Ramanujam CGK. 1955. Fossil wood of Dipterocarpaceae from the Tertiary of South Arcot district, India. – The Palaeobotanist 4: 45-56.
Ramayya N, Shanmukha Rao SR. 1976. Morphology, phylesis and biology of the peltate, scale, stellate, and tufted hair in some Malvaceae. – J. Indian Bot. 55: 75-79.
Randrianasolo A, Miller JS. 1994. Sarcolaena isaloensis, a new species of Sarcolaenaceae from Isalo, south-central Madagascar. – Novon 4: 290-292.
Randrianasolo A, Miller JS. 1999. Taxonomic revision of the genus Sarcolaena (Sarcolaenaceae). – Ann. Missouri Bot. Gard. 86: 702-722.
Randrianasolo A, Lowry II PP, Schatz GE, Phillipson PB, Wahlert GA. 2013. The lianescent species of Grewia L. (Malvaceae s.l., formerly Tiliaceae) in Madagascar. – Adansonia 35: 73-85.
Rao AN. 1953. Embryology of Shorea talura Roxb. – Phytomorphology 3: 476-484.
Rao AN. 1955. A contribution to the embryology of Vateria indica. – Proc. Indian Natl. Inst. Sci., N. S., 21: 247-255.
Rao AN. 1956. Life history of Shorea robusta. – Curr. Sci. 25: 128-129.
Rao CV. 1949. Floral anatomy of some Sterculiaceae with special reference to the position of the stamen. – J. Indian Bot. Soc. 28: 237-245.
Rao CV. 1950. Pollen grains of Sterculiaceae. – J. Indian Bot. Soc. 29: 130-137.
Rasoamanana E, Razanamaro O, Ramavovololona P, Ramamonjisoa R, Verdeil J, Danthu P, Suárez-Cervera M. 2015. Pollen wall ultrastructure of the genus Adansonia L. species. – Plant Syst. Evol. 301: 541-554.
Ravenna P. 1998. On the identity, validity, and actual placement in Ceiba of several Chorisia species (Bombacaceae), and description of two new South American species. – Onira 3: 42-51.
Ray MF. 1994. A contribution to the systematics of Lavatera and Malva (Malvaceae) and related genera. – Ph.D. diss., University of California, Berkeley, California.
Ray MF. 1995. Systematics of Lavatera and Malva (Malvaceae, Malveae) – a new perspective. – Plant Syst. Evol. 198: 29-53.
Ray MF. 1998. New combinations in Malva. – Novon 8: 288-295.
Record SJ. 1939. American woods of the family Bombacaceae. – Trop. Woods 59: 1-20.
Reeves RG. 1936. Comparative anatomy of the seeds of cottons and other malvaceous plants II. Hibisceae. – Amer. J. Bot. 23: 394-405.
Refaat J, Desoky SY, Ramadan MA, Kamel MS. 2013. Bombacaceae: a phytochemical review. – Pharm. Biol. 51: 100-130.
Reiche K. 1895. Cistaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(6), W. Engelmann, Leipzig, pp. 299-306.
Ren Z. 1989. The cladistic analysis of the systematic position of Craigia. – Acta Bot. Yunnan. 11: 17-23.
Ricci I. 1957. Morphologia e costituzione chimica dei peli nel genere Cistus e loro importanza nella systematic di alcune specie. – Ann. Bot. (Roma) 25: 540-566.
Rizk AM, El-Missiry MM. 1986. Non-diterpenoid constituents of Euphorbiaceae and Thymelaeaceae. – In: Evans FJ (ed), Naturally occurring phorbol esters, CRC Press, Boca Raton, Florida, pp. 107-138.
Robyns A. 1960. Contribution à l’étude monographique du genre Bombax s.l. I. – Bull. Jard. Bot. État (Bruxelles) 30: 473-484.
Robyns A. 1963. Essai de monographie du genre Bombax L. s.l. (Bombacaceae). – Bull. Jard. Bot. État (Bruxelles) 33: 1-315.
Robyns A. 1964a. Family 114. Tiliaceae. – In: Woodson RE, Schery RW (eds), Flora of Panama VI, Ann. Missouri Bot. Gard. 51: 1-35.
Robyns A. 1964b. Family 115. Bombacaceae. – In: Woodson RE, Schery RW (eds), Flora of Panama VI, Ann. Missouri Bot. Gard. 51: 37-68.
Robyns A. 1970. Revision of the genus Cullenia Wight (Bombacaceae – Durioneae). – Bull. Jard. Bot. Natl. Belg. 40: 241-254.
Robyns A. 1971. On pollen morphology of Bombacaceae. – Bull. Jard. Bot. Nat. Belg. 41: 451-456.
Robyns A, Nilsson S. 1972. Bombacaceae neotropicae novae IV. Two new species of Quararibea from Amazonia. – Bull. Jard. Bot. Nat. Belg. 42: 347-352.
Robyns A, Nilsson S, Dechamps R. 1977. Sur la position systématique du genre Maxwellia Baillon. – Bull. Jard. Bot. Natl. Belg. 47: 145-153.
Rodrigo A del P. 1944. Las species argentinas y uruguayas del género Sida (Malvaceae). – Rev. Mus. La Plata, Secc. Bot. 6: 81-212.
Rogers ZS. 2004. A revision of Stephanodaphne Baill. (Thymelaeaceae). – Adansonia, sér. III, 26: 7-35.
Rogers ZS. 2005. A revision of Octolepis Oliv. (Thymelaeaceae, Octolepidoideae). – Adansonia, sér. III, 27: 89-111.
Rogers ZS. 2006. A new species of Malagasy Gnidia and the lectotypification of Octolepis decalepis (Thymelaeaceae). – Adansonia, sér. III, 28: 155-160.
Rogers ZS. 2009. A revision of Malagasy Gnidia (Thymelaeaceae, Thymelaeoideae). – Ann. Missouri Bot. Gard. 96: 324-369.
Rohweder O. 1972. Das Andröcium der Malvales und der ”Konservatismus” des Leitgewebes. – Bot. Jahrb. Syst. 92: 155-167.
Ronse De Craene LP. 1989. Floral development of Cochlospermum tinctorium and Bixa orellana with special emphasis on the androecium. – Amer. J. Bot. 76: 1344-1359.
Ronse De Craene LP, Smets EF. 1995. The floral development of Neurada procumbens L. (Neuradaceae). – Acta Bot. Neerl. 44: 439-451.
Roquet C, Coissac É, Cruaud C, Boleda M, Boyer F, Alberti A, Gielly L, Taberlet P, Thuiller W, Van Es J, Lavergne S. 2016. Understanding the evolution of holoparasitic plants: the complete plastid genome of the holoparasite Cytinus hypocistis (Cytinaceae). – Ann. Bot. 118: 885-896.
Rosello EF, Melhem TS. 1998. Palinotaxonomia de espécies Brasileiras de Thymelaeaceae Juss. – Bot. Bot. Univ. São Paulo 17: 1-24.
Rosenberg O. 1898. Studien über die Membranschleime der Pflanzen II. Vergleichende Anatomie der Samenschale der Cistaceen. – Bih. Kungl. Sv. Vetensk.-Akad. Handl. 24, Afd. III, 1: 1-60.
Roth I, Lindorf H. 1990. Blatt- und Rindenstruktur von Tepuianthus auyantepuiensis, einer neueren Familie aus Venezuela. – Bot. Jahrb. Syst. 111: 403-421.
Roy RP, Jha RP. 1965. Cytological studies in Dipterocarpaceae 1. – J. Indian Bot. Soc. 44: 387-397.
Rye BL. 1984. Four new names for Pimelea species (Thymelaeaceae) represented in the Perth region. – Nuytsia 5: 1-11.
Rye BL. 1988. A revision of western Australian Thymelaeaceae. – Nuytsia 6: 129-278.
Rye BL. 1994. Thymelaeaceae – the family. – Australian Plants 17: 297-327.
Rye BL, Heads MJ. 1990. Thymelaeaceae. – In: George AS (ed), Flora of Australia 18, Australian Government Publ. Service, Canberra, pp. 122-215.
Saad SI. 1960. The sporoderm stratification in the Malvaceae. – Pollen Spores 2: 13-41.
Sáenz de Rivas C. 1979. Pollen morphology of Spanish Cistaceae. – Grana 18: 91-98.
Sakai S, Momose K, Yumoto T, Kato M, Inoue T. 1999. Beetle pollination of Shorea parvifolia (section Mutica, Dipterocarpaceae) in a general flowering period in Sarawak, Malaysia. – Amer. J. Bot. 86: 62-69.
Santharam V. 1996. Visitation patterns of birds and butterflies at a Helicteres isora Linn. (Sterculiaceae) clump. – Curr. Sci. 70: 316-319.
Saunders ER. 1936. On certain features of floral construction and arrangement in the Malvaceae. – Ann. Bot. 50: 247-282.
Saunders ER. 1937. The vascular ground-plan as a guide to the floral ground-plan: illustrated from Cistaceae. – New Phytol. 35: 47-67.
Saunders JG. 1993. Four new distylous species of Waltheria (Sterculiaceae) and a key to the Mexican and Central American species and species groups. – Syst. Bot. 18: 356-376.
Saunders JG. 1995. Systematics and evolution of Waltheria (Sterculiaceae-Hermannieae). – Ph.D. diss., University of Texas, Austin, Texas.
Saunders JH. 1961. The wild species of Gossypium and their evolutionary history. – London.
Sawidis T, Eleftheriou EP, Teskos I. 1989. The floral nectaries of Hibiscus rosa-sinensis III. A morphometric and ultrastructural approach. – Nord. J. Bot. 9: 63-71.
Sazima M, Sazima I. 1988. Helicteres ovata (Sterculiaceae), pollinated by bats in southeastern Brazil. – Bot. Acta 101: 269-271.
Sazima M, Fabián ME, Sazima I. 1982. Polinização de Luehea speciosa (Tiliaceae) por Glossophaga soricina (Chiroptera, Phyllostomidae). – Rev. Brasil. Biol. 42: 505-513.
Schatz GE, Lowry II PP. 2006. Endemic families of Madagascar X. Two new species of Rhopalocarpus Bojer (Sphaerosepalaceae). – Adansonia, sér. III, 28: 329-336.
Schatz GE, Lowry II PP, Wolf A-E. 1999. Endemic families of Madagascar II. A synoptic revision of Sphaerosepalaceae. – Adansonia, sér. III, 21: 107-123.
Schatz GE, Lowry II PP, Wolf A-E. 2000. Endemic families of Madagascar VI. A synoptic revision of Rhodolaena (Sarcolaenaceae). – Adansonia, sér. III, 22: 239-252.
Schatz GE, Lowry II PP, Wolf A-E. 2001. Endemic families of Madagascar VII. A synoptic revision of Leptolaena Thouars sensu stricto (Sarcolaenaceae). – Adansonia, sér. III, 23: 171-189.
Schinz H. 1901. Rosaceae. Neurada austrafricana Schinz. – Bull. Herb. Boissier, sér. II, 1: 874.
Schmid R, Carlquist SJ, Hufford LD, Webster GL. 1985. Systematic anatomy of Oceanopapaver: a monotypic genus of the Capparaceae from New Caledonia. – Bot. J. Linn. Soc. 89: 119-152.
Schmidt JH, Wells R. 1990. Evidence for the presence of gossypol in malvaceous plants other than those in the cotton tribe. – J. Agric. Food Chem. 38: 505-508.
Schmidt RJ. 1986a. Biosynthetic and chemosystematic aspects of the Euphorbiaceae and Thymelaeaceae. – Evans FJ (ed), Naturally occurring phorbol esters, CRC Press, Boca Raton, Florida, pp. 87-106.
Schmidt RJ. 1986b. The daphnane polyol esters. – In: Evans FJ (ed), Naturally occurring phorbol esters, CRC Press, Boca Raton, Florida, pp. 217-244.
Schnarf K. 1931. Ein Beitrag zur Kenntnis der Samenentwicklung der Gattung Cochlospermum. – Österr. Bot. Zeitschr. 80: 45-50.
Schneider JV. 2013. The Peruvian species of Cristaria (Malveae, Malvaceae): taxonomic revision, chromosome counts, and breeding system. – Phytotaxa 110: 31-47.
Schröter C. 1883. Beitrag zur Kenntnis des Malvaceen-Androeceums. – Jahrb. Königl. Bot. Gart. Mus. Berlin 2: 153-165.
Schultes RE. 1957. The genus Quararibea in Mexico and the use of its flowers as a spice for chocolate. – Bot. Mus. Leafl. 17: 247-264.
Schultes RE. 1958. A synopsis of the genus Herrania. – J. Arnold Arbor. 39: 216-295.
Schumann K. 1886. Vergleichende Blütenmorphologie der cucullaten Sterculiaceae. – Jahrb. Königl. Bot. Gart. Mus. Berlin 4: 286-332.
Schumann K. 1895a. Tiliaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(6), W. Engelmann, Leipzig, pp. 8-30; Schumann K. 1897. Nachträge zu III(6), pp. 232-234.
Schumann K. 1895b. Malvaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(6), W. Engelmann, Leipzig, pp. 30-53; Schumann K. 1897. Nachträge zu III(6), pp. 235-239.
Schumann K. 1895c. Bombacaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(6), W. Engelmann, Leipzig, pp. 53-68; Schumann K. 1897. Nachträge zu III(6), p. 240.
Schumann K. 1895d. Sterculiaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(6), W. Engelmann, Leipzig, pp. 69-99; Schumann K. 1897. Nachträge zu III(6), pp. 240-242.
Schuann K. 1895e. Chlaenaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(6), W. Engelmann, Leipzig, pp. 168-175.
Schumann K. 1900. Eine neue Familie der Malvales. – Engl. Bot. Jahrb. Syst. 28: 330-331.
Seelanan T, Schnabel A, Wendel JF. 1997. Congruence and consensus in the cotton tribe (Malvaceae). – Syst. Bot. 22: 259-290.
Seelanan T, Brubaker CL, Stewart J McD, Craven LA, Wendel JF. 1999. Molecular systematics of Australian Gossypium Section Grandicalyx (Malvaceae). – Syst. Bot. 24: 183-208.
Seilmeier A. 2000. Structural variation of Tertiary Grewia woods (Tiliaceae) from East Bavarian Molasse, Germany. – Feddes Repert. 111: 465-480.
Sensarma P. 1957. On the vascularisation of the leaf and its associated structures in Muntingia calabura. – Bot. Gaz. 119: 116-119.
Shanmukha Rao SR. 1987. Structure, distribution and classification of plant trichomes in relation to taxonomy: Sterculiaceae. – Feddes Repert. 98: 127-135.
Shanmukha Rao SR. 1990. Trichome ontogenesis in some Tiliaceae. – Beitr. Biol. Pflanzen 65: 363-375.
Shanmukha Rao SR, Ramayya N. 1984. Structure and taxonomic distribution of the epidermal idioblasts in the Malvales. – Indian J. Bot. 7: 117-123.
Shanmukha Rao SR, Ramayya N. 1987. Trichome types and their taxonomic importance in the Tiliaceae. – Indian J. Bot. 10: 65-73.
Sharma AK, Sharma A. 1962. Polyploidy and chromosome evolution in Hibiscus. – Cellule 62: 283-300.
Sharma BD. 1969a. Pollen morphology of Tiliaceae in relation to plant taxonomy. – J. Palynol. (Lucknow) 5: 7-29.
Sharma BD. 1969b. Studies of Indian pollen grains in relation to plant taxonomy – Sterculiaceae. – Proc. Indian Natl. Inst. Sci., Sect. B, 35: 320-359.
Sharma BD. 1970. Contribution to the pollen morphology and plant taxonomy of the family Bombacaceae. – Proc. Indian Natl. Acad. Sci., Sect. B, 36: 175-191.
Sharma R. 1990a. Trichomes in some Tiliaceae. – J. Indian Bot. Soc. 69: 11-14.
Sharma R. 1990b. Floral anatomy of Grewia. – J. Indian Bot. Soc. 69: 277-280.
Shenstone FS, Vickery JR. 1961. Occurrence of cyclopropene acids in some plants of the order Malvales. – Nature 190: 168-169.
Singh HB, Dube VP. 1993. Taxonomic significance of foliar epidermal features of Muntingia Linn. (Tiliaceae). – J. Plant Anat. Morphol. 6: 123-128.
Sivarajan VV, Pradeep AK. 1994. Taxonomy of the Sida rhombifolia (Malvaceae) complex in India. – Sida 16: 63-78.
Sivarajan VV, Pradeep AK. 1996. Malvaceae of Southern Peninsular India. – Daya Publ. House, Delhi.
Skema C. 2012. Toward a new circumscription of Dombeya (Malvales: Dombeyaceae): a molecular phylogenetic and morphological study of Dombeya of Madagascar and a new segregate genus, Andringitra. – Taxon 61: 612-628.
Skovsted A. 1935. Chromosome numbers in the Malvaceae I. – J. Genet. 31: 263-296.
Skovsted A. 1941. Chromosome numbers in the Malvaceae II. – Compt. Rend. Trav. Labor. Carlsberg, sér. Physiol. 23: 195-242.
Slooten DF van. 1941. Sertulum Dipterocarpaceum Malayensium II. – Bull. Jard. Bot. Buitenz. III, 17: 96-138.
Slooten DF van. 1956. Sertulum Dipterocarpaceum Malayensium VI. – Reinwardtia 3: 315-346.
Slotta TAB. 2000. Phylogenetic analysis of Iliamna (Malvaceae) using the Internal Transcribed Spacer region. – Ph.D. diss., Virginia Polytechnic Institute and State University, Virginia.
Small RL. 2004. Phylogeny of Hibiscus sect. Muenchusia (Malvaceae) based on chloroplast rpl16 and ndhF, and nuclear ITS and GBSSI sequences. – Syst. Bot. 29: 385-392.
Smith CC. 1934. A case of ‘pollinia’. – Phytologia 1: 83-88.
Smith WW, Evans WE. 1921. Craigia, a new genus of Sterculiaceae. – Trans. Proc. Bot. Soc. Edinb. 28: 69-71.
Smithies SJ, Burgoyne PM. 2010. 659. Cytinus visseri. – Curtis’s Bot. Mag. 26: 322-332.
Smits WTM. 1983. Dipterocarps and mycorrhiza. An ecological adaptation and a factor in forest regeneration. – Flora Malesiana Bull. 36: 3926-3927.
Smits WTM. 1994. Dipterocarpaceae: mycorrhizae and regeneration. – Tropenbos Series 9, Tropenbos Foundation, Wageningen.
Soepadmo E, Eow BK. 1977. The reproductive biology of Durio zibethinus Murr. – Gard. Bull. (Singapore) 29: 25-33.
Solheim SL. 1991. Reevesia and Ungeria (Sterculiaceae): a taxonomic and biogeographic study. – Ph.D. diss., University of Wisconsin, Madison, Wisconsin.
Somego M. 1978. Cytogenetical study of Dipterocarpaceae. – Malaysian For. 41: 358-366.
Souèges R. 1937. Développement de l’embryon chez l’Helianthemum guttatum. – Bull. Soc. Bot. France 84: 400.
Soulebeau A, Pellens R, Lowry PP II, Aubriot X, Evans M, Haevermans T. 2016. Conservation of phylogenetic diversity in Madagascar’s largest endemic plant family, Sarcolaenaceae. – In: Pellens R, Grandcolas P (eds), Biodiversity conservation and phylogenetic systematics: preserving our evolutionary heritage in an extinction crisis, Springer, New York, pp. 355-374.
Sousa Silva CR, Figueira A. 2005. Phylogenetic analysis of Theobroma (Sterculiaceae) based on Kunitz-like trypsin inhibitor sequences. – Plant Syst. Evol. 250: 93-104.
Standley PC. 1929. Studies of American plants I. – Field Mus. Nat. Hist., Bot. 4: 197-299.
Standley PC. 1937. Flora of Costa Rica II. Malvaceae. – Field Mus. Nat. Hist., Bot. 18: 664-677.
Standley PC, Steyermark J. 1949. Flora of Guatemala. Malvaceae. – Fieldiana: Bot. 24: 324-386.
Stevens WD. 1987. On the identity and recognition of the genus Pochota Ramirez Goyena (Bombacaceae). – Taxon 36: 458-464.
Steyermark JA. 1987. Notes on Catostemma and Scleronema (Bombacaceae). – Ann. Missouri Bot. Gard. 74: 636-646.
Steyermark JA, Stevens WD. 1988. Notes on Rhodognaphalopsis and Bombacopsis (Bombacaceae) in the Guyanas. – Ann. Missouri Bot. Gard. 75: 396-398.
St. John H. 1983. Melochia (Sterculiaceae) of Makatea Island, Tuamotu Archipelago (Pacific plant studies 42). – Syst. Bot. 8: 427-429.
Straka H. 1963. Betrachtungen zur Phylogenie der Sarcolaenaceae (Chlaenaceae). – Ber. Deutsch. Bot. Ges. 76: 55-62.
Straka H. 1964a. Palynologia madagassica et mascarenica. Fam. 126: Sarcolaenaceae (Chlaenaceae). – Pollen Spores 6: 239-301.
Straka H. 1964b. Palynologia madagassica et mascarenica. Errata et addenda. – Pollen Spores 6: 641-643.
Straka H. 1965. Über die Pollenmorpholigie der Gattung Eremolaena (Sarcolaenaceae). – Beitr. Biol. Pflanzen 41: 65-68.
Straka H. 1971. Über das System der madagassichen Sarcolaenaceae. – Ber. Deutsch Bot. Ges. 84: 731-735.
Straka H, Albers F. 1978. Die Pollenmorphologie von Diegodendron humbertii R. Capuron (Diegodendraceae, Ochnales bzw. Theales). – Bot. Jahrb. Syst. 99: 363-369.
Straka H, Friedrich B. 1983. Palynologia madagassica et mascarenica. Fam. 121-127. Microscopie électronique à balayage et addenda. – Pollen Spores 25: 49-73.
Ströhler A. 1997. Frucht- und Samenanatomie der Aquilarioideae und Gonystyloideae. – Thesis, Universität Hamburg, Germany.
Supprian K. 1894. Beiträge zur Kenntnis der Thymelaeaceae und Penaeaceae. – Engl. Bot. Jahrb. Syst. 18: 306-353.
Suzuki E, Ashton PS. 1996. Sepal and nut size ratio of fruits of Asian Dipterocarpaceae and its implications for dispersal. – J. Trop. Ecol. 12: 853-870.
Swarupanandan K. 1986. Late embryogenesis and morphology of mature embryos in three species of Dipterocarpaceae. – Can. J. Bot. 64: 2582-2587.
Symington CF. 1933. Notes on Malayan Dipterocarpaceae I. – Gard. Bull. Straits Settlem. (Singapore) 7: 129-159.
Symington CF. 1934. Notes on Malayan Dipterocarpaceae II. – Gard. Bull. Straits Settlem. (Singapore) 8: 1-40.
Symington CF. 1943. Foresters manual of dipterocarps. – Malayan For. Rec. 16, Caxton, Kuala Lumpur.
Szyszylowicz I von. 1885. Zur Systematik der Tiliaceen I. – Engl. Bot. Jahrb. Syst. 6: 427-457.
Takayama K, Ohi-toma T, Kudoh H, Kato H. 2005. Origin and diversification of Hibiscus glaber, species endemic to the oceanic Bonin Islands, revealed by chloroplast DNA polymorphism. – Mol. Ecol. 14: 1059-1071.
Takeuchi C, Esteves GL. 2012. Synopsis of Abutilon (Malvoideae, Malvaceae) in the state of São Paulo, Brazil. – Phytotaxa 44: 39-57.
Takeuchi C, Kano CH, Tate JA, Esteves GL. 2018. Molecular phylogenetics and character evolution of Gaya and related genera (Malvoideae, Malvaceae). – Syst. Bot. 43: 676-688.
Talavera S, Gibbs PE, Herrera J. 1993. Reproductive biology of Cistus ladanifer (Cistaceae). – Plant Syst. Evol. 186: 123-134.
Talavera S, Gibbs PE, Arista M. 1997. Reproductive biology of Halimium atriplicifolium (Lam.) Spach and H. halimifolium (L.) Willk. (Cistaceae). – Lagascalia 19: 571-578.
Talip N, Greenham J, Cutler DF, Keith-Lucas M. 2008. The utility of leaf flavonoids as taxonomic markers for some Malaysian species of the tribe Shoreae (Dipterocarpaceae). – Bot. J. Linn. Soc. 157: 755-762.
Tan K. 1980a. Studies in the Thymelaeaceae I. Germination, seedlings, fruits, and seeds. – Notes Roy. Bot. Gard. Edinb. 38: 149-164.
Tan K. 1980b. Studies in the Thymelaeaceae II. A revision of the genus Thymelaea. – Notes Roy. Bot. Gard. Edinb. 38: 189-264.
Tang Y. 1990. The systematic position of Corchoropsis Sieb. et Zucc. and Paradombeya Stapf in relation to the delimitation between Tiliaceae and Sterculiaceae. – Ph.D. diss., Institute of Botany, Academica Sinica, Kunming, Peoples Republic of China.
Tang Y. 1992a. On the affinities of Pterospermum Schreb. (Sterculiaceae). – Guihaia 12: 8-14. [In Chinese]
Tang Y. 1992b. The systematic position of Corchoropsis Sieb. et Zucc. – Cathaya 4: 131-150. [In Chinese]
Tang Y. 1992c. A study of Melhania hamiltoniana in relation to the systematic position of the genus. – Acta Bot. Yunnan. 14: 13-20. [In Chinese]
Tang Y. 1993. On the systematic position of Paradombeya Stapf. – Acta Phytotaxon. Sin. 31: 297-308. [In Chinese]
Tang Y. 1998. Floral morphology and embryo sac development in Burretiodendron kydiifolium Y. C. Hsu et R. Zhuge (Tiliaceae) and their systematic significance. – Bot. J. Linn. Soc. 128: 149-158.
Tang Y, Gao X-F. 1993. Pollen morphology of Burretiodendron sensu lato (Tiliaceae) and its systematic significance. – Cathaya 5: 81-88. [In Chinese]
Tang Y, Pan KY. 1994. Gametophytic development of Melhania hamiltoniana Wall. (Sterculiaceae) and its systematic implications. – Cathaya 6: 67-74. [In Chinese]
Tang Y, Zhuge R. 1996. Geographical distribution of Tilia Linn. – Acta Phytotaxon. Sin. 34: 244-264. [In Chinese]
Tang Y, Xie J-S, Gao H. 2005. A study of wood anatomy in Burretiodendron and Excentrodendron and its systematic implications. – Acta Bot. Yunnan. 27: 235-246. [In Chinese]
Tang Y, Xie J-S, Gao H, Sun H. 2005. Tile cells: their occurrence and systematic implications in Malvaceae s.l. – Guihaia 25: 441-446. [In Chinese]
Tang Y, Gao H, Wang C-M, Chen J-Z. 2006. Microsporogenesis and microgametogenesis of Excentrodendron hsienmu (Malvaceae s.l.) and their systematic implications. – Bot. J. Linn. Soc. 150: 447-457.
Tang Y, Gao H, Xie J-S. 2009. An embryological study of Eriolaena candollei Wallich (Malvaceae) and its systematic implications. – Flora 204: 569-580.
Taroda N, Gibbs PE. 1982. Floral biology and breeding system of Sterculia chichi St. Hil. (Sterculiaceae). – New Phytol. 90: 735-743.
Tate JA. 2003. Andeimalva, a new genus of Malvaceae from Andean South America. – Lundellia 6: 10-18.
Tate JA. 2011. The status of Urocarpidium (Malvaceae): insight from nuclear and plastid-based phylogenies. – Taxon 60: 1330-1338.
Tate JA, Simpson BB. 2003. Paraphyly of Tarasa (Malvaceae) and diverse origins of the polyploid species. – Syst. Bot. 28: 723-737.
Tate JA, Aguilar JF, Wagstaff SJ, La Duke JC, Slotta TAB, Simpson BB. 2005. Phylogenetic relationships within tre tribe Malveae (Malvaceae, subfamily Malvoideae) as inferred from ITS sequence data. – Amer. J. Bot. 92: 584-602.
Tawan CS. 1999. A new species of Gonystylus (Thymelaeaceae) from Sarawak, Borneo. – Bot. J. Linn. Soc. 130: 65-68.
Taylor EL. 1989. Systematic studies in the tribe Sterculieae: a taxonomic revision of the neotropical species of Sterculia L. (Sterculiaceae). – Ph.D. diss., Harvard University, Cambridge, Massachusetts.
Terada K, Suzuki M. 1998. Revision of the so-called ‘Reevesia’ fossil woods from the Tertiary in Japan – a proposal of the new genus Wataria (Sterculiaceae). – Rev. Palaeobot. Palynol. 103: 235-251.
Terracino A. 1899. I nettarii estranuziali nelle “Bombacacee”. – Contr. Biol. Veg. Palermo 2(2): 1-55.
Thanos CA, Georghiou K, Kadis C, Pantazi C. 1993. Cistaceae: a plant family with hard seeds. – Israel J. Bot. 41: 251-263.
Thirumalachar MJ, Razi BA. 1941. Megasporogenesis and endosperm formation in Eriodendron anfractuosum DC. – Proc. Indian Acad. Sci., Sect. B, 14: 461-465.
Threlfall S. 1982. The genus Pimelea (Thymelaeaceae) in eastern mainland Australia. – Brunonia 5: 113-201.
Threlfall S. 1984. Pimelea: the eastern mainland species. – Australian Plants 12: 246-258.
Thulin M. 1984. A new species of Helianthemum (Cistaceae) from N. Somalia. – García de Orta, Sér. Bot. 6: 69-72.
Thulin M. 1998. New names in Abutilon and Hibiscus (Malvaceae) from Somalia. – Kew Bull. 53: 1013-1014.
Tieghem PEL van. 1893. Recherches sur la structure et les affinités des Thyméléacées et des Pénéacées. – Ann. Sci. Nat., Bot. VII, 17: 185-294.
Tieghem PEL van. 1900. Sur les Bixacées, les Cochlospermacées et les Sphérosépalacées. – J. Bot. (Morot) 14: 32-54.
Tirel C. 1996. Tiliaceae. – In: Müller IH, Barlow BA, Tirel C, Jérémie J (eds), Flore de la Nouvelle-Calédonie et Dépendances 20, Muséum National d’Histoire Naturelle, Paris, pp. 112-140.
Tirel C, Jérémie J, Lobreau-Callen D. 1996. Corchorus neocaledonicus (Tiliaceae), véritable identité de l’énigmatique Oceanopapaver. – Bull. Mus. Natl. Hist. Nat. Paris, sér. IV, sect. B, Adansonia 18: 35-43.
Toledo VM. 1975. Cheiranthodendron pentadactylon Larreategui (Sterculiaceae): una especie polinizada por aves percheras. – Bol. Soc. Bot. Mexic. 35: 59-67.
Tsukada M. 1964. Pollen morphology and identification III. Modern and fossil tropical pollen with emphasis on Bombacaceae. – Pollen Spores 6: 393-462.
Tsumura Y, Kawahara T, Wickneswari R, Yoshimura K. 1996. Molecular phylogeny of Dipterocarpaceae in Southeast Asia using RFLP of PCR-amplified chloroplast genes. – Theor. Appl. Gen. 93: 22-29.
Tsumura Y, Kado T, Yoshida K, Abe H, Ohtani M, Taguchi Y, Fukue Y, Tani N, Ueno S, Yoshimura K, Kamiya K, Harada K, Takeuchi Y, Diway B, Finkeldey R, Na’iem M, Indrioko S, Ng KK, Muhammad N, Lee SL. 2011. Molecular database for classifying Shorea species (Dipterocarpaceae) and techniques for checking the legitimacy of timber and wood products. – J. Plant Res. 124: 35-48.
Ukraintseva VV. 1993. Pollen morphology of the family Cistaceae in relation to its taxonomy. – Grana [Suppl.] 2: 33-36.
Ulbrich E. 1916. Malvaceae andinae novae vel criticae imprimis Weberbauerianae II. – Engl. Bot. Jahrb. Syst. 54, Beibl. 117: 48-77.
Ulbrich E. 1922. Monographie der afrikanischen Pavonia-Arten nebst Übersicht über die ganze Gattung. – Engl. Bot. Jahrb. Syst. 57: 54-184.
Ulbrich E. 1935. Über eine neue Gattung der Malvaceae Papuasiens Cephalohibiscus Pekeelii Ulbrich nov. gen., n. sp. – Notizbl. Bot. Gart. Mus. Berlin-Dahlem 12: 494-500.
Vaudois-Miéja N. 1988. Un nouveau genre de fruit fossile de Tiliacées des grès à palmiers (Eocène) de l’ouest de France. – Tert. Res. 9: 31-44.
Veldkamp JF, Flipphi RCH. 1987. A revision of Leptonychia (Sterculiaceae) in Southeast Asia. – Blumea 32: 443-457.
Venkata Rao C. 1949a. Floral anatomy of some Sterculiaceae with special reference to the position of stamens. – J. Indian Bot. Soc. 28: 237-245.
Venkata Rao C. 1949b. Contributions to the embryology of Sterculiaceae I. – J. Indian Bot. Soc. 28: 180-197.
Venkata Rao C. 1950a. Pollen grains of Sterculiaceae. – J. Indian Bot. Soc. 29: 130-137.
Venkata Rao C. 1950b. Contributions to the embryology of Sterculiaceae II. Waltheria indica Linn. – J. Indian Bot. Soc. 29: 163-176.
Venkata Rao C. 1951a. Contributions to the embryology of Sterculiaceae 3. – J. Indian Bot. Soc. 30: 122-131.
Venkata Rao C. 1951b. Life history of Muntingia calabura L. – Curr. Sci. 20: 47-48.
Venkata Rao C. 1952a. The embryology of Muntingia calabura L. – J. Indian Bot. Soc. 31: 87-101.
Venkata Rao C. 1952b. Floral anatomy of some Malvales and its bearing on the affinities of families included in the order. – J. Indian Bot. Soc. 31: 171-203.
Venkata Rao C. 1952c. Contributions to the embryology of Sterculiaceae IV. Development of the gametophytes in Pterospermum suberifolium Lam. – J. Indian Bot. Soc. 31: 251-260.
Venkata Rao C. 1953. Contributions to the embryology of Sterculiaceae V. – J. Indian Bot. Soc. 32: 208-238.
Venkata Rao C. 1954a. A contribution to the embryology of Bombacaceae. – Proc. Indian Natl. Inst. Sci. Acad., Sect. B, 39: 51-75.
Venkata Rao C. 1954b. Embryological studies in Malvaceae I. Development of gametophytes. – Proc. Indian Natl. Inst. Sci., Sect. B, 20: 127-150.
Venkata Rao C. 1955. Embryological studies in Malvaceae II. Fertilization and seed development. – Proc. Indian Natl. Inst. Sci., Sect. B, 21: 53-67.
Venkata Rao C, Sambasiva Rao KV. 1952. A contribution to the embryology of Triumfetta rhomboidea Jacq. and Corchorus acutangulus L. – J. Indian Bot. Soc. 31: 56-68.
Venkatesh CS. 1956. The curious anther of Bixa – its structure and dehiscence. – Amer. Midl. Natur. 55: 473-476.
Venkatesvarlu J. 1945. Embryological studies in the Thymelaeaceae I. Thymelaea arvensis. – J. Indian Bot. Soc. 24: 45-66.
Venkatesvarlu J. 1946. A case of polyembryony in Daphne cannabina Wall. – Curr. Sci. 15: 169.
Venkatesvarlu J. 1947. Embryological studies in the Thymelaeaceae II. Daphne cannabina Wall. and Wikstroemia canescens Meissn. – J. Indian Bot. Soc. 26: 13-39.
Venturieri GA, Silva MB. 1997. Fenologia floral do Cacaujacaré (Herrania mariae) – Sterculiaceae. – Bol. Mus. E. Goeldi, Bot. 13: 31-47.
Verdcourt B. 1975. Cochlospermaceae. – In: Polhill RM (ed), Flora of tropical East Africa, Crown Agents for Oversea Governments and Administrations, London, pp. 1-3.
Verdcourt B. 1989. Dipterocarpaceae. – In: Polhill RM (ed), Flora of tropical East Africa, A. A. Balkema, Rotterdam, pp. 1-11.
Vischer W. 1919 [1920]. Sur les Quararibea Aubl., un genre de Bombacées à ovaire infère. – Bull. Soc. Bot. Genève, sér. II, 11: 199-210.
Vogel S. 2000. The floral nectaries of Malvaceae sensu lato – a conspectus. – Kurtziana 28: 155-171.
Vollesen K. 1986a. The Sida cuneifolia-complex (Malvaceae) in Africa. – Kew Bull. 41: 91-98.
Vollesen K. 1986b. Aethiocarpa (Sterculiaceae-Dombeyeae), a new genus from Somalia. – Kew Bull. 41: 959-961.
Vollesen K. 1987. The native species of Gossypium (Malvaceae) in Africa, Arabia and Pakistan. – Kew Bull. 42: 337-349.
Waddle B. 1970. The nectaries of cotton. – Ark. Univ. Ext. Misc. Publ. 127: 25-27.
Wagner R. 1915. Über die Sympodienbildung von Octolepis dinklagei Gilg. – Österr. Bot. Zeitschr. 65: 297-304.
Warburg O. 1894. Flacourtiaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(6a), W. Engelmann, Leipzig, pp. 1-56.
Warburg O. 1895. Bixaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(6), W. Engelmann, Leipzig, pp. 307-314.
Webb CJ. 1984. Flower and fruit movements in Muntingia calabura: a possible mechanism for avoidance of pollinator-disperser interference. – Biotropica 16: 37-42.
Webber IE. 1934. Systematic anatomy of the woods of the Malvaceae. – Trop. Woods 38: 15-36.
Webber JM. 1936. Chromosomes in Sphaeralcea and related genera. – Cytologia 7: 313-323.
Weberling F, Herkommer U. 1989. Untersuchungen zur Infloreszenzmorphologie der Thymelaeaceae. – Akad. Wiss. Abh. Math.-Naturwiss. Kl. 68: 1-124.
Weibel R. 1945. La placentation chez les Tiliacées. – Candollea 10: 155-177.
Wendel JF. 1989. New World tetraploid cottons contain Old World cytoplasm. – Proc. Natl. Acad. Sci. U.S.A. 86: 4132-4136.
Wendel JF, Albert VA. 1992. Phylogenetics of the cotton genus (Gossypium): character-state weighted parsimony analysis of chloroplast-DNA restriction site data and its systematic and biogeographic implications. – Syst. Bot. 17: 115-143.
Wendel JF, Percival AE. 1990. Molecular divergence in the Galapagos Islands-Baja California species pair Gossypium klotzschianum and G. davidsonii (Malvaceae). – Plant Syst. Evol. 171: 99-115.
Wendel JF, Stewart JM, Rettig JH. 1991. Molecular evidence for homoploid reticulate evolution among Australian species of Gossypium. – Evolution 45: 694-711.
Wendel JF, Schnabel A, Seelanan T. 1995a. Bidirectional interlocus concerted evolution following allopolyploid speciation in cotton (Gossypium). – Proc. Natl. Acad. Sci. U.S.A. 92: 280-284.
Wendel JF, Schnabel A, Seelanan T. 1995b. An unusual ribosomal DNA sequence from Gossypium gossypioides reveals ancient, cryptic, intergenomic introgression. – Mol. Phylogen. Evol. 4: 298-313.
West WC, Gunkel JE, Johnson MA. 1970. Morphology of the shoot apex in Elaeocarpaceae. – Phytomorphology 20: 58-67.
Westerkamp C, Aparecida Soares A, Amaral Neto LP do. 2006. Male and female booths with separate entrances in the tiny flowers of Guazuma ulmifolia (Malvaceae-Byttnerioideae) I. Structural integration. – Flora 201: 389-395.
Wheeler EA, Lehman TM, Gasson P. 1994. Javelinoxylon, a new genus of malvalean tree from the Upper Cretaceous of Big Bend National Park, Texas. – Amer. J. Bot. 1: 703-710.
White CT. 1946. Papuodendron, a new genus of arborescent Malvaceae from New Guinea. – J. Arnold Arbor. 27: 272-273.
Whitlock BA, Baum DA. 1999. Phylogenetic relationships of Theobroma and Herrania (Sterculiaceae) based on sequences of the nuclear gene Vicilin. – Syst. Bot. 24: 128-138.
Whitlock BA, Hale AM. 2011. The phylogeny of Ayenia, Byttneria, and Rayleya (Malvaceae s.l.) and its implications for the evolution of growth forms. – Syst. Bot. 36: 129-136.
Whitlock BA, Bayer C, Baum DA. 2001. Phylogenetic relationships and floral evolution of the Byttnerioideae (”Sterculiaceae“ or Malvaceae s.l.) based on sequences of the chloroplast gene, ndhF. – Syst. Bot. 26: 420-437.
Whitlock BA, Karol KG, Alverson WS. 2003. Chloroplast DNA sequences confirm the placement of the enigmatic Oceanopapaver within Corchorus (Grewioideae: Malvaceae s.l., formerly Tiliaceae). – Intern. J. Plant Sci. 164: 35-41.
Whitlock BA, Hale AM, Indorf JL, Wilkins CF. 2011. Polyphyly of Rulingia and Commersonia (Lasiopetaleae, Malvaceae s.l.). – Aust. Syst. Bot. 24: 215-225.
Whitmore TC. 1962a. Studies in systematic bark morphology I. Bark morphology in Dipterocarpaceae. – New Phytol. 61: 191-207.
Whitmore TC. 1962b. Studies of systematic bark morphology II. General features of bark construction in Dipterocarpaceae. – New Phytol. 61: 208-220.
Whitmore TC. 1962c. Studies in systematic bark morphology III. Bark taxonomy in Dipterocarpaceae. – Gard. Bull. (Singapore) 19: 321-371.
Wickens GE. 1982. The baobab – Africa’s upside-down tree. – Kew Bull. 37: 173-209.
Wiggins IL. 1936. A resurrection and revision of the genus Iliamna Greene. – Contr. Dudley Herb. 1: 213-229.
Wilbur RL, Perry JD. 1967. Palynological notes on American species of Helianthemum (Cistaceae). – Rhodora 69: 184-194.
Wild H. 1961. 29. Bombacaceae; 30. Sterculiaceae. – In: Exell AW, Wild H (eds), Flora Zambesiaca 1 (Part 2), Crown Agents for Oversea Governments and Administrations, London, pp. 511-564.
Wild H. 1963. 31. Tiliaceae. – In: Exell AW, Fernandes A, Wild H (eds), Flora Zambesiaca 2 (Part 1), Crown Agents for Oversea Governments and Administrations, London, pp. 33-91.
Wilkie P, Clark A, Pennington RT, Cheek M, Bayer C, Wilcock CC. 2006. Phylogenetic relationships within the subfamily Sterculioideae (Malvaceae/Sterculiaceae-Sterculieae) using the chloroplast gene ndhF. – Syst. Bot. 31: 160-170.
Wilkins CF. 2002. A systematic study of Lasiopetaleae (Malvaceae s.l. or Sterculiaceae s.l.. – Ph.D. diss., the University of Western Australia, Perth.
Wilkins CF, Chappill JA. 2001. Taxonomic revision of Hannafordia (Lasiopetaleae: Sterculiaceae (Malvaceae s.l.)). – Aust. Syst. Bot. 14: 101-124.
Wilkins CF, Chappill JA. 2002a. New chromosome numbers for Lasiopetaleae: Malvaceae s.l. (or Sterculiaceae). – Aust. Syst. Bot. 15: 1-8.
Wilkins CF, Chappill JA. 2002b. Seed and seedling morphology, and seed anatomy of Lasiopetaleae (Malvaceae s.l. or Sterculiaceae). – Aust. Syst. Bot. 15: 545-563.
Wilkins CF, Chappill JA. 2003. Taxonomic revision of Guichenotia (Lasiopetaleae: Malvaceae s.l. or Sterculiaceae). – Aust. Syst. Bot. 16: 323-360.
Wilkins CF, Whitlock BA. 2011a. A revision of Commersonia including Rulingia (Malvaceae s.l. or Byttneriaceae). – Aust. Syst. Bot. 24: 226-283.
Wilkins CF, Whitlock BA. 2011b. A new Australian genus, Androcalva, separated from Commersonia (Malvaceae s.l. or Byttneriaceae). – Aust. Syst. Bot. 24: 284-349.
Wilkins CF, Whitlock BA. 2016. Seringia revised to include Keraudrenia (Lasiopetaleae: Malvaceae s.l.). – Aust. Syst. Bot. 28: 265-325.
Wilkins CF, Copeland LM, Whitlock BA. 2008. Two new species of Commersonia (Malvaceae sensu lato) from south-eastern Australia. – Telopea 12: 59-69.
Williams LO, Standley PC. 1952. Pentaplaris, a new genus of Tiliaceae from Costa Rica. – Ceiba 3: 139-142.
Williams RC. 1962. The comparative anatomy and morphology of the Bixaceae. – M.Sc. thesis, University of Cincinnati, Ohio.
Wilson FD. 1999. Revision of Hibiscus section Furcaria (Malvaceae) in Africa and Asia. – Bull. Nat. Hist. Mus. London, Bot. 29: 47-79.
Wojciechowska B. 1969. Seed morphology and anatomy of some Helianthemum species. – Monogr. Bot. 29: 121-135.
Wood JRI. 1984. Eight new species and taxonomic notes on the flora of Yemen. – Kew Bull. 39: 123-139.
Woon C, Keng H. 1979. Observations on stamens of the Dipterocarpaceae. – Gard. Bull. (Singapore) 32: 1-55.
Yen T-K. 1932. Carpel dehiscence in Firmiana simplex. – Bot. Gaz. 93: 205-212.
Young AM.1989. Comparative attractiveness of floral fragrance oils of “RIM” and “Catongo” cultivars of cacao (Theobroma cacao L.) to Diptera in a CostaRican cacao plantation. – Turrialba 39: 137-142.
Young AM, Schaller M, Strand M. 1984. Floral nectaries and trichomes in relation to pollination in some species of Theobroma and Herrania. – Amer. J. Bot. 1: 466-480.
Young AM, Erickson EH Jr Strand MA, Erickson BJ. 1987. Pollination biology of Theobroma and Herrania (Sterculiaceae) I. Floral biology. – Insect Sci. Applic. 8: 151-164.
Yulita KS, Bayer RJ, West JG. 2005. Molecular phylogenetic study of Hopea and Shorea (Dipterocarpaceae): evidence from the trnL-trnF and internal transcribed spacer regions. – Plant Species Biol. 20: 167-182.
Zaborsky JG. 2009. Hildegardia dauphinensis (Malvaceae, Sterculioideae): a new species from southeastern Madagascar. – Adansonia, sér. III, 31: 143-148.
Zavada MS, Lowrey TK. 1995. Floral heteromorphism in Dais cotinifolia L. (Thymelaeaceae): a possible case of heterostyly. – Bull. Mus. Natl. Hist. Nat., Sect. B, sér. IV, Adansonia 17: 11-20.
Zebe V. 1915. Monographie der Sterculiaceen-Gattungen Kleinhovia, Helicteres, Reevesia, Ungeria und Pterospermum. Allgemeiner Teil. – Ph.D. diss., Friedrich-Wilhelm-Universität, Breslau, Poland.
Zeeuw C de. 1977. Pakaraimoideae: Dipterocarpaceae of the Western Hemisphere III. Stem anatomy. – Taxon 26: 368-380.
Zhang Y, Sun H, Boufford DE. 2007. Two new species of Wikstroemia (Thymelaeaceae) from western Sichuan, China. – Rhodora 109: 448-455.
Zhuge R. 1989. The cladistic analysis of the systematic position of Craigia. – Acta Bot. Yunnan. 11: 17-23. [In Chinese]
Zhuge R. 1990. On the genus Burretiodendron sensu lato (Tiliaceae). – J. Arnold Arbor. 71: 371-380.
Zietsman PC. 1991. Reproductive biology of Grewia occidentalis L. (Tiliaceae). – South Afr. J. Bot. 57: 348-351.