”The Nitrogen Fixing
clade”
[Polygalales+[Rosales+[Cucurbitales+Juglandales]]]
POLYGALALES Juss. ex Bercht. et
J. Presl
Berchtold et Presl, Přir. Rostlin: 228. Jan-Apr
1820 [‘Polygaleae’]
Habit Usually bisexual (rarely
monoecious, andromonoecious or polygamomonoecious), evergreen or deciduous
trees, shrubs, lianas or suffrutices, perennial, biennial or annual herbs.
Vegetative anatomy Root
nodules containing nitrogen fixing bacteria usually present. Phellogen ab
initio superficially or deeply seated. Primary vascular tissue cylinder without
separate vascular bundles, or cylinder of bundles. Secondary lateral growth
normal or anomalous from concentric cambia, or absent. Vessel elements usually
with simple (rarely scalariform) perforation plates; lateral pits alternate,
simple or bordered pits. Vestured pits present. Imperforate tracheary xylem
elements libriform fibres or tracheids (sometimes fibre tracheids) with usually
simple (sometimes bordered) pits, septate or non-septate (also vasicentric
tracheids). Wood rays uniseriate or multiseriate, homocellular or
heterocellular. Axial parenchyma apotracheal diffuse or diffuse-in-aggregates,
or paratracheal scanty, aliform, lozenge-aliform, winged-aliform, confluent,
reticulate, unilateral, vasicentric, or banded. Sieve tube plastids Ss, Pc or
Pcs type (rarely Pfs type). Nodes 1:1 or 1:3, unilacunar with one or three leaf
traces, or 3:3, trilacunar with three traces (sometimes 5:5, pentalacunar with
five traces). Secretory cavities often present. Heartwood often with resins or
resin-like substances. Silica bodies present or absent. Calciumoxalate as
prismatic or acicular crystals, druses, styloids, or crystal sand.
Trichomes Hairs unicellular or
multicellular, uniseriate or multiseriate, simple or branched, furcate or
stellate, sometimes dendritic; pearl glands, stalked multicellular glands
and/or glandular hairs (sometimes lepidote) often present; stinging hairs
rare.
Leaves Usually alternate
(spiral or distichous; rarely opposite or verticillate), paripinnate,
imparipinnate or trifoliolate (sometimes bipinnate or multifoliolate, rarely
unifoliolate), usually with entire (rarely lobate or serrate) opposite
conduplicate leaflets (rarely alternate), or scales, simple, entire, with
conduplicate ptyxis. Stipules intrapetiolar, often foliaceous or modified into
spines or glands, caducous or persistent; leaf sheath absent. Petiole vascular
bundle transection arcuate or annular. Venation pinnate, usually
brochidodromous, or palmate. Stomata anomocytic, paracytic, anisocytic,
tetracytic, parallelocytic or cyclocytic. Cuticular wax crystalloids usually as
rosettes of platelets (Fabales type; sometimes as scales or granules).
Epidermis with or without mucilaginous idioblasts. Mesophyll often with
sclerenchymatous idioblasts (with fibres or branched sclereids). Secretory
cavities with ethereal oils, mucilage or resin often present. Leaf margin and
leaflet margins serrate or entire.
Inflorescence Terminal or
axillary, corymb, panicle, botryoid, raceme, spike, head, or flowers solitary
axillary.
Flowers Usually zygomorphic
(sometimes actinomorphic). Hypogyny. Sepals (three to) five (or six), with
valvate or imbricate aestivation, free or more or less connate, with helical
initiation. Petals (one to) three or five (rarely four), with valvate,
imbricate or cochlear-descending aestivation, often clawed, free or more or
less connate. Nectariferous disc intrastaminal, annular or unilateral, or
absent.
Androecium Stamens (two or)
five to ten (to numerous), in one or two (or three) whorls. Filaments free or
more or less connate into tube, usually free from tepals (sometimes
epipetalous). Anthers basifixed or dorsifixed, versatile or non-versatile,
usually tetrasporangiate (rarely disporangiate), introrse or latrorse, usually
longicidal (dehiscing by longitudinal slits) or poricidal (dehiscing by apical
or subapical pores or very short slits). Tapetum usually secretory. Staminodia
usually absent (sometimes four or five or more).
Pollen grains
Microsporogenesis simultaneous. Pollen grains (2–)3–23(–33)-colporate
(sometimes porate, colporoidate, colpate, rugate or inaperturate, rarely
syncolporate), usually shed as monads (sometimes tetrads or polyads), usually
bicellular (sometimes tricellular) at dispersal. Exine tectate or semitectate,
with usually columellate infratectum, perforate, microreticulate, reticulate,
punctate, striate, or rugulate, gemmate, verrucate, granulate, fossulate,
foveolate or psilate.
Gynoecium Pistil composed of
usually one carpel (sometimes two to 16 free carpels), or two to eight connate
(rarely entirely or partially free) carpels. Ovary superior, usually unilocular
(monomerous) or bilocular to quinquelocular. Style single, simple or bilobate,
often hollow (rarely five free stylodia). Stigma capitate or bilobate
(sometimes complex), papillate or non-papillate, Dry or Wet type. Pistillodium
usually absent (male flowers sometimes with pistillodium).
Ovules Placentation
marginal-ventral, axile or parietal. Ovules one to more than 20 per carpel,
usually anatropous, anacampylotropous or campylotropous (sometimes
hemianatropous or amphitropous, rarely pleurotropous), pendulous, horizontal or
ascending, epitropous, usually bitegmic (rarely unitegmic), usually
crassinucellar (rarely tenuinucellar). Micropyle bistomal or endostomal (rarely
exostomal). Nucellar cap sometimes present. Megagametophyte usually
monosporous, Polygonum type (rarely disporous, Allium type).
Synergids often with a filiform apparatus. Endosperm development ab initio
nuclear. Endosperm haustorium usually chalazal (sometimes also micropylar;
rarely lateral) or absent. Embryogenesis onagrad, asterad or caryophyllad.
Fruit Usually a loculicidal
capsule (often a legume; sometimes a nut, samara, drupe, berry, follicle,
schizocarp, or syncarp).
Seeds Aril often present.
Elaiosome or carunculus sometimes present. Seed coat exotestal or endotestal.
Testa sometimes multiplicative. Exotesta usually palisade (often with lignified
malpighian cells, polygonal in cross-section, with linea lucida (light line)
separating heavily thickened outer anticlinal cell walls). Mesotesta consisting
of stellate cells or crushed. Endotestal cells palisade, crystalliferous, or
crushed. Tegmen usually crushed. Perisperm not developed. Endosperm absent or
sparse (sometimes copious). Embryo straight or curved, well differentiated,
usually with chlorophyll. Cotyledons two, often well developed and nutritious.
Germination phanerocotylar or cryptocotylar.
Cytology x = 5–7 (11)
DNA Plastid gene infA
lost/defunct. Mitochondrial intron coxII.i3 lost.
Phytochemistry Flavonols
(kaempferol, quercetin, myricetin), flavone-C-glycosides, afzelechin,
5-deoxyflavonoids, isoflavonoids (pterocarpanes, isoflavanes), cyanidin,
epigallocatechin-3-gallate, oleanolic acid derivatives, dammarane, surianol,
methylated ellagic acids, ellagitannins (e.g. geraniin), proanthocyanidins
(prodelphinidins), pyridine alkaloids, quinolizidine alkaloids, indole
alkaloids, loline alkaloids, pyrrolizidine alkaloids as macrocyclic diesters,
triterpene saponins, tyrosine-, leucine- or phenylalanine-derived cyanogenic
compounds, anthraquinones, pinitol (cyclitol), simmondsin-like compounds,
lignans (syringaresinol), toxic non-protein amino acids, and lectins
(hemagglutinins, especially in seeds) present. Non-methylated ellagic acids not
found.
Systematics Polygalales are sister-group to
the clade [Rosales+[Cucurbitales+Juglandales]]
(Soltis & al. 2011), or the clade [Cucurbitales+Juglandales] (Wang
& al. 2009) or to Rosales (Magallón &
Castillo 2009).
The sister-group relationships within Polygalales are not
unambiguously resolved and the bootstrap or bayesian support for any branch is
more or less weak. Hence, Fabaceae, Polygalaceae, Quillajaceae or Surianaceae may be identified as
sister to the remainder, depending on the characters and analysis methods
used.
Bello & al. (2007, 2009) found the
following topologies based on matK, rbcL or combined
matK and rbcL, respectively: [Polygalaceae+[Fabaceae+[Quillajaceae+Surianaceae]]], [Fabaceae+[Quillajaceae+[Polygalaceae+Surianaceae]]] and [Quillajaceae+[Surianaceae+ [Fabaceae+Polygalaceae]]]. Persson (2001)
received the topology [Polygalaceae+[Surianaceae+ [Fabaceae+Quillajaceae]]]. Qiu & al.
(2010) found the following topology: [Quillajaceae+ [Fabaceae+[Polygalaceae+Surianaceae]]]. Soltis & al.
(2011) received [[Quillajaceae+Polygalaceae]+[Fabaceae+Surianaceae]]. Finally, Moore
& al. (2011) found [Surianaceae+ [Quillajaceae+[Fabaceae+Polygalaceae]]].
Lindley in Edwards’s Bot. Reg. 22: ad t. 1845.
1 Apr 1836 [‘Leguminosae, or Fabaceae’], nom. cons.
LeguminosaeJuss.,
Gen. Plant.: 345. 4 Aug 1789, nom. cons. et nom. alt.;
PapilionaceaeGiseke, Prael. Ord. Nat. Plant.: 415.
Apr 1792, nom. cons. et nom. alt.; CaesalpiniaceaeR.
Br. in M. Flinders, Voy. Terra Austral. 2: 551. 19 Jun 1814
[’Lomentaceaevel Caesalpineae’], nom. cons.;
MimosaceaeR. Br. in M. Flinders, Voy. Terra Austral.
2: 551. 19 Jul 1814 [’Mimoseae’], nom. cons.;
CassiaceaeVest, Anleit. Stud. Bot.: 270, 291. 1818
[’Cassioideae’]; RobiniaceaeVest,
Anleit. Stud. Bot.: 270, 291. 1818 [’Robinioideae’];
AspalathaceaeMartinov, Tekhno-Bot. Slovar: 51. 3 Aug
1820 [’Aspalathoides’];
AstragalaceaeBercht. et J. Presl, Přir. Rostlin:
229. Jan-Apr 1820 [’Astragalides’];
BauhiniaceaeMartinov, Tekhno-Bot. Slovar: 67. 3 Aug
1820 [’Bauhineae’];
CoronillaceaeMartinov, Tekhno-Bot. Slovar: 162. 3 Aug
1820 [’Coronillae’]; CytisaceaeBercht.
et J. Presl, Přir. Rostlin: 229. Jan-Apr 1820 [‘Citiseae’];
DaleaceaeBercht. et J. Presl, Přir. Rostlin: 230.
Jan-Apr 1820 [‘Daleae’];
GaledupaceaeMartinov, Tekhno-Bot. Slovar: 277. 3 Aug
1820 [’Galedupeae’]; HedysaraceaeBercht.
et J. Presl, Přir. Rostlin: 230. Jan-Apr 1820 [‘Hedysareae’];
SophoraceaeBercht. et J. Presl, Přir. Rostlin: 229.
Jan-Apr 1820; TamarindaceaeMartinov, Tekhno-Bot.
Slovar: 622. 3 Aug 1820 [‘Tamarindi’];
TamarindalesBercht. et J. Presl, Přir. Rostlin: 230.
Jan-Apr 1820 [‘Tamaryndeae’];
TrifoliaceaeBercht. et J. Presl, Přir. Rostlin: 230.
Jan-Apr 1820 [‘Trifoliae’];
VicialesBercht. et J. Presl, Přir. Rostlin: 229.
Jan-Apr 1820 [‘Viciae’]; LotaceaeOken,
Lehrb. Naturgesch. 2(2.2): xv, 727. 1826 [’Loteae’];
ViciaceaeOken, Lehrb. Naturgesch. 2(2.2): xv. 1826
[’Vicieae’]; CassialesLink, Handbuch 2:
135. 4-11 Jul 1829 [’Cassiaceae’];
CeratoniaceaeLink, Handbuch 2: 135. 4-11 Jul 1829;
MimosalesLink, Handbuch 2: 131. 4-11 Jul 1829
[‘Mimoseae’]; Swartziaceae(DC.) Bartl.,
Ord. Nat. Plant.: 231, 413. Sep 1830 [’Swartzieae’];
Detariaceae(DC.) J. Hess, Übers. Phan. Nat.
Pfl.-Fam.: 46. 1832 [’Detarieae’];
Dalbergiaceae(DC.) Mart., Consp. Regn. Veg.: 34.
Sep-Oct 1835; Geoffroeaceae(DC.) Mart., Consp. Regn.
Veg.: 34. Sep-Oct 1835 [‘Geoffroeae’];
LathyraceaeBurnett, Outl. Bot.: 660, 1092, 1138. Feb
1835; LotalesBurnett, Outl. Bot.: 1138. Jun 1835
[‘Lotianae’], nom. illeg.;
Phaseolaceae(DC.) Mart., Consp. Regn. Veg.: 34.
Sep-Oct 1835 [‘Phaseoleae’];
AcaciaceaeE. Mey., Comm. Plant. Afr. Austr. 1: 164.
14 Feb-5 Jun 1836 [‘Acacieae’];
FabalesBromhead in Edinburgh New Philos. J. 25: 126.
Jul 1838; CiceraceaeSteele, Handb. Field Bot.: xx,
63. 1847 [’Cicereaevel Leguminosae’];
InocarpaceaeZoll., Syst. Verz. 2: 117. 1854-1855
[’Inocarpeae’]; FabanaeR. Dahlgren ex
Reveal in Phytologia 74: 179. 25 Mar 1993
Genera/species
717/19.555–>20.170
Distribution Cosmopolitan
except Antarctica.
Fossils Pollen grains are
known from the Paleocene and the Eocene of Europe, Africa and Texas. Late
Cretaceous fossils of Fabaceae are questionable.
Habit Usually bisexual (rarely
monoecious, andromonoecious or polygamomonoecious), evergreen or deciduous
trees, shrubs, lianas or suffrutices, perennial, biennial or annual herbs.
Numerous species are xerophytes, whereas some are aquatic. Petiole or branch
sometimes modified into photosynthesizing phyllodia or phyllocladia,
respectively. Many species are spiny. Sometimes with large plank buttresses.
Vegetative anatomy Mycorrhiza
sometimes absent. Root nodules (usually originating in cortex, sometimes as
modified lateral roots) containing nitrogen-fixing bacteria
(Azorhizobium, Bradyrhizobium, Rhizobium, and
Sinorhizobium) present in most species. Nodules usually with bacterial
colonies in centre and with vascular tissue in peripheral parts. Nodule-forming
nitrogen-fixing bacteria usually α-2-proteobacteria (in some Mimoseae
and Papilionoideae also certain β-proteobacteria). Infection threads
permanent or absent, nodules long-lived or short-lived. Nitrogen metabolism
usually very specific: free amino acids frequent and nitrogen in xylem juice
transported as mixture of different amino acids, amids and sometimes also
ureids (very small amount transported as nitrate). Ectomycorrhiza present in
many (perhaps especially in non-nodulated?) species (i.a. in
Cynometreae and Detarieae). Phellogen ab initio superficially
or deeply seated (in Papilionoideae sometimes outer-cortical). Primary
vascular tissue cylinder without separate vascular bundles, or ring of bundles.
Endodermis in Cercis conspicuous. Secondary lateral growth normal or
anomalous from concentric cambia or absent. Cambium and wood elements sometimes
storied. Vessel elements with simple perforation plates; lateral pits
alternate, bordered pits. Vestured pits often present. Imperforate tracheary
xylem element libriform fibres with usually simple (sometimes bordered) pits,
septate or non-septate (also vasicentric tracheids). Wood rays uniseriate or
multiseriate, homocellular or heterocellular. Axial parenchyma usually
abundant, apotracheal diffuse or diffuse-in-aggregates, or paratracheal scanty,
aliform, lozenge-aliform, winged-aliform, confluent, reticulate, vasicentric,
unilateral, or banded. Secondary phloem often stratified into hard fibrous
layers alternating with soft parenchymatous layers. Sieve tube plastids usually
Pcs type (in Mimoseae Pc type) with protein crystalloids (rarely Ss
type, with starch and proteins or only starch). Nodes usually 3:3, trilacunar
with three leaf traces (sometimes 5:5, pentalacunar with five traces).
Secretory cells and cavities present or absent. Heartwood often with resins and
resin-like substances. Silica bodies present or absent. Prismatic
calciumoxalate crystals and styloids abundant (especially in axial parenchyma),
druses, navicular, acicular (organic) and spherical crystals sometimes present
as well as crystal sand.
Trichomes Hairs unicellular or
multicellular (usually tricellular), uniseriate or multiseriate, simple or
branched, furcate or stellate, sometimes dendritic; pearl glands (pearl
bodies), stalked multicellular glands and/or glandular hairs (sometimes
lepidote) often present; microhairs sometimes present; stinging hairs rare.
Leaves Usually alternate
(spiral or distichous; rarely opposite), usually pinnately or palmately
compound (paripinnate, imparipinnate or trifoliolate, sometimes bipinnate or
multifoliolate, rarely unifoliolate or simple), usually with entire (rarely
lobed or serrate) opposite conduplicate leaflets (often pulvinate, rarely
alternate) usually with entire margins, sometimes with foliar tendrils,
sometimes with stipulules, sometimes coriaceous, sometimes reduced and
scale-like, usually with conduplicate ptyxis? Stipules intrapetiolar, often
foliaceous or modified into spines, scales or glands (extrafloral nectaries?),
caducous or persistent; leaf sheath absent. Colleters often frequent. Petiole
vascular bundles of various shape. Leaflets often pulvinate. Stipulules
sometimes present. Venation pinnate or palmate. Stomata anomocytic, paracytic,
anisocytic, tetracytic, parallelocytic, or cyclocytic. Cuticular wax
crystalloids as rosettes of platelets (Fabales type), scales or granules.
Epidermis with or without mucilaginous idioblasts. Mesophyll with or without
sclerenchymatous idioblasts (with fibres or branched sclereids). Secretory
cavities with oils, mucilage or resin present or absent. Silica sometimes
frequent. Leaf and leaflet margins serrate or entire. Extrafloral nectaries
often present on stipules, petiole or lamina.
Inflorescence Terminal or
axillary, racemose, corymb, panicle, fascicle, raceme, spike, or head, or
flowers solitary axillary. Lateral inflorescence branches sometimes with
extrafloral nectaries. Floral prophylls (bracteoles) in Papilionoideae
sometimes suppressed.
Flowers Usually zygomorphic
(in Mimosoideae actinomorphic; actinomorphic flowers in Cadia
is a reversal, due to dorsalization of flower). Hypanthium often well
developed. Hypogyny. Sepals (three to) five (or six), with valvate or imbricate
(in Schwartzieae irregular) aestivation, usually persistent, free or
more or less connate (rarely strongly reduced); median sepal abaxial. Petals
usually five (rarely one, three or four), with valvate (Mimoseae),
imbricate, imbricate-ascending or cochlear-descending (adaxial-median petal
internal) aestivation, often clawed, persistent or caducous, free or two front
petals connate at base into carina (especially in Papilionoideae,
sometimes four petals connate) or all petals connate (especially in
Mimoseae); median petal adaxial (vexillum in Papilionoideae;
lateral petals alae in Papilionoideae; median petal sometimes absent.
Nectariferous disc intrastaminal or absent. Development of floral parts largely
centripetalous; gynoecium initiated prior to petals.
Androecium Stamens usually ten
(sometimes two to nine; in Mimoseae sometimes numerous, in
Maniltoa to more than 100), usually in one whorl (at initiation
usually in two whorls, diplostemonous; sometimes in three to six? whorls),
unidirectionally initiated. Filaments free or more or less connate into tube
(often with one adaxial stamen free), usually free from (sometimes adnate to)
petals. Anthers basifixed and/or dorsifixed, usually versatile,
tetrasporangiate, introrse or latrorse, usually longicidal (dehiscing by
longitudinal slits; in some Cassieae poricidal, with apical pores);
connective sometimes with caducous apical gland; placentoid sometimes present.
Tapetum usually secretory, with uninucleate or quadrinucleate (to
septanucleate) cells. Staminodia sometimes five or more.
Pollen grains
Microsporogenesis simultaneous. Pollen grains (2–)3(–6)-colporate
(sometimes 2–6-porate, -colpate, -rugate or inaperturate; sometimes with
pseudocolpi), usually shed as monads (sometimes tetrads or polyads with eight,
18 or more pollen grains, especially in Mimoseae), usually bicellular
(in some Mimoseae tricellular) at dispersal. Exine tectate or
semitectate, with columellate or acolumellate? (granular?) infratectum,
perforate, microreticulate, reticulate or striate-reticulate to striate,
rugulate, verrucate or psilate.
Gynoecium Pistil composed of
usually one carpel (in some Mimoseae two to 16 free carpels,
[apocarpy]), often on gynophore. Ovary superior, usually unilocular
(monomerous; rarely appearing bilocular by secondary septum), sometimes
stipitate. Style single, simple, usually curved upwards, often hollow. Stigma
capitate (sometimes widened), sometimes hollow, often papillate, Dry or Wet
type. Male flowers sometimes with pistillodium? Enantiostyly present in some
species (flowers then asymmetric).
Ovules Placentation
marginal-ventral (along abaxial suture). Ovules (one or) two to numerous per
carpel, usually anatropous, anacampylotropous or campylotropous (sometimes
hemianatropous or amphitropous), ascending to pendulous, usually bitegmic
(rarely unitegmic), usually crassinucellar (rarely incompletely tenuinucellar).
Funicle often long or stout. Micropyle endostomal or bistomal, often Z-shaped
(zig-zag). Outer integument two to ten cell layers thick, with vascular strand.
Inner integument two or three cell layers thick. Parietal tissue one or two
cell layers thick. Nucellar cap approx. three cell layers thick. Archespore
often multicellular. Megagametophyte usually monosporous, Polygonum
type (rarely disporous, Allium type). Synergids often with a filiform
apparatus. Porogamy. Endosperm development ab initio nuclear. Endosperm
haustorium usually chalazal (sometimes also micropylar; rarely
[Adesmieae] lateral; sometimes elongate). Embryogenesis onagrad,
asterad or caryophyllad.
Fruit A legume (in some genera
modified into nut, drupe, berry, abaxially dehiscing follicle, or lomentum, in
some species of Mimoseae a syncarp), sometimes with accrescent
calyx.
Seeds Aril often present.
Funicle often thick and fleshy (arilloid). Elaiosome sometimes present. Seed
usually without hilar sulcus. Seed coat usually exotestal (sometimes
indistinct). Testa sometimes undefined or ruminate. Exotesta often with
palisade, more or less lignified Malpighian cells, polygonal in cross-section,
often with pleurogram with deeply situated linea lucida (light line, separating
very thick outer anticlinal cell walls from thin inner cell walls) and rounded
linea fissura demarcating pleurogram. Mesotesta consisting of stellate cells.
Endotesta and tegmen collapsed. Perisperm not developed. Endosperm usually
absent (sometimes sparse, rarely copious, rarely thick-walled mucilaginous,
with galactomannans). Embryo usually large, straight or curved, well
differentiated (especially in Papilionoideae), with chlorophyll.
Suspensor with very various structure; suspensor haustoria often present.
Cotyledons two, well developed and nutritious, fatty, proteinaceous and often
starchy, in Amherstieae, Dearieae and Sclerolobieae
with amyloid (xyloglucans). Radicula straight, oblique or curved and lateral
(Papilionoideae). Germination phanerocotylar or cryptocotylar.
Cytology x = 7, 8, 12, 14
DNA The plastid genome is
extensively rearranged in Fabaceae. Examples are as follows.
Plastid gene rpl22 transferred from plastid genome to nuclear genome.
Plastid inverted repeat lost in six tribes and Wisteria. Plastid
genome in most Papilionoideae with inversion of c. 50 kb. Plastid
genome in most Phaseoleae with inversion of 78 kb. Robinieae
(except Sesbania) with inversion of c. 30 kb. Plastid genome in
Medicago with several inversions (62 kb inversion etc.). At least
eight inversions present in Pisum (in P. humile inversion of
4 kb). Two or three inversions present in Vicia faba. Plastid gene
rps16 lost in Moldenhawera and 16 tribes of
Papilionoideae. Plastid gene ycf4 lost in most
Papilionoideae. Plastid gene ndhF lost in Hebestigma
cubense. Plastid gene accD (zpfA, ORF512) lost in
Trifolium. Plastid gene clpP lost in Cicer and other
genera. Plastid ORF224 lost in Pisum sativum and ORF84 lost multiple
times. Intron lost in plastid gene rpoC1 in Medicago
suffruticosa. Intron in plastid gene rpl12 transferred to nucleus
in, i.a., Bauhinia and Desmodium (present in, e.g.,
Brya, Soemmeringia and Mucuna), mitochondrial gene
srp12 in, i.a., Bauhinia. Numerous additional structural
rearrangements, intron losses, indels etc. present in different clades.
Phytochemistry Flavonols
(kaempferol, quercetin, myricetin), flavone-C-glycosides, afzelechin,
5-deoxyflavonoids, isoflavonoids (pterocarpans, genistein, pachyrrhizin,
coumestrol, rotenone and other rotenoids, munduserone, angolensin, etc. in
Papilionoideae), cyanidin, epigallocatechin-3-gallate, oleanolic acid
derivatives, dammarane, phytoalexins, methylated ellagic acids, ellagitannins,
geraniin, proanthocyanidins (prodelphinidins), pyrrolizidine alkaloids as
macrocyclic diesters (abundant at least in Crotalaria), lupine
alkaloids (cytisine, N-methylcytisine, anagyrine, thermopsine,
lupanine, sparteine, etc.), loline alkaloids (in Genisteae, but not in
Crotalaria), physostigmine and similar alkaloids, pyridine,
quinolizidine and indole alkaloids, Erythrina alkaloids, triterpene
saponins, tyrosine-, leucine- or phenylalanine-derived cyanogenic compounds,
anthraquinones, pinitol (cyclitol), simmondsin-like compounds, lignans
(syringaresinol), toxic free non-protein amino acids, and lectins
(hemagglutinins, especially in seeds) present. Gums often frequent (especially
in seeds). Storage polysaccharides (especially in seeds) present as
galactomannans (galactose/mannose relationship possibly phylogenetically
informative). Numerous secondary metabolites synthesized by endophytic fungi or
bacteria.
Use Ornamental plants, edible
fruits and seeds, vegetables, forage plants, spices and flavours, dragant and
gums (Astragalus gummifer, Acacia spp.), dyeing substances
(Indigofera, Genista tinctoria, etc.), medicinal plants,
poisons (rotenonoids from Derris, Lonchocarpus and other
genera), timber, tanning, fertilizers.
Systematics There is no
undisputable sister-group to the Fabaceae, although there are
several candidates.
The presentation below mainly follows that in
LPWG (2013, 2017). Duparquetioideae or Cercideae are
sister-group to all other Fabaceae.
Duparquetioideae
Legume Phylogeny Working Group in Taxon 66(1): 69. Feb. 2017
Duparquetiinae H. S. Irwin et Barneby
in R. M. Polhill et P. H. Raven, Adv. Legume System. 1: 102. 1981
1/1. Duparquetia (1; D.
orchidacea; tropical West and Central Africa). – Liana, unarmed. Root
nodules absent. Vestured pits absent. Stipules lateral, free. Leaves pinnately
compound, imparipinnate. Leaflets/pinnae opposite (lamina entire). Extrafloral
nectaries absent. Terminal raceme. Flowers not papilionate. Hypanthium absent.
Floral development acropetal. Sepals four, free, unequal, abaxial and adaxial
sepals cucullate and sepaloid, lateral sepals petaloid. Petals five, free,
imbicate, with stalked glands along margins; adaxial petal outermost. Stamens
four, antesepalous. Filaments free. Anthers basifixed, poricidal (dehiscing by
pores), with pointed appendages, post-genitally fused into synandrium. Pollen
grains asymmetrical. Apertures with ectoaperture encircling equator and with
two equatorial endoapertures. Gynoecium unicaprellate, stipitate, with free
stipe. Gynoecial initiation not advanced (relative to other organs). Ovary bi-
to quinque-ovulate. Fruit a woody pod with spirally coiling valves. Seeds
without pleurograms. Embryo straight. n = ? Chemistry unknown.
Cercidoideae Legume
Phylogeny Working Group in Taxon 66(1): 68. Feb. 2017
Cercideae Bronn, Form. Plant. Legumin.:
ad Sect. 134, 131. 1822
14/300–460. Cercis (12; the
Mediterranean, eastern Asia, North America to northeastern Mexico);
Adenolobus (2; A. garipensis, A. pechuelii; southern
Angola, Namibia, northern Botswana, Northern Cape); Bauhinia
(150–300; tropical and subtropical regions of both hemispheres, especially
tropical South America), Piliostigma (5; P. foveolatum,
P. malabaricum, P. reticulatum, P. thonningii,
P. tortuosum; Africa incl. South Africa to southern Asia and
Australia), Brenierea (1; B. insignis; Madagascar);
Griffonia (4; G. physocarpa, G. simplicifolia,
G. speciosa, G. tessmannii; tropical West Africa);
Gigasiphon (3; G. gossweileri, G. humblotianum,
G. macrosiphon; tropical Africa), Tylosema (5; T.
angolense, T. argentea, T. esculentum, T.
fassoglensis, T. humifusa; Africa incl. South Africa),
Barklya (1; B. syringifolia; northeastern Queensland),
Schnella (30–35; tropical South America), Lysiphyllum (8;
Malesia to tropical Australia), Phanera (60–65; pantropical, East
Asia from eastern Himalayas to Japan, Indochina, Java), Lasiobema (12;
India to Southeast Asia and Malesia; in Phanera?), Cheniella
(10; India to southern China, Southeast Asia, West and Central Malesia). –
Mainly tropical and subtropical regions, few species in warm temperate areas.
Trees, shrubs or lianas, often with branch tendrils and/or prickles or
infrastipular spines. Root nodules absent. Vestured pits absent. Stipules
lateral, free. Petiole with a single joined pulvinus. Leaves simple
alternatively unifoliolate (or bifoliolate), usually bilobate or entire (rarely
with two leaflets). Leaflets/pinnae opposite when lamina bifoliolate. Lamina
when unifoliolate entire or bilobate. Stomata usually anomocytic (rarely
paracytic). Extrafloral nectaries usually absent. Raceme or pseudoraceme.
Flowers sometimes papilionate. Hypanthium present (often very elongated) to
almost absent. Sepals five, connate into spathaceous or bi- to quinquelobate
calyx, or free. Petals usually five (rarely two, six or absent), free,
imbricate, adaxial petal innermost. Stamens usually ten (sometimes fewer) in
two whorls of alternate length. Filaments more or less connate or free. Anthers
dorsifixed, longicidal or poricidal. Staminodia sometimes present. Pollen
grains tricolporate, 3–6-colpate, 3-porate, 3-pororate, 3–4-colporoidate or
inaperturate, rarely in tetrads. Exine striate. Zwischenkörper (pectic
substances) present below apertures at least in Cercis. Gynoecium
unicarpellate, stipitate, with free or adnate stipe. Ovary uni- to
multiovulate. Fruit an often explosively dehiscent pod, with twisted valves, or
indehiscent and usually samaroid. Seeds with apical usually crescent-shaped
(rarely circular) hilum, without pleurograms, pseudopleurograms, wing or aril.
Embryo usually straight. x = 7. Introns in mitochondrial genes
srp12 and rpl2 absent in Bauhinia.
Coumarins and cyanogenic glucosides present. Non-protein aminoacids
(5-hydroxy-L-tryptophan) frequent. – Cercis is sister to the
remaining Cercidoideae and Adenolobus successive sister to
the two major clades.
Fabaceae except
Duparquetia and Cercideae
[Detarioideae+[Dialioideae+[Caesalpinioideae+Papilionoideae]]]
Nitrogen fixation sometimes occurring.
Vestured pits usually present. Fruit sometimes a drupe, a samara, a schizocarp,
etc. Dumbbell-shaped cells present below palisade exotesta. Free amino acids,
especially in seeds.
Detarioideae
Burmeist., Handb. Naturgesch.: 319. 1837 [‘Detarieae’]
78/760–>805. Trees or sometimes
shrubs (rarely suffruticose). Root nodules absent. Ectomycorrhiza often
present. Vestured pits present in secondary xylem. Resinous ducts often present
in stem. Stipules usually fused partly or entirely, intrapetiolar (between
petiole and axillary bud), often caducous. Leaf phloem transfer cells present.
Leaves usually paripinnate or bifoliolate (rarely unifoliolate).
Leaflets/pinnae opposite or alternate. Leaflets often with translucent
crater-like glands on abaxial surface. Extrafloral nectaries often present on
lower side of leaves, rarely on margins of leaflets or on leaf rachis. Raceme
or panicle. Floral prophylls (bracteoles) often well developed (often petaloid,
valvate, imbricate or partially fused with each other or with hypanthium,
partially or completely enclosing bud), caducous. Flowers not papilionate.
Hypanthium present (often elongated) to almost absent. Sepals often four or
five, with two adaxial sepals often connate (rarely some or all absent or up to
seven). Petals up to five (to seven), free, imbricate, equal or unequal,
adaxial petal outermost. Stamens usually ten (sometimes two to numerous).
Filaments free or more or less connate. Anthers dorsifixed or basifixed,
longicidal. Pollen grains usually tricolporate. Exine sometimes striate.
Gynoecium unicarpellate, stipitate, with free or adnate stipe. Ovary uni- to
multiovulate. Style abaxially curved. Fruit usually a woody dehiscent pod
(sometimes indehiscent samaroid). Seeds occasionally with pseudopleurograms,
sometimes arillate. Embryo straight. Endosperm absent. Cotyledons with
thick-walled cells, with amyloid (xyloglucans). x = 12. Coumarins present.
Bicyclic diterpenes (resins) and non-protein amino acids often present. – Two
major clades, (1) the resin-producing Detarieae and (2)
Amherstieae, comprise the major part of the Detarioideae and these two
clades form a polytomy together with a number of small groups. – The clade
[Barnebydendreae+Schotieae] is sister-group to
Detarieae in a wide sense, according to Estrella & al. (2018).
Barnebydendreae
Estrella, L. P. Queiroz et Bruneae in Sci. Rep. 8: 6884. 2018
2/2. Barnebydendron (1; B.
riedelii; tropical Central and South America), Goniorrhachis (1;
G. marginata; southeastern Brazil). – Zwischenkörper (pectic
substances) present below apertures.
Schotieae Estrella, L.
P. Queiroz et Bruneae in Sci. Rep. 8: 6884. 2018
1/4–5. Schotia (4–5; S.
afra, S. brachypetala, S. capitata, S.
latifolia; Africa south of the Zambesi River). – Zwischenkörper (pectic
substances) present below apertures.
[[Prioria
clade+Daniellieae]+Detarieae]
Prioria clade
3/16. Colophospermum (1; C.
mopane; tropical southern Africa), Hardwickia (1; H.
binata; drier regions of India), Prioria (14; tropical Africa,
tropical Asia to islands in the Pacific, Central America, Jamaica, northwestern
South America). – Tropical.
Daniellieae Nakai,
Chosakuronbun Mokuroku [Ord. Fam. Trib. Nov.]: 252. 20 Jul 1943
[’Danielleae’]
2/6. Brandzeia (2; B.
filicifolia, B. rubriflora; Madagascar), Daniellia (4;
D. klainei, D. oblonga, D. ogea, D.
oliveri; tropical Africa). – Tropical Africa, Madagascar.
Brandzeia and Daniellia may be sister-groups, although the
support is weak.
Detarieae DC., Prodr.
2: 521. Nov (med.) 1825
14/163–170. Peltogyne (23;
Central America, tropical South America), Guibourtia (16; tropical
Africa, tropical South America), Stemonocoleus (1; S.
micranthus; Central Africa), Augouardia (1; A. letestui;
Gabon), Hymenaea (14; southern Mexico, Central America, the West
Indies, tropical South America, one species, H. verrucosa, in eastern
Africa); Neoapaloxylon (3; N. madagascariense, N.
mandrarense, N. tuberosum; Madagascar), Baikiaea (7;
B. fragrantissima, B. ghesquiereana, B. insignis,
B. plurijuga, B. robynsii, B. suzannae, B.
zenkeri; tropical to southern Africa in Namibia and Botswana),
Copaifera (40–45; tropical Africa, Borneo, Central America, tropical
South America), Detarium (3; D. letestui, D.
microcarpum, D. senegalense; tropical and subtropical West Africa
to Sudan), Gilletiodendron (5; G. glandulosum, G.
kisantuense, G. mildbraedii, G. ogoouense, G.
pierreanum; tropical Africa), Hylodendron (1; H.
gabunense; coasts of the Gulf of Guinea), Sindora (18–20;
Southeast Asia, Malesia), Sindoropsis (1; S. letestui;
Gabon), Tessmannia (12; tropical Africa), Eperua (c 15;
northeastern South America), Eurypetalum (3; E. batesii,
E. tessmannii, E. unijugum; Cameroon to Gabon). – Tropical
and subtropical. Flowers bisymmetric or actinomorphic. Petals absent or one to
five. Stamens three to ten (when fewer, then staminodia as well).
Zwischenkörper (pectic substances) present below apertures. Bicyclic
diterpenes present in resinous ducts.
Amherstieae Benth. in
J. Bot. (Hooker) 2: 73. Mar 1840
Cynometreae Benth. in J. Bot. (Hooker)
2: 74. Mar 1840
c 56/570–>605.
Saraceae Estrella, L. P. Queiroz et Bruneae in Sci.
Rep. 8: 6884. 2018. Endertia (1; E. spectabilis; Borneo),
Lysidice (2; L. brevicalyx, L. rhodostegia; southern
China, Vietnam), Saraca (11; India, Sri Lanka, southern China,
Southeast Asia, Malesia to Sulawesi), Leucostegane (2; L.
grandis, L. latistipulata; the Malay Peninsula, Borneo). –
Afzelieae Estrella, L. P. Queiroz et Bruneae in Sci.
Rep. 8: 6884. 2018. Afzelia (13; tropical Africa, tropical Asia),
Brodriguesia (1; B. santosii; eastern Brazil),
Intsia (4; I. acuminata, I. bijuga, I.
moeleri, I. palembanica; Southeast Asia to Pacific coasts). –
Amherstieae sensu stricto. Ecuadendron (1;
E. acostasolisianum; western Ecuador), Brownea (c 40; Costa
Rica to Peru, the West Indies), Macrolobium (75–80; Central America,
tropical South America), ‘Heterostemon’ (1–7; H.
mimosoides; tropical South America; diphyletic), ‘Elizabetha’
(6; E. bicolor, E. coccinea, E. durissima, E.
paraensis, E. princeps, E. speciosa; tropical South
America; polyphyletic), Paloue (4; P. brasiliensis, P.
guianensis, P. induta, P. riparia; the Guayana Shield in
northern South America), Paloveopsis (1; P. emarginata;
northeastern South America); Crudia (50–55; tropical Africa,
tropical Asia, tropical America), Lebruniodendron (1; L.
leptanthum; coasts of the Gulf of Guinea), Normandiodendron (2;
N. becquaertii, N. romii; western Central Africa),
‘Cynometra’ pro parte (c 85 + 20–25; pantropical; paraphyletic),
Plagiosiphon (5; P. discifer, P. emarginatus, P.
gabonensis, P. longitubus, P. multijugus; Central
Africa), Zenkerella (6; Z. capparidacea, Z. citrina,
Z. egregia, Z. grotei, Z. perplexa, Z.
schliebenii; coast of Guinea, tropical East Africa),
Neochevalierodendron (1; N. stephanii; Gabon),
Gabonius (1; G. ngouniensis; Gabon), Micklethwaitia
(1; M. carvalhoi; Mozambique), Annea (2; A. afzelii,
A. laxiflora; tropical West and Central Africa),
Scorodophloeus (2; S. fischeri, S. zenkeri; coasts
of Guinea and East Africa), ‘Hymenostegia’ pro parte (7–13?;
along the Gulf of Guinea; diphyletic); Librevillea (1; L.
klainei; Gabon), ‘Gilbertiodendron’ (c 30; tropical and
subtropical Africa; non-monophyletic), Didelotia (10–11; Central
Africa), ‘Anthonotha’ (c 30; tropical Africa; polyphyletic),
Oddoniodendron (6; O. gambanum, O. gilletii, O.
micranthum, O. normandii, O. reitsmarum, O.
romeroi; along the Gulf of Guinea), Englerodendron (1?; E.
usambarensis; Usambara Mountains in Tanzania), ‘Isoberlinia’
(5; I. angolensis, I. dalzielii, I. doka, I.
scheffleri, I. stolzii; tropical Africa; polyphyletic),
‘Berlinia’ (17; tropical Africa; polyphyletic),
Microberlinia (2; M. bisulcata, M. brazzavillensis;
coasts of the Gulf of Guinea), Brachystegia (25–30; central tropical
Africa to Botswana), Icuria (1; I. dunensis; Central
Mozambique), Aphanocalyx (14; tropical West and Central Africa),
Julbernardia (11; tropical to southern Africa), ‘Bikinia’
(c 10; western Central Africa; paraphyletic; incl. Tetraberlinia?),
Tetraberlinia (7; T. baregarum, T. bifoliata, T.
korupensis, T. longiracemosa, T. moreliana, T.
polyphylla, T. tubmaniana; Central Africa; in Bikinia?);
Cryptosepalum (12; tropical Africa), Humboldtia (7; H.
bourdillonii, H. brunonis, H. decurrens, H.
laurifolia, H. trijuga, H. unijuga, H.
vahliana; southern India, Sri Lanka), Paramacrolobium (1; P.
coeruleum; tropical Africa), Cynometra sensu stricto (tropical
Africa), Dicymbe (c 20; the Guayana Shield to western Amazonia in
Brazil), Polystemonanthus (1; P. dinklagei; tropical West
Africa); Tamarindus (1; T. indica; tropical Africa),
Loesenera (2; L. kalantha, L. talbotii; tropical
West Africa), Talbotiella (5–8; T. bakossiensis, T.
batesii, T. breteleri, T. ebo, T. eketensis,
T. gentii, T. korupensis, T. velutina; tropical West
Africa), Leonardoxa (1; L. africana; Cameroon),
Hymenostegia sensu stricto (3?; H. floribunda; coast of the
Gulf of Guinea); unplaced: Amherstia (1; A. nobilis; Burma,
extinct?), Brachycylix (1; B. vageleri; northern Central
Colombia), Michelsonia (1; M. microphylla; eastern Congo),
Pseudomacrolobium (1; P. mengei; Congo). – Pantropical. –
The sister-group of Amherstieae is not yet clarified.
[Dialioideae+[Caesalpinioideae+Papilionoideae]]
Dialioideae Legume
Phylogeny Working Group in Taxon 66(1): 69. Feb. 2017
Dialiinae H. S. Irwin et Barneby in
Polhill et Raven, Adv. Legume Syst. 1: 100. 1981
16/c 83. Poeppigia (1; P.
procera; southern Mexico, Central America, tropical South America);
Dialium (c 40; tropical to southern Africa, Madagascar, South and
Southeast Asia, one species, one species, D. guianense, in tropical
America); Petalostylis (2; P. cassioides, P.
labicheoides; drier regions in Australia), Labichea (14;
Australia except central parts), Baudouinia (6; B. capuronii,
B. fluggeiformis, B. louvelii, B. orientalis, B.
rouxevillei, B. sollyaeformis; Madagascar),
Distemonanthus (1; D. benthamianus; tropical West Africa),
Storckiella (4; S. australiensis: northeastern Queensland;
S. neocaledonica, S. pancheri: New Caledonia; S.
vitiensis: Fiji), Zenia (1; Z. insignis; southern China,
Thailand, Vietnam), Androcalymma (1; A. glabrifolium; upper
Amazon Basin in Brazil), Kalappia (1; K. celebica; Malili in
Sulawesi), Apuleia (1; A. leiocarpa; northeastern Peru,
southeastern Brazil, northern Argentina), Dicorynia (2; D.
guianensis, D. paraensis; tropical South America),
Eligmocarpus (1; E. cynometroides; southeastern Madagascar),
Koompassia (3; K. excelsa, K. grandiflora, K.
malaccensis; Malesia), Martiodendron (4; M. elatum,
M. excelsum, M. mediterraneum, M. parviflorum;
tropical South America), Mendoravia (1; M. dumaziana;
southeastern Madagascar). – Tropical. Trees or shrubs (rarely suffruticose),
unarmed. Root nodules absent. Vestured pits rarely present in secondary xylem.
Stipules lateral, free, or absent. Leaves imparipinnate (rarely paripinnate,
unifoliolate or palmately compound). Leaflets/pinnae usually alternate (rarely
opposite). Extrafloral nectaries absent. Inflorescence usually cymose,
primarily simple or compound cymes, thyrsoid or dichasia (sometimes racemes
with distichous floral arrangement or flowers solitary). Flowers sometimes
papilionate. Hypanthium usually absent (rarely present). Sepals (3–)5(–6),
free. Petals usually five or fewer (rarely six), free, imbricate, adaxial petal
innermost. Stamens usually five or fewer (rarely six to ten). Filaments free.
Anthers usually basifixed (rarely dorsifixed), longicidal to poricidal.
Staminodia often present. Pollen grains tricolporate. Exine punctate or finely
reticulate (rarely striate). Gynoecium usually unicarpellate (sometimes
bicarpellate), stipitate with free stipe, or sessile. Ovary usually biovulate
(rarely uni- to multiovulate). Fruit usually drupe or samara (rarely dehiscent
or drupe with endocarp indurating into one-seeded segments). Seeds without
pleurograms. n = 14. Chemistry unknown. – Many representatives have lost one
or several floral organs during evolution.
[Caesalpinioideae+Papilionoideae]
Vestured pits usually present (absent in
Cassieae). Non-protein amino acids sometimes present (especially in
seeds).
Caesalpinioideae DC.,
Prodr. 2: 473. Nov (med.) 1825 [‘Caesalpineae’]
148/4.100–>4.200. Trees, shrubs, lianas,
suffruticose or functionally herbaceous, unarmed or often armed with prickles
or spines. Root nodules often present. Vestured pits present in secondary
xylem. Stipules lateral, free, or absent. Leaves bipinnate or pinnate (then
usually paripinnate, rarely imparipinnate or bifoliolate, modified into
phyllodes or absent). Leaflets/pinnae usually opposite (rarely alternate).
Extrafloral nectaries often present on petiole and/or on primary and secondary
rachises, usually between pinnae or leaflet pairs (sometimes on stipules or
bracts). Inflorescence globose, spike, panicle, raceme or flowers in fascicles.
Flowers sometimes papilionate or asymmetric. Hypanthium absent or cupular
(rarely tubular). Sepals (3–)5(–6), free. Petals (3–)5(–6), free or
connate, with valvate or imbricate aestivation, then adaxial petal innermost.
Stamens diplo- or haplostemonous (sometimes 3–5), often numerous (up to more
than 100). Filaments free or connate. Anthers basifixed or dorsifixed,
longicidal or poricidal (apically or basally), often with stipitate or sessile
apical gland. Staminodia present or absent. Pollen grains shed as monads,
tetrads, bitetrads or polyads, tricolporate or porate. Exine never striate.
Gynoecium usually unicarpellate (rarely polycarpellate), stipitate, with free
stipe, or sessile. Ovary uni- to multiovulate. Fruit a one- to many-seeded pod,
dehiscent along one or two sutures, often a lomentum, a craspedium, or thick,
woody and indehiscent or explosively dehiscent. Seeds usually with open or
closed pleurogram on both sides (sometimes with aril och sarcotesta, sometimes
winged), hilum usually apical. Embryo straight. x = 7, 12. Non-protein amino
acids, coumarins, cyanogenic glucosids, phenylethylamine, traptamines and
β-carboline alkaloids present.
Ceratonieae Reichb.,
Flora Germ. Excurs. 2(2): 544. 1832
7/24–27. Ceratonia (2; C.
oreothauma, C. siliqua; southeastern Mediterranean to Somalia and
the Arabian Peninsula), Acrocarpus (1; A. fraxinifolius;
India, Sri Lanka, Southest Asia, Malesia), Tetrapterocarpon (2; T.
geayi, T. septentrionalis; Madagascar); Arcoa (1; A.
gonavensis; Hispaniola); Gymnocladus (5–6; G.
angustifolius, G. assamicus, G. burmanicus, G.
chinensis, G. dioicus; East and Southeast Asia, eastern North
America), Umtiza (1; U. listeriana; Eastern Cape), Gleditsia
(14; the Caspian area, China, Japan to New Guinea, eastern North America, South
America). – Subtropical and tropical regions. Most species are usually
dioecious (not so in Acrocarpus and Umtiza). The perianth is
often greenish and more or less reduced. – Ceratonieae may be
sister-group to the remainder of Caesalpinioideae.
[[Pterogyne+Caesalpinieae+Cassieae]+[[Dimorphandra
clade
A+Peltophoreae+Tachigaleae]+Dimorphandra
clade B]]
[Pterogyne+Caesalpinieae+Cassieae]
Pterogyne (1; P. nitens;
tropical South America). – Pterogyne is sister to either
Caesalpinieae or Cassieae.
Caesalpinieae Rchb.,
Fl. Germ. Excurs. 2(2): 544. 1832 [‘Caesalpineae s.
Cassieae’]
27/224. Cordeauxia (1; C.
edulis; Somalia, Ethiopia), Stuhlmannia (1; S. moavi;
coast of Kenya and Tanzania, northern Madagascar), Lophocarpinia (1;
L. aculeatifolia; Paraguay, northern Argentina, southern Brazil?),
Haematoxylum (5; H. brasiletto, H. calakmulense,
H. campechianum, H. dinteri, H. sousanum; Mexico,
Central America, the West Indies, northwestern South America),
Hererolandia (1; H. pearsonii; Ababes in Namibia),
Denisophytum (8; the Arabian Peninsula, Somalia, Kenya, northern
Madagascar, Florida, Mexico, the West Indies, Paraguay, northern Argentina),
Coulteria (7; C. cubensis, C. glabra, C.
mollis, C. platyloba, C. pringlei, C. pumila,
C. velutina; Mexico, Central America, the West Indies, Colombia),
Tara (3; T. cacalaco, T. spinosa, T.
vesicaria; Mexico, Central America, the West Indies, northwestern South
America), Gelrebia (8; northern Kenya, Ethiopia, Somalia, Congo,
southern Africa), Hultholia (1; H. mimosoides; India,
Bangladesh, Burma, Yunnan, Thailand, Indochina), Guilandina (19;
warmer regions on both hemispheres), Moullava (4; M. digyna,
M. spicata, M. tortuosa, M. welwitschiana; India,
Sri Lanka, Burma, Yunnan, Hainan, Southeast Asia, Malesia, one species, M.
welwitschiana, in Central Africa), Biancaea (6; B.
decapetala, B. godefroyana, B. millettii, B.
oppositifolia, B. parviflora, B. sappan; India, Burma,
Southeast Asia, Malesia to the Philippines and Borneo), Ticanto (15;
southern China, Southeast Asia, Malesia), Pterolobium (10; India,
southern China, Thailand, Indochina, Malesia, one species, P.
stellatum, in southern and eastern tropical Africa and the Arabian
Peninsula), Mezoneuron (c 25; tropical Africa, Madagascar, tropical
and subtropical Asia to tropical Australia and the Hawaiian Islands),
Paubrasilia (1; P. echinata; eastern Brazil),
Caesalpinia (9; Mexico, Central America, the West Indies, tropical
South America), Cenostigma (14; southern Mexico, Central America, the
West Indies, tropical South America), Stenodrepanum (1; S.
bergii; central and western Argentina), Hoffmanseggia (23;
tropical to southern Africa, southwestern United States to Chile and
Argentina), Balsamocarpon (1; B. brevifolium; northern
Chile), Zuccagnia (1; Z. punctata; the Andes in northern and
central Chile and western central Argentina), Libidibia (7; L.
coriaria, L. ferrea, L. glabrata, L.
monosperma, L. paraguariensis, L. punctata, L.
sclerocarpa; Mexico, Central America, the West Indies, tropical South
America), Pomaria (16; southern Africa, southern United States,
Mexico, southeastern South America), Arquita (5; A.
ancashiana, A. celendiniana, A. grandiflora, A.
mimosifolia, A. trichocarpa; the Andes in Ecuador, Peru, Bolivia
and northwestern Argentina), Erythrostemon (31; southern United
States, Mexico, Central America, the West Indies, South America). – Tropical
and subtropical regions, few species in warm-temperate parts of the world.
Trees or shrubs. Sieve tube plastids also with fibres. Leaves often bicompound.
Ovules usually campylotropous. Outer integument usually with vascular bundle.
Aril often present. – Cordeauxia and Stuhlmannia form a
clade sister to the remaining Caesalpinieae.
Cassieae Bronn, Form.
Plant. Legumin.: 78, 127, 130. 1822
8/600–780. Cassia
(30–90 or more?; tropical and subtropical regions on both hemispheres; incl.
Latrobea?), Latrobea (4–6; L. colophona, L.
diosmifolia, L. genistoides, L. hirtella;
southwesternmost Western Australia; in Cassia?),
Senna
(260–350; warm-temperate to tropical regions, especially America);
Batesia (1; B. floribunda; Amazonas), Chamaecrista
(300–330?; Africa, East Asia, temperate and tropical regions in America),
Melanoxylum (1; M. brauna; Amazonas), Recordoxylon
(1; R. speciosum; Amazonas); Vouacapoua (3; V.
americana, V. macropetala, V. pallidior; tropical South
America). – Mainly tropical and subtropical regions, few species in
warm-temperate regions. Trees or shrubs (rarely herbs). Vestured pits absent.
– Chamaecrista,
Melanoxylon and Recordoxylon have the ability to form
bacterial nodules. Rhizobia in Chamaecrista
remain in infection threads.
[[Dimorphandra clade
A+Schizolobieae+Tachigaleae]+Diptychandra
clade B]
[Dimorphandra clade
A+Schizolobieae+Tachigalieae]
Dimorphandra clade
A
6/43–63. Burkea (1–2;
B. africana, B. caperangau; tropical and subtropical Africa
to Namibia and northern South Africa), Campsiandra (c 20; tropical
South America, with their highest diversity in Amazonas), Dimorphandra
pro parte (c 25; tropical America), Dinizia (1; D. excelsa;
Guayana, Brazil), Mora (7–10; M. abbottii, M.
ekmanii, M. excelsa, M. gonggrijpii, M.
megistosperma, M. oleifera, M. paraensis; Central
America, the West Indies, northern tropical South America),
Stachyothyrsus (3; S. stapfiana, S. staudtii, S.
tessmannii; tropical West Africa). – Tropical Africa and America. The
Dimorphandra clade is weakly supported.
Schizolobieae Nakai,
Chosakuronbun Mokuroku [Ord. Fam. Trib. Nov.]: 251. 20 Jul 1943
8/45–49. Bussea (7; B.
eggelingii, B. gossweileri, B. massaiensis, B.
occidentalis, B. perrieri, B. sakalava, B.
xylocarpa; tropical Africa, Madagascar), Peltophorum (11;
tropical and subtropical regions); Schizolobium (2; S.
amazonicum, S. parahyba; tropical South America), Delonix
(10–11; eastern Africa, Madagascar, the Arabian Peninsula, India; incl.
Colvillea and Lemuropisum?), Colvillea (1; C.
racemosa; Madagascar; in Delonix?),
Lemuropisum (1; L. edule; Madagascar; in Delonix?),
Conzattia (2; C. multiflora, C. sericea; Mexico), Parkinsonia
(12–15; drier warmer regions in America, northeastern Africa, Namibia and
Northern Cape, and from Mexico to Argentina). – Tropical and subtropical.
Leaves bipinnate. Petals usually yellow. Seeds narrow. –
Schizolobium is sister to the Delonix-Parkinsonia
clade.
Tachigalieae Nakai,
Chosakuronbun Mokuroku [Ord. Fam. Trib. Nov.]: 252. 20 Jul 1943
3/100–105. Arapatiella (2;
A. emarginata, A. psilophylla; southeastern Brazil),
Jacqueshuberia (7; J. amplifolia, J. brevipes,
J. loretensis, J. purpurea, J. pustulata, J.
quinquangulata, J. splendens; Colombia, Brazil),
Tachigali (c 95; tropical America, especially Amazonas). – Tropical
America.
Dimorphandra clade
B
[Dimorphandra pro
parte+[Diptychandra+Moldenhawera]+Pachyelasma+Erythrophleum+Sympetalandra+Mimoseae]
Dimorphandra pro parte
Diptychandra (1; D.
aurantiaca; Brazil, Bolivia, Paraguay), Moldenhawera (10;
Venezuela, eastern Brazil). – Tropical South America. Diptychandra
and Moldenhawera may be sister-groups, but the support is weak.
Pachyelasma (1; P. tessmannii;
tropical West Africa).
Erythrophleum (10; tropical to southern
Africa, Madagascar, southern and eastern Asia to northern Australia). –
Tropical regions in the Old World. Erythrophleum may be sister to
Mimoseae, although the support is weak.
Sympetalandra (1; S.
schmutzii; Flores).
Mimoseae Bronn, Form.
Plant. Legumin.: 78, 127, 130. 1822 (incl. Acacieae Dumort., Anal.
Fam. Plant.: 40. 1829 and Ingeae Benth. et Hook. f., Gen. Plant. 1:
437. 19 Oct 1865)
82/3.035–3.150. Adenanthera
(13; tropical Asia and Australia, islands in the Pacific), Tetrapleura
(1–2; T. chevalieri, T. tetraptera; tropical Africa),
Amblygonocarpus (1; A. andongensis; tropical Africa to
Namibia and Botswana), Calpocalyx (11; tropical West Africa),
Pseudoprosopis (7; P. bampsiana, P. claessensii,
P. euryphylla, P. fischeri, P. gilletii, P.
sericea, P. uncinata; tropical Africa), Xylia (9–10;
tropical to southern Africa, tropical Asia); Pentaclethra (3; P.
eetveldeana, P. macroloba, P. macrophylla; tropical West
and Central Africa, Central America, tropical South America);
‘Entada’ (c 30; tropical regions; non-monophyletic; incl.
Elephantorrhiza?), Elephantorrhiza (9; tropical to southern
Africa; in Entada?), Piptadeniastrum (1; P.
africanum; Central Africa); Newtonia (14–15; tropical Africa,
tropical America), Fillaeopsis (1; F. discophora; western and
southwestern Africa), Indopiptadenia (1; I. oudhensis; India,
Nepal), Lemurodendron (1; L. capuronii; northeastern
Madagascar); Plathymenia (1; P. reticulata; Brazil,
Argentina); ‘Prosopis’ (26; Africa, Southwest Asia, southern
United States to South America; non-monophyletic), Xerocladia (1;
X. viridiramis; Namibia, Northern Cape); Cylicodiscus (1;
C. gabunensis; Central Africa); Prosopidastrum (5; P.
angusticarpum, P. globosum, P. gracile, P.
mexicanum, P. striatum; Mexico to southern South America);
Piptadeniopsis (1; P. lomentifera; Paraguay),
Mimozyganthus (1; M. carinatus; southeastern Bolivia,
southwestern Paraguay, Argentina); Desmanthus (c 25; southern United
States, Mexico, Central America, the West Indies, tropical South America),
Leucaena (c 25; Texas to Peru), Schleinitzia (2; S.
fosbergii, S. insularum; Malesia, Vanuatu to Tahiti, Guam);
Calliandropsis (1; C. nervosus; Mexico),
Dichrostachys (c 25; tropical Africa, Madagascar, southern and
southeastern Asia to Australia), Gagnebina (5; G. bakoliae,
G. calcicola, G. commersoniana, G. icrocephala,
G. pterocarpa; Madagascar, the Comoros, the Mascarene Islands);
Neptunia (8; tropical and subtropical regions); Aubrevillea
(2; A. kerstingii, A. platycarpa; tropical West and Central
Africa); Chidlowia (1; C. sanguinea; western tropical
Africa); Parkia (c 35; tropical Africa and Asia, Madagascar, tropical
Australia, tropical and subtropical America), Anadenanthera (2; A.
colubrina, A. peregrina; Hispaniola, Puerto Rico, tropical South
America); Adenopodia (7; A. gymnantha, A. oaxacana,
A. patens, A. rotundifolia, A. scelerata, A.
schlechteri, A. spicata; tropical Africa, Mexico, Central
America), Mimosa (c 400?; tropical and subtropical regions, with their
largest diversity in Central and tropical South America),
’Piptadenia’ (c 30; southern Mexico, Central America, tropical
South America; non-monophyletic), Pityrocarpa (4; P.
leucoxylon, P. moniliformis, P. obliqua,
‘Pseudopiptadenia’ brenanii; southern Mexico, Central
America, tropical South America), Stryphnodendron duckeanum
(Brazil), Parapiptadenia (3; P. excelsa, P.
pterosperma, P. rigida; tropical South America),
Pseudopiptadenia (5–10; P. contorta, P.
leptostachya, P. psilostachya, P. suaveolens, P.
warmingii; Central America, tropical South America),
‘Stryphnodendron’ (c 12; Central America, tropical South America;
polyphyletic), Vachellia (163; tropical Africa, Madagascar, the
Mascarene Islands, tropical Asia, tropical Australia, islands in the Pacific),
‘Senegalia’ (c 225; tropical and subtropical regions in Africa and
Asia, Mexico, Central America, tropical South America; polyphyletic),
Pseudosenegalia (2; P. feddeana, P. riograndensis;
Bolivia), Parasenegalia (7; P. lundellii, P.
muricata, P. rurrenabaqueana, P. santosii, P.
skleroxyla, P. visco, P. vogeliana; Central America, the
West Indies, tropical South America), Mariosousa (13; southwestern
United States, Mexico, Central America), Lachesiodendron (1; L.
viridiflorum; Mexico, Guatemala, Colombia and Venezuela to eastern Brazil,
Bolivia, Paraguay and northern Argentina), Acaciella (15; southern and
central United States to Argentina), Acacia (900–1.000; Madagascar,
tropical Asia, Australia, islands in the Pacific); Pseudosamanea (1;
P. cubana; Cuba), Cedrelinga (1; C. cateniformis;
Brazil), Hesperalbizia (1; H. occidentalis; southwestern
Mexico), ‘Enterolobium’ (12; tropical America; polyphyletic),
‘Archidendron’ (95–100; tropical Asia, with their highest
diversity on Borneo; non-monophyletic), Archidendropsis (14; islands
in southwestern Pacific), Wallaceodendron (1; W. celebicum;
the Philippines, Sulawesi), Samanea (5; S. dinklagei, S.
guineensis, S. inopinata, S. leptophylla, S.
tubulosa; Central America, the West Indies, tropical South America),
‘Abarema’ (c 45; New Caledonia, Mexico, Central America, tropical
South America; polyphyletic), Balizia (2; B. elegans, B.
leucocalyx; southern Mexico, Central America, tropical South America),
Blanchetiodendron (1; B. blanchetii; eastern Brazil),
Leucochloron (2; L. foederale, L. minarum; Brazil),
Macrosamanea (8; tropical South America), Zygia (14; southern
Mexico, Central America, tropical South America), Inga (c 300;
tropical and subtropical regions in South America), Ebenopsis (2;
E. confinis, E. ebano; southern Texas, Mexico),
Sphinga (3; S. acatlensis, S. platyloba, S.
prehensilis; southern Mexico, Central America, the West Indies, tropical
South America), Pithecellobium (c 75; tropical and subtropical
regions), Havardia (6; H. albicans, H.
campylacanthus, H. elachistophylla, H. mexicana, H.
pallens, H. sonorae; Texas to Central America),
Thailentadopsis (3; T. nitida, T. tenuis, T.
vietnamensis; Sri Lanka, Thailand, southern Vietnam),
Viguieranthus (18; Madagascar, tropical Asia), Zapoteca (c
20; southwestern North America to northern Argentina), Cojoba (c 20;
southern Mexico, Central America, the West Indies, tropical South America),
Afrocalliandra (2; A. gilbertii, A. redacta;
tropical to southern Africa), Calliandra (c 140; pantropical),
Pararchidendron (1; P. pruinosum; Java, northern Australia),
Falcataria (3; F. moluccana: East Malesia to New Guinea, the
Bismarck Archipelago, Solomon Islands; F. pullenii: Papua New Guinea;
F. toona: Queensland), Paraserianthes (1; P.
lophantha; Malesia), ‘Acacia’ pro parte, Lysiloma
(11; Florida, Mexico, the West Indies), Faidherbia (1; F.
albida; eastern Mediterranean, tropical to southern Africa),
‘Albizia’ (c 150; pantropical; paraphyletic), Cathormion
(4; C. altissimum, C. berteroanum, C. rhombifolium,
C. umbellatum; tropical West and Central Africa), Serianthes
(18; Thailand, Southeast Asia, Malesia to New Guinea, Melanesia, especially New
Caledonia, Micronesia, western Polynesia). – Tropical, subtropical and
warm-temperate. Bisexual, monoecious, andromonoecious or polygamomonoecious,
evergreen or deciduous trees or shrubs (rarely lianas or perennial, biennial or
annual herbs). Often with root nodules containing nitrogen fixing bacteria.
Ectomycorrhiza sometimes present. Fibres usually non-septate. Axial parenchyma
sometimes aliform. Wood rays usually 20 or more cells high. Sieve tube plastids
PIVa type, also with fibres. Leaves usually bipinnately compound (sometimes
pinnately compound or modified into phyllodia). Stipules intrapetiolar (often
scale-like or modified into spines or glands). Petiolar extrafloral nectaries
frequent (sometimes present on petiolules). Stomata usually anomocytic or
paracytic (sometimes anisocytic, tetracytic or cyclocytic). With or without
mucilaginous idioblasts and secretory cavities containing mucilage, resins or
oils. Inflorescence terminal or axillary, usually capitate. Flowers often
synchronously developing and opening simultaneously. Floral prophylls
(bracteoles) absent. At least corolla actinomorphic, usually small. Hypanthium
often absent. Sepals (three to) five (or six), usually with valvate (in the
Parkia and Mimozyganthus clades imbricate; in Calliandra
cochlear-descending) aestivation, usually more or less connate. Petals (three
or) four or five (or six), sessile, usually with valvate (in Dinizia
imbricate) aestivation, not clawed, in lower part usually connate into tube;
median petal usually abaxial. Stamens as many or twice as many as petals or
numerous (in Calliandra
spirally initiated), often showy. Filaments free or connate at base, free or
adnate to petals, often much exserted. Anthers dorsifixed, versatile,
tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits);
connective often with caducous apical gland. Tapetal cells uninucleate. Pollen
grains usually shed in tetrads or polyads (sometimes monads), usually
bicellular (sometimes tricellular) at dispersal. Pistil composed of usually one
carpel (in Affonsea, Archidendron and Klugiodendron
two to 16 free carpels, sometimes antesepalous), usually stipitate (gynophore).
Ovary unilocular. Stigma usually cup-shaped (sometimes peltate), Dry type.
Placentation marginal (along abaxial suture). Ovules usually anatropous,
ascending or pendulous. Micropyle bistomal or endostomal. Synergids often with
a filiform apparatus. Arillus sometimes present. Funicle filiform. Testa with
vascular bundle. Pleurogram usually present; linea fissura U-shaped (sometimes
absent). Endosperm haustorium usually chalazal (rarely lateral). Embryogenesis
onagrad, asterad or caryophyllad? Fruit usually a legume (rarely a follicle, a
samaroid or a lomentum). Funicular aril sometimes present. Testa with vascular
strand. Pleurogram usually present, as well as a linea fissura (a thin line
delimitating pleurogram). Funicle often long and thin. Seed usually without
hilum fissure. Endosperm thin, with thickened cell walls, with or without
starch, or absent. Embryo straight, with chlorophyll. Radicula short and thick,
straight. Cotyledons usually without starch grains, without amyloid.
Germination phanerocotylar or cryptocotylar. Often with x = 13, 14. Seed often
with rare free (non-protein) amino acids (e.g. albizziine; canavanine absent).
– The relationships among the different lineages within Mimoseae are
largely unresolved.
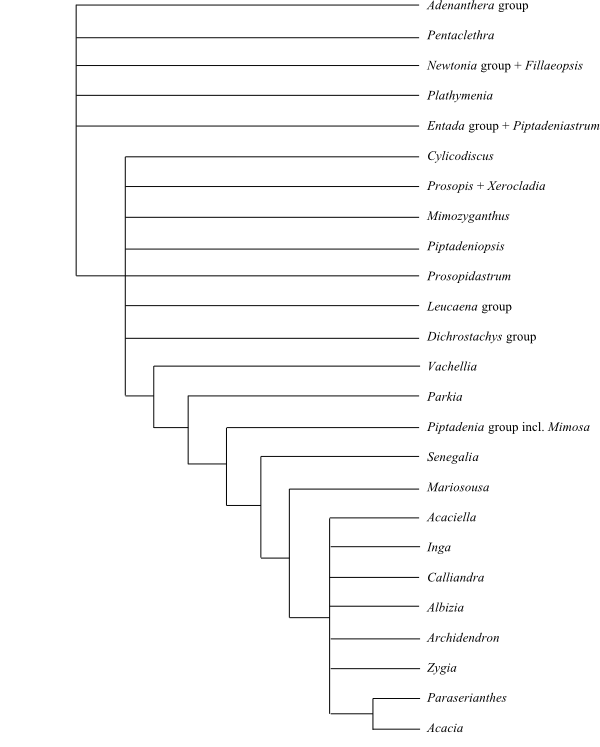 |
Phylogeny (somewhat simplified) of
Mimosoideae based on DNA sequence data (LPWG 2013).
|
Papilionoideae L. ex
DC., Prodr. 2: 94. Nov (mid) 1825
Faboideae (Giseke) Rudd in Rhodora 70:
496. 31 Dec 1968
460/14.315–14.700. Cosmopolitan except
Antarctica. Bisexual evergreen or deciduous trees, shrubs, lianas, suffrutices,
or perennial, biennial or annual herbs, often twining with foliar tendrils,
usually unarmed. Root nodules with nitrogen fixing bacteria usually present.
Nodules usually arising in association with lateral roots. Ectomycorrhiza
sometimes present. Phellogen superficially or deeply seated. Vestured pits
present in secondary xylem. Sieve tube plastids PIVb type or S type, with
spindle-shaped non-dispersive protein bodies (forisomes). Stipules usually
intrapetiolar, often scale-like or modified into spines or glands, free or
absent (very rarely interpetiolar). Leaves usually paripinnate, imparipinnate
or palmately compound, often unifoliolate or trifoliolate (rarely bifoliolate
or tetrafoliolate). Leaflets/pinnae opposite or alternate, sometimes modified
into tendrils (rarely in phyllodes). Stomata usually anomocytic or paracytic
(sometimes anisocytic, tetracytic or cyclocytic). Often with secretory chambers
containing mucilage, resins or oils. Extrafloral nectaries absent from petiole
and leaf rachis (occasionally present on stipules, stipels, bracts, or swollen
and nectar-secreting peduncles or sepals). Inflorescence terminal or axillary,
panicle, fascicle, raceme or pseudoraceme (sometimes cyme, spicate or capitate,
or flowers solitary). Flowers usually zygomorphic and papilionate (rarely
actinomorphic or asymmetrical). Hypanthium present or absent (in some species
of Amorpheae stemonozone instead of hypanthium). Sepals (three to)
five, with imbricate aestivation, in lower parts usually connate into tube
(sometimes entire and splitting into irregular lobes or lobes dimorphic and
some petaloid). Petals (1–)5(–6) (absent in some Amorpheae, in
other Amorpheae more or less uniform), with imbricate-ascending
aestivation; two lower petals usually connate into carina; median adaxial
(upper) petal vexillum, enclosing remaining petals in bud; lateral petals alae,
enclosing carina in bud (in some genera only adaxial petal free). Petals in
Mucuna holtoni functioning as reflectors for ultrasounds
(cheiropterophily with nectar guide). Sepals, corolla and stamens with
unidirectional (abaxial to adaxial) initiation. Stamens (nine or) ten (to
numerous). All filaments connate into tube surrounding pistil or adaxial
(posterior) stamen free (rarely all filaments free), usually free from petals
(in, e.g., Dalbergieae, Genisteae, Mirbelieae, and
Trifolieae often adnate to petals). Anthers basifixed and/or
dorsifixed, often versatile, tetrasporangiate, introrse or latrorse,
longicidal; connective sometimes with apical gland. Tapetal cells uninucleate.
When androecium monadelphic, then pollinator reward often pollen grains
liberated by secondary pollen presentation (pollen pump). When androecium
diadelphic, then pollinator reward often nectar from glands present between
filament tube and gynoecium. Pollen grains shed as monads, usually
tricolporate, tricolpate or triporate, bicellular at dispersal. Pollen grains
in Lupinus shed as threads. Pistil composed of usually one carpel
(monomery; in Swartzia often more than one), often stipitate
(gynophore). Ovary unilocular (rarely bilocular due to secondary septum).
Pollen grains in Vicia presented on stigmatic brush. Secondary pollen
display explosive in theMedicago clade. Placentation
marginal (along abaxial suture). Ovules usually campylotropous, ascending or
pendulous. Micropyle bistomal (often Z-shaped) or endostomal. Megagametophyte
sometimes protruding into micropyle. Synergids often with a filiform apparatus.
Endosperm haustorium usually chalazal (rarely lateral). Funicle short.
Embryogenesis onagrad, asterad or caryophyllad. Fruit usually a legume,
dehiscing along dorsal and ventral sutures simultaneously, valves twisting
(often explosively dehiscent; rarely follicle, nut, samaroid, lomentum, or
drupe); two layers of lignified fibres present, one or two oblique to long axis
of legume. Aril often present. Seed with hilar furrow; hilum often with group
of tracheids immediately below hilar surface. Raphe shorter than antiraphe.
Testa sometimes multiplicative. Exotesta palisade, cells beneath exotesta
hour-glass. Pleurograms and linea fissura usually absent. Endosperm often
absent; cell walls sometimes thick. Embryo usually curved (rarely straight),
with chlorophyll, sometimes starchy. Suspensor well developed. Cotyledons
accumbent, with margins against but not investing radicula, without amyloid
(xyloglucans); cotyledon areoles frequent. Radicula usually long, curved (in
Sophoreae short and straight). Germination phanerocotylar or
cryptocotylar. Duplication of gene CYC. 25 kb plastid IR absent.
Inversion of 50 kb in trnL intron in plastid LSC region present in
numerous Papilionoideae (absent in Swartzieae and in
Sophoreae). Isoflavonoids (pterocarpans, isoflavanes), prenylated
flavonoids, pyrrolizidine, indolizidine and quinolizidine alkaloids, canavanine
and other non-protein amino acids present. – Forisomes providing a regulatory
mechanism of phloem transport, are a synapomorphy of Papilionoideae,
which has been lost several times (i.a. in the astragalean clade).
Swartzioideae DC.,
Prodr. 2: 422. Nov (med.) 1825 [‘Svarzieae’]
8/210–230. Fairchildia (1;
F. panamensis; Central America, northwestern South America),
Swartzia (180–200; southern Mexico, Central America, the West
Indies, tropical South America), Bobgunnia (2; B.
fistuloides: tropical and southern Africa; B. madagascariensis:
Madagascar), Bocoa (4; B. marionii, B. prouacensis,
B. ratteri, B. viridiflora; tropical South America,
especially the Guayana Shield and northern Brazil), Candolleodendron
(1; C. brachystachyum; northeastern South America);
Trischidium (5; T. alternum, T. decipiens, T.
limae, T. molle, T. racemulosum; South America),
Ateleia (18; Central America, the West Indies, tropical South
America), Cyathostegia (1–2; C. mathewsii, C.
weberbaueri; Ecuador, Peru). – Tropical and subtropical America and
Africa. Trees or shrubs. Root nodules often formed. Floral prophylls
(bracteoles) absent. Sepals in Swartzia connate, early caducous. Petal
one. Nectary in at least Swartzia absent. Stamens numerous, free,
developing from annular meristem. Pistil composed of usually one carpel
(sometimes several carpels). Ovary in Swartzia stipitate. Arillus
usually present. Embryo in Swartzia straight. –
Swartzioideae (at least Swartzia) may be sister-group to the
remaining Papilionoideae except Dipterygeae. Some molecular
analyses (using the trnL intron) suggested that Swartzioideae
are instead sister to the remaining Papilionoideae (including the
Dipterygeae), although the support is weak.
Dipterygeae Polhill in
R. M. Polhill et P. H. Raven, Adv. Legume System. 1: 231. 1981
[‘Dipteryxeae’]
16/59. Angylocalyceae
clade Angylocalyx (6; A. boutiqueanus, A.
braunii, A. oligophyllus, A. pynaertii, A.
schumannianus, A. talbotii; tropical Africa),
Xanthocercis (3; X. madagascariensis, X. rabiensis,
X. zambesiaca; Gabon and Zambia to southern Africa, Madagascar),
Castanospermum (1; C. australe; western New Britain, eastern
Queensland, eastern New South Wales, New Caledonia, Vanuatu),
Uleanthus (1; U. erythrinoides; Amazonian Brazil). –
Dipterygeae clade Monopteryx (3; M.
angustifolia, M. inpae, M. uaucu; tropical South
America), Taralea (5; T. cordata, T. crassifolia,
T. oppositifolia, T. phaeophylla, T. reticulata;
southern Central America, Hispaniola, tropical South America),
Dipteryx (9; Central America, the West Indies, tropical South
America), Pterodon (2; P. abruptus, P. emarginatus;
Brazil, Bolivia). – Amburaneae clade
Dussia (10; southern Mexico, Central America, the West Indies,
tropical South America), Petaladenium (1; P. urceoliferum;
Rio Negro in Brazil), Myrospermum (1; M. frutescens; Mexico,
Central America, the West Indies, northern tropical South America),
Myrocarpus (4; M. fastigiatus, M. frondosus, M.
leprosus, M. venezuelensis; tropical South America),
Myroxylon (2; M. balsamum, M. peruiferum; Central
America, tropical South America), Dupuya (2; D. haraka,
D. madagascariensis; Madagascar), Amburana (3; A.
acreana, A. cearensis, A. erythrosperma; tropical South
America), Cordyla (6; C. africana, C. densiflora,
C. excelsa, C. pinnata, C. richardii, C.
somalensis; tropical Africa to South Africa, Madagascar). – Tropical
America and Africa, Madagascar, Papuasia to Melanesia. Adaxial two sepals in
Dipteryx, Pterodon and Taralea enlarged petaloid;
abaxial three sepals forming tridentate lobe. – Dipterygeae are
possibly sister-group to the remaining Papilionoideae.
Angylocalyceae seem to be sister to
[Dipterygeae+Amburaneae]. Some molecular analyses (using
matK with low bootstrap support) suggest that the clade
[Swartzioideae+Dipterygeae] is sister-group to the remaining
Papilionoideae.
[cladrastidoids+50 kb inversion clade]
The bootstrap support of this clade is
weak.
Cladrastidoids
3/18. ’Cladrastis’
(9; East Asia, one species, C. kentukea, in southeastern United
States; paraphyletic; incl. Pickeringia and Styphnolobium?),
Pickeringia (1; P. montana; California; in Cladrastis?),
Styphnolobium (8; southern United States, Mexico, Central America; in
Cladrastis?).
– East Asia, North to Central America. – The cladrastidoids may be
sister-group to the remaining Papilionoideae, but the bootstrap
support is weak.
50 kb inversion clade
(meso-papilionoids)
Inversion of 50 kb usually present in
the plastid Large Single Copy Region (situated in the intron between the genes
accD and trnL). Root nodules usually formed. – Unplaced:
Amphimas (4; A. ferrugineus, A. klaineanus, A.
pterocarpoides, A. tessmannii; western Central Africa).
Exostyleae Nakai,
Chosakuronbun Mokuroku [Ord. Fam. Trib. Nov.]: 254. 20 Jul 1943
6/17–19. Holocalyx (1; H.
balansae; tropical South America), Lecointea (4; L.
amazonica, L. ovalifolia, L. peruviana, L.
tango; tropical South America), Zollernia (8; Central America,
tropical South America); Harleyodendron (1; H. unifoliolatum;
northeastern Brazil), Exostyles (2–4; E. glabra, E.
venusta; southeastern Brazil); Uribea (1; U.
tamarindoides; Central America). – Tropical America.
[Dermatophyllum+Ormosieae+[Brongniartieae+Bowdichia
clade+[[Sophoreae
+Euchresteae+Thermopsideae]+[Podalyrieae+[Crotalarieae+Genisteae
s.str.]]]]]
Pyrrolizidine or quinolizidine alkaloids
present. x = 9.
Dermatophyllum
clade
1/6. Dermatophyllum (6; D.
arizonicum, D. gypsophilum, D. guadalupense, D.
juanhintonianum, D. secundiflorum, D. purpusii;
southwestern United States, northern Mexico). – Trees or shrubs.
Ormosieae Yakovlev in
Bobovye Zemnogo Shara: 72. 1991
4/c 140. Ormosia (c 130; East
Asia to Queensland, Central and eastern South America), Clathrotropis
(6; C. brachypetala, C. brunnea, C. glaucophylla,
C. macrocarpa, C. nitida, C. paradoxa; tropical
South America), Panurea (1; P. longifolia; Colombia, northern
Brazil), Spirotropis (2; S. candollei, S.
longifolia; northeastern South America). – Pantropical, few species in
temperate East Asia.
Genistoideae F.
Schwarz, Forstl. Bot.: 347. Oct 1891 [‘Genisteae’]
80/2.075–2.130. –
Brongniartieae and Podalyrieae form well supported clades,
whereas the
Sophoreae/Crotalarieae/Thermopsideae/Genisteae
clade is still largely unresolved. 50 kb inversion absent in
Sophoreae. – Unplaced Genistoideae: Pericopsis (4;
P. angolensis, P. elata, P. laxiflora, P.
mooniana; tropical Africa, Sri Lanka to Micronesia). –
Pericopsis may be closely allied to Camoensia (see below).
Brongniartieae Hutch.,
Gen. Fl. Plants 1: 393. 3 Dec 1964
c 16/150–155. Haplormosia (1;
H. monophylla; tropical West and Central Africa); Poecilanthe
(7; P. falcata, P. grandiflora, P. itapuana, P.
ovalifolia, P. parviflora, P. subcordata, P.
ulei; tropical South America); Harpalyce (c 30; Mexico, Cuba,
Brazil), Tabaroa (1; T. caatingicola; southwestern Bahia in
Brazil), Amphiodon (1; A. effusus; tropical South America),
Limadendron (2; L. amazonicum, L. hostmannii;
Colombia, southern Venezuela, the Guianas, Amazonian Brazil), Behaimia
(1; B. cubensis; Cuba), Cyclolobium (1; C.
brasiliense; Brazil, Paraguay, Bolivia), ‘Brongniartia’
ulbrichiana (Bolivia), Brongniartia (50–55; Mexico, Central
America, Ecuador, Peru), ‘Templetonia’ (13; Australia;
diphyletic), Hovea (35–40; southwestern, southeastern and eastern
Australia, Tasmania, Arnhem Land), Cristonia (2; C. biloba,
C. stenophylla; western Western Australia), Plagiocarpus (1;
P. axillaris; northeastern Western Australia, northwestern Northern
Australia), Lamprolobium (2; L. fruticosum, L.
grandiflorum; northeastern Queensland), Thinicola (1; T.
incana; northern Western Australia). – Tropical and subtropical America,
Australia. – Haplormosia is sister to the remaining
Brongniartieae and Poecilanthe successive sister to the rest
(Cardoso & al. 2017).
Leptolobieae (Benth.)
Cardoso et al. in South Afr. J. Bot. 89: 70. Nov
2013
6/27–32. Orphanodendron
(1–2; O. bernalii, O. grandiflorum; northwestern Colombia);
Diplotropis (10–12; Amazonas to northern Argentina),
Guianodendron (1; G. praeclarum; the Guayana Shield in Guyana
and northern Brazil), Staminodianthus (3; S. duckei, S.
racemosus, S. rosae; Amazonas), Bowdichia (2; B.
nitida, B. virgilioides; tropical South America),
Leptolobium (10–12; Mexico, Central America, tropical South
America). – Tropical America. – The position of Orphanodendron is
uncertain. Leptolobieae may be sister-group to the remaining
Genistoideae, although the branch support is low.
[Camoensieae+[[Sophoreae+Euchresteae+Thermopsideae]+[Podalyrieae+[Crotalarieae+Genisteae
s.str.]]]]
Camoensieae Yakovlev
in Bot. Zhurn. (Moscow & Leningrad) 53: 1477. 12 Jul 1968
1/2. Camoensia (2; C.
brevicalyx, C. scandens; coasts of the Gulf of Guinea and south
to Angola).
[[Sophoreae+Euchresteae+Thermopsideae]+[Podalyrieae+[Crotalarieae+Genisteae]]]
[Sophoreae+Euchresteae+Thermopsideae]
Sophoreae Spreng. ex
DC., Prodr. 2: 94. Nov (med.) 1825
6–11/64–76. Ammodendron
(5–10; A. bifolium, A. conollyi, A. eichwaldii,
A. karelinii, A. maxima; West and Central Asia to
northwestern China), Ammothamnus (3; A. gibbosus, A.
lehmannii, A. songoricus; Central Asia), Maackia
(10–11; East Asia), Salweenia (1–2; S. bouffordiana,
S. wardii; southeastern Tibet), Sophora (45–50;
southeastern Europe, South Asia to tropical Australia and islands in the
Pacific incl. the Hawaiian Islands, western United States, Florida, Puerto
Rico, western South America), Ammopiptanthus (2; A.
mongolicus, A. nanus; Central Asia to Mongolia and northwestern
China). – Tropical Africa, Asia, South America. – Panurea
(Ormosieae) and Bowdichia (Leptolobieae) have
similar pollen morphology. – Cardoso & al. (2013b) separated
Camoensia as a tribe (Camoensieae) sister to
[Sophoreae
s.lat.+[Podalyrieae+[Crotalarieae+Genisteae
s.str.]]]. Sophoreae in a wider sense include Thermopsideae
and Euchresteae. – Unplaced Sophoreae s.lat.:
Bolusanthus (1; B. speciosus; Northern Province, Mpumalanga,
Swaziland), Dicraeopetalum (3; D. stipulare: southeastern
Ethiopia, southern Somalia, northeastern Kenya; D. capuronianum,
D. mahafaliense: Madagascar), Neoharmsia (2; N.
baronii, N. madagascariensis; western and northwestern
Madagascar), Platycelyphium (1; P. voense; drier regions in
northeastern and eastern Africa), Sakoanala (2; S.
madagascariensis, S. villosa; northwestern and eastern
Madagascar).
Thermopsideae
Yakovlev in Bot. Žurn. (Moscow et Leningrad) 57: 592. 26 Jun 1972
5/61–62. Anagyris (1–2;
A. foetida, A. latifolia; the Canary Islands, the
Mediterranean, the Middle East to Iran), Piptanthus (2; P.
nepalensis, P. tomentosus; the Himalayas), Baptisia (27;
eastern and southern United States), Thermopsis (c 30; East Asia,
southern Canada, United States; incl. Vuralia?), Vuralia (1;
V. turcica; near Lake Aksehir in Turkey; in Thermopsis?). –
Macaronesia and the Mediterranean to Central and East Asia, North America.
Euchresteae (Nakai)
Ohashi in J. Jap. Bot. 48: 229. 1973
1/4. Euchresta (4; E.
formosana, E. horsfieldii, E. japonica, E.
tubulosa; India, the Himalayas, China, the Korean Peninsula, Japan,
Southeast Asia, Java, Taiwan).
[Podalyrieae+[Crotalarieae+Genisteae]]
Podalyrieae Benth. in
S. F. L. Endlicher, E. Fenzl, G. Bentham et H. W. Schott, Enum. Plant.: 27. Apr
1837
9/138–152. Cyclopia
(23; Western and Eastern Cape), ’Amphithalea’
(c 42; Western and Eastern Cape; paraphyletic?), Coelidium (c 20;
Northern, Western and Eastern Cape; in Amphithalea?),
Xiphotheca (9; Western and Eastern Cape; in Amphithalea?),
Stirtonanthus (3; S. chrysanthus, S. insignis,
S. taylorianus; Western Cape), Podalyria
(25–30; Western Cape to KwaZulu-Natal), Liparia (c
20; Western and Eastern Cape), Virgilia
(2; V. divaricata, V. oroboides; Western Cape, western
Eastern Cape), ’Calpurnia’ (7 or 16?; C. aurea, C.
capensis, C. glabrata, C. robinioides, C.
sericea, C. villosa, C. woodii; Africa incl. South
Africa; paraphyletic), Cadia (7; C. commersoniana, C.
ellisiana, C. emarginatior, C. pedicellata, C.
pubescens, C. purpurea, C. rubra; Madagascar, one
species in northeastern tropical Africa to southern Arabian Peninsula). –
South Africa, few species to northeastern Africa and the Arabian Peninsula.
Methylated anthocyanins, carboxylic acid esters of quinolizidine alkaloids,
3’-hydroxydaidzein, and canavanine (in seeds) present. Alkaloids not found.
– The position of Cadia is uncertain. Its species possess radially
symmetrical flowers, showing complete expression of the Cycloidea
genes (Citerne & al. 2006).
Oberholzeria (1; O.
etendekaensis; northwestern Namibia). – Oberholzeria is sister
to [Crotalarieae+Genisteae], according to Swanepoel & al.
(2015).
[Crotalarieae+Genisteae]
Crotalarieae Hutch.,
Gen. Fl. Plants 1: 364. 3 Dec 1964
16/1.095? Euchlora (1; E.
hirsuta; Northern and Western Cape), Bolusia (5; B.
acuminata, B. amboensis, B. ervoides, B.
grandis, B. resupinata; arid and semiarid regions of Africa south
of the equator), Crotalaria
(c 500?; tropical and subtropical regions on both hemispheres, with their
largest diversity in Africa and Madagascar), ’Wiborgiella’ (9;
Northern, Western and Eastern Cape; polyphyletic), Ezoloba (1; E.
macrocarpa; Western Cape), Calobota
(15; North and South Africa), ’Lebeckia’
(22; Namibia, Northern, Western and Eastern Cape, KwaZulu-Natal; paraphyletic),
Wiborgia
(10; Northern and Western Cape), Rafnia (19;
Western and Eastern Cape, KwaZulu-Natal), Aspalathus
(c 290; Western Cape to KwaZulu-Natal), ’Lotononis’
(c 150; the Mediterranean, Africa, southwestern Asia to India, with their
largest diversity in South Africa; paraphyletic), Listia (7; L.
angolensis, L. bainesii, L. heterophylla, L.
marlothii, L. minima, L. solitudinis, L.
subulata; southeastern Africa), Pearsonia (c 12; tropical to
southern Africa), Rothia (2; R. hirsuta, R. indica;
Africa to South Africa, India and Australia), Robynsiophyton (1;
R. vanderystii; southern Central Africa), Leobordea (51;
tropical and southern Africa, northeastern Africa, southwestern Asia). –
Mainly South Africa, few species in eastern and northeastern Africa and
southwestern Asia.
Genisteae Bronn in B.
C. J. Dumortier, Fl. Belg.: 98. 1827
9/525–545. Cytisus (c
140; Europe, Africa, Asia), Genista (c
130; Europe, Macaronesia, the Mediterranean, Asia), ’Adenocarpus’
(c 15; the Canary Islands, the Mediterranean, tropical African mountains;
paraphyletic), Lupinus
(200–220; the Mediterranean, North and East Africa, America, with their
largest diversity in western North and western South America),
Anarthrophyllum (15; the Andes), Sellocharis (1; S.
paradoxa; southeastern Brazil), Dichilus (5; D.
gracilis, D. lebeckioides, D. pilosus, D.
reflexus, D. strictus; southern Africa), Polhillia (6;
P. brevicalyx, P. canescens, P. connata, P.
ignota, P. obsoleta, P. pallens; Western Cape), Melolobium
(15; southern Africa). – Eurasia, Africa, North and South America.
α-pyridone-type quinolizidine alkaloids present.
[Vataireoid
clade+[Andirinae+Dalbergioideae]]
Vataireoid clade
4/26. Luetzelburgia (13;
northeastern South America to Bolivia, with their highest diversity in Brazil);
Sweetia (1; S. fruticosa; tropical South America),
Vataireopsis (4; V. araroba, V. iglesiasii, V.
speciosa, V. surinamensis; northern South America),
Vatairea (8; tropical America). – Tropical America. Root nodules not
formed. – Luetzelburgia is sister to
[Sweetia+[Vataireopsis+Vatairea]], according to
Cardoso & al. (2013).
[Andirinae+Dalbergioideae]
Andirinae Baill. in M.
A. Hartog [trans.], Nat. Hist. Plant. 2: 217. Sep-Dec 1872
[‘Andireae’]
3/c 70. Aldina (17; the Guayana
Shield to northern Amazonia in Brazil), Hymenolobium (13; tropical
South America), Andira (c 40; Central America, tropical South America,
one species, A. inermis, also in tropical West Africa). – Tropical
Central and South America, tropical West Africa. – Aldina has
ectomycorrhiza.
Dalbergioideae
Burnett, Outlines Bot.: 661. Feb 1835 [‘Dalbergidae’]
Amorpheae Boriss. in
Novit. Syst. Plant. Vasc. 1964: 224. 14 Oct 1964
8/245–250. Amorpha
(16; southern Canada, United States, northern Mexico, with their highest
diversity in southeastern United States), Parryella (1; P.
filifolia; Mexico), Apoplanesia (2; A. cryptopetala,
A. paniculata; drier regions in Central America), Errazurizia
(4; E. benthamii, E. megacarpa, E. multifoliolata,
E. rotundata; arid regions in southwestern United States, coastal
Chile), Eysenhardtia (13; Central America), ’Psorothamnus’
(c 10; arid regions in southwestern North America; paraphyletic),
Marina (c 40; southwestern United States, Mexico, Central America,
Venezuela), Dalea (c
160; arid and semiarid regions in Canada to Argentina, especially Mexico and
the Andes). – America, with their highest diversity in drier regions. Petals
often absent. Filaments often adnate to petals (epipetalous) forming tubular
stemonozone (hypanthium absent).
Dalbergieae DC.,
Prodr. 2: 415. Med Nov 1825
45/1.290–1.335. Adesmia
(210–220; South America), Poiretia (11; tropical America),
Amicia (8; Mexico, Central America, tropical South America),
Zornia (80–85; warmer regions on both hemispheres, with their
largest diversity in Brazil), Nissolia (14; Mexico, Central America,
the West Indies, tropical South America), Chaetocalyx (c 15; southern
Mexico, Central America, Hispaniola, tropical South America);
Riedeliella (3; R. graciliflora, R. magalhaesii,
R. sessiliflora; Brazil, Paraguay); Platypodium (2; P.
elegans, P. viride; Central America, tropical South America),
Paramachaerium (5; P. gruberi, P. krukovii, P.
ormosioides, P. schomburgkii, P. schunkei; Central
America, tropical South America), Pterocarpus (c 35; tropical and
subtropical regions), Ramorinoa (1; R. girolae; western
Argentina), Centrolobium (7; C. microchaete, C.
ochroxylum, C. paraense, C. robustum, C.
sclerophyllum, C. tomentosum, C. yavizanum; southern
Central America, tropical South America), Tipuana (1; T.
tipu; Brazil, Bolivia, Argentina), Inocarpus (3; I.
fagifer, I. glabellus, I. papuanus; Malesia to New
Guinea, islands in the Pacific), Discolobium (8; eastern South
America), Platymiscium (c 20; Mexico, Central America, tropical South
America), Acosmium (3; A. cardenasii: southwestern Brazil,
Bolivia; A. diffusissimum, A. lentiscifolium: southeastern
Brazil), Maraniona (1; M. lavinii; northern Peru),
Chapmannia (7; C. floridana, C. gracilis, C.
prismatica, C. reghidensis, C. sericea, C.
somalensis, C. tinireana; Somalia, Yemen, Socotra, one species,
C. floridana, in Florida, southern Mexico, Guatemala, northern
Venezuela), Arachis (c 70; South America), Stylosanthes
(40–45; tropical and subtropical regions), Grazielodendron (1;
G. riodocensis; Brazil), Cranocarpus (3; C.
gracilis, C. martii, C. mezii; Brazil), Brya
(6; B. buxifolia, B. chrysogonii, B. ebenus, B.
hirsuta, B. microphylla, B. subinermis; the West
Indies), Cascaronia (1; C. astragalina; Bolivia, northern
Argentina), Geoffroea (2; G. decorticans, G.
spinosa; South America), Fissicalyx (1; F. fendleri;
Venezuela, Guyana), Fiebrigiella (1; F. gracilis; Bolivia);
Machaerium (c 155; southern Mexico, Central America, the West Indies,
tropical South America, one species, M. lunatum, also in coastal
tropical West Africa), Steinbachiella (1; S. leptoclada;
Bolivia), Dalbergia (250–270; tropical Africa, Madagascar, tropical
Asia, Central America, tropical South America), ’Aeschynomene’ (c
175; tropical and subtropical regions; polyphyletic), Cyclocarpa (1;
C. stellaris; tropical Africa, tropical Australia), Kotschya
(c 30; tropical to southern Africa, Madagascar), Soemmeringia (1;
S. semperflorens; tropical South America), Smithia (c 22;
tropical and subtropical regions in the Old World), Humularia
(30–35; Central Africa), Bryaspis (2; B. humularioides,
B. lupulina; tropical West Africa), Geissaspis (4; G.
cristata, G. keilii, G. psittacorhyncha, G.
tenella; tropical and subtropical Asia), Pictetia (10; the West
Indies), Diphysa (c 20; Mexico, Central America, the West Indies,
tropical South America), Zygocarpum (6; Z. coeruleum, Z.
dhofarense, Z. gillettii, Z. rectangulare, Z.
somalense, Z. yemenense; Somalia, southern Arabian Peninsula,
Socotra), Ormocarpum (16; tropical and subtropical regions in the Old
World), Ormocarpopsis (7–8; O. aspera, O.
calcicola, O. itremoensis, O. mandrarensis, O.
nitida, O. parvifolia, O. tulearensis; Madagascar; in
Ormocarpum?), Weberbauerella (1–2; W.
brongniartioides, W. raimondiana; coast of Peru). – Tropical
and subtropical with their largest diversity in tropical South America.
Desmodioid (aeschynomenoid) root nodules with determinate growth and associated
with lateral roots and glandular-based trichomes are synapomorphies of
Dalbergieae (Chaetocalyx and Nissolia produce no
nodules; glandular-based trichomes are present in most species).
Old World clade
[Baphieae+Canavanine-accumulating
clade]
Baphieae Yakovlev in
Bot. Žurn. (Moscow et Leningrad) 53: 1477. 1968
5/58–63. Baphia (45–50;
tropical to southern Africa, Madagascar), Baphiopsis (1; B.
parviflora; tropical Africa), Leucomphalos (6; L.
brachycarpus, L. callicarpa, L. capparideus, L.
discolor, L. insignis, L. mildbraedii; tropical Africa,
Madagascar), Airyantha (3; A. borneensis, A.
confusum, A. schweinfurthii; Central Africa), Dalhousiea
(3; D. africana, D. bracteata, D. paucisperma;
tropical West Africa, northeastern India, Bangladesh). – Tropical Africa to
East Asia. Trees, shrubs, lianas. – Baphia is sister to the
remaining Papilionoideae, the Canavanine-accumulating clade.
Canavanine-accumulating clade
(non-protein amino acid-accumulating clade, NPAAA clade)
Canavanine (non-protein amino acid)
present. – The peptide pea albumin is present in Phaseoleae and
Hologalegina (absent in Robinieae).
Hypocalypteae
(Yakovlev) A. L. Schutte in A. L. Schutte et B.-E. van Wyk, Novon 8: 180.
1998
1/3. Hypocalyptus
(3; H. coluteoides, H. oxalidifolius, H.
sophoroides; Western and Eastern Cape).
Mirbelieae (Benth.)
Polhill in R. M. Polhill et P. H. Raven, Adv. Legume System. 1: 391. 1981
32/715–>745. Giant
antipodals group 1 Daviesia
(c 135; Australia, with their highest diversity in southwestern Australia),
Erichsenia (1; E. uncinata; southwestern Western Australia),
Viminaria (1; V. juncea; southwestern Western Australia,
coastal areas of southeastern Australia). – Giant antipodals group
2 Gompholobium
(44; Australia, with their highest diversity in southwestern Australia),
Sphaerolobium (18–22; Australia, with their highest diversity in
southwestern Australia), Goodia (2; G. lotifolia, G.
medicaginea; southwestern and southeastern Australia), Bossiaea
(c 60; temperate Australia, with their highest diversity in southwestern
Australia), Platylobium (4; P. alternifolium, P.
formosum, P. obtusangulum, P. triangulare; southeastern
South Australia to southeasternmost Queensland, Tasmania),
Muelleranthus (2; M. stipularis, M. trifoliolatus;
Australia), Paragoodia (1; P. crenulata; southwestern Western
Australia), Ptychosema (2; P. anomalum, P. pusillum;
Central and Western Australia), Aenictophyton (1; A.
reconditum; northwestern Australia). – Multiple embryo-sacs
group Isotropis
(14–16; Australia); Jacksonia
(35–40; Australia); Leptosema
(6; L. aculeatum, L. anomalum, L. bossiaeoides,
L. chambersii, L. daviesioides, L. uniflorum;
northern, central and southwestern Australia); Chorizema
(c 20; southwestern and eastern Australia), ‘Mirbelia’ (25–30;
Australia; polyphyletic), Oxylobium (17; Australia, with their highest
diversity in southwestern Australia), ‘Podolobium’ (6?;
southwestern and southeastern Australia; polyphyletic); Callistachys
(6; C. aciculifera, C. cordifolia, C. hamulosa,
C. lanceolata, C. microphylla, C. procumbens;
southwestern Western Australia), Gastrolobium
(100–110; Australia); Euchilopsis (1; E. linearis;
southwestern Western Australia), Phyllota (10–11; southwestern and
eastern Australia), Latrobea (5; L. brunonis, L.
diosmifolia, L. genistoides, L. hirtella, L.
tenella; southwestern Western Australia), Urodon (2; U.
capitatus, U. dasyphyllus; southwestern Western Australia),
Otion (8; northern, central and southwestern Australia)?,
Aotus (14–18; Australia), Stonesiella (1; S.
selaginoides; Tasmania), Almaleea (5; A. cambagei,
A. capitata, A. incurvata, A. paludosa, A.
subumbellata; eastern New South Wales, northeasternmost Victoria), Eutaxia (c
25; Australia, with their highest diversity in southwestern Australia),
Dillwynia (c 22; Australia), ‘Pultenaea’ (>110;
southwestern and southeastern Australia; non-monophyletic). – Australia, with
their highest diversity in southwestern and southern parts. –
Mirbelieae are an exclusively Australian clade. Aberrant types of
megagametophyte development or antipodal cells of unusual size are peculiar
features characterizing subclades in this group.
[[Indigofereae+Phaseoleae]+Hologalegina]
[Indigofereae+Phaseoleae]
Floral development with early expression
of monosymmetry.
Indigofereae Benth.
in C. F. von Martius, S. Endlicher et I. Urban, Fl. Bras. 15(1): 5-6, 35-36. 30
Jul 1859
6/>800. Phylloxylon (7;
P. arenicola, P. decipiens, P. perrieri, P.
phillipsonii, P. spinosa, P. xiphoclada, P.
xylophylloides; Madagascar); Indigofera
(>750; tropical and subtropical regions), Cyamopsis (4; C.
dentata, C. senegalensis, C. serrata, C.
tetragonoloba; drier regions in Africa south to South Africa and east to
India), Indigastrum (9; tropical and southern Africa, one pantropical
species), Microcharis (c 35; Africa south to South Africa, Madagascar,
the Arabian Peninsula), Rhynchotropis (2; R. marginata,
R. poggei; southern Central Africa). – Pantropical, with their
largest diversity in Africa and Madagascar. Inflorescence racemose.
Disynstemon (1; D.
paullinioides; southwestern Madagascar) may be sister to the millettioids
(Phaseoleae).
Phaseoleae DC.,
Prodr. 2: 381. mid Nov 1825 (the millettioids)
164/3.210–3.300.
Clitoriinae DC., Prodr. 2: 216. Nov (med.) 1825
[‘Clitoriae’]. Clitoria (c 45; tropical and subtropical
regions, with their largest diversity in South America), Centrosema (c
35; tropical and subtropical regions in America), Periandra (7; P.
berteriana, P. coccinea, P. densiflora, P.
gracilis, P. heterophylla, P. mediterranea, P.
pujalu; Hispaniola, Brazil, Bolivia), Clitoriopsis (1; C.
mollis; Congo, Sudan). – Austrosteenisia (4; A.
blackii, A. glabristyla, A. mollitricha, A.
stipularis; New Guinea, northern Australia). –
Abrinae Wight et Arn. ex Endl., Gen. Plant.: 1301.
Oct 1840 [‘Abrineae’]. Abrus (13–18; tropical and
southern Africa, Madagascar, the Arabian Peninsula, tropical Asia to China and
tropical Australia). – Diocleinae Benth., Comm.
Legum. Gen.: 113. Jun 1837 [‘Diocleae’]. Canavalia (c 50;
warmer regions of both hemispheres incl. the Hawaiian Islands, with their
highest diversity in tropical and subtropical America); ’Dioclea’
(c 50; tropical America, few species in the Old World; non-monophyletic; incl.
Cleobulia, Cymbosema, Macropsychanthus),
Macropsychanthus (3; M. dolichobotrys, M.
lauterbachii, M. mindanaensis; New Guinea, New Britain, Solomon
Islands; in Dioclea), Cymbosema (2; C. apurense,
C. roseum; tropical America; in Dioclea), Cleobulia
(3–5; C. multiflora; Mexico, Brazil; in Dioclea);
Rhodopis (2; R. lowdenii, R. planisiliqua;
Hispaniola), Neorudolphia (1; N. volubilis; Puerto Rico);
Bionia (5; B. bella, B. coccinea, B.
coriacea, B. pedicellata, B. tomentosa; Brazil,
especially Minas Gerais), Cratylia (4; C. argentea, C.
bahiensis, C. hypargyraea, C. mollis; South America),
Lackeya (1; L. multiflora; southeastern United States),
‘Camptosema’ (5–10; C. coriaceum, C.
paraguariense, C. pedicellatum, C. rubicundum, C.
scarlatinum; South America; polyphyletic), ’Galactia’ (c 55;
tropical and subtropical regions, with their largest diversity in South
America; non-monophyletic), Luzonia (1; L. purpurea; the
Philippines). – Millettieae Miq., Fl. Ned. Ind.
1(1): 137. 2 Aug 1855. Dalbergiella (2; D. nyasae, D.
welwitschii; tropical Africa), ’Millettia’ (c 150; tropical
and subtropical regions in the Old World, especially Africa and Madagascar;
polyphyletic), Deguelia (15–17; Panamá to Amazonas),
Leptoderris (c 40; tropical Africa), Philenoptera (13;
tropical Africa, Madagascar), Ophrestia (15–20; Africa, southern
Asia), Apurimacia (2; A. boliviana, A. dolichocarpa;
drier regions in South America), Mundulea (13–14; southern Africa,
Madagascar), Ptycholobium (3; P. biflorum, P.
contortum, P. plicatum; drier regions in northeastern and
southern Africa, the Arabian Peninsula), Tephrosia (>350; tropical
and subtropical regions on both hemispheres, especially Africa),
Piscidia (4; P. carthagenensis, P. grandifolia,
P. mollis, P. piscipula; Florida, Central America, the West
Indies), Lonchocarpus (120–130; tropical America, one species,
L. sericeus, also in tropical West Africa; paraphyletic?),
Pongamiopsis (3; P. amygdalina, P. pervilleana,
P. viguieri; Madagascar), ‘Fordia’ (18; Southeast Asia,
West Malesia; non-monophyletic), Burkilliodendron (1; B.
album; the Malay Peninsula), ‘Solori’ (9?; Southeast Asia;
non-monophyletic), ’Derris’ (c 65; tropical regions of the Old
World to New Guinea, with their largest diversity in Southeast Asia;
non-monophyletic); Platycyamus (1; P. regnellii; Brazil,
Peru); Kunstleria (11; Kerala in southwestern India, West and Central
Malesia, tropical Australia); Apios (8–10; East Asia, southern
Canada, United States); Craibia (2; C. atlantica, C.
brevicaudata; tropical to southern Africa), Ostryocarpus (2;
O. riparius, O. zenkerianus; tropical West and Central
Africa; incl. Xeroderris?), Xeroderris (1; X.
stuhlmannii; tropical to southern Africa; in Ostryocarpus?),
Platysepalum (7–8; tropical Africa), Schefflerodendron
(3–4; S. usambarense; tropical Africa), Sylvichadsia (4;
S. grandidieri, S. grandifolia, S. macrophylla,
S. perrieri; Madagascar), Hesperothamnus (5; H.
ehrenbergii, H. grandis, H. littoralis, H.
pentaphyllus, H. purpusii; Mexico), Dahlstedtia (1;
D. pinnata; southern Brazil, northern Argentina), Aganope
(3–6; A. gabonica, A. heptaphylla, A. thyrsiflora;
tropical Africa, Southeast Asia), Pyranthus (6; P. alasoa,
P. ambatoana, P. lucens, P. monantha, P.
pauciflora, P. tulearensis; Madagascar), Chadsia (c 17?;
Madagascar), Requienia (3; R. obcordata, R.
pseudosphaerosperma, R. sphaerosperma; western to northeastern
Africa, southern Africa), Dewevrea (1; D. bilabiata; tropical
West Africa). – Kennediinae Benth., Comm. Legum.
Gen.: 112. Jun 1837 [‘Kennedyeae’]. Shuteria (3; S.
densiflora, S. hirsuta, S. involucrata; tropical Asia),
Kennedia (14–16; New Guinea, Australia), Hardenbergia (3;
H. comptoniana, H. perbrevidens, H. violacea;
Australia), Vandasina (1; V. retusa; New Guinea, northeastern
Queensland). – Desmodiinae Benth. et Hook. f., Gen.
Plant. 1: 449. 19 Oct 1865 [‘Desmodieae’]. Craspedolobium
(2; C. schochii, C. unijugum; western China);
Campylotropis (c 37; the Himalayas, China, the Korean Peninsula,
Taiwan), ‘Lespedeza’ (c 40; tropical and eastern Asia, Australia,
temperate North and South America; paraphyletic), Kummerowia (2;
K. stipulacea, K. striata; East Asia; in
Lespedeza?); Hylodesmum (16; tropical Asia),
Pseudarthria (6; P. confertiflora, P. crenata,
P. fagifolia, P. hookeri, P. macrophylla, P.
viscida; southern Africa, Madagascar, Mauritius, Réunion, tropical Asia),
‘Desmodium’ (c 275; tropical and subtropical regions;
polyphyletic), Grona (41; tropical and subtropical regions),
‘Alysicarpus’ (30–35; tropical and subtropical Africa, southern
Asia; paraphyletic), Melliniella (1; M. micrantha; western central
Africa; in Alysicarpus?), Leptodesmia (3; L.
bojeriana, L. congesta, L. perrieri; Madagascar,
India?), Codariocalyx (2; C. gyroides, C. motorius;
India, Sri Lanka, China, Indochina, Malesia to tropical Australia, Taiwan),
‘Uraria’ (c 20; tropical and subtropical regions in the Old World;
non-monophyletic), Mecopus (1; M. nidulans; India, Hainan,
Indochina, Java), Christia (2; C. obcordata, C.
vespertilionis; India to China and Indochina, Malesia to northern
Australia), Eleiotis (2; E. monophyllos, E.
rottleri; India and Sri Lanka to Burma), Ototropis (13?; tropical
Asia, southern China, Taiwan), Pycnospora (1; P. lutescens;
tropical East Africa to Somalia, India, East and Southeast Asia to northern
Australia), Trifidacanthus (1; T. unifoliolatus; Hainan,
southern Vietnam, the Philippines, Lombok, Flores); Ougeinia (1;
O. oojeinensis; India, western Nepal), Aphyllodium (4; A.
australiense, A. biarticulatum, A. hispidum, A.
novoguineense; India, Sri Lanka, Hainan, Indochina, Malesia to New Guinea,
northern Australia), Tadehagi (4; T. pseudotriquetrum, T.
robustum, T. rodgei, T. triquetrum; tropical Asia),
Akschindlium (1; A. godefroyanum; Southeast Asia),
Droogmansia (6; D. chevalieri, D. megalantha, D.
munamensis, D. pteropus, D. scaettaiana, D.
wheptei; tropical Africa), Dendrolobium (c 20; tropical Asia,
Indian Ocean islands, Australia), Phyllodium (8; tropical and
subtropical Asia to northern Australia), Ohwia (1; O.
caudata; India, China, Japan, Indochina, Malesia, Taiwan),
Hanslia (2; H. hentyi, H. ormocarpoides; Malesia to
New Guinea, northern Queensland, Vanuatu), ‘Nephrodesmus’ (5–7;
N. albus, N. ferrugineus, N. francii, N.
hochreutineri, N. macrobotryosus, N. parvifolius, N.
sericeus; New Caledonia; polyphyletic), Arthroclianthus (c 20;
New Caledonia, one species also in Vanuatu), Verdesmum (1; V.
hentyi; Yunnan). – Cajaninae Benth., Comm.
Legum. Gen.: 113. Jun 1837 [‘Cajaneae’]. Butea (2; B.
monosperma, B. superba; tropical Asia), Cajanus (37;
tropical regions in the Old World to Australia), Rhynchosia (c 250;
tropical and subtropical regions), Eriosema (>150; tropical and
subtropical regions), Adenodolichos (22; tropical Africa),
Paracalyx (6; P. balfourii, P. microphyllus, P.
nogalensis, P. scariosus, P. schweinfurthii, P.
somalorum; Ethiopia, Somalia, Socotra, India, Indochina),
Bolusafra (1; B. bituminosa; Western Cape),
Carrissoa (1; C. angolensis; Angola), Dunbaria
(20–25; India, Southeast Asia to China, Malesia to New Guinea, northern
Australia), Flemingia (c 30; tropical regions in the Old World). –
Erythrininae Benth., Comm. Legum. Gen.: 113. Jun 1837
[‘Erythrineae’]. Otoptera (2; O. burchelli:
tropical Africa; O. madagascariensis: Madagascar), Erythrina
(c 130; tropical and subtropical regions on both hemispheres),
Psophocarpus (7; P. grandiflorus, P. lancifolius,
P. lukafuensis, P. monophyllus, P. palustris, P.
scandens, P. tetragonolobus; tropical and subtropical regions in
the Old World), Spatholobus (c 30; Southeast Asia to Central Malesia),
Meizotropis (2; M. buteiformis, M. pellita; India,
the Himalayas, western Indochina), Cochlianthus (2; C.
gracilis, C. montanus; Nepal, western China). –
Psoraleeae Benth. in C. F. von Martius, S. Endlicher
et I. Urban, Fl. Bras. 15(1): 3–4, 31–34. 1859 [‘Psoraleae’].
Calopogonium (4; C. caeruleum, C. galactioides,
C. mucunoides, C. velutinum; Mexico, Central America,
the West Indies, tropical South America, one species, C. mucunoides,
also in the Old World tropics), Pseudovigna (1; P. argentea;
tropical Africa), Neorautanenia (3; N. amboensis, N.
ficifolius, N. mitis; southern tropical Africa),
Pachyrhizus (5; P. ahipa, P. erosus, P.
ferrugineus, P. panamensis, P. tuberosus; Mexico,
Central America, the West Indies, tropical South America); Neonotonia
(2; N. verdcourtii, N. wightii; tropical and southern Africa,
tropical Asia from southern Arabian Peninsula to Malesia);
’Pueraria’ (17–20; southern and eastern Asia; non-monophyletic);
Amphicarpaea (5; A. africana, A. bracteata, A.
edgeworthii, A. ferruginea, A. linearis; Africa, East
Asia, North America), Teramnus (7; T. flexilis, T.
labialis, T. micans, T. mollis, T. repens,
T. uncinatus, T. volubilis; tropical and subtropical regions
on both hemispheres), ’Glycine’ (c 25; Africa, southern Asia to
Australia; non-monophyletic?), Sinodolichos (2; S. lagopus,
S. oxyphyllus; Burma, southern China; in Glycine?),
Bituminaria (4; B. acaulis, B. bituminosa, B.
flaccida, B. morisiana; the Mediterranean to the Caucasus and the
Middle East), ’Otholobium’ (50–55; eastern and southeastern
Africa, South Africa, South America; non-monophyletic), Orbexilum (11;
the United States, Mexico), Psoralea (30–35; southern Africa, with
their largest diversity in Western Cape), Cullen (c 35; the
Mediterranean, Africa, southern Asia to New Guinea and Australia),
Pediomelum (20–25; North America), Ladeania (2; L.
juncea, L. lanceolata; western United States), Rupertia
(3; R. hallii, R. physodes, R. rigida; southwestern
Canada, western United States), Herpyza (1; H. grandiflora;
western Cuba), Teyleria (3; T. koordersii; Southeast Asia,
West Malesia), Dumasia (c 10; Africa to southern Asia), Nogra
(4; N. dalzellii, N. filicaulis, N. grahamii, N.
guangxiensis; India to southern China and Thailand; non-monophyletic?),
Eminia (4; E. antennulifera, E. benguellensis,
E. harmsiana, E. holubii; Zambesian region in Africa),
Pseudeminia (4; P. benguellensis, P. comosa, P.
mendoncae, P. muxiria; tropical Africa), Phylacium (2;
P. bracteosum, P. majus; Southeast Asia to Queensland),
Neocollettia (1; N. wallichii; Burma, Java),
Mastersia (1; M. bakeri; Assam, Central Malesia),
Diphyllarium (1; D. mekongense; Indochina). –
Phaseolinae J. Presl in Nowočeská Bibl. [Wšobecný
Rostl.] 7: 346, 402. 1846 [‘Phaseolaceae’]. Cologania
(12; tropical America, with their highest diversity in Mexico); Wajira
(5; W. albescens, W. danissana, W. grahamiana,
W. praecox, W. virescens; Africa, southwestern Arabian
Peninsula, India, Sri Lanka); Sphenostylis (5; S.
angustifolia, S. erecta, S. marginata, S.
schweinfurthii, S. stenocarpa; Africa, India), Decorsea
(6; D. dinteri, D. galpinii, D. grandidieri, D.
livida, D. meridionalis, D. schlechteri; tropical to
southern Africa, Madagascar), Macrotyloma (c 25; tropical and
subtropical Africa and Asia), Alistilus (3; A. bechuanicus,
A. jumellei, A. magnificus; southern tropical Africa,
Madagascar), Dolichos (c 60; tropical and subtropical Africa to South
Africa to India and East Asia), Nesphostylis (4; N.
bracteata, N. holosericea, N. junodii, N.
lanceolata; tropical Africa, tropical Asia); Dipogon (1; D.
lignosus; Western and Eastern Cape), Lablab (1; L.
purpureus; tropical and subtropical Africa), Spathionema (1;
S. kilimandscharicum; tropical Africa), Vatovaea (1; V.
pseudolablab; tropical East Africa to Oman), Physostigma (2–4;
P. mesoponticum, P. venenosum; tropical Africa),
’Vigna’ (90–100; tropical and subtropical regions on both
hemispheres; non-monophyletic), Phaseolus (c 70; tropical and
subtropical regions in America), Oxyrhynchus (4; O. papuanus,
O. populneus, O. trinervus, O. volubilis; East
Malesia, Central America), Ramirezella (7; R. calcoma, R.
crassa, R. lozanii, R. micrantha, R. nitida,
R. penduliflora, R. strobilophora; Mexico, Central America),
Cochliasanthus (1; C. caracalla; northern South America,
Bolivia, Argentina), Leptospron (2; L. adenanthum, L.
gentryi; Mexico, Central America, Colombia), Sigmoidotropis (9;
Mexico, Central America, Hispaniola, tropical South America to Peru),
Helicotropis (4; H. hookeri, H. linearis, H.
spectabilis, H. stenoloba; Mexico, Central America, tropical
South America to Bolivia), Ancistrotropis (6; A. arrabidae,
A. clitorioides, A. formula, A. peduncularis, A.
robusta, A. subhastata; Mexico, Central America, tropical South
America to Bolivia), Condylostylis (4; C. candida, C.
latidenticulata, C. venusta, C. vignoides; Mexico,
Central America, tropical South America to Argentina), Macroptilium
(19; Mexico, Central America, the West Indies, tropical South America),
Mysanthus (1; M. uleanus; Brazil), Dolichopsis (2;
D. ligulata, D. paraguariensis; southern Brazil, Paraguay,
northern Argentina), Strophostyles (3; S. helvola, S.
leiosperma, S. umbellata; southern Canada, United States),
Mucuna (100–105; tropical and subtropical regions on both
hemispheres), Pseudoeriosema (5; P. andongense, P.
borianii, P. homblei, P. longipes, P.
moeroense; tropical Africa), Oryxis (1; O. monticola;
Minas Gerais in Brazil), Austrodolichos (1; A. errabundus;
northern Australia), Dysolobium (6; D. apioides, D.
dolichoides, D. grande, D. lucens, D. pilosum,
D. tetragonum; eastern India, southwestern China, Indochina, Malesia),
Strongylodon (14; Madagascar, tropical Asia to Polynesia). –
Tropical to warm-temperate regions. Inflorescence often pseudoracemose. –
Phaseoleae are still in a state of flux. The relationships among the many
lineages are far from understood and the clades have low bootstrap support.
However, Clitoriinae may be sister-group to the remaining
Phaseoleae, and the clade
[Abrinae+[Dioclinae+Millettieae]] (the core
millettioids) sister to the rest (the phaseoloids).
Hologalegina
(galegoids)
Hologalegina consist of two
mainly northern hemispherical major lineages, the Robinioid clade and the
Inverted Repeat-Lacking Clade.
[Robinioid clade+IRLC]
Robinioid clade
Loteae DC., Prodr. 2:
115. mid Nov 1825
19/290–300. Antopetitia (1;
A. abyssinica; mountains in tropical East Africa),
Pseudolotus (1; P. villosus; Oman to Pakistan),
Podolotus (1; P. hosackioides; Oman to western India),
Dorycnopsis (2; D. abyssinica, D. gerardii; western
Mediterranean, northeastern Africa, the Arabian Peninsula), Ornithopus
(5; O. compressus, O. micranthus, O. perpusillus,
O. pinnatus, O. sativus; Europe, the Mediterranean, western
Asia, Atlantic islands, temperate South America), Hosackia (14;
southwestern Canada to Guatemala), Acmispon (17;
southern Canada to Mexico, one species, A. subpinnatus, in Chile;
incl. Kebirita, Ottleya and Syrmatium?),
Kebirita (1; K. roudairei; northwestern Africa; in Acmispon?),
Ottleya (10; southwestern North America; in Acmispon?),
Syrmatium (12; western North America; in Acmispon?),
Hippocrepis
(34; Europe, the Mediterranean, western Asia), Scorpiurus
(2; S. muricatus, S. vermiculatus; Macaronesia, the
Mediterranean, the Near East to Iran), Coronilla (c
20; Europe, the Mediterranean, Atlantic islands; incl. Securigera?),
Securigera
(12; Europe, the Mediterranean, northeastern Africa to Somalia; in Coronilla?),
Anthyllis
(22; Europe, Macaronesia, the Mediterranean, northeastern Africa),
Hammatolobium (2; H. kremerianum, H. lotoides; the
Mediterranean), Tripodion
(1; T. tetraphyllum; the Mediterranean), Cytisopsis (2;
C. dorycniifolia, C. pseudocytisus; eastern Mediterranean,
North Africa), Lotus
(130–140; temperate regions in the Old World, Macaronesia, the Mediterranean,
southwestern Asia). – Temperate regions on the Northern Hemisphere, temperate
South America.
[Sesbanieae+Robinieae]
The support of this clade is fairly
weak.
Sesbanieae Hutch.,
Gen. Flow. Pl. 1: 401. 3 Dec 1964
1/c 60. Sesbania
(c 60; tropical and subtropical regions on both hemispheres). – Sesbania
is sister to either Robinieae or Loteae.
Robinieae Hutch.,
Gen. Fl. Plants 1: 366. 3 Dec 1964
11/64–76. Robinia (5–10;
southern Canada, United States), Genistidium (1; G. dumosum;
Texas, Mexico), Peteria (3; P. glandulosa, P.
scoparia, P. thompsoniae; southwestern North America),
Coursetia (35; southwestern United States, Mexico, Central America,
the West Indies, tropical South America to Brazil and Peru), Olneya
(1; O. tesota; southwestern United States, northwestern Mexico),
Sphinctospermum (1; S. constrictum; western Mexico),
Poissonia (4; P. eriantha, P. heterantha, P.
orbicularis, P. weberbaueri; Peru, Bolivia, Argentina),
Lennea (3–5; L. melanocarpa, L. modesta, L.
viridiflora; Central America), Hebestigma (1; H.
cubense; Cuba), Gliricidia (5; G. brenningii, G.
ehrenbergii, G. maculata, G. robustum, G.
sepium; tropical America), Poitea (5–10; P. carinalis,
P. florida, P. galegoides, P. paucifolia, P.
punicea; the West Indies). – Warm-temperate to tropical regions in
America. Loss of plastid inverted repeat.
IRLC (inverted repeat-lacking
clade)
Leaves without pulvini, with somewhat
different development. Flower with CA primordia. Stamens with bidirectional
initiation. Initiation of petals, stamens and carpels overlapping. Plastid
inverted repeat absent (lost). Introns in plastid genes clpP and
rps12 absent (lost).
Glycyrrhizeae Rydb.,
Fl. Rocky Mts.: 454. 31 Dec 1917
1/19. Glycyrrhiza
(19; Europe, Asia, Australia, North America, temperate South America; incl.
‘Callerya’ atropurpurea).
[Wisterieae+[[Hedysareae+Galegeae]+Fabeae]]
Wisterieae X. Y. Zhu
in Cathaya 6: 121. 1994
3/14. Wisteria
(8; East and Southeast Asia to northeastern Australia and New Caledonia,
eastern United States), Endosamara (1; E. racemosa; India,
Sri Lanka, Southeast Asia, Malesia), Antheroporum (5; A.
banaense, A. glaucum, A. harmandii, A. pierrei,
A. vidalii; southwestern China, Indochina). – Eastern North America,
East, South and Southeast Asia, New Guinea, Australia, New Caledonia. n = 8.
rps12 intron present.
[[Hedysareae+Galegeae]+Fabeae]
Hedysareae DC.,
Prodr. 2: 307. mid Nov 1825
14/475–520. Chesneya
clade Chesneya (c 35; Southwest and Central Asia to Mongolia
and the Himalayas), Chesniella (5; C. ferganensis, C.
gansuensis, C. gracilis, C. mongolica, C.
villosa; Central Asia and Mongolia to northwestern China),
Gueldenstaedtia (5–10; G. himalaica, G. henryi,
G. monophylla, G. taihangensis, G. verna; Siberia
and Mongolia to the Himalayas and western China), Tibetia (5; T.
forrestii, T. himalaica, T. tongolensis, T.
yadongensis, T. yunnanensis; Central Asia, the Himalayas, Tibet,
western China). – Caragana clade Caragana
(80–100; eastern Europe, Turkey and the Caucasus to Siberia, Mongolia, West
and Central Asia to China). – Hedysarum
clade Alhagi (6; A. canescens, A.
graecorum, A. kirghisorum, A. maurorum, A.
nepalensis, A. sparsifolia; the Mediterranean to Nepal),
Sulla (6; S. aculeolata, S. capitata, S.
carnosa, S. coronaria, S. pallida, S.
spinosissima; the Mediterranean and North Africa to Siberia),
Ebenus (c 20; the Mediterranean to Baluchistan), Taverniera
(15; northeastern Africa, southwestern Asia), Hedysarum (c 160;
temperate regions on the Northern Hemisphere), Onobrychis (130–150;
Europe, the Mediterranean, Asia, Ethiopia), Greuteria (1; G.
argyrea; Morocco, West Sahara), Eversmannia (4; E.
botschantzevii, E. sarytavica, E. sogdiana, E.
subspinosa; southeastern Russia, northern Iran, Central Asia),
Corethrodendron (5; C. fruticosum, C. krasnowii,
C. lignosum, C. multijugum, C. scoparium; Russia,
Kazakhstan, Mongolia, China). – Temperate regions in Eurasia, North Africa to
Ethiopia.
Galegeae Bronn in B.
C. J. Dumortier, Fl. Belg.: 101. 1827
14/3.685–3.740. Carmichaelia
clade Carmichaelia (c 25; New Zealand, Lord Howe),
Streblorrhiza (1; S. speciosa; Philip Island near Norfolk
Island, extinct), Swainsona (c 85; drier regions in Australia),
Clianthus (2; C. maximus, C. puniceus; northeastern
New Zealand). – Colutea clade Erophaca (1;
E. baetica; the Mediterranean), Oxytropis (300–350;
temperate regions on the Northern Hemisphere, especially Central Asia),
Astragalus (>3.280; mainly temperate and subtropical regions on the
Northern Hemisphere, tropical African mountains, northern India, the Andes to
Chile, one species in southern Africa), Colutea (25–28; the
Mediterranean, northeastern and eastern Africa, Asia to China and the
Himalayas), Oreophysa (1; O. microphylla; Iran),
Eremosparton (3; E. aphyllum, E. flaccidum, E.
songoricum; southeastern Russia to Central Asia), Sphaerophysa
(2; S. kotschyana: Central Anatolia; S. salsula: eastern
Mediterranean to Central Asia), Smirnowia (1; S. turkestana;
Turkestan), Lessertia (c 55; tropical eastern and southern Africa).
– Galega clade Galega (6–8;
G. albiflora, G. battiscombei, G. lindblomii, G.
officinalis, G. orientalis; eastern Europe, East African
mountains, temperate Asia). – Temperate and subtropical regions on the
northern hemisphere, the Andes south to Chile, tropical African mountains,
South Africa, Australia, Norfolk Island, Lord Howe, New Zealand.
Fabeae Reichenb., Fl.
Germ. Excurs. 2(2): 525. 1832
9/755–825.
Trifoliinae Bronn, Form. Plant. Legumin.: 127, 132.
1822 [‘Trifolieae’]. Trifolium
(240–300; temperate and subtropical regions on both hemispheres except
Australia), Ononis (c 30;
Europe, the Canary Islands, the Mediterranean, Ethiopia, Iran), Medicago
(85–90; Europe, the Mediterranean, Ethiopia, southern Africa, Asia), Trigonella
(35–40; Macaronesia, the Mediterranean, southern Africa, Australia), Melilotus
(19; Europe, the Mediterranean, North Africa, Ethiopia, temperate and
subtropical Asia). – Parochetinae Yakovlev, Bobovye
Zemnogo Shara: 117. 1991. Parochetus
(1; P. communis; mountains of tropical Africa and Asia to Java). –
Viciinae Bronn, Form. Plant.
Legumin.: ad Sect. 134, 133. 1822 [‘Vicieae’]. Cicer (c 45;
Greece, the Canary Islands, Morocco, Ethiopia, West and Central Asia), Lathyrus
(c 160; temperate regions on the Northern Hemisphere, tropical East African
mountains, temperate South America); Vicia (c
140; temperate regions on the Northern Hemisphere, tropical East Africa, South
America, the Hawaiian Islands). – Subcosmopolitan. – Parochetus
may be sister to the remaining Fabeae. A peculiar type of microhairs
is a synapomorphy of Fabeae. Vicia has
secondary pollen display by stigmatic pollen brush.
 |
Phylogeny (somewhat simplified) of Fabaceae based on DNA
sequence data (LPWG 2013). Dotted branches are weakly supported.
|
 |
Phylogeny (simplified) of Fabaceae based on DNA
sequence data (LPWG 2013). Dotted branches are weakly supported.
|
POLYGALACEAE Hoffmanns.
et Link
|
( Back to Polygalales )
|
Hoffmannsegg et Link, Fl. Portug. 1: 62. 1 Sep
1809 [‘Polygalinae’], nom. cons.
Polygalopsida Endl.,
Gen. Plant.: 1076. Apr 1840 [’Polygalineae’];
Diclidantheraceae J. Agardh, Theoria Syst. Plant.:
195. Apr-Sep 1858 [‘Diclidanthereae’], nom. cons.;
Moutabeaceae (Endl.) Endl. in H. Pfeiffer, Nomencl.
Bot. 2(1): 364. 24 Jan 1873; Polygalineae Bessey in
C. K. Adams, Johnson’s Universal Cyclop. 8: 461. 15 Nov 1895;
Xanthophyllaceae (Baill. ex Engl.) Gagnep. ex Reveal
et Hoogland in Bull. Mus. Natl. Hist. Nat., sér. 4, sect. B, Adansonia, 12:
206. 24 Nov 1990; Polygalanae Doweld, Tent. Syst.
Plant. Vasc.: xxxviii. 23 Dec 2001
Genera/species
28/1.025–>1.100
Distribution Cosmopolitan
except polar regions, Polynesia and New Zealand.
Fossils
Polycolporopollenites is stephanocolpate pollen from the Palaeogene
and Neogene of New Zealand and the Falkland Islands. Paleosecuridaca
curtisii comprises fossilized fruits (composed of two-seeded carpels) of
Polygalaceae from the
Paleocene of North Dakota.
Habit Usually bisexual (in
Balgoya functionally unisexual), perennial or annual herbs, evergreen
or deciduous? shrubs, trees (rarely lianas; Salomonia and
Epirixanthes comprise chlorophyllous hemimycotrophs versus
achlorophyllous holomycotrophs). Some species are xerophytes. Often with paired
crateriform glands (extrafloral nectaries) or spines at nodes (sometimes
elsewhere).
Vegetative anatomy Arbuscular
mycorrhiza present in at least some genera (e.g. Polygala,
Polygaloides, Salomonia, and Epirixanthes).
Phellogen ab initio superficial. Medulla with lignified and unlignified cells.
Primary vascular tissue cylinder, without separate vascular bundles. Secondary
lateral growth anomalous (from concentric cambia) present in lianas, or absent.
Vessel elements (usually solitary) usually with simple perforation plates;
lateral pits usually alternate, simple or bordered pits. Imperforate tracheary
xylem elements usually tracheids (in Securidaca fibre tracheids) with
bordered pits, non-septate (also vasicentric tracheids). Wood rays usually
uniseriate (sometimes biseriate or multiseriate), heterocellular. Axial
parenchyma usually paratracheal aliform, lozenge-aliform, winged-aliform
confluent, vasicentric, reticulate, or banded (sometimes apotracheal diffuse or
diffuse-in-aggregates). Tyloses present in some species. Intraxylary
(concentric) phloem present in lianas. Sieve tube plastids S type. Nodes 1:1,
unilacunar with one leaf trace. Calciumoxalate as druses, crystal sand or
prismatic or acicular crystals.
Trichomes Hairs unicellular,
simple, or absent.
Leaves Usually alternate
(rarely opposite or verticillate), simple, entire, often coriaceous, sometimes
ericoid or scale-like or absent, with ? ptyxis. Stipules intrapetiolar
(sometimes scale-like or modified into spines or paired glands) or absent; leaf
sheath absent. Leaf base or abaxial side of lamina in Xanthophyllum
with nectariferous glands (extrafloral nectaries; rarely on petiole). Petiole
vascular bundle transection? Venation pinnate, usually brochidodromous. Stomata
usually anomocytic or paracytic (in Xanthophyllum anisocytic or
paracytic; in Balgoya cyclocytic). Cuticular wax crystalloids as
rosettes of platelets (Fabales type). Lamina sometimes with lysigenous
secretory cavities containing ethereal oils, often with glands or domatia. Leaf
margin usually entire (rarely with nectariferous glandular teeth).
Inflorescence Terminal or
axillary, usually spike or raceme (sometimes panicle; flowers rarely solitary
axillary).
Flowers Usually zygomorphic
(rarely actinomorphic). Pedicel often articulated. Hypogyny. Sepals five, with
imbricate-quincuncial aestivation, persistent or caducous, free or partially or
entirely connate (two lower sepals connate at base; sepals sometimes connate
into tube); median sepal adaxial; two adaxial lateral sepals often enlarged
into wing-like alae and petaloid, two abaxial lateral sepals small. Petals
three or five (in Xanthophyllum rarely four), with imbricate or
contorted aestivation, free or connate at base; vexillum consisting of two
connate adaxial petals, carina consisting of abaxial petal (often fimbriate),
two abaxial-lateral petals small. Alternatively: sepals almost uniform, carina
consisting of abaxial petal. Alternatively: flowers more or less actinomorphic,
with sepals and petals almost uniform, without distinct carina. Nectariferous
disc intrastaminal, annular or unilateral (sometimes excentric), or absent.
Some species of Polygala with pollination mechanisms (also secondary
pollen display) similar to Faboideae.
Androecium Stamens (two to)
five to eight (to ten); median stamen in each whorl usually absent. Filaments
usually connate in lower part (often into tube) around pistil, usually more or
less adnate at base to petals (epipetalous). Anthers usually basifixed (in
Xanthophyllum dorsifixed), usually non-versatile, disporangiate or
tetrasporangiate, usually dehiscing by apical or subapical pores or very short
slits (rarely by longitudinal introrse? slits). Tapetum secretory. Female
flowers with staminodia?
Pollen grains
Microsporogenesis simultaneous. Pollen grains (5–)7–23(–33)-polycolporate
(sometimes heteropolar and asymmetrical; rarely syncolporate; in
Balgoya tricolporate), shed as monads, bicellular or tricellular at
dispersal. Exine tectate, with columellate infratectum, perforate (to
microreticulate), punctate, or finely rugulate, verrucate, granulate,
fossulate, foveolate, or psilate.
Gynoecium Pistil composed of
two to eight connate carpels; when two carpels then adaxial carpel reduced.
Ovary superior, usually bilocular to quinquelocular (sometimes unilocular,
pseudomonomerous, with one carpel reduced; in Xanthophyllum
stipitate). Style single, long, erect or curved, simple or bifid (one branch
with apical stigma, second branch with hair tuft). Stigma capitate or bilobate,
often complex, asymmetrical, non-papillate, Dry type. Male flowers with
pistillodium?
Ovules Placentation axile or
parietal. Ovule usually one per carpel (in Xanthophyllum four to more
than 40 per unilocular ovary composed of two carpels), anatropous or
hemianatropous, pendulous, usually epitropous (in Xanthophyllum
apotropous), bitegmic, crassinucellar. Micropyle usually bistomal, Z-shaped
(zig-zag) (sometimes exostomal or endostomal). Outer integument usually two to
five (in Xanthophyllum four to twelve) cell layers thick. Inner
integument (one or) two to three (in Securidaca up to nine) cell
layers thick. Hypostase enlarged. Parietal tissue approx. two cell layers
thick. Nucellar cap present. Megagametophyte 8-nucleate (probably monosporous,
Polygonum type; rarely tetrasporous). Synergids sometimes with a
filiform apparatus. Endosperm development ab initio nuclear. Endosperm
haustoria? Embryogenesis asterad.
Fruit Usually a loculicidal
capsule with membranous or fleshy pericarp (often flattened; rarely a berry, a
drupe or a nut, often a samara; in, e.g., Polygala with persistent
green calyx).
Seeds Hairs (sometimes
containing juice, sometimes as coma near hilum), exostomal elaiosome or
carunculus, or other arilloid structures often present (funicular aril present
in Moutabeeae). Seed coat endotestal. Testa multiplicative. Exotesta
subsclerotic. Endotestal cells isodiametric or palisade with narrowly elongate
cells, with U-shaped thickenings, usually with crystals. Tegmen? Perisperm not
developed. Endosperm copious or sparse, starchy (oily?), or absent. Embryo
straight, at least in some species with chlorophyll. Cotyledons two,
planoconvex. Germination phanerocotylar or cryptocotylar.
Cytology n = 5–14, 16, 17,
19, 20, 22, 23, 28–30 – Polyploidy and aneuploidy frequently occurring.
DNA Gene rpl22
present in nuclear genome (not in plastid genome; at least in
Polygala). Plastid gene rps16 absent (lost) in
Polygala (P. lindheimeri investigated) and
Securidaca (S. diversifolia investigated).
Phytochemistry Flavonols
(kaempferol, quercetin), indole and ergoline alkaloids and triterpene saponins
present. Ellagic acid, tannins, proanthocyanidins, and cyanogenic compounds not
found. Starch often replaced by saccharose or polygalite (1,5-anhydrosorbite).
Aluminium accumulated in Xanthophyllum and Moutabeeae.
Use Ornamental plants
(Polygala), medicinal plants (e.g. Polygala senega).
Systematics
Xanthophyllum is sister to the remaining Polygalaceae.
Xanthophyllum
1/c 95. Xanthophyllum (c 95;
southern India, Sri Lanka, Southeast Asia, Malesia, New Guinea, the Solomon
Islands, Queensland). – Trees or shrubs. Axial parenchyma apotracheal,
diffuse. Glands present at nodes. Hypogyny. Sepals unequal, caducous. Petals
five, with contorted aestivation. Stamens (seven or) eight (to ten),
diplostemonous. Filaments expanded and hairy below, not adnate to petals.
Anthers dorsifixed, introrse, longicidal. Pollen grains 5–11-colporate.
Pistil composed of two connate carpels. Ovary unilocular, stipitate. Style
single. Stigma small, usually bilobate (sometimes capitate). Placentation
parietal. Ovules two to 16 per carpel, apotropous, inserted in two rows. Outer
integument four to twelve cell layers thick. Fruit sometimes an irregularly
loculicidal capsule. Testa multiplicative, often indistinct. Hypostase massive.
Aluminium accumulated. n = ?
Polygaloideae Eaton,
Bot. Dict., ed. 4: 46. Apr-Mai 1836 [‘Polygaleae’]
27/935–>1.000. Hypogyny. Petals
three or five, large. Filaments connate, often adnate to petals, often
monadelphous. Anthers dehiscing by short apical slits. Pistil composed of two
to five (to eight) carpels. Ovule one per carpel, epitropous. Funicular or
exostomal aril often present.
Moutabeeae Chodat in
Engler et Prantl, Nat. Pflanzenfam. III, 4: 329. Jul 1896
5/16. ’Moutabea’ (8;
tropical America; paraphyletic), Balgoya (1; B. pacifica; New
Caledonia), Eriandra (1; E. fragrans; New Guinea),
Barnhartia (1; B. floribunda; tropical South America),
Diclidanthera (5; D. bolivarensis, D. laurifolia,
D. octandra, D. penduliflora, D. wurdackiana;
tropical South America). – Tropical America, New Guinea to New Caledonia.
Axial parenchyma apotracheal banded (Moutabea). Leaves (and sometimes
nodes) with glands. Sepals adnate to petals. Petals five, abaxial petal not
keeled. Stamens six to ten. Anthers dehiscing by confluent short apical slits.
Pistil composed of three to eight connate carpels. Stigma capitate. Funicular
aril present. n = 14. Aluminium accumulated.
[Carpolobieae+Polygaleae]
Carpolobieae B.
Eriksen in Plant Syst. Evol. 186: 47. 1993
2/7–8. Atroxima (2–3;
A. afzeliana, A. congolana, A. liberica; tropical
West and Central Africa), Carpolobia (5; C. alba, C.
gabonica, C. goetzei, C. gossweileri, C. lutea;
tropical Africa, Madagascar). – Tropical Africa, Madagascar. Petals five,
sometimes with contorted aestivation; abaxial petal keeled. Stamens (four or)
five. Anthers dehiscing by confluent short apical slits. Pistil composed of
three connate carpels. Stigma capitate. n = 9–11.
Polygaleae Fr., Fl.
Scan.: 35, 38. 1835
20/915–>1.000.
’Bredemeyera’ (c 60; New Guinea, tropical Australia, tropical
America; polyphyletic), Acanthocladus (8; Central America, tropical
South America), Gymnospora (2; P. blanchetii, P.
violoides; South America), Badiera (16; the West Indies),
Hebecarpa (c 30?; southern United States to South America),
Securidaca (c 80?; tropical regions on both hemispheres), Comesperma
(c 40; Australia), Ancylotropis (2; A. insignis, A.
malmeana; Brazil), Monnina
(c 150; New Mexico to Chile, with their highest diversity in the Andes; incl.
Pteromonnina?), Pteromonnina (c 30; the Andes; in Monnina?),
Phlebotaenia (2–3; P. cowellii, P. cuneata; Cuba,
Puerto Rico), Rhinotropis (17?; southwestern United States, Mexico),
Asemeia (28; southern United States, Mexico, Central America, the West
Indies, South America), Caamembeca (13; tropical South America), Muraltia
(c 120; tropical and southern Africa, with their largest diversity in the Cape
Provinces), Polygala
(300–400; almost cosmopolitan), Epirixanthes (5; E.
cylindrica, E. elongata, E. kinabaluensis, E.
pallida, E. papuana; tropical Asia), Salomonia (2;
S. cantoniensis, S. ciliata; tropical Asia, tropical
Australia to eastern Queensland), Heterosamara (5; H. cabrae,
H. carrissoana, H. caudata, H. resinosa, H.
wattersii; tropical and southern Africa, tropical and subtropical Asia),
Polygaloides
(6; P. balansae, P. chamaebuxus, P. munbyana, P.
paucifolia, P. vayredae, P. webbiana; Europe, the
Mediterranean, North Africa, western Asia, eastern North America). –
Cosmopolitan except polar areas, Polynesia and New Zealand. Vestured pits
present. Axial parenchyma paratracheal banded. Two abaxial lateral sepals
minute, two adaxial lateral sepals alae. Two abaxial lateral petals minute
(sometimes absent), two connate adaxial petals velum, abaxial petal carina
(often fimbriate). Stamens (two to) four or six to eight (in Polygala
myrtifolia, with eight stamens, two median stamens on opposite sides of
flower probably lost). Anthers dehiscing by apical pores. Pistil composed of
two connate carpels (adaxial carpel sometimes reduced; gynoecium sometimes
pseudomonomerous). Stylar canal present. Stigma bilobate, asymmetric.
Carunculus often present. Chalazal aril sometimes present. Fruit a capsule
(often flattened), a samara or a drupe. n = 6 or more. –
Epirixanthes consists of holomycoheterotrophs,
whereasSalomonia comprises root holoparasites.
 |
Cladogram of Polygalaceae based on
DNA sequence data (Persson 2001).
|
 |
Cladogram of Polygalaceae based on
DNA sequence data (Forest & al. 2007; Banks & al. 2008). The
generic nomenclature is updated.
|
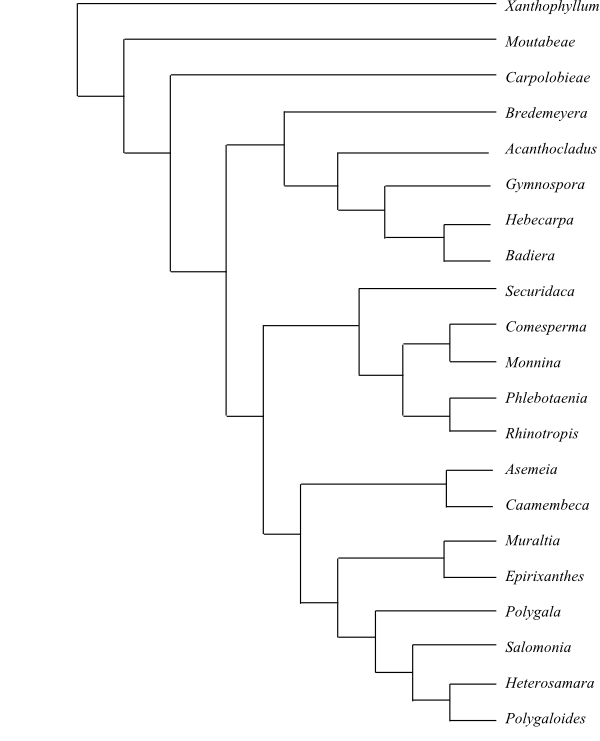 |
Phylogeny (Bayesian inference) of Polygalaceae based on
DNA sequence data (Pastore 2012; Epirixanthes added here;
Nylandtia synonymized with Muraltia).
|
Don in Edinburgh New Philos. J. 10: 299. 1831
[‘Quillajeae’]
Quillajales Doweld,
Tent. Syst. Plant. Vasc.: xxxviii. 23 Dec 2001
Genera/species 1/2
Distribution Central Chile to
southern Brazil, Uruguay and northern Argentina.
Fossils Unknown.
Habit Bisexual or unisexual,
evergreen small trees.
Vegetative anatomy Phellogen
ab initio subepidermal. Endodermis absent. Vessel elements (solitary) with
simple or scalariform perforation plates; lateral pits?, simple pits. Vestured
pits absent. Imperforate tracheary xylem elements? Wood rays ?-seriate,
heterocellular. Axial parenchyma apotracheal. Secondary phloem diffusely
expanded (intraxylary?). Sieve tube plastids S type? Nodes 1:3, unilacunar with
three leaf traces. Mucilage cells present. Calciumoxalate styloids present.
Trichomes Hairs verrucose.
Leaves Leaves alternate
(spiral), simple, entire, coriaceous, with conduplicate ptyxis. Stipules small,
petiolar, caducous; leaf sheath absent. Petiole vascular bundle transection
arcuate; pericyclic fibres absent. Venation pinnate. Stomata anomocytic?
Cuticular wax crystalloids as rosettes of platelets? Leaf margin usually
serrate, with hydathodes? (sometimes entire).
Inflorescence Terminal or
axillary, botryoid, cymose. Terminal flower bisexual, lateral flowers male.
Flowers Actinomorphic.
Hypogyny. Sepals five, with valvate aestivation, persistent and accrescent in
fruit, with nectary on lower half, free. Petals five, with contorted
aestivation, clawed, free. Disc intrastaminal, wide, thick and fleshy,
quinquelobate, with lobes adnate to stamens.
Androecium Stamens ten,
diplostemonous; five antesepalous stamens inserted on abaxial margin of disc
lobes a distance up sepals (above nectary), and five antepetalous stamens
inserted near ovary base (below nectary), unidirectionally developing.
Filaments subulate, free from each other and from tepals. Anthers dorsifixed,
versatile?, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal
slits). Tapetum secretory? Staminodia absent.
Pollen grains
Microsporogenesis simultaneous? Pollen grains tricolporate, shed as monads,
?-cellular at dispersal. Exine tectate, with columellate infratectum, striate;
exine protruding at apertures.
Gynoecium Pistil composed of
five antesepalous carpels connate in lower part (laterally largely free). Ovary
superior, quinquelocular. Stylodia five, free. Stigmas decurrent, type?
Pistillodium absent.
Ovules Placentation axile.
Ovules numerous per carpel (inserted in two marginal rows), pleurotropous,
horizontal, bitegmic, crassinucellar? Micropyle bistomal? Outer integument
approx. three cell layers thick. Inner integument ? cell layers thick.
Megagametophyte monosporous, Polygonum type. Endosperm development?
Endosperm haustoria? Embryogenesis?
Fruit A strongly asymmetrical
follicular capsule with stellately radiating lobes and accrescent calyx,
dehiscing downwards dorsally and ventrally by two coriaceous valves.
Seeds Aril absent. Seed with
long apical wing. Seed coat exotestal. Exotesta three-layered; layers
thickened, sclerotic. Endotesta? Tegmen degenerated. Perisperm not developed.
Endosperm one cell layer thick. Embryo? Chlorophyll? Cotyledons two,
conduplicate. Germination?
Cytology n = 14, 17
DNA Gene rpl22
present in nuclear or plastid genome?
Phytochemistry Insufficiently
known. Flavone-C-glycosides, prodelphinidins (in leaves), and
triterpene saponins (high concentrations in bark) present.
Use Medicinal plants,
detergents, food industry.
Systematics Quillaja
(2; Q. saponaria: central Chile from 31oS to 38oS; Q. brasiliensis: Peru?, southeastern
Brazil to Uruguay and northern Argentina).
Arnott in Wight et Arnott, Prodr. Fl. Ind.
Orient. 1: 360. 22 Sep 1834 [’Surianeae’], nom. cons.
Stylobasiaceae J.
Agardh, Theoria Syst. Plant.: 169. Apr-Sep 1858 [’Stylobasieae’]);
Surianales Doweld, Tent. Syst. Plant. Vasc.: xxxviii.
23 Dec 2001
Genera/species 5/8
Distribution Tropical beaches,
Australia, Mexico.
Fossils Wood fossil with the
name of Suriana inordinata have been described from the Eocene of
Wyoming.
Habit Usually bisexual (in
Stylobasium polygamomonoecious), evergreen small trees or shrubs. Some
species are xerophytes.
Vegetative anatomy Phellogen
ab initio subepidermal or inner-cortical. Medullary vascular bundles present in
Recchia. Vessels in radial multiples. Vessel elements with simple
perforation plates; lateral pits alternate, simple or bordered pits.
Imperforate tracheary xylem elements fibre tracheids or libriform fibres with
simple pits, non-septate (in Stylobasium also vasicentric tracheids).
Wood rays usually uniseriate (rarely biseriate), homocellular or
heterocellular. Axial parenchyma usually absent (in Suriana
apotracheal diffuse or diffuse-in-aggregates, or paratracheal scanty
vasicentric, or usually with crystalliferous strands). Wood elements in
Suriana and Stylobasium storied. Sieve tube plastids S type
(Suriana) or Pfs type (in Stylobasium). Nodes usually 3:3,
trilacunar with three leaf traces (in Suriana 1:1, unilacunar with one
trace). Secretory cavities present or absent. Sclereids present. Heartwood
often with red or brown resin. Parenchyma cells often with acicular
calciumoxalate crystals, styloids, crystal sand, and other types of
crystals.
Trichomes Hairs unicellular or
multicellular, simple or absent; glandular hairs, with multicellular head and
stalk, often present.
Leaves Alternate (spiral or
distichous), usually simple, entire (in some species of Recchia
pinnately compound, with alternate leaflets, with articulated petiolules),
usually coriaceous, with ? ptyxis. Stipules usually replaced by pseudostipules
and/or metastipules (absent in Suriana); leaf sheath absent. Petiole
vascular bundle transection arcuate or annular. Petiole or mid-vein in
Cadellia with extrafloral nectaries. Venation pinnate-reticulate.
Stomata anomocytic or anisocytic (in Stylobasium paracytic?).
Cuticular wax crystalloids usually as rosettes of platelets? (sometimes as
scales). Epidermis without mucilage cells. Mesophyll and epidermis usually with
druses or single crystals of calciumoxalate. Leaf margin entire.
Inflorescence Terminal or
axillary, cymose, raceme-like or panicle, or flowers solitary axillary.
Flowers Actinomorphic. Pedicel
articulated. Hypogyny. Sepals five (to seven), with imbricate-quincuncial
aestivation, usually persistent, free or connate at base. Petals usually five
(absent in Stylobasium), with contorted aestivation, caducous, in
Recchia and Suriana shortly clawed, free (rarely absent).
Nectary usually absent. Disc usually absent (present in Recchia).
Androecium Stamens 5+5 or five
(and up to five outer staminodia; in Suriana antepetalous inner
stamens shorter or staminodial), obdiplostemonous, as many as sepals,
antesepalous, persistent or caducous, sometimes articulated, antesepalous
stamens usually longer than antepetalous stamens. Filaments filiform or
subulate, free from each other and from tepals. Anthers basifixed or
dorsifixed, sometimes versatile, tetrasporangiate, usually introrse (rarely
latrorse; in Stylobasium extrorse?), longicidal (dehiscing by
longitudinal slits). Tapetum secretory, with binucleate cells. Staminodia in
bisexual and male flowers four or five, intrastaminal or extrastaminal, or
absent; female flowers in Stylobasium with staminodia.
Pollen grains
Microsporogenesis simultaneous. Pollen grains tricolpor(oid)ate, shed as
monads, bicellular at dispersal. Exine tectate or semitectate, with columellate
infratectum, perforate, microreticulate, striate or finely rugulate, sometimes
gemmate to verrucate; exine sometimes protruding at apertures.
Gynoecium Carpels one to five
(in Stylobasium, Guilfoylia and Recchia usually one
carpel [monomery], in Cadellia and Suriana five antepetalous
carpels), secondarily free (apocarpy), antepetalous (in Recchia on
short nectariferous gynophore). Ovaries superior. Stylodia one to five,
gynobasic (ventrobasic). Stigmas peltate to capitate or clavate, papillate,
type? Male flowers in Stylobasium with pistillodium.
Ovules Placentation
(supra)basal (basal-marginal or subbasal?). Ovules (one or) two (to five) per
carpel, collateral, anatropous to campylotropous, ascending, unitegmic,
crassinucellar, pachychalazal. Integument three to seven cell layers thick.
Obturator absent. Hypostase present. Parietal tissue four or five cell layers
thick. Nucellar cap sometimes present. Archespore often multicellular.
Megagametophyte monosporous, Polygonum type. Antipodal cells
ephemeral, degenerating. Endosperm development ab initio nuclear. Endosperm
haustorium chalazal. Embryogenesis onagrad.
Fruit A nut, a drupe or a
berry, in Cadellia and Suriana an assemblage of nutlets or
drupelets, usually with persistent and accrescent calyx. Exocarp in
Cadellia and Recchia with thickened cell walls. Mesocarp
usually hard (in Stylobasium fleshy), parenchymatic, with innermost
cell layer crystalliferous. Endocarp usually hard, usually three-layered, with
outer layer lignified, palisade-sclerenchymatous.
Seeds Aril absent. Seed coat
exotestal. Exotesta in Suriana green, with cuboid, enlarged,
tanniniferous cells. Mesotestal and endotestal cells crushed. Perisperm not
developed. Endosperm usually absent (in Stylobasium sparse). Embryo
curved (often hippocrepomorphic) or plicate (conduplicate, transversely
induplicate or globular), with chlorophyll. Cotyledons two, usually thick,
incumbent, oily or starchy. Germination in Stylobasium
phanerocotylar.
Cytology n = ?
DNA Gene rpl22
present in nuclear or plastid genome?
Phytochemistry Insufficiently
known. Surianol (triterpene diol) present in Suriana.
Proanthocyanidins sometimes present. Ellagic acids, saponins and cyanogenic
compounds not found.
Use Unknown.
Systematics Recchia
(3; R. connaroides, R. mexicana, R. simplicifolia;
Mexico), Cadellia (1; C. pentastylis; southeastern
Queensland, northeastern New South Wales), Suriana (1; S.
maritima; pantropical, sea-shores), Guilfoylia (1; G.
monostylis; eastern Queensland, northeastern New South Wales), Stylobasium
(2; S. australe, S. spathulatum; mainly western and central
Australia).
Analyses of matK (Bello & al.
2009) resulted in the topology
[[Recchia+Cadellia]+[Suriana+[Guilfoylia+Stylobasium]]].
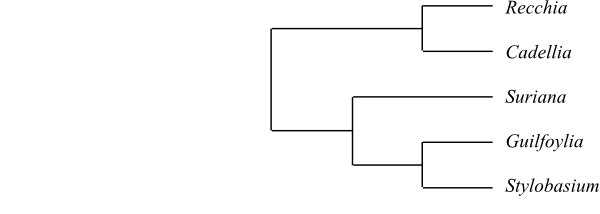 |
Nelsen strict consensus tree of Surianaceae based on DNA
sequence data (Bello & al. 2009).
|
Literature
Abbott JR. 2009. Phylogeny of the Polygalaceae and a revision of
Badiera. – Ph.D. diss., University of Florida, Gainesville,
Florida.
Abbott JR. 2011. Notes on the disintegration
of Polygala (Polygalaceae), with four new genera
for the Flora of North America. – J. Bot. Res. Inst. Texas 5: 125-137.
Abbott JR, Judd WS. 2011. Badiera
subrhombifolia (Polygalaceae), a new species from
Hispaniola. – Brittonia 63: 161-170.
Abbott JR, Pastore JFB. 2015. Preliminary
synopsis of the genus Hebecarpa (Polygalaceae). – Kew Bull. 70: 39
DOI 10.1007/S12225-015-9589-2
Abdou-El-Enain MM. 1999. Chromosomal criteria
and taxonomic relationships in the genus Lathyrus (Leguminosae). –
Taeckholmia 19: 193-201.
Abdou-El-Enain MM. 2002. Chromosomal criteria
and their phylogenetic implications in the genus Onobrychis Mill.
sect. Lophobrychis (Leguminosae), with special reference to Egyptian
species. – Bot. J. Linn. Soc. 139: 409-414.
Abdou-El-Enain MM, Loufty MHA, Shehata AA.
2007. Seed surface characters and their systematic significance in the genus
Lathyrus (Leguminosae, Papilionoideae, Vicieae). – Feddes Repert.
118: 269-285.
Adema F. 1966. A review of the herbaceous
species of Polygala in Malesia (Polygalaceae). – Blumea 14:
253-356.
Adema F. 1969. Identities of the herbaceous
Australian species of Polygala represented in the Brisbane Herbarium.
– Proc. Roy. Soc. Queensland 80: 125-130.
Adema F. 1998. Notes on Malesian Fabaceae (Leguminosae-Papilionoideae) 3.
The genera Dioclea, Luzonia, and Macropsychanthus.
– Blumea 43: 233-239.
Adema F. 2000. Notes on Malesian Fabaceae (Leguminosae-Papilionoideae) 6.
The rare genus Burkilliodendron. – Gard. Bull. (Singapore) 52:
5-10.
Adema F, Ohashi H, Sunarno B. 2016. Notes on
Malesian Fabaceae
(Leguminosae-Papilionoideae) 17. The genus Dalbergia. – Blumea 61:
186-206.
Adomou AC, Maesen LJG van der. 2001 [2002].
Millettia sect. Efulgentes and sect. Opacae
(Leguminosae, Papilionoideae), an overview. – Syst. Geogr. Plants 71:
445-448.
Agerer-Kirchhoff C, Agerer R. 1976. Revision
von Astragalus L. sect. Astragalus (Leguminosae). –
Boissiera 25: 1-197.
Agerer-Kirchhoff C, Agerer R. 1977. Eine neue
Sektion der Gattung Astragalus L.: Laxiflori
Agerer-Kirchhoff. – Mitt. Bot. Staatssamml. München 13: 203-234.
Aguiar ACA, Marques MCM, Yamamoto K. 2008.
Taxonomía das espécies de Polygala L. subg. Hebeclada
(Chodat) Blake (Polygalaceae)
ocorrentes no Brasil. – Rev. Bras. Bioci. 6: 91-109.
Aguiar-Dias ACA de, Yamamoto K, Castro M de
M. 2011. Stipular extranuptial nectaries new to Polygala: morphology
and ontogeny. – Bot. J. Linn. Soc. 166: 40-50.
Ahangarian S, Kazempour Osaloo S, Maassoumi
AA. 2007. Molecular phylogeny of the tribe Hedysareae with special reference to
Onobrychis (Fabaceae) as
inferred from nrDNA ITS sequences. – Iran. J. Bot. 13: 64-74.
Aïnouche A, Bayer RJ. 1999. Phylogenetic
relationships in Lupinus (Fabaceae: Papilionoideae) based on
internal transcribed spacer sequences (ITS) of nuclear ribosomal DNA. – Amer.
J. Bot. 86: 590-607.
Aïnouche A, Bayer RJ, Cubas P, Misset M-T.
2003. Phylogenetic relationships within tribe Genisteae (Papilionoideae) with
special reference to genus Ulex. – In: Klitgaard BB, Bruneau A
(eds), Advances in legume systematics 10: higher level systematics, Royal
Botanic Gardens, Kew, pp. 239-252.
Aïnouche A, Bayer RJ, Misset M-T. 2004.
Molecular phylogeny, diversification and character evolution in
Lupinus (Fabaceae) with
special attention to Mediterranean and African lupines. – Plant Syst. Evol.
246: 211-222.
Akan H, Firat M, Ekici M. 2008.
Astragalus bahcesarayensis (Leguminosae-Papilionoideae), a new species
of section Alopecuroidei DC. from Turkey. – Bot. J. Linn. Soc. 156:
439-444.
Alexander IJ. 1989. Systematics and ecology
of ectomycorrhizal legumes. – Monogr. Syst. Missouri Bot. Gard. 29:
607-624.
Antonio R, Sousa M. 1991. Contribuciónes al
conocimiento del género Diphysa (Leguminosae) para la flora
Mesoamericana. – Anal. Inst. Biol. Univ. Nac. Auton. México, Ser. Bot. 62:
115-120.
Alexander JA. 2005. The taxonomic status of
Astragalus mokiacensis (Fabaceae), a species endemic to the
southwestern United States. – Brittonia 57: 320-333.
Ali SI. 1958. Notes on the genus
Astragalus Linn. from W. Pakistan and N.W. Himalayas. – Kew Bull.
1958: 303-318.
Ali SI. 1961. Revision of the genus
Astragalus L. from W. Pakistan and N.W. Himalayas. – Biologia 7:
7-92.
Ali SI. 1962. A taxonomic revision of the
genus Gueldenstaedtia Fisch. – Candollea 18: 137-159.
Ali SI. 1965. Contributions to the genus
Astragalus L. from West Pakistan I. Subgenera Pogonophace and
Astragalus. – Bot. Not. 118: 87-96.
Ali SI. 1967. Revision of the genus
Vicia Linn. from West Pakistan. – Bot. Not. 120: 46-56.
Ali SI. 1994. A new combination in the genus
Lotus L. – Pakistan J. Bot. 26: 477.
Allan GJ. 1998. Molecular systematic and
biogeographic studies of the temperate herbaceous papilionoid tribes Loteae and
Coronilleae (Fabaceae). – Ph.D.
diss., Claremont Graduate School, Claremont, California.
Allan GJ, Porter JM. 2000. Tribal
delimitation and phylogenetic relationships of Loteae and Coronilleae
(Faboideae: Fabaceae) with special
reference to Lotus: evidence from nuclear ribosomal ITS sequences. –
Amer. J. Bot. 87: 1871-1881.
Allan GJ, Zimmer EA, Wagner WL, Sokoloff DD.
2003. Molecular phylogenetic analyses of tribe Loteae (Leguminosae):
implications for classification and biogeography. – In: Klitgaard BB, Bruneau
A (eds), Advances in legume systematics 10: higher level systematics, Royal
Botanic Gardens, Kew, pp. 371-393.
Allan GJ, Francisco-Ortega J, Santos-Guerra
A, Boerner E, Zimmer EA. 2004. Molecular phylogenetic evidence for the
geographic origin and classification of Canary Island Lotus (Fabaceae: Loteae). – Mol. Phylogen.
Evol. 32: 123-138.
Almada RD, Davina JR, Seijo JG. 2006.
Karyotype analysis and chromosome evolution in southernmost South American
species of Crotalaria (Leguminosae). – Bot. J. Linn. Soc. 150:
329-341.
Al-Mayah A-R A, Al-Shehbaz IA. 1977.
Chromosome numbers for some Leguminosae from Iraq. – Bot. Not. 130:
437-440.
Altschul SR. 1964. A taxonomic study of the
genus Anadenanthera. – Contr. Gray Herb. Harvard Univ. 193: 3-65.
Amirahmadi A, Kazempour Osaloo S, Moein F,
Kaveh A, Maassoumi A. 2014. Molecular systematics of the tribe Hedysareae (Fabaceae) based on nrDNA ITS and plastid
trnL-F and matK sequences. – Plant Syst. Evol. 300:
729-747.
Amirahmadi A, Kazempour-Osaloo S, Kaveh A,
Maassoumi AA, Naderi R. 2016. The phylogeny and new classification of the genus
Onobrychis (Fabaceae-Hedysareae): evidence from
molecular data. – Plant Syst. Evol. 302: 1445-1456.
Amshoff GJH. 1939. On South American
Papilionaceae. – Meded. Utrecht 52: 1-72.
Anantaswamy Rau M. 1951. The endosperm in
some of the Papilionaceae. – Phytomorphology 1: 153-158.
Anantaswamy Rau M. 1953. Some observations on
the endosperm in Papilionaceae. – Phytomorphology 3: 209-222.
Ancibor E. 1969. Los nectarios florales en
Leguminosas-Mimosóideas. – Darwiniana 15: 128-140.
Anderson JL, Porter JM. 1994. Astragalus
tortipes (Fabaceae), a new
species from desert badlands in southwestern Colorado and its phylogenetic
relationships within Astragalus. – Syst. Bot. 19: 116-125.
Ansari HA, Ellison NW, Griffiths AG, Williams
WM. 2004. A lineage-specific centromeric satellite sequence in the genus
Trifolium. – Chromosome Res. 12: 357-367.
Antonio R, Sousa M. 1991. Contribuciónes al
conocimiento del género Diphysa (Leguminosae) para la flora
Mesoamericana. – Anal. Inst. Biol. Univ. Nac. Auton. México, Ser. Bot. 62:
115-120.
Aparicio A, Albaladejo RG, Ceballos GL. 2002.
Genetic differentiation in silicicolous Echinospartum (Leguminosae)
indicated by allozyme variability. – Plant Syst. Evol. 230: 189-201.
Arce LR, Banks H. 2001. A preliminary survey
of pollen and other morphological characters in neotropical Acacia
subgenus Aculeiferum (Leguminosae: Mimosoideae). – Bot. J. Linn.
Soc. 135: 263-270.
Archambault A, Bruneae A. 2006. Phylogenetic
utility of the LEAFY/FLORICAULA gene in the Caesalpinioideae
(Leguminosae): gene duplication and a novel insertion. – Syst. Bot. 29:
609-626.
Ariati SR, Murphy DJ, Udovicic F, Ladiges
Pauline Y. 2006. Molecular phylogeny of three groups of acacias
(Acacia subgenus Phyllodineae) in arid Australia based on the
internal and external transcribed spacer regions of nrDNA. – Syst. Biodiv. 4:
417-426.
Arndt RR, Du Plessis LM. 1968. The alkaloids
of Coelidium fourcadei Compt. – J. South Afr. Chem. Inst. 21:
54-57.
Arreguín-Sánchez ML, Palacios-Chávez R,
Quiroz-García DL, Ramos-Zamora D. 1988. Morfología de los granos de pollen de
la familia Polygalaceae del Valle
de Mexico. – Acta Bot. Mex. 4: 21-27.
Arroyo MTK. 1976. The systematics of the
legume genus Harpalyce (Leguminosae: Lotoideae). – Mem. New York
Bot. Gard. 26: 1-80.
Arroyo MTK. 1981. Breeding systems and
pollination biology in Leguminosae. – In: Polhill RM, Raven PH (eds),
Advances in legume systematics 2, Royal Botanic Gardens, Kew, pp. 723-769.
Ashfield T, Egan AN, Pfeil BE, Chen NWG,
Podicheti R, Ratnaparkhe MB, Ameline-Torregrosa C, Denny R, Cannon S, Doyle JJ,
Geffroy V, Roe BA, Saghai Maroof MA, Young ND, Innes RW. 2012. Evolution of a
complex disease resistance gene cluster in diploid Phaseolus and
tetraploid Glycine. – Plant Physiol. 159: 336-354.
Asmussen CA, Liston A. 1998. Chloroplast DNA
characters, phylogeny, and classification of Lathyrus (Fabaceae). – Amer. J. Bot. 85:
387-401.
Assis Ribeiro dos Santos F de, Oliveira RC
de. 2008. Pollen morphology of some species of Calliandra Benth.
(Leguminosae-Mimosoideae) from Bahia, Brazil. – Grana 47: 101-116.
Atahuachi B M, Hughes CE. 2006. Two new
species of Mimosa (Fabaceae)
endemic to Bolivia. – Brittonia 58: 59-65.
Aubréville A. 1968. Leonardoxa
Aubréville, genre nouveau de césalpinioidées Guinéo-Congolaise. –
Adansonia, sér. 2, 8: 177-179.
Awmack CS, Lock JM. 2002. The genus
Alhagi (Leguminosae: Papilionoideae) in the Middle East. – Kew Bull.
57: 435-443.
Aymard GAC, Pastore JFB. 2009. Una nueva
especie de Acanthocladus (Polygalaceae) de Colombia. –
Caldasia 31: 13-17.
Aymard GAC, Gerardo A, Campbell LM. 2007. A
new species of Securidaca (Polygalaceae) from sandstone
outcrops in the Venezuelan Andes. – Brittonia 59: 328-333.
Aytac Z, Ekici M, Acik L. 2001. A new species
of Astragalus L. (Leguminosae) from South Anatolia. – Bot. J. Linn.
Soc. 136: 131-135.
Azani N, Bruneau A, Wojciechowski MF, Zarre
S. 2017. Molecular phylogenetics of annual Astragalus (Fabaceae) and its systematic
implications. – Bot. J. Linn. Soc. 184: 347-365.
Azani N, Babineau M, Bailey CD, Banks H,
Barbosa AR, Barbosa Pinto R, Boatwright JS et al. 2017. The Legume Phylogeny
Working Group (LPWG). A new subfamily classification of the Leguminosae based
on a taxonomically comprehensive phylogeny. – Taxon 66: 44-77.
Azevedo-Tozzi AMG de. 1995. New species of
Lonchocarpus Kunth (Leguminosae: Papilionoideae: Millettieae) from
Brazil. – Kew Bull. 50: 173-177.
Baas P. 1991. Leaf anatomy of Balgoya
pacifica (Polygalaceae) from
New Caledonia. – Bull. Mus. Natl. Hist. Nat., B, Adansonia 13: 13-15.
Babineau M, Bruneau A. 2017. Phylogenetic and
biogeographical history of the Afro-Madagascan genera Delonix,
Colvillea and Lemuropisum (Fabaceae: Caesalpinioideae). – Bot. J.
Linn. Soc. 184: 59-78.
Bacchetta G, Brullo S. 2006. Taxonomic
revision of the Astragalus genargenteus complex (Fabaceae). – Willdenowia 36: (Spec.
issue): 157-167.
Badr A. 1995. Electrophoretic studies of seed
proteins in relation to chromosomal criteria and the relationships of some taxa
of Trifolium. – Taxon 44: 183-191.
Badr A, El-Shazly HH, Abdou-El-Enain MM.
2000. Seed protein diversity and its implications on the relationships in the
genus Lathyrus. – Proc. 1st Intern. Conf. Bio. Sci.,
Tanta University, Egypt, pp. 1-15.
Badr A, El-Shazly HH, El-Rabey H, Watson L.
2002. Systematic relationships in Lathyrus Sect. Lathyrus (Fabaceae) based on amplified fragment
length polymorphism (AFLP) data. – Can. J. Bot. 80: 962-969.
Bagheri A, Ghahremaninejad F, Maassoumi AA,
Rahiminejad MR, Blattner FR. 2017. Nine new species of the species-rich genus
Astragalus (Leguminosae). – Novon 25: 266-281.
Bailey CD, Doyle JJ, Kajita T, Nemoto T,
Ohashi H. 1997. The chloroplast rpl2 intron and ORF184 as phylogenetic
markers in the legume tribe Desmodieae. – Syst. Bot. 22: 133-138.
Bailey CD, Hughes CE, Harris SA. 2004. Using
RAPDs to identify DNA sequence loci for species level phylogeny reconstruction:
an example from Leucaena (Fabaceae). – Syst. Bot. 29: 4-14.
Baker EG. 1914. The African species of
Crotalaria. – Bot. J. Linn. Soc. 42: 241-425.
Baker EG. 1926. The Leguminosae of tropical
Africa 1. – Erasmus Press, Gent.
Baker EG. 1929. The Leguminosae of tropical
Africa 2. – Ostende.
Baker EG. 1930. The Leguminosae of tropical
Africa 3. – Ostende.
Bamert U. 1990. Aspekte der Struktur,
Funktion und Diversität der Blüten bei Polygalaceae. – Ph.D. diss,
Universität Zürich, Switzerland.
Banfi E, Galasso G. 2008. New combinations in
Vachellia Wight & Arn., formerly Acacia Mill. s.s. (Fabaceae). – Atti della Soc. Ital.
Sci. Nat. Mus. Civico Stor. Nat. Milano 149: 149-150.
Bank M van der, Bank FH van der, Wyk B-E van.
1996. Speciation in Virgilia (Fabaceae): allopatric divergence
followed by introgression? – Plant Syst. Evol. 201: 57-73.
Bank M van der, Bank FH van der, Wyk B-E van.
1999. Evolution of sprouting versus seeding in Aspalathus linearis.
– Plant Syst. Evol. 219: 27-38.
Bank M van der, Chase MW, Wyk B-E van, Fay
MF, Bank FH van der, Reeves G, Hulme A. 2002. Systematics of the tribe
Podalyrieae (Fabaceae) based on DNA,
morphological and chemical data. – Bot. J. Linn. Soc. 139: 159-170.
Banks H. 1997. The pollen of Delonix
(Leguminosae: Caesalpinioideae: Caesalpinieae). – Kew Bull. 52: 417-434.
Banks H. 2003. Structure of pollen apertures
in the Detarieae sensu stricto (Leguminosae: Caesalpinioideae), with particular
reference to underlying structures (Zwischenkörper). – Ann. Bot. 92:
425-435.
Banks H, Gasson P. 2000. Pollen morphology
and wood anatomy of the Crudia group (Leguminosae, Caesalpinioideae,
Detarieae). – Bot. J. Linn. Soc. 134: 19-59.
Banks H, Gwilym G. 2018. Phylogenetically
informative pollen structures of ‘caesalpinioid’ pollen (Caesalpinioideae,
Cercidoideae, Detarioideae, Dialioideae and Duparquetioideae: Fabaceae). – Bot. J. Linn. Soc. 187:
59-86.
Banks H, Klitgaard BB. 2000. Palynological
contribution to the systematics of detarioid legumes (Leguminosae:
Caesalpinioideae). – In: Herendeen P, Bruneau A (eds), Advances in legume
systematics 9, Royal Botanic Gardens, Kew, pp. 79-106.
Banks H, Lewis GP. 2009. Pollen morphology of
the Dimorphandra group (Leguminosae, Caesalpinioideae). – Grana 48:
19-26.
Banks H, Rudall PJ. 2016. Pollen structure
and function in caesalpinioid legumes. – Amer. J. Bot. 103: 423-436.
Banks H, Rico L. 1999. Pollen morphology and
phylogenetic analysis of Eperua Aublet (Detarieae: Caesalpinioideae:
Leguminosae). – Grana 38: 261-276.
Banks H, Klitgaard BB, Lewis GP, Crane PR,
Bruneau A. 2003. Pollen and the systematics of the tribes Caesalpinieae and
Cassieae. – In: Klitgaard BB, Bruneau A (eds), Advances in legume systematics
10: Higher level systematics, Royal Botanic Gardens, Kew, pp. 95-122.
Banks H, Feist-Burkhart S, Klitgaard BB.
2006. The unique pollen morphology of Duparquetia (Leguminosae:
Caesalpinioideae): developmental evidence of aperture orientation using
confocal microscopy. – Ann. Bot. 98: 107-115.
Banks H, Klitgaard BB, Claxton F, Forest F,
Crane PR. 2008. Pollen morphology of the family Polygalaceae (Fabales). – Bot. J.
Linn. Soc. 156: 253-289.
Banks H, Himanen I, Lewis GP. 2010. Evolution
of pollen, stigmas and ovule numbers at the caesalpinioid-mimosoid interface
(Fabaceae). – Bot. J. Linn. Soc.
162: 594-615.
Banks H, Forest F, Lewis G. 2014. Evolution
and diversity of pollen morphology in tribe Cercideae (Leguminosae). – Taxon
63: 299-314.
Barbosa-Fevereiro VP. 1986.
Macroptilium (Bentham) Urban do Brasil
(Leguminosae-Faboideae-Phaseoleae-Phaseolinae). – Arq. Jard. Bot. Rio de
Janeiro 28: 109-180.
Baretta-Kuipers T. 1981. Wood anatomy of
Leguminosae: its relevance to taxonomy. – In: Polhill RM, Raven PH (eds),
Advances in legume systematics 2, Royal Botanic Gardens, Kew, pp. 677-705.
Barneby RC. 1962. A synopsis of
Errazurizia. – Leafl. Western Bot. 9: 209-214.
Barneby RC. 1964. Atlas of North American
Astragalus. – Mem. New York Bot. Gard. 13: 1-1188.
Barneby RC. 1977. Daleae imagines: an
illustrated revision of Errazurizia Philippi, Psorothamnus
Rydberg, Marina Liebmann, and Dalea Lucanus emend. Barneby,
including all species of Leguminosae tribe Amorpheae Borissova ever referred to
Dalea. – Mem. New York Bot. Gard. 27: 1-891.
Barneby RC. 1980. Three new species of
Dalea sect. Parosela (Leguminosae: Amorpheae) from western
and southern Mexico. – Brittonia 32: 392-396.
Barneby RC. 1981. New species of
Dalea section Parosela (Leguminosae: Amorpheae) from Peru and
Mexico. – Brittonia 33: 508-511.
Barneby RC. 1986. A contribution to the
taxonomy of Piptadenia (Mimosaceae) in South America. – Brittonia
38: 222-229.
Barneby RC. 1988. The genus Dalea
(Fabaceae tribe Amorpheae) in
Departamento de Cajamarca, Peru, with description of three new species. –
Brittonia 40: 1-6.
Barneby RC. 1990. Two new taxa in
Dalea (Fabaceae: Amorpheae)
from southern Mexico and northern Chile. – Brittonia 42: 89-91.
Barneby RC. 1991. Sensitivae censitae: a
description of the genus Mimosa L. (Mimosaceae) in the New World. –
Mem. New York Bot. Gard. 65: 1-835.
Barneby RC. 1996. Neotropical Fabales at NY:
asides and oversights. Phyllocarpus Riedel ex Endlicher, a monotypic
genus. – Brittonia 48: 183-186.
Barneby RC. 1997. Toward a census of genus
Mimosa (Mimosaceae) in the Americas: a new species from Mexico (Baja
California Sur) and two from planaltine Brazil (Goiás, Minas Gerais). –
Brittonia 49: 452-457.
Barneby RC. 1998. Silk tree, guanacaste,
monkey’s earring. A generic system for the synandrous Mimosaceae of the
Americas III. Calliandra. – Mem. New York Bot. Gard. 74: 1-223.
Barneby RC. 1999. Increments to genus
Chamaecrista (Caesalpiniaceae: Cassiinae) from Bolivia and from
Atlantic and Planaltine Brazil. – Brittonia 51: 331-339.
Barneby RC, Grimes JW. 1984. Two leguminous
forest trees new to the flora of French Guiana. – Brittonia 36: 45-48.
Barneby RC, Grimes JW. 1996. Silk tree,
guanacaste, monkey’s earring. A generic system for the synandrous Mimosaceae
of the Americas I: Abarema, Albizia, and allies. – Mem. New
York Bot. Gard. 74: 1-292.
Barneby RC, Grimes JW. 1997. Silk tree,
guanacaste, monkey’s earring. A generic system for the synandrous Mimosaceae
of the Americas II: Pithecellobium, Cojoba and
Zygia. – Mem. New York Bot. Gard. 74: 1-292.
Barron AR, Purves DW, Hedin LO. 2011.
Facultative nitrogen fixation by canopy legumes in a lowland tropical forest.
– Oecologia 165: 511-520.
Barros MJF, Morim MP. 2014.
Senegalia (Leguminosae, Mimosoideae) from the Atlantic Domain, Brazil.
– Syst. Bot. 39: 452-477.
Barros TC de, Teixeira SP. 2016. Revisited
anatomy of anther glands in mimosoids (Leguminosae). – Intern. J. Plant Sci.
177: 18-33.
Barykina RP, Kramina TE. 2006. A comparative
morphological and anatomical study of the model legume Lotus japonicus
and related species. – Wulgenia 13: 33-56.
Basconsuelo S, Weberling F, Kraus T. 2002.
Transition zone between root and stem vascular system in seedlings of members
of the tribe Phaseoleae (Fabaceae).
– Feddes Repert. 113: 224-230.
Bässler M. 1966. Die Stellung des Subgenus
Orobus (L.) Baker in der Gattung Lathyrus L. und seine
systematische Gliederung. – Feddes Repert. 72: 69-97.
Bässler M. 1971. Beiträge zur Nomenklatur
der Gattung Lathyrus L. – Feddes Repert. 82: 433-439.
Bässler M. 1973. Revision der eurasiatischen
Arten von Lathyrus L. Sect. Orobus (L.) Gren. et. Godr. –
Feddes Repert. 84: 329-447.
Bässler M. 1981. Revision von
Lathyrus L. Sect. Lathyrostylis (Griseb.) Bässler (Fabaceae). – Feddes Repert. 92:
179-254.
Bässler M. 1985. Die Gattung Mimosa
L. (Leguminosae-Mimosoideae) in Cuba. – Feddes Repert. 96: 581-611.
Bässler M. 1990a. Die Gattung
Calliandra Benth. (Leguminosae-Mimosoideae). – Gleditschia 18:
187-210.
Bässler M. 1990b. Die Gattung
Schrankia Willd. (Leguminosae-Mimosoideae) – neu für Kuba. –
Feddes Repert. 101: 7-8.
Bässler M. 1992. Die Gattung Inga
Mill. (Leguminosae-Mimosoideae) in Kuba. – Gleditschia 20: 3-14.
Bate-Smith EC. 1965. Investigation of the
chemistry and taxonomy of subtribe Quillajeae of the Rosaceae using comparisons of fresh
and herbarium material. – Phytochemistry 4: 535-539.
Bauchan GR, Elgin JH. 1984. A new chromosome
number for the genus Medicago. – Crop Sci. 24: 193-195.
Baudet JC. 1978. Prodrome d’une
classification générique des Papilionaceae-Phaseoleae. – Bull. Jard. Bot.
Natl. Belg. 48: 183-220.
Baudet JC, Maréchal R. 1976. Signification
taxonomique de la presence de poils uncinulés chez certains genres de
Phaseoleae et d’Hedysareae (Papilionaceae). – Bull. Jard. Bot. Natl. Belg.
46: 419-426.
Baum BR. 1968. A clarification of the generic
limits of Trigonella and Medicago. – Can. J. Bot. 46:
741-749.
Beaumont AJ, Beckett RP, Edwards TJ, Stirton
CH. 1999. Revision of the genus Calpurnia (Sophoreae: Leguminosae).
– Bothalia 29: 5-23.
Bechara MD, Moretzsohn MC, Palmieri DA,
Monteiro JP, Bacci M, Martins J, Valls JFM, Lopes CR, Gimenes MA. 2010.
Phylogenetic relationships in genus Arachis based on ITS and 5.8S rDNA
sequences. – BMC Evolutionary Biology 10: 255.
Becht R. 1978. Revision der Sektion
Alopecuroidei DC. der Gattung Astragalus L. –
Phanerogamarum Monogr. 10.
Beentje HJ. 1998. Surianaceae. – In: Beentje HJ,
Whitehouse CM (eds), Flora of tropical East Africa, A. A. Balkema, Rotterdam,
pp. 1-4.
Behnke H-D. 1981. Swartzia: phloem
ultrastructure supporting its inclusion into Leguminosae. Papilionoideae. –
Iselya 2: 13-16.
Behnke H-D, Pop L. 1981. Sieve-element
plastids and crystalline p(hloem)-protein in Leguminosae: micromorphological
characters as an aid to the circumscription of the family and subfamilies. –
In: Polhill RN, Raven PH (eds), Advances in legume systematics 2, Royal Botanic
Gardens, Kew, pp. 707-715.
Behnke H-D, Kiritsis U, Patrick SJ, Kenneally
KF. 1996. Form-Pfs plastids, stem anatomy and systematic affinities of
Stylobasium Desf. (Stylobasiaceae). A contribution to the knowledge of
sieve-element plastids in the Rutales and Sapindales. – Botanica
Acta 109: 346-359.
Bell EA. 1958. Canavanine and related
compounds in the Leguminosae. – Biochem. J. 70: 617-619.
Bell EA. 1962. Associations of
ninhydrin-reacting compounds in the seed of 49 species of Lathyrus.
– Biochem. J. 83: 225-229.
Bell EA. 1981. Non-protein amino acids in the
Legminosae. – In: Polhill RM, Raven PH (eds), Advances in legume systematics
2, Royal Botanic Gardens, Kew, pp. 489-499.
Bell EA, Tirimanna ASL. 1965. Associations of
amino acids and related compounds in the seeds of forty-seven species of
Vicia: their taxonomic and nutritional significance. – Biochem. J.
97: 104-111.
Bell EA, Lackey JA, Polhill RM. 1978.
Systematic significance of canavanine in the Papilionoideae (Fabaceae). – Biochem. Syst. Ecol. 6:
201-212.
Bello MA, Hawkins JA, Rudall PJ. 2007. Floral
morphology and development in Quillajaceae and Surianaceae (Fabales), the
species-poor relatives of Leguminosae and Polygalaceae. – Ann. Bot. 100:
1491-1505.
Bello MA, Bruneau A, Forest F, Hawkins JA.
2009. Elusive relationships within order Fabales: phylogenetic analyses using
matK and rbcL sequence data. – Syst. Bot. 34: 102-114.
Bello A, Stirton CH, Chimphango SBM, Muasya
AM. 2017. Taxonomic revision of African Psoralea pinnata species
complex (Psoraleeae, Leguminosae). – South Afr. J. Bot. 112: 128-179.
Bello MA, Hawkins JA, Rudall PJ. 2010. Floral
ontogeny in Polygalaceae and its
bearing on the homologies of keeled flowers in Fabales. – Intern. J. Plant
Sci. 171: 482-498.
Bello MA, Rudall PJ, Hawkins JA. 2012.
Combined phylogenetic analyses reveal interfamilial relationships and patterns
of floral evolution in the eudicot order Fabales. – Cladistics 28:
393-421.
Bena G. 2001. Molecular phylogeny supports
the morphologically based taxonomic transfer of the “medicagoid”
Trigonella species to the genus Medicago L. – Plant Syst.
Evol. 229: 217-236.
Bena G, Jubier M-F, Oliviera I, Lejeune B.
1998. Ribosomal external and internal transcribed spacers: combined use in the
phylogenetic analysis of Medicago (Leguminosae). – J. Mol. Evol. 46:
299-306.
Bena G, Prosperi J-M, Lejeune B, Olivieri I.
1998. Evolution of annual species of the genus Medicago: a molecular
phylogenetic approach. – Mol. Phylogen. Evol. 9: 552-559.
Bentham G. 1871. Revision of the genus
Cassia. – Trans. Linn. Soc. London 27: 503-591.
Bentham G. 1875. Revision of the suborder
Mimoseae. – Trans. Linn. Soc. London 30: 335-664.
Bernardi L. 2000. Consideraciones
taxonómicas y fitogeográficas acerca de 101 Polygalae americanas.
– Cavanillesia Altera 1: 1-456.
Bessega C, Fortunato RH. 2011. Section
Mimadenia: its phylogenetic relationships within the genus
Mimosa (Leguminosae, Mimosoideae) using plastid trnL-F
sequence data. – Aust. Syst. Bot. 24: 104-110.
Bessega C, Hopp HE, Fortunato RH. 2008.
Toward a phylogeny of Mimosa (Leguminosae: Mimosoideae): a preliminary
analysis of southern South American species based on chloroplast DNA sequence.
– Ann. Missouri Bot. Gard. 95: 567-579.
Beukes CW, Stępkowski T, Venter SN, Cłapa
T, Phalane FL, le Roux MM, Steenkamp ET. 2016. Crotalarieae and Genisteae of
the South African Great Escarpment are nodulated by novel
Bradyrhizobium species with unique and diverse symbiotic loci. –
Mol. Phylogen. Evol. 100: 206-218.
Beuselinck PR, Li B, Steiner JJ. 1996.
Rhizomatous Lotus corniculatus L. I. Taxonomic and cytological study.
– Crop Sci. 36: 179-185.
Beyra-Matos Á. 1998. Las leguminosas (Fabaceae) de Cuba II. Tribus
Crotalarieae, Aeschynomeneae, Millettieae y Robinieae. – Collect- Bot. 24:
149-332.
Beyra-Matos Á, Lavin M. 1999. Monograph of
Pictetia (Leguminosae-Papilionoideae) and review of the
Aeschynomeneae. – Syst. Bot. Monogr. 56: 1-93.
Beyra-Matos Á, Herrera P, Reyes G,
Hernández L. 2005. Revisión taxonómica del género Galactia P. Br.
(Leguminosae-Papilionoideae) en Cuba. – Rev. Acad. Colomb. Ci. 29:
467-494.
Bezuidenhoudt BCB, Brandt EV, Ferreira D.
1987. Flavonoid analogues from Pterocarpus species. – Phytochemistry
26: 531-535.
Bhattacharyya B, Maheshwari JK. 1971a.
Studies on extrafloral nectaries of the Leguminales I. Papilionaceae, with a
discussion on the system of the Leguminales. – Proc. Indian Natl. Acad. Sci.
37: 11-30.
Bhattacharyya B, Maheshwari JK. 1971b.
Studies on extrafloral nectaries of the Leguminales II. The genus
Cassia Linn. (Caesalpinaceae). – Proc. Indian Natl. Acad. Sci. 37:
74-90.
Bir SS, Kumari S. 1977. Evolutionary status
of Leguminosae from Pachmarhi, central India. – Nucleus 20: 94-98.
Bisby FA. 1981. Genisteae. – In: Polhill
RM, Raven PH (eds), Advances in legume systematics 1, Royal Botanic Gardens,
Kew, pp. 409-425.
Bisby FA, Nicholls KW. 1977. Effects of
varying character definitions on classification of Genisteae (Leguminosae). –
Bot. J. Linn. Soc. 74: 97-121.
Bisby FA, Buckingham J, Harborne JB (eds).
1994. Phytochemical dictionary of the Leguminosae. – Chapman & Hall,
Cambridge.
Blake SF. 1916. A revision of the genus
Polygala in Mexico, Central America, and the West Indies. – Contr.
Gray Herb. 47: 1-122.
Blanco A, Perrino P. 1974. Analisi citologica
di alcune specie de genere Vicia. – Giorn. Bot. Ital. 108:
123-133.
Boatwright JS. 2010. A rare new species of
Polhillia (Genisteae, Fabaceae). – South Afr. J. Bot. 76:
142-145.
Boatwright JS, Wyk B-E van. 2007a. A new
species of Rafnia (Crotalarieae, Fabaceae). – South Afr. J. Bot. 73:
471-473.
Boatwright JS, Wyk B-E van. 2007b. The
identity of Lebeckia lotononoides (Crotalarieae, Fabaceae). – South Afr. J. Bot. 73:
664-666.
Boatwright JS, Wyk B-E van. 2009. A revision
of the African genus Robynsiophyton (Crotalarieae, Fabaceae). – South Afr. J. Bot. 75:
367-370.
Boatwright JS, Wyk B-E van. 2011. The
systematic position of Sophora inhambanensis (Sophoreae, Fabaceae). – South Afr. J. Bot. 77:
249-250.
Boatwright JS, Wyk B-E van, Tilney PM. 2007.
Systematic studies in the genus Lebeckia and related genera: a
revision of Lebeckia section Stiza (Crotalarieae, Fabaceae). – South Afr. J. Bot. 73:
280.
Boatwright JS, Savolainen V, Wyk B-E van,
Schutte-Vlok AL, Forest F, Bank M van der. 2008. Systematic position of the
anomalous genus Cadia and the phylogeny of the tribe Podalyrieae (Fabaceae). – Syst. Bot. 33:
133-147.
Boatwright JS, Le Roux MM, Wink M, Tilney PM,
Wyk B-E van. 2008. Systematic studies in the genus Lebeckia and
related genera (Crotalarieae, Fabaceae): a new generic classification
for Lebeckia. – South Afr. J. Bot. 74: 362.
Boatwright JS, Le Roux MM, Wink M, Morozova
T, Wyk B-E van. 2008. Phylogenetic relationships of tribe Crotalarieae (Fabaceae) inferred from DNA sequences
and morphology. – Syst. Bot. 33: 752-761.
Boatwright JS, Le Roux MM, Wink M, Morozova
T, Wyk B-E van. 2008. Phylogenetic relationships of tribe Crotalarieae (Fabaceae) inferred from DNA sequences
and morphology. – Syst. Bot. 33: 752-761.
Boatwright JS, Tilney PM, Wyk B-E van. 2008.
A taxonomic revision of the genus Rothia (Crotalarieae, Fabaceae). – Aust. Syst. Bot. 21:
422-430.
Boatwright JS, Wink M, Tilney PM, Wyk B-E
van. 2009. New generic circumscriptions in the tribe Crotalarieae (Fabaceae). – South Afr. J. Bot. 75:
393-394.
Boatwright JS, Tilney PM, Wyk B-E van. 2009.
The generic concept of Lebeckia (Crotalarieae, Fabaceae): reinstatement of the genus
Calobota and the new genus Wiborgiella. – South Afr. J.
Bot. 75: 546-556.
Boatwright JS, Tilney PM, Wyk B-E van. 2010.
Taxonomy of Wiborgiella (Crotalarieae, Fabaceae), a genus endemic to the
Greater Cape Region of South Africa. – Syst. Bot. 35: 325-340.
Boatwright JS, Wink M, Wyk B-E van. 2011. The
generic concept of Lotononis (Crotalarieae, Fabaceae): reinstatement of the genera
Euchlora, Leobordea and Listia and the new genus
Ezoloba. – Taxon 60: 161-177.
Boatwright JS, Maurin O, van der Bank M.
2015. Phylogenetic position of Madagascan species of Acacia s.l. and
new combinations in Senegalia and Vachellia (Fabaceae, Mimosoideae, Acacieae). –
Bot. J. Linn. Soc. 179: 288-294.
Bobrov EG. 1967. On the span of the genus
Trifolium s.l. – Bot. Žurn. 52: 1593-1599. [In Russian]
Bocage AL du, Queiroz LP. 2006. New
combinations in Senegalia Raf. (Leguminosae: Mimosoideae). –
Neodiversity 1: 11-12.
Boeke JH. 1973. The postgenital fusion in the
gynoecium of Trifolium repens L.: light and electron microscopical
aspects. – Acta Bot. Neerl. 22: 503-509.
Bontemps C, Elliott GN, Simon MF, Dos Reis FB
Jr, Gross E, Lawton RC, Neto NE, Loureiro M de F, Faria SM de, Sprent JI, James
EK, Young JPW. 2010. Burkholderia species are ancient symbionts of
legumes. – Mol. Ecol. 19: 44-52.
Boonkerd T, Saengmanee S, Baum BR. 2002. The
varieties of Bauhinia pottsii G. Don in Thailand
(Leguminosae-Caesalpinioideae). – Plant Syst. Evol. 232: 51-62.
Borissova AG. 1964. De genere
Chesneya Lindl. et genere novo Chesniella Boriss. (Familia
Leguminosae) notula. – Nov. Sist. Nizsh. Rast. 1964: 178-190.
Bos JJ. 1967. The genus Liparia L.
(Pap.). – J. South Afr. Bot. 33: 269-292.
Bosser J, Rabevohitra R. 2005. Espèces
nouvelles dans le genre Dalbergia (Fabaceae, Papilionoideae) à Madagascar.
– Adansonia, sér. III, 27: 209-216.
Bouchenak-Khelladi Y, Maurin O, Hurter J,
Bank M van der. 2010. The evolutionary history and biogeography of Mimosoideae
(Leguminosae): an emphasis on African acacias. – Mol. Phylogen. Evol. 57:
495-508.
Boulos L. 1995. Studies in the Leguminosae of
Arabia I. Notes on Acacia Mill. – Kew Bull. 50: 327-337.
Brand A. 1898. Monographie der Gattung
Lotus. – Engl. Bot. Jahrb. Syst. 25: 166-232.
Brantjes NBM. 1982. Pollen placement and
reproductive isolation between two Brazilian Polygala species (Polygalaceae). – Plant Syst. Evol.
141: 41-52.
Brantjes NBM, Pijl L van der. 1980.
Pollination mechanisms in Polygalaceae. – Acta Bot. Neerl.
29: 56-57.
Bravo L. 1978. El género Cassia en
la Argentina. – Darwiniana 21: 343-391.
Brehm BG, Alston RE. 1964. A chemotaxonomic
study of Baptisia leucophaea var. laevicaulis (Leguminosae).
– Amer. J. Bot. 51: 644-650.
Brenan JPM. 1955. Notes on Mimosoideae. –
Kew Bull. 10: 161-192.
Brenan JPM. 1958. New and noteworthy cassias
from tropical Africa. – Kew Bull. 13: 231-252.
Brenan JPM. 1959. Leguminosae Subfamily
Mimosoideae. – In: Hubbard CE, Milne-Redhead E (eds), Flora of tropical East
Africa, Crown Agents for Oversea Governments and Administrations, London, pp.
1-173.
Brenan JPM. 1963a. Notes on African
Caesalpinioïdeae. – Kew Bull. 17: 197-214.
Brenan JPM. 1963b. A further note on
Piptadenia. – Kew bull. 17: 227-228.
Brenan JPM. 1967. Leguminosae Subfamily
Caesalpinioideae. – In: Milne-Redhead E, Polhill RM (eds), Flora of tropical
East Africa, Crown Agents for Oversea Governments and Administrations, London,
pp. 1-230.
Brenan JPM. 1968. Notes on Mimosoideae 11.
– Kew Bull. 21: 477-483.
Brenan JPM. 1970. 61. Leguminosae (incl.
Mimosaceae, Caesalpiniaceae and Papilionaceae). – In: Brenan JPM (ed), Flora
Zambesiaca 3 (Part 1), Crown Agents for Oversea Governments and
Administrations, London.
Brenan JPM. 1986. The genus
Adenopodia (Leguminosae). – Kew Bull. 41: 73-90.
Breteler FJ. 1960. Revision of Abrus
Adanson (Papilionoideae) with special reference to Africa. – Blumea 10:
607-624.
Breteler FJ. 1995. The boundary between
Amherstieae and Detarieae (Caesalpinioideae). – In: Crisp MD, Doyle JJ (eds),
Advances in legume systematics 7: phylogeny, Royal Botanic Gardens, Kew, pp.
53-61.
Breteler FJ. 2006. Novitates Gabonenses 56.
Two Anthonotha species from Gabon transferred to
Englerodendron (Fabaceae,
Caesalpinioideae). – Adansonia, sér. III, 28: 106-111.
Breteler FJ. 2011. Revision of the African
genus Isomacrolobium (Leguminosae, Caesalpinioideae). – Plant Ecol.
Evol. 144: 64-81.
Breteler FJ, Smissaert-Houwing AAS. 1977.
Revision of Atroxima Stapf and Carpolobia G. Don (Polygalaceae). – Meded. Landbouwh.
Wageningen 77(18): 1-45.
Bridgewater SGM, Baas P. 1982. Wood anatomy
of Xanthophyllum Roxb. – IAWA Bull., N. S., 3: 115-125.
Bridgewater SGM, Stirton CH. 1997. A
morphological and biogeographic study of the Acosmium dasycarpum
complex (Leguminosae: Papilionoideae, Sophoreae). – Kew Bull. 52: 471-475.
Briquet J. 1894. Études sur les Cytises des
Alpes Maritimes. – Georg & Co., Genève et Bâle.
Britten EJ. 1963. Chromosome numbers in the
genus Trifolium. – Cytologia 28: 428-449.
Britton NL, Killip EP. 1936. Mimosaceae and
Caesalpiniaceae of Colombia. – Ann. New York Acad. Sci. 35: 167-169.
Britton NL, Rose JN. 1928. Mimosaceae. –
North American Flora 23: 1-194.
Brizicky GK. 1960. A new species of
Paramachaerium from Panama. – Trop. Woods 112: 58-64.
Broich SL. 1987. Revision of the Lathyrus
vestitus-laetiflorus complex (Fabaceae). – Syst. Bot. 12:
139-153.
Brouillet L. 2008. The taxonomy of North
American Loti (Fabaceae: Loteae): new
names in Acmispon and Hosackia. – J. Bot. Res. Inst. Texas
2: 387-394.
Brown GK. 2008. Systematics of the tribe
Ingeae (Leguminosae-Mimosoideae) over the past 25 years. – Muelleria 26:
27-42.
Brown GK, Ariati SR, Murphy DJ, Miller JTH,
Ladiges PY. 2006. Bipinnate acacias (Acacia subg.
Phyllodineae sect. Botrycephalae) of eastern Australia are
polyphyletic based on DNA sequence data. – Aust. Syst. Bot. 19: 315-326.
Brown GK, Murphy DJ, Miller JT, Ladiges PY.
2008. Acacia s.s. and its relationship among tropical legumes, tribe
Ingeae (Leguminosae: Mimosoideae). – Syst. Bot. 33: 739-751.
Brown GK, Murphy DJ, Ladiges PY. 2011.
Relationships of the Australo-Malesian genus Paraserianthes
(Mimosoideae: Leguminosae) identifies the sister group of Acacia sensu
stricto and two biogeographical tracks. – Cladistics 27: 380-390.
Brown GK, Murphy DJ, Kidman J, Ladiges PY.
2013. Phylogenetic connections of phyllodinous species of Acacia
outside Australia are explained by geological history and human-mediated
dispersal. – Aust. Syst. Bot. 25: 390-403.
Brummer EC, Bouton JH, Kochert G. 1995.
Analysis of annual Medicago species using RAPD markers. – Genome 38:
362-367.
Brummitt RK. 1968. The genus Baphia
(Leguminosae) in east and north-east tropical Africa. – Kew Bull. 22:
513-536.
Brummitt RK. 1980. Reconsideration of the
genera Ptycholobium, Caulocarpus, Lupinophyllum and
Requienia in relation to Tephrosia
(Leguminosae-Papilionoideae). – Kew Bull. 35: 459-473.
Brummitt RK. 2011. (292) Acacia: a
solution that should be acceptable to everybody. – Taxon 59: 1925-1926.
Bruneau A. 1993. Systematics of
Erythrina (Leguminosae: Phaseoleae) and implications for the evolution
of pollination systems. – Ph.D. diss., Cornell University, Ithaca, New
York.
Bruneau A. 1996. Phylogenetic and
biogeographical patterns in Erythrina (Leguminosae: Phaseoleae) as
inferred from morphological and chloroplast DNA characters. – Syst. Bot. 21:
587-605.
Bruneau A. 1997. Evolution and homology of
bird pollination syndromes in Erythrina (Leguminosae). – Amer. J.
Bot. 84: 54-71.
Bruneau A, Doyle JJ. 1993. Cladistic analysis
of chloroplast DNA restriction site characters in Erythrina
(Leguminosae: Phaseoleae). – Syst. Bot. 18: 229-247.
Bruneau A, Doyle JJ, Palmer JD. 1990. A
chloroplast DNA inversion as a subtribal character in the Phaseoleae
(Leguminosae). – Syst. Bot. 15: 378-386.
Bruneau A, Doyle JJ, Doyle JL. 1995.
Phylogenetic relationships in Phaseoleae: evidence from chloroplast restriction
site characters. – In: Crisp MD, Doyle JJ (eds), Advances in legume
systematics 7: phylogeny, Royal Botanic Gardens, Kew, pp. 309-330.
Bruneau A, Breteler FJ, Wieringa JJ, Gervais
GYF, Forest F. 2000. Phylogenetic relationships in tribes Macrolobieae and
Detarieae as inferred from chloroplast trnL intron sequences. – In:
Herendeen PS, Bruneau A (eds), Advances in legume systematics 9, Royal Botanic
Gardens, Kew, pp. 121-149.
Bruneau A, Forest F, Herendeen PS, Klitgaard
BB, Lewis GP. 2001. Phylogenetic relationships in the Caesalpinioideae
(Leguminosae) as inferred from chloroplast trnL intron sequences. –
Syst. Bot. 26: 487-514.
Bruneau A, Mercure M, Lewis GP, Herendeen PS.
2008. Phylogenetic patterns and diversification in the caesalpinioid legumes.
– Can. J. Bot. 86: 697-718.
Bruneau A, Klitgaard BB, Prenner G,
Fougère-Danezan M, Tucker SC. 2014. Floral evolution in the Detarieae
(Leguminosae): phylogenetic evidence for labile floral development in an
early-diverging legume lineage. – Intern. J. Plant Sci. 175: 392-417.
Brunsberg K. 1977. Biosystematics of the
Lathyrus pratensis complex. – Opera Bot. 41: 1-78.
Buckeridge MS, Panagassi VR, Rocha DC,
Dietrich SMC. 1995. Seed galactomannan in the classification and evolution of
the Leguminosae. – Phytochemistry 38: 871-875.
Buckeridge MS, Dietrich SMC, Lima DU de.
2000. Galactomannans as the reserve carbohydrate in legume seeds. – In: Gupta
AK, Kaur N (eds), Carbohydrate reserves in plants – synthesis and
application, Elsevier, Amsterdam, pp. 283-316.
Bunsupa S, Katayama K, Ikeura E, Oikawa A,
Toyooka K, Saito K, Yamazaki M. 2012. Lysine cecarboxylase catalyzes the first
step of quinolizidine alkaloid biosynthesis and coevolved with alkaloid
production in Leguminosae. – Plant Cell 24: 1202-1216.
Burck W. 1893. Contributions à la flore de
l’archipel Malais I. Les espèces du genre Mucuna de l’archipel
Malais et de la Nouvelle Guinée. – Ann. Jard. Bot. Buitenzorg 11:
181-217.
Burghardt AD, Espert SM. 2007. Phylogeny of
Prosopis (Leguminosae) as shown by morphological and biochemical
evidence. – Aust. Syst. Bot. 20: 332-339.
Burgt XM van der, Eyakwe MB, Newbery DM.
2007. Englerodendron korupense (Fabaceae, Caesalpinioideae), a new tree
species from Korup National Park, Cameroon. – Adansonia, sér. III, 29:
59-65.
Burkart A. 1935. Revisión de las especies de
Lathyrus de la Républica Argentina. – Rev. Fac. Agron. Vet. 8:
41-127.
Burkart A. 1939. Descripción de
Mimozyganthus, nuevo género de Leguminosas y sinopsis preliminar de
los géneros argentinos de Mimosóideas. – Darwiniana 3: 445-469.
Burkart A. 1943. Las leguminosas Argentinas.
– Acme Agency, Buenos Aires.
Burkart A. 1944. Tres nuevas Leguminosas del
Paraguay coleccionadas por el Señor Teodoro Rojas. – Darwiniana 6:
477-493.
Burkart A. 1946. Leguminosas nuevas o
críticas. – Darwiniana 7: 216-239.
Burkart A. 1949. Contribución al studio del
género Adesmia (Leguminosae). – Lilloa 15: 1-17.
Burkart A. 1952. Las Leguminosas Argentinas.
2nd ed. – ACME Agency, Buenos Aires.
Burkart A. 1954. Contribución al estudio del
género Adesmia (Leguminosae) II. – Darwiniana 10: 465-546.
Burkart A. 1957a. Leguminosas nuevas o
criticas V. – Darwiniana 2: 256-271.
Burkart A. 1957b. El género
Pachecoa (Leguminosae). – Darwiniana 11: 261-268.
Burkart A. 1960. Contribución al estudio del
género Adesmia (Leguminosae) III. – Darwiniana 12: 81-136.
Burkart A. 1962. Contribución al estudio del
género Adesmia (Leguminosae) IV. – Darwiniana 12: 309-364.
Burkart A. 1964a. Contribución al estudio
del género Adesmia (Leguminosae) V. – Darwiniana 13: 9-66.
Burkart A. 1964b. Leguminosas nuevas o
criticas VI. – Darwiniana 13: 428-448.
Burkart A. 1966. Contribución al estudio del
género Adesmia (Leguminosae) VI. – Darwiniana 14: 195-248.
Burkart A. 1967. Sinopsis del género
sudamericano de Leguminosas Adesmia DC. – Darwiniana 14: 463-568.
Burkart A. 1969. Leguminosas nuevas o
críticas. – Darwiniana 15: 501-549.
Burkart A. 1970. Las Leguminosas Faséolas
Argentinas de los géneros Mucuna, Dioclea y
Camptosema. – Darwiniana 16: 175-218.
Burkart A. 1971. El género Galactia
(Leg. Phaseoleae) en Sudamérica con especial referencia a la Argentina y
paises vecinos. – Darwiniana 16: 663-796.
Burkart A, Vilchez O. 1971. Valoración e
ilustración del género Fiebrigiella Harms (Leguminosae-Hedysareae).
– Darwiniana 16: 659-662.
Burnham RJ. 1995. A new species of winged
fruit from the Miocene of Ecuador: Tipuana ecuatoriana (Leguminosae).
– Amer. J. Bot. 82: 1599-1607.
Burtt BL. 1973. Polygala arvensis,
chinensis and glomerata. – Not. Roy. Bot. Gard. Edinb. 32:
403-404.
Buss PA, Lersten NR. 1975. Survey of tapetal
nuclear number as a taxonomic character in Leguminosae. – Bot. Gaz. 136:
388-395.
Butcher R, Chappill JA. 2001.
Sphaerolobium macranthum (Leguminosae: Mirbelieae) complex revised.
– Aust. Syst. Bot. 14: 155-173.
Butcher R, Chappill JA. 2004. A revision of
the Sphaerolobium fornicatum complex (Leguminosae: Mirbelieae) from
south-west Western Australia. – Aust. Syst. Bot. 17: 423-439.
Butler A. 1986. Studies in the seed coat of
Lathyrus. – In: Kaul A, Combes D (eds), Lathyrus and
lathyrism, Proc. Intern. Symp., IBEAS, Univ. Paul, Sept. 1985, New York, pp.
25-38.
Caccavari M. 2002. Pollen morphology and
structure of tropical and subtropical American genera of the
Piptadenia-group (Leguminosae: Mimosoideae). – Grana 41: 130-141.
Caccavari M, Dome E. 2000. Subpseudocolpi in
polyads of Acacia, subgenus Aculeiferum. – Grana 39:
32-38.
Cai Z, Guisinger M, Kim HG, Ruck E, Blazier
JC, McMurtry V, Kuehl JV, Boore J, Jansen RK. 2008. Extensive reorganization of
the plastid genome of Trifolium subterraneum (Fabaceae) is associated with numerous
repeated sequences and novel DNA insertions. – J. Mol. Ecol. 67: 696-704.
Callen EO. 1959. Studies in the genus
Lotus (Leguminosae) I. Limits and subdivisions of the genus. – Can.
J. Bot. 37: 157-165.
Calvillo-Canadell L, Cevallos-Ferriz SRS.
2005. Diverse assemblage of Eocene and Oligocene Leguminosae from Mexico. –
Intern. J. Plant Sci. 166: 671-692.
Calvo J, Hantson S, Paiva J. 2014.
Polygala webbiana (Polygalaceae), the first record for
Europe and a synopsis of Polygala subgen. Chamaebuxus. –
Nord. J. Bot. 32: 38-41.
Camargo RA, Azevedo Tozzi AM G de. 2014. A
synopsis of the genus Deguelia (Leguminosae, Papilionoideae,
Millettieae) in Brazil. – Brittonia 66: 12-32.
Camp RH op den, De Mita S, Lillo A, Cao Q,
Limpens E, Bisseling T, Geurts R. 2011. A phylogenetic strategy based on a
legume-specific whole genome duplication yields symbiotic cytokinin type-a
response regulators. – Plant Physiol. 157: 2013-2022.
Campbell GJ, Wyk B-E van. 2001. A taxonomic
revision of Rafnia (Fabaceae, Crotalarieae). – South Afr.
J. Bot. 67: 90-149.
Campo M van, Guinet P. 1961. Les pollens
composés. L’exemple des Mimosacées. – Pollen Spores 3: 201-218.
Cannon SB, Ilut D, Farmer AD, Maki SL, May
GD, Singer SR, Doyle JJ. 2010. Polyploidy did not predate the evolution of
nodulation in all legumes. – PloS One 5(7):e11630.
Cantó P, Sánchez MJ. 1988. Revisión del
agregado Genista cinerea (Leguminosae). – Candollea 43: 73-92.
Cardoso DBOS. 2008. Taxonomia da tribo
Sophoreae (Leguminosae, Papilionoideae) na Bahia, Brasil. – M.Sc. thesis,
Universidade Estadual de Feira de Santana, Brazil.
Cardoso DBOS, Queiroz LP de. 2010.
Ormosia limae (Leguminosae, Papilionoideae): a new species from the
Atlantic Forest of Southern Bahia, Brazil. – Syst. Bot. 35: 272-276.
Cardoso DBOS, Lima HC de, Rodrigues RS,
Queiroz LP de, Pennington RT, Lavin M. 2012a. The realignment of
Acosmium sensu stricto with the dalbergioid clade (Leguminosae,
Papilionoideae) reveals a proneness for independent evolution of radial floral
symmetry among early-branching papilionoid legumes. – Taxon 61: 1057-1073.
Cardoso DBOS, Lima HC de, Rodrigues RS,
Queiroz LP de, Pennington RT, Lavin M. 2012b. The Bowdichia clade of genistoid
legumes: phylogenetic analysis of combined molecular and morphological data and
a recircumscription of Diplotropis. – Taxon 61: 1074-1087.
Cardoso DBOS, Queiroz LP de, Pennington RT,
Lima HC de, Fonty É, Wojciechowski MF, Lavin M. 2012c. Revisiting the
phylogeny of papilionoid legumes: new insights from comprehensively sampled
early-branching lineges. – Amer. J. Bot. 99: 1991-2013.
Cardoso DBOS, Queiroz LP de, Lima HC de,
Suganuma E, Berg C van den, Lavin M. 2013a. A molecular phylogeny of the
vataireoid legumes underscores floral evolvability that is general to many
early-branching papilionoid lineages. – Amer. J. Bot. 100: 403-421.
Cardoso DBOS, Pennington RT, de Queiroz LP,
Boatwright JS, Van Wyk B-E, Wojciechowski MF, Lavin M. 2013b. Reconstructing
the deep-branching relationships of the papilionoid legumes. – South Afr. J.
Bot. 89: 58-75.
Cardoso DBOS, Lima HC de, Queiroz LP de.
2013c. Staminodianthus, a new neotropical genistoid legume genus
segregated from Diplotropis. – Phytotaxa 110: 1-16.
Cardoso DBOS, Stirton CH, Torke BM. 2014.
Taxonomy of South American Ormosia (Leguminosae, Papilionoideae):
recircumscription of O. costulata, reinstatement of O.
trifoliolata, and the new species O. lewisii from the Brazilian
Atlantic forest. – Syst. Bot. 39: 1132-1141.
Cardoso DBOS, Queiroz LP de, Lima HC de.
2014. A taxonomic revision of the South American papilionoid genus
Luetzelburgia (Fabaceae).
– Bot. J. Linn. Soc. 175: 328-375.
Cardoso DBOS, São-Mateus WMB, Cruz DT da,
Zartman CE, Komura DL, Kite G, Prenner G, Wieringa JJ, Clark A, Lewis G,
Pennington RT, Queiroz LP de. 2015. Filling in the gaps of the papilionoid
legume phylogeny: the enigmatic Amazonian genus Petaladenium is a new
branch of the early-diverging Amburaneae clade. – Molec. Phylogen. Evol. 84:
112-124.
Cardoso MB, Schifino-Wittmann MT,
Bodanese-Zanettini MH. 2000. Taxonomic and evolutionary implications of
intraspecific variability in chromosome numbers of species of Leucaena
Benth. (Leguminosae). – Bot. J. Linn. Soc. 134: 549-556.
Cardoso DBOS, São-Mateus WMB, Cruz DT da,
Zartman CE, Komura DL, Kite G, Prenner G, Wieringa JJ, Clark A, Lewis G,
Pennington RT, Queiroz LP de. 2015. Filling in the gaps of the papilionoid
legume phylogeny: the enigmatic Amazonian genus Petaladenium is a new
branch of the early-diverging Amburaneae clade. – Mol. Phylogen. Evol. 84:
112-124.
Cardoso DBOS, Harris DJ, Wieringa JJ,
São-Mateus WMB, Batalha-Filho H, Torke BM, Prenner G, Queiroz LP de. 2017. A
molecular-dated phylogeny and biogeography of the monotypic legume genus
Haplormosia, a missing African branch of the otherwise
American-Australian Brongniartieae clade. – Mol. Phylogen. Evol. 107:
431-442.
Cardoso MB, Schifino-Wittmann MT,
Bodanese-Zanettini MH. 2000. Taxonomic and evolutionary implications of
intraspecific variability in chromosome numbers of species of Leucaena
Benth. (Leguminosae). – Bot. J. Linn. Soc. 134: 549-556.
Carlquist SJ. 1989. Wood anatomy of
Cercidium (Fabaceae), with
emphasis on vessel wall sculpture. – Aliso 12: 235-255.
Carmo-Bastos M de N. 1987. Contribuição ao
estudo sistemático de algumas espécies do gênero Machaerium Persoon
(Leguminosae-Papilionoideae) ocorrentes na Amazônia Brasileira. – Bol. Mus.
Paraense Emílio Goeldi, Sér. Bot. 3: 183-279.
Carstairs SA, Buirchell BJ, Cowling WA. 1992.
Chromosome number, size and intercrossing ability of three Old World lupines,
Lupinus princei Harms, L. atlanticus Gladstones and L.
digitatus Forskal, and implications for cytosystematic relationships among
the rough-seeded lupins. – J. Roy. Soc. West. Aust. 75: 83-88.
Carter S, Reynolds T. 1990. Aloe
penduliflora and Aloe confusa. – Kew Bull. 45: 647-651.
Carulli JP, Fairbrothers DE. 1988. Allozyme
variation in three eastern United States species of Aeschynomene (Fabaceae), including the rare A.
virginica. – Syst. Bot. 13: 559-566.
Carvalho AM de. 1997. A synopsis of the genus
Dalbergia (Fabaceae:
Dalbergieae) in Brazil. – Brittonia 49: 87-109.
Casimiro-Soriguer R, Talavera M, Balao F,
Terrab A, Herrera J, Talavera S. 2010. Phylogeny and genetic structure of
Erophaca (Leguminosae), a East-West Mediterranean disjunct genus from the
Tertiary. – Molec. Phylogen. Evol. 56: 441-450.
Castellanos C, Steeves R, Lewis GP, Bruneau
A. 2017. A settled sub-family for the orphan tree: the phylogenetic position of
the endemic Colombian genus Orphanodendron in the Leguminosae. –
Brittonia 69: 62-70.
Castro C de. 1945. Alguns dados cariológicos
para a sistemática dos géneros Echinospartum,
Stauracanthus, Nepa e Ulex. – Bol. Soc. Brot.,
ser. II, 19: 525-539.
Castro S, Silveira P, Coutinho AP, Figueiredo
E. 2005. Systematic studies in Tylosema (Leguminosae). – Bot. J.
Linn. Soc. 147: 99-115.
Castro S, Silveira P, Coutinho AP, Paiva J.
2007. Heterosamara sect. Villososperma comb. nov. (Polygalaceae) from eastern Asia. –
Nord. J. Bot. 25: 286-293.
Castro S, Silveira P, Navarro L. 2008. How
does secondary pollen presentation affect the fitness of Polygala
vayredae (Polygalaceae)? –
Amer. J. Bot. 95: 706-712.
Castro S, Silveira P, Navarro L, Paiva J,
Coutinho AP. 2009. Pollen morphology of Chamaebuxus (DC.) Schb.,
Chodatia Paiva and Rhinotropis (Blake) Paiva
(Polygala L., Polygalaceae). – Grana 48:
179-192.
Catalano SA, Vilardi JC, Tosto D, Saidman BO.
2008. Molecular phylogeny and diversification history of Prosopis (Fabaceae: Mimosoideae). – Biol. J.
Linn. Soc. 93: 621-640.
Čechov VP. 1932. Kariosistematičeskij
analiz triby Trifolieae D.C. sem. Leguminosae Juss. – Trudy Prikl. Bot., Ser.
II, Genet. Rast. 1: 119-146.
Čelakovský L. 1874. Über den Aufbau der
Gattung Trifolium. – Österr. Bot. Zeitschr. 24: 37-44, 75-82.
Ceolin GB, Miotto STS. 2013. Synopsis of the
genus Galactia (Phaseoleae, Papilionoideae, Leguminosae) in Brazil.
– Phytotaxa 134: 1-26.
Cervantes-Martinez T, Horner HT, Palmer RG,
Hymowitz T, Brown AHD. 2005. Calcium oxalate crystals macropatterns in leaves
of species from groups Glycine and Shuteria (Glycininae;
Phaseoleae; Papilionoideae; Fabaceae). – Can. J. Bot. 83:
1410-1421.
Champagne CEM, Goliber TE, Wojciechowski MF,
Mei RW, Townsley BT, Wang K, Paz MM, Geeta R, Sinha NR. 2007. Compound leaf
development and evolution in the legumes. – Plant Cell 19: 3369-3378.
Chandler GT, Crisp MD. 1998. Morphometric and
phylogenetic analysis of the Daviesia ulicifolia complex (Fabaceae, Mirbelieae). – Plant Syst.
Evol. 209: 93-122.
Chandler GT, Bayer RJ, Crisp MD. 2001. A
molecular phylogeny of the endemic Australian genus Gastrolobium (Fabaceae: Mirbelieae) and allied genera
using chloroplast and nuclear markers. – Amer. J. Bot. 88: 1675-1687.
Chandler GT, Crisp MD, Cayzer LW, Bayer RJ.
2002. Monograph of Gastrolobium (Fabaceae: Mirbelieae). – Aust. Syst.
Bot. 15: 619-739.
Chang ZY. 2008. A taxonomical study of
Caragana Fabr. from China. – Ph.D. thesis, Northeast Forestry
University, China.
Chappill JA. 1995. Cladistic analysis of
Leguminosae: the development of an explicit phylogenetic hypothesis. – In:
Crisp MD, Doyle JJ (eds), Advances in legume systematics 7: phylogeny, Royal
Botanic Gardens, Kew, pp. 1-9.
Chappill JA, Maslin BR. 1995. A phylogenetic
assessment of the tribe Acacieae. – In: Crisp MD, Doyle JJ (eds), Advances in
legume systematics 7: phylogeny, Royal Botanic Gardens, Kew, pp. 77-99.
Chappill JA, Wilkins CF, Crisp MD. 2007.
Taxonomic revision of Jacksonia (Leguminosae: Mirbelieae). – Aust.
Syst. Bot. 20: 473-623.
Chappill JA, Wilkins CF, Crisp MD. 2008.
Taxonomic revision of Gompholobium (Leguminosae: Mirbelieae). –
Aust. Syst. Bot. 21: 67-151.
Chaudhary LB, Sanjappa M. 1998. Parochetinae:
a new subtribe of Trifolieae (Leguminosae, Papilionoideae). – Taxon 47:
829-831.
Chaudhary LB, Rana TS, Narzary D, Verma S.
2007. A new species of Astragalus L. (Leguminosae) from India based on
morphological and molecular markers. – Bot. J. Linn. Soc. 154: 27-34.
Chauhan V, Pandey AK. 2014. Structure and
evolution of the pod in Indigofera (Fabaceae) reveals a trend towards small
thin indehiscent pods. – Bot. J. Linn. Soc. 176: 260-276.
Chen C-C, Gibson PB. 1982. Chromosome
relationships of Trifolium uniflorum to T. repens and T.
occidentale. – Can. J. Genet. Cytol. 14: 591-595.
Chen C-J, Mendenhall MG, Turner BL. 1994.
Taxonomy of Thermopsis (Fabaceae) in North America. – Ann.
Missouri Bot. Gard. 81: 714-742.
Chen L. 1939. The Chinese species of
Bauhinia. – Lingnan Sci. J. 18: 261-281, 475-494. [In Chinese]
Chen Y-F, Zhang D-X. 2005. Bauhinia
larsenii, a fossil legume from Guangxi, China. – Bot. J. Linn. Soc. 147:
437-440.
Chiara Moço M de, Araujo Mariath JE de.
2009. Comparative floral ontogeny in Adesmia (Leguminosae:
Papilionoideae: Dalbergieae). – Aust. J. Bot. 57: 65-75.
Chodat RH. 1887. Notice sur les Polygalacées
& synopsis des Polygala d’Europe et d’Orient. – Compt. Rend.
Séances Soc. Phys. Hist. Nat. Genève/Arch. Sci. Phys. Nat. Genève, sér. 3,
18: 281-299.
Chodat RH. 1889. Polygalacées. – In:
Micheli M (ed), Contributions à la flore du Paraguay. – Mém. Soc. Phys.
Genève 30: 101-114.
Chodat RH. 1891a. Sur la distribution et
l’origine de l’espèce et des groupes chez les Polygalacées. – Arch.
Sci. Phys. Nat., sér. 3, 25: 695-714.
Chodat RH. 1891b. Monographia polygalacearum
I. – Mém. Soc. Phys. Hist. Nat. Genève suppl. 1890, 7: 1-143.
Chodat RH. 1893. Monographia polygalacearum
II. – Mém. Soc. Phys. Hist. Nat. Genève 31: 1-500.
Chodat RH. 1896a. Polygalaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien III(4), W. Engelmann,
Leipzig, pp. 323-345.
Chodat RH. 1896b. Polygalaceae novae vel parum
cognitae 5. – Bull. Herb. Boiss. 4: 233-237.
Chodat RH. 1908. Polygalaceae andinae. – In: Urban
I (ed), Plantae novae andinae imprimis Weberbauerianae IV. – Bot. Jahrb.
Syst. 42: 97-104.
Choi B-H. 1994. Foliar flavonoids of the
genus Hedysarum and related genera (tribe Hedysareae-Leguminosae). –
Kor. J. Plant Taxon. 24: 259-264.
Choi B-H, Kim JH. 1997. ITS sequences and
speciation on Far Eastern Indigofera (Leguminosae). – J. Plant Res.
110: 339-346.
Choi B-H, Ohashi H. 1996. Pollen morphology
and taxonomy of Hedysarum and its related genera of the tribe
Hedysareae (Leguminosae-Papilionoideae). – J. Jap. Bot. 71: 191-213.
Choi B-H, Ohashi H. 1998. Proposal to
conserve the name Hedysarum (Leguminosae: Papilionoideae) with a
conserved type. – Taxon 47: 877.
Choi B-H, Ohashi H. 2003. Generic criteria
and an infrageneric system for Hedysarum and related genera
(Papilionoideae-Leguminosae). – Taxon 52: 567-576.
Choi B-H, Nemoto T, Ohashi H. 1999. Anatomy
of nodal regions and leaves in Hedysarum and related genera
(Leguminosae). – J. Jap. Bot. 74: 236-250.
Choi B-H, Seok DI, Endo Y, Ohashi H. 2006.
Phylogenetic significance of stylar features in genus Vicia
(Leguminosae); an analysis with molecular phylogeny. – J. Plant Res. 119:
513-523.
Choi H-K, Kim D, Uhm T, Limpens E, Lim H, Mun
J-H, Kalo P, Penmetsa RV, Seres A, Kullkova O, Roe BA, Bisseling T, Kiss GB,
Cook DR. 2004. A sequence-based genetic map of Medicago truncatula and
comparison of marker colinearity with M. sativa. – Genetics 166:
1463-1502.
Choudhary P, Choudhary SS. 1989. Karyotypic
studies and trend of speciation in some species of Caesalpiniaceae. – J.
Cytol. Genet. 23: 183-189.
Chrtek J, Chrtková A. 1983. Bemerkungen zu
einigen balkanischen Oxytropis-Arten. – Folia Geobot. Phytotaxon.
18: 309-319.
Chrtková-Zertová A. 1966. Bemerkungen zur
Taxonomie von Lotus uliginosus Schkuhr und L. pedunculatus
Cav. – Folia Geobot. Phytotax. Bohemoslov. 1: 78-87.
Chu NM, Tewari KK. 1982. Arrangement of the
ribosomal RNA genes in chloroplast DNA of Leguminosae. – Mol. Gen. Genet.
1886: 23-32.
Chuxanova NA. 1967. Chromosome numbers of
some species of Leguminosae Juss. indigenous to the U.S.S.R. – Bot. Žurn.
52: 1124-1131. [In Russian]
Citerne HL, Pennington RT, Cronk QCB. 2006.
An apparent reversal in floral symmetry in the legume Cadia is a
homeotic transformation. – Proc. Natl. Acad. Sci. U.S.A. 103: 12017-12020.
Clark RP, Mackinder BA, Banks H. 2017.
Cheniella gen. nov. (Leguminosae: Cercidoideae) from southern China,
Indochina and Malesia. – European J. Taxon. 360: 1-37.
Clarke AE. 1934. The number and morphology of
chromosomes in the genus Melilotus. – Univ. Calif. Publ. Bot. 17:
435-443.
Clarke HD, Seigler DS, Ebinger JE. 1989.
Acacia farnesiana (Fabaceae:
Mimosoideae) and related species from Mexico, the southwestern U.S., and the
Caribbean. – Syst. Bot. 14: 549-564.
Clarke HD, Downie SR, Seigler DS. 2000.
Implications of chloroplast DNA restriction site variation for systematics of
Acacia (Fabaceae:
Mimosoideae). – Syst. Bot. 25: 618-632.
Clarke HD, Seigler DS, Ebinger JE. 2009.
Taxonomic revision of the Vachellia acuifera species group (Fabaceae: Mimosoideae) in the Caribbean.
– Syst. Bot. 34: 84-101.
Clausen RT. 1945. A botanical study of the
yam beans (Pachyrrhizus). – Mem. Cornell Univ. Agric. Exp. Stat.
264: 1-38.
Clawson ML, Bourret A, Benson DR. 2004.
Assessing the phylogeny of Frankia-actinorhizal plant nitrogen-fixing
root nodule symbioses with Frankia 16SrRNA and glutamine synthetase
gene sequences. – Mol. Phylogen. Evol. 31: 131-138.
Claxton F, Banks H, Klitgaard BB, Crane PR.
2005. Pollen morphology of families Quillajaceae and Surianaceae (Fabales). – Rev.
Paleobot. Palyn. 133: 221-233.
Coetzee H, Robbertse PJ. 1985. Pollen and
tapetal development in Securidaca longipedunculata. – South Afr. J.
Bot. 51: 111-124.
Compton RH. 1912. An investigation of the
seedling structure in the Leguminosae. – Bot. J. Linn. Soc. 41: 1-222.
Conceição A de S, Queiroz LP de, Lambert
SM, Pereira ACS, Borba EL. Biosystematics of Chamaecrista sect.
Absus subsect. Baseophyllum (Leguminosae-Caesalpinioideae)
based on allozyme and morphometric analyses. – Plant Syst. Evol. 270:
183-207.
Conceição A de S, Queiroz LP de, Borba EL.
2008. Natural hybrids in Chamaecrista sect. Absus subsect.
Baseophyllum (Leguminosae-Caesalpinioideae): genetic and morphological
evidence. – Plant Syst. Evol. 271: 19-27.
Conceição A de S, Queiroz LP de, Lewis GP,
Andrade MJG de, Almeida PRM de, Schnadelbach AS, Berg C van den. 2009.
Phylogeny of Chamaecrista Moench (Leguminosae-Caesalpinioideae) based
on nuclear and chloroplast DNA regions. – Taxon 58: 1168-1180.
Conn BJ, Tame TM. 1996. A revision of the
Acacia uncinata group (Fabaceae-Mimosoideae). – Aust. Syst.
Bot. 9: 827-857.
Conte L, Cristofolini G. 2000. Infraspecific
diversity of Cytisus emeriflorus Reichenb., an endemic plant with
disjunct distribution. – Plant Biosystems 134: 373-384.
Conte L, Troìa A, Cristofolini G. 1998.
Genetic diversity in Cytisus aeolicus Guss. (Leguminosae), a rare
endemite of the Italian flora. – Plant Biosystems 132: 239-249.
Conterato IF, Schifino-Wittmann MT. 2006. New
chromosome numbers, meiotic behaviour and pollen fertility in American taxa of
Lupinus (Leguminosae): contributions to taxonomic and evolutionary
studies. – Bot. J. Linn. Soc. 150: 229-240.
Conterato IF, Sfoggia MST, Schifino-Wittmann
MT. 2007. Chromosome number, karyotype, and taxonomic considerations on the
enigmatic Sellocharis paradoxa Taubert (Leguminosae, Papilionoideae,
Genisteae). – Bot. J. Linn. Soc. 155: 223-226.
Cooper WE. 2015. A taxonomic revision of
Cynometra L. (Fabaceae) in
Australia with a new species from the Wet Tropics of Queensland and a range
extension to the mainland. – Austrobaileya 9: 393-403.
Corby HDL. 1981. The systematic value of
leguminous root nodules. – In: Polhill RN, Raven PH (eds), Advances in legume
systematics 2, Royal Botanic Gardens, Kew, pp. 657-670.
Corby HDL. 1988. Types of rhizobial nodules
and their distribution among the Leguminosae. – Kirkia 13: 53-123.
Corner EJH. 1951. The leguminous seed. –
Phytomorphology 1: 117-150.
Costa MFB, Paulino JV, Marinho CR, Leite VG,
Pedersoli GD, Teixeira SP. 2014. Stigma diversity in tropical legumes with
consideration on stigma classification. – Bot. Rev. 80: 1-29.
Coutinho ÍAC, Francino DMT, Meira RMSA.
2015. New records of colleters in Chamaecrista (Leguminosae,
Caesalpinioideae s.l.): structural diversity, secretion, functional role, and
taxonomic importance. – Intern. J. Plant Sci. 176: 72-85.
Cowan RS. 1975. A monograph of the genus
Eperua (Leguminosae: Caesalpinioideae). – Smithsonian Institution
Press 28: 1-45.
Cowan RS. 1981. Swartzieae. – In: Polhill
RM, Raven PH (eds), Advances in legume systematics 1, Royal Botanic Gardens,
Kew, pp. 209-212.
Cowan RS, Polhill RM. 1981a. Detarieae DC.
(1825). – In: Polhill RM, Raven PH (eds), Advances in legume systematics 1,
Royal Botanic Gardens, Kew, pp. 117-134.
Cowan RS, Polhill RM. 1981b. Amherstieae
Benth. emend. J. Léon. (1957). – In: Polhill RM, Raven PH (eds), Advances in
legume systematics 1, Royal Botanic Gardens, Kew, pp. 135-142.
Cozzo D. 1949. Estudio anatomico sobre la
posicion sistematica de algunos géneros Argentinos de Leguminosas
Papilionoideas. – Lilloa 16: 97-124.
Cozzo D. 1950. Anatomia del leno secundario
de las Leguminosas Papilionoideas Argentinas silvestres y cultivadas. Revista
del Instituto Nacional de Investigacion de las Ciencias Naturales “Bernadino
Rivadavia”. – Cienc. Bot. I(7): 223-361.
Cranmer MF, Mabry TJ. 1966. The lupine
alkaloids of the genus Baptisia (Leguminosae). Phytochemistry 5:
1133-1138.
Crayn DM, Fernando ES, Gadek PA, Quinn CJ.
1995. A reassessment of the familial affinity of the Mexican genus
Recchia Mociño & Sessé ex DC. – Brittonia 47: 397-402.
Crepet WL, Dilcher DL. 1977. Investigations
of angiosperms from the Eocene of North America: a mimosoid inflorescence. –
Amer. J. Bot. 64: 714-725.
Crepet WL, Herendeen PS. 1992. Papilionoid
flowers from the early Eocene of southeastern North America. – In: Herendeen
PS, Dilcher DL (eds), Advances in legume systematics 4: the fossil record,
Royal Botanic Gardens, Kew, pp. 43-55.
Crepet WL, Taylor DW. 1985. The
diversification of the Leguminosae: first fossil evidence of Mimosoideae and
Papilionoideae. – Science 288: 1087-1089.
Crepet WL, Taylor DW. 1986. Primitive
mimosoid flowers from the Paleocene-Eocene and the systematic and evolutionary
implications. – Amer. J. Bot. 73: 548-563.
Crisp MD. 1990. Contributions towards a
revision of Daviesia (Fabaceae: Mirbelieae) I. The D.
squarrosa group. – Aust. Syst. Bot. 3: 241-251.
Crisp MD. 1991. Contributions towards a
revision of Daviesia Smith (Fabaceae: Mirbelieae) II. The D.
latifolia group. – Aust. Syst. Bot. 4: 229-298.
Crisp MD. 1994. Evolution of bird pollination
in some Australian legumes (Fabaceae). – In: Eggleton P,
Vane-Wright R (eds), Phylogenetics and ecology, London, pp. 281-309.
Crisp MD. 1995a. Revision of
Brachysema (Fabaceae:
Mirbelieae). – Aust. Syst. Bot. 8: 307-353.
Crisp MD. 1995b. Contributions towards a
revision of Daviesia (Fabaceae: Mirbelieae) III. A synopsis of
the genus. – Aust. Syst. Bot. 8: 1155-1249.
Crisp MD. 1999. Revision of
Leptosema (Fabaceae:
Mirbelieae). – Aust. Syst. Bot. 12: 1-54.
Crisp MD, Chandler GT. 1997a. Contributions
towards a revision of Daviesia (Fabaceae: Mirbelieae) IV. D.
ulicifolia sens. lat. – Aust. Syst. Bot. 10: 31-48.
Crisp MD, Chandler GT. 1997b. Contributions
towards a revision of Daviesia (Fabaceae: Mirbelieae) V. D.
cardiophylla sens. lat. – Aust. Syst. Bot. 10: 321-329.
Crisp MD, Cook LG. 2003a. Molecular evidence
for definition of genera in the Oxylobium group (Fabaceae: Mirbelieae). – Syst. Bot.
28: 705-713.
Crisp MD, Cook LG. 2003b. Phylogeny and
embryo sac evolution in the endemic Australasian papilionoid tribes Mirbelieae
and Bossiaeeae. – In: Klitgaard BB, Bruneaeu A (eds), Advances in legume
systematics 10: higher level systematics, Royal Botanic Gardens, Kew, pp.
253-268.
Crisp MD, Cook LG. 2003. Phylogeny and
evolution of anomalous roots in Daviesia (Fabaceae: Mirbelieae). – Intern. J.
Plant Sci. 164: 603-612.
Crisp MD, Doyle JJ (eds). 1995. Advances in
legume systematics 7: phylogeny. – Royal Botanic Gardens, Kew.
Crisp MD, Weston PH. 1987. Cladistics and
legume systematics, with an analysis of the Bossiaeeae, Brongniartieae, and
Mirbelieae (Papilionoideae, Leguminosae). – In: Stirton CH (ed) Advances in
legume systematics 3, Royal Botanic Gardens, Kew, pp. 65-130.
Crisp MD, Weston PH. 1991. Almaleea,
a new genus of Fabaceae from
south-eastern Australia. – Telopea 4: 307-311.
Crisp MD, Weston PH. 1995. Mirbelieae. –
In: Crisp MD, Doyle JJ (eds), Advances in legume systematics 7: phylogeny,
Royal Botanic Gardens, Kew, pp. 245-282.
Crisp MD, Gilmore SR, Weston PH. 1999.
Phylogenetic relationships of two anomalous species of Pultenaea (Fabaceae: Mirbelieae), and description
of a new genus. – Taxon 48: 701-714.
Crisp MD, Gilmore S, Wyk B-E van. 2000.
Molecular phylogenetics of the genistoid tribes of papilionoid legumes. – In:
Herendeen PS, Bruneau A (eds), Advances in legume systematics 9, Royal Botanic
Gardens, Kew, pp. 249-276.
Crisp MD, Chappill JA, Kok RPJ de, Jobson PC.
2005. Tribe Mirbelieae. – In: Lewis GP, Schrire BD, Mackinder B, Lock JM
(eds), Legumes of the world, Royal Botanic Gardens, Kew, pp. 338-354.
Cristofolini G. 1974. Contributo preliminare
alla sistematica di Chamaecytisus hirsutus e Chamaecytisus
supinus. – Giorn. Bot. Ital. 108: 55-73.
Cristofolini G. 1976. I Citisi italiani della
sezione Tubocytisus DC. – Webbia 30: 257-283.
Cristofolini G. 1987. Serological
relationships among Sophoreae, Thermopsideae and Genisteae (Fabaceae). – Bot. J. Linn. Soc. 94:
421-432.
Cristofolini G. 1989. A serological
contribution to the systematics of the genus Lupinus (Fabaceae). – Plant Syst. Evol. 166:
265-278.
Cristofolini G. 1991. Taxonomic revision of
Cytisus Desf. sect. Tubocytisus DC. (Fabaceae). – Webbia 45: 187-219.
Cristofolini G. 1997. The biodiversity of
Leguminosae-Genisteae and its genesis. – Lagascalia 19: 121-128.
Cristofolini G, Conte L. 2002. Phylogenetic
patterns and endemism genesis in Cytisus Desf. (Leguminosae-Cytiseae)
and related genera. – Israel J. Plant Sci. 50: 37-50.
Cristofolini G, Feoli-Chiapella L. 1977.
Serological systematics of the tribe Genisteae (Fabaceae). – Taxon 26: 43-56.
Cristofolini G, Feoli-Chiapella L. 1984a.
Origin and diversification of the Genisteae (Fabaceae): a serosystematic purview. –
Webbia 38: 105-122.
Cristofolini G, Feoli-Chiapella L. 1984b.
Serotaxonomy and systematic relationships of the genus Adenocarpus
(Genisteae – Fabaceae). – Nord.
J. Bot. 4: 457-461.
Cristofolini G, Jarvis CE. 1991. On the
status of Cytisus hirsutus and C. supinus (Fabaceae). – Taxon 40: 495-498.
Cristofolini G, Troìa A. 2006. A
reassessment of the sections of the genus Cytisus Desf. (Cytiseae,
Leguminosae). – Taxon 55: 733-746.
Crompton CW, Grant WF. 1993. Pollen
morphology in Loteae (Leguminosae) with particular reference to the genus
Lotus L. – Grana 32: 129-153.
Cubas P. 1986. Números cromosomáticos en
Ulex L. y Stauracanthus Link (Genisteae, Papilionaceae). –
An. Jard. Bot. Madrid 43: 217-233.
Cubas P, Tahiri H, Pardo C. 2001.
Karyological and taxonomic notes on Cytisus Desf. Sect.
Spartopsis Dumort. and Sect. Alburnoides DC. (Genisteae,
Leguminosae) from the Iberian Peninsula and Morocco. – Bot. J. Linn. Soc.
135: 43-50.
Cubas P, Pardo C, Tahiri H. 2002. Molecular
approach to the phylogeny and systematics of Cytisus (Leguminosae) and
related genera based on nucleotide sequences of nrDNA (ITS region) and cpDNA
(trnL-trnF intergenic spacer). – Plant Syst. Evol. 233:
223-242.
Cubas P, Pardo C, Tahiri H, Castroviejo S.
2010. Phylogeny and evolutionary diversification of Adenocarpus DC.
(Leguminosae). – Taxon 59: 720-732.
Cumbie BG. 1960. Anatomical studies in the
Leguminosae. – Trop. Woods 113: 1-47.
Curtis WF. 1976. Chromosome counts in
Grielum and Cercis. – Ann. Missouri Bot. Gard. 63:
379-380.
Czefranova Z. 1971. Conspectus systematis
generis Lathyrus L. – Nov. Syst. Plant. Vasc. 8: 191-201.
Dahlgren RMT. 1960. Revision of the genus
Aspalathus I. The species with flat leaflets. – Opera Bot. 4:
1-393.
Dahlgren RMT. 1961. Revision of the genus
Aspalathus II. The species with ericoid and pinoid leaflets 1-2. –
Opera Bot. 6(2): 1-118.
Dahlgren RMT. 1963a. Revision of the genus
Aspalathus II. The species with ericoid and pinoid leaflets 3. The
Aspalathus ciliaris group and some related groups. – Opera Bot.
8(1): 1-183.
Dahlgren RMT. 1963b. Studies on
Aspalathus and some related genera in South Africa. – Opera Bot.
9(1): 1-301.
Dahlgren RMT. 1963c. The genus
Borbonia L. incorporated in Aspalathus L. – Bot. Not. 116:
185-192.
Dahlgren RMT. 1964. The genus
Euchlora Eckl. & Zeyh. as distinguished from Lotononis
Eckl. & Zeyh. – Bot. Not. 117: 371-388.
Dahlgren RMT. 1965. The riddle of
Walpersia Harv. – Bot. Not. 118: 97-103.
Dahlgren RMT. 1965. Revision of the genus
Aspalathus II. The species with ericoid and pinoid leaflets 4. The
Aspalathus ericifolia, parviflora, calcarata,
desertorum, macrantha, pinea, rostrata,
filicaulis, laricifolia, and longifolia groups. –
Opera Bot. 10(1): 1-231.
Dahlgren RMT. 1966. Revision of the genus
Aspalathus II. The species with ericoid and pinoid leaflets 5. The
Aspalathus carnosa, aciphylla, pachyloba,
arida, pinguis, spinosa, and sanguinea
groups and some other groups. – Opera Bot. 11(1): 1-266.
Dahlgren RMT. 1967a. Some new and
rediscovered species of Aspalathus (Leguminosae). – Bot. Not. 120:
26-40.
Dahlgren RMT. 1967b. Chromosome numbers in
some South African genera of the tribe Genisteae s. lat. (Leguminosae). –
Bot. Not. 120: 149-160.
Dahlgren RMT. 1967c. A new species of
Lebeckia (Leguminosae) from the Cape Province. – Bot. Not. 120:
268-271.
Dahlgren RMT. 1968a. Revision of the genus
Aspalathus II. The species with ericoid and pinoid leaflets 6. The
Aspalathus frankenioides, nivea, juniperina,
rubens, and divaricata groups and some other groups. –
Opera Bot. 21: 1-309.
Dahlgren RMT. 1968b. Revision of the genus
Aspalathus II. The species with ericoid and pinoid leaflets 7.
Subgenus Nortieria. With remarks on Rooibos tea cultivation. – Bot.
Not. 121: 165-208.
Dahlgren RMT. 1968c. Revision of the genus
Aspalathus III. The species with flat and simple leaves. – Opera
Bot. 22: 1-126.
Dahlgren RMT. 1969. Comprehensive key to the
species of Aspalathus (Leguminosae). – Bot. Not. 122: 512-548.
Dahlgren RMT. 1970a. Wiborgia
apterophora R. Dahlgr., a new species of Leguminosae from the Cape
Province. – Bot. Not. 123: 112-114.
Dahlgren RMT. 1970b. Current topics.
Parallelism, convergence, and analogy in some South African genera of
Leguminosae. – Bot. Not. 123: 551-568.
Dahlgren RMT. 1971a. Multiple similarity of
leaf between two genera of Cape plants, Cliffortia L. (Rosaceae) and Aspalathus
L. (Fabaceae). – Bot. Not. 124:
292-304.
Dahlgren RMT. 1971b. Chromosome numbers in
the South African genus Aspalathus L. (Fabaceae). – Bot. Not. 124:
383-398.
Dahlgren RMT. 1972. The genus
Hypocalyptus Thunb. (Fabaceae). – Bot. Not. 125:
102-125.
Dahlgren RMT. 1975. Studies on
Wiborgia Thunb. and related species of Lebeckia Thunb. (Fabaceae). – Opera Bot. 38: 1-83.
D’Amato-Avanzi MG. 1956. Attuali conoscenze
sulla citotassonomia del genre Cassia (Caesalpiniaceae-Cassieae). –
Caryologia 9: 16-173.
Damerval C. 1983. Comparaison de six espèces
de luzernes annuelles à l’aide de caractères biométriques et enzymatiques.
– Agronomie 3: 971-982.
D’Arcy WG. 1980. Pachyrhizus. –
Ann. Missouri Bot. Gard. 67: 743-746.
Dasuki UA, Schot AM. 1991. Taxonomy of
Fordia Hemley (Papilionaceae: Millettieae). – Blumea 36: 191-204.
Dave YS, Menon ARS. 1987. Structure, origin
and development of the extra floral nectaries of Pithecellobium dulce
Benth. (Mimosaceae). – Acta Bot. Hung. 33: 117-125.
Davis CC, Fritsch PW, Li J, Donoghue MJ.
2002. Phylogeny and biogeography of Cercis (Fabaceae): evidence from nuclear
ribosomal ITS and chloroplast ndhF sequence data. – Syst. Bot. 27:
289-302.
Dayanandan S, Bawa KS, Kesseli RV. 1997.
Conservation of microsatellites among tropical trees (Leguminosae). – Amer.
J. Bot. 84: 1658-1663.
De Aguiar ACA, Moreira GV, Gonçalves-Esteves
V, Yamamoto K. 2008. Palynotaxonomy of Brazilian species of Polygala
subgenus Hebeclada. – Bot. J. Linn. Soc. 157: 609-619.
De Castro O, Cozzolino S, Jury SL, Caputo P.
2002. Molecular relationships in Genista L. Sect.
Spartocarpus Spach (Fabaceae). – Plant Syst. Evol. 231:
91-108.
De Faria SM, Sprent JI. 1994. Legume nodule
development: an evolutionary hypothesis. – In: Sprent JI, McKey D (eds),
Advances in legume systematics 5: the nitrogen factor, Royal Botanic Gardens,
Kew, pp. 33-39.
Degtjareva GV, Valiejo-Roman CM, Kramina TE,
Mironov EM, Samigullin TH, Sokoloff DD. 2003. Taxonomic and phylogenetic
relationships between Old World and New World members of the tribe Loteae
(Leguminosae): new insights from molecular and morphological data, with special
emphasis on Ornithopus. – Wulfenia 10: 15-50.
Degtjareva GV, Valiejo-Roman CM, Samigullin
TH, Kramina TE, Sokoloff DD. 2004. Phylogenetic relationships of North American
Loteae (Leguminosae): analyses of nrITS sequences and morphological data,
implications for taxonomy and evolution of basic chromosome number. –
Chromosome Sci. 8: 119-122.
Degtjareva GV, Kramina TE, Sokoloff DD,
Samigullin TH, Valiejo-Roman CM, Antonov AS. 2006. Phylogeny of the genus
Lotus (Leguminosae, Loteae): evidence from nrITS sequences and
morphology. – Can. J. Bot. 84: 813-830.
Degtjareva GV, Kramina TE, Sokoloff DD,
Samigullin TH, Sandral G, Valiejo-Roman CM. 2008. New data on nrITS phylogeny
of Lotus (Leguminosae, Loteae). – Wulfenia 15: 35-49.
Degtjareva GV, Samigullin TH, Vallejo-Roman
CM, Sokoloff DD. 2010. Phylogenetic placement of Podolotus suggests
independent origin of lomentaceous fruits in Coronilla and
Hippocrepis (Leguminosae: Loteae). – Pakistan J. Bot., Spec. Iss.
(S.I. Ali Festschrift) 42: 11-25.
Degtjareva GV, Valiejo-Roman CM, Samigullin
TH, Guara-Requena M, Sokoloff DD. 2012. Phylogenetics of Anthyllis
(Leguminosae: Papilionoideae: Loteae): partial incongruence between nuclear and
plastid markers. A long branch problem and implications for morphological
evolution. – Mol. Phylogen. Evol. 62: 693-707.
Delgado-Salinas A. 1985. Systematics of the
genus Phaseolus (Leguminosae), in Mexico and Central America. –
Ph.D. diss., University of Texas, Austin, Texas.
Delgado-Salinas A. 2000. New species of
Mexican Phaseolus (Fabaceae). – Syst. Bot. 25:
414-436.
Delgado-Salinas A, Lewis GP. 1997.
Oryxis, a new genus in tribe Phaseoleae (Leguminosae: Papilionoideae)
from Brazil. – Kew Bull. 52: 221-225.
Delgado-Salinas A, Bruneau A, Doyle JJ. 1993.
Chloroplast DNA phylogenetic studies in New World Phaseolinae (Leguminosae:
Papilionoideae: Phaseoleae). – Syst. Bot. 18: 6-17.
Delgado-Salinas A, Turley T, Richman A, Lavin
M. 1999. Phylogenetic analysis of the cultivated and wild species of
Phaseolus (Fabaceae). –
Syst. Bot. 24: 438-460.
Delgado-Salinas A, Bibler R, Lavin M. 2006.
Phylogeny of the genus Phaseolus (Leguminosae): a recent
diversification in an ancient landscape. – Syst. Bot. 31: 779-791.
Delgado-Salinas A, Thulin M, Pasquet R,
Weeden N, Lavin M. 2011. Vigna (Leguminosae) sensu lato: the names and
identities of the American segregate genera. – Amer. J. Bot. 98:
1694-1715.
Deml I. 1972. Revision der Sektionen
Acanthophace Bunge und Aegacantha Bunge der Gattung
Astragalus L. – Boissiera 21: 1-235.
De Nysschen AM, Wyk B-E van, Heerden FR van.
1998. Seed flavonoids of the Podalyrieae and Liparieae (Fabaceae). – Plant Syst. Evol. 212:
1-11.
De Padua Teixeira S, Carmello-Guerreiro SM,
Machado SR. 2004. Fruit and seed ontogeny related to the seed behaviour of two
tropical species of Caesalpinia (Leguminosae). – Bot. J. Linn. Soc.
146: 57-70.
De-Paula OC, Oliveira DMT. 2008. Multiple
pleurograms in Chamaecrista Moench (Leguminosae, Caesalpinioideae).
– Bot. J. Linn. Soc. 157: 487-492.
De-Paula OC, Oliveira DMT. 2012. Seed
ontogeny of Chamaecrista and its systematic implications in Cassiinae
(Leguminosae, Caesalpinioideae). – Plant Syst. Evol. 298: 1659-1669.
Deshpande PK, Bhasin RK. 1974. Embryological
studies in Phaseolus aconitifolius Jacq. – Bot. Gaz. 135:
104-113.
Détienne P. 1991. Anatomie du bois de
Balgoya pacifica (Polygalaceae) de Nouvelle
Calédonie. – Bull. Mus. Natl. Hist. Nat., sect. B, Adansonia, sér. IV, 13:
17-20.
Devesa JA. 1988. Estudio taxonomico del
gênero Ononis L. (Leguminosae) en Portugal. – Mem. Soc. Brot. 28:
5-53.
De Wilde WJJO, Duyfjes BEE. 2005. New taxa
and taxonomic status in Xanthophyllum Roxb. (Polygalaceae) from Borneo. – Gard.
Bull. Singapore 57: 47-61.
Dharani N. 2006. Field guide to Acacias of
East Africa. – Struik Publ., Cape Town, Republic of South Africa.
Dhillon M. 1981. Floral anatomy of two
species of Swartzia and its bearing on its systematic position. –
Feddes Repert. 92: 689-693.
Dickison WC. 1973. Nodal and leaf anatomy of
Xanthophyllum (Polygalaceae). – Bot. J. Linn.
Soc. 67: 103-115.
Dickison WC. 1981. The evolutionary
relationships of the Leguminosae. – In: Polhill PM, Raven PH (eds), Advances
in legume systematics 1, Royal Bot. Gardens, Kew, pp. 35-54.
Diez MJ, Ferguson IK. 1990. Studies of the
pollen morphology and taxonomy of the tribes Loteae and Coronilleae
(Leguminosae: Faboideae) 1, Anthyllis L. and related genera. –
Lagascalia 16: 77-94.
Diez MJ, Ferguson IK. 1994. The pollen
morphology of the tribes Loteae and Coronilleae (Papilionoideae: Leguminosae)
2, Lotus L. and related genera. – Rev. Palaeobot. Palynol. 81:
233-255.
Diez MJ, Ferguson IK. 1996. Studies of the
pollen morphology and taxonomy of the tribes Loteae and Coronilleae
(Papilionoideae: Leguminosae) 3, Coronilla L. and related genera and
systematic conclusions. – Rev. Palaeobot. Palynol. 94: 239-257.
Dilcher DL, Herendeen PS, Hueber FM. 1992.
Fossil Acacia flowers with attached anther glands from Dominican
Republic amber. – In: Herendeend PS, Dilcher DL (eds), Advances in legume
systeamtics 4. The fossil record, Royal Botanic Gardens, Kew, pp. 34-42.
Dinc M, Bagci Y, Duran A. 2009. Retraction: a
macro-micromorphological study on the Genista sessilifolia complex,
with some taxonomic notes on Genista (Fabaceae) in Turkey. – Nord. J. Bot.
26: 384.
Ding Hou, Larsen K, Larsen SS. 1996.
Caesalpiniaceae (Leguminosae-Caesalpinioideae). – In: Kalkman C et al. (eds),
Flora Malesiana I, 12(2), Flora Malesiana Foundation, Rijksberbarium/Hortus
Botanicus, Leiden, pp. 409-730.
Ding S-Y, Gu H-Y, Qu L-J, Chen Z-L. 1995. A
preliminary study on the use of RFLP analysis of the PCR amplified products in
the systematic investigation of the subtribe Astragalinae (Fabaceae). – Acta Bot. Sin. 37:
97-102.
Dion DJ. 1997. A taxonomic revision of the
genus Austrosteenisia R. Geesink (Fabaceae: Millettieae). –
Austrobaileya 5: 79-91.
Ditsch F, Patha H, Barthlott W. 1994.
Micromorphology of epicuticular waxes in Fabales s.l. and its systematic
significance. – Beitr. Biol. Pfl. 68: 297-310.
Dnyansagar VR. 1955. Embryological studies in
the Leguminosae XI. Embryological features and formula and taxonomy of the
Mimosaceae. – J. Indian Bot. Soc. 34: 362-374.
Dnyansagar VR. 1960. Comparative embryology
of angiosperms: Leguminosae. – Bull. Indian Natl. Sci. Acad. 41: 93-103.
Dogan M, Kence A, Tigin C. 1992. Numerical
taxonomic study of Turkish Lathyrus (Leguminosae). – Edinburgh J.
Bot. 49: 333-341.
Domenech-Ramirez JI, Tucker SC. 1990.
Comparative ontogeny of the perianth in mimosoid legumes. – Amer. J. Bot. 77:
624-635.
Domínguez E. 1976. Revision de las especies
anuales del género Hippocrepis L. – Lagascalia 5: 225-261.
Domínguez E, Galiano EF. 1974a. Revision del
género Scorpiurus L. I. Parte experimental. – Lagascalia 4:
61-84.
Domínguez E, Galiano EF. 1974b. Revision del
género Scorpiurus L. II. Parte sistematica. – Lagascalia 4:
259-280.
Dominguez E, Galiano EF. 1979. Revision del
genero Tetragonolobus Scop. (Fabaceae). – Lagascalia 8: 189-214.
Donkpegan ASL, Doucet J-L, Migliore J,
Duminil J, Dainou K, Piñeiro R, Wieringa JJ, Champluvier D, Hardy OJ. 2017.
Evolution in African tropical trees displaying ploidy-habitat association: the
genus Afzelia (Leguminosae). – Mol. Phylogen. Evol. 107: 270-281.
Dorado O, Arias DM. 2006. Brongniartia
papyracea (Fabaceae: Faboideae):
a new species from the tropical deciduous forest of southern Jalisco and
southwestern Michoacán, Mexico. – Brittonia 58: 357-361.
Dormer KJ. 1945. On the absence of a plumule
in some leguminous seedlings. – New Phytol. 44: 25-28.
Dormer KJ. 1946. Vegetative morphology as a
guide to the classification of the Papilionatae. – New Phytol. 45:
145-161.
Downie SR, Katz-Downie DS, Roger EJ, Zujewski
HL, Small E. 1998. Multiple independent losses of the plastid rpoC1
intron in Medicago (Fabaceae) as inferred from phylogenetic
analyses of nuclear ribosomal DNA internal transcribed spacer sequences. –
Can. J. Bot. 76: 791-803.
Doyle JJ. 1994. Phylogeny of the legume
family: an approach to understanding the origins of nodulation. – Ann. Rev.
Ecol. Syst. 25: 325-349.
Doyle JJ. 1995. DNA data and legume
phylogeny: a progress report. – In: Crisp MD, Doyle JJ (eds), Advances in
legume systematics 7: phylogeny, Royal Botanic Gardens, Kew, pp. 11-30.
Doyle JJ. 2011. Phylogenetic perspectives on
the origins of nodulation. – Mol. Plant-Microbe Interact. 24: 1289-1295.
Doyle JJ, Beachy RN. 1985. Ribosomal gene
variation in soybean (Glycine) and its relatives. – Theor. Appl.
Gen. 70: 369-376.
Doyle JJ, Brown AHD. 1989. 5S nuclear
ribosomal gene variation in the Glycine tomentella polyploid complex
(Leguminosae). – Syst. Bot. 14: 398-407.
Doyle JJ, Doyle JL. 1993. Chloroplast DNA
phylogeny of the papilionoid legume tribe Phaseoleae. – Syst. Bot. 18:
309-327.
Doyle JJ, Doyle JL. 1999. Nuclear
protein-coding genes in phylogeny reconstruction and homology assessment: some
examples from Leguminosae. – In: Hollingsworth P, Bateman R, Gornall R (eds),
Molecular systematics and plant evolution, Taylor and Francis, London, pp.
229-254.
Doyle JJ, Luckow MA. 2003. The rest of the
iceberg: legume diversity and evolution in a phylogenetic context. – Plant
Physiol. 131: 900-910.
Doyle JJ, Doyle JL, Brown AHD. 1990a. A
chloroplast DNA phylogeny of the wild perennial relatives of the soybean
(Glycine subgenus Glycine): congruence with morphological and
crossing groups. – Evolution 44: 371-389.
Doyle JJ, Doyle JL, Brown AHD. 1990b.
Chloroplast DNA phylogenetic affinities of newly described species in
Glycine (Leguminosae: Phaseoleae). – Syst. Bot. 15: 466-471.
Doyle JJ, Doyle JL, Brown AHD. 1990c.
Chloroplast DNA polymorphism and phylogeny in the B genome of Glycine
subgenus Glycine (Leguminosae). – Amer. J. Bot. 77: 772-782.
Doyle JJ, Doyle JL, Brown AHD. 1990d.
Analysis of a polyploid complex in Glycine with chloroplast and
nuclear DNA. – Aust. Syst. Bot. 3: 125-136.
Doyle JJ, Lavin M, Bruneau A. 1992.
Contributions of molecular data to papilionoid legume systematics. – In:
Soltis PS, Soltis DE, Doyle JJ (eds), Molecular systematics of plants, Chapman
and Hall, New York, pp. 223-251.
Doyle JJ, Doyle JL, Palmer JD. 1995. Multiple
independent losses of two genes and one intron from legume chloroplast genomes.
– Syst. Bot. 20: 272-294.
Doyle JJ, Doyle JL, Ballenger JA, Palmer JD.
1996. The distribution and phylogenetic significance of a 50-kb chloroplast DNA
inversion in the flowering plant family Leguminosae. – Mol. Phylogen. Evol.
5: 429-438.
Doyle JJ, Doyle JL, Ballenger JA, Dickson EE,
Kajita T, Ohashi H. 1997. A phylogeny of the chloroplast gene rbcL in
the Leguminosae: taxonomic correlations and insights into the evolution of
nodulation. – Amer. J. Bot. 84: 541-554.
Doyle JJ, Chappill JA, Bailey CD, Kajita T.
2000. Towards a comprehensive phylogeny of legumes: evidence from rbcL
sequences and non-molecular data. – In: Herendeen PS, Bruneau A (eds),
Advances in legume systematics 9: phylogeny, Royal Botanic Gardens, Kew, pp.
1-20.
Doyle JJ, Doyle JL, Harbison C. 2003.
Chloroplast-expressed glutamine synthetase in Glycine and related
Leguminosae: phylogeny, gene duplication, and ancient polyploidy. – Syst.
Bot. 28: 567-577.
Doyle JJ, Doyle JL, Rauscher JT, Brown AHD.
2004. Diploid and polyploid reticulate evolution throughout the history of the
perennial soybeans (Glycine subgenus Glycine). – New
Phytol. 161: 121-132.
Doyle JJ, Doyle JL, Rauscher JT, Brown AHD.
2004. Evolution of the perennial soybean polyploidy complex (Glycine
subgenus Glycine): a study of contrasts. – Biol. J. Linn. Soc. 82:
583-597.
Drummond CS. 2008. Diversification of
Lupinus (Leguminosae) in the western New World: derived evolution of
perennial life history and colonization of montane habitats. – Mol. Phylogen.
Evol. 48: 408-421.
Drummond CS, Eastwood RJ, Miotto STS, Hughes
CE. 2012. Multiple continental radiations and correlates of diversification in
Lupinus (Leguminosae): testing for key innovations with incomplete
taxon sampling. – Syst. Biol. 61: 443-460.
Duan L, Wen J, Yang X, Liu P-L, Arslan E,
Ertuğrul K, Chang Z-Y. 2015. Phylogeny of Hedysarum and tribe
Hedysareae (Leguminosae: Papilionoideae) inferred from sequence data of ITS,
matK, trnL-F and psbA-trnH. – Taxon 64:
49-64.
Duan L, Yang X, Liu P, Johnson G, Wen J,
Chang Z. 2016. A molecular phylogeny of Caraganeae (Leguminosae,
Papilionoideae) reveals insights into new generic and infrageneric
delimitations. – PhytoKeys 70: 111-137.
Dube VP. 1962. Morphological and anatomical
studies in Polygalaceae and its
allied families. – Agra Univ. J. Res., Sci. 11: 109-112.
Dube VP, Awasthi DK. 1985. Morphological and
anatomical studies of floral nectaries in Polygalaceae. – J. Indian Bot.
Soc. 64: 231-235.
Dudik NM. 1981. Morphology of the pods of
Leguminales (Fabales). – In: Polhill RM, Raven PH (eds), Advances in legume
systematics 2, Royal Botanic Gardens Kew, pp. 897-901.
Duke JA, Polhill RM. 1981. Seedlings of the
Leguminosae. – In: Polhill RM, Raven PH (eds), Advances in legume systematics
2, Royal Botanic Gardens, Kew, pp. 941-950.
Duley ML, Vincent MA. 2003. A synopsis of the
genus Cladrastis (Leguminosae). – Rhodora: 205-239.
Duman H, Akan H. 2003. New species of
Astragalus (sect. Alopecuroidei: Leguminosae) from Turkey.
– Bot J. Linn. Soc. 143: 201-205.
Dunn DB. 1971. A case of long range dispersal
and “rapid” speciation in Lupinus. – Trans. Missouri Acad. Sci.
5: 26-38.
Dunn DB. 1984. Genetic resources:
cytotaxonomy and distribution of New World lupine species. – In: Proceedings
of the 3rd International Lupine Congress, La Rochelle, France, pp.
68-85.
Dunn DB, Gillett JM. 1966. The lupines of
Canada and Alaska. – Queen’s Princter, Ottawa, Canada.
Dunn DB, Harmon WE. 1977. The Lupinus
montanus complex of Mexico and Central America. – Ann. Missouri Bot.
Gard. 64: 340-365.
Dunn ST. 1912. A revision of the genus
Millettia Wight et Arn. – Bot. J. Linn. Soc. 41: 123-243.
Duno-de-Stefano R, Fernández-Concha GC,
Can-Itza LL, Lavin M. 2010. The morphological and phylogenetic distinctions of
Courseta greenmanii (Leguminosae): taxonomic and ecological
implications. – Syst. Bot. 35: 289-295.
Du Puy DJ. 1995. Pyranthus, a new
genus of the tribe Millettieae (Leguminosae: Papilionoideae) from Madagascar.
– Kew Bull. 50: 73-84.
Du Puy DJ, Ireland H. 1997. Two species of
Crotalaria L. (Leguminosae: Papilionoideae: Crotalarieae) from Central
Madagascar. – Kew Bull. 52: 443-449.
Du Puy DJ, Labat J-N, Schrire BD. 1993. The
separation of two previousy confused species in the Indigofera spicata
complex (Leguminosae: Papilionoideae). – Kew Bull. 48: 727-733.
Du Puy DJ, Labat J-N, Shrire BD. 1994.
Révision du genre Vaughania S. Moore
(Leguminosae-Papilionoideae-Indigofereae). – Bull. Mus. Natl. Hist. Nat., B,
Adansonia 16: 75-102.
Du Puy DJ, Phillipson PB, Rabevohitra R.
1995. The genus Delonix (Leguminosae: Caesalpinioideae: Caesalpinieae)
in Madagascar. – Kew Bull. 50: 445-475.
Du Puy DJ, Labat J-N, Schrire BD. 1995. A
revision of Phylloxylon (Leguminosae: Papilionoideae: Indigofereae).
– Kew Bull. 50: 477-494.
Du Puy DJ, Labat J-N, Rabevohitra R, Villiers
J-F, Bosser J, Moat J. 2002. The Leguminosae of Madagascar. – Royal Botanic
Gardens, Kew.
Ebinger JE, Seigler DS, Clarke HD. 2000.
Taxonomic revision of South American species of the genus Acacia
subgenus Acacia (Fabaceae:
Mimosoideae). – Syst. Bot. 25: 588-617.
Edwards D, Hawkins JA. 2007. Are Cape floral
clades the same age? Contemporaneous origins of two lineages in the genistoids
s.l. (Fabaceae). – Mol. Phylogen.
Evol. 45: 952-970.
Edwards D, Horn A, Taylor D, Savolainen V,
Hawkins JA. 2008. DNA barcoding of a large genus, Aspalathus L. (Fabaceae). – Taxon 57: 1317-1327.
Edwards TJ. 1994. A revision of
Argyrolobium (Crotalarieae, Fabaceae) in South Africa. – Ph.D.
diss., University of Natal, Pietermaritzburg, Republic of South Africa.
Egan AN, Crandall KA. 2008. Incorporating
gaps as phylogenetic characters across eight DNA regions: ramifications for
North American Psoraleeae (Leguminosae). – Mol. Phylogen. Evol. 46:
532-546.
Egan AN, Reveal JL. 2009. A new combination
in Pediomelum and a new genus, Ladeania, from Western North
America (Fabaceae, Psoraleeae). –
Novon 19: 310-314. http://dx.doi.org/10.3417/2008074
Ekici M, Aytac Z, Akan H, Pinar M. 2008. A
new species of Astragalus L. (section Onobrychoidei DC.: Fabaceae) from Turkey. – Bot. J. Linn.
Soc. 157: 741-747.
El-Gazzar A, El-Fiki MA. 1977. The main
subdivisions of Leguminosae. – Bot. Not. 129: 371-375.
El-Gazzar A. 1981. Systematic implication of
susceptibility to Uromyces rusts in Leguminosae. – In: Polhill RN,
Raven PH (eds), Advances in legume systematics 2, Royal Botanic Gardes, Kew,
pp. 979-994.
Elias TS. 1972. Morphology and anatomy of
foliar nectaries of Pithecellobium macradenium (Leguminosae). – Bot.
Gaz. 133: 38-42.
Elias TS. 1980. Foliar nectaries of unusual
structure in Leonardoxa africana (Leguminosae), an African obligate
myrmecophyte. – Amer. J. Bot. 67: 423-425.
Elias TS. 1981. Mimosoideae. – In: Polhill
PM, Raven PH (eds), Advances in legume systematics 1, Royal Botanic Gardens,
Kew, pp. 143-151.
Elliott GN, Chen W-M, Chou J-H, Wang H-C,
Sheu S-Y, Perin L, Reis VM, Moulin L, Simon MF, Bontemps C, Sutherland JM,
Bessi R, Faria SM de, Trinick MJ, Prescott AR, Sprent JI, James EK. 2007.
Burkholderia phymatum is a highly effective nitrogen-fixing symbiont
of Mimosa spp. and fixes nitrogen ex planta. – New Phytol.
173: 168-180.
Ellison NW, Liston A, Steiner JJ, Williams
WM, Taylor NL. 2006. Molecular genetics of the clover genus (Trifolium
– Leguminosae). – Mol. Phylogen. Evol. 39: 688-705.
Elmer ADE. 1907. Some new Leguminosae. –
Leafl. Philipp. Bot. 1: 220-232.
Endo Y. 1994. Pistil morphology of Vicia
amurensis (Legminosae) and its allied species. – J. Jap. Bot. 69:
379-382.
Endo Y. 2012. Anatomical diversity of fruits
in Leguminosae. – J. Plant Res. 125: 41-53.
Endo Y, Ohashi H. 1995. The morphology of
styles and stigmas in Vicia (Leguminosae), and its systematic
implications. – J. Plant Res. 108: 17-24.
Endo Y, Ohashi H. 1996. The infrageneric
positions of East Asian species of Vicia (Leguminosae). – J. Jap.
Bot. 71: 254-262.
Endo Y, Ohashi H. 1997. The morphology and
anatomy of styles in Vicieae (Leguminosae). – J. Jap. Bot. 72: 9-18.
Endo Y, Ohashi H. 1998. The features of
cotyledon areoles in Leguminosae and their systematic utility. – Amer. J.
Bot. 85: 753-759.
Endo Y, Ohashi H. 1999. Morphological
variation and distribution of the cotyledon areoles in Papilionoideae
(Leguminosae) and their systematic utility. – J. Jap. Bot. 74: 296-306.
Endo Y, Ohashi H. 2001. Phylogenetic
relationships among East Asian species of Vicia (Leguminosae). – In:
Cho DS (ed), Proceedings of the 19th symposium on plant biology,
biodiversity-status, conservation and restoration, The Catholic University of
Korea, Puchon, pp. 153-158.
Endo Y, Choi B-H, Ohashi H, Delgado-Salinas
A. 2008. Phylogenetic relationships of New World Vicia (Leguminosae)
inferred from nrDNA internal transcribed spacer sequences and floral
characters. – Syst. Bot. 33: 356-363.
Engel T. 1990. Dornenanatomie und
Samenmikromorphologie der kleinasiatischen Vertreter der Gattung
Astracantha Podlech sowie der dornigen Vertreter der Gattung
Astragalus L. (Fabaceae).
– Diss. Bot. 151.
Engel T. 1991. The evolution of rachis thorns
in Astragalus and Astracantha (Leguminosae) and the
systematic applicability of thorn anatomy. – Fl. Veg. Mundi 9: 17-27.
Engel T. 1992. Petiolar anatomy of North
American Astragalus species (Fabaceae) with persistent petioles. –
Aliso 2: 339-345.
Engler A (†). 1931. Simarubaceae. – In:
Engler A (†), Harms H, Pax F (eds), Die natürlichen Pflanzenfamilien, 2.
Aufl., Bd. 19a, W. Engelmann, Leipzig, pp. 359-405.
Erdtman G. 1944. The systematic position of
the genus Diclidanthera Mart. – Bot. Not. 97: 80-84.
Eren O, Parolly G, Raus T, Kurschner H. 2008.
A new species of Polygala L. (Polygalaceae) from South-West
Anatolia. – Bot. J. Linn. Soc. 158: 82-86.
Eriksen B. 1993a. Floral anatomy and
morphology in the Polygalaceae.
– Plant Syst. Evol. 186: 17-32.
Eriksen B. 1993b. Phylogeny of the Polygalaceae and its taxonomic
implications. – Plant Syst. Evol. 186: 33-55.
Eriksen B. 1993c. A revision of
Monnina subg. Pterocarya (Polygalaceae) in northwestern South
America. – Ann. Missouri Bot. Gard. 80: 191-207.
Eriksen B. 1997. Heterogeneity in
Monnina sect. Stipulatae – evidence from fruit anatomy and
morphology. – BioLlania ed. esp. no. 6: 311-323.
Eriksen B, Persson C. 2006. Polygalaceae. – In: Kubitzki K
(ed), The families and genera of vascular plants IX. Flowering plants.
Eudicots. Berberidopsidales,
Buxales, Crossosomatales, Fabales p. p., Geraniales, Gunnerales, Myrtales p. p., Proteales, Saxifragales, Vitales, Zygophyllales, Clusiaceae Alliance, Passifloraceae
Alliance, Dilleniaceae, Huaceae, Picramniaceae, Sabiaceae, Springer, Berlin,
Heidelberg, New York, pp. 345-363.
Eriksen B, Ståhl B, Persson C. 2000. 102. Polygalaceae. – In: Harling G,
Andersson L (eds), Flora of Ecuador 65, Botanical Institute, Göteborg
University, pp. 1-132.
Espert SM, Sede SM, Ruiz LK, Fortunato RH,
Poggio L. 2008. New chromosome reports in the subtribes Diocleinae and
Glycininae (Phaseoleae: Papilionoideae: Fabaceae). – Bot. J. Linn. Soc. 158:
336-341.
Estada-C AE, Villarreal-Q JA, Gonzalez-E M.
2004. A new species of Dalea sect. Parosela (Fabaceae: Amorpheae) from Mexico. –
Brittonia 56: 67-71.
Estrada-C AE, Villarreal-Q JA, Yen-M C. 2005.
Astragalus mario-sousae (Fabaceae: Galegeae), a new species from
northeastern Mexico. – Brittonia 57: 314-319.
Estrella M de la, Devesa JA. 2014. The genus
Gilbertiodendron (Leguminosae-Caesalpinioideae) in Western Africa. –
Syst. Bot. 39: 160-192.
Estrella M de la, Aedo C, Velayos M. 2009. A
morphometric analysis of Daniellia (Fabaceae-Caesalpinioideae). – Bot. J.
Linn. Soc. 159: 268-279.
Estrella M de la, Devesa JA, Wieringa JJA.
2012. Morphological re-evaluation of the taxonomic status of the genus
Pellegriniodendron (Harms) J. Leonard
(Leguminosae-Caesalpinioideae-Detarieae) and its inclusion in
Gilbertiodendron J. Leonard. – South Afr. J. Bot. 78: 257-265.
Estrella M de la, Wieringa JJ, Mackinder B,
Burgt X van der, Devesa JA, Bruneau A. 2014. Phylogenetic analysis of the
African genus Gilbertiodendron J. Léonard and related genera
(Leguminosae-Caesalpinioideae-Detarieae). – Intern. J. Plant Sci. 175:
975-985.
Estrella M de la, Forest F, Klitgård B,
Lewis GP, Mackinder BA, Queiroz LP de, Wieringa JJ, Bruneau A. 2018. A new
phylogeny-based tribal classification of subfamily Detarioideae, an early
branching clade of florally diverse tropical arborescent legumes. – Sci. Rep.
8: 6884.
Evans JA, Gasson PE, Lewis GP. 2006. Wood
anatomy of the Mimosoideae (Leguminosae). – IAWA J. [Suppl. 5: 1-117],
Nationaal Herbarium Nederland, Leiden.
Evans SV, Fellows LE, Bell EA. 1985.
Distribution and systematic significance of basic non-protein amino acids and
amines in the Tephrosieae (Leguminosae). – Biochem. Syst. Ecol. 13:
271-302.
Exell AW. 1960. 20. Polygalaceae. – In: Exell AW, Wild
H (eds), Flora Zambesiaca 1 (Part 1), Crown Agents for Oversea Governments and
Administrations, London, pp. 303-336.
Exell AW, Robson NKB. 1960. New species of
Polygala and Garcinia from tropical Africa. – Bol. Soc.
Brot., ser. II, 34: 93-97.
Fahn A, Zohary M. 1955. On the pericarpial
structure of the legumen, its evolution and relation to dehiscence. –
Phytomorphology 5: 99-111.
Faria MA, Harris DJ, Visnevschi-Necrasov T,
Sousa MT de, Nunes E. 2012. The correct phylogenetic position of Lotus
conimbricensis Brot. (Leguminosae, Loteae) based on nuclear ribosomal ITS
sequences. – Acta Bot. Croat. 71: 87-94.
Faria SM de, Lima HC de. 1998. Additional
studies of the nodulation status of legume species in Brazil. – Plant and
Soil 200: 185-192.
Faria SM de, Franco AA, Jesus RM de, Menandro
M de S, Baitello JB, Mucci ESF, Dobereiner J, Sprent JI. 1984. New nodulating
legume trees from South-East Brazil. – New Phytol. 98: 317-328.
Faria SM de, Lewis GP, Sprent JI, Sutherland
JM. 1989. Occurrence of nodulation in the Leguminosae. – New Phytol. 111:
607-619.
Faria SM de, Lima HC de, Carvalho AM,
Concalves VF, Sprent JI. 1994. Occurrence of nodulation in legume species from
Bahia, Minas Gerais, and Espirito Santo states of Brazil. – In: Sprent JI,
McKey D (eds), Advances in legume systematics 5, Royal Botanic Gardens, Kew,
pp. 17-23.
Farruggia FT, Howard JH. 2011. Examination of
five nuclear markers for phylogenetic study of Hologalegina (Leguminosae). –
Brittonia 63: 489-499.
Farruggia FT, Lavin M, Wojciechowski MF.
2018. Phylogenetic systematics and biogeography of the pantropical genus
Sesbania (Leguminosae). – Syst. Bot. 43: 414-429.
Fasbender MV. 1959. Pollen grain morphology
and its taxonomic significance in Amherstieae, Cynometreae, and Sclerolobieae
(Caesalpiniaceae) with special reference to American genera. – Lloydia 22:
1-161.
Faugeras C, Paris R. 1968. Chemitaxinomie des
Papilionacées-Genistées. – Bull. Soc. Bot. France, Mém. 1965: 75-102.
Fearing OS. 1959. A cytotaxonomic study of
the genus Cologania and its relationship to Amphicarpaea
(Leguminosae-Papilionoideae). – Ph.D. diss., University of Texas, Austin,
Texas.
Fedtschenko BA. 1899. Liste provisoire des
espèces du genre Hedysarum. – Bull. Herb. Boissier 7: 254-261.
Fedtschenko BA. 1902. The genus
Hedysarum. – Acta Hort. Petrop. 19: 185-342.
Fedtschenko BA. 1927. Revision préliminaire
du genre “Güldenstaedtia” Fisch. – Nuovo G. Bot. Ital. 2:
1435-1442.
Fehrenbach S, Barthlott W. 1988.
Mikromorphologie der Epicuticular-Wachse der Rosales s.l. und deren systematische
Gliederung. – Bot. Jahrb. Syst. 109: 407-428.
Feng X, Zhao Z, Tian Z, Xu S, Luo Y, Cai Z,
Wang Y, Yang J, Wang Z, Weng L, Chen J, Zheng L, Guo X, Luo J, Sato S, Tabata
S, Ma W, Cao X, Hu X, Sun C, Luo D. 2006. Control of petal shape and floral
zygomorphy in Lotus japonicus. – Proc. Natl. Acad. Sci. U.S.A. 103:
4970-4975.
Fenster CB. 1995. Mirror image flowers and
their effect on the outcrossing rate in Chamaecrista fasciculata
(Leguminosae). – Amer. J. Bot. 82: 46-50.
Feoli-Chiapella L, Cristofolini G. 1980.
Sero-systematics of Cytisus sect. Trianthocytisus (Fabaceae). – Plant Syst. Evol. 136:
209-216.
Feoli-Chiapella L, Cristofolini G. 1981.
Serological contributions to the systematics of Ulex (Genisteae-Fabaceae) and allied genera. – Nord.
J. Bot. 1: 723-729.
Ferguson IK. 1981. The pollen morphology of
Macrotyloma (Leguminosae: Phaseoleae). – Kew Bull. 36: 455-461.
Ferguson IK. 1984. Pollen morphology and
biosystematics of the subfamily Papilionoideae (Leguminosae). – In: Gran WF
(ed), Plant biosystematics, Academic Press, Toronto, pp. 377-394.
Ferguson IK. 1987. A preliminary survey of
the pollen exine stratification in the Caesalpinioideae. – In: Stirton CH
(ed), Advances in legume systematics 3, Royal Botanic Gardens, Kew, pp.
355-379.
Ferguson IK. 1990. The significance of some
pollen morphological characters of the tribe Amorpheae and of the genus
Mucuna (tribe Phaseoleae) in the biology and systematics of subfamily
Papilionoideae (Leguminosae). – Rev. Palaeobot. Palyn. 64: 129-136.
Ferguson IK, Banks H. 1994. Tetrad pollen in
the subfamily Caesalpinioideae (Leguminosae) and its significance. – Rev.
Palaeobot. Palynol. 83: 31-42.
Ferguson IK, Pearce KJ. 1986. Observations on
the pollen morphology of the genus Bauhinia L. (Leguminosae:
Caesalpinioideae) in the neotropics. – In: Blackmore S, Ferguson IK (eds),
Pollen and spores, form and function, Academic Press, London, pp. 283-296.
Ferguson IK, Schrire BD. 1994. A cladistic
analysis of the pollen morphology of the tribe Swartzieae. – Acta Bot. Gall.
141: 207-215.
Ferguson IK, Skvarla JJ. 1981. The pollen
morphology of the subfamily Papilionoideae (Leguminosae). – In: Polhill RN,
Raven PH (eds), Advances in legume systematics 2, Royal Botanic Gardens, Kew,
pp. 859-896.
Ferguson IK, Skvarla JJ. 1982. Pollen
morphology in relation to pollinators in Papilionoideae (Legminosae). – Bot.
J. Linn. Soc. 84: 183-193.
Ferguson IK, Skvarla JJ. 1988. Pollen
morphology of the tribe Swartzieae (subfamily Papilionoideae: Leguminosae) 1.
Introduction and all genera excluding Aldina and Swartzia.
– Amer. J. Bot. 75: 1884-1897.
Ferguson IK, Skvarla JJ. 1991. Pollen
morphology of the tribe Swartzieae (subfamily Papilionoideae: Leguminosae) 2.
The genera Aldina Endlicher and Swartzia Schreber. – Rev.
Paleobot. Palynol. 67: 153-178.
Ferguson IK, Stirton CH. 1993. Pollen
morphology of the genera Panurea and Bowdichia (Leguminosae:
Papilionoideae: Sophoreae. – Grana 32, Suppl. 2: 19-26.
Ferguson IK, Tucker SC (eds). 1994. Advances
in legume systematics 6: structural botany. – Royal Botanic Gardens, Kew.
Ferguson IK, Schrire BD, Shepperson R. 1994.
Pollen morphology of the tribe Sophoreae and relationships between subfamilies
Caesalpinioideae and Papilionoideae (Leguminosae). – In: Ferguson IK, Tucker
SC (eds), Advances in legume systematics 6, Structural botany, Royal Botanic
Gardens, Kew, pp. 11-32.
Ferguson ME, Maxted N, Slageren MV, Robertson
LD. 2000. A re-assessment of the taxonomy of Lens Mill. (Leguminosae,
Papilionoideae, Vicieae). – Bot. J. Linn. Soc. 133: 41-59.
Fernanda Silva N, De Oliveira Arruda R do C,
Maced Oalves F, Bagnatori Sartori ÂL. 2018. Leaflet anatomy of the Dipterygeae
clade (Faboideae: Fabaceae):
evolutionary implications and systematics. – Bot. J. Linn. Soc. 187:
99-117.
Fernandes A. 1982. Contribution à la
connaissance des lotiers du groupe corniculatus de la Péninsule
Ibérique et des Îles Baléares. – Bol. Soc. Brot. 55: 29-86.
Fernandes A. 1996. O taxon
Aeschynomene no Brasil. – Edicões UFC, Fortaleza, Brazil.
Fernandes A, Queirós M. 1978. Contribution
à la connaissance cytotaxonomique des Spermatophyta du Portugal IV.
Leguminosae (Suppl. 3). – Bol. Soc. Brot., ser. II, 52: 79-164.
Fernandes A, Santos M de F. 1971.
Contribution à la connaissance cytotaxonomique des Spermatophyta du Portugal
IV. Leguminosae. – Bol. Soc. Brot., ser. II, 45: 177-225.
Fernandes A, Santos M de F. 1975.
Contribution à la connaissance cytotaxonomique des Spermatophyta du Portugal
IV. Leguminosae (Suppl. 1). – Bol. Soc. Brot., ser. II, 49: 173-196.
Fernandes A, Santos M de F, Queirós M. 1977.
Contribution à la connaissance cytotaxonomique des Spermatophyta du Portugal
IV. Leguminosae (Suppl. 2). – Bol. Soc. Brot., ser. II, 51: 137-186.
Fernando ES, Quinn CJ. 1992. Pericarp anatomy
and systematics of the Simaroubaceae sensu
lato. – Aust. J. Bot. 40: 263-289.
Fernando EL, Gadek PA, Crayn DM, Quinn CJ.
1993. Rosid affinities of Surianaceae: molecular evidence. –
Mol. Phylogen. Evol. 2: 344-350.
Fernando ES, Gadek PA, Quinn CJ. 1995. Simaroubaceae, an
artificial construct: evidence from rbcL sequence variation. – Amer.
J. Bot. 82: 92-103.
Ferrara LS, Quinn JA. 1985. Cleistogamous and
chasmogamous flower and fruit production in New Jersey populations of
Polygala paucifolia Willd. – Amer. J. Bot. 72: 851-852.
Ferreyra R. 1951. Una nueva Leguminosae del
Peru. – Publ. Mus. Hist. Nat. ”Javier Prado”, Ser. B, Bot. 3: 1-5.
Filardi FLR, Lima HC de. 2014. The diversity
of Machaerium (Leguminosae: Papilionoideae) in the Atlantic forest:
three new species, nomenclatural updates, and a revised key. – Syst. Bot. 39:
145-159.
Fiori A. 1949. Chiave analitica ed
illustrazione delle specie di Trifolium dell’Abissinia. – Nuov.
Giorn. Bot. Ital. 55: 335-346.
Fletcher R. 2002. Ooline Cadellia
pentastylis F. Muell.: a survivor. – Victorian Natur. (Blackburn) 119:
235-236.
Flores AS, Correa AM, Forni-Martins ER, Tozzi
AMGA. 2006. Chromosome numbers in Brazilian species of Crotalaria
(Leguminosae, Papilionoideae) and their taxonomic significance. – Bot. J.
Linn. Soc. 151: 271-277.
Flores AS, Tozzi AMGA, Trigo JR. 2009.
Pyrrolizidine alkaloid profiles in Crotalaria species from Brazil:
chemotaxonomic significance. – Biochem. Syst. Ecol. 37: 459-469.
Flores-Cruz M, Santana-Lira HD, Koch SD,
Grether R. 2004. Taxonomic significance of leaflet anatomy in Mimosa
series Quadrivalves (Leguminosae, Mimosoideae). – Syst. Bot. 29:
892-902.
Focke WO. 1894. Rosaceae. – In: Engler A, Prantl
K (eds), Die natürlichen Pflanzenfamilien III(3), W. Engelmann, Leipzig, pp.
1-61.
Ford AJ, Halford DA, Van Der Merwe M,
Mathieson MT. 2017. A revision of the tropical white-flowered species of
Comesperma (Polygalaceae) in Australia. –
Aust. Syst. Bot. 30: 159-182.
Forero E. 1972. Studies in
Stryphnodendron (Leguminosae-Mimosoideae) including two new taxa. –
Brittonia 24: 143-147.
Forest F, Manning JC. 2006. Evidence for
inclusion of South African endemic Nylandtia in Muraltia (Polygalaceae). – Syst. Bot. 31:
525-532.
Forest F, Nänni I, Chase MW, Crane PR,
Hawkins JA. 2007. Diversification of a large genus in a continental
biodiversity hotspot: temporal and spatial origin of Muraltia (Polygalaceae) in the Cape of South
Africa. – Mol. Phylogen. Evol. 43: 60-74.
Forest F, Chase MW, Persson C, Crane PR,
Hawkins JA. 2007. The role of biotic and abiotic factors in evolution of ant
dispersal in the milkwort family (Polygalaceae). – Evolution 61:
1675-1694.
Förther H, Podlech D. 1991. Revision der
Ononis natrix-Gruppe (Leguminosae) von Makaronesien, Nordafrika und
dem angrenzenden Westasien. – Mitt. Bot. Staatssamml. München 30:
197-296.
Fortuna-Perez AP, Silva MJ da, Queiroz LP de,
Lewis GP, Simões AO, Azevedo Tozzi AMG de, Sarkinen T, Souza AP de. 2013.
Phylogeny and biogeography of the genus Zornia (Leguminosae:
Papilionoideae: Dalbergieae). – Taxon 62: 723-732.
Fortunato RH. 1984. Dos Leguminosas nuevas
para la flora Argentina. – Darwiniana 25: 367-369.
Fortunato RH. 2005. Mimozygantheae. – In:
Lewis GP, Schrire BD, Mackinder B, Lock M (eds), Legumes of the world, Royal
Botanic Gardens, Kew, pp. 184-185.
Fortunato RH, Cialdella AM. 1996. Una especie
nueva del género Acacia (Acacieae, Mimosoideae, Fabaceae) para el Chaco
boliviano-paraguayo: A. emilioana Fortunato et Ciald. Discusión sobre
su ubicación infragenérica. Contribución al estudio de la flora y
vegetación del Chaco X. – Candollea 51: 215-224.
Fortunato RH, Queiroz LP de, Lewis GP. 1996.
Lackeya, a new genus in tribe Phaseoleae subtribe Diocleinae
(Leguminosae: Papilionoideae) from North America. – Kew Bull. 51: 365-370.
Fougère-Danezan M, Maumont S, Bruneau A.
2003. Phylogenetic relationships in resin-producing Detarieae inferred from
molecular data and preliminary results for a biogeographic hypothesis. – In:
Klitgaard BB, Bruneau A (eds), Advances in legume systematics 10: higher level
systematics, Royal Botanic Gardens, Kew, pp. 161-180.
Fougère-Danezan M, Maumont S, Bruneau A.
2007. Relationships among resin-producing Detarieae s.l. (Leguminosae) as
inferred by molecular data. – Syst. Bot. 32: 748-761.
Fougère-Danezan M, Herendeen PS, Maumont S,
Bruneau A. 2010. Morphological evolution in the variable resin-producing
Detarieae (Fabaceae): Do
morphological characters retain a phylogenetic signal? – Ann. Bot. 105:
311-325.
Frahm-Leliveld JA. 1957. Observations
cytologiques sur quelques Léguminoeuses tropicales et subtropicales. – Rev.
Cytol. Biol. Vég. 8: 273-287.
Frahm-Leliveld JA. 1960a. Observations on
chromosomes in the genus Indigofera L. – Acta Bot. Neerl. 9:
286-293.
Frahm-Leliveld JA. 1960b. Chromosome numbers
in leguminous plants. – Acta Bot. Neerl. 9: 327-329.
Frahm-Leliveld JA. 1969. Cytotaxonomic notes
on African Papilionaceae. – Acta Bot. Neerl. 18: 67-73.
Francino DMT, Coutinho ÍAC, Dalvi VC,
Azevedo AA, Conceição A de S, Meira RMSA. 2015. Anatomical interpretations of
the taxonomy of Chamaecrista (L.) Moench sect. Absus
(Legumonosae-Caesalpinioideae). – Plant Syst. Evol. 301: 2087-2103.
Francisco-Ortega J, Jackson MT, Catty JP,
Ford-Lloyd BV. 1992. Genetic diversity in the Chamaecytisus proliferus
complex (Fabaceae: Genisteae) in the
Canary Islands in relations in relation to in situ conservation. – Genet.
Res. Crop Evol. 39: 149-158.
Francisco-Ortega J, Newbury HJ, Ford-Lloyd
BV. 1993. Numerical analyses of RAPD data highlight the origin of cultivated
tagasaste (Chamaecytisus proliferus ssp. palmensis) in the
Canary Islands. – Theor. Appl. Gen. 87: 264-270.
Freitas Mansano V de, Pádua Teixeira S.
2008. Floral anatomy of the Lecointea clade (Leguminosae,
Papilionoideae, Swartzieae sensu lato). – Plant Syst. Evol. 273: 201-209.
Freitas Mansano V de, Bittrich V, Azevedo
Tozzi AMG de, Souza AP de. 2003. Composition of the Lecointea clade
(Leguminosae, Papilionoideae, Swartzieae), a re-evaluation based on combined
evidence from morphology and molecular data. – Taxon 53: 1007-1018.
Friend SA, Quandt D, Tallury SP, Stalker HT,
Hilu KW. 2010. Species, genomes, and section relationships in the genus
Arachis (Fabaceae): a
molecular phylogeny. – Plant Syst. Evol. 290: 185-199.
Fritsch PW, Cruz BC. 2012. Phylogeny of
Cercis based on DNA sequences of nuclear ITS and four plastid regions:
implications for transatlantic historical biogeography. – Mol. Phylogen.
Evol. 62: 816-825.
Fukii T, Baas P, Gasson P, Ridder Numan JWA.
1994. Wood anatomy of the Sophora group (Leguminosae). – In:
Ferguson IK, Tucker S (eds), Advances in legume systematics 6, Royal Botanic
Gardens, Kew, United Kingdom, pp. 205-249.
Fuks R. 1983. O genero Quillaja
Molina (Rosaceae) no Brasil. –
Arq. Jard. Bot. Rio de Janeiro 26: 61-67.
Fukuda T, Tokoyama J, Maki M. 2003. Molecular
evolution of cycloidea-like genes in Fabaceae. – J. Molec. Evol. 57:
588-597.
Furness CA, Stafford PJ. 1995. Polygalaceae. – Rev. Palaeobot.
Palyn. 88: 61-82.
Gadek PA, Quinn CJ. 1992. Pericarp anatomy
and systematics of the Surianaceae
sensu lato. – Aust. J. Bot. 40: 263-289.
Gagnepain F. 1911. Mimosées nouvelles. –
Notul. Syst. (Paris) 2: 56-62.
Gagnon E, Hughes CE, Lewis GP, Bruneau A.
2015. A new cryptic species in a new cryptic genus in the Caesalpinia
group (Leguminosae) from the seasonally dry inter-Andean valleys of South
America. – Taxon 64: 468-490.
Gagnon E, Bruneau A, Hughes CE, Queiroz LP
de, Lewis GP. 2016. A new generic system for the pantropical
Caesalpinia group (Leguminosae). – PhytoKeys 71: 1-160.
Gale SW, Pennington TD. 2004.
Lysiloma (Leguminosae: Mimosoideae) in Mesoamerica. – Kew Bull. 59:
453-467.
Galiana A, Tibok A, Duhoux E. 1991.
Nitrogen-fixing potential of micropropagated clones of Acacia mangium
inoculated with different Bradyrhizobium spp. strains. – Plant and
Soil 135: 161-166.
Gandhi KN, Vincent MA, Reveal JA. 2011.
Dermatophyllum, the correct name for Calia (Fabaceae). – Phytoneuron 57: 1-4.
Gao T, Sun Z, Yao H, Song J, Zhu Y, Ma X,
Chen S. 2011. Identification of Fabaceae plants using the DNA barcode
matK. – Planta medica 77: 92-94.
Gardner SK, Murphy DJ, Newbigin E, Drinnan
AN, Ladiges PY. 2005. An investigation of phyllode variation in Acacia
verniciflua and A. leprosa (Mimosaceae), and implications for
taxonomy. – Aust. Syst. Bot. 18: 383-398.
Gasson PE. 1994. Wood anatomy of the tribe
Sophoreae and related Caesalpinioideae and Papilionoideae. – In: Ferguson IK,
Tucker SC (eds), Advances in legume systematics 6, Royal Botanic Gardens, Kew,
pp. 165-203.
Gasson PE. 1996. Wood anatomy of the tribe
Swartzieae with comments on related papilionoid and caesalpinioid Leguminosae.
– IAWA J. 17: 45-75.
Gasson PE. 1999. Wood anatomy of the tribe
Dipterygeae with comments on related papilionoid and caesalpinioid Leguminosae.
– IAWA J. 20: 361-375.
Gasson PE. 2000. Does wood anatomy support
tribal and generic classification in papilionoid Leguminosae? – In: Herendeen
PS, Bruneau A (eds), Advances in legume systematics 9, Royal Botanic Gardens,
Kew, pp. 201-215.
Gasson PE, Webley P. 1999. Wood anatomy of
Exostyles venusta (Swartzieae, Papilionoideae, Leguminosae). – IAWA
J. 20: 59-66.
Gasson PE, Trafford C, Matthews B. 2003. Wood
anatomy of Caesalpinioideae. – In: Klitgaard BB, Bruneau A (eds), Advances in
legume systematics 10: higher level systematics, Royal Botanic Gardens, Kew,
pp. 63-93.
Gasson PE, Wray E, Schrire BD. 2004. Wood
anatomy of the tribe Millettieae with comments on related papilionoid
Leguminosae. – IAWA J. 25: 485-545.
Gasson PE, Warner K, Lewis G. 2009. Wood
anatomy of Caesalpinia s.s., Coulteria,
Erythrostemon, Guilandina, Libidibia,
Mezoneuron, Poincianella, Pomaria and Tara
(Leguminosae, Caesalpinioideae, Caesalpinieae). – IAWA J. 30: 247-276.
Gazer M. 1993. Revision of
Astragalus L. sect. Sesamei DC. (Leguminosae). – Sendtnera
1: 69-155.
Geesink R. 1981. Tephrosieae. – In: Polhill
RM, Raven PH (eds), Advances in legume systematics 1, Royal Botanic Gardens,
Kew, pp. 245-260.
Geesink R. 1984. Scala Millettiearum. A
survey of the genera of the tribe millettieae (Leguminosae-Papilionoideae) with
methodological considerations. – Ph.D. diss., Leiden Botanical Series 8, E.
J. Brill and Leiden University Press, Leiden, The Netherlands.
Gemmeke V. 1982. Entwicklungsgeschichtliche
Untersuchungen an Mimosaceen-Blüten. – Bot. Jahrb. Syst. 103: 185-210.
Genc H, Sahin A. 2008. A new species of
Lathyrus L. (section Cicercula; Fabaceae) from Turkey. – Bot. J. Linn.
Soc. 158: 301-305.
George SM, Bhavandan KV. 1993. Cytological
studies in some species of Cassia from south India. – J. Cytol.
Genet. 28: 1-5.
Gervais GYF, Bruneau A. 2002. Phylogenetic
analysis of a polyphyletic African genus of Caesalpinioideae (Leguminosae):
Monopetalanthus Harms. – Plant Syst. Evol. 235: 19-34.
Ghouse AKM, Yunus M. 1974a. A new record on
the occurrence of stratified cambium in the family Mimosaceae. – Geobios 1:
138.
Ghouse AKM, Yunus M. 1974b. Cambial structure
in Dalbergia. – Phytomorphology 24: 152-158.
Gibbs PE. 1966. A revision of the genus
Genista. – Notes Roy. Bot. Gard. Edinb. 27: 11-99.
Gibbs PE. 1967. A revision of the genus
Adenocarpus. – Bol. Soc. Brot., sér. II, 41: 67-121.
Gibbs PE, Dingwall I. 1971. A revision of the
genus Teline. – Bol. Soc. Brot., sér. II, 45: 269-316.
Gill LS, Husaini SWH. 1981. Cytomorphological
investigations of some species of the genus Cassia L. in Nigeria. –
Bull. Mus. Natl. Hist. Nat. Paris, sér. IV, sect. B, Adansonia 3: 461-472.
Gill LS, Husaini SWH. 1982. Cytology of some
arborescent Leguminosae of Nigeria. – Silvae Genetica 31: 117-124.
Gill LS, Husaini SWH. 1984. Cytomorphology of
the genus Millettia Wight & Arn. (Leguminosae) from Nigeria. –
Bol. Soc. Brot., sér. II, 57: 237-249.
Gill LS, Husaini SWH. 1985. Cytology of some
arborescent Papilionoideae (Leguminosae) of southern Nigeria. – Bol. Soc.
Brot., sér. II, 58: 187-200.
Gill LS, Husaini SWH. 1986. Cytological
observations in Leguminosae from southern Nigeria. – Willdenowia 15:
521-527.
Gillespie DJ, McComb JA. 1991. Morphology and
distribution of species in the Medicago murex complex. – Can. J.
Bot. 69: 2655-2662.
Gillett JB. 1952. The genus
Trifolium in southern Arabia and in Africa south of the Sahara. –
Kew Bull. 7: 367-404.
Gillett JB. 1958a. Notes on
Tephrosia in tropical Africa. – Kew Bull. 13: 111-132.
Gillett JB. 1958b. Indigofera
(Microcharis) in tropical Africa with the related genera
Cyamopsis and Rhynchotropis. – Kew Bull., Add. Ser. 1.
Gillett JB. 1958c. Lotus in Africa
south of the Sahara (excluding the Cape Verde Islands and Socotra) and its
distinction from Dorycnium. – Kew Bull. 13: 361-381.
Gillett JB. 1964. Coronilla L.
(Leguminosae) in Somaliland. – Kew Bull. 17: 563.
Gillett JB. 1966. The species of
Ormocarpum Beauv. and Arthrocarpum Balf. f. (Leguminosae) in
South-Western Asia and Africa (excluding Madagascar). – Kew Bull. 20:
323-355.
Gillett JB. 1969. Taxonomy of
Trifolium (Leguminosae) II. The T. longipes complex in North
America. – Can. J. Bot. 7: 93-113.
Gillett JB. 1970. Additions to our knowledge
of Indigofera L. in East Tropical Africa. – Kew Bull. 24:
465-506.
Gillett JB, Taylor NL. 200. The world of
clovers. – Iowa State University Press, Ames, Iowa.
Gillett JB, Polhill RM, Verdcourt B. 1971a.
Leguminosae (Part 3) Subfamily Papilionoideae (1). – In: Milne-Redhead E,
Polhill RM (eds), Flora of tropical East Africa, Crown Agents for Oversea
Governments and Administrations, London, pp. 1-501.
Gillett JB, Polhill RM, Verdcourt B. 1971b.
Leguminosae (Part 4) Subfamily Papilionoideae (2). – In: Milne-Redhead E,
Polhill RM (eds), Flora of tropical East Africa, Crown Agents for Oversea
Governments and Administrations, London, pp. 503-1108.
Gillies ACM, Abbott RJ. 1998. Evaluation of
random amplified polymorphic DNA for species identification and phylogenetic
analysis in Stylosanthes (Fabaceae). – Plant Syst. Evol. 211:
201-216.
Gillis WT. 1975. Bahama Polygalaceae and their Greater
Antillean affinities – a preliminary treatment. – Phytologia 32: 35-44.
Giraud E, Moulin L, Vallenet D, Barbe V,
Cytryn E, Avarre J-C, Jaubert M, Simon D, Cartieaux F, Prin Y, Bena G, Hannibal
L, Fardious J, Kojadinovic M, Vuillet L, Lajus A, Cruveiller S, Rouy Z,
Mangenot S, Segurens B, Dossat C, Franck WL, Chang W-S, Sauders E, Bruce D,
Richardson P, Normand P, Dreyfus B, Pignol D, Stacey G, Emerich D, Verméglio
A, Médigue C, Sadowsky M. 2007. Legume symbioses: absence of Nod
genes in photosynthetic bradyrhizobia. – Science 316: 1307-1312.
Gladstones JS. 1974. Lupins of the
Mediterranean region and Africa. – Dept. Agricult. West Australia, Techn.
Bull. 26: 1-48.
Glass CE, Seigler DS. 2006. A new combination
in Senegalia and typification of six New World Acacia names.
– Taxon 55: 993-995.
Gledhill D. 1968. The Geissaspis,
Bryaspis, Humularia complex. – Bol. Soc. Brot., ser. II:
305-319.
Goel S, Raina SN, Ogihara Y. 2002. Molecular
evolution and phylogenetic implications of Internal Transcribed Spacer
sequences of nuclear ribosomal DNA in the Phaseolus-Vigna
complex. – Mol. Phylogen. Evol. 22: 1-1-19.
Goldblatt P. 1976. Cytotaxonomic studies in
the tribe Quillajeae (Rosaceae).
– Ann. Missouri Bot. Gard. 63: 200-206.
Goldblatt P. 1981a. Cytology and the
phylogeny of Leguminosae. – In: Polhill PM, Raven PH (eds), Advances in
legume systematics 2, Royal Botanic Gardens, Kew, pp. 427-463.
Goldblatt P. 1981b. Chromosome numbers in
Leguminosae II. – Ann. Missouri Bot. Gard. 68: 551-557.
Gomes CMR, Gottlieb OR, Gottlieb RC, Salatino
A. 1981. Phytochemistry in perspective: chemosystematics of the Papilionoideae.
– In: Polhill RM, Raven PH (eds), Advances in legume systematics 2, Royal
Botanic Gardens, Kew, pp. 465-488.
Gonzalez AM, Marazzi B. 2018. Extrafloral
nectaries in Fabaceae: filling gaps
in structural and anatomical diversity in the family. – Bot. J. Linn. Soc.
187: 26-45.
González PM, Crespo MB. 2009. Machaerium
meridanum Meléndez (Fabaceae,
Papilionoideae, Dalbergieae), a new species from Venezuela: amended key for the
species identification. – Candollea 64: 89-90.
González-Andrés F, Ortiz J-M. 1996.
Morphometrical characterization of Cytisus and allies (Genisteae:
Leguminosae) as an aid in taxonomic discrimination. – Israel J. Plant Sci.
44: 95-114.
González-Orozco CE, Laffan SW, Miller JT.
2011. Spatial distribution of species richness and endemism of the genus
Acacia in Australia. – Aust. J. Bot. 59: 601-609.
González-Pérez MA, Sosa PA, Batista FJ.
2009. Genetic variation and conservation of the endangered endemic Anagyris
latifolia Brouss. ex Willd. (Leguminosae) from the Canary Islands. –
Plant Syst. Evol. 279: 59-68.
Gorbunova NN. 1984. De generis
Caragana Lam. (Fabaceae)
notae systematicae. – Novost. Sist. Vyssh. Rast. 21: 92-101.
Görts-van Rijn ARA. 1974. Notes on Polygalaceae from Suriname. – Acta
Bot. Neerl. 23: 189-191.
Gottsberger G, Silberbauer-Gottsberger I.
1988. Evolution of flower structures and pollination in Neotropical Cassiinae
(Caesalpiniaceae) species. – Phyton 28: 293-320.
Govindarajulu R, Hughes CE, Bailey CD. 2011.
Phylogenetic and population genetic analyses of diploid Leucaena
(Leguminosae-Mimosoideae) reveal cryptic species diversity and patterns of
allopatric divergent speciation. – Amer. J. Bot. 98: 2049-2063.
Graham A, Barker G. 1981. Palynology and
tribal classification in the Caesalpinioideae. – In: Polhill RM, Raven PH
(eds), Advances in legume systematics 2, Royal Botanic Gardens, Kew, pp.
801-834.
Graham A, Barker G, Freitas da Silva M. 1980.
Unique pollen types in the Caesalpinioideae (Leguminosae). – Grana 19:
79-84.
Granby R. 1980. Revision of the genus
Coelidium (Liparieae-Fabaceae). – Opera Bot. 54: 1-47.
Granby R. 1985. Revision of the genus
Amphithalea (Liparieae-Fabaceae). – Opera Bot. 80: 1-34.
Granby R. 1987. Coelidium flavum, a
new species of Fabaceae-Liparieae
from the Cape Province. – Nord. J. Bot. 7: 51-52.
Grant WF. 1965. A chromosome atlas and
interspecific hybridization index for the genus Lotus (Leguminosae).
– Can. J. Genet. Cytol. 7: 457-471.
Grant WF, Sidhu BS. 1967. Basic chromosome
number, cyanogenetic glucoside variation and geographic distribution of
Lotus species. – Can. J. Bot. 45: 639-647.
Grant WF, Whetter JM. 1966. The cytogenetics
of Lotus XI. The use of thin-layer chromatography in the separation of
secondary phenolic compounds in Lotus (Leguminosae). – J. Chromat.
21: 247-256.
Grant WF, Zandstra II. 1968. The
biosystematics of the genus Lotus (Leguminosae) in Canada II.
Numerical chemotaxonomy. – Can. J. Bot. 46: 585-589.
Greene EL. 1890. Enumeration of the North
American Loti. – Pittonia 2: 133-150.
Greinwald R. 1988. Untersuchungen zur
chemotaxonomischen Bedeutung von Leguminosenalkaloiden und zum
Alkaloidstoffwechsel in transformierten Geweben und Zellkulturen. – Ph.D.
diss., Universität Würzburg, Germany.
Greinwald R, Cantó P, Bachmann P, Witte L,
Czygan F-C. 1992. Distribution and taxonomic significance of alkaloids in the
Genista cinerea aggregat. – Biochem. Syst. Ecol. 20: 75-81.
Greinwald R, Bachmann P, Lewis GP, Witte L,
Czygan F. 1995. Alkaloids of the genus Poecilanthe (Leguminosae:
Papilionoidae). – Biochem. Syst. Ecol. 23: 547-553.
Greinwald R, Rensen I van, Veit M, Cantó P,
Witte L. 1995. A chemical dichotomy in quinolizidine alkaloid accumulation
between the section Spartioides of the genus Genista (Fabaceae: Genisteae). – Biochem. Syst.
Ecol. 23: 89-97.
Greinwald R, Henrichs C, Veen G, Ross JH,
Witte L, Czygan F-C. 1995. A survey of alkaloids in Templetonia biloba
(Benth.) Polhill (Fabaceae:
Brongniartieae). – Biochem. Syst. Ecol. 23: 649-654.
Greinwald R, Ross JH, Witte L, Czygan F-C.
1995. The alkaloid pattern of Plagiocarpus axillaris (Fabaceae: Brongniartieae). – Biochem.
Syst. Ecol. 23: 645-646.
Greinwald R, Reyes CR, Ross JH, Witte L,
Czygan FC. 1996. A survey of alkaloids in the genera Harpalyce and
Brongniartia (Fabaceae:
Brongniartieae). – Biochem. Syst. Ecol. 24: 749-755.
Greinwald R, Ross JH, Witte L, Czygan F-C.
1996. Alkaloids of Templetonia incana. – Biochem. Syst. Ecol. 24:
423-426.
Greissl R. 2006. Ontogeny of the
Calliandra-massulae (Mimosaceae: Ingeae), and the associated viscin
body. – Flora 201: 570-587.
Gresser G. 1992. Aspekte zum Stoffwechsel und
zur Chemotaxonomie der Chinolizidinalkaloide in Fabaceae und Berberidaceae.
– Ph.D. diss., Universität Würzburg, Germany.
Grether R. 1990. Two new taxa of
Mimosa (Leguminosae) from Oaxaca, Mexico. – Syst. Bot. 15:
435-441.
Grether R, Martinez-Bernal A. 1996.
Mimosa tejupilcana, a new species of Series Plurijugae
(Leguminosae) from the State of Mexico, Mexico. – Syst. Bot. 21: 617-621.
Grimes JW. 1986. A reevaluation of the
discontinuity plate in Psoraleeae and Amorpheae (Leguminosae). – Amer. J.
Bot. 73: 1222-1229.
Grimes JW. 1995. Generic relationships of
Mimosoideae tribe Ingeae, with emphasis on the New World
Pithecellobium complex. – In: Crisp MD, Doyle JJ (eds), Advances in
legume systematics 7: Phylogeny, Royal Botanic Gardens, Kew, pp. 101-122.
Grimes JW. 1996. Branch apices, heterochrony,
and inflorescence morphology in some mimosoid legumes (Leguminosae:
Mimosoideae). – Telopea 6: 729-748.
Grimes JW. 1997. A revision of
Cullen (Leguminosae: Papilionoideae). – Aust. Syst. Bot. 10:
565-648.
Grimes JW. 1999. Inflorescence morphology,
heterochrony, and phylogeny in the mimosoid tribes Ingeae and Acacieae
(Leguminosae: Mimosoideae). – Bot. Rev. 65: 317-347.
Grondona EM. 1948. Las especies Argentinas
del género Polygala. – Darwiniana 2-3: 1-405.
Guern M, Gorenflot R. 1966. Caryologie du
genre Hippocrepis L. – Compt. Rend. Acad. Sci. Paris, sér. D, 263:
509-512.
Guinet P. 1965. Étude des charactères du
pollen dans le genre Calliandra (Mimosaceae). – Pollen Spores 7:
157-173.
Guinet P. 1969. Les Mimosacées, étude de
palynologie fondamentale, corrélations, évolution. – Trav. Sci. Techn.
Inst. Franç. Pondichéry 9: 1-293.
Guinet P. 1981. Mimosoideae: the characters
of their pollen grains. – In: Polhill RM, Raven PH (eds), Advances in legume
systematics 2, Royal Botanic Gardens, Kew, pp. 835-855.
Guinet P. 1986. Geographic patterns of the
main pollen characters in the genus Acacia (Leguminosae), with
particular reference to subgenus Phyllodineae. – In: Blackmore S,
Ferguson IK (eds), Pollen and spores: form and function, Linnean Society
Symposium Series 12, Academic Press, London, pp. 297-311.
Guinet P, Caccavari MA. 1992. Pollen
morphology of the genus Stryphnodendron (Leguminosae, Mimosoideae) in
relation to its taxonomy. – Grana 31: 101-112.
Guinet P, Ferguson IK. 1989. Structure,
evolution, and biology of pollen in Leguminosae. – In: Stirton CH, Zarucchi
JL (eds), Advances in legume biology, Monogr. Syst. Bot. Missouri Bot. Gard.
29: 77-103.
Guinet P, Lugardon B. 1976. Diversité des
structures de l’exine dans le genre Acacia (Mimosaceae). – Pollen
Spores 18: 483-511.
Guinet P, Salard-Cheboladaeff M. 1975. Grain
de pollen du tertiaire du Cameroun pouvant être rapportés aux Mimosacées.
– Boissiera 24a: 21-28.
Guinet P, El Sabrouty N, Soliman HAS, Omran
AM. 1987. Étude des caractères du pollen des Légumineuses-Mimosoidées des
sediments tertiaires du Nord-Ouest de l’Egypte. – In: Cambon G, Richard P,
Suc JP (eds), Palynologie et milieux tropicaux, Mém. Trav. E.P.H.E., Inst.
Montpellier 17: 159-171.
Guinet P, Vassal J. 1978. Hypotheses on the
differentiation of the major groups in the genus Acacia (Leguminosae).
– Kew Bull. 32: 509-527.
Güneş F. 2012. Pollen morphology of
Lathyrus (Leguminosae) taxa belonging to Lathyrus,
Orobastrum and Cicercula sections from Turkey. – Plant
Syst. Evol. 298: 1777-1794.
Gunn CR. 1970. A key and diagrams for the
seeds of one hundred species of Vicia (Leguminosae). – Proc. Intern.
Seed Testing Assoc. 35: 773-790.
Gunn CR. 1979. Genus Vicia with
notes about tribe Vicieae (Fabaceae)
in Mexico and Central America. – U.S. Dept. Agric., Techn. Bull. Washington
1601.
Gunn CR. 1984. Fruits and seeds of genera in
the subfamily Mimosoideae (Fabaceae).
– U. S. Dept. Agric. Techn. Bull. Washington 1681: 50-57.
Gunn CR. 1991. Fruits and seeds of genera in
the subfamily Caesalpinioideae (Fabaceae). – U.S. Dept. Agric., Techn.
Bull. Washington 1755.
Gunn CR, Kluve J. 1976. Androecium and pistil
characters for tribe Vicieae (Fabaceae). – Taxon 25: 563-575.
Gunn CR, Norman EM, Lassetter JS. 1980.
Chapmannia floridana Torrey & Gray (Fabaceae). – Brittonia 32: 178-185.
Guo S, Kenne L, Lundgren LN, Rönnberg B,
Sundquist BG. 1998. Triterpenoid saponins from Quillaja saponaria. –
Phytochemistry 48: 175-180.
Gutzwiller MA. 1961. Die phylogenetische
Stellung von Suriana maritima L. – Bot. Jahrb. Syst. 81: 1-49.
Haddad RS, Barnett JR. 1989. Variation in
petiole anatomy of the European spiny species of Astragalus L.
(Leguminosae-Papilionoideae-Galegeae). – Bot. J. Linn. Soc. 101: 241-247.
Hakoyama T, Niimi K, Watanabe H, Tabata R,
Matsubara J, Sato S, Nakamura Y, Tabata S, Jichun L, Matsumoto T, Tatsumi K,
Nomura M, Tajima S, Ishizaka M, Yano K, Imaizumi-Anraku H, Kawaguchi M, Kouchi
H, Suganuma N. 2009. Host plant genome overcomes the lack of a bacterial gene
for symbiotic nitrogen fixation. – Nature 462: 514-517.
Hanelt P, Mettin D. 1989. Biosystematics of
the genus Vicia L. (Leguminosae). – Ann. Rev. Ecol. Syst. 20:
199-223.
Hannah B, Gwilym L. 2009. Pollen morphology
of the Dimorphandra group (Leguminosae, Caesalpinioideae). – Grana
48: 19-26.
Hanson L. 1995. Some new chromosome counts in
the genus Inga (Leguminosae: Mimosoideae). – Kew Bull. 50:
801-804.
Hao G, Zang D-X, Zhang M-Y, Guo L-X, Li S-J.
2003. Phylogenetics of Bauhinia subgenus Phanera
(Leguminosae: Caesalpinioideae) based on ITS sequences of nuclear ribosomal
DNA. – Bot. Bull. Acad. Sin. 44: 223-228.
Harborne JB. 1969. Chemosystematics of the
Leguminosae: flavonoid and isoflavonoid patterns in the tribe Genisteae. –
Phytochemistry 8: 1449-1456.
Harborne JBD, Boulter D, Turner BL (eds).
1971. Chemotaxonomy of the Leguminosae. – London, New York.
Harder DK. 1992. Chromosome counts in
Psophocarpus. – Kew Bull. 47: 529-534.
Harley RM. 1978. A study of
Cranocarpus in Brazil. – Bradea, Bol. Herb. Bradeanum 2: 265-272.
Harley RM. 1980. A new Endostemon
(Labiatae) in Kenya. – Kew Bull. 34: 547-549.
Harms HAT. 1897. Leguminosae. – In: Engler
A, Prantl K (eds), Die natürlichen Pflanzenfamilien, Nachträge zu III(3), W.
Engelmann, Leipzig, pp. 190-204.
Harms HAT. 1911. Über die Verbreitung der
Leguminosen-Gattung Mastersia. – Feddes Repert. 9: 367-369.
Harms HAT. 1912. Neue Arten der Gattung
Melolobium Eckl. und Zeh. aus Deutsch-Südwestafrika. – Feddes
Repert. 6: 84-88.
Harms HAT. 1915. Leguminosae africanae 8. –
Engl. Bot. Jahrb. Syst. 53: 455-476.
Harms HAT. 1920. Drei neue
Mucuna-Arten aus Papuasien. – Notizbl. Bot. Gart. Mus. Berlin-Dahlem
7: 372-374.
Harms HAT. 1934. Leguminosae. – Notizbl.
Bot. Gart. Mus. Berlin-Dahlem 12: 84-85.
Harms H. 1938-1939. Leguminosae. – Notizbl.
Bot. Gart. Mus. Berlin-Dahlem 14: 30-32.
Harrier LA. 1995. Non-nodulating African
species of Acacia. – Ph.D. diss., University of Dundee, Scotland.
Harrier LA, Whitty PW, Sutherland JM, Sprent
JI. 1997. Phenetic investigation of non-nodulating African species of
Acacia (Leguminosae) using morphological and molecular markers. –
Plant Syst. Evol. 205: 27-51.
Harriman NA. 1998. Proposal to conserve the
name Gymnocladus (Leguminosae: Caesalpinioideae) with a conserved
gender and type. – Taxon 47: 875-876.
Harris SA. 1995. Systematics and randomly
amplified polymorphic DNA in the genus Leucaena (Leguminosae,
Mimosoideae). – Plant Syst. Evol. 197: 195-208.
Haston EM, Lewis GP, Hawkins JA. 2003. A
phylogenetic investigation of the Peltophorum group (Caesalpinieae:
Leguminosae). – In: Bruneaeu A, Klitgaard BB (eds), Advances in legume
systematics 10: Higher level systematics, Royal Botanic Gardens, Kew, pp.
149-159.
Haston EM, Lewis GP, Hawkins JA. 2005. A
phylogenetic reappraisal of the Peltophorum group (Caesalpinieae:
Leguminosae) based on the chloroplast trnL-F, rbcL and
rps16 sequence data. – Amer. J. Bot. 92: 1359-1371.
Hatfield GM, Keller WJ, Rankin JM. 1980.
Quinolizidine alkaloids of Clathrotropis brachypetala. – J. Nat.
Prod. 43: 164-167.
He H, Bleby TM, Veneklaas EJ, Lambers H, Kuo
J. 2012. Precipitation of calcium, magnesium, strontium and barium in tissues
of four Acacia species (Leguminosae: Mimosoideae). – PLoS ONE 7:
1-12.
Heel WA van. 1993. Floral ontogeny of
Archidendron lucyi (Mimosaceae), with remarks on Amherstia
nobilis (Caesalpiniaceae). – Bot. Jahrb. Syst. 109: 17-23.
Heenan PB. 2001. Relationships of
Streblorrhiza (Fabaceae), an
extinct monotypic genus from Phillip Island, South Pacific Ocean. – New
Zealand J. Bot. 39: 9-15.
Heenan PB, Dawson MI, Wagstaff SJ. 2004. The
relationship of Sophora sect. Edwardsia (Fabaceae) to Sophora tomentosa,
the type species of the genus Sophora, observed from DNA sequence data
and morphological characters. – Bot. J. Linn. Soc. 146: 439-446.
Hegnauer R, Grayer-Barkmeijer RJ. 1993.
Relevance of seed polysaccharides and flavonoids for the classification of the
Leguminosae: a chemotaxonomic approach. – Phytochemistry 34: 3-16.
Hemsley AJ, Ferguson IK. 1985. Pollen
morphology of the genus Erythrina (Leguminosae: Papilionoideae) in
relation to floral structure and pollinators. – Ann. Missouri Bot. Gard. 72:
570-590.
Hendrych R. 1976. Vorläufige Mitteilung zur
Gattung Chrysaspis Desvaux (1818). – Preslia 48: 216-224.
Hendrych R. 1978. Ein Versuch, die
Arealentwicklung der Gattung Chrysaspis zu erläutern. – Preslia 50:
119-137.
Hendrych R. 1988. Die ersten
nomenklatorischen Ergänzungen zur Trifolium – Monographie von
Zohary und Heller (taxa supraspecifica). – Preslia 60: 215-236.
Henkel TW, Terborgh J, Vilgalys RJ. 2002.
Ectomycorrhizal fungi and their leguminous hosts in the Pakaraima Mountains of
Guyana. – Mycol. Res. 106: 515-531.
Heo K, Tobe H. 1994. Embryology and
relationships of Suriana maritima L. (Surianaceae). – J. Plant Res. 107:
29-37.
Hepper FN. 1958. Papilionaceae. – In: Keay
RWJ (ed), Flora of West Tropical Africa. 2nd ed., Crown Agents,
London.
Herendeen PS. 1992. The fossil record of the
Leguminosae from the Eocene of southeastern North America. – In: Herendeen
PS, Dilcher DL (eds), Advances in legume systematics 4: the fossil record,
Royal Botanic Gardens, Kew, pp. 85-160.
Herendeen PS. 1995. Phylogenetic
relationships of the tribe Swartzieae. – In: Crisp MD, Doyle JJ (eds),
Advances in legume systematics 7: Phylogeny, Royal Botanic Gardens, Kew, pp.
123-132.
Herendeen PS. 2000. Structural evolution in
the Caesalpinioideae (Leguminosae). – In: Herendeen PS, Bruneau A (eds),
Advances in legume systematics 9: phylogeny, Royal Botanic Gardens, Kew, pp.
45-64.
Herendeen PS, Crane PR. 1992. Early
caesalpinioid fruits from the Palaeogene of southern England. – In: Herendeen
PS, Dilcher DL (eds), Advances in legume systematics 4: the fossil record,
Royal Botanic Gardens, Kew.
Herendeen PS, Dilcher DL. 1990.
Diplotropis (Leguminosae, Papilionoideae) from the Middle Eocene of
southeastern North America. – Syst. Bot. 15: 526-533.
Herendeen PS, Dilcher DL (eds). 1992.
Advances in legume systematics 4. The fossil record. – The Royal Botanic
Gardens, Kew.
Herendeen PS, Crepet WL, Dilcher DL. 1992.
The fossil history of the Leguminosae: phylogenetic and biogeographic
implications. – In: Herendeen PS, Dilcher DL (eds), Advances in legume
systematics 4: the fossil record, Royal Botanic Gardens, Kew, pp. 303-315.
Herendeen PS, Lewis GP, Bruneau A. 2003.
Floral morphology in caesalpinioid legumes: testing the monophyly of the
“Umtiza clade”. – Intern. J. Plant Sci. 164(Suppl.):
S393-S407.
Herendeen PS, Bruneau A, Lewis GP. 2003.
Phylogenetic relationships in caesalpinioid legumes: a preliminary analysis
based on morphological and molecular data. – In: Klitgaard BB, Bruneau A
(eds), Advances in legume systematics 10: Higher level systematics, Royal
Botanic Gardens, Kew, pp. 37-62.
Hernández HM. 1989. Systematics of
Zapoteca (Leguminosae). – Ann. Missouri Bot. Gard. 76: 781-862.
Hernández HM. 1990. A new subgenus and a new
species of Zapoteca (Leguminosae). – Syst. Bot. 15: 226-230.
Hernández HM. 2008. Calliandra
dolichopoda and C. cualensis (Leguminosae, Mimosoideae), two new
species from Mexico. – Brittonia 60: 245-251.
Hernández HM, Guinet P. 1990.
Calliandropsis: a new genus of Leguminosae: Mimosoideae from Mexico.
– Kew Bull. 45: 609-620.
Hernández HM, Sousa S M. 1988. Two new
species of Calliandra (Leguminosae: Mimosoideae) from southern Mexico.
– Syst. Bot. 13: 519-524.
Heubl GR. 1984. Systematische Untersuchungen
an mitteleuropäischen Polygala-Arten. – Mitt. Bot. Staatssamml.
München 20: 205-428.
Heyn CC. 1956. Some chromosome counts in the
genus Medicago. – Caryologia 9: 160-165.
Heyn CC. 1963. The annual species of
Medicago. – Magnes Press, Jerusalem.
Heyn CC. 1968. An evolutionary study of fruit
morphology in the tribe Trigonelleae (Leguminosae). – Phytomorphology 13:
54-59.
Heyn CC. 1970. Studies in Lotus III.
The L. angustissimus group. – Israel J. Bot. 19: 271-292.
Heyn CC. 1981. Trifolieae. – In: Polhill
RM, Raven PH (eds), Advances in legume systematics 1, Royal Botanic Gardens,
Kew, pp. 383-385.
Heyn CC, Herrnstadt I. 1978. Seed coat
structure of Old World Lupinus species. – Bot. Not. 130: 427-435.
Heyn CC, Raviv V. 1966. Experimental
taxonomic studies in the genus Scorpiurus (Papilionaceae). – Bull.
Torrey Bot. Club 93: 259-280.
Heywood VH. 1968. A synopsis of the European
species of Cytisus and allied genera. – Feddes Repert. 79: 20-23.
Hillcoat D, Lewis G, Verdcourt B. 1980. A new
species of Ceratonia (Leguminosae-Caesalpinioideae) from Arabia and
the Somali republic. – Kew Bull. 35: 261-271.
Hindmarsh GJ. 1964. Gametophyte development
in Trifolium pratense L. – Aust. J. Bot. 12: 1-14.
Hoehne FC. 1941. Leguminosas-Papilionadas,
gêneros Dalbergia e Cyclolobium. – Flora Brasilica
XXV(III): 34-39.
Hofer J, Turner L, Hellens R, Ambrose M,
Matthews P, Michael A, Ellis N. 1997. UNIFOLIATA regulates leaf and
flower morphogenesis in pea. – Curr. Biol. 7: 581-587.
Hoffman DL, Soltis DE, Muehlbauer FJ,
Ladizinsky G. 1986. Isozyme polymorphism in Lens (Leguminosae). –
Syst. Bot. 11: 392-402.
Hoffman DL, Muehlbauer FJ, Ladizinsky G.
1988. Morphological variation in Lens (Leguminosae). – Syst. Bot.
13: 87-96.
Holm T. 1929. Morphology of North American
species of Polygala. – Bot. Gaz. 88: 167-185.
Holmen K. 1962. Chromosome studies in some
arctic Alaskan Leguminosae. – Bot. Not. 115: 87-92.
Holubova-Klaskova A. 1964. Bemerkungen zur
Gliederung der Gattung Cytisus L. s.l. – Acta Univ. Carol. Praha,
Biol., Suppl. 2: 1-24.
Hooker JD. 1897. Quillaja saponaria.
– Curtis’s Bot. Mag. III, 53, t. 7568.
Hopkins HCF. 1994. The Indo-Pacific species
of Parkia (Leguminosae: Mimosoideae). – Kew Bull. 49: 181-234.
Horjales M. 1974. Numeros cromosomicos en
Genisteas. – Anal. Inst. Bot. Cavanilles 31: 175-178.
Hosamani KN. 1995. A minor source of
vernolic, malvalic, and sterculic acids in Pithecellobium dulce (syn.
Inga dulcis) seed oil. – J. Amer. Oil. Chemists’s Soc. 72:
489-492.
Hossain M. 1961. A revision of
Trifolium in the Nearer East. – Notes Roy. Bot. Gard. Edinb. 23:
387-481.
Hou X, Liu J-E, Zhao Y-Z. 2008. Molecular
phylogeny of Caragana (Fabaceae) in China. – J. Syst. Evol.
46: 600-607.
Howell JT, Porter DM. 1968. The plant genus
Polygala in the Galapagos Islands. – Proc. Calif. Acad. Sci., Ser.
IV, 32(19): 581-586.
Hu J-M. 2000. Phylogenetic relationships of
the tribe Millettieae and allies – the current status. – In: Herendeen PS,
Bruneau A (eds), Advances in legume systematics 9: phylogenetics, Royal Botanic
Gardens, Kew, pp. 299-310.
Hu J-M, Chang S-P. 2003. Two new members of
the Callerya group (Fabaceae) based on phylogenetic analysis
of rbcL sequences: Endosamara racemosa (Roxb.) Geesink and
Callerya vasta (Kosterm.) Schot. – Taiwania 48: 118-128.
Hu J-M, Lavin M, Wojciechowski MF, Sanderson
MJ. 2000. Phylogenetic systematics of the tribe Millettieae (Leguminosae) based
on chloroplast trnK/matK sequences and its implications for
evolutionary patterns in Papilionoideae. – Amer. J. Bot. 87: 418-430.
Hu J-M, Lavin M, Wojciechowski MF, Sanderson
MJ. 2002. Phylogenetic analysis of nuclear ribosomal ITS/5.8S sequences in the
tribe Millettieae (Fabaceae):
Poecilanthe-Cyclolobium, the core Millettieae, and the
Callerya group. – Syst. Bot. 27: 722-733.
Hughes CE. 1997. Proposal to conserve the
name Leucaena (Leguminosae) with a conserved type. – Taxon 46:
355-356.
Hughes CE. 1998. A monograph of
Leucaena (Leguminosae-Mimosoideae). – Syst. Bot. Monogr. 55:
1-244.
Hughes CE, Bailey CD, Harris SA. 2002.
Divergent and reticulate species relationships in Leucaena (Fabaceae) inferred from multiple data
sources: insights into polyploid origins and nrDNA polymorphism. – Amer. J.
Bot. 89: 1057-1073.
Hughes CE, Bailey DC, Krosnick S, Luckow M.
2003. Relationships among genera of the informal Dichrostachys and
Leucaena groups (Mimosoideae) inferred from nuclear ribosomal ITS
sequences. – In: Klitgaard BB, Bruneaeu A (eds), Advances in legume
systematics 10: Higher level systematics, Royal Botanic Gardens, Kew, pp.
221-238.
Hughes CE, Lewis GP, Yomona AD, Reynel C.
2004. Maraniona. A new dalbergioid legume genus (Leguminosae,
Papilionoideae) from Peru. – Syst. Bot. 29: 366-374.
Hühner P. 1902. Vergleichende Untersuchungen
über die Blatt- und Achsenstruktur einiger australischer Podalyrieen-Gattungen
(Gastrolobium, Pultenaea, Latrobea, Eutaxia
and Dillwynia). – Beih. Bot. Centralbl. 11: 143-217.
Hunde A. 1983. Acacia. – In:
Thulin M (ed), Leguminosae of Ethiopia, Opera Bot. 68: 38-55.
Husaini SWH, Gill LS. 1985. Cytomorphology of
some arborescent species of Caesalpinoideae (Leguminosae) from Nigeria. – J.
Tree Sci. 4: 7-14.
Husaini SWH, Gill LS. 1986. Cytology of the
tribe Dalbergieae (Leguminosae) from Nigeria. – Feddes Repert. 97:
469-473.
Iganci JRV, Morim MP. 2009a. Three new
species of Abarema (Leguminosae, Mimosoideae) from south-eastern
Brazil. – Kew Bull. 64: 271-277.
Iganci JRV, Morim MP. 2009b. Abarema
(Leguminosae, Mimosoideae) no estado do Rio de Janeiro, Brasil. – Rodriguesia
60: 581-594.
Iganci JRV, Morim MP. 2012. Abarema
(Leguminosae, Mimosoideae) in the Atlantic Domain, Brazil. – Bot. J. Linn.
Soc. 168: 473-486.
Iganci JRV, Miotto STS, Souza-Chies TT,
Särkinen TE, Simpson BB, Simon MF, Pennington RT. 2013. Diversification
history of Adesmia ser. Psoraleoides (Leguminosae):
evolutionary processes and the colonization of the southern Brazilian highland
grasslands. – South Afr. J. Bot. 89: 257-264.
Iganci JRV, Soares MV, Guerra E, Morim MP.
2016. A preliminary molecular phylogeny of the Abarema alliance
(Leguminosae) and implications for taxonomic rearrangement. – Intern. J.
Plant Sci. 177: 34-43.
Imhof S. 2007. Specialized mycorrhizal
colonization pattern in achlorophyllous Epirixanthes spp. (Polygalaceae). – Plant Biol. 9:
786-792.
Ingham JL. 1979. Isoflavonoid phytoalexins of
the genus Medicago. – Biochem. Syst. Ecol. 7: 29-34.
Ingham JL. 1981a. Phytoalexin induction and
its chemosystematic significance in the genus Trigonella. – Biochem.
Syst. Ecol. 9: 275-281.
Ingham JL. 1981b. Phytoalexin induction and
its taxonomic significance in the Leguminosae (subfamily Papilionoideae). –
In: Polhill RM, Raven PH (eds), Advances in legume systematics 2, Royal Botanic
Gardens, pp. 599-626.
Ingham JL. 1990. Systematic aspects of
phytoalexin formation within tribe Phaseoleae of the Leguminosae (subfamily
Papilionoideae). – Biochem. Syst. Ecol. 5: 329-343.
Ireland HE. 2007. Taxonomic changes in the
South American genus Bocoa (Leguminosae-Swartzieae): reinstatement of
the name Trischidium, and a synopsis of both genera. – Kew Bull. 62:
333-350.
Ireland HE, Pennington RT. 1999. A revision
of Geoffroea (Leguminosae-Papilionoideae). – Edinburgh J. Bot. 56:
329-347.
Ireland HE, Pennington RT, Preston J. 2000.
Molecular systematics of the Swartzieae. – In: Herendeen PS, Bruneau A (eds),
Advances in legume systematics 9: Phylogeny, Royal Botanic Gardens, Kew, pp.
217-231.
Ireland HE, Kite GC, Veitch NC, Chase MW,
Schrire B, Lavin M Linares J, Pennington RT. 2010. Biogeographical, ecological
and morphological structure in a phylogenetic analysis of Ateleia
(Swartzieae, Fabaceae) derived from
combined molecular, morphological and chemical data. – Bot. J. Linn. Soc.
162: 39-53.
Irwin HS, Barneby RC. 1981. Cassieae. – In:
Polhill RM, Raven PH (eds), Advances in legume systematics 1, Royal Botanic
Gardens, Kew, pp. 97-106.
Irwin HS, Barneby RC. 1982. The American
Cassiinae. A synoptical revision of Leguminosae tribe Cassieae subtribe
Cassiinae in the New World. – Mem. New York Bot. Gard. 35: 1-918.
Irwin HS, Turner BL. 1960. Chromosomal
relationships and taxonomic considerations on the genus Cassia. –
Amer. J. Bot. 47: 309-318.
Isaacs MJH, Weitz, FM, Johnson CT. 1993.
Seed-coat characteristics of selected southern African species of
Polygala L. (Polygalaceae). – South Afr. J.
Bot. 59: 592-596.
Isely D. 1955. Observations on seeds of the
Leguminosae: Mimosoideae and Caesalpinioideae. – Proc. Iowa Acad. Sci. 62:
142-149.
Isely D. 1962. Leguminosae of the
north-central states IV: Psoraleeae. – Iowa State J. Sci. 37: 103-162.
Isely D. 1975. Leguminosae of the United
States II. Subfamily Caesalpinioideae. – Mem. New York Bot. Gard. 25:
1-228.
Isely D. 1981. Leguminosae of the United
States III. Subfamily Papilionoideae: tribes Sophoreae, Podalyrieae, Loteae.
– Mem. New York Bot. Gard. 25: 1-264.
Isely D. 1983. New combinations and two new
varieties in Astragalus, Orophaca, and Oxytropis
(Leguminosae). – Syst. Bot. 8: 420-426.
Isely D. 1998. Native and naturalized
Leguminosae (Fabaceae) of the United
States (exclusive of Alaska and Hawaii). – Monte L. Bean Life Science Museum,
Brigham Young University, Provo.
Izaguirre P, Mérola S, Beyhaut R. 1994. Seed
ontogeny in Adesmia securigerifolia (Fabaceae-Adesmieae). – Nord. J. Bot.
14: 547-556.
Jaaska V. 1996. Isozyme diversity and
phylogenetic affinities among the Phaseolus beans (Fabaceae). – Plant Syst. Evol. 200:
233-252.
Jaaska V. 1997. Isoenzyme diversity and
phylogenetic affinities in Vicia subgenus Vicia (Fabaceae). – Genet. Res. Crop Evol. 4:
557-574.
Jaaska V. 2005. Isozyme variation and
phylogenetic relationships in Vicia subgenus Cracca (Fabaceae). – Ann. Bot. 96:
1085-1096.
Jaaska V, Jaaska V. 1988. Isoenzyme variation
in the genera Phaseolus and Vigna (Fabaceae) in relation to their
systematics: aspartate aminotransferase and superoxide dismutase. – Plant
Syst. Evol. 159: 145-159.
Jabbour F, Gaudeul M, Lambourdière J,
Ramstein G, Hassanin A, Labat J-N, Sarthou C. 2018. Phylogeny, biogeography and
character evolution in the tribe Desmodieae (Fabaceae: Papilionoideae), with special
emphasis on the New Caledonian endemic genera. – Mol. Phylogen. Evol. 118:
108-121.
Jaca TP, Boatwright JS, Moteetee AN. 2018.
Taxonomic studies of the genus Rhynchosia Lour. (Phaseoleae, Fabaceae) in South Africa: a review of
section Chrysoscias. – South Afr. J. Bot. 117: 119-133.
Jacot AP. 1927. The genus
Gueldenstaedtia (Leguminosae). – J. North China Branch Roy As. Soc.
58: 85-126.
Jadin F. 1901. Contribution à l’étude des
Simarubacées. – Ann. Sci. Nat., sér. VIII, Bot. 13: 201-304.
Jahan B, Vahidy AA, Ali SI. 1994. Chromosome
numbers in some taxa of Fabaceae
mostly native to Pakistan. – Ann. Missouri Bot. Gard. 81: 792-799.
James EK, Sprent JI, Sutherland JM, McInroy
SG, Minchin FR. 1992. The structure of nitrogen fixing root nodules on the
aquatic mimosoid legume Neptunia plena. – Ann. Bot. 69: 173-180.
Jansen RK, Wojciechowski MF, Sanniyasi E, Lee
S-B, Daniell H. 2008. Complete plastid genome sequence of the chickpea
(Cicer arietinum) and the phylogenetic distribution of rps12
and clpP intron losses among legumes (Leguminosae). – Mol.
Phylogenet. Evol. 48: 1204-1217.
Janzen DH. 1974. Swollen-thorn acacias of
Central America. – Smithsonian Contr. Bot. 13: 1-131.
Jauch B. 1918. Quelque points de l’anatomie
et de la biologie des Polygalacées. – Bull. Soc. Bot. Genève 10: 47-84.
Javadi F, Wojciechowski MF, Yamaguchi H.
2007. Geographical diversification of the genus Cicer (Leguminosae:
Papilionoideae) inferred from molecular phylogenetic analyses of chloroplast
and nuclear DNA sequences. – Bot. J. Linn. Soc. 154: 175-186.
Jawad JT, Seigler DS, Ebinger JE. 2000. A
systematic treatment of Acacia coulteri (Fabaceae, Mimosoideae) and similar
species in the New World. – Ann. Missouri Bot. Gard. 87: 528-548.
Jha SS, Pandey AK. 1989. Seed coat structure
in Melilotus (Fabaceae). –
Phytomorphology 39: 222-229.
Jia H, Manchester SR. 2014. Fossil leaves and
fruits of Cercis L. (Leguminosae) from the Eocene of western North
America. – Intern. J. Plant Sci. 175: 601-612.
Jobson RW, Luckow M. 2007. Phylogenetic study
of the genus Piptadenia (Mimosoideae: Leguminosae) using plastid
trnL-F and trnK/matK sequence data. – Syst. Bot.
32: 569-575.
Jobson PC, Weston PH. 1999. Two new species
of Dillwynia (Fabaceae:
Mirbelieae) from the Southern Tablelands of New South Wales. – Telopea 8:
363-369.
Jobson PC, Weston PH. 2001. Dillwynia
rupestris (Fabaceae:
Mirbelieae), a new species from the New England Tableland of New South Wales.
– Telopea 9: 323-327.
Johnson CD. 1989. Adaptive radiation of
Acanthoscelides in seeds: examples of legume-buchid interactions. –
In: Stirton CH, Zarucchi JL (eds), Advances in legume biology, Missouri
Botanical Garden, St. Louis, pp. 747-779.
Johnson CD. 1990. Coevolution of Bruchidae
and their hosts: evidence, conjecture, and conclusions. – In: Fuji K,
Gategouse AMR, Johnson CD, Mitchel R, Yoshida T (eds), Bruchids and legumes:
economics, ecology and coevolution, Kluwer, Dordrecht, pp. 27-51.
Johnson CT. 1987. Taxonomy of the African
species of Securidaca (Polygalaceae). – South Afr. J.
Bot. 53: 5-11.
Johri BM, Garg S. 1959. Development of
endosperm haustorium in some Leguminosae. – Phytomorphology 9: 34-46.
Jones GN. 1955. Leguminales: a new ordinal
name. – Taxon 4: 188-189.
Jongkind CCH. 2003. Leptoderris
sassandrensis and Leptoderris fasciculata (Leguminosae,
Dalbergieae), two out of one. – Syst. Geogr. Plants 73: 95-98.
Jordaan A, Wessels DCJ, Kruger H. 2002.
Structure of the style and wet non-papillate stigma of Colophospermum
mopane, Caesalpinioideae: Detarieae. – Bot. J. Linn. Soc. 139:
295-304.
Juan A, Crespo MB. 2009. Scanning electron
microscope characters and their systematic significance within
Medicago L. sect. Dendrotelis (Fabaceae: Trifolieae). – Feddes
Repert. 120: 158-168.
Juzysta M, Nowacki E. 1979. Saponins of the
genus Medicago. – Acta Agrobot. 32: 13-17.
Jurzysta M, Burda S, Oleszek WA, Ploszynski
M. 1988. The chemotaxonomic significance of laricytrin and medicagenic acid in
the tribe Trigonelleae Schulz. – Can. J. Bot. 66: 363-367.
Jurzysta M, Small E, Nozzolillo C. 1988.
Hemolysis, a synapomorphic discriminator of an expanded genus Medicago
(Leguminosae). – Taxon 37: 354-363.
Kaamian R, Ranjbar M. 2005.
Astragalus sect. Astragalus (Fabaceae) in Iran. – Bot. J. Linn.
Soc. 147: 363-368.
Kajita T, Ohashi H, Tateishi Y, Bailey CD,
Doyle JJ. 2001. rbcL and legume phylogeny, with particular reference
to Phaseoleae, Millettieae, and allies. – Syst. Bot. 26: 515-536.
Kang Y, Zhang M-L. 2004. Study of pollen
brush in selected species of Astragalus L. subgenus
Pogonophace Bunge (Leguminosae). – Plant Syst. Evol. 249: 1-8.
Kanis A. 1980. The Malesian species of
Serianthes Bentham (Fabaceae-Mimosoideae). – Brunonia 2:
289-320.
Kant Y, Zhang M-L. 2009. Pollen brush of
Astragalus L. subgenus Pogonophace Bunge (Leguminosae) and
its systematic significance. – Plant Syst. Evol. 280: 167-174.
Kang Y, Zhang M-L, Chen Z-D. 2003. A
preliminary phylogenetic study of the subgenus Pogonophace
(Astragalus) in China based on ITS sequence data. – Acta Bot. Sin.
45: 140-145.
Kaplan DR. 1980. Heteroblastic leaf
development in Acacia. – La Cellule 73: 135-203.
Käss E. 1995. Molekulare Phylogenie der
Schmetterlingsblütler (Familie Leguminosae). – Ph.D. diss., Universität
Heidelberg, Germany.
Käss E, Wink M. 1995. Molecular phylogeny of
the Papilionoideae (family Leguminosae): rbcL gene sequences versus
chemical taxonomy. – Bot. Acta 108: 149-162.
Käss E, Wink M. 1996. Molecular evolution of
the Leguminosae: phylogeny of the three subfamilies based on rbcL
sequences. – Biochem. Syst. Ecol. 24: 365-378.
Käss E, Wink M. 1997a. Phylogenetic
relationships in the Papilionoideae (family Leguminosae) based on nucleotide
sequences of cpDNA (rbcL) and ncDNA (ITS1 and 2). – Mol. Phylogen.
Evol. 8: 65-88.
Käss E, Wink M. 1997b. Molecular phylogeny
and phylogeography of Lupinus (Leguminosae) inferred from nucleotide
sequences of rbcL gene and ITS 1+2 regions of rDNA. – Plant Syst.
Evol. 208: 139-167.
Kassau E. 1931. Beiträge zur Systematik
einiger Polygalaceen-Gattungen unter Berücksichtigung des Vorkommens von
Saponin. – Ph.D. diss., Friedrich-Wilhelms-Universität, Berlin, Germany.
Katznelson J, Morley FHW. 1965. A taxonomic
revision of sect. Calycomorphum of the genus Trifolium 1. The
geocarpic species. – Israel J. Bot. 14: 112-134.
Kaur H, Pal A. 1989. Structure, anatomy and
spermoderm pattern of seeds in some Vicia species (Papilionoideae).
– Phytomorphology 39: 363-370.
Kazemi M, Kazempour Osaloo S, Maassoumi AA,
Pouyani ER. 2009. Molecular phylogeny of selected Old World Astragalus
(Fabaceae): incongruence among
chloroplast trnL-F, ndhF and nuclear ribosomal DNA ITS
sequences. – Nord. J. Bot. 27: 425-436.
Kazempour Osaloo SH, Maassoumi AA, Murakami
N. 2003. Molecular systematics of the genus Astragalus L. (Fabaceae): phylogenetic analyses of
nuclear ribosomal DNA internal transcribed spacers and chloroplast gene
ndhF sequences. – Plant Syst. Evol. 242: 1-32.
Kazempour Osaloo SH, Maassoumi AA, Murakami
N. 2005. Molecular systematics of the Old World Astragalus (Fabaceae) as inferred from nrDNA ITS
sequence data. – Brittonia 57: 367-381.
Kenicer GJ, Kajita T, Pennington R, Murata J.
2005. Systematics and biogeography of Lathyrus (Leguminosae) based on
internal transcribed spacer and cpDNA sequence data. – Amer. J. Bot. 92:
1199-1209.
Kenrick J. 2003. Review of pollen-pistil
interactions and their relevance to the reproductive biology of
Acacia. – Aust. Syst. Bot. 16: 119-130.
Kenrick J, Knox RB. 1979. Pollen development
and cytochemistry in some Australian species of Acacia. – Aust. J.
Bot. 27: 413-427.
Kergoat GJ, Silvain J-F, Buranapanichpan S,
Tuda M. 2007. When insects help to resolve plant phylogeny: Evidence for a
paraphyletic genus Acacia from the systematics and host-plant range of
their seed-predators. – Zool. Scripta 36: 143-152.
Kerrigan RA. 2008. A taxonomic revision of
Polygala in Australia. – M.Sc. thesis, James Cook University,
Cairns, Queensland.
Kerrigan RA. 2012. A treatment for
Polygala of northern Australia. – Aust. Syst. Bot. 25: 83-137.
Khatoon S, Ali SI. 1991. Chromosome numbers
in subfamily Papilionoideae (Leguminosae) from Pakistan. – Willdenowia 20:
159-165.
Khokhrjakov AP. 1998. Bobrovia A.
Khokhr.: genus novum e familia Fabaceae. – Novost. Sist. Vysš. Rast.
1998: 137-139. [In Russian]
Kies P. 1951. Revision of the genus
Cyclopia and notes on some other sources of bush tea. – Bothalia 6:
161-176.
Kim YJ, Wang PF, Navarro-Villalobos M, Rohde
BD, Derryberry J, Gin DY. 2006. Synthetic studies of complex immunostimulants
from Quillaja saponaria: synthesis of the potent clinical
immunoadjuvant QS-21A(api). – J. Amer. Chem. Soc. 128: 11906-11915.
Kinghorn AD, Balandrin MF. 1984.
Quinolizidine alkaloids of the Leguminosae: structural types, analysis,
chemotaxonomy, and biological activites. – In: Pelletier SW (ed), Alkaloids:
chemical and biological perspectives, John Wiley and Sons, New York, pp.
105-148.
Kinghorn AD, Hussain RA, Robbins EF,
Balandrin MF, Stirton CH, Evans SV. 1988. Alkaloid distribution in seeds of
Ormosia, Pericopsis and Haplormosia. –
Phytochemistry 27: 439-444.
Kirkbride JH Jr. 1999.
Barnebydendron, a new generic name (Fabaceae, Caesalpinioideae, Detarieae,
Brownea group). – Sida 18: 815-818.
Kirkbride JH Jr. 1999b. Lotus
systematics and distribution. – In: Beuselinck PR (ed), Trefoil: the science
and technology of Lotus, CSSA Spec. Publ. 28, American Society of
Agronomy; Crop Science Society of America, Madison, Wisconsin, pp. 1-20.
Kirkbride JH Jr. 2003. Proposal to conserve
the name Chloroleucon against Chloroleucum (Fabaceae). – Taxon 52: 141-142.
Kirkbride JH Jr. 2005. Dupuya, a new
genus of Malagasy legumes (Fabaceae).
– Novon 15: 305-314.
Kirkbride JH Jr. 2010. Lotus
alianus, a new species from Cabo Verde and nomenclatural notes on
Lotus section Pedrosia (Fabaceae). – Pakistan J. Bot. 42:
1-10.
Kirkbride JH Jr, Wiersema JH. 1997.
Bobgunnia, a new African genus of the tribe Swartzieae (Fabaceae, Faboideae). – Brittonia 49:
1-23.
Kirkbride JH Jr, Gunn CR, Weitzman AL. 2003.
Fruits and seeds of genera in the subfamily Faboideae (Fabaceae) I-II. – Techn. Bull. 1890,
United States Dept. Agric., Agricultural Res. Div., Washington, D.C.
Kite GC, Pennington RT. 2003. Quinolizidine
alkaloid status of Styphnolobium and Cladrastis
(Leguminosae). – Biochem. Syst. Ecol. 31: 1409-1416.
Kite GC, Wieringa JJ. 2003. Hydroxypipecolic
acids and hydroxyprolines as chemical characters in Aphanocalyx, Bikinia and
Tetraberlinia (Leguminosae: Caesalpinioideae): support for the segregation of
Monopetalanthus. – Biochem. Syst. Ecol. 31: 279-292.
Klitgaard BB. 1991. Ecuadorian
Brownea and Browneopsis (Leguminosae-Caesalpinioideae):
taxonomy, palynology, and morphology. – Nord. J. Bot. 11: 433-449.
Klitgaard BB. 1999a. Floral ontogeny in tribe
Dalbergieae (Leguminosae: Papilionoideae): Dalbergia brasiliensis,
Machaerium villosum sens. lat., Platymiscium floribundum, and
Pterocarpus rotundifolius. – Plant Syst. Evol. 21: 1-25
Klitgaard BB. 1999b. New species and
nomenclatural changes in neotropical Platymiscium (Leguminosae:
Papilionoideae: Dalbergieae). – Kew Bull. 54: 967-973.
Klitgaard BB. 2005. Platymiscium
(Leguminosae: Dalbergieae): biogeography, systematics, morphology, taxonomy and
uses. – Kew Bull. 60: 321-400.
Klitgaard BB, Ferguson IK. 1992. Pollen
morphology of Browneopsis (Leguminosae: Caesalpinioideae), and its
evolutionary significance. – Grana 31: 285-290.
Knoblauch M, Peters WS, Ehlers K, Bel AJE
van. 2001. Reversible calcium-regulated stopcocks in legume sieve tubes. –
Plant Cell 13: 1221-1230.
Kodela PG, Wilson PG. 2006. New combinations
in the genus Vachellia (Fabaceae: Mimosoideae) from Australia.
– Telopea 11: 233-244.
Kok RPJ de, West JG. 2002. A revision of
Pultenaea (Fabaceae) 1.
Species with ovaries glabrous and/or with tufted hairs. – Aust. Syst. Bot.
15: 81-113.
Kok RPJ de, West JG. 2003. A revision of the
genus Pultenaea (Fabaceae)
2. Eastern Australian species with velutinous ovaries and incurved leaves. –
Aust. Syst. Bot. 16: 229-273.
Kok RPJ de, West JG. 2004. A revision of the
genus Pultenaea (Fabaceae)
3. The eastern species with recurved leaves. – Aust. Syst. Bot. 17:
273-326.
Komarov VL. 1908. Generis Caragana
monographia. – Trudy Glavn. Bot. Sada. 29: 179-388.
Kopooshian H, Isely D. 1966. Seed character
relationships in the Leguminosae. – Proc. Iowa Acad. Sci. 73: 59-67.
Koptur S. 1983. Flowering phenology and
floral biology of Inga (Fabaceae: Mimosoideae). – Syst. Bot.
8: 354-368.
Kostermans AJGH. 1954. A monograph of the
Asiatic, Malaysian, Australian and Pacific species of Mimosaceae formerly
included in Pithecellobium Mart. – Bull. Org. Natuurw. Onderz. 20:
1-122.
Kostermans AJGH. 1956. Additional notes on
Mimosaceae. The genera Mammea and Ochrocarpus Thou. –
Commun. Forest Res. Inst. 54: 1-15.
Kotina EL, Oskolski AA, Stepanova AV, Wyk B-E
van. 2013. Notes on the taxonomic and ecological significance of bark structure
in the genus Virgilia (Fabaceae, Podalyrieae). – South Afr.
J. Bot. 89: 240-243.
Kotina EL, Stepanova AV, Oskolski AA, Tilney
PM, van Wyk B-E. 2015. Crystal types and their distribution in the bark of
African genistoid legumes (Fabaceae
tribes Sophoreae, Podalyrieae, Crotalarieae and Genisteae). – Bot. J. Linn.
Soc. 178: 620-632.
Koyama H. 1995. A revision of the genus
Salomonia (Polygalaceae). – Bull. Natl. Sci.
Mus. Tokyo, Ser. B, 21: 1-12.
Kramina TE. 1999. A contribution to the
taxonomic revision of the Lotus corniculatus complex (Leguminosae,
Loteae) in the European part of the former USSR. – Syst. Geogr. Plants 68:
265-279.
Kramina TE. 2006. A contribution to the
taxonomic revision of the Lotus angustissimus complex (Leguminosae,
Loteae). – Wulfenia 13: 57-92.
Kramina TE, Sokoloff DD. 1997. Lotus
roudairei Bonnet and taxonomic relationships between African and North
American species of the tribe Loteae (Papilionaceae). – Adansonia, sér. 3,
19: 321-328.
Kramina TE, Sokoloff DD. 1999. Taxonomic
bearing of stylodium tooth in the genus Lotus (Papilionaceae) with
special reference to Lotus creticus L. – Feddes Repert. 110:
527-533.
Kramina TE, Sokoloff DD. 2001.
Kebirita, a new genus of Leguminosae-Loteae from NW Africa. – Byull.
Moskovsk. Obshch. Isp. Prir., Otd. Biol. 106: 58-63. [in Russian]
Kramina TE, Sokoloff DD. 2003. On
Lotus sect. Erythrolotus and related taxa (Leguminosae). –
Byull. Moskovsk. Obshch. Isp. Prir., Otd. Biol. 108: 59-62. [in Russian]
Kramina TE, Sokoloff DD. 2004. A taxonomic
study of the Lotus australis complex (Leguminosae), with special
emphasis on plants from Pacific Ocean islands. – Adansonia, sér. III, 26:
171-197.
Kramina TE, Degtjareva GV, Samigullin TH,
Valiejo-Roman CM, Kirkbride Jr JH, Volis S, Deng T, Sokoloff DD. 2016.
Phylogeny of Lotus (Leguminosae: Loteae): partial incongruence between
nrITS, nrETS and plastid markers and biogeographic implications. – Taxon 65:
997-1018.
Krapovickas A. 1965. Recuentos cromosomicos
de Leguminosae. – Kurtziana 2: 91-94.
Krapovickas A, Gregory WC. 1994. Taxonomía
del género Arachis (Leguminosae). – Bonplandia 8: 1-186.
Krapovickas A, Gregory WC. 2007. Taxonomy of
the genus Arachis (Leguminosae). – Bonplandia 16(suppl.): 1-205.
Krüger H, Pretorius WE. 1997. Notes on the
structure of the stigma of Polygala virgata var. virgata (Polygalaceae). – South Afr. J.
Bot. 63: 261-266.
Krüger H, Robbertse PJ. 1988. Floral
ontogeny of Securidaca longepedunculata Fresen. (Polygalaceae), including
inflorescence morphology. – In: Leins P, Tucker SC, Endress PK (eds), Aspects
of floral development, J. Cramer, Berlin, pp. 159-167.
Krüger H, Merwe MJ van der, Robbertse PJ.
1988. Floral organogenesis in Securidaca longepedunculata and
Polygala virgata var. decora (Polygalaceae). – South Afr. J.
Sci. 84: 308-313.
Krüger H, Tiedt LR, Wessels DCJ. 1999.
Floral development in the legume tree Colophospermum mopane,
Caesalpinioideae: Detarieae. – Bot. J. Linn. Soc. 131: 223-233.
Krukoff BA, Barneby RC. 1974. Conspectus of
the genus Erythrina. – Lloydia 37: 332-459.
Kubitzki K. 2006. Quillajaceae. – In: Kubitzki K
(ed), The families and genera of vascular plants IX. Flowering plants.
Eudicots. Berberidopsidales,
Buxales, Crossosomatales, Fabales p. p., Geraniales, Gunnerales, Myrtales p. p., Proteales, Saxifragales, Vitales, Zygophyllales, Clusiaceae Alliance, Passifloraceae
Alliance, Dilleniaceae, Huaceae, Picramniaceae, Sabiaceae, Springer, Berlin,
Heidelberg, New York, pp. 407-408.
Kumari S, Bir SS. 1990. Karyomorphological
evolution in Papilionaceae. – J. Cytol. Genet. 25: 173-219.
Kumar S, Sane PV. 2003. Legumes of South
Asia: a checklist. – Kew Publishing.
Kuntze CEO. 1891. Polygalaceae Revisio Generum
Plantarum 1: 45-49. – A. Felix, Leipzig.
Kupicha FK. 1975. Observations on the
vascular anatomy of the tribe Vicieae (Leguminosae). – Bot. J. Linn. Soc. 70:
231-242.
Kupicha FK. 1976. The infrageneric structure
of Vicia. – Notes Roy. Bot. Gard. Edinb. 34: 287-326.
Kupicha FK. 1981. Vicieae. – In: Polhill
RM, Raven PH (eds), Advances in legume systematics 1, Royal Botanic Gardens,
Kew, pp. 377-381.
Kupicha FK. 1983. The infrageneric structure
of Lathyrus. – Notes Roy. Bot. Gard. Edinb. 41: 209-244.
Kuriakose ME. 2007. Palynological studies of
some south Indian members of Phaseoleae (Papilionaceae) and its bearing on the
systematics of the tribe. – J. Palynol. 43: 129-164.
Kwon DJ, Bae YS. 2009. Flavonoids from the
aerial parts of Lespedeza cuneata. – Biochem. Syst. Ecol. 37:
46-48.
Kyalangalilwa B, Boatwright JS, Daru BH,
Maurin O, Bank M van der. 2013. Phylogenetic position and revised
classification of Acacia s.l. (Fabaceae: Mimosoideae) in Africa,
including new combinations in Vachellia and Senegalia. –
Bot. J. Linn. Soc. 172: 500-523.
Labat J-N, Du Puy DJ. 1996. Two new species
of Ormocarpopsis R. Viguier and a new combination in
Ormocarpum P. Beauvois (Leguminosae-Papilionoideae) from Madagascar.
– Novon 6: 54-58.
Labat J-N, Du Puy DJ. 1997. A revision of
Peltiera, a new, poorly known and probably extinct genus of
Leguminosae (Papilionoideae-Aeschynomeneae) from Madagascar. – Adansonia,
sér. III, 19: 85-91.
Labat J-N, Du Puy DJ. 1998.
Sylvichadsia, a new genus of Leguminosae-Papilionoideae-Millettieae
endemic to Madagascar. – Adansonia, sér. 3, 20: 163-171.
Lack AJ, Kay QON. 1987. Genetic structure,
gene flow and reproductive ecology in sand-dune populations of Polygala
vulgaris. – J. Ecol. 75: 259-276.
Lackey JA. 1977a. A synopsis of the
Phaseoleae (Leguminosae, Papilionoideae). – Ph.D. diss., Iowa State
University, Ames, Iowa.
Lackey JA. 1977b. A revised classification of
the tribe Phaseoleae (Leguminosae: Papilionoideae), and its relation to
canavanine distribution. – Bot. J. Linn. Soc. 74: 163-178.
Lackey JA. 1977c. Neonotonia, a new
generic name to include Glycine wightii (Arnott) Verdcourt
(Leguminosae, Papilionoideae). – Phytologia 37: 209-212.
Lackey JA. 1978. Leaflet anatomy of
Phaseoleae (Leguminosae: Papilionoideae) and its relation to taxonomy. – Bot.
Gaz. 139: 436-446.
Lackey JA. 1979. Sketches of flower
dissections in Phaseoleae (Fabaceae,
Faboideae). – Isleya 1: 19-53.
Lackey JA. 1980. Chromosome numbers in the
Phaseoleae (Fabaceae: Faboideae) and
their relation to taxonomy. – Amer. J. Bot. 67: 595-602.
Lackey JA. 1981. Tribe 19. Phaseoleae DC.
(1825). – In: Polhill RM, Raven PH (eds), Advances in legume systematics 1,
Royal Botanic Gardens, Kew, pp. 301-327.
Lackey JA. 1983. A review of generic concepts
in American Phaseolinae (Fabaceae,
Faboideae). – Isleya 2: 21-64.
Lackey JA. 2009. Cotyledon areoles in
Bobgunnia (Fabaceae:
Swartzieae). – Bot. J. Linn. Soc. 159: 287-291.
Lackey JA. 2011. Endosperm and cotyledon
areole correlation in Leguminosae subfamily Papilionoideae. – J. Bot. Res.
Inst. Texas 5: 219-236.
Lai M, Sceppa J, Ballenger JA, Doyle JJ,
Wunderlin RP. 1997. Polymorphism for the presence of the rpl2 intron
in chloroplast genomes of Bauhinia (Leguminosae). – Syst. Bot. 22:
519-528.
Lamarque AL, Fortunato RH. 2003. Taxonomic
significance of seed protein profiles in Acacia, with special
reference to Acacia emilioana. – Aust. Syst. Bot. 16: 35-39.
Lamarque AL, Labuckas DO, Greppi J, Fortunato
RH. 2009. Electrophoretic analysis of Geoffroea (Leguminosae,
Papilionoideae): taxonomic inferences in Argentinean populations. – Aust.
Syst. Bot. 22: 137-142.
Landsmann J, Dennis ES, Higgins TJV, Appleby
CA, Kortt AA, Peacock WJ. 1986. Common evolutionary origin of legume and
non-legume plant haemoglobins. – Nature 324: 166-168.
Langenheim JH. 1981. Terpenoids in the
Leguminosae. – In: Polhill RM, Raven PH (eds), Advances in legume systematics
2, Royal Botanic Gardens, Kew, pp. 627-656.
Lapcík O. 2007. Isoflavonoids in
non-leguminous taxa: a rarity or rule? – Phytochemistry 68: 2909-2916.
Larkin RA, Graumann HO. 1954. Anatomical
structure of the alfalfa flower and an explanation of the tripping mechanism.
– Bot. Gaz. 116: 40-52.
Larsen K. 1955a. Cyto-taxonomical studies in
Lotus II. – Bot. Tidsskr. 52: 8-17.
Larsen K. 1955b. Cyto-taxonomical studies on
the Mediterranean flora. – Bot. Not. 108: 263-275.
Larsen K. 1959. On the cytological pattern of
the genus Polygala. – Bot. Not. 112: 369-371.
Larsen K. 1964. The chromosomes of
Monnina xalapensis. – Phyton (Buenos Aires) 21: 45-46.
Larsen K. 1967. Cytological studies on
Monnina. – Feddes Repert. 75: 43-46.
Larsen K. 1971. Chromosome numbers of some
Thai Leguminosae. – Bot. Tidsskr. 66: 38-50.
Larsen K, Larsen SS. 1973. The genus
Bauhinia in Thailand. – Nat. Hist. Bull. Siam Soc. 25: 1-22.
Larsen K, Larsen SS. 1982. Notes on some
Asian Bauhinia. – Nord. J. Bot. 2: 329-332.
Larsen K, Larsen SS. 1983. The genus
Bauhinia in Australia. Taxonomy and palynology. – Bot. Helvetica 93:
213-220.
Larsen K, Larsen SS. 1991. Notes on the genus
Bauhinia (Leguminosae-Caesalpinioideae) in SE Asia. – Nord. J. Bot.
11: 629-634.
Larsen K, Larsen SS. 1993. New taxa and
nomenclatural combinations in Malesian Bauhinia
(Leguminosae-Caesalpinioideae). – Nord. J. Bot. 13: 657-665.
Larsen K, Larsen SS, Vidal JE. 1980.
Lègumineuses-Césalpinioidées. – In: Flore du Cambodge, du Laos et du
Viêtnam 18, Muséum National d’Histoire Naturelle, Paris, pp. 1-226.
Larsen SS. 1975. Pollen morphology of the
Thai species of Bauhinia (Caesalpiniaceae). – Grana 14: 114-131.
Larsen SS. 1994. A new species of
Bauhinia (Fabaceae-Caesalpinioideae) from Brunei.
– Nord. J. Bot. 14: 289-292.
Lassen P. 1989. A new delimitation of the
genera Coronilla, Hippocrepis, and Securigera (Fabaceae). – Willdenowia 19: 49-62.
Lavin M. 1987. A cladistic analysis of the
tribe Robinieae (Papilionoideae, Leguminosae). – In: Stirton CH (ed),
Advances in legume systematics 3, Royal Botanic Gardens, Kew, pp. 31-64.
Lavin M. 1990. The genus
Sphinctospermum (Leguminosae): taxonomy and tribal relationships as
inferred from a cladistic analysis of traditional data. – Syst. Bot. 15:
544-559.
Lavin M. 1993. Biogeography and systematics
of Poitea (Leguminosae). – Syst. Bot. Monogr. 37: 1-87.
Lavin M. 1995. Tribe Robinieae and allies:
model groups for assessing early Tertiary northern latitude diversification of
tropical legumes. – In: Crisp M, Doyle JJ (eds), Advances in legume
systematics 7: phylogeny, Royal Botanical Gardens, Kew, pp. 141-160.
Lavin M, Delgado-Salinas A. 1990. Pollen
brush of Papilionoideae (Leguminosae): morphological variation and systematic
utility. – Amer. J. Bot. 77: 1294-1312.
Lavin M, Doyle JJ. 1991. Tribal relationships
of Sphinctospermum (Leguminosae): integration of traditional and
chloroplast DNA data. – Syst. Bot. 16: 162-172.
Lavin M, Marriott H. 1997. Astragalus
molybdenus s.l. (Leguminosae): higher taxonomic relationships and identity
of constituent species. – Syst. Bot. 22: 199-217.
Lavin M, Sousa S M. 1987. The
Madrensis group of Coursetia (Leguminosae: Robinieae). –
Syst. Bot. 12: 106-115.
Lavin M, Sousa S M. 1996. Phylogenetic
systematics and biogeography of the tribe Robinieae (Leguminosae). – Syst.
Bot. Monogr. 45: 1-165.
Lavin M, Doyle JJ, Palmer JD. 1990.
Evolutionary significance of the loss of the chloroplast-DNA inverted repeat in
the Leguminosae subfamily Papilionoideae. – Evolution 44: 390-402.
Lavin M, Mathews S, Hughes C. 1991.
Chloroplast DNA variation in Gliricidia sepium (Leguminosae):
intraspecific phylogeny and tokogeny. – Amer. J. Bot. 78: 1576-1585.
Lavin M, Eshbaugh E, Hu JM, Mathews S,
Sharrok RA. 1998. Monophyletic subgroups of the tribe Millettieae (Leguminosae)
as revealed by phytochrome nucleotide sequence data. – Amer. J. Bot. 85:
412-433.
Lavin M, Thulin M, Labat J-N, Pennington RT.
2000. Africa, the odd man out: molecular biogeography of dalbergioid legumes
(Fabaceae) suggests otherwise. –
Syst. Bot 25: 449-467.
Lavin M, Pennington RT, Klitgaard BB, Sprent
JI, Lima HC de, Gasson PE. 2001. The dalbergioid legumes (Fabaceae): delimitation of a pantropical
monophyletic clade. – Amer. J. Bot. 88: 503-533.
Lavin M, Wojciechowski MF, Richman A, Rotella
J, Sanderson MJ, Matos AB. 2001. Identifying Tertiary radiations of Fabaceae in the Greater Antilles:
alternatives to cladistic vicariance analysis. – Intern. J. Plant Sci.
162(Suppl.): S53-S76.
Lavin M, Wojciechowski MF, Gasson P, Hughes
C, Wheeler E. 2003. Phylogeny of robinioid legumes (Fabaceae) revisited: Coursetia
and Gliricidia recircumscribed, and a biogeographical appraisal of the
Caribbean endemics. – Syst. Bot. 28: 387-409.
Lavin M, Schrire BP, Lewis G, Pennington RT,
Delgado-Salinas A, Thulin M, Hughes CE, Beyra Matos A, Wojciechowski MF. 2004.
Metacommunity process rather than continental tectonic history better explains
geographically structured phylogenies in legumes. – Philos. Trans. Roy. Soc.
London, Ser. B, 359: 1509-1522.
Lavin M, Herendeen PS, Wojciechowski MF.
2005. Evolutionary rates analysis of Leguminosae implicates a rapid
diversification of lineages during the Tertiary. – Syst. Biol. 54:
575-594.
Lecompte-Barbet O. 1981. Étude de
l’ornamentation du tégument externe des graines d’Ononis L. au
microscope électronique à balayage. – Bull. Mus. Natl. Hist. Nat. Paris,
sér. IV, 3: 19-36.
Ledingham GF. 1960. Chromosome numbers in
Astragalus and Oxytropis. – Can. J. Genet. Cytol. 2:
119-128.
Ledingham GF, Rever BM. 1963. Chromosome
numbers of some southwest Asian species of Astragalus and
Oxytropis (Leguminosae). – Can. J. Genet. Cytol. 5: 18-32.
Lee AT. 1978. Some species of
Crotalaria in Australia. – Telopea 1: 319-356.
Lee AT. 1980. The Psoralea patens
complex. – Telopea 2: 129-141.
Lee J. 2000. Molecular genetics on the genus
Glycine I. Phylogenetic study of the subtribe Glycininae. II.
Development of a universal soybean genetic map. – Ph.D. diss., University of
Illinois at Urbana-Champaign, Urbana, Illinois.
Lee J, Hymowitz T. 2001. A molecular
phylogenetic study of the subtribe Glycininae (Leguminosae) derived from the
chloroplast DNA rps16 intron sequences. – Amer. J. Bot. 88:
2064-2073.
Lee KW, Tokuoka T, Heo K. 2004. Molecular
evidence for the inclusion of the Korean endemic genus
‘Echinosophora’ in Sophora (Fabaceae), and embryological features of
the genus. – J. Plant Res. 117: 209-219.
Lee YS, Seigler DS, Ebinger JE. 1989.
Acacia rigidula (Fabaceae)
and related species in Mexico and Texas. – Syst. Bot. 14: 91-100.
Leelavathi P, Prabhakar M, Ramayya N. 1984.
Structure, distribution, ontogeny and taxonomic significance of crystalliferous
cells in Leguminosae. – Ind. J. Bot. 7: 125-131.
Legume Phylogeny Working Group; Bruneaeu A,
Doyle JJ, Herendeen P, Hughes CE, Kenicer G, Lewis G, Mackinder B, Pennington
RT, Sanderson MJ, Wojciechowski MF, Koenen E et al. 2013. Legume phylogeny and
classification in the 21st century: progress, prospects and lessons
for other species-rich clades. – Taxon 62: 217-248.
Leht M. 2005. Cladistic and phenetic analyses
of relationships in Vicia subgenus Cracca (Fabaceae) based on morphological data.
– Taxon 54: 1023-1032.
Leht M. 2009a. Phylogeny of Old World
Lathyrus L. (Fabaceae) based
on morphological data. – Feddes Repert. 120: 59-74.
Leht M. 2009b. Phylogenetics of
Vicia (Fabaceae) based on
morphological data. – Feddes Repert. 120: 379-393.
Leht M, Jaaska V. 2002. Cladistic and
phenetic analysis of relationships in Vicia subgenus Vicia
(Fabaceae) by morphology and
isozymes. – Plant Syst. Evol. 232: 237-260.
Leinfellner W. 1970. Zur Kenntnis der
Karpelle der Leguminosen 2. Caesalpiniaceae and Mimosaceae. – Österr. Bot.
Zeitschr. 118: 108-120.
Leinfellner W. 1971. Das Gynözeum von
Krameria und sie Vergleich mit jenem der Leguminosae und der Polygalaceae. – Österr. Bot.
Zeitschr. 119: 102-117.
Leinfellner W. 1972. Zur Morphologie des
Gynözeums der Polygalaceen. – Österr. Bot. Zeitschr. 120: 51-76.
Leins P, Merxmüller H. 1966. Zur Gliederung
der Oxytropis campestris-Gruppe. – Mitt. Bot. Staatssamml. München
6: 19-31.
Leite VG, Mansano VF, Teixeira SP. 2014.
Floral ontogeny in Dipterygeae (Fabaceae) reveals new insights into one
of the earliest branching tribes in papilionoid legumes. – Bot. J. Linn. Soc.
174: 529-550.
Leite VG, Teixeira SP, Mansano VF, Prenner G.
2015. Floral development of the early-branching papilionoid legume Amburana
cearensis (Leguminosae) reveals rare and novel characters. – Intern. J.
Plant Sci. 176: 94-106.
Léonard J. 1951. Les Cynometra et
les genres voisins en Afrique tropicale. – Bull. Jard. Bot. Natl. Belg. 21:
373-450.
Léonard J. 1954. Aeschynomene. –
Bull. Jard. Bot. État Bruxelles 24: 63-84.
Léonard J. 1957. Genera des Cynometreae et
des Amherstieae africaines, Leguminosae-Caesalpinioideae. Éssai de
blastogénie appliquée à la systématique. – Mém. Acad. Roy. Belg., Cl.,
30: 1-314.
Leon-de la Luz JL, Perez-Navarro JJ,
Dominguez-Cadena R. 2002. Two new Marina (Leguminosae) from the
southern Baja California peninsula, Mexico. – Brittonia 54: 72-77.
Le Roux MM, Wyk B-E van. 2007. A revision of
Lebeckia sect. Lebeckia: the L. sepiaria group. –
South Afr. J. Bot. 73: 118-130.
Le Roux MM, Wyk B-E van. 2008. A revision of
Lebeckia sect. Lebeckia: the L. plukenetiana group
(Fabaceae, Crotalarieae). – South
Afr. J. Bot. 74: 660-676.
Le Roux MM, Wyk B-E van, Bank M van der.
2007. A taxonomic study of the type section of the genus Lebeckia
Thunb. (Fabaceae, Crotalarieae). –
South Afr. J. Bot. 73: 297.
Le Roux MM, Wyk B-E van, Boatwright JS,
Tilney PM. 2011. The systematic significance of morphological and anatomical
variation in fruits of Crotalaria and related genera of tribe
Crotalarieae (Fabaceae). – Bot. J.
Linn. Soc. 165: 84-106.
Le Roux MM, Boatwright JS, Wyk B-E van. 2013.
A global infrageneric classification system for the genus Crotalaria
(Leguminosae) based on molecular and morphological evidence. – Taxon 62:
957-971.
Lersten NR. 1981. Testa topography in
Leguminosae, Subfamily Papilionoideae. – Proc. Iowa Acad. Sci. 88:
180-191.
Lersten NR. 1983. Suspensors in Leguminosae.
– Bot. Rev. 49: 233-257.
Lersten NR, Gunn C. 1981. Seed morphology and
testa topography in Cicer (Fabaceae: Faboideae). – Syst. Bot. 6:
223-230.
Lersten NR, Gunn C. 1982. Testa characters in
tribe Vicieae, with notes about tribes Abreae, Cicereae, and Trifolieae (Fabaceae). – Techn. Bull. 1667:
1-40.
Lersten NR, Horner HT. 2005. Macropattern of
styloid and druse crystals in Quillaja (Quillajaceae) bark and leaves. –
Intern. J. Plant Sci. 166: 705-711.
Lersten NR, Horner HT. 2007. Calcium oxalate
crystals in tribe Galegeae (Leguminosae) including foliar crystal micropattern
development in Caragana frutex. – Can. J. Bot. 85: 394-403.
Lesins KA, Lesins I. 1979. Genus
Medicago (Leguminosae). A taxogenetic study. – W. Junk Publ., The
Hague.
Lesins KA, Lesins I, Gillies CB. 1970.
Medicago murex with 2n = 16 and 2n = 14 chromosome complements. –
Chromosoma 30: 109-122.
Levyns MR. 1949. The floral morphology of
some South African members of Polygalaceae. – J. South Afr. Bot.
15: 79-92.
Levyns MR. 1954. The genus Muraltia.
– J. South Afr. Bot. [Suppl.] 2: 1-247.
Lewis GP. 1985. Two new taxa and one
little-known species of Aeschynomene (Leguminosae-Papilionoideae) from
Brazil. – Kew Bull. 40: 599-605.
Lewis GP. 1986. A nomenclatural and taxonomic
update of the tribe Mimoseae (Leguminosae-Mimosoideae). – Bull. IGSM 14:
49-52.
Lewis GP. 1987. Legumes of Bahia. – Royal
Botanic Gardens, Kew, United Kingdom.
Lewis GP. 1988. Four little-known species of
Leguminosae from Cuba. – Willdenowia 18: 223-229.
Lewis GP. 1991. Five new taxa of
Piptadenia (Leguminosae: Mimosoideae) from Brazil. – Kew Bull. 46:
159-168.
Lewis GP. 1992. Two new species of
Aeschynomene (Leguminosae: Papilionoideae) from Brazil. – Kew Bull.
47: 141-145.
Lewis GP. 1993. Two new taxa of
Aeschynomene (Leguminosae: Papilionoideae) from Brazil. – Kew Bull.
49: 93-97.
Lewis GP. 1996a. Two new species of
Acacia (Leguminosae: Mimosoideae) from Brazil. – Kew Bull. 51:
371-375.
Lewis GP. 1996b. Notes on
Stuhlmannia Taub. and the correct placement of Caesalpinia
insolita (Harms) Brenan & J. B. Gillett (Leguminosae:
Caesalpinioideae: Caesalpinieae). – Kew Bull. 51: 377-379.
Lewis GP. 2005. Caesalpinieae. – In: Lewis
G, Schrire B, Mackinder B, Lock M (eds), Legumes of the world, Royal Botanic
Gardens, Kew, pp. 127-161.
Lewis GP. 2016. New insights into the
systematics and biology of Brazilian Leguminosae (Fabaceae). – Intern. J. Plant Sci.
177: 1-2.
Lewis GP, Champluvier D. 1999.
Caesalpinia. A revision of the
Poincianella-Erythrostemon group. – Syst. Geogr. Plants 69:
140-141.
Lewis GP, Delgado Salinas A. 1994.
Mysanthus, a new genus in tribe Phaseoleae (Leguminosae:
Papilionoideae) from Brazil. – Kew Bull. 49: 343-351.
Lewis GP, Elias T. 1981. Mimoseae. – In:
Raven PH, Polhill RM (eds), Advances in legume systematics 1, Royal Botanic
Gardens, Kew, pp. 155-168.
Lewis GP, Forest F. 2005. Cercideae. – In:
Lewis G, Schrire B, Mackinder B, Lock M (eds), Legumes of the world, Royal
Botanic Gardens, Kew, pp. 57-67.
Lewis GP, Guinet P. 1986. Notes on
Gagnebina (Leguminosae: Mimosoideae) in Madagascar and neighbouring
islands. – Kew Bull. 41: 463-470.
Lewis GP, Lima MPM. 1991.
Pseudopiptadenia Rauschert no Brasil (Leguminosae: Mimosoideae). –
Arq. Jard. Bot. Rio J. 30: 43-67.
Lewis GP, Mannetje L ‘t. 1982. Two new
species of Leguminosae: Papilionoideae from Bahia, Brazil. – Kew Bull. 37:
123-127.
Lewis GP, Salinas AD. 1994.
Mysanthus, a new genus in tribe Phaseoleae (Leguminosae:
Papilionoideae) from Brazil. – Kew Bull. 49: 343-351.
Lewis GP, Schrire BD. 2003. Leguminosae or Fabaceae? – In: Klitgaard B, Bruneaeu
A (eds), Advances in legume systematics 10: Higher level systematics, Royal
Botanic Gardens, Kew, pp. 1-3.
Lewis GP, Schrire BD. 2004.
Micklethwaitia, a new name for Brenaniodendron J. Léonard
(Leguminosae: Caesalpinioideae: Detarieae). – Kew Bull. 59: 166.
Lewis GP, Simpson BB, Neff JL. 2000. Progress
in understanding the reproductive biology of the Caesalpinioideae
(Leguminosae). – In: Herendeen PS, Bruneau A (eds), Advances in legume
systematics 9: phylogeny, Royal Botanic Gardens, Kew, pp. 65-78.
Lewis GP, Schrire B, Mackinder B, Lock M
(eds). 2005. Legumes of the world. – Royal Botanic Gardens, Kew.
Lewis GP, Wood JRI, Lavin M. 2012.
Steinbachiella (Leguminosae: Papilionoideae: Dalbergieae), endemic to
Bolivia, is re-instated as an accepted genus. – Kew Bull. 67: 789-796.
Lewis GP, Siqueira GS, Banks H, Bruneau A.
2017. The majestic canopy-emergent genus Dinizia (Leguminosae:
Caesalpinioideae), including a new species endemic to the Brazilian state of
Espírito Santo. – Kew Bull. 72: 48. doi 10.1007/S12225-017-9720-7
Lewis WH, Davis SA. 1962. Cytological
observations of Polygala in eastern North America. – Rhodora 64:
102-113.
Li J, Gao Y, Wu J, Guo H. 2007. Vessel
features of three Caragana species in their natural habitats in the
inner Mongolia Plateau of China. – Nord. J. Bot. 25: 342-350.
Li J, Jiang J, Stel HV, Homkes A, Corajod J,
Brown K, Chen Z. 2014. Phylogenetics and biogeography of Apios (Fabaceae) inferred from sequences of
nuclear and plastid genes. – Intern. J. Plant Sci. 175: 764-780.
Li J, Jiang J-H, Fu C-X, Tang S-Q. 2014.
Molecular systematics and biogeography of Wisteria inferred from
nucleotide sequences of nuclear and plastid genes. – J. Syst. Evol. 52:
40-50.
Lima HC de. 1982a. Considerações
taxonômicas sobre o gênero Hymenolobium Bentham
(Leguminosae-Faboideae). – Acta Amazonica 12: 41-48.
Lima HC de. 1982b. Revisão taxonômica do
gênero Vatairea Aublet (Leguminosae-Faboideae). – Arq. Jard. Bot.
Rio de Janeiro XXVI: 173-214.
Lima HC de. 1983. Novos taxa de Leguminosae
Papilionoideae (tribo Dalbergieae) do Brasil. – Bradea, Bol. Herb. Bradeanum
3: 399-405.
Lima HC de. 1985. Centrolobium
Martius ex Bentham (Leguminosae-Papilionoideae) estudo taxonômico das
espécies Brasileiras extra-Amazônicas. – Arq. Jard. Bot. Rio de Janeiro
XXVII: 177-191.
Lima HC de. 1990. Tribo Dalbergieae
(Leguminosae Papilionoideae) – Morfología do frutos sementes e plântulas e
sua aplicação na sistemática. – Arq. Jard. Bot. Rio de Janeiro 30:
1-42.
Lima HC de, Studart da Fanseca Vaz AM. 1984.
Revisão taxonômica do gênero Riedeliella Harms
(Leguminosae-Faboideae). – Rodriguésia 36: 9-16.
Lima MPM, Lima HC. 1984.
Parapiptadenia Brenan (Leguminosae-Mimosoideae): estudo taxonômico
das espécies brasileiras. – Rodriguesia 36: 23-30.
Linder HP, Crisp M. 2011. The fate of
Acacia. – Taxon 60: 570-571.
Liston A. 1992a. Variation in the chloroplast
genes rpoC1 and rpoC2 of the genus Astragalus (Fabaceae): evidence from restriction
site mapping of a PCR-amplified fragment. – Amer. J. Bot. 79: 953-961.
Liston A. 1992b. Isozyme systematics of
Astragalus Sect. Leptocarpi Subsect. Californici (Fabaceae). – Syst. Bot. 17:
367-379.
Liston A. 1995. Use of the polymerase chain
reaction to survey for the loss of the inverted repeat in the legume
chloroplast genome. – In: Crisp MD, Doyle JJ (eds), Advances in legume
systematics 7: phylogeny, Royal Botanic Gardens, Kew, pp. 31-40.
Liston A, Wheeler JA. 1994. The phylogenetic
position of the genus Astragalus (Fabaceae): evidence from the chloroplast
genes rpoC1 and rpoC2. – Biochem. Syst. Ecol. 22:
377-388.
Liu C-C, Huang T-C. 1999. Microsporogenesis
and exine substructure in Uraria crinita (Fabaceae). – Grana 38: 277-283.
Lock JM. 1988. Cassia sens. lat.
(Leguminosae: Caesalpinioideae) in Africa. – Kew Bull. 43: 333-342.
Lock JM. 1989. Legumes of Africa: a
check-list. – Royal Botanic Gardens, Kew.
Lock JM, Maxted N. 2005. Fabeae. – In:
Lewis G, Schrire B, Mackinder B, Lock M (eds), Legumes of the world, Royal
Botanic Gardens, Kew, pp. 504-509.
Loesener T, Solereder H. 1905. Über die
bisher wenig bekannte südmexikanische Gattung Rigiostachys. – Verh.
Bot. Ver. Prov. Brandenburg 47: 35-62.
Lombardi JA. 2003. Martiodendron
fluminense (Leguminosae, Caesalpinioideae), a new species from the
Atlantic coast rainforest of Brazil. – Brittonia 54: 327-330.
Lopez J, Devesa JA, Ortega-Olivencia A, Ruiz
T. 2000. Production and morphology of fruit and seeds in Genisteae (Fabaceae) of south-west Spain. – Bot.
J. Linn. Soc. 132: 97-120.
Lorence DH, Wood KR. 1994. Kanaloa:
a new genus of Fabaceae (Mimosoideae)
from Hawaii. – Novon 4: 137-145.
Lotova LI, Timonin AC. 1999. Anatomy of
cortex and secondary phloem in Rosaceae III. Quillajoideae. –
Bot. Žurn. 84: 34-41.
Louis S, Delobel B, Gressent B, Duport G,
Diol O, Rahioui I, Charles H, Rahbé Y. 2007. Broad screening of the legume
family for variability in seed insecticidal activities and for the occurrence
of the A1b-like knottin peptide entomotoxins. – Phytochemistry 68:
521-535.
Lozano P, Klitgaard BB. 2006. The genus
Machaerium (Leguminosae: Papilionoideae: Dalbergieae) in Ecuador. –
Brittonia 58: 124-150.
Luckow M. 1993. A monograph of
Desmanthus (Mimosoideae: Leguminosae). – Syst. Bot. Monogr. 38:
1-166.
Luckow M. 1995. A phylogenetic analysis of
the Dichrostachys group (Mimosoideae: Mimoseae). – In: Crisp MD,
Doyle JJ (eds), Advances in legume systematics 7: Phylogeny, Royal Botanic
Gardens, Kew, pp. 63-75.
Luckow M. 1997. Generic relationships in the
Dichrostachys group (Leguminosae: Mimosoideae): evidence from
chloroplast DNA restriction sites and morphology. – Syst. Bot. 22:
189-198.
Luckow M. 2002. Anatomical features of the
leaves in the Dichrostachys group (Leguminosae: Mimosoideae) and their
utility for phylogenetic studies. – Syst. Bot. 27: 29-40.
Luckow M, Grimes J. 1997. A survey of anther
glands in the mimosoid tribes Parkieae and Mimoseae. – Amer. J. Bot. 84:
285-297.
Luckow M, Hopkins HCF. 1995. A cladistic
analysis of Parkia (Leguminosae: Mimosoideae). – Amer. J. Bot. 82:
1300-1320.
Luckow M, White PJ, Bruneau A. 2000.
Relationships among the basal genera of mimosoid legumes. – In: Herendeen PS,
Bruneau A (eds), Advances in legume systematics 9: Phylogeny, Royal Botanic
Gardens, Kew, pp. 165-180.
Luckow M, Miller JT, Murphy DJ, Livshultz T.
2003. Phylogenetic analysis of the Mimosoideae (Leguminosae) based on
chloroplast DNA sequence data. – In: Klitgaard BB, Bruneau A (eds), Advances
in legume systematics 10: higher level systematics, Royal Botanic Gardens, Kew,
pp. 197-220.
Luckow M, Fortunato RH, Sede S, Livshultz T.
2005. The phylogenetic affinities of two mysterious monotypic mimosoids from
southern South America. – Syst. Bot. 30: 585-602.
Lüdtke R, Teixeira de Souza-Chies T, Miotto
STS. 2013. Ogênero Polygala L. (Polygalaceae) na região Sul do
Brasil. – Hoehnea 40. http://dx.doi.org/10.1590/S2236-89062013000100001
Luo M-C, Hwu K-K, Huang T-C. 2000. Taxonomic
study of Taiwan Astragalus based on genetic variation. – Taxon 49:
35-46.
Lyshede OB. 1977. Structure and function of
trichomes in Spartocytisus filipes. – Bot. Not. 129: 395-404.
Maassoumi A-A. 1987. Notes on the genus
Astragalus in Iran I. Cytotaxonomic studies on some species. –
Iranian J. Bot. 3: 117-128.
Maassoumi A-A. 1998. New findings on the
genus Astragalus in Iran. – Iranian J. Bot. 7: 221-226.
Maassoumi A-A, Ranjbar M. 1996. Notes on the
genus Astragalus sect. Alopecuroidei DC. in Iran. – Iran.
J. Bot. 7: 39-43.
Maassoumi A-A, Ghahreman A, Ghahremani-Nejad
F, Matin F. 1999. Astragalus gigantirostratus (Fabaceae), a remarkable new species from
N Iran and supplementary notes on A. sect. Cytisodes Bunge.
– Willdenowia 29: 221-225.
McDermott LF. 1910. An illustrated key to the
North American species of Trifolium. – San Francisco.
McDonald MW. 2003. Revision of Acacia
tumida (Leguminosae: Mimosoideae) and close allies, including the
description of three rare taxa. – Aust. Syst. Bot. 16: 139-164.
McDonald MW, Maslin BR. 1997. A reappraisal
of Acacia cowleana and allied taxa, including the description of a new
species, A. elachantha, from the tropical dry-zone of Australia. –
Aust. Syst. Bot. 10: 303-320.
McDonald MW, Maslin BR. 2000. Taxonomic
revision of the salwoods: Acacia aulacocarpa Cunn. ex Benth. and its
allies (Leguminosae: Mimosoideae: section Juliflorae). – Aust. Syst.
Bot. 13: 21-78.
McDougall KL. 2009. Four new species related
to Bossiaea bracteosa F. Muell. ex Benth. in south-eastern Australia.
– Telopea 12: 347-360.
Maciel HS, Schifino-Wittmann MT. 2002. First
chromosome number determinations in south-eastern South American species of
Lupinus L. (Leguminosae). – Bot. J. Linn. Soc. 139: 395-400.
McKey D. 1994. Legumes and nitrogen: the
evolutionary ecology of a nitrogen-demanding lifestyle. – In: Sprent JI,
McKey D (eds), Advances in legume systematics 5: the nitrogen factor, Royal
Botanic Gardens, Kew, pp. 211-228.
Mackinder BA. 1999. Three new taxa and a new
name in Dolichos L. (Leguminosae: Papilionoideae: Phaseoleae). – Kew
Bull. 54: 415-423.
Mackinder BA. 2001 [2002]. Further systematic
studies in Berlinia (Leguminosae, Caesalpinioideae, Detarieae sensu
lato). – Syst. Geogr. Plants 71: 433-441.
Mackinder BA. 2005. Tribe Detarieae. – In:
Lewis G, Schrire B, Mackinder B, Lock M (eds), Legumes of the world, Royal
Botanic Gardens, Kew, pp. 68-109.
Mackinder BA, Lewis GP. 1990. Two new species
of Calliandra (Leguminosae-Mimosoideae) from Brazil. – Kew Bull. 45:
681-684.
Mackinder BA, Pennington RT. 2011. Monograph
of Berlinia (Leguminosae). – Syst. Bot. Monogr. 91: 1-117.
Mackinder BA, Wieringa JJ. 2013.
Annea gen. nov. (Detarieae, Caesalpinioideae, Leguminosae): a home for
two species long misplaced in Hymenostegia sensu lato. – Phytotaxa
142: 1-14.
Mackinder BA, Wieringa JJ, Lunenburg I, Banks
H. 2010. Challenges and progress in clarifying the generic limits of
Talbotiella and Hymenostegia (Detarieae: Caesalpinioideae:
Leguminosae). – In: Burgt XM van der (ed), Proceedings of the 18th
AETFAT Congress, Royal Botanic Garden, Kew, pp. 43-56.
Mackinder BA, Wieringa JJ, Biurgt XM van der.
2011. A revision of the genus Talbotiella Baker f. (Caesalpinioideae:
Leguminosae). – Kew Bull. 65: 401-420.
Mackinder BA, Saslis-Lagoudakis H, Wieringa
JJ, Devey D, Forest F, Bruneau A. 2013. The tropical African
Scorodophloeus clade includes two undescribed Hymenostegia
segregate genera and Micklethwaitia, a rare, monospecific genus from
Mozambique. – South Afr. J. Bot.
http://dx.doi.org/10.1016/j.sajb.2013.07.002
McMahon MM. 2005. Phylogenetic relationships
and floral evolution in the papilionoid legume clade Amorpheae. – Brittonia
57: 397-411.
McMahon MM, Hufford L. 2002. Developmental
morphology and structural homology of corolla-androecium synorganisation in the
tribe Amorpheae (Fabaceae:
Papilionoideae). – Amer. J. Bot. 89: 1884-1898.
McMahon MM, Hufford L. 2004. Phylogeny of
Amorpheae (Fabaceae: Papilionoideae).
– Amer. J. Bot. 91: 1219-1230.
McMahon MM, Hufford L. 2005. Evolution and
development in the amorphoid clade (Amorpheae: Papilionoideae: Leguminosae):
petal loss and dedifferentiation. – Intern. J. Plant Sci. 166: 383-396.
McMahon MM, Sanderson MJ. 2006. Phylogenetic
supermatrix analysis of GenBank sequences from 2228 papilionoid legumes. –
Syst. Biol. 55: 818-836.
McNeill J. 1968. Taxonomic and nomenclatural
notes on Polygala in Europe. – Feddes Repert. 79: 23-34.
McNeill J, Turland NJ. 2010. The conservation
of Acacia with A. penninervis as conserved type. – Taxon
59: 613-616.
Macqueen DJ. 1997. A revision of
Calliandra Series Racemosae (Leguminosae: Mimosoideae). –
Kew Bull. 52: 1-50.
Maesen LJG van der. 1970. Primitiae Africanae
8. A revision of the genus Cadia Forsskål (Caesalpinioideae) and some
remarks regarding Dicraeopetalum Harms (Papilionoideae) and
Platycelyphium Harms (Papilionoideae). – Acta Bot. Neerl. 19:
227-248.
Maesen LJG van der. 1985. Revision of the
genus Pueraria DC. with some notes on Teyleria Backer
(Leguminosae). – Agric. Univ. Wageningen Papers 85(1): 1-132.
Maesen LJG van der. 2003. Cajaninae of
Australia (Leguminosae: Papilionoideae). – Aust. Syst. Bot. 16: 219-227.
Magee AM, Aspinall S, Rice DW, Cusack BP,
Sémon M, Perry AS, Stefanovic S, Milbourne D, Barth S, Palmer JD, Gray JC,
Kavanagh TA, Wolfe KH. 2010. Localized hypermutation and associated gene losses
in legume chloroplast genomes. – Genome Res. 20: 1700-1710.
Maldonado S. 1982. Estudios embriológicos y
palinológicos en Astragalus (Leguminosae). – Bol. Soc. Arg. Bot.
20: 201-225.
Mangotra R, Koul AK. 1991. Base number in
genus Crotalaria. Evidences from meiosis. – Nucleus 34: 158-161.
Mansano V de Freitas. 1997. Estudos
taxonômicos da tribo Swartzieae (DC.) Benth. (Leguminosae: Papilionoideae) no
sudeste do Brasil. – M.Sc. diss., Universidad Estadual de Campinas,
Brazil.
Mansano V de Freitas, Bruneau A. 2012.
Phylogeny reconstruction in the Caesalpinieae grade (Leguminosae) based on
duplicated copies of the sucrose synthase gene and plastid markers. – Mol.
Phylogen. Evol. 65: 149-162.
Mansano V de Freitas, Lewis GP. 2004. A
revision of the genus Exostyles Schott (Leguminosae: Papilionoideae).
– Kew Bull. 59: 521-529.
Mansano V de Freitas, Souza AL de. 2005. A
new Swartzia (Leguminosae: Papilionoideae: Swartzieae) species with
trimorphic stamens from Amazonian Brazil. – Bot. J. Linn. Soc. 147:
235-238.
Mansano V de Freitas, Teixeira S de P. 2008.
Floral anatomy of the Lecointea clade (Leguminosae, Papilionoideae,
Swartzieae sensu lato). – Plant Syst. Evol. 273: 201-209.
Mansano V de Freitas, Tozzi AMG de A. 1999a.
The taxonomy of some Swartzieae (Leguminosae, subfam. Papilionoideae) from
southeastern Brazil. – Brittonia 51: 149-158.
Mansano V de Freitas, Tozzi AMG de A. 1999b.
Distribuição geográfica, ambiente preferencial e centros de diversidade dos
membros da tribo Swartzieae na região sudeste do Brasil. – Rev. Brasil. Bot.
22: 249-257.
Mansano V de Freitas, Tozzi AMG de A. 2001.
Swartzia Schreb. (Leguminosae: Papilionoideae: Swartzieae): a
taxonomic study of the Swartzia acutifolia complex including a new
name and a new species from southeastern Brazil. – Kew Bull. 56: 917-929.
Mansano V de Freitas, Tucker SC, Tozzi AMG de
A. 2002. Floral ontogeny of Lecointea, Zollernia,
Exostyles, and Harleyodendron (Leguminosae: Papilionoideae:
Swartzieae s.l.). – Amer. J. Bot. 89: 1553-1569.
Mansano V de Freitas, Bittrich V, Tozzi AMG
de A, De Souza AL. 2004. Composition of the Lecointea clade
(Leguminosae, Papilionoideae, Swartzieae), a re-evaluation based on combined
evidence from morphology and molecular data. – Taxon 53: 1007-1018.
Mansano V de Freitas, Tozzi AMG de A, Lewis
GP. 2004. A revision of the South American genus Zollernia Wied-Neux.
& Nees (Leguminosae, Papilionoideae, Swartzieae). – Kew Bull. 59:
497-520.
Marazzi B, Endress PK. 2008. Patterns and
development of floral asymmetry in Senna (Leguminosae, Cassiinae). –
Amer. J. Bot. 95: 22-40.
Marazzi B, Sanderson M. 2010. Large-scale
patterns of diversification in the widespread legume genus Senna and
the evolutionary role of extrafloral nectaries. – Evolution 64: 3570-3592.
Marazzi B, Endress PK, Queiroz LP de, Conti
E. 2006. Phylogenetic relationships within Senna (Leguminosae,
Cassiinae) based of three chloroplast DNA regions: patterns in the evolution of
floral symmetry and extrafloral nectaries. – Amer. J. Bot. 93: 288-303.
Marazzi B, Fortunato RH, Endress PK,
Spichiger R. 2006. Senna (Cassiinae, Leguminosae) in Paraguay:
synopsis, occurrence, ecological role and ethnobotany. – Candollea 61:
315-329.
Marazzi B, Cécile A, Simon MF, Luckow M,
Delgado Salinas A, Sanderson MJ. 2012. Locating evolutionary precursors on a
phylogenetic tree. – Evolution 66: 3918-3930.
Maréchal R. 1970. Données cytologiques sur
les espèces de la sous-tribu des Papilionaceae-Phaseoleae-Phaseolinae.
Deuxième série. – Bull. Jard. Bot. Natl. Belg. 40: 307-348.
Maréchal R. 1982. Arguments for a global
conception of the genus Vigna. – Taxon 31: 280-283.
Maréchal R, Mascherpa J-M, Stainier F. 1978.
Étude taxonomique d’un groupe complexe d’espèces des genres
Phaseolus et Vigna (Papilionaceae) sur la base de données
morphologiques et polliniques, traitées par l’analyse informatique. –
Boissiera 28: 1-273.
Maréchal R, Mascherpa JM, Stainier F. 1981.
Taxonometric study of the Phaseolus-Vigna complex and related
genera. – In: Polhill RM, Raven PH (eds), Advances in legume systematics 1,
Royal Botanic Gardens, Kew, pp. 329-335.
Mariani A, Pupilli F, Calderini O. 1996.
Cytological and molecular analysis of annual species of the genus
Medicago. – Can. J. Bot. 74: 299-307.
Marinho CR, Oliveira RB, Teixeira SP. 2016.
The uncommon cavitated secretory trichomes in Bauhinia s.s. (Fabaceae): the same roles in different
organs. – Bot. J. Linn. Soc. 180: 104-122.
Marques MCM. 1980. Revisão das espécies do
gênero Bredemeyera Willd. (Polygoalaceae) do Brasil. – Rodriguésia
32: 269-321.
Marques MCM. 1984a. Polígalas do Brasil I.
Seção Acanthocladus (Kl. ex Hassk.) Chod. (Polygalaceae). – Rodriguésia 36:
3-10.
Marques MCM. 1984b. Polígalas do Brasil III.
Seção Gymnospora Chod. do gênero Polygala L. (Polygalaceae). – Rodriguésia 36:
31-34.
Marques MCM. 1989. Monnina Ruiz et
Pavón (Polygalaceae) no Brasil.
– Rodriguésia 67: 3-33.
Marques MCM. 1996. Securidaca, L.
(Polygalaceae) do Brasil. –
Arq. Jard. Bot. Rio de Janeiro 34: 7-144.
Marques MCM, Guimarães EF. 2003. Espécie e
variedads novas de Polygala L. (Polygalaceae) do Brasil. – Bradea
9: 45-50.
Marques MCM, Martins HF. 1997. Polygalaceae. – Albertoa 4:
130-199.
Marques MCM, Peixoto AL. 2004. Polygala
warmingiana A. W. Benn. (Polygalaceae) taxonomia e
nomenclatura. – Bradea 10: 13-16.
Marques MCM, Peixoto AL. 2007. Estudo
taxonômico de Polygala subgenera Ligustrina. –
Rodriguésia 58: 95-146.
Martin JN, Watt JR. 1944. The strophiole and
other seed structures associated with hardness in Melilotus alba L.
and M. officinalis Willd. – Iowa St. Coll. J. Sci. 18: 457-469.
Martin PG, Dowd JM. 1990. A protein sequence
study of the dicotyledons and its relevance to the evolution of the legumes and
nitrogen fixation. – Aust. Syst. Bot. 3: 91-100.
Martin PG, Dowd JM. 1993. Partial sequences
of ribosomal RNA of Papilionaceae and related families. – Phytochemistry 33:
361-363.
Maslin BR. 1989. Wattle become of
Acacia? – Aust. Syst. Bot. Soc. Newsl. 58: 1-13.
Maslin BR. 1994. Notes on Acacia
adunca and A. linearifolia and the description of a new
subspecies of A. junciflora (Leguminosae: Mimosoideae) from eastern
Australia. – Telopea 6: 43-51.
Maslin BR, Reid JE. 2012. A taxonomic
revision of mulga (Acacia aneura and its close relatives: Fabaceae) in Western Australia. –
Nuytsia 22: 129-267.
Maslin BR, Stirton CH. 1997. Generic and
infrageneric classification in Acacia (Leguminosae: Mimosoideae): a
list of critical species on which to build a comparative data set. – Bull.
Intern. Group for the Study of Mimosoideae 20: 22-44.
Maslin BR, Thomson LAJ. 1992. Re-appraisal of
the taxonomy of Acacia holosericea, including the description of a new
species, A. colei, and the reinstatement of A. neurocarpa.
– Aust. Syst. Bot. 5: 729-743.
Maslin BR, Miller JT, Seigler DS. 2003.
Overview of the generic status of Acacia (Leguminosae: Mimosoideae).
– Aust. Syst. Bot. 16: 1-18.
Massoud R. 2010. Taxonomic notes on the genus
Astragalus (Fabaceae) in
Iran, with the description of a new species. – Phytotaxa 7: 46-51.
Maureira-Butler I, Pfeil B, Muangprom A,
Osborn T, Doyle J. 2008. The reticulate history of Medicago (Fabaceae). – Syst. Biol. 57:
466-482.
Maxted N. 1991. Intergeneric relationships
between Psophocarpus Necker ex DC. (Phaseolae, Leguminosae) and its
allies. – Candollea 46: 367-382.
Maxted N. 1993a. A phenetic analysis of
Vicia section Atossa series Truncatulae
(Leguminosae: Vicieae). – Kew Bull. 48: 739-753.
Maxted N. 1993b. A phenetic investigation of
Vicia L. subgenus Vicia (Leguminosae, Vicieae). – Bot. J.
Linn. Soc. 111: 155-182.
Maxted N. 1994. A phenetic investigation of
Vicia section Peregrinae Kupicha (Leguminosae,
Papilionoideae, Vicieae). – Edinburgh J. Bot. 51: 75-97.
Maxted N, Douglas C. 1997. A phenetic
investigation of Vicia section Hyperchusa (Alef.) Aschers.
& Graebner (Leguminosae, Papilionoideae, Vicieae). – Lagascalia 19:
345-370.
Maxwell RH. 1970. The genus
Cymbosema (Leguminosae): notes and distribution. – Ann. Missouri
Bot. Gard. 57: 252-257.
Maxwell RH. 1977. A resumé of the genus
Cleobulia (Leguminosae) and its relation to the genus
Dioclea. – Phytologia 38: 51-65.
Maxwell RH. 2011. New species and notes in
the genus Dioclea s.l. (Fabaceae, subtribe Diocleinae). –
Novon 21: 226-243.
Maxwell RH, Taylor DW. 2003. Phylogenetic
relationships of the Diocleinae with particular emphasis on the subgroups of
Dioclea. – In: Klitgaard BB, Bruneau A (eds), Advances in legume
systematics 10, Higher level systematics, Royal Botanic Gardens, Kew, United
Kingdom, pp. 325-353.
Mayer MS, Bagga SK. 2002. The phylogeny of
Lens (Leguminosae): new insight from ITS sequence analysis. – Plant
Syst. Evol. 232: 145-154.
Mears JA, Mabry TJ. 1971. Alkaloids in the
Leguminosae. – In: Harborne JB, Boulter D, Turner BL (eds), Chemotaxonomy of
the Leguminosae, Academic Press, London, pp. 73-178.
Meijden R van der. 1982. Systematics and
evolution of Xanthophyllum (Polygalaceae). – Leiden Bot. Ser.
7, E. J. Brill, Leiden, pp. 1-157.
Meijden R van der. 1986. Polygalaceae. – In: Steenis CGGJ
van (†), Wilde WJJO de (eds), Flora Malesiana I, 10(3), Kluwer Academic
Publ., Dordrecht, Boston, London, pp. 455-539.
Meireles JEC. 2007. Revisão taxonômica e
filogenia de Poecilanthe s.l. (Leguminosae, Papilionoideae,
Brongniartieae). – M.Sc. thesis, Universidade Estadual de Campinas, Campinas,
São Paulo, Brazil.
Meireles JEC, Azevedo Tozzi AMG de. 2008.
Seed and embryo morphology of Poecilanthe (Fabaceae, Papilionoideae,
Brongniartieae). – Bot. J. Linn. Soc. 158: 249-256.
Meireles JEC, Azevedo Tozzi AMG de. 2014.
Taxonomic revision of Amphiodon (Leguminosae, Papilionoideae, Brongniartieae).
– Syst. Bot. 39: 1150-1153.
Meireles JEC, Azevedo Tozzi AMG de. 2015.
Limadendron: a new genus of Leguminosae (Papilionoideae,
Brongniartieae) from South America. – Plant Syst. Evol. 301: 701-707.
Meireles JEC, Azevedo Tozzi AMG de, Lavin M.
2014. A phylogenetic analysis of molecular and morphological data reveals a
paraphyletic Poecilanthe (Leguminosae, Papilionoideae). – Syst. Bot.
39: 1142-1149.
Mennes CB, Moerland MS, Rath M, Smets EF,
Merckx VSFT. 2015. Evolution of mycoheterotrophy in Polygalaceae: the case of
Epirixanthes. – Amer. J. Bot. 102: 598-608.
Mercado-Ruaro P, Delgado-Salinas A. 1996.
Karyoogical studies in several Mexican species of Phaseolus L. and
Vigna Sav (Phaseolinae, Fabaceae). – In: Pickersgill B, Lock
JM (eds), Advances in legume systematics 8, Royal Botanic Gardens, Kew, pp.
83-87.
Mercado-Ruaro P, Delgado-Salinas A. 1998.
Karyotypic studies on species of Phaseolus (Fabaceae-Phaseolinae). – Amer. J. Bot.
85: 1-9.
Merxmüller H, Heubl GR. 1983. Karyologische
und palynologische Studien zur Verwandtschaft der Polygala chamaebuxus
L. – Bot. Helv. 93: 133-144.
Mettin D, Hanelt P. 1964. Cytosystematische
Untersuchungen in der Artengruppe um Vicia sativa L. I. –
Kulturpflanze 12: 163-225.
Mettin D, Hanelt P. 1968. Bemerkungen zur
Karyologie und Systematik einiger Sippen der Gattung Vicia L. –
Feddes Repert. 77: 11-30.
Milby TH. 1976. Studies in the floral anatomy
of Polygala (Polygalaceae). – Amer. J. Bot. 63:
1319-1326.
Miller JT, Bayer RJ. 2000. Molecular
systematics of the tribe Acacieae (Leguminosae: Mimosoideae). – In: Herendeen
P, Bruneau A (eds), Advances in legume systematics 9: phylogeny, Royal Botanic
Gardens, Kew, pp. 181-200.
Miller JT, Bayer RJ. 2001. Molecular
phylogenetics of Acacia (Fabaceae: Mimosoideae) based on
chloroplast matK coding sequence and flanking trnK intron
spacer regions. – Amer. J. Bot. 88: 697-705.
Miller JT, Bayer RJ. 2003. Molecular
phylogenetics of Acacia subgenera Acacia and
Aculeiferum (Fabaceae:
Mimosoideae) based on the chloroplast matK coding sequence and
flanking trnK intron spacer regions. – Aust. Syst. Bot. 16:
27-33.
Miller JT, Seigler DS. 2012. Evolutionary and
taxonomic relationships of Acacia s.l. (Leguminosae: Mimosoideae). –
Aust. Syst. Bot. 25: 217-224.
Miller JT, Grimes JW, Murphy DJ, Bayer RJ,
Ladiges PY. 2003. A phylogenetic analysis of the Acacieae and Ingeae
(Mimosoideae: Fabaceae) based on
trnK, matK, psbA-trnH, and
trnL/trnF sequence data. – Syst. Bot. 28: 558-566.
Miller JT, Murphy DM, Brown GK, Richardson
DM, González-Orozco CE. 2011. The evolution and phylogenetic placement of
invasive Acacia species. – Divers. Distrib. 17: 848-860.
Miller JT, Terra V, Riggins C, Ebinger JE,
Seigler DS. 2017. Molecular phylogenetics of Parasenegalia and
Pseudosenegalia (Fabaceae:
Mimosoideae). – Syst. Bot. 42: 465-469.
Minamioka H, Imai H. 2009. Sphingoid
long-chain base composition of glucosylceramides in Fabaceae: a phylogenetic interpretation
of Fabeae. – J. Plant Res. 122: 415-419.
Mironov EM. 2000. Pericarp anatomy of East
European species of the genus Hedysarum L. (Papilionaceae): sections
Gamotion and Multicaulia. – Bull. Mosc. Soc. Nat., Bot.
105: 50-53. [In Russian]
Misset MT. 1990. Données caryologiques chez
le genre Ulex L. (Papilionoideae) dans le Massif Armoricain. – Taxon
39: 630-635.
Mitchell AD, Heenan PB. 2002.
Sophora sect. Edwardsia (Fabaceae): further evidence from nrDNA
sequence data of a recent and rapid radiation around the Southern Oceans. –
Bot. J. Linn. Soc. 140: 435-441.
Mitchell RE, Geissman TA. 1971. Constituents
of Suriana maritima: a triterpene diol of novel structure and a new
flavonol glycoside. – Phytochemistry 10: 1559-1567.
Mohlenbrock RH. 1957. A revision of the genus
Stylosanthes. – Ann. Missouri Bot. Gard. 44: 299-355.
Mohlenbrock RH. 1960. Recent studies in the
Leguminosae genus Stylosanthes. – Rhodora 62: 340-346.
Mohlenbrock RH. 1961. A monograph of the
Leguminous genus Zornia. – Webbia 16: 1-141.
Mohlenbrock RH. 1962. Additional collections
of the Leguminous genus Zornia. – Webbia 16: 649-655.
Mohlenbrock RH. 1963a. Further considerations
in Stylosanthes (Leguminosae). – Rhodora 65: 245-258.
Mohlenbrock RH. 1963b. A revision of the
leguminous genus Sweetia. – Webbia 17: 223-263.
Mohlenbrock RH. 1966. A revision of
Pithecellobium sect. Archidendron. – Webbia 21: 653-724.
Moiloa NA, Chimphango SBM, Muasya AM. 2018. A
phylogenetic study of the genus Wiborgia (Crotalarieae, Fabaceae). – South Afr. J. Bot. 115:
179-193.
Monro A. 2003. Systematics of the Australian
Polygalaceae and
Xanthophyllaceae. – Ph.D. diss., Australian National University, Canberra.
Monteiro R, Gibbs PE. 1986. A taxonomic
revision of the unifoliolate species of Lupinus (Leguminosae) in
Brazil. – Notes Roy. Bot. Gard. Edinb. 44: 71-104.
Moore G, Smith GF, Figueiredo E, Demissew S,
Lewis G, Schrire B, Rico L, Wyk AE van. 2010. Acacia, the 2011
nomenclature section in Melbourne, and beyond. – Taxon 59: 1188-1195.
Moore RJ. 1968. Chromosome numbers and
phylogeny in Caragana (Leguminosae). – Can. J. Bot. 46:
1513-1522.
Morat P, Meijden R van der. 1991.
Balgoya (Polygalaceae
trib. Moutabeeae), a new genus from New Caledonia. – Bull. Mus. Natl. Hist.
Nat., sect. B, Adansonia 13: 3-8.
Morgan DR, Soltis DE, Robertson KR. 1994.
Systematic and evolutionary implications of rbcL sequence variation in
Rosaceae. – Amer. J. Bot. 81:
890-903.
Moteetee AN. 2003. A taxonomic study of
Melolobium and related African genera of the tribe Genisteae (Fabaceae). – Ph.D. diss., Rand
Afrikaans University (University of Johannesburg), Johannesburg, Republic of
South Africa.
Moteetee AN, Wyk B-E van. 2006a. A revision
of the genus Melolobium (Genisteae, Fabaceae). – South Afr. J. Bot. 72:
51-98.
Moteetee AN, Wyk B-E van. 2006b. A revision
of the genus Bolusafra (tribe Phaseoleae, Fabaceae). – South Afr. J. Bot. 72:
604-608.
Moteetee AN, Wyk B-E van. 2007. A taxonomic
revision of the genus Millettia in South Africa. – South Afr. J.
Bot. 73: 304.
Moteetee AN, Wyk B-E van, Tilney PM. 2002.
The taxonomic significance of trichome type and distribution in
Melolobium (Fabaceae). –
Bothalia 32: 85-89.
Moteetee AN, Boatwright JS, Jaca TP. 2014. A
review of Rhynchosia Section Polytropia (Phaseoleae, Fabaceae) and a new species from the
Western Cape Province, South Africa. – Syst. Bot. 39: 1127-1131.
Motsi MC. 2004. Molecular phylogenetics of
the genus Rafnia Thunb. (Fabaceae, Crotalarieae). – Masters
diss., Rand Afrikaans University (University of Johannesburg), Johannesburg,
Republic of South Africa.
Motzkus B. 1973. Beiträge zur Kenntnis
einiger Chromosomenzahlen der Gattung Coronilla L. – Feddes Repert.
84: 741-746.
Moulin L, Munive A, Dreyfus B, Boivin-Masson
C. 2001. Nodulation of legumes by members of the β-subclass of Proteobacteria.
– Nature 411: 948-950.
Moura TM de, Mansano VF, Torke BM, Lewis GP,
Tozzi AMGA. 2013. A taxonomic revision of Mucuna (Fabaceae: Papilionoideae: Phaseoleae) in
Brazil. – Syst. Bot. 38: 631-637.
Moura TM de, Wilmot-Dear M, Vatanparast M,
Fortuna-Perez AP, Tozzi AMGA, Lewis GP. 2016. A new infrageneric classification
of Mucuna (Leguminosae-Papilionoideae): supported by morphology,
molecular phylogeny and biogeography. – Syst. Bot. 41: 606-616.
Moura TM de, Vatanparast M, Tozzi AMGA,
Forest F, Wilmot-Dear CM, Simon MF, Mansano VF, Kajita T, Lewis GP. 2016. A
molecular phylogeny and new infrageneric classification of Mucuna
Adans. (Leguminosae-Papilionoideae) including insights from morphology and
hypotheses about biogeography. – Intern. J. Plant Sci. 177: 76-89.
Moura TM de, Lewis GP, Tozzi AMGA. 2016. A
revision of the South American genus Platycyamus Benth. (Leguminosae).
– Kew Bull. 71: 9 DOI 10.1007/S12225-016-9617-X
Mourad MM, Sharawy SM. 2009. The
interspecific relationships of Astragalus species in Egypt assessed by
the morpho-anatomical characters of the pod. – Feddes Repert. 120:
307-316.
Murphy B, Estrella M de la, Schley R, Forest
F, Klitgård B. 2018. On the monophyly of Macrolobium Schreb., an
ecologically diverse Neotropical tree genus (Fabaceae-Detarioideae). – Intern. J.
Plant Sci. 179: 75-86.
Murphy DJ. 2008. A review of the
classification of Acacia (Leguminosae, Mimosoideae). – Muelleria 26:
10-26.
Murphy DJ, Udovicic F, Ladiges PY. 2000.
Phylogenetic analysis of Australian Acacia (Leguminosae: Mimosoideae)
by using sequence variations of an intron and two intergenic spacers of
chloroplast DNA. – Aust. Syst. Bot. 13: 745-754.
Murphy DJ, Miller JT, Bayer RJ, Ladiges PY.
2003. Molecular phylogeny of Acacia subgenus Phyllodinae
(Mimosoideae: Leguminosae) based on DNA sequences of the internal transcribed
spacer region. – Aust. Syst. Bot. 16: 19-26.
Murphy DJ, Brown GK, Miller JT, Ladiges PY.
2010. Molecular phylogeny of Acacia Mill. (Mimosoideae: Leguminosae):
evidence for major clades and informal classification. – Taxon 59: 7-19.
Naganowska B, Wolko B, Śliwińska E,
Kaczmarek Z, Schifino-Wittmann MT. 2005 [2006]. 2C DNA variation and
relationships among New World species of the genus Lupinus (Fabaceae). – Plant Syst. Evol. 256:
147-157.
Naghiloo S, Dadpur MR. 2010. Floral ontogeny
in Wisteria sinensis (Fabaceae: Faboideae: Millettieae) and
its systematic implications. – Aust. Syst. Bot. 23: 393-400.
Naghiloo S, Dadpour MR, Movafeghi A. 2012.
Floral ontogeny in Astragalus compactus (Leguminosae: Papilionoideae:
Galegeae): variable occurrence of bracteoles and variable ptterns of sepal
initiation. – Planta 235: 793-805.
Nakai T. 1923. Genera nova Rhamnacearum et
Leguminosarum ex Asia orientali. – Jap. Bot. Mag. 37: 29-36.
Narayan RK. 1982. Discontinuous DNA variation
in the evolution of plant species, the genus Lathyrus. – Evolution
36: 877-891.
Neill DA. 1988. Experimental studies on
species relationships in Erythrina (Leguminosae: Papilionoideae). –
Ann. Missouri Bot. Gard. 75: 886-969.
Neill DA. 1998. Ecuadendron (Fabaceae: Caesalpinioideae: Detarieae):
a new arborescent genus from western Ecuador. – Novon 8: 45-49.
Nemoto T, Yokoyama J, Fukuda T, Iokawa Y,
Ohashi H. 2010. Phylogeny of Lespedeza (Leguminosae) based on
chloroplast trnL-trnF sequences. – J. Jap. Bot. 85:
213-229.
Newcomb W, Wood SM. 1986. Fine structure of
nitrogen-fixing leguminous root nodules from the Canadian Arctic. – Nord. J.
Bot. 6: 609-626.
Ngok Banak L, Breteler FJ. 2004. Novitates
Gabonenses 50. Le genre Oddoniodendron (Leguminosae, Caesalpinioideae)
de Basse Guinée: une révision taxonomique du genre avec description de deux
espèces nouvelles du Gabon. – Adansonia, sér. III, 26: 241-250.
Nicholls KW, Bohm BA. 1987. Flavonoids of
Lupinus Sects. Platycarpos and Lupinellus (Fabaceae). – Syst. Bot. 12:
320-323.
Nie ZL, Gu ZJ, Sun H. 2002. Cytological study
of Tibetia (Fabaceae) in the
Hengduan Mountains region, China. – J. Plant Res. 115: 17-22.
Nielsen IC. 1979. Notes on the genera
Archidendron F. v. Mueller and Pithecellobium Martius in
mainland S.E. Asia. – Adansonia, sér. II, 19: 3-37.
Nielsen IC. 1980. Notes on Indo-Chinese
Mimosaceae. – Adansonia, sér. II, 19: 339-363.
Nielsen IC. 1981a. Ingeae Benth. – In:
Polhill RM, Raven PH (eds), Advances in legume systematics 1, Royal Botanic
Gardens, Kew, pp. 173-190.
Nielsen IC. 1981b.
Légumineuses-Mimosoidées. – In: Flore du Cambodge, du Laos et du Viêtnam
19, Muséum National d’Histoire Naturelle, Paris.
Nielsen IC. 1982. The Australian species of
Archidendron. – Nord. J. Bot. 2: 479-490.
Nielsen IC. 1985. Leguminosae-Mimosoideae.
– In: Larsen K, Smitinand T (eds), Flora of Thailand 4, 2: 131-222.
Nielsen IC. 1992. Mimosaceae
(Leguminosae-Mimosoideae). – In: Wilde WJJO de, Nooteboom HP, Kalkman C
(eds), Flora malesiana I, 11(1), Rijksherbarium/Hortus Botanicus, Leiden, pp.
1-226.
Nielsen IC, Guinet P. 1992. Synopsis of
Adenanthera (Leguminosae-Mimosoideae). – Nord. J. Bot. 12:
85-114.
Nielsen IC, Baretta-Kuipers T, Guinet P.
1984. The genus Archidendron (Leguminosae-Mimosoideae). – Opera Bot.
76: 1-120.
Nielsen IC, Labat J-N, Munzinger J. 2005.
Synopsis of Storckiella Seem. (Fabaceae, Caesalpinioideae) with
description of a new species and a new subspecies from New Caledonia. –
Adansonia, sér. III, 27: 217-230.
Nikiforova OD. 1985. The system of the genus
Vicia (Fabaceae) in Siberia.
– Bot. Žurn. 70: 604-611. [In Russian].
Niyomdham C. 1992. Notes on Thai and
Indo-Chines Phaseoleae (Leguminosae-Papilionoideae). – Nord. J. Bot. 12:
339-346.
Nooteboom HP. 1962. Simaroubaceae. – In:
Steenis CGGJ van (ed), Flora Malesiana I, 6, Noordhoff, Leiden, pp. 193-226.
Nooteboom HP. 1966. Flavonols,
leuco-anthocyanins, cinnamic acids, and alkaloids in dried leaves of some
Asiatic and Malesian Simaroubaceae. –
Blumea 14: 309-315.
Nores MJ, Simpson BB, Hick P, Anton AM,
Fortunato RH. 2012. The phylogenetic relationships of four monospecific
caesalpinioids (Leguminosae) endemic to southern South America. – Taxon 61:
790-802.
Norton LB. 1943. Rotenone in the yam bean
(Pachyrrhizus). – J. Amer. Chem. Soc. 65: 2259-2260.
Norton LB, Hansberry R. 1945. Constituents of
the insecticidal resin of the yam bean (Pachyrrhizus erosus). – J.
Amer. Chem. Soc. 67: 1609-1614.
Norverto CA,González-Andrés F, Ortiz J-M.
1994. Leaf and stem anatomy of species of Cytisophyllum,
Cytisus, Chamaecytisus, Genista and Genista
sect. Teline (Fabaceae:
Genisteae) as an aid for taxonomy. – Israel J. Plant Sci. 42: 213-225.
Oballa PO, Olng’otie PAS. 1993. Chromosome
numbers in two African Acacia species. – Kew Bull. 49: 107-113.
Oberprieler C, Vogt R. 1996. Chromosome
numbers of North African phanerogams VI. Some counts in Leguminosae. –
Willdenowia 25: 669-680.
Obiang-Mbomio D, Breteler FJ. 2007. Révision
du genre Eurypetalum Harms (Fabaceae, Caesalpinioideae). –
Adansonia, sér. III, 29: 67-76.
Occhioni EML. 1990. Considerações
taxonômicas no gênero Stryphnodendron Mart.
(Leguminosae-Mimosoideae) e distribuição geográfica das espécies. – Acta
Bot. Bras. 4: 153-158.
Occhioni-Martins EM. 1974.
Stryphnodendron Mart. (Leg. Mim.): as espécies do nordeste, sudeste e
sul do Brasil II. – Leandra 4-5: 53-66.
Occhioni-Martins EM. 1975.
Stryphnodendron Mart. (Leg. Mimosoideae): as espécies da região
centro-oeste do Brasil. – Leandra 5: 47-54.
Occhioni-Martins EM. 1981.
Stryphnodendron Mart. (Leguminosae-Mimosoideae) com especial
refeência aos taxa amazônicos. – Leandra 10-11: 3-100.
Occhioni-Martins EM, Martins AG Jr. 1972.
Stryphnodendron Mart. (Leg. Mim.): as espécies da Amazônia
Brasileira. – Leandra 2: 11-40.
Ochoterena-Booth H. 1991. Revisión
taxonómica del género Ramirezella Rose (Fabaceae, Papilionoideae). – Tesis de
Licenciatura, Facultad de Ciencias, U.N.A.M., México.
Ohashi H. 1971. A taxonomic study of the
tribe Coronilleae (Leguminosae), with a special reference to pollen morphology.
– J. Fac. Sci. Univ. Tokyo, Sect. III (Botany), 11: 25-92.
Ohashi H. 1999. The genera, tribes, and
subfamilies of Japanese Leguminosae. – Sci. Rep. Tôhoku Univ., ser. IV
(Biol.) 40: 187-268.
Ohashi H, Mill RR. 2000. Hylodesmum,
a new name for Podocarpium (Leguminosae). – Edinburgh J. Bot. 57:
171-188.
Ohashi H, Mill RR. 2004. Proposal to reject
the name Papilionopsis Steenis (Fabaceae). – Taxon 53: 564-565.
Ohashi H, Ohashi K. 2012a.
Ototropis, a genus separated from Desmodium (Leguminosae).
– J. Jap. Bot. 87: 108-118.
Ohashi H, Ohashi, K. 2012b.
Verdesmium, a new genus of Leguminosae: tribe Desmodieae. – J. Jap.
Bot. 87: 299-306.
Ohashi H, Polhill RM, Schubert BG. 1981.
Desmodieae. – In: Polhill RN, Raven PH (eds), Advances in legume systematics
1, Royal Botanic Gardens, Kew, pp. 292-300.
Ohashi H, Tateishi Y, Nemoto T, Endo Y. 1988.
Taxonomic studies on the Leguminosae of Taiwan III. – Sci. Rep. Tôhoku
Univ., ser. IV (Biology) 39: 191-248.
Ojeda DI, Santos-Guerra A, Jaén-Molina R,
Oliva-Tejera F, Caujapé-Castelis J, Cronk Q. 2012. The origin of bird
pollination in Macaronesian Lotus (Loteae, Leguminosae). – Mol.
Phylogen. Evol. 62: 306-318.
Ojeda DI, Santos-Guerra A, Oliva-Tejera F,
Jaen-Molina R, Caujapé-Castells J, Marrero-Rodíguez A, Cronk Q. 2014. DNA
barcodes successfully identified Macaronesian Lotus (Leguminosae)
species within early diverged lineages of Cape Verde and mainland Africa. –
AoB Plants 6: plu050.
Onyilagha JC, Islam S, Ntamatungiro S. 2009.
Comparative phytochemistry of eleven species of Vigna (Fabaceae). – Biochem. Syst. Ecol. 37:
16-19.
Oono R, Schmitt I, Sprent JI, Denison RF.
2010. Multiple evolutionary origins of legume traits leading to extreme
rhizobial differentiation. – New Phytol. 187: 508-520.
Oostermeijer JGB. 1989. Myrmecochory in
Polygala vulgaris L., Luzula campestris (L.) DC., and
Viola curtisii Forster in a Dutch dune area. – Oecologia 78:
302-311.
Op den Camp RH, De Mita S, Lillo A Cao Q,
Limpens E, Bisseling T, Geurts R. 2011. A phylogenetic strategy based on a
legume-specific whole genome duplication yields symbiotic cytokinin type-a
response regulators. – Plant Physiology 157: 2013-2022.
Orchard AE, Maslin BR. 2003. Proposal to
conserve the name Acacia (Leguminosae: Mimosoideae) with a conserved
type. – Taxon 52: 362-363.
Ortega J. 1976. Citogenética del género
Lotus en Macaronesia I. Números de cromosomas. – Bot. Macaronésica
1: 17-24.
Ortega-Olivencia A, Catalán P. 2009.
Systematics and evolutionary history of the circum-Mediterranean genus
Anagyris L. (Fabaceae) based
on morphological and molecular data. – Taxon 58: 1290-1306.
Orthia LA, Crisp MD, Cook LG, Kok RPJ de.
2005. Bush peas: a rapid radiation with no support for monophyly of
Pultenaea (Fabaceae:
Mirbelieae). – Aust. Syst. Bot. 18: 133-147.
Orthia LA, Kok RPJ de, Crisp MD. 2005. A
revision of Pultenaea (Fabaceae: Mirbelieae) 4. Species
occurring in Western Australia. – Aust. Syst. Bot. 18: 149-206.
Orthia LA, Cook LG, Crisp MD. 2005. Generic
delimitation and phylogenetic uncertainty: an example from a group that has
undergone an explosive radiation. – Aust. Syst. Bot. 18: 41-47.
Ortiz S, Pulgar I, Iglesias I. 2001. A new
species of Cytisus Desf. (Fabaceae) from islands off the west
coast of Galicia (North-West Iberian Peninsula). – Bot. J. Linn. Soc. 136:
339-344.
Osaloo SK, Kazemi NM, Maassoumi AA, Pouyani
ER. 2006. Phylogenetic status of Oreophysa microphylla (Fabaceae-Galegeae) based on nrDNA (ITS
region) and cpDNA (trnL intron/trnL-trnF intergenic
spacer) sequences. – Rostaniha 7: 177-188.
Osaloo SK, Maassoumi AA, Murakami N. 2003.
Molecular systematics of the genus Astragalus L. (Fabaceae): phylogenetic analyses of
nuclear ribosomal DNA internal transcribed spacers and chloroplast gene
ndhF sequences. – Plant Syst. Evol. 242: 1-32.
Oskolski AA, Stepanova AV, Boatwright JS,
Tilney PM, Van Wyk B-E. 2014. A survey of wood anatomical characters in the
tribe Crotalarieae (Fabaceae). –
South Afr. J. Bot. 94: 155-165.
Ottley AM. 1923. A revision of the California
species of Lotus. – Univ. Calif. Publ. Bot. 10: 189-305.
Ottley AM. 1944. The American Loti with
special consideration of a proposed new section, Simpeteria. –
Brittonia 5: 81-123.
Owens SA. 2000. Secondary and tertiary
pulvini in the unifoliolate leaf of Cercis canadensis L. (Fabaceae) with comparison to
Bauhinia purpurea L. – Intern. J. Plant Sci. 161: 583-597.
Owens SA, Ewers FW, Flegler SL, Klomparens
KL. 1995. Architecture of cauliflory in the genus Cercis (Fabaceae: Caesalpinioideae). – Can. J.
Bot. 73: 1270-1282.
Owens SJ. 1989. Stigma, style, pollen, and
the pollen-stigma interaction in the Caesalpinioideae. – Monogr. Syst. Bot.
Missouri Bot. Gard. 29: 113-126.
Owens SJ. 1990. The morphology of the wet,
non-papillate (WN) stigma form in the tribe Caesalpinieae (Caesalpinioideae:
Leguminosae). – Bot. J. Linn. Soc. 104: 293-302.
Owens SJ, Lewis GP. 1996. Stigma morphology
in the Leguminosae: the wet, papillate (WP) stigma in Caesalpinioideae. – Kew
Bull. 51: 119-131.
Padua Teixeira S de, Forni-Martins ER, Ranga
NT. 2002. Development and cytology of pollen in Dahlstedtia Malme
(Leguminosae: Papilionoideae). – Bot. J. Linn. Soc. 138: 461-471.
Paiva EAS, Machado SR. 2008. The floral
nectary of Hymenaea stigonocarpa (Fabaceae, Caesalpinioideae): structural
aspects during floral development. – Ann. Bot. 101: 125-133.
Paiva JAR. 1988. El género Polygala
(Polygalaceae) en el
Mediterráneo Occidental. – Coll. Bot. (Barcelona) 17: 191-203.
Paiva JR. 1998. Polygalarum Africanarum et
Madagascariensium prodromus atque gerontogaei generis Heterosamara
Kuntze, a genere Polygala L. segregati et a nobis denuo recepti,
synopsis monographica. – Fontqueria 50: 1-346.
Paiva JAR, Santos Dias JD. 1990. The pollen
grain of Polygala fruticosa Berg (Polygalaceae). – An. Jard. Bot.
Madrid 47: 377-385.
Palacios RA, Hoc PS. 2001. Three new species
of Prosopidastrum (Mimosaceae) from Argentina. – Novon 11: 79-87.
Palevitz BA, Newcomb EH. 1971. The
ultrastructure and development of tubular and crystalline P-protein in the
sieve elements of certain papilionaceous legumes. – Protoplasma 72:
399-425.
Palmer JD, Singh GP, Pillay DTN. 1983.
Structure and sequence evolution of three legume chloroplast DNAs. – Mol.
Gen. Genet. 190: 13-19.
Palmer JD, Jorgensen RA, Thompson WF. 1985.
Chloroplast DNA variation and evolution in Pisum: patterns of change
and phylogenetic analysis. – Genetics 109: 195-213.
Palmer JD, Osorio B, Aldrich J, Thompson WF.
1987. Chloroplast DNA evolution among legumes: loss of a large inverted repeat
occurred prior to other sequence rearrangements. – Curr. Genet. 11:
275-286.
Palmer JD, Osorio B, Thompson WF. 1988.
Evolutionary significance of inversions in legume chloroplast DNAs. – Curr.
Genet. 14: 65-74.
Palomino G, Martinez P, Bernal C, Sousa S M.
1993. Differencias cromosomicas entre algunas especies de los géneros
Sophora L. y Styphnolobium Schott. – Ann. Missouri Bot.
Gard. 80: 284-290.
Pan AD, Currano ED, Jacobs BF, Feseha M,
Tabor N, Herendeen PS. 2012. Fossil Newtonia (Fabaceae: Mimoseae) seeds from the Early
Miocene (22-21 MA) Mush Valley in Ethiopia. – Intern. J. Plant Sci. 173:
290-296.
Pantulu JV. 1940. A note on the chromosome
numbers of Cassia. – Curr. Sci. 9: 416-417.
Pardo C, Tahiri H, Cubas P, El Alaooui-Faris
FE. 2000. Pollen morphology in Cytisus (Papilionoideae, Leuminosae)
from Morocco and the Iberian Peninsula. – Grana 39: 159-168.
Pardo C, Cubas P, Tahiri H. 2004. Molecular
phylogeny and systematics of Genista (Leguminosae) and related genera
based on nucleotide sequences of nrDNA (ITS region) and cpDNA
(trnL-trnF intergenic spacer). – Plant Syst. Evol. 244:
93-119.
Pardo C, Cubas P, Tahiri H. 2008. Genetic
variation and phylogeography of Stauracanthus (Fabaceae, Genisteae) from the Iberian
Peninsula and northern Morocco assessed by chloroplast microsatellite (cpSSR)
markers. – Amer. J. Bot. 95: 98-109.
Pardo C, Tahiri H, Cubas P, El Alaoui-Faris
FE. 2000. Pollen morphology in Cytisus (Papilionoideae, Leguminosae)
from Morocco and the Iberian Peninsula. – Grana 39: 159-168.
Parker MA. 2012. Legumes select symbiosis
island sequence variants in Bradyrhizobium. – Mol. Ecol. 21:
1769-1778.
Pascal LM, Motte-Florac EF, McKey DB. 2000.
Secretory structures on the leaf rachis of Caesalpinioideae and Mimosoideae
(Leguminosae): implications for the evolution of nectary glands. – Amer. J.
Bot. 87: 327-338.
Pascal O. 1999. Two new species of
Cynometra (Leguminosae: Caesalpinioideae) from Mayotte in the Comoro
Archipelago. – Kew Bull. 54: 163-169.
Pasquet RS. 2001. Notes on the genus
Vigna (Leguminosae-Papilionoideae). – Kew Bull. 56: 223-227.
Pasquet RS. 2002. Proposal to conserve
Vigna nom. cons. (Leguminosae) against an additional name,
Cadelium. – Taxon 51: 819.
Pastore JFB. 2012. Caamembeca:
generic status and new name for Polygala subgenus Ligustrina
(Polygalaceae). – Kew Bull. 67:
435-442.
Pastore JFB, Abbott JR. 2012. Taxonomic notes
and new combinations for Asemeia (Polygalaceae). – Kew Bull. 67:
801-813.
Pastore JFB, Morães PLR de. 2013. Generic
status and lectotypifications for Gymnospora (Polygalaceae). – Novon 22:
304-306.
Pastore JFB, Silva Cardoso DBO, Aymard C GA.
2010. A synopsis, new combinations, and synonyms in Acanthocladus (Polygalaceae). – Novon 20:
317-324.
Pastore JFB, Abbott JR, Neubig KM, Whitten
WM, Mascarenhas RB, Mota MCA, Berg C van den. 2017. A molecular phylogeny and
taxonomic notes in Caamembeca (Polygalaceae). – Syst. Bot. 42:
54-62.
Pastore JFB, Morães PLR de. 2013. Generic
status and lectotypifications for Gymnospora (Polygalaceae). – Novon 22:
304-306.
Paulino JV, Groppo M, Texeira S de P. 2011.
Floral developmental morphology of three Indigofera species and its
systematic significance within Papilionoideae. – Plant Syst. Evol. 292:
165-176.
Paulino JV, Prenner G, Mansano VF, Teixeira S
de P. 2014. Comparative development of rare cases of a polycarpellate gynoecium
in an otherwise monocarpellate family, Leguminosae. – Amer. J. Bot. 101:
572-586.
Pazy B, Heyn CC, Herrnstadt I, Plitmann U.
1977. Studies in populations of the Old World Lupinus species I.
Chromosomes of the East Mediterranean lupins. – Israel J. Bot. 26:
115-127.
Péchoutre F. 1902. Contribution à
l’étude du développement de l’ovule et des graines des Rosacées. –
Ann. Sci. Nat., sér. VIII, Bot. 16: 1-158.
Pedersoli GD, Paulino JV, Leite VG, Teixeira
SP. 2010. Elucidating enigmatic floral issues in Copaifera
langsdorffii Desf. (Leguminosae, Caesalpinioideae). – Intern. J. Plant
Sci. 171: 834-846.
Pedley L. 1984. A revision of
Comesperma (Polygalaceae) in Queensland. –
Austrobaileya 2: 7-14.
Pedley L. 1986. Derivation and dispersal of
Acacia (Leguminosae), with particular reference to Australia, and the
recognition of Senegalia and Racosperma. – Bot. J. Linn.
Soc. 92: 219-254.
Pellegrin F. 1908. Recherches anatomiques sur
la classification des Genêts et des Cytises. – Ann. Sci. Nat., sér. IX, 7:
129-320.
Pellegrin F. 1943. Un genre nouveau de
Caesalpiniées du Gabon. – Boissiera 7: 296-300.
Pellegrin F. 1949. Les Légumineuses du
Gabon. – Mém. Inst. Études Centrafricaines 1: 1-284.
Peltier M. 1972. Les Sophorées de
Madagascar. – Adansonia, sér. III, 12: 137-154.
Pendry CA. 1999. A new combination of
Polygala (Polygalaceae)
for Southeast Asia. – Novon 9: 545.
Peng J, Yu J, Wang H, Guo Y, Li G, Bai G,
Chen R. 2011. Regulation of compound leaf development in Medicago
truncatula by Fused Compound Leaf1, a class M KNOX gene.
– Plant Cell 23: 3929-3943.
Pennington RT. 1995. Cladistic analysis of
chloroplast DNA restriction site characters in Andira (Leguminosae:
Dalbergieae). – Amer. J. Bot. 82: 526-534.
Pennington RT. 1996. Molecular and
morphological data provide phylogenetic resolution at different hierarchical
levels in Andira. – Syst. Biol. 45: 496-515.
Pennington RT. 2003. A monograph of
Andira. – Syst. Bot. Monogr. 64: 1-143.
Pennington RT, Aymard G, Cuello N. 1997. A
new species of Andira (Leguminosae, Papilionoideae) from the
Venezuelan Guyana. – Novon 7: 72-74.
Pennington RT, Gemeinholzer B. 2000. Cryptic
clades, fruit wall morphology and biology of Andira (Leguminosae:
Papilionoideae). – Bot. J. Linn. Soc. 134: 267-286.
Pennington RT, Lima HC de. 1995. Two new
species of Andira (Leguminosae) from Brazil and the influence of
dispersal in determining their distributions. – Kew Bull. 50: 557-566.
Pennington RT, Klitgaard BB, Ireland H, Lavin
M. 2000. New insight into floral evolution of basal Papilionoideae from
molecular phylogenis. – In: Herendeen PS, Bruneau A (eds), Advances in Legume
Systematics 9: Phylogeny, Royal Botanic Gardens, Kew, pp. 233-248.
Pennington RT, Lavin M, Ireland H, Klitgaard
B, Preston J, Hu J-M. 2001. Phylogenetic relationships of basal papilionoid
legumes based upon sequences of the chloroplast trnL intron. – Syst.
Bot. 26: 537-556.
Pennington TD. 1997. The genus Inga:
Botany. – Royal Botanic Gardens, Kew.
Pennington TD. 1999. The genus Inga:
a correction. – Kew Bull. 54: 982.
Penzig O. 1901. Beiträge zur Kenntnis der
Gattung Epirrhizanthes Bl. – Ann. Jard. Bot. Buitenzorg 17:
142-170.
Percy DM, Cronk QCB. 2002. Different fates of
island brooms: contrasting evolution in Adenocarpus, Genista,
and Teline (Genisteae, Fabaceae) in the Canary Islands and
Madeira. – Amer. J. Bot. 89: 854-864.
Persson C. 2001. Phylogenetic relationships
in Polygalaceae based on plastid
DNA sequences from the trnL-F region. – Taxon 50: 763-779.
Perveen S, Khatoon S. 1989. Chromosome
numbers in Papilionaceae from Pakistan. – Pakistan J. Bot. 21: 247-251.
Peters WS, Haffer D, Hanakam CB, Bel AJE van,
Knoblauch M. 2010. Legume phylogeny and the evolution of a unique contractile
apparatus that regulates phloem transport. – Amer. J. Bot. 97: 797-808.
Peter-Stibel E. 1938. Revision der
chinesischen Astragalus- und Oxytropis-Arten. – Acta Horti
Gothoburg. 12: 21-85.
Pettigrew CJ, Watson L. 1977. On the
classification of Caesalpinioideae. – Taxon 26: 57-64.
Pfeil BE, Tindale MD, Craven LA. 2001. A
review of the Glycine clandestina species complex (Fabaceae: Phaseoleae) reveals two new
species. – Aust. Syst. Bot. 14: 891-900.
Pfeil BE, Schlueter JA, Shoemaker RC, Doyle
JJ. 2005. Placing paleopolyploidy in relation to taxon divergence: a
phylogenetic analysis in legumes using 39 gene families. – Syst. Biol. 54:
441-454.
Pfeil BE, Craven LA, Brown AHD, Murray BG,
Doyle JJ. 2006. Three new species of northern Australian Glycine (Fabaceae, Phaseoleae), G.
gracei, G. montis-douglas and G. syndetika. – Aust.
Syst. Bot. 19: 245-258.
Pigg KB, DeVore ML, Wojciechowski MF. 2008.
Paleosecuridaca curtisii gen. et sp. nov., Securidaca-like
samaras (Polygalaceae) from the
late Paleocene of North Dakota and their significance to the divergence of
families within the Fabales. – Intern. J. Plant Sci. 169: 1304-1313.
Pijl L van der 1941. Flagelliflory and
cauliflory ad adaptions to bats in Mucuna and other plants. – Ann.
Bot. Gard. Buitenzorg 51: 63-93.
Pijl L van der. 1956. Classification of the
leguminous fruits according to their ecological and morphological properties.
– Proc. Kon. Nederl. Akad. Wet., C, 58: 301-313.
Pina FJ, Valdés B. 2009. A new species of
Lotus (Leguminosae, Loteae) from the L. angustissimus (sect.
Lotus) complex. – Syst. Bot. 34: 709-714.
Pinto RB, Torke BM, Mansano V de F. 2012.
Updates to the taxonomy of Swartzia (Leguminosae) in extra-Amazonian
Brazil, with descriptions of five new species and a regional key to the genus.
– Brittonia 64: 119-138.
Pinto RB, Lusa MG, Mansano V de F, De Azevedo
Tozzi AMG, Sampaio Mayer JL. 2018. Morphoanatomy of the leaflets of the
Hymenaea clade (Fabaceae:
Detarioideae) reveals their potential for taxonomic and phylogenetic studies.
– Bot. J. Linn. Soc. 187: 87-98.
Piper CV. 1921. Two new legumes from Mexico
and Costa Rica. – Proc. Biol. Soc. Washington 34: 41-42.
Piper CV. 1925. The American species of
Canavalia and Wenderothia. – Contr. U.S. Natl. Herb. 20:
555-588.
Piper CV. 1926. Studies in American
Phaseolinae. – Contr. U. S. Natl. Herb. 22: 663-701.
Pirani A, Zarre SH, Tillich H-J, Podlech D,
Niknam V. 2006. Spine anatomy and its systematic application in
Astragalus sect. Rhacophorus s.l. (Fabaceae) in Iran. – Flora 201:
240-247.
Pirie MD, Klitgaard BB, Pennington RT. 2009.
Revision and biogeography of Centrolobium
(Leguminosae-Papilionoideae). – Syst. Bot. 34: 345-359.
Pittier H. 1916. Caesalpiniaceae. The genera
Brownea and Browneopsis as represented in Panama, Colombia
and Venezuela. Need of a new treatment. – Contr. U. S. Natl. Herb. 18:
145-157.
Pittier H. 1922. On the species of
Dalbergia of Mexico and Central America. – J. Washington Acad. Sci.
12: 54-64.
Planchuelo Ravelo AM. 1984. Taxonomic studies
of Lupinus in South America. – In: Proceedings of the 3rd
International Lupine Congress, La Rochelle, France, pp. 40-54.
Planchuelo Ravelo AM, Dunn DB. 1984. The
simple leaved lupines and their relatives in Argentina. – Ann. Missouri Bot.
Gard. 71: 92-103.
Playford J. 1997. The 5SDNA units of
Acacia species. – Bull. Intern. Group for the Study of the
Mimosoideae 20: 19-21.
Plitman U. 1981. Evolutionary history of Old
World lupines. – Taxon 30: 430-437.
Plitman U, Pazy B. 1984. Cytogeographical
distribution of Old World Lupinus. – Webbia 38: 531-539.
Podlech D. 1982. Neue Aspekte zur Evolution
und Gliederung der Gattung Astragalus L. – Mitt. Bot. Staatssamml.
München 18: 359-378.
Podlech D. 1983. Zur Taxonomie und
Nomenklatur der tragacanthoiden Astragali. – Mitt. Bot. Staatssaml.
München 19: 1-23.
Podlech D. 1988. Revision von
Astragalus L. sect. Caprini DC. (Leguminosae). – Mitt. Bot.
Staatssamml. München 25: 1-924.
Podlech D. 1991. The systematics of the
annual species of the genus Astragalus L. (Leguminosae). – Flora et
Vegetatio Mundi IX: 1-8.
Podlech D. 1994. Revision der altweltlichen
annuellen Arten der Gattung Astragalus L. (Leguminosae). – Sendtnera
2: 39-170.
Podlech D. 2008. The genus
Astragalus L. (Fabaceae) in
Europe with exclusion of the former Soviet Union. – Feddes Repert. 119:
310-387.
Podlech D. 2009. Some new species and
combinations in Astragalus and Phyllolobium (Fabaceae). – Feddes Repert. 120:
48-58.
Podlech D, Ekici M. 2008. Some new and
interesting Astragalus species (Fabaceae) from Turkey. – Feddes
Repert. 119: 24-36.
Podlech D, Maassoumi AA. 2003. New species of
Astragalus L. (Fabaceae)
from Iran, mainly of sects. Incani and Malacothrix. –
Feddes Repert. 114: 320-349.
Podlech D, Sytin A. 2002. New species of
Astragalus L. (Leguminosae) sect. Hololeuce,
Onobrychoidei, Ornithopodium and Synochreati and a
new section Baldaccia. – Sendtnera 8: 155-166.
Podlech D, Zarre S. 2003. New species of
Astragalus sect. Ammodendron (Fabaceae) with a key for the species of
the Flora Iranica area. – Willdenowia 33: 341-352.
Poggio L, Espert SM, Fortunato RH. 2008.
Citogenética evolutiva en Leguminosas Americanas. – Rodriguésia 59:
423-433.
Polhill RM. 1968a. Argyrolobium
(Leguminosae) in tropical Africa. – Kew Bull. 22: 145-168.
Polhill RM. 1968b. Miscellaneous notes on
African species of Crotalaria L. 2. – Kew Bull. 22: 169-348.
Polhill RM. 1969. Notes on East African
Dalbergieae Bronn (Leguminosae). – Kew Bull. 23: 483-490.
Polhill RM. 1971. Some observations on
generic limits in Dalbergieae-Lonchocarpinae Benth. (Leguminosae). – Kew
Bull. 25: 259-273.
Polhill RM. 1974. A revision of
Pearsonia (Leguminosae-Papilionoideae). – Kew Bull. 29: 383-410.
Polhill RM. 1976. Genisteae (Adans.) Benth.
and related tribes (Leguminosae). – Bot. Syst. 1: 143-368.
Polhill RM. 1981a. Papilionoideae. – In:
Polhill PM, Raven PH (eds), Advances in legume systematics 1, Royal Botanic
Gardens, Kew, pp. 191-208.
Polhill RM. 1981b. Sophoreae. – In: Polhill
RN, Raven PH (eds), Advances in legume systematics 1, Royal Botanic Gardens,
Kew, pp. 213-230.
Polhill RM. 1981c. Dipterygeae. – In:
Polhill RN, Raven PH (eds), Advances in legume systematics 1, Royal Botanic
Gardens, Kew, pp. 231-232.
Polhill RM. 1981d. Dalbergieae. – In:
Polhill RM, Raven PH (eds), Advances in legume systematics 1, Royal Botanic
Gardens, Kew, pp. 233-242.
Polhill RM. 1981e. Abreae. – In: Polhill
RM, Raven PH (eds), Advances in legume systematics 1, Royal Botanic Gardens,
Kew, pp. 243-244.
Polhill RM. 1981f. Amorpheae. – In: Polhill
RN, Raven PH (eds), Advances in legume systematics 1, Royal Botanic Gardens,
Kew, pp. 244-246.
Polhill RM. 1981g. Adesmieae. – In: Polhill
RM, Raven PH (eds), Advances in legume systematics 1, Royal Botanic Gardens,
Kew, pp. 355-356.
Polhill RM. 1981h. Galegeae. – In: Polhill
RM, Raven PH (eds), Advances in legume systematics 1, Royal Botanic Gardens,
Kew, pp. 357-363.
Polhill RM. 1981i. Carmichaelieae. – In:
Polhill RM, Raven PH (eds), Advances in legume systematics 1, Royal Botanic
Gardens, Kew, pp. 364-366.
Polhill RM. 1981j. Loteae and Coronilleae.
– In: Polhill RM, Raven PH (eds), Advances in legume systematics 1, Royal
Botanic Gardens, Kew, pp. 371-375.
Polhill RM. 1981k. Liparieae. – In: Polhill
RM, Raven PH (eds), Advances in legume systematics 1, Royal Botanic Gardens,
Kew, pp. 398.
Polhill RM. 1982. Crotalaria in
Africa and Madagascar. – A. A. Balkema, Rotterdam.
Polhill RM. 1986. Notes on
Indigofera (Leguminosae) for ‘Flore des Mascareignes’. – Kew
Bull. 41: 343-346.
Polhill RM. 1994a. Classification of the
Leguminosae and complete synopsis of legume genera. – In: Bisby FA,
Buckingham J, Harborne JB (eds), Phytochemical dictionary of the Leguminosae,
Chapman and Hall, New York, pp. xxxv-lvii.
Polhill RM. 1994b. Complete synopsis of
legume genera. – In: Bisby FA, Buckingham J, Harborne JB (eds), Phytochemical
dictionary of the Leguminosae, Chapman and Hall, New York, pp. xlix-liv.
Polhill RM. 2003. Two new species of
Crotalaria (Fabaceae) from
metalliferous sites in Zimbabwe. – Syst. Geogr. Plants 73: 285-288.
Polhill RM, Raven PH (eds). 1981. Advances in
legume systematics 1-2. – Royal Botanic Gardens, Kew.
Polhill RM, Verdcourt B. 2002. Proposal to
change the authorship of Eriosema, nom. cons.
(Leguminosae-Papilionoideae) and to delete Euriosma Desv., nom. rej.
– Taxon 51: 817-818.
Polhill RM, Vidal JE. 1981. Caesalpinieae.
– In: Polhill RM, Raven PH (eds), Advances in legume systematics 1, Royal
Botanic Gardens, Kew, pp. 81-96.
Polhill RM, Bisby FA, Cristofolini G, Frodin
DG, Meikle RD. 1978. Proposal to conserve and retypify the name
Cytisus (Leguminosae). – Taxon 27: 556-559.
Polhill RM, Raven PH, Stirton CH. 1981.
Evolution and systematics of the Leguminosae. – In: Polhill PM, Raven PH
(eds), Advances in legume systematics 1, Royal Botanic Gardens, Kew, pp.
1-26.
Poncy O. 1985. Studies on the flora of the
Guianas 13. Le genre Inga (Légumineuses, Mimosoideae) en Guyane
Française. Systématique, morphologie des formes juvéniles, écologie. –
Mém. Mus. Natl. Hist. Nat. Paris, sect. B, Bot. 31.
Poncy O. 2007. A new species of Inga
Mill. (Fabaceae, Mimosoideae) from
the Guianas. – Adansonia, sér. III, 29: 249-254.
Poole MM. 1979. Pollen morphology of
Psophocarpus (Leguminosae) in relation to its taxonomy. – Kew Bull.
34: 211-220.
Potokina E, Tomooka N, Vaughan DA,
Alexandrova T, Xu R-Q. 1999. Phylogeny of Vicia subgenus
Vicia (Fabaceae) based on
analysis of RAPDs and RFLP of PCR-amplified chloroplast genes. – Genet. Res.
Crop Evol. 46: 149-161.
Potter D, Doyle JJ. 1994. Phylogeny and
systematics of Sphenostylis and Nephostylis (Leguminosae:
Phaseoleae) based on morphological and chloroplast DNA data. – Syst. Bot. 19:
389-406.
Povydysh MN, Goncharov MY, Yakovlev GP. 2011.
On the genus Uleanthus (Fabaceae). – Bot. Žurn. 96:
423-432.
Prain D. 1904. The species of
Dalbergia of south-eastern Asia. – Ann. Roy. Bot. Gard. Calcutta 10:
1-114.
Prakash N. 1987. Embryology of the
Leguminosae. – In: Stirton CH (ed), Advances in legume systematics 3, Royal
Botanic Gardens, Kew, pp. 241-262.
Prakash N, Chan Y-Y. 1976. Embryology of
Glycine max. – Phytomorphology 25: 302-309.
Prakash S. 1960. The endosperm of Arachis
hypogaea Linn. – Phytomorphology 10: 60-64.
Prance GT. 1965. The systematic position of
Stylobasium Desf. – Bull. Jard. Bot. État Bruxelles 35: 435-448.
Prenner G. 2004a. New aspects in floral
development of Papilionoideae: initiated but suppressed bracteoles and variable
initiation of sepals. – Ann. Bot. 93: 537-545.
Prenner G. 2004b. Floral development in
Polygala myrtifolia (Polygalaceae) and its similarities
with Leguminosae. – Plant Syst. Evol. 249: 67-76.
Prenner G. 2004c. Floral ontogeny in
Calliandra angustifolia (Leguminosae: Mimosoideae: Ingeae) and its
systematic implications. – Intern. J. Plant Sci. 165: 417-426.
Prenner G. 2004d. The asymmetric andreoecium
in Papilionoideae (Leguminosae): definition, occurrence, and possible
systematic value. – Intern. J. Plant Sci. 165: 499-510.
Prenner G. 2004e. Floral development in
Daviesia cordata (Leguminosae: Papilionoideae: Mirbelieae) and its
systematic implications. – Aust. J. Bot. 52: 285-291.
Prenner G. 2011. Floral ontogeny of
Acacia celastrifolia: an enigmatic mimosoid legume with pronounced
polyandry and multiple carpels. – In: Wanntorp L, Ronse de Craene LP (eds),
Flowers on the Tree of Life, Systematics Association Spec. Vol. 80, Cambridge
University Press, Cambridge, pp. 256-278.
Prenner G. 2013a. Papilionoid inflorescences
revisited (Leguminosae-Papilionoideae). – Ann. Bot. 112: 1567-1576.
Prenner G. 2013b. Flower development in
Abrus precatorius (Leguminosae: Papilionoideae: Abreae) and a review
of androecial characters in Papilionoideae. – South Afr. J. Bot. 89:
210-218.
Prenner G, Klitgaard BB. 2008. Towards
unlocking the deep nodes of Leguminosae: floral development and morphology of
the enigmatic Duparquetia orchidacea (Leguminosae, Caesalpinioideae).
– Amer. J. Bot. 95: 1349-1365.
Pretel A, Sañudo A. 1978. Estudios
cariologicos en especies españolas del género Astragalus L. I.
Numero y comportamiento de los cromosomas durante la meiosis. – Lagascalia 8:
25-38.
Pritchard AJ. 1962. Number and morphology of
chromosomes in African species in the genus Trifolium L. – Aust. J.
Agric. Res. 13: 1023-1029.
Pryor BM, Creamer R, Shoemaker RA,
McLain-Romero J, Hambleton S. 2009. Undifilum, a new genus for
endophytic Embellisia oxytropis and parasitic Helmintosporium
bornmuelleri on legumes. – Botany 87: 178-194.
Pueyo JJ, Delgado-Salinas A. 1997. Presence
of alfa-amylase inhibitor in some members of the subtribe Phaseolinae
(Phaseoleae: Fabaceae). – Amer. J.
Bot. 84: 79-84.
Qian ZG. 1998. A revision of the genus
Chesneya from East Himalayas. – Acta Bot. Yunn. 20: 399-402.
Queiroz LP de. 2008a. Re-establishment,
synopsis and new combinations in the genus Bionia Mart. Ex Benth.
(Leguminosae: Papilionoideae). – Neodiversity 3: 13-18.
Queiroz LP de 2008b. Leguminosas da Caatinga.
– Universidad Estadual de Feira de Santana, Feira de Santana, Brazil.
Queiroz LP de, Cardoso DBOS. 2008. A new
species of Aeschynomene L. (Leguminosae, Papilionoideae) from a
continental sand dune area in north-eastern Brazil. – Bot. J. Linn. Soc. 157:
749-753.
Queiroz LP de, Lavin M. 2011.
Coursetia (Leguminosae) from Eastern Brazil: nuclear ribosomal and
chloroplast DNA sequence analysis reveal the monophyly of three
Caatinga-inhabiting species. – Syst. Bot. 36: 69-79.
Queiroz LP de, Lewis GP, Allkin R. 1999. A
revision of the genus Moldenhawera Schrad.
(Leguminosae-Caesalpinioideae). – Kew Bull. 54: 817-852.
Queiroz LP de, Fortunato RH, Giulietti AM.
2003. Phylogeny of the subtribe Diocleinae (Leguminosae: Papilionoideae) based
on morphological characters. – In: Klitgaard BB, Bruneau A (eds), Advances in
legume systematics 10: Higher level systematics, Royal Botanic Gardens, Kew,
pp. 105-128.
Queiroz LP de, Lewis GP, Wojciechowski MF.
2010. Tabaroa, a new genus of Leguminosae tribe Brongniartieae from
Brazil. – Kew Bull. 65: 189-203.
Queiroz LP de, Pastore JFB, Cardoso D, Snak
C, Lima AL de C, Gagnon E, Vatanparast M, Holland AE, Egan AN. 2015. A
multilocus phylogenetic analysis reveals the monophyly of a recircumscribed
papilionoid legume tribe Diocleae with well-supported generic relationships.
– Mol. Phylogen. Evol. 90: 1-19.
Queiroz LP de, São-Mateus W, Delgado-Salinas
A, Torke BM, Lewis GP, Dorado Ó, Ardley JK, Wojciechowski MF, Cardoso D. 2017.
A molecular phylogeny reveals the Cuban enigmatic genus Behaimia as a
new piece in the Brongniartieae puzzle of papilionoid legumes. – Mol.
Phylogen. Evol. 109: 191-202.
Queiroz R, Goulart de Azevedo Tozzi A, Lewis
G. 2013. Seed morphology: an addition to the taxonomy of Tephrosia
(Leguminosae, Papilionoideae, Millettieae) from South America. – Plant Syst.
Evol. 299: 459-470.
Quirk JT, Miller RB. 1983. Nonvestured pits
in Koompassia Maingay (Leguminosae). – IAWA Bull., n.s., 4: 191-195.
Raabe U, Tan K, Iatroú G, Vold G, Parolly G.
2009. Polygala rausiana (Polygalaceae), a new species from
the northern Peloponnese, Greece. – Willdenowia 39: 69-75.
Radcliffe-Smith A. 1989. Notes on Madagascan
Euphorbiaceae II.
Claoxylon sect. Luteobrunnea. – Kew Bull. 44: 333-340.
Radosavljevic A, Mackinder BA, Herendeen PS.
2017. Phylogeny of the detarioid legume genera Cynometra and
Maniltoa (Leguminosae). – Syst. Bot. 42: 670-679.
Radzhi AD. 1971a. Conspectus systematis
specierum caucasicarum generis Vicia L. – Nov. Syst. Plant. Vasc. 7:
228-240. [In Russian]
Radzhi AD. 1971b. Evolution of the genera of
the tribe Vicieae Adans. – Bot. Žurn. 56: 978-981. [In Russian]
Radzhi AD. 1972. De genere Vicia L.
Notula systematica. – Nov. Syst. Plant. Vasc. 9: 215-223. [In Russian]
Ralphs MH, Creamer R, Baucom D, Gardner DR,
Welsh SL, Graham JD, Hart C, Cook D, Stegelmeier BL. 2008. Relationship between
the endophyte Embellisia spp. and the toxic alkaloid swainsonine in
major locoweed species (Astragalus and Oxytropis). – J.
Chem. Ecol. 34: 32-38.
Ramdhani S, Behenna M, Cowling RM, Barker NP.
2008. Phylogenetic studies in southern African thicket: Schotia Jacq.
(Fabaceae). – South Afr. J. Bot.
74: 376.
Ramdhani S, Cowling RM, Barker NP. 2010.
Phylogeography of Schotia (Fabaceae): recent evolutionary processes
in an ancient thicket biome lineage. – Intern. J. Plant Sci. 171: 626-640.
Ramírez N. 1995. Revisión taxonómica del
género Alexa Moq. (Fabaceae, Sophoreae). – Ann. Missouri
Bot. Gard. 82: 549-569.
Ramírez-De Anda MP, Colín RT. 2007.
Revisión taxonómica del complejo Bauhinia macranthera (Fabaceae: Caesalpinioideae: Cercideae),
un grupo endémico del centro y noreste de México. – Brittonia 59:
357-369.
Ramirez-Domenech J, Tucker SC. 1988. Patterns
of organ development in mimosoid legume flowers. – In: Leins P, Tucker SC,
Endress PK (eds), Aspects of floral development, Berlin and Stuttgart, pp.
171-180.
Ramírez-Domenech J, Tucker SC. 1990.
Comparative ontogeny of the perianth in mimosoid legumes. – Amer. J. Bot. 77:
624-635.
Ramos G, Lima HC de, Prenner G, Queroz LP de,
Zartman CE, Cardoso D. 2016. Molecular systematics of the Amazonian genus
Aldina, a phylogenetically enigmatic ectomycorrhizal lineage of
papilionoid legumes. – Mol. Phylogen. Evol. 97: 11-18.
Ramp E. 1988. Struktur, Funktion und
systematische Bedeutung des Gynoeciums bei den Rutaceae und Simaroubaceae. – Ph.D.
diss., Universität Zürich, Switzerland.
Ranabahu P, Harborne J. 1993. The flavonoids
of the genus Lathyrus and a comparison of flavonoid pattern within the
tribe Vicieae. – Biochem. Syst. Ecol. 21: 715-722.
Randell BR. 1988. Revision of the Cassiinae
in Australia I. Senna Mill. sect. Chamaefistula (Colladon)
Irwin and Barneby. – J. Adelaide Bot. Gard. 11: 19-49.
Randell BR. 1989. Revision of the Cassiinae
in Australia I. Senna Mill. sect. Psilorhegma (J. Vogel)
Irwin and Barneby. – J. Adelaide Bot. Gard. 12: 165-272.
Randell BR. 1990. Revision of the Cassiinae
in Australia I. Senna Mill. sect. Senna. – J. Adelaide Bot.
Gard. 13: 1-16.
Rando JG, Zuntini AR, Conceição AS, Berg C
van den, Pirani JR, Queiroz LP de. 2016. Phylogeny of Chamaecrista
ser. Coriaceae (Leguminosae) unveils a lineage recently diversified in
Brazilian Campo Rupestre vegetation. – Intern. J. Plant Sci. 177: 3-17.
Ranjbar M. 2009. Onobrychis
oshnaviyehensis sp. nov. (sect. Hymenobrychis, Fabaceae) from Iran. – Nord. J. Bot.
27: 115-119.
Ranjbar M, Karamian R. 2002.
Astragalus sect. Astragalus (Fabaceae) in Iran, complementary notes
with a key to the species. – Nord. J. Bot. 22: 177-181.
Ranjbar M, Karamian R. 2003a. Astragalus
neo-assadianus (Fabaceae), a new
species in sect. Alopecuroidei from Iran. – Bot. J. Linn. Soc. 143:
197-200.
Ranjbar M, Karamian R. 2003b. Some remarks on
the genus Astragalus sect. Incani in Iran. – Bot. J. Linn.
Soc. 143: 443-447.
Ranjbar M, Karamian R. 2003c. Caraganeae, a
new tribe with notes on the genus Chesneya Lindl. ex Endl. (Fabaceae)
from flora of Iran. – Thaiszia J. Bot. 13: 67-75.
Ranjbar M, Maassoumi A.-A. 1998. New species
and new records of the genus Astragalus L. (Leguminosae) from Iran.
– Iran. J. Bot. 7: 235-238.
Ranjbar M, Maassoumi A-A, Podlech D. 2002.
Astragalus sect. Alopecuroidei (Fabaceae) in Iran, complementary notes
with a key to the species. – Willdenowia 32: 85-91.
Ranjbar M, Amirabadizadeh H, Karamian R,
Ghahremani MA. 2004. Notes on Onobrychis sect. Heliobrychis
(Fabaceae) in Iran. – Willdenowia
34: 187-190.
Ranjbar M, Rahiminejad M-R, Assadi M. 2005. A
contribution to Astragalus sect. Incani (Fabaceae) in Iran. – Willdenowia 35:
117-124.
Ranjbar M, Rahiminejad M-R, Maassoumi A-A.
2007. Notes on the short-stemmed species of Astragalus sect.
Onobrychoidei (Fabaceae) in
Iran. – Willdenowia 37: 305-312.
Ranjbar M, Karamian R, Olanj N. 2007. A new
species of Hedysarum (Fabaceae) in Iran and other new
Hedysarum records. – Bot. J. Linn. Soc. 155: 505-512.
Ranjbar M, Karamian R, Olanj N, Johartchi MR.
2008. A key and four new species of Hedysarum (Fabaceae) in Iran. – Nord. J. Bot. 26:
10-20.
Ranjbar M, Karamian R, Hadadi A, Joharchi M.
2012. Taxonomic notes on Onobrychis sect. Onobrychis subsect.
Macropterae (Fabaceae) from
Iran. – Phytotaxa 39: 51-60.
Ranjbar M, Hajmoradi F, Waycott M, Van Dijk
KJ. 2014. A phylogeny of the tribe Caraganeae (Fabaceae) based on DNA sequence data
from ITS. – Feddes Repert. 125: 1-7.
Rankin-Rodríguez R, Greuter W. 2001.
Humboldt, Willdenow, and Polygala (Polygalaceae). – Taxon 50:
1231-1247.
Rao AN. 1964. An embryological study of
Salomonia contoniensis Lour. – New Phytol. 63: 281-288.
Rao CK. 1983. Distribution of canavanine in
some Indian Galegeae (Fabaceae) and
its systematic significance. – Curr. Sci. 52: 824-825.
Rao VS, Sirdeshmukh K, Sardar MG. 1958. The
floral anatomy of the Leguminosae. – J. Univ. Bombay, N. S., 26(5B):
65-138.
Rath M, Weber HC, Imhof S. 2013.
Morpho-anatomical and molecular characterization of the mycorrhizas of European
Polygala species. – Plant Biol. 15: 548-557.
Rau MA. 1940. An embryological study of
Suriana maritima L. – Proc. Indian Acad. Sci. 11: 100-106.
Rau MA. 1953. Some observations on the
endosperm in Papilionaceae. – Phytomorphology 3: 209-222.
Ravi V, Khurana JP, Tyagi AK, Khurana P.
2007. Rosales sister to Fabales:
towards resolving the rosid puzzle. – Mol. Phylogen. Evol. 44: 488-493.
Rechinger KH, Dulfer H, Patzak A. 1958.
Širjaevii fragmenta astragalogica IV. Astragalus sect.
Onobrychium. – Österr. Akad. Wiss., Math.-Naturwiss. Kl.
Sitzungsber., Abt. I, Biol. 167: 321-361.
Rechinger KH, Dulfer H, Patzak A. 1961.
Širjaevii fragmenta astragalogica XV. Astragalus sect.
Ammodendron. – Sitzungsber. Österr. Akad. Wiss., Math.-naturw. Kl.,
Abt. I, 170: 35-53.
Redden KM, Herendeen PS. 2006. Morphology and
phylogenetic analysis of Paloue and related genera in the
Brownea clade (Detarieae, Caesalpinioideae). – Intern. J. Plant Sci.
167: 1229-1246.
Redden KM, Herendeen PS, Wurdack KJ, Bruneau
A. 2010. Phylogenetic relationships of the northeastern South American
Brownea clade of tribe Detarieae (Leguminosae: Caesalpinioideae) based
on morphology and molecular data. – Syst. Bot. 35: 524-533.
http://dx.doi.org/10.1600/036364410792495863
Ree RH, Citerne HL, Lavin M, Cronk QCB. 2004.
Heterogeneous selection on LEGCYC paralogs in relation to flower
morphology and the phylogeny of Lupinus (Leguminosae). – Mol. Biol.
Evol. 21: 321-331.
Reer U, Podlech D. 1986. Die europäischen
Vertreter ger Gattung Astracantha Podl. (Leguminosae). – Mitt. Bot.
Staatssamml. München 22: 513-569.
Rees H, Hazarika M. 1969. Chromosome
evolution in Lathyrus. – Chrom. Tod. 2: 157-165.
Renvoize SA. 1981. The genus
Calliandra (Leguminosae) in Bahia, Brazil. – Kew Bull. 36: 63-83.
Riahi M, Zarre S, Chehregani A,
Shahsavan-Behboudi B. 2003. Seed development in two species of medifixed hairy
Astragalus (Fabaceae). –
Flora 198: 211-219.
Riahi M, Zarre S, Maassoumi AA, Kazempour
Osaloo S, Wojciechowski MF. 2011. Towards a phylogeny for Astragalus
section Caprini (Fabaceae)
and its allies based on nuclear and plastid DNA sequences. – Plant Syst.
Evol. 293: 119-133.
Ribeiro RA, Lavin M, Lemos-Filho JP,
Mendonça Filho CV, Santos FR dos, Lovato MB. 2007. The genus
Machaerium (Leguminosae) is more closely related to
Aeschyomene sect. Ochopodium than to Dalbergia:
inferences from combined sequence data. – Syst. Bot. 32: 762-771.
Ribeiro PG, Luckow M, Lewis GP, Simon MF,
Cardoso D, Souza ÉR de, Silva APC, Jesus MC, Santos FAR dos, Azevedo V,
Queiroz LP de. 2018. Lachesiodendron, a new monospecific genus
segregated from Piptadenia (Leguminosae: Caesalpinioideae: mimosoid
clade): evidence from morphology and molecules. – Taxon 67: 37-54.
Ricker M, Veen G, Daly DC, Wltte L, Sinta-V
M, Chota-I J, Czygan F-C. 1994. Alkaloid diversity in eleven species of
Ormosia and in Clathrotropis macrocarpa
(Leguminosae-Papilionoideae). – Brittonia 46: 355-371.
Ricker M, Daly DC, Eugene GV, Robbins F, Sint
V M, Hota I J, Franz-C C, Kinghorn AD. 1999. Distribution of quinolizidine
alkaloid types in nine Ormosia species (Leguminosae-Papilionoideae).
– Brittonia 51: 34-43.
Rico-Arce M de L. 1991. New species,
combinations and synonyms for Zygia, Cojoba,
Marmaroxylon and Pithecellobium (Leguminosae: Mimosoideae,
Ingeae). – Kew Bull. 46: 493-521.
Rico-Arce M de L. 1992. New chromosome counts
in Neotropical Albizia, Havardia and Pithecellobium
and a new combination for Albizia (Leguminosae-Mimosoideae-Ingeae).
– Bot. J. Linn. Soc. 108: 269-274.
Rico-Arce M de L. 1994. Four new species of
Zygia (Leguminosae: Mimosoideae). – Kew Bull. 49: 547-554.
Rico-Arce M de L. 1995. Note on Neotropical
Acacia (Leguminosae: Mimosoideae). – Kew Bull. 50: 178.
Rico-Arce M de L. 1999. New combinations in
Mimosaceae. – Novon 9: 554-556.
Rico-Arce M de L. 2003. Geographical patterns
in neotropical Acacia (Leguminosae: Mimosoideae). – Aust. Syst. Bot.
16: 41-48.
Rico-Arce M de L, Bachman S. 2006. A
taxonomic revision of Acaciella (Leguminosae, Mimosoideae). – An.
Jard. Bot. Madrid 63: 189-244.
Rico-Arce M de L, Banks H. 2001. A
preliminary survey of pollen and other morphological characters in neotropical
Acacia subgenus Aculeiferum (Leguminosae: Mimosoideae). –
Bot. J. Linn. Soc. 135: 263-270.
Rico-Arce M de L, Sousa S M, Fuentes S S.
1999. Guinetia: a new genus in the tribe Ingeae (Leguminosae:
Mimosoideae) from Mexico. – Kew Bull. 54: 975-981.
Ridder-Numan JWA. 1995. Phylogeny and
biogeography of Spatholobus, Butea, Meizotropis and
Kunstleria (Leguminosae-Papilionoideae). – In: Crisp M, Doyle JJ
(eds), Advances in legume systematics 7: Phylogeny, Royal Botanic Gardens, Kew,
pp. 133-139.
Ridder-Numan JWA. 1996. Phylogeny and
biogeography of Spatholobus and allies (Leguminosae-Papilionoideae):
just another case history. – Aust. Syst. Bot. 9: 127-132.
Ridder-Numan JWA, Kornet DJ. 1994. A revision
of the genus Kunstleria (Legumonosae-Papilionoideae). – Blumea 38:
465-485.
Riggins R. 1988. A comparison of the North
and South American Lupinus group Microcarpi (Leguminosae).
– Madroño 35: 92-104.
Rikli M. 1901. Die Gattung
Dorycnium. – Engl. Bot. Jahrb. Syst. 31: 314-404.
Riley-Hulting E, Delgado-Salinas A, Lavin M.
2004. Phylogenetic systematics of Strophostyles (Fabaceae): a North American temperate
genus within a neotropical diversification. – Syst. Bot. 29: 627-653.
Rivals P. 1953. Sur quelques légumineuses
géocarpes et amphicarpes. – Rev. Intern. Bot. Appl. 33: 244-249.
Rivers MC, Brummitt NA, Nic Lughadha E,
Meagher TR. 2011. Genetic variation in Delonix s.l. (Leguminosae) in
Madagascar revealed by AFLPs: fragmentation, conservation status and taxonomy.
– Conserv. Genet. 12: 1333-1344.
Rizzi Longo L, Feoli Chiapella L. 1994.
Contribution to the systematics of Genista L. sect.
Spartocarpus Spach (Genisteae, Fabaceae) with emphasis of palynological
data. – Studia Geobot. 14. 41-62.
Rizzini CT, Heringer EP. 1987. As espécies
anãs de Stryphnodendron Mart. (Leguminosae-Mimosoideae). – Rev.
Bras. Biol. 47: 447-454.
Rizzini CT, Mattos Filho A. 1977. Sobre
Luetzelburgia Harms (Leg.). – Rodriguésia 29: 7-24.
Robbertse PJ. 1974. The genus Acacia
in South Africa II. With special reference to the morphology of the flower and
inflorescence. – Phytomorphology 24: 1-15.
Robbertse PJ, Schijff HP van der. 1971. The
genus Acacia Miller in South Africa. – Mitt. Bot. Staatssamml.
München 10: 170-177.
Robeson DJ, Harborne JB. 1980. A chemical
dichotomy in phytoalexin induction within the tribe Vicieae of the Leguminosae.
– Phytochemistry 19: 2359-2365.
Robinson J. 1996. A cpDNA phylogeny of the
genus Acacia and related genera in the Mimosoideae. – Ph.D. diss.,
University of St. Andrews, England.
Robinson J, Harris SA. 2000. A plastid DNA
phylogeny of the genus Acacia Miller (Acacieae, Leguminosae). – Bot.
J. Linn. Soc. 132: 195-222.
Rodrigue A. 1893. Structure du tégument
séminal des Polygalacées. – Bull. Herb. Boissier 1: 450-463, 513-541,
571-583.
Rodrigues RS. 2009. Novos sinônimos e
lectotipificação em Sweetia Spreng. (Leguminosae, Papilionoideae).
– Rev. Brasil, Bioci. 7: 134-137.
Rodrigues RS, de Azevedo Tozzi AMG. 2006.
Guianodendron, a new genus of Leguminosae from South America. –
Novon 16: 129-132.
Rodrigues RS, de Azevedo Tozzi AMG. 2007.
Morphological analysis and re-examination of the taxonomic circumscription of
Acosmium (Leguminosae, Papilionoideae, Sophoreae). – Taxon 56:
439-452.
Rodrigues RS, de Azevedo Tozzi AMG. 2008a.
Systematic relevance of seedling morphology in Acosmium,
Guianodendron, and Leptolobium (Leguminosae, Papilionoideae).
– Brittonia 60: 287-296.
Rodrigues RS, de Azevedo Tozzi AMG. 2008b.
Reinstatement of the name Leptolobium Vogel (Leguminosae,
Papilionoideae, Sophoreae). – Taxon 57: 980-984.
Rodrigues RS, de Azevedo Tozzi AMG. 2009.
Revisão taxonômica de Acosmium Schott (Leguminosae, Papilionoideae,
Sophoreae). – Acta Bot. Brasil. 23: 164-174.
Rodrigues RS, de Azevedo Tozzi AMG. 2010. Two
new species of Leptolobium (Leguminosae, Papilionoideae) from Brazil.
– Brittonia 62: 20-25.
Rodrigues RS, de Azevedo Tozzi AMG. 2012.
Revisão taxonômica de Leptolobium (Papilionoideae, Legminosae). –
Acta Bot. Brasil. 26: 146-164.
Rodrigues TM, Machado SR. 2007. The pulvinus
endodermal cells and their relation to leaf movement in legumes of the
Brazilian cerrado. – Plant Biol. 9: 469-477.
Rodriguez-Pontes M. 2007. Development of
megagametophyte, embryo, and seed in Senna corymbosa (Lam.) H. S.
Irwin & Barneby (Leguminosae-Caesalpinioideae). – Bot. J. Linn. Soc. 153:
169-179.
Rodriguez-Pontes M. 2009. Seed formation in
two species of Adesmia (Fabaceae): co-occurrence of micropylar
and lateral endosperm haustoria in legumes and its taxonomic value. – Bot. J.
Linn. Soc. 158: 602-612.
Roig FA. 1986. The wood of Adesmia
horrida and its modifications by climatic conditions. – IAWA Bull. 7:
129-135.
Rojo JP. 1972. Pterocarpus
(Leguminosae-Papilionaceae) revised for the world. – J. Cramer, Lehre.
Rollins RC. 1940. Studies in the genus
Hedysarum in North America. – Rhodora 42: 217-239.
Rosato M, Castro M, Rosselló JA. 2008.
Relationships of the woody Medicago species (section
Dendrotelis) assessed by molecular cytogenetic analyses. – Ann. Bot.
102: 15-22.
Rose RJ, Schlarbaum SE, Small E, Johnson LB.
1988. Chloroplast genomic variation and phylogeny in Medicago section
Intertextae. – Can. J. Bot. 66: 1352-1358.
Roskov YR. 1990. The new species and the new
nomenclature combinations in the genera Lupinaster,
Chrysaspis, Trifolium and Amoria (Fabaceae). – Bot. Žurn. 75:
715-720.
Roskov YR, Bisby FA, Zarucchi JL, Schrire BD,
White RJ (eds). 2005. ILDIS world database of legumes: draft checklist, version
10 (November 2005). http://www.ildis.org/International Legume Database &
Information Service, Reading, United Kingdom.
Ross JH. 1974a. Notes on Acacia
species in Southern Africa: IV. – Bothalia 11: 231-234.
Ross JH. 1974b. Notes on Acacia
species from north-east tropical Africa. – Bothalia 11: 299-303.
Ross HJ. 1975. The Acacia senegal
complex. – Bothalia 11: 453-462.
Ross JH. 1979. A conspectus of the African
Acacia species. – Mem. Bot. Survey South Afr. 44: 1-155.
Ross JH. 1981. An analysis of the African
Acacia species: their distribution, possible origins and
relationships. – Bothalia 13: 389-413.
Ross JH. 2001. Two new endemic Australian
genera in the tribe Brongniartieae (Fabaceae) to accommodate two species
formerly included in Templetonia R. Br. – Muelleria 15: 7-14.
Ross JH. 2004. Proposal to conserve the name
Bossiaea against Platylobium (Leguminosae). – Taxon 53:
1075-1076.
Ross JH, Crisp MD. 2005a. Tribe
Brongniartieae. – In: Lewis GP, Schrire BD, Mackinder B, Lock JM (eds),
Legumes of the World, Royal Botanic Gardens, Kew, pp. 252-259.
Ross JH, Crisp MD. 2005b. Tribe Bossiaeeae.
– In: Lewis GP, Schrire BD, Mackinder B, Lock JM (eds), Legumes of the World,
Royal Botanic Gardens, Kew, pp. 354-359.
Rothmaler W. 1941. Revision der Genisteen I.
Monographien der Gattungen um Ulex. – Bot. Jahrb. Syst. 72:
69-116.
Rothmaler W. 1944. Die Gliederung der Gattung
Cytisus L. – Feddes Rep. 53: 137-150.
Roti-Michelozzi G, Serrato-Valenti G. 1989.
Seed characteristics in Italian species of genus Vicia section
Ervum and their diagnostic value. – Seed Sci. & Techn. 14:
391-402.
Roti-Michelozzi G, Riggio-Bevilacqua L, Boca
P. 1989. Development of the tissues surrounding the embryo in Melilotus
alba Med. – Phytomorphology 39: 157-160.
Roux MM le, Wyk B-E van. 2007. A revision of
Lebeckia sect. Lebeckia: the L. sepiaria group (Fabaceae, Crotalarieae). – South Afr.
J. Bot. 73: 118-130.
Roux MM le, Wyk B-E van. 2008. A revision of
Lebeckia sect. Lebeckia: the L. plukenetiana group
(Fabaceae, Crotalarieae). – South
Afr. J. Bot. 74: 660-676.
Roux MM le, Wyk B-E van. 2009. A revision of
Lebeckia sect. Lebeckia: the L. pauciflora and
L. wrightii groups (Fabaceae, Crotalarieae). – South Afr.
J. Bot. 75: 83-96.
Roux MM le, Wyk B-E van. 2013. A taxonomic
revision of Amphitrichae, a new section of Crotalaria (Fabaceae). – Syst. Bot. 38:
638-652.
Roy B. 1933. Studies in the development of
the female gametophyte in some leguminous crop plants of India. – Indian J.
Agric. Sci. 3: 1098-1107.
Royen P van, Steenis CGGJ van. 1952.
Eriandra, a new genus of Polygalaceae from New Guinea. – J.
Arnold Arbor. 33: 91-96.
Rudd VE. 1954. Centrolobium
(Leguminosae). Validation of a specific name and a brief review of the genus.
– J. Washington Acad. Sci. 44: 284-288.
Rudd VE. 1955. The American species of
Aeschynomene. – Contr. U.S. Natl. Herb. 32: 1-172.
Rudd VE. 1956. A revision of the genus
Nissolia. – Contr. U.S. Natl. Herb. 32: 173-206.
Rudd VE. 1958. A revision of the genus
Chaetocalyx. – Contr. U.S. Natl. Herb. 32: 207-243.
Rudd VE. 1959. The genus
Aeschynomene in Malaysia (Leguminosae-Papilionatae). – Reinwardtia
5: 23-36.
Rudd VE. 1965. The American species of
Ormosia (Leguminosae). – Contr. U. S. Natl. Herb. 32: 279-384.
Rudd VE. 1967. Supplementary studies in
Aeschynomene II: Series Pleuronerviae. – Phytologia 15:
114-119.
Rudd VE. 1968. Leguminosae of Mexico
(Faboideae) I. Sophoreae and Podalyrieae. – Rhodora 70: 492-532.
Rudd VE. 1970a. Revival of Nissolia
microptera (Leguminosae). – Phytologia 20: 324.
Rudd VE. 1970b. Etaballia dubia
(Leguminosae), a new combination. – Phytologia 20: 426-428.
Rudd VE. 1972a. Reduction of
Balisaea to Aeschynomene (Leguminosae). – Phytologia 23:
321.
Rudd VE. 1972b. Supplementary studies in
Chaetocalyx (Leguminosae) I. Including a new species from Brazil. –
Phytologia 24: 295-297.
Rudd VE. 1972c. A new variety of Poiretia
latifolia and a brief resume of the genus Poiretia Vent.
(Leguminosae). – Phytologia 23: 141-148.
Rudd VE. 1973. New taxa and combinations in
Machaerium (Leguminosae) III. – Phytologia 25: 398-403.
Rudd VE. 1974. A resume of the genus
Tipuana (Leguminosae). – Phytologia 28: 475-478.
Rudd VE. 1975a. Nissolia chiapensis,
a new species of Leguminosae from Mexico. – Phytologia 31: 427-430.
Rudd VE. 1975b. Supplementary studies in
Aeschynomene III: Series Scopariae in Mexico and Central
America. – Phytologia 31: 431-434.
Rudd VE. 1977. The genus Machaerium
(Leguminosae) in Mexico. – Bol. Soc. Bot. Mexico 37: 119-146.
Rudd VE. 1981a. Two new species of
Paramachaerium (Leguminosae) and a brief resume of the genus. –
Brittonia 33: 435-440.
Rudd VE. 1981b. Aeschynomeneae. – In:
Polhill RM, Raven PH (eds), Advances in legume systematics 1, Royal Botanic
Gardens, Kew, pp. 347-354.
Rudd VE. 1986. A new species of
Machaerium (Leguminosae) from Nicaragua. – Phytologia 60: 93-94.
Rudd VE. 1987a. Studies in
Machaerium (Leguminosae) VI. – Phytologia 62: 282-302.
Rudd VE. 1987b. Studies in
Machaerium (Leguminosae) VII. Section II. Lineata I. Species
with wingless fruit. – Phytologia 64: 1-12.
Rudd VE. 1996. Chaetocalyx longiloba
(Fabaceae, Papilionoideae), a new
species from Peru. – Novon 6: 119.
Ruiz E, Crawford DJ, Stuessy TF, Conzález F,
Samuel R, Becerra J, Silva M. 2004. Phylogenetic relationships and genetic
divergence among endemic species of Berberis, Gunnera,
Myrceugenia and Sophora of the Juan Fernández Islands
(Chile) and their continental progenitors based on isozymes and nrITS
sequences. – Taxon 53: 321-332.
Rundel RW. 1989. Ecological success in
relation to plant form and function in the soody legumes. – In: Stirton CH,
Zarucchi JL (eds), Advances in legume biology, Monogr. Syst. Bot. Missouri Bot.
Gard. 29: 377-398.
Russell A. 2004. The distribution and
taxonomic significance of a 50 kb inversion in the Papilionoideae
(Leguminosae). – M.Sc. thesis, University of Edinburgh, Edinburgh.
Rydberg PA. 1919. Fabaceae: Psoraleeae, part 1. – North
American Flora 24: 1-34, 40-64.
Rydberg PA. 1920. Fabaceae: Psoraleeae, part 2. – North
American Flora 24: 65-136.
Rydberg PA. 1928a. Genera of North American
Fabaceae III: tribe Psoraleeae. –
Amer. J. Bot. 15: 195-203.
Rydberg PA. 1928b. Genera of North American
Fabaceae IV: tribe Psoraleeae
(continued). – Amer. J. Bot. 15: 425-432.
Sabaii T, Zarre S, Podlech D. 2007. Two new
species of Astragalus sect. Anthylloidei (Fabaceae). – Willdenowia 37:
297-304.
Sagen AL, Gertsch J, Becker R, Heilmann J,
Sticher O. 2002. Quinolizidine alkaloids from the curare adjuvant
Clathrotropis glaucophylla. – Phytochemistry 61: 975-978.
Saini A, Jawali N. 2009. Molecular evolution
of 5S rDNA region in Vigna subgenus Ceratotropis and its
phylogenetic implications. – Plant Syst. Evol. 280: 187-206.
Saint-Martin M. 1978. Observations
séminologiques au microscope électronique à balayage de diverses espèces de
Papilionacées. – Compt. Rend. Acad. Sci. Paris 287D: 927-930.
Saint-Martin M. 1982. Biosystématique des
Papilionaceae. Ontogénie, phytodermologie, séminologie. – Ph.D. diss.,
Université de P. Sabatier, Toulouse, France.
Saint-Martin M. 1984. Ontogénie des
plantules et phytodermologie chez les Papilionaceae. – Gaussenia 1: 19-44.
Saint-Martin M. 1986. Micromorphologie
tégumentaire des graines de Papilionaceae. – Bull. Soc. Bot. France 133,
Lettr. Bot.: 137-153.
Saint-Martin M. 1992. Phytodermology and
testa topography in Ononis (Papilionaceae). – Willdenowia 22:
37-47.
Salatino A, Gottlieb OR. 1983.
Chemogeographical evolution of quinolizidines in Papilionoideae. – Plant
Syst. Evol. 143: 167-174.
Sampaio G. 1924. Revisão das Ulicíneas
portuguesas. – Broteria, ser. bot. 21: 142-168.
Sanchir C. 1980. Outline of Caragana
Lam. Species. – Institute of Botany, People’s Republic of Mongolia Academy,
Ulan Bator, vol. 4, pp. 106-123.
Sanchir C. 1999. System of the genus
Caragana Lam. (Fabaceae).
– Acta Sci. Nat. Univ. Inner Mongol. 30: 501-512.
Sanchir C. 2000. Systema generis
Caragana Lam. (Fabaceae).
– Nov. Sist. Vyssh. Rast. 32: 76-90.
Sanderson MJ. 1991. Phylogenetic
relationships within North American Astragalus L. – Syst. Bot. 16:
414-430.
Sanderson MJ, Doyle JJ. 1993. Phylogenetic
relationships in North American Astragalus (Fabaceae) based on chloroplast DNA
restriction site variation. – Syst. Bot. 18: 395-408.
Sanderson MJ, Liston A. 1995. Molecular
phylogenetic systematics of Galegeae, with special reference to
Astragalus. – In: Crisp MD, Doyle JJ (eds), Advances in legume
systematics 7: Phylogeny, Royal Botanic Gardens, Kew, pp. 331-350.
Sanderson MJ, Wojciechowski MF. 1996.
Diversification rates in a temperate legume clade: are there “so many species
of Astragalus (Fabaceae)?”. – Amer. J. Bot. 83:
1488-1502.
Sandral G, Remizowa MV, Sokoloff DD. 2006. A
taxonomic survey of Lotus section Pedrosia (Leguminosae,
Loteae). – Wulfenia 13: 97-192.
Sandra G, Degtjareva GV, Kramina TE, Sokoloff
DD, Samigullin TH, Hughes S, Valiejo-roman CM. 2010. Are Lotus
creticus and Lotus cytisoides (Leguminosae) closely related
species? Evidence from nuclear ribosomal ITS sequence data. – Gent. Resources
Crop Evol. 57: 501-514.
Sands VE. 1975. The cytoevolution of the
Australian Papilionaceae. – Proc. Linn. Soc. New South Wales 100: 118-155.
Sanjappa M. 1994. Crudia
(Leguminosae: Caesalpinioideae), a new generic record for India with a new
species of the genus. – Kew Bull. 49: 565-568.
San Miguel-Chávez R, Soto-Hernández M,
Terrazas T, Kite G. 2006. Morphology and alkaloidal profile of the seedlings of
Erythrina americana Mill. and E. coralloides A. DC. –
Feddes Repert. 117: 232-239.
Santos AC dos 1945. Algunas contagenas de
cromosomas nos géneros Genista L. & Cytisus L. – Bol.
Soc. Brot. 19: 519-522.
Santos-Silva J, Azevedo Tozzi AMG de, Simon
MF, Urquiza NG, Morales M. 2013. Evolution of trichome morphology in
Mimosa (Leguminosae-Mimosoideae). – Phytotaxa 119: 1-20.
Santos-Silva J, Simon MF, De Azevedo Tozzi
AMG. 2013. Pollen diversity and its phylogenetic implications in
Mimosa ser. Leiocarpae Benth. (Leguminosae, Mimosoideae). –
Grana 52: 15-25.
Sañudo A. 1973a. Variabilidad cromosómica
de las genisteas españolas en relación con su ecología 1. Número y
comportamiento de los cromosomas durante la meiosis. E. Género
Cytisus L. – Bol. Roy. Soc. Española Hist. Nat. (Biol.) 71:
341-355.
Sañudo A. 1973b. Variabilidad cromosómica
de las Genisteas de la flora española en relación con su ecología. –
Lagascalia 3: 205-210.
Sañudo A. 1974. Variabilidad cromosómica de
las Genisteas de la flora española en relación con su ecología. – An.
Inst. Cavanilles 31: 165-174.
Sañudo A. 1979. Chromosome variability in
the Genisteae (Adans.) Benth. (Leguminosae). – Webbia 34: 363-408.
Sañudo A, Ruiz Rejon M, Pretel A. 1979.
Variabilité chromosomique chez les espèces d’Ononis de la flore
espagnole. Note préliminaire. – Webbia 34: 535-542.
Sartori ALB, Tozzi AMGA. 2004. Revisão
taxonômica de Myrocarpus Allemão (Leguminosae, Papilionoideae,
Sophoreae). – Acta Bot. Brasil. 18: 521-535.
Sirichamorn Y, Adema FACB, Welzen PC van.
2012. The genera Aganope, Derris, and Paraderris (Fabaceae, Millettieae) in Thailand. –
Syst. Bot. 37: 404-436.
Särkinen TE, Marcelo-Peña JL, Yomona AD,
Simon MF, Pennington RT, Hughes CE. 2011. Unterestimated endemic species
diversity in the dry inter-Andean valley of the Río Marañón, northern Peru:
an example from Mimosa (Leguminosae, Mimosoideae). – Taxon 60:
139-150.
Sartori ALB. 2000. Revisão taxonômica e
estudos morfológicos de Myrocarpus Allemão, Myroxylon L. f.
e Myrospermum Jacq. (Leguminosae, Papilionoideae, Sophoreae). –
Ph.D. diss., Universidad Estadual de Campinas, Brazil.
Sartori ALB, Tozzi AMGA. 2004. Revisão
taxonômica de Myrocarpus Allemão (Leguminosae, Papilionoideae,
Sophoreae). – Acta Bot. Brasil. 18: 521-535.
Sartori ALB, Azevedo Tozzi AMG de. 2002.
Comparative leaflet anatomy in Myrocarpus Allemao, Myroxylon
L. f. and Myrospermum Jacq. (Leguminosae-Papilionoideae-Sophoreae)
species. – Bot. J. Linn. Soc. 140: 249-259.
Sartori ALB, Lewis GP, Mansano V de F,
Azevedo Tozzi AMG de. 2015. A revision of the genus Myroxylon
(Leguminosae: Papilionoideae). – Kew Bull. 70: 48 DOI
10.1007/S12225-015-9604-7
Saski C, Lee SB, Daniell H, Wood TC, Tomkins
J, Kim H-G, Jansen RK. 2005. Complete chloroplast genome sequence of
Glycine max and comparative analyses with other legume genomes. –
Plant Mol. Biol. 59: 309-322.
Saslis-Lagoudakis CH, Chase MW, Robinson DN,
Russell SJ, Klitgaard BB. 2008. Phylogenetics of neotropical
Platymiscium (Leguminosae: Dalbergieae): systematics, divergence
times, and biogeography inferred from nuclear ribosomal and plastid DNA
sequence data. – Amer. J. Bot. 95: 1270-1286.
Saslis-Lagoudakis CH, Klitgaard BB, Forest F,
Francis L, Savolainen V, Williamson EM, Hawkins JA. 2011. The use of phylogeny
to interpret cross-cultural patterns in plant use and guide medicinal plant
discovery: an example from Pterocarpus (Leguminosae). – PloS ONE
6(7): e22275.
Sauer J. 1964. Revision of
Canavalia. – Brittonia 16: 106-181.
Scalon VR. 2007. Revisão taxonômica do
gênero Stryphnodendron Mart. (Leguminosae-Mimosoideae). – Ph.D.
thesis, Universidade de São Paulo, Brazil.
Scherson RA, Choi H-K, Cook DR, Sanderson MJ.
2005. Phylogenetics of New World Astragalus: screening of novel
nuclear loci for the reconstruction of phylogenies at low taxonomic levels. –
Brittonia 57: 354-366.
Scherson RA, Vidal R, Sanderson MJ. 2008.
Phylogeny, biogeography and rates of diversification of New World
Astragalus (Leguminosae) with an emphasis on South American
radiations. – Amer. J. Bot. 95: 1030-1039.
Scheublin TR, Heijden MGA van der. 2006.
Arbuscular mycorrhizal fungi colonize nonfixing root nodules of several legume
species. – New Phytol. 172: 732-738.
Schley RJ, Estrella M de la, Pérez-Escobar
OA, Bruneau A, Barraclough T, Forest F, Klitgård B. 2018. Is Amazonia a
‘museum’ for Neotropical trees? The evolution of the Brownea clade
(Detarioideae, Leguminosae). – Mol. Phylogen. Evol. 126: 279-292.
Schmatz Maciel H, Shiffino-Wittmann MT. 2002.
First chromosome number determinations in south-eastern South American species
of Lupinus L. (Leguminosae). – Bot. J. Linn. Soc. 139: 395-400.
Schmidt B. 1979. Beiträge zur Kenntnis der
Sippenstruktur der Gattung Coronilla L. – Feddes Repert. 90:
257-361.
Schmidt P-A. 2011. Evolution of Macaronesian
Lotus L. – M.Sc. thesis, Philipps-Universität Marburg, Germany.
Schnabel A, Wendel JF. 1998. Cladistic
biogeography of Gleditsia (Leguminosae) based on ndhF and
rpl16 chloroplast gene sequences. – Amer. J. Bot. 85: 1753-1765.
Schnabel A, McDonel PE, Wendel JF. 2003.
Phylogenetic relationships in Gleditsia (Leguminosae) based on ITS
sequences. – Amer. J. Bot. 90: 310-320.
Schneider JV. 2006. Surianaceae. – In: Kubitzki K (ed),
The families and genera of vascular plants IX. Flowering plants. Eudicots. Berberidopsidales,
Buxales, Crossosomatales, Fabales p. p., Geraniales, Gunnerales, Myrtales p. p., Proteales, Saxifragales, Vitales, Zygophyllales, Clusiaceae Alliance, Passifloraceae
Alliance, Dilleniaceae, Huaceae, Picramniaceae, Sabiaceae, Springer, Berlin,
Heidelberg, New York, pp. 449-455.
Schot AM. 1991. Phylogenetic relations and
historical biogeography of Fordia and Imbralyx
(Papilionaceae: Millettieae). – Blumea 36: 205-234.
Schot AM. 1994. A revision of
Callerya Endl. (including Padbruggea and
Whitfordiodendron) (Papilionaceae: Millettieae). – Blumea 39:
1-40.
Schrire BD. 1987. A synopsis of
Tephrosia subgenus Barbistyla (Fabaceae) in southern Africa. –
Bothalia 17: 7-15.
Schrire BD. 1995. Evolution of the tribe
Indigofereae (Leguminosae-Papilionoideae). – In: Crisp MD, Doyle JJ (eds),
Advances in legume systematics 7: Phylogeny, Royal Botanic Gardens, Kew, pp.
161-244.
Schrire BD. 1997. Notes relating to the genus
Indigofera (Leguminosae-Papilionoideae) for Flora Zambesiaca 1.
Section Psiloceratiae (J. B. Gillett) Schrire. – Kew Bull. 52:
153-160.
Schrire BD. 1998. Notes relating to the genus
Indigofera (Leguminosae-Papilionoideae) for Flora Zambesiaca 2.
Section Indigofera. – Kew Bull. 53: 651-668.
Schrire BD. 2000a. A synopsis of the genus
Philenoptera (Leguminosae-Millettieae) from Africa and Madagascar. –
Kew Bull. 55: 81-94.
Schrire BD. 2000b. A synopsis of the genus
Philenoptera (Leguminosae-Millettieae): a correction. – Kew Bull.
55: 498.
Schrire BD. 2005. Tribe Phaseoleae. – In:
Lewis GP, Schrire BD, Mackinder B, Lock M (eds), Legumes of the world, Royal
Botanic Gardens, Kew, United Kingdom, pp. 393-431.
Schrire BD. 2008. The Madagascan genus
Vaughania is reduced to synonymy under Indigofera
(Leguminosae-Papilionoideae-Indigofereae). – Kew Bull. 63: 477-479.
Schrire BD, Sims JR. 1997. A re-evaluation of
pollen morphology and taxonomy in the tribe Indigofereae
(Leguminosae-Papilionoideae). – Kew Bull. 52: 841-878.
Schrire BD, Lavin M, Barker NP, Cortes-Burns
H, Senger I von, Kim J-H. 2003. Towards a phylogeny of Indigofera
(Leguminosae-Papilionoideae): identification of major clades and relative-ages.
– In: Klitgaard BB, Bruneau A (eds), Advances in legume systematics 10:
Higher level systematics, Royal Botanic Gardens, Kew, pp. 269-302.
Schrire BD, Lavin M, Lewis GP. 2005. Global
distribution patterns of the Leguminosae: insights from recent phylogenies. –
Biol. Skr. 55: 375-422. [Friies I, Balslev H (eds), Proceedings of a symposium
on plant diversity and complexity patterns: Local, regional and global
dimensions, Danish Academy of Sciences and Letters, Copenhagen]
Schrire BD, Lavin M, Barker NP, Forest F.
2009. Phylogeny of the tribe Indigofereae (Leguminosae-Papilionoideae):
geographically structured more in succulent-rich and temperate settings than in
grass-rich environments. – Amer. J. Bot. 96: 816-852.
Schulz OE. 1901. Monographie der Gattung
Melilotus. – Engl. Bot. Jahrb. Syst. 29: 660-728.
Schutte AL. 1995a. A taxonomic study of the
tribes Podalyrieae and Liparieae (Fabaceae). – Ph.D. diss., Rand
Afrikaans University (University of Johannesburg), Johannesburg, Republic of
South Africa.
Schutte AL. 1995b. Five new species of the
genus Liparia (Fabaceae)
from South Africa. – Nord. J. Bot. 15: 149-156.
Schutte AL. 1997a. A revision of the genus
Xiphotheca (Fabaceae). –
Ann. Missouri Bot. Gard. 84: 90-102.
Schutte AL. 1997b. Systematics of the genus
Liparia (Fabaceae). –
Nord. J. Bot. 17: 11-37.
Schutte AL. 1997c. Systematics of the genus
Cyclopia Vent. (Fabaceae,
Podalyrieae). – Edinburgh J. Bot. 54: 125-170.
Schutte AL. 1998. A re-evaluation of the
generic status of Amphithalea and Coelidium (Fabaceae). – Taxon 47: 55-65.
Schutte AL, Wyk B-E van. 1988. A synopsis of
the genus Dichilus (Fabaceae-Crotalarieae). – South Afr.
J. Bot. 54: 182-184.
Schutte AL, Wyk B-E van. 1990. Taxonomic
relationships in the genus Dichilus (Fibaceae-Crotalarieae). – South
Afr. J. Bot. 56: 244-256.
Schutte AL, Wyk B-E van. 1991. A new species
of Amphithalea (Liparieae). – Bothalia 21: 58-59.
Schutte AL, Wyk B-E van. 1992. A new species
of Coelidium (Fabaceae,
Liparieae). – Bothalia 22: 188-189.
Schutte AL, Wyk B-E van. 1993. The
reinstatement of the genus Xiphotheca (Fabaceae). – Taxon 42: 43-49.
Schutte AL, Wyk B-E van. 1994. A reappraisal
of the generic status of Liparia and Priestleya (Fabaceae). – Taxon 43: 573-582.
Schutte AL, Wyk B-E van. 1998a. Evolutionary
relationships in the Podalyrieae and Liparieae (Fabaceae) based on morphological,
cytological and chemical evidence. – Plant Syst. Evol. 209: 1-31.
Schutte AL, Wyk B-E van. 1998b. The tribal
position of Hypocalyptus Thunberg (Fabaceae). – Novon 8: 178-182.
Schutte AL, Vlok JHJ, Wyk B-E van. 1995.
Fire-survival strategy – a strategy of taxonomic, ecological and evolutionary
importance in fynbos legumes. – Plant Syst. Evol. 195: 243-259.
Schutte-Vlok AL, Wyk B-E van. 2011. A
taxonomic revision of Podalyria (Fabaceae). – Syst. Bot. 36:
631-660.
Schwarz EN, Ruhlman TA, Sabir JSM, Hajrah NH,
Alharbi NS, Al-Malki AL, Bailey CD, Jansen RK. 2015. Plastid genome sequences
of legumes reveal parallel inversions and multiple losses of rps16 in
papilionoids. – J. Syst. Evol. 53: 458-468.
Sede SM. 2005. Estudios multidisciplinarios
en el complejo Galactia-Camptosema-Collaea (Leguminosae). – Ph.D.
thesis, Universidad de Buenos Aires, Argentina.
Sede SM, Dezzi R, Greizerstein E, Fortunato
RH, Poggio L. 2003. Chomosome studies in the complex
Galactia-Collaea-Camptosema (Diocleinae, Phaseoleae, Papilionoideae,
Fabaceae). – Caryologia 56:
295-301.
Sede SM, Fortunato RH, Poggio L. 2006.
Chromosome evaluation of southern South American species of Camptosema
and allied genera (Diocleinae-Phaseoleae-Papilionoideae-Leguminosae). – Bot.
J. Linn. Soc. 152: 235-243.
Sede SM, Tosto DS, Gottlieb AM, Poggio L,
Fortunato RH. 2008. Genetic relationships in the
Galactia-Camptosema-Collaea complex (Leguminosae)
inferred from AFLP markers. – Plant Syst. Evol. 276: 261-270.
Sede SM, Tosto D, Talia P, Luckow M, Poggio
L, Fortunato RH. 2009. Phylogenetic relationships among southern South American
species of Camptosema, Galactia and Collaea
(Diocleinae: Papilionoideae: Leguminosae) on the basis of molecular and
morphological data. – Aust. J. Bot. 57: 76-86.
Seigler DS. 2003. Phytochemistry of
Acacia – sensu lato. – Biochem. Syst. Ecol. 31: 845-873.
Seigler DS, Ebinger JE. 1988. Acacia
macracantha, A. pennatula, and A. cochliacantha (Fabaceae: Mimosoideae) species complexes
in Mexico. – Syst. Bot. 13: 7-15.
Seigler DS, Ebinger JE. 2005. New
combinations in the genus Vachellia (Fabaceae: Mimosoideae) from the New
World. – Phytologia 87: 139-178.
Seigler DS, Ebinger JE. 2009. New
combinations in the genus Senegalia (Fabaceae: Mimosoideae). – Phytologia
91: 25-29.
Seigler DS, Ebinger JE. 2010. New
combinations in Senegalia and Vachellia (Fabaceae: Mimosoideae). – Phytologia
92: 90-93.
Seigler DS, Ebinger JE, Miller JT. 2006a. The
genus Senegalia (Fabaceae:
Mimosoideae) from the New World. – Phytologia 88: 38-93.
Seigler DS, Ebinger JE, Miller JT. 2006b.
Mariosousa, a new segregate genus from Acacia s.l. (Fabaceae, Mimosoideae) from Central and
North America. – Novon 16: 413-420.
Seigler DS, Ebinger JE, Riggins CW, Terra V,
Miller JT. 2017. Parasenegalia and Pseudosenegalia (Fabaceae): new genera of the
Mimosoideae. – Novon 25: 180-205.
Seijo J, Fernandez A. 2003. Karyotype
analysis and chromosome evolution in South American species of
Lathyrus (Leguminosae). – Amer. J. Bot. 90: 980-987.
Seijo JG, Solis Neffa VG. 2004. The
cytological origin of the polyads and their significance in the reproductive
biology of Mimosa bimucronata. – Bot. J. Linn. Soc. 144: 343-349.
Senn HA 1938. Chromosome number relationships
in the Leguminosae. – Bibliogr. Genet. 12: 175-345.
Sepet H, Özdemir C, Bozdağ B. 2011.
Cytological study of the genus Chesneya Lindl. (Fabaceae) in Turkey. – Caryologia 64:
184-188.
Serrato Valenti G. 1971. Adumbratio Florae
Aethiopicae 22. Caesalpiniaceae – Gen. Cassia. – Webbia 26:
1-99.
Shanmughasundaram K, Subramanian D. 1992.
Cytotaxonomical studies on 9 taxa of Macroptilium and
Strophostyles helvola. – Cell Chromosome Res. 15: 23.
Shavvon RS, Osaloo SK, Maassoumii AA,
Moharrek F, Erkul SK, Lemmon AR, Lemmon EM, Michalak I, Muellner-Riehl AN,
Favre A. 2017. Increasing phylogenetic support for explosively radiating taxa:
the promise of high-throughput sequencing for Oxytropis (Fabaceae). – J. Syst. Evol. 55:
385-404.
Shi W, Su ZH, Liu PL, Pan BR, Zhao YF, Wang
JC. 2017. Molecular, karyotypic, and morphological evidence for
Ammopiptanthus (Fabaceae)
taxonomy. – Ann. Missouri Bot. Gard. 102: 559-573.
Shue LZ. 1985. The ecological differentiation
of the Hedysarum L. and geographical distribution in China. – Acta
Bot. Bor.-Occid. Sin. 4: 275-285. [in Chinese]
Shue LZ, Hao XY. 1989. Studies in the
taxonomy and the floristic geography of Caragana Fabr. (Leguminosae)
in Loess Plateau and Qinling mountains. – Acta Bot. Bor.-Occid. Sin. 9:
92-101.
Sibichen MT. 2004. Morphologic and taxonomic
studies on Fabaceae (tribe
Crotalarieae) in South India. – Ph.D. diss., University of Calicut, India.
Silva MJ da, Tozzi AMG de A. 2008. Four new
Brazilian species of Lonchocarpus (Leguminosae, Papilionoideae,
Millettieae). – Brittonia 60: 318-328.
Silva MJ da, Queiroz LP de, Tozzi AMG de A,
Lewis GP, Sousa AP de. 2012. Phylogeny and biogeography of
Lonchocarpus sensu lato and its allies in the tribe Millettieae
(Leguminosae, Papilionoideae). – Taxon 61: 93-108.
Simola HA. 1986. Structural and chemical
aspects of evolution of Lathyrus species. – In: Kaul A, Combes D
(eds), Lathyrus and lathyrism, Proc. Intern. Symp., IBEAS, Univ. Pau,
Sept. 1985, New York, pp. 225-239.
Simon JP, Simon A. 1965. Relationship in
annual species of Medicago I. Number and morphology of chromosomes.
– Aust. J. Agric. Res. 16: 37-50.
Simon MF. 2008. Systematics and evolution of
Mimosa L. (Leguminosae) and the assembly of a Neotropical plant
diversity hotspot. – Ph.D. diss., University of Oxford, Oxford, England.
Simon MF, Grether R, Queiroz LP de, Särkinen
TE, Dutra VF, Hughes CE. 2011. The evolutionary history of Mimosa
(Leguminosae): toward a phylogeny of the sensitive plants. – Amer. J. Bot.
98: 1201-1221.
Simon MF, Pastore JFB, Souza AF, Borges LM,
Scalon VR, Ribeiro PG, Santos-Silva J, Souza VC, Queiroz LP de. 2016. Molecular
phylogeny of Stryphnodendron (Mimosoideae, Leguminosae) and generic
delimitations in the Piptadenia group. – Intern. J. Plant Sci. 177:
44-59.
Simpson BB, Ulibarri EA. 2006. A synopsis of
the genus Hoffmannseggia (Leguminosae). – Lundellia 9: 7-33.
Simpson BB, Larkin LL, Weeks A. 2003.
Progress towards resolving relationships of the Caesalpinia group
(Caesalpinieae: Caesalpinioideae: Leguminosae). – In: Klitgaard B, Bruneaeu A
(eds), Advances in legume systematics 10: Higher level systematics, Royal
Botanic Gardens, Kew, pp. 123-148.
Simpson BB, Tate JA, Weeks A. 2004. Phylogeny
and character evolution of Hoffmannseggia (Caesalpinieae:
Caesalpinioideae: Leguminosae). – Syst. Bot. 29: 933-946.
Simpson BB, Tate JA, Weeks A. 2005. The
biogeography of Hoffmannseggia (Leguminosae, Caesalpinioideae:
Caesalpinieae): a tale of many travels. – J. Biogeography 32: 15-27.
Simpson BB, Larkin L, Weeks A, McDill J.
2006. Phylogeny and biogeography of Pomaria (Caesalpinioideae:
Leguminosae). – Syst. Bot. 31: 792-804.
Singh V. 2001. Monograph on Indian subtribe
Cassiinae (Caesalpiniaceae). – Scientific Publ., Jodhpur.
Singhal VK, Gill BS, Dishu MS. 1990.
Cytological explorations of Indian woody legumes. – Proc. Indian Acad. Sci.,
Plant Sci. 100: 319-331.
Sinha SSN, Prasad R. 1973. Meiotic studies in
some Indian Leguminosae. – Proc. Indian Sci. Congr. Assoc. 60: 320-321.
Sinou C, Forest F, Lewis GP, Bruneau A. 2009.
The genus Bauhinia s.l. (Leguminosae): a phylogeny based on the
plastid trnL-trnF region. – Botany 87: 947-960.
Sirichamorn Y, Adema FACB, Welzen PC van.
2012. The genera Aganope, Derris, and Paraderris (Fabaceae, Millettieae) in Thailand. –
Syst. Bot. 37: 404-436.
Sirichamorn Y, Adema FA, Gravendeel B, Welzen
PC van. 2012. Phylogeny of palaeotropic Derris-like taxa (Fabaceae) based on chloroplast and
nuclear DNA sequences shows reorganization of (infra)generic classifications is
needed. – Amer. J. Bot. 99: 1793-1808.
Sirichamorn Y, Adema FACB, Roos MC, Welzen PC
van. 2014. Molecular and morphological phylogenetic reconstruction reveals a
new generic delimitation of Asian Derris (Fabaceae): reinstatement of
Solori and synonymisation of Paraderris with Derris.
– Taxon 63: 522-538.
Širjaev G. 1925. Onobrychis generis
revisio critica I. – Spisy Přír. Fak. Masarykovy Univ. 56: 1-195.
Širjaev G. 1932. Generis Ononis L.
Revisio critica. – Beih. Bot. Centralbl. 49: 381-665.
Širjaev G. 1934. Generis Trigonella
L. Revisio critica II(1). – Fac. Sci. Univ. Masaryk, Brno.
Širjaev G. 1939. Conspectus Tragacantharum
(Astragalus L. subgenus Tragacantha Bge.) I-II. – Feddes
Repert. 47: 194-208, 225-261.
Skalická A. 1967. Taxonomische Studie über
die Arten der Gattung Corothamnus (W. D. J. Koch) C. B. Presl. –
Preslia 39: 10-29.
Skalická A. 1969. Taxonomische Revision der
Gattung Lembotropis Griseb. – Acta Univ. Carol. Praha (Biol.) 1968:
263-267.
Small E. 1981. A numerical analysis of major
groupings in Medicago employing traditionally used characters. –
Can. J. Bot. 59: 1553-1577.
Small E. 1987a. Reduction of Ursia
to Trifolium. – Taxon 36: 578-583.
Small E. 1987b. Generic changes in Trifolieae
subtribe Trigonellinae. – In: Stirton CH (ed), Advances in legume systematics
3, Royal Botanic Gardens, Kew, pp. 169-181.
Small E. 1987c. A taxonomic study of the
“medicagoid” Trigonella. – Can. J. Bot. 65: 1199-1211.
Small E. 1990. Medicago syriaca, a
new species. – Can. J. Bot. 68: 1473-1478.
Small E, Brookes BS. 1983. The systematic
value of stigma morphology in the legume tribe Trifolieae with particular
reference to Medicago. – Can. J. Bot. 61: 2388-2404.
Small E, Brookes BS. 1984. Reduction of the
geocarpic Factorovskya to Medicago. – Taxon 33: 622-635.
Small E, Jomphe M. 1989. A synopsis of the
genus Medicago (Leguminosae). – Can. J. Bot. 67: 3260-3294.
Small E, Bassett IJ, Crompton CW. 1981.
Pollen variation in tribe Trigonelleae (Leguminosae) with special reference to
Medicago. – Pollen Spores 23: 296-320.
Small E, Crompton CW, Brookes BS. 1981. The
taxonomic value of floral characters in tribe Trigonelleae (Leguminosae), with
special reference to Medicago. – Can. J. Bot. 59: 1578-1598.
Small E, Lefkovitch LP, Classen D. 1982.
Character set incongruence in Medicago. – Can. J. Bot. 60:
2505-2510.
Small E, Lassen P, Brookes BS. 1987. An
expanded circumscription of Medicago (Leguminosae, Trifolieae) based
on explosive flower tripping. – Willdenowia 16: 415-437.
Smith CP. 1918a. Studies in the genus
Lupinus II. The Microcarpi, exclusive of Lupinus
densiflorus. – Bull. Torrey Bot. Club 45: 1-22.
Smith CP. 1918b. Studies in the genus
Lupinus III. Lupinus densiflorus. – Bull. Torrey Bot. Club
45: 167-202.
Smith CP. 1938-1951. Species lupinorum. –
Signatures, Sarasota.
Smith DL. 1981. Cotyledons of Leguminosae.
– In: Polhill RM, Raven PH (eds), Advances in legume systematics 2, Royal
Botanic Gardens, Kew, pp. 927-940.
Smith GF, Wyk AE van, Luckow M, Schrire B.
2006. Conserving Acacia Mill. with a conserved type. What happened in
Vienna? – Taxon 55: 223-225.
Smith PP, Timberlake JR, Wyk AE van. 1998.
Proposal to conserve the name Colophospermum against
Hardwickia (Leguminosae, Caesalpinioideae). – Taxon 47: 751-752.
Snak C, Vatanparast M, Silva C, Lewis GP,
Lavin M, Kajita T, Queiroz LP de. 2016. A dated phylogeny of the papilionoid
legume genus Canavalia reveals recent diversification by a pantropical liana
lineage. – Mol. Phylogen. Evol. 98: 133-146.
Soares-Silva LH, Freitas Mansano, V de. 2004.
A new species of Exostyles (Leguminosae, Papilionoideae, Swartzieae
s.l.), from Parana State, Brazil. – Bot. J. Linn. Soc. 146: 103-106.
Soják J. 1986. Nové jméno pro
Galearia Presl. – Cas. Nár. Muz. (Praha) 154: 35.
Sokoloff DD. 1999a. Typification of several
specific and generic names in the tribe Loteae (Papilionaceae). – Taxon 48:
57-59.
Sokoloff DD. 1999b. Ottleya, a new
genus of Papilionaceae–Loteae from North America. – Feddes Repert. 110:
89-97.
Sokoloff DD. 2000. New combinations in
Acmispon (Leguminosae, Loteae). – Ann. Bot. Fenn. 37: 125-131.
Sokoloff DD. 2001. New records of
Lotus (Leguminosae: Papilionoideae: Loteae) from Africa and southwest
Asia. – Kew Bull. 56: 715-720.
Sokoloff DD. 2003a. Morphology and
classification of the tribe Loteae DC. of the family Leguminosae. – Ph.D.
diss., Moscow State Unviersity, Russia. [in Russian]
Sokoloff DD. 2003b. On system and phylogeny
of the tribe Loteae DC. (Leguminosae). – Byull. Moskovsk. Obshch. Isp. Prir.,
Otd. Biol. 108: 35-48. [in Russian]
Sokoloff DD. 2005. Cladistic analysis of the
tribe Loteae (Leguminosae) based on morphological characters. – In: Pandey
AK, Wen J, Dogra JVV (eds), Plant taxonomy: advances and relevance, CBS Publ.
& Distr., New Delhi, pp. 45-81.
Sokoloff DD, Degtjareva GV, Endress PK,
Remizowa MV, Samigullin TH, Valiejo-Roman CM. 2007. Inflorescence and early
flower development in Loteae (Leguminosae) in a phylogenetic and taxonomic
context. – Intern. J. Plant Sci. 168: 801-833.
Soladoye MO. 1982. New species of
Baphia (Leguminosae: Papilionoideae) from Lower Guinea. – Kew Bull.
37: 295-303.
Soladoye MO. 1985. A revision of
Baphia (Leguminosae-Papilionoideae). – Kew Bull. 40: 291-386.
Song J, Clemens J, Jameson PE. 2011.
Expression of floral identity genes in Clianthus maximus during mass
inflorescence abortion and floral development. – Ann. Bot. 107: 1501-1509.
Sørensen M. 1988. A taxonomic revision of
the genus Pachyrhizus (Fabaceae-Phaseoleae). – Nord. J. Bot.
8: 167-192.
Sorsa P. 1969. Pollen morphological studies
on the Mimosaceae. – Ann. Bot. Fenn. 6: 1-34.
Sotuyo S, Lewis GP. 2007. A new species of
Caesalpinia from the Río Balsas Depression, Mexico, and an updated
taxonomic circumscription of the Caesalpinia hintonii complex
(Leguminosae: Caesalpinioideae: Caesalpinieae: Poincianella Group).
– Brittonia 59: 33-36.
Sousa ER de, Lewis GP, Forest F, Schadebach
AS, van den Berg C, de Queiroz LP. 2013. Phylogeny of Calliandra
(Leguminosae: Mimosoideae) based on nuclear and plastid molecular markers. –
Taxon 62: 1200-1219.
Sousa F de, Bertrand YJK, Pfeil BE. 2016.
Patterns of phylogenetic incongruence in Medicago found among six
loci. – Plant Syst. Evol. 302: 493-513.
Sousa M, Andrade G. 1992. Identidad de
Microlobius y Goldmania (Leguminosae: Mimosoideae: Mimoseae)
y nuevas combinaciones. – An. Inst. Biol. Univ. Nac. Auton Mex. Ser. Bot. 63:
101-107.
Sousa M, Rudd VE. 1993. Revision del género
Styphnolobium (Leguminosae: Papilionoideae: Sophoreae). – Ann.
Missouri Bot. Gard. 80: 270-283.
Sousa Sanchez M. 2005. Heteroflorum:
un nuevo género del grupo Peltophorum (Leguminosae: Caesalpinioideae:
Caesalpinieae), endémico para México. – Novon 15: 213-218.
Sousa Sanchez M, Andrade G. 1992. Identidad
de Microlobius y Goldmania (Leguminosae: Mimosoideae:
Mimoseae) y nuevas combinaiçones. – An. Inst. Biol. Univ. Nac. Autón.
México, Bot 73: 101-107.
Sousa Sanchez M, Delgado A. 1993. Mexican
Leguminosae: phytogeography, endemism, and origins. – In: Ramamoorthy TP, Bye
R, Lot A, Fa J (eds), Biological diversity of Mexico: origins and diversity,
Oxford University Press, Oxford, pp. 459-511.
Sousa Sanchez M, Rudd VE. 1993. Revisión del
género Styphnolobium (Leguminosae: Papilionoideae: Sophoreae). –
Ann. Missouri Bot. Gard. 80: 270-283.
Sousa Sanchez M, Sousa MP de. 1981. New World
Lonchocarpinae. – In: Polhill RM, Raven PH (eds), Advances in legume
systematics 1, Royal Botanic Gardens, Kew, pp. 261-281.
Southon IW (compiler). 1994. Phytochemical
dictionary of the Leguminosae 1. Plants and their constituents, phytochemical
dictionary of the Leguminosae; 2. Chemical constituents. – Chapman and Hall,
London.
Souza ER, Lewis GP, Forest F, Schnadelbach
AS, Van den Berg C, Queiroz LP. 2014. Phylogeny of Calliandra
(Leguminosae: Mimosoideae) based on nuclear and plastid molecular markers. –
Taxon 62: 1200-1219.
Souza F, Meireles J, Mendonça C,
Gonçalves-Esteves V. 2014. Pollen diversity and its implications to the
systematics of Poecilanthe (Fabaceae, Papilionoideae,
Brongniartieae). – Plant Syst. Evol. 300: 1759-1770.
Souza MGC, Benko-Iseppon AM. 2004.
Cytogenetics and chromosome banding patterns in Caesalpinioideae and
Papilionoideae species of Pará, Amazonas, Brazil. – Bot. J. Linn. Soc. 144:
181-191.
Spegazzini CL. 1921. Acacieas Argentinas. –
Bol. Acad. Nac. Ci. 26: 161-334.
Spellenberg R. 1976. Chromosome numbers and
their cytotaxonomic significance for North American Astragalus (Fabaceae). – Taxon 25: 463-476.
Sprent JI. 1981. Functional evolution in some
papilionoid root nodules. – In: Polhill RM, Raven PH (eds), Advances in
legume systematics 2, Royal Botanic Gardens, Kew, pp. 671-676.
Sprent JI. 1989. Which steps are essential
for the formation of functional legume nodules? – New Phytol. 111:
129-153.
Sprent JI. 1994a. Evolution and diversity in
the legume-rhizobium symbiosis: chaos theory? – Plant and Soil 161: 1-10.
Sprent JI. 1994b. Nitrogen acquisition
systems in the Leguminosae. – In: Sprent JI, McKey D (eds), Advances in
legume systematics 5: the nitrogen factor, Royal Botanic Gardens, Kew, pp.
1-16.
Sprent JI. 2000. Nodulation as a taxonomic
tool. – In: Herendeen PS, Bruneaeu A (eds), Advances in legume systematics 9:
Phylogeny, Royal Botanic Gardens, Kew, pp. 21-44.
Sprent JI. 2001. Nodulation in legumes. –
Royal Botanic Gardens, Kew.
Sprent JI. 2005. West African legumes: the
role of nodulation and nitrogen fixation. – New Phytol. 167: 326-330.
Sprent JI. 2007. Evolving ideas of legume
evolution and diversity: a taxonomic perspective on the occurrence of
nodulation. – New Phytol. 174: 11-25.
Sprent JI. 2009. Legume nodulation: a global
perspective. – Wiley-Blackwell, London, New York.
Sprent JI, James EK. 2007. Legume evolution:
where do nodules and mycorrhizas fit in? – Plant Physiol. 144: 575-581.
Sprent JI, Sutherland JM, Faria SM de. 1989.
Structure and function of root nodules from woody legumes. – In: Stirton CH,
Zarucchi JL (eds), Advances in legume biology, Monogr. Syst. Bot. Missouri Bot.
Gard. 29: 559-578.
Sirichamorn Y, Adema FACB, Gravendeel B,
Welzen PC van. 2012. Phylogeny of palaeotropic Derris-like taxa (Fabaceae) based on chloroplast and
nuclear DNA sequences shows reorganization of (infra)generic classifications is
needed. – Amer. J. Bot. 99: 1793-1808.
Sirichamorn Y, Adema FACB, Roos MC, Welzen PC
van. 2014. Molecular and morphological phylogenetic reconstruction reveals a
new generic delimitation of Asian Derris (Fabaceae): reinstatement of
Solori and synonymisation of Paraderris with Derris.
– Taxon 63: 522-538.
Stankevich AK. 1970. On clarification of the
systematics of the genus Vicia L. – Bull. Appl. Bot. Genet. Plant
Breeding 43: 110-125. [in Russian]
Stankevich AK. 1982. On taxonomic position of
some sections in the genus Vicia L. – Bull. Appl. Bot. Genet. Plant
Breeding 72: 21-27. [in Russian]
Stankevich AK. 1983a. The genus
Vicia L. and its position in the tribe Fabeae (Fabaceae). – Bull. Appl. Bot. Genet.
Plant Breeding 79: 3-10. [in Russian]
Stankevich AK. 1983b. Remarks on Vicia
subvillosa (Ledeb.) Boiss. and its relation to genus Orobus L.
– Bull. VIR 131: 35-38. [in Russian]
Steele KP, Wojciechowski MF. 2003.
Phylogenetic analyses of tribes Trifolieae and Vicieae based on sequences of
the plastid gene matK (Papilionoideae: Leguminosae). – In: Klitgaard
BB, Bruneau A (eds), Advances in legume systematics 10: Higher level
systematics, Royal Botanic Gardens, Kew, pp. 355-370.
Steele KP, Ickert-Bond SM, Zarre S,
Wojciechowski MF. 2010. Phylogeny and character evolution in Medicago
(Leguminosae): evidence from analyses of plastid trnK/matK
and nuclear GA3ox1 sequences. – Amer. J. Bot. 97: 1142-1155.
Steenis CGGJ van. 1968. Notes on
Bredemeyera (Comesperma) with a new Papuan species and the
Australian species listed (Polygalaceae). – Acta Bot. Neerl.
17: 377-384.
Steenis CGGJ van. 1975. A review of the genus
Sympetalandra and its position in Caesalpinioideae. – Blumea 22:
159-167.
Stefanović S, Pfeil BE, Palmer JD, Doyle JJ.
2009. Relationships among phaseoloid legumes based on sequences from eight
chloroplast regions. – Syst. Bot. 34: 115-128.
Stenglein SA, Arambarri AM. 2005. Epidermal
features of Lotus oroboides=Ottleya oroboides (Leguminosae: Loteae).
– Lotus Newsletter 35: 174-181.
Stepanova AV, Oskolski AA, Tilney PM, Wyk B-E
van. 2013. Wood anatomy of the tribe Podalyrieae (Fabaceae, Papilionoideae): diversity and
evolutionary trends. – South Afr. J. Bot. 89: 244-256.
Stepkowski T, Watkin E, McInnes A, Gurda D,
Gracz J, Steenkamp ET. 2012. Distinct Bradyrhiz[o]bium communities
nodulate legumes native to temperate and tropical monsoon Australia. – Mol.
Phylogen. Evol. 63: 265-277.
Sterling C. 1966. Comparative morphology of
the carpel in the Rosaceae IX.
Spiraeoideae: Quillajeae, Sorbarieae. – Amer. J. Bot. 53: 951-960.
Stirton CH. 1981a. Psoraleeae. – In:
Polhill RM, Raven PH (eds), Advances in legume systematics 1, Royal Botanic
Gardens, Kew, pp. 337-343.
Stirton CH. 1981b. Petal sculpturing in
papilionoid legumes. – In: Polhill RM, Raven PH (eds), Advances in legume
systematics 1, Royal Botanic Gardens, Kew, pp. 771-788.
Stirton CH. 1981c. Studies in the
Leguminosae-Papilionoideae of southern Africa. – Bothalia 13: 317-325.
Stirton CH. 1986. Polhillia, a new
genus of papilionoid legumes endemic to South Africa. – South Afr. J. Bot.
52: 167-180.
Stirton CH (ed). 1987. Advances in legume
systematics 3. – Royal Botanic Gardens, Kew.
Stirton CH, Muasya AM. 2016. Seven new
species and notes on the genus Aspalathus (Crotalarieae, Fabaceae). – South Afr. J. Bot. 104:
35-46.
Stirton CH, Muasya AM. 2017. Ten new species
and a new record for the genus Otholobium (Psoraleeae, Leguminosae)
from South Africa. – Kew Bull. 72: 50. doi 10.1007/S12225-017-9722-5
Straub SCK, Doyle JJ. 2014. Molecular
phylogenetics of Amorpha (Fabaceae): an evaluation of monophyly,
species relationships, and poyploid origins. – Mol. Phylogen. Evol. 76:
49-66.
Styer CH. 1977. Comparative anatomy and
systematics of Moutabeae (Polygalaceae). – J. Arnold Arbor.
58: 109-145.
Subramaniam S, Pandey AK, Rather SA. 2015. A
revised circumscription of the species in Bracteatae complex (section
Calycinae) in the genus Crotalaria L.: evidence from nuclear
and chloroplast markers. – Plant Syst. Evol. 301: 2261-2290.
Swanepoel W, Roux MM le, Wojciechowski MF,
Wyk AE van. 2015. Oberholzeria (Fabaceae subfam. Faboideae), a new
monotypic legume genus from Namibia. – PloS One 10(3): e0122080.
Taeb F, Zarre S, Podlech D, Tillich H-J,
Kazempour Osaloo S, Maassoumi AA. 2007. A contribution to the phylogeny of
annual species of Astragalus (Fabaceae) in the Old World using hair
micromorphology and other morphological characters. – Feddes Repert. 118:
206-227.
Tahiri H, Ouyahya A, El Alaoui-Faris F-E.
1999. Étude du tegument des graines des genres Cytisus L.,
Argyrocytisus (Maire) Raynaud, Chamaecytisus Link et
Genista L. (Section Teline Medik.) (Fabaceae) au Maroc. – Acta Bot. Malac.
24: 53-61.
Talavera S, Gibbs P. 1996. Novedades
taxonómicas, nomenclaturales y corológicas de Genisteae (Adans.) Benth. de
Marruecos. – Lagascalia 18: 266-271.
Talavera S, Salgueiro FJ. 1999. Sobre el
tratamiento de la tribu Cytiseae Bercht. & J. Presl (Papilionoideae,
Leguminosae) en “Flora Iberica”. – An. Jard. Bot. Madrid 57: 200-218.
Tang HL. 2005. Studies on systematics of the
genus Hedysarum L. from China. – M.Sc. thesis, Northwest A&F
University, China.
Tateishi H, Ohashi H. 1981. Eastern Asiatic
species of Mucuna (Leguminosae). – Bot. Mag. (Tokyo) 94: 91-105.
Tateishi Y, Maxted N. 2002. New species and
combinations in Vigna subgenus Ceratotropis (Piper) Verdc.
(Leguminosae, Phaseoleae). – Kew Bull. 57: 625-633.
Taubert P. 1894. Leguminosae. – In: Engler
A, Prantl K (eds), Die natürlichen Pflanzenfamilien, III(3), W. Engelmann,
Leipzig, pp. 70-388.
Taubert P. 1896. Leguminosae africanae 1. –
Engl. Bot. Jahrb. Syst. 23: 172-196.
Taylor JM, Crips MD. 1992. A revision of
Chorizema (Leguminosae: Mirbelieae). – Aust. Syst. Bot. 5:
249-335.
Taylor NL, Quesenberry KH, Anderson MK. 1979.
Genetic system relationships in Trifolium. – Econ. Bot. 33:
431-441.
Taylor NL, Quarles RF, Anderson MK. 1980.
Methods of overcoming interspecific barriers in Trifolium. –
Euphytica 33: 431-441.
Taylor RA. 1937. Studies on the genera
Cytisus and Genista. – Trans. Bot. Soc. Edinb. 32:
278-286.
Teppner H. 2007. Polyad development and
karyology in Inga and Calliandra (Mimosaceae-Ingeae): a reply
to a recent paper in Flora. – Phyton 47: 1-46.
Teppner H, Stabentheiner E. 2007. Anther
opening, polyad presentation, pollenkitt and pollen adhesive in four
Calliandra species (Mimosaceae-Ingeae). – Phyton 47: 291-320.
Teppner H, Stabentheiner E. 2010.
Pararchidendron pruinosum (Mimosaceae-Ingeae): ESEM investigations on
anther opening, polyad presentation and stigma. – Phyton 50: 109-126.
Terra V, Garcia FCP, Queiroz LP de, Bank M
van der, Miller JT. 2017. Phylogenetic relationships in Senegalia
(Leguminosae-Mimosoideae) emphasizing the South American lineages. – Syst.
Bot. 42: 458-464.
The Legume Phylogeny Working Group (LPWG).
2017. A new subfamily classification of the Leguminosae based on a
taxonomically comprehensive phylogeny. – Taxon 66: 44-77.
Thiele KR, Funk VA, Iwatsuki K, Morat P, Peng
CI, Raven PH, Sarukhan J, Seberg O. 2011. The controversy over the
retypification of Acacia Mill. with an Australian type: a pragmatic
view. – Taxon 60: 194-198.
Thompson IR. 2001. Morphometric analysis and
revision of eastern Australian Hovea (Brongniartieae-Fabaceae). – Aust. Syst. Bot. 14:
1-99.
Thompson IR. 2010a. A revision of
Plagiocarpus (Brongniartieae: Fabaceae). – Muelleria 28: 40-52.
Thompson IR. 2010b. A revision of the
leafless species of Templetonia (Brongniartieae: Fabaceae). – Muelleria 28: 53-65.
Thompson IR. 2010c. A revision of
Cristonia (Brongniartieae: Fabaceae). – Muelleria 28: 66-73.
Thompson IR. 2011. A revision of
Muelleranthus, Ptychosema and Aenictophyton (Fabaceae: Bossiaeeae). – Muelleria 29:
173-189.
Thompson IR, Ladiges PY, Ross JH. 2001.
Phylogenetic studies of the tribe Brongniartieae (Fabaceae) using nuclear DNA (ITS-1) and
morphological data. – Syst. Bot. 26: 557-570.
Thompson J. 1990. New species and new
combinations in the genus Swainsona (Fabaceae) in New South Wales. –
Telopea 4: 1-5.
Thompson J. 1993. A revision of the genus
Swainsona (Fabaceae). –
Telopea 5: 427-581.
Thulin M. 1976. Two new species of
Trifolium from Ethiopia. – Bot. Not. 129: 167-171.
Thulin M. 1978. New records of Ethiopian
Leguminosae. – Bot. Not. 131: 71-74.
Thulin M. 1981. Notes on some species of
Rhynchosia (Leguminosae-Papilionoideae) from NE Africa and Yemen. –
Nord. J. Bot. 1: 37-42.
Thulin M. 1982a. New and noteworthy species
of Indigofera (Leguminosae) from NE Africa. – Nord. J. Bot. 2:
41-50.
Thulin M. 1982b. New species of
Crotalaria (Leguminosae-Papilionoideae) from Ethiopia. – Nord. J.
Bot. 2: 115-119.
Thulin M. 1982c. Wajira gen. et sp.
nov. (Leguminosae) from Kenya. – Nord. J. Bot. 2: 475-478.
Thulin M. 1983. Leguminosae of Ethiopia. –
Opera Bot. 68: 1-23.
Thulin M. 1985. Revision of
Taverniera (Leguminosae-Papilionoideae). – Symb. Bot. Ups. 25(1):
44-95.
Thulin M. 1986. Comparison of the legume
flora in Ethiopia and Somalia. – Symb. Bot. Ups. 26(2): 114-119.
Thulin M. 1989. New or noteworthy species of
Leguminosae in NE tropical Africa. – Nord. J. Bot. 8: 457-488.
Thulin M. 1990a. Two new species of
Ormocarpum (Leguminosae) from Somalia. – Nord. J. Bot. 10:
433-436.
Thulin M. 1990b. Seven new species and one
new record of Polygala (Polygalaceae) from Somalia. –
Nord. J. Bot. 10: 465-475.
Thulin M. 1991. Two new species of
Vigna (Leguminosae) from Somalia. – Nord. J. Bot. 11: 543-547.
Thulin M. 1992. Eight new species and a new
record of Indigofera and Microcharis
(Leguminosae-Indigofereae) from Somalia. – Nord. J. Bot. 12: 315-326.
Thulin M. 1994. Two new species of
Abrus (Leguminosae-Papilionoideae) from Somalia. – Nord. J. Bot. 14:
55-58.
Thulin M. 1995. Indigofera
rubromarginata sp. nov. (Leguminosae) from southern Arabia, with a note on
I. eremophila. – Nord. J. Bot. 15: 519-521.
Thulin M. 1996. A new species of
Microcharis (Leguminosae) from Somalia. – Nord. J. Bot. 16:
295-296.
Thulin M. 1999 [2000]. Chapmannia
(Leguminosae-Stylosanthinae) extended. – Nord. J. Bot. 19: 597-607.
Thulin M. 2007. Acacia fumosa sp.
nov. (Fabaceae) from eastern
Ethiopia. – Nord. J. Bot. 25: 272-274.
Thulin M, Hassan AS. 1996. A new species of
Acacia (Leguminosae) from the Horn of Africa. – Nord. J. Bot. 16:
303-306.
Thulin M, Lavin M. 2001. Phylogeny and
biogeography of the Ormocarpum group (Fabaceae): a new genus
Zygocarpum from the Horn of Africa region. – Syst. Bot. 26:
299-317.
Thulin M, Guinet P, Hunde A. 1981.
Calliandra (Leguminosae) in continental Africa. – Nord. J. Bot. 1:
27-34.
Thulin M, Lavin M, Pasquet R, Delgado-Salinas
A. 2004. Phylogeny and biogeography of Wajira (Leguminosae): a
monophyletic segregate of Vigna centered in the Horn of Africa region.
– Syst. Bot. 29: 903-920.
Thulin M, Phillipson PB, Lavin M. 2013.
Peltiera (Fabaceae), the
coming and going of an “extinct” genus in Madagascar. – Adansonia 35:
61-71.
Tietz S. 1988. Revision von
Astragalus L. sect. Campylanthus Bunge, sect.
Microphysa Bunge und sect. Poterion Bunge. – Mitt. Bot.
Staatssamml. München 27: 135-380.
Tietz S, Zarre S. 1994. Revision von
Astragalus L. sect. Megalocystis Bunge (Fabaceae). – Sendtnera 2: 287-363.
Tikhomirov VN, Sokoloff DD. 1996. On the
genera Hammatolobium Fenzl and Tripodion Medik.
(Papilionaceae, Loteae s.l.). – Feddes Repert. 107: 209-217.
Tindale MD. 1975. Notes on Australian taxa of
Acacia 4. – Telopea 1: 68-83.
Tindale MD. 1978. Notes on Australian taxa of
Acacia 5. – Telopea 1: 371-386.
Tindale MD. 1980a. Notes on Australian taxa
of Acacia 6. – Telopea 1: 429-449.
Tindale MD. 1980b. Notes on Australian taxa
of Acacia 7. – Telopea 2: 113-125.
Tindale MD. 1984. Two new Eastern Australian
species of Glycine Willd. (Fabaceae). – Brunonia 7: 207-213.
Tindale MD. 1986. Taxonomic notes on three
Australian and Norfolk Island species of Glycine Willd. (Fabaceae: Phaseoleae) including the
choice of a neotype for G. clandestina Wendl. – Brunonia 9:
179-191.
Tindale MD, Craven LA. 1988. Three new
species of Glycine (Fabaceae: Phaseoleae) from north-western
Australia, with notes on amphicarpy in the genus. – Aust. Syst. Bot. 1:
399-410.
Tindale MD, Kodela PG. 1991. Acacia
tessellata, A. cangaiensis and A. dangarensis (Fabaceae, Mimosoideae), three new
species from northern New South Wales, Australia. – Aust. Syst. Bot. 4:
579-589.
Tindale MD, Kodela PG. 1992. New species of
Acacia (Fabaceae,
Mimosoideae) from tropical Australia. – Telopea 5: 53-66.
Tindale MD, Kodela PG, Davis SJ. 1992.
Acacia bulgansis and A. matthewii, two new species of
Acacia section Juliflorae (Fabaceae, Momosoideae) allied to A.
cheelii, eastern New South Wales, Australia. – Aust. Syst. Bot. 5:
645-655.
Tindale MD, Bedward M, Kodela PG. 1996.
Acacia multistipulosa and A. rigescens (Fabaceae: Mimosoideae, Acacia
sect. Juliflorae), two new species from the Northern Territory,
Australia. – Aust. Syst. Bot. 9: 8959-866.
Tissot M, Weberling F. 1993.
Infloreszenzuntersuchungen an Leguminosae-Caesalpinioideae. – Trop. Subtrop.
Pflanzenwelt 84: 1-59.
Tiwari RD, Bajpai M. 1981. A new
flavonol-8-C-glycoside form the leaves of Cassia sophora
Linn. – Indian J. Chem., Sect. B, 20: 437-438.
Tjepkema JD. 1979. Oxygen relations in
leguminous and actinorhizal nodules. – In: Gordon JC, Wheeler CT, Perry DA
(eds), Symbiotic nitrogen fixation in the management of temperate forests,
Forest Research Laboratory, Oregon State University, Corvallis, Oregon, pp.
175-186.
Toon A, Cook LG, Crisp MD. 2014. Evolutionary
consequences of shifts to bird-pollination in the Australian pea-flowered
legumes (Mirbelieae and Bossiaeeae). – BMC Evol. Biol. 2014, 14: 43.
Torke BM. 2004. Two new species of
Swartzia (Leguminosae) from the Amazon Basin of Brazil, with notes on
the genus and a key to the unifoliolate species. – Syst. Bot. 29: 358-365.
Torke BM. 2007. Three new species of
Swartzia (Leguminosae-Papilionoideae) from northern South America. –
Bot. J. Linn. Soc. 153: 343-355.
Torke BM, Mansano V de F. 2009. A
phylogenetically based sectional classification of Swartzia
(Leguminosae-Papilionoideae). – Taxon 58: 913-924.
Torke BM, Schaal BA. 2008. Molecular
phylogenetics of the species-rich neotropical genus Swartzia
(Leguminosae, Papilionoideae) and related genera of the swartzioid clade. –
Amer. J. Bot. 95: 215-228.
Tosso F, Hardy OJ, Doucet J-L, Daïnou K,
Kaymak E, Migliore J. 2018. Evolution in the Amphi-Atlantic tropical genus
Guibourtia (Fabaceae,
Detarioideae), combining NGS phylogeny and morphology. – Mol. Phylogen. Evol.
120: 83-93.
Tozzi AMGA, Agostini K, Sazima M. 2005. A new
species of Mucuna Adans. (Leguminosae, Papilionoideae, Phaseoleae)
from southeastern Brazil, with a key to Brazilian species. – Taxon 54:
451-455.
Trautvetter ER. 1875. Catalogus viciearum
rossicarum. – Acta Horti Petropol. 3: 33-83.
Trenchard LJ, Harris PJC, Smith SJ,
Pasiecznik NM. 2008. A review of ploidy in the genus Prosopis
(Leguminosae). – Bot. J. Linn. Soc. 156: 425-438.
Trindade R de, Silva JK da, Setzer WN. 2018.
Copaifera of the Neotropics: a review of the phytochemistry and
pharmacology. – Intern. J. Mol. Sci. 19: 1511. doi: 10.3390/ijms19051511
Trusty JL, Johnson KJ, Lockaby BG, Goertzen
LR. 2007. Bi-parental cytoplasmic DNA inheritance in Wisteria (Fabaceae): evidence from a natural
experiment. – Plant Cell Phys. 48: 662-665.
Tschunko AH, Nickerson NH. 1976. The
androecium of Suriana maritima. – Rhodora 78: 162-164.
Tsutsupa TA. 2003. Biomorphological analysis
of some species of Leguminosae tribus Loteae. – Ph.D. diss., Moscow
University, Russia. [In Russian]
Tucker SC. 1984. Unidirectional organ
initiation in leguminous flowers. – Amer. J. Bot. 71: 1139-1146.
Tucker SC. 1985. Floral development in
Caesalpinia (Leguminosae). – Amer. J. Bot. 72: 1424-1434.
Tucker SC. 1987a. Floral initiation and
development in legumes. – In: Stirton CH (ed), Advances in legume systematics
3, Royal Botanic Gardens, Kew, pp. 183-239.
Tucker SC. 1987b. Pseudoracemes in
papilionoid legumes: their nature, development, and variation. – Bot. J.
Linn. Soc. 95: 181-206.
Tucker SC. 1988. Dioecy in Bauhinia
resulting from organ suppression. – Amer. J. Bot. 75: 1584-1597.
Tucker SC. 1989a. Evolutionary implications
of floral ontogeny in legumes. – In: Stirton CH, Zarucchi JL (eds), Advances
in legume biology, Missouri Botanical Garden, St Louis, Monogr. Syst. Bot.
Missouri Bot. Gard. 29: 59-75.
Tucker SC. 1989b. Overlapping organ
initiation and common primordia in flowers of Pisum sativum
(Leguminosae: Papilionoideae). – Amer. J. Bot. 76: 714-729.
Tucker SC. 1990. Loss of floral organs in
Ateleia (Leguminosae: Papilionoideae: Sophoreae). – Amer. J. Bot.
77: 750-761.
Tucker SC. 1991. Helical floral organogenesis
in Gleditsia, a primitive caesalpinioid legume. – Amer. J. Bot. 78:
1130-1149.
Tucker SC. 1992. The developmental basis for
sexual expression in Ceratonia siliqua (Leguminosae: Cassieae). –
Amer. J. Bot. 79: 318-327.
Tucker SC. 1996a. Trends in evolution of
floral ontogeny in Cassia sensu stricto, Senna, and
Chamaecrista. – Amer. J. Bot. 83: 687-711.
Tucker SC. 1996b. Stamen structure and
development in legumes, with emphasis on poricidal stamens of caesalpinioid
tribe Cassieae. – In: D’Arcy WG, Keating RC (eds), The anther: form,
function and phylogeny, Cambridge University Press, Cambridge, pp. 236-254.
Tucker SC. 1998. Floral ontogeny in legume
genera Petalostylis, Labichea, and Dialum
(Caesalpinioideae: Cassieae), a series in floral reduction. – Amer. J. Bot.
85: 184-208.
Tucker SC. 2000a. Evolutionary loss of sepals
and/or petals in Detarioid legume taxa Aphanocalyx,
Brachystegia, and Monopetalanthus (Leguminosae:
Caesalpinioideae). – Amer. J. Bot. 87: 608-624.
Tucker SC. 2000b. Floral development in tribe
Detarieae (Leguminosae: Caesalpinioideae): Amherstia,
Brownea, and Tamarindus. – Amer. J. Bot. 87: 1385-1407.
Tucker SC. 2000c. Floral development and
homeosis in Saraca (Leguminosae: Caesalpinioideae: Detarieae). –
Intern. J. Plant Sci. 161: 537-549.
Tucker SC. 2001a. The ontogenetic basis for
missing petals in Crudia (Leguminosae: Caesalpinioideae: Detarieae).
– Intern. J. Plant Sci. 162: 83-89.
Tucker SC. 2001b. Floral development in
Schotia and Cynometra (Leguminosae: Caesalpinioideae:
Detarieae). – Amer. J. Bot. 88: 1164-1180.
Tucker SC. 2002a. Floral ontogeny of
Cercis (Leguminosae: Caesalpinoideae: Cercideae): does it show
convergence with papilionoids? – Intern. J. Plant Sci. 163: 75-87.
Tucker SC. 2002b. Comparative floral
development in Detarieae (Leguminosae: Caesalpinioideae) 1. Radially
symmetrical taxa lacking organ suppression. – Amer. J. Bot. 89: 875-887.
Tucker SC. 2002c. Comparative floral
development in Detarieae (Leguminosae: Caesalpinioideae) 2. Zygomorphic taxa
with petal and stamen suppression. – Amer. J. Bot. 89: 888-907.
Tucker SC. 2002d. Floral ontogeny in
Sophoreae (Leguminosae: Papilionoideae) 3. Radial symmetry and random petal
aestivation in Cadia purpurea. – Amer. J. Bot. 89: 748-757.
Tucker SC. 2003a. Floral development in
legumes. – Plant Physiol. 131: 911-926.
Tucker SC. 2003b. Floral ontogeny in
Swartzia (Leguminosae: Papilionoideae: Swartzieae): distribution and
role of the ring meristem. – Amer. J. Bot. 90: 1271-1292.
Tucker SC. 2003c. Comparative floral ontogeny
in Detarieae (Leguminosae: Caesalpinioideae) III. Adaxially initiated whorls in
Julbernardia and Sindora. – Intern. J. Plant Sci. 164:
275-286.
Tucker SC. 2006. L. A. S. Johnson review 6.
Floral ontogeny of Hardenbergia violacea (Fabaceae: Faboideae: Phaseoleae) and
taxa of tribes Bossiaeeae and Mirbelieae, with emphasis on presence of
pseudoraceme inflorescences. – Aust. Syst. Bot. 19: 193-210.
Tucker SC, Douglas AW. 1994. Ontogenetic
evidence and phylogenetic relationships among basal taxa of legumes. – In:
Ferguson IK, Tucker S (eds), Advances in legume systematics 6: structural
botany, Royal Botanic Gardens, Kew, pp. 11-32.
Tucker SC, Kantz KE. 1997. Comparative floral
development and evolution in tribe Caesalpinieae (Leguminosae:
Caesalpinioideae). Haematoxylum. – Amer. J. Bot. 84: 1047-1063.
Tucker SC, Kantz KE. 2001. Open carpels with
ovules in Fabaceae. – Intern. J.
Plant Sci. 162: 1065-1073.
Tucker SC, Stirton 1991. Development of the
cymose inflorescence, cupulum and flower of Psoralea pinnata
(Leguminosae: Papilionoideae: Psoraleeae). – Bot. J. Linn. Soc. 106:
209-227.
Turini FG, Bräuchler C, Heubl G. 2010.
Phylogenetic relationships and evolution of morphological characters in
Ononis L. (Fabaceae). –
Taxon 59: 1077-1090.
Turner BL. 1956. Chromosome numbers in the
Leguminosae. – Amer. J. Bot. 43: 577-582.
Turner BL. Revision of the genus
Piptanthus (Fabaceae:
Thermopsideae). – Brittonia 32: 281-285.
Turner BL. 1981. Thermopsideae. – In:
Polhill RM, Raven PH (eds), Advances in legume systematics 1, Royal Botanic
Gardens, Kew, pp. 403-407.
Turner BL, Fearing OS. 1959. Chromosome
numbers in the Leguminosae II. African species including phyletic
interpretations. – Amer. J. Bot. 46: 49-57.
Turner BL, Fearing OS. 1964. A taxonomic
study of the genus Amphicarpaea (Leguminosae). – Southw. Natur. 9:
207-218.
Turner GW. 1986. Comparative development of
secretory cavities in the tribes Amorpheae and Psoraleeae (Leguminosae:
Papilionoideae). – Amer. J. Bot. 73: 1178-1192.
Ulibarri EA. 1978. Novedades en
Adesmia (Leguminosae). – Hickenia 1: 121-124.
Ulibarri EA. 1980. Notas sobre
Adesmia I (Leguminosae-Papilionoideae). – Darwiniana 22: 493-498.
Ulibarri EA. 1982a. Notas sobre
Adesmia DC. II (Leguminosae-Papilionoideae). – Darwiniana 24:
267-281.
Ulibarri EA. 1982b. Nueva especie de
Adesmia DC. (Leguminosae-Papilionoideae) de Chile. – Hickenia 1:
277-290.
Ulibarri EA. 1984. Notas sobre
Adesmia DC. III (Leguminosae-Papilionoideae). – Darwiniana 25:
355-360.
Ulibarri EA. 1987. Las especies de
Adesmia de la serie Microphyllae
(Leguminosae-Papilionoideae). – Darwiniana 27: 315-388.
Ulibarri EA. 1990. Notas sobre
Adesmia IV (Leguminosae-Papilionoideae). – Darwiniana 30:
291-292.
Uribe-Echevarria PM, Urrutia P. 1992. Nuevos
datos sobre la sección Erinacoides Spach del género Genista
L. en la Península Ibérica. – Est. Mus. Ci. Nat. Alava 7: 103-114.
Uysal T, Ertuğrul K, Bozkurt M. 2014. A new
genus segregated from Thermopsis (Fabaceae: Papilionoideae):
Vuralia. – Plant Syst. Evol. 300: 1627-1637.
Vaillancourt RE, Weeden NF, Bruneau A, Doyle
JJ. 1993. Chloroplast DNA phylogeny of Old World Vigna (Leguminosae).
– Syst. Bot. 18: 642-651.
Valizadeh M, Kang KK, Kanno A, Kameya T.
1996. Analysis of genetic distance among nine Medicago species by
using DNA polymorphisms. – Breeding Sci. 46: 7-10.
Valsecchi F. 1993. Il genere Genista
L. in Italia I. Le specie delle sezioni Erinacoides Spach,
Ephedrospartum Spach, Aureospartum sect. nova. – Webbia 48:
779-824.
Vander Stappen J, De Laet J, Gama-López S,
Campenhout S van, Volckaert G. 2002. Phylogenetic analysis of
Stylosanthes (Fabaceae)
based on the internal transcribed spacer region (ITS) of nuclear ribosomal DNA.
– Plant Syst. Evol. 234: 27-51.
Varela ES, Lima JPMS, Galdino AS, Pinto LS,
Bezerra WM, Nunes EP, Alves MAO, Grangeiro TB. 2004. Relationships in subtribe
Diocleinae (Leguminosae; Papilionoideae) inferred from internal transcribed
spacer sequences from nuclear ribosomal DNA. – Phytochemistry 65: 59-69.
Vasilčenko IT. 1953. A review of the species
of the genus Trigonella L. – Tr. Bot. Inst. Akad. Nauk SSR, Ser. 1,
Flora Sist. Vysš. Rast. 14: 124-269. [in Russian]
Vasilčenko IT. 1965. K voprosu o genezise
roda ostrolodočnik Oxytropis DC. – Bot. Žurn. 50: 313-322.
Vassal J. 1969. Contribution à l’étude de
la morphologie des plantules d’Acacia. Acacias africains. – Bull.
Soc. Hist. Nat. Toulouse 105: 55-111.
Vassal J. 1972. Apport des recherches
ontogéniques et seminologiques à l’étude morphologique, taxonomique et
phylogénique du genre Acacia. – Bull. Soc. Hist. Nat. Toulouse 108:
125-247.
Vatanparast M, Takayama K, Sousa MS, Tateishi
Y, Kajita T. 2011. Origin of Hawaiian endemic species of Canavalia (Fabaceae) from sea-dispersed species
revealed by chloroplast and nuclear DNA sequences. – J. Jap. Bot. 86:
15-25.
Velásquez DC, Agostini G. 1981. Dos nuevas
especies Venezolanas de Brownea (Leguminosae-Caesalpinioideae). –
Ernstia 5: 1-16.
Venkatesh CS. 1956. The special mode of
dehiscence of anthers of Polygala and its significance in autogamy.
– Bull. Torrey Bot. Club 83: 19-26.
Verdcourt B. 1968. The identities of
Dolichos trilobus L. and Dolichos trilobatus L. – Taxon 17:
170-173.
Verdcourt B. 1970a. Studies in the
Leguminosae-Papilionoideae for the “Flora of tropical East Africa” II. –
Kew Bull. 24: 235-307.
Verdcourt B. 1970b. Studies in the
Leguminosae-Papilionoideae for the “Flora of tropical East Africa” III. –
Kew Bull. 24: 379-447.
Verdcourt B. 1970c. Studies in the
Leguminosae-Papilionoideae for the “Flora of tropical East Africa” IV. –
Kew Bull. 24: 507-569.
Verdcourt B. 1971a. Studies in the
Leguminosae-Papilionoideae for the “Flora of tropical East Africa” V. –
Kew Bull. 25: 65-169.
Verdcourt B. 1971b. Aeschynomene.
– In: Gillett JB, Polhill RM, Verdcourt B (eds), Flora of tropical East
Africa, Leguminosae, Papilionoideae, Royal Botanic Gardens, Kew, pp.
364-406.
Verdcourt B. 1971c. Phaseoleae. – In:
Milne-Redhead E, Polhill RM (eds), Flora of tropical East Africa, Leguminosae,
part 3-4, Crown Agents, London, pp. 503-807.
Verdcourt B. 1974. Summary of the
Leguminosae-Papilionoideae-Hedysareae (sensu lato) of Flora Zambesiaca. –
Kirkia 9: 359-556.
Verdcourt B. 1978a. The demise of two
geocarpic legume genera. – Taxon 27: 219-222.
Verdcourt B. 1978b. New taxa of Leguminosae
from Papua. – Kew Bull. 32: 455-473.
Verdcourt B. 1978c. Miscellaneous notes on
New Guinea. Legumonosae: Archidendron and Mucuna. – Kew
Bull. 33: 125-126.
Verdcourt B. 1979. A manual of New Guinea
legumes. – Bot. Bull. 11, Office of Forests, Divison of Botany, Lae, Papua
New Guinea, pp. 1-645.
Verdcourt B. 1980. The classification of
Dolichos L. emend. Verdc., Lablab Adans., Phaseolus
L., Vigna Savi and their allies. – In: Summerfield RJ, Bunting AH
(eds), Advances in legume science, Royal Botanic Gardens, Kew, pp. 45-48.
Verdcourt B. 1981. New taxa of
Mucuna (Legminosae-Phaseoleae) from East Africa and Australia. – Kew
Bull. 35: 743-752.
Verdcourt B. 1982. A revision of
Macrotyloma (Leguminosae). – Hooker’s Icones Plant. 38, 4: 1-138,
Tab. 3776-3800, Entham-Moxon Trustees, Kew.
Verdcourt B, Halliday P. 1978. A revision of
Psophocarpus (Leguminosae-Papilionoideae-Phaseoleae). – Kew Bull.
33: 191-227.
Verdoorn IC. 1928. A revision of the
Crotalarias of South and South-East tropical Africa. – Bothalia 2:
371-420.
Verkerke W. 1984. Ovule and seed of
Xanthophyllum (Polygalaceae). – Blumea 29:
409-421.
Verkerke W. 1985. Ovules and seeds of the Polygalaceae. – J. Arnold Arbor.
66: 353-394.
Verkerke W. 1991. Fruits and seeds of
Balgoya pacifica (Polygalaceae) from New Caledonia.
– Bull. Mus. Natl. Hist. Nat. Paris, sect. B, Adansonia, sér. IV, 13:
9-12.
Verkerke W, Bouman F. 1980. Ovule ontogeny
and its relations to seed-coat structure in some species of Polygala
(Polygalaceae). – Bot. Gaz.
141: 277-282.
Verma DPS, Stanley J. 1989. The
legume-Rhizobium equation: a coevolution of two genomes. – In:
Stirton CH, Zarucchi JL (eds), Advances in legume biology, Missouri Botanical
Garden, St. Louis, Missouri, pp. 545-557.
Verma RC, Raina SN. 1980. Cytogenetics of
Crotalaria II. Male meiosis in 8 species of Crotalaria. –
Cytologia 45: 297-306.
Vessey JK, Pawlowski K, Bergman B. 2004.
Root-based N-2-fixing symbioses: legumes, actinorrhizal plants,
Parasponia sp. and cycads. – Plant and Soil 266: 205-230.
Vicioso C. 1953. Genisteas españolas I.
Genista-Genistella. – Bol. Inst. Forest. Invest. Exper.
Madrid 67: 1-153.
Vicioso C. 1962. Revisión del género
Ulex en España. – Bol. Inst. Forest. Invest. Exper. Madrid 80:
1-57.
Villanueva E, Ramos A. 1986. Contribución al
estudio polínico de Polygala L. (Polygalaceae) en la Península
Ibérica. – An. Jard. Bot. Madrid 42: 377-388.
Villiers JF. 1983. Le genre
Pseudoprosopis Harms (Mimosaceae) en Afrique. – Bull. Jard. Bot.
État Bruxelles 53: 417-436.
Vioque J, Pastor J. 1991. Aportaciones al
conocimeiento cariológico de la tribu Loteae (Fabaceae). – Lazaroa 12: 9-19.
Viviani T, Conte L, Cristofolini G, Speranza
M. 1991. Sero-systematic and taximetric studies on the Phaseoleae (Fabaceae) and related tribes. – Bot.
J. Linn Soc. 105: 113-136.
Voronchikhin VV. 1981. The identification of
Vicia species by fruits and seeds. – Herald Moscow State Univ.,
Biology 2: 22-29. [In Russian]
Vural C, Ekici M, Akan H, Aytaç Z. 2008.
Seed morphology and its systematic implications for genus Astragalus
L. sections Onobrychoidei DC., Uliginosi Gray and
Ornithopodium Bunge (Fabaceae). – Plant Syst. Evol. 274:
255-263.
Wagstaff SJ, Heenan PB, Sanderson MJ. 1999.
Classification, origins, and patterns of diversification in New Zealand
Carmichaelinae (Fabaceae). – Amer.
J. Bot. 86: 1346-1356.
Wang H, Chen J, Wen J, Tadege M, Li G, Liu Y,
Mysore KS, Ratet P, Chen R. 2008. Control of compound leaf development by
FLORICAULA/LEAFY ortholog SINGLE LEAFLET1 in Medicago
truncatula. – Plant Physiology 146: 1759-1772.
Wang HC, Sun H, Compton JA, Yang JB. 2006. A
phylogeny of Thermopsideae (Leguminosae: Papilionoideae) inferred from nuclear
ribosomal internal transcribed spacer (ITS) sequences. – Bot. J. Linn. Soc.
151: 365-373.
Wang Q, Song Z, Chen Y, Shen S, Li Z. 2014.
Leaves and fruits of Bauhinia (Leguminosae, Caesalpinioideae,
Cercideae) from the Oligocene Ningming Formation of Guangxi, South China and
their biogeographic implications. – BMC Evol. Biol. 14: 1-13.
Warwick MC, Pennington RT. 2002. Revision of
Cyclolobium (Leguminosae-Papilionoideae). – Edinburgh J. Bot. 59:
247-257.
Warwick MC, Lewis GP. 2009. A revision of
Cenostigma (Leguminosae-Caesalpinioideae-Caesalpinieae), a genus
endemic to Brazil. – Kew Bull. 64: 135-146.
Warwick MC, Lewis GP, Lima HC de. 2008. A
reappraisal of Barnebydendron (Leguminosae: Caesalpinioideae:
Detarieae). – Kew Bull. 63: 143-149.
Waterman PG. 1994. Costs and benefits of
secondary metabolites to the Leguminosae. – In: Sprent JI, McKey D (eds),
Advances in legume systematics 5: the nitrogen factor, Royal Botanic Gardens,
Kew, pp. 129-149.
Waterman PG, Faulkner DF. 1982.
Quinolizidine/indolizidine alkaloids from the seed of Camoensia
brevicalyx. – Phytochemistry 21: 215-218.
Watson L, Dallwitz MJ. 1983. The genera of
Leguminosae-Caesalpinioideae. Anatomy, morphology, classification and keys. –
Australian National University, Canberra.
Watson LE, Hanan SA, Badr A. 2000. Molecular
phylogeny of Old World Trifolium (Fabaceae). – Plant Syst. Evol. 224:
153-171.
Webber IE. 1936. Systematic anatomy of the
woods of the Simarubaceae. – Amer. J. Bot. 23: 577-587.
Weberling F. 1974. Weitere Untersuchungen zur
Morphologie des Unterblattes bei den Dikotylen VII. Polygalales; VIII.
Koeberlinia Zucc. – Beitr. Biol. Pflanzen 50: 277-289.
Weberling F. 1989. Structure and evolutionary
tendencies of inflorescences in the Leguminosae. – In: Stirton CH, Zarucchi
JL (eds), Advances in legume biology, Monogr. Syst. Bot, Missouri Bot. Gard.,
pp. 35-58.
Weberling F, Lörcher H, Böhnke E. 1980. Die
Stipeln der Irvingioideae und Recchioideae und ihre systematische Wertung nebst
Bemerkungen zur Holzanatomie und Palynologie. – Plant Syst. Evol. 133:
261-283.
Weeden NF. 2007. Genetic changes accompanying
the domestication of Pisum sativum: Is there a common basis to the
‘domestication syndrome’ in legumes? – Ann. Bot. 100: 1017-1025.
Weekley CW, Brothers A. 2006. Failure of
reproductive assurance in chasmogamous flowers of Polygala lewtonii
(Polygalaceae), an endangered
sandhill herb. – Amer. J. Bot. 93: 245-253.
Welzen PCV, Hengst SD. 1994. The genus
Mastersia (Papilionaceae: Phaseoleae). – Blumea 30: 77-87.
Wendelbo P. 1980. New species of
Dionysia, Kickxia and Onobrychis from Iran. –
Notes Roy. Bot. Gard. Edinb. 38: 105-110.
Wendt TL. 1978. A systematic study of
Polygala Section Rhinotropis (Polygalaceae). – Ph.D. diss.,
Universitys of Texas, Austin, Texas.
Wenninger J. 1991. Revision von
Astragalus L. sect. Chlorostachys Bunge, sect.
Phyllolobium Bunge und sect. Skythropos Bunge (Leguminosae).
– Mitt. Bot. Staatssamml. München 30: 1-196.
Werff H van der. 2008. A synopsis of the
genus Tachigali (Leguminosae: Caesalpinioideae) in northern South
America. – Ann. Missouri Bot. Gard. 95: 618-661.
Westerkamp C, Weber A. 1997. Secondary and
tertiary pollen presentation in Polygala myrtifolia and allies (Polygalaceae, South Africa). –
South Afr. J. Bot. 63: 254-258.
Westerkamp C, Weber A. 1999. Keel flowers of
the Polygalaceae and Fabaceae: a functional comparison. –
Bot. J. Linn. Soc. 129: 207-221.
Wheeler E, Baas P. 1992. Fossil wood of the
Leguminosae: a case study in xylem evolution and ecological anatomy. – In:
Herendeen PS, Dilcher DL (eds), Advances in legume systematics 4: the fossil
record, Royal Botanic Gardens, Kew, pp. 281-301.
Wieringa JJ. 1999. Monopetalanthus
exit: a systematic study of Aphanocalyx, Bikinia,
Icuria, Michelsonia and Tetraberlinia (Leguminosae,
Caesalpinioideae). – Ph.D. diss. Wageningen Agricultural University Papers,
Wageningen 9-4: I-XVI, 1-320.
Wieringa JJ, Gervais GYF. 2003. Phylogenetic
analyses of combined morphological and molecular data sets on the
Aphanocalyx-Bykinia-Tetraberlinia group (Leguminosae,
Caesalpinioideae, Detarieae s.l.). – In: Klitgaard B, Bruneau A (eds),
Advances in legume systematics 10: Higher level systematics, Royal Botanic
Gardens, Kew, pp. 181-196.
Wieringa JJ, Mackinder BA, Proosdij ASJ van.
2013. Gabonius gen. nov. (Leguminosae, Caesalpinioideae, Detarieae), a
distant cousin of Hymenostegia endemic to Gabon. – Phytotaxa 142:
15-24.
Wilbur RL. 1975. A revision of the genus
Amorpha (Leguminosae-Psoraleeae). – Rhodora 77: 337-409.
Wilkinson HP. 1983. The anatomy of the
pulvinus in various Mimosoideae in relation to function. – Notes Jodrell Lab.
(Kew) 10: 1-11.
Williams AV, Miller JT, Small I, Nevill PG,
Boykin LM. 2016. Integration of complete chloroplast genome sequences with
small amplicon datasets improves phylogenetic resolution in Acacia.
– Mol. Phylogen. Evol. 96: 1-8.
Wilmot-Dear CM. 1984. A revision of
Mucuna (Leguminosae-Phaseoleae) in China and Japan. – Kew Bull. 39:
23-65.
Wilmot-Dear CM. 1987. A revision of
Mucuna (Leguminosae: Phaseoleae) in the Indian Subcontinent and Burma.
– Kew Bull. 42: 23-46.
Wilmot-Dear CM. 1990. A revision of
Mucuna (Leguminosae: Phaseoleae) in the Pacific. – Kew Bull. 45:
1-35.
Wilmot-Dear CM. 1991. A revision of
Mucuna (Leguminosae-Phaseoleae) in the Philippines. – Kew Bull. 46:
213-251.
Wilmot-Dear CM. 1992. A revision of
Mucuna (Leguminosae: Phaeoleae) in Thailand, Indochina and the Malay
Peninsula. – Kew Bull. 47: 203-245.
Wilson PG. 1994. Two new species of
Indigofera (Fabaceae:
Indigofereae) from south-western Queensland. – Telopea 5: 631-635.
Wilson PG. 2001. Some name changes in
Indigofera (Fabaceae). –
Taxon 50: 491-493.
Wilson PG, Rowe RRM. 1994. The Indigofera
trita complex (Fabaceae:
Indigofereae) in Australia. – Telopea 5: 637-645.
Wilson PG, Rowe RRM. 2004. A revision of the
Indigofereae (Fabaceae) in Australia
1. Indigastrum and the simple or unifoliolate species of
Indigofera. – Telopea 10: 651-682.
Wilson PG, Rowe RRM. 2008a. Three new species
of Indigofera (Fabaceae:
Faboideae) from Cape York Peninsula. – Telopea 12: 285-292.
Wilson PG, Rowe RRM. 2008b. A revision of the
Indigofereae (Fabaceae) in Australia
2. Indigofera species with trifoliolate and alternately pinnate
leaves. – Telopea 12: 293-307.
Wink M, Mohamed GIA. 2003. Evolution of
chemical defense traits in the Leguminosae: mapping of distribution patterns of
secondary metabolites on a molecular phylogeny inferred from nucleotide
sequences of the rbcL gene. – Biochem. Syst. Ecol. 31: 897-917.
Wink M, Meißner C, Witte L. 1995. Patterns
of quinolizidine alkaloids in 56 species of the genus Lupinus. –
Phytochemistry 38: 139-153.
Wiriadinata H, Ohashi H. 1990. Four new
species of Mucuna (Legumonosae) of the Lesser Sunda Islands. – J.
Jap. Bot. 65: 97-108.
Wiriadinata H, Ohashi H, Adema F. 2016. Notes
on Malesian Fabaceae
(Leguminosae-Papilionoideae). 16. The genus Mucuna. – Blumea 61: 90-124.
Wirz H. 1910. Beiträge zur
Entwicklungsgeschichte von Sciaphila spec. und von Epirrhizanthes
elongata Bl. – Flora 101: 395-446.
Wit HCD de. 1942. Conspectus of the genus
Archidendron F. von Mueller. – Bull. Bot. Gard. Buitenzorg 17:
256-272.
Wit HCD de. 1952. A revision of the genus
Archidendron F. Muell. (Mimosaceae). – Reinwardtia 2: 69-96.
Wit HCD de. 1956. A revision of the Malaysian
Bauhinieae. – Reinwardtia 3: 381-547.
Wojciechowski MF. 2003. Reconstructing the
phylogeny of legumes (Leguminosae): an early 21st century
perspective. – In: Klitgaard BB, Bruneau A (eds), Advances in legume
systematics 10: Higher level systematics, Royal Botanic Gardens, Kew, pp.
5-35.
Wojciechowski MF. 2005. Astragalus
(Fabaceae): a molecular phylogenetic
perspective. – Brittonia 57: 382-396.
Wojciechowski MF. 2013. The origin and
phylogenetic relationships of the Californian chaparral ‘paleoendemic’
Pickeringia (Leguminosae). – Syst. Bot. 38: 132-142.
Wojciechowski MF, Sanderson MJ, Baldwin BG,
Donoghue MJ. 1993. Monophyly of aneuploid Astragalus (Fabaceae): evidence from nuclear
ribosomal DNA internal transcribed spacer sequences. – Amer. J. Bot. 80:
711-722.
Wojciechowski MF, Sanderson MJ, Hu J-M. 1999.
Evidence on the monophyly of Astragalus (Fabaceae) and its major subgroups based
on nuclear ribosomal DNA ITS and chloroplast DNA trnL intron data. –
Syst. Bot. 24: 409-437.
Wojciechowski MF, Sanderson MJ, Steele KP,
Liston A. 2000. Molecular phylogeny of the “temperate herbaceous tribes” of
papilionoid legumes: a supertree approach. – In: Herendeen PS, Bruneau A
(eds), Advances in legume systematics 9: Phylogeny, Royal Botanic Gardens, Kew,
pp. 277-298.
Wojciechowski MF, Lavin M, Sanderson MJ.
2004. A phylogeny of legumes (Leguminosae) based on analysis of the plastid
matK gene resolves many well-supported subclades within the family.
– Amer. J. Bot. 91: 1846-1862.
Wood CE. 1949. The American barbistyled
species of Tephrosia (Leguminosae). – Rhodora 51: 193-384.
Wood JRI, Beck SG. 2013. Una revisión de
Polygala L. sensu lato en Bolivia. – Rev. Soc. Boliv. Bot. 7:
5-53.
Wouw M van de, Maxted N, Chabane K,
Ford-Lloyd BV. 2001. Molecular taxonomy of Vicia ser. Vicia
based on amplified fragment length polymorphisms. – Plant Syst. Evol. 229:
91-105.
Wouw M van de, Maxted N, Ford-Lloyd BV. 2003.
A multivariate and cladistic study of Vicia L. ser. Vicia (Fabaceae) based on analysis of
morphological characters. – Plant Syst. Ecol. 237: 19-39.
Wu S-H, Rejmánek M, Grotkopp E, DiTomaso JM.
2005. Herbarium records, actual distribution, and critical attributes of
invasive plants: genus Crotalaria in Taiwan. – Taxon 54: 133-138.
Wunderlin RP. 2010a. Reorganization of the
Cercideae (Fabaceae:
Caesalpinioideae). – Phytoneuron 48: 1-5.
Wunderlin RP. 2010b. New combinations in
Schnella (Fabaceae:
Caesalpinioideae: Cercideae). – Phytoneuron 49: 1-5.
Wunderlin RP, Larsen K, Larsen SS. 1981.
Cercideae. – In: Polhill RM, Raven PH (eds), Advances in legume systematics
1, Royal Botanic Gardens, Kew, pp. 107-116.
Wunderlin RP, Larsen K, Larsen SS. 1987.
Reorganization of the Cercideae (Fabaceae: Caesalpinioideae). – Biol.
Skr. 28: 1-40.
Wurdack JJ. 1974. Notes on Brazilian Polygalaceae. – Phytologia 28:
10-14.
Wurdack JJ. 1979. Duas Poligaláceas novas da
Bahía. – Bradea 3: 17-19.
Wyk B-E van. 1986. A revision of the genus
Virgilia (Fabaceae). –
South Afr. J. Bot. 52: 347-353.
Wyk B-E van. 1991a. A review of the tribe
Crotalarieae (Fabaceae). – Contr.
Bolus Herb. 13: 265-288.
Wyk B-E van. 1991b. A synopsis of the genus
Lotononis (Fabaceae:
Crotalarieae). – Contr. Bolus Herb. 14: 1-292.
Wyk B-E van. 2003. The value of
chemosystematics in clarifying relationships in the genistoid tribes of
papilionoid legumes. – Biochem. Syst. Ecol. 31: 875-884.
Wyk B-E van, Kolberg H. 2008. A new species
of Lotononis section Oxydium (Fabaceae, Crotalarieae). – South Afr.
J. Bot. 74: 750-752.
Wyk B-E van, Schutte AL. 1989. Taxonomic
relationships amongst some genera of Leguminosae tribe Crotalarieae and
Argyrolobium (Genisteae). – Kew Bull. 44: 397-423.
Wyk B-E van, Schutte AL. 1994.
Stirtonia, a new genus of the tribe Podalyrieae (Leguminosae) from
South Africa. – Nord. J. Bot. 14: 319-325.
Wyk B-E van, Schutte AL. 1995a.
Stirtonanthus, a new name for Stirtonia (Leguminosae, tribe
Podalyrieae). – Nord. J. Bot. 15: 67.
Wyk B-E van, Schutte AL. 1995b. Phylogenetic
relationships in the tribes Podalyrieae, Liparieae, and Crotalarieae. – In:
Crisp MD, Doyle JJ (eds), Advances in legume systematics 7: Phylogeny, Royal
Botanic Gardens, Kew, pp. 283-308.
Wyk B-E van, Verdoorn GH. 1990. Alkaloids as
taxonomic characters in the tribe Crotalarieae (Fabaceae). – Biochem. Syst. Ecol 18:
503-515.
Wyk B-E van, Verdoorn GH, Schutte AL. 1988.
Chemotaxonomic value of alkaloids in the genus Dichilus. – Biochem.
Syst. Ecol. 16: 471-474.
Wyk B-E van, Verdoorn GH, Greinwald R,
Bachmann P. 1991. Taxonomic significance of major alkaloids in the genus
Priestleya. – Biochem. Syst. Ecol. 19: 595-598.
Wyk B-E van, Verdoorn GH, Greinwald R. 1991.
Taxonomic significance of alkaloids in the genus Liparia (Fabaceae-Liparieae). – South Afr. J.
Bot. 57: 344-347.
Wyk B-E van, Verdoorn GH, Schutte AL. 1992.
Distribution and taxonomic significance of major alkaloids in the genus
Podalyria. – Biochem. Syst. Ecol. 20: 163-172.
Wyk B-E van, Greinwald R, Witte L. 1993. The
taxonomic significance of alkaloids in the South American genus
Anarthrophyllum. – Biochem. Syst. Ecol. 21: 705-709.
Wyk B-E van, Venter M, Boatwright JS. 2010. A
revision of the genus Bolusia (Fabaceae, Crotalarieae). – South Afr.
J. Bot. 76: 86-94.
Xu B, Wu N, Gao XF, Zhang LB. 2012. Analysis
of DNA sequences of six chloroplast and nuclear genes suggests incongruence,
introgression, and incomplete lineage sorting in the evolution of
Lespedeza (Fabaceae). –
Mol. Phylogen. Evol. 62: 346-358.
Xu LR, Podlech D. 2006. Some new taxa of
Astragalus L. (Leguminosae) from China and Nepal. – Feddes Repert.
117: 225-231.
Yakovlev GP. 1969. A review of
Sweetia and Acosmium. – Notes Roy. Bot. Gard. Edinburgh 29:
347-355.
Yakovlev GP. 1972. A contribution to the
system of the order Fabales Nakai (Leguminosales Jones). – Bot. Žurn. 57:
585-594. [In Russian]
Yakovlev GP. 1975. Changes in the system of
Fabales. – Bot. Žurn. 60: 701-703. [In Russian]
Yakovlev GP. 1977. Notes on the taxonomy of
the genera Baphiopsis Baker, Spirotropis Tul.,
Panurea Benth., and Dicraeopetalum Harms – Novosti Sist.
Vyssh. Rast. 14: 137-139. [In Russian]
Yakovlev GP, Sviazeva OA. 1987. On the
taxonomy of the new genus Spongiocarpella (Fabaceae). – Bot. Žurn. 72:
249-260.
Yakovlev GP, Sytin AK, Roskov YR. 1996.
Legumes of Northern Eurasia, a check-list. – Kew.
Yamamoto K, Fujiwara T, Blumenreich I. 1984.
Karyotypes and morphological characteristics of some species of the genus
Lathyrus. – Jap. J. Breed. 34: 273-284.
Yeh MS, Yuasa H, Maekawa F. 1986. Chromosome
numbers in the Leguminosae. – Sci. Rep. Res. Inst. Evol. Biol. 3: 57-71.
Yoder JB, Briskine R, Mudge J, Farmer A,
Paape T, Steele K, Weiblen GD, Bhart AK, Zhou P, May GD, Young ND, Tiffin P.
2013. Phylogenetic signal variation in the genomes of Medicago (Fabaceae). – Syst. Biol. 62:
424-438.
Young JPW, Johnston AWB. 1989. The evolution
of specificity in the legume-Rhizobium symbiosis. – Trends Ecol.
Evol. 4: 341-349.
Yue X-K, Yue J-P, Yang L-E, Li Z-M, Sun H.
2011. Systematics of the genus Salweenia (Leguminosae) from Southwest
China with discovery of a second species. – Taxon 60: 1366-1374.
Zamora N. 2000. Nuevas especies y
combinaciones en leguminosas de Mesoamerica. – Novon 10: 175-180.
Zandee M, Geesink R. 1987. Phylogenetics and
legumes: a desire for the impossible? – In: Stirton CH (ed), Advances in
legume systematics 3, Her Majesty’s Stationery Office, London, pp.
131-167.
Zarre S. 2000. Systematic revision of
Astragalus sect. Adiaspastus, sect. Macrophyllium
and sect. Pterophorus. – Englera 18: 1-219.
Zarre S. 2003. Hair micromorphology and its
phylogenetic application in thorny species of Astragalus (Fabaceae). – Bot. J. Linn. Soc. 143:
323-330.
Zarre S, Podlech D. 1996. Taxonomic revision
of Astragalus L. sect. Hymenostegis Bunge (Leguminosae). –
Sendtnera 3: 255-312.
Zarre S, Podlech D. 1997. Problems in the
taxonomy of tragacanthic Astragalus. – Sendtnera 4: 243-250.
Zarre S, Podlech D. 2001a. Taxonomic revision
of Astragalus sect. Acanthophace (Fabaceae). – Sendtnera 7: 233-255.
Zarre S, Podlech D. 2001b. A short
contribution to genus Astragalus L. (Fabaceae) in Turkey. – Pakistan J.
Bot. 33: 153-155.
Zarre S, Podlech D. 2001c.
Astragalus sect. Semnanenses (Fabaceae): a new monotypic section from
Iran. – Nord. J. Bot. 21: 485-491.
Zarre S, Podlech D, Taeb F. 2005. New species
of the genus Astragalus L. (Fabaceae), mainly from the “Flora
Iranica” area. – Feddes Repert. 116: 54-79.
Zhang D. 1993. Some additional taxa of
Bauhinia (Leguminosae) from China. – Nord. J. Bot. 13: 399-402.
Zhang M-L. 1997. A reconstructing phylogeny
in Caragana (Fabaceae). –
Acta Bot. Yunnan. 19: 331-341. [In Chinese]
Zhang M-L. 1998. A preliminary analytic
biogeography in Caragana (Fabaceae). – Acta Bot. Yunnan. 20:
1-11.
Zhang M-L. 2000. Studies on geographical
distribution pattern of the subgenus Pogonophace (Astragalus:
Fabaceae) in China using GIS
technique. – Acta Bot. Sin. 42: 849-854.
Zhang M-L. 2002. Systematics of
Astragalus subgenus Pogonophace (Leguminosae). – Acta Bot.
Yunnan. 24: 543-553.
Zhang M-L. 2003. Biogeography of
Astragalus subgenus Pogonophace (Leguminosae). – Acta Bot.
Yunnan. 25: 25-32.
Zhang M-L, Fritsch PW. 2010. Evolutionary
response of Caragana (Fabaceae) to Qinghai-Tibetan Plateau
uplift and Asian interior aridification. – Plant Syst. Evol. 288: 191-199.
Zhang M-L, Podlech D. 2005. Revision of the
genus Phyll[ol]obium Fisch.
(Leguminosae-Papilionoideae). – Feddes Repert. 117: 1-2, 41-64. – Erratum:
Feddes Repert. 117 (2006): 318.
Zhang M-L, Tian X-Y, Ning J-C. 1996. Pollen
morphology and its taxonomic significance of Caragana Fabr. (Fabaceae) from China. – Acta
Phytotaxon. Sin. 34: 397-409. [In Chinese]
Zhang M-L, Huang Y-M, Kang Y, Wang Y-W. 2002.
Biodiversity and biogeography of legumes in Ordos Plateau. – China Bull. Bot.
Res. 22: 497-502. [In Chinese]
Zhang M-L, Fritsch PW, Cruz BC. 2009.
Phylogeny of Caragana (Fabaceae) based on DNA sequence data
from rbcL, trnS-trnG, and ITS. – Mol. Phylogen.
Evol. 50: 547-559.
Zhang M-L, Wen ZB, Fritsch PW, Sanderson SC.
2015. Spatiotemporal evolution of Calophaca (Fabaceae) reveals multiple dispersals in
central Asian mountains. – PLoS ONE 10(4). doi:
10.1371/journal.pone.0123228
Zhang M-L, Wen ZB, Hao XL, Byalt VV,
Sukhorukov AP, Sanderson SC. 2015. Taxonomy, phylogenetics and biogeography of
Chesneya (Fabaceae),
evidenced from data of three sequences, ITS, trnS-trnG, and
rbcL. – Biochem. Syst. Ecol. 63: 80-89.
Zhang S-Y. 1992. Systematic wood anatomy of
the Rosaceae. – Blumea 37:
81-158.
Zhao Y-Z. 1993. Taxonomic study of the genus
Caragana from China. – Acta Sci. Nat. Univ. Nei Mongol. 24: 631-653.
[In Chinese]
Zhao Y-Z. 2009. Classification and its
floristic geography of Caragana Fabr. in the world. – Inner Mongolia
Univ. Press, Hohhot, pp. 14-91.
Zhou DW. 1996. Study on taxonomy of the genus
Caragana Fabr. – J. Northeast Mormal Univ. 4: 69-76.
Zhou Q-X, Yang Y-P, Zhang M-L. 2002.
Karyotypes of fourteen species in Caragana. – Bull. Bot. Res. 22:
492-496. [In Chinese]
Zhu X-Y. 1996. Note on the genus
Chesneya Lindl. ex Endl. (Fabaceae). – Acta Phytotaxon. Sin. 34:
558-562.
Zhu X-Y. 2004. A revision of the genus
Gueldenstaedtia (Fabaceae).
– Ann. Bot. Fenn. 41: 283-291.
Zhu X-Y. 2005a. A taxonomic revision of
Tibetia (Leguminosae; Papilionoideae; Galegeae). – Bot. J. Linn.
Soc. 148: 475-488.
Zhu X-Y. 2005b. Pollen and seed morphology of
Gueldenstaedtia Fisch. & Tibetia (Ali) H. P. Tsui
(Leguminosae) – with a special reference to their taxonomic significance. –
Nord. J. Bot. 23: 373-384.
Zhu X-Y, Ohashi H. 2000. Systematics of
Chinese Oxytropis DC. (Leguminosae). – Cathaya 11-12.
Zimmerman E, Prenner G, Bruneau A. 2013.
Floral morphology of Apuleia leiocarpa (Caesalpinioideae: Cassieae),
an unusual andromonoecious legume. – Intern. J. Plant Sci. 174: 154-160.
Zimmerman E, Herendeen PS, Lewis GP, Bruneau
A. 2017. Floral evolution and phylogeny of the Dialioideae, a diverse subfamily
of tropical legumes. – Amer. J. Bot. 104: 1019-1041.
Zindler-Frank E. 1987. Calcium oxalate
crystals in legumes. – In: Stirton CH (ed), Advances in legume systematics 3,
Royal Botanic Gardens, Kew, pp. 279-316.
Zohary M. 1971. A revision of the species of
Trifolium sect. Trifolium (Leguminosae) I. Introduction. –
Candollea 26: 297-308.
Zohary M. 1972a. A revision of the species of
Trifolium sect. Trifolium (Leguminosae) II. Taxonomic
treatment. – Candollea 27: 99-158.
Zohary M. 1972b. Origins and evolution in the
genus Trifolium. – Bot. Not. 125: 501-511.
Zohary M, Heller D. 1970. The
Trifolium species of sect. Vesicaria Crantz. – Israel J.
Bot. 19: 314-335.
Zohary M, Heller D. 1984. The genus
Trifolium. – Israel Academy of Sciences and Humanities, Jerusalem,
Israel.
Zoric L, Merkulov L, Lukovic J, Boza P. 2012.
Comparative analysis of qualitative anatomical characters of Trifolium
L. (Fabaceae) and their taxonomic
implications: preliminary results. – Plant Syst. Evol. 298: 205-219.
Zou P, Liao J, Zhang D. 2008. Leaf epidermal
micromorphology of Cercis (Fabaceae: Caesalpinioideae). – Bot. J.
Linn. Soc. 158: 539-547.






