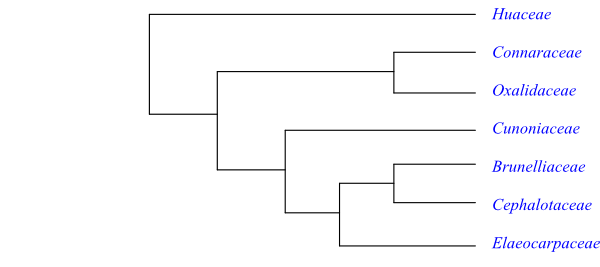
Cladogram of Oxalidales based on DNA sequence data (Wurdack & Davis 2009). Huaceae are sister to the rest (with a bootstrap support of 100%), these being split into two likewise well supported major clades.
[Celastrales+[Oxalidales+Malpighiales]]
Oxalidanae Doweld, Tent. Syst. Plant. Vasc.: xxx. 23 Dec 2001
Habit Usually bisexual (rarely monoecious, andromonoecious, polygamomoneicous, dioecious, androdioecious, gynodioecious, or polygamodioecious), usually evergreen (rarely deciduous) trees, shrubs or suffrutices (rarely lianas), or perennial (rarely annual) herbs.
Vegetative anatomy Phellogen ab initio superficial or absent. Secondary lateral growth normal or absent. Vessel elements usually with simple (rarely scalariform or reticulate) perforation plates; lateral pits usually alternate or opposite (rarely scalariform or intermediate), simple or bordered pits. Imperforate tracheary xylem elements fibre tracheids or libriform fibres (rarely tracheids) with simple or bordered pits, septate or non-septate (often also vasicentric tracheids). Wood rays uniseriate, homocellular or heterocellular, or absent. Axial parenchyma apotracheal diffuse, or paratracheal scanty vasicentric, scalariform, reticulate, or banded, or absent. Tyloses sometimes frequent. Sieve tube plastids Ss, Pcs, or Pcfs type (rarely Pc type). Nodes 1:1, unilacunar with one leaf trace, or 3:3, trilacunar with three traces (rarely 5:5, pentalacunar with five traces, or 7:7, septalacunar with seven traces). Heartwood sometimes with gum-like substances. Calciumoxalate as prismatic, acicular, cuboid or elongate crystals, styloids, druses or crystal sand.
Trichomes Hairs unicellular or multicellular, simple or branched, stellate or furcate (sometimes dendritic, vesicular or as peltate scales); multicellular glandular hairs often abundant.
Leaves Alternate (usually spiral, sometimes distichous) or opposite (rarely verticillate), pinnately or palmately compound, or simple and entire or lobed (sometimes strongly reduced), often coriaceous, usually with circinate ptyxis. Stipules intrapetiolar or lateral, persistent or caducous, or absent; leaf sheath absent. Colleters sometimes present. Petiole vascular bundle transection annular, D-shaped or complex; petiole sometimes with medullary or flange bundles. Venation pinnate, craspedodromous, semicraspedodromous, brochidodromous, palmate or subpalmate (leaves sometimes one-veined, rarely reticulodromous). Stomata paracytic, anomocytic or cyclocytic (rarely anisocytic, anomocyclocytic, bicyclic, diacytic or brachyparacytic). Cuticular wax crystalloids as rodlets, platelets or rosettes of platelets (Fabales type), or absent. Domatia as pits, pockets or hair tufts, or absent. Epidermis often with mucilaginous idioblasts. Secretory cavities present or absent. Mesophyll sometimes with tannins and calciumoxalate as druses or solitary crystals, sometimes with sclerenchymatous idioblasts. Calciumoxalate crystals often abundant, as one prismatic or cuboid crystal per cell or as druses. Leaf margin or leaflet margins entire, serrate, biserrate or glandular serrate; leaf teeth often cunonoid.
Inflorescence Terminal or axillary, simple or compound, panicle, thyrsoid, thyrso-paniculate, dichasial, raceme-, head- or umbel-like, or racemose, or flowers solitary axillary.
Flowers Actinomorphic. Usually hypogyny (rarely epigyny or half epigyny). Sepals three to five (to ten), with imbricate or valvate aestivation, persistent, free or more or less connate. Petals (three or) four or five (to ten), with contorted, valvate, induplicate-valvate or imbricate (sometimes open) aestivation, often clawed, early caducous, usually free (sometimes more or less connate; sometimes absent). Nectariferous disc or nectaries extrastaminal or intrastaminal, often as antepetalous glands on filament bases outside antepetalous staminal whorl (sometimes absent). Disc present or absent.
Androecium Stamens (four or five or) eight or ten (to more than 300), in one to several whorls, obdiplostemonous (outer alternisepalous, inner antesepalous; sometimes five, haplostemonous, antesepalous or antepetalous). Filaments often with uniseriate somewhat moniliform hairs, free or connate at base into ring, free from tepals. Anthers basifixed or dorsifixed, versatile or non-versatile, tetrasporangiate, introrse or latrorse, longicidal (dehiscing by longitudinal slits; rarely at apex), poricidal (dehiscing by one or two usually apical pores) or dehiscing by transverse valve. Tapetum secretory. Staminodia five, extrastaminal or intrastaminal, or absent.
Pollen grains Microsporogenesis simultaneous. Pollen grains usually tricolpate or tricolporoidate (sometimes dicolpate or dicolporate, rarely triporate, tricolporate, tetracolp[or]ate, pantocolp[or]ate or syncolpate), shed as monads, usually bicellular (rarely tricellular) at dispersal. Exine tectate or semitectate, with columellate infratectum, perforate, microperforate, punctate, reticulate, striate, rugulate, microverrucate or smooth.
Gynoecium Pistil composed of (one to) five to eight (to 18) usually connate antepetalous carpels (rarely free, apocarpy). Ovary usually superior (rarely inferior or semi-inferior), (bilocular to) quinquelocular to octalocular (sometimes unilocular, rarely up to 18-locular), locules sometimes divided by secondary septa. Style single, simple, or stylodia (two to) five to eight (to 18), usually free (rarely connate at base). Stigmas spatulate, capitate or punctate (rarely decurrent), papillate, usually Dry (occasionally Wet) type. Pistillodium absent or male flowers with pistillodia.
Ovules Placentation usually axile (sometimes apical, rarely parietal or basal). Ovules (one or) two (to more than 30) per carpel, anatropous or hemianatropous, ascending, horizontal or pendulous, apotropous or epitropous, bitegmic, usually weakly crassinucellar or incompletely tenuinucellar. Integument (at least outer) often multiplicative. Micropyle usually bistomal (sometimes exostomal or endostomal). Obturator sometimes present. Archespore usually unicellular (rarely multicellular). Chalazal appendages present. Megagametophyte usually monosporous, Polygonum type (sometimes disporous, Allium type). Endosperm development ab initio nuclear. Endosperm haustoria? Embryogenesis asterad.
Fruit A loculicidal and/or septicidal capsule, often fleshy (rarely finally schizocarpous usually with five capsule-like mericarps, sometimes one or several follicles, a drupe, rarely a berry, nut, or pyxidium). Fruit often elastically dehiscent (including aril-like epidermis of seeds).
Seeds Aril rarely present. Seed coat endotestal-exotegmic (usually exotegmic). Testa often multiplicative, sometimes mucilaginous. Exotesta unspecialized or with elongate cells, sometimes palisade. Endotestal cells with calciumoxalate crystals and sometimes thickened walls, sometimes palisade. Tegmen often multiplicative or crushed. Exotegmen palisade, with lignified cell walls, or fibrous/tracheidal, well developed, sometimes two-layered. Endotegmen unspecialized or lignified. Perisperm not developed. Endosperm usually copious, fleshy, oily and usually starchy, usually ruminate. Embryo usually straight (rarely curved), well differentiated, usually with chlorophyll. Cotyledons two. Germination phanerocotylar or cryptocotylar.
Cytology x = (5–)7(–12)
DNA Plastid gene infA lost/defunct. Mitochondrial intron coxII.i3 lost.
Phytochemistry Flavonols (kaempferol, quercetin, myricetin), flavonol sulfates, flavanols, cyanidin, epigallocatechine-3-gallate (polyphenol), triterpenes, cucurbitacins, geraniin, ellagic and gallic acids, hydrolyzable and non-hydrolyzable tannins based on ellagic, gallic and methylated ellagic acids, proanthocyanidins (prodelphinidins), pyrrolizidine and tropane alkaloids, saponins, cyanogenic compounds, bergenin, benzoquinone rapanone, benzoquinones (embelin), β-sitosterol and its glycoside, hentriacontane, and mesoinosite present.
Systematics Oxalidales may be sister-group to Malpighiales.
Huaceae are sister to the remaining Oxalidales (Zhang & Simmons 2006).
A probable topology of Oxalidales is the following: [Huaceae+[[Connaraceae+Oxalidaceae]+ [Cunoniaceae+[Elaeocarpaceae+[Brunelliaceae+Cephalotaceae]]]]].
On the other hand, Soltis & al. (2011) recovered Brunellia as sister to the clade [Elaeocar-paceae+[Cephalotus+Cunoniaceae]].
Oxalidales except Huaceae are have the following potential synapomorphies, according to Stevens (2001 onwards): presence of mucilage cells; leaves compound, imparipinnate, trifoliolate or unifoliolate; stylodia separate; stigma secretory (Wet type); micropyle bistomal; integument multiplicative; endotesta crystalliferous, palisade; and exotegmen tracheidal or non-tracheidal.
Connaraceae and Oxalidaceae share the advanced characters (Stevens 2001 onwards): plant with sympodial shoot arrangement; roots diarch (lateral roots tetrastichous); vessel elements with simple perforation plates; wood rays uniseriate; sieve tube plastids with protein crystalloids; absence of calciumoxalate druses; cuticular wax crystalloids as rosettes of platelets (Fabales type); absence of stipules; petiolules articulated; venation pinnate to palmate; leaflets with entire margins; petals postgenitally subbasally connate, with uniseriate glandular hairs; nectaries extrastaminal; stamens in two whorls of different lengths, connate at base, with uniseriate glandular hairs; stylodia two or three; ovule with endothelium; sepals persistent in fruit; exotesta carnose; presence of benzoquinone rapanone; and absence of ellagic acid.
The clade [Cunoniaceae+[Elaeocarpaceae+[Brunelliaceae+Cephalotaceae]]] has the potential synapomorphies: sepals with valvate aestivation and postgenitally coherent by hairs. The clade [Elaeocarpaceae+[Brunelliaceae+Cephalotaceae]] is characterized by: inner integument three to five cell layers thick. Finally, Brunelliaceae and Cephalotaceae share the synapomorphies: inflorescence cymose; petals absent; carpels free or mostly free; stylodia recurved, stigma decurrent; placentation basal; ovules two per carpel; and fruit a follicle.

|
|
Cladogram of Oxalidales based on DNA sequence data (Wurdack & Davis 2009). Huaceae are sister to the rest (with a bootstrap support of 100%), these being split into two likewise well supported major clades. |
BRUNELLIACEAE Engl. |
Genera/species 1/c 60
Distribution Mainly mountain areas in tropical America.
Fossils Unknown.
Habit Usually dioecious or gynodioecious (sometimes bisexual), evergreen trees. Branches with angular internodes and distinct nodes. Densely brown-hairy.
Vegetative anatomy Phellogen ab initio superficial? Primary medullary rays alternately wide and narrow, or exclusively narrow. Vessel elements with simple and/or scalariform perforation plates; lateral pits scalariform to opposite, simple pits. Imperforate tracheary xylem elements libriform fibres with simple or bordered pits, often septate. Wood rays uniseriate, heterocellular. Axial parenchyma absent. Tyloses frequent. Sieve tube plastids S type? Nodes usually 3:3, trilacunar with three leaf traces (rarely 5:5, pentalacunar with five traces). Crystals absent.
Trichomes Hairs unicellular, simple, brown to yellow or red, abundant (often with lignified cell walls).
Leaves Opposite or verticillate (ternate), usually imparipinnate with stipulules (sometimes simple and entire or lobate, alternatively unifoliolate), coriaceous, with conduplicate ptyxis. Stipules lateral (cauline), small, pairwise connate into interpetiolar stipules or free, early caducous; leaf sheath absent. Petiole vascular bundle transection annular or D-shaped; petiole with or without adaxial wing bundles. Venation pinnate, craspedodromous; secondary veins prominent, proceeding to leaf margins. Stomata actinocytic or anomocytic. Cuticular wax crystalloids as rosettes of platelets (Fabales type). Epidermis with mucilaginous idioblasts? Domatia? Sclerenchymatous idioblasts? Leaf margin and leaflet margins usually serrate or biserrate (rarely entire).
Inflorescence Terminal or axillary, thyrso-paniculate.
Flowers Actinomorphic, small. Usually hypogyny (sometimes epigyny?). Sepals four to six (to eight), with valvate aestivation, persistent, free or somewhat connate at base. Petals absent. Nectariferous disc (androgynophore?) intrastaminal, annular, octa- to decemlobate, adnate to calyx.
Androecium Stamens 4+4 or 5+5 or more, usually twice (sometimes three times) as many as sepals (inner staminal whorl sometimes with twice as many stamens as sepals), obdiplostemonous. Filaments inserted between nectariferous disc lobes, free from each other and from tepals. Anthers dorsifixed, versatile, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits); connective somewhat prolonged. Tapetum secretory? Female flowers with staminodia.
Pollen grains Microsporogenesis simultaneous? Pollen grains tricolporate, shed as monads, bicellular at dispersal. Exine tectate, with columellate infratectum, striate-reticulate to reticulate(-rugulate) or punctate.
Gynoecium Carpels two to eight, alternisepalous or antesepalous, free, partially adnate to surrounding disc (ventral carpel not entirely closed). Ovaries superior, unilocular (apocarpous), with adaxial furrows. Stylodia two to eight, upright, reflexed at apex. Stigmas linear, decurrent along sulcus, type? Male flowers with pistillodia.
Ovules Placentation marginal (near middle of ventral suture). Ovules two per carpel, collateral, anatropous, pendulous, epitropous, bitegmic, crassinucellar. Micropyle bistomal?, upright, not Z-shaped. Outer integument ? cell layers thick. Inner integument three to five cell layers thick. Obturator present. Megagametophyte monosporous, Polygonum type. Endosperm development ab initio nuclear. Endosperm haustoria? Embryogenesis?
Fruit Usually an assemblage of densely red-, yellow- or brown-hairy follicles (rarely drupes or nutlets), with persistent sepals and stylodia usually directed outwards to downwards. Endocarp hard, separating from soft mesocarp/exocarp during maturation. Hairs often lignified.
Seeds Seed often winged or tomentose, long persistent on exposed placental and exocarpous tissue (prolongations of funicles). Aril absent. Raphe erect, aril-like. Testa shining, with subepidermal sclerenchymatous layer and innermost layer palisade. Exotegmen and endotegmen finally fused. Perisperm not developed. Endosperm copious, carnose, mealy. Embryo large, straight, well differentiated, chlorophyll? Cotyledons two, flat. Germination phanerocotylar.
Cytology n = 14
DNA
Phytochemistry Unknown.
Use Medicinal plants.
Systematics Brunellia (c 60; southern Mexico, Central America, the West Indies, northern South America, especially in the Andes southwards to 18°S in Bolivia).
Brunellia is sister to Cephalotus (Cephalotaceae).
CEPHALOTACEAE Dumort. |
Cephalotales Lindl. in C. F. P. von Martius, Consp. Regn. Veg.: 37. Sep-Oct 1835 [‘Cephaloteae’]
Genera/species 1/1
Distribution Southwestern Western Australia.
Fossils Unknown.
Habit Bisexual, perennial herb with thick and richly branched rhizome, and heterophylly. Hygrophytic. Carnivorous. Rhizome with numerous scale-like leaves. Roots fibrous. Main root ephemeral, early replaced by adventitious roots.
Vegetative anatomy Roots with starchy cortex and medulla. Phellogen absent. Young stem with vascular tissue as cylinder. Secondary lateral growth absent. Vessel elements with simple (scalariform?) perforation plates; lateral pits scalariform or alternate. Imperforate tracheary xylem elements tracheids with simple? pits. Wood rays usually uniseriate. Axial parenchyma diffuse ‘to more abundant states’. Sieve tube plastids Ss type. Nodes 1:1, unilacunar with one leaf trace. Raphides absent. Crystals?
Trichomes Hairs unicellular or multicellular, uniseriate, simple or branched; sessile multicellular glands frequent.
Leaves Alternate (spiral), simple, entire, with ? ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle transection annular. Lamina (or rhachis) of outer (lower) rosette leaves modified into insect-catching ascidiate pitcher-shaped stalked structure with lid-like (operculate) upper part and two distinct ridges on front side; lamina of inner (upper) rosette leaves flat (normal in shape), on abaxial side with multicellular glands. Lower side of lid and upper part of inner side of pitcher slippery and covered with overlapping outgrowths directed downwards. Pitcher mouth surrounded by folded margin, each fold claw-shaped and directed inwards and downwards. Abaxial side of pitcher nectar-secreting and with sunken multicellular glands; adaxial side of pitcher with numerous hydathodes; upper part of adaxial side of pitcher with numerous small sunken glands, and lower part with large bottle-shaped glands (especially on strongly coloured cushion-shaped appendages). Venation pinnate? Stomata paracytic (anomocytic?). Cuticular waxes absent? Margin of normal flat leaves entire.
Inflorescence Terminal, scapose, branched, spike-like, racemose thyrse with scorpioid lateral partial inflorescences.
Flowers Actinomorphic, small. Hypanthium wide, persistent. Hypogyny to half epigyny (androecium). Sepals six, with imbricate aestivation, cucullate, connate at base. Petals absent. Nectariferous disc intrastaminal, with glandular outgrowth, usually alternisepalous.
Androecium Stamens 6+6. Filaments free from each other and from sepals, inserted at apex of calyx tube, on inner margin of nectariferous disc. Anthers dorsifixed, versatile, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits); connective gland-tipped; loculi filled with secretions. Tapetum secretory. Staminodia absent.
Pollen grains Microsporogenesis simultaneous? Pollen grains tricolpate, shed as monads, bicellular at dispersal. Exine semitectate, with columellate infratectum, reticulate.
Gynoecium Carpels six, plicate, alternisepalous, free or connate at base. Ovary superior to semi-inferior, unilocular (apocarpy), with secrete-filled loculi. Stylodia six, terminal, upright, free, reflexed. Stigmatic areas apical, with ventral papillae, type? Pistillodium absent.
Ovules Placentation basal. Ovules one (or two) per carpel, anatropous, ascending, epitropous, bitegmic, crassinucellar. Micropyle endostomal or bistomal. Outer integument ? cell layers thick. Inner integument three to five cell layers thick. Megagametophyte monosporous, Polygonum type. Endosperm development cellular? Endosperm haustoria? Embryogenesis?
Fruit An assemblage of usually one-seeded (rarely two-seeded) hairy indehiscent follicle-like fruits with persistent and accrescent calyx and hypanthium.
Seeds Aril absent. Testa and tegmen largely collapsed. Testa membranous. Exotesta papillate. Tegmen? Perisperm not developed. Endosperm copious, granular. Embryo small, straight, well differentiated, accumbent, chlorophyll? Cotyledons two, planoconvex. Germination phanerocotylar. Seedling with widened hypocotylar non-vascularized appendage, probably functioning as nutritional reserve.
Cytology n = 10
DNA
Phytochemistry Insufficiently known. Flavonols (quercetin, myricetin), flavanols (biflavanoids), and ellagic and gallic acids present. Iridoids and tannins not found.
Use Ornamental plant.
Systematics Cephalotus (1; C. follicularis; southwestern Western Australia).
Cephalotus is sister to Brunellia (Brunelliaceae).
CONNARACEAE R. Br. |
Connarales Link, Handbuch 2: 129. 4-11 Jul 1829 [’Connaraceae’]; Cnestidaceae (Raf.) Raf., Med. Fl. 2: 113. Mai-Dec 1830 [’Cnestides’]
Genera/species 12/175–195
Distribution Pantropical, southwards to Uruguay, South Africa and Queensland.
Fossils Unknown.
Habit Usually bisexual (sometimes monoecious, rarely dioecious), usually evergreen (sometimes deciduous) shrubs or lianas (sometimes trees). Many species are poisonous.
Vegetative anatomy Phellogen ab initio superficial. Secondary lateral growth usually normal (in Rourea anomalous, from concentric cambia). Vessel elements with simple perforation plates; lateral pits alternate, usually simple (sometimes bordered) pits. Imperforate tracheary xylem elements libriform fibres (rarely fibre tracheids?) with simple pits, septate or non-septate (also vasicentric tracheids). Wood rays usually uniseriate (sometimes biseriate), homocellular or heterocellular. Axial parenchyma apotracheal diffuse, or paratracheal scanty vasicentric, or banded, or absent. Tyloses often frequent. Intraxylary phloem present in Rourea. Sieve tube plastids Pcs or Pcfs type (closely similar to those in Oxalidaceae). Nodes usually 3:3, trilacunar with three leaf traces (sometimes 5:5 or 7:7, quinquelacunar or septalacunar with five or seven traces, respectively). Often with secretory cavities and mucilaginous ducts. Wood often with silica incrustations (sometimes as grains). Prismatic calciumoxalate crystals abundant; crystal sand, acicular or elongate crystals and/or styloids present in some species.
Trichomes Hairs unicellular or multicellular, uniseriate, simple or branched, furcate (sometimes dendritic or stellate); unicellular or multicellular, uniseriate glandular hairs often present.
Leaves Alternate (spiral or distichous), pinnately compound, trifoliolate or unifoliolate, often coriaceous, with ? ptyxis. Stipules and leaf sheath absent. Petiole and petiolules pulvinate at base, often transversely ridged. Petiole vascular bundle transection? Stipulules absent. Venation pinnate. Stomata paracytic, anisocytic, anomocyclocytic or bicyclic (rarely diacytic). Cuticular wax crystalloids as rosettes of platelets (Fabales type). Upper epidermis sometimes with mucilaginous idioblasts. Leaflet margins entire.
Inflorescence Usually axillary (sometimes subterminal), usually panicle (sometimes raceme-like or capitate).
Flowers Actinomorphic or somewhat zygomorphic, small. Pedicel usually articulated. Hypogyny. Androgynophore sometimes present. Sepals (four or) five, with imbricate or valvate aestivation, persistent or caducous, free or connate at base. Petals (four or) five, usually with imbricate (rarely valvate) aestivation, free or connate at base, with uniseriate glandular hairs. Nectaries extrastaminal (sometimes absent). Disc small or absent. Flowers usually diheterostylous or triheterostylous (sometimes homostylous).
Androecium Stamens usually 5+5, obdiplostemonous, with outer antesepalous longer and inner antepetalous shorter (sometimes five, haplostemonous). Filaments free or connate at base, free from tepals. Anthers dorsifixed, versatile, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory? Staminodia one to five, intrastaminal and/or alternating with fertile stamens of inner staminal whorl; female flowers with staminodia.
Pollen grains Microsporogenesis simultaneous? Pollen grains tri- or tetracolpate or -colporate, shed as monads, ?-cellular at dispersal. Exine semitectate, with columellate? infratectum, reticulate.
Gynoecium Carpels usually one or five (rarely three, seven, or eight; often five, four of which degenerating), free (apocarpy) or connate at base. Ovary superior, usually unilocular or quinquelocular (rarely tri-, septa- or octalocular), with adaxial furrows, sometimes stipitate. Stylodia terminal, free. Stigmas capitate, type? Male flowers with pistillodium.
Ovules Placentation subbasal, axile or subapical (or marginal). Ovules two per carpel, collateral (one ovule often degenerating), anatropous to hemianatropous or orthotropous, ascending, bitegmic, crassinucellar. Micropyle exostomal, upright. Outer integument five to seven cell layers thick. Inner integument three to five cell layers thick. Funicle absent. Parietal tissue approx. one cell layer thick. Megagametophyte monosporous, Polygonum type. Endosperm development ab initio nuclear. Endosperm haustoria? Embryogenesis?
Fruit One or several follicles, keeled or ridged, usually single-seeded (in Jollydora two-seeded; sometimes also abaxially dehiscent; rarely a drupe, nutlet or, in some species of Rourea, a pyxidium), during maturation often fusing, indurated, with persistent and sometimes accrescent sepals.
Seeds Aril present or absent. Testa black and orange-red, vascularized, non-lignified. Exotesta variable, often palisade (sometimes with lignified cell walls); sarcoexotesta developed in raphe-chalazal region or in larger part of seed. Endotesta? Tegmen multiplicative, often largely crushed or early degenerating. Perisperm not developed. Endosperm copious or sparse, oily, or absent. Embryo?, chlorophyll? Cotyledons two, thin and flat to planoconvex. Germination cryptocotylar.
Cytology n = 14, 16
DNA Plastid gene rpl22 present in nuclear but not in plastid genome. Plastid gene rpl16 absent.
Phytochemistry Flavonols (kaempferol, quercetin, myricetin), cyanidin, delphinidin, bergenin, benzoquinone rapanone, benzoquinones (embelin), β-sitosterol and its glycoside, hentriacontane, and mesoinosite present. Poisonous substances frequent (e.g. glabrin: methionin sulfoxymine at least in Cnestis). Ellagic acid, alkaloids, and saponins not found.
Use Timber, medicinal plants, seed oil (Connarus).
Systematics Connarus (75–80; tropical regions on both hemispheres), Ellipanthus (6; coastal regions in tropical East Africa, Madagascar, tropical Asia), Hemandradenia (2; tropical West and Central Africa), Burttia (1; B. prunoides; central Tanzania), Vismianthus (2; V. punctatus: Mlinguru in southeastern Tanzania; V. sterculiifolius: southwestern Burma); Jollydora (3; eastern Nigeria to Angola); Manotes (4–5; tropical Africa); Cnestis (13; tropical Africa, Madagascar, one species in tropical Asia), Pseudoconnarus (5; tropical South America), Agelaea (8; tropical Africa, Madagascar, tropical Asia), Cnestidium (2; tropical America), Rourea (55–70; tropical regions on both hemispheres).
Connaraceae are sister to Oxalidaceae.
There is no available phylogeny of Connaraceae.
CUNONIACEAE R. Br. |
Baueraceae Lindl., Intr. Nat. Syst. Bot.: 50. 27 Sep 1830; Bauerales Lindl. in C. F. P. von Martius, Consp. Regn. Veg.: 48. Sep-Oct 1835 [‘Bauraceae’]; Cunoniales R. Br. in C. F. P. von Martius, Consp. Regn. Veg.: 48. Sep-Oct 1835 [‘Cunoniaceae’]; Eucryphiaceae C. Gay in Bot. Zeitung (Berlin) 6: 130. 18 Feb 1848 [’Eucrifiaceas’], nom. cons.; Belangeraceae J. Agardh, Theoria Syst. Plant.: 337. Apr-Sep 1858 [’Belangereae’]; Callicomaceae J. Agardh, Theoria Syst. Plant.: 146. Apr-Sep 1858 [’Callicomeae’]; Davidsoniaceae Bange in Blumea 7: 294. 1 Sep 1952; Spiraeanthemaceae Doweld, Tent. Syst. Plant. Vasc.: xxxi. 23 Dec 2001
Genera/species 27/315–325
Distribution Mainly the Southern Hemisphere between 13ºS and 35ºS, with their highest diversity in Australia, New Caledonia and New Guinea, few species northwards to the Philippines, Mexico and the West Indies.
Fossils Leaves, inflorescences, flowers, pollen grains and fruits have been found in the Maastrichtian onwards mainly of Australia and New Zealand. Platydiscus peltatus is represented by tetramerous flowers with a conspicuous disc from the Late Santonian to the Early Campanian of Sweden. The stamens are obdiplostemonous and the tricolporate pollen has a reticulate exine. The ovary is quadrilocular and semi-inferior with basally connate carpels containing numerous anatropous ovules inserted on an involute placenta. Fossil wood of the genus Weinmannioxylon has been recorded from Late Cretaceous layers in Antarctica and of Cunonioxylon from the Eocene and the Oligocene of Europe. Flowers assigned to Ceratopetalum are known from the Late Eocene to the mid-Miocene of Australia.
Habit Usually bisexual (rarely andromonoecious, polygamomonoecious, dioecious, androdioecious, gynodioecious, or polygamodioecious), usually evergreen (rarely deciduous) trees or shrubs (some species of Weinmannia are lianas or hemi-epiphytes). Some species are xerophytes. Bark provided with numerous lignified cells and usually with lenticels.
Vegetative anatomy Phellogen ab initio superficial. Cortex sometimes with sclereids and often ring of fibres. Primary medullary rays narrow. Young stem with vascular tissue as cylinder, without separate vascular bundles. Vessel elements usually with simple and/or scalariform (sometimes reticulate) perforation plates; lateral pits scalariform, opposite or alternate; simple or bordered pits. Imperforate tracheary xylem elements tracheids (at least in Eucryphia), fibre tracheids or libriform fibres with simple or bordered pits, septate or non-septate (also vasicentric tracheids). Wood rays uniseriate or multiseriate, homocellular or heterocellular. Axial parenchyma apotracheal diffuse or diffuse-in-aggregates, or paratracheal scanty, or banded, or absent. Tyloses often frequent. Sieve tube plastids very small, usually S type (in Eucryphia Pc type, very small); sieve tubes with non-dispersive protein bodies. Nodes usually 3:3, trilacunar with three leaf traces (sometimes 5:5, pentalacunar with five traces; in Bauera 1:3, unilacunar with three traces; sometimes with split lateral traces). Wood with prismatic calciumoxalate crystals.
Trichomes Hairs unicellular or multicellular, uniseriate or multiseriate, usually simple (sometimes stellate or peltate); glandular hairs sometimes present (in Davidsonia long, rigid, stinging).
Leaves Usually opposite (sometimes verticillate; in Davidsonia alternate, spiral), simple or pinnately (usually imparipinnate) or palmately compound, entire or pinnately or palmately lobed, often coriaceous, with ? ptyxis. Stipules free or connate in pairs, usually interpetiolar (in some species of Geissois one axillary, intrapetiolar; in Lamanonia two, cauline-intrapetiolar; in Davidsonia two, lateral), usually rounded (rarely acicular; in Bauera sometimes absent); leaf sheath absent. Colleters present. Petiole vascular bundle transection usually annular (rarely arcuate); petiole sometimes with adaxial or medullary bundles. Stipulules sometimes present. Venation usually pinnate (rarely palmate), craspedodromous or semicraspedodromous (rarely brochidodromous; in some species of Eucryphia reticulodromous); lower branch of secondary veins often proceeding into leaf teeth (upper branch continuing above teeth). Stomata anomocytic, brachyparacytic, cyclocytic, paracytic or anisocytic. Cuticular wax crystalloids as platelets, polygonal rodlets, or rosettes (Fabales type). Domatia (sometimes along midvein) as pits, pockets or hair tufts. Epidermis with mucilaginous idioblasts. Mesophyll with tannins and calciumoxalate as druses or single crystals, with or without sclerenchymatous idioblasts. Leaflet margins serrate or glandular-serrate (sometimes entire).
Inflorescence Terminal or axillary, usually panicle, or thyrsoid, capitate or racemose? (flowers rarely solitary axillary).
Flowers Actinomorphic, usually small. Usually hypogyny (sometimes epigyny or half epigyny). Sepals (three or) four or five (to ten), with valvate or imbricate aestivation, free or connate at base. Petals (three or) four or five (to ten), with valvate or imbricate aestivation, usually free (in i.a. Davidsonia and Hooglandia absent; in Bauera more numerous than sepals). Nectariferous disc extrastaminal to intrastaminal, annular or consisting of intrastaminal and interstaminal parts (sometimes absent). Tannins and druses abundant in all floral parts.
Androecium Stamens usually 4+4 or 5+5 (rarely four, five, or numerous centripetally developing), usually in one or two whorls (sometimes spiral?), obdiplostemonous (in Spiraeanthemum haplostemonous, antesepalous); in Eucryphia numerous stamens inserted on androphore (receptacular prolongation). Filaments usually articulated, often longer than petals, inflexed in bud, free from each other and from tepals. Anthers usually dorsifixed (sometimes basifixed), usually versatile, tetrasporangiate, usually introrse (in Eucryphia latrorse?), usually longicidal (dehiscing by longitudinal slits; in Bauera short apical slits; in Davidsonia pores widening into longitudinal slits); connective often somewhat prolonged. Tapetum secretory. Staminodia absent?
Pollen grains Microsporogenesis simultaneous. Pollen grains usually dicolpate, dicolporate or tricolporate (rarely syncolpate), usually very small, shed as monads, bicellular at dispersal. Exine tectate or semitectate, with columellate infratectum, perforate or reticulate, usually smooth (rarely rugulate).
Gynoecium Pistil composed of usually two to five (in Eucryphia four to 14[-18]) usually connate antepetalous carpels (in Spiraeanthemeae apocarpy; in Hooglandia one fertile carpel, ovary pseudomonomerous). Ovary usually superior (sometimes inferior or semi-inferior), usually bilocular to quinquelocular (locules usually as many as carpels; ovary in Spiraeanthemeae and Hooglandia unilocular; ovary in Eucryphia quadrilocular to 14[–18]-locular without coherent columella). Stylodia usually two to five (in Eucryphia four to 14[–18]), usually free (rarely connate at base), usually hollow (with stylar canal; in, e.g., Aistopetalum solid). Stigmas usually terminal, punctate to capitate (rarely decurrent), usually papillate (in Eucryphia non-papillate), Dry type. Pistillodium absent?
Ovules Placentation axile to apical, often intruding. Ovules usually two to numerous (rarely one) per carpel, anatropous or hemianatropous, ascending, horizontal or pendulous, usually apotropous (rarely epitropous), bitegmic, crassinucellar. Micropyle exostomal, endostomal or bistomal, in, e.g., Bauera Z-shaped (zig-zag). Outer integument ? cell layers thick. Inner integument ? cell layers thick. Obturator present. Hypostase present at least in Ceratopetalum. Megagametophyte monosporous, Polygonum type. Endosperm development ab initio nuclear. Endosperm haustoria? Embryogenesis?
Fruit Usually a septicidal (in Bauera a loculicidal) capsule (sometimes an assemblage of follicles), usually dehiscent from apex (in Cunonia from base), rarely a nut, drupe (Hooglandia), or schizocarp (in Davidsonia drupaceous schizocarp); sepals in Ceratopetalum and Pullea persistent, wing-like; exocarp in Codia and Pseudoweinmannia hairy, in Platylophus inflated.
Seeds Aril absent. Testa often winged or hairy. Exotestal cells tangentially elongate. Endotesta? Exotegmen? Endotegmic cells tangentially elongate. Perisperm not developed. Endosperm usually copious (absent in Davidsonia), starchy. Embryo usually straight, well differentiated, chlorophyll? Cotyledons two (in Eucryphia large). Germination usually phanerocotylar (in Davidsonia cryptocotylar).
Cytology n = (12, 14–)16
DNA
Phytochemistry Flavonols (kaempferol, quercetin, myricetin) and their glycosides, flavonol sulphates, cyanidin, methylated and non-methylated ellagic acids, gallic acid, epigallocatechine-3-gallate, hydrolyzable and condensed tannins (often abundant), and proanthocyanidins (prodelphinidins), alkaloids, and cyanogenic compounds present. Aluminium accumulated in many species.
Use Ornamental plants, timber, carpentries.
Systematics Spiraeanthemeae Engl. in Engler et Prantl, Nat. Pflanzenfam., ed. 2, 18a: 235, 237. 3 Mai 1930. Acsmithia (14; the Moluccas, New Guinea, northeastern Queensland, New Caledonia, Fiji), Spiraeanthemum (6; New Guinea?, New Britain, the Solomon Islands, Vanuatu, Fiji, Samoa). – Hooglandiaclade Hooglandia (1; H. ignambiensis; New Caledonia). – Bauereae DC., Prodr. 4: 13. late Sep 1830. Bauera (4; southeastern Australia, Tasmania). – Davidsonia clade Davidsonia (3; eastern Queensland, eastern New South Wales). – Aistopetalumclade Aistopetalum (2; New Guinea). – Schizomerieae J. C. Bradford et R. W. Barnes in Syst. Bot. 26: 373. 2001.Schizomeria (3; the Moluccas, New Guinea, eastern Queensland, northeastern New South Wales, the Solomon Islands, New Zealand), Ceratopetalum (9; New Guinea, eastern Queensland, northeastern New South Wales), Anodopetalum (1; A. biglandulosum; western Tasmania), Platylophus (1; P. trifoliatus; southern Western Cape, Eastern Cape). – Eucryphieae Cambess. ex G. Don, Gen. Hist. 1: 599, 613. prim. Aug 1831.Eucryphia (7; Chile, southeastern Australia, Tasmania). – Acrophyllum clade Acrophyllum (1; A. australe; central eastern New South Wales). – Gillbeeaclade Gillbeea (3; New Guinea, northeastern and western Queensland). – Geissoieae Endl. ex Meisn., Plant. Vasc. Gen.: Tab. Diagn. 138, Comm. 101. 16-22 Sep 1838 [‘Geissoideae’]. Lamanonia (5; Brazil, Paraguay, Argentina), Pseudoweinmannia (2; eastern Queensland, northeastern New South Wales), Karrabina (2; eastern Queensland, northeastern New South Wales), Geissois (16; New Caledonia, Vanuatu, Fiji, Santa Cruz Islands). – Caldcluvieae J. C. Bradford et R. W. Barnes in Syst. Bot. 26: 372. 8 Jun 2001.Caldcluvia (1; C. paniculata; Chile), Opocunonia (1; O. nymanii; East Malesia to New Guinea, the Solomon Islands), Ackama (4; eastern Queensland, New South Wales, North Island of New Zealand), Spiraeopsis (6; Sulawesi, the Philippines, the Moluccas, New Guinea, the Solomon Islands). – Codieae G. Don, Gen. Hist. 3: 197, 202. 8-15 Nov 1834.Pullea (≥3; the Moluccas?, New Guinea, northeastern Queensland, Fiji), Codia (12; New Caledonia), Callicoma (1; C. serratifolia; southeastern Queensland, northeastern New South Wales). – Cunonieae (R. Br.) Schrank et Mart., Hort. Reg. Monac.: 125. 1829. Vesselowskya (2; southeasternmost Queensland, northeastern New South Wales), Pancheria (c 30; New Caledonia), Cunonia (c 25; New Caledonia, one species, Cunonia capensis, in Western and Eastern Cape and KwaZulu-Natal), Weinmannia (150–160; Madagascar, the Mascarene Islands, Malesia to New Guinea, eastern Australia, New Caledonia, Fiji, New Zealand, the Austral Islands, the Marquesas, Mexico, the West Indies, South America).
Cunoniaceae are sister-group to the clade [Elaeocarpaceae+[Brunelliaceae+Cephalotaceae]].
Acsmithia and Spiraeanthemum form a sister-group to the remaining Cunoniaceae. They have synchronously maturing flowers, apocarpous gynoecium producing follicles, epidermal glands, pocket domatia along midvein, and vessel elements with scalariform perforation plates. These characters are present also in other parts of Cunoniaceae, but the combination is unique to Spiraeanthemeae.
Hooglandia is successive sister above Spiraeanthemeae in the analyses by Sweeney & al. (2004). The same authors identified a clade consisting of [Bauera+Davidsonia] and [Ceratopetalum+[Platylophus+Anodopetalum]] immediately as sister to the remainder. Above these basal clades a polytomy formed the crown-clade comprising all other investigated Cunoniaceae.
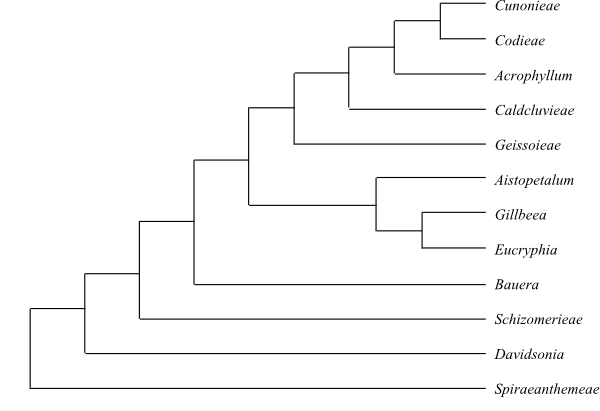 |
|
Cladogram (simplified) of Cunoniaceae based on DNA sequence data and morphology (Bradford & Barnes 2001). |
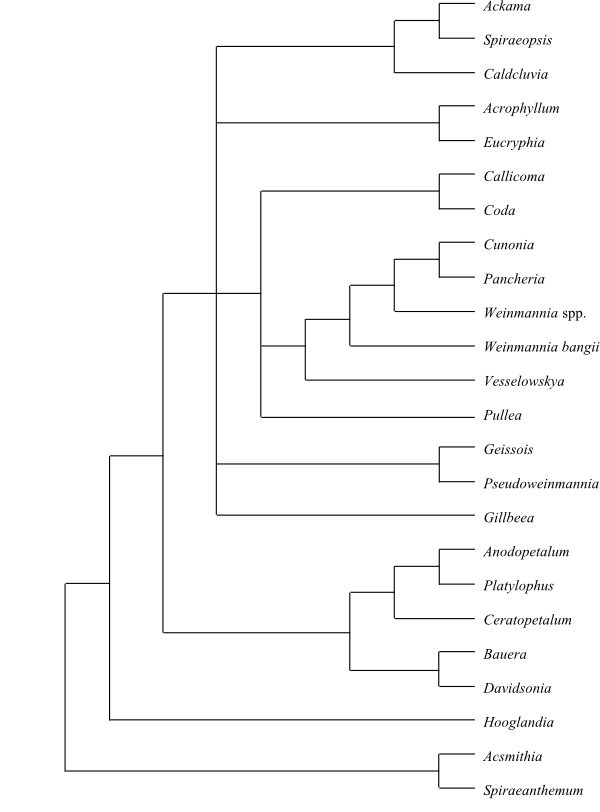 |
|
50% bootstrap consensus tree of Cunoniaceae based on DNA sequence data (Sweeney & al. 2004). |
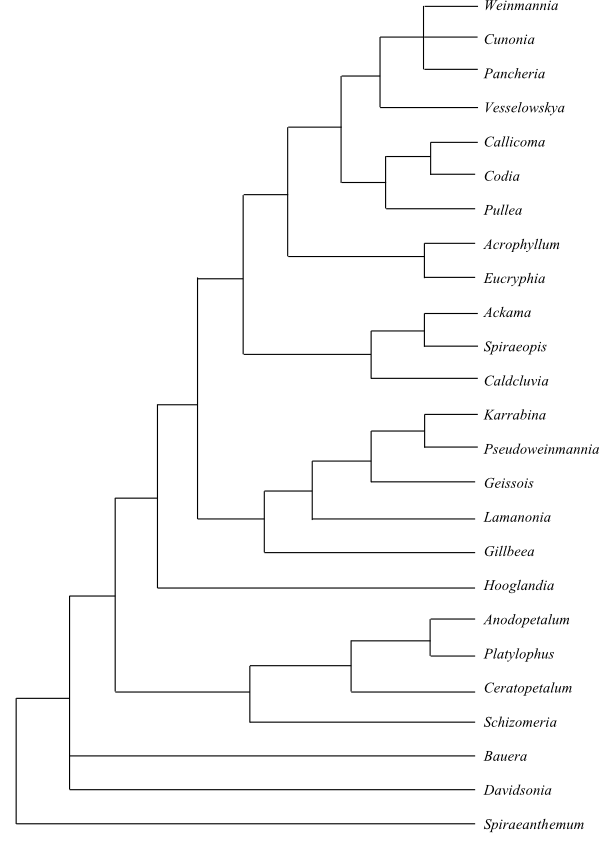 |
|
Bayesian inference tree (simplified) of Cunoniaceae based on plastid DNA sequence data (Hopkins & al. 2013). |
ELAEOCARPACEAE Juss. |
Tetrathecaceae R. Br. in M. Flinders, Voy. Terra Austr. 2: 544. 19 Jul 1814; Elaeocarpales Juss. ex Bercht. et J. Presl, Přir. Rostlin: 223. Jan-Apr 1820 [‘Elaeocarpeae’]; Tremandraceae R. Br. ex DC., Prodr. 1: 343. med Jan 1824 [‘Tremandreae’], nom. cons.; Aristoteliaceae Dumort., Anal. Fam. Plant.: 37, 41. 1829; Tremandrales R. Br. in C. F. P. von Martius, Consp. Regn. Veg.: 43. Sep-Oct 1835 [‘Tremandreae’]
Genera/species 12/c 600
Distribution Madagascar, Mauritius, Socotra, eastern and southern India, Sri Lanka, eastern Himalayas, East Asia to Japan, Southeast Asia, Malesia to New Guinea, Australia, Tasmania, Melanesia, New Zealand, Samoa, Tonga, and other islands in the Pacific, tropical America southwards to southern Chile.
Fossils Pollen grains and leaves of Elaeocarpaceae have been reported from Eocene onwards in Australia and from the Eocene London Clay (possibly also from North America and Greenland).
Habit Usually bisexual (rarely monoecious or dioecious), evergreen trees, shrubs or suffrutices. A large number of species are xerophytes.
Vegetative anatomy Phellogen ab initio superficial. Primary medullary rays narrow (in Tremandra, Platytheca, and Tetratheca). Vessel elements in radial multiples, usually with simple (rarely scalariform) perforation plates; lateral pits usually opposite or alternate (rarely scalariform or intermediate between opposite and alternate), simple pits. Imperforate tracheary xylem elements fibre tracheids or libriform fibres with simple or bordered pits, septate or non-septate (also vasicentric tracheids). Wood rays uniseriate or multiseriate, heterocellular. Axial parenchyma paratracheal scanty vasicentric or banded, or absent. Tyloses sometimes frequent. Wood elements and secondary phloem not storied. Sieve tube plastids Ss type. Nodes usually 3:3, trilacunar with three leaf traces (in Tremandra, Platytheca, and Tetratheca 1:1, unilacunar with one trace). Secretory cavities usually absent. Heartwood sometimes with gum-like substances. Calciumoxalate as druses or single prismatic crystals.
Trichomes Hairs multicellular, uniserate, usually simple (sometimes with glandular tip), in Tremandra, Platytheca, and Tetratheca also stellate.
Leaves Leaves alternate (spiral or distichous) or opposite (rarely verticillate), usually simple (rarely pinnately compound), entire, often coriaceous, often small, sometimes ericoid or highly reduced, with various ptyxis. Stipules lateral (sometimes absent), persistent or caducous; leaf sheath absent. Colleters (modified stipules?) present in some species without stipules. Petiole pulvinate at one or both ends. Petiole vascular bundle transection annular; petiole often with medullary (sometimes flange) bundles. Venation pinnate or palmate; secondary veins proceeding into caducous, opaque and often glandular leaf teeth apices (leaves sometimes single-veined). Stomata anomocytic, paracytic, cyclocytic or actinocytic. Cuticular wax crystalloids as rodlets. Domatia as pits, pockets or hair tufts. Epidermis often with mucilaginous idioblasts. Secretory cavities absent. Mesophyll often with calciumoxalate as druses or solitary prismatic crystals. Hydathodes sometimes present. Leaf margin usually serrate (rarely entire).
Inflorescence Terminal or axillary, simple or compound, panicle, dichasial, raceme-like or racemose, or flowers solitary axillary (in, i.a., Tremandra, Platytheca, and Tetratheca). Flowers often pendant. Bracts usually early caducous.
Flowers Actinomorphic. Pedicel usually articulated (not in Tremandra, Platytheca, and Tetratheca; sometimes absent). Hypogyny. Receptacle sometimes prolonged into andro(gyno)phore. Sepals (three or) four or five (to nine), usually with valvate (rarely imbricate) aestivation, usually free (in, e.g., Crinodendron connate), sometimes petaloid. Petals usually three to six (absent in some species of Sloanea), with valvate, valvate-induplicate or cochlear aestivation, usually fimbriate, dentate or lobate (sometimes entire and sepaloid), usually free (rarely connate). Nectariferous disc usually extrastaminal, annular or lobate, usually large (absent in Platytheca and Tetratheca).
Androecium Stamens four or five to more than 300, usually numerous (rarely as many as or twice as many as petals; rarely in four or five antesepalous or antepetalous fascicles; in Sloanea up to c. 300), centrifugally developing. Filaments free from each other and from tepals, inserted on or above nectariferous disc or around free disc lobes. Anthers basifixed, non-versatile, tetrasporangiate, introrse or latrorse, with adaxial lignified hairs, poricidal (dehiscing by one or two usually apical pores; sometimes at apex of tubular elongation of connective) or longicidal (dehiscing by short to long longitudinal lateral slits, Crinodendron) or dehiscing by transverse valve (in Elaeocarpus); connective sometimes prolonged into bristle or tube. Tapetum secrectory. Staminodia absent.
Pollen grains Microsporogenesis simultaneous. Pollen grains tricolpor(oid)ate (sometimes syncolpate), shed as monads, bicellular at dispersal. Exine tectate, with columellate infratectum, indistinct to weakly psilate, rugulate or finely reticulate to microperforate.
Gynoecium Pistil composed of two to eight (or nine) connate carpels (rarely one carpel, monomerous?). Ovary superior, (unilocular or) bilocular to octalocular (or novemlocular); locules sometimes divided by secondary septa. Style usually single, simple or bilobate to octalobate (or novemlobate) at apex (stylodia rarely two to nine, free). Stigma(s) punctate, papillate, Dry type. Pistillodium absent?
Ovules Placentation usually axile (in Tremandra, Platytheca, and Tetratheca apical). Ovules one to more than 30 per carpel, hairy, anatropous, pendulous or ascending, apotropous or epitropous, bitegmic, crassinucellar, with curved chalazal appendage. Micropyle bistomal, Z-shaped (zig-zag). Outer integument two to six cell layers thick. Inner integument usually three to seven cell layers thick (in Tremandra, Platytheca, and Tetratheca up to c. 25 cell layers thick). Hypostase sometimes present. Endothelium present in Tremandra, Platytheca, and Tetratheca). Megagametophyte monosporous, Polygonum type. Endosperm development ab initio nuclear. Endosperm haustoria? Embryogenesis asterad.
Fruit A loculicidal (sometimes also septicidal) capsule (in some species of Sloanea with prickles or bristles), or a drupe (rarely berry).
Seeds Funicular aril absent. Testa glabrous or hairy, multiplicative, with chalazal, raphal, exostomal or integumentary aril/arilloid, or sometimes with apical chalazal strophiole. Testal cells elongate, thickened and lignified (sarcotesta sometimes present). Endotesta usually crystalliferous. Tegmen multiplicative, vascularized. Exotegmen fibrous. Endotegmen sometimes lignified. Perisperm not developed. Endosperm copious, sometimes oily, ab initio with starch (in, e.g., Tremandra, Platytheca, and Tetratheca). Embryo usually straight (in Sericolea and some species of Elaeocarpus curved), well differentiated, at least sometimes with chlorophyll. Cotyledons two. Germination phanerocotylar.
Cytology n = 12 (Elaeocarpus), 14 (Aristotelia), 15 (Elaeocarpus), 21 (Crinodendron hookerianum), c. 90 (Dubouzetia elegans)
DNA
Phytochemistry Flavonols (kaempferol, quercetin, myricetin), cyanidin, triterpenes, cucurbitacins, geraniin, ellagic and gallic acids, condensed procyanidin-based tannins, hydrolyzable tannins (based on ellagic and gallic acid and methylated ellagic acids), proanthocyanidins (prodelphinidins), pyrrolizidine alkaloids, tropane alkaloids (e.g. tropacocaine, oxytropanols and oxynortropanols, indole and elaeocarpidine alkaloids), saponins, and cyanogenic compounds (phenylalanine-derived?) present.
Use Ornamental plants, fruits, timber, carpentry, medicinal plants.
Systematics Sloaneeae Endl., Gen. Plant.: 1005. 1-14 Feb 1840. Sloanea (c 150; tropical regions on both hemispheres), Vallea (1–2; Colombia to Bolivia), Aristotelia (5; northeastern New South Wales, Tasmania, New Zealand, Peru to Chile). – Elaeocarpeae Bartl., Ord. Nat. Plant.: 340. Sep 1830 [‘Elaeocarpea’]. Crinodendron (5; Chile, Argentina), Peripentadenia (2; northeastern Queensland), Dubouzetia (11; the Moluccas, New Guinea, northern Northern Territory, northeastern Queensland, New Caledonia), Tremandra (2; southwestern Western Australia), Platytheca (2; southwestern Western Australia), Tetratheca (c 40; southwestern Western Australia, southeastern South Australia to southeastern Queensland, Tasmania), Elaeocarpus (c 350; Madagascar, tropical and subtropical Asia and eastwards to New Caledonia, New Zealand and the Hawaiian Islands), Sericolea (16; East Malesia), Aceratium (c 20; East Malesia and eastwards to northeastern Queensland, the Solomon Islands and Vanuatu).
Elaeocarpaceae are sister to the clade [Brunelliaceae+Cephalotaceae].
The clade consisting or Tremandra, Platytheca, and Tetratheca (the former Tremandraceae) is nested deep inside Elaeagnaceae.
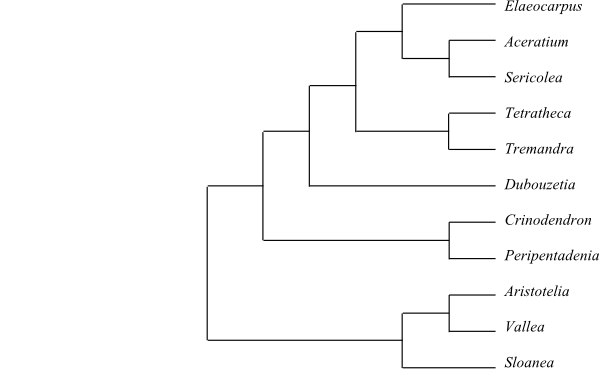 |
|
Cladogram of Elaeocarpaceae based on DNA sequence data (Crayn & al. 2006). |
HUACEAE A. Chev. |
Huales Doweld, Tent. Syst. Plant. Vasc.: xxx. 23 Dec 2001
Genera/species 2/3–4
Distribution Tropical West and Central Africa.
Fossils Unknown.
Habit Bisexual, evergreen trees or shrubs. With strong garlic-like smell.
Vegetative anatomy Phellogen? Bark with widened rays, not divided into fibrous and non-fibrous layers. Parenchyma confluent. Vessel elements usually with simple (sometimes scalariform) perforation plates; lateral pits alternate, (simple? or) bordered pits. Imperforate tracheary xylem elements very thick-walled libriform fibres with simple or bordered pits, non-septate. Wood-rays multiseriate, heterocellular, very wide. Axial parenchyma paratracheal scalariform, reticulate, or narrowly banded. Phloem with wide rays, not stratified. Sieve tube plastids S type. Nodes 3:3, trilacunar with three leaf traces. Cristarque cells present in many tissues. Mucilaginous idioblasts absent. Vessel elements in heartwood with gum-like? substances. Prismatic calciumoxalate crystals often abundant.
Trichomes Hairs unicellular or multicellular, simple or branched (sometimes non-uniformly furcate), stellate or peltate-lepidote; glands present on leaves.
Leaves Alternate (distichous), simple, entire, with ? ptyxis. Stipules intrapetiolar, not connate, usually early caducous; leaf sheath absent. Petiole bundle transection complex. Venation pinnate, often very finely reticulate (sometimes with one pair of coarse veins from leaf base). Stomata usually paracytic. Cuticular waxes absent. Secretory cavities absent. Scattered rounded glands present on abaxial side of lamina near leaf base (sometimes along leaf margin). Leaf margin entire.
Inflorescence Axillary, few-flowered fascicle (flowers rarely solitary).
Flowers Actinomorphic, small. Hypogyny. Sepals adaxially glandular, in Hua five, with valvate aestivation, free, in Afrostyrax three to five, partially or entirely connate (calyptrate calyx in Afrostyrax opening by three or five irregular lobes). Petals four or five, with induplicate-valvate aestivation, adaxially hairy, clawed and peltate (Hua), or sessile and strongly obovate (Afrostyrax), free. Nectary? Disc absent.
Androecium Stamens ten (or eight; twice as many as petals), in one whorl. Filaments free from each other and from tepals. Anthers basifixed, non-versatile, tetrasporangiate (inner microsporangia smaller than outer), introrse, longicidal (dehiscing in Afrostyrax by longitudinal lateral slits and in Hua at apex); connective in Afrostyrax somewhat prolonged at apex. Tapetum secretory? Staminodia absent.
Pollen grains Microsporogenesis simultaneous? Pollen grains triporate, shed as monads, ?-cellular at dispersal. Three aperture-like furrows (pseudocolpi?) present at each pole. Exine tectate, with columellate? infratectum, microverrucate.
Gynoecium Pistil composed of five or six connate carpels. Ovary superior, unilocular. Style single, simple. Stigma punctate, type? Pistillodium absent.
Ovules Placentation basal. Ovules one (Hua) or four to six (Afrostyrax) per ovary, anatropous, ascending, bitegmic, crassinucellar? Micropyle ?-stomal. Outer integument ? cell layers thick. Inner integument ? cell layers thick. Megagametophyte monosporous, Polygonum type. Endosperm development? Endosperm haustoria? Embryogenesis?
Fruit A drupe (Afrostyrax) or a one- or two-seeded (septicidal?) capsule (Hua). Pericarp with stony (sclerenchymatous?) central layer.
Seeds Aril absent. Testa usually glabrous (in Hua finely hairy), vascularized. Exotegmen palisade, with lignified cell walls. Endotegmen? Perisperm not developed. Endosperm copious, starchy and oily, ruminate, with garlic-like scent. Embryo straight, well differentiated, chlorophyll? Cotyledons two, flattened. Germination?
Cytology n = ?
DNA
Phytochemistry Very insufficiently known. Ellagic acid, hydrolyzable and condensed tannins, and proanthocyanidins not found.
Use Timber, spices, medicinal plants.
Systematics Afrostyrax (2–3; tropical West and Central Africa), Hua (1; H. gabonensis; Central Africa).
Huaceae are possibly sister-group to the remaining Oxalidales (Wurdack & Davis 2009).
OXALIDACEAE R. Br. |
Oxydaceae Rupr., Fl. Ingr. 1: 236. Mai 1860 [’Oxydeae’], nom. illeg.; Averrhoaceae Hutch., Fam. Fl. Pl., ed. 2: 356. 4 Jun 1959
Genera/species 5/500–600?
Distribution Tropical and subtropical regions, few species of Oxalis in temperate regions.
Fossils Unknown.
Habit Usually bisexual (in Dapania androdioecious), evergreen shrubs or trees (rarely lianas) or perennial (rarely annual) herbs, often with root tubers, sometimes succulent. Some species are xerophytes or helophytes. Juice often bitter. Some species of Oxalis have CAM physiology.
Vegetative anatomy Phellogen ab initio superficial. Secondary lateral growth normal or absent. Vessel elements with simple perforation plates; lateral pits alternate, simple pits. Imperforate tracheary xylem elements in woody species libriform fibres with simple or bordered pits, septate or non-septate. Wood rays uniseriate, homocellular or heterocellular. Axial parenchyma apotracheal diffuse, or paratracheal scanty vasicentric. Tyloses sometimes frequent. Sieve tube plastids usually Pc or Pcfs type (very similar to those in Connaraceae; in Biophytum S type; in Averrhoa Pcfs type). Nodes 3:3, trilacunar with three leaf traces. Prismatic calciumoxalate crystals abundant.
Trichomes Hairs unicellular or multicellular, usually simple, often vesicular; multicellular glandular hairs often present.
Leaves Alternate (spiral; in Averrhoa carambola and Sarcotheca distichous), usually pinnately or palmately compound with articulated petiolules (rarely unifoliolate), sometimes strongly reduced, usually with circinate ptyxis. Stipules usually absent (rarely present, small); leaf sheath absent. Colleters present in Oxalis. Petiole articulated, sometimes expanded to a phyllodium, or woody. Petiole vascular bundle transection annular; petiole sometimes with medullary bundles. Venation pinnate or subpalmate. Stomata paracytic. Cuticular wax crystalloids as rosettes of platelets (Fabales type). Abaxial side of lamina often with vesicular hairs. Secretory cavities usually present. Calciumoxalate crystals abundant, usually as one prismatic or cuboid crystal per cell. Hydathodes present in some species. Leaflet margins entire. Leaves sometimes (in, e.g., Biophytum) sensitive to touch or light (folding up petiole or lamina).
Inflorescence Axillary, thyrso-paniculate, umbel-like or racemose, or flowers solitary.
Flowers Actinomorphic. Pedicel articulated. Hypogyny. Sepals five, with imbricate quincuncial aestivation, persistent, free or connate at base. Petals five, usually with contorted (sometimes imbricate or open) aestivation, often clawed, early caducous (often absent in cleistogamous flowers), usually free (sometimes connate at base), often with uniseriate glandular hairs. Nectaries extrastaminal, often as antepetalous glands at filament bases outside antepetalous staminal whorl. Disc present or absent. Flowers usually triheterostylous or diheterostylous.
Androecium Stamens usually 5+5, obdiplostemonous (sometimes five, haplostemonous, antesepalous or, in Averrhoa, antepetalous), antepetalous outer stamens shorter than antesepalous inner stamens. Filaments with uniseriate somewhat moniliform hairs, connate at base into ring, free from tepals. Anthers dorsifixed, versatile?, tetrasporangiate, extrorse or introrse, longicidal (dehiscing by longitudinal slits); connective somewhat prolonged. Tapetum secretory. Staminodia five, extrastaminal or intrastaminal, or absent.
Pollen grains Microsporogenesis simultaneous. Pollen grains usually tricolpate or tricolporoidate (rarely tricolporate, tetracolp[or]ate or pantocolp[or]ate), often starchy, shed as monads, usually bicellular (in Biophytum tricellular) at dispersal. Exine tectate or semitectate, with columellate? infratectum, perforate or finely reticulate.
Gynoecium Pistil composed of (three to) five usually connate antepetalous carpels (in Biophytum free, apocarpy). Ovary superior, usually (trilocular to) quinquelocular (in Biophytum unilocular, apocarpous). Stylodia (three to) five, free. Stigmas spatulate to capitate or punctate, papillate, Dry type. Pistillodium absent.
Ovules Placentation usually axile (in Oxalis aberrans and Biophytum parietal). Ovules usually (one or) two (to six) per carpel, anatropous or hemianatropous, pendulous, epitropous, bitegmic, crassinucellar (Averrhoa) or tenuinucellar (Oxalis, Biophytum). Micropyle usually bistomal (sometimes exostomal, sometimes Z-shaped), upright (in Averrhoa and Biophytum furrow-shaped, in Oxalis infundibuliform). Outer integument three to five cell layers thick. Inner integument three to six cell layers thick. Archespore rarely multicellular. Chalazal appendages present. Megagametophyte usually monosporous, Polygonum type (sometimes disporous, octacellular, Allium type). Antipodal cells often degenerating. Endosperm development ab initio nuclear. Endosperm haustoria? Embryogenesis asterad.
Fruit A loculicidal capsule, ridged or angular, often fleshy (in Biophytum finally schizocarpous usually with five capsule-like mericarps), with persistent calyx, or a quinquangular berry (Averrhoa, Sarcotheca). Fruit often explosively or elastically dehiscent (mucilaginous epidermis of seeds often also dehiscing).
Seeds Aril present in Dapania. Seed sometimes subruminate. Seed coat endotestal-exotegmic. Endothelium present. Testal often mucilaginous, aril-like, usually multiplicative. Exotesta unspecialized. Endotestal cells with calciumoxalate crystals and sometimes thickened walls, not palisade. Exotegmen fibrous, well developed, sometimes two-layered. Endotegmen unspecialized. Tegmen absent in Biophytum. Testa and tegmen poorly differentiated, when fruit baccate. Perisperm not developed. Endosperm usually copious, fleshy, usually ruminate, oily and usually starchy, sometimes absent. Embryo large, straight, well differentiated, with or without chlorophyll. Cotyledons two. Germination phanerocotylar.
Cytology n = (5–)7(–42) (Oxalis), 7–16 (Biophytum), 11–12 (Averrhoa)
DNA Plastid gene infA lost/defunct (Oxalis).
Phytochemistry Flavonols, cyanidin, gallic acid, tannins, proanthocyanidins (prodelphinidins), saponins benzoquinone rapanone, and free soluble oxalic acid present. Ellagic acid, alkaloids, and cyanogenic compounds not found.
Use Ornamental plants, fruits (Averrhoa), vegetables (e.g. tubers of Oxalis tuberosa, O. deppei etc.), timber.
Systematics Oxalis (500–600?; nearly cosmopolitan, with their largest diversity in the Cape Provinces and the Andes), Biophytum (c 50; tropical regions on both hemispheres), Averrhoa (2; orig. in Malesia or eastern Brazil), Dapania (3; Madagascar, Malesia), Sarcotheca (<11; West Malesia).
Oxalidaceae are sister to Connaraceae.
There is no available phylogeny of Oxalidaceae.
Literature
Adlassnig W, Peroutka M, Lendl T. 2011. Traps of carnivorous pitcher plants as a habitat: composition of the fluid, biodiversity and mutualistic activities. – Ann. Bot. 107: 181-194.
Agababian VS. 1964. Evolution of pollen in the order Cunoniales and Saxifragales in relation to some questions of their systematics and phylogeny. – Bull. Armenian Acad. Sci., Biol., 17: 59-72.
Albert VA, Williams SE, Chase MW. 1992. Carnivorous plants: phylogeny and structural evolution. – Science 257: 1491-1495.
Alford JJ. 1995. Two new species of Tetratheca (Tremandraceae), from the Coolgardie and Austin Botanical Districts, Western Australia. – Nuytsia 10: 143-149.
Arber A. 1941. On the morphology of the pitcher-leaves in Heliamphora, Sarracenia, Darlingtonia, Cephalotus, and Nepenthes. – Ann. Bot., N. S., 5: 563-578.
Azanza JJ. 1988. Notes on Ecuadorean Vallea (Elaeocarpaceae) with the description of a new species. – Nord. J. Bot. 8: 19-23.
Azkue DD. 2000. Chromosome diversity of South American Oxalis (Oxalidaceae). – Bot. J. Linn. Soc. 132: 143-152.
Baas P. 1972. Anatomical contributions to plant taxonomy II. The affinities of Hua Pierre and Afrostyrax Perkins et Gilg. – Blumea 20: 161-192.
Balgooy MMJ van. 1976. A note on Aceratium ferrugineum C. T. White (Elaeocarpaceae). – Blumea 23: 49-50.
Balgooy MMJ van. 1982. A revision of Sericolea Schlechter (Elaeocarpaceae). – Blumea 28: 103-141.
Bange GGJ. 1952. A new family of dicotyledons: Davidsoniaceae. – Blumea 7: 293-296.
Barker PCJ, Brown MJ. 1994. Anodopetalum biglandulosum: growth form and abundance in Tasmanian rainforest. – Aust. J. Ecol. 19: 435-443.
Barnes RW. 1999. Palaeobiogeography, extinctions and evolutionary trends in the Cunoniaceae. A synthesis of the fossil record. – PhD. diss., School of Plant Science, University of Tasmania, Hobart, Tasmania.
Barnes RW, Hill RS. 1999a. Ceratopetalum fruits from Australian Cainozoic sediments and their significance for petal evolution in the genus. – Aust. Syst. Bot. 12: 635-645.
Barnes RW, Hill RS. 1999b. Macrofossils of Callicoma and Codia (Cunoniaceae) from Australian Cainozoic sediments. – Aust. J. Bot. 12: 647-670.
Barnes RW, Jordan GJ. 2000. Eucryphia (Cunoniaceae) reproductive and leaf macrofossils from Australian Cainozoic sediments. – Aust. Syst. Bot. 13: 373-394.
Barnes RW, Rozefelds AC. 2000. Comparative morphology of Anodopetalum (Cunoniaceae). – Aust. Syst. Bot. 13: 267-282.
Barnes RW, Hill RS, Bradford JC. 2001. The history of Cunoniaceae in Australia from macrofossil evidence. – Aust. J. Bot. 49: 301-320.
Barth OM, Barbosa AF. 1973. Catálogo sistemático das pólens das plantas arbóreas do Brasil meridional XVII. Elaeocarpaceae e Tiliaceae. – Mem. Inst. Oswaldo Cruz 71: 203-217.
Bate-Smith EC. 1977. Chemistry and taxonomy of the Cunoniaceae. – Biochem. Syst. Ecol. 5: 95-105.
Bate-Smith EC, Davenport SM, Harborne JB. 1967. Comparative biochemistry of flavonoids: a correlation between chemistry and plant geography in the genus Eucryphia. – Phytochemistry 6: 1407-1413.
Bauert U, Bohn HF, Federle W. 2008. Harmless nectar source or deadly trap: Nepenthes pitchers are activated by rain, condensation and nectar. – Proc. Roy. Soc., Sect. B, 275: 259-265.
Bausch J. 1938. A revision of the Eucryphiaceae. – Kew Bull. 1938: 317-349.
Bayer C. 2006. Huaceae. – In: Kubitzki K (ed), The families and genera of vascular plants IX. Flowering plants. Eudicots. Berberidopsidales, Buxales, Crossosomatales, Fabales p. p., Geraniales, Gunnerales, Myrtales p. p., Proteales, Saxifragales, Vitales, Zygophyllales, Clusiaceae Alliance, Passifloraceae Alliance, Dilleniaceae, Huaceae, Picramniaceae, Sabiaceae, Springer, Berlin, Heidelberg, New York, pp. 191-193.
Bayer MB. 1992. Salter’s revision of South African Oxalis (Oxalidaceae) and some new combinations. – Herbertia 48: 58-69.
Bean AR, Jessup LW. 1997. Dubouzetia saxatilis (Elaeocarpaceae), a new species from north Queensland, Australia. – Austrobaileya 4: 673-675.
Behnke H-D. 1982. Sieve-element plastids of Connaraceae and Oxalidaceae. A contribution to the knowledge of P-type plastids in dicotyledons and their significance. – Bot. Jahrb. Syst. 103: 1-8.
Behnke H-D. 1985. Contributions to the knowledge of P-type sieve-element plastids in dicotyledons II. Eucryphiaceae. – Taxon 34: 607-610.
Beijersbergen A. 1972. Notes on the chemotaxonomy of Huaceae. – Blumea 20: 160.
Bensel CR, Palser BF. 1975. Floral anatomy in the Saxifragaceae sensu lato IV. Baueroideae and conclusions. – Amer. J. Bot. 62: 688-694.
Biddle JA, Christophel DC. 1978. Intergynoecial development in Tremandraceae. – Phytomorphology 28: 411-418.
Boesewinkel FD. 1985. Development of ovule and seed-coat in Averrhoa (Oxalidaceae) with notes on some related genera. – Acta Bot. Neerl. 34: 413-424.
Boesewinkel FD. 1999. Ovules and seeds of Tremandraceae. – Aust. J. Bot. 47: 769-781.
Bouman F. 1974. Developmental studies of the ovule, integuments, and seeds in some angiosperms. – Ph.D. diss., University of Amsterdam, LOS, Naarden, The Netherlands.
Bouquet A. 1969. Féticheurs et medicines traditionelles du Congo (Brazzaville). – Mém. O.R.S.T.O.M. 36.
Bradford JC. 1998. A cladistic analysis of species-groups in Weinmannia (Cunoniaceae) based on morphology and inflorescence architecture. – Ann. Missouri Bot. Gard. 85: 565-593.
Bradford JC. 2000. Phylogenetic systematics of Cunoniaceae (Oxalidales), with an emphasis on species-groups and inflorescence evolution in Weinmannia and related genera. – Ph.D. diss., Washington University, St. Louis, Missouri.
Bradford JC. 2001. The application of a cladistic analysis to the classification and identification of Weinmannia (Cunoniaceae) in Madagascar and the Cormoro Islands. – Adansonia, sér. III, 23: 237-246.
Bradford JC. 2002. Molecular phylogenetics and morphological evolution in Cunonieae (Cunoniaceae). – Ann. Missouri Bot. Gard. 89: 491-503.
Bradford JC, Barnes RW. 2001. Phylogenetics and classification of Cunoniaceae (Oxalidales) using chloroplast DNA sequences and morphology. – Syst. Bot. 26: 354-385.
Bradford JC, Berry PE. 1998. Cunoniaceae. – In: Berry PE, Holst BK, Yatskievych K (eds), Flora of the Venezuelan Guayana 4, Missouri Botanical Garden Press, St. Louis.
Bradford JC, Jaffré T. 2004. Plant species microendemism and conservation of montane maquis in New Caledonia: two new species of Pancheria (Cunoniaceae) from the Roche Ouaïème. – Biodiv. Conserv. 13: 2253-2273.
Bradford JC, Miller JS. 2001. New taxa and nomenclatural notes on the flora of the Marojejy massif, Madagascar V. Cunoniaceae: Weinmannia. – Adansonia, sér. III, 23: 219-236.
Bradford JC, Fortune Hopkins HC, Barnes RW. 2004. Cunoniaceae. – In: Kubitzki K (ed), The families and genera of vascular plants VI. Flowering plants. Dicotyledons. Celastrales, Oxalidales, Rosales, Cornales, Ericales, Springer, Berlin, Heidelberg, New York, pp. 91-111.
Breteler FJ (ed). 1989. The Connaraceae. A taxonomic study with emphasis on Africa. – Belmontia, Miscell. Publ. Bot., N. S. 21(136), Agric. Univ. Wageningen Pap. 89(6): 1-403.
Bricker JS. 1991. A revision of the genus Crinodendron (Elaeocarpaceae). – Syst. Bot. 16: 77-88.
Bricker JS. 1992. Pollination biology of the genus Crinodendron (Elaeocarpaceae). – J. Arizona-Nevada Acad. Sci. 24-25: 51-54.
Brongniart AD, Gris A. 1864. Sur les Saxifragées-Cunoniées, et description du nouveau genre Pancheria. – Ann. Sci. Nat. Bot. Biol. Veg., sér. V, 1: 365-378.
Butcher R. 2007. New taxa of ‘leafless’ Tetratheca (Elaeocarpaceae, formerly Tremandraceae) from Western Australia. – Aust. Syst. Bot. 20: 139-160.
Butcher R, Byrne M, Crayn DM. 2007. Evidence for convergent evolution among phylogenetically distant rare species of Tetratheca (Elaeocarpaceae, formerly Tremandraceae) from Western Australia. – Aust. Syst. Bot. 20: 126-138.
Capuron R. ca. 1965. Études sur les essences forestières de Madagascar: Voanama, Sloanea rhodantha (Baker) R. Cap. – Elaeocarpacées. – Centre technique forestier tropical (Section de Madagascar). [Mimeographed]
Carlquist SJ. 1977. Wood anatomy of the Tremandraceae: phylogenetic and ecological implications. – Amer. J. Bot. 64: 704-713.
Carlquist SJ. 1981. Wood anatomy of Cephalotaceae. – IAWA Bull., N. S., 2: 175-178.
Carniel K. 1967. Licht- und elektronenmikroskopische Untersuchungen der Ubischkörper in der Gattung Oxalis. – Österr. Bot. Zeitschr. 114: 490.
Carpenter RJ, Buchanan AM. 1993. Oligocene leaves, fruits and flowers of the Cunoniaceae from Cethana, Tasmania. – Aust. Syst. Bot. 6: 91-109.
Chambers KL, Poinar G Jr, Buckley R. 2010. Tropidogyne, a new genus of early Cretaceous eudicots (Angiospermae) from Burmese amber. – Novon 20: 23-29.
Chevalier A. 1947a. La famille des Huacacées et ses affinités. – Rev. Intl. Bot. Appl. Agric. Trop. 27: 26-29.
Chevalier A. 1947b. Arbres à ail, Huacacées et Styrax à benjoin. – Rev. Intrl. Bot. Appl. Agric. Trop. 27: 401-407.
Chodat R. 1896. Tremandraceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(4), W. Engelmann, Leipzig, pp. 320-323.
Chung RCK, Lim AL. 1998. The embryology of Averrhoa (Oxalidaceae). – Sandakania 12: 37-55.
Cocucci AA. 2006. Oxalidaceae. – In: Kubitzki K (ed), The families and genera of vascular plants VI. Flowering plants. Dicotyledons. Celastrales, Oxalidales, Rosales, Cornales, Ericales, Springer, Berlin, Heidelberg, New York, pp. 285-290.
Conn BJ, Richards PG. 1994. A new species of Oxalis section Corniculatae (Oxalidaceae) from Australasia. – Aust. Syst. Bot. 7: 171-181.
Conran JG. 2004. Cephalotaceae. – In: Kubitzki K (ed), The families and genera of vascular plants VI. Flowering plants. Dicotyledons. Celastrales, Oxalidales, Rosales, Cornales, Ericales, Springer, Berlin, Heidelberg, New York, pp. 65-68.
Conran JG, Denton MD. 1996. Germination in the Western Australian Pitcher Plant Cephalotus follicularis and its unusual early seedling development. – West. Aust. Natur. 21: 37-42.
Coode MJE. 1978. A conspectus of Elaeocarpaceae in Papuasia. – Brunonia 1: 131-302.
Coode MJE. 1983. A conspectus of Sloanea (Elaeocarpaceae) in the Old World. – Kew Bull. 38: 347-427.
Coode MJE. 1984. Elaeocarpus in Australia and New Zealand. – Kew Bull. 39: 509-586.
Coode MJE. 1985. Aristotelia and Vallea, closely related in Elaeocarpaceae. – Kew Bull. 40: 479-507.
Coode MJE. 1987a. Crinodendron, Dubouzetia and Peripentadenia, closely related in Elaeocarpaceae. – Kew Bull. 42: 777-814.
Coode MJE. 1987b. 55. Eléocarpacées. – In: Bosser et al. (eds), Flore des Mascareignes, fams. 51. Malvacées – 62. Oxalidacées, MSIRI, Mauritius, ORSTOM, Paris, Royal Botanical Garden, Kew.
Coode MJE. 1995. Elaeocarpus in the Flora Malesiana area: E. kraengensis and ten new species from Sulawesi. – Kew Bull. 50: 267-294.
Coode MJE. 1996a. Elaeocarpus for Flora Malesiana: notes, new taxa and combinations in sect. Elaeocarpus: 2. – Kew Bull. 51: 83-101.
Coode MJE. 1996b. Elaeocarpus for Flora Malesiana: notes, new taxa and combinations in the Acronodia group. – Kew Bull. 51: 267-300.
Coode MJE. 1996c. Elaeocarpus for Flora Malesiana: the ‘Polystachyus’ group. – Kew Bull. 51: 649-666.
Coode MJE. 1998. Elaeocarpus for Flora Malesiana: notes, new taxa, names and combinations for Borneo. – Kew Bull. 53: 83-128.
Coode MJE. 2001a. Contributions to the flora of Mt Jaya V. Elaeocarpus in New Guinea: new taxa in the Debruynii subgroup of the Monocera group. – Kew Bull. 56: 449-460.
Coode MJE. 2001b. Elaeocarpus for Flora Malesiana: the Fissipetalum group in Central Malesia. – Kew Bull. 56: 461-463.
Coode MJE. 2001c. Elaeocarpus for Flora Malesiana: the E. stipularis complex, E. nitidus group & E. barbulatus. – Kew Bull. 56: 513-565.
Coode MJE. 2001d. Elaeocarpus for Flora Malesiana: the Coilopetalum group in Sulawesi & Maluku. – Kew Bull. 56: 769-836.
Coode MJE. 2001e. Elaeocarpus for Flora Malesiana: the Coilopetalum group in the Lesser Sunda Islands. – Kew Bull. 56: 875-883.
Coode MJE. 2001f. Elaeocarpus for Flora Malesiana: the Verticillatae subgroup of the Monocera group and a new Philippine species. – Kew Bull. 56: 885-901.
Coode MJE. 2002. Contributions to the flora of Mt. Jaya IX. Elaeocarpus in New Guinea: E. sericoloides (Fissipetalum group), E. royenii & E. multisectus (sect. Elaeocarpus). – Kew Bull. 57: 925-935.
Coode MJE. 2003. Elaeocarpus for Flora Malesiana: two new taxa in the Coloides subgroup of the Monocera group from New Guinea. – Kew Bull. 58: 453-458.
Coode MJE. 2004. Elaeocarpaceae. – In: Kubitzki K (ed), The families and genera of vascular plants VI. Flowering plants. Dicotyledons. Celastrales, Oxalidales, Rosales, Cornales, Ericales, Springer, Berlin, Heidelberg, New York, pp. 135-144.
Coode MJE. 2005. Elaeocarpus for Flora Malesiana: E. crenulatus, E. myrtoides and E. amabilis from New Guinea, reconsidered. – Kew Bull. 60: 305-311.
Coode MJE, Weibel R. 1994. Elaeocarpus for Flora Malesiana: notes, new taxa and combinations in sect. Elaeocarpus 1. – Kew Bull. 49: 235-259.
Corner EJH. 1939. Elaeocarpus. – Gard. Bull. Straits’ Settlem. (Singapore) 10: 308-329.
Crayn DM, Rossetto M, Maynard DJ. 2006. Molecular phylogeny and dating reveals an Oligo-Miocene radiation of dry-adapted shrubs (former Tremandraceae) from rainforest tree progenitors (Elaeocarpaceae) in Australia. – Amer. J. Bot. 93: 1328-1342.
Cuatrecasas J. 1970. Flora Neotropica. Monograph 2. Brunelliaceae. – Hafner, Darien, Connecticut.
Cuatrecasas J. 1985. Flora Neotropica. Monograph 2. Brunelliaceae (Suppl.). – New York Botanical Garden, Bronx, New York, pp. 29-103.
Dadswell HE, Eckersley AM. 1938. The wood structure of some Australian Cunoniaceae with methods for their identification. – Council for Scientific and Industrial Research, No. 119.
Dakin WJ. 1919. The West Australian pitcher plant (Cephalotus follicularis), and its physiology. – J. Roy. Soc. West. Aust. 4: 37-53.
De Azkue D. 2000. Chromosome diversity of South American Oxalis (Oxalidaceae). – Bot. J. Linn. Soc. 132: 143-152.
DeBuhr LE. 1976. Field notes on Cephalotus follicularis in Western Australia. – Carn. Plants Newslett. 5: 8-9.
Dehay C. 1961. Remarques sur l’anatomie comparée des Elaeocarpacées. – Bull. Soc. Bot. France 14: 89-96.
Denton MF. 1973. A monograph of Oxalis, section Ionoxalis (Oxalidaceae) in North America. – Publ. Michigan State Univ. Mus., Biol. Ser. 4: 455-615.
Dettmann ME, Clifford HT. 2001. The fossil record of Elaeocarpus L. fruits. – Mem. Queensland Mus. 46: 461-497.
Dickison WC. 1971. Anatomical studies in the Connaraceae I. Carpels. – J. Elisha Mitchell Sci. Soc. 87: 77-86.
Dickison WC. 1972. Anatomical studies in the Connaraceae II. Wood anatomy. – J. Elisha Mitchell Sci. Soc. 88: 120-136.
Dickison WC. 1973a. Anatomical studies in the Connaraceae III. Leaf anatomy. – J. Elisha Mitchell Sci. Soc. 89: 121-138.
Dickison WC. 1973b. Anatomical studies in the Connaraceae IV. The bark and young stem. – J. Elisha Mitchell Sci. Soc. 90: 166-171.
Dickison WC. 1975a. Floral morphology and anatomy of Bauera. – Phytomorphology 25: 69-76.
Dickison WC. 1975b. Studies on the floral anatomy of the Cunoniaceae. – Amer. J. Bot. 62: 433-447.
Dickison WC. 1975c. Leaf anatomy of Cunoniaceae. – Bot. J. Linn. Soc. 71: 275-294.
Dickison WC. 1977. Wood anatomy of Weinmannia (Cunoniaceae). – Bull. Torrey Bot. Club 104: 12-23.
Dickison WC. 1978. Comparative anatomy of Eucryphiaceae. – Amer. J. Bot. 65: 722-735.
Dickison WC. 1979. A survey of pollen morphology of the Connaraceae. – Pollen Spores 21: 31-79.
Dickison WC. 1980a. Diverse nodal anatomy of the Cunoniaceae. – Amer. J. Bot. 67: 975-981.
Dickison WC. 1980b. Comparative wood anatomy and evolution of the Cunoniaceae. – Allertonia 2: 281-321.
Dickison WC. 1984. Fruits and seeds of the Cunoniaceae. – J. Arnold Arbor. 65: 149-190.
Dickison WC, Rutishauser R. 1990. Developmental morphology of stipules and systematics of the Cunoniaceae and presumed allies II. Taxa without interpetiolar stipules and conclusions. – Bot. Helvet. 100: 75-95.
Dickson A. 1878. The structure of the pitcher of Cephalotus follicularis. – J. Bot. (London) 16: 1-5.
Dickson A. 1883. On the morphology of the pitcher of Cephalotus follicularis. – Trans. Proc. Bot. Soc. Edinb. 14: 172-181.
Diels L. 1930. Cephalotaceae. – In: Engler A, Harms H (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 18a, W. Engelmann, Leipzig, pp. 71-74.
Doweld AB. 1998. The carpology and taxonomic relationships of Davidsonia (Davidsoniaceae). – Edinburgh J. Bot. 55: 13-25.
Downing TL, Ladiges PY, Duretto MF. 2008. Trichome morphology provides phylogenetically informative characters for Tremandra, Platytheca and Tetratheca (former Tremandraceae). – Plant Syst. Evol. 271: 199-221.
Dreyer LL. 1996. A palynological review of Oxalis (Oxalidaceae) in Southern Africa. – Ph.D. diss., University of Pretoria, Republic of South Africa.
Dreyer LL, Johnson C. 2000. New chromosome number records of South African Oxalis species. – South Afr. J. Bot. 66: 130-132.
Dreyer LL, Oberlander KC. 2007. Morphological diversity of southern African members of Oxalis. – South Afr. J. Bot. 73: 285.
Dreyer LL, Roets F, Oberlander KC. 2009. Oxalis saltusbelli: a new Oxalis (Oxalidaceae) species from the Oorlogskloof Nature Reserve, Nieuwoudtville, South Africa. – South Afr. J. Bot. 75: 110-116.
Earl Smith C. 1954. The New World species of Sloanea (Elaeocarpaceae). – Contr. Gray Herb. 175: 1-114.
Ehrendorfer F, Morawetz W, Dawe J. 1984. The neotropical angiosperm families Brunelliaceae and Caryocaraceae: first karyosystematical data and affinities. – Plant Syst. Evol. 145: 183-191.
Emshwiller E. 2002. Biogeography of the Oxalis tuberosa alliance. – Bot. Rev. 68: 128-152.
Emshwiller E, Doyle JJ. 1999. Chloroplast-expressed glutamine synthetase (ncpGS): potential utility for phylogenetic studies with an example from Oxalis (Oxalidaceae). – Mol. Phylogen. Evol. 12: 310-319.
Emswhiller E, Doyle JJ. 2002. Origin of domestication and polyploidy in oca (Oxalis tuberosa: Oxalidaceae) 2. Chloroplast expressed glutamine synthetase data. – Amer. J. Bot. 89: 1042-1056.
Engler A. 1891a. Cephalotaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(2a), W. Engelmann, Leipzig, pp. 39-40.
Engler A. 1891b. Saxifragaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(2a), W. Engelmann, Leipzig, pp. 41-93.
Engler A. 1891c. Cunoniaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(2a), W. Engelmann, Leipzig, pp. 94-103.
Engler A. 1897. Brunelliaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien, Nachträge zu III(2a), W. Engelmann, Leipzig, pp. 182-184.
Engler A. 1930a. Saxifragaceae. – In: Engler A, Harms H (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 18a, W. Engelmann, Leipzig, pp. 74-226.
Engler A. 1930b. Brunelliaceae. – In: Engler A, Harms H (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 18a, W. Engelmann, Leipzig, pp. 226-229.
Engler A. 1930c. Cunoniaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 18a, W. Engelmann, Leipzig, pp. 229-262.
Exell AW. 1963. 38. Oxalidaceae. – In: Exell AW, Fernandes A, Wild H (eds), Flora Zambesiaca 2 (Part 1), Crown Agents for Oversea Governments and Administrations, London, pp. 149-162.
Eyde RH. 1970. Anatomy. – In: Cuatrecasas J (ed), Flora Neotropica Monograph No. 2. Brunelliaceae, Organization for Flora Neotropica, Darien, Connecticut, pp. 32-43.
Fogliani B, Bouraima-Madjebi S, Medevielle V, Pineau R. 2002. Screening of 50 Cunoniaceae species from New Caledonia for antimicrobial properties. – New Zealand J. Bot. 40: 511-520.
Fogliani B, Hopkins HCF, Bouraïma-Madjèbi S, Medevielle V. 2009. Morphological development of Geissois pruinosa (Cunoniaceae) from seed to adult stage and the expression of plesiomorphic characters in seedlings. – Flora 204: 7-16.
Forero E. 1983. Flora Neotropica. Monograph 36. Connaraceae. – New York Botanical Garden, New York.
Forero E. 1996. 81. Connaraceae. – In: Harling G, Andersson L (eds), Flora of Ecuador 56, Nord. J. Bot., Copenhagen, pp. 153-167.
Forster PI, Hyland BPM. 1997. Two new species of Eucryphia Cav. (Cunoniaceae) from Queensland. – Austrobaileya 4: 589-596.
Froebe HA, Baur N. 1988. Die Morphogenese der Kannenblätter von Cephalotus follicularis Labill. – Akad. Wiss. Lit. Mainz, Abh. Math.-Naturwiss. Kl. 1988, 3: 1-19.
Fryns-Claessens E, Van Cotthem W. 1966. L’appareil stomatique des Pandacées et Davidsoniacées. – Rev. Gén. Bot. 63: 783-789.
Gardner AG, Vaio M, Guerra M, Emswhiller E. 2012. Diversification of the American bulb-bearing Oxalis (Oxalidaceae): dispersal to North America and modification of the tristylous breeding system. – Amer. J. Bot. 99: 152-164.
Garnock-Jones PJ, Timmerman GM, WagstaffSJ. 1996. Unknown New Zealand angiosperm assigned to Cunoniaceae using sequence of the chloroplast rbcL gene. – Plant Syst. Evol. 202: 211-218.
Gasson P. 1996. Wood anatomy of the Elaeocarpaceae. – In: Donaldson LA, Singh AP, Butterfield BG, Whitehouse LJ (eds), Recent advances in wood anatomy, New Zealand Forest Research Institute, Rotorua, pp. 47-71.
Germain R. 1963. 89. Huaceae. – In: Robyns W (ed), Flore du Congo, du Rwanda et du Burundi 10, Nat. Plantentuin Belg., Meise, pp. 317-319.
Gibbs PE, Marshall D, Brunton D. 1978. Studies on the cytology of Oxalis tuberosa and Tropaeolum tuberosum. – Notes Bot. Gard. Edinb. 37: 215-220.
Gilg E. 1894. Connaraceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(3), W. Engelmann, Leipzig, pp. 61-70, 388; Gilg E. 1897. Nachträge zu III(3), pp. 189-190.
Gilg E. 1925. Eucryphiaceae. – In: Engler A, Gilg E (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 21, W. Engelmann, Leipzig, pp. 47-50.
Godley EJ. 1983. The fruit in Ackama, Caldcluvia, and Weinmannia (Cunoniaceae). – New Zealand J. Bot. 21: 455-456.
Govil CM, Saxena NP. 1976. Anatomy and embryology of Weinmannia fraxinea Sm. (Cunoniaceae). – J. Indian Bot. Soc. 55: 219-226.
Govindappa DA, Boriah G. 1956. The development of female gametophyte in Biophytum sensitivum. – Curr. Sci. 25: 403-404.
Guillaumin A. 1940. Matériaux pour la Flore de la Nouvelle-Calédonie LVI. Révision des Cunoniacées. – Bull. Soc. Bot. France 87: 242-256.
Guillaumin A. 1948. Famille LXXX. Cunoniacées. – In: Flore analytique et synoptique de la Nouvelle-Calédonie. Phanérogames, Office de la Recherche Scientifique Coloniale, Paris, pp. 137-142.
Hallier H. 1923. Beiträge zur Kenntnis der Linaceae (DC. 1819) Dumort. 25. Lepidobotrys Engl., die Oxalidaceen und die Geraniaceen. – Beih. Bot. Centralbl. 39: 1-178.
Hamel JL. 1959. Contribution à l’étude caryo-taxinomique des Eucryphiacées. – Bull. Mus. Hist. Nat. Paris 31: 526-535.
Hamilton AG. 1904. Notes on the West Australian pitcher plant (Cephalotus follicularis La Bill.). – Proc. Linn. Soc. New South Wales 29: 36-53.
Harden GJ, Williams JB. 2000. A revision of Davidsonia (Cunoniaceae). – Telopea 8: 413-428.
Harling G. 1999. 78. Cunoniaceae. – In: Harling G, Andersson L (eds), Flora of Ecuador 61, Nord. J. Bot., Copenhagen, pp. 1-72.
Hartl D. 1957. Die Pseudosympetalie von Correa speciosa (Rutaceae) und Oxalis tubiflora (Oxalidaceae). – Abh. Akad. Wiss. Lit. Mainz, Abh. Math. Naturwiss. Kl. 2: 53-63.
Hemsley JH.1956. Connaraceae. – In: Turrill WB, Milne-Redhead E (eds), Flora of tropical East Africa, Crown Agents for Oversea Governments and Aministration, London, pp. 1-27.
Herr JM, Dowd ML. 1968. Development of the ovule and megagametophyte in Oxalis corniculata L. – Phytomorphology 18: 43-53.
Hideux M, Ferguson IK. 1976. The stereostructure of the exine and its evolutionary significance in Saxifragaceae sensu lato. – In: Ferguson IK, Muller J (eds), The evolutionary significance of the exine, Linn. Soc. Symposium, No. 1, London and New York, pp. 327-377.
Hill RS. 1991. Leaves of Eucryphia (Eucryphiaceae) from Tertiary sediments in south-eastern Australia. – Aust. Syst. Bot. 4: 481-497.
Holmes WBK, Holmes FM. 1992. Fossil flowers of Ceratopetalum Sm. (family Cunoniaceae) from the Tertiary of Australia. – Proc. Linn. Soc. New South Wales 113: 265-270.
Hoogland RD. 1960. Studies in the Cunoniaceae I. The genera Ceratopetalum, Gillbeea, Aistopetalum, and Calycomis. – Aust. J. Bot. 8: 318-341.
Hoogland RD. 1979. Studies in the Cunoniaceae II. The genera Caldcluvia, Pillea, Acsmithia, and Spiraeanthemum. – Blumea 25: 481-505.
Hoogland RD. 1981. Studies in the Cunoniaceae III. Additional notes on Ceratopetalum and Acrophyllum. – Brunonia 4: 213-216.
Hoogland RD, Jérémie J. Hopkins HCF. 1997. Le genre Cunonia (Cunoniaceae) en Nouvelle-Calédonie. Description de cinq espèces nouvelles. – Adansonia, sér. III, 19: 7-19.
Hopkins HCF. 1998a. A revision of Weinmannia (Cunoniaceae) in Malesia and the Pacific 1. Introduction and an account of the species of Western Malesia, the Lesser Sundas and the Moluccas. – Adansonia, sér. III, 20: 5-41.
Hopkins HCF. 1998b. A revision of Weinmannia (Cunoniaceae) in Malesia and the Pacific 2. Sulawesi and the Philippines. – Adansonia, sér. III, 20: 43-66.
Hopkins HCF. 1998c. A revision of Weinmannia (Cunoniaceae) in Malesia and the Pacific 3. New Guinea, Solomon Islands, Vanuatu and Fiji, with notes on the species of Samoa, Rarotonga, New Caledonia and New Zealand. – Adansonia, sér. III, 20: 67-106.
Hopkins HCF. 2005. Nomenclature and typification in the endemic genus Codia (Cunoniaceae) from New Caledonia. – Adansonia, sér. III, 27: 243-254.
Hopkins HCF. 2006. Nomenclature and typification in Geissois (Cunoniaceae) in the South-West Pacific. – Adansonia, sér. III, 28: 311-327.
Hopkins HCF. 2007. Geissois bradfordii, a new species of Cunoniaceae from New Caledonia. – Kew Bull. 62: 275-280.
Hopkins HCF. 2008. The morphology of stipules and inflorescences in Geissois sensu stricto (Cunoniaceae). – Kew Bull. 63: 625-638.
Hopkins HCF, Bradford JC. 2009. Nomenclature and typification of names in the endemic genus Pancheria (Cunoniaceae) from New Caledonia. – Adansonia, sér. III, 31: 103-135.
Hopkins HCF, Bradford JC. 1998. A revision of Weinmannia (Cunoniaceae) in Malesia and the Pacific 1. Introduction and an account of the species of Western Malesia, the Lesser Sunda Islands and the Moluccas. – Adansonia, sér. 3, 20: 5-41.
Hopkins HCF, Florence J. 1998. A revision of Weinmannia (Cunoniaceae) Malesia and the Pacific 4. The Society, Marquesas and Austral Islands. – Adansonia, sér. III, 20: 107-130.
Hopkins HCF, Hoogland RD. 2002. Cunoniaceae. – In: Nooteboom HP (ed), Flora Malesiana, I, 16, Flora Malesiana Foundation, Nationaal Herbarium Nederland, Leiden, pp. 53-165.
Hopkins HCF, Pillon Y. 2011. Further new endemic taxa of Cunoniaceae from New Caleonia. – Kew Bull. 66: 405-423.
Hopkins HCF, Pillon Y, Bradford JC. 2009. The endemic genus Pancheria (Cunoniaceae) in New Caledonia: notes on morphology and the description of three new species. – Kew Bull. 64: 429-446.
Hopkins HCF, Rozefelds AC, Pillon Y. 2013. Karrabina gen. nov. (Cunoniaceae), for the Australian species previously placed in Geissois, and a synopsis of genera in the tribe Geissoieae. – Aust. Syst. Bot. 26: 167-185.
Hufford L, Dickison WC. 1992. A phylogenetic analysis of Cunoniaceae. – Syst. Bot. 17: 181-200.
Huynh K-L. 1969a. Étude du pollen des Oxalidacées I. Morphologie générale – palynotaxonomie des Oxalis américains. – Bot. Jahrb. Syst. 89: 272-303.
Huynh KL. 1969b. Étude du pollen des Oxalidacées II. Palynotaxonomie des Oxalis sud-africains. Considerations générales. – Bot. Jahrb. Syst. 89: 305-334.
Hyland BPM, Coode MJE. 1982. A second species for the Australian genus Peripentadenia (Elaeocarpaceae). – Kew Bull. 36: 741-745.
Ingle HD, Dadswell HE. 1956. The anatomy of the timbers of the Southwest Pacific area IV. Cunoniaceae, Davidsoniaceae, and Eucryphiaceae. – Aust. J. Bot. 4: 125-151.
Jaffré T, Brooks RR, Trow JM. 1979. Hyperaccumulation of nickel by Geissois species. – Plant and Soil 51: 157-161.
Jaramillo Azanza J. 1988. Note on Ecuadorean Vallea (Elaeocarpaceae) with the description of a new species. – Nord. J. Bot. 8: 19-23.
Jay M. 1968. Distribution des flavonoïdes chez les Cunoniacées. – Taxon 17: 489-495.
Jay M, Lebreton P. 1973. Recherches chimiotaxinomiques sur les plantes vasculaires XXVI. Les flavonoïdes des Sarracéniacées, Nepenthacées, Droséracées, et Céphalotacées; étude critique de l’ordre des Sarracéniales. – Nat. Canad. (Québec) 91: 607-613.
Jeannoda VLR, Valisolalao J, Creppy EE, Dirheimer G. 1985. Identification of the toxic principle of Cnestis glabra as methionine sulphoximine. – Phytochemistry 24: 854-855.
Johns SR, Lamberton JA, Sioumis AA. 1971. New tropane alkaloids, (+)-(3R, 6R)-3α-acetoxy-6β-hydroxytropane and (+)-2α-benzoyloxy-3β-hydroxynortropane from Peripentadenia mearsii (Elaeocarpaceae). – Aust. J. Chem 24: 2399-2405.
Johnson MAT. 1979. Chromosome numbers in Akania and Cephalotus. – Kew Bull. 34: 37-38.
Jongkind CCH. 1991. A new section and a new species in Agelaea Sol. ex Planch. (Connaraceae). – Bull. Jard. Bot. Nat. Belg. 61: 71-75.
Jongkind CCH, Lemmens RHJ. 1989. The Connaraceae: a taxonomic study with special emphasis on Africa. – Landbouwuniversiteit te Wageningen, The Netherlands.
Kabuye CHS. 1971. Oxalidaceae. – In: Milne-Redhead E, Polhill RM (eds), Flora of tropical East Africa, Crown Agents for Oversea Governments and Administrations, London, pp. 1-20.
Kennedy MJ, Prakash N. 1981. A morphological and embryological study of Callicoma serratifolia Andr. (Cunoniaceae). – Aust. J. Bot. 29: 721-731.
Knuth R. 1931. Oxalidaceae. – In: Engler A (†), Harms H, Pax F (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 19a, W. Engelmann, Leipzig, pp. 11-42.
Kress A. 1970. Zytotaxonomische Untersuchungen an einigen Insektenfängern (Droseraceae, Byblidaceae, Cephalotaceae, Roridulaceae, Sarraceniaceae). – Ber. Deutsch. Bot. Ges. 83: 55-62.
Kubitzki K. 2004. Brunelliaceae. – In: Kubitzki K (ed), The families and genera of vascular plants VI. Flowering plants. Dicotyledons. Celastrales, Oxalidales, Rosales, Cornales, Ericales, Springer, Berlin, Heidelberg, New York, pp. 26-28.
Kvacek Z, Hably L, Manchester SR. 2001. Sloanea (Elaeocarpaceae) fruits and foliage from the Early Oligocene of Hungary and Slovenia. – Palaeontographica, Abt. B, 259: 113-124.
Lack AJ, Kevan PG. 1987. The reproductive biology of a distylous tree, Sarcotheca celebica (Oxalidaceae) in Sulawesi, Indonesia. – Bot. J. Linn. Soc. 95: 1-8.
Lee DW. 1991. Ultrastructural basis and function of iridescent blue colour of fruits in Elaeocarpus. – Nature 349: 260-263.
Leenhouts PW. 1958. Connaraceae. – In: Steenis CGGJ van (ed), Flora Malesiana I, 5(4), Noordhoff-Kolff N. V., Djakarta, pp. 495-541.
Leinfellner W. 1970. Über die Karpelle der Connaraceen. – Österr. Bot. Zeitschr. 118: 542-559.
Leite NA da S. 1983. Estudos taxonômicas e morphológicos sobre Lamanonia ternata (Cunoniaceae). – Arq. Univ. Fed. Rural Rio de janeiro (Itaguaí) 6: 49-64.
Lemmens RHMJ. 1992. A reconsideration of Ellipanthus (Connaraceae) in Madagscar and continental Africa, and a comparison with the species in Asia. – Bull. Mus. Natl. Hist. Nat., sect. B, Adansonia 14: 99-108.
Lemmens RHMJ, Breteler FJ, Jongkind CCH. 2004. Connaraceae. – In: Kubitzki K (ed), The families and genera of vascular plants VI. Flowering plants. Dicotyledons. Celastrales, Oxalidales, Rosales, Cornales, Ericales, Springer, Berlin, Heidelberg, New York, pp. 74-81.
Llorens L, Gil L, Cardona C, Franquesa M, Boi M. 2005. A new species of Oxalis section Corniculatae (Oxalidaceae) from the Balearic islands. – Bot. J. Linn. Soc. 148: 489-493.
Lloyd FE. 1942. The carnivorous plants. – Waltham, Massachusetts.
López A, Panseri AF, Urtubey E. 2013. Revision of Oxalis section Palmatifoliae DC. (Oxalidaceae). – Phytotaxa 138: 1-14.
López Naranjo H, Huber H. 1971. Anatomía comparada de las semillas de Brunellia y Weinmannia con respeto a su posición sistemática. – Pittiera 3: 19-28.
Lourteig A. 1970. The correct name for Oxalis “siliquosa” (Oxalidaceae). – Baileya 17: 38-39.
Lourteig A. 1975. Oxalidaceae extra-austroamericanae I. Oxalis L. Sectio Thamnoxys Planchon. – Phytologia 29: 449-471.
Lourteig A. 1979. Oxalidaceae extra-austroamericanae II. Oxalis L. Sectio Corniculatae DC. – Phytologia 42: 57-198.
Lourteig A. 1981. Oxalidaceae extra-austroamericanae IV. Oxalis L. Sectio Articulatae Knuth. – Phytologia 50: 130-142.
Lourteig A. 1994. Oxalis L. subgenera Thamnoxys (Endl.) Reiche emend. Lourteig. – Bradea 7: 1-199.
Lourteig A. 1995. Oxalis L. subgenus Trifidus Lourt. subgen. nov. – Bradea 6: 389-392.
Lourteig A. 2000. Oxalis L. subgenera Monoxalis (Small) Lourteig, Oxalis y Trifidus Lourteig. – Bradea 7: 201-629.
Luffitz F. 1966. The West Australian pitcher plant (Cephalotus follicularis Labill.). – Aust. Plants 12: 34-35.
Lusk CH. 1999. Long-lived light-demanding emergents in southern temperate forests: the case of Weinmannia trichosperma (Cunoniaceae) in Chile. – Plant Ecol. 140: 111-115.
McPherson G, Lowry PP II. 2004. Hooglandia, a newly discovered genus of Cunoniaceae from New Caledonia. – Ann. Missouri Bot. Gard. 91: 260-265.
Manchester SR, Kvacek Z. 2009. Fruits of Sloanea (Elaeocarpaceae) in the Paleogene of North America and Greenland. – Intern. J. Plant Sci. 170: 941-950.
Matthews ML, Endress PK. 2002. Comparative floral structure and systematics in Oxalidales (Oxalidaceae, Connaraceae, Brunelliaceae, Cephalotaceae, Cunoniaceae, Elaeocarpaceae, Tremandraceae). – Bot. J. Linn. Soc. 140: 321-381.
Matthews ML, Endress PK, Schönenberger J, Friis EM. 2001. A comparison of floral structures of Anisophylleaceae and Cunoniaceae and the problem of their systematic position. – Ann. Bot. 88: 439-455.
Mauritzon J. 1934. Zur Embryologie der Elaeocarpaceae. – Ark. f. Bot. 26A(10): 1-8.
Maynard DJ. 2004. A molecular phylogeny for Australian Elaeocarpus (Elaeocarpaceae) and the affinities of a putative new taxon. – B.Sc. (Hons.) thesis, University of New South Wales, Kensington, New South Wales.
Mayura Devi P, Hashim M. 1966. Homostyly in heterostyled Biophytum sensitivum DC. – J. Genet. 59: 245-249.
Miers J. 1868. On the Tricuspidariae, a subtribe of the Elaeocarpae. – Ann. Mag. Nat. Hist., Ser. IV, 2: 39-54.
Mildbraed J. 1913. Über die Gattungen Afrostyrax Perk. et Gilg und Hua Pierre und die ”Knoblauch-Rinden” Westafrikas. – Engl. Bot. Jahrb. Syst. 49: 552-559.
Miranda-Esquivel DR. 2001. Sobre la posición sistemática de Brunellia Ruiz & Pavon: un reanálisis de Orozco (1997). – Caldasia 22: 337-340.
Moody M, Hufford L. 2000. Floral development and structure of Davidsonia (Cunoniaceae). – Can. J. Bot. 78: 1034-1043.
Mosebach G. 1934. Die Fruchtstielschwellung der Oxalidaceen und Geraniaceen. – Jahrb. Wiss. Bot. 79: 353-384.
Narayana LL. 1966. A contribution to the floral anatomy of Oxalidaceae. – J. Jap. Bot. 41: 321-328.
Narayana LL. 1970. Oxalidaceae. – In: Proceedings of the symposium on comparative embryology of angiosperms, Indian National Science Academy, New Delhi, pp. 114-116.
Nicholls KW, Bohm BA, Ornduff R. 1985. Flavonoids and affinities of Cephalotaceae. – Biochem. Syst. Ecol. 13: 261-264.
Oberlander KC. 2009. Molecular systematicstudy of Southern African Oxalis (Oxalidaceae). – Ph.D. diss., University of Stellenbosch, Republic of South Africa.
Oberlander KC, Dreyer LL, Bellstedt DU, Reeves G. 2003. Systematic relationships in southern African Oxalis L. (Oxalidaceae): congruence between palynological and plastid trnL-F evidence. – Taxon 53: 977-985.
Oberlander KC, Dreyer LL, Bellstedt DU. 2007. Molecular phylogeny supports model of bulb evolution in southern African Oxalis: morphological and taxonomic implications. – South Afr. J. Bot. 73: 305-306.
Oberlander KC, Dreyer LL, Bellstedt DU. 2008. Bulb evolution in Oxalis revisited: the link between anatomy and phylogeny. – South Afr. J. Bot. 74: 374.
Oberlander KC, Dreyer LL, Curran HR. 2009. An unusual new species of Oxalis (Oxalidaceae) from the Knersvlakte, South Africa. – South Afr. J. Bot. 75: 239-245.
Oberlander KC, Emshwiller E, Bellstedt DU, Dreyer LL. 2009. A model of bulb evolution in the eudicot genus Oxalis (Oxalidaceae). – Mol. Phylogen. Evol. 51: 54-63.
Oberlander KC, Dreyer LL, Bellstedt DU. 2011. Molecular phylogenetics and origins of southern African Oxalis. – Taxon 60: 1667-1677.
Oliver EGH. 1993. A new species of Oxalis from the western Cape. – Bothalia 23: 72-74.
Ornduff R. 1964. The breeding system of Oxalis suksdorfii. – Amer. J. Bot. 51: 307-314.
Ornduff R. 1972. The breakdown of trimorphic incompatibility in Oxalis section Corniculatae. – Evolution 26: 52-65.
Orozco CI. 1985. Dos especies nuevas de Brunellia de Colombia. – Flora Neotropica 2(Suppl.): 20-24.
Orozco CI. 1997. Sobre la posición sistemática de Brunellia Ruiz & Pavon. – Caldasia 19: 145-164.
Orozco CI. 2001a. Reanálisis de Brunellia Ruiz & Pavon: una repuesta a Miranda. – Caldasia 22: 341-346.
Orozco CI. 2001b. Pollen morphology of Brunellia (Brunelliaceae) and related taxa in the Cunoniaceae. – Grana 40: 245-255.
Orozco CI. 2002. Evolutionary biology of Brunellia Ruiz & Pavón (Brunelliaceae, Oxalidales). – Bogotá, Colombia.
Orozco CI, Coba B. 2003. Leaf anatomy in Brunellia Ruiz & Pavón. – In: Orozco-Pardo CI (ed), Evolutionary biology of Brunellia Ruiz & Pavón (Brunelliaceae, Oxalidales), Bogotá, Colombia, pp. 59-79.
Orozco CI, Weberling F. 1999. A comparative study of inflorescences in Brunellia Ruiz & Pav. (Brunelliaceae) and related taxa. – Beitr. Biol. Pflanzen 71: 261-279.
Overbeck F. 1923. Zur Kenntnis des Mechanismus der Samenausschleuderung von Oxalis. – Jahrb. Wiss. Bot. 62: 258-282.
Palacios Duque L. 2007. Sloanea chocoana, nueva especie de Elaeocarpaceae (subgen. Sloanea, sect. Brevispicae) para Colombia. – Darwiniana 45: 83-87.
Parkes DM, Hallam ND. 1984. Adaptation for carnivory in the West Australian pitcher plant (Cephalotus follicularis). – Aust. J. Bot. 32: 595-604.
Patel RN. 1990. Wood anatomy of the dicotyledons indigenous to New Zealand 20. Cunoniaceae. – New Zealand J. Bot. 28: 347-355.
Peng C-I, Goldblatt P. 1983. Confirmation of the chromosome number in Cephalotaceae and Roridulaceae. – Ann. Missouri Bot. Gard. 70: 197-198.
Perkins J. 1909. Eine neue Gattung der Styracaceae aus dem tropischen Afrika. – Engl. Bot. Jahrb. Syst. 43: 214-217.
Peroutka M, Adlassnig W, Lendl T, Pranic K, Lichtscheidl IK. 2008. Functional biology of carnivorous plants. – In: Texeira da Silva JA (ed), Floriculture, ornamental and plant biotechnology, Vol. 4, Advances and topical issues, Global Science Books, Isleworth, pp. 266-287.
Piliciauskas E. 1989. Cephalotus follicularis and how to grow them from seed. – Vic. C. P. Soc. Newslett. 6: 12-15.
Pillon Y. 2011. Focus on Geissois (Cunoniaceae): another example of the Melanesian connection. – In: Bouchet P, Le Guyader H, Pascal O (eds), The natural history of Santo, Muséum National d’Histoire Naturelle, Paris, pp. 93-94.
Pillon Y, Hopkins HCF, Bradford JC. 2008. Two new species of Cunonia (Cunoniaceae) from New Caledonia. – Kew Bull. 63: 419-431.
Pillon Y, Hopkins HCF, Munzinger J, Chase MW. 2009. A molecular and morphological survey of generic limits of Acsmithia and Spiraeanthemum (Cunoniaceae). – Syst. Bot. 34: 141-148.
Pillon Y, Munzinger J, Amir H, Hopkins HCF, Chase MW. 2009. Reticulate evolution on a mosaic of soils: diversification of the New Caledonian endemic genus Codia (Cunoniaceae). – Mol. Ecol. 18: 2263-2275.
Pole M. 1993. Early Miocene flora of the Manuherikia Group, New Zealand 5. Smilacaceae, Polygonaceae, Elaeocarpaceae. – J. Roy. Soc. New Zealand 23: 289-302.
Poole I, Cantrill DJ, Hayes P, Francis F. 2000. The fossil record of Cunoniaceae: new evidence from Late Cretaceous wood of Antarctica? – Rev. Palaeobot. Palynol. 111: 127-144.
Prakash N, McAlister EJ. 1977. An embryological study of Bauera capitata with comments on the systematic position of Bauera. – Aust. J. Bot. 25: 615-622.
Prakash U, Dayal R. 1963. Fossil wood resembling Elaeocarpus and Leea from Deccan Intertrappean Beds of Mahurzari near Nagpur. – Palaeobotanist 12: 121-127.
Price RA, Palmer JD. 1993. Phylogenetic relationships of Geraniaceae and Geraniales from rbcL sequence comparisons. – Ann. Missouri Bot. Gard. 80: 661-671.
Radlkofer L. 1886. Über die durchsichtigen Punkte und andere anatomische Charaktere der Connaraceae. – Sitzungsber. Bayer. Akad. Wiss. (Math.-Phys. Kl.) 16: 345-378.
Rama Devi D. 1991. Floral anatomy of Hypseocharis (Oxalidacee) with a discussion on its systematic position. – Plant Syst. Evol. 177: 161-164.
Rao TA, Dickison WC. 1985a The veinsheath syndrome in Cunoniaceae I. Pancheria Brongn. et Gris. – Proc. Indian Acad. Sci. (Plant Sci.) 95: 87-94.
Rao TA, Dickison WC. 1985b. The veinsheath syndrome in Cunoniaceae II. The genera Acsmithia, Codia, Cunonia, Geissois, Pullea and Weinmannia. – Proc. Indian Acad. Sci. (Plant Sci.) 95: 247-261.
Reddy B Bal, Narayana LL. 1982. Systematic position of Averrhoaceae. – J. Econ. Taxon. Bot. 3: 343-348.
Reiche K. 1896a. Oxalidaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(4), W. Engelmann, Leipzig, pp. 15-23.
Reiche K. 1896b. Linaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(4), W. Engelmann, Leipzig, pp. 27-35.
Robyns A. 1976. Huaceae. – In: Flore d’Afrique Centrale (Zaire-Rwanda-Burundi), Nat. Plantentuin Belg., Meise.
Rogers ZS, Bradford JC. 2004. Weinmannia magnifica and W. aggregata (Cunoniaceae): two distinctive new species from Madagascar. – Adansonia, sér. III, 26: 83-91.
Rozefelds AC, Barnes RW. 2002. The systematic and biogeographical relationships of Ceratopetalum (Cunoniaceae) in Australia and New Guinea. – Intern. J. Plant Sci. 163: 651-673.
Rozefelds AC, Christophel C. 1996a. Elaeocarpus (Elaeocarpaceae) endocarps from the Oligo-Miocene of Eastern Australia. – Papers & Proc. Roy. Soc. Tasmania 130: 41-48.
Rozefelds AC, Christophel C. 1996b. Elaeocarpus (Elaeocarpaceae) from the Early to Middle Miocene Yallourn Formation of Eastern Australia. – Muelleria 9: 229-237.
Rozefelds AC, Christophel DC. 2002. Cenozoic Elaeocarpus (Elaeocarpaceae) fruits from Australia. – Alcheringa 26: 261-274.
Rozefelds AC, Pellow B. 2000. A new species of Gillbeea (Cunoniaceae) from north-eastern Queensland, Australia. – Nord. J. Bot. 20: 435-441.
Rozefelds AC, Pellow B. 2011. A taxonomic revision of Pseudoweinmannia Engl. (Cunoniaceae: Geissoieae). – Austrobaileya 8: 252-266.
Rozefelds AC, Barnes RW, Pellow B. 2001. A new species and comparative morphology of Vesselowskya (Cunoniaceae). – Aust. Syst. Bot. 14: 175-192.
Rutishauser R, Dickison WC. 1989. Developmental morphology of stipules and systematics of the Cunoniaceae and presumed allies I. Taxa with interpetiolar stipules. – Bot. Helvet. 99: 147-169.
Salter TS. 1944. The genus Oxalis in South Africa. – J. South Afr. Bot. [Suppl.] 1: 1-355.
Sauer H. 1933. Blüte und Frucht der Oxalidaceen, Linaceen, Geraniaceen, Tropaeolaceen und Balsaminaceen. Vergleichend-entwicklungsgeschichtliche Untersuchungen. – Planta 19: 417-481.
Schellenberg G. 1910. Beiträge zur vergleichenden Anatomie und zur Systematik der Connaraceen. – Mitt. Bot. Mus. Univ. Zürich 50: 1-158.
Schellenberg G. 1925. Die phylogenetische Entwicklung und die Wanderungen der Connaraceen. – Engl. Bot. Jahrb. Syst. 60: 207-251.
Schimanski LJ, Rozefelds AC. 2002. Comparative morphology of the Australian species of Geissois (Cunoniaceae). – Aust. Syst. Bot. 15: 221-236.
Schlechter R. 1916. Die Elaeocarpaceen Papuasiens. – Engl. Bot. Jahrb. Syst. 54: 92-155.
Schönenberger J, Friis EM, Matthews ML, Endress PK. 2001. Cunoniaceae in the Cretaceous of Europe: evidence from fossil flowers. – Ann. Bot. 88: 423-437.
Schrödinger R. 1927. Die Stipeln der Cunoniaceen. – Verh. Zool.-Bot. Ges. Wien 77: 5-38.
Schulze W, Schulze ED, Pate JS, Gillison AN. 1997. The nitrogen supply from soils and insects during growth of the pitcher plants Nepenthes mirabilis, Cephalotus follicularis and Darlingtonia californica. – Oecologia 112: 464-471.
Schumann K. 1895. Elaeocarpaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(6), W. Engelmann, Leipzig, pp. 1-8.
Schweiger J. 1909. Vergleichende Untersuchungen über Sarracenia und Cephalotus follicularis betreffs ihrer etwaigen systematischen Verwandtschaft. – Beih. Bot. Centralbl. 25: 490-539.
Smith AC. 1952. Studies of Pacific Island plants XII. The Cunoniaceae of Fiji and Samoa. – J. Arnold Arbor. 33: 119-149.
Smith AC. 1953. Studies of Pacific island plants XV. The genus Elaeocarpus in the New Hebrides, Fiji, Samoa, and Tonga. – Contr. U. S. Natl. Herb. 30: 523-573.
Smith CE. 1954. The New World species of Sloanea (Elaeocarpaceae). – Contr. Gray Herbarium Harvard Univ. 175: 1-114.
Smith CE. 1965. Family 113. Elaeocarpaceae. – In: Woodson RE, Schery RW (eds), Flora of Panama VI, Ann. Missouri Bot. Gard. 52: 487-495.
Smith DA. 1996. Three previously undescribed Central American species of Sloanea (Elaeocarpaceae). – Novon 6: 120-127.
Sprague TA. 1907. A revision of Dubouzetia. – Kew Bull. 1907: 125-127.
Sweeney PW, Bradford JC. 2004. Phylogenetic position and of the New Caledonian endemic genus Hooglandia (Cunoniaceae) as determined by maximum parsimony analysis of chloroplast DNA. – Ann. Missouri Bot. Gard. 91: 2676-274.
Sweeney PW, Bradford JC, Lowry PP II. 2004. Phylogenetic position of the New Caledonian endemic genus Hooglandia (Cunoniaceae) as determined by maximum parsimony analysis of chloroplast DNA. – Ann. Missouri Bot. Gard. 91: 266-274.
Tang Y, Wu Z-Y. 1990. Study on the pollen morphology of Chinese Elaeocarpaceae. – Acta Bot. Yunnan. 12: 397-403. [In Chinese with English summary]
Taylor F, Hill RS. 1996. A phylogenetic analysis of the Eucryphiaceae. – Aust. Syst. Bot. 9: 735-748.
Thompson J. 1976. A revision of the genus Tetratheca (Tremandraceae). – Telopea 1: 139-215.
Tirel C. 1982. Eléocarpacées. – In: Hallé N (ed), Flore de la Nouvelle Calédonie et Dépendances 11, Muséum National d’Histoire Naturelle, Paris, pp. 3-124.
Tirel C. 1985. Fam. 125. Eléocarpacées. – In: Badré F (ed), Flore de Madagascar et des Comores, Muséum National d’Histoire Naturelle, Paris, pp. 3-54.
Tirel C, McPherson G. 2006. Elaeocarpus tremulus Tirel & McPherson, nouvelle espèce d’Elaeocarpaceae de Nouvelle-Calédonie. – Adansonia, sér. III, 28: 137-141.
Tirel C, Raynal J. 1980. Recherches bibliographiques sur trios espèces d’Elaeocarpus (Elaeocarpaceae). – Adansonia, sér. II, 20: 169-177.
Veldkamp JF. 1967. A revision of Sarcotheca Bl. and Dapania Korth. (Oxalidaceae). – Blumea 15: 519-543.
Venkata Rao C. 1953. Floral anatomy and embryology of two species of Elaeocarpus. – J. Indian Bot. Soc. 32: 21-33.
Watson MF. 1989. Nomenclatural aspects of Oxalis section Corniculatae in Europe. – Bot. J. Linn. Soc. 101: 347-362.
Webb CJ, Simpson MJA. 1991. Seed morphology in relation to taxonomy in New Zealand species of Weinmannia, Ackama, and the related South American Caldcluvia paniculata (Cunoniaceae). – New Zealand J. Bot. 29: 451-453.
Weberling F. 1976. Weitere Untersuchungen zur Morphologie des Unterblattes bei den Dikotylen IX. Saxifragaceae s.l., Brunelliaceae, und Bruniaceae. – Beitr. Biol. Pflanzen 52: 163-181.
Weibel R. 1968. Morphologie de l’embryon et de la graine des Elaeocarpus. – Candollea 23: 101-108.
Weller SG,Domínguez CA, Molina-Freaner FE, Fornoni J, LeBuhn G. 2007. The evolution of distyly from tristyly in populations of Oxalis alpina (Oxalidaceae) in the Sky Islands of the Sonoran Desert. – Amer. J. Bot. 94: 972-985.
West WC, Gunkel JE, Johnson MA. 1970. Morphology of the shoot apex in Elaeocarpaceae. – Phytomorphology 20: 58-67.
Wollenweber E, Dörr M, Rozefelds AC, Minchin P, Forster PI. 2000. Variation in flavonoid exudates in Eucryphia species from Australia and South America. – Biochem. Syst. Ecol. 28: 111-118.
Wurdack KJ, Davis CC. 2009. Malpighiales phylogenetics: gaining ground on one of the most recalcitrant clades in the angiosperm tree of life. – Amer. J. Bot. 96: 1551-1570.
Zhang L-B, Simmons MP. 2006. Phylogeny and delimitation of the Celastrales inferred from nuclear and plastid genes. – Syst. Bot. 31: 122-137.
Zickel CS, Leitão Filho H da F. 1993. Revisão taxonômica de Lamanonia Vell. (Cunoniaceae). – Rev. Brasil. Bot. 16: 73-91.
Zmarzty S. 2001. Revision of Elaeocarpus (Elaeocarpaceae) Section Elaeocarpus in Southern India and Sri Lanka. – Kew Bull. 56: 405-447.