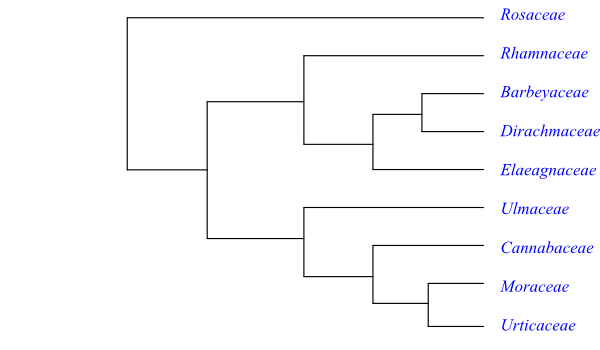
Cladogram of Rosales based on DNA sequence data (Zhang & al. 2011).
[Polygalales+[Rosales+[Cucurbitales+Juglandales]]]
Fossils Fossil Rosales are known from the mid-Eocene (c. 44 Mya).
Habit Usually bisexual (sometimes monoecious, polygamomonoecious, dioecious, androdioecious, or gynodioecious), evergreen or deciduous trees, shrubs or suffrutices (sometimes lianas), or perennial (sometimes annual) herbs.
Vegetative anatomy Ectomycorrhiza sometimes present. Roots diarch (lateral roots tetrastichous) or tetrarch. Root nodules with nitrogen-fixing bacteria (Frankia) often present. Frankia infection sometimes taking place through intercellular penetration. Phellogen subepidermal, cortical or outer-cortical. Vessel elements usually with simple (sometimes reticulate or scalariform) perforation plates; lateral pits alternate or opposite, simple or bordered pits. Vestured pits often present. Imperforate tracheary xylem elements tracheids, fibre tracheids or libriform fibres with simple or bordered pits, usually non-septate (also vasicentric tracheids). Wood rays uniseriate to multiseriate, homocellular or heterocellular. Axial parenchyma apotracheal diffuse or diffuse-in-aggregates, or usually paratracheal scanty, aliform, lozenge-aliform, winged-aliform, scalariform, reticulate, confluent, unilateral, vasicentric or banded (sometimes absent). Fibres often present in secondary phloem. Sieve tube plastids Ss or S0 type (rarely Pc type); sieve tubes at least sometimes with non-dispersive protein bodies. Nodes 1:1, unilacunar with one leaf trace, or 3:3, trilacunar with three traces (sometimes ≥5:≥5, penta- to multilacunar with five or more traces). Heartwood often with gum-like substances. Laticiferous cavities or ducts sometimes present. Calciumoxalate usually as solitary prismatic crystals (sometimes also druses or acicular crystals, rarely raphides) or absent. Prismatic crystals usually present in wood ray cells.
Trichomes Hairs unicellular or multicellular, simple or branched, furcate, stellate, lepidote, or peltate; glandular hairs unicellular or multicellular (sometimes peltate-lepidote or pearl glands); prickles or stinging hairs sometimes present.
Leaves Usually alternate (spiral or distichous; rarely opposite or verticillate), pinnately or palmately compound, or simple and entire or lobed, with conduplicate, supervolute, involute, plicate, curved or flat ptyxis. Stipules lateral, axillary, inserted on branch or adnate to petiole (rarely interpetiolar), small to foliaceous, usually persistent (sometimes caducous, rarely absent); leaf sheath absent. Petiole vascular bundle transection arcuate, U-shaped or annular. Venation usually pinnate (sometimes palmate, rarely single-veined), craspedodromous or camptodromous (sometimes acrodromous). Stomata usually anomocytic (sometimes cyclocytic, rarely anisocytic, helicocytic or paracytic). Cuticular wax crystalloids as tubuli or rosettes of platelets (Fabales type), or absent. Domatia as pits, pockets or hair tufts, or absent. Epidermis with or without mucilaginous idioblasts. Tanniniferous or mucilaginous cells often present. Cystoliths sometimes present. Laticiferous cavities or ducts sometimes present. Leaf margins and leaflet margins usually serrate or biserrate (sometimes crenate or entire, rarely glandular serrate); teeth often rosoid or urticoid.
Inflorescence Terminal or axillary, cymes, panicle, thyrse, raceme-, spike-, catkin-, head-, spadix- or umbel-like, corymb, raceme, umbellate, spicate or fasciculate (sometimes pseudanthia), or flowers solitary. Bracts and floral prophylls (bracteoles) sometimes absent.
Flowers Usually actinomorphic (occasionally zygomorphic). Hypanthium usually present (rarely absent). Hypogyny, epigyny or half epigyny. Sepals (one to) four or five (to ten), with valvate, induplicate-valvate or imbricate (rarely open) aestivation, persistent, free, or absent. Petals (three or) four or five (to ten), usually with imbricate (sometimes induplicate-valvate) aestivation, often clawed, caducous, free, or absent. Nectaries usually on adaxial side of hypanthium or on staminal bases, or absent. Nectariferous disc intrastaminal, annular or divided, inserted around hypanthial orifice, or absent.
Androecium Stamens one to more than 20. Filaments free or connate in lower part, free from tepals, usually inserted on hypanthium. Anthers basifixed or dorsifixed, versatile or non-versatile, usually tetrasporangiate (rarely disporangiate), usually introrse (sometimes latrorse or extrorse), longicidal (dehiscing by longitudinal slits) or poricidal (dehiscing by apical pores). Tapetum secretory. Staminodia usually absent (sometimes extrastaminal, petaloid; female flowers often with staminodia).
Pollen grains Microsporogenesis simultaneous. Pollen grains tricolpor(oid)ate or (1–)2–6(–15)-porate (sometimes stephanoporate), shed as monads, bicellular at dispersal, often starchy. Exine tectate or semitectate, with columellate or granular infratectum, perforate, reticulate, microreticulate, striate, rugulate, fossulate, scabrate, spinulate, echinulate, verrucate, pilate, or psilate.
Gynoecium Pistil composed of usually two to five more or less connate carpels, or carpels one (sometimes pseudomonomerous) or numerous (sometimes spiral), free or more or less connate or adnate to hypanthium (rarely somewhat stipitate, on gynophore). Ovary superior, inferior or semi-inferior, unilocular (often apocarpy) to quinquelocular (to octalocular). Style single, simple, or stylodia few to numerous, usually terminal (sometimes lateral or subbasal), free or more or less connate, or absent. Stigmas capitate, clavate, punctate, widened, decurrent or bilobate (rarely fimbriate or penicillate), papillate or non-papillate, Dry or Wet type. Pistillodium usually absent (male flowers sometimes with pistillodium).
Ovules Placentation axile, basal, subbasal, apical or subapical. Ovules usually one or two (sometimes several, rarely numerous) per carpel, usually anatropous (sometimes hemianatropous, campylotropous, or orthotropous), usually ascending or pendulous (rarely erect), apotropous or epitropous, bitegmic or unitegmic, crassinucellar. Micropyle bistomal or endostomal (sometimes exostomal, rarely almost absent). Obturator (sometimes funicular) sometimes present. Archespore unicellular or multicellular. Nucellar cap often present. Nucellar beak sometimes present. Megagametophyte usually monosporous, Polygonum type (rarely disporous, 8-nucleate, Allium type, or tetrasporous, Drusa or Adoxa type). Synergids sometimes with a filiform apparatus. Antipodal cells sometimes proliferating. Endosperm development usually nuclear (rarely helobial). Endosperm haustorium chalazal or absent. Embryogenesis usually asterad (sometimes onagrad, rarely solanad).
Fruit An assemblage of follicles, drupelets or achenes, a drupe, nut, samara, pome, loculicidal or septicidal(-septifragal) capsule (sometimes a cyconium or other types of fleshy syncarp, or a cynarrhodium, rarely schizocarp or anthocarp), often with persistent hypanthium and/or calyx.
Seeds Funicular aril sometimes present. Elaiosome rarely present. Seed coat usually mesotestal (sometimes exotestal, occasionally reduced). Testa sometimes multiplicative. Exotesta often palisade (sometimes tabular); exotestal cells often malpighiaceous with thickened walls (often spiral or with reticulate thickenings), sometimes periclinally elongate. Mesotesta usually sclerotic, sometimes aerenchymatous. Endotesta sometimes tanniniferous. Exotegmen crushed. Endotegmic cells somewhat thickened, sometimes tanniniferous, sometimes cuboid. Perisperm usually not developed. Endosperm copious, sparse or absent, oily. Embryo straight or curved (rarely spirally twisted), with or without chlorophyll. Cotyledons usually two (rarely one). Germination phanerocotylar or cryptocotylar.
Cytology x = 7–17, 19, 21
DNA Plastid gene infA lost/defunct. Mitochondrial intron coxII.i3 lost. Cis-spliced (Rosaceae) or trans-spliced intron in mitochondrial gene nad1 (Rosales except Rosaceae).
Phytochemistry Flavonols (kaempferol, quercetin, myricetin) and their glycosides, isoflavonoids, afzelechin, flavone-C-glycosides, flavanone glycosides, biflavonoids (biflavonyls), cyanidin, epigallocatechin-3-gallate, dihydrochalcones, dammaranes, cucurbitacins, oleanolic acid derivatives, methylated and non-methylated ellagic acids, gallic acid, hydrolyzable ellagitannins (casuaricitin, geraniins, pedunculagin, stachyurins, tellimagrandin I, tellimagrandin II), non-hydrolyzable tannins, proanthocyanidins (prodelphinidins), chlorogenic acid, caffeic acid derivatives, hydroxycinnamate derivatives, indole alkaloids, isoquinoline alkaloids (e.g. liriodenine and benzylisoquinoline alkaloids), peptide alkaloids, phenylalanine-, tyrosine- or leucine-derived cyanogenic glucosides (amygdalin, cardiospermin, dhurrin, heterodendrin, prunasin), triterpene saponins, anthraquinones, naphthoquinones, arbutin, benzophenones, xanthones, polyacetate-derived arthroquinones, sesquiterpene lactones, coumarins, furanocoumarins, p-coumaric acid, acetophenones, L-quebrachitol, cannabinoids (tetrahydrocannabinol etc.), simple indole bases, humulones, lupulones, chelidonic acid, sinapic acid, ursolic acid, eleostearic acid, barbeyol, naphthalene and lupeolin derivatives, and lignans present.
Systematics Rosales are probably sister to [Cucurbitales+Juglandales] (Soltis & al. 2011).
A possible topology of Rosales is the following: [Rosaceae+[[Rhamnaceae+[Elaeagna-ceae+[Barbeyaceae+Dirachmaceae]]]+[Ulmaceae+[Cannabaceae+[Moraceae+Urticaceae]]]]].
Rosales except Rosaceae and Cynomoriaceae are characterized by trans-spliced intron in mitochondrial nad1 gene.
The clade [Rhamnaceae+[Elaeagnaceae+[Barbeyaceae+Dirachmaceae]]] has the following potential synapomorphies (Stevens 2001 onwards): petiole bundle transection arcuate; stamens as many as petals, alternitepalous/antepetalous; placentation basal; ovule erect; parietal tissue five or six cell layers thick; capsule septicidal; seed coat multiplicative; exotesta palisade, with thick-walled cells; and cotyledons large. Barbeyaceae and Dirachmaceae share the features: anther connective prolonged; and outer integument three to five cell layers thick. Rhamnaceae and Dirachma differ from the Ulmaceae-Urticaceae clade by pits of wood ray parenchyma resembling lateral pits of vessel elements.
The clade [Ulmaceae+[Cannabaceae+[Moraceae+Urticaceae]]] is characterized by the following potential synapomorphies (Stevens 2001 onwards): presence of watery exudate; hairs unicellular and multicellular-glandular; cambium storied; presence of libriform fibres; phloem stratified; sieve tubes with non-dispersive protein bodies; presence of globose and usually calciumcarbonate cystoliths; epidermal and hair cell walls silicified and calcified; presence of at least one prominent prophyllar bud; stipules cauline; presence of urticoid teeth (secondary leaf veins proceeding straight to non-glandular teeth, higher-order veins convergent on those teeth); flowers small; absence of corolla and nectary; stamens as many as tepals, antetepalous; pollen grains porate; infratectum granular; carpels two; only abaxial carpel fertile; stigmas sessile, diverging, with pollen receptive area extending down adaxial surface and confluent; placentation apical; ovule epitropous; fruit a drupe; testa perforated; endosperm sparse; polyembryony frequent; x = 14; centromeres median and subterminal; presence of flavonols and their glycosides, including myricetin; and absence of ellagic acid.
The clade [Cannabaceae+[Moraceae+Urticaceae]] has the potential synapomorphies (Stevens 2001 onwards): flowers unisexual; sieve tube plastids sometimes Ss type (with starch grains); unicellular hairs usually micropapillate; stipules cauline-intrapetiolar; venation palmate; embryo curved; and presence of flavone-C-glycosides. Finally, Moraceae and Urticaceae share the following potential synapomorphies: presence of laticifers and latex (usually white, milky); venation sometimes pinnate; stamens inflexed in bud; presence of pistillodium; and absence of polyembryony.

|
Cladogram of Rosales based on DNA sequence data (Zhang & al. 2011). |
BARBEYACEAE Rendle |
( Back to Rosales ) |
Barbeyales Takht. et Reveal in Phytologia 74: 172. 25 Mar 1993; Barbeyanae Takht. ex Reveal et Doweld in Novon 9: 549. 30 Dec 1999
Genera/species 1/1
Distribution Ethiopia, Somalia, Yemen.
Fossils Unknown.
Habit Dioecious, evergreen trees with silvery indumentum on abaxial side of lamina.
Vegetative anatomy Phellogen? Primary medullary strands narrow, alternating with wide strands. Vessel elements with simple perforation plates; lateral pits alternate, simple pits. Non-vestured pits present. Imperforate tracheary xylem elements libriform fibres with simple or bordered pits, non-septate. Wood rays usually uniseriate or biseriate, heterocellular. Axial parenchyma paratracheal scanty, or absent. Tyloses abundant in vessels. Secondary phloem with numerous peripheral fibres and sclereids. Sieve tube plastids S type, with up to ten starch grains; sieve tubes with compound perforations. Nodes 1:1, unilacunar with one leaf trace. Mucilage cells? Laticifers and latex absent. Cystoliths absent. Wood ray cells without prismatic crystals.
Trichomes Hairs unicellular, spirally twisted; glandular hairs absent.
Leaves Opposite, simple, entire, with supervolute-curved ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle transection arcuate. Venation pinnate, camptodromous. Stomata anomocytic or laterocytic. Cuticular wax crystalloids? Mucilaginous idioblasts absent. Leaf margin entire.
Inflorescence Axillary, fasciculate or umbel-like, cymose. Bracts and floral prophylls (bracteoles) absent.
Flowers Actinomorphic. Hypanthium absent. Hypogyny. Sepals three or four, in male flowers with valvate aestivation, in female flowers with imbricate aestivation, persistent, in female flowers free, in male flowers somewhat connate at base. Petals absent. Nectary absent. Disc absent.
Androecium Stamens six to nine (to twelve). Filaments short, free from each other and from tepals. Anthers basifixed, non-versatile, tetrasporangiate, latrorse, longicidal (dehiscing by longitudinal slits); connective apically prolonged. Tapetum secretory? Staminodia absent.
Pollen grains Microsporogenesis simultaneous? Pollen grains tricolpor(oid)ate, shed as monads, ?-cellular at dispersal. Exine tectate to semitectate, with granular to intermediate infratectum, perforate or finely reticulate, rugulate, spinulate or verrucate.
Gynoecium Carpels one or two (or three), free (apocarpy) or connate at base. Ovary superior, usually unilocular (monomerous; sometimes bilocular or trilocular), sometimes slightly stipitate. Stylodia one or two (or three), free or connate at base. Stigmas clavate, decurrent, papillate, type? Pistillodium absent.
Ovules Placentation subapical. Ovule one per carpel, anatropous, pendulous, epitropous, bitegmic, crassinucellar. Micropyle endostomal. Outer integument ? cell layers thick. Inner integument five or six cell layers thick. Hypostase present. Nucellar cap approx. five cell layers thick. Megagametophyte monosporous, Polygonum type. Endosperm development nuclear? Endosperm haustoria? Embryogenesis?
Fruit One to three nuts surrounded by persistent and accrescent sepals.
Seeds Aril absent? Testa indistinct, unspecialized, non-multiplicative. Exotesta perforated, aerenchymatous, not palisade, with sinuous anticlinal cell walls. Endotesta?, tanniniferous. Exotegmen? Endotegmic cells tanniniferous, with sinuous anticlinal walls. Perisperm not developed. Endosperm absent. Embryo straight, well differentiated, chlorophyll? Cotyledons two, fleshy. Germination?
Cytology n = ?
DNA Trans-spliced intron present in mitochondrial gene nad1.
Phytochemistry Insufficiently known. Flavonols (kaempferol, quercetin), ellagic acid, and barbeyol (phenolic indane) present. Alkaloids possibly present? Myricetin and glycoflavonols not found.
Use Unknown.
Systematics Barbeya (1; B. oleoides; eastern Ethiopia, northern Somalia, Yemen).
Barbeya is sister-group to Dirachmaceae.
CANNABACEAE Martinov |
( Back to Rosales ) |
Humulaceae Bercht. et J. Presl, Přir. Rostlin: 258. Jan-Apr 1820; Lupulaceae Schultz Sch., Nat. Syst. Pflanzenr.: 370. 30 Jan-10 Feb 1832 [’Lupulinae’]; Celtidaceae Endl., Ench. Bot.: 171. 15-21 Aug 1841 [’Celtideae’]
Genera/species 9/c 95
Distribution Tropical and subtropical regions, temperate regions on the Northern Hemisphere.
Fossils Endocarps assigned to Aphananthe cretacea and Gironniera gonnensis have been found in the Maastrichtian of Germany. Eoceltis dilcheri, a fossil male flower of proposed affinity to Celtidoideae, has been described from the mid-Eocene of Texas. The pollen grains are triporate and scabrate. Celtoidanthus pseudorobustus is another male flower of questioned affinity with numerous stamens and triporate pollen grains from the Miocene of Germany. Cenozoic fossil Cannabaceae comprise leaves, pollen grains and endocarps from the Northern Hemisphere.
Habit Usually monoecious, polygamomonoecious or dioecious (rarely bisexual), usually evergreen (sometimes deciduous) trees or shrubs (some species of Celtis are lianas), perennial or annual herbs (Humulus is twining and climbing by means of specialized hairs).
Vegetative anatomy Ectomycorrhiza sometimes (Gironniera) present. Root nodules with nitrogen-fixing bacteria (Parasponia only known genus outside Fabaceae with other types of nitrogen-fixing bacteria, Rhizobium, than actinobacteria; rhizobia persisting inside infection threads). Phellogen ab initio superficial. Medulla in Celtis septated by diaphragms. Vessel elements usually with simple (rarely scalariform) perforation plates; lateral pits alternate, usually simple pits. Imperforate tracheary elements tracheids or libriform fibres with usually simple (sometimes bordered) pits, septate or non-septate (in, e.g., Ampelocera septate). Wood rays uniseriate to multiseriate, homocellular or heterocellular. Axial parenchyma usually paratracheal scanty, aliform, lozenge-aliform, winged-aliform, confluent, vasicentric, unilateral, or banded (sometimes apotracheal diffuse), or absent. Tyloses often frequent (sometimes sclerotic). Secondary phloem stratified into hard fibrous and soft parenchymatous non-fibrous layers. Sieve tube plastids Ss type or S0 type; sieve tube elements with extruded nucleoli and non-dispersive protein bodies. Nodes 3:3, trilacunar with three leaf traces (in Humulus split laterals or commissural bundle). Secretory ducts usually without latex (coloured latex rare?). Mucilage cells absent. Cystoliths (of calciumcarbonate?) globose, usually with pegs (cellulose expansions of cell walls, covered with crystals). Epidermal cell walls often with calciumcarbonate or silica. Prismatic calciumoxalate crystals abundant; druses or crystal sand present in some species.
Trichomes Hairs rigid or soft, unicellular (usually micropapillate) or multicellular, usually simple, often with inflated base (sometimes furcate); unicellular or multicellular glandular hairs (also peltate-lepidote) often abundant.
Leaves Usually alternate (usually distichous, sometimes spiral; in Humulus and Lozanella opposite), usually simple (in, e.g., Cannabis palmately compound), usually entire (rarely palmately lobed), with conduplicate-plicate (sometimes laterally; sometimes conduplicate or supervolute) ptyxis. Stipules usually lateral and axillary, intrapetiolar (in Lozanella interpetiolar; in Parasponia intrapetiolar), sometimes more or less connate, persistent or caducous; leaf sheath absent. Petiole vascular bundle transection? Venation pinnate or palmate, usually brochidodromous or eucamptodromous (in Aphananthe and Parasponia craspedodromous; in Trema acrodromous), usually with three main-veins from base; secondary veins usually not reaching into teeth (secondary and tertiary veins in Cannabis and Humulus proceeding into lobe tips and teeth: urticoid teeth). Stomata anomocytic. Cuticular wax crystalloids? Domatia? Epidermis and mesophyll often with mucilaginous idioblasts. Leaf margin serrate or entire. Leaves often with unicellular hairs with inflated base, often with glands with bitter-tasting substances (lupulin etc. in Humulus) or glandular hairs with aromatic resins (tetrahydrocannabinols in Cannabis).
Inflorescence Axillary, cymose (often thyrsoid, sometimes spicate; female inflorescence in Humulus cone-like; flowers solitary in Chaetachme). Prophylls usually basal (in Humulus absent).
Flowers Actinomorphic, small to minute. Hypogyny. Sepals (four or) five (to seven), usually with imbricate (in Trema induplicate-valvate) aestivation, whorled, sometimes persistent, usually connate at base (rarely free). Petals absent. Nectary absent. Disc absent.
Androecium Stamens usually (four or) five (to seven; in Ampelocera four to 16), antesepalous. Filaments usually filiform, sometimes inflexed in bud, free, adnate to sepal bases. Anthers dorsifixed, versatile, tetrasporangiate, usually introrse (sometimes extrorse), longicidal (dehiscing by longitudinal slits). Tapetum secretory, with binucleate to quadrinucleate cells. Female flowers sometimes (especially in Celtis) with staminodia.
Pollen grains Microsporogenesis simultaneous. Pollen grains (2–)3–5(–6)-porate, shed as monads, bicellular at dispersal. Exine tectate, with granular infratectum, perforate scabrate, spinulate, verrucate or echinulate.
Gynoecium Pistil composed of two partially connate carpels. Ovary superior, unilocular. Stylodia two, sometimes connate at base. Stigmas decurrent, ventral (in Lozanella bilobate), papillate, Dry type. Male flowers often with pistillodium.
Ovules Placentation subapical. Ovules one per ovary, anatropous to orthotropous or campylotropous, pendulous, epitropous, bitegmic, crassinucellar (to tenuinucellar?). Micropyle usually endostomal? (in Celtis bistomal). Outer integument two to four (to eight) cell layers thick. Inner integument two or three cell layers thick. Hypostase present. Parietal tissue approx. six cell layers thick. Nucellar cap approx. two cell layers thick. Megagametophyte monosporous, Polygonum type, with haustorium. Endosperm development ab initio nuclear. Endosperm haustoria absent. Embryogenesis onagrad (at least in Cannabis and Humulus).
Fruit Usually a drupe (in Pteroceltis a samara; in Cannabis and Humulus a nutlet enclosed by calyx).
Seeds Aril absent. Exotestal cells periclinally elongate, with arm-shaped processes, with unthickened walls (anticlinal walls in Humulus sinuous). Endotesta? Tegmen? Perisperm not developed. Endosperm usually sparse or absent (in Lozanella and Parasponia carnose), oily. Embryo curved (in Humulus spirally twisted), usually without chlorophyll (in Humulus with chlorophyll). Cotyledons two, folded, inrolled. Germination phanerocotylar.
Cytology n = 8–11, 13–15 (20) (30) (42) (c. 60) – Polyploidy occurring. Centromeres medial/submedial, simple. Cannabis and Humulus with X-autosome balance system (sex chromosomes, X or Y).
DNA Trans-spliced intron present in mitochondrial gene nad1.
Phytochemistry Flavonols (kaempferol, quercetin, myricetin; in Aphananthe, Ampelocera, Gironniera, Cannabis, and Humulus) and their glycosides, flavone-C-glycosides, prodelphinidins, sesquiterpene lactones, alkaloids, triterpene saponins, tyrosine-derived cyanogenic compounds, quebrachitol, cannabinoids (in Cannabis), humulones (bitter-tasting α-lupulic acids, in Humulus), and lupulones (bitter-tasting β-lupulic acids, in Humulus) present. Ellagic acid, sesquiterpenes and lignans not found. Raffinose and stachyose present in phloem exudates.
Use Ornamental plants, fibre plants (ropes and paper from phloem of male plants of Cannabis sativa), beer spices (Humulus lupulus), seed oils, medicinal plants and narcotics (Cannabis sativa), timber, carpentries.
Systematics Aphananthe (5–6; A. aspera, A. cuspidata, A. monoica, A. philippinensis, A. sakalava; Madagascar, East and Southeast Asia to Japan, eastern Queensland, northeastern New South Wales, Mexico), Gironniera (6; G. celtidifolia, G. hirta, G. nervosa, G. parvifolia, G. rhamnifolia, G. subaequalis; Sri Lanka, Southeast Asia, southern China to islands in the Pacific), Lozanella (2; L. enantiophylla, L. permollis; tropical America), Cannabis (1; C. sativa; Central Asia), Humulus (2–3; temperate regions on the Northern Hemisphere), Celtis (c 60; tropical to warm-temperate regions on both hemispheres), Pteroceltis (1; P. tatarinowii; northern and central China), Chaetachme (2; C. aristata, C. madagascariensis; tropical and southern Africa, Madagascar), Trema (c 15; tropical and subtropical regions on both hemispheres).
Cannabaceae are sister to [Moraceae+Urticaceae].
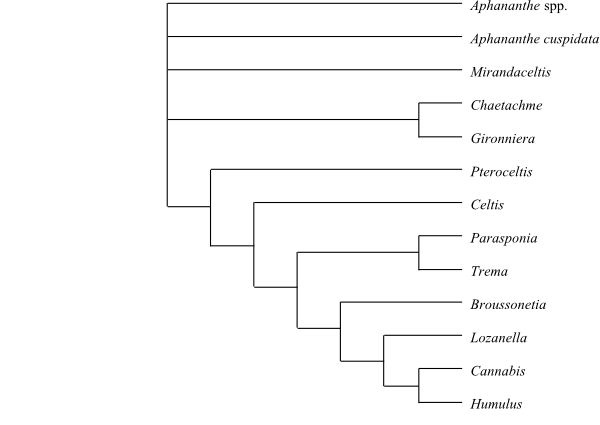 |
Phylogeny (simplified) of Cannabaceae based on morphology, phytochemistry and cytology (Zavada & Kim 1996). The placement of Broussonetia here is mysterious, since it is a member of the Moraceae. |
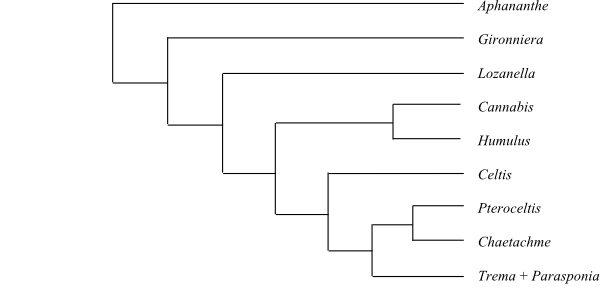 |
Bayesian inference tree (simplified) of Cannabaceae based on plastid DNA data (Yang & al. 2013). |
DIRACHMACEAE Hutch. |
( Back to Rosales ) |
Genera/species 1/2
Distribution Socotra, Somalia.
Fossils Unknown.
Habit Dioecious, evergreen shrubs or small trees. Long and short shoots well differentiated.
Vegetative anatomy Phellogen ab initio subepidermal. Vessel elements with simple perforation plates; lateral pits alternate, simple pits. Vestured pits absent. Imperforate tracheary xylem elements tracheids with more or less simple pits, non-septate (also vasicentric tracheids). Wood rays usually uniseriate or biseriate, heterocellular. Axial parenchyma apotracheal banded, or paratracheal scanty vasicentric. Tyloses or gum-like substances frequent in heartwood. Secondary phloem stratified into hard fibrous and soft parenchymatous non-fibrous layers. Sieve tube plastids S type? Nodes? Prismatic calciumoxalate crystals abundant; druses present in cortical and medullary parenchyma cells.
Trichomes Hairs unicellular, uniseriate; glandular hairs present or absent.
Leaves Alternate (spiral), simple, entire, with ? ptyxis. Stipules intrapetiolar, linear-triangular (subulate), persistent; leaf sheath absent. Petiole vascular bundle transection arcuate. Venation pinnate. Stomata anomocytic or cyclocytic. Cuticular wax crystalloids? Epidermis with mucilaginous idioblasts. Leaf margin coarsely serrate to crenate.
Inflorescence Flowers terminal, solitary (reduced cymose inflorescence?).
Flowers Actinomorphic, large. Hypanthium-like structure present between sepals and petals. Pedicel with ’epicalyx’ (’hypocalyx’) consisting of four to eight bracts. Half epigyny. Sepals five or six (Dirachma somalensis) or eight (Dirachma socotrana), with valvate aestivation, caducous above hypanthium-like structure (consisting of perianth and sometimes also androecium), free. Petals five or six (Dirachma somalensis) or eight (Dirachma socotrana), with contorted aestivation, with ventral appendages at base, free. Nectariferous glands present at petal bases (petal nectaries) or on subbasal (ventral) appendages, caducous. Disc absent.
Androecium Stamens five or six (Dirachma somalensis) or eight (Dirachma socotrana), obhaplostemonous, alternisepalous, antepetalous. Filaments free, adnate at base to petals. Anthers basifixed, non-versatile, tetrasporangiate, extrorse, longicidal (dehiscing from apex by longitudinal slits). Tapetum secretory, with binucleate cells. Staminodia absent.
Pollen grains Microsporogenesis simultaneous? Pollen grains tricolporate, shed as monads, ?-cellular at dispersal, with starch grains. Exine semitectate, with columellate (granular?) infratectum, finely reticulate.
Gynoecium Pistil composed of five or six (Dirachma somalensis) or eight (Dirachma socotrana) connate antesepalous carpels. Ovary semi-inferior, quinquelocular or sexalocular (Dirachma somalensis) or octalocular (Dirachma socotrana), and lobate. Style single, simple. Stigmas five or six (Dirachma somalensis) or eight (Dirachma socotrana), clavate to cylindrical or punctate, type? Pistillodium absent.
Ovules Placentation basal to axile. Ovule one per carpel, anatropous, ascending, apotropous, bitegmic, crassinucellar. Micropyle bistomal, Z-shaped (zig-zag). Outer integument six to ten cell layers thick. Inner integument two or three cell layers thick. Hypostase present? Nucellar cap minute or absent. Megagametophyte monosporous, probably Polygonum type. Endosperm development ab initio nuclear, later cellular. Endosperm haustorium chalazal. Embryogenesis?
Fruit A septicidal and septifragal capsule with columella, dehiscing adaxially from base to apex (a schizocarp with five or six [Dirachma somalensis] or eight [Dirachma socotrana] ventricidal follicular mericarps).
Seeds Seed flattened, with small funicular aril. Seed coat exotestal-endotegmic. Testa multiplicative, with median antiraphal vascular bundle. Exotesta palisade with thick anticlinal cell walls, tanniniferous. Endotesta and exotegmen crushed. Endotegmic cells thick-walled, tanniniferous. Perisperm not developed. Endosperm scarse or absent. Embryo straight, well differentiated, chlorophyll? Cotyledons two, large. Germination?
Cytology n = ?
DNA Trans-spliced intron present in mitochondrial gene nad1.
Phytochemistry Very insufficiently known. Flavonoids present.
Use Unknown.
Systematics Dirachma (2; D. socotrana: Socotra; D. somalensis: central Somalia).
Dirachma is member of a trichotomy also comprising Elaeagnaceae and Rhamnaceae.
ELAEAGNACEAE Juss. |
( Back to Rosales ) |
Elaeagnales R. Br. ex Bercht. et J. Presl, Přir. Rostlin: 234. Jan-Apr 1820 [‘Elaeagneae’]; Hippophaëaceae G. Mey., Chloris Han.: 456, 460. Jul-Aug 1836 [’Hippophaëae’]
Genera/species 3/50–55
Distribution Temperate and subtropical regions on the Northern Hemisphere, Southeast Asia, Queensland.
Fossils Pollen grains similar to Elaeagnaceae have been reported from Cenozoic and even Late Cretaceous layers in Asia and Europe (e.g. Slowakipollis from the Oligocene of eastern Europe). Pollen of Shepherdia was recorded from the Miocene of Oregon and Idaho. Fossil leaves of Elaeagnus were reported from Miocene layers on the Qinghai-Tibet Plateau.
Habit In Elaeagnus monoecious, androdioecious or gynodioecious, in Hippophae and Shepherdia dioecious, usually deciduous (sometimes evergreen) trees or shrubs (rarely climbing), often spiny and with silvery leaves. Some species are xerophytes.
Vegetative anatomy Ectomycorrhiza often present. Root nodules with nitrogen-fixing bacteria (Frankia etc.) usually present. Cluster roots present in Hippophae. Phellogen ab initio superficial. Cambium storied. Vessel elements with simple perforation plates; lateral pits alternate, bordered pits. Vestured pits present. Imperforate tracheary xylem elements tracheids or fibre tracheids (libriform fibres?) with bordered pits, non-septate (also vasicentric tracheids). Wood rays uniseriate or multiseriate, homocellular or heterocellular. Axial parenchyma apotracheal diffuse or diffuse-in-aggregates, or absent (Shepherdia). Wood elements often more or less storied. Tyloses frequent. Phloem usually tangentially stratified into hard fibrous and soft parenchymatous layers. Sieve tube plastids S0 type; sieve tube nuclei with non-dispersive protein bodies. Nodes 1:1, unilacunar with one leaf trace. Heartwood often with gum-like substances. Calciumoxalate as crystal sand often present. Wood ray cells without prismatic crystals.
Trichomes Hairs lepidote, peltate or stellate; glandular hairs absent.
Leaves Usually alternate (spiral; rarely opposite), simple, entire, often coriaceous, with conduplicate-flat ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle transection arcuate or annular. Venation pinnate. Stomata anomocytic. Cuticular wax crystalloids? Mucilaginous idioblasts absent. Leaf margin entire.
Inflorescence Axillary, usually raceme, spike or fascicle (flowers rarely solitary).
Flowers Actinomorphic, usually small. Receptacle flat (often in male flowers) or tubular hypanthium (in female and bisexual flowers). Hypogyny. Sepals (two to) four (to six), usually with valvate (rarely open) aestivation, sometimes petaloid, connate in lower part into tubular hypanthium. Petals absent. Receptacular nectaries present. Disc consisting of separate parts.
Androecium Stamens (two to) four (to six) (Elaeagnus, Hippophae) or 4+4 (rarely 6+6) (Shepherdia), haplostemonous or obdiplostemonous, antesepalous and/or alternisepalous. Filaments free, adnate to upper part of hypanthium. Anthers basifixed or dorsifixed, usually non-versatile, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory. Staminodia absent.
Pollen grains Microsporogenesis simultaneous. Pollen grains (2–)3(–4)-colporate, shed as monads, bicellular or tricellular at dispersal. Exine tectate, with columellate infratectum, perforate, punctate or somewhat rugulate.
Gynoecium Pistil composed of one carpel (pseudomonomery?). Ovary superior, unilocular. Style (stylulus) single, simple, long. Stigma decurrent or capitate, non-papillate, Dry type. Pistillodium absent.
Ovules Placentation basal. Ovule one per ovary, anatropous, ascending, bitegmic, crassinucellar. Funicle short. Micropyle endostomal? Outer integument five to 16 cell layers thick. Inner integument three or four cell layers thick. Obturator funicular. Archespore multicellular. Megagametophyte usually monosporous, Polygonum type (in Shepherdia disporous, 8-nucleate, Allium type). Endosperm development ab initio nuclear. Endosperm haustorium inElaeagnus chalazal. Embryogenesis?
Fruit An achene surrounded by accrescent and fleshy hypanthium (baccate or drupaceous anthocarp). Pericarp thin.
Seeds Aril absent. Testa very thick, hard. Exotesta often palisade, with malpighiaceous cells with thick, at least partially sinuous anticlinal cell walls. Mesotesta aerenchymatous, with thick-walled cells. Endotesta? Tegmen? Perisperm not developed. Endosperm sparse, sometimes starchy, or absent. Embryo straight, well differentiated, oily and with aleuron (starch absent), without chlorophyll. Cotyledons two, fleshy, plano-convex. Germination phanerocotylar.
Cytology n = 6, 10, 11, 13, 14 (x = 12 in Hippophae, x =14 in Elaeagnus, x = 11, 13 in Shepherdia)
DNA Trans-spliced intron present in mitochondrial gene nad1.
Phytochemistry Flavonols (kaempferol, quercetin) and other O-methylflavonoids, pentacyclic triterpenes, ellagic and gallic acids, hydrolyzable ellagitannins, condensed tannins, simple indole bases, indole alkaloids, saponins, p-coumaric acid, L-quebrachitol, and sinapic acid present. Dihydroflavonols? Myricetin, cyanidin, proanthocyanidins (prodelphinidins), caffeic acid, and cyanogenic compounds not found.
Use Ornamental plants, fruits, stabilization of soil (Hippophae).
Systematics Hippophae (6–7; H. goniocarpa, H. gyantsensis, H. litangensis, H. neurocarpa, H. rhamnoides, H. salicifolia, H. tibetana; Europe, temperate Asia); Elaeagnus (40–45; temperate and subtropical regions in Asia and North America, Southeast Asia, one species, E. triflora, in Queensland), Shepherdia (3; S. argentea, S. canadensis, S. rotundifolia; Canada, United States incl. Alaska).
Elaeagnaceae may be sister-group to [Barbeyaceae+Dirachmaceae]
A probable topology of Elaeagnaceae is [Hippophae+[Elaeagnus+Shepherdia]].
MORACEAE Gaudich. |
( Back to Rosales ) |
Artocarpaceae Bercht. et J. Presl, Přir. Rostlin: 260. Jan-Apr 1820 [‘Artocarpeae’]; Ficaceae Bercht. et J. Presl, Přir. Rostlin: 260. Jan-Apr 1820 [‘Ficeae’]; Dorsteniaceae Chevall., Fl. Gén. Env. Paris 2: 376. 1827; Ficales Dumort., Anal. Fam. Plant.: 15. 1829 [‘Ficarieae’]; Artocarpales DC. in C. F. P. von Martius, Consp. Regn. Veg.: 14. Sep-Oct 1835 [’Artocarpeae’]; Morales Endl. in C. F. P. von Martius, Consp. Regn. Veg.: 13. Sep-Oct 1835 [‘Moreae’]
Genera/species 36/1.140–1.210
Distribution Tropical and subtropical regions, few species in temperate regions.
Fossils Apart from numerous Cenozoic fossils of Moraceae, there are leaves and reproductive organs of presumed Artocarpus from the Late Cretaceous of Greenland.
Habit Monoecious or dioecious (rarely gynodioecious), usually evergreen (sometimes deciduous) trees, shrubs, lianas, suffrutices or perennial herbs (Dorstenia and other tuberous geophytes or succulents; in Fatoua annual herbs). Often with large plank buttresses. Certain species of Ficus are epiphytes, some of which, the ’stranglers’, anchor in the soil by means of adventitious roots and then ‘strangle’ the host tree by the growth of these roots causing the death of the host.
Vegetative anatomy Phellogen ab initio usually superficial (sometimes outer-cortical). Vessel elements with simple perforation plates; lateral pits usually alternate (sometimes opposite), usually simple (sometimes bordered) pits. Imperforate tracheary xylem elements fibre tracheids or libriform fibres (often very long) usually with simple (sometimes bordered) pits, septate (in Castilleae) or non-septate (also vasicentric tracheids). Wood rays usually multiseriate (sometimes uniseriate), usually heterocellular (sometimes homocellular). Axial parenchyma usually paratracheal scanty, aliform, lozenge-aliform, winged-aliform, confluent, vasicentric, scalariform, reticulate, unilateral, or banded (rarely apotracheal banded). Wood elements storied. Tyloses frequent (sometimes sclerotic). Secondary phloem often stratified into hard fibrous and soft parenchymatous non-fibrous layers. Sieve tube plastids Ss type or S0 type; sieve tube elements with extruded nucleoli and non-dispersive protein bodies. Nodes usually 3:3, trilacunar with three leaf traces (in Ficus 5:5, pentalacunar with five traces). Laticifers usually with abundant white (sometimes colourless) latex (absent in Fatoua; in Fatoua and Malaisia coloured juice); growth and branching of laticiferous cells not accompanied by cell wall formation. Secretory mucilaginous cavities usually present. Heartwood sometimes with gum-like substances. Cystoliths abundant, globose (calciumcarbonate?), usually with pegs? (cellulose expansions of cell wall, covered with crystals). Epidermal cell walls sometimes with calciumcarbonate or silica. Prismatic calciumoxalate crystals abundant; druses present in some species.
Trichomes Hairs unicellular (usually micropapillate) or multicellular, simple, often capitate (with unicellular stalk and uni- or multicellular head), sometimes uncinate, sometimes calcified or silicified; pearl glands often frequent.
Leaves Usually alternate (spiral or distichous; rarely opposite or verticillate), usually simple (rarely pinnately or palmately compound), entire or pinnately or palmately lobed, often coriaceous, with various ptyxis. Stipules often large, intrapetiolar or interpetiolar, sometimes sheathing (in Ficus open in leaf axils) or reduced, persistent or deciduous (sometimes connate; stipules absent in Fatoua); leaf sheath absent. Petiole vascular bundle transection? Venation pinnate or palmate; secondary and tertiary veins proceeding into non-glandular teeth (urticoid teeth)? Extrafloral nectaries present in Ficus on abaxial or adaxial side of lamina. Stomata usually anomocytic (rarely anisocytic or cyclocytic). Cuticular wax crystalloids? Domatia usually absent (sometimes as pockets or hair tufts). Mesophyll often with sclerenchymatic idioblasts with tannins, gum, mucilage or crystals of calciumcarbonate, calciumoxalate or silica. Hydathodes sometimes present. Leaf margin serrate, crenate or entire (rarely glandular-serrate).
Inflorescence Terminal or axillary, simple cymose (often raceme-, spike-, umbel- or headlike) or racemose, or compound open or almost closed, often spicate male inflorescence and globose female inflorescence, often pseudanthium (flowers rarely solitary). Receptacle often accrescent and fleshy in fruit, in some genera urceolate and dome-shaped with flowers on adaxial side (Ficus), or open and discoid (Dorstenia etc.). Inflorescence in Ficus concave, almost closed syconium, with apical pore, ostiole. Prophylls usually basal.
Flowers Actinomorphic, small to minute. Usually hypogyny (sometimes half epigyny). Sepals (one to) four or five (to ten), with valvate or imbricate aestivation, persistent or caducous, free or (often in female flowers) connate at base (sometimes adnate to sepals of adjacent flowers), or absent. Petals absent. Nectary absent (possibly present in some genera). Disc absent.
Androecium Stamens (one to) four (to eight), antesepalous, usually as many as sepals. Filaments usually inflexed in bud (in Moreae recurved with lightning rapidity at anthesis; rarely straight), usually free (sometimes connate), usually free from (sometimes adnate to?) sepals. Anthers basifixed or dorsifixed, often versatile, usually tetrasporangiate (rarely disporangiate), introrse or extrorse, longicidal (dehiscing by longitudinal slits, sometimes explosively dehiscent). Tapetum secretory. Female flowers often with staminodia.
Pollen grains Microsporogenesis simultaneous. Pollen grains (1–)2–3(–5)-porate (rarely ≥6-pantoporate), shed as monads, bicellular at dispersal. Exine tectate, with granular infratectum, smooth or scabrate.
Gynoecium Pistil composed of two (or three) connate carpels, one or two of which usually degenerating (in Broussonetia modified into pseudostyle). Ovary usually superior (sometimes semi-inferior), unilocular (or bilocular). Style single, simple, or stylodia two, free or connate at base. Stigmas decurrent, papillate or non-papillate, Dry type. Male flowers often with pistillodium.
Ovules Placentation usually apical or subapical (sometimes almost lateral, rarely basal). Ovule one per ovary, anatropous or campylotropous, usually pendulous (rarely erect and basal), epitropous, bitegmic, crassinucellar. Micropyle endostomal. Outer integument three or four cell layers thick. Inner integument approx. three cell layers thick. Hypostase present or absent. Nucellar cap approx. five cell layers thick. Megagametophyte usually monosporous, Polygonum type (sometimes disporous, 8-nucleate, Allium type). Antipodal cells usually not proliferating (in at least Dorstenia proliferating). Endosperm development ab initio nuclear. Endosperm haustoria? Embryogenesis usually asterad.
Fruit Usually a drupe with dehiscing exocarp (rarely an indehiscent drupe or achene) or enclosed by fleshy perianth and/or sunken into accrescent fleshy floral receptacle and/or fleshy inflorescence axis; single fruits often connate into fleshy syncarp (in, e.g., Ficus, Morus and Artocarpus; sepals, bracts and receptacle in Artocarpus and Parartocarpus not distinctly separate; syncarp in Artocarpus as ripe weighing up to c. 50 kg; syncarp in Ficus a syconium with nutlets enclosed within fleshy inflorescence axis).
Seeds Aril absent. Testa indistinct, poorly differentiated, usually vascularized (in Prainea with several thickened cell layers). Exotesta tanniniferous. Tegmen? Perisperm not developed. Endosperm usually sparse, often oily, or absent. Embryo usually large, straight to curved, usually without chlorophyll. Cotyledons single (one degenerating) or two, flattened or folded. Germination phanerocotylar or cryptocotylar.
Cytology n = 12–16, 18, 20, 21, 24, 28, 32 (large variation in Dorstenia) – Polyploidy occurring; centromeres terminal and median.
DNA Trans-spliced intron present in mitochondrial gene nad1.
Phytochemistry Flavonols (kaempferin, quercetin) and their glycosides, flavone-C-glycosides and other flavonoids, isoflavonoids, cyanidin, alkaloids, triterpene saponins, cyanogenic compounds, hydroxycinnamate derivatives, benzophenones, xanthones, coumarins, and furanocoumarins (in Ficus) present. Ellagic acid and tannins not found. Latex containing gums, proteins (e.g. papain-like enzyme), waxes, triterpenoid resins, polyphenols, cardenolids, etc. Raffinose and stachyose present in phloem exudates.
Use Ornamental plants, fruits (Ficus carica, Artocarpus altilis, Artocarpus heterophyllus, Morus nigra), textile (tapa) and paper (Antiaris, Broussonetia papyrifera), nutrient substrate of silk moth larvae (Bombyx mori on Morus alba), narcotics (Brosimum acutifolium), medicinal plants, arrow poison, rubber (Castilla elastica, Ficus elastica), timber.
Systematics Moraceae are sister-group to Urticaceae.
The clade [Artocarpeae+Moreae] is sister to the remaining Moraceae.
[Artocarpeae+Moreae]
Female flowers with peltate bracts.
Artocarpeae Lam. et DC., Syn. Plant. Fl. Gall.: 183. 30 Jun 1806
3/65–70. Batocarpus (3; B. amazonicus, B. costaricensis, B. orinocensis; Costa Rica to Bolivia; paraphyletic?), Clarisia (3; C. biflora, C. ilicifolia, C. racemosa; southern Mexico to Bolivia; in Batocarpus?), Artocarpus (c 60; tropical Asia east to islands in western Pacific). – Tropical Asia, tropical America. Male flowers dimerous. Stamen usually single (sometimes three). Filaments straight.
Moreae Dumort., Anal. Fam. Plant.: 17. 1829
6/50–55. Sorocea (14; southern Mexico, Central America, tropical South America), Bagassa (1; B. guianensis; northeastern South America), Trophis (9; Madagascar, Malesia to New Caledonia, southern Mexico, Central America, the West Indies, tropical South America), Morus (10–15; tropical and southern Africa, Southwest, East and Southeast Asia, eastern United States, Texas, Mexico, Central America, tropical America), Milicia (2; M. excelsa, M. regia; tropical Africa), Streblus (14; tropical Africa, Madagascar, Southeast Asia, eastern Queensland, eastern New South Wales, the Solomon Islands, Norfolk Island, New Zealand, Micronesia, Polynesia). – Pantropical, few species in temperate regions. – Sorocea may be sister to the remaining Moreae.
[Maclureae+[[Parartocarpus+Hullettia]+[Dorstenieae+[Castilleae+Ficeae]]]]
Maclureae W. L. Clement et Weiblen in Syst. Bot. 34: 545. 2009
1/11–12. Maclura (11–12; tropical and subtropical regions on both hemispheres). – Dioecy. Axillary spines often present. Inflorescence often with glands with yellow dye. Bracts of male flowers not peltate. Bracts of female flowers sometimes peltate. Stigmas usually two, often unequal in length.
Parartocarpus (4; P. bracteatus, P. triandrus, P. venenosus, P. woodii; Malesia) and Hullettia (2; H. dumosa, H. griffithiana; southern Burma, Peninsular Thailand, the Malay Peninsula, Sumatra) form a clade sister to [Dorstenieae+[Castilleae+Ficeae]] in the analyses by Gardner & al. (2017).
[Dorstenieae+[Castilleae+Ficeae]]
Monoecy (inflorescences bisexual). Radial laticiferous ducts present. Pistillodium conical.
Dorstenieae Dumort., Anal. Fam. Plant.: 16. 1829
12/145–150. Fatoua (2; F. pilosa, F. villosa; Madagascar, East and Southeast Asia, northern and easternmost Australia, New Caledonia), Broussonetia (8; Madagascar, tropical and subtropical Asia), Malaisia (1; M. aculeata; Malesia), Bleekrodea (4; B. insignis, B. madagascariensis, B. malayana, B. tonkinensis; Madagascar, the Malay Peninsula, Borneo), Sloetia (1; S. elongata; West Malesia, the Philippines, Sulawesi), Dorstenia (c 105; tropical regions on both hemispheres), Utsetela (2; U. gabonensis, U. neglecta; Gabon, Congo), Brosimum (15–18; southern Mexico, Central America, tropical South America), Trilepisium (1; T. madagascariense; tropical and southern Africa, Madagascar, the Mascarene Islands), Treculia (3; T. africana, T. lamiana, T. obovoidea; tropical West and Central Africa, Madagascar), Bosqueiopsis (1; B. gilletii; tropical Africa)?, Scyphosyce (2; S. manniana, S. pandurata; tropical West and Central Africa)? – Tropical and subtropical regions on both hemispheres, with their highest diversity in the Old World. Male flowers sometimes trimerous, often with connate sepals. Female flowers sometimes dimerous, sometimes sunken into receptacle. – Fatoua is sister to the remaining Dorstenieae.
[Castilleae+Ficeae]
Inflorescence axis urceolate, with insects (thrips in Castilleae; fig wasps, Agaonidae in Ficeae) reproducing among flowers and simultaneously pollinating these. Filaments straight. Bracts of female flowers not peltate. Sepals free.
Castilleae C. C. Berg in Acta Bot. Neerl. 26: 78. 15 Feb 1977
11/65–70. Sparattosyce (1; S. dioica; New Caledonia), Antiaropsis (2; A. decipiens, A. uniflora; New Guinea), Poulsenia (1; P. armata; southern Mexico to Bolivia), Antiaris (1; A. toxicaria; tropical regions in the Old World), Mesogyne (1; M. insignis; tropical Africa), Naucleopsis (16–20; tropical America; non-monophyletic?), ’Perebea’ (9–10; tropical America; polyphyletic), Maquira (4; M. calophylla, M. coriacea, M. guianensis, M. sclerophylla; tropical South America), Helicostylis (17; tropical America), Castilla (3; C. elastica, C. tunu, C. ulei; Central America, tropical South America), Pseudolmedia (10–12; southern Mexico, Central America, the West Indies, tropical South America). – Tropical and subtropical regions on both hemispheres, with their largest diversity in tropical America. Wood fibres septate. Cystoliths absent. Inflorescence axis involucrate, discoid to urceolate. Bracts absent in male flowers. Bracts usually present in female flowers. Pistillodium absent. – The clade [Antiaropsis+Sparattosyce] (Antiaropsineae) is sister to the remaining Castilleae.
Ficeae Dumort., Fl. Belg.: 24. 1827 [‘Ficineae’]
1/800–850. Ficus (800–850; tropical and subtropical regions on both hemispheres, few species in warm-temperate regions). – Latex usually present. Leaves sometimes opposite. Inflorescence syconium. Flowers trimerous. Bracts of male flowers not peltate. Stamens two.
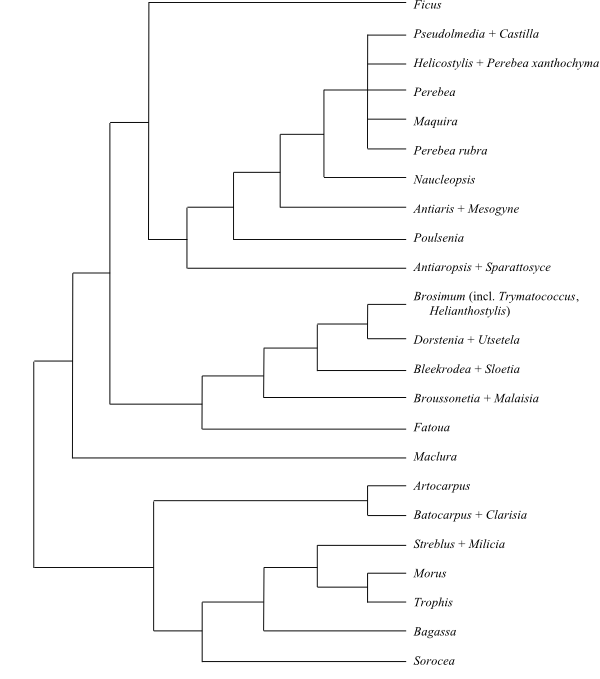 |
Phylogeny (Baysian inference, simplified) of Moraceae based on DNA sequence data (Clement & Weiblen 2009). |
RHAMNACEAE Juss. |
( Back to Rosales ) |
Frangulaceae DC. in de Lamarck et A. P. de Candolle, Fl. Franç., ed. 3, 4(2): 619. 17 Sep 1805; Rhamnales Link, Handbuch 2: 118. 4-11 Jul 1829 [‘Rhamneae’]; Gouaniaceae Raf., Fl. Tellur. 2: 73. Jan-Mar 1837 [’Guanidia’]; Frangulopsida Endl., Gen. Plant.: 1081. Apr 1840; Rhamnopsida Brongn., Enum. Plant. Mus. Paris: xxxi, 121. 12 Aug 1843 [’Rhamnoideae’]; Phylicaceae J. Agardh, Theoria Syst. Plant.: 186. Apr-Sep 1858; Frangulales Wirtg., Anleit. Pflanzenk. 2: 36. 17-18 Sep 1860 [‘Frangulaceae’]; Ziziphaceae Adans. ex Post et Kuntze, Lex. Gen. Phan.: 665, 714. 20-30 Nov 1903 [’Zizyphaceae’]; Rhamnanae Takht. ex Reveal in Novon 2: 236. 13 Oct 1992
Genera/species 53–54/1.020–1.110
Distribution Cosmopolitan except polar areas, with their largest diversity in tropical and subtropical regions.
Fossils Coahuilanthus belinda from the Late Campanian of Mexico is represented by pentamerous bisexual flowers with antepetalous stamens and a prominent nectariferous disc. Floral fossils from Eocene strata of the Claiborne Formation (North America) and assigned to Solanites pusillus are apparently of Rhamnaceae origin (see Millan & Crepet 2014). Fossil leaves, pollen grains and fruits of Rhamnaceae are known from Oligocene strata onwards (e.g. fruits of Paliurus from North America and Europe).
Habit Usually bisexual (rarely monoecious, dioecious or androdioecious), evergreen or deciduous trees, shrubs or lianas (sometimes with tendrils or hooks; Crumenaria decumbens is a perennial herb), often with spines, often xeromorphic. Some species have phyllocladia and reduced leaves.
Vegetative anatomy Ectomycorrhiza present at least in Pomaderris and Rhamnus. Root nodules with nitrogen-fixing actinobacteria (e.g. Frankia) present in, i.a., Ceanothus and numerous species of Colletieae (Discaria, Colletia, Kentrothamnus, Retanilla and Trevoa). Phellogen ab initio superficial. Primary vascular tissue cylinder, without separate vascular bundles. Vessel elements usually with simple (rarely scalariform or reticulate) perforation plates; lateral pits usually alternate (sometimes opposite), often confluent, simple or bordered pits. Vestured and non-vestured pits present. Imperforate tracheary xylem elements tracheids or libriform fibres with simple or bordered pits, usually non-septate (also vasicentric tracheids). Wood rays uniseriate or multiseriate, homocellular or heterocellular. Axial parenchyma usually paratracheal scanty, aliform, lozenge-aliform, winged-aliform, confluent, vasicentric, or banded (rarely apotracheal diffuse), or absent. Secondary phloem often stratified into hard fibrous and soft parenchymatous layers. Sieve tube plastids S type. Nodes 3:3, trilacunar with three leaf traces. Heartwood often with brown gum-like deposits. Secretory lysigenous mucilaginous cavities present or absent. Silica bodies present in wood ray cells of some species. Prismatic to diamond-shaped or acicular (sometimes styloids or crystal sand) calciumoxalate crystals abundant.
Trichomes Hairs unicellular or multicellular, usually simple (in Sageretia bifid, in Pomaderreae also or only stellate); glandular hairs?
Leaves Alternate (spiral or distichous) or opposite, simple, entire, sometimes strongly reduced, with conduplicate (-plicate) or involute ptyxis. Stipules small, persistent or caducous (sometimes intra- or interpetiolarly connate or modified into spines; petiolar in Colletia; absent in most species of Phylica); leaf sheath absent. Colleters present. Petiole vascular bundle transection arcuate or U-shaped. Venation usually pinnate (rarely palmate or leaves one-veined); secondary veins often parallel and prominent; tertiary veins scalariform. Stomata usually anomocytic (sometimes paracytic or anisocytic), sometimes sunken into cavities or furrows. Cuticular wax crystalloids as rosettes of platelets (Fabales type), cuticular wax as crust or smooth layer. Domatia usually absent (sometimes as pits, pockets or hair tufts). Pellucid dots present in lamina in Karwinskia. Epidermis often with lysigenous mucilaginous cavities. Mesophyll usually with mucilaginous idioblasts, often with calciumoxalate as druses or single prismatic crystals (in Gouanieae acicular styloids). Leaf margin serrate or entire. Extrafloral nectaries reported as rarely present as glands at leaf base in Gouania.
Inflorescence Usually axillary (sometimes terminal), umbellate, corymbose, raceme- or spike-like or panicle, thyrse or fascicle (flowers rarely solitary terminal).
Flowers Actinomorphic, small. Hypanthium present (sometimes long, tubular) or absent. Hypogyny to epigyny. Sepals (three or) four or five (or six), with valvate aestivation, with adaxial median ridge, persistent or caducous, usually free (rarely connate). Petals (three or) four or five (or six), with cucullate (induplicate-valvate) aestivation, often shortly clawed, often enclosing stamens, free (rarely absent). Nectariferous disc intrastaminal, entire or lobed, often adnate to hypanthium (receptacular nectaries; rarely absent).
Androecium Stamens (three or) four or five (or six), alternisepalous, antepetalous. Filaments thin, free, free from tepals or adnate to petal bases. Anthers very small, dorsifixed, often versatile, tetrasporangiate, usually introrse, longicidal (dehiscing by longitudinal slits or valves). Tapetum secretory, with binucleate cells. Staminodia absent.
Pollen grains Microsporogenesis simultaneous. Pollen grains usually tricolporate (rarely tetracolporate), shed as monads, bicellular at dispersal. Exine usually tectate, with columellate (or granular) infratectum, microreticulate or perforate, striate, rugulate, fossulate, verrucate, pilate or psilate.
Gynoecium Pistil composed of two to five connate antesepalous carpels; median carpel adaxial. Ovary superior to inferior, bilocular to quinquelocular (sometimes unilocular by degeneration of carpels). Style single, simple, or stylodia several, more or less free, usually with two to four (rarely one, five or no) canals. Stigmas terminal, papillate, Dry type. Pistillodium absent. Common hypanthial and stylar indumentum sometimes forming secondary pollen display.
Ovules Placentation basal. Ovules usually one (in Karwinskia two median) per carpel, anatropous, ascending, apotropous or epitropous, bitegmic, crassinucellar. Micropyle usually exostomal (sometimes bistomal). Outer integument four to ten cell layers thick. Inner integument three or four cell layers thick. Obturator funicular. Hypostase sometimes present (in Ziziphus epistase). Parietal tissue four to seven cell layers thick. Archespore unicellular or multicellular. Nucellar cap (formed by apical cells of megasporangial epidermis) five or six cell layers thick; nucellar beak sometimes present; megasporangium often protruding through micropyle. Megagametophyte usually monosporous, Polygonum type (rarely disporous, 8-nucleate, Allium type). Synergids usually with a filiform apparatus. Antipodal cells degenerating. Endosperm development ab initio nuclear. Chalazal endosperm haustorium possibly present? Embryogenesis usually asterad (in Ziziphus solanad).
Fruit A drupe or a septicidal and/or loculicidal capsule (sometimes explosively dehiscent; rarely a nut; in Paliurus and Ventilago a samara; in most Gouanieae a schizocarp with two to five mericarps and often persistent sepals). Pericarp often distinctly two-layered (inner layer often lignified and finally spiralized), with layers often separating during maturation. Endocarp sclerenchymatous.
Seeds Funicular aril present in ziziphoid clade. Elaiosome present in some species. Seed often laterally flattened. Seed coat exotestal. Testa, usually multiplicative, often with median antiraphal vascular bundle. Exotesta often palisade, with malpighian cells with thickened walls. Mesotesta sometimes with sclerotic cells. Endotesta? Tegmen crushed. Endotegmen consisting of cuboid cells, with scalariform thickenings, usually not lignified (sometimes poorly lignified). Perisperm not developed. Endosperm sparse, usually oily (sometimes starchy; in Reynosia ruminate), or absent. Embryo large, usually straight (rarely curved), well differentiated, oily, with chlorophyll. Cotyledons two, large. Polyembryony common (in Ziziphus synergid polyembryony). Germination phanerocotylar or cryptocotylar.
Cytology n = (6, 8–)10–13
DNA Trans-spliced intron present in mitochondrial gene nad1. Mitochondrial coxI intron present in numerous genera.
Phytochemistry Flavonols (kaempferol, quercetin), biflavonyls (biflavonoids), cyanidin, dammaranes, oleanolic acid derivatives, gallic acid, condensed tannins, proanthocyanidins (prodelphinidins), benzylisoquinoline alkaloids, liriodenine, peptide alkaloids, anthraquinone glycosides (in Rhamnus), saponins, naphthoquinones, arbutin, polyacetate derived arthroquinones, chelidonic acid, and naphthalene and lupeolin derivatives present. Myricetin, ellagic acids and cyanogenic compounds not found.
Use Ornamental plants, medicinal plants, fruits, timber, carpentries, dyeing substancies.
Systematics Rhamnaceae may be sister-group to the clade [Elaeagnaceae+[Barbeyaceae+Dirachmaceae]] (Zhang & al. 2011).
Three main clades can be recognized in Rhamnaceae (Richardson & al. 2000a): (1) rhamnoids (Ventilagineae, Maesopsideae and Rhamneae) with epigyny; (2) ampeloziziphoids (Bathiorhamnus, Ampeloziziphus and Doerpfeldia) with palmate foliar venation; and (3) ziziphoids (including Gouanieae, Paliureae, Colletieae, Phyliceae and Pomaderreae). Fay & al. (2001) received different results in their analyses. The classification below is based mainly on Richardson & al. (2000b).
A plausible topology (Richardson & al. 2000a, b) followed here is: [[Ventilagineae+[Maesopsideae+Rhamneae]]+[[Bathiorhamneae+[Ampelozizipheae+Doerpfeldieae]]+[Gouanieae+[Paliureae+[Lasiodiscus+[Emmenosperma+[Alphitonia+Granitites+Colubrina]+[[Schistocarpaea+Colletieae]+[Phyliceae+[Ceanothus+Pomaderreae]]]]]]]]]
[Ventilagineae+[Maesopsideae+Rhamneae]]
Ventilagineae Benth. et Hook. f., Gen. Plant. 1: 372. 7 Aug 1862
2/20–45. Ventilago (9–35; tropical regions in the Old World, with one species, V. africana, in continental Africa and one species in Madagascar), Smythea (c 10; the Seychelles, Southeast Asia to Fiji and the Caroline Islands). – Tropical regions in the Old World.
[Maesopsideae+Rhamneae]
Maesopsideae Engl. et Weberb. in Engler et Prantl, Nat. Pflanzenfam. Nachtr.: 229. 4 Oct 1897
1/1. Maesopsis (1; M. eminii; tropical Africa).
Rhamneae Horan., Char. Ess. Fam.: 138. 17 Jun 1847
13/265–270. Rhamnus (c 120; temperate to tropical regions on the Northern Hemisphere south to South Africa and Brazil), Scutia (4; S. arenicola, S. buxifolia, S. myrtina, S. spicata; tropical regions in the Old World south to southern Africa, tropical South America), Sageretia (c 35; Somalia and southwestern Asia to Japan and Taiwan, tropical and subtropical Asia), Berchemiella (2; B. berchemiifolia: Japan; B. wilsonii: Hubei in China), Rhamnella (c 10; northern Pakistan to China, the Korean Peninsula and Japan, New Guinea, eastern Queensland, Fiji and Tonga), Dallachya (1; D. vitiensis; New Guinea, islands in western Pacific), Berchemia (c 20; East Africa to East Asia, New Caledonia, western North America), Rhamnidium (12; Central America, the West Indies, tropical South America), Karwinskia (16; southwestern United States and the West Indies to Bolivia), Condalia (18–20; southwestern United States, Mexico, Central America, tropical and subtropical South America), Auerodendron (10; the West Indies), Reynosia (c 15; Florida, Central America, the West Indies), Krugiodendron (2; K. acuminatum, K. ferreum; Mexico, Costa Rica, the West Indies). –
[[Bathiorhamneae+[Ampelozizipheae+Doerpfeldieae]]+[Gouanieae+[Paliureae+[Lasiodiscus+[Emmenosperma+[Alphitonia+Granitites+Colubrina]+[[Schistocarpaea+Colletieae]+[Phyliceae+[Ceanothus+Pomaderreae]]]]]]]]
[Bathiorhamneae+[Ampelozizipheae+Doerpfeldieae]]
Bathiorhamneae J. E. Richardson in Kew Bull. 55: 335. 15 Aug 2000
1/7. Bathiorhamnus (7; B. capuronii, B. cryptophorus, B. dentatus, B. louvelii, B. macrocarpus, B. reticulatus, B. vohemarensis; Madagascar).
[Ampelozizipheae+Doerpfeldieae]
Ampelozizipheae J. E. Richardson in Kew Bull. 55: 335. 15 Aug 2000
1/2. Ampelozizyphus (2; A. amazonicus, A. guaquirensis; northern tropical South America).
Doerpfeldieae J. E. Richardson in Kew Bull. 55: 335. 15 Aug 2000
1/1. Doerpfeldia (1; D. cubensis; Cuba).
[Gouanieae+[Paliureae+[Lasiodiscus+[Emmenosperma+[Alphitonia+Granitites+Colubrina]+[[Schistocarpaea+Colletieae]+[Phyliceae+[Ceanothus+Pomaderreae]]]]]]]
Gouanieae Rchb., Handb. Nat. Pfl.-Syst.: 222. 1-7 Oct 1837 [‘Gouaniaceae’]
5/c 63. Gouania (c 50; tropical and subtropical regions on both hemispheres), Helinus (5; H. brevipes, H. integrifolius, H. lanceolatus, H. mystacinus, H. spartioides; tropical and southern Africa, Madagascar, northwestern India), Reissekia (1; R. smilacina; Brazil), Alvimiantha (1; A. tricamerata; Brazil), Crumenaria (6; C. decumbens, C. dffusa, C. erecta, C. glaziovii, C. lilloi, C. steyermarkii; Central America, Colombia to Argentina). – Tropical and subtropical regions on both hemispheres.
[Paliureae+[Lasiodiscus+[Emmenosperma+[Alphitonia+Granitites+Colubrina]+[[Schistocarpaea+Colletieae]+[Phyliceae+[Ceanothus+Pomaderreae]]]]]]
Paliureae Reissek ex Endl., Gen. Plant.: 1095. Apr 1840
3/110–160? Hovenia (3; H. acerba, H. dulcis, H. trichocarpa; the Himalayas, northern Burma, China, the Korean Peninsula, Japan), Paliurus (5; P. hemsleyanus, P. hirsutus, P. orientalis, P. ramosissimus, P. spina-christi; the Mediterranean to Japan; in Ziziphus?), ‘Ziziphus’ (100–150?; tropical and subtropical regions on both hemispheres; paraphyletic; incl. Paliurus?). – Tropical and subtropical regions on both hemispheres.
[Lasiodiscus+[Emmenosperma+[Alphitonia+Granitites+Colubrina]+[[Schistocarpaea+Colletieae]+[Phyliceae+[Ceanothus+Pomaderreae]]]]]
Lasiodiscus clade
1/12. Lasiodiscus (12; tropical Africa, Madagascar).
[Emmenosperma+[Alphitonia+Granitites+Colubrina]+[[Schistocarpaea+Colletieae]+[Phyliceae+[Ceanothus+Pomaderreae]]]]
Emmenosperma clade
1/5. Emmenosperma (5; E. alphitonoides, E. cunninghamii, E. micropetalum, E. pancherianum, E. papuanum; New Guinea, northern and eastern Australia, New Caledonia, Fiji).
Colubrina clade
3/c 52. Jaffrea (2; J. erubescens, J. xerocarpa; New Caledonia), Alphitonia (c 15; Malesia to New Guinea, northern and eastern Australia, islands in western Pacific, the Hawaiian Islands), Granitites (1; G. intangendus; southwestern Western Australia), Colubrina (34; tropical and subtropical regions on both hemispheres, with their highest diversity in tropical America). – Tropical and subtropical regions on both hemispheres.
[[Schistocarpaea+Colletieae]+[Phyliceae+[Ceanothus+Pomaderreae]]]
[Schistocarpaea+Colletieae]
Schistocarpaea clade
1/1. Schistocarpaea (1; S. johnsonii; northeastern Queensland).
Colletieae Reissek ex Endl., Gen. Plant.: 1099. Apr 1840
6/29. Colletia (5; C. hystrix, C. paradoxa, C. spartioides, C. spinosissima, C. ulicina; southern South America), Discaria (12; southeastern Queensland, eastern New South Wales, eastern Victoria, Tasmania, New Zealand, South America), Adolphia (2; A. californica, A. infesta; southwestern Unites States, northwestern Mexico), Kentrothamnus (1; K. weddellianus; Bolivia, Argentina), Retanilla (4; R. ephedra, R. patagonica, R. stricta, R. trinervia; Peru, Chile, western Argentina), Trevoa (5; T. campanulata, T. closiana, T. glauca, T. quinquenervia, T. spinifer; the Andes). – Southeastern Australia, Tasmania, New Zealand, southwestern Unites States, northwestern Mexico, South America.
[Phyliceae+[Ceanothus+Pomaderreae]]
Phyliceae Reissek ex Endl., Gen. Plant: 1100. Apr 1840
3–4/190–195. Phylica (c 190; southern Africa, Madagascar, the Mascarene Islands, Amsterdam Island, Tristan da Cunha, Gouth Island; incl. Nesiota?), Nesiota (1; N. elliptica; St. Helena; in Phylica?), Trichocephalus (1; T. stipularis; Western Cape), Noltea (1; N. africana; Western and Eastern Cape, KwaZulu-Natal). – Southern Africa, Madagascar, the Mascarene Islands, Amsterdam Island, St. Helena, Tristan da Cunha, Gouth Island.
[Ceanothus+Pomaderreae]
Ceanothus clade
1/c 55. Ceanothus (c 55; North America, Mexico, with their highest diversity in California and northwestern Mexico).
Pomaderreae Reissek ex Endl., Gen. Plant.: 1101. Apr 1840
10/210–220. Pomaderris (c 75; Southeast Asia, southern and eastern Australia, Tasmania, New Zealand), Siegfriedia (1; S. darwinioides; southwestern Western Australia; in Pomaderris?), Trymalium (14; southwestern Western Australia, southeastern South Australia), Spyridium (c 30; southern Australia, Tasmania), Stenanthemum (30–35?; southern and central Australia, Tasmania, with their highest diversity in southwestern Western Australia; non-monophyletic?), Cryptandra (55–60; Australia, Tasmania, with their largest diversity in southwestern Western Australia and southeastern Australia), Blackallia (1; B. nudiflora; southwestern Western Australia), Serichonus (1; S. gracilipes; southwestern Western Australia), Papistylus (2; P. grandiflorus, P. intropubens; southwestern Western Australia), Polianthion (4; P. bilocularis, P. collinum, P. minutiflorum, P. wichurae; southwestern Western Australia, southeastern Queensland). – Australia, Tasmania, New Zealand, with their highest diversity in southwestern Western Australia.
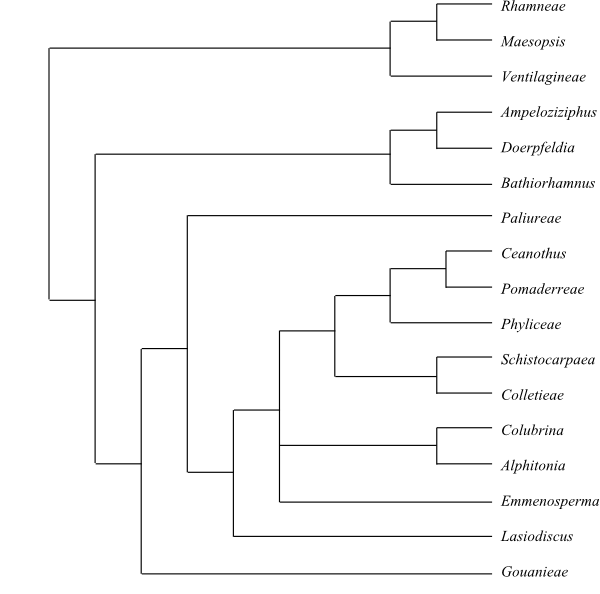 |
Cladogram (successive weighting) of Rhamnaceae based on DNA sequence data (Richardson & al. 2000a). According to Fay & al. (2001), [Alphitonia+Granitites] is sister-group to [Stenanthemum+Cryptandra+ [Siegfriedia+[Trymalium+[Spyridium+Pomaderris]]]] and Colubrina is sister to all other Rhamnaceae except [Bathiorhamnus+[Ampeloziziphus+Doerpfeldia]]. |
ROSACEAE Juss. |
( Back to Rosales ) |
Spiraeaceae Bertuch, Taf. Allg. Naturgesch. Gewächs-Reich: Enum. 10, Syn. Tab. 3. 1801 [’Spiraeae’]; Poteriaceae Raf., Anal. Nat.: 173. Apr-Jul 1815 [’Poteria’]; Fragariaceae Nestl., Monogr. Potentilla: 14. Jun 1816; Pyraceae Vest, Anleit. Stud. Bot.: 268, 286. 1818 [’Pyroideae’]; Alchemillaceae Martinov, Tekhno-Bot. Slovar: 17. 3 Aug 1820 [’Alchemilleae’]; Amygdalaceae Marquis, Esq. Règne Vég.: 49. 15-22 Jul 1820 [’Amygdaleae’], nom. cons.; Potentillaceae Bercht. et J. Presl, Přir. Rostlin: 231. Jan-Apr 1820 [‘Potentilleae’]; Prunaceae Martinov, Tekhno-Bot. Slovar: 511. 3 Aug 1820 [‘Pruniferae’]; Prunales Bercht. et J. Presl, Přir. Rostlin: 230. Jan-Apr 1820 [‘Prunaceae’]; Sanguisorbaceae Bercht. et J. Presl, Přir. Rostlin: 231. Jan-Apr 1820 [‘Sanguisorbeae’]; Tormentillaceae Martinov, Tekhno-Bot. Slovar: 636. 3 Aug 1820 [’Tormentillae’]; Agrimoniaceae Gray, Nat. Arr. Brit. Pl. 2: 395, 574. 10 Jan 1822; Dryadaceae Gray, Nat. Arr. Brit. Pl. 2: 395, 577. 10 Jan 1822 [’Dryadeae’]; Ulmariaceae Gray, Nat. Arr. Brit. Pl. 2: 395, 588. 10 Jan 1822 [’Ulmariae’]; Amygdalales Link, Handbuch 2: 72. 4-11 Jul 1829 [’Amygdaleae’]; Dryadales Link, Handbuch 2: 98. 4-11 Jul 1829 [’Dryadeae’]; Sanguisorbales Link, Handbuch 2: 113. 4-11 Jul 1829 [‘Sanguisorbeae’]; Spiraeales Link, Handbuch 2: 94. 4-11 Jul 1829 [‘Spiraeaceae’]; Mespilaceae Schultz Sch., Nat. Syst. Pflanzenr.: 509. 30 Jan-10 Feb 1832 [’Mespileae’]; Rosineae Rchb., Deutsch. Bot. Herb.-Buch: lxvi. Jul 1841; Neilliaceae Miq., Fl. Ned. Ind. 1(1): 390. 20 Dec 1855; Cydoniaceae Schnizl., Anal. Nat. Ordn. Gew.: 14. 1856; Cercocarpaceae J. Agardh, Theoria Syst. Plant.: 287. Apr-Sep 1858 [’Cercocarpeae’]; Coleogynaceae J. Agardh, Theoria Syst. Plant.: 171. Apr-Sep 1858 [’Coleogyneae’]; Lindleyaceae J. Agardh, Theoria Syst. Plant.: 166. Apr-Sep 1858; Rhodotypaceae J. Agardh, Theoria Syst. Plant.: 172. Apr-Sep 1858 [’Rhodotypeae’]; Chamaemoraceae Lilja, Skånes Fl., ed. 2: 349, 980. Apr-Dec 1870 [’Chamaemoreae’]; Sorbaceae Brenner, Florist. Handb.: 94. 1886; Malaceae Small, Fl. S.E. U.S.: 529. 22 Jul 1903, nom. cons.
Genera/species 77/3.140–>4.190
Distribution Cosmopolitan except continental Antarctica, with their largest diversity in temperate regions on the Northern Hemisphere.
Fossils Paleorosa similkameenensis is a flower (similar to those in Pyrodae) from the mid-Eocene of Canada and numerous other fossil Rosaceae have been recorded from the Eocene onwards in Europe and North America.
Habit Usually bisexual (sometimes monoecious, dioecious or polygamous), evergreen or deciduous trees, shrubs or suffrutices, or usually perennial (rarely annual) herbs. Some species are xerophytes.
Vegetative anatomy Ectomycorrhiza often present. Root nodules with nitrogen-fixing actinobacteria (e.g. Frankia) present at least in Dryadoideae. Phellogen usually deeply seated (in Exochorda subepidermal). Polydermis commonly present. Endodermis often prominent. Medulla without lysigenous mucilage canals, in some species septate by diaphragms. Vessel elements usually with simple (sometimes also reticulate or scalariform) perforation plates; lateral pits alternate or opposite, bordered pits. Vestured pits present. Imperforate tracheary xylem elements tracheids or fibre tracheids (rarely libriform fibres) with simple or bordered pits, non-septate (also vasicentric tracheids). Wood rays uniseriate to multiseriate, homocellular or heterocellular. Axial parenchyma usually apotracheal diffuse or diffuse-in-aggregates (sometimes paratracheal scanty or banded, rarely unilateral or vasicentric, or absent). Tyloses absent. Sieve tube plastids Ss type; cell nuclei with non-dispersive protein bodies. Nodes usually 3:3, trilacunar with three leaf traces (sometimes unilacunar or penta- to multilacunar with one or several traces, respectively). Heartwood often with gum-like substances. Calciumoxalate usually as solitary prismatic crystals (in Pruneae also druses) or absent (in Pyrinae prismatic crystals in axial parenchyma).
Trichomes Hairs unicellular or multicellular, uniseriate or multiseriate, simple or branched, sometimes stellate; multicellular glandular hairs often frequent; prickles present in several genera.
Leaves Usually alternate (spiral; in Lyonothamnus, Coleogyne and Rhodotypos opposite), simple or pinnately or palmately compound, entire or lobed, sometimes coriaceous, usually with conduplicate ptyxis. Stipules inserted on branch or adnate to petiole, small to foliaceous, usually persistent (absent in Oemleria; in some species of Rosa with extrafloral nectaries); leaf sheath short? (with free margins) or absent. Petiole vascular bundle transection arcuate or annular. Venation usually pinnate (sometimes palmate), craspedodromous or camptodromous. Stomata anomocytic. Cuticular wax crystalloids often as rodlets or ribs (sometimes as granules or clustered tubuli of Berberis type, chemically dominated by nonacosan-10-ol). Domatia as pits, pockets or hair tufts, or absent. Epidermis with or without mucilaginous idioblasts. Cystoliths absent. Hydathodes sometimes present. Leaf margin and leaflet margins usually serrate (sometimes crenate or entire). Extrafloral nectariferous glands present on distal part of petiole or on base of lamina (sometimes on stipules), especially in Rosa and Prunus.
Inflorescence Terminal or axillary, usually cymose (sometimes racemose) of various shapes (cyme, panicle, raceme, corymb, umbel, fascicle, etc.), or flowers solitary axillary or terminal.
Flowers Usually actinomorphic (rarely zygomorphic). Hypanthium usually cupular, campanulate, tubular or discoid (rarely absent). Epicalyx, consisting of sepalous stipules of calyx lobes inserted on top of hypanthium, present in certain Rosoideae. Hypogyny, epigyny or half epigyny. Sepals (three or) four or five (to ten), with valvate or imbricate aestivation, persistent, free. Petals (three or) four or five (to ten), usually with imbricate aestivation, often clawed, caducous, free (rarely absent). Nectaries usually on adaxial side of hypanthium or on staminal bases. Nectariferous disc intrastaminal, annular or divided, inserted around hypanthial orifice (sometimes absent).
Androecium Stamens usually numerous (often 10+5+5, outer stamens antesepalous; rarely one [Aphanes clade of Alchemilla] or three, or five to ten), inflexed in bud, centripetally developing. Filaments free or connate below, free from tepals, usually inserted on hypanthium. Anthers dorsifixed, usually versatile, tetrasporangiate, usually introrse (in, e.g., Potentilla latrorse), longicidal (dehiscing by longitudinal slits) or poricidal (dehiscing by apical pores). Tapetum secretory. Staminodia usually absent (sometimes extrastaminal, petaloid).
Pollen grains Microsporogenesis simultaneous. Pollen grains usually tricolpor(oid)ate (rarely triporate or, in Sanguisorbinae, pantoporate), shed as monads, bicellular at dispersal. Exine tectate or semitectate, with columellate infratectum, perforate, reticulate or striate.
Gynoecium Carpels one to numerous (sometimes spiral), often antesepalous or antepetalous, secondarily free or more or less connate or adnate to hypanthium (rarely somewhat stipitate, on gynophore). Ovary superior, inferior or semi-inferior, unilocular (apocarpy, monomery, sometimes epigyny + apocarpy), or bilocular to quinquelocular (syncarpy). Style single, simple, or stylodia few to numerous, usually terminal (in Rosoideae sometimes lateral or subbasal), free or more or less connate, or absent. Stigmas capitate, punctate, widened, decurrent or bifid (rarely fimbriate or penicillate), papillate or non-papillate, Dry or Wet type. Pistillodium?
Ovules Placentation usually basal or subbasal (rarely apical; when syncarpy then axile). Ovules usually one or two (sometimes several, rarely numerous) per carpel, usually anatropous (sometimes hemianatropous or campylotropous), usually ascending, apotropous (sometimes pendulous, epitropous), usually bitegmic (sometimes unitegmic), crassinucellar. Micropyle usually endostomal (rarely bistomal or almost absent). Outer integument five to 14 cell layers thick. Inner integument three to six cell layers thick. Obturator (in Pyrodae funicular) present in some genera. Parietal tissue two to four cell layers thick. Archespore multicellular. Nucellar cap approx. four cell layers thick. Megagametophyte monosporous, Polygonum type. Antipodal cells sometimes proliferating. Endosperm development usually ab initio nuclear (in Lyonothamnus helobial). Endosperm haustorium chalazal (in Prunus). Embryogenesis usually asterad.
Fruit An assemblage of follicles, an achene, an achenetum (multiple achenes from a single flower), a coccetum (multiple carpels from a single flower, separating at maturity and each dehiscing along a pair of sutures), a drupe, a drupetum (multiple drupelets from a single flower), a nuculanium (similar to drupe or drupetum but mesocarp not fleshy), a polyprenous drupe, a pome (carpels adnate to hypanthium, with fleshy layer consisting of hypanthial and sometimes carpellary tissue, often with numerous sclereids, and with unlignified endocarp); in Lindleya a capsule; in Rosa a cynarrhodium (multiple achenes enclosed by an urceolate carnose hypanthium); in Fragaria multiple achenes on surface of fleshy swollen floral receptacle; in Rubus an assemblage of partially or entirely connate drupelets.
Seeds Aril absent. Seed coat usually mesotestal (sometimes reduced and indistinct). Testa occasionally winged. Exotestal cells unspecified, periclinally elongate, with thickened radial walls, or palisade or tabular; cell walls spiral or with reticulate thickenings; outer wall often eventually mucilaginous. Mesotesta often sclerotic. Endotesta? Exotegmen? Endotegmic cells somewhat thickened. Perisperm usually not developed. Endosperm usually sparse or absent (in, e.g., Physocarpus and Rhodotypos copious). Embryo straight or curved, usually without chlorophyll. Cotyledons two, fleshy or flattened. Germination phanerocotylar or cryptocotylar.
Cytology x = 9 (Rosoideae, Dryadoideae, Spiraeoideae pro parte); x = 7 (8) (Rosoideae, Spiraeoideae pro parte); x = (9, 15–)16–17 (Pyrinae); n = 27 (Lyonothamnus) – A special type of maternal inheritance present in the Rosa canina-group (2n = 28 + 7). Agamospermy (diplospory, apogamety, apospory, pseudogamy etc.) occurring in several genera (e.g. Alchemilla, Crataegus, Potentilla, Rubus, and Sorbus).
DNA Cis-spliced intron present in mitochondrial gene nad1. Ancient duplication of GBSSI gene (gene coding for granule-bound starch synthase) resulting in two copies in all diploid Rosaceae with x = 7, 8 or 9, and another duplication (due to paleopolyploidization) resulting in two additional copies in diploid taxa with x = 15 or 17 (i.e. GBSSI-1A, GBSSI-1B, GBSSI-2A, andGBSSI-2B in Pyreae).
Phytochemistry Flavonols (kaempferol, quercetin), afzelechin, flavanone glycosides (especially in Prunus), biflavonoids, cyanidin, epigallocatechin-3-gallate, phlorizin and other dihydrochalcones, dammaranes, triterpenes, cucurbitacins, methylated and non-methylated ellagic acids, gallic acid, hydrolyzable ellagitannins (casuaricitin, geraniins, pedunculagin, stachyurins, tellimagrandin I, tellimagrandin II), proanthocyanidins (prodelphinidins), chlorogenic acid, phenylalanine-, tyrosine- or leucine-derived cyanogenic glucosides (amygdalin, cardiospermin, dhurrin, heterodendrin, prunasin), triterpene saponins, arbutin, acetophenones, eleostearic acid, condensed tannins (hydrolyzable tannins rare), indole alkaloids, and ursolic acid present. Sorbitol often replacing saccharose as transport carbohydrate.
Use Ornamental plants, fruits, perfumes (Rosa), medicinal plants, timber, carpentries.
Systematics Rosaceae are sister-group to the remaining Rosales.
Rosoideae are sister to [Dryadoideae+Spiraeoideae].
Rosoideae Arn., Botany: 107. 9 Mar 1832 [’Roseae’]
28–31/2.080–>2.840. Subcosmopolitan, with their highest diversity in temperate regions on the Northern Hemisphere. Usually perennial (rarely annual) herbs or shrubs (rarely trees). Wood rays often narrow. Leaves alternate, usually compound (sometimes simple). Cuticular wax crystalloids as narrow riblets and triangular rodlets. Hypanthium absent or more or less developed. Epicalyx (possibly modified stipules associated with sepals) present in Potentilleae. Receptacle sometimes enlarged. Hypanthium free from the separate ovaries. Carpels usually numerous. Ovule one per carpel, usually anatropous (rarely orthotropous), epitropous, unitegmic. Fruit an achenetum or drupetum. x = 7 (8). Ellagic acid and 2-pyrone-4,6-dicarboxylic acid present. Cyanogenic glycosides and sorbitol absent.
Filipendula clade
1/12. Filipendula (12; Europe, temperate Asia, northeastern North America). – Herbs. Receptacle enlarged. Ovules two per carpel. – Filipendula is sister to the remaining Rosoideae.
Rosodae T. Eriksson, Smedmark et M. S. Kerr in D. Potter et al. in Plant Syst. Evol. 266: 36. 2007
27–30/2.070–>2.830. Subcosmopolitan, with their highest diversity in temperate regions on the Northern Hemisphere.
Roseae Lam. et DC., Syn. Plant. Fl. Gall.: 331. 30 Jun 1806 [‘Rosae’]
1/100–150. Rosa (100–150; temperate regions on the Northern Hemisphere, tropical mountains in Ethiopia, the Philippines and Mexico). – Prickly arching shrubs. Hypanthium carnose, urceolate. Ovules two per carpel, collateral. Integuments approx. eight cell layers thick.
Rubeae Dumort., Anal. Fam. Plant.: 39. 1829
1/>250(–700). Rubus (>250(–700). sexual species; cosmopolitan except polar regions, with their highest diversity in temperate regions on the Northern Hemisphere). – Prickly scrambling shrubs. Receptacle enlarged. Ovules two per carpel. Integuments approx six cell layers thick. Fruit a drupetum.
Sanguisorbeae DC., Prodr. 2: 588. Nov (med.) 1825
12/300–310. Agrimoniinae J. Presl in Nowočeská Bibl. [Wšobecný Rostl.] 7: 502. 1846 [‘Agrimonieae’]. Agrimonia (12–15; temperate regions on the Northern Hemisphere south to Central and southern Africa, West Malesia, Hispaniola and Brazil), Leucosidea (1; L. sericea; South Africa, Lesotho, Zimbabwe), Aremonia (1; A. agrimonioides; southern and southeastern Europe), Spenceria (1; S. ramalana; western China), Hagenia (1; H. abyssinica; Central African mountains, Sudan, Ethiopia to Zimbabwe). – Sanguisorbinae Torr. et A. Gray, Fl. N. Amer. 1: 428. Jun 1840 [‘Sanguisorbeae’]. Cliffortia (c 120; southern Africa to Angola and Kenya, with their highest diversity in the Cape Provinces), 'Acaena' (c 100; temperate and subtropical regions on the Southern Hemisphere including Antarctic Islands and Macquarie Island, one species, A. pinnatifida, in California, one possibly extinct species, A. exigua, on Puu Kukui on Maui in the Hawaiian Islands; paraphyletic; incl. Margyricarpus and Tetraglochin?), Margyricarpus (c 8; South America), Tetraglochin (8; Peru to southern Argentina and central Chile), Polylepis (c 20; the Andes), Poterium (13; Europe, Madeira, the Canary Islands, the Mediterranean, North Africa, temperate Asia), Poteridium(1; P. annuum; western United States, northwestern Mexico), Sanguisorba (c 15; temperate regions on the Northern Hemisphere). – Subcosmopolitan, few in tropical Asia and tropical America. Carpels one to five. Integuments six to eight cell layers thick.
Potentilleae Sweet, Brit. Fl. Gard. 2: 124. 1 Sep 1825 [‘Potentillae’]
11–13/>1.360. Potentillinae J. Presl, Wšobecný Rostl. 1: 491. 1846 [‘Potentilleae’]. Potentilla (c 250; temperate and arctic regions on the Northern Hemisphere, few species in temperate regions on the Southern Hemisphere). – Fragariinae Torr. et A. Gray, Fl. N. Amer. 1: 435. Jun 1840 [’Fragarieae’]. Argentina (c 65; Eurasia to New Guinea), Alchemilla (>1.000; temperate regions on the Northern Hemisphere, tropical mountains in the Old World, southeastern New South Wales, eastern Victoria, South America), Comarum (1; C. palustre; temperate regions on the Northern Hemisphere), Farinopsis (1; F. salesoviana; the Himalayas, Central Asia, Siberia, Mongolia, China), Sibbaldia (2–4; S. procumbens, S. retusa; arctic and alpine regions on the Northern Hemisphere), Sibbaldianthe (2; S. adpressa, S. bifurca; eastern Europe to Siberia and western China; in Sibbaldia?), ‘Sibbaldiopsis’ cuneifolia (Pakistan, Kashmir, northern India, Nepal, Bhutan, Sikkim, Xizang in China; in Sibbaldia?); Dasiphora (3; D. davurica, D. fruticosa, D. parvifolia; temperate regions on the Northern Hemisphere), Chamaerhodos (7; C. altaica, C. canescens, C. corymbosa, C. erecta, C. grandiflora, C. sabulosa, C. trifida; Central and East Asia, western North America), Drymocallis (3; D. arguta, D. glandulosa, D. rupestris; Europe, the Mediterranean, temperate Asia), Chamaecallis (1; C. perpusilloides; Afghanistan, the Himalayas, southwestern China, Burma); Fragaria (c 20; temperate regions on both hemispheres). – Temperate, arctic and alpine regions on the Northern Hemisphere, tropical mountains, temperate regions on the Southern Hemisphere. Epicalyx sometimes present. Receptacle enlarged. Anther thecae in Fragariinae confluent. Style in Potentillinae often lateral/gynobasic. Integuments approx. four cell layers thick. Parietal tissue in Fragariinae approx. two cell layers thick and nucellar cap approx. seven cell layers thick.
Colurieae Rydb., N. Amer. Fl. 22. 240. 12 Jun 1908
2–3/c 60. Geum (c 50; temperate regions on both hemispheres), Oncostylus (7; Tasmania, New Zealand and adjacent islands, subantarctic South America; in Geum?), Fallugia (1; F. paradoxa; southwestern United States, northwestern Mexico). – Temperate regions on both hemispheres, tropical mountains, Chile. Ovule in Geum apotropous.
[Dryadoideae+Spiraeoideae]
Sorbitol present as transport carbohydrate. Cyanogenic glycosides present.
Dryadoideae Sweet, Brit. Fl. Gard. 1: ad t. 43. Apr 1830 [‘Dryadeae’]
4/21. Dryas (2; D. integrifolia: eastern Siberia, Greenland, northern and western North America; D. octopetala: arctic and alpine regions from eastern Greenland through Europe, the Caucasus and northern Asia to western North America), Cercocarpus (9; western United States, northwestern Mexico), Chamaebatia (2; C. australis: southern California, northern Baja California in northwestern Mexico; C. foliolosa: mountains in California), Purshia (8; southwestern Canada, western United States, northwestern Mexico). – Temperate, arctic and alpine regions on the Northern Hemisphere. Shrubs, shrublets or small trees. Ectomycorrhiza sometimes present (at least in Cercocarpus). Symbiosis with root-dwelling nitrogen fixing Frankia. Leaves compound in Chamaebatia, otherwise simple. Stipules present. Hypanthium free from ovary. Carpels one to numerous. Pistil single (Cercocarpus, Chamaebatia, Purshia) or four to many (Dryas, Purshia). Carpel in Cercocarpus and Chamaebatia single. Placentation in Cercocarpus and Chamaebatia basal. Ovules usually orthotropous (in Dryas anatropous, apotropous). Obturator absent in Cercocarpus and Chamaebatia. Fruits achenes or achenetum with hairy styles. x = 9. Polyploidy not known. Cyanogenic glycosides present. Sorbitol present in trace amounts.
Spiraeoideae Arn., Botany: 107. 9 Mar 1832 [‘Spiraeeae’]
45/1.040–>1.330. Usually shrubs or trees. Leaves usually alternate, simple. Stipules usually present. Cuticular wax crystalloids as tubules or platelets. Hypanthium usually free from ovary/ovaries. Carpels one to five, antepetalous, usually free. Stigma usually Wet type. Ovules two or more per carpel. Papillate funicular obturator present. Fruit a follicetum, an achene, an achenetum, a coccetum, a drupe, a drupetum, a polyprenous drupe, a nuculanium, or a pome (in Pyrinae). x = 8, 9, 15, 17. Flavones, cyanogenic glycosides and sorbitol (often abundant) present. Ellagic acid not found.
Lyonothamneae Brouillet in J. Bot. Res. Inst. Texas 2: 385. 24 Jul 2008
1/1. Lyonothamnus (1; L. floribundus; Channel Islands off southern California). – Leaves opposite, compound. Stipules deciduous. Half epigyny. Placentation apical. Ovules four to six per carpel, apical, epitropous. Cyanogenic glycosides not found. – Relictual endemic. Three fossil species of Lyonothamnus have been described and the group had a significantly larger distribution during the Neogene (Erwin & Shorn 2000).
Amygdaleae DC., Prodr. 2: 529. Nov (med.) 1825
1/200–430. Prunus (200–430; temperate regions on both hemispheres, tropical mountains). – Usually bisexual (dioecious in ‘Maddenia clade’) Ectomycorrhiza often present. Phellogen superficial. Tracheids absent. Leaves with vertically or laterally conduplicate ptyxis. Extrafloral nectaries present on petiole or abaxial side of lamina. Petals absent in ‘Maddenia clade’. Carpel single. Ovules two per carpel, hemianatropous, unitegmic. Outer integument six to eight cell layers thick. Inner integument three to six cell layers thick. Obturator developed from ovary wall. Fruit a drupe. Seed coat usually pachychalazal. x = 8.
Neillieae Maxim. in Trudy Imp. S.-Peterburgsk. Bot. Sada 6: 164, 216. Jul-Aug 1879
2/25–32. Neillia (15–17; eastern Himalayas, China, mountains in West Malesia), Physocarpus (10–15; northeastern Asia, eastern and western North America). – Eastern Himalayas and China to northeastern Asia, mountains in West Malesia, North America. Placentation apical. Ovule one per carpel, apotropous (sometimes up to five per carpel, pleurotropous). Micropyle in Physocarpus bistomal. Fruitlets hard, shiny.
Sorbarieae Rydb., N. Amer. Fl. 22: 239, 256. 12 Jun 1908
4/13. Adenostoma (2; A. fasciculatum: western United States, northern Baja California in northwestern Mexico; A. sparsifolium: southern California, northern Baja California), Chamaebatiaria (1; C. millefolium; western United States), Sorbaria (c 9; temperate East Asia), Spiraeanthus (1; S. schrenkianus; Kazakhstan, Kyrgyzstan?). – Central to East Asia, western United States, northwestern Mexico. Leaves usually compound (in Adenostoma simple). Cuticular wax crystalloids in Adenostoma, Chamaebatiaria and Sorbaria transversely ridged, as rodlets. Carpel in Adenostoma single. Placentation apical. Ovules usually epitropous. Micropyle in Adenostoma, Chamaebatiaria and Sorbaria bistomal. Obturator (of different types) usually present. Fruit in Adenostoma an achene.
Spiraeeae DC., Prodr. 2: 541. Nov (med.) 1825 [‘Spiraeaceae’]
8/90–110. Aruncus (1; A. dioicus; temperate regions on the Northern Hemisphere), Holodiscus (1; H. discolor; western Canada, western United States, Mexico, Central America, Colombia), Kelseya (1; K. uniflora; Rocky Mountains in western United States), Eriogynia (1; E. pectinata; Rocky Mountains in northwestern North America), Petrophytum (3; P. caespitosum, P. cinerascens, P. hendersonii; Rocky Mountains in western North America), Sibiraea (4; S. angustata, S. laevigata, S. tianschanica, S. tomentosa; the Balkan Peninsula, Siberia, Central and East Asia), Spiraea (80–100; temperate regions on the Northern Hemisphere south to the Himalayas and Mexico), Xerospiraea (1; X. hartwegiana; Mexico). – Temperate regions on the Northern Hemisphere south to mountains in Colombia. Vestured pits present. Nodes 1:1 (unilacunar with one leaf trace). Stipules absent. Placentation in Spiraea, Holodiscus and Aruncus apical. Ovules six to eight per carpel, epitropous, unitegmic. Obturator present in Spiraea, Holodiscus and Aruncus. Fruit in Holodiscus an achene.
Kerriodae D. Potter, S. H. Oh et K. R. Robertson in D. Potter et al. in Plant Syst. Evol. 266: 38. 2007
7/10. Central to East Asia, western and southeastern North America, northwestern Mexico.
Exochordeae Schulze-Menz ex Reveal J. Bot. Res. Inst. Texas 4(1): 215. 29 Jul 2010 (Osmaronieae Rydb., N. Amer. Fl. 22: 240. 1918, nom. illeg.)
3/5. Oemleria (1; O. cerasiformis; southwestern Canada, western United States), Exochorda (1; E. racemosa; Central Asia to China), Prinsepia (3; P. sinensis, P. uniflora, P. utilis; the Himalayas to northern China and Taiwan). – Central Asia to the Himalayas and East Asia, western North America. Phellogen superficial. Medulla septated. Stipules caducous. Stylodia lateral. Obturator developed from ovary wall. Fruit a drupe or a septicidal capsule (in Exochorda also adaxially dehiscing). x = 8.
Kerrieae Focke in Engler et Prantl, Nat. Pflanzenfam. III, 3: 12, 27. Dec 1888
4/5. Coleogyne (1; C. ramosissima; southwestern United States, northwestern Mexico), Rhodotypos (1; R. scandens; China, the Korean Peninsula, Japan), Neviusia (3; N. alabamensis: southeastern United States; N. cliftonii: Shasta County in northern California), Kerria (1; K. japonica; temperate East Asia). – Temperate East Asia, southwestern and southeastern United States, northwestern Mexico. Carpels one to five. Megasporangium in Rhodotypos protruding. Fruit achenetum-like. n = 8 or 9 (Neviusia). Sorbitol and flavones present in Neviusia. Ellagic acid not found in Neviusia.
Pyrodae C. S. Campbell, R. C. Evans, D. R. Morgan et T. A. Dickinson in D. Potter et al., Plant Syst. Evol. 266: 39. 2007
22/700–>730. Ectomycorrhiza present. Phellogen superficial. Wood rays often narrow. Colleters present. Carpels (one or) two to five, antesepalous or median carpel abaxial, adnate to base of hypanthium, often connate. Gynoecial ring primordium present. Placentation basal (not when ovules numerous). Ovules (one or) two per carpel, anatropous, apotropous, bitegmic. Micropyle sometimes bistomal. Funicular obturator papillate. Exotestal cells thickened, with lignified walls, often mucilaginous. Mesotesta thick, sclerotic. x = (9, 15–)16–17. Flavone-C-glycosides present.
Gillenieae Maxim. in Trudy Imp. S.-Peterburgsk. Bot. Sada 6: 164, 222. Jul-Dec 1879
1/2. Gillenia (2; G. stipulata, G. trifoliata; southeastern United States). – Perennial herbs. Leaves compound. x = 9. – Gillenia is sister to the remaining Pyrodae.
Pyreae Baill., Hist. Plant. 1: 442, 475. Jan-Jul 1869
21/695–>725. x = 15, 17 (aneuploid reduction from x = 18). GBSSI present in four copies.
Kageneckia clade
2/4. Kageneckia (3; K. angustifolia, K. lanceolata, K. oblonga; Peru, Chile), Lindleya (1; L. mespiloides; Mexico). – Mexico, Peru. Chile. Ovules four to numerous per carpel, pleurotropous.
Vauquelinia clade
1/2. Vauquelinia (2; V. californica, V. corymbosa; southwestern United States, northwestern Mexico). – Tanniniferous cells pervasive. Capsule septicidal; carpels dehiscing adaxially (and partially abaxially).
Pyrinae Dumort., Fl. Belg.: 92. 1827 [‘Pyreae’]
18/690–>720. Dichotomanthes (1; D. tristaniicarpa; southwestern China), Eriobotrya (11–15; the Himalayas, East Asia, mountains in West Malesia), Rhaphiolepis (5; R. ferruginea, R. indica, R. lanceolata, R. major, R. salicifolia; East and Southeast Asia), Sorbus (130–140; temperate regions on the Northern Hemisphere), Amelanchier (c 20; temperate regions on the Northern Hemisphere south to Mexico and Guatemala), Pyrus (15–20; Europe, the Mediterranean, temperate Asia), Aronia (c 40; the Himalayas to Japan and Sumatra, eastern North America, Central America), Malus (c 40; temperate regions on the Northern Hemisphere), Cydonia (1; C. oblonga, the Caucasus, Kurdistan), Docynia (1; D. indica; eastern Himalayas, southern China, northern Thailand), Pseudocydonia (1; P. sinensis; China), Chaenomeles (3; C. cathayensis, C. japonica, C. speciosa; China, the Korean Peninsula, Japan), Cotoneaster (>260; temperate regions in the Old World), Pyracantha (7; P. coccinea: northeastern Spain to northern Iran; P. crenulata: the Himalayas; P. angustifolia, P. atalantioides, P. crenatoserrata, P. rogersiana: southwestern to central China; P. koidzumii: Taiwan), Crataegus (140–150; temperate regions on the Northern Hemisphere), Osteomeles (1–3; O. anthyllidifolia, O. schwerinae, O. subrotunda; China, the Ryukyu islands, Taiwan, the Cook Islands, Tonga, Pitcairn Island, Rapa Iti, the Hawaiian Islands), Hesperomeles (11; Central America, Colombia to Peru and Bolivia), Chamaemeles (1; C. coriacea; Madeira). – Temperate, alpine and arctic regions on the Northern Hemisphere and southwards to mountains in Sumatra and Bolivia. Leaves usually simple (in Osteomeles and species of Sorbus compound). Stipules deciduous. Usually half epigyny (in Dichotomanthes hypogyny). Carpels sometimes laterally free (in Dichotomanthes single). Outer integument five to 14 cell layers thick. Inner integument three to six cell layers thick. Hypantium carnose in fruit. Endocarp usually absent.
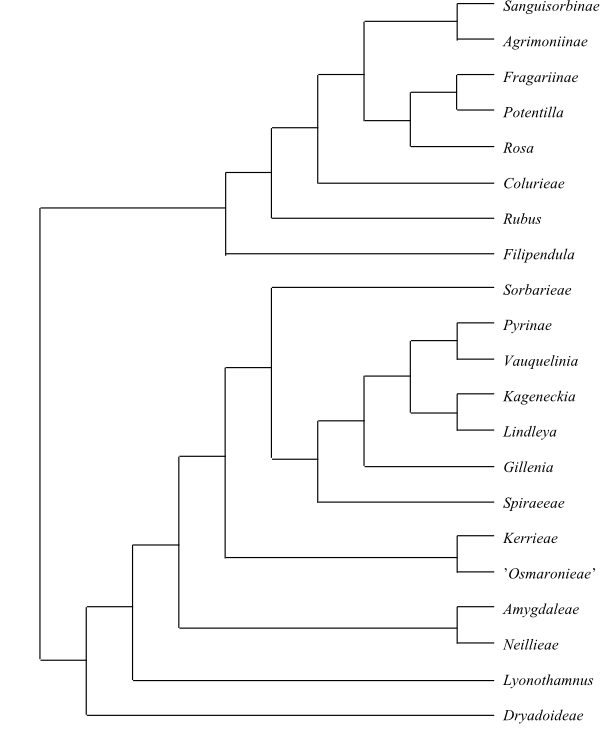 |
Cladogram (simplified) of Rosaceae based on DNA sequence data (Potter & al. 2007). |
ULMACEAE Mirb. |
( Back to Rosales ) |
Ulmales Link, Handbuch 2: 445. 4-11 Jul 1829 [‘Ulmaceae’]
Genera/species 7/52–57
Distribution Temperate regions in North America and Eurasia, tropical Africa, India, Central and northern South America, with their highest diversity in temperate regions.
Fossils Leaves, pollen grains and fruits of Ulmaceae are abundant in Cenozoic layers from the Paleocene onwards in the Northern Hemisphere. Cedrelospermum, consisting of fossilized samaras, has been described from the Eocene of North America and from the Late Eocene to mid-Miocene of Europe. Late Cretaceous records of Ulmaceae are questioned.
Habit Bisexual, monoecious, andromonoecious or polygamomonoecious, evergreen or deciduous trees or shrubs. Prophylls usually basal.
Vegetative anatomy Ectomycorrhiza often present. Phellogen ab initio superficial. Vessel elements usually with simple (sometimes scalariform) perforation plates; lateral pits alternate, simple or bordered pits. Imperforate tracheary xylem elements sometimes very long libriform fibres usually with simple (sometimes bordered) pits, septate (also vasicentric tracheids). Wood rays uniseriate to multiseriate, usually homocellular. Axial parenchyma usually paratracheal scanty, aliform, winged-aliform, confluent, vasicentric, or banded (sometimes apotracheal diffuse). Wood sometimes fluorescent. Wood elements often storied. Tyloses frequent. Secondary phloem stratified into hard fibrous and soft parenchymatous non-fibrous layers. Sieve tube plastids usually S type (in Ulmus Pc type with protein crystalloids); sieve tube elements with extruded nucleoli and non-dispersive protein bodies. Nodes 3:3, trilacunar with three leaf traces. Laticifers and latex absent. Heartwood sometimes with gum-like substances. Mucilaginous idioblasts and canals present. Cystoliths globose (calciumcarbonate?), usually without pegs (cellulose expansions of cell wall, covered by crystals; in Hemiptelea with pegs). Epidermal cell walls often with calciumcarbonate or silica. Prismatic calciumoxalate crystals frequent.
Trichomes Hairs usually rigid, unicellular (not micropapillar) or multicellular, simple, often with swollen base or calcified/silicified (hairs in Ampelocera smooth); glandular hairs?
Leaves Usually alternate (usually distichous; rarely opposite), simple, entire, usually with laterally (rarely vertically) conduplicate-plicate ptyxis. Stipules usually lateral and non-axillary, usually extrapetiolar (one of two stipules in Ulmus intrapetiolar), usually caducous; leaf sheath absent. Petiole vascular bundle transection? Leaf base often asymmetrical. Venation pinnate, usually craspedodromous; secondary and tertiary veins usually proceeding into leaf teeth (urticoid teeth; not in Ampelocera). Stomata anomocytic or paracytic. Cuticular wax crystalloids? Domatia as pockets or hair tufts, or absent. Epidermis and mesophyll often with mucilaginous idioblasts. Leaf margin usually coarsely serrate (rarely entire). Leaf usually with rigid unicellular hairs with swollen base, or glabrous.
Inflorescence Axillary, usually cymose (often fascicle; rarely racemose, or flowers solitary).
Flowers Actinomorphic, small to very small. Hypanthium present. Hypogyny. Sepals (three to) five (to nine), usually with imbricate (sometimes valvate) aestivation, usually spiral, persistent, free or partially connate. Petals absent. Nectary absent. Disc absent.
Androecium Stamens (four or) five (rarely eight, ten, or twelve), antesepalous. Filaments filiform, usually free, often adnate to sepal bases. Anthers dorsifixed, often versatile, tetrasporangiate, extrorse, longicidal (dehiscing by longitudinal slits). Tapetum secretory, with binucleate to quadrinucleate cells. Female flowers sometimes with staminodia.
Pollen grains Microsporogenesis simultaneous. Pollen grains 4–5(–7)-stephanoporate, shed as monads, usually bicellular (in Ulmus tricellular) at dispersal. Exine tectate, with granular infratectum, rugulate, spinulate.
Gynoecium Pistil composed of two connate carpels. Ovary superior, usually unilocular (due to pseudomonomery; in Ulmus bilocular). Stylodia usually two (in some species of Zelkova sometimes one). Stigmas decurrent, ventral, papillate, Dry type. Male flowers often with pistillodium.
Ovules Placentation subapical. Ovule one per ovary, usually anatropous (sometimes orthotropous), pendulous, epitropous, bitegmic, crassinucellar (to tenuinucellar?). Micropyle usually bistomal (in Ulmus endostomal), not Z-shaped. Outer integument four to six cell layers thick. Inner integument approx. four cell layers thick. Hypostase present. Megagametophyte usually monosporous, Polygonum type (in Ulmus tetrasporous, usually Drusa type, rarely Adoxa type). Antipodal cells sometimes proliferating (forming up to ten cells in Holoptelea). Endosperm development ab initio nuclear. Endosperm haustoria? Embryogenesis solanad (Ulmus) or onagrad (Holoptelea).
Fruit A samara (in Ulmus with reticulately veined wings; in Planera with coriaceous appendages; in Zelkova an unwinged nut).
Seeds Aril absent. Seed flattened. Testa thin, indistinct. Exotestal cells elongate, usually not thickened (in Holoptelea with thickened walls). Endotesta? Tegmen? Perisperm not developed. Endosperm sparse or absent. Embryo straight (in Zelkova curved), well differentiated, without chlorophyll. Cotyledons two, flattened or longitudinally folded. Germination phanerocotylar.
Cytology n = 14, 28, (30) 42 (x = 14) – Polyploidy occurring. Chromosomes often with terminal or subterminal diffuse and complex centromers.
DNA Trans-spliced intron present in mitochondrial gene nad1. Deletion of 69 bp in plastid gene ndhF.
Phytochemistry Flavonols (kaempferol, quercetin, myricetin) and their glycosides, cyanidin, proanthocyanidins (prodelphinidins), alkaloids (in Ulmus), sesquiterpene lactones, and lignans present. Saponins sometimes present? Ellagic acid and cyanogenic compounds not found. Raffinose and stachyose present in phloem exudates. Aluminium accumulated in some species.
Use Ornamental plants, timber, carpentries.
Systematics Hemiptelea (1; H. davidii; northern China, the Korean Peninsula), Holoptelea (2; H. grandis: western, central and southwestern tropical Africa; H. integrifolia: India), Phyllostylon (3; P. brasiliense, P. orthopterum, P. rhamnoides; tropical South America), Planera (1; P. aquatica; southeastern United States), Ulmus (25–30; temperate regions on the Northern Hemisphere and southwards to northern Mexico), Zelkova (6; Z. abelicea: Crete; Z. carpinifolia: eastern Turkey, the Caucasus, northern Iran; Z. serrata: China, the Korean Peninsula, Japan, Kuril Islands, Taiwan; Z. sicula: Sicily; Z. schneideriana, Z. sinica: China), Ampelocera (14; Mexico, Central America, tropical South America to Brazil).
Ulmaceae are sister-group to the clade [Cannabaceae+[Moraceae+Urticaceae]].
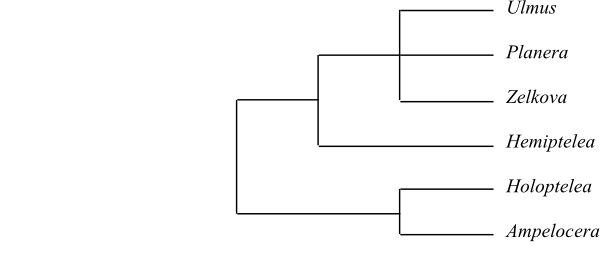 |
Cladogram of Ulmaceae based on plastid DNA restriction site analysis (Wiegrefe & al. 1998). Morphological analyses sometimes recover Ampelocera within Cannabaceae (Zavada & Kim 1996). |
URTICACEAE Juss. |
( Back to Rosales ) |
Parietariaceae Bercht. et J. Presl, Přir. Rostlin: 261. Jan-Apr 1820 [’Parietariae’]; Urticales Juss. ex Bercht. et J. Presl, Přir. Rostlin: 260. Jan-Apr 1820 [‘Urticeae’]; Urticopsida Bartl., Ord. Nat. Plant: 91, 102. Sep 1830 [’Urticinae’]; Cecropiaceae C. C. Berg in Taxon 27: 39, 23 Mar 1978; Urticanae Takht. ex Reveal in Novon 2: 237. 13 Oct 1992
Genera/species 48–49/1.590–>1.700
Distribution Cosmopolitan except polar areas, with their largest diversity in tropical Asia.
Fossils Leaves and achenes of Urticaceae are known from the Cenozoic. Many Late Cretaceous fossils have been attributed to the Urticaceae (from Central Europe etc.), although none of them is unambiguous.
Habit Usually monoecious, polygamomonoecious or dioecious (rarely bisexual), usually evergreen (sometimes deciduous) trees, shrubs, lianas or suffrutices, perennial or annual herbs (in Coussapoa and Poikilospermum usually epiphytes). Young stems and branches often quadrangular in cross-section. Some arborescent species have buttresses. Cecropia often live in symbiosis with ants.
Vegetative anatomy Phellogen ab initio usually superficial (in, e.g., Urtica cortical). Primary medullary rays often narrow, alternating with wide ones. Vessel elements with simple perforation plates; lateral pits alternate, usually simple (sometimes bordered) pits. Imperforate tracheary xylem elements very long and strong libriform fibres, usually with simple (sometimes bordered) pits, usually septate. Wood rays usually multiseriate (sometimes uniseriate), usually heterocellular (sometimes homocellular). Axial parenchyma usually paratracheal scanty, aliform, lozenge-aliform, winged-aliform, confluent, vasicentric, or banded. Wood sometimes fluorescent. Wood elements usually storied. Tyloses often abundant. Secondary phloem stratified into hard fibrous and soft parenchymatous non-fibrous layers. Sieve tube plastids usually Ss type, with one to three starch grains, or S0 type (cell nuclei in Laportea with non-dispersive P-protein bodies); sieve tube elements with extruded nucleoli and non-dispersive protein bodies. Nodes usually 3:3, trilacunar with three leaf traces (sometimes bilacunar or pentalacunar?). Laticifers and latex cells usually absent (rarely present in bark); latex usually absent (white latex rarely present). Cystoliths usually present (absent in, e.g., Cecropia), globose (calciumcarbonate?) or elongate (with pegs?). Prismatic calciumoxalate crystals abundant; druses sometimes present; raphides present in Dendrocnide.
Trichomes Hairs unicellular (usually micropapillate) or multicellular, simple, bristle-like and non-stinging, often curled, arachnoid or hooked, or stinging (in Urticeae with, e.g., 5-hydroxytryptamine); pearl glands rarely present.
Leaves Alternate (spiral or distichous) or opposite, simple, entire or (sometimes deeply) trilobate to septalobate, sometimes anisophyllous (in some species of Pilea peltate), with laterally or vertically conduplicate ptyxis. Stipules lateral, intrapetiolar or interpetiolar, often sheathingly connate or branch-enclosing, in Cecropia and Musanga very large; absent in several genera of Parietarieae); leaf sheath absent. Petiole vascular bundle transection arcuate or annular. Leaf base often asymmetrical. Venation pinnate or palmate; secondary and tertiary veins proceeding into non-glandular teeth (urticoid teeth). Stomata usually anomocytic or anisocytic (sometimes helicocytic or paracytic). Cuticular wax crystalloids? Epidermal cells often with elongate to punctiform cystoliths (cystoliths sometimes absent); epidermal cell walls often with calciumcarbonate or silica. Mesophyll usually with mucilaginous idioblasts and ducts, often with calciumoxalate druses. Leaf margin serrate, sinuate or entire.
Inflorescence Axillary, panicle, spicate, capitate, umbellate or catkin- or spadix-like cymose (flowers rarely solitary), often with male inflorescence raceme- or catkin-like and female inflorescence capitate, often with involucroid bracts. Inflorescence axis in Elatostemateae flattened, discoid and fleshy. Inflorescence sometimes composed of spikes and surrounded by large involucroid caducous bract. Prophylls usually basal.
Flowers Usually actinomorphic (female flowers often zygomorphic), small to minute. Pedicel often articulated. Usually hypogyny (rarely half epigyny). Sepals in male flowers (one to) four or five (or six), with valvate aestivation, persistent, free or connate below (rarely entirely connate); in female flowers three to five, valvate, often unequal in size, often accrescent in fruit, free or entirely or almost entirely connate (rarely rudimentary or absent). Petals absent. Nectary absent. Disc absent.
Androecium Stamens (one to) four or five (or six), antesepalous, usually as many as sepals. Filaments usually inflexed in bud and at anthesis recurved with lightning rapidity (in Cecropia etc. usually erect), usually free from each other, free from tepals. Anthers basifixed to dorsifixed, usually non-versatile, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits), usually explosively dehiscent. Tapetum secretory. Female often with staminodia, sometimes scale-like (in Pilea and other Elatostemateae often enhancing fruit dispersal by discharging ripe achenes through instantaneous straightening of staminodial filaments).
Pollen grains Microsporogenesis simultaneous. Pollen grains (1–)2–6(–15)-porate, shed as monads, bicellular at dispersal. Exine tectate, with granular infratectum, spinulate.
Gynoecium Pistil composed of two connate carpels (one of which entirely or almost entirely degenerating and sterile). Ovary usually superior, unilocular (pseudomonomery), often oblique or asymmetrical. Style single, simple, short, or absent. Stigma capitate, penicillate, ligulate or filiform (rarely peltate), papillate, Dry type. Male flowers usually with pistillodium.
Ovules Placentation basal or subbasal. Ovule one per carpel, anatropous, hemianatropous or orthotropous, ascending to upright, epitropous, bitegmic, crassinucellar. Funicle present or absent. Micropyle usually endostomal (in Parietaria bistomal). Outer integument approx. two cell layers thick. Inner integument approx. two cell layers thick, sometimes obturator. Hypostase present in at least Urtica. Parietal tissue four to six cell layers thick. Nucellar cap two to four cell layers thick. Megagametophyte monosporous, Polygonum type. Antipodal cells sometimes proliferating. Endosperm development ab initio nuclear. Endosperm haustorium chalazal (at least in Urtica). Embryogenesis asterad.
Fruit Usually a nutlet or an achene (rarely a drupe), often surrounded by (sometimes adnate to) persistent and accrescent (sometimes carnose) calyx; often a syncarp consisting of connate ovaries from several flowers.
Seeds Aril absent. Testa thin, crushed. Exotesta usually perforated. Some testal and tegmic layers persistent. Perisperm not developed. Endosperm copious to sparse, often oily or proteinaceous (sometimes starchy), or absent. Embryo small, straight, well differentiated, without chlorophyll. Cotyledons two, usually flattened (sometimes thick). Germination? Adventitious embryony and polyembryony occurring in Elatostema.
Cytology x = 6–14, 16, 19, 21 – Polyploidy frequently occurring. Centromers median and subterminal? Protein bodies present in nucleus.
DNA Trans-spliced intron present in mitochondrial gene nad1. Mitochondrial coxI intron present in Pilea.
Phytochemistry Flavonols (kaempferol, quercetin), flavone-C-glycosides, cyanidin, alkaloids, caffeic acid derivatives, polyacetate-derived arthroquinones, and furanocoumarins present. Ellagic acid, saponins and cyanogenic compounds not found.
Use Ornamental plants, textile plants (fibres of Boehmeria nivea, Girardinia, Laportea, Urtica, etc.), medicinal plants (Parietaria etc.), fruits (Cecropia, Pourouma), vegetables, timber.
Systematics Urticaceae are sister to Moraceae.
Cecropieae are sister-group to the clade [Urticeae+Elatostemateae] (Hadiah & al. 2008).
Cecropieae Dumort., Anal. Fam. Plant.: 17. 1829
29/435–485. Leucosyke (c 50; Malesia to Polynesia), Coussapoa (50–55; tropical South America), Cecropia (c 60; southern Mexico, Central America, the West Indies, tropical South America), Musanga (2; M. cecropioides, M. leo-errerae; tropical Africa), Myrianthus (4–7; M. arboreus, M. holstii, M. preussii, M. serratus; tropical Africa), Pourouma (25–30; Central America, tropical South America); ‘Parietaria’ (c 20; tropical to temperate regions on both hemispheres; paraphyletic; incl. Gesnouinia?, Soleirolia?), Gesnouinia (1; G. arborea; the Canary Islands; in Parietaria?), Soleirolia (1; S. soleirolii; Italy, islands in western Mediterranean; in Parietaria?); Forsskaolea (6; F. angustifolia, F. candida, F. hereroensis, F. procridifolia, F. tenacissima, F. viridis; southeastern Spain, the Canary Islands, the Cape Verde Islands, Africa, the Arabian Peninsula, India), Didymodoxa (3; D. caffra, D. capensis, D. catira; southern and eastern tropical Africa to northern Ethiopia), Droguetia (7; D. ambigua, D. debilis, D. gaudichaudiana, D. hildebrandtii, D. humbertii, D. iners, D. leptostachys; tropical to subtropical Africa, northeastern India to Yunnan, Taiwan and Java; incl. Australina?), Australina (2; A. flaccida, A. pusilla; Ethiopia, Kenya, southeastern Australia, New Zealand; in Droguetia?); Oreocnide (c 18; China, Japan, tropical Asia to New Guinea); Phenax (14; tropical America); Chamabainia (1; C. cuspidata; tropical Asia, Taiwan), Gonostegia (5; G. hirta, G. parvifolia, G. pentandra, G. quinquenervis, G. triandra; southern China, Southeast Asia to northern Australia), ‘Pouzolzia’ (c 35; tropical regions on both hemispheres; non-monophyletic), Neodistemon (1; N. indicum; tropical Asia), Hemistylus (4; H. boehmerioides, H. brasiliensis, H. macrostachya, H. odontophylla; tropical South America), Rousselia (2; R. guilhoti, R. humilis; Central America, the West Indies, Colombia), Pipturus (35–40; the Mascarene Islands, tropical Asia to New Guinea, Northern Territory and Queensland, Melanesia, Polynesia incl. the Hawaiian Islands), Neraudia (5; N. angulata, N. kauaiensis, N. melastomifolia, N. ovata, N. sericea; the Hawaiian Islands); ’Boehmeria’ (65–80; tropical regions on both hemispheres, subtropical regions on the Northern Hemisphere; polyphyletic), Debregeasia (3; D. longifolia, D. orientalis, D. saeneb; Ethiopia, tropical and subtropical Asia), Astrothalamus (1; A. reticulatus; West and Central Malesia), Archiboehmeria (1; A. atrata; Southeast Asia, southern China), Sarcochlamys (1; S. pulcherrima; tropical Asia), ’Cypholophus’ (15–30; Malesia to New Guinea and islands in western Pacific, Taiwan; polyphyletic). – Warm-temperate to tropical regions on both hemispheres.
[Urticeae+Elatostemateae]
Urticeae Lam. et DC., Syn. Plant. Fl. Gall.: 184. 30 Jun 1806
10/205–210. Nanocnide (2; N. japonica, N. lobata; China, northern Vietnam, the Korean Peninsula, Japan, Taiwan), Zhengyia (1; Z. shennongensis; Hubei province in central China), Urtica (c 80; nearly cosmopolitan), Dendrocnide (37; tropical Asia to New Guinea, eastern Queensland, eastern New South Wales and islands in the Pacific), Discocnide (1; D. mexicanus; Mexico, Guatemala), Girardinia (3; G. bullosa, G. diversifolia, G. palmata; East and Northeast Africa, tropical and subtropical regions in Africa and Asia); ‘Laportea’ (22; tropical and subtropical regions on both hemispheres, East Asia, eastern North America; diphyletic), ‘Urera’ (c 35; tropical and southern Africa, Madagascar, the Hawaiian Islands, tropical America; non-monophyletic; incl. Obetia?, Poikilospermum?), Obetia (8; tropical and southern Africa, Madagascar, the Mascarene Islands; in Urera?), Poikilospermum (c 20; eastern Himalayas, Southeast Asia, Malesia; in Urera?). – Subcosmopolitan. Stinging hairs are a synapomorphy of Urticeae. – Laportea is diphyletic, according to Deng & al. (2013). Zhengyia is sister-group to Urtica. The clade [Urtica pilulifera + U. neubaueri] is sister to all other species of Urtica. Urera is paraphyletic.
Elatostemateae Gaudich. in H. L. C. de Saulces de Freycinet, Voy. Uranie, Bot.: 493. 6 Mar 1830 [’Elatostemeae’]
7–8/950–>1.000. ’Elatostema’ (c 300; tropical regions in the Old World, one species, E. rugosum, in New Zealand; non-monophyletic; incl. Procris?), Procris (c 15; tropical regions in the Old World; in Elatostema?); Gyrotaenia (5; G. crassifolia, G. microcarpa, G. myriocarpa, G. spicata, G. trujilloana; the West Indies), Myriocarpa (18; southern Mexico, Central America, tropical South America), Meniscogyne (1; M. thorelii; Laos, Vietnam), Lecanthus (3; L. peduncularis, L. petelotii, L. pileoides; tropical regions in the Old World), Petelotiella (1; P. tonkinensis; Indochina), Pilea (600–>700; tropical and subtropical regions on both hemispheres). – Pantropical. – Water-storing hydrenchyma present in succulent species of Pilea.
Unplaced Urticaceae
Haroldiella (2; H. rapaensis, H. sykesii; French Polynesia, Rapa Island), Metatrophis (1; M. margaretae; Rapa Island).
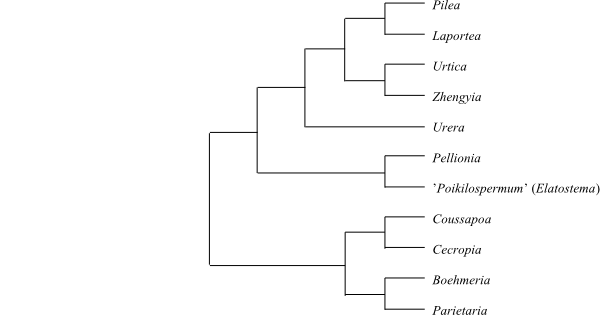 |
Cladogram of Urticaceae based on DNA sequence data (Sytsma & al. 2002). |
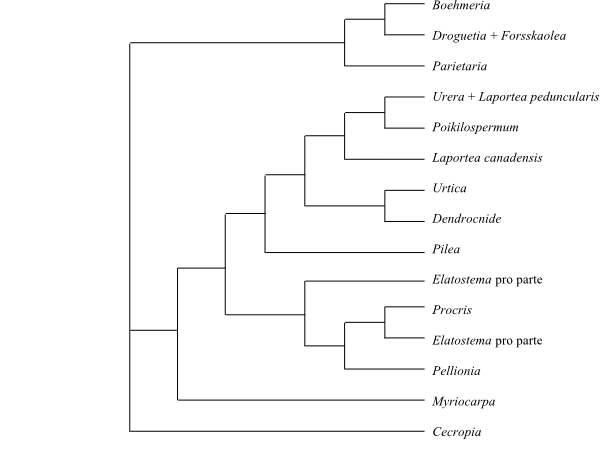 |
Cladogram (simplified) of Urticaceae based on DNA sequence data (Hadiah & al. 2008). |
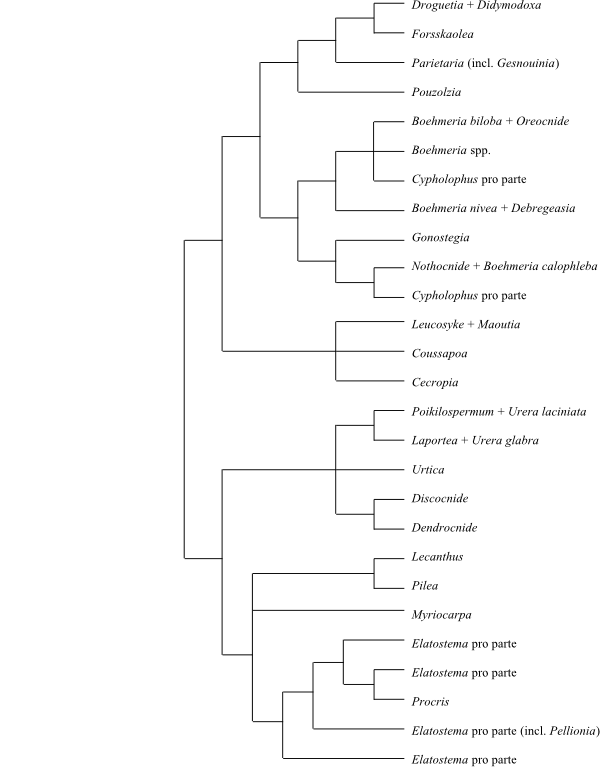 |
Cladogram (simplified) of Urticaceae based on trnL/F sequence data (Hadiah & al. 2008). Girardinia is sister to Dendrocnide, according to Deng & al. (2013). |
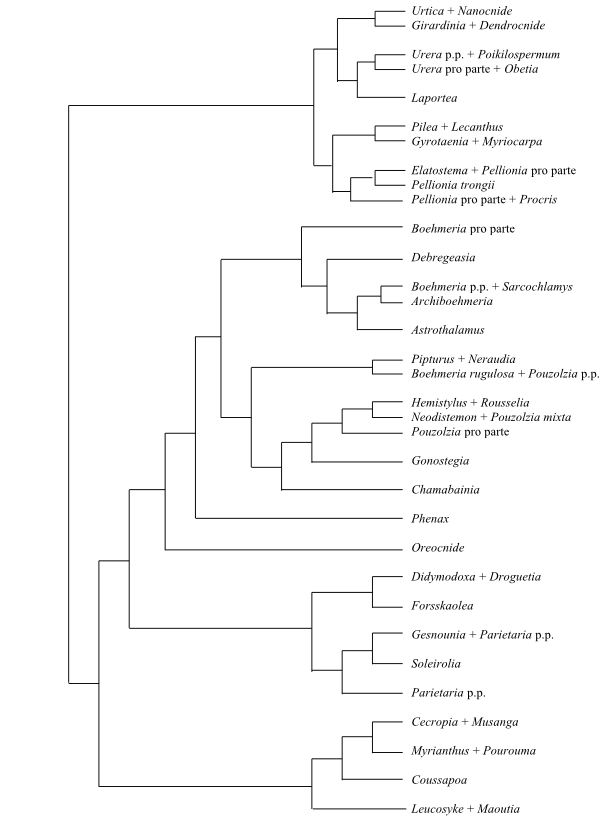 |
Phylogeny (simplified) of Urticaceae based on DNA sequence data (Wu & al. 2013, Treiber & al. 2016) |
Literature
Aagesen L. 1999. Phylogeny of the tribe Colletieae, Rhamnaceae. – Bot. J. Linn. Soc. 131: 1-43.
Aagesen L. 2004. The information content of an ambiguously alignable region, a case study of the trnL intron from the Rhamnaceae. – Organisms Divers. Evol. 4: 35-49.
Aagesen L, Medan D, Kellermann J, Hilger HH. 2005. Phylogeny of the tribe Colletieae (Rhamnaceae) – a sensitivity analysis of the plastid region trnL-trnF combined with morphology. – Plant Syst. Evol. 250: 197-214.
Acosta JM, Salariato DL, Cialdella AM. 2016. Molecular phylogeny and morphological analysis of Tetraglochin (Rosaceae: Rosoideae: Sanguisorbeae) and recognition of the new species T. andina. – Syst. Bot. 41: 839-850.
Ahmed B, Al-Rehaily AJ, Mossa JS. 2002. Barbeyol: a new phenolic indane type component from Barbeya oleoides. – Zeitschr. Naturforsch., Sekt. C., 57: 17-20.
Ahmed S, Compton SG, Butlin RK, Gilmartin PM. 2009. Wind-borne insects mediate directional pollen transfer between desert fig trees 160 kilometers apart. – Proc. Natl. Acad. Sci. U.S.A. 106: 20342-20347.
Akkermans ADL, Roelofsen W, Blom J, Huss-Danell K, Harkink R. 1983. Utilization of carbon and nitrogen compounds by Frankia in synthetic media and in root nodules of Alnus glutinosa, Hippophae rhamnoides, and Datisca cannabina. – Can. J. Bot. 61: 2793-2800.
Akkermans ADL, Hafeez F, Roelofsen W, Chaudhary AH, Baas R. 1983. Ultrastructure and nitrogenase activity of Frankia grown in pure culture and in actinorrhizae of Alnus, Colletia and Datisca. – In: Veeger C, Newton WE (eds), Advances in nitrogen fixation research, Nijhoff/Dr. W. Junk, The Hague, pp. 311-319.
Alaniya M, Kavtaradze NS, Skhirtladze A, Pizza C, Piacente S. 2010. Flavonol glycoside from Humulus lupulus. – Chem. Nat. Comp. 46: 641-642.
Albarouki E, Peterson A. 2007. Molecular and morphological characterization of Crataegus L. species (Rosaceae) in southern Syria. – Bot. J. Linn. Soc. 153: 255-263.
Albornoz P, Arias M, Castagnaro A, Díaz Ricci JC. 2007. Comparative root anatomy of Duchesnea indica, Fragaria vesca and Potentilla tucumanensis (Rosaceae) in Tucumán province, Argentina. – Adansonia, sér. III, 29: 255-267.
Aldasoro JJ, Aedo C, Navarro C, Garmendia FM. 1998. The genus Sorbus (Maloideae, Rosaceae) in Europe and in North Africa: morphological analysis and systematics. – Syst. Bot. 23: 189-212.
Aldasoro JJ, Aedo C, Garmendia FM, Pando de la Hoz F, Navarro C. 2004. Revision of Sorbus subgenera Aria and Torminaria (Maloideae – Rosaceae). – Syst. Bot. Monogr. 69: 1-148.
Aldasoro JJ, Aedo C, Navarro C. 2005. Phylogenetic and phytogeographical relationships in Maloideae (Rosaceae) based on morphological and anatomical characters. – Blumea 50: 3-32.
Alice LA. 1997. Molecular phylogenetic systematics of Rubus (Rosaceae). – Ph.D. diss., University of Maine, Orono, Maine.
Alice LA, Campbell CS. 1999. Phylogeny of Rubus based on nuclear ribosomal DNA internal transcribed spacer region sequences. – Amer. J. Bot. 86: 81-97.
Alice LA, Eriksson T, Eriksen B, Campbell CS. 2001. Hybridization and gene flow between distantly related species of Rubus (Rosaceae): evidence from nuclear ribosomal DNA internal transcribed spacer sequences. – Syst. Bot. 26: 769-778.
Allen GA. 1986. Flowering pattern and fruit production in the dioecious shrub Oemleria cerasiformis (Rosaceae). – Can. J. Bot. 64: 1216-1220.
Anderson LC. 1974. A study of systematic wood anatomy in Cannabis. – Bot. Mus. Leafl. Harv. Univ. 24: 29-36.
Antos JA, Allen GA. 1990. Habitat relationships of the Pacific coast shrub Oemleria cerasiformis (Rosaceae). – Madroño 37: 249-260.
Appleby CA, Tjepkema JD, Trinick MJ. 1983. Hemoglobin in a nonleguminous plant, Parasponia: possible genetic origin and function in nitrogen fixation. – Science 220: 951-953.
Arohonka T, Rousi A. 1980. Karyotypes and C-bands in Shepherdia and Elaeagnus. – Ann. Bot. Fenn. 17: 258-263.
Aronne G, Wilcock CC. 1995. Reproductive lability in predispersal biology of Rhamnus alaternus L. (Rhamnaceae). – Protoplasma 187: 49-59.
Arora N. 1953. The embryology of Zizyphus rotundifolia Lamk. – Phytomorphology 3: 88-98.
Arruda VLV de, Sazima M. 1988. Polinização e reprodução de Celtis iguanaea (Jacq.) Sarg. (Ulmaceae), una espécie anemófila. – Rev. Bras. Bot. 11: 113-122.
Avdeyev VI. 1983. Novaya taxonomiya roda oblepikha: Hippophae L. – Izvestiya Akademii Nauk Tadzhikskoy SSR, Otdeleniye Biologicheskih Nauk 93: 11-17.
Aweke g. 1979. Revision of the genus Ficus L. (Moraceae) in Ethiopia. – Meded. Landbouwhog. Wageningen 79(3): 1-115.
Baas P, Jansen S, Smets E. 2001. Vegetative anatomy and affinities of Dirachma socotrana (Dirachmaceae). – Syst. Bot. 26: 231-241.
Badenes ML, Parfitt DE. 1995. Phylogenetic relationships of cultivated Prunus from an analysis of chloroplast DNA variation. – Theor. Appl. Gen. 90: 1035-1041.
Bailey LH. 1941-1945. The genus Rubus in North America. – Gentes Herb. 5.
Baker HG, Baker I. 1967. The cytotaxonomy of Filipendula (Rosaceae) and its implications. – Amer. J. Bot. 54: 1027-1034.
Balfour B. 1888. Botany of Socotra: Dirachma. – Trans. Roy. Soc. Edinb. 31: 45-46.
Banerjee SP, Mukerjee PK. 1970. Studies in the Rhamnaceae 3, a taxonomic revision of Indian Ventilagineae. – Indian Forester 96: 203-217.
Banerji I. 1953. A contribution to the life history of Artocarpus lakooche Roxb. – Proc. Indian Acad. Sci., Sect. B, 39: 128-132.
Barth MO. 1984. Surface morphology of Brazilian Moraceae pollen grains. – Bol. IG-USP, Ser. Ci. 15: 142-150.
Bartish IV, Swenson U. 2004. Elaeagnaceae. – In: Kubitzki K (ed), The families and genera of vascular plants VI. Flowering plants. Dicotyledons. Celastrales, Oxalidales, Rosales, Cornales, Ericales, Springer, Berlin, Heidelberg, New York, pp. 131-134.
Bartish IV, Jeppsson N, Nybom H. 1999. Population genetic structure in the dioecious pioneer plant species Hippophae rhamnoides investigated by random amplified polymorphic DNA (RAPD) markers. – Mol. Ecol. 8: 791-802.
Bartish IV, Jeppsson N, Bartish GI, Lu R, Nybom H. 2000. Inter- and intraspecific genetic variation in Hippophae (Elaeagnaceae) investigated by RAPD markers. – Plant Syst. Evol. 225: 85-101.
Bartish IV, Hylmö B, Nybom H. 2001. RAPD analysis of interspecific relationships in presumably apomicic Cotoneaster species. – Euphytica 120: 273-280.
Bartish IV, Jeppsson N, Nybom H, Swenson U. 2002. Phylogeny of Hippophaë (Elaeagnaceae) inferred from parsimony analysis of chloroplast DNA and morphology. – Syst. Bot. 27: 41-54.
Basinger JF. 1976. Paleorosa similkameenensis, gen. et sp. nov., permineralized flowers (Rosaceae) from the Eocene of British Columbia. – Can. J. Bot. 54: 2293-2305.
Bate-Smith EC. 1961. Chromatography and taxonomy in the Rosaceae, with special reference to Potentilla and Prunus. – Bot. J. Linn. Soc. 58: 39-54.
Bate-Smith EC. 1965. Investigation of the chemistry and taxonomy of subtribe Quillajeae of the Rosaceae using comparisons of fresh and herbarium material. – Phytochemistry 4: 535-539.
Bate-Smith EC, Creasy LL. 1969. Luteoforol in strawberry leaves. – Phytochemistry 8: 1811-1813.
Bate-Smith EC, Richens RH. 1973. Flavonoid chemistry and taxonomy in Ulmus. – Biochem. Syst. 1: 141-146.
Bayer C. 2004. Dirachmaceae. – In: Kubitzki K (ed), The families and genera of vascular plants VI. Flowering plants. Dicotyledons. Celastrales, Oxalidales, Rosales, Cornales, Ericales, Springer, Berlin, Heidelberg, New York, pp. 122-124.
Bazara’a M, Guarino L, Miller A, Obadi N. 1991. Dirachma socotrana – back from the brink? – Oryx 25: 229-232.
Beaman RS. 2000. Phylogeny and biogeography of Elatostema (Urticaceae) from Mount Kinabalu, Sabah, Malaysia. – Ph.D. diss., University of Florida, Boca Raton, Florida.
Bean AR. 2004. New species of Cryptandra Sm. and Stenanthemum Reissek (Rhamnaceae) from northern Australia. – Austrobaileya 6: 917-940.
Bechtel AR. 1921. Floral anatomy of the Urticales. – Amer. J. Bot. 8: 386-407.
Beck NG, Lord EM. 1988a. Breeding system in Ficus carica, the common fig 1. Floral diversity. – Amer. J. Bot. 75: 1904-1912.
Beck NG, Lord EM. 1988b. Breeding system in Ficus carica, the common fig 2. Pollination events. – Amer J. Bot. 75: 1913-1922.
Becking JH. 1979. Nitrogen fixation by Rubus ellipticus J. E. Smith. – Plant and Soil 53: 541-545.
Becking JH. 1983. The Parasponia parviflora-Rhizobium symbiosis: host specificity, growth and nitrogen fixation under various conditions. – Plant Soil 75: 309-342.
Behnke H-D. 1973. Sieve-tube plastids of Hamamelididae. Electron microscopic investigation with special reference to Urticales. – Taxon 22: 205-210.
Behnke H-D. 1974. P- und S-Typ Siebelement-Plastiden bei Rhamnales. – Beitr. Biol. Pflanzen 50: 457-464.
Benedict JC, DeVore ML, Pigg KB. 2011. Prunus and Oemleria (Rosaceae) flowers from the late Early Eocene Republic flora of northeastern Washington State, U.S.A. – Intern. J. Plant Sci. 172: 948-958.
Bennek C. 1958. Die morphologische Beurteilung der Staub- und Blumenblätter der Rhamnaceen. – Bot. Jahrb. Syst. 77: 423-457.
Berg CC. 1972. Flora Neotropica. Monograph 7. Olmedieae and Brosimeae (Moraceae). – Hafner, New York.
Berg CC. 1973. Some remarks on the classification and differentiation of Moraceae. – Meded. Bot. Mus. Herb. Rijksuniv. Utrecht 386: 1-10.
Berg CC. 1977a. The Castilleae, a tribe of the Moraceae, renamed and redefined due to the exclusion of the type genus Olmedia from the “Olmedieae”. – Acta Bot. Neerl. 26: 73-82.
Berg CC. 1977b. Urticales, their differentiation and systematic position. – Plant Syst. Evol. [Suppl.] 1: 349-374.
Berg CC. 1977c. Revisions of African Moraceae (excluding Dorstenia, Ficus, Musanga, and Myrianthus). – Bull. Jard. Bot. Natl. Belg. 47: 267-407.
Berg CC. 1978a. Cecropiaceae, a new family of the Urticales. – Taxon 27: 39-44.
Berg CC. 1978b. Espécies de Cecropia de Amazônia Brasileira. – Acta Amazonica 8: 149-182.
Berg CC. 1978c. Revision of Dorstenia sect. Nothodorstenia (Moraceae). – Bot. Not. 131: 53-66.
Berg CC. 1980. Moraceae. – In: Stoffers AL (ed), Flora of the Netherlands Antilles 2: 111-120.
Berg CC. 1981. An exceptional new species of Cecropia (Moraceae) from Ecuador. – Nord. J. Bot. 1: 485-487.
Berg CC. 1982. The reinstatement of the genus Milicia Sim (Moraceae). – Bull. Jard. Bot. Natl. Belg. 52: 225-229.
Berg CC. 1983. Dispersal and distribution in the Urticales – an outline. – In: Kubitzki K (ed), Dispersal and distribution: an international symposium, Sonderb. Naturwiss. Ver. Hamburg 7: 219-229.
Berg CC. 1986. The delimitation and subdivision of the genus Maclura (Moraceae). – Proc. Kon. Nederl. Akad. Wet. Amsterdam, ser. C, 89: 241-247.
Berg CC. 1988a. The genera Trophis and Streblus (Moraceae) remodelled. – Proc. Kon. Nederl. Akad. Wet. Amsterdam, ser. C, 91: 345-362.
Berg CC. 1988b. New taxa and combinations in Ficus (Moraceae) of Africa. – Kew Bull. 43: 77-97.
Berg CC. 1989. Systematics and phylogeny of the Urticales. – In: Crane PR, Blackmore S (eds), Evolution, systematics, and fossil history of the Hamamelidae, Vol. 2, ‘Higher’ Hamamelidae, Syst. Assoc. Spec. Vol. 40B, Clarendon Press, Oxford, pp. 193-220.
Berg CC. 1990a. Differentiation of flowers and inflorescences of Urticales in relation to their protection against breeding insects and to pollination. – Sommerfeltia 11: 13-24.
Berg CC. 1990b. Reproduction and evolution in Ficus (Moraceae): traits connected with the adequate rearing of pollinators. – Mem. New York Bot. Gard. 55: 169-185.
Berg CC. 1998. 27B. Moraceae (excl. Ficus). – In: Harling G, Andersson L (eds), Flora of Ecuador 60, Nord. J. Bot., Copenhagen, pp. 1-126.
Berg CC. 2001. Moreae, Artocarpeae, and Dorstenia (Moraceae) with introductions to the family and Ficus and with additions and corrections to Flora Neotropica Monograph 7. – New York Botanical Garden, Bronx, New York.
Berg CC. 2002. Ficus baola, a new species of Ficus subgenus Urostigma section Malvanthera (Moraceae) from the Solomon Islands. – Blumea 47: 315-317.
Berg CC. 2003. Flora Malesiana precursor for the treatment of Moraceae 1: the main subdivision of Ficus: the subgenera. – Blumea 48: 167-178.
Berg CC. 2004a. A new species of Ficus (Moraceae) of uncertain provenance. – Brittonia 56: 54-57.
Berg CC. 2004b. Two new species of Pourouma (Cecropiaceae) from South America. – Brittonia 56: 255-259.
Berg CC. 2005a. Moraceae diversity in a global perspective. – In: Friis I, Balslev H (eds), Proceedings of a Symposium on Plant Diversity and Complexity Patterns – Local, Regional and Global Dimensions. Danish Academy of Sciences and Letters, Copenhagen, Biol. Skr. 55: 423-440.
Berg CC. 2005b. Flora Malesiana precursor for the treatment of Moraceae 8: other genera than Ficus. – Blumea 50: 535-550.
Berg CC, Akkermans RWAP. 1985. Studies on the flora of the Guianas 14: new taxa and combination in Sorocea (Moraceae) and a key to its species. – Proc. Kon. Nederl. Akad. Wet. Amsterdam, ser. C, 88: 381-394.
Berg CC, Carauta JPP. 2003. New species of Ficus (Moraceae) from Brazil. – Brittonia 54: 236-250.
Berg CC, Corner EJH (†). 2005. Moraceae – Ficus. – In: Nooteboom HP (ed), Flora Malesiana I, 17, Foundation Flora Malesiana, Nationaal Herbarium of Nederland, Leiden, The Netherlands, pp. 1-727.
Berg CC, Dahlberg SV. 2001. A revision of Celtis subg. Mertensia (Ulmaceae). – Brittonia 53: 66-81.
Berg CC, Dewolf GP. 1975. Moraceae. – E. J. Brill, The Netherlands.
Berg CC, Hijman MEE. 1977. A precursor to the treatment of Dorstenia for the floras of Cameroun and Gabon. – Adansonia, sér. II, 16: 415-443.
Berg CC, Hijman MEE. 1999. The genus Dorstenia (Moraceae). – Ilicifolia 2: 1-211.
Berg CC, Rosselli PF. 1993. 27A. Cecropiaceae. – In: Harling G, Andersson L (eds), Flora of Ecuador 48, Nord. J. Bot., Copenhagen, pp. 1-107.
Berg CC, Akkermans RWAP, Heusden ECH van. 1990. Flora Neotropica. Monograph 51. Cecropiaceae: Coussapoa and Pourouma, with an introduction to the family. – New York Botanical Garden, Bronx, New York, pp. 1-208.
Berg CC, Corner EJH (†), Jarrett FM. 2006. Moraceae – genera other than Ficus. – In: Nooteboom HP (ed), Flora Malesiana I, 17, Foundation Flora Malesiana, Nationaal Herbarium of Nederland, Leiden, The Netherlands, pp. 1-152.
Bernbeck F. 1932. Vergleichende Morphologie der Urticaceen- und Moraceen-Infloreszenzen. – Bot. Abh. 19: 1-100.
Bigalke H. 1933. Die Blattspodogramme der Urticaceae und ihre Verwendbarkeit für die Systematik. – Beitr. Biol. Pflanzen 21: 1-56.
Bitter G. 1911a. Revision der Gattung Polylepis. – Engl. Bot. Jahrb. Syst. 45: 564-656.
Bitter G. 1911b. Die Gattung Acaena. Vorstudien zu einer Monographie. – Bibl. Bot. 17: 1-336.
Boesewinkel FD, Bouman F. 1997. Ovules and seeds of Dirachma socotrana (Dirachmaceae). – Plant Syst. Evol. 205: 195-204.
Bolle F. 1933. Eine Übersicht über die Gattung Geum L. und die ihr nahestehenden Gattungen. – Feddes Repert. Beih. 72: 1-119.
Bolle F. 1935. Über eine bemerkenswerte Missbildung bei Geum. – Notizbl. Bot. Gart. Mus. Berlin-Dahlem 12(113): 349-354.
Bolmgren K, Oxelman B. 2004. Generic limits in Rhamnus L. s.l. (Rhamnaceae) inferred from nuclear and chloroplast DNA sequence phylogenies. – Taxon 53: 383-390.
Bond G, MacConnel JT, McCallum AH. 1956. The nitrogen nutrition of Hippophaë rhamnoides L. – Ann. Bot., N. S., 20: 501-512.
Bonne G. 1925. Sur les faiscaux de rebroussement dans la coupe florale de certains Rosacées. – Comp. Rend. Acad. Sci. Paris 219: 181-191.
Bonne G. 1928. Recherches sur la pédicelle et la fleur des Rosacées. – Jouve et Cie, Paris.
Bonsen KJ, Welle BJH ter. 1983. Comparative wood and leaf anatomy of the Cecropiaceae (Urticales). – Bull. Mus. Natl. Hist. Nat. Paris, sér. IV, 5, sect. B, Adansonia, 2: 151-177.
Bonsen KJ, Welle BJH ter. 1984. Systematic wood anatomy and affinities of the Urticaceae. – Bot. Jahrb. Syst. 105: 49-71.
Boratyńska K. 1995. Chromosome numbers of Polish brambles (Rubus, Rosaceae). – Willdenowia 25: 267-271.
Bortiri E, Oh S, Jiang J, Baggett S, Granger A, Weeks C, Buckingham M, Potter D, Parfitt D. 2001. Phylogeny and systematics of Prunus (Rosaceae) as determined by sequence analysis of ITS and the chloroplast trnL-trnF spacer DNA. – Syst. Bot. 26: 797-807.
Bortili E, Oh S, Gao F, Potter D. 2002. The phylogenetic utility of nucleotide sequences of sorbitol 6-phosphate dehydrogenase in Prunus (Rosaceae). – Amer. J. Bot. 89: 1697-1708.
Bortiri E, Heuvel B Vanden, Potter D. 2006. Phylogenetic analysis of morphology in Prunus reveals extensive homoplasy. – Plant Syst. Evol. 259: 53-71.
Bosco M, Fernandez MP, Simonet P, Materassi R, Normand P. 1992. Evidence that some Frankia sp. strains are able to cross boundaries between Alnus and Elaeagnus host specificity groups. – Appl. Env. Microbiol. 58: 1569-1576.
Bouchet P. 1974. Étude ultrastructurale des cellules constituant les poches “lysigènes“ à mucilage de la bourdaine: Rhamnus frangula L. – Compt. Rend. Acad. Sci. Paris D, 279: 1073.
Bouman F, Boesewinkel FD. 1997. Ovules and seeds of Barbeya with additional arguments for an urticalean affinity of the Barbeyaceae. – Acta Bot. Neerl. 46: 255-261.
Braid KW. 1925. Revision of the genus Alphitonia. – Kew Bull. 1925: 171-186.
Bramwell D. 1978. The endemic genera of Rosaceae (Poterieae) in Macaronesia. – Bot. Macaronesica 6: 67-73.
Brantjes NBM. 1981. Nectar and the pollination of bread fruit, Artocarpus altilis (Moraceae). – Acta Bot. Neerl. 30: 345-352.
Brittingham HA, Koski MH, Ashman T-L. 2018. Higher ploidy is associated with reduced range breadth in the Potentilleae tribe. – Amer. J. Bot. 105: 700-710.
Bronstein JL, McKey D (coordinators). 1989. Multi-author review: the comparative biology of figs. – Experientia 45: 601-680.
Brown HB. 1910. The genus Crataegus with some theories of the origin of its species. – Bull. Torrey Bot. Club 37: 251-260.
Bruneaeu A, Starr JR, Joly S. 2007. Phylogenetic relationships in the genus Rosa: new evidence from chloroplast DNA sequences and an appraisal of current knowledge. – Syst. Bot. 32: 366-378.
Bruun-Lund S, Clement WL, Kjellberg F, Rønsted N. 2017. First plastid phylogenomic study reveals potential cyto-nuclear discordance in the evolutionary history of Ficus L. (Moraceae). – Mol. Phylogen. Evol. 109: 93-104.
Burge DO, Zhukovsky K. 2013. Taxonomy of the Ceanothus vestitus complex (Rhamnaceae). – Syst. Bot. 38: 406-417.
Burge DO, Erwin DM, Islam MB, Kellermann J, Kembel SW, Wilken DH, Manos PS. 2011. Diversification of Ceanothus (Rhamnaceae) in the Californian floristic province. – Intern. J. Plant Sci. 172: 1137-1164.
Burge DO, Zhukovsky K, Wilken DH. 2015. A taxonomic conspectus of Ceanothus Subgenus Cerastes (Rhamnaceae). – Syst. Bot. 40: 950-961.
Burge DO, Rebman JP, Mulligan MR, Wilken DH. 2017. Three edaphic-endemic Ceanothus (Rhamnaceae) new to science. – Syst. Bot. 42: 529-542.
Burger WC. 1962. Studies in New World Moraceae: Trophis, Clarisia, Acanthinophyllum. – Ann. Missouri Bot. Gard. 49: 1-34.
Burger WC. 1967. Families of flowering plants of Ethiopia. – Experimental Station Bull. No. 45, Oklahoma State University Press.
Burger WC. 1977a. Flora Costaricensis. Fam. 52. Moraceae. – Fieldiana Bot. 40: 94-215.
Burger WC. 1977b. Flora Costaricensis. Fam. 53. Urticaceae. – Fieldiana Bot. 40: 218-283.
Burger WC, Lanjouw J, Boer JGW. 1962. The genus Sorocea St. Hil. (Morac.). – Meded. Bot. Mus. Herb. Rijksuniv. Utrecht 193: 428-474.
Burgess AH. 1964. Hops. Botany, cultivation, and utilization. – Interscience Publ., New York.
Burgess MB, Cushman KR, Doucette ET, Talent N, Frye CT, Campbell CS. 2014. Effects of apomixis and polyploidy on diversification and geographic distribution in Amelanchier (Rosaceae). – Amer. J. Bot. 101: 1375-1387.
Burn MJ, Mayle FE. 2008. Palynological differentiation between genera of the Moraceae family and implications for Amazonian palaeoeology. – Rev. Palaeobot. Palynol. 149: 187-201.
Byatt JI. 1976. The genus Crataegus (Rosaceae) in Greece. – Candollea 31: 283-301.
Cahen D, Utteridge TMA. 2018. A synopsis of the genus Smythea (Rhamnaceae). – Kew Bull. 73: 2. doi 10.1007/S12225-017-9724-3
Callmander MW, Phillipson PB, Buerki S. 2008. Révision du genre Bathiorhamnus Capuron (Rhamnaceae) endémique de Madagascar. – Adansonia, sér. III, 30: 151-170.
Calvillo-Canadell L, Cevallos-Ferriz SRS. 2007. Reproductive structures of Rhamnaceae from the Cerro del Pueblo (Late Cretaceous, Coahuila) and Coatzingo (Oligocene, Puebla) formations, Mexico. – Amer. J. Bot. 94: 1658-1669.
Camp WH. 1942. The Crataegus problem. – Castanea 7: 51-55.
Campbell CS, Dickinson TA. 1990. Apomixis, patterns of morphological variation, and species concepts in subfam. Maloideae (Rosaceae). – Syst. Bot. 15: 124-135.
Campbell CS, Greene CW, Dickinson TA. 1991. Reproductive biology in the Maloideae (Rosaceae). – Syst. Bot. 16: 333-349.
Campbell CS, Donoghue MJ, Baldwin BG, Wojciechowski MF. 1995. Phylogenetic relationships in Maloideae (Rosaceae): evidence from sequences of the internal transcribed spacers of nuclear ribosomal DNA and its congruence with morphology. – Amer. J. Bot. 82: 903-918.
Campbell CS, Wojciechowski MF, Baldwin BG, Alice LA, Donoghue MJ. 1997. Persistent nuclear ribosomal DNA sequence polymorphism in the Amelanchier agamic complex (Rosaceae). – Mol. Biol. Evol. 14: 81-90.
Campbell CS, Alice LA, Wright WA. 1999. Comparisons of within-population genetic variation in sexual and agamospermous Amelanchier (Rosaceae) using RAPD markers. – Plant Syst. Evol. 215: 157-167.
Campbell CS, Evans RC, Morgan DR, Dickinson TA, Arsenault MP. 2007. Phylogeny of subtribe Pyrinae (formerly the Maloideae, Rosaceae): limited resolution of a complex evolutionary history. – Plant Syst. Evol. 266: 119-145.
Capuron R. 1966. Notes sur quelques Rhamnacées arbustive ou arborescentes de Madagascar. – Adansonia 6: 116-141.
Catling PM, McKay-Kuja SM, Mitrow G. 1999. Rank and typification in North American dwarf cherries, and a key to the taxa. – Taxon 48: 483-488.
Celotti N. 1995. The pollen tube pathway and obturator in hawthorn sexual reproduction. – B.Sc. Honours thesis, Biology Department, Queen’s University, England.
Cevallos-Ferriz SRS, Erwin DM, Stockey RA. 1993. Further observations of Paleorosa similkameenensis (Rosaceae) from the middle Eocene Princeton chert of British Columbia, Canada. – Rev. Paleobot. Palynol. 78: 277-291.
Chakass MA, Verhille A-M, d’Amico N, Boussioud-Corbières F. 2008. Palynologie de deux varieties d’une espèce utile du Liban: le néflier du Japon (Eriobotrya japonica). – Adansonia, sér. III, 30: 171-175.
Challice JS. 1973. Phenolic compounds of the subfamily Pomoideae: a chemotaxonomic survey. – Phytochemistry 12: 1095-1101.
Challice JS. 1974. Rosaceae chemotaxonomy and the origins of the Pomoideae. – Bot. J. Linn. Soc. 69: 239-259.
Challice JS. 1981. Chemotaxonomic studies in the family Rosaceae and the evolutionary origins of the subfamily Maloideae. – Preslia 53: 289-304.
Challice JS, Kovanda M. 1981. Chemotaxonomic studies in the family Rosaceae and the evolutionary origins of the subfamily Maloideae. – Preslia 53: 289-304.
Chang K-S, Chang C-S, Park TY, Roh MS. 2007. Reconsideration of the Prunus serrulata complex (Rosaceae) and related taxa in eastern Asia. – Bot. J. Linn. Soc. 154: 35-54.
Chantarasuwan B, Berg CC, Welzen PC van. 2013. A revision of Ficus Subsection Urostigma (Moraceae). – Syst. Bot. 38: 653-686.
Chauhan JS, Kumari G. 1979. A new glycoflavonol from the root bark of Artocarpus lakoocha. – Planta Medica 37: 86-88.
Chauveaud LG. 1891. Recherches embryogénique sur l’appareil laticifère des Euphorbiacées, Urticacées, Apocynées et Asclepiadées. – Ann. Sci. Nat. Bot. VII, 14: 1-161.
Chen C-J. 1982. A monograph of Pilea (Urticaceae) in China. – Bull. Bot. Res. (Harbin) 2: 1-132. [In Chinese]
Chen C-J. 1983. A revision of the genus Urtica in China. – Bull. Bot. Res., Harbin 3: 104-125.
Chen C-J, Wilmot-Dear CM, Friis I. 2005. Notes on Chinese and Indochinese Boehmeria (Urticaceae). – Kew Bull. 60: 449-453.
Chen Y-S, Meseguer AS, Godefroid M, Zhou Z, Zhang J-W, Deng T, Kim J-H, Nie Z-L, Liu CY-S, Sun H. 2017. Out-of India dispersal of Paliurus (Rhamnaceae) indicated by combined molecular phylogenetic and fossil evidence. – Taxon 66: 78-90.
Chernik VV. 1975. Arrangement and reduction of perianth and androecium parts in representatives of the Ulmaceae Mirbel and Celtidaceae Link. – Bot. Žurn. 60: 1561-1573. [In Russian with English summary]
Chernik VV. 1980. Peculiarities of structure and development of the pericarp of the representatives of the families Ulmaceae and Celtidaceae. – Bot. Žurn. 65: 521-531. [In Russian]
Chernik VV. 1981. Pseudomonomeric gynoecium of the Ulmaceae and Celtidaceae representatives. – Bot. Žurn. 66: 958-962. [In Russian with English summary]
Chernik VV. 1982. Characteristics of the structural development of sporoderm in some representatives of Ulmaceae and Celtidaceae. – Bot. Žurn. 67: 1216-1220. [In Russian with English summary]
Chew W-L. 1963. Florae Malesianae precursores XXXIV. A revision of the genus Poikilospermum (Urticaceae). – Gard. Bull. (Singapore) 20: 1-103.
Chew W-L. 1965. Laportea and allied genera (Urticaceae). – Gard. Bull. (Singapore) 21: 195-208.
Chew W-L. 1969a. A monograph of Dendrocnide (Urticaceae). – Gard. Bull. (Singapore) 25: 1-104.
Chew W-L. 1969b. A monograph of Laportea (Urticaceae). – Gard. Bull. (Singapore) 25: 111-177.
Chew W-L. 1989a. Moraceae. – In: George AS (ed), Flora of Australia 3, Australian Government Publ. Service, Canberra, pp. 15-68.
Chew W-L. 1989b. Urticaceae. – In: George AS (ed), Flora of Australia 3, Australian Government Publ. Service, Canberra, pp. 68-93.
Chin S-W, Wen J, Johnson G, Potter D. 2010. Merging Maddenia with the morphologically diverse Prunus (Rosaceae). – Bot. J. Linn. Soc. 164: 236-245.
Chin S-W, Shaw J, Haberle R, Wen J, Potter D.
2014. Diversification of almonds, peaches, plums and cherries – molecular
systematics and biogeographic history of Prunus (Rosaceae). – Molec. Phylogen. Evol. 76:
34-48.
Christen HR. 1950. Untersuchungen über die Embryologie pseudogamer und sexueller Rubus-Arten. – Ber. Schweiz. Bot. Ges. 60: 153-198.
Christenhusz MJM, Väre H. 2012. New combinations in Potentilla (Rosaceae) for the Nordic Flora. – Phytotaxa 57: 1-5.
Christensen KI. 1985. A taxonomic study of Crataegus ser. Kyrtostylae Pojark. ex Botschantzev in Europe. – Feddes Repert. 96: 363-385.
Christensen KI. 1992. Revision of Crataegus sect. Crataegus and nothosect. Crataeguineae (Rosaceae-Maloideae) in the Old World. – Syst. Bot. Monogr. 35: 1-199.
Chrtek J. 1979. Bemerkungen zur Gliederung der Gattung Urtica L. – Folia Geobot. Phytotaxon. 14: 265-266.
Chun W-Y, Tsiang Y. 1939. A new species of Hovenia. – Sunyatsenia 4: 16-17.
Chung K-S, Elisens WJ, Skvarla JJ. 2010. Pollen morphology and its phylogenetic significance in tribe Sanguisorbeae (Rosaceae). – Plant Syst. Evol. 285: 139-148.
Cialdella AM, Pometti CL. 2017. Taxonomic revision of the genus Tetraglochin (Rosaceae, Rosoideae) and morphometric analysis of its species. – Phytotaxa 296(3). DOI: http://dx.doi.org/10.11646/phytotaxa.296.3.1
Clark MN. 1978. A study of infraspecific flavonoid variation of Cannabis sativa L. (Cannabaceae). – Ph.D. diss., University of British Columbia, Vancouver, Canada.
Clarke RC. 1981. Marijuana botany. – And/or Press, Berkeley, California.
Clawson ML, Bourret A, Benson DR. 2004. Assessing the phylogeny of Frankia-actinorhizal plant nitrogen-fixing root nodule symbioses with Frankia 16SrRNA and glutamine synthetase gene sequences. – Mol. Phylogen. Evol. 31: 131-138.
Clement WL. 2008. Phylogeny and pollination ecology of Castilleae (Moraceae): investigating the evolutionary history of the figs’ closest relatives. – Ph.D. thesis, University of Minnesota – Twin Cities, Saint Paul.
Clement WL, Weiblen GD. 2009. Morphological evolution in the mulberry family (Moraceae). – Syst. Bot. 34: 530-552.
Colenso W. 1886. Newly discovered indigenous plants: order LXXI, Genus 4. Australina. – Trans. New Zealand Inst. 18: 266.
Collinson ME. 1989. The fossil history of the Moraceae, Urticaceae (including Cecropiaceae), and Cannabaceae. – In: Crane PR, Blackmore S (eds), Evolution, systematics andfossil history of the Hamamelidae, vol. 2, ‘Higher’ Hamamelidae, Syst. Assoc. Spec. Vol. 40B, Clarendon Press, Oxford, pp. 319-339.
Compton SG (ed). 1996. Fig trees and their associated animals. – J. Biogeogr. 23: 405-607.
Conn BJ, Hadiah JT. 2009. Nomenclature of tribes within the Urticaceae. – Kew Bull. 64: 349-352.
Considine MJ, Wan Y, D’Antuono MF, Zhou Q, Han M, Gao H, Wang M. 2012. Molecular genetic features of polyploidization and aneuploidization revel unique patterns for genome duplication in diploid Malus. – PLoS One 7:e29449.
Cook JM, Rasplus JY. 2003. Mutualists with attitude: coevolving fig wasps and figs. – Trends Ecol. Evol. 18: 241-248.
Cooper DC. 1932. The development of the peltate hairs of Shepherdia canadensis. – Amer. J. Bot. 19: 423-428.
Corner EJH. 1962. The classification of Moraceae. – Gard. Bull. (Singapore) 19: 187-252.
Cook JM, Rasplus JY. 2003. Mutualists with attitude: coevolving fig wasps and figs. – Trends Ecol. Evol. 18: 241-248.
Corner EJH. 1959. Taxonomic notes on Ficus Linn., Asia and Australasia I. Subgen. Urostigma (Gasp.) Miq. – Gard. Bull. (Singapore) 17: 368-404.
Corner EJH. 1962. The classification of Moraceae. – Gard. Bull. (Singapore) 19: 187-252.
Corner EJH. 1965. Checklist of Ficus in Asia and Australasia with keys to identification. – Gard. Bull. (Singapore) 21: 1-186.
Corner EJH. 1967. Ficus in the Solomon Islands and its bearing on the post-Jurassic history of Melanesia. – Philos. Trans. Roy. Soc. London, Ser. B, 253: 23-159.
Corner EJH. 1970a. Ficus subgen. Pharmacosycea with reference to the species of New Caledonia. – Philos. Trans. Roy. Soc. London, Ser. B, 259: 383-433.
Corner EJH. 1970b. New species of Streblus and Ficus (Moraceae). – Blumea 18: 393-411.
Corner EJH. 1975. The evolution of Streblus Lour. (Moraceae): with a new species of sect. Bleekrodea. – Phytomorphology 25: 1-12.
Corner EJH. 1978. Ficus dammaropsis Bl. and the pedunculate species of Ficus subgen. Sycocarpus. – Phil. Trans. Roy. Soc. London, ser. B, 281: 373-406.
Correa E, Jaramillo C, Manchester S, Gutierrez M. 2010. A fruit and leaves of rhamnaceous affinities from the late Cretaceous (Maastrichtian) of Columbia. – Amer. J. Bot. 97: 71-79.
Corsi G, Masini A. 1997. Anatomical and ecological aspects in Italian taxa of the genus Urtica. – Atti Soc. Tosc. Sci. Nat. Pisa Mem., Ser. B, 104: 1-8.
Corsi G, Garbari F, Maffei F. 1999. Il genere Urtica L. (Urticaceae) in Italia. Revisione biosistematica. – Webbia 53: 193-239.
Côte B, Carlson RW, Dawson JO. 1988. Leaf photosynthetic characteristics of seedlings of actinorhizal Alnus spp. and Elaeagnus spp. – Photosynth. Res. 16: 211-218.
Cowan RS. 1949. A taxonomic revision of the genus Neraudia Gaud. (Urticaceae). – Pacific Sci. 3: 231-270.
Crawford DJ, Brauner S, Cosner MB, Stuessy TF. 1993. Use of RAPD markers to document the origin of the intergeneric hybrid x Margyracaena skottsbergii (Rosaceae) on the Juan Fernandez Islands. – Amer. J. Bot. 80: 89-92.
Cruz Cisneros R, Valdes M. 1991. Actinorhizal root nodules on Adolphia infesta (H.B.K.) Messner (Rhamnaceae). – Nitrogen Fixing Tree Res. Rep. 9: 87-89.
Cuellar HS. 1967. Description of a pollen release mechanism in the flower of the Mexican hackberry tree, Celtis laevigata. – Southw. Natur. 12: 471-474.
Curtis JD, Lersten NR. 1986. Hydathode anatomy in Potentilla palustris (Rosaceae). – Nord. J. Bot. 6: 793-796.
Cushman KR, Burgess MB, Doucette ET, Nelson GA, Campbell CS. 2017. Species delimitation in tetraploid, apomictic Amelanchier (Rosaceae). – Syst. Bot. 42: 234-256.
Czapik 1981a. Elementary apomictic processes in Rubus L. – Acta Soc. Bot. Poloniae 50: 201-204.
Czapik R. 1981b. Embryology of Rubus saxatilis L. – Acta Biol. Cracov., Ser. Bot. 23: 7-13.
Czapik r. 1983. The secondary nucleus in four species of the genus Rubus. – Acta Biol. Cracov., Ser. Bot. 25: 179-188.
Czapik R. 1996. Problems of apomictic reproduction in the families Compositae and Rosaceae. – Folia Geobot. Phytotaxon. 3: 381-387.
Czerepanov S. 1957. Revisio specierum generum Zelkova Spach et Hemiptelea Planchon. – Bot. Mater. Gerb. Bot. Inst. Komarova Akad. Nauk. SSSR (Not. Syst. Leningrad) 18: 58-72.
Dahlgren RMT. 1971. Multiple similarity of leaf between two genera of Cape plants, Cliffortia L. (Rosaceae) and Aspalathus L. (Fabaceae). – Bot. Not. 124: 292-304.
D’Ambrogio AC, Medan D. 1993. Comportamiento reproductivo de Colletia paradoxa (Rhamnaceae). – Darwiniana 32: 1-14.
Danet F. 2003. Deux nouvelles espèces de Potentilla (Rosaceae) de Nouvelle-Guinée. – Adansonia, sér. III, 25: 239-246.
Darlington CD, Moffett AA. 1930. Primary and secondary chromosome balance in Pyrus. – J. Genet. 22: 129-151.
Datwyler SL, Weiblen GD. 2004. On the origin of the fig: phylogenetic relationships of Moraceae from ndhF sequences. – Amer. J. Bot. 91: 767-777.
Dayanandan P, Kaufman PB. 1976. Trichomes of Cannabis sativa L. (Cannabaceae). – Amer. J. Bot. 63: 578-591.
De Cock K, Vander Mijnsbrugge K, Breyne P, Bockstaele E van, Slycken J van. 2008. Morphological and AFLP-based differentiation within the taxonomical complex section Caninae (subgenus Rosa). – Ann. Bot. 102: 685-697.
Delgado L, Gallego F, Rico E. 2000. Karyosystematic study of Potentilla L. subgen. Potentilla (Rosaceae) in the Iberian Peninsula. – Bot. J. Linn. Soc. 132: 263-280.
Deng T, Kim C, Zhang D-G, Zhang J-W, Li Z-M, Nie Z-L, Sun H. 2013. Zhengyia shennongensis: a new bulbiliferous genus and species of the nettle family (Urticaceae) from central China exhibiting parallel evolution of the bulbil trait. – Taxon 62: 89-99.
Denk T, Dillhoff RM. 2005. Ulmus leaves and fruits from the Early-Middle Eocene of northwestern North America: systematics and implications for character evolution within Ulmaceae. – Can. J. Bot. 83: 1663-1681.
Denk T, Grimm GW. 2005. Phylogeny and biogeography of Zelkova (Ulmaceae sensu stricto) as inferred from leaf morphology, ITS sequence data and the fossil record. – Bot. J. Linn. Soc. 147: 129-157.
De Pasquale A. 1974. Ultrastructure of the Cannabis sativa glands. – Planta Medica 25: 238-248.
Depypere L, Chaerle P, Breyne P, Mijnsbrugge K, Goetghebeur P. 2009. A combined morphometric and AFLP based diversity study challenges the taxonomy of the European members of the complex Prunus L. section Prunus. – Plant Syst. Evol. 279: 219-231.
Dermen H. 1949. Are the pomes amphiploid? – J. Heredity 40: 221-222.
De Rooij MJM. 1975. Urticaceae. – In: Lanjouw J, Stoffers AL (eds), Flora of Suriname 5(1), E. J. Brill, Leiden, pp. 300-318.
DeVore ML, Pigg KB. 2007. A brief review of the fossil history of the family Rosaceae with a focus on the Eocene Okanogan Highlands of eastern Washington State, USA, and British Columbia, Canada. – Plant Syst. Evol. 266: 45-57.
De Wildeman E. 1921. Documents pour une monographie des Alchemilla d’Afrique. – Bull. Jard. Bot. État 6: 207-221, 7: 317-386.
Diapulis C. 1933. Beiträge zur Kenntnis der orientalischen Pomaceen. Crataegus Linn. – Feddes Repert. 34: 49-66.
Dickinson TA, Campbell CS. 1991. Population structure and reproductive ecology in the Maloideae (Rosaceae). – Syst. Bot. 16: 350-362.
Dickinson TA, Belaoussoff S, Love RM, Muniyamma M. 1996. North American black-fruited hawthorns I. Variation in floral construction, breeding system correlates, and their possible evolutionary significance in Crataegus sect. Douglasii Loudon. – Folia Geobot. Phytotaxon. 31: 355-371.
Dickinson TA, Lo E, Talent N. 2007. Polyploidy, reproductive biology, and Rosaceae: understanding evolution and making classifications. – Plant Syst. Evol. 266: 59-78.
Dickison WC, Sweitzer EM. 1970. The morphology and relationship of Barbeya oleoides. – Amer. J. Bot. 57: 468-476.
Dickson EE. 1995. Systematic studies of Malus section Chloromeles (Maloideae, Rosacae). – Ph.D. diss., Department of Botany, Cornell University, Ithaca, New York.
Dickson EE, Kresovich S, Weeden NF. 1991. Isozymes in North American Malus (Rosaceae): hybridization and species differentiation. – Syst. Bot. 16: 363-375.
Dickson EE, Arumuganathan K, Kresovich S, Doyle JJ. 1992. Nuclear DNA content variation within the Rosaceae. – Amer. J. Bot. 79: 1081-1086.
Dikshit BK, Panigrahi G. 1981. Revision of the genus Sibbaldia L. (Rosaceae) in India. – Proc. Indian Acad. Sci., Sect. B, 90: 253-272.
Dikshit BK, Panigrahi G. 1998. The family Rosaceae in India: revisionary studies on Potentilla L., Sibbaldia L. and Brachycaulos Dikshit et Panigr. – Bishen Singh Mahendra Pal Singh, Dehra Dun.
Dixon DJ. 2001a. Figs, wasps and species concepts: a re-evaluation of the infraspecific taxa of Ficus macrophylla (Moraceae: Urostigma sect. Malvanthera). – Aust. Syst. Bot. 14: 125-132.
Dixon DJ. 2001b. Figuring out the figs: the Ficus obliqua-Ficus rubiginosa complex (Moraceae: Urostigma sect. Malvanthera). – Aust. Syst. Bot. 14: 133-154.
Dixon DJ. 2001c. A chequered history: the taxonomy of Ficus platypoda and F. leucotricha (Moraceae: Urostigma sect. Malvanthera) unravelled. – Aust. Syst. Bot. 14: 535-563.
Dixon DJ. 2001d. Ficus lilliputiana (Moraceae), a new species from the Kimberley region of Western Australia and the Northern Territory. – Nuytsia 13: 457-464.
Dixon DJ. 2003. A taxonomic revision of the Australian Ficus species in the section Malvanthera (Ficus subg. Urostigma: Moraceae). – Telopea 10: 125-153.
Dobes C, Paule J. 2010. A comprehensive chloroplast DNA-based phylogeny of the genus Potentilla (Rosaceae): implications for its geographic origin, phylogeography and generic circumscription. – Mol. Phylogen. Evol. 56: 156-175.
Doll R. 1974. Zur Kenntnis der Gattung Crataegus. – Gleditschia 2: 9-16.
Donmez AA. 2005. A new species of Crataegus (Rosaceae) from Turkey. – Bot. J. Linn. Soc. 148: 245-249.
Donmez AA. 2007. Taxonomic notes on the genus Crataegus (Rosaceae) in Turkey. – Bot. J. Linn. Soc. 155: 231-240.
Drummond RB. 1966. 53. Rhamnaceae. – In: Exell AW, Fernandes A, Wild H (eds), Flora Zambesiaca 2 (Part 2), Crown Agents for Oversea Governments and Administrations, London, pp. 419-439.
Duman H, Mill RR. 1999. Two new species of Potentilla L. (Rosaceae) from SW Turkey. – Edinburgh J. Bot. 56: 349-354.
Dunn DW, Segar ST, Ridley J, Chan R, Crozier RH, Yu DW, Cook JM. 2008. A role for parasites in stabilising the fig-pollinator mutualism. – PloS Biol. 6(3): e59.
Duse E. 1905. Revisioni delle Acaena degli Erbari di Firenze, Roma e Monaco. – Nuovo Giorn. Bot. Ital., Ser. II, 12: 349-362.
Dute R, Patel J, Jansen S. 2010. Torus-bearing pit membranes in Cercocarpus. – IAWA J. 31: 53-66.
Edees ES, Newton A. 1988. Brambles of the British Isles. – London.
Eide F. 1981. Key for Northwest European Rosaceae pollen. – Grana 20: 101-118.
Ekdahl I. 1941. Die Entwicklung von Embryosack und Embryo bei Ulmus glabra Huds. – Svensk Bot. Tidskr. 35: 143-156.
Emboden WA. 1974. Cannabis – a polytypic genus. – Econ. Bot. 28: 304-310.
Emmelin N, Feldberg W. 1947. The mechanism of the sting of the common nettle (Urtica urens). – J. Physiol. 106: 440-455.
Engler A. 1889a. Ulmaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(1), W. Engelmann, Leipzig, pp. 59-66.
Engler A. 1889b. Moraceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(1), W. Engelmann, Leipzig, pp. 66-98; Engler A. 1897. Nachträge zu III(1), pp. 119-122.
Engler A. 1889c. Urticaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(1), W. Engelmann, Leipzig, pp. 98-118.
Engler A. 1897. Rosaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien, Nachträge zu III(3), W. Engelmann, Leipzig, pp. 186-189.
Engler A. 1902. Moraceae africanae II. – Engl. Bot. Jahrb. Syst. 33: 114-119.
Eriksson T, Donoghue MJ, Hibbs MS. 1998. Phylogenetic analysis of Potentilla using DNA sequences of nuclear ribosomal internal transcribed spacers (ITS), and implications for the classification of Rosoideae (Rosaceae). – Plant Syst. Evol. 211: 155-179.
Eriksson T, Hibbs MS, Yoder AD, Delwiche CF, Donoghue MJ. 2003. The phylogeny of Rosoideae (Rosaceae) based on sequences of the internal transcribed spacers (ITS) of nuclear ribosomal DNA and the trnL/F region of chloroplast DNA. – Intern. J. Plant Sci. 164: 197-211.
Eriksson T, Lundberg M, Töpel M, Östensson
P, Smedmark J. 2015. Sibbaldia: a molecular phylogenetic study of a
remarkably polyphyletic genus in Rosaceae. – Plant Syst. Evol. 301:
171-184.
Erlanson EW, Boulenger GA. 1937. Revision des roses d’Asie. – J. Indian Bot. Soc. 16: 241-244.
Ertter B. 1989. Revisionary studies in Ivesia (Rosaceae: Potentilleae). – Syst. Bot. 14: 231-244.
Ertter B. 1993. A re-evaluation of the Horkelia bolanderi (Rosaceae) complex, with the new species Horkelia yadonii. – Syst. Bot. 18: 137-144.
Erwin DM, Schorn HE. 2000. Revision of Lyonothamnus A. Gray (Rosaceae) from the Neogene of western North America. – Intern. J. Plant Sci. 161: 179-193.
Escarré A. 1969. Aportaciones al conocimento de la flora de Fernando Po II: Piperaceae, Urticaceae. – Acta Phytotaxon. Barcinonensia 3(5).
Evans RA, Biswel HH, Palmqvist DE. 1987. Seed dispersal in Ceanothus cuneatus and C. leucodermis in a sierran oakwoodland savanna. – Madroño 34: 283-293.
Evans RC. 1999. Molecular, morphological, and ontogenetic evaluation of relationships and evolution in the Rosaceae. – Ph.D. diss., University of Toronto, Ontario.
Evans RC, Campbell CS. 2002. The origin of the apple subfamily (Maloideae; Rosaceae) is clarified by DNA sequence data from duplicated GBSSI genes. – Amer. J. Bot. 89: 1478-1484.
Evans RC, Dickinson TA. 1996. North American black-fruited hawthorns II. Floral development of 10- and 20-stamen morphotypes in Crataegus section Douglasii (Rosaceae: Maloideae). – Amer. J. Bot. 83: 961-978.
Evans RC, Dickinson TA. 1999a. Floral ontogeny and morphology in subfamily Amygdaloideae T. & G. (Rosaceae). – Intern. J. Plant Sci. 160: 955-979.
Evans RC, Dickinson TA. 1999b. Floral ontogeny and morphology in subfamily Spiraeoideae Endl. (Rosaceae). – Intern. J. Plant Sci. 160: 981-1012.
Evans RC, Dickinson TA. 2005. Floral ontogeny and morphology in Gillenia (‘Spiraeoideae’) and subfamily Maloideae C. Weber (Rosaceae). – Intern. J. Plant Sci. 166: 427-447
Evans RC, Alice LA, Campbell CS, Dickinson TA, Kellogg EA. 2000. The granule-bound starch synthase (GBSSI) gene in the Rosaceae: multiple loci and phylogenetic utility. – Mol. Phylogen. Evol. 17: 388-400.
Evert RF, Deshpande BP. 1969. Electron microscope investigation of sieve-element ontogeny and structure in Ulmus americana. – Protoplasma 68: 403-432.
Exell AW. 1928. Two new hybrid Cotoneasters. – Gard. Chron., Ser. 3(84): 44.
Fagerlind F. 1944. Die Samenbildung und die Zytologie bei agamospermischen und sexuellen Arten von Elatostema und einigen nahestehenden Gattungen nebst Beleuchtung einiger damit zusammenhängender Probleme. – Kungl. Sv. Vetensk.-Akad. Handl., Ser. III, 21(4): 1-130.
Fay MF, Lledó MD, Richardson JE, Rye BL, Hopper SD. 2001. Molecular data confirm the affinities of the South-West Australian endemic Granitites with Alphitonia (Rhamnaceae). – Kew Bull. 56: 669-675.
Fehrenbach S, Barthlott W. 1988. Mikromorphologie der Epicuticular-Wachse der Rosales s.l. und deren systematische Gliederung. – Bot. Jahrb. Syst. 109: 407-428.
Feng T, Moore MJ, Sun Y, Meng A, Chu H, Li J, Wang H. 2015. A new species of Argentina (Rosaceae, Potentilleae) from Southeast Tibet, with reference to the taxonomic status of the genus. – Plant Syst. Evol. 301: 911-921.
Feng T, Moore MJ, Yan M-H, Sun Y-X, Zhang H-J, Meng A-P, Li X-D, Jian S-G, Li J-Q, Wang H-C. 2017. Phylogenetic study of the tribe Potentilleae (Rosaceae), with further insight into the disintegration of Sibbaldia. – J. Syst. Evol. 55: 177-191.
Figueiredo E. 1995. A revision of Lasiodiscus (Rhamnaceae). – Kew Bull. 50: 495-526.
Flinck KE, Hylmö B. 1966. A list of series and species in the genus Cotoneaster. – Bot. Not. 119: 445-463.
Focke WO. 1889. Anmerkungen zur Gattung Potentilla. – Abh. Naturwiss. Ver. Bremen 10: 43-420.
Focke WO. 1894. Rosaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(3), W. Engelmann, Leipzig, pp. 1-61.
Focke WO. 1911. Species Ruborum. Monographia Generis Rubi Prodromus I-II. – Bibl. Bot. 72: 1-223.
Fosberg FR. 1942. The genus Batocarpus Karst. (Moraceae). – Proc. Biol. Soc. Washington 55: 99-101.
Fosberg FR. 1960. Introgression in Artocarpus in Micronesia. – Brittonia 12: 101-113.
Fougère-Danezan M, Joly S, Bruneau A, Gao XF, Zhang LB. 2015. Phylogeny and biogeography of wild roses with specific attention to polyploids. – Ann. Bot. 115: 275-291.
Freisleben R. 1933. Untersuchungen über Bildung und Auflösung von Cystolithen bei den Urticales. – Flora 127: 1-45.
Frett JJ. 1989. Germination requirements of Hovenia dulcis seeds. – Hort. Sci. 24: 152.
Friis I. 1981. A synopsis of Girardinia (Urticaceae). – Kew Bull. 36: 143-157.
Friis I. 1982. The identity of Urera longifolia and U. oligoloba – a supplement to Chew’s monograph of Laportea (Urticaceae). – Nord. J. Bot. 2: 231-233.
Friis I. 1983a. A synopsis of Obetia (Urticaceae). – Kew Bull. 38: 221-228.
Friis I. 1983b. The acaulescent and succulent species of Dorstenia sect. Kosaria (Moraceae) from NE tropical Africa and Arabia. – Nord. J. Bot. 3: 533-538.
Friis I. 1984. Studies in tropical African Urticaceae and Moraceae. – Ph.D. diss., Acta Univ. Upsal. 761.
Friis I. 1985a. Notes on Somalian species of Ficus (Moraceae). – Nord. J. Bot. 5: 331-333.
Friis I. 1985b. The genus Urera (Urticaceae) in eastern tropical Africa. – Nord. J. Bot. 5: 547-553.
Friis I. 1985c. Two new taxa and a new combination in the Urticaceae for the Flora Zambesiaca. – Bol. Soc. Brot. 58: 201-214.
Friis I. 1986. The typification and identity of three species of Parietaria L. (Urticaceae) ostensibly described from South Africa. – Taxon 35: 701-705.
Friis I. 1988a. Distribution patterns and biological observations in the Urticaceae of Sub-Saharan Africa, Madagascar and the Mascarenes. – Monogr. Syst. Bot. Missouri Bot. Gard. 25: 527-542.
Friis I. 1988b. New taxa and combinations in tropical African Pilea (Urticaceae). – Kew Bull. 43: 648.
Friis I. 1989a. The Urticaceae: a systematic review. – In: Crane PR, Blackmore S (eds), Evolution, systematics, and fossil history of the Hamamelidae 2, ‘Higher’ Hamamelidae, Syst. Assoc. Spec. Vol. 40B, Clarendon Press, Oxford, pp. 285-308.
Friis I. 1989b. A revision of Pilea (Urticaceae) in Africa. – Kew Bull. 44: 557-600.
Friis I. 1990. An additional note on the genus Droguetia (Urticaceae). – Nord. J. Bot. 10: 431-432.
Friis I. 1993a. Barbeyaceae. – In: Kubitzki K, Rohwer JG, Bittrich V (eds), The families and genera of vascular plants II. Flowering plants. Dicotyledons. Magnoliid, hamamelid and caryophyllid families, Springer, Berlin, Heidelberg, New York, pp. 141-143.
Friis I. 1993b. Urticaceae. – In: Kubitzki K, Rohwer JG, Bittrich V (eds), The families and genera of vascular plants II. Flowering plants. Dicotyledons. Magnoliid, hamamelid and caryophyllid families, Springer, Berlin, Heidelberg, New York, pp. 612-630.
Friis I, Jellis S. 1984. A synopsis of Pouzolzia Gaud. (Urticaceae) in tropical Africa, with an identification of P. erythraeae and P. piscicelliana. – Kew Bull. 39: 587-601.
Friis I, Marais W. 1982. Name changes for well known species of Boehmeria (Urticaceae). – Kew Bull. 37: 163-164.
Friis I, Vollesen K. 1980. The identity of the Ethiopian monotypic genus Tzellemtinia Chiov. – Bot. Not. 133: 347-349.
Friis I, Wilmot-Dear CM. 1988. A revision of the tribe Forsskaoleae (Urticaceae). – Nord. J. Bot. 8: 25-59.
Friis I, Wilmot-Dear CM. 1997. Proposal to reject the name Pentocnide so as to maintain Phenax (Urticaceae). – Taxon 46: 773-774.
Friis I, Immelman K, Wilmot-Dear CM. 1987. New taxa and combinations in Old World Urticaceae. – Nord. J. Bot. 7: 125-126.
Fröhner SE. 1986. Zur infragenerischen Gliederung der Gattung Alchemilla L. in Eurasien. – Gleditschia 14: 3-49.
Fröhner SE. 2008. Auf dem Weg zu einer Monographie der Gattung Alchemilla L. (Rosaceae). – Feddes Repert. 119: 253-271.
Fuks R. 1987. O gênero Agrimonia L. (Rosaceae) no Brasil. Albertoa 1: 73-84.
Fukuoka N. 1982. On pseudomonomerous pistil of the Ulmaceae. – Acta Phytotaxon. Geobot. 32: 84-91.
Furr M, Mahlberg PG. 1981. Histochemical analysis of laticifers and glandular trichomes in Cannabis sativa. – J. Natur. Prod. (Lloydia) 44: 153-159.
Gagnepain F. 1926. Quelques Artocarpus nouveaux d’Indo-Chine. – Bull. Soc. Bot. France 73: 86-91.
Gajewski W. 1957. A cytogenetic study of the genus Geum. – Monogr. Bot. (Warszawa) 4: 1-414.
Gajewski W. 1959. Evolution in the genus Geum. – Evolution 13: 378-388.
Galatis B. 1988. Microtubules and epithem-cell morphogenesis in hydatodes of Pilea cadierei. – Planta 176: 287-297.
Galil J, Zeroni M. 1967. On the pollination of Zizyphus spina-christi (L.) Willd. in Israel. – Israel J. Bot. 16: 71-77.
Ganeva T, Uzunova K, Koleva D. 2009. Comparative leaf epidermis investigation in species of genus Crataegus L. (Rosaceae) from Bulgaria. – Feddes Repert. 120: 169-184.
Gangadhara MK, Inamdar JA. 1977. Trichomes and stomata and their taxonomic significance in the Urticales. – Plant Syst. Evol. 127: 121-137.
García MA, Nicholson EH, Nickrent DL. 2004. Extensive intraindividual variation in plastid rDNA sequences from the holoparasite Cynomorium coccineum (Cynomoriaceae). – J. Mol. Evol. 58: 322-332.
Gardner EM, Sarraf P, Williams EW, Zerega NJC. 2017. Phylogeny and biogeography of Maclura (Moraceae) and the origin of an anachronistic fruit. – Mol. Phylogen. Evol. 117: 49-59.
Gardner IC. 1958. Nitrogen fixation in Elaeagnus root nodules. – Nature 181: 717-718.
Gardner IC, Bond G. 1957. Observations on the root nodules of Shepherdia. – Can. J. Bot. 35: 305-314.
Gebauer R. 1994. Some taxa of Parietaria (Urticaceae) from tropical Africa. – Nord. J. Bot. 14: 501-514.
Gehrke B, Bräuchler C, Romoleroux K, Lundberg M, Heubl G, Eriksson T. 2008. Molecular phylogenetics of Alchemilla, Aphanes and Lachemilla (Rosaceae) inferred from plastid and nuclear intron and spacer DNA sequences, with comments on generic classification. – Mol. Phylogen. Evol. 47: 1030-1044.
Geltman DV. 1982. The genus Urtica (Urticaceae) in the flora of East Siberia and the Far East of the USSR. – Bot. Žurn. 67: 194-207.
Geltman DV. 1998. New species and new combinations of Urtica (Urticaceae) from South America. – Novon 8: 15-17.
Gemoll K. 1902. Anatomisch-systematische Untersuchung des Blattes der Rhamneen aus der Triben: Rhamneen, Colletieen und Gouanieen. – Beih. Bot. Centralbl. 12: 351-421.
Gentcheff G, Gustafsson Å. 1940. Parthenogenesis and pseudogamy in Potentilla. – Bot. Not. 93: 109-132.
Gentry AH. 1983. Plagioceltis (Ulmaceae) – a superfluous genus. – Taxon 32: 460-461.
Gerlach D von. 1965. Befruchtung und Autogamie bei Rubus caesius. – Biol. Zentralbl. 84: 611-633.
Gerstner E, Mätzke V, Pfeil E. 1968. Zur chemischen und biologischen Systematik der Rosaceen: Untersuchungen des Flavynsystems D-Oxynitrilase. – Naturwissenschaften 55: 561-563.
Giannasi DE. 1978. Generic relationships in the Ulmaceae based on flavonoid chemistry. – Taxon 27: 331-344.
Giannasi DE. 1986. Phytochemical aspects of phylogeny in Hamamelidae. – Ann. Missouri Bot. Gard. 73: 417-437.
Gilg E. 1894. Elaeagnaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(6a), W. Engelmann, Leipzig, pp. 246-251.
Gladkova VN. 1969. On the systematic position of the genus Dichotomanthus Kurz. – Bot. J. U.S.S.R. Bot. Soc. 54: 431-436.
Gladkova VN. 1972. On the origin of the subfamily Maloideae. – Bot. Žurn. 57: 42-49. [In Russian]
Godwin H. 1967. Pollen-analytic evidence fort he cultivation of Cannabis in England. – Rev. Palaeobot. Palynol. 4: 71-80.
Goldblatt P. 1976. Cytotaxonomic studies in the tribe Quillajeae (Rosaceae). – Ann. Missouri Bot. Gard. 63: 200-206.
Golubkova EI. 1991. New subfamily Coleogynoideae (Rosaceae). – Sci. Reports Higher School, Biol. Sci., 3(327): 102-109.
Godt MJW, Race T, Hamrick JL. 1997. A population genetic analysis of Ziziphus celata, an endangered Florida shrub. – J. Heredity 88: 531-533.
Gotelli MM, Galati BG, Zarlavsky G. 2016. Pollen development and anther morphology in 14 species of Rhamnaceae. – Plant Syst. Evol. 302: 1433-1444.
Gottlieb OR, Lima RA de, Mendes PH, Magalhães MT. 1975. Constituents of Brazilian Moraceae. – Phytochemistry 14: 1674-1675.
Graham RA. 1958a. Notes on African Rosaceae II. – Kew Bull. 12: 405-407.
Graham RA. 1958b. Notes on African Rosaceae III. – Kew Bull. 12: 428.
Graham RA. 1960. Rosaceae. – In: Hubbard CE, Milne-Redhead E (ed), Flora of tropical East Africa, Crown Agents for Oversea Governments and Administrations, London, pp. 1-61.
Grandoso e. 1964. Las especies Argentinas del género Acaena (Rosaceae). – Darwiniana 13: 209-342.
Greene EL. 1887. West American phases of the genus Potentilla. – Pittonia 1: 95-106.
Greene EL. 1906. Segregates from Sieversia. – Leafl. Bot. Observ. Crit. 1: 174-179.
Grey-Wilson C. 1978. Alvimiantha, a new genus of Rhamnaceae from Bahia, Brazil. – Bradea 2: 287-290.
Grice AC. 1996. Seed production, dispersal and germination in Cryptostegia grandiflora and Ziziphus mauritiana, two invasive shrubs in tropical woodlands in northern Australia. – Aust. J. Ecol. 21: 324-331.
Grice AC. 1998. Ecology in the management of Indian jujube (Ziziphus mauritiana). – Weed Scil. 46: 467-474.
Grondóna E. 1964. Las especies argentinas del género Acaena (Rosaceae). – Darwiniana 13: 209-342.
Grosbard S. 1924. Développement du pistil chez l’urticée Girardinia zeylanica. – Bull. Acad. Pol., B 1924: 437-443.
Grosse-Veldmann B, Nürk NM, Smissen R, Breitwieser I, Quandt D, Weigend M. 2016. Pulling the sting out of nettle systematics – a comprehensive phylogeny of the genus Urtica L. (Urticaceae). – Mol. Phylogen. Evol. 102: 9-19.
Grosse-Veldmann B, Conn BJ, Weigend M. 2016. Weeding the nettles IV: a redefinition of Urtica incisa and allies in New Zealand and Australia, including the segregation of two new species Urtica sykesii and U. perconfusa. – Phytotaxa 245: 251-261.
Grudzinskaya IA. 1967. The Ulmaceae and reasons for distinguishing Celtidoideae as a separate family Celtidaceae Link. – Bot. Žurn. 52: 1723-1748. [In Russian with English summary]
Guérin P. 1923. Les urticacées: cellules à mucilage, lactifères et canaux sécréteurs. – Bull. Bot. Soc. France 70: 125-136, 207-215, 255-263.
Guillaumet JL. 1965. Un nouveau Dorstenia (Moraceae) en Côte d’Ivoire. – Adansonia, sér. II, 5: 99-102.
Gustafsson Å. 1933. Chromosomenzahlen in der Gattung Rubus. – Hereditas 18: 77-80.
Gustafsson Å. 1939. Differential polyploidy within the blackberries. – Hereditas 25: 33-47.
Gustafsson Å. 1943. The genesis of the European blackberry flora. – Lunds Univ. Årsskr., N. F., Avd. II, 39(6).
Gustafsson Å. 1944. The constitution of the Rosa canina complex. – Hereditas 30: 405-428.
Gustafsson CE. 1934. Rubi africani. – Ark. f. Bot. 26A(7): 1-68.
Guymer GP. 1995. Elaeagnaceae. – In: Orchard AE (ed), Flora of Australia 16, CSIRO, Melbourne, Australia, pp. 1-3.
Haas K, Rentschler I. 1984. Discrimination between epicuticular and intracuticular wax in blackberry leaves: ultrastructural and chemical evidence. – Plant Sci. Letters 36: 143-147.
Hadiah JT, Conn BJ. 2009. Usefulness of morphological characters for infrageneric classification of Elatostema (Urticaceae). – Blumea 54: 181-191.
Hadiah JT, Quinn CJ, Conn BJ. 2003. Phylogeny of Elatostema (Urticaceae) using chloroplast DNA data. – Telopea 10: 235-246.
Hadiah JT, Conn BJ, Quinn CJ. 2008. Infra-familial phylogeny of Urticaceae, using chloroplast sequence data. – Aust. Syst. Bot. 21: 375-385.
Håkansson A. 1946. Untersuchungen über die Embryologie einiger Potentilla-Formen. – Lunds Univ. Årsskr., N. F., Avd. II, 42(5): 1-70.
Hall KFM, Parsons RF. 1987. Ecology of Discaria (Rhamnaceae) in Victoria. – Proc. Roy. Soc. Victoria 99: 99-108.
Hallé N, Aké Assi L. 1967. Le Dorstenia djettii J. L. Guillaumet est un Craterogyne. – Adansonia, sér. II, 7: 390.
Hamilton AC. 1976. Identification of East African Urticales pollen. – Pollen Spores 18: 27-66.
Hammond CT, Mahlberg PG. 1973. Morphology of glandular hairs of Cannabis sativa from scanning electron microscopy. – Amer. J. Bot. 60: 524-528.
Hammond CT, Mahlberg PG. 1977. Morphogenesis of capitate glandular hairs of Cannabis sativa L. (Cannabaceae). – Amer. J. Bot. 64: 1023-1031.
Hammond CT, Mahlberg PG. 1978. Ultrastructural development of capitate glandular hairs of Cannabis sativa L. (Cannabaceae). – Amer. J. Bot. 65: 140-151.
Hanácková Z, Piñeyro López A. 1999. The Karwinskia parvifolia flower. – Biologia (Bratislava) 54: 85-90.
Hans AS. 1972. Cytomorphology of arborescent Moraceae. – J. Arnold Arbor. 53: 216-225.
Hans AS. 1981. Compatibility and crossability studies in Ulmus. – Silvae Genet. 30: 149-152.
Hardig TM, Soltis PS, Soltis DE. 2000. Diversification of the North American shrub genus Ceanothus (Rhamnaceae): conflicting phylogenies from nuclear ribosomal DNA and chloroplast DNA. – Amer. J. Bot. 87: 108-123.
Hardig TM, Soltis PS, Soltis DE, Hudson RB. 2002. Morphological and molecular analysis of putative hybrid speciation in Ceanothus (Rhamnaceae). – Syst. Bot. 27: 734-746.
Hardin JW. 1973. The enigmatic chokeberries (Aronia, Rosaceae). – Bull. Torrey Bot. Club 100: 178-184.
Hardin JW. 1981. Atlas of foliar surface features in woody plants II. Broussonetia, Morus, and Maclura of North America. – Bull. Torrey Bot. Club 108: 338-346.
Harrison JE, Beveridge T. 2002. Fruit structure of Hippophae rhamnoides cv. Indian Summer (sea buckthorn). – Can. J. Bot. 80: 399-409.
Harrison RD. 2005. Figs and the diversity of tropical rainforests. – Bioscience 55: 1053-1064.
Harvey CF, Braggins JE. 1985. Reproduction of the New Zealand taxa of Pomaderris Labill. (Rhamnaceae). – New Zealand J. Bot. 23: 151-156.
Hassler É. 1919. Enumeratio Urticacearum Paraguariensium. – Ann. Cons. Jard. Bot. Genève 21: 141-143.
Hauman L. 1948. Moraceae (excl. Ficus). – In: Flore du Congo Belge et du Ruana-Urundi 1, Bruxelles, pp. 52-98.
Hauman L, Balle S. 1934. Les ”Alchemilla” du Congo Belge. – Rev. Zool. Bot. Afr. 24: 301-368.
Hauman L, Balle S. 1936a. Les Alchemilla de l’Abyssinie et de Madagascar. – Bull. Jard. Bot. État 14: 1-55.
Hauman L, Balle S. 1936b. Les Alchemilla de l’Afrique australe. – Mém. Acad. Roy. Belg. (Cl. Sci.) Coll. 8, sér. II, 16: 1-29.
Hayırlıoğlu-Ayaz S, Beyazoğlu O. 1996. Chromosome numbers in species of Alchemilla L. belonging to the series Sericeae Bus. and Pubescentes Bus. (section Alchemilla) in Turkey. – Caryologia 49: 9.
Hayırlıoğlu-Ayaz S, Beyazoğlu O. 1997. New chromosome numbers in Alchemilla L. (Rosaceae) from Turkey. – Willdenowia 27: 191-194.
Hebda RJ, Chinnappa CC. 1990. Studies on the pollen morphology of Rosaceae in Canada. – Rev. Palaeobot. Palynol. 64: 103-108.
Hebda RJ, Chinnappa CC. 1994. Studies on pollen morphology of Rosaceae. – Acta Bot. Gall. 141: 183-193.
Hedberg O. 1986. Taxonomic notes on Ethiopian Rosaceae. – Nord. J. Bot. 6: 573-579.
Hedlund JT. 1901. Monographie der Gattung Sorbus. – Kongl. Sv. Vetensk.-Akad. Handl. 35(1).
Heide F. 1927. Observations on the pollination of some flowers in Dutch East Indies. – Dansk Bot. Ark. 5: 1-42.
Helfgott DM, Francisco-Ortega J, Santos-Guerra A, Jansen RK, Simpson BB. 2000. Biogeography and breeding system evolution of the woody Bencomia alliance (Rosaceae) in Macaronesia based on ITS sequence data. – Syst. Bot. 25: 82-97.
Henning T, Quandt D, Grosse-Veldmann B, Monro A, Weigend M. 2014. Weeding the nettles II: a delimitation of “Urtica dioica L.” (Urticaceae) based on morphological and molecular data, including a rehabilitation of Urtica gracilis Ait. – Phytotaxa 162: 61-83.
Henrickson J. 1986a. Notes on Rosaceae. – Phytologia 60: 468.
Henrickson J. 1986b. Xerospiraea, a generic segregate of Spiraea (Rosaceae) from Mexico. – Aliso 11: 199-211.
Henrickson J. 1987. Two new species of Cercocarpus (Rosaceae) from Mexico. – Syst. Bot. 12: 293-298.
Herre EA, Machado CA, Bermingham E, Nason JD, Windsor DM, McCafferty SS, Van Houten W, Bachmann K. 1996. Molecular phylogenies of figs and their pollinating wasps. – J. Biogeogr. 23: 521-530.
Herre EA, Jandér KC, Machado CA. 2008. Evolutionary ecology of figs and their associates: recent progress and outstanding puzzles. – Ann. Rev. Ecol. Syst. 39: 439-458.
Herrera M, Arbeloa A. 1989. Influence of the pistil on pollen tube kinetics in peach (Prunus persica). – Amer. J. Bot. 76: 1441-1447.
Herzog T. 1903. Anatomisch-systematische Untersuchung des Blattes der Rhamnaceen aus den Triben: Ventilagineen, Zizypheen und Rhamneen. – Beih. Bot. Centralbl. 15: 95-207.
Heslop-Harrison Y. 1953. Cytological studies in the genus Rubus L. I. Chromosome numbers in the British Rubus flora. – New Phytol. 52: 22-39.
Hess WJ, Henrickson J. 1987. A taxonomic revision of Vauquelinia (Rosaceae). – Sida Contr. Bot. 12: 101-163.
Hewson HJ. 1989. Ulmaceae. – In: George AS (ed), Flora of Australia 3, Australan Government Publ. Service, Canberra, pp. 4-13.
Hijman MEE. 1990. New taxa and combinations in Dorstenia (Moraceae) of Africa. – Kew Bull. 45: 361-368.
Hjelmqvist H. 1959. Studien über Embryologie und Variabilität bei einigen Aphanes-Arten. – Bot. Not. 112: 17-64.
Hjelmquist H. 1962. The embryo sac development of some Cotoneaster species. – Bot. Not. 115: 208-236.
Hoen PP, Punt W. 1989. Pollen morphology of the tribe Dorstenieae (Moraceae). – Rev. Palaeobot. Palyn. 57: 187-220.
Hoffmann P. 1991. Blütenmorphologische Untersuchungen an Vertretern der Rhamnaceae unter besonderer Berücksichtigung der Gattung Reynosia Griseb. – M.Sc. thesis, Humboldt-Universität, Berlin, Germany.
Holm L. 1979. Some problems in angiosperm taxonomy in light of the rust data. – In: Hedberg I (ed), Parasites as plant taxonomists, Uppsala, pp. 177-181.
Holm T. 1927. Boehmeria cylindrica (L.) Sw.: a morphological study. – Amer. J. Sci., 5th ser., 13: 115-122.
Holmgren K, Oxelman B. 2004. Generic limits in Rhamnus L. s.l. (Rhamnaceae) inferred from nuclear and chloroplast DNA sequence phylogenies. – Taxon 53: 383-390.
Holthuijzen AMA, Boerboom JHA. 1982. The Cecropia seedbank in the Surinam lowland rain forest. – Biotropica 14: 62-68.
Holub J. 1992. A preliminary checklist of Rubus species occurring in the Czech Republic. – Preslia 64: 97-132.
Hopkins HCF, Pillon Y, Stacy EA, Kellermann J. 2015. Jaffrea, a new genus of Rhamnaceae endemic to New Caledonia, with notes on Alphitonia and Emmenosperma. – Kew Bull. 70: 42 DOI 10.1007/S12225-015-9593-6
Howarth DG, Gardner DE, Morden CW. 1997. Phylogeny of Rubus Subgenus Idaeobatus (Rosaceae) and its implications toward colonization of the Hawaiian Islands. – Syst. Bot. 22: 433-441.
Huber H. 1963. Die Verwandtschaftsverhältnisse der Rosifloren. – Mitt. Bot. Staatssamml. München 5: 1-48.
Hull P, Smart GJB. 1984. Variation in two Sorbus species endemic to the Isle of Arran, Scotland. – Ann. Bot., N. S., 53: 641-648.
Hummer KE, Janick J. 2009. Rosaceae: taxonomy, economic importance, genomics. – In: Folta KM, Gardiner SE (eds), Genetics and Genomics of Rosaceae, Springer, New York, pp. 1-17.
Humphries CJ, Blackmore S. 1989. A review of the classification of the Moraceae. – In: Crane PR, Blackmore S (eds), Evolution, systematics, and fossil history of the Hamamelidae, Vol. 2, ‘Higher’ Hamamelidae, Syst. Assoc. Spec. Vol. 40B, Clarendon Press, Oxford, pp. 267-277.
Hurst CC. 1931-1932. Embryo-sac formation in diploid and polyploid species of Rosa. – Proc. Roy. Soc. London, Sect. B, 109: 126-148.
Hurusawa I. 1943. Cotoneaster Asiae Orient. – Acta Phytotaxon. Geobot. 13: 225.
Hurusawa I. 1972. Taxonomische Untersuchung der Gattung Cotoneaster (Rosaceen) auf karpologischer Grundlage. – Inform. Ann. Hort. Bot. Fac. Sci. Univ. Tokyoensis.
Hutchinson J. 1915. New tropical African species of Ficus. – Kew Bull. 1915: 313-344.
Hylmö B, Fryer J. 1999. Cotoneasters in Europe. – Acta Bot. Fenn. 162: 179-184.
Hyvönen J. 1996. On phylogeny of Hippophae (Elaeagnaceae). – Nord. J. Bot. 16: 51-62.
Ikeda H. 1989. Chromosome numbers of the Himalayan Potentilla (Rosaceae). – J. Jap. Bot. 64: 361-367.
Ikeda H, Ohba H. 1999. A systematic revision of Potentilla L. section Leptostylae (Rosaceae) in the Himalaya and adjacent regions. – Bull. Univ. Mus. Univ. Tokyo 39: 31-117.
Iketani H, Ohashi H. 1991a. Anatomical structure of fruits and evolution of the tribe Sorbeae in the subfamily Maloideae (Rosaceae). – J. Jap. Bot. 66: 319-351.
Iketani H, Ohashi H. 1991b. Pourthiaea (Rosaceae) distinct from Photinia. – J. Jap. Bot. 66: 352-355.
Iltis H. 1913. Über das Gynophor und die Fruchtausbildung bei der Gattung Geum. – Sitz.-Ber. Math.-Naturwiss. Kl. Kais. Akad. Wiss. 122: 1177-1212.
Islam MB, Simmons MP. 2006. A thorny dilemma: testing alternative intrageneric classifications within Zizyphus (Rhamnaceae). – Syst. Bot. 31: 826-842.
Izmaiłow R. 1981. Karyological studies in species of Alchemilla L. from the series Calycinae Bus. (section Brevicaulon Rothm.). – Acta Biol. Cracov., Ser. Bot. 23: 117-130.
Izmaiłow R. 1982. Further karyological studies in species of Alchemilla L. from the series Calycinae Bus. (section Brevicaulon Rothm.). – Acta Biol. Cracov., Ser. Bot. 24: 127-141.
Izmaiłow R. 1986. Cyto-embryological studies on Alchemilla L. (series Calycinae Buser) II. Apomictic processes in ovules. – Acta Biol. Cracov., Ser. Bot. 28: 39-64.
Izmaiłow R. 1994. Embryo and endosperm relations at early stages of their development in Alchemilla subsect. Heliodrosium (Rosaceae). – Polish Bot. Stud. 8: 61-67.
Jackson AP. 2004. Cophylogeny of the Ficus microcosm. – Biol. Rev. 79: 751-768.
Jackson AP, Machado CA, Robbins N, Herre EA. 2008. Multi-locus phylogenetic analysis of neotropical figs does not support co-speciation with the pollinators: the importance of systematic scale in fig/wasp cophylogenetic studies. – Symbiosis 45: 57-72.
Jackson G. 1934. The morphology of the flowers of Rosa and certain closely related genera. – Amer. J. Bot. 21: 453-466.
Jacobsen P. 1957. The sex chromosomes in Humulus. – Hereditas 43: 357-370.
Jacobsson-Stiasny E. 1914. Versuch einer embryologische-phylogenetischen Bearbeitung der Rosaceae. – Sitz.-Ber. Akad. Wiss. Wien 123: 1-38.
Jankun A, Kovanda M. 1988. Embryological studies in Sorbus III. Apomixis at the diploid level in Sorbus eximia. – Preslia 60: 193-213.
Jansen S, Piessschaert F, Smets E. 2000. Wood anatomy of Elaeagnaceae, with comments on vestured pits, helical thickenings, and systematic relationships. – Amer. J. Bot. 87: 20-28.
Jansen S, Sano Y, Choat B, Rabaey D, Lens F, Dute RR. 2007. Pit membranes in tracheary elements of Rosaceae and related families: new records of tori and pseudotori. – Amer. J. Bot. 94: 503-514.
Janzen DH. 1973. Dissolution of mutualism between Cecropia and its Azteca ants. – Biotropica 5: 15-28.
Janzen DH, McKey D. 1977. Musanga cecropioides is a Cecropia without ants. – Biotropica 9: 57.
Jarrett FM. 1959a. Studies in Artocarpus and allied genera I. General considerations. – J. Arnold Arbor. 40: 1-29.
Jarrett FM. 1959b. Studies in Artocarpus and allied genera II. A revision of Prainea. – J. Arnold Arbor. 40: 30-37.
Jarrett FM. 1959c. Studies in Artocarpus and allied genera III. A revision of Artocarpus subgenus Artocarpus. – J. Arnold Arbor. 40: 113-155, 298-368.
Jarrett FM. 1960a. Studies in Artocarpus and allied genera IV. A revision of Artocarpus subgenus Pseudojaca. – J. Arnold Arbor. 41: 73-139.
Jarrett FM. 1960b. Studies in Artocarpus and allied genera V. A revision of Parartocarpus and Hullettia. – J. Arnold Arbor. 41: 320-340.
Jarrett FM. 1975. Four new Artocarpus species from Indo-Malesia (Moraceae). – Blumea 22: 409-410.
Jarrett FM. 1976. The syncarp of Artocarpus – a unique biological phenomenon. – Gard. Bull (Singapore) 24: 35-39.
Jeong SC, Liston A, Myrold DD. 1997. Molecular phylogeny of the genus Ceanothus (Rhamnaceae) using rbcL and ndhF sequences. – Theor. Appl. Gen. 94: 852-857.
Jestrow B, Valdés JJ, Jiménez Rodríguez F, Francisco-Ortega J. 2012. Phylogenetic placement of the Dominican Republic endemic genus Sarcopilea (Urticaceae). – Taxon 61: 592-600.
Johnston MC. 1962. Revision of Condalia including Microrhamnus (Rhamnaceae). – Brittonia 14: 332-368.
Johnston MC. 1963a. The species of Ziziphus indigenous to the United States and Mexico. – Amer. J. Bot. 50: 1020-1027.
Johnston MC. 1963b. Novelties in Colubrina including Cormonema and Hybosperma (Rhamnaceae). – Wrightia 3: 91-92.
Johnston MC. 1964. The fourteen species of Ziziphus including Sarcomphalus (Rhamnaceae) indigenous to the West Indies. – Amer. J. Bot. 51: 1113-1118.
Johnston MC. 1971. Revision of Colubrina (Rhamnaceae). – Brittonia 23: 2-53.
Johnston MC. 1972. Rhamnaceae. – In: Milne-Redhead E, Polhill RM (eds), Flora of tropical East Africa, Crown Agents for Oversea Governments and Administrations, London, pp. 1-40.
Johnston MC. 1973. Revision of Kentrothamnus (Rhamnaceae). – J. Arnold Arb. 54: 471-473.
Johnston MC. 1974. Revision of Scutia (Rhamnaceae). – Bull. Torrey Bot. Club 101: 64-71.
Johnston MC. 1988. Gouania axilliflora (Rhamnaceae), a new species from Peru. – Syst. Bot. 13: 493-495.
Johri BM, Konar RN. 1956. The floral morphology and embryology of Ficus relgiosa Linn. – Phytomorphology 6: 97-111.
Joly S, Bruneau A. 2007. Delimiting species boundaries in Rosa Sect. Cinnamomeae (Rosaceae) in eastern North America. – Syst. Bot. 32: 819-836.
Joly S, Starr JR, Lewis WH, Bruneau A. 2006. Polyploid and hybrid evolution in roses east of the Rocky Mountains. – Amer. J. Bot. 93: 412-425.
Jones GN. 1946. American species of Amelanchier. – Illinois Biol. Monogr. 20: 1-126.
Jossang A, Zahir A, Diakite D. 1996. Mauritine J, a cyclopeptide alkaloid from Zizyphus mauritiana. – Phytochemistry 42: 565-567.
Jousselin E, Rasplus J-Y, Kjellberg F. 2003. Convergence and coevolution in a mutualism: evidence from a molecular phylogeny of Ficus. – Evolution 57: 1255-1269.
Juel HO. 1902. Entwicklungsgeschichte des Samens von Cynomorium. – Beih. Bot. Centralbl. 13: 194-202.
Juel HO. 1910. Cynomorium und Hippuris. – Svensk Bot. Tidskr. 4: 151-159.
Juel HO. 1918. Beiträge zur Blütenanatomie und zur Systematik der Rosaceen. – Kungl. Sv. Vetensk.-Akad. Handl. 58: 1-81.
Juel HO. 1927. Über die Blütenanatomie einiger Rosaceen. – Nova Acta Reg. Soc. Sci. Upsal., vol. extr., Uppsala.
Juel HO. 1929. Beiträge zur Morphologie und Entwicklungsgeschichte der Rhamnaceen. – Kungl. Sv. Vetensk.-Akad. Handl. 7(3): 1-13.
Kachroo P, Bhat MM. 1981. Leaf anatomy of Urticales. – J. Econ. Taxon. Bot. 2: 45-64.
Kalkman C. 1965. The Old World species of Prunus subg. Laurocerasus including those formerly referred to Pygeum. – Blumea 13: 1-115.
Kalkman C. 1968. Potentilla, Duchesnea, and Fragaria in Malesia (Rosaceae). – Blumea 16: 325-354.
Kalkman C. 1973. The Malesian species of subfamily Maloideae (Rosaceae). – Blumea 21: 413-442.
Kalkman C. 1988. The phylogeny of the Rosaceae. – Bot. J. Linn. Soc. 98: 37-59.
Kalkman C. 1993. Rosaceae. – In: Kalkman C et al. (eds), Flora Malesiana I, 11(2), Rijksherbarium/Hortus Botanicus, Leiden, pp. 227-351.
Kalkman C. 2004. Rosaceae. – In: Kubitzki K (ed), The families and genera of vascular plants VI. Flowering plants. Dicotyledons. Celastrales, Oxalidales, Rosales, Cornales, Ericales, Springer, Berlin, Heidelberg, New York, pp. 343-386.
Kania W. 1973. Entwicklungsgeschichtliche Untersuchungen an Rosaceenblüten. – Bot. Jahrb. Syst. 93: 175-246.
Kanzaki S, Yonemori K, Sugiura A, Subhadrabandhu S. 1997. Phylogenetic relationships between jackfruit, the breadfruit and nine other Artocarpus spp. from RFLP analysis of an amplified region of cpDNA. – Sci. Horticult. 70: 57-66.
Katiyar K. 1982. On the pollen morphology in relation to the taxonomic position of the unknown tribe of Rosaceae. – J. Palynol. 16: 63-69.
Keighery GJ. 1978. Siegfriedia. – Aust. Plants 11: 176.
Kellermann J. 2006a. New combinations for two species of Spyridium (Rhamnaceae: Pomaderreae) from the Grampians, Victoria. – Muelleria 22: 97-104.
Kellermann J. 2006b. Cryptandra triplex K. R. Thiele ex Kellermann, a new species of Rhamnaceae (Pomaderreae) from Arnhem Land, Northern Territory. – Austrobaileya 7: 299-303.
Kellermann J. 2007a. Re-instatement of the name Spyridium waterhousei from Kangaroo Island, South Australia, with a short history of the tribe Pomaderreae (Rhamnaceae). – J. Adelaide Bot. Gard. 21. 55-62.
Kellermann J. 2007b. The Australian stellate-haired Rhamnaceae: a systematic study of the tribe Pomaderreae. – Ph.D. diss., School of Botany, The University of Melbourne, Parkville, Melbourne, Victoria.
Kellermann J, Udovicic F. 2007. A revision of the Cryptandra propinqua complex (Rhamnaceae: Pomaderreae). – Proc. Linn. Soc. New South Wales 128: 81-98.
Kellermann J, Udovicic F. 2008. Large indels obscure phylogeny in analysis of chloroplast DNA (trnL-F) sequence data: Pomaderreae (Rhamnaceae) revisited. – Telopea 12: 1-22.
Kellermann J, Udovicic F, Ladiges PY. 2005. Phylogenetic analysis and generic limits of the tribe Pomaderreae (Rhamnaceae) using internal transcribed spacer DNA sequences. – Taxon 54: 619-631.
Kellermann J, Medan D, Aagesen L, Hilger HH. 2005. Rehabilitation of the South American genus Ochetophila Poepp. ex Endl. (Rhamnaceae: Colletieae). – New Zealand J. Bot. 43: 865-869.
Kellermann J, Rye BL, Thiele KR. 2006. Polianthion, a new genus of Rhamnaceae (Pomaderreae) from Western Australia and Queensland. – Aust. Syst. Bot. 19: 169-181.
Kellermann J, Rye BL, Thiele KR. 2007. Blackallia, Serichonus and Papistylus: three closely related genera of Rhamnaceae (Pomaderreae) from south-western Australia. – Nuytsia 16: 299-316.
Keogh JA, Bannister P. 1993. Transoceanic dispersal in the amphiantarctic genus Discaria: an evaluation. – New Zealand J. Bot. 31: 427-430.
Kerr MS. 2004. A phylogenetic and biogeographic analysis of Sanguisorbeae (Rosaceae), with emphasis on the Pleistocene radiation of the high Andean genus Polylepis. – Ph.D. diss., Cell Biology & Molecular Genetics Department, University of Maryland, College Park, Maryland.
Kessler M. 1995a. Polylepis-Wälder Boliviens: Taxa, Ökologie, Verbreitung und Geschichte. – Diss. Bot. 246, J. Cramer, Berlin.
Kessler M. 1995b. The genus Polylepis (Rosaceae) in Bolivia. – Candollea 50: 131-171.
Kessler M, Schmidt-Lebuhn AN. 2006. Taxonomical and distributional notes on Polylepis (Rosaceae). – Organisms Divers. Evol. 6: 67-69.
Killip EP. 1923. New species of Urticaceae from Colombia. – J. Washington Acad. Sci. 13: 354-360.
Killip EP. 1925. New tropical American species of Urticaceae. – J. Washington Acad. Sci. 15: 289-299.
Killip EP, Morton CV. 1931. The genus Lozanella. – J. Washington Acad. Sci. 21: 336-339.
Kim C, Deng T, Chase M, Zhang D-G, Nie Z-L, Sun H. 2015. Generic phylogeny and character evolution in Urticeae (Urticaceae) inferred from nuclear and plastid DNA regions. – Taxon 64: 65-78.
Kjellberg F, Jousselin E, Bronstein JL, Patel A, Yokoyama J, Rasplus J-Y. 2001. Pollination mode in fig wasps: the predictive power of correlated traits. – Proc. Roy. Soc. London, B, 268: 113-121.
Klackenberg J. 1983. The holarctic complex Potentilla fruticosa (Rosaceae). – Nord. J. Bot. 3: 181-191.
Klášterská I. 1969. Cytology and some chromosome numbers of Czechoslovak roses I. – Folia Geobot. Phytotaxon. 4: 175-189.
Klášterská I, Klášterský I. 1974. Cytology and some chromosome numbers of Czechoslovak roses II. – Bot. Not. 127: 328-337.
Klášterská I, Natarajan AT. 1974. Studies on the cytology of the genus Rosa with special reference to the section Caninae. – Hereditas 76: 97-108.
Kline GJ, Sørensen PD. 2008. A revision of Agrimonia (Rosaceae) in North and Central America. – Brittonia 60: 11-33.
Klotz G. 1963. Neue oder kritische Cotoneaster-Arten. – Wiss. Zeitschr. Univ. Jena, Math.-Naturwiss. Reihe 12: 769-786.
Klotz G. 1970. Die Hybridisation, ein wichtiger Evolutionsfaktor der Gattung Cotoneaster Med. – Wiss. Zeitschr. Univ. Jena, Math.-Naturwiss. Reihe 19: 329-344.
Klotz G. 1978. Neue oder kritische Cotoneaster-Arten VIII. – Wiss. Zeitschr. Univ. Jena, Math.-Naturwiss. Reihe 27: 19-26.
Klotz G. 1982. Synopsis der Gattung Cotoneaster I. – Beitr. Phytotaxon. 10.
Klotz G. 1996. Neue oder kritische Cotoneaster-Arten IX. – Mitt. Deutsch. Dendrol. Gesellsch. 82: 67-85.
Klotz G. 2008. Neue oder kritische Cotoneaster-Arten X (Rosaceae). – Feddes Repert. 119: 272-280.
Knuth R. 1931. Geraniaceae. – In: Engler A (†), Harms H, Pax F (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 19a, W. Engelmann, Leipzig, pp. 43-66.
Koehler DL, Smith DM. 1981. Hybridization between Cowania mexicana var. stansburiana and Purshia glandulosa (Rosaceae). – Madroño 28: 13-25.
Koehne E. 1890. Die Gattungen der Pomaceen. – Wiss. Beil. Progr. Falk-Realgymn. Berlin, Gaertner, Berlin.
Koehne E. 1891. Die Gattungen der Pomaceen. – Gartenflora 40: 4-7, 35-38, 59-61.
Koek-Noorman J, Ter Welle BJH. 1976. The anatomy of branch abscission layers in Perebea mollis and Naucleopsis guianensis (Castilleae, Moraceae). – Leiden Bot. Ser. 3: 196-203.
Koek-Noorman J, Topper SMC, Welle BJH ter. 1984a. The systematic wood anatomy of the Moraceae (Urticdales) I. Tribe Castilleae. – IAWA Bull. 5: 183-195.
Koek-Noorman J, Topper SMC, Welle BJH ter. 1984b. The systematic wood anatomy of the Moraceae (Urticdales) II. Tribe Dorstenieae. – IAWA Bull. 5: 317-329.
Koek-Noorman J, Topper SMC, Welle BJH ter. 1984c. The systematic wood anatomy of the Moraceae (Urticdales) III. Tribe Ficeae. – IAWA Bull. 5: 330-334.
Kohls SJ, Thimmapuram J, Buschena CA, Paschke MW, Dawson JO. 1994. Nodulation patterns of actinorhizal plants in the family Rosaceae. – Plant and Soil 162: 229-239.
Koller G. 1981. Sorbaria sorbifolia, Ural false spirea. – Arnoldia 541: 190-191.
Kollmann J, Steinger T, Roy BA. 2000. Evidence of sexuality in European Rubus (Rosaceae) species based on AFLP and allozyme analysis. – Amer. J. Bot. 87: 1592-1598.
Koopman WJM, Wissemann V, Cock K de, Huylenbroeck J van, Riek J de, Sabatino GJH, Visser D, Vosman B, Ritz CM, Maes B, Werlemark G, Nybom H, Debener T, Linde M, Smulders MJM. 2008. AFLP markers as a tool to reconstruct complex relationships: a case study in Rosa (Rosaceae). – Amer. J. Bot. 95: 353-366.
Korine C, Kalko EKV, Herre EA. 2000. Fruit characters and factors affecting fruit removal in a Panamanian community of strangler figs. – Oecologia 123: 560-568.
Kovanda M. 1965. On the generic concepts in the Maloideae. – Preslia 37: 27-34.
Kovanda M, Challice J. 1981. The genus Micromeles revisited. – Folia Geobot. Phytotaxon. 16: 181-193.
Krahulcová A. 1994. Cytogeography of Geum montanum (Rosaceae). – Folia Geobot. Phytotaxon. 29: 85-90.
Kravtsova TI. 2003. Seed coat structure in the Urticaceae and relations of the Urticales. – Bot. Žurn. 88: 11-41. [In Russian]
Kravtsova TI. 2006. Cells with wall ingrowths in the pericarp and seed coat of the representatives of the Urticaceae. – Bot. Žurn. 91: 1369-1378. [In Russian]
Kravtsova TI. 2007. A system of the family Urticaceae. – Bot. Žurn. 92: 3-28.
Kravtsova TI, Oskolski AA. 2007. Cladistic analysis of the Urticaceae, Cecropiaceae and Moraceae (Urticales) based on carpological characters. – Bot. Žurn. 92: 613-640. [In Russian]
Kravtsova TI, Friis I, Wilmot-Dear CM. 2000. Morphology and anatomy of fruits in New World Boehmeria in relation to taxonomy. – Kew Bull. 55: 43-62.
Kravtsova TI, Friis I, Wilmot-Dear CM. 2003. Morphology and anatomy of fruits in Pouzolzia (Urticaceae) in relation to taxonomy. – Kew Bull. 58: 297-327.
Krügel T. 1992a. Zur zytologischen Struktur der Gattung Cotoneaster (Rosaceae, Maloideae) III. – Beitr. Phytotaxon. Univ. Jena 15: 69-86.
Krügel T. 1992b. Zur zytologischen Struktur von x Sorbocotoneaster pozdnjakovii Pojark. – Beitr. Phytotaxon. Univ. Jena 15: 87-92.
Kubitzki K. 1993a. Cannabaceae. – In: Kubitzki K, Rohwer JG, Bittrich V (eds), The families and genera of vascular plants II. Flowering plants. Dicotyledons. Magnoliid, hamamelid and caryophyllid families, Springer, Berlin, Heidelberg, New York, pp. 204-206.
Kubitzki K. 1993b. Cecropiaceae. – In: Kubitzki K, Rohwer JG, Bittrich V (eds), The families and genera of vascular plants II. Flowering plants. Dicotyledons. Magnoliid, hamamelid and caryophyllid families, Springer, Berlin, Heidelberg, New York, pp. 243-246.
Kumar A, Panigrahi G. 1995. Revisionary studies on Coptoneaster Medik. – In: The family Rosaceae in India, Dehra Dun.
Kumar D, Kumar K, Gupta J, Bishnoi N, Kumar S. 2012. A mini review on chemistry and biology of Holoptelea integrifolia Roxb. Planch (Ulmaceae). – Asian Pac. J. Trop. Biomed. doi: 10.1016/S2221-1691(12)60384-0
Kuprianova LA. 1962. Palynological data for the systematics of the orders Fagales and Urticales. – In: Proceedings of the First International Conference on Palynology 1962, Tucson, Arizona; Publ. House of USSR, Academy of Sciences, Moskow, pp. 17-25. [In Russian]
Kyle NE, Jakobek JL, Backhaus RA, Stutz JC, Righetti TL. 1986. Micrografting between N-fixing and non-N-fixing genera of the Rosaceae. – Bot. Gaz. 147: 243-246.
Łańcucka-Środoniowa M. 1967. Two new genera: Hemiptelea Planch. and Weigela Thunb. in the younger Tertiary of Poland. – Acta Palaeobot. 8: 1-19.
Langenfeld W. 1971. Die Evolutionder Gattung Malus Mill – Wiss. Zeitschr. Univ. Rostock 20, Math.-Naturwiss. Reihe 1: 49-51.
Lanjouw J. 1935. Studies in Moraceae I. The genera Trymatococcus Poepp. et Endl. and Craterogyne Lanj. – Rec. Trav. Bot. Néerl. 32: 262-278.
Leandri J. 1950. Les Urticacées de Madagascar. – Ann. Mus. Colon. Marseille, sér. VI, 7-8: 1-93.
Leandri J. 1965. 56. Urticacées. – In: Humbert H (ed), Flore du Madagascar 56, Muséum National d’Histoire Naturelle, Paris, pp. 1-107.
Lebègue A. 1956a. Embryogénie des urticacées. Développement de l’embryon chez l’Urtica dioica. – Compt. Rend. Acad. Sci. Paris 242: 923-926.
Lebègue A. 1956b. Embryogénie des urticacées. Développement de l’embryon chez le Parietaria officinalis. – Compt. Rend. Acad. Sci. Paris 243: 817-820.
Lebègue A. 1956c. Développement de l’embryon chez l’Urtica urens. – Bull. Soc. Bot. France 103: 587-590.
Leberton P. 1965. Eléments de chimiotaxonomie botanique 2. Cas de flavonoides chez les Urticales; conclusions générales. – Bull. Soc. Bot. France 111: 80-93.
Le Coq C. 1963. Contribution à l’étude cytotaxonomique des moracées et des urticacées. – Rev. Gén. Bot. 70: 385-426.
Lee S, Hong S-P. 2011. Phylogenetic relationships of the rare Korean monotypic endemic genus Pentactina Nakai in the tribe Spiraeeae (Rosaceae) based on molecular data). – Plant Syst. Evol. DOI 10.1007/200606-011-0457-8.
Lee S, Wen J. 2001. A phylogenetic analysis of Prunus and the Amygdaloideae (Rosaceae) using ITS sequences of nuclear ribosomal DNA. – Amer. J. Bot. 88: 150-160.
Leins P. 1967. Morphologische Untersuchungen an Elaeagnaceen-Pollenkörnern. – Grana Palynol. 7: 390-399.
Leins P, Orth C. 1979. Zur Entwicklungsgeschichte männlicher Blüten von Humulus lupulus (Cannabaceae). – Bot. Jahrb. Syst. 100: 372-378.
Leliveld JA. 1935. Cytological studies in the genus Ulmus II. The embryo sac and seed development in the common Dutch Elm. – Recl. Trav. Bot. Néerl. 32: 543-573.
Leroy J-F. 1961. Un deuxième Aphananthe (Ulmacée) du Mexique. – J. Agricult. Trop. Bot. Appl. 8: 72-74.
Lersten NR, Curtis JD. 1982. Hydathodes in Physocarpus (Rosaceae: Spiraeoideae). – Can. J. Bot. 60: 850-855.
Lersten NR, Horner HT. 2000. Calcium oxalate crystal types and trends in their distribution patterns in leaves of Prunus (Rosaceae: Prunoideae). – Plant Syst. Evol. 224: 83-96.
Letouzey R. 1967. Notes sur diverses espèces d’Afrique et de Madagascar du genre Urera Gaudich. (Urticaceae). – Adansonia 7: 295-300.
Letouzey R. 1968. Urticaceae. – In: Flore du Cameroun 8, Muséum National d’Histoire Naturelle, Paris, pp. 67-216.
Lewis WH. 1958. The roses of Virginia and West Virginia. – Castanea 23: 77-88.
Li F, Fan Q, Li Q, Chen S, Guo W, Cui D, Liao
W. 2014. Molecular phylogeny of Cotoneaster (Rosaceae) inferred from nuclear ITS and
multiple chloroplast sequences. – Plant Syst. Evol. 300: 1533-1546.
Li H-Q, Wang S, Chen J-Y, Gui P. 2012. Molecular phylogeny of Ficus section Ficus in China based on four DNA regions. – J. Syst. Evol. 50: 422-432.
Li M, Ohi-Toma T, Gao Y-D, Xu B, Zhu Z-M, Ju W-B, Gao X-F. 2017. Molecular phylogenetics and historical biogeography of Sorbus sensu stricto (Rosaceae). – Mol. Phylogen. Evol. 111: 76-86.
Li Q-Y, Guo W, Liao W-B, Macklin JA, Li J-H. 2012. Generic limits of Pyrinae: insights from nuclear ribosomal DNA sequences. – Bot. Stud. 53: 151-164.
Li X-H, Shao J-W, Lu C, Zhang X-P, Qiu Y-X. 2012. Chloroplast phylogeography of a temperate tree Pteroceltis tatarinowii (Ulmaceae) in China. – J. Syst. Evol. 50: 325-333.
Li Y, Smith T, Liu C-J, Awasthi N, Yang J, Wang Y-F, Li C-S. 2011. Endocarps of Prunus (Rosaceae: Prunoideae) from the early Eocene of Wutu, Shandong Province, China. – Taxon 60: 555-564.
Lian Y-S, Chen X-L. 1993. Study on the germplasm resource of the genus Hippophae L. – In: International Symposium on Sea Buckthorn (Hippophae rhamnoides L.), Novosibirsk, Russia, pp. 157-161.
Lian Y-S, Chen X-L, Sun K. 1995. New discoveries of the genus Hippophae L. – In: Proceedings of International Workshop on Seabuckthorn, China Science and Technology Press, Beijing, pp. 60-66.
Lian Y-S, Chen X-L, Lian H. 1998. Systematic classification of the genus Hippophae L. – Seabuckthorn Research 1: 13-23.
Liljefors A. 1953. Studies on propagation, embryology and pollination in Sorbus. – Acta Horti Berg. 16: 277-329.
Liljefors A. 1955. Cytological studies in Sorbus. – Acta Horti Berg. 17: 47-113.
Lian Y-S, Chen X-L, Sun K, Ma R-J. 2003. Clarification of the systematic position of Hippophae goniocarpa (Elaeagnaceae). – Bot. J. Linn. Soc. 142: 425-430.
Lin Q. 2008. A revision of Elatostema section Weddelia series Salvinioida (Urticaceae). – Bot. J. Linn. Soc. 158: 62-66.
Lindenhofer A, Weber A. 1999a. Polyandry in Rosaceae: evidence for a spiral origin of the androecium in Spiraeoideae. – Bot. Jahrb. Syst. 121: 553-582.
Lindenhofer A, Weber A. 1999b. The spiraeoid androecium of Pyroideae and Amygdaloideae (Rosaceae). – Bot. Jahrb. Syst. 121: 583-605.
Lindenhofer A, Weber A. 2000. Structural and developmental diversity in the androecium of Rosoideae (Rosaceae). – Bot. Jahrb. Syst. 122: 63-91.
Link DA. 1991. Dirachma somaliensis D. A. Link sp. nov. A new species of a remarkable and highly endangered monogeneric family. – Bull. Jard. Bot. Nat. Belg. 61: 3-13.
Link DA. 1993. Dirachmaceae. – In: Thulin M (ed), Flora of Somalia 1, Royal Botanic Gardens, Kew, pp. 191-192.
Link DA. 1994. Dirachma Schweinf. (Dirachmaceae) a highly remarkable and endangered bispecific genus. – In: Seyani JH, Chikuni AC (eds), Proceedings of the 13th Plenary Meeting A.E.T.F.A.T., Zomba, Malawi, 2-11 April 1991. Vol. 2. Plants for the people, National Herbarium and Botanic Gardens of Malawi, Zomba, pp. 1229-1238.
Litav M, Orshan G. 1971. Biological flora of Israel 1. Sarcopoterium spinosum (L.) Sp. – Israel J. Bot. 20: 48-64.
Liu Q, Berry AM. 1991. The infection process and nodule initiation in the Frankia-Ceanothus root nodule symbiosis. – Protoplasma 163: 82-92.
Lo EYY, Donoghue MJ. 2012. Expanded phylogenetic and dating analyses of the apples and their relatives. – Mol. Phylogen. Evol. 63:230-243.
Lo EYY, Stefanović S, Dickinson TA. 2007. Molecular reappraisal of relationships between Crataegus and Mespilus (Rosaceae, Pyreae) – two genera or one? – Syst. Bot. 32: 596-616.
Lo EYY, Stefanović S, Christensen KI, Dickinson TA. 2009. Evidence for genetic association between East Asian and western North American Crataegus L. (Rosaceae) and rapid divergence of the eastern North American lineages based on multiple DNA sequences. – Mol. Phylogen. Evol. 51: 157-168.
Lo EYY, Stefanovic S, Dickinson TA. 2010. Reconstructing reticulation history in a phylogenetic framework and the potential of allopatric speciation driven by polyploidy in an agamic complex in Crataegus (Rosaceae). – Evolution 64: 3593-3608.
Lobin W, Rössler H. 1985. Die Gattung Forsskaolea Linnaeus 1764 auf den Kanarischen und Kapverdischen Inseln. – Senckenbergiana Biol. 65: 373-390.
Lomáscolo SB, Speranza P, Kimball RT. 2008. Correlated evolution of fig size and color supports the disperser syndromes hypothesis. – Oecologia 156: 783-796.
Lomáscolo SB, Levey DJ, Kimball RT, Bolker BM, Alborn HT. 2010. Dispersers shape fruit diversity in Ficus (Moraceae). – Proc. Natl. Acad. Sci. U.S.A. 107: 14668-14672.
Long AA. 1989. Disjunct populations of the rare shrub Neviusia alabamensis Gray (Rosaceae). – Castanea 54: 29-39.
Longley AE. 1924. Cytological studies in the genus Rubus. – Amer. J. Bot. 11: 249-283.
Lotova LI, Timonin AC. 1998. Anatomy of cortex and secondary phloem of Rosaceae 2. Spiraeoideae except Spiraeeae and Lyonothamneae. – Bot. Žurn. 83: 14-27.
Lotova LI, Timonin AC. 2002. Anatomy of cortex and secondary phloem of Rosaceae 13. Maloideae. – Bot. Žurn. 87: 31-53.
Lu R. 1992. Seabuckthorn: a multipurpose plant species for fragile mountains. – ICIMOD Publ. Unit, Katmandu.
Lundberg M. 2011. Systematics and polyploid evolution in Potentilleae (Rosaceae). – Ph.D. diss., Stockholm University, Stockholm, Sweden.
Lundberg M, Töpel M, Eriksen B, Nylander JAA, Eriksson T. 2009. Allopolyploidy in Fragariinae (Rosaceae): comparing four DNA sequence regions, with comments on classification. – Mol. Phylogen. Evol. 51: 269-280.
Lux A, Lišková D, Piñeyro-López A, Ruiz-Ordóñez J, Kákoniová D. 1998. Micropropagation of Karwinskia parvifolia and the transfer of plants to ex vitro conditions. – Biol. Plantarum 40: 143-147.
Lye KA. 1991. Strange flowering plants from the Mediterranean area: Cynomorium coccineum. – Svensk Bot. Tidskr. 85: 1-6. [In Swedish with English summary]
Ma CM, Nakamura N, Miyashiro H, Hattori M, Shimotohno K. 1999. Inhibitory effects of constituents from Cynomorium songaricum and related triterpene derivatives on HIV-1 protease. – Chem. Pharm. Bull. 47: 141-145.
Mabberley DJ. 2002. Potentilla and Fragaria (Rosaceae) reunited. – Telopea 9: 793-801.
McAllister HA. 1986. The rowan and its relatives (Sorbus spp.). – Ness Gardens (University of Liverpool Botanic Gardens), Ness, Neston, South Wirral.
McArthur ED, Sanderson SC. 1985. A cytotaxonomic contribution to the Western North American rosaceous flora. – Madroño 32: 24-28.
MacDaniels LH. 1940. The morphology of the apple and other pome fruits. – Mem. Cornell Univ. Agr. Exp. Sta. 230: 1-32.
Macdonald AD. 1974. Theoretical problems of interpreting floral organogenesis of Laportea canadensis. – Can. J. Bot. 52: 639-644.
McDonald KR, Pennell J, Frank JL, Southworth D. 2010. Ectomycorrhizas of Cercocarpus ledifolius (Rosaceae). – Amer. J. Bot. 97: 1867-1872.
Machado AFP, Rønsted N, Bruun-Lund S, Pereira RAS, Queiroz LP de. 2018. Atlantic forests to the all Americas: biogeographical history and divergence times of Neotropical Ficus (Moraceae). – Mol. Phylogen. Evol. 122: 46-58.
Machado CA, Jousselin E, Kjellberg F, Compton SG, Herre EA. 2001. Phylogenetic relationships, historical biogeography and character evolution of fig-pollinating wasps. – Proc. Roy. Soc. London, ser. B, 268: 685-694.
Machado CA, Robbins N, Gilbert MTP, Herre EA. 2005. A critical review of host-specificity and its co-evolutionary implications in the fig/fig-wasp mutualism. – Proc. Natl. Acad. Sci. U.S.A. 102(Suppl. 1): 6558-6565.
Macklin JA. 2001. Systematics of Crataegus ser. Coccineae I. Delimitation of series. – Ph.D. diss., Department of Plant Sciences, University of Western Ontario, Canada.
McPherson JK, Chou C-H, Muller CH. 1971. Allelopathic constituents of the chaparral shrub Adenostoma fasciculatum. – Phytochemistry 10: 2925-2933.
Maeda E. 1977. Scanning electron microscope studies on lupulin glands in Humulus lupulus L. – Jap. J. Crop Sci. 46: 249-253.
Mahlberg PG, Hammond CT, Turner JC, Hemphyll JK. 1984. Structure, development and composition of glandular trichomes of Cannabis sativa L. – In: Rodriguez E, Healey PL, Mehta I (eds), Biology and chemistry of plant trichomes, Plenum Press, New York, pp. 23-51.
Mai DH. 1984. Karpologische Untersuchungen der Steinkerne fossiler und rezenter Amygdalaceae (Rosales). – Feddes Repert. 95: 299-329.
Manchester SR. 1987. Extinct ulmaceous fruits from the Tertiary of Europe and western North America. – Rev. Palaeobot. Palynol. 52: 119-129.
Manchester SR. 1989a. Systematics and fossil history of the Ulmaceae. – In: Crane PR, Blackmore S (eds), Evolution, systematics and fossil history of the Hamamelidae 2: ‘Higher’ Hamamelidae, Syst. Assoc. Spec. Vol. 40B, Clarendon Press, Oxford, pp. 221-251.
Manchester SR. 1989b. Attached reproductive and vegetative remains of the extinct American-European genus Cedrelospermum (Ulmaceae) from the early Tertiary of Utah and Colorado. – Amer. J. Bot. 76: 256-276.
Manchester SR, Akhmetiev MA, Kodrul TM. 2002. Leaves and fruits of Celtis aspera (Newberry) comb. nov. (Celtidaceae) from the Paleocene of North America and Eastern Asia. – Intern. J. Plant Sci. 163: 725-736.
Mandryk VY. 1994. Embryologic investigation of Cotoneaster melanocarpus Fisch. ex Blytt (Rosaceae). – Ukrayins’k. Bot. Žurn. 51: 86-93.
Marais W. 1982. Notes on Urticaceae of the Mascarene Islands. – Kew Bull. 37: 273-657.
Marais W. 1985. Urticacées. – In: Flore des Mascareignes. 161. Urticacées, Mauritius, Paris, Kew, pp. 1-36.
Markham JH. 2009. Does Dryas integrifolia fix nitrogen? – Botany 87: 1106-1109.
Marticorena A. 2006. Revisión del género Acaena (Rosaceae) en Chile. – Ann. Missouri Bot. Gard. 93: 412-454.
Martinez-Cabrera HI, Cevallos-Ferriz SRS. 2006. Maclura (Moraceae) wood from the Miocene of the Baja California Peninsula, Mexico: fossil and biogeographic history of its closer allies. – Rev. Palaeobot. Palynol. 140: 113-122.
Massagetov PS. 1946. Alkaloids in plants of the family Elaeagnaceae. – Žurn. Gen. Chem. 16: 139-140. [In Russian]
Mauritzon J. 1939. Contribution to the embryology of the orders Rosales and Myrtales. – Acta Univ. Lund. 35: 1-121.
Maximowicz CJ. 1879. Adnotationes de Spiraeaceis. Sorbaria. – Acta Horti Petropol. 6: 222-225.
Medan D. 1985. Fruit morphogenesis and seed dispersal in the Colletieae (Rhamnaceae) I. The genus Discaria. – Bot. Jahrb. Syst. 105: 205-262.
Medan D. 1986. Anatomía y arquitectura foliares de Discaria (Rhamnaceae). – Kurtziana 18: 133-151.
Medan D. 1988. Gynoecium ontogenesis in the Rhamnaceae. A comparative study. – In: Leins P, Tucker SC, Endress PK (eds), Aspects of floral development, J. Cramer, Berlin, pp. 133-141.
Medan D. 1989. Diaspore diversity in the anemochorous Gouanieae (Rhamnaceae). – Plant Syst. Evol. 168: 149-158.
Medan D, Aagesen L. 1995. Comparative flower and fruit structure in the Colletieae (Rhamnaceae). – Bot. Jahrb. Syst. 117: 531-564.
Medan D, Arce ME. 2000. Reproductive biology of the Andean-disjunct genus Retanilla (Rhamnaceae). – Plant Syst. Evol. 218: 281-298.
Medan D, D’Ambrogio AC. 1998. Reproductive biology of the andromonoecious shrub Trevoa quinquenervia (Rhamnaceae). – Bot. J. Linn. Soc. 126: 191-206.
Medan D, Hilger HH. 1992. Comparative flower and fruit morphogenesis in Colubrina (Rhamnaceae) with special reference to C. asiatica. – Amer. J. Bot. 79: 809-819.
Medan D, Schirarend C. 2004. Rhamnaceae. – In: Kubitzki K (ed), The families and genera of vascular plants VI. Flowering plants. Dicotyledons. Celastrales, Oxalidales, Rosales, Cornales, Ericales, Springer, Berlin, Heidelberg, New York, pp. 320-338.
Mehra PN, Gill BS. 1974. Cytological studies in Ulmaceae, Moraceae, and Urticaceae. – J. Arnold Arbor. 55: 663-677.
Mendes EJ, Kupicha FK. 1978. 62. Rosaceae. – In: Launert E (ed), Flora Zambesiaca 4, Flora Zambesiaca Managing Committee, London, pp. 7-33.
Mennega AWM, Lanzing-Vikenborg M. 1977. On the wood anatomy of the tribe ”Olmedieae” (Moraceae) and the position of the genus Olmedia R. & P. – Acta Bot. Neerl. 26: 1-27.
Merxmüller H, Roessler H. 1980. Merkmals-Introgressionen bei Forsskaolea (Urticaceae). – Landbouwhogesch. Wageningen Misc. Pap. 19: 263-280.
Měsiček J, Soják J. 1992. Chromosome counts of some Mongolian Potentilla species. – Preslia 27: 167-176.
Metcalfe CR, Chalk L. 1950. Anatomy of the dicotyledons 2. Moraceae. – Clarendon Press, Oxford, pp. 1259-1271.
Mildbread J, Burret M. 1911. Die afrikanischen Arten der Gattung Ficus L. Beiträge zur Flora von Afrika XXXVIII. – Engl. Bot. Jahrb. Syst. 46: 163-269.
Millan M, Crepet W. 2014. The fossil record
of the Solanaceae
revisited and revised – the fossil record of Rhamnaceae enhanced. – Bot. Rev. 80:
73-106.
Miller IM, Baker DD. 1985. The initiation, development and structure of root nodules in Elaeagnus angustifolia L. (Elaeagnaceae). – Protoplasma 128: 107-119.
Mishima M, Ohmido N, Fukui K, Yahara T. 2002. Trends in site-number change of rDNA loci during polyploid evolution in Sanguisorba (Rosaceae). – Chromosoma 110: 550-558.
Moe AM, Rossi DR, Weiblen GD. 2011. Pollinator sharing in dioecious figs (Ficus: Moraceae). – Biol. J. Linn. Soc. 103: 546-588.
Moffett AA. 1931a. The chromosome constitution of the Pomoideae. – Proc. Roy. Soc. London, Ser. B, Biol. Sci. 108: 423-446.
Moffett AA. 1931b. A preliminary account of chromosome behaviour in the Pomoideae. – J. Pomology Horticult. Sci. 9: 100-110.
Mohan Ram HY, Nath R. 1964. The morphology and embryology of Cannabis sativa Linn. – Phytomorphology 14: 414-429.
Momose K, Hatada A, Yamaoka R, Inoue T. 1998. Pollination biology of the genus Artocarpus, Moraceae. – Tropics 7: 165-172.
Monasterio-Huelin E. 1991. Avance del estudio del género Rubus L. (Rosaceae) en la Península Ibérica. – An. Jard. Bot. Madrid 48: 274-281.
Monasterio-Huelin 1993. Rubi Discolores de la Pinínsula Ibérica. – Candollea 48: 61-82.
Monasterio-Huelin E. 1995. Taxonomy and distribution of the genus Rubus (Rosaceae) series Radula on the Iberian Peninsula. – Nord. J. Bot. 15: 365-373.
Moncur MW. 1985. Floral ontogeny of the jackfruit, Artocarpus heterophyllus Lam. (Moraceae). – Aust. J. Bot. 33: 585-593.
Monro AK. 2001. Synopsis of Mesoamerican Pilea (Urticaceae), including eighteen typifications and a key to the species. – Bull. Nat. Hist. Mus. Lond., Bot. Ser. 31: 9-25.
Monro AK. 2004. Contributions tot he flora of Mt Jaya XV. Three new species, and three new names in Pilea (Urticaceae) from New Guinea. – Kew Bull. 59: 573-579.
Monro AK. 2006. The revision of species-rich genera: a phylogenetic framework for the strategic revision of Pilea (Urticaceae) based on cpDNA, nrDNA, and morphology. – Amer. J. Bot. 93: 426-441.
Monro AK, Rodríguez A. 2009. Three new species and a nomenclatural synopsis of Urera (Urticaceae) from Mesoamerica. – Ann. Missouri Bot. Gard. 96: 268-285.
Morales-Briones DF, Romoleroux K. Kolář, Tank DC. 2018. Phylogeny and evolution of the Neotropical radiation of Lachemilla (Rosaceae): uncovering a history of reticulate evolution and implications for infrageneric classification. – Syst. Bot. 43: 17-34.
Moretti C, Gaillard Y, Grenand P, Bevalot F, Prevosto JM. 2006. Identification of 5-hydroxy-tryptamine (bufotenine) in takini (Brosimum acutifolium Huber subsp. acutifolium C. C. Berg, Moraceae), a shamanic potion used in the Guiana Plateau. – J. Ethnopharmac. 106: 198-202.
Morgan DR, Soltis DE, Robertson KR. 1994. Systematic and evolutionary implications of rbcL sequence variation in Rosaceae. – Amer. J. Bot. 81: 890-903.
Mowrey BD, Werner DJ. 1990. Phylogenetic relationships among species of Prunus as inferred by isozyme markers. – Theor. Appl. Gen. 80: 129-133.
Muniyamma M, Phipps JB. 1979. [Studies in Crataegus (Rosceae: Maloideae) I] Cytological proof of apomixis in Crataegus (Rosaceae). – Amer. J. Bot. 66: 149-155.
Müntzing A. 1928. Pseudogamie in der Gattung Potentilla. – Hereditas 11: 267-283.
Murbeck S. 1941. Untersuchungen über das Androeceum der Rosaceen. – Lunds Univ. Årsskr., N. F., Avd. II, 37(7): 1-56.
Naeger JA, Golenberg EM. 2016. Mode and tempo of sequence and floral evolution within the Anserineae. – Plant Syst. Evol 302: 385-398.
Nagarajan GR, Parmar VS. 1977a. Chemical examination of the heartwood of Prunus domestica. – Planta Medica 31: 146-150.
Nagarajan GR, Parmar VS. 1977b. Flavonoids of Prunus cerasus. – Planta Medica 32: 50-53.
Nair NC. 1970. Comparative embryology of angiosperms: Meliaceae, Rhamnaceae, Vitaceae, Leeaceae. – Bull. Natl. Sci. Acad. India 41: 151-155, 168-173, 174-179, 180-184.
Nair NC, Sharma VS. 1961. Organography and floral anatomy of some members of the Rhamnaceae. – J. Indian Bot. Soc. 40: 47-55.
Nair PKK, Sharma M. 1965. Pollen morphological studies in Indian Urticales. – Bot. Not. 118: 177-186.
Nakai T. 1923. Genera nova Rhamnacearum et Leguminosarum ex Asia orientali. – Jap. Bot. Mag. 37: 29-36.
Namdaung U, Aroonrerk N, Suksamrarn S, Danwisetkanjana K, Saenboonrueng J, Arjchomphu W, Suksamrarn A. 2006. Bioactive constituents of the root bark of Artocarpus rigidus subsp. rigidus. – Chem. Pharmaceut. Bull. 54: 1433-1436.
Naruhashi N, Toyoshima Y. 1979. Pollen morphology of Japanese Rosaceae. – J. Phytogeogr. Taxon. 27: 46-50.
Nathorst AG. 1890. Ueber die Reste eines Brotfruchtbaums, Artocarpus dicksoni n.sp., aus den cenomanen Kreideablagerungen Grönlands. – Kongl. Sv. Vetensk.-Akad. Handl. 24: 1-10.
Nebel B. 1929. Zur cytology von Malus und Vitis. – Die Gartenbauwissenschaft 1: 549-592.
Nelson-Jones EB, Briggs D, Smith AG. 2002. The origin of intermediate species of the genus Sorbus. – Theor. Appl. Gen. 105: 953-963.
Nepal MP, Ferguson CJ. 2012. Phylogenetics of Morus (Moraceae) inferred from ITS and trnL-trnF sequence data. – Syst. Bot. 37: 442-450.
Newcomb W, Heisey RM: 1984. Ultrastructure of actinorhizal root nodules of Chamaebatia foliolosa (Rosaceae). – Can. J. Bot. 62: 1697-1707.
Nickrent DL, Der JP, Anderson FE. 2005. Discovery of the photosynthetic relatives of the ‘Maltese mushroom’ Cynomorium. – BMC Evol. Biol. 5: 38.
Niezgoda CJ, Nowaczyk J. 1976. Palynological studies in Acanthinophyllum, Clarisia, Sorocea, and Trophis (Moraceae). – Pollen Spores 18: 513-522.
Nordborg G. 1963. Studies in Sanguisorba officinalis L. – Bot. Not. 116: 267-288.
Nordborg G. 1966. Sanguisorba L., Sarcopoterium Spach, and Bencomia Webb et Berth. Delimitation and subdivision of the genera. – Opera Bot. 11(2): 1-103.
Nordborg G. 1967a. Embryological studies in the Sanguisorba minor complex (Rosaceae). – Bot. Not. 120: 109-119.
Nordborg G. 1967b. The genus Sanguisorba section Poterium. Experimental studies and taxonomy. – Opera Bot. 16: 1-166.
Nordborg G. 1968. Pubescence in the Sanguisorba group (Rosaceae). – Bot. Not. 121: 641-651.
Notov AA, Kusnetzova TV. 2004. Architectural units, axiality and their taxonomic implications in Alchemillinae. – Wulfenia 11: 85-130.
Nybom H. 1988. Apomixis versus sexuality in blackberries (Rubus subg. Rubus, Rosaceae). – Plant Syst. Evol. 160: 207-218.
Nylehn J, Hamre E, Nordal I. 2003. Facultative apomixis and hybridization in arctic Potentilla section Niveae (Rosaceae) from Svalbard. – Bot. J. Linn. Soc. 142: 373-381.
Oginuma K, Tobe H. 1995. Karyomorphology of some Moraceae and Cecropiaceae (Urticales). – J. Plant Res. 108: 313-326.
Oginuma K, Raven PH, Tobe H. 1990. Karyomorphology and relationships of Celtidaceae and Ulmaceae (Urticales). – Bot. Mag. (Tokyo) 103: 113-131.
Ogunkunle ATJ, Oladele FA. 2008. Leaf epidermal studies in some Nigerian species of Ficus L. (Moraceae). – Plant Syst. Evol. 274: 209-221.
Oh S-H. 2006. Neillia includes Stephanandra (Rosaceae). – Novon 16: 91-95.
Oh S-H. 2013. Phylogenetic analysis of PISTILLATA sequences in Neillia (Rosaceae). – J. Plant Biol. 56: 145-151.
Oh S-H, Potter D. 2003. Phylogenetic utility of the second intron of LEAFY in Neillia and Stephanandra (Rosaceae) and implications for the origin of Stephanandra. – Mol. Phylogen. Evol. 29: 203-215.
Oh S-H, Potter D. 2005. Molecular phylogenetic systematics and biogeography of tribe Neillieae (Rosaceae) using DNA sequences of cpDNA, rDNA and LEAFY. – Amer. J. Bot. 92: 179-192.
Oh S-H, Chen L, Kim S-H, Kim Y-D, Shin H. 2010. Phylogenetic relationship of Physocarpus insularis (Rosaceae) endemic on Ulleung Island: implications for conservation biology. – J. Plant Biol. 53: 94-105.
Ohashi H. 1988. Rhaphiolepis (Rosaceae) of Japan. – J. Jap. Bot. 63: 1-7.
Ohta S, Yamamoto T, Nishitani C, Katsuki T, Iketani H, Omura M. 2007. Phylogenetic relationships among Japanese flowering cherries (Prunus subgenus Cerasus) based on nucleotide sequences of chloroplast DNA. – Plant Syst. Evol. 263: 209-225.
Okabe S. 1963. Cytological studies of the apomixes in Angiosperms 1. Apomixis in the genus Boehmeria. – Sci. Rep. Tôhoku Univ. IV, Biol. 29: 207-215.
Okamoto M, Kosuge K, Fukuoka N. 1992. Pistil development and parietal placentation in the pseudomonomerous ovary of Zelkova serrata (Ulmaceae). – Amer. J. Bot. 79: 921-927.
Okuda T, Yoshida T, Hatano T, Iwatsuki M, Kubo M, Orime T, Yoshizaki M, Naruhashi N. 1992. Hydrolysable tannins as chemotaxonomic markers in the Rosaceae. – Phytochemistry 31: 3091-3096.
Oliveira MM, Pais MSS. 1988. Glandular trichomes of Humulus lupulus var. Brewer’s Gold: ontogeny and histochemical characterization of the secretion. – Nord. J. Bot. 8: 349-359.
Olsson Å, Nybom H, Prentice HC. 2000. Relationships between Nordic dogroses (Rosa L. sect. Caninae, Rosaceae) assessed by RAPDs and elliptic Fourier analysis of leaflet shape. – Syst. Bot. 25: 511-521.
Omori Y, Terabayashi S. 1993. Gynoecial vascular anatomy and its systematic implications in Celtidaceae and Ulmaceae (Urticales). – J. Plant Res. 106: 249-258.
Paclt J. 1999. Proposal to conserve the name Zizyphus (Rhamnaceae) with that spelling and with feminine gender. – Taxon 48: 173-174.
Palhares D, Paula JED, Pereira LAR, Silveira CED. 2007. Comparative wood anatomy of stem, root and stylopodium of Brasimum gaudichaudii (Moraceae). – IAWA J. 28: 83-94.
Palmer EJ. 1943. The species concept in Crataegus. – Chron. Bot. 7: 373-375.
Palmer EJ. 1946. Crataegus in the northeastern and central U. S. and adjacent Canada. – Brittonia 5: 471-490.
Panigrahi G, Dikshit BK. 1987. Systematics of the genus Potentilla (Rosaceae Juss.) – its infrageneric classification and evolutionary trends. – Bull. Bot. Surv. India 27: 177-196.
Panigrahi G, Kumar A. 1988. A conspectus of the 74 taxa of Cotoneaster Erhardt ex Medik. (Rosaceae) in India. – Bull. Bot. Surv. India 28: 63-80.
Parolly G, Nordt B. 2002. A new chasmophytic species of Potentilla (Rosaceae) from S Anatolia, including some taxonomic remarks on P. subg. Fragariastrum in the E Mediterranean. – Willdenowia 32: 73-84.
Paule J, Soják J. 2009. Taxonomic comments on the genus Sibbaldiopsis Rydb. (Rosaceae). – J. Natl. Mus. (Prague), Nat. Hist. Ser. 178: 15-16.
Pawłowski B. 1965. De generis Potentilla L. serie Crassinerviae (Th. Wolf) B. Pawł. – Fragm. Florist. Geobot. 11: 53-91.
Pearce RD. 1989. Cannabaceae. – In: George AS (ed), Flora of Australia 3, Australan Government Publ. Service, Canberra, pp. 14-15.
Péchoutre F. 1902. Contribution à l’étude du développement de l’ovule et de la graine des Rosacées. – Ann. Sci. Nat., sér. VIII, Bot. 16: 1-158.
Pederneiras LC, Carauta JPP, Neto SR, Mansano V de F. 2015. An overview of the infrageneric nomenclature of Ficus (Moraceae). – Taxon 64: 589-594.
Pederneiras LC, Romaniuc-Neto S, Mansano V de F. 2015. Molecular phylogenetics of Ficus section Pharmacosycea and the description of Ficus subsection Carautaea (Moraceae). – Syst. Bot. 40: 504-509.
Pedersen A, Schou JC. 1989. Nordiske Brombær. – AAU Reports 21: 1-216.
Pedersen A, Weber HE. 1993. Atlas der Brombeeren von Niedersachsen und Bremen (Gattung Rubus L. subgenus Rubus). – Naturschutz Landschaftspflege Niedersachsen 28: 1-204.
Persson Hovmalm HA, Jeppsson N, Bartish IV, Nybom H. 2004. RAPD analysis of diploid and tetraploid populations of Aronia points to different reproductive strategies within the genus. – Hereditas 141: 301-312.
Phelouzat R. 1963. Morphologie, phyllotaxie, ontogénie de trois Rosacées: Agrimonia eupatoria L., Poterium Sanguisorba L., Geum urbanum L. – Rev. Cyt. Biol. Vég. Fr. 26:101-362.
Phelouzat R. 1965. La gamétogénèse et la fertilité dans les fleurs femelles et les fleurs hermaphrodites du Poterium sanguisorba L. – Bull. Soc. Bot. France 112: 370-378.
Phipps JB. 1983. Biogeographic, taxonomic, and cladistic relationships between East Asiatic and North American Crataegus. – Ann. Missouri Bot. Gard. 70: 667-700.
Phipps JB. 1990. Mespilus canescens, a new rosaceous endemic from Arkansas. – Syst. Bot. 15: 26-32.
Phipps JB. 1992. Heteromeles and Photinia (Rosaceae, subfam. Maloideae) of Mexico and Central America. – Can. J. Bot. 70: 2138-2162.
Phipps JB. 2005. A review of hybridization in North American hawthorns. – Ann. Missouri Bot. Gard. 92: 113-126.
Phipps JB, Muniyamma M. 1980. [Studies in Crataegus (Rosaceae: Maloideae) III.] A taxonomic revision of Crataegus (Rosaceae) in Ontario. – Can. J. Bot. 58: 1621-1699.
Phipps JB, Robertson KR, Smith PG, Rohrer JR. 1990. A checklist of the subfamily Maloideae (Rosaceae). – Can. J. Bot. 68: 2209-2269.
Phipps JB, Robertson KR, Rohrer JR, Smith PG. 1991. Origin and evolution of subfam. Maloideae (Rosaceae). – Syst. Bot. 16: 303-332.
Phipps JB, O’Kennon RJ, Lane RW. 2003. Hawthorns and medlars. – Timber Press, Portland, Oregon.
Pillans NS. 1942. The genus Phylica Linn. – J. South Afr. Bot. 8: 1-164.
Plouvier V. 1955. Sur le sorbitol des Rosacées. – Compt. Rend. Acad. Sci. Paris 241: 1220-1222.
Polhill RM. 1964. Enumeration of the Ulmaceae in Africa south of the Sahara. – Kew Bull. 19: 139-145.
Polhill RM. 1966. Ulmaceae. – In: Hubbard CE, Milne-Redhead E (eds), Flora of tropical East Africa, Crown Agents for Oversea Governments and Administrations, London, pp. 1-15.
Pollard BJ, Cheek M, Bygrave P. 2003. New Dorstenia (Moraceae) discoveries in Western Cameroon. – Kew Bull. 58: 185-193.
Pool A. 2014. Taxonomic revision of
Gouania (Rhamnaceae) for North
America. – Ann. Missouri Bot. Gard. 99: 490-552.
Popoff A. 1935. Über die Fortpflanzungsverhältnisse der Gattung Potentilla. – Planta 24: 510-522.
Potter D. 2003. Molecular phylogenetic studies in Rosaceae. – In: Sharma AK, Sharma A (eds), Plant genome: biodiversity and evolution I, A: Phanerogams, Scientific Publ., Enfield, New Hampshire, pp. 319-351.
Potter D, Luby JJ, Harrison RE. 2000. Phylogenetic relationships among species of Fragaria (Rosaceae) inferred from non-coding nuclear and chloroplast DNA sequences. – Syst. Bot. 25: 337-348.
Potter D, Gao F, Bortiri PE, Oh S-H, Baggett S. 2002. Phylogenetic relationships in Rosaceae inferred from chloroplast matK and trnL-trnF nucleotide sequence data. – Plant Syst. Evol. 231: 77-89.
Potter D, Eriksson T, Evans RC, Oh S, Smedmark JEE, Morgan DR, Kerr M, Robertson KR, Arsenault M, Dickinson TA, Campbell CS. 2006 [2007]. Phylogeny and classification of Rosaceae. – Plant Syst. Evol. 266: 5-43.
Potter D, Still SM, Grebenc T, Ballian D, Bozic G, Franjiæ J, Kraigher H. 2006 [2007]. Phylogenetic relationships in tribe Spiraeeae (Rosaceae) inferred from nucleotide sequence data. – Plant Syst. Evol. 266: 105-118.
Primack RB. 1983. Forester’s guide to the Moraceae of Sarawak. – Forest Department, Sarawak, Malaysia.
Proksch M, Proksch P, Weissenbock G, Rodriguez E. 1982. Flavonoids from the leaf resin of Adenostoma sparsifolium. – Phytochemistry 21: 1835-1836.
Ptak K. 1989. Cyto-embryological investigations on the Polish representatives of the genus Crataegus L. II. Embryology of triploid species. – Acta Biol. Cracov., Ser. Bot. 31: 97-112.
Punt W. 1978. On the pollen morphology of Scyphosyce and Dorstenia. – Grana 17: 77-79.
Punt W, Eetgerink E. 1982. On the pollen morphology of some genera of the tribe Moreae (Moraceae). – Grana 21: 15-19.
Punt W, Malotaux M. 1984. Cannabaceae, Moraceae, and Urticaceae. – Rev. Palaeobot. Palynol. 42: 23-44.
Qian G-Z, Liu L-F, Hong D-Y, Tang G-G. 2008. Taxonomic study of Malus section Florentinae (Rosaceae). – Bot. J. Linn. Soc. 158: 223-227.
Racette S, Torrey JG. 1989b. Root nodule initiation in Gymnostoma (Casuarinaceae) and Shepherdia (Elaeagnaceae) induced by Frankia strain HFPGpI1. – Can. J. Bot. 67: 2873-2879.
Rahn K. 1989. A survey of the genus Sorbaria (Rosaceae). – Nord. J. Bot. 8: 557-563.
Rajput MT, Tahir SS, Hussain SZ, Spongberg S. 1997. The genus Sibbaldia (Rosaceae). – Pakistan J. Bot. 29: 1-38.
Ramirez BW. 1994. Coevolution of Ficus and Agaonidae. – Ann. Missouri Bot. Gard. 61: 770-780.
Rao VS. 1974. The nature of the perianth in Elaeagnus on the basis of floral anatomy, with comments on the systematic position of Elaeagnaceae 1. – J. Indian Bot. Soc. 53: 156-161.
Raspé O, Jacquemart A-L, De Sloover J. 1998. Isozymes in Sorbus aucuparia (Rosaceae: Maloideae): genetic analysis and evolutionary significance of zymograms. – Intern. J. Plant Sci. 159: 627-636.
Rastogi S, Kulshreshtha DK, Rawat AKS. 2006. Streblus asper Lour. (Shakhotaka): a review of its chemical, pharmacological and ethnomedicinal properties. – Evidence-Based Complem. Altern. Medicine 3: 217-222.
Rauh W, Reznik H. 1951. Histogenetische Untersuchungen an Blüten- und Infloreszenzachsen. I. Teil. Die Histogenese becherförmiger Blüten- und Infloreszenzachsen, sowie der Blütenachsen einiger Rosoideen. – Sitz.-Ber. Heidelberger Akad. Wiss. Math.-naturwiss. Kl.: 139-207.
Ravi V, Khurana JP, Tyagi AK, Khurana P. 2007. Rosales sister to Fabales: towards resolving the rosid puzzle. – Mol. Phylogen. Evol. 44: 488-493.
Record SJ, Hess RW. 1940. American woods of the family Moraceae. – Trop. Woods 61: 11-54.
Rehder AA. 1945. Moraceae, Hippocastanaceae et Vitaceae, nomina conservanda. – J. Arnold Arbor. 26: 277-279.
Reiche C. 1898. Ramnáceas. Estudios críticos sobre la flora de Chile. – An. Univ. Chile 97: 41-58.
Reiche K. 1896. Geraniaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(4), W. Engelmann, Leipzig, pp. 1-14.
Reitsma T. 1966. Pollen morphology of some European Rosaceae. – Acta Bot. Neerl. 15: 290-307.
Rendle AB. 1916. New species of Urera from tropical Africa. – J. Bot. 54: 368-371.
Rendle AD. 1917. Tropical African Urticaceae. – J. Bot. 55: 201-203.
Renner O. 1907. Beiträge zur Anatomie und Systematik der Artocarpeen und Conocephaleen, insbesondere der Gattung Ficus. – Engl. Bot. Jahrb. Syst. 39: 319-448.
Rensselaer M van, McMinn HE. 1942. Ceanothus. – Santa Barbara Botanic Garden, California.
Richardson JE. 1999. Molecular systematics of the genus Phylica L. with an emphasis on the island species. – Ph.D. diss., University of Edinburgh, Scotland.
Richardson JE, Fay MF, Cronk QCB, Bowman D, Chase MW. 2000a. A phylogenetic analysis of Rhamnaceae using rbcL and trnL-F plastid DNA sequences. – Amer. J. Bot. 87: 1309-1324.
Richardson JE, Fay MF, Cronk QCB, Chase MW. 2000b. A revision of the tribal classification of Rhamnaceae. – Kew Bull. 55: 311-340.
Richardson JE, Weitz FM, Fay MF, Cronk QCB, Linder HP, Reeves G, Chase MW. 2001. Phylogenetic analysis of Phylica L. (Rhamnaceae) with an emphasis on island species: evidence from plastid trnL-F and nuclear internal transcribed spacer (ribosomal) DNA sequences. – Taxon 50: 405-427.
Richardson JE, Fay MF, Cronk QCB, Chase MW. 2003. Species delimitation and the origin of populations in island representatives of Phylica (Rhamnaceae). – Evolution 57: 816-827.
Richardson JE, Chatrou LW, Mols JB, Erkens RHJ, Pirie MD. 2004. Historical biogeography of two cosmopolitan families of flowering plants: Annonaceae and Rhamnaceae. – Phil. Trans. Roy. Soc. London, Ser. B, 359: 1495-1508.
Rickson FR. 1976. Anatomical development of the leaf trichilium and Mullerian bodies of Cecropia peltata L. – Amer. J. Bot. 64: 1266-1271.
Rivières R. 1958. Fleurs et inflorescence de quelques Urticacées. – Natur. Monspeliensia, sér. Bot., 8: 189-204.
Robertson KR, Phipps JB, Rohrer JR, Smith PG. 1991. A synopsis of genera in Maloideae (Rosaceae). – Syst. Bot. 16: 376-394.
Robertson KR, Phipps JB, Rohrer JR. 1992. Summary of leaves in the genera of Maloideae (Rosaceae). – Ann. Missouri Bot. Gard. 79: 81-94.
Robertson KR, Weeden NF, Rohrer JR. 1995. The current status of Chamaemeles (Rosaceae: Maloideae), a Madeiran endemic. – Bot. Mus. Mun. Funchal, suppl. 4: 621-636.
Robinson WA, Partanen CR. 1980. Expeimental taxonomy in the genus Amelanchier 1. A new look at the chromosome numbers of the Amelanchier species growing in the Northeast United States. – Rhodora 822: 483-493.
Rohrer JR. 1994. Floral morphology of Maloideae (Rosaceae) and its systematic relevance. – Amer. J. Bot. 81: 574-581.
Rohrer JR, Robertson KR, Phipps JB. 1991. Variation in structure among fruits of Maloideae (Rosaceae). – Amer. J. Bot. 78: 1617-1635.
Rohrer JR, Robertson KR, Phipps JB. 1994. Floral morphology of Maloideae (Rosaceae) and its systematic relevance. – Amer. J. Bot. 81: 574-581.
Rohrer JR, Ahmad R, Southwick SM, Potter D. 2004. Microsatellite analysis of relationships among North American plums (Prunus sect. Prunocerasus, Rosaceae). – Plant Syst. Evol. 244: 69-75.
Rohwer JG, Berg CC. 1993. Moraceae. – In: Kubitzki K, Rohwer JG, Bittrich V (eds), The families and genera of vascular plants II. Flowering plants. Dicotyledons. Magnoliid, hamamelid and caryophyllid families, Springer, Berlin, Heidelberg, New York, pp. 438-453.
Romaniuc-Neto S. 1999. Cecropioideae (C. C. Berg) Romaniuc Neto stat. nov. (Moraceae-Urticales). – Albertoa, nov. ser. 4: 13-16.
Romoleroux K. 1996. 79. Rosaceae. – In: Harling G, Andersson L (eds), Flora of Ecuador 56, Nord. J. Bot., Copenhagen, pp. 1-151.
Romoleroux K, Frost-Olsen P. 1996. A new species of Aphanes (Rosaceae) from Volcán Cotopaxi, Ecuador. – Nord. J. Bot. 16: 473-475.
Ronse De Craene L-P, Miller AG. 2004. Floral development and anatomy of Dirachma socotrana (Dirachmaceae): a controversial member of the Rosales. – Plant Syst. Evol. 249: 111-127.
Rønsted N, Weiblen GD, Cook JM, Salamin N, Machado CA, Savolainen V. 2005. 60 million years of co-divergence in the fig-wasp symbiosis. – Proc. Roy. Soc. London, Ser. B, 272: 2593-2599.
Rønsted N, Salvo G, Savolainen V. 2007. Biogeographical and phylogenetic origins of African fig species (Ficus section Galoglychia). – Mol. Phylogen. Evol. 43: 190-201.
Rønsted N, Weiblen GD, Clement WL, Zerega NJC, Savolainen V. 2008. Reconstructing the phylogeny of figs (Ficus, Moraceae) to reveal the history of the fig pollination mutualism. – Symbiosis 45: 45-56.
Rønsted N, Weiblen GD, Savolainen V, Cook JM. 2008. Phylogeny, biogeography, and ecology of Ficus section Malvanthera (Moraceae). – Mol. Phylogen. Evol. 48: 12-22.
Rothmaler W. 1934. Systematische Vorarbeiten zu einer Monographie der Gattung Alchemilla (L.) Scop. I. – Feddes Repert. 33: 342-350.
Rothmaler W. 1936. Systematische Vorarbeiten zu einer Monographie der Gattung Alchemilla (L.) Scop. IV. – Feddes Repert. 40: 208-212.
Rothmaler W. 1937. Systematische Vorarbeiten zu einer Monographie der Gattung Alchemilla (L.) Scop. V. – Feddes Repert. 42: 164-173.
Rothmaler W. 1938. Systematik und Geographie der Subsection Calycanthum der Gattung Alchemilla. – Feddes Repert. Beih. 100: 57-93.
Rothmaler W. 1939. Sobre algunas Rosáceas sudamericanas I. Sinopsis de Tetraglochin. – Darwiniana 3: 429-437.
Rousi A. 1965. Observations on the cytology and variation of European and Asiatic populations of Hippophae rhamnoides. – Ann. Bot. Fenn. 2: 1-18.
Rousi A. 1971. The genus Hippophaë L. A taxonomic study. – Ann. Bot. Fenn. 8: 177-227.
Rousseau-Gueutin M, Gaston A, Ainouche A, Ainouche ML, Olbricht K, Staudt G, Richard L, Denoyes-Rothan B. 2009. Tracking the evolutionary history of polyploidy in Fragaria L. (strawberry): new insights from phylogenetic analyses of low-copy nuclear genes. – Mol. Phylogen. Evol. 51: 515-530.
Rowe JW, Seikel MK, Roy DN, Jorgensen E. 1972. Chemotaxonomy of Ulmus. – Phytochemistry 11: 2513-2517.
Rowley JR, Rowley JS. 1986. Ontogenetic development of microspores of Ulmus (Ulmaceae). – In: Blackmore S, Ferguson IK (eds), Pollen and spores: form and function, The Linnean Society of London, pp. 19-33.
Rudolf PO. 1974. Sorbaria sorbifolia (L.) A. Br. Ural false-spiraea. – Agric. Handb. U.S. Dept. Agric. 450: 779.
Ruiter G de. 1976. Revision of the genera Myrianthus and Musanga (Moraceae). – Bull. Jard. Bot. Belg. 46: 471-510.
Rydberg PA. 1896a. Notes on Potentilla II. – Bull. Torrey Bot. Club 23: 259-265.
Rydberg PA. 1896b. Notes on Potentilla III. – Bull. Torrey Bot. Club 23: 301-306.
Rydberg PA. 1898. A monograph of the North American Potentilleae. – Mem. Dept. Bot. Columbia Coll. 2: 1-223.
Rye BL. 1995a. New and priority taxa in the genera Spyridium and Trymalium (Rhamnaceae) of Western Australia. – Nuytsia 10: 119-140.
Rye B. 1995b. New and priority taxa in the genera Cryptandra and Stenanthemum (Rhamnaceae) of Western Australia. – Nuytsia 10: 255-305.
Rye B. 1996a. Granitites, a new genus of Rhamnaceae from the south-west of Western Australia. – Nuytsia 10: 451-457.
Rye B. 1996b. A synopsis of the genera Pomaderris, Siegfriedia, Spyridium and Trymalium (Rhamnaceae) in Western Australia. – Nuytsia 11: 109-131.
Rye BL. 1997. The Rhamnaceae of the Kimberley region of Western Australia. – Nuytsia 11: 287-292.
Rye BL. 2001. A taxonomic update of Stenanthemum (Rhamnaceae: Pomaderreae) in Western Australia. – Nuytsia 13: 495-507.
Rye BL. 2007. New species and keys for Cryptandra and Stenanthemum (Rhamnaceae) in Western Australia. – Nuytsia 16: 325-382.
Saïd C. 1979. Quelques aspects de l’écologie florale chez les Rosaceae: étude morphologique et histologique comparée chez Sanguisorba offiinalis L. et Poterium sanguisorba L. – Bull. Soc. Bot. France 126, Lettr. Bot.: 311-324.
Sakai S. 2001. Thrips pollination of androdioecious Castilla elastica (Moraceae) in a seasonal tropical forest. – Amer. J. Bot. 88: 1527-1534.
Sakai S, Kato M, Nagamasu H. 2000. Artocarpus (Moraceae)-gall midge pollination mutualism mediated by a male-flower parasitic fungus. – Amer. J. Bot. 87: 440-445.
Santamour FS Jr. 1972. Flavonoid distribution in Ulmus. – Bull. Torrey Bot. Club 99: 127-131.
Sasakawa H, Hiyoshi T, Sugiyama T. 1988. Immuno-gold localization of nitrogenase in root nodules of Elaeagnus pungens Thunb. – Plant Cell Physiol. 29: 1147-1152.
Satake Y. 1931. Systematic and anatomical studies of some Japanese plants I. Systematic importance of spodograms in the Urticales. – J. Fac. Sci. Tokyo Univ. 3: 485-507.
Sattarian A, Maesen LJG van der. 2006. Endocarp morphology of African Celtis (Celtidaceae/Ulmaceae). – Blumea 51: 389-397.
Sattarian A, Berg RG van den, Maesen LJG van der. 2006. Pollen morphology of African Celtis (Celtidaceae). – Feddes Repert. 117: 34-40.
Savile DBO. 1968. Parasite relationships and disposition of Filipendula. – Brittonia 20: 230-231.
Sax K. 1931. The origin and relationships of the Pomoideae. – J. Arnold Arbor. 12: 3-22.
Sax K. 1932. Chromosome relationships in Pomoideae. – J. Arnold Arbor. 13: 363-367.
Sax K. 1933. The origin of the Pomoideae. – Proc. Amer. Horticult. Soc. 30: 147-150.
Schaeppi H. 1953. Kelch und Aussenkelch von Rhodotypus kerrioides. – Vierteljahresschr. Naturf. Ges. Zürich 98: 30-36.
Schaeppi H. 1977. Über den “doppelten Fruchtknoten” von Rhodotypos. – Beitr. Biol. Pflanzen 53: 165-179.
Schaeppi H, Steindl F. 1950. Vergleichend-morphologische Untersuchungen am Gynoeceum der Rosoideen. – Ber. Schweiz. Bot. Ges. 60: 15-30.
Schirarend C. 1984. Holzanatomische Untersuchungen als Beiträge zur Systematik der Familie Rhamnaceae Jussieu. – Ph.D. diss., Humboldt-Universität, Berlin, Germany.
Schirarend C. 1987. Zur Holz- und Blattanatomie der neotropischen Gattung Krugiodendron Urban (Rhamnaceae). – Feddes Repert. 98: 515-519.
Schirarend C. 1991. The systematic wood anatomy of the Rhamnaceae Juss. (Rhamnales) I. Tribe Zizipheae. – IAWA Bull., N. S., 12: 359-388.
Schirarend C, Hoffmann P. 1993. Untersuchungen zur Blütenmorphologie der Gattung Reynosia Griseb. (Rhamnaceae). – Flora 188: 275-286.
Schirarend C, Köhler E. 1993. Rhamnaceae Juss. – In: Nilsson S, El Ghazaly G (eds), World Pollen and Spore Flora 17/18: 1-53.
Schirarend C, Olabi MN. 1994. Revision of the genus Paliurus Tourn. ex Mill. (Rhamnaceae). – Bot. Jahrb. Syst. 116: 333-359.
Schirarend C, Süss H. 1985. Zur Holzanatomie und systematischen Stellung der Gattung Maesopsis Engler (Rhamnaceae). – Gleditschia 13: 41-45.
Schleuss G. 1958. Über die Fruchtentwicklung der Gattung Dorstenia, insbesondere über ihren Turgeszenz-Schleuder-mechanismus. – Planta 52: 276-319.
Schmidt-Lebuhn AN, Kessler M, Kumar M. 2006. Promiscuity in the Andes: species relationships in Polylepis (Rosaceae, Sanguisorbeae) based on AFLP and morphology. – Syst. Bot. 31: 547-559.
Schmidt-Lebuhn AN, Kumar M, Kessler M. 2006. An assessment of the genetic population structure of two species of Polylepis Ruiz & Pav. (Rosaceae) in the Chilean Andes. – Flora 201: 317-325.
Schmidt-Lebuhn AN, Seltmann P, Kessler M. 2007. Consequences of the pollination system on genetic structure and patterns of species distribution in the Andean genus Polylepis (Rosaceae): a comparative study. – Plant Syst. Evol. 266: 91-103.
Schmidt-Lebuhn AN, Fuchs J, Hertel D, Hirsch H, Toivonen J, Kessler M. 2010. An Andean radiation: polyploidy in the tree genus Polylepis (Rosaceae, Sanguisorbeae). – Plant Biol. 12: 917-926.
Schönenberger J, Balthazar M von. 2006. Reproductive structures and phylogenetic framework of the rosids – progress and prospects. – Plant Syst. Evol. 260: 87-106.
Schorn HE. 1998. Holodiscus lisii (Rosaceae): a new species of ocean spray from the late Eocene Florissant Formation, Colorado, USA. – PaleoBios 13: 21-24.
Schröter H, Winkler H. 1935. Monographie der Gattung Elatostema s.l. Allgemeiner Teil. – Feddes Repert. Beih. 831-71.
Schultes RE, Klein WM, Plowman T, Lockwood TE. 1974. Cannabis: an example of taxonomic neglect. – Bot. Mus. Leafl. Harv. Univ. 23: 337-367.
Schweinfurth C. 1891. Barbeya Schwf. gen. nov. urticacearum. – Malpighia 5: 332-340.
Senanayake YDA, Bringhurst RS. 1967. Origin of Fragaria polyploids I. Cytological analysis. – Amer. J. Bot. 54: 221-228.
Servettaz C. 1909. Monographie des Eléagnacées. – Beih. Bot. Centralbl. 25: 1-420.
Setoguchi H, Tobe H, Ohba H, Okazaki M. 1993. Silicon-accumulating idioblasts in leaves of Cecropiaceae (Urticales). – J. Plant Res. 106: 327-335.
Shah AM, Kachroo P. 1975. Comparative anatomy in Urticales I. The trichomes in Moraceae. – J. Indian Bot. Soc. 54: 138-153.
Shah AM, Wilcock CC. 1991. Notes on the genus Potentilla (Rosaceae) from Pakistan and Kashmir. – Willdenowia 21: 195-199.
Shah AM, Wilcock CC. 1993. Infrageneric classification of the genus Potentilla L. (Rosaceae) in Pakistan and Kashmir. – Edinburgh J. Bot. 50: 173-179.
Shanahan M, So S, Compton SG, Corlett R. 2001. Fig-eating by vertebrate frugivores: a global review. – Biol. Rev. 76: 529-572.
Sharawy SM. 2004. Numerical taxonomic evaluation of calcium oxalate and calcium carbonate crystals in the leaves of certain Ficus species (Moraceae). – Feddes Repert. 115: 441-452.
Sharma MR. 1965. Morphological and anatomical investigations on Artocarpus Forst. III. The flower. – Phytomorphology 15: 185-201.
Sharp AJ. 1958. Mirandaceltis, a new genus from Mexico. – Bol. Soc. Bot. Mexico 23: 38-42.
Sharpe FR, Laws DRJ. 1981. The essential oil of hops – a review. – J. Inst. Brew. 87: 96-107.
Shaw J, Small RL. 2004. Addressing the ‘hardest puzzle in American pomology’: phylogeny of Prunus sect. Prunocerasus (Rosaceae) based on seven noncoding chloroplast DNA regions. – Amer. J. Bot. 91: 985-996.
Sheng H-M, An L-Z, Chen T, Xu S-J, Liu G-X, Zheng X-L, Pu L-L, Liu Y-J, Lian Y-S. 2006. Analysis of the genetic diversity and relationships among and within species of Hippophae (Elaeagnaceae) based on RAPD markers. – Plant Syst. Evol. 260: 25-37.
Shi W, Wen J, Lutz S. 2013. Pollen morphology of the Maddenia clade of Prunus and its taxonomic and phylogenetic implications. – J. Syst. Evol. 51: 164-183.
Shimotomai N. 1935. Zur Kenntnis der Pseudogamie bei Potentilla. – Proc. Imp. Acad. Japan 11: 338-339.
Shulaev V, Sargent DJ, Crowhurst RN, Mockler TC, Folkerts O, Delcher AL, Jaiswal P, Mockaitis K, Liston A, Mane SP, Burns P, Davis TM, Slovin JP, Bassil N, Hellens RP, Evans C, Harkins T, Kodira C, Desany B, Crasta OR, Jensen RV, Allan AC, Michael TP, Setubal JC, Celton JM, Rees DJ, Williams KP, Holt SH, Ruiz Rojas JJ, Chatterjee M, Liu B, Silva H, Meisel L, Adato A, Filichkin SA, Troggio M, Viola R, Ashman TL, Wang H, Dharmawardhana P, Elser J, Raja R, Priest HD, Bryant DW Jr, Fox SE, Givan SA, Wilhelm LJ, Naithani S, Christoffels A, Salama DY, Carter J, Lopez Girona E, Zdepski A, Wang W, Kerstetter RA, Schwab W, Korban SS, Davik J, Monfort A, Denoyes-Rothan B, Arus P, Mittler R, Flinn B, Aharoni A, Bennetzen JL, Salzberg SL, Dickerman AW, Velasco R, Borodovsky M, Veilleux RE, Folta KM. 2011. The genome of woodland strawberry (Fragaria vesca). – Nature Genet. 43: 109-16.
Silvieus SI, Clement WL, Weiblen GD. 2008. Cophylogeny of figs, pollinators gallers, and parasitoides. – In: Tilmon KJ (ed), Specialization, speciation, and radiation: the evolutionary biology of herbivorous insects, University of California Press, Berkeley, pp. 225-239.
Simpson BB. 1979. A revision of the genus Polylepis (Rosaceae: Sanguisorbeae). – Smithsonian Contr. Bot. 43: 1-62.
Singh SP. 1956. Floral anatomy of Cannabis sativa L. – Agra Univ. J. Res. Sci. 5: 155-162.
Sinnott QP, Phipps JB. 1983. Variation patterns in Crataegus series Pruinosae (Rosaceae) in southern Ontario. – Syst. Bot. 8: 59-70.
Skalicky V. 1971. Amerikansiche Odermennige, Agrimonia L. ser. Parviflora ser. n. – Nov. Bot. Inst. Bot. Univ. Carol. Prag 1970: 9-16.
Small ES, Cronquist A. 1976. A practical and natural taxonomy for Cannabis. – Taxon 25: 405-435.
Smedmark JEE. 2006. Recircumscription of Geum L. (Rosaceae, Colurieae). – Bot. Jahrb. Syst. 126: 409-417.
Smedmark JEE, Eriksson T. 2002. Phylogenetic relationships of Geum (Rosaceae) and relatives inferred from the nrITS and trnL-trnF regions. – Syst. Bot. 27: 303-317.
Smedmark JEE, Eriksson T. 2006. Early stages of development shed light on fruit evolution in allopolyploid species of Geum (Rosaceae). – Intern. J. Plant Sci. 167: 791-803.
Smedmark JEE, Eriksson T, Evans RC, Campbell CS. 2003. Ancient allopolyploid speciation in Geinae (Rosaceae): evidence from nuclear granule-bound starch synthase (GBSSI) gene sequences. – Syst. Biol. 52: 374-385.
Smedmark JEE, Eriksson T, Bremer B. 2005. Allopolyploid evolution in Geinae (Colurieae: Rosaceae) – building reticulate species trees from bifurcating gene trees. – Organisms Divers. Evol. 5: 275-283.
Smith JMB. 1988. Prunus (Amygdalaceae) in New South Wales. – Telopea 3: 145-157.
Soepadmo E. 1977. Ulmaceae. – In: Steenis CGGJ van (ed), Flora Malesiana I, 8(2), Sijthoff & Noordhoff International Publ., Alphen aan den Rijn, The Netherlands, pp. 31-76.
Soják J. 1969. Nomenklatorische Anmerkungen zur Gattung Potentilla. – Folia Geobot. Phytotaxon. 4: 205-209.
Soják J. 1986a. Notes on Potentilla I. Hybridogenous species derived from intersectional hybrids of sect. Niveae x sect. Multifidae. – Bot. Jahrb. Syst. 106: 145-210.
Soják J. 1986b. Notes on Potentilla (Rosaceae) II. Some new species from Mongolia. – Willdenowia 16: 125-142.
Soják J. 1987a. Notes on Potentilla IV. Classification of Wolf’s group ‘Potentillae trichocarpae’. – Candollea 42: 491-500.
Soják J. 1987b. Notes on Potentilla V. Potentilla pensylvanica group in the Old World. – Preslia 59: 289-305.
Soják J. 1989. Generická problematika Potentilla s.l. – Die generische Problematik von Potentilla s.l. – Čas. Nár. Muz. Praze, Řada Přir. 154: 117-118.
Soják J. 1991. Notes on Potentilla L. (Rosaceae) IX. New species from Turkey, the Caucasus, Iran and Turkmeniya. – Willdenowia 20: 117-124.
Soják J. 1993. Taxonomische Bemerkungen zu einigen mediterranen Potentilla-Sippen. – Preslia 65: 117-130.
Soják J. 1994. Notes on Potentilla (Rosaceae) X. The section Dumosae; XI. The P. microphylla and P. stenophylla groups (sect. Pentaphylloides); XII. Key to the taxa of P. sect. Pentaphylloides (Anserina). – Bot. Jahrb. Syst. 116: 11-81.
Soják J. 2003. Notes on Potentilla XV. Some new taxa of Potentilla (Rosaceae) from New Guinea, Asia and Canada. – Willdenowia 33: 409-423.
Soják J. 2004. Notes on Potentilla XVI. Potentilla L. (Rosaceae) and related genera in the former USSR (identification key, checklist and figures). – Bot. Jahrb. Syst. 125: 253-340.
Soják J. 2005. Notes on Potentilla XVIII. Potentilla L. s.l. (Rosaceae) in Flora Europae Orientalis. – Candollea 60: 59-78.
Soják J. 2006. Notes on Potentilla XXII. New taxa and nomenclatural combinations in Potentilla L. (Rosaceae). – Feddes Repert. 117: 486-500.
Soják J. 2007. Notes on Potentilla XIX. Potentilla (Rosaceae) in China. – Harvard Pap. Bot. 12: 285-323.
Soják J. 2008a. Notes on Potentilla XX. Potentilla compacta in Tibet. – Bot. Jahrb. Syst. 127: 343-347.
Soják J. 2008b. Notes on Potentilla XXI. A new division of the tribe Potentilleae (Rosaceae) and notes on generic delimitations. – Bot. Jahrb. Syst. 127: 349-358.
Soják J. 2009. Notes on Potentilla XXIV. Potentilla L. (Rosaceae) in the former USSR; second part: comments. – Feddes Repert. 120: 185-217.
Soják J. 2010. Argentina Hill, a genus distinct from Potentilla (Rosaceae). – Thaiszia – J. Bot. 20: 91-97.
Soják J. 2011. Synopsis of Drymocallis Fourr. ex Rydb. (Rosaceae – Potentilleae) in the Old World. – Ann. Naturhist. Mus. Wien, B, 112: 319-328.
Soják J. 2012. Notes on Potentilla XXVIII. Potentilla L. (Rosaceae) and related genera in Asia (excluding the former USSR), Africa and New Guinea. – Plant Divers. Evol. 130: 7-157.
Song B, Li F-Z. 2002. The utility of trnK intron 5’ region in phylogenetic analysis of Ulmaceae s. l. – Acta Phytotaxon. Sin. 40: 125-132.
Song B-H, Wang X-Q, Li F-Z, Hong D-Y. 2001. Further evidence for paraphyly of the Celtidaceae from the chloroplast gene matK. – Plant Syst. Evol. 228: 107-115.
Song J-H, Hong S-P. 2018. Comparative petiole anatomy of the tribe Sorbarieae (Rosaceae) provide new taxonomically informative characters. – Nord. J. Bot. 36: e01702
Song J-H, Moon H-K, Hong S-P. 2016. Pollen morphology of the tribe Sorbarieae (Rosaceae). – Plant Syst. Evol. 302: 853-869.
Song J-H, Moon H-K, Oak M-K, Hong S-P. 2017. Phylogenetic evaluation of pollen and orbicule morphology in Rosaceae tribe Neillieae (subfamily Amygdaloideae). – Bot. J. Linn. Soc. 183: 439-453.
Sorsa P. 1971. Pollen morphological study of the genus Hippophae L., including the new taxa recognized by A. Rousi. – Ann. Bot. Fenn. 8: 228-236.
Sorsa P, Huttunen P. 1975. On the pollen morphology of the Urticaceae. – Ann. Bot. Fenn. 12: 165-182.
Spies JJ, Stirton CH, Du Plessis H. 1987. The genus Rubus (Rosaceae) in South Africa IV. Natural hybridization. – Bothalia 17: 105-119.
Spitaler R, Gurschler S, Ellmerer E, Schubert B, Sgarbossa M, Zidorn C. 2009. Flavonoids from Celtis australis (Cannabaceae). – Biochem. Syst. Ecol. 37: 120-121.
Stahl E. 1953. Untersuchungen an den Drüsenhaaren der Schabgarbe. – Zeitschr. Bot. 41: 123-146.
Staudt G. 2003. Notes on Asiatic Fragaria species III. Fragaria orientalis Losinsk. and Fragaria mandshurica spec. nov. – Bot. Jahrb. Syst. 124: 397-419.
Staudt G. 2006. Himalayan species of Fragaria (Rosaceae). – Bot. Jahrb. Syst. 126: 483-508.
Staudt G, Dickoré WB. 2001. Notes on Asiatic Fragaria species: Fragaria pentaphylla Losinsk. and Fragaria tibetica spec. nov. – Bot. Jahrb. Syst. 123: 341-354.
Steeves TA, Steeves MW, Olson AR. 1991. Flower development in Amelanchier alnifolia (Maloideae). – Can. J. Bot. 69: 844-857.
Sterling C. 1953. Developmental anatomy of the fruit of Prunus domestica L. – Bull. Torrey Bot. Club 80: 457-477.
Sterling C. 1963. The affinities of Prinsepia (Rosaceae). – Amer. J. Bot. 50: 693-699.
Sterling C. 1964a. Comparative morphology of the carpel in the Rosaceae I. Prunoideae: Prunus. – Amer. J. Bot. : 36-44.
Sterling C. 1964b. Comparative morphology of the carpel in the Rosaceae II. Prunoideae: Maddenia, Pygeum, Osmaronia. – Amer. J. Bot. 51: 354-360.
Sterling C. 1964c. Comparative morphology of the carpel in the Rosaceae III. Pomoideae: Crataegus, Hesperomeles, Mespilus, Osteomeles. – Amer. J. Bot. 51: 705-712.
Sterling C. 1965a. Comparative morphology of the carpel in the Rosaceae IV. Pomoideae: Chamaemeles, Cotoneaster, Dichotomanthes, Pyracantha. – Amer. J. Bot. 52: 47-54.
Sterling C. 1965b. Comparative morphology of the carpel in the Rosaceae V. Pomoideae: Amelanchier, Aronia, Malacomeles, Malus, Peraphyllum, Pyrus, Sorbus. – Amer. J. Bot. 52: 418-426.
Sterling C. 1965c. Comparative morphology of the carpel in the Rosaceae VI. Pomoideae: Eriobotrya, Heteromeles, Photinia, Pourthiaea, Raphiolepis, Stranvaesia. – Amer. J. Bot. 52: 938-946.
Sterling C. 1966a. Comparative morphology of the carpel in the Rosaceae VII. Pomoideae: Chaenomeles, Cydonia, Docynia. – Amer. J. Bot. 53: 225-231.
Sterling C. 1966b. Comparative morphology of the carpel in the Rosaceae VIII. Spiraeoideae: Holodisceae, Neillieae, Spiraeeae, Ulmarieae. – Amer. J. Bot. 53: 521-530.
Sterling C. 1966c. Comparative morphology of the carpel in the Rosaceae IX. Spiraeoideae: Quillajeae, Sorbarieae. – Amer. J. Bot. 53: 951-960.
Sterling C. 1969. Comparative morphology of the carpel in the Rosaceae X. Evaluation and summary. – Österr. Bot. Zeitschr. 116: 46-54.
St-Laurent L, Bousquet J, Simon L, Lalonde M. 1987. Separation of various Frankia strains in the Alnus and Elaeagnus host specificity groups using sugar analysis. – Can. J. Microbiol. 33: 764-772.
St-Laurent L, Baum BR, Akpagana K, Arnason JT. 2000. A numerical taxonomic study of Trema (Ulmaceae) from Togo, West Africa. – Syst. Bot. 25: 399-413.
Strasburger E. 1905. Die Apogamie der Eualchemillen und allgemeine Gesichtspunkte die sich aus ihr ergeben. – Jahrb. Wiss. Bot. 41: 88-164.
Sturms R, Kakar S, Trent III J, Hargrove MS. 2010. Trema and Parasponia hemoglobins reveal convergent evolution of oxygen transport in plants. – Biochemistry 49: 4085-4093.
Su T, Wilf P, Xu H, Zhou Z-K. 2014. Miocene
leaves of Elaeagnus (Elaeagnaceae) from the Qinghai-Tibet
Plateau, its modern center of diversity and endemism. – Amer. J. Bot. 101:
1350-1361.
Suessenguth K. 1953. Rhamnaceae. – In: Engler A (†), Harms H (†), Mattfeld J (†), Melchior H, Werdermann E (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 20d, Duncker & Humblot, Berlin, pp. 7-173.
Sun K, Chen X, Ma R, Li C, Wang Q, Ge S. 2002. Molecular phylogenetics of Hippophae L. (Elaeagnaceae) based on the internal transcribed spacer (ITS) sequences of nrDNA. – Plant Syst. Evol. 235: 121-134.
Sventenius ERS. 1948. Estudio taxonómico en el género Bencomia. – Bol. Inst. Nac. Investig. Agron. 18: 1-19.
Sweitzer EM. 1971. Comparative anatomy of Ulmaceae. – J. Arnold Arbor. 52: 523-585.
Swenson U, Bartish IV. 2002. Taxonomic synopsis of Hippophae (Elaeagnaceae). – Nord. J. Bot. 22: 369-374.
Sytsma KJ, Morawetz J, Pires JC, Nepokroeff M, Conti E, Zihra M, Hall JC, Chase MW. 2002. Urticalean rosids: circumscription, rosid ancestry, and phylogenetics based on rbcL, trnL-F, and ndhF sequences. – Amer. J. Bot. 89: 1531-1546.
Täckholm G. 1922. Zytologische Studien über die Gattung Rosa. – Acta Horti Berg. 7: 97-381.
Takahashi M. 1989. Pollen morphology of Celtidaceae and Ulmaceae: a reinvestigation. – In: Crane PR, Blackmore S (eds), Evolution, systematics and fossil history of the Hamamelidae 2, ‘Higher’ Hamamelidae, Syst. Assoc. Spec. Vol. 40B, Clarendon Press, Oxford, pp. 253-265.
Takaso T, Tobe H. 1990. Seed coat morphology and evolution in Celtidaceae and Ulmaceae (Urticales). – Bot. Mag. (Tokyo) 103: 25-41.
Talent N, Dickinson TA. 2005. Polyploidy in Crataegus and Mespilus (Rosaceae, Maloideae): evolutionary inferences from flow cytometry of nuclear DNA amounts. – Can. J. Bot. 83: 1268-1304.
Talent N, Dickinson TA. 2007a. Endosperm formation in aposporous Crataegus L. (Rosaceae, Spiraeoideae, tribe Pyreae): parallels to Ranunculaceae and Poaceae. – New Phytol. 173: 231-249.
Talent N, Dickinson TA. 2007b. Apomixis and hybridization in Rosaceae subtribe Pyrinae Dumort.: a new tool promises new insights. – In: Grossniklaus U, Hörandl E, Sharbel T, Dijk P van (eds), Apomixis: evolution, mechanisms and perspectives, Gantner, Ruggell, Liechtenstein.
Tandang DN, Tadiosa ER, Gardner EM. 2017. Sloetia (Moraceae): a new generic record for the Philippines. – Telopea 20: 69-73.
Taylor PE, Card G, House J, Dickinson MH, Flagan RC. 2006. High-speed pollen release in the white mulberry, Morus alba L. – Sex. Plant Reprod. 19: 19-24.
Teppner H. 1966. Zur Kenntnis der Gattung Waldsteinia I. – Phyton 11: 224-238.
Terabayashi S. 1991. Vernation patterns in Celtidaceae and Ulmaceae (Urticales), and their evolutionary and systematic implications. – Bot. Mag. (Tokyo) 104: 1-13.
Teryokhin ES, Yakovlev MS, Nikiticheva ZI. 1975. Development of microsporangia, pollen grains, ovule, and embryo sac of Cynomorium songaricum Rupr. (Cynomoriaceae). – Bot. Žurn. 60: 153-162. [In Russian]
Thiele KR. 2007. Two new species of Australian Stenanthemum (Rhamnaceae), with a conspectus and key to species outside Western Australia. – J. Adelaide Bot. Gard. 21: 63-70.
Thiele KR, West JG. 2004. Spyridium burragorang (Rhamnaceae), a new species from New South Wales, with new combinations for Spyridium buxifolium and Spyridium scortechinii. – Telopea 10: 823-829.
Thulin M, Bremer B, Richardson J, Niklasson J, Fay MF, Chase MW. 1998. Family relationships of the enigmatic rosid genera Barbeya and Dirachma from the Horn of Africa region. – Plant Syst. Evol. 213: 103-119.
Tippo O. 1938. Comparative anatomy of the Moraceae and their presumed allies. – Bot. Gaz. 100: 1-99.
Tobe H, Takahashi M. 1990. Trichome and pollen morphology of Barbeya (Barbeyaceae) and its relationships. – Taxon 39: 561-567.
Tobe H, Takaso T. 1996. Trichome morphology in Celtidaceae and Ulmaceae (Urticales). – Acta Phytotaxon. Geobot. 47: 153-168.
Todzia CA. 1989. A revision of Ampelocera (Ulmaceae). – Ann. Missouri Bot. Gard. 76: 1087-1102.
Todzia CA. 1993. Ulmaceae. – In: Kubitzki K, Rohwer JG, Bittrich V (eds), The families and genera of vascular plants II. Flowering plants. Dicotyledons. Magnoliid, hamamelid and caryophyllid families, Springer, Berlin, Heidelberg, New York, pp. 603-611.
Todzia CA, Panero JL. 1998. A new species of Ulmus (Ulmaceae) from southern Mexico and a synopsis of the species in Mexico. – Brittonia 50: 343-347.
Tong Y-H, Xia N-H. 2016. New combinations for Chinese Argentina Hill (Rosaceae). – J. Trop. Subtrop. Bot. 24: 426-428. [In Chinese]
Töpel M. 2010. Phylogenetic and phyloclimatic inference of the evolution of Potentilleae (Rosaceae). – Ph.D. thesis, University of Gothenburg, Gothenburg, Sweden.
Töpel M, Lundberg M, Eriksson T, Eriksen B. 2011. Molecular data and ploidal levels indicate several putative allopolyploidization events in the genus Potentilla (Rosaceae). – PloS Currents 3: RRN1237.
Töpel M, Antonelli A, Yesson C, Eriksen B. 2012. Past climate change and plant evolution in western North America: a case study in Rosaceae. – PloS One 7:e50358.
Topper SMC, Koek-Noorman J. 1980. The occurrence of axial latex tubes in the secondary xylem of some species of Artocarpus J. R. & G. Forster (Moraceae). – IAWA Bull. 1: 113-119.
Tortosa RD. 1983a. El género Discaria (Rhamnaceae). – Bol. Soc. Argent. Bot. 22: 301-335.
Tortosa RD. 1983b. Una especie polimorfa de Discaria: D. chacaye (G. Don) comb. nov. (Rhamnaceae) y sus híbridos presuntivos. – Parodiana 2: 79-89.
Tortosa RD. 1984. El gineceo de Condalia (Rhamnaceae) y su relación con el de otros géneros afines. – Kurtziana 17: 49-54.
Tortosa RD. 1989. El género Colletia (Rhamnaceae). – Parodiana 5: 279-332.
Tortosa RD. 1992. El complejo Retanilla-Talguenea-Trevoa (Rhamnaceae). – Darwiniana 31: 223-252.
Tortosa RD. 1993. Revisión del género Adolphia (Rhamnaceae-Colletieae). – Darwiniana 32: 185-189.
Tortosa RD. 2008. A dubious resurrection of Ochetophila Poepp. ex Endl. (Rhamnaceae). – Feddes Repert. 119: 622-624.
Tortosa RD, Medan D. 1992. Rhamnaceae with multiple lateral buds: an architectural analysis. – Bot. J. Linn. Soc. 108: 275-286.
Tortosa RD, Aagesen L, Tourn GM. 1996. Morphological studies in the tribe Colletieae (Rhamnaeae): analysis of architecture and inflorescences. – Bot. J. Linn. Soc. 122: 353-367.
Tourn GM, Bartoli A, Tortosa RD. 1991. The morphology and growth of Gouania ulmifolia Triana et Planch. (Rhamnaceae): an architectural analysis. – Naturalia Monspeliensia, h. s., 7: 666-667.
Tourn GM, Tortosa RD, Medan D. 1992. Rhamnaceae with multiple lateral buds: an architectural analysis. – Bot. J. Linn. Soc. 108: 275-286.
Treiber EL, Gaglioti AL, Romaniuc-Neto S, Madriñán S, Weiblen GD. 2016. Phylogeny of the Cecropieae (Urticaceae) and the evolution of an ant-plant mutualism. – Syst. Bot. 41: 56-66.
Ueda K, Kosuge K, Tobe H. 1997. A molecular phylogeny of Celtidaceae and Ulmaceae (Urticales) based on rbcL nucleotide sequences. – J. Plant Res. 110: 171-178.
Ungricht S, Rasplus J-Y, Kjellberg F. 2003. Nomenclature of the endemic monoecious fig trees (Moraceae: Ficus L.) of New Caledonia and Vanuatu (Pacific Ocean). – Taxon 52: 319-325.
Unruh M. 1943. Monographie der Gattung Leucosyce Zoll. & Mor. – Bot. Jahrb. Syst. 73: 191-258.
Vamosi JC, Dickinson TA. 2006. Polyploidy and diversification: a phylogenetic investigation in Rosaceae. – Intern. J. Plant Sci. 167: 349-358.
Vanden Heuvel BD, Benson DR, Bortiri E, Potter D. 2004. Low genetic diversity among Frankia spp. strains nodulating sympatric populations of actinorhizal species of Rosaceae, Ceanothus (Rhamnaceae) and Datisca glomerata (Datiscaceae) west of the Sierra Nevada (California). – Can. J. Microbiol. 50: 989-1000.
Vanstraten J, Akkermans ADL, Roelofsen W. 1977. Nitrogenase activity of endophyte suspensions derived from root-nodules of Alnus, Hippophae, Shepherdia and Myrica spp. – Nature 266: 257-258.
Vassilyev AE. 1994a. Developmental and comparative ultrastructure of glandular hairs in the two Urticaceae I. Urtica dioica. – Nord. J. Bot. 14: 531-545.
Vassilyev AE. 1994b. Developmental and comparative ultrastructure of glandular hairs in the two Urticaceae II. Dendrocnide morioides. – Nord. J. Bot. 14: 657-670.
Veenendal WLH van, Outer RW den 1990. Distribution and development of the non-articulated branched laticifers of Morus nigra L. (Moraceae). – Acta Bot. Neerl. 39: 285-296.
Veldkamp JF. 1986. Elaeagnaceae. – In: Steenis CGGJ van, Wilde WJJO de (eds), Flora malesiana I, 10(2), Martinus Nijhoff, The Hague, Boston, London, pp. 151-156.
Velzen R van, Bakker FT, Sattarian A, Maesen van der LJG. 2006. Evolutionary relationships of Celtidaceae. – In: Sattarian A (ed), Contribution to the biosystematics of Celtis L. (Celtidaceae) with special emphasis on the African species, Ph.D. diss., Universiteit Wageningen, Wageningen, the Netherlands, pp. 7-30.
Venkataraman K. 1972. Wood phenolics in the chemotaxonomy of the Moraceae. – Phytochemistry 11: 1571-1586.
Vent W. 1962. Monographie der Gattung Oreoherzogia W. Vent, gen. nov. – Feddes Repert. 65: 3-132.
Verdcourt B. 1975. Cannabaceae. – In: Polhill RM (ed), Flora of tropical East Africa, Crown Agents for Oversea Governments and Administrations, London, pp. 1-3.
Verkerke W. 1989. Structure and function of the fig. – Experientia 45: 612-622.
Vidal J. 1963. Le genre Neillia (Rosaceae). – Adansonia 3: 142-166.
Vikhireva VV. 1952. Morphological and anatomical investigation of fruits of Rhamnaceae. – In: Alexandrova VG (ed), Proceedings of the VL Komarov Botanical Institution of the Academy of Sciences of the USSR, Series VII, Plant Morphology and Anatomy, 3, Bot. Inst. Trudy, ser. 7, Academia Nauk, Moscow, pp. 241-292. [In Russian]
Waksman N, Martinez L, Fernández R. 1989. Chemical and toxicological screening in genus Karwinskia (Mexico). – Rev. Latinoamer. Quím. 20: 27-29.
Walker RI. 1950. Megasporogenesis and development of megagametophyte in Ulmus. – Amer. J. Bot. 37: 47-52.
Wallaart RAM. 1980. Distribution of sorbitol in Rosaceae. – Phytochemistry 19: 2603-2610.
Walsh NG. 1988. Two new species of Pomaderris Labill. (Rhamnaceae) from south-eastern New South Wales. – Muelleria 6: 429-435.
Walsh NG. 1990. The Pomaderris oraria F. Muell. complex in Australia. – Muelleria 7: 267-287.
Walsh NG, Coates F. 1997. New taxa, new combinations and an infrageneric classification in Pomaderris (Rhamnaceae). – Muelleria 10: 27-56.
Wang Q, Manchester SR, Li C, Geng B. 2010. Fruits and leaves of Ulmus from the Paleogene of Fushun, northeastern China. – Intern. J. Plant Sci. 171: 221-226.
Wang W-T. 1980. Classificatio Specierum Sinensium Pellionae (Urticaceae). – Bull Bot. Lab. North-East. For. Inst. 6: 45-66.
Wang W-T. 2012. Five new species of Elatostema (Urticaceae) from China. – J. Syst. Evol. 50: 574-576.
Wang W-T. 2013. Five new series, eleven new species, and one new variety of Elatostema (Urticaceae) from China. – J. Syst. Evol. 51: 225-228.
Wang Y-F, Ferguson DK, Zetter R, Denk T, Garfi G. 2001. Leaf architecture and epidermal characters in Zelkova, Ulmaceae. – Bot. J. Linn. Soc. 136: 255-265.
Warburg O. 1894. Moraceae africanae II. Ficus. Beiträge zur Flora von Afrika IX. – Engl. Bot. Jahrb. Syst. 20: 151-175.
Waugh R, Ven GTW van de, Phillips MS, Powell W. 1990. Chloroplast DNA diversity in the genus Rubus (Rosaceae) revealed by Southern hybridization. – Plant Syst. Evol. 172: 65-75.
Webb JE. 1902. A morphological study of flower and embryo of Spiraea. – Bot. Gaz. 33: 451-460.
Weber C. 1964. The genus Chaenomeles (Rosaceae). – J. Arnold Arbor. 45: 161-295, 302-345.
Weber HE. 1986. Rubi Westfalici. – Abh. Westfal. Mus. Naturk. 3(47): 1-452.
Weber HE. 1999. The present state of taxonomy and mapping of blackberries (Rubus) in Europe. – Acta Bot. Fenn. 162: 161-168.
Weber JE, Campbell CS. 1989. Breeding systems in a hybrid between a sexual and an apomictic Amelanchier, shadbush (Rosaceae: Maloideae). – Amer. J. Bot. 76: 341-347.
Weberbauer A. 1896. Rhamnaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(5), W. Engelmann, Leipzig, pp. 393-427.
Wegener KA. 1967. Chromosomenzahlen aus Wurzelspitzen von Alchemilla-Arten der Sektionen Pentaphyllon Rothm. und Brevicaulon Rothm. – Biol. Zentralbl. 86: 771-792.
Weiblen GD. 2000. Phylogenetic relationships of functionally dioecious Ficus (Moraceae) based on ribosomal DNA sequences and morphology. – Amer. J. Bot. 87: 1342-1357.
Weiblen GD. 2002. How to be a fig wasp. – Ann. Rev. Entomology 47: 299-330.
Weiblen GD. 2004. Correlated evolution in fig pollination. – Syst. Biol. 53: 128-139.
Weiblen GD, Bush GL. 2002. Speciation in fig pollinators and parasites. – Mol. Ecol. 11: 1573-1578.
Weigend M. 2005. Die Erben Pokornys – Ein Beitrag zur Abgrenzung der enigmatischen Sippen Urtica galeopsifolia und Urtica pubescens in Mittel- und Osteuropa. – Hoppea 66: 101-117.
Weigend M, Luebert F. 2009. Weeding the nettles I: clarifying species limits in perennial, rhizomatous Urtica (Urticaceae) from southern and central Chile and Argentina. – Phytotaxa 2: 1-12.
Weimarck H. 1934. Monograph of the genus Cliffortia. – Lund.
Weimarck H. 1948. The genus Cliffortia, a taxonomical survey. – Bot. Not. 101: 167-203.
Welle BJH ter, Koek-Noorman J, Topper SMC. 1986a. The systematic wood anatomy of the Moraceae (Urticales) IV. Genera of the tribe Moreae with urticaceous stamens. – IAWA Bull. 7: 91-128.
Welle BJH ter, Koek-Noorman J, Topper SMC. 1986b. The systematic wood anatomy of the Moraceae (Urticales) V. Genera of the tribe Moreae without urticaceous stamens. – IAWA Bull. 7: 175-193.
Wen J, Shi W. 2012. Revision of the Maddenia clade of Prunus (Rosaceae). – PhytoKeys 11: 39-59.
Wen J, Berggen ST, Lee C-H, Ickert-Bond S, Yi T-S, Yoo K-O, Xie L, Shaw J, Potter D. 2008. Phylogenetic inferences in Prunus (Rosaceae) using chloroplast ndhF and nuclear ribosomal ITS sequences. – J. Syst. Evol. 46: 322-332.
Werlemark G. 2000. Evidence of apomixis in hemisexual dogroses, Rosa section Caninae. – Sexual Plant Repr. 12: 353-359.
Weyland W, Pflug HD, Jähnischen H. 1958. Celtoidanthus pseudorobustus n. gen., n. sp., eine Ulmaceen-Blüte aus Braunkohle der Niederlausitz. – Palaeontographica, Abt. B, 105: 67-74.
Whitmore TC. 1981. Studies in Macaranga X. Novitates Sumatranae. – Kew Bull. 36: 423-424.
Whittemore AT. 2005. Genetic structure, lack of introgression, and taxonomic status in the Celtis laevigata-C. reticulata complex (Cannabaceae). – Syst. Bot. 30: 809-817.
Wickens GE. 1982. The baobab: Africa’s upside-down tree. – Kew Bull. 37: 173-209.
Wieffering J. 1979. Het basis-chromosoomgetal en de taxonomische positie van de tribus Quillajeae binnen de Rosaceae. – Danseria 16: 122-123.
Wiegrefe SJ. 1992. Molecular genetic variation in the Ulmaceae: phylogenetic implications. – Ph.D. diss., University of Wisconsin, Madison, Wisconsin.
Wiegrefe SJ, Sytsma KJ, Guries RP. 1994. Phylogeny of elms (Ulmus, Ulmaceae): molecular evidence for a sectional classification. – Syst. Bot. 19: 590-612.
Wiegrefe SJ, Sytsma KJ, Guries RP. 1998. The Ulmaceae, one family or two? Evidence from chloroplast DNA restriction site mapping. – Plant Syst. Evol. 210: 249-270.
Wilkes S, Glasl H. 2001. Isolation, characterisation and systematic significance of 2-pyrone-4,6-dicarboxylic acid in Rosaceae. – Phytochemistry 58: 441-449.
Williams AH. 1979. Dibenzoylmethanes and flavones of Malus. – Phytochemistry 18: 1897-1898.
Williams AH. 1982. Chemical evidence from the flavonoids relevant to the classification of Malus species. – Bot. J. Linn. Soc. 84: 31-39.
Williams EW, Gardner EM, Harris III R, Chaveerach A, Pereira JT, Zerega NJC. 2017. Out of Borneo: biogeography, phylogeny and divergence date estimates of Artocarpus (Moraceae). – Ann. Bot. 119: 611-627.
Willis CL. 1969. Toxic constituents of the stinging nettle. – M.Sc. thesis, Iowa State University, Ames, Iowa.
Wilmot-Dear CM. 1988. An account of the genus Debregeasia (Urticaceae-Boehmerieae). – Kew Bull. 43: 673-692.
Wilmot-Dear CM, Friis I. 1996. The New World species of Boehmeria and Pouzolzia (Urticaceae, tribus Boehmerieae). A taxonomic revision. – Opera Bot. 129: 1-103.
Wilmot-Dear CM, Friis I. 2012. New World Pouzolzia and Boehmeria (Urticaceae): a new species and new generic record for Paraguay, Pouzolzia amambaiensis, and additional observations on already described species of both genera. – Nord. J. Bot. 29: 691-695.
Wilmot-Dear CM, Friis I. 2013. The old World species of Boehmeria (Urticaceae, tribus Boehmerieae). A taxonomic revision. – Blumea 58: 85-216.
Wissemann V. 2000. Epicuticular wax morphology and the taxonomy of Rosa (section Caninae, subsection Rubiginosae). – Plant Syst. Evol. 221: 107-112.
Wissemann V, Ritz CM. 2005. The genus Rosa (Rosoideae, Rosaceae) revisited: molecular analysis of nrITS-1 and atpB-rbcL intergenic spacer (IGS) versus conventional taxonomy. – Bot. J. Linn. Soc. 147: 275-290.
Wissemann V, Ritz CM. 2007. Evolutionary patterns and processes in the genus Rosa (Rosaceae) and their implications for host-parasite co-evolution. – Plant Syst. Evol. 266: 78-89.
Wolf CB. 1938. The North American species of Rhamnus. – Rancho Santa Ana Botanic Garden, Claremont, California.
Wolf T. 1908. Monographie der Gattung Potentilla. – Bibl. Bot. 16(71): 1-714.
Wolfe JA, Wehr W. 1988. Rosaceous Chamaebatiaria-like foliage from the Paleogene of western North America. – Aliso 12: 177-200.
Woodland DW. 1982. Biosystematics of the perennial North American taxa of Urtica II. Taxonomy. – Syst. Bot. 7: 282-290.
Woodland DW. 1989. Biology of temperate Urticaceae (nettle) family. – In: Crane PR, Blackmore S (eds), Evolution, systematics, and fossil history of the Hamamelidae 2, ‘Higher’ Hamamelidae, Systematics Assoc., Spec. Vol. 40B, Clarendon Press, Oxford, pp. 309-318.
Woodland DW, Bassett IJ, Crompton C, Forget S. 1982. Biosystematics of the perennial North American taxa of Urtica I. Chromosome number, hybridization, and palynology. – Syst. Bot. 7: 269-281.
Wu C-Y, Chang S-S. 1989. Taxa nova nonnulla Moracearum Sinensium. – Acta Bot. Yunnan. 11: 24-34.
Wu Z-Y, Monro AK, Milne RI, Wang H, Yi T-S, Liu J, Li D-Z. 2013. Molecular phylogeny of the nettle family (Urticaceae) inferred from multiple loci of three genomes and extensive generic sampling. – Mol. Phylogen. Evol. 69: 812-827.
Xiang Y, Huang C-H, Hu Y, Wen J, Li S, Yi T-S, Chen H, Xiang J, Ma H. 2017. Evolution of Rosaceae fruit types based on nuclear phylogeny in the context of geological times and genome duplication. – Mol. Biol. Evol. 34: 262-281.
Xu L, Harrison RD, Yang P, Yang D-R. 2011. New insight into the phylogenetic and biogeographic history of genus Ficus: vicariance played a relatively minor role compared with ecological opportunity and dispersal. – J. Syst. Evol. 49: 546-557.
Xu M. 1994. The medical research and exploitation of sea buckthorn. – Hippophae 7: 32-34.
Yakovleva OV. 1994. The ultrastructure of mucilage cells in the leaf epidermis of Dirachma socotrana (Dirachmaceae). – Bot. Žurn. 79: 52-58.
Yamada H, Yoshida O. 1979. Embryological study of the family Ulmaceae I. Embryology of Zelkova serrata Makino. – J. Coll. Arts Chiba Univ. 12B: 27-43.
Yamazaki T. 1973. On Rhamnella and its allied genera. – J. Jap. Bot. 48: 30-32.
Yamazaki T. 1975. Embryology of Elaeagnus umbellata Thunb. – J. Jap. Bot. 50: 281-284.
Yao X, Li C, Dick CW. 2013. Exon-primed intron-crossing (EPIC) markers for evolutionary studies of Ficus and other taxa in the fig family (Moraceae). – Applic. Plant Sci. 1: 1300037.
Yao Y, Tigerstedt PMA. 1993. Isozyme studies of genetic diversity and evolution in Hippophae. – Genet. Res. Crop Evol. 40: 153-164.
Yarnell SH, Blackhurst HT. 1941. Rose investigations – cytological studies. – Texas Agricult. Exp. Sta. 54 Ann. Rep. 53.
Yazbek M, Oh S-H. 2013. Peaches and almonds: phylogeny of Prunus subg. Amygdalus (Rosaceae) based on DNA sequences and morphology. – Plant Syst. Evol. 299: 1403-1418.
Yesson C, Russell SJ, Parrish T, Dalling JW, Garwood NC. 2004. Phylogenetic framework for Trema (Celtidaceae). – Plant Syst. Evol. 248: 85-109.
Yu T-T. 1984. Origin and evolution of Rosaceae. – Acta Phytotaxon. Sin. 22: 431-444. [In Chinese with English summary]
Zardini EM. 1973. Los géneros de Rosáceas espontáneos en la Repuública Argentina. – Bol. Soc. Argentina Bot. 15: 209-228.
Zavada MS. 1983. Pollen morphology of Ulmaceae. – Grana 22: 23-30.
Zavada MS, Crepet WL. 1981. Investigations of angiosperms from the middle Eocene of North America: flowers of the Celtidoideae. – Amer. J. Bot. 68: 924-933.
Zavada MS, Kim M. 1996. Phylogenetic analysis of Ulmaceae. – Plant Syst. Evol. 200: 13-20.
Zeilinga AE. 1964. Polyploidy in Cotoneaster. – Bot. Not. 117: 262-279.
Zerega NJC. 2003. Molecular phylogenetic and genome-wide analyses of Artocarpus (Moraceae): implications for the systematics, origins, human-mediated dispersal, and conservation of breadfruit. – Ph.D. diss., New York University, New York.
Zerega NJC, Clement WL, Datwyler SL, Weiblen GD. 2005. Biogeography and divergence times in the mulberry family (Moraceae). – Mol. Phylogen. Evol. 37: 402-416.
Zerega NJC, Ragone D, Motley TJ. 2005. Systematics and species limits of breadfruit (Artocarpus, Moraceae). – Syst. Bot. 30: 603-615.
Zerega NJC, Nur Supardi MN, Motley TJ. 2010. Phylogeny and recircumscription of Artocarpeae (Moraceae) with a focus on Artocarpus. – Syst. Bot. 35: 766-782.
Zhang S-D, Soltis DE, Yang Y, Li D-Z, Yi T-S. 2011. Multi-gene analysis provides a well-supported phylogeny of Rosales. – Mol. Phylogen. Evol. 60: 21-28.
Zhang S-Y. 1992. Systematic wood anatomy of the Rosaceae. – Blumea 37: 81-158.
Zhang S-Y, Baas P. 1992. Wood anatomy of trees and shrubs from China III. Rosaceae. – IAWA Bull., N. S., 13: 21-91.
Zhang S-Y, Baas P, Zandee M. 1992. Wood structure of the Rosaceae in relation to ecology, habit and phenology. – IAWA Bull., N. S., 13: 307-349.
Zhang Z-H, Li C-Q, Li J. 2009. Phylogenetic placement of Cynomorium in Rosales inferred from sequences of the inverted repeat region of the chloroplast genome. – J. Syst. Evol. 47: 297-304.
Zhang Z-Y, Sun H, Gu Z-J. 2002. Karyomorphological study of the Spiraea japonica complex (Rosaceae). – Brittonia 54: 168-174.
Zhao L, Jiang X-W, Zuo Y-J, Liu X-L, Chin S-W, Haberle R, Potter D, Chang Z-Y, Wen J. 2016. Multiple events of allopolyploidy in the evolution of the racemose lineages in Prunus (Rosaceae) based on integrated evidence from nuclear and plastid data. – PLoS One 11: e0157123
Zhao W-G, Zhou Z-H, Miao X-X, Zhang Y, Wang S-B, Huang J-H, Xiang H, Pan Y-L, Huang Y-P. 2007. A comparison of genetic variation among wild and cultivated Morus species (Moraceae: Morus) as revealed by ISSR and SSR markers. – Biodiv. Cons. 16: 275-290.
Zheng X, Cai D, Potter D, Postman J, Liu J,
Teng Y. 2014. Phylogeny and evolutionary histories of Pyrus L.
revealed by phylogenetic trees and networks based on data from multiple DNA
sequences. – Molec. Phylogen. Evol. 80: 54-65.
Zhong Y, Baas P, Wheeler EA. 1992. Wood anatomy of trees and shrubs from China IV. Ulmaceae. – IAWA Bull., N. S., 13: 419-453.
Zhu ZM, Gao XF, Fougere-Danezan M. 2015. Phylogeny of Rosa sections Chinenses and Synstylae (Rosaceae) based on chloroplast and nuclear markers. – Mol. Phylogen. Evol. 87: 50-64.
Zieliński J. 1980. Distribution of Rosa persica Michx ex Juss. and its hybrids. – Arbor. Korn. 25: 41-51.
Zieliński J. 1990. The genus Rosa L. in Greece. – Arbor. Kórnickie 35: 3-45.
Zietsman PC. 1990. Pollination of Ziziphus mucronata subsp. mucronata (Rhamnaceae). – South Afr. J. Bot. 56: 350-355.
Zietsman PC, Botha FC. 1992. Flowering of Ziziphus mucronata subsp. mucronata (Rhamnaceae): anthesis, pollination and protein synthesis. – Bot. Bull. Acad. Sin. 33: 33-42.