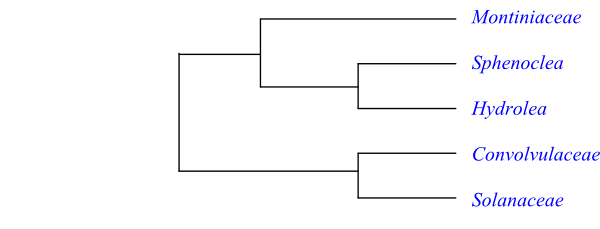
Cladogram of Solanales based on DNA sequence data (Bremer & al. 2002; etc.). The clades have a bootstrap support of 100% or almost so.
Solananae R. Dahlgren ex Reveal in Novon 2: 236. 13 Oct 1992
Habit Usually bisexual (sometimes monoecious, andromonoecious or dioecious), evergreen trees, shrubs or lianas, perennial, biennial or annual herbs (sometimes climbing).
Vegetative anatomy Roots diarch. Phellogen ab initio superficially or deeply seated. Secondary lateral growth sometimes anomalous (via cylindrical cambium or concentric cambia) or absent. Vessel elements usually with simple (rarely scalariform) perforation plates; lateral pits usually alternate (sometimes scalariform), simple or bordered pits. Imperforate tracheary xylem elements fibre tracheids, libriform fibres or tracheids with simple or bordered pits, usually non-septate (sometimes also vasicentric tracheids). Wood rays uniseriate or multiseriate, homocellular or heterocellular. Axial parenchyma apotracheal diffuse or diffuse-in-aggregates, or paratracheal scanty vasicentric, aliform, lozenge-aliform, confluent, reticulate, unilateral or banded (sometimes absent). Wood often fluorescent. Intraxylary phloem usually present. Sieve tube plastids S type. Nodes 1:1–3, unilacunar with one to three leaf traces (rarely multilacunar with several traces). Latex cells and articulated laticifers sometimes frequent. Resins present or absent. Cystoliths often present. Prismatic or acicular calciumoxalate crystals, druses, styloids and crystal sand often abundant.
Trichomes Hairs unicellular or multicellular, uniseriate, simple, furcate, dendritic or stellate (sometimes peltate-lepidote); multicellular glandular hairs often abundant.
Leaves Usually alternate (spiral; sometimes opposite), usually simple (sometimes pinnately or palmately compound or), entire or pinnately lobed, sometimes coriaceous, often with conduplicate ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle transection arcuate or annular. Venation pinnate or palmate. Stomata usually anomocytic or paracytic (sometimes anisocytic, diacytic, tetracytic or parallelocytic). Cuticular wax crystalloids? Domatia as hair tufts or absent. Mesophyll without mucilage cells, with or without sclerenchymatous idioblasts. Leaf margin and leaflet margins serrate or entire.
Inflorescence Terminal or axillary, usually simple or compound dichasia or cincinni (rarely corymbose or paniculate cymose, capitate or spicate), or flowers solitary terminal or axillary. Floral prophylls (bracteoles) rarely absent.
Flowers Actinomorphic to obliquely zygomorphic. Usually hypogyny (rarely epigyny or half epigyny). Sepals (three to) five (to seven), usually with imbricate or plicate (rarely valvate or open) aestivation, often persistent, free or connate. Petals (three to) five (to seven), with contorted, plicate, imbricate, induplicate or valvate aestivation, usually connate into infundibuliform, campanulate or tubular corolla. Nectariferous disc intrastaminal, annular or cupular (sometimes tuberculate, discoid or pulvinate, rarely absent).
Androecium Stamens (three to) five (to seven), haplostemonous, antesepalous, alternipetalous. Filaments free from each other, from tepals or adnate to corolla (epipetalous), sometimes of two different lengths. Anthers often connivent around style, usually dorsifixed to basifixed (rarely ventrifixed), usually non-versatile, tetrasporangiate, usually introrse (rarely extrorse), longicidal (dehiscing by longitudinal slits or sometimes by two apical slits) or poricidal (dehiscing by one apical pore, or two separate apical pores). Tapetum secretory. Female flowers sometimes with staminodia.
Pollen grains Microsporogenesis simultaneous. Pollen grains (2–)3–5(–8)-colpate or (2–)3–5(–6)-colpor(oid)ate (sometimes polycolpate, pantoporate or inaperturate), usually shed as monads (rarely tetrads), usually bicellular (sometimes tricellular) at dispersal. Exine tectate or semitectate, with usually columellate (occasionally granular) infratectum, perforate, reticulate, punctate or striate, psilate, spinulate or echinate. Pollen tube with callose plugs.
Gynoecium Pistil composed of usually two (sometimes five, rarely one or three) connate antepetalous carpels, often displaced relative to floral symmetry. Ovary usually superior (rarely inferior or semi-inferior), usually unilocular or bilocular (rarely trilocular, quinquelocular or multilocular [due to secondary septa]). Style single, simple, usually terminal (rarely gynobasic), or stylodia two (to five) usually more or less connate. Stigma capitate, truncate, punctate, filiform, peltate, infundibuliform or lobate, papillate or non-papillate, Dry or Wet type. Male flowers sometimes with pistillodium.
Ovules Placentation usually axile to basal (often with large placentae; rarely parietal). Ovules one to several hundred per carpel, usually anatropous or hemianatropous (sometimes amphitropous, campylotropous or pleurotropous), ascending to pendulous, apotropous, unitegmic or bitegmic, usually tenuinucellar (sometimes weakly crassinucellar). Megagametophyte usually monosporous, Polygonum type (sometimes disporous, Allium type, or tetrasporous, Adoxa type). Synergids sometimes with a filiform apparatus. Antipodal cells sometimes persistent. Endosperm development cellular or nuclear (rarely [seemingly?] helobial). Endosperm haustoria micropylar and/or chalazal (sometimes absent). Embryogenesis usually solanad or caryophyllad (rarely onagrad).
Fruit A loculicidal or septicidal (rarely irregularly dehiscent) capsule, a pyxidium or berry (rarely a drupe, nut or schizocarp with five to numerous nutlike mericarps), often with persistent and accrescent calyx.
Seeds Aril absent. Exotesta and endotesta persistent. Theoidal exotestal thickenings often present. Perisperm not developed. Endosperm sometimes ruminate, usually copious, oily (rarely starchy). Embryo straight to curved (rarely annular, U-shaped or spirally twisted), usually well differentiated, with or without chlorophyll. Cotyledons usually two (rarely four). Germination phanerocotylar.
Cytology x = (6–)7–13
DNA
Phytochemistry Flavonols (kaempferol, quercetin, myricetin), O-methyl flavones, O-methyl flavonols and flavone glycosides, cyanidin, acylated anthocyanins, coumarins, ursolic acid, esters of caffeic acid, glycine betaines, nicotinic acid compounds, hygroline alkaloids (hygrines), nortropane-3α-ols, tropane-3α-ols (e.g. hyoscyamine, apoatropine, littorine), tropane-3β-ols (e.g. tigloidine), hydroxyl tropines (e.g. scopolamine, daturamine), tropane alkaloids (e.g. teloidines, tropinone, cuscohygrine, convolvine, convolamine), pyrrolizidine and pyrrolidine alkaloids (e.g. nicotinic pyrrolidine), saponins, cyanogenic compounds, withanolide steroidal lactones, and polyacetate-derived arthroquinones present. Tannins rare. Montinioside (iridoid gentiobioside) present in Montiniaceae. Iridoids and ellagic acid not found. Carbohydrates stored as oligosaccharides. Cell walls with arabinoxyloglucanes. Pyrrolidine, pyrrolizidine and tropane alkaloids synthesized from ornithine precursor.
Systematics Solanales may be sister-group to Plantaginales, although the support is fairly low.
A possible topology is [[Montiniaceae+[Hydrolea+Sphenoclea]]+[Convolvulaceae+Sola-naceae]].
The clade [Montiniaceae+[Sphenoclea+Hydrolea]] has the potential synapomorphies: petiole bundle transection arcuate; and presence of alkaloids (Stevens 2001 onwards). Sphenoclea and Hydrolea share the characters: swollen placentae; numerous ovules per carpel; cellular endosperm development; multicellular micropylar and chalazal haustoria; and endosperm at most scanty.
Convolvulaceae and Solanaceae form a highly supported clade, which is characterized by the following potential synapomorphies, according to Stevens (2001 onwards): presence of intraxylary phloem; leaves with conduplicate ptyxis; floral symmetry oblique; corolla aestivation usually contorted-plicate or induplicate-valvate; corolla tube formation late; carpels antepetalous; ovules numerous per carpel; integument (5–)9–20(–40) cell layers thick; presence of integumentary tapetum; persistent calyx conspicuous in fruit; testa often multiplicative; young seeds with starch; cotyledons incumbent; presence of flavonol and flavone glycosides, acylated anthocyanins, coumarins, caffeic acid esters, tropane (polyhydroxy nortropanes), pyrrolizidine and pyrrolidine alkaloids (synthesized from ornithine precursor); and absence of tannins.

|
Cladogram of Solanales based on DNA sequence data (Bremer & al. 2002; etc.). The clades have a bootstrap support of 100% or almost so. |
CONVOLVULACEAE Juss. |
( Back to Solanales ) |
Convolvulales Juss. ex Bercht. et J. Presl, Přir. Rostlin: 247. Jan-Apr 1820 [‘Convolvulaceae’]; Cuscutales Bercht. et J. Presl, Přir. Rostlin: 247. Jan-Apr 1820 [‘Cuscutae’]; Cressaceae Raf. in Ann. Gén. Sci. Phys. Bruxelles 8: 270. 1821 [’Cressaria’]; Evolvulaceae Bercht. et J. Presl, Přir. Rostlin 2: 130, 132. 1825; Cuscutaceae (Dumort.) Dumort., Anal. Fam. Pl.: 20, 25. 1829, nom. cons.; Dichondraceae Dumort., Anal. Fam. Plant.: 20, 24. 1829, nom. cons.; Cuscutineae Link, Handbuch 1: 594. 4-11 Jul 1829 [‘Cuscutinae’]; Erycibaceae Endl. ex Meisn., Plant. Vasc. Gen.: Tab. Diagn. 272, Comm. 185. 5-11 Apr 1840 [’Erycibeae’]; Convolvulopsida Brongn., Enum. Plant. Mus. Paris: xviii, 54. 12 Aug 1843 [’Convolvulineae’]; Evolvulineae J. Presl in Nowočeská Bibl. [Wšobecný Rostl.] 7: 1088, 1097. 1846 [‘Evolvulaceae‘]; Poranaceae J. Agardh, Theoria Syst. Plant.: 364. Apr-Sep 1858; Convolvulineae Engl., Syllabus, ed. 2: 175. Mai 1898; Humbertiaceae Pichon in Notul. Syst. (Paris) 13: 23. Jul-Sep 1947, nom. cons.
Genera/species c 60/1.590–1.625
Distribution Cosmopolitan except polar areas, with their largest diversity in subtropical regions in Asia and America.
Fossils Pollen fossils (Perfotricolpites digitatus) resemble Convolvulus and other extant Convolvulaceae. Other pollen fossils (Calystegiapollis microechinatus, Perfotricolpites digitatus, Tricolpites trioblatus, Xenostegia tridentata) are known from the Eocene of Africa, southern Australia, Brazil and Europe.
Habit Usually bisexual (in Hildebrandtia dioecious), usually climbing and winding perennial or annual herbs (rarely evergreen trees, shrubs or lianas). Some species are xerophytic. Cuscuta are achlorophyllous, winding root-less stem holoparasites with haustoria and often filiform stems and branches.
Vegetative anatomy Main root in Cuscuta ephemeral, without mycorrhiza. Phellogen ab initio usually superficial (sometimes pericyclic). Medulla often septated through diaphragms. Secondary lateral growth usually anomalous (usually from concentric/successive cambia) or absent. Vessel elements with simple perforation plates; lateral pits alternate, simple or bordered pits. Imperforate tracheary xylem elements tracheids or fibre tracheids with bordered pits, non-septate (sometimes also vasicentric tracheids). Wood rays uniseriate or multiseriate, homocellular or somewhat heterocellular. Axial parenchyma apotracheal diffuse, or paratracheal scanty vasicentric, unilateral or banded. Wood sometimes fluorescent. Tyloses sometimes abundant. Intraxylary (concentric) phloem usually present (absent in Cuscuta). Sieve tube plastids S type. Nodes 1:1, unilacunar with one leaf trace. Latex cells and articulated laticifers abundant. Fibres and sclereids sometimes present. Heartwood sometimes with gum-like substances. Calciumoxalate present as acicular or prismatic crystals, styloids, druses and/or elongate crystals, crystal sand etc. in some representatives.
Trichomes Hairs unicellular or multicellular, uniseriate, furcate or multi-armed (sometimes stellate or dendritic); glandular hairs multicellular (also lepidote).
Leaves Alternate (spiral), usually simple (sometimes pinnately or palmately compound), entire or lobed, sometimes coriaceous (in Cuscuta very small, scale-like, or absent), often with conduplicate ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle transection usually arcuate (in Humbertia annular). Venation usually palmate (sometimes pinnate). Stomata usually paracytic (sometimes anomocytic, anisocytic or parallelocytic). Cuticular wax crystalloids? Mesophyll with or without sclerenchymatous idioblasts. Leaf margin serrate or entire (occasionally lobed). Extrafloral nectaries present on petiole and lamina in numerous species.
Inflorescence Terminal or axillary, usually simple or compound dichasia or cincinni (rarely dense head or spike), or flowers solitary axillary. Floral prophylls (bracteoles) sometimes large and forming involucre.
Flowers Usually actinomorphic (rarely zygomorphic; in Humbertia with oblique irregular corolla), often large. Hypogyny. Sepals (three to) five, with imbricate quincuncial aestivation, often large (sometimes unequal in size), persistent, usually free. Petals (three to) five, with induplicate-valvate or contorted-plicate (in Cuscuta imbricate [valvate?]) aestivation, connate into usually infundibuliform (sometimes campanulate or tubular) corolla, in Cuscuta with fimbriate corona especially below and behind stamens; corolla tube formation late. Nectariferous disc intrastaminal, annular or cupular (absent in Humbertia).
Androecium Stamens (three to) five, haplostemonous, antesepalous, alternipetalous, often unequal in size. Filaments free from each other, adnate to corolla tube (epipetalous). Anthers basifixed (in Humbertia) or dorsifixed, non-versatile, tetrasporangiate, usually introrse, longicidal (dehiscing by longitudinal slits; anthers rarely septate). Anther placentoid absent. Tapetum usually secretory (in Cuscuta sometimes amoeboid-periplasmodial), with multinucleate cells. Staminodia usually absent (staminodia in Cuscuta five, intrastaminal, scale-like, alternating with fertile stamens).
Pollen grains Microsporogenesis simultaneous. Pollen grains tri- to zonopolycolpate or pantoporate, shed as monads, usually bicellular (sometimes tricellular) at dispersal. Exine tectate or semitectate, with columellate infratectum, imperforate, perforate, reticulate, punctate, psilate, echinate or spinulate.
Gynoecium Pistil composed of two (to five) connate median antepetalous carpels (gynophore present in Humbertia). Ovary superior, usually unilocular or bilocular (rarely trilocular or quinquelocular; in Dichondra and Falkia deeply bilobate or quadrilobate, with lobes fused at base of bifid gynobasic style; in Mina with secondary septa). Style single or stylodia two (to five), usually more or less connate (in Cuscuta simple or two free; style sometimes, e.g. in Dichondreae, gynobasic). Stigma capitate, punctate, filiform or lobate (rarely peltate, truncate, infundibuliform etc.), papillate (with multicellular papillae), Dry type. Pistillodium absent.
Ovules Placentation usually subbasal to basal (in Humbertia axile). Ovules (one or) two (to four; in Humbertia numerous) per carpel, usually anatropous, ascending, apotropous, unitegmic, tenuinucellar (reduced tenuinucellar, with meiocyte semi-inferior) to weakly crassinucellar (in Cuscuta incompletely tenuinucellar). Integument usually 9–20 (in Cuscuta 15–17) cell layers thick. Placental obturator often present. Parietal tissue one or two cell layers thick. Megagametophyte usually monosporous, Polygonum type (in Cuscuta usually disporous, octacellular, Allium type). Endosperm development ab initio nuclear. Endosperm haustoria? Embryogenesis usually caryophyllad (in Cuscuta sometimes solanad).
Fruit Usually a loculicidal capsule or a pyxidium (sometimes irregularly dehiscing; rarely a nut or, e.g. in Erycibe, a berry); sepals usually persistent and often lignified in fruit.
Seeds Aril absent. Testa usually multiplicative (not in Cuscuta), with complex anatomy. Outer three cell integument layers specially differentiated. Exotesta often with papillae or hairs, somewhat thickened. Outer hypodermis consisting of small cells, little thickened. Inner hypodermis consisting one or more palisade layers. Inner hypodermal cells often elongate, thickened, or somewhat elongate. Inner layers two to eight, consisting of sclereidal cells. Endotesta? Perisperm not developed. Endosperm copious, often chartaceous, oily. Embryo usually large, straight or curved, with chlorophyll (in Cuscuta filiform, spirally coiled, poorly differentiated). Suspensor haustorium present. Cotyledons two, often plicate or coiled, bifid (almost absent in Cuscuta). Germination phanerocotylar.
Cytology n = 7–15 or more – Protein crystals often present in nucleus.
DNA Deletion of 6–15 bp present in plastid gene atpB. Deletion of 150 bp present in plastid gene trnF. Plastid gene rpl2 intron lost. Plastid gene ndhF pseudogene in Cuscuta and probably in many other Convolvulaceae. Plastid inverted repeat reduced by 7 kb in Cuscuta reflexa: hence genes rpl2, rpl23, trnI, region homologous to Nicotiana ORF2280, and one rps12 intron lost. Pseudogene ψndhB in Cuscuta europaea reduced and cis-spliced intron of rps12 absent. Plastid gene ycf15 lost. Plastid ORF244 and intron in rpl2 lost in numerous Convolvulaceae, including Cuscuta. Plastid gene infA lost (Convolvulus). Mitochondrial coxI intron present (Ipomoea).
Phytochemistry Flavonols (kaempferol, quercetin), flavonol glycosides, flavones, O-methyl flavonols, O-methyl flavones, cyanidin (in Cuscuta), acylated anthocyanins, coumarins, caffeic acid esters, ornithin-derived tropane alkaloids (polyhydroxy nortropanes, e.g. convolvine, convolamine, cuscohygrine; not found in Cuscuta and Humbertia), ergoline alkaloids, pyrrolidine alkaloids (not found in Cuscuta), glycine betaines, saponins, cyanogenic compounds (derived from phenylalanine?) latex, and glucoretinous resin present. Ellagic acid and tannins not found. Some secondary metabolites are synthesized by endophytic fungi or bacteria in, e.g., Ipomoea and Turbina.
Use Ornamental plants, starch source (Ipomoea batatas), vegetables (Ipomoea aquatica), forage plants, medicinal plants.
Systematics Convolvulaceae are sister-group to Solanaceae.
Humbertia is sister to the remaining Convolvulaceae.
Humbertioideae Roberty in Candollea 14: 22. Oct 1952
1/1. Humbertia (1; H. madagascariensis; Madagascar). – Large tree. Wood with scent similar to sandalwood. Vascular bundles collateral. Secondary lateral growth normal. Wood rays uniseriate, homocellular, with exclusively procumbent cells. Usually with latex cells only in flowers or with articulated laticifers. Petiole vascular bundle transection annular. Flowers solitary, axillary, strongly obliquely zygomorphic. Sepals with five traces. Nectariferous disc absent. Filaments curved in bud, adnate to corolla base (epipetalous). Anthers basifixed. Gynophore present. Style clavate. Placentation axile. Ovules numerous per carpel. Fruit a few-seeded drupe. Endosperm copious. n = ? Ornithin-derived tropane alkaloids absent. – The phytochemistry of Humbertia is very insufficiently known.
Convolvuloideae Burnett, Outl. Bot.: 1002, 1095, 1105. Feb 1835 [‘Convolvulidae’]
c 60/1.590–1.625. Cardiochlamyeae Stafanović et D. F. Austin in S. Stefanović, D. F. Austin et R. G. Olmstead in Syst. Bot. 28: 796. 13 Nov 2003. Cordisepalum (2; C. phalanthopetalum, C. thorelii; Southeast Asia), Cardiochlamys (2; C. madagascariensis, C. velutina; Madagascar), Dinetus (6; D. decorus, D. dinetoides, D. duclouxii, D. grandiflorus, D. racemosus, D. truncatus; the Himalayas to Burma, southern China and Southeast Asia, Malesia), Porana (2; P. nutans, P. volubilis; Southeast to the Philippines, Mexico), Duperreya (3; D. commixta, D. halfordii, D. sericea; western and southern Australia), Poranopsis (3; P. discifera, P. paniculata, P. sinensis; India, Tibet and Burma to southern China and Southeast Asia, Malesia), Tridynamia (4; T. bialata, T. megalantha, T. sinensis, T. spectabilis; India and Burma to Hainan, Southeast Asia and West Malesia). – Erycibeae Hogg, Veg. Kingd.: 535. 1858. Erycibe (67; tropical Asia to Japan and tropical Australia). – Dichondreae Choisy ex G. Don, Gen. Hist. 4: 253, 302. 1837-8 Apr 1838. Dichondra (14; tropical and subtropical regions on both hemispheres), Falkia (3; F. canescens, F. oblonga, F. repens; Africa), Nephrophyllum (1; N. abyssinicum; Ethiopia), Petrogenia (1; P. repens; the Chihuahuan Desert in Mexico), Metaporana (5; M. conica, M. densiflora, M. parvifolia, M. sericosepala, M. verdcourtii; East Africa, Madagascar, Socotra), Calycobolus (c 30; tropical Africa, Madagascar, tropical America), Dipteropeltis (3; D. macrantha, D. mayumbensis, D. poranoides; tropical West and Central Africa), Rapona (1; R. tiliifolia; Madagascar). – Cresseae C. B. Clarke in J. D. Hooker, Fl. Brit. India 4: 180. Jun 1883. Hildebrandtia (13; Africa, Madagascar, the Arabian Peninsula), Seddera (c 20; tropical and subtropical regions in Africa, Madagascar, the Arabian Peninsula), Evolvulus (c 100; southern United States, Mexico, Central America, the West Indies, tropical South America, two species, E. alsinoides and E. nummularius, also in the Old World tropics), Cressa (4–5; C. aphylla, C. australis, C. cretica, C. nudicaulis, C. truxillensis; tropical to mediterranean regions on both hemispheres), Bonamia (c 45; tropical and subtropical regions on both hemispheres), Stylisma (8; southern and eastern United States), Wilsonia (3; W. backhousei, W. humilis, W. rotundifolia; southern Australia, Tasmania), Itzaea (1; I. sericea; Central America), Neuropeltis (18; tropical Africa, tropical Asia), Neuropeltopsis (1; N. alba; Borneo), Keraunea (1; K. brasiliensis; Bahia in Brazil). – Maripeae Webb et Berthel., Hist. Nat. Iles Canaries 3(2,3): 27. Apr 1844. Dicranostyles (15; tropical America), Maripa (20; tropical and subtropical America, with their highest diversity in northern South America), Lysiostyles (1; L. scandens; tropical South America). – Jacquemontieae Stefanović et D. F. Austin in S. Stefanović, D. F. Austin et R. G. Olmstead in Syst. Bot. 28: 802. 13 Nov 2003. Jacquemontia (80–100; tropical and subtropical regions on both hemispheres, with their largest diversity in the Neotropics). – Cuscuteae Dumort., Fl. Belg.: 50. 1827. Cuscuta (c 170; almost cosmopolitan). – Aniseieae Stefanović et D. F. Austin in S. Stefanović, D. F. Austin et R. G. Olmstead in Syst. Bot. 28: 796. 13 Nov 2003. Aniseia (6; A. argentina, A. cernua, A. harmandii, A. heterophylla, A. martinicensis, A. minor; southern Mexico, Central America, the West Indies, tropical South America), Odonellia (2; O. eriocephala, O. hirtiflora; tropical America), Tetralocularia (1; T. pennellii; Colombia). – Convolvuleae Dumort., Fl. Belg.: 50. 1827.Convolvulus (c 125; cosmopolitan, with their highest diversity in temperate regions). – Ipomoeeae Hallier f. in Engl. Bot. Jahrb. Syst. 16: 562, 583. 27 Jun 1893. Polymeria (10; East Malesia to northern and eastern Australia and New Caledonia); ‘Merremia’ sibirica, ‘Meremia’ poranoides, Remirema (1; R. bracteata; Thailand), Distimake (35; tropical Africa, Madagascar, the Seychelles, India and Sri Lanka to southern China, tropical Australia and islands in the Pacific), Camonea (5; C. bambusetorum, C. kingii, C. pilosa, C. umbellata, C. vitifolia; tropical Asia, one species also in tropical West and East Africa, Madagascar and tropical America from southern Mexico and the West Indies to northern Argentina), ‘Merremia’ caloxantha, Operculina (c 15; tropical regions on both hemispheres), ‘Merremia’ pro parte, Hyalocystis (2; H. popovii, H. viscosa; tropical Africa), Hewittia (1; H. malabarica; tropical Africa, Madagascar, tropical Asia to New Guinea), Xenostegia (5; X. filiformis, X. media, X. pinnata, X. sapinii, X. tridentata; tropical Africa, Madagascar, tropical Asia to tropical Australia and Solomon Islands), Decalobanthus (c 13; Southeast Asia, Malesia to islands in the Pacific; one species, D. peltatus, in tropical East Africa and Madagascar to islands in the Pacific), Merremia (10–20?; South, East and Southeast Asia, Malesia to New Guinea and northern Australia), Daustinia (1; D. montana; Brazil), ’Ipomoea’ (c 650; warm-temperate to tropical regions on both hemispheres; non-monophyletic), Stictocardia (12; tropical regions in the Old World; paraphyletic), Turbina (15; tropical and southern Africa, New Caledonia, tropical America; in Stictocardia?), Lepistemon (10; tropical regions in the Old World), ‘Ipomoea’ pro parte; unplaced Ipomoeeae: Argyreia (90–95; tropical Asia to Australia), Astripomoea (12; tropical and southern Africa), Blinkworthia (2; B. convolvuloides, B. lycioides; Burma, southern China), Lepistemonopsis (1; L. volkensii; tropical East Africa), Paralepistemon (1; P. shirensis; southern tropical Africa). – Unplaced Convolvuloideae Saccia (1; S. elegans; Colombia, Bolivia). – Distribution as for Convolvulaceae. Herbaceous (to woody), dextrorsely twining vines and lianas (rarely trees). Secondary lateral growth anomalous. Fibres and sclereids sometimes present. Hairs often unicellular, T-shaped (sometimes stellate). Leaf margin usually entire (sometimes lobed or serrate). Bract sometimes adnate to pedicel and accrescent in fruit (in Cardiochlamyeae often foliaceous); floral prophylls (bracteoles) in Convolvulus sometimes strongly enlarged. Flowers sometimes somewhat obliquely disymmetrical. Sepals with less than five traces. Pollen grains pantoporate or tri- to polycolpate. Exine in Cuscuta sometimes reticulate. Gynophore absent. Ovary in Mina with secondary septum. Placentation subbasal to basal. Ovules (one or) two (to four) per carpel, tenuinucellar to weakly crassinucellar. Integument vascularized, with unbranched bundle. Archespore in Cuscuta multicellular. Megagametophyte in at least Ipomoea strongly elongated. Fruit in Cardiochlamyeae one-seeded, utriculate, with papery pericarp. Testal cells in Erycibeae and Maripeae little thickened and elongated. Fruit usually a capsule with varying types of dehiscence. Endosperm usually with galactomannans. Ergoline alkaloids (produced by Clavicipitales fungi) sometimes present.
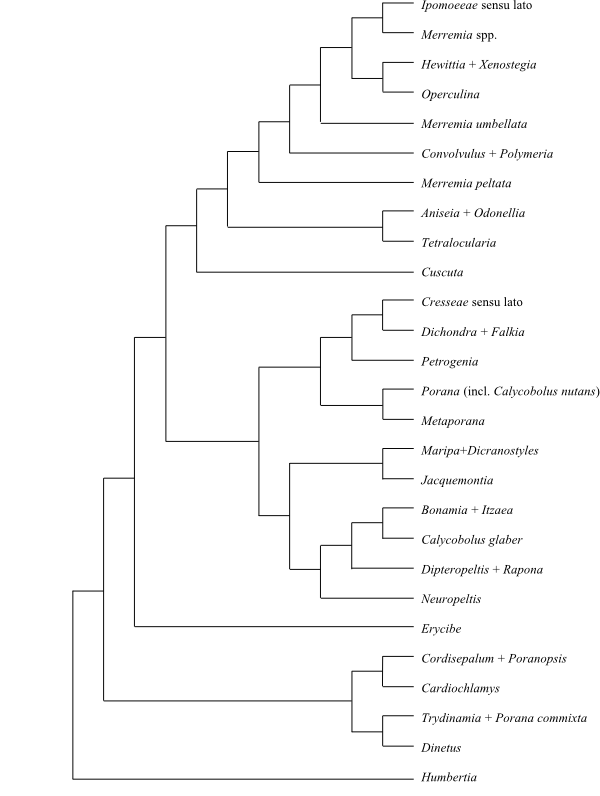 |
Phylogeny (simplified) of Convolvulaceae based on DNA sequence data (Stefanović, Krueger & Olmstead 2002; Stefanović, Austin & Olmstead 2003). |
HYDROLEACEAE R. Br. ex Edwards |
( Back to Solanales ) |
Hydroleales Bercht. et J. Presl, Přir. Rostlin: 247. Jan-Apr 1820 [‘Hydroleae’]
Genera/species 1/11
Distribution Almost pantropical.
Fossils Unknown.
Habit Bisexual, annual or perennial herbs or shrubs, sometimes with axillary-sublateral spines. Helophytic.
Vegetative anatomy Mycorrhiza absent. Phellogen? Vessel elements with usually simple perforation plates (rarely scalariform); lateral pits? Vestured pits occasionally present. Imperforate tracheary xylem elements fibre tracheids, usually non-septate. Wood rays? Axial parenchyma? (diffuse parenchyma absent). Sieve tube plastids S type? Nodes? Crystals clustered in cells surrounding air-canals in primary cortex. Calcium oxalate druses in leaf veins.
Trichomes Hairs unicellular or multicellular, uniseriate; stalked glandular hairs abundant.
Leaves Alternate (spiral), simple, entire, with ? ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle transaction arcuate. Venation pinnate, brochidodromous. Stomata anomocytic. Cuticular wax crystalloids? Leaf margin serrate or entire.
Inflorescence Terminal or axillary, cymose.
Flowers Actinomorphic (with diagonally inserted carpels). Hypogyny. Sepals four or five, with valvate aestivation, connate at base; first sepal adaxial, second sepal abaxial (median sepal abaxial in Hydrolea palustris). Petals four or five, with imbricate aestivation, postgenitally? connate into campanulate or tubular perigone; corolla tube formation late. Nectariferous disc intrastaminal, consisting of four or five interstaminal tubercles, or absent.
Androecium Stamens four or five, haplostemonous, antesepalous, alternipetalous. Filaments free from each other, swollen at base, adnate to corolla tube in lower part (epipetalous). Anthers basifixed, versatile, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory? Staminodia absent.
Pollen grains Microsporogenesis simultaneous? Pollen grains tricolporate, shed as monads?, ?-cellular at dispersal. Exine semitectate, with columellate infratectum, reticulate, without supratectal sculpturing.
Gynoecium Pistil composed of two (to four) connate, diagonally arranged/initiated carpels; carpel synascidiate (in upper part symplicate). Ovary superior, usually bilocular (sometimes trilocular or quadrilocular). Stylodia two (to four), incurved, largely free, spreading. Stigmas somewhat infundibuliform or capitate, Wet type? Pistillodium absent.
Ovules Placentation axile, with large bilobate placentae. Ovules c. 200 to c. 300 per carpel, usually anatropous, unitegmic, tenuinucellar. Integument six to eight cell layers thick. Megagametophyte monosporous, Polygonum type. Antipodal cells early degenerating. Endosperm development cellular. Endosperm haustoria micropylar and chalazal, multicellular. Embryogenesis solanad.
Fruit A usually septicidal (sometimes also loculicidal) capsule (rarely irregularly dehiscing).
Seeds Aril absent. Seeds ruminate, with longitudinal ridges. Exotestal cells thin-walled. Endotestal cells tanniniferous, with cuticle. Perisperm not developed. Endosperm sparse, with oil and aleuron. Embryo straight, well differentiated, chlorophyll? Cotyledons two. Germination phanerocotylar.
Cytology n = (9) 10 (12) – Nuclear protein inclusions absent.
DNA
Phytochemistry Virtually unknown. Alkaloids? O-methyl flavonols? O-methyl flavones? Inulin not found.
Use Unknown.
Systematics Hydrolea (11; tropical West and Central Africa, tropical Asia, northern Australia, southeastern United States, Mexico, Central America, the West Indies, tropical South America).
Hydrolea is sister to Sphenoclea (Sphenocleaceae).
MONTINIACEAE Nakai |
( Back to Solanales ) |
Kaliphoraceae Takht. in Bot. Žurn. 81(2): 86. Mai-Jun 1996
Genera/species 3/5
Distribution Eastern and southern Africa, Madagascar.
Fossils Unknown.
Habit Dioecious (Montinia, Grevea) or monoecious (Kaliphora), evergreen trees or shrubs (Grevea bosseri a liana), often with peppery scent. Nodes with axillary hair tufts (in Kaliphora weakly developed).
Vegetative anatomy Phellogen? Medulla in Grevea with vascular bundles (medullary bundles). Pericyclic fibres usually absent (sometimes weakly developed). Young stem with vascular tissue usually as cylinder (sometimes as separate bundles). Cambium sometimes storied. Vessel elements with simple or scalariform perforation plates; lateral pits alternate, simple or bordered pits. Vestured pits present (Grevea). Imperforate tracheary xylem elements fibre tracheids (Grevea) or libriform with simple or bordered pits, usually non-septate (in Grevea septate). Wood rays uniseriate or multiseriate, usually heterocellular (in Grevea homocellular). Axial parenchyma paratracheal scanty (Montinia, Grevea), or absent (Kaliphora). Sieve tube plastids S type. Nodes unilacunar or multilacunar with one (Kaliphora), three (Montinia) or four to eleven (Grevea) leaf traces (1–11:1–11). Calciumoxalate as crystal sand, acicular crystals and styloids usually present.
Trichomes Hairs unicellular or multicellular, usually uniseriate (sometimes biseriate or multiseriate); glandular hairs absent.
Leaves Alternate (spiral) or (in Grevea) more or less opposite, simple, entire, usually coriaceous (in Grevea thin), with ? ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle transection arcuate (sometimes rounded); petiole sometimes with additional vascular strands. Venation pinnate. Stomata usually anomocytic (in some species of Grevea anisocytic). Cuticular wax crystalloids? Leaf margin entire.
Inflorescence Terminal or axillary, cyme, or corymbose to paniculate cymose (female flowers in Montinia and Grevea solitary terminal). Floral prophylls (bracteoles) absent.
Flowers Actinomorphic. Epigyny (Montinia, Grevea) or half epigyny (Kaliphora). Sepals in male flowers three or four (or five), in female flowers four or five, with open? aestivation, connate. Petals in male flowers three or four (or five), in female flowers four or five, with imbricate (Montinia, Grevea) or valvate (Kaliphora) aestivation, caducous, free. Nectariferous disc present at least in female flowers, pulvinate to discoid, vascularized.
Androecium Stamens three or four (or five), haplostemonous, antesepalous, alternipetalous. Filaments short, thick, free from each other and from tepals. Anthers dorsifixed (Montinia, Grevea) or basifixed (Kaliphora), non-versatile, tetrasporangiate, extrorse, longicidal (dehiscing by longitudinal slits). Tapetum secretory? Female flowers often with staminodia (absent in Kaliphora).
Pollen grains Microsporogenesis simultaneous? Pollen grains usually tricolporate (sometimes tetracolporate), shed as monads, bicellular at dispersal. Exine semitectate, with columellate infratectum, reticulate or retipilate (Kaliphora).
Gynoecium Pistil composed of usually two connate carpels (sometimes one carpel). Ovary inferior or semi-inferior, bilocular. Stylodia two, recurved (Kaliphora) or style single, short, stout, bifid, hollow, persistent in fruit (Montinia, Grevea). Stigmas decurrent (Kaliphora) or stigma large and bilobate (Montinia, Grevea), type? Male flowers sometimes with rudimentary pistillodium.
Ovules Placentation intrusively parietal to subaxile or axile. Ovules one (Kaliphora), four to six (Grevea) or up to twelve (Montinia) per carpel, anatropous (Grevea, Montinia) or campylotropous (Kaliphora), ascending, apotropous (Kaliphora) to pendulous, unitegmic, tenuinucellar? Integument ? cell layers thick. Parietal tissue approx. one cell layer thick. Megasporangial base in Montinia thin. Endothelium? Megagametophyte monosporous, Polygonum type. Endosperm development cellular? Endosperm haustoria? Embryogenesis?
Fruit A loculicidal capsule with persistent style and stigma (Montinia, Grevea) or a drupe with two pyrenes (Kaliphora).
Seeds Aril absent. Testa winged or unwinged, thin-walled, in Grevea juicy when moistened. Exotesta lignified, with thickened periclinal cell walls (exotesta in Grevea and Kaliphora thin), usually persistent (in Grevea ephemeral). Adjacent mesotestal cell walls thickened in Montinia. Endotesta? Perisperm not developed. Endosperm copious (Grevea) or thin (Kaliphora) with hemicellulose and stratified often thick cell walls, or absent (Montinia). Embryo straight, well differentiated, chlorophyll? Cotyledons two, accumbent, foliaceous (cotyledons in Montinia with connate petioles, laterally penetrated by plumule). Radicula often oblique. Germination phanerocotylar.
Cytology n = 16 (Kaliphora), 34 (Montinia)
DNA
Phytochemistry Route I iridoids, Route II secoiridoids, montinioside (iridoid glucoside [iridoid gentiobioside] related to valepotriates), tannins, and proanthocyanidins present. Flavonoids?
Use Medicinal plants.
Systematics Kaliphora (1; K. madagascariensis; eastern Madagascar); Montinia (1; M. caryophyllacea; southwestern Angola, Namibia, Northern, Western and Eastern Cape, Botswana), Grevea (3; G. bossei, G. eggelingii, G. madagascariensis; eastern Africa, Madagascar).
Montiniaceae are presumably sister to the clade [Hydroleaceae+Sphenocleaceae].
Kaliphora is most probably sister to [Montinia+Grevea].
SOLANACEAE Juss. |
( Back to Solanales ) |
Hyoscyamaceae Vest, Anleit. Stud. Bot.: 272, 294. 1818 [’Hyoscyamoideae’]; Atropaceae Martinov, Tekhno-Bot. Slovar: 57. 3 Aug 1820; Browalliaceae Bercht. et J. Presl, Přir. Rostlin: 243. Jan-Apr 1820 [’Brovalliae’]; Daturaceae Bercht. et J. Presl, Přir. Rostlin: 243. Jan-Apr 1820 [’Datureae’]; Nicotianaceae Martinov, Tekhno-Bot. Slovar: 416. 3 Aug 1820 [’Nicotianeae’]; Nolanaceae Bercht. et J. Presl, Přir. Rostlin: 244. Jan-Apr 1820 [’Nolaneae’], nom. cons.; Cestraceae Schlechtend. in Linnaea 8: 250. Jan-Jul 1833 [’Cestrineae’]; Nolanales Lindl., Nix. Pl.: 18. 17 Sep 1833; Cestrales Schltdl. in C. F. P. von Martius, Consp. Regn. Veg.: 22. Sep-Oct 1835 [‘Cestrineae’]; Lyciaceae Raf., Autik. Bot. 1: 15. 1840 [’Lycioides’]; Solanopsida Brongn., Enum. Plant. Mus. Paris: xix, 57. 12 Aug 1843 [’Solanineae’]; Daturineae J. Presl in Nowočeská Bibl. [Wšobecný Rostl.] 7: 1117, 1130. 1846 [‘Datureae’]; Hyoscyamineae J. Presl in Nowočeská Bibl. [Wšobecný Rostl.] 7: 1117, 1134. 1846; Nolanineae J. Presl in Nowočeská Bibl. [Wšobecný Rostl.] 7: 1117, 1118. 1846; Solanineae J. Presl in Nowočeská Bibl. [Wšobecný Rostl.] 7: 1117, 1118. 1846 [‘Solaneae’]; Sclerophylacaceae Miers in London J. Bot. 7: 57, 58. Feb 1848 [‘Sclerophylaceae’]; Goetzeaceae Miers in Trans. Linn. Soc. London 27: 191. 1870 [’Goetziaceae’]; Duckeodendraceae Kuhlm. in Arq. Serv. Florest. 3: 7. 1950; Salpiglossidaceae (Benth.) Hutch., Evol. Phylog. Fl. Pl.: 631. 28 Aug 1969
Genera/species 93/2.635–2.800
Distribution Tropical, subtropical and temperate regions on both hemispheres, with their highest diversity in tropical and subtropical America.
Fossils There are only few unambiguous Solanaceae fossils known. Some macrofossils from Eocene and later layers (North America etc.) have been assigned to Solanites and they may belong in Solanaceae. However, there are several fossils that fairly well fit into other lineages of Solanales. A fossil fruit similar to Physalis were found in Eocene layers of Argentina.
Habit Usually bisexual (sometimes monoecious, andromonoecious or dioecious), evergreen trees, shrubs or lianas, perennial, biennial or annual herbs. Some species are xerophytes. Some species are succulent. Often evil-smelling (foetid).
Vegetative anatomy Roots diarch (lateral roots tetrastichous). Phellogen ab initio superficially or deeply seated. Endodermis sometimes prominent. Secondary lateral growth sometimes anomalous (from cylindrical cambium). Vessel elements with simple perforation plates; lateral pits usually alternate (sometimes scalariform), simple and/or bordered pits. Vestured pits present. Imperforate tracheary xylem elements fibre tracheids or (sometimes very long) libriform fibres (rarely tracheids) with simple or bordered pits, non-septate (also vasicentric tracheids). Wood rays uniseriate or multiseriate, homocellular or heterocellular. Axial parenchyma apotracheal diffuse or diffuse-in-aggregates, or paratracheal scanty, aliform, lozenge-aliform, confluent, vasicentric, reticulate, or banded. Wood often fluorescent. Stem and petioles usually with intraxylary phloem (absent in, e.g., Tsoala). Sieve tube plastids S type. Nodes 1:1–3, unilacunar with one to three leaf traces. Secretory cavities present in Duckeodendron. Resins present or absent. Cystoliths present. Prismatic calciumoxalate crystals, druses and crystal sand abundant. Raphides present in Sclerophylax.
Trichomes Hairs unicellular or multicellular, usually uniseriate, often dendritic (sometimes peltate-lepidote, echinoid or stellate); glandular hairs frequent.
Leaves Usually alternate (spiral; sometimes opposite especially in inflorescence), usually simple (sometimes pinnately compound or trifoliolate), entire or pinnately lobed (in Goetzeoideae usually coriaceous), often with conduplicate ptyxis. Stipules and leaf sheath absent. Petiole vascular bundles arcuate or annular. Venation pinnate. Stomata usually anomocytic or anisocytic (sometimes paracytic or diacytic; in Duckeodendron diacytic). Cuticular wax crystalloids? Domatia as hair tufts or absent. Mesophyll usually without mucilaginous idioblasts. Leaf margin and leaflet margins serrate or entire. Extrafloral nectaries present on lamina in, e.g., some species of Solanum.
Inflorescence Terminal or axillary, thyrsoid with various shape and with usually monochasial cymes, or flowers solitary axillary.
Flowers Actinomorphic to more or less (obliquely) zygomorphic (in Schizanthus resupinate and strongly zygomorphic, 3:2 type). Usually hypogyny (in Sclerophylax sometimes epigyny). Sepals (four or) five (to seven), usually with imbricate (rarely valvate) aestivation, often persistent, more or less connate (sometimes more or less inflated). Petals (four or) five (to seven), with valvate, valvate-induplicate, valvate-plicate, valvate-conduplicate, valvate-supervolute, cochleate, cochleate-conduplicate, cochleate-plicate, contorted-induplicate, contorted-conduplicate, contorted-plicate, quincuncial or reciprocative aestivation, connate into infundibuliform, campanulate or tubular perigone (odd or median petal adaxial); corolla tube formation late. Nectariferous disc intrastaminal (rarely absent). Extrafloral nectaries often present on abaxial side of corolla or on abaxial side of leaves (rarely on calyx).
Androecium Stamens (three to) five (to seven; in Schizanthus two fertile), haplostemonous, antesepalous, alternipetalous. Filaments free from each other, adnate to corolla tube (epipetalous), sometimes of two different lengths. Anthers often densely connivent around style, usually dorsifixed to basifixed (rarely ventrifixed), sometimes versatile, tetrasporangiate, usually introrse (sometimes extrorse or latrorse), longicidal (dehiscing by longitudinal slits or by two apical slits) or poricidal (with separate apical pores or with common apical pore due to anthers connivent or connate). Tapetum secretory, with often quadrinucleate cells. Staminodia usually absent (in Salpiglossis one, corresponding to adaxial median stamen; in Schizanthus three, corresponding to adaxial median stamen and adaxial lateral staminal pair), sometimes with four fertile stamens or with two fertile stamens and two staminodia, or sometimes with five staminodia or with two lateral or dorsal mobile fertile stamens and three staminodia.
Pollen grains Microsporogenesis simultaneous. Pollen grains (2–)3–5(–9)-colpate or (2–)3–5(–6)-colpor(oid)ate (sometimes inaperturate, rarely pantoporate), usually shed as monads (in Bouchetia, Nierembergia, Reyesia and Salpiglossis as tetrads; rarely as massulae), usually bicellular (sometimes tricellular) at dispersal. Exine usually tectate to semitectate (sometimes intectate), with usually columellate (occasionally granular) infratectum, perforate, reticulate, scabrate, striate, regulate, punctate-foveolate, striate-reticulate or striate-rugulate (sometimes echinate, psilate, microcechinate or granulate, rarely regulate-reticulate, regulate-striate, microgranulate, scabrate-gemmate, fossulate or smooth).
Gynoecium Pistil composed of usually two (sometimes five, rarely one or three; in Nicandra three to five; in Nolana primarily five, often secondarily transversely and/or longitudinally septate) connate antepetalous carpels, usually displaced relative to floral symmetry with oblique orientation (odd carpel often displaced to adaxial position; not so in Nicandra). Ovary usually superior (rarely inferior), usually bilocular (sometimes quinquelocular, rarely unilocular or trilocular, sometimes multilocular due to secondary septa; in Nolana usually quinquelocular, often with secondary septa; in Goetzeoideae occasionally unilocular and pseudomonomerous; in, e.g., Capsicum apically unilocular). Style single, simple, usually terminal (in Nolana sometimes gynobasic). Stigma usually bifid (sometimes capitate; in Nolana peltate), papillate or non-papillate, usually Wet type (in Solandra Dry type). Pistillodium?
Ovules Placentation axile (with large placentae). Ovules usually several to numerous (in, e.g., Duckeodendron one, in Goetzeoideae two) per carpel, usually anatropous or hemianatropous (sometimes amphitropous, anacampylotropous or hemicampylotropous), unitegmic, tenuinucellar. Integument usually 9–20 cell layers thick. Megagametophyte monosporous, Polygonum type, disporous, Allium type, or tetrasporous, Adoxa type. Synergids sometimes with a filiform apparatus. Antipodal cells sometimes persistent. Endosperm development usually cellular (rarely nuclear or helobial). Endosperm haustorium chalazal (sometimes absent). Embryogenesis usually solanad (rarely onagrad).
Fruit Usually a septicidal (sometimes a loculicidal or septicidal-loculicidal) capsule, a pyxidium or many-seeded berry (rarely diclesium; in Goetzeoideae except Metternichia drupe; in Duckeodendron single-seeded drupe; in Nolana schizocarp with five to numerous nutlike mericarps), often with persistent and accrescent calyx.
Seeds Aril absent. Exotestal cells usually with thickened inner periclinal and anticlinal walls. Endotesta persistent, with lignified cell walls. Perisperm not developed. Endosperm usually copious (in Duckeodendron and Goetzeoideae sparse or absent), usually oily (rarely starchy). Embryo straight or curved (sometimes annular, rarely spirally coiled, in Duckeodendron U-shaped), usually well differentiated, without chlorophyll. Cotyledons usually two (in Goetzeoideae sometimes four). Germination phanerocotylar.
Cytology x = 10 (Schizanthoideae); x = 12, 13 (Goetzeoideae); x = 11 (Benthamielleae); x = 8, 10–12 (Cestroideae); x = 7 (Calibrachoa), 8 (Bouchetia, Hunzikeria, Nierembergia), 9 (Petunia, Fabiana, Nierembergia), 10 (Leptoglossis), 11 (Brunfelsia); x = 12 (Schwenckioideae); x = 9–23 (Solanoideae); x = 8–12 (Nicotianoideae) – Protein bodies present in nuclei. Cestreae without Arabidopsis type telomeres, and with much larger chromosomes than in remaining Solanaceae.
DNA Genome duplication supposed to have taken place c. 50 Mya (Schlueter & al. 2004). Duckeodendron and Goetzeoideae share unique deletion in trnL/F spacer. Plastid gene infA lost (present in nuclear genome).
Phytochemistry Flavonols (kaempferol, quercetin, myricetin), flavones, acylated anthocyanins, coumarins, ursolic acid, caffeic acid esters, hygroline alkaloids (hygrines), tropane-3α-ols (e.g. hyoscyamine, apoatropine, littorine), tropane-3β-ols (e.g. tigloidine), nortropane-3α-ols, hydroxyl tropines (e.g. scopolamine, daturamine), teloidines, tropinone, cuscohygrine and other tropane alkaloids, nicotinic pyrrolidine and other pyrrolidine alkaloids, steroidal alkaloids (e.g. tomatine, solanine, solanocapsine), saponins, cyanogenic compounds, polyacetate-derived arthroquinones (in Fabiana), withanolides (steroidal lactones), and nicotinic acid compounds present. Ellagic acid, tannins and proanthocyanidins not found. Aluminium accumulated in some species. Carbohydrates stored as oligosaccharides. Cell walls containing arabinoxyloglucanes and sometimes galactoxyloglucan hemicelluloses.
Use Ornamental plants, spices (Capsicum), fruits and vegetables (Solanum, Physalis, Capsicum annuum, Espadaea), starch sources (tubers of Solanum tuberosum), medicinal plants, stimulants (tobacco from Nicotiana spp., ethanol from tubers of Solanum tuberosum, narcotics from Scopolia carniolica, Atropa belladonna, Hyoscyamus, Datura, Brugmansia), insecticides (Nicotiana), timber.
Systematics Solanaceae are sister to Convolvulaceae.
Analyses of the nuclear gene SAMT (coding for salicylic acid methyltransferase; Martins & Barkman 2005) and the plastid genes ndhF and trnL/F (e.g. Olmstead & al. 2008) indicate that Schizanthus (x = 10) is sister to the remaining Solanaceae.
According to Olmstead & al. (2008), Sclerophylax is recovered in the same subclade within Atropeae as Lycium and Nolana. The morphology of Sclerophylax differs considerably from other Solanaceae and the genus has often formed a monogeneric family, Sclerophylacaceae. Crystal sand present in some species. Leaves opposite, entire, succulent, usually asymmetrical. Petiole sometimes absent. Stomata diacytic. Flowers solitary, axillary. Sometimes epigyny. Sepals five, unequal in size, connate at base into asymmetrical calyx. Petals five, with conduplicate-contorted aestivation, connate into somewhat zygomorphic infundibuliform corolla. Stamens five. Filaments epipetalous. Anthers dehiscing by longitudinal slits. Pollen grains tricolporate. Exine perforate to reticulate. Pistil composed of two oblique carpels. Ovary bilocular, with septum oblique relative to median floral plane. Style single, terminal. Stigma simple, papillate. Placentation subapical-axile. Ovules two or three per carpel, pendulous. Fruit one- to three-seeded, dry, indehiscent, with membranous pericarp, and surrounded by persistent and accrescent spiny calyx. Endosperm present. Embryo straight or curved. n = 12.
A possible topology of Solanaceae is the following: [Schizanthoideae+[[Duckeodendron+Goetzeoideae]+[[Nicotianoideae+Solanoideae]+Schwenckieae+Petunioideae+[Benthamielleae+Cestroideae]]]] (Olmstead & al. 2008).
Schizanthoideae Hunz. in Kurtziana 28: 56. Jul 2000
1/12. Schizanthus (12; Chile, Argentina). – Annual herbs. Phellogen pericyclic. Pericyclic fibres absent. Flowers strongly zygomorphic, resupinate. Abaxial pair of petals connate into keel. Stamens two abaxial-lateral fertile and three staminodial. Endosperm development ab initio nuclear. Fruit a septicidal capsule. Embryo curved. n = 10. Typical tropane alkaloids present.
[[Duckeodendron+Goetzeoideae]+[[Petunioideae+[Schwenckieae+[Nicotianoideae+Solanoideae]]]+[Benthamielleae+Cestroideae]]]
[Duckeodendron+Goetzeoideae]
Endosperm sparse or absent. Unique deletion in trnL/F spacer region.
Duckeodendron
1/1. Duckeodendron (1; D. cestroides; Amazonian Brazil). – Tree. Wood with large open radial canals. Secretory cavities present. Stomata diacytic. Carpels oblique. Ovule one per carpel. Fruit a single-seeded drupe with thick fibrous mesocarp. Endosperm sparse. Embryo U-shaped. Cotyledons small. n = ? – Duckeodendron is sister to Goetzeoideae, according to Santiago-Valentin & Olmstead (2003), or sister to the remaining Solanaceae above Goetzeoideae, according to some analysis by Olmstead (2013).
Goetzeoideae (Miers ex Airy Shaw) Thorne et Reveal in Bot. Rev. (Lancaster) 73: 132. 29 Jun 2007
6/7. Metternichia (1; M. principis; eastern Brazil), Coeloneurum (1; C. ferrugineum; Hispaniola), Henoonia (1; H. myrtifolia; Cuba), Espadaea (1; E. amoena; Cuba), Goetzea (2; G. elegans: Puerto Rico; G. ekmanii: Hispaniola), Tsoala (1; T. tubiflora; Madagascar, probably extinct). – Madagascar, Cuba, Hispaniola, Puerto Rico, eastern Brazil. Trees or shrubs. Pollen grains tricolpate. Ovules two per carpel. Exine tectate-perforate, echinate. Fruit usually a drupe (in Metternichia capsule). Endosperm sparse to almost absent. Cotyledons often large, fleshy, sometimes four. x = 12, 13. – A probable topology is, according to Santiago-Valentin & Olmstead (2003): [Metternichia+[Coeloneurum+[Henoonia+[Espadaea+Goetzea]]]]. The position of Tsoala tubiflora is unknown (the species has not been observed since 1959 and may be extinct).
[[Petunioideae+[Schwenckieae+[Nicotianoideae+Solanoideae]]]+[Benthamielleae+Cestroideae]]
[Petunioideae+[Schwenckieae+[Nicotianoideae+Solanoideae]]]
Petunioideae (Horan.) Thorne et Reveal in Bot. Rev. (Lancaster) 73: 132. 29 Jun 2007
8/135–140. Petunia (40; tropical and subtropical South America), Fabiana (c 15; warm-temperate South America, especially in the Andes); Brunfelsia (45–50; tropical America); Leptoglossis (6; L. acutiloba, L. albiflora, L. darcyana, L. ferreyraei, L. lomana, L. schwenckioides; coastal Peru and Chile, Argentina), Nierembergia (c 20; Mexico, South America south to Chile and Argentina), Bouchetia (4; B. anomala, B. arniatera, B. erecta, B. procumbens; southern United States to Brazil), Hunzikeria (3; H. coulteri, H. steyermarkiana, H. texana; southwestern United States, Mexico, Venezuela), Plowmania (1; P. nyctaginoides; Mexico, Guatemala). – Southern United States to southern South America. Phellogen usually superficial (rarely deeply seated). Bordered pits present. Pericyclic fibres usually present. Druses usually absent. Flowers sometimes zygomorphic. Stamens usually four (rarely five), usually didynamous. Embryo straight or slightly curved. x = 7–9, 11. Tropane alkaloids (calystegines) sometimes present. – The position of Petunioideae is unresolved and sometimes they are placed as sister to [Nicotianoideae+ Solanoideae] (e.g. Olmstead & al. 1999).
[Schwenckieae+[Nicotianoideae+Solanoideae]]
Schwenckieae Hunz. in Kurtziana 10: 42. 25 Apr 1977
3/25–30. Heteranthia (1; H. decipiens; Brazil), Melananthus (7; M. cubensis, M. dipyrenoides, M. fasciculatus, M. guatemalensis, M. luetzelburgii, M. multiflorus, M. ulei; Central America, the West Indies, tropical South America), Schwenckia (c 20; tropical America, one species, S. americana, also in tropical West Africa). – Tropical America, tropical West Africa. Annual herbs. Pericyclic fibres present. Flowers zygomorphic. Each corolla lobe in Melananthus and Schwenckia trilobate. Stamens two longer and two shorter, or two fertile and two or three staminodia. Embryo straight, short. x = 12 (Schwenckia).
[Nicotianoideae+Solanoideae]
Stigma Wet type. Cotyledons sometimes with margins against radicula. x = 12. Tropane alkaloids and nicotine (a pyridine alkaloid) sometimes present.
Nicotianoideae Miers in London J. Bot. 7: 58. 1848 [‘Nicotianeae’]
8/c 110. Nicotiana (c 75; southwestern United States, northern Mexico, southern South America east of the Andes, Australia, islands in the South Pacific, one species, N. africana, in Namibia), Symonanthus (2; S. aromaticus, S. bancroftii; southwestern Western Australia), Anthocercis (c 15; southwestern Western Australia, southern South Australia), Grammosolen (2; G. dixonii, G. truncatus; southeastern Western Australia, southern South Australia),’Cyphanthera’ (9; southern Australia; polyphyletic), Anthotroche (3; A. myoporoides, A. pannosa, A. walcottii; southwestern Western Australia), Crenidium (1; C. spinescens; continental southwestern Western Australia), 'Duboisia' (4; D. arenitensis, D. hopwoodii, D. leichhardtii, D. myoporoides; eastern Australia, New Caledonia; probably non-monophyletic). – Namibia, Australia, New Caledonia, America. Phellogen superficial. Pericyclic fibres often present. Stamens four (staminodium sometimes present) or five, sometimes didynamous. Embryo usually straight (sometimes curved). Radicula short. x = 8–12. Tropane alkaloids (calystegines) sometimes present. Nicotine often present.
Solanoideae Burnett, Outlines Bot.: 985, 1095, 1106. Feb 1835 [‘Solanidae’]
57/2.170–2.270. Atropeae Kitt. in A. Richard, Nouv. Elém. Bot., ed. 3, Germ. transl.: 796. 1840. Latua (1; L. pubiflora; southern Chile); Jaborosa (23; Chile, southern Argentina); Sclerophylax (14; Uruguay, Paraguay, Argentina), Nolana (89; Peru to Chile and western Argentina, the Galápagos Islands), Lycium (90–95; warm-temperate and subtropical regions on both hemispheres), Atrichodendron (1; A. tonkinense; Vietnam; in Lycium?); Atropa (5; A. acuminata, A. baetica, A. belladonna, A. komarovii, A. pallidiflora; northern Morocco, Europe to the Caucasus, northern Iran, Central Asia and the Himalayas, Mongolia), Jaborosa (22; southern Peru, Chile, southern Argentina), Anisodus (5; A. acutangulus, A. carniolicoides, A. luridus, A. mariae, A. tanguticus; the Himalayas in Nepal, India and Bhutan to western China), Atropanthe (1; A. sinensis; China), Hyoscyamus (c 17; Europe, the Mediterranean, Madeira, the Canary Islands, North Africa to Somalia, southwestern and Central Asia to China), Przewalskia (1; P. tangutica; western China), Scopolia (2; S. carniolica: the Alps, the Carpathians, the Caucasus; S. japonica: the Korean Peninsula, Japan), Physochlaina (6; P. capitata, P. infundibularis, P. macrocalyx, P. macrophylla, P. physaloides, P. praealta; the Caucasus to India and China). – Exodeconus clade Exodeconus (6; E. flavus, E. integrifolius, E. maritimus, E. miersii, E. prostratus, E. pusillus; South America, the Galápagos Islands). – Nicandreae Lowe, Man. Fl. Madeira 2: 98. Jan-Apr 1872. Nicandra (1; N. physalodes; Peru to northern Argentina). – Solandreae Miers in Ann. Mag. Nat. Hist., ser. 2, 3: 166. Mar 1849. Solandra (10; Mexico, Central America, the West Indies, tropical South America south to Bolivia and southeastern Brazil), Schultesianthus (7; S. coriaceus, S. crosbyanus, S. dudleyi, S. leucanthus, S. megalandrus, S. uniflorus, S. venosus; southern Mexico, Central America, Venezuela and Colombia to Peru), ‘Markea’ (20; Central America, northern South America; polyphyletic), Trianaea (5; T. brevipes, T. naeka, T. nobilis, T. speciosa, T. spectabilis; Venezuela, Colombia, Ecuador, Peru), ‘Merinthopodium’ (3; M. neuranthum, M. pendulum, M. vogelii; southern Mexico, Central America to Venezuela and Colombia; non-monophyletic), Poortmannia (1; P. speciosa; Colombia, Ecuador), Ectozoma (2; E. pavonii, E. ulei; southwestern Colombia, Ecuador, northern Peru), Dyssochroma (4; D. albidoflava, D. eximia, D. longipes, D. viridiflora; southeastern Brazil), Juanulloa (8–11; Mexico, Central America, the West Indies, tropical South America to Bolivia), Hawkesiophyton (4; H. klugii, H. ochraceum, H. panamense, H. ulei; Panamá to Colombia and Amazonia in Brazil). – Mandragoreae Rchb., Handb. Nat. Pfl.-Syst.: 201. 1-7 Oct 1837. Mandragora (4; M. autumnalis, M. caulescens, M. officinarum, M. turcomanica; the Mediterranean, Central Asia, the Himalayas). – Datureae Dumort., Anal. Fam. Plant.: 24. 1829. Trompettia (1; T. cardenasiana; Bolivia), Datura (12; southern United States, Mexico), Brugmansia (6; B. arborea, B. longifolia, B. pittieri, B. sanguinea, B. suaveolens, B. versicolor; the Andes in Venezuela to Bolivia). – Salpichroa clade Salpichroa (22; southern United States and Mexico to southern Andes). – Physalideae Miers in Ann. Mag. Nat. Hist., ser. 2, 3: 179. Mar 1849 [‘Physaleae’]. Cuatresia clade: Cuatresia (13; tropical America); Withaniinae Bohs et Olmstead in R. G. Olmstead et al. in Taxon 57: 1171. 26 Nov 2008: Deprea (c 50; Central America, tropical South America, with their highest diversity in northern and central Andes), Aureliana (15; eastern Brazil, central South America), Withania (c 20; warm-temperate to tropical regions in the Old World, one species, W. begoniifolia, on St. Helena), Nothocestrum (4; N. breviflorum, N. latifolium, N. longifolium, N. peltatum; the Hawaiian Islands), Tubocapsicum (1; T. anomalum; southern and eastern Asia), Discopodium (1; D. penninervium; tropical African mountains); Iochromeae Miers in Ann. Mag. Nat. Hist., ser. 2, 3: 178. Mar 1849: Dunalia (8; the Andes), ’Iochroma’ (c 25; western South America south to north-western Argentina; non-monophyletic), Saracha (3; S. andina, S. punctata, S. quitensis; the Andes from Venezuela to Bolivia), Acnistus (1; A. arborescens; tropical America; in Iochroma?), Eriolarynx (3; E. fasciculata, E. iochromoides, E. lorentzii; Bolivia, Argentina), Vassobia (3; V. breviflora, V. dichotoma, V. fasciculata; central South America); Physalis clade: Witheringia (c 14; southeastern Mexico, Central America to Bolivia, the West Indies), Leucophysalis (5; L. grandiflora, L. kimurai, L. nana, L. savatieri, L. viscosa; North America, Mexico, Central America, Colombia, Venezuela), ’Physalis’ (c 90; nearly cosmopolitan, with their highest diversity in Mexico to northern South America; paraphyletic), Schraderanthus (1; S. viscosus; Mexico, Guatemala, Venezuela; in Physalis?), Capsicophysalis (1; C. potosina, southern Mexico to Guatemala and Honduras; in Physalis?), Calliphysalis (1; C. carpenteri; southeastern United States), Darcyanthus (1; D. spruceanus; Peru, Bolivia), Oryctes (1; O. nevadensis; southwestern United States), Quincula (1; Q. lobata; southwestern United States to northern Mexico), Chamaesaracha (13; North America, northern South America). – Capsiceae Dumort., Fl. Belg.: 38. 1827. Lycianthes (c 140; East Asia, tropical America; paraphyletic?), Capsicum (c 32; southern United States to central Argentina; incl. Lycianthes?). – Solaneae Dumort., Anal. Fam. Plant.: 24. 1829. Jaltomata (c 60; tropical and subtropical America), Solanum (1.300–1.400; nearly cosmopolitan, with their largest diversity in Australia and South America). – Subcosmopolitan. Herbs or shrubs (sometimes small trees). Vestured pits absent. Crystal sand sometimes present. Stamens (four or) five, sometimes didynamous. Filament base often enlarged, lobed etc. Pistil sometimes composed of three carpels or carpels sometimes divided into single-seeded units (e.g. Nolana with primarily five antepetalous carpels). Style sometimes gynobasic. Endosperm development cellular. Fruit usually a berry (rarely drupe or schizocarp, in Hyoscyameae etc. pyxidium). Calyx sometimes strongly accrescent in fruit. Seeds flattened. Exotestal cells sometimes anticlinally elongate. Embryo curved, often coiled. x = 9–23. Tropane alkaloids (calystegines), nicotine and steroidal alkaloids sometimes present. Glycine betaines present in Lycium.
[Benthamielleae+Cestroideae]
Benthamielleae Hunz. in Kurtziana 28: 61. Jul 2000
3/15. Benthamiella (13; southern Patagonia in Chile and Argentina), Combera (2; C. minima, C. paradoxa; the Andes in temperate Chile and Argentina), Pantacantha (1; P. ameghinoi; Patagonia in central Argentina). – Southern South America. Exine with special irregular ornamentation. x = 11. – Benthamielleae are sister to Cestroideae, according to Olmstead & al. (2008).
Cestroideae Burnett, Outlines Bot.: 985, 1095, 1106. Feb 1835 [‘Cestridae’]
6/160–210. Salpiglossideae Benth. in Edwards’s Bot. Reg. 21: ad t. 1770. 1 Feb 1835. Salpiglossis (1; S. sinuata; southern Andes), Reyesia (4; R. cactorum, R. chilensis, R. juniperoides, R. parviflora; northern Chile)? x = 11. – Browallieae Hunz. in Lorentzia 8: 6. 31 Mai 1995. Browallia (5; B americana, B. demissa, B. eludens, B. speciosa, B. viscosa; southeastern Arizona to central South America, the West Indies). x = 10–12. – Cestreae Dumort., Anal. Fam. Plant.: 24. 1829 [‘Cestrineae’]. Cestrum (150–200; tropical and subtropical America), Vestia (1; V. foetida; Chile), Protoschwenckia (1; P. mandonii; Brazil, the Andes in Bolivia). x = 8. – Southern United States to southern Andes. Phellogen superficial or deeply seated. Bordered pits present. Pericyclic fibres present. Stamens four or five, often didynamous. Staminodia often present. Pollen grains in Salpiglossis shed as tetrads. Fruit sometimes fleshy. Exotesta in Cestrum with all cell walls somewhat thickened. x = 8, 10–12. Chromosomes larger than other Solanaceae. Arabidopsis type telomeres absent at least in some Cestroideae. Steroid alkaloids present in Cestrum. – A probable topology of Cestroideae is [Salpiglossideae+[Browallieae+Cestreae]]. A plausible topology of Cestreae is [Protoschwenckia+[Vestia+[Cestrum+Sessea]]] (Olmstead & al. 2008).
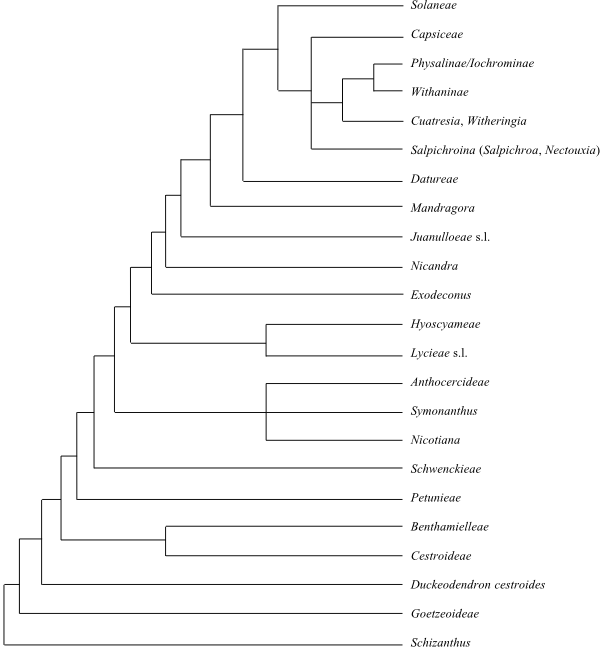 |
Cladogram (simplified) of Solanaceae based on DNA sequence data (Olmstead 2013). |
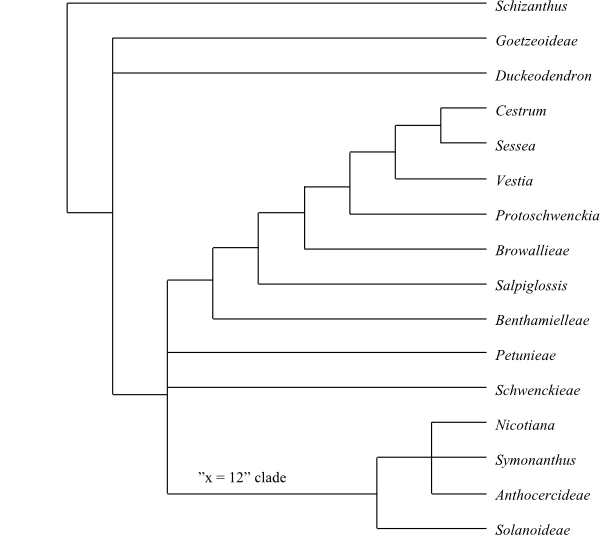 |
Cladogram (simplified) of Solanaceae based on DNA sequence data (Olmstead & al. 2008). |
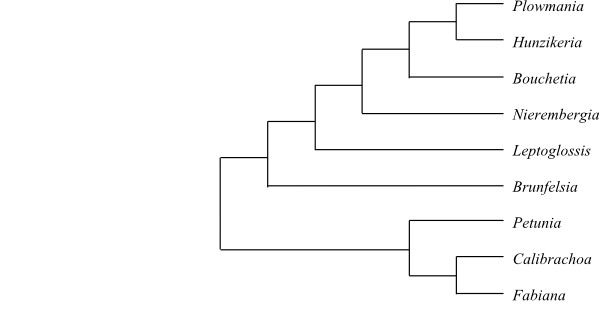 |
Cladogram of Petunioideae based on DNA sequence data (Olmstead & al. 2008). |
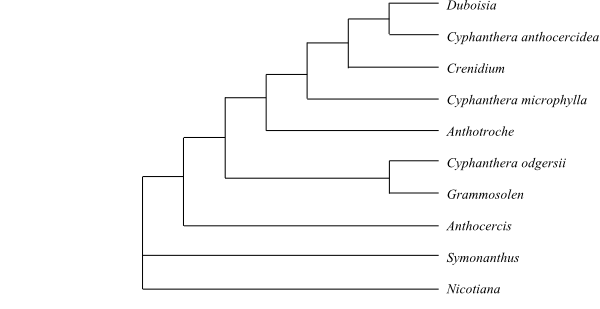 |
Cladogram of Nicotianoideae based on DNA sequence data (Olmstead & al. 2008). |
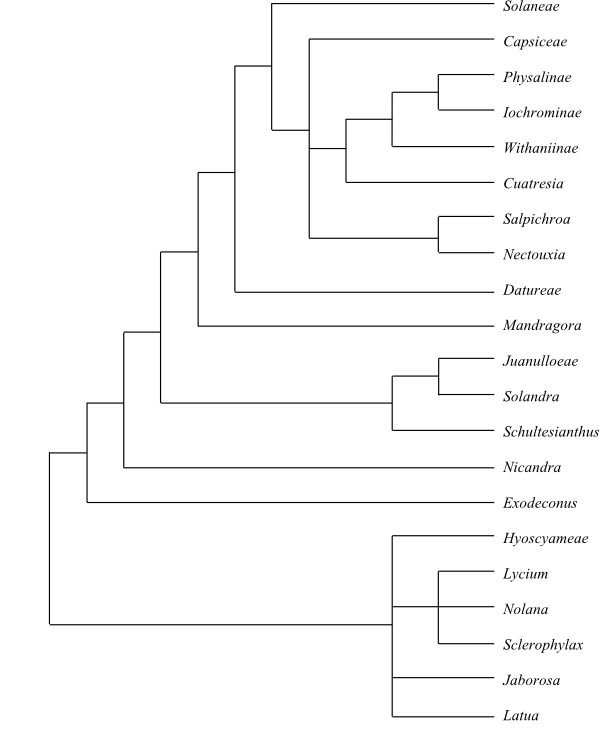 |
Cladogram of Solanoideae based on DNA sequence data (Olmstead & al. 2008). |
SPHENOCLEACEAE (Lindl.) Baskerville |
( Back to Solanales ) |
Pongatiaceae Endl. ex Meisn., Plant. Vas. Gen.: Tab. Diagn.: 242, Comm.: 151. 18-24 Aug 1839 [’Pongatieae’], nom. illeg.; Sphenocleales Doweld, Tent. Syst. Plant. Vasc.: xlvii. 23 Dec 2001
Genera/species 1/2
Distribution Pantropical.
Fossils Unknown.
Habit Bisexual, annual herbs. Relatively succulent. Helophytic. Stem hollow.
Vegetative anatomy Phellogen mid-cortical? Large vertical cortical air canals (aerenchyma) present in stem. Vessel elements with simple perforation plates; lateral pits scalariform or alternate, simple pits? Imperforate tracheary xylem elements tracheids? with simple pits. Wood rays absent? Axial parenchyma paratracheal scanty. Pericyclic sclerenchyma present. Sieve tube plastids S type? Nodes? Laticifers and latex absent. Calciumoxalate druses present.
Trichomes Hairs unicellular?
Leaves: Alternate (spiral), simple, entire, with ? ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle transection arcuate. Venation pinnate. Stomata tetracytic. Cuticular wax crystalloids? Mesophyll with calciumoxalate druses. Leaf margin entire.
Inflorescence Terminal, densely spicate.
Flowers Actinomorphic, small. Epigyny or partial epigyny (upper parts of ovary free). Sepals five, with imbricate aestivation, persistent, connate. Petals five, with imbricate quincuncial aestivation, connate in lower part, inflexed, caducous; corolla tube formation early. Nectary absent. Disc absent.
Androecium Stamens five, haplostemonous, antesepalous, alternipetalous. Filaments short, free from each other, adnate to base of corolla tube (epipetalous). Anthers dorsifixed, non-versatile?, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory, with binucleate cells. Staminodia absent.
Pollen grains Microsporogenesis simultaneous. Pollen grains tricolporate to tricolporoidate, shed as monads, tricellular at dispersal. Exine tectate, with columellate infratectum, imperforate to finely perforate, smooth or almost so.
Gynoecium Pistil composed of two connate carpels. Ovary inferior or semi-inferior, bilocular. Style single, simple, short, or absent. Stigma capitate, Wet type. Pistillodium absent.
Ovules Placentation axile, with large placentae. Ovules numerous per carpel, anatropous, pendulous?, unitegmic, tenuinucellar. Integument ? cell layers thick. Hypostase absent. Megagametophyte monosporous, Polygonum type. Synergids elongated. Antipodal cells degenerating, ephemeral. Endosperm development cellular. Endosperm haustoria chalazal and micropylar, multicellular. Embryogenesis onagrad.
Fruit A circumscissilely dehiscing capsule (pyxidium).
Seeds Aril absent. Exotesta with thickened inner cell walls; Exotestal cells polygonal, with thickened inner walls and spine-shaped radial processes. Endotesta developing into endothelium? Perisperm not developed. Endosperm very sparse, with thick cell walls, or absent. Embryo straight, well differentiated, chlorophyll? Cotyledons two. Germination phanerocotylar.
Cytology n = 12, 16, 20, 21, 24 (chromosome numbers uncertain)
DNA
Phytochemistry Very insufficiently known. Cyclic thiosulphinates (zeylanoxides) present. O-methylflavonols? O-methylflavones? Alkaloids? Iridoids not found. Secoiridoids sometimes present. Fructose with isokestose linkages present (inulin has been reported).
Use Unknown.
Systematics Sphenoclea (2; S. pongatium, S. zeylanica; tropical and subtropical regions on both hemispheres).
Sphenoclea is sister to Hydrolea (Hydroleaceae), although Gustafsson & Bremer (1995), in analyses based on morphological features, placed Sphenoclea in Campanulales. However, Sphenoclea lacks laticifers and latex and the flowers do not have secondary pollen presentation, features frequently present in Campanulales.
Literature
Acosta MC, Moscone EA. 2000. Estudio cariotípico en Dyssochroma viridiflora (Solanaceae). – Bol. Soc. Argent. Bot. 35: 227-336.
Acosta MC, Moscone EA. 2011. B chromosomes in Nierembergia aristata (Solanaceae): nucleolar activity and competition with the A chromosomes. – Cytogenet. Genome Res. 132: 105-112.
Acosta MC, Bernardello G, Guerra M, Moscone EA. 2005. Karyotype analysis in several South American species of Solanum and Lycianthes rantonnei (Solanaceae). – Taxon 54: 713-723.
Acosta MC, Ordóñez A del V, Cocucci AA, Moscone EA. 2006. Chromosome reports in South American Nicotianeae (Solanaceae), with particular reference to Nierembergia. – Ann. Missouri Bot. Gard. 93: 634-646.
Adam G, Hesse M. 1971. Ein neues C28-steroidlacton von withaferon-Typ aus Dunalia australis (Griseb.) Sleum. – Tetrahedron Letters 17: 1199-1204.
Adam G, Hesse M. 1972. Strukturaufklärung ein C28-Steroidlacton von Withaferin-typ aus Dunalia australis (Griseb.) Sleumer. – Tetrahedron 26: 3527-3534.
Adam G, Chien NQ, Khoi NH. 1981. Dunawithanine A and B, first plant withanolide glycosides from Dunalia australis. – Naturwiss. 68: 425-426.
Adam G, Chien NG, Khoi NH. 1984. Dunawithanines A and B, the first withanolide glycosides from Dunalia australis. – Phytochemistry 23: 2293-2297.
Agra M de F. 2000. Revisão taxonômica de Solanum section Erythrotrichum Child (Solanaceae). – Ph.D. diss., Universidade de São Paulo, São Paulo, Brazil.
Agra M de F. 2001. Diversity and biogeography of Solanum sect. Erythrotrichum Child. – In: Berg R G van der, Barendese GWM, Weerden GM van der, Mariani C (eds), Solanaceae V: advances in taxonomy and utilization, Nijmegen University Press, Nijmegen, pp. 53-60.
Agra M de F. 2004. Synopsis of Solanum sect. Erythrotrichum Child (Solanaceae). – In: Rangel-C JO, Aguirre-C J, Andrade-C MG, Cañas DG (eds), Memorias octavo congresso latinoamericano e segundo colombiano de botânica, Universidad Nacional de Colombia, Bogotá, pp. 192-211.
Agra M de F. 2008. Four new species of Solanum section Erythrotrichum (Solanaceae) from Brazil and Peru, and a key to the species of the section. – Syst. Bot. 33: 556-565.
Aguilar-Meléndez A, Morrell PL, Roose ML, Kim S-C. 2009. Genetic diversity and structure in semiwild and domesticated chiles (Capsicum annuum; Solanaceae) from Mexico. – Amer. J. Bot. 96: 1190-1202.
Ahimsa-Müller AA, Markert A, Hellwig S, Knoop V, Steiner U, Drewke C, Leistner E. 2007. Clavicipitaceous fungi associated with ergoline-containing Convolvulaceae. – J. Nat. Prod. 70: 1955-1960.
Ahmad KJ. 1972. Cuticular studies in some Acanthaceae and Solanaceae. – Ph.D. diss., Lucknow University, India.
Ahmad VU, Anwar SM. 1980. Isolation of betaine from Lycium barbarum. – J. Chem. Soc. Pakistan 2: 213-214.
Ahmad VU, Baqai FT, Fatima I, Ahmad R. 1991. A spirostanol glycoside from Cestrum nocturnum. – Phytochemistry 30: 3057-3061.
Ahmad VU, Baqai FT, Fatima I, Ahmad R. 1993. A tigogenin pentasaccharide from Cestrum diurnum. – Phytochemistry 34: 511-515.
Ahmad VU, Baqai FT, Fatima I, Ahmad R. 1995. A diosgenin tetrasaccharide from Cestrum nocturnum. – Zeitschr. Naturforsch., Ser. B, 50: 1104-1110.
Ahmad S, Malik A, Yasmin R, Ullah N et al. 1999. Withanolides from Physalis peruviana. – Phytochemistry 50: 647-651.
Airapetian AM. 1995. The aperture types of pollen and possible ways of their evolution in the family Solanaceae. – Bot. Žurn. 80: 1-10. [In Russian with English summary]
Airy Shaw HK. 1948. Sphenocleaceae. – In: Steenis CGGJ van (ed), Flora Malesiana, ser I, 4(1), Noordhoff-Kolff N. V., Djakarta, pp. 27-28.
Airy Shaw HK. 1968. Sphenocleaceae. – In: Milne-Redhead E, Polhill RM (eds), Flora of tropical East Africa, Crown Agents for Oversea Governments and Administrations, London, pp. 1-3.
Akers CR, Weybrew JA, Long RC. 1978. Ultrastructure of glandular trichomes of leaves of Nicotiana tabacum L., cv. Xanthi. – Amer. J. Bot. 65: 282-292.
Alemany J. 1985. Flor, esporogénesis y gametogénesis de Nierembergia hippomanica (Solanaceae). – Bol. Soc. Argent. Bot. 24: 49-69.
Alfaro ME, Mesa A. 1979. El origin morfológico del floema in traxylar en Nolanaceae y la posición systemática de esta familia. – Bol. Soc. Argent. Bot. 18: 123-126.
Alfonso D, Kaptanidis I. 1991. Iochromolide: a new acetylated withanolide from I. coccineum. – J. Nat. Prod. 54: 1576-1582.
Alfonso D, Kaptanidis I. 1994. Withanolides from Iochroma gesnerioides. – Phytochemistry 36: 179-183.
Alfonso D, Belardinelli G, Kapetanidis I. 1993. Withanolides from Iochroma coccineum. – Phytochemistry 34: 517-521.
Alvarez A. 1996. Systematics of Saracha (Solanaceae). – Thesis, Graduate School of University of Missouri, St. Louis, Missouri.
Ames M, Spooner DM. 2010. Phylogeny of Solanum series Piurana and related species in Solanum section Petota based on five conserved ortholog sequences. – Taxon 1091-1101.
Ames M, Salas A, Spooner DM. 2008. A morphometric study of species boundaries of the wild potato Solanum Series Piurana (Solanaceae) and putatively related species from seven other series in Solanum Sect. Petota. – Syst. Bot. 33: 566-578.
Ampornpan L, Armstrong JE. 2002. Floral ontogeny of Salpiglossis (Solanaceae) and the oblique gynoecium. – J. Torrey Bot. Soc. 129: 85-95.
Amshoff GJH. 1954. Notes on some South American Solanaceae. – Mem. Soc. Cubana Hist. Nat. 22: 419-425.
Anderson DE. 1964. Documented chromosome numbers in plants. Quincula lobata. – Madroño 17: 266-268.
Anderson GJ. 1975. The variation and evolution of selected species of Solanum sect. Basarthrum I. – Brittonia 27: 209-222.
Anderson GJ. 1977. The variation and evolution of selected species of Solanum sect. Basarthrum II. – Brittonia 29: 116-128.
Anderson GJ. 1979a. Dioecious Solanum species of hermaphroditic origin is an example of broad convergence. – Nature 282: 836-838.
Anderson GJ. 1979b. Systematic and evolutionary consideration of species of Solanum, section Basarthrum. – In: Hawkes JG, Lester RN, Skelding AD (eds), The biology and taxonomy of the Solanaceae, Linn. Soc. Symp. Ser. 7, Linnean Society and Academic Press, London, pp. 549-562.
Anderson GJ, Gensel PG. 1976. Pollen morphology and the systematics of Solanum section Basarthrum. – Pollen Spores 18: 533-552.
Anderson GJ, Levine DH. 1982. Three taxa constitute the sexes of a single dioecious species of Solanum. – Taxon 31: 667-672.
Anderson GJ, Symon DE. 1985. Extrafloral nectarines in Solanum. – Biotropica 17: 40-45.
Anderson GJ, Symon DE. 1989. Functional dioecy an andromonoecy in Solanum. – Evolution 43: 204-219.
Anderson GJ, Bernardello LM. 1991. The relationships of Solanum cochoae (Solanaceae), a new species from Peru. – Novon 1: 127-133.
Anderson GJ, Steinharter TP, Cooper-Driver G. 1987. Foliar flavonoids and the systematics of Solanum sect. Basarthrum. – Syst. Bot. 12: 534-540.
Anderson GJ, Bernardello G, Schlehofer M. 1999. Continuous variation among three species of Solanum sect. Anarrhichomenum (Solanaceae): the synonymy of S. carchiense and S. tetrapetalum with S. sodiroi. – Kurtziana 27: 233-242.
Anderson GJ, Bernardello G, Opel MR, Santos-Guerra A, Anderson M. 2006. Reproducive biology of the dioecious Canary Islands endemic Withania aristata (Solanaceae). – Amer. J. Bot. 93: 1295-1305.
Ando T, Hashimoto G. 1993. Two new species of Petunia (Solanaceae) from southern Brazil. – Bot. J. Linn. Soc. 111: 265-280.
Ando T, Hashimoto G. 1994. A new Brazilian species of Petunia (Solanaceae) from the Serra da Mantiqueira. – Brittonia 46: 340-343.
Ando T, Hashimoto G. 1995. Petunia guarapuvensis (Solanaceae): a new species from planalto of Paraná and Santa Catarina, Brazil. – Brittonia 47: 328-334.
Ando T, Hashimoto G. 1996. A new Brazilian species of Petunia, Solanaceae from interior Santa Catarina and Rio Grande do Sul, Brazil. – Brittonia 48: 217-223.
Ando T, Hashimoto G. 1998. Two new species of Petunia (Solanaceae) from southern Rio Grande do Sul, Brazil. – Brittonia 50: 483-492.
Ando T, Saito N, Tatsuzawa F, Kakefuda T, Yamakage K, Ohtani E, Koshi-ishi M, Matsusake Y, Kokubun H, Watanabe H, Tsukamoto T, Ueda Y, Hashimoto G, Marchesi E, Asakura K, Hara R, Seki H. 1999. Floral anthocyanins in wild taxa of Petunia (Solanaceae). – Biochem. Syst. Ecol. 27: 623-650.
Ando T, Kokubun H, Watanabe H, Tanaka N, Yukawa T, Hashimoto G, Marchesi E, Suárez E, Basualdo LL. 2005. Phylogenetic analysis of Petunia sensu Jussieu (Solanaceae) using chloroplast DNA RFLP. – Ann. Bot. 96: 289-297.
Andrews J. 1984. Peppers. The domesticated Capsicums I-XI. – University of Texas Press, Austin, Texas.
Anozie VC. 1987. Pharmacognostic studies on Datura metel L.: macro- and micromorphology of fruits and seeds. – Phytomorphology 37: 39-46.
Aoki S, Ito M. 2000. Molecular phylogeny of Nicotiana (Solanaceae) based on the nucleotide sequence of the matK gene. – Plant Biol. 2: 316-324.
Armstrong JE. 1986. Comparative floral anatomy of Solanaceae: a preliminary survey. – In: D’Arcy WG (ed), Solanaceae: biology and systematics, Columbia University Press, New York, pp. 101-113.
Arroyo S. 1976. Novedades en el género Fabiana (Solanaceae). – Hickenia 1: 49-54.
Arroyo S. 1980. The genus Benthamiella (Solanaceae). – Bot. Not. 133: 67-76.
Athiê-Souza SM, Staples G, Zickel CS, Buril MT. 2017. Towards a better understanding of the tribe Aniseieae: revisiting Aniseia and Iseia (Convolvulaceae). – Syst. Bot. 42: 590-605.
Atta-Ur-Rahman, Jamel A, Choudhary MI. 1992. Two new withanolides from Withania somnifera. – Heterocyces 34: 689-698.
Atta-Ur-Rahman, AbbaS, Dur-E-Shahwar, Jamas SA et al. 1993. New withanolides from Withania spp. – J. Natur. Prod. 56: 1000-1006.
Atta-Ur-Rahman, Shabir M, Yousaf M, Qureschi S et al. 1999. Three withanolides from Withania coagulans. – Phytochemistry 52: 1361-1364.
Aubriot X, Singh P, Knapp S. 2016. Tropical Asian species show that the Old World clade of ’spiny solanums’ (Solanum subgenus Leptostemonum pro parte: Solanaceae) is not monophyletic. – Bot. J. Linn. Soc. 181: 199-223.
Austin DF. 1973a. The American Erycibeae (Convolvulaceae): Maripa, Dicranostyles, and Lysiostyles I. Systematics. – Ann. Missouri Bot. Gard. 60: 306-412.
Austin DF. 1973b. The American Ericibeae (Convolvulaceae): Maripa, Dicranostyles, and Lyciostyles II. Palynology. – Pollen Spores 15: 203-226.
Austin DF. 1976. Varieties of Ipomoea trichocarpa. – Sida 6: 216-220.
Austin DF. 1979. An infrageneric classification of Ipomoea (Convolvulaceae). – Taxon 28: 359-361.
Austin DF. 1982. 165. Convolvulaceae. – In: Harling G, Sparre B (eds), Flora of Ecuador 15, Swedish Natural Science Research Council, Stockholm, pp. 1-98.
Austin DF. 1995. Merremia discoidesperma (Convolvulaceae) seeds as medicines in Mexico. – Econ. Bot. 49: 330-332.
Austin DF. 1996. A synopsis of Ipomoea (Convolvulaceae) in the Americas. – Taxon 45: 3-38.
Austin DF. 1997. Dissolution of Ipomoea series Anisomerae (Convolvulaceae). – J. Torrey Bot. Soc. 124: 140-159.
Austin DF. 1998a. Parallel and convergent evolution in the Convolvulaceae. – In: Mathews P, Sivadasan M (eds), Biodiversity and taxonomy of tropical flowering plants, Mentor Books, Calicut, pp. 201-234.
Austin DF. 1998b. Xixicamatic or wood rose (Merremia tuberosa, Convolvulaceae): origins and dispersal. – Econ. Bot. 52: 412-422.
Austin DF. 1998c. Additions and corrections in American Ipomoea (Convolvulaceae). – Taxon 47: 833-838.
Austin DF. 1999. The genus Aniseia (Convolvulaceae). – Syst. Bot. 23: 411-420.
Austin DF. 2000. A revision of Cressa L. (Convolvulaceae). – Bot. J. Linn. Soc. 133: 27-39.
Austin DF, Demissew S. 1997. Unique fruits and generic status of Stictocardia (Convolvulaceae). – Kew Bull. 52: 161-169.
Austin DF, Eich E. 2001. Synopsis of Stictocardia with another Madagscan species, S. mojangensis (Convolvulaceae). – Willdenowia 31: 79-85.
Austin DF, Huáman Z. 1996. A synopsis of Ipomoea (Convolvulaceae) in the Americas. – Taxon 45: 3-38.
Austin DF, Sebsebe D. 1997. Unique fruits and generic status of Stictocardia (Convolvulaceae). – Kew Bull. 57: 161-168.
Austin DF, Staples GW. 1980. Xenostegia, a new genus of Convolvulaceae. – Brittonia 32: 533-536.
Austin DF, Staples GW. 1983. Additions and changes in the Neotropical Convolvulaceae. Notes on Merremia, Operculina, and Turbina. – J. Arnold Arbor. 64: 483-489.
Austin DF, Staples G. 1985. Petrogenia as a synonym of Bonamia (Convolvulaceae), with comments on allied species. – Brittonia 37: 310-316.
Austin DF, Staples GW. 1991. A revision of the neotropical species of Turbina Raf. (Convolvulaceae). – Bull. Torrey Bot. Club 118: 265-280.
Austin DF, Powell DA, Nicolson DH. 1978. Stictocardia tiliifolia (Convolvulaceae) re-evaluated. – Brittonia 30: 195-198.
Averett JE. 1971. New combinations in the Solaneae (Solanaceae) and comments regarding the taxonomy of Leucophysalis. – Ann. Missouri Bot. Gard. 57: 380-382.
Averett JE. 1973. Biosystematic study of Chamaesaracha (Solanaceae). – Rhodora 75: 325-365.
Averett JE. 1977. Taxonomic notes and new combinations in Leucophysalis (Solanaceae). – Ann. Missouri Bot. Gard. 64: 141-142.
Averett JE. 1979. Biosystematics of the physaloid genera of the Solaneae in North America. – In: Hawkes JG, Lester RN, Skelding AD (eds), The biology and taxonomy of the Solanaceae, Linn. Soc. Symp. Ser. 7, Linnean Society and Academic Press, London, pp. 493-503.
Averett JE. 2009a. Taxonomy of Leucophysalis (Solanaceae, tribe Physaleae). – Rhodora 111: 209-217.
Averett JE. 2009b. Schraderanthus, a new genus of Solanaceae. – Phytologia 91: 54-61.
Averett JE, Martínez M. 2009. Capsicophysalis: a new genus of Solanaceae (Physaleae) from Mexico and Central America. – J. Bot. Res. Inst. Texas 3: 71-75.
Avery AG, Satina S, Rietsema J. 1959. Blakeslee: the genus Datura I-XLI, Ronald Press Co., New York.
Avery GS. 1933a. Structure and germination of tobacco seed and the developmental anatomy of the seedling plant. – Amer. J. Bot. 20: 309-327.
Avery GS. 1933b. Structure and development of the tobacco leaf. – Amer. J. Bot. 20: 565-592.
Avetisian EM. 1986. Palynomorphology of the families Campanulaceae, Sphenocleaceae, and Pentaphragmataceae. – Bot. Žurn. 71: 1003-1010. [In Russian with English summary]
Axelius B. 1992. Testa patterns in some species of Physalis L. and some other genera in the tribe Solaneae (Solanaceae). – Intern. J. Plant Sci. 153: 488-502.
Axelius B. 1993. The correct names of two Japanese species of Physaliastrum Makino. – J. Jap. Bot. 68: 138-141.
Axelius B. 1995. A new combination in Physalis. – Phytologia 79: 10-11.
Axelius B. 1996. The phylogenetic relationships of the physaloid genera (Solanaceae) based on morphological data. – Amer. J. Bot. 83: 118-124.
Axelius B, D’Arcy WG. 1993. A new species of Deprea (Solanaceae) from Venezuela. – Novon 3: 11-13.
Badr A, Khalifa SF, Aboel-Atta AI, Abou-El-Enain MM. 1997. Chromosomal criteria and taxonomic relationships in the Solanaceae. – Cytologia 62: 103-113.
Baehni C.1943. Henoonia, type d’une famille nouvelle? – Boisiera 7: 346-358.
Baehni C. 1944. Organogénie de la fleur chez l’Anthocercis littorea Labill. (Solanée). – Bull. Soc. Bot. Suisse 54.
Baehni C. 1946. L’ouverture du bouton chez les fleurs des Solanées. – Candollea 10: 399-492.
Bakolimalala-Ramamonjiarisoa R. 1989. Wood anatomy of Malagasy and African Montiniaceae. – Service de Botanique, B. P. 906, Antananarivo.
Baldwin JT Jr, Spese BM. 1951. Cytogeography of Physalis in West Africa. – Bull. Torrey Bot. Club 78: 254-257.
Banks P. 1984. A new diploid chromosome number for tomato (Lycopersicon esculentum)? – Can. J. Gen. Cytol. 26: 636-639.
Baquar SR. 1967. Cytomorphological studies in the family Solanaceae from West Pakistan. – Genetica 38: 388-397.
Barboza GE. 1986. Estudios palinológicos en Jaborosa Juss. y Trechonaetes Miers (Solanaceae). – Bol. Acad. Nac. Ci. Córdoba 57: 357-376.
Barboza GE. 1989. Sobre la naturaleza tricelular de los granos de pollen en la tribu Jaboroseae (Solanaceae). – Kurtziana 20: 19-145.
Barboza GE. 1991. El sistema reproductivo en Jaborosa (Solanaceae) I. Esporogénesis, gametogénesis y fecundación. – Kurtziana 21: 39-71.
Barboza GE, Hunziker AT. 1987. Estudios sobre Solanaceae XXV. Revisión de Jaborosa. – Kurtziana 19: 77-153.
Barboza GE, Hunziker AT. 1989. Estudios sobre Solanaceae XXXIV. Sinopsis taxonómica de Athenaea. – Bol. Soc. Argent. Bot. 26: 91-105.
Barboza GE, Hunziker AT. 1991. Estudios sobre Solanaceae. XXXI. Peculiaridades del androceo de interés taxonómico en Solanum. – Kurtziana 21: 185-194.
Barboza GE, Hunziker AT. 1992. Estudios sobre Solanaceae XXXIII. El género Lycianthes en la Argentina. – Darwiniana 31: 17-34.
Barboza GE, Hunziker AT. 1993. Estudios sobre Solanaceae XXXIV. Revision taxonómica de Fabiana. – Kurtziana 22: 109-153.
Barboza GE, Hunziker AT. 1994. Estuios sobre Solanaceae XXXVII. Sinopsis taxonómica de Deprea. – Kurtziana 23: 43-65.
Barboza GE, Hunziker AT. 2005. Revision of Solanum sect. Campanulisolanum. – Ann. Missouri Bot. Gard. 79: 346-360.
Barboza GE, Carrizo GC, Hunziker AT. 1997. Estudios sobre Solanaceae XLIV. Exodeconus: anatomía del fruto e implicancias sobre su posición sistemática. – Kurtziana 25: 123-139.
Barboza GE, Bianchetti L De Bem. 2005. Three new species of Capsicum (Solanaceae) and a key to the wild species from Brazil. – Syst. Bot. 30: 863-871.
Barboza GE, Agra MF, Romero MV, Scaldaferro MA Moscone EA. 2011. New endemic species of Capsicum (Solanaceae) from the Brazilian caatinga: comparison with the re-circumscribed C. parvifolium. – Syst. Bot. 36: 768-781.
Barclay AS. 1959a. New considerations in an old genus: Datura. – Bot. Mus. Leafl. 18: 1-272.
Barclay AS. 1959b. Studies in the genus Datura (Solanaceae) I. Taxonomy of subgenus Datura. – Ph.D. diss., Harvard University Library, Cambridge, Massachusetts.
Barkley FA. 1953. Lycium in Argentina. – Lilloa 26: 177-228.
Barnard C. 1949. Microsporogenesis, macrosporogenesis and development of the macrogametophyte and seeds of Duboisia leichhardtii and D. myoporoides. – Aust. J. Sci. Res., ser. B, 2: 241-248.
Barnard C. 1952. The Duboisias of Australia. – Econ. Bot. 6: 3-17.
Basak RK. 1967. The pollen grains of Solanaceae. – Bull. Bot. Soc. Bengal 21: 49-58.
Basey K, Woolley JG, 1973. Alkaloids of Physalis alkekengi. – Phytochemistry 12: 2557-2559.
Basey K, McGaw BA, Woolley JG. 1992. Phygrine, an alkaloid from Physalis species. – Phytochemistry 31: 4173-4176.
Baylis GTS. 1954. Chromosome number and distribution of Solanum aviculare Forst. and S. laciniatum Ait. – Trans. Proc. Roy. Soc. New Zealand 82: 639-643.
Baylis GTS. 1963. A cytogenetical study of the Solanum aviculare species complex. – Aust. J. Bot. 11: 168-177.
Bean AR. 2001. A revision of Solanum brownii Dunal (Solanaceae) and its allies. – Telopea 9: 639-661.
Bean AR. 2012. A taxonomic revision of the Solanum echinatum group (Solanaceae). – Phytotaxa 57: 33-50.
Beckman RL Jr, Stucky JM. 1981. Extrafloral nectaries and plant guarding in Ipomoea pandurata (L.) G. F. W. Mey. (Convolvulaceae). – Amer. J. Bot. 68: 72-79.
Begley MJ. 1976. A new class of natural steroids, with ring D aromatic, from Nicandra physaloides (Solanaceae). X-ray analysis of Nic-10, and the structures of Nic-1 (Nicandrenone), -12, and -17. – J. Chem. Soc. Perkin I: 304-308.
Bell AD, Dines TD. 1995. Branching patterns in the Solanaceae. – In: Hoch PC, Stephenson AG (eds), Experimental and molecular approaches to plant biosystematics, Missouri Botanical Garden, St. Louis, pp. 157-172.
Benítez de Rojas CE. 1974. Los géneros de las Solanaceae de Venezuela. – Rev. Fac. Agron. (Aracay) 7: 25-108.
Benítez de Rojas CE. 1989. El género Hawkesiophyton Hunz. (Solanaceae) en Venezuela. – Ernstia 56: 15-18.
Benítez de Rojas CE. 1993. La tribu Schwenckieae A. Hunz. (Solanaceae). – Universidad Central de Venezuela, Caracas.
Benítez de Rojas CE, D’Arcy WG. 1997. The genus Lycianthes (Solanaceae) in Venezuela. – Ann. Missouri Bot. Gard. 84: 167-200.
Benítez de Rojas CE, D’Arcy WG. 1998. The genera Cestrum and Sessea (Solanaceae: Cestreae) in Venezuela. – Ann. Missouri Bot. Gard. 85: 273-351.
Benítez de Rojas CE, Júregui D. 2005. Morphology of foliar trichomes in species of Sessea (Solanaceae). – Monogr. Syst. Bot. Missouri Bot. Gard. 104: 321-338.
Benítez de Rojas CE, Magallanes NA. 1998. El género Physalis (Solanaceae) de Venezuela. – Acta Bot. Venezuelica 21: 11-42.
Benítez de Rojas CE, Martínez M.1995. Larnax hunzikeriana (Solanaceae, Solanoideae). Una nueva especie y la primera mención del género para Venezuela. – Phytologia 78: 353-356.
Benítez de Rojas CE, Nee M. 2001. The neotropical genus Sessea (Solanaceae): a preliminary survey. – In: Berr RG van de, Barendse GWM, Werden GM van der, Mariani C (eds), Solanaceae V: advances in taxonomy and utilization, Nijmegen University Press, Nijmegen, pp. 153-159.
Benítez de Rojas CE, Sawyer NW. 1999. A new species of Cestrum (Solanaceae) from the Cordillera de Mérida, Venezuela. – Brittonia 51: 163-165.
Benoist R. 1938. Nouvelles espèces du genre Salpichroa. – Bull. Soc. Bot. France 85: 53-56.
Beresford PJ, Woolley JG. 1974a. (±)-6β-propanoyloxy-3α-tigloyloxytropane, a new alkaloid from Datura innoxia. – Phytochemistry 13: 1249-1251.
Beresford PJ, Woolley JG. 1974b. Biosystematics of tigloidine in Physalis peruviana. – Phytochemistry 13: 2143-2144.
Beresford PJ, Woolley JG. 1974c. (+)-6β-[2-methylbutanoyloxy]-tropan-3α-ol, a new alkaloid from Datura ceratocaula. Structure and biosynthesis. – Phytochemistry 13: 2511-2513.
Berg RG van den, Groendijk-Wilders N. 1999. Numerical analysis of the taxa of series Circaeifolia (Solanum sect. Petota). – In: Nee, Symon, Lester, Jessop (eds), Solanaceae IV, Royal Botanic Gardens, Kew, pp. 213-226.
Berg RG van den, Miller JT, Ugarte ML, Kardolus JP, Villand J, Nienhuis J, Spooner DM. 1998. Collapse of morphological species in the wild potato Solanum brevicaule complex (sect. Petota). – Amer. J. Bot. 85: 92-109.
Berg RG van den, Barendse GWM, Werden GM van der, Mariani C (eds). 2001. Solanaceae V: advances in taxonomy and utilization. – Nijmegen University Press, Nijmegen.
Berg RG van den, Bryan G, Rio A del, Spooner DM. 2002. Reduction of species in the wild potato Solanum section Petota series Longipedicellata: AFLP, RAPD and chloroplast SSR data. – Theor. Appl. Gen. 105: 1109-1114.
Bergner AD. 1943.Chromosomal interchange among six species of Datura in nature. – Amer. J. Bot. 30: 431-440.
Bernardello G, Stiefkens L, Las Peñas ML. 2008. Karyotype studies in Grabowskia and Phrodus (Solanaceae). – Plant Syst. Evol. 275: 265-269.
Bernardello LM. 1982a. Estudios en Lycium (Solanaceae) I. Anatomía de hoja y tallo, y su diferencias con Grabowskia. – Bot. Soc. Argent. Bot.21: 153-185.
Bernardello LM. 1982b. Estudios en Lycium (Solanaceae) II. Recuentos cromosómicos en entidades argentinas. – Hickenia I: 321-328.
Bernardello LM. 1983a. Estudios en Lycium (Solanaceae) III. Estructura y desarrollo de fruto y semilla en Lycium y Grabowskia. – Bol. Soc. Argent. Bot. 22: 147-176.
Bernardello LM. 1983b. Estudios en Lycium (Solanaceae) IV. Biología reproductiva de L. cestroides, con especial referencia a la dehiscencia de la antera en el género. – Kurtziana 16: 33-70.
Bernardello LM. 1985a. The nectary exudate of Lycium cestroides (Solanaceae). – Biotropica 18: 241-243.
Bernardello LM. 1985b. El número cromosómico de Phrodus microphyllus (Solaaceae). – Bol. Soc. Argent. Bot. 24: 196-197.
Bernardello LM. 1986a. Estudios en Lycium (Solanaceae) V. El gineceo de Lycieae. – Kurtziana 18: 23-45.
Bernardello LM. 1986b. Revisión taxonómica de las especies sudamericanas de Lycium (Solanaceae). – Bol. Acad. Nac. Ci. Córdoba 57: 173-356.
Bernardello LM. 1987. Comparative floral morphology in Lycieae (Solanaceae). – Brittonia 39: 112-129.
Bernardello LM, Anderson GJ. 1990. Karyotypic studies in Solanum sect. Basarthrum (Solanaceae). – Amer. J. Bot. 77: 420-431.
Bernardello LM, Bonzani N. 1991. Sobre la naturaleza del endosperma en Lycium (Solanaceae). – Kurtziana 21: 177-184.
Bernardello LM, Chiang-Cabrera F. 1998. A cladistic study on the American species of Lycium (Solanaceae) based on morphological variation. – Monogr. Syst. Bot. Missouri Bot. Gard.: 33-46.
Bernardello LM, Hunziker AT. 1987a. Estudios sobre Solanaceae XXVI. Revisión taxonómica de Phrodus. – Kurtziana 19: 69-76.
Bernardello LM, Hunziker AT. 1987b. A synoptical revision of Solandra (Solanaceae). – Nord. J. Bot. 7: 639-652.
Bernardello LM, Hunziker AT. 1991. The genus Schultesianthus (Solanaceae): leaf anatomy and taxonomic synopsis. – In: Hawkes JG, Lester RN, Nee M, Estrada N (eds), Solanaceae III. Taxonomy, chemistry, evolution, Royal Botanic Gardens, Kew, Linnean Society, London, pp. 1-17.
Bernardello LM, Luján MC. 1997. Pollen morphology of tribe Lycieae: Grabowskia, Lycium, Phrodus. – Rev. Paleobot. Palynol. 96: 305-315.
Bernardello LM, Heiser CB, Piazzano M. 1994. Karyotypic studies in Solanum sect. Lasiocarpa (Solanaceae). – Amer. J. Bot. 81: 95-103.
Berra J. 2000. Withania somnifera Dunal. – Fitociencia 3: 20-22.
Bessis J, Guyot M. 1979. An attempt to use stomatal characters in systematic and phylogenetic studies of Solanaceae. – In: Hawkes JG, Lester RN, Skelding AD (eds), The biology and taxonomy of the Solanaceae. Linn. Soc. Symp. Ser. 7, Academic Press, London, pp. 321-326.
Bhaduri PN. 1933. Chromosome numbers of some solanaceous plants of Bengal. – J. Indian Bot. Soc. 12: 56-63.
Bhaduri PN, Sharma AK. 1946. Cytogenetics of Datura fastuosa L. – Bull. Torrey Bot. Club 73: 438-450.
Bhattacharyya J, Basilio IJLD, Morais LCSL, de Fatima Agra M, Majetich G. 2009. Alkaloids of the root-bark of Solanum paludosum Moric. – Biochem. Syst. Chem. 37: 228-229.
Bick IRC, Bremner JB., Gillard JW, Winzenberg KN. 1974. Alkaloids of Anthocercis tasmanica (Solanaceae). – Aust. J. Chem. 27: 2525-2518.
Bitter G. 1903. Die Rassen von Nicandra physaloides. – Bot. Centralbl. 14: 145-176.
Bitter G. 1911. Steinzellkonkretionen im Fruchtfleisch beerentragender Solanaceen und deren systematische Bedeutung. – Engl. Bot. Jahrb. Syst. 45: 483-507.
Bitter G. 1914. Weitere Untersuchungen über das Vorkommen von Steinzellkonkretionen im Fruchtfleisch beerentragenden Solanaceen. – Abhandl. Naturw. Ver. Bremen 23: 114-163.
Bitter G. 1919. Die Gattung Lycianthes. – Abhandl. Naturw. Ver. Bremen 24: 292-520.
Bitter G. 1921a. Zur Gattung Cacabus. – Feddes Repert. 17: 243-246.
Bitter G. 1921b. Eine verkannte Hebecladus-Art und ihre Bedeutung für die Stellung der Gattung in der tribus der Solaneae. – Feddes Repert. 17: 246-251.
Bitter G. 1921c. Zur Gliederung der Gattung Saracha I. – Feddes Repert. 17: 338-346.
Bitter G. 1921d. Aufteilung der Gattung Bassovia (im Dunalschen Sinne) zwischen Solanum, Capsicum und Lycianthes. – Feddes Repert. 17: 328-335.
Bitter G. 1922a. Zur Gliederung der Gattung Saracha II. – Feddes Repert. 18: 99-112.
Bitter G. 1922b. Zur Gatung Sessea. – Feddes Repert. 18: 199-225.
Bitter G. 1923. Zur Gliederung der Gattung Saracha III. – Feddes Repert. 19: 265-270.
Bitter G. 1924. Weitere Untersuchungen über Hebecladus. – Feddes Repert. 20: 372-376.
Blakeslee AF. 1932. The species problem in Datura. – Proc. 6th Internat. Congr. Genetics 1, pp. 104-120.
Bodendorf K, Kummer H. 1962. Über die Alkaloide in Latua venenosa. – Pharm. Zentralh. 101: 620-622.
Bogani P, Lio P, Intrieri MC, Buiatti M. 1997. A physiological and molecular analysis of the genus Nicotiana. – Mol. Phylogen. Evol. 7: 62-70.
Bohs L. 1986. The generic delimitation of Cyphomandra (Solanaceae). – Amer. J. Bot. 73: 751.
Bohs L. 1988. Four new species of Cyphomandra (Solanaceae) from South America. – Syst. Bot. 13: 265-275.
Bohs L. 1989. Solanum allophyllum (Miers) Standl. and the generic delimitation of Cyphomandra and Solanum (Solanaceae). – Ann. Missouri Bot. Gard. 76: 1129-1140.
Bohs L. 1990. The systematics of Solanum section Allophyllum (Solanaceae). – Ann. Missouri Bot. Gard. 7: 398-409.
Bohs L. 1995a. Transfer of Cyphomandra (Solanaceae) and its species to Solanum. – Taxon 44: 583-587.
Bohs L. 1995b. Major clades in Solanum based on ndhF sequence data. – In: Keating RC, Hollowell VC, Croat TB (eds), A festschrift for William G. D’Arcy: the legacy of a taxonomist, Monogr. Syst. Bot. Missouri Bot. Gard. 104: 27-49.
Bohs L. 1997. Solanum maternum (Solanaceae), a new Bolivian relative of the tree tomato. – Novon 7: 341-345.
Bohs L. 2001. Revision of Solanum
Section Cyphomandropsis (Solanaceae). – Syst. Bot. Monogr. 61:
1-85.
Bohs L. 2004. A chloroplast DNA phylogeny of Solanum section Lasiocarpa. – Syst. Bot. 29: 177-187.
Bohs L. 2005. Major clades in Solanum based on ndhF sequence data. – In: Keating RC, Hollowell VC, Croat TB (eds), A Festschrift for William G. D’Arcy. The legacy of a taxonomist, Monogr. Syst. Bot. Missouri Bot. Gard. 104, Missouri Botanical Garden Press, St. Louis, pp. 27-49.
Bohs L. 2007. Phylogeny of the Cyphomandra clade of the genus Solanum (Solanaceae) based on ITS sequence data. – Taxon 56: 1012-1026.
Bohs L, Olmstead RG. 1997. Phylogenetic relationships in Solanum (Solanaceae) based on ndhF sequences. – Syst. Bot. 22: 5-17.
Bohs L, Olmstead RG. 1999. Solanum phylogeny inferred from chloroplast DNA sequence data. – In: Nee M, Symon D, Lester RN, Jessop J (eds), Solanaceae IV: advances in biology and utilization, Royal Botanic Gardens, Kew, pp. 97-110.
Bohs L, Olmstead RG. 2001. A reassessment of Normania and Triguera (Solanaceae). – Plant Syst. Evol. 228: 33-48.
Bohs L, Weese T, Myers N, Lefgren V, Thomas N, Wagenen A van, Stern S. 2007. Zygomorphy and heteranthy in Solanum in a phylogenetic context. – In: Spooner DM, Bohs L, Giovannoni J, Olmstead RG, Shibata D (eds), Solanaceae VI: Genomics Meets Biodiversity, ISHA Section Root and Tuber Crops, pp. 201-223. [Acta Hortic. 745]
Bömmer D, Haberhausen G, Zetsche K. 1993. A large deletion in the plastid DNA of the holoparasitic flowering plant Cuscuta reflexa concerning two ribosomal proteins (rpl2, rpl23), one transfer RNA (trnI) and an ORF2280 homologue. – Curr. Genet. 24: 171-176.
Bondeson WE. 1986.Gynoecial morphology and funicular germination plugs in the Nolanaceae. – Nord. J. Bot. 6: 183-198.
Bosland PW. 1994. Chiles: history, cultivation and uses. – In: Charalambous G (ed), Spices, herbs and edible fungi, Elsevier, pp. 347-366.
Bosland PW, Votava EJ. 1999. Peppers: vegetable and spice capsicums I-XI. – New York.
Bosser J. 1990. Les sous-espèces de Grevea madagascariensis Baillon (Montiniaceae) à Madagascar et en Afrique. – Bull. Mus. Natl. Hist. Nat. Paris, sér. IV, sect. B, Adansonia 12: 109-112.
Bosser J, D’Arcy WG, Lobreau-Callen D. 1992. Découverte d’un genre nouveau de Solanaceae à Madagascar. – Bull. Mus. Natl. Hist. Nat. Paris, sér. IV, sect. B, Adansonia 14: 3-12.
Bouquet J. 1952. La mandragore en Afrique du Nord. – Bull. Soc. Sci. Nat. Tunisie 5: 29-44.
Bowers KAW. 1975. The pollination biology of Solanum rostratum (Solanaceae). – Amer. J. Bot. 62: 633-638.
Bradley MV. 1936. Comparative anatomy of leaf, stem and pedicel of the genus Nicotiana, with special reference to the relationships of species and hybrids. – Ph.D. diss., Library of the University of California.
Bradley V, Collins DJ, Eastwood FW, Irvine MC, Swan JM. 1979. Distribution of steroidal alkaloids in Australian species of Solanum. – In: Hawkes JG, Lester RN, Skelding AD (eds), The biology and taxonomyof the Solanaceae, Linn. Soc. Symp. Ser. 7, Linnean Soc. And Academic PRESS; London, pp. 203-209.
Bremer B, Bremer K, Heidari N, Erixon P, Anderberg AA, Olmstead RG, Källersjö M, Barkhordarian E. 2002. Phylogenetics of asterids based on three coding and three non-coding chloroplast DNA markers and the utility of non-coding DNA at higher taxonomic levels. – Mol. Phylogen. Evol. 24: 274-301.
Bremner JB, Cannon JR. 1968. Isolation of (-)-hyoscyamine from Anthotroche pannosa Endl. – Aust. J. Chem. 21: 1369-1370.
Bristol ML. 1966. Notes on the species of tree ‘daturas’. – Bot. Mus. Leafl. 21: 229-247.
Bristol ML. 1969. Tree datura drugs of the Colombian Sibundoy. – Bot. Mus. Leafl. 24: 165-227.
Brown GD. 1994. The sesquiterpenes of Fabiana imbricata. – Phytochemistry 35: 425-433.
Brown KS Jr. 1987. Chemistry at the Solanaceae/Ithomiinae interface. – Ann. Missouri Bot. Gard. 74: 359-397.
Brücher H. 1954. Cytologische und ökologische Beobachtungen an nordargentinischer Solanum-Arten der Sektion Tuberarium. – Der Züchter 10: 281-295.
Brücher H. 1960. Problematisches zur Ursprung der Kultur-kartoffel aus Chiloé. – Zeitschr. f. Pflanzenzücht. 43: 241-265.
Bruneau A, Dickson EE, Knapp S. 1995. Congruence of chloroplast DNA restriction site characters with morphological and isozyme data in Solanum sect. Lasiocarpa. – Can. J. Bot. 73: 1151-1167.
Bruno GB. 1994. Organización y vasculature del gineceo de Nolana crassulifolia y N. rostrata. – Bol. Soc. Argent. Bot. 30: 51-57.
Buchholz JT, Williams L, Blakeslee F. 1935. Pollen tube growth of ten species of Datura in interspecific pollinations. – Proc. Natl. Acad. Sci. U.S.A. 21: 651-656.
Bukasov SM. 1939. The origin of potato species. – Physis (Buenos Aires) 1: 41-46.
Bukenya ZR. 1995. Solanum (Solanaceae) in Uganda. – Bothalia 25: 43-59.
Bukenya ZR, Hall B. 1988. Solanum (Solanaceae) in Ghana. – Bothalia 18: 79-88.
Bukovits GJ, Gros EG. 1979. Acnistoferin, a new withanolide from Acnistus breviflorus. – Phytochemistry 18: 1237-1239.
Burbidge N. 1960. The Australian species of Nicotiana L. (Solanaceae). – Aust. J. Bot. 8: 342-380.
Buril MT. 2013. Sistemática e filogenia de Jacquemontia Choisy (Convolvulaceae). – Ph.D. diss., Universidade Federal de Pernambuco, Brazil.
Buril MT, Simões AR, Carine M, Alves M. 2013
[2014]. Austinia, a new genus of Convolvulaceae from Brazil. –
Phytotaxa 186: 254-260.
Buril MT, Simões AR, Carine M, Alves M.
2015. Daustinia, a replacement name for Austinia (Convolvulaceae). – Phytotaxa 194.
doi: http://dx.doi.org/10.11646/phytotaxa.197.1.8
Buril MT, Oliveira PP, Rodrigues R, Ribeiro
Dos Santos F de A, Alves M. 2015. Pollen morphology and taxonomic implications
in Jacquemontia Choisy (Convolvulaceae). – Grana 54:
1-11.
Burns JA. 1982. The chromosomes of Nicotiana africana Merxm., a recently discovered species. – J. Heredity 73: 115-118.
Burton G, Oberti JC. 2000. Withanólidos en Solanaceae. – Kurtziana 28: 81-93.
Buschi CA, Pomilio AM. 1987. Pyrrole-3-carbamidine: a letal principle from Nierembergia hippomanica. – Phytochemistry 26: 863-865.
Bye R. 1986. Datura lanosa, a new species of Datura from Mexico. – Phytologia 61: 204-206.
Bye R, Sosa V. 2013. Molecular phylogeny of the jimsonweed genus Datura (Solanaceae). – Syst. Bot. 38: 818-829.
Cabrera AL. 1952. El género Melananthus (Solanaceae) en la República Argentina. – Bol. Soc. Argent. Bot. 4: 192-194.
Cabrera AL, Juliani HJ. 1981. Na cinamoilhistamina a partir de Lycium cestroides Schltdl. (Solanáceas). – Anal. Asoc. Quím. Argent. 69: 357-358.
Campin MG. 1924. A cytological study of pollen formation in Solandra grandiflora Sw. – New Phytol. 23: 282-287.
Campin MG. 1925. A cytological study of pollen development in Nolana. – New Phytol. 24: 17-23.
Cannon JR, Joshi KR, Meehan GV, Williams JR. 1969. The tropane alkaloids from three Western Australian Anthocercis species. – Aust. J. Chem. 22: 221-229.
Capuron R. 1974. Contributions à l’étude de la flore forestière de Madagascar. Sur la place du genre Kaliphora Hook. f. – Adansonia, sér. II, 9: 395-397.
Cárcamo C, Fajardo V, Tojo E. 1993. (-)-Jaboromagellonine: new withanolide from seeds of Jaborosa magellanica. – Heterocycles 36: 1771-1774.
Carine MA, Robba L. 2010. Taxonomy and evolution of the Convolvulus sabatius complex (Convolvulaceae). – Phytotaxa 14: 1-21.
Carine MA, Russell SJ, Santos-Guerra A, Francisco-Ortega J. 2004. Relationships of the Macaronesian and Mediterranean floras: molecular evidence for multiple colonizations into Macaronesia and back-colonization of the continent in Convolvulus (Convolvulaceae). – Amer. J. Bot. 91: 1070-1085.
Carine MA, Robba L, Little R, Russell S, Guerra AS. 2007. Molecular and morphological evidence for hybridization between endemic Canary Island Convolvulus. – Bot. J. Linn. Soc. 154: 187-204.
Carle R. 1981. Investigations on the content of steroidal alkaloids and sapogenins, within Solanum sect. Solanum (= sect. Morella), Solanaceae. – Plant Syst. Evol. 138: 61-71.
Carlquist SJ. 1987. Wood anatomy of Nolanaceae. – Aliso 11: 463-471.
Carlquist SJ. 1988. Wood anatomy and relationships of Duckeodendraceae and Goetzeaceae. – IAWA Bull., N. S., 9: 3-12.
Carlquist SJ. 1989. Wood anatomy and relationships of Montinia. – Aliso 12: 369-378.
Carlquist SJ. 1992. Wood anatomy of Solanaceae. A survey. – Allertonia 6: 279-326.
Carlquist S, Eckhart VM. 1984. Wood anatomy of Hydrophyllaceae II. Genera other than Eriodictyon, with comments on parenchyma bands containing vessels with large pits. – Aliso 10: 527-546.
Carlquist SJ, Hanson MA. 1991. Wood and stem anatomy of Convolvulaceae: a survey. – Aliso 13: 51-94.
Carrizo García C. 1998. Sobre el andreoceo y el gineceo en la tri Datureae (Solanaceae) y su implicancia taxonómica. – Kurtziana 26: 33-53.
Carrizo García C. 1999. Studio morfo-anatómico de los verticilos florales fértiles en Nicandra physalodes (Solanaceae). – Kurtziana 27: 173-185.
Carrizo García C. 2000. Histología de la antera en la tribu Jaboroseae (Solanaceae). – Kurtziana 28: 195-204.
Carrizo García C. 2002. An approach to the diversity of endothecial thickenings in Solanaceae. – Flora 197: 214-223.
Carrizo García C. 2003. Combination of sequences of cell divisions in the anther wall formation in Solanaceae species. – Flora 198: 243-246.
Carrizo García C, Matesevach M, Barboza G. 2008. Features related to anther opening in Solanum species (Solanaceae). – Bot. J. Linn. Soc. 158: 344-354.
Carrizo García C, Wahlert G, Orozco CI, Barboza GE, Bohs L. 2015. Phylogeny of the Andean genus Deprea (Physalideae, Solanaceae): testing the generic circumscription. – Phytotaxa 238: 71-81.
Carvalho LAF. 1986. The genus Metternichia in Brazil. – In: D’Arcy WG (ed), Solanaceae: biology and systematics, Columbia University Press, New York, pp. 5-14.
Carvalho LAF, Schnoor A. 1997. Sessea Carv. et Schnoor, uma nova seçao para o genero Cestrum. – Rodriguésia 45: 15-24.
Casero CN, Oberti JC, Orozco CI, Cárdenas A, Brito I, Barboza GE, Nicotra VE. 2015. Withanolides from three species of the genus Deprea (Solanaceae). Chemotaxonomical considerations. – Phytochemistry 110: 83-90.
Castel R, Kusters E, Koes R. 2010. Inflorescence development in petunia: through the maze of botanical terminology. – J. Experim. Bot. 61: 2235-2246.
Castillo R, Spooner DM. 1997. Phylogenetic relationships of wild potatoes, Solanum Series Conicibaccata (Sect. Petota). – Syst. Bot. 22: 45-83.
Catalan CAN, Tomasini SP. 1992. Triterpenoids and sterols from Cestrum kunthii. – Fitoterapia 63: 551.
Cavazos ML, Jiao M, Bye R. 2000. Phenetic analysis of Datura section Dutra (Solanaceae) in Mexico. – Bot. J. Linn. Soc. 133: 493-507.
Chakravarti RN, Datta S, Mitra MN. 1964. Tigogenin and ursolic acid from Cestrum diurnum. – Experientia 20: 200.
Chandra Sekhar KN, Sawhney VK. 1984. A scanning electron microscope study of the development and surface features of floral organs of tomato (Lycopersicon esculentum). – Can. J. Bot. 62: 2403-2413.
Chao JM, Der Marderosian AH. 1973. Identification of ergoline alkaloids in the genus Argyreia and related genera and their chemotaxonomic implication in the Convolvulaceae. – Phytochemistry 12: 2435-2440.
Chase MW, Knapp S, Cox AV, Clarkson JJ, Butsko Y, Joseph J, Savolainen V, Parokonny AS. 2003. Molecular systematics, GISH and the origin of hybrid taxa in Nicotiana (Solanaceae). – Ann. Bot. 92: 107-127.
Cheek M, Simão-Bianchini R. 2013. Keraunea gen. nov. (Convolvulaceae) from Brazil. – Nord. J. Bot. 31: 453-457.
Chen C, Chen C-L. 1977. On the Chinese genera Scopolia Jacq., Anisodus Link et Otto and Atropanthe Pascher. – Acta Phytotaxon. Sin. 15: 57-68.
Chen L-X, He H, Qiu F. 2011. Natural withanolides: an overview. – Nat. Prod. Rep. 28: 705-740.
Chiale CA, Cabrera JL, Juliani HR. 1990. Cinnamoylhistamine derivates from Lycium cestroides. – Anal. Asoc. Quim. Argent. 29: 688-689.
Chiang-Cabrera F. 1981. A taxonomic study of the North American species of Lycium (Solanaceae). – Ph.D. diss., University of Texas, Austin, Texas.
Chiang-Cabrera F. 1982. Estudios cromosómicos en Lycium (Solanaceae) de Norteamérica. – Bol. Soc. Bot. Mexico 43: 9-23.
Chiang-Cabrera F. 1983a. Nomenclatural changes for new sectional delimitation in Lycium (Solanaceae) of the new world. – Taxon 32: 456-458.
Chiang-Cabrera F. 1983b. El número cromosómico de Grabowskia geniculata (Solanaceae). – Bol. Soc. Bot. Mexico 45: 141-142.
Chiarini FE. 2003. Las cromosomas somáticos de Schwenckia americana (Solanaceae). – Bol. Soc. Argent. Bot. 38: 325-327.
Chiarini FE. 2004. A new species of Solanum subgen. Leptostemonum (Solanaceae) from Argentina. – Brittonia 56: 284-287.
Chiarini FE, Barboza GE. 2008. Karyological studies in Jaborosa (Solanaceae). – Bot. J. Linn. Soc. 156: 467-478.
Chiarini FE, Moreno NC, Barboza GE, Bernardello G. 2010. Karyotype characterization of Andean Solanoideae (Solanaceae). – Caryologia 63: 278-291.
Chiarini FE, Santiñaque FF, Urdampilleta JD, Las Peñas ML. 2014. Genome size and karyotype diversity in Solanum sect. Acanthophora (Solanaceae). – Plant Syst. Evol. 300: 113-125.
Chiarini FE, Moreno NC, Moré M, Barboza GE. 2017. Chromosomal changes and recent diversification in the Andean genus Jaborosa (Solanaceae). – Bot. J. Linn. Soc. 183: 57-74.
Child A. 1983. Taxonomic studies in Solanum L. – Feddes Repert. 94: 35-42.
Child A. 1984a. Taxonomic studies in Solanum L.: two new infrageneric taxa for the genus Solanum. – Feddes Repert. 95: 141-150.
Child A. 1984b. Studies in Solanum (and related genera) 3. A provisional conspectus of the genus Cyphomandra Mart. ex Sendt. – Feddes Repert. 95: 283-298.
Child A. 1986. Taxonomic studies in Solanum L. and related genera 4. Cyphomandra casana Child sp. nov. and Solanum sect Glaucophyllum Child sect. nov. – Feddes Repert. 97: 143-146.
Child A. 1990. A synopsis of Solanum subgenus Potatoe (G. Don) D’Arcy. – Feddes Repert. 101: 209-235.
Child A. 1998. Studies in Solanum and related genera 6. New infrageneric taxa for the genus Solanum L. (Solanaceae). – Feddes Repert. 109: 407-427.
Child A, Lester RN. 1991. Life form and branching within the Solanaceae. – In: Hawkes JG, Lester RN, Nee M, Estrada-Ramos N (eds), Solanaceae III: Taxonomy, chemistry, evolution, Royal Botanic Gardens, Kew, pp. 151-160.
Christen P, Kapetanidis I. 1988. Isolation and characterization of a withanolide from the leaves of Lycium halimifolium Miller (Solanaceae). – Pharm. Acta Helv. 63: 263-265.
Cirigliano AM, Veleiro AS, Bonetto GM, Oberti JC et al. 1996. Spiranoid withanolides from Jaborosa runcinata and Jaborosa araucana. – J. Nat. Prod. 59: 717-721.
Clark JL, Nee M, Bohs L, Knapp S. 2015. A revision of Solanum Section Aculeigerum (the Solanum wendlandii Group, Solanaceae). – Syst. Bot. 40: 1102-1136.
Clarkson JJ, Knapp S, Garcia VF, Olmstead RG, Leitch AR, Chase MW. 2004. Phylogenetic relationships in Nicotiana (Solanaceae) inferred from multiple plastid DNA regions. – Mol. Phylogen. Evol. 33: 75-90.
Clausen AM, Spooner DM. 1998. Molecular support for the hybrid origin of the wild potato species Solanum x rechei (Solanum sect. Petota). – Crop Science 38: 858-865.
Cochran HL. 1938. A morphological study of flower and seed development in pepper. – J. Agric. Res. 56: 395-419.
Cocucci AA. 1989a. El mecanismo floral de Schizanthus (Solanaceae). – Kurtziana 20: 113-132.
Cocucci AA. 1989b. Sobre el diagrama floral de Schizanthus (Solanaceae) y su interpretación. – Kurtziana 20: 133-137.
Cocucci AA. 1991. Pollination biology of Nierembergia (Solanaceae). – Plant Syst. Evol. 174: 17-35.
Cocucci AA. 1995. Floral mechanisms in the tribe Salpiglossideae (Solanaceae). – Plant Syst. Evol. 194: 207-230.
Cocucci AA. 1996. El osmóforo de Cyphomandra (Solanaceae). Estudio con microscopio electrónico de barrido. – Darwiniana 34: 145-150.
Cocucci AA. 1999. Evolutionary radiation in neotropical Solanaceae. – In: Nee M, Symon D, Lester RN, Jessop J (eds), Solanaceae IV, Royal Botanic Gardens, Kew, pp. 9-22.
Cocucci AA, Hunziker AT. 1993. Estudios sobre Solanaceae XXXV. Novedades en Nierembergia. – Lorentzia 7: 5-15.
Comes O. 1899. Monographie du genre Nicotiana. – Naples.
Constance L. 1963. Chromosome numbers and classification in Hydrophyllaceae. – Brittonia 15: 273-285.
Contreras-M, A, Spooner DM. 1999. Revision of Solanum section Etuberosum (subgenus Potatoe). – In: Nee M, Symon D, Lester RN, Jessop J (eds), Solanaceae IV, Royal Botanic Gardens, Kew, pp. 227-245.
Cooper DC. 1927. Anatomy and development of tomato flower. – Bot. Gaz. 83: 399-411.
Cooper DC. 1931. Macrosporogenesis and the development of the macrogametophyte of Lycopersicon esculentum. – Amer. J. Bot. 18: 739-748.
Correll DS. 1952. Section Tuberarium of the genus Solanum of North America and Central America. – Agricult. Monogr. 11, U.S. Dept. Agric., Washington, D.C.
Correll DS. 1962. The potato and its wild relatives. – Contr. Texas Res. Found., Bot. Stud. 4: 1-606.
Cosa de Gastiazoro MT. 1991. Estudio morfo-anatómico de órganos vegetativos en Cestroideae (Solanaceae) I. Tribu Nicotianeae. – Kurtziana 21: 111-152.
Cosa de Gastiazoro MT. 1993. Estudio morfo-anatómico de órganos vegetativos en Cestroideae (Solanaceae) II. Tribu Salpiglosdideae. – Kurtziana 22: 47-72.
Cosa de Gastiazoro MT. 1994. Estudio morfo-anatómico de órganos vegetativos en Cestroideae (Solanaceae) III. Tribu Schwenckieae. – Kurtziana 23: 9-25.
Cosa de Gastiazoro MT. 1997. Anatomía de órganos vegetativos en Heteranthia y su relación con los géneros de Schwenckieae (Solanaceae). – Kurtziana 25: 115-122.
Cosa de Gastiazoro MT, Bruno G, Dottori N. 1998. Anatomía de los órganos vegetativos en Solanum juvenale y su comparación con S. elaeagnifolium (Solanaceae). – Anal. Inst. Biol. Univ. Nac. Autón. Mexico, Ser. Bot. 69: 9-22.
Costea M, Stefanović S. 2009a. Molecular phylogeny of the Cuscuta californica complex (Convolvulaceae) and a new species from New Mexico and Trans-Pecos. – Syst. Bot. 34: 570-579.
Costea M, Stefanović S. 2009b. Cuscuta jepsonii (Convolvulaceae): an invasive weed or an extinct endemic? – Amer. J. Bot. 96: 1744-1750.
Costea M, Nesom GL, Tardif FJ. 2005. Taxonomic status of Cuscuta nevadensis and C. veatchii (Convolvulaceae) in North America. – Brittonia 57: 264-272.
Costea M, Wright MAR, Stefanović S. 2009. Untangling the systematics of salt marsh dodders: Cuscuta pacifica a new segregate species from Cuscuta salina (Convolvulaceae). – Syst. Bot. 34: 787-795.
Costea M, García MA, Stefanović S. 2015. A phylogenetically based infrageneric classification of the parasitic plant genus Cuscuta (dodders, Convolvulaceae). – Syst. Bot. 40: 269-285.
Coulson JF, Griffin WJ. 1967. The alkaloids of Duboisia myoporoides I. Aerial parts. – Planta Medica 15: 459-466.
Creté P. 1959. Embryogénie du Nicandra physaloides (Solanacées). – Phytomorphology 9: 163-167.
Cuatrecasas J. 1958a. Notes on American Solanaceae. – Feddes Repert. 61: 74-86.
Cuatrecasas J. 1958b. The Colombian species of Juanulloa. – Brittonia 10: 146-150.
Cuatrecasas J. 1959. New chiropterophilous Solanaceae from Colombia. – J. Washington Acad. Sci. 49: 269-272.
Czaja AT. 1963. Neue Untersuchungen an der Testa der Tomatensamen. – Planta 59: 262-279.
Dahlgren RMT, Jensen SR, Nielsen BJ. 1977. Seedling morphology and iridoid occurrence in Montinia caryophyllacea (Montiniaceae). – Bot. Not. 130: 329-332.
Dammer U. 1913. Solanaceae africanae II. – Engl. Bot. Jahrb. Syst. 48: 224-260.
Danert S. 1955. Ein Beitrag zur kenntnis der Gattung Datura L. – Feddes Repert. 57: 231-242.
Danert S. 1958. Die Verzweigung der Solanaceen im reproduktiven Bereich. – Abh. Deutsche Akad. Wiss. Berlin, Math.-Naturwiss. Kl. 1957, 6: 1-183.
Danert S. 1961. Zur Systematik von Nicotiana tabacum. – Kulturpflanze 9: 287-363.
Danert S. 1967. Die Verzweigung als infragenerisches Gruppenmerkmal in der Gattung Solanum L. – Kulturpflanze 15: 275-292.
Danert S. 1970. Infragenerische Taxa der Gattung Solanum L. – Kulturpflanze 18: 253-297.
D’Arcy WG. 1973. Solanaceae. Flora of Panama. – Ann. Missouri Bot. Gard. 60: 573-780.
D’Arcy WG. 1976 [1977]. New names and taxa in the Solanaceae. – Ann. Missouri Bot. Gard. 63: 363-369.
D’Arcy WG. 1978. A preliminary synopsis of Salpiglossis and other Cestreae (Solanaceae). – Ann. Missouri Bot. Gard. 65: 698-724.
D’Arcy WG. 1979. The classification of the Solanaceae. – In: Hawkes JG, Lester RN, Skelding AD (eds), The biology and taxonomy of the Solanaceae, Linn. Soc. Symposium, No. 7, Academic Press, London, pp. 3-48.
D’Arcy WG. 1986a. The calyx in Lycianthes and some other genera. – Ann. Missouri Bot. Gard. 73: 117-127.
D’Arcy WG. 1986b. The genera of Solanaceae and their types. – Solanaceae Newsletter 2: 10-33.
D’Arcy WG (ed). 1986c. Solanaceae: biology and systematics. – Columbia University Press, New York.
D’Arcy WG. 1987. The circumscription of Lycopersicon. – Solanaceae Newsletter 2: 60-61.
D’Arcy WG. 1991. The Solanaceae since 1976, with a review of its biogeography. – In: Hawkes JG, Lester RN, Nee M, Estrada-Ramos N (eds), Solanaceae III: taxonomy, chemistry and evolution, Royal Botanic Gardens, Kew, pp. 75-137.
D’Arcy WG. 1992. Solanaceae of Madagascar: form and geography. – Ann. Missouri Bot. Gard. 79: 29-45.
D’Arcy WG, Averett J. 1996. Recognition of tribes Capsiceae and Physaleae, subfamily Solanoideae, Solanaceae. – Phytologia 80: 273-275.
D’Arcy WG, Benitez de Rojas C. 1991. Biogeographical mapping: the Schwenckieae example. – In: Hawkes JG, Lester RN, Nee M, Estrada N (eds), Solanaceae III: taxonomy, chemistry, evolution, Royal Botanic Gardens, Kew, pp. 169-179.
D’Arcy WG, Keating RC. 1973. The affinities of Lithophyton: a transfer from Solanaceae to Verbenaceae. – Brittonia 25: 213-225.
D’Arcy WG, Zhang Z-Y. 1992. Notes on the Solanaceae of China and neighboring areas. – Novon 2: 124-128.
D’Arcy WG, Gentry JL, Averett JE. 1981. Recognition of Brachistus (Solanaceae). – Ann. Missouri Bot. Gard. 68: 226-227.
D’Arcy WG, Mione T, Davis IV T. 1992. Jaltomata grandiflora (Solanaceae): a rare Mexican species. – Novon 2: 190-192.
Datta S. 1933. Embryological and cytological studies in Nolana atriplicifolia and Nolana prostrata. – J. Indian Bot. Soc. 12: 131-152.
Daunay M-C, Janick J, Laterrot H. 2007. Iconography of the Solanaceae from antiquity to the XVIIth century: a rich source of information on genetic diversity and uses. – In: Spooner DM, Bohs L, Giovannoni J, Olmstead RG, Shibata D (eds), Solanaceae VI: genomics meets biodiversity. Proceedings of the sixth international Solanaceae conference, Acta Horticulturae 745, International Society for Horticultural Science, Leuven, pp. 59-88.
Dave YS, Patel ND, Rao KS. 1980. Origin, development and structure of spiny projections on the pericarp of Datura innoxia Mill. – Feddes Repert. 91: 89-93.
Davenport LJ. 1988. A monograph of Hydrolea (Hydrophyllaceae). – Rhodora 90: 169-208.
Davis IV T. 1980. The generic relationship of Saracha and Jaltomata (Solanaceae). – Rhodora 82: 345-352.
Dawson JH. 1987. Cuscuta (Convolvulaceae) and its control. – In: Weber HC, Forstreuter W (eds), Parasitic flowering plants, Philipps-Universität, Marburg, pp. 137-149.
Dawson JH, Musselman LJ, Wolswinkel P, Dörr I. 1994. Biology and control of Cuscuta. – Rev. Weed Sci. 6: 265-317.
Dean EA. 1994. Lycianthes starbuckii and Lycianthes rzedowskii (Solanaceae). Two new species of perennial herbs from Mexico. – Novon 4: 324-329.
Dean EA. 1998. Lycianthes jalicensis (Solanaceae), a new species from Jalisco, Mexico. – Novon 8: 133-136.
Dean EA. 2004. A taxonomic revision of Lycianthes series Meizonodontae (Solanaceae). – Bot. J. Linn. Soc. 145: 385-424.
Deanna R, Barboza GE, Scaldaferro MA. 2014. First karyological report in Larnax and Deprea. – Aust. J. Bot. 62: 251-261.
Deanna R, Leiva González S, Barboza GE. 2014. Four new species and eighteen lectotypifications of Larnax from Ecuador and Peru and a new synonym of Deprea orinocensis (Solanaceae: Solanoideae, Physalideae). – Phytotaxa 167: 1-34.
Deanna R, Leiva González S, Barboza GE. 2015. Changes in the circumscription of Deprea (Physalideae, Solanaceae): thirty two new combinations. – PhytoKeys 46: 73-87.
Deanna R, González SL, Barboza GE. 2016. A key and three new species for the re-circumscribed genus Deprea (Solanaceae). – Syst. Bot. 41: 1028-1041.
Deanna R, Barboza GE, García CC. 2018. Phylogenetic relationships of Deprea: new insights into the evolutionary history of physaloid groups. – Mol. Phylogen. Evol. 119: 71-80.
Deb DB. 1979. Solanaceae in India. – In: Hawkes, Lester, Skelding (eds), The biology and taxonomy of the Solanaceae, Linn. Soc. Symp. Ser. 7, Linnean Society and Academic Press, London, pp. 87-112.
De la Fuente G, Reina M, Muñoz O, San Martín A et al 1988. Tropane alkaloids from Schizanthus pinnatus. – Heterocycles 27: 1887-1897.
Del Vitto LA, Petenatti EM. 1999. Notas en Solanum (Solanaceae) de Argentina II. – Kurtziana 27: 319-326.
Demissew S. 1995. The genus Nephrophyllum (Convolvulaceae, tribe Dichondreae) in Ethiopia. – Kew Bull. 50: 103-108.
Demissew S. 1996a. The genus Cladostigma (Convolvulaceae). – Kew Bull. 51: 405-411.
Demissew S. 1996b. The genus Hildebrandtia (Convolvulaceae) from mainland Africa and Arabia. – Kew Bull. 51: 525-541.
Demissew S. 1999. A synopsis of the genus Convolvulus (Convolvulaceae) in Ethiopia and Eritrea. – Kew Bull. 54: 63-79.
Demissew S. 2001. A synopsis of the genus Merremia (Convolvulaceae) in the Flora of Ethiopia and Eritrea. – Kew Bull. 56: 931-943.
Demissew S, Austin DF. 1995. The genus Nephrophyllum (Convolvulaceae, tribe Dichondreae) in Ethiopia. – Kew Bull. 50: 103-108.
Demissew S, Austin DF. 1996. Generic delimitation and relationships in the tribe Hildebrandtieae (Convolvulaceae). – In: Maesen LJG van der, Burgt XM van der, Medenbach de Rooy JM (eds), The biodiversity of African plants, Kluwer Academic Publ., Dordrecht, The Netherlands, pp. 409-420.
Demissew S, Mill RR. 2009. Revision of the genus Seddera (Convolvulaceae). – Kew Bull. 64: 197-233.
Deroin T. 1992. Espèces nouvelles de Convolvulaceae du Sud de Madagascar. – Bull. Mus. Natl. Hist. Nat. Paris, sect. B, Adansonia 14: 335-346.
Deroin T. 1992 [1993]. Anatomie florale de Humbertia madagascariensis Lam. Contribution à la morphologie comparée de la fleur et du fruit des Convolvulaceae. – Bull. Mus. Natl. Hist. Nat. Paris, sér IV, sect. B., Adansonia 2: 235-255.
Deroin T. 1996. Deux espèces malgaches nouvelles du genre Hildebrandtia Vatke (Convolvulaceae). – Candollea 51: 147-155.
Deroin T. 1997. Additions au genre Metaporana N. E. Br. (Convolvulaceae) à Madagascar. – Bull. Jard. Bot. Belg. 66: 31-38.
Deroin T. 1999. Ontogeny and phylogeny in Convolvulaceae-Ipomoeeae: preliminary comparative remarks on ovary morphology. – Syst. Geogr. Plants 68: 225-232.
Deroin T. 2002. Anatomie florale de Maripa (Convolvulaceae-Erycibeae). – Adansonia, sér. III, 24: 93-106.
Deroin T. 2004. Anatomie florale comparée de Cardiochlamys et Cordisepalum. Illustration de l’hétérobathmie des Convolvulaceae-Cardiochlamydeae. – Adansonia, sér. III, 26: 199-212.
Deroin T, Demissew S. 2009. The genus Seddera Hochst. (Convolvulaceae) in Madagascar. – Adansonia, sér. III, 31: 207-214.
Desai SR.1970. Chromosome numbers and morphology in three Solanaceae. – Phyton 27: 199-200.
Deshpande BD, Singh A. 1967. Seedling and floral anatomy of Withania somnifera Dunal. – J. Indian Bot. Soc. 46: 76-81.
Dewender K van, Jenkins PD. 1993. A new species of Browallia (Solanaceae) from the southwestern United States and northwestern Mexico. – Madroño 40: 24-223.
Dewolf GP. 1955. Notes on cultivated Solanaceae 1. Solandra. – Baileya 3: 172-175.
Dewolf GP. 1956. Notes on cultivated Solanaceae 2. Datura. – Baileya 4: 13-23.
Dharamadhaj P, Prakash N. 1978. Development of the anther and ovule in Capsicum L. – Aust. J. Bot. 26: 433-439.
Diez MJ, Ferguson IK. 1984. Pollen morphology of Mandragora autumnalis Bertol. (Solanaceae). – Pollen Spores 26: 151-160.
Di Fulvio TE. 1961. El género Sclerophylax (Solanaceae): estudio anatómico, embriológico, y caryológico con especial referencia a la taxonomica. – Kurtziana 1: 9-103.
Di Fulvio TE. 1969. Embriología de Nolana paradoxa (Nolanaceae). – Kurtziana 5: 39-54.
Di Fulvio TE. 1971. Morfología floral de Nolana paradoxa (Nolanaceae) con especial referencia a la organización del gineceo. – Kurtziana 6: 41-51.
Di Fulvio TE. 1976a. Sobre el polen de Nierembergia. – Kurtziana 9: 87-91.
Di Fulvio TE. 1976b. Recuentos cromosómicos en Nierembergia (Solanaceae). – Kurtziana 9: 141-142.
Di Fulvio TE. 1983. Los ‘tipos’ de endosperma y de haustorios endospérmicos. Su clasificación. – Kurtziana 16: 7-31.
Di Fulvio TE. 1984. Poliploidía en Nierembergia (Solanaceae). – Kurtziana 17: 25-30.
Di Fulvio TE. 1987. La endospermogenesis en Hydrophylleae (Hydrophyllaceae) con relación a la taxonomia. – Kurtziana 19: 13-34.
Di Fulvio TE. 1989. Observaciones embriológicas en especies Argentinas de Hydrolea (Hydrophyllaceae) con especial referencia a la endospermogénesis. – Kurtziana 20: 33-64.
Di Fulvio TE. 1990. Endospermogenesis y taxonomía de la familia Hydrophyllaceae y su relación con las demas Gamopetalas. – Acad. Nac. Ci. Exact. Físic. Nat. Buenos Aires 5: 73-82.
Di Fulvio TE. 1991. Inclusiones nucleares en Hydrophyllaceae. – Kurtziana 21: 9-21.
Di Fulvio TE. 1999. Morfología y vascularización floral en Hydroleae e Hydrophylleae (Hydrophyllaceae). – Kurtziana 25: 7-34.
Diggle PK. 1994. The expression of andromonoecy in Solanum hirtum (Solanaceae): phenotypic plasticity and ontogenetic contingency. – Amer. J. Bot. 81: 1354-1365.
Dillon MO, Tu T, Soejima A, Yi T, Nie Z, Tye A, Wen J. 2007. Phylogeny of Nolana (Nolaneae, Solanoideae, Solanaceae) as inferred from granule-bound starch synthase I (GBSSI) sequences. – Taxon 56: 1000-1011.
Dillon MO, Tu T, Xie L, Quipuscoa Silvestre V, Wen J. 2009. Biogeographic diversification in Nolana (Solanaceae), a ubiquitous member of the Atacama and Peruvian Deserts along the western coast of South America. – J. Syst. Evol. 47: 457-476.
Dodds KS. 1962. Classification of cultivated potatoes. – In: Correll DS (ed), The potato and its wild relatives, Contr. Texas Res. Found., Bot. Stud. 4, Remer, Texas, pp. 516-539.
Doerk-Schmitz K, Witte L, Alfermann AW. 1994. Tropane alkaloid patterns in plants and hairy roots of Hyoscyamus albus. – Phytochemistry 35: 107-110.
Doncheva T, Berkov S, Philipov S. 2006. Comparative study of the alkaloids in tribe Datureae and their chemosystematic significance. – Biochem. Syst. Ecol. 34: 478-488.
Dorofeev PI. 1963. Treticnye Flory Zapadnoj Sibiri. – Akademia Nauk SSSR, Moscow and Leningrad. [In Russian]
Dörr I. 1987. The haustorium of Cuscuta – new structural results. – In: Weber HC, Forstreuter W (eds), Parasitic flowering plants, Philipps-Universität, Marburg, pp. 163-170.
Dottori N. 1995. Desarrollo y estructura de fruto y semilla en Solanum Sect. Cyphomandropsis (Solanaceae) de Argentina. – Kurtziana 24: 83-104.
Dottori N. 1998. Anatomía y ontogenía de fruto y semilla de Solanum juvenale (Solanaceae). – Kurtziana 26: 13-22.
Dottori N, Cosa MT. 1999. Anatomía y ontogenia de fruto y semilla en Solanum hieronymi (Solanaceae). – Kurtziana 27: 293-302.
Dottori N, Bruno G, Cosa MT. 2000. Anatomía comparada de las plántulas de Solanum elaeagnifolium y S. juvenale (Solanaceae). – Bol. Soc. Argent. Bot. 35: 115-123.
Dulberger R, Levy A, Palevitch D. 1981. Andromonoecy in Solanum marginatum. – Bot. Gaz. 142: 259-266.
Dunbar A. 1975. On pollen of Campanulaceae and related families with special reference to the surface ultrastructure II. Campanulaceae subfam. Cyphioideae and subfam. Lobelioideae; Goodeniaceae; Sphenocleaceae. – Bot. Not. 128: 102-118.
Dupin J, Smith SD. 2018. Phylogenetics of Datureae (Solanaceae), including description of the new genus Trompettia and re-circumscription of the tribe. – Taxon 67: 359-375.
Dupraz JM, Christen P, Kapetanidis I. 1994. Tropane alkaloids in transformed roots of Datura quercifolia. – Planta Medica 60: 158-162.
Durbin ML, Learn GH, Huttley GA, Clegg MT. 1995. Evolution of the chalcone synthase gene family in the genus Ipomoea. – Proc. Natl. Acad. Sci. U.S.A. 92: 3338-3342.
Dyki BL, Jankiewicz S, Staniaszek M. 1998. Anatomical structure and surface micromorphology of tomatillo leaf and flower [Physalis ixocarpa Brot. (Solanaceae)]. – Acta Soc. Bot. Poloniae 67: 181-191.
Edmonds JM. 1972. A synopsis of the taxonomy of Solanum Sect. Solanum (Maurella) in South America. – Kew Bull. 27: 95-114.
Edmonds JM. 1977. Taxonomic studies on Solanum section Solanum (Maurella). – Bot. J. Linn. Soc. 75: 141-178.
Edmonds JM. 1978. Numerical taxonomic studies on Solanum L. section Solanum (Maurella). – Bot. J. Linn. Soc. 76: 27-51.
Edmonds JM. 1984. Pollen morphology of Solanum L. section Solanum. – Bot. J. Linn. Soc. 88: 237-351.
Eich E. 2008. Solanaceae and Convolvulaceae: secondary metabolites. – Springer, Berlin.
El Imam YMA, Evans WC. 1984. Tropane alkaloids of species of Anthocercis, Cyphanthera and Crenidium. – Planta Medica 50: 86-87.
Engelmann G. 1895. Systematic arrangement of the species of the genus Cuscuta with critical remarks on old species and descriptions of new ones. – Trans. Acad. Sci., St. Louis 1: 453-523.
Engler A. 1897. Montiniaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(2a), Nachträge zu III(2a), W. Engelmann, Leipzig, p. 181.
Engler A. 1930. Saxifragaceae. – In: Engler A, Harms H (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 18a, W. Engelmann, Leipzig, pp. 74-226.
Erazo Guiffra S, Galeffi C, Ciasca Rendina A, Delle Monache EM. 1971. Nuovo glucosido flavonico della Vestia lycioides. – Ann. Inst. Super. Sanita 7: 23-30.
Erbar C. 1995. On the floral development of Sphenoclea zeylanica (Sphenocleaceae, Campanulales) – SEM-investiations on herbarium material. – Bot. Jahrb. Syst. 117: 469-483.
Erbar C, Porembski S, Leins P. 2005. Contributions to the systematic position of Hydrolea (Hydroleaceae) based on floral development. – Plant Syst. Evol. 252: 71-83.
Eserman LA, Tiley GP, Jarret RL, Leebens-Mack
JH, Miller RE. 2014. Phylogenetics and diversification of morning glories
(tribe Ipomoeeae, Convolvulaceae)
based on whole plastome sequences. – Amer. J. Bot. 101: 92-103.
Eshbaugh WH. 1970. A biosystematic and evolutionary study of Capsicum baccatum (Solanaceae). – Brittonia 22: 31-43.
Eshbaugh WH. 1980. The taxonomy of the genus Capsicum (Solanaceae). – Phytologia 47: 153-166.
Eshbaugh WH, Smith PG, Nickrent D. 1983. Capsicum tovari (Solanaceae) a new species of pepper from Peru. – Brittonia 35: 55-60.
Estrada E, Martínez M. 1998. Physalis (Solanaceae) and allied genera: Tzeltalia, a new genus from the highlands of southern Mexico and northwestern Guatemala. – Brittonia 50: 285-295.
Estrada E, Martínez M. 1999. Physalis L. (Solanoideae: Solaneae) and allied genera I. A morphology-based cladistic analysis. – In: Nee M, Symon DE, Lester RN, Jessop JP (eds), Solanaceae IV: advances in biology and utilization, Royal Botanic Gardens, Kew, pp. 139-159.
Evans WC. 1973. Distribution of alkaloids in Anthocercis littorea and A. viscosa. – Phytochemistry 12: 2505-2507.
Evans WC. 1979. Tropane alkaloids of the Solanaceae. – In: Hawkes JG, Lester RN, Skelding AD (eds), The biology and taxonomy of the Solanaceae, Ser. 7, Academic Press, London, pp. 241-254.
Evans WC. 1983. Alkaloids of the Solanaceae tribe Anthocercideae. – Phytochemistry 22: 2219-2225.
Evans WC, Griffin WJ. 1963. The alkaloids of the genus Datura, section Brugmansia II. New monotigloyl esters of the leaves of D. cornigera Hook. – J. Chem. Soc. 1963: 4348-4363.
Evans WC, Lampard JF. 1972. Alkaloids of Datura suaveolens. – Phytochemistry 11: 3293-3298.
Evans WC, Major VA. 1966. The alkaloids of the genus Datura, section Brugmansia IV. New alkaloids of D. sanguinea R. and P. – J. Chem. Soc. 1966: 1621-1623.
Evans WC, Major VA. 1968. The alkaloids of the genus Datura, section Brugmansia V. Alkaloids of D. sanguinea R. and P. and related esters of tropane-3α,6β,7β-triol. – J. Chem. Soc. (C): 2775-2779.
Evans WC, Ramsey KPA. 1981. Tropane alkaloids from Anthocercis and Anthotroche. – Phytochemistry 20: 497-499.
Evans WC, Somanabandhu A. 1974. Cuscohygrine, a constituent of the roots of some Convolvulaceae. – Phytochemistry 13: 519-520.
Evans WC, Somanabandhu A. 1980. Nitrogen-containing non-steroidal secondary metablites of Solanum, Cyphomandra, Lycianthes and Margaranthus. – Phytochemistry 19: 2351-2356.
Evans WC, Than MP. 1962. The alkaloids of the genus Datura, section Brugmansia I. D. cornigera Hook. – J. Pharm. Pharmacol. 14: 147-156.
Evans WC, Treagust PG. 1973. Distribution of alkaloids in Anthocercis littorea and A. viscosa. – Phytochemistry 12: 2505-2508.
Evans WC, Woolley JG. 1965. The alkaloids of Datura meteloides D. C. – J. Chem. Soc. 1965: 4936-4939.
Evans WC, Woolley VA. 1978. 3-α-Tigloyloxynortropan-6β-ol. A new alkaloid from Datura. – Phytochemistry 17: 171.
Evans WC, Ghani A, Wooley VA. 1972a. Alkaloids of Salpichroa origanifolia. – Phytochemistry 11: 469.
Evans WC, Ghani A, Wooley VA. 1972b. Alkaloids of Solandra species. – Phytochemistry 11: 470-472.
Evans WC, Ghani A, Wooley VA. 1972c. Alkaloids of Cyphomandra betacea Sendt. – J. Chem. Soc., Perkin Transact. I: 2017-2019.
Evans WC, Ghani A, Treagust PG. 1974. Metabolism of hyoscine and hyosciamine in the Solanaceae. – J. Pharm. Pharmacol. 26 [Suppl.]: 112.
Evans WC, Ghani A, Woolley VA. 1972a. Alkaloids of Salpichroa origanifolia. – Phytochemistry 11: 469.
Evans WC, Ghani A, Woolley VA. 1972b. Alkaloids in Solandra species. – Phytochemistry 11: 470-472.
Evans WC, Ghani A, Woolley VA. 1972c. Alkaloids of Cyphomandra betacea. – J. Chem. Soc. Perkin Trans. I: 2017-2019.
Fagherazzi Abgrall M, Meyer J. 1963. Développement de l’albumen avec differentiation d’un tissue chalazien d’aspect haustorial chez deux Schizanthus. – Phytomorphology 13: 22-29.
Fægri K. 1986. The solenoid flower. – In: Alexander IJ, Gregory NM (eds), Bot. Soc. Edinburgh 150th Anniversary Supple, Transactions, Edinburgh, pp. 51-59.
Faini F, Castillo M, Torres R. 1978. A new β-carboline alkaloid from Vestia lycioides. – Phytochemistry 17: 338.
Faini F, Torres R, Castillo M. 1984. (25 R)-isonuatigenin, an unusual steroidal sapogenin from Vestia lycioides. – Phytochemistry 23: 1301-1303.
Fajardo D, Castillo R, Salas A, Spooner DM. 2008. A morphometric study of species boundaries of the wild potato Solanum series Conicibaccata: a replicated field trial in Anean Peru. – Syst. Bot. 33: 183-192.
Fajardo V, Podesta F, Shamma M, Freyer AJ. 1991. New withanolides from Jaborosa magellanica. – J. Natur. Prod. 54: 554-563.
Farooqui SM, Bahadur B. 1985. Studies on seed morphology (L. M. & SEM) of American Nicotiana L. (Solanaceae). – Indian J. Bot. 8: 191-197.
Fay MF, Olmstead RG, Richardson JE, Santiago E, Prance GT, Chase MW. 1998. Molecular data support the inclusion of Duckeodendron cestroides in Solanaceae. – Kew Bull. 53: 203-212.
Fay MF, Thomas VE, Knapp S. 2007. Mellissia begoniifolia (Solanaceae). – Curtis’s Bot. Mag. 24: 243-250.
Fedde F. 1896. Beiträge zur vergleichende Anatomie der Solanaceae. – Breslau.
Feinbrun N. 1970. A taxonomic review of European Cuscutae. – Israel J. Bot. 19: 16-29.
Ferguson MC, Coolidge EB. 1932. A cytological and genetical study of Petunia IV. Pollen grains and the method of studying them. – Amer. J. Bot. 19: 644-658.
Ferguson MC, Ottley AM. 1932. Studies on Petunia III. A redescription and additional discussion of certain species of Petunia. – Amer. J. Bot. 19: 385-405.
Fernandes RS. 1969. Os gêneros Nicandra, Physalis e Withania no ultramar português. – Garcia de Orta (Lisboa) 17: 275-278.
Fernandez-Hilario R, Smith SD. 2017. A new species of Saracha (Solanaceae) from the Central Andes of Peru. – PhytoKeys 85: 31-43.
Ferreyra R. 1961. Revisión de las especies peruanas del género Nolana (Nolanacee). – Mem. Mus. Hist. Nat. Javier Prado 12: 1-70.
Filipowicz N, Renner SS. 2012. Brunfelsia (Solanaceae): a genus evenly divided between South America and radiations on Cuba and other Antillean islands. – Mol. Phylogen. Evol. 64: 1-11.
Filippa EM. 1992. Estructura y desarrollo de fruto y semilla en especies de Athenaea, Aureliana y Capsicum (Solaneae, Solanaceae). – Darwiniana 31: 137-150.
Filippa EM, Bernardello LM. 1987. Sobre la anatomía de órganos vegetativos y el análisis de la variabilidad en Phrodus (Solanaceae). – Kurtziana 19: 57-67.
Finn VV. 1937. Vergleichende Embryologie und Karyologie einiger Cuscuta-Arten. – Žurn. Inst. Bot. Vseukrainsk. Akad. Nauk 12: 83-99. [In Ukrainian with German and Russian summaries]
Fosberg FR. 1959. Nomenclatural notes on Datura L. – Taxon 8: 52-57.
Fosberg FR, Sachet M-H. 1977. Flora of Micronesia 3: Convolvulaceae. – Smithsonian Contr. Bot. 36: 1-34.
Francey P. 1933. Beitrag zur Kenntnis der Gattung Sessea. – Notizbl. Bot. Gart. Mus. Berlin 11: 879-883.
Francey P. 1934. Übersicht über die Gattung Sessea. – Notizbl. Bot. Gart. Mus. Berlin 11: 978-990.
Francey P. 1935-1936. Monographie du genre Cestrum L. – Candollea 6: 46-398.
Francey P. 1936. Monographie du genre Cestrum L., partie II. – Candollea 7: 1-132.
Francisco-Ortega J, Hawkes JG, Lester RN, Acebes-Genovés JR. 1993. Normania, an endemic Makaronesian genus distinct from Solanum. – Plant Syst. Evol. 185: 189-205.
Fregonezi JN, de Freitas LB, Bonatto SL, Semir J, Stehmann JR. 2012. Infrageneric classification of Calibrachoa (Solanaceae) based on morphological and molecular evidence. – Taxon 61: 120-130.
Fregonezi JN, Turchetto C, Bonatto SL, Freitas LB. 2013. Biogeographical history and diversification of Petunia and Calibrachoa (Solanaceae) in the Neotropical Pampas grassland. – Bot. J. Linn. Soc. 171: 140-153.
Freire de Carvalho L d’A. 1966.O gênero Melananthus no Brasil (Solanaceae). – Sellowia 18: 51-66.
Freire de Carvalho L d’A. 1966b. O gênero Protoschwenckia no Brasil (Solanaceae). – Sellowia 18: 67-72.
Freire de Carvalho L d’A. 1978. O gênero Schwenckia D. van Rooyejn ex Linnaeus no Brasil (Solanaceae). – Rodriguésia 44: 307-524.
Freire de Carvalho L d’A. 1986. The genus Metternichia in Brazil. – In: D’Arcy WG (ed), Solanaceae. Biology and systematics, Columbia University Press,New York, pp. 5-14.
Freire de Carvalho LF, Machado RD, Bovni MG. 1999. Seed coat micromorphology of Brazilian species of Schwenckia. –In: Nee M, Symon D, Lester RN, Jessop J (eds), Solanaceae IV, Royal Botanic Gardens, Kew, pp. 23-31.
Fries RE. 1911. Die Arten der Gattung Petunia. – Kungl. Sv. Vetensk.-Akad. Handl. 46(5): 3-72.
Freyer R, Neckermann K, Maier RM, Kössel H. 1995. Structural and functional analysis of plastid genomes from parasitic plants: loss of an intron within the genus Cuscuta. – Curr. Genet. 27: 580-586.
Fuentes VR. 1980. Solanáceas de Cuba I. Datura L. – Rev. Jard. Bot. Nac. 1: 61-81.
Fuentes VR. 1982. El género Espadaea A. Richard. – Rev. Jard. Bot. Nac. 3: 51-67.
Fuentes VR. 1983. Solanáceas de Cuba III. Margaranthus Schltdl. – Rev. Jard. Bot. Nac. 4: 51-57.
Fuentes VR. 1989. Solanáceas de Cuba V. Acnistus Schott. – Rev. Jard. Bot. Nac 10: 99-101.
Fuentes VR, Rodriguez N. 1984. Estudios en el género Henoonia Griseb. I. Morfología y biometrica de las hojas. – Rev. Jard. Bot. Nac. 5: 29-40.
Fukuda T, Yokoyama J, Ohashi H. 2001. Phylogeny and biogeography of the genus Lycium (Solanaceae): inferences from chloroplast DNA sequences. – Mol. Phylogen. Evol. 19: 246-258.
Funayama S, Zhang G-R, Nozoe S. 1995. Kukoamine B, a spermine alkaloid from Lycium chinense. – Phytochemistry 38: 1529-1531.
Galetto L. 1991. Sobre el nectar y los nectarios de algunas especies de Nicotiana (Solanaceae). – Kurtziana 21: 165-176.
Gambaro B, Labée C, Castillo M. 1983. Angeloyl, tigloyl and senecioyloxytropane alkalods from Schizanthus hookeri. – Phytochemistry 22: 1838-1839.
García CC, Matesevach M, Barboza G. 2008. Features related to anther opening in Solanum species (Solanaceae). – Bot. J. Linn. Soc. 158: 344-354.
García CC, Barfuss MHJ, Sehr EM, Barboza GE. 2016. Phylogenetic relationships, diversification and expansion of chili peppers (Capsicum, Solanaceae). – Ann. Bot. 118: 35-51.
García CC, Basso AV, González SL, Gonzáles P, Barboza GE. 2018. Unraveling the phylogenetic relationships of Nectouxia (Solanaceae): its position relative to Salpichroa. – Plant Syst. Evol. 304: 177-183.
García MA. 2001. A new western Mediterranean species of Cuscuta (Convolvulaceae) confirms the presence of holocentric chromosomes in subgenus Cuscuta. – Bot. J. Linn. Soc. 135: 169-178.
García MA, Martín MP. 2007. Phylogeny of Cuscuta subgenus Cuscuta (Convolvulaceae) based on nrDNA ITS and chloroplast trnI intron sequences. – Syst. Bot. 32: 899-916.
García MA, Costea M, Kuzmina M, Stefanović
S. 2014. Phylogeny, character evolution, and biogeography of Cuscuta
(dodders; Convolvulaceae)
inferred from coding plastid and nuclear sequences. – Amer. J. Bot. 101:
670-690.
García VF, Olmstead RG. 2003. Phylogenetics of tribe Anthocercideae (Solanaceae) based on ndhF and trnL/F sequence data. – Syst. Bot. 28: 609-615.
Gates DJ, Pilson D, Smith SD. 2018. Filtering of target sequence capture individuals facilitates species tree construction in the plant subtribe Iochrominae (Solanaceae). – Mol. Phylogen. Evol. 123: 26-34.
Geeta R, Gharaibeh W. 2007. Historical evidence for a pre-Columbian presence of Datura in the Old world and implications for a first millennium transfer from the New World. – J. Biosci. 32: 1227-1244.
Gemeinholzer B, Wink M. 2001. Solanaceae: occurrence of secondary compounds versus molecular phylogeny. – In: Berg RG van den, Barendse GWM, Weerden GM van der, Mariani C (eds), Solanaceae: advances in taxonomy and utilisation, Nijmegen University Press, Nijmegen, pp. 165-178.
Gentry JL. 1973. Restoration of the genus Jaltomata (Solanaceae). – Phytologia 27: 286-288.
Gentry JL. 1974. The generic name Saracha Ruiz & Pavón (Solanaceae). – Fieldiana (Botany) 36: 69-71.
Gentry JL. 1979. Pollen morphology of the Salpiglossideae (Solanaceae). – In: Hawkes JG, Lester JG, Skelding AD (eds), The biology and taxonomy of the Solanaceae, Linn. Soc. Symp. 7, Academic Press, London, pp. 327-332.
Gentry JL. 1986. Pollen studies in the Cestreae (Solanaceae). – In: D’Arcy W (ed), Solanaceae: biology and systematics, Columbia University Press, New York, pp. 138-158.
Gentry JL, Child A, 1979. Studies in Solanum section Jasminosolanum: a new species from Guatemala. – Brenesia 16: 147-149.
Gentry JL, Standley PC. 1974. Flora of Guatemala: Solanaceae. – Fieldiana, Bot. 24, X: 1-151.
Gerard DJ. 1930. Beiträge zur Geschichte einigen Solaneen. – Inaugural Diss., Colmar.
Gerasimenko II. 1970. Conspectus subgeneris Archaeosolanum Marzell generis Solanum L. – Novosti Syst. Vyssh. Rast. 7: 270-275. [In Russian]
Gerasimenko II, Reznikova SA. 1968. A cytological investigation of the genus Solanum L. – Bot. Žurn. 53: 505-513. [In Russian]
Gerstenaberger P, Leins P. 1978. REM-Untersuchungen an Blütenknospen von Physalis philadelphica (Solanaceae). – Ber. Deutsch Bot. Ges. 91: 381-387.
Ghani A, Evans WC, Woolley VA. 1972. Alkaloids of Hyoscyamus species. – Bangladesh Pharm. J. 1: 12-14.
Ghareman A, Khatamsaz M. 1996. The genus Hyoscyamus L. (Solanaceae) in Iran. – Iran. J. Bot. 7: 31-37.
Ghareman A, Khatamsaz M, Ganj-Karimi M. 1999. Leaf epidermal studies in the genus Hyoscyamus L. (Solanaceae) in Iran. – Iran. J. Bot. 8: 81-90.
Giannattasio R, Spooner DM. 1994a. A reexamination of species boundaries between Solanum megistacrolobum and S. toralapanum (Solanum sect. Petota, series Megistacroloba): morphological data. – Syst. Bot. 19: 89-105.
Giannattasio R, Spooner DM. 1994b. A reexamination of species boundaries and hypotheses of hybridization concerning Solanum megistacrolobum and S. toralapanum (Solanum sect. Petota, series Megistacroloba): molecular data. – Syst. Bot. 19: 106-115.
Gil RR, Lin L, Chai H, Pezzuto JM, Cordell GA. 1995. Cardenolides from Nierembergia aristata. – J. Natur. Prod. 58: 848-856.
Gil RR, Misico RI, Sotes IR, Oberti JC. 1997. 16-hydroxylated withanolides from Exodeconus maritimus. – J. Natur. Prod. 60: 568-572.
Gilli A. 1970. Bestimmungsschlüssel der Subgenera und Sektionen der Gattung Solanum. – Feddes Repert. 81: 429-435.
Gizicki F, Kotitschke K, 1951. Über das Nicandrin, einen Bitterstoff aus der Solanaceae Nicandra physaloides Gaertn. – Arch. Pharm. 284: 129-132.
Glišić LJM. 1928. Zur Entwicklungsgeschichte der Solanaceen. Die Endospermbildung von Datura metel L. – Bull. Inst. Bot. Univ. Belgrade 1: 82-85.
Godwin H, Echlin P, Chapman B. 1967. The development of the pollen grain wall in Ipomoea purpurea (L.) Roth. – Rev. Palaeobot. Palynol. 3: 181-195.
Gonçalves AE. 1997. Two new species of Solanum (Solanaceae) from Mozambique. – Kew Bull. 52: 703-709.
Gonzalez MD, Pomilio AB. 1982. Two acylated flavonone glycosides from Nierembergia hippomanica. – Phytochemistry 2: 757-759.
Gonzalez MD, Pomilio AB, Gros EG. 1981a. An acylated flavonoid from Nierembergia hippomanica. – Phytochemistry 20: 1174-1175.
Gonzalez MD, Pomilio AB, Gros EG. 1981b. Terpenoids and alkaloids from Nierembergia hippomanica. – Anal. Soc. Quim. Argent. 69: 297-299.
Goodspeed TH. 1933. Chromosome number and morphology in Nicotiana VI. Chromosome numbers of forty species. – Proc. Natl. Acad. Sci. U.S.A. 19: 649-653.
Goodspeed TH. 1947. Maturation of the gametes and fertilization in Nicotiana. – Madroño 9: 110-120.
Goodspeed TH. 1954. The genus Nicotiana: origins, relationships, and evolution of its species in the light of their distribution, morphology, and cytogenetics. – Chron. Bot. 16: 1-536.
Gorinova NI, Atanassov AI, Velcheva MP. 1999. Physochlaina species: in vitro culture and the production of physochlaine and other tropane alkaloids. – In: Bajaj YPS (ed), Medicinal and aromatic plants XI, Springer, Berlin, pp. 350-363.
Gottschalk W. 1954. Die Chromosomenstruktur der Solanaceen unter Berücksichtigung phylogenetischer Fragestellungen. – Chromosoma 6: 539-626.
Gottschalk W. 1956. Die Cytologie der Kulturtomate und ihrer wildwachsenden Verwandten. – Bibliogr. Genet. 17: 1-109.
Govil CM. 1971. Morphological studies in the family Convolvulaceae I. Development and structure of the seed coat. – J. Indian Bot. Soc. 50: 32-38.
Govil CM. 1972. Morphological studies in the family Convolvulaceae IV. Vascular anatomy of the flower. – Proc. Indian Acad. Sci., Sect. B, 75: 271-282.
Grau J, Gronbach E. 1984. Untersuchungen zur Variabilität in der Gattung Schizanthus (Solanaceae). – Mitt. Bot. Staatssamml. München 20: 111-203.
Griffin WJ. 1967. The alkaloids of Duboisia leichhardtii: tetramethylputrescine. – Australas. J. Pharm. 48: 567.
Gunn CR. 1977. Merremia discoidesperma: its taxonomy and capacity of its seeds for ocean drifting. – Econ. Bot. 31: 237-252.
Gunn CR, Gaffney FB. 1974. Seed characteristics of 42 economically important species of Solanaceae in the United States. – Techn. Bull. Agric. Res. Serv. U.S. Dept. Agric., Washington, D.C., 1471: 1-33.
Gupta DP. 1959. Vascular anatomy of the flower of Sphenoclea zeylanica Gaertn. and some other related species. – Proc. Indian Natl. Inst. Sci., Sect. B, 25: 55-64.
Gupta SK, Roy SK. 1979. A new basic number in the genus Physalis Linn. – Chromosome Inform. Serv. 27: 4-5.
Haberhausen G, Zetsche K. 1994. Functional loss of all ndh genes in an otherwise relatively unaltered plastid genome of the holoparasitic flowering plant Cuscuta reflexa. – Plant Mol. Biol. 24: 217-222.
Haberhausen G, Valentin K, Zetsche K. 1992. Organisation and sequence of photosynthetic genes from the plastid genome of the holoparasitic flowering plant Cuscuta reflexa. – Mol. Gen. Genet. 232: 154-161.
Habtemariam S, Gray AI, Waterman PG. 1998. Withanolides from the roots of Discopodium penninervium. – Phytochemistry 64. 275-276.
Haccius B, Troll W. 1961. Über die sogenannten Wurzelhaare an den Keimpflanzen von Drosera- und Cuscuta-Arten. – Beitr. Biol. Pflanzen 36: 139-157.
Haegi LAR. 1976. Taxonmic account of Datura L. (Solanaceae) in Australia, with a note on Brugmansia Pers. – Aust. J. Bot. 24: 415-435.
Haegi LAR. 1979. Australian genera of the Solanaceae. – In: Hawkes JG, Lester RN, Skelding AD (eds), The biology and taxonomy of the Solanaceae, Ser. 7, Academic Press, London, pp. 121-124.
Haegi LAR. 1981. A conspectus of Solanaceae tribe Anthocercideae. – Telopea 2: 173-180.
Haegi LAR. 1983. Systematic and evolutionary studies in the Australian Solanaceae. – Ph.D. diss., Flinders University, Adelaide, Australia.
Haegi LAR. 1986. The affinities of Anthocercis (Solanaceae) and related genera. – In: D’Arcy WG (ed), Solanaceae: biology and systematics, Columbia University Press, New York, pp. 27-40.
Haegi LAR. 1991. Trichomes of Solanaceae, tribe Anthocercideae. – In: Hawkes JG, Lester RN, Nee M, Estrada N (eds), Solanaceae III: taxonomy, chemistry, evolution, Royal Botanic Gardens, Kew, pp. 181-195.
Halim AF, Collins RP, Berigari MS. 1971. Alkaloids produced by Cestrum nocturnum and Cestrum diurnum. – Planta Medica 20: 44-49.
Hallier H. 1893a. Versuch einer natürlichen Gliederung der Convolvulaceen auf morphologischer und anatomischer Grundlage. – Engl. Bot. Jahrb. Syst. 16: 453-591.
Hallier H. 1893b. Convolvulaceae Africanae. – Engl. Bot. Jahrb. Syst. 18: 81-160.
Hammer K, Romeike A, Tittel C. 1983. Vorarbeiten zur monographischen Darstellung von Wildpflanzensortimenten: Datura L., sect. Dutra Bernh., Ceratocaulis Bernh. et Datura. – Kulturpflanze 31: 13-75.
Hansen A, Hansen C. 1973. Verbascum osbeckii L. and the Triguera species (Solanaceae). A revision. – Lagascalia 3: 183-193.
Harborne JB. 1986. Systematic significance of variation in defence chemistry in the Solanaceae. – In: D’Arcy WG (ed), Solanaceae: biology and systematics, Columbia University Press, New York, pp. 328-344.
Harborne JB, Khn MB. 1993. Variations in the alkaloidal and phenolic profiles in the genus Atropa (Solanaceae). – Bot. J. Linn. Soc. 111: 47-53.
Hartl D. 1963. Das Placentoid der Pollensäcke, ein Merkmal der Tubifloren. – Ber. Deutsch. Bot. Ges. 76: 70-72.
Hartmann R, San Martin A, Muñoz O, Breitmaier E. 1990. Grahamine, an unusual tropane alkaloid from Schizanthus grahamii. – Angew. Chem. 29: 385-386.
Hassler E. 1917. Solanaceae austro-americanae imprimis paraguarienses. – Ann. Cons. Jard. Bot. Genève 20: 173-189.
Hawkes JG. 1954. New Solanum species in sub-section Hyperbasarthrum Bitt. – Ann. Mag. Nat. Hist. London, Ser. 12(7): 689-710.
Hawkes JG. 1956. A revision of the tuber-bearing solanums. – Scott. Plant Bred. Stat. Rec. 1956: 37-109.
Hawkes JG. 1963. A revision of the tuber-bearing solanums. 2nd ed. – Scott. Plant Bred. Stat. Rec. 1963: 76-181.
Hawkes JG. 1972. Solanaceae [Mandragora]. – Bot. J. Linn. Soc. 65: 356-357.
Hawkes JG. 1989. Nomenclatural and taxonomic notes on the infrageneric taxa of the tuber-bearing solanums (Solanaceae). – Taxon 38: 489-492.
Hawkes JG. 1990. The potato: evolution, biodiversity and genetic resources I-VIII. – Smithsonian Institution Press, Washington, D.C.
Hawkes JG. 1999. The economic importance of the familiy Solanaceae. – In: Nee M, Symon DE, Lester RN, Jessop JP (eds), Solanaceae IV. Advances in biology and utilization, Royal Botanic Gardens, Kew, pp. 1-8.
Hawkes JG, Hjerting JP. 1969. The potatoes of Argentina, Brazil, Paraguay and Uruguay. A biosystematic study. – Oxford University Press, Oxford.
Hawkes JG, Hjerting JP. 1989. The potatoes of Bolivia. Their breeding value and evolutionary relationships. – Oxford University Press, Oxford.
Hawkes JG, Tucker WG. 1968. Serological assessment of relationships in a flowering plant family (Solanaceae). – In Hawkes JG (ed), Chemotaxonomy and serotaxonomy, Syst. Assoc. Spec. Vol. 2: 77-88.
Hawkes JG, Lester RN, Skelding AD (eds). 1979. The biology and taxonomy of the Solanaceae. – Linn. Soc. Symposium, No. 7, Academic Press, London.
Hawkes JG, Lester RN, Nee M, Estrada N (eds). 1991. Solanaceae III: taxonomy, chemistry, evolution. – The Royal Botanic Gardens, Kew.
He C, Saedler H. 2005. Heterotopic expression of MPF2 is the key to the evolution of the Chinese lantern of Physalis, a morphological novelty in Solanaceae. – Proc. Natl. Acad. Sci. U.S.A. 102: 5779-5784.
Heide-Jørgensen HS. 1987. Changes in cuticle structure during development and attachment of the upper haustorium of Cuscuta L., Cassytha L., and Viscum L. – In: Weber HC, Forstreuter W (eds), Parasitic flowering plants, Philipps-Universität, Marburg, pp. 319-334.
Heine H. 1960. Notes on Solanum. – Kew Bull. 14: 245-249.
Heiser CB. 1971. Notes on some species of Solanum (sect. Leptostemonum) in Latin America. – Baileya 18: 59-65.
Heiser CB. 1972. The relationship of the naranjilla, Solanum quitoënse. – Biotropica 4: 77-84.
Heiser CB, Smith PG. 1953. The cultivated Capsicum peppers. – Econ. Bot. 7: 214-224.
Heltmann H. 1979. Morpologische und phytochemische Untersuchungen an Sippen der Gattung Atropa. – Herba Hungarica 18: 101-110.
Hemsley WB. 1892. The genus Melananthus Walpers. – Ann. Bot. 6: 145-146.
Hepper FN. 1991. Old World Withania (Solanaceae): a taxonomic review and key to the species. – In: Hawkes JG, Lester RN, Nee M, Estrada N (eds), Solanaceae III. Taxonomy, chemistry, evolution, Royal Botanic Gardens, Kew, pp. 211-227.
Hideux MJ, Ferguson IK. 1976. The stereostructure of the exine and its evolutionary significance in Saxifragaceae sensu lato. – In: Ferguson IK, Muller J (eds), The evolutionary significance of the exine, Academic Press, London, pp. 327-377.
Hijmans R, Gavrilenko T, Stephenson S, Bamberg J, Salas A, Spooner DM. 2007. Geographic and environmental range expansion through polyploidy in wild potatoes (Solanum section Petota). – Global Ecol. Biogeogr. 16: 485-495.
Hitchcock CL. 1932. A monographic study of the genus Lycium of the Western Hemisphere. – Ann. Missouri Bot. Gard. 19: 179-374.
Hoare AL, Knapp S. 1997. A phylogenetic conspectus of the tribe Hyoscyameae. – Bull. Nat. Hist. Mus. London (Bot.) 27: 11-29.
Hogstad A. 1923. A morphological and chemical study of Nicandra physaloides. – J. Amer. Pharm. Assoc. 12: 576-581.
Horton P. 1979. Taxonomic account of Nicandra (Solanaceae) in Australia. – J. Adelaide Bot. Gard. 1: 351-356.
Horton P. 1981. A taxonomic revision of Nicotiana (Solanaceae). – J. Adelaide Bot. Gard. 3: 1-56.
Hosaka K. 1986. Who is the mother of the potato? – Restriction endonuclease analysis of chloroplast DNA of cultivated potatoes. – Theor. Appl. Gen. 72: 606-618.
Hosaka K, Ogihara Y, Matsubayashi M, Tsunewaki K. 1984. Phylogenetic relationship between the tuberous Solanum species as revealed by restriction endonuclease analysis of chloroplast DNA. – Jap. J. Gen. 59: 349-369.
House HD. 1906. Studies in the North American Convolvulaceae. – Bull. Torrey Bot. Club 33: 313-318.
Hsiao PK, Hsia KC, Ho LY. 1973. Occurrence of some important tropane alkaloids in Chinese solanaceous plants. – Acta Bot. Sin. 15: 187-194.
Hsiao TH. 1986. Specificity of certain chrysomelid beetles for Solanaceae. – In: D’Arcy WG (ed), Solanaceae: biology and systematics, Columbia University Press, New York, pp. 345-363.
Hu C, Saedler H. 2007a. Molecular evolution of a morphological novelty in Solanaceae: the inflated-calyx-syndrome (ICS) in Physalis. – In: Spooner DM, Bohs L, Giovannoni J, Olmstead RG, Shibata D (eds), Solanaceae VI. Genomics meets biodiversity, ISHA Section Root and Tuber Crops., Acta Hortic. 745: 171-181.
Hu J-Y, Saedler H. 2007b. Evolution of the inflated calyx syndrome in Solanaceae. – Mol. Biol. Evol. 24: 2443-2453.
Huber KA. 1980. Morphologische und entwicklungsgeschichtliche Untersuchungen an Blüten und Blütenständen von Solanaceen und von Nolana paradoxa Lindl. (Nolanaceae). – Diss. Bot. 55: 1-252, Cramer, Vaduz.
Hülsbruch M. 1961. Strobulierende Drüsenköpfchen bei Atropa belladonna L. – Flora 150: 572-599.
Humbert H. 1948. Au sujet de l’Humbertia madagascariensis Lam. – Not. Syst. 13: 303.
Hunziker AT. 1950. Estudios sobre Solanaceae I. Sinopsis de las especies silvestres de Capsicum de Argentina y Paraguay. – Darwiniana 9: 225-247.
Hunziker AT. 1954 [1956]. Synopsis of the genus Capsicum. – Compt. Rend. Comm., Sect. 3-6, 8e Congr. Intern. Bot. Paris.
Hunziker AT. 1969. Estudios sobre Solanaceae V. Contribución al conocimiento de Capsicum y géneros afines (Witheringia, Acnistus, Athenaea, etc.). – Kurtziana 5: 101-179.
Hunziker AT. 1977. Estudios sobre Solanaceae VII. – Kurtziana 10: 7-50.
Hunziker AT. 1977. Estudios sobre Solanaceae VIII. Novedades varias sobre tribus, géneros, secciones y especies de Sud América. – Kurtziana 10: 7-59.
Hunziker AT. 1979b. South American Solanaceae: a synoptic survey. – In: Hawkes JG, Lester RN, Skelding AD (eds), The biology and taxonomy of the Solanaceae, Linn. Soc. Symp. 7, Academic Press, London, pp. 49-85.
Hunziker AT. 1979a. The Solanaceae in the Neotropics: a critical appraisal. – In: Larsen K, Holm-Nielsen LB (eds), Tropical botany, Academic Press, London, pp. 355-364.
Hunziker AT. 1982. Revisión sinóptica de Acnistus. – Kurtziana 15: 81-102.
Hunziker AT. 1984. Estudios sobre Solanaceae XIX. Sinopsis de Vassobia. – Kurtziana 17: 91-128.
Hunziker AT. 1985. Estudios sobre Solanaceae XX. Markea lopezii, nueva especie de Colombia. – Lorentzia 5: 9-12.
Hunziker AT. 1987. Studies on Solanaceae XXI. A preliminary synopsis of Cuatresia. – Opera Bot. 92: 73-82.
Hunziker AT. 1989. Estudios sobre Solanaceae XXVIII. Novedades sobre las especies argentinas de Solanum. – Kurtziana 20: 181-194.
Hunziker AT. 1995. Studies on Solanaceae XXXVIII. Miscellaneous novelties in the taxonomy of Solanaceae. – Lorentzia 8: 5-8.
Hunziker AT. 1997. Estudios sobre Solanaceae LXIII. Revisión de las especies de Markea. – Kurtziana 25: 67-113.
Hunziker AT. 1998. Estudios sobre Solanaceae XLVI. Los ajíes silvestres de Argentina (Capsicum). – Darwiniana 36: 201-203.
Hunziker AT. 2000a. Miscellaneous novelties in the taxonomy of Solanaceae (Part II). – Kurtziana 28: 55-64.
Hunziker AT. 2000b. The tribe Solaneae (Solanaceae): key for its genera and description of Darcya gen. nov. – Bol. Soc. Argent. Bot. 35: 163-169.
Hunziker AT. 2000c. Darcyanthus nom. nov. substitutes Darcya (Solanaceae). – Bol. Soc. Argent. Bot. 35: 345.
Hunziker AT. 2001. Genera solanacearum. The genera of Solanaceae illustrated, arranged according to a new system. – A. R. G. Gantner Verlag, Ruggell, Liechtenstein.
Hunziker AT, Barboza GE. 1991. Estudios sobre Solanaceae XXX. Revisión de Aureliana. – Darwiniana 30: 95-113.
Hunziker AT, Barboza GE. 1995. Estudios sobre Solanaceae XXXIX. Sobre la verdadera identidad de Parabouchetia brasiliensis y su exclusión de la familia. – Lorentzia 8: 9-12.
Hunziker AT, Barboza GE. 1998. Estudios sobre Solanaceae XLV. Sobre la presencia de Exodeconus en Argentina y una novedad en Capsicum baccatum. – Kurtziana 26: 23-31.
Hunziker AT, Bernardello LM. 1989. Sobre la posición sistemática de Trianaea (Solanaceae). – Kurtziana 20: 215.
Hunziker AT, Subils R. 1979. Observaciones preliminaries sobre el género Leptoglossis (Solanaceae). – Lorentzia 3: 13-17.
Hunziker AT, Subils R. 1980. Salpiglossis, Leptoglossis and Reyesia (Solanaceae). A synoptical survey. – Bot. Mus. Leafl. (Harvard University) 27: 1-43.
Hunziker AT, Subils R. 1983. Estudios sobre Solanaceae XVIII. Sinopsis taxonómica de Buchetia. – Bol. Soc. Argent. Bot. 22: 275-295.
Hunziker AT, Subils R. 1991. Estudios sobre Solanaceae XXXII. Sinopsis taxonómica de Juanulloa. – Kurtziana 21: 209-235.
Husaini SWH, Iwo GA. 1990. Cytomorphological studies in some weedy species of the family Solanaceae from Joe Plateau, Nigeria. – Feddes Repert. 101: 41-47.
Huttley GA, Durbin ML, Glover DE, Clegg MT. 1997. Nucleotide polymorphism in the chalcone synthase-A locus and evolution of the chalcone synthase multigene family of common morning glory Ipomoea purpurea. – Mol. Ecol. 6: 549-558.
Inamdar JA, Murthy SR. 1977. Vessels in some Solanaceae. – Flora, Sect. B, 166: 441-447.
Inamdar JA, Patel RC. 1969. Development of stomata in some Solanaceae. – Flora, Sect. B, 158: 462-472.
Intrieri MC, Buiatti M. 2001. The horizontal transmission of Agrobacterium rhizogenes genes and the evolution of the genus Nicotiana. – Mol. Phylogen. Evol. 20: 100-110.
Irish HC. 1898. Revision of the genus Capsicum with special reference to garden varieties. – Ann. Rep. Missouri Bot. Gard. 9: 53-110.
Jackson BP, Berry MI. 1973. Hydroxytropane triglates in the roots of Mandragora species. – Phytochemistry 12: 1165-1166.
Jackson BP, Berry MI. 1979. Mandragora: taxonomy and chemistry of the European species. – In: Hawkes JG, Lester RN, Skelding AD (eds), The biology and taxonomy of the Solanaceae, Linn. Soc. Symp. Ser. 7, Linnean Society and Academic Press, London, pp. 505-512.
Jackson JF. 1999. Phytic acid in the seeds of Solanum species. – In: Nee, Symon, Lester, Jessop (eds), Solanaceae IV, Royal Botanic Gardens, Kew, pp. 45-49.
Jaeger P-ML. 1986. Systematic studies in the genus Solanum in Africa. – Ph.D. diss., University of Birmingham, England.
Jaeger P-ML, Hepper FN. 1986. A review of the genus Solanum in Africa. – In: D’Arcy WG (ed), Solanaceae, biology and systematics, Columbia University Press, New York, pp. 41-55.
Jagannadham D. 1988. Embryological studies in Nicotiana tabacum and N. rustica. – J. Indian Bot. Soc. 67: 135-141.
Janaki-Ammal EK. 1932. Chromosome studies in Nicandra physaloides. – Cellule 41: 89-110.
Japan Tobacco Inc. 1994. The genus Nicotiana illustrated. – Japan Tobacco Inc., Tokyo.
Jayasuriya KMGG, Baskin JM, Geneve RL, Baskin CC. 2009. Phylogeny of seed dormancy in Convolvulaceae, subfamily Convolvuloideae (Solanales). – Ann. Bot. 103: 45-63.
Jenett-Siems K. 1996. Phytochemische Untersuchungen an Windengewächsen der gattungen Calystegia, Convolvulus, Ipomoea und Merremia unter besonderer Berücksichtigung des Alkaloidvorkommens. – Ph.D. diss., Fachbereich Pharmazie, Freie Universität Berlin, Germany.
Jenett-Siems K, Schimming T, Kaloga M, Eich E, Siems K, Gupta MP, Witte L, Hartmann T. 1998. Pyrrolizidine alkaloids of Ipomoea hederifolia and related species. – Phytochemistry 47: 1551-1560.
Jenkins JA. 1948. The origin of the cultivated tomato. – Econ. Bot. 2: 379-392.
Jeppesen S. 1981. 189A. Sphenocleaceae. – In: Harling G,Sparre B (eds), Flora of Ecuador 14, Swedish Natural Science Research Council, Stockholm, pp. 173-174.
Jiao M, Luna-Cavazos M, Bye R. 2002. Allozyme variation in Mexican species and classification of Datura (Solanaceae). – Plant Syst. Evol. 232: 155-166.
Johnson RW. 2009. A conspectus of Merremia Dennst. ex Endl. (Convolvulaceae) in Australia with the addition of two species. – Austrobaileya 8: 55-63.
Johnson RW. 2010. Davenportia R. W. Johnson, a new genus of Convolvulaceae (Merremieae) from central Australia. – Austrobaileya 8: 171-175.
Johnson RW. 2014. Six new species of Bonamia Thouars. (Convolvulaceae) from northern Australia. – Austrobaileya 9: 292-310.
Johnston IM. 1936. A study of the Nolanaceae. – Proc. Amer. Acad. Arts Sci. 71: 1-87 (Contr. Gray Herb. 112: 1-83).
Johri BM. 1934. The development of the male and female gametophytes in Cuscuta reflexa Roxb. – Proc. Indian Acad. Sci., Sect. B, 1: 283-289.
Johri BM, Tiagi B. 1952. Floral morphology and seed formation in Cuscuta reflexa Roxb. – Phytomorphology 2: 162-180.
Jos JS. 1967. Studies on floral anatomy of Nicotiana. – J. Indian Bot. Soc. 46: 82-89.
Juel O. 1894. Ueber den Mechanismus der Schizanthus-Blüte. – Kongl. Sv. Vetensk.-Akad. Förhandl. 1894(2): 67-72.
Juel O. 1911. Om blommans byggnad hos Browallia. – Biologiske Arbejde Tilegnede E. Warming paa hans 70 aars Fødselddag, København, pp. 109-188.
Kadereit JW, Bittrich V (eds). 2016. The families and genera of vascular plants XIV. Flowering plants – eudicots – Aquifoliales, Boraginales, Bruniales, Dipsacales, Escalloniales, Garryales, Paracryphiales, Solanales (except Convolvulaceae), Icacinaceae, Metteniusaceae, Vahliaceae. Springer, 412 pp.
Kalin Arroyo MT, Squeo F. 1990. Genetic incompatibility in the endemic Patagonian genus Benthamiella (Solanaceae). – Gayana 47: 51-55.
Kaniewski K. 1966. Sclereidal concretions in the fruit of Physalis alkekengi L. – Acad. Polon. Sci. Bull. Ser. Sci. Biol. 14: 569-573.
Kapundu M, Delaude C. 1988. Sapogenins of Schwenckia americana L. – Bull. Soc. Roy. Sci. Liège 57: 561-565.
Kardolus JP. 1999. Morphological variation within series Acaulia Juz. (Solanum sect. Petota). – In: Nee M, Symon JD, Lester RN, Jessop J (eds), Solanaceae IV, Royal Botanic Gardens, Kew, pp. 257-274.
Kaur H. 1969. Structure and development of seed in Ipomoea obscura Ker Gawl. – J. Indian Bot. Soc. 48: 346-351.
Kaur H, Singh RP. 1970. Structure and development of seeds in three Ipomoea species. – J. Indian Bot. Soc. 49: 168-174.
Kaur H, Singh RP. 1987. Development and structure of seed and fruit in some Convolvulaceae. – Phytomorphology 37: 145-154.
Kausik SB, Subramanyam K. 1946. A contribution to the life-history of Sphenoclea zeylanica Gaertn. – Proc. Indian Acad. Sci., Sect. B, Biol. Sci. 23: 274-280.
Kawai M, Opgura T, Makino B, Matsumoto A et al. 1992. Physalins N and O from Physalis alkekengi. – Phytochemistry 31: 4299-4302.
Keeler KH. 1977. The extrafloral nectaries of Ipomoea carnea (Convolvulaceae). – Amer. J. Bot. 64: 1182-1188.
Keeler KH. 1980. The extrafloral nectaries of Ipomoea leptophylla (Convolvulaceae). – Amer. J. Bot. 67: 216-222.
Kennedy GS. 1971. (-)-hyoscyamine in Duboisia hopwoodii. – Phytochemistry 10: 1335-1337.
Kennedy PB, Crafts AS. 1931. The anatomy of Convolvulus arvensis, wild morning-glory or field bindweed. – Hilgardia 4: 591-622.
Kenton A, Parokonny AS, Gleba YY, Bennett MD. 1993. Characterization of the Nicotiana tabacum L. genome by molecular cytogenetics. – Mol. Gen. Genet. 240: 159-169.
Kereselide EV, Pkheidze TA, Kemeterlidze EP. 1970. Steroid sapogenins from Cestrum parqui and C. elegans. – Khim. Prir. Soedin 6: 379. [In Russian]
Khalik KAN. 2006. Seed morphology of Cuscuta L. (Convolvulaceae) in Egypt and its systematic significance. – Feddes Repert. 117: 217-224.
Khalik KNA, Osman AK. 2007. Seed morphology of some species of Convolvulaceae from Egypt (identification of species and systematic significance). – Feddes Repert. 118: 24-37.
Khan MR, Hu JY, He C. 2012. Plant hormones including ethylene are recruited in calyx inflation in solanaceous plants. – J. Plant Physiol. 169: 940-948.
Khan MR, Hu JY, Riss S, He C, Saedler H. 2009. MPF2-like-A MADS-box genes control the inflated calyx syndrome in Withania (Solanaceae): roles of Darwinian selection. – Mol. Biol. Evol. 26: 2463-2473.
Khan R. 1979. Solanum melongena and its ancestral forms. – In: Hawkes JG, Lester RN, Skelding AD (eds), The biology and taxonomy of Solanaceae, Linn. Soc. Symp. Ser. 7, Linnean Society and Academic Press, London, pp. 629-636.
Khanna KL, Schwarting AE, Rother A, Bobbitt JM. 1961. Occurrence of tropine and pseudotropine in Withania somnifera. – Lloydia 24: 179-181.
Khanna KL, Schwarting AE, Bobbitt JM. 1962. The occurrence of isopelletierine in Withania somnifera. – J. Pharm. Sci. 51: 1194.
Khatamsaz M, Zangirian E. 1998. SEM survey of pollen morphology in Iranian species of Hyoscyamus L. (Solanaceae). – Iranian J. Bot. 7: 153-163.
Kilmer FB. 1925. Rambling among the nightshades. – Amer. J. Pharm. 94: 1-37.
Kirson I, Lavie D, Albonico M, Juliani HR. 1970. The withanolides of Acnistus australis (Griseb.) Griseb. – tetrahedron 26: 5063.
Kite GC, Leon C. 1995. Volatile compounds emitted from flowers and leaves of Brugmansia candida. – Phytochemistry 40: 1093-1095.
Knapp S. 1983. Sectional nomenclature in Solanum (Solanaceae). – Taxon 32: 635-636.
Knapp S. 1985. New species of Solanum section Geminata (G. Don) Walp. (Solanaceae) from South and Central America. – Ann. Missouri Bot. Gard. 72: 558-569.
Knapp S. 1986. New species of Solanum section Geminata (Solanaceae) from South America. – Brittonia 38: 273-301.
Knapp S. 1989a. A revision of the Solanum nitidum group (section Holophylla pro parte: Solanaceae). – Bull. Nat. Hist. Mus. London (Bot.) 19: 63-102.
Knapp S. 1989b. Six new species of Solanum section Geminata from South America. – Bull. Nat. Hist. Mus. London (Bot.) 19: 103-112.
Knapp S. 1991a. A revision of the Solanum sessile species group (section Geminata pro parte: Solanaceae). – Bot. J. Linn. Soc. 105: 179-210.
Knapp S. 1991b. A cladistic analysis of the Solanum sessile species group (section Geminata pro parte: Solanaceae). – Bot. J. Linn. Soc. 106: 73-89.
Knapp S. 1992a. Five new species of Solanum section Geminata (Solanaceae) from South America. – Brittonia 44: 61-68.
Knapp S. 1992b. Two new species of Solanum section Geminata (Solanaceae) from Cerro del Torrá in western Colombia. – Bull. Nat. Hist. Mus. London (Bot.) 22: 147-152.
Knapp S. 1995. New data and combinations in the tribe Juanulloeae (Solanaceae). – Novon 5: 281-283.
Knapp S. 1997. A revision of Solanum section Pteroidea: Solanaceae. – Bull. Nat. Hist. Mus. London (Bot.) 27: 31-73.
Knapp S. 1998. New species and notes on the natural history of Markea (Solanaceae) from Colombia and Ecuador. – Novon 8: 152-161.
Knapp S. 2000. A revision of Solanum thelopodium species group (section Anthoresis sensu Seithe pro parte: Solanaceae). – Bull. Nat. Hist. Mus. London (Bot.) 30: 13-30.
Knapp S. 2002. Floral diversity and evolution in the Solanaceae. – In: Cronk QCB, Bateman RM, Hawkins JA (eds), Developmental genetics and plant evolution, Taylor and Francis, London, pp. 267-297.
Knapp S 2002. Tobacco to tomatoes: a phylogenetic perspective on fruit diversity in the Solanaceae. – J. Exp. Bot. 53: 2001-2022.
Knapp S. 2008. A revision of the Solanum havanense species group and new taxonomic additions to the Geminata clade (Solanum, Solanaceae). – Ann. Missouri Bot. Gard. 95: 405-458.
Knapp S. 2010. On ‘various contrivances’: pollination, phylogeny and flower form in the Solanaceae. – Phil. Trans. Roy. Soc., Sect. B, 365: 449-460.
Knapp S. 2013. A revision of the dulcamaroid clade of Solanum L. (Solanaceae). – PhytoKeys 22: 1-432.
Knapp S, D’Arcy WG. 1993. Rahowardiana globifera (Solanaceae), a new species from Colombia. – Novon 3: 429-430.
Knapp S, Helgason T. 1997. A revision of Solanum sect. Pteroidea: Solanaceae. – Bull. Nat. Hist. Mus. London (Bot.) 27: 31-73.
Knapp S, Vorontsova M. 2016. A revision of the “African Non-Spiny” Clade of Solanum L. (Solanum sections Afrosolanum Bitter, Benderianum Bitter, Lemurisolanum Bitter, Lyciosolanum Bitter, Macronesiotes Bitter, and Quadrangulare Bitter: Solanaceae). – PhytoKeys 66: 1-142.
Knapp S, Persson V, Blackmore S. 1997. A phylogenetic conspectus of the tribe Juanulloeae (Solanaceae). – Ann. Missouri Bot. Gard. 84: 67-89.
Knapp S, Persson V, Blackmore S. 1998. Pollen morphology and functional dioecy in Solanum (Solanaceae). – Plant Syst. Evol. 210: 113-139.
Knapp S, Stafford P, Persson V. 2000. Pollen morphology in the Anthocercideae (Solanaceae). – Kurtziana 28: 7-18.
Knapp S, Chase MW, Clarkson JJ. 2004. Nomenclatural changes and a new sectional classification in Nicotiana (Solanaceae). – Taxon 53: 73-82.
Knapp S, Bohs L, Nee M, Spooner DM. 2004. Solanaceae – a model for linking genomics with biodiversity. – Comp. Funct. Genom. 5: 285-291.
Knapp S, Barboza GE, Romero MV, Vignoli-Silva M, Giacomin LL, Stehmann JR. 2015. Identification and lectotypification of the Solanaceae from Vellozo’s Flora Fluminensis. – Taxon 64: 822-836.
Komarova NY, Grimm GW, Hemleben V, Volkov RA. 2008. Molecular evolution of 35S rDNA and taxonomic status of Lycopersicon within Solanum sect. Petota. – Plant Syst. Evol. 276: 59-71.
Konar RN, Linskens HF. 1966a. The morphology and anatomy of the stigma of Petunia hybrida. – Planta 71: 356-371.
Konar RN, Linskens HF. 1966b. Physiology and biochemistry of the stigmatic fluid of Petunia hybrida. – Planta 71: 372-387.
Kostoff D. 1941-1943. Cytogenetics of the genus Nicotiana. Karyosystematics, genetics, cytology, cytogenetics and phylesis of tobaccos. – State Printing House, Sophia.
Krause K. 2011. Piecing together the puzzle of parasitic plant plastome evolution. – Planta 234: 647-656.
Krause K, Berg S, Krupinska K. 2003. Plastid transcription in the holoparasitic plant genus Cuscuta: parallel loss of the rrn16 PEP-promoter and of the rpoA and rpoB genes coding for the plastid-encoded RNA polymerase. – Planta 5: 815-823.
Kuang K-Z. 1966. Archiphysalis Kuang, genus novum solanacearum Asiae Orientalis. – Acta Phytotaxon. Sin. 11: 60-63.
Kuang K-Z, Lu A-M. 1965. Revisio Physaliastrorum Makino. – Acta Phytotaxon. Sin. 10: 347-355.
Kuang K-Z, Lu A-M. 1974. De speciebus sinensibus generis Physochlainae. – Acta Phytotaxon. Sin. 12: 407-411.
Kuhlmann JG. 1934. Notas sôbre o gênero Duckeodendron. – Arq. Inst. Biol. Veg. (Rio de Janeiro) 1: 35-37.
Kuhlmann JG. 1947. Duckeodendraceae Kuhlmann (nova familia). – Arq. Serv. Forest. Brasil 3: 7-8.
Kulcheski FR, Muschner VC, Lorenz-Lemke AP, Stehmann JR, Bonatto SL, Salzano FM, Freitas LB. 2006. Molecular phylogenetic analysis of Petunia Juss. (Solanaceae). – Genetica 126: 3-14.
Kuriachan P. 1981. A cytogenetic study of the wild and cultivated varieties of Capsicum baccatum L. – Indian J. Bot. 4: 27-32.
Lagerheim G. 1891. Zur Biologie der Iochroma macrocalyx Benth. – Ber. Deutsch. Bot. Ges. 9: 348-351.
Lagerheim G. 1895. Monographie der ecuadorianischen Arten der Gattung Brugmansia Pers. – Bot. Jahrb. 20: 655-668.
Lara-Cabrera S, Spooner DM. 2004. Taxonomy of North and Central American diploid wild potato (Solanum sect. Petota) species: AFLP data. – Plant Syst. Evol. 248: 129-142.
Lara-Cabrera S, Spooner DM. 2005. Taxonomy of Mexican diploid wild potatoes (Solanum sect. Petota): morphological and microsatellite data. – Monogr. Syst. Bot. Missouri Bot. Gard. 104: 199-205.
Las Penas ML, Chiarini FE, Bernardello G, Benítez de Rojas C. 2006. Karyotypes of some species of Cestrum, Sessea, and Vestia (tribe Cestreae, Solanaceae). – Caryologia 59: 131-137.
Lavie D, Greenfield S, Glotter E. 1966. constituents of Withania somnifera Dun. VI. The stereochemistry of withaferin A. – J. Chem Soc. (C) 1966: 1753-1756.
Lavie D, Gottlieb HE, Pestchanker MJ, Giordano OS. 1986. Jaborosa lactone-1, a withanolide from Jaborosa leucotricha. – Phytochemistry 25: 1765-1766.
Lavie D, Besalle R, Pestchanker MJ, Gottlieb HE et al. 1987. Trechonolide A, a new withanolide type from Trechonaetes laciniata. – Phytochemistry 26: 1791-1795.
Lawrence GHM. 1960. The cultivated species of Solanum. – Baileya 8: 20-35.
Leary JD, Khanna KL, Schwarting AE, Bobbitt JM. 1963. Occurrence of cuscohygrine and 3α-tigloyloxytropane in Withania somnifera. – Lloydia 26: 44-48.
Lee KB. 2007. Structure and development of the upper haustorium in the parasitic flowering plant Cuscuta japonica (Convolvulaceae). – Amer. J. Bot. 94: 737-745.
Lee KB, Lee CD. 1989. The structure and development of the haustorium in Cuscuta australis. – Can. J. Bot. 67: 2975-2982.
Lee TM, Chao JM, Der Marderosian AH. 1979. Isolation and identification of ergoline alkaloids from seeds of Stictocardia campanulata (L.) Merrill. – Planta Medica 35: 247-252.
Lee Y-N, Oh Y-C. 1971. A cytological study of genus Scopolia in South East Asia. – J. Korean Res. Inst. Better Living 6: 69-75.
Lefort M. 1951. Contribution à l’étude de quelques convolvulacées tropicales. – Ann. Sci. Nat. Bot., sér. XI, 12: 193-215.
Leiva González S. 1996. Dos nuevas especies de Larnax (Solanaceae: Solaneae) del norte del Peru. – Arnaldoa 4: 15-22.
Leiva González S, Mione T. 1999. Dos nuevas especies de Jaltomata Schltdl. (Solanaceae: Solaneae) del norte de peru. – Arnaldoa 6: 65-74.
Leiva González S, Rodríguez Ridríguez E. 2006. Tres nuevas species de Larnax (Miers) Hunziker (Solanaceae) del Departamento Amazonas, Perú. – Arnaldoa 13: 290-304.
Leiva González S, Silvestre VQ. 1998. Iochroma nitidum y I. schjellerupii (Solanaceae), dos nuevas especies andinas del norte de Peru. – Arnaldoa 5: 171-178.
Leiva González S, Quipuscoa SV, Sawyer NW. 1998. Nuevas especies andinas de Larnax (Solanaceae) de Ecuador y Peru. Arnaldoa 5: 83-92.
Leiva González S, Rodriguez JE, Campos de la Cruz J. 1998. Cinco nuevas especies de Larnax (Solanaceae: Solaneae) de los bosques montanos del norte del Peru. – Arnaldoa 5: 193-210.
Leiva González S, Mione T, Quipuscoa SV. 1998. Cuatro nuevas especies de Jaltomata (Solanaceae) del norte de Perú. – Arnoldoa 5: 179-192.
Leiva González S, Deanna R, Barboza GE, Cueva Manchego M. 2013. Sobre la presencia del género Larnax (Solanaceae) en Bolivia. – Arnaldoa 20: 291-300.
Lejoly J, Lisowski S. 1986. Paralepistemon, nouveau genre de Convolvulaceae d’Afrique tropicale. – Bull. Jard. Bot. Natl. Belg. 56: 195-197.
Lejoly J, Lisowski S. 1987. Paralepistemon curtoi (Rendle) Lejoly & Lisowski comb. nov. (Convolvulaceae). – Bull. Jard. Bot. Natl. Belg. 57: 271-272.
Lepschi BJ, Symon DF. 1999. A preliminary cladistic analysis of Australasian Solanum and Lycianthes. – In: Nee M, Symon D, Lester RN, Jessop J (eds), Solanaceae IV, Royal Botanic Gardens, Kew, pp. 161-170.
Lester RN. 1979. The use of protein characters in the taxonomy of Solanum and other Solanaceae. – In: Hawkes JG, Lester RN, Skelding AD (eds), The biology and taxonomy of the Solanaceae, Academic Press, London, pp. 285-304.
Levin RA, Miller JS. 2005. Relationships within tribe Lycieae (Solanaceae): paraphyly of Lycium and multiple origins of gender dimorphism. – Amer. J. Bot. 92: 2044-2053.
Levin RA, Watson K, Bohs L. 2005. A four gene study of evolutionary relationships in Solanum section Acanthophora. – Amer. J. Bot. 92: 603-612.
Levin RA, Myers NR, Bohs L. 2006. Phylogenetic relationships among the “spiny solanums” (Solanum subgenus Leptostemonum, Solanaceae). – Amer. J. Bot. 93: 157-169.
Levin RA, Shak JR, Miller JS, Bernardello G, Venter AM. 2007. Evolutionary relationships in tribe Lycieae (Solanaceae). – In: Spooner DM, Bohs L, Giovannoni J, Olmstead RG, Shibata D (eds), Solanaceae VI: genomics meets biodiversity, Proceedings of the sixth international Solanaceae conference, Acta Horticulturae 745, International Society for Horticultural Science, Leuven, pp. 225-239.
Levin RA, Blanton J, Miller JS. 2009. Phylogenetic utility of nuclear nitrate reductase: a multi-locus comparison of nuclear and chloroplast sequence data for inference of relationships among American Lycieae (Solanaceae). – Mol. Phylogen. Evol. 50: 608-617.
Levin RA, Whelan A, Miller JS. 2009. The utility of nuclear conserved ortholog set II (COSII) genomic regions for species-level phylogenetic inference in Lycium (Solanaceae). – Mol. Phylogen. Evol. 53: 881-890.
Levin RA, Bernardello G, Whiting C, Miller JS. 2011. A new generic circumscription in tribe Lycieae (Solanaceae). – Taxon 60: 681-690.
Levine DA, Anderson GJ. 1986. Evolution of dioecy in an American Solanum. – In: D’Arcy (ed), Solanaceae – Biology and systematics, Colombia University Press, New York, pp. 264-273.
Lewis WH. 1961. Chromosomes of North American Lycium (Solanaceae). – Texas J. Sci. 13: 45-48.
Lim KY, Matyasek R, Lichtenstein CP, Leitch AR. 2000. Molecular cytogenetic analyses and phylogenetic studies in the Nicotiana section Tomentosae. – Chromosoma 109: 245-258.
Lischevski M, Hang NTB, Porzel A, Adam G et al. 1991. Withanolides from Dunalia australis. – Phytochemistry 30: 4184-4186.
Lischevski M, Hang NTB, Porzel A, Adam G et al. 1992. Withanolide glucosides from Dunalia australis. – Phytochemistry 31: 393-942.
Liscovsky IJ, Cosa MT, Dottori N. 2002. Estudio anatómico de órganos vegetativos en representantes de Datureae (Solanaceae). – Bol. Soc. Argent. Bot. 37: 171-180.
Liscovsky IJ, Cosa MT, Barboza GE. 2009. Flower vascularisation in Solanaceae: a particular pattern in Metternichia J. G. Mikan. – Adansonia, sér. III, 31: 413-425.
Lisowski S, Wiland-Szymañska J. 1999. Nouvelle espèce du genre Ipomoea (Convolvulaceae) du Haut-Katanga (Congo-Kinshasa). – Syst. Geogr. Plants 69: 135-136.
Lloyd HA, Fales HM, Goldman ME, Jerina DM et al. 1985. Brunfelsamidine: a novel convulsant from the medicinal plant Brunfelsia grandiflora. – Tetrahedron Letters 26: 2623-2624.
Lockwood TE. 1973. Generic recognition of Brugmansia. – Bot. Mus. Leafl. 23: 273-281.
Lu A-M. 1986. Solanaceae in China. – In: D’Arcy WG (ed), Solanaceae: biology and systematics, Columbia University Press, New York, pp. 79-85.
Lu A-M, Zhang Z-Y. 1986. Studies of the subtribe Hyoscyaminae in China. – In: D’Arcy WG (ed), Solanaceae: biology and systematics, Columbia University Press, New York, pp. 56-78.
Lu A-M, Zhang Z-Y, Chen Z-D, Pan Y-K. 1999. Embryology and adaptive ecology in the genus Przewalskia. – In: Nee M, Symon D, Lester RN, Jessop J (eds), Solanaceae IV, Royal Botanic Gardens, Kew, pp. 71-79.
Luckwill LC. 1943. The genus Lycopersicon. – Aberdeen Univ. Stud. 120: 1-44.
Luis JG, Echeverri F, Gonzalez AG. 1994a. Acnistins F-H, withanolides from Dunalia solanacea. – Phytochemistry 36: 769-772.
Luis JG, Echeverri F, Gonzalez AG. 1994b. Acnistins C and D, withanolides from Dunalia solanacea. – Phytochemistry 36: 1297-1301.
Lujea NC, Chiarini FE. 2017. Differentiation of Nolana and Sclerophylax (Solanaceae) by means of heterochromatin and rDNA patterns. – New Zealand J. Bot. 55: 163-177.
Lundgren L, Norelius G, Stenhagen G. 1985. Leaf volatiles from some wild tomato species. – Nord. J. Bot. 5: 315-320.
Ma C-Y, Williams ID, Che C-T. 1999. Withanolides from Hyoscyamus niger seeds. – J. Nat. Prod. 62: 1445-1447.
McDonald JA. 1982. Biosystematics of the Ipomoea tricolor complex. – Ph.D. diss., University of Texas, Austin, Texas.
McDonald JA. 1987. Three new species of Convolvulaceae from northeast México. – Brittonia 39: 106-111.
McDonald JA. 1991. Origin and diversity of Mexican Convolvulaceae. – An. Inst. Biol. Univ. Nac. Autónoma México, Ser. Bot., 62: 65-82.
McDonald JA. 2008. Merremia cielensis (Convolvulaceae: Merremieae) : a new species and narrow endemic from tropical Northeast Mexico. – Syst. Bot. 33: 552-555.
McDonald JA, Austin DF. 1990. Changes and additions in Ipomoea section Batatas (Convolvulaceae). – Brittonia 42: 116-120.
McDonald JA, Mabry TJ. 1992. Phylogenetic systematics of New World Ipomoea (Convolvulaceae) based on chloroplast DNA restriction site variation. – Plant Syst. Evol. 180: 243-259.
McDonald JA, Hansen DR, McDill JR, Simpson BB. 2011. A phylogenetic assessment of breeding systems and floral morphology of North American Ipomoea (Convolvulaceae). – J. Bot. Res. Inst. Texas 5: 159-177.
Macfarlane TD, Wardell-Johnston G. 1996. Anthocercis sylvicola (Solanaceae), a rare new species from the tingle forests of Walpole, south-western Australia. – Nuytsia 11: 71-78.
Machado MA, Zetsche K. 1990. A structural, functional, and molecular analysis of plastids of the holoparasites Cuscuta reflexa and Cuscuta europaea. – Planta 181: 91-96.
Maciel Pacheco J. 1980. Estudo anatomico e indices diagnósticos da especie Datura arborea L. (Solanaceae). – Arq. Jard. Bot. Rio de Janeiro 24: 153-178.
McNeal JR, Kuehl JV, Boore JL, dePamphilis CW. 2007. Complete plastid genome sequences suggest strong selection for retention of photosynthetic genes in the parasitic plant genus Cuscuta. – BMC Plant Biol. 7: 57.
McPherson GD. 1979. Studies in the genus Ipomoea. – Ph.D. diss., University of Michigan, Ann Arbor, Michigan.
Machado AS, Cosa MT, Barboza GE. 2011. Anatomía de fruto y semilla de Duckeodendron cestroides, una enigmática especie de Solanaceae. – Bol. Soc. Argent. Bot. 46 (Suppl.): 106.
Madhavadian P. 1967. The cytology of Iochroma tubulosa Benth. – Caryologia 20: 309-315.
Madhavadian P. 1968. Chromosome numbers in South Indian Solanaceae. – Caryologia 21: 343-347.
Mahmood T, Salman S, Siddiqui S. 1988. Daturinol. A new withanolide from the leaves of Datura metel. – Heterocycles 27: 101-103.
Makino B, Kawai M, Iwata Y, Yamamura H et al. 1995. Physalins from Physalis alkekengi var. franchetii. – Bull. Chem. Soc. Japan 68: 219-226.
Maldoni BE. 1984. Alcaloides en dos especies de Lycium. – Rev. Latinoamer. Quím. 15: 83.
Manoko MLK, Berg RG, Feron RMC, Weerden GM, Mariani C. 2007. AFLP markers support separation of Solanum nodiflorum from Solanum americanum sensu stricto (Solanaceae). – Plant Syst. Evol. 267: 1-11.
Manos PS, Miller RE, Wilken P. 2001. Phylogenetic analysis of Ipomoea, Argyreia, Stictocardia, and Turbina suggests a generalized model of morphological evolution of morning glories. – Syst. Bot. 26: 585-602.
Mansilla MS, Cosa MT, Dottori N. 1999. Estudio morfo-anatómico de órganos vegetativos en representantes de los géneros Solanum Sect. Cyphomandropsis y Cyphomandra. – Kurtziana 27: 271-284.
Markert A, Steffan N, Ploss K, Hellwig S, Steiner U, Drewke C, Li S-M, Boland W, Leistner E. 2008. Biosynthesis and accumulation of ergoline alkaloids in a mutualistic association between Ipomoea asarifolia (Convolvulaceae) and a clavicipitalean fungus. – Plant Physiol. 147: 296-305.
Marks CE, Newbigin E, Ladiges PY. 2011. Comparative morphology and phylogeny of Nicotiana section Suaveolentes (Solanaceae) in Australia and the South Pacific. – Aust. Syst. Bot. 24: 61-86.
Martin JN, Erwin AT, Lounsberry CC. 1932. Nectaries of Capsicum. – Iowa St. Coll. J. Sci. 6: 277-285.
Martin PG, Dowd JM, Morris C, Symon DE. 1984. The study of plant phylogeny using amino acid sequences of ribulose-1,5-bisphosphate carboxylase VI. Some Solanum and allied species from different continents. – Aust. J. Bot. 34: 187-195.
Martine CT, Vanderpool D, Anderson GJ, Les DH. 2006. Phylogenetic relationships of andromonoecious and dioecious Australian species of Solanum subgenus Leptostemonum section Melongena: inferences from ITS sequence data. – Syst. Bot. 32: 410-420.
Martine CT, Anderson GJ, Les DH. 2009. Gender-bending aubergines: molecular phylogenetics of cryptically dioecious Solanum in Australia. – Aust. Syst. Bot. 22: 107-120.
Martínez M. 1998. Revision of Physalis section Epateiorhiza (Solanaceae). – Anal. Inst. Biol. Univ. Nac. Autón. Mexico, Ser. Bot., 69: 71-117.
Martínez M. 1999. Infrageneric taxonomy of Physalis. – In: Nee M, Symon D, Lester RN, Jessop J (eds), Solanaceae IV, Royal Botanic Gardens, Kew, pp. 275-283.
Martínez M. 1967. Las ‘solandras’ de Mexico, con una especie nueva. – Anal. Inst. Biol. Univ. Nac. Autón. Mexico, Ser. Bot., 37: 97-106.
Martínez M, Vargas O. 2005. A new species of Tzeltalia (Solanaceae) from Mexico. – Brittonia 57: 35-38.
Martins TR, Barkman TJ. 2005. Reconstruction of Solanaceae phylogeny using the nuclear gene SAMT. – Syst. Bot. 30: 435-447.
Martins TR, Stout JT, Todd SE, Kuipers K, Barkman TJ. 2007. Molecular phylogenetic tests of floral scent evolution in the Solanaceae. – In: Spooner DM, Bohs L, Giovannoni J, Olmstead RG, Shibata D (eds), Solanaceae VI. Genomics Meets Biodiversity, ISHA Section Root and Tuber Crops, Acta Hortic. 745: 183-200.
Matuda E. 1964. El género de Ipomoea en México II. – An. Inst. Biol. Univ. Nac. México 35: 45-76.
Mechler E, Kohlenbach HW. 1978. Alkaloid content in leaves of diploid and haploid Datura species. – Planta Med. 33: 350-355.
Meeuse ADJ. 1957. The South African Convolvulaceae. – Bothalia 6: 641-792.
Mendes EJ. 1978. 65. Montiniaceae. – In: Launert E (ed), Flora Zambesiaca 4, Flora Zambesiaca Managing Committee, London, pp. 54-58.
Mendozer-Heuer I. 1971. Aportación al conocimiento del género Convolvulus en la zona Macaronesica. – Cuad. Bot. Canar. 12: 22-34.
Mentz LA, de Oliveira PL. 2004. Solanum (Solanaceae) na região Sul do Brasil. – Pesquisas, Bot. 54: 9-327.
Menzel MY. 1950. Cytotaxonomic observations on some genera of the Solanaceae: Margaranthus, Saracha and Quincula. – Amer. J. Bot. 37: 25-30.
Menzel MY. 1951. The cytotaxonomy and genetics of Physalis. – Proc. Amer. Philos. Soc. 95: 132-183.
Merxmüller H, Butler KP. 1975. Nicotiana in der afrikanischen Namib. – Mitt. Bot. Staatssamml. München 12: 91-104.
Merz KW, Stolte H. 1960. Constituents of Lycium species. – Planta Medica 8: 121-126.
Mesa A. 1986. The classification of the Nolanaceae. – In: A’Arcy WG (ed), Solanaceae: biology and systematics, New York, pp. 86-90.
Millan AR. 1931. Solanáceas argentinas; claves para la determinación de los géneros. – Bol. Minist. Agric. Nac. 30: 3-21.
Millan AR. 1941. Revisión de las especies del género Nierembergia (Solanaceae). – Darwiniana 5: 487-547.
Millan M, Crepet W. 2014. The fossil record
of the Solanaceae revisited and
revised – the fossil record of Rhamnaceae enhanced.
– Bot. Rev. 80: 73-106.
Miller JS. 2002. Phylogenetic relationships and the evolution of gender dimorphism in Lycium (Solanaceae). – Syst. Bot. 27: 416-428.
Miller JS, Diggle PK. 2003. Diversification of andromonoecy in Solanum section Lasiocarpa (Solanaceae): the roles of phenotypic plasticity and architecture. – Amer. J. Bot. 90: 707-715.
Miller JS, Venable DL. 2002. The transition to gender dimorphism on an evolutionary background of self-incompatibility: an example from Lycium (Solanaceae). – Amer. J. Bot. 89: 1907-1915.
Miller JS, Levin RA, Feliciano NM. 2008. A tale of two continents: Baker’s Rule and the maintenance of self-incompatibility in Lycium (Solanaceae). – Evolution 62: 1052-1065.
Miller JS, Kamath A, Levin RA. 2009. Do multiple tortoises equal a hare? The utility of nine noncoding plastid regions for species-level phylogenetics in tribe Lycieae (Solanaceae). – Syst. Bot. 34: 796-804.
Miller JT, Spooner DM. 1999. Collapse of species boundaries in the wild potato Solanum brevicaule complex (Solanaceae, S. sect. Petota): molecular data. – Plant Syst. Evol. 214: 103-130.
Miller RE, Rausher MD, Manos PS. 1999. Phylogenetic systematics of Ipomoea (Convolvulaceae) based on ITS and waxy sequences. – Syst. Bot. 24: 209-227.
Miller RE, Buckley TR, Manos PS. 2002. An examination of the monophyly of morning glory taxa using Bayesian phylogenetic inference. – Syst. Biol. 51: 740-753.
Miller RE, McDonald JA, Manos PS. 2004. Systematics of Ipomoea subgenus Quamoclit (Convolvulaceae) based on ITS sequence data and a Bayesian phylogenetic analysis. – Amer. J. Bot. 91: 1208-1218.
Miller RJ, Mione T, Phan H-L, Olmstead RG. 2011. Color by numbers: nuclear gene phylogeny of Jaltomata (Solanaceae), sister genus to Solanum, supports three clades differing in fruit color. – Syst. Biol. 36: 153-162.
Milne-Redhead E, Metcalfe CR. 1955. Montiniaceae. – Hooker’s Icon. Plant. Ser. 5, 6(2): tab. 3541-3544, 1-10.
Minne L, Spies JJ, Venter AJT, Venter AM. 1994. Breeding systems in some representatives of the genus Lycium (Solanaceae). – Bothalia 24: 107-110.
Mione T. 1999. Jaltomata II. New combinations for five South American species. – Brittonia 51: 31-33.
Mione T, Leiva S. 1997. A new Peruvian species of Jaltomata (Solanaceae) with blood-red floral nectar. – Rhodora 99: 283-286.
Mione T, Serazo LA. 1999. Jaltomata lojae (Solanaceae). Description and floral biology of a new Andean species. – Rhodora 101: 136-142.
Mione T, Anderson GJ, Nee M. 1993. Jaltomata I: circumscription, description, and new combinations for five South American species (Solaneae, Solanaceae). – Brittonia 45: 138-145.
Mione T, Olmstead RC, Jansen RK, Anderson GJ. 1994. Systematic implications of chloroplast DNA variation in Jaltomata and selected physaloid genera (Solanaceae). – Amer. J. Bot. 81: 912-918.
Mione T. Leiva S, Yacher L. 2000. Three new species of Jaltomata (Solanaceae) from Ancash (Peru). – Novon 10: 53-59.
Mirzamatov RT, Lutfullin KL, Malikov VM, Junusov SJ. 1974. Fizochlain – novyj alkaloid iz Physochlaina alaica. – Chim. Rirod. Soed. 1974: 415-417.
Misico RI, Oberti JC, Veleiro AS, Burton G. 1996. New 19-hydroxywithanolides from Jaborosa leucotricha. – J. Natur. Prod. 59: 66-68.
Misico RI, Veleiro AS, Burton G, Oberti JC. 1997. Withanolides from Jaborosa leucotricha. – Phytochemistry 45: 1045-1048.
Misico RI, Oberti JC, Nicotra V, Barboza GE, Gil RR, Burton G. 2011. Withanolides and related steroids. – In: Kinghorn AD et al. (eds), Progress in the chemistry of organic natural products 94: 127-229.
Mitchell CD, Eshbaugh WH, Wilson KG, Pittman BK. 1989. Patterns of chloroplast DNA variation in Capsicum (Solanaceae): a preliminary study. – Newslett. Intern. Organ. Plant Biosyst. 12: 3-11.
Mitra JN. 1947. A contribution to the life-history of Hydrolea zeylanica Vahl. J. Indian Bot. 26: 51-61.
Modilewski J. 1935. Cytological investigation of the genus Nicotiana. Cytology and embryology of the amphi-diploid Nicotiana ditagla. – Vseukr. Akad. Nauk. Inst. Bot. Ž. 7: 7-29.
Mogensen HL, Suthar HK. 1979. Ultrastructure of the egg apparatus of Nicotiana tabacum (Solanaceae) before and after fertilization. – Bot. Gaz. 140: 168-179.
Mohan K. 1968. Morphology, development and structure of seed of Browallia demissa L. – Proc. Nat. Inst. Sci. India (Part B: Biol. Sci.) 34: 142-148.
Mohan K, Singh B. 1969. Morphological studies in Solanaceae III. Structure and development of seed of Solanum torvum Sw. – J. Indian Bot. Soc. 48: 338-345.
Mohan Ram HY, Kamini I. 1964. Embryology and fruit development in Withania somnifera Dunal. – Phytomorphology 14: 574-587.
Momin AR. 1975a. Bearing of embryological data on taxonomy of Convolvulaceae. – J. Univ. Bombay 44: 50-65.
Momin AR. 1975b. On embryological formulae of some Convolvulaceae members. – Sci. Cult. 41: 318.
Moncada M, Fuentes VR. 1993. Palinología de Goetzeaceae. – Rev. Jard. Bot. Nac. Univ. Habana 12: 75-79.
Monod T. 1980. À propos du Sphenoclea zeylanica (Sphenocleaceae). – Adansonia, sér. II, 20: 147-164.
Monteagudo ES, Oberti JC, Gros EG, Burton G. 1990. A spiranic withanolide from Jaborosa odonelliana. – Phytomorphology 29: 933-935.
Montero-Castro JC, Delgado-Salinas A, De Luna E, Equiarte LE. 2006. Phylogenetic analysis of Cestrum section Habrothamnus (Solanaceae) based on plastid and nuclear DNA sequences. – Syst. Bot. 31: 843-850.
Moré M, Cocucci AA, Sérsic AN, Barboza GE. 2015. Phylogeny and floral trait evolution in Jaborosa (Solanaceae). – Taxon 64: 523-534.
Mors WB, Ribeiro O. 1957. Occurrence of scopoletin in the genus Brunfelsia. – J. Org. Chem. 22: 978.
Morton CV. 1936. The genus Cestrum in Guatemala. – J. Arnold Arbor. 17: 341-349.
Morton CV. 1976. A revision of the Argentine species of Solanum. – Academia Nacional de Ciencias, Córdoba, Argentina.
Moscone EA. 1986. Sobre el gineceo de Vassobia. – Bol. Soc. Argent. Bot. 24: 319-331.
Moscone EA. 1989. Karyotype analysis in three Patagonian and Andean endemic genera of Nicotianeae (Solanaceae). – Plant Syst. Evol. 166: 31-39.
Moscone EA. 1990. Chromosome studies on Capsicum (Solanaceae) I. Karyotype analysis in C. chacoënse. – Brittonia 42: 147-154.
Moscone EA. 1991. Análisis cariotípico en algunos representantes de Solanum sect. Cyphomandropsis y Cyphomandra (Solanaceae) y su implicancia en la delimitación de ambos géneros. Resúmenes XXIII. – Jorn. Argent. Bot.: 209.
Moscone EA. 1992. Estudios sobre cromosomas meióticos en Solanaceae de Argentina. – Darwiniana 31: 261-297.
Moscone EA, Lambrou M, Hunziker AT, Ehrendorfer F. 1993. Giemsa C-banded karyotypes in Capsicum (Solanaceae). – Plant Syst. Evol. 186: 213-229.
Moscone EA, Lambrou M, Ehrendorfer F. 1996. Fluorescent chromosome banding in the cultivated species of Capsicum (Solanaceae). – Plant Syst. Evol. 202: 37-63.
Moscone EA, Ordonez A del V, Freire de Carvalho L d’A, Hunziker AT. 2005. Thr chromosomes of Metternichia principis (Solanaceae) and their significance in the systematic position of the genus. – In: Keating RC, Holowell VC, Croat TB (eds), A Festschrift for William G. D’Arcy: the legacy of a taxonomist, Monogr. Syst. Bot. Missouri Bot. Gard. 104, Missouri Botanical Garden Press, St. Louis, Missouri, pp. 315-320.
Moscone EA, Scaldaferro MA, Grabiele M, Romero MV, Debat H, Seijo JG, Acosta MC, Daviña JR, Barboza GE, Ducasse DA. 2011. Genomic characterization of the chili peppers (Capsicum, Solanaceae) germplasm by classical and molecular cytogenetics. – In: Physical mapping technologies for the identification and characterization of mutated genes to crop quality, International Atomic Energy Agency, Vienna, Austria, TECDOC 1664, pp. 97-104.
Muller CH. 1940. A revision of the genus Lycopersicon. – U.S. Dept. Agric. Misc. Publ. 382, Washington, D.C.
Müller W. 1905. Beiträge zur Entwickelungsgeschichte der Infloreszenzen der Boraginaceen und Solaneen. – Flora 94: 385-419.
Muñoz O. 1991. Schizanthine X, a new alkaloid from Schizanthus grahamii. – J. Natur. Prod. 54: 1094-1096.
Muñoz O, Piovano M, Garbarino J, Hellwig V, Breitmaier E. 1996. Tropane alkaloids from Schizanthus littoralis. – Phytochemistry 43: 709-713.
Murray MA. 1945. Carpellary and placental structure in the Solanaceae. – Bot. Gaz. (Crawfordsville) 107: 243-260.
Murry LE, Eshbaugh WH. 1971. A palynological study of the Solaninae (Solanaceae). – Grana 11: 65-78.
Myint T. 1966. Revision of the genus Stylisma (Convolvulaceae). – Brittonia 18: 97-117.
Nagels L, Dongen W van, Parmentier F. 1982. Cestric acid, a caffeic acid ester from Cestrum euanthes. – Phytochemistry 21: 743-746.
Nahrstedt A, Sattar EA, El-Zalabani SMH. 1990. Amygdalin-acyl-derivatives, cyanogenic glycosides from the seeds of Merremia dissecta. – Phytochemistry 29: 1179-1181.
Nalbandov O, Yamamoto RT, Fraenkel GS. 1964. Nicandrenone, a new compound with insecticidal properties, isolated from Nicandra physaloides. – Agric. Food Chem. 12: 55-59.
Namoff S, Thornton HEB, Lewis CE, Oviedo R, Francisco-Ortega J. 2007. Molecular evidence for phylogenetic relationships of Jacquemontia reclinata House (Convolvulaceae) – a critically endangered species from South Florida. – Bot. J. Linn. Soc. 154: 443-454.
Natarajan AT. 1957. Studies in the morphology of pollen grains – Tubiflorae. – Phyton (Buenos Aires) 8: 21-42.
Nee M. 1979. Patterns in biogeography in Solanum, section Acanthophora. – In: Hawkes JG, Lester RN, Skelding AD (eds), The biology and taxonomy of the Solanaceae, Linn. Soc. Symp. Ser. 7, Linnean Society and Academic Press, London, pp. 569-580.
Nee M. 1986. Placentation patterns in the Solanaceae. – In: D’Arcy W (ed), Solanaceae: biology and systematics, Columbia University Press, New York, pp. 169-175.
Nee M. 1991a. Synopsis of Solanum Section Acanthophora: A group of interest for glycoalkaloids. – In: Hawkes JG, Lester RN, Nee M, Estrada N (eds), Solanaceae III. Taxonomy, chemistry, evolution, Royal Botanic Gardens, Kew, pp. 257-266.
Nee M. 1991b. Datura and Brugmansia: two genera or one? – Solanaceae Newsletter : 27-35.
Nee M. 1999. Synopsis of Solanum in the New World. – In: Nee M, Symon DE, Lester RN, Jessop JP (eds), Solanaceae IV: advances in biology and utilization, Royal Botanic Gardens, Kew, pp. 285-334.
Nee M, Symon DE, Lester RN, Jessop JP (eds). 1999. Solanaceae IV: advances in biology and utilization. – Royal Botanic Gardens, Kew.
Nee M, Bohs L, Knapp S. 2006. New species of Solanum and Capsicum (Solanaceae) from Bolivia, with clarification of nomenclature in some Bolivian Solanum. – Brittonia 58: 322-356.
Newbigin E, Anderson M, Clarke A. 1999. Molecular basis of self-incompatibility in the Solanaceae. – In: Nee M, Symon DE, Lester RN, Jessop JP (eds), Solanaceae IV. Advances in biology and utilization, Royal Botanic Gardens, Kew, Richmond, pp. 189-195.
Neyland R. 2001. A phylogeny inferred from large ribosomal subunit (26S) rDNA sequences suggests that Cuscuta is a derived member of Convolvulaceae. – Brittonia 53: 108-115.
Ng J, Smith SD. 2016. Widespread flower color convergence in Solanaceae via alternate biochemical pathways. – New Phytol. 209: 407-417.
Nha NT, Danert S. 1973. Ueber die Fruchtentwicklung und über zentrische Leitbundel in der Gattung Datura (Solanaceae). – Kulturpflanzen 21: 119-193.
Nishino E. 1983. Corolla tube formation in the Tubiflorae and Gentianales. – Bot. Mag. (Tokyo) 96: 223-243.
Nurit-Silva K, Agra MF. 2011. Leaf epidermal characters of Solanum sect. Polytrichum (Solanaceae) as taxonomic evidence. – Microsc. Res. Tech. 74: 1186-1191.
Oberti JC. 1998. Withanolides en Solanaceae, con especial referencia a la tribu Jaboroseae. – Proc. VI. Congr. Latinoamer. Bot., Missour Bot. Gard. Press, St. Louis, Missouri, pp. 47-52.
Ochoa CM. 1950. Papas del subgrupo Trigonohypsa en el Central del Peru. – Bol. Soc. Peruana Bot. 2: 5-8.
Ochoa CM. 1962. Los solanum tuberiferos del Perú (Secc. Tuberarium, Sub-secc. Hyperbasarthrum). – Privately publ., Lima, Peru.
Ochoa CM. 1990. The potatoes of South America: Bolivia. – Cambridge University Press, Cambridge.
Ochoa CM. 1999. Las papas de Sudamérica: Peru (Parte I). – Centro Internacional de la papa, Lima, Peru.
O’Donell CA. 1941. Revisión de las especies americanas de Merremia (Convolvulaceae). – Lilloa 6: 467-554.
O’Donell CA. 1953. Un nuevo género de Convolvuláceas: Iseia O’Donell. – Bol. Soc. Argentina Bot. 5: 75-80.
O’Donell CA. 1957. Convolvuloideas Chilenas. – Bol. Soc. Argent. Bot. 6: 143-184.
O’Donell CA. 1960. Notas sobre Convolvuláceas americanas. – Lilloa 30: 39-69.
Ogunwenmo KO. 2003. Cotyledon morphology: an aid in identification of Ipomoea taxa (Convolvulaceae). – Feddes Repert. 115: 198-203.
Ogunwenmo KO. 2006. Variation in fruit and seed morphology, germination and seedling behaviour of some taxa of Ipomoea L. (Convolvulaceae). – Feddes Repert. 117: 207-216.
Okada KA Carrillo BJ, Tilley M. 1977. Solanum malacoxylon Sendt.: a toxic plant in Argentina. – Econ. Bot. 31: 225-236.
Olmstead RG. 2013. Phylogeny and biogeography in Solanaceae, Verbenaceae and Bignoniaceae: a comparison of continental and intercontinental diversification patterns. – Bot. J. Linn. Soc. 171: 80-102.
Olmstead RG, Bohs L. 2007. A summary of molecular systematic research in Solanaceae: 1982-2006. – In: Spooner DM, Bohs L, Giovannoni J, Olmstead RG, Shibata D (eds), Solanaceae VI: genomics meets biodiversity, ISHA Section Root and Tuber Crops, Acta Horticulturae 745, International Science for Horticultural Science, Leuven, pp. 255-268.
Olmstead RG, Palmer JD. 1991. Chloroplast DNA and systematics of the Solanaceae. – In: Hawkes JG, Lester RN, Nee M, Estrada N (eds), Solanaceae III: taxonomy, chemistry, evolution, Royal Botanic Gardens, Kew, pp. 161-168.
Olmstead RG, Palmer JD. 1992. A chloroplast DNA phylogeny of the Solanaceae: subfamilial relationships and character evolution. – Ann. Missouri Bot. Gard. 79: 346-360.
Olmstead RG, Palmer JD. 1997. Implications for the phylogeny, classification, and biogeography of Solanum from cpDNA restriction site variation. – Syst. Bot. 22: 19-29.
Olmstead RG, Sweere JA. 1994. Combining data in phylogenetic systematics: an empirical approach using three molecular data sets in the Solanaceae. – Syst. Biol. 43: 467-481.
Olmstead RG, Sweere JA, Spangler RE, Bohs L, Palmer JD. 1999. Phylogeny and provisional classification of the Solanaceae based on chloroplast DNA. – In: Nee M, Symon DE, Lester RN, Jessop JP (eds), Solanaceae IV: advances in biology and utilization, Royal Botanic Gardens, Kew, pp. 111-137.
Olmstead RG, Bohs L, Migid HA, Santiago-Valentin E, Garcia VF, Collier SM. 2008. A molecular phylogeny of the Solanaceae. – Taxon 57: 1159-1181.
Ono M. 1967a. The systematic position of Scalesia from the viewpoint of chromosome number. – Not. Galápagos 9-10: 16-17.
Ono M. 1967b. Chromosome number of Scalesia (Compositae), an endemic genus of the Galápagos Islands. – J. Jap. Bot. 42: 353-360.
Ooststroom SJ van. 1939. The Convolvulaceae of Malaysia II. The genera Jacquemontia, Aniseia, Convolvulus, Calystegia, Shutereia, Merremia, Operculina and Decalobanthus. – Blumea 3: 267-371.
Ooststroom SJ van. 1940. The Convolvulaceae of Malaysia III. The genus Ipomoea. – Blumea 3: 481-582.
Ooststroom SJ van. 1943. The Convolvulaceae of Malaysia IV. The genera Mina, Lepistemon, Stictocardia and Argyreia. – Blumea 5: 339-393.
Ooststroom SJ van, Hoogland RD. 1953. Convolvulaceae. – In: Steenis CGGJ van (ed), Flora Malesiana I, 4(4), Noordhoff-Kolff N. V., Batavia, pp. 458-512.
Orejuela A. 2016. Filogenia de Markea Rich. (Solanaceae: Juanulloeae) con base en datos de secuencia de ADN nuclear y del cloroplasto. – M.Sc. thesis, Universidad Nacional de Colombia, Bogotá, Colombia.
Orejuela A, Orozco CI, Barboza GE. 2014. Three new species of Markea (Solanaceae, Juanulloeae) from Colombia. – Phytotaxa 167: 151-165.
Orejuela A, Wahlert GA, Orozco CI, Barboza GE, Bohs L. 2017. Phylogeny of the tribes Juanulloeae and Solandreae (Solanaceae). – Taxon 66: 379-392.
Ortega AT. 1974. Estudio biosistemático de cinco especies de Datura existentes en el Ecuador. – Ciencia y Naturaleza 15: 12-54.
Ortiz-Ramírez CI, Plata-Arboleda S, Pabón-Mora N. 2018. Evolution of genes associated with gynoecium patterning and fruit development in Solanaceae. – Ann. Bot. 121: 1211-1230.
OshimaY, Hikino H, Sahai M, Ray A. 1989. Withaperuvin H, a withanolide of Physalis peruviana roots. – J. Chem. Soc. 1989: 628-629.
Paez DM, Gil BA. 1971. Estudio de los alcaloides de Nicotiana noctiflora. – Rev. Asoc. Bioq. Arg. 36: 86-92.
Palmer JD, Zamir D. 1982. Chloroplast DNA evolution and phylogenetic relationships in Lycopersicon. – Proc. Natl. Acad. U.S.A. 79: 5006-5010.
Palomino G, Viveros R, Bye RA Jr. 1988. Cytology of five Mexican species of Datura L. (Solanaceae). – Southw. Natur. 33: 85-90.
Pant DD, Banerji R. 1965. Epidermal structure and development of stomata in some Convolvulaceae. – Senck. Biol. 46: 155-173.
Parokonny AS, Kenton AY. 1995. Comparative physical mapping and evolution of the Nicotiana tabacum L. karyotype. – In: Brandham PE, Bennett MD (eds), Kew Chromosome Conference IV. Royal Botanic Gardens, Kew, Richmond, pp. 301-320.
Parr A, Payne J, Eagles J, Chapman BP et al. 1990. Variation in tropane alkaloid accumulation within the Solanaceae and strategies for its exploitation. – Phytochemistry 29: 2545-2550.
Pascher A. 1909. Species novae generis Physochlainae. – Feddes Repert. 7: 166-167.
Pascher A. 1910a. Der Aufbau des Sprosses bei Przewalskia tangutica. – Flora 100: 295-304.
Pascher A. 1910b. Ueber Gitterkelche, einen neuen biologischen Kelchtypus der Nachtschattengewächse. – Flora 101: 273-278.
Pascher A. 1959. Ueber Atropa. – Flora 148: 84-109.
Passarelli LM. 1999. Morphology, reserves and pollen viability of some Solanum sect. Cyphomandropsis species. – Grana 38: 284-288.
Patel RC, Inamdar JA. 1971. Structure and ontogeny of stomata in some Polemoniales. – Ann. Bot. 35: 389-409.
Pavari F. 1957. Ricerche embriologiche e cariologiche su Cestrum elegans L. (Solanaceae). – Caryologia 9: 437-452.
Pearce C, Skelton MNJ, Naylor S, Kanaan R. et al. 1992. Parquin and carboxyparquin, toxic kaurene glycosides from the shrub Cestrum parqui. – J. Chem. Soc. Perkin Trans. 1, 1992: 593-600.
Peralta IE, Spooner DM. 2000. Classification of wild tomatoes: a review. – Kurtziana 28: 45-54.
Peralta IE, Spooner DM. 2001. Granule-bound starch synthase (GBSSI) gene phylogeny of wild tomatoes (Solanum L. section Lycopersicon [Mill.] Wettst. subsection Lycopersicon). – Amer. J. Bot. 88: 1888-1902.
Peralta IE, Spooner DM. 2005. Morphological characterization and relationships of wild tomatoes (Solanum L. Section Lycopersicon). – Monogr. Syst. Bot., Missouri Bot. Gard. 104: 227-257.
Peralta IE, Knapp S, Spooner DM. 2005. New species of wild tomatoes (Solanum section Lycopersicon: Solanaceae) from northern Peru. – Syst. Bot. 30: 424-434.
Peralta IE, Spooner DM, Knapp S. 2008.
Taxonomy of wild tomatoes and their relatives (Solanum sect.
Lycopersicoides, sect. Juglandifolia, sect.
Lycopersicon; Solanaceae).
– Syst. Bot. Monogr. 84: 1-186.
Pérez F, Arroyo MTK, Medel R, Hershkovitz MA. 2006. Ancestral reconstruction of flower morphology and pollination systems in Schizanthus (Solanaceae). – Amer. J. Bot. 93: 1029-1038.
Perry G, McNeill J. 1987. Nomenclatural notes on Salpichroa (Solanaceae). – Taxon 36: 132-135.
Persson V, Knapp S, Blackmore S. 1994. Pollen morphology and systematics of tribe Juanulloeae A. Hunziker. – Rev. Paleobot. Palynol. 83: 1-30.
Persson V, Knapp S, Blackmore S. 1999. Pollen morphology and the phylogenetic analysis of Datura and Brugmansia. – In: Nee M, Symon DE, Lester RN, Jessop JP (eds), Solanaceae IV: advances in biology and utilization, Royal Botanic Gardens, Kew, pp. 171-188.
Peter A. 1897a. Convolvulaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien IV(3a), W. Engelmann, Leipzig, pp. 1-40, 375-377.
Peter A. 1897b. Hydrophyllaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien IV(3a), W. Engelmann, Leipzig, pp. 54-71.
Pfleger R. 1964. Daturamin, ein neues Scopinalkaloid. – Arch. Pharm. 297: 182-183.
Phillipson JD, Handa SS. 1975. N-oxides of hyoscyamine and hyoscine in the Solanaceae. – Phytochemistry 14: 999-1003.
Pichon M. 1947. Le genre Humbertia. – Not. Syst. (Paris) 13: 13-25.
Pickersgill B. 1977. Chromosomes and evolution in Capsicum. – In: Pochard E (ed), Capsicum 77, C. R. 3ème Congr. Eucarpia, pp. 27-37.
Pickersgill B. 1988. The genus Capsicum: a multidisciplinary approach to the taxonomy of cultivate and wild plants. – Biol. Zentralbl. 107: 381-389.
Piovano MA. 1989. El cariótipo de Dyssochroma longipes (Solanaceae). – Kurtziana 20: 207-212.
Pistone A. 1894. Le liane del genere Solandra Sw. Ricerche anatomo-biologiche. – Contr. Biol. Veg. 1: 98-122.
Pitrez SR, Andrade LA, Alves LIF, Felix LP. 2008. Karyology of some Convolvulaceae species occurring in NE Brazil inselbergs. – Plant Syst. Evol. 276: 235-241.
Plowman T. 1973. Four new ‘brunfelsias’ from northwestern Souh America. – Bot. Mus. Leafl. 23: 14-20.
Plowman T. 1979. The genus Brunfelsia. A conspectus of taxonomy and biogeography. – In: Hawkes JG, Lester RN, Skelding AD (eds), The biology and taxonomy of the Solanaceae, Linn. Soc. Symp. Ser. 7, Linnean Society and Academic Press, London, pp. 475-482.
Plowman T. 1981. Five new species of Brunfelsia from South America (Solanaceae). – Fieldiana, Bot., N. S., 8: 1-16.
Plowman T. 1982. Brugmansia (tree-daturas) in South America. – In: Voelger G, Welck K von (eds), Rausch und Realität 2, Rowohlt Verlag, Hamburg, pp. 770-784.
Plowman T. 1998. A revision of the South American species of Brunfelsia. – Fieldiana, Bot., N. S., 39: 1-135.
Plowman T, Gyllenhaal LO, Lindgren JE. 1971. Latua pubiflora, magic plant from southern Chile. – Bot. Mus. Leafl. 23: 61-92.
Poczai P, Taller J, Szabó I. 2008. Analysis of phylogenetic relationships in the genus Solanum (Solanaceae) as revealed by RAPD markers. – Plant Syst. Evol. 275: 59-67.
Pomilio AB, Gros EG. 1979. Pinocembrin 7-neohesperidoside from Nierembergia hippomanica. – Phytochemistry 18: 1410-1411.
Porembski S, Koch M. 1999. Inulin occurrence in Sphenoclea (Sphenocleaceae). – Plant Biol. 1: 288-289.
Powell AM, Averett JE. 1967. Chromosome numbers of Chamaesaracha (Solanaceae) in Trans-Pecos Texas and adjacent regions. – Sida 3: 156-162.
Prasad T, Singh D. 1978. Gametophytes and seed development in Nicandra physaloides Gaertn. – J. Indian Bot. Soc. 57: 76-83.
Prenner G, Deutsch G, Harvey P. 2002. Floral development and morphology in Cuscuta reflexa Roxb. (Convolvulaceae). – Stapfia 80: 311-322.
Pringle GJ, Murray BG. 1993. Karyotypes and C-banding patterns in species of Cyphomandra Mart. ex Sendt. – Bot. J. Linn. Soc. 111: 331-342.
Prohens J, Anderson GJ, Blanca JM, Canizares J, Zuriaga E, Nuez F. 2006. The implications of AFLP data for the systematics of the wild species of Solanum section Basarthrum. – Syst. Bot. 31: 208-216.
Punt W, Monna-Brands M. 1977. the northwest European pollen flora 8. Solanaceae. – Rev. Paleobot. Palyn. 23: 1-30.
Purdie RW, Symon DE, Haegi L. 1982. Solanaceae. – In: George AS (ed), Flora of Australia 29, Australian Government Publ. Service, Canberra, pp. 1-197.
Quesada-Aguilar A, Kalisz S, Ashman T-L. 2008. Flower morphology and pollinator dynamics in Solanum carolinense (Solanaceae): implications for the evolution of andromonoecy. – Amer. J. Bot. 95: 974-984.
Rabaey D, Lens F, Smets E, Jansen S. 2010. The phylogenetic significance of vestured pits in Boraginaceae. Taxon 59: 510-516.
Radlkofer L. 1888. Über die Versetzung der Gattung Henoonia van der Sapotaceen zu den Solanaceen. – Sitzungsber. Bayer. Akad. Wiss. München, Math.-Phys. Cl. 18: 405-421.
Raffauf RF, Shemluck MJ, Le Quesne PW. 1991. The withanolides of Iochroma fuchsioides. – J. Natur. Prod. 54: 1601-1606.
Rajapakse S, Nilmalgoda SD, Molnar M, Ballard RE, Austin DF, Bohac JR. 2004. Phylogenetic relationships of the sweetpotato in Ipomoea series Batatas (Convolvulaceae) based on nuclear β-amylase gene sequences. – Mol. Phylogen. Evol. 30: 623-632.
Rajput KS, Raole VM, Gandhi D. 2008. Radial secondary growth and formation of successive cambia and their products in Ipomoea hederifolia L. (Convolvulaceae). – Bot. J. Linn. Soc. 158: 30-40.
Rao GR, D’Arcy WG. 1989. Cyto-morphology of Schwenckia browallioides. – Solanaceae Newsletter 3: 74-75.
Ratera EL. 1943. Número de cromosomas de algunas Solanáceas Argentinas. – Revista Fac. Agron. Veterin. 10: 318-325.
Ratera EL. 1961. Estudios cariológicos en Solanáceas. – Rev. Inst. Munic. Bot. 1: 61-65.
Ratera EL. 1969. Estudios cariológicos en Solanáceas II. – Rev. Inst . Munic. Bot. 3: 59-66.
Raud G. 1963. Organographie de la capsule du Datura stramonium L. – Bull. Soc. Bot. France 110: 216-237.
Ray AB, Sahai M, Sethi PD. 1976. Physoperuvine, a new alkaloid of Physalis peruviana Linn. – Chem. Ind. 1976: 454-455.
Reck-Kortmann M, Silva-Arias GA, Segatto ALA,
Mäder G, Bonatto SL, Freitas LB de. 2014. Multilocus phylogeny reconstruction:
new insights into the evolutionary history of the genus Petunia. –
Molec. Phylogen. Evol. 81: 19-28.
Record SJ. 1933. The woods of Rhabdodendron and Duckeodendron. – Trop. Woods 33: 6-10.
Rego LNAA, da Silva CRM, Torezan JMD, Gaeta ML, Vanzela ALL. 2009. Cytotaxonomical study in Brazilian species of Solanum, Lycianthes and Vassobia (Solanaceae). – Plant Syst. Evol. 279: 93-102.
Reis dos C, Das Graças Sajo M, Stehmann JR. 2002. Leaf structure and taxonomy of Petunia and Calibrachoa (Solanaceae). – Brazil. Arch. Biol. Technol. 45: 59-66.
Rendle AB. 1921. Elisia, an overlooked genus-name. – J. Bot. 59: 261-264.
Ribeiro O, Machado A. 1951. The alkaloid of Solanum lycocarpum St. Hil. – Engenh. e Quimica 3: 166-167.
Ricardi M. 1962. Notas botánicas I. El género Combera. – Gayana 4: 3-13.
Rick CM. 1963. Biosystematic studies on Galápagos tomatoes. – Occas. Pap. Calif. Acad. Sci. 44: 59-77.
Rick CM. 1979. Biosystematic studies in Lycopersicon and closely related species of Solanum. – In: Hawkes JG, Lester RN, Skelding AD (eds), The biology and taxonomy of Solanaceae, Linn. Soc. Symp. Ser. 7, Linnean Society and Academic Press, London, pp. 667-677.
Rick CM, Laterrot H, Philouze J. 1990. A revised key for the Lycopersion pecies. – TGO Report 40.
Ripperger H. 1979 Schizanthin A und B, zwei neue tropanalkaloide aus Schizanthus pinnatus. – Phytochemistry 18: 171-173.
Ripperger H. 1981. 4’’-O-Acetylsarotanoside, a novel flavanone glycoside from Nierembergia hippomanica. – Phytochemistry 20: 1757-1758.
Ripperger H, Porzel A. 1992. 2a-hydroxysoladulcidine from Lycianthes biflora. – Phytochemistry 31: 725-726.
Rivera C, Piñeyro E, Giral F. 1976. Dehydroacetic acid in anthers of Solandra nitida (Solanaceae). – Experientia 32: 1490.
Robertson KR. 1971. A revision of the genus Jacquemontia (Convolvulaceae) in North and Central America and West Indies. – Ph.D. diss., Washington University, St. Louis, Missouri.
Robertson KR. 1982. Odonellia, a new genus of Convolvulaceae from tropical America. – Brittonia 34: 417-423.
Roberty G. 1952. Genera Convolvulacearum. – Candollea 14: 11-60.
Roberty G. 1964. Les genres de Convolvulacées (esquisse). – Boissiera 10: 129-156.
Robyns W. 1931. L’organisation florale des Solanacées zygomorphes. – Mém. Acad. Roy. Sci. Belg., Cl. Sci., 11: 1-82.
Robyns W. 1932. L’étude detaillée des formes florales et son importance pour la systématique (Solanacées, Labiatées). – Bull. Assoc. Franç.: 264-270.
Robyns W. 1936. Sur des phénomènes de pleiomérie et de synanthie dans Salpiglossis sinuata. – Bull. Acad. Roy. Belg., Cl. Sci., 5a Sér. 22: 1080-1098.
Roddick JG. 1979. Distribution of steroidal glycoalkaloids in cells of Solanum and Lycopersicon. – In: Hawkes JG, Lester RN, Skelding AD (eds), The biology and taxonomy of the Solanaceae, Linn. Soc. Symp. Ser. 7, Linnean Society and Academic Press, London, pp. 223-229.
Roddick JG. 1986. Steroidal alkaloids of the Solanaceae. – In: D’Arcy (ed), Solanaceae biology and systematic, Columbia University Press, New York, pp. 201-222.
Rodríguez A, Spooner DM. 1997. Chloroplast DNA analysis of Solanum bulbocastanum and S. cardiophyllum, and evidence for the distinctiveness of S. cardiophyllum subsp. ehrenbergii (sect. Petota). – Syst. Bot. 22: 31-43.
Rodríguez A, Spooner DM. 2002. Subspecies boundaries of the wild potatoes Solanum bulbocastanum and S. cardiophyllum based on morphological and nuclear RFLP data. – Acta Mexicana 61: 9-25.
Rodríguez F, Spooner DM. 2009. Nitrate reductase phylogeny of potato (Solanum sect. Petota) genomes with emphasis on the origins of the polyploid species. – Syst. Bot. 34: 207-219.
Rodríguez I. 1995. Estudio morfoanatómico de hoja y tallo en Exodeconus maritimus (Solanaceae). – Arnaldoa 3: 9-18.
Rodríguez I. 1999a. Anatomía floral de Deprea orinocensis, Physalis minima y P. viscosa (Solanacae: Solaneae). – Kurtziana 28: 19-34.
Rodríguez I. 1999b. Flower anatomy and morphology of Exodeconus maritimus (Solanaceae, Solaneae) and Nicandra physalodes (Solanaceae, Nicandreae): importance for their systematic relationnsips. – Adansonia, sér. III, 22: 187-199.
Rodríguez I. 2000. Flower anatomy and morphology of Exodeconus maritimus (Solanaceae, Solaneae) and Nicandra physalodes (Solanaceae, Nicandreae): importance for their systematic relationships. – Adansonia, sér. III, 22: 187-199.
Roe KE. 1967a. Chromosome size in Solanum and Cyphomandra: taxonomic and phylogenetic implications. – Amer. Natur. 101: 295-297.
Roe KE. 1967b. A revision of Solanum sect. Brevantherum (Solanaceae) in North and Central America. – Brittonia 19: 353-373.
Roe KE. 1971. Terminology of hairs in the genus Solanum. – Taxon 20: 501-508.
Roe KE. 1972. A revision of Solanum sect. Brevantherum (Solanaceae). – Brittonia 24: 239-278.
Romeike A. 1962. Über das Vorkommen von 6-Hydroxyhyoscyamin in Datura. – Naturwissenschaften 49: 281.
Romeike A. 1965. Hygrin, das Hauptalkaloid in Nicandra-Wurzeln. – Naturwissenschaften 52: 619.
Romeike A. 1966. Vorkommen von Tropinon in Nicandra-Wurzeln. – Naturwissenschaften 53: 82.
Roney JK, Khatibi PA, Westwood JH. 2007. Cross-species translocation of mRNA from host plants into the parasitic plant dodder. – Plant Physiol. 143: 1037-1043.
Ronse De Craene L-P, Linder PH, Smets EF. 2000. The questionable relationship of Montinia (Montiniaceae): evidence from a floral ontogenetic and anatomical study. – Amer. J. Bot. 87: 1408-1424.
Rusby HH. 1926. Additions to the genus Lycianthes Dunal. – Bull. Torrey Bot. Club 53: 209-213.
Rydberg PA. 1896. The north American species of Physalis and related geera. – Mem. Torrey Bot. Club : 297-372.
Rydberg PA. 1924. The section Tuberarium of the genus Solanum in Mexico and Central America. – Bull. Torrey Bot. Club 51: 127-165.
Sa’ad F. 1967. The Convolvulus species of the Canary Islands, the Mediterranean region and the Near and Middle East. – Meded. Bot. Mus. Herb. Rijksuniv. Utrecht 281: 1-287.
Safford WE. 1921. Synopsis of the genus Datura. – J. Washington Acad. Sci. 11: 173-189.
Sahai M, Ray AB. 1980. Secotropane alkaloids of Physalis peruviana. – J. Org.Chem. 45: 3265-3268.
Sahai M, Singh M, Singh AK, Hara N, Fujimoto Y. 1994. Novel glucosides from the leaves of Cestrum nocturnum. – J. Chem. Res. (5) 1994, 1: 22-23.
Saitoh F, Noma M, Kawashima N. 1985. The alkaloid contents of sixty Nicotiana species. – Phytochemistry 24: 477-480.
Salas AR, Spooner DM, Huamán Z, Torres-Maita RV, Hoekstra R, Schüler K, Hijmans RJ. 2001. Taxonomy and new collections of wild potato species in central and southern Peru in 1999. – Amer. J. Potato Res. 78: 197-207.
Salts Y, Herrmann RG, Peleg N, Lavi U, Izhar S, Frankel R, Bechmann JS. 1984. Physical mapping of plastid DNA variation among eleven Nicotiana species. – Theor. Appl. Gen. 69: 1-14.
Sampathkumar R. 1982. Studies on the cotyledonary leaves of some Convolvulaceae. – Taxon 31: 53-56.
Sandina LB. 1980. A critical analysis of the genus Scopolia (Solanaceae). – Bot. Žurn. 65: 485-496.
Sandina LB, Taraevich VF. 1982. Some palynological data on the study of the genera Whiteleya, Atropanthe and Scopolia s. str. (Solanaceae). – Bot. Žurn. 67: 146-154.
San Martin A, Rovirosa J, Gambaro V, Castillo M. 1980. Tropane alkaloids from Schizanthus hookeri. – Phytochemistry 19: 2007-2008.
San Martin A, Labbé C, Muñoz O, Castillo M et al. 1987. Tropane alkaloids from Schizanthus grahamii. – Phytochemistry 26: 819-822.
Santiago-Valentin E. 1995. Reproductive and population ecology of Goetzea elegans Wydler (Solanaceae or Goetzeaceae). – Masters thesis, University of Puerto Rico, Mayagüez Campus, Puerto Rico.
Santiago-Valentin E, Olmstead RG. 2003. Phylogenetics of the Antillean Goetzeoideae (Solanaceae) and their relationships within the Solanaceae based on chloroplast and ITS DNA sequence data. – Syst. Bot. 28: 452-460.
Särkinen T, Bohs L, Olmstead RG, Knapp S. 2013. A phylogenetic framework study of the nightshades (Solanaceae): a dated 1000-tip tree. – BMC Evol. Biol. 13: 214.
Särkinen T, Barboza GE, Knapp S. 2015. True black nightshades: phylogeny and delimitation of the morelloid clade of Solanum. – Taxon 64: 945-958.
Sarmento da Silva TM, de Carvalho MG, Braz-Filho R, Agra MF. 2003. Occurrence of flavones, flavonols and its glycosides in species of the genus Solanum (Solanaceae). – Quim. Nova 26: 517-522.
Sassen MMA. 1964. Fine structure of Petunia pollen grain and pollen tube. – Acta Bot. Neerl. 13: 175-181.
Saunders ER. 1936. On certain unique features of the gynoecium in Nolanaceae. – New Phytol. 35: 423-431.
Sawyer NW. 1998. Two new species of Larnax (Solanaceae) from Ecuador. – Novon 8: 72-76.
Sawyer NW. 2001. New species and new combinations in Larnax (Solanaceae). – Novon 11: 460-471.
Sawyer NW. 2005. Systematics of Deprea and Larnax (Solanaceae) based on morphological evidence. – In: Keating RC, Holowell VC, Croat TB (eds), A Festschrift for William G. D’Arcy: the legacy of a taxonomist, Monogr. Syst. Bot. Missouri Bot. Gard. 104, Missouri Botanical Gadeen Press, St. Louis, Missouri, pp. 259-285.
Sawyer NW, Anderson GJ. 2000. Dioecy in South American Deprea. – Biotropica 33: 291-298.
Sawyer NW, Benítez de Rojas C. 1998. Morphological analysis of three equivocal sibling species of Deprea (Solanaceae). – Brittonia 50: 524-535.
Sayeedud Din M. 1953. Observations on the anatomy of some of the Convolvulaceae. – Proc. Indian Acad. Sci., Sect. B, 37: 106-109.
Sazima M, Vogel S, Cocucci A, Hausner G. 1993. The perfume flowers of Cyphomandra (Solanaceae): pollination by euglossine bees, bellows mechanism, osmophores and volatiles. – Plant Syst. Evol. 187: 51-88.
Sazima M, Buzato S, Sazima I. 2003. Dyssochroma viridiflorum (Solanaceae): a reproductively bat-dependent epiphyte from the Atlantic rainforest in Brazil. – Ann. Bot. (Oxford) 92: 725-730.
Schäfer H. 1969. Der Bau des Gynoeceum be Nicandra physaloides (Solanaceae). – Kulturpflanze 17: 187-189.
Schilling EE. 1981. Systematics of Solanum sect. Solanum (Solanaceae) in North America. – Syst. Bot. 6: 172-185.
Schimming T. 2001. Beiträge zur Phytochemie und Chemotaxonomie der Convolvulaceen unter besonderer Berücksichtigung des Calysteginvorkommens. – Ph.D. diss., Fachbereich Biologie, Chemie, Pharmazie, Freie Universität Berlin, Germany.
Schimming T, Tofern B, Mann P, Richter A, Jennett-Siems K, Dräger B, Asano M, Gupta MP, Correa MD, Eich E. 1998. Distribution and taxonomic significance of calystegins in the Convolvulaceae. – Phytochemistry 49: 1989-1995.
Schönland S. 1894. Campanulaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien IV(5), W. Engelmann, Leipzig, pp. 40-70.
Schreiber K. 1963a. Ueber die Alkaloidgykoside kollentragender Solanum-arten. – Kulturpflanze 11: 422-450.
Schreiber K. 1963b. Isolieren von Solasodin-glyosiden aus Pflanzen der Gattung Solanum L. – Kulturpflanze 11: 451-501
Schreiber K. 1968. Steroid alkaloids: the Solanum group. – In: Manske RHF (ed), The alkaloids 10, Academic Press, New York, pp. 1-191.
Schreiber K. 1979. The steroid alkaloids of Solanum. – In: Hawkes JG, Lester RN, Skelding AD (eds), The biology and taxonomy of the Solanaceae, Linn. Soc. Symp. Ser. 7, Linnean Society and Academic Press, London, pp. 193-202.
Schröter H-B. 1958. Alkaloide von Salpiglossis sinuata. – Naturwissenschafen 45: 338.
Schröter H-B, Neumann D. 1966. Withasomnine. A pyrazole alkaloid from Withania somnifera Dun. – Tetrahedron 22: 2895-2897.
Schultes RE. 1979. Solanaceous hallucinogens and their role in the development of New World cultures. – In: Hawkes JG, Lester RN, Skelding AD (eds), The biology and taxonomy of the Solanaceae, Linn. Soc. Symp. Ser. 7, Linnean Society and Academic Press, London, pp. 137-160.
Schulz G. 1926. Scopolia carniolica Jacquin. Eine botanisch-pharmakognostische Stue. – Bot. Arch. 13: 433-488.
Schumann K. 1910. Le Metternichia wercklei. – Rev. Hortic. 82: 149.
Schwarting AE, Bobbitt JM, Rother A, Attal CK et al. 1963. The alkaloids of Withania somnifera. – Lloydia 26: 258-273.
Scolnik R. 1954. Las especies de Cestrum de la Argentina, Chile y Uruguay. – Facultad Cienc. Ex. Fís. Nat. Ser. Cienc. Nat. (Córdoba) 3: 3-104.
Sebsebe D, Austin DF. 1996. Generic delimitation and relationships in the tribe Hildebrandtieae (Convolvulaceae). – In: Maesen LJG van der (ed), The biodiversity of African plants, Dordrecht, etc., pp. 409-420.
Seithe A. 1962. Die Haararten der Gattung Solanum und ihre taxonomische Verwertung. – Bot. Jahrb. Syst. 81: 261-336.
Seithe A, Anderson GJ. 1982. Hair morphology and the relationship of species in Solanum sect. Basarthrum. – Plant Syst. Evol. 139: 229-256.
Seithe A, Sullivan JR. 1990. Hair morphology and systematics of Physalis. – Plant Syst. Evol. 170: 193-204.
Sengupta S. 1972. On the pollen morphology of Convolvulaceae, with special reference to taxonomy. – Rev. Palaeobot. Palynol. 13: 157-212.
Ser NA. 1988. Flavonoids from Physalis minima. – Phytochemistry 27: 3708-3709.
Sharma AK, Datta PC. 1958. Cytological investigations on the genus Ipomoea and its importance in the study of phylogeny. – The Nucleus 1: 89-122.
Sharma AK, Sharma A. 1957. Karyotype studies in Cestrum as an aid in taxonomy. – Genetica 29: 83-100.
Sharma M. 1972. Studies in the flower of Datura stramonium L. in relation to bee-botany. – J. Palynol. 8: 17-21.
Sharma M. 1974. Contribution to the palinotaxonomy of genus Solanum Linn. – J. Palynol. 10: 51-68.
Sharma RC, Raghuvanshi RK, Singh D. 1987. Embryological studies in Atropa L. and Hyoscyamus L. – J. Indian Bot. Soc. 66: 311-316.
Sheidai M, Mosallanejad M, Khatamsaz M. 1999. Karyological studies in Hyoscyamus species of Iran. – Nord. J. Bot. 19: 369-373.
Sheidai M, Narengi Z, Khatamsaz M. 1999. Karyotype and seed protein analyses in Lycium (Solanaceae). – Edinburgh J. Bot. 56: 253-264.
Sherman TD, Bowling AJ, Barger TW, Vaughn KC. 2008. The vestigial root of dodder (Cuscuta pentagona) seedlings. – Intern. J. Plant Sci. 169: 998-1012.
Shingu K, Yahara S, Nohara T. 1990. New withanolides, daturataturins A and B from Datura tatula L. – Chem. Pharm. Bull. 38: 3485-3487.
Shingu K, Marubayashi N, Ueda I, Yahara S et al. 1990. Two new ergostane derivatives from Tubocapsicum anomalum. – Chem. Pharm. Bull. 38: 1107-1109.
Shingu K, Yahara S, Nohara. 1994. Five new ergostane-related ompounds from Nicandra physaloides. – Chem. Pharm. Bull. 42: 318-321.
Shingu K, Fujii, Mizuki K, Ueda I et al. 1994. Ergostane glycosides from Petunia hybrida. – Phytochemistry 36: 1307-1314.
Siddiqui S, Sultana N, Ahmed SS, Haider SI. 1986. Isolation and structure of a new alkaloid datumetine from the leaves of Datura metel. – J. Natur. Prod. 49: 511-513.
Siddiqui SA, Khan FA. 1988. Ontogeny and dehiscence of anther in Solanaceae. – Bull. Soc. Bot. France 135, Lettr. Bot. 2: 101-109.
Simões AR. 2013. Disentangling the bindweeds: systematics and evolution of tribe Merremieae (Convolvulaceae). – Ph.D. diss., University of Reading, United Kingdom.
Simões AR, Culham A, Carine M. 2015. Resolving the unresolved tribe: a molecular phylogenetic framework for the Merremieae (Convolvulaceae). – Bot. J. Linn. Soc. 179: 374-387.
Sing S, Khanna NM, Dhar MM. 1974. Solanoplumbin, a new anticancer glycoside from Nicotiana plumbaginifolia. – Phytochemistry 13: 2020-2022.
Sink KC (ed). 1984. Petunia. – Springer, Berlin.
Skottsberg C. 1916. Benthamiella Speg. und Saccardophytum Speg. – Engl. Bot. Jahrb. Syst. 54: 44-50.
Sleumer H. 1950. Estudios sobre el género Dunalia H. B. K. – Lilloa 23: 117-142.
Smith SD, Baum DA. 2006. Phylogenetics of the florally diverse Andean clade Iochrominae (Solanaceae). – Amer. J. Bot. 93: 1140-1153.
Smith SD, Baum DA. 2007. Systematics of Iochrominae (Solanaceae): patterns in floral diversity and interspecific crossability. – In: Spooner DM, Bohs L, Giovannoni J, Olmstead RG, Shibata D (eds), Solanaceae VI: genomics meets biodiversity. Proceedings of the Sixth International Solanaceae Conference, Acta Horticulturae 74, International Society for Horticulatural Science, Leuven, pp. 241-254.
Solereder H. 1891. Über die Versetzung der Gattung Melananthus von der Phrymaceen zu den Solanaceen. – Ber. Deutsch. Bot. Gesellsch. 9: (65)-(84).
Solereder H. 1898. Zwei Beiträge zur Systematik der Solanaceen. – Ber. Deutsch. Bot. Ges. 16: 242-260.
Solereder H. 1915. Über die Versetzung der Gattung Heteranthia von den Scrophulariaceen zu den Solanaceen. – Beih. Bot. Centralbl. 33, Abt. II, 1: 113-117.
Solms-Laubach HG zu. 1876. Die Entwickelung der Blüthe bei Brugmansia Zippelii Bl. und Aristolochia Clematitis L. – Bot. Zeitung (Berlin) 31: 481-489.
Soriano A. 1948. El género Benthamiella (Solanaceae). – Darwiniana 8: 233-262.
Souèges MR. 1907. Développement et structure du tégument séminal chez les Solanacées. – Thèses Fac. Sci. Paris, sér. A, 556, Masson et Cie, Paris, pp. 1-124.
Souèges MR. 1920. Embryogénie des Solanacées. Développement de l’embryon chez les Nicotiana. – Compt. Rend. Acad. Sci. 70: 125-127.
Souèges MR. 1937. Développement de l’embryon chez les Schizanthus et les Petunia. – Bull. Soc. Bot. France 83: 570-577.
Spies JJ, Minne L, Venter HJT, Venter AM. 1993. A cytogeneic study of the functionally dioecious species in the genus Lycium. – South Afr. J. Bot. 59: 535-540.
Spooner DM, Berg RG van den. 1992a. An analysis of recent taxonomic concepts in wild potatoes (Solanum sect. Petota). – Gen. Res. Crop Evol. 39: 23-37.
Spooner DM, Berg RG van den. 1992b. Species limits and hypotheses of hybridization of Solanum berthaultii Hawkes and S. tarijense Hawkes: morphological data. – Taxon 41: 685-700.
Spooner DM, Castillo R. 1997. Reexamination of series relationships of South American wild potatoes (Solanaceae: Solanum sect. Petota): evidence from chloroplast DNA restriction site variation. – Amer. J. Bot. 84: 671-685.
Spooner DM, Salas A. 2006. Structure, biosystematics, and genetic resources. – In: Gopal J, Khurana SMP (eds), Handbook of potato production, improvement, and post-harvest management, Haworth’s Press, Inc., Binghampton, New York, pp. 1-39.
Spooner DM, Sytsma KJ. 1992. Reexamination of series relationships of Mexican and Central American wild potatoes (Solanum sect. Petota): evidence from chloroplast DNA restriction site variation. – Syst. Bot. 17: 432-448.
Spooner DM, Anderson GA, Jansen RK. 1993. Chloroplast DNA evidence for the interrelationships of tomatoes, potatoes, and pepinos (Solanaceae). – Amer. J. Bot. 80: 676-688.
Spooner DM, Castillo T R, Lopez J LE. 1993. Synonymy within wild potatoes (Solanum sect. Petota: Solanaceae): the case of Solanum andreanum. – Syst. Bot. 18: 209-217.
Spooner DM, Berg RG van den, Bamberg JB. 1995. Examination of species boundaries of Solanum series Demissa and potentially related species in series Acaulia and series Tuberosa (sect. Petota). – Syst. Bot. 20: 295-314.
Spooner DM, Berg RG van den, Miller JT. 2001. Species and series boundaries of Solanum series Longipedicellata (Solanaceae) and phenetically similar species in series Demissa and series Tuberosa: implications for a practical taxonomy of sect. Petota. – Amer. J. Bot. 88: 113-130.
Spooner DM, Berg RG van den, Rivera-Peña A, Velguth P, Rio A del, Salas A. 2001. Taxonomy of Mexican and Central American members of Solanum Series Conicibaccata (sect. Petota). – Syst. Bot. 26: 743-756.
Spooner DM, Berg RG van den, Rodríguez A, Bamberg J, Hijmans RJ, Lara-Cabrera SI. 2004. Wild potatoes (Solanum section Petota) of North and Central America. – Syst. Bot. Monogr. 68: 1-209.
Spooner DM, Peralta I, Knapp S. 2005. AFLP phylogeny of wild tomatoes (Solanum L. section Lycopersicon [Mill.] Wettst. subsection Lycopersicon. – Taxon 54: 43-61.
Spooner DM, McLean K, Ramsay G, Waugh R, Bryan GJ. 2005. A single domestication for potato based on multilocus AFLP genotyping. – Proc. Natl. Acad. Sci. U.S.A. 120: 14694-14699.
Spooner DM, Fajardo D, Bryan GJ. 2007. Species limits of Solanum berthaultii Hawkes and S. tarijense Hawkes and the implications for species boundaries in Solanum sect. Petota. – Taxon 56: 987-999.
Spooner DM, Bohs L, Giovannoni J, Olmstead RG, Shibata D (eds). 2007. Solanaceae VI: genomics meets biodiversity. Proceedings of the Sixth International Solanaceae Conference. – Acta Horticulturae 745, International Society for Horticultural Science, Leuven.
Spooner DM, Fajardo D, Salas A. 2008. Revision of the Solanum medians complex (Solanum section Oetota). – Syst. Bot. 33: 579-588.
Spooner DM, Ghislain M, Simon R, Jansky SH,
Gavrilenko T. 2014. Systematics, diversity, genetics, and evolution of wild and
cultivated potatoes. – Bot. Rev. 80: 283-383.
Spurna V, Jojova M, Jirmanova E, Sustackova A. 1981. Chromosomal characteristics and occurrence of main alkaloids in Datura stramonium and Datura wrightii. – Planta Med. 41: 366-373.
Sripleng A, Smith FH. 1960. Anatomy of the seed of Convolvulus arvensis. – Amer. J. Bot. 47: 386-392.
Stafford P, Knapp S. 2006. Pollen morphology and systematics of the zygomorphic-flowered nightshades (Solanaceae; Salpiglossidae sensu D’Arcy, 1978 and Cestroideae sensu D’Arcy, 1991, pro parte): a review. – Syst. Biodiv. 4: 173-201.
Standley PC, Morton CV. 1938. Solanaceae. Flora of Costa Rica. – Field Mus. Nat. Hist., Bot. Ser. 18: 1035-1099.
Staples GW. 1987. A revision of Porana Burman f. (Convolvulaceae) and an evaluation of the Poraneae. – Ph.D. diss., Harvard University, Cambridge, Massachusetts.
Staples GW. 1990. Preliminary taxonomic consideration of the Poraneae (Convolvulaceae). – J. Arnold Arbor. 71: 251-258.
Staples GW. 2006. Revision of Asiatic Poraneae (Convolvulaceae) – Cordisepalum, Dinetus, Duperreya, Porana, Poranopsis, and Tridynamia. – Blumea 51: 403-491.
Staples GW. 2007. Checklist of Pacific Operculina (Convolvulaceae), including a new species. – Pacific Sci. 61: 587-593.
Staples GW. 2010. A checklist of Merremia (Convolvulaceae) in Australasia and the Pacific. – Gard. Bull. (Singapore) 61: 483-522.
Staples GW, Austin DF. 1981. Changes in the West Indian Operculina (Convolvulaceae). – Brittonia 33: 591-596.
Staples GW, Austin DF. 2009. Revision of neotropical Calycobolus and Porana (Convolvulaceae). – Edinburgh J. Bot. 66: 133-153.
Staples GW, Noltie HJ. 2007. Proposal to conserve the name Hewittia against Shutereia Choisy (Convolvulaceae). – Taxon 56: 262.
Staples GW, Na Songkhla B, Khunwasi C, Traiperm P. 2005. Annotated checklist of Thai Convolvulaceae. – Thai Forest Bull., Bot. 33: 171-184.
Staub H. 1942. Über nichtalkaloidische Inhaltsstoffe der Mandragorawurzel. – Helv. Chim. Acta 25: 649-683.
Staub H. 1962. Über die chemischen Bestandteile der Mandragorawurzel 2. Die Alkaloide. – Helv. Chim. Acta 45: 2297-2305.
Staub M. 1997. Tropane alkaloids. Datura and Brugmansia. – Schweiz. Lab. Zeitschr. 54: 239-244.
Steenis CGGJ van. 1930. Brugmansia or Pseudodatura. – Bull. Jard. Bot. Buitenzorg, sér. III, 11: 15-18.
Stefanović S, Costea M. 2008. Reticulate evolution in the parasitic genus Cuscuta (Convolvulaceae): over and over again. – Botany 86: 791-808.
Stefanović S, Olmstead RG. 2004. Testing the phylogenetic position of a parasitic plant (Cuscuta, Convolvulaceae, Asteridae): Bayesian inference and the parametric bootstrap on data drawn from three genomes. – Syst. Biol. 53: 384-399.
Stefanović S, Krueger L, Olmstead RG. 2002. Monophyly of the Convolvulaceae and circumscription of their major lineages based on DNA sequences of multiple chloroplast loci. – Amer. J. Bot. 89: 1510-1522.
Stefanović S, Austin DF, Olmstead RG. 2003. Classification of Convolvulaceae: a phylogenetic approach. – Syst. Bot. 28: 791-806.
Stefanović S, Kuzmina M, Costea M. 2007. Delimitation of major lineages within Cuscuta subgenus Grammica (Convolvulaceae) using plastid and nuclear DNA sequences. – Amer. J. Bot. 94: 568-589.
Stehmann JR, Giacomin L. 2012. Markea atlantica (Solanaceae): a new species of tribe Juanulloeae disjunct from its core distribution. – Syst. Bot. 37: 1035-1042.
Stehmann JR, Semir J. 1997. A new species and combinations in Calibrachoa (Solanaceae). – Novon 7: 417-419.
Stern SR, Bohs LA. 2012. An explosive innovation: phylogenetic relationships of Solanum section Gonatotrichum (Solanaceae). – PhytoKeys 8: 83-98.
Stern SR, Bohs LA. 2016. An eight marker phylogeny of Solanum sect. Micracantha (Solanaceae). – Syst. Bot. 41: 120-127.
Stern SR, Weese T, Bohs LA. 2010. Phylogenetic relationships in Solanum section Androceras (Solanaceae). – Syst. Bot. 35: 885-893.
Stern SR, Agra M de F, Bohs LA. 2011. Molecular delimiation of clades within New World species of the “spiny solanums” (Solanum subg. Leptostemonum). – Taxon 60: 1429-1441.
Stern SR, Bohs LA, Giacomin L, Stehmann J, Knapp S. 2013. A revision of Solanum Section Gonatotrichum. – Syst. Bot. 38: 471-496.
Stiefkens L, Bernardello L. 1996. Karyotypic studies in South American Lycium (Solanaceae). – Cytologia 61: 395-402.
Stiefkens L, Bernardello G. 2000. Karyotypes and DNA content in diploid and polyploid Lycium (Solanaceae). – Bol. Soc. Argent. Bot. 35: 237-244.
Stiefkens L, Bernardello G. 2002. Karyotypic studies in Lycium section Mesocope (Solanaceae) from South America. – Caryologia 55: 199-206.
Stiefkens L, Bernardello G. 2006. Karyotypic studies in Lycium sections Schistocalyx and Sclerocarpellum (Solanaceae). – Edinburgh J. Bot. 62: 53-67.
St. John H. 1986. Nothocestrum inconcinnum sp. nov. (Solanaceae). – Phytologia 61: 343-344.
Stout AB. 1952. Reproduction in Petunia. – Mem. Torrey Bot. Club 20: 1-202.
Su BN, Park EJ, Nikolic D, Vigo JS, Graham JG, Cabieses F, Breemen RB van, Fong HHS, Farnsworth NR, Pezzuto JM, Kinghorn AD. 2003. Isolation and characterization of miscellaneous secondary metabolites of Deprea subtriflora. – J. Nat. Prod. 66: 1089-1093.
Subils R. 1979. Cromosomas gaméticos de Leptoglossis linifolia (Solanaceae). – Lorentzia 3: 19-20.
Subramanyam K. 1950. A contribution to our knowledge of the systematic position of the Sphenocleaceae. – Proc. Indian Acad. Sci., Sect. B, Biol. Sci. 31: 60-65.
Subramanyam K. 1970. Comparative embryology of angiosperms: Crassulaceae, Campanulaceae, Sphenocleaceae, Pentaphragmataceae, Stylidiaceae. – Bull. Natl. Sci. Acad. India 41: 84-89, 306-312, 313-316, 317-320, 321-324.
Subramanyam K, Mahadevan S. 1994. The cDNA sequence of cytochrome b5 associated with cytokinin-induced haustoria formation in Cuscuta reflexa. – Gene 149: 375-376.
Sudhakaran S, Ganapathi A. 1999. Biosystematics of South Indian Physalis. – In: Nee M, Symon DE, Lester RN, Jessop JP (eds), Solanaceae IV, Royal Botanic Gardens, Kew, pp. 335-340.
Sullivan JR. 1985. Systematics of the Physalis viscosa complex (Solanaceae). – Syst. Bot. 10: 426-444.
Svensson HG. 1925. Zur Embryologie der Hydrophyllaceen, Borraginaceen und Heliotropiaceen mit besonderer Rücksicht auf die Endospermbildung. – Ph.D. diss., Uppsala Univ. Årsskr., Matem. Naturvet., 2: 1-176.
Svensson HG. 1926. Zytologische-embryologische Solanaceen-Studien I. Über die Samenentwicklung von Hyoscyamus niger L. – Svensk Bot. Tidskr. 20: 420-434.
Sýkorová E, Lim KY, Chase MW, Knapp S, Leitch IJ, Leitch AR, Fajkus J. 2003. The absence of Arabidopsis-type telomeres in Cestrum and closely related genera Vestia and Sessea (Solanaceae): first evidence from eudicots. – Plant J. 34: 283-291.
Sýkorová E, Lim KY, Fajkus J, Leitch AR. 2003. The signature of the Cestrum genome suggests an evolutionary response to the loss of (TTTAGGG)n telomeres. – Chromosoma 112: 164-172.
Symon DE. 1970. Dioecious solanums. – Taxon 19: 909-910.
Symon DE. 1979. The genus Solanum in Australia. –In: Hawkes JG, Lester RN, Skelding AD (eds), The biology and taxonomy of the Solanaceae, Linn. Soc. Symp. Ser. 7, Linnean Society and Academic Press, London, pp. 125-131.
Symon DE. 1981a. A revision of the genus Solanum in Australia. – J. Adelaide Bot. Gard. 4: 1-367.
Symon DE. 1981b. The solanaceous genera Browallia, Capsicum, Cestrum, Cyphomandra and Withania, naturalised in Australia. – J. Adelaide Bot. Gard. 3: 133-166.
Symon DE. 1985. The Solanaceae of New Guinea. – J. Adelaide Bot. Gard. 8: 1-171.
Symon DE. 1991a. Fruits of Brugmansia. – Solanaceae Newsletter 3: 22-24.
Symon DE. 1991b. Gondwanan elements of the Solanaceae. – In: Hawkes JG, Lester RN, Nee M, Estrada N (eds), Solanaceae III: taxonomy, chemistry, evolution, Royal Botanic Gardens, Kew, pp. 139-150.
Symon DE. 1994. Kangaroo apples. Solanum sect. Archaeosolanum. – Publ. by the author, South Australia.
Symon DE. 1995. Four new species of Solanum L. (Solanaceae) from south east Queensland. – Austrobaileya 4: 429-437.
Symon DE, Haegi L. 1989. Datura is a New World genus. – Solanaceae Newsletter 3: 58-59.
Symon DE, Haegi L. 1991a. Datura (Solanaceae) is a New World genus. – In: Hawkes JG, Lester RN, Nee M, Estrada N (eds), Solanaceae III: taxonomy, chemistry, evolution, Royal Botanic Gardens, Kew, pp. 197-210.
Symon DE, Haegi L. 1991b. Brugmansia: to be or not to be. – Solanaceae Newsletter 3: 25-26.
Tago-Nakazawa M, Dillon MO. 1999. Biogeografía y evolución del clado Nolana (Nolaneae-Solanaceae). – Arnaldoa 6: 81-115.
Takhtajan A, Trifonova VI. 1999. Fruit and seed anatomy of the genus Kaliphora (Kaliphoraceae) in relation to its taxonomic position. – Bot. Žurn. 84: 1-8. [In Russian]
Talianova M, Janousek B. 2011. What can we learn from tobacco and other Solanaceae about horizontal DNA transfer. – Amer. J. Bot. 98: 1231-1242.
Tanksley SD. 2004. The genetic, developmental, and molecular bases of fruit size and shape variation in tomato. – Plant Cell 16(Suppl. 2004): 181-189.
Tate JA, Acosta MC, McDill J, Moscone EA, Simpson BB, Cocucci AA. 2009. Phylogeny and character evolution in Nierembergia (Solanaceae): molecular, morphological, and cytogenetic evidence. – Syst. Bot. 34: 198-206.
Telek L, Delpin H, Cabanillas E. 1977. Solanum mammosum as a source of solasodine in the lowland tropics. – Econ. Bot. 31: 120-122.
Tepe EJ, Bohs L. 2010. A molecular phylogeny of Solanum sect. Pteroidea (Solanaceae) and the utility of COSII markers in resolving relationships among closely related species. – Taxon 59: 733-743.
Tepe EJ, Farruggia FT, Bohs L. 2011. A 10-gene phylogeny of Solanum section Herpystichum (Solanaceae) and a comparison of phylogenetic methods. – Amer. J. Bot. 98: 1356-1365.
Terrazaz T, Aguilar-Rodríguez A, Ojanguren CT. 2011. Development of successive cambia, cambial activity, and their relationship to physiological traits in Ipomoea arborescens (Convolvulaceae) seedlings. – Amer. J. Bot. 98: 765-774.
Terrell EE. 1977. The name for the tomato. – Taxon 26: 129-148.
Tétényi P. 1987. A chemotaxonomic classification of the Solanaceae. – Ann. Missouri Bot. Gard. 74: 600-608.
Thulin M. 1983. 100. Sphenocleaceae. – In: Launert E (ed), Flora Zambesiaca 7 (Part 1), Flora Zambesiaca Managing Committee, London, pp. 114-116.
Tiagi B. 1951. A contribution to the morphology of Cuscuta hyalina Roth and C. planiflora Tenore. – Phytomorphology 1: 9-21.
Tiagi B. 1966. Floral morphology of Cuscuta reflexa Roxb. and C. lupuliformis Krocker with a brief review of the literature on the genus Cuscuta. – Bot. Mag. (Tokyo) 79: 89-97.
Timmerman HA. 1927. Stramonium and other species of Datura: a comparative study of the structure of their leaves. – Pharmaceut. J. Pharmacist 1927: 241-243.
Tobe H, Morin NR. 1996. Embryology and circumscription of Campanulaceae and Campanulales: a review of the literature. – Intern. J. Plant Res. 109: 425-435.
Toledo JF. 1941. Sobre a presença, no Brasil, do gênero Sessea. – Arq. Bot. S. Pâulo, N. S., 1: 65-70.
Torres R, Modak B, Faini F. 1988. (25 R)-isonuatigenin, an unusual steroidal sapogenin as taxonomic marker in Cestrum parqui and Vestia lycioides. – Bol. Soc. Chil. Quím. 33: 239-241.
Tru’c Nhã N, Danert S. 1973. Ueber die Fruchtentwicklung und über zentrische Leitbündel in der Gattung Datura (Solanaceae). – Kulturpflanze 21: 119-193.
Tu T-Y, Sun H, Gu Z-J, Yue J-P. 2005. Cytological studies on the Sino-Himalayan endemic Anisodus and four related genera from the tribe Hyoscyameae (Solanaceae) and their systematic and evolutionary implications. – Bot. J. Linn. Soc. 147: 457-468.
Tu T, Dillon MO, Sun H, Wen J. 2008. Phylogeny of Nolana (Solanaceae) of the Atacama and Peruvian deserts inferred from sequences of four plastid markers and the nuclear LEAFY second intron. – Mol. Phylogen. Evol. 49: 561-573.
Tu T, Volis S, Dillon MO, Sun H, Wen J. 2010. Dispersals of Hyoscyameae and Mandragoreae (Solanaceae) from the New World to Eurasia in the early Miocene and their biogeographic diversification within Eurasia. – Mol. Phylogen. Evol. 57: 1225-1237.
Tucker WG. 1969. Serotaxonomy of the Solanaceae: a preliminary survey. – Ann. Bot., N. S., 33: 1-23.
Turner BL. 2015. Taxonomy of Chamaesaracha (Solanaceae). – Phytologia 97: 226-245.
Ungricht S, Knapp S, Press JR. 1988. A revision of the genus Mandragora. – Bull. Nat. Hist. Mus. London (Bot.) 28: 17-40.
Unzelman JM, Healey PL. 1974. Development, structure and occurrence of secretory trichomes of Pharbitis. – Protoplasma 80: 285-303.
Urdampilleta JD, Chiarini F, Stiefkens L, Bernardello G. 2015. Chromosomal differentiation of Tribe Cestreae (Solanaceae) by analyses of 18-5.8-26S and 5S rDNA distribution. – Plant Syst. Evol. 301: 1325-1334.
Usubillaga A, Castellano G de. 1980. Acnistins, a new class of steroidal lactones from Acnistus ramiflorus Miers… etc. – J. C. S. Chem. Comm. 1980: 854.
Valencia MLC de. 1985. Anatomía del fruto de la uchuva. – Acta Biol. Colomb. 1: 63-89.
Valencia Ávalos S, Martínez Gordillo M. 1995. Merremia macdonaldii (Convolvulaceae), especie nueva del estado de Guerrero. – An. Inst. Biol. Univ. Nac. Autónoma México, Ser. Bot., 66: 107-111.
Vales MA, Fuentes V. 1990. Caracteristicas de la epidermis foliar de Goetzeaceae. – Acta Bot. Hung. 36: 255-265.
Vargas O, Martínez M, Dávila A P. 2001. Two new species of Physalis (Solanaceae) endemic to Jalisco, Mexico. – Brittonia 53: 505-510.
Veleiro AS, Burton G, Gros EG. 1985. 2-3-dihydrojaborosalactone A, a withanolide from Acnistus breviflorus. – Phytochemistry 24: 1799-1802.
Veleiro A, Oberti JC, Burton G. 1992. A ring-D aromatic withanolide from Salpichroa origanifolia. – Phytochemistry 31: 935-937.
Veleiro AS, Cirigliano AM, Oberti JC, Burton G. 1999. 7-hydroxywithanolides from Datura ferox. – J. Natur. Prod. 62: 1010-1012.
Venter AM. 2007. Lycium hantamense (Solanaceae), a new species from the Hantam-Roggeveld centre of plant endemism, South Africa. – South Afr. J. Bot. 73: 214-217.
Verdcourt B. 1961. Notes from the East African herbarium XII. Notes on African Convolvulaceae V. – Kew Bull. 15: 1-18.
Verdcourt B. 1963. Convolvulaceae. – In: Hubbard CE, Milne-Redhead E (eds), Flora of tropical East Africa, Crown Agents for Oversea Governments and Administrations, London, pp. 1-161.
Verdcourt B. 1971. The genus Cardiochlamys Oliv. (Convolvulaceae). – Kew Bull. 26: 137-140.
Verdcourt B. 1973a. Notes on the genus Grevea Baill. (Montiniaceae). – Kew Bull. 28: 147-150.
Verdcourt B. 1973b. Montiniaceae. – In: Polhill RM (ed), Flora of tropical East Africa, Crown Agents for Oversea Governments and Administrations, London, pp. 1-7.
Verdcourt B. 1990. A new subgenus of Stictocardia (Convolvulaceae). – Kew Bull. 45: 583-585.
Verdcourt B. 1996a. Proposal to conserve the name Falkia (Convolvulaceae) with a conserved spelling. – Taxon 45: 559-560.
Verdcourt B. 1996b. A reappraisal of the Grevea (Montiniaceae) growing on the Kenya coast. – Kew Bull. 51: 771-773.
Vesprini JL, Galetto L. 2000. The reproductive biology of Jaborosa integrifolia (Solanaceae). Why its fruits are so rare? – Plant Syst. Evol. 225: 15-28.
Vilcapoma-Segovia G. 1987. Las Solanáceas del Valle de Chillón (Lima). – Bol. Lima 52: 63-82.
Vilmorin R de, Simonet M. 1927. Variations du nombre des chromosomes chez quelques Solanées. – Compt. Rend. Acad. Sci. 184: 164-166.
Vilmorin R de, Simonet M. 1928. Recherches sur le nombre des chromosomes chez les Solanées. – Zeitschr. Induct. Abstamm. Vererbungsl., Suppl. 2: 1520-1536.
Volkov RA, Komarova NY, Panchuk II, Hemleben V. 2003. Molecular evolution of rDNA external transcribed spacer and phylogeny of sect. Petota (genus Solanum). – Mol. Phylogen. Evol. 29: 187-202.
Volkova EM. 1991. Comparative anatomy and ultrastructure of the testa in species of subgenus Archaeosolanum Bitter ex Marz. of the genus Solanum L. – Nauchnye Doklady Vysshei Shkoly, Biologischeskie Naubi 2: 104-110. [In Russian]
Vorontsova MS, Stern S, Bohs L, Knapp S. 2013. African spiny Solanum (subgenus Leptostemonum, Solanaceae): a thorny phylogenetic tangle. – Bot. J. Linn. Soc. 173: 176-193.
Voss R, Turner M, Inoye R, Fisher M et al. 1980. Floral biology of Markea neurantha Hemsley (Solanaceae), a bat-pollinated epiphyte. – Amer. Midl. Natur. 103: 262-268.
Wahlert GA, Chiarini FE, Bohs L. 2015. A revision of Solanum Section Lathyrocarpum (the Carolinense Clade, Solanaceae). – Syst. Bot. 40: 853-887.
Wallis TE, Butterfield R. 1939. The flower of Atropa belladonna. – Pharmaceutical Society of Great Britain.
Walsh BM, Hoot SB. 2001. Phylogenetic relationships of Capsicum (Solanaceae) using DNA sequences from two noncoding regions: the chloroplast atpB-rbcL spacer region and nuclear waxy introns. – Intern. J. Plant Sci. 162: 1409-1418.
Walters DR. 1969. A revision of the genus Schizanthus. – Ph.D. diss., Indiana University.
Wang M, Berg R, Linden G, Vosman B. 2008. The utility of NBS profiling for plant systematics: a first study in tuber-bearing Solanum species. – Plant Syst. Evol. 276: 137-148.
Wang Q, Xu C, Pan K-Y, Hong D-Y. 2017. Which family? – morphological and phylogenetic analyses of the enigmatic genus Numaeacampa (Campanulaceae). – Kew. Bull. 72: 72. DOI 10.1007/S12225-017-9701-X
Waterfall UT. 1958. A taxonomic study of the genus Physalis in North America north of Mexico. – Rhodora 60: nos. 712-714.
Waterfall UT. 1967. Physalis in Mexico, Central America and the West Indies. – Rhodora 69: no. 677.
Weberling F. 1956. Weitere Untersuchungen zur Morphologie des Unterblattes bei den Dikotylen III. Convolvulaceae; IV. Zygophyllaceae. – Beitr. Biol. Pflanzen 33: 149-161.
Weese TL, Bohs L. 2007. A three-gene phylogeny of the genus Solanum (Solanaceae). – Syst. Bot. 32: 445-463.
Weinert E. 1972. Zur Taxonomie und Chorologie der Gattung Scopolia Jacq. – Feddes Repert. 82: 617-628.
Welsh M, Stefanovic S, Costea M. 2010. Pollen evolution and its taxonomic significance in Cuscuta (dodders, Convolvulaceae). – Plant Syst. Evol. 285: 83-101.
Went JL van. 1970. The ultrastructure of the synergids of Petunia. – Acta Bot. Neerl. 19: 121-127.
Werdermann E. 1928. Übersicht über die in Chile vorkommenden Arten der Gattung Salpiglossis Ruiz et Pav. (Solanaceae). – Notizbl. Bot. Gart. Mus. Berlin-Dahlem 10: 472-475.
Wessely I. 1960. Die mitteleuropäischen Sippen der Gattung Solanum, Sektion Morella. – Feddes Repert. 63: 290-321.
Wettstein R von. 1895a. Nolanaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien IV(3b), W. Engelmann, Leipzig, pp. 1-4.
Wettstein R von. 1895b. Solanaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien IV(3b), W. Engelmann, Leipzig, pp. 4-38; Wettstein R von. 1897. Nachträge zu IV(3b), pp. 292-293.
Whalen MD. 1978a. Reproductive character displacement and floral diversity in Solanum Section Androceras. – Syst. Bot. 3: 77-86.
Whalen MD. 1978b. Foliar flavonoids of Solanum Section Androceras: a systematic survey. – Syst. Bot. 3: 257-276.
Whalen MD. 1979a. Reports [on chromosomes of Solanaceae]. – Taxon 28: 276.
Whalen MD. 1979b. Taxonomy of Solanum Section Androceras. – Gentes Herb. 11: 359-426.
Whalen MD. 1979c. Allozyme variation and evolution in Solanum Section Androceras. – Syst. Bot. 4: 203-222.
Whalen MD. 1984a. Conspectus of species groups in Solanum subgenus Leptostemonum. – Gentes Herb. 12: 179-282.
Whalen MD. 1984b. Nectouxia, a Mexican genus with South American affinities. – Solanaceae Newsletter 2: 15-18.
Whalen MD, Anderson GJ. 1981. Distribution of gametophytic self-incompatibility and infrageneric classification in Solanum. – Taxon 30: 761-767.
Whalen MD, Caruso EE. 1983. Phylogeny in Solanum sect. Lasiocarpa (Solanaceae): congruence of morphological and molecular data. – Syst. Bot. 8: 369-380.
Whalen MD, Costich DE. 1986. Andromonoecy in Solanum. – In: D’Arcy WG (ed), Solanaceae: biology and systematics, Columbia University Press, New York, pp. 284-302.
Whalen MD, Costich DE, Heiser CG. 1981. Taxonomy of Solanum section Lasiocarpa. – Gentes Herb. 12: 41-129.
Whitson M. 2012. Calliphysalis (Solanaceae): a new genus from the southeastern USA. – Rhodora 114: 133-147.
Whitson M, Manos PS. 2005. Untangling Physalis (Solanaceae) from the physaloids: a two-gene phylogeny of the Physalinae. – Syst. Bot. 30: 216-230.
Wijsman HJW. 1982. On the interrelationships of certain species of Petunia I: taxonomic notes on the parental species of Petunia hybrida. – Acta Bot. Neerl. 31: 477-490.
Wijsman HJW. 1983. On the interrelationships of certain species of Petunia II: experimental data: crosses between different taxa. – Acta Bot. Neerl. 32: 97-107.
Wijsman HJW. 1990. On the inter-relationships of certain species of Petunia VI: new names for the species of Calibrachoa formerly included into Petunia (Solanaceae). – Acta Bot. Neerl. 39: 101-102.
Wijsman HJW, Jong JH de, Pedersen TM. 1983. On the interrelationships of certain species of Petunia III: the position of P. linearis and P. calycina. – Acta Bot. Neerl. 32: 323-332.
Wijsman HJW, Jong JH de. 1985. On the interrelationships of certain species of Petunia IV: hybridization between P. linearis and P. calycina and nomenclatural consequences in the Petunia group. – Acta Bot. Neerl. 34: 337-349.
Wilkin P. 1999. A morphological cladistic analysis of the Ipomoeeae (Convolvulaceae). – Kew Bull. 54: 853-876.
Williams E. 1975. A new chromosome number in the Australian species Nicotiana cavicola L. – New Zealand J. Bot. 13: 811-812.
Willis JC. 1895. Contibutions to the natural history of the flower II. Fertilization methods of various flowers (Hydrolea spinosa). – Bot. J. Linn. Soc. 30: 293-294.
Witasek J. 1910. Solanaceae. – In: Wettstein R von (ed), Botanische Expedition nach Südbrasilien, 1901, Denkschr. Akad. Wiss. 79, II, Wien, pp. 313-375.
Wojciechowska B. 1972. Systematic studies on the seeds of the Solanaceae Pers. family. – Monogr. Bot. 36: 117-179.
Wollenweber E, Dörsan M, Dörr M, Roitman JN, Valant-Vetschera KM. 2005. Chemodiversity of surface flavonoids in Solanaceae. – Zeitschr. Naturf., C 60: 661-670.
Wood JRI. 2013. Bonamia (Convolvulaceae) in Bolivia. – Kew Bull. 68: 249-260.
Wood JRI. 2015. A foundation monograph of Convolvulus L. (Convolvulaceae). – PhytoKeys 51: 1-282.
Wright MAR, Welsh M, Costea M. 2011. Diversity and evolution of the gynoecium in Cuscuta (dodders, Convolvulaceae) in relation to their reproductive biology: two styles are better than one. – Plant Syst. Evol. 296: 51-76.
Wu F, Tanksley SD. 2010. Chromosome evolution in the plant family Solanaceae. – BMC Genomics 11: 182.
Xiqués X, Román MI, Licourt T, Fuentes VR, Pinillo J. 1994. Estudio citotaxonómico de Espadaea amoena A. Rich. – Sagra. Revista Biol. (La Habana) 3: 119-126.
Yamaguchi H, Nishimoto K. 1965. Studies on the alkaloids of the root of Physalis alkekengi. Isolation of 3α-tigloyloxytropane. – Chem. Pharm. Bull. 13: 217-221.
Yamaguchi H, Numata A, Hokimoto K. 1974. Studies on the alkaloids of the root of Physalis alkekengi II. – J. Pharm. Soc. Japan 94: 1115-1123.
Yamamoto K, Sakai K. 1932. On the chromosome number in some Solanaceae. – Jap. J. Genet. 8: 27-33.
Yang D-Z, Zhang Z-Y, Lu A-M, Sun K, Liu J-Q. 2002. Floral organogenesis and development of two taxa in tribe Hyoscyameae (Solanaceae) – Przewalskia tangutica and Hyoscyamus niger. – Acta Bot. Sin. 44: 889-894.
Yoshida K, Shingu K, Yahara S, Nohara T. 1988. A new class of ergostane glycosides from Tubocapsicum anomalum. – Tetrahedron Letters 29: 673-674.
Yuan Y, Zhang Z-Y, Olmstead RG. 2006. A retroposon insertion to the waxy gene: defining monophyly of the tribe Hyoscyameae (Solanaceae), and revealing the allopolyploid origin of Atropa belladonna. – Mol. Biol. Evol. 23: 2263-2267.
Yuan Y, Zhang Z-Y, Chen Z-D, Olmstead RG. 2006. Tracking ancient polyploids: a retroposon reveals an extinct diploid ancestor in the polyploid ancestry of Belladonna. – Mol. Biol. Evol. 23: 2263-2267.
Yukawa M, Tsudzuki T, Sugiura M. 2005. The 2005 version of the chloroplast DNA sequence from tobacco (Nicotiana tabacum). – Plant Mol. Biol. Rep. 23: 359-365.
Yuncker TG. 1921. Revision of the North American and West Indian species of Cuscuta. – Illinois Biol. Monogr. 6: 1-142.
Yuncker TG. 1932. The genus Cuscuta. – Mem. Torrey Bot. Club 18: 109-331.
Zamberlan PM, Rodrigues IMC, Mäder G, Castro L, Stehmann JR, Bonatto SL, Freitas LB. 2015. Re-evaluation of the generic status of Athenaea and Aureliana (Withaniinae, Solanaceae) based on molecular phylogeny and morphology of the calyx. – Bot. J. Linn. Soc. 177: 322-334.
Zamora-Tavares M del P, Martínez M, Magallón S, Guzmán-Dávalos L, Vargas-Ponce O. 2016. Physalis and physaloids: a recent and complex evolutionary history. – Mol. Phylogen. Evol. 100: 41-50.
Zhang J, Khan MR, Tian Y, Li Z, Riss S, He C. 2012. Divergences of MPF2-like MADS-domain proteins have an association with the evolution of the inflate calyx syndrome in Solanaceae. – Planta 236: 1247-1260.
Zhang J, Stevens PF, Zhang W. 2017. Evolution of floral zygomorphy in androecium and corolla in Solanaceae. – J. Syst. Evol. 55: 581-590.
Zhang Z-Y, Lu A-M. 1984. Pollen morphology of the subtribe Hyoscyaminae (Solanaceae). – Acta Phytotaxon. Sin. 22: 175-180.
Zhang Z-Y, Lu A-M. 1995. Pollen morphology of Physalis (Solanaceae) in China and its systematic significance. – Cathaya 7: 63-74.
Zhang Z-Y, Lu A-M. 1999. A comparative study of Physalis, Capsicum and Tubocapsicum. – In: Nee M, Symon DE, Lester RN, Jessop JP (eds), Solanaceae IV, Royal Botanic Gardens, Kew, pp. 81-96.
Zhang Z-Y, Yang D-Z, Lu A-M, Knapp S. 2005. Seed morphology of the tribe Hyoscyameae (Solanaceae). – Taxon 54: 71-83.
Zhang Z-Y, Yang D-Z, Joongku L, Li L-Q. 2009. Supplemental study of the pollen morphology of the tribe Hyoscyameae (Solanaceae) and its systematic significance. – Guihaia 29: 285-295. [In Chinese]
Zito SW, Leary JD. 1966. Alkaloids of Scopolia carniolica. – J. Pharmaceut. Sci. 55: 1150-1151.
Zöllner O. 1987. El género Fabiana en Chile. – Bot. Staatssamml. München 23: 291-319.
Zona S. 1989. Leaf anatomy of the Goetzeaceae. – Aliso 12: 303-312.