Cladogram of Dipsacales based on DNA sequence data.
Dipsacanae Takht., Divers. Classif. Fl. Pl.: 396. 24 Apr 1997
Habit Usually bisexual (rarely polygamomonoecious or dioecious), annual, biennial or perennial herbs, evergreen or deciduous trees or shrubs, lianas (sometimes suffrutices, rarely woody and creeping).
Vegetative anatomy Phellogen ab initio superficial or pericyclic. Pericyclic fibres absent. Secondary lateral growth normal or absent. Vessel elements with simple or scalariform (sometimes reticulate or foraminate) perforation plates; lateral pits alternate, scalariform or opposite, usually bordered (sometimes simple) pits. Imperforate tracheary xylem elements tracheids, libriform fibres or fibre tracheids with simple and/or bordered pits, septate or non-septate (sometimes also vasicentric tracheids). Wood rays uniseriate or multiseriate, heterocellular, or absent. Axial parenchyma apotracheal diffuse or diffuse-in-aggregates, or paratracheal scanty, aliform, confluent, vasicentric, unilateral, banded, or absent. Wood elements sometimes partially storied. Tile cells often present. Intraxylary phloem sometimes present. Sieve tube plastids S type. Nodes usually 3:3, trilacunar with three leaf traces (sometimes 1:1, unilacunar with one trace, or 5:5, pentalacunar with five traces). Oil cells and/or mucilage cells frequent. Prismatic or rhomboidal calciumoxalate crystals or crystal sand often frequent.
Trichomes Hairs unicellular or multicellular, uniseriate or branched (often stellate or peltate, rarely hair tufts); glandular hairs often present.
Leaves Usually opposite (rarely verticillate or alternate), simple or compound (pinnately compound or trifoliolate), entire or lobed, with involute, supervolute or conduplicate (rarely plicate) ptyxis. Stipules usually absent (stipitate extrafloral nectaries sometimes present in stipular position); leaf sheath absent. Petiole vascular bundle transection arcuate, petiole sometimes also with adaxial inverted bundles (sometimes closed C-shaped and petiole with wing bundles). Foliaceous to scale-like appendages or nectariferous glands sometimes present on petiole or leaf base. Venation pinnate or palmate. Stomata usually anomocytic (sometimes anisocytic or paracytic). Cuticular wax crystalloids as rodlets or tubuli, or absent. Domatia usually absent. Leaf margin and leaflet margins serrate (sometimes glandular serrate) or entire; glandular leaf teeth with main vein and two accessory veins, or one accessory vein proceeding above tooth.
Inflorescence Terminal or axillary, dichasial, cymose, head-, spike-, raceme- or umbel-like, corymbose, paniculate, or thyrsoid (flowers often paired, sometimes solitary axillary), sometimes pseudanthia with large sterile marginal flowers. Supernumerary bracts often present (number of bracts larger than number of flowers due to floral abortions, forming epicalyx).
Flowers Actinomorphic or zygomorphic (sometimes asymmetric). Epicalyx (consisting of supernumerary bracts) sometimes present. Usually epigyny (sometimes partial epigyny). Sepals (two or) three to five, with imbricate or open aestivation, small, usually persistent, usually connate; median sepal adaxial. Petals (three or) four or five (or six), with imbricate or valvate aestivation, caducous, connate into a disc-shaped, infundibuliform, campanulate or tubular and bilabiate corolla. Unicellular or multicellular nectariferous hairs or glands often present near corolla lobe bases (sometimes at stylar base). Disc usually absent (sometimes intrastaminal).
Androecium Stamens (one to) three to five, more or less bifid, haplostemonous, antesepalous, alternipetalous. Filaments free from each other and from tepals, or epipetalous. Anthers basifixed or dorsifixed, versatile or non-versatile, usually tetrasporangiate (rarely split into two disporangiate halves), extrorse or introrse, longicidal (dehiscing by longitudinal slits). Tapetum usually amoeboid-periplasmodial (sometimes secretory). Staminodia absent.
Pollen grains Microsporogenesis simultaneous. Pollen grains usually (2–)3(–4)-colporate (sometimes colpate or porate, rarely pororate), shed as monads, usually tricellular (rarely bicellular) at dispersal. Exine tectate or semitectate, with columellate (rarely acolumellate) infratectum, perforate, reticulate or microreticulate, spinulate (sometimes echinate or psilate, rarely intectate).
Gynoecium Pistil composed of two to five (to eight) connate carpels, one or several of which often sterile and degenerating. Ovary usually inferior (sometimes semi-inferior), (unilocular or) bilocular to quinquelocular; ovaries often more or less connate. Style single, simple, or stylodia three to five, free (sometimes absent). Stigmas entire or lobate, papillate or non-papillate, Dry or Wet type. Pistillodium absent.
Ovules Placentation axile to apical. Ovules one to numerous per carpel, anatropous, pendulous, apotropous or epitropous, unitegmic, usually tenuinucellar (sometimes crassinucellar through multiple divisions of parietal cell). Megagametophyte usually monosporous, Polygonum type (sometimes disporous, Allium type, or tetrasporous, Adoxa type). Endosperm development ab initio cellular. Endosperm haustoria sometimes present. Embryogenesis asterad, solanad or piperad.
Fruit A berry-like drupe, many-seeded berry, drupaceous syncarp, septicidal capsule or cypseloid nut, often with persistent calyx and/or epicalyx. Endocarp often with two layers of different cell types, with fibrous layers perpendicular to each other. Endocarp sclereids thick-walled, crystalliferous.
Seeds Aril absent. Testa sometimes vascularized, sometimes crushed. Exotestal cells often palisade, often enlarged, cuboud to rectangular, often lignified and with theoidal thickenings. Endotesta? Perisperm not developed. Endosperm usually copious (sometimes sparse or absent), often with thin-walled undifferentiated cells, rich in fatty oils, sometimes ruminate. Embryo large or small, straight, well differentiated, with or without chlorophyll. Cotyledons two. Germination phanerocotylar.
Cytology n = (5–)9–18(–20, 36) (x = 9)
DNA Several CYCLOIDEA and DIVARICATA genes duplicated (duplications possibly correlated with evolution of zygomorphy, according to Howarth & Donoghue 2005, 2009, and Howarth & al. 2011). Mitochondrial intron coxII.i3 often absent.
Phytochemistry Flavonols (kaempferol, quercetin), biflavonoids, cyanidin, Route I secoiridoids, Group V carbocyclic iridoids (valtrate, villoside, patrinoside), Group VI secoiridoids (secologanin, morroniside, kingiside), Group VII secoiridoids (sweroside, gentiopicroside), Group X secoiridoids (e.g. loganin, adoxoside, iridoid pyridine alkaloids), iridoid glucosides (i.a. dimethyl-acetal of cantleyoside), valepotriates (triesters of Route I secoiridoids), oleanolic acid derivatives, monoterpenoids, sesquiterpenoids (foetid ethereal oils), ursolic acid, caffeic acid, chlorogenic acid, indole alkaloids, triterpene saponins, cyanogenic glycosides (holocalin, sambunigrin, zierin), phenylalanine- or tyrosine-derived cyanogenic compounds, ethereal oils (rare), arbutin, eleostearic acid, valerianic acid, davidigenin, and actinidin present. Ellagic acid not found.
Systematics Dipsacales are sister-group to Paracryphiaceae.
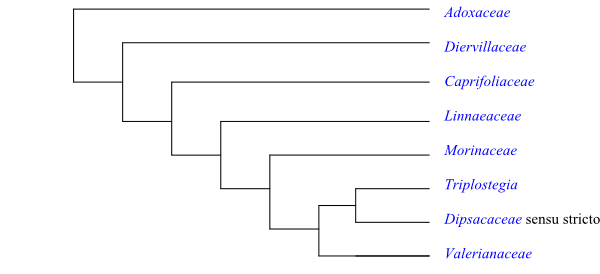
A strongly supported topology is the following: [Adoxaceae+[Diervillaceae+[Caprifoliaceae+ [Linnaeaceae+[Morinaceae+[Dipsacaceae+Valerianaceae]]]]]].
The clade [Diervillaceae+[Caprifoliaceae+[Linnaeaceae+[Morinaceae+[Dipsacaceae+ Valerianaceae]]]]], in other words Dipsacales except Adoxaceae, is supported by the potential synapomorphies: bark papery-flaky; phellogen deeply seated; pericyclic fibres moderately developed; wood often fluorescent; glandular foliar tooth with mid-vein and two lateral veins or lateral vein running above tooth; corolla zygomorphic, often two-lipped, tubular; epigyny; nectary as densely spaced adaxial unicellular or multicellular glandular hairs near base of corolla; tapetum amoeboid-periplasmodial; pollen grains large, spheroidal; tectum echinate; lateral and dorsal carpellary bundles absent, ventral bundles in axis; style long; stigma capitate, Wet type; chromosomes small; duplication of nuclear genes dipsCYC2 and dipsCYC3; horizontal transfer of mitochondral gene rps11; and presence of O-methylated flavonols and flavones (Stevens 2001 onwards).
[Caprifoliaceae+[Linnaeaceae+[Morinaceae+[Dipsacaceae+Valerianaceae]]]] is characterized by indehiscent fruit. Leaf bases often connate (in particular inflorescence bracts).
The clade [Linnaeaceae+[Morinaceae+[Dipsacaceae+Valerianaceae]]] has the following potential synapomorphies, according to Stevens (2001 onwards): supernumerary bracts (bracts more numerous than flowers) due to numerous aborted flowers; stamens four, didynamous; pistil composed of three carpels, two of which aborted; fruit a cypsela; seed coat flattened; and exotesta not lignified.
The clade [Morinaceae+[Dipsacaceae+Valerianaceae]] is characterized by the synapomorphies: perennial herbs with taproot; presence of flank bridge in stem between lateral vascular bundles (two bundles on each side seem to form split lateral branches and one branch from each bundle fusing in middle, forming bridging bundles); bundles innervating petiole arise from bridging bundle (not in Morina); presence of libriform fibres; absence of bud scales; nectary present at corolla base; filaments glabrous; anthers dorsifixed; ovary with much reduced sterile loculi; placentation apical; ovule one per carpel; endothelium prominent, with crystalliferous layer; chalazal nuclei proliferating; exotesta not thickened; endosperm sparse; embryo large; plastid inverted repeat significantly expanded; presence of monoterpenoids, cathecolic tannins and alkaloids; and absence of ellagic acid.
Finally, Dipsacaceae and Valerianaceae share the following potential synapomorphies: fairly small flowers; uniform staminal length (stamens equal in length); tapetum with cells at least quadrinucleate; exine with prominent and branched infratectal columellae; stigma Dry type; megagametophyte with binucleate to quadrinucleate chalazal cells; embryo with chlorophyll; presence of saccharose; and absence of quercetin.

|
Cladogram of Dipsacales based on DNA sequence data. |
ADOXACEAE E. Mey. |
( Back to Dipsacales ) |
Sambucaceae Batsch ex Borkh., Bot. Wörterb. 2: 322. 1797 [‘Sambuci’]; Sambucales Bercht. et J. Presl, Přir. Rostlin: 258. Jan-Apr 1820 [‘Sambucinae’]; Tinaceae Martinov, Tekhno-Bot. Slovar: 635. 3 Aug 1820 [‘Tiniaceae’]; Viburnaceae Raf. in Ann. Gén. Sci. Phys. Bruxelles 6: 87. Oct-Dec 1820 [’Viburnidia’]; Viburnales Dumort., Anal. Fam. Plant.: 29. 1829 [’Viburnarieae’]; Adoxales Nakai in J. Jap. Bot. 24: 14. Dec 1949
Genera/species 4–5/180–205
Distribution Mainly temperate regions on the Northern Hemisphere; some species in subtropical regions, few species on tropical mountains, southeastern Australia and Tasmania, with their largest diversity in Himalayas and China.
Fossils Numerous Cenozoic fossilized pollen, fruits and seeds of Sambucus and Viburnum are known from Europe and North America. Endocarps of Viburnum are known from the Miocene onwards and seeds of Sambucus are recorded from Eocene and later strata. Pollen fossils of Viburnum are known from Eocene to Miocene layers in, e.g., North America and Iceland (Manchester & al. 2015).
Habit Usually bisexual (in Viburnum rarely polygamomonoecious), perennial herbs (Adoxa, Sinadoxa, Tetradoxa, some species of Sambucus), evergreen or deciduous trees or shrubs (Sambucus, Viburnum).
Vegetative anatomy Phellogen ab initio superficial. Pericyclic fibrous envelope poorly developed or absent. Secondary lateral growth normal or absent. Vessel elements usually with scalariform (in Sambucus simple) perforation plates; lateral pits alternate, scalariform or opposite, bordered pits. Imperforate tracheary xylem elements tracheids, libriform fibres (Sambucus) or fibre tracheids (Viburnum) with simple or bordered pits, septate or non-septate; tracheids absent in Adoxa, Sinadoxa and Tetradoxa. Vestured pits absent. Wood rays uniseriate or multiseriate, heterocellular. Axial parenchyma apotracheal diffuse or diffuse-in-aggregates, or paratracheal scanty, aliform, confluent, vasicentric, unilateral, or banded. Wood elements in Sambucus partially storied. Tile cells present. Intraxylary phloem present in Sambucus. Sieve tube plastids S type. Nodes usually 3:3, trilacunar with three leaf traces (sometimes 1:1, unilacunar with one trace, or 5:5, pentalacunar with five traces). Coenocytic canals in internodes in Sambucus secreting tannins. Oil cells and/or mucilage cells frequent. Prismatic calciumoxalate crystals abundant. Crystal sand frequent in Sambucus. Calcium oxalate (crystal sand and druses) abundant in Sambucus and Viburnum.
Trichomes Hairs unicellular or multicellular, uniseriate or branched (in Viburnum often stellate or peltate); glandular hairs abundant. Hairs absent in Adoxa, Sinadoxa and Tetradoxa.
Leaves Usually opposite (rarely verticillate or alternate spiral), simple or compound (pinnately compound or trifoliolate), entire or lobed, usually with involute or conduplicate (rarely plicate) ptyxis. Stipules absent (Sambucus usually with stipitate extrafloral nectaries in stipular position); leaf sheath absent. Petiole vascular bundle transection arcuate (sometimes closed C-shaped); petiole also with adaxial inverted bundles (sometimes with wing bundles). In Sambucus and Viburnum foliaceous to scale-like appendages or nectariferous glands sometimes present on petiole or leaf base. Venation pinnate or palmate. Stomata usually anomocytic (sometimes paracytic). Cuticular wax crystalloids? Leaf margin and leaflet margins usually serrate. Mesophyll without mucilage cells.
Inflorescence Usually terminal (rarely axillary), dichasial, of various shape: in Adoxa capitate; in Sinadoxa spicate; in Tetradoxa raceme-like; in Sambucus and Viburnum umbel-like, corymbose or paniculate, thyrsoid; in Viburnum sometimes pseudanthium with large sterile marginal flowers.
Flowers Usually actinomorphic (sometimes slightly zygomorphic), small. Epigyny to partial epigyny. Sepals (two or) three to five, with open aestivation, very small, usually persistent, connate; median sepal adaxial. Petals (three to) five (or six), with imbricate or valvate aestivation, caducous, connate into disc-shaped, infundibuliform, campanulate or tubular corolla. Nectary in Viburnum epigynous disc-like structure on ovary (absent in Sambucus and some species of Viburnum). Nectaries in Adoxa, Sinadoxa and Tetradoxa multicellular nectariferous glands at corolla lobe bases. Disc absent.
Androecium Stamens three to five, more or less bifid (in Sinadoxa bifid to base), haplostemonous, antesepalous, alternipetalous. Filaments free from each other and from tepals or adnate to corolla tube (epipetalous, in Sambucus adnate to base of corolla tube). Anthers basifixed or dorsifixed (Viburnum), non-versatile, usually tetrasporangiate (in Adoxa, Sinadoxa and Tetradoxa more or less split into two disporangiate monothecal anther halves, in Tetradoxa peltate), extrorse (Adoxa, Sinadoxa, Tetradoxa, Sambucus) or introrse (Viburnum), longicidal (dehiscing by longitudinal slits). Tapetum usually secretory (at least in Adoxa and sometimes in Viburnum amoeboid-periplasmodial). Staminodia absent.
Pollen grains Microsporogenesis simultaneous. Pollen grains usually tricolporate (sometimes tricolpate or triporate), shed as monads, tricellular at dispersal. Exine semitectate, with columellate infratectum, reticulate, or intectate (Viburnum).
Gynoecium Pistil composed of two to five connate carpels (often only one carpel developed; in Viburnum three, two of which usually sterile). Ovary inferior to semi-inferior (in Tetradoxa superior?), usually bilocular to quinquelocular (in Sinadoxa unilocular, in Viburnum unilocular to trilocular); ovary cells in Sambucus with calciumoxalate druses. Stylodia in Adoxa and Tetradoxa three to five, free; absent in Sinadoxa; in Sambucus and Viburnum very short or absent. Stigmas capitate or lobate, papillate, Dry or Wet type. Pistillodia absent.
Ovules Placentation axile to apical. Ovule one median per carpel, anatropous, pendulous, apotropous (Viburnum) or epitropous (Sambucus), unitegmic, usually tenuinucellar (in Viburnum crassinucellar by division of parietal cell). Integument ? cell layers thick. Parietal tissue present. Endothelium present (in Adoxa poorly developed). Megagametophyte usually tetrasporous, octanucleate, Adoxa type (in Viburnum monosporous, Polygonum type, disporous, Allium type, or tetrasporous, Adoxa type). Endosperm development cellular. Endosperm haustoria? Embryogenesis asterad.
Fruit A berry-like drupe with one (Viburnum) or three to five (Sambucus) single-seeded pyrenes, or a drupaceous syncarp (Adoxa, Sinadoxa, Tetradoxa), often with persistent calyx.
Seeds Aril absent. Seeds often ruminate. Exotestal cells often palisade, unlignified. Endotesta? Perisperm not developed. Endosperm copious, oily, in Viburnum with small amorphous crystals. Embryo large or small, straight, usually well differentiated, with (Sambucus) or without (Viburnum) chlorophyll. Cotyledons two. Germination phanerocotylar.
Cytology n = (8) 9 (10, 18) – Chromosomes large, continuously condensed from prophase to metaphase.
DNA Mitochondrial intron coxII.i3 lost in Viburnum.
Phytochemistry O-methylated flavonols, Group VI secoiridoids present in Adoxa and Viburnum (secologanin, morroniside), Group X secoiridoids (in Viburnum adoxoside), Group III, V and VII secoiridoids present in Viburnum, alkaloids, caffeic acid, p-coumaric acid, sinapic acid, ferulic acid, ursolic acid (Sambucus), tannins, and cyanogenic compounds present. Ellagic acid and proanthocyanidins not found.
Use Ornamental plants, medicinal plants, liqueurs, wines, tea and jam (fruits of Sambucus nigra), timber.
Systematics Adoxaceae are sister to the remaining Dipsacales.
Viburnum is sister to the remaining Adoxaceae and Sambucus sister to the clade [Sinadoxa+[Adoxa+Tetradoxa]].
1/150–175. Viburnum (150–175; temperate regions on the Northern Hemisphere, tropical mountains; absent from Africa). – Usually shrubs (rarely trees). Hairs often stellate or peltate. Foliaceous to scale-like appendages or nectariferous glands sometimes present on petiole or leaf base. Nectary epigynous disc-like epithelial structure on ovary. Anthers dorsifixed, introrse. Pistil composed of three connate carpels, with odd carpel abaxial, and two carpels aborting. Ovary unilocular to trilocular. Ovule apotropous, crassinucellar by division of parietal cell. Parietal tissue approx. one cell layer thick. Fruit a single-seeded baccate drupe. Endocarp with fibrous layers orientated in parallel, with one or more sclereidal layers. Endosperm crystalliferous, with differentiated outer layer and thickened cell walls. – Viburnum clemensae on Borneo is obviously sister to the other species in Viburnum.
Adoxoideae Syme in Smith et Soweby, Engl. Bot., ed. 3(B), 4: 197. 1865 [‘Adoxeae’]
3–4/29–30. Sambucus (c 25; temperate regions on both hemisphere, few species in subtropical regions and tropical mountains in South America, southeastern Australia and Tasmania), Adoxa (2; A. moschatellina,A. xizangensis; temperate regions on the Northern Hemisphere south to the Himalayas and central United States; incl. Tetradoxa?), Tetradoxa (1; T. omeiensis; China; in Adoxa?), Sinadoxa (1; S. corydalifolia; China). – Temperate regions on the Northern Hemisphere, few species in subtropical regions and on tropical mountains. Shrubs or herbs. Cambium in Sambucus storied. Vessel elements with simple perforations. Cortex and phloem in Sambucus with crystal sand. Nodes in Sambucus 1:1. Leaves usually compound (sometimes simple and deeply lobed). Sambucus usually with stipitate extrafloral nectaries in stipular position. Nectary epigynous, on corolla associated with multicellular hairs, or absent. Anthers extrorse (sometimes eight, monothecal). Pistil composed of one or five connate antepetalous fertile carpels. Stylodia free. Stigmas capitate. Megagametophyte tetrasporic, 8-nucleate, Adoxa type. Endocarp with three layers of various cell types: two fibrous cell layers and one sclereid cell layer. Embryo in Sambucus large.
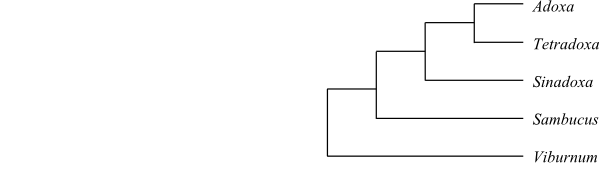 |
Cladogram of Adoxaceae based on DNA sequence data (Winkworth & al. 2008). |
CAPRIFOLIACEAE Juss. |
( Back to Dipsacales ) |
Loniceraceae Vest, Anleit. Stud. Bot.: 272, 296. 1818 [’Loniceroideae’]; Caprifoliales Bercht. et J. Presl, Přir. Rostlin: 257. Jan-Apr 1820 [‘Caprifoliae’]; Caprifoliopsida Endl., Gen. Plant.: 520. Jun 1838 [’Caprifolia’]; Loniceropsida Brongn., Enum. Plant. Mus. Paris: xvii, 47. 12 Aug 1843 [’Loniceroideae’]; Lonicerales T. Liebe, Grund. Spec. Bot.: 71. 31 Jan-1 Feb 1866 [’Loniceroideae’]
Genera/species 5/c 210
Distribution Mainly temperate regions on the Northern Hemisphere, with their highest diversity in the Himalayas and China; few species in tropical mountains south to Mexico and West Malesia.
Fossils Pollen of Lonicera were reported from the Late Eocene of western North America.
Habit Usually bisexual (rarely polygamomonoecious), evergreen or deciduous shrubs or lianas (rarely trees; in Triosteum perennial rhizomatous herbs).
Vegetative anatomy Phellogen ab initio usually pericyclic (in Heptacodium in centre of cortex). Medulla in some species of Lonicera septated by diaphragms. Cortical fibres in Heptacodium well developed. Stem endodermis absent. Vessel elements with simple or scalariform (sometimes reticulate) perforation plates; lateral pits alternate, scalariform or opposite, usually bordered (sometimes simple) pits. Imperforate tracheary xylem elements tracheids with simple and/or bordered pits, non-septate (also vasicentric tracheids). Wood rays uniseriate or multiseriate, heterocellular. Axial parenchyma apotracheal diffuse, or paratracheal scanty vasicentric or unilateral, or absent. Tile cells? Sieve tube plastids S type. Nodes usually 3:3, trilacunar with three leaf traces (rarely 1:1?, unilacunar with one? trace). Prismatic calciumoxalate crystals (druses) often abundant.
Trichomes Hairs unicellular or multicellular, usually simple (sometimes stellate); sometimes peltate-lepidote glandular hairs.
Leaves Usually opposite (rarely verticillate), simple, usually entire (in, e.g., Leycesteria and often in Lonicera pinnately lobed), usually with supervolute or conduplicate (in Heptacodium involute) ptyxis. Stipules usually absent (present in, e.g., Leycesteria); leaf sheath absent. Petiole vascular bundles? Venation camptodromous or brochidodromous, pinnate or palmate (lamina in Heptacodium three-veined from base). Stomata usually anomocytic (rarely paracytic). Cuticular wax crystalloids in Leycesteria, Lonicera and Symphoricarpos as tubuli (sparsely branched; in some species of Lonicera platelets), dominated by nonacosan-10-ol (absent in Heptacodium and Triosteum). Domatia usually absent (rarely as pits, pockets or hair tufts?). Leaf margin serrate or entire. Extrafloral nectaries present or absent.
Inflorescence Terminal or axillary, dichasial or corymbose, raceme-, spike- or head-like; flowers often paired (in Triosteum solitary axillary). Supernumerary bracts absent (cf. Linnaeaceae).
Flowers Usually zygomorphic (rarely actinomorphic). Epigyny. Sepals four or five, with imbricate or open aestivation, small, usually persistent, connate; median sepal adaxial. Petals four or five, with imbricate aestivation, caducous, connate to tubular to infundibuliform corolla (corolla often bilabiate, in Lonicera with quadrilobate upper and unilobate lower lip). Corolla at base with swelling covered by nectariferous unicellular hairs. Disc present or absent.
Androecium Stamens four or five, approximately equal in length, haplostemonous, antesepalous, alternipetalous. Filaments glabrous, free from each other, adnate to corolla tube (epipetalous). Anthers dorsifixed, versatile, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits). Tapetum usually amoeboid-periplasmodial. Staminodia absent.
Pollen grains Microsporogenesis simultaneous. Pollen grains (2–)3(–4)-colporate, -colpate or -porate?, shed as monads, tricellular at dispersal. Exine tectate, with columellate infratectum, microreticulate, spinulate.
Gynoecium Pistil composed of usually two to five (to eight?) connate carpels, all or two, three or four of which fertile; odd carpel adaxial (in Leycesteria antepetalous). Ovary inferior, usually bilocular to quinquelocular (to octalocular; ovaries in Lonicera often connate); one or two locules often compressed and sterile. Style single, simple. Stigma capitate to truncate, or bilobate to quinquelobate, papillate, Wet type. Pistillodium absent.
Ovules Placentation axile to apical. Ovules one to numerous per carpel, anatropous, pendulous, apotropous, unitegmic, tenuinucellar. Integument ? cell layers thick. Megagametophyte monosporous, Polygonum type, or disporous, Allium type, or tetrasporous, Adoxa type. Endosperm development cellular. Endosperm haustoria? Embryogenesis asterad (also other types?).
Fruit A one- to three-seeded drupe (in Symphoricarpus externally with thick layer of narrow vertical fibres and internal to this a layer of horizontal fibres), or a many-seeded berry or syncarp (Lonicera).
Seeds Aril absent. Testa in Symphoricarpus crushed. Exotestal cells? Endotesta? Perisperm not developed. Endosperm copious, oily. Embryo straight, without chlorophyll. Cotyledons two. Germination phanerocotylar.
Cytology n = (8–)9(–12, 16, 18)
DNA Plastid gene infA lost/defunct. Deletion in plastid gene clpP present in at least some species of Lonicera. Mitochondrial intron coxII.i3 lost. Duplication of nuclear genes dipsCYC2 and dipsCYC3?
Phytochemistry Flavonols (kaempferol, quercetin; O-methylated?), flavones (dominating), Group VI secoiridoids (secologanin, morroniside, kingiside), Group VII secoiridoids (sweroside), and Group X secoiridoids (loganin) present. Proanthocyanidins (cyanidin), alkaloids, and phenylalanine-derived cyanogenic compounds sometimes present. Coumarins present in Lonicera and Symphoricarpos. Ellagic acid not found.
Use Ornamental plants, medicinal plants, berries (Lonicera edulis).
Systematics Heptacodium (1; H. miconioides; central and eastern China); Triosteum (6; T. angustifolium, T. aurantiacum, T. himalayanum, T. perfoliatum, T. pinnatifidum, T. sinuatum; eastern Himalayas to China, the Korean Peninsula and Japan, eastern United States), Lonicera (c 180; temperate and subtropical regions on the Northern Hemisphere south to the Philippines and Mexico), Leycesteria (6–7; L. crocothyrsos, L. formosa, L. glaucophylla, L. gracilis, L. sinensis, L. stipulata, L. thibetica; western Himalayas to southwestern China), Symphoricarpos (16; Canada, United States, Mexico, Central America, one species, S. sinensis, in western China).
Caprifoliaceae are sister to [Linnaeaceae+[Morinaceae+[Dipsacaceae+Valerianaceae]]].
Heptacodium is sister to the remaining Caprifoliaceae.
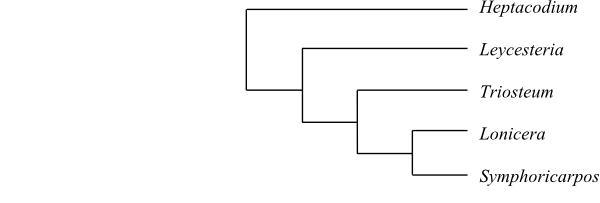 |
Cladogram of Caprifoliaceae based on DNA sequence data (Winkworth & al. 2008). In analyses by Theis & al. (2008) Leycesteria is sister to Symphoricarpos and Lonicera sister to [Leycesteria+Symphoricarpos]. |
DIERVILLACEAE (Raf.) Pyck |
( Back to Dipsacales ) |
Genera/species 2/13–14
Distribution East Asia, eastern North America.
Fossils Weigela fossils have been found in Miocene and Pliocene layers in Europe and Siberia.
Habit Bisexual, deciduous shrubs.
Vegetative anatomy Phellogen ab initio pericyclic. Vessel elements usually with scalariform (sometimes reticulate) perforation plates; lateral pits alternate or scalariform?, bordered pits. Imperforate tracheary xylem elements tracheids with bordered pits, non-septate. Wood rays uniseriate or multiseriate, heterocellular. Axial parenchyma apotracheal diffuse, or paratracheal scanty or banded, or absent. Sieve tube plastids S type. Nodes 3:3, trilacunar with three leaf traces. Crystals?
Trichomes Hairs unicellular or multicellular, uniseriate, simple.
Leaves Opposite, simple, entire, with involute ptyxis (Weigela). Stipules and leaf sheath absent. Petiole vascular bundle arcuate. Venation pinnate. Stomata anomocytic. Cuticular wax crystalloids as platelets (Weigela) or absent (Diervilla). Leaf margin serrate or entire.
Inflorescence Terminal or axillary, cymose; flowers often paired or solitary axillary. Supernumerary bracts usually absent (present in some species).
Flowers Zygomorphic (in Weigela almost actinomorphic). Epigyny. Sepals four or five, with open or imbricate? aestivation, free (Weigela) or connate (Diervilla); median sepal adaxial. Petals four or five, with imbricate aestivation, connate to tubular to infundibuliform corolla (in Diervilla with quadrilobate upper lip). Corolla at base with swelling covered by nectar secreting unicellular hairs. Disc absent.
Androecium Stamens four or five, antesepalous, alternipetalous. Filaments hairy, free from each other, adnate to corolla tube (epipetalous). Anthers dorsifixed, versatile, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits). Tapetum amoeboid-periplasmodial? Staminodia absent.
Pollen grains Microsporogenesis simultaneous. Pollen grains usually tripororate, shed as monads, tricellular at dispersal. Exine tectate, with acolumellate to columellate infratectum (with little developed columellae), spinulate.
Gynoecium Pistil composed of two connate carpels, one of which often aborted and sterile. Ovary inferior, unilocular or bilocular. Style single, simple. Stigma capitate, papillate, Dry type. Pistillodium absent.
Ovules Placentation axile. Ovules numerous per carpel, anatropous, pendulous, apotropous, unitegmic, tenuinucellar. Integument ? cell layers thick. Megagametophyte monosporous, Polygonum type? Endosperm development cellular. Endosperm haustoria? Embryogenesis?
Fruit A septicidal capsule (with beak-shaped processes and two valves), dehiscing downwards along its sides.
Seeds Aril absent. Testa with (Weigela) or without (Diervilla) comb-shaped wing. Exotestal cells? Endotesta? Perisperm not developed. Endosperm copious, oily. Embryo straight, well developed, without chlorophyll. Cotyledons two. Germination phanerocotylar?
Cytology n = 9
DNA Deletion in plastid gene clpP? Duplication of nuclear genes dipsCYC2 and dipsCYC3?
Phytochemistry Insufficiently known. Flavonols (O-methylated?; dominating) and Group VI secoiridoids (secologanin) present. Coumarins present. Ellagic acid not found?
Use Ornamental plants.
Systematics Diervilla (3; D. lonicera, D. rivularis, D. sessilifolia; southeastern Canada, eastern United States), Weigela (10–11; China, the Korean Peninsula, Japan).
Diervillaceae are sister to the remaining Dipsacales except Adoxaceae.
Diervilla may be paraphyletic.
DIPSACACEAE Juss. |
( Back to Dipsacales ) |
Scabiosaceae Martinov, Tekhno-Bot. Slovar: 563. 3 Aug 1820 [’Scabioseae’]; Triplostegiaceae (Höck) A. E. Bobrov ex Airy Shaw in Kew Bull. 18: 269. 8 Dec 1965; Dipsacineae Shipunov in A. Shipunov et J. L. Reveal in Phytotaxa 16: 63. 4 Feb 2011
Genera/species 12/300–305
Distribution Temperate and subtropical regions of Eurasia, North Atlantic islands, and Africa, with their largest diversity in the Mediterranean and western Asia; some species on mountains in southern India, Sri Lanka, Southeast Asia, Central Malesia, and New Guinea; Triplostegia: eastern Himalayas, southern China, Taiwan, Sulawesi, New Guinea.
Fossils Unknown.
Habit Bisexual, usually perennial, biennial or annual herbs (sometimes suffrutices, rarely shrubs).
Vegetative anatomy Phellogen ab initio usually pericyclic (at least in Knautia often superficial). Pericyclic fibrous envelope often well developed. Flank bridge in stem between lateral vascular bundles? Vessel elements usually with simple (sometimes scalariform) perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids with bordered pits. Wood rays uniseriate or multiseriate, heterocellular, or absent. Axial parenchyma? Sieve tube plastids S type. Endodermis sometimes with thick-walled cells (e.g. Dipsacus). Nodes 3: 3, trilacunar with three leaf traces (lateral traces bifurcating, median trace not ramifying). Calcium oxalate crystals usually in clusters (wood ray cells each with one rhomboidal calciumoxalate crystal).
Trichomes Hairs unicellular, usually lignified, or multicellular (in Cephalaria hair tufts); glandular hairs often present (in Knautia capitate glandular hairs).
Leaves Usually opposite (rarely verticillate), simple or pinnately compound, entire or pinnately lobed, sometimes coriaceous, with ? ptyxis. Stipules and leaf sheath absent. Petiole vascular bundles?; vascular flank bridge connecting vascular strands leading into petioles. Leaf bases sometimes connate. Venation pinnate. Stomata usually anomocytic (sometimes anisocytic). Cuticular wax crystalloids? Leaf margin and leaflet margins serrate or entire.
Inflorescence Terminal, usually head-like cymose, usually as pseudanthia (polythelic capitula), often with involucre consisting of bracts (in Triplostegia few-flowered open paniculate thyrse with glanduliferous branches). Supernumerary bracts (forming epicalyx) present (number of bracts larger than number of flowers, due to numerous floral abortions). Usually persistent (in Cephalaria, Dipsacus, and Knautia caducous) eight-ribbed epicalyx, consisting of usually two connate floral prophylls (bracteoles), usually surrounding calyx (epicalyx absent from some species of Cephalaria and Succisa; in Triplostegia two epicalyces present: one outer/lower epicalyx, consisting of four basally connate glandular acute bracts, and one inner/upper epicalyx, consisting of cupularly connate floral prophylls with eight ridges); epicalyx tube with usually eight ribs (in Knautia four ribs).
Flowers Usually zygomorphic (corolla in Triplostegia actinomorphic), usually small. Epigyny. Sepals usually five (often bilobate, in Triplostegia four), with valvate aestivation, usually bristle-like or membranous, usually persistent, free (when bristle-like) or connate (two sepals often entirely connate). Petals four or five (in Triplostegia four), with imbricate aestivation, unequal in size, caducous, connate into tubular or infundibuliform, often bilabiate corolla (two petals often entirely connate; lateral abaxial corolla lobes overlapping adaxial lobes). Nectaries usually present at stylar base (in Triplostegia at abaxial-basal part of corolla tube), as unicellular hairs. Disc intrastaminal.
Androecium Stamens usually four (rarely three; in Pterocephalidium diandrum two adaxial), antesepalous, alternipetalous; adaxial stamen absent; two abaxial stamens often shorter. Filaments free from each other, adnate to corolla lobe (epipetalous). Anthers dorsifixed, versatile?, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits). Tapetum amoeboid-periplasmodial, with multinucleate cells. Staminodia usually absent (sometimes one or two?).
Pollen grains Microsporogenesis simultaneous. Pollen grains 3(–4)-colpate or 3(–4)-porate (in Triplostegia tricolpor[oid]ate), with costae surrounding apertures, shed as monads, tricellular at dispersal. Exine tectate, with columellate infratectum (with branched columellae), perforate to microperforate, spinulate (with spines or microspines) or echinate.
Gynoecium Pistil composed of usually two (in Triplostegia three) connate carpels, one of which (adaxial-lateral) fertile. Ovary inferior, unilocular, pseudomonomerous. Style single, simple, narrow. Stigma entire or bilobate (with unequally sized lobes; in Triplostegia small), capitate, non-papillate, Dry type. Pistillodium absent.
Ovules Placentation apical. Ovule one per ovary, anatropous, pendulous, apotropous, unitegmic, tenuinucellar. Micropyle very long. Integument ten to 15 cell layers thick, non-vascularized? Hypostase usually present (absent from Triplostegia), with cavities containing yellowish liquid. Megagametophyte monosporous, Polygonum type. Chalazal cells binucleate to quadrinucleate, polyploid. Endosperm development cellular. Endosperm haustoria present in Triplostegia. Embryogenesis piperad.
Fruit A single-seeded cypseloid nut with persistent epicalyx and with remnants of calyx as bristles or prickles (in Triplostegia with two epicalyces).
Seeds Aril absent. Elaiosome present in Knautia. Testa membranous, consisting of outer epidermis and hypodermis, remaining parts crushed. Exotestal cells? crushed? Endotesta? Perisperm not developed. Endosperm sparse, oily (starch almost absent), not ruminate. Embryo large, straight, well differentiated, with chlorophyll. Cotyledons two. Germination phanerocotylar.
Cytology x = (5), 7–10(–17)
DNA Elongation of plastid inverted repeat. Duplication of nuclear genes dipsCYC2 and dipsCYC3. Horizontal transfer of mitochondrial gene rps11.
Phytochemistry Flavonoids, Group VI secoiridoids (secologanin), Group VII secoiridoids (gentiopicroside), monoterpenoid glucoindole alkaloids, triterpenoid glycosides, alkaloids, coumarins, phenolic compounds, caffeoyl guinic acid derivatives, lignin glycoside, and saponins present. Valepotriate iridoids present in Triplostegia. Quercetin, ellagic acid, proanthocyanidins and cyanogenic compounds not found. Inulin? (in Cephalaria?) Catechol tannins? O-methylated flavones?
Use Ornamental plants, carding of wool (species of Dipsacus).
Systematics Dipsacaceae are sister-group to Valerianaceae.
Triplostegia is sister to the remaining Dipsacaceae.
Triplostegia
1/2. Triplostegia (2; T. glandulifera, T. grandiflora; eastern Himalayas, southern China, Taiwan, Sulawesi, New Guinea). – Herbs. Inflorescence few-flowered open paniculate thyrse with glanduliferous branches. Flowers tetramerous, with floral prophylls (bracteoles) and double epicalyx: one outer/lower epicalyx, consisting of four basally connate glandular acute bracts, and one inner/upper epicalyx, consisting of cupularly connate floral prophylls with eight ridges. Corolla actinomorphic. Stamens four. Pollen grains tricolpor[oid]ate, apertures with halo and granular colpus. Pistil composed of three carpels. Hypostase absent. Endosperm haustoria present. Fruit surrounded by capitate-glandular outer epicalyx and with hooked tips. Endosperm reduced. n = ? Valepotriate iridoids present. – Valerianaceae also have pollen aperture with halo and granulate colpus, and valepotriates are present (apart from several other morphological similarities).
Dipsacoideae Eaton, Bot. Dict., ed. 4: 36. Apr-Mai 1836 [‘Dipsaceae’]
12/300–305. Bassecoia (3; B. bretschneideri, B. hookeri, B. siamensis; the Himalayas, western China, Thailand); Pseudoscabiosa (5; P. africana, P. diandra, P. grosii, P. saxatilis, P. limoniifolia; western and central Mediterranean), Succisa (3; S. pratensis: Europe, the Mediterranean, North Africa, western Siberia; S. pinnatifida: northwestern Iberian Peninsula; S. trichotocephala: Cameroon), Succisella (5; S. andreae-molinae, S. carvalhoana, S. inflexa, S. microcephala, S. petteri; the Iberian Peninsula, eastern Europe from the Balkan Peninsula to Lithuania); Knautia (c 60; Europe, the Mediterranean), Pterocephalidium (1; P. diandrum; central Spain, Portugal), Pterothamnus (1; P. centennii; Mozambique); Cephalaria (c 80; Europe, the Mediterranean to Central Asia, Ethiopia, southern Africa), Dipsacus (c 20; Europe, the Mediterranean, mountains in tropical Africa, temperate Asia, southern India, Sri Lanka); Lomelosia (>50; the Mediterranean to the Caucasus), Pterocephalus (c 30; Macaronesia, the Mediterranean to Central Asia, the Himalayas and western China, tropical Africa), Scabiosa (c 40; Europe, the Mediterranean, temperate Asia, East African mountains, southern Africa). – Temperate regions in Europe, Africa and Asia, eastern and southern Africa and southern Asia to New Guinea. Inflorescence capitulum with involucre. Epicalyx single, eight-ribbed. Flowers usually pentamerous. Stamens sometimes two or three. Fruit with calycine awns or bristles. n = 5, 7–9 (10). – The epicalyx morphology varies strongly among Dipsacoideae. The epicalyx is often provided with wings or spiny bristles. In species with reduced or without corona, the number of calyx bristles is often increased to form a pappus-like structure adapted to long-distance wind dispersal. Some groups are marked by additional epicalyx structures often associated with elaborate wing-like appendages. The ‘diaphragma’ is formed by a secondary meristem on the inner side of the epicalyx, and encloses the stalk of the calyx-like collar. The ‘epi-diaphragma’ is a small attachment present between the diaphragma and the ‘corona’, which is a membranous wing-like structure that forms the apex of the epicalyx.
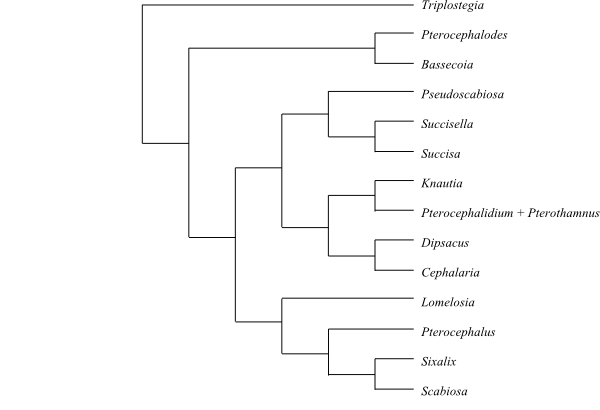 |
Phylogeny (founded on Maximum parsimony and Bayesian inference trees) of Dipsacaceae s. str. based on DNA sequence data (Avino & al. 2009; Carlson & al. 2009; Mayer & Ehrendorfer 2013). Triplostegia is usually sister-group to the remaining Dipsacaceae (sometimes sister to Valerianaceae, or to the clade [Dipsacaceae+Valerianaceae]). |
LINNAEACEAE (Raf.) Backlund |
( Back to Dipsacales ) |
Genera/species 1/19
Distribution Temperate regions on the Northern Hemisphere south to the Himalayas and Mexico, with their highest diversity in China.
Fossils Winged fruits from North American Late Eocene have been described as Diplodipelta, and fossils assigned to ‘Dipelta’ (Linnaea) are also known from the Late Eocene of England. Pollen fossils were reported from the Eocene in Greenland and Central Europe.
Habit Bisexual, evergreen or deciduous shrubs or small trees, or creeping and partially woody.
Vegetative anatomy Phellogen ab initio pericyclic. Pericyclic fibrous envelope often well developed. Vessel elements usually with simple (sometimes scalariform) perforation plates; lateral pits alternate, bordered pits. Imperforate tracheary xylem elements tracheids with bordered pits, non-septate. Wood rays uniseriate or multiseriate, heterocellular. Axial parenchyma apotracheal diffuse or diffuse-in-aggregates, or paratracheal scanty, or absent. Sieve tube plastids S type. Nodes usually 3:3, trilacunar with three leaf traces. Crystals?
Trichomes Hairs unicellular or multicellular?; glandular hairs often present.
Leaves Opposite, simple, entire, with supervolute (to curved) ptyxis. Stipules and leaf sheath absent. Petiole vascular bundles? Venation pinnate. Stomata anomocytic. Cuticular wax crystalloids as platelets (in Dipelta and Kolkwitzia absent) Leaf margin serrate or entire.
Inflorescence Axillary, few-flowered cymose (flowers often paired), or flowers solitary axillary. Supernumerary bracts often present (cf. Caprifoliaceae).
Flowers Zygomorphic. Epigyny. Sepals four or five, with open aestivation, entirely or partially connate; median sepal adaxial. Petals four or five, with imbricate aestivation, connate into infundibuliform corolla. Abaxial petal with nectary as basal swelling with nectariferous unicellular hairs. Disc absent.
Androecium Stamens four or five, antesepalous, alternipetalous. Filaments hairy, free from each other, adnate to corolla tube (epipetalous). Anthers dorsifixed, versatile?, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits). Tapetum amoeboid-periplasmodial. Staminodia absent.
Pollen grains Microsporogenesis simultaneous. Pollen grains usually tricolporate (in ‘Abelia’ sometimes zonorate), shed as monads, tricellular at dispersal. Exine tectate, with columellate infratectum, psilate to spinulate.
Gynoecium Pistil composed of two (to four) connate carpels, one or two (adaxial-lateral) of which fertile. Ovary inferior, usually bilocular (sometimes trilocular or quadrilocular, with two locules compressed and sterile). Style single, simple. Stigma capitate, papillate?, Wet type? Pistillodium absent.
Ovules Placentation axile to apical? Ovule one or several? per carpel, anatropous, pendulous, apotropous?, unitegmic, tenuinucellar. Integument ? cell layers thick. Megagametophyte monosporous, Polygonum type? Endosperm development cellular. Endosperm haustoria? Embryogenesis?
Fruit Usually a nut or drupe (rarely berry), often with calyx and/or bracts persistent and modified (cypseloid?; L. dipelta and L. yunnanensis have more or less fleshy two-seeded fruit with two accrescent bracts).
Seeds Aril absent. Exotestal cells? Endotesta? Perisperm not developed. Endosperm copious, oily. Embryo straight, well developed, without chlorophyll. Cotyledons two. Germination phanerocotylar?
Cytology n = 8, 9
DNA Deletion in plastid gene clpP? Duplication of nuclear genes dipsCYC2 and dipsCYC3?
Phytochemistry Very insufficiently known. Group VI secoiridoids (e.g. secologanin) present. Flavonols (O-methylated?) dominating, flavones.
Use Ornamental plants.
Systematics Linnaea (19; temperate regions on the Northern Hemisphere south to northern Vietnam, Taiwan and central Mexico, with their highest diversity in China and Mexico).
Linnaeaceae are sister-group to [Morinaceae+[Dipsacaceae+Valerianaceae]].
Vesalea is sister to the remaining Linnaeaceae (Jacobs & al. 2010; Landrein 2010).
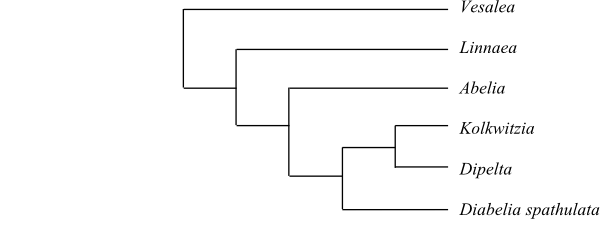 |
Phylogeny of Linnaeaceae based on DNA sequence data (Jacobs & al. 2010; Landrein 2010). |
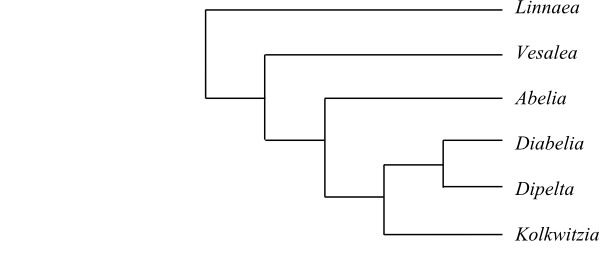 |
Phylogeny of Linnaeaceae based on DNA sequence data (Landrein & al. 2012). |
MORINACEAE Raf. |
( Back to Dipsacales ) |
Genera/species 4/22–23
Distribution The Balkan Peninsula, southwestern Asia to Afghanistan, Central Asia, the Himalayas, East Asia.
Fossils Unknown.
Habit Bisexual, perennial herbs. Often prickly and spinose. Stem hollow.
Vegetative anatomy Phellogen ab initio pericyclic? Flank bridge present in stem between lateral vascular bundles. Vessel elements with simple perforation plates; lateral pits alternate. Imperforate tracheary xylem elements libriform fibres with bordered? pits. Wood rays absent? Axial parenchyma? Sieve tube plastids S type. Nodes 5:5, quinquelacunar with five leaf traces. Calcium oxalate crystals as druses.
Trichomes Hairs usually unicellular; corolla with stalked multicellular glands.
Leaves Usually opposite (sometimes verticillate), simple, usually pinnately lobed (rarely entire), with involute ptyxis. Stipules and leaf sheath absent. Petiole vascular bundles?; petiole with vascular flank bridge connecting vascular strands leading into petioles. Leaf bases often sheathing and connate. Venation palmate. Stomata anomocytic. Cuticular wax crystalloids? Epidermis with or without mucilage cells, with secretory cells containing ethereal oils. Leaf margin usually serrate (often serrate-dentate; rarely entire).
Inflorescence Axillary, in cymose whorls along vertical stem; partial inflorescences consisting of sessile cymes. Supernumerary bracts absent. Epicalyx campanulate, 12-ribbed, consisting of two connate serrate-dentate floral prophylls (bracteoles, bracts?) enclosing flower.
Flowers Zygomorphic. Epigyny. Sepals two or four, with imbricate? aestivation, persistent, connate and bilabiate, with lips entire or bilobate. Petals five, with imbricate aestivation, connate into more or less tubular bilabiate corolla. Nectariferous disc intrastaminal, annular. Nectaries as unicellular hairs.
Androecium Stamens two fertile adaxial stamens and two staminodia (didynamous; adaxial stamen absent), haplostemonous, antesepalous, alternipetalous. Filaments long, thick, free from each other, adnate to corolla tube (epipetalous. Anthers dorsifixed or basifixed?, non-versatile, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits). Tapetum amoeboid-periplasmodial. Staminodia two (corresponding to abaxial two stamens) or absent.
Pollen grains Microsporogenesis simultaneous. Pollen grains usually 3(–4)-colporate (sometimes 3(–4)-pororate), shed as monads, bicellular at dispersal. Exine tectate, with acolumellate to columellate infratectum (with poorly developed columellae), imperforate?, spinulate? Pollen tube-like structures covered by intine formed prior to germination of pollen grains.
Gynoecium Pistil composed of two connate carpels, one of which degenerating (adaxial-lateral carpel fertile). Ovary inferior, unilocular. Style single, simple, narrow. Stigma capitate, type? Pistillodium absent.
Ovules Placentation apical. Ovule one per ovary, anatropous, pendulous, unitegmic, tenuinucellar. Integuments 14 to 18 cell layers thick, non-vascularized. Hypostase well developed, without cavity. Megagametophyte monosporous, Polygonum type. Antipodal cells proliferating. Chalazal cells producing multicellular structures. Endosperm development cellular. Endosperm haustoria absent. Embryogenesis solanad.
Fruit A single-seeded cypseloid nut with persistent remains of calyx. Pericarp thick, rugose.
Seeds Aril absent. Seed coat exotestal-epidermal. Testa thin, multiplicative, non-vascularized, consisting of outer epidermis. Exo- and endotestal cells used up during endosperm development. Perisperm not developed. Endosperm sparse, ruminate, oily. Embryo large, straight, well differentiated, chlorophyll? Cotyledons two. Germination phanerocotylar?
Cytology n = 17
DNA Duplication of nuclear genes dipsCYC2 and dipsCYC3. Horizontal transfer of mitochondrial gene rps11? Elongation of plastid inverted repeat?
Phytochemistry Very insufficiently known. Alkaloids, saponins and polyphenols present. Ethereal oils present. Monoterpenoids? Iridoids, ellagic acid, proanthocyanidins, alkaloids, and cyanogenic compounds not found. Cathecole tannins?
Use Ornamental plants.
Systematics Zabelia (12–13; Central Asia, Afghanistan, northwestern India, Nepal, China, the Korean Peninsula, Japan, the Russian Far East); Cryptothladia (4; C. chinensis, C. chlorantha, C. kokonorica, C. polyphylla; the Himalayas, western China), Morina (3; M. couteriana, M. longifolia, M. persica; Greece, Bulgaria, Romania, Turkey, Lebanon, Israel, Iraq, Iran, Central Asia, eastern Himalayas, western China).
Morinaceae are sister to [Dipsacaceae+Valerianaceae].
Zabelia, with psilate exine and endocingulum at inner nexine wall layer, is ususally sister to the remaining Morinaceae (but see Landrein & al. 2012). The generic limits and sister-group relationships among the majority of Morinaceae are still fairly confusing.
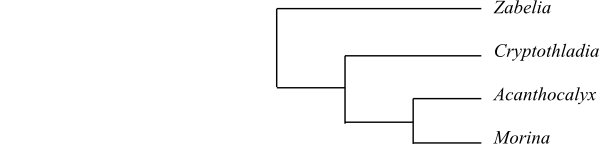 |
Cladogram of Morinaceae based on DNA sequence data (Bell 2004, Winkworth & al. 2008; Jacobs & al. 2010). Acanthocalyx is sister to [Dipsacaceae+Valerianaceae] and Zabelia sister to the clade [[Cryptothladia+Morina]+[Acanthocalyx+[Dipsacus clade+Valeriana clade]]] in some analyses by Landrein & al. (2012). However, in other most of their analyses Zabelia is part of a trichotomy also comprising Linnaeaceae and [Dipsacaceae+Valerianaceae] and Morinaceae sister-group to this trichotomy (Landrein & al. 2012). |
VALERIANACEAE Batsch |
( Back to Dipsacales ) |
Valerianales DC. ex Bercht. et J. Presl, Přir. Rostlin: 256. Jan-Apr 1820 [‘Valerianeae’]
Genera/species 5?/270–400
Distribution Temperate regions on the Northern Hemisphere, Mexico, Central America, the West Indies, South America (c. 50% of the species occurring in the Andes), mountain areas in eastern and southern Africa, and South Asia to Sumatra and Java, with their largest diversity in the Mediterranean, West Asia and the Andes.
Fossils Unknown.
Habit Usually bisexual (sometimes polygamomonoecious or dioecious), usually perennial, biennial or annual herbs (rarely shrubs). Often evil-smelling.
Vegetative anatomy Phellogen ab initio pericyclic. Flank bridge in stem between lateral vascular bundles? Primary vascular tissue cylinder of vascular bundles. Vessel elements extremely small, usually with simple (in Patrinia scalariform) perforation plates; lateral pits alternate. Imperforate tracheary xylem elements libriform fibres and/or fibre tracheids (in Patrinia tracheids) with simple or bordered pits. Wood rays multiseriate or absent. Axial parenchyma usually scanty paratracheal (rarely vasicentric or confluent) or absent. Sieve tube plastids S type. Nodes usually 3:3–5, trilacunar with three to five leaf traces (rarely 1:1 or 5–7:5–7). Rhizomal and root cortex and cork tissue with idioblastic cells containing strongly smelling ethereal oils. Calcium oxalate crystals sometimes present.
Trichomes Hairs unicellular or multicellular, simple; glandular hairs often present.
Leaves Opposite, simple or pinnately compound, entire or pinnately lobed, with ? ptyxis. Stipules and leaf sheath absent. Petiole bases often sheathingly connate. Petiole vascular bundles?; vascular flank bridge connecting vascular strands leading into petioles. Venation pinnate. Stomata anomocytic. Cuticular wax crystalloids? Hydathodes sometimes present. Leaf margin and leaflet margins serrate or entire.
Inflorescence Terminal, umbel-like, corymbose, capitate or paniculate cymose. Supernumerary bracts absent. Epicalyx absent. Floral prophylls (bracteoles) present.
Flowers Zygomorphic or asymmetrical (in Patrinia and Nardostachys almost actinomorphic), usually small. Epigyny. Sepals usually minute and reduced (in Patrinia and Nardostachys five, well developed), persistent, connate, at anthesis often as whorl of up to 20 inrolled parts, later unrolled, expanded and bristle-like or plumose (pappus-like). Petals usually five (sometimes three), with imbricate aestivation, connate into infundibuliform or tubular (sometimes bilabiate) structure; abaxial petal often with nectariferous spur at base (corolla in Centranthus divided by vertical septum). Nectaries as unicellular hairs. Disc absent?
Androecium Stamen one (in Centranthus adaxial), two (adaxial, in ‘Fedia’), three (in ‘Fedia’ adaxial and central stamens absent), four (adaxial stamen absent; in Patrinia and Nardostachys) or five, haplostemonous, antesepalous, alternipetalous, asymmetrically inserted. Filaments usually free from each other (two stamens in ‘Fedia’ connate), adnate to corolla tube (epipetalous). Anthers dorsifixed, versatile?, usually tetrasporangiate (in Valeriana subgenus Phyllactis disporangiate), introrse, longicidal (dehiscing by longitudinal slits). Tapetum amoeboid-periplasmodial, with multinucleate cells. Staminodia absent.
Pollen grains: Microsporogenesis simultaneous. Pollen grains 3(–4)-colpate or 3(–4)-colpor(oid)ate, shed as monads, tricellular at dispersal. Exine tectate, with columellate infratectum (with branched columellae), perforate (to microperforate) spinulate or echinate.
Gynoecium Pistil composed of three connate carpels. Ovary inferior, trilocular (two locules reduced, sterile, adaxial-lateral carpel fertile; sometimes unilocular, pseudomonomerous). Style single, simple, narrow. Stigma capitate or bi- or trilobate, Dry type. Pistillodium absent.
Ovules Placentation apical. Ovule one per ovary, anatropous, pendulous, apotropous?, unitegmic, tenuinucellar. Integument ? cell layer thick, non-vascularized? Hypostase sometimes present. Megagametophyte monosporous, Polygonum type. Chalazal cells binucleate to quadrinucleate. Antipodal cells persistent. Endosperm development cellular. Endosperm haustoria? Embryogenesis asterad.
Fruit A single-seeded cypseloid nutlet, usually with persistent and accrescent calyx as pappus-like (winged, plumose, etc.) appendages, hooks or membranous margins (sometimes reduced).
Seeds Aril absent. Testa one-layered. Testal cells? Perisperm not developed. Endosperm absent or almost absent (sometimes sparse, starchy). Embryo large, straight, well differentiated, oily, sometimes with chlorophyll. Cotyledons two. Germination phanerocotylar.
Cytology n = (7) 8, 9(–16)
DNA Elongation of plastid inverted repeat. Duplication of nuclear genes dipsCYC2 and dipsCYC3. Horizontal transfer of mitochondrial gene rps11 to nuclear genome. Mitochondrial intron coxII.i3 lost.
Phytochemistry Flavonols (kaempferol), O-methylated flavonols, Route I iridoids and secoiridoids, valepotriates (triesters of Route I secoiridoids), Group V carbocyclic iridoids (valtrate, villoside, patrinoside), Group VI secoiridoids (morroniside), Group X secoiridoids (loganin, iridoid pyridine alkaloids), foetid ethereal monoterpenoids sesquiterpenoids, alkaloids, triterpene saponins, and valeric acid present. Quercetin, ellagic acid, proanthocyanidins, and cyanogenic compounds not found. Catechol tannins?
Use Ornamental plants, medicinal plants, perfumery (Nardostachys), vegetables (Valerianella).
Systematics Patrinia (c 17; Ural and western Asia to the Himalayas and East Asia); Nardostachys (1; N. jatamansi; the Himalayas, western China); Centranthus (9; southern Europe, the Mediterranean; in Valeriana?), ’Valeriana’ (c 270; temperate regions on the Northern Hemisphere, mountain areas in eastern and southern Africa, South Asia to Sumatra and Java, Mexico, Central America, the West Indies, South America with their highest diversity in the Andes; polyphyletic), Valerianella (c 70; temperate regions on the Northern Hemisphere south to North Africa).
Valerianaceae are sister-group to Dipsacaceae.
Patrinia is sister to the remaining Valerianaceae, and Nardostachys successive sister to the remainder. Sometimes Patrinia and Nardostachys form a joint sister-group to the remaining Valerianaceae. Patrinia contains villoside and patrinoside. Valerianella (including Fedia) and Valeriana celtica + V. hardwickii are successive sister-groups to Valeriana (including Centranthus) (Hidalgo & al. 2010).
Triplostegia is sometimes placed as sister-group to Valerianaceae (morphological or both morphological and molecular data). Both Triplostegia and Valerianaceae have valepotriate iridoids, a pollen aperture with halo and granulate colpus, and also a reduced endosperm, apart from similarities in epicalyx morphology.
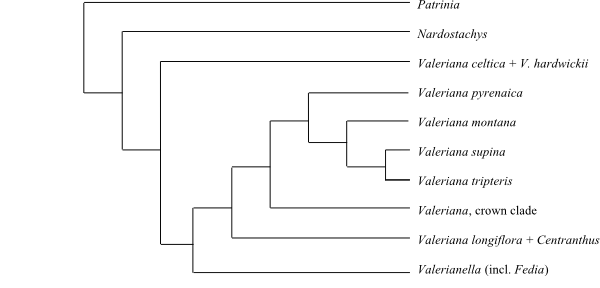 |
Phylogeny of Valerianaceae based on DNA sequence data (Hidalgo & al. 2010; Bell & al. 2012). A similar topology was obtained by Bell & al. (2015), although with a larger number of taxa. |
Literature
Airy Shaw HK. 1932. A revision of the genus Leycesteria. – Kew Bull. 4: 161-176.
Airy Shaw HK. 1952. A second species of the genus Heptacodium Rehd. (Caprifoliaceae). – Kew Bull. 7: 245-246.
Adsoy N, Süleyman GR, Acik L, Celebi A. 2007. Cephalaria duzceensis (Dipsacaceae), a new species from the western Black Sea region, Turkey. – Nord. J. Bot. 25: 64-69.
Akimaliev A, Alimbaeva PK, Mzhel’skaya LG, Abubakirov NK. 1971. Triterpene glycosides of Scabiosa soongorica II. The structure of songorosides C, G and I. – Chem. Nat. Comp. 12: 415-418.
Amich F, Devesa JA, Bernardos S. 2004. Taxonomic revision of the genus Succisella (Dipsacaceae) in the Iberian Peninsula. – Bot. J. Linn. Soc. 144: 351-364.
An M-T, Gou G-Q. 2009. Abelia lipoensis, a new species of the genus Abelia from Guizhou, China. – Bull. Bot. Res. (North-East. For. Univ.) 29: 129-130.
Artyushenko ZT. 1951. Development of the flowers and fruits in Caprifoliaceae. – Trudy Bot. Inst. Akad. Nauk SSSR, ser. VII, 2: 131-169.
Asplund E. 1920. Studien über die Entwicklungsgeschichte der Blüten einiger Valerianaceen. – Kungl. Sv. Vetensk.-Akad. Handl. 61(3): 1-65.
Avino M, Tortoriello G, Caputo P. 2009. A phylogenetic analysis of Dipsacaceae based on four DNA regions. – Plant Syst. Evol. 279: 69-86.
Backlund A. 1996. Phylogeny of the Dipsacales. – Ph.D. diss., Department of Systematic Botany, University of Uppsala, Acta Universitatis Upsaliensis, Uppsala, pp. 1-34.
Backlund A, Bremer B. 1997. Phylogeny of the Asteridae s. str. based on rbcL sequences, with particular reference to the Dipsacales. – Plant Syst. Evol. 207: 225-254.
Backlund A, Bremer K. 1998. To be or not to be – principles of classification and monotypic plant families. – Taxon 47: 391-400.
Backlund A, Donoghue MJ. 1996. Morphology and phylogeny of the order Dipsacales. – In: Backlund A, Phylogeny of the Dipsacales, Acta Universitatis Upsaliensis, Uppsala.
Backlund A, Moritz T. 1998. Phylogenetic implications of an expanded valepotriate distribution in the Valerianaceae. – Biochem. Syst. Evol. 26: 309-335.
Backlund A, Nilsson S. 1997. Pollen morphology and the systematic position of Triplostegia (Dipsacales). – Taxon 46: 21-31.
Backlund A, Pyck N. 1998. Diervillaceae and Linnaeaceae, two new families of caprifolioids. – Taxon 47: 657-661.
Bailey LH. 1929. The case of Diervilla and Weigela. – Gentes Herb. 2: 39-54.
Baksay L. 1952. Monographie der Gattung Succisa. – Ann. Hist. Nat. Mus. Natl. Hung., n. s., 2: 237-259.
Barabe D. 1991. Analysis of the phyllotaxis of the genus Dipsacus (Dipsacaceae). – Saussurea 22: 95-101.
Bassett IJ, Compton CW. 1970. Pollen morphology of the family Caprifoliaceae in Canada. – Pollen Spores 12: 365-380.
Bate-Smith EC. 1978. Astringent tannins of Viburnum and Hydrangea species. – Phytochemistry 17: 267-270.
Bedi YS, Bir SS, Gill BS. 1982. Cytological studies in certain woody members of family Caprifoliaceae. – J. Tree Sci. 1: 27-34.
Bell CD. 2003. Phylogeny and biogeography of Valeriana. – Ph.D. diss., Yale University, New Haven, Connecticut.
Bell CD. 2004. Preliminary phylogeny of Valerianaceae (Dipsacales) inferred from nuclear and chloroplast DNA sequence data. – Mol. Phylogen. Evol. 31: 340-350.
Bell CD. 2007. Phylogenetic placement and biogeography of the North American species of Valerianella (Valerianaceae: Dipsacales) based on chloroplast and nuclear DNA. – Mol. Phylogen. Evol. 44: 929-941.
Bell CD, Donoghue MJ. 2003. Phylogeny and biogeography of Morinaceae (Dipsacales) based on nuclear and chloroplast DNA sequences. – Organisms Divers. Evol. 3: 227-237.
Bell CD, Donoghue MJ. 2005a. Dating the Dipsacales: comparing models, genes, and evolutionary implications. – Amer. J. Bot. 92: 284-296.
Bell CD, Donoghue MJ. 2005b. Phylogeny and biogeography of Valerianaceae (Dipsacales) with special reference to the South American valerians. – Organisms Divers. Evol. 5: 147-159.
Bell CD, Gonzalez LA. 2018. Exploring the utility of “next-generation” sequence data on inferring the phylogeny of the South American Valeriana (Valerianaceae). – Mol. Phylogen. Evol. 123: 44-49.
Bell CD, Edwards EJ, Kim S-T, Donoghue MJ. 2001. Dipsacales phylogeny based on chloroplast DNA sequences. – Harvard Pap. Bot. 6: 481-499.
Bell CD, Kutschker A, Arroyo MTK. 2012. Phylogeny and diversification of Valerianaceae (Dipsacales) in the southern Andes. – Mol. Phylogen. Evol. 63: 724-737.
Bell CD, Calderon G, Gonzalez L, Scholz A, Liede-Schumann S. 2015. Resolving relationships within Valerianaceae (Dipsacales): new insights and hypotheses from low-copy nuclear regions. – Syst. Bot. 40: 327-335.
Benko-Iseppon AM. 1992. Karyologische Untersuchung der Caprifoliaceae s.l. und möglicher verwandter Familien. – Ph.D. diss., Universität Wien, Austria.
Benko-Iseppon AM, Morawetz W. 1993. Cold-induced chromosome regions and karyosystematics in Sambucus and Viburnum. – Bot. Acta 106: 183-191.
Benko-Iseppson AM, Morawetz W. 2000. Viburnales: cytological features and a new circumscription. – Taxon 49: 5-16.
Blackmore S, Cannon MJ. 1983. Palynology and systematics of Morinaceae. – Rev. Palaeobot. Palynol. 40: 207-226.
Blankenhorn B. 1978. Pollenmorphologisch-systematische Untersuchungen an Valerianaceae. – Bot. Jahrb. Syst. 99: 108-138.
Bohm BA, Glennie CWM. 1971. A chemosystematic study of the Caprifoliaceae. – Can. J. Bot. 49: 1799-1807.
Böhnke-Gütlein E, Weberling F. 1981. Palynologische Untersuchungen an Caprifoliaceaee I. Sambuceae, Viburneae, und Diervilleae. – Akad. Wiss. Lit. (Mainz), Trop. Subtrop. Pflanzenwelt 34: 131-189.
Bolli R. 1994. Revision of the genus Sambucus. – Diss. Bot. 223: 1-227.
Borsini OE. 1966a. Sobre la identidad del genero Astrephia Dufr. con Valeriana L. (Valerianaceae). – Lilloa Rev. Bot. 32: 369-374.
Borsini OE. 1966b. Valerianáceas de Chile. – Lilloa Rev. Bot. 32: 375-476.
Bos R, Woerdenbag HJ, Hendriks H, Smit HF, Wikstrom HV, Scheffer JJ. 1997. Composition of the essential oil from roots and rhizomes of Valeriana wallichii DC. – Flavour Fragrance J. 12: 123-131.
Bounthanh C, Bergmann C, Beck JP, Haag-Berrurier M, Anton R. 1981. Valepotriates, a new class of cytotoxic and antitumor agents. – Planta Medica 41: 21-28.
Braun R, Dittmar W, Machut M, Weickmann S. 1982. Valepotriate mit Epoxidstruktur – beatliche Alkylantien. – Deutsche Apotheker-Zeitung 122: 1109-1113.
Briquet J. 1914. Decades plantarum novarum vel minus cognitarum, Décades 8-16 Valerianaceae. – Ann. Conserv. Jard. Bot. Genève 19: 326-356.
Burtt BL. 1999. The importance of some far Eastern species of Dipsacaceae in the history of the family. – In: Tandon RK, Prithipalsingh (eds), Biodiversity, taxonomy, and ecology, Scientific Publ., Jodhpur, pp. 131-139.
Cannon JFM. 1983. 95. Valerianaceae. – In: Launert E (ed), Flora Zambesiaca 7 (Part 1), Flora Zambesiaca Managing Committee, London, pp. 75-77.
Cannon MJ, Cannon JFM. 1983. 96. Dipsacaceae. – In: Launert E (ed), Flora Zambesiaca 7 (Part 1), Flora Zambesiaca Managing Committee, London, pp. 77-85.
Cannon MJ, Cannon JFM. 1984. A revision of the family Morinaceae (Magnoliophyta-Dipsacales). – Bull. Brit. Mus. (Nat. Hist.), Bot. Ser., 12: 1-35.
Caputo P, Cozzolino S. 1994. A cladistic analysis of Dipsacaceae (Dipsacales). – Plant Syst. Evol. 189: 41-61.
Caputo P, Cozzolino S. 1995. Cladogeny in Dipsacaceae: dispersal and vicariance models. – Boll. Soc. Sarda Sci. Nat. 30: 233-243.
Caputo P, Cozzolino S, Moretti A. 2004. Molecular phylogenetics of Dipsacaceae reveals parallel trends in seed dispersal syndromes. – Plant Syst. Evol. 246: 163-175.
Carey K, Ganders FR. 1987. Patterns of isoenzyme variation in Plectritis (Valerianaceae). – Syst. Bot. 12: 125-132.
Carlquist SJ. 1982. Wood anatomy of Dipsacaceae. – Taxon 31: 443-450.
Carlquist SJ. 1983. Wood anatomy of Calyceraceae and Valerianaceae, with comments on aberrant perforation plates in predominantly herbaceous groups of dicotyledons. – Aliso 10: 413-425.
Carlson SE, Mayer V, Donoghue MJ. 2009. Phylogenetic relationships, taxonomy, and morphological evolution in Dipsacaceae (Dipsacales) inferred by DNA sequence data. – Taxon 58: 1075-1091.
Chapelle J-P, Denoel A. 1972. Contribution à l’étude des valépotriates dans la racine de valériane officinale. – Plantes Méd. Phytothér. 6: 91.
Chen H-B, Cheng C-R. 1991. Two new species of Morina L. from China. – Acta Phytotaxon. Sin. 29: 190-192.
Chevalier J. 1907. Pharmacodynamic action of a new alkaloid contained in the roots of fresh valerian. – Compt. Rend. Soc. Biol. 144: 154-157.
Chiarugi A. 1927. Poliploidia nel genere Knautia (Dipsacaceae). – Nuovo Giorn. Bot. Ital. 34: 864-871.
Christenhusz MJM. 2013. Twins are not alone: a recurcumscription of Linnaea (Caprifoliaceae). – Phytotaxa 125: 25-32.
Christopoulou C, Graikou K, Chinou I. 2008. Chemosystematic value of chemical constituents from Scabiosa hymettia (Dipsacaceae). – Chem. Biodiv. 5: 318-322.
Clarke GCS. 1978. Pollen morphology and generic relationships in the Valerianaceae. – Grana 17: 61-75.
Clarke GCS. 1981. The Northwest European pollen flora 21. Dipsacaceae. – Rev. Palaeobot. Palynol. 33: 1-26.
Clarke GCS, Jones MR. 1977. The Northwest European pollen flora 16. Valerianaceae. – Rev. Palaeobot. Palynol. 24: 155-179.
Clement WL, Donoghue MJ. 2011. Dissolution of Viburnum section Megalotinus (Adoxaceae) of southeast Asia and its implications for morphological evolution and biogeography. – Intern. J. Plant Sci. 172: 559-573.
Clement WL, Arakaki M, Sweeney PW, Edwards
EJ, Donoghue MJ. 2014. A chloroplast tree for Viburnum (Adoxaceae) and its implications for
phylogenetic classification and character evolution. – Amer. J. Bot. 101:
1029-1049.
Coombes AJ. 1990. Heptacodium jasminoides – the Chinese seven-son flower in Britain. – Kew Mag. 7: 133-138.
Cooper TB. 1938. A study of the pericycle in the Caprifoliaceae. – Trans. Bot. Soc. Edinb. 32: 548-555.
Corsi G, Lokar L, Pagni A-M. 1984. Biological and phytochemical aspects of Valeriana officinalis. – Biochem. Syst. Ecol. 12: 57-62.
Devesa JA. 1984a. Revisión del género Scabiosa en la Peninsula Ibérica e Islas Baleares. – Lagascalia 12: 143-212.
Devesa JA. 1984b. Pseudoscabiosa, género nuevo de Dipsacaceae. – Lagascalia 12: 213-221.
DeVos F. 1951. The stem anatomy of some species of the Caprifoliaceae with reference to phylogeny and identification of the species. – Ph.D. diss., Cornell University, Ithaca, New York.
Doll W. 1927. Beitrag zur Kenntnis der Dipsaceen und dipsaceenähnlichen Pflanzen. – Bot. Arch. 17: 107-146.
Donoghue MJ. 1983a. A preliminary analysis of phylogenetic relationships in Viburnum (Caprifoliaceae s.l.). – Syst. Bot. 8: 45-58.
Donoghue MJ. 1983b. The phylogenetic relationships of Viburnum. – In: Platnick NI, Funk VA (eds), Advances in cladistics 2. Proceedings of the Willi Hennig Society, Columbia University Press, New York, pp. 143-166.
Donoghue MJ. 1985. Pollen diversity and exine evolution in Viburnum and the Caprifoliaceae sensu lato. – J. Arnold Arbor. 66: 421-469.
Donoghue MJ, Olmstead RG, Smith JF, Palmer JD. 1992. Phylogenetic relationships of Dipsacales based on rbcL sequences. – Ann. Missouri Bot. Gard. 79: 333-345.
Donoghue MJ, Eriksson T, Reeves PA, Olmstead RG. 2001. Phylogeny and phylogenetic taxonomy of Dipsacales, with special reference to Sinadoxa and Tetradoxa (Adoxaceae). – Harvard Papers Bot. 6: 459-479.
Donoghue MJ, Bell CD, Winkworth RC. 2003. The evolution of reproductive characters in Dipsacales. – Intern. J. Plant Sci. 164(Suppl.): S453-S464.
Donoghue MJ, Baldwin BG, Li J, Winkworth RC. 2004. Viburnum phylogeny based on chloroplast trnK intron and nuclear ribosomal ITS DNA sequences. – Syst. Bot. 29: 188-198.
Dyal SC. 1938. Valerianella in North America. – Rhodora 40: 185-213.
Egger K. 1962. Astragalin und Paeonosid – die Hauptglykoside des Schneeballs Viburnum opulus L. – Zeitung für Naturforschung 17: 139-141.
Eggers DM. 1969. A revision of Valerianella in North America. – Ph.D. diss., Vanderbilt University, Nashville, Tennessee.
Eggers Ware DM. 1983. Genetic fruit polymorphisms in North American Valerianella (Valerianaceae) and its taxonomic implications. – Syst. Bot. 8: 33-44.
Egolf DR. 1962. A cytological study of the genus Viburnum. – J. Arnold Arbor. 43: 132-172.
Ehrendorfer F. 1962. Beiträge zur Phylogenie der Gattung Knautia (Dipsacaceae) I. Cytologische Grundlagen und allgemeine Hinweise. – Österr. Bot. Zeitschr. 109: 276-343.
Ehrendorfer F. 1964a. Über stammesgeschichtliche Differenzierungsmuster bei den Dipsacaceen. – Ber. Deutsch. Bot. Ges. 77: 83-94.
Ehrendorfer F. 1964b. Evolution and karyotype differentiation in a family of flowering plants: Dipsacaceae. – In: Genetics Today. Proceedings of the 11th International Congress of Genetics, The Hague, September 1963, vol. 2, pp. 399-407.
Ehrendorfer F. 1965. Evolution and karyotype differentiation in a family of flowering plants: Dipsacaceae. – Genetics Today (Proc. XI International Congress of Genetics, The Hague, The Netherlands, 1963) 2: 399-407.
Ehrendorfer F. 1981. Neue Beiträge zur Karyosystematik und Evolution der gattung Knautia (Dipsacaceae) in den Balkanländern. – Bot. Jahrb. Syst. 102: 225-238.
Elvers I. 1932. Chromosomenzahlen in der Gattung Valerianella nebst einigen systematischen Bemerkungen. – Acta Horti Berg. 11: 81-87.
Engel K. 1976. Beiträge zur Systematik der Valerianaceae unter besonderer Berücksichtigung cytosystematischer Ergebnisse. – Justus Liebig Universität, Gießen.
Erbar C. 1994. Contributions to the affinities of Adoxa from the viewpoint of floral development. – Bot. Jahrb. Syst. 116: 259-282.
Erdtman G. 1945. Pollen morphology and plant taxonomy III. Morina L. With an addition on pollenmorphological terminology. – Svensk Bot. Tidskr. 39: 187-191.
Eriksen B. 1989a. Note on generic and infrageneric delimitation in the Valerianaceae. – Nord. J. Bot. 9: 179-187.
Eriksen B. 1989b. 186. Valerianaceae. – In: Harling G, Andersson L (eds), Flora of Ecuador 34, Nord. J. Bot., Copenhagen, pp. 1-59.
Eriksen B. 1991. Two new species of Valeriana (Valerianaceae) from the northern Peruvian Andes. – Nord. J. Bot. 11: 619-622.
Eriksen B. 1993. Taxonomical studies in the Polygalaceae and Valerianaceae. – Ph.D. diss., University of Gothenburg, Sweden.
Eriksson T, Donoghue MJ. 1997. Phylogenetic relationships of Sambucus and Adoxa (Adoxoideae, Adoxaceae) based on nuclear ribosomal ITS sequences and preliminary morphological data. – Syst. Bot. 22: 555-573.
Ernet D. 1977a. Sproßaufbau und Lebensform von Valerianella und Fedia (Valerianaceae). – Plant Syst. Evol. 127: 243-276.
Ernet D. 1977b. Blütenbau und Fortpflanzungsbiologie von Valerianella und Fedia (Valerianaceae). – Plant Syst. Evol. 128: 1-22.
Ernet D. 1978. Fruchtbau und Verbreitungsbiologie von Valerianella und Fedia (Valerianaceae). – Plant Syst. Evol. 130: 85-126.
Fahn A. 1978. The extrafloral nectaries of Sambucus nigra. – Ann. Bot., N. S., 60: 299-308.
Fanlo R. 1986. El género Centranthus DC. en España I. Sección Calcitrapa Lange. – Lagascalia 14: 3-8.
Farmer JB. 1888-1889. On the development of the endocarp in Sambucus nigra. – Ann. Bot. 2: 389-392.
Franchet MA. 1896. Araliaceae, Cornaceae et Caprifoliaceae novae e flora sinensi. – J. Bot. 10: 301-308.
Franzén R. 1986. Materials for the Mountain Flora of Greece 28. The Valeriana crinii-group (Valerianaceae) in Greece. – Willdenowia 15: 351-357.
Fritsch K. 1891a. Caprifoliaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien IV(4), W. Engelmann, Leipzig, pp. 156-169.
Fritsch K. 1891b. Adoxaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien IV(4), W. Engelmann, Leipzig, pp. 170-171, 190.
Fukuoka N. 1968. Phylogeny of the tribe Linnaeeae. – Acta Phytotaxon. Geobot. 23: 82-94.
Fukuoka N. 1969. Inflorescence of Linnaeeae (Caprifoliaceae). – Acta Phytotaxon. Geobot. 23: 153-162.
Fukuoka N. 1972. Taxonomic study of the Caprifoliaceae. – Mem. Fac. Sci. Kyoto Univ., Ser. Biol., 6: 15-58.
Fukuoka N. 1974. Floral morphology of Adoxa moschatellina. – Acta Phytotaxon. Geobot. 26: 65-76.
Fukuoka N. 1975. Studies in the systematics of Caprifoliaceae 2. – Acta Phytotaxon. Geobot. 26: 133-139.
Fukuoka N. 1987. Classification and distribution of Sambucus. – Acta Phytotaxon. Geobot. 39: 303-310.
Ganders FR, Carey K, Griffiths AJF. 1976. Outcrossing rates in natural populations of Plectritis brachystemon (Valerianaceae). – Can. J. Bot. 55: 2070-2074.
Giger E. 1912. Linnaea borealis L., eine monographische Studie. – Beih. Bot. Centralbl. 30: 1-78.
Golubkova VF. 1965. De genere Heptacodium Rehd. e familia Caprifoliaceae. – Nov. Syst. Plant. Vasc. 2: 230-236.
Golubkova VF. 1973. De speciebus nonnullis generis Abelia R. Br.(Caprifoliaceae Juss.). – Nov. Syst. Plant. Vascul. (Nov. Sist. Vysshch. Rast.) 10: 241-248.
Gould KR, Donoghue MJ. 2000. Phylogeny and biogeography of Triosteum (Caprifoliaceae). – Harvard Pap. Bot. 5: 157-166.
Gräbner P. 1899. Beiträge zur Kenntnis der süd- und centalamerikanischen Valerianaceae. – Engl. Bot. Jahrb. Syst. 26: 425-436.
Gräbner P. 1900. Die Gattung Linnaea (einschließlich Abelia). – Engl. Bot. Jahrb. Syst. 29: 120-145.
Gräbner P. 1906a. Valerianaceae andinae. – Engl. Bot. Jahrb. Syst. 37: 436-451.
Gräbner P. 1906b. Die Gattungen der natürlichen Familie der Valerianaceae. – Engl. Bot. Jahrb. Syst. 37: 464-480.
Gräbner P. 1918. Aretiastrum maximum, eine neue Valerianacee aus Peru. – Feddes Repert. 15: 323.
Graikou K, Aligiannis N, Ioanna B, Chinou IB, Harvala C. 2002. Cantleyoside-dimethyl-acetal and other iridoid glucosides from Pterocephalus perennis – Antimicrobial activities. – Zeitschr. Naturforsch. 57c: 95-99.
Graikou K, Aligiannis N, Chinou IB. 2006. Chemical constituents from Pterocephalus perennis subsp. perennis (Dipsacaceae). – Biochem. Syst. Ecol. 34: 438-441.
Greger H, Ernet D. 1971. Flavonoide und Systematik der Valerianaceae. – Naturwissenschaften 58: 416-417.
Greger H, Ernet D. 1973. Flavonoid-Muster, Systematik und Evolution bei Valerianella. – Phytochemistry (Oxford) 12: 1693-1699.
Greilhuber J. 1979. C-band distribution, DNA-content, and base composition in Adoxa moschatellina (Adoxaceae): a plant with cold-sensitive chromosome segments. – Plant Syst. Evol. 131: 243-259.
Gülcemal D, Masullo M, Alankus-Calıskan O, Karayıldırım T, Senol SG, Piacente S, Bedir E. 2010. Monoterpenoid glucoindole alkaloids and iridoids from Pterocephalus pinardii. – Magn. Reson. Chem. 48: 239-243.
Gundersen A-L. 1910. Recherches anatomique sur les Caprifoliacées. – L’Université de Paris.
Günthart A. 1904. Blütenbiologische Untersuchungen 2. Beiträge zur Blütenbiologie der Dipsaceen. – Flora 93: 199-250.
Gütlein R, Weberling F. 1982. Fruchtanatomische Untersuchungen an Valerianaceae. – Ber. Deutsch. Bot. Ges. 95: 35-43.
Hara H. 1981. A new species of the genus Adoxa from Mt. Omei of China. – J. Jap. Bot. 56: 271-274.
Hara H. 1983. A revision of Caprifoliaceae of Japan with reference to allied plants in other districts and the Adoxaceae. – Ginkgoana 5: 1-336.
Hedberg I, Hedberg O. 1977. The genus Dipsacus in tropical Africa. – Bot. Not. 129: 383-389.
Hemsley WB. 1908. New or noteworthy plants – the genus Dipelta. – Gard. Chron., ser. 3, 44: 101-103.
Hidalgo O, Garnatje T, Susanna A, Mathez J. 2004. Phylogeny of Valerianaceae based on matK and ITS markers, with reference to matK individual polymorphism. – Ann. Bot. 93: 283-294.
Hidalgo O, Mathez J, Garcia S, Garnatje T, Pellicer J, Vallès J. 2010. Genome size study in the Valerianaceae: first results and new hypotheses. – J. Bot., Article ID 797246, pp. 1-19, doi:10.1155/2010/797246.
Hilger HH, Hoppe M. 1984. Die Entwicklung des Außenkelchs von Scabiosa Sekt. Sclerostemma und Trochocephalus (Dipsacaceae). – Beitr. Biol. Pflanzen 59: 55-73.
Hillebrand GR. 1969. A serological investigation of intrageneric relationships of Viburnum (Caprifoliaceae). – Bull. Torrey Bot. Club 96: 556-567.
Hillebrand GR, Fairbrothers DE. 1970a. Phytoserological systematic survey of the Caprifoliaceae. – Brittonia 22: 125-133.
Hillebrand GR, Fairbrothers DE. 1970b. Serological investigation of the systematic position of the Caprifoliaceae I. Correspondence with selected Rubiaceae and Cornaceae. – Amer. J. Bot. 57: 810-815.
Höck F. 1882. Beiträge zur Morphologie, Gruppierung und geographischen Verbreitung der Valerianaceen. – Engl. Bot. Jahrb. Syst. 3: 1-73.
Höck F. 1891a. Valerianaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien IV(4), W. Engelmann, Leipzig, pp. 172-182.
Höck F. 1891b. Dipsacaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien IV(4), W. Engelmann, Leipzig, pp. 182-189.
Höck F. 1892. Zur systematischen Stellung von Sambucus. – Beih. Bot. Centralbl. 51: 233-234.
Höck F. 1902. Verwandtschaftsbeziehungen der Valerianaceen und Dipsacaceen. – Engl. Bot. Jahrb. Syst. 31: 403-411.
Hofmann U, Göttmann J. 1990. Morina Wall. und Triplostegia Wall. ex DC. im Vergleich mit Valerianaceae und Dipsacaceae. – Bot. Jahrb. Syst. 111: 499-553.
Hölzl J von, Jurcic K. 1975. Valepotriate in den Blättern von Valeriana jatamansii. – Planta Medica 27: 133-139.
Hong DY. 2010. Nomenclatural notes on the Morinaceae and Valerianaceae in China. – Novon 20: 418-419.
Horne AL. 1914. A contribution to the study of the evolution of the flower, with special reference to the Hamamelidaceae, Caprifoliaceae, and Cornaceae. – Trans. Linn. Soc. London, Bot., 2nd ser., 8: 239-309.
Houghton PJ. 1988. The biological activity of valerian and related plants. – J. Ethnopharmacology 22: 121-142.
Houghton PJ. 1999. The scientific basis for the reputed activity of Valerian. – J. Pharm. Pharmacol. 51: 505-512.
Hounsel RW. 1968. Cytological studies in Sambucus. – Can. J. Genet. Cytol. 10: 237-247.
Howarth DG, Donoghue MJ. 2005. Duplications in CYC-like genes from Dipsacales correlate with floral form. – Intern. J. Plant Sci. 166: 357-370.
Howarth DG, Donoghue MJ. 2009. Duplications and expression of DIVARICATA-like genes in Dipsacales. – Mol. Biol. Evol. 26: 1245-1258.
Howarth DG, Martins T, Chimney L, Donoghue MJ. 2011. Diversification of CYCLOIDEA expression in the evolution of bilateral flower symmetry in Caprifoliaceae and Lonicera (Dipsacales). – Ann. Bot. 107: 1521-1532.
Hsu P-S. 1983. A preliminary numerical taxonomy of the family Caprifoliaceae. – Acta Phytotaxon. Sin. 21: 26-32.
Ikuse M, Kurosawa S. 1954. Notes on sect. Zabelia Rehder of the genus Abelia. – J. Jap. Bot. 29: 107-110.
Jacobs B, Donoghue MJ, Bouman F, Huysmans S, Smets E. 2008. Evolution and phylogenetic importance of endocarp and seed characters in Viburnum (Adoxaceae). – Intern. J. Plant Sci. 169: 409-431.
Jacobs B, Pyck N, Smets E. 2010. Phylogeny of the Linnaea clade: are Abelia and Zabelia closely related? – Mol. Phylogen. Evol. 57: 741-752.
Jacobs B, Bell C, Smets E. 2010. Fruits and seeds of the Valeriana clade (Dipsacales): diversity and evolution. – Intern. J. Plant Sci. 171: 421-434.
Jacobs B, Huysmans S, Smets E. 2010. Evolution and systematic value of fruit and seed characters in Adoxaceae (Dipsacales). – Taxon 59: 850-866.
Jacobs B, Geuten K, Pyck N, Huysmans S, Jansen S, Smets E. 2011. Unravelling the phylogeny of Heptacodium and Zabelia (Caprifoliaceae): an interdisciplinary approach. – Syst. Bot. 26: 231-252.
Jensen SR, Lyse-Pedersen SE, Nielsen BJ. 1979. Novel bis-iridoid glucosides from Dipsacus sylvestris. – Phytochemistry 18: 273-277.
Jordheim M, Giske NH, Andersen ØM. 2006. Anthocyanins in Caprifoliaceae. – Biochem. Syst. Ecol. 35: 153-159.
Jorica HS. 1921. Development of head and flower of Dipsacus sylvestris. – Bot. Gaz. 71: 138-145.
Kachidze N. 1929. Karyologische Studien über die Familie der Dipsacaceae. – Planta 7: 482-502.
Kadereit JW, Bittrich V (eds). 2016. The families and genera of vascular plants XIV. Flowering plants – eudicots – Aquifoliales, Boraginales, Bruniales, Dipsacales, Escalloniales, Garryales, Paracryphiales, Solanales (except Convolvulaceae), Icacinaceae, Metteniusaceae, Vahliaceae. – Springer, 412 pp.
Kamelina OP. 1980. Sravnitelnaja embriologija semejstv Dipsacaceae i Morinaceae [Comparative embryology of the families Dipsacaceae and Morinaceae]. – Nauka, Leningrad.
Kamelina OP. 1983. Basic results of the comparative embryological investigation of Dipsacaceae and Morinaceae. – In: Edelská O, Ciamporová A, Lux A, Pret’ová J, Tup’y J (eds), Fertilization and embryogenesis in ovulated plants. Proceedings from the 7th International Cytoembryological Symposium, Bratislava, Czechoslovakia 1982, June 14-17, Slovak Academy of Sciences, pp. 343-346.
Kamelina OP, Yakovlev MS. 1974. Development of the embryo sac in the genus Morina. – Bot. Žurn. 59: 1609-1617. [In Russian]
Kamelina OP, Yakovlev MS. 1976. Development of the anther and microgametogenesis in the representatives of Dipsacaceae and Morinaceae. – Bot. Žurn. 61: 932-945.
Kao M-T, Devol C. 1973. The Valerianaceae of Taiwan. – Taiwania 18: 146-159.
Karcz J. 1996. Fruit micromorphology and anatomy of Valeriana officinalis s. str. (Valerianaceae). – Nord. J. Bot. 16: 409-419.
Kern JM. 1951. The genus Viburnum (Caprifoliaceae) in Malaysia. – Reinwardtia 1: 107-170.
Kern JH, Steenis CGGJ van. 1951. Caprifoliaceae. – In: Steenis CGGJ van (ed), Flora Malesiana I, 4(3), Noordhoff-Kolff N. V., Batavia, pp. 175-194.
Killip EP. 1925. Twelve new species of Valeriana from the Andes of South America. – J. Washington Acad. Sci. 15: 450-456.
Killip EP. 1928. Seven new species of Valeriana from Colombia and Peru. – J. Washington Acad. Sci. 18: 498-501.
Killip EP, Smith AC. 1931. The South American species of Viburnum. – Bull. Torrey Bot. Club 57: 245-258.
Kim T. 1998. Phylogenetic studies of tribe Linnaeeae (Caprifoliaceae). – Ph.D. thesis, Chonbuk National University.
Kocsis Á, Szabó LF, Podányi B. 1993. New bis-iridoids from Dipsacus laciniatus. – J. Nat. Prod. (Lloydia) 56: 1486-1499.
Kokwaro JO. 1968. Valerianaceae. – In: Milne-Redhead E, Polhill RM (eds), Flora of tropical East Africa, Crown Agents for Oversea Governments and Administrations, London, pp. 1-9.
Koller GL. 1986. Seven-son flower from Zhejiang: introducing the versatile ornamental shrub Heptacodium jasminoides. – Arnoldia 46: 3-13.
Kutschker A. 2009. Valeriana L. (Valerianaceae) en Sudamérica Austral: taxonomía, aspectos biogeográficos y fitoquímicos. – Ph.D. diss., Universidad Nacional de la Patagonia SJB, Comodoro Rivadavia, Argentina.
Kutschker A, Morrone JJ. 2012. Distributional patterns of the species of Valeriana (Valerianaceae) in southern South America. – Plant Syst. Evol. 298: 535-547.
Lagerberg T. 1909. Studien über die Entwicklungsgeschichte und systematische Stellung von Adoxa moschatellina L. – Kungl. Sv. Vetensk.-Akad. Handl., ser. II, 44: 1-86.
Łańcucka-Środoniowa M. 1967. Two new genera: Hemiptelea Planch. and Weigela Thunb. in the younger Tertiary of Poland. – Acta Palaeobot. 8: 1-19.
Landrein S. 2010a. Diabelia, a new genus of the tribe Linnaeeae subtribe Linnaeinae (Caprifoliaceae). – Phytotaxa 3: 34-38.
Landrein S. 2010b. Abelia and its relatives. – The Plantsman (New Series) 9: 40-47.
Landrein S, Prenner G. 2013. Unequal twins? Inflorescence evolution in the twinflower tribe Linnaeeae (Caprifoliaceae s.l.). – Inter. J. Plant Sci. 174: 200-233.
Landrein S, Prenner G. 2016. Structure, ultrastructure and evolution of floral nectaries in the twinflower tribe Linnaeeae and related taxa (Caprifoliaceae). – Bot. J. Linn. Soc. 181: 37-69.
Landrein S, Prenner G, Chase MW, Clarkson JJ. 2012. Abelia and relatives: phylogenetics of Linnaeeae (Dipsacales-Caprifoliaceae s.l.) and a new interpretation of their inflorescence morphology. – Bot. J. Linn. Soc. 169: 692-713.
Larsen BB. 1986. A taxonomic revision of Phyllactis and Valeriana sect. Bracteata (Valerianaceae). – Nord. J. Bot. 6: 427-446.
Läufer JL, Seckel BJ, Zwaving JH. 1970. Onderzoek naar de samenstelling van de werkzame bestanddelen van verschillende valeriaan- en kentranthussoorten. – Pharmaceutisch Weekblad 105: 609-625.
Lavaille P. 1925a. Sur les antipodes et la région chalazienne de l’ovule des Dipsacées. – Compt. Rend. Acad. Sci. Paris 180: 1606-1608.
Lavaille P. 1925b. Sur le sac embryonnaire des Dipsacacées. – Compt. Rend. Acad. Sci. Paris 180: 1127-1129.
Lewis WH, Fantz PR. 197. Tribal classification of Triosteum (Caprifoliaceae). – Rhodora 75: 120-121.
Li S-H, Ning Z-H. 1987. Studies on the family Adoxaceae. – Bull. Bot. Res. 7: 93-109.
Liang H-X. 1993. The chromosome numbers of Adoxaceae and their systematic significance. – Acta Bot. Yunnan. 15: 260-262.
Liang X-Q, Li Y, Kvaečk Z, Wilde V, Li C-S. 2013. Seeds of Weigela (Caprifoliaceae) from the Early Miocene of Weichang, China, and the biogeographical history of the genus. – Taxon 62: 1009-1018.
Landrein S, Prenner G. 2013. Unequal twins? Inflorescence evolution in the twinflower tribe Linnaeeae (Caprifoliaceae s.l.). – Intern. J. Plant Sci. 174: 200-233.
Liu JJ, Wang XL, Guo BL, Huang WH, Xiao PG, Huang CQ, Zheng LZ, Zhang G, Qin L, Tu GZ. 2011. Triterpenoid saponins from Dipsacus asper and their activities in vitro. – J. Asian Nat. Prod. Res. 13: 851-860.
Liu JQ, Chen ZD, Lu AM. 2000. The phylogenetic relationships of the Qinghai-Tibet endemic Sinadoxa, revealed by ITS data. – Acta Phytotaxon. Sin. 42: 301-308.
Lokar LC, Moneghini M. 1989. Geographical variation in the monoterpenes of Valeriana officinalis leaf. – Biochem. Syst. Ecol. 17: 563-568.
López Gonzáles G. 1987. Pterocephalidium, un nuevo género ibérico de la familia Dipsacaceae. – An. Jard. Bot. Madrid 43: 245-252.
Lörcher H. 1990. Achsenverdickung und Sproβanatomie bei Valerianaceae. – Akad. Wiss. Lit. Mainz, Trop. u. subtrop. Pflanzenwelt 74.
Lörcher H, Weberling F. 1982. Zur Achsenverdickung hochandiner Valerianaceen. – Ber. Deutsch. Bot. Ges. 95: 57-74.
Lörcher H, Weberling F. 1984. Anatomie und Achsenverdickung brasilianischer Valerianaarten Series Polystachyae. – In: Rauh W (ed), Tropische und subtropische Pflanzenwelt 47, Franz Steiner Verlag, Weisbaden, pp. 315-341.
Lörcher H, Weberling F. 1985. Zur Achsenanatomie hochandiner Valeriana-Arten (Valeriana micropterina Wedd. und Valeriana thalictroides Graebn.). – Flora 176: 197-212.
Lörcher H, Weberling F. 1990. Anatomical and morphological investigations of Phyllactis spp. – Flora 184: 231-254.
Lys P. 1954. Recherches biochimiques sur les Dipsacacées du Liban et de la Syrie. – In: Dubertret ML (ed), Notes et mémoires sur le Moyen-Orient 5, Muséum National d’Histoire Naturelle, Paris, pp. 1-137.
Ma WG, Wang DZ, Zeng YL, Yang CH. 1991. Three triterpenoid saponins from Triplostegia grandiflora. – Phytochemistry (Oxford) 30: 3401-3404.
Ma WG, Wang DZ, Zeng YL, Yang CR. 1992. Triterpenoid saponins from Triplostegia grandiflora. – Phytochemistry (Oxford) 31: 1343-1347.
McAtee WL. 1921. Notes on Viburnum and the assemblage Caprifoliaceae. – Bull. Torrey Bot. Club 48: 149-154.
Maciejewska I. 1997. Pollen morphology of the Polish species of the family Caprifoliaceae 1. – Acta Soc. Bot. Poloniae 66: 133-142.
Maheshwari P. 1946. The Adoxa type embryo sac: a critical review. – Lloydia 9: 73-113.
Manchester SR, Donoghue MJ. 1995. Winged fruits of Linnaeeae (Caprifoliaceae) in the Tertiary of western North America: Diplodipelta gen. nov. – Intern. J. Plant Sci. 15: 709-722.
Manning JC, Goldblatt P, Johns A. 2014. A
taxonomic review of Cephalaria (Dipsacaceae) in the Cape Floristic
Region. – South Afr. J. Bot. 94: 195-203.
Martinovsky JO. 1931. Einige interessante Blätter- und Blütenabnormität an Adoxa moschatellina L. – Österr. Bot. Zeitschr. 80: 250-264.
Matveeva AV, Abubakirov NK. 1964. Saponins of Patrinia intermedia. – Khimicheskii Žurnal 8: 43-46. [in Russian]
Mayer V. 1995. The epicalyx in fruits of Scabiosa and Tremastelma (Dipsacaceae): anatomy and ecological significance. – Bot. Jahrb. Syst. 117: 211-238.
Mayer V, Ehrendorfer F. 1999. Fruit differentiation, palynology, and systematics in the Scabiosa group of genera and Pseudoscabiosa (Dipsacaceae). – Plant Syst. Evol. 216: 135-166.
Mayer V, Ehrendorfer F. 2000. Fruit differentiation, palynology, and systematics in Pterocephalus Adanson and Pterocephalodes, gen. nov. (Dipsacaceae). – Bot. J. Linn. Soc. 132: 47-78.
Mayer V, Ehrendorfer F. 2013. The phylogenetic position of Pterocephalidium and the new African genus Pterothamnus within an improved classification of Dipsacaceae. – Taxon 62: 112-126.
Mayer V, Svoma E. 1998. Development and function of the elaiosome in Knautia (Dipsacaceae). – Bot. Acta 111: 402-410.
Metcalfe CR. 1952. Notes on the anatomy of Heptacodium. – Kew Bull. 7: 247-248.
Meyer FG. 1951. Valeriana in North America and the West Indies (Valerianaceae). – Ann. Missouri Bot. Gard. 38: 377-503.
Moissle HE. 1941. Vergleichende embryologische Studien über die Familie der Caprifoliaceae. – Österr. Bot. Zeitschr. 90: 153-212.
Moore BR, Donoghue MJ. 2007. Correlates of diversification in the plant clade Dipsacales: geographic movement and evolutionary innovations. – Amer. Natur. 170 (Suppl.) 2: S28-S55.
Morvai-Vitany M, Games DE. 1993. Analysis of supercritical fluid extracts of valerian roots by combined chromatography/mass spectrometry. – In: Palevitch D, Putievsky E (eds), Acta Horticulturae, Tiberias, Israel.
Morton CV. 1933. The Mexican and Central American species of Viburnum. – Contr. U.S. Natl. Herb. 26: 339-366.
Movsumov IS, Garaev EA, Isaev MI. 2006. Flavonoids from Cephalaria gigantea flowers. – Chem. Nat. Comp. 42: 677-680.
Murai F, Tagawa M, Matsuda S, Kikuchi T, Uesato S, Inouye H. 1985. Abeliosides A and B, secoiridoid glucosides from Abelia grandiflora. – Phytochemistry 24: 2329-2335.
Napper DM. 1968a. Notes on some tropical and South African Dipsacaceae. – Kew Bull. 21: 463-470.
Napper DM. 1968b. Dipsacaceae. – In: Milne-Redhead E, Polhill RM (eds), Flora of tropical East Africa, Crown Agents for Oversea Governments and Administrations, London, pp. 1-10.
Nepomnyashchaya OA. 1984. On a new species of the genus Adoxa (Adoxaceae) from the Far East. – Bot. Žurn. 69: 259-262.
Nepomnyashchaya OA. 1984b. Structure of the flower and evolutionary trends in species of the genus Adoxa (Adoxaceae). – Bot. Žurn. 69: 1030-1039.
Neubauer HF. 1977. Morphologische Beobachtungen an Semlingen von Sambucus nigra. – Phyton 18: 57-69.
Neubauer HF. 1978 [1979]. On nodal anatomy and petiole vascularization of some Valerianaceae and Dipsacaceae. – Phytomorphology 28: 431-436.
Nielsen SD. 1949. Systematic studies in the Valerianaceae. – Amer. Midl. Natur. 42: 480-501.
Nilova MV. 2001. Comparative bark anatomy of representatives of the family Caprifoliaceae s.l. – Bot. Žurn. 86: 37-48, pp. 1-3. [In Russian]
Norn V. 1978. En phytokemisk undersøgelse af Viburnum. – Licentiatafhandling, Danmarks Tekniske Højskole, København, Denmark.
Novak J, Novak S, Bitsch C, Franz CM. 2000. Essential oil composition of underground parts of Valeriana celtica ssp. from Austria and Italy. – Flavour Fragrance J. 15: 40-42.
Ogata K. 1988. Wood anatomy of the Caprifoliaceae of Japan. – IAWA Bull., N. S., 9: 299-316.
Ogata K. 1991. Wood anatomy of Zabelia (Caprifoliaceae): evidence for generic recognition. – IAWA Bull., N. S., 12: 111-121.
Pasi S, Aligiannis N, Skaltsounis AL, Chinou IB. 2002. A new lignin glycoside and other constituents from Cephalaria ambrosioides. – Nat. Prod. Lett. 16: 365-370.
Patel VC, Skvarla JJ. 1979. Valerianaceae pollen morphology. – Pollen Spores 21: 81-103.
Peng C-I, Tobe H, Takahashi M. 1995. Reproductive morphology and relationships of Triplostegia (Dipsacales). – Bot. Jahrb. Syst. 116: 505-516.
Perdetzoglou DK, Skaltsa H, Tzakou O, Harvala C. 1994. Comparative phytochemical and morphological study of two species of the Scabiosa L. genus. – Feddes Repert. 105: 157-165.
Periasamy K. 1966. Studies on seeds with ruminate endosperm VI. Rumination in the Araliaceae, Aristolochiaceae, Caprifoliaceae and Ebenaceae. – Proc. Indian Acad. Sci., Sect. B, 60: 127-134.
Persidsky D. 1939. Gynoecium evolution in the family Caprifoliaceae. – J. Inst. Bot. Acad. Sci. Ukraine 21-22: 45-75. [In Ukrainian with Russian and English summaries]
Philipson WR. 1947a. Studies in the development of the inflorescence II. The capitula of Succisa pratensis Moench. and Dipsacus fullonum L. – Ann. Bot., N. S., 11: 285-297.
Philipson WR. 1947b. Studies in the development of the inflorescence III. The thyrse of Valeriana officinalis L. – Ann. Bot., N. S., 11: 409-416.
Plouvier V. 1992-1993. Chemotaxonomy of the Caprifoliaceae and relationship with some allied families. – Bull. Mus. Natl. Hist. Nat. (Paris), Sect. B, Adansonia, Bot. Phytoch. 14: 461-472.
Poddubnaja-Arnoldi P. 1933. Spermazellen in der Familie Dipsacaceae. – Planta 21: 381-386.
Popov SS, Handijieva NV, Marekov N. 1974. A new valepotriate: 7-epideacetylisovaltrate from Valeriana officinalis. – Phytochemistry 13: 2815-2818.
Prima VM. 1975. De specie Pseudobetckea caucasica (Boiss.) Lincz. (Valerianaceae) in Caucasi orientalis. – Nov. Syst. Plant. Vasc. 12: 250-252.
Punt W, Reitsma T, Reuvers AAML. 1974. The northwest European pollen flora. Caprifoliaceae. – Rev. Palaeobot. Palynol. 17: 5-29.
Pyck N. 2001. Phylogenetic relations within Dipsacales: a combined molecular and morphological approach. – Ph.D. diss., Katholieke Universiteit Leuven, Louvain, Belgium.
Pyck N, Smets E. 2000. A search for the phylogenetic position of the seven-son flower (Heptacodium, Dipsacales): combining molecular and morphological evidence. – Plant Syst. Evol. 225: 185-199.
Pyck N, Smets E. 2001. Dipsacales phylogeny: combining chloroplast sequences with morphological evidence. – Lab. Plant Syst., Leuven, Belgium.
Pyck N, Smets E. 2004. On the systematic position of Triplostegia (Dipsacales): a combined molecular and morphological approach. – Belg. J. Bot. 137: 125-139.
Pyck N, Roels P, Smets E. 1999. Tribal relationships of Caprifoliaceae: evidence from a cladistic analysis using ndhF sequences. – Syst. Geogr. Plants 69: 145-159.
Pyck N, Lysebetten A van, Stessens J, Smets E. 2002. The phylogeny of Patrinieae sensu Graebner (Valerianaceae) revisited: additional evidence from ndhF sequence data. – Plant Syst. Evol. 233: 29-46.
Rader LL. 1976. A biosystematic study of Viburnum prunifolium and Viburnum rufidulum (Caprifoliaceae). – M.Sc. diss., University of Tennessee, Knoxville, Tennessee.
Rauh W, Weberling F. 1959. Morphologische und anatomische Untersuchungen an der Valerianaceengattung Stangea Graebner. – Akad. Wissensch. Literatur, Mainz, Abhandl. Math.-Naturwiss. Kl. 10: 799-839.
Raymundez MB, Mathez J, Xena de Enrech N, Duduisson J-Y. 2002. Coding of insertion-deletion events of the chloroplast intergene atpB-rbcL for the phylogeny of the Valerianeae tribe (Valerianaceae). – Compt. Rend. Biol. 8: 131-139.
Razi BA, Subramanyam K. 1952. Embryology of the Dipsacaceae. – Proc. Indian Acad. Sci., Sect. B, 3: 249-257.
Reese-Krug H, Weberling F. 1991. Zur Taxonomie hochandiner Valeriana-Arten (Valerianaceae) I. Valeriana nivalis Wedd. und Valeriana pycnantha A. Gray. – Bot. Jahrb. Syst. 112: 399-410.
Rehder A. 1908. The viburnums of eastern Asia. – In: Sargent CS (ed), Trees and shrubs 2, Houghton Mifflin, Boston, Massachusetts.
Rehder A. 1911. Caprifoliaceae, Abelia. – In: Sargent CS (ed), Plantae Wilsonianae 1, Cambridge University Press, Cambridge, pp. 118-129.
Rehder A. 1916. Caprifoliaceae, Heptacodium. – In: Sargent CS (ed), Plantae Wilsonianae 2, Cambridge University Press, Cambridge, pp. 617-619.
Reidt G, Leins P. 1994. Das Initialstadium der sympetalen Krone bei Sambucus racemosa L. und Viburnum farreri Stearn. – Bot. Jahrb. Syst. 116: 1-9.
Reitsma TJ, Reuvers AAMC. 1975. The Northwest European pollen flora 19. Adoxaceae. – Rev. Palaeobot. Palynol. 4: 71-74.
Rešetnik I, Frajman B, Bogdanović S,
Ehrendorfer F, Schönswetter P. 2014. Disentangling relationships among the
diploid members of the intricate genus Knautia (Caprifoliaceae, Dipsacoideae). –
Molec. Phylogen. Evol. 74: 97-110.
Reveal JL. 2008. Proposals to conserve the name Viburnaceae (Magnoliophyta), the name Adoxaceae against Viburnaceae, a “superconservation” proposal, and, as an alternative, the name Sambucaceae. – Taxon 57: 303.
Richardson IBK. 1975. A revision of the genus Centranthus DC. (Valerianaceae). – Bot. J. Linn. Soc. 71: 211-234.
Riße K. 1926. Chromosomenzahlen und Periplasmodiumbildung in der Familie der Dipsacaceen. – Ber. Deutsch. Bot. Ges. 46: 296-297.
Roels P. 1993. Lengtepolymorfisme van chloroplast-DNA-restrictiefragmenten en bloemontogeinie in de Dipsacales. – Thesis, Katholieke Universiteit Leuwen, Belgium.
Roels P. 1994. A comparative floral ontogenetic study between Adoxa moschatellina and Sambucus ebulus. – Belg. J. Bot. 127: 157-170.
Roels P. 1998. Phylogenetic position and delimitation of the order Dipsacales: a multidisciplinary approach. – Ph.D. diss., Katholieke Universiteit, Leuwen, Belgium.
Roels P, Smets E. 1995. A comparative floral ontogenetical study between Adoxa moschatellina and Sambucus ebulus. – Belg. J. Bot. 127: 157-170.
Roels P, Smets E. 1996. A floral ontogenetic study in Dipsacales. – Intern. J. Plant Sci. 157: 203-218.
Rossow RA. 1993. Novedades en el género Valeriana (Valerianaceae). – Parodiana 8: 1-8.
Rücker G. 1969. Neue Inhaltsstoffe des “chinesischen Spikenardeöles – Nardostachys jatamansi”. – Planta Medica 17: 32-34.
Samutina ML. 1986. Comparative-morphological analysis of the pollen of the genus Sambucus (Caprifoliaceae). – Bot. Žurn. 71: 168-174.
Sarıkahya NB, Pekmez M, Arda N, Kayce P, Yavaşoğlu NUK, Kırmızıgül S. 2011. Isolation and characterization of biologically active glycosides from endemic Cephalaria species in Anatolia. – Phytochem. Letters 4: 415-420.
Sastry SD, Maheswari ML, Chakravarti KK, Bhattacharyya SC. 1967. Chemical constituents of Nardostachys jatamansii. – Perfumery Essential Oil Record 58: 154-156.
Sati S, Mathela CS. 2004. Essential oil composition of Valeriana hardwickii var. arnottiana from the Himalayas. – Flavour Fragrance J. 20: 299-301.
Sax K, Kribs DA. 1930. Chromosomes and phylogeny in Caprifoliaceae. – J. Arnold Arbor. 11: 147-153.
Schild W, Seitz W. 1971. Studien an Valerianaceen II. Valepotriate in südamerikanischen Arten. – Ber. Deutsch. Bot. Ges. 84: 225-241.
Schmale F. 1937. Valerianaceae americanae novae. – Feddes Repert. 41: 292-295.
Schmale F. 1938. Die Gattung Belonanthus Graebn. – Notizbl. Bot. Gart. Berlin-Dahlem 13: 23-26.
Schulte KE, Reisch J, Busch P. 1964. Ein Acetylenkohlenwasserstoff als Inhaltsstoff der Baldrianwurzel. – Arzneimittel-Forschung 14: 844-845.
Schwerin FG von. 1909. Monographie der Gattung Sambucus. – Mitt. Deutsch. Dendrol. Ges. 18: 1-56.
Schwerin FG von. 1920. Revisio generic Sambucus. – Mitt. Deutsch. Dendrol. Ges. 29: 57-94.
Seitz W. 1969. Zytotaxonomische Studien an Valerianaceen I. – Ber. Deutsch. Bot. Ges. 82: 651-655.
Smith SA. 2009. Taking into account phylogenetic and divergence-time uncertainty in a parametric biogeographical analysis of a Northern Hemisphere plant clade Caprifolieae. – J. Biogeogr. 36: 2324-2337.
Souèges ECR. 1957. Embryogénie des Dipsacacées. Développement de l’embryon chez le Scabiosa columbaria L. – Compt. Rend. Acad. Sci. Paris 245: 465-469.
Souèges ECR. 1963a. Embryogénie des Dipsacacées. Développement de l’embryon chez le Cephalaria tatarica Schrad. – Compt. Rend. Acad. Sci. Paris 256: 45-48.
Souèges ECR. 1963b. Embryogénie des Dipsacacées. Développement de l’embryon chez le Knautia arvensis Coult. – Compt. Rend. Acad. Sci. Paris 256: 1190-1194.
Souèges ECR. 1963c. Embryogénie des Dipsacacées. Développement de l’embryon chez le Dipsacus sylvestris Mill. – Compt. Rend. Acad. Sci. Paris 256: 2268-2273.
Sprague TA. 1927. The morphology and taxonomy of the Adoxaceae. – Bot. J. Linn. Soc. 47: 471-487.
Stabbetorp OE. 1989. Gynoecial anatomy in Sambucus callicarpa (Caprifoliaceae) with emphasis on meiotic divisions in a special tissue. – Nord. J. Bot. 9: 73-79.
Stahl E von, Schild W. 1969. Ein charakteristisches chromogenes Valepotriat ohne Dienstruktur in Valerianaceen. – Tetrahedron Letters 13: 1053-1056.
Stahl E von, Schild W. 1971. Über die Verbreitung der aequilibrierend wirkende Valepotriate in der Familie der Valerianaceen. – Phytochemistry 10: 147-153.
Steenis CGGJ van. 1951. Dipsacaceae [Triplostegia]. – In: Steenis CGGJ van (ed), Flora Malesiana I, 4(3), Noordhoff-Kolff N. V., Batavia, pp. 290-292.
Stoll A, Seebeck E, Staffacher D. 1957. New investigations on valerian. – Schweiz. Apotheker-Zeitung 95: 115-120.
Stroh H-H, Schafter H, Haschke E. 1962. D-Glukosester von Hydroxysimtsäuren in Sambucus nigra L. – Zeitschr. Chemie 2: 373-374.
Sturm K. 1910. Monographische Studien über Adoxa moschatellina L. – Vierteljahrsschr. Naturforsch. Ges. Zürich 55: 391-462.
Sunesson S. 1933. Zur Embryologie der Gattung Viburnum. – Bot. Not. 1933: 181-194.
Szabó Z von. 1905. Monographie der Gattung Knautia. – Engl. Bot. Jahrb. Syst. 36: 389-442.
Szabó Z von 1911. A Knautia gènusz monográfiája. – Magyar Tudományos Akadémia, Budapest.
Szabó Z von. 1923. The development of the flower of the Dipsacaceae. – Ann. Bot. 37: 325-334.
Szabó Z von. 1930. Entwicklungsgeschichtliche Deutung des Blütenstandes der Dipsacaceen. – Szent István Akadémia Ertesítöje 1: 55-72.
Szabó Z von. 1940a. Eine neue Gattung der Familie der Dipsacaceen. – Matemat. Termeszettud. Ertes. 49: 397-410.
Szabó Z von. 1940b. A Cephalaria génusz monográphiája. – Mat. Természettud. Közlem 38: 1-352.
Szentpétery RG, Kovács A, Sárkány S. 1969. Volatile oil excretion by the differentiating epidermis on the developing leaf of Valeriana collina Wallr. – Acta Agron. Hung. 18: 287-296.
Taguchi H, Yokokawa Y, Endo T. 1973. Studies on the constituents of Patrinia villosa. – Yakagaku Zasshi [J. Pharmacol. Soc. Japan] 93: 607-611.
Takamatsu S. 1977. Powdery mildew fungi parasitic on Nelumbo nucifera and Viburnum plicatum var. glabrum. – Trans. Mycol. Soc. Jap. 18: 197-201.
Tanaka K, Komatsu K. 2008. Comparative study on volatile components of Nardostachys rhizome. – J. Nat. Med. 62: 112-116.
Tang Y-C, Li L-Q. 1994. The phytogeography of Caprifoliaceae s. str. with its implications for understanding eastern Asiatic flora. – Acta Phytotaxon. Sin. 32: 197-218.
Theis N, Donoghue MJ, Li J. 2008. Phylogenetics of the Caprifolieae and Lonicera (Dipsacales) based on nuclear and chloroplast DNA sequences. – Syst. Bot. 33: 776-783.
Theisen I, Barthlott W. 1994. Mikromorphologie der Epicuticularwachse und die Systematik der Gentianales, Rubiales, Dipsacales und Calycerales. – Trop. Subtrop. Pflanzenwelt 89: 7-62.
Theisen I, Barthlott W. 1996. Die Mikromorphologie epicuticularer Wachse bei Caprifoliaceae. – Feddes Repert. 107: 31-35.
Thies PW von. 1966. Über die Wirkstoffe des Baldrians 2. Zur Konstitution der Isovaleriansäureester Valepotriat, Acetoxylvalepotriat und Dihydrovalepotriat. – Tetrahedron Letters 11: 1163-1170.
Thies PW von. 1968. Die Konstitution der Valepotriate. Mitteilung über die Wirkstoffe des Baldrians. – Tetrahedron Letters 24: 313-347.
Thies PW von, Funke S. 1966. Über die Wirkstoffe des Baldrians 1. Mitteilung Nachweis und Isolierung von sedativ wirksamen Isovaleriansäureestern aus Wurzeln und Rhizomen von verschiedenen Valeriana- und Kentranthus-arten. – Tetrahedron Letters 11: 1155-1162.
Thomas PJ, Daniel M, Sabnis SD. 1988. Chemosystematics of some members of Caprifoliaceae. – Acta Bot. Indica 16: 213-215.
Tieghem P van. 1909. Remarques sur les Dipsacacées. – Ann. Sci. Nat., Bot. Biol. Vég., sér. IX, 10: 148-200.
Tokô E. 1980. Embryology of Sambucus racemosa L. – Acta Biol. Crakov. (Bot.) 2(2): 173-188.
Tomassini L, Foddai S, Nicoletti M. 2004. Iridoids from Dipsacus ferox (Dipsacaceae). – Biochem. Syst. Ecol. 32: 1083-1085.
Torssell K, Wahlberg K. 1967. Isolation, structure and synthesis of alkaloids from Valeriana officinalis L. – Acta Chem. Scand. 21: 53-62.
Troll W, Weberling F. 1966. Die Infloreszenzen der Caprifoliaceae und ihre systematische Bedeutung. – Abh. Akad. Wiss. Lit. Mainz. Math.-Naturw. Kl. 4: 455-605.
Ulubelen A, Öksüz S, Aynehchi Y, Siami A. 1978. Flavonoids of Cephalaria procera. – Lloydia 41: 435-436.
Vatke W. 1872. Über die Gattung Abelia R. Br. – Österreich. Bot. Zeitschr. 22: 290-291.
Verdcourt B. 1968. Caprifoliaceae. – In: Milne-Redhead E, Polhill RM (eds), Flora of tropical East Africa, Crown Agents for Oversea Governments and Administrations, London, pp. 1-4.
Verlaque R. 1975. Contribution à l’étude caryologique des Dipsacaceae de la Méditerranée orientale. – Biol. Gallo-Hellen. 6: 75-100.
Verlaque R. 1977a. Rapports entre les Valerianaceae, les Morinaceae et les Dipsacaceae. – Bull. Soc. Bot. France 124: 475-482.
Verlaque R. 1977b. Importance du fruit dans la détermination des Dipsacaceae. – Bull. Soc. Bot. France 124: 515-527.
Verlaque R. 1983. Contribution à l’étude du genre Morina L. – Pollen et Spores 25: 143-162.
Verlaque R. 1984a. Étude biosystématique et phylogénétique des Dipsacaceae I. Délimitation des Dipsacaceae à l’intérieur des Dipsacales, rapports avec les autres familles de l’ordre. – Rev. Gén. Bot. 91: 81-121.
Verlaque R. 1984b. A biosystematic and phylogenetic study of the Dipsacaceae. – In: Grant R (ed), Plant biosystematics, Academic Press, Toronto, pp. 307-320.
Verlaque R. 1985a. Étude biosystématique et phylogénétique des Dipsacaceae II. Caractères généraux des Dipsacaceae. – Rev. Cytol. Biol. Végét. Bot. 8: 117-168.
Verlaque R. 1985b. Étude biosystématique et phylogénétique des Dipsacaceae III. Tribu des Knautieae et Dipsaceae. – Rev. Cytol. Biol. Végét. Bot. 8: 171-243.
Verlaque R. 1986a. Étude biosystématique et phylogénétique des Dipsacaceae IV. Tribu des Scabioseae (phylum 1, 2, 3). – Rev. Cytol. Biol. Végét. Bot. 9: 5-72.
Verlaque R. 1986b. Étude biosystématique et phylogénétique des Dipsacaceae V. Tribu des Scabioseae (phylum 4) et conclusion. – Rev. Cytol. Biol. Végét. Bot. 9: 97-176.
Vidal L. 1903. Contribution à l’anatomie des Valérianacées. – Ann. Univ. Grenoble 15: 561-605.
Vieth J. 1958. Quelques observations nouvelles sur la fleur et l’inflorescence de Morina longifolia. – Bull. Sci. Bourgogne (Toulouse) 19: 71-89.
Vieth J. 1965. Étude morphologique et anatomique de morphoses induites par voie chimique sur quelques Dipsacacées. – L’Université de Dijon, France.
Vijayaraghavan MR, Sarveshwari GS. 1968. Embryology and systematic position of Morina longifolia Wall. – Bot. Not. 121: 383-402.
Villareal JA. 1997. A new species of Abelia (Caprifoliaceae) from western Mexico. – Brittonia 49: 84-86.
Villareal JA, La Rosa I M de. 2000. Two new species of Abelia (Caprifoliaceae) from Mexico. – Brittonia 52: 172-176.
Vinokurova LV. 1959. Palynological data on the systematic position of the Dipsacaceae and the Morinaceae. – Probl. Bot. 4: 51-67. [In Russian]
Wagenitz G, Laing D. 1984. Die Nektarien der Dipsacales und ihre systematische Bedeutung. – Bot. Jahrb. Syst. 104: 483-507.
Weberling D, Weberling F. 1981. Zur Morphologie und Anatomie der Gattung Belonanthus Graebn. (Valerianaceae). – Trop. Subtrop. Pflanzenwelt, Abhandl. Akad. Wiss. Litt. Mainz 36: 1-41.
Weberling F. 1957. Morphologische Untersuchungen zur Systematik der Caprifoliaceen. – Abhandl. Akad. Wiss. Litt. Mainz, Math.-Naturwiss. Kl., 1957(1): 1-50.
Weberling F. 1960. Zur systematische Stellung der Valerianaceengattung Astrephia Dufr. – Bot. Jahrb. Syst. 79: 394-404.
Weberling F. 1961. Die Infloreszenzen der Valerianaceen und ihre systematische Bedeutung. – Akad. Wiss. Abhandl., Math.-Naturwiss. Kl. 5: 151-281.
Weberling F. 1966. Zur systematischen Stellung der Gattung Heptacodium Rehd. – Bot. Jahrb. Syst. 85: 253-258.
Weberling F. 1975. On the systematics of Nardostachys (Valerianaceae). – Taxon 24: 443-452.
Weberling F. 1977. Vergleichende und entwicklungsgeschichtliche Untersuchungen über die Haarformen der Dipsacales. – Beitr. Biol. Pflanzen 53: 61-89.
Weberling F. 1978a. Über das Vorkommen von “Sternhaaren” bei Scabiosa. Ein Nachtrag. – Beitr. Biol. Pflanzen 54: 29-32.
Weberling F. 1978b. Monographie der Gattung Nardostachys (Valerianaceae). – Bot. Jahrb. Syst. 99: 188-221.
Weberling F. 1982. Stangea, Belonanthus und Phyllactis – ein Vergleich. – Ber. Deutsch. Bot. Ges. 95: 165-179.
Weberling F. 2003. Notes on South American Valerianaceae I. – Feddes Repert. 114: 437-453.
Weberling F. 2004. Notes on South American Valerianaceae II. – Feddes Repert. 115: 453-482.
Weberling F. 2005. Notes on South American Valerianaceae III. – Feddes Repert. 116: 294-312.
Weberling F. 2007 [2008]. Notes on South American Valerianaceae IV. – Feddes Repert. 118: 7-8, 327-351.
Weberling F, Engel K. 1975. Zur Morphologie und Entwicklungsweise von Nardostachys jatamansi (D. Don) DC. (Valerianaceae). – Flora 164: 377-391.
Weberling F, Hildenbrand M. 1982. Tapetumentwicklung bei Triosteum L., Leycesteria Wall., und Kolkwitzia Graebn. (Caprifoliaceae). – Beitr. Biol. Pflanzen 57: 481-486.
Weberling F, Hildenbrand M. 1986. Weitere Untersuchungen der Tapetumentwicklung der Caprifoliaceae. – Beitr. Biol. Pflanzen 61: 3-20.
Weberling F, Uhlarz H. 1977. Morphologische, anatomische und palynologische Untersuchungen an der Gattung Aretiastrum (Valerianaceae). – Plant Syst. Evol. 127: 217-242.
Weberling F, Ronse De Craene L. 2002. Reappraisal of our current knowledge of the genus Stangea Graebn. (Valerianaceae). – Syst. Geogr. Plants 72: 243.
Weberling F, Salzer E. 2009. Tufted stellate hairs in Valeriana tomentosa Kunth. – Feddes Repert. 120: 139-144.
Wilkinson AM. 1948a. Floral anatomy and morphology of some species of the tribe Lonicereae of the Caprifoliaceae. – Amer. J. Bot. 35: 261-271.
Wilkinson AM. 1948b. Floral anatomy and morphology of some species of the tribes Linnaeeae and Sambuceae of the Caprifoliaceae. – Amer. J. Bot. 35: 365-371.
Wilkinson AM. 1948c. Floral anatomy and morphology of the genus Viburnum of the Caprifoliaceae. – Amer. J. Bot. 35: 455-465.
Wilkinson AM. 1949. Floral anatomy and morphology of Triosteum and the Caprifoliaceae sensu stricto. – Amer. J. Bot. 36: 481-489.
Willer K-H. 1962. Beitrag zur Morphologie und Anatomie der Vegetationsorgane der Valerianaceae. – Heidelberg.
Winkworth RC, Donoghue MJ. 2004. Viburnum phylogeny: evidence from the duplicated nuclear gene GBSSI. – Mol. Phylogen. Evol. 33: 109-126.
Winkworth RC, Donoghue MJ. 2005. Viburnum phylogeny based on combined molecular data: implications for taxonomy and biogeography. – Amer. J. Bot. 92: 653-666.
Winkworth RC, Lundberg J, Donoghue MJ. 2008. Towards a resolution of campanulid phylogeny, with special reference to the placement of Dipsacales. – Taxon 57: 53-65.
Winkworth RC, Bell CD, Donoghue MJ. 2008. Mitochondrial sequence data and Dipsacales phylogeny: mixed models, partitioned Bayesian analysis, and model selection. – Mol. Phylogen. Evol. 46: 830-843.
Wittrock VB. 1907. Linnaea borealis L. species polymorpha et polychrome. Linnaea borealis L. en mångformig art. – Acta Horti Berg. 4(7): 5-178.
Wu C-Y. 1981. Another new genus of Adoxaceae, with special reference on the intrafamiliar evolution and the systematic position of the family. – Acta Bot. Yunnan. 3: 383-388. [In Chinese with English summary]
Wu C-Y, Wu ZL, Huang RF. 1981. Sinadoxa C.-Y. Wu, Z. L. Wu, et R. F. Huang, genus novum familiae Adoxacearum. – Acta Phytotaxon. Sin. 19: 203-210.
Xena de Enrech N, Mathez J. 1998. Genetic control of fruit polymorphism in the genus Fedia (Valerianaceae) in the light of dimorphic and trimorphic populations of F. pallescens. – Plant Syst. Evol. 210: 199-210.
Xena de Enrech N, Cardona MÀ, Mathez J. 1991. Estudio citotaxonómico del género Fedia Gaertn. (Valerianaceae). – An. Jard. Bot. Madrid 48: 157-169.
Xu L, Lu L, Li D-Z, Wang H. 2011. Evolution of pollen in Dipsacales. – Plant Divers. Res. 33: 249-259. [In Chinese]
Yokoyama J, Fukuda T, Yokoyama A, Maki M. 2002. The intersectional hybrid between Weigela hortensis and W. maximowiczii (Caprifoliaceae). – Bot. J. Linn. Soc. 138: 369-380.
Zabel H. 1893. Über die Gattng Abelia. – Mitt. Deutsch. Dendrol. Ges. 2: 29-31.
Zhang W-H, Chen Z-D, Chen H-B, Tang Y-C. 2001. Phylogenetic relationships of the disputed genus Triplostegia based on trnL-F sequences. – Acta Phytotaxon. Sin. 39: 337-344.
Zhang W-H, Chen Z-D, Li JH, Chen H-B, Tang Y-C. 2003. Phylogeny of the Dipsacales s.l. based on chloroplast trnL-F and ndhF sequences. – Mol. Phylogen. Evol. 26: 176-189.
Zhang Z-Y, Zhou Z-K, Gu Z-J. 2002. Karyomorphology of Heptacodium (Caprifoliaceae s.str.) and its phylogenetic implications. – Taxon 51: 499-505.
Zobel AM. 1986. Ontogenesis of tannin-containing coenocytes in Sambucus racemosa L. III. The mature coenocyte. – Ann. Bot. 58: 849-858.