Phylogeny of Araliales based on DNA sequence data (Tank & Donoghue 2010).
[Araliales+Dipsidae]
Habit Usually bisexual (sometimes monoecious, andromonoecious, polygamomonoecious, gynomonoecious, dioecious or gynodioecious), evergreen or deciduous trees, shrubs or lianas, suffrutices, perennial, biennial or annual herbs. Some representatives are xerophytes, helophytes or aquatic. Internodes often hollow. Leaf scars often large and distinct.
Vegetative anatomy Phellogen ab initio superficially or deeply seated. Peripheral collenchyma usually well developed. Medulla large and wide (as old shrinking and disappearing). Medullary and/or cortical vascular bundles frequent, often inverted. Secondary lateral growth normal or anomalous (via cylindrical cambium). Vessel elements often solitary; often at least some vessels in groups. Vessel elements with simple or scalariform (sometimes reticulate etc.) perforation plates; lateral pits alternate, scalariform or opposite, usually bordered pits. Vestured pits often present. Imperforate tracheary xylem elements usually libriform fibres (sometimes fibre tracheids or tracheids) with usually bordered pits (often reduced bordered pits), septate or non-septate (sometimes also vasicentric tracheids). Wood rays uniseriate or multiseriate, usually heterocellular. Axial parenchyma usually paratracheal scanty vasicentric or banded (sometimes apotracheal diffuse or diffuse-in-aggregates), or absent. Tyloses sometimes frequent. Palisade mesophyll often with arm cells. Sieve tube plastids Ss type. Nodes usually ≥5:≥5, multilacunar with five or more leaf traces (sometimes 1:3 or 3:3, unilacunar or trilacunar with three traces). Parenchyma and other tissues usually with schizogenous secretory canals and cavities containing ethereal oils, resins, gums, or mucilage. Prismatic calciumoxalate crystals or crystal sand sometimes frequent.
Trichomes Hairs unicellular or multicellular, uniseriate or multiseriate, simple or branched, often vesicular, sometimes dendritic, stellate, T-shaped, peltate or lepidote; glandular hairs multicellular uniseriate, or absent.
Leaves Usually alternate (spiral or rarely distichous; rarely opposite or verticillate), simple or compound (often several times pinnately compound), usually pinnately lobed (sometimes palmately lobed or entire), with conduplicate, curved or supervolute ptyxis (rarely linear, terete, articulated leaves with hydathodes). Stipules absent or cauline (rarely intrapetiolar); lower part of petiole usually sheathingly expanding and enclosing stem. Petiole vascular bundles with very various and complex anatomy. Venation pinnate or palmate (rarely parallelodromous). Stomata usually anomocytic or paracytic (sometimes anisocytic, parallelocytic, cyclocytic or diacytic). Cuticular wax crystalloids as rodlets (Pittosporaceae). Domatia rarely present. Secretory canals and cavities with ethereal oils, resins, gums, or mucilage. Epidermis sometimes with mucilage cells. Mesophyll sometimes with sclerenchymatous idioblasts. Mesophyll cells sometimes with calciumoxalate druses. Leaf margin and leaflet margins usually serrate (rarely entire, crenate or sinuate).
Inflorescence Terminal or axillary, panicle, thyrsoid, corymb, or simple or compound umbels (of dichasial origin), or capitate (sometimes with whorls of flowers below terminal umbel), rarely racemose (flowers sometimes solitary). Floral prophylls (bracteoles, involucel bracts) subtending flowers of partial umbels, or absent. Bracts (involucral bracts) subtending partial inflorescences (partial umbels), or absent. Inflorescences sometimes pseudanthia with petaloid involucral bracts (and sometimes sterile or female peripheral flowers).
Flowers Usually actinomorphic (marginal flowers sometimes zygomorphic), often small. Pedicel sometimes articulated. Usually epigyny (sometimes hypogyny or half epigyny). Sepals five, usually minute (sometimes absent), usually with open (sometimes imbricate, rarely valvate) aestivation, persistent or caducous, free or more or less connate; median sepal adaxial. Petals (three to) five (rarely ten or twelve), usually with valvate (sometimes imbricate) aestivation, often clawed, often caducous, usually (secondarily) free (sometimes connate at base, rarely absent), with apex often inflexed. Nectariferous disc intrastaminal, annular to angular, usually present on stylopodium (nectary and disc rarely absent).
Androecium Stamens (three to) five to ten (to twelve, rarely numerous), usually haplostemonous, antesepalous, alternipetalous (rarely diplostemonous), often unequal in length. Filaments often inflexed in bud, free from each other, from petals and from pistil, inserted on or outside annular nectariferous disc. Anthers basifixed or dorsifixed, versatile or non-versatile, usually tetrasporangiate (rarely octosporangiate), usually introrse (rarely latrorse), usually longicidal (dehiscing by longitudinal slits; rarely poricidal, dehiscing by apical pores). Tapetum secretory. Staminodia absent.
Pollen grains Microsporogenesis simultaneous. Pollen grains (2–)3(–6)-colporate or (2–)3(–6)-colpate, shed as monads, usually tricellular (rarely bicellular) at dispersal. Exine tectate or semitectate, with columellate infratectum, reticulate, perforate, scabrate or striate (sometimes imperforate), with various supratectal processes or smooth.
Gynoecium Pistil composed of two (to five; rarely numerous) connate carpels (sometimes three carpels, with abaxial carpel fertile); vascular bundles of ventral carpels usually consisting of connate bundles from common placenta; when fused ventral bundles opposite carpels, then two bundles arising from common carpel; when fused ventral bundles present in septal radia, then two bundles arising from adjacent carpel. Ovary usually inferior (sometimes superior or semi-inferior), usually bilocular to quinquelocular (rarely unilocular, multilocular or pseudomonomerous). Stylodia two (to five; style sometimes single), short, usually free (rarely connate at base), usually inserted on epigynous stylopodium (on top of ovary). Stigmas capitate to punctate (rarely somewhat lobate), papillate or non-papillate, usually Wet (sometimes Dry) type. Pistillodium usually absent.
Ovules Placentation usually axile to apical (occasionally parietal). Ovules usually one (sometimes two, rarely numerous) per carpel, anatropous, pendulous (rarely horizontal or ascending), usually epitropous (rarely apotropous), unitegmic, tenuinucellar or crassinucellar (sometimes pseudocrassinucellar). Hypostase present or absent. Funicular obturator rarely present. Nucellar cap present or absent. Megagametophyte usually monosporous, Polygonum type (rarely Oenothera type, or disporous, Allium type, or tetrasporous, Penaea, or Drusa types). Antipodal cells often proliferating. Endosperm development ab initio nuclear. Endosperm haustoria absent. Embryogenesis solanad, onagrad, asterad or chenopodiad.
Fruit Usually a drupe (often dry) or dry schizocarp with persistent carpophore (formed by ventral vascular bundles supporting carpels and surrounding tissues) and two nutlike mericarps (sometimes a berry, a loculicidal capsule or a syncarp). Vallecular and intrajugal vittae, often with elongate secretory oil canals and oil cavities.
Seeds Aril usually absent. Exotestal cell walls thick or thin, often tangentially elongate, tanniniferous. Endotesta unspecialized. Perisperm not developed. Endosperm copious, oily (sometimes ruminate), with petroselinic acid. Embryo usually small, straight, well differentiated, without chlorophyll. Cotyledons (one or) two. Germination phanerocotylar.
Cytology x = (4–)8–12 (or more); x = 6? for Araliales (Yi & al. 2004)
DNA Mitochondrial gene rpl2 absent (lost). Deletion of 92 bp in plastid gene rpl16.
Phytochemistry Flavonols (kaempferol, quercetin, myricetin?), flavones, sulfated or O-methylated flavonoids, Route I carbocyclic iridoids (griselinoside, aralioside, verbascosides) and Route II decarboxylated iridoids (rare), simple coumarins (e.g. umbelliferone), hydroxycoumarins, pyranocoumarins, dihydropyranocoumarins, furanocoumarins, dihydrofuranocoumarins etc., oleanolic acid derivatives, dammaranes, sesquiterpene lactones, triterpenoid ethereal oils and resins, caffeic acid, chlorogenic acid, ellagic acid, tannins (rare), proanthocyanidins (prodelphinidins), alkaloids (incl. benzylisoquinoline and hemlock alkaloids), triterpene saponins, acetate-derived arthroquinones, phenantrenes, phenylpropenes (e.g. myristicin), acetophenones, germacrane-like compounds, asarone, syringaresinol, pinoresinol, polyacetylenes (e.g. falcarinone) derived from fatty acids, mostly aliphatic C17 acetylenes (substitute for iridoids?), petroselinic acid (in endosperm), and mannitol. Cyanogenic compounds not found. Carbohydrates stored as trisaccharide umbelliferose.
Systematics Araliales are sister-group to Dipsidae, i.e the clade [Paracryphiaceae+Dipsacales].
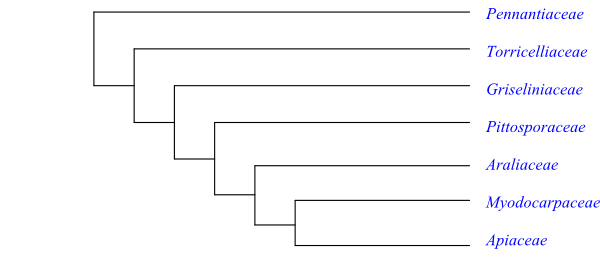
A well supported topology of Araliales is the following: [Pennantiaceae+[Torricelliaceae+ [Griseliniaceae+[Pittosporaceae+[Araliaceae+[Myodocarpaceae+Apiaceae]]]]]].
Pennantia has hypogynous flowers. The pistil is composed of three connate carpels, although it is seemingly consisting of a single carpel and often only the abaxial carpel is fertile. The anatomy, pollen morphology, embryology and phytochemistry are very poorly known in Pennantia, which makes the character polarization difficult in Araliales.
Potential synapomorphies for the Araliales with the exception of Pennantia include (Stevens 2001 onwards): young stems with peripheral collenchyma; pericyclic fibres few or absent; vessel elements mainly occurring in groups and with simple perforations; pits usually simple or only narrowly bordered; paratracheal axial parenchyma scanty; nodes ≥5:≥5; leaf base broad; epigyny; petals secondarily free, with imbricate aestivation; nectary inserted on top of ovary; styles/stigmas recurved; and polyacetylenes present.
Griselinia and Torricelliaceae share many features. Both groups have vessel elements arranged in clusters; septate fibres with simple pits; wood rays sometimes more than ten cell layers wide and with square or upright cells; ovary with transseptal vascular bundles; only the abaxial carpel fertile; ovule apotropous; and presence of griselinoside. Since these characters are virtually unknown in Pennantia, it cannot be established whether they are apomorphies or plesiomorphies in Araliales.
The clade [Griseliniaceae+[Pittosporaceae+[Araliaceae+[Myodocarpaceae+Apiaceae]]]] has the potential synapomorphies: glandular hairs rare; petals single-veined; nectaries present; ovule with endothelium; and petroselinic acid present in seeds.
The clade [Pittosporaceae+[Araliaceae+[Myodocarpaceae+Apiaceae]]] has a large number of characters in common (Stevens 2001 onwards): lateral roots originating from either side of xylem poles (one resinous canal running down stem in pole apex); parenchyma in secondary phloem surrounding secretory canals and arranged in groups with sieve tubes; schizogenous resinous canals present in pericycle, phloem etc.; nodes sometimes 3:3; shoot with reduced bract-like leaves at base; leaves with conduplicate ptyxis; leaf margins serrate or otherwise incised; leaf teeth often with broad glandular apex with one main and two accessory veins, or one vein proceeding above tooth; ultimate inflorescence units umbels; corolla tube formation early; pollen grains tricellular at dispersal; carpels two, connate, both fertile, with nectariferous stylopodium delimited from style by basal furrow; stigma Wet type; ovules two per carpel, epitropous; seeds often with hemicellulose as carbohydrate reserve; embryo tiny; x = 12; presence of furanocoumarins, triterpenoid ethereal oils, falcarinone, polyacetylenes (mainly aliphatic, including C17 acetylenes, etc.), and acetate-derived anthraquinones; and absence of iridoids, flavonols and tannins.
The [Araliaceae+[Myodocarpaceae+Apiaceae]] clade is supported by, i.a., the following characters: inflorescence terminal; epigyny; calyx minute, with open aestivation; presence of stylopodium; nectary (on stylopodium) divided; one of two ovules much reduced; fruit a schizocarp; fruit laterally compressed; wings or “pseudo-wings”, when present, with mesocarp and endocarp, with vascular bundles at wing margin; deletion comprising 92 bp in plastid gene rpl16; petroselinic acid and polyacetylene C18 tariric fatty acid present in seeds; and trisaccharide umbelliferose as carbohydrate reserve.
Finally, Myodocarpaceae and Apiaceae both have inflorescence panicle or raceme; and furanocoumarins.
The floral “disc” in Apiaceae and Araliaceae, and probably in Myodocarpaceae, is in reality a carpellary margin nectary displaced due to intercalary growth. The axile placentation in these three clades may be equivalent to the parietal placentation in Pittosporaceae, in which there is a short basal zone of separate locules, the ovules being inserted above this zone. The ovules are principally inserted on corresponding sites in Apiaceae and Araliaceae.

|
Phylogeny of Araliales based on DNA sequence data (Tank & Donoghue 2010). |
APIACEAE Lindl. |
( Back to Araliales ) |
Umbelliferae Juss., Gen. Plant.: 218. 4 Aug 1789, nom. cons. et nom. alt.; Ammiaceae Bercht. et J. Presl, Přir. Rostlin: 259. Jan-Apr 1820; Angelicaceae Martinov, Tekhno-Bot. Slovar: 29. 3 Aug 1820 [’Angeliceae’]; Bupleuraceae Bercht. et J. Presl, Přir. Rostlin: 258. Jan-Apr 1820 [’Bupleureae’]; Caucalidaceae Bercht. et J. Presl, Přir. Rostlin: 259. Jan-Apr 1820 [‘Caucalideae’]; Daucaceae Augier ex Martinov, Tekhno-Bot. Slovar: 183. 3 Aug 1820 [’Daucoideae’]; Eryngiaceae Bercht. et J. Presl, Přir. Rostlin: 259. Jan-Apr 1820 [‘Eryngieae’]; Imperatoriaceae Martinov, Tekhno-Bot. Slovar: 328. 3 Aug 1820 [‘Imperatoriae’]; Lagoeciaceae Bercht. et J. Presl, Přir. Rostlin: 259. Jan-Apr 1820 [‘Lagoeciae’]; Pastinacaceae Martinov, Tekhno-Bot. Slovar: 457. 3 Aug 1820 [‘Pastinaceae’]; Pimpinellaceae Bercht. et J. Presl, Přir. Rostlin: 258. Jan-Apr 1820 [‘Pimpinelleae’]; Saniculaceae Bercht. et J. Presl, Přir. Rostlin: 259. Jan-Apr 1820 [’Saniculeae’]; Scandicaceae Bercht. et J. Presl, Přir. Rostlin: 259. Jan-Apr 1820 [’Scandicinae’]; Selinaceae Bercht. et J. Presl, Přir. Rostlin: 259. Jan-Apr 1820 [’Selineae’]; Sileraceae Bercht. et J. Presl, Přir. Rostlin: 259. Jan-Apr 1820; Ammiineae Link, Handbuch 1: 327. 4-11 Jul 1829 [‘Ammieae’]; Bupleurineae Link, Handbuch 1: 317. 4-11 Jul 1829 [‘Bupleurinae’]; Pimpinellineae Link, Handbuch 1: 506. 4-11 Jul 1829 [‘Pimpinelleae’]; Angelicales Burnett, Outl. Bot.: 1127. Jun 1835 [‘Angelicinae’]; Coriandraceae Burnett, Outl. Bot.: 773, 783, 1093, 1128. Feb 1835; Smyrniaceae Burnett, Outl. Bot.: 773, 1093, 1128. Feb 1835; Angelicineae Burnett, Outlines Bot.: 616, 762, 1093, 1127. Feb 1835 [‘Angelicosae’]; Caucalidineae J. Presl in Nowočeská Bibl. [Wšobecný Rostl.] 7: 702, 753. 1846 [‘Caucalideae’]; Coriandrineae J. Presl in Nowočeská Bibl. [Wšobecný Rostl.] 7: 702, 766. 1846 [‘Coriandreae’]; Daucineae J. Presl in Nowočeská Bibl. [Wšobecný Rostl.] 7: 702, 752. 1846; Saniculineae J. Presl in Nowočeská Bibl. [Wšobecný Rostl.] 7: 701, 707. 1846 [‘Saniculeae’]; Scandicineae J. Presl in Nowočeská Bibl. [Wšobecný Rostl.] 7: 702, 755. 1846 [‘Scandicinae’]; Silerineae J. Presl in Nowočeská Bibl. [Wšobecný Rostl.] 7: 701, 748. 1846; Smyrniineae J. Presl in Nowočeská Bibl. [Wšobecný Rostl.] 7: 702, 759. 1846 [‘Smyrneae’]; Ferulaceae Sacc. in Nuovo Giorn. Bot. Ital. 4: 214. 31 Jul 1872; Ammiales Small, Fl. S.E. U.S.: 851. 22 Jul 1903; Mackinlayaceae Doweld, Tent. Syst. Plant. Vasc.: lii. 23 Dec 2001; Apiineae G. M. Plunkett et Lowry in G. M. Plunkett et al., S. African J. Bot. 70: 379. 7 Oct 2004; Actinotaceae Konstant. et Melikjan in Bot. Žurn. 90: 1763. 15 Nov 2005
Genera/species 437–478/3.475–3.645
Distribution Cosmopolitan except polar areas.
Fossils Fossil fruits (Carpites ulmiformis) of Apiaceae are reported from the Late Cretaceous (Maastrichtian) of North America. Fruit fossils have also been found in Eocene layers in western North America.
Habit Usually bisexual (sometimes andromonoecious or polygamomonoecious, rarely dioecious), usually perennial, biennial or annual herbs (sometimes suffrutices or shrubs, rarely trees). Some representatives are xerophytic, aquatic or helophytic. Internodes usually hollow.
Vegetative anatomy Phellogen ab initio superficially or cortical. Peripheral collenchyma usually well developed. Medulla large and wide (shrinking and disappearing as old). Medullary and cortical vascular bundles abundant. Secondary lateral growth normal or anomalous (from cylindrical cambium). Vessel elements usually with simple (sometimes scalariform; in Mackinlayoideae also reticulate or foraminate) perforation plates; lateral pits alternate, scalariform or opposite, bordered pits. Vestured pits? Imperforate tracheary xylem elements libriform fibres with simple or (reduced) bordered pits, septate or non-septate. Wood rays uniseriate or multiseriate, heterocellular. Axial parenchyma usually paratracheal scanty vasicentric or banded (rarely apotracheal diffuse or diffuse-in-aggregates). Sieve tube plastids S type. Nodes usually ≥5:≥5, multilacunar with five or more leaf traces (sometimes 3:3, trilacunar with three traces). Parenchyma and other tissues usually with schizogenous secretory canals and cavities containing ethereal oils, resins, gums, or mucilage. Druses sometimes frequent. Prismatic calciumoxalate crystals sometimes frequent (rhomboidal or druses in, e.g., Mackinlayoideae).
Trichomes Hairs unicellular or multicellular, often vesicular, uniseriate or branched, sometimes dendritic, digitiform, equisetiform, or stellate; glandular hairs frequent.
Leaves Usually alternate (spiral; rarely opposite or verticillate), simple or compound (often several times pinnately compound), usually pinnately lobed (sometimes palmately lobed or entire; rarely linear-terete and articulated with hydathodes at articulations), with ? or supervolute ptyxis. Stipules usually absent (present in some genera in Azorelloideae); lower part of petiole usually sheathingly expanding and enclosing stem (leaf sheath often long). Petiole vascular bundles with very various and complex anatomy. Venation usually pinnate (sometimes palmate, rarely parallelodromous). Stomata usually anomocytic or paracytic (sometimes anisocytic, parallelocytic, or diacytic). Cuticular wax crystalloids as platelets, irregular structures, etc. Secretory canals and cavities with ethereal oils, resins, gums, or mucilage. Leaf margin and leaflet margins, respectively, usually serrate (rarely entire); each tooth with broad glandular apex with one main vein and two lateral veins, or with one vein running above tooth.
Inflorescence Usually terminal (sometimes axillary), simple or usually compound umbels or capitate (occasionally with whorls of flowers below terminal umbel; flowers sometimes solitary). Floral prophylls (bracteoles) subtending flowers of partial umbels, or absent. Bracts subtending partial inflorescences (partial umbels), or absent. Inflorescences sometimes pseudanthia with petaloid involucral bracts (and possibly sterile peripheral flowers).
Flowers Usually actinomorphic (marginal flowers often zygomorphic), small. Epigyny. Sepals usually minute or entirely reduced (in Saniculoideae five, larger), with valvate or open aestivation, persistent, free or more or less connate; median sepal adaxial. Petals usually five (rarely four), with valvate aestivation, usually clawed, free (rarely absent), with apex usually inflexed. Nectariferous disc intrastaminal, annular, usually on stylopodium.
Androecium Stamens five, haplostemonous, antesepalous, alternipetalous, often unequal in length. Filaments inflexed in bud, free from each other and from petals, inserted on nectariferous disc. Anthers basifixed, non-versatile, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory. Staminodia absent.
Pollen grains Microsporogenesis simultaneous. Pollen grains usually tricolporate (rarely dicolporate), shed as monads, tricellular at dispersal. Exine tectate or semitectate, with columellate infratectum, perforate, reticulate, or striate, with various supratectal processes or smooth.
Gynoecium Pistil composed of two connate carpels; ventral carpel vascular bundles consisting of connate bundles from common placenta. Ovary inferior, usually bilocular (rarely unilocular, pseudomonomerous). Stylodia two, short, free, usually inserted on stylopodium on top of ovary. Stigmas capitate to punctate, non-papillate, Wet type. Pistillodium?
Ovules Placentation axile to apical. Ovules one or two (one fertile and one sterile) per carpel, anatropous, pendulous, epitropous, unitegmic, usually tenuinucellar (sometimes pseudocrassinucellar). Integument approx. seven cell layers thick. Nucellar cap present or absent. Hypostase present or absent. Megagametophyte usually monosporous, Polygonum type (rarely disporous or tetrasporous, Oenothera, Allium, Penaea, or Drusa type). Antipodal cells often proliferating. Endosperm development ab initio nuclear. Endosperm haustoria absent. Embryogenesis solanad.
Fruit Usually a dry schizocarp with persistent carpophore (formed from ventral vascular bundles supporting carpels and surrounding tissues; rarely absent) and two nutlike mericarps, often with four or five ridges or wings, and furrows (fruit rarely fleshy). Mesocarp usually lignified. Vallecular and intrajugal vittae with elongate secretory oil canals and oil cavities usually present. Crystals solitary, rhomboidal, in small-celled inner layer. Endocarp usually lignified, two cell layers thick, sclereidal-fibrous. Testa sometimes adnate to endocarp.
Seeds Aril absent. Exotestal cells thin-walled. Endotesta unspecialized. Perisperm not developed. Endosperm copious, oily, with petroselinic acid. Embryo usually small, straight, well differentiated, without chlorophyll. Cotyledons usually two (rarely one). Germination phanerocotylar.
Cytology x = (4–)8–12 (13 or more)
DNA Mitochondrial gene rpl2 absent. Deletion of 92 bp present in plastid gene rpl16. Plastid inverted repeat in Coriandrum comprising less than 12 kb.
Phytochemistry Flavonols (kaempferol, quercetin, myricetin?), sulphated or O-methylated flavonoids (in Apioideae), simple coumarins (e.g. umbelliferone), pyranocoumarins, dihydropyranocoumarins, furanocoumarins, dihydrofuranocoumarins etc., sesquiterpene lactones, triterpenoid ethereal oils and resins, caffeic acid, hemlock alkaloids, benzylisoquinoline alkaloids, triterpene saponins, polyols, acetate-derived arthroquinones, phenylpropenes (e.g. myristicin), polyacetylenes (e.g. falcarinone) derived from fatty acids, mostly aliphatic C17 acetylenes, petroselinic acid (in endosperm), lignans, and mannitol present. Flavones, hydroxycoumarins, ellagic acid, tannins, proanthocyanidins and cyanogenic compounds not found. Carbohydrates stored as umbelliferose (trisaccharide, raffinose isomer).
Use Ornamental plants, spices, flavours, perfumes, vegetables, medicinal plants, poisons, gum resins (Ferula spp.).
Systematics Apiaceae are sister-group to Myodocarpaceae.
Mackinlayoideae G. M. Plunkett et Lowry in South Afr. J. Bot 70(3): 379. 2004
10/105–110. Actinotus (c 20; Australia, New Zealand), Apiopetalum (2; A. glabratum, A. velutinum; New Caledonia), Mackinlaya (5; M. celebica, M. confusa, M. macrosciadea, M. radiata, M. schlechteri; Central Malesia to islands in western Pacific); Chlaenosciadium (1; C. gardneri; Western Australia), Brachyscias (1; B. verecundus; Western Australia), Xanthosia (c 25; Australia, with their highest diversity in southwestern Western Australia); Pentapeltis (1; P. peltigera; southwestern Western Australia), Schoenolaena (1; S. juncea; Western Australia), Micropleura (2; M. flabellifolia, M. renifolia; Mexico, Colombia, Chile), Centella (c 50; southern Africa to Zimbabwe and Malawi, one species, C. asiatica, pantropical). – Africa, East Malesia, Australia, western South America, with their highest diversity in Western Australia, Melanesia and South Africa. Usually trees or shrubs (rarely herbs). Phellogen in Centella subepidermal. Vessel elements often with scalariform perforation plates. Axial parenchyma usually apotracheal (sometimes also paratracheal). Leaves in Mackinlaya irregularly palmately compound. Inflorescence paniculate or raceme-like, often consisting of compound umbels. Pedicel usually articulated. Sepals in Pentapeltis and Schoenolaena petaloid with ribs containing indistinct oil ducts; sepals absent in Centella and Micropleura. Petals with valvate aestivation, distinctly clawed. Nectary usually divided (in Actinotus present on style). Pistil composed of two (to four) carpels. Fruit usually laterally compressed (not so in Apiopetalum). Wings (“pseudo-wings”), if present, formed by compression or folding of carpel and composed of both mesocarp and endocarp; vascular bundle present at margin. Endocarp lignified. Vittae absent. Carpophore absent; single or paired ventral bundles often present and fused with mericarps. Ribs with distinct oil ducts in, e.g., Actinotus, Chlaenosciadium, Centella, Micropleura, and Xanthosia (in Centella distinct or indistinct). Fruit in Actinotus monocarpellate with lignified endocarp, but without vittae. Centellose (oligosaccharide) present in Centella. x = 10. – Ventral bundles fused with the mericarps represent a feature homologous to the carpophore (Liu & al. 2016).
[Platysace+[[Klotzschia+[Diposis+Azorelloideae]]+[Hermas+[Saniculoideae+[[Lichtensteinia+[Choritaenia+Marlothiella]]+Apioideae]]]]]
Vessel elements usually with simple perforation plates. Umbels usually compound. Fruit usually dorsally compressed. Carpophore present. Schizocarp with mericarps separating at maturity.
Platysace clade
1/c 25. Platysace (c 25; Australia, with their highest diversity in southwestern Western Australia). – Perennial herbs and suffrutices. Ribs sometimes with oil ducts. Sepals absent. Cotyledons rounded, serrate. n = 8.
[[Klotzschia+[Diposis+Azorelloideae]]+[Hermas+[Saniculoideae+[[Lichtensteinia+[Choritaenia+Marlothiella]]+Apioideae]]]]
Mesocarp vittae irregular, anastomosing and/or branching.
[Klotzschia+[Diposis+Azorelloideae]]
Klotzschia clade
1/3. Klotzschia (3; K. brasiliensis, K. glaziovii, K. rhizophylla; Brazil). – Pollen grains resembling those in Araliaceae: small size, long colpus, short os, thin exine, similar sexine stratification, and weak differentiation into tectum and short columellae. Fruit without wings or “pseudo-wings”. Ribs without distinct oil ducts. Single fused ventral bundle replacing carpophore.
[Diposis+Azorelloideae]
Diposis clade
1/3. Diposis (3; D. bulbocastanum, D. patagonica, D. saniculifolia; southern Chile and Argentina). – Carpophore free. Ribs without distinct oil ducts.
Azorelloideae G. M. Plunkett et Lowry in South Afr. J. Bot. 70(3): 379. 2004
15/110–115. Homalocarpus (6; H. bowlesioides, H. dichotomus, H. digitatus, H. dissectus, H. integerrimus, H. nigripetalus; Chile), Bolax (2; B. caespitosa, B. gummifera; temperate regions in Chile and Argentina), Dichosciadium (1; D. ranunculaceum; Australia), Bowlesia (c 15; South America; incl. Drusa?), Drusa (1; D. glandulosa; the Canary Islands, Somalia; in Bowlesia?), Domeykoa (4; D. amplexicaulis, D. oppositifolia, D. perennis, D. saniculifolia; Peru, northern Chile), Eremocharis (9; Peru, Chile), Oschatzia (2; O. cuneifolia, O. saxifraga; southeastern Australia, Tasmania), Pozoa (2; P. coriacea, P. volcanica; the Andes in Chile and Argentina), Asteriscium (8; Chile, Argentina), Gymnophyton (6; G. flexuosum, G. foliosum, G. isatidicarpum, G. polycephalum, G. robustum, G. spinosissimum; the Andes in Chile and Argentina), Spananthe (1; S. paniculata; the Andes), Dickinsia (1; D. hydrocotyloides; southwestern China), Diplaspis (3; D. cordifolia, D. hydrocotylea, D. nivis; southeastern Australia, Tasmania), Azorella (58; southeastern Australia, Macquarie Island, New Zealand including Stewart Island and adjacent islands, the Antipode Islands, Campbell Island and Auckland Islands, from the northern Andes to southern South America, Falkland Islands, subantarctic islands). – Southwestern China, Australia, Tasmania, New Zealand, South America, subAntarctic islands, with their highest diversity in the southern Andes; Drusa glandulosa (Bowlesia glandulosa) in the Canary Islands and Somalia. Phellogen in Azorella Sect. Spinosae deeply seated. Leaves usually simple (rarely compound). Stipules present. Megasporocytes two to four. Megasporangium large and relatively persistent. Megagametophyte tetrasporous, 16-nucleate, Drusa type (and other developmental types). Fruit with wings (“pseudo-wings”) and/or ribs. Wings (“pseudo-wings”), if present, formed by compression or folding of carpel and composed of both mesocarp and endocarp; vascular bundle present at margin. Carpophore present in some species of Azorella. Carpophores free in Drusa, Homalocarpus, Asteriscium, Gymnophyton, Spananthe, Dickinsia, and Diplaspis (several other genera have fused ventral bundles). Outer mesocarp in Azorella with druses. Companion cells (oil canals associated with vascular bundles) present. Inner layer of endocarp fibres longitudinally arranged. n = 8–10. Petroselinic acid absent at least in Bowlesia (in Drusa glandulosa?). – The clade [Dickinsia+Diplaspis] is sister to the Azorella clade (Nicolas & Plunkett 2012).
[Hermas+[Saniculoideae+[[Lichtensteinia+[Choritaenia+Marlothiella]]+Apioideae]]]
Stipules absent. Ovules incompletely tenuinucellate. Nucellar cap present. Funicle long. Fruit with lateral secondary ribs. Mesocarp non-lignified. Endocarp consisting of single cell layer. Intrajugal vittae (oil ducts in ribs) present.
Hermas clade
1/9. Hermas (9; Western and Eastern Cape). – Perennial herbs or suffrutices. Umbels congested. Carpophore present. Single rhomboidal crystals present in mesocarp cells. Druses absent. Intrajugal vittae present. Vallecular vittae absent. Rib oil ducts present. Endocarp woody. n = 7.
[Saniculoideae+[[Lichtensteinia+[Choritaenia+Marlothiella]]+Apioideae]]
Stipules absent. Basal leaf with pinnate venation. Ovules more or less tenuinucellar. Nucellar cap present. Fruit wings, if present, formed by expansion of mesocarp, consisting only of mesocarp tissue; vascular bundle usually at base. Secondary (lateral) ribs sometimes present. Mesocarp cell walls lignified. Endocarp one cell layer thick, parenchymatous. Cell walls sometimes lignified. Calciumoxalate druses present in mesocarp and around commissure. Rhomboidal crystals absent.
Saniculoideae Burnett Outl. Bot.: 774. Jun 1835
10/360–365. Mainly temperate and subtropical regions; some species in tropical areas. Phellogen sometimes outer cortical. Peripheral stem collenchyma often very well developed. Leaves usually simple (rarely compound). Flowers usually in capitula or simple umbel (inflorescence probably consisting of reduced compound umbels). Nectary sometimes extrastaminal. Style separated from disc by narrow groove. Fruit sessile, usually spiny or scaly. Carpophore not free, or absent. Intrajugal, irregular, vittae (secretory canals/cavities, branched or anastomosing) usually present. Regular vittae absent. Druses usually frequent, present not only in commissural side. Mesocarp vittae present or absent. Ribs with large oil ducts or cavities. Endocarp often lignified. Cotyledons round. n = 8 (9, 11, 12). Kaurene type terpenoids, 3’-O-β-D-glucopranosyl rosmarinic acid (rosmarinic acid glucoside), and cardenolides (in Eryngium) present.
Saniculeae W. D. J. Koch in Nova Acta Phys.-Med. Acad. Caes. Leop.-Carol. Nat. Cur. 12(1): 66, 138. 1824
7/355–360. Alepidea (c 40; eastern tropical and southern Africa), Actinolema (2; A. eryngioides, A. macrolema; eastern Mediterranean), Arctopus (3; A. dregei, A. echinatus, A. monacanthus; Northern, Western and Eastern Cape), Astrantia (11; Central and South Europe, western Asia), Eryngium (255–260; temperate to tropical regions on both hemispheres; paraphyletic?), Petagnaea (1; P. gussonii; Sicily), Sanicula (c 45; almost cosmopolitan, absent from New Guinea and Australia). – Cosmopolitan. Usually perennial herbs (rararely shrubs or small trees). Leaf margin often spinose-dentate (with hairy or spiny tooth apices). Inflorescence often with large (involucral) bracts; pseudanthium often present. Flowers (at least female flowers) usually sessile. Fruit usually scaly or spiny (in Petagnaea glabrous). Free carpophore absent. Regular vittae rarely present (few species in Alepidea). Rib oil ducts sometimes absent. Druses usually scattered throughout mesocarp. Endocarp not lignified. n = 8 (9, 11, 12). – Eryngium may be divided in one New World and one Old World clade. Some species of Eryngium have fruit wings entirely consisting of exocarp.
[Phlyctidocarpa+Steganotaenieae]
Phlyctidocarpeae Magee, C. I. Calviño, M. Liu, S. R. Downie, P. M. Tilney et B.-E. van Wyk in Taxon 59: 578. 4 Apr 2010
1/1. Phlyctidocarpa (1; P. flava; Outjo and Kaokoveld in Namibia). – Herbs. Umbels pedunculate, compound. Fruit with bristles and surface vesicles. Ribs bifurcate in cross-section. Regular vittae present. Rib oil ducts large. Druses scattered throughout mesocarp. Ventral bundles weakly developed. n = ?
Steganotaenieae C. I. Calviño et S. R. Downie in Mol. Phylogen. Evol. 44(1): 187. Jan 2007
2/3. Steganotaenia (2; S. araliacea, S. hockii; Ethiopia to South Africa), Polemanniopsis (1; P. marlothii; Northern and Western Cape). – Ethiopia to South Africa. Shrubs. Phelloderm with chambered crystalliferous cells; phelloderm in Steganotaenia with axial secretory canals. Dilatation of secondary phloem by expansion of axial parenchyma. Leaves proteranthous (developing prior to floral shoots). Fruit heteromericarpous, with two or three exocarp and mesocarp wings. Rib oil ducts forming cavities. Secretory (oil) cavities (intrajugal cavities) associated with ribs strongly expanded. Carpophore present. Mesocarp usually with calciumoxalate druses. Endocarp slightly lignified. n = ?
[[Lichtensteinia+[Choritaenia+Marlothiella]]+Apioideae]
[Lichtensteinia+[Choritaenia+Marlothiella]]
Ribs with oil ducts or cavities.
Lichtensteinieae Magee, C. I. Calviño, M. Liu, S. R. Downie, P. M. Tilney et B.-E. van Wyk in Taxon 59: 578. 4 Apr 2010
1/8. Lichtensteinia (8; L. crassijuga, L. globosa, L. interrupta, L. lacera, L. latifolia, L. obscura, L. trifida: Western and Eastern Cape, KwaZulu-Natal; L. burchellii: St. Helena). – Annual or perennial herbs, sometimes woody at base. Leaves deciduous, often proteranthous (developing prior to floral shoots). Inflorescence compound umbel. Fruit usually heteromericarpous. Calciumoxalate crystals present in pericarp. Rib oil ducts very large. Irregular vittae present below veins in ribs. Intrajugal (vallecular) vittae absent. Druses scattered throughout mesocarp.
[Choritaenia+Marlothiella]
Choritaenieae Magee, C. I. Calviño, M. Liu, S. R. Downie, P. M. Tilney et B.-E. van Wyk in Taxon 59: 578. 4 Apr 2010
1/1. Choritaenia (1; C. capensis; southern Africa). – Annual herb. Inflorescence compound umbel. Fruit hairy, dorsally compressed, with “inter-rib” wings; marginal wings composed entirely of mesocarp. Mericarps not separating. Mesocarp lignified. Endocarp woody, with globular oil vesicles in wings (instead of normal vittae or rib oil ducts). Druses absent. Carpophore very short, hygroscopic.
Marlothielleae Magee, C. I. Calviño, M. Liu, S. R. Downie, P. M. Tilney et B.-E. van Wyk in Taxon 59: 578. 4 Apr 2010
1/1. Marlothiella (1; M. gummifera; coastal areas in Namibia). – Small shrub. Inflorescence compound umbel. Fruit heteromericarpous, with unicellular stellate hairs. Wings absent. Rib oil ducts very large. Regular vittae absent. Druses scattered throughout mesocarp. Ventral vascular bundles poorly developed or absent.
Apioideae Seem., Fl. Vit.: 112. Jan 1866 [‘Apiaceae’] (mainly according to Downie & al. 2010)
395–435/2.850–3.010. Cosmopolitan, although mainly in northerly temperate regions. Secretory canals/cavities distinct. Leaves usually compound. Stipules usually absent (Foeniculum and some other genera with stipules). Inflorescence usually compound umbel. Outer flowers of umbel often zygomorphic. Stylopodium without groove. Ovule tenuinucellar. Hypostase present. Carpophore free, bifid, with mericarps attached to apex. Regular vittae present, often distinctly vallecular and commissural, or cyclic. Intrajugal oil ducts small or absent. Endocarp one cell layer thick, unlignified. Druses sometimes only on commissural side, or absent. x = 11. Flavones, methylated flavonoids, furanocoumarins, and phenylpropenes present. – The phylogeny and subdivision below follows Downie & al. (2010).
Annesorhizeae Magee, C. I. Calviño, M. Liu, S. R. Downie, P. M. Tilney et B.-E. van Wyk in Taxon 59: 578. 4 Apr 2010
6/24. ’Annesorhiza’ (15; southern Africa; non-monophyletic), Astydamia (1; A. latifolia; the Canary Islands, Madeira), ’Chamarea’ (5; C. capensis, C. esterhuyseniae, C. gracillima, C. longipedicellata, C. snijmaniae; western, central and southern South Africa to Eastern Cape; non-monophyletic), Ezosciadium (1; E. capense; Eastern Cape), Itasina (1; I. filifolia; Western Cape, southwestern Eastern Cape), Molopospermum (1; M. peloponnesiacum; the Pyrenees, the Cevennes, the Alps). – Southern and Central Europe (Molopospermum), Macaronesia (Astydamia), Africa, with their largest diversity in the Cape Provinces. Usually perennial (in Ezosciadium annual) herbs (sometimes lignified). Leaves compound, proteranthous (developing prior to floral shoots) or deciduous. Vascular bundles of fruit strongly lignified. Fruit in Annesorhiza and Molopospermum heteromericarpous. Druses usually scattered throughout mesocarp (in Ezosciadium restricted to commissure). n = 11 (Annesorhiza), 12 (Itasina).
[Heteromorpheae+the remaining Apioideae]
Tanniniferous epidermal cells absent. Druses present only on commissural side of mericarp, or absent.
Heteromorpheae M. F. Watson et S. R. Downie in Amer. J. Bot. 87(2): 289. 2000
11/39. Heteromorpha clade Anginon (13; southern Namibia, Northern, Western and Eastern Cape), Heteromorpha (9; Africa south of Sahara, Socotra, Yemen), Polemannia (4; P. rossulariaefolia, P. marlothii, P. montana, P. simplicior; mountains in South Africa and Lesotho). – Malagasy clade Andriana (3; A. coursii, A. marojejyensis, A. taratananensis; Madagascar), Anisopoda (1; A. bupleuroides; Madagascar), Cannaboides (2; C. andohahelensis, C. betsileensis; Madagascar), Dracosciadium (2; D. italae, D. saniculifolium; KwaZulu-Natal), Oreofraga (1; O. morrisiana; Socotra), Pseudocannaboides (1; P. andingitrensis; Madagascar), Pseudocarum (2; P. eminii, P. laxiflorum; tropical East Africa, Madagascar), Tana (1; T. bojerianum; Madagascar). – Africa, Madagascar, Socotra, Yemen, with their largest diversity in southwestern Africa. Trees, shrubs, suffrutices or lianas (Dracosciadium herbs with persistent leaves). Walls of vessel elements with helical thickenings. Fibres septate. Sepals sometimes well developed. Fruit not or only slightly dorsiventrally or laterally compressed, in Heteromorpha heteromericarpous. Vittae in Pseudocarum branching and anastomosing. Druses scattered throughout mesocarp.
[Bupleureae+the crown Apioideae]
Druses absent.
Bupleureae Spreng. in J. J. Roemer et J. A. Schultes, Syst. Veg. 6: xxxiii. Aug-Dec 1820 [‘Bupleurinae’]
1/c 210. Bupleurum (c 210; Europe, the Canary Islands, the Mediterranean, northern Africa, temperate Asia, one species, B. mundii, in South Africa, one species, B. americanum, in arctic North America). – Usually annual or perennial herbs (rarely shrubs with secondary lateral growth). Walls of vessel elements with helical thickenings. Fibres septate. Leaves simple, often grass-like. Leaf margin entire. Venation often parallel. Pollen grains usually rhomboidal. Vittae branching and anastomosing. Cotyledons linear, with single vein, glabrous. n = 7, 8.
The “Crown Apioideae”
Pericarp without druses.
[Pleurospermopsis clade+the rest]
Pleurospermopsis clade
1/1. Pleurospermopsis (1; P. sikkimense; the Himalayas, western China). – Biennial or perennial herb. Leaves pinnately compound. Inflorescence compound umbel. Bracts (involucral bracts) and bracteoles (involucel bracts) prominent. Fruit laterally slightly compressed. Ribs prominent, narrowly winged. Carpophore bipartite.
[Chamaesium clade+the rest]
Chamaesium clade
1/6. Chamaesium (6; C. delavayi, C. novem-jugum, C. paradoxum, C. thalictrifolium, C. viridiflorum, C. wolffianum; Central Asia, northern India, the Himalayas, Tibet, Yunnan). – Perennial herbs. Leaf pinnately compound. Bracts and bracteoles few or absent. Fruit smooth. Ribs prominent to narrowly winged. Carpophore divided to base.
[Diplolophium clade+the rest]
Diplolophium clade
1/7. Diplolophium (7; D. africanum, D. boranense, D. buchananii, D. diplolophioides, D. marthozianum, D. somaliense, D. zambesianum; tropical to southern Africa). – Perennial herbs. Leaves pinnately compound. Bracts and bracteoles present. Carpophore divided to base.
[Pleurospermeae+the rest]
Pleurospermeae M. F. Watson et S. R. Downie in Amer. J. Bot. 87: 289. 15 Feb 2000
9–11/c 69. Aulacospermum (c 16; Europe, temperate Asia), Pleurospermum (2; P. austriacum, P. uralense; Europe, temperate Asia), Trachydium (1; T. roylei; the Himalayas), Pseudotrachydium (5; P. depressum, P. dichotomum, P. kotschyi, P. pauciradiatum, P. vesiculosoalatum; southwestern and Central Asia), Hymenidium (c 35; Pakistan to China), Eleutherospermum (1; E. cicutarium; the Caucasus, southwestern Asia), Physospermum (2; P. cornubiense, P. verticillatum; Europe, temperate Asia), Eremodaucus (1; E. lehmannii; the Caucasus to Central Asia and Afghanistan), Korshinskya (4; K. assyriaca, K. bupleuroides, K. kopetdaghensis, K. olgae; southwestern and Central Asia), ‘Physospermopsis’ muliensis (southwestern Sichuan, northwestern Yunnan), ‘Physospermopsis’ shaniana (Sichuan, Xizang, Yunnan, Burma). – Temperate and alpine regions in Eurasia.
[[Physospermopsis clade+Komarovieae]+rest]
Physospermopsisclade (East Asia clade)
10–15/>52. Hansenia (1; H. mongholica; northern and Central Asia), Haplosphaera (2; H. himalayensis, H. phaea; southern and eastern Asia), Heptaptera (4; H. angustifolia, H. colladonioides, H. macedonica, H. triquetra; eastern Mediterranean, southwestern Asia), Hymenolaena (3; H. badachschanica, H. candollei, H. pimpinellifolia; Central Asia, the Himalayas), Keraymonia (4; K. cortiformis, K. nipaulensis, K. pinnatifolia, K. triradiata; the Himalayas), ‘Notopterygium’ (6; N. forrestii, N. franchetii, N. incisum, N. oviforme, N. pinnatiivolucellatum, N. tenuifolium; China; non-monophyletic), ‘Physospermopsis’ (9; Central to East Asia; non-monophyletic), ‘Pimpinella’ pro parte (3),‘Sinocarum’ cruciatum (western China, northern Burma), ‘Sinocarum’ dolichopodum (western Sichuan, northwestern Yunnan), Sinolimprichtia (1; S. alpina; eastern Tibet), ‘Spuriopimpinella’ brachycarpa (southeastern Russia, China, northern Korean Peninsula), ‘Tongoloa’ (16; Central Asia to northern India; non-monophyletic), ‘Trachydium’ simplicifolium (northwestern Yunnan), ‘Vicatia’ bipinnata (northwestern Sichuan, Yunnan). – Temperate and alpine regions in Asia.
Komarovieae J. Zhou et S. R. Downie in Mol. Phylogenet. Evol. 53(1): ?. 6 Jun 2009
7/9. Parasilaus (1; P. asiaticus; southwestern and Central Asia), Komarovia (1; K. anisoptera; Central Asia), Calyptrosciadium (1; C. polycladum; Iran, Afghanistan), Changium (2; C. angustilobum, C. smyrnioides; Tibet, eastern China), Chuanminshen (1; C. violaceus; China), Cyclorhiza (2; C. peucedanifolia, C. waltonii; southwestern China), Sphaerosciadium (1; S. denaense; Central Asia). – Southwestern Asia to China.
[Erigenieae+the rest]
Erigenieae Rydb. ex Pimenov et Constance in Taxon 34: 497. 19 Aug 1985
1/1. Erigenia (1; E. bulbosa; eastern United States). – Small glabrous perennial herb. Corm present. Vesicular-arbuscular mycorrhiza absent. Leaves ternately compound. Involucral bracts absent. Bracteoles (involucel bracts) present. Fruit slightly compressed.
[Oenantheae+the rest]
Oenantheae Dumort., Fl. Belg.: 79. 1827
20/c 150. ’Sium’ (9; the Northern Hemisphere, Africa; polyphyletic), Berula (5; B. bracteata, B. burchellii, B. erecta, B. imbricata, B. repanda; temperate regions on the Northern Hemisphere, eastern and southern Africa, St. Helena), Cryptotaenia (5; C. calycina, C. canadensis, C. flahaultii, C. japonica, C. polygama; temperate regions on the Northern Hemisphere, East African mountains), Helosciadium (c 45; temperate regions in the Old World), Naufraga (1; N. balearica; Majorca; in Helosciadium?), Cicuta (5; C. bulbifera, C. curtissii, C. douglasii, C. maculata, C. virosa; temperate regions on the Northern Hemisphere), Oenanthe (c 28; temperate regions on the Northern Hemisphere, tropical African mountains, India to Malesia and Australia), Oxypolis (9; southern Canada, United States), Neogoezia (2; N. gracilipes, N. planipetala; Mexico), Atrema (1; A. americanum; southern United States), Trepocarpus (1; T. aethusae; southern United States), Daucosma (1; D. laciniatum; the United States), Lilaeopsis (9; Mauritius, Australia, New Zealand, North and South America), Cynosciadium (2; C. digitatum, C. pinnatum; southern United States), Limnosciadium (2; L. innatum, L. pumilum; southern central United States), Harperella (1; H. nodosa; estern United States), Tiedemannia (1; T. rigida; eastern United States, Cuba), Ptilimnium (7; P. capillaceum, P. costatum, P. fluviatile, P. nodosum, P. nuttallii, P. texense, P. viviparum; eastern United States), Perideridia (15; southwestern Canada, western United States, northern Mexico), Trocdaris (1; T. verticillata; southwestern Europe, Morocco). – Cosmopolitan.
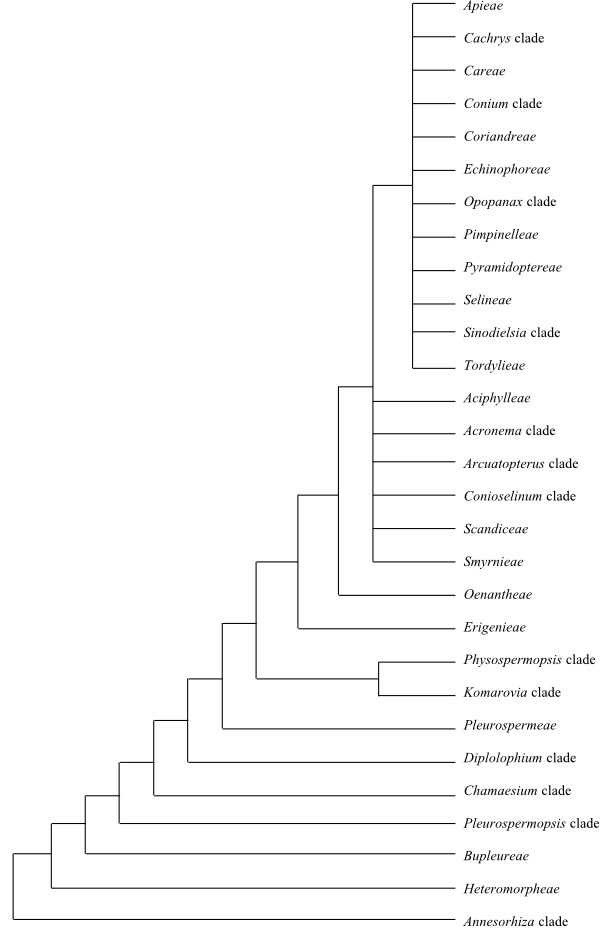
[apioid superclade+Aciphylleae+Acronema clade+Arcuatopterus clade+Conioselinum chinense clade+Scandiceae+Smyrnieae]
Aciphylleae M. F. Watson et S. R. Downie in Amer. J. Bot. 87: 288. 15 Feb 2000
1–5/c 75. ‘Aciphylla’ (c 45; eastern Australia, Tasmania, New Zealand; non-monophyletic; incl. Anisotome?),‘Anisotome’ (16; New Zealand, subAntarctic islands; non-monophyletic; in Aciphylla?), Lignocarpa (2; L. carnosula, L. diversifolia; New Zealand; in Aciphylla?), Scandia (2; S. geniculata, S. rosaefolia; New Zealand; in Aciphylla?), Gingidia (10; Australia, Tasmania, New Zealand; in Aciphylla?). – Mainly New Zealand, some species of ‘Aciphylla’ in eastern Australia.
Acronema clade
16–21/160–170. Acronema (c 40; the Himalayas, western China), ’Angelica’ anomala (Siberia, China, the Korean Peninsula), Halosciastrum (1; H. melanotilingia; East Asia), Haloselinum (1; H. falcaria; southern Siberia, Mongolia), Harrysmithia (2; H. franchetii, H. heterophylla; China), Kitagawia (5; K. baicalensis, K. eryngiifolia, K. litoralis, K. praeruptora, K. terebinthacea; Central Asia), ‘Ligusticum’ (50–60; temperate regions on the Northern Hemisphere; non-monophyletic), Meeboldia (5; M. achilleifolia, M. digitata, M. scariosa, M. selinoides, M. yunnanensis; the Himalayas), Oreocomopsis (2; O. aromatica, O. xizangensis; the Himalayas, southern China), Ostericum grosseserratum (Mongolia, China, the Korean Peninsula), Ostericum scaberulum (Yunnan), Pachypleurum (5; P. dolichostylum, P. lhasanum, P. muliense, P. nyalamense, P. xizangense; temperate regions in Europe and Asia), ‘Pleurospermum’ hookeri (eastern Himalayas, China), ‘Pleurospermum’ yunnanense (western Sichuan, northwestern Yunnan, northeastern Burma), Pternopetalum (c 25; eastern Himalayas, China, southern Japan), Pterygopleurum (1; P. neurophyllum; the Korean Peninsula, southern Japan), Rupiphila (1; R. tachiroei; China), Sinocarum (7; China), Spuriopimpinella (4; S. arguta, S. calycina, S. koreana, S. nikoensis; China), Tilingia (2; T. ajanensis, T. limprichtii; China, northeastern Asia, Alaska), Xyloselinum (3; X. laoticum: northern Laos; X. leonidii, X. vietnamense: northern Vietnam). – Temperate and alpine regions in Eurasia, especially Central Asia to China.
Arcuatopterus clade
2/6. Arcuatopterus (5; A. harae, A. linearifolius, A. ramosissimus, A. sikkimensis, A. thalictrioideus; eastern Himalayas, southwestern China), Sillaphyton (1; S. podagraria; the Korean Peninsula). – Glabrous perennial herbs. Leaves two- or three-pinnatisect. Bracts usually absent (sometimes one, caducous). Bracteoles absent. Fruit dorsally strongly compressed. Dorsal ribs indistinct. Lateral ribs broadly winged; wings thin to corky. Carpophore divided.
Rivasmartinezia clade
5–9/10. Rivasmartinezia (2; R. cazorlana, R. vazquezii; Spain), ‘Ligusticum’ canadense (North America); ‘Conioselinum’ chinense (Russia, China, Japan, North America), ‘Conioselinum’ scopulorum (western United States), ‘Ligusticum’ porteri (western United States, northern Mexico); Mutellina (1; M. caucasica; temperate regions in Europe and Asia), Trochiscanthes (1; T. nodiflora; southern Europe), Meum (1; M. athamanticum; Europe, northern Africa), Dethawia (1; D. splendens; the Pyrenees, Cordillera Cantabrica). – Temperate regions on the Northern Hemisphere.
Scandiceae Spreng. in J. J. Roemer et J. A. Schultes, Syst. Veg. 6: xliii. Aug-Dec 1820 [‘Candicinae’]
c 38/422. Scandicinae Tausch in Flora 17: 342. 14 Jun 1834. Conopodium (8; Europe, the Mediterranean, temperate regions in Asia), Athamanta (11; Europe, the Canary Islands, the Mediterranean), Todaroa (1; T. aurea; the Canary Islands), Sphallerocarpus (1; S. gracilis; southern Asia), ’Chaerophyllum’ (c 45; temperate regions on the Northern Hemisphere; paraphyletic), Scandix (4; S. australis, S. iberica, S. pecten-veneris, S. stellata; Europe, the Mediterranean), Osmorhiza (c 15; East Asia, America), Myrrhis (1; M. odorata; Europe), Anthriscus (c 15; Europe, mountains in Africa, temperate Asia), Geocaryum (8; eastern Mediterranean), Kozlovia (4; K. capnoides, K. laseroides, K. longiloba, K. paleacea; Central Asia to India), Chaerophyllopsis (1; C. huai; western China). – Ferulinae Drude in H. G. A. Engler et K. A. E. Prantl, Nat. Pflanzenfam. III, 8: 115. Dec 1897. Ferula (c 210; the Canary Islands, the Mediterranean to Central Asia), Leutea (1; L. petiolaris; southwestern and central Asia), Autumnalia (2; A. botschantzevii, A. innopinata; southwestern and Central Asia)?, Fergania (1; F. polyantha; Central Asia)?, Kafirnigania (1; K. hissarica; Central Asia)? – Torilidinae Dumort., Fl. Belg.: 81. 1827. Astrodaucus (3; A. littoralis, A. orientalis, A. persicus; Europe, temperate regions in Asia), Glochidotheca (1; G. foeniculacea; Europe, western Asia), Szovitsia (1; S. callicarpa; the Caucasus, Armenia, Iran), Torilis (8; Europe, the Canary Islands, the Mediterranean to East Asia, tropical and southern Africa), Yabea (1; Y. microcarpa; western United States, northwestern Mexico), Caucalis (1; C. platycarpos; southern Europe), Turgenia (1; T. latifolia; Central Europe, the Mediterranean to Central Asia), Lisaea (2; L. heterocarpa, L. papyracea; eastern Mediterranean, southwestern Asia). – Daucinae Dumort., Fl. Belg.: 81. 1827. Cuminum (4; C. borszczowii, C. cyminum, C. setifolium, C. sudanense; the Mediterranean to Sudan and Central Asia), Ammodaucus (1; A. leucotrichus; the Canary Islands, northwestern Africa), Ekimia (1; E. bornmuelleri; southern Turkey), Laserpitium (8; L. emilianum, L. gallicum, L. halleri, L. krapfii, L. latifolium, L. longiradium, L. nitidum, L. peucedanoides; Europe, the Canary Islands, the Mediterranean, southwestern Asia), Thapsia (c 24; the Iberian Peninsula, the Mediterranean, the Canary Islands, northern to tropical Africa), Laser (7; central, southern and eastern Europe, the Mediterranean, the Canary Islands, the Caucasus, western and southwestern Asia), Siler (3; S. montanum, S. ochridanum, S. zernyi; mountains in central and southern Europe), ‘Laserpitium’ pseudomeum (mountains in southern Greece), Orlaya (4; O. daucorlaya, O. grandiflora, O. kochii, O. topaliana; southeastern Europe to Central Asia), Silphiodaucus (2; S. hispidus, S. prutenicus; central, southern and eastern Europe), Daucus (28; Europe, the Mediterranean, Macaronesia, North and tropical Africa, southwestern and Central Asia, Australia, New Zealand, America). – Daucinae have fruits with prominent secondary ridges projecting into wings or spines. – Artedia cladeArtedia (1; A. squamata; eastern Mediterranean). – Glaucosciadium clade Glaucosciadium (1; G. cordifolium; southern Turkey, Cyprus). – Scandicinae is distributed mainly in warm-temperate regions on the Northern Hemisphere; the Ferula clade in southwestern to Central Asia, with few species in the Mediterranean and Macaronesia; Torilidinae in temperate and warmer regions in the Old World, with one species in western North America; Daucinae mainly in warm-temperate and subtropical regions on the Northern Hemisphere, with their highest diversity in Macaronesia, the Mediterranean and southwestern Asia; and the Artedia and the Glaucosciadium clades in the eastern Mediterranean.
Smyrnieae Spreng. in J. J. Roemer et J. A. Schultes, Syst. Veg. 6: xxxvii. Aug-Dec 1820 [’Smyrniae’].
2/6. Smyrnium (5; S. apiifolium, S. olusatrum, S. orphanidis, S. perfoliatum, S. rotundifolium; southern and southeastern Europe, the Mediterranean), Lecokia (1; L. cretica; Crete to Iran). – Southern and southeastern Europe, the Mediterranean, southwestern Asia.
The Apioid Superclade
Apieae Takht. ex V. M. Vinogr., Fl. Vostochnoĭ Evropy 11: 339. 2004
12/40. Apium (c 11; cosmopolitan), Anethum (1; A. graveolens; southwestern Asia?), Ridolfia (1; R. segetum; the Mediterranean), Pseudoridolfia (1; P. fennanei; Morocco), Foeniculum (3; F. scoparium, F. subinodorum, F. vulgare; temperate regions in Asia), Deverra (8; arid and semi-arid regions in North Africa to southern Africa, the Arabian Peninsula and southwestern Asia), Ammi (6; A. crinitum, A. huntii, A. majus, A. topalii, A. trifoliatum, A. visnaga; Macaronesia, the Mediterranean, northern Africa, western Asia), Petroselinum (2; P. crispum, P. segetum; Europe, the Mediterranean), Billburttia (2; B. capensoides, B. vaginoides; Madagascar), Sclerosciadium (1; S. nodiflorum; Europe, the Mediterranean, Africa), ‘Seseli’ webbii (the Canary Islands), Stoibrax (3; S. dichotomum, S. hanotei, S. pomelianum; the Iberian Peninsula, North Africa). – Cosmopolitan, with their highest diversity in warm-temperate regions in Africa to southwestern Asia. – Naufraga balearica lacks free carpophore and probably has lignified endocarp.
Cachrydinae Meisn., Plant. Vasc. Gen.: Tab. Diagn. 150, Comm. 108. 16-22 Sep 1838 [’Cachrydeae’]
8/c 82. Alococarpum (1; A. erianthum; Iran), Azilia (1; A. eryngioides; Iran), Bilacunaria (4; B. boissieri, B. caspia, B. microcarpa, B. scabra; southwestern Asia), Cachrys (5; C. crassiloba, C. cristata, C. libanotis, C. pungens, C. sicula; Europe, North Africa), Diplotaenia (2; D. cachrydifolia, D. damavandica; Turkey, Iran), Ekimia (1; E. bornmuelleri; Turkey), Eriocycla (4; E. albescens, E. eriocarpa, E. pelliotii, E. stewartii; northern Iran, western Himalayas to northern and western China), Ferulago (c 45; southeastern Europe, the Mediterranean to Central Asia), Prangos (c 20; eastern Europe, eastern Mediterranean, temperate Asia). – Warm-temperate regions in Europe, North Africa and Asia, with their largest diversity in the eastern Mediterranean to southwestern Asia. – Bilacunaria has oil vesicles dispersed in mesocarp.
Careae Baill., Hist. Plant. 7: 174, 219. Apr 1879
11/33. 'Carum' pro parte (5; Europe, the Caucasus, southwestern Asia), Fuernrohria (1; F. setifolia; the Caucasus, Armenia; in Carum?), ‘Grammosciadium’ (8; eastern Mediterranean; non-monophyletic; in Carum?), Chamaesciadium (1; C. acaule; western Asia; in Carum?), Rhabdosciadium (5; R. aucheri, R. microcalycinum, R. petiolare, R. stenophyllum, R. straussii; eastern Europe, western Asia), Falcaria (1; F. vulgaris; Central Europe, the Mediterranean, western and Central Asia), Aegokeras (1; A. caespitosa; Turkey), ‘Aegopodium’ (8; temperate regions in Europe and Asia; non-monophyletic), Gongylosciadium (1; G. falcarioides; Turkey, the Caucasus, Iran), Hladnikia (1; H. pastinacifolia; Slovenia), Pseudopimpinella (1; P. anthriscoides; Iran). – Temperate regions in Eurasia, with the higest diversity in southwestern to Central Asia.
Coniinae Rouy, Consp. Fl. France: 116. 15 Aug 1927 [‘Conieae’]
1/4. Conium (6; C. chaerophylloides, C. divaricatum, C. fontanum, C. hillburttorum, C. maculatum, C. sphaerocarpum; southern Europe, the Mediterranean, temperate Asia, southern Africa).
Coriandreae W. D. J. Koch in Nova Acta Phys.-Med. Acad. Caes. Leop.-Carol. Nat. Cur. 12(1): 65, 82. 1824
2/5. Coriandrum (2; C. sativum, C. tordylium; southeastern Europe, southwestern Asia), Bifora (3; B. americana, B. radians, B. testiculata; the Mediterranean to Central Asia, southern United States, northern Mexico?). – Southeastern Europe, the Mediterranean, southwestern to Central Asia, one species of Bifora (B. americana) in the southern United States and northern Mexico?
Echinophoreae Benth. et Hook. f., Gen. Plant. 1: 862, 865. Sep 1867
7–9/33. Rughidia (2; R. cordata, R. milleri; Yemen), Dicyclophora (1; D. persica; Iran), Pycnocycla (12; tropical West Africa to northwestern India), ‘Echinophora’ (11; the Mediterranean, southwestern Asia to Iran; non-monophyletic), Anisosciadium (3; A. isosciadium, A. lanatum, A. orientale; southwestern Asia), Mediasia (1; M. macrophylla; southwestern and Central Asia), Nirarathamnos (1; N. asarifolius; Haggeher Mountains on Socotra), ’Pimpinella’ heyneana (Sri Lanka), ’Trachyspermum’ aethusifolium (Somalia). – Warm-temperate to subtropical regions in West Africa and the Mediterranean to Central and South Asia.
Opopanax clade
7/12. Smyrniopsis (1; S. aucheri; eastern Mediterranean, southwestern Asia to Iran), Opopanax (3; O. chironius, O. hispidus, O persicus; the Balkan Peninsula to Iran), Crenosciadium (1; C. siifolium; Turkey), Krubera (1; K. peregrina; western Mediterranean), Magydaris (2; M. panacifolia, M. pastinacea; the Mediterranean), Petroedmondia (1; P. syriaca; southwestern Asia), Stefanoffia (3; S. aurea, S. daucoides, S. insoluta; eastern Mediterranean). – The Mediterranean to southwestern Asia. – Smyrniopsis has oil vesicles dispersed in mesocarp. Krubera is sister to Coriandreae (with Levisticum sister to these three genera) in the analyses by Magee & al. (2009). The exact placement of Krubera is very uncertain at this moment and has to await phylogenetic investigations including a wide spectrum of taxa.
Pimpinelleae Spreng. in J. J. Roemer et J. A. Schultes, Syst. Veg. 6: xxxiv. Aug-Dec 1820
12–17/130–135. ’Psammogeton’ (3; P. biternatum, P. canescens, P. stocksii; southwestern Asia; non-monophyletic), ’Aphanopleura’ (5; A. breviseta, A. capillifolia, A. leptoclada, A. trachysperma, A. zangelanica; Central Asia to Afghanistan; non-monophyletic), ’Pimpinella’ (c 100; Europe, Macaronesia, the Mediterranean, temperate Asia, East African mountains; non-monophyletic), Arafoe (1; A. aromatica; the Caucasus), Bubon (1; B. macedonicum; southern Italy, southern Balkan), ‘Cryptotaenia’ africana (Central and East Africa), Demavendia (1; D. pastinacifolia; southwestern and Central Asia), Frommia (1; F. ceratophylloides; southern Central Africa), Haussknechtia (1; H. elymaitica; southwestern Iran), Nothosmyrnium (2; N. japonicum, N. xizangense; East Asia), Opsicarpium (1; O. insignis; Iran), Phellolophium (1; P. madagascariense; Madagascar), ’Physospermopsis’ cuneata (Sichuan, Yunnan), ’Seseli’ diffusum (northern India), ’Trachyspermum’ scaberulum (western China), ’Trachyspermum’ triradiatum (Sichuan), ’Zeravschania’ (11; southwestern to Central Asia; non-monophyletic). – Temperate to tropical regions in Eurasia and Africa.
Pyramidoptereae Boissier, Fl. Orient. 2: 1089. 1872 [‘Pyramidopterae’]
30–32/165–175. Ammoides (3; A. arabica, A. atlantica, A. pusilla; the Mediterranean), Astomaea (2; A. galiocarpa, A. seselifolia; eastern Mediterranean to Central Asia), ‘Bunium’ (45–50; Europe, the Mediterranean, North Africa, southwestern and Central Asia; non-monophyletic), ‘Carum’ (c 25?, e.g. C. heldreichii, C. rupestre and C. rupicola; C. buriaticum; C. appuanum and C. graecum; temperate regions on the Northern Hemisphere; polyphyletic), Crithmum (1; C. maritimum; Atlantic coasts of Europe, the Mediterranean), Cyclospermum (1; C. leptophyllum; Central America, the West Indies), Elaeosticta (25–30; eastern Europe, western and Central Asia), Galagania (4; G. ferganensis, G. fragrantissima, G. gracilis, G. neglecta; eastern Europe to Central Asia), Gongylotaxis (1; G. rechingeri; Afghanistan), Hellenocarum (3; H. depressum, H. multiflorum, H. strictum; southern Italy, Greece, western Turkey), Hyalolaena (4; H. bupleuroides, H. jaxartica, H. lipskyi, H. trichophylla; southwestern and Central Asia), Indoschulzia (2; I. garhwalica, I. hameliana; the Himalayas), Kosopoljanskia (2; K. hebecarpa, K. turkestanica; Central Asia), Lagoecia (1; L. cuminoides; the Mediterranean), Lipskya (1; L. insignis; Central Asia), Mogoltavia (2; M. narynensis, M. severtzovii; Central Asia), Muretia (4; M. amplifolia, M. aurea, M. oeroilanica, M. transcaspica; southern Russia, western and Central Asia), Neomuretia (2; N. amplifolia, N. pisidica; Turkey, northern Iran), Notiosciadium (1; N. pampicola; Argentina),‘Oedibasis’ (3; O. apiculata, O. platycarpa, O. tamerlanii; Central Asia; non-monophyletic), Oreoschimperella (2; O. aberdarensis, O. verrucosa; mountains in Ethiopia, Kenya and Yemen), Ormopterum (2; O. tuberosum, O. turcomanicum; Central Asia, Pakistan), ‘Pimpinella’ siifolia (western Pyrenees, northern Spain), Postiella (1; P. capillifolia; Turkey), Pyramidoptera (1; P. cabulica; Afghanistan), Scaligeria (7; S. alziarii, S. cretica, S. halophila, S. moreana, S. napiformis, S. setacea, S. stewartiana; the Balkan Peninsula, southwestern Asia), Schrenkia (12?; Central Asia), Schtschurowskia (1; S. meifolia; Central Asia), Schulzia (4; S. albiflora, S. crinita, S. dissecta, S. prostrata; Central Asia, northwestern India), Sison (4; S. amomum, S. exaltatum, S. segetum, S. trinervium; western and southern Europe, the Mediterranean), Tamamschjanella (1; T. rubella; the Balkan Peninsula, the Caucasus, southwestern Asia), ‘Trachyspermum’ ammi (northeastern Africa). – Temperate to subtropical regions on the Northern Hemisphere and one species in Argentina, with the largest diversity in the Mediterranean to Central Asia. – Lagoecia has well developed sepals and single-seeded fruit. Notiosciadium pampicola has compound umbels, laterally compressed fruits and entire carpophores.
Selineae Spreng. in J. J. Roemer et J. A. Schultes, Syst. Veg. 6: xlvi. Aug-Dec 1820
c 67/745–810. Aethusa (1; A. cynapium; Europe, North Africa, western Asia), Ammoselinum (5; A. butleri, A. giganteum, A. occidentale, A. popei, A. rosengurtii; North America, temperate South America), ‘Angelica’ (c 115; temperate regions on the Northern Hemisphere; non-monophyletic), Apiastrum (1; A. angustifolium; North America), Cervaria (5–6; C. aegopodioides, C. alsatica, C. cervariifolia, C. glauca, C. latifolia, C. rivini; temperate regions in Europe and Asia), ‘Cortia’ (3; C. depressa, C. lhasana, C. staintoniana; Central Asia, the Himalayas, Tibet; non-monophyletic), Cortiella (4; C. caespitosa, C. cortioides, C. hookeri, C. lamondiana; Central and South Asia), Dichoropetalum (27?; southern and southeastern Europe, the Mediterranean, North Africa, southwestern Asia), Dimorphosciadium (1; D. gayoides; Central Asia), Dystaenia (2; D. ibukiensis, D. takesimana; the Korean Peninsula, Japan), Endressia (2; E. castellana, E. pyrenaica; the Pyrenees, northern Spain), Exoacantha (1; E. heterophylla; southwestern Asia), Ferulopsis (2; F. hystrix, F. mongolica; northern and Central Asia), Glehnia (1; G. littoralis; northeastern Asia, western North America), Imperatoria (27?; Europe, North Africa), Johrenia (4; J. aurea, J. distans, J. selinoides, J. thessala; Greece, Turkey, southwestern and Central Asia), Kailashia (2; K. robusta, K. xizangensis; northern India, Tibet), Karatavia (1; K. kultiassovii; Central Asia), Kedarnatha (5; K. garhwalica, K. hameliana, K. meifolia, K. sanctuarii, K. vaginata; the Himalayas, northern Burma), Ledebouriella (2; L. multiflora, L. seseloides; Central Asia), Ligusticopsis (2–15; L. glaucescens, L. pseudodaucoides; temperate East Asia), ‘Ligusticum’ pro parte (7; temperate regions), Lomatocarpa (4; L. afghanica, L. albomarginata, L. korovinii, L. steineri; southwestern and Central Asia, Afghanistan), Lomatocarum (1–2; L. alpinum; the Caucasus), Magadania (2; M. olaensis, M. victoris; northeastern Asia), Oligocladus (1; O. patagonicus; Argentina), Oreocome (2; O. candollei, O. stelliphora; Pakistan to western China), Oreoselinum (1; O. nigrum; Europe east to the Ural), Ormosolenia (1; O. alpina; eastern Mediterranean), ‘Pachypleurum’ mutellinoides (Central Europe, Arctic Russia, northern Ural), Paraligusticum (1; P. discolor; the Altai Mountains), ’Peucedanum’ (100–120; temperate regions in Europe and Asia, mountains in tropical and southern Africa; polyphyletic), Pilopleura (2; P. goloskokovii, P. tordyloides; Central Asia), Pteroselinum (11; Europe, temperate Asia), Rumia (1; R. crithmifolia; Crimea), Sajanella (1; S. monstrosa; southern Siberia), ‘Selinum’ (2–5; S. capitellatum, S. carvifolia, S. cryptotaenium, S. longicalycinum, S. wallichianum; Europe to Central Asia; non-monophyletic; incl. Cnidium?), Cnidium (4?; C. cnidiifolium, C. monnieri, C. officinale, C. salinum; Europe, Africa, North America; in Selinum?), ’Seseli’ (100–120; Europe to Central Asia, northern tropical Africa; non-monophyletic), Siculosciadium (1; S. nebrodense; Sicily), Spermolepis (5; S. castellanosi, S. divaricata, S. echinata, S. inermis, S. patens; North America, Argentina, the Hawaii Islands), Stenocoelium (3; S. athamantoides, S. popovii, S. trichocarpum; Central Asia), Thecocarpus (2; T. carvifolius, T. meifolius; Turkey, Iran), Tommasinia (1; T. verticillaris; Central Europe), Trinia (10; the Mediterranean to Central Asia), Vicatia (2; V. coniifolia, V. wolffiana; southern Siberia, Central Asia, the Himalayas to western China), Xanthoselinum (2; X. alsaticum; southern and southeastern Europe, the Caucasus to western Siberia). – Neotropical clade Cotopaxia (2; C. asplundii, C. whitei; Colombia, Ecuador), 'Niphogeton' (18; northern Andes; non-monophyletic), Perissocoeleum (3; P. crinoideum, P. phylloideum, P. purdiei; Colombia), ’Arracacia’ (c 40; tropical America; polyphyletic), Coaxana (2; C. bambusioides, C. purpurea; Mexico), Mathiasella (1; M. bupleuroides; Mexico), 'Prionosciadium' (11; Mexico; polyphyletic), Ottoa (1; O. oenanthoides; Mexico), Myrrhidendron (4; M. chirripoense, M. donnellsmithii, M. glaucescens, M. maxonii; Central America, Colombia), Dahliaphyllum (1; D. almedae; Mexico), Neonelsonia (1; N. acuminata; Mexico, Central and northern South America), Coulterophytum (4; C. holwayi, C. laxum, C. macrophyllum, C. pubescens; Mexico), Enantiophylla (1; E. heydeana; Central America; in Coulterophytum?), Donnellsmithia (c 20; Mexico, Central America), 'Rhodosciadium' (9; Mexico; polyphyletic). – North American clade ’Lomatium’ (c 90; western North America; polyphyletic), ’Cymopterus’ (50–55; western North America; polyphyletic; partially in Lomatium), Eurytaenia (2; E. hinckleyi, E. texana; Oklahoma, New Mexico, Texas), Podistera (4; P. eastwoodiae, P. macounii, P. nevadensis, P. yukonensis; western Canada, western Unites States incl. Alaska), Shoshonea (1; S. pulvinata; Wyoming). – Mainly temperate and alpine regions on both hemispheres. The Arracacia clade is distributed in Mexico, Central and South America. The Perennial endemic North American clade is centered in the Rocky Mountains. – Myrrhidendron, in Central America and Colombia, is woody with secondary lateral growth. . – Oreoselinum nigrum may be closely allied to Imperatoria (e.g. Pimenov & al. 2016b).
Sinodielsia clade
11–20/47–48. ‘Angelica’ pro parte (3; A. paeoniifolia, A. sinensis, A. tianmuensis; China), Cenolophium (1; C. denudatum; eastern Europe, temperate Asia), ‘Cnidium’ dahuricum (Siberia), ‘Cnidium’ officinale (China), Conioselinum (18; eastern Europe, temperate regions in Asia and North America), Levisticum (1; L. officinale; eastern Mediterranean), ‘Ligusticum’ acuminatum (China), ‘Ligusticum’ jeholense (China), ‘Ligusticum’ sinense (China), ‘Ligusticum’ tenuissimum (China, the Korean Peninsula), Lithosciadium (1; L. kamelinii; Central and northern Asia to western China), Paulita (2; P. alpina, P. ovczinnikovii; Central Asia), ‘Pleurospermum’ wrightianum (western China), ‘Prangos’ haussknechtii (Iran), Pterocyclus (4; P. angelicoides, P. forrestii, P. rivulorum, P. rotundatus; the Himalayas, southwestern China), Seselopsis (1; S. tianschanica; Central Asia), Silaum (1; S. silaus; Europe, temperate Asia), Sinodielsia (3–4; S. delavayi, S. digitata, S. yunnanensis; southeastern Tibet, Yunnan), Sphaenolobium (3; S. kultiassovii, S. tenuisectum, S. tianschanicum; Central Asia), ‘Vicatia’ ('Sinodielsia') thibetica (Nepal, eastern and southern Tibet, western China). – Temperate and alpine regions on the Northern Hemisphere, with their highest diversity in Central Asia, the Himalayas and China.
Tordylieae W. D. J. Koch in Nova Acta Phys.-Med. Acad. Caes. Leop.-Carol. Nat. Cur. 12(1): 65, 85. 1824.
28–31/215–240. Tordyliinae Drude in H. G. A. Engler et K. A. E. Prantl, Nat. Pflanzenfam. III, 8: 115. Dec 1897. ‘Angelica’ likiangensis (Guizhou, Yunnan), ‘Angelica’ oncosepala (northwestern Yunnan), ‘Heracleum’ (c 50; temperate regions on the Northern Hemisphere, East African tropical mountains, with their largest diversity in the Himalayas and western China; incl. Mandenovia?; non-monophyletic), Mandenovia (1; M. komarovii; the Caucasus; in Heracleum?), Kandaharia (1; K. rechingerorum; Afghanistan), Lalldhwojia (4; L. acronemifolia, L. cooperi, L. pastinacifolia, L. staintonii; the Himalayas), Malabaila (4; M. aurea, M. graveolens, M. involucrata, M. secacul; the Balkan Peninsula, Turkey, the Caucasus to Iran), Leiotulus (6–8; L. alexandrinus, L. dasyanthus, L. isfahanicus, L. kotschyi, L. pastinacifolius, L. porphyrodiscus; Europe, temperate Asia), ‘Pastinaca’ (14; P. hirsuta, P. latifolia, P. lucida, P. sativa, P. umbrosa; Europe, temperate Asia; non-monophyletic), ‘Semenovia’ (6–30; Asia; non-monophyletic), Tetrataenium (6; T. aquilegifolium, T. cardiocarpum, T. lasiopetalum, T. leucocarpum, T. nephrophyllum, T. pasquieri; southwestern to Central Asia), Tordyliopsis (1; T. brunonis; the Himalayas, Tibet), Tordylium (18; southern Europe, the Mediterranean, southwestern Asia), Trigonosciadium (6; T. brachytaenium, T. intermedium, T. komarovii, T. lasiocarpum, T. tuberosum, T. viscidulum; Turkey, Iraq, Iran), Vanasushava (1; V. pedata; southern India), Zosima (4–15; southwestern and Central Asia). – Cymbocarpum clade Ducrosia (6; D. anethifolia, D. areysiana, D. assadii, D. flabellifolia, D. inaccessa, D. ismaelis; Egypt to northwestern India), Kalakia (1; K. marginata; Iran), Cymbocarpum (5; C. amanum, C. anethoides, C. erythraeum, C. marginatum, C. wiedemannii; southwestern Asia). – Lefebvrea clade Afrosciadium (18; eastern and southeastern Africa), Afroligusticum (13; tropical Africa), Cynorhiza (3; C. bolusii, C. meifolia, C. typica; Western Cape), Capnophyllum (4; C. africanum, C. leiocarpon, C. lutzeyeri, C. macrocarpum; coastal areas of Northern and Western Cape), Dasispermum (7; D. capense, D. grandicarpum, D. hispidum, D. humile, D. perennans, D. suffruticosum, D. tenue; coastal areas in South Africa), Scaraboides (1; S. manningii; Vanrhynsdorp and Tanqua karoo in Northern and Western Cape), Nanobubon (3; N. capillaceum, N. strictum; Western Cape, western Eastern Cape), Notobubon (12; Western and Eastern Cape), Stenosemis (2; S. angustifolia, S. caffra; Eastern Cape to southern KwaZulu-Natal), ‘Notobubon’ pearsonii (western Northern Cape), Lefebvrea (10; tropical and southwestern Africa). – Tordyliinae are distributed in temperate and alpine regions on the Northern Hemisphere, tropical African mountains and southern India, with their largest diversity in southwestern to Central Asia and the Himalayas; the Cymbocarpum clade occurs in southwestern Asia; and the Lefebvrea clade in tropical to southern Africa, with their highest diversity in the Cape Provinces.
Unplaced Apioideae Adenosciadium (1; A. arabicum; southwestern Arabian Peninsula), Aframmi (2; A. angolense, A. longiradiatum; Angola), Afrosison (3; A. djurense, A. gallabatense, A. schweinfurthii; tropical Africa), Agasyllis (1; A. gummifera; the Caucasus), Angoseseli (2; A. mazzocchii-alamannii, A. mossamedensis; Angola), Apodicarpum (1; A. ikenoi; eastern Japan), Asciadium (1; A. coronopifolium; Cuba), Austropeucedanum (1; A. oreopansii; northwestern Argentina), Bonannia (1; B. graeca; southern Italy, southern Greece), Caropsis (1; C. verticillatoinundata; Europe), Cephalopodum (3; C. afghanicum, C. badachshanicum, C. hissaricum; Central Asia), Coristospermum (1–2; C. huteri, C. lucidum; Europe), Cyathoselinum (1; C. tomentosum; southeastern Europe), Ergocarpon (1; E. cryptanthum; eastern Iraq, western Iran), Eriosynaphe (1; E. longifolia; southeastern Russia to Central Asia), Erythroselinum (1; E. atropurpureum; Ethiopia), Froriepia (2; F. gracillima, F. subpinnata; Turkey to Iran), Grafia (1; G. golaka; Italy, northwestern Balkan Peninsula), Haplosciadium (1; H. abyssinicum; Ethiopia to East African mountains), Horstrissea (1; H. dolinicola; Crete), Karnataka (1; K. benthamii; southern India), Kelussia (1; K. odoratissima; Iran), Kundmannia (1; K. sicula; southern Europe, the Mediterranean), Ladyginia (3; L. afghanica, L. bucharica, L. gigantea; southwestern and Central Asia), Macroselinum (1; M. latifolium; southern Europe, the Caucasus), Mastigosciadium (1; M. hysteranthum; Afghanistan), Microsciadium (1; M. minutum; eastern Greece, Turkey), Oliveria (1; O. decumbens; Syria to Iran), Palimbia (2; P. defoliata, P. rediviva; southern and eastern Russia to Central Asia), Paraselinum (1; P. weberbaueri; Peru), Pastinacopsis (1; P. glacialis; Central Asia), Pedinopetalum (1; P. domingense; Hispaniola), Physotrichia (4; P. atropurpurea, P. heracleoides, P. muriculata, P. welwitschii; tropical Africa), Pinacantha (1; P. porandica; Afghanistan), Pinda (1; P. concanensis; Western Ghats, southern India), Polyzygus (1; P. tuberosus; southern India), Portenschlagiella (1; P. ramosissima; southern Italy, western Balkan Peninsula), Pseudoselinum (1; P. angolense; Angola), Ptychotis (1; P. saxifraga; Central and southwestern Europe), Registaniella (1; R. hapaxlegomena; Afghanistan), Rhopalosciadium (1; R. stereocalyx; Iran; in Torilis?), Rutheopsis (1; R. herbanica; Lanzarote and Fuerteventura in the Canary Islands), Sclerochorton (1; S. haussknechtii; Iran), Sclerotiaria (1; S. pentaceros; Central Asia), Scrithacola (1; S. kuramensis; Afghanistan, Pakistan), Selinopsis (2; S. foetida, S. montana; the Mediterranean), Spuriodaucus (2; S. asper, S. quarrei; tropical Africa), Stenotaenia (3; S. daralaghezica, S. macrocarpa, S. nudicaulis; southwestern Asia), Stewartiella (1; S. baluchistanica; Pakistan), Taeniopetalum (3; T. neumayeri, T. peucedanoides, T. urbani; the Balkan Peninsula, Turkey), Tamamschjania (2; T. rhizomatica, T. rubella; northern Greece, Turkey), Thamnosciadium (1; T. junceum; Greece), Trachyspermum p.p. (8; T. ammi, T. roxburghianum, T. scaberulum, T. triradiatum, T. villosum; tropical and northeastern Africa to Central Asia, India and western China), Tricholaser (3; T. afghanicum, T. cachemiricum, T. ovatilobum; southern and southwestern Asia), Xatardia (1; X. scabra; eastern Pyrenees).
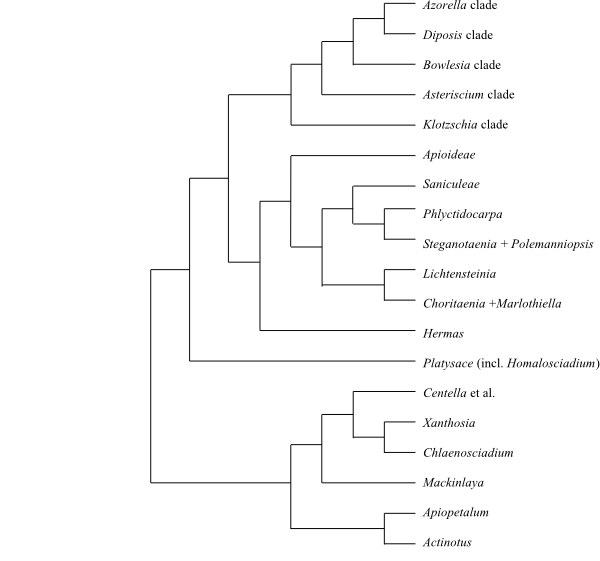 |
Cladogram (simplified) of Apiaceae based on DNA sequence data (Nicolas & Plunkett 2009). |
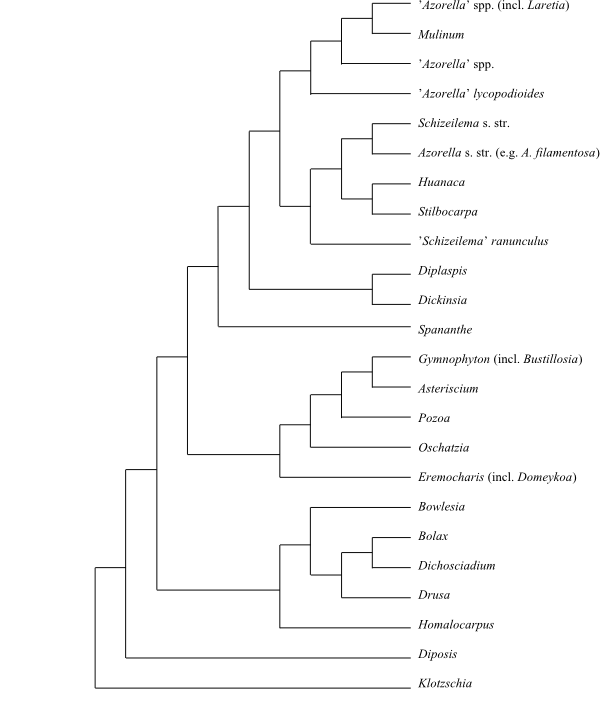 |
Cladogram (simplified) of Azorelloideae and some other Apiaceae based on DNA sequence data (Nicolas & Plunkett 2009). |
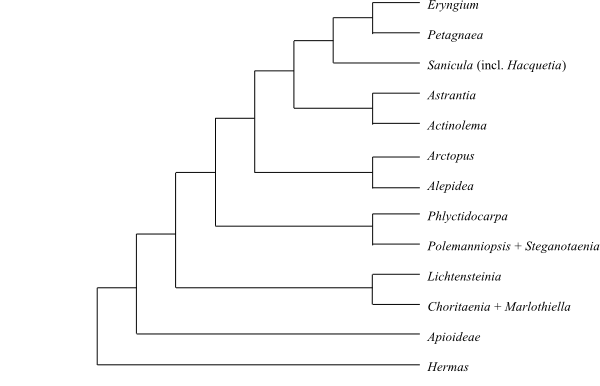 |
Cladogram of Saniculoideae based on DNA sequence data (Nicolas & Plunkett 2009; Magee & al. 2010). |
 |
Phylogeny of Apioideae based on DNA sequence data (Downie & al. 2010). |
ARALIACEAE Juss. |
( Back to Araliales ) |
Hederaceae Giseke, Prael. Ord. Nat. Plant.: 519. Apr 1792; Hydrocotylaceae Bercht. et J. Presl, Přir. Rostlin: 258. Jan-Apr 1820 [’Hydrocotyleae’], nom. cons.; Hederales Link, Handbuch 2: 5. 4-11 Jul 1829 [’Hederaceae’]; Hydrocotylineae Link, Handbuch 1: 314. 4-11 Jul 1829 [’Hydrocotylinae’]; Botryodendraceae J. Agardh, Theoria Syst. Plant.: 231. Apr-Sep 1858 [’Botryodendreae’]
Genera/species 37–38?/1.495–1.550
Distribution Mainly tropical and subtropical regions in the Southern and Northern Hemispheres; a few genera in temperate areas.
Fossils Leaves, pollen grains and endocarps of Araliaceae have been reported from Cenozoic strata in Europe and Asia. The oldest known macrofossils of Araliaceae – endocarps of Acanthopanax and Aralia – have been found in the Maastrichtian of Germany. Fossilized endocarps are also recorded from North America. Pollen were reported from Late Cretaceous (Early Campanian) layers in Wyoming, but also from the Paleocene and the Eocene of North America, Greenland and Europe.
Habit Usually bisexual (sometimes monoecious, andromonoecious, gynomonoecious, polygamomonoecious, or dioecious), evergreen or deciduous trees, shrubs or lianas (sometimes perennial herbs or suffrutices). Some species are aquatic or helophytes. Leaf scars large and distinct.
Vegetative anatomy Phellogen ab initio superficial. Peripheral collenchyma? Medullary and cortical vascular bundles often present. Vessel elements with simple and/or scalariform (sometimes reticulate etc.) perforation plates; lateral pits scalariform, opposite or alternate, simple and/or bordered pits. Vestured pits present. Imperforate tracheary xylem elements usually libriform fibres (in Boerlagiodendron fibre tracheids) with simple or bordered pits, septate or non-septate (also vasicentric tracheids). Wood rays uniseriate or multiseriate, usually heterocellular (rarely homocellular). Axial parenchyma usually paratracheal scanty, often vasicentric (sometimes apotracheal diffuse), or absent. Tyloses often abundant. Sieve tube plastids S type. Nodes usually ≥5:≥5, multilacunar with five or more leaf traces (sometimes 3:3, trilacunar with three traces). Parenchyma and other tissues with schizogenous secretory canals and cavities containing ethereal oils, resins and gum. Prismatic calciumoxalate crystals sometimes frequent.
Trichomes Hairs unicellular or multicellular, uniseriate or branched, often stellate or dendritic (sometimes T-shaped or peltate-lepidote).
Leaves Usually alternate (rarely distichous; rarely opposite [Cheirodendron] or verticillate), simple, pinnately compound (sometimes two or three times) or palmately compound (leaflets usually articulated), usually pinnately or palmately lobed (sometimes entire), often coriaceous, with ? or supervolute ptyxis. Stipules cauline or petiolar, or one intrapetiolar or absent; lower part of petiole usually sheathing and expanding. Petiole vascular bundles? Venation pinnate or palmate. Stomata anomocytic, paracytic or anisocytic. Cuticular wax crystalloids as irregular platelets and threads. Secretory canals and cavities abundant. Leaf margin and leaflet margins usually serrate (rarely entire); each tooth with broad glandular apex with one main vein and two lateral veins, or with one vein running above tooth.
Inflorescence Terminal or axillary, panicle, thyrsoid etc., or umbellate, spicate or capitate, often compound; partial inflorescences umbels.
Flowers Actinomorphic, small. Usually epigyny (rarely hypogyny or half epigyny). Sepals five, usually minute (sometimes absent), usually with open aestivation, persistent, usually connate. Petals (three to) five to ten (to twelve), with valvate aestivation, caducous, usually free (in, e.g., Osmoxylon connate at base; in, e.g., Boerlagiodendron connate and tubular; in, e.g., ‘Tupidanthus’ [Schefflera] calyptrate); corolla tube initiation early. Nectariferous disc intrastaminal, annular, distally grading into stylopodium.
Androecium Stamens (three to) five to ten (to twelve; rarely twice as many as petals, or numerous; in ‘Tupidanthus’ [Schefflera]calyptratus up to 172 in one whorl; in ‘Plerandra’ [Schefflera] up to c. 500 in four whorls), antesepalous, alternipetalous. Filaments free from each other, from petals and from pistil, inserted outside annular nectariferous disc. Anthers dorsifixed, versatile?, usually tetrasporangiate (rarely octosporangiate), introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory. Staminodia absent.
Pollen grains Microsporogenesis simultaneous. Pollen grains (2–)3(–6)-colporate or (2–)3(–6)-colpate, shed as monads, usually tricellular (rarely bicellular) at dispersal. Exine tectate or semitectate, with columellate infratectum, imperforate to finely perforate or reticulate.
Gynoecium Pistil composed of usually two to five (rarely numerous; in ‘Tupidanthus’ [Schefflera] calyptratus up to 132) connate carpels; vascular bundles of ventral carpels consisting of connate bundles of adjacent placentae. Ovary usually inferior (rarely superior or semi-inferior), usually bilocular to quinquelocular (rarely pseudomonomerous or multilocular; in Seemannaralia unilocular). Stylodia two to five, usually free (sometimes connate into single style), inserted on epigynous stylopodium. Stigmas punctate, papillate, usually Wet (sometimes Dry) type. Pistillodium absent.
Ovules Placentation apical. Ovules usually one (rarely two) per carpel, anatropous, pendulous, epitropous, unitegmic, usually crassinucellar (sometimes tenuinucellar). Funicle with short hairs. Integument five to ten cell layers thick. Oburator funicular. Nucellar cap sometimes present. Hypostase sometimes present. Endothelium sometimes present. Megagametophyte usually monosporous, Polygonum type. Endosperm development ab initio nuclear. Endosperm haustoria absent. Embryogenesis onagrad or chenopodiad.
Fruit Usually a drupe with pyrenes of the same number as locules, often dry, with thick-walled lignified mesocarp containing solitary rhomboidal crystals in cell layers immediately outside sclerified bony endocarp (sometimes a berry or syncarp consisting of more or less connate ovaries; rarely [in, e.g., Harmsiopanax with two carpels and carpophore] drupaceous schizocarp with two to five mericarps). “Pseudo-wings”, if present, formed by compression or folding of carpel and composed of both mesocarp and endocarp; vascular bundle present at margin. Vallecular and intrajugal vittae with secretory oil canals and oil cavities usually present.
Seeds Aril absent. Seed coat exotestal. Exotestal cell walls sometimes slightly thickened. Endotesta? Testal cells sometimes with stellate calcium oxalate crystals. Perisperm not developed. Endosperm copious, oily (sometimes ruminate), with petroselinic acid. Embryo small, straight?, well differentiated, without chlorophyll. Cotyledons two. Germination phanerocotylar.
Cytology x = (9) (11) 12 (18)
DNA Mitochondrial gene rpl2 lost. Deletion of 92 bp in plastid gene rpl16. Mitochondrial coxI intron present in Hydrocotyle.
Phytochemistry Flavonols (kaempferol, quercetin), flavonoid sulphates, hydroxycoumarins, oleanolic acid derivatives, dammaranes, sesquiterpene lactones, triterpenoid ethereal oils and resins, caffeic acid, chlorogenic acid, ellagic acid, proanthocyanidins (prodelphinidins), alkaloids, triterpene saponins, acetate derived arthroquinones?, phenantrenes, phenylpropenes (e.g. myristicin), acetophenones, germacrane-like compounds, asarone, syringaresinol, pinoresinol, polyacetylenes (e.g. falcarinone) derived from fatty acids, mostly aliphatic C17-acetylenes, and petroselinic acid (in endosperm) present. Furanocoumarins, tannins and cyanogenic compounds not found. Carbohydrates stored as trisaccharide umbelliferose.
Use Ornamental plants, medicinal plants (Acanthopanax, Aralia, Panax etc.), paper (Tetrapanax papyrifera), timber.
Systematics Araliaceae are sister-group to [Myodocarpaceae+Apiaceae]].
Hydrocotyloideae Link, Handbuch 1: 314. Jan-Aug 1829
2/115–145. Trachymene (40–45; Southeast Asia, Malesia to New Guinea, Australia, New Caledonia, Fiji; Hydrocotyle (75–100; almost cosmopolitan). – Cosmopolitan, with their highest diversity on the Southern Hemisphere. More or less herbaceous. Nodes 3:3, trilacunar with three leaf traces. Stipules cauline or basal on leaves. Lamina rounded-peltate. Leaf margin crenate. Inflorescence usually terminal (sometimes axillary). Sepals usually absent in Hydrocotyle. Pistil composed of two connate carpels. Integument approx. five cell layers thick (Hydrocotyle). Funicle short. Fruit a schizocarp, laterally compressed. Endocarp sclerified. Carpophore in Trachymene undivided (carpophores not free). n = 6 or higher. Polyploidy frequently occurring in Hydrocotyle. – Hydrocotyloideae may be sister to the remaining Araliaceae, although in some analyses it takes a position as an ingroup in Aralioideae.
Aralioideae Eaton, Bot. Dict., ed. 4: 37. Apr-Mai 1836 [‘Araliaceae’] (under construction)
c 34/1.380–1.400? Harmsiopanax (3; H. aculeata, H. harmsii, H. ingens; Malesia to New Guinea), Osmoxylon (c 60; Taiwan, Central and East Malesia to islands in western Pacific); Astrotricha (c 20; Australia, with their highest diversity in the southeastern parts); Cheirodendron (6; C. dominii, C. fauriei, C. forbesii, C. platyphyllum, C. trigynum: the Hawaiian Islands; C. bastardianum: the Marquesas Islands), 'Raukaua' (6; R. gunnii: Tasmania; R. anomalus, R. edgerleyi, R. simplex: New Zealand; R. laetevirens, R. valdiviensis: southern Chile, southern Argentina; polyphyletic), Motherwellia (1; M. haplosciadea; northeastern Queensland), Cephalaralia (1; C. cephalobotrys; eastern Queensland, eastern New South Wales); Cussonia (c 20; tropical and southern Africa, Madagascar, the Mascarene Islands), Seemannaralia (1; S. gerrardii; South Africa); Aralia (c 70; East and Southeast Asia, Malesia, southern Canada, United States); Polyscias (c 160; tropical regions in the Old World east to New Caledonia, with their largest diversity in Madagascar), Meryta (28–30; eastern Australia, New Caledonia, Vanuatu, New Zealand, Three Kings Islands and Hen and Chickens Islands off New Zealand, Norfolk Island, Pitcairn Island, Society Islands, the Marquesas Islands and other islands in southwestern Pacific; incl. Pseudopanax?), Pseudopanax (11–12; Tasmania, New Zealand, Chile?; in Meryta?), Plerandra (33; New Guinea, Solomon Islands, Vanuatu, Fiji); Heteropanax (8; southern Asia, China), Fatsia (3; F. japonica, F. oligocarpella, F. polycarpa; southern Japan, southern Korean Peninsula, Taiwan), Oplopanax (3; O. elatus, O. horridus, O. japonicus; Japan, northwestern North America), Metapanax (2; M. davidii, M. delavayi; southern China, northern Vietnam), Macropanax (17; the Himalayas, China, Southeast Asia, West Malesia), Kalopanax (1; K. septemlobus; eastern Siberia, China, the Korean Peninsula, Japan), Tetrapanax (1; T. papyrifer; southern China, Taiwan), Heteropanax (9; South and Southeast Asia, China), ’Schefflera’ (c 600?; tropical and subtropical regions on both hemispheres; polyphyletic), Astropanax (18–19; tropical and subtropical Africa, Madagascar, the Seychelles), Neocussonia (31; Tanzania to southern Africa, Madagascar, with their highest diversity in Madagascar), Sinopanax (1; S. formosanus; Taiwan; in Schefflera?), Oreopanax (80–85; Mexico, Central America, the West Indies, South America to Brazil and Argentina), Dendropanax (90–95; southern China, Southeast Asia, the Malay Peninsula, Central and South America), Merrilliopanax (3; M. alpinus, M. listeri, M. membranifolius; eastern Himalayas, northern Burma, Yunnan), Hedera (12–15; Europe, Macaronesia, the Mediterranean, northwestern Africa, central and southern Asia to Japan and Taiwan), Trevesia (7; T. arborea, T. beccarii, T. burckii, T. lateospina, T. palmata, T. sundaica, T. valida; India, Southeast Asia, Malesia; incl. Brassaiopsis?), Brassaiopsis (40–45; the Himalayas, China, Thailand, Indochina, West and Central Malesia; in Trevesia?), Eleutherococcus (c 40; the Himalayas, southeastern Siberia, China, the Korean Peninsula, Japan, Southeast Asia, Malesia to the Philippines). – Unplaced Aralioideae Anakasia (1; A. simplicifolia; western New Guinea), Woodburnia (1; W. penduliflora; Burma). – Mainly tropical and subtropical areas, few species in temperate regions. Usually trees, shrubs or lianas (sometimes herbs). Leaves simple, or pinnately or palmately compound. Pistil composed of one to five (to more than 130) connate carpels (when five, then carpels antepetalous; when three, then median carpel abaxial). Stylopodium undivided. Integument approx. ten cell layers thick. Parietal tissue sometimes present. Hypostase sometimes present. Testa multiplictive. Exotestal cells tangentially elongated. – Harmsiopanax, with two carpels and carpophore, may be sister to the remaining Aralioideae, although Plunkett & al. (2004) found it to be nested deeply inside Aralioideae, as sister to Aralia and the Polyscias clade. Osmoxylon may be successive sister to Aralioideae above Harmsiopanax. Astrotricha is possibly also basal, although the support is weak.
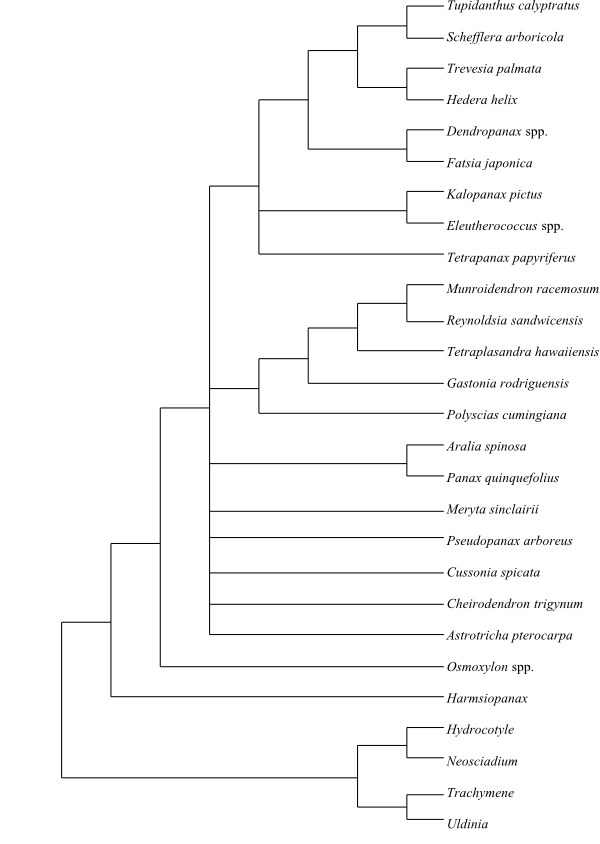 |
Cladogram of Araliaceae based on DNA sequence data (Plunkett & Lowry 2001; Nicolas & Plunkett 2009). |
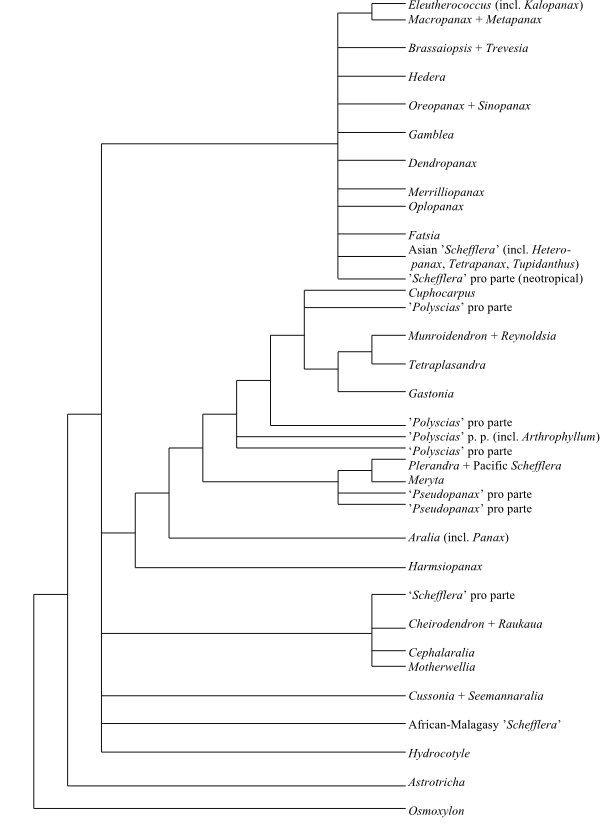 |
Bayesian inference tree of Araliaceae based on DNA sequence data (Plunkett & al. 2004). |
GRISELINIACEAE J. R. Forst. et G. Forst. ex A. Cunn. |
( Back to Araliales ) |
Griseliniales (J.R. Forst. et G. Forst. ex Cunn.) Takht. ex Reveal et Doweld in Novon 9: 550. 30 Dec 1999
Genera/species 1/7
Distribution New Zealand incl. Stewart Island, southeastern Brazil, Paraguay, Chile.
Fossils Unknown.
Habit Dioecious, evergreen trees or shrubs (sometimes lianas or epiphytes).
Vegetative anatomy Phellogen ab initio superficial. Peripheral collenchyma? Vessel elements (solitary) with scalariform perforation plates; lateral pits scalariform or opposite, simple pits. Imperforate tracheary xylem elements ? with bordered pits, septate or non-septate. Wood rays multiseriate, heterocellular. Axial parenchyma usually very rare (apotracheal diffuse or diffuse-in-aggregates, or paratracheal scanty, often vasicentric) or absent. Sieve tube plastids S type? Nodes 3:3, trilacunar with three leaf-traces, or ≥5:≥5, multilacunar with five or more traces. Prismatic calciumoxalate crystals frequent.
Trichomes Hairs unicellular, with granular, tuberous or verrucate outgrowths.
Leaves Alternate (distichous), simple, entire, coriaceous, often asymmetrical, with conduplicate ptyxis. Stipules absent; leaf base often with petiolar margins and adaxially expanded flange enclosing stem/branch, somewhat sheathing. Petiole vascular bundle transection incurved-arcuate. Venation pinnate? Stomata cyclocytic. Cuticular wax crystalloids? Epidermis with mucilage cells; epidermal cells rhomboidal, small. Mesophyll with sclerenchymatous idioblasts. Leaf margin serrate (often spinose-dentate) or entire.
Inflorescence Terminal or axillary, panicle or raceme. Floral prophylls (bracteoles) absent.
Flowers Actinomorphic, small. Pedicel articulated. Epigyny. Sepals five, with open aestivation, connate, in male flowers minute, in female flowers larger. Petals five, with imbricate aestivation, free (absent in female flowers of Griselinia lucida). Nectary absent. Disc fleshy, quinquangular.
Androecium Stamens five, haplostemonous, antesepalous, alternipetalous. Filaments free from each other and from petals. Anthers dorsifixed, versatile, tetrasporangiate, latrorse, longicidal (dehiscing by longitudinal slits). Tapetum secretory? Staminodia absent?
Pollen grains Microsporogenesis simultaneous? Pollen grains tricolporate, shed as monads, ?-cellular at dispersal. Exine tectate, with ? infratectum, striate.
Gynoecium Pistil composed of usually three (sometimes four) connate carpels (sometimes only two carpels fertile). Ovary inferior, usually unilocular (pseudomonomerous; rarely bilocular); transseptal vascular bundles present. Stylodia usually three (sometimes four), short, free or connate below. Stigmas apical or adaxial, type? Pistillodium absent.
Ovules Placentation apical. Ovule one per carpel, anatropous, pendulous, unitegmic, weakly crassinucellar. Integument ? cell layers thick. Obturator funicular. Megagametophyte monosporous, Polygonum type. Endosperm development ab initio nuclear. Endosperm haustoria? Embryogenesis?
Fruit Usually a single-seeded berry.
Seeds Aril absent. Testa multilayered?, with outer two layers (and sometimes especially additional third testal layer) with thickened cell walls. Perisperm not developed. Endosperm copious. Embryo small, straight?, narrowly elongate, chlorophyll? Cotyledons two. Germination?
Cytology n = 18
DNA Mitochondrial gene rpl2 absent?
Phytochemistry Insufficiently known. Route I carbocyclic iridoids (griselinoside), caffeic acid, chlorogenic acid, and petroselinic acid (in endosperm) present. Flavonols? Route II decarboxylated iridoids? Polyacetylenes? Ellagic and gallic acids, tannins, proanthocyanidins, and cyanogenic compounds not found. Umbelliferose?
Use Ornamental plants, timber.
Systematics Griselinia (7; G. littoralis, G. lucida: New Zealand, Stewart Island; G. carlomunozii, G. jodinifolia, G. racemosa, G. ruscifolia, G. scandens: southeastern Brazil, Paraguay, western Argentina, Chile).
Griselinia is sister to the clade [Pittosporaceae+[Araliaceae+[Myodocarpaceae+Apiaceae]]].
MYODOCARPACEAE Doweld |
( Back to Araliales ) |
Genera/species 2/15
Distribution East Malesia to New Guinea, Melanesia, Queensland, Norfolk Island.
Fossils Unknown.
Habit Usually bisexual, evergreen trees or shrubs.
Vegetative anatomy Phellogen ab initio superficial? Peripheral collenchyma? Vessel elements with simple or scalariform perforation plates; lateral pits alternate, bordered pits? Vestured pits? Imperforate tracheary xylem elements very thick-walled libriform fibres with bordered pits, non-septate (vasicentric tracheids present in Delarbrea). Wood rays multiseriate?, homocellular. Axial parenchyma apotracheal diffuse or diffuse-in-aggregates, or paratracheal scanty. Sieve tube plastids S type? Nodes multilacunar? Parenchyma and other tissues usually with schizogenous secretory canals and cavities containing ethereal oils, resins, gum or mucilage? Phelloderm sometimes with sclereids. Crystals?
Trichomes Hairs?
Leaves Alternate (spiral), usually pinnately compound (sometimes simple, entire), with ? ptyxis. Stipules present (Myodocarpus) or absent; petiolar base sheathing. Petiole vascular bundles? Venation usually pinnate, brochidodromous. Stomata? Cuticular wax crystalloids? Leaf margins entire or serrate.
Inflorescence Terminal, panicle (with umbel-like partial inflorescences), or compound umbels.
Flowers Actinomorphic, small. Pedicels articulated. Epigyny. Sepals five, with valvate aestivation, free. Petals five, usually with imbricate (in Delarbrea rarely valvate) aestivation, free, often clawed, apically inflexed. Nectariferous disc intrastaminal, fleshy, present on stylopodium.
Androecium Stamens five, haplostemonous, antesepalous, alternipetalous. Filaments incurved in bud, free from each other and from tepals. Anthers dorsifixed, non-versatile?, tetrasporangiate, introrse?, longicidal (dehiscing by longitudinal slits). Tapetum secretory? Staminodia absent.
Pollen grains Microsporogenesis simultaneous? Pollen grains usually tricolporate?, shed as monads, ?-cellular at dispersal. Exine semitectate, with columellate infratectum, reticulate or finely reticulate to scabrate.
Gynoecium Pistil composed of two connate carpels; vascular bundles of ventral carpels adnate to bundles of adjacent placentae. Ovary inferior, bilocular. Stylodia two, free, on a stylopodium. Stigmas non-papillate?, Wet type? Pistillodium absent.
Ovules Placentation axile? Ovule one? per carpel, ?-tropous, unitegmic, tenuinucellar? Integument ? cell layers thick. Megagametophyte monosporous, Polygonum type. Endosperm development nuclear? Endosperm haustoria absent? Embryogenesis?
Fruit A drupe (Delarbrea) or schizocarp with free carpophores and two samaroid laterally flattened mericarps (Myodocarpus). Wings (“pseudo-wings”) in Myodocarpus formed by compression or folding of carpel and composed of both mesocarp and endocarp; vascular bundle present at margin. Mesocarp with secretory vesicles. Endocarp reduced, with scattered large circular schizogenous oil ducts (different in morphology from vittae in Apiaceae).
Seeds Seeds in Myodocarpus laterally flattened. Aril absent. Exotestal cells? Endotesta? Perisperm not developed. Endosperm copious, oily. Embryo small, straight?, chlorophyll? Cotyledons two. Germination phanerocotylar?
Cytology n = 12 (Delarbrea)
DNA
Phytochemistry Virtually unknown. Flavonols? Furanocoumarins? Triterpenoid ethereal oils and resins? Sesquiterpene lactones? Polyacetylenes? Petroselinic acid? Umbelliferose?
Use Unknown.
Systematics Myodocarpus (8; M. crassifolius, M. fraxinifolius, M. gracilis, M. involucratus, M. lanceolatus, M. pinnatus, M. simplicifolius, M. vieillardii; New Caledonia), Delarbrea (7; D. balansae, D. collina, D. harmsii, D. longicarpa, D. michieana, D. montana, D. paradoxa; the Moluccas, Lesser Sunda Islands, New Guinea, Solomon Islands, northeastern Queensland, New Caledonia, Vanuatu, Norfolk Island).
Myodocarpaceae are sister to Apiaceae.
PENNANTIACEAE J. Agardh |
( Back to Araliales ) |
Pennantiales Doweld, Tent. Syst. Plant. Vasc.: lii. 23 Dec 2001
Genera/species 1/4
Distribution Eastern Australia, New Zealand, Norfolk Island.
Fossils Unknown.
Habit Dioecious, usually evergreen trees (Pennantia baylisiana shrub).
Vegetative anatomy Phellogen? Collenchyma poorly developed. Pericyclic envelope present. Vessel elements usually with scalariform (rarely also reticulate) perforation plates; lateral pits opposite and/or scalariform, simple or bordered pits. Imperforate tracheary xylem elements tracheids with bordered pits, non-septate. Wood rays uniseriate or multiseriate, heterocellular. Axial parenchyma apotracheal diffuse or diffuse-in-aggregates, or paratracheal scanty, or absent. Tyloses absent. Sieve tube plastids ? type. Nodes 3:3, trilacunar with three leaf traces. Crystals and crystal complexes absent.
Trichomes Hairs unicellular (also uncinate); glandular hairs multicellular with uniseriate stalk.
Leaves Alternate (spiral), simple, entire, often coriaceous, with ? ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle transection annular; petiole often with wing bundles and sometimes with medullary bundle. Venation pinnate. Stomata paracytic. Cuticular wax crystalloids? Domatia as pockets or hair tufts, or absent. Foliar sclereids absent. Leaf margin serrate, crenate or entire.
Inflorescence Usually terminal (sometimes axillary), panicle.
Flowers Actinomorphic, small. Pedicel articulated. Hypogyny. Sepals four or five, with open? aestivation, very small, free. Petals five, with valvate aestivation, connate at base, inflexed at apex. Nectary absent. Disc absent.
Androecium Stamens four or five?, haplostemonous, antesepalous, alternipetalous. Filaments free from each other, folded in bud, sometimes adnate to corolla (epipetalous). Anthers dorsifixed, versatile, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory? Female flowers with or without staminodia.
Pollen grains Microsporogenesis simultaneous? Pollen grains?, shed as monads, ?-cellular at dispersal. Exine?, with ? infratectum, sculpturing?
Gynoecium Pistil composed of two or three connate carpels. Ovary superior, unilocular?; transseptal vascular bundles present? Stylodia three, very short (when stigmas punctate), or styles absent. Stigmas three, either punctate or hippocrepomorphic forming sinuate ring, papillate, type? Male flowers with pistillodium.
Ovules Placentation apical. Ovules one per carpel, anatropous, unitegmic, tenuinucellar or thinly crassinucellar. Integument ? cell layers thick, vascularized. Megagametophyte monosporous, Polygonum type. Endosperm development? Endosperm haustoria? Embryogenesis?
Fruit A drupe with inner cells transversely elongate.
Seeds Aril absent. Testa thin. Exotestal cells? Endotesta? Perisperm? Endosperm copious, oily?, chlorophyll? Embryo minute, straight? Cotyledons two. Germination?
Cytology n = 25
DNA Mitochondrial gene rpl2 absent?
Phytochemistry Unknown. Iridoids? Umbelliferose?
Use Timber.
Systematics Pennantia (4; P. baylisiana: Three Kings Islands; P. corymbosa: New Zealand; P. cunninghamii: eastern Queensland, eastern New South Wales; P. endlicheri: Norfolk Island).
PITTOSPORACEAE R. Br. |
( Back to Araliales ) |
Pittosporales Link, Handbuch 2: 222. 4-11 Jul 1829 [’Pittosporeae’]
Genera/species 8/265–270
Distribution Madeira, Africa south of Sahara, Madagascar, Yemen, India, Sri Lanka, eastern Himalayas, East Asia to the Korean peninsula and southern Japan, Southeast Asia, Malesia to New Guinea, Melanesia, Australia, Tasmania, New Zealand, islands in the Pacific and the Indian Ocean, with their largest diversity in Australia.
Fossils Unknown.
Habit Usually bisexual (sometimes polygamomonoecious, dioecious, or gynodioecious), evergreen trees, shrubs or lianas (sometimes spiny). Some species are xerophytic. Many species are aromatic.
Vegetative anatomy Phellogen ab initio superficial. Peripheral collenchyma? Young stem with vascular cylinder (in Pittosporum septated). Vessel elements usually with simple (in one species of Pittosporum scalariform) perforation plates (in primary xylem with scalariform perforation plates only); lateral pits alternate, bordered pits. Vestured pits? Imperforate tracheary xylem elements fibre tracheids and/or libriform fibres with simple or bordered pits, septate to non-septate (also vasicentric tracheids). Wood rays multiseriate, heterocellular (almost homocellular). Axial parenchyma paratracheal scanty, often vasicentric, or absent. Sieve tube plastids Ss type. Nodes usually 3:3, trilacunar with three leaf traces (sometimes 1:3, unilacunar with three traces). Parenchyma and other tissues (including pericycles of stem, secondary cortex and secondary phloem of older stems, roots and leaves) with schizogenous secretory canals and cavities with ethereal oils? and/or resins and/or gum-like substances? Prismatic calciumoxalate crystals often frequent.
Trichomes Hairs unicellular or multicellular, uniseriate or branched, short-stalked T-shaped with two or three basal cells and long terminal cell (terminal cell vertically or transversely elongate); glandular hairs clavate or absent.
Leaves Alternate (spiral), simple (leaves of seedlings in Pittosporum sometimes pinnately compound), usually entire (rarely lobate), often coriaceous, with supervolute to curved ptyxis. Stipules absent; leaf base narrow to sheathing and decurrent. Petiole vascular bundle transection arcuate. Venation pinnate. Stomata usually paracytic (rarely cyclocytic). Cuticular wax crystalloids as rodlets. Schizogenous secretory canals and cavities (around phloem) with resin. Mesophyll cells with calciumoxalate usually as druses. Leaf margin usually entire (rarely serrate or sinuate).
Inflorescence Terminal or axillary, corymb, panicle, thyrse etc., or flowers solitary. Partial inflorescences not umbels.
Flowers Usually actinomorphic (sometimes slightly obliquely zygomorphic). Hypogyny (probably secondary). Sepals five, with imbricate aestivation, caducous, usually free (sometimes connate at base); median sepal adaxial. Petals five, with imbricate aestivation, usually connate at base. Nectariferous disc intrastaminal.
Androecium Stamens five, haplostemonous, antesepalous, alternipetalous. Filaments free from each other or somewhat connate at base, free from petals. Anthers usually dorsifixed (sometimes basifixed), non-versatile, tetrasporangiate, introrse, usually longicidal (dehiscing by longitudinal or sometimes short slits; in Cheiranthera poricidal, with apical pores). Placentoid present. Tapetum secretory. Staminodia absent.
Pollen grains Microsporogenes simultaneous. Pollen grains 3(–4)-colporate, shed as monads, usually tricellular (sometimes bicellular) at dispersal. Exine tectate, with columellate? infratectum, imperforate to finely perforate.
Gynoecium Pistil composed of two (to five) connate carpels. Ovary superior (probably secondarily), usually unilocular (rarely completely or incompletely bilocular to quinquelocular), with lateral nectaries. Style one, entire. Stigma capitate or somewhat lobate, non-papillate, Wet type. Pistillodium absent.
Ovules Placentation parietal (when ovary unilocular) or axile (when ovary multilocular); ovules inserted above basal zone of separate loculi (comparable to Araliaceae and Apiaceae). Ovules several to numerous per carpel, anatropous or somewhat campylotropous, horizontal or ascending, usually epitropous (sometimes apotropous), unitegmic, tenuinucellar. Integument ? cell layers thick. Funicular obturator probably absent. Endothelium absent. Megagametophyte monosporous, Polygonum type. Endosperm development ab initio nuclear. Endosperm haustoria? Embryogenesis asterad.
Fruit A loculicidal (sometimes also septicidal) capsule or a berry. Seeds surrounded by resinous, juicy and viscid tissue.
Seeds Aril present or absent. Seed coat exotestal, fleshy. Testa multilayered. Exotestal cell walls thickened, non-lignified. Endotesta? Perisperm not developed. Endosperm copious, oily. Embryo small, straight, well differentiated, without chlorophyll. Cotyledons usually two (in Pittosporum sometimes up to five). Germination phanerocotylar.
Cytology x = 12
DNA Plastid gene infA lost/defunct (Pittosporum). Mitochondrial gene rpl2 lost?
Phytochemistry Furanocoumarins, hydroxycoumarins, oleanolic acid derivatives, non-hydrolyzable tannins, triterpenoid ethereal oils and resins, caffeic acid, alkaloids, triterpene saponins, polyacetate derived arthroquinones, C20- and C22-fatty acids, polyacetylenes (e.g. falcarinone) derived from fatty acids, mostly aliphatic C17-acetylenes, and chinic acid present. Ellagic acid, proanthocyanidins, cyanogenic compounds and petroselinic acid not found. Flavonols (kaempferol, quercetin)? Carbohydrates stored as trisaccharide umbelliferose. Aluminium accumulated.
Use Ornamental plants, carpentries.
Systematics Bursaria (7; B. calcicola, B. incana, B. longisepala, B. occidentalis, B. reevesii, B. spinosa, B. tenuifolia; eastern, southeastern and southwestern Australia, Tasmania), Auranticarpa (6; A. edentata, A. ilicifolia, A. melanosperma, A. papyracea, A. resinosa, A. rhombifolia; northern Australia to eastern New South Wales), Pittosporum (c 200; Madeira [Pittosporum coriaceum], tropical and southern Africa, Madagascar, the Arabian Peninsula, southern and eastern Asia, Australia, Tasmania, New Zealand, islands in the Pacific incl. the Hawaiian Islands), Hymenosporum (1; H. flavum; New Guinea, eastern Queensland, northeastern New South Wales), Bentleya (2; B. diminuta, B. spinescens; southwestern Western Australia), Billardiera (c 25; Australia, Tasmania), Marianthus (18; southwestern and southeastern Australia, Tasmania), Cheiranthera (7; C. alternifolia, C. cyanea, C. filifolia, C. linearis, C. preissiana, C. telfordii, C. volubilis; southwestern and southeastern Australia).
Pittosporaceae are sister-group to [Myodocarpaceae+Apiaceae].
Bursaria may be sister to the remaining Pittosporaceae, although the support is weak and the basal clade usually a trichotomy.
 |
Cladogram of Pittosporaceae based on morphological data (Cayzer & al. 2004). |
TORRICELLIACEAE (Wang) H. H. Hu |
( Back to Araliales ) |
Melanophyllaceae Takht. ex Airy Shaw in Kew Bull. 26: 492. 1972; Aralidiaceae Philipson et B. C. Stone in Taxon 29: 402. 25 Aug 1980; Aralidiales Takht. ex Reveal in Novon 2: 238. 13 Oct 1992; Torricelliales Takht. ex Reveal et Doweld in Novon 9: 550. 30 Dec 1999
Genera/species 3/11
Distribution Madagascar, eastern Himalayas to northern Burma and China, southern Thailand, West Malesia.
Fossils Fossilized fruits attributed to Torricellia has been described from the Late Paleocene to Eocene of North America and from the Eocene of England and Central Europe. Numerous additional macrofossils have been assigned to Torricelliaceae.
Habit Bisexual (Melanophylla), monoecious (Torricellia) or dioecious (Aralidium), evergreen or deciduous trees or shrubs.
Vegetative anatomy Phellogen ab initio superficial (Aralidium). Primary cork tissue in Torricellia with collenchyma. Cortical vascular bundles present in Aralidium. Endodermis absent (Melanophylla). Vessel elements with simple (Melanophylla, Torricellia) or scalariform (Aralidium, Melanophylla) perforation plates; lateral pits in Aralidium opposite or scalariform, in Melanophylla alternate or scalariform, in Torricellia alternate or opposite, simple pits. Imperforate tracheary xylem elements libriform fibres with simple (Aralidium) or bordered (Melanophylla, Torricellia) pits, septate or non-septate. Wood rays multiseriate, heterocellular. Axial parenchyma apotracheal (Melanophylla, Torricellia) or paratracheal scanty, vasicentric (Aralidium, Melanophylla). Tyloses frequent. Sieve tube plastids S type? Nodes ≥5:≥5, multilacunar with five or more leaf traces (Aralidium, Torricellia). Secretory canals absent. Medulla in Aralidium heterogeneous, with groups of sclereids. Calciumoxalate as crystal sand present in Aralidium.
Trichomes Hairs unicellular or multicellular, uniseriate (absent in Melanophylla); glandular hairs in Aralidium multicellular asymmetrical, in Torricellia tricellular or quadricellular.
Leaves Alternate (spiral), simple, entire (Melanophylla), pinnately (Aralidium) or palmately (Torricellia) lobed, with ? ptyxis. Stipules absent (Aralidium, Torricellia) or present as glandular hairs (Melanophylla); petiole sheathing at base. Ligule sometimes present. Petiole vascular bundles scattered (Aralidium). Leaf base with petiolar flange (and sometimes small adaxial flange), often enclosing branch. Venation pinnate (Aralidium, Melanophylla) or palmate (Torricellia). Stomata anomocytic (Melanophylla, Torricellia) or anisocytic (Aralidium). Cuticular wax crystalloids? Mucilage cells present. Leaf margin usually serrate and/or sinuate (sometimes entire).
Inflorescence Terminal or axillary, usually panicle (sometimes thyrsoid; Aralidium, Torricellia, Melanophylla) or raceme (Melanophylla).
Flowers Actinomorphic, small. Pedicel usually articulated (in female flowers of Torricellia inarticulated). Epigyny. Sepals three to five, with imbricate or open aestivation (Torricellia), free (Aralidium) or connate (Torricellia, Melanophylla). Petals five, with imbricate (Aralidium, Melanophylla) or induplicate-valvate (male flowers in Torricellia) aestivation, free (absent in female flowers of Torricellia). Nectaries in Melanophylla present on stout stylar discs. Disc absent in Torricellia; vascularized intrastaminal nectariferous disc present in female flowers of Aralidium as usually three (rarely four) convex stylopodia.
Androecium Stamens five, haplostemonous, antesepalous, alternipetalous. Filaments short, thin, free from each other and from tepals. Anthers basifixed (Melanophylla, Torricellia) or dorsifixed (Aralidium, Melanophylla), versatile?, tetrasporangiate, latrorse to introrse (in Torricellia latero-introrse), longicidal (dehiscing by longitudinal slits). Tapetum secretory? Female flowers in Aralidium with staminodia.
Pollen grains Microsporogenesis simultaneous? Pollen grains 3(–4)-colporate, usually shed as monads (in Melanophylla usually as tetrads), ?-cellular at dispersal. Exine tectate or semitectate, with columellate infratectum, perforate, reticulate, rugulate or smooth.
Gynoecium Pistil composed of usually three (sometimes two or four) connate carpels; transseptal vascular bundles present. Ovary inferior, trilocular (sometimes bilocular or quadrilocular; Torricellia, Melanophylla) or secondarily unilocular (pseudomonomerous, only one locule fertile in Melanophylla and Aralidium). Stylodia usually three (sometimes two or four), subulate, erect or reflexed, free (in Aralidium on stylopodia), or absent. Stigmas linear or punctate (in Torricellia bilobate, persistent), type? Male flowers in Torricellia with pistillodium.
Ovules Placentation apical (in Melanophylla axile to apical). Ovules one per carpel, anatropous, pendulous, apotropous, unitegmic, tenuinucellar. Integument ? cell layers thick (massive in Aralidium). Funicular obturator present in Torricellia. Megagametophyte monosporous, Polygonum type. Endosperm development nuclear? Endosperm haustoria? Embryogenesis?
Fruit A single-seeded drupe with one fertile locule and two to four degenerated sterile locules (often with persistent styles and stigmas). Endocarp with sclereids.
Seeds Aril present (in Torricellia formed by funicular obturator). Testa very thin (in Aralidium vascularized; in Torricellia somewhat sclerified). Exotestal cells with scalariform thickened walls, non-lignified (Melanophylla). Endotesta? Perisperm absent (Aralidium). Endosperm copious, fleshy, usually ruminate. Embryo small, well differentiated, straight (Aralidium, Melanophylla) or curved and thin (Torricellia), chlorophyll? Cotyledons two. Germination?
Cytology n = 12 (Torricellia), 20±2
DNA Mitochondrial gene rpl2 absent?
Phytochemistry Insufficiently known. Flavonols (kaempferol, rare), Route I iridoid glucosides (griselinoside in Torricellia, aralioside and verbascosides in Aralidium), ellagic acid, tannins (Torricellia), and C11 polyacetylenes with chiral centre (Torricellia) present. Route II iridoids? Proanthocyanidins not found.
Use Unknown.
Systematics Torricellia (3; T. anguita, T. intermedia, T. tiliaefolia; the Himalayas in western Nepal to Bhutan, northern Burma, China), Aralidium (1; A. pinnatifidum; southern Thailand, the Malay Peninsula, Sumatra, Borneo), Melanophylla (7; M. alnifolia, M. angustior, M. aucubifolia, M. crenata, M. madagascariensis, M. modestei, M. perrieri; Madagascar).
Torricelliaceae are sister to the clade [Griseliniaceae+[Pittosporaceae+[Araliaceae+[Myodocarpaceae+Apiaceae]]]].
Aralidium is sister to [Melanophylla+Torricellia] (Soltis & al. 2011).
Literature
Abebe D. 1989. New species of Pimpinella (Umbelliferae) from Ethiopia. – Kew Bull. 44: 341-348.
Abebe D. 1992. Systematic studies in the genus Pimpinella L. (Umbelliferae) from tropical Africa. – Bot. J. Linn. Soc. 110: 327-372.
Ackerfield A. 2001. Trichome morphology in Hedera (Araliaceae). – Edinburgh J. Bot. 58: 259-267.
Ackerfield JR, Wen J. 2002. A morphometric analysis of Hedera L. (the ivy genus, Araliaceae) and its taxonomic implications. – Adansonia 24: 197-212.
Ackerfield JR, Wen J. 2003. Evolution of Hedera (the ivy genus, Araliaceae): insights from chloroplast DNA data. – Intern. J. Plant Sci. 164: 593-602.
Adamson RS. 1951. A revision of the subgenus Solandra of Centella. – J. South Afr. Bot. 17: 1-48.
Affolter JM. 1985. A monograph of the genus Lilaeopsis (Umbelliferae). – Syst. Bot. Monogr. 6: 1-140.
Airy Shaw HK. 1972. A new species of Melanophylla Baker (Melanophyllaceae). – Kew Bull. 26: 491-493.
Ajani Y, Ajani A, Cordes JM, Watson MF, Downie SR. 2008. Phylogenetic analysis of nrDNA ITS sequences reveals relationships within five groups of Iranian Apiaceae subfamily Apioideae. – Taxon 57: 383-401.
Akalin E, Pimenov MG. 2004. Ferulago trojana (Umbelliferae), a new species from western Turkey. – Bot. J. Linn. Soc. 146: 499-504.
Al-Attar A. 1974. Studies in the systematic anatomy, embryology and morphology of the Umbelliferae tribe Caucalideae. – Ph.D. diss., University of Reading, England.
Alava R. 1972. The genus Ainsworthia (Umbelliferae). – Not. Roy. Bot. Gard. Edinb. 31: 109-118.
Alava R. 1975. A new genus of Umbelliferae (Tordyliinae) from Caucasia. – Notes Roy. Bot. Gard. Edinb. 32: 189-193.
Al-Eisawi DM. 1989. Chromosome counts of Umbelliferae of Jordan. – Ann. Bot. 47: 201-214.
Al-Eisawi DM, Jury SL. 1988. A taxonomic revision of the genus Tordylium L. (Apiaceae). – Bot. J. Linn. Soc. 97: 357-403.
Allison I, Wyk B-E van. 1997. A revision of the genus Anginon (Apiaceae). – Nord. J. Bot. 17: 561-577.
Alkhatib R, Hennebelle T, Roumy V, Sahpaz S, Suzgec S, Akalin E, Mericli AH, Bailleul F. 2009. Coumarins, caffeoyl derivatives and a monoterpenoid glycoside from Ferulago asparagifolia. – Biochem. Syst. Ecol. 37: 230-233.
Andersen TB, López C, Manczak T, Martinez K, Simonsen HT. 2015. Thapsigargin – From Thapsia L. to Mipsagargin. – Molecules 20: 6113-6127.
Andersen TB, Hansen NB, Laursen T, Weitzel C, Simonsen HT. 2016. Evolution of NADPH-cytochrome P450 oxidoreductases (POR) in Apiales – POR 1 is missing. – Mol. Phylogen. Evol. 98: 21-28.
Andersson L, Kocsis M, Eriksson R. 2006. Relationships of the genus Azorella (Apiaceae) and other hydrocotyloids inferred from sequence variation in three plastid markers. – Taxon 55: 270-280.
Arano H, Saito H. 1980. Cytological studies in the family Umbelliferae 5. Karyotypes of seven species in subtribe Seselinae. – Kromosomo, ser. II, 17: 471-480.
Arbizu C, Reitsma KR, Simon PW, Spooner DM. 2014. Morphometrics of Daucus (Apiaceae): a counterpart to a phylogenomic study. – Amer. J. Bot. 101: 2005-2016.
Arbizu C, Ruess H, Senalik D, Simon PW, Spooner DM. 2014. Phylogenomics of the carrot genus (Daucus, Apiaceae). – Amer. J. Bot. 101: 1666-1685.
Arenas Posada JA, García Martín F. 1993. Atlas carpológico y corológico de la subfamilia Apioideae Drude (Umbelliferae) en España peninsular y Baleares. – Ruizia 12: 1-245.
Avato P, Trabace G, Smitt UW. 1996. Essential oils from fruits of three types of Thapsia villosa. – Phytochemistry 43: 609-612.
Babu TD, Kuttan G, Padikkala J. 1995. Cytotoxic and antitumour properties of certain taxa of Umbelliferae with special reference to Centella asiatica (L.) Urban. – J. Ethnopharm. 48: 53-57.
Badoc A, Lamarti A. 1991. A chemotaxonomic evaluation of Anethum graveolens L. (Dill) of various origins. – J. Ess. Oil Res. 3: 269-278.
Baillon H. 1878. Recherches nouvelles sur les Araliées et sur la famille des Ombellifères en general. – Adansonia 12: 125-178.
Bakker K, Steenis CGGJ van. 1957. Pittosporaceae. – In: Steenis CGGJ van (ed), Flora Malesiana I, 5(3), Noordhoff-Kolff N. V., Djakarta, pp. 345-362.
Ball PW. 1968. A revision of the genus Huetia Boiss. – In: Heywood VH (ed), Flora Europaea. Notulae systematicae ad floram europaeam spectantes, No. 7, Feddes Repert. 79: 3-18.
Ball PW. 1979. Thaspium trifoliatum (meadow-parsnip) in Canada. – Can. Field Natur. 93: 306-307.
Banasiak L, Piwcyński M, Uliński T, Downie SR, Watson MF, Shakya B, Spalik K, Carine M. 2013. Dispersal patterns in space and time: a case study of Apiaceae subfamily Apioideae. – J. Biogeogr. 40: 1324-1335.
Banasiak Ł, Wojewódzka A, Baczyński J, Reduron J-P, Piwczyński M, Kurzyna-Młynik R, Gutaker R, Czarnocka-Cieciura A, Kosmala-Grzechnik S, Spalik K. 2016. Phylogeny of Apiaceae subtribe Daucinae and the taxonomic delineation of its genera. – Taxon 65: 563-585.
Banerjee RN. 1973. Pseudobrassaiopsis – a new genus of Araliaceae with a note on the status of Euaraliopsis Hutch. – J. Bombay Nat. Hist. Soc. 72: 71-73.
Bani B, Ulusoy F, Karakaya MA, Koch MA. 2016. Taxonomic implications from morphological and anatomical studies in the section Stenodiptera from the genus Grammosciadium (Apiaceae). – PhytoKeys 68: 73-89.
Barclay EL, Watson MF. 1998. A revision of Carum and Trachyspermum (Umbelliferae) in the Socotran Archipelago. – Kew Bull. 53: 897-907.
Barrett SCH, Helenurm K. 1981. Floral sex ratios and life history in Aralia nudicaulis (Araliaceae). – Evolution 35: 752-762.
Barrett SCH, Thomson JD. 1982. Spatial pattern, floral sex ratios, and fecundity in dioecious Aralia nudicaulis (Araliaceae). – Can. J. Bot. 60: 1662-1670.
Bartgis RL. 1997. The distribution of the endangered plant Ptilimnium nodosum (Rose) Mathias (Apiaceae) in the Potomac River drainage. – Castanea 62: 55-59.
Bate-Smith EC. 1980. The systematic position of Aralidium Miq. – a multidisciplinary study 5. A note on the phenolic constituents. – Taxon 29: 412.
Baumann MG. 1946. Umbellifloren-Studien I. Myodocarpus und die Phylogenie der Umbelliferen-Fruchte. – Ber. Schweiz. Bot. Ges. 56: 13-112.
Baumann-Bodenheim MG. 1955. Ableitung und Bau bicarpellat-monospermer und pseudomonocarpellater Araliaceen- und Umbelliferen-Früchte. – Ber. Schweiz. Bot. Ges. 65: 481-510.
Bawa KS, Keegan CR, Voss RH. 1982. Sexual dimorphism in Aralia nudicaulis L. (Araliaceae). – Evolution 36: 371-378.
Baylis GTS. 1977. Pennantia baylisiana (Oliver) Baylis comb. nov. – New Zealand J. Bot. 15: 511-512.
Baylis GTS. 1997. Pennantia baylisiana, New Zealand’s rarest tree – its discovery and propagation. – New Zealand Gard. J. 2: 12-13.
Bean AR. 1991. Notes on Astrotricha DC. (Araliaceae) in Queensland. – Austrobaileya 3: 523-528.
Beddie AD. 1958. Precocious fruiting in Pennantia corymbosa. – Wellington Bot. Soc. Bull. 30: 12-14.
Bell CR. 1971. Breeding systems and floral biology of the Umbelliferae, or evidence for specialization in unspecialized flowers. – In: Heywood VH (ed), The biology and chemistry of the Umbelliferae, Bot. J. Linn. Soc. 64 [Suppl. 1]: 93-108.
Bell CR, Constance L. 1957. Chromosome numbers in Umbelliferae. – Amer. J. Bot. 44: 565-572.
Bell CR, Constance L. 1960. Chromosome numbers in Umbelliferae II. – Amer. J. Bot. 47: 24-32.
Bell CR, Constance L. 1966. Chromosome numbers in Umbelliferae III. – Amer. J. Bot. 53: 512-520.
Berenbaum MR. 1981. Patterns of furanocoumarin distribution and insect herbivory in the Umbelliferae: plant chemistry and community structure. – Ecology 62: 1254-1266.
Berenbaum MR. 1990. Evolution of specialization in insect-umbellifer associations. – Ann. Rev. Entomol. 35: 319-343.
Berenbaum MR. 2001. Chemical mediation of coevolution: phylogenetic evidence for Apiaceae and associates. – Ann. Missouri Bot. Gard. 88: 45-59.
Bernardi L. 1969. Araliacearum Madagascariae et Comores exordium 1. Revisio et taxa nova Schefflerarum. – Candollea 24: 89-122.
Bernardi L. 1971. Araliacearum Madagascariae et Comores propositum 2. Revisio et taxa nova Polysciadim. – Candollea 26: 12-89.
Bernardi L. 1979a. Tentamen revisionis generis Ferulago. – Boissiera 30: 1-182.
Bernrdi L. 1979b. The New Caledonian genera of Araliaceae and their relationships with those of Ocenia and Indonesia. – In: Larsen K, Holm-Nielsen LB (eds), Tropical Botany, Academic Press, London, pp. 315-325.
Bernardi L. 1980. Synopsis Araliacearum Madagascariae et Comorarum Insularum (auxilio methodi “Ferulago”). – Candollea 35: 117-132.
Bidgood S, Vollesen K. 2006. A new species of Diplolophium (Apiaceae) from Ethiopia. – Kew Bull. 61: 239-242.
Bohlmann F. 1971. Acetylenic compounds in the Umbelliferae. – In: Heywood VH (ed), The biology and chemistry of the Umbelliferae, Bot. J. Linn. Soc. 64 [Suppl. 1]: 279-291.
Boissieu H de. 1906. Note sur quelques Ombellifères de la Chine, d’après les collections du Muséum d’histoire naturelle de Paris. – Bull. Soc. Bot. France 53: 418-437.
Boissieu H de. 1909. Note complementaire sur quelques Ombellifères nouvelles ou peux connues d’Extrême-Orient, d’après les collections du Muséum d’Histoire Naturelle de Paris. – Bull. Soc. Bot. France 56: 348-355.
Bone TS. 2007. A phylogenetic and biogeographic study of the umbellifer genus Lilaeopsis. – M.Sc. thesis, University of Illinois at Urbana-Champaign, Illinois.
Bone TS, Downie SR, Affolter JM, Spalik K. 2011. A phylogenetic and biogeographic study of the genus Lilaeopsis (Apiaceae tribe Oenantheae). – Syst. Bot. 36: 789-805.
Bornmüller J. 1930. Kritische Bemerkungen über einige orientalische Arten der Gattung Johrenia nebst Beschreibung zweier neuer Typen. – Feddes Repert. 28: 33-53.
Bornmüller J, Wolff H. 1921. Pimpinella cruciata spec. nov. – Feddes Repert. 17: 44.
Borre A van den, Henwood MJ. 1998. A revision of the genus Diplaspis (Mulinae-Hydrocotyloideae-Apiaceae). – Aust. Syst. Bot. 11: 1-12.
Brace LJK. 1929. Notes on the occurrence of Oxypolis filiformis in the Bahamas. – Torreya 1: 16-17.
Bremer G. 1915. Reliquiae Treubianae: 2. The development of the ovule and embryo of Pittosporum ramiflorum and Pittosporum timorense. – Ann. Jard. Bot. Buitenzorg 14: 161-164.
Briquet J. 1924. L’anatomie du fruit et le comportement des bandelettes dans le genre Heracleum. – Candollea 2: 1-62.
Brochmann C, Rustan OH, Lobin W, Kilian N, Schmidt KHA. 1997. The endemic vascular plants of the Cape Verde. Tornabenea Parl. – Sommerfeltia 24: 77-91.
Bruck M. 1988. Untersuchungen über die Morphologie und Anatomie von Peucedanum carvifolia Vill. (Apiaceae). – Bull. Soc. Natur. Luxemburg 88: 69-80.
Brullo C, Brullo S, Downie SR, Danderson CA, Giusso del Galdo G. 2013. Siculosciadium, a new monotypic genus of Apiaceae from Sicily. – Ann. Missouri Bot. Gard. 99: 1-18.
Bull-Hereñu K, Claßen-Bockhoff R. 2010. Developmental conditions for terminal flower production in apioid umbellets. – Plant Divers. Evol. 128: 221-232.
Burtt BL. 1988. A new shrubby genus of African Umbelliferae. – Notes Roy. Bot. Gard. Edinb. 45: 493-501.
Burtt BL. 1989. The adoption of Stoibrax for Tragiopsis and Brachyapium (Umbelliferae), and its N-S African disjunction. – In: Tan K (ed), The Davis and Hedge Festschrift, Edinburgh University Press, Edinburgh, pp. 143-147.
Burtt BL. 1991. Umbelliferae of southern Africa: an introduction and annotated check-list. – Edinburgh J. Bot. 48: 133-282.
Burtt BL, Davis PH. 1949. Glaucosciadium: a new Mediterranean genus of Umbelliferae. – Kew Bull. 1948: 225-230.
Burtt BL, Dickison WC. 1975. The morphology and relationships of Seemannaralia (Araliaceae). – Notes Roy. Bot. Gard. Edinb. 33: 449-464.
Busch EA. 1931. A new species of the genus Eleutherospermum C. Koch from Caucasus. – Trudy Bot. Muz. 23: 58-64. [In Russian]
Buwalda P. 1949. Umbelliferae. – In: Steenis CGGJ (ed), Flora Malesiana, I, 4(2), Noordhoff-Kolff N. V., Batavia, pp. 113-140.
Calestani V. 1905a. Conspectus specierum europaearum generis Peucedani. – Bull. Soc. Bot. Ital. Ann. 1905: 193-201.
Calestani V. 1905b. Contributo alla sistematica delle Ombrellifere d’Europa. – Webbia 1: 89-280.
Calviño CI, Downie SR. 2007. Circumscription and phylogeny of Apiaceae subfamily Saniculoideae based on chloroplast DNA sequences. – Mol. Phylogen. Evol. 44: 175-191.
Calviño CI, Tilney PM, Wyk B-E van, Downie SR. 2006. A molecular phylogenetic study of southern African Apiaceae. – Amer. J. Bot. 93: 1828-1847.
Calviño CI, Martínez SG, Downie SR. 2008a. Morphology and biogeography of Apiaceae subfamily Saniculoideae as inferred by phylogenetic analysis of molecular data. – Amer. J. Bot. 95: 196-214.
Calviño CI, Martínez SG, Downie SR. 2008b. The evolutionary history of Eryngium (Apiaceae, Saniculoideae): rapid radiations, long distance dispersals, and hybridizations. – Mol. Phylogen. Evol. 46: 1129-1150.
Calviño CI, Martínez SG, Downie SR. 2010. Unravelling the taxonomic complexity of Eryngium L. (Apiaceae, Saniculoideae): phylogenetic analysis of 11 non-coding cpDNA loci corroborates rapid radiation. – Plant Divers. Evol. 128: 137-149.
Calviño CI, Fernández M, Martinez SG. 2016. Las especies de Azorella (Azorelloideae, Apiaceae) con distribución extra-Argentina. – Darwiniana 4: 57-82.
Cannon JFM. 1967. The generic limits of Caucalis and Torilis. – In: Heywood VH (ed), Flora Europaea, Notulae Systematicae 6, Feddes Repert. 74: 36-37.
Cannon JFM. 1973. Studies in tropical African Umbelliferae I: Frommia ceratophylloides and the Diplolophium buchananii complex. – Edinburgh J. Bot. 32: 195-201.
Cannon JFM. 1978a. 90. Umbelliferae. – In: Launert E (ed), Flora Zambesiaca 4, Flora Zambesiaca Managing Committee, London, pp. 555-621.
Cannon JFM. 1978b. 91. Araliaceae. – In: Launert E (ed), Flora Zambesiaca 4, Flora Zambesiaca Managing Committee, London, pp. 621-632.
Cannon JFM, Theobald WL. 1967. Phlyctidocarpa – a new monotypic genus of the Umbelliferae from S.W. Africa. – Mitt. Bot. Staatssamml. München 6: 479-482.
Cannon MJ, Cannon FM. 1989. Central American Araliaceae – a precursory study for the Flora Mesoamericana. – Bull. Brit. Bus. (Nat. Hist.), Bot. 19: 5-61.
Carbonnier J, Fatianoff O, Molho D. 1982. Phytochimie comparée des taxons rattachés à la tribu des Peucedaneae (Umbelliferae-Apioideae). – In: Cauwet-Marc A-M, Carbonnier J (eds), Les Ombellifères. Actes de 2ème Symp. Int. Ombellifères, Monogr. Syst. Bot. Missouri Bot. Gard. 6: 387-513.
Carlquist SJ. 1981. Wood anatomy of the Pittosporaceae. – Allertonia 2: 355-392.
Carlquist SJ. 1982. Wood anatomy of Daphniphyllaceae: ecological and phylogenetic considerations, review of pittosporalean families. – Brittonia 34: 252-266.
Carolin RC, Bittrich V. 2018. Pittosporaceae. – In: Kadereit JW, Bittrich V (eds). 2018. The families and genera of vascular plants XV. Flowering plants – eudicots – Apiales, Gentianales (except Rubiaceae). Springer, Berlin, pp. 539-547.
Castro O, Cennamo P, Luca P. 2009. Analysis of the genus Petagnaea Caruel (Apiaceae), using new molecular and literature data. – Plant Syst. Evol. 278: 239-249.
Cauwet A-M. 1967a. Contribution à l’étude caryologique de quelques ombellifères d’Espagne. – Natur. Monspel. Sér. Bot. 18: 201-210.
Cauwet A-M. 1967b. Contribution à l’étude caryosystématique du genre Bupleurum L. I. – Bull. Soc. Bot. France 114: 371-386.
Cauwet A-M. 1969. Contribution à l’étude caryosystématique du genre Bupleurum L. II. – Bull. Soc. Bot. France 116: 19-28.
Cauwet-Marc A-M. 1976. Biosystématique des espèces vivaces de Bupleurum L. (Umbelliferae) du Bassin Méditerranéen occidental. – Ph.D. diss., Perpignan, France.
Cauwet-Marc A-M, Carbonnier J. 1982. Les Ombellifères. Actes du 2e Symposium International sur les Ombellifères “Contributions Pluridisciplinaires à la Systématique”. – Monogr. Syst. Bot. Missouri Bot. Gard. 6, Braun-Brumfield, Ann Arbor, Michigan.
Cauwet-Marc A-M, Carbonnier J, Cerceau-Larrival M-T, Dodin R, Guyot M. 1978. Contribution à l’étude multidisciplinaire du genre Bupleurum L. – In: Cauwet-Marc A-M, Carbonnier J (eds), Actes du 2e Symp. Intern. Ombellif., Centre Univ. Perpignan.
Cauwet-Marc A-M, Carbonnier J, Farille M. 1982. Contribution à l’étude caryologique des Ombellifères de Nepal I. – Candollea 35: 497-510.
Cayzer LW, Crisp MD. 2004. Reinstatement and revision of the genus Marianthus (Pittosporaceae). – Aust. Syst. Bot. 17: 127-144.
Cayzer LW, Crisp MD, Telford IRH. 1999a. Bursaria (Pittosporaceae): a morphometric analysis and revision. – Aust. Syst. Bot. 12: 117-143.
Cayzer LW, Crisp MD, Telford IRH. 1999b. Revision of Rhytidosporum (Pittosporaceae). – Aust. Syst. Bot. 12: 689-708.
Cayzer LW, Crisp MD, Telford IRH. 2000a. Revision of Pittosporum (Pittosporaceae) in Australia. – Aust. Syst. Bot. 13: 845-902.
Cayzer LW, Crisp MD, Telford IRH. 2000b. Auranticarpa, a new genus of Pittosporaceae from northern Australia. – Aust. Syst. Bot. 13: 903-917.
Cayzer LW, Crisp MD, Telford IRH. 2004. Cladistic analysis and revision of Billardiera (Pittosporaceae). – Aust. Syst. Bot. 17: 83-125.
Cayzer LW, Crisp MD, Donaldson S. 2007. Cheiranthera (Pittosporaceae). – Aust. Syst. Bot. 20: 340-354.
Cerceau-Larrival M-T. 1959. Clé de determination d’Ombellifères de France et d’Afrique du Nord d’après leurs grains de pollen. – Pollen Spores 1: 145-190.
Cerceau-Larrival M-T. 1962. Plantules et pollens d’ombellifères. Leur intérêt systématique et phylogénétique. – Thèse, Mém. Mus. Natl. Hist. Nat. Paris, sér. B, Bot. 14: 1-166.
Cerceau-Larrival M-T. 1963. Le pollen d’Ombellifères Méditerranéennes II. Tordylinae Drude. – Pollen Spores 5: 297-323.
Cerceau-Larrival M-T. 1965a. Le pollen d’Ombellifères Méditerranéennes III. Scandicineae Drude; IV. Dauceae Drude. – Pollen Spores 7: 35-62.
Cerceau-Larrival M-T. 1965b. Involucre et involucelle chez les Ombellifères. – Bull. Soc. Bot. France 112: 252-267.
Cerceau-Larrival M-T. 1967. Corrélations de caractères chez les grains de pollen d’Ombellifères. – Rev. Palaeobot. Palynol. 4: 311-324.
Cerceau-Larrival M-T. 1971. Morphologie pollinique et correlations phylogénétiques chez les Ombellifères. –In: Heywood VH (ed), The biology and chemistry of the Umbelliferae, Bot. J. Linn. Soc. 64 [Suppl. 1]: 109-135.
Cerceau-Larrival M-T. 1979. Intérêt de l’ontogénie pour la classification évolutive d’une famille: série foliaire des Ombellifères. – Bull. Bot. Soc. France 126, Actual. Bot. 3: 39-53.
Chandler GT, Plunkett GM. 2004. Evolution in Apiales: nuclear and chloroplast markers together in (almost) perfect harmony. – Bot. J. Linn. Soc. 144: 123-147.
Chandler GT, Plunkett GM, Pinney SM, Cayzer LW, Gemmill CEC. 2007. Molecular and morphological agreement in Pittosporaceae: phylogenetic analysis with nuclear ITS and plastid trnL-trnF sequence data. – Aust. Syst. Bot. 20: 390-401.
Chang H-T. 1975. Revision of Notopterygium (Umbelliferae). – Acta Phytotaxon. Sin. 13: 83-87.
Choi H-K, Kim Y, Sun B-Y, Shin H. 1998. Phylogeny of Dystenia in subfamily Apioideae (family Apiaceae) based on ITS sequences. – Korean J. Plant Taxon. 28: 139-149.
Choi H-K, Kim C, Shin H. 2000. Molecular re-examination of Korean Umbelliferae based on internal transcribed spacer sequences of rDNA: Ligusticum tenuissimum (Nakai) Katagawa and Libanotis coreana (Wolff) Kitagawa. – J. Plant Biol. 43: 128-135.
Chu X-F, Liu Q-X. 2007. Morphological features and anatomical structures of Angelica acutiloba mericarp in Apiaceae. – J. Plant Res. Envir. 16: 53-55.
Chung KF. 2007. Inclusion of the South Pacific alpine genus Oreomyrrhis (Apiaceae) in Chaerophyllum based on nuclear and chloroplast DNA sequences. – Syst. Bot. 32: 671-681.
Chung KF, Ping C-I, Downie SR, Spalik K, Schaal BA. 2005. Molecular systematics of the trans-Pacific genus Oreomyrrhis (Apiaceae): phylogenetic affinities and biogeographic implications. – Amer. J. Bot. 92: 2054-2071.
Constance L. 1971. History of the classification of Umbelliferae (Apiaceae). – In: Heywood VH (ed), The biology and chemistry of the Umbelliferae, Bot. J. Linn. Soc. 64 [Suppl. 1]: 1-11.
Constance L. 1980. Four new species of Eryngium (Umbelliferae) from Mexico. – Brittonia 32: 118-127.
Constance L. 1987. Neogeozia (Apiaceae), a very distinct and elegant genus of Mexican Umbelliferae. – Opera Bot. 92: 59-71.
Constance L. 1990. Tardy transfers from Apium to Ciclospermum (Umbelliferae). – Brittonia 42: 276-278.
Constance L. 1997. An instance of East-West confusion in Chinese Umbelliferae, or Arracacia out of Asia! – Edinburgh J. Bot. 54: 99-104.
Constance L, Affolter JM. 1987. Aa trio of new tauschias (Umbelliferae) from eastern Mexico. – Syst. Bot. 12: 286-292.
Constance L, Affolter JM. 1997. A pair of species of Tauschia Schltdl. (Umbelliferae/Apiaceae) from Mexico. – Brittonia 49: 458-462.
Constance L, Cannon JMF. 1967. Naufraga – a new genus of Umbelliferae from Mallorca. – Feddes Repert. 74: 1-4.
Constance L, Chuang T-I. 1982. Chomosome numbers of Umbelliferae (Apiaceae) from Africa south of the Sahara. – Bot. J. Linn. Soc. 85: 195-208.
Constance L, Hitchcock CL. 1954. Mathiasella, a new genus of North American Umbelliferae. – Amer. J. Bot. 41: 56-58.
Constance L, Shan RH. 1948. The genus Osmorhiza (Umbelliferae), a study in geographic affinities. – Univ. Calif. Publ. Bot. 23: 111-156.
Constance L, Chuang T-I, Bell CR. 1971. Chromosome numbers in Umbelliferae IV. – Amer. J. Bot. 58: 577-587.
Constance L, Chuang T-I, Bell CR. 1976. Chromosome numbers in Umbelliferae V. – Amer. J. Bot. 63: 608-625.
Coode MJE. 1976. Notes on Pittosporaceae and Myrsinaceae of the Mascarenes. – Kew Bull. 31: 221-225.
Cooper RC. 1956. The Australian and New Zealand species of Pittosporum. – Ann. Missouri Bot. Gard. 43: 87-187.
Cooperrider TS. 1985. Thaspium and Zizia (Umbelliferae) in Ohio. – Castanea 50: 116-119.
Cordes JM. 2009. A systematic study of poison hemlock (Conium, Apiaceae). – M.Sc. thesis, University of Illinois at Urbana-Champaign, Urbana, Illinois.
Costello A. 2002. Molecular and morphological systematics of the Tetraplasandra group (Araliaceae) and the development of the superior ovary in Tetraplasandra. – Ph.D. diss., New York University, New York.
Costello A, Motley TJ. 2001. Molecular systematics of Tetraplasandra, Munroidendron, and Reynoldsia sandwicensis (Araliaceae) and the evolution of superior ovaries in Tetraplasandra. – Edinburgh J. Bot. 58: 229-242.
Costello A, Motley TJ. 2004. The development of the superior ovary in Tetraplasandra (Araliaceae). – Amer. J. Bot. 91: 644-655.
Costello A, Motley TJ. 2007. Phylogenetics of the Tetraplasandra group (Araliaceae) inferred from ITS, 5S-NTS, and morphology. – Syst. Bot. 32: 464-477.
Costion CM, Plunkett G. 2016. A revision of the genus Osmoxylon (Araliaceae) in Palau, including two new species. – PhytoKeys 58: 49-64.
Coulter JM, Rose JN. 1887. Notes on Umbelliferae of the E. United States III. – Bot. Gaz. (Chicago) 12: 73-76.
Coulter JM, Rose JN. 1888. Revision of North American Umbelliferae. – Wabash College, Crawfordsville, Indiana.
Coulter JM, Rose JN. 1900. Monograph of the North American Umbelliferae. – Contr. U.S. Natl. Herb. 7: 9-256.
Coulter JM, Rose JN. 1909. Supplement to the monograph of the North American Umbelliferae. – Contr. U.S. Natl. Herb. 12: 441-451.
Crawford DJ, Hartman RL. 1972. Chromosome numbers and taxonomic notes for Rocky Mountain Umbelliferae. – Amer. J. Bot. 59: 386-392.
Crisp MD, Taylor JM. 1990. A new species of Bentleya E. Bennett (Pittosporaceae) from southern Western Australia. – Bot. J. Linn.Soc. 103: 309-315.
Cronquist A. 1982. Reduction of Pseudotaenidia to Taenidia (Apiaceae). – Brittonia 34: 365-367.
Crowden RK, Harborne JB, Heywood VH. 1969. Chemotaxonomics of the Umbelliferae – a general survey. – Phytochemistry 8: 1963-1984.
Cufodontis G. 1960. 19. Pittosporaceae. – In: Exell AW, Wild H (eds), Flora Zambesiaca 1 (Part 1), Crown Agents for Oversea Governments and Administrations, London, pp. 298-303.
Cufodontis G. 1966. Pittosporaceae. – In: Milne-Redhead E, Polhill RM (eds), Flora of tropical East Africa, Crown Agents for Oversea Governments and Administrations, London, pp. 1-14.
Danderson CA. 2011. A phylogenetic study of the Arracacia clade (Apiaceae). – Ph.D. diss., University of Illinois at Urbana-Champaign, Urbana, Illinois.
Danderson CA, Downie SR, Hermann M. 2018. Rampant polyphyly in the Arracacia clade (Apiaceae) and an assessment of the phylogenetic utility of 20 noncoding plastid loci. – Mol. Phylogen. Evol. 118: 286-305.
Dawson JW. 1971. Relationships of the New Zealand Umbelliferae. – In: Heywood VH (ed), The biology and chemistry of the Umbelliferae, Supplement 1, Bot. J. Linn. Soc. 64: 43-62.
Dawson JW, Webb CJ. 1982. Generic problems in Australasian Apioideae (Umbelliferae). – In: Cauwet-Marc A-M, Carbonnier J (eds), Les Ombellifères, Actes du 2e Symposium International sur les Ombellifères “Contributions Pluridisciplinaires à la Systématique”, Monogr. Syst. Bot. Missouri Bot. Gard. 6: 21-32.
Day J. 1998. Architecture of juvenile Pennantia corymbosa, a divaricate shrub from New Zealand. – New Zealand J. Bot. 36: 141-148.
Debonte LR, Matthews BF, Wilson KG. 1984. Variation of plastid and mitochondrial DNAs in the genus Daucus. – Amer. J. Bot. 71: 932-940.
Dechamps R. 1977. Comparaison anatomique d’une espèce fossile arborescente d’Afrique à Steganotaenia araliacea (Ombellifère). – Bull Jard. Bot. Natl. Belg. 47: 473-482.
Degtjareva GV, Kljukov EV, Samigullin TH, Valiejo-Roman CM, Pimenov MG. 2009. Molecular appraisal of Bunium and some related arid and subarid geophilic Apiaceae-Apioideae taxa of the ancient Mediterranean. – Bot. J. Linn. Soc. 160: 149-170.
Degtjareva GV, Logacheva MD, Samigullin TH, Terentieva EI, Valiejo-Roman CM. 2012. Organization of chloroplast psbA-trnH intergenic spacer in dicotyledonous angiosperms of the family Umbelliferae. – Biochemistry (Moscow) 77: 1056-1064.
Degtjareva GV, Kljuykov EV, Samigullin TH, Valiejo-Roman CM, Pimenov MG. 2013. ITS phylogeny of Middle Asian geophilic Umbelliferae-Apioideae genera with comments on their morphology and utility of psbA-trnH sequences. – Plant Syst. Evol. 299: 985-1010.
De Leonardis W, De Santis C, Ferrauto G, Fichera G, Zizza A. 2008. Chiave palinologica di identificazione di tre generi appartenenti alla famiglia delle Apiacee. – Boll. Accad. Gioenia Sci. Nat. 41: 81-90.
Dilcher DL, Dolph GE. 1970. Fossil leaves of Dendropanax from Eocene sediments of southeastern North America. – Amer. J. Bot. 57: 153-160.
Dillon MO. 2018. Griseliniaceae. – In: Kadereit JW, Bittrich V (eds). 2018. The families and genera of vascular plants XV. Flowering plants – eudicots – Apiales, Gentianales (except Rubiaceae). Springer, Berlin, pp. 505-509.
Dillon MO, Muñoz-Schick M. 1993. A revision of the dioecious genus Griselinia (Griseliniaceae), including a new species from the coastal Atacama Desert of northern Chile. – Brittonia 45: 261-274.
Dirmenci T. 2008. A new species of Pastinaca L. (Apiaceae) from Turkey. – Bot. J. Linn. Soc. 158: 296-300.
Doğru-Koca A. 2016. Phylogeny of the genus Tordylium (Tordylineae, Apioideae, Apiaceae) inferred from morphological data. – Nord. J. Bot. 34: 111-119.
Domin K. 1908. Über eine neue austral-antarktische Umbelliferen-Gattung. – Bot. Jahrb. 40: 573-585.
Downie SR, Jansen RK. 2015. A comparative analysis of whole plastid genomes from the Apiales: expansion and contraction of the inverted repeat, mitochondrial to plastid transfer of DNA, and identification of highly divergent noncoding regions. – Syst. Bot. 40: 336-351.
Downie SR, Katz-Downie DS. 1996. A molecular phylogeny of Apiaceae subfamily Apioideae: evidence from nuclear ribosomal DNA internal transcribed spacer sequences. – Amer. J. Bot. 83: 234-251.
Downie SR, Katz-Downie DS. 1999. Phylogenetic analysis of chloroplast rps16 intron sequences reveals relationships within the woody southern African Apiaceae subfamily Apioideae. – Can. J. Bot. 77: 1120-1135.
Downie SR, Katz-Downie DS, Cho K-J. 1996. Phylogenetic analysis of Apiaceae subfamily Apioideae using nucleotide sequences from the chloroplast rpoC1 intron. – Mol. Phylogen. Evol. 6: 1-18.
Downie SR, Ramanath S, Katz-Downie DS, Llanas E. 1998. Molecular systematics of Apiaceae subfamily Apioideae: phylogenetic analyses of nuclear ribosomal DNA internal transcribed spacer and plastid rpoC1 intron sequences. – Amer. J. Bot. 85: 563-591.
Downie SR, Watson MF, Spalik K, Katz-Downie DS. 1999 [2000]. Molecular systematics of Old World Apioideae (Apiaceae): relationships among some members of tribe Peucedaneae sensu lato, the placement of several island-endemic species, and resolution within the apioid superclade. – Can. J. Bot. 78: 506-528.
Downie SR, Katz-Downie DS, Spalik K. 2000. A phylogeny of Apiaceae tribe Scandiceae: evidence from nuclear ribosomal DNA internal transcribed spacer sequences. – Amer. J. Bot. 87: 76-95.
Downie SR, Katz-Downie DS, Watson MF. 2000. A phylogeny of the flowering plant family Apiaceae based on chloroplast DNA rpl16 and rpoC1 intron sequences: towards a suprageneric classification of subfamily Apioideae. – Amer. J. Bot. 87: 273-292.
Downie SR, Plunkett GM, Watson MF, Spalik K, Katz-Downie DS, Valiejo-Roman CM, Terentieva EI, Troitsky AV, Lee B-Y, Lahham J, El-Oqlah A. 2001. Tribes and clades within Apiaceae subfamily Apioideae: the contribution of molecular data. – Edinburgh J. Bot. 58: 301-330.
Downie SR, Hartman RL, Sun F-J, Katz-Downie DS. 2002. Polyphyly of the spring-parsleys (Cymopterus): molecular and morphological evidence suggests complex relationships among the perennial endemic genera of western North American Apiaceae. – Can. J. Bot. 80: 1295-1324.
Downie SR, Sun F-J, Katz-Downie DS, Colletti GJ. 2004. A phylogenetic study of Perideridia (Apiaceae) based on nuclear ribosomal DNA ITS sequences. – Syst. Bot. 29: 737-751.
Downie SR, Katz-Downie DS, Sun F-J, Lee C-S. 2008. Phylogeny and biogeography of Apiaceae tribe Oenantheae inferred from nuclear rDNA ITS and cpDNA psbI-5’trnK(UUU) sequences, with emphasis on the North American endemics clade. – Botany 86: 1039-1064.
Downie SR, Spalik K, Katz-Downie DS, Reduron J-P. 2010. Major clades within Apiaceae subfamily Apioideae as inferred by phylogenetic analysis of nrDNA ITS sequences. – Plant Divers. Evol. 128: 111-136.
Drew DP, Dueholm B, Weitzel C, Zhang Y, Sensen C, Simonsen HT. 2013. Transcriptome analysis of Thapsia laciniata Rouy provides insights into terpenoid biosynthesis and diversity in Apiaceae. – Intern. J. Mol. Sci. 14: 9080-9098.
Drude O. 1898. Umbelliferae (Apiaceae, Doldengewächse). – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(8), W. Engelmann, Leipzig, pp. 63-250.
Ducamp L. 1902. Recherches sur l’embryogénie des Araliacées. – Ann. Sci. Nat., Bot. 15: 311-402.
Duman H, Sagiroglu M. 2005. A new species of Ferula (Apiaceae) from South Anatolia, Turkey. – Bot. J. Linn. Soc. 147: 357-361.
Duman H, Watson MF. 1999. Ekimia, a new genus of Umbelliferae, and two new taxa of Prangos Lindl. (Umbelliferae) from Southern Turkey. – Edinburgh J. Bot. 56: 199-209.
Dümmer R. 1913. A revision of the genus Alepidea. – Trans. Roy. Soc. South Afr. 3: 1-20.
Duran A, Duman H. 1999. Two new species of Umbelliferae from southern Turkey. – Edinburgh J. Bot. 56: 47-53.
Durrieu G. 1982. Les champignons parasites et leur apport à la systématique des Ombellifères. – In: Cauwet-Marc A-M, Carbonnier J (eds), Les Ombellifères “Contributions pluridisciplinaires à la systématique”, Actes du 2ème Symposium International sur les Ombellifères, Monogr. Syst. Bot. Missouri Bot. Gard. 6, Braun-Brumfield, Ann Arbor, Michigan, pp. 525-533.
Easterly NW. 1957a. A morphological study of the genus Ptilimnium. – Ph.D. diss., University of West Virginia, Morgantown, West Virginia.
Easterly NW. 1957b. A morphological study of Ptilimnium. – Brittonia 9: 136-145.
Eibl J, Plunkett GM, Lowry II PP. 2001. Evolution of Polyscias sect. Tieghemopanax (Araliaceae) based on nuclear and chloroplast DNA sequence data. – Adansonia, sér. III, 23: 23-48.
El Alaoui-Faris FE. 2005. Étude comparative de quelques espèces marocaines rattachées au genre Carum (Apiaceae). – Flora Medit. 15: 599-609.
El-Eisawi D, Jury SL. 1988. A taxonomic revision of the genus Tordylium L. (Apiaceae). – Bot. J. Linn. Soc. 97: 357-403.
El-Gamal AA. 2001. Sesquiterpene lactones from Smyrnium olusatrum. – Phytochemistry 57: 1197-1200.
El-Moghazi AM, Ross SA, Halim AF, Abou-Rayya A. 1980. Flavonoids of Daucus carota. – Planta Medica 40: 382-385.
Engler A. 1896. Icacinaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(5), W. Engelmann, Leipzig, pp. 233-257.
Engstrand L. 1970a. Studies in the Aegean flora XVIII. Notes and chromosome numbers in Aegean Umbelliferae. – Bot. Not. 123: 384-393.
Engstrand L. 1970b. The European species of Scaligeria (Umbelliferae). – Bot. Not. 123: 505-511.
Engstrand L. 1973. Generic delimitation of Bunium, Conopodium and Geocaryum (Umbelliferae). – Bot. Not. 126: 146-154.
Engstrand L. 1977. Biosystematics and taxonomy in Geocaryum Cosson (Umbelliferae). – Ph.D. diss., University of Lund, Sweden.
Erbar C, Leins P. 1985. Studien zur Organsequenz in Apiaceen-Blüten. – Bot. Jahrb. Syst. 105: 379-400.
Erbar C, Leins P. 1988. Blütenentwicklungsgeschichtliche Studien an Aralia und Hedera (Araliaceae). – Flora 180: 391-406.
Erbar C, Leins P. 1995. An analysis of the early floral development of Pittosporum tobira (Thunb.) Aiton and some remarks on the systematic position of the family Pittosporaceae. – Feddes Rep. 106: 463-473.
Erbar C, Leins P. 1997. Different patterns of floral development in whorled flowers, exemplified by Apiaceae and Brassicaceae. – Intern. J. Plant Sci. 158(Suppl.): S49-S64.
Erbar C, Leins P. 2004. Sympetaly in Apiales (Apiaceae, Araliaceae, Pittosporaceae). – South Afr. J. Bot. 70: 458-467.
Erbar C, Leins P. 2010. Nectaries in Apiales and related groups. – Plant Divers. Evol. 128: 269-295.
Evert EF, Constance L. 1982. Shoshonea pulvinata, a new genus and species of Umbelliferae from Wyoming. – Syst. Bot. 7: 471-475.
Eyde RH, Tseng CC. 1969. Flower of Tetraplasandra gymnocarpa. Hypogyny with epigynous ancestry. – Science 166: 506-508.
Eyde RH, Tseng CC. 1971. What is the primitive floral structure of Araliaceae? – J. Arnold Arbor. 52: 205-239.
Fairbairn JW. 1971. The alkaloids of hemlock (Conium maculatum L.) (or Conium maculatum L.: the odd man out). – In: Heywood VH (ed), The biology and chemistry of the Umbelliferae, Bot. J. Linn. Soc. 64 [Suppl. 1]: 361-368.
Fairbrothers DE. 1980. The systematic position of Aralidium Miq. – a multidisciplinary study 6. Comparative serological investigations. – Taxon 29: 412-416.
Farille MA. 1984. Apiaceae himalayenses II. – Rev. Gén. Bot. 91: 27-34.
Farille MA, Cauwet-Marc A-M, Malla SB. 1985. Apiaceae himalayenses III. – Candollea 40: 509-562.
Feist MAE. 2009. Clarifications concerning the nomenclature and taxonomy of Oxypolis ternata (Apiaceae). – J. Bot. Res. Inst. Texas 3: 661-666.
Feist MAE. 2010. The reinstatement of Ptilimnium texense (Apiaceae) and a new key to the genus. – J. Bot. Res. Inst. Texas 4: 641-651.
Feist MAE, Downie SR. 2008. A phylogenetic study of Oxypolis and Ptilimnium (Apiaceae) based on nuclear rDNA ITS sequences. – Syst. Bot. 33: 447-458.
Feist MAE, Downie SR, Magee AR, Liu MR. 2012. Revised generic delimitations for Oxypolis and Ptilimnium (Apiaceae) based on leaf morphology, comparative fruit anatomy, and phylogenetic analysis of nuclear rDNA ITS and cpDNA trnQ-trnK intergenic spacer sequence data. – Taxon 61: 402-418.
Feng T, Downie SR, Yu Y, Zhang X-M, Chen W-W, He X-J, Liu S. 2009. Molecular systematics of Angelica and allied genera (Apiaceae) from the Hengduan Mountains of China based on nrDNA ITS sequences: phylogenetic affinities and biogeographic implications. – J. Plant Res. 122: 403-414.
Ferguson IK, Hideux MJ. 1978 [1980]. Some aspects of the pollen morphology and its systematic significance in Cornaceae sens. lat. – 4th Intern. Palynol. Conf., Lucknow, 1976-1977, 1: 240-249.
Fernándes F, Carvalho JA. 2014. An historical review and new taxa in the Madeiran endemic genus Monizia (Apiaceae, Apioideae). – Webbia 69: 13-37.
Fernández M, Ezcurra C, Calviño CI. 2016.
Morphology, fruit anatomy and taxonomy of the South Andean genus
Laretia (Azorelloideae, Apiaceae). – Syst. Bot. 41: 807-812.
Fernández-Mazuecos M, Jiménez-Mejías P,
Rotllan-Puig X, Vargas P. 2014. Narrow endemics to Mediterranean islands:
moderate genetic diversity but narrow climatic niche of the ancient, critically
endangered Naufraga (Apiaceae). – Persp. Plant Ecol. Evol.
Syst. 16: 190-202.
Fernández-Prieto JA, Cires E. 2014. Phylogenetic placement of Dethawia, Meum, and Rivasmartinezia (Apioideae, Apiaceae): evidence from nuclear and plastid DNA sequences. – Plant Biosyst. 148: 975-987.
Fiaschi P. 2004. Schefflera aurata, a new species of Araliaceae from southern Bahia, Brazil. – Brittonia 56: 357-360.
Fiaschi P. 2005a. Three new species of Dendropanax (Araliaceae) from Bahia, Brazil. – Brittonia 57: 240-247.
Fiaschi P. 2005b. Four new species of Schefflera (Araliaceae) from Espirito Santo State, Brazil. – Kew Bull. 60: 77-85.
Fiaschi P, Jung-Mendaçolli SL. 2006. Three new species of Dendropanax Decne. & Planch. (Araliaceae) from São Paulo state, Brazil. – Candollea 61: 457-466.
Fiaschi P, Plunkett GM. 2011. Monophyly and phylogenetic relationships of Neotropical Schefflera (Araliaceae) based on plastid and nuclear markers. – Syst. Bot. 36: 806-817.
Fiaschi P, Plunkett GM. 2018. Revision of the Didymopanax group of Neotropical Schefflera (Araliaceae). – Ann. Missouri Bot. Gard. 103: 24-105.
Fiaschi P, Frodin DG, Plunkett GM. 2008. Four new species of the Didymopanax group of Schefflera (Araliaceae) from the Brazilian Amazon. – Brittonia 60: 274-286.
Fiaschi P, Santos F de Assis R dos, Westbrook E, Plunkett GM. 2010. Taxonomic significance of pollen morphology in Neotropical Schefflera (Araliaceae). – Plant Divers. Evol. 128: 297-323.
Flanagan LB, Moser W. 1985. Flowering phenology, floral display and reproductive success in dioecious Aralia nudicaulis L. (Araliaceae). – Oecologia 68: 23-28.
Franchet MA. 1894. Notes sur quelques Ombellifères du Yunnan. – Bull. Soc. Philom. Paris, sér. (6): 106-146.
Franchet MA. 1896. Araliaceae, Cornaceae et Caprifoliaceae novae e flora sinensi. – J. Bot. (Morot) 10: 301-308.
French DH. 1971. Ethnobotany of the Umbelliferae. – In: Heywood VH (ed), The biology and chemistry of the Umbelliferae, Bot. J. Linn. Soc. 64 [Suppl. 1]: 385-412.
Frey R. 1989. Taxonomische Revision der Gattung Peucedanum: Sektion Peucedanum und Sektion Palimbioidea (Umbelliferae). – Candollea 44: 257-327.
Fridlender A, Boisselier-Dubayle M-C. 2000. Comparison of thegenetic diversity (RAPD) of ex situ collections and natural populations of Naufraga balearica Constance & Cannon. – Compt. Rend. Acad. Sci., sér. III, Sci. Vie 323: 399-406.
Friis I. 1987. A reconsideration of Pittosporum in Africa and Arabia. – Kew Bull. 42: 319-335.
Frodin DG. 1970. The complex of Cephaloschefflera in Schefflera (Araliaceae). – PhD. Thesis, University of Cambridge, Cambridge, Massachusetts.
Frodin DG. 1975. Studies in Schefflera (Araliaceae): the Cephaloschefflera complex. – J. Arnold Arbor. 56: 427-448.
Frodin DG. 1982. Systematics of Araliaceae and inflorescence morphology. – Aust. Syst. Bot. Soc. Newsl. 30: 43-55.
Frodin DG. 1986. Studies in Schefflera (Araliaceae) II. Northern Luzon (Philippines) species of the Heptapleurum group. – Proc. Acad. Nat. Sci. Philadelphia 138: 403-425.
Frodin DG. 1989. Studies in Schefflera (Araliaceae) IV. Synopsis of the Formenkreis comprised of Didymopanax attenuatus (Sw.) El. Marchal and allied species, with nomenclatural changes. – Proc. Acad. Nat. Sci. Philadelphia 141: 313-319.
Frodin DG. 1990. Identity of Aralia bastardiana Decaisne. – Pacific Sci. 44: 265-276.
Frodin DG. 1993. Studies in Schefflera (Araliaceae) VI. New species and subordinate taxa in the Venezuelan Guayana and immediately adjacent areas. – Novon 3: 367-403.
Frodin DG. 1995. Neotropical montane Araliaceae: an overview. – In: Churchill SP, Balslev H, Forero E, Luteyn JL (eds), Biodiversity and conservation of neotropical montane forests, New York Botanical Garden, Bronx, New York, pp. 421-430.
Frodin DG, Govaerts R. 2003. World checklist and bibliography of Araliaceae. – Royal Botanic Gardens, Kew, United Kingdom.
Frodin DG, Lowry II PP, Plunkett GM. 2010. Schefflera (Araliaceae): taxonomic history, overview and progress. – Plant Div. Evol. 128: 561-595.
Froebe HA. 1964. Die Blütenstände der Saniculoideen (Umbelliferae). Eine vergleichend-morphologische und entwicklungsgeschichtliche Untersuchung. – Beitr. Biol. Pflanzen 40: 325-388.
Froebe HA. 1971a. Wuchsform und Infloreszenzgestaltung in den Gattungen Sanicula, Hacquetia und Astrantia (Umbelliferae). – Bot. Jahrb. Syst. 91: 1-38.
Froebe HA. 1971b. Inflorescence structure and evolution in Umbelliferae. – In: Heywood VH (ed), The biology and chemistry of the Umbelliferae, Supplement 1, Bot. J. Linn. Soc. 64: 31-41.
Froebe HA. 1979. Die Infloreszenzen der Hydrocotyloideen (Apiaceae). – Trop. Subtrop. Pflanzenwelt 29: 501-679.
Froebe HA. 1980. Randmusterbildung und Synorganisation bei strahlenden Apiaceendolden. – Plant Syst. Evol. 133: 223-237.
Froebe HA, Ulbrich G. 1978. Pseudanthien bei Umbelliferen. – Beitr. Biol. Pfl. 54: 175-206.
García Martín F, Silvestre S. 1985. Revisión de los géneros Elaeoselinum Koch ex DC., Margotia Boiss. y Distichoselinum García Martín & Silvestre (Umbelliferae). – Lagascalia 13: 205-237.
Gardé A, Malheiros-Gardé N. 1949. Contribuïção para o estudo cariológico da familia Umbelliferae I. – Agron. Lusit. 11: 99-140.
Gardé A, Malheiro-Gardé N. 1954. Contribuïção para o estudo cariológico da familia Umbelliferae III. – Brotéria, Ser. Ci. 23: 5-35.
Gardner RO, Lange PJ de. 2002. Revision of Pennantia (Icacinaceae), a small isolated genus of Southern Hemisphere trees. – J. Roy. Soc. New Zealand 32: 669-695.
Gawlowska M. 1967. Pimpinella nigra Willd. in Poland III. Numbers of chromosomes in Pimpinella nigra Willd. and related species. – Diss. Pharm. 19: 439-450.
Gemmill CEC, Allan GJ, Wagner WL, Zimmer EA. 2001. Evolution of insular Pacific Pittosporum (Pittosporaceae): origin of the Hawaiian radiation. – Mol. Phylogen. Evol. 22: 31-42.
George EE, Mansfield DH, Smith JF, Hartman
RL, Downie SR, Hinchliff CE. 2014. Phylogenetic analysis reveals multiple cases
of morphological parallelism and taxonomic polyphyly in Lomatium (Apiaceae). – Syst. Bot. 39: 662-675.
Gianguzzi L, La Mantia A, Lo Presti RM. 2004. Distribuzione, ecologia e status conservative delle stazioni di Petagnaea gussonei (Sprengel) Rauschert (Apiaceae) nell’area dei Monti Nebrodi (Sicilia nord-orientale). – Naturalista Sicil. 28: 205-242.
Gilmartin AJ, Simmons KS. 1987. Relationships of Lomatium among other genera of Apiaceae. – Plant Syst. Evol. 157: 95-103.
Gonzalez AG, Galindo A. 1982. Lactonas sesquiterpenicas en Umbeliferas. – In: Cauwet-Marc A-M, Carbonnier J (eds), Les Ombellifères. Actes du 2e Symp. Int. Ombellifères, Monogr. Syst. Bot. Missouri Bot. Gard. 6: 365-377.
Goodrich S. 1986. Utah flora: Apiaceae (Umbelliferae). – Great Basin Natur. 46: 66-106.
Gopinath DM. 1944. Gametogenesis and embryogenesis in a few members of the Araliaceae. – Proc. Indian Acad. Sci., Sect. B, 20: 239-309.
Grintzesco J. 1910. Monographie du genre Astrantia. – Ann. Cons. Jard. Bot. Genève: 66-194.
Gros JP. 1965. Contribution à l’étude cytotaxinomique des Pittosporacées. – Mém. Mus. Natl. Hist. Nat. Paris, sér. B, Botanique 16: 61-90.
Grosso C, Teixeira G, Gomes I, Martins ES, Barroso JG, Pedro LG, Figueiredo AC. 2009. Assessment of the essential oil composition of Tornabenea annua, Tornabenea insularis and Tornabenea tenuissima fruits from Cape Verde Islands. – Biochem. Syst. Ecol. 37: 474-478.
Gruas-Cavagnetto C, Cerceau-Larrival M-T. 1982. Presence de pollens d’Ombellifères fossils dans le Paleogène du Bassin Anglo-Parisien: premiers resultats. – In: Cauwet-Marc A-M, Carbonnier J (eds), Les Ombellifères, Actes du 2e Symposium International sur les Ombellifères “Contributions Pluridisciplinaires à la Systématique”, Monographs in Systematic Botany from the Missouri Botanical Garden, Vol. 6, Braun-Brumfield, Ann Arbor, Michigan, pp. 255-267.
Grushvitzky IV, Tikhomirov VN, Aksenov ES, Shibakina GV. 1969. Succulent fruit with carpophore in species of the genus Stilbocarpa Decne. et Planch. (Araliaceae). – Bull. Mos. Soc. Nat., Biol. Ser. 74: 64-76. [In Russian]
Grushvitzky IV, Skvortsova NT, Arkhangelsky DB, Chistyakova LD. 1984. The species of the genus Trevesia (Araliaceae) in the flora of Vietnam. – Bot. Žurn. 69: 1019-1029. [In Russian]
Grushvitzky IV, Skvortsova NT, Ha TD. 1987. Vidy roda Macropanax (Araliaceae) vo flore V’etnama. – Bot. Žurn. SSSR 17: 380-389.
Guenot JF. 1906. Contributions à l’étude anatomique des Pittosporacées. – M.Sc. thesis, l’Université de Paris.
Guha S. 1971. Cytotaxonomy of Araliaceae. – Proc. Indian Sci Congr. Assoc., Sect. VI, 58: 471.
Gupta SC. 1970. Umbelliferae. – Bull. Indian Natl. Sci. Acad. 41: 233-238.
Gustafsson MHG, Bremer K. 1995. Morphology and phylogenetic interrelationships of the Asteraceae, Calyceraceae, Campanulaceae, Goodeniaceae, and related familes (Asterales). – Amer. J. Bot. 82: 250-265.
Guymer GP. 1984. Icacinaceae. – In: George AS (ed), Flora of Australia 22, Australian Government Publ. Service, Canberra, pp. 204-212.
Guyot M. 1966. Les stomates des Ombellifères. – Bull. Soc. Bot. France 113: 244-273.
Guyot M. 1971. Phylogenetic and systematic value of stomata of the Umbelliferae. – In: Heywood VH (ed), The biology and chemistry of the Umbelliferae, Bot. J. Linn. Soc. 64 [Suppl. 1]: 199-214.
Guyot M. 1984. Les types stomatiques dans les espèces françaises du genre Peucedanum (Ombellifères). – Rev. Cytol. Biol. Vég. Bot. 7: 17-30.
Guyot M, Cerceau-Larrival M-T, Carbonnier-Jarreau M-C, Derouet L, Relot J. 1980. Corrélations entre types stomatiques et types polliniques dans la tribu des Caucalidées (Ombellifères). – Bull. Mus. Natl. Hist. Nat. Paris 2: 341-385.
Ha TD. 1974. Florae Vietnamensis taxa nova e tribu Aralieae Benth. (fam. Araliaceae). – Novosti systematikj Vysshikh Rasteni 11: 226-240.
Haas JE. 1977. The Pacific species of Pittosporum Banks ex Gaertn. (Pittosporaceae). – Allertonia 1: 73-167.
Haccius B. 1952. Verbreitung und Ausbildung der Einkeimblättrigkeit bei den Umbelliferen. – Österr. Bot. Zeitschr. 99: 483-505.
Hadaček F. 1989. Vergleichende phytochemische Untersuchungen in der Gattung Peucedanum (Apiaceae-Apioideae). – Stapfia 18: 1-186.
Hadaček F, Samuel R. 1994. Chromosome counts and chemotaxonomy in Peucedanum sect. Peucedanum (Apiaceae, Apioideae) from the Balkan Peninsula. – Willdenowia 24: 33-48.
Hadaček F, Werner A, Greger H. 1988. Computerized HPLC-diode array screening on characteristic acetylene patterns within Peucedanum (Umbelliferae-Apioideae). – In: Lam J et al. (eds), Chemistry and biology of naturally-occurring acetylenes and related compounds, Amsterdam, pp. 107-114.
Hair JB, Beuzenberg EJ. 1959. Contributions to a chromosome atlas of the New Zealand flora 2. Miscellaneous families. – New Zealand J. Bot. 2: 148-156.
Håkansson A. 1953. Some chromosome numbers in Umbelliferae. – Bot. Not. 1953: 301-307.
Halim AF, Saad H-E A, Lahloub MF, Ahmed AF. 1995. Pituranthoside from Pituranthos triradiatus. – Phytochemistry 40: 927-929.
Hamada H, Mohamed B, Massiot G, Long C, Lavaud C. 2004. Alkylated isocoumarins from Pituranthos scoparius. – Nat. Prod. Res. 18: 409-413.
Hansen L, Boll PM. 1986. Polyacetylenes in Araliaceae: their chemistry, biosynthesis and biological significance. – Phytochemistry 25: 285-293.
Hara H. 1970. On the Asiatic species of the genus Panax. – J. Jap. Bot. 45: 197-212.
Harborne JB. 1971. Flavonoid and phenylpropanoid patterns in the Umbelliferae. – In: Heywood VH (ed), The biology and chemistry of the Umbelliferae, Bot. J. Linn. Soc. 64 [Suppl. 1]: 293-314.
Harborne JB, King L. 1976. Flavonoid sulphates in the Umbelliferae. – Biochem. Syst. Ecol. 4: 111-115.
Harborne JB, Williams CA. 1972. Flavonoid patterns in the fruits of the Umbelliferae. – Phytochemistry 11: 1741-1750.
Harborne JB, Heywood VH, Williams CA. 1969. Distribution of myristicin in seeds of the Umbelliferae. – Phytochemistry 8: 1729-1732.
Harborne JB, Heywood VH, Chen X-Y. 1986. Separation of Ostericum from Angelica on the basis of leaf and mericarp flavonoids. – Biochem. Syst. Ecol. 14: 81-83.
Hardig TM, Soltis PS. 1999. An ITS-based phylogenetic analysis of the Euryptera species group in Lomatium (Apiaceae). – Plant Syst. Evol. 219: 65-78.
Hardway TM. 2001. A phylogenetic study of Apiaceae tribe Oenantheae. – M.Sc. thesis, University of Illinois at Urbana-Champaign, Urbana, Illinois.
Hardway TM, Spalik K, Watson MF, Katz-Downie DS, Downie SR. 2004. Circumscription of Apiaceae tribe Oenantheae. – South Afr. J. Bot. 70: 393-406.
Harms H. 1897. Zur Kenntnis der Gattungen Aralia und Panax. – Engl. Bot. Jahrb. Syst. 23: 1-23.
Harms H. 1898. Araliaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(8), W. Engelmann, Leipzig, pp. 1-62.
Harms H. 1918. Übersicht über die Arten der Gattung Acanthopanax. – Mitt. Deutsch. Dendrol. Ges. 27: 1-39.
Harms H. 1940. Araliaceae. – In: Diels L, Neue Arten aus Ecuador III. – Notizbl. Bot. Gart. Berlin-Dahlem 15: 50-53.
Harn C, Whang J. 1963. Development of female gametophyte of Panax ginseng. – Korean J. Bot. 6: 3-6.
Hart JM. 1998. Systematics of Xanthosia and allied genera (Apiaceae). – Ph.D. diss., University of Sydney, Australia.
Hart JM. 2000. The taxonomy of Xanthosia huegelii and closely related species (Apiaceae: Hydrocotyloideae). – Telopea 8: 441-453.
Hart JM, Henwood MJ. 1999. Brachyscias (Apiaceae): a new genus from south-west Western Australia. – Aust. Syst. Bot. 12: 175-179.
Hart JM, Henwood MJ. 2000. Systematics of the Xanthosia pilosa complex (Apiaceae: Hydrocotyloideae). – Aust. Syst. Bot. 13: 245-266.
Hart JM, Henwood MJ. 2006. A revision of Australian Trachymene (Apiaceae: Hydrocotyloideae). – Aust. Syst. Bot. 19: 11-57.
Hartman RL. 1985. A new species of Cymopterus (Umbelliferae) from southern Idaho. – Brittonia 37: 102-105.
Hartman RL. 2000. A new species of Cymopterus (Apiaceae) from the Rocky Mountain region, U.S.A. – Brittonia 52: 136-141.
Hartman RL, Constance L. 1985. Two new species of Cymopterus (Umbelliferae) from western North America. – Brittonia 37: 88-95.
Hartman RL, Constance L. 1988. A new Lomatium (Apiaceae) from the Sierran crest of California. – Madroño 35: 121-125.
Hartvig P. 1983. Two new species of Apiaceae from Greece. – Willdenowia 13: 289-293.
Hauman L. 1919. Notes sur les espèces argentines des genres “Azorella” et “Bolax”. – Physis 4: 468-520.
He X-J, Wang Y-P, Pu F-T, Wang P-L, Xu J-M. 1998. Anatomical studies on fruits of the genus Heracleum from China and its revision of systematics. – Acta Bot. Yunnan. 20: 295-302.
Hedge IC, Lamond JM. 1973. A review of the tribe Echinophoreae (Umbelliferae). – Notes Roy. Bot. Gard. Edinb. 32: 167-188.
Hedge IC, Lamond JM. 1980. Notes on Umbelliferae: some Asiatic Scandiceae. – Notes Roy. Bot. Gard. Edinb. 38: 251-257.
Hedge IC, Lamond JM, Rechinger KH et al. 1987. Umbelliferae. – In: Rechinger KH (ed), Flora Iranica 162, Akademische Druck- und Verlagsanstalt, Graz, pp. 1-555.
Heenan PB, Telford IRH, Bruhl JJ. 2013. Three new species of Gingidia (Apiaceae: Apioideae) from Australia and New Zealand segregated from G. montana. – Aust. Syst. Bot. 26: 196-209.
Hegnauer R. 1971. Chemical patterns and relationships of Umbelliferae. – In: Heywood VH (ed), The biology and chemistry of the Umbelliferae, Bot. J. Linn. Soc. 64 [Suppl. 1]: 267-277.
Hegnauer R. 1982. Phytochemie und Klassifikation der Umbelliferen, eine Neubewertung im Lichte der seit 1972 bekannt gewordenen phytochemischen Tatsachen. – In: Cauwet-Marc A-M, Carbonnier J (eds), Les Ombellifères. Actes du 2e Symp. Intern. Ombellifères, Monogr. Syst. Bot. Missouri Bot. Gard. 6: 335-363.
Heintzelman CE Jr, Howard RA. 1948. The comparative morphology of the Icacinaceae V. The pubescence and the crystals. – Amer. J. Bot. 35: 42-52.
Henwood MJ. 1991. Pollen morphology of Polyscias (Araliaceae): the Malesian and Australian species. – Grana 30: 559-576.
Henwood MJ. 2000. Actinotus periculosus (Apiaceae): a new perennial species from eastern Australia. – Telopea 8: 455-459.
Henwood MJ, Hart JM. 2001. Towards an understanding of the phylogenetic relationships of Australian Hydrocotyloideae (Apiaceae). – Edinburgh J. Bot. 58: 269-289.
Henwood MJ, Lu-Irving P, Perkins AJ. 2010. Can molecular systematics provide insights into aspects of the reproductive biology of Trachymene Rudge (Araliaceae)? – Plant Divers. Evol. 128: 85-110.
Herrnstadt I, Heyn CC. 1975. New combinations in Cachrys L. as a genus distinct from Hippomarathrum Link. – Notes Roy. Bot. Gard. Edinb. 33: 441-443.
Herrnstadt I, Heyn CC. 1977. A monographic study of the genus Prangos (Umbelliferae). – Boissiera 26.
Heywood VH. 1968. Scanning electron microscopy and micro-characters in the fruits of the Umbelliferae-Caucalideae. – Proc. Linn. Soc. London 179: 287-289.
Heywood VH. 1971a. Systematic survey of Old World Umbelliferae. – In: Heywood VH (ed), The biology and chemistry of the Umbelliferae, Bot. J. Linn. Soc. 64 [Suppl. 1]: 31-41.
Heywood VH (ed). 1971b. The biology and chemistry of the Umbelliferae. – Bot. J. Linn. Soc. 64 [Suppl. 1], Academic Press, London, New York.
Heywood VH. 1971c. Chemosystematic studies in Daucus and allied genera. – Boissiera 19: 289-295.
Heywood VH. 1973. The taxonomic position of Agrocharis Hochst. and allied genera. – Notes Roy. Bot. Gard. Edinb. 32: 211-215.
Heywood VH. 1978a. Introduction to the taxonomy of the Umbelliferae. – In: Cauwet AM, Carbonnier J (eds), Actes du 2e Symposium International sur les Ombellifères. Contributions Pluridisciplinaires à la Systématique, Missouri Botanical Garden, St. Louis, Missouri, pp. 107-112.
Heywood VH. 1978b. Multivariate taxonomic synthesis of the tribe Caucalideae. – In: Cauwet AM, Carbonnier J (eds), Actes du 2e Symposium International sur les Ombellifères. Contributions Pluridisciplinaires à la Systématique, Missouri Botanical Garden, St. Louis, Missouri, pp. 727-736.
Heywood VH. 1982a. General introduction to the taxonomy of the Umbelliferae. – In: Cauwet-Marc A-M, Carbonnier J (eds), Actes du 2e Symposium International sur les Ombellifères. Contributions pluridisciplinaires à la Systématique, Perpignan, France, 18-21 May 1977, Monogr. Syst. Bot. Missouri Bot. Gard. 6: 107-112.
Heywood VH. 1982b. Multivariate taxonomic synthesis of the tribe Caucalideae. – In: Cauwet-Marc A-M, Carbonnier J (eds), Les Ombellifères, Actes du 2ème Symposium International sur les Ombellifères “Contributions Pluridisciplinaires à la Systématique”, Monographs in Systematic Botany from the Missouri Botanical Garden, Vol. 6, Braun-Brumfield, Ann Arbor, Michigan, pp. 727-736.
Heywood VH. 1983. Relationships and evolution in the Daucus carota complex. – Israel J. Bot. 32: 51-65.
Heywood VH. 1986. The Umbelliferae – an impossible family? – Symb. Bot. Ups. 26: 173-180.
Heywood VH, Dakshini KMM. 1971. Fruit structure in the Umbelliferae-Caucalideae. – In: Heywood VH (ed), The biology and chemistry of the Umbelliferae, Bot. J. Linn. Soc. 64 [Suppl. 1]: 217-232.
Heywood VH, Saenz de Rivas C. 1974. Estudio preliminar sobre los Daucus de la España peninsular. – Anal. Inst. Bot. Cavanilles 31: 97-118.
Hiller K. 1971. Chemosystematics of the Saniculoideae. – In: Heywood VH (ed), The biology and chemistry of the Umbelliferae, Bot. J. Linn. Soc. 64 [Suppl. 1]: 369-384.
Hiraoka N, Chang J-I, Bohm LR, Bohm BA. 2002. Furanocoumarin and polyacetylenic compound composition of wild Glehnia littoralis in North America. – Biochem. Syst. Ecol. 30: 321-325.
Hiroe M. 1958. Umbelliferae of Asia (excluding Japan). – Eicodo (Akira Imagawa), Maruzen Ltd., Kyoto.
Hiroe M. 1962. Supplementary notes on the genus Glehnia (Umbelliferae). – Acta Phytotaxon. Geobot. 19: 39-44.
Hiroe M. 1979. Umbelliferae of the World. – Ariake Book Co., Tokyo.
Hiroe M, Constance L. 1958. Umbelliferae of Japan. – Univ. Calif. Publ. Bot. 30: 1-444.
Hoar CS. 1915. A comparison of the stem anatomy of the cohort Umbelliflorae. – Ann. Bot. 29: 55-63.
Holland AE. 1989. Notes on Trachymene Rudge (Apiaceae) in Queensland 1. – Austrobaileya 3: 135-139.
Holland AE. 1991. Notes on Trachymene Rudge (Apiaceae) in Queensland 2. – Austrobaileya 3: 401-407.
Holub M, Budesinsky M. 1986. Sesquiterpene lactones of the Umbelliferae. – Phytochemistry 25: 2015-2026.
Holub M, Toman J, Herout V. 1987. The phylogenetic relationships of the Asteraceae and Apiaceae based on phytochemical characters. – Biochem. Syst. Ecol. 15: 321-326.
Hoo G. 1961. The systematics, relationship and distribution of the Araliaceae of China. – Bull. Amoi Univ. (Nat. Sci.) 8: 1-11.
Hoo G, Tseng C-J. 1965. Contributions to the Araliaceae of China. – Acta Phytotaxon. Sin., Addit. 1: 129-176.
Horn af Rantzien H. 1946. Om Pleurospermum austriacum (L.) Hoffm. emend. Turcz., dess taxonomi, utbredning och ekologi. – Svensk Bot. Tidskr. 40: 179-213.
Hu S-Y. 1980. Eleutherococcus vs. Acanthopanax. – J. Arnold Arbor. 61: 107-111.
Ikeda R, Nagao T, Okabe H, Nakano Y, Matsunaga H, Katano M, Mori M. 1998a. Antiproliferative constituents in Umbelliferae plants III. Constituents in the root and the ground part of Anthriscus sylvestris Hoffm. – Chem. Pharmaceut. Bull. (Tokyo) 46: 871-874.
Ikeda R, Nagao T, Okabe H, Nakano Y, Matsunaga H, Katano M, Mori M. 1998b. Antiproliferative constituents in Umbelliferae plants IV. Constituents in fruits of Anthriscus sylvestris Hoffm. – Chem. Pharmaceut. Bull. (Tokyo) 46: 875-878.
Iorizzo M, Senalik D, Szklarczyk M, Grzebelus D, Spooner D, Simon P. 2012. De novo assembly of the carrot mitochondrial genome using next generation sequencing of whole genomic DNA provides first evidence of DNA transfer into an angiosperm plastid genome. – BMC Plant Biol. 12: 61.
Jackson G. 1933. A study of the carpophore of the Umbelliferae. – Amer. J. Bot. 20: 121-144.
Jacques-Félix H. 1970. Contribution à l’étude des Umbellifloreae du Cameroun. – Adansonian, sér. II, 10: 35-94.
Jarvis CE, Knees SG. 1988. Linnaean names in the genus Athamanta L. (Umbelliferae: Apioideae) and their typification. – Taxon 37: 472-477.
Jay M. 1969. Chemotaxonomic researches in vascular plants XIX. Flavonoid distribution in the Pittosporaceae. – Bot. J. Linn. Soc. 62: 423-429.
Jean F-I, Deslaurier H, Collin GJ, Gagnon M, Hachey J-M, Paré JR, Bélanger A. 1990. The essential oil of Ligusticum scoticum L. – J. Ess. Oil Res. 2: 37-44.
Jebb MHP. 1998. A revision of the genus Trevesia (Araliaceae). – Glasra 3: 85-113.
Jensen SR, Nielsen BJ. 1980a. Iridoid glucosides in Griselinia, Aralidium and Toricellia. – Phytochemistry 19: 2685-2688.
Jensen SR, Nielsen BJ. 1980b. The systematic position of Aralidium Miq. – a multidisciplinary study 4. Iridoid glucosides. – Taxon 29: 409-411.
Jiménez-Mejias P, Vargas P. 2015. Taxonomy of the tribe Apieae (Apiaceae) revisited as revealed by molecular phylogenies and morphological characters. – Phytotaxa 212: 057-079.
Judd WS. 1982. The taxonomic status of Oxypolis greenmanii (Apiaceae). – Rhodora 84: 265-279.
Jurica HS. 1922. A morphological study of the Umbelliferae. – Bot. Gaz. 74: 292-307.
Jury SL. 1978. Tuberculate fruit in the Umbelliferae. – In: Cauwet AM, Carbonnier J (eds), Actes du 2e Symposium International sur les Ombellifères. Contributions Pluridesciplinaires à la Systématique, Missouri Botanical Garden, St. Louis, Missouri, pp. 149-160.
Jury SL. 1986. Fruit and leaf variation in the African species of the Umbelliferae tribe Caucalideae. – Symb. Bot. Ups. 26: 181-188.
Kadereit JW, Bittrich V (eds). 2018. The families and genera of vascular plants XV. Flowering plants – eudicots – Apiales, Gentianales (except Rubiaceae). Springer, 570 pp.
Kadereit JW, Repplinger M, Schmalz N, Uhink CH, Wörz A. 2008. The phylogeny and biogeography of Apiaceae subf. Saniculoideae tribe Saniculeae: from south to north and south again. – Taxon 57: 365-382.
Kaplan DR. 1970. Comparative development and morphological interpretation of ‘rachis leaves’ in Umbelliferae. – In: Robson NKB, Cutler DF, Gregory M (eds), New research in plant anatomy, Bot. J. Linn. Soc. Suppl., Academic Press, London, pp. 101-126.
Kårehed J. 2001. Multiple origin of the tropical forest tree family Icacinaceae. – Amer. J. Bot. 88: 2259-2274.
Kårehed J. 2002. Not just hollies – the expansion of Aquifoliales. – In: Evolutionary studies in asterids emphasising euasterids II, Acta Universitatis Upsaliensis, Uppsala, pp. 1-14.
Kårehed J. 2003. The family Pennantiaceae and its relationships to Apiales. – Bot. J. Linn. Soc. 141: 1-24.
Karpunina PV, Oskolski AA, Nuraliev MS, Lowry II PP, Degtjareva GV, Samigullin TH, Valiejo-Roman CM, Sokoloff DD. 2016. Gradual vs. abrupt reduction of carpels in syncarpous gynoecia: a case study from Polyscias subg. Arthrophyllum (Araliaceae: Apiales). – Amer. J. Bot. 103: 2028-2057.
Katz-Downie DS, Valiejo-Roman CM, Terentieva EI, Troitsky AV, Pimenov MG, Lee B-Y, Downie SR. 1999. Towards a molecular phylogeny of Apiaceae subfamily Apioideae: additional information from nuclear ribosomal DNA ITS sequences. – Plant Syst. Evol. 216: 167-195.
Kaul MK. 1975. Contribution to the umbellifers of Kashmir. – J. Bombay Nat. Hist. Soc. 72: 692-715.
Keighery GJ. 1989. Taxonomy of the Xanthosia fruticulosa group (Apiaceae). – Nord. J. Bot. 8: 445-446.
Keighery GJ. 1996. A new species of Platysace (Apiaceae) from Northern Australia. – Nord. J. Bot. 16: 135-137.
Kim C-H. 1997. Systematics of Eleutherococcus and related genera (Araliaceae). – Ph.D. diss., Chonbuk National University, Korea.
Kim C-H, Sun B-Y. 2000. New taxa and combinations of Eleutherococcus (Araliaceae) from Eastern Asia. – Novon 10: 209-214.
Kim K-J, Lee H-L. 2004. Complete chloroplast genome sequences from Korean ginseng (Panax schinseng Nees) and comparative analysis of sequence evolution among 17 vascular plants. – DNA Res. 11: 247-261.
Kitagawa M. 1960. Synoptical review of Umbelliferae from Japan, Korea & Manchuria. – Bull. Nat. Sci. Mus. 5: 1-35.
Kleiman R, Spencer GF. 1982. Search for new industrial oils XVI. Umbelliflorae-seed oils rich in petroselenic acid. – J. Amer. Oil Chem. Soc. 59: 29-38.
Kljuykov EV. 1983. Conspectus specierum generis Elaeosticta Fenzl (Apiaceae). – Nov. Syst. Plant. Vasc. 12: 140-154. [In Russian]
Kljuykov EV. 1985. Note on Muretia amplifolia Boiss. & Hausskn. and the genus Hellenocarum Wolff (Umbelliferae-Apioideae). – Nauchnye Dokl. Vysshei Shkoly Biol. Nauki 8: 60-63. [In Russian]
Kljuykov EV. 1986a. A taxonomic survey of the genus Sinodielsia Wolff including a description of two new species. – Feddes Repert. 97: 753-757.
Kljuykov EV. 1986b. De generibus Stefanoffia H. Wolff and Carum L. (Apiaceae) in flora Turciae notulae systematicae. – Nov. Sist. Vyssh. Rast. 23: 89-92. [In Russian]
Kljuykov EV. 1988. A survey of the genus Bunium L. Revision of the generic system. – Bull. Soc. Nat. Mosc., div. boil. 93: 76-89. [In Russian]
Kljuykov EV, Lavrova TV. 1994. On systematic position of some species of the genera Pleurospermum and Trachydium (Umbelliferae). – Bot. Žurn. 79: 102-108. [In Russian]
Kljuykov EV, Ukrainskaja U. 2010. Distribution of the Umbelliferae in Middle Asia and Kazakhstan. – Plant Div. Evol. 128: 547-559.
Kljuykov EV, Pimenov MG, Tikhomirov VN. 1976a. Elaeosticta Fenzl, a self-contained genus of the family Umbelliferae, distinct from Scaligeria DC. – Bull. Soc. Nat. Mosc, div. biol. 81: 83-94. [In Russian]
Kljuykov EV, Pimenov MG, Tikhomirov VN. 1976b. Revision of the genus Aulacospermum Ledeb. 1. Limits and system of the genus. – Biull. Mosk. Obshch. Ispyt. Prir. Otdel Biol. 81: 75-89. [In Russian]
Kljuykov EV, Pimenov MG, Tikhomirov VN. 1976c. Revision of the genus Aulacospermum Ledeb. 2. Review of species. – Biull. Mosk. Obshch. Ispyt. Prir. Otdel Biol. 81: 61-68. [In Russian]
Kljuykov EV, Pimenov MG, Tikhomirov VN. 1978. Additions and specifications to the systematics of the genus Elaeosticta Fenzl and related taxa. – Bull. Soc. Nat. Mosc., div. biol. 83: 100-107. [In Russian]
Kljuykov EV, Liu M, Ostroumova TA, Pimenov MG, Tilney PM, Wyk B-E van. 2004. Towards a standardized terminology for taxonomically important morphological characters in the Umbelliferae. – South Afr. J. Bot. 70: 488-496.
Knees SG. 1996. Tinguarra Benth. & Hook. in Morocco. – Lagascalia 18: 286-287.
Komissarenko NF, Pimenov MG. 1977. Coumarins of the roots of Heracleum carpaticum, H. ligusticifolium, and Symphyoloma graveolens. – Chem. Natur. Comp. 13: 110-111.
Kondo K, Terabayashi S, Okada M, Yuan C, He S. 1996. Phylogenetic relationships of medicinally important Cnidium officinale and Japanese Apiaceae based on rbcL sequences. – J. Plant Res. 109: 21-27.
Konstantinova AI, Suchorukow AP. 2010. Die Karpologie der asiatischen Schefflera-Sippen (Araliaceae). – Ann. Naturhist. Mus. Wien, B, 111:149-170.
Kordyum EL. 1967. Cytoembryology of the family Umbelliferae. – Kiev. [In Russian]
Korovin EP. 1927. The genus Bunium and its Middle-Asian representatives. – Byull. Sredne-Asiatsk. Gosud. Univ. 15: 117-129. [In Russian]
Korovin EP. 1928a. Le genre Scaligeria DC. (Umbelliferae) et sa philogénie. – Acta Univ. Asiae Mediae, Ser. VIII-b Botanica II, 9: 3-92.
Korovin EP. 1928b. The genus Scaligeria DC. (Umbelliferae) and its phylogeny. Experience of the application of ecology to phylogeny of small taxonomic groups. – Trudy Sredne-Aziatsk Univ., ser. VIII-b, Bot. 2: 1-92. [In Russian]
Korovin EP. 1947a. Generis Ferula (Tourn.) L. monographia illustrata. – Acad. Scient. UzRSS, Taschkent, Uzbekistan, pp. 7-10.
Korovin EP. 1947b. New species of Umbelliferae of Uzbekistan flora 1. – Bot. Mater. Gerb. Inst. Bot. Zool. Akad. Nauk Uzbeksk SSR 8: 3-24. [In Russian]
Koso-Poljansky BM. 1914. Essay on the phylogeny of the Caucasian Umbelliferae. – Věstn. Tiflissk. Bot. Sada 16: 172-229. [In Russian]
Koso-Poljansky BM. 1915. Species Umbelliferarum minus cognitae. – Vestn. Tiflissk. Bot. Sada 11: 136-170. [In Russian]
Koso-Poljansky BM. 1916. Sciadophytorum systematis lineamenta. – Bull. Soc. Imp. Natur. Moscou 29: 93-221.
Koso-Poljansky BM. 1917. Sciadophytorum systematis lineamenta. Mantissa prima. – Bull. Soc. Imp. Natur. Moscou 30: 277-290.
Koso-Poljansky BM. 1923. Systema Chaerophyllorum Rossiae. – Bot. Mater. Gerb. Glavn. Bot. Sada RSFSR 4: 189-190.
Koso-Poljansky BM. 1924. Hydrocotyloidearum revisio. – Bot. Mater. Gerb. Glavn. Bot. Sada RSFSR 5: 17-24.
Kotina EL, Oskolski AA. 2010. Survey of the bark anatomy of Araliaceae and related taxa. – Plant Divers. Evol. 128: 455-489.
Kotina EL, Wyk B-E van, Tilney PM, Oskolski AA. 2012. The systematic significance of bark structure in southern African genera of tribe Heteromorpheae (Apiaceae). – Bot. J. Linn. Soc. 169: 677-691.
Kotina EL, Fiaschi P, Plunkett GM, Oskolski AA. 2013. Systematic and ecological wood anatomy of Neotropical Schefflera (Araliaceae), with an emphasis on the Didymopanax group. – Bot. J. Linn. Soc. 173: 452-475.
Kozawa M, Morita N, Hata K. 1978. Structure of anthriscusin, a new phenyl propanoid ester from the roots of Anthriscus sylvestris. – Chem. Pharmac. Bull. (Tokyo) 26: 1337-1338.
Krähenbühl M, Küpfer P. 1992. Nombre chromosomique de base et position systématique du genre Molopospermum Koch au sein des Umbelliferae. – Bauhinia 10: 75-84.
Kral R. 1981. Notes on some ”quill-leaved” umbellifers. – SIDA Contr. Bot. 9: 124-134.
Kress WJ, Maddox GD, Roesel CS. 1994. Genetic variation and protection priorities in Ptilimnium nodosum (Apiaceae), an endangered plant of the eastern United States. – Cons. Biol. 8: 271-276.
Kubeczka K-H, Rohde A. 1984. Qualitative und quantitative Analyse von Cumarinen in ausgewählten Apiaceen. – Fresenius Zeitschr. Analyt. Chem. 318: 245-246.
Kupchan SM, Britton RW, Ziegler MF, Gilmore CJ, Restivo RJ, Bryan RF. 1973. Steganacin and steganangin, novel antileukemic lignan lactones from Steganotaenia araliacea. – J. Amer. Chem. Soc. 95: 1335-1336.
Kurihara T, Kikuchi M. 1979. Constituents of Anthriscus sylvestris 2. Components of the flowers and leaves. – Yakugaku Zasshi 99: 602-606.
Kurihara T, Kikuchi M, Suzuki S, Hisamichi S. 1978. Studies on the constituents of Anthriscus sylvestris 1. On the components of the root. – Yakugaku Zasshi 98: 1586-1591.
Kurzyna-Młynik R, Oskolski AA, Downie SR, Kopacz R, Wojewódzka A, Spalik K. 2008. Phylogenetic position of the genus Ferula (Apiaceae) and its placement in tribe Scandiceae as inferred from nrDNA ITS sequence variation. – Plant Syst. Evol. 274: 47-66.
Lancucka-Srodoniowa M. 1975. Hydrangea L. (Saxifragaceae) and Schefflera Forst. (Araliaceae) in the Tertiary of Poland. – Acta Palaeobot. 17: 103-112.
Lavaud C, Massiot G, Le Men-Olivier L, Viari A, Vigny P, Delaude C. 1992. Saponins from Steganotaenia araliacea. – Phytochemistry 31: 3177-3181.
Lavrova TV. 1994. Sistematicheskoe polozhenie Ligusticum caucasicum Somm. et Levier (Umbelliferae). – Byull. Moskovsk Obshch. Isp. Prir. Otd. Biol. 98: 93-98.
Lavrova TV, Pimenov MG, Tikhomirov VN. 1982. Petiole anatomy as a source of taxonomical information in Ligusticeae (Umbelliferae-Apioideae). – Bjull. Moskovsk. Obšč. Isp. Prir., Otd. Biol. 87: 99-111. [In Russian]
Lavrova TV, Pimenov MG, Tikhomirov VN. 1983. Description and analysis of the Umbelliferae fruit structure in the tribe Ligusticeae. – Bull. Soc. Nat. Moscou, Sect. Biol. 88: 107-122. [In Russian]
Lavrova TV, Pimenov MG, Deviatkova GN. 1987. The usage of cluster analysis in the elucidation of the taxonomic relations of species of subtribe Foeniculinae (Umbelliferae) of the flora of the USSR. – Bot. Žurn. 72: 25-38. [In Russian]
Le Claire E, Schwaiger S, Banaigs B, Stuppner H, Gafner F. 2005. Distribution of a new rosmarinic acid derivative in Eryngium alpinum L. and other Apiaceae. – J. Agric. Food Chem. 53: 4367-4372.
Lee B-Y. 1998. A phylogenetic study of Apiaceae tribe Caucalideae. – Ph.D. diss., University of Illinois at Urbana-Champaign, Urbana, Illinois.
Lee B-Y. 2002. Taxonomic review on the African Umbelliferous genus Agrocharis: inferences based on molecular data. – Israel J. Plant Sci. 50: 211-216.
Lee B-Y, Downie SR. 1999. A molecular phylogeny of Apiaceae tribe Caucalideae and related taxa: inferences based on ITS sequence data. – Syst. Bot. 24: 461-479.
Lee B-Y, Downie SR. 2000. Phylogenetic analysis of cpDNA restriction sites and rps16 intron sequences reveals relationships among Apiaceae tribes Caucalideae, Scandiceae and related taxa. – Plant Syst. Evol. 221: 35-60.
Lee B-Y, Park C-W. 2014. Molecualr phylogeny of Daucus (Apiaceae): evidence from nuclear ribosomal DNA ITS sequences. – J. Spec. Res. 3: 39-52.
Lee B-Y, Levin GA, Downie SR. 2001. Relationships within the spiny-fruited umbellifers (Scandiceae subtribes Daucinae and Torilidinae) as assessed by phylogenetic analysis of morphological characters. – Syst. Bot. 26: 622-642.
Lee C-S, Downie SR. 2006. Phylogenetic relationships within Cicuta (Apiaceae tribe Oenantheae) inferred from nuclear rDNA ITS and cpDNA sequence data. – Can. J. Bot. 84: 453-468.
Lee C, Wen J. 2004. Phylogeny of Panax using chloroplast trnC-trnD intergenic region and the utility of trnC-trnD in interspecific studies of plants. – Mol. Phylogen. Evol. 31: 894-903.
Lee S-B, Rasmussen SK. 1998. Phylogenetic analysis of rDNA internal transcribed spacer of Angelica, Bupleurum and Peucedanum species. – Available as Risø-R-1091(EN) from the Risø National Laboratory, Denmark.
Lee S-B, Rasmussen SK. 2000. Molecular markers in some medicinal plants of the Apiaceae family. – Euphytica 114: 87-91.
Leins P, Erbar C. 1985. Zur frühen Entwicklungsgeschichte des Apiaceen-Gynöceums. – Bot. Jahrb. Syst. 106: 53-60.
Leins P, Erbar C. 2004. Floral organ sequences in Apiales (Apiaceae, Araliaceae, Pittosporaceae). – South Afr. J. Bot. 70: 468-474.
Lens F, Kårehed J, Baas P, Jansen S, Rabaey D, Huysmans S, Hamann T, Smets E. 2008. The wood anatomy of polyphyletic Icacinaceae s.l., and their relationships within asterids. – Taxon 57: 525-552.
Leute G-H. 1966. Die Gattungen Imperatoria L. und Tommasinia Bertol. (Apiaceae). – Ann. Naturhist. Mus. Wien 69: 69-73.
Leute G-H. 1969. Untersuchungen über den Verwandtschaftskreis der Gattung Ligusticum L. (Umbelliferae) I. – Ann. Naturhist. Mus. Wien 73: 55-98.
Leute G-H. 1970. Untersuchungen über den Verwandtschaftskreis der Gattung Ligusticum L. (Umbelliferae) II. – Ann. Naturhist. Mus. Wien 74: 457-519.
Leute G-H. 1971. Die Arten der Gattung Conium L. (Umbelliferae). – Ann. Naturhist. Mus. Wien 75: 91-98.
Leute G-H. 1972. Bemerkungen zur Gattung Peucedanum L. in Griechenland. – Österr. Bot. Zeitschr. 120: 29-32.
Leute G-H, Speta F. 1972. Umbelliferen-Studien zur ’Flora Iranica’ 1. – Österr. Bot. Zeitschr. 120: 289-311.
Li H-L. 1942. The Araliaceae of China. – Sargentia 2: 1-134.
Li H-L. 1944. The phytogeographical division of China, with special reference to Araliaceae. – Proc. Acad. Nat. Sci. Philadelphia 96: 249-277.
Li H-L. 1949. A new genus of the Araliaceae. – J. Arnold Arbor. 30: 231-232.
Li L, Yuan C, Ting C, Cheo T. 1993. A taxonomic study on the genus Cnidium in China. – Acta Bot. Boreali-Occid. Sin. 13: 63-69. [In Chinese]
Li R, Wen J. 2013. Phylogeny and biogeography of Dendropanax (Araliaceae), an amphi-Pacific disjunct genus between tropical/subtropical Asia and the Neotropics. – Syst. Bot. 38: 536-551.
Li R, Wen J. 2016. Phylogeny and diversification of Chinese Araliaceae based on nuclear and plastid DNA sequence data. – J. Syst. Evol. 54: 453-467.
Li Z-Y, Zhu G. 2005. Proposal to conserve the name Acanthopanax against Eleutherococcus (Araliaceae). – Taxon 54: 194-195.
Liang G, Xu B, Pan W, Cao P, Lu Y, Wu Y, Hao X. 2009. A novel iridoid from Torricellia angulata var. intermedia. – Nat. Prod. Res. 23: 1-4.
Liao C-Y, He X-J. 2012. Angelica dabashanensis (Apiaceae), a new species from Shaanxi, China. – Ann. Bot. Fenn. 49: 125-133.
Liao C-Y, Downie SR, Yu Y, He, X-J. 2012. Historical biogeography of the Angelica group (Apiaceae tribe Selineae) inferred from analyses of nrDNA and cpDNA sequences. – J. Syst. Evol. 50: 206-217.
Liao C-Y, Downie SR, Li Q, Yu Y, He X-J, Zhou B. 2013. New insights into the phylogeny of Angelica and its allies (Apiaceae) with emphasis on East Asian species, inferred from nrDNA, cpDNA, and morphological evidence. – Syst. Bot. 38: 266-281.
Liao C-Y, He X-J, Ma X-G. 2012. Anatomical studies on petiole of Angelica sensu lato and its allies from China. – Acta Bot. Bor.-Occid. Sin. 32: 90-98.
Lim A-L. 1986. The comparative inflorescence structure in five Malaysian species of Arthrophyllum (Araliaceae). – Kew Bull. 41: 769-780.
Lindsey AH. 1982. Floral phenology patterns and breeding systems in Thaspium and Zizia (Apiaceae). – Syst. Bot. 7: 1-12.
Liu MR. 2004. A taxonomic evaluation of fruit structure in the family Apiaceae. – Ph.D. diss., Rand Afrikaans University, Auckland Park, Republic of South Africa.
Liu MR, Downie SR. 2017. The phylogenetic significance of fruit anatomical and micromorphological structures in Chinese Heracleum species and related taxa (Apiaceae). – Syst. Bot. 42: 313-325.
Liu MR, Wyk B-E van, Tilney PM. 2003a. The taxonomic value of fruit structure in the subfamily Saniculoideae and related African genera (Apiaceae). – Taxon 52: 261-270.
Liu MR, Wyk B-E van, Tilney PM. 2003b. The taxonomic value of fruit structure in the Chinese endemic genus Dickinsia (Apiaceae). – Nord. J. Bot. 22: 603-607.
Liu MR, Wyk B-E van, Tilney PM. 2004. Ontogeny of the fruits of two anomalous African woody genera, Polemanniopsis and Steganotaenia (Apiaceae), and their phylogenetic relationship. – Edinburgh J. Bot. 60: 249-257.
Liu MR, Plunkett GM, Lowry PP II, Wyk B-E van, Tilney PM. 2006. The taxonomic value of fruit wing types in the order Apiales. – Amer. J. Bot. 93: 1357-1368.
Liu MR, Wyk B-E van, Tilney PM. 2007a. A revision of the genus Choritaenia (Apiaceae). – South Afr. J Bot. 73: 184-189.
Liu MR, Wyk B-E van, Tilney PM. 2007b. A revision of the genus Marlothiella (Apiaceae). – South Afr. J. Bot. 73: 208-213.
Liu MR, Wyk B-E van, Tilney PM. 2007c. Irregular vittae and druse crystals in Steganotaenia fruit support a taxonomic affinity with the subfamily Saniculoideae (Apiaceae). – South Afr. J. Bot. 73: 252-255.
Liu MR, Wyk B-E van, Tilney PM, Plunkett GM, Lowry II PP. 2009. Evidence from fruit structure supports in general the circumscription of Apiaceae subfamily Azorelloideae. – Plant Syst. Evol. 280: 1-13.
Liu MR, Plunkett GM, Lowry II PP. 2010. Fruit anatomy provides structural synapomorphies to help define Myodocarpaceae (Apiales). – Syst. Bot. 35: 1-7.
Liu MR, Plunkett GM, Wyk B-E van, tilney PM, Lowry II PP. 2012. The phylogenetic significance of the carpophore in Apiaceae. – Ann. Bot. 110: 1531-1543.
Liu MR, Plunkett GM, Lowry II PP, Van Wyk B-E, Tilney PM, Nicolas AN. 2016. The phylogenetic significance of fruit and trichome structures in Apiaceae subfamily Mackinlayoideae. – Syst. Bot. 41: 685-699.
Liu MR, Wyk B-E van, Tilney PM, Plunkett GM, Lowry PP II, Magee AR. 2016. The phylogenetic significance of fruit structural variation in the tribe Heteromorpheae (Apiaceae). – Pak. J. Bot. 48: 201-210.
Llorens L. 1982. Un nuevo endemismo de la isla de Menorca: Apium bermejoi. – Fol. Bot.Misc. 3: 27-33.
Logacheva MD, Valiejo-Roman CM, Pimenov MG. 2008. ITS phylogeny of West Asian Heracleum species and related taxa of Umbelliferae-Tordylieae W. D. J. Koch, with notes on evolution of their psbA-trnH sequences. – Plant Syst. Evol. 270: 139-157.
Logacheva MD, Valiejo-Roman CM, Degtjareva GV, Stratton JM, Downie SR, Samigullin TH, Pimenov MG. 2010. A comparison of nrDNA ITS and ETS loci for phylogenetic inference in the Umbelliferae: an example from the tribe Tordylieae. – Mol. Phylogen. Evol. 57: 471-476.
Long C, Oskolski A. 2018. Wood and bark anatomy of Andriana (Heteromorpheae, Apiaceae) with phylogenetic implications. – South Afr. J. Bot. 115: 138-142.
Lovett Doust J. 1980. Floral sex ratios in andromonoecious Umbelliferae. – New Phytol. 85: 265-273.
Lovett Doust J, Harper JL. 1980. The resource costs of gender and maternal support in an andromonoecious umbellifer, Smyrnium olusatrum L. – New Phytol. 85: 251-264.
Lowry II PP. 1986a. A systematic study of three genera of Araliaceae endemic to or centered on New Caledonia: Delarbrea, Myodocarpus, and Pseudosciadium. – Ph.D. diss., Washington University, St. Louis, Missouri.
Lowry II PP. 1986b. A systematic study of Delarbrea Vieill. (Araliaceae). – Allertonia 4: 169-201.
Lowry II PP. 1989. A revision of Araliaceae from Vanuatu. – Bull. Mus. Natl. Hist. Nat., Paris, sér. IV, sect. B, Adansonia 11: 117-155.
Lowry II PP, Jones AG. 1979. Biosystematic investigations and taxonomy of Osmorhiza Rafinesque section Osmorhiza (Apiaceae) in North America. – Amer. Midl. Natur. 101: 21-27.
Lowry II PP, Jones AG. 1984. Systematics of Osmorhiza Raf. (Apiaceae: Apioideae). – Ann. Missouri Bot. Gard. 71: 1128-1171.
Lowry II PP, Plunkett GM. 2010. Recircumscription of Polyscias (Araliaceae) to include six related genera, with a new infrageneric classification and a synopsis of species. – Plant Divers. Evol. 128: 55-84.
Lowry II PP, Plunkett GM. 2018. Myodocarpaceae. – In: Kadereit JW, Bittrich V (eds). 2018. The families and genera of vascular plants XV. Flowering plants – eudicots – Apiales, Gentianales (except Rubiaceae). Springer, Berlin, pp. 527-532.
Lowry II PP, Wood KR. 2000. A new, threatened species of Tetraplasandra (Araliaceae) from Kaua’i, Hawaiian Islands, and notes on its conservation status. – Novon 10: 40-44.
Lowry II PP, Miller JS, Frodin DG. 1989. New combinations and name changes for some cultivated tropical Old World and Pacific Araliaceae. – Baileya 23: 5-13.
Lowry II PP, Pascal O, Labat J-N. 1999. A new species of Polyscias (Araliaceae) from Mayotte, Comoro Islands. – Adansonia, sér. III, 21: 67-73.
Lowry II PP, Plunkett GM, Oskolski AA. 2001. Early lineages in Apiales: insights from morphology, wood anatomy and molecular data. – Edinburgh J. Bot. 58: 207-220.
Lowry II PP, Plunkett GM, Wen J. 2004. Generic relationships in Araliaceae: looking into the crystal ball. – South Afr. J. Bot. 70: 382-392.
Lowry II PP, Plunkett GM, Raquet V, Sprenkle TS, Jérémie J. 2004. Inclusion of the endemic New Caledonian genus Pseudosciadium in Delarbrea (Apiales, Myodocarpaceae). – Adansonia, sér. III, 26: 251-256.
Lowry II PP, Plunkett GM, Frodin DG. 2013. Revision of Plerandra (Araliaceae). I. A synopsis of the genus with an expanded circumscription and a new infrageneric classification. – Brittonia 65: 42-61.
Lowry II PP, Plunkett GM, Gostel MR, Frodin DG. 2017. A synopsis of the Afro-Malagasy species previously included in Schefflera (Araliaceae): resurrection of the genera Astropanax and Neocussonia. – Candollea 72: 265-282.
Lu J-L, Duan J-A, Tang Y-P, Yang N-Y, Zhang L-B. 2009. Phthalide mono- and dimmers from the radix of Angelica sinensis. – Biochem. Syst. Ecol. 37: 405-411.
Lu M (R), Plunkett GM, Lowry II PP. 2010. Fruit anatomy provides structural synapomorphies to help define Myodocarpaceae (Apiales). – Syst. Bot. 35: 675-681.
Lutomski J, Nham NT. 1977. Studies on the saponin fraction from the root of Aralia mandshurica Rupr. et Maxim. I. Chromatographic investigations. – Herba Polonica 23: 5-11.
Lyskov DF, Degtjareva GV, Samigullin TH, Pimenov MG. 2015. Systematic placement of the Turkish endemic genus Ekimia (Apiaceae) based on morphological and molecular data. – Turkish J. Bot. 39: 673-680.
Lyskov DF, Degtjareva GV, Samigullin TH, Pimenov MG. 2017. The revision of Prangos subsections Koelzella and Fedtschenkoana (Apiaceae) with some note sto phylogeny and biogeography of the genus: molecular and morphological evidences. – Plant Syst. Evol. 303: 815-826.
McGillivray DJ. 1975. Billardiera Sm. and Rhytidosporum F. Muell. (Pittosporaceae) in New South Wales. – Telopea 1: 55-57.
McNeill J, Parker PF, Heywood VH. 1969. A taximetric approach to the classification of the spiny-fruited members (tribe Caucalideae) of the flowering-plant family Umbelliferae. – In: Cole AJ (ed), Numerical taxonomy, Academic Press, London, pp. 129-147.
McPherson G, Rabenantoandro J. 2002. Melanophylla angustior (Melanophyllaceae), a new species from southeastern Madagascar. – Adansonia, sér. III, 24: 263-265.
Magee AR, Clark VR. 2017. Mzansi’s mountain hemlocks: the identities of Hilliard and Burtt’s Conium species 3 and 4 (Apiaceae) and a revised key for the genus in sub-Saharan Africa. – South Afr. J. Bot. 108: 243-247.
Magee AR, Wyk B-E van, Vuuren SF van. 2007. Ethnobotany and antimicrobial activity of sieketroos (Arctopus species). – South Afr. J. Bot. 73: 159-162.
Magee AR, Wyk B-E van, Tilney PM, Downie SR. 2007. New generic circumscriptions of Cape peucedanoid species (Apiaceae). – South Afr. J. Bot. 73: 298-299.
Magee AR, Wyk B-E van, Tilney PM. 2008a. A taxonomic revision of the genus Nanobubon (Apiaceae: Apioideae). – South Afr. J. Bot. 74: 713-719.
Magee AR, Wyk B-E van, Tilney PM. 2008b. A taxonomic revision of the genus Cynorhiza (Apiaceae: Apioideae). – South Afr. J. Bot. 74: 726-734.
Magee AR, Wyk B-E, van, Tilney PM. 2008c. The identity of the type of Bubon gummiferum (Peucedanum gummiferum) (Apiaceae) – Taxon 57: 615-618.
Magee AR, Wyk B-E van, Tilney PM, Bank M van der. 2008. A taxonomic revision of the South African endemic genus Arctopus (Apiaceae, Saniculoideae). – Ann. Missouri Bot. Gard. 95: 471-486.
Magee AR, Wyk B-E van, Tilney PM, Downie SR. 2008. Ezosciadium (Apiaceae): a taxonomic revision of yet another early diverging South African apioid genus. – Plant Syst. Evol. 276: 167-175.
Magee AR, Wyk B-E van, Tilney PM, Downie SR. 2009. A taxonomic revision of Capnophyllum (Apiaceae: Apioideae). – South Afr. J. Bot. 75: 283-291.
Magee AR, Calvino CI, Liu M, Downie SR, Tilney PM, Wyk B-E van. 2009. New tribal delimitations in African Apiaceae. – South Afr. J. Bot. 75: 410-411.
Magee AR, Wyk B-E van, Tilney PM, Sales F, Hedge I, Downie SR. 2009. Billburttia, a new genus of Apiaceae (tribe Apieae) endemic to Madagascar. – Plant Syst. Evol. 283: 237-245.
Magee AR, Wyk B-E van, Tilney PM. 2009. A taxonomic revision of the woody South African genus Notobubon (Apiaceae: Apioideae). – Syst. Bot. 34: 220-242.
Magee AR, Wyk B-E van, Tilney PM, Downie SR. 2009. Generic delimitations and relationships of the Cape genera Capnophyllum, Dasispermum, and Sonderina, the North African genera Krubera and Stoibrax, and a new monotypic genus of the subfamily Apioideae (Apiaceae). – Syst. Bot. 34: 580-594.
Magee AR, Wyk B-E van, Tilney PM, Downie SR. 2010. A taxonomic revision of the South African endemic genus Dasispermum (Apiaceae, Apioideae). – South Afr. J. Bot. 76: 308-323.
Magee AR, Calviño CI, Liu M(R), Downie SR, Tilney PM. 2010. New tribal delimitations for the early diverging lineages of Apiaceae subfamily Apioideae. – Taxon 59: 567-580.
Magee AR, Wyk B-E van, Tilney PM, Downie SR. 2010. Phylogenetic position of African and Malagasy Pimpinella species and related genera (Apiaceae, Pimpinelleae). – Plant Syst. Evol. 288: 201-211.
Magee AR, Wyk B-E van, Tilney PM, Sales F, Hedge I, Downie SR. 2012. Billburttia, a new genus of Apiaceae (tribe Apieae) endemic to Madagascar. – Plant Syst. Evol. 283: 237-245.
Magee AR, Villiers BJ de Wyk B-E van, Tilney PM. 2015. A revision of the South African genus Hermas (Apiaceae). – Syst. Bot. 40: 352-365.
Maggi F, Lucarini D, Tirillini B, Sagratini G, Papa F, Vittori S. 2009. Chemical analysis of the essential oil of Ferula glauca L. (Apiaceae) growing in Marche (central Italy). – Biochem. Syst. Ecol. 37: 432-441.
Magin N. 1977. Das Gynoecium der Apiaceae: Modell und Ontogenie. – Ber. Deutsch. Bot. Ges. 90: 53-66.
Magin N. 1980. Eine blütenmorphologische Analyse der Lagoecieae (Apiaceae). – Plant Syst. Evol. 133: 239-259.
Maguire B, Steyermark JA, Frodin DG. 1984. Araliaceae. – In: Maguire B, Cowan RS, Wurdack JJ et al. (eds), The botany of the Guyana Highlands XII, Mem. New York Bot. Gard. 38: 46-82.
Makinson RO. 1991. Two new species of Astrotricha (Araliaceae) from New South Wales. – Telopea 4: 313-319.
Malheiros-Garde N, Garde A. 1951. Contribuicão para o estudo cariologico da familia Umbelliferae II. – Genét. Ibér. 3: 23-35.
Manchester SR. 1999. Biogeographical relationships of North American Tertiary floras. – Ann. Missouri Bot. Gard. 86: 472-522.
Manchester SR, O’Leary EL. 2010. Phylogenetic distribution and identification of fin-winged fruits. – Bot. Rev. 76: 1-82.
Manchester SR, Collinson ME, Soriano C, Sykes D. 2017. Homologous fruit characters in geographically separated genera of extant and fossil Torricelliaceae (Apiales). – Intern. J. Plant Sci. 178: 567-579.
Mandenova IP. 1940. On a new species of the genus Tordylium from Daghestan. – Zametki Sist. Geogr. Rast. 9: 41-46. [In Russian]
Mandenova IP. 1950. Caucasian species of the genus Heracleum. – Tbilisi. [In Russian]
Mandenova IP. 1951. On the genus Symphyoloma C. A. Meyer [De genere Symphyoloma C. A. Meyer]. – Zametki Sist. Geogr. Rast. [Not. Syst. Geogr. Inst. Bot. Thbilissiensis] 16: 81-88. [In Russian]
Mandenova IP. 1959. Materials on systematics of the tribe Pastinaceae K.-Pol. emend. Manden. (Umbelliferae-Apioideae). – Trudy Tbilissk. Bot. Inst. 20: 3-57. [In Russian]
Mandenova IP. 1962. Taxonomic review of Turkish species of Heracleum. – Notes Roy. Bot. Gard. Edinb. 24: 173-181.
Mandenova IP. 1981. Conspectus systematis generis Heracleum L. (Umbelliferae). – Zametki Sist. Geogr. Rast. 37: 19-20.
Mandenova IP, Carbonnier J, Carbonnier-Jarreau M-C, Cauwet-Marc A-M, Cerceau-Larrival M-T, Guyot M, Molho D, Reduron J-P. 1982. Contribution à l’étude du genre Tetrataenium (D.) Manden. – In: Cauwet-Marc A-M, Carbonnier J (eds), Les Ombellifères, Actes du 2e Symposium International sur les Ombellifères “Contributions Pluridisciplinaires à la Systématique”, Perpignan, Monogr. Syst. Bot. Missouri Bot. Gard. 6: 675-725.
Marais W. 1984. Notes on Mascarene Araliaceae. – Kew Bull. 39: 809-816.
Marais W. 1990. 106. Araliaceae. – In: Bosser J, Cadet T, Guého J, Marais W (eds), Flore de Mascareignes, Mauritius Sugar Industry Research Institute, Royal Botanic Gardens, Kew, pp. 1-20.
Martínez S. 1989. El género Azorella (Apiaceae, Hydrocotyloideae) en Argentina. – Darwiniana 29: 13-178.
Martínez S. 1993a. Relaciones fenéticas entre las especies del género Azorella (Apiaceae, Hydrocotyloideae). – Darwiniana 32: 159-170.
Martínez S. 1993b. Sinopsis del género Azorella (Apiaceae, Hydrocotyloideae). – Darwiniana 32: 171-184.
Mastrogiuseppe JD, Gill SJ, Simmons KS, Brown GK. 1985. Morphologic and cytotaxonomic evaluation of Lomatium tuberosum (Apiaceae). – Brittonia 37: 252-260.
Mathias ME. 1928. Studies in the Umbelliferae I. – Ann. Missouri Bot. Gard. 15: 91-109.
Mathias ME. 1930. Studies in the Umbelliferae III. A monograph of Cymopterus including a critical study of related genera. – Ann. Missouri Bot. Gard. 17: 213-476.
Mathias ME. 1936a. Notes on the genus Ptilimnium. – Brittonia 2: 244.
Mathias ME. 1936b. Studies in the Umbelliferae V. – Brittonia 3: 239-245.
Mathias ME. 1938. A revision of the genus Lomatium. – Ann. Missouri Bot. Gard. 25: 225-297.
Mathias ME. 1965. Distribution patterns of certain Umbelliferae. – Ann. Missouri Bot. Gard. 52: 387-398.
Mathias ME. 1971. Systematic survey of New World Umbelliferae. – In: Heywood VH (ed), The biology and chemistry of the Umbelliferae, Bot. J. Linn. Soc. 64 [Suppl. 1]: 13-29.
Mathias ME, Constance L. 1941. A synopsis of the North American species of Eryngium. – Amer. Midl. Natur. 25: 361-387.
Mathias ME, Constance L. 1942. A synopsis of the American species of Cicuta. – Madroño 6: 145-176.
Mathias ME, Constance L. 1944-1945. Umbelliferae. – In: North American Flora 28B, New York Botanical Garden, New York, pp. 43-295.
Mathias ME, Constance L. 1951. A revision of the Andean genus Niphogeton (Umbelliferae). – Univ. Calif. Publ. Bot. 23: 405-426.
Mathias ME, Constance L. 1955. The genus Oreomyrrhis (Umbelliferae). – Univ. Calif. Publ. Bot. 27: 347-416.
Mathias ME, Constance L. 1957. Four notable Umbelliferae from Peru. – Bull. Torrey Bot. Club 84: 189-198.
Mathias ME, Constance L. 1962a. Four new or renamed South American Umbelliferae. – Bull. Torrey Bot. Club 89: 371-380.
Mathias ME, Constance L. 1962b. A revision of Asteriscium and some related hydrocotyloid Umbelliferae. – Univ. Calif. Publ. Bot. 33: 99-184.
Mathias ME, Constance L. 1965. A revision of the genus Bowlesia Ruiz & Pav. (Umbelliferae-Hydrocotyloideae) and its relatives. – Univ. Calif. Publ. Bot. 38: 1-73.
Mathias ME, Constance L. 1971. A first revision of Huanaca (Umbelliferae-Hydrocotyloideae). – Kurtziana 6: 7-23.
Mathias ME, Constance L. 1976. 145. Umbelliferae. – In: Harling G, Sparre B (eds), Flora of Ecuador 5 Swedish Natural Science Research Council, Stockholm, pp. 1-71.
Mathias ME, Constance L. 1981. Two new Umbelliferae of the Chihuahuan Desert. – Brittonia 33: 342-346.
Melek FR, Miyase T, Abdel-Khalik SM, Mahmoud II, Mina SA. 2004. Saponins and acylated saponins from Dizygotheca kerchoveana. – Phytochemistry 65: 3089-3095.
Melikian AP, Konstantinova AI. 2006. Possible phylogenetic connections of the genus Actinotus Labill. (Umbelliferae-Hydrocotyloideae) according to the data of comparative carpology. – Bot. Žurn. 90: 1753-1764. [In Russian]
Meller B. 2006. Comparative investigation of modern and fossil Toricellia fruits – a disjunctive element in the Miocene and Eocene of Central Europe and the U.S.A. – Beitr. Paläont. 30: 315-327.
Mendes MD, Trindade H, Figueiredo AC, Barroso JG, Fontinha SS, Pedro LG. 2009. Volatile and molecular characterization of two Portuguese endemic species: Angelica lignescens and Melanoselinum decipiens. – Biochem. Syst. Ecol. 37: 98-105.
Mendoza M, Watson MF. 2008. Four new species of Eryngium L. (Apiaceae) from the inter-Andean dry valleys of Bolivia. – Candollea 63: 5-16.
Menemen Y, Jury SL. 2001a. A systematic study of the genus Malabaila Hoffm. (Umbelliferae) comparing with the closely related genera. – In: The Proceedings of the 2nd Balkan Botanical Congress, Istanbul, pp. 299-312.
Menemen Y, Jury SL. 2001b. A taxonomic revision of the genus Pastinaca L. (Umbelliferae). – Israel J. Plant Sci. 49: 67-77.
Menemen Y, Jury SL. 2001c. Taxonomic studies on the genus Zosima Hoffm. (Umbelliferae). – Ann. Naturhist. Mus. Wien 103B: 557-571.
Menitsky GL. 1991. Synopsis of the species of the family Apiaceae (Umbelliferae) from the Caucasus. – Bot. Žurn. 76: 1749-1764. [In Russian]
Meragelman KM, McKee TC, Boyd MR. 2001. 10-Demethoxystegane, a new lignan from Steganotaenia araliacea. – J. Nat. Prod. 64: 1480-1482.
Meylan BA, Butterfield BG. 1978. The structure of New Zealand woods. – New Zealand Dept. Sci. Ind. Res., Wellington.
Mitchell AD, Wagstaff SJ. 1997. Phylogenetic relationships of Pseudopanax species (Araliaeae) inferred from parsimony analysis of rDNA sequence data and morphology. – Plant Syst. Evol. 208: 121-138.
Mitchell AD, Wagstaff SJ. 2000. Phylogeny and biogeography of the Chilean Pseudopanax laetevirens. – New Zealand J. Bot. 38: 409-414.
Mitchell AD, Wen J. 2004. Phylogenetic utility and evidence for multiple copies of granule-bound starch synthase I (GBSSI) in Araliaceae. – Taxon 53: 29-41.
Mitchell AD, Wen J. 2005. Phylogeny of Brassaiopsis (Araliaceae) in Asia based on nuclear ITS and 5S-NTS DNA sequences. – Syst. Bot. 30: 872-886.
Mitchell AD, Frodin DG, Heads MJ. 1997. Reinstatement of Raukaua, a genus of Araliaceae centred in New Zealand. – New Zealand J. Bot. 35: 309-315.
Mitchell AD, Webb CJ, Wagstaff SJ. 1998. Phylogenetic relationships of species of Gingidia and related genera (Apiaceae, subfamily Apioideae). – New Zealand J. Bot. 36: 417-424.
Mitchell AD, Meurk CD, Wagstaff SJ. 1999. Evolution of Stilbocarpa, a megaherb from New Zealand’s sub-antarctic islands. – New Zealand J. Bot. 37: 205-211.
Mitchell AD, Wagstaff SJ. 2000. Phylogeny and biogeography of the Chilean Pseudopanax laetevirens. – New Zealand J. Bot. 38: 409-414.
Mitchell AD, Li R, Brown JW, Schönberger I, Wen J. 2013. Ancient divergence and biogeography of Raukaua (Araliaceae) and close relatives in the southern hemisphere. – Aust. Syst. Bot. 25: 432-446.
Mittal SP. 1961. Studies in the Umbellales II. The vegetative anatomy. – J. Indian Bot. Soc. 40: 424-443.
Mohana Rao PR. 1972. Morphology and embryology of Tieghemopanax sambucifolius with comments on the affinities of the family Araliaceae. – Phytomorphology 22: 75-86.
Mole RH. 1989. Pennantia ‘Otari Debut’. – Wellington Bot. Soc. Bull. 45: 54-55.
Moore DM. 1971. Chromosome studies in the Umbelliferae. – In: Heywood VH (ed), The biology and chemistry of the Umbelliferae, Bot. J. Linn. Soc. 64 [Suppl. 1], Academic Press, London, New York, pp. 233-255.
Moorsel RCMJ van, Baudewijn O. 2000. Apium, Berula en Sium. – Gorteria 26: 244-245.
Motley TJ. 2005. Tetraplasandra lydgatei (Araliaceae): taxonomic recognition of a rare, endemic species from O’ahu, Hawaiian Islands. – Pacific Sci. 59: 105-110.
Mozaffarian V. 1983. The family of Umbelliferae in Iran. Keys and distribution. – Research Institute of Forests and Rangelands, Tehran.
Mozaffarian V. 2003. New species and new records of Iranian Umbelliferae. – Bot. Žurn. 88: 104-124.
Muckensturm B, Diyani F. 1995. Rapport préliminaire sur les constituants volatils de Selinum pyrenaeum, Selinum carvifolia. – Univ. Haute-Alsace, Mulhouse.
Mukherjee PK. 1978. A resume of Indian umbellifers. – In: Cauwet-Marc AM, Carbonnier J (eds), Les Ombellifères, Actes du 2e Symposium International sur les Ombellifères “Contributions Pluridisciplinaires à la Systématique”, Perpignan, Monogr. Syst. Bot. Missouri Bot. Gard. 6: 47-70.
Mukherjee PK. 1982. Nomenclatural transfers in Indian Umbelliferae. – Bull. Bot. Soc. India 24: 42-45.
Mukherjee PK, Constance L. 1986. Two new genera of Indian Umbelliferae. – Brittonia 38: 145-149.
Mukherjee PK, Constance L. 1991. New taxa and transfers in Indian Umbelliferae. – Edinburgh J. Bot. 48: 41-44.
Mukherjee PK, Constance L. 1993. Umbelliferae (Apiaceae) of India. – American Institute of Indian Studies and Oxford & IBH Publ. Co., New Delhi.
Mulligan GA. 1980. The genus Cicuta in North America. – Can. J. Bot. 58: 1755-1767.
Murray BG, De Lange PJ. 1995. Chromosome numbers in the rare endemic Pennantia baylisiana (W. R. B. Oliv.) G. T. S. Baylis (Icacinaceae) and related species. – New Zealand J. Bot. 33: 563-564.
Nagata KM, Gon III SM. 1987. Sanicula mariversa (Apiaceae), a new species from ’Ōhikilolo Ridge, Wai’anae Mountains, O’ahu in the Hawaiian Archipelago. – Syst. Bot. 12: 406-409.
Nakai T. 1924. Araliaceae imperii japonica. – J. Arnold Arbor. 5: 1-36.
Nannfeldt JA. 1924. Revision des Verwandtschaftskreises von Centella asiatica (L.) Urb. – Svensk Bot. Tidskr. 18: 397-426.
Narayana LL, Radhakrishnaiah M. 1976. Floral anatomy of the Pittosporaceae: 1. – J. Jap. Bot. 51: 278-282.
Narayana LL, Radhakrishnaiah M. 1978. Floral anatomy of the Pittosporaceae: 2. – Acta Bot. Indica 6: 104-107.
Narayana LL, Radhakrishnaiah M. 1979. Floral anatomy of the Pittosporaceae: 3. – J. Jap. Bot. 54: 324-349.
Narayana LL, Radhakrishnaiah M. 1980. Floral anatomy of the Pittosporaceae: 4. – Indian J. Bot. 3: 6-12.
Narayana LL, Radhakrishnaiah M. 1981. Floral anatomy of the Pittosporaceae: 5. – J. Jap. Bot. 56: 137-141.
Narayana LL, Radhakrishnaiah M. 1982. Floral anatomy of the Pittosporaceae: 6. – Can. J. Bot. 60: 1859-1867.
Narayana LL, Radhakrishnaiah M. 1984. Floral anatomy of the Pittosporaceae: a discussion. – J. Indian Bot. 63: 53-56.
Narayana LL, Sundari KT. 1977. Embryology of Pittosporaceae 1. – J. Jap. Bot. 52: 204-209.
Nasir E. 1972. Stewartiella E. Nasir, gen. nov. – Fl. West Pakistan 20: 152-153.
Neves SS, Watson MF. 2004. Phylogenetic relationships in Bupleurum (Apiaceae) based on nuclear ribosomal DNA ITS sequence data. – Ann. Bot. 93: 379-398.
Ng N, Walker JRL. 1975. A chemotaxonomic study of the New Zealand Araliaceae: phenolic compounds. – Mauri Ora 3: 3-10.
Nicolas AN, Plunkett GM. 2009. The demise of subfamily Hydrocotyloideae (Apiaceae) and the re-alignment of its genera across the entire order Apiales. – Mol. Phylogen. Evol. 53: 134-151.
Nicolas AN, Plunkett GM. 2012. Untangling generic limits in Azorella, Laretia, and Mulinum (Apiaceae: Azorelloideae): insights from phylogenetics and biogeography. – Taxon 61: 826-840.
Nicolas AN, Plunkett GM. 2014.
Diversification times and biogeographic patterns in Apiales. – Bot. Rev. 80:
30-58.
Nielsen BE. 1971. Coumarin patterns in the Umbelliferae. – In: Heywood VH (ed), The biology and chemistry of the Umbelliferae, Bot. J. Linn. Soc. 64 [Suppl. 1]: 325-336.
Norman C. 1922. On Cotylonia, a new genus of Umbelliferae. – J. Bot. (London) 60: 166-167.
Norman C. 1929. Umbelliferae from Nepal. – J. Bot. (London) 67: 245-247.
Norman C. 1938. On the genus Trachydium. – J. Bot. (London) 76: 229-233.
Norman C. 1940. Notes on the Sino-Himalayan Umbelliferae. – J. Bot. (London) 78: 229-232.
Nuraliev MS, Beer AS, Oskolski AA. 2009. Vascular anatomy of flower of Tupidanthus calyptratus and related species of Schefflera in the context of the origin of floral polymery in Araliaceae. – Bot. Žurn. 94: 625-642. [In Russian]
Nuraliev MS, Oskolski AA, Sokoloff DD, Remizowa MV. 2010. Flowers of Araliaceae: structural diversity, developmental and evolutionary aspects. – Plant Divers. Evol. 128: 247-268.
Nuraliev MS, Sokoloff DD, Oskolski AA. 2011. Floral anatomy of Asian Schefflera (Araliaceae, Apiales): comparing variation of flower groundplan and vascular patterns. – Intern. J. Plant Sci. 172: 735-762.
Nuraliev MS, Degtjareva GV, Sokoloff DD, Oskolski AA, Samigullin TH, Valiejo-Roman CM. 2014. Flower morphology and relationships of Schefflera subintegra (Araliaceae, Apiales): an evolutionary step towards extreme floral polymery. – Bot. J. Linn. Soc. 175: 553-597.
Ohashi H. 1987a. Eleutherococcus (Araliaceae) – a new system and new combinations. – J. Jap. Bot. 62: 353-361.
Ohashi H. 1987b. New combination in Chinese Evodiopanax (Araliaceae). – J. Jap. Bot. 62: 362.
Ohashi H. 1994. Nomenclature of Kalopanax septemlobus (Thunberg ex Murray) Koidzumi and classification of its infraspecific taxa (Araliaceae). – J. Jap. Bot. 69: 28-31.
Okeke SE. 1982. Morphological variation of bracts, bracteoles and fruits in Daucus L. – In: Cauwet-Marc A-M, Carbonnier J (eds), Les Ombellifères, Actes du 2e Symposium International sur les Ombellifères “Contributions Pluridisciplinaires à la Systématique”, Monogr. Syst. Bot. Missouri Bot. Gard. 6: 161-174.
Olivier DK, Wyk B-E van, Heerden FR van. 2008. The chemotaxonomic and medicinal significance of phenolic acids in Arctopus and Alepidea (Apiaceae subfamily Saniculoideae). – Biochem. Syst. Ecol. 36: 724-729.
Orchard AE. 1989. Azorella Lamarck (Apiaceae) on Heard and Macquerie Islands, with description of a new species, A. macquariensis. – Muelleria 7: 15-20.
Oskolski AA. 1994. Wood anatomy of Araliaceae. – Proc. Komarov Bot. Inst. 10, Russian Academy of Sciences, St. Petersburg. [In Russian]
Oskolski AA. 1995. Wood anatomy of Schefflera and related taxa (Araliaceae). – IAWA J. 16: 159-215.
Oskolski AA. 1996. A survey of the wood anatomy of the Araliaceae. – In: Donaldson LA, Singh AP, Butterfield BG, Whitehouse LJ (eds), Recent advances in wood anatomy, Forest Research Institute, Rotorua, New Zealand, pp. 99-119.
Oskolski AA. 2001. Phylogenetic relationships within Apiales: evidence from wood anatomy. – Edinburgh J. Bot. 58: 201-206.
Oskolski AA, Jansen S. 2009. Distribution of scalariform and simple perforation plates within the vessel network in secondary xylem of Araliaceae and its implications for wood evolution. – Plant Syst. Evol. 278: 43-51.
Oskolski AA, Lowry II PP. 2000. Wood anatomy of Mackinlaya and Apiopetalum (Araliaceae) and its systematic implications. – Ann. Missouri Bot. Gard. 87: 171-182.
Oskolski AA, Lowry II PP. 2001. Wood anatomy of Schefflera and related taxa (Araliaceae) II. Systematic wood anatomy of New Caledonian Schefflera. – IAWA J. 22: 301-330.
Oskolski AA, Wyk B-E van. 2009. Systematic and phylogenetic value of wood anatomy in Heteromorpheae (Apiaceae, Apioideae). – Bot. J. Linn. Soc. 158: 569-583.
Oskolski AA, Wyk B-E van. 2010. Wood and bark anatomy of Centella: scalariform perforation plates support an affinity with the subfamily Mackinlayoideae (Apiaceae). – Plant Syst. Evol. 289: 127-135.
Oskolski AA, Lowry II PP, Richter HG. 1997. Systematic wood anatomy of Myodocarpus, Delarbrea, and Pseudosciadium (Araliaceae). – Adansonia, sér. III, 19: 61-75.
Oskolski AA, Kotina EL, Fomichev IV, Tronchet F, Lowry PP. 2007. Systematic implications of wood and bark anatomy in the Pacific Island genus Meryta (Araliaceae). – Bot. J. Linn. Soc. 153: 363-379.
Oskolski AA, Rossouw AS, Wyk B-E van. 2010. Wood and bark anatomy of Steganotaenia and Polemanniopsis (tribe Steganotaenieae, Apiaceae) with notes on phylogenetic implications. – Bot. J. Linn. Soc. 163: 55-69.
Oskolski AA, Sokoloff DD, Wyk B-E van. 2010. False paracarpy in Seemannaralia (Araliaceae): from bilocular ovary to unilocular fruit. – Ann. Bot. 106: 29-36.
Ostroumova TA. 1987. The types of stomata in representatives of the Apiaceae family. – Bot. Žurn. 72: 1479-1488.
Ostroumova TA. 1990. Stomatal types in the Umbelliferae in relation to taxonomy. Tribes Coriandreae and Scandiceae. – Feddes Repert. 101: 409-417.
Ostroumova TA, Kljuykov EV. 2007. Stomatal types in Chinese and Himalayan Umbelliferae. – Feddes Repert. 118: 84-102.
Ostroumova TA, Oskolski AA. 2010. Survey of the leaf anatomy of Araliaceae and some related taxa. – Plant Div. Evol. 128: 423-441.
Ostroumova TA, Pimenov MG. 1997. Carpological diversity of African Peucedanum s.l. (Umbelliferae) I. The species of southern Africa. – Feddes Repert. 108: 299-318.
Ozcan T. 2002. SEM observations on petals and fruits of some Turkish endemic Bupleurum L. (Umbelliferae) species. – Bot. J. Linn. Soc. 138: 441-449.
Ozcan T. 2004. Analysis of the fruit surfaces in Bupleurum L. (Umbelliferae) with SEM. – Plant Syst. Evol. 247: 61-74.
Ozhatay N, Akalin E. 2000. A new species of Ferulago W. Koch (Umbelliferae) from North-West Turkey. – Bot. J. Linn. Soc. 133: 535-542.
Pan W, Zhang Y, Xu B, Cao P, Liang G. 2006. Two new naturally occurring optical polyacetylene compounds from Torricellia angulata var. intermedia and the determination of their absolute configuration. – Nat. Prod. Res. 20: 1098-1104.
Pan Z-H, Liu X-T, Sheh M-L, Xu L-R. 1991. A study on karyotypes of eight species and geographical distribution of Angelica (Umbelliferae) in Sichuan. – Acta Phytotaxon. Sin. 29: 431-438.
Pan Z-H, Wu Z-J, Pu F-T. 1992. Anatomical studies of petiole in Ligusticum from China. – Acta Bot. Yunnan. 14: 143-149. [In Chinese]
Pan Z-H, Zhuang T-D, Yao X-M, Sheng N. 1994. A study on karyotypes and geographical distribution of Angelica and relatied genera (Umbelliferae) in China. – Acta Phytotaxon. Sin. 32: 419-424.
Pan Z-H, Sheh M-I, Liu X-T, Yao X-M. 1995. On karyotypes and geographical distribution of endemic genera in Umbelliferae from China. – J. Plant Res. Envir. 4: 1-8.
Panahi M, Banasiak Ł, Piwczyński M, Puchałka R, Oskolski AA, Spalik K. 2015. Phylogenetic relationships among Dorema, Ferula and Leutea (Apiaceae: Scandiceae: Ferulinae) inferred from nrDNA ITS and cpDNA noncoding sequences. – Taxon 64: 770-783.
Papini A. 2006. The systematic position of Chamaesciadium C. A. Meyer (Umbelliferae) on the basis of nuclear ITS sequence. – Flora Medit. 16: 45-55.
Papini A, Banci F, Nardi E. 2007. Molecular evidence of polyphyletism in the plant genus Carum L. (Apiaceae). – Gen. Mol. Biol. 30: 475-482.
Park WC, Lee S. 1989. A palynotaxonomic study of the Korean Araliaceae. – Korean J. Plant Taxon. 19: 103-121.
Patel RN. 1973. Wood anatomy of the dicotyledons indigenous to New Zealand I. Cornaceae. – New Zealand J. Bot. 11: 3-22.
Patel RN, Bowles A. 1978. Wood anatomy of the dicotyledons indigenous to New Zealand XII. Icacinaceae. – New Zealand J. Bot. 16: 7-12.
Pax F. 1891. Pittosporaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(2a), W. Engelmann, Leipzig, pp. 106-114.
Peng F,Sheh M-I. 1991. Approaches to the systematic position and origin of Dickinsia Franch. (Umbelliferae). – Bull. Nanjing Bot. Gard. 1991: 23-30.
Periasamy K. 1966. Studies on seeds with ruminate endosperm VI. Rumination in the Araliaceae, Aristolochiaceae, Caprifoliaceae and Ebenaceae. – Proc. Indian Acad. Sci., Sect. B, 60: 127-134.
Perkins, A. J. 2019a. Molecular phylogenetics and species delimitation in annual species of Hydrocotyle (Araliaceae) from South Western Australia. – Mol. Phylogen. Evol. 134: 129-141.
Perkins, A. J. 2019b. Nomenclatural updates and a new species of annual Hydrocotyle (Araliaceae) from Western Australia. – Nuytsia 30: 253-277.
Perrie LR, Shepherd LD. 2009. Reconstructing the species phylogeny of Pseudopanax (Araliaceae), a genus of hybridising trees. – Mol. Phylogen. Evol. 52: 774-783.
Petersen G, Affolter J. 1999. A new species of Lilaeopsis (Apiaceae) from Mauritius. – Novon 9: 92-94.
Petersen G, Seberg O, Larsen S. 2002. The phylogenetic and taxonomic position of Lilaeopsis (Apiaceae), with notes on the applicability of ITS sequence data for phylogenetic reconstruction. – Aust. Syst. Bot. 15: 181-191.
Pfosser M, Jakubowsky G, Schlüter PM, Fer T, Kato H, Stuessy TF, Syn B-Y. 2005 [2006]. Evolution of Dystaenia takesimana (Apiaceae), endemic to Ullung Island, Korea. – Plant Syst. Evol. 256: 159-170.
Philipson WR. 1951. Contributions to our knowledge of Old World Araliaceae. – Bull. Brit. Mus. (Nat. Hist.), Bot. 1: 3-20.
Philipson WR. 1965. The New Zealand genera of the Araliaceae. – New Zealand J. Bot. 3: 333-341.
Philipson WR. 1967. Griselinia Forst. fil. – anomaly or link? – New Zealand J. Bot. 5: 134-165.
Philipson WR. 1970a. Constant and variable features of the Araliaceae. – In: Robson NKB, Cutler DF, Gregory M (eds), New research in plant anatomy, Bot. J. Linn. Soc. 63[Suppl. 1], Academic Press, London, pp. 87-100.
Philipson WR. 1970b. A redefinition of Gastonia and related genera (Araliaceae). – Blumea 18: 497-505.
Philipson WR. 1973. A revision of Harmsiopanax (Araliaceae). – Blumea 21: 81-86.
Philipson WR. 1976. A synopsis of the Malesian species of Osmoxylon (including Boerlagiodendron), Araliaceae. – Blumea 23: 99-119.
Philipson WR. 1978a. Araliaceae: growth forms and shoot morphology. – In: Tomlinson PB, Zimmermann MH (eds), Tropical trees as living systems, Cambridge University Press, Cambridge, pp. 269-284.
Philipson WR. 1978b. A synopsis of the Malesian species of Polyscias (Araliaceae). – Blumea 24: 169-172.
Philipson WR. 1978c. The identity of Arthrophyllum and Eremopanax (Araliaceae). – Adansonia, sér. II, 17: 329-333.
Philipson WR. 1979. Araliaceae I. – In: Steenis CGGJ van (ed), Flora Malesiana I, 9(1), Sijthoff & Noordhoff International Publ., Alphen aan den Rijn, The Netherlands, pp. 1-105.
Philipson WR, Butterfield BG. 1980. The systematic position of Aralidium Miq. – a multidisciplinary study 2. Wood anatomy. – Taxon 29: 404-406.
Philipson WR, Stone BC. 1980. The systematic position of Aralidium Miq. – a multidisciplinary study 1. Introduction and floral and general anatomy. – Taxon 29: 391-403.
Philipson WR, Stone BC, Butterfield BG, Tseng CC, Jensen SR, Nielsen BJ, Bate-Smith EC, Fairbrothers DE. 1980. The systematic position of Aralidium Miq. – a multidisciplinary study. – Taxon 29: 391-416.
Pickel B, Drew DP, Manczak T, Weitzel C, Simonsen HT, Ro DK. 2012. Identification and characterization of a kunzeaol synthase from Thapsia garganica: implications for the biosynthesis of the pharmaceutical thapsigargin. – Biochem. J. 448: 261-271.
Pickering JL, Fairbrothers DE. 1970. A serological comparison of Umbelliferae subfamilies. – Amer. J. Bot. 57: 988-992.
Pickering JL, Fairbrothers DE. 1971. The use of serological data in a comparison of tribes in the Apioideae. – In: Heywood VH (ed), The biology and chemistry of the Umbelliferae, Bot. J. Linn. Soc. 64 [Suppl. 1]: 315-324.
Pimenov MG. 1968a. Systematic grouping of species of Angelica L. occurring in the USSR on the basis of coefficients of similarity. – Bull. Soc. Nat. Moscou, Sect. Biol. 73: 124-139.
Pimenov MG. 1968b. The analysis of the distribution of Angelica species occurring in the Soviet Far East. – Bot. Žurn. 53: 932-946. [In Russian]
Pimenov MG. 1975. Sphaenolobium M. Pimen. Genus novum Umbelliferarum ex Asia Media. – Nov. Sist. Vysš. Rast. 12: 238-245. [In Russian]
Pimenov MG. 1977. Vicatia DC., a new genus of the family Umbelliferae in the flora of the USSR. – Bot. Žurn. 62: 1321-1326. [In Russian]
Pimenov MG. 1978. De generis Seseli L. notulae systematicae II. Adumbratio specierum florae URSS. – Nov. Sist. Vyssh. Rast. 15: 188-200. [In Russian]
Pimenov MG. 1982. Les Ombellifères d’Asie Moyenne. – In: Cauwet-Marc A-M, Carbonnier J (eds), Les Ombellifères, Actes du 2e Symposium International sur les Ombellifères “Contributions Pluridisciplinaires à la Systématique”, Monogr. Syst. Bot. Missouri Bot. Gard. 6: 33-45.
Pimenov MG. 1986. Kitagawia – a new Asiatic genus of the family Umbelliferae. – Bot. Žurn. 71: 942-949.
Pimenov MG. 1988. The genus Zeravschania Korov.: the species and the taxonomic position in the family Umbelliferae. – Byull. Moskovsk. Obshch. Isp. Prir., Otd. Biol. 93: 75-80. [In Russian]
Pimenov MG. 1992. Ormosolenia restored. – Edinburgh J. Bot. 49: 219-223.
Pimenov MG, Constance L. 1985. Nomenclature of suprageneric taxa in Umbelliferae/Apiaceae. – Taxon 34: 493-501.
Pimenov MG, Kljuykov EV. 1982. Critical analysis of the genera Hyalolaena and Hymenolyma and the related taxa of Umbelliferae-Apioideae. – Bot. Žurn. 67: 873-889. [In Russian]
Pimenov MG, Kljuykov EV. 1987. Neoconopodium – a new genus of the Umbelliferae from the Himalaya. – Feddes Repert. 98: 373-378.
Pimenov MG, Kljuykov EV. 1995a. Korshinskya extended westwards. – Edinburgh J. Bot. 52: 337-342.
Pimenov MG, Kljuykov EV. 1995b. Notes on Himalayan Schulzia (Umbelliferae). – Kew Bull. 50: 637-644.
Pimenov MG, Kljuykov EV. 1996a. Taxonomic and floristic novelties in Chinese Umbelliferae from Qomolangma regions (Xizang, the Himalayas). – Act Phytotaxon. Sin. 34: 1-11.
Pimenov MG, Kljuykov EV. 1996b. Gongylotaxis, a new genus of the Umbelliferae, endemic to Afghanistan. – Edinburgh J. Bot. 53: 187-191.
Pimenov MG, Kljuykov EV. 1998. Proposal to conserve the name Sinocarum against Dactylaea (Umbelliferae). – Taxon 47: 477-478.
Pimenov MG, Kljuykov EV. 1999. New nomenclatural combinations for Chinese Umbelliferae. – Feddes Repert. 110: 481-491.
Pimenov MG, Kljuykov EV. 2000a. Taxonomic revision of Pleurospermum Hoffm. and related genera of Umbelliferae II. The genera Pleurospermum, Pterocyclus, Trachydium, Keraymonia, Pseudotrachydium, Aulacospermum, and Hymenolaena. – Feddes Repert. 111: 517-534.
Pimenov MG, Kljuykov EV. 2000b. Taxonomic revision of Pleurospermum Hoffm. and related genera of Umbelliferae III. The genera Physospermopsis and Hymenidium. – Feddes Repert. 111: 535-552.
Pimenov MG, Kljuykov EV. 2001. Floristic novelties in the Umbelliferae of Xinjiang, China. – Acta Phytotaxon. Sin. 39: 193-202.
Pimenov MG, Kljuykov EV. 2002a. The Umbelliferae of Kirghyzia. – KMK Scientific Press, Moscow. [In Russian]
Pimenov MG, Kljuykov EV. 2002b. Broadening the genus Cephalopodum Korovin (Umbelliferae). – Candollea 57: 261-269.
Pimenov MG, Kljuykov EV. 2003a. Notes on some Sino-Himalayan species of Angelica and Ostericum (Umbelliferae). – Willdenowia 33: 121-137.
Pimenov MG, Kljuykov EV. 2003b. What is Sium frigidum Hand.-Mazz. (Umbelliferae)? – Feddes Repert. 114: 350-357.
Pimenov MG, Kljuykov EV. 2004a. A new look at Kedarnatha P. K. Mukh. & Constance (Umbelliferae). – Feddes Repert. 115: 230-238.
Pimenov MG, Kljuykov EV. 2004b. Critical notes on the genus Calyptrosciadium Rech. f. & Kuber (Umbelliferae). – Candollea 59: 95-101.
Pimenov MG, Kljuykov EV. 2004c. Hymenidium (Umbelliferae) in China and India: a new species, a new combination and some corrections. – Bot. Žurn. 89: 1652-1665.
Pimenov MG, Kljuykov EV. 2005. New West Himalayan genus of the Umbelliferae, with notes on Tibetan species, described in Pachypleurum. – Feddes Repert. 116: 80-91.
Pimenov MG, Kljuykov EV. 2009. Towards a clarification in the taxonomy of Sino-Himalayan species of Selinum s.l. (Umbelliferae) 2. Further studies in Oreocome in the Himalayas and adjacent areas. – Willdenowia 39: 93-99.
Pimenov MG, Kljuykov EV. 2013. Two new species of Zeravschania (Umbelliferae) and some additional notes on the genus. – Phytotaxa 130: 25-33.
Pimenov MG, Lavrova TV. 1994. Second species of the genus Tamamschjania M. Pimen. et Kljuykov (Umbelliferae). – Feddes Repert. 105: 433-436.
Pimenov MG, Leonov MV. 1993. The genera of the Umbelliferae. A nomenclator. – Royal Botanic Gardens, Kew, United Kingdom.
Pimenov MG, Leonov MV. 2004a. Asia, the continent with the highest Umbelliferae biodiversity. – South Afr. J. Bot. 70: 417-419.
Pimenov MG, Leonov MV. 2004b. The Asian Umbelliferae biodiversity database (ASIUM) with particular reference to South-West Asian taxa. – Turk. J. Bot. 28: 139-145.
Pimenov MG, Ostroumova TA. 1994. The genus Malabaila Hoffm. (Umbelliferae: Tordylieae): a carpological investigation and taxonomic implications. – Feddes Repert. 105: 141-155.
Pimenov MG, Ostroumova TA. 2000. Closest relatives of Chinese genus Arcuatopterus (Umbelliferae) in the Indian subcontinent. – Feddes Repert. 111: 553-558.
Pimenov MG, Sdobnina LI. 1975. On the taxonomy of the genus Seseli L. I. Revision of the genus Libanotis Hill (Umbelliferae). – Bot. Žurn. 60: 1108-1122. [In Russian]
Pimenov MG, Sdobnina LI. 1984. Nodal anatomy as a taxonomic character in the family Umbelliferae. – Bot. Žurn. 69: 283-294. [In Russian]
Pimenov MG, Tikhomirov VN. 1983. The taxonomic problems in the genera Prangos Lindl., Cachrys L., Cryptodiscus Schrenk and Hippomarathrum Hoffmg. & Link. – Feddes Repert. 94: 145-164.
Pimenov MG, Kljuykov EV, Teriochin AT, Deviatkova GN. 1981. The delimitation of the genera of the geophytic Umbelliferae of Middle Asia by the methods of multivariate statistics. – Bot. Žurn. 66: 328-340. [In Russian]
Pimenov MG, Tikhomirov VN, Lavrova TV. 1986. Revision of the genus Cnidium Cuss. ex Jussieu (Umbelliferae-Apioideae). – Bjull. Moskovsk. Obšč. Isp. Prir., Otd. Biol. 91: 90-98. [In Russian]
Pimenov MG, Mukherjee PK, Kljuykov EV, Leonov MV. 1991. Notes on the genera Vicatia DC., Sinodielsia H. Wolff and Tongoloa H. Wolff (Umbelliferae). – Feddes Repert. 102: 375-384.
Pimenov MG, Schneyer VS, Valiejo-Roman KM, Terentieva EI, Troitsky AV. 1999. Komarovia Korovin (Umbelliferae): a multidisciplinary study of a genus of uncertain taxonomic position. – Komarovia 1: 61-73.
Pimenov MG, Kljuykov EV, Leonov MV. 2000. Taxonomic revision of Pleurospermum Hoffm. and related genera of Umbelliferae I. General part. – Feddes Repert. 111: 499-515.
Pimenov MG, Kljuykov EV, Dickoré WB, Miehe G. 2000. Four Himalayan Umbelliferae new to the flora of China, with critical notes on Tordyliopsis DC. and Keraymonia Farille. – Willdenowia 30: 361-367.
Pimenov MG, Kljuykov EV, Ostroumova TA. 2001. Towards a clarification in the taxonomy of Sino-Himalayan species of Selinum L. s.l. (Umbelliferae). The genus Oreocome Edgew. – Willdenowia 31: 101-124.
Pimenov MG, Kljuykov EV, Ostroumova TA. 2003. A revision of Conioselinum Hoffm. (Umbelliferae) in the Old World. – Willdenowia 33: 353-377.
Pimenov MG, Vasil’eva MG, Leonov MV, Daushkevich JV. 2003. Karyotaxonomical analysis in the Umbelliferae. – Science Publ., Inc., Enfield, New Hampshire.
Pimenov MG, Kljuykov EV, Ostroumova TA. 2007. Critical taxonomic analysis of Dichoropetalum, Johrenia, Zeravschania and related genera of Umbelliferae-Apioideae-Peucedaneae. – Willdenowia 37: 465-502.
Pimenov MG, Kljuykov EV, Ostroumova TA. 2008. Reduction of Notopterygium to Hansenia (Umbelliferae). – Willdenowia 38: 155-172.
Pimenov MG, Ostroumova TA, Degtjareva GV, Samigullin TH. 2016a. Sillaphyton, a new genus of the Umbelliferae, endemic to the Korean Peninsula. – Bot. Pacifica 5: 31-41.
Pimenov MG, Degtjareva GV, Ostroumova TA, Samigullin TH, AVeryanov LV. 2016b. Xyloselinum laoticum (Umbelliferae), a new species from Laos, and taxonomic placement of the genus in the light of nrDNA ITS sequence analysis. – Phytotaxa 244: 248-262.
Piwczyński M, Puchałka R, Spalik K. 2015. The infrageneric taxonomy of Chaerophyllum (Apiaceae) revisited: new evidence from nuclear ribosomal DNA ITS sequences and fruit anatomy. – Bot. J. Linn. Soc. 178: 298-313.
Plouvier V. 1982. Ombellifères et familles voisines: leurs analogies et leurs distinctions biochimiques. – In: Cauwet-Marc A-M, Carbonnier J (eds), Les Ombellifères, Actes du 2e Symposium International sur les Ombellifères “Contributions Pluridisciplinaires à la Systématique”, Monogr. Syst. Bot. Missouri Bot. Gard. 6: 535-548.
Plunkett GM. 1994. A molecular-phylogenetic approach to the “family-pair dilemma” in Apiales and Cyperales. – Ph.D. diss., Washington State University, Pullman, Washington.
Plunkett GM. 2001. Relationships of the order Apiales to subclass Asteridae: a re-evaluation of morphological characters based on insights from molecular data. – Edinburgh J. Bot. 58: 183-200.
Plunkett GM, Downie SR. 1999. Major lineages within Apiaceae subfamily Apioideae: a comparison of chloroplast restriction site and DNA sequence data. – Amer. J. Bot. 86: 1014-1026.
Plunkett GM, Downie SR. 2000. Expansion and contraction of the chloroplast inverted repeat in Apiaceae subfamily Apioideae. – Syst. Bot. 25: 648-667.
Plunkett GM, Lowry II PP. 2001. Relationships among ‘ancient araliads’ and their significance for the systematics of Apiales. – Mol. Phylogen. Evol. 19: 259-276.
Plunkett GM, Lowry II PP. 2010. Paraphyly and polyphyly in Polyscias sensu lato: molecular evidence and the case for recircumscribing the “pinnate genera” of Araliaceae. – Plant Divers. Evol. 128: 23-54.
Plunkett GM, Lowry II PP. 2012. Phylogeny and diversification in the Melanesian Schefflera clade (Araliaceae) based on evidence from nuclear rDNA spacers. – Syst. Bot. 37: 279-291.
Plunkett GM, Nicolas AN. 2017. Assessing Azorella (Apiaceae) and its allies: phylogenetics and a new classification. – Brittonia 69: 31-61.
Plunkett GM, Soltis DE, Soltis PS. 1996a. Evolutionary patterns in Apiaceae: inferences based on matK sequence data. – Syst. Bot. 21: 477-495.
Plunkett GM, Soltis DE, Soltis PS. 1996b. Higher level relationships of Apiales (Apiaceae and Araliaceae) based on phylogenetic analysis of rbcL sequences. – Amer. J. Bot. 83: 499-515.
Plunkett GM, Soltis DE, Soltis PS. 1997. Clarification of the relationship between Apiaceae and Araliaceae based on matK and rbcL sequence data. – Amer. J. Bot. 84: 565-580.
Plunkett GM, Lowry II PP, Burke MK. 2001. The phylogenetic status of Polyscias (Araliaceae) based on nuclear ITS sequence data. – Ann. Missouri Bot. Gard. 88: 213-230.
Plunkett GM, Chandler GT, Lowry II PP, Pinney SM, Sprenkle TS. 2004. Recent advances in understanding Apiales and a revised classification. – South Afr. J. Bot. 70: 371-381.
Plunkett GM, Lowry II PP, Vu NV. 2004. Phylogenetic relationships among Polyscias (Araliaceae) and close relatives from the Western Indian Ocean Basin. – Intern. J. Plant Sci. 165: 861-873.
Plunkett GM, Wen J, Lowry II PP. 2004. Infrafamilial classifications and characters in Araliaceae: insights from the phylogenetic analysis of nuclear (ITS) and plastid (trnL-trnF) sequence data. – Plant Syst. Evol. 245: 1-39.
Plunkett GM, Lowry II PP, Frodin DG, Wen J. 2005. Phylogeny and geography of Schefflera: pervasive polyphyly in the largest genus of Araliaceae. – Ann. Missouri Bot. Gard. 92: 202-224.
Plunkett GM, Pimenov MG, Reduron J-P, Kljuykov EV, van Wyk B-E, Ostroumova TA, Henwood MJ, Tilney PM, Spalik K, Watson MF, Lee B-Y, Pu F-D, Webb CJ, Hart JM, Mitchell AD, Muckensturm B. 2018. Apiaceae. – In: Kadereit JW, Bittrich V (eds). 2018. The families and genera of vascular plants XV. Flowering plants – eudicots – Apiales, Gentianales (except Rubiaceae). Springer, Berlin, pp. 9-206.
Plunkett GM, Wen J, Lowry II PP, Mitchell AD, Henwood MJ, Fiaschi P. 2018. Araliaceae. – In: Kadereit JW, Bittrich V (eds). 2018. The families and genera of vascular plants XV. Flowering plants – eudicots – Apiales, Gentianales (except Rubiaceae). Springer, Berlin, pp. 413-446.
Plunkett GM, Xiang Q-Y, Lowry II PP, Schatz GE. 2018. Torricelliaceae. – In: Kadereit JW, Bittrich V (eds). 2018. The families and genera of vascular plants XV. Flowering plants – eudicots – Apiales, Gentianales (except Rubiaceae). Springer, Berlin, pp. 549-556.
Potgieter MJ. 2018. Pennantiaceae. – In: Kadereit JW, Bittrich V (eds). 2018. The families and genera of vascular plants XV. Flowering plants – eudicots – Apiales, Gentianales (except Rubiaceae). Springer, Berlin, pp. 533-538.
Press JR, Dias E. 1998. The genera Melanoselinum Hoffm. and Angelica L. (Umbelliferae) in Macaronesia. – Arquipélago. Life and Marine Sciences 16A: 1-10.
Prieto JAF, Cires E. 2014. Phylogenetic placement of Dethawia, Meum, and Rivasmartinezia (Apioideae, Apiaceae): evidence from nuclear and plastid DNA sequences. – Plant Biosyst. 148: 975-987.
Pritzel E. 1930. Pittosporaceae. – In: Engler A, Harms H (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 18a, W. Engelmann, Leipzig, pp. 265-286.
Pu F-T. 1991. A revision of the genus Ligusticum (Umbelliferae) in China. – Acta Phytotaxon. Sin. 29: 385-393, 525-548. [In Chinese]
Pu F-T. 1998. New names in Chinese Apiaceae. – Novon 8: 70-71.
Pu F-T, Peng Y-L. 2005. Taxonomic notes on Meeboldia H. Wolff (Umbelliferae). – Acta Phytotaxon. Sin. 43: 552-556.
Pu F-T, Wang Y-P. 1994. A new species of Notopterygium (Umbelliferae) from Sichuan. – J. Sichuan Univ., Nat. Sci. Ed. 31: 386-388. [In Chinese]
Pu F-T, He X-J, Wang P-L, Wang Y-P, Liu X-T. 1993. New taxa of Heracleum (Umbelliferae) from China. – Acta Phytotaxon. Sin. 31: 368-373.
Pu F-T, Wang P-L, Zheng Z-H, Wang Y-P. 2000. A reclassification of Notopterygium Boissieu (Umbelliferae). – Acta Phytotaxon. Sin. 38: 430-436. [In Chinese]
Pu G-Z, Liu Q-X. 2005. Comparative anatomical study on the genus Physospermopsis fruit from China and its systematic significance. – J. Plant Res. Envir. 14: 1-6.
Pujadas-Salva AJ, Plaza-Arregui L. 2003. Studies on Thapsia (Apiaceae) from north-western Africa: a forgotten and a new species. – Bot. J. Linn. Soc. 143: 433-442.
Qi C-J. 1988. Hunaniopanax – a new genus of Araliaceae from China. – Acta Phytotaxon. Sin. 26: 47-49.
Qin H-Z, Li B-Y, Wu Z-J, Pan Z-H. 1995. On the fruit anatomy of Angelica L. (s.l.) of East Asia and North America and its evolution. – Acta Bot. Bor.-Occid. Sin. 15: 48-54.
Radford EA, Watson MF, Preston J. 2001. Phylogenetic relationships of species of Aciphylla (Apiaceae subfamily Apioideae) and related genera using molecular, morphological and combined data sets. – New Zealand J. Bot. 39: 183-208.
Raquet V. 2004. Phylogénie morphologique des Myodocarpaceae: famille sub-endémique de Nouvelle-Calédonie. – Thesis, DEA de Systématique Animale et Végétale, Muséum National d’Histoire Naturelle, Paris.
Rasmussen SK, Avatgo P. 1998. Characterization of chromosomes and genome organization of Thapsia garganica L. by localizations of rRNA genes using fluorescent in situ hybridization. – Hereditas 129: 231-239.
Rechinger KH. 1952. Umbelliferae novae iranicae III. – Anz. Österr. Akad. Wiss., Math.-Naturwiss. Kl. 11: 240-244.
Rechinger KH, Riedl H. 1963. Symbolae Afghanicae V: Umbelliferae. – Biol. Skr. 13: 27-135.
Reduron J-P. 1982.Contribution à l’étude morphologique de petale chez les Ombellifères. – In: Cauwet-Marc A-M, Carbonnier J (eds), Les Ombellifères “Contributions pluridisciplinaires à la systématique”. Actes du 2e Symposium International sur les Ombellifères, Monogr. Syst. Bot. Missouri Bot. Gard. 6: 121-131.
Reduron J-P. 2007. Ombellifères de France 2. – Bull. Soc. Bot. Centre-Ouest, nouv. sér., num. spec. 27.
Reduron J-P, Spalik K. 1995. Le genre Anthriscus (Apiaceae) dans la flore française. – Acta Bot. Gall. 142: 55-96.
Reduron J-P, Charpin A, Pimenov MG. 1997. Contribution à la nomenclature générique des Apiaceae (Ombellifères). – J. Bot. Soc. France 1: 91-104.
Reduron J-P, Mathez J, Downie SR, Danderson CA, Ostroumova T. 2009. Pseudoridolfia, nouveau genre d’Apiaceae découvert au Maroc. – Acta Bot. Gallica 156: 487-500.
Reiche KF. 1899. Estudios críticos sobre la flora de Chile, 52, Umbelliferas. – An. Univ. Chile 104: 767-847.
Reuther K, Claßen-Bockhoff R. 2010. Diversity behind uniformity – inflorescence architecture and flowering sequence in Apiaceae-Apioideae. – Plant Divers. Evol. 128: 181-220.
Roberts HA. 1979. Periodicity of seedling emergence and seed survival in some Umbelliferae. – J. Appl. Ecol. 16: 195-201.
Rodríguez CRL. 1957. Systematic anatomical studies on Myrrhidendron and other woody Umbellales. – Univ. Calif. Publ. Bot. 29: 145-318.
Rodríguez CRL. 1971. The relationships of the Umbellales. – In: Heywood VH (ed), The biology and chemistry of the Umbelliferae, Bot. J. Linn. Soc. 64 [Suppl. 1]: 63-91.
Rogers CL. 1950. The Umbelliferae of North Carolina and their distribution in the southeast. – J. Elisha Mitchell Sci. Soc. 66: 195-266.
Roland-Heydacker F, Cerceau-Larrival M-T. 1978. Ultrastructure du tectum de pollen d’Ombellifères. – Grana Palynol. 17: 81-89.
Rompel J. 1895. Krystalle von Calciumoxalat in der Fruchtwand der Umbelliferen und ihre Verwerthung für die Systematik. – Sitzungsber. Kaiserl. Akad. Wiss. Math.-Naturwiss. Cl., Abt. 1, 104: 417-473.
Ronse AC, Popper ZA, Preston JC, Watson MF. 2011. Taxonomic revision of European Apium L. s.l.: Helosciadium W. D. J. Koch restored. – Plant Syst. Evol. 287: 1-17.
Rose JN. 1905. Two new umbelliferous plants from the coastal plain of Georgia. – Proc. U.S. Natl. Mus. 29: 441-445.
Rose JN. 1906. New names for two recently described genera of plants. – Proc. Biol. Soc. Washington 19: 96-98.
Rosendal Jensen S, Juhl Nielsen B. 1980. The systematic position of Aralidium Miq. – a multidisciplinary study 4. Iridoid glucosides. – Taxon 29: 409-411.
Rubal JJ, Moreno-Dorado FJ, Guerra FM, Jorge ZD, Galán Mdel C, Salido GM, Christensen SB, Søhoel H, Massanet GM. 2010. A phenylpropanoid, a slovenolide, two sulphur-containing germacranes and Ca2+-ATPase inhibitors from Thapsia villosa. – Planta Med. 76: 284-90.
Rüffle L. 2000. Beitrag zu einigen Araliaceae-Blättern des Alttertiärs. – Feddes Repert. 111: 445-448.
Ruhlman T, Lee S-B, Jansen RK, Hostetler JB, Tallon LJ, Town CD, Daniell H. 2006. Complete plastid genome sequence of Daucus carota: Implications for biotechnology and phylogeny of angiosperms. – BMC Genomics 7: 222. http://www.biomedcentral.com/1471-2164-7-222
Runemark H. 1968. Studies in the Aegean flora XIII. Tordylium L. (Umbelliferae). – Bot. Not. 121: 233-258.
Rutherford A, McAllister HA, Mill RR. 1993. New ivies from the Mediterranean area and Macaronesia. – Plantsman 15: 115-128.
Saenz de Rivas C, Heywood VH, Jury S, Al-Attar A. 1982. Étude micromorphologique et anatomique du fruit des Caucalideae Bentham (Umbelliferae). – In: Cauwet-Marc A-M, Carbonnier J (eds), Les Ombellifères, Actes du 2ème Symposium International sur les Ombellifères “Contributions Pluridisciplinaires à la Systématique”, Monogr. Syst. Bot. Missouri Bot. Gard. 6: 175-193.
Sáenz Laín C. 1981. Research on Daucus L. (Umbelliferae). – An. Jard. Bot. Madrid 37: 481-534.
Sagiroglu M, Duman H. 2007. Ferula mervynii (Apiaceae), a distinct new species from North-East Anatolia, Turkey. – Bot. J. Linn. Soc. 153: 357-362.
Saleh NAM, El-Negoumy SI, El-Hadidi MN, Hosni HA. 1983. Comparative study of the flavonoids of some local members of the Umbelliferae. – Phytochemistry 22: 1417-1420.
Sales F, Hedge IC. 2010. Flore de Madagascar et des Comores. Fam. 157. Ombellifères. – Muséum National d’Histoire Naturelle, Paris.
Sales F, Hedge IC, Coutinho AXP, Marques A. 2004. Apiaceae subfamily Apioideae in Madagascar. – South Afr. J. Bot. 70: 446-448.
Schaeppi H. 1971. Zur Gestaltung des Gynoeceums von Pittosporum tobira. – Ber. Schweiz. Bot. Ges. 81: 40-51.
Schatz GE, Lowry PP II, Wolf A-E. 1998. Endemic families of Madagascar I. A synoptic revision of Melanophylla Baker (Melanophyllaceae). – Adansonia, sér. III, 20: 233-242.
Schlessman MA. 1984. Systematics of tuberous lomatiums (Umbelliferae). – Syst. Bot. Monogr. 4: 1-55.
Schlessman MA. 1985. Floral biology of American ginseng (Panax quinquefolium). – Bull. Torrey Bot. Club 112: 129-133.
Schlessman MA. 1987. Gender modification in North American ginsengs: dichotomous choice versus adjustment. – BioScience 37: 469-475.
Schlessman MA. 1990. Phenotypic gender in sex changing dwarf ginseng, Panax trifolium (Araliaceae). – Amer. J. Bot. 77: 1125-1131.
Schlessman MA. 1991. Size, gender, and sex change in dwarf ginseng, Panax trifolium (Araliaceae). – Oecologia 87: 588-595.
Schlessman MA. 2010. Major events in the evolution of sexual systems in Apiales: ancestral andromonoecy abandoned. – Plant Divers. Evol. 128: 239-245.
Schlessman MA, Graceffa LM. 2002. Protogyny, pollination, and sex expression of andromonoecious Pseudocymopterus montanus (Apiaceae, Apioideae). – Intern. J. Plant Sci. 163: 409-417.
Schlessman MA, Lloyd DG, Lowry PP II. 1990a. Functional dioecism in the New Caledonian endemic Polyscias pancheri (Araliaceae). – Biotropica 22: 133-139.
Schlessman MA, Lloyd DG, Lowry PP II. 1990b. Evolution of sexual systems in New Caledonian Araliaceae. – In: Gottsberger G, Prance GT (eds), Reproductive biology and evolution of tropical woody angiosperms, Mem. New York Bot. Gard. 55: 105-117.
Schlessman MA, Plunkett GM, Lowry PP II, Lloyd DG. 2001. Sexual systems of New Caledonian Araliaceae: a preliminary phylogenetic reappraisal. – Edinburgh J. Bot. 58: 221-228.
Schmidt KHA, Lobin W. 1999. Tornabenea ribeirensis (Apiaceae): a new species from Sao Nicolau, Cape Verde Islands (West Africa). – Feddes Repert. 110: 7-11.
Schnepf E. 1969. Über den Feinbau von Öldrüsen IV. Die Ölgänge von Umbelliferen: Heracleum sphondylium und Dorema ammoniacum. – Protoplasma 73: 375-390.
Schneyer VC, Borschtchenko GP, Pimenov MG, Leonov MV. 1991. The serological investigation of intergeneric relationships in the subfamily Apioideae (Apiaceae). – Bot. Žurn. 76: 245-257. [In Russian]
Schneyer VC, Borschtchenko GP, Pimenov MG, Leonov MV. 1992. The tribe Smyrnieae (Umbelliferae) in the light of serotaxonomical analysis. – Plant Syst. Evol. 182: 135-148.
Schneyer VC, Borschtchenko GP, Pimenov MG. 1995. Immunochemical appraisal of relationships within the tribe Peucedaneae (Apiaceae). – Plant Syst. Evol. 198: 1-16.
Schneyer VC, Kutyavina NG, Pimenov MG. 2003. Systematic relationships within and between Peucedanum and Angelica (Umbelliferae-Peucedaneae) inferred from immunological studies of seed protein. – Plant Syst. Evol. 236: 175-194.
Schodde R. 1972. A review of the family Pittosporaceae in Papuasia. – Aust. J. Bot., Suppl. Ser. 3: 1-60.
Schubert MTR, Wyk B-E van. 1995a. Two new species of Centella (Umbelliferae) with notes on the infrageneric taxonomy. – Nord. J. Bot. 15: 167-171.
Schubert MTR, Wyk B-E van. 1995b. A taxonomic study of the Centella rupestris group. – Nord. J. Bot. 15: 263-268.
Schulz-Gaebel H-H. 1930. Entwicklungsgeschichtlich-zytologische Studien an der Umbelliferen-Unterfamilie der Apioideen. – Beitr. Biol. Pflanzen 18: 345-398.
Schürhoff NN. 1929. Über die systematische Stellung der Pittosporaceae. – Beitr. Biol. Pflanzen 17: 72-86.
Shan R-H. 1940. Studies of Umbelliferae of China III. – Sinensia 11: 1327-174. [In Chinese]
Shan R-H. 1941. Studies on Umbelliferae of China IV. – Sinensia 12: 163-183. [In Chinese]
Shan R-H, Constance L. 1951. The genus Sanicula (Umbelliferae) in the Old World and the New. – Univ. Calif. Publ. Bot. 25: 1-78.
Shan R-H, Pu F-T. 1978. On Chinese genus Pternopetalum Franch. (Umbelliferae). – Acta Phytotaxon. Sin. 16: 65-78. [In Chinese]
Shan R-H, Sheh M-L, Yuan C-C, Wang T-S, Pu F-T, Chang H-T. 1980. New taxa of the Umbelliferae from Xizang (Tibet). – Acta Phytotaxon. Sin. 18: 374-379. [In Chinese]
Shang C-B. 1983. Révision du genre Macropanax Miq. (Araliaceae). – Bull. Mus. Natl. Hist. Nat., sect. B, sér. II, Adansonia 1: 33-52.
Shang C-B. 1984. Le genre Schefflera (Araliacées) en Chine et en Indochine. – Candollea 39: 453-486.
Shang C-B. 1985a. The study of the genus Macropanax Miq. (Araliaceae). – J. Nanjing Inst. Forestry 1985: 12-29. [In Chinese with English summary]
Shang C-B. 1985b. New taxa and some revisions about the Araliaceae of China. – J. Nanjing Inst. Forestry 1985(2): 15-28. [In Chinese with English summary]
Shang C-B, Callen D. 1988. Pollen morphology of the family Araliaceae in China. – Bull. Bot. Res. (China) 8: 13-35. [In Chinese with English summary]
Shang C-B, Huang J-Y. 1993a. Chengiopanax – a new genus of Araliaceae. – Bull. Bot. Res. (China) 13: 44-49. [In Chinese with English summary]
Shang C-B, Huang J-Y. 1993b. Revision of the genus Evodiopanax. – J. Nanjing Forestry Univ. 17: 32-36. [In Chinese]
Shang C-B, Lowry II PP, Frodin DG. 2000. A taxonomic revision and re-definition of the genus Gamblea (Araliaceae). – Adansonia, sér. III, 22: 45-55.
Sheh M-L, Pu F-T. 1997. A new species of Notopterygium de Boiss. from China. – J. Plant Resources Environm. 6: 41-42.
Sheh M-L, Shan R-H. 1980. Cyclorhiza and Chuanminshen – two newly proposed genera in Umbelliferae (Apiaceae). – Acta Phytotaxon. Sin. 18: 45-49. [In Chinese]
Sheh M-L, Shan R-H. 1986. Arcuatopterus, a new genus of the Umbelliferae. – Bull. Bot. Res. (China) 6: 11-20.
Sheh M-L, Su P. 1987. The floristic analysis of endemic genera in Chinese Umbelliferae. – Bull. Nanjing Bot. Gard. 1987: 14-26. [In Chinese]
Sheh M-L, Su P. 1992. Pollen morphology of Hydrocotyloideae and Saniculoideae (Apiaceae) in China. – Acta Phytotaxon. Sin. 30: 126-136. [In Chinese]
Sheh M-L, Su P, Pan Z-H. 1997. A comparative study of pollen morphology of Angelica L. between East Asia and North America. – J. Plant Res. Environm. 6: 41-47.
Sherff EE. 1955. Revision of the Hawaiian members of the genus Tetraplasandra A. Gray. – Fieldiana Bot. 29: 49-142.
Shevock JR, Constance L. 1979. A new species of Oreonana, a genus of snow-adapted Umbelliferae. – Madroño 26: 128-134.
Shner JV, Alexeeva TV, Pimenov MG, Wyk B-E van. 2011. Chromosome numbers of South African Umbelliferae (Apiaceae). – South Afr. J. Bot. 77: 497-502.
Shneyer VS. 2010. On some cases of conflict between serological and DNA sequence data in Apiaceae studies. – Plant Divers. Evol. 128: 173-180.
Shneyer VS, Borschtschenko GP, Pimenov MG, Leonov MV. 1992. The tribe Smyrnieae (Umbelliferae) in the light of serotaxonomical analysis. – Plant Syst. Evol. 182: 135-148.
Shneyer VS, Borschtschenko GP, Pimenov MG. 1995. Immunochemical appraisal of relationships within the tribe Peucedaneae (Apiaceae). – Plant Syst. Evol. 198: 1-16.
Shneyer VS, Kutyavina NG, Pimenov MG. 2003. Systematic relationships within and between Peucedanum and Angelica (Umbelliferae-Peucedaneae) inferred from immunological studies of seed protein. – Plant Syst. Evol. 236: 175-194.
Short PS. 1979. Apium L. sect. Apium (Umbelliferae) in Australasia. – J. Adelaide Bot. Gard. 1: 205-235.
Shoup JR, Tseng C-C. 1977a. Pollen of Klotzschia (Umbelliferae): a possible link to Araliaceae. – Amer. J. Bot. 64: 461-463.
Shoup JR, Tseng C-C. 1977b. A palynological study of Schefflera paraensis Huber ex Duke (Araliaceae). – Grana 16: 81-84.
Shu P, Sheh M-L. 2001. Pollen photographs and flora of Umbelliferae in China. – Shanghai Scientific & Technical Publ., Shanghai. [In Chinese]
Shu P, Sheh M-L. 2004. Ultrastructure of pollen exine in Peucedaneae Drude with reference to its systematic significance. – Acta Bot. Sin. 46: 311-318.
Silvestre S. 1973. Estudio taxonómico de los géneros Conopodium Koch y Bunium L. en la Península Ibérica II. Parte sistemática. – Lagascalia 3: 3-48.
Silvestre S. 1978. Contribución al studio cariológicos de la familia Umbelliferae en la Península Iberica II. – Lagascalia 7: 163-172.
Simmons KS. 1985. Systematic studies in Lomatium. – Ph.D. diss., Washington State University, Pullman, Washington.
Singh D. 1954. Floral morphology and embryology of Hedera nepalensis K. Koch. – Agra Univ. J. Res. Sci. 3: 289-299.
Skovsted A. 1935. Chromosome numbers in the Malvaceae I. – J. Genet. 31: 263-296.
Skvortsova NT, Averyanov LV. 1994. New genus and species – Grushvitzkya stellata (Araliaceae) from the North Vietnam. – Bot. Žurn. 79: 108-112. [In Russian with English summary]
Sleumer H. 1942. Icacinaceae. – In: Engler A (†), Harms H, Mattfeld J (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 20b, W. Engelmann, Leipzig, pp. 322-396.
Sleumer H. 1969. Materials towards the knowledge of the Icacinaceae of Asia, Malesia and adjacent areas. – Blumea 17: 181-264.
Sleumer H. 1970. The identity of Plectomirtha Oliv. with Pennantia J. R. & G. Forster (Icacinaceae). – Blumea 18: 217-218.
Small E. 1978. A numerical taxonomic analysis of the Daucus carota complex. – Can. J. Bot. 56: 248-276.
Smith AC, Stone BC. 1968. Studies of Pacific Island plants XIX. The Araliaceae of the New Hebrides, Fiji, Samoa, and Tonga. – J. Arnold Arbor. 49: 431-493.
Snogerup S. 1962. Studies in the Aegaean flora IV. Bupleurum flavum Forsk. and related species. – Bot. Not. 115: 357-375.
Snogerup S. 1971. New species of Bupleurum from Turkey. – Bot. Not. 124: 359-375.
Snogerup S, Snogerup B. 2001. Bupleurum L. (Umbelliferae) in Europe 1. The annuals, B. sect. Bupleurum and sect. Aristata. – Willdenowia 31: 205-308.
Soine TO et al. 1973. Natural coumarines VII. Isolation and structure of a new coumarin, peuruthenicin, from Peucedanum ruthenicum. – J. Pharm. Sci. 62: 1879-1880.
Sokoloff DD, Oskolski AA, Remizowa MV, Nuraliev MS. 2007. Flower structure and development in Tupidanthus calyptratus (Araliaceae): an extreme case of polymery among asterids. – Plant Syst. Evol. 268: 209-234.
Sokoloff DD, Karpunina PV, Nuraliev MS, Oskolski AA. 2018. Flower structure and development in Melanophylla (Torricelliaceae: Apiales): lability in direction of corolla contortion and orientation of pseudomonomerous gynoecium in a campanulid eudicot. – Bot. J. Linn. Soc. 187: 247-271.
Solov’eva NM, Pimenov MG, Vasil’eva MG, Zigareva NN, Turkov VD. 1985. Karyotaxonomic study of some species of Peucedanum (Umbelliferae). – Plant Syst. Evol. 151: 89-101.
Soltis PS, Kuzoff RK. 1993. ITS sequence variation within and among populations of Lomatium grayi and L. laevigatum (Umbelliferae). – Mol. Phylogen. Evol. 2: 166-170.
Soltis PS, Novak SJ. 1997. Polyphyly of the tuberous Lomatiums (Apiaceae): cpDNA evidence for morphological convergence. – Syst. Bot. 22: 99-112.
Spalik K. 1996. Species boundaries, phylogenetic relationships and ecological differentiation in Anthriscus (Apiaceae). – Plant Syst. Evol. 199: 13-32.
Spalik K. 1997. Revision of Anthriscus (Apiaceae). – Polish Bot. Stud. 13: 1-69.
Spalik K, Downie SR. 2001. The utility of morphological characters for inferring phylogeny in Scandiceae subtribe Scandicinae (Apiaceae). – Ann. Missouri Bot. Gard. 88: 270-301.
Spalik K, Downie SR. 2006. The evolutionary history of Sium sensu lato (Apiaceae): dispersal, vicariance, and domestication as inferred from ITS rDNA phylogeny. – Amer. J. Bot. 93: 747-761.
Spalik K, Downie SR. 2007. Intercontinental disjunctions in Cryptotaenia (Apiaceae, Oenantheae): an appraisal using molecular data. – J. Biogeogr. 34: 2039-2054.
Spalik K, Wojewódzka A, Downie SR. 2001a. The delimitation of genera in Apiaceae with examples from Scandiceae subtribe Scandicinae. – Edinburgh J. Bot. 58: 331-346.
Spalik K, Wojewódzka A, Downie SR. 2001b. The evolution of fruit in Scandiceae subtribe Scandicinae (Apiaceae). – Can. J. Bot. 79: 1358-1374.
Spalik K, Reduron J-P, Downie SR. 2004. The phylogenetic position of Peucedanum sensu lato and allied genera and their placement in tribe Selineae (Apiaceae, subfamily Apioideae). – Plant Syst. Evol. 243: 189-210.
Spalik K, Downie SR, Watson MF. 2009. Generic delimitations within the Sium alliance (Apiaceae tribe Oenantheae) inferred from cpDNA rps16-5’trnK(UUU) and nrDNA ITS sequences. – Taxon 58: 735-748.
Spalik K, Piwczynski M, Danderson CA, Kurzyna-Mlynik R, Bone TS, Downie SR. 2010. Amphitropic amphiantarctic disjunctions in Apiaceae subfamily Apioideae. – J. Biogeogr. 37: 1977-1994.
Spooner D, Rojas P, Bonierbale M, Mueller LA, Srivastav M, Senalik D, Simon P. 2013. Molecular phylogeny of Daucus (Apiaceae). – Syst. Bot. 38: 850-857.
Spooner D, Ruess H, Iorizzo M, Senalik D, Simon P. 2017. Entire plastid phylogeny of the carrot genus (Daucus, Apiaceae): concordance with nuclear data and mitochondrial and nuclear DNA insertions to the plastid. – Amer. J. Bot. 104: 296-312.
Stepanova AV, Oskolski AA II. 2010. Wood anatomy of Bupleurum L. (Apioideae, Apiaceae) in relation to habit, phylogenetic relationships, and infrageneric taxonomy. – Plant Divers. Evol. 128: 501-516.
Stone BC. 1965a. Notes on Polyscias (Araliaceae) from Micronesia. – Micronesica 2: 51-59.
Stone BC. 1965b. Notes on the type species of Polyscias J. R. and G. Forst. (Araliaceae). – Taxon 14: 281-285.
Stone BC, Loo AH. 1969. Cytotaxonomic notes on some species of Polyscias (Araliaceae). – J. Jap. Bot. 44: 321-327.
St. Pierre MD, Bayer RJ, Weis IM. 1990. An isozyme-based assessment of the genetic variability within the Daucus carota complex (Apiaceae: Caucalideae). – Can. J. Bot. 68: 2449-2457.
Strid A. 1980. New species of Aethionema and Peucedanum from the Greek mountains. – Bot. Not. 133: 521-526.
Stuhlfauth T, Fock H, Huber H, Klug K. 1985. The distribution of fatty acids including petroselinic and tariric acids in the fruit and seed oils of the Pittosporaceae, Araliaceae, Umbelliferae, Simarubaceae and Rutaceae. – Phytochemistry 13: 447-453.
Sun B-Y, Kim C-H, Soh W-Y. 1988. Chromosome numbers of Araliaceae in Korea. – Korean J. Plant Taxon. 18: 291-296.
Sun F-J. 2003. A phylogenetic study of Cymopterus and related genera (Apiaceae). – Ph.D. diss., University of Illinois at Urbana-Champaign, Illinois.
Sun F-J, Downie SR. 2004. A molecular systematic investigation of Cymopterus and its allies (Apiaceae) based on phylogenetic analyses of nuclear (ITS) and plastid (rps16 intron) DNA sequences. – South Afr. J. Bot. 70: 407-416.
Sun F-J, Downie SR. 2010a. Phylogenetic relationships among the perennial, endemic Apiaceae subfamily Apioideae of western North America: additional data from the cpDNA trnF-trnL-trnT region continue to support a highly polyphyletic Cymopterus. – Plant Divers. Evol. 128: 151-172.
Sun F-J, Downie SR. 2010b. Phylogenetic analyses of morphological and molecular data reveal major clades within the perennial, endemic western North American Apiaceae subfamily Apioideae. – J. Torrey Bot. Soc. 137: 133-156.
Sun F-J, Downie SR, Hartman RL. 2004. An ITS-based phylogenetic analysis of the perennial, endemic Apiaceae subfamily Apioideae of western North America. – Syst. Bot. 29: 419-431.
Sun N, He X-J, Zhou S-D. 2008. Morphological cladistic analysis of Ligusticum (Umbelliferae) in China. – Nord. J. Bot. 26: 118-128.
Tabanca N, Douglas AW, Bedir E, Dayan FE, Kirimer N, Baser KHC, Aytac Z, Khan IA, Scheffler BE. 2005. Patterns of essential oil relationships in Pimpinella (Umbelliferae) based on phylogenetic relationships using nuclear and chloroplast sequences. – Plant Genet. Resour. 3: 149-163.
Tamamschian SG. 1933a. Beiträge zur Morphologie der Gruppe Eryngieae. – Trudy Tiflissk. Bot. Sada [Acta Tifl. Bot. Gard.] 1: 153-173.
Tamamschian SG. 1933b. Materialen zur Caryosystematik der kultivierten und wilden Umbelliferen. – Bull. Appl. Bot. Plantbreed. (Leningrad) 2: 137-164.
Tamamschian SG. 1945. About a little-known genus Smyrniopsis Boiss. from the family Umbelliferae. – Bull. Acad. Sci. Armenian S.S.R. 5-6: 47-62. [In Russian]
Tamamschian SG. 1946. The genus Hohenackeria Fisch. et Mey., and its place in the Umbelliferae system. – Soviet Bot. 14: 219-238. [In Russian]
Tamamschian SG. 1947. Carpological characterization of the genus Austrodaucus Drude and some Caucasian Caucalinae and Daucinae. – Soviet Bot. 4: 198-212. [In Russian]
Tamamschian SG. 1950. On systematics of the genus Symphyoloma C. A. M. (Umbelliferae). – Bot. Žurn. 35: 335-342. [In Russian]
Tamamschian SG. 1951. De generibus Phlojodicarpus Turcz. et Stenocoelium Ldb. notae criticae. – Bot. Mater. Gerb. Bot. Inst. Komarova Akad. Nauk S.S.S.R. 14: 265-276.
Tamamschian SG, Vinogradova VM. 1969. A contribution to the taxonomy of the genus Grammosciadium DC. – Bot. Žurn. 54: 1197-1212. [In Russian]
Tennant JR. 1968. Araliaceae. – In: Milne-Redhead E, Polhill RM (eds), Flora of tropical East Africa, Crown Agents for Oversea governments and Administrations, London, pp. 1-23.
Teo SP, Haron NW. 1999. Anatomical studies in West Malaysian Icacinaceae. – Aust. Syst. Bot. 11: 729-738.
Terentieva EI, Valiejo-Roman CM, Samigullin TH, Pimenov MG, Kljuykov EV. 2008. Phylogenetic analysis of nuclear rDNA ITS and morphological data reveal relationships among some endemic Umbelliferae taxa of Middle Asia, Kazakhstan and Siberia. – In: Pimenov MG, Tilney PM (eds), Apiales 2008. The programme and proceedings of the 6th International Symposium on Apiales, KMK Sci. Press, Ltd., Moscow, pp. 134-137.
Theobald WL. 1967a. Anatomy and systematic position of Uldinia (Umbelliferae). – Brittonia 19: 165-173.
Theobald WL. 1967b. Comparative morphology and anatomy of Chlaenosciadium (Umbelliferae). – Bot. J. Linn. Soc. 60: 75-84.
Theobald WL. 1971. Comparative anatomical and developmental studies in the Umbelliferae. – In: Heywood VH (ed), The biology and chemistry of the Umbelliferae, Bot. J. Linn. Soc. 64 [Suppl. 1]: 177-197.
Theobald WL, Cannon JFM. 1973. A survey of Phlyctidocarpa (Umbelliferae) using the light and scanning electron microscope. – Notes Roy. Bot. Gard. Edinb. 32: 203-210.
Theobald WL, Tseng CC, Mathias ME. 1963. A revision of Aletes and Neoparrya (Umbelliferae). – Brittonia 16: 296-315.
Thomson JD, Barrett SCH. 1981. Temporal variation of gender in Aralia hispida Vent. (Araliaceae). – Evolution 35: 1094-1107.
Thorne RF. 1973. Inclusion of the Apiaceae (Umbelliferae) in the Araliaceae. – Notes Roy. Bot. Gard. Edinb. 32: 161-165.
Thulin M. 1991. Another arborescent umbellifer: a new species of Steganotaenia from North-East tropical Africa. – Bot. J. Linn. Soc. 107: 164-167.
Tieghem P van. 1884. Sur la structure et les affinités des Pittosporées. – Bull. Soc. Bot. France 31: 383-385.
Tikhomirov VN. 1961. On the systematic position of the genera Hydrocotyle L. and Centella L. emend. Urban. – Bot. Žurn. 46: 584-586. [In Russian]
Tikhomirov VN. 1973. Paraligusticum V. Tichom. gen. nov. – a new genus of the family Umbelliferae. – Bjull. Moskovsk. Obšč. Isp. Prir., Otd. Biol. 78: 100-108. [In Russian]
Tikhomirov VN, Konstantinova AI. 1995. On the phylogenetic value of some characters of fruit structure in Umbelliferae-Hydrocotyloideae. – Bull. Moscow Soc. Natur. (Biol.) 100: 61-73. [In Russian]
Tilney PM, Wyk B-E van. 1995. Unusual structural variation in the fruit of Dasispermum suffruticosum (Apiaceae): a new record of heteromorphic fruits in the family. – South Afr. J. Bot. 61: 245-248.
Tilney PM, Wyk B-E van. 2001. A revision of the genus Annesorhiza (Apiaceae). – Nord. J. Bot. 21: 615-649.
Tilney PM, Wyk B-E van, Downie SR, Calvino CI. 2009. Phylogenetic relationships in the genus Lichtensteinia (Apiaceae) based on morphological, anatomical and DNA sequence data. – South Afr. J. Bot. 75: 64-82.
Ting W-S, Tseng C-C, Mathias ME. 1964. A survey of pollen morphology of Hydrocotyloideae (Umbelliferae). – Pollen Spores 6: 479-514.
Tomkovich LP, Pimenov MG. 1989. Botanico-geographical analysis of the genus Ferulago. – Feddes Repert. 100: 119-129.
Tomlinson PB, Fisher JB, Hallé F, Villalobos R. 2005. Development of woody branch attachments in Schefflera (Araliaceae or Apiaceae). – Amer. J. Bot. 92: 1765-1773.
Townsend CC. 1964. Notes on the Umbelliferae of Iraq I. – Kew Bull. 17: 427-439.
Townsend CC. 1968. Notes on the Umbelliferae of Iraq IV. – Kew Bull. 22: 429-433.
Townsend CC. 1983a. The genus Cryptotaenia (Umbelliferae) in East Africa. – Kew Bull. 38: 57-60.
Townsend CC. 1983b. Notes on East African Umbelliferae: Oenanthe, and a genus new for Tanzania. – Kew Bull. 38: 311-315.
Townsend CC. 1985a. Notes on Pimpinella (Umbelliferae) in Central and East Africa. – Kew Bull. 40: 759-780.
Townsend CC. 1985b. Some notes on species of Heteromorpha (Umbelliferae). – Kew Bull. 40: 843-850.
Townsend CC. 1986. Oreoschimperella and Trachyspermum (Umbelliferae) in East Africa and Arabia. – Kew Bull. 41: 453-458.
Townsend CC. 1987. Notes on Peucedanum (Umbelliferae) and allied genera in Central and East Africa. – Kew Bull. 42: 587-604.
Townsend CC. 1989. Umbelliferae. – In: Polhill RM (ed), Flora of tropical East Africa, A. A. Balkema, Rotterdam, pp. 1-127.
Trifonova VI. 1998. Fruit and seed anatomy of the genus Melanophylla (Melanophyllaceae) in relation to its taxonomical position. – Bot. Žurn. 83: 97-103. [In Russian]
Tronchet F, Plunkett GM, Jérémie J, Lowry PP. 2005. Monophyly and major clades of Meryta (Araliaceae). – Syst. Bot. 30: 657-670.
Tseng C-C. 1967. Anatomical studies of flowers and fruits in the Hydrocotyloideae (Umbelliferae). – Univ. Calif. Publ. Bot. 42: 1-58.
Tseng C-C. 1971. Light and scanning electron microscopic studies on pollen of Tetraplasandra (Araliaceae) and relatives. – Amer. J. Bot. 58: 505-516.
Tseng C-C. 1973. Systematic palynology of Tupidanthus and Plerandra (Araliaceae). – Grana 13: 51-56.
Tseng C-C. 1974. Pollen of Boerlagiodendron: a unique type in the Araliaceae. – Amer. J. Bot. 61: 717-721.
Tseng C-C. 1980. The systematic position of Aralidium Miq. – a multidisciplinary study 3. Pollen morphology. – Taxon 29: 407-409.
Tseng C-C, Hoo G. 1982. A new classification for the family Araliaceae. – Acta Phytotaxon. Sin. 20: 125-129. [In Chinese]
Tseng C-C, Shoup JR. 1978. Pollen morphology of Schefflera (Araliaceae). – Amer. J. Bot. 65: 384-394.
Tseng C-C, Shoup JR, Chuang T-I, Hsieh W-C. 1983. Pollen morphology of Acanthopanax (Araliaceae). – Grana 22: 11-17.
Tseng C-C, Wen J, Hedges KL, Chen Y-C. 1993. Computer-based protein profile analysis in Aralia (Araliaceae). – Cathaya 5: 69-79.
Tseng C-J, Hoo G. 1982. A new classification scheme for the family Araliaceae. – Acta Phytotaxon. Sin. 20: 125-130.
Tucker AO, Dill NH, Pizzolato TD, Kral RD. 1983. Nomenclature, distribution, chromosome numbers, and fruit morphology of Oxypolis canbyi and O. filiformis (Apiaceae). – Syst. Bot. 8: 299-304.
Turmel JM. 1950. Évolution des Saniculoidées II. Évolution du genre Alepidea (Ombellifères). – Bull. Mus. Natl. Hist. Nat. Paris, sér. I, 2: 120-126.
Tutin TG. 1968. Notes on the genus Athamanta (Umbelliferae) in Europe. – In: Heywood VH (ed), Flora Europaea. Notulae systematicae ad floram europaeam spectantes 7, Feddes Repert. 79: 18-20.
Utteridge TMA. 2000. Contributions to the flora of Mt Jaya II. The subalpine members of Pittosporum (Pittosporaceae) from Mt Jaya, New Guinea. – Kew Bull. 55: 699-710.
Valcárcel V, Fiz O, Vargas P. 2003. Chloroplast and nuclear evidence for multiple origins of polyploids and diploids of Hedera (Araliaceae) in the Mediterranean basin. – Mol. Phylogen. Evol. 27: 1-20.
Valcárcel V, Fiz-Palacios O, Wen J. 2014.
The origin of the early differentiation of ivies (Hedera L.) and the
radiation of the Asian palmate group (Araliaceae). – Molec. Phylogen. Evol.
70: 492-503.
Valiejo-Roman CM, Pimenov MG, Terentieva EI, Downie SR, Katz-Downie DS, Troitsky AV. 1998. Molecular systematics of the Umbelliferae: using nuclear rDNA internal transcribed spacer sequences to resolve issues of evolutionary relationships. – Bot. Žurn. 83: 1-22.
Valiejo-Roman CM, Terentieva EI, Samigullin TH, Pimenov MG. 2002a. Relationships among genera in Saniculoideae and selected Apioideae (Umbelliferae) inferred from nrITS sequences. – Taxon 51: 91-101.
Valiejo-Roman CM, Terentieva EI, Samigullin TH, Pimenov MG. 2002b. nrDNA ITS sequences and affinities of Sino-Himalayan Apioideae (Umbelliferae). – Taxon 51: 685-701.
Valiejo-Roman CM, Terentieva EI, Samigullin TH, Pimenov MG, Ghahremani-Nejad F, Mozaffarian V. 2006. Molecular data (nrITS-sequencing) reveal relationships among Iranian endemic taxa of the Umbelliferae. – Feddes Repert. 117: 367-388.
Valiejo-Roman CM, Shneyer VS, Samigullin TH, Terentieva EI, Pimenov MG. 2006. An attempt to clarify taxonomic relationships in ”Verwandtschaftskreis der Gattung Ligusticum” (Umbelliferae-Apioideae) by molecular analysis. – Plant Syst. Evol. 257: 25-43.
Valiejo-Roman CM, Terentieva EI, Pimenov MG, Kljuykov EV, Samigullin TH. 2008. Broad polyphyly in Pleurospermum s.l. (Umbelliferae-Apioideae) or broad homoplasy in nrDNA ITS and chloroplast sequences? – In: Pimenov MG, Tilney PM (eds), Apiales 2008. The programme and proceedings of the 6th International Symposium on Apiales, KMK Sci. Press, Ltd., Moscow, pp. 140-143.
Valiejo-Roman CM, Terentieva EI, Pimenov MG, Kljuykov EV, Samigullin TH, Tilney PM. 2012. Broad polyphyly in Pleurospermum s. l. (Umbelliferae-Apioideae) as inferred from nrDNA ITS and chloroplast sequences. – Syst. Bot. 37: 573-581.
Vargas P, Baldwin BG, Constance L. 1998. Nuclear ribosomal DNA evidence for a western North American origin of Hawaiian and South American species of Sanicula (Apiaceae). – Proc. Natl. Acad. Sci. U.S.A. 95: 235-240.
Vargas P, Baldwin BG, Constance L. 1999. A phylogenetic study of Sanicula sect. Sanicoria and S. sect. Sandwicenses (Apiaceae) based on nuclear rDNA and morphological data. – Syst. Bot. 24: 228-248.
Vargas P, McAllister HA, Morton C, Jury SL, Wilkinson MJ. 1999. Polyploid speciation in Hedera (Araliaceae): phylogenetic and biogeographic insights based on chromosome counts and ITS sequences. – Plant Syst. Evol. 219: 165-179.
Vasil’eva MG, Pimenov MG. 1991. Karyotaxonomical analysis in the genus Angelica (Umbelliferae). – Plant Syst. Evol. 177: 117-138.
Vasil’eva MG, Solovieva NM, Pimenov MG, Vaulina EL. 1984. The karyotypes of some endemic taxa of the Umbelliferae of Middle Asia. – Biol. Nauki 3: 66-74. [In Russian]
Vasil’eva MG, Kljuykov EV, Pimenov MG. 1985. Karyotaxonomic analysis of the genus Bunium (Umbelliferae). – Plant Syst. Evol. 149: 71-88.
Vessio N. 2001. The generic affinities of deciduous species of the genera Annesorhiza Cham. & Schlechtd., Chamarea Eckl. & Zeyh. and Peucedanum L. (Apiaceae). – Ph.D. diss., Rand Afrikaans University, Johannesburg, Republic of South Africa.
Viguier R. 1905. Note sur le genre Dizygotheca. – J. Bot. (Morot) 19: 21-27.
Viguier R. 1906. Recherches anatomiques sur la classification des Araliacées. – Ann. Sci. Nat., Bot., sér. IX, 4: 1-210.
Viguier R. 1909. Nouvelles recherches sur les Araliacées. – Ann. Sci. Nat., Bot., sér. IX, 9: 305-405.
Viguier R. 1910-1913 [1925]. Contribution à l’étude de la flore de la Nouvelle-Calédonie: Araliacées. – J. Bot. (Morot), sér. II, 3: 38-101.
Villiers BJ de, Wyk B-E van. 2008. A new species of the genus Hermas (Apiaceae) from South Africa. – Novon 18: 29-32.
Villiers BJ de, Plunkett GM, Tilney PM, Wyk B-E van. 2009. A phylogenetic study of the genus Cussonia (Araliaceae) based on morphological, anatomical and molecular data. – South Afr. J. Bot. 75: 398.
Villiers BJ de, Tilney PM, Wyk B-E van. 2010. The taxonomic significance of leaf anatomical characters in Cussonia and related genera (Araliaceae). – Bot. J. Linn. Soc. 164: 248-263.
Vinogradova VM. 1995. The new data on the genus Grammosciadium and the systematic position of Fuernrohria setifolia (Apiaceae). – Bot. Žurn. 80: 91-99.
Vu NV. 2001. Molecular systematics of Indian Ocean Basin Polyscias (Araliaceae). – M.Sc. thesis, Virginia Commonwealth University, Richmond, Virginia.
Wang C-B, Xiang-Guang MA, Xing-Jin HE. 2011. A taxonomic re-assessment in the Chinese Bupleurum (Apiaceae): insights from morphology, nuclear ribosomal internal transcribed spacer, and chloroplast (trnH-psbA, matK) sequences. – J. Syst. Evol. 49: 558-589.
Wang L-S. 2007. Phenetic analysis of the genus Pternopetalum (Apiaceae). – Acta Bot. Yunnan. 29: 13-25.
Wang L-S. 2012. A revision of the genus Pternopetalum Franch. – J. Syst. Evol. 50: 550-572.
Wang P-L, Pu F-D. 1992. Pollen morphology diversity and evolution trend of the genus Physospermopsis in the Hengduan Mountains of China. – Acta Bot. Yunnan. 14: 413-417. [In Chinese]
Wang YP, Pu FT, Wang PL, He X-J. 1996. Studies on the systematics of the Chinese endemic genus Notopterygium. – Acta Bot. Yunnan. 18: 424-430.
Wanscher JH. 1931. Studies on the chromosome numbers of the Umbelliferae. – Hereditas 15: 179-184.
Wanscher JG. 1932. Studies on the chromosome numbers of the Umbelliferae II. – Bot. Tidsskr. 42: 49-58.
Wanscher JH. 1933. Studies on the chromosome numbers of the Umbelliferae III. – Bot. Tidsskr. 42: 384-399.
Wardle P. 1968. The taxonomy and distribution of the stipulate species of Pseudopanax in New Zealand. – New Zealand J. Bot. 6: 226-235.
Watson MF. 1996. Notes relating to the flora of Bhutan XXXIII. Umbelliferae I. – Edinburgh J. Bot. 53: 127-144.
Watson MF. 1998. Notes relating to the flora of Bhutan XXXVI. Umbelliferae II. – Edinburgh J. Bot. 55: 367-415.
Watson MF, Plunkett GM, Downie SR, Lowry II PP. 2001. Evolution, biogeography, and systematics of the Apiales (Araliaceae and Apiaceae). – Edinburgh J. Bot. 58: 179-181.
Weakley AS, Nesom GL. 2004. A new species of Ptilimnium (Apiaceae) from the Atlantic coast. – SIDA Contr. Bot. 21: 743-752.
Webb CJ. 1979. Breeding systems and the evolution of dioecy in New Zealand apioid Umbelliferae. – Evolution 33: 662-672.
Webb CJ. 1980. The status of New Zealand Actinotus (Umbelliferae). – New Zealand J. Bot. 18: 343-345.
Webb CJ. 1981. Andromonoecism, protandry, and sexual selection in Umbelliferae. – New Zealand J. Bot. 19: 335-338.
Webb CJ. 1984. Pollination specialization and protogyny in Myrrhidendron donnellsmithii (Umbelliferae). – Syst. Bot. 9: 240-246.
Webb CJ. 1986. Breeding systems and relationships in Gingidia and related Australasian Apiaceae. – In: Barlow BA (ed), Flora and fauna of alpine Australasia: ages and origins, Commonwealth Scientific and Industrial Research Organization (CSIRO), Australia, pp. 383-399.
Webb CJ. 1996. The breeding system of Pennantia baylisiana (Icacinaceae). – New Zealand J. Bot. 34: 421-422.
Webb CJ, Druce AP. 1984. A natural intergeneric hybrid, Aciphylla squarrosa x Gingidia montana, and the frequency of hybrids among other New Zealand apioid Umbelliferae. – New Zealand J. Bot. 22: 403-411.
Webb CJ, Hair JB. 1984. Chromosome numbers for Australian Gingidia (Apiaceae). – Brunonia 7: 215-216.
Weimarck H. 1949. A revision of the genus Alepidea. – Bot. Not. 4: 217-268.
Weitzel C, Rønsted N, Spalik K, Simonsen HT.
2014. Resurrecting deadly carrots: towards a revision of Thapsia (Apiaceae) based on phylogenetic analysis
of nrITS sequences and chemical profiles. – Bot. J. Linn. Soc. 174:
620-636.
Wen J. 1991. Systematics of Aralia (Araliaceae). – Ph.D. diss., Ohio State University, Columbus, Ohio.
Wen J. 1993. Generic delimitation of Aralia L. (Araliaceae). – Brittonia 45: 47-55.
Wen J. 2000. Internal transcribed spacer phylogeny of the Asian and eastern North American disjunct Aralia sect. Dimorphanthus (Araliaceae) and its biogeogaphic implications. – Intern. J. Plant Sci. 161: 959-966.
Wen J. 2001. Evolution of the Aralia-Panax complex (Araliaceae) as inferred from nuclear ITS ribosomal ITS sequences. – Edinburgh J. Bot. 58: 243-257.
Wen J. 2002. Revision of Aralia sect. Pentapanax (Seem.) J. Wen (Araliaceae). – Cathaya 13-14: 1-116.
Wen J. 2004. Systematics and biogeography of Aralia L. sect. Dimorphanthus (Miq.) Miq. (Araliaceae). – Cathaya 15-16: 1-187.
Wen J. 2011. Systematics and biogeography of Aralia L. (Araliaceae): revision of Aralia sects. Aralia, Humiles, Nanae, and Sciadodendron. – Contr. U.S. Natl. Herb. 57: 5-35.
Wen J, Frodin DG. 2001. Metapanax, a new genus of Araliaceae from China and Vietnam. – Brittonia 53: 116-121.
Wen J, Lowry II PP. 2006. New species and new combinations in Brassaiopsis (Araliaceae) from Vietnam and southwestern China. – Adansonia, sér. III, 28: 181-190.
Wen J, Nowicke JW. 1999. Pollen ultrastructure of Panax (the ginseng genus, Araliaceae), an eastern Asian and eastern North American disjunct genus. – Amer. J. Bot. 86: 1624-1636.
Wen J, Zimmer EA. 1996. Phylogeny and biogeography of Panax L. (the ginseng genus, Araliaceae): inferences from ITS sequences of nuclear ribosomal DNA. – Mol. Phylogen. Evol. 6: 167-177.
Wen J, Shi S, Jansen RK, Zimmer EA. 1998. Phylogeny and biogeography of Aralia sect. Aralia (Araliaceae). – Amer. J. Bot. 85: 866-875.
Wen J, Plunkett GM, Mitchell AD, Wagstaff SJ. 2001. The evolution of Araliaceae: a phylogenetic analysis based on ITS sequences of nuclear ribosomal DNA. – Syst. Bot. 26: 144-167.
Wen J, Lowry II PP, Esser H-J. 2001. Aralia kingdon-wardii J. Wen, Lowry & Esser, a new name for an Asian Araliaceae. – Adansonia, sér. III, 23: 307-310.
Wen J, Lowry II PP, Walck JL, Yoo K-O. 2002. Phylogenetic and biogeographic diversification in Osmorhiza (Apiaceae). – Ann. Missouri Bot. Gard. 89: 414-428.
Wen J, Deng L, Shi X. 2002. Aralia lihengiana J. Wen, L. Deng and X. Shi, a new species of Araliaceae from China. – Adansonia, sér. III, 24: 217-220.
Wen J, Lee C, Lowry II PP, Hiep NT. 2003. Inclusion of the Vietnamese endemic genus Grushvitzkya in Brassaiopsis (Araliaceae): evidence from nuclear ribosomal ITS and chloroplast ndhF sequences. – Bot. J. Linn. Soc. 142: 455-463.
Wen J, Lôc PK, Hiep NT, Regalado J Jr, Averyanov LV, Lee C. 2007. An unusual new species of Trevesia from Vietnam and its implications on generic delimitation in Araliaceae. – Taxon 56: 1261-1268.
Wen J, Zhu YP, Lee CH, Widjaja E, Saw LG. 2008. Evolutionary relationships of Araliaceae in the Malesian region: a preliminary analysis. – Acta Bot. Yunnan. 30: 391-399.
Wickens GE. 1995. Llareta (Azorella compacta, Umbelliferae): a review. – Econ. Bot. 49: 201-212.
Wickramaratne DB, Pengsuparp T, Mar W, Chai HB, Chagwedera TE, Beecher CW, Farnsworth NR, Kinghorn AD, Pezzuto JM, Cordell GA. 1993. Novel antimitotic dibenzocyclo-octadiene lignan constituents of the stem bark of Steganotaenia araliacea. – J. Nat. Prod. 56: 2083-2090.
Wilkinson HP. 1992. Leaf anatomy of the Pittosporaceae R. Br. – Bot. J. Linn. Soc. 110: 1-59.
Williams CA, Harborne JB. 1972. Essential oils in the spiny-fruited Umbelliferae. – Phytochemistry 11: 1981-1987.
Wilm BW, Taft JB. 1998. Trepocarpus aethusae Nutt. (Apiaceae) in Illinois. – Trans. Illinois State Acad. Sci. 91: 53-56.
Winkworth RC, Lundberg J, Donoghue MJ. 2008. Toward a resolution of campanulid phylogeny, with special reference to the placement of Dipsacales. – Taxon 57: 53-65.
Winter PJD, Wyk B-E van. 1994. The taxonomic value of epidermal characters in the leaf of Heteromorpha and some related genera (Apiaceae). – Bothalia 24: 187-194.
Winter PJD, Wyk B-E van. 1996. A revision of the genus Heteromorpha. – Kew Bull. 51: 225-265.
Winter PJD, Tilney PM. 1993. The morphology and development of the fruit of Heteromorpha (Apiaceae). – South Afr. J. Bot. 59: 336-341.
Winter PJD, Magee AR, Phephu N, Tilney PM, Downie SR, Wyk B-E van. 2008. A new generic classification for African peucedanoid species (Apiaceae). – Taxon 57: 347-364.
Winter PJD, Wyk B-E van, Magee AR, Downie SR, Tilney PM. 2008. The demise of Peucedanum (Apiaceae) in Africa. – South Afr. J. Bot. 74: 383.
Wolff H. 1924. Meeboldia, genus novum Umbelliferarum himalaycum. – Feddes Repert. 19: 313.
Wolff H. 1925a. Neue Umbelliferen-Gattungen aus Ostasien. – Notizbl. Bot. Gart. Berlin 9: 276-280.
Wolff H. 1925b. Stefanoffia, eine neue Umbelliferen-Gattung von der Balkanhalbinsel und aus Kleinasien. – Notizbl. Bot. Gart. Mus. Berlin-Dahlem 9: 281-282.
Wolff H. 1925c. Umbelliferae novae asiaticae II. – Feddes Repert. 21: 244-249.
Wolff H. 1926. Plantae sinenses a Dr. H. Smith annis 1921-22 lectae XVI. Umbelliferae. – Acta Horti Gothoburg. 2: 288-328.
Wolff H. 1929a. Umbelliferae asiaticae novae relictae I. – Feddes Repert. 27: 112-128.
Wolff H. 1929b. Umbelliferae asiaticae novae relictae II. – Feddes Repert. 27: 179-192.
Wolff H. 1930. Umbelliferae asiaticae novae relictae III. – Feddes Repert. 27: 301-335.
Wörz A. 1999a. A taxonomic index of the species of Eryngium (Apiaceae: Saniculoideae). – Stuttgarter Beitr. Naturk., Ser. A, 596: 48.
Wörz A. 1999b. Systematics and evolution of the genus Astrantia L. (Apiaceae-Saniculoideae). – Bot. Jahrb. Syst. 121: 507-536.
Wörz A. 1999c. Distribution patterns in the genus Astrantia (Apiaceae, Saniculoideae). – Acta Bot. Fenn. 162: 141-143.
Wörz A. 2004. On the distribution and relationships of the South-West Asian species of Eryngium L. (Apiaceae-Saniculoideae). – Turk. J. Bot. 28: 85-92.
Wörz A. 2005. A new subgeneric classification of the genus Eryngium L. (Apiaceae, Saniculoideae). – Bot. Jahrb. Syst. 126: 253-259.
Wörz A. 2011. Revision of Eryngium (Apiaceae-Saniculoideae): general part and palaearctic species. – Bibl. Bot. 159: 1-498.
Wyk B-E van. 2001. A preliminary analysis of evolution of African and Madagascan Apiaceae. – Edinburgh J. Bot. 58: 291-299.
Wyk B-E van. 2010. A new species of Polemanniopsis (Apiacee) from Namibia. – South Afr. J. Bot. 76: 153-157.
Wyk B-E van, Tilney PM. 2004. Diversity of Apiaceae in Africa. – South Afr. J. Bot. 70: 433-445.
Wyk B-E van, Tilney PM. 2008. A new species of Lichtensteinia (Apiaceae). – South Afr. J. Bot. 74: 757-760.
Wyk B-E van, Allison I, Tilney PM. 1997. Morphological variation and phylogenetic relationships in the genus Anginon (Apiaceae). – Nord. J. Bot. 17: 511-526.
Wyk B-E van, Tilney PM, Winter PJD. 1999. Four new genera of woody Apiaceae of Madagascar. – Taxon 48: 737-745.
Wyk B-E van, Castro A de Tilney PM, Winter PJD, Magee AR. 2008. A new species of Alepidea (Apiaceae, subfam. Saniculoideae). – South Afr. J. Bot. 74: 740-745.
Xue H-J, Yan M-H, Lu C-M, Wang N-H, Wu G-R. 2007. Taxonomic study of Angelica from East Asia: inferences from ITS sequences of nuclear ribosomal DNA. – Acta Phytotaxon. Sin. 45: 783-795.
Yabe Y. 1902. Revisio Umbelliferarum japonicarum . – J. Coll. Sci. Imp. Univ. Tokyo 16: 1-108.
Yang D-Q. 1981. The cyto-taxonomic studies on some species of Panax L. – Acta Phytotaxon. Sin. 19: 298-303. [In Chinese with English summary]
Yi T-S, Lowry PP II, Plunkett GM, Wen J. 2004. Chromosomal evolution in Araliaceae and close relatives. – Taxon 53: 987-1005.
Yi D-K, Lee H-L, Sun B-Y, Chung M-Y, Kim K-J. 2012. The complete chloroplast DNA sequence of Eleutherococcus senticosus (Araliaceae); comparative evolutionary analyses with other three asterids. – Molec. Cells 33: 497-508.
Yoo KO, Malla KJ, Wen J. 2001. Chloroplast DNA variation of Panax (Araliaceae) in Nepal and its taxonomic implications. – Brittonia 53: 447-453.
Yi T-S, Jin G-H, Wen J. 2015. Chloroplast capture and intra- and inter-continental biogeographic diversification in the Asian – New World disjunct plant genus Osmorhiza (Apiaceae). – Mol. Phylogen. Evol. 85: 10-21.
Yıldırımlı Ş. 1997. The chorology of the Turkish species of Apiaceae family. – OT Sist. Bot. Dergisi 4: 105-128.
Yoo KO, Lowry PP II, Wen J. 2002. Discordance of chloroplast and nuclear ribosomal DNA data in Osmorhiza (Apiaceae). – Amer. J. Bot. 89: 966-971.
Yu Y, Downie SR, He X, Deng X, Yan L. 2011. Phylogeny and biogeography of Chinese Heracleum (Apiaceae tribe Tordylieae) with comments on their fruit morphology. – Plant Syst. Evol. 296: 179-203.
Yuan CC, Shan RH. 1985. On the genera Angelica L. and Ostericum Hoffm. (Umbelliferae) in China. – Bull. Nanjing Bot. Gard. 1984-1985: 1-17.
Yurtseva OV. 1988. A cytologic study of some species of the genus Pimpinella L. (Umbelliferae-Apioideae). – Biol. Nauki 11: 78-85. [In Russian]
Yurtseva OV, Tikhomirov VN. 1998. Morphological diversity and taxonomy of the Pimpinella tragium Vill. group (Umbelliferae-Apioideae) in the Mediterranean. – Feddes Repert. 109-479-500.
Zakharova EA. 2010. Morphological evidence of polyphyletic nature of traditional Carum (Apiaceae-Apioideae). – Plant Div. Evol. 128: 409-421.
Zakharova EA, Degtjareva GV, Pimenov MG. 2012. Redefined generic limits of Carum (Umbelliferae, Apioideae) and new systematic placement of some of its taxa. – Willdenowia 42: 149-168.
Zakharova EA, Degtjareva GV, Kljuykov EV, Downie SR. 2015. A taxonomic study of the genus Hellenocarum H. Wolff (Umbelliferae-Apioideae) based on morphology, fruit anatomy, and molecular data. – Turk. J. Bot. 39. doi: 10.3906/bot-1504-40
Zech JC. 1992. Systematics of the genus Mulinum Pers. (Apiaceae, Hydrocotyloideae, Mulineae). – Ph.D. diss., The Ohio State University, Columbus, Ohio.
Zech JC. 1995. Nomenclatural novelties on Mulinum (Apiaceae). – Kurtziana 24: 192.
Zeuske D, Schaat A, Weber HC. 1999. Progressive series related to mycotrophy in Araliaceae: ginseng (Panax spec.) as a final stage? – Syst. Geogr. Plants 68: 85-93.
Zhang Q-Y, He X-J, Zhang Y-C, Luo P, Wu N. 2005. Study on karyotypes of six species in Angelica from Sichuan, China. – Acta Bot. Yunnan. 27: 539-544.
Zhou J, Huang W-G, Wu M-Z, Yang C-R, Feng K-M, Wu Z-Y. 1975. Triterpenoids from Panax Linn. and their relationship with taxonomy and geographical distribution. – Acta Phytotaxon. Sin. 13: 29-45. [In Chinese with English summary]
Zhou J, Peng H, Downie SR, Liu Z-W, Gong X. 2008. A molecular phylogeny of Chinese Apiaceae subfamily Apioideae inferred from nuclear ribosomal DNA internal transcribed spacer sequences. – Taxon 57: 402-416.
Zhou J, Gong X, Downie SR, Peng H. 2009. Towards a more robust molecular phylogeny of Chinese Apiaceae subfamily Apioideae: additional evidence from nrDNA ITS and cpDNA intron (rpl16 and rps16) sequences. – Mol. Phylogen. Evol. 53: 56-68.
Zijlstra G. 1996. Proposal to conserve the name Conopodium (Umbelliferae) with a conserved type. – Taxon 45: 557-558.
Zohary M. 1941. Taxonomical studies, Umbelliferae. – Palestine J. Bot. 2: 171.
Zuo Y-J, Wen J, Ma J-S, Zhou S-L. 2015. Evolutionary radiation of the Panax bipinnatifidus species complex (Araliaceae) in the Sino-Himalayan region of eastern Asia as inferred from AFLP analysis. – J. Syst. Evol. 15: 210-220.
Zuo Y-J, Wen J, Zhou S-L. 2017. Intercontinental and intracontinental biogeography of the eastern Asian – Eastern North American disjunct Panax (the ginseng genus, Araliaceae), emphasizing its diversification processes in eastern Asia. – Mol. Phylogen. Evol. 117: 60-74.