
Figurtext här
[Garryidae+Campanulidae]
[Apodytes clade+Cassinopsis+Emmotaceae+Garryales+Icacinaceae+Lamiidae]
 |
|
Figurtext här |
APODYTES CLADE |
( Back to Basal Garryidae ) |
Genera/species 3/23–24
Distribution Tropical regions in the Old World; tropical South America?
Fossils Unknown.
Habit Bisexual, evergreen trees, shrubs or lianas.
Vegetative anatomy Phellogen ab initio superficial? Secondary lateral growth in Rhaphiostylis anomalous via successive (concentric) cambia. Vessel elements with simple (Rhaphiostylis) or scalariform (Apodytes, rarely reticulate) perforation plates; lateral pits opposite (Apodytes) or alternate to opposite (Rhaphiostylis), bordered pits. Imperforate tracheary xylem elements tracheids (Rhaphiostylis) with bordered pits, non-septate. Wood rays uniseriate or multiseriate, homocellular or heterocellular. Axial parenchyma apotracheal diffuse-in-aggregates (in Rhaphiostylis sometimes diffuse), or paratracheal scanty or banded. Sieve tube plastids S type? Nodes 1:1, unilacunar with one leaf trace, or 3:3, trilacunar with three traces. Prismatic (calciumoxalate?) crystals frequent. Styloid-like crystals and crystal groups present in wood ray cells of hos Apodytes.
Trichomes Hairs unicellular or absent.
Leaves Alternate (spiral or distichous), simple, entire, with ? ptyxis. Stipules and leaf sheath absent. Petiole vascular bundles? Venation pinnate, brochidodromous. Stomata usually anomocytic (sometimes cyclocytic). Cuticular wax crystalloids? Domatia absent. Leaf margin entire.
Inflorescence Terminal (Apodytes) or axillary (Apodytes, Rhaphiostylis), panicle or fasciculate to corymbose cyme.
Flowers Actinomorphic. Pedicel articulated. Hypogyny. Sepals four or five, with imbricate aestivation, connate. Petals four or five, with valvate aestivation, free (or connate at base). Nectary? Disc absent.
Androecium Stamens four or five, antesepalous, alternipetalous. Filaments free from each other and from tepals. Anthers dorsifixed, versatile, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory? Staminodia absent.
Pollen grains Microsporogenesis simultaneous? Pollen grains tricolporate?, shed as monads, bicellular? at dispersal. Exine semitectate, with columellate? infratectum, reticulate (Apodytes).
Gynoecium Pistil composed of two? connate carpels (in Apodytes pseudomonomery, with one carpel modified into fleshy dispersal structure). Ovary superior, unilocular, with or without a fleshy appendage. Style single, simple, narrow. Stigma subcapitate to bilobate, type? Pistillodium absent.
Ovules Placentation subapical to axile (pseudoparietal). Ovules two per ovary, orthotropous, pendulous, unitegmic, crassinucellar? Integument ? cell layers thick. Megagametophyte monosporous, Polygonum type? Endosperm development nuclear? Endosperm haustoria? Embryogenesis?
Fruit A drupe, very asymmetrical, with ridge and persistent lateral-basal style.
Seeds Aril absent. Testa? Perisperm not developed. Endosperm oily? Embryo straight, chlorophyll? Cotyledons two. Germination?
Cytology n = 20, 22?
DNA
Phytochemistry Virtually unknown. Route I iridoids (e.g. daphylloside), cyanogenic compounds?
Use Unknown.
Systematics Apodytes (c 15; tropical and subtropical regions of eastern and southern Africa, Madagascar, southern India, Sri Lanka, Indochina, Malesia, Queensland, New Caledonia), Rhaphiostylis (6; tropical West Africa), Dendrobangia (2–3; tropical South America)?
The Apodytes clade is part of a basal polytomy also comprising Emmotaceae, Oncothecaceae, Metteniusaceae, and Lamiidae.
Rhaphiostylis is very similar to Icacinaceae in its anatomy, with the exception of the banded parenchyma present in Icacinaceae.
Dendrobangia is sister to [Apodytes+Rhaphiostylis], according to Byng & al. (2014). Dendrobangia has cyclocytic stomata.
CASSINOPSIS CLADE |
( Back to Basal Garryidae ) |
Genera/species 1/6
Distribution Southern and eastern Africa, Madagascar.
Habit Bisexual, evergreen shrubs, often scrambling. Sometimes spiny.
Vegetative anatomy Vessel elements with scalariform perforation plates. Imperforate tracheary xylem elements tracheids. Nodes 3:3, trilacunar with three leaf traces.
Trichomes Hairs unicellular, unbranched, or absent.
Leaves Opposite, simple, entire. Vennation pinnate, brochidodromous. Stomata cyclocytic. Leaf margin spinose-serrate to entire.
Inflorescence Terminal or axillary, corymbose cymose.
Flowers Actinomorphic. Hypogyny. Petals five, connate at base. Disc absent.
Androecium Stamens five, haplostemonous, antesepalous, alternipetalous. Filaments free. Anthers dorsifixed, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits).
Fruit A drupe.
Phytochemistry Virtually unknown. Verbascosides present.
Use Medicinal plants.
Systematics Cassinopsis (6; southern and eastern Africa, Madagascar).
Cassinopsis is very
similar to, e.g., Icacinaceae, Emmotaceae and
the Apodytes clade. However, DNA phylogenies often
place Cassinopsis in a lineage of its own at the very
base of Garryidae. In analyses by Byng & al.
(2014), on the other hand, Cassinopsis is sister to
Icacinaceae.
EMMOTACEAE Tiegh. |
( Back to Basal Garryidae ) |
Emmotales Doweld, Tent. Syst. Pl. Vasc.: li. 23 Dec 2001
Genera/species 5 (or 6)/26 (or 33)
Distribution Indochina, Malesia eastwards to New Guinea, Mexico, Central America, the West Indies, tropical South America.
Fossils Unknown.
Habit Usually bisexual (Platea dioecious), evergreen trees.
Vegetative anatomy Phellogen ab initio superficial? Vessel elements with scalariform perforation plates; lateral pits usually scalariform to opposite (sometimes alternate), usually bordered (in at least Poraqueiba simple) pits. Imperforate tracheary xylem elements tracheids with bordered pits, non-septate. Wood rays uniseriate or multiseriate, homocellular or heterocellular. Axial parenchyma apotracheal usually diffuse-in-aggregates (rarely diffuse), or paratracheal scanty or banded. Tyloses sclerotic sometimes abundant. Sieve tube plastids S type? Nodes 3:3, trilacunar with three leaf traces. Heartwood with gum-like substances. Prismatic calciumoxalate crystals frequent.
Trichomes Hairs usually absent (sometimes T-shaped or, in Platea, stellate or lepidote, sometimes caducous).
Leaves Alternate (spiral), simple, entire, in Platea with conduplicate ptyxis. Petiole vascular bundle transection? Venation pinnate, brochidodromous or eucamptodromous. Stomata usually cyclocytic (sometimes anomocytic). Cuticular waxes? Domatia absent. Leaf margin serrate to entire.
Inflorescence Axillary, panicle, cymose, or compound or simple racemes.
Flowers Actinomorphic, small. Pedicel articulated. Hypogyny. Sepals four or five, with imbricate aestivation, connate. Petals four or five, with valvate aestivation, free (in female flowers of Platea absent), sometimes with adaxial hairs. Nectary? Disc absent.
Androecium Stamens four or five, antesepalous, alternipetalous? Filaments free from each other, free from or adnate at base to petals. Anthers basifixed to dorsifixed, versatile?, tetrasporangiate, latrorse to extrorse, longicidal (dehiscing by longitudinal slits); connective often enlarged. Tapetum secretory? Staminodia absent.
Pollen grains Microsporogenesis simultaneous? Pollen grains tricolporate?, shed as monads, bicellular? at dispersal. Exine?, with ? infratectum, sculpturing?
Gynoecium Pistil composed of three connate carpels, two of which sterile. Ovary superior, unilocular (pseudomonomerous) or (in Emmotum) bilocular or trilocular, without fleshy appendage. Style single, simple, often short. Stigma capitate, slightly trifid or discoid, type? Pistillodium absent.
Ovules Placentation apical, collateral or superposed. Ovules two per fertile carpel, anatropous?, pendulous?, unitegmic, ?-nucellar? Integument ? cell layers thick. Megagametophyte monosporous, Polygonum type? Endosperm development nuclear? Endosperm haustoria? Embryogenesis?
Fruit A usually one-seeded drupe, flattened, often asymmetrical. Pyrene sometimes ribbed.
Seeds Aril absent. Exotesta? Endotesta? Perisperm not developed. Endosperm oily? Embryo straight, short to long, with chlorophyll? Cotyledons two. Germination?
Cytology n = ?
DNA
Phytochemistry Insufficiently known. Monoterpene secoiridoids and emmotins (eudesmane sesquiterpenes) present. Cyanogenic compounds? Aluminium accumulated in Platea.
Use Fruits (Poraqueiba).
Systematics Emmotum (12; tropical South America), Poraqueiba (3; tropical South America), Oecopetalum (3; Mexico, Central America), Ottoschulzia (3; Guatemala, the West Indies), Platea (5; Indochina, Malesia eastwards to New Guinea), Calatola (7; Mexico to Ecuador)?
Emmotaceae are part of a basal polytomy also comprising the Apodytes clade, Cassinopsis, Icacinaceae, Garryales, and Lamiidae.
Calatola has vessel elements with scalariform perforation plates, nodes 3:3, trilacunar with three leaf traces, leaves with conduplicate ptyxis and serrate leaf margin. Emmotum and Ottoschulzia possess vessel elements with scalariform perforation plates with less than eleven cross-ribs.
Emmotum is somewhat
similar to Oncotheca (Oncothecaceae) in its
monosporangiate thecae and in the gynoecium having both fertile
and sterile carpels.
Platea occupies an isolated position in Emmotaceae on behalf of indumentum morphology and presence of aluminium accumulation.
The clade
[Calatola+Platea] is part of a trichotomy
also including the weakly supported clades
[Emmotaceae+Metteniusaceae etc.] and
[Apodytes group+Oncothecaceae] in analyses by
Byng & al. (2014). In the same analyses,
Metteniusa is sister to
[Oecopetalum+Ottoschulzia], whereas
Emmotum is sister to Poraqueiba (see also
Duno De Stefano & Fernández-Concha 2011).
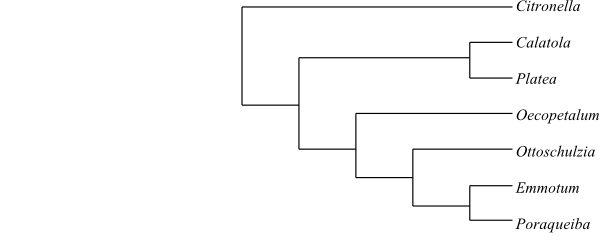 |
|
One of numerous most-parsimonious cladograms of Emmotaceae based on DNA sequence data and morphology (Kårehed 2001). Citronella is usually placed in Cardiopteridaceae (Aquifoliales). |
GARRYALES Lindl. |
( Back to Basal Garryidae ) |
Eucommiales Nèmejc ex Cronq., Integr. Syst. Class. Fl. Pl.: 182. 10 Aug 1981; Eucommianae Takht. ex Reveal in Phytologia 74: 179. 25 Mar 1993; Aucubales Takht., Divers. Classif. Fl. Pl.: 385. 24 Apr 1997
Habit Dioecious, usually evergreen (rarely deciduous) trees or shrubs or perennial herbs. Young stems and branches quadrangular in cross-section.
Vegetative anatomy Phellogen ab initio superficial. Vessel elements with simple or scalariform perforation plates; lateral pits alternate, scalariform or opposite, bordered pits; vessel elements with helical thickenings. Imperforate tracheary xylem elements tracheids with simple or bordered pits, usually non-septate. Wood rays uniseriate or multiseriate, homocellular or heterocellular. Axial parenchyma usually apotracheal diffuse or diffuse-in-aggregates, or paratracheal scanty or banded. Sieve tube plastids S type. Nodes usually 3:3, trilacunar with three leaf traces (rarely 1:1, unilacunar with one trace). Phloem and cortex sometimes with articulated, unicellular, gutta-percha secreting laticifers. Often with calciumoxalate crystal sand.
Trichomes Hairs unicellular, simple, with ridges running counter-clock-wise (sometimes micropapillate), or absent.
Leaves Usually opposite (sometimes alternate), usually simple (occasionally somewhat pinnately compound), entire or slightly lobed, coriaceous, with conduplicate, supervolute or curved ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle transection arcuate. Venation pinnate. Stomata laterocytic, paracytic or anomocytic. Cuticular wax crystalloids as tubuli (and sometimes platelets) or absent. Mesophyll often with sclerenchymatous idioblasts. Sometimes with laticifers containing gutta-percha. Leaf margin serrate or entire.
Inflorescence Terminal or axillary, cymose, catkin-like or dichotomously thyrsoid. Male inflorescences axillary catkin-like cymose; female flowers solitary axillary. Floral prophylls (bracteoles) sometimes absent.
Flowers Actinomorphic. Epigyny (sometimes hypogyny?). Sepals two or four with valvate aestivation, strongly reduced, free or more or less connate, caducous or persistent (sometimes absent). Petals four, with valvate aestivation, free, or absent. Nectariferous disc intrastaminal, quadrangular, bilobate or absent.
Androecium Stamens four (to twelve, rarely more than twelve), usually haplostemonous, alternisepalous or antesepalous. Filaments free from each other and from tepals. Anthers basifixed or dorsifixed, non-versatile, tetrasporangiate, introrse or latrorse, longicidal (dehiscing by longitudinal slits). Tapetum amoeboid-periplasmodial or secretory. Staminodia absent.
Pollen grains Microsporogenesis simultaneous. Pollen grains 2–3(–15)-colporate, shed as monads, bicellular at dispersal. Exine semitectate, with columellate infratectum, reticulate, or exine intectate with smooth, spinulate or verrucate surface.
Gynoecium Pistil composed of two (or three) connate carpels (gynoecium finally pseudomonomerous) or one carpel. Ovary usually inferior (sometimes superior?), unilocular. Stylodia two (or three), free, or style single, simple, short, or absent. Stigmatic surfaces elongate, decurrent, non-papillate, Dry type, or stigma one, capitate, slightly bifid. Male flowers with pistillodium or pistillodium absent.
Ovules Placentation apical. Ovules one or two (or three) per ovary, anatropous, pendulous, apotropous, unitegmic, usually crassinucellar (rarely tenuinucellar). Archespore sometimes multicellular. Large placental obturator sometimes present. Megagametophyte monosporous, Polygonum type. Fertilization often delayed. Antipodal cells sometimes early degenerating. Endosperm development nuclear or cellular. Endosperm haustoria? Embryogenesis solanad.
Fruit A one- or two-seeded and gradually drying berry with persistent calyx or style/stylodia, or a single-seeded samara.
Seeds Aril absent. Arilloid formed from placental obturator or absent. Testa usually thick, multiplicative (sometimes membranous); outer part sarcotesta, inner part with tangentially elongate cells. Exotestal cells large, palisade, fleshy. Endotesta? Perisperm not developed. Endosperm copious, fleshy, with oil, petroselinic acid, starch and hemicellulose. Suspensor sometimes very long. Embryo small or large, straight, well differentiated, with chlorophyll. Cotyledons two. Germination phanerocotylar.
Cytology n = 8, 11, 17
DNA Plastid gene rps16 and ORF184 absent in Eucommia.
Phytochemistry Flavonols (kaempferol, quercetin), O-methylated flavonoids, cyanidin, Route II decarboxylated iridoids, Group I carbocyclic iridoids (e.g. scandoside, aucubin, ipolamiide, ajugol, ajugoside, and harpagide), Group II decarboxylated iridoids, oleanolic acid derivatives, ellagic acid, caffeic acid, chlorogenic acid, strongly toxic diterpene alkaloids, saponins, petroselinic (cis-6-octadecenoic) acid, eucommin A, lignans (pinoresinol, syringaresinol), gutta-percha, and acetylenes present. Gallic acid and cyanogenic compounds not found. Carbohydrates sometimes stored as inulin.
Systematics Garryales comprise Eucommia (Eucommiaceae) and Garryaceae.
Literature on Garryales is found under Basal Garryidae (Literature).
EUCOMMIACEAE Engl. |
( Back to Garryales ) |
Genera/species 1/1
Distribution Temperate central China.
Fossils Leaves and samaras of Eucommia is frequently recorded in Cenozoic layers in North America, Europe and East Asia from the Eocene of eastern North America and Japan onwards. Eucommia is known from Central and North America in the Oligocene and the Miocene and in Europe (also by typical tricolpate pollen grains) from the Oligocene to the Pleistocene.
Habit Dioecious, deciduous tree.
Vegetative anatomy Phellogen ab initio superficial. Medulla lamellated. Vessel elements with simple perforation plates (earliest secondary xylem scalariform); lateral pits alternate or opposite, bordered pits. Imperforate tracheary xylem elements tracheids with bordered pits, non-septate. Wood rays uniseriate or multiseriate, homocellular or somewhat heterocellular. Axial parenchyma apotracheal diffuse, or paratracheal banded. Sieve tube plastids Ss type (with approx. ten starch grains). Nodes 1:1, unilacunar with one leaf trace. Phloem fibres present. Phloem and cortex with articulated, unicellular laticifers secreting gutta percha. Crystals?
Trichomes Hairs micropapillate, unicellular, unbranched. Buds perulate.
Leaves Alternate (spiral to distichous), simple, entire, with supervolute (involute?) to curved ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle transection arcuate. Venation pinnate. Stomata anomocytic. Cuticular wax crystalloids absent. Laticifers with gutta-percha. Leaf margin coarsely serrate; single vein proceeding into each glandular-tipped tooth.
Inflorescence Male inflorescences axillary catkin-like cymose; female flowers solitary axillary. Floral prophylls (bracteoles) absent.
Flowers Actinomorphic, small. Hypogyny? Tepals absent. Nectary absent. Disc absent.
Androecium Stamens usually five to twelve (rarely four or more than twelve). Filaments short, free. Anthers basifixed, non-versatile, tetrasporangiate, introrse?, longicidal (dehiscing by longitudinal slits); connective prolonged at apex. Tapetum secretory. Staminodia absent.
Pollen grains Microsporogenesis simultaneous. Pollen grains tricolporate with indistinct ora, shed as monads, bicellular at dispersal. Exine intectate. Pollen surface spinulate to verrucate.
Gynoecium Pistil composed of two connate carpels, one fertile and one aborted. Ovary superior?, unilocular due to pseudomonomery. Stylodia two, short, unequally sized, diverging. Stigmas adaxially decurrent, type? Pistillodium absent.
Ovules Placentation apical. Ovules two (one fertile) per ovary, anatropous, collateral, pendulous, apotropous, unitegmic, tenuinucellar to weakly crassinucellar. Micropyle very long. Integument five to nine cell layers thick. Parietal tissue approx. three cell layer thick. Nucellar cap present. Megagametophyte monosporous, Polygonum type. Endosperm development cellular. Endosperm haustoria? Embryogenesis solanad.
Fruit A one-seeded samara.
Seeds Aril absent. Testa membranous. Exotestal cells? Endotesta? Perisperm not developed. Endosperm copious, oily? Embryo large, straight, well differentiated, with chlorophyll? Cotyledons two. Germination phanerocotylar.
Cytology n = 17
DNA Plastid gene rps16 and ORF184 absent.
Phytochemistry Flavonols (kaempferol, quercetin), O-methylated flavonols, cyanidin, Route II decarboxylated iridoids, Group I carbocyclic iridoids (e.g. aucubin, ipolamiide, ajugol, ajugoside, and harpagide), oleanolic acid derivatives, ellagic acid, condensed tannins (in bark), saponins, eucommin A, lignans (pinoresinol, syringaresinol), gutta percha, and acetylenes present. Alkaloids and cyanogenic compounds not found. Carbohydrates stored as inulin. Oxalate sometimes accumulated.
Use Ornamental plant, medicinal plant, latex (gutta percha).
Systematics Eucommia (1; E. ulmoides; temperate central China; probably extinct in the wild).
Eucommia is sister to Garryaceae.
GARRYACEAE Lindl. |
( Back to Garryales ) |
Aucubaceae Bercht. et J. Presl, Přir. Rostlin: 2: 91. 1825 [’Aucubeae’]
Genera/species 2/16–22
Distribution Eastern Himalayas, northern Burma, China, southern Korean Peninsula, Japan, the Ryukyu Islands, Taiwan, western North America, Mexico, Central America, the West Indies.
Fossils Unknown.
Habit Dioecious, evergreen trees or shrubs (Garrya); shrubs or perennial herbs (Aucuba). Young stems and branches quadrangular in cross-section.
Vegetative anatomy Phellogen ab initio superficial. Cauline pericyclic envelope absent in Aucuba. Vessel elements with simple or scalariform perforation plates; lateral pits alternate, scalariform or opposite, bordered pits. Imperforate tracheary xylem elements tracheids (vascular tracheids present in Aucuba) with simple (Aucuba) and/or bordered pits (in Garrya with spiral wall thickenings), usually non-septate (in Aucuba sometimes septate). Wood rays uniseriate or multiseriate, heterocellular. Axial parenchyma usually apotracheal diffuse or diffuse-in-aggregates, or paratracheal scanty or banded. Sieve tube plastids S type. Nodes 3:3, trilacunar with three leaf traces. Often with calciumoxalate crystal sand.
Trichomes Hairs unicellular, simple, with ridges running counter-clock-wise, or absent.
Leaves Opposite, usually simple (occasionally somewhat pinnately compound), entire or slightly lobed, coriaceous, with conduplicate ptyxis. Stipules and leaf sheath absent. Petioles more or less connate at base. Petiole vascular bundle transection arcuate. Venation pinnate. Stomata in Garrya laterocytic or paracytic, in Aucuba anomocytic. Cuticular wax crystalloids as tubuli (and sometimes platelets). Mesophyll often with sclerenchymatous idioblasts. Leaf margin serrate (Aucuba) or entire (in Garrya cartilaginous).
Inflorescence Terminal or axillary, cymose; in Garrya catkin-like, silk-hairy, with bracts more or less connate; in Aucuba dichotomously thyrsoid. Floral prophylls (bracteoles) absent? in Garrya and in male flowers of Aucuba.
Flowers Actinomorphic, small. Epigyny. Sepals four in male flowers of Garrya, small, with valvate aestivation, strongly reduced, connate at apex, early caducous, in female flowers usually two (rarely four; prophylls?), almost entirely connate, strongly reduced or absent; sepals in Aucuba four, free, persistent in fruit, or absent. Petals absent in Garrya; petals in Aucuba four, with valvate aestivation, free. Nectariferous disc intrastaminal, in Aucuba fleshy, quadrangular, on ovary apex, in male flowers of Garrya heavily reduced (bilobate), in female flowers absent.
Androecium Stamens four, alternisepalous (Garrya) or antesepalous, alternipetalous (Aucuba). Filaments free from each other and from tepals. Anthers basifixed (Garrya) or dorsifixed (Aucuba), non-versatile, tetrasporangiate, introrse or latrorse, longicidal (dehiscing by longitudinal slits). Tapetum amoeboid-periplasmodial. Staminodia absent.
Pollen grains Microsporogenesis simultaneous. Pollen grains 2–3(–15)-colporate, shed as monads, bicellular at dispersal. Exine semitectate with columellate? infratectum and reticulate tectum (Garrya), or exine intectate with smooth surface (Aucuba).
Gynoecium Pistil composed of usually two (rarely three) connate carpels (gynoecium finally pseudomonomerous, Garrya), or one carpel (seemingly? monomerous, Aucuba). Ovary inferior, unilocular, in Aucuba nectariferous. Stylodia usually two (rarely three), subulate, free, diverging (Garrya) or style single, simple, short, thick (Aucuba). Stigmatic surfaces elongate, decurrent, non-papillate, Dry type (Garrya), or stigma one, capitate, slightly bifid, type? (Aucuba). Male flowers with pistillodium.
Ovules Placentation apical. Ovules usually one or two (sometimes three) per ovary, anatropous, pendulous, epitropous (Garrya), unitegmic, crassinucellar. Integument twelve to c. 30 cell layers thick (Garrya). Parietal tissue in Garrya four or five cell layers thick, in Aucuba approx. eight cell layers thick. Hypostaste present in Garrya. Nucellar cap sometimes two cell layers thick. Archespore sometimes multicellular (Aucuba). Megasporocytes in Aucuba sometimes several. Large placental obturator closing upper part of locule in Garrya. Megagametophyte monosporous, Polygonum type. Antipodal cells in Garrya early degenerating or persistent. Endosperm development nuclear (Garrya) or cellular (Aucuba). Endosperm haustoria? Embryogenesis solanad (Garrya).
Fruit A one- or two-seeded and gradually drying berry with persistent calyx or style (stylodia).
Seeds Aril absent. Arilloid in Garrya formed from placental obturator. Testa thick, multiplicative; outer part sarcotesta, inner part with tangentially elongate cells. Exotestal cells in Garrya large, palisade, fleshy. Endotesta not persistent (Garrya). Perisperm not developed. Endosperm copious, fleshy, with oil, petroselinic acid, starch and hemicellulose. Suspensor in Garrya very long. Embryo small, straight, well differentiated, with chlorophyll. Cotyledons two. Germination phanerocotylar (Garrya).
Cytology n = 8 (Aucuba), 11 (Garrya)
DNA
Phytochemistry Flavonols (kaempferol, quercetin, in Aucuba), flavonoid glycosides, Route II decarboxylated iridoids, Group I carbocyclic iridoids (e.g., scandoside and aucubin), strongly toxic diterpene alkaloids (e.g. garryfoline in Garrya), chlorogenic acids, and petroselinic acid present. Caffeic acid? Gutta percha in Aucuba? Ellagic and gallic acids, tannins, proanthocyanidins, saponins, and cyanogenic compounds not found.
Use Ornamental plants, medicinal plants.
Systematics Aucuba (3–4; Sikkim, northern Burma, China, southern Korean Peninsula, Japan, the Ryukyu Islands, Taiwan), Garrya (13–18; western North America northwards to Washington, Mexico, Central America to Panamá, the Greater Antilles).
Garryaceae are sister group to Eucommia (Eucommiaceae).
ICACINACEAE (Benth.) Miers |
( Back to Basal Garryidae ) |
Phytocrenaceae Arn. ex R. Br. in J. J. Bennett et R. Brown, Plant. Jav. Rar.: 244. 13 Mar 1852 [’Phytocreneae’]; Iodaceae Tiegh. in Bot. Jahresber. (Just) 25(2): 406. 19 Jan 1900 [’Jodaceae’]; Pleurisanthaceae Tiegh. in Bot. Jahresber. (Just) 25(2): 406. 19 Jan 1900; Sarcostigmataceae Tiegh. in Bot. Jahresber. (Just) 25(2): 406. 19 Jan 1900; Icacinales Tiegh. in Bot. Jahresber. (Just) 25(2): 406. 19 Jan 1900; Icacinanae Doweld, Tent. Syst. Plant. Vasc.: li. 23 Dec 2001
Genera/species c 24/c 150
Distribution Tropical Africa, Madagascar, tropical Asia, southern China, Japan, Taiwan, islands of the western Pacific, Malesia, New Guinea, northern Queensland, Melanesia, tropical America.
Fossils Flattened grooved unilocular endocarps, assigned to Icacinaceae, with deeply foveolate-reticulate surface and a papillate inner lining have been found in the Late Turonian to the Santonian of Central Europe and were described as Icacinicarya budvarensis. Similar endocarps from the Maastrichtian of Germany have been attributed to Icacinicarya papillaris and Iodes germanica. From the Palaeogene of eastern North America are many reports of fossil Icacinaceae and the extinct Stizocaryopsis was described from the Paleocene of Egypt. Fossilized wood, leaves and additional endocarps assigned to Icacinaceae are also known, e.g. from the Eocene of England (Icacinicarya and Palaeophytocrene described from the London Clay) and Germany. Fossil fruits of Palaeophytocrene (Phytocreneae) from Mid and Late Paleocene have been found in western North America and Colombia (Stull & al. 2012).
Habit Usually bisexual (sometimes andromonoecious?, gynomonoecious?, polygamomonoecious?, dioecious, androdioecious?, or gynodioecious?), evergreen trees, shrubs or lianas with non-axillary branch tendrils.
Vegetative anatomy Phellogen ab initio superficial. Medullary vascular bundles present in Iodes. Secondary lateral growth in lianas (e.g. Sarcostigma with interxylary phloem) often anomalous (via concentric/successive cambia or from simple cylindrical cambium). Vessel elements with simple perforation plates; lateral pits alternate to opposite, bordered pits. Imperforate tracheary xylem elements tracheids, fibre tracheids or libriform fibres with simple or bordered pits, usually non-septate. Wood rays uniseriate or multiseriate, homocellular or heterocellular. Axial parenchyma apotracheal diffuse or diffuse-in-aggregates, or paratracheal scanty, aliform, winged-aliform, confluent, scalariform, vasicentric, or banded. Tyloses sometimes present in Icacina and Mappia. Secondary phloem stratified. Intraxylary phloem often present in lianas (e.g. Sarcostigma). Sieve tube plastids S type. Nodes usually 1:1, unilacunar with one leaf trace (sometimes 3:3, trilacunar with three traces). Secretory cavities (with latex) present or absent? Cortex with or without cristarque cells? Sclereids present. Prismatic calciumoxalate crystals or groups of crystals usually in parenchyma cells; crystal sand sometimes present in wood rays; druses present in many species; styloid-like crystals present in some species.
Trichomes Hairs unicellular, often adpressed and T-shaped, sometimes globular.
Leaves Usually alternate (spiral; in Iodes opposite), simple, usually entire (rarely palmately lobed), often coriaceous, usually with conduplicate (rarely conduplicate-plicate) ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle transection arcuate and petiole with wing bundles, or bundle transection annular (petiole in Iodes also with medullary bundles). Venation usually pinnate (in Hosiea palmate). Stomata usually cyclocytic (sometimes anomocytic or anisocytic). Cuticular wax crystalloids? Domatia usually absent (sometimes as pits or pockets). Secretory cavities (with latex) present or absent? Mesophyll with or without sclerenchymatous idioblasts? Leaf margin usually entire (sometimes serrate, in Hosiea with long teeth).
Inflorescence Axillary, panicle, spike or raceme.
Flowers Actinomorphic, small. Pedicel articulated. Hypogyny. Sepals (three to) five (or six)?, usually with imbricate (rarely valvate?) aestivation, persistent in fruit, usually more or less connate (in Phytocrene free or almost free; absent in Pyrenacantha). Petals (three to) five (or six)?, usually with valvate aestivation, usually free (sometimes connate at base; rarely absent), with incurved apex and sometimes adaxial keel. Nectariferous disc usually absent (sometimes discoid, columellate or lobed).
Androecium Stamens (three to) five (or six)?, haplostemonous, antesepalous, alternipetalous. Filaments free from each other and usually from tepals (rarely adnate to corolla tube, epipetalous). Anthers dorsifixed, versatile?, tetrasporangiate, usually introrse (sometimes latrorse?), usually longicidal (dehiscing by longitudinal slits; rarely poricidal, dehiscing by apical pores?). Tapetum secretory, with multinucleate cells. Female flowers often with staminodia.
Pollen grains Microsporogenesis simultaneous. Pollen grains usually tricolporate or triporate (rarely tricolpate?; in Stachyanthus inaperturate), shed as monads, bicellular at dispersal. Exine tectate, with ? infratectum, echinate.
Gynoecium Pistil composed of usually two (rarely one or three?) connate carpels. Ovary superior, unilocular (pseudomonomerous?), without fleshy appendage. Style single, simple, short, or absent. Stigma wide (when style absent) or punctate, type? Male flowers with pistillodium.
Ovules Placentation usually apical. Ovules usually two (rarely one) per ovary, anatropous, pendulous, apotropous, unitegmic (in Phytocrene apically bitegmic, with integuments free in micropyle), usually (thinly) crassinucellar (sometimes tenuinucellar). Integument ? cell layers thick. Funicular obturator present. Megagametophyte monosporous, Polygonum type. Synergids often with a filiform apparatus. Antipodal cells ephemeral. Endosperm development usually nuclear (in Nothapodytes cellular). Endosperm haustoria present or absent (in Nothapodytes chalazal, often elongate). Embryogenesis?
Fruit A one-seeded drupe, often flattened and/or ridged, with persistent calyx. Endocarp cells sometimes papillate.
Seeds Aril absent. Exotesta present. Endotesta present. Perisperm not developed. Endosperm copious or sparse, oily (in Merrilliodendron starchy), sparse or absent (sometimes ruminate). Embryo straight or curved?, usually long, well differentiated, with chlorophyll. Cotyledons two, foliaceous. Germination phanerocotylar or cryptocotylar.
Cytology n = 10, 12
DNA
Phytochemistry Flavonols (quercetin), cyanidin, monoterpene secoiridoids, diterpenoids, ipecacalkaloids, camptothecin (monoterpene indole alkaloid), saponins, and cyanogenic compounds present. Route I iridoids? Ellagic acid not found. Aluminium accumulated in some species.
Use Timber, medicinal plants (seed oil from Sarcostigma), starch sources (tubers of Casimirella).
Systematics Nothapodytes (5; East Asia, tropical Asia), Mappia (4; southern Mexico, Central America, the West Indies), Icacina (6; tropical Africa), Casimirella (7; tropical America), Leretia (1; L. cordata; tropical South America), Lavigeria (1; L. macrocarpa; Cameroun, Gabon, Congo), Merrilliodendron (1; M. rotense; the Philippines, western Pacific islands), Alsodeiopsis (11; tropical Africa), Desmostachys (7; tropical Africa, Madagascar), Mappianthus (2; southern China, Borneo), Pleurisanthes (5–6; tropical South America), Natsiatum (1; N. herpeticum; eastern Himalayas to Southeast Asia), Sleumeria (1; S. auriculata; northern Borneo), 'Iodes' (28; tropical regions in the Old World; paraphyletic; incl. Polyporandra?), Polyporandra (1; P. scandens; East Malesia, New Guinea, Melanesia; in Iodes?), Sarcostigma (2; tropical Asia), Miquelia (8; tropical Asia), Phytocrene (11; Southeast Asia, Malesia), Rhyticaryum (12; East Malesia and eastwards to western Pacific islands), Stachyanthus (6; tropical Africa), Pyrenacantha (c 20; tropical regions in the Old World). – Unplaced Icacinaceae Hosiea (2; western and central China, Japan), Natsiatopsis (1; N. thunbergiifolia; Burma), Pittosporopsis (1; P. kerrii; Southeast Asia).
Icacinaceae are part of a basal polytomy also comprising the Apodytes clade, Cassinopsis, Emmotaceae, Garryales, and Lamiidae.
The Icacina clade is morphologically homogenous (almost all species are lianas) and is characterized by, e.g., vessel elements with simple perforation plates, lateral pits usually alternate, vessel elements and fibres relatively short, axial parenchyma banded and vasicentric, wood rays multiseriate, high and wide, and variation in the shape of the cambium.
Sarcostigma has interxylary phloem, few septated libriform fibres occurring together with normal fibres with bordered pits, and sparse biseriate and low multiseriate wood rays. Even the foliar anatomy and the pollen morphology are different from the other lianous Icacinaceae.
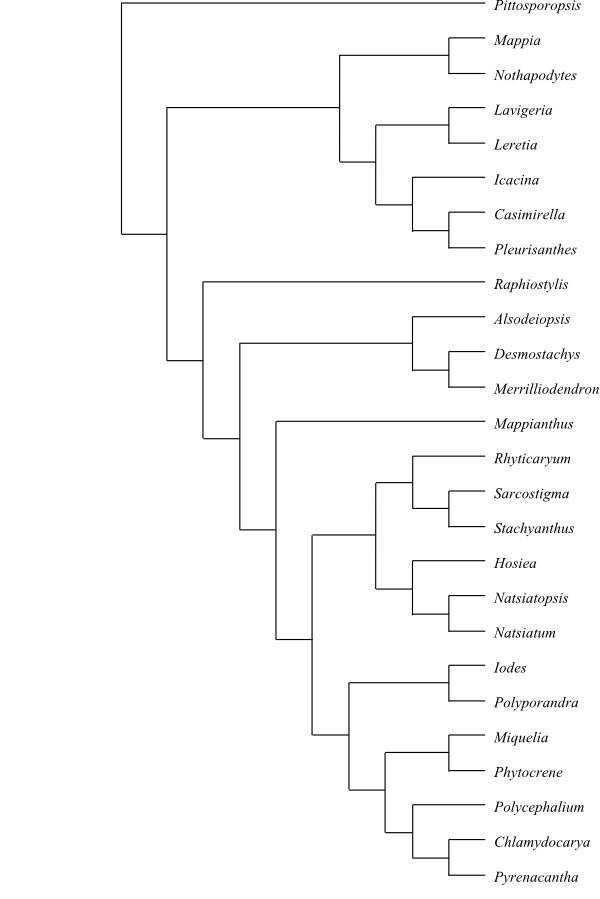 |
|
One out of numerous most-parsimonious cladograms of Icacinaceae based on DNA sequence data and morphology (Kårehed 2001). Raphiostylis is usually placed in the Apodytes clade as sister to Apodytes. According to Angulo & al. (2013), Leretia, Icacina and Casimirella form a clade which is more related to other genera than to [Mappia+Nothapodytes]. |
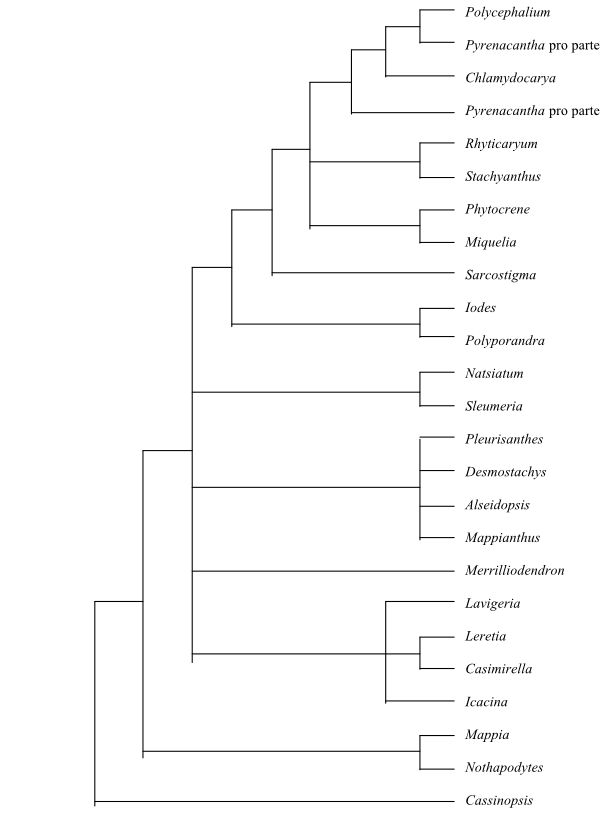 |
|
Phylogeny of Icacinaceae based on Byng & al. (2014). |
LAMIIDAE Takht. ex Reveal |
( Back to Basal Garryidae ) |
[Metteniusa+Oncotheca+[Boraginaceae+Vahlia+[Rubiales+[Plantaginales+Solanales]]]]
METTENIUSACEAE H. Karst. ex Schnizl. |
( Back to Lamiidae ) |
Metteniusales Takht., Divers. Classif. Fl. Pl.: 352. 24 Apr 1997
Genera/species 1/7
Distribution Central America, northwestern South America.
Fossils Unknown.
Habit Bisexual, evergreen trees.
Vegetative anatomy Phellogen? Vessel elements with scalariform perforation plates; lateral pits opposite to somewhat alternate, simple pits. Imperforate tracheary xylem elements ? with bordered pits, non-septate. Wood rays multiseriate, heterocellular. Axial parenchyma apotracheal diffuse-in-aggregates, or paratracheal scanty? or banded. Tyloses absent. Sieve tube plastids S type? Nodes 5:5, pentalacunar with five leaf traces. Prismatic calciumoxalate crystals abundant; druses frequent in flowers.
Trichomes Hairs unicellular, unbranched or T-shaped.
Leaves Alternate (spiral), simple, entire, with ? ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle transection arcuate, complex. Venation pinnate. Stomata anomocytic or cyclocytic (anomocyclocytic). Cuticular wax crystalloids? Mesophyll with fibres. Leaf margin entire.
Inflorescence Axillary, cymose thyrsoid. Floral prophylls (bracteoles) (two or) three per flower, thick, triangular.
Flowers Actinomorphic, large. Hypogyny. Sepals five, with imbricate quincuncial aestivation, persistent, connate. Petals five, with valvate aestivation, in late stage connate in lower part (late sympetaly). Nectary absent. Disc absent. Secretory hairs present.
Androecium Stamens five, haplostemonous, antesepalous, alternipetalous. Filaments long, subulate, free, adnate to corolla tube (epipetalous). Anthers basifixed, versatile, polysporangiate, septate, latrorse, longicidal (dehiscing by longitudinal slits, 17 or fewer partial microsporangia – locelli – in four vertical rows opening individually), long and moniliform; thecae strongly recurved following dehiscence; connective massive. Tapetum secretory. Staminodia absent.
Pollen grains Microsporogenesis simultaneous? Pollen grains tricolporate, shed as monads, ?-cellular at dispersal. Exine ?, with ? infratectum, sculpturing?
Gynoecium Pistil composed of five paracarpous and connate carpels, one fertile and four strongly reduced leading to pseudomonomery; carpel initials congenitally fused by their margins; gynoecium zygomorphic throughout ontogeny (indicating pseudomonomery). Ovary superior, unilocular (due to pseudomonomery?). Style single, long, filiform. Stigma punctate, slightly papillate, type? Pistillodium absent.
Ovules Placentation parietal. Ovules two per ovary, anatropous, pendulous, unitegmic, tenuinucellar. Integument more than 20 cell layers thick, massive, highly vascularized. Megagametophyte monosporous, Polygonum type? Endosperm development? Endosperm haustoria? Embryogenesis?
Fruit A one-seeded (one ovule aborted) drupe with asymmetrically arranged ridges.
Seeds Aril absent. Funicle massive. Testa membranous, vascularized. Exotestal cells? Endotesta? Perisperm not developed. Endosperm copious. Embryo curved, large, well differentiated, with chlorophyll? Cotyledons two, foliaceous. Germination?
Cytology n = ?
DNA
Phytochemistry Virtually unknown. Tannins present.
Use Edible seeds (Metteniusa edulis).
Systematics Metteniusa (7; Costa Rica, Panamá, the northern Andes to Peru and western Venezuela).
Metteniusa is member of a polytomy also comprising Oncotheca (Oncothecaceae) and the clade [Boraginaceae+Vahlia+[Rubiales+[Plantaginales+Solanales]]] (González & al. 2007). In some trees Oncotheca is sister to Metteniusa. Late sympetaly and unitegmic ovules support placement of Metteniusaceae among the lamiids. The combination of polysporangiate, transversely septate anthers and dehiscence by four (rather than two) longitudinal grooves per anther, appears to be unique for Metteniusa. The five unequal carpels form a single locule, in which two ovules arise from the two smallest and most poorly developed lateral carpels. For features common to both Metteniusa and Oncotheca, see under Oncothecaceae.
Metteniusa forms a
weakly supported clade together with
[Oecopetalum+Ottoschulzia] (here in
Emmotaceae) in analyses by Byng & al.
(2014).
ONCOTHECACEAE Kobuski ex Airy Shaw |
( Back to Lamiidae ) |
Oncothecales Doweld, Tent. Syst. Plant. Vasc.: li. 23 Dec 2001
Genera/species 1/2
Distribution New Caledonia.
Fossils Unknown.
Habit Bisexual, evergreen trees or shrubs.
Vegetative anatomy Phellogen ab initio in outer cortex. Vessel elements with scalariform perforation plates; lateral pits scalariform?, simple pits. Imperforate tracheary xylem elements tracheids or fibre tracheids with bordered pits?, non-septate. Wood rays multiseriate?, heterocellular? Axial parenchyma apotracheal diffuse or diffuse-in-aggregates, or paratracheal scanty. Secondary phloem in young stems stratified. Sieve tube plastids S type. Nodes usually 5:5, pentalacunar with five leaf traces (sometimes 3:3, trilacunar with three traces). Asterosclereids present. Parenchyma cells with aggregations of calciumoxalate crystals. Druses abundant.
Trichomes Hairs absent.
Leaves Alternate (spiral), simple, entire, coriaceous, with convolute? ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle transection arcuate. Venation pinnate. Stomata anomocytic, paracytic, tetracytic or cyclocytic. Cuticular wax crystalloids? Mesophyll with sclerenchymatous idioblasts. Calciumoxalate druses present. Leaf margin serrate or entire, with small caducous glands on teeth.
Inflorescence Axillary, usually panicle or thyrse. Floral prophylls (bracteoles) triangular, two or three per flower.
Flowers Actinomorphic, small. Hypogyny. Sepals five, with imbricate quincuncial aestivation, persistent, more or less free. Petals five, with imbricate aestivation, caducous, connate. Nectary? Disc absent.
Androecium Stamens five, haplostemonous, antesepalous, alternipetalous. Filaments free, adnate to corolla tube (epipetalous). Anthers basifixed, non-versatile, disporangiate (monothecal), extrorse, longicidal (dehiscing by longitudinal slits); connective in Oncotheca balansae (not in O. humboldtiana) acute and incurved above gynoecium. Tapetum secretory? Staminodia absent.
Pollen grains Microsporogenesis simultaneous? Pollen grains (2–)3-colporate, shed as monads, bicellular? at dispersal. Exine tectate, with columellate infratectum, perforate, smooth.
Gynoecium Pistil composed of five syncarpous and connate antepetalous carpels. Ovary superior, quinquelocular, with five ridges, apically somewhat quinquelobate (with lobes incompletely closed). Stylodia five, free, recurved, conduplicate. Stigmas ventral, punctate, type? Pistillodium absent.
Ovules Placentation apical to axile. Ovules usually two (sometimes one) per carpel, campylotropous, pendulous, epitropous, unitegmic, tenuinucellar (crassinucellar?). Funicle long. Integument ? cell layers thick. Megagametophyte monosporous, Polygonum type? Endosperm development nuclear? Endosperm haustoria? Embryogenesis?
Fruit A two- to five-seeded drupe with thick quinquelocular pyrene and persistent calyx.
Seeds Aril absent. Testa thin. Exotesta? Endotesta? Perisperm not developed. Endosperm copious. Embryo straight, cylindrical, well differentiated, with chlorophyll? Hypocotyl long. Cotyledons usually two (rarely three), short. Germination?
Cytology n = 25
DNA
Phytochemistry Virtually unknown. Tannins present.
Use Unknown.
Systematics Oncotheca (2; New Caledonia).
Oncotheca is member of a polytomy also comprising Metteniusa (Metteniusaceae) and the clade [Boraginaceae+Vahlia+[Rubiales+[Plantaginales+Solanales]]] (González & al. 2007). However, Oncotheca might be sister to Metteniusa. Vessel elements with scalariform perforation plates, pentalacunar nodes, presence of druses and tanniniferous cells in vegetative and floral organs, spiral leaves with anomocytic to cyclocytic stomata, similarities in inflorescence architecture, cymes with thick, triangular bracts, flowers with two or three triangular floral prophylls (bracteoles), alternipetalous stamens with basifixed anthers, quinquecarpellate gynoecium, biovulate carpels, long funicle, drupe with persistent perianth, and copious endosperm are common features and plausible synapomorphies (González & Rudall 2010).
Oncotheca forms a very
weakly supported clade together with the Apodytes
clade in analyses by Byng & al. (2014).
Literature
Abe T. 2001. Flowering phenology, display size, and fruit set in an understory dioecious shrub, Aucuba japonica (Cornaceae). – Amer. J. Bot. 88: 455-461.
Angulo DF, Stefano RD de, Stull GW. 2013. Systematics of Mappia (Icacinaceae), an endemic genus of tropical America. – Phytotaxa 116: 1-18.
Baas P. 1973. Epidermal leaf characters of the Malesian Icacinaceae. – Acta Bot. Neerl. 22: 329-359.
Baas P. 1974. Stomatal types in Icacinaceae. Additional observations on genera outside Malesia. – Acta Bot. Neerl. 23: 193-200.
Baas P. 1975. Vegetative anatomy and the affinities of Aquifoliaceae, Sphenostemon, Phelline, and Oncotheca. – Blumea 22: 311-407.
Bailey IW, Howard RA. 1941a. The comparative morphology of the Icacinaceae I. Anatomy of the node and internode. – J. Arnold Arbor. 22: 125-132.
Bailey IW, Howard RA. 1941b. The comparative morphology of the Icacinaceae II. Vessels. – J. Arnold Arbor. 22: 171-187.
Bailey IW, Howard RA. 1941c. The comparative morphology of the Icacinaceae III. Imperfect tracheary elements and xylem parenchyma. – J. Arnold Arbor. 22: 432-442.
Bailey IW, Howard RA. 1941d. The comparative morphology of the Icacinaceae IV. Rays of the secondary xylem. – J. Arnold Arbor. 22: 556-568.
Bâthie HP de la. 1952. Icacinacées (Icacinaceae). – In: Humbert H (ed), Flore de Madagascar et des Comores 119, Typographie Firmin-Didot et Cie, Paris.
Bautista PB, De Andrade TAP. 1975. O pólen em plantas da Amazônia V. Contribuição ao estudo da familia Icacinaceae. – Bol. Mus. Paraense “Emilio Goeldi”, N. S., Bot. 47: 1-11.
Braga de Oliveira A. 1995. Terpenoids from Amazonian Icacinaceae. – In: Chemistry of the Amazon, ACS Symposium Ser., Vol. 588, pp. 99-115.
Breteler FJ, Villiers J-F. 2000. Novitates Gabonenses 39. Une nouvelle espèce de Pyrenacantha (Icacinaceae) du Gabon. – Adansonia, sér. III, 22: 201-204.
Byng JW, Bernardini B, Joseph
JA, Chase MW, Utteridge TMA. 2014. Phylogenetic relationships of
Icacinaceae focusing on the vining genera. – Bot. J. Linn. Soc.
176: 277-294.
Call VB, Dilcher DL. 1997. The fossil record of Eucommia (Eucommiaceae) in North America. – Amer. J. Bot. 894: 798-814.
Cameron KM. 2003. On the phylogenetic position of the New Caledonian endemic families Paracryphiaceae, Oncothecaceae, and Strasburgeriaceae: a comparison of molecules and morphology. – Bot. Rev. 68: 428-443.
Capuron R. 1970. Notes sur les Icacinaceae. – Adansonia, n. s., 10: 507-510.
Carpenter CS. 1975. The morphology and relationships of Oncotheca balansae. – Ph.D. diss., Master of Arts, University of North Carolina, Chapel Hill, North Carolina.
Carpenter CS, Dickison WC. 1976. The morphology and relationships of Oncotheca balansae. – Bot. Gaz. 137: 141-153.
Coulter JM, Evans WH. 1890. Garrya. – Bot. Gaz. 15: 93-97.
Dahl AO. 1952. The comparative morphology of the Icacinaceae VI. The pollen. – J. Arnold Arbor. 33: 252-295.
Dahl AO. 1955. The pollen morphology of several genera excluded from the family Icacinaceae. – J. Arnold Arbor. 36: 159-163.
Dahling GV. 1978. Systematics and evolution of Garrya. – Contr. Gray Herb. 209: 1-104.
Deyama T, Ikawa T, Nishibe S. 1985. The constituents of Eucommia ulmoides Oliv. II. Isolation and structures of three new lignan glycosides. – Chem. Pharm. Bull. 33: 3651-3657.
Dickison WC. 1982. Vegetative anatomy of Oncotheca macrocarpa: a newly described species of Oncothecaceae. – Bull. Mus. Natl. Hist. Nat. (Paris), sér. IV, sect. B, Adansonia 3: 177-181.
Dickison WC. 1986. Further observations on the floral anatomy and pollen morphology of Oncotheca (Oncothecaceae). – Brittonia 38: 249-259.
Duno De Stefano R. 2007. Tratamiento taxonómico del género Dendrobangia Rusby (Cardiopteridaceae o Icacinaceae). – Candollea 62: 91-103.
Duno De Stefano R, Fernández-Concha GC. 2011. Morphology-inferred phylogeny and a revision of the genus Emmotum (Icacinaceae). – Ann. Missouri Bot. Gard. 98: 1-27.
Eckardt T. 1956. Zur systematischen Stellung von Eucommia ulmoides. – Ber. Deutsch. Bot. Ges. 69: 487-498.
Eckardt T. 1963. Some observations on the morphology and embryology of Eucommia ulmoides. – J. Indian Bot. Soc., Sect. A, 42: 27-34.
Endress PK, Rapini A. 2014.
Floral structure of Emmotum (Icacinaceae sensu stricto
or Emmotaceae), a phylogenetically isolated genus of lamiids with
a unique pseudotrimerous gynoecium, bitegmic ovules and
monosporangiate thecae. – Ann. Bot. 114: 945-959.
Engler A. 1896. Icacinaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(5), W. Engelmann, Leipzig, pp. 233-257, 459-460; Engler A. 1897. Nachträge zu III(5), pp. 225-227.
Eyde RH. 1964. Inferior ovary and generic affinities of Garrya. – Amer. J. Bot. 51: 1083-1092.
Fagerlind F. 1945. Bau des Gynöceums, der Samenanlage, und des Embryosackes bei einigen Repräsentanten der Familie Icacinaceae. – Svensk Bot. Tidskr. 39: 346-364.
González FA, Rudall PJ. 2010. Flower and fruit characters in the early-divergent lamiid family Metteniusaceae, with particular reference to the evolution of pseudomonomery. – Amer. J. Bot. 97: 191-206.
González FA, Betancur J, Maurin O, Freudenstein JV, Chase MW. 2007. Metteniusaceae, an early-diverging family in the lamiid clade. – Taxon 56: 795-800.
Graebner IB, Mostardeiro MA, Ethur EM, Burrow RA, Dessoy EC, Morel AF. 2000. Diterpenoids from Humirianthera ampla. – Phytochemistry 53: 955-959.
Guillaumin A. 1938. Observations morphologiques et anatomiques sur le genre Oncotheca. – Rev. Gén. Bot. 50: 629-635.
Guymer GP. 1984. Icacinaceae. – In: George AS (ed), Flora of Australia 22, Australian Government Publ. Service, Canberra, pp. 204-212.
Hallock FA. 1930. The development of the flowers and seeds of Garrya and its bearing on the phylogenetic position of the genus. – Ann. Bot. 44: 771-812.
Harms H. 1898. Cornaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(8), W. Engelmann, Leipzig, pp. 250-270.
Harms H. 1930. Eucommiaceae. – In: Engler A, Harms H (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 18a, W. Engelmann, Leipzig, pp. 348-351.
Haron NW, Ping ST. 1997. Distribution and taxonomic significance of flavonoids in the Olacaceae and Icacinaceae. – Biochem. Syst. Ecol. 25: 265-263.
Heads M. 2010. The endemic plant families and the palms of New Caledonia: a biogeographical analysis. – J. Biogeogr. 37: 1239-1250.
Heintzelmann CE, Howard RA. 1948. The comparative morphology of the Icacinaceae V. The pubescence and the crystals. – Amer. J. Bot. 35: 42-52.
Herissey H, Lebas C. 1910. Présence de l’aucubine dans plusier espèces du genre Garrya. – Beih. Bot. Centralbl., pp. 117-176.
Howard RA. 1940. Studies of the Icacinaceae I. Preliminary taxonomic notes. – J. Arnold Arbor. 21: 461-489.
Howard RA. 1942a. Studies of the Icacinaceae II. Humirianthera, Leretia, Mappia and Nothapodytes, valid genera of the Icacineae. – J. Arnold Arbor. 23: 55-78.
Howard RA. 1942b. Studies of the Icacinaceae III. A revision of Emmotum. – J. Arnold Arbor. 23: 479-494.
Howard RA. 1942c. Studies of the Icacinaceae IV. Considerations of the New World genera. – Contr. Gray Herb. Harvard Univ. 142: 3-60.
Howard RA. 1943. Studies of the Icacinaceae VIII. Brief notes of some Old World genera. – Lloydia 6: 144-154.
Howard RA. 1992. A revision of Casimirella, including Humirianthera (Icacinaceae). – Brittonia 44: 166-172.
Huzioka K. 1961. A new Paleogene species of the genus Eucommia from Hokkaido, Japan. – Trans. Proc. Palaeontol. Soc. Japan, New Series, 41: 9-12.
Iwashina T, Kamenosono K, Hatta H. 1997. Flavonoid glycosides from leaves of Aucuba japonica and Helwingia japonica (Cornaceae): phytochemical relationship with the genus Cornus. – J. Jap. Bot. 72: 337-346.
Kapil RN, Mohana Rao PR. 1966 [1967]. Studies of the Garryaceae II. Embryology and systematic position of Garrya Douglas ex Lindl. – Phytomorphology 16: 564-578.
Kaplan MAC, Ribeiro J, Gottlieb OR. 1991. Chemographical evolution of terpenoids in Icacinaceae. – Phytochemistry 30: 2672-2676.
Kårehed J. 2001. Multiple origin of the tropical forest tree family Icacinaceae. – Amer. J. Bot. 88: 2259-2274.
Kårehed J. 2002. Not just hollies – the expansion of Aquifoliales. – In: Evolutionary studies in asterids emphasising euasterids II, Ph.D. diss., Acta Universitatis Upsaliensis, Uppsala, Sweden, pp. 1-14.
Karsten H. 1859. Metteniusa Karst. – Flora of Colombia 1: 79-80, t. 39.
Kronfeld M. 1896. Aquifoliaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(5), W. Engelmann, Leipzig, pp. 183-189.
Labat J-N, Rabevohitra R, El-Achkar E. 2006. Révision synoptique du genre Apodytes (Icacinaceae) à Madagascar et aux Comores. – Adansonia, sér. III, 28: 379-387.
Labat J-N, El-Achkar E, Rabevohitra R. 2006. Révision synoptique du genre Pyrenacantha (Icacinaceae) à Madagascar. – Adansonia, sér. III, 28: 399-404.
Lens F, Kårehed J, Baas P, Jansen S, Rabaey D, Huysmans S, Hamann T, Smets E. 2008. The wood anatomy of polyphyletic Icacinaceae s.l., and their relationships within asterids. – Taxon 57: 525-552.
Liston A. 2003. A new interpretation of floral morphology in Garrya (Garryaceae). – Taxon 52: 271-276.
Lobreau-Callen D. 1972. Le pollen des Icacinaceae I. Atlas (1). – Pollen Spores 14: 345-388.
Lobreau-Callen D. 1973. Le pollen des Icacinaceae II. Observations en microscopie électronique, corrélations, conclusions. – Pollen Spores 15: 47-89.
Lobreau-Callen D. 1980. Caractères comparés du pollen des Icacinaceae et des Olacaceae. – Adansonia, sér. II, 20: 29-89.
Loesener T. 1942. Aquifoliaceae. – In: Engler A (†), Harms H, Mattfeld J (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 20b, W. Engelmann, Leipzig, pp. 36-86.
Lorence A, Nessler CL. 2004. Camptothecin, over four decades of surprising findings. – Phytochemistry 65: 2735-2749.
Lozano-Contreras G, de Lozano NB. 1988. Metteniusaceae. – In: Pinto P, Lozano-Contreras G (eds), Flora de Colombia, Monografia 11, Instituto de Ciencias Naturales, Universidad Nacional de Colombia, Bogotá, pp. 1-53.
Lozano-Contreras G, Lozano NB de. 1994. Una nueva especie de Metteniusa Karsten (Metteniusaceae) de Colombia. – Novon 4: 266-270.
Lucas GL. 1968. Icacinaceae. – In: Milne-Redhead E, Polhill RM (eds), Flora of tropical East Africa, Crown Agents for Overseas Governments and Administrations, London, pp. 1-18.
McPherson G, Morat P, Veillon JM. 1982. Existence d’une deuxième espèce appartenant au genre Oncotheca endémique de la Nouvelle-Calédonie et nouvelle données concernant les Oncothécacées. – Bull. Mus. Natl. Hist. Nat. (Paris), sér. IV, sect. B, Adansonia 3: 305-311.
Mauritzon J. 1936. Embryologische Angaben über Stackhousiaceae, Hippocrateaceae und Icacinaceae. – Svensk Bot. Tidskr. 30: 541-550.
Mendes EJ. 1963. 50. Icacinaceae. – In: Exell AW, Fernandes A, Wild H (eds), Flora Zambesiaca 2 (Part 1), Crown Agents for Oversea Governments and Administrations, London, pp. 340-351.
Meurman O. 1929. Association and types of chromosomes in Aucuba japonica. – Hereditas 12: 179-209.
Moseley MF, Beeks RM. 1955. Studies of the Garryaceae I. The comparative morphology and phylogeny. – Phytomorphology 5: 314-346.
Paliwal GS, Kakkar L. 1970. Leaf anatomy of some Garrya species. – Bot. J. Linn. Soc. 63: 81-90.
Perrier de la Bâthie H. 1944. Révision des Icacinacées de Madagascar et des Comores. – Mém. Mus. Natl. Hist. Nat. 18: 289-308.
Pigg KB, Manchester SR, DeVore ML. 2008. Fruits of Icacinaceae (tribe Iodeae) from the Late Paleocene of western North America. – Amer. J. Bot. 95: 824-832.
Pittier H. 1925. Árboles y arbustos nuevos de Venezuela. – Bol. Sci. Técn. Mus. Comercial Venezuela 1: 45-47.
Potgieter MJ, Wyk AE van. 1994a. Fruit structure of the genus Cassinopsis Sond. (Icacinaceae) in Africa. – South Afr. J. Bot. 60: 117-122.
Potgieter MJ, Wyk AE van. 1994b. Two new species of Apodytes (Icacinaceae) from southern Africa. – South Afr. J. Bot. 60: 231-239.
Potgieter MJ, Wyk AE van. 1994c. Fruit structure of the southern African species of Apodytes E. Meyer ex Arn. (Icacinaceae). – Bot. J. Linn. Soc. 115: 221-233.
Potgieter MJ, Wyk AE van. 1999. Leaf anatomy of the southern African Icacinaceae and its taxonomic significance. – South Afr. J. Bot. 65: 153-162.
Puri SC, Verma V, Amna T, Qazi GN, Spiteller M. 2005. An endophytic fungus from Nothapodytes foetida that produces camptothecin. – J. Nat. Prod. 68: 1717-1719.
Rankin BD, Stockey RA, Beard G. 2008. Fruits of Icacinaceae from the Eocene Appian Way locality of Vancouver Island, British Columbia. – Intern. J. Plant Sci. 169: 305-314.
Rasoanaivo P, Ratsimmamanga-Urverg S, Messana I, DeVicente Y, Galeffi C. 1990. Cassinopin, a kaempferol trirhamnoside from Cassinopsis madagascariensis. – Phytochemistry 29: 2040-2043.
Reeve RM. 1943. Comparative ontogeny of the inflorescence and the axillary vegetative shoot in Garrya elliptica. – Amer. J. Bot. 30: 608-619.
Roth WB, Carr ME, Davis EA, Bagby MO. 1985. New sources of gutta-percha in Garrya flavescens and Garrya wrightii. – Phytochemistry 24: 183-184.
Shiklina IA. 1977. Comparative anatomy of the wood of the genus Oncotheca Baill. (Theales). – Bot. Žurn. 62: 1273-1275. [In Russian]
Shweta S, Zuehlke S, Ramesha BT, Priti V, Kumar PM, Ravikanth G, Spiteller M, Vasudeva R, Shaanker RU. 2010. Endophytic fungal strains of Fusarium solani, from Apodytes dimidiata E. Mey. ex Arn. (Icacinaceae) produce camptothecin, 10-hydroycamptothecin and 9-methoxycamptothecin. – Phytochemistry 71: 117-122.
Sleumer H. 1934. Eine neue Art der Gattung Aveledoa Pittier. – Notizbl. Bot. Gart. Berlin-Dahlem 12: 148-150.
Sleumer H. 1936. Über die Gattung Metteniusa Karsten (=Aveledoa Pittier). – Notizbl. Bot. Gart. Berlin-Dahlem 13: 359-361.
Sleumer H. 1942. Icacinaceae. – In: Engler A (†), Harms H, Mattfeld J (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 20b, W. Engelmann, Leipzig, pp. 322-396.
Sleumer H. 1969. Materials towards the knowledge of the Icacinaceae of Asia, Malesia, and adjacent areas. – Blumea 17: 181-264.
Sleumer H. 1971. Icacinaceae. – In: Steenis CGGJ van (ed), Flora Malesiana I, 7, Wolters-Noordhoff, Groningen, pp. 1-87.
Sogo A, Tobe H. 2006. Mode of pollen tube growth in pistils of Eucommia ulmoides (Eucommiaceae, Garryales). – Intern. J. Plant Sci. 167: 933-941.
Solereder H. 1899. Zur Morphologie und Systematik der Gattung Cercidiphyllum Sieb. et Zucc. mit Berücksichtigung der Gattung Eucommia Oliv. – Ber. Deutsch. Bot. Ges. 17: 387-406.
Spichiger R, Savolainen V, Manen J-F. 1993. Systematic affinities of Aquifoliaceae and Icacinaceae from molecular data analysis. – Candollea 48: 459-464.
Staveren MGC van, Baas P. 1973. Epidermal leaf characters of Malesian Icacinaceae. – Acta Bot. Neerl. 22: 329-359.
Stull GW, Moore RW, Manchester SR. 2011. Fruits of Icacinaceae from the Eocene of southeastern North America and their biogeographic implications. – Intern. J. Plant Sci. 172: 935-947.
Stull GW, Herrera F, Manchester SR, Jaramillo C, Tiffney BH. 2012. Fruits of an “Old World” tribe (Phytocreneae; Icacinaceae) from the Paleogene of North and South America. – Syst. Bot. 37: 784-794.
Tang SH. 1962. Sporogenesis and gametophyte development in Eucommia ulmoides. – Acta Bot. Sin. 10: 29-34.
Teo SP, Haron NW. 1999. Anatomical studies in West Malaysian Icacinaceae. – Aust. Syst. Bot. 11: 729-738.
Tippo O. 1940. The comparative anatomy of the secondary xylem and the phylogeny of the Eucommiaceae. – Amer. J. Bot. 27: 832-838.
Utteridge TMA. 2010. A new species of Platea (Icacinaceae) from Peninsular Malaysia: Platea malayana. – Kew Bull. 65: 345-348.
Utteridge TMA, Nagamasu H, Teo SP, White LC, Gasson P. 2005. Sleumeria (Icacinaceae): a new genus from northern Borneo. – Syst. Bot. 30: 635-643.
Varossieau WW. 1942. On taxonomic position of Eucommia ulmoides Oliv. (Eucommiaceae). – Blumea 5: 81-92.
Villiers J-F. 1973. Icacinaceae. – In: Aubréville A, Leroy J-F (eds), Flore de Gabon 20, Muséum National d’Histoire Naturelle, Paris, pp. 3-100.
Villier J-F. 1980. Icacinaceae. – In: Aubréville A, Leroy J-F (eds), Flore de la Nouvelle Calédonie 9, Muséum National d’Histoire Naturelle (Paris), pp. 159-174.
Wagner R. 1923. Über Vorkommnisse von Domatien bei Icacinaceen. – Anz. Akad. Wiss. Wien., Math.-Naturw. Kl. 60: 189-193.
Wang Y-F, Li C-S, Collinson ME, Lin J, Sun Q-J. 2003. Eucommia (Eucommiaceae), a potential biothermometer for the reconstruction of paleoenvironments. – Amer. J. Bot. 90: 1-7.
Zhang Y-L, Wang F-S, Chien N-F. 1988. A study on pollen morphology of Eucommia ulmoides. – Acta Phytotaxon. Sin. 26: 367-370. [In Chinese with English summary]
Zhang Z-Y, Lu A-M, Pan K-Y, Wen-Jie. 1990. The anatomy, embryology, and systematic relationships of Eucommiaceae. – Acta Phytotaxon. Sin. 28: 430-441. [In Chinese with English summary]