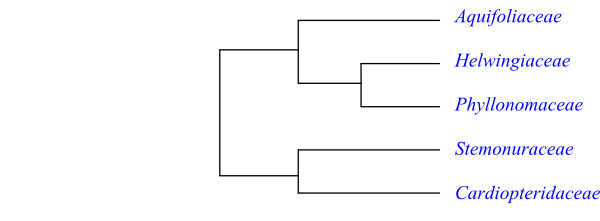
Phylogeny of Aquifoliales based on DNA sequence data (maximum parsimony analyses of ndhF, rbcL, atpB and 18S rDNA) and morphology (Kårehed 2001; Soltis & al. 2011).
[Aquifoliales+Apiidae]
Ilicales Bercht. et J. Presl, Přir. Rostlin: 226. Jan-Apr 1820 [‘Illicinae’]; Aquifolianae Doweld, Tent. Syst. Plant. Vasc.: lii. 23 Dec 2001
Habit Usually dioecious or bisexual (occasionally andromonoecious, gynomonoecious, polygamomonoecious, androdioecious or gynodioecious), usually evergreen (rarely deciduous) trees or shrubs (rarely lianas or climbing herbs).
Vegetative anatomy Phellogen ab initio superficial. Vessel elements usually with scalariform (sometimes simple, rarely reticulate) perforation plates; lateral pits opposite, scalariform or alternate, usually bordered (rarely simple) pits. Vestured pits present. Imperforate tracheary xylem elements fibre tracheids (sometimes tracheids, rarely libriform fibres) with bordered pits, usually non-septate. Wood rays usually multiseriate (sometimes uniseriate), usually heterocellular (sometimes homocellular). Axial parenchyma apotracheal diffuse or diffuse-in-aggregates (sometimes paratracheal scanty or banded, rarely vasicentric). Sieve tube plastids Ss type. Nodes usually 3:3, trilacunar with three leaf traces (sometimes 1:1, unilacunar with one trace). Resiniferous secretory canals usually present. Silica bodies rarely frequent. Prismatic calciumoxalate crystals (sometimes styloids, druses or crystal sand) often abundant.
Trichomes Hairs usually unicellular (rarely multicellular), uniseriate, or absent.
Leaves Usually alternate (spiral or distichous, rarely opposite), simple, entire to pinnately (rarely palmately) lobed, usually coriaceous, usually with supervolute (rarely conduplicate or curved) ptyxis. Stipules very small, often caducous, or absent; leaf sheath absent. Petiole vascular bundle transection arcuate, annular or complex. Venation usually pinnate (rarely palmate). Stomata usually anomocytic (rarely paracytic, helicocytic or cyclocytic). Cuticular wax crystalloids as rodlets (rarely absent). Domatia usually absent (sometimes as pits or pockets). Epidermis with or without mucilage cells. Mesophyll often with resiniferous or laticiferous idioblasts; idioblasts often with sclereids. Leaf margin usually serrate (sometimes serrate-dentate or entire); leaf teeth with simple vein and translucent caducous apex.
Inflorescence Usually axillary, panicle, fascicle, or raceme-like (rarely umbel-like; flowers rarely solitary axillary). Inflorescence sometimes epiphyllous.
Flowers Actinomorphic, small. Usually hypogyny (rarely epigyny). Sepals usually four or five (sometimes eight, rarely nine), usually with imbricate (rarely open) aestivation, usually caducous, usually more or less connate below (rarely absent). Petals usually four or five (sometimes eight, rarely nine), usually with imbricate (rarely valvate) aestivation, usually more or less connate (sometimes free, rarely absent). Nectaries usually present at petal bases (sometimes absent). Disc usually absent or poorly developed (sometimes with annular or lobate nectariferous disc).
Androecium Stamens (three or) four, five or eight (to twelve), haplostemonous, antesepalous, alternipetalous, to triplostemonous. Filaments free from each other, usually adnate to petals (epipetalous). Anthers usually basifixed (sometimes dorsifixed), non-versatile, tetrasporangiate, usually introrse (rarely latrorse to extrorse), usually longicidal (dehiscing by longitudinal slits; rarely poricidal, dehiscing by small pores). Tapetum secretory, with multinucleate cells. Female flowers usually with staminodia (sometimes petaloid).
Pollen grains Microsporogenesis simultaneous. Pollen grains usually 3(–4)-colpor(oid)ate (sometimes inaperturate, sometimes porate), shed as monads, bicellular at dispersal. Exine tectate or semitectate, with columellate infratectum, reticulate or perforate, clavate, gemmate, spinulate, verrucate or echinate.
Gynoecium Pistil composed of two to six (to 24) connate carpels. Ovary usually superior (rarely inferior), usually quadri- to sexalocular (to 24-locular, sometimes unilocular, bilocular or trilocular, rarely with incomplete septa). Style usually single, simple, short, or absent (stylodia rarely two, free). Stigma capitate, truncate or lobate, persistent in fruit, non-papillate, usually Wet (rarely Dry) type. Male flowers usually with pistillodium.
Ovules Placentation usually apical (sometimes axile, rarely parietal). Ovules usually one (sometimes two, rarely numerous) per carpel, usually anatropous (sometimes campylotropous), pendulous, apotropous, usually unitegmic (sometimes bitegmic), usually crassinucellar (rarely tenuinucellar). Funicular obturator papillate (sometimes absent). Endothelium present (Ilex). Hypostase present (Ilex). Parietal cell dividing (Ilex). Megagametophyte monosporous, Polygonum type. Synergids sometimes with a filiform apparatus. Antipodal cells persistent after fertilization (Ilex). Endosperm development usually cellular (sometimes nuclear). Endosperm haustoria absent. Embryogenesis caryophyllad (Ilex).
Fruit Usually a drupe with (two to) four to six (to 24) separate single-seeded pyrenes and persistent stigma (rarely a samara or berry).
Seeds Aril absent. Testa often thick. Exotestal cells cuboid, tangentially elongate, with lignified inner walls (theoid exotestal thickenings), elsewhere crushed. Endotesta with tannins. Perisperm not developed. Endosperm usually copious (rarely ruminate), with hemicellulose, oil and proteins (starch absent). Embryo usually small and straight, usually without chlorophyll. Cotyledons two (sometimes foliaceous). Germination phanerocotylar.
Cytology n = 9, 10, 14, 19 (Helwingia), 22
DNA
Phytochemistry Flavonols (kaempferol, quercetin), flavones, secoiridoids, ursolic acid, caffeic acid, tannins, alkaloids, saponins, cyanogenic compounds, simmondsinoid compounds, chlorogenic acid, arbutin, and acetophenones present. Ellagic acid, gallic acid and proanthocyanidins not found. Aluminium rarely accumulated.
Systematics Aquifoliales are sister to the remaining Campanulidae.
A probable topology of Aquifoliales is [[Aquifoliaceae+[Helwingia+Phyllonoma]]+[Stemonuraceae+Cardiopteridaceae]].
The clade [Stemonuraceae+Cardiopteridaceae] has the following potential synapomorphies (Stevens 2001 onwards): pits usually simple; axial parenchyma usually apotracheal or similar types; nodes 3:3; hairs unicellular, sometimes adpressed; leaves spiral or distichous; stipules absent; stomata cyclocytic to anisocytic; leaf margin entire; petal inflexed at apex; anthers basifixed; carpels two; only adaxial carpel fertile; ovary unilocular; placentation apical; ovules two per carpel, thinly crassinucellate; integument vascularized; funicular obturator present; fruit a single-seeded drupe; testa vascularized; and embryo very short. Furthermore, according to Tobe (2011), the pseudomonomerous gynoecium present in, e.g., Cardiopteris, may be a synapomorphy for the [Cardiopteridaceae+Stemonuraceae] clade.
The following features may be synapomorphies for the clade [Aquifoliaceae+[Helwingia+ Phyllonoma]], according to Stevens (2001 onwards): nodes 1:1; leaves spiral; stipules small, cauline; petiole bundle transection arcuate; leaf margin usually serrate; style absent; and fruit a drupe with separate pyrenes. Helwingia and Phyllonoma share the synapomorphies: hairs absent; stipules fimbriate; leaf venation brochidodromous; inflorescence epiphyllous, inserted on adaxial side of lamina; nectary annular; and stigmas elongated and recurved.

|
Phylogeny of Aquifoliales based on DNA sequence data (maximum parsimony analyses of ndhF, rbcL, atpB and 18S rDNA) and morphology (Kårehed 2001; Soltis & al. 2011). |
AQUIFOLIACEAE Bercht. et J. Presl |
( Back to Aquifoliales ) |
Ilicaceae Dumort., Comment. Bot.: 59. 1822 [’Iliceae’]; Ilicineae J. Presl in Nowočeská Bibl. [Wšobecný Rostl.] 7: 295, 299. 1846; Aquifoliineae Shipunov in A. Shipunov et J. L. Reveal in Phytotaxa 16: 63. 4 Feb 2011
Genera/species 1/450–500
Distribution Nearly cosmopolitan.
Fossils Ribbed endocarps of Ilex antiqua from the Maastrichtian have been described from Germany and fossilized endocarps of Ilex have been found at numerous sites from the Cenozoic of Europe. Pollen grains, which could possibly be attributed to Aquifoliaceae, are known from the Turonian and the Coniacian of southeastern Australia, Gabon and Egypt, and Ilexpollenites has been described from Late Cretaceous layers in California. Fossil pollen is also frequently found in Paleogene and Neogene layers.
Habit Usually dioecious or bisexual (rarely polygamomonoecious), usually evergreen (rarely deciduous) trees or shrubs (rarely lianas or epiphytes).
Vegetative anatomy Phellogen ab initio superficial. Vessel elements with scalariform perforation plates; lateral pits opposite, scalariform or alternate, bordered pits. Vestured pits present. Imperforate tracheary xylem elements fibre tracheids with bordered pits, usually non-septate. Wood rays multiseriate, heterocellular. Axial parenchyma apotracheal diffuse or diffuse-in-aggregates. Sieve tube plastids S type. Nodes usually 3:3, trilacunar with three leaf traces (sometimes 1:1, unilacunar with one trace). Secretory canals with resins present. Prismatic calciumoxalate crystals often abundant.
Trichomes Hairs unicellular or absent.
Leaves Usually alternate (sometimes distichous, rarely opposite), simple, entire to pinnately lobed, coriaceous, usually with supervolute (rarely conduplicate) ptyxis. Stipules minute, black, often caducous, or absent; leaf sheath absent. Petiole vascular bundle transection arcuate to annular, complex. Venation pinnate. Stomata anomocytic. Cuticular wax crystalloids as rodlets. Domatia usually absent (in one species present as pockets). Abaxial side of lamina often with corky warts. Epidermis with or without mucilaginous idioblasts. Mesophyll often with resiniferous or laticiferous idioblasts; often with sclereids. Leaf margin usually serrate (sometimes spinose-dentate or entire); leaf teeth with simple vein and translucent, caducous, palisade glandular apex with secretions rich in proteins.
Inflorescence Usually axillary, panicle, fascicle, or umbel- or raceme-like (flowers rarely solitary axillary).
Flowers Actinomorphic, small. Hypogyny. Sepals usually four or five (sometimes eight, rarely nine), with imbricate aestivation, usually caducous, more or less connate in lower part (rarely absent). Petals usually four or five (sometimes eight, rarely nine), with imbricate aestivation, usually connate at base (rarely absent). Nectaries present at petal bases. Disc absent or poorly developed.
Androecium Stamens four, five or eight (to twelve), haplostemonous and antesepalous to triplostemonous, in one to three whorls. Filaments free, usually adnate at base to petals (epipetalous). Anthers basifixed, non-versatile, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory, with multinucleate cells. Female flowers with staminodia (sometimes petaloid).
Pollen grains Microsporogenesis simultaneous. Pollen grains usually 3(–4)-colpor(oid)ate (sometimes inaperturate), shed as monads, bicellular at dispersal. Exine tectate, with columellate? infratectum, covered with gemmae, verrucae and clavi.
Gynoecium Pistil composed of (two to) four to six (to 24) connate antepetalous carpels. Ovary superior, (bilocular to) quadri- to sexalocular (to multilocular). Style single, simple, very short, or absent. Stigma capitate or broadly lobate, blackening, persistent in fruit, non-papillate, Wet type. Male flowers with pistillodium.
Ovules Placentation ab initio axile, later free central. Ovules usually one (rarely two) per carpel, usually anatropous (sometimes campylotropous), pendulous, apotropous, unitegmic, usually crassinucellar (parietal cell dividing; rarely tenuinucellar). Integument twelve to 15 cell layers thick. Funicular obturator papillate (sometimes absent). Hypostase present. Endothelium present. Parietal tissue approx. one cell-layer thick. Megagametophyte monosporous, Polygonum type. Antipodal cells persistent after fertilization. Endosperm development cellular. Endosperm haustoria absent. Embryogenesis caryophyllad.
Fruit A drupe with (two to) four to six (to numerous) separate pyrenes and persistent stigma (and often calyx).
Seeds Aril probably absent. Exotestal cells cuboid, tangentially elongate, with lignified inner walls (theoid exotestal thickenings), other walls crushed. Endotesta with tannins. Perisperm not developed. Endosperm copious, with hemicellulose, oil and proteins (starch absent). Embryo minute, straight, without chlorophyll. Cotyledons two. Germination phanerocotylar.
Cytology n = 9, 10
DNA Mitochondrial intron coxII.i3 lost. Mitochondrial coxI intron present. PI duplication absent. I copy of nuclear gene RPB2 present. Introns 18–23 of d copy of RPB2 lost.
Phytochemistry Flavonols (kaempferol, quercetin), ursolic acid, tannins, alkaloids (caffeine, theobromine), saponins, cyanogenic compounds, simmondsinoid compounds, chlorogenic acid, arbutin, and acetophenones present. Iridoids, ellagic acid and proanthocyanidins not found.
Use Ornamental plants, medicinal plants, tea (’yerba maté’ from Ilex paraguensis), timber, carpentries.
Systematics Ilex (450–500; western, central and southern Europe, Macaronesia, tropical and subtropical parts of Africa (one species), Madagascar, Turkey to northern Iran, India and the Himalayas to Japan and the Russian Far East, Southeast Asia, Malesia to New Guinea, northern Australia, Melanesia, Tahiti, the Hawaiian Islands, eastern North America, Mexico, the West Indies, Central America, tropical South America, one species, I. mitis, in tropical and subtropical parts of Africa).
Ilex is sister to [Helwingiaceae+Phyllonomaceae].
’Nemopanthus’ mucronatus (Ilex mucronatus) is sister to Ilex amelanchier (Powell & al. 2000).
CARDIOPTERIDACEAE Blume |
( Back to Aquifoliales ) |
Peripterygiaceae(Engl.) G. King, Mat. Fl. Malay Penins.: 620. Apr 1895; Leptaulaceae Tiegh. in Bot. Jahresber. (Just) 25(2): 406. 19 Jan 1900; Cardiopteridales Takht., Divers. Classif. Fl. Pl.: 352. 24 Apr 1997
Genera/species 5/43
Distribution Southeast Asia, Taiwan, Malesia to New Guinea, eastern Australia, Melanesia, islands in the Pacific, mountain regions in tropical America; tropical Africa and Madagascar.
Fossils Unknown.
Habit Usually bisexual (sometimes andromonoecious, gynomonoecious, polygamomonoecious, dioecious, androdioecious, or gynodioecious), evergreen trees or shrubs, or climbing and twining herbs (Cardiopteris).
Vegetative anatomy Phellogen ab initio superficial. Vessel elements usually with scalariform (sometimes with up to 40 cross-bars) or simple perforation plates (rarely reticulate); lateral pits usually opposite or alternate (sometimes scalariform), usually bordered (sometimes simple) pits. Imperforate tracheary xylem elements tracheids (Cardiopteris) or fibre tracheids? with bordered pits, non-septate. Wood rays uniseriate or multiseriate (Pseudobotrys without uniseriate wood rays, but with very wide multiseriate rays), homocellular or heterocellular. Axial parenchyma apotracheal diffuse or diffuse-in-aggregates, or paratracheal scanty or banded. Tyloses absent. Sieve tube plastids S type. Nodes 3:3, trilacunar with three leaf traces. Prismatic calciumoxalate crystals, styloid-like and druses usually present (sometimes also rhombic crystals and/or crystal sand).
Trichomes Hairs unicellular, normal, papillar or malpighiaceous (rarely absent).
Leaves Alternate (spiral), simple, entire or palmately lobed (Cardiopteris), with ? ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle transection annular; petiole sometimes also with medullary bundles. Venation usually pinnate (in Cardiopteris palmate). Stomata anomocytic, paracytic or intermediate between cyclocytic and anomocytic. Cuticular wax crystalloids? Domatia usually absent (sometimes as pits or pockets). Articulated laticifers with latex present in Cardiopteris. Leaf margin usually entire (rarely serrate).
Inflorescence Axillary or terminal, cymose (in Cardiopteris scorpioid) or raceme (in one species of Leptaulus “epiphyllous”). Bracts usually present (absent in, e.g., Cardiopteris).
Flowers Actinomorphic, small. Hypogyny. Sepals (four or) five, with imbricate quincuncial aestivation, usually connate in lower part. Petals (four or) five, sometimes (in, e.g., Cardiopteris) with imbricate quincuncial aestivation, usually connate (in Citronella not or only slightly connate). Nectariferous disc usually absent (in Cardiopteris annular and flat or cushion-shaped).
Androecium Stamens (four or) five, haplostemonous, antesepalous, alternipetalous. Filaments free from each other, usually adnate to corolla tube (epipetalous). Anthers dorsifixed, versatile?, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory. Staminodia present (female flowers in Gonocaryum) or absent.
Pollen grains Microsporogenesis simultaneous. Pollen grains in Cardiopteris, Citronella and Pseudobotrys tricolpor(oid)ate (in Cardiopteris heteropolar and often tetracolporate, in Leptaulus tri- or tetraporate, in Gonocaryum cryptoaperturate), shed as monads, bicellular at dispersal. Exine tectate to semitectate, with columellate infratectum, reticulate, microreticulate, perforate or microperforate, echinate or clavate.
Gynoecium Pistil composed of two or three connate carpels. Ovary superior, usually unilocular (in Citronella and Pseudobotrys with pseudoloculi due to formation of secondary septa; in Cardiopteris pseudomonomerous, consisting of one adaxial carpel and two lateral-abaxial ovuliferous carpels); nectary present at base of ovary. Style usually single, simple, thin (stylodia sometimes two, free; in Cardiopteris stylodia heteromorphous: one thin style arising from apex of adaxial carpel and with capitate stigma, whereas apices of two lateral-abaxial carpels elongate and develop into fleshy appendage in fruit), sometimes absent. Stigma capitate or truncate, type? Male flowers sometimes with reduced pistillodium.
Ovules Placentation apical. Ovules usually two (rarely one) per ovary, anatropous or orthotropous (Cardiopteris), pendulous (Cardiopteris), usually unitegmic (in Cardiopteris ategmic), crassinucellar (in Cardiopteris tenuinucellar?). Micropyle ?-stomal. Integument few cell layers thick. Funicular obturator present. Megagametophyte monosporous, Polygonum type. Endosperm development ab initio nuclear (Gonocaryum) or cellular (Cardiopteris). Endosperm haustorium present at least in Cardiopteris. Egg apparatus in Cardiopteris present in chalazal end. Embryogenesis asterad or solanad (Cardiopteris).
Fruit Usually a flat and/or ridged drupe (in Cardiopteris a two-winged samara, with horizontally striated wings and accrescent style forming apical appendage).
Seeds Aril absent. Testa vascularized, in Cardiopteris formed from nucellar tissue. Perisperm not developed. Endosperm copious, in Gonocaryum and Leptaulus ruminate. Embryo usually small (sometimes relatively large), well differentiated, at least sometimes with chlorophyll. Cotyledons two, usually foliaceous, usually unfolded. Germination phanerocotylar.
Cytology n = 14 (Leptaulus)
DNA
Phytochemistry Virtually unknown. Violet flavonoid present in Leptaulus. Secoiridoids present in at least Gonocaryum. Aluminium accumulated.
Use Timber.
Systematics Cardiopteris (3; C. antecedens, C. moluccana, C. quinqueloba; eastern Himalayas and Yunnan to Southeast Asia, Malesia to New Guinea, the Bismarck Archipelago and Solomon Islands), Citronella (21; Malesia to New Guinea, Queensland and Solomon Islands, New Caledonia, Fiji, Samoa, Tonga, Central America, tropical South America, Chile), Pseudobotrys (2; P. cauliflora, P. dorae; New Guinea), Gonocaryum (11; Hainan, Indochina, the Malay Peninsula, Malesia to New Guinea and the Bismarck Archipelago, Taiwan), Leptaulus (6; L. citrioides, L. congolanus, L. daphnoides, L. grandifolius, L. holstii, L. madagascariensis; tropical Africa, Madagascar).
Stemonuraceae are sister to Cardiopteridaceae.
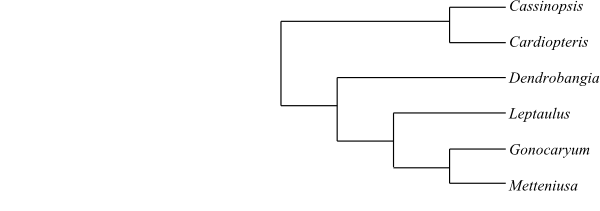 |
One of numerous most-parsimonious cladograms of Cardiopteridaceae based on DNA sequence data and morphology (Kårehed 2001). Cassinopsis and Metteniusa were later shown to belong in the basal Garryidae grade. Dendrobangia is possibly closely allied to the Apodytes clade (basal Garryidae). |
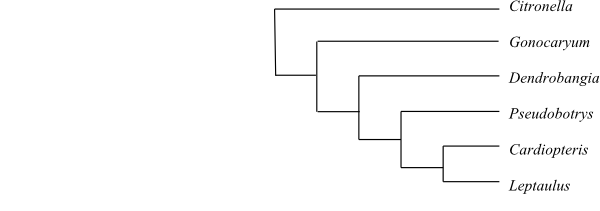 |
One of numerous most-parsimonious cladograms of Cardiopteridaceae based on DNA sequence data and morphology (Kårehed 2002). |
HELWINGIACEAE Decne. |
( Back to Aquifoliales ) |
Helwingiales Takht., Divers. Classif. Fl. Pl.: 389. 24 Apr 1997
Genera/species 1/4
Distribution Eastern Himalayas, northeastern India to northern Burma and Thailand, continental China, Japan, the Ryukyu Islands, Taiwan, northern Vietnam.
Fossils Unknown.
Habit Dioecious, evergreen shrubs or small trees.
Vegetative anatomy Phellogen ab initio superficial? Pericyclic fibres absent. Vessel elements with scalariform perforation plates; lateral pits scalariform or opposite, simple pits. Imperforate tracheary xylem elements libriform fibres (fibre tracheids absent) with simple or bordered pits, septate or non-septate. Wood rays multiseriate, heterocellular. Axial parenchyma paratracheal scanty. Sieve tube plastids? Nodes 1:1, unilacunar with one leaf trace. Secretory canals and cavities absent. Silica bodies frequent in ray cells and axial parenchyma (sometimes also in septate fibres). Crystals?
Trichomes Hairs absent.
Leaves Alternate (spiral), simple, entire, with supervolute to curved ptyxis. Stipules small, intrapetiolar, fimbriate, caducous; leaf sheath absent. Petiole vascular bundle transection arcuate; petiole with two small additional inverted bundles. Venation pinnate, brochidodromous. Stomata anomocytic. Cuticular wax crystalloids absent. Mesophyll without calciumoxalate. Secretory cavities absent. Leaf margin serrate.
Inflorescence Axillary, fasciculate or umbel-like cymose, seemingly epiphyllous (inflorescence seemingly arising from meristem on adaxial surface of leaf) and apparently inserted adaxially on mid-vein.
Flowers Actinomorphic, small. Hypogyny. Sepals three to five, with valvate to imbricate aestivation, connate. Petals absent. Nectariferous disc intrastaminal, edged.
Androecium Stamens three to five, alternitepalous, obhaplostemonous. Filaments free from each other and from sepals. Anthers basifixed, non-versatile, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory, with binucleate cells. Staminodia absent.
Pollen grains Microsporogenesis simultaneous. Pollen grains tricolporate (to hexacolporate), with diffuse endoapertures, shed as monads, bicellular at dispersal. Exine semitectate, with columellate? infratectum, reticulate to almost regulate, often striate and microechinate. Orbicules present.
Gynoecium Pistil composed of usually three to five (rarely two) connate alternitepalous carpels. Ovary inferior, usually trilocular or quinquelocular (rarely bilocular). Style single, simple, short. Stigmas usually three to five (rarely two), recurved, Dry type. Pistillodium absent.
Ovules Placentation apical. Ovule one per carpel, anatropous, pendulous, epitropous, unitegmic, incompletely tenuinucellar, pachychalazal. Integument eight to more than twelve cell layers thick. Megagametophyte monosporous, Polygonum type. Antipodal cells early degenerating. Nucellar cap absent. Hypostase not developing. Obturator absent. Endosperm development ab initio cellular. Endosperm haustoria? Embryogenesis?
Fruit A drupe with usually three or four separate single-seeded pyrenes.
Seeds Aril absent. Testa thin, multiplicative. Exotestal cells and endotestal cells collapsed at maturity. Perisperm not developed. Endosperm copious, weakly ruminate, rich in lipids. Embryo small, straight, well differentiated, chlorophyll? Cotyledons two. Germination?
Cytology n = 19 (polyploidy occurring)
DNA
Phytochemistry Insufficiently known. Flavones, iridoids (unidentified), caffeic acid, and chlorogenic acid present. Ellagic and gallic acids not found.
Use Unknown.
Systematics Helwingia (4; H. chinensis, H. himalaica, H. japonica, H. omeiensis; the Himalayas in central Nepal to Bhutan, Assam, Manipur, Tibet, northern Burma, Thailand, China, the Korean Peninsula, Japan, the Ryukyu Islands, Taiwan, northern Vietnam).
Helwingia is sister to Phyllonoma (Phyllonomaceae).
The wood anatomy is very similar to that in Ilex (Aquifoliaceae), especially to the subgenus Prinus.
The meristem of the lateral shoot is an example of ontogenetic displacement. It is produced in axillary position and displaced onto the subtending leaf during the development (Weber 2003).
PHYLLONOMACEAE Small |
( Back to Aquifoliales ) |
Dulongiaceae J. Agardh, Theoria Syst. Plant.: 315. Apr-Sep 1858 [’Dulongieae’], nom. illeg.
Genera/species 1/4
Distribution Southern Mexico to northwestern Bolivia.
Fossils Unknown.
Habit Bisexual, evergreen trees or shrubs.
Vegetative anatomy Phellogen ab initio superficial. Endodermis absent. Young stem and branches with separate vascular bundles. Vessel elements with scalariform perforation plates; lateral pits opposite or scalariform. Imperforate tracheary xylem elements ? with simple? or bordered pits, septate? Wood rays multiseriate?, heterocellular. Axial parenchyma apotracheal diffuse. Sieve tube plastids S type. Nodes 1:1, unilacunar with one leaf trace. Druses absent. Crystals?
Trichomes Hairs absent.
Leaves Alternate (usually distichous), simple, entire, with ? ptyxis. Stipules minute, fimbriate, early caducous; leaf sheath absent. Petiole vascular bundle transection annular. Venation pinnate, brochidodromous. Stomata anomocytic? Cuticular wax crystalloids? Leaf margin serrate or entire.
Inflorescence Axillary, fasciculate or umbel-like, seemingly epiphyllous (inflorescence seemingly arising from meristem on adaxial surface of leaf) and apparently inserted adaxially on mid-vein. Bracteoles absent.
Flowers Actinomorphic, small. Epigyny. Sepals four or five, with valvate or slightly quincuncially imbricate aestivation, persistent, free. Petals three to five, with valvate aestivation, free. Nectariferous disc intrastaminal, annular, flattened.
Androecium Stamens four or five, antesepalous, alternipetalous. Filaments short, subulate, free from each other and from tepals. Anthers basifixed?, bifid, non-versatile, tetrasporangiate, latrorse to extrorse, longicidal (dehiscing by longitudinal slits). Tapetum secretory. Staminodia absent.
Pollen grains Microsporogenesis simultaneous. Pollen grains (2–)3-colpor(oid)ate, shed as monads, bicellular at dispersal. Exine tectate, with columellate infratectum, microechinate to smooth.
Gynoecium Pistil composed of usually two (sometimes three) connate carpels. Ovary inferior, incompletely bilocular. Stylodia usually two (sometimes three), very short, free. Stigmas usually two (sometimes three), elongate, recurved, type? Pistillodium absent.
Ovules Placentation parietal. Ovules numerous per carpel, campylotropous, possibly bitegmic, weakly crassinucellar. Outer integument several cell-layers thick. Inner integument two cell-layers thick. Parietal tissue approx. one cell-layer thick. Megagametophyte monosporous, Polygonum type. Endosperm development cellular? Endosperm haustoria? Embryogenesis?
Fruit A three- to six-seeded incompletely bilocular berry with persistent calyx.
Seeds Aril absent. Testa multilayered, rugose. Exotesta with two or three layers of flattened cells; exotestal cells large, thick-walled, mucilaginous. Endotesta? Perisperm not developed. Endosperm copious, fleshy, with hemicellulose. Embryo relatively large, straight, chlorophyll? Cotyledons two. Germination?
Cytology n = ?
DNA
Phytochemistry Virtually unknown. Aluminium accumulated.
Use Unknown.
Systematics Phyllonoma (4; P. laticuspis, P. ruscifolia, P. tenuidens, P. weberbaueri; Durango in southern Mexico, Central America, the northern Andes to Peru and northwestern Bolivia).
Phyllonoma is sister to Helwingia (Helwingiaceae).
The inflorescence in Phyllonoma is primarily developing from the leaf axil. However, it has several times been erroneously referred to as truly epiphyllous, since the meristem seemed to originate on top of the leaf blade instead of in the axil of the leaf. According to Weber (2003, using SEM and clearing preparations), the axillary meristem (leading to the inflorescence shoot) is continuous to the leaf axil. The primordium developing into the inflorescence forms a meristem, which extends from the axillary region and along the leaf. The floral primordia, bracts etc. arise from the distal part of the meristem. Hence, this is the same principle of inflorescence development as in Helwingia.
STEMONURACEAE (M. Roem.) Kårehed |
( Back to Aquifoliales ) |
Genera/species 12/95–100
Distribution Tropical West Africa, southern India (Western Ghats), Sri Lanka, Assam, Burma, Indochina to New Guinea, Queensland and Melanesia, Central America, tropical South America.
Fossils Unknown.
Habit Usually dioecious (sometimes bisexual), evergreen trees or shrubs.
Vegetative anatomy Phellogen ab initio superficial. Vessel elements with simple or scalariform perforation plates; lateral pits usually opposite to scalariform (sometimes alternate), simple or bordered pits. Imperforate tracheary xylem elements tracheids and/or fibre tracheids with simple or bordered pits, non-septate. Wood rays uniseriate or multiseriate, homocellular or heterocellular. Axial parenchyma apotracheal diffuse or diffuse-in-aggregates, or paratracheal scanty, scalariform, unilateral or banded (rarely vasicentric). Tyloses (sclerotic) sometimes frequent. Sieve tube plastids S type. Nodes 3:3, trilacunar with three leaf traces. Cortex with articulated laticifers. Calciumoxalate as druses, styloids and/or elongate or prismatic crystals usually present (sometimes crystal sand or rhombic crystals).
Trichomes Hairs unicellular or multicellular (sometimes icacinaceous or malpighiaceous type).
Leaves Alternate (spiral), simple, entire or lobate, often coriaceous, with conduplicate ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle transection arcuate and wing bundles present, or annular and medullary bundles present. Venation pinnate, brochidodromous. Stomata anisocytic or cyclocytic (rarely helicocytic). Cuticular wax crystalloids? Epidermis with or without mucilage cells? Mesophyll often with sclerenchymatous idioblasts. Domatia absent. Tannin crystals sometimes present. Leaf margin entire.
Inflorescence Usually axillary (in Gomphandra sometimes terminal), often raceme-like cymose.
Flowers Usually actinomorphic, small. Pedicel articulated. Hypogyny. Sepals four or five (to seven), with open to valvate aestivation, connate. Petals four or five (to seven), with valvate aestivation, sometimes keeled, usually connate (sometimes free). Nectariferous disc annular, hippocrepomorphic or squamiform (rarely absent).
Androecium Stamens four or five (to seven), antesepalous, alternipetalous. Filaments often stout, widened above, free from each other and from tepals, usually with long clavate hairs. Anthers usually basifixed (sometimes dorsifixed), non-versatile, tetrasporangiate, usually introrse (rarely extrorse), longicidal (dehiscing by longitudinal slits); connective well developed. Tapetum secretory, with multinucleate cells. Female flowers with staminodia (in Gomphandra one staminodium often reflexed).
Pollen grains Microsporogenesis simultaneous. Pollen grains usually triporate (occasionally tetra- or pentaporate, rarely more), shed as monads, bicellular at dispersal. Exine tectate to semitectate, with columellate infratectum, reticulate, echinate, striate, rugulate or smooth (sometimes echinulate, microperforate, fossulate-verrucate, microgemmate or microechinate.
Gynoecium Pistil composed of usually three (sometimes two or five) connate carpels. Ovary superior, unilocular, pseudomonomerous with one fertile carpel, with or without fleshy appendage close to placental bundle. Style single, simple, short, or absent. Stigma capitate, lobate or punctate, type? Male flowers with pistillodium.
Ovules Placentation apical. Ovules two per ovary or carpel, anatropous, pendulous, unitegmic, crassinucellar. Integument up to ten cell layers thick. Funicular obturator present. Megagametophyte monosporous, Polygonum type. Synergids with a filiform apparatus. Endosperm development nuclear. Endosperm haustoria? Embryogenesis?
Fruit A usually dorsiventrally flattened drupe (sometimes round in transverse view). The two sides sometimes with very unequal appearance, one side with fleshy outgrowth above sulcus (Cantleya with pseudolocule) or with ridges on one side of chalaza. Inner mesocarp fibrous or with ridges and grooves on sides of fleshy outgrowth.
Seeds Aril absent. Testa thin, vascularized, elongate (cell walls unthickened). Perisperm not developed. Endosperm copious to sparse. Embryo small, straight or curved, with chlorophyll? Cotyledons two. Germination phanerocotylar (Gomphandra).
Cytology n = 22
DNA inserted repeat region in trnL-F spacer (possibly unique)
Phytochemistry Group VI secoiridoids (secologanin), Group X secoiridoids (loganin), cantleyoside, and cyanogenic compounds present. Flavonols (quercetin), cyanidin, and alkaloids? Ellagic acid not found.
Use Timber, medicine.
Systematics Lasianthera (1; L. africana; tropical West Africa); Cantleya (1; C. corniculata; Malesia), Codiocarpus (2; C. andamanicus, C. merrittii; the Andaman and Nicobar Islands, the Philippines, Aru Islands), Discophora (2; D. guianensis, D. montana; Costa Rica to Bolivia), Gastrolepis (2; G. alticola, G. austrocaledonica; New Caledonia), Gomphandra (60–65; Southeast Asia, Malesia to New Guinea and Solomon Islands), Grisollea (2; G. myriantha, G. thomasetii; Madagascar, the Comoros, the Seychelles), Hartleya (1; H. inopinata; New Guinea), Irvingbaileya (1; I. australis; northeastern Queensland), Medusanthera (10; Malesia to islands in western Pacific), Stemonurus (14; Sri Lanka, the Andaman Islands, Vietnam, Malesia), Whitmorea (1; W. grandiflora; Solomon Islands).
Stemonuraceae are sister to Cardiopteridaceae.
Lasianthera is sister to the remaining Stemonuraceae.
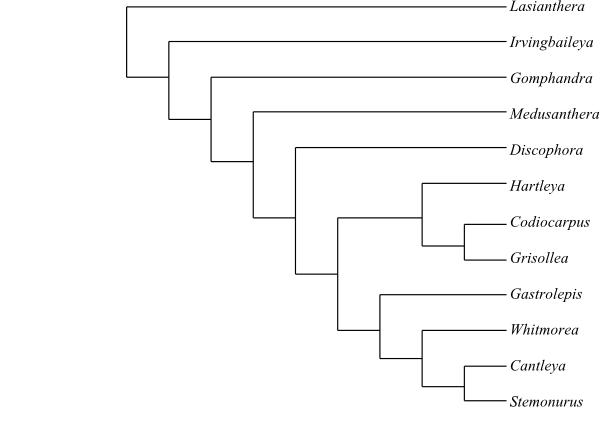 |
One of numerous most-parsimonious cladograms of Stemonuraceae based on DNA sequence data and morphology (Kårehed 2001). |
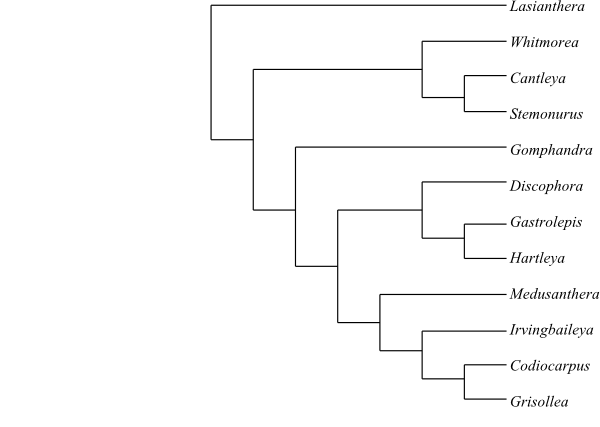 |
One of numerous most-parsimonious cladograms of Stemonuraceae based on DNA sequence data and morphology (Kårehed 2002). |
Literature
Andrews S. 1984. A reappraisal of Ilex aquifolium and I. perado (Aquifoliaceae). – Kew Bull. 39: 141-155.
Ao C, Tobe H. 2015. Floral morphology and
embryology of Helwingia (Helwingiaceae, Aquifoliales): systematic and
evolutionary implications. – J. Plant Res. 128: 161-175.
Baas P. 1973a. The wood anatomical range in Ilex (Aquifoliaceae) and its ecological and phylogenetic significance. – Blumea 21: 193-258.
Baas P. 1973b. Epidermal leaf characters of the Malesian Icacinaceae. – Acta Bot. Neerl. 22: 329-359.
Baas P. 1974. Stomatal types in Icacinaceae. Additional observations on genera outside Malesia. – Acta Bot. Neerl. 23: 193-200.
Baas P. 1975. Vegetative anatomy and the affinities of Aquifoliaceae, Sphenostemon, Phelline, and Oncotheca. – Blumea 22: 311-407.
Baas P. 1984. Vegetative anatomy and the taxonomic status of Ilex collina and Nemopanthus (Aquifoliaceae). – J. Arnold Arbor. 65: 243-250.
Bailey IW, Howard RA. 1941a. The comparative morphology of the Icacinaceae I. Anatomy of the node and internode. – J. Arnold Arbor. 22: 125-132.
Bailey IW, Howard RA. 1941b. The comparative morphology of the Icacinaceae II. Vessels. – J. Arnold Arbor. 22: 171-187.
Bailey IW, Howard RA. 1941c. The comparative morphology of the Icacinaceae III. Imperfect tracheary elements and xylem parenchyma. – J. Arnold Arbor. 22: 432-442.
Bailey IW, Howard RA. 1941d. The comparative morphology of the Icacinaceae IV. Rays of the secondary xylem. – J. Arnold Arbor. 22: 556-568.
Bâthie HP de la. 1952. Icacinacées (Icacinaceae). – In: Humbert H (ed), Flore de Madagascar et des Comores 119, Typographie Firmin-Didot et Cie, Paris.
Bautista PB, De Andrade TAP. 1975. O pólen em plantas da Amazônia V. Contribuição ao estudo da familia Icacinaceae. – Bol. Mus. Paraense “Emilio Goeldi”, N. S., Bot. 47: 1-11.
Breuer B, Stuhlfauth T, Fock H, Huber H. 1987. Fatty acids of some Cornaceae, Hydrangeaceae, Aquifoliaceae, Hamamelidaceae and Styracaceae. – Phytochemistry 26: 1441-1445.
Britton NL. 1894. Ilicioides Dumont. – Mem. Torrey Bot. Club 5: 217.
Byng JW, Bernardini B, Joseph JA, Chase MW, Utteridge TMA. 2014. Phylogenetic relationships of Icacinaceae focusing on the vining genera. – Bot. J. Linn. Soc. 176: 277-294.
Chan YY, Leu YL, Lin FW, Li CY, Wu YC, Shi LS, Liou MJ, Wu TS. 1998. A secoiridoid and other constituents of Gonocaryum calleryanum. – Phytochemistry 47: 1073-1077.
Chen X-Q, Zan K, Liu H, Yang J, Lai M-X, Wang Q. 2009. Triterpenes and flavonoids from Ilex hainanensis Merr. (Aquifoliaceae). – Biochem. Syst. Ecol. 37: 678-682.
Clark RC. 1974. Ilex collina, a second species of Nemopanthus in the southern Appalachians. – J. Arnold Arbor. 55: 435-440.
Copeland HF. 1963. Structural notes on hollies (Ilex aquifolium and I. cornuta, family Aquifoliaceae). – Phytomorphology 13: 455-464.
Cuénoud P, Del Pero Martinez MA, Loizeau P-A, Spichiger R, Andrews S, Manen J-F. 2000. Molecular phylogeny and biogeography of the genus Ilex L. (Aquifoliaceae). – Ann. Bot. 85: 111-122.
Dahl AO. 1952. The comparative morphology of the Icacinaceae VI. The pollen. – J. Arnold Arbor. 33: 252-295.
Dahl AO. 1955. The pollen morphology of several genera excluded from the family Icacinaceae. – J. Arnold Arbor. 36: 159-163.
Dickinson TA, Sattler R. 1974. Development of the epiphyllous inflorescence of Phyllonoma integerrima (Turcz.) Loes.: Implications for comparative morphology. – Bot. J. Linn. Soc. 69: 1-13.
Dickinson TA, Sattler R. 1975. Development of the epiphyllous inflorescence of Helwingia japonica (Helwingiaceae). – Amer. J. Bot. 62: 962-973.
Druce GC. 1914. Nemopanthus mucronatus. – In: Supplement to report for 191, new combinations. Bot. exch. Club Brit. Isles 3: 422.
Edwin G. 1967 [1968]. Part VI. Family 102. Aquifoliaceae. – In: Woodson Jr RE, Schery RW (eds), Flora of Panama, Ann. Missouri Bot. Gard. 75: 381-387.
Engler A. 1896. Icacinaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(5), W. Engelmann, Leipzig, pp. 233-257.
Engler A. 1930. Saxifragaceae. – In: Engler A, Harms H (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 18a, W. Engelmann, Leipzig, pp. 74-226.
Fagerlind F. 1945. Bau des Gynöceums, der Samenanlage, und des Embryosackes bei einigen Repräsentanten der Familie Icacinaceae. – Svensk Bot. Tidskr. 39: 346-364.
Fang WP. 1951. A study on Helwingia Willd. – Syml. Sin. 7(3): 685-688.
Ferguson IK. 1977. Cornaceae Dum. – World Pollen and Spore Flora 6, Almqvist & Wiksell, Stockholm.
Ferguson IK, Hideux MJ. 1978 [1980]. Some aspects of the pollen morphology and its taxonomic significance in Cornaceae sens. lat. – 4th Intern. Palynol. Conf., Lucknow, 1976-1977, 1: 240-249.
Freire Fierro A. 2004. Phyllonomaceae. – In: Harling G, Andersson L (eds), Flora of Ecuador 73, Botanical Institute, Göteborg University, pp. 83-86.
Galle FC. 1997. Hollies: the genus Ilex. – Timber Press, Portland, Oregon.
Giberti GC. 1979. Las especies Argentinas del género Ilex L. (Aquifoliaceae). – Darwiniana 22: 217-240.
Giberti GC. 1994. Aquifoliaceae. – In: Spichiger R, Ramela L (eds), Flora de Paraguay 24, Conservatoire et Jardin Botaniques de la Ville de Genève, Geneva, pp. 1-34.
González AM, Tarragó JR. 2009. Anatomical structure and secretion compounds in nine Ilex species from southern South America. – Bot. J. Linn. Soc. 160: 197-210.
González-Gutiérrez PA, Sierra-Calzado J. 2004. Aquifoliaceae. – In: Greuter W, Rankin-Rodríguez R (eds), Flora de la República de Cuba, ser. A, Plantas vasculares, Fasc. 9, Koeltz Scientific Books, Königstein, Germany.
Groppo M. 2007. A new species of Ilex (Aquifoliaceae) fron Espinhaco Range, Bahia, Brazil. – Bot. J. Linn. Soc. 155: 153-156.
Guymer GP. 1984. Icacinaceae. – In: George AS (ed), Flora of Australia 22, Australian Government Publ. Service, Canberra, pp. 204-212.
Hahn WL. 1993. A synopsis of the Panamanian species of Ilex (Aquifoliaceae). – Novon 3: 34-45.
Hahn WL. 2001. Aquifoliaceae. – In: Stevens WD, Ulloa U C, Pool A, Montiel OM (eds), Flora de Nicaragua. Angiospermas: Acanthaceae-Euphorbiaceae, Monogr. Syst. Bot. Missouri Bot. Gard. 85: 133-136.
Hara H, Kurosawa S. 1975. A revision of the genus Helwingia. – Univ. Mus., Univ. Tokyo, Bull. 8: 393-413.
Harms H. 1898. Cornaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(8), W. Engelmann, Leipzig, pp. 250-270.
Haron NW, Ping ST. 1997. Distribution and taxonomic significance of flavonoids in the Olacaceae and Icacinaceae. – Biochem. Syst. Ecol. 25: 265-263.
Heintzelmann CE, Howard RA. 1948. The comparative morphology of the Icacinaceae V. The pubescence and the crystals. – Amer. J. Bot. 35: 42-52.
Herr JM. 1959. The development of the ovule and megagametophyte in the genus Ilex L. – J. Elisha Mitchell Sci. Soc. 75: 107-128.
Herr JM. 1961. Endosperm development and associated ovule modifications in the genus Ilex. – J. Elisha Mitchell Sci. Soc. 77: 26-32.
Hewson HJ. 1984. Cardiopteridaceae. – In: George AS (ed), Flora of Australia 22, Australian Government Publ. Service, Canberra, p. 213.
Howard RA. 1940. Studies of the Icacinaceae I. Preliminary taxonomic notes. – J. Arnold Arbor. 21: 461-489.
Howard RA. 1942a. Studies of the Icacinaceae IV. Considerations of the New World genera. – Contr. Gray Herb. Harvard Univ. 142: 3-59.
Howard RA. 1942b. Studies of the Icacinaceae V. A revision of the genus Citronella D. Don. – Contr. Gray Herb. Harvard Univ. 142: 60-89.
Howard RA. 1943a. Studies of the Icacinaceae VI. Irvingbaileya and Codiocarpus, two new genera of the Icacineae. – Brittonia 5: 47-57.
Howard RA. 1943b. Studies of the Icacinaceae VII. A revision of the genus Medusanthera Seeman. – Lloydia 6: 133-143.
Howard RA. 1943c. Studies of the Icacinaceae VIII. Brief notes of some Old World genera. – Lloydia 6: 144-154.
Hu SY. 1949. The genus Ilex in China. – J. Arnold Arbor. 30: 233-344.
Hu SY. 1950. The genus Ilex in China. – J. Arnold Arbor. 31: 39-80.
Iwashina T, Kamenosono K, Hatta H. 1997. Flavonoid glycosides from leaves of Aucuba japonica and Helwingia japonica (Cornaceae): phytochemical relationship with the genus Cornus. – J. Jap. Bot. 72: 337-346.
Jahnke P. 1986. Der Infloreszenzbau der Cornaceen sensu lato und seine systematischen Konsequenzen. – Trop. Subtrop. Pflanzenwelt 57, Steiner, Stuttgart.
Johnson MA. 1958. The epiphyllous flowers of Turnera and Helwingia. – Bull. Torrey Bot. Club 85: 313-323.
Kadereit JW, Bittrich V (eds). 2016. The families and genera of vascular plants XIV. Flowering plants – eudicots – Aquifoliales, Boraginales, Bruniales, Dipsacales, Escalloniales, Garryales, Paracryphiales, Solanales (except Convolvulaceae), Icacinaceae, Metteniusaceae, Vahliaceae. – Springer, 412 pp.
Kaneko T, Sakamoto M, Ohtani K, Ito A, Kasai R, Yamasaki K, Padorina WG. 1995. Secoiridoid and flavonoid glycosides from Gonocaryum calleryanum. – Phytochemistry 39: 115-120.
Kaplan MAC, Ribeiro J, Gottlieb OR. 1991. Chemographical evolution of terpenoids in Icacinaceae. – Phytochemistry 30: 2672-2676.
Kårehed J. 2001. Multiple origin of the tropical forest tree family Icacinaceae. – Amer. J. Bot. 88: 2259-2274.
Kårehed J. 2002. Not just hollies – the expansion of Aquifoliales. – In: Evolutionary studies in asterids emphasising euasterids II, Acta Universitatis Upsaliensis, Uppsala, pp. 1-14.
Kong D-R, Peng H, Liang H-X. 2002. A new type of embryo sac in Cardiopteris and its systematic implication. – Acta Bot. Sin. 44: 496-498.
Krach JE. 1976. Samenanatomie der Rosifloren I. Die Samen der Saxifragaceae. – Bot. Jahrb. Syst. 97: 1-60.
Kronfeld M. 1896. Aquifoliaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(5), W. Engelmann, Leipzig, pp. 183-189.
Lens F, Kårehed J, Baas P, Jansen S, Rabaey D, Huysmans S, Hamann T, Smets E. 2008. The wood anatomy of polyphyletic Icacinaceae s.l., and their relationships within asterids. – Taxon 57: 525-552.
Lobreau-Callen D. 1972. Pollen des Icacinaceae I. Atlas. – Pollen Spores 14: 345-388.
Lobreau-Callen D. 1973. Le pollen des Icacinaceae II. Observations en microscopie électronique, corrélations, conclusions. – Pollen Spores 15: 47-89.
Lobreau-Callen D. 1977. Les pollens des Celastrales: illustrations, commentaires. – École Pratique des Hautes Études, Institut de Montpellier, Montpellier.
Lobreau-Callen D. 1980. Caractères comparés du pollen des Icacinaceae et des Olacaceae. – Adansonia, sér. II, 20: 29-89.
Lobreau-Callen D. 1982. Structures et affinités polliniques des Cardiopterygaceae, Dipentodontaceae, Erythropalaceae et Octoknemataceae. – Bot. Jahrb. Syst. 103: 371-412.
Loesener T. 1897. Aquifoliaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien, Nachträge zu III(5), W. Engelmann, Leipzig, pp. 217-221.
Loesener T. 1901. Monographia Aquifoliacearum I. –Nova Acta Acad. Caes. Leop.-Carol. German. Nat. Cur. 78: 1-567.
Loesener T. 1908. Monographia Aquifoliacearum II. – Nova Acta Acad. Caes. Leop.-Carol. German. Nat. Cur. 89: 5-314.
Loesener T. 1942. Aquifoliaceae. – In: Engler A (†), Harms H, Mattfeld J (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 20b, W. Engelmann, Leipzig, pp. 36-86.
Loizeau P-A. 1994. Les Aquifoliaceae péruviennes (eléments pour une revision des Aquifoliaceae néotropicales). – Boissiera 48: 1-306.
Loizeau P-A, Spichiger R. 1992. Proposition d’une classification des inflorescences d’Ilex L. (Aquifoliaceae). – Candollea 47: 99-112.
Loizeau P-A, Barriera G, Manen J-F, Broennimann O. 2005. Towards an understanding of the distribution of Ilex L. (Aquifoliaceae) on a worldwide scale. – Biol. Skr. 55: 501-520. [In: Friis I, Balslev H (eds), Proceedings of a symposium on plant diversity and complexity patterns: local, regional and global dimensions, Danish Academy of Sciences and Letters, Copenhagen]
Lucas GL. 1968. Icacinaceae. – In: Milne-Redhead E, Polhill RM (eds), Flora of tropical East Africa, Crown Agents for Overseas Governments, London, pp. 1-18.
Manen J-F, Boulter MC, Naciri-Graven Y. 2002. The complex history of the genus Ilex L. (Aquifoliaceae): evidence from the comparison of plastid and nuclear DNA sequences and from fossil data. – Plant Syst. Evol. 235: 79-98.
Manen J-F, Barriera G, Loizeau P-A, Naciri Y. 2010. The history of extant Ilex species (Aquifoliaceae): evidence of hybridization within a Miocene radiation. – Mol. Phylogen. Evol. 57: 961-977.
Martin HA. 1977. The history of Ilex (Aquifoliaceae) with special reference to Australia: evidence from pollen. – Aust. J. Bot. 25: 655-673.
Mauritzon J. 1933. Studien über die Embryologie der Familien Crassulaceae und Saxifragaceae. – Håkan Ohlsson, Lund.
Mauritzon J. 1936. Embryologische Angaben über Stackhousiaceae, Hippocrateaceae und Icacinaceae. – Svensk Bot. Tidskr. 30: 541-550.
Mendes EJ. 1966. 51. Aquifoliaceae. – In: Exell AW, Fernandes A, Wild H (eds), Flora Zambesiaca 2 (Part 2), Crown Agents for Oversea Governments and Administrations, London, pp. 353-355.
Miers J. 1864. On the genus Villaresia, with a description of a new species. – J. Bot. 11: 257-266.
Mori SA, Kallunki JA. 1977. A revision of the genus Phyllonoma (Grossulariaceae). – Brittonia 29: 69-84.
Munzinger J, McPherson G, Lowry PP. 2008. A second species in the endemic New Caledonian genus Gastrolepis (Stemonuraceae) and its implications for the conservation status of high-altitude maquis vegetation: coherent application of the IUCN Red List criteria is urgently needed in New Caledonia. – Bot. J. Linn. Soc. 157: 775-783.
Nidia L, Cuello A, Gerardo A, Aymard C. 2008. Ilex guaramacalensis (Aquifoliaceae), a new species from the Ramal de Guaramacal in the Venezuelan Andes. – Novon 18: 319-324.
Ogle DW. 1992. Notes on the type locality and ecology of Ilex collina Alexander (Aquifoliaceae). – Castanea 57: 213-215.
Padmanabhan D. 1961. A contribution to the embryology of Gomphandra polymorpha. – Proc. Natl. Inst. Sci. India, Sect. B, 27: 389-398.
Pedley L. 1984. Aquifoliaceae. – In: George AS (ed), Flora of Australia 22, Australian Government Publ. Service, Canberra, pp. 199-204.
Powell M, Savolainen V, Cuénoud P, Manen J-F, Andrews S. 2000. The mountain holly (Nemopanthus mucronatus: Aquifoliaceae) revisited with molecular data. – Kew Bull. 55: 341-347.
Rusby HH. 1897. The affinities of Dendrobangia Rusby. – Mem. Torrey Bot. Club 24: 79-81.
Sattler R. 1984. Homology – a continuing challenge. – Syst. Bot. 9: 382-394.
Sattler R. 1986. Biophilosophy. Analytic and holistic perspectives. – Springer, Berlin and New York.
Sattler R. 1990. Towards a more dynamic plant morphology. – Acta Biotheor. 38: 303-315.
Schori M. 2010. A systematic revision of Gomphandra (Stemonuraceae). – Ph.D. diss., University of Ohio.
Schori M, Furness CA. 2014. Pollen diversity
in Aquifoliales. – Bot. J.
Linn. Soc. 175: 169-190.
Schori M, Utteridge TMA. 2012. Six new species and a new subspecies of Gomphandra (Stemonuraceae) from the Philippines. – Kew Bull. 67: 713-729.
Schori M, Lowry II PP, Schatz GE. 2013. A revision of the genus Grisollea (Stemonuraceae). – Syst. Bot. 38: 497-506.
Selbach-Schnadelbach A, Smith Cavalli S, Manen J-F, Coelho GC, Souza-Chies TT de. 2009. New information for Ilex phylogenetics based on the plastid psbA-trnH intergenic spacer (Aquifoliaceae). – Bot. J. Linn. Soc. 159: 182-193.
Setoguchi H, Watanabe I. 2000. Intersectional gene flow between insular endemics of Ilex (Aquifoliaceae) on the Bonin Islands and the Ryukyu Islands. – Amer. J. Bot. 87: 793-810.
Sévenet T, Thal C, Potier P. 1971. Isolement et structure du cantleyoside, nouveau glucoside terpénique de Cantleya corniculata (Becc.) Howard (Icacinacées). – Tetrahedron 27: 663-668.
Sleumer H. 1942. Icacinaceae. – In: Engler A (†), Harms H, Mattfeld J (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 20b, W. Engelmann, Leipzig, pp. 322-396.
Sleumer H. 1969. Materials towards the knowledge of the Icacinaceae of Asia, Malesia, and adjacent areas. – Blumea 17: 181-264.
Sleumer H. 1971. Icacinaceae. – In: Steenis CGGJ van (ed), Flora Malesiana I, 7, Wolters-Noordhoff, Groningen, The Netherlands, pp. 1-87.
Spichiger R, Savolainen V, Manen J-F. 1993. Systematic affinities of Aquifoliaceae and Icacinaceae from molecular data analysis. – Candollea 48: 459-464.
Staveren MGC van, Baas P. 1973. Epidermal leaf characters of Malesian Icacinaceae. – Acta Bot. Neerl. 22: 329-359.
Stefano RD de. 2008. El género Discophora Miers (Stemonuraceae) en el Neotrópico. – Candollea 63: 177-187.
Steyermark JA. 1988. Aquifoliaceae. – In: Steyermark JA (ed), Flora of the Venezuelan Guayana IV. – Ann. Missouri Bot. Gard. 75: 320-333.
Steyermark JA, Berry PE. 1995. Aquifoliaceae. – In: Steyermark JA, Berry PE, Holst BK (eds), Flora of the Venezuelan Guayana 2, Timber Press, Portland, Oregon, and Missouri Botanical Garden Press, St. Louis, Missouri, pp. 571-599.
Teo SP, Haron NW. 1999. Anatomical studies in West Malaysian Icacinaceae. – Aust. Syst. Bot. 11: 729-738.
Thevénard M. 1906. Recherches histologiques sur les Ilicacées. – Ph.D. diss., École Supérieure de Pharmacie, l’Université de Paris.
Thouvenin M. 1890. Recherches sur la structure des Saxifragacées. – Ann. Sci. Nat. Bot. sér. 7, 12: 1-174.
Thouvenin M. 1891. Sur la présence de laticifères dans une Olacacée, le Cardiopteris lobata. – Bull. Soc. Bot. France 38: 129-130.
Tobe H. 2011. Floral structure of Cardiopteris (Cardiopteridaceae) with special emphasis on the gynoecium: systematic and evolutionary implications. – J. Plant Res. 125: 361-369.
Trelease W. 1892. Revision of North American Ilicineae and Celastraceae. – Trans. Acad. Sci. St. Louis 5: 343-349.
Utteridge TMA. 2001. Contributions to the flora of Mt Jaya IV. A new species of Medusanthera Seem. (Icacinaceae) from New Guinea: Medusanthera inaequalis Utteridge. – Kew Bull. 56: 233-237.
Utteridge TMA 2011. A revision of the genus Medusanthera (Stemonuraceae, Icacinaceae s.l.. – Kew Bull. 66: 1-31.
Verdcourt B. 1968. Aquifoliaceae. – In: Milne-Redhead E, Polhill RM (eds), Flora of tropical East Africa, Crown Agents for Oversea Governments and Administrations, pp. 1-4.
Villiers J-F. 1973. Icacinaceae. – In: Aubréville A, Leroy J-F (eds), Flore de Gabon 20, Muséum National d’Histoire Naturelle, Paris, pp. 3-100.
Villier J-F. 1980. Icacinaceae. – In: Aubréville A, Leroy J-F (eds), Flore de la Nouvelle Calédonie 9, Muséum National d’Histoire Naturelle, Paris, pp. 159-174.
Wagner R. 1923. Über Vorkommnisse von Domatien bei Icacinaceen. – Anz. Akad. Wiss. Wien., Math.-Naturw. Kl. 60: 189-193.
Walther H, Kvacek Z. 2008. Die Gattung Ilex L. (Aquifoliaceae) im Paläogen von Mitteleuropa. – Feddes Repert. 119: 172-190.
Wangerin W. 1906. Die Umgrenzung und Gliederung der Familie der Cornaceae. – Bot. Jahrb. Syst. 38, Beibl. 86: 1-88.
Weber A. 2003. What is morphology and why is it time for its renaissance in plant systematics? – In: Stuessy TF, Mayer V, Hörandl E (eds), Deep morphology. Toward a renaissance of morphology in plant systematic, A. R. G. Gantner Verlag K. G., Ruggell, Liechtenstein, pp. 3-32.