
Cladogram of Santalales based on DNA sequence data (Su & al. 2015). The sister-group relationship of Octoknema has not yet been resolved.
[Santalales+[[Berberidopsidales+Caryophyllales]+Asteridae]]
Santalopsida Brongn., Enum. Plant. Mus. Paris: xxix, 115. 12 Aug 1843 [’Santalieae’]; Santalanae Thorne ex Reveal in Novon 2: 236. 13 Oct 1992
Fossils Leaves and reproductive organs are known from the Eocene of North America. Fruits resembling those in Erythropalaceae and Olacaceae have been found in Eocene layers in England. Accuratipollis, Cranwellia and Gothanipollis are fossil pollen grains of the Late Cretaceous and the Cenozoic, which may be assigned to Santalales.
Habit Bisexual, monoecious or dioecious (rarely androdioecious), usually evergreen (sometimes deciduous) trees or shrubs, or perennial herbs (sometimes lianas). Usually root- or stem-hemiparasites (sometimes leafless holoparasites or autotrophic). Sometimes spiny or xeromorphic. Branches sometimes as phyllocladia. Normal roots often absent.
Vegetative anatomy Mycorrhiza absent (except, e.g., Ongokea, Coula, and Strombosia). With or without epicortical roots; roots usually modified into intruding and branching haustoria. Root hairs often absent. Granuliferous tracheary elements usually present in haustoria; granules usually consisting of proteins (in, e.g., Ximenia starch). Phellogen ab initio usually subepidermal (sometimes epidermal). Secondary lateral growth usually normal. Vessel elements usually with simple (sometimes scalariform, rarely reticulate or foraminate) perforation plates; lateral pits alternate, scalariform or opposite, simple or bordered pits. Imperforate tracheary xylem elements tracheids, fibre tracheids or libriform fibres with usually simple pits, non-septate (also vasicentric tracheids). Wood rays uniseriate or multiseriate, homocellular or heterocellular. Axial parenchyma apotracheal diffuse or diffuse-in-aggregates, or paratracheal scanty vasicentric or banded (rarely reticulate, scalariform, aliform, winged-aliform, lozenge-aliform, or confluent), or absent. Sieve tube plastids Ss or S0 type. Pericyclic fibres present or absent. Nodes 1:1, usually unilacunar with one leaf trace (sometimes 3:3, trilacunar with three traces, or ≥5:≥5, multilacunar with five or more traces). Laticifers sometimes present. Secretory cavities present or absent. Schizogenous resiniferous ducts occasionally present. Wood cystoliths absent. Cristarque cells often present. Heartwood often with gum-like substances. Silica bodies often frequent. Prismatic (rhomboidal) calciumoxalate crystals often abundant; druses sometimes present.
Trichomes Hairs unicellular or multicellular, uniseriate or branched (dendritic, candelabra-shaped or stellate), or absent.
Leaves Alternate (spiral or distichous) or opposite (rarely verticillate), simple, entire, often coriaceous, sometimes isobilateral, sometimes scale-like or modified into spines, with conduplicate ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle simple or bundle transection arcuate or annular. Venation pinnate, eucamptodromous, brochidodromous, or parallelodromous (rarely acrodromous; leaves sometimes uninerved). Stomata usually paracytic (sometimes anomocytic, anisocytic, parallelocytic or cyclocytic). Cuticular wax crystalloids as platelets, clustered tubuli (sometimes Berberis type, with nonacosan-10-ol as main wax) or annular rodlets, chemically often dominated by palmitone. Lamina often gland-dotted. Epidermal cells often sclerified, with druses, tannins or mucilage. Mesophyll sometimes with sclerenchymatous idioblasts (sometimes with asterosclereids). Cell walls sometimes silicified. Leaf margin usually entire (sometimes serrate).
Inflorescence Terminal or axillary, spike-, raceme-, catkin-, umbel-, head- or corymb-like, panicle or fasciculate, spikes, spadices or racemes, or flowers solitary axillary.
Flowers Actinomorphic or zygomorphic. Hypanthium present or absent. Usually epigyny (sometimes hypogynyor half epigyny). Sepals (three to) five or six (to nine), with open, imbricate or valvate aestivation, connate (often reduced to a narrow border on ovary), usually very small or absent. Petals (two to) four to six (to nine; rarely absent), with usually valvate (sometimes imbricate) aestivation, free or more or less connate, often adaxially hairy. Nectaries intrastaminal or extrastaminal or often alternating with stamens. Disc annular (often nectariferous) intrastaminal, extrastaminal or absent.
Androecium Stamens (one to) four to six (to 20), haplostemonous, antepetalous. Filaments usually free from each other (sometimes connate at base), free from or adnate to tepals/petals. Anthers basifixed or dorsifixed, usually non-versatile, usually tetrasporangiate (rarely disporangiate or monosporangiate), introrse, extrorse or latrorse, usually longicidal (dehiscing by longitudinal slits; sometimes poricidal or with one or several short transverse slits; anthers rarely connate into a synandrium). Tapetum secretory. Female flowers often with staminodia.
Pollen grains Microsporogenesis usually simultaneous (rarely successive). Pollen grains 3(–19)-colp(or)(oid)ate or 3(–5)-porate (rarely tetraperturate, dipororate to octopororate, or inaperturate), shed as monads, bicellular or tricellular at dispersal. Exine tectate or semitectate, with granular, intermediate or columellate infratectum, reticulate, microreticulate, perforate, microperforate or imperforate, striate, scabrate, verrucate, spinulate, echinate, echinulate or smooth.
Gynoecium Pistil composed of (one to) three to five (to numerous) connate carpels (carpels rarely absent). Ovary usually inferior (sometimes superior or semi-inferior), unilocular or multilocular. Style single, simple, or stylodia two or three (to five), or absent. Stigmas one to three, lobate or capitate, type? Male flowers often with pistillodium.
Ovules Placentation free central or basal (often as a mamelon; sometimes axile or apical, with ovules pendulous from apex of central column) or ovary with undifferentiated placenta with sporogenous tissue, in which one or two megagametophytes develop. Ovules one to numerous per carpel or ovary, usually anatropous (sometimes hemianatropous, or orthotropous), pendulous (rarely horizontal), often apotropous, usually unitegmic or ategmic (rarely bitegmic), tenuinucellar; megasporangium sometimes undifferentiated; ovule often poorly or indistinctly differentiated. Mamelon, spherical structure consisting of basal placenta, megagametophyte and all remaining tissue, sometimes formed by fusion of megasporangium and ovary wall. Micropyle usually endostomal (rarely bistomal). Megagametophyte monosporous, Polygonum type, or disporous, Allium type. Often growth of megagametophyte cells outside ovule, and sometimes for considerable distances within style (e.g. in Olax, many Loranthaceae and Santalaceae). Synergids often with a filiform apparatus. Antipodal cells one uninucleate or trinucleate, or three uninucleate (sometimes two uninucleate), or absent. Endosperm development ab initio usually cellular (sometimes nuclear, helobial or anomalous). Endosperm haustorium chalazal or formed from lateral endosperm cells. Embryogenesis piperad or irregular and complex. Single megagametophytes sometimes strongly prolonged reaching apex of mamelon (or even stigma at apex of a long style). Embryo often transferred to base of mamelon by development of a prolonged suspensor after fertilization.
Fruit A berry-like fruit with sticky layers of tissue, a nut or a one-seeded drupe with stony mesocarp (rarely a syncarp).
Seeds Seed often surrounded by sticky viscin tissue. Aril absent. Testa and tegmen usually absent (rarely present, crushed). Perisperm not developed. Endosperm copious, scarce or absent, usually oily (sometimes starchy), with or without chlorophyll. Embryo minute to large, straight, well or little differentiated (sometimes rudimentary), with or without chlorophyll. Cotyledons one, two (rarely up to six) or absent. Germination phanerocotylar or cryptocotylar.
Cytology n = 5–13
DNA Duplication of the nuclear gene PI. I copy of nuclear gene RPB2 lost. Intron absent from mitochondrial gene coxII.i3 (Comandra).
Phytochemistry Flavonols (quercetin, myricetin), cyanidin, triterpenes, oleanolic acid derivatives, sesquiterpene alcohols, proanthocyanidins (prodelphinidins), pyrrolizidine alkaloids as esters of arylic and aralkylic acids, triglycerides of polyacetylenic fatty acids (triglycerides with C18 acetylenic acids, in some clades), long-chain polyunsaturated fatty acids (e.g. minquartynoic, ximeninic or santalbic acids, in other angiosperms rare or absent), gallic acid, tannins, tyrosine derived cyanogenic glycosides (sambunigrin etc.), triterpene sapogenins, myo-inisitol, quebrachitol, pinitol, and acetylenes present. Saponins rare. Ellagic acid not found.
Systematics Santalales are sister-group to [Asteridae+[Berberidopsidales+Caryophyllales]].
Hemiparasitism is an apomorphy in Santalales. Erythropalaceae, sister to the remaining Santalales, are non-parasitic. Misodendraceae are often recovered as sister-group to [Schoepfiaceae+Loranthaceae], especially in analyses comprising numerous taxa, otherwise often as sister to Schoepfia. The sister-group relationships of the holoparasitic Balanophoraceae are unresolved.
The basal monophyletic groupings in Santalales are characterized entirely by molecular data.
The clade [Ximeniaceae+Aptandraceae+Olacaceae+[Octoknemaceae+[[Loranthaceae+ [Misodendraceae+Schoepfiaceae]]+[Opiliaceae+Santalaceae]]]] has the following potential synapomorphies (Stevens 2001 onwards): root hemiparasites; vessel elements with usually simple perforation plates; axial parenchyma strands seven or more cell layers wide; nodes usually unilacunar with a single leaf trace; petiole and median vein without sclerenchyma fibres; petiole vascular bundle transection arcuate; cuticular thickening present (also in Coulaceae); guard cell chamber small (also in Coulaceae); stomata usually paracytic; some foliar mesophyll and/or epidermal cells silicified; ovules unitegmic (also in Strombosiaceae) or ategmic (in Ximeniaceae usually bitegmic); megagametophyte with chalazal caecum (also in Strombosiaceae) and developed micropylar prolongation.
The clade [[Loranthaceae+[Misodendraceae+Schoepfiaceae]]+[Opiliaceae+Santalaceae]] has, according to Stevens (2001 onwards), guard cell walls not lignified; carpels non-septate; ovules not differentiated, ategmic; testa absent; and endosperm oily, without starch. The [Loranthaceae+ [Misodendraceae+Schoepfiaceae]] clade may have the following synapomorphies: cambium storied; petiole without asterosclereids; epidermal cells sclerified, with druses; calyx minute; and carpels three. Remaining monophyletic groups are characterized by molecular synapomorphies.

|
Cladogram of Santalales based on DNA sequence data (Su & al. 2015). The sister-group relationship of Octoknema has not yet been resolved. |
APTANDRACEAE Miers |
( Back to Santalales ) |
Cathedraceae Tiegh. in Bot. Jahresber. (Just) 25(2): 406. 19 Jan 1900; Chaunochitonaceae Tiegh. in Bot. Jahresber. (Just) 25(2): 406. 19 Jan 1900 [’Chaunochitaceae’]; Harmandiaceae Tiegh. in Bot. Jahresber. (Just) 24(2): 279. 1898
Genera/species 8/33
Distribution Tropical Africa, Madagascar, Indochina, the Malay Peninsula to islands in the Pacific, tropical America.
Fossils Unknown.
Habit Usually bisexual (Harmandia and Hondurodendron dioecious), evergreen or deciduous trees or shrubs. At least the majority probably root hemiparasites. Some specis are xerophytes.
Vegetative anatomy Arbuscular mycorrhiza present in Ongokea. Phellogen ab initio subepidermal. Vessel elements with scalariform perforation plates; lateral pits alternate, simple or bordered pits. Imperforate tracheary xylem elements fibre tracheids or libriform fibres with simple or bordered pits, non-septate (also vasicentric tracheids). Vascular bundle fibres present in some species. Wood rays uniseriate, homocellular or heterocellular. Axial parenchyma apotracheal diffuse or diffuse-in-aggregates, or paratracheal scanty or in narrow bands (sometimes reticulate, scalariform or aliform). Tyloses sometimes abundant. Vessel elements and/or axial parenchyma sometimes storied. Sieve tube plastids ? type; sieve tube nuclei with non-dispersive protein bodies. Nodes 1:1, unilacunar with one leaf trace. Laticifers present in some species. Cristarque cells sometimes present. Wood ray cells with rhomboidal crystals, without silica bodies. Cystoliths absent. Prismatic calciumoxalate? crystals abundant.
Trichomes Hairs unicellular, simple, or absent.
Leaves Alternate (spiral or distichous), simple, entire, coriaceous, with conduplicate ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle simple, sometimes with bundle fibres. Venation pinnate, brochidodromous. Stomata paracytic, with small guard cell chamber. Cuticular wax crystalloids usually absent (sometimes present as platelets). Cuticular thickenings present. Lamina often gland-dotted. Epidermal cells with tannins or mucilage. Mesophyll sometimes with sclerenchymatous idioblasts containing sclereids (spicula fibres, present in Cathedra). Asterosclereids often present. Laticifers present in Aptandra, Chaunochiton and Harmandia, otherwise absent. Epidermal and mesophyll cell walls often silicified. Epidermal cells without druses. Leaf margin entire.
Inflorescence Axillary, fasciculate, raceme-, head- or corymb-like panicles, or flowers solitary axillary. Floral prophylls (bracteoles) usually connate.
Flowers Actinomorphic, small. Hypanthium present or absent. Hypogyny. Sepals four to six, with open or imbricate aestivation, sometimes persistent, usually entirely or almost entirely connate. Petals four to six, usually with valvate (rarely imbricate) aestivation, free or connate at base (in Harmandia connate for most of their length), often with adaxial hairs and/or thickenings. Nectariferous disc extrastaminal (in Aptandra, Harmandia and Ongokea) or intrastaminal (in Phanerodiscus), annular, or absent (in Aptandra as four extrastaminal glands, alternating with stamens).
Androecium Stamens four to six (as many as petals), haplostemonous, antepetalous. Filaments usually free (in Aptandra, Harmandia and Ongokea connate in lower part) and surrounding style, free from or adnate to petals. Anthers dorsifixed or basifixed (anthers in Chaunochiton very short, with each loculus dehiscing by separate slit), often versatile, usually tetrasporangiate (rarely disporangiate), usually extrorse (in Chaunochiton introrse), usually dehiscing by membranous longitudinal slits or valves (in Hondurodendron with three valves; in Anacolosa, Cathedra, Chaunochiton and Phanerodiscus poricidal, dehiscing by apical pores); connective in Cathedra and Phanerodiscus massive. Tapetum secretory. Staminodia usually absent (present in female flowers of Harmandia; in Ongokea often a staminodium-like disc).
Pollen grains Microsporogenesis simultaneous. Pollen grains usually tricolpate or tetracolpate (sometimes hexaporate), heteropolar, shed as monads, bicellular or tricellular at dispersal. Apocolpium often concave. Garside’s rule? Exine tectate, with granular, columellate or intermediate infratectum, microperforate or smooth.
Gynoecium Pistil composed of two or three connate carpels; carpels usually septate, usually antesepalous (rarely antepetalous) or median carpel adaxial. Ovary superior, usually bilocular (rarely unilocular or trilocular; sometimes bilocular or trilocular in lower part and unilocular in upper part), with or without ridges. Style single, simple, short to long, or absent. Stigma clavate, capitate, bilobate or trilobate, type? Pistillodium usually absent (present in male flowers of Harmandia).
Ovules Placentation free central (when ovary unilocular) or axile to apical (when ovary at least in upper part bilocular or plurilocular). Ovule one per carpel, anatropous, pendulous, usually unitegmic or ategmic (sometimes bitegmic), tenuinucellar. Integument ? cell layers thick. Megagametophyte monosporous, Polygonum type. Synergids with a filiform apparatus. Endosperm development ab initio usually cellular. Endosperm haustorium chalazal. Embryogenesis?
Fruit A drupe or nut, usually with persistent and strongly accrescent calyx and/or disc etc. (not in Phanerodiscus). Fruit in Phanerodiscus surrounded by a membranous envelope formed from receptacular cupule (not disc cupule). Fruit in Cathedra tightly enclosed by growing disc cupule.
Seeds Aril absent. Seed coat testal? Testa present. Exotesta? Tegmen? Perisperm not developed. Endosperm copious, oily (and often starchy?). Embryo very small, straight, with chlorophyll? Cotyledons one, two (sometimes fused) or absent. Germination phanerocotylar or cryptocotylar.
Cytology n = ?
DNA
Phytochemistry Insufficiently known. Alkaloids, cyanogenic compounds, and triglycerides of polyacetylenic fatty acids present. Saponins usually absent. Aluminium accumulated in Cathedra.
Use Fruits, timber.
Systematics Aptandraceae are subdivided into two monophyletic groups (Ulloa Ulloa & al. 2010).
Aptandreae Engl. in H. G. A. Engler et K. A. E. Prantl, Nat. Pflanzenfam. Nachtr.: 145, 146. 2 Aug 1897
5/10. Aptandra (4; A. caudata, A. liriosmoides, A. tubicina, A. zenkeri; tropical Africa, tropical South America), Chaunochiton (3; C. angustifolium, C. kappleri, C. loranthoides; tropical South America), Harmandia (1; H. mekongensis; Indochina, the Malay Peninsula), Hondurodendron (1; H. urceolatum; Honduras), Ongokea (1; O. gore; tropical West Africa). – Pantropical. Filaments connate. Anthers with valvicidal dehiscence. Nectariferous disc extrastaminal. Pollen grains with concave mesocolpium and apocolpium. Calyx accrescent in fruit.
Anacoloseae Engl. in H. G. A. Engler et K. A. E. Prantl, Nat. Pflanzenfam. III, 1: 233, 234. Mai 1889
3/23. Anacolosa (16; tropical Africa, Madagascar, tropical Asia), Cathedra (5; C. acuminata, C. bahiensis, C. grandiflora, C. paraensis, C. rubricaulis; Brazil), Phanerodiscus (2; P. capuronii, P. diospyroidea; Madagascar). – Tropical Africa, Madagascar, tropical Asia, Brazil. Guard cell walls lignified. Anthers with poricidal dehiscence; connective prolonged and massive. Pollen grains diploporate. Disc and/or extradiscal area accrescent in fruit.
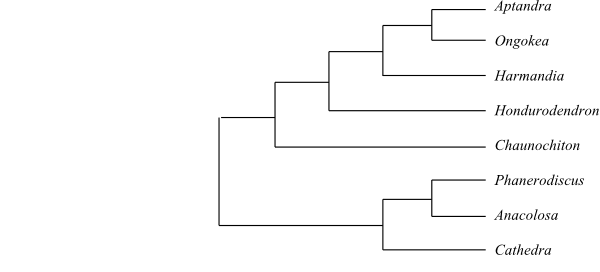 |
Cladogram of Aptandraceae based on DNA sequence data (Ulloa Ulloa & al. 2010; Nickrent & al. 2010; Su & al. 2015). |
BALANOPHORACEAE L. C. et A. Rich. |
( Back to Santalales ) |
Balanophorales Dumort., Anal. Fam. Plant.: 65. 1829 [‘Balanophorarieae’]; Helosaceae (Schott et Endl.) Bromhead in Mag. Nat. Hist., n.s., 4: 336, 338. Jul 1840 [‘Helosiaceae’]; Lophophytaceae (Schott et Endl.) Bromhead in Mag. Nat. Hist., n.s., 4: 336, 338. Jul 1840; Sarcophytaceae A. Kern., Pflanzenleben 2: 708, 709. 8 Aug 1891; Scybaliaceae A. Kern., Pflanzenleben 2: 708, 709. 8 Aug 1891; Balanophorineae Engl., Syllabus, ed. 2: 108. Mai 1898; Langsdorffiaceae Tiegh. ex Pilger in Engler et Prantl, Nat. Pflanzenfam. Nachtr. 4: 76. Apr 1914; Balanophoranae R. Dahlgren ex Reveal in Novon 2: 235. 13 Oct 1992
Genera/species 13–14/41–43
Distribution Tropical and southern Africa, Madagascar, the Comoros, the Himalayas, South and Southeast Asia to Japan, tropical northeastern Australia, New Caledonia, New Zealand, islands in the Pacific, Mexico to tropical and subtropical South America.
Fossils Unknown.
Habit Monoecious or dioecious, perennial, whitish, yellow, brown or red, achlorophyllous herbaceous root holoendoparasites with branched or simple subterranean tuber-like structures partly of root nature, partly consisting of host tissue. Succulents.
Vegetative anatomy Mycorrhiza absent. Normal roots absent. Stem endogenous. Phellogen absent? Vascular tissue strongly reduced. Secondary lateral growth absent. Vessel elements with simple or scalariform perforation plates, or absent. Imperforate tracheary xylem elements? Wood rays absent. Axial parenchyma absent? Sieve tube plastids S type. Nodes? Calciumoxalate crystals abundant.
Trichomes Epidermal hairs unicellular on tubers (Langsdorffia), simple.
Leaves Usually alternate (spiral or distichous; rarely opposite or verticillate), simple, entire, membranous and scale-like, or absent. Stipules and leaf sheath absent. Venation absent. Stomata absent. Cuticular wax crystalloids absent. Leaf margin entire.
Inflorescence Terminal, raceme-, spike- or spadix-like. Inflorescence usually developing endogenously inside tuber (or lobes of tuber; in some genera exogenously). Tuber rupturing during inflorescence development leaving an annular collar-like volva at base of peduncle. Extrafloral nectaries sometimes present on pedicel bases in Balanophora.
Flowers Actinomorphic, usually small to extremely minute (female flowers in Balanophora sometimes consisting of only 50 to 100 cells, resembling an archegonium). Epigyny to half epigyny (hypogyny?). Tepals sepaloid, in male flowers two to four (to eight), in one whorl, with usually valvate (sometimes imbricate) aestivation, free or connate at base, or absent; tepals in female flowers extremely small (sometimes comprising only few cells) or absent.
Androecium Stamens usually three or four (sometimes two; in Sarcophyte and Chlamydophytum sometimes seven or eight), as many as tepals (one or two when perianth absent), antetepalous, free or connate into synandrium, usually free from tepals. Anthers usually free, basifixed, non-versatile, tetrasporangiate, extrorse, usually longicidal (dehiscing by longitudinal slits; rarely poricidal or with a transversal slit), or connate into a synandrium, irregularly dehiscing. Tapetum secretory? Staminodia absent.
Pollen grains Microsporogenesis usually simultaneous (in Corynaea successive). Pollen grains usually 3(–5)-colpate, 3(–6)-colporate, or 3(–5)-porate (rarely di- or tripororate, hepta- or octapororate or inaperturate), shed as monads, bicellular or tricellular at dispersal. Exine tectate, with usually columellate (sometimes granular) infratectum, usually smooth (rarely microreticulate or finely striate).
Gynoecium Pistil composed of two or three (to five) connate carpels (in Balanophora of seemingly one carpel; in Rhopalocnemis of two transversely orientated), or carpels absent (flowers acarpellate). Ovary inferior or semi-inferior, unilocular. Stylodia two or three (to five), free, or style single, simple or bi- or trilobate (absent in Sarcophyte and Chlamydophytum). Stigmas one to three, more or less widened or capitate to punctate, type? Pistillodium?
Ovules Placentation usually apical (sometimes basal; placenta in Balanophora largely absent). ‘Ovule’ consisting of usually a single megagametophyte per ovary (sometimes several), more or less fused with surrounding pericarp tissue (in Balanophora, Langsdorffia and Thonningia fused with ovary wall), sometimes orthotropous or anatropous, pendulous, ategmic, extremely tenuinucellar. Megagametophyte usually disporous, Allium type (in Balanophora, Langsdorffia and Thonningia monosporous, Polygonum type), often hooked (chalazal caecum sometimes present). Antipodal cells one or two, ephemeral, or absent. Endosperm development usually cellular or nuclear (rarely helobial). One out of two cells formed at first endosperm division not dividing further and possibly corresponding to chalazal endosperm haustorium in other Santalales. Embryogenesis piperad (first zygote division vertical).
Fruit A drupelet, nut or achene, sometimes many together forming a fleshy syncarp.
Seeds Aril absent. Seed coat sometimes absent. Perisperm not developed. Endosperm copious, oily and starchy or with balanophorins. Embryo short or rudimentary, undifferentiated (four- to twelve-celled), without chlorophyll? Cotyledons absent. Germination via germination tube.
Cytology n = 14, 16?, 18
DNA
Phytochemistry Insufficiently known. Tannins present. Carbohydrates usually stored as starch, yet in Balanophora, Langsdorffia and Thonningia mostly as a wax-like substances, balanophorins (palmitate and lupeol palmitate), especially in tuber. Alkaloids and cyanogenic compounds not found.
Use Wax for lighting fuel (balanophorin from Balanophora and Langsdorffia), medicinal plants.
Systematics Sarcophyteae are sister-group to the remaining Balanophoraceae.
Sarcophyteae Endl., Gen. Plant.: 73. Aug 1836
2/2. Chlamydophytum (1; C. aphyllum; tropical West Africa), Sarcophyte (1; S. sanguinea; tropical East Africa to Eastern Cape). – Tropical and southern Africa. Stylodia absent.
Helosideae Schott et Endl., Melet. Bot.: 11. 1832 [‘Helosieae’]
6–7/17. Corynaea (1; C. crassa; Central America and the Andes from Costa Rica to Bolivia), Ditepalanthus (2; D. afzelii, D. malagasicus; Madagascar), Helosis (1; H. cayennensis; Mexico, Central America, tropical South America; incl. Exorhopala?), Exorhopala (1; E. ruficeps; northwestern Malay Peninsula; in Helosis?), Ombrophytum (4; O. microlepis, O. peruvianum, O. subterraneum, O. violaceum; western Brazil, Peru, Bolivia, northern Argentina, the Galápagos Islands), Lophophytum (4; L. leandrii, L. mirabile, L. rizzoi, L. weddellii; tropical South America), Scybalium (4; S. depressum, S. fungiforme, S. glaziovii, S. jamaicense; the Great Antilles, Colombia, Ecuador, southeastern Brazil). – Madagascar, the Malay Peninsula, Mexico, Central America, the West Indies, South America. Microsporogenesis in Coronaea successive.
Balanophoreae Engl. in H. G. A. Engler et K. A. E. Prantl, Nat. Pflanzenfam. III, 1: 250, 260. Aug 1889
5/22–24. Rhopalocnemis (1; R. phalloides; India, the Himalayas, southern China, Thailand, Vietnam, West Malesia), Lathrophytum (1; L. peckoltii; Atlantic forests in southeastern Brazil, central Brazil), Lophophytum (3–4; tropical South America), Balanophora (17–19; Congo, Madagascar, the Comoros, tropical Asia to southern Japan, Malesia, tropical northeastern Australia and islands in the Pacific), Langsdorffia (2; L. hypogaea, L. malagasica; Madagascar, New Guinea, Mexico, Central America, tropical South America), Thonningia (1; T. sanguinea; tropical Africa from Senegal to southwestern Ethiopia and south to Zambia). – Pantropical. Megagametophyte monosporous, Polygonum type, fused with ovary wall. Balanophorins as storage carbohydrates.
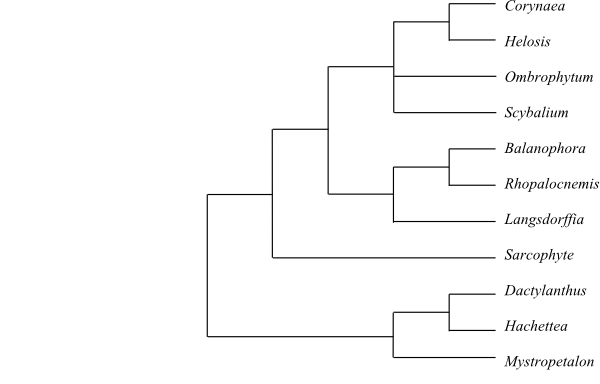 |
Cladogram of Balanophoraceae based on DNA data (according to Nickrent, The Parasitic Plant Connection website, http://www.parasiticplants.siu.edu/). Dactylanthus, Hachettea and Mystropetalon are now transferred to Mystropetalaceae (Su & al. 2015). |
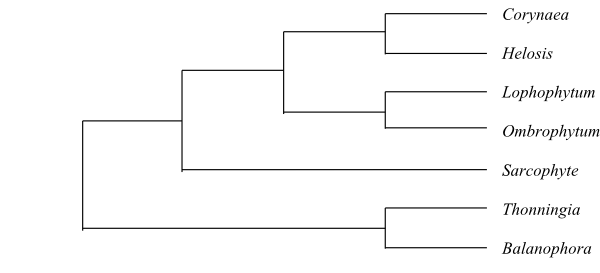 |
Cladogram of Balanophoraceae based on DNA data (Su & al. 2015). |
COULACEAE Tiegh. |
( Back to Santalales ) |
Genera/species 3/3
Distribution Tropical West Africa, West Malesia, northern tropical South America.
Fossils Unknown.
Habit Bisexual, evergreen trees. Haustoria have never been observed and the three genera are probably non-parasitic.
Vegetative anatomy Arbuscular mycorrhiza present at least in Coula. Phellogen ab initio subepidermal. Vessel elements with simple perforation plates; lateral pits alternate, simple pits. Imperforate tracheary xylem elements libriform fibres with simple or bordered pits, non-septate. Wood rays multiseriate, heterocellular. Axial parenchyma apotracheal diffuse or diffuse-in-aggregates, paratracheal scanty or in narrow bands. Tyloses (also sclerotic) abundant. Sieve tube plastids ? type; sieve tube nuclei with non-dispersive protein bodies. Nodes 3:3, trilacunar with three leaf traces. Laticifers, often articulated, present in Minquartia. Wood ray cells without silica bodies. Cystoliths absent. Prismatic calciumoxalate? crystals abundant.
Trichomes Hairs unicellular, or multicellular and dendritic.
Leaves Alternate (spiral), simple, entire, coriaceous, with conduplicate ptyxis. Stipules and leaf sheath absent. Petiole often swollen distally; petiole vascular bundle transection arcuate or annular. Venation pinnate, eucamptodromous. Stomata paracytic or anomocytic, with small guard cell chamber; guard cell walls not lignified. Cuticular waxes usually absent (sometimes present as platelets). Cuticular thickenings present. Lamina often gland-dotted, often with ‘cork warts’ of stomatal complexes. Epidermal cells with tannins or mucilage, with lignified walls, with druses. Mesophyll with lignified cell walls, without sclerenchymatous idioblasts. Asterosclereids present. Cristarque cells have been reported. Laticiferous canals and schizogenous secretory cavities resiniferous. Cell walls not silicified. Leaf margin entire. Foliar hairs multicellular, dendritic.
Inflorescence Axillary, spike-like thyrse. Peduncles and pedicels usually ferrugineously tomentose.
Flowers Actinomorphic, small. Hypanthium present or absent. Hypogyny. Sepals usually four or five (rarely three, six or seven), with open or imbricate aestivation, sometimes persistent, almost entirely connate. Petals usually four or five (rarely three, six or seven), usually with valvate (rarely imbricate) aestivation, free or connate at base, usually with adaxial hairs on distal part. Nectary absent. Disc absent.
Androecium Stamens usually one to three at each petal (four or five, eight or ten, in Coula twelve or 20), in one to three whorls. Filaments free from each other and from petals. Anthers dorsifixed, often versatile, usually tetrasporangiate, introrse or latrorse, longicidal (dehiscing by longitudinal slits); connective usually massive. Tapetum secretory. Staminodia sometimes present.
Pollen grains Microsporogenesis simultaneous. Pollen grains usually tricolporate or tetracolporate, shed as monads, bicellular or tricellular at dispersal. Apocolpium convex. Garside’s rule? Exine tectate, without infratectum, smooth or microperforate.
Gynoecium Pistil composed of (three or) four or five connate carpels; carpels mostly septate, antesepalous, or median carpel adaxial. Ovary superior, trilocular to quadrilocular, with or without ridges. Style single, simple, short to long. Stigma capitate, type? Pistillodium absent.
Ovules Placentation free central (if ovary unilocular) or axile to apical (if ovary multilocular). Ovule one per carpel, anatropous, pendulous, bitegmic, tenuinucellar? Micropyle ?-stomal. Outer integument five or six cell layers thick. Inner integument five or six cell layers thick. Megagametophyte monosporous, Polygonum type. Synergids with a filiform apparatus. Endosperm development ab initio cellular. Endosperm haustorium chalazal. Embryogenesis?
Fruit A drupe with thin pericarp and lignified endocarp.
Seeds Aril absent. Testa present. Exotesta? Endotesta? Tegmen? Perisperm not developed. Endosperm copious, oily and/or starchy (amyloid). Embryo very small, straight, with chlorophyll? Cotyledons two. Germination phanerocotylar?
Cytology n = ?
DNA
Phytochemistry Virtually unknown. Triglycerides of polyacetylenic fatty acids present.
Use Fruits (Coula), timber.
Systematics Minquartia (1; M. guianensis; northern tropical South America), Coula (1; C. edulis; tropical West Africa), Ochanostachys (1; O. amentacea; West Malesia).
Minquartia, Coula and Ochanostachys form an unresolved trichotomy in the analyses by Malécot & Nickrent (2008). On the other hand, Su & al. (2015) recovered Ochanostachys as sister to [Coula+Minquartia].
ERYTHROPALACEAE Planch. ex Miq. |
( Back to Santalales ) |
Erythropalales Tiegh. in Bot. Jahresber. (Just) 25(2): 406. 19 Jan 1900; Heisteriaceae Tiegh. in Bot. Jahresber. (Just) 25(2): 406. 19 Jan 1900; Heisteriales Tiegh. in Bot. Jahresber. (Just) 25(2): 406. 19 Jan 1900
Genera/species 4/c 40
Distribution Tropical West Africa, eastern Himalayas to West Malesia, tropical America.
Fossils Unknown.
Habit Usually bisexual (androdioecious in Erythropalum), usually evergreen trees or shrubs (in Erythropalum liana climbing by axillary branch tendrils). At least Erythropalum and Heisteria are autotrophic (haustoria absent).
Vegetative anatomy Arbuscular mycorrhiza present? Phellogen ab initio subepidermal. Medulla with or without diaphragms (in Brachynema septated by diaphragms). Inner side of xylem cylinder in Brachynema provided with distinct grooves. Vessel elements usually with simple or scalariform (sometimes reticulate or foraminate; in Brachynema scalariform) perforation plates; lateral pits alternate, scalariform or opposite (in Brachynema scalariform or opposite), usually simple (sometimes bordered) pits (in Brachynema simple pits or reduced bordered pits). Imperforate tracheary xylem elements fibre tracheids or libriform fibres with simple or bordered pits, usually non-septate. Wood rays multiseriate, heterocellular. Axial parenchyma apotracheal diffuse or diffuse-in-aggregates, or paratracheal scanty or in narrow bands, or absent. Tyloses sometimes abundant. Sieve tube plastids ? type; sieve tube nuclei with non-dispersive protein bodies. Pericyclic fibres present (Brachynema). Nodes usually 3:3, trilacunar with three leaf traces (in, e.g., Brachynema 5:5, pentalacunar with five traces). Sclereids and sclereid nests present in Brachynema and sometimes forming a continuous layer on outer side of pericycle. Wood with idioblasts containing ethereal oils. Wood cystoliths absent. Cristarque cells present in Brachynema. Wood ray cells without silica bodies. Cortex with or without cristarque cells. Prismatic crystals abundant; druses sometimes present.
Trichomes Hairs usually absent (in Brachynema unicellular; in Maburea multicellular, uniseriate).
Leaves Alternate (spiral or distichous), simple, entire, with conduplicate ptyxis. Stipules and leaf sheath absent. Petiole in Brachynema with proximal and distal pulvini, and diaphragms with sclereids. Petiole vascular bundle transection annular (sometimes with adaxial plate: vascular cylinder with one or more adaxial or enclosed strands). Venation usually pinnate (lamina three- to five-veined at base; in Erythropalum and Maburea palmate), brochidodromous. Stomata cyclocytic, anisocytic or paracytic, usually with large guard cell chamber. Cuticular waxes? Epidermal cells sometimes with lignified walls, sometimes with druses. Cuticular thickenings present. Cells with tannins or mucilage. Laticifers (often articulated) and idioblastic sclereids present in mesophyll in Heisteria. Cell walls not silicified. Leaf margin usually entire (in Brachynema glandular-serrate).
Inflorescence Axillary, often compound, raceme-like or panicle, or flowers solitary axillary (in Erythropalum large loose slender compound cymose; in Heisteria fascicles; in Maburea often spike-like fascicles; in Brachynema ramiflorous or axillary, fascicular or corymbose). Peduncles sometimes transformed into tendrils. Bracts and floral prophylls (bracteoles) possibly absent in Brachynema.
Flowers Actinomorphic, usually small. Hypanthium absent? Usually hypogyny (in Erythropalum half epigyny). Sepals (three or) four five (or six), small, with valvate aestivation, usually partially connate at base (sometimes absent; in Brachynema connate). Petals (three or) four or five (or six), with valvate aestivation, usually caducous, often connate at base (in Brachynema connate into a tube), often with adaxial hairs. Nectary absent. Disc usually intrastaminal (rarely extrastaminal or absent), entire or lobed, in Heisteria adnate to lower part of ovary.
Androecium Stamens (three or) 4+4 or 5+5, antesepalous and antepetalous, usually twice the number of petals (in Heisteria sometimes inner ones fertile and outer ones staminodial; stamens in Erythropalum as many as petals, often six, haplostemonous; in Brachynema four or five, haplostemonous, antesepalous, alternipetalous). Filaments short, wide, free, usually adnate to petals (epipetalous; in Brachynema adnate to base of perianth tube), with two lateral hairy scales at base. Anthers basifixed to subbasifixed, non-versatile, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits); connective usually massive (in Brachynema prolonged). Tapetum secretory? Staminodia present in Erythropalum and some species of Heisteria.
Pollen grains Microsporogenesis simultaneous? Pollen grains tri- or tetracolpate or tri- or tetracolpor(oid)ate (in Brachynema ramiflorum triporate), shed as monads, bicellular at dispersal. Apocolpium convex. Exine tectate, with granular or columellate infratectum, microperforate or smooth.
Gynoecium Pistil composed of two or three (to five) connate carpels, usually septate, usually antesepalous. Ovary usually superior (in Erythropalum semi-inferior), trilocular (finally often unilocular by disintegration of septa), often with ten ridges. Largest carpels? (stamens?) antesepalous, smallest carpels? (stamens?) antepetalous or odd carpel? (stamen?) adaxial. Style single, simple, very short to long (in Brachynema absent). Stigma capitate or somewhat (bi- or) tri-(to quinque-)lobate, type? Pistillodium absent.
Ovules Placentation usually apical (in Erythropalum free central). Ovule one per carpel, at least in Maburea anatropous, usually pendulous (rarely horizontal), epitropous?, usually bitegmic (sometimes unitegmic), tenuinucellar. Micropyle usually exostomal. Outer integument ? cell layers thick. Inner integument ? cell layers thick. Megagametophyte monosporous, Polygonum type. Endosperm development at least in Strombosia cellular. Endosperm haustorium chalazal? Embryogenesis?
Fruit A one-seeded drupe, often enclosed by persistent and in Brachynema and Heisteria often accrescent calyx; endocarp in Erythropalum dehiscing from apex and downwards into (three to) five or six valves.
Seeds Aril absent. Seed in Brachynema with vertical canals and with vascular strands running down canals. Testa thin. Exotesta? Endotesta? Tegmen? Perisperm not developed. Endosperm copious, occasionally somewhat ruminate, oily and starchy (in Brachynema with yellow globuli consisting of a viscid fat substance). Embryo very small, well differentiated, with chlorophyll? Cotyledons one to six, free or fused (sometimes foliaceous). Radicula long. Germination phanerocotylar.
Cytology n = 16 (Heisteria parvifolia)
DNA Insertion comprising two to four amino acid triplets (codons) in the 3’-end of the plastid gene rbcL immediately 5’ of the stop codon present in Maburea.
Phytochemistry Virtually unknown. Scopolamine and gallic acid present in Heisteria.
Use Timber.
Systematics Erythropalum (1; E. scandens; eastern Himalayas to Sulawesi and Java), Maburea (1; M. trinervis; Guyana),Heisteria (36; tropical West Africa, tropical America), Brachynema (2; B. axillare: southern Venezuela and northernmost Brazil, Amazonian Peru; B. ramiflorum: central Amazonian Brazil).
Erythropalaceae are sister-group to the remaining Santalales. However, Erythropalaceae have sometimes been recovered as sister to Coulaceae. The leaf anatomy is intermediate between Coulaceae and Aptandraceae and the pollen morphology in Heisteria is similar to that of Coulaceae.
Erythropalum may be basal within Erythropalaceae, and a sister-group relationship between Maburea and Heisteria was recovered by Su & al. (2015). The position of Brachynema is uncertain. However, its wood anatomy is similar to that in Heisteria and molecular data indicate close relationship between Brachynema and Maburea.
LORANTHACEAE Juss. |
( Back to Santalales ) |
Loranthales Link, Handbuch 2: 1. 4-11 Jul 1829 [’Lorantheae’]; Loranthopsida Bartl., Ord. Nat. Plant.: 219, 231. Sep 1830 [’Lorantheae’]; Elytranthaceae Tiegh. in Österr. Bot. Zeitschr. 46: 368. Oct 1896; Nuytsiaceae Tiegh. in Österr. Bot. Zeitschr. 46: 368. Oct 1896; Dendrophthoaceae Tiegh. in Bot. Jahresber. (Just) 24(2): 291. 1898; Treubellaceae Tiegh. in Bot. Jahresber. (Just) 24(2): 291. 1898, nom. illeg.; Gaiadendraceae Tiegh. ex Nakai in Bull. Natl. Sci. Mus. Tokyo 31: 45. Mar 1952 [’Giadendraceae’]; Psittacanthaceae (Horan.) Nakai in Bull. Natl. Sci. Mus. Tokyo 31: 46. Mar 1952
Genera/species c 74/895–915
Distribution Tropical and subtropical regions, with their largest diversity in the Southern Hemisphere, and a few species in temperate parts in the Northern or Southern Hemispheres (southeastern Europe, Japan, New Zealand, temperate South America).
Fossils Leaves have been described from Eocene sediments in Germany and Australia. Ephedrites johnianus, representing shoots with decussately arranged leaves and branches, may also be assigned to Loranthaceae. Fossil pollen are known from Early Eocene onwards (Manchester & al. 2015).
Habit Usually bisexual (rarely dioecious; in Nuytsia monoecious), usually evergreen shrubs (sometimes lianas; Atkinsonia, Gaiadendron and Nuytsia are root parasitic trees) with distinctly sympodial growth. Usually hemiparasites (rarely leafless holoparasites) and stem parasites (although root parasitism is probably a plesiomorphy in Loranthaceae). Some species are hyperparasites on stem hemiparasitic shrubs. Branches rarely transformed into photosynthezising phyllocladia. Shoots rarely resembling Cuscuta (Convolvulaceae) in appearance.
Vegetative anatomy Mycorrhiza absent. Normal roots usually absent, modified into primary or secondary haustoria; usually with one or several haustoria in apices of epicortical roots. Phellogen ab initio subepidermal. Secondary lateral growth normal, or anomalous from concentric cambia. Vessel elements with simple perforation plates; lateral pits alternate, scalariform or opposite, simple or bordered pits. Imperforate tracheary xylem elements short or very short fibre tracheids or libriform fibres usually with bordered pits, non-septate (in Nuytsia also vasicentric tracheids). Wood rays multiseriate, homocellular or heterocellular. Axial parenchyma apotracheal diffuse or diffuse-in-aggregates, or paratracheal scanty vasicentric, or banded. Wood elements often storied. Sieve tube plastids Ss type. Nodes 1:1, unilacunar with one leaf trace. Stem in Nuytsia with gum ducts containing slimy material. Wood cystoliths absent. Cristarque cells often frequent. Silica bodies often abundant. Prismatic calciumoxalate crystals often frequent.
Trichomes Hairs unicellular or multicellular, uniseriate or branched, candelabra-shaped (sometimes dendritic or stellate).
Leaves Usually opposite (rarely spiral or verticillate), simple, entire, usually coriaceous, sometimes isobilateral, rarely scale-like, with ? ptyxis. Stipules and leaf sheath absent. Petiole without asterosclereids. Petiole vascular bundle transection arcuate? Venation pinnate or parallel, camptodromous or parallelodromous. Stomata usually paracytic (sometimes anomocytic), sometimes with transverse orientation. Cuticular waxes? Epidermal cells sclerified, with druses, tannins or mucilage. Mesophyll sometimes with sclerenchymatous idioblasts (e.g. branched stone cells) containing asterosclereids, etc. Leaf margin entire.
Inflorescence Terminal or axillary, usually compound raceme-, umbel-, spike- or head-like cymose, or fasciculate of various shape (flowers in Ixocactus solitary). Bract or floral prophylls (bracteoles) often present at base and immediately to one side of ovary.
Flowers Zygomorphic (often slit-monosymmetric) or actinomorphic, often large, often opening explosively. Uppermost part of receptacle together with two connate and often calyculus-shaped floral prophylls? surrounding and adnate to ovary. Epigyny. Sepals (three to) five to seven (to nine), with open aestivation, small, persistent, often reduced, connate (sometimes absent). Petals (three to) five to seven (to nine), with valvate aestivation, free or connate at base into a tube (corolla tube often curved and split on one side). Nectary absent. Disc present or absent.
Androecium Stamens (three to) five to seven (to nine), haplostemonous or diplostemonous, alternisepalous, antepetalous, usually non-septate. Filaments free, usually adnate to petals (epipetalous). Anthers usually basifixed or dorsifixed, versatile or non-versatile, usually tetrasporangiate (rarely di- or monosporangiate), often with septate microsporangia, introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory. Staminodia usually absent (in Passovia rarely three).
Pollen grains Microsporogenesis simultaneous. Pollen grains usually tricolpate or tricolporate (rarely tetracolpate or tetracolporate, in Atkinsonia inaperturate), often with fused apertures, shed as monads, bicellular at dispersal. Apocolpium convex (pollen grains sometimes tridentate). Exine tectate, with granular to columellate infratectum, perforate or imperforate, scabrate, verrucate, spinulate, echinate, or smooth.
Gynoecium Pistil composed of (one to) three to five (to twelve?) connate carpels. Ovary inferior, unilocular (sometimes seemingly multilocular in lower part). Style single, simple, short to long. Stigma small, type? Pistillodium often present in male flowers.
Ovules Placentation free central or basal (in Lysiana axile). ’Ovules’ four to twelve per carpel, indistinctly differentiated, ategmic, extremely tenuinucellar (integument and megasporangium rudimentary and diffuse). Megasporocytes numerous. Megagametophyte usually disporous, 8-nucleate, Allium type (sometimes monosporous, Polygonum type), developing within a mamelon. Synergids with a filiform apparatus. Megagametophyte sometimes growing to stylar apex (in Moquiniella up to 48 mm long, growing along style way up to stigma and back downwards). Mamelon formed by adnation of megasporangium to ovary wall; mamelon sometimes growing out from ovary base where megagametophyte developes (mamelon sometimes reduced or absent). Endosperm development ab initio cellular, endosperms from all megagametophytes in one ovary developing simultaneously and gradually fusing into a homogeneous mass. ’Hypostase’ formed as a collenchymatous zone below megagametophytes. First division of zygote vertical. Endosperm haustoria developing from basal endosperm cells. Embryogenesis piperad. Polyembryony present.
Fruit Usually a one-seeded (sometimes two- or three-seeded) berry-like or drupaceous fruit (in, e.g., Nuytsia a nut). Mesocarp viscid, with gum in laticifers outside vascular bundles (cf. Santalaceae).
Seeds ’Seed’ covered by sticky viscin tissue, secreted from mesocarp. Aril absent. Testa and tegmen absent. Perisperm not developed. Endosperm copious, scarce or absent (in Aetanthus), oily (and starchy?), formed from several ‘ovules’. Suspensor very long. Embryo large, well differentiated, with chlorophyll, at least sometimes without distinct radicle. Cotyledons two, in many Old World species connate (except at base). Germination phanerocotylar or cryptocotylar.
Cytology x = 8–12, 16
DNA Mitochondrial genes from a hypothetical extinct? root parasitic species of Loranthaceae (with mycorrhiza) may have been uptaken by Botrychium (Ophioglossaceae; in Asia?), perhaps via a common mycorrhizal fungus.
Phytochemistry Flavonols (kaempferol, quercetin), flavones, dehydroxyflavones, C-glycoflavones, chalcones, cyanidin, ellagic acid (in Nuytsia), gallic acid (in Taxillus), condensed tannins, alkaloids, and rubber (in fruits) present. Tyramine and phenylethylamine often abundant. Saponins and cyanogenic compounds not found.
Use Medicinal plants, the sticky substance of the fruits used for bird-lime.
Systematics Loranthaceae are sister to [Misodendraceae+Schoepfiaceae].
The three root parasitic genera Nuytsia (southwestern Western Australia), Atkinsonia (eastern Australia) and Gaiodendron (Central and South America) form a basal grade and are successive sister-groups to the remaining Loranthaceae. The stem parasitic genus Notanthera (x = 12) may also be basal.
Nuytsioideae Tiegh. in Bot. Centralbl. 62: 294. 1895
1/1. Nuytsia (1; N. floribunda; southwestern Western Australia). – Root parasitic tree. Unique haustorial behavior occurring: haustoria wearing sclerenchymatous tips functioning as scissors and cutting host root transversely. Leaves spiral. Stomata transversely arranged. Monoecious with bisexual flower central in dichasium and lateral male flowers. Calyculus with vascular bundles. Petals six to eight, free. Stamens six, antepetalous. Fruit a dry three-winged samara (achene). Cotyledons three or four, foliaceous. Germination phanerocotylar. n = 12
Atkinsonia
1/1. Atkinsonia (1; A. ligustrina; the Blue Mountains in New South Wales). – Root parasitic shrub. Axillary raceme with hexamerous (to octomerous) open flowers in monads. Calyculus with vascular bundles. Stamens inserted at two levels on corolla. Anthers dorsifixed, versatile. Fruit a drupe. n = 12
1/1. Gaiadendron (1; G. punctatum; Central and South America). – Tree or shrub. Root parasitic or epiphytic shrub (perhaps root parasite on epiphytes). Racemose or paniculate inflorescences with dichasial partial inflorescences bearing open flowers in triads. Flowers hexamerous (to octomerous). Calyculus with vascular bundles. Stamens inserted at two levels on corolla. Anthers dorsifixed, versatile. Fruit a drupe. Seedling without primary haustoria. n = 12
Loranthoideae Eaton, Bot. Dict., ed. 4: 37. Apr-Mai 1836 [‘Lorantheae’]
c 71/895–915. Stem and branch parasites forming a ’burl’ at insertion point and with epicortical roots running above surface and forming secondary ’burls’. Leaves usually opposite (rarely spiral). Flowers (3–)5–6(–9)-merous. Calyculs usually unvascularized (often absent). Stamens usually biseriate (one whorl sometimes staminodial). Anthers sometimes septate. Megagametophyte disporous, 8-nucleate, Allium type. Fruit a drupe, berry or nut. Endosperm sometimes absent. Embryo plug like (cotyledons sometimes connate). Radicula absent. Primary haustorium present. Germination sometimes cryptocotylar.
Elytrantheae Danser in Verh. Kon. Ned. Akad. Wetensch., Afd. Natuurk., Tweede Sect. 29(6): 4. 1933
14/140–145. Alepis (1; A. flavida; New Zealand), Peraxilla (2; P. colensoi, P. tetrapetala; New Zealand). – Elytranthinae Engl. in Engler et Prantl, Nat. Pflanzenfam. Nachtr.: 125. 2 Aug 1897. Amylotheca (5; A. acuminatifolia, A. densiflora, A. dictyophleba, A. duthieana, A. subumbellata; Southeast Asia to New Guinea, tropical Australia, New Caledonia and Vanuatu), Cyne (6; C. baetorta, C. banahaensis, C. monotrias, C. papuana, C. perfoliata, C. quadriangula; the Philippines to New Guinea), Decaisnina (c 25; Java and the Philippines to northern Australia, Tahiti and the Marquesas), Elytranthe (8; eastern India to Vietnam, West Malesia), Lampas (1; L. elmeri; northern Borneo), Lepeostegeres (9; West Malesia to New Guinea), Lepidaria (1; L. tetrantha; southern Thailand, North and West Malesia), Loxanthera (1; L. speciosa; West Malesia), Lysiana (1; L. casuarinae; Australia), Macrosolen (80–85; southern China, South and Southeast Asia to New Guinea, with their highest diversity on Borneo), Thaumasianthes (1; T. amplifolia; the Philippines), Trilepidea (1; T. adamsii; North Island of New Zealand). – Epicortical roots usually present (absent in Lysiana). Flowers bisexual, actinomorphic, usually hexamerous (tetramerous in Alepis and Peraxilla). Anthers usually basifixed (dorsifixed in Alepis and Loxanthera). Placentation in Lysiana axile. x = 12.
Psittacantheae Horan., Char. Ess. Fam.: 86. 17 Jun 1847
17–18/285–290. Central and South America, the West Indies. Epicortical roots present or absent. Partial inflorescences monads, dyads or triads forming spikes, racemes, umbels, or capitula. Flowers actinomorphic, usually bisexual (sometimes unisexual), usually hexamerous (sometimes tetra- or pentamerous). Anthers basifixed or dorsifixed, often dimorphic, in Psittacanthinae with an apiculate connective prolongation. x = 8, 10, 12, 16 – Tristerix, in South America, is pollinated by tree-dwelling marsupials. In Tristerix aphyllus the axis of the seedling degenerates and is replaced by adventitious shoots from the endophyte.
Tupeinae Nickrent et Vidal-Russell in D. L. Nickrent et al., Taxon 59: 546. 4 Apr 2010
1/1. Tupeia (1; T. antarctica; New Zealand). – n = 12.
Notantherinae Nickrent et Vidal-Russell in D. L. Nickrent et al., Taxon 59: 547. 4 Apr 2010
2/2. Desmaria (1; D. mutabilis; Chile from c 38° to c 40°S), Notanthera (1; N. heterophylla; temperate South America). – Temperate South America. n = 12 (Notanthera), n = 16 (Desmaria).
Ligarinae Nickrent et Vidal-Russell in D. L. Nickrent et al., Taxon 59: 547. 4 Apr 2010
2/>13. Ligaria (≥2; L. cuneifolia, L. teretiflora; central Brazil), Tristerix (11; the Andes from Colombia and Ecuador to Chile and Argentina). – South America. n = 10 (Ligaria), n = 12 (Tristerix).
Psittacanthinae Engl. in Engler et Prantl, Nat. Pflanzenfam. Nachtr.: 125, 135. 2 Aug 1897
12–13/270–275. Aetanthus (c 15; northern Andes), Cladocolea (c 35; Mexico, Central America, tropical South America), Dendropemon (c 40; the West Indies, with their highest diversity on Hispaniola), Ixocactus (5; I. clandestinus, I. hutchisonii, I. inconspicuus, I. inornus, I. macrophyllus; Colombia, Venezuela), Oryctanthus (14; tropical America; incl. Oryctina?), Oryctina (6; O. atrolineata, O. eubrachioides, O. myrsinites, O. quadrangularis, O. scabrida, O. subaphylla; South America; in Oryctanthus?), Panamanthus (1; P. panamensis; Panamá), Passovia (23; tropical South America), Phthirusa (23; tropical South America), Psittacanthus (57; Mexico, Central America, the West Indies, tropical South America), Struthanthus (c 50; tropical America), Peristethium (2; P. aequatoris, P. lojae; Costa Rica to northern Bolivia), Tripodanthus (2; T. acutifolius, T. flagellaris; South America). – Tropical America. x = 8.
Lorantheae Rchb., Fl. Germ. Excurs. 1(3): 203. Jul-Dec 1831
c 40/470–480. Tropical regions in the Old World. x = 9 (11)
Ileostylinae Nickrent et Vidal-Russell in D. L. Nickrent et al., Taxon 59: 548. 4 Apr 2010
2/2. Ileostylus (1; I. micranthus; New Zealand, Stewart Island), Muellerina (1; M. celastroides; southeastern Queensland, eastern New South Wales, Victoria). – Southeastern Australia, New Zealand. Epicortical roots present. Raceme- or umbel-like inflorescence consisting of monads or triads. Petals four or five. Anthers basifixed or dorsifixed. x = 11
Loranthinae Engl. in Engler et Prantl, Nat. Pflanzenfam. Nachtr.: 125, 127. 2 Aug 1897
2/2. Cecarria (1; C. obtusifolia; the Philippines, Flores, Timor to northeastern Queensland), Loranthus (1; L. europaeus; southeastern Europe). – Europe, North and East Malesia to Queensland. x = 9.
Amyeminae Nickrent et Vidal-Russell in D. L. Nickrent et al., Taxon 59: 548. 4 Apr 2010
c 9/c 110. ’Amyema’ (c 95; Southeast Asia, Malesia to New Guinea and tropical Australia; polyphyletic), Baratranthus (4; B. axanthus, B. mabioides, B. nodiflorus, B. productus; Sri Lanka, West Malesia), Benthamina (1; B. alyxifolia; eastern Queensland, eastern New South Wales), Dactyliophora (3; D. novaeguineae, D. salomonia, D. verticillata; Ceram, New Guinea, northestern Queensland), Diplatia (4; D. alberticii, D. furcata, D. grandibractea, D. tomentosa; tropical Australia), Distrianthes (1; D. molliflora; New Guinea), Helicanthes (1; H. elastica; India), Papuanthes (1; P. albertisii; New Guinea), Sogerianthe (1; S. sogerensis; eastern New Guinea to Solomon Islands). – Tropical Asia to eastern Australia, Solomon Islands. x = 9.
Scurrulinae Nickrent et Vidal-Russell in D. L. Nickrent et al., Taxon 59: 549. 4 Apr 2010
2/c 30. Scurrula (10; southern China, Southeast Asia, Malesia to the Moluccas), Taxillus (c 20; tropical Asia to Central Malesia, one species, T. wiensii, on the coast of Kenya). – Tropical Asia, Kenya. x = 9.
Dendrophthoinae Nickrent et Vidal-Russell in D. L. Nickrent et al., Taxon 59: 549. 4 Apr 2010
4/90–95. Dendrophthoe (c 60; tropical Asia to tropical Australia), ’Helixanthera’ (c 25; tropical Africa, tropical Asia to Sulawesi and the Philippines; non-monophyletic), Tolypanthus (2; T. esquirolii, T. maclurei; India to southeastern China), Trithecanthera (5; T. flava, T. scortechinii, T. sparsa, T. superba, T. xiphostachya; West Malesia). – Tropical regions in the Old World. x = 9.
Emelianthinae Nickrent et Vidal-Russell in D. L. Nickrent et al., Taxon 59: 549. 4 Apr 2010
7/c 70. Emelianthe (1; E. panganensis; eastern and northeastern tropical Africa), Erianthemum (16; eastern and southern Africa), Globimetula (13; tropical Africa), Moquiniella (1; M. rubra; Northern, Western and Eastern Cape), Oliverella (3; O. bussei, O. hildebrandtii, O. rubroviridis; eastern and south-central Africa), Phragmanthera (34; tropical Africa to Namibia, the Arabian Peninsula), Spragueanella (1; S. rhamnifolia; eastern and south-central Africa). – Tropical and southern Africa, the Arabian Peninsula. x = 9.
Tapinanthinae Nickrent et Vidal-Russell in D. L. Nickrent et al., Taxon 59: 550. 4 Apr 2010
14/165–170. Actinanthella (2; A. menyharthii, A. wyliei; southeastern and southern Africa), Agelanthus (c 60; Africa, the Arabian Peninsula), Bakerella (16; Madagascar), Berhautia (1; B. senegalensis; Senegal, Gambia), Englerina (c 25; tropical Africa), Oedina (2; O. erecta, O. pendens; Tanzania, northern Malawi), Oncella (2; O. ambigua, O. curviramea; tropical East Africa), Oncocalyx (11; eastern and southern Africa, the Arabian Peninsula), Pedistylis (1; P. galpinii; southern Mozambique, Swaziland, northeastern South Africa), Plicosepalus (11; arid and semiarid regions in eastern and southern Africa), Septulina (2; S. glauca, S. ovalis; southern Namibia, Northern and Western Cape), Socratina (1; S. bemarivensis; southwestern Madagascar), Tapinanthus (33; tropical and southern Africa, one species also in northern Yemen), Vanwykia (1; V. remota; eastern and southeastern Africa). – Africa, Madagascar, the Arabian Peninsula. x = 9.
 |
Cladogram (simplified) of Loranthaceae based on DNA sequence data (Nickrent & al. 2010). |
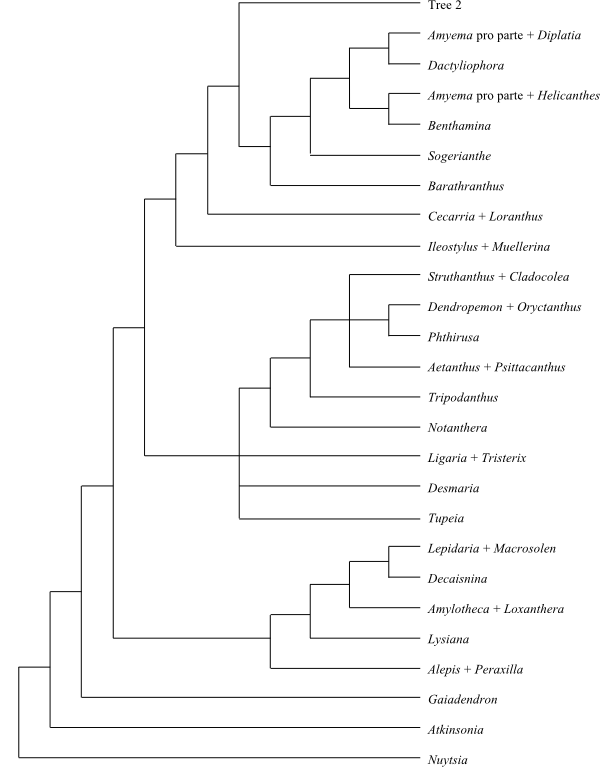 |
Majority rule consensus tree 1 of a Bayesian analysis of Loranthaceae based on DNA sequence data (Vidal-Russell & Nickrent 2008). |
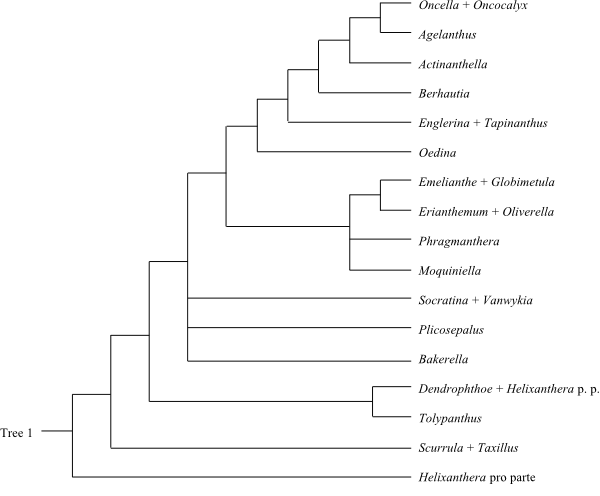 |
Majority rule consensus tree 2 of a Bayesian analysis of Loranthaceae based on DNA sequence data (Vidal-Russell & Nickrent 2008). |
MISODENDRACEAE J. Agardh |
( Back to Santalales ) |
Genera/species 1/8
Distribution Southern Chile and adjacent parts of Argentina.
Fossils Unknown.
Habit Usually dioecious (rarely bisexual or monoecious), evergreen shrubs with distinct sympodial growth and even as young a thick stem. Stem/branch hemiparasites almost exclusively on Nothofagus (Nothofagaceae). Branches coarse. Stem apex aborting annually, one or several lateral branches continuing the sympodial vegetative growth the following year.
Vegetative anatomy Mycorrhiza absent. Normal roots absent. Roots modified into haustoria; haustorial region radially expanding. Phellogen ab initio subepidermal? Primary medullary rays narrow, uniseriate. Secondary lateral growth normal or anomalous from concentric (successive) cambia; sometimes an inner secondary cylinder of bundle-like fascicular areas. Vessel elements very short, with simple perforation plates; lateral pits alternate to almost scalariform, simple or bordered pits. Imperforate tracheary elements libriform fibres usually with simple pits, non-septate (also vasicentric tracheids). Wood rays absent or consisting of fibres or thin-walled cells. Axial parenchyma apotracheal diffuse, with fusiform cells. Vessel elements, fibres and/or axial parenchyma storied. Sieve tube plastids S0 type, without starch or protein inclusions. Nodes probably 1:1, unilacunar with one leaf trace. Wood ray cells without silica bodies. Wood without cystoliths. Parenchyma with calciumoxalate crystals.
Trichomes Hairs unicellular.
Leaves Alternate (spiral), simple, entire, often coriaceous, sometimes scale-like, with ? ptyxis. Stipules and leaf sheath absent. Foliar dimorphism occurring: leaves on floral shoots different from leaves on vegetative shoots. Petiole without asterosclereids. Petiole vascular bundles? Venation pinnate, camptodromous. Stomata usually paracytic (sometimes anomocytic). Cuticular waxes? Cystoliths present. Epidermal cells sclerified (with druse?)? Cell walls often silicified. Leaf margin entire.
Inflorescence Axillary, raceme-, catkin- or spike-like (sometimes compound) with pubescent pedicels. Floral prophylls (bracteoles) present or absent.
Flowers Actinomorphic, very small. Possibly hypogyny (to slightly half epigyny). Tepals absent in male flowers; tepals in female flowers three, in one whorl, with open aestivation, sepaloid, reduced, connate at base. Nectariferous disc intrastaminal, annular, lobate.
Androecium Stamens two or three, haplostemonous, antepetalous. Filaments free from each other and from tepals. Anthers basifixed, non-versatile, disporangiate (monothecal), introrse, dehiscing by short apical tangential slits. Tapetum secretory? Staminodia in female flowers short, bristle-like, alternitepalous, each inserted in furrow in ovary (to one side of tepal attachment point), persistent and accrescent.
Pollen grains Microsporogenesis simultaneous? Pollen grains (3–)4–12(–19)-porate, shed as monads, ?-cellular at dispersal. Apocolpium convex. Exine intectate, acolumellate, with granular, psilate-scabrate and echinate endexine beset with scattered spinulae.
Gynoecium Pistil composed of three connate carpels. Ovary possibly superior (or slightly semi-inferior), unilocular, with three furrows. Style single, simple, very short, stout. Stigma trilobate, type? Pistillodium absent.
Ovules Placentation free central. Ovules three per ovary, orthotropous, pendulous, ategmic, tenuinucellar. Megasporangium indistinctly defined. Megagametophyte monosporous, Polygonum type. Endosperm development cellular. Endosperm haustorium chalazal, elongate. Embryogenesis complex, unusual type.
Fruit A dry, one-seeded, nut-like fruit (sometimes a samara), inserted at three strongly accrescent, plume- or bristle-like staminodia, each one inserted at a furrow in ovary to other side of perianth base. Setae of pistillate flower elongating following anthesis to become elongated plume-like organs 10–85 mm long and covered by numerous uniseriate trichomes (setae in some species with hooked apex); these organs possibly modified stamens alternating with three perianth segments.
Seeds Aril absent. Seed coat sparsely sclereidal. Testa and tegmen absent. Perisperm not developed. Endosperm copious to sparse, oily, or absent. Embryo small to large, straight, with chlorophyll. Cotyledons two, connate. Germination phanerocotylar.
Cytology n = ?
DNA
Phytochemistry Unknown.
Use Unknown.
Systematics Misodendrum (8; cold-temperate Chile and Argentina from 33°S to Magellan’s Strait).
Misodendrum is sister to Schoepfiaceae.
Antidaphne possibly belongs in Misodendraceae (here provisionally placed in Santalaceae). The cotyledons in Antidaphne probably have three vascular strands.
The wood anatomy of Misodendrum resembles that in Loranthaceae (Carlquist 1985).
MYSTROPETALACEAE J. D. Hooker |
( Back to Santalales ) |
Genera/species 3/3
Distribution Southwestern South Africa, North Island of New Zealand, mountains on New Caledonia.
Fossils Unknown.
Habit Monoecious or dioecious, perennial, yellow, brown or red, achlorophyllous herbaceous root holoendoparasites with branched or simple subterranean tuber-like structures partly of root nature, partly consisting of host tissue. Succulents.
Vegetative anatomy Mycorrhiza absent. Normal roots absent. Stem endogenous. Phellogen absent? Vascular tissue strongly reduced. Secondary lateral growth absent. Vessel elements with simple or scalariform perforation plates, or absent. Imperforate tracheary xylem elements? Wood rays absent. Axial parenchyma absent? Sieve tube plastids S type. Nodes? Calciumoxalate crystals abundant.
Trichomes Epidermal hairs unicellular, simple.
Leaves Usually alternate (spiral or distichous; rarely opposite or verticillate), simple, entire, membranous and scale-like, or absent. Stipules and leaf sheath absent. Venation absent. Stomata absent. Cuticular wax crystalloids absent. Leaf margin entire.
Inflorescence Terminal, raceme-like. Inflorescence usually developing endogenously inside tuber (or lobes of tuber; in some genera exogenously). Tuber rupturing during inflorescence development leaving an annular collar-like volva at base of peduncle. Male inflorescence branches of first order on elongated upper part of stem, subtended by bracts (male inflorescence branches of first order in Dactylanthus inserted on slightly swollen apical part of stem, not subtended by bracts). Female flower single on thin elongated branch of first order. Extrafloral nectaries?
Flowers Usually actinomorphic (in Mystropetalon zygomorphic), very small. Epigyny to half epigyny. Tepals sepaloid, in male flowers usually three (in Dactylanthus two filiform or absent), in one whorl, with usually valvate (sometimes imbricate) aestivation, free or connate at base, or absent; tepals in female flowers three, very small, connate and cupuliform, persistent, inserted on an articulate pedicel later becoming a cushion-like elaiosome. Floral parts in Dactylanthus and Hachettea surrounded and more or less covered by peduncle bracts. Nectariferous disc somewhat lobed (Mystropetalon).
Androecium Stamens one or two, antetepalous, free or connate into synandrium, usually free from tepals (in Mystropetalon adnate to lower part of adaxial tepals). Anthers usually free, basifixed, non-versatile, tetrasporangiate, extrorse (in Mystropetalon finally introrse), usually longicidal (dehiscing by longitudinal slits; rarely poricidal or with a transversal slit), or connate into a synandrium, irregularly dehiscing. Tapetum secretory? Staminodia absent.
Pollen grains Microsporogenesis usually simultaneous. Pollen grains usually 5–12(–15)-colpate, in Dactylanthus and Hachettea annulate and (3–)4(–5)-angular, shed as monads, bicellular or tricellular at dispersal. Exine tectate, with usually columellate (sometimes granular) infratectum, usually smooth (rarely microreticulate or finely striate).
Gynoecium Pistil composed of two or three connate carpels, or carpels absent (flowers acarpellate). Ovary inferior or semi-inferior, adnate to calyx tube, basally encircled by irregularly crenate disk, usually unilocular (in Mystropetalon bilocular or trilocular). Style single, simple. Stigmas one to three, capitate?, type? Male flowers in Mystropetalon with pistillodium.
Ovules Placentation apical. ‘Ovule’ consisting of usually a single megagametophyte per ovary (sometimes several; in Mystropetalon one per carpel), more or less fused with surrounding pericarp tissue, sometimes orthotropous or anatropous, pendulous, ategmic, extremely tenuinucellar. Megagametophyte usually disporous, Allium type, often hooked (chalazal caecum sometimes present). Antipodal cells one or two, ephemeral, or absent. Endosperm development usually cellular or nuclear (rarely helobial). One out of two cells formed at first endosperm division not dividing further and possibly corresponding to chalazal endosperm haustorium in other Santalales. Embryogenesis piperad (first zygote division vertical).
Fruit A drupelet, nut or achene (in Mystropetalon surrounded by fleshy perianth and basal disc-like elaiosome consisting of modified pedicel).
Seeds Aril absent. Seed coat sometimes absent. Perisperm not developed. Endosperm copious, oily and starchy or with balanophorins. Embryo short or rudimentary, undifferentiated (four- to twelve-celled), without chlorophyll? Cotyledons absent. Germination via germination tube.
Cytology n = 14, 16?, 18
DNA
Phytochemistry Insufficiently known. Tannins present (in Mystropetalon reddish-brown mystrin). Carbohydrates usually stored as starch, balanophorins (palmitate and lupeol palmitate), especially in tuber. Alkaloids and cyanogenic compounds not found.
Use Medicinal plants.
Systematics Mystropetalon (1; M. thomii; Western Cape), Dactylanthus (1; D. taylorii; North Island of New Zealand), Hachettea (1; H. austrocaledonica; New Caledonia). – New Caledonia, New Zealand. – The haustoria in Mystropetalon are provided with stolons which may form additional haustoria with graniferous tracheary cells. The flowers in Mystropetalon are epigynous and the pollen grains are cuboid with a pore in each of the eight corners.
Mystropetalaceae are sister-group to Balanophoraceae.
Monoecious, arising from subspherical lobed tuber. Peduncle imbricately leaved. Inflorescence spicate, lower part with female flowers, upper male flowers. Flowers slightly zygomorphic, subtended by one bract and two prophylls. Male flowers with thre perianth segments valvately arranged and two distinct stamens. Anthers dorsifixed, lengthwise dehiscing. Pollen grains (9-)12(-15)-colpate and (3)4(5)-angular. Disk short, lobed, encircling a rudimentary pistil. Female flowers inserted on an articulate pedicel later to become a cushion-like elaiosome. Perianth segments three. Staminodes two, ovary inferior, obviously trimerous. Style single, basally encircled by crenate disk. Fruit almost spherical, with hard exocarp and soft endocarp. Parasite on Leucadendron and Protea.
Dioecious, arising from spheroid tuber. Basal sheath not obvious. Peduncle covered by spiraly arranged scaly leaves which in upper third gradually become larger and function as bracts each subtending one inflorescence branch. Flowers subtended by short, reduced bracts, male flowers pedicellate. Tepals three, elliptic, valvate. Stamens two with very shorty filaments. Anthers terminal, dehiscing by transverse slits. Pollen grains asymmetric or centrosymmetric, (3)4(6)-porate-annulate. Female flowers sessile, perianth very small, superior, short tubular trilobate. Ovary inferior, narrowly ovoid, apparently three-celled. Style single. Parasite on Cunoniaceae.
Dioecious, arising from a large, irregularly lobed tuber. Basal sheath not obvious. Flowering shoots often more than 20 at a time from large tubers, with spirally arranged imbricate scaly leaves which increase in size distally in inflorescence and form an involucre around the flower-bearing branches, these 15-25, ebracteate, inserted at swollen apical part of peduncle, each with up to 50 male or 100 female flowers. Male flowers usually ebracteate, sessile. Tepals usually two, lateral. Stamen single with short and thick filament. Anther deeply lobed quadrilocular, dehiscing irregularly. Pollen grains asymmetric or centrosymmetric, (3-)10-11-porate-annulate. Female flowers sessile. Tepals two minute, linear. Ovary inferior, apparently two-celled. Style single. Parasite on many different families.
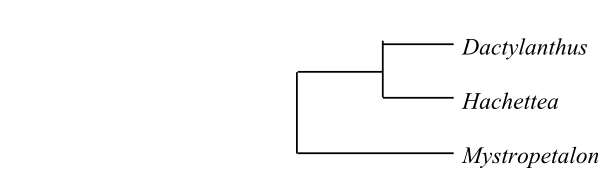 |
Cladogram of Mystropetalaceae based on DNA data (according to Nickrent, The Parasitic Plant Connection website at http://www.parasiticplants.siu.edu/, and Su & al. 2015) |
OCTOKNEMACEAE Solereder |
( Back to Santalales ) |
Genera/species 1/≥14
Distribution Tropical Africa.
Fossils Unknown.
Habit Dioecious, evergreen trees or shrubs. Probably root hemiparasites. Some representatives are xerophytes.
Vegetative anatomy Phellogen ab initio epidermal. Vessel elements with scalariform perforation plates; lateral pits opposite, simple pits. Imperforate tracheary xylem elements libriform fibres with simple or bordered pits, septate or non-septate. Wood rays multiseriate, heterocellular. Axial parenchyma usually absent (rarely diffuse). Sieve tube plastids ? type; sieve tube nuclei with non-dispersive protein bodies. Nodes ≥5:≥5, at least pentalacunar with five or more leaf traces. Wood ray cells without silica bodies. Cystoliths absent. Prismatic calciumoxalate crystals frequent.
Trichomes Hairs stellate to peltate.
Leaves Alternate (spiral or distichous), simple, entire, coriaceous, with conduplicate ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle transection annular. Venation pinnate, camptodromous. Stomata anisocytic or cyclocytic, with closed small guard cell chamber. Cuticular waxes? Cuticle thickenings occurring. Lamina often gland-dotted. Epidermal cells with tannins or mucilage. Mesophyll without sclerenchymatous idioblasts. Asterosclereids present. Cristarque cells present. Cell walls not silicified. Prismatic crystals abundant. Leaf margin entire.
Inflorescence Axillary, few-flowered, fasciculate, male flowers in raceme or flowers solitary, female flowers in simple spike.
Flowers Actinomorphic, small. Hypanthium present or absent. Epigyny. Sepals strongly reduced, with open or imbricate aestivation, sometimes persistent, usually entirely or almost entirely connate, or absent. Petals five, with valvate aestivation, free or connate at base (rarely largely connate?), often with adaxial hairs. Nectariferous disc lobate, with lobes alternating with stamens (male flowers) or staminodia (female flowers).
Androecium Stamens as many as petals, antepetalous. Filaments free from each other, free or adnate to petals. Anthers dorsifixed to basifixed, often versatile, usually tetrasporangiate (rarely disporangiate), introrse, longicidal (dehiscing by longitudinal slits); connective usually massive. Tapetum secretory. Female flowers with staminodia.
Pollen grains Microsporogenesis simultaneous. Pollen grains tricolporate or tetracolporate, shed as monads, bicellular or tricellular at dispersal. Apocolpium convex. Garside’s rule? Exine tectate, with granular and/or columellate infratectum, microperforate or smooth.
Gynoecium Pistil composed of usually three (sometimes four) connate carpels. Ovary inferior, usually trilocular (sometimes quadrilocular) in lower part and unilocular in upper part. Style single, simple, short. Stigma trilobate to quinquelobate, each lobe further divided (expanded stigmatic excrescences), type? Male flowers with pistillodium.
Ovules Placentation free central. Ovule one per carpel, anatropous, unitegmic or bitegmic, tenuinucellar. Micropyle ?-stomal. Integument ? cell layers thick. Megagametophyte monosporous, Polygonum type. Synergids with a filiform apparatus? Endosperm development usually cellular. Endosperm haustorium chalazal? Embryogenesis?
Fruit A drupe, often with persistent perianth. Endocarp laminated (with six to ten thin intrusions into seed furrows).
Seeds Aril absent. Testa present. Exotesta? Endotesta? Tegmen? Perisperm not developed. Endosperm copious, ruminate, oily and starchy. Embryo very small, straight, with chlorophyll? Cotyledons often six. Radicula elongated. Germination phanerocotylar, epigaeous.
Cytology n = ?
DNA
Phytochemistry Insufficiently known. Alkaloids, saponins, cyanogenic compounds, tannins (phenylpropanoids), triglycerides of polyacetylenic fatty acids present. Aluminium accumulated.
Use Fruits.
Systematics Octoknema (≥14; tropical Africa).
Octoknema is sister-group to the clade [[Loranthaceae+[Misodendraceae+Schoepfiaceae]]+[Opiliaceae+Santalaceae]] (Nickrent & al. 2010).
Octoknema is sister-group to all Santalales except Strombosiaceae and Erythropalaceae (Nickrent & al. 2010; Su & al. 2015). The wood anatomy resembles that in Ximeniaceae.
OLACACEAE Juss. ex R. Br. |
( Back to Santalales ) |
Olacales Mirb. in C. F. P. von Martius, Consp. Regn. Veg.: 40. Sep-Oct. 1835 [’Olacineae’]
Genera/species 2–3/45–50
Distribution Tropical and southern Africa, Madagascar, Indomalesia, Australia, New Caledonia;tropical South America.
Fossils Unknown.
Habit Usually bisexual (in Olax rarely monoecious or dioecious), evergreen trees or shrubs (rarely lianas). Olax (including ‘Dulacia’?) and Ptychopetalum are root hemiparasites. Some species are xerophytes. Branches in Olax sometimes strongly quadrangular (with wing-like edges).
Vegetative anatomy Mycorrhiza absent. Phellogen ab initio subepidermal. Vessel elements with scalariform perforation plates; lateral pits alternate, simple and bordered pits. Imperforate tracheary xylem elements fibre tracheids or libriform fibres with simple or bordered pits, non-septate (also vasicentric tracheids). Wood rays uniseriate, heterocellular. Axial parenchyma apotracheal diffuse or diffuse-in-aggregates, or paratracheal scanty or in relatively narrow bands. Sieve tube plastids ? type; sieve tube nuclei with non-dispersive protein bodies. Nodes in Olax usually 1:1, unilacunar with one leaf trace, in Ptychopetalum (and ‘Dulacia’) 3:3, trilacunar with three traces. Cystoliths absent. Silica bodies often abundant, also in wood ray cells. Prismatic calciumoxalate crystals often abundant.
Trichomes Hairs unicellular or absent.
Leaves Alternate (spiral or distichous; rarely opposite), simple, entire, coriaceous, with conduplicate ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle simple. Venation pinnate, brochidodromous. Stomata paracytic or anomocytic, with small guard cell chamber. Cuticular wax crystalloids usually absent (sometimes present as platelets). Cuticular thickenings present. Lamina often gland-dotted. Epidermal cells with tannins or mucilage. Mesophyll without sclerenchymatous idioblasts (spicula fibres). Asterosclereids absent. Epidermal and mesophyll cell walls often silicified. Cristarque cells have been reported. Cell walls often silicified. Leaf margin entire.
Inflorescence Axillary, panicle, raceme- or head-like, or flowers solitary axillary. Bracts usually caducous (sometimes persistent and foliaceous).
Flowers Actinomorphic, small. Hypanthium present or absent. Hypogyny to half epigyny. Sepals (four or) five or six, with open or imbricate aestivation, sometimes persistent, usually entirely or almost entirely connate (sometimes absent). Petals (three to) five or six, with valvate aestivation, connate at base or to half of their length, with adaxial hairs and in Olax sometimes a membranous ligule between filament base and petal. Nectary? Disc intrastaminal, annular or cupulate (absent in Ptychopetalum).
Androecium Stamens usually three to six (in Ptychopetalum up to ten or twelve), as many as or twice the number of petals, in one or two whorls, alternisepalous or antesepalous, alternipetalous or antepetalous (larger stamens antesepalous, smaller stamens antepetalous). Filaments free from each other, adnate to petals. Anthers dorsifixed or basifixed, often versatile, usually tetrasporangiate (rarely disporangiate), introrse, usually longicidal (dehiscing by longitudinal slits, rarely apically); connective usually massive. Tapetum secretory. Staminodia three to six, bifid or bilobate, or absent (three or five outer stamens in Olax sometimes staminodial; three outer stamens in Ptychopetalum staminodial).
Pollen grains Microsporogenesis simultaneous. Pollen grains usually 3–5(–8)-colpate or 3–5(–8)-colporate (in some species 3–5-porate), shed as monads, bicellular or tricellular at dispersal. Apocolpium convex. Garside’s rule? Exine tectate, with granular acolumellate infratectum, microperforate or smooth.
Gynoecium Pistil composed of three connate carpels. Ovary superior to semi-inferior, usually unilocular (sometimes trilocular at base), with or without ridges. Style single, simple, short to long (heterostyly present in ‘Dulacia’). Stigma slightly trilobate, type? Pistillodium?
Ovules Placentation free central (axile when ovary trilocular). Ovule one per carpel, anatropous, unitegmic or ategmic, tenuinucellar. Integument five or six cell layers thick. Nucellar cap sometimes present in Olax. Megagametophyte in Olax disporous, 8-nucleate, Allium type. Synergids with a filiform apparatus. Endosperm development at least in Olax helobial. Endosperm haustorium chalazal, often very long (in Olax sometimes reaching into pedicel). Embryogenesis?
Fruit A drupe or nut-like fruit, often with persistent calyx (in Olax sometimes surrounded by strongly accrescent calyx).
Seeds Aril absent. Testa present. Exotesta? Endotesta? Perisperm not developed. Endosperm copious, oily (in ‘Dulacia’ also starchy). Embryo very small, straight, with chlorophyll? Cotyledons one or absent. Germination phanerocotylar or cryptocotylar.
Cytology n = 12, (24?) (Olax)
DNA
Phytochemistry Insufficiently known. Alkaloids, saponins, cyanogenic compounds and triglycerides of polyacetylenic fatty acids present. Manicol (a hydroxytropolon) and tannins (phenylpropanoids) present in Olax.
Use Fruits, medicinal plants.
Systematics Ptychopetalum (2; P. olacoides, P. uncinatum; Amazonas), Olax (40–55; tropical and southern Africa, Madagascar, tropical Asia, Australia, New Caledonia; tropical America [‘Dulacia’]; probably incl. Dulacia with 13 species in tropical South America).
OPILIACEAE (Benth.) Val. |
( Back to Santalales ) |
Anthobolaceae Dumort., Anal. Fam. Plant.: 15, 17. 1829 [’Anthoboleae’]; Anthobolales Dumort., Anal. Fam. Plant.: 15. 1829 [‘Anthobolariae’]; Cansjeraceae J. Agardh, Theoria Syst. Plant.: 238. Apr-Sep 1858 [’Cansiereae’]
Genera/species 12/c 40
Distribution Mexico to tropical and subtropical South America (Agonandra), Madagascar, South and Southeast Asia, southeastern China, Taiwan, Malesia to New Guinea, Solomon Islands, northern Australia, with their highest diversity in tropical Asia.
Fossils Unknown.
Habit Usually bisexual (in Agonandra and Gjellerupia dioecious), evergreen trees and shrubs (sometimes lianas). Root hemiparasites.
Vegetative anatomy Mycorrhiza absent. Phellogen ab initio subepidermal. Secondary lateral growth normal. Vessel elements with simple perforation plates; lateral pits alternate, bordered pits. Imperforate tracheary xylem elements usually tracheids (in Lepionurus fibre tracheids) with simple or bordered pits, non-septate (also vasicentric tracheids). Wood rays uniseriate or multiseriate, heterocellular. Axial parenchyma diffuse. Wood often fluorescent. Sieve tube plastids ? type. Nodes usually 1:3 or 1:5 unilacunar with three or five leaf traces (sometimes 1:1, unilacunar with one trace). Wood ray cells without silica bodies. Wood cystoliths often present. Prismatic crystals present. Druses present in some species.
Trichomes Hairs unicellular or multicellular, uniserial or branched (sometimes dendritic), or absent.
Leaves Alternate (spiral or distichous), simple, entire, with ? ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle transection arcuate? Venation pinnate, camptodromous. Stomata usually paracytic, in Anthobolus transversely orientated. Cuticular waxes? Mesophyll often with mucilage cells, without sclerified cell walls. Cystoliths usually present (visible as small bulges on the lamina; possibly absent in Anthobolus). Cell walls sometimes silicified. Calciumoxalate crystals absent. Leaf margin entire.
Inflorescence Axillary, spike-, raceme-, umbel- or catkin-like, or paniculate. Bracts in Agonandra and Opilia large and peltate.
Flowers Actinomorphic, small. Hypanthium present or absent. Usually hypogyny (possibly secondary; sometimes half epigyny). Calyculus usually present (absent in Lepionurus). Sepals four or five, with open to valvate aestivation, very small, persistent, largely connate, or absent (especially in female flowers). Petals (three or) four or five (or six), with valvate aestivation, usually free (sometimes connate at base into a tube; in female flowers of Gjellerupia absent). Nectaries free or connate only at base, alternating with stamens, or absent. Disc consisting of separate parts, as many as and alternating with stamens.
Androecium Stamens four or five, haplostemonous, usually alternisepalous, antepetalous. Filaments free from each other, free from or adnate in lower part to petals. Anthers dorsifixed, usually non-versatile, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory. Staminodia absent.
Pollen grains Microsporogenesis simultaneous. Pollen grains usually tricolporate (sometimes tricolporoidate), shed as monads, bicellular at dispersal. Apocolpium convex. Exine usually semitectate, usually with columellate infratectum, reticulate (rarely microperforate), psilate to microechinate.
Gynoecium Pistil composed of two to five connate carpels. Ovary usually superior (sometimes semi-inferior), unilocular. Style single, simple, short, or absent. Stigma capitate, type? Pistillodium absent.
Ovules Placentation usually free central (in Agonandra basal). Ovule usually one (rarely two) per ovary, anatropous to orthotropous, usually pendulous (in Agonandra ascending), ategmic or unitegmic, tenuinucellar, or megasporangium undifferentiated. Integument ? cell layes thick or absent. Megagametophyte monosporous, Polygonum typ (megagametophytes sometimes several). Synergids with a filiform apparatus. Endosperm development ab initio cellular. Endosperm haustorium chalazal (often elongate). Embryogenesis?
Fruit A one-seeded drupe.
Seeds Aril absent. Testa and tegmen absent. Perisperm not developed. Endosperm copious, oily (and starchy?). Embryo sometimes relatively small, well differentiated, chlorophyll? Cotyledons usually three or four (sometimes two). Radicula minute. Germination cryptocotylar.
Cytology n = 10
DNA Plastid gene infA lost/defunct (Lepionurus). Mitochondrial coxI intron present (Lepiurus).
Phytochemistry Virtually unknown. Tannins? Saponins not found.
Use Timber, edible fruits, medicinal plants.
Systematics Agonandra (c 10; Central America, tropical South America), Gjellerupia (1; G. papuana; New Guinea); Anthobolus (3; A. filifolius, A. foveolatus, A. leptomerioides; central and northern Australia); Champereia (1; C. manillana; Yunnan, Guangxi, Taiwan, the Andaman and Nicobar Islands, Burma, Thailand, Vietnam, Malesia to the Philippines and New Guinea), Melientha (1; M. suavis; Yunnan, Southest Asia to the Philippines), Yunnanopilia (1; Y. longistaminea; Yunnan, Guangxi, Laos, northern Vietnam); Lepionurus (1; L. sylvestris; Nepal, Bhutan, northeastern India, southern Yunnan, Burma, Thailand, Indochina, Malesia to New Guinea), Urobotrya (7; U. congolana, U. floresensis, U. latisquama, U. longipes, U. parviflora, U. siamensis, U. sparsiflora; tropical Africa, Southeast Asia, Malesia to Flores), Cansjera (3; C. leptostachya, C. parvifolia, C. rheedei; India, Sri Lanka, Nepal, Andaman and Nicobar Islands, southern China, South and Southeast Asia, Malesia to New Guinea, the Bismarck Archipelago, Solomon Islands, tropical Australia), Rhopalopilia (3; R. altescandens, R. hallei, R. pallens; Central Africa), Pentarhopalopilia (4; P. madagascariensis, P. marquesii, P. perrieri, P. umbellulata; tropical Central and coastal East Africa, Madagascar), Opilia (5; O. afzelii, O. amentacea, O. campestris, O. congolana, O. fragrans; tropical Africa, Madagascar, India, Sri Lanka, Yunnan, Southeast Asia, the Philippines, New Guinea, tropical Australia).
The division of the endosperm nucleus results at least in species of Cansjera in one tubular chalazal and one micropylar chamber. The chalazal chamber functions as a haustorium, extending to the ovary base; the apex proliferates and forms branches between the cells. The micropylar chamber develops into the endosperm.
 |
Cladogram of Opiliaceae based on DNA sequence data (Der & Nickrent 2008; Su & al. 2015). Yang & al. (2018) recovered Agonandra as sister to the remaining Opiliaceae (Anthobolus was not included) and Lepionurus as sister to Cansjera; Champereia was sister to Melientha+Yunnanopilia. |
 |
Cladogram of Opiliaceae based on DNA sequence data (Le & al. 2018). The support for Anthobolus as sister to the [Champereia+[Melientha+Yunnanopilia]] clade and the [Agonandra+Gjellerupia] clade as sister to the rest are weak |
SANTALACEAE R. Br. |
( Back to Santalales ) |
Viscaceae Batsch, Tab. Affin. Regni Veg.: 240. 2 Mai 1802 [’Viscinae’]; Thesiaceae Vest, Anleit. Stud. Bot.: 270, 289. 1818 [’Thesioideae’]; Osyridaceae Raf. in Ann. Gén. Sci. Phys. Bruxelles 5: 348. Jul-Sep 1820 [’Osyridia’]; Viscales Bercht. et J. Presl, Přir. Rostlin: 257. Jan-Apr 1820 [‘Viscaceae’]; Osyridales Link, Handbuch 1: 371. 4-11 Jul 1829 [’Osyrinae’]; Canopodaceae C. Presl, [Epimel. Bot.: 248] in Abh. Königl. Böhm. Ges. Wiss., ser. 5, 6: 608. ante Oct 1851 [’Canopiaceae’]; Exocarpaceae J. Agardh, Theoria Syst. Plant.: 317. Apr-Sep 1858 [’Exocarpeae’]; Phoradendraceae H. Karsten, Fl. Columb. 1: 73. 13 Feb 1860 [’Phoradendreae’]; Arceuthobiaceae Tiegh. in Bot. Jahresber. (Just) 23(2): 290. Oct-Dec 1897; Ginalloaceae Tiegh. in Bot. Jahresber. (Just) 25(2): 406. 19 Jan 1900; Bifariaceae Nakai in Bull. Natl. Sci. Mus. Tokyo 31: 46. Mar 1952; Eremolepidaceae Tiegh. ex Nakai in Bull. Natl. Sci. Mus. Tokyo 31: 48. Mar 1952; Lepidocerataceae Nakai in Bull. Natl. Sci. Mus. Tokyo 31: 45. Mar 1952 [’Lepidoceraceae’]
Genera/species c 41/950–1.030
Distribution Mostly tropical and subtropical regions, temperate parts in the Northern and Southern Hemispheres, with their largest diversity in semiarid climates.
Fossils Flowers similar to Thesium and male flowers of Osyris were found in Baltic amber.
Habit Bisexual, monoecious or dioecious, usually evergreen trees or shrubs, or perennial herbs, sometimes with distinct sympodial growth. Root or stem hemiparasites (Phacellaria and some species of Viscum are hyperparasites, parasitizing on Loranthaceae or Amphorogynaceae). Sometimes spiny or xeromorphic. Branches sometimes photosynthesizing phyllocladia. Viscum minimum (Eastern Cape province) has aerial stems with a length of c. 3 mm and with a single internode; these stems arise from the endophytic part of the plant.
Vegetative anatomy Mycorrhiza usually absent. Normal roots often absent. With or without epicortical roots; roots modified into haustoria (sometimes hypha-like) often intruding into and branching within host tissue. Phellogen ab initio subepidermal (absent when cuticular epithelium present). Secondary lateral growth normal. Vessel elements usually with simple (sometimes scalariform) perforation plates; lateral pits alternate or opposite, simple and/or bordered pits. Imperforate tracheary xylem elements tracheids, fibre tracheids or libriform fibres (sometimes very long) with simple or bordered pits, non-septate (also vasicentric tracheids). Wood rays uniseriate or multiseriate, homocellular or heterocellular. Axial parenchyma apotracheal diffuse or diffuse-in-aggregates, or paratracheal scanty or banded, or absent. Axial parenchyma bundles absent. Wood elements often storied. Tyloses sometimes common. Sieve tube plastids Ss type. Nodes 1:1, unilacunar with one leaf trace. Wood ray cells usually without silica bodies. Wood cystoliths absent. Cuticular epithelium (without suberin and lenticels) abundant. Stomata of stem transversely oriented. Secretory cavities present or absent. Gum-like substances present in heartwood in, e.g., Santalum. Prismatic calciumoxalate crystals often abundant. Druses present in parenchyma cells in some species.
Trichomes Hairs usually unicellular (rarely multicellular, uniseriate, dendritic or stellate).
Leaves Alternate (spiral) or opposite, simple, entire, often coriaceous, sometimes isobilateral, sometimes scale-like or modified into spines, with ? ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle transection arcuate? Venation usually pinnate (sometimes parallel or leaves one-veined), eucamptodromous or brochidodromous. Stomata usually paracytic (sometimes anomocytic); guard cell thickenings? Cuticular wax crystalloids usually as platelets, often with irregular edges (sometimes as transversely ridged annular rodlets), chemically characterized by presence of palmitone (hentriacontan-16-one) etc. Lamina often gland-dotted. Epidermal cells often sclerified, with druses, tannins or mucilage. Mesophyll cells sometimes with sclereids. Cell walls sometimes silicified. Leaf margin entire (sometimes serrate, in Ionidium with spines).
Inflorescence Terminal or axillary, spike-, raceme- or head-like, or fasciculate, or spikes or racemes, or flowers solitary axillary.
Flowers Actinomorphic, usually small. Hypanthium present or absent (calyculus absent in Nanodeaceae). Usually epigyny (rarely hypogyny or half epigyny). Sepals very small, with open or valvate aestivation, or absent (often as a narrow border on top of ovary). Petals (two to) four or five (to eight; rarely absent), with valvate aestivation, free or connate into a tube or a cup, often with adaxial hairs. Nectaries often present on adaxial side of hypanthium or corolla, lobed (staminodial?), often alternating with stamens (sometimes in Thesiaceae). Disc usually intrastaminal (sometimes absent).
Androecium Stamens (two to) four or five (to eight), haplostemonous, antepetalous. Filaments free from each other, free from or adnate to tepals in lower part. Anthers basifixed or dorsifixed, sometimes versatile, usually tetrasporangiate (rarely disporangiate), introrse or latrorse, longicidal (usually dehiscing by longitudinal slits; sometimes poricidal or with several short transverse slits; anthers rarely, in, e.g., Korthalsella, connate into a synandrium). Tapetum secretory. Female flowers often with staminodia.
Pollen grains Microsporogenesis simultaneous. Pollen grains tricolpate, tricolpor(oid)ate or triporate, shed as monads, bicellular or tricellular at dispersal. Apocolpium convex. Exine tectate or semitectate, in at least Okoubaka with granular acolumellate infratectum, perforate or reticulate, sometimes echinate.
Gynoecium Pistil composed of (two or) three (to five) connate carpels; median carpel abaxial. Ovary usually inferior (rarely superior or semi-inferior), usually unilocular (sometimes multilocular below). Style single, simple, with stylar canal, or absent. Stigma lobate or capitate, type? Male flowers often with pistillodium.
Ovules Placentation free central or basal, often as mamelon (placental megasporangial complex), or ovary with undifferentiated placenta with sporogeneous tissue, one or two megagametophytes being disporous, Allium type. Ovules one to five per ovary, usually orthotropous or anatropous (sometimes hemianatropous), pendulous, ategmic or unitegmic, tenuinucellar or without differentiated megasporangium (ovule often poorly differentiated). Integument ? cell layers thick or absent. Megagametophyte monosporous, Polygonum type, or disporous, Allium type. Antipodal cells one trinucleate, or three uninucleate. Endosperm development usually cellular (sometimes helobial). Endosperm haustorium chalazal or from lateral endosperm cells (sometimes micropylar). Embryogenesis of various types, often irregular, complex and of unusual type (sometimes as transverse cell divisions).
Fruit A berry-like fruit with viscid layers of tissue, a nut or a one-seeded drupe with stony mesocarp (in Viscaceae elastic or passively dehiscing, usually without gum).
Seeds Seed surrounded by sticky viscin tissue (or in radicle end). Aril absent. Testa and tegmen absent. Perisperm not developed. Endosperm copious, fleshy, oily or sometimes starchy, with or without chlorophyll. Suspensor short or absent. Embryo small to large, straight, often well differentiated (in Viscaceae poorly differentiated), usually with chlorophyll (absent in Lepidoceras). Cotyledons two or absent. Germination phanerocotylar or cryptocotylar.
Cytology n = 5–7, 10, 13
DNA The mitochondrial gene coxII.i3 lacks an intron in at least Comandra.
Phytochemistry Flavonols (quercetin, myricetin), cyanidin, proanthocyanidins (prodelphinidins), gallic acid (Okoubaka), pyrrolizidine alkaloids as esters of arylic and aralkylic acids (at least in Thesium) and other alkaloids, sesquiterpene alcohols, polyacetylenes, and triglycerides of acetylenic fatty acids present. Tannins (phenylpropanoids) present in Santalaceae. Tyramine and phenylethylamine as well as glycoproteins (viscotoxins, phoratoxins) often abundant in Viscaceae. Ellagic acid, cyanogenic compounds and saponins not found.
Use Perfumes (sandal oil from wood and roots of Santalum album etc.), incense (Santalum), bird-lime (Viscum etc.), medicinal plants, fruits (Acanthosyris, Exocarpos), timber, carpentries, carvings, decoration (Phoradendron, Viscum).
Systematics Santalaceae are sister-group to Opiliaceae.
The Comandraceae clade [Comandra+Geocaulon] may be basal within the Santalaceae, although further DNA sequence analyses are needed before this hypothesis can be confirmed.
Stem/branch parasites have probably evolved three times: in Santalaceae, Amphorogynaceae and Viscaceae.
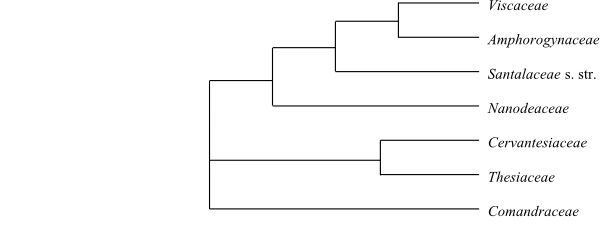 |
Cladogram (simplified) of Santalaceae sensu lato based on DNA sequence data (Nickrent & al. 2010; Su & al. 2015). |
Comandraceae Nickrent et Der in D. L. Nickrent et al., Taxon 59: 550. 4 Apr 2010
2/2. Comandra (1; C. umbellata; the Balkan Peninsula to Romania, Canada, United States, northern Mexico), Geocaulon (1; G. lividum; Alaska, Canada, northeastern United States). – North America, the Balkan Peninsula to Romania. Bisexual (Comandra), or monoecious or andromonoecious (Geocaulon). Root hemiparasitic perennial herbs. Leaves alternate, monomorphic. Inflorescence terminal or axillary, simple or compound dichasia arranged in panicles, cymes or corymbs. Floral prophyll (bracteole) single. Hypanthium sometimes present. Epigyny. Calyx and calyculus absent. Petals (three to) five (to seven), connate at base into a campanulate or urceolate corolla, in bisexual and male flowers with adaxial hairs. Nectariferous disc intrastaminal, lobate; lobes as nectariferous glands alternating with stamens. Stamens (three to) five (to seven), antepetalous. Anthers dorsifixed, tetrasporangiate, longicidal. Female flowers of Geocaulon with staminodia. Ovary inferior, unilocular. Style filiform or short conical. Stigma subcapitate or punctate. Placentation free central. Ovules two to four, anatropous, pendulous, unitegmic. Megagametophyte with lateral caecum. Endosperm development cellular. Endosperm haustoria micropylar. First zygote division vertical. Fruit a one-seeded, dry nutlet or pseudodrupe with persistent perianth parts, in Comandra with coriaceous exocarp and bony mesocarp (endocarp absent, consumed by endosperm), in Geocaulon with fleshy mesocarp. Suspensor absent from embryo. n = 13, 14, (26). Mitochondrial intron coxII.i3 lost (Comandra).
Thesiaceae Vest, Anleit. Stud. Bot.: 270, 289. 1818 [’Thesioideae’]
3/c 350. Buckleya (4; B. angulosa, B. graebneriana, B. lanceolata: China, Japan; B. distichophylla: southeastern United States), Osyridicarpos (1; O. schimperianus; tropical and southern Africa), Thesium (c 340; temperate and subtropical regions in the Old World). – Temperate and subtropical regions. Bisexual or dioecious. Root hemiparasitic shrubs or perennial or annual herbs. Leaves alternate or opposite, monomorphic, often scale-like or ericoid. Inflorescence terminal or axillary, monochasium, spike-, umbel- or raceme-like, simple or compound dichasia, or flowers solitary. Epigyny. Calyx usually absent or as calyculus (rarely lobed). Petals four or five, sometimes connate in lower part, in bisexual and male flowers usually with adaxial hairs. Nectariferous disc usually present in bisexual and female flowers. Stamens four or five. Anther dorsifixed, tetrasporangiate, longicidal. Staminodia absent. Pollen grains heteropolar. Pistil composed of three connate carpels. Ovary inferior, unilocular, or multilocular in lower part and unilocular in upper part. Stigma capitate or bilobate to quadrilobate. Pistillodium absent. Ovules two to five, unitegmic or ategmic. Endosperm haustoria chalazal. Fruit a one-seeded achene or pseudodrupe with persistent perianth parts; mesocarp bony; endocarp crustaceous. Fruiting pedicel in Thesium sometimes modified into an elaiosome-like structure. Endosperm starchy in Thesium. Suspensor present. n = 6- 9, 12.
 |
Cladogram of Thesiaceae (Der & Nickrent 2008; Su & al. 2015). |
Cervantesiaceae Nickrent et Der in D. L. Nickrent et al., Taxon 59: 551. 4 Apr 2010
8/c 21. Cervantesia (2; C. bicolor, C. tomentosa; the northern Andes in Colombia to Bolivia), Jodina (1; J. rhombifolia; southern Brazil, Uruguay, Argentina), Acanthosyris (6; A. annonagustata, A. asipapote, A. falcata, A. glabrata, A. paulo-alvinii, A. spinescens; northwestern South America to southern Brazil, Uruguay, Paraguay and northern Argentina); Pyrularia (2; P. edulis: the Himalayas, China, P. pubera: eastern United States), Pilgerina (1; P. madagascariensis; Madagascar), Staufferia (1; S. capuronii; Madagascar), Scleropyrum (c 6; S. aurantiacum, S. leptostachyum, S. maingayi, S. moschiferum, S. pentandrum, S. ridleyi; tropical Asia), Okoubaka (2; O. aubrevillei, O. michelsonii; tropical Africa). – Tropical Africa, Madagascar, tropical Asia to China, southeastern United States, South America. Bisexual, dioecious or polygamous. Root hemiparasitic trees and shrubs. Axillary branches sometimes modified into spines. Leaves alternate, simple. Inflorescence monochasia, spike- or raceme-like, panicle, fasciculate, or flowers solitary. Calyx and calyculus absent. Hypogyny, epigyny or half epigyny. Petals four or five, with adaxial hairs. Nectariferous disc lobate; lobes as nectariferous glands alternating with stamens. Stamens four or five. Anthers dorsifixed or basifixed, tetrasporangiate, longicidal. Female flowers with staminodia. Pistil composed of three connate carpels. Ovary superior, inferior or semi-inferior, unilocular. Style long, short or absent. Stigma capitate, infundibuliform, peltate, scutiform, or bilobate to quinquelobate. Male flowers with pistillodium. Ovules one to three, unitegmic or ategmic. Endosperm haustoria chalazal. Fruit a one-seeded pseudodrupe with persistent perianth parts (outer parts of exocarp dehiscing in Iodina); mesocarp stony; endocarp wall absent (consumed by endosperm). Suspensor absent. n = 36.
 |
Cladogram of Cervantesiaceae (Der & Nickrent 2008). Jodina was recovered as sister to [Acanthosyris+Cervantesia] by Su & al. (2015). |
Nanodeaceae Nickrent et Der in D. L. Nickrent et al., Taxon 59: 552. 4 Apr 2010
2/2. Mida (1; M. salicifolia; Juan Fernandez Islands, North Island of New Zealand), Nanodea (1; N. muscosa; Chile). – New Zealand, Chile, Juan Fernadez Islands. Bisexual (Nanodea) or gynodioecious (Mida). Root hemiparasitic tree (Mida) or small shrub (Nanodea). Leaves alternate or opposite. Inflorescence axillary, few-flowered raceme-like (Mida), or flowers solitary axillary (Nanodea). Epigyny (Nanodea) or half epigyny (Mida). Calyculus absent. Calyx reduced or absent. Petals (four or) five (or six), caducous (Mida), with adaxial hairs. Nectariferous disc lobate; lobes as nectariferous glands alternating with stamens. Stamens (four or) five (or six), antepetalous. Anthers dorsifixed, tetrasporangiate, longicidal. Female flowers in Mida with staminodia. Ovary inferior or semi-inferior, unilocular. Stigma in Mida bilobate to quadrilobate. Ovules two or three, hemianatropous, ategmic. Endosperm haustorium chalazal. Fruit a one-seeded pseudodrupe with persistent perianth parts; exocarp and endocarp parenchymatous; mesocarp stony or lignified. Suspensor present. n = 33.
Santalaceae Brown, Prodr. Fl. Nov.-Holl.: 350. 27 Mar 1810, nom. cons., sensu stricto
Eremolepidaceae Tiegh. ex Nakai in Bull. Natl. Sci. Mus. Tokyo 31: 48. Mar 1952
10–11/c 65. Omphacomeria (1; O. acerba; eastern New South Wales, eastern Victoria, southern Tasmania?; in Exocarpos?), Exocarpos (27; Southeast Asia, Malesia to New Guinea, Australia, New Caledonia, the Hawaiian Islands; incl. Omphacomeria?), Myoschilos (1; M. oblongum; Chile), Antidaphne (9; western tropical South America), Eubrachion (2; E. ambiguum, E. gracile; the West Indies, South America), Lepidoceras (2; L. peruvianum: Peru; L. chilense: Chile), Santalum (12; tropical Asia, Australia to the Hawaiian Islands, one species, S. fernandezianum, extinct in Juan Fernández Islands), Osyris (3; O. alba, O. lanceolata, O. speciosa; the Mediterranean, Africa, southwestern Asia to India), Nestronia (1; N. umbellula; southeastern United States), Colpoon 6–7; C. compressum, C. speciosum; Africa to India), Rhoiacarpos (1; R. capensis; eastern Western Cape, Eastern Cape, KwaZulu-Natal). – The Mediterranean, Africa, southwestern and tropical Asia, Australia, New Caledonia, the Hawaiian Islands, southeastern United States and the West Indies to Chile. Bisexual, monoecious, andromonoecious, dioecious, or androdioecious. Root hemiparasitic trees or shrubs, or stem parasitic shrubs (Antidaphne, Eubrachion, Lepidoceras). Leaves alternate or opposite, persistent or deciduous, sometimes dimorphic, sometimes scale-like. Foliar cystoliths absent. Inflorescence terminal or axillary, monochasia, spike- or umbel-like, simple or compound dichasia, panicle, fasciculate, or flowers solitary. Epigyny or half epigyny. Calyx usually absent (calyculus present in Myoschilos). Petals three to six, free or connate into a campanulate or urceolate corolla, often with adaxial hairs. Nectariferous disc usually indistinct or absent (in Osyris lobate, with lobes as nectariferous glands alternating with stamens). Stamens three to five, antepetalous. Anthers dorsifixed or basifixed, tetrasporangiate, longicidal. Female flowers sometimes with staminodia. Secondary pollen display present in Santalum (pollen collecting hairs on filaments). Ovary inferior or semi-inferior, unilocular, or multilocular in lower part and unilocular in upper part. Stigma capitate, or bilobate to quinquelobate. Male flowers sometimes with pistillodium. Ovules one to five, ategmic, consisting of megagametophyte. Megagametophyte developing from placenta or mamelon, with long-protruding apex. Endosperm haustorium chalazal (in Exocarpus multicellular). Fruit a one-seeded drupe or pseudo-drupe, or a one-seeded viscid berry, often with persistent perianth parts; mesocarp stony. Upper part of fruiting pedicel in Exocarpos and Omphacomeria fleshy and swollen. Suspensor present. n = 10, 11, 13, 15, 20, 20+B.
The clade [Exocarpos+Omphacomeria], with hypogyny and epigyny, respectively, is basal. The clade [Antidaphne+Eubrachion+Lepidoceras] (‘Eremolepidaceae’) is characterized by: cuticular epithel developing; stem stomata transversely orientated; leaves spiral; inflorescence raceme-like; pollen grains spheroidal; placenta indistinct; ovules basal; megagametophyte disporous; fruit often completely covered with viscin; and endosperm green. Nestronia is sister to the clade [Colpoon+Rhoiacarpus].
Some Santalaceae in the strict sense have vascular bundles running backwards inside the gynoecium, possibly indicating receptacular epigyny. Periderm is absent during development of the cuticular epithel, and the epithel (having various origins) lacks suberine and lenticels; it occurs in Viscaceae and some Santalaceae (e.g. Antidaphne).
 |
Cladogram of Santalaceae sensu stricto (Der & Nickrent 2008). |
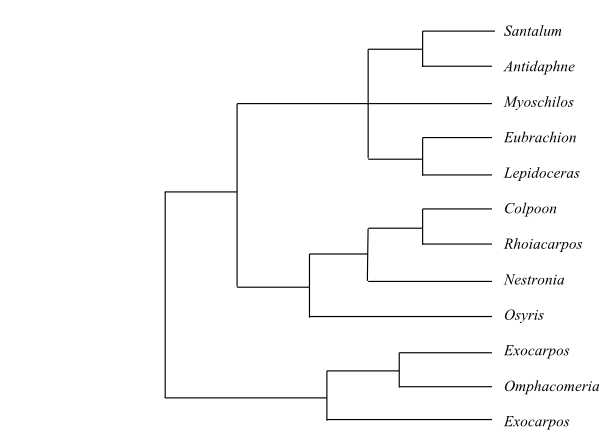 |
Cladogram of Santalaceae sensu stricto (Su & al. 2015). |
Amphorogynaceae (Stauffer ex Stearn) Nickrent et Der in D. L. Nickrent et al., Taxon 59: 552. 4 Apr 2010
9/54. Amphorogyne (3; A. celastroides, A. spicata, A. staufferi; New Caledonia), Daenikera (1; D. corallina; New Caledonia), Choretrum (7; C. candollei, C. chrysanthum, C. glomeratum, C. lateriflorum, C. pauciflorum, C. pritzelii, C. spicatum; southwestern Western Australia), Spirogardnera (1; S. rubescens; southwestern Western Australia), Leptomeria (17; Western Australia to southeastern Queensland and Tasmania, with their highest diversity in southwestern Western Australia), Phacellaria (6; P. caulescens, P. compressa, P. fargesii, P. glomerata, P. rigidula, P. tonkinensis; Burma, southern and southwestern China, Southeast Asia), Dufrenoya (7; D. aurantiaca, D. longicuneata, D. papillosa, D. poilanei, D. pruinosa, D. robusta, D. sessilis; tropical Asia), Dendrotrophe (7; D. amorpha, D. buxifolia, D. granulata, D. platyphylla, D. polyneura, D. umbellata, D. varians; the Himalayas, southern China, Southeast Asia, Malesia to tropical Australia), Dendromyza (9; Southeast Asia, Malesia to Queensland). – South and Southeast Asia, Malesia to Australia, New Caledonia, with their largest diversity in Australia. Bisexual or dioecious. Root hemiparasitic trees or root and stem hemiparasitic (amphiphagous) shrubs, stem hemiparasitic lianas, or stem hemiparasitic shrubs (Phacellaria comprises hyperparasites on Loranthaceae and Dendrophthoe). Leaves alternate, sometimes dimorphic, persistent or deciduous, sometimes scale-like. Inflorescence terminal or axillary, monochasia, spike-, umbel- or raceme-like, panicle, or flowers solitary. Epigyny. Calyx and calyculus absent. Petals four to six, usually with adaxial hairs, sometimes connate in lower part. Nectariferous disc present, often lobate (lobes as nectariferous glands alternating with stamens). Stamens four to six. Filaments short or absent. Anthers dorsifixed, tetrasporangiate, longicidal; loculi pairwise above one another, anisomerous: two anterior loculi and two posterior loculi, each loculus dehiscing separately. Female flowers sometimes with staminodia. Ovary inferior, unilocular to sexalocular in lower part, unilocular in upper part. Style short (sometimes hollow) or absent. Stigma trilobate to quinquelobate. Male flowers sometimes with pistillodium. Placentation free central. Ovules two to five, ategmic, consisting of megagametophyte. Fruit a one-seeded drupe or pseudo-drupe, with persistent perianth; exocarp forming seed attachment structures in aerial parasites; mesocarp fleshy, fibrous, forming seed attachment structures in aerial parasites; endocarp stony, with crustaceous, bony, woody, or cartilaginous wall. Suspensor present. n = ? Pyrrolizidine alkaloids present.
Amphorogynaceae – with root or stem parasites – are sister-group to Viscaceae. Leptomeria drupacea (Labill.) Druce (Australia) develops megagametophyte-like structures in its microsporangia.
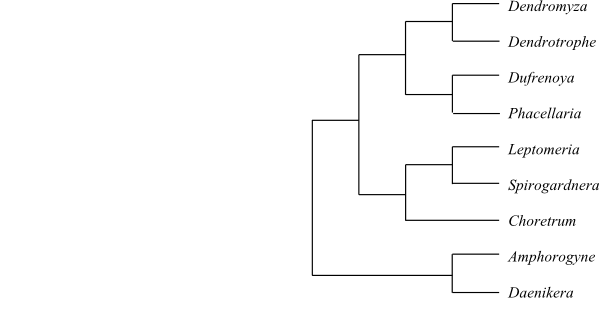 |
Cladogram of Amphorogynaceae (Der & Nickrent 2008; Su & al. 2015). |
Viscaceae Batsch, Tab. Affin. Regni Veg.: 240. 2 Mai 1802 [’Viscinae’]
c 7/460–540. Korthalsella (11–15; Northeast and East Africa, Madagascar, the Mascarene Islands, the Himalayas, East Asia to Japan, New Zealand, the Hawaiian Islands), Arceuthobium (40; the Mediterranean, northeastern tropical Africa, eastern Himalayas, China, Southeast Asia, West Malesia, North America, Central America, the West Indies), Notothixos (8; Sri Lanka, Burma, Southeast Asia, Malesia to the Philippines and southeastern Australia, the Santa Cruz Islands), Viscum (c 75; temperate to tropical regions in the Old World), Ginalloa (9; tropical Asia), ‘Phoradendron’ (200–270; warm-temperate to tropical America; non-monophyletic), ‘Dendrophthora’ (120–130; tropical America non-monophyletic). – Subcosmopolitan. Monoecious, andromonoecious or dioecious. Stem (aerial) hemiparasitic shrubs (some species of ‘Phoradendron’ parasitize other species of ‘Phoradendron’). Some species are endophytic. Often with haustoria branching through host; no roots running on host surface. Cuticular epithelium developing. Stems articulated, with transversely orientated stomata. Leaves opposite, often scale-like. Secondary veins usually palmate. Cuticular wax crystalloids usually as platelets with irregular margins. Mesophyll with idioblasts containing sclereids and silicified cell walls. Inflorescence terminal or axillary, spike, simple or compound dichasium, fasciculate. Female flowers usually lateral in monoecious inflorescences, solitary in reduced dichasia of dioecious species; male flowers usually central in monoecious inflorescences, in triads in dichasia of dioecious species. Bracts and floral prophylls (bracteoles) not inserted immediately below ovary. Epigyny. Calyx and calyculus absent. Petals usually three in female flowers and four (to six) in male flowers, with valvate aestivation. Male flowers with nectariferous disc (absent or nearly so in female flowers). Stamens three or four, antepetalous, adnate at base to petal. Filaments short or absent. Anthers tetrasporangiate and longicidal, or disporangiate and poricidal (dehiscing by terminal pores or pore-like slits), or transversely septate and dehiscing by several transverse slits, or connate into a synandrium (Viscum). Pistil composed of three or four connate carpels. Ovary inferior, unilocular or without distinct locule due to massive mamelon. Style short or absent. Normal ovules absent, consisting of two megagametophytes per flower. Megagametophyte embedded in mamelon. Megagametophytes disporic, 8-nucleate, Allium type. First zygote division vertical (Arceuthobium, Korthalsella). Fruit a usually one-seeded (sometimes two-seeded) berry (in Arceuthobium explosively dehiscent), with usually thin endocarp. Testa absent. Incomplete viscid layer present inside vascular bundles of seed; viscin consisting of polysaccharide seed-attaching threads interspersed with mucilaginous pectic/mucopolysaccharide substances. Endosperm with chlorophyll, starchy, developing from primary endosperm nucleus of one of megagametophytes. Suspensor short or absent. Embryos one to four per seed, with chlorophyll. Cotyledon seemingly one (in reality two connate). n = 10, 12-14(-17), 20. Toxic polypeptides present. Several dioecious species of Viscum have translocation heterozygosity determining sexuality and sex ratios. A very large genome with a C value of c. 350 pg or more is only known in Viscum.
Expanded wood roses may be produced by the host, although only where the parasite is infecting. Arceuthobium are notorious parasites on conifers, causing large witches’ brooms and often killing the host. Some Viscaceae are wind pollinated.
Viscaceae have the most obvious reductions of the placental column, without a real placenta but with a placental nucellar complex, mamelon, in which a single functional megasporocyte is embedded. A similar reductional trend can be found in the placenta of many Loranthaceae, e.g. Helixanthera, in which the placenta is entirely absent and the archesporial cells differentiate at the base of the ovarian cavity.
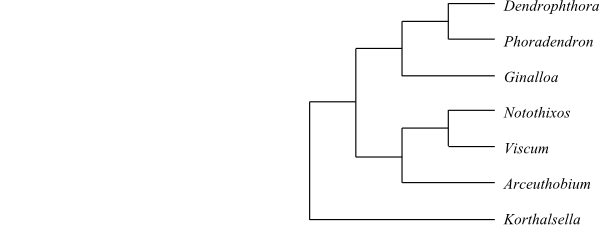 |
Cladogram of Viscaceae (Der & Nickrent 2008). |
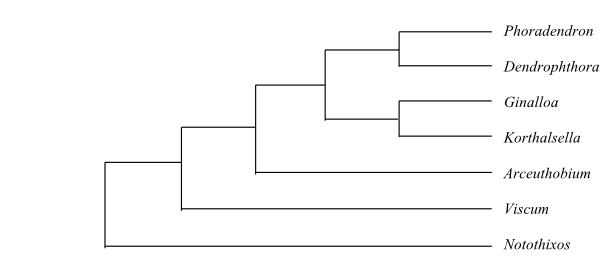 |
Cladogram of Viscaceae (Su & al. 2015). |
SCHOEPFIACEAE Blume |
( Back to Santalales ) |
Arjonaceae Tiegh. in Bot. Jahresber. (Just) 24(2): 279. 1898
Genera/species 3/38–45
Distribution Southeastern China, Hainan, southern Japan, the Korean Peninsula, Taiwan, Indochina, northern Sumatra, Mexico, Central America, the West Indies, tropical and temperate South America.
Fossils Unknown.
Habit Usually bisexual (sometimes unisexual), evergreen trees or shrubs, or perennial herbs (many species of Quinchamalium). Root hemiparasites.
Vegetative anatomy Mycorrhiza absent. Phellogen ab initio subepidermal? Vessel elements with simple perforation plates; lateral pits alternate, simple or bordered pits. Imperforate tracheary xylem elements tracheids or libriform fibres with simple or bordered pits, non-septate (also vasicentric tracheids). Wood rays uniseriate or multiseriate, homocellular. Axial parenchyma apotracheal diffuse or diffuse-in-aggregates, or paratracheal aliform, confluent, winged-aliform or lozenge-aliform. Cambium, vessel elements and/or axial parenchyma storied. Sieve tube plastids ? type. Nodes 1:1, unilacunar with one leaf trace. Cystoliths absent. Crystals?
Trichomes Hairs unicellular or absent.
Leaves Alternate (spiral), simple, entire, often coriaceous, with ? ptyxis. Stipules and leaf sheath absent. Petiole without asterosclereids. Petiole vascular bundle simple. Venation pinnate, brochidodromous. Stomata paracytic, parallelocytic, cyclocytic, anisocytic or anomocytic. Cuticular waxes? Epidermal cells with tannins or mucilage, sclerified. Epidermal cell walls often silicified, not lignified. Leaf margin entire.
Inflorescence Terminal, capitate or spicate (in Quinchamalium and Arjona), or (in Schoepfia) axillary head-, raceme-, umbel- or spike-like, cymose, or flowers solitary. Bracts and floral prophylls (bracteoles) arising immediately below ovary (resembling those in Loranthaceae).
Flowers Actinomorphic. Uppermost part of receptacle together with two connate floral prophylls in Quinchamalium and Schoepfia modified into calyculus (epicalyx, in Quinchamalium persistent), surrounding and adnate to ovary. Epigyny to half epigyny. Sepals (three or) four or five (or six), very small (reduced), with open aestivation, connate (resembling those in Loranthaceae), persistent in fruit. Petals (three or) four or five (or six), with valvate? aestivation, connate into a tube, often with adaxial hairs, in Arjona usually persistent. Nectariferous disc intrastaminal, cushion-shaped or annular.
Androecium Stamens (three or) four or five (or six), haplostemonous, antepetalous. Filaments free, largely adnate to petals (epipetalous). Anthers dorsifixed (in Arjona and Schoepfia) or basifixed (in Quinchamalium), non-versatile, tetrasporangiate, introrse or extrorse, longicidal (dehiscing by longitudinal slits). Tapetum secretory? Staminodia absent.
Pollen grains Microsporogenesis simultaneous? Pollen grains (resembling those in Loranthaceae) tri- or tetracolpate or -colporate, heteropolar, tetrahedral, with fused apertures, shed as monads, bicellular at dispersal. Apocolpium convex. Apertural development in Schoepfia according to Garside’s rule (three pores at four points in tetrad). Exine tectate, with granular acolumellate infratectum, psilate or smooth.
Gynoecium Pistil composed of usually three (in some species of Schoepfia two) connate carpels. Ovary inferior to semi-inferior, septate at base, bilocular or trilocular in lowermost part and unilocular in upper part. Style usually single, simple, usually filiform (occasionally distylous). Stigma capitate or trilobate, type? Pistillodium absent.
Ovules Placentation free central. Ovule one per carpel, hemitropous, pendulous, unitegmic, crassinucellar? Integument ? cell layers thick. Megagametophyte monosporous, Polygonum type? Synergids and antipodal cells in Quinchamalium often very long and haustorial (antipodal haustoria sometimes multinucleate). Endosperm development cellular? Endosperm haustoria? Embryogenesis?
Fruit In Schoepfia a one-seeded drupe with parchment-like endocarp; in Arjona a nut-like fruit; in Quinchamalium a nut-like pseudocarp, without pericarp, endosperm being surrounded by sclerotic bracts. Calyculus (epicalyx) in Quinchamalium persistent and cupular.
Seeds Aril absent. Testa and tegmen absent. Perisperm not developed. Endosperm copious, oily? Embryo very small, with chlorophyll? Cotyledons two? Germination?
Cytology n = 12, 14
DNA
Phytochemistry Unknown.
Use Edible tubers (Arjona tuberosa).
Systematics Schoepfia (c 10; southeastern China, Hainan, the Korean Peninsula, southern Japan, Taiwan, Indochina, northern Sumatra, Mexico, Central America, the West Indies, tropical South America), Quinchamalium (20–25; the Andes), Arjona (8–10; southern tropical and temperate South America).
Schoepfiaceae are sister-group to Misodendrum (Misodendraceae). Schoepfia is sister to [Arjona+Quinchamalium], according to Der & Nickrent (2008). Nut-like fruit and accrescent bracts with sclerenchymatous layer in fruit may be synapomorphies of [Arjona+Quinchamalium]. However, Quinchamalium was recovered as sister to [Arjona+Schoepfia] by Su & al. (2015).
STROMBOSIACEAE Tiegh. |
( Back to Santalales ) |
Scorodocarpaceae Tiegh. in Bot. Jahresber. (Just) 25(2): 406. 19 Jan 1900; Tetrastylidiaceae Tiegh. in Bot. Jahresber. (Just) 25(2): 406. 19 Jan 1900
Genera/species 6/c 20
Distribution Tropical Africa, India, Sri Lanka, Burma to Central Malesia, southern Brazil.
Fossils Unknown.
Habit Usually bisexual (rarely dioecious), evergreen or deciduous trees or shrubs. At least Scorodocarpus and Strombosia autotrophic (haustoria absent).
Vegetative anatomy Arbuscular mycorrhiza present at least in Strombosia. Phellogen ab initio subepidermal. Medulla with or without diaphragms. Vessel elements with scalariform or simple perforation plates; lateral pits alternate, opposite or scalariform, simple pits. Imperforate tracheary xylem elements fibre tracheids or libriform fibres with simple or bordered pits, usually non-septate. Wood rays uniseriate or multiseriate, heterocellular. Axial parenchyma apotracheal diffuse or diffuse-in-aggregates, or paratracheal scanty or in narrow bands. Tyloses, sclerotic or non-sclerotic, abundant. Sieve tube plastids ? type; sieve tube nuclei with non-dispersive protein bodies. Nodes 3:3, trilacunar with three leaf traces, or 5:5, pentalacunar with five traces (sometimes 1:1, unilacunar with one trace). Wood cystoliths absent. Wood with idioblasts containing ethereal oils. Wood ray cells without silica bodies. Cortex with or without cristarque cells. Heartwood in Scorodocarpus with gum-like substances. Prismatic calciumoxalate crystals or druses abundant.
Trichomes Hairs multicellular, uniseriate or multiseriate, often stellate or dendritic, or absent.
Leaves Alternate (spiral or distichous), simple, entire, with conduplicate ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle transection annular (sometimes with adaxial bundles; foliar bundles sometimes connected with groups of thin fibres). Venation pinnate (lamina sometimes palmate, three- to five-veined at base), camptodromous. Stomata usually cyclocytic or anisocytic (rarely anomocytic, helicocytic or paracytic), with large guard cell chamber. Cuticular waxes? Epidermal cells usually with calciumoxalate druses (not in Scorodocarpus). Petiole and median veins with asterosclereids. Mesophyll sclereids present in e.g. Scorodocarpus. Cells with tannins or mucilage. Cell walls not silicified; epidermal cells often silica sand. Leaf margin entire; in Diogoa, Strombosia and Strombosiopsis with special type of unlignified thin fibres near leaf margin.
Inflorescence Axillary or extra-axillary, fasciculate, raceme-like or panicle (often highly contracted), or flowers solitary axillary. Peduncles sometimes modified into tendrils.
Flowers Actinomorphic, small. Hypanthium sometimes present. Usually hypogyny (in Strombosia and Strombosiopsis half epigyny). Sepals four or five, small, with valvate (imbricate?) aestivation, connate, sometimes accrescent. Petals four or five, with valvate aestivation, usually caducous, connate at base. Nectariferous disc usually intrastaminal (in Engomegoma extrastaminal), entire, trilobate or quinquelobate.
Androecium Stamens usually four or five (in Scorodocarpus 4+4 or 5+5), as many as (in Scorodocarpus twice the number of) petals, alternisepalous, antepetalous. Filaments short, wide, free, usually adnate to petals (epipetalous), with two lateral hairy scales at base. Anthers dorsifixed or basifixed, versatile?, tetrasporangiate, introrse or latrorse, longicidal (dehiscing by longitudinal slits; in Tetrastylidium transversely multiseptate; loculi in Engomegoma dehiscing separately); connective in Engomegoma, Strombosiopsis and Tetrastylidium massive, somewhat prolonged. Tapetum secretory? Female flowers with staminodia.
Pollen grains Microsporogenesis simultaneous? Pollen grains usually tricolpate or tricolpor(oid)ate (endoaperture more or less rectangular), shed as monads, bicellular at dispersal. Apocolpium convex. Exine tectate to semitectate, with usually granular (sometimes columellate) infratectum, microperforate to reticulate or smooth.
Gynoecium Pistil composed of three to six connate carpels, usually septate at base. Ovary usually superior (sometimes semi-inferior), ab initio trilocular to quinquelocular, finally often partially or entirely unilocular by disintegration of septa. Style single, simple, very short to long. Stigma capitate or trilobate to quinquelobate, type? Male flowers with pistillodium.
Ovules Placentation apical. Ovule one per carpel, anatropous, usually pendulous (rarely horizontal), usually unitegmic (sometimes ategmic; in Scorodocarpus occasionally bitegmic), tenuinucellar. Integument approx. six cell layers thick. Micropyle prolonged. Megasporocytes sometimes several. Megagametophyte monosporous, Polygonum type, usually with caecum. Endosperm development at least in Strombosia ab initio cellular. Endosperm haustoria chalazal, unicellular. Embryogenesis?
Fruit A one-seeded drupe, often enclosed by persistent and sometimes accrescent hypanthium and calyx; fruit often dehiscing by three to five valves.
Seeds Aril absent. Exotesta? Endotesta? Tegmen? Perisperm not developed. Endosperm copious, sometimes slightly ruminate, oily and starchy. Embryo very small, well differentiated, with chlorophyll. Cotyledons one to six, free or connate. Radicula long. Germination?
Cytology n = 16, 19, 20 (36?, 40?)
DNA Insertion comprising two to four amino acid triplets (codons) in the 3’-end of the plastid gene rbcL immediately 5’ of the stop codon present in Diogoa, Scorodocarpus, Strombosia, Strombosiopsis, and Tetrastylidium (and Engomegoma?).
Phytochemistry Virtually unknown. Gallic acid present in Tetrastylidium.
Use Timber.
Systematics Scorodocarpus (1; S. borneensis; West Malesia from Peninsular Thailand to Borneo), Strombosia (9–10; tropical Africa, India, Sri Lanka, Burma, Southeast Asia, Malesia to the Philippines an northern Moluccas), Diogoa (2; D. retivenia, D. zenkeri; tropical Africa), Engomegoma (1; E. gordonii; Gabon), Strombosiopsis (3; S. nana, S. sereinii, S. tetrandra; tropical Africa), Tetrastylidium (3; T. brasiliense, T. grandifolium, T. peruvianum; Peru, southern Brazil).
There is fairly weak bootstrap support for a sister-group relationship between Strombosiaceae and Erythropalaceae. The anisocytic, cyclocytic or anisocytic stomata may be a synapomorphy for this clade.
Strombosia, Strombosiopsis, Tetrastylidium and Diogoa have similar foliar epidermal crystals, petiolar vascularization and wood rays. They may form a monophyletic group, but a phylogeny of Strombosiaceae is still lacking.
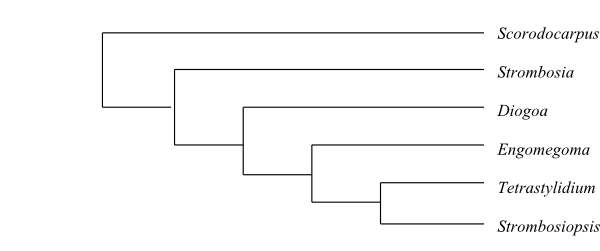 |
Maximum likelihood tree of Strombosiaceae based on DNA sequence data (Su & al. 2015). |
XIMENIACEAE Horan. |
( Back to Santalales ) |
Ximeniales Tiegh. in Bot. Jahresber. (Just) 25(2): 406. 19 Jan 1900
Genera/species 4/≥13
Distribution Pantropical, southern Africa, southern China.
Fossils Fossil pollen of Ximenia were found in Lower Pleistocene layers in East Africa.
Habit Usually bisexual (in one species of Ximenia often functionally unisexual), evergreen trees or shrubs (species of Ximenia). At least Ximenia are root hemiparasites (probably also Curupira, Douradoa and Malania, albeit haustoria have not yet been reported from these genera). Some representatives of Ximenia are xerophytes. Axillary shoots in Ximenia often modified into spines.
Vegetative anatomy Mycorrhiza absent. Phellogen ab initio subepidermal. Vessel elements usually with scalariform perforation plates; lateral pits alternate, simple and bordered pits. Imperforate tracheary xylem elements fibre tracheids with simple or bordered pits, non-septate (also vasicentric tracheids). Wood rays uniseriate, heterocellular. Axial parenchyma apotracheal diffuse or diffuse-in-aggregates, paratracheal scanty or in narrow bands, or absent. Tyloses (also sclerotic) abundant. Sieve tube plastids ? type; sieve tube nuclei with non-dispersive protein bodies. Nodes 3:3, trilacunar with three leaf traces. Laticifers absent. Heartwood in Ximenia with resins. Cystoliths absent. Wood ray cells with rhomboidal calciumoxalate? crystals and sometimes with silica bodies. Prismatic calciumoxalate crystals abundant.
Trichomes Hairs unicellular or multicellular; glandular hairs sometimes present on inflorescence parts.
Leaves Alternate (spiral or distichous), simple, entire, often coriaceous, with conduplicate ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle transection arcuate. Venation usually pinnate, acrodromous, eucamptodromous or brochidodromous (occasionally palmate). Stomata paracytic or anomocytic, with small guard cell chamber. Cuticular wax crystalloids usually absent (sometimes present as platelets). Cuticular thickenings occurring. Lamina often gland-dotted. Epidermal cells with tannins or mucilage, with non-lignified walls. Mesophyll with or without sclerenchymatous idioblasts (specula fibres). Asterosclereids present in primary vein. Cystoliths absent. Epidermal and mesophyll cell walls often silicified. Laticiferous canals and schizogenous cavities absent. Cristarque cells have been reported. Cell walls sometimes silicified. Druses absent. Leaf margin entire.
Inflorescence Axillary, umbel-like, cymose (in Malania compound, raceme-like), or flowers solitary axillary.
Flowers Actinomorphic, small. Hypanthium present or absent. Hypogyny. Sepals four or five, with open or imbricate aestivation, sometimes persistent, usually entirely or almost entirely connate. Petals four or five, usually with valvate (rarely imbricate) aestivation, free, usually caducous, with adaxial hairs. Nectariferous disc absent or in Ximenia intrastaminal, annular, encircling ovary base.
Androecium Stamens usually eight, ten or twelve, diplostemonous (sometimes triplostemonous; in Douradoa four or five, haplostemonous, antepetalous). Filaments free from each other and from petals. Anthers dorsifixed (in Malania and Ximenia) or basifixed (in Curupira and Douradoa), often versatile, usually tetrasporangiate (rarely disporangiate), introrse, usually longicidal (dehiscing by longitudinal slits; in Curupira poricidal, dehiscing by an elongated pore); connective usually massive. Tapetum secretory. Staminodia usually absent (present in functionally female flowers of Ximenia parviflora).
Pollen grains Microsporogenesis simultaneous. Pollen grains usually tricolporate or tetracolporate, shed as monads, bicellular or tricellular at dispersal. Apocolpium convex. Garside’s rule? Exine tectate, with usually granular (sometimes columellate) infratectum, smooth or microperforate.
Gynoecium Pistil composed of two to four connate carpels; carpels usually septate, antesepalous (rarely antepetalous), or median carpel adaxial. Ovary superior, bilocular to quadrilocular, with or without ridges. Style single, simple, short to long, or absent. Stigma usually capitate, type? Pistillodium usually absent (present in male flowers of one species of Ximenia).
Ovules Placentation free central (when ovary unilocular) or axile to apical (when ovary multilocular). Ovule one per carpel, anatropous, pendulous, usually bitegmic (sometimes unitegmic), at least in Ximenia americana pseudocrassinucellar. Micropyle ?-stomal. Outer integument ? cell layers thick. Inner integument ? cell layers thick. Megagametophyte monosporous, Polygonum type. Synergids with a filiform apparatus. Endosperm development ab initio cellular. Endosperm haustorium chalazal. Embryogenesis?
Fruit A drupe with often persistent and sometimes strongly accrescent calyx or hypanthium, and with woody or crustaceous endocarp.
Seeds Aril absent. Testa present. Exotesta? Endotesta? Tegmen? Perisperm not developed. Endosperm copious, oily. Embryo very small, straight, with chlorophyll? Cotyledons two, sometimes connate. Germination in Ximenia cryptocotylar with two lowest phyllomes recurved and inserted between cotyledons.
Cytology n = 12, 13, (24?), 26
DNA
Phytochemistry Unsufficiently known. Triglycerides of polyacetylenic fatty acids present. Alkaloids, saponins, tannins (phenylpropanoids) and cyanogenic compounds sometimes present.
Use Timber, medicinal plants, seed oils for food or lubricants (Malania), soap (Curupira).
Systematics Malania (1; M. oleifera; western Guangxi, eastern Yunnan), Curupira (1; C. tefeensis; Amazonian Brazil), Ximenia (≥10; tropical and southern Africa, Madagascar, tropical Asia to Queensland, islands in southwestern Pacific, Florida, Mexico, Central America, the West Indies, tropical South America), Douradoa (1; D. consimilis; Amapa and Pará in Brazil).
Synapomorphies for Ximeniaceae are, e.g., inflorescence type, stamen number and wood characters.
Malania was found to be sister to [Curupira+Ximenia] by Su & al. (2015). Douradoa was not included in their analyses.
Literature
Abbiatti D. 1943. Sinopsis de las Loranthaceas Argentinas. – Rev. Mus. La Plata, N. S., 7.
Agarwal S. 1962. Embryology of Quinchamalium chilense Lam. – Proc. Symp. Plant Embryology, 1960, Counc. Sci. & Industr. Res., New Delhi, pp. 162-169.
Agarwal S. 1963a. Morphological and embryological studies in the family Olacaceae I. Olax L. – Phytomorphology 13: 185-196.
Agarwal S. 1963b. Morphological and embryological studies in the family Olacaceae II. Strombosia Blume. – Phytomorphology 13: 348-356.
Aitzetmüller K. 2012. Santalbic acid in the plant kingdom. – Plant Syst. Evol. 298: 1609-1617.
Amico GC, Vidal-Russell R, Nickrent DL. 2007. Phylogenetic relationships and ecological speciation in the mistletoe Tristerix (Lorantaceae): the influence of pollinators, dispersers, and hosts. – Amer J. Bot. 94: 558-567.
Amico GC, Vidal-Russel R, Garcia MA, Nickrent DL. 2012. Evolutionary history of the South American mistletoe Tripodanthus (Loranthaceae) using nuclear and plastid markers. – Syst. Bot. 37: 218-225.
Andersen H. 1976. Ombrophytum peruvianum (Balanophoraceae) found in the Galapagos Islands. – Bot. Not. 129: 113-117.
Anselmino E. 1932a. Die Stammpflanzen der Droge Muira-puama. – Notizbl. Bot. Gart. Mus. Berlin-Dahlem 11: 623-626.
Anselmino E. 1932b. Die Stammpflanzen von Muira-puama. – Arch. Pharm. Ber. Deutsch. Pharm. Ges. 271: 296-314.
Arekal GD, Shivamurthy GR. 1976. “Seed” germination in Balanophora abbreviata. – Phytomorphology 26: 135-138.
Ashworth VETM. 1999. Phylogenetic relationships in Phoradendreae (Viscaceae) inferred from DNA sequence data. – Ph.D. diss., Claremont Graduate University, Claremont, California.
Ashworth VETM. 2000a. Phylogenetic relationship in Phoradendreae (Viscaceae) inferred from three regions of the nuclear ribosomal cistron I. Major lineages and paraphyly of Phoradendron. – Syst. Bot. 25: 349-370.
Ashworth VETM. 2000b. Phylogenetic relationship in Phoradendreae (Viscaceae) inferred from three regions of the nuclear ribosomal cistron II. The North American species of Phoradendron. – Aliso 19: 41-53.
Ashworth VETH, Dos Santos G. 1997. Wood anatomy of four Californian mistletoe species (Phoradendron, Viscaceae). – IAWA J. 18: 229-245.
Azuma J-I, Kim N-H, Heux L, Vuong R, Chanzy H. 2000. The cellulose system in viscin from mistletoe berries. – Cellulose 7: 3-19.
Baas P, Kool R. 1983. Comparative leaf anatomy of Heisteria (Olacaceae). – Blumea 28: 367-388.
Baas P, Oosterhoud E van, Scholtes CJL. 1982. Leaf anatomy and classification of the Olacaceae, Octoknema, and Erythropalum. – Allertonia 3: 155-210.
Baillon HM. 1862. Deuxième mémoire sur les Loranthacées. – Adansonia 3: 50-128.
Balle S. 1954. Sur quelques Loranthoidées d’Afrique. – Bull. Séances Acad. Roy. Sci. Colon Bruxelles 25: 1619-1635.
Balle S. 1955. À propos de la morphologie des “Loranthus” d’Afrique. – Webbia 11: 541-585.
Balle S. 1956. West African Loranthaceae. – Kew Bull. 11: 168.
Balle S. 1968. Les Loranthacées de l’Afrique du Sud-Ouest. – Mitt. Bot. Staatssamml. München 7: 119-209.
Balle S. 1970. Loranthaceae des environs du Lac Tana et des montagnes du Demien. – Stuttgarter Beitr. Naturk. 221: 1-8.
Balle S. 1982. Loranthacées. – In: Satabié B, Leroy J-F (eds), Flore du Cameroun 23, Yaoundé.
Balle S, Hallé N. 1961. Loranthacées de Côte d’Ivoire. – Adansonia, sér. II, 1: 208-265.
Banwell MG, Cameron JM. 1996. Enantiospecific construction of the carbon skeleton associated with manicol, an antineoplastic sesquiterpene from Dulacia guianensis (Olacaceae). – Tetrahedron Letters 37: 525-526.
Barber CA. 1906. Studies in root-parasitism. The haustorium of Santalum album 1. Early stages, up to penetration. – Mem. Dept. Agric. India 1: 1-30.
Barber CA. 1907a. Studies in root parasitism. The haustorium of Olax scandens. – Mem. Dept. Agric. Indian, Bot. Ser. 2: 1-47.
Barber CA. 1907b. Parasitic trees of southern India. – Proc. Cambridge Phil. Soc. 14: 246-256.
Barkman TJ, McNeal JR, Lim S-H, Coat G, Croom HB, Young ND, dePamphilis CW. 2007. Mitochondrial DNA suggests at least 11 origins of parasitism in angiosperms and reveals genomic chimerism in parasitic plants. – B. M. C. Evol. Biol. 7: 248. http://dx.doi.org/10.1186/1471-2148-7-248
Barlow BA. 1964. Classification of the Loranthaceae and Viscaceae. – Proc. Linn. Soc. New South Wales, ser. II, 89: 268-272.
Barlow BA. 1966. A revision of the Loranthaceae of Australia and New Zealand. – Aust. J. Bot. 14: 421-499.
Barlow BA. 1974. A revision of the Loranthaceae of New Guinea and the south-western Pacific. – Aust. J. Bot. 22: 531-621.
Barlow BA. 1982. Supplement to a revision of the Loranthaceae of Australia. – Brunonia 5: 203-212.
Barlow BA. 1983a. A revision of the genus Notothixos (Viscaceae). – Brunonia 6: 1-24.
Barlow BA. 1983b. A revision of the Viscaceae of Australia. – Brunonia 6: 25-57.
Barlow BA. 1983c. Biogeography of Loranthaceae and Viscaceae. – In: Calder DM, Bernhardt P (eds), The biology of mistletoes, Academic Press, Sydney, New York, pp. 19-45.
Barlow BA. 1984a. Loranthaceae. – In: George AS (ed), Flora of Australia 22, Australian Government Publ. Service, Canberra, pp. 68-131.
Barlow BA. 1984b. Viscaceae. – In: George AS (ed), Flora of Australia 22, Australian Government Publ. Service, Canberra, pp. 131-145.
Barlow BA. 1990. Biogeographical relationships of Australia and Malesia: Loranthaceae as a model. – In: Baas P, Kalkman K, Geesink R (eds), The plant diversity of Malesia, Kluwer Academic Publ., Dordrecht, pp. 273-292.
Barlow BA. 1991. Conspectus of the genera Scurrula L. and Taxillus Tieghem (Loranthaceae) in the Malesian region. – Blumea 36: 63-85.
Barlow BA. 1995. New and noteworthy Malesian species of Loranthaceae. – Blumea 40: 15-31.
Barlow BA. 1996. Advances in systematic knowledge of Australian Loranthaceae and Viscaceae: a review. – Telopea 6: 851-862.
Barlow BA. 1997. Loranthaceae. – In: Kalkman C et al. (eds), Flora Malesiana I, 13, Flora Malesiana Foundation, Rijksherbarium/Hortus Botanicus, Leiden, pp. 209-401.
Barlow BA. 1997. Viscaceae. – In: Kalkman C et al. (eds), Flora Malesiana I, 13, Flora Malesiana Foundation, Rijksherbarium/Hortus Botanicus, Leiden, pp. 403-442.
Barlow BA, Wiens D. 1971. The cytogeography of the loranthaceous mistletoes. – Taxon 20: 291-312.
Barlow BA, Wiens D. 1973. The classification of the generic segregates of Phrygilanthus (=Notanthera) of the Loranthaceae. – Brittonia 25: 26-39.
Barlow BA, Wiens D. 1977. Host-parasite resemblance in Australian mistletoes: the case for cryptic mimicry. – Evolution 31: 69-84.
Barroso GM. 1969. Acanthosyris Paulo-alvinii – una nova espécie de Santalaceae. – An. Congr. Soc. Bot. Brasil (XIX Congresso Nacional de Botânica Fortaleza, 21-29 january 1968): 107-109.
Bernhardt P. 1983. The floral biology of Amyema in southeastern Australia. – In: Calder M, Bernhardt P (eds), The biology of mistletoes, Academic Press, Sydney, pp. 87-100.
Beyer C, Forstreuter W, Weber HC. 1989. Anatomical studies of haustorium ontogeny and remarkable mode of penetration of the haustorium in Nuytsia floribunda (Labill.) R. Br. – Bot. Acta 102: 229-235.
Bhandari NN. 1969. Ontogeny and marginal growth in the leaf of Eubrachion ambiguum. – Ann. Bot. 33: 537-540.
Bhandari NN, Indira K. 1969. Studies in the Viscaceae IV. Embryology of Eubrachion (Hook. et Arn.) Engl. – Bot. Not. 122: 183-203.
Bhandari NN, Nanda K. 1968a. Studies in the Viscaceae I. Morphology and embryology of the Indian dwarfmistletoe – Arceuthobium minutissimum Hook. f. – Phytomorphology 18: 435-450.
Bhandari NN, Nanda K. 1968b. Studies in the Viscaceae II. A reinvestigation of the female gametophyte of Arceuthobium douglasii. – Amer. J. Bot. 55: 1028-1030.
Bhandari NN, Vohra SCA. 1983. Embryology and affinities of Viscaceae. – In: Calder DM, Bernhardt P (eds), The biology of mistletoes, Academic Press, Sydney, New York, pp. 69-86.
Bhatnagar SP. 1960. Morphological and embryological studies in the family Santalaceae IV. Mida salicifolia A. Cunn. – Phytomorphology 10: 198-207.
Bhatnagar SP. 1965. Studies in angiospermous parasites 2. Santalum album – the Sandalwood Tree. – Bull. Natl. Bot. Gard. 112: 1-190.
Bhatnagar SP, Agarwal S. 1961. Morphological and embryological studies in the family Santalaceae VI. Thesium. – Phytomorphology 11: 273-282.
Bhatnagar SP, Johri BM. 1983. Embryology of the Loranthaceae. – In: Calder DM, Bernhardt P (eds), The biology of mistletoes, Academic Press, Sydney, New York, pp. 47-66.
Bhatnagar SP, Joshi PC. 1965. Morphological and embryological studies in the family Santalaceae VII. Exocarpus bidwellii Hook. f. – Proc. Natl. Inst. Sci. India, Sect. B, 31: 34-44.
Bhatnagar SP, Sabharwal G. 1969. Morphology and embryology of Iodina rhombifolia Hook. & Arn. – Beitr. Biol. Pflanzen 45: 465-479.
Billings FH. 1932. Microsporogenesis in Phoradendron. – Ann. Bot. 46: 979-992.
Billings FH. 1933. Development of the embryo-sac in Phoradendron. – Ann. Bot. 47: 261-278.
Black GA, Murça Pires J. 1948. Dois gêneros novos Curupira e Froesia, cinco espécies novas, uma nova combinação, chaves e observações sobre plantas da região amazônica. – Bol. Técn. Inst. Agron. Norte 15: 16-19.
Blakely WF. 1922. The Loranthaceae of Australia. – Proc. Linn. Soc. New South Wales 47: 1-25, 199-222, 391-414.
Bonneville R, Lobreau D, Riollet G. 1982. Pollen fossile de Ximenia (Olacaceae) dans le Pléistocène Inférieur d’Oldouvai en Tanzanie: implications paléoécologiques. – J. Biogeogr. 9: 469-486.
Brenan JPM. 1985. New species of Selago and Thesium from South Africa. – Kew Bull. 40: 81-83.
Breteler FJ. 2001. Novitates Gabonenses 40. A new species of Strombosiopsis (Olacaceae) from Gabon. – Adansonia, sér. III, 23: 303-306.
Breteler FJ. 2007. A reconsideration of the species delimitation in Diogoa (afrotropical Olacaceae). – Syst. Geogr. Plants 77: 239-245.
Breteler FJ, Baas P, Boesewinkel FD, Bouman F, Lobreau-Callen D. 1996. Engomegoma Breteler (Olacaceae): a new monotypic genus from Gabon. – Bot. Jahrb. Syst. 118: 113-132.
Brown RH, Nickrent DL, Gasser CS. 2010. Expression of ovule and integument-associated genes in reduced ovules of Santalales. – Evol. Developm. 12: 231-240.
Butaud JF, Raharivelomanana P, Bianchini JP, Gaydou EM. 2008. Santalum insulare acetylenic fatty acid seed oils: comparison within the Santalum genus. – J. Amer. Oil Chem. Soc. 85: 353-356.
Cabrera JF. 2002. Molecular phylogeny and historical biogeography of Lorantaceae (Santalales) inferred from matK sequence data. – M.Sc. thesis, Dept. of Plant Biology, Southern Illinois University, Carbondale, Illinois.
Calder M, Bernhardt P (eds). 1983. The biology of mistletoes. – Academic Press, Sydney.
Calladine A, Pate JS. 2000. Haustorial structure and functioning of the root hemiparasitic tree Nuytsia floribunda (Labill.) R. Br. and water relationships with its hosts. – Ann. Bot. 85: 723-731.
Calladine A, Pate JS, Dixon KW. 2000. Haustorial development and growth benefit to seedlings of the root hemiparasitic tree Nuytsia floribunda (Labill.) R. Br. in association with various hosts. – Ann. Bot. 85: 733-740.
Callmander MW, Luino I, Da-Giau S, Rakotovao
C, Gautier L. 2014. A synoptic revision of the Malagasy endemic genus
Socratina Balle (Loranthaceae). – Candollea 69:
65-73.
Calvin CL. 1967. Anatomy of the endophytic system of the mistletoe Phoradendron flavescens. – Bot. Gaz. 128: 117-137.
Calvin CL, Wilson CA. 1998. Comparative morphology of haustoria within African Loranthaceae. – In: Polhill R, Wiens D (eds), Mistletoes of Africa, Royal Botanic Gardens, Kew, pp. 17-36.
Calvin CL, Wilson CA. 2006. Comparative morphology of epicortical roots in Old and New World Loranthaceae with reference to root types, origin, patterns of longitudinal extension and potential for clonal growth. – Flora 201: 51-64.
Calvin CL, Wilson CA. 2009. Epiparasitism in Phoradendron durangense and P. falcatum (Viscaceae). – Aliso 27: 1-12.
Capuron R. 1968. Matériaux pour l’étude de la flore forestière de Madagascar: Olacacées, Opiliacées et Santalacées arbustives ou arborescentes de Madagascar. – Centre Technique Forestier Tropical Madagascar, Section Botanique, Antananarivo.
Cardoso LJT, Braga JMA. 2015. A new Caribbean species of Helosis (Balanophoraceae) with a revised key to the genus. – Syst. Bot. 40: 597-603.
Carlquist SJ. 1985. Wood and stem anatomy of Misodendraceae: systematic and ecological conclusions. – Brittonia 37: 58-75.
Carvell WN, Eshbaugh WH. 1982. A systematic study of the genus Buckleya (Santalaceae). – Castanea 47: 17-37.
Cavaco A. 1954. Sur le genre Phanerodiscus gen. nov. (Olacacées) de Madagascar. – Not. Syst., Paris 15:10-14.
Cavaco A, Keraudren M. 1955a. Sur le Ximenia (Olacacées) de Madagascar, une nouvelle espèce d’Olax. – Bull. Soc. Bot. France 102: 117-119.
Cavaco A, Keraudren M. 1955b. Fam. 58. Santalacées, 1-9. – In: Humbert H (ed), Flore de Madagascar et des Comores (plantes Vasculaires), Firmin-Didot, Paris.
Cavaco A, Keraudren M. 1956. Sur le Turraea thouarsiana (Baill.) Cav. & Ker., comb. nov. (Olacacées) et une nouvelle espèce d’Olax. – Bol. Soc. Brot., ser. II, 29: 23-25.
Chibnall AC, Piper SH, Mangouri HAE, Williams EF, Iyengar AVV. 1937. The wax from the leaves of sandal (Santalum album). – Biochem. J. 31: 1981-1986.
Cocucci AF. 1983. New evidence from embryology in angiosperm classification. – Nord. J. Bot. 3: 67-73.
Cocucci AF, Venturelli M. 1982. El ovulo y el gineceo en Loranthaceae. – Bol. Soc. Argentina Bot. 21: 131-141.
Coetzee J, Fineran BA. 1987. The apoplastic continuum, nutrient absorption and plasma tubules in the dwarf mistletoe Korthalsella lindsayi (Viscaceae). – Protoplasma 136: 145-153.
Cohen LI. 1954. The anatomy of the endophytic system of the dwarf mistletoe, Arceuthobium campylopodum. – Amer. J. Bot. 41: 840-847.
Cohen LI. 1963. Studies on the ontogeny of the dwarf mistletoes, Arceuthobium I. Embryogeny and histogenesis. – Amer. J. Bot. 50: 400-407.
Cohen LI. 1968. Development of the staminate flower in the dwarf mistletoe, Arceuthobium. – Amer. J. Bot. 55: 187-193.
Cohen LI. 1970. The development of the pistillate flower in the dwarf mistletoe, Arceuthobium. – Amer. J. Bot. 57: 477-485.
Correa JP. 1958. Morphological and embryological studies in the family Loranthaceae-Viscoideae. – Delhi.
Danser BH. 1929. On the taxonomy and the nomenclature of the Loranthaceae of Asia and Australia. – Bull. Jard. Bot. Buitenzorg 10: 291-374.
Danser BH. 1931. The Loranthaceae of the Netherlands Indies. – Bull. Jard. Bot. Buitenzorg 11: 233-519.
Danser BH. 1933. A new system for the genera of Loranthaceae-Loranthoideae, with a nomenclator for the Old World species of this subfamily. – Verh. Kon. Akad. Wetensch. Amsterdam Afd. Natuurk., Sect. 2, 29: 1-128.
Danser BH. 1935. A revision of the Philippine Loranthaceae. – Philipp. J. Sci. 58: 1-149.
Danser BH. 1939. A revision of the genus Phacellaria (Santalaceae). – Blumea 3: 212-235.
Danser BH. 1940. On some genera of Santalaceae Osyrideae from the Malay Archipelago, mainly from New Guinea. – Nova Guinea, N. S., 4: 133-149.
Danser BH. 1955. Supplementary notes on the Santalaceous genera Dendromyza and Cladomyza. – Nova Guinea, N. S., 6: 261-277.
Datta RM. 1951. Occurrence of a hermaphrodite flower in Arceuthobium minutissimum Hook. f. the smallest known dicotyledonous plant. – Nature 167: 203-204.
Davidar P. 1983. Similarity between flowers and fruits in some flowerpecker pollinated mistletoes. – Biotropica 15: 32-37.
Davis CC, Anderson WR, Wurdack KJ. 2005. Gene transfer from a parasitic flowering plant to a fern. – Proc. Roy. Soc., Ser. B, 272: 2237-2242.
Davreux M, Delaude C, Huls R. 1971. Contribution à l’étude de la saponine extraite d’une Olacacées africaine: Olax subscorpioidea Oliv. – Bull. Soc. Roy. Sci. Liège 40: 493-497.
Davreux M, Delaude C. 1972. Contribution à l’étude des saponines extraites des Olacacées: identification de la saponine d’Olax gombecola Baill. – Bull. Soc. Roy. Sci. Liège 41: 510-572.
Dawson G. 1944. Las Santaláceas Argentinas. – Extr. Rev. Mus. La Plata (N. S.), Secc. Bot. 6: 5-80.
DeFilipps RA. 1969. Parasitism in Ximenia (Olacaceae). – Rhodora 71: 439-443.
Delaude C, Davreux M. 1973. Contribution à l’étude phytochimique des Olacacées. Identification de la saponine d’Olax latifolia Engl. – Bull. Soc. Roy. Sci. Liège 42: 241-244.
Delprete PG. 2004. A new species of Lophophytum and the first report of Lathrophytum (Balanophoraceae) from the State of Goiás, Central Brazil. – Kew Bull. 59: 291-295.
Der JP. 2005. Molecular phylogenetics and classification Santalaceae. – M.Sc. thesis, Southern Illinois University, Carbondale, Illinois.
Der JP, Nickrent DL. 2008. A molecular phylogeny of Santalaceae (Santalales). – Syst. Bot. 33: 107-116.
Dixit SN. 1958a. Morphological and embryological studies of the family Loranthaceae IV. Amyema Van Tiegh. – Phytomorphology 8: 346-364.
Dixit SN. 1958b. Morphological and embryological studies of the family Loranthaceae V. Lepeostegeres gemmiflorus (Bl.) Bl. – Phytomorphology 3: 365-376.
Dixit SN. 1961. Morphological and embryological studies of the family Loranthaceae VIII. Tolypanthus Bl. – Phytomorphology 11: 335-345.
Dixit SN. 1962. Rank of the subfamilies Loranthoideae and Viscoideae. – Bull. Bot. Soc. India 4: 49-55.
Dobbins DR, Kuijt J. 1974a. Anatomy and fine structure of the mistletoe haustorium (Phthirusa pyrifolia) I. Development of the young haustorium. – Amer. J. Bot. 61: 535-543.
Dobbins DR, Kuijt J. 1974b. Anatomy and fine structure of the mistletoe haustorium (Phthirusa pyrifolia) II. Penetration attempts and formation of the gland. – Amer. J. Bot. 61: 544-550.
Docters van Leeuwen WM. 1954. On the biology of some Javanese Loranthaceae and the role birds play in their life histories. – Beaufortia Misc. Publ. 41: 105-207.
Dowding ES. 1931. Floral morphology of Arceuthobium americanum. – Bot. Gaz. 41: 42-54.
Downey PO, Wilson CA. 2004. Muellerina flexialabastra (Loranthaceae): a new species of mistletoe from south-eastern Australia. – Aust. Syst. Bot. 17: 441-445.
Eberwein RK, Weber A. 2004. Exorhopala ruficeps (Balanophoraceae): morphology and transfer to Helosis. – Bot. J. Linn. Soc. 146: 513-517.
Eberwein R, Nickrent DL, Weber A. 2009. Development and morphology of flowers and inflorescences in Balanophora papuana and B. elongata (Balanophoraceae). – Amer. J. Bot. 96: 1055-1067.
Ekambaran T, Panje RR. 1935. Contributions to our knowledge of Balanophora 2. Life history of B. dioica. – Proc. Indian Acad. Sci., Sect. B, 1: 522-543.
Engell K. 1979. Morphology and embryology of Scybalioideae (Balanophoraceae) I. Corynaea crassa Hook. f. var. sprucei (Eichl.) B. Hansen. – Bot. Tidsskr. 73: 155-166.
Engler A. 1889a. Loranthaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(1), W. Engelmann, Leipzig, pp. 156-198.; Engler A. 1897. Nachträge zu III(1), pp. 124-140.
Engler A. 1889b. Olacaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(1), W. Engelmann, Leipzig, pp. 231-242; Engler A. 1897. Nachträge zu III(1), pp. 144-149.
Engler A. 1889c. Balanophoraceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(1), W. Engelmann, Leipzig, pp. 243-263; Engler A. 1897. Nachträge zu III(1), pp. 149-150.
Engler A. 1897. Opiliaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien, Nachträge zu III(1), W. Engelmann, Leipzig, pp. 142-143.
Engler A. 1909. Olacaceae africanae. – Engl. Bot. Jahrb. 43: 161-170.
Engler A (†), Krause K. 1935. Loranthaceae. – In: Engler A (†), Harms H (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 16b, W. Engelmann, Leipzig, pp. 98-203.
Fagerlind F. 1938a. Ditepalanthus, eine neue Balanophoraceen-Gattung aus Madagascar. – Ark. f. Bot. 29A(7): 1-15.
Fagerlind F. 1938b. Bau und Entwicklung der floralen Organe von Helosis cayannensis. – Svensk Bot. Tidskr. 32: 139-159.
Fagerlind F. 1940. Beobachtungen über die Kletterorgane bei Olax. – Svensk Bot. Tidskr. 34: 26-34.
Fagerlind F. 1945a. Blüte und Blütenstand der Gattung Balanophora. – Bot. Not. 1945: 330-350.
Fagerlind F. 1945b. Bildung und Entwicklung des Embryosacks bei sexuellen und agamospermischen Balanophora-Arten. – Svensk Bot. Tidskr. 39: 65-82.
Fagerlind F. 1945c. Bau der floralen Organe der Gattung Langsdorffia. – Svensk Bot. Tidskr. 39: 197-210.
Fagerlind F. 1946. Gynöceummorphologie, Embryologie und systematische Stellung der Gattung Erythropalum. – Svensk Bot. Tidskr. 40: 9-14.
Fagerlind F. 1947. Gynöceummorphologische und embryologische Studien in der Familie Olacaceae. – Bot. Not. 1947: 207-230.
Fagerlind F. 1948a. Bau und Entwicklung der vegetativen Organe von Balanophora. – Kungl. Sv. Vetensk.-Akad. Handl. 25(3): 1-72.
Fagerlind F. 1948b. Beiträge zur Kenntnis der Gynöceummorphologie und Phylogenie der Santalales-Familien. – Svensk Bot. Tidskr. 42: 195-229.
Fagerlind F. 1959. Development and structure of the flower and gametophytes in the genus Exocarpus. – Svensk Bot. Tidskr. 53: 257-282.
Feehan J. 1985. Explosive flower opening in ornithophily: a study of pollination mechanisms in some central African Loranthaceae. – Bot. J. Linn. Soc. 90: 129-144.
Fernald ML. 1928. Contributions from the Gray Herbarium of Harvard University LXXIX. I. Geocaulon, a new genus of the Santalaceae. – Rhodora 30: 21-24.
Feuer SM. 1977. Pollen morphology and evolution in the Santalales s.s., a parasitic order of flowering plants. – Ph.D. diss., University of Massachusetts, Amherst, Massachusetts.
Feuer SM. 1978. Aperture evolution in the genus Ptychopetalum Benth. (Olacaceae). – Amer. J. Bot. 65: 759-763.
Feuer SM. 1981. Pollen morphology and relationships of the Misodendraceae (Santalales). – Nord. J. Bot. 1: 731-734.
Feuer SM, Kuijt J. 1978. Fine structure of mistletoe pollen I. Eremolepidaceae, Lepidoceras, and Tupeia. – Can. J. Bot. 56: 2853-2864.
Feuer SM, Kuijt J. 1979. Fine structure of mistletoe pollen II. Pollen morphology and evolution in Psittacanthus (Loranthaceae). – Bot. Not. 132: 295-309.
Feuer SM, Kuijt J. 1980. Fine structure of mistletoe pollen III. Large-flowered neotropical Loranthaceae and their Australian relatives. – Amer. J. Bot. 67: 34-50.
Feuer SM, Kuijt J. 1985. Fine structure of mistletoe pollen VI. Small flowered neotropical Loranthaceae. – Ann. Missouri Bot. Gard. 72: 187-212.
Fineran BA. 1963. Studies on the root parasitism of Exocarpus bidwillii Hook. f. III. Primary structure of the haustorium. – Phytomorphology 13: 42-54.
Fineran BA. 1964. Studies on the root parasitism of Exocarpus bidwillii Hook. f. IV. Structure of the mature haustorium. – Phytomorphology 13: 249-267.
Fineran BA. 1991. Root hemi-parasitism in the Santalales. – Bot. Jahrb. Syst. 113: 277-308.
Fineran BA, Calvin CL. 2000. Transfer cells and flange cells in sinkers of the mistletoe Phoradendron macrophyllum (Viscaceae), and their novel combination. – Protoplasma 211: 76-93.
Fineran BA, Hocking PJ. 1983. Features of parasitism, morphology and haustorial anatomy in loranthaceous root parasites. – In: Calder M, Bernhardt P (eds), The biology of mistletoes, Academic Press, Sydney, pp. 205-227.
Fineran BA, Ingerfeld M. 1982. Graniferous tracheary elements in the haustorium of Atkisona ligustrina, a root hemi-parasite of the Loranthaceae. – Protoplasma 113: 150-160.
Fineran BA, Ingerfeld M, Patterson WD. 1987. Inclusions of graniferous tracheary elements in the root hemi-parasite Olax phyllanthi (Olacaceae). – Protoplasma 136: 16-28.
Forest F, Manning JC. 2013. The minor genera Kunkeliella and Thesidium included in Thesium. – Bothalia 43: 214-216.
Forgacs P, Provost J. 1981. Olaxoside, a saponine from Olax andronensis, Olax glabriflora and Olax psittacorum. – Phytochemistry 20: 1689-1691.
Gagnepain F. 1910. Comment faut-il comprendre la famille des Olacacées? – Bull. Soc. Bot. France 57: 373-380.
Gagnepain F, Boureau E. 1947. Une nouvelle famille de Gymnospèrmes: les Sarcopodacées. – Bull. Soc. Bot. France 93: 313-320.
Gambetta B, Martinelli EM, Mustich G. 1974. Plants of Mozambique V. Triterpenes of Olax dissitiflora. – Fitoterapia 45: 3-5.
Garcia JG. 1963. 48. Olacaceae; 49. Opiliaceae. – In: Exell AW, Fernandes A, Wild H (eds), Flora Zambesiaca 2 (Part 1), Crown Agents for Oversea Governments and Administrations, London, pp. 328-340.
Gedalovich E, Kuijt J. 1987. An ultrastructural study of the viscin tissue of Phthirusa pyrifolia (H.B.K.) Eichler (Loranthaceae). – Protoplasma 137: 145-155.
Gedalovich-Shedletzky E, Kuijt J. 1990. An ultrastructural study of the tuber strands of Balanophora (Balanophoraceae). – Can. J. Bot. 1990 68: 1271-1279.
Gedalovich-Shedletzky E, Delmer DP, Kuijt J. 1989. Chemical composition of viscin mucilage from three mistletoe species – a comparison. – Ann. Bot. 64: 249-252.
George AS. 1984. Olacaceae. – In: George AS (ed), Flora of Australia 22, Australian Government Publ. Service, Canberra, pp. 13-26.
Germishuizen G, Jaarsveld EJ van, Condy G. 2007. Viscum crassulae (Viscaceae). – Flowering Plants of Africa 60: 58-62.
Gilbert MG, Polhill RM, Wiens D. 1985. Ethiopian mistletoes: new species and combinations. – Nord. J. Bot. 5: 221-224.
Gómez-Sánchez M, Sánchez-Fuentes LJ, Salazar-Olivo LA. 2011. Anatomía de especies mexicanas de los géneros Phoradendron y Psittacanthus, endémicos del Nuevo Mundo. – Rev. Mex. Biodiversidad 82: 1203-1208.
González AM, Mauseth JD. 2010. Morphogenesis is highly aberrant in the vegetative body of the holoparasite Lophophytum leandri (Balanophoraceae): all typical vegetative organs are absent and many tissues are highly modified. – Intern. J. Plant Sci. 171: 499-508.
González F, Pabón-Mora N. 2017. On the supposed polycotyledony and lack of endosperm in Psittacanthus (Loranthaceae). – Brittonia 69: 176-185.
Govindappa DA, Shivamurthy GR. 1976. “Seed” germination in Balanophora abbreviata. – Phytomorphology 26: 135-138.
Graves GR. 1982. Pollination of a Tristerix mistletoe (Loranthaceae) by Diglossa (Aves, Thraupidae). – Biotropica 14: 316-317.
Hallé N. 1978. Illustrations de deux rares Balanophoracées d’Afrique équatoriale appartenant aux genres Chlamydophytum Mildbr. et Balanophora Forst. – Adansonia II, 17: 249-261.
Hallé N. 1987. Okoubaka Pellegrin & Normand est vraiment un genre de Santalaceae. – Bull. Mus. Natl. Hist. Nat. Paris, sect. B, Adansonia: Bot., Phytoch. 9: 355-363.
Hambali GG. 1977. On mistletoe parasitism. – Proc. 6th Conf. Asian-Pacific Weed Sci. Soc., pp. 58-66.
Hamilton SG, Barlow BA. 1963. Studies in Australian Loranthaceae II. Attachment structures and their interrelationships. – Proc. Linn. Soc. New South Wales 88: 74-90.
Hansen B. 1972. The genus Balanophora J. R. & G. Forster. A taxonomic monograph. – Dansk Bot. Ark. 28(1): 1-188.
Hansen B. 1976. Pollen and stigma conditions in the Balanophoraceae s. lat. – Bot. Not. 129: 341-345.
Hansen B. 1980. Flora Neotropica. Monograph 23. Balanophoraceae. – New York Botanical Garden, Bronx, New York.
Hansen B. 1982. The Balanophoraceae of the Pacific. – Acta Phytotaxon. Geobot. 33: 92-102.
Hansen B. 1983. 33. Balanophoraceae. – In: Harling G, Sparre B (eds), Flora of Ecuador 19, Swedish Natural Science Research Council, Stockholm, pp. 1-16.
Hansen B. 1984. Balanophoraceae. – Flore de Madagascar et des Comores. Famille 61: 1-10, Paris.
Hansen B. 1986. The Balanophoraceae of continental Africa. – Bot. Jahrb. Syst. 106: 359-377.
Hansen B, Engell K. 1978. Inflorescences in Balanophoroideae, Lophophytoideae, and Scybalioideae (Balanophoraceae). – Svensk Bot. Tidskr. 72: 177-187.
Hansen B, Kubitzki K. 2014. Balanophorales. – In: Kuijt J, Hansen B (eds), the families and genera of vascular plants 12, Flowering plants: Eudicots; Santalales, Balanophorales, Springer, Cham, Switzerland, pp. 190-207.
Harbaugh DT. 2007. A taxonomic revision of Australian northern sandalwood (Santalum lanceolatum, Santalaceae). – Aust. Syst. Bot. 20: 409-416.
Harbaugh DT, Baldwin BG. 2007. Phylogeny and biogeography of the sandalwoods (Santalum, Santalaceae): repeated dispersals throughout the Pacific. – Amer. J. Bot. 94: 1028-1040.
Harms H. 1935. Balanophoraceae. – In: Engler A (†), Harms H (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 16b, W. Engelmann, Leipzig, pp. 296-339.
Haron NW, Ping ST. 1997. Distribution and taxonomic significance of flavonoids in the Olacaceae and Icacinaceae. – Biochem. Syst. Ecol. 25: 265-263.
Hawksworth FG, Wiens D. 1972. Biology and classification of dwarf mistletoes (Arceuthobium). – Agriculture Handbook 401, Forest Service, United States Dept. Agric., Washington, D.C.
Hawksworth FG, Estabrook GF, Roges DJ. 1968. An application of an information theory model for character analysis in the genus Arceuthobium (Viscaceae). – Taxon 17: 605-619.
Heckel E. 1899. Sur le processus germinatif dans la graine de Ximenia americana L. et sur la nature des écailles radiciformes propres à cette espèce. – Rev. Gén. Bot. 11: 401-408.
Heckel E. 1900. Sur le parasitisme du Ximenia americana L. – Compt. Rend. Acad. Sci. 131: 764-765.
Heckel E. 1901. Sur le processus germinatif dans les genres Onguekoa et Strombosia de la famille des Olacacées. – Ann. Inst. Bot.-Géol. Col. Marseille 8: 17-27.
Heckel E. 1902. Sur la germination des Onguekoa et des Strombosia – Compt. Rend. Acad. Sci. 134: 489-490.
Heide-Jørgensen HS. 1987. Changes in cuticle structure during development and attachment of the upper haustorium of Cuscuta L., Cassytha L., and Viscum L. – In: Weber HC, Forstreuter W (eds), Parasitic flowering plants, Philipps-Universität, Marburg, pp. 319-334.
Heil H. 1926. Haustorialstudien an Struthanthus-arten. – Flora 121: 40-76.
Heinricher E. 1915a. Die Keimung und Entwicklungsgeschichte der Wacholdermistel, Arceuthobium oxycedri, auf Grund durchgeführter Kulturen geschildert. – Sitz.-Ber. Akad. Wiss. Wien 124: 319-352.
Heinricher E. 1915b. Über Bau und Biologie der Blüten von Arceuthobium oxycedri (DC.) M.B. – Sitz.-Ber. Akad. Wiss. Wien 124: 481-504.
Hendrych R. 1966. Thesium hispanicum sp. nova, a new plant for Spain. – Folia Geobot. Phytotaxon. 1: 70-77.
Hendrych R. 1968. A treatise of Thesium arvense. – Acta Univ. Carol.-Biol. 1968: 243-262.
Hendrych R. 1969. The outline of the taxonomy and chorology of Thesium linophyllon. – Acta Univ. Carol.-Biol. 1969: 119-170.
Hendrych R. 1970. The natural history and systematics of the genus Thesium L. – Acta Univ. Carol., Biol., 1970: 293-358.
Herbert DA. 1918-1919. The West Australian Christmas Tree, Nuytsia floribunda – (The Christmas Tree) – its structure and parasitism. – J. Roy. Soc. W. Austr. 5: 72-88.
Herbert DA. 1922. The parasitism of Olax imbricata. – Philipp. Agric. 11: 17-18.
Hewson HJ. 1984. Balanophoraceae. – In: George AS (ed), Flora of Australia 22, Australian Government Publ. Service, Canberra, pp. 146-147.
Hewson HJ, George AS. 1984. Santalaceae. – In: George AS (ed), Flora of Australia, Australian Government Publ. Service, Canberra, pp. 29-67.
Hiepko P. 1979. A revision of Opiliaceae I. Genera of the eastern Old World, excluding Opilia. – Willdenowia 9: 13-56.
Hiepko P. 1972. Zwei neue Urobotrya-Arten (Opiliaceae) aus Südostasien. – Willdenowia 6: 471-477.
Hiepko P. 1979. A revision of Opiliaceae I. Genera of the eastern Old World, excluding Opilia. – Willdenowia 9: 13-56.
Hiepko P. 1982. A revision of Opiliaceae II. Opilia Roxb. – Willdenowia 12: 161-182.
Hiepko P. 1984a. Opiliaceae. – In: Steenis CGGJ van (ed), Flora Malesiana I, 10(1), Martinus Nijhoff, The Hague, Boston, London, pp. 31-52.
Hiepko P. 1984b. Opiliaceae. – In: George AS (ed), Flora of Australia 22, Australian Government Publ. Service, Canberra, pp. 27-29.
Hiepko P. 1985. A revision of Opiliaceae III. Urobotrya Stapf. – Bot. Jahrb. Syst. 107: 137-152.
Hiepko P. 1987. A revision of Opiliaceae IV. Rhopalopilia Pierre and Pentarhopalopilia (Engler) Hiepko gen. nov. – Bot. Jahrb. Syst. 108: 271-291.
Hiepko P. 1997. A new name and a new combination in the neotropical genus Agonandra (Opiliaceae). – Willdenowia 27: 225-226.
Hiepko P. 2000. Flora Neotropica. Monograph 82. Opiliaceae. – New York Botanical Garden Press, Bronx, New York.
Hiepko P. 2002. 30A. Opiliaceae. – In: Harling G, Andersson L (eds), Flora of Ecuador 69, Botanical Institute, Göteborg University, pp. 49-57.
Hiepko P. 2008. Opiliaceae. – In: Hiepko P (ed), Species plantarum: Flora of the world Part 12. Conservatoire et Jardin botaniques de la Ville de Genève, pp. 1-71.
Hieronymus G. 1889a. Myzodendraceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(1), W. Engelmann, Leipzig, pp. 198-202.
Hieronymus G. 1889b. Santalaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(1), W. Engelmann, Leipzig, pp. 202-227.
Hilliard OM. 1994. A note on Colpoon (Santalaceae). – Edinb. J. Bot. 51: 391-392.
Holzapfel S. 2001. Studies of the New Zealand root-parasite Dactylanthus taylorii (Balanophoraceae). – Englera 22: 1-176.
Hopkins CY, Chisholm MJ. 1969. Fatty acid components of some Santalaceae seed oils. – Phytochemistry 8: 161-165.
Hopper SD. 2010. 660. Nuytsia floribunda Loranthaceae. – Curtis’s Bot. Mag. 26: 333-368.
Hsiao SC, Mauseth JD, Gomez LD. 1993. Growth and anatomy of the vegetative body of the parasitic angiosperm Helosis cayennensis (Balanophoraceae). – Bull. Torrey Bot. Club 120: 295-309.
Hsiao SC, Mauseth JD, Gomez LD. 1994. Growth and anatomy of the vegetative body of the parasitic angiosperm Langsdorffia hypogaea (Balanophoraceae). – Bull. Torrey Bot. Club 121: 24-39.
Hsiao SC, Mauseth JD, Peng CI. 1995. Composite bundles, the host/parasite interface in the holoparasitic angiosperms Langsdorffia and Balanophora. – Amer. J. Bot. 82: 81-91.
Hürlimann H, Stauffer HU. 1957. Santalales-Studien II. Daenikera, eine neue Santalaceen-Gattung. – Vierteljahrsschr. Naturforsch. Ges. Zürich 102: 332-336.
Iwasa J, Kimura Y. 1969. Studies on the constituents of Muira puama. – J. Pharm. Soc. Japan 89: 1172-1174.
Jarzen DM. 1977. Aquilapollenites and some Santalalean genera. A botanical comparison. – Grana 16: 29-39.
Johnson T. 1888. Arceuthobium oxycedri. – Ann. Bot. 2: 137-160.
Johri BM, Agarwal S. 1965. Morphological and embryological studies in the family Santalaceae VIII. Quinchamalium chilense Lam. – Phytomorphology 15: 360-372.
Johri BM, Bhatnagar SP. 1960. Embryology and taxonomy of the Santalales I. – Proc. Natl. Inst. Sci. India, Biol. Sci., 26B (Suppl.): 199-220.
Johri BM, Agrawal JS, Garg S. 1957. Morphological and embryological studies in the family Loranthaceae I. Helicanthes elastica (Desr.) Dans. – Phytomorphology 7: 336-354.
Johri BM, Raj B. 1965. Embryo sac development in Moquiniella. – Nature 205(4969): 415-416.
Johri BM, Raj B. 1969. Morphological and embryological studies in the family Loranthaceae XII. Moquiniella rubra (Spreng. f.) Balle. – Österr. Bot. Zeitschr. 116: 475-485.
Jones BL, Gordon CC. 1965. Embryology and development of the endosperm haustorium of Arceuthobium douglasii. – Amer. J. Bot. 52: 127-132.
Jørgensen PM, Ulloa C. 2002. 30B. Olacaceae. – In: Harling G, Andersson L (eds), Flora of Ecuador 69, Botanical Institute, Göteborg University, pp. 61-103.
Joshi PC. 1960. Morphological and embryological studies in the family Santalaceae V. Oxyris wightiana Wall. – Phytomorphology 10: 239-248.
Kirkup D. 1998. Pollination mechanisms in African Loranthaceae. – In: Polhill R, Wiens D (eds), Mistletoes of Africa, Royal Botanic Gardens, Kew, pp. 37-60.
Koek-Noorman J, Rijckevorsel P van. 1983. Wood and leaf anatomy of Opiliaceae. – Willdenowia 13: 147-174.
Kubat R. 1987. Report of the first investigations of parasitism in Opiliaceae (Santalales). – In: Weber HC, Forstreuter W (eds), Parasitic flowering plants. Proceedings of the 4th ISPFP, Kempkes Offset, Marburg, pp. 489-492.
Kubitzki K. 2014. Chemosystematics. – In: Kuijt J, Hansen B (eds), The families and genera of vascular plants 12, Flowering plants: Eudicots; Santalales, Balanophorales, Springer, Cham, Switzerland, pp. 43-48.
Kuijt J. 1960. Morphological aspects of parasitism in the dwarf mistletoes (Arceuthobium). – Univ. Calif. Publ. Bot. 30: 337-436.
Kuijt J. 1961. Notes on the anatomy of the genus Oryctanthus (Loranthaceae). – Can. J. Bot. 39: 1809-1816.
Kuijt J. 1963. On the ecology and parasitism of the Costa Rican tree mistletoe, Gaiadendron punctatum (Ruiz and Pavón) G. Don. – Can. J. Bot. 41: 927-938.
Kuijt J. 1964a. A revision of the Loranthaceae of Costa Rica. – Bot. Jahrb. Syst. 83: 251-326.
Kuijt J. 1964b. Critical observations on the parasitism of New World mistletoes. – Can. J. Bot. 42: 1243-1278.
Kuijt J. 1965. On the nature and action of the santalalean haustorium, as exemplified by Phthirusa and Antidaphne (Loranthaceae). – Acta Bot. Neerl. 14: 278-307.
Kuijt J. 1967. On the structure and origin of the seedling of Psittacanthus schiedeanus (Loranthaceae). – Can. J. Bot. 45: 1497-1506.
Kuijt J. 1968. Mutual affinities of Santalalean families. – Brittonia 20: 136-147.
Kuijt J. 1969. The biology of parasitic flowering plants. – University of California Press, Berkeley and Los Angeles, California.
Kuijt J. 1970. Seedling establishment in Psittacanthus (Loranthaceae). – Can. J. Bot. 48: 705-711.
Kuijt J. 1973. Further evidence for the systematic position of Psittacanthus sonorae (Loranthaceae). – Madroño 22: 177-185.
Kuijt J. 1975. The genus Cladocolea (Loranthaceae). – J. Arnold Arb. 56: 265-335.
Kuijt J. 1976. Revision of the genus Oryctanthus (Loranthaceae). – Bot. Jahrb. Syst. 95: 478-534.
Kuijt J. 1977. Haustoria of phanerogamic parasites. – Ann. Rev. Phytopathol. 17: 91-118.
Kuijt J. 1978. Germination of Comandra (Santalaceae). – Madroño 25: 202-204.
Kuijt J. 1979. Host selection by parasitic angiosperms. – Symb. Bot. Upsal. 22: 194-199.
Kuijt J. 1980a. A note on heterophylly and branching patterns in the Amyema complex (Loranthaceae). – Blumea 26: 403-410.
Kuijt J. 1980b. Miscellaneous mistletoe notes 1–9. – Brittonia 32: 518-529.
Kuijt J. 1981. Inflorescence morphology of Loranthaceae: an evolutionary synthesis. – Blumea 27: 1-73.
Kuijt J. 1982a. Seedling morphology and its systematic significance in Loranthaceae of the New World, with supplementary comments on Eremolepidaceae. – Bot. Jahrb. Syst. 103: 305-342.
Kuijt J. 1982b. Epicortical roots and vegetative reproduction in Loranthaceae (s.s.) of the New World. – Beitr. Biol. Pflanzen 56: 307-316.
Kuijt J. 1983. Status of the genera Aetanthus and Psathyranthus (Loranthaceae). – Candollea 38: 661-672.
Kuijt J. 1985. Morphology, biology, and systematic relationships of Desmaria (Loranthaceae). – Plant Syst. Evol. 151: 121-130.
Kuijt J. 1986a. 32A. Eremolepidaceae, 32B. Viscaceae, 32C. Loranthaceae. – In: Harling G, Sparre B (eds), Flora of Ecuador 24, Swedish Natural Science Research Council, Stockholm, pp. 1-197.
Kuijt J. 1986b. Observations on establishment and early shoot emergence of Viscum minimum (Viscaceae). – Acta Bot. Neerl. 35: 449-456.
Kuijt J. 1988a. Monograph of the Eremolepidaceae. – Syst. Bot. Monogr. 18: 1-60.
Kuijt J. 1988b. Revision of Tristerix (Loranthaceae). – Syst. Bot. Monogr. 19: 1-61.
Kuijt J. 1989. Additional notes on the parasitism of New World Loranthaceae. – Beitr. Biol. Pflanzen 64: 115-125.
Kuijt J. 1990a. A second species of Ligaria (Loranthaceae). – Brittonia 42: 66-69.
Kuijt J. 1990b. Correlations in the germination patterns of Santalacean and other mistletoes. – In: Baas P et al. (eds), The plant diversity of Malesia, pp. 63-72.
Kuijt J. 1991a. Panamanthus, a new monotypic genus of Neotropical Loranthaceae. – Ann. Missouri Bot. Gard. 78: 172-176.
Kuijt J. 1991b. Inflorescence structure and generic placement of some small-flowered species of Phthirusa (Loranthaceae). – Syst. Bot. 16: 283-291.
Kuijt J. 1991c. Two new species of Ixocactus (Loranthaceae) and a reformulation of the genus. – Syst. Bot. 16: 292-298.
Kuijt J. 2000. Two new Brazilian species of Oryctina (Loranthaceae) with a revised key to the genus. – Novon 10: 391-397.
Kuijt J. 2003a. Monograph of Phoradendron (Viscaceae). – Syst. Bot. Monogr. 66: 1-643.
Kuijt J. 2003b. Two new South American species of Struthanthus (Loranthaceae) posing a challenge to circumscription of neotropical genera. – Bot. J. Linn. Soc. 142: 469-474.
Kuijt J. 2003c. A new species of Oryctina (Loranthaceae) from Guyana. – Brittonia 55: 169-172.
Kuijt J. 2009a. Monograph of Psittacanthus (Loranthaceae). – Syst. Bot. Monogr. 86: 1-361.
Kuijt J. 2009b. Miscellaneous mistletoe notes, 48-60: descriptions of twelve new species of Loranthaceae and Viscaceae. – Brittonia 61: 144-162.
Kuijt J. 2010. A note on stamen position and petal number in Loranthaceae. – Blumea 55: 224-225.
Kuijt J. 2011a. Pulling the skeleton out of the closet: resurrection of Phthirusa Martius and the consequent revival of Passovia (Loranthaceae). – Plant Divers. Evol. 129: 159-211.
Kuijt J. 2011b. Monograph of Dendropemon (Loranthaceae). – Syst. Bot. Monogr. 92: 1-110.
Kuijt J. 2012. Reinstatement and expansion of the genus Peristethium (Loranthaceae). – Ann. Missouri Bot. Gard. 98: 542-577.
Kuijt J. 2012. Prophyll, calyculus, and perianth in Santalales. – Blumea 57: 248-252.
Kuijt J. 2014a. A monograph of the genus
Aetanthus (Loranthaceae).
– Plant Div. Evol. 131: 1-51.
Kuijt J. 2014b. Five new species, one new
name, and transfers in Neotropical mistletoes (Loranthaceae), miscellaneous notes,
61-68. – Novon 23: 176-186.
Kuijt J. 2014c. Santalales. – In: Kuijt J, Hansen B (eds), The families and genera of vascular plants 12, Flowering plants: Eudicots; Santalales, Balanophorales, Springer, Cham, Switzerland, pp. 1-189.
Kuijt J. 2016. Measurements and taxonomy in Arceuthobium (Viscaceae). – Phytologia 98: 186-189.
Kuijt J, Bruns D. 1987. Roots in Corynaea (Balanophoraceae). – Nord. J. Bot. 7: 539-542.
Kuijt J, Dong WX. 1990a. Surface features of the leaves of Balanophoraceae. A family without stomata? – Plant Syst. Evol. 170: 29-35.
Kuijt J, Dong WX. 1990b. The systematic significance of the surface features of the Balanophoraceae tuber. – Plant Syst. Evol. 171: 129-134.
Kuijt J, Hansen B (eds). 2015. The families and genera of vascular plants XII. Flowering plants – eudicots – Santalales, Balanophorales. – Springer, 213 pp.
Kuijt J, Lye D. 2005a. A preliminary survey of foliar sclerenchyma in neotropical Loranthaceae. – Blumea 50: 323-355.
Kuijt J, Lye D. 2005b. Gross xylem structure of the interface of Psittacanthus ramiflorus (Loranthaceae) with its hosts and with a hyperparasite. – Bot. J. Linn. Soc. 147: 197-201.
Kuijt J, Weberling F. 1972 (1973). The flower of Phthirusa pyrifolia (Loranthaceae). – Ber. Deutsch. Bot. Ges. 85: 467-480.
Kuo J, Pate JS, Davidson NJ. 1989. Ultrastructure of the haustorial interface and apoplastic continuum between host and the root hemiparasite Olax phyllanthi (Labill.) R. Br. (Olacaceae). – Protoplasma 150: 27-39.
Kusano S. 1902. Studies on the parasitism of Buckleya quadriala, B. et H., a Santalaceous parasite, and on the structure of its haustorium. – J. Coll. Sci., Imp. Univ. Tokyo 17: 1-42.
Kuwada Y. 1928. An occurrence of restitution-nuclei in the formation of the embryosacs in Balanophora japonica Mak. – Bot. Mag. (Tokyo) 42: 117-129.
Ladley JJ, Kelly D, Robertson AW. 1997. Explosive flowering, nectar production, breeding systems, and pollinators of New Zealand mistletoes (Loranthaceae). – New Zealand J. Bot. 35: 345-360.
Laphitz RML, Ezcurra C, Vidal-Russell R. 2015. Morphological variation in Quinchamalium (Schoepfiaceae) is associated with climatic patterns along its Andean distribution. – Syst. Bot. 40: 1045-1052.
Le C-T, Liu B, Barrett RL, Lu L-M, Wen J, Chen Z-D. 2018. Phylogeny and a new tribal classification of Opiliaceae (Santalales) based on molecular and morphological evidence. – J. Syst. Evol. 56: 56-66.
Lee S-K. 1980. Malania, a new genus of oil-yielding plant. – Bull. Bot. Lab. North-East For. Inst. 6: 67-72.
Léonard J, Troupin G. 1950. Observations sur le genre Okoubaka Pellgr. et Normand (Octoknemaceae). – Bull. Jard. Bot. État Bruxelles 20: 11-14.
Leopold DJ, Muller RN. 1983. Hosts of Pyrularia pubera Michx. (Santalaceae) in the field and in culture. – Castanea 48: 138-145.
Lepschi BJ. 1999. Taxonomic revision of Leptomeria (Santalaceae). – Aust. Syst. Bot. 12: 55-100.
Li J, Boufford DE, Donoghue MJ. 2001. Phylogenetics of Buckleya (Santalaceae) based on its sequences of nuclear ribosomal DNA. – Rhodora 103: 137-150.
Li WZ. 1989. A new species of Melientha (a new recorded genus of Opiliaceae from China). – Acta Bot. Yunnan. 11: 407-408.
Ling Y-R. 1982. A revision and addition of Olacaceae from China and a primitive discussion for the taxonomy and floristics of the family. – Bull. Bot. Res. 2: 7-36.
Liu B, Le CT, Barrett RL, Nickrent DL, Chen Z, Lu L, Vidal-Russell R. 2018. Historical biogeography of Loranthaceae (Santalales): diversification agrees with emergence of tropical forests and radiation of songbirds. – Mol. Phylogen. Evol. 124: 199-212.
Lobreau-Callen D. 1980. Caractères comparées du pollen des Icacinaceae et des Olacaceae. – Adansonia, sér. II, 20: 29-89.
Lobreau-Callen D. 1982. Structures et affinités polliniques des Cardiopterygaceae, Dipentodontaceae, Erythropalaceae et Octoknemataceae. – Bot. Jahrb. Syst. 103: 371-412.
Lotsy JP. 1901. Rhopalocnemis phalloides Jungh. A morphological-systematical study. – Ann. Jard. Bot. Buitenzorg 17: 73-101.
Louis J, Léonard J. 1948. Octoknemaceae. – In: Comite Executif de la Flore du Congo-Belge & Jardin Botanique de l’État (eds), Flore du Congo Belge et du Ruanda-Urundi. Spermatophytes 1, Jardin Botanique de l’État, Bruxelles.
Lucas GL. 1968a. Olacaceae. – In: Milne-Redhead E, Polhill RM (eds), Flora of tropical East Africa, Crown Agents for Oversea Governments and Administrations, London, pp. 1-16.
Lucas GL. 1968b. Opiliaceae. – In: Milne-Redhead E, Polhill RM (eds), Flora of tropical East Africa, Crown Agents for Oversea Governments and Administrations, London, pp. 1-7.
Maas PJM, Baas P, Boesewinkel FD, Hiepko P, Lobreau-Callen D, Oever L van den, Welle BJH ter. 1992. The identity of “unknown Z”: Maburea Maas, a new genus of Olacaceae in Guyana. – Bot. Jahrb. Syst. 114: 275-291.
Macklin J. 2000. A systematic revision of the Santalaceae R. Br. of Southeast Asia. – Ph.D. diss., Trinity College, Dublin, Ireland.
Macklin J, Parnell J. 2000. An introduction to the Santalaceae R. Br. of Thailand. – Thai For. Bull. 28: 112-122.
Macklin J, Parnell J. 2002. An account of the Santalaceae of Thailand. – Thai For. Bull. 30: 75-115.
Maguire B, Wurdack JJ, Huang Y-C. 1974. Pollen grains of some American Olacaceae. – Grana Palynol. 14: 26-38.
Maheshwari P, Singh B. 1952. Embryology of Macrosolen cochinchinensis. – Bot. Gaz. 113: 20-32.
Maheshwari P, Johri BM, Dixit SN. 1957. The floral morphology and embryology of the Loranthoideae (Loranthaceae). – J. Madras Univ., Ser. B, 27: 121-136.
Malécot V. 2002. Histoire, classification et phylogénie des Olacaceae R. Brown (Santalales). – Ph.D. diss., l’Université de Pierre et Marie Curie, Paris.
Malécot V, Nickrent DL. 2008. Molecular phylogenetic relationships of Olacaceae and related Santalales. – Syst. Bot. 33: 97-106.
Malécot V, Schatz GE, Bosser J. 2003. Révision synoptique du genre Phanerodiscus Cavaco (Olacaceae) à Madagascar. – Adansonia, sér. III, 25: 119-128.
Malécot V, Nickrent DL, Baas P, Oever L van den, Lobreau-Callen D. 2004. A morphological cladistic analysis of Olacaceae. – Syst. Bot. 29: 569-586.
Mangenot G. 1947. Recherches sur l’organisation d’une Balanophoracée: Thonningia coccinea Vahl. – Rev. Gén. Bot. 54: 201-244, 271-294.
Mathiasen RL, Kenaley SC. 2017. Contrasting perspectives on the measurements and taxonomy of Arceuthobium (Viscaceae): a long standing controversy. – Phytologia 99: 95-110.
Mathiasen RL, Nickrent DL, Shaw DC, Watson DM. 2008. Mistletoes: pathology, systematics, ecology, and management. – Plant Dis. 92 933-1006.
Mauseth JD, Montenegro G, Walckowiak AM. 1985. Host infection and flower formation by the parasite Tristerix aphyllus (Loranthaceae). – Can. J. Bot. 63: 567-581.
Mauseth JD, Hsiao SC, Montenegro G. 1992. Vegetative body of the parasitic angiosperm Ombrophytum subterraneum (Balanophoraceae). – Bull. Torrey Bot. Club 119: 407-417.
Metcalfe CR. 1935. The structure of some sandalwoods and their substitutes and some other little known scented woods. – Kew Bull. 1935: 165-195.
Michaud M. 1962. Contribution à l’étude des Olacacées d’Afrique tropicale. – DESS diss., Université de Jussieu Paris VI, Paris.
Michaud M. 1966. Contribution à l’étude des Olacacées d’Afrique tropicale. – Mém. Inst. Fond. d’Afrique Noire 75: 157-290. [or: Schnell R, Grout de Beaufort F, Bernhard F, Michaud M. Mélanges Botaniques. – Institut fondamental d’Afrique noire.]
Mildbraed J. 1935. Octoknemaceae. – In: Engler A (†), Harms H (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 16b, W. Engelmann, Leipzig, pp. 42-45.
Molvray M. 1997. A synopsis of Korthalsella (Viscaceae). – Novon 7: 268-273.
Molvray M, Kores PJ, Chase MW. 1999. Phylogenetic relationships within Korthalsella (Viscaceae) based on nuclear ITS and plastid trnL-F sequence data. – Amer. J. Bot. 86: 249-260.
Moore LB. 1940. The structure and life-history of the root parasite Dactylanthus taylori Hook. f. – New Zealand J. Sci. Techn. 21: 206B-224B.
Moore TE, Verboom GA, Forest F. 2010. Phylogenetics and biogeography of the parasitic genus Thesium (Santalaceae), with an emphasis on the Cape of South Africa. – Bot. J. Linn. Soc. 162: 435-452.
Musselman LJ, Mann WF. 1977. Cataphyll behavior in Ximenia americana seedlings (Olacaceae). – Beitr. Biol. Pflanzen 53: 121-125.
Narayana R. 1958a. Morphological and embryological studies in the family Loranthaceae II. Lysiana exocarpi (Behr.) van Tieghem. – Phytomorphology 8: 146-169.
Narayana R. 1958b. Morphological and embryological studies in the family Loranthaceae III. Nuytsia floribunda (Labill.) R. Br. – Phytomorphology 8: 306-323.
Naumann J, Salomo K, Der JP, Wafula EK, Bolin JF, Maass E, Frenzke L, Samain M-S, Neinhuis C, dePamphilis CW, Wanke S. 2013. Single-copy nuclear genes place haustorial Hydnoraceae within Piperales and reveal a Cretaceous origin of multiple parasitic angiosperm lineages. – PLoS ONE 8: e79204. http://dx.doi.org/10.1371/journal.pone.0079204
Nee M. 1996. A new species of Acanthosyris (Santalaceae) from Bolivia and a key to the woody South American Santalaceae. – Brittonia 48: 574-579.
Nickrent DL. 2002. Mistletoe phylogenetics: current relationships gained from analysis of DNA sequences. – In: Angwin P (ed), Proceedings of the forty-eighth western international forest disease work conference, USDA Forest Service, Redding, California, pp. 48-57.
Nickrent DL. 1997–. The Parasitic Plant Connection. http.//www.parasiticplants.siu.edu/
Nickrent DL, Duff RJ. 1996. Molecular studies of parasitic plants using ribosomal RNA. – In: Moreno MT, Cubero JI, Berner D, Joel D, Musselman LJ, Parker C (eds), Advances in parasitic plant research, Junta de Andalucia, Dirección General de Investigación Agraria, Cordoba, pp. 28-52.
Nickrent DL, Franchina CR. 1990. Phylogenetic relationships of the Santalales and relatives. – J. Mol. Evol. 31: 294-301.
Nickrent DL, García MA. 2009. On the brink of holoparasitism: plastome evolution in dwarf mistletoes (Arceuthobium, Viscaceae). – J. Mol. Evol. 68: 603-615.
Nickrent DL, Malécot V. 2001. A molecular phylogeny of the Santalales. – In: Fer A, Thalouarn P, Joel DM, Musselman LJ, Parker C, Verkleij JAC (eds), Proceedings of the 7th international parasitic weed symposium. Faculté des Sciences, l’Université de Nantes, Nantes, pp. 69-74.
Nickrent DL, Starr EM. 1994. High rates of nucleotide substitution in nuclear small-subunit (18S) rDNA from holoparasitic flowering plants. – J. Mol. Evol. 39: 62-70.
Nickrent DL, Schuette KP, Starr EM. 1994. A molecular phylogeny of Arceuthobium (Viscaceae) based on nuclear ribosomal DNA internal transcribed spacer sequences. – Amer. J. Bot. 81: 1149-1160.
Nickrent DL, Duff RJ, Colwell AE, Wolfe AD, Young ND, Steiner KE, dePamphilis C. 1998. Molecular phylogenetic and evolutionary studies of parasitic plants. – In: Soltis D, Soltis P, Doyle J (eds), Molecular systematics of plants II. DNA sequencing, Kluwer Academic Publ., Boston, pp. 211-241.
Nickrent DL, Malécot V, Vidal-Russell R, Der JP. 2010. A revised classification of Santalales. – Taxon 59: 538-558.
Normand D. 1944. Note sur l’anatomie du genre nouveau Okoubaka. – Bull. Soc. Bot. France 91: 20-25.
Norverto CA. 1993. Wood anatomy and relationships of Santalaceae I. Acanthosyris, Jodina, and Myoschilos. – Aliso 13: 499-511.
Norverto CA. 2004. Evolución de las Santalaceae. – Revist. Fac. Ci. Agrar. 22: 1668-1940.
Norverto CA. 2011. Study of the comparative wood anatomy of the species of Amphorogynaceae, Cervantesiaceae, Nanodeaceae, Santalaceae and Thesiaceae. – J. Bot. Res. Inst. Texas 5: 643-659.
Oever L van den. 1984. Comparative wood anatomy of the Olacaceae. – In: Sudo S (ed), Proceedings of the Pacific Regional Wood Anatomy Conference, Tsukuba University, Tsukuba, pp. 177-178.
Oever L van den. 1990. Phylogenetic wood anatomy of the Olacaceae and related families. – IAWA Bull., N. S., 11: 133.
Oever L van den, Welle BJH ter, Koek-Noorman J. 1993. Olacaceae woods and timber. – In: Gorts-van Rijn ARA (ed), Flora of Guiana, Koeltz Scientific Books, Koenigstein, Germany, pp. 44-64.
Orfilia EN. 1976. Sinopsis de las Misodendraceae de la Argentina y Chile. – Rev. Fac. Agron. Univ. Nac. La Plata 12: 37-62.
Orfilia EN. 1978. Misodendraceae de Argentina y Chile. – Buenos Aires.
Orhan DD, Orhan I. 2006. Fatty acid composition of Viscum album subspecies from Turkey. – Chem. Nat. Compounds 42: 641-644.
Paliwal RL. 1956. Morphological and embryological studies in some Santalaceae. – Agra Univ. J. Res. Sci. 5: 193-284.
Pankow E, Auterhoff H. 1969. Inhaltsstoffe von Muira puama. – Arch. Pharm. (Berlin) 302: 209-212.
Pate JS, Davidson NJ, Kuo J, Milburn JA. 1990. Water relations of the root hemiparasite Olax phyllanthi (Labill.) R. Br. (Olacaceae) and its multiple hosts. – Oecologia 84: 186-193.
Pate JS, Kuo J, Davidson NJ. 1990. Morphology and anatomy of the haustorium of the root hemiparasite Olax phyllanthi (Olacaceae), with special reference to the haustorial interface. – Ann. Bot. 65: 425-436.
Pate JS, Pate SP, Kuo J, Davidson NJ. 1990. Growth, resource allocation, and haustorial biology of the root hemiparasite Olax phyllanthi (Olacaceae). – Ann. Bot. 65: 437-449.
Pate JS, Woodall G, Jeschke W, Stewart G. 1994. Root xylem transport of amino-acids in the root hemiparasitic shrub Olax phyllanthi (Labill.) R. Br. (Olacaceae) and its multiple hosts. – Plant Cell Environm. 17: 1263-1273.
Patil DA, Pai RM. 1984. The floral anatomy of Olax scandens Roxb. (Olacaceae). – Indian Bot. Rep. 3: 10-14.
Piehl MA. 1965. The natural history and taxonomy of Comandra (Santalaceae). – Mem. Torrey Bot. Club 22: 1-97.
Piehl MA. 1973. Root parasitism in Schoepfia chrysophylloides (A. Rich.) Planchon and Ximenia americana L. (Santalales, Olacaceae). – ASB Bull. 20: 75.
Pierre JBL. 1897. Sur le genre Ongokea et la famille des Aptandracées. – Bull. Mens. Soc. Linn. Paris, sér. II, 2: 1313-1315.
Pilger R. 1924. Die Santalaceae von Neu-Guinea, mit bemerkungen über die Gattung Exocarpus im Allgemeinen. – Engl. Bot. Jahrb. Syst. 59: 118-128.
Pilger R. 1935. Santalaceae. – In: Engler A (†), Harms H (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 16b, W. Engelmann, Leipzig, pp. 52-91.
Ping ST. 1997. Root hemi-parasitism in Malayan Olacaceae. – Gard. Bull. (Singapore) 49: 7-13.
Pisek A. 1924. Antherenentwicklung und meiotische Teilung bei der Wacholdermistel (Arceuthobium oxycedri DC.) M.B. Antherenbau und Chromosomenzahlen von Loranthus europaeus Jacq. – Sitz.-Ber. Akad. Wiss. Wien 133: 1-15.
Pizzoni P. 1906. Contribuzione alla conoscenza degli austori dell’Osyris alba. – Ann. Botanica 4: 79-98.
Polhill RM, Wiens D. 1998. Mistletoes of Africa. – Royal Botanic Gardens, Kew, Richmond, England.
Polhill RM, Wiens D. 1999a. Loranthaceae. – In: Beentje HJ, Whitehouse CM (eds), Flora of tropical East Africa, A. A. Balkema, Rotterdam, pp. 1-121.
Polhill RM, Wiens D. 1999b. Viscaceae. – In: Beentje HJ, Whitehouse CM (eds), Flora of tropical East Africa, A. A. Balkema, Rotterdam, pp. 1-24.
Polonsky J, Beloeil JC, Prange T, Pascard C, Jacquemin H, Donnelly DMX, Kenny PTM. 1983. Manicol: a sesquiterpenoid hydroxytropolone from Dulacia guianensis: a revised structure (X-ray analysis). – Tetrahedron 39: 2647-2656.
Prakash S. 1960. Morphological and embryological studies in the family Loranthaceae VI. Peraxilla tetrapetala (Linn. f.) Van Tiegh. – Phytomorphology 10: 224-234.
Prakash S. 1963. Morphological and embryological studies in the family Loranthaceae X. Barathranthus axanthus (Korth.) Miq. – Phytomorphology 13: 97-103.
Raj B. 1970. Morphological and embryological studies in the family Loranthaceae XIII. Amylotheca dictyophleba Van Tiegh. – Österr. Bot. Zeitschr. 118: 417-430.
Ram M. 1957. Morphological and embryological studies in the family Santalaceae I. Comandra umbellata (L.) Nutt. – Phytomorphology 7: 24-35.
Ram M. 1959a. Morphological and embryological studies in the family Santalaceae II. Exocarpus with a discussion on its systematic position. – Phytomorphology 9: 4-19.
Ram M. 1959b. Morphological and embryological studies of the family Santalaceae III. Leptomeria R. Br. – Phytomorphology 9: 20-33.
Ram M. 1959c. Occurrence of embryo sac-like structures in the microsporangia of Leptomeria billardierii R. Br. – Nature 184: 914-915.
Ram M. 1970. Comparative ambryology of angiosperms: Olacaceae, Opiliaceae, Grubbiaceae, Myzodendraceae, Loranthaceae. – Bull. Natl. Sci. Acad. India 41: 4-8, 9-12, 13-14, 19-21, 22-28.
Rao LN. 1942a. Parasitism in the Santalaceae. – Ann. Bot., N. S., 6: 131-150.
Rao LN. 1942b. Studies in the Santalaceae. – Ann. Bot., N. S., 6: 151-175.
Rao TA, Kelkar SS. 1951. Studies on foliar sclereids in dicotyledons III. On sclereids in species of Loranthus (Loranthaceae) and Niebuhria apetala (Capparidaceae). – J. Univ. Bombay, N. S., Sect. B, 20: 16-20.
Record SJ. 1938. The American woods of the orders Celastrales, Olacales, and Santalales. – Trop. Woods 53: 11-38.
Reed CF. 1955. The comparative morphology of the Olacaceae, Opiliaceae, and Octoknemaceae. – Mem. Soc. Brot. 10: 29-79.
Reid N. 1987. Ramifying haustoria in Australian mistletoes: an adaptation to aridity, or for vegetative persistence on long-lived hosts? – Golden Bough 9: 1-2.
Reid N. 1988. The mistletoebird and Australian mistletoe: coevolution or coincidence? – Emu 87: 130-131.
Reid N. 1991. Coevolution of mistletoes and frugivorous birds. – Aust. J. Ecol. 16: 457-469.
Restrepo C, Sargent S, Levey D, Watson D. 2002. The role of vertebrates in the diversification of New World mistletoes. – In: Levey D, Silva W, Galetti M (eds), Seed dispersal and frugivory: ecology, evolution and conservation, CABI Publ., New York, pp. 83-98.
Rodrigues WA. 1961. Ensaios preliminaries de germinação da castanha Curupira em laboratorio. – Publ. Inst. Natl. Pesquisas Amazonia, Bot., 12: 1-22.
Rogers ZS, Malécot V, Sikes KG. 2006. A synoptic revision of Olax L. (Olacaceae) in Madagascar and the Comoro Islands. – Adansonia, sér. III, 28: 71-100.
Rogers ZS, Nickrent DL, Malécot V. 2008. Staufferia and Pilgerina: two new endemic monotypic arborescent genera of Santalaceae from Madagascar. – Ann. Missouri Bot. Gard. 95: 391-404.
Ross CM, Sumner MJ. 2004. Development of the unfertilized embryo sac and pollen tubes in the dwarf mistletoe Arceuthobium americanum (Viscaceae). – Can. J. Bot. 82: 1566-1575.
Ross CM, Sumner MJ. 2005a. Early endosperm and embryo development in the dwarf mistletoe Arceuthobium americanum (Viscaceae). – Intern. J. Plant Sci. 166: 901-927.
Ross CM, Sumner MJ. 2005b. Ultrastructure of the fertilized embryo sac in the dwarf mistletoe Arceuthobium americanum (Viscaceae) and development of the caecum. – Can. J. Bot. 83: 459-466.
Ross Friedman CM, Sumner MJ. 2009. Maturation of the embryo, endosperm, and fruit of the dwarf mistletoe Arceuthobium americanum (Viscaceae). – Intern. J. Plant Sci. 170: 290-300.
Rossow RA. 1982. Sinopsis de las Misodendraceae. – Parodiana 1: 245-270.
Rüffle L, Knappe H. 1988. Ökologische und paläogeographische Bedeutung der Oberkreideflora von Quedlinburg, besonders einiger Loranthaceae und Monimiaceae. – Hallesches Jahrb. Geowiss. 13: 49-65.
Rutishauser A. 1935. Entwicklungsgeschichtliche und zytologische Untersuchungen an Korthalsella Dacrydii (Ridl.) Danser. – Ber. Schweiz. Bot. Ges. 44: 389-436.
Rutishauser A. 1937. Blütenmorphologische und embryologische Untersuchungen an der Viscoideen Korthalsella opuntia und Ginalloa linearis Danser. – Ber. Schweiz. Bot. Ges. 47: 5-28.
Sallé GC. 1979. Le système endophytique du Viscum album: anatomie et fonctionnement des suçoirs secondaires. – Can. J. Bot. 57: 435-449.
Sato HS, Gonzalez AM. 2013. Anatomía y desarrollo de la flor estaminada, microsporogénesis y microgametogénesis en especies de Lophophytum (Balanophoraceae) en la Argentina. – Bol. Soc. Argent. Bot. 48: 59-72.
Schaeppi H. 1942. Morphologische und entwicklungsgeschichtliche Untersuchungen an den Blüten von Thesium. – Mitt. Naturwiss. Ges. Winterhur 23: 41-61.
Schaeppi H, Steindl F. 1937. Blütenmorphologische und embryologische Untersuchungen an Osyris alba L. – Ber. Schweiz. Bot. Ges. 47: 369-392.
Schaeppi H, Steindl F. 1942. Blütenmorphologische und embryologische Untersuchungen an Loranthoideen. – Vierteljahrsschr. Naturf. Ges. Zürich 87: 301-372.
Schaeppi H, Steindl F. 1945. Blütenmorphologische und embryologische Untersuchungen an einigen Viscoideen. – Vierteljahrsschr. Naturf. Ges. Zürich 90: 1-46.
Shamanna SA. 1954. A contribution to the embryology of Olax wightiana Wall. – Proc. Indian Acad. Sci., sect. B, 39: 249-256.
Shamrov II, Anisimova GM, Batygina TB, Lakshmi Sita G. 2001. The types and morphological evolution of the ovule in the order Santalales. – Bot. Žurn. 86: 1-14. [In Russian]
Shivamurthy GR, Swamy BGL, Arekal GD. 1981. Ontogeny and organization in the inflorescence in Balanophora. – Ann. Bot., N. S., 48: 853-859.
Singh V, Ratnakar G. 1974. Contribution to the floral anatomy of the Loranthaceae I. Subfamily Loranthoideae. – J. Indian Bot. Soc. 53: 162-169.
Siqueira I, Lara D, Silva D, Gaieski F, Nunes D, Elisabetsky E. 1998. Psychopharmacological properties of Ptychopetalum olacoides Bentham (Olacaceae). – Pharmac. Biol. 36: 327-334.
Siqueira IR, Cordova CAS, Creczynski-Pasa TB, Elisabetsky E, Nunes DS, Netto CA. 2002. Antioxidant action of an ethanol extract of Ptychopetalum olacoides. – Pharmac. Biol. 40: 374-379.
Siqueira IR, Fochesatt C, Silva AL da, Nunes DS, Battastini AM, Netto CA, Elisabetsky E. 2003. Ptychopetalum olacoides, a traditional Amazonian ’nerve tonic’, possesses anticholinesterase activity. – Pharmac. Biochem. Behav. 75: 645-650.
Skottsberg C. 1913a. Bemerkungen zur Systematik der Gattung Myzodendron. – Engl. Bot. Jahrb. Syst. 50: 384-391.
Skottsberg C. 1913b. Morphologische und embryologische Studien über die Myzodendraceen. – Kongl. Sv. Vetensk.-akad. Handl. 51(4): 1-34.
Skottsberg C. 1916. Zur Morphologie und Systematik der Gattung Arjona Cav. – Svensk Bot. Tidskr. 10: 520-528.
Skottsberg C. 1930. The geographic distribution of the sandal-woods and its significance. – In: Vries O de, Lam HJ, Bijlmer HJT (eds), Proceedings of the fourth Pacific Science congress 3, Pacific Science Assoc., Batavia and Bandoeng, Java, pp. 435-442.
Skottsberg C. 1935. Myzodendraceae. – In: Engler A (†), Harms H (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 16b, W. Engelmann, Leipzig, pp. 92-97.
Sleumer HO. 1935a. Olacaceae. – In: Engler A (†), Harms H (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 16b, W. Engelmann, Leipzig, pp. 5-32.
Sleumer HO. 1935b. Opiliaceae. – In: Engler A (†), Harms H (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 16b, W. Engelmann, Leipzig, pp. 33-41.
Sleumer HO. 1942. Erythropalaceae. – In: Engler A (†), Harms H, Mattfeld J (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 20b, W. Engelmann, Leipzig, pp. 401-403.
Sleumer HO. 1980. A taxonomic account of the Olacaceae of Asia, Malesia, and the adjacent areas. – Blumea 8: 145-168.
Sleumer HO. 1984a. Flora Neotropica. Monograph 38. Olacaceae. – In: Luteyn JL (ed), New York Botanical Garden, Bronx, New York.
Sleumer HO. 1984b. Olacaceae. – In: Steenis CGGJ van (ed), Flora malesiana I, 10(1), Martinus Nijhoff, The Hague, Boston, London, pp. 1-29.
Smith AC. 1937. A Colombian species of Cervantesia R. & P. – Trop. Woods 51: 12-14.
Smith FH, Smith EC. 1943. Floral anatomy of the Santalaceae and some related forms. – Oregon State Monogr. Stud. Bot. 5: 1-93.
Solms-Laubach H zu. 1875. Das Haustorium der Loranthaceen und der Thallus der Rafflesiaceen und Balanophoraceen. – Abh. Naturf. Ges. Halle 13(3): 1-40.
Soukup J. 1965. Opiliaceae, Balanophoraceae, Aristolochiaceae, Rafflesiaceae, ...Polygonaceae of Peru. – Biota 5: 315-339.
Spitzer V, Bordignon S, Schenkel E, Marx F. 1994. Identification of 9-acetylenic fatty-acids, 9-hydroxystearic acid and 9,10-epoxystearic acid in the seed oil of Jodina rhombifolia Hook. et Arn. (Santalaceae). – J. Amer. Oil Chem. Soc. 71: 1343-1348.
Spitzer V, Tomberg W, Hartmann R, Aichholz R. 1997. Analysis of the seed oil of Heisteria silvanii (Olacaceae): a rich source of a novel C-18 acetylenic fatty acid. – Lipids 32: 1189-1200.
Standley PC. 1920. The North American species of Agonandra. – J. Washington Acad. Sci. 10: 505-508.
Stauffer HU. 1957. Santalales-Studien I. Zur Stellung der Gattung Okoubaka Pellegrin et Normand. – Ber. Schweiz. Bot. Ges. 67: 422-427.
Stauffer HU. 1959. Santalales-Studien IV. Revisio Anthobolearum; eine morphologische Studie mit Einschluss der Geographie, Phylogenie und Taxonomie. – Mitt. Bot. Mus. Univ. Zürich 213: 1-260.
Stauffer HU. 1961a. Santalales-Studien VII. Südamerikansche Santalaceae I. Acanthosyris, Cervantesia und Jodina. – Vierteljahrsschr. Naturf. Ges. Zürich 106: 406-412.
Stauffer HU. 1961b. Santalales-Studien VIII. Zur Morphologie und Taxonomie des Olacaceae – Tribus Couleae. – Vierteljahrsschr. Naturf. Ges. Zürich 106: 412-418.
Stauffer HU. 1961c. Beiträge zum Blütendiagramm der Santalales. – Verh. Schweiz. Naturf. Ges. 141: 123-125.
Stauffer HU. 1968. Santalales-Studien IX. Spirogardnera, eine neue Santalaceen-Gattung aus West-Australien. – Mitt. Bot. Mus. Univ. Zürich 113: 305-309.
Stauffer HU. 1969. Santalales-Studien X. Amphorogyneae, eine neue Tribus der Santalaceae. – Vierteljahrsschr. Naturf. Ges. Zürich 114: 49-76.
Stauffer HU, Hürlimann H. 1957. Santalales-Studien III. Amphorogyne, eine weitere Santalaceen-Gattung aus Neukaledonien. – Vierteljahrsschr. Naturf. Ges. Zürich 102: 337-349.
Stearn WT. 1972. Kunkeliella, a new genus of Santalaceae in the Canary Islands. – Cuad. Bot. Canar. 16: 11-26.
Steenis CGGJ van. 1933. Het geslacht Henslowia op Java. – De Trop. Natuur 22: 97-99.
Steenis CGGJ van. 1955. Reduction of the genera Schizopremna Baill. (Verb.), and Worcesterianthus Merr. (Olacac.). – Acta Bot. Neerl. 4: 477-480.
Stefano RD de, Berry PE, Velásquez GO. 1995. A new species of Brachynema (Olacaceae) from South America. – Novon 5: 238-240.
Steindl F. 1935. Pollen- und Embryosackentwicklung bei Viscum album L. und Viscum articulatum Burm. – Ber. Schweiz. Bot. Ges. 44: 343-388.
Stevenson GB. 1934. The life history of the New Zealand species of the parasitic genus Korthalsella. – Trans. Roy. Soc. New Zealand 64: 175-190.
Su HJ, Hu JM. 2012. Rate heterogeneity in six protein-coding genes from the holoparasite Balanophora (Balanophoraceae) and other taxa of the Santalales. – Ann. Bot. 110: 1137-1147.
Su HJ, Hu JM, Anderson FE, Der JP, Nickrent DL. 2015. Phylogenetic relationships of Santalales with insights into the origins of holoparasitic Balanophoraceae. – Taxon 64: 491-506.
Suaza-Gaviria V, Pabón-Mora N, González F. 2016. Development and morphology of flowers in Loranthaceae. – Intern. J. Plant Sci. 177: 559-578.
Suaza-Gaviria V, González F, Pabón-Mora N. 2017. Comparative inflorescence development in selected Andean Santalales. – Amer. J. Bot. 104: 24-38.
Swamy BGL. 1949. The comparative morphology of the Santalaceae: node, secondary xylem, and pollen. – Amer. J. Bot. 36: 661-673.
Swamy BGL. 1960. Contributions to the embryology of Cansjera rheedii. – Phytomorphology 10: 397-409.
Tennakoon KU, Cameron DD. 2006. The anatomy of Santalum album (sandalwood) haustoria. – Can. J. Bot. 84: 1608-1616.
Teo SP. 1997. Root hemi-parasitism in Malayan Olacaceae. – Gard. Bull. (Singapore) 49: 7-13.
Thoday D. 1956a. Modes of union and interaction between parasite and host in the Loranthaceae I. Viscoideae, not including Phoradendreae. – Proc. Roy. Soc. B, 145: 531-548.
Thoday D. 1956b. Modes of union and interaction between parasite and host in the Loranthaceae II. Phoradendreae. – Proc. Roy. Soc. B, 146: 320-338.
Thoday D. 1958. Modes of union and interaction between parasite and host in the Loranthaceae III. Further observations on Viscum and Korthalsella. – Proc. Roy. Soc. B, 148: 188-206.
Thoday D. 1960. Modes of union and interaction between parasite and host in the Loranthaceae V. Some South African Loranthoideae. – Proc. Roy. Soc. London, Ser. B, Biol. Sci. 152: 143-162.
Thoday D. 1961. Modes of union and interaction between parasite and host in the Loranthaceae VI. A general survey of the Loranthoideae. – Proc. Roy. Soc. B, 155: 1-25.
Thoday D. 1963. Modes of union and interaction between parasite and host in the Loranthaceae VII. Some Australian Loranthoideae with exceptional features. – Proc. Roy. Soc. London, Ser. B, Biol. Sci. 157: 507-516.
Thoday D, Johnson ET. 1930. On Arceuthobium pusillum Beck. II. Flowers and fruit. – Ann. Bot. 44: 813-824.
Thumfort PD, Pate JS, Rasins E, Ghisalberti EL. 1993. S-ethenil cysteine: an amino acid from Olax phyllanthi. – Phytochemistry 34: 657-659.
Tieghem P van. 1894. Sur le groupement des espèces en genres dans les Loranthacées a calice dialysépale et anthères basifixes. – Bull. Soc. Bot. France 41: 497-511.
Tieghem P van. 1895a. Sur le groupement des espèces en genres dans les Loranthacées à calice gamosépale et anthères basifixes ou dendrophthoées. – Bull. Soc. Bot. France 42: 241-269.
Tieghem P van. 1895b. Sur le groupement des espèces en genres dans la tribu des Psittacanthées. Aetanthus, Macrocalyx, Phyllostephanus, Desrousseauxia, Alveolina, Solenocalyx, Siphanthemum. – Bull. Bot. Soc. France 42: 356-361.
Tieghem P van. 1895c. Sur le groupement des espèces en genres dans la tribu des Gaiadendrées de la famille des Loranthacées. – Bull. Soc. Bot. France 42: 455-460.
Tieghem P van. 1896a. Sur l’organisation florale des Balanophoracées et sur la place de cette famille dans la sous-classe de Dicotylédones inovulées ou Loranthinées. – Bull. Soc. Bot. France 43: 295-310.
Tieghem P van. 1896b. Sur les phanérogames à ovule sans nucelle, formant le groupe des innucellées ou Santalinées. – Bull. Soc. Bot. France 43: 543-577.
Tieghem P van. 1897. Sur les inseminées à ovules sans nucelle formant la subdivision des innucellées ou Santalinées. – Compt. Rend. Hebd. Séances Acad. Sci. 124: 723-728.
Tieghem P van. 1899a. Deux genres nouveaux pour la famille des Coulacées. – Bull. Mus. Natl. Hist. Nat. Paris 5: 97-100.
Tieghem P van. 1899b. Sur les Coulacées. – Ann. Sci. Nat. Bot., sér. VIII, 10: 125-136.
Tieghem P van. 1905. Sur le genre Octocnème considéré comme type d’une famille distincte, les Octocnémacées. – J. Bot. 19: 45-58.
Toth R, Kuijt J. 1976. Anatomy and ultrastructure of the young haustorial gland in Comandra (Santalaceae). – Can. J. Bot. 54: 2315-2327.
Toth R, Kuijt J. 1977. Anatomy and ultrastructure of the haustorium in Comandra (Santalaceae). – Can. J. Bot. 55: 455-469.
Treub M. 1881. Observations sur les Loranthacées. – Ann. Jard. Bot. Buitenzorg 2: 54-76.
Ulloa Ulloa C, Jørgensen PM. 1998. Acanthosyris annonagustata (Santalaceae), a new species from eastern Ecuador. – Novon 8: 84-86.
Ulloa Ulloa C, Jørgensen PM. 2002. 31. Santalaceae. – In: Harling G, Andersson L (eds), Flora of Ecuador 69, Botanical Institute, Göteborg University, pp. 105-121.
Ulloa Ulloa C, Nickrent DL, Whitefoord C, Kelly DL. 2010. Hondurodendron, a new monotypic genus of Aptandraceae from Honduras. – Ann. Missouri Bot. Gard. 97: 457-467.
Ultée AJ. 1926. Ueber das sogenannte Balanophorin. – Bull. Jard. Bot. Buitenzorg III, 8: 32-34.
Umiker O. 1920. Entwicklungsgeschichtlich-cytologische Untersuchungen an Helosis guyanensis Rich. – Ph.D. diss., Arb. Inst. Allgem. Bot. Pfl.-Physiol. Univ. Zürich, no. 23.
Urban I. 1898. Additamenta ad cognitionem florae Indiae occidentalis IV. Loranthaceae. – Engl. Bot. Jahrb. Syst. 24: 10-77.
Valeton T. 1886. Critisch overzicht der Olacineae B. et H. – Ph.D. diss., Plant- en Dierkunde, Rijks Universiteit, Groningen.
Veenendaal EM, Abebrese IK, Walsh MF, Swaine MD. 1996. Root hemiparasitism in a West African rainforest tree, Okoubaka aubrevillei (Santalaceae). – New Phytol. 134: 487-493.
Venkata Rao C. 1963. On the morphology of the calyculus. – J. Indian Bot. Soc. 42: 618-628.
Venturelli M. 1981. Embriología de Struthanthus vulgaris (Loranthaceae-Loranthoideae). – Kurtziana 14: 73-100.
Venturelli M. 1983. Estudos embriológicos em Loranthaceae: gênero Tripodanthus. – Kurtziana 16: 71-90.
Vidal-Russell R. 2007. The first aerial parasites in the sandalwood order (Santalales): molecular phylogenetic and biogeographic investigations. – Ph.D. dss., Southern Illinois University, Carbondale, Illinois.
Vidal-Russell R, Nickrent DL. 2007. A molecular phylogeny of the feathery mistletoe Misodendrum. – Syst. Bot. 32: 560-568.
Vidal-Russell R, Nickrent DL. 2008a. The first mistletoes: origin of aerial parasitism in Santalales. – Mol. Phylogen. Evol. 47: 523-527.
Vidal-Russell R, Nickrent DL. 2008b. Evolutionary relationships in the showy mistletoe family (Loranthaceae). – Amer. J. Bot. 95: 1015-1029.
Visser J. 1981. South African parasitic flowering plants. – Juta and Co., Ltd., Capetown.
Wanntorp L, Ronse De Craene LP. 2009. Perianth evolution in the sandalwood order Santalales. – Amer. J. Bot. 96: 1361-1371.
Watson DM. 2001. Mistletoe – a keystone resource in forests and woodlands worldwide. – Ann. Rev. Ecol. Syst. 32: 219-249.
Watson DM. 2011. Mistletoes of Southern Australia. – CSIRO Publ., Collingwood, Australia.
Weber HC. 1986. Granulahaltige Xylem-Leitbahnen und andere den Santalales ähnliche anatomische Strukturen in den haustorialen Knollen von Mystropetalon thomii (Balanophoraceae). – Flora 178: 315-328.
Weber HC, Sunaryo. 1990. Kolbenträgerschmarotzer (Balanophoraceae): extreme Blütenpflanzen mit pilzlichem Charakter. – Biol. Rundschau 28: 83-86.
Werth CR, Baird WV, Musselman LJ. 1979. Root parasitism in Schoepfia Schreb. (Olacaceae). – Biotropica 11: 140-143.
Wiens D. 1964. Chromosome numbers in the North American Loranthaceae: Arceuthobium, Phoradendron, Psittacanthus and Struthanthus. – Amer. J. Bot. 51: 1-6.
Wiens D. 1975. Chromosome numbers in African and Madagascan Loranthaceae and Viscaceae. – Bot. J. Linn. Soc. 71: 295-310.
Wiens D, Barlow BA. 1971. The cytogeography and relationships of the viscaceous and eremolepidaceous mistletoes. – Taxon 20: 313-332.
Wilson CA, Calvin CL. 2003. Development, taxonomic significance and ecological role of the cuticular epithelium in the Santalales. – IAWA J. 24: 129-138.
Wilson CA, Calvin CL. 2006a. Character divergences and convergences in canopy-dwelling Loranthaceae. – Bot. J. Linn. Soc. 150: 101-113.
Wilson CA, Calvin CL. 2006b. An origin of aerial branch parasitism in the mistletoe family, Loranthaceae. – Amer. J. Bot. 93: 787-796.
Wu ZY, Li DZ. 2000. Yunnanopilia – a primitive new genus of Opiliaceae from Yunnan Plateau, China, and its biogeographic significance. – Acta Bot. Yunnan. 22: 248-250.
Yang L, Yang G-S, Ma H-Y, Wang Y-H, Shen S-K. 2018. Phylogenetic placement of Yunnanopilia (Opiliaceae) inferred from molecular and morphological data. – J. Syst. Evol. 56: 48-55.
York HH. 1909. The anatomy and some of the biological aspects of the “American mistletoe” Phoradendron flavescens (Pursh.). – Bull. Univ. Texas 20.
York HH. 1913. The origin and development of the embryo sac and embryo of Dendrophthora opuntioides and D. gracile I. – Bot. Gaz. 56: 89-111.
Yoshida O, Kawaguchi H. 1971. Embryology of Korthalsella japonica (Thunb.) Engler. – J. Coll. Arts Chiba Univ., Sect. B, 4: 37-47.
Zaki M, Kuijt J. 1994. Ultrastructural studies on the embryo sac of Viscum minimum II. Megagametogenesis. – Can. J. Bot. 72: 1613-1628.
Zaki M, Kuijt J. 1995. Ultrastructural studies on the embryo sac of Viscum minimum I. Megasporogenesis. – Protoplasma 185: 93-105.
Zavaro CA, Crisci JV, Morrone JJ. 1997. Synopsis and cladistics of the genus Misodendrum (Misodendraceae, Santalales). – Fontqueria 48: 225-239.
Zweifel R. 1939. Cytologisch-embryologische Untersuchungen an Balanophora abbreviata Blume und Balanophora indica Wall. – Vierteljahrsschr. Naturf. Ges. Zürich 84: 245-306.