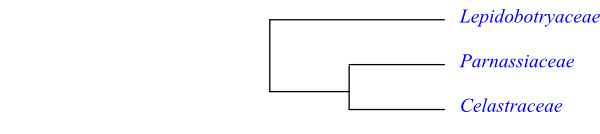
Cladogram of Celastrales based on DNA sequence data (Zhang & Simmons 2006).
[Celastrales+[Oxalidales+Malpighiales]]
Fossils Uncertain. Wuyunanthus hexapetalus, a hexamerous flower from the Paleocene of northeastern China, has been assigned to Celastraceae, although its systematic affiliation is problematic.
Habit Bisexual, monoecious, andromonoecious, polygamomonoecious?, dioecious, gynodioecious, or polygamodioecious?, evergreen or deciduous trees, shrubs, suffrutices, or lianas, perennial or annual herbs.
Vegetative anatomy Phellogen ab initio usually subepidermal (sometimes epidermal or cortical). Secondary lateral growth normal or anomalous (from concentric cambia). Vessel elements usually with simple (rarely scalariform) perforation plates; lateral pits alternate or scalariform, simple or bordered pits. Imperforate tracheary xylem elements tracheids, fibre tracheids or libriform fibres with simple or bordered pits, septate or non-septate (also vasicentric tracheids). Wood rays uniseriate or multiseriate, homocellular or heterocellular. Axial parenchyma apotracheal, diffuse or diffuse in aggregates, or paratracheal scanty vasicentric, reticulate, unilateral, or banded (rarely aliform, lozenge-aliform, winged-aliform, or confluent; sometimes absent). Intraxylary phloem (concentric or diffuse) sometimes present. Sieve tube plastids S type. Nodes usually 1:1, unilacunar with one leaf trace (rarely 3:3, trilacunar with three traces, or 1:5–7, unilacunar with five to seven traces). Usually with laticifers containing gutta between cortex and phloem. Cortex with or without cristarque cells. Calciumoxalate druses sometimes present; prismatic crystals often abundant; solitary and aggregates of crystals rarely present.
Trichomes Hairs usually absent (rarely unicellular, simple, furcate or stellate); glandular hairs rarely present.
Leaves Usually alternate (spiral or distichous) or opposite (rarely verticillate), usually simple rarely palmately compound and unifoliolate, entire, usually with involute (rarely conduplicate) ptyxis. Stipules usually small, cauline and caducous; leaf sheath absent. Petiole vascular bundle transection arcuate or annular. Venation usually pinnate (rarely campylodromous or acrodromous, palmate). Stomata usually anomocytic or anisocytic (rarely laterocytic, cyclocytic, tetracytic, or paracytic). Cuticular wax crystalloids usually absent (rarely as platelets). Domatia as pockets or absent. Epidermis with or without mucilaginous idioblasts; epidermal cells sometimes with calciumoxalate as druses or rhomboidal crystals. Mesophyll with or without secretory cavities, with or without sclerenchymatous idioblasts. Laticifers with gutta present in association with vascular strands and sometimes in mesophyll. Leaf margin entire, serrate, glandular-serrate or spinose-serrate.
Inflorescence Terminal or axillary, cymose, compound thyrsoid, fasciculate, paniculate, umbellate, raceme-like or spicate, or flowers solitary.
Flowers Usually actinomorphic (rarely zygomorphic). Usually hypogyny (rarely epigyny, with stamens at ovary apex, or half epigyny with hypanthium surrounded by nectary). Sepals (two to) four or five (or six), usually with imbricate quincuncial (rarely valvate or open) aestivation, free. Petals (two to) four or five (or six), usually with imbricate quincuncial (rarely contorted or valvate) aestivation, persistent or caducous, usually free. Nectariferous disc annular, intrastaminal to extrastaminal (rarely absent), or nectaries intrastaminal at bases of staminodia. Floral tissues often with calciumoxalate druses.
Androecium Stamens usually three to five (rarely two, 5+5, or c. 30 to more than 100). Filaments usually free from each other (rarely connate at base into tube), free from tepals. Anthers basifixed to dorsifixed (rarely ventrifixed), usually non-versatile, tetrasporangiate or disporangiate, usually introrse to latrorse (sometimes extrorse), usually longicidal (usually dehiscing by longitudinal slits; rarely with transverse or oblique orifice, or with apical confluent horizontal slits). Tapetum secretory. Staminodia (two or) three to five, intrastaminal, or absent; female flowers with or without staminodia.
Pollen grains Microsporogenesis simultaneous. Pollen grains usually tricolpor(oid)ate (rarely dicolporate, tetracolporate, triporate or syncolpate), shed as monads or tetrads (rarely polyads with eight or sixteen pollen grains), bicellular or tricellular at dispersal. Exine usually semitectate (rarely tectate), with columellate infratectum, usually reticulate (rarely psilate or microreticulate).
Gynoecium Pistil composed of usually two to five (rarely more than five) connate antepetalous carpels (when three then median carpel adaxial; rarely three paracarp and connate carpels); carpels basically synascidiate; postgenital carpel closure through much elongated cells. Ovary usually superior (rarely inferior or semi-inferior), partially or entirely bilocular to quinquelocular (all locules except one usually degenerating; rarely primarily unilocular, or with eight to twelve locules each locule secondarily transversely divided by secondary septa). Style usually single, simple (stylodia sometimes two to five, free or connate at base), or absent. Stigmas two to five or one, capitate or lobate, commissural, non-papillate, Dry type. Male flowers with or without pistillodium.
Ovules Placentation usually axile (rarely parietal, basal or apical). Ovules one to twelve (to more than 50) per carpel, usually anatropous (rarely amphitropous), ascending to pendulous, usually apotropous (rarely epitropous), bitegmic, usually weakly crassinucellar (sometimes incompletely tenuinucellar). Micropyle usually bistomal (rarely endostomal or exostomal). Megagametophyte monosporous, Polygonum type. Synergids sometimes with a filiform apparatus. Antipodal cells usually ephemeral. Endosperm development usually nuclear (rarely cellular). Endosperm haustoria absent. Embryogenesis caryophyllad or solanad (sometimes asterad).
Fruit A loculicidal capsule, a berry, drupe or samara (rarely a septicidal or loculicidal-septicidal capsule, nut, or schizocarp with two to five nutlike mericarps).
Seeds Seed with or without funicular aril or arilloid structure from hilum or exostoma (sometimes winged; wings in at least some cases possibly homologous to an aril). Seed coat usually exotegmic (occasionally reduced). Testa multiplicative, usually lignified (sometimes vascularized). Exotesta with thick cuticle, often palisade (rarely tanniniferous and with thickened outer wall). Mesotesta with sclerotic cells (rarely absent or fibrous). Endotesta unspecialized? Exotegmen usually fibrous (consisting of tall lignified fibres). Endotegmen rarely persistent, tanniniferous. Perisperm not developed. Endosperm copious to sparse, oily, or absent. Embryo straight, well differentiated, usually with chlorophyll. Cotyledons two. Germination phanerocotylar or cryptocotylar.
Cytology n = 8–10, 12, 14–18, 23, 30, 32
DNA Plastid gene infA present. Mitochondrial intron coxII.i3 lost.
Phytochemistry Flavonols (kaempferol, quercetin, myricetin), cyanidin, Ouratea catechins, diterpene and triterpene derivatives (celastrol, pristimerin, tingenin), gutta (trans-1,4-polyisoprene), tannins, proanthocyanidins (prodelphinidins), benzylisoquinoline alkaloids, pyrrolizidine alkaloids as aliphatic monocarboxylic esters, sesquiterpene alkaloids (i.a. mayteine and 7-epi-mayteine, euonymine and 7-epi-euonymine, mekongensines, peritassines), d-norpseudoephedrine (cathine), saponins, narcotic L(S)-(-)-α-aminopropiophenone (cathinone), maytansine (ansamycin macrolide), hexitols (dulcitol etc.), and salacinol present. Ellagic acid and cyanogenic compounds not found.
Systematics Celastrales may be sister-group to [Malpighiales+Oxalidales] (Soltis & al. 2011).
Celastraceae are sister to Parnassiaceae with very high support. They share the following potential synapomorphies (Stevens 2001 onwards): nodes 1:1; leaves with veins proceeding to congested deciduous tooth; stamens as many as petals; carpels antepetalous; stigma commissural; ovules tenuinucellar; presence of flavonols and hexitols (dulcitol etc.); and absence of ellagic acid. The leaf traces in both Brexia and Parnassia arise in the central part of the stem at a significant distant below the petiole.

|
Cladogram of Celastrales based on DNA sequence data (Zhang & Simmons 2006). |
CELASTRACEAE R. Br. |
( Back to Celastrales ) |
Hippocrateaceae Juss. in Ann. Mus. Natl. Hist. Nat. 18: 486. Jul-Aug 1811 [‘Hippocraticeae’], nom. cons.; Stackhousiaceae R. Br. in M. Flinders, Voy. Terra Austr. 2: 555. 19 Jul 1814 [‘Stackhouseae’], nom. cons.; Euonymales Bercht. et J. Presl, Přir. Rostlin: 226. Jan-Apr 1820 [‘Evonymeae’] Hippocrateales Juss. ex Bercht. et J. Presl, Přir. Rostlin: 225. Jan-Apr 1820 [‘Hippocrateae’]; Euonymaceae Juss. ex Bercht. et J. Presl, Přir. Rostlin: 2(110): 438, 439. 1825 [‘Evonymeae’]; Brexiaceae Loudon, Hort. Brit.: 524. 30 Aug 1830 [‘Brexieae’]; Brexiales Lindl., Nix. Plant.: 18. 17 Sep 1833; Salaciaceae Raf., Fl. Tellur. 4: 101. med 1838 [‘Salacides’]; Celastropsida Brongn., Enum. Plant. Mus. Paris: xxiv, 87. 12 Aug 1843 [’Celastoideae’]; Chingithamnaceae Hand.-Mazz. in Sinensia 2: 126. Apr-Mai 1932; Stackhousiales R. Br. in C. F. P. von Martius, Consp. Regn. Veg.: 55. Sep-Oct 1835 [’Stackhousieae’]; Siphonodontaceae (Croizat) Gagnep. et Tardieu ex Tardieu in Notul. Syst. (Paris) 14: 102. Jul-Sep 1951, nom. cons.; Canotiaceae Airy Shaw in Kew Bull. 18: 255. 8 Dec 1965; Plagiopteraceae Airy Shaw in Kew Bull. 18: 266. 8 Dec 1965; Celastranae Takht., Sist. Filog. Cvetk. Rast. [Syst. Phylog. Magnolioph.]: 371. 4 Feb 1967; Pottingeriaceae (Engl.) Takht., Sist. Magnoliof. [Systema Magnoliophytorum]: 212. 24 Jun 1987
Genera/species 102/1.180–>1.210
Distribution Mainly tropical and subtropical regions in the Northern and Southern Hemispheres; some species in temperate areas.
Fossils Uncertain.
Habit Bisexual, monoecious, andromonoecious, polygamomonoecious?, dioecious, gynodioecious, or polygamodioecious?, usually evergreen or deciduous trees, shrubs or lianas (rarely herbs, suffrutices, or ericoid or epiphytic shrubs; Stackhousia usually annual or perennial herbs, sometimes succulent). With or without spines (Acanthothamnus and Canotia with glandular stems). Branches rarely photosynthesizing phyllocladia. Bark often yellow (due to triterpenic compounds).
Vegetative anatomy Phellogen ab initio usually subepidermal (sometimes epidermal or cortical). Medulla homogeneous or heterogeneous. Young stem with vascular cylinder. Secondary lateral growth normal or anomalous (via concentric cambia). Vessel elements usually with simple (rarely scalariform) perforation plates; lateral pits alternate, usually bordered (in, e.g., Hippocratea and Tripterygium sometimes also simple) pits. Imperforate tracheary xylem elements tracheids, fibre tracheids or libriform fibres with simple and/or bordered pits, septate or non-septate (also vasicentric tracheids). Wood rays uniseriate or multiseriate, heterocellular. Axial parenchyma apotracheal diffuse, or paratracheal scanty, reticulate, vasicentric, unilateral, or banded (rarely aliform, lozenge-aliform, winged-aliform, or confluent; sometimes, e.g. in Pottingeria, absent). Tyloses sometimes frequent (sometimes sclerotic). Intraxylary phloem (concentric or diffuse) present in Salacioideae. Sieve tube plastids S type. Endodermal cells in Stackhousia thick-walled and with granular content (endodermis absent in Pottingeria). Nodes usually 1:1, unilacunar with one leaf trace (in Brexia 3:3, trilacunar with three traces; in Lophopetalum 1:5–7, unilacunar with five to seven traces). Usually with latex sacs or laticifers containing gutta between cortex and phloem. Phloem in Pottingeria with sclereids. Cortex with or without cristarque cells. Colleters often blackening. Calciumoxalate druses present in flowers of some species. Prismatic crystals often abundant. Solitary and aggregated crystals present in cortex and phloem of Pottingeria.
Trichomes Hairs usually absent (rarely unicellular or multicellular, simple or furcate; in Plagiopteron stellate, ferrugineous); glandular hairs present in Stackhousioideae.
Leaves Usually alternate (spiral or distichous) or opposite (rarely verticillate), simple, entire, sometimes coriaceous, rarely reduced and scale-like, usually with involute (in Brexia and Plagiopteron conduplicate to flat) ptyxis. Stipules usually small, cauline and caducous (sometimes fringed or absent; sometimes persistent); colleters often present; leaf sheath absent. Petiole vascular bundle transection usually arcuate or annular (sometimes complex with several bundles). Venation usually pinnate (rarely acrodromous, palmate); lateral veins often proceeding into congested caducous foliar teeth. Stomata usually anomocytic or anisocytic (rarely laterocytic, cyclocytic or tetracytic). Cuticular wax crystalloids usually absent (rarely as platelets). Domatia as pockets or absent. Epidermis with or without mucilaginous idioblasts. Epidermal cells in some genera with calciumoxalate as druses or rhomboidal crystals. Mesophyll with or without secretory cavities, with or without sclerenchymatic idioblasts. Laticifers with gutta in association with vascular strands and sometimes in mesophyll (often visible as elastic threads when leaf cut). Leaf margin entire, serrate, glandular-serrate, or spinose-serrate.
Inflorescence Terminal or axillary (in ‘Polycardia’ sometimes epiphyllous), compound thyrsoid, fascicle, panicle, umbellate, raceme-like or spicate cymose, or flowers solitary.
Flowers Usually actinomorphic (rarely zygomorphic), usually small. Usually hypogyny (rarely epigyny, with stamens at ovary apex; in Stackhousioideae half epigyny with hypanthium surrounded by nectary; in Pottingeria half epigyny). Perianth in Plagiopteron with epicalyx. Sepals (two to) four or five (or six), usually with imbricate (rarely valvate; in Plagiopteron open) aestivation, free. Petals (two to) four or five (or six), usually with imbricate (rarely contorted or valvate) aestivation, persistent or caducous, usually free (in Stackhousia clawed; absent in Plagiopteron). Nectariferous disc usually wide, massive and often fleshy, annular (in Brexia with stiff outgrowths), intrastaminal to extrastaminal (absent in Plagiopteron). Floral tissues often with calciumoxalate druses.
Androecium Stamens (two or) three to five (in Stackhousioideae three longer and two shorter; in Plagiopteron c. 30 to more than 100), antesepalous, alternipetalous. Filaments usually free (in Plagiopteron connate at base into infundibuliform tube), free from tepals (in Pottingeria subulate, inserted at disc). Anthers basifixed or dorsifixed, sometimes versatile, tetrasporangiate or disporangiate, usually introrse to latrorse (in Hippocrateoideae usually extrorse), usually longicidal (usually dehiscing by longitudinal slits; rarely with transverse or oblique orifice; in Plagiopteron with apical confluent horizontal slits). Tapetum secretory, with binucleate (in, e.g., Stackhousia) to multinucleate cells. Staminodia (two or) three to five, intrastaminal, alternating with stamens, or absent (staminodia in Brexia fringed; in Pottingeria absent); female flowers with or without staminodia (stamens in Siphonodon alternating with staminodia).
Pollen grains Microsporogenesis simultaneous. Pollen grains usually tricolporate (rarely dicolporate, tetracolporate or triporate), shed as monads or tetrads (rarely as polyads of eight or sixteen pollen grains), bicellular or tricellular at dispersal. Exine usually semitectate (in Plagiopteron tectate?), with columellate infratectum, usually reticulate (in Plagiopteron psilate; in Pottingeria microreticulate or reticulate), usually with endexinal folds in aperture.
Gynoecium Pistil composed of usually two to five (rarely more than five) connate antepetalous carpels (when three then median carpel adaxial; in Siphonodon numerous; in Pottingeria three paracarp and connate carpels); carpels sunken into disc. Ovary usually superior (rarely inferior or semi-inferior), partially or entirely usually bilocular to quinquelocular (all locules except one usually degenerating; rarely, e.g. in Empleuridium and Pottingeria, primarily unilocular; in Siphonodon with eight to twelve locules each locule secondarily transversely divided by secondary septa); dorsal bulge present in ovaries with apical septum. Gynophore sometimes present. Style usually single, simple (stylodia in Stackhousioideae sometimes two to five, free or connate at base), short (rarely long), hollow, or absent. Stigmas two to five, or one (in Pottingeria trilobate, with long spreading lobes), capitate or lobate, not or only little widened, commissural, non-papillate, Dry type. Male flowers with or without pistillodium.
Ovules Placentation usually axile to apical (rarely parietal or basal; in Pottingeria intrusively parietal). Ovules one to twelve (to numerous; in Pottingeria numerous) per carpel, usually anatropous (rarely amphitropous), ascending to pendulous (in Pottingeria sometimes horizontal), usually apotropous to pleurotropous (in Tripterygium epitropous), bitegmic, usually crassinucellar (rarely tenuinucellar). Micropyle usually bistomal (in Empleuridium endostomal; in Stackhousia exostomal; in Plagiopteron Z-shaped, zig-zag). Outer integument three to eight cell layers thick. Inner integument two to four cell layers thick. Megagametophyte monosporous, Polygonum type. Synergids sometimes with a filiform apparatus. Antipodal cells usually ephemeral (in Plagiopteron and Stackhousia proliferating to six to c. 20 cells). Endosperm development usually nuclear (in Pottingeria cellular). Endosperm haustoria absent. Embryogenesis caryophyllad or solanad (rarely asterad). Polyembryony (adventitious embryony) common in some species (embryos developing from inner integument).
Fruit Usually a loculicidal capsule, berry, drupe or samara (in Pottingeria a septicidal capsule with persistent stamens and placental vascular bundles; in Canotia loculicidal-septicidal; in Plagiopteron finally septicidal; in Pleurostylia a nut; in Stackhousioideae a schizocarp with two to five nutlike mericarps).
Seeds Seeds smooth or furrowed, with or without funicular aril or arilloid structure from hilum or exostoma (sometimes winged; wings in at least some species possibly homologous to aril; aril absent in Pottingeria). Seed coat usually exotegmic (occasionally reduced). Testa multiplicative (to 16 layers), usually lignified (sometimes vascularized). Exotesta with thick cuticle, often palisade (rarely tanniniferous and with thickened outer wall). Mesotestal cells sclerotic (rarely absent; in Pottingeria fibrous). Endotesta unspecialized? Exotegmen usually fibrous (consisting of tall lignified fibres). Endotegmen rarely persistent, tanniniferous. Perisperm not developed. Endosperm copious to sparse (in Brexia thin; in Pottingeria copious), oily, or absent. Embryo straight, well differentiated, usually with chlorophyll. Cotyledons two, large, sometimes connate. Germination phanerocotylar or cryptocotylar.
Cytology n = 8–10, 12, 14–17, 20, 23 (in Brexia n = 30, 32) – Polyploidy frequently occurring.
DNA Plastid gene infA present. Mitochondrial coxI intron present in Brexia.
Phytochemistry Flavonols (kaempferol, quercetin, myricetin), cyanidin, Ouratea catechins, diterpene and triterpene derivatives (quinone methides, celastrol, pristemerin, tingenin, etc.), gutta (trans-1,4-polyisoprene), tannins, proanthocyanidins (prodelphinidins), benzylisoquinoline alkaloids, pyrrolizidine alkaloids as aliphatic monocarboxylic esters, monoamine alkaloids (narcotic L(S)-(-)-alpha-aminopropiophenone, e.g. cathinone, cathine), sesquiterpene alkaloids (mayteine and 7-epi-mayteine, euonymine and 7-epi-euonymine, mekongensines, peritassines, etc., possessing cancer-inhibiting and other effects), saponins, d-norpseudoephedrin (cathin, in Catha edulis), maytansine (ansamycin macrolide; maytansinoids probably produced by endophytic actinobacteria), hexitols (dulcitol etc.), lupane lactones, and salacinol present. Ellagic acid and cyanogenic compounds not found. Nickel accumulated in species of Stackhousia.
Use Ornamental plants, medicinal plants, narcotics (Catha edulis), seed oils, fruits and seeds for food, insecticides, arrow poison, timber, carpentry, basketry.
Systematics Celastraceae are sister to Parnassiaceae.
Mortonia and Pottingeria (Pottingerioideae) form a sister-group to the remaining Celastraceae (Zhang & Simmons 2006). A provisional topology of Celastraceae is the following: [Pottingerioideae+[Microtropis clade+[Monimopetalum+[Stackhousioideae+[[Schaefferia clade+Celastroideae]+[American ‘Maytenus‘ clade+[[Gymnosporia clade+Old World ‘Maytenus‘ clade]+[Salaciopsis+[Brexioideae+[Hippocrateoideae+[Sarawakodendroideae+Salacioideae]]]]]]]]]]] (Bacon & al. 2016).
Nicobariodendron has dioecy, distichous leaves, no stipules, mucilage cells, racemose inflorescence, intrastaminal disc, two stamens, basal placentation and drupe (Vasudeva Rao & Chakrabarty 1985). It is known only from the type material from the Nicobar Islands.
Pottingerioideae Airy Shaw in Kew Bull. 28: 100. 1973
2/12. Pottingeria (1; P. acuminata; Naga Hills in Assam, northern Burma, northwestern Thailand), Mortonia (11; southwestern United States, northern Mexico). – Southeast Asia, southwestern United States, northern Mexico. Description of Pottingeria: Vessel elements in young stem with scalariform perforation plates. Leaves spiral. Stipules minute, cauline. Venation palmate. Leaf margin entire. Inflorescence fasciculate. Sepals and petals inserted abaxial on disc, free. Pistil composed of three connate carpels. Placentation intrusively parietal. Ovules numerous per carpel. Fruit a septicidal capsule with persistent stamens. Mesotesta fibrous. Endosperm copious. – Pottingeria was resolved as sister-group of Parnassiaceae on the rDNA tree (jacknife support 77%), but sister to Mortonia on the cpDNA tree (jacknife support 80%) of Zhang & Simmons (2006).
[Microtropis clade+[Monimopetalum+[Stackhousioideae+[[Schaefferia clade+Celastroideae]+[American ‘Maytenus‘ clade+[[Gymnosporia clade+Old World ‘Maytenus‘ clade]+[Salaciopsis+[Brexioideae+[Hippocrateoideae+[Sarawakodendroideae+Salacioideae]]]]]]]]]]
Microtropis clade
3/94. Zinowiewia (17; Mexico, Central America, Colombia, Venezuela), Microtropis (66; tropical Asia), Quetzalia (11; southern Mexico, Central America). – Tropical Asia, Mexico to Venezuela. – This clade was sister to the remaining Celastraceae in the combined analysis by Simmons, McKenna & al. (2012; see also Simmons, Bacon & al. 2012 and Simmons & Cappa 2013, etc.).
[Monimopetalum+[Stackhousioideae+[[Schaefferia clade+Celastroideae]+[American ‘Maytenus‘ clade+[[Gymnosporia clade+Old World ‘Maytenus‘ clade]+[Salaciopsis+[Brexioideae+[Hippocrateoideae+[Sarawakodendroideae+Salacioideae]]]]]]]]]
Monimopetalum clade
1/1. Monimopetalum (1; M. chinense; China). – Scandent shrub. Hairs absent. Leaves alternate. Leaf margin entire. Stipules pairwise, persistent. Inflorescence axillary, cymose. Flowers tetramerous. Disc globose, compressed. Stamens inserted on disc. Ovary quadrilocular. Ovules two per carpel, erect. Fruit a deeply quadrilobate loculicidal capsule with persistent wing-like petals. Aril small. – Monimopetalum chinense was sister to the remaining Celastraceae, according to Simmons & Cappa (2013).
[Stackhousioideae+[[Schaefferia clade+Celastroideae]+[American ‘Maytenus‘ clade+[[Gymnosporia clade+Old World ‘Maytenus‘ clade]+[Salaciopsis+[Brexioideae+[Hippocrateoideae+[Sarawakodendroideae+Salacioideae]]]]]]]]
Stackhousioideae Burnett, Outlines Bot.: 618, 1092, 1140. Feb 1835 [‘Stackhousidae’]
18/89. Wilczekra (1; W. congolensis; Central Africa); Hexaspora (1; H. pubescens; northeastern Queensland); Menepetalum (4; M. cassinoides, M. cathioides, M. salicifolium, M. schlechteri; New Caledonia), Dinghoua (1; D. globularis; northeastern Queensland), Apatophyllum (5; A. constablei, A. flavovirens, A. macgillivrayi, A. olsenii, A. teretifolium; southeastern Queensland, eastern New South Wales), Psammomoya (4; P. choretroides, P. ephedroides, P. grandiflora, P. implexa; southwestern Western Australia); Tripterococcus (4; T. brachystigma, T. brunonis, T. simplex, T. spathulatus; southwestern Western Australia), Macgregoria (1; M. racemigera; central Australia), Stackhousia (16; Malesia to New Guinea, Australia, Tasmania, New Zealand, Micronesia); Denhamia (10; northern and eastern Australia), Brassiantha (1; B. pentamera; New Guinea), Hedraianthera (1; H. porphyropetala; eastern Queensland, northeastern New South Wales), Dicarpellum (4; D. baillonianum, D. pancheri, D. paucisepalum, D. pronyense; New Caledonia), Hypsophila (3; H. dielsiana, H. halleyana, H. oppositiflora; northeastern Queensland). – Siphonodon (6; S. annamensis, S. australis, S. celastrineus, S. membranaceus, S. peltatus, S. pendulum; tropical Asia to New Guinea, eastern Queensland, northeastern New South Wales), Peripterygia (1; P. marginata; New Caledonia). Crossopetalum (c 25; tropical America), Xenodrys (1; X. micranthum; Madagascar). – Madagascar, Malesia to New Guinea, Australia, Tasmania, New Caledonia, New Zealand, Micronesia, tropical America. – The main part of Stackhousioideae s.lat. comprises “The Austral-Pacific clade”, according to Simmons & al. (2012) and Bacon & al. (2016). Hexaspora is sister to the remaining Stackhousioideae in Simmons & Cappa (2013) and the clade [[Menepetalum+Dinghoua]+[Apatophyllum+Psammomoya]] is successive sister-group to the remaining Stackhousioideae. [Menepetalum+[Apatophyllum+Psammomoya]] appears as a clade in Coughenour & al. (2010). [Macgregoria+Stackhousia] is sister to Tripterococcus. Brassiantha, Dicarpellum and Hypsophila form a Melanesian-NE Australian clade, perhaps together with Hedraianthera. [Peripterygia+Siphonodon] is sister-group to [Crossopetalum+Xenodrys] (Bacon & al. 2016).
[[Schaefferia clade+Celastroideae]+[American ‘Maytenus‘ clade+[[Gymnosporia clade+Old World ‘Maytenus‘ clade]+[Salaciopsis+[Brexioideae+[Hippocrateoideae+[Sarawakodendroideae+Salacioideae]]]]]]]
[Schaefferia clade+Celastroideae]
Schaefferia clade
5/32. Haydenoxylon (3; H. gentryi, H. haberianum, H. urbanianum; tropical South America), Gyminda (4; G. fimbrillata, G. latifolia, G. orbicularis, G. tonduzii; southern Mexico, Central America, the West Indies), Orthosphenia (1; O. mexicana; Mexico), Rzedowskia (1; R. tolantonguensis; Mexico), Schaefferia (23; tropical America). – Mexico, tropical America.
Celastroideae Burnett, Outlines Bot.: 621, 1140. Feb 1835 [‘Celastridae’] (under construction)
10/c 200. Celastrus (31; warm-temperate to tropical regions on both hemispheres), Tripterygium (1; T. wilfordii; eastern China, Taiwan); Paxistima (2; P. canbyi, P. myrsinites; North America), Wimmeria (12; Central America); Acanthothamnus (1; A. aphyllus; Mexico), Canotia (2; C. holacantha, C. wendtii; southwestern United States); Euonymus (c 130; temperate regions on the Northern Hemisphere, eastern Australia, Tasmania; non-monophyletic; incl. Glyptopetalum?, Torralbasia?, Xylonymus?), Glyptopetalum (c 20; tropical Asia), Torralbasia (1; T. cuneifolia; the West Indies), Xylonymus (1; X. versteeghii; western New Guinea).
[American ‘Maytenus‘ clade+[[Gymnosporia clade+Old World ‘Maytenus‘ clade]+[Salaciopsis+[Brexioideae+[Hippocrateoideae+[Sarawakodendroideae+Salacioideae]]]]]]
American Maytenus clade
5/145–160. Maytenus (15–24; Costa Rica, Panama, tropical and subtropical South America), Tricerma (6; T. obovatum, T. octogonum, T. orbiculare, T. texanum, T. viscifolium, T. vitis-idaeum; Florida, Texas, Mexico, Central America, the West Indies, tropical South America), Fraunhofera (1; F. multiflora; northeastern Brazil), Plenckia (3; P. bahiensis, P. microcarpa, P. populnea; Brazil, Paraguay, Bolivia, northwestern Argentina), Monteverdia (120–125; southern United States, Mexico, Central America, the West Indies, tropical South America). – Tropical and subtropical America.
[[Gymnosporia clade+Old World ‘Maytenus‘ clade]+[Salaciopsis+[Brexioideae+[Hippocrateoideae+[Sarawakodendroideae+Salacioideae]]]]]
[Gymnosporia clade+Old World ‘Maytenus‘ clade]
Gymnosporia clade
8?/>113? ‘Gymnosporia’ (>100; southern Spain, Madeira, the Canary Islands, northern to southern Africa, Madagascar, southwestern India, Sri Lanka, Malesia to eastern Queensland and islands in the Pacific; diphyletic), Lydenburgia (2; L. abbottii, L. cassinoides; Eastern Cape, Northern Province), Platypterocarpus (1; P. tanganyikensis; western Usambara Mountains in Uganda; possibly extinct), Allocassine (1; A. laurifolia; eastern Zimbabwe, southern Mozambique, Eastern Cape, KwaZulu-Natal, Swaziland), Cassine (3?; South Africa, Swaziland), Lauridia (2; L. reticulata, L. tetragona; South Africa, Swaziland), Maurocenia (1; M. frangula; Western Cape), Catha (3; C. abbottii, C. edulis, C. transvaalensis; eastern tropical and southern Africa, Madagascar, Yemen). – Tropical and subtropical regions in the Old World, tropical Asia to tropical Australia and New Caledonia, the West Indies, with their highest diversity on Madagascar.
Old World 'Maytenus' clade
6?/? Empleuridium (1; E. juniperinum; Caledon District in Western Cape), Pterocelastrus (4; P. echinatus, P. galpinii, P. rostratus, P. tricuspidatus; southeastern Africa, Zimbabwe, Mozambique, Malawi), ‘Maytenus’ pro parte (?; tropical and subtropical regions in the Old World), Mystroxylon (1; M. aethiopicum; eastern and southern Africa, Madagascar, the Mascarene Islands), Pseudosalacia (1; P. streyi; southern KwaZulu-Natal), Robsonodendron (2; R. eucleiforme, R. maritimum; South Africa, Swaziland).
[Salaciopsis+[Brexioideae+[Hippocrateoideae+[Sarawakodendroideae+Salacioideae]]]]
Salaciopsis
Salaciopsis (6; S. glomerata, S. longistyla, S. neocaledonica, S. sparsiflora, S. tapeinospermophylla, S. tiwakae; New Caledonia).
[Brexioideae+[Hippocrateoideae+[Sarawakodendroideae+Salacioideae]]]
Brexioideae Burnett, Outl. Bot.: 896, 1093, 1127. Jun 1835
15/108–123. Brexia (1 or 12; coastal regions in tropical East Africa, Madagascar, the Seychelles), ‘Polycardia’ (4; P. aquifolium, P. lateralis, P. libera, P. phyllanthoides; Madagascar; paraphyletic); Kokoona (10; Sri Lanka to Malesia), Lophopetalum (18–20; tropical Asia to tropical Australia); ‘Brexiella’ (c 10; Madagascar; non-monophyletic), ‘Euonymopsis’ (8; Madagascar; non-monophyletic), Elaeodendron (c 40; tropical and subtropical regions in the Old World, the West Indies), Pseudocatha (1; P. mandenensis; Madagascar), Halleriopsis (1; H. cathoides; Madagascar), Erythrostegia (2; E. grandis, E. integrifolia; Madagascar), Astrocassine (2–4; A. glomerata, A. pleurostylioides; Madagascar), Salvadoropsis (4; S. arenicola, S. grevicola, S. ludiifolia, S. paniculata; Madagascar), Pleurostylia (5; P. africana, P. capensis, P. opposita, P. pachyphlaea, P. serrulata; tropical and southern Africa, Madagascar, the Mascarene Islands, tropical Asia to New Guinea, northern Australia, New Caledonia), Hartogiopsis (1–2; H. trilobocarpa; Madagascar), Macrynodrys (1; M. mcphersonii; Madagascar).
[Hippocrateoideae+[Sarawakodendroideae+Salacioideae]]
Hippocrateoideae Lindl., Intr. Nat. Syst. Bot.: 120. 27 Sep 1830 [‘Hippocrateae’]
18/106. Plagiopteron (1; P. suaveolens; southern China, southern Burma, Thailand); Helictonema (1; H. velutinum; tropical Africa); Pristimera (21; tropical regions on both hemispheres); Cuervea (7; C. crenulata, C. hawkesii, C. integrifolia, C. isangiensis, C. jamaicensis, C. kappleriana, C. macrophylla; tropical West Africa, tropical America), Anthodon (4; A. decussatum, A. panamense, A. selloanum, A. trinerve; Central America, tropical South America), Arnicratea (3; A. cambodiana, A. ferruginea, A. grahamii; India to Southeast Asia), Bequaertia (1; B. mucronata; tropical Africa), Elachyptera (8; tropical Africa, Madagascar, tropical America), Apodostigma (1; A. pallens; tropical Africa, Madagascar), Reissantia (7; R. angustipetala, R. arborea, R. buchananii, R. cassinoides, R. indica, R. parviflora, R. setulosa; tropical regions in the Old World), Hylenaea (3; H. comosa, H. praecelsa, H. unguiculata; Central America, tropical South America), Semialarium (2; S. mexicanum, S. paniculatum; southern Mexico, Central America, tropical South America), Loeseneriella (16; tropical regions in the Old World), Hippocratea (16; tropical Africa, tropical America), Campylostemon (10; tropical Africa), Tristemonanthus (2; T. mildbraedianus, T. nigrisilvae; tropical West and Central Africa), Simicratea (1; S. welwitschii; Angola), Trochantha (2; T. graciliflora, T. preussii; tropical Africa). – Tropical regions on both hemispheres, with their highest diversity in tropical Africa. Flowers bisexual, pentamerous. Nectariferous disc extrastaminal. Stamens usually three. Anthers transversely dehiscent. Fruit a transversely flattened, deeply trilobate capsule. Testa with basal membranous wings or narrow stipes. – Plagiopteron was sister to the remaining Hippocrateoideae in the combined analysis by Simmons & al. (2001). Helictonema is sister to the remaining Hippocrateoideae “above” Plagiopteron (Coughenour 2010, 2011).
[Sarawakodendroideae+Salacioideae]
Sarawakodendroideae Savinov et Melikyan in Bot. Žurn. (Moscow and Leningrad) 87(7): 109. 29 Jul 2002
1/1. Sarawakodendron (1; S. filamentosum; Borneo). – Sarawakodendron is sister to Salacioideae, according to Coughenour & al. (2010). Median sepal abaxial. Two filaments shorter than remainder. Aril filaments supposedly homologous to spiral filaments in mucilaginous pulp in Salacioideae.
Salacioideae N. Hallé ex Thorne et Reveal in Bot. Rev. (Lancaster) 73: 181. 29 Jun 2007
5/c 270. Cheiloclinium (15; tropical America), Peritassa (c 20; Tobago, tropical South America), ‘Salacia’ (c 200; tropical and subtropical regions on both hemispheres; non-monophyletic; incl. Tontelea?), Tontelea (c 30; tropical America; in Salacia?), Salacighia (2; S. letestuana, S. linderi; tropical West and Central Africa to Angola), Thyrsosalacia (2; T. nematobrachion, T. viciflora; Central Africa). – Pantropical. Bisexual. Flowers pentamerous. Nectariferous disc extrastaminal. Stamens usually three. Anthers transversely dehiscent. Fruit a berry with arillate mucilaginous pulp.
Unplaced Celastraceae
Goniodiscus (1; G. elaeospermus; Brazil), Katafa (1; K. crassisepalum; Madagascar; in Brexioideae?), Ptelidium (2; P. ovatum, P. scandens; Madagascar; in Brexioideae?), Tetrasiphon (1; T. jamaicensis; Jamaica).
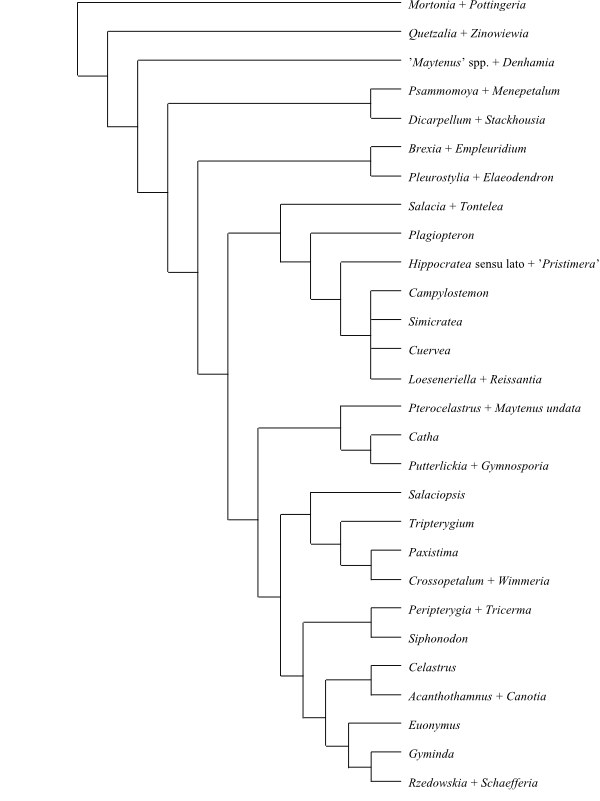 |
Phylogeny (simplified) of Celastraceae based on DNA sequence data and morphology (Simmons & al. 2001; Zhang & Simmons 2006). |
LEPIDOBOTRYACEAE J. Léonard |
( Back to Celastrales ) |
Genera/species 2/2
Distribution Tropical Africa, Costa Rica, northern South America.
Fossils Unknown.
Habit Dioecious, evergreen trees (or shrubs?).
Vegetative anatomy Phellogen? Vessel elements with simple perforation plates; lateral pits alternate or scalariform, simple or bordered pits. Vestured pits present in Ruptiliocarpon. Imperforate tracheary xylem elements thin-walled fibre tracheids? (tracheids absent) with simple (or bordered?) pits, non-septate. Wood rays uniseriate, homocellular. Axial parenchyma apotracheal, diffuse or diffuse-in-aggregates (or paratracheal?). Wood fluorescent. Sieve tube plastids Ss type. Nodes in Lepidobotrys 2:2, bilacunar with two leaf traces. Prismatic calciumoxalate crystals abundant. Druses absent (at least in flowers).
Trichomes Hairs unicellular, simple.
Leaves Alternate (distichous), basically palmately compound with one entire leaflet (unifoliolate), with ? ptyxis. Rhachis usually with long adaxial caducous stipulule (stipel; in Lepidobotrys two-veined) and articulated petiol(ul)e. Stipules usually caducous (rarely fused with petiole); leaf sheath absent. Petiole vascular bundle transection? Venation pinnate. Stomata laterocytic or paracytic. Cuticular wax crystalloids? Cristarque cells enveloped by vascular bundles. Leaf margin entire.
Inflorescence Terminal (often appearing leaf-opposite), raceme-like or spicate (sometimes congested).
Flowers Actinomorphic, small. Hypogyny. Sepals five, with imbricate quincuncial aestivation, in female flowers persistent, usually slightly connate at base. Petals five, with imbricate quincuncial aestivation, free. Nectariferous disc intrastaminal, in Lepidobotrys cupular (from receptacle), in Ruptiliocarpon tubular (from basally connate stamens).
Androecium Stamens 5+5, antepetalous longer than antesepalous. Filaments free (Lepidobotrys) or connate at base into tube (Ruptiliocarpon), free from tepals. Anthers basifixed to dorsifixed, versatile?, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory. Female flowers with 5+5 staminodia.
Pollen grains Microsporogenesis simultaneous. Pollen grains tricolporoidate (Lepidobotrys), shed as monads, ?-cellular at dispersal. Exine in Lepidobotrys crassitegillate-intectate, with intraluminar columellae; exine in Ruptiliocarpon tectate, with columellate infratectum, verrucate to fossulate/foveolate.
Gynoecium Pistil composed of two or three connate carpels. Ovary superior, bilocular or trilocular. Stylodia two or three more or less free and stigmas capitate, or style single simple short and stigma bilobate or trilobate, stigma type? Male flowers with pistillodium.
Ovules Placentation apical-axile. Ovules two per carpel, pachychalazal, collateral, anatropous, pendulous, epitropous, bitegmic, tenuinucellar. Micropyle bistomal. Outer integument seven to ten cell layers thick. Inner integument approx. four cell layers thick (Ruptiliocarpon). Integument multiplicative. Funicular obturator present above micropyle. Parietal tissue approx. ten cell layers thick. Megagametophyte monosporous, 8-nucleate (Polygonum-type?). Endosperm development? Endosperm haustoria? Embryogenesis?
Fruit A usually single-seeded septicidal capsule with mesocarp/exocarp separating from endocarp. Mesocarp with radially arranged scalariform fibres. Endocarp corneous, distinctly delimited. Columella persistent.
Seeds Carunculus inserted at apex of ovary locule. Seed coat exotegmic. Testa and tegmen multiplicative. Exotegmic cells in Ruptiliocarpon fibrous (not in Lepidobotrys), heavily thickened; remaining cells more or less crushed (in Lepidobotrys?). Endotegmen? Perisperm not developed. Endosperm absent. Embryo straight, oily, well differentiated, chlorophyll? Cotyledons two. Germination?
Cytology n = ?
DNA The plastid gene infA is present or absent?
Phytochemistry Spirotriterpenoids present (Ruptiliocarpon).
Use Timber.
Systematics Lepidobotrys (1; L. staudtii; Cameroon to Ethiopia), Ruptiliocarpon (1; R. caracolito; Costa Rica, Colombia, Suriname, Peru).
Lepidobotryaceae are sister to [Parnassiaceae+Celastraceae].
PARNASSIACEAE Martinov |
( Back to Celastrales ) |
Parnassiales J. Presl in Nowočeská Bibl. [Wšobecný Rostl.] 7: 110. 1846 [’Parnassieae’]; Lepuropetalaceae (Engl.) Nakai, Chosakuronbun Mokuroku [Ord. Fam. Trib. Nov.]: 243. 20 Jul 1943
Genera/species 2/c 70
Distribution Temperate regions on the Northern Hemisphere, southeastern United States, Mexico, Uruguay, central Chile.
Fossils Unknown.
Habit Bisexual, annual succulent (Lepuropetalon) or perennial (Parnassia) herbs.
Vegetative anatomy Rhizome in Parnassia with cortex and medulla consisting of starch-filled parenchymatic cells. Phellogen? Young stems with separate vascular bundles. Pericycle six-layered, fibrous (Parnassia), or one- or two-layered, sclerenchymatic (Lepuropetalon). Secondary lateral growth absent? Vessel elements with simple perforation plates; lateral pits? Imperforate tracheary xylem elements? Wood rays? Axial parenchyma? Sieve tube plastids S type? Nodes 1:1, unilacunar with one leaf trace. Crystals? Druses absent (at least in flowers).
Trichomes Hairs usually absent.
Leaves Alternate (spiral), simple, entire, with ? ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle transection arcuate or annular. Venation palmate or almost palmate, campylodromous (Parnassia) or acrodromous (Lepuropetalon), with veins proceeding to congested caducous foliar teeth. Stomata anomocytic. Cuticular wax crystalloids? Leaves without crystals. Young leaves in Parnassia with mucilaginous fringed appendages. Epidermis with distinct tanniniferous secretory cells (Lepuropetalon). Leaf margin serrate or entire.
Inflorescence Terminal, monochasium, or flowers solitary (basically reduced cyme).
Flowers Actinomorphic (Lepuropetalon) or weakly zygomorphic (Parnassia). Hypogyny (Parnassia) or half epigyny (Lepuropetalon). Floral receptacle distinctly hollow. Sepals (four or) five (to seven), with imbricate quincuncial aestivation, persistent, connate at base. Petals in Parnassia (four or) five (to seven), with imbricate quincuncial aestivation, persistent, free, inLepuropetalon rudimentary or absent. Nectaries intrastaminal, inserted at staminodial bases. Disc absent.
Androecium Stamens (four or) five (to seven), obdiplostemonous, antesepalous, alternipetalous. Filaments free from each other and from tepals. Anthers basifixed to ventrifixed, often versatile, usually tetrasporangiate (in Lepuropetalon disporangiate), extrorse (introrse as unripe and bending inwards one after the other during maturation), longicidal (dehiscent by longitudinal slits), often glandular, lobate (with apical glands on lobes). Tapetum secretory, with uninucleate or binucleate cells. Staminodia five, alternisepalous, antepetalous, intrastaminal trilobate to 15-lobate usually with apical glands on lobes (Parnassia) or extrastaminal simple (Lepuropetalon).
Pollen grains Microsporogenesis simultaneous. Pollen grains usually tricolporate (rarely tetracolporate or syncolpate), shed as monads, bicellular at dispersal. Exine semitectate, with columellate? infratectum, reticulate.
Gynoecium Pistil composed of three or four (or five) connate antepetalous carpels, syncarpous at base and paracarpous above base; odd carpel abaxial. Ovary in Lepuropetalon semi-inferior, unilocular, in Parnassia superior, unilocular in upper part and multilocular in lower part. Style single, simple, very short or absent (Parnassia), or stylodia three or four (or five), very short, free (Lepuropetalon). Stigmas commissural (in Lepuropetalon capitate), papillate, Dry type. Pistillodium absent.
Ovules Placentation parietal (Lepuropetalon) or axile with T-shaped placentae (Parnassia). Ovules c. 25 to more than 100 per ovary, anatropous, horizontal, bitegmic (Parnassia) or unitegmic (Lepuropetalon), tenuinucellar. Micropyle bistomal (Parnassia). Outer integument two or three cell layers thick (Parnassia). Inner integument three or four cell layers thick. Integument in Lepuropetalon two cell layers thick. Parietal tissue one cell layer thick or absent. Nucellar cap absent? Megagametophyte monosporous, Polygonum type. Synergids with a filiform apparatus. Antipodal cells three, proliferating to approx. five cells or non-proliferating. Endosperm development ab initio nuclear. Endosperm haustoria? Embryogenesis asterad.
Fruit A loculicidal capsule with persistent calyx.
Seeds Aril absent. Testa in Parnassia winged. Raphe absent. Exotestal cells in Parnassia with thickened anticlinal walls. Endotestal cells in Lepuropetalon with heavily thickened walls. Tegmen multiplicative. Exotegmic cells tanniniferous, in Parnassia with U-shaped wall thickenings. Air spaces present in Parnassia between testa and embryo. Perisperm not developed. Endosperm one-layered or absent. Embryo straight, chlorophyll? Cotyledons two, short. Germination phanerocotylar?
Cytology n = 8, 9, 18 (Parnassia; Parnassia palustris: 2n = 17, 18, 27, 36, 32–37, 43–45 och 54); n = 8, 9, 23 (Lepuropetalon) – Allopolyploidy and autopolyploidy occurring in Parnassia.
DNA Plastid gene infA present.
Phytochemistry Insufficiently known. Flavonols and hexitols (dulcitol etc.) present. Ellagic acid and cyanogenic compounds not found.
Use Ornamental plants (Parnassia).
Systematics Parnassia (c 70; cold-temperate regions on the Northern Hemisphere south to Morocco, Sumatra and Mexico, with their largest diversity in the Himalayas, western China and northwestern North America), Lepuropetalon (1; L. spathulatum; southeastern United States, Mexico, Uruguay, central Chile).
Parnassiaceae are sister to Celastraceae.
Literature
Adatia RD, Gavde SG. 1962. Embryology of the Celastraceae. – In: Plant embryology. A symposium I-II, Council of Scientific & Industrial Research, New Delhi.
Airy Shaw HK, Cutler DE, Nilsson S. 1973. Pottingeria, its taxonomic position, anatomy and palynology. – Kew Bull. 28: 97-104.
Alvarenga SM, Lombardi JA. 2010. Leaf anatomy as a contribution to the taxonomy of Salacioideae N. Hallé ex Thorne & Reveal (Celastraceae). – Plant Syst. Evol. 289: 13-33.
Arber A. 1913. On the structure of the androecium in Parnassia and its bearing on the affinities of the genus. – Ann. Bot. 27: 491-510.
Arber A. 1915. The anatomy of the stamens in certain Indian species of Parnassia. – Ann. Bot. 29: 159-160.
Archer RH. 1990. The taxonomic status of Cassine L. s.l. (Celastraceae) in southern Africa. – M.Sc. thesis, University of Pretoria, Republic of South Africa.
Archer RH, Wyk AE van. 1992. Palynology and intergeneric relationships in some southern African species of subfamily Cassinoideae (Celastraceae). – Grana 31: 241-252.
Archer RH, Wyk AE van. 1993a. Bark structure and intergeneric relationships of some Southern African Cassinoideae (Celastraceae). – IAWA J. 14: 35-53.
Archer RH, Wyk AE van. 1993b. Wood structure and generic status of some southern African Cassinoideae (Celastraceae). – IAWA J. 14: 373-389.
Archer RH, Wyk AE van. 1997. A taxonomic revision of Cassine L. s. str. (Cassinoideae: Celastraceae). – South Afr. J. Bot. 63: 146-157.
Archer RH, Wyk AE van. 1998a. A taxonomic revision of Maurocenia (Celastraceae), a Western Cape monotypic endemic. – Bothalia 28: 7-10.
Archer RH, Wyk AE van. 1998b. A taxonomic revision of Elaeodendron Jacq. (Cassinoideae: Celastraceae) in Africa. – South Afr. J. Bot. 64: 93-109.
Archer RH, Wyk AE van. 1998c. A taxonomic revision of Allocassine N. Robson (Celastraceae). – South Afr. J. Bot. 64: 189-191.
Asim M, Hussein H, Poveda L, Arnason JT, Durst T. 2010. Triterpenoids from the bark of Ruptiliocarpon caracolito. – Phytochemistry 71: 1418-1422.
Baas P, Geesink R, Heel WA van, Muller J. 1979. The affinities of Plagiopteron suaveolens Griff. (Plagiopteraceae). – Grana 18: 69-89.
Bacon CD, Simmons MP, Archer RH, Zhao L-C, Andriantiana J. 2016. Biogeography of the Malagasy Celastraceae: multiple independent origins followed by sidespread dispersal of genera from Madagascar. – Mol. Phylogen. Evol. 94: 365-382.
Barker WR. 1977. Taxonomic studies in Stackhousia Sm. (Stackhousiaceae) in South Australia. – J. Adelaide Bot. Gard. 1: 69-82.
Barker WR. 1984. Stackhousiaceae. – In: George AS (ed), Flora of Australia 22, Australian Government Publ. Service, Canberra, pp. 186-199.
Barker WR, Cockerton GTB. 2011. Stackhousia stratfordii (Celastraceae: Stackhousioideae), a remarkable new species from a remote location near Norseman, south-west Western Australia. – Nuytsia 21: 69-74.
Bean AR, Jessup LW. 2000. Two new species of Apatophyllum McGillivray (Celastraceae) from Queensland. – Austrobaileya 5: 691-697.
Begley MJ, Crombie L, Crombie WML, Toplis D, Whiting DA. 1990. Isolation and structure of vaalens-1, -3, -5, and -7. Sesquiterpene esters of Catha transvaalensis Codd (Celastraceae): X-ray molecular structure of vaalens-5. – J. Chem. Soc., Perkin Trans. 1: 2841-2846.
Bennett AW. 1871. Note on the structure and affinites of Parnassia palustris L. – Bot. J. Linn. Soc. 11: 24-31.
Bensel CR, Palser BF. 1975. Floral anatomy in the Saxifragaceae sensu lato I. Introduction, Parnassioideae and Brexioideae. – Amer. J. Bot. 62: 176-185.
Berkeley F. 1953. Morphological studies in the Celastraceae. – J. Elisha Mitchell Sci. Soc. 69: 185-208.
Biral L, Simmons MP, Smidt EC, Tembrock LR, Bolson M, Archer RH, Lombardi JA. 2017. Systematics of New World Maytenus (Celastraceae) and a new delimitation of the genus. – Syst. Bot. 42: 680-693.
Blakelock RA. 1951. A synopsis of the genus Euonymus L. – Kew Bull. 2: 210-290.
Blakelock RA. 1956. Notes on African Celastraceae. – Kew Bull. 11: 237-247.
Bohm BA, Collins FW, Bose R. 1977. Flavonoids of Bergenia, Francoa, and Parnassia. – Biochem. Syst. Ecol. 13: 221-233.
Bohm BA, Donevan LS, Bhat UG. 1986. Flavonoids of some species of Bergenia, Francoa, Parnassia and Lepuropetalon. – Biochem. Syst. Ecol. 14: 75-77.
Boole JAJ. 1955. Studies in the anatomy of the family Celastraceae. – Department of Botany, University of North Carolina, Chapel Hill, North Carolina.
Borgen L, Hultgård U-M. 2003. Parnassia palustris: a genetically diverse species in Scandinavia. – Bot. J. Linn. Soc. 142: 347-372.
Brizicky GK. 1964. Polyembryony in Euonymus (Celastraceae). – J. Arnold Arbor. 45: 251-259.
Brüning R, Wagner H. 1978. Übersicht über die Celastraceen-Inhaltsstoffe: Chemie, Chemotaxonomie, Biosynthese, Phamakologie. – Phytochemistry 17: 1821-1858.
Burge DO, Barker WR. 2010. Evolution of nickel hyperaccumulation by Stackhousia tryonii (Celastraceae), a serpentinite-endemic plant from Queensland, Australia. – Aust. Syst. Bot. 23: 415-430.
Bye RA, Soltis DE. 1979. Parnassia townsendii (Saxifragaceae), a Mexican endemic. – South West. Natur. 24: 209-222.
Carlquist SJ. 1987. Wood anatomy and relationships of Stackhousiaceae. – Bot. Jahrb. Syst. 108: 473-480.
Carvalho-Okano RM. 1992. Estudos taxonomicos do genero Maytenus Mol. emend. Mol. (Celastraceae) no Brasil extra-amazônico. – Universidade Estadual de Campinas, Campinas, Brazil.
Casady JM, Chan KK, Floss HG, Leistner E. 2004. Recent developments in maytansinoid antitumor agents. – Chem. Pharm. Bull. 52: 1-26.
Chakrabarty T, Gangopadhyay M. 1990. The Celastraceae of Andaman-Nicobar Islands. – J. Econ. Taxon. Bot. 14: 115-129.
Copeland HF. 1966. Morphology and embryology of Euonymus japonica. – Phytomorphology 16: 326-334.
Coughenour JM, Simmons MP, Lombardi JA, Cappa JJ. 2010. Phylogeny of Celastraceae subfamily Salacioideae and tribe Lophopetaleae inferred from morphological characters and nuclear and plastid genes. – Syst. Bot. 35: 358-367.
Coughenour JM, Simmons MP, Lombardi JA, Yakobson K, Archer RH. 2011. Phylogeny of Celastraceae subfamily Hippocrateoideae inferred from morphological characters and nuclear and plastid loci. – Mol. Phylogen. Evol. 59: 320-330.
Croizat L. 1947. A study in the Celastraceae: Siphonodonoideae subf. nov. – Lilloa 13: 31-43.
Cunnell GJ. 1959. The arrangement of sepals and petals in Parnassia palustris L. – Ann. Bot., N. S., 23: 441-453.
Daumann E. 1935. Über die Bestäubungsökologie der Parnassia-Blüte II. Ein weiterer Beitrag zur experimentellen Blütenforschung. – Jahrb. Wiss. Bot. 81: 705-717.
Daumann E. 1960. Über die Bestäubungsökologie der Parnassia-Blüte: ein weiterer Beitrag zur experimentellen Blütenökologie. – Biol. Pl. 2: 113-125.
David E. 1938. Embryologische Untersuchungen an Myoporaceen, Salvadoraceen, Sapindaceen und Hippocrateaceen. – Planta 28: 680-703.
Davison JD. 1927. Celastraceae R. Br. – Bothalia 2: 289-346.
Demissew S. 1985. The genus Maytenus (Celastraceae) in NE tropical Africa and tropical Arabia. – Symb. Bot. Upsal. 25(2): 1-98.
Ding Hou. 1955. A revision of the genus Celastrus. – Ann. Missouri Bot.Gard. 42: 215-302.
Ding Hou. 1962. Celastraceae I. – In: Steenis CGGJ van (ed), Flora Malesiana I, 6, Wolters-Noordhoff, Groningen, pp. 227-291.
Ding Hou. 1963a. Florae Malesianae precursores XXXIV: notes on some genera of Celastraceae in Malaysia. – Blumea 12: 31-38.
Ding Hou. 1963c. Two additional Asiatic species of Glyptopetalum (Celastraceae). – Blumea 12: 57-60.
Ding Hou. 1964. Celastraceae II. – In: Steenis CGGJ van (ed), Flora Malesiana I, 6, Wolters-Noordhoff, Groningen, pp. 389-421.
Ding Hou. 1967. Sarawakodendron, a new genus of Celastraceae. – Blumea 15: 139-143.
Ding Hou. 1969. Pollen of Sarawakodendron (Celastraceae) and some related genera, with notes on techniques. – Blumea 17: 97-120.
Ding Hou. 1975. A new species of Euonymus (Celastraceae) from Australia. – Blumea 22: 271-274.
Drennan PM, Drewes SE, Dtaden J van, MacRae S, Dickens CWS. 1987. An anatomical, phytochemical and ultrastructural characterization of the elastic threads of Maytenus acuminata. – South Afr. J. Bot. 53: 17-24.
Dreyer GD, Baird LM, Fickler C. 1987. Celastrus scandens and Celastrus orbiculatus: comparisons of reproductive potential between a native and an introduced woody vine. – Bull. Torrey Bot. Club 114: 260-264.
Drude O. 1875. Ueber die Blüthengestaltung und die Verwandtschaftsverhältnisse des Genus Parnassia, nebst einer systematischen Revision seiner Arten. – Linnaea 39: 239-324.
Duan H, Takaishi Y, Imakura Y, Jia Y, Li D, Cosentino M, Lee K-H. 2000. Sesquiterpene alkaloids from Tripterygium hypoglaucum and Tripterygium wilfordii: a new class of potent anti-HIV agents. – J. Nat. Prod. 63: 357-361.
Dunkley HL, Brenan JPM. 1948. Platypterocarpus, a new tree genus of Celastraceae from Tanganyika. – Kew Bull. 1948: 47-50.
Edgell T. 2004. Floral studies of Brexia madagascariensis Thouars (Celastraceae). – M.Sc. thesis, Royal Botanic Garden, Edinburgh, United Kingdom.
Edwin G, Ding Hou. 1975. Flora of Panama: family 103. Celastraceae. – Ann. Missouri Bot. Gard. 62: 45-56.
Eichinger A. 1908. Beitrag zur Kenntnis und systematischen Stellung der Gattung Parnassia. – Beih. Bot. Centralbl. 23: 298-317.
Engler A. 1891. Saxifragaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(2a), W. Engelmann, Leipzig, pp. 41-93.
Engler A. 1930. Saxifragaceae. – In: Engler A, Harms H (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 18a, W. Engelmann, Leipzig, pp. 74-226.
Espinosa-Osornio G, Engleman EM. 1993. Anatomía del desarrollo de la semilla de Hippocratea celastroides. – Bol. Soc. Bot. Mex. 53: 43-53.
Espinosa-Osornio G, Engleman EM. 1994. Anatomía del desarrollo de la semilla de cuatro especies mexicanas de Hippocratea (Celastraceae). – Bol. Soc. Bot. Mex. 54: 57-67.
Exell AW. 1953. Tropical African plants XXIII: Celastraceae. – Kew Bull. 23: 103-104.
Funamoto T, Kondo K, Hong D-Y, Yang Q-E, Ge S, Hizume M, Shimada T. 1996. Karyomorphological studies in Chinese Parnassia II: three species in Qinghai Province. – La Kromosomo II, 82: 2845-2854.
Funamoto T, Kondo K, Hong D-Y, Zhou S-L, Deguchi H. 1998. A chromosome study of three Parnassia species collected in the Qin Ling Mountains, Shaanxi Province, China. – Chromosome Sci. 2: 111-115.
Gamlath CB, Gunatilaka AAL. 1988. Two phenolic friedo-23,24-dinoroleanane triterpenes from Kokoona zeylanica. – Phytochemistry 27: 3221-3224.
Gastony GJ, Soltis DE. 1977. Chromosome studies of Parnassia and Lepuropetalon (Saxifragaceae) from the eastern United States. A new base number for Parnassia. – Rhodora 79: 573-578.
Gibson AC. 1979. Anatomy of Koeberlinia and Canotia revisited. – Madroño 26: 1-12.
Goldblatt PH, Tobe H, Carlquist S, Patel VC. 1985. Familial position of the Cape genus Empleuridium. – Ann. Missouri Bot. Gard. 72: 167-183.
Gomes SMA, Lombardi JA. 2010. Leaf anatomy as a contribution to the taxonomy of Salacioideae N. Hallé ex Thorne & Reveal (Celastraceae). – Plant Syst. Evol. 289: 13-33.
Gomes SMA, Lombardi JA. 2013. Anatomy of the floral nectaries of some neotropical Salacioideae (Celastraceae). – Plant Syst. Evol. 299: 515-528.
Gonzàlez AG, López I, Ferro EA, Ravelo AG, Gutiérrez J, Aguilar MA. 1986. Taxonomy and chemotaxonomy of some species of Celastraceae. – Biochem. Syst. Ecol. 14: 479-480.
Gonzalez-Medrano F. 1981. Rzedowskia, un nuevo género de Celastraceae de México. – Bol. Soc. Bot. México 41: 41-46.
Gornall RJ, Wendworth JE. 1993. Variation in the chromosome number of Parnassia palustris L. in the British Isles. – New Phytol. 123: 383-388.
Gornall RJ, Al-Shammary KIA. 198. Parnassiaceae. – In: Cutler DF, Gregory M (eds), Anatomy of the dicotyledons: Saxifragales, 4, Clarendon Press, Oxford, pp. 245-247.
Görts-van Rijn ARA, Mennega AMW. 1994. 110. Hippocrateaceae. – In: Görts-van Rijn ARA (ed), Flora of the Guianas 16, Koeltz Scientific Books, Königstein, pp. 3-81.
Gris MA. 1868. Sur le movement des étamines dans la Parnassie des marais. – Compt. Rend. Acad. Sci. Paris 67: 913-916.
Groppo M, Simmons MP, Cappa JJ, Biral L,
Lombardi JA. 2014. A new species of Maytenus (Celastraceae) with fleshy fruits
from eastern Brazil, with notes on the delimitation of Maytenus. –
Syst. Bot. 39: 478-484.
Gunatilaka AAL. 1996. Triterpenoid quinonemethiodes and related compounds (celastroloids). – Progr. Chem. Nat. Compounds 67: 1-114.
Hallé N. 1958. Hippocrateacées nouvelles d’Afrique occidentale. – Bull. Mus. Natl. Hist. Nat. Paris 30: 464-471.
Hallé N. 1960. Essai de clé pour la détermination des pollens des Hippocratéacées Ouest-Africaines. – Pollen Spores 2: 5-12.
Hallé N. 1962. Monographie des Hippocratéacées d’Afrique occidentale. – Mém. Inst. Franç. Afrique Noire 64: 1-245.
Hallé N. 1978. Révision monographique des Hippocrateae (Celastr.) 1. Les espèces de Madagascar. – Adansonia 17: 397-414.
Hallé N. 1981. Révision des Hippocrateae (Celastraceae) 2. Le genre Pristimera Miers en Afrique et en Indonésie. – Bull. Mus. Natl. Hist. Nat. Paris, sect. B, Adansonia 3: 5-14.
Hallé N. 1983. Révision des Hippocrateae (Celastreae) 3. Fruits, graines et structures placentaires. – Bull. Mus. Natl. Hist. Nat. Paris, sect. B, Adansonia IV, 5: 11-26.
Hallé N. 1984. Révision des Hippocrateae (Celastraceae) 4. Les genres Simirestis et Arnicratea (gen. nov.). – Bull. Mus. Natl. Hist. Nat. Paris, sect. B, Adansonia 6: 3-18.
Hallé H. 1986. Celastraceae Hippocrateoideae. – In: Morat P (ed), Flore du Gabon (avec complements pour d’autres pays d’Afrique et Madagascar), Muséum National d’Histoire Naturell, Paris, Laboratoire de Phanérogamie 29: 1-287.
Hallé H. 1990. Celastracées (Hippocratéoidées). – In: Satabie B, Morat P (eds), Flore du Cameroun 32, Ministère de l’Enseignement Supérieur de l’Informatique et de la Recherche Scientifique Mesires, Yaoundé, pp. 3-243.
Hallier H. 1923. Beiträge zur Kenntnis der Linaceae (DC. 1819) Dumort. 25. Lepidobotrys Engl., die Oxalidaceen und die Geraniaceen. – Beih. Bot. Centralbl. 39: 1-178.
Hammel BE. 1997. Three new species of Celastraceae from Costa Rica, one disjunct from Mexico. – Novon 7: 147-155.
Hammel BE, Zamora N. 1993. Ruptiliocarpon (Lepidobotryaceae): a new arborescent genus and tropical American link to Africa, with a reconsideration of the family. – Novon 3: 408-417.
Hartog née van ter Tholen RM den, Baas P. 1978. Epidermal characters of the Celastraceae sensu lato. – Acta Bot. Neerl. 27: 355-388.
Hedin JPT. 1999. Systematic studies of the neotropical species of Salacia L. (Hippocrateaceae) and its relatives. – Ph.D. diss., Washington University.
Hultgård U-M. 1987. Parnassia palustris L. in Scandinavia. – Symb. Bot. Upsal. 28: 1-128.
Islam MB, Simmons MP, Archer RH. 2006. Phylogeny of the Elaeodendron group (Celastraceae) inferred from morphological characters and nuclear and plastid genes. – Syst. Bot. 31: 512-524.
Jansen WT, Baas P. 1973. Comparative leaf anatomy of Kokoona and Lophopetalum (Celastraceae). – Blumea 21: 153-178.
Jessup LW. 1984a. Celastraceae. – In: George AS (ed), Flora of Australia 22, Australian Government Publ. Service, Canberra, pp. 150-180.
Jessup LW. 1984b. Hippocrateaceae. – In: George AS (ed), Flora of Australia 22, Australian Government Publ. Service, Canberra, pp. 180-184.
Joffily A, Vieira RC. 2006. Lectotypification of Goniodiscus elaeospermus and new synonyms for Maytenus (Celastroideae-Celastraceae) from Brazil. – Kew Bull. 61: 265-267.
Joffily A, Vieira RC. 2010. Cork-warts on the leaf epidermis of four genera of Celastroideae-Celastraceae. – Flora 205: 313-318.
Johnston MC. 1975. Synopsis of Canotia (Celastraceae) including a new species from the Chihuahuan Desert. – Brittonia 27: 119-122.
Jordaan M, Wyk B-E van. 1998a. Systematic studies in subfamily Celastroideae (Celastraceae) in southern Africa 1: Goveria, a new monotypic genus. – South Afr. J. Bot. 64: 299-302.
Jordaan M, Wyk B-E van. 1998b. Systematic studies in subfamily Celastroideae (Celastraceae) in southern Africa 3: the genus Putterlickia. – South Afr. J. Bot. 64: 322-329.
Jordaan M, Wyk B-E van. 1999. Systematic studies in subfamily Celastroideae (Celastraceae) in southern Africa 2: reinstatement of the genus Gymnosporia. – South Afr. J. Bot. 65: 177-181.
Jordaan M, Wyk B-E van. 2003a. Reinstatement of Gymnosporia (Celastraceae): implications for the Flora Malesiana region. – Telopea 10: 155-167.
Jordaan M, Wyk B-E van. 2003b. A taxonomic revision of the Gymnosporia mossambicensis group (Celastraceae: Celastroideae). – Kew Bull. 58: 833-866.
Jordaan M, Wyk B-E van. 2006. Sectional classification of Gymnosporia (Celastraceae), with notes on the nomenclatural and taxonomic history of the genus. – Taxon 55: 515-525.
Kalix P. 1992. Cathinone, a natural amphetamine. – Parmacol. Toxicol. 70: 77-86.
Kamelina OP. 1988. Sporo-, gametogenesis, and fertilization of Escallonia and Brexia with comments on their taxonomy. – In: Cresti M, Gori P, Pacini E (eds), Sexual reproduction in higher plants. Proceedings of the 10th international symposium on the sexual reproduction in higher plants, 30 May – 4 June 1988, University of Siena, Siena, Italy, Springer, Berlin, pp. 431-435.
Keighery GJ. 2002. Psammomoya (Celastraceae), a taxonomic review. – Nuytsia 14: 385-392.
Klopfer K. 1972. Beiträge zur floralen Morphogenese und Histogenese der Saxifragaceae 7. Parnassia palustris und Francoa sonchifolia. – Flora, Sekt. B, 161: 320-332.
Knuth R. 1931. Oxalidaceae. – In: Engler A (†), Harms H, Pax F (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 19a, W. Engelmann, Leipzig, pp. 11-42.
Koontz JA, Soltis DE. 1999. DNA sequence data reveal polyphyly of Brexioideae (Brexiaceae; Saxifragaceae sensu lato). – Plant Syst. Evol. 219: 199-208.
Korta J. 1972. Anatomical analysis of Parnassia palustris L. – Acta Biol. Cracov. 15: 31-37.
Kostermans AJGH. 1986. Notes on Asiatic Cassine L. (Celastraceae). – Gard. Bull. (Singapore) 39: 179-191.
Krikorian AD. 1985. Growth mode and leaf arrangement in Catha edulis (Kat). – Econ. Bot. 39: 514-521.
Ku T. 1987. A revision of the genus Parnassia (Saxifragaceae) in China. – Bull. Bot. Res. (Harbin) 7: 1-59.
Kubitzki K. 2004. Lepidobotryaceae. – In: Kubitzki K (ed), The families and genera of vascular plants VI. Flowering plants. Dicotyledons. Celastrales, Oxalidales, Rosales, Cornales, Ericales, Springer, Berlin, Heidelberg, New York, pp. 233-235.
Kuhlmann JG. 1933. Novo genero de Celastraceas da flora amazonica. – Arch. Jard. Bot. Rio de Janeiro 6: 109-110.
Kupchan SM, Court WA, Dailey RG, Gilmore CJ, Bryan RF. 1972. Triptolide and tripdiolide, novel antileukemic diterpenoid triepoxides from Tripterygium wilfordii. – J. Amer. Chem. Soc. 94: 7194-7195.
Lander NS, Johnson LAS. 1975. Australian species of Celastrus. – Telopea 1: 33-39.
Law SK-Y, Simmons MP, Techen N, Khan IA, He M-F, Shaw P-C, But P P-H. 2011. Molecular analyses of the Chinese herb leigongteng (Tripterygium wilfordii Hook.f.). – Phytochemistry 72: 21-26.
Lebègue A. 1953. Embryogénie des Parnassiacées: Développement de l’embryon chez le Parnassia palustris L. – Compt. Rend. Acad. Sci. Paris 236: 1693-1695.
Léonard J. 1950. Lepidobotrys Engl., type d’une famille nouvelle de Spermatophytes: les Lepidobotryaceae. – Bull. Jard. Bot. État, Bruxelles 20: 31-40.
Lhinhatrakool T, Prabpai S, Kongsaeree P, Sutthivaiyakit S. 2011. Antiplasmodial sesquiterpene alkaloids from the roots of Maytenus mekongensis. – J. Nat. Prod. 74: 1386-1391.
Li Y-L, Zhang X-Y. 1990. Studies on comparative wood anatomy of 16 species of vines and trees in Celastraceae. – Acta Bot. Sin. 32: 252-261, pl. 1-2.
Li Y-N, Xie L, Li J-Y, Zhang Z-X. 2014.
Phylogeny of Euonymus inferred from molecular and morphological data.
– J. Syst. Evol. 52: 149-160.
Link DA. 1991. The floral nectaries of Geraniales III. Lepidobotryaceae J. Léonard. – Bull. Jard. Bot. Natl. Belg. 61: 347-354.
Lobreau D. 1969. Les limites de l’”ordre” des Célastrales d’après le pollen. – Pollen Spores 11: 499-555.
Lobreau-Callen D. 1975a. Les pollens des Célastrales et groupes apparantés. – Ph.D. diss., l’Université de Science et Technique de Languedoc, Montpellier, C. N. R. S.: no. A.08071, France.
Lobreau-Callen D. 1975b. Les pollens colpes dans les Célastrales: interprétation nouvelle de l’aperture simple. – Compt. Rend. Acad. Sci. Paris 280: 2547-2550.
Lobreau-Callen D. 1975c. Deux genres de Celastraceae Cassine L. et Maytenus Mol. révus à la lumière de la palynologie. – Adansonia 15: 215-223.
Lobreau-Callen D. 1977a. Les pollens des Célastrales (illustrations, commentaires). – Mém. Trav. Inst. Montpellier École Prat. Hautes Études 3: 1-116.
Lobreau-Callen D. 1977b. Nouvelle interpretation de l’”ordre” des Celastrales à l’aide de la palynologie. – Compt. Rend. Acad. Sci. Paris, sér. D, 284: 915-918.
Loesener T. 1896a. Celastraceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(5), W. Engelmann, Leipzig, pp. 189-222; Loesener T. 1897. Nachträge zu III(5), pp. 221-225.
Loesener T. 1896b. Hippocrateaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(5), W. Engelmann, Leipzig, pp. 222-230.
Loesener T. 1908. Celastraceae africanae 4. – Engl. Bot. Jahrb. Syst. 41: 298-312.
Loesener T. 1937. Celastraceae novae vel melius cognoscendae III. – Notizbl. Bot. Gart. Berlin-Dahlem 13: 563-581.
Loesener T. 1942. Celastraceae. – In: Engler A (†), Harms H, Mattfeld J (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 20b, W. Engelmann, Leipzig, pp. 87-197.
Loesener T. 1942. Hippocrateaceae. – In: Engler A (†), Harms H, Mattfeld J (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 20b, W. Engelmann, Leipzig, pp. 198-231.
Lombardi JA. 2000. Two new species of Hippocrateaceae from Brazil. – Brittonia 52: 337-340.
Lombardi JA. 2004. Three new species of Celastraceae (Hippocrateoideae) from southeastern Brazil and a new combination in Peritassa. – Novon 14: 315-321.
Lombardi JA. 2006. A new species of Tontelea from Amazonian Peru and Ecuador, and notes on the Tontelea attenuata species group (Celastraceae, Hippocrateoideae). – Brittonia 58: 52-58.
Lombardi JA. 2007. Three new South American species of Salacia with fasciculate inflorescences (Celastraceae, Hippocrateoideae). – Novon 17: 33-39.
Lombardi JA. 2009. Three new species of Salacia from Mesoamerica (Celastraceae, Salacioideae). – Novon 19: 372-379.
Lourteig A, O’Donnell CA. 1955. Las Celastráceas de Argentina y Chile. – Natura (Buenos Aires) 1: 181-233.
Lundell CL. 1939. Revision of the American Celastraceae I. Wimmeria, Microtropis, and Zinowiewia. – Contr. Univ. Michigan Herb. 3: 5-46.
Lundell CL. 1985. Two species of the genus Gymnosporia (Celastraceae) in South America. – Phytologia 57: 313-314.
Ma J-S. 2001. A revision of Euonymus (Celastraceae). – Thaiszia 11: 1-264.
Ma J-S. 2002. A synopsis of Euonymus (Celastraceae). – Belg. Dendrol. Belge 2002: 31-83.
Ma J-S, Brach AR, Liu Q-R. 1999. A revision of the genus Tripterygium (Celastraceae). – Edinb. J. Bot. 56: 33-46.
McGillivray DJ. 1971. Apatophyllum: an interesting new Australian genus in the family Celastraceae. – Kew Bull. 25: 401-406.
McKenna MJ, Simmons MP, Bacon CD, Lombardi JA. 2011. Delimitation of segregate genera of Maytenus s.l. (Celastraceae) based on molecular and morphological characters. – Syst. Bot. 36: 922-932.
Marais W. 1981. A new species of Pleurostylia (Celastraceae) from Rodrigues. – Kew Bull. 36: 229-230.
Martens P. 1936. Pollination et biologie florale chez Parnassia palustris. – Bull. Soc. Bot. Belg. 68: 183-221.
Matsuda H, Murakami T, Yashiro K, Yamahar J, Yoshikawa M. 1999. Antidiabetic principles of natural medicines IV. Aldose reductase and a-glucosidase inhibitors from the roots of Salacia oblonga Wall. (Celastraceae): structure of a new friedelane-type triterpene, kotalagenin 16-acetate. – Chem. Pharmaceut. Bull. 47: 1725-1729.
Mattfeld J. 1942. Stackhousiaceae. – In: Engler A (†), Harms H, Mattfeld J (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 20b, W. Engelmann, Leipzig, pp. 240-254.
Matthews ML, Endress PK. 2005. Comparative floral structure and systematics in Celastrales (Celastraceae, Parnassiaceae, Lepidobotryaceae). – Bot. J. Linn. Soc. 149: 129-194.
Mauritzon J. 1936a. Zur Embryologie und systematischen Abgrenzung der Reihen Terebinthales und Celastrales. – Bot. Not. 1936: 161-212.
Mauritzon J. 1936b. Embryologische Angaben über Stackhousiaceae, Hippocrateaceae und Icacinaceae. – Svensk Bot. Tidskr. 30: 541-550.
Mehotra RC, Prakash U, Bande MB. 1984. Fossil woods of Lophopetalum and Artocarpus from the Deccan Intertrappean Beds of Mandla District, Madhya Pradesh, India. – Palaeobotanist 32: 310-320.
Mennega AMW. 1972. A survey of the wood anatomy of the New World Hippocrateaceae. – In: Ghouse AKM (ed), Research trends in plant anatomy. K. A. Chowdhury commemoration volume, Tata McGraw-Hill, New Delhi, pp. 61-72.
Mennega AMW. 1993. Comparative wood anatomy of Ruptiliocarpon caracolito (Lepidobotryaceae). – Novon 3: 418-422.
Mennega AMW. 1994. Wood and timber: Hippocrateaceae. – In: Görts-van Rijn ARA (ed), Flora of the Guianas 16, Koeltz Scientific Books, Königstein, pp. 110-140.
Mennega AMW. 1997. Wood anatomy of the Hippocrateoideae (Celastraceae). – IAWA J. 18: 331-368.
Merrill ED, Freeman FL. 1940. The Old World species of the celastraceous genus Microtropis Wallich. – Proc. Amer. Acad. Arts 73: 271-310.
Miers J. 1872. On the Hippocrateaceae of South America. – Trans. Linn. Soc. London 28: 319-432.
Morgan DR, Soltis DE. 1993. Phylogenetic relationships among members of Saxifragaceae sensu lato based on rbcL sequence data. – Ann. Missouri Bot. Gard. 80: 631-660.
Mory B. 1992. Zur Blattnervatur antillanischer Arten der Gattung Crossopetalum P. Br. (Celastraceae). – Flora 187: 17-36.
Mory B. 2001. Notes on Crossopetalum, Myginda and Gyminda (Celastraceae) from Cuba. – Willdenowia 31: 129-135.
Muhwezi O. 1999. The use of Loeseneriella apocynoides around Bwindi Impenetrable National Park, southwest Uganda. – In: Timberlake J, Kativu S (eds), African plants: biodiversity, taxonomy and uses, Royal Botanic Gardens, Kew, pp. 523-527.
Müller IH. 1995. Systematics and leaf anatomy of the Celastraceae sensu stricto of New Caledonia. – Ph.D. diss., Universität Zürich, Switzerland.
Murbeck S. 1918. Über die Organisation und verwandtschaftlichen Beziehungen der Gattung Lepuropetalon. – Ark. f. Bot. 15(10): 1-12.
Narang N. 1953. The life-history of Stackhousia linariaefolia A. Cunn. with a discussion of its systematic position. – Phytomorphology 3: 485-493.
Navaro AM, Blackwell WH. 1990. A revision of Paxistima (Celastraceae). – Sida 14: 231-249.
Ngassapa OD, Soejarto DD, Che C-T, Pezzuto JM, Farnsworth NR. 1991. New cytotoxic lupane lactones from Kokoona ochracea. – J. Nat. Prod. 54: 1353-1359.
Oltmann O. 1971. Pollenmorphologisch-systematische Untersuchungen innerhalb der Geraniales. – Diss. Bot. 11: 1-163.
Pace L. 1912. Parnassia and some allied genera. – Bot. Gaz. 54: 306-328.
Paclt J. 1998a. Proposal to amend the gender of Euonymus, nom. cons. (Celastraceae), to feminine. – Taxon 47: 473-474.
Paclt J. 1998b. Proposal to conserve the name Celastrus (Celastraceae) as being of feminine gender. – Taxon 47: 879-880.
Pant DD, Kidwai PF. 1966. Epidermal structure and stomatal ontogeny in some Celastraceae. – New Phytol. 65: 288-295.
Pax F. 1896. Stackhousiaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(5), W. Engelmann, Leipzig, pp. 231-233.
Pei S-J, Li Y-H. 1981. New materials for Yunnan Maytenus Molina. – Acta Bot. Yunnan. 3: 239-248.
Perrier de la Bâthie H. 1933. Les Brexiées de Madagascar. – Bull. Soc. Bot. France 80: 198-214.
Perrier de la Bâthie H. 1942a. Au sujet des affinités des Brexia, des Célastracées, et des deux Brexia nouveaux de Madagascar. – Bull. Soc. Bot. France 89: 219-221.
Perrier de la Bâthie H. 1942b. Révision des Célastracées de Madagascar et des Comores. – Not. Syst. 10: 173-206.
Perrier de la Bâthie H. 1944. Salvadoropsis nouveau genre de Célastracées de Madagascar. – Bull. Soc. Bot. France 91: 96-97.
Perrier de la Bâthie H. 1946a. Célastracées (Celastraceae). – In: Humbert H (ed), Flore de Madagascar et des Comores (Plantes Vasculaires) 116, Muséum National d’Histoire Naturelle (Paris), Laboratoire de Phanérogamie, pp. 1-76.
Perrier de la Bâthie H. 1946b. Hippocratéacées (Hippocrateaceae). – In: Humbert H (ed), Flore de Madagascar et des Comores (Plantes Vasculaires) 117, Muséum National d’Histoire Naturelle (Paris), Laboratoire de Phanérogamie, pp. 1-27.
Plouvier V. 1956. Sur la presence d’aspéruloside chez les Escallonia et de dulcitol chez de la Brexia madagascariensis Thou. (Saxifragacées). – Compt. Rend. Acad. Sci. Paris 242: 1643-1645.
Poole I, Wilkinson HP. 1999. A celastraceous twig from the Eocene London Clay of south-east England. – Bot. J. Linn. Soc. 129: 165-176.
Prigge BA. 1984. Studies on Acanthothamnus, Mortonia, and Orthosphenia (Celastraceae): anatomy, ecology, and systematics. – Ph.D. diss., Claremont Graduate School, Claremont, California.
Pullen CB, Schmitz P, Hoffmann D, Meurer K, Boettcher T, Bamberg D van, Pereira AM, Castro França S de, Hauser M, Geertsema H, Wyk A van, Mahmud T, Floss HG, Leistner E. 2003. Occurrence and non-detectability of maytansinoids in individual plants of the genera Maytenus and Putterlickia. – Phytochemistry 62: 377-387.
Qin X-S, Zhang R-J, Xing F-W. 2008. A new species of Maytenus section Gymnosporia (Celastraceae) from Hainan Island, China. – Bot. J. Linn. Soc. 158: 534-538.
Raju DCS. 1965. Enumeration of the Indian species of Hippocrateaceae. – J. Biol. Sci. 8: 55-59.
Rao PSN, Sreekumar PV. 1992. Hydnocarpus sharmae (Flacourtiaceae), a new species from Andaman Islands, India. – Nord. J. Bot. 12: 225-226.
Record SJ. 1938. The American woods of the orders Celastrales, Olacales, and Santalales. – Trop. Woods 53: 11-38.
Rehder A, Wilson EH. 1916. Celastraceae. – In: Sargent CS (ed), Plantae Wilsonianae: an enumeration of the woody plants collected in western China for the Arnold Arboretum of Harvard University during the years 1907, 1908, and 1909 by E. H. Wilson, vol. 2, Cambridge University Press, Cambridge, pp. 346-359.
Robson NKB. 1965. New and little known species from the Flora Zambesiaca area XVI: taxonomic and nomenclatural notes on Celastraceae. – Bol. Soc. Brot. 39: 5-55.
Robson NKB. 1966. 52. Celastraceae (incl. Hippocrateaceae). – In: Exell AW, Fernandes A, Wild H (eds), Flora Zambesiaca 2 (Part 2), Crown Agents for Oversea Governments and Administrations, London, pp. 355-418.
Robson NKB. 1978. 66. Brexiaceae. – In: Launert E (ed), Flora Zambesiaca 4, Flora Zambesiaca Managing Committee, London, pp. 59-61.
Robson NKB, Demissew S. 1987. New and little-known species of Maytenus (Celastraceae) in East Africa. – Kew Bull. 42: 423-427.
Robson NKB, Sousa EP. 1969. Celastraceae. – In: Fernandes A (ed), Flora de Moçambique 48, Centro de Botânica, Lisboa, pp. 1-66.
Robson NKB, Hallé N, Mathew B, Blakelock R. 1994. Celastraceae. – In: Polhill RM (ed), Flora of tropical East Africa 108, A. A. Balkema, Rotterdam, pp. 1-78.
Rogers CB, Abbott ATD, Wyk AE van. 1999. A convenient thin layer chromatographic technique for chemotaxonomic application in Maytenus (Celastraceae). – South Afr. J. Bot. 65: 174-176.
Rudolf PO. 1974. Euonymus L. – In: Schopmeyer CS (ed), Seeds of woody plants in the United States, Forest Service, U.S. Department of Agriculture, Washington, D.C., pp. 393-397.
Rydberg PA. 1905. Family 4. Parnassiaceae. – North American Flora 22: 77-80.
Savinov IA. 2002. Sarawakodendroideae – a new subfamily from the Celastraceae family. – Bot. Žurn. 87: 108-109.
Savinov IA. 2004. The comparative morphology of reproductive organs in the context of taxonomy of Celastraceae family. – Bot. Žurn. 89: 1385-1402. [in Russian]
Savinov IA. 2006. Some morphological basics for a revision of the tribe Celastreae Loes. (Celastraceae R. Br.). – Wulfenia 13: 207-215.
Savinov IA. 2007. Systematics and phylogeny of some problematic taxa from the Celastraceae R. Br. family which are endemics for Madagascar, south-eastern Asia and Oceania. – In: Perspectives of development and problems of modern botany: materials of I (III) Russian young scientific – practical conference of botanists at Novosibirsk, Siberian Branch of RAS, Novosibirsk, pp. 282-285.
Savinov IA. 2008. Floral evolution in the Celastrales order. – Bot. Žurn. 93: 1544-1555. [in Russian]
Savinov IA. 2010. Comparative morphological study of some Celastraceae from Madagascar and their relationships with other African representatives of the family. – Scripta Bot. Belg. 46: 451.
Savolainen V, Manen JF, Douzery E, Spichiger R. 1994. Molecular phylogeny of families related to Celastrales based on rbcL 5’ flanking sequences. – Mol. Phylogen. Evol. 3: 27-37.
Savolainen V, Spichiger R, Manen J-F. 1997. Polyphyletism of Celastrales deduced from a chloroplast noncoding DNA region. – Mol. Phylogen. Evol. 7: 145-157.
Saxena NP. 1964. Studies in the family Saxifragaceae II. Development of ovule and megagametophyte in Parnassia nubicola Wall. – Proc. Indian Acad. Sci., Sect. B, 60: 196-202.
Saxena NP. 1976. Studies in the family Saxifragaceae X. Floral morphology and systematic position of Parnassia. – J. Indian Bot. Soc. 55: 282-288.
Schatz GE, Lowry PP II. 2004. A synoptic revision of Brexia (Celastraceae) in Madagascar. – Adansonia, sér. III, 26: 67-81.
Schorno X, Brenneisen R, Steinegger E. 1982. Qualitative und quantitative Untersuchungen über das Vorkommen ZNS-aktiver Phenylpropylamine in Handelsdrogen und über deren Verteilung in verschiedenen Organen von Catha edulis. – Pharm. Acta Helv. 57: 168-176.
Sebsebe D. 1985. The genus Maytenus (Celastraceae) in NE tropical Africa and tropical Arabia. – Symb. Bot. Upsal. 25: 1-98.
Sha W-L, Luo J-Y, Chen X-X, Cheng C-Y. 1981. New species of genus Maytenus from Guangxi. – Acta Phytotaxon. Sin. 19: 232-234.
Sharma VK. 1968. Morphology, floral anatomy, and embryology of Parnassia nubicola Wall. – Phytomorphology 18: 193-204.
Simmons MP. 2004a. Celastraceae. – In: Kubitzki K (ed), The families and genera of vascular plants VI. Flowering plants. Dicotyledons. Celastrales, Oxalidales, Rosales, Cornales, Ericales, Springer, Berlin, Heidelberg, New York, pp. 29-64.
Simmons MP. 2004b. Parnassiaceae. – In: Kubitzki K (ed), The families and genera of vascular plants VI. Flowering plants. Dicotyledons. Celastrales, Oxalidales, Rosales, Cornales, Ericales, Springer, Berlin, Heidelberg, New York, pp. 291-296.
Simmons MP, Barrie FR. 2014. Haydenoxylon, a replacement name for Haydenia (Celastraceae). – Novon 23: 224-225.
Simmons MP, Cappa JJ. 2013. Wilczekra, a new genus of Celastraceae for a disjunct lineage of Euonymus. – Syst. Bot. 38: 148-153.
Simmons MP, Hedin JP. 1999. Relationships and morphological character change among genera of Celastraceae sensu lato (including Hippocrateaceae). – Ann. Missouri Bot. Gard. 86: 723-757.
Simmons MP, Clevinger CC, Savolainen V, Archer RH, Mathews S, Doyle JJ. 2001. Phylogeny of the Celastraceae inferred from phytochrome B gene sequence and morphology. – Amer. J. Bot. 88: 313-325.
Simmons MP, Savolainen V, Clevinger CC, Archer RH, Davis JI. 2001. Phylogeny of the Celastraceae inferred from 26S nuclear ribosomal DNA, phytochrome B, rbcL, atpB, and morphology. – Mol. Phylogen. Evol. 19: 353-366.
Simmons MP, Mabberley DJ, De Kok RPJ. 2004. Hippocrateaceae, Labiatae, Vitaceae. – In: Tirel C (ed), Flore de la Nouvelle-Calédonie et Dépendances 25, Muséum National d’Histoire Naturelle, Paris.
Simmons MP, Cappa JJ, Archer RH, Ford AJ, Eichstedt D, Clevinger CC. 2008. Phylogeny of the Celastreae (Celastraceae) and the relationships of Catha edulis (qat) inferred from morphological characters and nuclear and plastid genes. – Mol. Phylogen. Evol. 48: 745-757.
Simmons MP, Bacon CD, Cappa JJ, McKenna MJ. 2012. Phylogeny of Celastraceae subfamilies Cassinoideae and Tripterygioideae inferred from morphological characters and nuclear and plastid loci. – Syst. Bot. 37: 456-467.
Simmons MP, McKenna MJ, Bacon CD, Yakobsen K, Cappa JJ, Archer RH, Ford AJ. 2012. Phylogeny of Celastraceae tribe Euonymeae inferred from morphological characters and nuclear and plastid genes. – Mol. Phylogen. Evol. 62: 9-20.
Sleumer H. 1942. Peripterygiaceae. – In: Engler A (†), Harms H, Mattfeld J (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 20b, W. Engelmann, Leipzig, pp. 397-400.
Smith AC. 1940. The American species of Hippocrateaceae. – Brittonia 3: 341-555.
Smith AC. 1941. Notes on Old World Hippocrateaceae. – Amer. J. Bot. 28: 438-443.
Smith AC. 1946. Studies of South American plants XI. Noteworthy species of Hippocrateaceae and Vacciniaceae. – J. Arnold Arbor. 27: 86-120.
Smith AC, Bailey IW. 1941. Brassiantha, a new genus of Hippocrateaceae from New Guinea. – J. Arnold Arbor. 22: 389-394.
Soltis DE, Soltis PS. 1997. Phylogenetic relationships in Saxifragaceae sensu lato: a comparison of topologies based on 18S rDNA and rbcL sequences. – Amer. J. Bot. 84: 504-522.
Stant MY. 1952. Notes on the systematic anatomy of Stackhousia. – Kew Bull. 1951: 309-318.
Staszkiewicz J. 1997. The variability of seeds of Euonymus europaeus and E. verrucosus (Celastraceae). – Fragm. Florist. Geobot. Series Polon. 10: 151-159.
Szendrei K. 1981. The chemistry of khat. – Bull. Narcotics 32: 5-36.
Tang Y. 1994. Embryology of Plagiopteron suaveolens (Plagiopteraceae) and its systematic implications. – Bot. J. Linn. Soc. 116: 145-157.
Tieghem P van. 1899. Sur les Parnassiacées. – J. Bot. (Morot) 13: 326-332.
Tobe H, Hammel BE. 1993. Floral morphology, embryology, and seed anatomy of Ruptiliocarpon caracolito (Lepidobotryaceae). – Novon 3: 423-428.
Tobe H, Raven PH. 1993. Embryology of Acanthothamnus, Brexia and Canotia (Celastrales): a comparison. – Bot. J. Linn. Soc. 112: 17-32.
Trelease W. 1892. Revision of North American Ilicineae and Celastraceae. – Trans. Acad. Sci. St. Louis 5: 343-349.
Uttal LJ. 1986. Once and for all it is Paxistima. – Castanea 59: 67-68.
Vasudeva Rao MK. 1994. Hydnocarpus sharmae (Flacourtiaceae) is Siphonodon celastrineus (Celastraceae). – Nord. J. Bot. 14: 303-305.
Vasudeva Rao MK, Chakrabarty T. 1985. Nicobariodendron Vasud. & T. Chakrab. (Celastraceae): a new genus from the Nicobar Islands, India. – J. Econ. Taxon. Bot. 7: 513-516.
Verdcourt B. 1968. Brexiaceae. – In: Milne-Redhead E, Polhill, RM (ed), Flora of tropical East Africa, Crown Agents for Oversea Governments and Administrations, London, pp. 1-4.
Wanders GL, Skvarla JJ, Pyle CC. 1968. Fine structure of Hippocrateaceae pollen walls. – Pollen Spores 10: 189-196.
Wang Y-F, Li C-S, Li Z-Y, Fu D-Z. 2001. Wuyunanthus gen. nov., a flower of Celastraceae from the Palaeocene of North-East China. – Bot. J. Linn. Soc. 136: 323-327.
Warburg O. 1894. Flacourtiaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(6a), W. Engelmann, Leipzig, pp. 1-56.
Ward DB, Gholson AK. 1987. The hidden abundance of Lepuropetalum spathulatum (Saxifragaceae) and its first reported occurrence in Florida. – Castanea 52: 59-67.
Watari S. 1939. Anatomical studies on the leaves of some Saxifragaceous plants, with special reference to the vascular system. – J. Fac. Sci. Univ. Tokyo, Sect. III, Bot. 5: 195-316.
Webb CJ. 1979. Breeding system and seed set in Euonymus europaeus (Celastraceae). – Plant Syst. Evol. 132: 299-303.
Welzen P van, Baas P. 1984. A leaf anatomical contribution to the classification of the Linaceae complex. – Blumea 29: 453-479.
Wendel GW. 1974. Celastrus scandens L. – In: Schopmeyer CS (ed), Seeds of woody plants in the United States, Forest Service, U.S. Department of Agriculture, Washington, D.C., pp. 295-297.
Wentworth JE, Gornall RJ. 1996. Cytogenetic evidence for autopolyploidy in Parnassia palustris. – New Phytol. 134: 641-648.
Wheeler LC. 1943. History and orthography of the Celastraceous genus “Pachystima” Rafinesque. – Amer. Midl. Natur. 29: 792-795.
White OE, Bowden WM. 1947. Oriental and American bittersweet hybrids. – J. Heredity 38: 125-127.
Wilczek R. 1956. Novitates Africanae II. Hippocrateaceae du Congo Belge et du Ruanda-Urundi. – Bull. Jard. Bot. État Bruxelles 26: 399-428.
Wilczek R. 1959. Novitates Africanae IV. Celastraceae – Euonymus. – Bull. Jard. Bot. État Bruxelles 29: 183-184.
Wu D, Wang H, Li D-Z, Blackmore S. 2005. Pollen morphology in Parnassia L. (Parnassiaceae) and its systematic implications. – J. Integ. Plant Biol. 47: 2-12.
Wyk AE van, Prins M. 1987. A new species of Catha (Celastraceae) from southern Natal and Pondoland. – South Afr. J. Bot. 53: 202-205.
Yoshikawa M, Murakami T, Shimada H, Matsuda H, Yamahara J, Tanabe G, Muraoka O. 1997. Salacinol, potent antidiabetic principle with unique thiosugar sulfonium sulphate structure from the Ayurvedic traditional medicine Salacia reticulata in Sri Lanka and India. – Tetrahedron Letters 38: 8367-8370.
Zelger JL, Schorno HX, Carlini EA. 1981. Behavioural effects of cathinone, an amine obtained from Catha edulis Forsk.: comparisons with amphetamine, norpseudoephedrine, apomorphine and nomifensine. – Bull. Narcotics 32: 67-82.
Zhang L-B, Simmons MP. 2006. Phylogeny and delimitation of the Celastrales inferred from nuclear and plastid genes. – Syst. Bot. 31: 122-137.