ROSIDAE
Takht.
Takhtajan, Sist. Filog. Cvetk. Rast. [Syst.
Phylog. Magnolioph.]: 264. 4 Feb 1967
[Vitaceae+Rosanae]
VITACEAE Juss.
de Jussieu, Gen. Plant.: 267. 4 Aug 1789
[’Vites’], nom. cons.
Vitales Juss. ex Berchtold et
J. Presl, Přir. Rostlin: 225. Jan-Apr 1820 [‘Vites’];
Leeaceae (DC.) Dumort., Anal. Fam. Plant.: 21, 27.
1829, nom. cons.; Ampelopsidaceae Kostel., Allg.
Med.-Pharm. Fl.: 1194. Jan-Oct 1835 [’Ampelopsideae’];
Leeales DC. in C. F. P. von Martius, Consp. Regn.
Veg.: 26. Sep-Oct 1835 [‘Leeaceae’];
Cissaceae Drejer, Comp. Medic. Bot.: 45. 1840
[’Cisseae’]; Pterisanthaceae J. Agardh,
Theoria Syst. Plant.: 268. Apr-Sep 1858 [’Pterisantheae’];
Vitanae Takht. ex Reveal in Novon 2: 237. 13 Oct
1992
Genera/species c
13/725–>960
Distribution Mainly in
tropical and subtropical regions, some species in warm-temperate areas;
Leea: Southeast Asia, Malesia, New Guinea and Australia, two species in
Africa and Madagascar.
Fossils Fruits assigned to
Vitaceae are known from c 66 My
old sediments in India (Manchester & al. 2013). Seeds are abundant in
Cenozoic layers from the Paleocene onwards, and the clade was well
differentiated during the early Eocene. Palaeogene fossils have been assigned
to several extant genera.
Habit Bisexual, monoecious,
polygamomonoecious, dioecious or polygamodioecious, usually evergreen
(sometimes deciduous) lianas (sometimes climbing, perennial herbs) with
leaf-opposed, simple or branched branch-tendrils with small adhesive cushions
attaching plant to trees or cliffs etc. (rarely small succulent trees with
swollen stem; Leea consists of evergreen trees, shrubs or perennial
herbs without tendrils). Lenticels often abundant and significant.
Vegetative anatomy CAM
physiology sometimes occurring? Phellogen ab initio superficially or deeply
seated. Medulla continuous or interrupted by diaphragms at nodes. Primary
medullary strands wide. Secondary lateral growth normal or anomalous (in
Tetrastigma from concentric cambia). Cambium often stratified. Vessel
elements usually with simple (rarely scalariform) perforation plates; lateral
pits scalariform, opposite or alternate, simple pits. Imperforate tracheary
xylem elements libriform fibres with simple or bordered pits, usually septate.
Wood rays multiseriate, homocellular or heterocellular. Axial parenchyma
apotracheal diffuse, or paratracheal scanty vasicentric, or banded, or absent.
Wood partially storied. Tension wood absent. Secondary phloem often stratified
into hard fibrous and soft parenchymatic Tyloses present. zones. Sieve tube
plastids Pcs type (P2b type), with several polygonal protein crystals and
starch. Nodes 3–7:3–7, trilacunar to multilacunar with three to seven leaf
traces, often swollen. Calciumoxalate as druses, raphides, or single prismatic
crystals (raphides in Leea with filiform appendages). Parenchyma with
raphide sacs each containing often hundreds of raphides. Shoot apex producing
foliar primordia and primordia developing into inflorescences or tendrils.
Trichomes Hairs unicellular or
multicellular, uniseriate or branched, sometimes furcate, often lepidote,
peltate, and vesicular hairs; sugar- and oil-storing food bodies, pearl glands
(extrafloral nectaries; at least in Leea, Cissus and
Vitis): multicellular, spherical, with short multiseriate stalk,
caducous, sometimes with apical stoma.
Leaves Usually alternate
(usually distichous; in Leea usually spiral, rarely opposite), simple
or palmately compound, entire or palmately lobed (in Leea simply or
repeatedly compound), with conduplicate ptyxis. Stipules usually pairwise,
intrapetiolar, caducous or persistent (sometimes adaxially connate; rarely
absent; in Leea sheathing, as wing-like outgrowths along petiole
margin); leaf sheath absent. Petiole vascular bundle transection annular.
Venation usually palmate (rarely pinnate). Stomata anomocytic, staurocytic,
hemiparacytic or cyclocytic (Leea, or actinocytic?). Cuticular wax
crystalloids usually ? (sometimes as tubular rodlets). Domatia as pockets or
hair tufts, or absent. Mesophyll with numerous tanniniferous idioblasts, and
raphide cells (raphide sacs) usually with both mucilage and calciumoxalate
raphides, sometimes also mucilaginous idioblasts without raphides. Lamina often
gland-dotted (with caducous pearl glands) and with lepidote and peltate hairs.
Leaf margin serrate; teeth often coarse; leaf tooth with distally expanding
gland and with foramen, and with lateral veins running from above and below
tooth (in Leea with small glandular apex and one lateral vein running
further above tooth).
Inflorescence Usually
leaf-opposed (sometimes terminal or axillary; in Leea usually
terminal), cymose corymb or panicle (rarely spike-like; in Leea often
umbel-like). Flowers often replaced by tendrils (sometimes with specialized
‘sucking discs’). Inflorescence in Leea often densely beset with
ferrugineous hairs.
Flowers Actinomorphic, small.
Hypanthium present or absent (absent in, e.g., Leea). Usually hypogyny
(in Leea rarely half epigyny). Sepals (three or) four or five (to
seven), usually with open (in Leea valvate) aestivation, often as
teeth or scales or reduced to ring, caducous (or sometimes persistent?),
connate. Petals (three or) four or five (to seven), with valvate aestivation,
usually free (in Leea connate at base), in Vitis calyptrate
(at apex connate forming hood, calyptra, detached at anthesis). Gynoecial
nectaries present. Nectariferous disc intrastaminal, usually annular (nectary
sometimes enclosing ovary, or as four or five nectariferous glands alternating
with stamens, or absent; in Leea nectariferous disc absent, nectar not
produced).
Androecium Stamens (three or)
four or five (to seven), obhaplostemonous, alternisepalous, antepetalous;
stamens and petals developed from common primordium. Filaments usually free
from each other and from tepals (in Leea basally adnate to corolla
tube [epipetalous] and upwards connate into anther tube, sometimes together
with entire or bifid nectary lobes alternating with anthers, sometimes with
pendant tubular membrane near centre of anther tube). Anthers dorsifixed,
sometimes versatile, usually tetrasporangiate (rarely disporangiate), introrse,
longicidal (dehiscing by longitudinal slits). Tapetum usually secretory
(sometimes amoeboid-periplasmodial). Female flowers sometimes with
staminodia?
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually tricolporate (in
Leea also triporate), shed as monads, usually bicellular at dispersal
(in Leea usually tricellular). Exine semitectate, with columellate
infratectum, usually reticulate (in Leea psilate).
Gynoecium Pistil composed of
usually two (in Leea two or three [or four]) connate carpels,
vertically or transversely orientated (odd carpel in Leea abaxial).
Ovary usually superior (in Leea rarely semi-inferior), bilocular or
trilocular (in Leea sometimes quadrilocular, each locule divided by
secondary septum). Style single, usually very short (rarely absent; in
Leea longer). Stigma usually capitate (in Tetrastigma
quadrilobate), usually non-papillate (in Leea papillate), Dry type.
Male flowers sometimes with pistillodium?
Ovules Placentation axile
(seemingly subbasal-marginal; in Leea basal?). Ovules usually two per
carpel (in Leea one per incompletely closed locule), anatropous,
ascending, apotropous, bitegmic, crassinucellar. Micropyle usually endostomal
(sometimes bistomal; in Leea exostomal). Outer integument usually four
to seven cell layers thick. Inner integument usually two or three cell layers
thick. Obturator placental or absent. Parietal tissue up to 17 cell layers
thick. Nucellar cap present. Megagametophyte monosporous, Polygonum
type. Egg apparatus in Cissus present outside? ovule. Endosperm
development ab initio nuclear. Endosperm haustoria present or absent.
Embryogenesis asterad.
Fruit A usually one- to
four-seeded berry (in Leea four- to six-seeded).
Seeds Aril absent. Seed coat
endotestal. Seeds perichalazal, with prominent adaxial raphe (flanked by two
deep furrows) and abaxial chalazal tubercle. Testa with deep furrow adjacent to
raphe. Vascular bundle more or less enclosing seed. Testa sometimes
multiplicative. Cells with bundles of calciumoxalate raphides. Exotesta fleshy
(sarcotesta). Mesotesta two to 17 cell layers thick. Endotesta two to five cell
layers thick, lignified, usually with calciumoxalate raphides (rarely
palisade). Exotegmen crossed tracheidal. Endotegmen mucilaginous. Perisperm not
developed? Endosperm copious, oily and proteinaceous, sometimes ruminate,
usually Y-, T-, M- or U-shaped in cross-section. Embryo minute, straight, well
differentiated, without chlorophyll. Cotyledons two. Germination
phanerocotylar.
Cytology n = 10–16, 19, 20
(22–27, 30, 40, c. 42, c. 47)
DNA d copy of nuclear
gene RPB2 lost. Mitochondrial genome in Vitis very large,
with expanded intergenic spacers. Mitochondrial intron coxII.i3?
Phytochemistry Flavonols
(kaempferol, quercetin, myricetin), cyanidin, oleanolic acid derivatives,
ellagic and gallic acid, hydrolyzable and non-hydrolyzable tannins,
proanthocyanidins (prodelphinidins), caffeic acid, chlorogenic acid, alkaloids,
naphthoquinones, tartric acid, and myo-inisitol present. Saponins and
cyanogenic compounds not found.
Use Ornamental plants, fruits
(grapes, raisins, juice, etc.), wines and liquors (Vitis), medicinal
plants.
Systematics Vitaceae are sister-group to the
remaining Rosidae with high support, according to multigene analyses
including 17 genes (Soltis & al. 2011). Vitaceae were recovered as sister to
[Saxifragales+Rosanae],
although with weak support, in analyses of plastid inverted repeats in 244 taxa
(Moore & al. 2011). On the other hand, Vitaceae were found to be sister-group
to Saxifragales
with relatively high support in analyses by Zhang & al. (2016).
Leeoideae Burmeist.,
Handb. Naturgesch.: 338. 1837 [‘Leeaceae’]
1/34–>70. Leea
(34–>70; Southeast Asia, Malesia to New Guinea, tropical Australia, one
species, L. guineensis, in Africa and Madagascar). – Evergreen
trees, shrubs or perennial herbs. Raphides with filiform appendages. Leaves
spirally arranged, simply or twice compound. Stipules sheathing, visible as
wing-like outgrowths along petiole margin. Leaf teeth with small glandular apex
and one lateral vein running further above tooth. Petals connate at base.
Nectariferous disc absent, nectar not produced. Filaments adnate at base to
corolla tube, upwards connate and forming tube together with “nectary”
lobes. Ovary locules divided by secondary septum. Micropyle exostomal. Fruit a
four- to six-seeded berry. Testa rarely with raphides. n = (10–)12, 24.
–Leea is sister to the remaining Vitaceae.
Vitoideae Eaton, Bot.
Dict., ed. 4: 40. Apr-Mai 1836 [‘Vites’]
c 12/690–890. 'Ampelopsis'
(c 16; temperate and subtropical regions of Asia and America; paraphyletic),
Nekemias (9; India, East and Southeast Asia, Java, Sulawesi, eastern
North America), Cyphostemma
(c 150; tropical and subtropical regions on both hemispheres, with their
highest diversity in tropical Africa), 'Cayratia' (50–63; tropical
regions in the Old World east to eastern Queensland; paraphyletic),
Acareosperma (1; A. spireanum; Laos)?, Tetrastigma
(90–95; tropical Asia to tropical Australia), ’Cissus’
(200–350; tropical and subtropical regions on both hemispheres;
polyphyletic), Pterocissus (1; P. mirabilis; Hispaniola; in
Cissus?),
Clematicissus (2; C. angustissima: western Western Australia;
C. opaca: eastern Queensland, eastern New South Wales),
‘Parthenocissus’ (10–18; temperate Asia, North America, Mexico;
non-monophyletic), 'Ampelocissus' (95–120; tropical regions on both
hemispheres; paraphyletic), Vitis (c 65;
temperate and subtropical regions on the Northern Hemisphere). – Temperate to
tropical regions on both hemispheres, with their largest diversity in tropical
regions. Usually lianas (rarely trees or perennial herbs, sometimes with a
swollen root), climbing with the aid of leaf-opposite tendrils evolved from
inflorescence branches. Raphides glabrous. Leaves alternate (often distichous)
or opposite. Cuticular wax crystalloids sometimes as tubular rods. Leaf teeth
with gland distally expanded and with foramen; lateral veins running above and
below tooth. Inflorescence usually leaf-opposite (rarely terminal). Hypogyny.
Disc usually annular or as five glands (rarely surrounding gynoecium or
absent). Pistil composed of two connate carpels, transversal or vertical. Style
usually short (rarely long). Micropyle usually endostomal (sometimes bistomal).
Outer integument four to seven cell layers thick. Inner integument (one or) two
or three (or four) cell layers thick. Nucellar cap up to eight cell layers
thick. Testa usually with raphides, sometimes multiplicative. n = 10–16, 19,
20. – In the plastid DNA sequence analysis by Ren & al. (2011), three
distinct monophyletic groups were identified: 1) a “pentamerous clade”
including Ampelocissus, Vitis and
Parthenocissus; 2) a “tetramerous clade” containing Cissus,
Cayratia and Tetrastigma; and 3) a clade sister to the two
previous clades comprising the Ampelopsis
lineage (including Rhoicissus and several species of Cissus, Lu
& al. 2018). Clematicissus (not included in the analyses by Ren
& al. 2011) is sister to Cissus striata and C. tweediana
(Rossetto & al. 2007), and seems to be closely allied to the Ampelopsis
lineage (Wen & al. 2007). Lu & al. (2018) discussed the Late Cretaceous
rapid radiation of Vitoideae. Three main lineages were identified
(supported by gynoecium morphology): 1) the Ampelopsis clade as sister
to the rest, 2) Parthenocissus-Yua +
Ampelocissus-Vitis, and 3) Cissus.
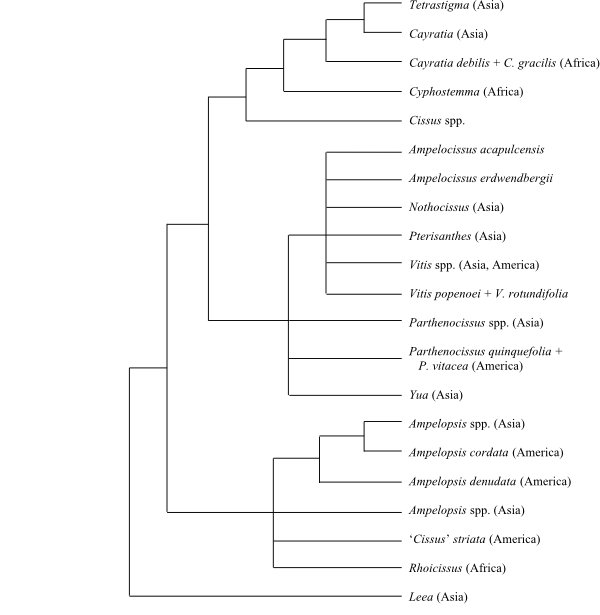 |
Phylogeny (simplified) of Vitaceae
based on DNA sequence data (Ren & al. 2011)
|
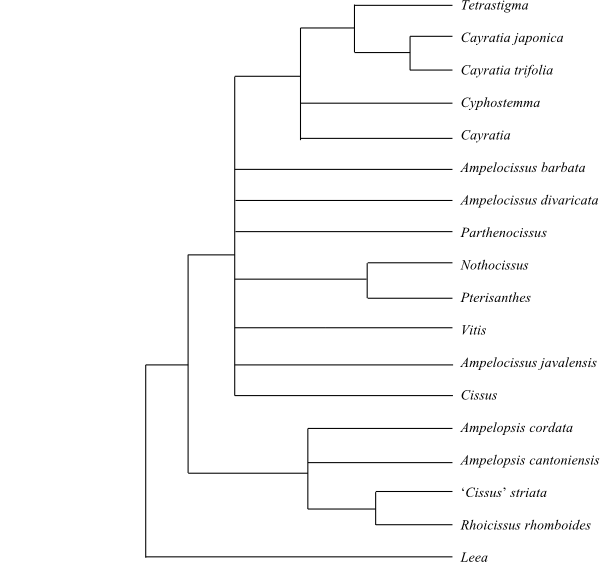 |
Phylogeny (simplified) of Vitaceae
based on DNA sequence data (Trias-Blasi & al. 2012)
|
Literature
Adkinson J. 1913. Some features of the
anatomy of the Vitaceae. – Ann. Bot.
27: 133-139.
Alquini Y, Bona C, Bueno NC, Cislinki J,
Contin A, Dunaiski A, Segecin S. 1995. Stem anatomy of four species of
Cissus (Vitaceae), typical in
Corumba (MS) – Brazil. – Arq. Biol. Tecn. (Curibita) 38: 815-827.
Aradhya M, Koehmstedt A, Prins BH, Dangl GS,
Stover E. 2008. Genetic structure, differentiation, and phylogeny of the genus
Vitis: implications for genetic conservation. – Acta Horticult. 799:
43-49.
Aradhya M, Wang Y, Walker M, Prins B,
Koehmstedt A, Velasco D, Gerrath J, Dangl G, Preece J. 2013. Genetic diversity,
structure, and patterns of differentiation in the genus Vitis. –
Plant Syst. Evol. 299: 317-330.
Arnott HJ, Webb MA. 2000. Twinned raphides of
calcium oxalate in grape (Vitis): implications for crystal stability
and function. – Intern. J. Plant Sci. 161: 133-142.
Behnke H-D. 1974. P- und S-typ
Siebelement-Plastiden bei Rhamnales. – Beitr. Biol. Pflanzen 50: 457-464.
Bernard AC. 1972-1973. Á propos du complex
axillaire chez certaines Vitacées. – Natur. Monspel., Sér. Bot. 23/24:
49-61.
Bouquet A. 1983. Contribution à l’étude
de l’espèce Muscadinia rotundifolia et de ses hybrides avec
Vitis vinifera. Applications en selection. – Ph.D. diss.,
l’Université de Bordeaux, France.
Calonje M, Cubas P, Martínez-Zapater JM,
Carmona MJ. 2002. Floral meristem identity genes are expressed during tendril
development in the grape vine. – Plant Physiol. 135: 1491-1501.
Cevallos-Ferriz SRS, Stockey RA. 1990.
Permineralized fruits and seeds from the Princeton chert (Middle Eocene) of
British Columbia: Vitaceae. – Can. J.
Bot. 68: 288-295.
Chen I. 2009. History of Vitaceae inferred from morphology-based
phylogeny and the fossil record of seeds. – Ph.D. diss., University of
Florida, Gainesville, Florida.
Chen I, Manchester SR. 2007. Seed morphology
of modern and fossil Ampelocissus (Vitaceae) and implications for
phytogeography. – Amer. J. Bot. 94: 1534-1553.
Chen I, Manchester SR. 2011. Seed morphology
of Vitaceae. – Intern. J. Plant Sci.
172: 1-35.
Chen PT, Chen L-Q, Wen J. 2011. The first
phylogenetic analysis of Tetrastigma (Miq.) Planch., the host of Rafflesiaceae. –
Taxon 60: 499-512.
Chen PT, Wen J, Chen L-Q. 2012. Spatial and
temporal diversification of Tetrastigma (Vitaceae). – Gard. Bull. (Singapore) 63:
307-327.
Comeaux BL, Nesbitt WB, Fantz PR. 1987.
Taxonomy of the native grapes of North Carolina. – Castanea 52: 197-215.
Critchfield WB. 1970. Shoot growth and leaf
dimorphism in Boston ivy Parthenocissus tricuspidata. – Amer. J.
Bot. 57: 535-542.
Descoings B. 1960. Un genre méconnu de
Vitacées: compréhension et distinction des genres Cissus L. et
Cyphostemma (Planch.) Alston. – Notulae Syst. 16: 113-125.
Díaz-Riquelme J, Lijavetzky D,
Martínez-Zapater JM, Carmona MJ. 2009. Genome-wide analysis of
MIKCc-type MADS-box genes in grapevine. – Plant Physiol. 149:
354-369.
Dorofeev PI. 1957. Seeds of
Ampelopsis from the Tertiary deposits of the territory of USSR. –
Bot. Žurn. 42: 643-648. [In Russian]
Dorofeev PI. 1963. Tretichnye flory zapadoni
Sibiri. – V. L. Komarov Bot. Inst., Izdat Nauka, Leningrad.
Dunaiski A Jr. 1992. Two new species of Vitaceae: Cissus cervii sp. n. and
Cissus macrocarpa sp. n. – Acta Biol. Paranaense 21: 135-141.
Esau K. 1948. Phloem structure in the
grapevine, and its seasonal changes. – Hilgardia 18: 217-296.
Gagnepain F. 1911. Classification des
Cissus et Cayratia. – Notulae Syst. 1: 339-343.
Gagnepain F. 1912. Leeacées. – In: Flore
générale de l’Indochine 1, Masson, Paris, pp. 934-944.
Gagnepain F. 1919. Acareosperma, un
genre nouveau d’Ampélidacées. – Bull. Mus. Natl. Hist. Nat. Paris 25:
131-132.
Gagnepain F. 1950a. Leeacées. – In: Flore
générale de l’Indochine, suppl. 7, Masson, Paris, pp. 844-855.
Gagnepain F. 1950b. Ampélidacées. – In:
Flore générale de l’Indochine, suppl. 7, Masson, Paris, pp. 855-915.
Galet P. 1967. Recherches sur les méthodes
d’identification et de classification des Vitacées des zones tempérées.
– Ph.D. diss., Faculté des Sciences, l’Université de Montpellier,
France.
Gerrath JM. 1992. Developmental morphology
and anatomy of grape flowers. – In: Janic J (ed), Horticultural Reviews 13,
John Wiley, New York, pp. 315-337.
Gerrath JM, Lacroix CR. 1997. Heteroblastic
sequence and leaf development in Leea guineensis. – Intern. J. Plant
Sci. 158: 747-756.
Gerrath JM, Posluszny U. 1988a. Morphological
and anatomical development in the Vitaceae I. Vegetative development in
Vitis riparia. – Can. J. Bot. 66: 209-224.
Gerrath JM, Posluszny U. 1988b. Morphological
and anatomical development in the Vitaceae II. Floral development in Vitis
riparia. – Can. J. Bot. 66: 1334-1351.
Gerrath JM, Posluszny U. 1988c. Comparative
floral development in some members of the Vitaceae. – In: Leins P, Tucker SC,
Endress PK (eds), Aspects of floral development, J. Cramer, Berlin, pp.
121-131.
Gerrath JM, Posluszny U. 1989a. Morphological
and anatomical development in the Vitaceae III. Vegetative development in
Parthenocissus inserta. – Can. J. Bot. 67: 803-816.
Gerrath JM, Posluszny U. 1989b. Morphological
and anatomical development in the Vitaceae IV. Floral development in
Parthenocissus inserta. – Can. J. Bot. 67: 1356-1365.
Gerrath JM, Posluszny U. 1989c. Morphological
and anatomical development in the Vitaceae V. Vegetative and floral
development in Ampelocissus brevipedunculata. – Can. J. Bot. 67:
2371-2386.
Gerrath JM, Posluszny U. 1994. Morphological
and anatomical development in the Vitaceae VI. Cissus antarctica. –
Can. J. Bot. 72: 635-643.
Gerrath JM, Posluszny U. 2007. Shoot
architecture in the Vitaceae. – Can. J.
Bot. 85: 691-700.
Gerrath JM, Lacroix CR, Posluszny U. 1990.
The developmental morphology of Leea guineensis II. Floral
development. – Bot. Gaz. 151: 210-220.
Gerrath JM, Lacroix CR, Posluszny U. 1998.
Phyllotaxis in the Vitaceae. – In: Jean
RV, Barabé D (eds), Symmetry in plants, World Scientific Press, Singapore, pp.
89-107.
Gerrath JM, Posluszny U, Dengler NG. 2001.
Primary vascular patterns in the Vitaceae. – Intern. J. Plant Sci. 162:
729-745.
Gerrath JM, Wilson T, Psoluszny U. 2004.
Morphological and anatomical development in the Vitaceae VII. Floral development in
Rhoicissus digitata with respect to other genera in the family. –
Can. J. Bot. 82: 198-206.
Gerrath JM, Posluszny U, Icket-Bond SM, Wen
J. 2017. Inflorescence morphology and development in the basal rosid lineage Vitales. – J. Syst. Evol. 55: 542-558.
Gilg E. 1896. Vitaceae (Ampelidaceae). – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien III(5), W. Engelmann,
Leipzig, pp. 427-456.
Gilg E, Brandt M. 1911-1912. Vitaceae Africanae. – Engl. Bot. Jahrb.
Syst. 46: 415-557.
Gong F, Karsai I, Liu YS. 2010. Vitis seeds
(Vitaceae) from the late Neogene Gray
fossil site, northeastern Tennessee, USA. – Rev. Palaeobot. Palynol. 162:
71-83.
Goremykin VV, Salamini F, Velasco R, Viola R.
2009. Mitochondrial DNA of Vitis vinifera and the issue of rampant
horizontal gene transfer. – Mol. Biol. Evol. 26: 99-110.
Harvey BT. 1915. The dissemination of
Virginia creeper seeds by English sparrows. – Plant World 18: 217-219.
Ickert-Bond SM, Gerrath JM, Wen J. 2014.
Gynoecial structure of Vitales and
implications for the evolution of placentation in the rosids. – Intern. J.
Plant Sci. 175: 998-1032.
Ickert-Bond SM, Gerrath JM, Posluszny U, Wen
J. 2015. Inflorescence development in the Vitis-Ampelocissus clade of
Vitaceae: the lamellate inflorescence of
Pterisanthes confirms the Ampelocissus Bauplan. – Bot. J.
Linn. Soc. 179: 725-741.
Ingrouille MJ, Chase MW, Fay MF, Bowman D,
Bank M van der, Bruijn ADE. 2002. Systematics of Vitaceae from the viewpoint of plastid
rbcL DNA sequence data. – Bot. J. Linn. Soc. 138: 421-432.
Jackes BR. 1984. Revision of the Australian
Vitaceae 1. Ampelocissus
Planchon. – Austrobaileya 2: 81-86.
Jackes BR. 1987a. Revision of the Australian
Vitaceae 2. Cayratia Juss. –
Austrobaileya 2: 365-379.
Jackes BR. 1987b. A study of the trichomes of
several frequently confused species of Cissus L. (Vitaceae). – Blumea 32: 143-148.
Jackes BR. 1988. Revision of the Australian
Vitaceae 3. Cissus L. –
Austrobaileya 2: 481-505.
Jackes BR. 1989a. Revision of the Australian
Vitaceae 4. Clematicissus
Planchon. – Austrobaileya 3: 11-19.
Jackes BR. 1989b. Revision of the Australian
Vitaceae 5. Tetrastigma (Miq.)
Planchon. – Austrobaileya 3: 149-158.
Jackes BR, Rossetto M. 2006. A new
combination in Clematicissus Planch. (Vitaceae). – Telopea 11: 390-391.
Jaillon O, Aury JM, Noel B, Policriti A,
Clepet C, Casagrande A, Choisne N, Aubourg S, Vitulo N, Jubin C, Vezzi A,
Legeai F, Hugueney P, Dasilva C, Horner D, Mica E, Jublot R, Poulain J,
Bruyère C, Billault A, Segurens B, Gouyvenoux M, Ugarte E, Cattonaro F,
Anthouard V, Vico V, Fabbro C del, Alaux M, Gaspero G di, Dumas V, Felice N,
Paillard S, Juman I, Moroldo M, Scalabrin S, Canaguier A, Le Clainche I,
Malacrida G, Durand E, Pesole G, Laucou V, Chatelet P, Merdinoglu D, Delledonne
M, Pezzotti M, Lecharny A, Scarpelli C, Artiguenave F, Pè ME, Valle G,
Morgante M, Caboche M, Adam-Blondon A-F, Weissenbach J, Quétier F, Wincker P.
2007. The grapevine genome sequence suggests ancestral hexaploidization in
major angiosperm phyla. – Nature 449: 463-467.
Jansen RK, Kaittanis C, Saski C, Lee S-B,
Tomkins J, Alverson AJ, Daniell H. 2006. Phylogenetic analysis of
Vitis (Vitaceae) based on
complete chloroplast genome sequences: effects of taxon sampling and
phylogenetic methods on resolving relationships among rosids. – BMC Evol.
Biol. 6: 32-45.
Kannabiran B, Pragasarn A. 1994. Foliar
epidermal features of Vitaceae and
taxonomic position of Leea. – J. Indian. Bot. Soc. 73: 81-87.
Karkamkar SP, Patil VP. 1992. Karyotype
variation in Leea L. – J. Indian Bot. Soc. 71: 217-220.
Kashyap G. 1955. Studies in the family Vitaceae I. Floral morphology of Vitis
trifolia L. – Agra Univ. J. Res. Sci. [Suppl.] 4: 777-783.
Kashyap G. 1957. Studies in the family Vitaceae II. Floral anatomy of Vitis
trifolia Linn., Vitis latifolia Roxb., and Vitis
himalayana Brandis. – J. Indian Bot. Soc. 36: 317-323.
Kashyap G. 1958. Studies in the family Vitaceae III. Floral morphology of Vitis
latifolia Roxb., Vitis himalayana Brandis, and Vitis
trifolia Linn. – J. Indian Bot. Soc. 37: 240-248.
Kevan PG, Longair RW, Gadawski RM. 1985.
Dioecy and pollen dimorphism in Vitis riparia (Vitaceae). – Can. J. Bot. 63:
2263-2267.
Kevan PG, Blades DCA, Posluszny U, Ambrose
JD. 1988. Pollen dimorphism and dioecy in Vitis aestivalis. – Vitis
27: 143-146.
Kirchheimer F. 1939. Rhamnales I: Vitaceae. – In: Fossil. Catl. II(24), pp.
1-174.
Kumbhojkar MS, Jadhav AS. 1980. Chromosome
numbers in the family Vitaceae. – Curr.
Sci. 49: 37-38.
Lacroix CR, Posluszny U. 1989a. Phyllotactic
patterns in some members of the Vitaceae.
– Bot. Gaz. 150: 303-313.
Lacroix CR, Posluszny U. 1989b. Stipules in
some members of the Vitaceae: relating
process of development to the mature structure. – Amer. J. Bot. 76:
1203-1215.
Lacroix CR, Gerrath JM, Posluszny U. 1990.
The developmental morphology of Leea guineensis I. Vegetative
development. – Bot. Gaz. 151: 204-209.
Latiff A. 1981. Studies in Malesian Vitaceae V. The genus Cayratia in
the Malay Peninsula. – Sains Malaysiana 10: 129-139.
Latiff A. 1982a. Studies in Malesian Vitaceae I. A revision of
Pterisanthes Bl. – Feder. Mus. J. (Perak) 27: 42-69.
Latiff A. 1982b. Studies in Malesian Vitaceae II. Nothocissus, a new
Malesian genus. – Feder. Mus. J. (Perak) 27: 70-74.
Latiff A. 1982c. Studies in Malesian Vitaceae III. Ampelocissus
complanata, a new species from Borneo. – Feder. Mus. J. (Perak) 27:
75-77.
Latiff A. 1982d. Studies in Malesian Vitaceae IV. The genera
Ampelocissus, Ampelopsis and Parthenocissus in the
Malay Peninsula. – Feder. Mus. J. (Perak) 27: 78-93.
Latiff A. 1983a. Studies in Malesian Vitaceae VII. The genus Tetrastigma
in the Malay Peninsula. – Gard. Bull. (Singapore) 36: 213-228.
Latiff A. 1983b. Numerical analysis and
classification of Malesian Vitaceae. –
Malays 12: 7-13.
Latiff A. 1984. The morphology and
systematics of Vitaceae in the Malay
Peninsula. – In: Sahid S & al. (eds), A collection of working papers 4,
University of Kebangsaan, Malaysia, pp. 33-41.
Latiff A. 1991. Studies in Malesian Vitaceae X. Two new species of
Tetrastigma from Borneo. – Blumea 35: 559-564.
Latiff A. 2001a. Studies in Malesian Vitaceae XII. Taxonomic notes on
Cissus, Ampelocissus, Nothocissus and
Tetrastigma and other genera. – Folia Malay. 2: 179-189.
Latiff A. 2001b. Diversity of the Vitaceae in the Malay Archipelago. –
Malayan Nat. J. 55: 29-42.
Lavie P. 1970. Contribution à l’étude
caryosystématique des Vitacées. – Ph.D. diss., Faculté des Sciences,
l’Université de Montpellier, France.
Lavie P. 1979. Caryosystématique des Vitaceae 1. Cissus L.,
Cyphostemma (Planch.) Alst., Rhoicissus Planch. –
Adansonia, sér. II, 19: 175-198.
Lavie P. 1990. Vitacées de l’ouest
Africain. Espèces sénégalaises. – Ministère de la cooperation et du
développement, Senegal.
Li C-L. 1990. Yua C. L. Li – a new
genus of Vitaceae. – Acta Bot. Yunnan.
12: 1-10.
Liu X-Q, Ickert-Bond SM, Chen L-Q, Wen J.
2013. Molecular phylogeny of Cissus L. of Vitaceae (the grape family) and evolution of
its pantropical intercontinental disjunctions. – Mol. Phylogen. Evol. 66:
43-53.
Liu X-Q, Ickert-Bond SM, Nie Z-L, Zhou Z,
Chen L-Q, Wen J. 2016. Phylogeny of the Ampelocissus-Vitis clade in Vitaceae supports the New World origin of
the grape genus. – Mol. Phylogen. Evol. 95: 217-228.
Lombardi JA. 1996. Eight new species of
Cissus (Vitaceae) from South
America. – Brittonia 48: 195-208.
Lombardi JA. 1997. Types of names in
Ampelocissus and Cissus (Vitaceae) referring to taxa in the
Caribbean, Central and N. America. – Taxon 46: 423-432.
Lombardi JA. 2000. Flora Neotropica.
Monograph 80. Vitaceae: Géneros
Ampelocissus, Ampelopsis e Cissus. – New York
Botanical Garden, Bronx, New York.
Lombardi JA. 2001. 115. Vitaceae. – In: Harling G, Andersson L
(eds), Flora of Ecuador 67, Botanical Institute, Göteborg University, pp.
1-37.
Lombardi JA. 2002. Cissus
pinnatifolia (Vitaceae), a new
species from the Atlantic coast of Brazil. – Brittonia 54: 175-177.
Lombardi JA. 2004. Cissus xerophila
(Vitaceae), a new species from the
xerophytic vegetation of northeastern Minas Gerais, Brazil. – Brittonia 56:
288-290.
Lombardi JA. 2005. Three new species of Vitaceae from Mesoamerica. – Novon 15:
562-567.
Lombardi JA. 2007. Systematics of Vitaceae in South America. – Can. J. Bot.
85: 712-721.
Lu L, Wen J, Chen Z. 2012. A combined
morphological and molecular phylogenetic analysis of Parthenocissus
(Vitaceae) and taxonomic implications.
– Bot. J. Linn. Soc. 168: 43-63.
Lu L, Wang W, Chen Z, Wen J. 2013. Phylogeny
of the non-monophyletic Cayratia Juss. (Vitaceae) and implications for character
evolution and biogeography. – Mol. Phylogen. Evol. 68: 502-515.
Lu L, Cox CJ, Mathews S, Wang W, Wen J, Chen
Z. 2018. Optimal data partitioning, multispecies coalescent and Bayesian
concordance analyses resolve early divergences of the grape family (Vitaceae). – Cladistics 34: 57-77.
Mabberley DJ. 1995. Vitaceae. – In: Dassanayake MD (ed), A
revised handbook to the flora of Ceylon IX, Amerind Publ. Co., New Delhi, pp.
446-482.
McAtee WL. 1906. Virginia creeper as a winter
food for birds. – Auk 23: 346-347.
Malaise F, Matamba M. 1990. Investigations on
the Vitaceae of Shaba, Zaire II.
Cyphostemma (new record) (Planch.) Alston for the flora of Zaire. –
Bull. Jard. Bot. Natl. Belg. Bruxelles 60: 307-316.
Manchester SR, Chen I, Lott TA. 2012. Seeds
of Ampelocissus, Cissus, and Leea (Vitales) from the Paleogene of western Peru
and their biogeographic significance. – Intern. J. Plant Sci. 173:
933-943.
Manchester SR, Kapgate DK, Wen J. 2013.
Oldest fruits of the grape family (Vitaceae) from the Late Cretaceous Deccan
Cherts of India. – Amer. J. Bot. 100: 1849-1859.
Miki S. 1956. Seed remains of Vitaceae in Japan. – J. Inst. Polytechn.
Osaka City Univ., Ser. D, 7: 247-271.
Millington WF. 1966. The tendril of
Parthenocissus inserta: determination and development. – Amer. J.
Bot. 53: 74-81.
Molina JE, Wen J, Struwe L. 2013. Systematics
and biogeography of the non-viny grape relative Leea (Vitaceae). – Bot. J. Linn. Soc. 171:
354-376.
Moore MO. 1987. A study of selected taxa of
Vitis (Vitaceae) in the
southeastern United States. – Rhodora 89: 75-91.
Moore MO. 1991. Classification and
systematics of eastern North American Vitis L. (Vitaceae) North of Mexico. – Sida 14:
339-367.
Moore MO, Giannasi DE. 1994. Foliar
flavonoids of eastern North American Vitis (Vitaceae) north of Mexico. – Plant Syst.
Evol. 193: 21-36.
Mulgera de Romero ME. 1978. Revision de la
vitaceas de la Argentina. – Darwiniana 2: 3-26.
Nair NC. 1968. Contribution to the floral
morphology and embryology of two species of Leea with a discussion on
the taxonomic position of the genus. – J. Indian Bot. Soc. 47: 193-205.
Nair NC. 1970. Comparative embryology of
angiosperms: Meliaceae,
Rhamnaceae, Vitaceae, Leeaceae. – Bull. Natl. Sci.
Acad. India 41: 151-155, 168-173, 174-179, 180-184.
Nair NC, Muni KV. 1960. Organography and
floral anatomy of some species of Vitaceae. – Phytomorphology 10:
138-144.
Nair NC, Nambisan PNN. 1957. Contribution to
the floral morphology and embryology of Leea sambucina Willd. – Bot.
Not. 110: 160-172.
Najmaddin C, Hussin K, Maideen H. 2011.
Comparative anatomical study between Cayratia mollissima,
Pterisanthes caudigera (Vitaceae) and Leea indica
(Leeaceae). – Amer. J. Appl. Sci. 8: 839-842.
Nebel B. 1929. Zur cytology von
Malus und Vitis. – Die Gartenbauwissenschaft 1: 549-592.
Nie Z-L, Sun H, Chen Z-D, Meng Y, Manchester
SR, Wen J. 2010. Molecular phylogeny and biogeographic diversification of
Parthenocissus (Vitaceae)
disjunct between Asia and North America. – Amer. J. Bot. 97: 1342-1353.
Nie Z-L, Sun H, Manchester SR, Meng Y, Luke
Q, Wen J. 2012. Evolution of the intercontinental disjunctions in sex
continents in the Ampelopsis clade of the grape family (Vitaceae). – BMC Evol. Biol. 12: 17.
http://dx.doi.org/10.1186/1471-2148-12-17
Op de Beck P, Bessière JM, Dijoux-Franca
M-G, David B, Mariotte A-M. 2000. Volatile constituents from leaves and wood of
Leea guineensis G. Don (Leeaceae) from Cameroon. – Flavour Fragrance
J. 15: 182-185.
Pavia EAS, Buono RA, Lombardi JA. 2009. Food
bodies in Cissus verticillata (Vitaceae): ontogenesis, structure and
functional aspects. – Ann. Bot. 103: 517-524.
Pelsy F. 2007. Untranslated leader region
polymorphism of Tvv1, a retrotransposon family, is a novel marker useful for
analyzing genetic diversity and relatedness in the genus Vitis. –
Theor. Appl. Genet. 116: 15-27.
Periasamy K. 1962. Studies on seeds with
ruminate endosperm 2. Development of rumination in the Vitaceae. – Proc. Indian Acad. Sci., Sect.
B, 56: 13-26.
Péros JP, Berger G, Portemont A, Boursiquot
JM, Lacombe T. 2011. Genetic variation and biogeography of the disjunct
Vitis subg. Vitis (Vitaceae). – J. Biogeogr. 38: 471-486.
Planchon JE. 1884. Les vignes des tropiques
du genre Ampelocissus. – Vigne Amér. Vitic. Eur. 8: 370-381.
Planchon JE. 1887. Monographie des
Ampélidées vraies. – In: Candolle AFPP de, Candolle C de (eds),
Monographiae Phanerogamarum 5, 2, Masson, Paris, pp. 306-654.
Poole I, Wilkinson HP. 2000. Two early Eocene
vines from south-east England. – Bot. J. Linn. Soc. 133: 1-26.
Posluszny U, Gerrath JM. 1986. The vegetative
and floral development of the hybrid grape cultivar ‘Ventura’. – Can. J.
Bot. 64: 1620-1631.
Prakash U, Dayal R. 1963. Fossil wood
resembling Elaeocarpus and Leea from Deccan Intertrappean
Beds of Mahurzari near Nagpur. – Palaeobotanist 12: 121-127.
Rehder AA. 1905. Die amerikanischen Arten der
Gattung Parthenocissus. – Mitt. Deutsch. Dendrol. Ges. 14:
129-136.
Rehder AA. 1908. The New England species of
Psedera. – Rhodora 10: 24-27.
Rehder AA. 1945. Moraceae, Hippocastanaceae et Vitaceae, nomina conservanda. – J. Arnold
Arbor. 26: 277-279.
Reille M. 1967. Contribution à l’étude
palynologique de la famille des Vitacées. – Pollen Spores 9: 279-303.
Ren H, Pan K-Y, Chen Z-D, Wang R-Q. 2003.
Structural characters of leaf epidermis and their systematic significance in Vitaceae. – Acta Phytotaxon. Sin. 41:
531-544.
Ren H, Lu L-M, Soejima A, Luke Q, Zhang D-X,
Chen Z-D, Wen J. 2011. Phylogenetic analysis of the grape family (Vitaceae) based on the noncoding plastid
trnC-petN, trnH-psbA, and trnL-F
sequences. – Taxon 60: 629-637.
Retief E. 2008. Systematics of
Rhoicissus Planch. (Vitaceae).
– South Afr. J. Bot. 74: 376.
Retief E, Wyk AE van. 1996. A new species of
Cyphostemma (Vitaceae) from
South Africa. – South Afr. J. Bot. 62: 183-187.
Ridsdale CE. 1974. A revision of the family
Leeaceae. – Blumea 22: 57-100.
Ridsdale CE. 1975. Leeaceae. – In: Steenis
CGGJ van (ed), Flora Malesiana I, 7, Noordhoff, Leiden, pp. 759-782.
Rodrigues JG, Lombardi JA, Lovato MB. 2014.
Phylogeny of Cissus (Vitaceae)
focusing of South American species. – Taxon 673: 287-298.
Rossetto M, McNally J, Henry RJ. 2001.
Evaluating the potential of SSR flanking regions for examining taxonomic
relationships in Vitaceae. – Theor.
Appl. Gen. 103: 61-66.
Rossetto M, Jackes BR, Scott KD, Henry RJ.
2001. Intergeneric relationships in the Australian Vitaceae: new evidence from cpDNA analysis.
– Gen. Res. Crop Evol. 48: 307-314.
Rossetto M, Jackes BR, Scott KD, Henry RJ.
2002. Is the genus Cissus (Vitaceae) monophyletic? Evidence from
plastid and nuclear ribosomal DNA. – Syst. Bot. 27: 522-533.
Rossetto M, Crayn DM, Jackes BR, Porter C.
2007. An updated estimate of intergeneric phylogenetic relationships in the
Australian Vitaceae. – Can. J. Bot. 85:
722-730.
Sandhu PS, Mann SK. 1989. SOCGI plant
chromosome number reports VIII. – J. Cytol. Genet. 24: 179-183.
Sarracino JM, Merritt R, Chin CK. 1992a.
Morphological and physiological characteristics of Leea coccinia and
Leea rubra in response to light-flux. – HortScience 27: 400-403.
Sarracino JM, Merritt R, Chin CK. 1992b.
Light acclimatization potential of Leea coccinia and Leea
rubra grown under low light-flux. – HortScience 27: 404-406.
Sax K. 1930. Chromosome counts in
Vitis and related genera. – Proc. Amer. Soc. Hort. Sci. 26:
32-33.
Shah JJ. 1959. Studies on the stipules of six
species of Vitaceae. – J. Arnold Arbor.
40: 398-412.
Shah JJ, Dave YS. 1966. Are tendrils of Vitaceae axillary? – Curr. Sci. 22:
559-561.
Shah JJ, Dave YS. 1970. Morpho-histogenetic
studies on the tendrils of Vitaceae. –
Amer. J. Bot. 57: 363-370.
Shetty BV. 1959. Cytotaxonomical studies in
Vitaceae. – Bibliogr. Genet. 18:
167-272.
Shetty BV, Singh P. 1989. Notes on Vitaceae in India and some neighbouring
regions. – Kew Bull. 44: 469-478.
Shiraishi S-I, Hsiung T-C, Shiraishi M. 1996.
Preliminary survey on stomatal density and length of grapevine. – J. Fac.
Agric. Kyushu Univ. 41: 11-15.
Soejima A, Wen J. 2006. Phylogenetic analysis
of the grape family (Vitaceae) based on
three chloroplast markers. – Amer. J. Bot. 93: 278-287.
Steenis CGGJ van, Bakhuizen van den Brink RC.
1967. Miscellaneous botanical notes XVI. 104: Two new Bornean
Pterisanthes (Vitaceae). –
Bot. Jahrb. Syst. 86: 385-390.
Steinbrecher T, Beuchle G, Melzer B, Speck T,
Kraft O, Schwaiger R. 2011. Structural development and morphology of the
attachment system of Parthenocissus tricuspidata. – Intern. J. Plant
Sci. 172: 1120-1129.
Sun Q, Rost TL, Matthews MA. 2008.
Wound-induced vascular occlusions in Vitis vinifera (Vitaceae): tyloses in summer and gels in
winter. – Amer. J. Bot. 95: 1498-1505.
Süssenguth K, Kirchheimer F, Scherz W (†),
Zimmermann J, Stellwaag F. 1953. Vitaceae. – In: Engler A (†), Harms H
(†), Mattfeld J (†), Melchior H, Werdermann E (eds), Die natürlichen
Pflanzenfamilien, 2. Aufl., Bd. 20d, Duncker & Humblot, Berlin, pp.
174-371.
Süssenguth K. 1953. Leeaceae. – In: Engler
A (†), Harms H (†), Mattfeld J (†), Melchior H, Werdermann E (eds), Die
natürlichen Pflanzenfamilien, 2. Aufl., Bd. 20d, Duncker & Humblot,
Berlin, pp. 372-390.
Tarasevich VF. 1984. Parthenocissus
and Ampelopsis pollen from the Miocene deposits of the Oka-Don River
plain. – Bot. Žurn. 69: 849-855.
Tarnavschi IT, Petria E. 1968. Contribution
to the knowledge of the microsporial structures in the family Leeaceae. –
Pollen Spores 10: 221-249.
Tiffney BH. 1979. Nomenclatural revision:
Brandon Vitaceae. – Rev. Palaeobot.
Palynol. 27: 91-92.
Tiffney BH, Barghoorn ES. 1976. Fruits and
seeds of the Brandon Lignite I. Vitaceae.
– Rev. Palaeobot. Palynol. 22: 169-191.
Timmons SA. 2006. Ontogeny and phylogenetic
trends in the grape family (Vitaceae):
Cissus and Vitis genera. – M.Sc. thesis, University of Guelph, Ontario.
Timmons SA, Posluszny U, Gerrath JM. 2007.
Morphological and anatomical development in the Vitaceae IX. Comparative ontogeny and
phylogenetic implications of Vitis rotundifolia Michx. – Can. J.
Bot. 85: 850-859.
Timmons SA, Posluszny U, Gerrath JM. 2007.
Morphological and anatomical development in the Vitaceae X. Comparative ontogeny and
phylogenetic implications of Cissus quadrangularis L. – Can. J. Bot.
85: 860-872.
Trias-Blasi A, Parnell JAN, Chayamarit K,
Teerawatananon A. 2011. Cayratia emarginata (Vitaceae), a new species from Thailand and
Vietnam. – Blumea 56: 16-17.
Trias-Blasi A, Parnell JAN, Hodkinson TR.
2012. Multi-gene region phylogenetic analysis of the grape family (Vitaceae). – Syst. Bot. 37: 941-950.
Tröndle D, Schröder S, Kassemeyer HH,
Kiefer C, Koch MA, Nick P. 2010. Molecular phylogeny of the genus
Vitis (Vitaceae) based on
plastid markers. – Amer. J. Bot. 97: 1168-1178.
Tucker SC, Hoefert LL. 1968. Ontogeny of the
tendril in Vitis vinifera. – Amer. J. Bot. 55: 1110-1119.
Vatsala P. 1960. Chromosome studies in the
Ampelidaceae. – Cellule 61: 191-206.
Viala P. 1910. Ampélographie générale. –
In: Viala P, Vermorel V (eds), Ampélographie 1, Masson, Paris, pp. 3-108.
Vivas N, Augustin M. 1996. About the genus
Parthenocissus: study of P. quinquefolia and P.
tricuspidata Planch. 1887 (Rhamnales, Vitaceae). – Bull. Soc. Linn. Bordeaux 24:
1-26.
Vollesen K. 1984. Notes on Ethiopian Vitaceae and Burseraceae. – Nord. J.
Bot. 4: 33-37.
Walter H. 1921. Über Perldrüsenbildung bei
Ampelideen. – Flora 13: 187-231.
Walters TW, Decker-Walters DS, Posluszny U,
Kevan PG. 1990. Understanding grape (Vitis, Vitaceae) cultivar phylogenies. – Econ.
Bot. 44: 129-131.
Wan Y, Schwaninger H, Simon CJ, Wang Y, He P.
2008. The eco-geographic distribution of wild grape germplasm in China. –
Vitis 47: 77-80.
Wan Y, Schwaninger H, Baldo AM, Labate JA,
Zhong GY, Simon CJ. 2013. A phylogenetic analysis of the grape genus
(Vitis L.) reveals broad reticulation and concurrent diversification
during Neogene and Quaternary climate change. – BMC Evol. Biol. 13: 141.
http://dx.doi.org/10.1186/1471-2148-13-141
Watari S. 1951. Studies on the fossil woods
from the Tertiary of Japan VII. Leea (Vitaceae) from the Miocene of Simane. –
Bot. Mag. (Tokyo) 64: 1-7.
Wen J. 2006a. Leeaceae. – In: Kubitzki K
(ed), The families and genera of vascular plants IX. Flowering plants.
Eudicots. Berberidopsidales,
Buxales, Crossosomatales, Fabales p. p., Geraniales, Gunnerales, Myrtales p. p., Proteales, Saxifragales, Vitales, Zygophyllales, Clusiaceae Alliance, Passifloraceae
Alliance, Dilleniaceae, Huaceae, Picramniaceae, Sabiaceae, Springer, Berlin,
Heidelberg, New York, pp. 221-225.
Wen J. 2006b. Vitaceae. – In: Kubitzki K (ed), The
families and genera of vascular plants IX. Flowering plants. Eudicots. Berberidopsidales,
Buxales, Crossosomatales, Fabales p. p., Geraniales, Gunnerales, Myrtales p. p., Proteales, Saxifragales, Vitales, Zygophyllales, Clusiaceae Alliance, Passifloraceae
Alliance, Dilleniaceae, Huaceae, Picramniaceae, Sabiaceae, Springer, Berlin,
Heidelberg, New York, pp. 467-479.
Wen J. 2008. Phylogenetic relationships and
biogeography of Vitaceae (the grape
family). – South Afr. J. Bot. 74: 382-383.
Wen J, Nie Z-L, Soejima A, Meng Y. 2007.
Phylogeny of Vitaceae based on the
nuclear GAI1 gene sequences. – Can. J. Bot. 85: 731-745. –
Erratum: 2007. Can. J. Bot. 85: 1018.
Wen J, Lu LM, Boggan JK. 2013. Diversity and
evolution of Vitaceae in the Philippines.
– Philipp. J. Sci. 142 (Spec. Issue): 223-244.
Wen J, Xiong ZQ, Nie ZL, Mao LK, Zhu YB, Kan
XZ, Ickert-Bond SM, Gerrath J, Zimmer EA, Fang XD. 2013. Transcriptome
sequences resolve deep relationships of the grape family. – PloS ONE 8:
e74394. http://dx.doi.org/10.1371/journal.pone.0074394
Wen J, Boggan J, Nie Z-L. 2014. Synopsis of
Nekemias Raf., a segregate genus from Ampelopsis Michx. (Vitaceae) disjunct between
eastern/southeastern Asia and eastern North America, with ten new combinations.
– PhytoKeys 42: 11-19.
Wheeler EA, LaPasha CA. 1994. Woods of the Vitaceae – fossil and modern. – Rev.
Palaeobot. Palynol. 80: 175-207.
Wild H. 1966. 55. Leeaceae. – In: Exell AW,
Fernandes A, Wild H (eds), Flora Zambesiaca 2 (Part 2), Crown Agents for
Oversea Governments and Administrations, London, pp. 492-494.
Wild H, Drummond RB. 1966. 54. Vitaceae. – In: Exell AW, Fernandes A,
Wild H (eds), Flora Zambesiaca 2 (Part 2), Crown Agents for Oversea Governments
and Administrations, London, pp. 439-492.
Wilson T, Posluszny U. 2003. Complex tendril
branching in two species of Parthenocissus: implications for the
vitaceous shoot architecture. – Can. J. Bot. 81: 587-597.
Wilson TC, Gerrath JM, Posluszny U. 2002.
Morphological and anatomical development in the Vitaceae VIII. Comparative development of
three Cyphostemma (Vitaceae)
species reveals important vegetative and reproductive differences among the
species. – Can. J. Bot. 84: 702-716.
Zecca G, Abbott JR, Sun WB, Spada A, Sala F,
Grassi F. 2012. The timing and the mode of evolution of wild grapes
(Vitis). – Mol. Phylogen. Evol. 62: 736-747.
Zhang N, Wen J, Zimmer EA. 2015a. Expression
patterns of AP1, FUL, FT and LEAFY orthologs in Vitaceae support the homology of tendrils
and inflorescences throughout the grape family. – J. Syst. Evol. 53:
469-476.
Zhang N, Wen J, Zimmer EA. 2015b. Congruent
deep relationships in the grape family (Vitaceae) based on sequences of chloroplast
genomes and mitochondrial genes via genome skimming. – PLoS ONE 10:
e0144701.
Zhang N, Wen J, Zimmer EA. 2016. Another look
at the phylogenetic position of the grape order Vitales: chloroplast phylogenomics with an
expanded sampling of key lineages. – Mol. Phylogen. Evol. 101: 216-223.

