”The COM
clade”
[Celastrales+[Oxalidales+Malpighiales]]
MALPIGHIALES Juss. ex Bercht. et
J. Presl
Berchtold et Presl, Přir. Rostlin: 225. Jan-Apr
1820 [’Malpighiaceae’]
Habit Bisexual, monoecious or
dioecious (rarely andromonoecious, gynomonoecious, polygamomonoecious,
androdioecious, or polygamodioecious), evergreen or deciduous trees, shrubs,
suffrutices or lianas, perennial, biennial or annual herbs (sometimes
climbing).
Vegetative anatomy Phellogen
ab initio epidermal or subepidermal, pericyclic, or outer- or inner-cortical.
Secondary lateral growth normal, anomalous (from cylindrical cambium or several
concentric cambia) or absent. Vessel elements with simple or scalariform
(sometimes reticulate) perforation plates; lateral pits alternate, scalariform
or opposite, simple or bordered pits. Vestured pits sometimes present.
Imperforate tracheary xylem elements tracheids, fibre tracheids or libriform
fibres with simple or bordered pits, septate or non-septate (often also
vasicentric tracheids). Wood rays uniseriate or multiseriate, usually
heterocellular (rarely homocellular), or absent. Axial parenchyma apotracheal
diffuse or diffuse-in-aggregates, or paratracheal scanty, vasicentric, aliform,
lozenge-aliform, winged-aliform, confluent, scalariform, reticulate,
unilateral, or banded, or absent. Intraxylary phloem sometimes present. Sieve
tube plastids Ss, S0, Pcs or Pc type. Nodes 1:1, unilacunar with one
leaf trace, 3:3, trilacunar with three traces, or ≥5:≥5, multilacunar with
five or more traces. Schizogenous secretory cells, canals or cavities or glands
with resin, balsam or other secretions often abundant. Laticifers sometimes
present. Heartwood often with gum-like substances. Silica bodies sometimes
present. Cristarque cells sometimes present. Calciumoxalate as prismatic,
rhomboidal or acicular crystals, crystal sand, druses, styloids or other
types.
Trichomes Hairs unicellular or
multicellular, uniseriate or multiseriate, simple or branched, furcate,
T-shaped, malpighiaceous hairs, stellate, candelabra-shaped, dendritic,
lepidote or peltate, or absent; glands and glandular hairs sometimes present;
stinging hairs occasionally present.
Leaves Usually alternate
(spiral or distichous) or opposite (rarely verticillate), pinnately or
palmately compound, or simple and entire or lobed, with conduplicate,
supervolute, convolute, involute, revolute, curved or flat ptyxis (rarely
absent). Stipules interpetiolar, intrapetiolar or petiolar, free or connate,
often rudimentary or absent (sometimes modified into spines or hair-like); leaf
sheath absent. Petiole often articulated, sometimes geniculate. Petiole
vascular bundle transection arcuate, annular or complex; petiole sometimes with
lateral flank bundles. Venation pinnate or palmate, eucamptodromous,
brochidodromous, reticulodromous or parallelodromous (rarely acrodromous,
actinodromous or campylodromous). Stomata anomocytic, paracytic or anisocytic
(rarely cyclocytic or tetracytic). Cuticular wax crystalloids usually as
rosettes of platelets (Fabales type; sometimes absent). Domatia as
pits or hair tufts (rarely myrmecodomatia). Epidermis with or without
mucilaginous idioblasts. Mesophyll often with sclerenchymatous idioblasts. Leaf
margin or leaflet margins entire, crenate or serrate; teeth with one vein
proceeding into congested caducous tooth apex; glandular teeth, salicoid or
violoid, rarely present on leaf margin.
Inflorescence Terminal or
axillary, panicle, fascicle, thyrsoid, corymb, raceme-, spike-, catkin- or
umbel-like cymose, or racemes, spikes or catkins (sometimes pseudanthium), or
flowers solitary axillary. Bracts and/or floral prophylls (bracteoles)
sometimes absent.
Flowers Actinomorphic or
zygomorphic. Pedicel often articulated. Hypanthium rarely present. Usually
hypogyny (rarely epigyny or half epigyny). Sepals (two or) three to five (to c.
20), with imbricate, valvate, decussate or open (sometimes truncate)
aestivation, usually whorled (rarely spiral and indistinctly separate from
petals), free or connate at base. Petals (two to) four to 15, usually whorled
(rarely spiral and indistinctly separate from sepals), with imbricate, valvate,
contorted, involute, decussate or crumpled (rarely cochlear or open)
aestivation, usually clawed, usually free (sometimes more or less connate, or
absent). Corona sometimes present at petal bases. Nectaries at filament bases
or absent. Disc slightly developed or absent (nectariferous disc sometimes
extrastaminal, lobate or cupular, usually annular).
Androecium Stamens one to more
than 750, usually in one or more whorls. Filaments free or more or less
connate, often in one or three fascicles, usually free from tepals (sometimes
epipetalous). Anthers basifixed or dorsifixed, usually non-versatile, usually
tetrasporangiate (rarely disporangiate), usually introrse (sometimes extrorse,
rarely latrorse), usually longicidal (dehiscing by longitudinal slits; rarely
poricidal, dehiscing by apical pores). Tapetum usually secretory (rarely
amoeboid-periplasmodial). Staminodia one to more than 50, extrastaminal or
intrastaminal, or absent.
Pollen grains
Microsporogenesis usually simultaneous (rarely successive). Pollen grains
(2–)3(–6)-colpor(oid)ate, tri- to pentacolp(oid)ate or tetra- to polyporate
(rarely syncolpate or inaperturate), usually shed as monads (sometimes
tetrads), usually bicellular (sometimes tricellular) at dispersal. Exine
tectate or semitectate (rarely intectate), with columellate or granular
infratectum, perforate, reticulate, microreticulate, or striate, rugulate,
fossulate, foveolate, scabrate, verrucate, spinulate, echinate, retipilate,
psilate or smooth.
Gynoecium Pistil composed of
two to ten (to 20) usually connate carpels. Ovary usually superior (rarely
inferior or semi-inferior), unilocular to quinquelocular (to 20-locular). Style
single, simple, or stylodia two to five (to more than twelve), usually free, or
absent; style sometimes unifacial. Stigma one, capitate to peltate, or stigmas
two to five, capitate, punctate or truncate, papillate, usually Dry (sometimes
Wet) type. Pistillodium usually absent (male flowers sometimes with
pistillodium).
Ovules Placentation axile,
apical, subbasal or parietal (rarely laminar). Ovules one to more than 50 per
carpel, anatropous or hemianatropous (sometimes amphitropous, orthotropous, or
anacampylotropous), ascending, horizontal or pendulous, apotropous or
epitropous, usually bitegmic, usually weakly crassinucellar or incompletely
tenuinucellar. Micropyle bistomal or endostomal (sometimes exostomal).
Funicular or placental obturator sometimes present. Archespore usually
unicellular (rarely bicellular or tricellular). Nucellar cap or nucellar beak
sometimes present. Megagametophyte usually monosporous, Polygonum type
(sometimes tetrasporous, 16-nucleate, Penaea type, or disporous,
Allium type, etc.). Antipodal cells sometimes proliferating, sometimes
absent. Endosperm development ab initio nuclear. Endosperm haustoria chalazal
or absent. Embryogenesis usually solanad (sometimes onagrad or asterad, rarely
piperad or caryophyllad).
Fruit A loculicidal and/or
septicidal capsule, berry, drupe, nut, or schizocarp (divided into two to five
nut-like, samaroid or drupaceous mericarps; rarely a secondary syncarp).
Seeds Aril or carunculus
sometimes present. Seed coat testal, exotegmic or endotegmic (usually
exotegmic). Testa sometimes vascularized, sometimes multiplicative. Sarcotesta
sometimes present. Exotesta sometimes palisade. Mesotesta and/or endotesta
sometimes lignified and/or sclerenchymatous. Tegmen sometimes multiplicative.
Exotegmen and/or endotegmen often fibrous or lignified (sometimes palisade).
Perisperm not developed. Endosperm copious or sparse, oily, or absent. Embryo
large or small, straight or curved (rarely hook-shaped, spirally twisted or
circinate), usually well differentiated, oily, with or without chlorophyll.
Cotyledons two (to four). Germination phanerocotylar or cryptocotylar.
Cytology x = 5–15, 17, 19,
21, 23
DNA Plastid gene
rps16 often entirely or partially(lost in Passifloraceae, Violaceae, Salicaceae and Turneraceae, and also in some
Linaceae and Malpighiaceae. Plastid gene
atpF lost several times. Plastid gene infA lost/defunct.
Mitochondrial intron coxII.i3 lost.
Phytochemistry Flavonols
(kaempferol, quercetin, myricetin), flavones, flavone-C-glycosides,
afzelechin, biflavonoids, biflavanoids, biflavonoyls, trihydroxyflavonoids,
flavonoid sulphates, cyanidin, cucurbitacins and other triterpenes, dammaranes,
phorbole ester diterpenes, oleanolic acid derivatives, ellagic acid, methylated
ellagic acids, gallic acid, non-hydrolyzable tannins (ellagitannins: geraniin,
mallotussinic acid), condensed tannins, tannins with proanthocyanidins and
catechin, proanthocyanidins (prodelphinidins), p-coumaric acid,
caffeic acid, chlorogenic acid, cinnamic acid derivatives, tropane (hygrolinic)
alkaloids (tropane-3α and tropane-3β-ols, tropacocaine, scopolamine oxides,
hydroxytropines, teloidines, ecgonines, norecgonines, phyllalbine, oxytrapanes,
brugine, etc.), indole alkaloids, pyrrolizidine alkaloids and other alkaloids
(securinine, phyllantine, phyllochrisine, etc.), triterpene saponins,
tyrosine-derived cyanogenic compounds, phenol glycosides (salicin, populin
etc.), cyclopentenoid (cyclopentenylic) cyanogenic glycosides (gynocardin)
and/or cyclopentenylic fatty acids, cyclopentenoid cyanhydrin glycosides
derived from non-protein amino acid 2-(2-cyclopentenyl)glycine (in families
near Achariaceae),
xanthones (euxanthone, bixanthones, macluraxanthone, mangiferin, norathyriol,
and anthraquinone xanthones), polyacetate-derived anthraquinones and
arthroquinones, anthraquinones (vismiones etc.), hypericin, pseudohypericin,
arbutin, emodin derivatives, biemodyles and closely allied compounds,
benzophenones, acetophenones, anthrones, naphthodianthrones, coumarin
derivatives substituted at position 4, syringaresinol, ferulic acid,
phytosterols (sitosterol, stigmasterol), ethereal oils, hyperforin,
picrotoxans, myo-inisitol, and nigracin present. Glucosinolates,
benzylisoquinoline alkaloids and fluoroacetic acid rare.
Systematics Malpighiales are possibly
sister-group to Oxalidales.
The phylogeny within Malpighiales is highly
unresolved (Korotkova & al. 2009; Wurdack & Davis 2009). Soltis &
al. (2011) present a fully resolved tree, yet with most of the basal nodes
weakly supported.
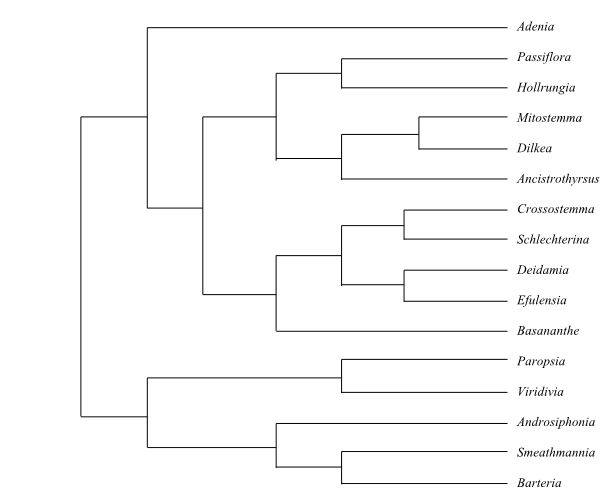
The clade [Achariaceae+[[Violaceae+[Passifloraceae+[Malesherbiaceae+Turneraceae]]]+ [Lacistemataceae+Salicaceae]+Goupiaceae]] have the following
potential synapomorphies (Stevens 2001 onwards): ray cells crystalliferous;
sieve tubes with non-dispersive protein bodies; cuticular waxes usually absent;
pedicels articulated; nectariferous tissue present; stamens as many as sepals,
antesepalous; median carpel abaxial; placentation parietal, with raised
placentae; aril present; endotegmen persistent; and endosperm oily, persistent.
Achariaceae, Malesherbiaceae, Turneraceae, and Passifloraceae often have
some kind of corona or scales on the petals; and cyclopentenoid cyanogenic
glycosides and/or cyclopentenylic fatty acids.
The clade [Passifloraceae+[Turneraceae+Malesherbiaceae]] has the
following advanced features (Stevens 2001 onwards): leaf teeth with vein
proceeding to opaque caducous apex; presence of colleters; sepals and petals
together forming tube; stamens five, antesepalous; stylodia well developed;
presence of funicular aril; endotestal cells large; exotegmen palisade;
endotegmen persistent; and presence of cyclopentenoid cyanogenic glycosides
and/or cyclopentenyl fatty acids. Turneraceae and Malesherbiaceae share the
characters: leaves spiral; micropyle bistomal; exotestal cells arranged in
lines; and x = 7. Moreover, Lacistemataceae and Salicaceae share the
synapomorphies: small flowers; anthers ellipsoid to subglobose; and copious
endosperm.
Lophopyxidaceae and Putranjivaceae have the
following characteristics in common: stomata paracytic; stylar branches short
or absent; placentation apical; ovules two per carpel; and fruit
single-seeded.
The clade [Ctenolophonaceae+[Erythroxylaceae+Rhizophoraceae]] is
characterized by: opposite leaves; stipules interpetiolar, enclosing terminal
bud; articulated pedicels; extrastaminal nectary; ten stamens, of two different
lengths; basifixed anthers, connate at base; postgenitally fused carpels;
capitate to lobate stigmas, papillate; placentation apical; ovules two per
carpel, collateral, pendulous, epitropous; megasporangium laterally thin,
disintegrating; presence of endothelium; presence of placental obturator;
sepals persistent in fruit; seed coat also exotestal; and presence of
endosperm.
Erythroxylaceae and Rhizophoraceae share the
synapomorphies, according to Stevens (2001 onwards): sieve tube plastids with
protein crystalloids; presence of abundant mucilage cells; leaves with involute
ptyxis; presence of colleters; stomata paracytic; inflorescence cymose; sepals
with valvate aestivation, postgenitally fused; petals clawed, with conduplicate
aestivation, enclosing stamen(s); antepetalous stamens longer than antesepalous
stamens; median carpel adaxial; style somewhat impressed; inner integument
approx. six cell layers thick; fruit a septicidal capsule; presence of aril;
exotestal cells enlarged, thick-walled, tanniniferous; endosperm starchy;
embryo with chlorophyll; presence of tropane (hygroline) and pyrrolidine
alkaloids; and presence of non-hydrolyzable tannins.
The clade [[Clusiaceae+Bonnetiaceae]+[Calophyllaceae+[Hypericaceae+Podostemaceae]]] is
characterized by: vessel elements with simple perforation plates; nodes
unilacunar with one leaf trace; presence of secretory ducts; presence of
schizogenous cavities; leaves with colleters; absence of stipules; stomata
paracytic; leaf margin entire; inflorescence cymose; petals with contorted
aestivation; absence of nectary; stamens numerous, often fasciculate, with
antepetalous fascicles; carpels antesepalous or median carpel adaxial; stigma
papillate; ovules numerous per carpel; micropyle bistomal; fruit a septicidal
or septifragal capsule; anticlinal exotegmic cell walls sinuous, low,
lignified; endosperm scarce or absent; embryo fusiform; and presence of
flavonols, flavones, biflavonoids, and abundant xanthones. The clade [Calophyllaceae+[Hypericaceae+Podoste-maceae]]
has leaves with gland dots or lines.
The clade [Centroplacaceae+[Elatinaceae+Malpighiaceae]] has fruits
with persistent sepals. Malpighiaceae and Elatinaceae have the following
potential synapomorphies in common: vessel elements with simple perforation
plates; sieve tube plastids without starch and protein inclusions; leaves
opposite (or verticillate); inflorescence cymose; flowers inverted; absence of
nectary; when three carpels, then median carpel adaxial; fruit a septifragal
capsule, with persistent calyx; endosperm scarce; and x = 6.
Ochnaceae, Medusagynaceae and Quiinaceae share the characters:
presence of vestured pits; presence of cristarque cells; presence of mucilage
cells and/or mucilage canals; leaves with secondary and tertiary venation well
developed; petals with contorted aestivation; absence of nectary; and ovules
tenuinucellate. Medusagyne and Quiinaceae have: separate
styloids; well developed ovary roof; expanded stigma; and often two ovules per
carpel. Moreover, Ochnaceae and Medusagyne
have medullary vascular bundles.
The clade [Peraceae+[Rafflesiaceae+Euphorbiaceae]] has the
following potential synapomorphies (Stevens 2001 onwards): vessel elements with
simple perforation plates; flowers small, unisexual; carpels three;
placentation apical; ovule one per carpel, pendulous, epitropous; presence of
nucellar cap (unknown in Peraceae); stylodia separate;
fruit a septicidal capsule or schizocarp, also splitting from columella and
loculicidally; exocarp/mesocarp often separating from endocarp; seeds large;
presence of micropylar carunculus; cotyledons longer and wider than
radicula.
Phyllanthaceae and Picrodendraceae share the
following synapomorphies: plant monoecious; stomata paracytic; flowers small;
presence of style; placentation apical; ovules two per carpel, apical,
epitropous; micropyle bistomal; parietal tissue at least ten cell layers thick;
presence of obturator and nucellar beak; fruit an explosively dehiscent
capsule, with fruit walls also splitting from persistent columella;
exocarp/mesocarp often separating from endocarp; and x = 13.
The clade [Balanopaceae+[[Trigoniaceae+Dichapetalaceae]+[Chrysobalanaceae+Euphro-niaceae]]]
is characterized by: hairs simple; ovules two per carpel, collateral; micropyle
bistomal; outer and inner integuments at least five cell layers thick each;
megasporangium evanescent by maturity; presence of endothelium; and endosperm
scarce or absence. The clade [[Trigoniaceae+Dichapetalaceae]+[Chrysobalanaceae+Euphroniaceae]] has the
following potential synapomorphies, according to Stevens (2001 onwards): vessel
elements with simple perforation plates; presence of vestured pits; presence of
mucilage cells; stomata paracytic; leaf margin entire; flowers obliquely
zygomorphic; pedicels articulated; presence of hypanthium; sepals congenitally
connate at base, with quincuncial aestivation, of unequal size (two outer
sepals shorter), with epidermal mucilage cells; fertile stamens abaxial,
connate; anthers much shorter than filaments, extremely introrse, with thecae
almost in one plane; connective well developed abaxially with endothecium
continuous over dorsal side of connective; presence of dorsal anther pit where
filament joins; staminodia adaxial, absent in posterior most antepetalous
position; gynoecium completely syncarpous up to stigma; carpel flanks slightly
bulged out transversely, carpels thus demarcated from each other by
longitudinal furrow; gynoecium and other floral parts with dense unlignified
unicellular hairs; presence of style; stigma commissural; ovules epitropous,
tenuinucellar; micropyle Z-shaped (zig-zag); outer integument two to five cell
layers thick; inner integument three to eight cell layers thick; and presence
of obturator.
Trigoniaceae and Dichapetalaceae share the
characters: petiole vascular bundle transection arcuate; secondary veins
strongly looping; inflorescence cymose; presence of mucilage cells in mesophyll
of sepals (in addition to epidermis); nectary semi-annular, with lobes or
scales; ovary and lower parts of style synascidiate; outer integument at most
five cell layers thick; and testa multiplicative. Chrysobalanaceae and
Euphroniaceae have the
following features in common: presence of spurred hypanthium; petals clawed,
with lignified hairs; and nectary present on adaxial side of hypanthium,
usually annular, without lobes or scales.

|
Maximum-likelihood majority-rule bootstrap
consensus tree of Malpighiales based on
information from 13 genes (Wurdack & Davis 2009). Clades that
receive less than 50% bootstrap support are not shown and the tree has
largely collapsed into a polytomy. The clade [Quiinaceae+Medusagyne]
has weak support (no sister-group relationship in Bayesian analysis). A
generally well-supported “parietal placentation clade” comprises
Achariaceae to
Violaceae
(Goupia has basal-axile placentation and its position has a
support of approx. 70%). The clusioid clade (Clusiaceae to Podostemaceae), the
ochnoid clade (Ochnaceae, Quiinaceae and
Medusagyne) and the euphorbioid clade [Peraceae+[Rafflesiaceae+Euphorbiaceae]] are
likewise well-supported (Ruhfel & al. 2011). A fifth well
circumscribed clade is the chrysobalanoid clade (Balanops to
Euphronia). Ctenolophon being sister to the
well-supported clade [Erythroxylaceae+Rhizophoraceae] has
a fairly low support. On the other hand, the clades [Phyllanthaceae+Picrodendraceae],
[Malpighiaceae+Elatinaceae] and
[Putranjivaceae+Lophopyxis]
are highly supported. Irvingiaceae may be
closely allied to either the clusioid or ochnoid clades.
|
Harms in Engler et Prantl, Nat. Pflanzenfam.,
Nachtr. 1: 256. Oct 1897, nom. cons.
Pangiaceae (Endl.)
Blume in J. K. Hasskarl, Cat. Hort. Bot. Bogor.: 186. Oct 1844;
Erythrospermaceae (DC.) Doweld, Tent. Syst. Plant.
Vasc.: xxxii. 23 Dec 2001
Genera/species 31/170–175
Distribution Pantropical, with
few species in southern Africa.
Fossils Unknown.
Habit Usually dioecious
(rarely monoecious), evergreen trees or shrubs (Acharieae consist of
climbing herbs).
Vegetative anatomy Phellogen
(in Lindackeria) ab initio superficial. Vessel elements with simple or
scalariform perforation plates; lateral pits alternate, scalariform or
opposite, simple or reduced bordered pits. Imperforate tracheary xylem elements
fibre tracheids with simple or bordered pits, septate or non-septate. Wood rays
uniseriate or multiseriate, heterocellular. Axial parenchyma absent (or very
rare). Tyloses sometimes abundant. Sieve tubes with non-dispersive protein
bodies?; sieve tube plastids S type. Nodes? Resinous substances etc. sometimes
present in heartwood. Silica bodies present in some species. Acicular crystals
and/or crystal sand present in some species. Prismatic crystals frequent;
druses sometimes present.
Trichomes Hairs unicellular or
multicellular, simple, stellate, peltate etc.
Leaves Alternate (spiral or
distichous), simple, entire or lobate, with ? ptyxis. Stipules caducous
(sometimes absent); leaf sheath absent. Petiole pulvinate, often geniculate.
Petiole vascular bundle transection annular, with two lateral/adaxial bundles
(in Lindackeria as inverted medullary plate). Venation pinnate.
Stomata anomocytic, paracytic or anisocytic. Cuticular wax crystalloids?
Domatia present in some species. Mesophyll sometimes with sclerenchymatous
idioblasts. Leaf margin usually entire (sometimes serrate or crenate; salicoid
teeth absent).
Inflorescence Terminal? or
axillary, fasciculate, spike-like cymose, or racemose to spicate, or flowers
solitary axillary.
Flowers Actinomorphic.
Hypogyny. Sepals two to five, with open to valvate (Acharieae)
aestivation, in one or two whorls or spiral, free or connate at base. Petals
four to 15, with valvate (Acharieae) aestivation, in one or two whorls
or spiral, usually free (in Acharieae three or four, connate into
tube; rarely absent). Some genera with corona of scales, hairs or lobes at
petal bases. Nectary usually absent. Disc absent.
Androecium Stamens three to
numerous, antesepalous, antepetalous or irregular, in one or more whorls or
groups, centripetally or almost synchronously developing. Filaments free; free
from or more or less adnate to petals. Anthers basifixed, non-versatile,
tetrasporangiate, introrse, usually longicidal (dehiscing by longitudinal
slits; in Chiangiodendron and Kiggelaria poricidal,
locellate, dehiscing by apical pores); connective sometimes widened. Tapetum
secretory? Staminodia three to five, intrastaminal, or absent.
Pollen grains
Microsporogenesis simultaneous? Pollen grains usually tricolpor(oid)ate, shed
as monads, bicellular at dispersal. Exine tectate or semitectate, with
columellate infratectum, perforate, reticulate or microreticulate, often
verrucate or psilate.
Gynoecium Pistil composed of
two to ten usually connate carpels (in Erythrospermeae secondarily
free). Ovary superior, unilocular. Style single, usually long (rarely short),
sometimes branched. Stigma one or two to five, capitate to peltate, type?
Pistillodium absent.
Ovules Placentation parietal.
Ovules usually numerous (sometimes three to c. 20) per ovary (rarely one per
carpel), usually anatropous (rarely orthotropous), bitegmic, crassinucellar.
Micropyle endostomal or bistomal (in Acharieae, Z-shaped, zig-zag).
Outer integument five or six cell layers thick, sometimes lobate. Inner
integument five or six cell layers thick. Archespore usually unicellular (in
Caloncoba bicellular). Nucellar cap and epistase present.
Megagametophyte usually monosporous, Polygonum type (in
Acharieae disporous, 8-nucleate), penetrating chalaza and forming
caecum below tracheid ring. Endosperm development ab initio nuclear. Endosperm
haustoria? Embryogenesis?
Fruit Usually a berry or
capsule (rarely a drupe).
Seeds Aril present or absent.
Seed coat thick, usually pachychalazal. Testa usually distinctly vascularized
(Acharieae lack vascular bundles in testa), in Acharieae with
stomata. Exotestal cells elongate, sclereidal. Sarcotesta present (in i.a.
Acharieae) or absent. Inner mesotesta sometimes sclereidal. Endotesta
lignified, with sclereidal cells (sometimes radially elongate). Exotegmen
usually non-fibrous, lignified (in Acharieae and
Erythrospermum fibrous). Endotegmen? Perisperm not developed.
Endosperm copious, oily. Suspensor absent. Embryo small, straight, well
differentiated, with chlorophyll. Cotyledons two. Germination?
Cytology x = 10, 12, 23
DNA
Phytochemistry Insufficiently
known. Ellagic acid (in Kiggelaria) and cyclopentenoid (cyclopentenyl)
cyanogenic glycosides (gynocardin) and/or cyclopentenyl fatty acids present.
Use Timber, medicinal plants
(seed oils).
Systematics
Pangieae Clos in Ann. Sci. Nat. Bot., sér. 4, 8:
267. 1857. Baileyoxylon (1; B. lanceolatum; northeastern
Queensland), Chiangiodendron (1; C. mexicanum; Mexico, Costa
Rica), Chlorocarpa (1; C. pentaschista; Sri Lanka),
Eleutherandra (1; E. pes-cervi; Malesia), Gynocardia
(1; G. odorata; Assam, Burma), Kiggelaria
(1; K. africana; tropical and southern Africa), Pangium (1;
P. edule; Malesia to New Guinea), Ryparosa (18; Malesia),
Scaphocalyx (2; S. parviflora, S. spathacea; the
Malay Peninsula, Sumatra), Trichadenia (2; T. zeylanica: Sri
Lanka; T. philippinensis: East Malesia to New Guinea and New Britain).
– Acharieae Benth. et Hook. f., Gen. Plant. 1: 809.
Sep 1867. Acharia (1; A. tragodes; Northern Province,
Mpumalanga, KwaZulu-Natal to Eastern Cape), Ceratiosicyos (1; C.
laevis; Namibia, Northern Province, Mpumalanga, KwaZulu-Natal, Western and
Eastern Cape), Guthriea (1; G. capensis; northeastern Western
Cape, Eastern Cape, KwaZulu-Natal, Lesotho). –
Lindackerieae Zmarzty in Chase et al., Kew Bull. 57:
172. 2002. Buchnerodendron (2; B. lasiocalyx, B.
speciosum; Central and tropical East Africa), Caloncoba (c 10;
tropical Africa), Camptostylus (2; C. mannii, C.
ovalis; tropical West and Central Africa), Carpotroche (12;
Central America, tropical South America), Grandidiera (1; G.
boivinii; tropical East Africa), Kuhlmanniodendron (1; K.
apterocarpum; Espírito Santo in Brazil), Lindackeria (14;
tropical Africa), Mayna (6; M. grandifolia, M.
hystricina, M. odorata, M. parvifolia, M.
pubescens, M. suaveolens; Central America, tropical South
America), Peterodendron (1; P. ovatum; tropical East Africa),
Poggea (4–6; P. alata, P. gossweileri, P.
kamerunensis, P. klaineana, P. longipedunculata, P.
stenura; tropical West and Central Africa), Prockiopsis (3;
P. calcicola, P. hildebrandtii, P. orientalis;
Madagascar), Xylotheca (10–13; eastern and southern Africa). –
Erythrospermeae DC., Prodr. 1: 257. Jan (med.) 1824.
Ahernia (1; A. glandulosa; Hainan, the Philippines),
Dasylepis (6; D. blackii, D. eggelingii, D.
integra, D. racemosa, D. seretii, D. thomasii;
tropical Africa), Erythrospermum (c 20; Mauritius, India, Sri Lanka,
Indochina, Malesia to Fiji), Rawsonia (2; R. burtt-davyi,
R. lucida; tropical Africa), Scottellia (3; S.
klaineana, S. leonensis, S. orientalis; tropical
Africa); Hydnocarpus (c 40; Southeast Asia, Malesia).
Achariaceae are sister-group to
a clade with the plausible topology [Goupiaceae+[Salicaceae+Lacistemataceae]+[Violaceae+[Malesherbiaceae+[Passifloraceae+Turneraceae]]]].
Acharieae were nested in
Pangieae in the rbcL tree in Sosa & al. (2003).
Hydnocarpus was sister to a unresolved clade comprising genera from
Lindackerieae and Erythrospermeae.
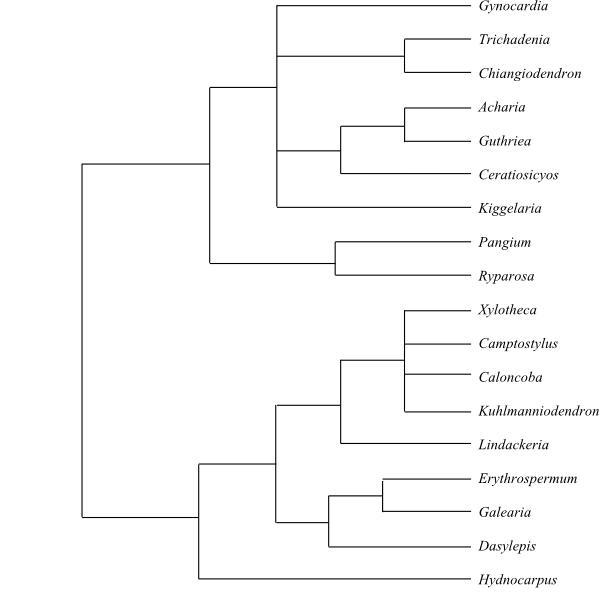 |
Phylogeny of Achariaceae based on
DNA sequence data (Groppo & al. 2013). Hydnocarpus,
Chiangiodendron and Trichadenia added from Sosa &
al. (2003). Galearia is usually included in Pandaceae.
|
BALANOPACEAE Benth. et
J. D. Hooker
|
( Back to Malpighiales )
|
Bentham et Hooker, Gen. Plant. 3: v, 341. 7 Feb
1880 [’Balanopseae’], nom. cons.
Balanopales Engl.,
Nat. Pflanzenfam. Nachtr. [1]: 345. Dec 1897
Genera/species 1/9
Distribution Queensland,
Melanesia, with their largest diversity in New Caledonia.
Fossils Unknown.
Habit Dioecious, evergreen
trees or shrubs.
Vegetative anatomy Phellogen
ab initio superficial. Vessel elements with usually scalariform to reticulate
(rarely simple) perforation plates; lateral pits alternate to almost opposite,
simple pits. Vestured pits absent. Imperforate tracheary xylem elements fibre
tracheids or thick-walled inconclusive libriform fibres with bordered pits.
Wood rays uniseriate or multiseriate, heterocellular. Axial parenchyma usually
apotracheal diffuse, or paratracheal scanty. Sieve tube plastids S type. Nodes
3:3, trilacunar with three leaf traces. Bark (and medulla?) with sclereids and
rhomboidal crystals. Cortex with cristarque cells present in some species.
Silica bodies present.
Trichomes Hairs unicellular,
simple, often caducous; glandular hairs absent.
Leaves Alternate (spiral),
simple, entire, coriaceous, with ? ptyxis. Stipules minute; leaf sheath absent.
Petiole vascular bundle transection? Venation pinnate. Stomata usually
anomocytic or laterocytic (sometimes cyclocytic). Cuticular waxes usually
absent (crystalloids sometimes present as platelets). Calciumoxalate crystals?
Leaf margin serrate; leaf teeth several cell layers thick, cells filled with
dark tannin-like content.
Inflorescence Male flowers in
axillary catkin-like cymose inflorescence; male flower with one bract. Female
flowers solitary, surrounded by cupule-like organ consisting of spiral bracts
with imbricate aestivation.
Flowers Actinomorphic, small.
Hypogyny. Tepals in male flowers as small rudimentary teeth; female flowers
without tepals. Nectary absent. Disc absent.
Androecium Stamens (one to)
three to six (to 14). Filaments very short, free from each other and from
tepals. Anthers basifixed, non-versatile, tetrasporangiate, latrorse to
introrse (latero-introrse), longicidal (dehiscing by longitudinal slits);
connective sometimes slightly prolonged. Tapetum secretory? Staminodia
absent.
Pollen grains
Microsporogenesis simultaneous? Pollen grains tri- to pentacolp(oid)ate, shed
as monads, bicellular at dispersal. Exine tectate, with columellate-granular
infratectum, microperforate, beset with small spinules.
Gynoecium Pistil composed of
(two or) three connate carpels. Ovary superior, (bilocular or) trilocular,
often incompletely septate. Stylodia (two or) three, free or connate at base,
once or twice bifid. Stigmas adaxial, long, type? Male flowers often with
pistillodium.
Ovules Placentation subbasal.
Ovules two per carpel, anatropous, intermediate between epitropous and
apotropous, ascending, at least partially apotropous, bitegmic, weakly
crassinucellar. Micropyle bistomal. Outer integument five to seven cell layers
thick. Inner integument five to nine cell layers thick. Obturator absent.
Parietal tissue approx. two cell layers thick. Megagametophyte monosporous,
Polygonum type. Endosperm development nuclear? Endosperm haustoria?
Embryogenesis?
Fruit A drupe with two or
three single-seeded pyrenes and surrounded in lower part by cupule consisting
of bracts.
Seeds Aril absent. Testa
vascularized, persistent, with slightly thickened cell walls. Perisperm not
developed. Endosperm sparse, thin. Embryo large, straight, with chlorophyll.
Cotyledons two, cordate. Hypocotyl elongate. Germination phanerocotylar.
Cytology n = 20 (21)
DNA
Phytochemistry Insufficiently
known. Tannins and triterpenes abundant (especially in bark). Alkaloids not
known. Ellagic acid?
Use Unknown.
BONNETIACEAE (Bartl.)
L. Beauvis. ex Nakai
|
( Back to Malpighiales )
|
Nakai in Bull. Natl. Sci. Mus. Tokyo 22: 25.
1948
Genera/species 3/45
Distribution Cambodia, Malesia
to New Guinea, Cuba, northern South America.
Fossils Unknown.
Habit Bisexual, evergreen
subpachycaul trees or shrubs.
Vegetative anatomy Phellogen
ab initio superfical (in roots cortical). Vessels with simple or
simple/transverse perforation plates; lateral pits alternate. Imperforate
tracheary xylem elements usually thick-walled tracheids with simple pits?,
non-septate? Wood rays uniseriate or multiseriate, heterocellular? Axial
parenchyma paratracheal scanty (or apotracheal?). Sieve tube plastids S type.
Nodes 1:1, unilacunar with one leaf trace (Archytaea,
Ploiarium), or 3:3, trilacunar with three traces (Bonnetia).
Mucilage cells frequent. Crystals?
Trichomes Hairs absent.
Leaves Alternate (spiral),
simple, entire, with involute or supervolute ptyxis. Stipules and leaf sheath
absent. Colleters present in leaf axils. Petiole vascular bundle transection
arcuate (Ploiarium) or complex. Venation pinnate, eucamptodromous,
brochidodromous or parallelodromous; secondary veins ascending. Stomata
paracytic. Cuticular wax crystalloids as rosettes. Epidermis in
Bonnetia with mucilaginous idioblasts. Leaves and bracts in
Archytaea and Ploiarium with vascularized disciform
structures, absent in Bonnetia. Endodermis present. Mesophyll in
Bonnetia with sclerenchymatous idioblasts; sclereids present
(Bonnetia) or absent (Archytaea, Ploiarium). Leaf
margin usually finely serrate (in young leaves with setae, associated with
vascular tissue in Archytaea and Ploiarium, not in
Bonnetia).
Inflorescence Axillary, cymose
panicle or raceme- to umbel-like, or flowers solitary axillary (receptacle in
Bonnetia ahogadoi developing into stolon with adventitious roots).
Flowers Actinomorphic.
Hypogyny. Sepals five, with imbricate quincuncial aestivation, unequal,
caducous or persistent, free, in Bonnetia and Ploiarium with
apical bristle. Petals five, with contorted aestivation, free. Buds with
long-pointed apex. Nectary and disc probably absent.
Androecium Stamens c. 40 to
more than 100. Filaments thin, free or connate in one group at base
(Bonnetia) or in five alternisepalous, antepetalous fascicles
(Archytaea, Ploiarium), free from petals (Bonnetia)
or adnate at base to petals (Archytaea, Ploiarium). Anthers
basifixed to somewhat dorsifixed, non-versatile, tetrasporangiate, introrse,
longicidal (dehiscing by longitudinal slits). Fasciclodium present or absent.
Tapetum secretory? Staminodia five in Archytaea and
Ploiarium, absent in Bonnetia.
Pollen grains
Microsporogenesis simultaneous? Pollen grains tricolporate (rarely syncolpate),
shed as monads, ?-cellular at dispersal. Exine semitectate, with columellate?
infratectum, finely reticulate.
Gynoecium Pistil composed of
three to five connate carpels. Ovary superior, tri- or quadrilocular
(Bonnetia), or quadri- or quinquelocular (Archytaea,
Ploiarium). Style single, simple or trilobate to quinquelobate, or
stylodia three to five, free. Stigmas papillate, type? Pistillodium absent.
Ovules Placentation axile.
Ovules c. 30 to c. 50 per carpel, anatropous, pendulous or horizontal,
bitegmic, tenuinucellar. Micropyle exostomal. Outer integument ? cell layers
thick. Inner integument ? cell layers thick. Hypostase absent. Megagametophyte
monosporous, Polygonum type. Endosperm development ab initio nuclear.
Endosperm haustoria? Embryogenesis?
Fruit A septicidal capsule (in
Archytaea, Ploiarium dehiscing from proximal end; in
Bonnetia dehiscing from distal end), usually with persistent central
columella.
Seeds Aril absent. Exotestal
cells thin-walled. Endotestal cells lignified (in Ploiarium elongate).
Exotegmen with sinuous anticlinal cell walls? Endotegmen? Perisperm not
developed. Endosperm sparse or absent. Embryo straight, well differentiated,
chlorophyll? Cotyledons two, small. Germination?
Cytology n = c. 150
(Bonnetia cubensis)
DNA
Phytochemistry Very
insufficiently known. Xanthones (euxanthone, in Ploiarium bixanthones
and anthraquinone xanthones) present.
Use Ornamental plants.
Systematics Bonnetia
(33; northern South America including the Guayana Highlands, the northern Andes
south to Peru, Brazilian Atlantic coast, Cuba); Archytaea (7; A.
alternifolia, A. angustifolia, A. multiflora, A.
pulcherrima, A. sessilis, A. triflora, A.
vahlii; northern South America including the roraimas), Ploiarium
(5; P. alternifolium, P. elegans, P. oblongifolium,
P. pulcherrimum, P. sessile; Cambodia, West Malesia,
Halmahera, New Guinea).
Bonnetiaceae are sister-group
to Clusiaceae.
Bonnetia is sister to
[Archytaea+Ploiarium] (Wurdack & Davis 2009).
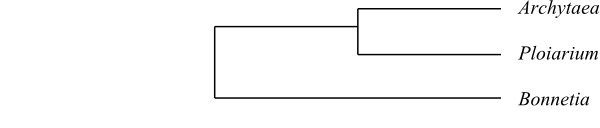 |
Maximum-likelihood majority-rule bootstrap
consensus tree of Bonnetiaceae based on
DNA sequence data (Wurdack & Davis 2009).
|
Agardh, Theoria Syst. Plant.: 121. Apr-Sep 1858
[’Calophylleae’]
Mesuaceae Bercht. et
J. Presl, Přir. Rostlin: 218. Jan-Apr 1820 [’Mesuae’];
Cambogiaceae Horan., Prim. Lin. Syst. Nat.: 98. 2 Nov
1834 [’Cambogiaceae (Guttiferae)’]
Genera/species 15/355–360
Distribution Pantropical.
Fossils Fossil pollen grains,
Kielmeyeropollenites, are known from the Eocene of India.
Symphonioxylon, fossil wood from Cretaceous and Miocene layers, may be
ascribed to Calophyllaceae or Clusiaceae.
Habit Usually bisexual
(occasionally cryptic-dioecious, rarely andromonoecious), usually evergreen
trees (sometimes shrubs or epiphytes).
Vegetative anatomy Phellogen
superficial or deeply seated. Endodermis in Kielmeyera often
significant. Secondary lateral growth usually normal (in Endodesmia
anomalous?). Vessel elements usually with simple (sometimes scalariform)
perforation plates; lateral pits alternate, usually simple pits. Vestured pits
often present. Imperforate tracheary xylem elements tracheids, fibre tracheids
or libriform fibres with usually simple (rarely bordered) pits, septate or
non-septate (also vasicentric tracheids). Wood rays uniseriate or multiseriate,
heterocellular. Axial parenchyma apotracheal or paratracheal. Sieve tube
plastids S type. Nodes 1:1, unilacunar with one leaf trace. Schizogenous
secretory ducts or cavities or glands with resin, balsam or yellow to red
secretions abundant (also in cortex and medulla). Colleters absent. Wood ray
cells sometimes with silica. Crystals?
Trichomes Hairs unicellular or
multicellular or absent (Caraipa and Marila with stellate
hairs; Marila with branched hairs).
Leaves Alternate (spiral or
distichous) or opposite, simple, entire, often coriaceous, usually with flat or
conduplicate (in Kielmeyera supervolute) ptyxis. Stipules and leaf
sheath absent. Paired modified colleters, “stipular glands”, sometimes
present. Petiole bundle transection arcuate, annular or complex. Venation
pinnate, eucamptodromous, brochidodromous or reticulodromous; tertiary venation
sometimes scalariform or absent. Stomata paracytic. Cuticular wax crystalloids
as rosettes. Lamina usually gland-dotted and/or with schizogenous secretory
cavities (sometimes canals) with resin, balsam or yellow to red secretions
(pellucid-punctate dots, resin/latex cavities). Leaf margin entire.
Inflorescence Terminal or
axillary, cymose, often thyrsoid (sometimes racemose), or flowers sometimes
solitary. Floral prophylls (bracteoles) sometimes (i.a. in Calophyllum
and Lebrunia) absent.
Flowers Usually actinomorphic
(in Marila asymmetralis obliquely zygomorphic), often large. Hypogyny.
Sepals (two to) four or five (to c. 20), with imbricate quincuncial or
decussate aestivation, usually free (rarely connate at base). Petals (three or)
four or five (to eight), with contorted or decussate aestivation, free (absent
in Calophyllum). Nectariferous disc usually absent (sometimes as
separate units).
Androecium Stamens (four to)
c. 20 to more than 100, not in distinct fascicles. Filaments usually in five
alternisepalous, antepetalous indistinct groups, usually free (rarely connate),
free from tepals. Anthers basifixed or dorsifixed, often versatile,
tetrasporangiate, usually introrse (sometimes extrorse), usually longicidal
(dehiscing by longitudinal slits, rarely poricidal, dehiscing by pores);
connective often with small single or paired, sometimes apical, complex or
simple glands (sometimes large and crateriform). Tapetum secretory. Staminodia
two to more than 50, extrastaminal, or absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually tricolporate (rarely
triporate or with several apertures), usually shed as monads (in
Kielmeyera often as tetrads), bicellular at dispersal. Exine tectate
or semitectate, with columellate? infratectum, reticulate, rugulate, fossulate,
foveolate, scabrate or psilate.
Gynoecium Pistil composed of
two to five connate antesepalous carpels (rarely monocarpellate?). Ovary
superior, bilocular to quinquelocular. Stylodia two to five, free, usually long
(longer than ovary), or style single, simple. Stigmas one to five, expanded to
punctate, usually non-papillate, Wet type. Pistillodium?
Ovules Placentation usually
axile (in Endodesmia clade apical, in Calophyllum and
Kayea basal, in Clusiella laminar). Ovules usually two to
numerous (in, e.g., Calophyllum, Endodesmia and
Lebrunia one) per carpel, usually anatropous, ascending or horizontal,
bitegmic, tenuinucellar. Micropyle bistomal. Outer integument ? cell layers
thick. Inner integument ? cell layers thick. Megagametophyte monosporous,
Polygonum type. Endosperm development ab initio nuclear. Endosperm
haustoria? Embryogenesis solanad. Polyembryony present at least in
Calophyllum and Kayea.
Fruit Usually a septicidal or
septifragal capsule (in Kayea often with persistent and strongly
accrescent calyx; sometimes a drupe; in Calophyllum,
Clusiella and Mammea a berry).
Seeds Aril absent. Seed coat
testal or exotegmic, sometimes winged. Testa with epidermis and exotegmen
sinuous and with lignified cell walls, or testa multi-layered, complex and
vascularized and exotegmen often absent. Endotegmen? Perisperm not developed.
Endosperm sparse. Embryo small to large, straight (fusiform) or curved,
rudimentary or well differentiated, with or without chlorophyll. Cotyledons
two, massive, medium-sized to very large (Calophyllum, Mesua
etc.; in Mammea connate). Germination phanerocotylar or cryptocotylar.
Radicula in large-seeded speces often ephemeral and replaced by adventitious
roots.
Cytology n = 16–21
DNA
Phytochemistry Flavonols
(kaempferol, quercetin, myricetin), flavones, biflavonoids, flavonoid
sulphates, dammaranes, cyanidin, oleanolic acid derivatives, ellagic and gallic
acids, proanthocyanidins (prodelphinidins), alkaloids, cyanogenic compounds
isoprenylated xanthones (euxanthone, macluraxanthone, norathyriol),
polyacetate-derived anthraquinones, coumarin derivatives substituted at
position 4, and syringaresinol present.
Use Ornamental plants, fruits
(Mammea americana etc), perfumes (Mammea siamensis),
medicinal plants, cosmetics, dyeing substances, seed oils, timber.
Systematics Calophyllaceae are
sister-group to [Hypericaceae+Podostemaceae].
Awaiting the name Calophyllaceae to become
conserved, I provisionally apply this name to the clade since it is now in
common use, although the name Mesuaceae is older.
Endodesmia and Lebrunia form
a sister-group to the remaining Calophyllaceae.
Endodesmia clade
2/2. Endodesmia (1; E.
calophylloides; tropical West Africa), Lebrunia (1; L.
bushaie; Congo). – Tropical West and Central Africa. Placentation
apical. Ovule one per carpel.
Calophylleae Choisy in
A. P. de Candolle, Prodr. 1: 561. Jan (med.) 1824.
13/355–360. Calophyllum
(185–190; tropical regions on both hemispheres), Mesua (c 40;
tropical Asia), Mammea (c 50; tropical regions on both hemispheres),
Kayea (7; K. coriacea, K. ferruginea, K.
macrophylla, K. megalocarpa, K. philippinensis, K.
punctulata, K. stylosa; Southeast Asia, Malesia to New Guinea),
Agasthiyamalaia (1; A. pauciflora; Western Ghats),
Poeciloneuron (2; P. indicum, P. pauciflorum;
southern India); Clusiella (9; Panamá to northern South America),
Marila (11; Central America, the West Indies, tropical South America),
Mahurea (3; M. exstipulata, M. palustris, M.
speciosa; tropical South America), Neotatea (3; N.
colombiana, N. longifolia, N. neblinae; northeastern
South America), Kielmeyera (c 20; Peru, southern Brazil),
Caraipa (21; tropical South America), Haploclathra (4; H.
cordata, H. leiantha, H. paniculata, H.
verticillata; Amazonia). – Pantropical. Placentation axile, basal or
laminar. Ovules usually numerous per carpel. – Genera with alternate leaves,
capsular fruit, often winged seeds, and cotyledons with a cordate base form a
monophyletic group.
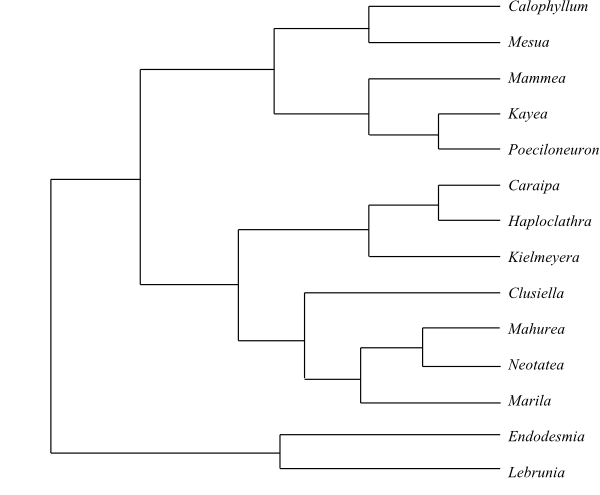 |
Optimal maximum likelihood tree (simplified)
of Calophyllaceae based
on morphological and DNA sequence data (Ruhfel & al. (2013).
|
Voigt, Hort. Suburb. Calcutt.: 88. Aug-Dec 1845,
nom. cons.
Rhizobolaceae DC.,
Prodr. 1: 599. med Jan 1824 [’Rhizoboleae’], nom. illeg.;
Rhizobolales DC. in C. F. P. von Martius, Consp.
Regn. Veg.: 60. Sep-Oct 1835 [‘Rhizoboleae’], nom. illeg.
Genera/species 2/c 25
Distribution Costa Rica to
Paraguay and the West Indies, with their largest diversity in Amazonia.
Fossils Uncertain. Fossil
pollen grains attributed to Caryocaraceae have been
described from the mid-Eocene.
Habit Bisexual, evergreen
trees or, sometimes, shrubs or suffrutices.
Vegetative anatomy Phellogen
ab initio superficial. Vessel elements with usually simple (sometimes
scalariform) perforation plates; lateral pits alternate, simple pits.
Non-vestured pits present. Imperforate tracheary xylem elements fibre tracheids
or libriform fibres with simple or bordered pits, septate or non-septate. Wood
rays uniseriate or multiseriate, heterocellular. Axial parenchyma apotracheal
diffuse or diffuse-in-aggregates, or paratracheal scanty vasicentric. Tyloses
abundant. Sieve tube plastids S type. Nodes ≥5:≥5, multilacunar with five
or more leaf traces. Parenchyma with idioblasts containing branched sclereids
(in medullary parenchyma) and solitary or groups of calciumoxalate crystals.
Prismatic crystals often present.
Trichomes Hairs simple or
absent.
Leaves Opposite
(Caryocar) or alternate (Anthodiscus), bipinnate or
trifoliolate to quinquefoliolate with articulated petiolules, coriaceous, with
? ptyxis. Stipules intrapetiolar (Anthodiscus) to interpetiolar, early
caducous, or absent; leaf sheath absent. Stipulules often present
(Caryocar), persistent or caducous. Colleters present. Petiole
vascular bundle transection? Venation palmate (leaflet venation pinnate).
Stomata usually anomocytic (sometimes anisocytic or paracytic). Cuticular wax
crystalloids as smooth to irregular rosettes of platelets. Domatia as hair
tufts. Epidermis with or without mucilaginous idioblasts. Mesophyll with
sclerenchymatous idioblasts containing branched sclereids. Hydathodes usually
present. Leaflet margins usually serrate (rarely almost entire).
Inflorescence Terminal,
racemose or corymbose. Bracts usually absent (rarely present, small and
caducous).
Flowers Actinomorphic, large
(Caryocar) or medium-sized (Anthodiscus). Pedicel articulated
at apex. Hypanthium sometimes present. Hypogyny. Sepals five (or six), with
quincuncial to truncate or open aestivation, in Anthodiscus lobate,
usually more or less connate. Petals five (or six), with quincuncial
aestivation, caducous, free or connate in lower parts (Caryocar), or
entirely connate forming caducous calyptra (Anthodiscus). Nectaries at
base of filaments and ovary, or absent. Disc absent. Extrafloral nectaries
often present on calyx.
Androecium Stamens 57 to at
least 750, subperigynous. Filaments very long, often glandular-tuberculate at
apex, connate at base or in five antesepalous fascicles, free from or adnate to
petal bases. Anthers dorsifixed (outer stamens) or basifixed (inner stamens),
versatile, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal
slits); connective usually not protruding. Tapetum secretory? Inner stamens
often with smaller anthers, or sometimes c 40 intrastaminal staminodia without
anthers present; innermost staminodia often markedly shorter than remainder and
with nectariferous glands at base. Staminodia fused with adjacent stamens into
tube encircling gynoecium.
Pollen grains
Microsporogenesis simultaneous? Pollen grains (2–)3(–6)-colporate
(sometimes parasyncolpate), shed as monads, bicellular at dispersal. Exine
semitectate, with columellate infratectum, reticulate, finely reticulate,
rugulate or verrucate.
Gynoecium Pistil composed of
three or four (to six) (Caryocar) or eight to c. 15(–20)
(Anthodiscus) connate carpels. Ovary superior, quadrilocular (to
sexalocular) (Caryocar) or 8- to c. 15(–20)-locular
(Anthodiscus), synascidiate. Stylodia three or four
(Caryocar) or eight to at least twelve (Anthodiscus), free,
long and filiform (Caryocar) or short (Anthodiscus); each
style in Anthodiscus supported by one vascular bundle from adjacent
carpels. Stigmas punctate, unicellular-papillate, type? Pistillodium absent.
Ovules Placentation axile.
Ovule one per carpel, hemianatropous to weakly campylotropous, ascending,
epitropous, bitegmic (Caryocar) or unitegmic (Anthodiscus),
weakly crassinucellar. Micropyle usually bistomal (sometimes endostomal). Outer
integument three to five cell layers thick. Inner integument five to seven cell
layers thick. Integument in Anthodiscus four or five cell layers
thick. Obturator absent. Endothelium absent. Megasporangium cytoplasm-rich and
filled with starch grains. Apical epidermal cells of megasporangium radially
elongate. Megagametophyte monosporous, Polygonum type? Endosperm
development ab initio nuclear. Endosperm haustoria? Embryogenesis?
Fruit A drupe or drupaceous
schizocarp (in Caryocar with radiating fibres) with single-seeded
pyrenes. Pericarp carnose to more or less lignified.
Seeds Aril absent. Testa
indistinct, vascularized, sometimes aerenchymatous. Exotegmen? Endotegmen?
Perisperm not developed. Endosperm thin or absent. Embryo well differentiated,
chlorophyll? Hypocotyl in Anthodiscus very large, oily and
proteinaceous, spirally twisted. Cotyledons two, small, inflexed. Germination
phanerocotylar.
Cytology n = 23
DNA
Phytochemistry Lupeol,
oleanolic acid derivatives, ellagic and gallic acids, and phytosterols
(sitosterol, stigmasterol) present. Cyanogenic compounds not found.
Use Seeds used for food and
cooking oil, fruits for fish poison, timber.
Systematics
Anthodiscus (c 10; Central America, tropical South America),
Caryocar (c 15; Central America, the West Indies, tropical South
America).
The sister-group relationship of Caryocaraceae is
unresolved.
CENTROPLACACEAE
(Radcl.-Sm.) Doweld et Reveal
|
( Back to Malpighiales )
|
Doweld et Reveal in Reveal in Bot. Rev.
(Lancaster) 71: 48. 20 Mai 2005
Genera/species 2/7
Distribution Central Africa,
southern India, Sri Lanka, the Andaman Islands, Southeast Asia, Malesia to New
Guinea, islands in southwestern Pacific.
Fossils Unknown.
Habit Bisexual
(Bhesa) or dioecious (Centroplacus), evergreen trees.
Vegetative anatomy
Ectomycorrhiza present in Bhesa. Phellogen ab initio usually
superficial? (sometimes cortical). Vessel elements with scalariform perforation
plates; lateral pits alternate, simple or bordered pits. Imperforate tracheary
xylem elements ? with simple or bordered pits, non-septate. Wood rays
multiseriate, heterocellular. Axial parenchyma apotracheal
diffuse-in-aggregates, or paratracheal scanty, reticulate, or banded. Sieve
tube plastids S type? Nodes 5:5, pentalacunar with five leaf traces
(Bhesa). Prismatic calciumoxalate crystals abundant.
Trichomes Hairs simple
(present in inflorescences only).
Leaves Alternate (in
Bhesa spiral; in Centroplacus distichous), simple, entire, in
Bhesa with conduplicate ptyxis. Stipules cauline (lateral), small, in
Centroplacus persistent, in Bhesa large and almost enclosing
stem/branch, caducous; leaf sheath absent. Colleters present in Bhesa
(absent in Centroplacus). Petiole in Bhesa with apical
pulvinus. Petiole vascular bundle transection in Bhesa U-shaped or
flat-annular; petiole with two or three medullary bundles and sometimes wing
bundles. Venation pinnate, in Centroplacus brochidodromous; secondary
veins in Bhesa stout, ascending (reticulate venation); tertiary veins
in Bhesa closely scalariform. Stomata anisocytic
(Centroplacus) or laterocytic (Bhesa). Cuticular wax
crystalloids? Mesophyll in Centroplacus with sclerenchymatous
idioblasts (containing different kinds of sclereids). Leaf margin in
Centroplacus indistinctly serrate, in Bhesa entire.
Inflorescence Male
inflorescences in Centroplacus axillary branched panicle, female
inflorescences racemiform-subpaniculate; in Bhesa terminal? racemose
simple or branched. Bracts very small.
Flowers Actinomorphic, small.
Pedicels articulated. Hypogyny. Sepals five, in Centroplacus with
imbricate aestivation, persistent, free. Centroplacus: petals in male
flowers five, with imbricate aestivation, free, absent in female flowers;
Bhesa: petals five, with contorted aestivation, free. Nectariferous
disc in Centroplacus extrastaminal, in male flowers cupular, in female
flowers acetabuliform, with five alternisepalous lobes; nectariferous disc in
Bhesa often lobate.
Androecium Stamens five,
haplostemonous, antesepalous, alternipetalous. Filaments free from each other
and from tepals. Anthers basifixed (Centroplacus), non-versatile?,
tetrasporangiate, extrorse (Bhesa) to introrse, longicidal (dehiscing
by longitudinal slits, in Centroplacus two obliquely apical slits);
connective in Centroplacus well developed. Tapetum secretory? Female
flowers in Centroplacus with very small antesepalous staminodia
(absent in Bhesa).
Pollen grains
Microsporogenesis simultaneous? Pollen grains tricolporate, shed as monads,
?-cellular at dispersal. Exine tectate (Centroplacus) or semitectate
(Bhesa), with brevicolumellate infratectum, psilate, in
Centroplacus finely reticulate or perforate, in Bhesa finely
striate.
Gynoecium Pistil composed of
three (Centroplacus) or two (Bhesa) connate carpels. Ovary
superior, trilocular (Centroplacus) or usually bilocular (rarely
unilocular; Bhesa). Style in Centroplacus single, short with
three diverging and somewhat recurved branches, stylodia in Bhesa two,
almost entirely free. Stigmas small, capitate, slightly widened, type? Male
flowers in Centroplacus with entire or trilobate hairy pistillodium;
pistillodium absent in Bhesa.
Ovules Placentation subapical
(Centroplacus) or basal (Bhesa). Ovules two per carpel,
collateral, anatropous, erect apotropous (Bhesa) or epitropous
(Centroplacus), bitegmic?, crassinucellar? Micropyle exostomal
(Bhesa). Outer integument six to eight cell layers thick
(Bhesa). Inner integument four or five cell layers thick
(Bhesa). Endostome lignified, more or less protruding
(Centroplacus). Obturator absent. Megagametophyte monosporous,
Polygonum type? Endosperm development nuclear? Endosperm haustoria?
Embryogenesis?
Fruit A septicidal and
sometimes loculicidal capsule with single-seeded locules (one seed aborting),
dehiscing from base and with persistent calyx (columella absent).
Seeds Aril
exostomal-funicular, fleshy, red to orange, completely or almost completely
enclosing seed (Bhesa). Carunculus narrowly elongate, red
(Centroplacus). Exotesta in Centroplacus with thickened outer
cell walls. Endotesta? Exotegmic cells ribbon-shaped with thick walls, in
Bhesa massive. Mesotegmic cells flattened and orientated at right
angles (Centroplacus). Endotegmic cells in Centroplacus more
or less thick-walled. Perisperm not developed. Endosperm in
Centroplacus copious, carnose. Embryo minute, short, chlorophyll?
Cotyledons two. Germination phanerocotylar?
Cytology n = ?
DNA
Phytochemistry Unknown.
Use Timber.
Systematics
Centroplacus (1; C. glaucinus; Cameroon, Equatorial Guinea,
Gabon), Bhesa (6; B. ceylanica, B. indica, B.
nitidissima, B. paniculata, B. robusta, B.
sinica; southern India, Sri Lanka, Assam, Burma, Indochina, the Andaman
Islands, Malesia to New Guinea, islands in southwestern Pacific).
Centroplacaceae are
sometimes placed with weak support as sister group to [Malpighiaceae+Elatinaceae] or as sister to
Pandaceae (Wurdack &
al. 2004).
Brown in J. H. Tuckey, Narr. Exped. Zaire: 433. 5
Mar 1818 [’Chrysobalaneae’], nom. cons.
Licaniaceae Martinov,
Tekhno-Bot. Slovar: 336. 3 Aug 1820 [’Licaneae’];
Chrysobalanales Link, Handbuch 2: 72. 4-11 Jul 1829
[’Chrysobalaneae’]; Hirtellaceae Horan.,
Char. Ess. Fam: 152. 30 Jun 1847
Genera/species 27/535–540
Distribution Pantropical.
Fossils Fossils of Chrysobalanaceae have been
found Eocene and later layers in North, Central and South America and tropical
Asia.
Habit Usually bisexual (rarely
andromonoecious or gynomonoecious), evergreen trees or shrubs. Lenticels
abundant.
Vegetative anatomy Phellogen
ab initio usually superficially (sometimes deeply) seated. Primary medullary
strands narrow (entirely or largely uniseriate). Vessel elements with simple
perforation plates; lateral pits alternate, usually with simple pits.
Imperforate tracheary xylem elements tracheids with bordered pits, non-septate
(also vasicentric tracheids). Wood rays uniseriate, usually heterocellular
(rarely homocellular). Axial parenchyma apotracheal, diffuse or
diffuse-in-aggregates, or paratracheal scanty, reticulate, or banded. Tyloses
abundant. Sieve tube plastids S type. Nodes 5:5, pentalacunar with five leaf
traces. Wood usually with silica bodies and grains. Prismatic calciumoxalate
crystals sometimes abundant.
Trichomes Hairs unicellular or
multicellular, uniseriate or multiseriate, simple or branched, stellate or
arachnoid; stalked and unstalked glands (also peltate-lepidote) present on
calyx and leaves.
Leaves Alternate (spiral or
distichous), simple, entire, with conduplicate (sometimes flat-conduplicate)
ptyxis. Stipules often petiolar or intrapetiolar; leaf sheath absent. Petiole
often pulvinate at one or both ends. Petiole vascular bundle transection
annular; petiole often with medullary plates and wing bundles; petiolar anatomy
often complex. Venation pinnate. Stomata paracytic. Cuticular wax crystalloids?
Lamina often with flattened abaxial glands (extrafloral nectaries), especially
near base. Myrmecodomatia (pouches formed when lamina rolls over onto itself
creating two spherical spaces at base) present in some species of
Hirtella; myrmecodomatia associated with enlarged extrafloral
nectaries on stipules and bracts, longer stomatal apertures, enlarged
parenchymatous and epidermal cells, and more numerous lignified sclerenchyma
fibres. Epidermis with or without silica bodies, with or without mucilaginous
idioblasts. Mesophyll sometimes with sclerenchymatous idioblasts. Leaf margin
usually entire (rarely serrate). Extrafloral nectaries sometimes present on
stipules, petiole and/or lamina.
Inflorescence Terminal or
axillary, cymose or racemose of various shape (simple or compound raceme etc.;
flowers rarely solitary). Extrafloral nectaries sometimes present on bracts or
pedicels.
Flowers Actinomorphic to
obliquely zygomorphic, usually small. Pedicel articulated. Hypogyny. Sepals
five, with imbricate quincuncial aestivation, connate in lower part into
tubular hypanthium-like structure (“floral cup”); median sepal adaxial.
Petals one to five, usually with imbricate quincuncial (sometimes cochlear)
aestivation, shortly clawed, free, adnate at margin of “hypanthium” (petals
rarely absent). Nectariferous disc annular or semicircular, intrastaminal,
inserted inside or along apex of “hypanthium”.
Androecium Stamens (two to)
five to c. 300, usually long exserted, in zygomorphic flowers concentrated to
one side of flower (lateral antesepalous stamens often larger than remainder);
abaxial stamens most developed. Filaments inflexed in bud, free or more or less
connate all together or in three to 20 staminal fascicles, free from tepals,
inserted at margin of hypanthium-like structure. Anthers dorsifixed, versatile,
tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits);
connective often dorsally thickened? Tapetum secretory. Intrastaminal
staminodia often present.
Pollen grains
Microsporogenesis simultaneous. Pollen grains 3(–4)-colp(or)ate, shed as
monads, bicellular at dispersal. Exine with very little patterning on walls,
with ? infratectum, usually scabrate to verrucate.
Gynoecium Pistil composed of
one to three carpels, fused only by common lateral to gynobasic style; usually
only abaxial carpel developed leading to pseudomonomery (remaining carpels
usually degenerated), often inserted on one side of “hypanthium”. Ovary
superior, usually unilocular; locule sometimes divided by secondary septa.
Style single, simple, lateral to gynobasic. Stigma usually simple, usually
punctate (rarely trilobate), papillate, Dry or Wet type. Pistillodium
absent.
Ovules Placentation
basal-axile. Ovules two per carpel, anatropous, ascending, epitropous
(antitropous), collateral or distributed over carpellary surface, bitegmic,
tenuinucellar. Micropyle bistomal, Z-shaped (zig-zag). Outer integument five to
twelve cell layers thick. Inner integument five to twelve cell layers thick.
Obturator seemingly basal (pollen tube transferring tissue perhaps functioning
as obturator) or absent. Archespore multicellular. Megagametophyte consisting
of an egg cell, two synergids and a central cell. Antipodal cells absent (early
degenerating). Endosperm development ab initio nuclear. Endosperm haustoria?
Embryogenesis?
Fruit A single-seeded, usually
juicy (rarely dry) drupe with persistent calyx. Endocarp often hairy inside.
Seeds Aril absent. Seeds
sometimes ruminate. Testa usually well developed, vascularized (sometimes
indistinct or mesotestal, sometimes multiplicative). Exotesta fibrous,
collapsed (sometimes with tannins). Tegmen multiplicative. Perisperm not
developed. Endosperm absent. Embryo large, well differentiated, chlorophyll?
Cotyledons two, sometimes thick. Germination cryptocotylar.
Cytology n = 10, 11
DNA
Phytochemistry Flavonols
(kaempferol, quercetin, myricetin), trihydroxyflavonoids, cyanidin,
delphinidin, cucurbitacins, and tannins present. Special unsaturated fatty
acids present in seeds. Ellagic acid, alkaloids, saponins, and cyanogenic
compounds not found.
Use Fruits (Chrysobalanus
icaco, Neocarya, Parinari), seed oil, timber.
Systematics
Kostermanthus (3; K. heteropetalus, K. malayanus,
K. robustus; West Malesia, Sulawesi), Neocarya (1; N.
macrophylla; tropical West Africa), Parinari (c 40; tropical and
subtropical regions on both hemispheres), Bafodeya (1; B.
benna; tropical West Africa), Geobalanus (2; G.
oblongifolius, G. pallidus; southeastern United States, Mexico,
Central America), Magnistipula (12; tropical Africa, Madagascar),
Parastemon (3; P. grandifructus, P. urophyllus,
P. versteeghii; the Nicobar Islands, Malesia to New Guinea),
Grangeria (2; G. porosa: Madagascar; G. borbonica:
Mauritius, Réunion), Dactyladenia (c 30; tropical Africa),
Atuna (8; tropical Asia to Samoa), Maranthes (12; ten species
in tropical Africa, one species, M. corymbosa, in tropical Asia and
east to islands in the Pacific, one species, M. panamensis, in
Nicaragua, Costa Rica and Panamá), Chrysobalanus (3; C.
cuspidatus: the Lesser Antilles; C. icaco: tropical Africa,
Florida, Mexico, Central America, the West Indies, tropical South America;
C. venezuelanus: southeastern Venezuela, northern Brazil),
Acioa (4; A. edulis, A. guianensis, A.
schultesii, A. somnolens; northern South America),
Exellodendron (5; E. barbatum, E. cordatum, E.
coriaceum, E. gardneri, E. gracile; tropical South
America), Angelesia (3; A. fusicarpa, A.
palawanensis, A. splendens; Southeast Asia, Malesia),
Hunga (11; New Guinea, New Caledonia, the Loyalty Islands),
‘Licania’ (c 100; southern Mexico, Central America, tropical South
America; polyphyletic), Gaulettia (4; G. canomensis, G.
elata, G. parillo, G. racemosa; tropical South America),
‘Hirtella’ (105–110; tropical America; polyphyletic),
Microdesmia (2; M. arborea, M. rigida; southern
Mexico, Central America, northern South America), Cordillera (1;
C. platycalyx; mountains in southern Central America and northern
South America), Afrolicania (1; A. elaeosperma; tropical West
and Central Africa; in Licania?), Parinariopsis (1; P.
licaniiflora; tropical South America), Hymenopus (28; Central
America, Trinidad and Tobago, northern South America), Leptobalanus
(31; Mexico, Central America, the West Indies, northern South America),
Moquilea (54; Mexico, Central America, tropical South America),
Couepia (c 70; Central America, tropical South America).
Chrysobalanaceae are
sister-group to Euphronia (Euphroniaceae).
Sothers & al. (2016) dissolved the
polyphyletic former Licania s.lat. and recognized instead the
following clade: [Hirtella+Licania
s.str.]+[[Microdesmia+Cordillera]+[[Afrolicania/Hymenopus
p.p.]+[[Parinariopsis/Hymenopus
p.p.+Leptobalanus]+[Moquilea+Couepia]]]].
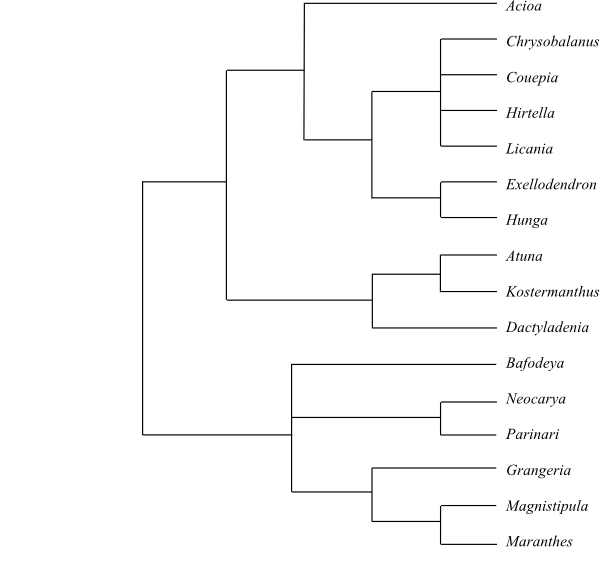 |
Bayesian consensus tree of Chrysobalanaceae
based on DNA sequence data (Yakandawala & al. 2010).
|
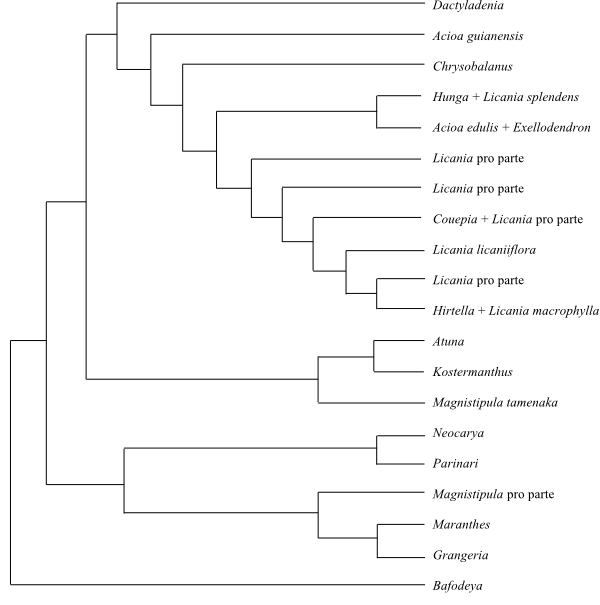 |
Phylogeny (simplified) of Chrysobalanaceae
based on DNA sequence data (Bardon & al. 2013).
Kostermanthus is sister to the remaining Chrysobalanaceae,
according to Bardon & al. (2016), although Bafodeya was
not included in their study.
|
Lindley, Intr. Nat. Syst. Bot., ed. 2: 74. 13 Jun
1836 [’Guttiferae, vel Clusiaceae’], nom. cons.
Guttiferae Juss.,
Gen. Plant: 255. 4 Aug 1789, nom. cons. et nom. alt.;
Garciniaceae Bartl., Ord. Nat. Plant.: 222, 292. Sep
1830 [’Garcinieae’]; Cambogiaceae
Horan., Prim. Lin. Syst. Nat.: 98. 2 Nov 1834 [’Cambogiaceae
(Guttiferae)’]; Garciniales DC. in C. F.
P. von Martius, Consp. Regn. Veg.: 60. Sep-Oct 1835
[‘Garcinieae’]
Genera/species
13–14/565–765
Distribution Pantropical.
Fossils Paleoclusia
chevalieri, pentamerous flowers from the Turonian of New Jersey, has been
attributed to Clusiaceae
and one of the few known Cretaceous representatives of Malpighiales. The stamens (or
staminodia) are grouped into five fascicles and a resin-like amorphous
substance is present in (and was perhaps secreted by) the anthers. The ovary is
quinquelocular and the stigma quinquelobate. The seeds are arillate.
’Pachydermites diederexii, fossil pollen of Symphonia, has
been used for stratigraphic dating by the oil industry.’
Symphonioxylon, fossil wood from the Cretaceous and the Miocene in
northeastern Africa and India, may be ascribed to Clusiaceae or Calophyllaceae.
Habit Usually bisexual (rarely
polygamomonoecious, in e.g. Clusia and Garcinia also
dioecious), evergreen trees or shrubs (in Clusia sometimes lianas or
epiphytes, also with CAM physiology).
Vegetative anatomy Phellogen
ab initio in roots (sub)epidermal or deeply seated; in stem superficial.
Secondary lateral growth usually normal. Vessel elements usually with simple
(sometimes also scalariform) perforation plates; lateral pits alternate,
usually simple pits. Vestured pits often present. Imperforate tracheary xylem
elements tracheids, fibre tracheids or libriform fibres usually with simple
(rarely bordered) pits, septate or non-septate (also vasicentric tracheids).
Wood rays uniseriate or multiseriate, heterocellular. Axial parenchyma
apotracheal or paratracheal. Sieve tube plastids S type. Nodes 1:1, unilacunar
with one leaf trace. Schizogenous secretory canals or cavities (and/)or glands
with resin, balsam or yellow to red secretions frequent (also in cortex and
medulla). Colleters numerous. Wood ray cells sometimes with silica.
Crystals?
Trichomes Hairs unicellular or
multicellular, or absent.
Leaves Usually opposite
(sometimes alternate; rarely whorled), simple, entire, often coriaceous, with
conduplicate or flat ptyxis. Stipules and leaf sheath absent. Paired modified
colleters, “stipular glands”, may occur. Petiole bundle transection
annular. Venation pinnate, usually eucamptodromous or brochidodromous (rarely
acrodromous). Stomata paracytic. Cuticular wax crystalloids as rosettes. Lamina
usually with schizogenous secretory glands or canals with resin, balsam or
yellowish to reddish secretions. Leaf margin entire.
Inflorescence Terminal or
axillary, cymose, often thyrsoid (flowers sometimes solitary).
Flowers Actinomorphic, often
large. Hypogyny. Sepals (two to) four or five (to 20), usually with imbricate
quincuncial or decussate (rarely valvate) aestivation, usually free (rarely
connate at base). Petals (three or) four or five (to eight), with contorted or
decussate aestivation, free (sometimes absent). Nectariferous disc usually
absent (sometimes as separate units at staminal bases; in Symphonia
extrastaminal nectariferous disc possibly representing antesepalous staminal
whorl). Resins often frequently secreted (in, i.a., Clusia).
Androecium Stamens (four to)
c. 20 to more than 100, often in distinct fascicles. Filaments stout, free, or
more or less connate into five alternisepalous, antepetalous fascicles, free
from tepals. Anthers basifixed or dorsifixed, often versatile,
tetrasporangiate, usually introrse (sometimes extrorse), usually longicidal
(dehiscing by longitudinal slits; rarely poricidal, dehiscing by pores);
connective usually without glands (sometimes with small glands). Tapetum
secretory. Staminodia two to more than 50, extrastaminal (sometimes producing
viscid triterpenoid resin), or absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually tricolporate (sometimes
triporate or with several apertures), usually shed as monads, bicellular at
dispersal. Exine tectate or semitectate, with columellate? infratectum,
reticulate, rugulate, fossulate, foveolate, scabrate, spinulate or psilate.
Gynoecium Pistil composed of
two to five (to more than twelve) connate, often antesepalous carpels (rarely
monocarpellate?). Ovary superior, bilocular to quinquelocular (to more than
duodecemlocular in Garcinieae, with single-seeded locules). Stylodia
two to five (to more than twelve), free, usually short (shorter than ovary), or
style single, simple, or absent. Stigmas usually several (sometimes one entire
stigma), usually widened (sometimes punctate) and non-papillate (rarely
papillate), Wet type (exposed stigmatic area absent in Symphonieae).
Pistillodium? Pollen grains in, e.g., Symphonia collected in droplet
secreted through pore at apex of stylar branches.
Ovules Placentation usually
axile (sometimes apical or basal; in Allanblackia parietal). Ovules
(one or) two to numerous per carpel, usually anatropous (sometimes amphitropous
or hemianatropous), ascending to horizontal, bitegmic, tenuinucellar. Micropyle
bistomal. Outer integument ? cell layers thick. Inner integument ? cell layers
thick. Megagametophyte monosporous, Polygonum type. Endosperm
development ab initio nuclear. Endosperm haustoria? Embryogenesis solanad.
Agamospermy present in Clusia and Garcinia mangostana.
Fruit Usually a drupe or berry
(in Garcinieae and Symphonieae many-seeded; in some species
of Garcinia drupe; sometimes a septicidal capsule).
Seeds Aril sometimes present.
Seed coat testal or exotegmic. Testa sometimes winged; testa with only
epidermis and exotegmen usually with lignified sinuous cell walls, or testa
multi-layered, vascularized and complex and exotegmen usually absent.
Endotegmen? Perisperm not developed. Endosperm sparse or absent. Embryo large
to small, straight and fusiform or curved, rudimentary or well differentiated,
with or without chlorophyll. Cotyledons two, very small or rudimentary.
Hypocotylar region very enlarged, forming tigellus. Germination phanerocotylar
or cryptocotylar. Radicula in species with large seeds often ephemeral and
replaced by adventitious roots.
Cytology n = 28–48
DNA Mitochondrial
coxI intron present in Montrouziera.
Phytochemistry Flavonols
(kaempferol, quercetin, myricetin), flavones, biflavonoids, flavonoid
sulphates, cyanidin, dammaranes, oleanolic acid derivatives, ellagic and gallic
acids, proanthocyanidins (prodelphinidins), alkaloids, cyanogenic compounds,
isoprenylated and other xanthones (euxanthone, macluraxanthone, norathyriol),
polyacetate-derived anthraquinones, polyisoprenylated benzophenones and fatty
acids (as resins), and syringaresinol present.
Use Ornamental plants, fruits
(Garcinia mangostana, Moronobea coccinea,
Platonia insignis), medicinal plants, gums and resins, seed oils,
timber.
Systematics
Clusieae Choisy in A. P. de Candolle, Prodr. 1: 557.
Jan (med.) 1824. Dystovomita (4; D. brasiliensis, D.
clusiifolia, D. paniculata, D. pittieri; tropical
America), Tovomitopsis (9?; T. centistaminibus, T.
croatii, T. faucis, T. guatemaltecana, T.
membranacea, T. myrcioides, T. paniculata, T.
spruceana, T. standleyana; Central America),
‘Tovomita’ (c 65; tropical America, with their highest diversity
in Venezuela; paraphyletic), Chrysochlamys (c 35; Central America, the
West Indies, tropical South America), Clusia (c 305; Florida, southern
Mexico, Central America, the West Indies, tropical South America). –
Garcinieae Choisy in A. P. de Candolle, Prodr. 1:
560. Jan (med.) 1824. ‘Garcinia’ (100–300; tropical and
subtropical regions on both hemispheres; non-monophyletic). –
Symphonieae Choisy in A. P. de Candolle, Prodr. 1:
563. Jan (med.) 1824. Symphonia (15–17; Madagascar, one species,
S. globulifera, in tropical Africa and tropical South America),
Pentadesma (c 15; tropical Africa), Moronobea (7; M.
coccinea, M. esculenta, M. intermedia, M.
jenmanii, M. ptaritepuiana, M. riparia, M.
rupicola; tropical South America), Platonia (1; P.
insignis; Guyana, Brazil), Montrouziera (5; M.
cauliflora, M. gabriellae, M. rhodoneura, M.
sphaeroidea, M. verticillata; New Caledonia),
‘Lorostemon’ (5; L. bombaciflorus, L. coelhoi,
L. colombianum, L. negrense, L. stipitatus; Brazil;
paraphyletic; incl. Thysanostemon?), Thysanostemon (2; T.
fanshawei, T. pakaraimae; Guyana; in Lorostemon?).
Clusiaceae are sister to Bonnetiaceae.
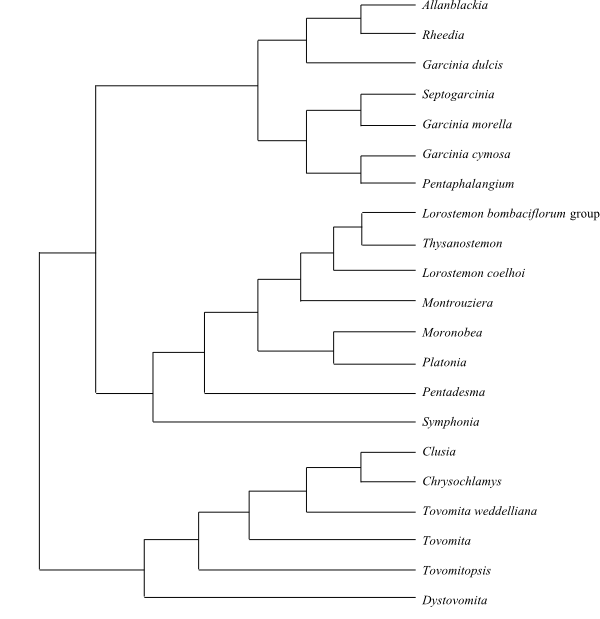 |
Optimal maximum likelihood tree (simplified)
of Clusiaceae
based on morphology and DNA sequence data (Ruhfel & al. 2013).
|
CTENOLOPHONACEAE (H.
Winkl.) Exell et Mendonça
|
( Back to Malpighiales )
|
Exell et Mendonça, Consp. Fl. Angol. 1: 248,
392. 20 Aug 1951
Genera/species 1/2
Distribution Tropical West
Africa, Malesia.
Fossils Fossil pollen assigned
to Ctenolophon has been found in South America, India and Malaysia,
and from Maastrichtian layers in Africa (Muller 1981, van der Ham 1989),
although these records are not cited by Friis & al. (2011).
Habit Bisexual, evergreen
trees. Excreting sticky resinous substance.
Vegetative anatomy Phellogen?
Vessel elements with scalariform or reticulate perforation plates; lateral pits
alternate, scalariform or opposite, bordered pits. Imperforate tracheary xylem
elements ? with bordered pits, non-septate (also vasicentric tracheids). Wood
rays uniseriate or multiseriate, heterocellular. Axial parenchyma apotracheal
diffuse, or paratracheal aliform, winged-aliform, confluent, vasicentric, or
unilateral. Sieve tube plastids ? type. Nodes 3:3, trilacunar with three leaf
traces. Cristarque cells present. Prismatic calciumoxalate crystals
frequent.
Trichomes Hairs
multicellular?, simple, fasciculate or stellate (stellate hairs present on
leaves, stipules and tepals).
Leaves Opposite, simple,
entire, coriaceous, with ? ptyxis. Stipules interpetiolar, early caducous,
without colleters; leaf sheath absent. Petiole vascular bundle transection
arcuate. Venation pinnate (arcuate and anastomosing). Stomata anomocytic or
anisocytic. Cuticular waxes absent. Leaf margin entire.
Inflorescence Terminal or
axillary, thyrsoid? or raceme-like.
Flowers Actinomorphic. Pedicel
articulated. Hypogyny. Sepals five, with imbricate quincuncial aestivation,
persistent, connate at base. Petals five, with contorted aestivation, fleshy,
spoon-shaped at base, caducous, free. Nectariferous disc extrastaminal,
cupular, with stomata.
Androecium Stamens 5+5,
diplostemonous. Filaments of two different lengths, inserted in lower part of
adaxial side of nectariferous disc, basally fused into tube, adnate at base to
petals (epipetalous). Ten lobes alternating with stamens and forming
corona-like tube at base on dorsal side of stamens. Anthers dorsifixed,
versatile, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal
slits); connective wide and thick, protruded. Tapetum secretory? Staminodia
absent.
Pollen grains
Microsporogenesis simultaneous? Pollen grains 3–9-stephanocolpate or
3–9-stephanocolpor(oid)ate, shed as monads, ?-cellular at dispersal. Exine
tectate, with columellate infratectum, perforate, spinulate. Pollen grains of
unique type, almost square in polar view and with extremely wide colpi.
Gynoecium Pistil composed of
two connate carpels. Ovary superior, bilocular, synascidiate. Style single,
bifid. Stigmas capitate, unicellular-papillate, type? Pistillodium absent.
Ovules Placentation lateral,
apical, axile. Ovules two per carpel, anatropous, pendulous, epitropous,
bitegmic, (weakly) crassinucellar. Micropyle bistomal, Z-shaped (zig-zag).
Integuments lobate. Outer integument approx. five cell layers thick. Inner
integument ten or eleven cell layers thick. Obturator placental. Endothelium
present. Megasporangium disintegrating. Megagametophyte monosporous,
Polygonum type. Endosperm development ab initio nuclear. Endosperm
haustoria? Embryogenesis?
Fruit A single-seeded nut
(nut-like capsule?) with lignified pericarp and persistent, accrescent, swollen
calyx. Seed persistent on thin central funicular columella.
Seeds Arillode hairy,
enclosing lower half of seed. Exotestal cells palisade, with thickened outer
wall. Endotesta? Exotegmen fibrous. Endotegmen? Perisperm not developed.
Endosperm copious. Embryo straight, well differentiated, chlorophyll?
Cotyledons two, very large, plicate. Germination?
Cytology n = ?
DNA
Phytochemistry Virtually
unknown. Ellagic acid? Alkaloids not found.
Use Timber.
Systematics
Ctenolophon (2; C. englerianus: Nigeria, Gabon, Congo,
Angola; C. parvifolius: peninsular Thailand and the Malay Peninsula to
New Guinea).
Ctenolophon may be sister to [Rhizophoraceae+Erythroxylaceae].
Baillon in von Martius, Fl. Bras. 12(1): 365. 1
Apr 1886 [‘Dichapetaleae’], nom. cons.
Chailletiaceae R. Br.
in J. H. Tuckey, Narr. Exped. Zaire: 442, 443. 5 Mar 1818
[’Chailleteae’]; Chailletiales Link,
Handbuch 2: 123. 4-11 Jul 1829 [‘Chailletiaceae’]
Genera/species 3/165–170
Distribution Pantropical,
south to southeastern and southern Africa.
Fossils Unknown.
Habit Usually at least
morphologically bisexual (at least sometimes functionally monoecious or
dioecious), evergreen trees, shrubs or lianas. Sometimes xerophytic. Lenticels
often numerous.
Vegetative anatomy Phellogen
ab initio superficial. Pericyclic envelope interrupted. Vessel elements with
simple and/or scalariform perforation plates; lateral pits usually alternate
(in Tapura often scalariform or intermediary), bordered pits.
Imperforate tracheary xylem elements fibre tracheids with simple or bordered
pits, non-septate. Wood rays uniseriate or multiseriate, heterocellular. Axial
parenchyma usually paratracheal aliform, lozenge-aliform, winged-aliform,
vasicentric, or confluent (sometimes apotracheal diffuse). Sieve tube plastids
S type?; sieve tubes with non-dispersive protein bodies. Nodes? Pericyclic
envelope interrupted. Resinous substances produced? Prismatic calciumoxalate
crystals abundant.
Trichomes Hairs unicellular,
simple, verrucose-papillate; glandular hairs (glands) sometimes present.
Leaves Alternate (spiral),
simple, entire, sometimes coriaceous, with ? ptyxis. Stipules intrapetiolar,
often fimbriate, usually caducous; leaf sheath absent. Petiole articulated.
Petiole vascular bundle transection arcuate. Venation pinnate, brochidodromous;
secondary veins strongly curved. Stomata usually paracytic (sometimes
anomocytic). Cuticular wax crystalloids? Domatia as pits. Epidermis with
mucilaginous idioblasts. Mesophyll with or without mucilaginous idioblasts.
Lamina sometimes with abaxial flattened glands and sometimes with mucilage
cells. Leaf margin entire. Extrafloral nectaries often present on adaxial or
abaxial side of lamina in Dichapetalum.
Inflorescence Axillary (to
petiolar; in two species of Dichapetalum and one species of
Tapura epiphyllous, arising from petiole), cymose (often
fasciculate).
Flowers Usually actinomorphic
(in Tapura obliquely zygomorphic), small. Pedicel often articulated.
Hypanthium-like structure (“floral cup”) present or absent. Usually
hypogyny to half epigyny (rarely epigyny). Sepals four or five, with imbricate
quincuncial aestivation, usually free (sometimes connate at base). Petals four
or five, with imbricate, involute or valvate to somewhat contorted (rarely
open) aestivation, usually deeply bifid, sometimes clawed, blackening when dry,
usually free (rarely more or less connate). Nectariferous disc annular when
petals connate or as four or five antepetalous scale-like nectariferous glands
between staminodia, inserted at petal bases (nectariferous disc in
Tapura lobate, semicircular).
Androecium Stamens usually
four or five (in Tapura three), haplostemonous, antesepalous,
alternipetalous (in Tapura three fertile stamens and two staminodia).
Filaments free or connate at base into tube, free from or adnate to tepals.
Anthers dorsifixed to almost basifixed, non-versatile, tetrasporangiate,
introrse, longicidal (dehiscing by longitudinal slits); connective often
dorsally thickened. Tapetum secretory. Female flowers with staminodia;
staminodia (intrastaminal?) in male flowers of Tapura two or five,
antesepalous.
Pollen grains
Microsporogenesis simultaneous. Pollen grains tricolporate, shed as monads,
bicellular? at dispersal. Exine semitectate, with columellate infratectum,
reticulate (sometimes microreticulate) to retipilate.
Gynoecium Pistil composed of
(two or) three (or four) connate carpels; carpel synascidiate. Ovary usually
superior to semi-inferior (rarely inferior), (bilocular or) trilocular (or
quadrilocular), with central columella and sometimes secondary septa. Style
usually single, simple or branched (stylodia rarely two to four, free). Stigma
capitate or trilobate, often with recurved lobes, papillate, Wet type. Male
flowers with pistillodium.
Ovules Placentation axile to
apical. Ovules two per carpel, anatropous, pendulous, epitropous (antitropous,
with micropyle above), bitegmic, tenuinucellar. Micropyle bistomal, Z-shaped
(zig-zag), or endostomal. Outer integument three to five cell layers thick.
Inner integument six to eight cell layers thick. Obturator apical, with long
multicellular papillae or hairs, or funicular. Hypostase absent.
Megagametophyte monosporous, Polygonum type. Endosperm development?
Endosperm haustoria? Embryogenesis?
Fruit An often flattened
usually unilocular (rarely bilocular or trilocular and lobed) dry or sometimes
juicy single-seeded drupe (sometimes a capsule) with dense, short, erect and
often golden yellow hairs and persistent calyx.
Seeds Aril or carunculus
present or absent. Testa multiplicative, vascularized, only consisting of
enlarged tanniniferous, sometimes divided, exotestal cells and remnants of
vascular bundles; testal cells often with stellate calcium oxalate crystals.
Endotesta? Exotegmen absent. Endotegmen? Perisperm not developed. Endosperm
absent. Embryo straight, well differentiated, oily, with chlorophyll.
Cotyledons two. Germination cryptocotylar.
Cytology n = 10, 12
DNA
Phytochemistry Insufficiently
known. Pyridine alkaloids sometimes present. Some genera (i.a.
Dichapetalum) with strongly toxic fluoroacetic acid (fluoroacetate).
Saponins not found.
Use Medicinal plants, arrow
poisons, mammal pesticides.
Systematics
Dichapetalum (c 135; tropical regions on both hemispheres; incl.
Tapura?), Stephanopodium (13; Costa Rica, northwestern South
America, coastal Brazil), Tapura (c 20; tropical Africa, Central
America, tropical South America; in Dichapetalum?).
Dichapetalaceae are sister
to Trigoniaceae.
A phylogeny is carried out by a research group
in Wageningen. Tapura is nested in Dichapetalum, according to
Yakandawala & al. (2010).
Dumortier, Anal. Fam. Plant.: 44, 49. 1829
[‘Elatinideae’], nom. cons
Cryptaceae Raf. in
Ann. Gén. Sci. Phys. Bruxelles 5: 349. Jul-Sep 1820
[‘Cryptinia’]; Elatinales Cambess. in C.
F. P. von Martius, Consp. Regn. Veg.: 54. Sep-Oct 1835
[‘Elatineae’]; Alsinastraceae Rupr., Fl.
Ingr. 1: 194. Mai 1860 [’Alsinastreae’], nom. illeg.
Genera/species 2/c 50
Distribution Cosmopolitan
except polar areas, with their largest diversity in tropical regions.
Fossils Curved seeds with
reticulate surface, resembling those in Elatine, are known from the
Pliocene of Europe.
Habit Bisexual, usually
perennial or annual herbs (Bergia suffruticosa is suffrutescent). A
large number of species are aquatic, whereas others are amphibious
helophytes.
Vegetative anatomy Phellogen
ab initio usually superficial (in Bergia inner-cortical). Endodermis
prominent in species of Elatine. Secondary lateral growth normal or
absent. Vessel elements with simple perforation plates; lateral pits alternate,
bordered pits? Imperforate tracheary xylem elements tracheids with bordered
pits, non-septate? (also vasicentric tracheids). Wood rays uniseriate to
multiseriate, heterocellular? Axial parenchyma apotracheal diffuse. Sieve tube
plastids S0 type, without starch or protein inclusions. Nodes 1:1,
unilacunar with one leaf trace. Mucilage cells and resin-producing cells
present. Secretory cells with tanniniferous substances (resinous latex) present
in Bergia. Druses and solitary crystals present.
Trichomes Hairs usually absent
(when present unicellular or uniseriate, simple, basifixed and sometimes
gland-tipped); glandular hairs abundant in Bergia
Leaves Usually opposite (in
Elatine alsinastrum verticillate), simple, entire, with ? ptyxis.
Stipules interpetiolar, minute, membranous; leaf sheath absent. Colleters
present. Petiole vascular bundle simple. Venation pinnate. Stomata usually
paracytic (rarely anomocytic or tetracytic), with four to eight irregularly
formed subsidiary cells. Cuticular wax crystalloids? Epidermis with or without
mucilaginous idioblasts. Lamina with or without secretory cavities. Leaf margin
serrate, crenate or entire, often with multicellular glands along margin.
Inflorescence Axillary,
few-flowered, cymose, or flowers solitary axillary.
Flowers Actinomorphic, small.
Hypogyny. Sepals (two or) three to five (or six), with imbricate aestivation,
free or connate at base; median sepal (when three sepals) abaxial. Petals (two
or) three to five (or six), with contorted or imbricate aestivation,
persistent, free. Nectary absent. Disc absent.
Androecium Stamens (two or)
three to five (or six), haplostemonous, antesepalous, or (four to) six to ten
(to twelve), in one or two whorls, diplostemonous, with outer stamens
antesepalous and inner stamens alternisepalous (inner staminal whorl sometimes
absent). Filaments free from each other and from tepals. Anthers dorsifixed,
versatile, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal
slits). Tapetum secretory. Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually tricolporate, shed as
monads, bicellular (Bergia) or tricellular (Elatine) at
dispersal. Exine semitectate, with columellate infratectum, reticulate.
Gynoecium Pistil composed of
two to five (or six) connate antesepalous carpels; median carpel (when three
carpels) abaxial. Ovary superior, bilocular to quinquelocular (or sexalocular;
septa sometimes not reaching apex). Stylodia two to five (or six), free.
Stigmas capitate, papillate, type? Pistillodium absent.
Ovules Placentation apical,
pendulous. Ovules (two to) numerous per carpel, anatropous, epitropous,
ascending or horizontal, bitegmic, weakly crassinucellar. Micropyle bistomal,
sometimes Z-shaped (zig-zag). Outer integument ? cell layers thick. Inner
integument ? cell layers thick. Archespore multicellular. Megagametophyte
monosporous, Polygonum type. Endosperm development ab initio nuclear.
Endosperm haustoria? Embryogenesis solanad.
Fruit A septicidal valvicidal
capsule.
Seeds Aril absent. Operculum
present. Seed coat exotegmic. Testa collapsed? Exotegmic cells with sinuous
anticlinal walls, lignified. Endotegmen? Perisperm not developed. Endosperm
thin (Bergia) or absent (Elatine). Embryo straight or
somewhat curved, large, fusiform, well differentiated, chlorophyll? Cotyledons
two, short. Germination phanerocotylar.
Cytology x = 6, 9, 10
DNA Duplication of
CYC genes.
Phytochemistry Flavonols,
ellagic acid, tannins, and proanthocyanidins (prodelphinidins) present.
Alkaloids and saponins not found.
Use Unknown.
Systematics Elatine
(c 25; almost cosmopolitan), Bergia (24–27; tropical and subtropical
regions on both hemispheres).
Elatinaceae are sister to
Malpighiaceae.
Potential synapomorphies shared by Elatinaceae and Malpighiaceae are, e.g.,
opposite leaves, multicellular glands often found along foliar margins, similar
type of inflorescence, septicidal capsule, and exalbuminous seeds (Davis &
Chase 2004).
Elatine alsinastrum is sister to the
remaining species of Elatine, based on morphological characters
(Razifard & al. 2017).
Kunth in von Humboldt, Bonpland et Kunth, Nov.
Gen. Sp. Plant. 5, ed. 4o: 175; ed. fol.: 135. 25 Feb 1822
[‘Erythroxyleae’], nom. cons.
Erythroxylales Link,
Handbuch 2: 339. 4-11 Jul 1829 [‘Erythroxyleae’];
Nectaropetalaceae (H. Winkl.) Exell et Mendonça in
Bol. Soc. Brot., ser. 2, 25: 105. 1951
Genera/species 4/235–240
Distribution Pantropical.
Fossils Unknown.
Habit Usually bisexual (rarely
dioecious), evergreen small trees or shrubs. Branches often covered with
distichous scale-like rudimentary leaves. Buds perulate.
Vegetative anatomy Mycorrhiza
absent. Phellogen ab initio superficial. Young stems and branches with cortical
vascular bundles. Primary vascular tissue cylinder, without separate bundles.
Vessel elements with simple perforation plates; lateral pits alternate, simple
pits. Vestured pits absent (Erythroxylum). Imperforate tracheary xylem
elements fibre tracheids with simple or bordered pits, non-septate (also
vasicentric tracheids). Wood rays uniseriate or multiseriate, heterocellular.
Axial parenchyma apotracheal diffuse or diffuse-in-aggregates, or paratracheal
scanty, aliform, winged-aliform, vasicentric, or confluent. Secondary phloem
often stratified into hard fibrous and soft parenchymatous layers. Sieve tube
plastids Pc (PV) type, with numerous square or polygonal protein crystalloids.
Nodes 3:3, trilacunar with three leaf traces, with lateral vascular bundles
originating relatively long before central bundle and forming cortical bundles.
Sclereids present. Wood usually with silica grains. Parenchyma and bundle
envelopes usually with cristarque cells (sometimes absent). Prismatic
calciumoxalate crystals frequent.
Trichomes Hairs absent.
Leaves Usually alternate
(spiral or distichous; in Aneulophus opposite), simple, entire, with
involute ptyxis. Stipules usually at least partially interpetiolar (in
Exythroxylum intrapetiolar), sheathing, in all species with basal
adaxial gum-secreting colleters; leaf sheath absent. Petiole vascular bundle
transection arcuate or annular; petiole with medullary and adaxial bundles.
Venation pinnate. Stomata paracytic. Cuticular wax crystalloids as rosettes of
platelets (Fabales type). Epidermis with or without mucilaginous
idioblasts. Mesophyll with or without sclerenchymatous idioblasts. Leaf margin
entire.
Inflorescence Axillary,
usually fasciculate, or flowers solitary axillary.
Flowers Usually actinomorphic
(in Aneulophus zygomorphic?), often small. Pedicel usually
articulated. Hypanthium present in Nectaropetalum. Hypogyny. Sepals
five, with imbricate quincuncial or valvate aestivation, persistent, connate at
base. Petals five, with imbricate or contorted aestivation, caducous, usually
with adaxial fimbriate bilobate ligule at base, free or connate at base.
Nectariferous glands present on abaxial side of staminal tube. Disc absent.
Heterostyly frequent.
Androecium Stamens 5+5,
usually obdiplostemonous (sometimes diplostemonous). Filaments connate into
tube around pistil at least at base, free from tepals, often with two different
lengths. Anthers dorsifixed to basifixed, versatile?, tetrasporangiate,
latrorse (or introrse?), longicidal (dehiscing by longitudinal slits);
connective often thickened. Tapetum secretory. Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually tricolporate (sometimes
tricolpate), shed as monads, tricellular at dispersal. Exine semitectate, with
columellate infratectum, finely reticulate to punctate.
Gynoecium Pistil composed of
(two or) three (or four) connate carpels; usually only adaxial carpel (rarely
all carpels) fertile. Ovary superior, usually with only one fertile locule,
synascidiate. Stylodia (two or) three (or four), free or more or less connate,
sometimes with canal. Stigmas capitate, unicellular-papillate, Dry type.
Pistillodium absent.
Ovules Placentation apical to
axile. Ovule one (or two) per carpel, anatropous to hemianatropous, pendulous,
epitropous, bitegmic, crassinucellar. Micropyle endostomal. Outer integument
two to five cell layers thick. Inner integument five to nine cell layers thick.
Hypostase absent. Parietal tissue two to four cell layers thick. Endothelium
usually present. Megagametophyte monosporous, Polygonum type.
Synergids with a filiform apparatus. Endosperm development ab initio nuclear.
Endosperm haustoria? Embryogenesis solanad.
Fruit Usually a single-seeded
drupe (in Aneulophus a septicidal capsule) with persistent calyx and
stamens.
Seeds Arilloid (carunculus?),
formed from exostome, present in Aneulophus. Seed coat exotegmic.
Exotestal cells thickened, elongate. Endotesta crushed. Tegmen often
multiplicative (in Aneulophus thin). Exotegmen fibrous. Endotegmen as
pigmented endothelium. Perisperm not developed. Endosperm very copious, starchy
(rarely absent). Embryo straight, well differentiated, with chlorophyll.
Cotyledons two. Germination phanerocotylar.
Cytology n = 12
DNA
Phytochemistry Flavonols
(kaempferol, quercetin), cyanidin, ethereal oils (in wood), non-hydrolyzable
tannins, chlorogenic acid, tropane (hygrolinic) alkaloids (tropane-3α and
tropane-3β-ols, tropacocaine, scopolamine oxides, hydroxytropines, teloidines,
ecgonines, norecgonines, etc.), pyrrolizidine alkaloids, and saponins present.
Ellagic acid and cyanogenic compounds not found.
Use Medicinal plants, cocaine
(Erythroxylum coca, E. novogranatense), timber, tar, dyeing
substances.
Systematics
Aneulophus (2; A. africanus, A. congoensis; tropical
West and Central Africa), Erythroxylum
(c 230; tropical regions on both hemispheres, east to eastern India, south to
central Chile and Argentina, with their highest diversity in Madagascar, the
Andes and Amazonas), Nectaropetalum (5; N. acuminatum, N.
capense, N. kaessneri, N. lebrunii, N.
zuluense; tropical and southern Africa, Madagascar), Pinacopodium
(2; P. congolense, P. gabonense; tropical Africa).
Erythroxylaceae are sister
to Rhizophoraceae.
 |
Cladogram of Erythroxylaceae
based on DNA sequence data and morphology (Schwarzbach & Ricklefs
2000).
|
de Jussieu, Gen. Plant.: 384. 4 Aug 1789
[’Euphorbiae’], nom. cons.
Tithymalaceae Vent., Tabl. Règne Vég. 3: 483. 5 Mai
1799 [’Tithymaloideae’]; Euphorbiales
Juss. ex Bercht. et J. Presl, Přir. Rostlin: 237. Jan-Apr 1820 [‘Euphorbiaceae’];
Mercurialaceae Bercht. et J. Presl, Přir. Rostlin:
237. Jan-Apr 1820 [’Mercurialideae’];
Ricinaceae Martinov, Tekhno-Bot. Slovar: 547. 3 Aug
1820 [’Ricini’]; Trewiaceae Lindl.,
Intr. Nat. Syst. Bot., ed. 2: 174. 13 Jun 1836;
Tragiaceae Raf., Fl. Tellur. 4: 111. med 1838
[’Tragides’]; Acalyphaceae Juss. ex
Menge., Cat. Plant. Grudent. Gedan.: 172. 1839 [’Acalyphinae’];
Crotonopsida Brong., Enum. Pl. Mus. Paris: xxiii, 79.
12 Aug 1843 [‘Crotonineae’]; Bertyaceae
J. Agardh, Theoria Syst. Plant.: 190. Apr-Sep 1858;
Crotonaceae J. Agardh, Theoria Syst. Plant.: 258.
Apr-Sep 1858 [’Crotoneae’];
Hippomanaceae J. Agardh, Theoria Syst. Plant.: 244.
Apr-Sep 1858 [’Hippomaneae’];
Ricinocarpaceae (Müll. Arg.) Hurus. in J. Fac. Sci.
Univ. Tokyo, ser. III, 6: 224. 15 Aug 1954;
Euphorbianae Takht. ex Reveal in Novon 2: 236. 13 Oct
1992; Cheilosaceae Doweld, Tent. Syst. Plant. Vasc.:
xxxi. 23 Dec 2001
Genera/species c
207/6.525–>6.600
Distribution Cosmopolitan
except polar areas, although mainly tropical, with their largest species
diversity (Euphorbia) in southern Africa, the Mediterranean and the
irano-turanian regions, and southern North America.
Fossils Fossil fruits and
seeds of Euphorbiaceae
from the Eocene of England (the London Clay and the Pipe-Clay in Dorset) have
been described (e.g. as Euphorbiospermum and Euphorbiotheca).
From Oligocene and younger layers in Australia there are likewise fossils
assigned to Euphorbiaceae.
Crepetocarpon from the Eocene of North America may belong in Euphorbiaceae-Euphorbioideae.
Fossil wood, probably of euphorbiacean origin, is frequently documented from
Palaeogene and Neogene layers on several continents (also New Zealand).
Habit Monoecious or dioecious,
evergreen or deciduous trees or shrubs, perennial or annual herbs (rarely
lianas; many species are stem succulents and xerophytes). CAM and C4
physiologies present in many species (i.a. Chamaesyce subclade of
Euphorbia).
Vegetative anatomy Phellogen
ab initio outer-cortical or pericyclic. Primary vascular tissue bicollateral or
centrifugal. Cortical and medullary vascular bundles sometimes present.
Secondary lateral growth usually normal (sometimes anomalous, from cylindrical
cambium) or absent. Vessel elements often in multiples. Vessel elements with
usually simple (sometimes scalariform) perforation plates; lateral pits
alternate, simple pits. Vestured pits sometimes present. Imperforate tracheary
xylem elements libriform fibres with simple or bordered pits, septate or
non-septate (in Bernardia also vasicentric tracheids). Wood rays
uniseriate or multiseriate, heterocellular. Axial parenchyma apotracheal
diffuse or diffuse-in-aggregates, eller paratracheal scanty, reticulate,
scalariform, vasicentric, or banded, or absent. Tyloses sometimes frequent.
Sieve tube plastids S type; sieve tubes with non-dispersive protein bodies.
Nodes usually 3:3, trilacunar with three leaf traces (rarely 1:1, unilacunar
with one trace, or ≥5:≥5, multilacunar with five or more traces).
Articulated or inarticulated laticifers with gums (rubber; usually absent in
Acalyphoideae). Secretory canals with tannins. Silica bodies present
in many species. Prismatic crystals often abundant (also in axial parenchyma
and/or wood ray cells); acicular crystals, druses, styloids, crystal sand or
other types of calciumoxalate crystals present or absent.
Trichomes Hairs unicellular or
multicellular, malpighiaceous hairs (unicellular T-shaped;
Argythamnia, Chiropetalum, Rhodothyrsus), simple,
furcate, stellate, candelabra-shaped, dendritic, peltate or lepidote; glandular
hairs often present; stinging hairs sometimes present (in, e.g.,
Cnidoscolus and Tragia).
Leaves Usually alternate
(spiral or distichous) or opposite (rarely verticillate), usually simple
(sometimes palmately compound), entire or lobed, with various ptyxis (sometimes
absent). Stipules usually cauline (sometimes hair-like or modified into glands;
in many species of Euphorbia modified into spines; rarely absent);
leaf sheath absent. Petiole often with distal pulvinus, sometimes with paired
or unpaired extrafloral nectaries. Petiole vascular bundle transection arcuate,
annular etc. Venation usually palmate (sometimes pinnate). Stomata usually
paracytic (sometimes anisocytic, parallelocytic or anomocytic). Cuticular wax
crystalloids as rosettes of platelets (Fabales type), or cuticular wax
as crust (especially in succulents). Epidermis with or without mucilaginous
idioblasts. Mesophyll with or without sclerenchymatous idioblasts. Styloids
present in some genera. Secretory cavities absent. Leaf margin entire or
serrate with simple veins proceeding into persistent transparent leaf teeth.
Subbasal glands present or absent; extrafloral nectaries sometimes present on
lamina.
Inflorescence Terminal or
axillary, cymose of various shapes (panicle, thyrsoid, fascicle, corymb,
raceme-, catkin- or spike-like, etc.) consisting of monochasia or dichasia.
Bracts sometimes involucral, sometimes large and showy, occasionally
resin-producing (e.g. Dalechampia). Inflorescence in
Euphorbia pseudanthial cyathium (thyrsoid), each with one terminal
female partial inflorescence with one female flower consisting of one
tricarpellate pistil, surrounded by four or five cincinni of male flowers, each
consisting of one stamen; five usually nectariferous bracts inserted on abaxial
side of each cyathium. Female flowers in Ricinus in distal part of
inflorescence.
Flowers Actinomorphic, small.
Hypogyny. Sepals (two or) three to six (to twelve), with valvate or imbricate
aestivation, usually free (sometimes connate at base; absent in some genera;
female flowers in Excoecaria with three tepals). Petals (two or) three
to six (to eight), with valvate or imbricate aestivation, usually free, or
absent. Nectariferous disc intrastaminal or extrastaminal, annular or
subdivided into separate glands, or absent.
Androecium Stamens one to more
than 50 (to more than 1.000). Filaments usually free (sometimes connate; in
Ricinus fused into branched fascicles), free from tepals, in some
genera apically split and branched. Anthers basifixed to dorsifixed, often
versatile, usually tetrasporangiate (rarely disporangiate), extrorse or
introrse, longicidal (dehiscing by longitudinal slits). Tapetum usually
secretory (sometimes amoeboid-periplasmodial). Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually tricolporate (sometimes
colpate, porate or inaperturate), shed as monads, bicellular or tricellular at
dispersal. Exine tectate, semitectate or intectate, with columellate
infratectum, perforate or reticulate, echinate, verruculate, spinulate or
smooth.
Gynoecium Pistil composed of
(two or) three (to numerous) connate carpels; median carpel usually abaxial.
Ovary superior, (bilocular or) trilocular (to multilocular). Stylodia (two or)
three (to numerous), free or connate at base, simple or branched. Stigmas
relatively large, often branched or with adaxial furrow, papillate or
non-papillate, Dry or Wet type. Pistillodium absent.
Ovules Placentation apical.
Ovules one (or two) per carpel, usually anatropous (sometimes hemianatropous or
amphitropous), pendulous, usually epitropous (sometimes apotropous), bitegmic,
crassinucellar. Micropyle usually exostomal (sometimes bistomal). Outer
integument three to many cell layers thick. Inner integument (three or) four to
25 cell layers thick. Nucellar cap present; megasporangium usually with
nucellar beak protruding through micropyle and reaching obturator. Placental
obturator present between stylar canal and micropyle forming roof above
micropyle. Hypostase present. Megagametophyte monosporous, disporous or
tetrasporous, Polygonum type, Acalypha type or other types
(Allium type?, Drusa type?, Fritillaria type?,
Penaea type?). Synergids sometimes with a filiform apparatus.
Antipodal cells sometimes proliferating (in at least Jatropha
sometimes up to five cells). Endosperm development ab initio nuclear. Endosperm
haustoria? Embryogenesis usually onagrad (sometimes solanad? or piperad?).
Fruit Usually a septicidal
(sometimes loculicidal) capsule (often explosively dehiscent) or usually
tripartite (rarely bipartite or multipartite) schizocarp (with carpels, cocci,
sometimes elastically dehiscing from central columella; mesocarps often
separating from endocarp; rarely a berry or drupe).
Seeds Seeds often large,
sometimes pachychalazal. Micropylar carunculus usually present. Aril present in
some species. Seed coat usually exotegmic (sometimes endotestal). Exotesta
usually palisade. Endotesta cells with amorphous calciumcarbonate. Exotegmen
often palisade, consisting of malpighian-like lignified cells
(Crotonoideae). Endotegmen absent. Perisperm not developed. Endosperm
usually copious, often oily (sometimes absent). Embryo straight or curved,
usually well differentiated, with or without chlorophyll. Cotyledons two,
usually longer and wider than radicula. Germination phanerocotylar or
cryptocotylar.
Cytology n = (5) 7 (8) 9–11
(or more); x = (7–)9(–11)
DNA Plastid gene infA
lost/defunct (Hevea). Mitochondrial coxI intron present.
Phytochemistry Flavonols
(kaempferol, quercetin), triterpenes (cucurbitacins), cocarcinogenic phorbol
esterditerpenes, ethereal oils and resins, ellagic and gallic acids,
ellagitannins (geraniin, mallotussic acid), p-coumaric acid, caffeic
acid, alkaloids (e.g. ricinine, in Croton benzylisoquinoline
alkaloids), cyanogenic glycosides derived from nicotinic acid or
valine/isoleucine, polyacetate-derived arthroquinones (Clutia),
ferulic acid, and lectins (hemagglutinins) present. Proanthocyanidins not
found. Saponins? Aluminium accumulated in some species.
Use Ornamental plants, starch
sources (Manihot), medicinal plants, rubber (Hevea), seed
oils (Ricinus), timber, poisons.
Systematics Euphorbiaceae are sister to
Rafflesiaceae.
The phylogenetic relationships among the genera
of Acalyphoideae, Crotonoideae and Euphorbioideae
are still very insufficiently known.
Cheilosoideae
(Müll.-Arg.) K. Wurdack et Petra Hoffm. in Amer. J. Bot. 92: 1413. 27 Jul
2005
2/7. Cheilosa (1; C.
montana; West Malesia), Neoscortechinia (6; N.
angustifolia, N. forbesii, N. kingii, N.
nicobarica, N. philippinensis, N. sumatrensis; southern
Burma, the Nicobar Islands, Malesia to New Guinea, the Bismarck Archipelago and
Solomon Islands). – Southern Burma, the Nicobar Islands, Malesia to New
Guinea, Solomon Islands. Stamens five to twelve. Pollen grains echinate. Pistil
composed of sometimes two connate carpels. Outer integument eight to ten cell
layers thick. Inner integument eight to twelve cell layers thick. Carunculus
absent. Testa with vascular bundles. Endosperm present.
[Suregada
clade+[Adenoclineae+[Acalyphoideae+[Crotonoideae+Euphorbioideae]]]]
Suregada clade
1–2/c 30. Suregada (c 30;
tropical Africa, Madagascar, tropical Asia to tropical Australia and islands in
the Pacific), Cladogelonium (1; C. madagascariense;
Madagascar; in Suregada?). – Tropical regions in the Old World.
Pollen grains pantoporate, acolumellate.
[Adenoclineae+[Acalyphoideae+[Crotonoideae+Euphorbioideae]]]
Adenoclineae
(Müll.-Arg.) G. L. Webster in Taxon 24: 598. 19 Dec 1975
6/67. Endospermum (11;
Southeast Asia, Malesia to Fiji); Klaineanthus (1; K.
gaboniae; Nigeria to Gabon), Tetrorchidium (23; tropical Africa,
Mexico, Central America, Jamaica, tropical South America), Adenocline
(8; southern Africa north to Malawi), Ditta (2; D.
maestrensis, D. myricoides; Cuba, Hispaniola, Puerto Rico);
Omphalea (22; tropical regions on both hemispheres). – Pantropical,
southern Africa. Endospermum may be sister to the remaining
Adenoclineae.
[Acalyphoideae+[Crotonoideae+Euphorbioideae]]
Outer integument six to approx. ten cell
layers thick. Phorbol esters (phorbol diterpenes) sometimes present.
Acalyphoideae (Kunth)
Beilschm. in Flora 16(Beibl. 7): 61, 104. 14 Jun 1833
[‘Acalypheae’]
c 100/2.000–>2.035.
Acalypheae Dumort., Anal. Fam. Plant.: 45. 1829. Acalypha
(450–455; tropical and subtropical regions on both hemispheres);
Claoxylon (c 115; Madagascar, tropical Asia, northern and eastern
Australia, Melanesia, the Hawaiian Islands), Crotonogynopsis (2;
C. akeassii, C. usambarica; tropical Africa),
Discoclaoxylon (4; D. hexandrum, D. occidentale,
D. pedicellare, D. pubescens; one species in western tropical
Africa, three species endemic to São Tomé); Erythrococca (c 40;
tropical and southern Africa, southern Arabian Peninsula), Micrococca
(12; tropical Africa, Madagascar, the Arabian Peninsula, tropical Asia to the
Malay Peninsula); Cleidion (30–35; tropical regions on both
hemispheres), Sampantaea (1; S. amentiflora; Thailand,
Cambodia), Wetria (2; W. insignis: southern Burma, Thailand,
West Malesia, the Philippines; W. australiensis: Papua New Guinea,
northeastern Queensland); Dysopsis (3; D. glechomoides,
D. hirsuta, D. paucidentata; the Andes, Juan Fernandez);
Lasiococca (5; L. brevipes, L. chanii, L.
comberi, L. locii, L. symphyllifolia; eastern Himalayas,
Hainan, Vietnam, the Malay Peninsula), Spathiostemon (2; S.
javensis, S. moniliformis; Peninsular Thailand, Malesia to New
Guinea), Homonoia (3; H. intermedia, H. retusa,
H. riparia; Malesia New Guinea); Lobanilia (8; Madagascar);
Macaranga (305–310; tropical regions in the Old World);
Mareya (4; M. acuminata, M. brevipes, M.
congolensis, M. micrantha; tropical West and Central Africa); Mercurialis
(12; Europe, the Mediterranean, temperate Asia to northern Thailand),
Seidelia (2; S. firmula, S. triandra; Northern and
Western Cape, Free State), Leidesia
(1; L. procumbens; southern Africa); Ricinus
(1; R. communis; eastern and northeastern Africa, the Arabian
Peninsula, southwestern Asia), Adriana (2; A. quadripartita,
A. urticoides; Australia); Avellanita (1; A.
bustillosii; central Chile), Mallotus (120–125; tropical Asia
to tropical Australia and islands in the Pacific, two species, M.
oppositifolius and M. subulatus, in tropical Africa and
Madagascar); Blumeodendron (5; B. bullatum, B.
calophyllum, B. concolor, B. kurzii, B.
tokbrai; Southeast Asia, the Andaman Islands, Malesia to New Guinea),
Podadenia (1; P. sapida; Sri Lanka), Ptychopyxis
(13; Peninsular Thailand, Malesia to New Guinea), Botryophora (1;
B. geniculata; Southeast Asia, West Malesia); Afrotrewia (1;
A. kamerunica; Cameroon, Gabon). –
Adelieae G. L. Webster in Taxon 24: 597. 19 Dec 1975.
Adelia (10; Mexico, Central America, the West Indies, tropical South
America), Enriquebeltrania (2; E. crenatifolia, E.
disjuncta; Mexico), Garciadelia (4; G. abbottii, G.
castilloae, G. leprosa, G. mejiae; Hispaniola),
Lasiocroton (c 25?; Cuba), Leucocroton (26–27; Cuba). –
Agrostistachydeae G. L. Webster in Taxon 24: 596. 19
Dec 1975. Agrostistachys (6; A. borneensis, A.
gaudichaudii, A. hookeri, A. indica, A.
sessilifolia, A. staminodiata; southern India, Sri Lanka,
Thailand, Malesia to New Guinea), Chondrostylis (2; C.
bancana, C. kunstleri; Peninsular Thailand, West Malesia),
Cyttaranthus (1; C. congolensis; Central Africa),
Pseudagrostistachys (2; P. africana, P. ugandensis;
tropical Africa). – Alchorneeae Hutch. in Amer. J.
Bot. 56: 752. Aug 1969. Alchornea (50–55; tropical regions on both
hemispheres), Aparisthmium (1; A. cordatum; southern Central
America, tropical South America), Bocquillonia
(14; New Caledonia), Orfilea (4; O. ankafinensis, O.
coriacea, O. multispicata, O. neraudiana; Madagascar,
Mauritius); Aubletiana (2; A. leptostachys, A.
macrostachys; Cameroon, Gabon), Conceveiba (14; Central America,
tropical South America), Mareyopsis (2; M. longifolia, M.
oligogyna; Central Africa). – Ampereae
Müll.-Arg. in Bot. Zeitung (Berlin) 22: 324. 14 Oct 1864. Monotaxis
(11; Australia), Amperea
(8; southwestern Western Australia, southeastern Australia, Tasmania). –
Bernardieae G. L. Webster in Taxon 24: 596. 19 Dec
1975. Bernardia (c 75; southern United States, Mexico, Central
America, tropical South America, with their highest diversity in Brazil),
Discocleidion (1; D. rufescens; central China, the Ryukyu
Islands), Adenophaedra (3; A. cearensis, A.
grandifolia, A. megalophylla; tropical South America). –
Caryodendreae G. L. Webster in Taxon 24: 596. 19 Dec
1975. Caryodendron (4; C. amazonicum, C.
angustifolium, C. janeirense, C. orinocense; Costa Rica
to Amazonian Brazil), Discoglypremna (1; D. caloneura;
tropical Africa), Alchorneopsis (2; A. floribunda; Central
America, tropical South America, one species, A. portoricensis, on
Puerto Rico). – Chrozophoreae Pax et K. Hoffm. in
H. G. A. Engler, Pflanzenr. 68(Addit. VI): 2. 6 Jun 1919. Chrozophora
(11; the Mediterranean, tropical East Africa, southwestern and southern Asia to
Thailand); Argythamnia (23; southern Mexico to Honduras, the West
Indies), Caperonia (c 35; tropical Africa, Madagascar, tropical
America), Chiropetalum (c 25; Texas, Mexico, tropical South America to
northern Chile), Ditaxis (c 50; Florida, southwestern United States,
Mexico, Central America, the West Indies, tropical South America),
Philyra (1; P. brasiliensis; southern Brazil, Paraguay,
northern Argentina); Doryxylon (1; D. spinosum; Luzon, the
Lesser Sunda Islands), Melanolepis (2; M. multiglandulosa,
M. vitifolia; Southeast Asia to islands in the Pacific, Taiwan),
Sumbaviopsis (1; S. albicans; Assam, Southeast Asia, West
Malesia), Thyrsanthera (1; T. suborbicularis; Southeast
Asia); Speranskia (3; S. cantonensis, S.
tuberculata, S. yunnanensis; southern China, northern Burma). –
Epiprineae Hurus. in J. Fac. Sci. Univ. Tokyo, ser.
3, Bot. 6: 309. 1954. Epiprinus (6; E. balansae, E.
lanceifolius, E. malayanus, E. mallotiformis, E.
poilanei, E. siletianus; India, Burma, southern China, Southeast
Asia, Sumatra), Cleidiocarpon (2; C. cavaleriei, C.
laurinum; Burma, southern China, western Thailand, northern Vietnam),
Koilodepas (12; southern India to Hainan and New Guinea),
Cladogynos (1; C. orientalis; Southeast Asia, Malesia),
Tsaiodendron (1; T. dioicum; Yunnan), Cephalocroton
(4; C. cordofanus, C. incanus, C. mollis, C.
polygynus; tropical Africa, Madagascar, Socotra, Sri Lanka);
Cephalomappa (5; C. beccariana, C. lepidotula,
C. malloticarpa, C. paludicola, C. penangensis;
southern China, West Malesia), Rockinghamia (2; R.
angustifolia, R. brevipes; northeastern Queensland). –
Erismantheae G. L. Webster in Taxon 24: 595. 19 Dec
1975. Erismanthus (2; E. obliquus, E. sinensis;
Southeast Asia, Hainan, West Malesia); Moultonianthus (1; M.
leembruggianus; Sumatra, Borneo), Syndyophyllum (2; S.
occidentale: northern Sumatra, Borneo; S. excelsum: northern
Papua New Guinea). – Plukenetieae Hutch. in Amer.
J. Bot. 56: 753. Aug 1969. Dalechampia
(120–125; tropical regions on both hemispheres, with their highest diversity
in tropical South America); Haematostemon (2; H. coriaceus,
H. guianensis; Guyana, Amazonian Venezuela), Plukenetia (c
20; tropical Africa, Madagascar, southern Mexico, Central America, tropical
South America, one species, P. corniculata, in tropical Asia),
Romanoa (1; R. tamnoides; eastern and southern Brazil,
Paraguay, Bolivia); Ctenomeria (2; C. capensis, C.
cordata; South Africa), Megistostigma (5; M. burmanicum,
M. cordatum, M. glabratum, M. peltatum, M.
yunnanense; Yunnan, Southeast Asia, West Malesia), Cnesmone (11;
Assam, Southeast Asia, West Malesia), ‘Tragia’ (150–155;
tropical, subtropical and warm temperate regions on both hemispheres;
polyphyletic), Bia (5; B. alienata, B. cordata,
B. fallax, B. fendleri, B. lessertiana; Costa Rica
to tropical South America), Platygyna (7; P. dentata, P.
hexandra, P. leonis, P. obovata, P. parvifolia,
P. triandra, P. volubilis; Cuba), Acidoton (6;
A. haitiensis, A. lanceolatus, A. microphyllus,
A. nicaraguensis, A. urens, A. variifolius;
Hispaniola and Jamaica), Zuckertia (2; Z. cordata, Z.
manuelii; Mexico, Central America), ‘Tragia’ pro parte,
Pachystylidium (1; P. hirsutum; India, Southeast Asia to
Central Malesia), Sphaerostylis (2; S. perrieri, S.
tulasneana; Madagascar), Gitara (1; G. nicaraguensis;
Central America to Venezuela), ‘Tragia’ pro parte; unplaced
Plukenetieae: Angostylis (2; A. longifolia, A.
tabulamontana; Amazonian Brazil), Astrococcus (1; A.
cornutus; Amazonian Venezuela, Amazonian Brazil). –
Pycnocomeae Reveal in Phytoneuron 2012-37:
218. 23 Apr 2012. Amyrea (11; Madagascar), Necepsia (3;
N. afzelii, N. castaneifolia, N. zairensis; tropical
Africa, Madagascar), Paranecepsia (1; P. alchorneifolia;
tropical East Africa); Pycnocoma(18; tropical Africa),
Droceloncia (1; D. rigidifolia; Madagascar, the Comoros),
Argomuellera (17; tropical Africa, Madagascar). –
Sphyranthereae Radcl.-Sm., Gen. Euphorbiacearum: 135.
2001. Sphyranthera (2; S. airyshawii, S. lutescens;
the Andaman Islands, the Nicobar Islands). – Pantropical, few species in
subtropical and warm-temperate regions. Pseudanthia present in Dalechampia.
Stigma in Acalypha
strongly branched. Outer integument three to six (to 16) cell layers thick.
Inner integument three to 24 cell layers thick. Testa sometimes with vascular
bundles.
[Crotonoideae+Euphorbioideae]
Laticifers and latex present. Large
variation among phorbol esters; cocarcinogens present.
Crotonoideae (Kunth)
Beilschm. in Flora 16(Beibl. 7): 61, 106. 14 Jun 1833
[’Crotoneae’]
60/2.010–>2.020.
Aleuritideae Hurus. in J. Fac. Sci. Univ. Tokyo, ser.
3, Bot., 6: 309. 15 Aug 1954. Aleurites
(2; A. moluccanus, A. rockinghamensis; tropical Asia to
islands in western Pacific), Vernicia (3; V. cordata, V.
fordii, V. montana; Burma, Southeast Asia, Malesia and southern
China to Japan); Benoistia (3; B. orientalis, B.
perrieri, B. sambiranensis; Madagascar); Cyrtogonone (1;
C. argentea; tropical West Africa), Crotonogyne (16; tropical
Africa), Manniophyton (1; M. fulvum; western and central
tropical Africa to Angola); Garcia (2; G. nutans, G.
parviflora; Mexico to Colombia); Grossera (8; tropical Africa,
Madagascar), Cavacoa (3; C. aurea, C. baldwinii,
C. quintasii; tropical Africa), Sandwithia (2; S.
guyanensis, S. heterocalyx; northeastern South America),
Tannodia (9; tropical Africa, Madagascar), Tapoides (1;
T. villamilii; Borneo); Neoboutonia (3; N.
macrocalyx, N. mannii, N. melleri; tropical Africa);
Deutzianthus (2; D. tonkinensis; northern Vietnam; D.
thyrsiflorus: Sumatra), Oligoceras (1; O. eberhardtii;
Vietnam), Paracroton (4; P. integrifolius, P.
pendulus, P. sterrhopodus, P. zeylanicus; southern
India, Sri Lanka, Malesia to New Guinea); Mildbraedia (5; M.
balboana, M. carpinifolia, M. fallax, M.
occidentalis, M. paniculata; western and central tropical Africa
to Mozambique). – Codiaeeae Hutch. in Amer. J. Bot.
56: 747. Aug 1969 [‘Codiaeae’]. Alphandia (3; A.
furfuracea, A. resinosa, A. verniciflua; New Guinea, New
Caledonia, Vanuatu), Anomalocalyx (1; A. uleanus; Amazonian
Brazil from near Manaos to Amapá), Baliospermum (5; B.
angustifolium, B. bilobatum, B. calycinum, B.
solanifolium, B. yui; the Himalayas, Tibet, Yunnan, Indochina,
the Malay Peninsula, Sumatra, Java, Sumbawa), Baloghia
(15; eastern Queensland, eastern New South Wales, Norfolk Island, New
Caledonia, with the largest diversity in New Caledonia), Blachia (11;
India, the Andaman Islands, southern China, Southeast Asia, the Philippines),
Codiaeum
(16–17; Malesia to New Guinea, tropical Australia and New Caledonia),
Dimorphocalyx (17; India, Sri Lanka to the Philippines, New Guinea and
tropical Australia), Dodecastigma (3; D. amazonicum, D.
integrifolium, D. uleanum; the Guianas, Amazonian Brazil),
Fontainea (9; New Guinea, Queensland, New Caledonia, Vanuatu),
Hylandia (1; H. dockrillii; northeastern Queensland),
Ostodes (2; O. kuangii, O. paniculata; Assam,
eastern Himalayas, Southeast Asia to Java and Borneo), Pantadenia (3;
P. chauvetiae and P. gervaisii: Madagascar; P.
adenanthera: Thailand, Indochina), Strophioblachia (1; S.
fimbricalyx; Yunnan, Hainan, Thailand, Cambodia, Vietnam, the Philippines,
Sulawesi); Trigonostemon (c 85; India and China to the Philippines,
New Guinea, Queensland and Fiji); Pausandra (8; Central America,
tropical South America). – Crotoneae Dumort., Anal.
Fam. Plant.: 45. 1829 [‘Crotonieae’]. Acidocroton (13;
southern Mexico, Central America to Colombia, Cuba, Hispaniola, Jamaica),
Brasiliocroton (1; B. mamoninha; northeastern Brazil), Croton
(>1.200; tropical and subtropical regions), Moacroton (>28;
tropical and subtropical North, Central and South America, the West Indies),
Sagotia (2; S. brachysepala, S. racemosa; Central
America, northern South America), Sandwithia (2; S.
guyanensis, S. heterocalyx; Amazonian South America). –
Elateriospermeae G. L. Webster in Taxon 24: 599. 19
Dec 1975. Elateriospermum (1; E. tapos; Peninsular Thailand,
West Malesia), Glycydendron (2; G. amazonicum, G.
espiritosantense; northern South America). –
Heveeae (Müll. Arg.) G. L. Webster in Kubitzki (ed)
2014 Fam. Gen. Vasc. Plants, p. 164. Hevea (9; the Amazon basin). –
Jatropheae Baill., Hist. Plant. 5: 156, 179.
Jan–Apr 1874. Jatropha
(>190; tropical and subtropical regions on both hemispheres, North America),
Joannesia (2; J. heveoides, J. princeps; Venezuela,
Amazonian and coastal Brazil), Vaupesia (1; V. cataractarum;
Colombia, western Brazil). – Manihoteae Pax in H.
G. A. Engler et K. A. E. Prantl, Nat. Pflanzenfam. III, 5: 14. Mai 1890.
Cnidoscolus (c 95; southern United States, Mexico, Central America,
the West Indies, tropical South America), Manihot
(105–110; southwestern United States, Mexico, Central America, the West
Indies, tropical South America). – Micrandreae G.
L. Webster in Taxon 24: 598. 19 Dec 1975. Hevea (c 10; the Amazon
basin); Micrandra (11–12; tropical South America),
Micrandropsis (1; M. scleroxylon; Amazonas, Amazonian
Colombia). – Ricinocarpeae Müll.-Arg. in Bot.
Zeitung (Berlin) 22: 324. 14 Oct 1864. Bertya (28; southern and
eastern Australia), Borneodendron (1; B. aenigmaticum;
ultrabasic soils on northern Borneo), Cocconerion
(2; C. balansae, C. minus; ultrabasic soils in New
Caledonia), Myricanthe (1; M. discolor; ultrabasic soils in
northwestern New Caledonia); Beyeria (c 25; southern and eastern
Australia, Tasmania), Ricinocarpos
(28; western and northern Australia, New Caledonia), Shonia (4; S.
bickertonensis, S. carinata, S. territorialis, S.
tristigma; Northern Territory, Queensland). –
Ricinodendreae Hutch. in Amer. J. Bot. 56: 749. Aug
1969. Annesijoa (1; A. novoguineensis; New Guinea),
Givotia (4; G. gosai, G. madagascariensis, G.
moluccana, G. stipularis; tropical East Africa, Madagascar,
India, Sri Lanka), Leeuwenbergia (2; L. africana, L.
letestui; Gabon to Cameroon and Congo), Ricinodendron (2; R.
heudelotii, R. lobatus; tropical Africa to Angola and
Mozambique); incertae sedis: Radcliffea (1; R. smithii;
western Madagascar). – Pantropical, few species in East Asia and North
America. Laticifers articulated or inarticulated. Hairs often stellate or
lepidote. Lamina sometimes with abaxial paired glands near petiole junction.
Petals present or absent. Pollen grains colpate, porate or inaperturate. Seeds
often pachychalazal. Aril or carunculus often present. Exotesta sometimes
palisade. Endotestal cells sometimes palisade, thin-walled, slightly lignified.
Tegmen usually vascularized. Cyanogenes via valine/isoleucine pathway. Deletion
of more than 100 bp in plastid trnL/F spacer in some species.
Euphorbioideae (Kunth)
Beilschm. in Flora 16(Beibl. 7): 61, 105. 14 Jun 1833
[‘Euphorbieae’]
38/2.410–2.420.
Euphorbieae Dumort., Anal. Fam. Plant.: 45. 1829.
Anthostema (3; A. aubryanum, A. madagascariense,
A. senegalense; tropical West Africa, Madagascar),
Dichostemma (2; D. glaucescens: Central Africa; D.
zenkeri: Cameroon); Neoguillauminia (1; N. cleopatra;
New Caledonia), Calycopeplus (5; C. casuarinoides, C.
collinus, C. marginatus, C. oligandrus, C.
paucifolius; northern Australia, southwestern Western Australia);
Euphorbia (c 2.050; almost cosmopolitan). –
Hippomaneeae Bartl., Ord. Nat. Plant.: 372. Sep 1830
[‘Hippomanea’]. Homalanthus (23; tropical Asia to New
Guinea, northern and eastern Australia, New Caledonia, New Zealand, Polynesia);
Colliguaja
(5; C. brasiliensis, C. dombeyana, C. integerrima,
C. odorifera, C. salicifolia; southern Brazil, central Chile
and adjacent Paraguay and Uruguay), Grimmeodendron (2; G.
eglandulosum, G. jamaicense; the West Indies), Bonania
(7; B. cubana, B. domingensis, B. elliptica, B.
emarginata, B. erythrosperma, B. linearifolia, B.
myricifolia; the West Indies), Adenopeltis (1; A.
serrata; Peru, Chile), Stillingia (c 30; Madagascar, the
Mascarene Islands, East Malesia, Fiji, southern United States, Mexico, Central
America, the West Indies, tropical South America), Gradyana (1; G.
franciscana; northeastern Brazil), Spegazziniophytum (1; S.
patagonicum; southern Argentina), Sapium
(23; Mexico, Central America, the West Indies, tropical South America),
Hippomane (3; H. horrida, H. spinosa: Hispaniola;
H. mancinella: Florida to Venezuela, the Galápagos Islands),
Senefelderopsis (2; S. chiribiquetensis, S.
croizatii; the Guayana Highlands and adjacent areas), Incadendron
(1; I. esseri; Ecuador, Peru), Pleradenophora (1; P.
longicuspis; Mexico, Guatemala, Belize), Balakata (2; B.
baccata, B. luzonica; southern China, tropical Asia, New Guinea,
tropical Australia), Falconeria (1; F. insignis; the
Himalayas, Southeast Asia, West Malesia), Sclerocroton (6; S.
carterianus, S. cornutus, S. integerrimus, S.
melanostictus, S. oblongifolius, S. schmitzii; tropical
Africa, Madagascar), Triadica (3; T. cochinchinensis, T.
rotundifolia, T. sebifera; East and tropical Asia),
Mabea (c 40; Mexico, Central America, tropical South America),
Gymnanthes (c 25; tropical and southern Africa, tropical Asia to
Malesia and islands in the Pacific, Florida, Mexico, Central America, the West
Indies, tropical South America, one species, G. japonica, in central
China, the Korean Peninsula and Japan), Actinostemon (c 20; the West
Indies, tropical South America), Senefeldera (6; S.
inclinata, S. macrophylla, S. multiflora, S.
testiculata, S. triandra, S. verticillata; tropical
South America), Dalembertia (4; D. hahniana, D.
platanoides, D. populifolia, D. triangularis; Mexico,
Guatemala), Maprounea (6; M. africana, M. amazonica,
M. brasiliensis, M. guianensis, M. membranacea,
M. obtusata; tropical Africa, tropical South America),
Excoecaria (c 40; Africa to Australia and Melanesia),
Sebastiania (70–75; southwestern United States, Mexico, Central
America, the West Indies, tropical South America), Algernonia (12;
Peru, eastern Brazil), Hura (2; H. polyandra: Mexico, Central
America; H. crepitans: Nicaragua to the West Indies, Peru and Brazil),
Ophthalmoblapton (4; O. crassipes, O. macrophyllum,
O. parviflorum, O. pedunculare; eastern Brazil);
Pachystroma (1; P. longifolium; southeastern Brazil, Bolivia,
Peru). – Stomatocalyceae G. L. Webster in Taxon 24:
600. 19 Dec 1975. Plagiostyles (2; P. africana, P.
pinnatus; southern Nigeria, Gabon, Congo), Pimelodendron (4;
P. amboinicum, P. griffithianum, P. macrocarpum,
P. zoanthogyne; Malesia to New Guinea, northeastern Queensland);
Hamilcoa (1; H. zenkeri; Nigeria, Cameroon),
Nealchornea (2; N. stipitata, N. yapurensis;
Colombia, Brazil, eastern Peru). – Subcosmopolitan. Laticifers inarticulated.
White caustic latex present. Starch grains often complex in latex (e.g. in Euphorbia).
Disc usually absent. Stamens not covered by tepals. Outer integument three to
six or eight to 22 cell layers thick. Inner integument three to five (to 22)
cell layers thick. Carunculus often present.
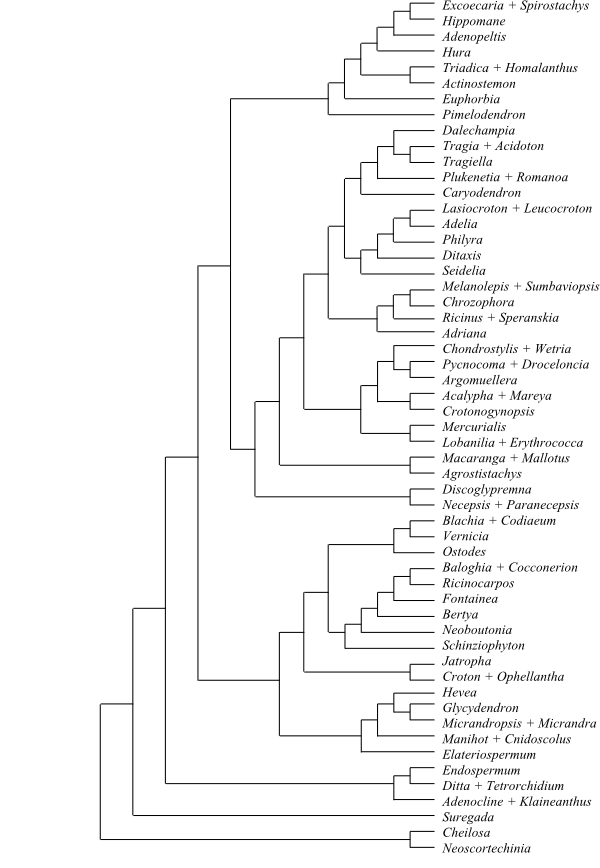 |
Cladogram of Euphorbiaceae based
on DNA sequence data (Tokuoka 2007).
|
Marcano-Berti in Pittieria 18: 16. Nov 1989
Genera/species 1/3
Distribution Northern and
northeastern tropical South America.
Fossils Unknown.
Habit Bisexual, evergreen tree
or shrub.
Vegetative anatomy Phellogen?
Vessel elements with simple perforation plates; lateral pits alternate,
bordered pits. Vestured pits absent. Imperforate tracheary xylem elements
tracheids? or libriform fibres with bordered pits, non-septate. Wood rays
uniseriate or multiseriate, heterocellular. Axial parenchyma paratracheal
aliform, winged-aliform, or confluent. Sieve tube plastids S type? Nodes?
Cortex and medulla with sclereids.
Trichomes Hairs simple,
unicellular; glands present on leaves.
Leaves Alternate (spiral),
simple, entire, coriaceous, with revolute ptyxis. Stipules small; leaf sheath
absent. Petiole vascular bundle transection annular; bundles not always
immediately fused with stele. Lamina densely tomentose on abaxial side.
Venation pinnate. Stomata paracytic? Cuticular wax crystalloids? Hypodermis
with mucilaginous idioblasts. Mesophyll with sclerenchymatous idioblasts. Leaf
margin entire, with abaxial glands at base.
Inflorescence Terminal,
cymose.
Flowers Obliquely zygomorphic.
Half epigyny. Sepals five, non-uniform, with imbricate quincuncial aestivation,
connate at base into short hypanthium-like structure (“floral cup”). Petals
three (abaxial-lateral and abaxial petals absent), with contorted aestivation,
free. Nectariferous disc annular, intrastaminal, inserted at adaxial side of
hypanthium-like structure. Calcium oxalate druses present in floral parts.
Androecium Stamens usually
four fertile (sometimes five to seven): two longer outer antesepalous and two
shorter inner alternisepalous, separated by one long acute abaxial-lateral
antesepalous staminodium on one side and (one to) three to five short dentate
adaxial staminodia on opposite side. Filaments connate at base into two groups,
adnate to petals (epipetalous). Anthers dorsifixed, versatile,
tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits);
connective often thickened dorsally? Tapetum secretory? Staminodia two to
six.
Pollen grains
Microsporogenesis simultaneous? Pollen grains tricolporate, shed as monads,
?-cellular at dispersal. Exine?, with ? infratectum, sculpturing?
Gynoecium Pistil composed of
three connate carpels; median carpel adaxial; carpel semi-synascidiate. Ovary
semi-inferior, trilocular, with central columella; locules filled by
unicellular hairs. Style single, simple. Stigma capitate, papillate, probably
Wet type. Pistillodium absent.
Ovules Placentation axile.
Ovules two per carpel, anatropous, apotropous, upper ovule with micropyle
directed downwards, lower ovule with micropyle directed upwards, bitegmic,
tenuinucellar. Micropyle bistomal. Outer integument three or four cell layers
thick. Inner integument six or seven cell layers thick. Obturator probably
absent. Megagametophyte monosporous, Polygonum type? Endosperm
development? Endosperm haustoria? Embryogenesis?
Fruit A septicidal capsule
with thin exocarp, persistent calyx and persistent central columella.
Seeds Aril absent. Testa
winged. Tegmen? Perisperm not developed. Endosperm thin and sparse or absent.
Embryo straight?, chlorophyll? Cotyledons two? Germination phanerocotylar?
Cytology n = ?
DNA
Phytochemistry Unknown.
Use Unknown.
Systematics Euphronia
(3; E. acuminatissima, E. guianensis, E.
hirtelloides; the Venezuelan and the Guayana Highlands, northeastern
tropical South America).
Euphronia is sister to Chrysobalanaceae.
Miers in Ann. Mag. Nat. Hist. ser. 3, 9: 292. Apr
1862
Genera/species 1/3
Distribution Northern tropical
South America.
Fossils Unknown.
Habit Bisexual, evergreen
trees or shrubs.
Vegetative anatomy Phellogen
ab initio superficial. Vascular cylinder and medulla quadrangular or
quinquangular. Vessel elements with simple or scalariform perforation plates;
lateral pits alternate, bordered pits. Imperforate tracheary xylem elements
tracheids with bordered pits, non-septate (also vasicentric tracheids). Wood
rays uniseriate or multiseriate, heterocellular. Axial parenchyma apotracheal
diffuse or diffuse-in-aggregates, or paratracheal scanty. Wood fluorescent.
Sieve tube plastids S type. Nodes 3:3, trilacunar with three leaf traces.
Heartwood with gum-like substances. Prismatic calciumoxalate crystals and
druses present.
Trichomes Hairs unicellular or
multicellular, uniseriate, simple, with thickened cell walls and pitted
base.
Leaves Alternate (distichous),
simple, entire, coriaceous, with ? ptyxis. Stipules narrowly elongate,
inflexed, caducous; leaf sheath absent. Petiole vascular bundle transection
annular; petiole with inverted medullary bundle. Venation pinnate to palmate,
acrodromous (actinodromous); secondary veins ascending; tertiary veins
scalariform. Stomata usually laterocytic (rarely anisocytic). Cuticular waxes
absent. Abaxial domatia present in vein axils. Sclereids simple or branched.
Epidermal cells mucilaginous. Leaf margin serrate or entire.
Inflorescence Axillary,
umbel-like, consisting of short racemes.
Flowers Actinomorphic. Pedicel
articulated? Hypogyny. Sepals five, with imbricate aestivation, connate
(free?). Petals five, with induplicate-valvate aestivation, long and subulate,
apically inflexed in bud (sometimes geniculate or sigmoid at anthesis), free.
Nectariferous disc annular, thin, intrastaminal, sinuate at margin.
Androecium Stamens five,
haplostemonous, antesepalous, alternipetalous. Filaments very short, free from
each other and from tepals, inserted at margin of nectariferous disc. Anthers
basifixed, adnate (locules short, somewhat separate), non-versatile,
tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits);
connective somewhat extended, with long apical hairs. Tapetum secretory.
Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains tricolpor(oid)ate or tricolpate,
shed as monads, ?-cellular at dispersal. Exine semitectate, with columellate?
infratectum, reticulate. Endexinal folds present.
Gynoecium Pistil composed of
five connate antepetalous carpels. Ovary superior, quinquelocular. Stylodia
five, separate, short, with adaxial furrows, inserted at outer carpellary
margins. Stigmas five, subulate, type? Pistillodium absent.
Ovules Placentation basal to
axile. Ovules few per carpel, anatropous, ascending, bitegmic, crassinucellar.
Micropyle ?-stomal. Outer integument approx. three cell layers thick. Inner
integument approx. three cell layers thick. Megagametophyte monosporous,
Polygonum type. Endosperm development ab initio nuclear. Endosperm
haustoria? Embryogenesis?
Fruit A many-seeded berry-like
bilocular or trilocular drupe.
Seeds Aril absent. Testa
reticulate, with mesotesta consisting of sclereids. Endotesta? Exotegmen
fibrous, little developed, ridged, sclereidal; exotegmic cells with U-shaped
wall thickenings. Endotegmen? Perisperm not developed. Endosperm copious,
fleshy. Embryo straight, well differentiated, chlorophyll? Cotyledons two.
Germination?
Cytology n = ?
DNA
Phytochemistry Virtually
unknown. Aluminium accumulated.
Use Timber (cupioba),
canoes.
de Jussieu in A. Saint-Hilaire, Fl. Bras. Merid.
2: 87. 10 Oct 1829, nom. cons.
Genera/species 8/55–60
Distribution Southern Mexico,
Central America, tropical South America; one species of Sacoglottis in
tropical West Africa.
Fossils Uncertain. Fossil
endocarps sometimes assigned to Sacoglottis and Vantanea have
been found in Miocene and Pliocene layers from Costa Rica in the north to
Bolivia in the south (Herrera & al. 2010).
Habit Bisexual, evergreen
trees or shrubs. Young branches angular in cross-section. Often with aromatic
juice.
Vegetative anatomy Phellogen
ab initio subepidermal. Vessel elements with simple or scalariform perforation
plates; lateral pits alternate, simple or bordered pits. Vestured pits present.
Imperforate tracheary xylem elements tracheids with bordered pits, non-septate.
Wood rays uniseriate or multiseriate, heterocellular. Axial parenchyma
apotracheal diffuse or diffuse-in-aggregates, or paratracheal aliform,
lozenge-aliform, winged-aliform, vasicentric, or unilateral. Tyloses sometimes
abundant. Sieve tube plastids Pcs type, with protein crystals and starch. Nodes
3:3, trilacunar with three leaf traces. Mucilage cells numerous. Secretory
cavities absent. Heartwood sometimes with gum-like substances. Silica bodies
present in some species. Prismatic calciumoxalate crystals abundant.
Trichomes Hairs simple or
absent.
Leaves Alternate (spiral or
distichous), simple, entire, coriaceous, usually with involute ptyxis. Stipules
small and caducous, or absent; leaf sheath absent. Petiole vascular bundle
transection? Venation pinnate. Stomata paracytic or anomocytic. Cuticular wax
crystalloids? Secretory cavities absent. Mesophyll with or without
sclerenchymatous idioblasts (with sclereids reaching from one epidermis to
opposite side of lamina) and with calciumoxalate as druses or single prismatic
crystals. Leaf margin usually serrate (sometimes crenate or entire).
Extrafloral nectaries rarely present on adaxial side of lamina (e.g. in
Vantanea).
Inflorescence Usually axillary
(rarely terminal), panicle or thyrse.
Flowers Actinomorphic or
somewhat zygomorphic. Hypogyny. Sepals (four or) five, two outer ones often
smaller, with imbricate aestivation, persistent, connate in lower part into
tube. Petals (four or) five, with usually quincuncial to cochlear (sometimes
imbricate or contorted) aestivation, thick, persistent or caducous, free.
Nectariferous disc intrastaminal, with stomata, usually cupular to tubular or
dentate to lobate (sometimes as ten to 20 free scales), often adnate to ovary
base or filament bases.
Androecium Stamens usually ten
to c. 30, in one to five whorls (sometimes in five antesepalous staminal
fascicles each with three stamens, and five antepetalous stamens; in
Vantanea c. 40 to more than 100 stamens in fascicles); filament
bundles alternipetalous. Filaments connate into tube at least in lower part,
free from tepals. Anthers dorsifixed or subbasifixed or inserted at connective
bases, versatile, (disporangiate or) tetrasporangiate, introrse, longicidal
(dehiscing by longitudinal slits), with thecae separated, superposed;
connective wide, apically prolongate. Tapetum secretory. Staminodia present in
some species.
Pollen grains:
Microsporogenesis simultaneous. Pollen grains usually 3(–4)-colporate (rarely
3–4-porate), shed as monads, ?-cellular at dispersal. Exine tectate, with
columellate infratectum, microreticulate, perforate or punctate.
Gynoecium Pistil composed of
(four or) five (to seven) connate usually antesepalous carpels. Ovary superior,
(quadrilocular or) quinquelocular (to septalocular; sometimes unilocular at
apex). Style single, simple. Stigma entire to slightly lobate, type?
Pistillodium absent.
Ovules Placentation apical to
axile. Ovules one (or two) per carpel, anatropous, pendulous, epitropous
(micropyle directed upwards-outwards), bitegmic, crassinucellar. Micropyle
usually bistomal or exostomal (rarely endostomal). Outer integument usually two
cell layers thick, tanniniferous. Inner integument three cell layers thick,
tanniniferous. Endothelium absent. Megagametophyte monosporous,
Polygonum type. Endosperm development ab initio nuclear. Endosperm
haustoria? Embryogenesis?
Fruit A one- or two-seeded
drupe, with uneven surface and operculate multilocular pyrene, sometimes with
numerous cavities filled with resinous? material (for floating on water
surface).
Seeds Aril absent. Exotestal
cell walls thick and lignified. Endotesta? Tegmen multiplicative (approx. five
cell layers thick). Exotegmen fibrous; cross layer present beneath exotegmen.
Endotegmen? Perisperm not or sparsely developed. Endosperm copious, oily.
Embryo straight or slightly curved, well differentiated, chlorophyll?
Cotyledons two. Germination?
Cytology x = 12
DNA
Phytochemistry Very
insufficiently known. Ellagic acid present. Bergenin and gallate present in
bark in Sacoglottis. Alkaloids not found.
Use Timber, fruits, medicinal
plants.
Systematics Vantanea
(16; Central America, tropical South America); Humiria (4; H.
balsamifera, H. crassifolia, H. fruticosa, H.
wurdackii; tropical South America), Duckesia (1; D.
verrucosa; Amazonian Brazil), Hylocarpa (1; H.
heterocarpa; Amazonian Brazil), Endopleura (1; E. uchi;
Amazonian Brazil), Humiriastrum (c 16; Central America to southeastern
Brazil), Sacoglottis (8–9; Central America, tropical South America,
one species, S. gabonensis, in tropical West and Central Africa),
Schistostemon (c 9; tropical South America).
The sister-group relationships of Humiriaceae are unresolved.
Vantanea, with three or more staminal
whorls, is sister to the remaining Humiriaceae, according to
Herrera & al. (2010).
 |
One of two most-parsimonious cladograms of
Humiriaceae
based on morphology (Herrera & al. 2010).
|
de Jussieu in Gen. Plant.: 254, 4 Aug 1789
[’Hyperica’], nom. cons.
Ascyraceae Plenck,
Elem. Termin. Bot.: 162. 1796 [’Asciroideae’];
Hypericales Juss. ex Bercht. et J. Presl, Přir.
Rostlin: 218. Jan-Apr 1820 [‘Hypericinae’]
Genera/species 6/475–485
Distribution Temperate regions
on both hemispheres, tropical mountains, tropical regions in Africa, Madagascar
and America.
Fossils Unknown.
Habit Bisexual, evergreen or
deciduous trees, shrubs or herbs. Species in dry areas sometimes with
lignotuber.
Vegetative anatomy Phellogen
ab initio superficial or pericyclic. Polyderm often present. Endodermis
sometimes prominent. Vessel elements usually with simple (sometimes
scalariform? or opposite) perforation plates; lateral pits alternate, bordered
pits present. Imperforate tracheary xylem elements fibre tracheids with simple
or bordered pits (also vasicentric tracheids), septate (Hypericum) or
non-septate. Wood rays uniseriate or multiseriate, homocellular or
heterocellular. Axial parenchyma paratracheal scanty, vasicentric, reticulate,
or banded, or absent. Tyloses sometimes abundant. Sieve tube plastids S type.
Nodes 1:1, unilacunar with one leaf trace. Schizogenous glands, canals and
cavities with resin, balsam or yellow to red secretions with hypericin and
closely allied compounds. Colleters often present. Silica bodies or prismatic
calciumoxalate crystals present in some species.
Trichomes Hairs unicellular or
multicellular, uniseriate or multiseriate, simple or branched, often dendritic
or stellate.
Leaves Usually opposite
(rarely verticillate or alternate), simple, entire, with ? ptyxis. Stipules and
leaf sheath absent. Colleters often present. Petiole vascular bundle
transection arcuate, annular or complex. Venation pinnate, usually
eucamptodromous or brochidodromous. Stomata usually paracytic (sometimes
anomocytic, anisocytic or cyclocytic). Cuticular wax crystalloids as parallel
grouped (usually non-entire) platelets (Hypericum type). Lamina with
glandular dots or glandular lines (pellucid-punctate dots, resin/latex
cavities). Idioblasts or schizogenous glands and canals and cavities present.
Leaf margin usually entire (rarely serrate).
Inflorescence Usually terminal
(rarely axillary), cymose (often thyrsoid, scorpioid?), or flowers solitary.
Flowers Actinomorphic.
Hypogyny. Sepals (two to) four or five, with decussate or quincuncial
aestivation, free. Petals (three or) four or five, often with contorted
aestivation, free. Nectary absent? Disc absent.
Androecium Stamens nine to
more than 650, centrifugally developing. Filaments thin, free or often connate
at base into three to five antepetalous fascicles, free from tepals. Anthers
basifixed or dorsifixed, often versatile, tetrasporangiate, usually introrse
(sometimes extrorse), longicidal (dehiscing by longitudinal slits); connective
often with apical glands. Tapetum secretory. Staminodia three or five
(nectaries?), alternipetalous, or absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains tri(col)porate, shed as monads,
bicellular at dispersal. Exine tectate or semitectate, with columellate
infratectum, perforate, reticulate or microreticulate, sometimes psilate.
Gynoecium Pistil composed of
three to five connate antesepalous carpels. Ovary superior, trilocular to
quinquelocular. Stylodia usually three to five, free or connate at base.
Stigmas expanded, punctate to widened, papillate or non-papillate, usually Dry
type. Pistillodium absent.
Ovules Placentation usually
axile (sometimes parietal). Ovules one to numerous per carpel, anatropous,
bitegmic, tenuinucellar. Micropyle bistomal. Outer integument ? cell layers
thick. Inner integument up to seven cell layers thick. Megagametophyte
monosporous, Polygonum type. Synergids sometimes with a filiform
apparatus? Antipodal cells often proliferating (in Hypericum; up to
seven cells). Endosperm development ab initio nuclear. Endosperm haustoria?
Embryogenesis solanad.
Fruit Usually a loculicidal
and/or septicidal capsule or a berry (rarely a drupe).
Seeds Aril absent. Seeds
sometimes winged. Operculum often? present. Seed coat exotegmic. Testa
sometimes glandular. Exotesta often with tanniniferous epidermal cells.
Endotesta? Exotegmen palisade, with anticlinal cell walls sinuate, low and
lignified (sometimes absent). Endotegmen? Perisperm not developed. Endosperm
sparse or absent. Embryo usually straight (fusiform, sometimes curved),
rudimentary or well differentiated, with or without chlorophyll. Cotyledons
two, often large. Germination phanerocotylar.
Cytology n = 6–12, 14, 16,
18–24
DNA
Phytochemistry Flavonols
(kaempferol, quercetin, myricetin), flavones, biflavonoids, xanthones (e.g.
mangiferin), benzophenones, and polyacetate-derived and other anthraquinones
(vismiones) present. In Hypericum also emodin derivatives, prenylated
phloroglucinol derivatives (hyperforin), and naphthodianthrones
(pseudohypericin and hypericin, possibly synthesized by endophytic relative to
Chaetomium). In Vismieae also anthrones, biemodyles and
closely allied compounds. Ellagic acid not found.
Use Ornamental plants,
medicinal plants, dyeing substances, timber.
Systematics
Hypericeae Choisy, Prodr. Monogr. Hypéric.: 32, 37.
9 Mar 1821. Hypericum
(c 370; temperate regions on both hemispheres, tropical mountains). –
Vismieae Choisy, Prodr. Monogr. Hypéric.: 32, 33. 9
Mar 1821. Vismia (60–65?; tropical Africa, southern Mexico, Central
America, tropical South America), Harungana (1; H.
madagascariensis; tropical Africa, Madagascar, Mauritius),
Psorospermum (40–45; tropical Africa, Madagascar). –
Cratoxyleae Benth. et Hook. f., Gen. Plant. 1: 164. 7
Aug 1862. Cratoxylum (6; C. arborescens, C.
cochinchinense, C. formosum, C. glaucum, C.
maingayi, C. sumatranum; Burma, southern China, Southeast Asia,
Malesia to the Lesser Sunda Islands), Eliea (1; E.
articulata; Madagascar).
Hypericaceae are sister to
Podostemaceae.
 |
Cladogram (simplified) of Hypericaceae based on
DNA sequence data (Ruhfel & al. 2011). Psorospermum and
Harungana are nested inside Vismia in the analyses by
Ruhfel & al. (2013).
|
IRVINGIACEAE (Engl.)
Exell et Mendonça
|
( Back to Malpighiales )
|
Exell et Mendonça, Consp. Fl. Angol. 1: 279,
395. 20 Aug 1951, nom. cons.
Irvingiales Doweld,
Tent. Syst. Plant. Vasc.: xxxi. 23 Dec 2001
Genera/species 3/12
Distribution Tropical West and
Central Africa, Madagascar, Southeast Asia, Malesia.
Fossils The fossil wood
Irvingiaceoxylon dechampsii was reported from the Upper Pliocene/Lower
Pleistocene of Ethiopia (Gros 1983).
Habit Bisexual, evergreen
trees.
Vegetatively anatomy Phellogen
ab initio deeply seated. Vessel elements with simple perforation plates;
lateral pits alternate, simple pits? Imperforate tracheary xylem elements
libriform fibres with simple or bordered pits, non-septate. Wood rays
uniseriate, homocellular. Axial parenchyma apotracheal diffuse?, or
paratracheal aliform, winged-aliform, confluent, reticulate, or banded. Tyloses
abundant. Sieve tube plastids S type. Nodes trilacunar? Secretory cavities with
mucilage; parenchyma without secretory cavities (secretory ducts absent from
stem in Allantospermum). Prismatic calciumoxalate crystals often
frequent.
Trichomes Hairs absent.
Leaves Alternate (distichous),
simple, entire, coriaceous, with revolute ptyxis. Stipules large,
intrapetiolar, enclosing terminal bud, early caducous; leaf sheath absent.
Petiole vascular bundle transection annular. Venation pinnate; secondary veins
subparallel; tertiary veins parallel. Stomata paracytic or anomocytic.
Cuticular wax crystalloids? Epidermis with mucilaginous idioblasts. Mesophyll
with mucilaginous idioblasts and canals, and with sclerenchymatous idioblasts
containing calciumoxalate druses or solitary prismatic crystals. Cristarque
cells abundant. Sclereids present in Irvingia. Leaf margin entire.
Inflorescence Terminal or
axillary, panicle.
Flowers Actinomorphic, small.
Pedicel articulated at base. Hypogyny. Sepals five, with imbricate aestivation,
recurved, persistent, free. Petals five, with cochlear or quincuncial
aestivation, free. Nectariferous disc intrastaminal, massive, lobate.
Androecium Stamens (nine or)
ten, not more than twice as many as petals, diplostemonous. Filaments
incurved-folded in bud, inserted below nectariferous disc, free from each other
and from tepals. Anthers basifixed to slightly dorsifixed, versatile,
tetrasporangiate, introrse (antesepalous stamens) or almost latrorse
(antepetalous stamens), longicidal (dehiscing by longitudinal slits);
connective not protruding. Tapetum secretory. Staminodia absent.
Pollen grains
Microsporogenesis simultaneous? Pollen grains tricolporate, shed as monads,
bicellular at dispersal. Tectum continuous. Exine?, with ? infratectum,
verrucate or striate-rugulate
Gynoecium Pistil composed of
two (Allantospermum, Desbordesia), four or five connate
carpels; carpels median (when two) or antesepalous. Ovary superior, bilocular,
quadrilocular or quinquelocular, synascidiate. Style single, simple, short.
Stigma capitate, unicellular-papillate, type? Pistillodium absent.
Ovules Placentation apical to
axile. Ovule one per carpel, anatropous to hemianatropous, pendulous,
epitropous, bitegmic, crassinucellar. Micropyle bistomal. Outer integument
three cell layers thick. Inner integument three or four cell layers thick,
non-multiplicative. Integumentary tapetum absent. Obturator placental.
Endothelium absent. Megasporangium with cytoplasm-rich peripheral cell layers
filled with starch grains. Megagametophyte monosporous, Polygonum
type. Endosperm development ab initio nuclear. Endosperm haustoria?
Embryogenesis?
Fruit A drupe with one, four
or five single-seeded (or five-seeded) pyrenes (often with radial fibres) or a
bilocular samara (Desbordesia) with persistent calyx.
Seeds Aril absent. Seed coat
testal. Testa thick, strongly sclerotic, multiplicative, highly vascularized.
Tegmen non-multiplicative. Exotegmen fibrous. Perisperm not developed.
Endosperm sparse to copious. Embryo?, chlorophyll? Cotyledons two, large,
cordate. Germination phanerocotylar, epigeal.
Cytology n = ?
DNA
Phytochemistry Very
insufficiently known. Ellagic and gallic acids present. Seed lipids with
myristic acid and lauric acid.
Use Timber, food (edible seeds
from Irvingia gabonensis).
Systematics
Allantospermum (2; A. multicaule: Madagascar; A.
borneense: Borneo), Klainedoxa (2; K. gabonensis, K.
trillesii; tropical West and Central Africa), Irvingia (8;
Central Africa, Southeast Asia, Malesia).
The sister-group relationships of Irvingiaceae are
unresolved.
Allantospermum is sister to
[Klainedoxa+Irvingia], according to Byng & al. (2016).
 |
Cladogram of Irvingiaceae
based on DNA sequence data (Byng & al. 2016)
|
Miquel, Fl. Ned. Ind. 1(2): viii, 494. 30 Sep
1858 [‘Ixionantheae’], nom. cons.
Genera/species 3/c 22
Distribution Tropical Africa,
northeastern India, eastern Himalayas, southern China, Southeast Asia, Malesia
to New Guinea, northeastern South America.
Fossils Unknown.
Habit Usually bisexual (rarely
unisexual), evergreen trees or shrubs.
Vegetative anatomy Phellogen?
Primary medullary strands narrow and wide, alternating. Vessel elements with
simple perforation plates; lateral pits alternate, scalariform or opposite,
simple or bordered pits. Imperforate tracheary xylem elements fibre tracheids
with simple or bordered pits, non-septate (also vasicentric tracheids). Wood
rays uniseriate or multiseriate, homocellular or heterocellular. Axial
parenchyma apotracheal diffuse-in-aggregates, or paratracheal scanty,
reticulate, or banded. Sieve tube plastids S type. Nodes 3:3, trilacunar with
three leaf traces. Mucilage cells and mucilage cavities absent. Tracheoidal
idioblasts often present. Most species with secretory ducts in stem? Silica
bodies sometimes abundant. Prismatic calciumoxalate crystals abundant.
Trichomes Hairs?
Leaves Alternate (usually
spiral, sometimes distichous), simple, entire, with involute ptyxis. Stipules
small lateral (sometimes cauline); leaf sheath absent. Petiole vascular bundle
transection arcuate. Venation pinnate. Stomata paracytic. Cuticular wax
crystalloids as platelets arranged in various ways. Mesophyll in
Ochthocosmus with sclerenchymatous idioblasts (some sclereids with
spiral thickenings). Leaf margin usually serrate (rarely entire). Extrafloral
nectaries sometimes present on lamina.
Inflorescence Terminal or
axillary, corymbose, paniculate or thyrsoid.
Flowers Actinomorphic, small.
Half epigyny. Sepals (four or) five, with imbricate or contorted aestivation,
usually connate at base. Petals (four or) five, with imbricate or contorted
aestivation, often marcescent, free. Nectariferous disc intrastaminal, usually
annular or cupular, free (in Ochthocosmus as interstaminal glands).
Androecium Stamens (four or)
five, antesepalous, or up to 20 (antepetalous stamens paired in
Ixonanthes, arising from common strand). Filaments widened at base,
sigmoid-folded in bud, free from each other and from tepals, free from or
adnate at base to nectariferous disc. Anthers dorsifixed, versatile,
tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits).
Tapetum secretory. Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains tricolporate, shed as monads,
bicellular at dispersal. Exine tectate, with columellate infratectum,
spinulate.
Gynoecium Pistil composed of
usually five (rarely two or four) connate carpels; carpels sometimes divided.
Ovary semi-inferior, sometimes unilocular at apex; locules sometimes divided
into locelli by incomplete secondary septa. Style single, simple, filiform,
folded in bud. Stigma single, capitate to discoid, type? Pistillodium
absent.
Ovules Placentation apical to
axile. Ovules usually two per carpel, anatropous, pendulous, bitegmic,
crassinucellar. Micropyle bistomal. Outer integument ? cell layers thick. Inner
integument approx. four cell layers thick. Obturator placental. Hypostase
present. Endothelium present. Megagametophyte monosporous, Polygonum
type? Endosperm development nuclear? Endosperm haustoria? Embryogenesis?
Fruit Usually a septicidal
(rarely also loculicidal, through secondary septa) capsule, also dehiscing
adaxially, with persistent sepals and petals and, sometimes, persistent central
columella.
Seeds Aril present between
hilum and micropyle, or testa winged at base (Ixonanthes,
Ochthocosmus). Exotegmen fibrous. Endotegmic cells with sinuous
anticlinal walls. Perisperm not developed. Endosperm usually sparse or absent.
Embryo straight, chlorophyll? Cotyledons two, large. Germination?
Cytology n = ?
DNA
Phytochemistry Very
insufficiently known. Ellagic acid and proanthocyanidins present. Alkaloids not
found.
Use Timber.
Systematics
Cyrillopsis (2; C. micrantha, C. paraensis;
northeastern Brazil), Ixonanthes (5; I. chinensis, I.
isocandra, I. khasiana, I. petiolaris, I.
reticulata; northeastern India, eastern Himalayas, southern China,
Southeast Asia, Malesia to New Guinea), Ochthocosmus (c 15; tropical
Africa, northeastern tropical South America, with their largest diversity in
Guayana Highlands).
The sister-group relationship of Ixonanthaceae is
unresolved.
There is no available phylogeny of Ixonanthaceae.
von Martius, Nov. Gen. Sp. Plant. 1: 154, 158.
Jan-Mar 1826 [’Lacistemeae’], nom. cons.
Lacistematales Mart.
in C. F. P. von Martius, Consp. Regn. Veg.: 49. Sep-Oct 1835
[’Lacistemeae’]
Genera/species 2/14–16
Distribution Southern Mexico,
Central America, Jamaica, Colombia to southeastern Brazil, Paraguay, Uruguay
and northern Argentina.
Fossils Unknown.
Habit Bisexual, evergreen
trees or shrubs.
Vegetative anatomy Phellogen
ab initio superficial. Vessel elements with scalariform perforation plates;
lateral pits alternate or opposite, bordered pits. Imperforate tracheary xylem
elements libriform fibres with simple pits, septate or non-septate. Wood rays
uniseriate or multiseriate, heterocellular. Axial parenchyma apotracheal
diffuse or diffuse-in-aggregates. Sieve tube plastids S type. Nodes 3:3,
trilacunar with three leaf traces. Prismatic calciumoxalate crystals
abundant.
Trichomes Hairs?
Leaves Alternate (distichous),
simple, entire, with ? ptyxis. Stipules small, caducous; leaf sheath absent.
Petiole vascular bundle transection D-shaped or deeply C-shaped; petiole also
with wing bundles. Venation pinnate, brochidodromous. Stomata anomocytic (to
anisocytic). Cuticular wax crystalloids? Mesophyll with calciumoxalate druses.
Leaf margin usually serrate (rarely entire); salicoid teeth absent.
Inflorescences Axillary,
racemose spike (Lozania) or catkin-like (Lacistema). Bracts
large and imbricate (Lacistema), or small (Lozania).
Flowers Actinomorphic, small.
Receptacle distinctly hollow, widened into fleshy concave disc. Hypogyny.
Sepals (one or) two to six, unequal in size, free (sometimes absent). Petals
absent. Nectariferous disc intrastaminal?, irregularly lobate, fleshy.
Androecium Stamen single.
Filament inserted at or in disc, free from tepal. Anther basifixed,
non-versatile, tetrasporangiate (thecae separated and sometimes individually
stipitate), introrse, longicidal (dehiscing by longitudinal slits); connective
widened. Tapetum secretory? Staminodia absent.
Pollen grains
Microsporogenesis simultaneous? Pollen grains tricolporate, shed as monads,
bicellular at dispersal. Exine semitectate, with columellate infratectum,
reticulate.
Gynoecium Pistil composed of
two or three connate carpels; median carpel adaxial. Ovary superior,
unilocular. Style single, shortly bifid or trifid. Stigmas two or three,
punctate, type? Pistillodium absent.
Ovules Placentation parietal.
Ovules one or two per carpel (two to six per ovary), anatropous, pendulous,
bitegmic, crassinucellar. Micropyle ?-stomal. Outer integument ? cell layers
thick. Inner integument ? cell layers thick. Funicle long, thick.
Megagametophyte monosporous, Polygonum type. Endosperm development ab
initio nuclear. Endosperm haustoria? Embryogenesis?
Fruit A usually single-seeded
(sometimes two- or three-seeded) loculicidal? capsule, in Lozania on
inner side with funicular? hair-like processes with thick unlignified cell
walls.
Seeds Aril present? Testa
sometimes carnose sarcotesta. Tegmen? Perisperm not developed. Endosperm
copious, oily. Embryo usually long (rarely short), straight, well
differentiated, chlorophyll? Cotyledons two, foliaceous. Germination?
Cytology n = 22, c. 31
DNA
Phytochemistry Virtually
unknown. Aluminium accumulated.
Use Timber.
Systematics Lacistema
(c 11; southern Mexico, Central America, Jamaica, tropical South America),
Lozania (3–5; L. glabrata, L. grandiflora, L.
klugii, L. mutisiana, L. pittieri; Central America,
tropical South America).
Lacistemataceae are
probably sister to Salicaceae.
Perleb, Vers. Arztneikr. Pfl.: 107. Mai 1818
[’Lineae’], nom. cons.
Linales Bercht. et J.
Presl, Přir. Rostlin: 239. Jan-Apr 1820 [‘Linicinae’];
Hugoniaceae Arn. in Wight et Arnott, Prodr. Fl. Ind.
Orient. 1: 71. 22 Sep 1834
Genera/species 7/250–270
Distribution
Subcosmopolitan.
Fossils Uncertain. Fossil
pollen grains assigned to Linaceae have been described from
Miocene layers (Muller 1981).
Habit Bisexual, evergreen
trees, shrubs, suffrutices or lianas, perennial, biennial or annual herbs. Some
species are xerophytes. Hugonieae are often lianas with tendrils
formed by basal branches of the inflorescences.
Vegetative anatomy Phellogen?
Vessel elements with simple or scalariform perforation plates; lateral pits
usually alternate, simple or bordered pits. Imperforate tracheary xylem
elements long tough tenacious tracheids or fibre tracheids with bordered pits,
non-septate (also vasicentric tracheids). Wood rays uniseriate or multiseriate,
heterocellular. Axial parenchyma apotracheal diffuse?, or paratracheal aliform,
lozenge-aliform, winged-aliform, or vasicentric (often difficult to define).
Tyloses frequent. Sieve tube plastids S type. Nodes 1:1, unilacunar with one
leaf trace (i.a. Linum), or 3:3, trilacunar with three traces. Cortex
in Hugonieae often with cristarque cells (in Lineae rarely).
Prismatic calciumoxalate crystals often frequent.
Trichomes Hairs unicellular or
multicellular, simple or branched, sometimes stellate; glandular hairs with
multicellular head sometimes present.
Leaves Alternate (spiral) or
opposite, simple, entire, usually with flat, involute or conduplicate ptyxis.
Stipules lateral, small, caducous, or absent, in Linum modified into
glands (extrafloral nectaries); leaf sheath absent. Petiole vascular bundle
transection arcuate. Venation pinnate. Stomata usually paracytic. Cuticular wax
crystalloids as parallel platelets. Epidermis in Lineae often with
mucilaginous idioblasts. Mesophyll at least in Hugonia with
sclerenchymatous idioblasts. Leaf margin serrate or entire. Extrafloral
nectaries sometimes (Linum) present on lamina.
Inflorescence Terminal or
axillary, cymose of various shape (in Anisadenia spicate), often with
partial inflorescences as cincinni or dichasia, or raceme, spike or panicle (in
Hugonieae).
Flowers Usually actinomorphic
(in Hugonieae often slightly zygomorphic). Pedicel articulated.
Hypogyny. Sepals (four or) five, with imbricate quincuncial aestivation,
persistent, free or connate at base. Petals (four or) five, with imbricate or
contorted aestivation, caducous, free or connate at base (in Lineae
usually clawed). Nectariferous glands extrastaminal, adnate to staminal tube or
petal bases, or absent. Disc extrastaminal, annular or consisting of separate
glands alternating with stamens. Flowers triheterostylous or diheterostylous in
numerous species of Lineae, triheterostylous sometimes in
Hugonieae (at least in Hugonia serrata).
Androecium Stamens in
Lineae (four or) five, usually haplostemonous, antesepalous,
alternipetalous (in Anisadenia obhaplostemonous, alternisepalous,
antepetalous); in Hugonieae 5+5, diplostemonous. Filaments in
Lineae often widened in lower part, connate at base into tube and
sometimes with staminodia, free from tepals; filaments in Hugonieae
connate into tube, free from tepals. Anthers dorsifixed, versatile,
tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits).
Tapetum secretory. Staminodia two to five, extrastaminal, tooth-like or
filiform or glandular, alternating with stamens, or absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually tricolpate or
tricolporate (rarely tetracolpate, tetracolporate, hexacolpate,
zonopolycolpate, zonopolyporate, zonopolyforate or inaperturate), starchy, shed
as monads, tricellular at dispersal. Exine tectate or semitectate, with
columellate or acolumellate infratectum, reticulate or microreticulate,
spinulate, gemmate or verrucate.
Gynoecium Pistil composed of
(two or) three to five (to ten; in Anisadenia two) connate carpels;
carpels antepetalous or median carpel adaxial. Ovary superior, unilocular or
(bilocular or) trilocular to quinquelocular (to decemlocular; ovary sometimes
unilocular only at apex); locules in Lineae usually (not in
Anisadenia) divided into locelli by an individual incomplete secondary
septum from carpellary midvein, synascidiate. Style single, simple, or stylodia
(two or) three to five, free or connate at base. Stigmas capitate, papillate,
Dry type. Pistillodium absent.
Ovules Placentation usually
axile (in Hugonieae sometimes apical). Ovules (one or) two per carpel,
anatropous, pendulous, epitropous? (micropyle directed upwards and outwards),
bitegmic, usually crassinucellar (in Linum partially tenuinucellar).
Micropyle bistomal or endostomal. Outer integument two or three cell layers
thick. Inner integument three to twelve cell layers thick (Lineae).
Obturator placental at least in Hugonieae. Parietal tissue four to six
cell layers thick. Archespore sometimes multicellular. Endothelium present.
Megagametophyte monosporous, Polygonum type. Synergids sometimes with
a filiform apparatus. Antipodal cells in Linum three ephemeral nuclei
(not cells). Endosperm development ab initio nuclear or helobial. Endosperm
haustorium chalazal. Embryogenesis solanad.
Fruit In Lineae
usually a septicidal capsule (rarely a drupe or nut; in Anisadenia a
bipartite schizocarp); in Hugonieae usually a drupe (sometimes with
septicidal dehiscence; sometimes schizocarp) with persistent calyx.
Seeds Aril absent; arillodium
present in some Hugonieae. Seed coat exotegmic. Exotesta in
Lineae with outer cell walls massively thickened. Mesotesta in
Hugonieae with sclerotic cells. Endotesta in Hugonieae
lignified. Tegmen highly multiplicative, sometimes reduced. Exotegmen fibrous.
Endotegmen pigmented endothelium. Perisperm not developed. Endosperm copious to
sparse or absent. Embryo usually straight (rarely somewhat curved), well
differentiated, in Lineae oily and with chlorophyll. Cotyledons two.
Germination phanerocotylar.
Cytology x = 6, 8–12
(Lineae); x = 6, 12, 13 (Hugonieae)
DNA Plastid genes
rps16 and clpP and ORF244 absent (lost) (Linum
grandiflorum). Mitochondrial coxI intron present
(Linum).
Phytochemistry Insufficiently
known. Ellagic and gallic acid present in at least Lineae. Saponins
and cyanogenic compounds present in Hugonieae. Alkaloids?
Proanthocyanidins? Flavonols not found.
Use Ornamental plants
(Linum, Reinwardtia), textile and paper (fibres from
Linum usitatissimum), seed oils (Linum usitatissimum, also as
medicine for constipation treatment and as forage), timber, medicinal plants,
fruits (Hugonia).
Systematics The sister-group
relationships of Linaceae
are unresolved.
Hugonieae Meisn.,
Plant. Vasc. Gen.: Tab. Diagn. 35, Comm. 27. 21-27 Mai 1837
[‘Hugoniaceae’]
3/50–57. 'Roucheria' (7;
tropical South America; non-monophyletic), Hebepetalum (3–10; H.
humiriifolium, H. neblinae, H. roraimense; northern
South America), Hugonia
(c 40; Africa and Madagascar to New Caledonia and Fiji). – Pantropical.
Trees, shrubs or often lianas with branch tendrils. Vessel elements with
scalariform perforation plates. Sclereids present. Leaves spiral or distichous.
Stipules sometimes pectinate. Stomata with usually lignified subsidiary cells
lobed beneath guard cells. Leaf margin serrate. Sepals often unequal. Petals
sometimes slightly clawed. Disc present at filament bases. Stamens five longer
and five shorter, diplostemonous. Carpels two to five. Ovary locules without
pseudosepta. Micropyle in ‘Roucheria’ endostomal. Outer integument
two or three cell layers thick. Inner integument three to five cell layers
thick. Hypostase present. Fruit a drupe (sometimes with septicidal dehiscence)
or a schizocarp. Arillode poorly developed or absent. Testa multiplicative.
Mesotesta with sclerotic cells. Endotesta lignified. Exotegmen poorly lignified
or tegmen reduced. Endosperm copious to sparse. Cotyledons large. x = 6, 12,
13. Ellagic acids?
Lineae Rchb., Fl.
Germ. Excurs. 2(2): 830, 831. 1832
4/200–210. Anisadenia (2–3;
A. khasyana, A. pubescens, A. saxatilis; the
Himalayas to central China and northern Thailand), Reinwardtia (1;
R. indica; northern Pakistan, northern India, southern Himalayas,
China, Southeast Asia), Tirpitzia (2–3; T. bilocularis,
T. ovoidea, T. sinensis; southwestern China, northern
Thailand, Vietnam), Linum (c
200; temperate and subtropical regions on both hemispheres). – Temperate and
subtropical regions on both hemispheres, Southeast Asia. Usually perennial or
annual herbs (rarely shrubs). Vessel elements with simple perforation plates.
Wood rays uniseriate. Nodes 1:1, unilacunar with one leaf trace (Linum).
Leaves opposite or alternate (spiral), usually with conduplicate ptyxis.
Stipules usually present. Leaf margin serrate or entire. Cuticular wax
crystalloids as parallel platelets. Sepals more or less equal. Petals clawed.
Nectaries extrastaminal or at petal bases. Stamens five, antesepalous,
alternating with staminodia. Pollen grains tripantocolpate, tripantocolporate
or inaperturate, at least sometimes starchy, tricellular at dispersal. Ovary
locules usually divided. Stigma Dry or Wet type. Ovules tenuinucellar.
Micropyle sometimes bistomal. Outer integument two or three cell layers thick.
Inner integument three to twelve cell layers thick. Integumentary endothelium
present. Obturator present. Archespore usually unisporangiate (rarely
multisporangiate). Endosperm development sometimes helobial. Endosperm
haustorium chalazal. Fruit usually a septicidal capsule (rarely a schizocarp
with two-seeded mericarps dehiscing adaxially along pseudosepta). Seeds often
with mucilage cells. Exotesta with outer cell walls massively thickened. Cross
cells present beneath exotegmen. Endosperm sparse. Embryo with chlorophyll (Linum). n =
6, (8) 9 (11–18 etc.). Ellagic acid sparsely present or absent.
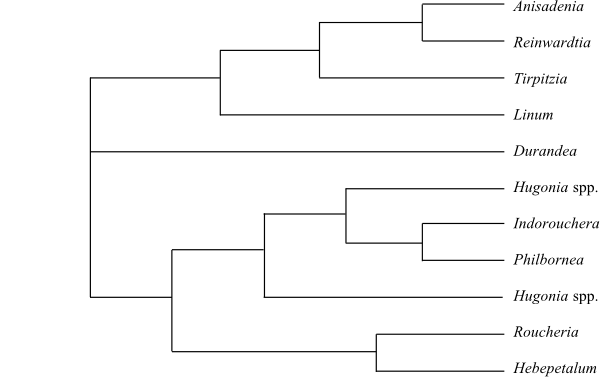 |
Maximum likelihood tree of Linaceae based on DNA
sequence data (McDill & al. 2009; McDill & Simpson 2011). Most
of the branches have low or relatively low support.
|
LOPHOPYXIDACEAE (Engl.)
H. Pfeiffer
|
( Back to Malpighiales )
|
Pfeiffer in Revista Sudamer. Bot. 10: 4. 1951
Genera/species 1/1
Distribution Malesia, islands
in western Pacific.
Fossils Unknown.
Habit Monoecious, usually
climbing evergreen shrub (rarely tree). Tendrils consisting of modified leaves?
(inflorescences?). Lateral bud present at branch bases.
Vegetative anatomy Phellogen
subepidermal. Vessel elements with simple perforation plates; lateral pits?
Imperforate tracheary xylem elements fibres with numerous minutely bordered
pits. Wood rays uniseriate to multiseriate. Axial parenchyma scarce and
paratracheal. Intraxylary phloem present. Phloem stratified. Sieve tube
plastids S type? Nodes unilacunar? Crystals as solitary rhomboids and
clusters.
Trichomes Hairs
unicellular.
Leaves Alternate (spiral),
simple, entire, with ? ptyxis. Stipules small; leaf sheath absent. Petiole
vascular bundle transection arcuate. Venation pinnate. Stomata paracytic.
Cuticular wax crystalloids? Leaf margin serrate or crenate.
Inflorescence Axillary,
panicle consisting of dense globular partial inflorescences.
Flowers Actinomorphic, small.
Hypogyny. Sepals five, with valvate aestivation, persistent, free or connate at
base. Petals five, minute, with open aestivation, inflexed in bud, free.
Nectariferous disc in female flowers as (staminodial?) glands, partially
covering petal bases; cordate nectariferous glands in male flowers adnate to
petals, in female flowers connate and forming quinquelobate nectariferous
disc.
Androecium Stamens five,
haplostemonous, antesepalous, alternipetalous. Filaments filiform, free from
each other and from tepals. Anthers basifixed, non-versatile, tetrasporangiate,
introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory?
Staminodia absent (or as nectariferous lobes?).
Pollen grains
Microsporogenesis simultaneous? Pollen grains tri- or tetracolporate, shed as
monads, bicellular? at dispersal. Exine?, with ? infratectum, sculpturing?
Gynoecium Pistil composed of
(four or) five connate antepetalous carpels. Ovary superior, synascidiate,
(quadrilocular or) quinquelocular. Style single, (quadrilobate or)
quinquelobate, very short or absent. Stigmas subulate, non-papillate, type?
Pistillodium, very small, present in male flowers.
Ovules Placentation (apical
to) axile. Ovules two per carpel, anatropous, pendulous, epitropous, bitegmic,
weakly crassinucellar or incompletely tenuinucellar. Micropyle endostomal.
Outer integument three cell-layers thick, without vascular bundles. Inner
integument five or six cell-layers thick, without vascular bundles. Obturator
funicular, small. Endothelium present. Megasporangium disintegrating. Nucellar
beak absent. Nucellar cap absent. Megagametophyte monosporous,
Polygonum type. Endosperm development nuclear? Endosperm haustoria?
Embryogenesis?
Fruit A single-seeded
five-winged samara.
Seeds Aril present. Testa?
Exotegmen fibrous. Endotegmen? Perisperm not developed. Endosperm copious.
Embryo straight, well differentiated, chlorophyll? Cotyledons two.
Germination?
Cytology n = ?
DNA
Phytochemistry Unknown.
Calciumoxalate druses present in floral organs.
Use Unknown.
Systematics
Lophopyxis (1; L. maingayi; the Malay Peninsula, Borneo, East
Malesia to New Guinea, Palau, Solomon Islands, the Caroline Islands).
Lophopyxis may be sister-group to
Putranjivaceae.
Don in Edinburgh New Philos. J. 2: 321. 1827,
nom. cons.
Malesherbiales D. Don
in C. F. P. von Martius, Consp. Regn. Veg.: 50. Sep-Oct 1835 [‘Malesherbiaceae’]
Genera/species 1/27
Distribution The Andes of
southern Peru, northern Chile and western Argentina.
Fossils Unknown.
Habit Bisexual, evergreen
shrubs or suffrutices, or usually perennial (rarely annual) herbs.
Evil-smelling. Often densely hairy.
Vegetative anatomy Phellogen
ab initio usually superficial (sometimes cortical). Primary medullary strands
narrow. Vessel elements usually with simple (sometimes scalariform) perforation
plates; lateral pits alternate, scalariform or pseudoscalariform. Imperforate
tracheary xylem elements libriform fibres with small pits (vascular tracheids
sometimes present in late wood; also vasicentric tracheids). Wood rays
uniseriate or multiseriate, heterocellular. Axial parenchyma paratracheal
vasicentric, scanty, more or less scarce or absent. Tyloses sometimes present.
Phloem fibres often present. Sieve tube plastids S type; sieve tubes with
non-dispersive protein bodies? Nodes 1:1, unilacunar with one leaf trace, or
3:3, trilacunar with three traces. Rhomboidal calciumoxalate crystals single,
few or absent; druses present in pits and cortex of some species.
Trichomes Hairs unicellular or
multicellular, multiseriate, simple or branched; glandular hairs excreting
nasty-smelling substance often present.
Leaves Alternate (spiral),
usually simple (sometimes pinnately compound), entire or often deeply lobed,
with ? ptyxis. Stipules absent (or foliaceous); small stipule-like leaves often
present (prophylls of axillary buds or stipules?) at bases of leaves and
bracts; leaf sheath absent. Stipules and/or prophylls and leaf primordia with
colleters? Venation pinnate. Stomata anomocytic. Cuticular wax crystalloids?
Leaf margin usually serrate or crenate (sometimes entire).
Inflorescence Axillary?,
simple or compound panicle (rarely fascicle) or raceme, or flowers solitary.
Flowers Actinomorphic.
Hypogyny. Sepals and petals connate into tubular, infundibuliform or
campanulate persistent chartaceous 0,5–5 cm long hypanthium-like perigone.
Sepals five, with valvate aestivation, free. Petals five, with valvate (to
cochlear) aestivation, clawed, sometimes with thin corona with denticulate
margin (as long as or slightly longer than remaining part of corolla), free.
Androgynophore present, lobate and hairy, with nectary at base. Disc absent.
Heterostyly present in almost all species.
Androecium Stamens five,
protruding, haplostemonous, antesepalous, alternipetalous. Filaments free from
each other and from tepals, inserted at androgynophore. Anthers dorsifixed,
versatile?, tetrasporangiate, introrse or extrorse, longicidal (dehiscing by
longitudinal slits). Tapetum secretory? Staminodia absent.
Pollen grains
Microsporogenesis simultaneous? Pollen grains usually tricolporate (rarely
syncolporate), shed as monads, ?-cellular at dispersal. Exine semitectate, with
columellate infratectum, reticulate.
Gynoecium Pistil composed of
three (or four) connate carpels. Ovary superior, unilocular, inserted at
androgynophore. Stylodia three (or four), filiform, free, inserted below ovary
apex. Stigmas capitate or clavate, type? Pistillodium absent.
Ovules Placentation parietal.
Ovules c. 30 to more than 100 per ovary, anatropous, bitegmic, crassinucellar.
Micropyle endostomal. Outer integument ? cell layers thick. Inner integument
one to three cell layers thick. Chalazal part well developed and forming large
outgrowth. Megagametophyte monosporous, Polygonum type. Endosperm
development? Endosperm haustoria? Embryogenesis?
Fruit A stalked capsule,
enclosed by persistent hypanthium-like perigonal tube.
Seeds Aril absent. Testa with
pores. Exotestal cells arranged in rows? Endotesta well developed, with inner
epidermis palisade of stone cells. Exotegmen palisade? Endotegmen persistent?
Perisperm not developed. Endosperm fleshy, oily and with aleurone. Embryo
straight, well differentiated, chlorophyll? Cotyledons two. Germination?
Cytology n = 7, 14
DNA
Phytochemistry Insufficiently
known. Cyclopentenoid cyanogenic glycosides and/or cyclopentenylic fatty acids
present. Tannins?
Use Medicinal plants.
Systematics
Malesherbia (27; arid and semiarid areas in the the Andes and lowlands
in southern Peru, northern and central Chile and western Argentina, with their
largest diversity in northern Chile).
Malesherbia is sister-group to [Passifloraceae+Turneraceae].
de Jussieu, Gen. Plant.: 252. 4 Aug 1789
[’Malpighiae’], nom. cons.
Malpighiopsida
Bartl., Ord. Nat. Plant.: 227, 357. Sep 1830 [’Malpighinae’];
Malpighiineae Engl., Syllabus, ed. 2: 139. Mai
1898
Genera/species c
74/1.385–1.445
Distribution Tropical and
subtropical regions on both hemispheres, with their highest diversity in
tropical South America.
Fossils Pollen grains have
been described from the mid-Eocene and leaves, Banisteriophyllum and
Malpighiastrum, are known from Early Cenozoic sites. Eoglandulosa
warmanensis comprises flowers from the Eocene of southeastern North
America and supposedly belong in Malpighiaceae.
Habit Usually bisexual (rarely
polygamomonoecious), evergreen or deciduous trees, shrubs, suffrutices or
lianas.
Vegetative anatomy Phellogen
ab initio usually superficial (sometimes deeply seated, almost near
endodermis). Secondary lateral growth normal or anomalous. Vessel elements with
simple perforation plates; lateral pits alternate, bordered pits. Vestured pits
present. Imperforate tracheary xylem elements libriform fibres with simple or
bordered pits, septate or non-septate (also vasicentric tracheids). Wood rays
uniseriate or multiseriate, heterocellular. Axial parenchyma apotracheal
diffuse or diffuse-in-aggregates, or paratracheal confluent, vasicentric,
scalariform, reticulate, or banded, or absent. Tyloses often abundant.
Secondary phloem often stratified into hard fibrous and soft parenchymatous
zones. Intraxylary phloem present or absent. Sieve tube plastids S0
type, without starch or protein inclusions. Nodes usually 3:3, trilacunar with
three leaf traces (sometimes 1:1, unilacunar with one trace). Secretory cells
abundant. Laticifers present in Galphimieae. Heartwood often with
gum-like substances. Crystals absent?
Trichomes Hairs unicellular,
furcate and often T-shaped – T-hairs, malpighiaceous hairs, balance hairs,
medifixed hairs – or multicellular and multi-armed (sometimes simple or
stellate); stinging hairs present in some genera (e.g. Malpighia);
glandular hairs often abundant.
Leaves Usually opposite
(rarely alternate [spiral, Acridocarpus] or verticillate), simple,
usually entire (in Stigmaphyllon lobed), with ? ptyxis. Stipules
cauline/interpetiolar (in e.g. Malpighia sometimes lobate),
intrapetiolar (in trees and shrubs) or petiolar (i.a. Hiraea), free or
connate (often rudimentary; in e.g. Acridocarpus absent); leaf sheath
absent. Petiole vascular bundle transection arcuate; petiole and/or abaxial
side of lamina often with usually two large flattened multicellular
nectariferous glands. Venation usually pinnate (in Stigmaphyllon
sometimes palmate). Stomata usually paracytic. Cuticular wax crystalloids as
rosettes of platelets (Fabales type). Epidermis with or without
mucilaginous idioblasts. Domatia in Acridocarpus as hair tufts. Leaf
margin usually entire (in Stigmaphyllon sometimes serrate);
nectariferous glandular teeth rarely present on leaf margin.
Inflorescence Terminal or
axillary, panicle or raceme- or umbel-like. Bracts often with extrafloral
nectaries.
Flowers Actinomorphic or
zygomorphic (often obliquely zygomorphic). Usually hypogyny (in
Acridocarpus epigyny). Sepals four or five, with imbricate
aestivation, free or slightly connate at base; usually with large paired
abaxial oil-producing glands (epithelial elaiophores) at base and sticking to
legs of neotropical oil-collecting bees. Petals five, with imbricate or
contorted aestivation, often crumpled in bud, usually clawed and often with
hairy, toothed or fringed margin, free; median petal adaxial or abaxial; one
adaxial-lateral petal often larger and differently shaped and coloured than
remaining petals. Nectariferous glands present at sepal bases in Old World
species, or absent. Disc poorly developed or absent.
Androecium Stamens usually
5+5, obdiplostemonous (rarely two or 15 stamens or five antesepalous stamens,
in one or three whorls). Filaments usually connate at base into tube, free from
tepals. Anthers basifixed, non-versatile, tetrasporangiate, introrse,
longicidal (usually dehiscing by longitudinal slits; rarely poricidal,
dehiscing by apical pores); connective sometimes enlarged. Tapetum secretory,
with multinucleate cells. Staminodia one to five, extrastaminal, or absent
(antepetalous stamens sometimes staminodial or absent).
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually tri- to pentacolporate or
tetra- to polyporate, shed as monads, bicellular at dispersal. Exine tectate to
semitectate, with columellate or granular infratectum (sometimes with
anastomosing elements), perforate, reticulate or microreticulate, often
verrucate.
Gynoecium Pistil composed of
usually three (sometimes four or five; in Acridocarpus two) usually
connate carpels; median carpel usually obliquely orientated (carpels rarely
almost free). Ovary usually superior (rarely inferior), usually trilocular
(sometimes quadrilocular or quinquelocular; in Acridocarpus bilocular;
rarely unilocular, apocarpous). Stylodia three (to five; in
Acridocarpus two), usually free (style sometimes single, simple,
subulate). Stigmas asymmetrically capitate or truncate, terminal or
non-terminal, papillate, Dry type. Pistillodium absent.
Ovules Placentation usually
axile to apical. Ovule one per carpel, hemianatropous, pendulous, apotropous,
bitegmic, crassinucellar. Micropyle bistomal or endostomal. Outer integument ?
cell layers thick. Inner integument ? cell layers thick. Nucellar beak present.
Megagametophyte usually tetrasporous, 16-nucleate, Penaea type (in
Galphimia glabra disporous, Allium type). Endosperm
development ab initio nuclear. Endosperm haustoria chalazal. Embryogenesis
solanad (or adventitious). Nucellar polyembryony frequently occurring.
Fruit In syncarpous species a
schizocarp (sometimes with carpophore, sometimes divided into two or three [to
five] nut-, samara- or drupe-like mericarps), a nut or drupe, often with
persistent (sometimes accrescent, sometimes wing-like) calyx and stamens;
septicidal capsule present in someGalphimieae; fruiting carpels in
some apocarpous species fusing and forming secondary syncarp.
Seeds Aril absent. Operculum?
Seed coat usually exotegmic (sometimes endotegmic). Testa? Exotegmen in at
least Byrsonima and Thryallis fibrous. Endotegmen often
fibrous; endotegmic cells lignified, sometimes elongate. Perisperm not
developed. Endosperm very sparse or absent. Embryo large, usually straight to
somewhat curved (rarely hook-shaped, spirally twisted or circinate), oily,
chlorophyll? Cotyledons two. Germination phanerocotylar or cryptocotylar.
Cytology x = 6, 9, 10, 12
(24)
DNA Plastid gene
rps16 lost in at least Malpighia coccigera. Duplication of
CYC genes. Mitochondrial coxI intron present
(Malpighia).
Phytochemistry Insufficiently
known. Condensed tannins present. Flavonols (kaempferol, quercetin), cyanidin,
indole (harmidine) alkaloids, and triterpene saponins sometimes present.
Iridoids? Cyanogenic compounds? Ellagic acid not found. Inulin sometimes
present as carbohydrate reserve.
Use Ornamental plants, fruits
(Malpighia), timber, narcotics (hallucinogens from
Banisteriopsis and Diplopterys).
SystematicsMalphighiaceae
are sister to Elatinaceae.
The clade
[Galphimieae+[Acmanthereae+Byrsonimeae]]
(Byrsonimoideae) is sister-group to the remaining Malpighiaceae (Davis &
Anderson 2010).
Byrsonimoideae W. R.
Anderson in Leandra 67(7): 6. Dec 1977 [1978]
10/215–240. Tropical America. Style
subulate. Stigma terminal.
Byrsonimeae W. R.
Anderson in Leandra 67(7): 7. Dec 1977 [1978]
3/150–175. Blepharandra (6;
B. angustifolia, B. cachimbensis, B. fimbriata,
B. heteropetala, B. hypoleuca, B. intermedia;
southern Venezuela, Guyana, Amazonian Brazil), Diacidia (11; Colombia,
Venezuela, Brazil, with their largest diversity in the Venezuelan Highlands),
Byrsonima (135–160; southeastern Florida, southern Mexico, Central
America, the West Indies, tropical South America to southeastern Brazil). –
Tropical America.
Acmanthereae W. R.
Anderson in Leandra 67(7): 11. Dec 1977 [1978]
3/c 23. Pterandra (c 15;
Panamá to Brazil), Coleostachys (1; C. genipifolia; southern
French Guiana, northern Brazil), Acmanthera (7; A. cowanii,
A. duckei, A. fernandesii, A. latifolia, A.
longifolia, A. minima, A. parviflora; Brazil). –
Tropical America.
Galphimieae Nied. in
Engler et Prantl, Nat. Pflanzenfam. III, 4: 53, 67. Dec 1890
4/41. Lophanthera (5; L.
hammelii, L. lactescens, L. longifolia, L.
pendula, L. spruceana; Costa Rica, Amazonian Brazil),
Spachea (7; S. correae, S. elegans, S.
herbert-smithii, S. martiana, S. membranacea, S.
perforata, S. tricarpa; southern Central America, Cuba, Trinidad,
northern tropical South America), Galphimia (27; southern Texas,
Mexico to Nicaragua, tropical South America, with their highest diversity in
Mexico), Verrucularia (2; V. glaucophylla, V.
piresii; Brazil). – Tropical America. Laticifers articulated.
Malpighioideae
Burnett, Outl. Bot.: 894, 1093, 1126. Jun 1835
c 63/1.170–1.205. Pollen grains
tetraporate to polyporate. Stigma usually not terminal. Fruit winged. – The
Acridocarpus clade is sister to the remaining Malpighioideae
(Davis & Anderson 2010).
Acridocarpus clade
2/c 33. Acridocarpus (c 30;
tropical and subtropical Africa, Madagascar, the Mascarene Islands, the Arabian
Peninsula, India, one species, A. austrocaledonicus, in New
Caledonia), Brachylophon (3; B. acuminatum, B.
anastomosans, B. curtisii, tropical regions in the Old World).
– Tropical regions in the Old World. Leaves spiral. Stipules absent. Epigyny.
Two carpels fertile.
Mcvaughia clade
3/7. Mcvaughia (1; M.
bahiana; Bahia in Brazil), Burdachia (3; B. duckei,
B. prismatocarpa, B. sphaerocarpa; Amazonian Colombia and
Venezuela, Guyana, Amazonian Brazil, eastern Peru), Glandonia (3;
G. macrocarpa, G. prancei, G. williamsii; Amazonian
Colombia, southern Venezuela, Amazonian Brazil). – Tropical South America.
Barnebya clade
1/2. Barnebya (2; B.
dispar, B. harleyi; eastern Brazil). – Trees or lianas.
Ptilochaeta clade
4/13. Lasiocarpus (5; L.
ferrugineus, L. multiflorus, L. ovalifolius, L.
salicifolius, L. triflorus; Mexico), Ptilochaeta (6;
P. bahiensis, P. densiflora, P. diodon, P.
elegans, P. glabra, P. nudipes; tropical and subtropical
South America), Dinemandra (1; D. ericoides; arid regions in
Peru and Chile), Dinemagonum (1; D. gayanum; Chile). –
Tropical and subtropical America.
Tristellateia clade
6/105–110. Echinopterys (2;
E. eglandulosa, E. setosa; Mexico), Tristellateia (c
20; Madagascar, one species, T. africana, in tropical Africa, one
species, T. australasiae, in tropical Asia and eastwards to tropical
Australia and New Caledonia), Heladena (1; H. multiflora;
southern Brazil, Paraguay, northeastern Argentina), Henleophytum (1;
H. echinatum; Cuba), Thryallis (3; T. brachystachys,
T. laburnum, T. parviflora; southern Brazil, Paraguay,
Bolivia), Bunchosia (c 80; Mexico, Central America, the West Indies,
tropical South America to southeastern Brazil and northern Argentina).
Hiraeeae Griseb. in C.
F. P. von Martius, Fl. Bras. 12(1): 4, 75. 1 Jun 1858
[‘Hiraeaceae’]
5/77–82. Lophopterys (6;
L. euryptera, L. floribunda, L. inpana, L.
peruviana, L. splendens, L. surinamensis; tropical South
America), Adelphia (4; A. hiraea, A. macrophylla,
A. mirabilis, A. platyrachis; Central America, the West
Indies, western South America), Hiraea (55–60; western Mexico,
Central America, Grenada, St. Lucia, tropical South America to southeastern
Brazil, Paraguay and northern Argentina), Psychopterys (8; southern
Mexico, Belize, Guatemala), Excentradenia (4; E. adenophora,
E. boliviana, E. primaeva, E. propinqua; northern
South America). – Tropical America.
Tetrapterys clade
15/320–335. ‘Tetrapterys’
(c 90; tropical America; non-monophyletic), Niedenzuella (16; Central
America, tropical South America), Dicella (7; D. bracteosa,
D. nucifera, D. aciculifera, D. conwayi, D.
julianii, D. macroptera, D. oliveirae; Costa Rica,
tropical South America), Tricomaria (1; T. usillo; western
Argentina), Carolus (6; C. anderssonii, C. chasei,
C. chlorocarpus, C. dukei, C. renidens, C.
sinemariensis; Mexico, Central America, the Lesser Antilles, tropical
South America), Hiptage (30–35; Mauritius, Sri Lanka, northern
Pakistan, southern Himalayas to southern China and Taiwan, Southeast Asia,
Malesia to Fiji), Flabellariopsis (1; F. acuminata; tropical
Africa),‘Heteropterys’ (130–140; Mexico, Central America, the
West Indies, tropical South America southwards to southeastern Brazil and
northern Argentina, one species, H. leona, in tropical West Africa;
non-monophyletic), Christianella (5; C. glandulifera, C.
mesoamericana, C. multiglandulosa, C. paludicola, C.
surinamensis; Central America, tropical South America), Jubelina
(6; J. grisebachiana, J. magnifica, J. riparia,
J. rosea, J. uleana, J. wilburii; Central America,
tropical South America), Alicia (2; A. anisopetala, A.
macrodisca; tropical South America), Callaeum (11; western Texas,
Mexico, Central America, tropical South America), Mezia (10; Panamá,
tropical South America), Flabellaria (1; F. paniculata;
tropical Africa), Malpighiodes (4; M. bracteosa, M.
guianensis, M. leucanthele, M. liesneri; southern
Venezuela, Guyana, Suriname, French Guiana, Amazonian Brazil).
Stigmaphyllon
clade
15/445–450. Stigmaphyllon
(113; the Ryukyu Islands, Taiwan, the Philippines to New Guinea, eastern
Queensland, Solomon Islands, New Caledonia, Vanuatu, Micronesia, Palau,
southern Mexico, Central America, the West Indies, tropical South America to
northern Argentina), Bronwenia (11; southern Mexico, Central America,
tropical South America), Diplopterys (c 45; Costa Rica, Panamá,
tropical South America), ‘Banisteriopsis’ (90–95; southern
Mexico, Central America, the West Indies, tropical South America;
non-monophyletic), ‘Sphedamnocarpus’ (18; tropical and southern
Africa, Madagascar; non-monophyletic; incl. Philgamia?),
Philgamia (4; P. brachystemon, P. denticulata,
P. glabrifolia, P. hibbertioides; Madagascar; in
Sphedamnocarpus?), Peixotoa (c 30; Brazil, Paraguay,
Bolivia), Gallardoa (1; G. fischeri; Argentina),
Mionandra (1; M. camareoides; Bolivia, Paraguay, Argentina),
Cordobia (2; C. argentea, C. fischeri; South
America), Cottsia (3; C. californica, C. gracilis,
C. linearis; southern United States, northern Mexico), ’Janusia’
(18; California to Argentina; paraphyletic), ’Aspicarpa’ (c 40;
southern United States, Mexico, Central America, the West Indies, tropical and
subtropical South America; paraphyletic; incl. Gaudichaudia?),
‘Gaudichaudia’ (c 60; Mexico to Bolivia; polyphyletic; in
Aspicarpa?), Camarea (9; eastern South America). –
Pantropical, southern United States.
Ectopopterys clade
1/1. Ectopopterys (1;
E. soejartoi; Colombia, Peru). – Liana.
Amorimia clade
1/10. Amorimia (10; tropical
South America). – Lianas.
Malpighieae DC.,
Prodr. 1: 577. Jan (med.) 1824
c 10/155–170. ’Mascagnia’
pro parte (35–50; Mexico to Argentina; non-monophyletic), Calcicola
(2; C. parvifolia, C. sericea; Mexico),
‘Malpighia’ (c 45; Florida, Mexico, Central America, the West
Indies, tropical South America; non-monophyletic), Aspidopterys (c 25;
tropical Asia), Caucanthus (3; C. albidus, C.
auriculatus, C. edulis; eastern and northeastern Africa, the
Arabian Peninsula), Triaspis (c 15; tropical and southern Africa),
Digoniopterys (1; D. microphylla; Madagascar),
Rhynchophora (2; R. humbertii, R. phillipsonii;
Madagascar), Madagasikaria (1; M. andersonii; southern
Madagascar), Microsteira (c 25; Madagascar). – Eastern Africa,
Madagascar, the Arabian Peninsula, tropical Asia, tropical America. The endemic
Malagasy genera Madagasikaria, Microsteira and
Rhynchophora form a well supported clade, according to Davis
(2002).
Unplaced Malpighiaceae
Rudolphia (1; R.
planisiliqua; Bolivia).
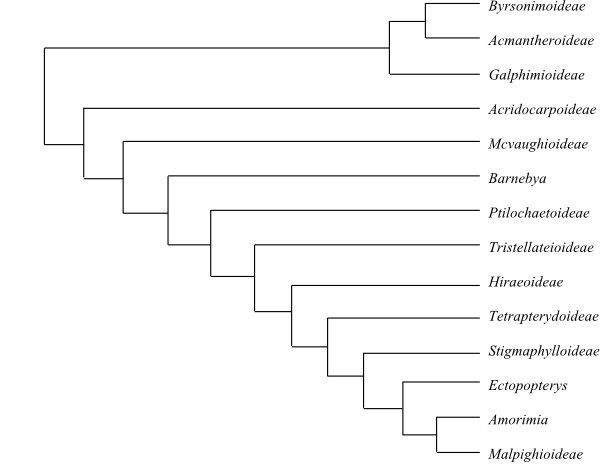 |
Cladogram (simplified) of Malpighiaceae based
on DNA sequence data (Davis & Anderson 2010).
|
MEDUSAGYNACEAE Engler
et Gilg, nom. cons.
|
( Back to Malpighiales )
|
Engler et Gilg, Syllabus, ed. 9 et 10: 280. 6 Nov
1924
Medusagynales Takht.
ex Reveal et Doweld in Novon 9: 551. 30 Dec 1999
Genera/species 1/1
Distribution The
Seychelles.
Fossils Unknown.
Habit Andromonoecious,
evergreen tree. Bark fibrous (resembling Juniperus bark). Flowers
evil-smelling. Buds perulate.
Vegetative anatomy Phellogen
ab initio subepidermal. Secondary lateral growth anomalous. Vessel elements
with simple perforation plates; lateral pits opposite or alternate, bordered
pits. Imperforate tracheary xylem elements tracheids or libriform fibres with
small bordered pits, non-septate? Wood rays multiseriate?, heterocellular.
Axial parenchyma apotracheal diffuse. Secondary phloem stratified into
concentric rings of alternating sieve tissue and sclerenchyma. Sieve tube
plastids S type. Nodes 5:5, pentalacunar with five leaf traces, and two phloic
vascular bundles. Secretory cavities absent. Cristarque cells absent.
Calciumoxalate druses present.
Trichomes Hairs absent.
Leaves Opposite, simple,
entire, coriaceous, with ? ptyxis. Stipules and leaf sheath absent. Paired
colleters present along leaf margin and in leaf axil outside axillary bud.
Petiole vascular bundle transection arcuate; petiole with numerous bundles,
variously orientated. Venation pinnate; tertiary venation highly reticulate.
Stomata anomocytic. Cuticular waxes absent. Mesophyll with mucilaginous
idioblasts, without sclerenchymatous idioblasts. Calciumoxalate druses present
adjacent to vascular strands of midvein. Leaf margin crenate.
Inflorescence Terminal,
panicle.
Flowers Actinomorphic, small.
Hypogyny. Sepals five, with imbricate aestivation, persistent, connate at base.
Petals five, with imbricate aestivation, recurved, caducous, free. Nectary
absent. Disc absent.
Androecium Stamens c. 50 to
more than 100, spirally arranged, arising from five vascular bundles. Filaments
thin, free from each other and from tepals. Anthers basifixed, non-versatile,
tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits).
Tapetum secretory? Staminodia absent.
Pollen grains
Microsporogenesis simultaneous? Pollen grains (2–)3(–4)-porate, shed as
monads, ?-cellular at dispersal. Exine tectate, with columellate infratectum,
finely striate.
Gynoecium Pistil composed of
16 to 19 (to 25) connate carpels, inserted on and adnate to central column;
ventral sutures open below ovular points of insertion. Ovary superior, 16- to
25-locular. Stylodia 16 to 25, free, marginal, inserted in characteristic way
on outer carpel margins (cf. the name Medusagyne). Stigmas capitate,
discoid, type? Pistillodium absent.
Ovules Placentation axile.
Ovules two to five per carpel, anatropous, upper ovules ascending to horizontal
and epitropous, lower ovules descending to pendulous and apotropous, bitegmic,
weakly crassinucellar. Micropyle ?-stomal. Outer integument three or four cell
layers thick. Inner integument three or four cell layers thick. Funicle long.
‘False endothelium’ present on surface of megasporangium. Megagametophyte
monosporous, Polygonum type? Endosperm development? Endosperm
haustoria? Embryogenesis?
Fruit An assemblage of ridged,
verrucose, septicidal follicles with persistent central columella, stigmas and
calyx and carpels acropetally detached – valves detaching from central
columella from base upwards except at apex, eventually umbrella-like with
ventricidal carpels –, adaxially dehiscing (fruit anatomy similar to Caryocaraceae), also
interpreted as a schizocarp with 16 to 25 follicle-like mericarps. Exocarp
without lacunae.
Seeds Aril absent. Testa
winged. Exotesta somewhat thickened. Endotesta crushed. Exotegmen fibrous.
Endotegmen crushed. Perisperm not developed. Endosperm thin. Embryo straight,
chlorophyll? Cotyledons two. Germination phanerocotylar, epigeal or
hypogeal.
Cytology n = ?
DNA
Phytochemistry Insufficiently
known. Tannins and phenolic compounds present.
Use Unknown.
Systematics
Medusagyne (1; M. oppositifolia; Mahé in the Seychelles).
Medusagyne, Quiinaceae and Ochnaceae usually form an
unresolved trichotomy.
According to Schneider & al. (2006), it
requires 16 additional steps (Bremer support) to force Medusagyne into
Ochnaceae.
de Candolle in Nouv. Bull. Sci. Soc. Philom.
Paris 2: 209. Jan 1811, nom. cons.
Ochnales DC. ex
Bercht. et J. Presl, Přir. Rostlin: 224. Jan-Apr 1820 [‘Ochnaceae’];
Sauvagesiaceae (Ging. ex DC.) Dumort., Anal. Fam.
Plant.: 44, 49. 1829; Lophiraceae Loudon, Hort.
Brit.: 513. 30 Aug 1830 [‘Lophireae’];
Sauvagesiales Lindl. in C. F. P. von Martius, Consp.
Regn. Veg.: 50. Sep-Oct 1835 [‘Sauvagesiaceae’];
Gomphiaceae DC. ex Schnizlein, Iconogr. Fam. Regni
Veg. 4: ad t. 248. 1843-1870; Luxemburgiaceae Soler.,
Syst. Anat. Dicot. Ergänz.: 77, 79. Mai 1908;
Ochnanae Doweld, Tent. Syst. Plant. Vasc.: xxxiii. 23
Dec 2001
Genera/species 28/500–505
Distribution Pantropical (few
subtropical species).
Fossils Unknown.
Habit Bisexual, usually
evergreen trees or shrubs (rarely perennial herbs).
Vegetative anatomy Phellogen
ab initio superficial. Cortical and medullary vascular bundles usually present.
Primary vascular tissue cylinder. Secondary lateral growth normal. Vessel
elements usually with simple (sometimes also scalariform) perforation plates;
lateral pits alternate, usually bordered pits. Vestured pits often present
(absent in Sauvagesia). Imperforate tracheary xylem elements usually
fibre tracheids (sometimes libriform fibres) with simple or bordered pits,
usually septate (occasionally non-septate; vasicentric tracheids rare). Wood
rays uniseriate or multiseriate, usually heterocellular (rarely homocellular).
Axial parenchyma apotracheal diffuse or diffuse-in-aggregates, or paratracheal
scanty, vasicentric, or unilateral, or metatracheal. Phloem sometimes (i.a. in
Godoya) stratified into hard fibrous and soft parenchymatous layers.
Sieve tube plastids S type. Nodes ≥3:≥3, trilacunar or multilacunar with
three or more? leaf traces. Mucilage cells and mucilage ducts often present.
Cortex often with cristarque cells. Hyaline latex present in some species.
Heartwood often with gum-like substances. Silica bodies present in some
species. Prismatic calciumoxalate crystals often abundant.
Trichomes Hairs usually absent
(simple hairs sometimes present); glandular hairs sometimes present.
Leaves Alternate (spiral or
distichous), usually simple (rarely pinnately compound), entire, often
coriaceous, usually with conduplicate to flat ptyxis. Stipules extrapetiolar,
scale-like, often fimbriate, in Ouratea with extrafloral nectaries;
leaf sheath absent. Petiole vascular bundle transection annular (sometimes
several bundles arcuately arranged); petiole sometimes with medullary bundles.
Venation pinnate, brochidodromous; secondary veins stout and often densely
parallel and/or tertiary veins parallel. Stomata usually paracytic (rarely
anomocytic). Cuticular waxes absent? Epidermis with or without mucilaginous
idioblasts. Mesophyll often with sclerenchymatous idioblasts, forming
continuous subepidermal layer on adaxial side. Cristarque cells often abundant.
Leaf margin serrate, crenate or entire.
Inflorescence Terminal or
axillary, panicle, fascicle, raceme or umbel, or flowers solitary.
Flowers Usually actinomorphic
(rarely somewhat zygomorphic due to non-uniformly developed androecium).
Pedicel articulated. Hypogyny. Sepals (three to) five (to ten), with imbricate
aestivation, almost membranous (in Lophira non-uniformly growing),
sometimes persistent, usually whorled (rarely spiral), free or connate at base.
Petals (four or) five (to ten), usually with contorted (rarely imbricate)
aestivation, sometimes clawed, caducous, usually whorled (rarely spiral), free.
Nectary absent. Disc absent.
Androecium Stamens five to 25
(to more than 300), in one to five whorls, often in five groups, sometimes on
androphore, sometimes centrifugally developing. Filaments free or more or less
connate, free from tepals. Anthers basifixed, non-versatile, tetrasporangiate,
often latrorse, usually poricidal (dehiscing by apical pores; sometimes
longicidal, dehiscing by longitudinal slits; sometimes locellate). Tapetum
secretory. Staminodia in Sauvagesioideae (five or) ten to c. 25, in
one or several whorls, sometimes petaloid (sometimes connate into tubular
corona), or absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually tricolporate, shed as
monads, usually bicellular (sometimes tricellular) at dispersal. Exine tectate,
columellate infratectum, microperforate or striate-rugulate.
Gynoecium Pistil composed of
(one to) five to ten (to 15) carpels free in upper part and connate in lower
part (in Ouratea secondarily free), or carpels paracarp and entirely
connate (antesepalous when three median carpels adaxial). Ovary superior,
usually unilocular to quinquelocular (rarely up to 15-locular by placental
intrusions), often deeply lobate. Style single, simple, usually long (sometimes
short), sometimes hollow, sometimes gynobasic (e.g. in Ouratea).
Stigmas punctate or somewhat lobate, non-papillate, Dry type. Pistillodium
absent.
Ovules Placentation axile,
parietal or intrusively parietal (marginal?). Ovules one, two or five to more
than 50 per carpel, anatropous to campylotropous, usually ascending (rarely
pendulous), apotropous or epitropous, usually bitegmic, usually more or less
connate (in Lophira unitegmic), incompletely tenuinucellar or weakly
crassinucellar. Micropyle bistomal or endostomal (or integuments connate),
often zigzag. Outer integument two to four cell layers thick. Inner integument
two to four cell layers thick. Hypostase often present. Endothelium absent?
Megagametophyte monosporous, Polygonum type. Synergids with a filiform
apparatus. Antipodal cells usually persistent, often enlarged. Endosperm
development ab initio nuclear. Endosperm haustoria? Embryogenesis? Polyembryony
may occur.
Fruit A berry, drupe
(sometimes an assemblage of drupes) or septicidal capsule (in Ouratea
schizocarp with three to ten drupaceous mericarps situated on fleshy
carpophore; in Lophira capsule with persistent calyx with two
wing-like sepals).
Seeds Aril absent. Testa well
developed or reduced and thin (Lophira), often winged, often
vascularized. Endotesta with small crystalliferous cells. Exotegmen often
fibrous. Endotegmen? Perisperm not developed. Endosperm sparse or absent, with
or without oils. Embryo usually straight (sometimes curved), chlorophyll?
Cotyledons two. Germination phanerocotylar or cryptocotylar.
Cytology n = 12, 14, 24
DNA
Phytochemistry
Biflavonyls/biflavonoids, C-glycoflavones, flavones, biflavones,
lophirone, Ouratea-catechins, cyanidin,vismiones, and anthrones
(3-O-geranylemodin anthrone etc.) present. Condensed tannins present.
Triterpenes and alkaloids rare. Flavonols, ellagic acid, saponins, and
cyanogenic compounds not found.
Use Ornamental plants,
medicinal plants, timber, roofing and basketing (Cespedesia).
Systematics Ochnaceae belong in a trichotomy
also comprising Medusagyne (Medusagynaceae) and Quiinaceae, although in some
analyses Medusagyne is sister to Quiinaceae.
A plausible topology of the Ochnaceae phylogeny is the
following, according to Schneider & al. (2014):
[Testulea+[Luxemburgioideae+[Ochnoideae+Sauvagesioideae]]]
Testulea clade
1/1. Testulea (1; T.
gabonensis; Gabon). – Testulea is sister-group to the remaining
Ochnaceae, according to
Schneider & al. (2014).
[Luxemburgioideae+[Ochnoideae+Sauvagesioideae]]
Luxemburgioideae
Planch. ex Endl., Gen. Plant. Suppl. 5: 98. 1850
[‘Luxembergieae’]
2/22. Luxemburgia (18; Brazil),
Philacra (4; P. auriculata, P. duidae, P.
longifolia, P. steyermarkii; Venezuela, northern Brazil). –
Venezuela, Brazil. Androecium and gynoecium obliquely zygomorphic in bud. One
adaxial stamen fertile; remaining (staminodial) stamens grouped on one side of
gynoecium. Filament connate. Anthers sometimes connate. Exine with small
perforations. Pistil composed of three connate carpels. n = ? –
Luxemburgioideae seem to be sister-group to the remaining Ochnaceae.
[Ochnoideae+Sauvagesioideae]
Staminodia separate, forming lobate disc
or corolla-like tube, or absent. Exine striate-rugulate.
Ochnoideae Burnett,
Outlines Bot.: 886, 1093, 1125. Feb 1835 [‘Ochnidae’]
9/c 385. Lophira (2; L.
alata, L. lanceolata; tropical West and Central Africa),
Elvasia (14–15; Central America, tropical South America),
Campylospermum (c 65; tropical central and East Africa, Madagascar,
tropical Asia), Idertia (2; I. axillaris, I.
morsonii; western and central tropical Africa, São Tomé),
Brackenridgea (10; tropical regions in the Old World),
Ouratea (c 200; tropical regions on both hemispheres),
Rhabdophyllum (4; R. letestui, R. reflexum,
R. ‘rigidum’, R. thonneri; western and central
tropical Africa), Ochna (c
85; tropical regions in the Old World). – Unplaced
Ochnoideae Perissocarpa (3; P. ondox,
P. steyermarkii, P. umbellifera; Venezuela, Peru, northern
Brazil). – Tropical, with their largest diversity in Brazil. Leaves
distichous. Vessel and parenchyma pits not unilaterally compound. Stipules in
Ouratea semi-intrapetiolar. Petiole sometimes with inverted medullary
vascular bundle and subepidermal fibres. Sepals almost membranous (in
Lophira non-uniformly growing). Petals with contorted aestivation.
Stamens diplostemonous or obdiplostemonous, centripetally developing. Anthers
sometimes longicidal (dehiscing by longitudinal slits). Pistil composed of (two
to) five (to 15; in Lophira two) connate carpels, sometimes present on
short gynophore. Style sometimes gynobasic. Floral receptacle sometimes
widened. Ovules usually one (in Lophira numerous) per locule,
apotropous. Integuments connate (except occasionally at apex), together seven
to 17 cell layers thick. Outer integument in Ochna
three or four cell layers thick. Inner integument in Ochna two
or three cell layers thick. Hypostase usually present. Fruit usually a drupe,
with persistent stamens (in Lophira with two sepals modified into
wings). Testa vascularized, without layer of small crystalliferous cells.
Fibrous exotegmen absent. Endosperm absent. Cotyledons massive, variously
arranged. x = 12–14.
Sauvagesioideae
Beilschm. in Flora 16(Beibl. 7): 88, 110. 14 Jun 1833
[‘Sauvagesieae’]
16/90–95. Blastemanthus (2;
B. gemmiflorus, B. grandifloras; northeastern South America),
Godoya (2; G. antioquiensis, G. obovata; the Andes
in Colombia, Peru and Bolivia), Rhytidanthera (2; R.
magnifica, R. splendida; Colombia, Venezuela),
Cespedesia (1–6; C. spathulata; Central America, tropical
South America), Krukoviella (1; K. disticha; Peru, Brazil),
Fleurydora (1; F. felicis; Guinea), Poecilandra (2;
P. pumila, P. retusa; northern tropical South America),
Wallacea (3; W. insignis, W. multiflora, W.
riparia; northern South America), Neckia (1; N. serrata;
West Malesia, the Philippines), Schuurmansia (18; Central Malesia to
New Guinea), Schuurmansiella (1; S. angustifolia;
northwestern Borneo), Euthemis (2; E. leucocarpa, E.
minor; Southeast Asia to Borneo), Tyleria (14; Venezuela, the
Guayana Highlands), Adenarake (2; A. macrocarpa, A.
muriculata; southern Venezuela, northern BrazilVenezuela),
Sauvagesia (c 40; tropical regions on both hemispheres, with their
highest diversity in tropical South America). – Unplaced
Sauvagesioidae Indosinia (1; I.
involucrata; southern Vietnam). – Pantropical, with their highest
diversity in tropical South America. Rarely perennial herbs. Vestured pits
absent in Sauvagesia. Leaves spiral, often with conduplicate-flat
ptyxis (in Rhytidanthera compound). Flowers rarely zygomorphic (with
late developing zygomorphy comprising androecium and gynoecium). Outer sepals
sometimes smaller than remainder. Sauvagesia with five petaloid
antepetalous staminodia and five antesepalous stamens. Exine sometimes with
small perforations. Pistil composed of two, three or five connate carpels; when
three, then median carpel adaxial. Outer integument approx. two cell layers
thick. Inner integument three or four cell layers thick. Testa often winged.
Exotestal cells large, detached. Endotestal cells crystalliferous. Endosperm
with aleurone. x = 18. – Blastemanthus may be sister to the
remaining Sauvagesioideae.
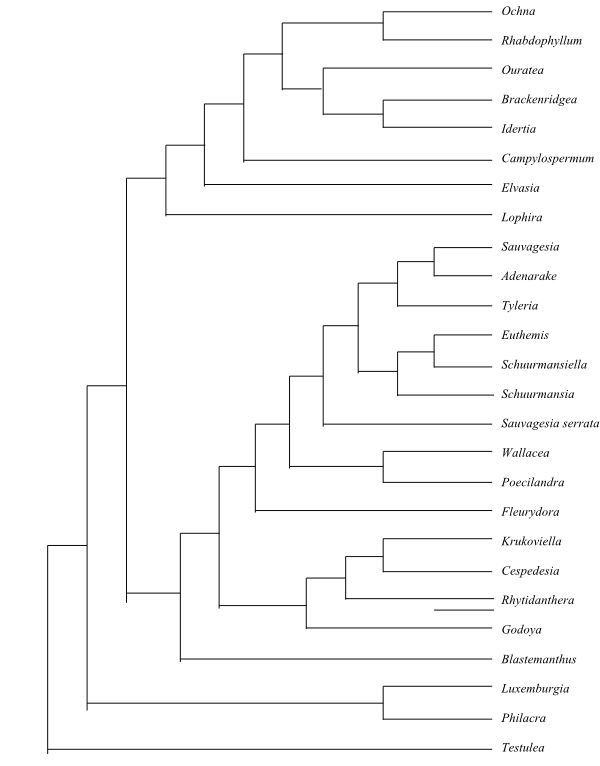 |
Cladogram (simplified) of Ochnaceae based on
Schneider & al. (2014).
|
Engler et Gilg in Engler’s Syllabus, ed. 7:
223. Oct 1912-Mar 1913, nom. cons.
Bennettiaceae R. Br.
ex Schnizlein, Iconogr. Fam. Regni Veg. 3: ad t. 172**. 1846-1866
[’Bennettieae’], nom. illeg.; Pandales
Engl. et Gilg in Engler, Syllabus, ed. 7: 223. Oct 1912-Mar 1913
Genera/species 3/16–17
Distribution Tropical regions
in the Old World.
Fossils Unknown.
Habit Dioecious, evergreen
trees or shrubs. Buds arising in axils of leafy short shoots, although often
not in true leaf axils.
Vegetative anatomy Phellogen?
Primary medullary strands alternately wide and narrow. Vessel elements usually
with scalariform (sometimes also simple) perforation plates; lateral pits
alternate or opposite, simple pits. Non-vestured pits present
(Microdesmis). Imperforate tracheary xylem elements tracheids or fibre
tracheids with simple or bordered pits, non-septate (also vasicentric
tracheids). Wood rays uniseriate or multiseriate, heterocellular. Axial
parenchyma apotracheal diffuse or diffuse-in-aggregates, or paratracheal
scanty, scalariform, or banded. Tyloses frequent. Sieve tube plastids S type?;
sieve tubes with non-dispersive protein bodies. Nodes? Latex and laticifers
absent. Cortex with cristarque cells. Prismatic calciumoxalate crystals
abundant.
Trichomes Hairs unicellular or
multicellular, uniseriate; glandular hairs sometimes present on floral
parts.
Leaves Alternate (spiral and
reduced on orthotropic shoots; distichous on plagiotropic short shoots
resembling pinnately compound leaves, especially in Galearia and
Panda), simple, entire or pinnately lobed, with involute ptyxis.
Stipules small (asymmetrical in Panda), usually persistent (caducous
in Panda); leaf sheath absent. Petiole vascular bundle transection
arcuate. Venation pinnate; one vein proceeding into persistent transparent apex
of leaf tooth. Stomata anomocytic (Microdesmis), paracytic
(Galearia) or cyclocytic (Panda). Cuticular waxes absent.
Lamina with or without glandular dots (pellucid-punctate in
Microdesmis). Mesophyll with sclerenchymatous idioblasts containing
calciumoxalate as druses or solitary prismatic crystals. Leaf margin serrate or
entire.
Inflorescence: Terminal or
axillary, fascicle, thyrsoid or panicle, or flowers solitary axillary.
Flowers Actinomorphic, small.
Hypogyny. Sepals five, with imbricate or open (Panda) aestivation,
free or connate (sepals of female flowers in Microdesmis usually
persistent, with basal glandular hairs). Petals five, with imbricate, contorted
or valvate aestivation, free (in Galearia each enclosing one or two
stamens). Nectary absent. Disc very small or absent.
Androecium Stamens five
antesepalous, or ten, 15 or 5+5 in one or two whorls. Filaments usually free
from each other, free from or partially adnate to tepals. Anthers basifixed,
non-versatile, tetrasporangiate, usually introrse (sometimes latrorse),
longicidal (dehiscing by longitudinal slits); connective sometimes prolonged.
Tapetum secretory. Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains tricolporate, shed as monads,
?-cellular at dispersal. Exine tectate or semitectate, with columellate
infratectum, reticulate or punctate.
Gynoecium Pistil composed of
two to five connate carpels. Ovary superior, bilocular to quinquelocular. Style
single, simple, or stylodia two to five, free or connate at base (rarely
absent). Stigmas not widened, capitate, stigma sometimes bilobate, type? Male
flowers with pistillodium.
Ovules Placentation
axile-apical. Ovule usually one per carpel, orthotropous (Panda) or
anatropous, pendulous, epitropous, bitegmic, weakly crassinucellar. Micropyle
endostomal. Outer integument three to five cell layers thick. Inner integument
three to five cell layers thick. Obturator absent. Megagametophyte monosporous,
Polygonum type. Endosperm development cellular? Endosperm haustoria?
Embryogenesis?
Fruit A drupe (often with
irregularly patterned surface).
Seeds Aril absent. Carunculus
absent. Seed coat exotegmic. Exotesta possibly absent. Endotesta possibly
absent. Exotegmen tracheoidal, fibrous. Endotegmen tanniniferous. Perisperm not
developed. Endosperm copious, oily. Embryo well differentiated, chlorophyll?
Cotyledons two, thin and flat, oily. Germination phanerocotylar.
Cytology n = 15
(Microdesmis)
DNA
Phytochemistry Very
insufficiently known. Alkaloids and/or saponins sometimes present.
Use Timber, carpentries, seed
oils for cooking.
Systematics
Microdesmis (10–11; tropical Africa, southern China, Southeast Asia,
West Malesia to the Philippines), Galearia (5; G. aristifera,
G. celebica, G. filiformis, G. fulva, G.
maingayi; Southeast Asia, Malesia to New Guinea, the Bismarck Archipelago
and Solomon Islands), Panda (1; P. oleosa; tropical West and
Central Africa).
The sister-group relationship of Pandaceae is unresolved.
Microdesmis is sister to
[Panda+Galearia]. Galearia celebica was sister to
Erythrospermum phytolaccoides in analyses by Groppo & al.
(2013).
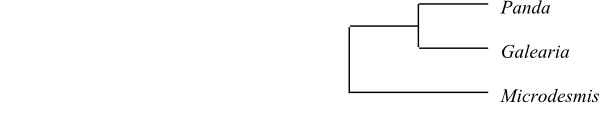 |
Cladogram of Pandaceae based on DNA
sequence data (Wurdack & Davis 2009).
|
Roussel, Fl. Calvados, ed. 2: 334. 1806
[’Passifloreae’], nom. cons.
Passiflorales Juss.
ex Bercht. et J. Presl, Přir. Rostlin: 237. Jan-Apr 1820
[‘Passifloreae’]; Paropsiaceae Dumort.,
Anal. Fam. Plant.: 37, 42. 1829; Smeathmanniaceae
Mart. ex Perleb, Clav. Class.: 33. Jan-Mar 1838
[‘Smeathmannieae’]; Passifloropsida
Brongn., Enum. Plant. Mus. Paris: xxix, 108. 12 Aug 1843
[’Passiflorineae’]; Modeccaceae Horan.,
Char. Ess. Fam: 146. 30 Jun 1847 [‘Turneraceae s.
Modeccaceae’]; Passiflorineae Bessey in C.
K. Adams, Johnson’s Universal Cyclop. 8: 462. 15 Nov 1895
Genera/species 16/620–625
Distribution Tropical,
subtropical and warm-temperate regions on both hemispheres, with their highest
diversity in tropical Africa and tropical America.
Fossils Seeds assigned to
Passiflora have been described from the Miocene of Europe.
Habit Usually bisexual (in,
e.g., Adenia dioecious), usually perennial or annual herbs, usually
climbing and twining with tendrils, or evergreen shrubs or lianas (rarely small
trees). Tendrils simple or branched, axillary, usually consisting of modified
pedicels or inflorescences, often oblique relative to the branch. Some species
are xerophytes.
Vegetative anatomy Phellogen?
Passiflora and its closely allied relatives possessing stem
collenchyma. Axillary buds usually superposed, with accessory bud originating
above axillary inflorescence/tendril bud. Secondary lateral growth usually
normal (in Adesmia anomalous, from concentric cambia). Vessel elements
usually with simple (rarely scalariform) perforation plates; lateral pits
alternate. Imperforate tracheary xylem elements fibre tracheids or libriform
fibres with bordered pits, non-septate (also vasicentric tracheids). Wood rays
multiseriate?, homocellular or heterocellular. Axial parenchyma usually
apotracheal diffuse? Wood often fluorescent. Intraxylary phloem present in some
lianas. Sieve tube plastids S type; sieve tubes with non-dispersive protein
bodies? Nodes 3:3, trilacunar with three leaf traces. Calciumoxalate as druses
and solitary prismatic crystals.
Trichomes Hairs unicellular or
multicellular, usually simple (sometimes stellate).
Leaves Alternate (spiral),
usually simple (rarely palmately compound), entire or palmately lobed, with
conduplicate ptyxis (sometimes modified conduplicate, V-shaped and at end of
each arm Λ-shaped). Stipules often small and caducous, sometimes foliaceous
(absent in Androsiphonia and Barteria); leaf sheath absent.
Stipules and/or prophylls and leaf primordia with colleters? Petiole and/or
stipules often with extrafloral nectaries without nectarostomata. Petiole
vascular bundle transection? Venation pinnate, pedate or palmate; vein
proceeding into transparent and caducous tooth apex? Stomata usually anomocytic
(sometimes paracytic). Cuticular wax crystalloids as rosettes of platelets
(Fabales type). Mesophyll with or without sclerenchymatous idioblasts.
Epidermis sometimes with mucilaginous idioblasts. Calciumoxalate druses and
solitary prismatic crystals frequent. Leaf margin usually serrate (sometimes
entire). Lamina sometimes with extrafloral nectaries.
Inflorescence Usually
axillary, racemose or cymose (flowers rarely solitary). Flowers usually
subtended by three bracts.
Flowers Usually actinomorphic
(rarely zygomorphic, e.g. Passiflora mucronata), usually large.
Hypanthium usually absent (present in some species of Adenia). Usually
half epigyny (rarely hypogyny). Sepals (three to) five (to eight), with
imbricate aestivation, often petaloid, persistent, often connate at base, often
adnate to petal bases. Petals (three to) five (to eight; rarely absent), with
imbricate aestivation, usually with staminodial corona at base consisting of
one to several whorls of c. 15 to more than 50 extrastaminal scales, hairs or
filiform lobes, usually free (rarely connate at base). Sepals and petals often
adnate to each other at base, sometimes forming common perianth tube. Annular
nectariferous disc or five nectaries, hypogynous, staminodial, extrastaminal or
alternating with stamens, inserted on perianth tube, or absent. Receptacle
often distinctly hollow, usually with androphore; androgynophore usually
present (in, e.g., Adenia gynophore).
Androecium Stamens usually
five or eight, antesepalous, alternipetalous (sometimes four or c. 20 to more
than 60). Filaments usually free (in, e.g., Adenia sometimes connate
at base; in Androsiphonia connate into tube surrounding gynophore),
sometimes adnate to and forming tube surrounding gynoecium. Anthers dorsifixed,
versatile, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal
slits). Tapetum secretory. Staminodia numerous (c. 15 to more than 50),
extrastaminal to intrastaminal, often petaloid, as showy corona or disc.
Pollen grains
Microsporogenesis simultaneous. Pollen grains 3–12-colporate to
3–12-colpoidorate (rarely hexaporate), shed as monads, bicellular at
dispersal. Exine semitectate, with columellate infratectum, reticulate,
sometimes echinate.
Gynoecium Pistil composed of
(two or) three (to seven) connate carpels. Ovary semi-inferior (rarely
superior), unilocular, stipitate. Stylodia (two or) three (to seven), free or
partially connate (rarely entirely connate into single, simple style). Stigmas
capitate to discoid (in Adenia split), sometimes lobate, usually with
multicellular (in Adenia unicellular) papillae or non-papillate, Dry
type. Pistillodium absent.
Ovules Placentation parietal.
Ovules usually numerous (in Dilkea few) per ovary, usually anatropous
(rarely orthotropous), bitegmic, crassinucellar. Funicle often long. Micropyle
bistomal, sometimes Z-shaped (zig-zag). Outer integument ? cell layers thick.
Inner integument ? cell layers thick. Megasporangium often pointed at apex.
Megagametophyte monosporous, Polygonum type. Synergids sometimes of
different shape, sometimes with a filiform apparatus. Endosperm development ab
initio nuclear. Endosperm haustoria? Embryogenesis usually onagrad (rarely
piperad).
Fruit A berry or a loculicidal
or irregularly dehiscent capsule.
Seeds Seeds with arilloid,
often flattened. Testa multiplicative, hard, often hairy (rarely winged).
Exotesta often sarcoexotesta. Endotestal cells large, with crystals and often
lignified walls. Exotegmen palisade. Endotegmen persistent? Perisperm not
developed. Endosperm copious, ruminate (Passiflora foetida) or
non-ruminate, oily and proteinaceous. Embryo straight, well differentiated,
without chlorophyll. Cotyledons two. Germination phanerocotylar or
cryptocotylar.
Cytology x = 6 (7), 9–12 –
Polyploidy occurring.
DNA Plastid ORF2280 absent
(lost). Plastid gene rps16 lost in Passiflora. Plastid gene
rpoC1 in Passiflora without intron. Plastid gene
rpl22 in Passiflora probably transferred from plastid genome
to nuclear genome.
Phytochemistry Flavonols,
flavone-C-glycosides, ellagic and gallic acids, alkaloids, valine- and
isoleucine-derived cyanogenic glycosides, cyclopentenoid cyanogenic glycosides
and/or cyclopentenyl fatty acids (from the gynocardic group), saponins, and
cinnamic acid derivatives present. Tannins and proanthocyanidins not found.
Use Ornamental plants, fruits
(Passiflora).
Systematics (under
construction) Passifloraceae are sister to
Turneraceae.
There is no available comprehensive phylogeny
of Passifloraceae.
Paropsioideae Burnett,
Outlines Bot.: 750, 1092, 1130. Feb 1835 [‘Paropsidae’]
6/20–27. Paropsia (11;
tropical Africa, Madagascar, East Malesia), Paropsiopsis (1–7;
P. africana, P. atrichogyna, P. decandra, P.
pulchra; tropical West and Central Africa), Viridivia (1; V.
suberosa; southwestern Tanzania, Zambia), Androsiphonia (1;
A. adenostegia; tropical Africa), Smeathmannia (2; S.
laevigata, S. pubescens, tropical West and Central Africa),
Barteria (4; B. dewevrei, B. fistulosa, B.
nigritana, B. solida; tropical Africa). – Tropical Africa,
Madagascar, East Malesia. Trees or shrubs. Vessel elements in multiples, with
scalariform perforation plates. Leaves spiral, reduced (orthotropic branches)
or distichous (plagiotropic branches). Stipules sometimes absent. Venation
pinnate. Leaf margin and apex glandular. Inflorescence racemose. Androgynophore
or gynophore often present. Nectary usually absent (sometimes annular). Stamens
rarely up to 30. Pistil composed of (two to) three to six connate carpels.
Style in Barteria single. Seeds scrobiculate. n = ?
Passifloroideae
Burnett, Outlines Bot.: 750, 1092, 1130. Feb 1835
[‘Passifloridae’]
10/600–605. Adenia
(90–95; tropical regions in the Old World, with their highest diversity in
tropical Africa); Basananthe (37; tropical and southern Africa),
Deidamia (5; D. alata, D. bicolor, D.
bipinnata, D. commersoniana, D. setigera; Madagascar),
Efulensia (2; E. clematoides, E. montana; tropical
Africa), Schlechterina (1; S. mitostemmatoides; tropical East
Africa), Crossostemma (1; C. laurifolium; tropical Africa);
Ancistrothyrsus (2; A. hirtellus, A. tessmannii;
western tropical South America), Dilkea (11; tropical South America),
Mitostemma (3; M. brevifilis, M. glaziovii, M.
jenmanii; tropical South America), ’Passiflora’
(c 450; tropical Asia to islands in the Pacific, tropical and subtropical
America; paraphyletic). – Pantropical, with their highest diversity in
tropical Africa (not Passiflora)
and tropical South America. Often herbaceous vines or lianas with simple
tendrils (modified branches). Secondary lateral growth often anomalous. Stem
collenchyma present. Vessel elements with simple perforation plates. Superposed
bud (developing into branch) often present. Leaves spiral, sometimes compound.
Venation often palmate. Petiole and/or stipules with glands (extrafloral
nectaries). Inflorescence cymose. Corona consisting of one to several rows of
filaments or membranes. Androgynophore or gynophore frequently present. Seeds
often hairy, often variously sculpted. – Adenia
has less developed corona, gynophore instead of androgynophore, sometimes
hypanthium, nectary often formed by separate glands, filaments sometimes
connate at base, tricolporate pollen grains, hollow style and stigma without
multicellular papillae.
PERACEAE (Baill.)
Benth. ex Klotsch
|
( Back to Malpighiales )
|
Klotsch in Monatsber. Königl. Preuss. Akad.
Wiss. Berlin 1859: 241, 246. 10-30 Mar 1859
Genera/species
4–5/105–115
Distribution Pantropical.
Fossils Unknown.
Habit Usually dioecious
(sometimes monoecious, especially in Pera), evergreen trees or shrubs
(sometimes perennial herbs, more or less lignified at base).
Vegetative anatomy Phellogen?
Cortical and medullary vascular bundles absent. Vessel elements with simple
perforation plates; lateral pits alternate, simple pits. Imperforate tracheary
xylem elements libriform fibres with simple or bordered pits, non-septate. Wood
rays uniseriate or multiseriate (in Pera uniseriate), homocellular or
heterocellular. Axial parenchyma apotracheal diffuse or diffuse-in-aggregates,
or paratracheal reticulate, vasicentric, or banded, abundant. Tyloses often
frequent (sometimes sclerotic). Intraxylary phloem present at least in
Pera. Sieve tube plastids S type? Nodes? Secretory cavities absent.
Wood in some species with lysigenous radial ducts. Laticifers present in some
species. Heartwood sometimes with gum-like substances. Prismatic calciumoxalate
crystals abundant.
Trichomes Hairs unicellular or
multicellular, uniseriate or multiseriate, simple (in Pera usually
stellate or lepidote; inflorescences in Pogonophora sometimes with
malpighiaceous hairs), or absent.
Leaves Usually alternate
(spiral or distichous; rarely opposite), simple, entire, with asymmetrical leaf
base, often coriaceous, with ? ptyxis. Stipules usually small (in
Chaetocarpus and Trigonopleura large), often caducous, or
absent; leaf sheath absent. Petiole vascular bundle transection interrupted
arcuate to annular or complete annular; petiole sometimes with central plate
and wing bundles. Venation pinnate; veins sometimes proceeding into transparent
caducous teeth or marginal spines. Stomata? Cuticular wax crystalloids?
Epidermis with mucilaginous idioblasts. Lamina without secretory cavities,
sometimes pellucid-punctate. Leaf margin entire to prickly dentate.
Inflorescence Axillary,
panicle, fascicle, thyrsoid or capitate glomerulus (female flowers sometimes
solitary); male flowers in Pera often surrounded by reduced sterile
female flowers. Bracts one or two small outer, free, and two larger involucrate
inner, more or less connate.
Flowers Actinomorphic, small?
Hypogyny. Sepals in male flowers (two to) four or five (to seven), with
imbricate aestivation, free or connate (in Pera sometimes absent;
sometimes absent in female flowers). Petals usually four or five, with
imbricate aestivation, sometimes unguiculate (absent in Pera and
Chaetocarpus; sometimes absent in female flowers), in
Pogonophora with rigid hairs on adaxial side. Nectary? Disc usually
consisting of five to numerous usually intrastaminal glands (nectariferous
glands?) in one to three whorls (disc in Chaetocarpus extrastaminal,
entire to somewhat lobate [crenellate]; disc absent in Pera).
Androecium Stamens (two to)
five to eight (to 20). Filaments connate into tube at least in lower part,
usually free from tepals. Anthers usually basifixed (sometimes dorsifixed),
non-versatile?, tetrasporangiate, introrse or extrorse (sometimes latrorse),
longicidal (dehiscing by longitudinal slits). Tapetum secretory? Female flowers
with or without staminodia.
Pollen grains
Microsporogenesis simultaneous? Pollen grains tricolporate, shed as monads,
?-cellular at dispersal. Exine tectate (rarely intectate), with columellate
infratectum, perforate or punctate to micropunctate-rugulate, sometimes
psilate.
Gynoecium Pistil composed of
three (to nine) connate carpels or one carpel. Ovary superior, trilocular (to
novemlocular) or unilocular; ovary septa membranous and not distinctly
vascularized. Style single, simple or trilobate (lobes often bifid), or absent.
Stigmas one or three, papillate, peltate to fimbriate (sometimes
valve-shaped?), type? Male flowers with or without pistillodium (entire,
bilobate or trilobate).
Ovules Placentation apical.
Ovules one per carpel, anatropous?, pendulous, epitropous, bistomal,
crassinucellar. Micropyle usually endostomal (rarely exostomal). Outer
integument three to six cell layers thick. Inner integument three to six cell
layers thick. Obturator placental. Archespore bicellular or tricellular.
Megasporangium approx. two cell layers thick, early degenerating.
Megagametophyte monosporous, Polygonum type? Endosperm development?
Endosperm haustoria? Embryogenesis?
Fruit A single-seeded drupe or
drupaceous usually septicidal capsule with fleshy or spongy mesocarp and
lignified endocarp, often with persistent tepals and stigmas; septicidal
capsule usually dehiscing into three mericarps, each partially dehiscing into
two valves (capsule rarely loculicidal and dehiscing into six valves). Fruit
septa membranous and not distinctly vascularized. Capsular valves dehiscing
along midvein and at base often remaining adnate to fruit stalk, central
columella (usually thin, in Trigonopleura three-winged) splitting
longitudinally into usually three parts.
Seeds Carunculus (in
Trigonopleura also aril) present. Seed coat exotestal-exotegmic. Testa
vascularized. Exotesta palisade, lignified, usually tanniniferous. Exomesotesta
sclereidal. Endotestal cells with calciumcarbonate? Exotegmen usually
tracheoidal (in Pogonophora palisade), sometimes U-shaped in
cross-section; exotegmic cells cuboid? Endotegmen absent. Perisperm not
developed. Endosperm usually copious, fleshy (rarely absent). Embryo straight,
well differentiated, chlorophyll? Cotyledons two, wide and flat.
Germination?
Cytology n = ?
DNA
Phytochemistry Unknown.
Use Timber.
Systematics
Pogonophora (2; P. letouzeyi: Gabon; P.
schomburgkiana: Colombia, Venezuela, Guyana, Suriname, French Guiana,
Brazil, Peru), Pera (30–35; southern Mexico, Central America, the
West Indies, tropical South America), Clutia (c
55; tropical and subtropical Africa to South Africa, the Arabian Peninsula),
Chaetocarpus (13–19; tropical West and Central Africa, Madagascar,
Sri Lanka, Bangladesh, Burma, Thailand, West Malesia, tropical America; incl.
Trigonopleura?), Trigonopleura (3; T. dubia, T.
macrocarpa, T. malayana; West and Central Malesia; in
Chaetocarpus?).
Peraceae are sister-group to the
clade [Euphorbiaceae+Rafflesiaceae].
Pogonophora is sister to the remaining
Peraceae (Wurdack & al.
2005).
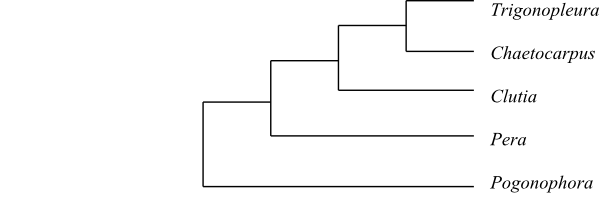 |
Cladogram (simplified) of Peraceae based on DNA
sequence data (Davis & al. 2007; Tokuoka 2007).
|
Martinov, Tekhno-Bot. Slovar: 369. 3 Aug 1820
[’Phyllanthoideae’]
Stilaginaceae C.
Agardh, Aphor. Bot.: 199. 13 Jun 1824 [‘Stilagineae’];
Antidesmataceae Loud., Hort. Brit.: 534. 30 Aug 1830
[’Antidesmeae’]; Stilaginales C. Agardh
in C. F. P. von Martius, Consp. Regn. Veg.: 14. Sep-Oct 1835
[‘Stilagineae’]; Scepaceae Lindl., Intr.
Nat. Syst. Bot., ed. 2: 171. 13 Jun 1836; Aporosaceae
Lindl. ex Planch. in Ann. Sci. Nat., Bot. Ser. 4, 2: 265. 1854
[’Aporoseae’]; Porantheraceae (Pax)
Hurus. in J. Fac. Sci. Univ. Tokyo, ser. III, 6: 224. 15 Aug 1954;
Bischofiaceae (Müll. Arg.) Airy Shaw in Kew Bull.
18: 252. 8 Dec 1965; Hymenocardiaceae Airy Shaw in
Kew Bull. 18: 261. 8 Dec 1965; Uapacaceae (Müll.
Arg.) Airy Shaw in Kew Bull. 18: 270. 8 Dec 1965;
Phyllanthales Doweld, Tent. Syst. Plant. Vasc.:
xxxiii. 23 Dec 2001
Genera/species 55/>2.100
Distribution Tropical and
subtropical regions on both hemispheres, with few species in warm-temperate
regions.
Fossils Uncertain.
Paraphyllanthoxylon, fossil wood from Cretaceous layers of South
Africa and North America, may represent Phyllanthaceae or Euphorbiaceae. A fossil
flower attributed to Antidesma has been found in Baltic amber
(Willemstein 1987).
Habit Usually monoecious or
dioecious (rarely bisexual), usually evergreen (sometimes deciduous) trees or
shrubs (sometimes climbing), perennial or annual herbs. Some species are
xeromorphic shrubs or herbs with ericoid leaves (some species of
Phyllanthus have phyllocladia; one species of Phyllanthus is
limnic and floating). Branches often dimorphic.
Vegetative anatomy
Ectomycorrhiza present in Uapaca. Phellogen ab initio superficial?
Primary medullary strands narrow (Hymenocardia). Vessel elements with
simple or scalariform perforation plates; lateral pits alternate, simple and/or
bordered pits. Vestured pits present in Bridelia and allies.
Imperforate tracheary xylem elements libriform fibres with simple or bordered
pits, septate or non-septate (in ‘Savia’ sometimes also
vasicentric tracheids). Wood rays uniseriate or multiseriate, heterocellular.
Axial parenchyma apotracheal diffuse or diffuse-in-aggregates, or paratracheal
scanty, reticulate, vasicentric, unilateral, or banded, or absent. Tyloses
sometimes abundant. Sieve tube plastids S type?; sieve tubes with
non-dispersive protein bodies (Bischofia). Nodes usually 3:3,
trilacunar with three leaf traces (sometimes 1:1, unilacunar with one trace).
Laticifers absent (resins excreted in Spondianthus and
Uapaca). Silica bodies present or absent. Prismatic calciumoxalate
crystals abundant; styloids and/or other types of crystals present or
absent.
Trichomes Hairs unicellular or
multicellular, usually simple (rarely stellate or lepidote); glands rarely
present (on leaves).
Leaves Usually alternate
(usually spiral, sometimes distichous; rarely opposite or whorled), usually
simple (in Bischofia trifoliolate or sometimes pinnately compound),
entire, sometimes coriaceous, with conduplicate or involute ptyxis; leaves on
orthotropic branches spiral and often reduced (sometimes scale-like), on
plagiotropic branches usually distichous, large and photosynthetic
(phyllanthoid branching, branches resembling compound leaves). Stipules often
scale-like, membranous, early caducous (rarely absent); leaf sheath absent.
Petiole in Bischofia pulvinate. Petiole vascular bundle transection?
Venation pinnate, brochidodromous or eucamptodromous. Stomata usually paracytic
(sometimes anisocytic). Cuticular wax crystalloids? Abaxial side of lamina in
Hymenocardia densely beset with red glandular dots. Epidermis
sometimes with mucilaginous idioblasts. Domatia, as pockets or pits, present in
some genera. Secretory cavities absent. Leaf margin usually entire (sometimes
serrate, rarely with glands; in Bischofia with caducous teeth).
Extrafloral nectaries often present on stipules, petiole and/or lamina.
Inflorescence Terminal or
axillary, fasciculate, raceme-, spike- or catkin-like, cymose,or racemose
inflorescence, or flowers solitary (usually female flowers). Inflorescenses
(with very short branches) often arising in axils of leaves on plagiotropic
shoots (phyllanthoid branching). Bracts sometimes involucrate (occasionally
showy; in Uapaca pseudanthium).
Flowers Actinomorphic, small.
Hypogyny. Sepals two to eight (to twelve), usually with imbricate or valvate
(sometimes open) aestivation, free or connate at base (sometimes absent in
female flowers). Petals (three to) five (or six), free, usually very small
(sometimes absent). Nectariferous disc extrastaminal or interstaminal, annular
or consisting of separate parts (sometimes central or absent), sometimes? with
glands.
Androecium Stamens two or
several (in Phyllanthus up to 15; in Tacarcuna 14 to 18; in
Lingelsheimia c. 15 to c. 35). Filaments free or more or less connate,
free from tepals. Anthers basifixed to dorsifixed, often versatile?,
tetrasporangiate, usually extrorse (sometimes introrse), longicidal (dehiscing
by usually longitudinal, sometimes transverse, slits; rarely apical, with
pore-like slits). Tapetum secretory, with binucleate to quinquenucleate
(Bischofia) cells. Female flowers often with staminodia; male flowers
rarely with staminodia (fertile stamens of male flowers in Uapaca
sometimes alternating with staminodia).
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually tri- to tetra- (rarely
penta-)colporate (sometimes porate or inaperturate; sometimes with diploporate
apertures; rarely pantosyncolpoidorate with up to c. 60 apertures; in
Phyllanthus polypantoporate), shed as monads, usually bicellular
(sometimes tricellular) at dispersal. Exine tectate or semitectate, with
columellate infratectum, usually reticulate or smooth (rarely echinate or
spinulate).
Gynoecium Pistil composed of
one carpel (pseudomonomery) or two to five (to 15) connate carpels. Ovary
superior, unilocular or bi- to quinquelocular (often? divided by
pseudophragmata). Style single (sometimes lateral-subterminal), simple or
branched, or stylodia two to four. Stigmas usually bilobate, fimbriate, with
adaxial furrow, papillate?, Wet type. Male flowers often with shortly
stipitate, peltate and entire or split pistillodium.
Ovules Placentation apical.
Ovules (one or) two per carpel, anatropous, anacampylotropous or
hemianatropous, pendulous, epitropous, bitegmic, crassinucellar. Micropyle
bistomal. Outer integument usually two to four (rarely five or more) cell
layers thick. Inner integument usually two or three (rarely up to ten or more)
cell layers thick. Megasporangium at least ten cell layers thick, protruding,
persistent. Placental obturator present between stylar canal and micropyle.
Hypostase present. Parietal tissue often protruding through micropyle. Nucellar
beak present. Megagametophyte monosporous, Polygonum type. Endosperm
development ab initio nuclear. Endosperm haustoria? Embryogenesis onagrad or
solanad.
Fruit Usually a septicidal
capsule with persistent columella (disintegrating into six valves or three
mericarps each with two valves), or a bi- to quadripartite schizocarp with
samaroid mericarps (rarely a berry or a single- to six-seeded drupe).
Seeds Aril? Carunculus usually
absent (sometimes present, more or less rudimentary). Seed coat usually
exotegmic. Testa sometimes vascularized (in Aporosa etc. sarcotestal).
Tegmen usually two to five (rarely up to 20 or more) cell layers thick.
Exotegmen with (sometimes also radially elongate) ribbon-shaped cells with
often sinuous cell walls, usually fibrous (sometimes palisade), often
tracheoidal (in Didymocistus and Hymenocardium collapsed
tracheoidal, also large endotegmic cells with tannins), sometimes (in, e.g.,
Aporosa) with sclerotic cells (exotegmen absent in
Poranthera). Perisperm not developed. Endosperm usually copious
(sometimes sparse or absent), oily. Embryo straight or curved, well
differentiated, with or without chlorophyll. Cotyledons two. Germination?
Cytology n = (6–9, 11) 13
(14); x = 13
DNA Mitochondrial maturase
gene matR lost in Croizatia and Lachnostylis.
Mitochondrial coxI intron present in Breynia and
Phyllanthus.
Phytochemistry Cyanidin,
delphinidin, cucurbitacins and other triterpenes, non-hydrolyzable tannins
(geraniin), tropane alkaloids (phyllalbin), pyrrolizidine alkaloids and other
alkaloids (securinine, phyllantine, phyllochrisine, etc.), and tyrosine-derived
cyanogenic compounds present. Ellagic acid not found. Flavonols? Saponins?
Aluminium accumulated in some species.
Use Ornamental plants, timber,
edible fruits, medicinal plants, fish poison.
Systematics Phyllanthaceae are sister to
Picrodendraceae.
Phyllanthaceae are
provisionally subdivided into Phyllanthoideae (the ’Fasciculate
clade’) and Antidesmatoideae (the ’Tanniniferous clade’) by
Kathriarachchi & al. (2005).
Phyllanthoideae
Beilschm. in Flora 16(Beibl. 7): 61, 109. 14 Jun 1833
[‘Phyllantheae’]
33/>1.660. Andrachne
(22; the Mediterranean, southwestern Asia, western North America, the West
Indies), Meineckia (c 30; southwestern and northeastern tropical
Africal, Madagascar, Socotra, southern Arabian Peninsula, southern India, Sri
Lanka, Assam, tropical America), Notoleptopus (1; N.
decaisnei; Malesia to New Guinea and northern Australia),
Poranthera (14; Australia, Tasmania, New Zealand),
Pseudophyllanthus (1; P. ovalis; southern Africa),
Phyllanthopsis (2; P. arida, P. phyllanthoides;
Texas, Mexico), Actephila (c 35; southern China, tropical Asia to
eastern Queensland, eastern New South Wales and islands in the Pacific),
Leptopus (10; the Caucasus, northern Iran and the Himalayas to China
and Southeast Asia), Heywoodia (1; H. lucens; tropical East
Africa, KwaZulu-Natal, Eastern Cape), Astrocasia (6; A.
austinii, A. diegoae, A. jacobinensis, A.
neurocarpa, A. peltata, A. tremula; southern Mexico,
Central America, the West Indies, Colombia, Venezuela), Chascotheca
(1; C. neopeltandra; Cuba, Hispaniola), Dicoelia (1; D.
beccariana; West Malesia), Wielandia (13; southeastern Kenya,
Madagascar, the Comoros, the Seychelles), Margaritaria (14; tropical
regions on both hemispheres), Lingelsheimia (6; L. abbayesii,
L. ambigua, L. fiherenensis, L. frutescens, L.
manongarivensis, L. sylvestris; Central Africa to Tanzania,
Madagascar), Flueggea (16; western Mediterranean, Turkey, tropical and
subtropical regions on both hemispheres), Plagiocladus (1; P.
diandrus; western Central Africa), Richeriella (1; R.
gracilis; northeastern India, Hainan, Thailand, West and Central Malesia;
in Flueggea?), Heterosavia (4; H. bahamensis, H.
erythroxyloides, H. laurifolia, H. maculata; Florida
Keys, Central America, Cuba, Bahamas, Grand Cayman), Phyllanthus
(>1.250; tropical and subtropical regions on both hemispheres), Securinega
(5; S. antsingyensis, S. capuronii, S. durissima,
S. perrieri, S. seyrigii; Madagascar, the Mascarene Islands),
Lachnostylis (3; L. bilocularis, L. hanekomii,
L. hirta; Western and Eastern Cape), Amanoa (16; tropical
Africa, Madagascar, tropical America), Keayodendron (1; K.
bridelioides; Ivory Coast to Cameroon), Savia (2; S.
dictyocarpa, S. sessiliflora; southern Mexico, the West Indies,
Venezuela, Paraguay, southern Brazil), Gonatogyne (1; G.
brasiliensis; São Paulo in Brazil), Croizatia (5; C.
brevipetiolata, C. cimalonia, C. naiguatensis, C.
neotropica, C. panamensis; Panamá, Venezuela),
Discocarpus (4; D. essequeboensis, D. gentryi,
D. pedicellatus, D. spruceanus; northeastern South America),
Tacarcuna (3; T. amanoifolia, T. gentryi, T.
tachirensis; Panamá, Colombia, Venezuela, Peru),
’Cleistanthus’ (c 140; tropical regions in the Old World;
paraphyletic), Pseudolachnostylis (1; P. maprouneifolia;
tropical and southern Africa), Pentabrachion (1; P.
reticulatum; Cameroon, Gabon), Bridelia (c 50; tropical Africa
and Madagascar to northern Australia and islands in the Pacific). – Tropical
and subtropical regions on both hemispheres, few species in the Mediterranean,
Turkey, the Caucasus, southwestern Asia and western North America. Pistil
composed of two to six (to 15) connate carpels. – Croizatia has five
petals, extrastaminal disc and bifid style.
Antidesmatoideae
Hurus. in J. Fac. Sci. Univ. Tokyo, ser. 3, Bot., 6: 321, 340. 15 Aug 1954
20/435–440. Bischofia (2;
B. polycarpa: central and southeastern China; B. javanica:
from India to eastern Asia, Melanesia and Polynesia to Samoa and Niue),
Spondianthus (1; S. preussii; tropical East and Central
Africa), Uapaca (c 50; tropical Africa, Madagascar),
Protomegabaria (3; P. macrophylla, P. meiocarpa,
P. stapfiana; tropical West and Central Africa), Richeria (5;
R. australis, R. dressleri, R. grandis, R.
obovata, R. tomentosa; tropical South America), Aporosa
(75–80; tropical Asia to Solomon Islands), Maesobotrya (c 20;
tropical Africa), Baccaurea (c 45; tropical Asia, islands in western
Pacific; incl. Distichirhops?, Nothobaccaurea?),
Distichirhops (3; D. megale, D. minor, D.
mitsemosik; Borneo, New Guinea; in Baccaurea?),
Nothobaccaurea (2; N. pulvinata, N. stylaris;
Solomon Islands, Fiji; in Baccaurea?), Celianella (1; C.
montana; sandstone tepuís in southern Venezuela), Jablonskia (1;
J. congesta; northern South America), Hieronyma (c 20;
southern Mexico, Central America, the West Indies, tropical South America),
Leptonema (2; L. glabrum, L. venosum; Madagascar),
Apodiscus (1; A. chevalieri; tropical West Africa),
Martretia (1; M. quadricornis; tropical West and Central
Africa), Antidesma (c 170; tropical and subtropical regions in the Old
World), Thecacoris (c 25; tropical Africa, Madagascar),
Hymenocardia (6; H. acida, H. heudelotii, H.
lyrata, H. punctata, H. ripicola, H. ulmoides;
tropical and southern Africa, Southeast Asia, Sumatra), Didymocistus
(1; D. chrysadenius; tropical South America). – Tropical and
subtropical. Petals often absent. Pistil composed of two to five connate
carpels, or one carpel. Tannins present.
Unplaced Phyllanthaceae
Ashtonia (2; A.
excelsa, A. praeterita; the Malay Peninsula, Borneo),
Chonocentrum (1; C. cyathophorum; Amazonia)?
 |
Cladogram of Phyllanthaceae based
on DNA sequence data mainly according to Wurdack & al. (2004). The
topology of the Poranthereae clade follows Vorontsova &
al. (2007) and Vorontsova & Hoffmann (2008).
|
 |
Cladogram of Phyllanthaceae based
on DNA sequence data (Kathriarachchi & al. 2005).
|
Small in J. New York Bot. Gard. 18: 184. Aug
1917, nom. cons.
Micrantheaceae J.
Agardh, Theoria Syst. Plant.: 182. Apr-Sep 1858 [‘Micrantheae’];
Pseudanthaceae Endl. in H. Pfeiffer, Nomencl. Bot.
2(2): 852. 3 Oct 1873; Androstachyaceae Airy Shaw in
Kew Bull. 18: 250. 8 Dec 1965 [‘Androstachydaceae’];
Paivaeusaceae (U. Köhler et G. L. Webster ex G. L.
Webster) A. Meeuse, Euphorbiaceae: 30. 1990
Genera/species 23/95–100
Distribution Tropical and
southern Africa, Madagascar, southern India, Sri Lanka, West Malesia, New
Guinea, Australia, Tasmania, New Caledonia, southwestern United States to
Argentina, with their highest diversity in Australia.
Fossils Rosenkrantzia
picrodendroides from the Danian (Early Paleocene) of western Greenland,
comprises fruits that resemble those in modern Picrodendraceae.
Habit Usually monoecious or
dioecious (rarely bisexual), evergreen or deciduous trees or shrubs, perennial
herbs; many species are stem succulents or twining.
Vegetative anatomy Phellogen
ab initio superficial? Vessel elements with simple or scalariform perforation
plates; lateral pits alternate or opposite, simple or bordered pits.
Imperforate tracheary xylem elements libriform fibres with simple or bordered
pits, non-septate (also vasicentric tracheids). Wood rays uniseriate or
multiseriate, homocellular or heterocellular. Axial parenchyma apotracheal
diffuse or diffuse-in-aggregates, or paratracheal scanty, aliform,
winged-aliform, confluent, vasicentric, unilateral, or banded, or absent.
Tyloses often frequent. Sieve tube plastids S type? Nodes? Laticifers absent.
Heartwood with gum-like substances. Silica bodies sometimes abundant.
Calciumoxalate as acicular crystals, styloids, crystal sand and other crystal
types present in some species. Wood parenchyma and/or wood ray cells with
prismatic crystals.
Trichomes Hairs unicellular or
multicellular, uniseriate, simple; glandular hairs?
Leaves Alternate (usually
spiral, sometimes distichous), opposite or verticillate, simple or palmately
compound, entire or lobed, or absent, with ? ptyxis. Stipules petiolar or
cauline (sometimes small, usually intrapetiolar; in Androstachys
ochreate, enclosing terminal bud) or absent; leaf sheath absent. Colleters
often present in leaf axils. Petiole vascular bundle transection? Venation
palmate or pinnate. Stomata paracytic; subsidiary cells present on top of guard
cells. Cuticular wax crystalloids? Epidermis usually with mucilaginous
idioblasts. Secretory cavities absent. Leaf margin serrate (teeth with caducous
apex) or entire. Foliar glands usually absent (sometimes with glands along leaf
margins).
Inflorescence Terminal or
axillary, catkin-like thyrse or fasciculate inflorescence (males), or flowers
solitary axillary (females).
Flowers Actinomorphic, small.
Hypogyny. Sepals in male flowers (two to) four to eight (to 13), with imbricate
aestivation, spiral, free, or absent; sepals in female flowers (three or) four
to eight (to 13), with valvate or imbricate aestivation, whorled, in some
genera unequally sized, free, or absent. Petals absent. Nectariferous disc
central or interstaminal, or absent (stamens rarely inserted in cavities on
disc).
Androecium Stamens two to more
than 100, alternate (sometimes on prolonged receptacle). Filaments usually free
(rarely connate at base), free from tepals. Anthers dorsifixed, versatile?,
apiculate, tetrasporangiate, extrorse to latrorse, longicidal (dehiscing by
longitudinal slits). Tapetum secretory? Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains 4–12-colporate, 5–6-porate to
zonaperturate or hexacolporoidate or inaperturate (with up to c. 60 very narrow
apertures), shed as monads, bicellular at dispersal. Exine tectate, with
columellate infratectum, spinulate, echinate or verruculate.
Gynoecium Pistil composed of
two to four (or five) connate carpels. Ovary superior, usually bilocular to
quadrilocular (or quinquelocular). Style usually single, simple, or stylodia
two to four (or five), free. Stigmas stout, sometimes lobate, papillate?,
usually Dry (sometimes Wet) type. Male flowers often with pistillodium.
Ovules Placentation axile to
apical. Ovules usually two (in Scagea one) per carpel, anatropous,
pendulous, epitropous, bitegmic, crassinucellar. Micropyle usually bistomal (in
Austrobuxus endostomal). Outer integument five or six cell layers
thick. Inner integument three to six cell layers thick. Funicular obturator
present between stylar canal and micropyle. Hypostase present. Nucellar cap
present. Nucellar beak often present. Megagametophyte monosporous,
Polygonum type. Endosperm development ab initio nuclear. Endosperm
haustoria? Embryogenesis onagrad?
Fruit Usually a septicidal
and/or loculicidal capsule with persistent column, or a tripartite schizocarp
(rarely a berry or a single- or two-seeded drupe with thin fleshy orange
pericarp containing bitter juice).
Seeds Aril present or absent.
Carunculus usually present (absent in, i.a., Podocalyx). Testa?
Exotegmen cuboid or fibrous (exotegmen in Oldfieldia palisade,
subprocumbent). Mesotegmen in Oldfieldia thickened. Endotegmen usually
absent (in Oldfieldia two-layered, with band-shaped cell wall
thickenings). Perisperm not developed. Endosperm usually copious, oily
(sometimes absent; in Picrodendron ruminate). Embryo curved, well
differentiated, with chlorophyll. Cotyledons two, sometimes strongly plicate
(in Picrodendron lobate). Germination?
Cytology n = 12
(Pseudanthus); x = 13
DNA
Phytochemistry Insufficiently
known. Flavones, oleanolic acids, triterpenes and toxic picrotoxans (e.g.
hyaenanchin) present. Saponins and cyanogenic compounds not found.
Use Ornamental plants,
medicinal plants, timber.
Systematics Podocalyx
(1; P. loranthoides; Amazonia); Tetracoccus (5; T.
capensis, T. dioicus, T. fasciculatus, T.
hallii, T. ilicifolius; southwestern United States, northwestern
Mexico), Hyaenanche (1; H. globosa; Vanrhynsdorp and
Clanwilliam Districts in Western Cape), Austrobuxus (c 28; West
Malesia, eastern Queensland, New Caledonia, Fiji), Dissiliaria (6;
D. baloghioides, D. indistincta, D. laxinervis,
D. muelleri, D. surculosa, D. tuckeri; eastern
Queensland), Sankowskya (1; S. stipularis; northeastern
Queensland), Whyanbeelia (1; W. terrae-reginae; eastern
Queensland), Choriceras (2; C. majus: Queensland; C.
tricorne: southern New Guinea, northern Northern Territory, northeastern
Queensland), Petalostigma (5; P. banksii, P.
pachyphyllum, P. pubescens, P. quadriloculare, P.
triloculare; Papua New Guinea, Queensland, New South Wales, Northern
Territory, Western Australia), Kairothamnus (1; K.
phyllanthoides; New Guinea), Scagea (2; S. depauperata,
S. oligostemon; New Caledonia), Neoroepera (2; N.
banksii, N. buxifolia; northeastern Queensland),
Micrantheum (4; M. demissum, M. ericoides, M.
hexandrum, M. serpentinum; southeastern South Australia to
Queensland, Tasmania), Stachystemon (9; southwestern Western
Australia), Pseudanthus (9; eastern Australia, Tasmania),
Piranhea (4; P. longipedunculata, P. mexicana,
P. securinega, P. trifoliata; western Mexico, Venezuela,
Guyana, Brazil), Parodiodendron (1; P. marginivillosum;
Bolivia, northern Argentina), Picrodendron (1; P. baccatum;
the West Indies), Oldfieldia (4; O. africana, O.
dactylophylla, O. macrocarpa, O. somalensis; tropical
Africa), Aristogeitonia (7; A. gabonica, A.
limoniifolia, A. lophirifolia, A. magnistipula, A.
monophylla, A. perrieri, A. uapacifolia; tropical
Africa, Madagascar), Mischodon (1; M. zeylanicus; southern
India, Sri Lanka), Voatamalo (2; V. capuronii, V.
eugenioides; Madagascar), Androstachys (1; A. johnsonii;
southeastern tropical Africa, Madagascar).
Picrodendraceae are
sister-group to Phyllanthaceae.
Podocalyx (carunculus absent) is a
plausible sister to the remaining Picrodendraceae (carunculus
present).
 |
Cladogram (simplified) of five of the genera
in Picrodendraceae
based on DNA sequence data (Wurdack & al. 2004).
|
Kunth in von Humboldt, Bonpland et Kunth, Nov.
Gen. Sp. 1: ed. 4°: 246. Mai 1816 [’Podostemeae’], nom. cons.
Marathraceae Dumort.,
Anal. Fam. Plant.: 60, 62. 1829 [‘Marathrineae’];
Marathrales Dumort., Anal. Fam. Plant.: 60. 1829
[‘Marathrarieae’]; Podostemales Lindl.,
Nix. Plant.: 17. 17 Sep 1833; Philocrenaceae Bongard,
Mém. Acad. Imp. Sci. St. Pétersb., sér. 6, Math. Nat. 3(2): 87. 13 Jun-13
Jul 1834; Podostemineae Engl., Syllabus, ed. 2: 124.
Mai 1898; Tristichaceae J. C. Willis in Bot. J. Linn.
Soc. 43: 51. 15 Mai 1915; Podostemopsida G. Cusset
& C. Cusset in Bull. Mus. Natl. Nat. Paris, Sect. B, Adansonia, sér. 4,
10: 210. 14 Oct 1988; Podostemanae R. Dahlgren ex
Reveal in Novon 2: 236. 13 Oct 1992 [‘Podostemonanae’]
Genera/species 57/290–300
Distribution Tropical,
subtropical and warm-temperate regions on both hemispheres.
Fossils Uncertain. Fossil
leaves under name of Nitophyllites zaisanica have been found in Late
Eocene layers in Russia and interpreted as Podostemaceae.
Habit Bisexual, usually annual
(sometimes perennial) herbs. Aquatic (rheophytes) in rapidly running fresh
water. Stems reduced or prolonged, simple or branched, sympodial, sometimes
dimorphic or developed during anthesis only, often with specialized thalloid
adventitious roots on the lower side and attached to the substrate by adhesive
usually unicellular hooked hairs, hapters, excreting viscid polysaccharides
(attaching to biofilm produced by cyanobacteria in the substrate). Adventitious
roots dorsiventrally or laterally flattened, filiform, strap-shaped or discoid,
creeping or partially floating, sometimes ephemeral or absent. Branching
extra-axillary. Leaves inserted on prolonged stems or on procumbent, often
discoid, stems. Podostemaceae with
ribbon-like roots bear opposite branches, whereas species with foliose or
crustose species develop single endogenous shoots from their upper surface
(sometimes from cortex).
Vegetative anatomy Main root
early withering. Adventitious roots photosynthesizing, usually endogenous
(sometimes exogenous). Shoots nearly always arising as endogenous lateral buds
from roots (in Cladopus both exogenous and endogenous lateral buds and
lateral adventitious roots). Phellogen absent. Secondary lateral growth absent.
Typical xylem and phloem elements (vessels, tracheids, libriform fibres, sieve
tubes etc.) usually absent. Wood rays absent. Parenchyma? Sieve tube plastids S
type. Nodes 1:1, unilacunar with one leaf trace. Laticifers and/or resinous
cells present in many Podostemoideae. Air canals and aerenchyma rare.
Silica bodies frequent in epidermis (and in subepidermal layers) in many
species. Calciumoxalate crystals?
Trichomes Hairs unicellular or
multicellular, simple, or absent.
Leaves Alternate (usually
distichous or tristichous, sometimes spiral, rarely tetrastichous to
hexastichous), opposite or absent, scale-like to hair-like, simple or compound,
entire or lobed, with ? ptyxis. Stipules petiolar or absent; leaf sheath simple
or double, with lobes sometimes prolonged into stipule-like appendages. Petiole
vascular bundle transection? Venation pinnate. Stomata at least usually absent.
Cuticular waxes absent. Schizogenous secretory canals present. Leaf margin
entire.
Inflorescence Terminal or
axillary, cymose or racemose of various shape, or flowers solitary. Floral buds
often endogenous. Usually only one floral bud naked (Weddellinoideae,
some Tristichoideae) or surrounded by vascularized cupule (other
Tristichoideae), or entirely enclosed within tubular or saccate
membranous and non-vascularized spathella formed by two connate leaves or part
of leaves (Podostemoideae).
Flowers Actinomorphic or
zygomorphic (sometimes inverted in bud). Hypogyny. Tepals (four or) five (or
six), with imbricate aestivation, sepaloid (Tristichoideae,
Weddellinoideae) or two to c. 25, thin (staminodial?), usually
alternating with stamens (Podostemoideae; in flowers with two basally
connate stamens sometimes with additional tepal at apex of andropodium), spiral
or in complete or incomplete whorl, often restricted to one side of flower, or
reduced to annular margin, or absent; when few then more or less connate.
Nectary absent. Disc absent.
Androecium Stamens one, few or
c. 40 in one or two complete whorls, or in one incomplete whorl, or restricted
to one side of flower and constisting of one to three free stamens, or Y-shaped
structure composed of andropodium with two (to seven) stamens (cf.
Podostemum, referring to Y-shaped androecium). Filaments free (in
Tulasneantha connate into tube) or connate at base into fascicles
(rarely paired), free from tepals. Anthers basifixed to subbasifixed,
non-versatile?, tetrasporangiate (usually with microsporangia in one row?),
usually introrse to latrorse (when two whorls then inner extrorse), longicidal
(dehiscing by longitudinal slits); connective sometimes prolonged. Tapetum
secretory. Staminodia absent.
Pollen grains
Microsporogenesis usually simultaneous (sometimes successive). Pollen grains
pantoporate with up to 16 pores (Tristichoideae), tricolporate
(Weddellinoideae), tricolpate to pentacolpate
(Podostemoideae) or rarely inaperturate, usually shed as monads or
dyads (in Diamantina tetrads), bicellular at dispersal. Exine tectate,
with granular infratectum, usually echinate (in Weddellinoideae
rugulo-areolate).
Gynoecium Pistil composed of
two to five connate carpels; antepetalous when carpels as many as tepals. Ovary
superior, usually bilocular or trilocular (in some Podostemoideae
unilocular). Gynophore usually present. Style single, usually simple (stylodia
in Diamantina two), or absent. Stigmas one to three, of various shape,
often linear, papillate?, type? Pistillodium absent.
Ovules Placentation usually
axile (sometimes free central, when ovary unilocular). Ovules usually numerous
(sometimes two or several) per carpel, anatropous, bitegmic, tenuinucellar.
Micropyle exostomal. Outer integument ? cell layers thick. Inner integument ?
cell layers thick. Apical part of megasporangium – part of megasporangium
below megasporocyte and surrounding developing megagametophyte –
significantly elongating and extending far beyond inner integument.
Megagametophyte development monosporous or disporous, quadrinucleate or
quinquenucleate, tricellular, quadricellular or quinquecellular,
Apinagia type, Dacraea, Podostemon or
Polypleurum subtypes. Meiosis II taking place in both dyad cells (no
cell division); micropylar cell reduced to cap-like structure above
megagametophyte. Chalazal megaspore nucleus dividing resulting in polar nuclei
or one or two antipodals, or entirely or partially degenerating. Micropylar
megaspore nucleus dividing vertically and chalazal nucleus horizontally
(Dacraea and Polypleurum types, polar cell micropylar), or
micropylar megaspore nucleus dividing horizontally and chalazal nucleus
vertically (Podostemon type, polar cell chalazal). Polar nuclei often
absent. Four micropylar nuclei forming megagametophyte, chalazal part
developing into multinucleate nucellar plasmodium, produced by hyponucellus
(part of megasporangium below megagametophyte). Antipodal cells usually absent
(sometimes one reduced antipodal cell present). Fertilization simple (double
fertilization not occurring). Endosperm not developing. Megasporangium
plasmodial after fertilization. Endosperm haustorium micropylar, usually as
well developed suspensor haustorium. Embryogenesis solanad.
Fruit Usually a septicidal
capsule (in Farmeria metzgerioides a berry or drupe?).
Seeds Aril absent. Exotesta
thick-walled, usually mucilaginous. Endotesta? Tegmen unspecialized. Exotegmic
cell walls sometimes lignified. Endotegmic cell walls lignified. Suspensor
present. Perisperm not developed. Endosperm present. Embryo straight
(fusiform?), chlorophyll? Cotyledons usually two, large (sometimes one).
Hypocotyl and plumule usually absent. Germination phanerocotylar. Radicula
ephemeral or absent.
Cytology n = 8, 10, 12–15,
17, 20
DNA
Phytochemistry Insufficiently
known. Isoprenylated and other xanthones usually present (not found in
Tristichoideae). Salts often accumulated.
Use Vegetables.
Systematics Podostemaceae are sister to
Hypericaceae.
Tristichoideae Warming
in Kong. Danske Vidensk. Natur. Math. Afl. 6(11): 53. 1901
[’Tristicheae’]
6/14–18. Tristicha (1; T.
trifaria: tropical and southern Africa, Madagascar, Mauritius, India,
tropical America); Indotristicha (3; I. malayana, I.
ramosissima, I. tirunelveliana; India), Dalzellia (6;
D. angustissima, D. gracilis, D. kailarsenii, D.
ranongensis, D. ubonensis, D. zeylanica; Sri Lanka to
southern China and Southeast Asia), Indodalzellia (1; I.
gracilis; southern India), Cussetia (2; C. carinata,
C. diversifolia; Thailand, Cambodia, Laos; possibly extinct);
Terniopsis (1–5; T. malayana; southern China, Thailand, the
Malay Peninsula). – Tropical Africa, Madagascar, tropical Asia and eastwards
to northern Australia, tropical America. Root apical meristem sometimes on both
adaxial and abaxial side. Roots sometimes without root cap. Spathella absent.
Hypogyny. Tepals three, usually free (sometimes connate). Stamens (one to)
three (stamen in Tristicha one, adaxial). Pollen grains pantoporate.
Pistil composed of three sometimes connate carpels. Integument developing
simultaneously. Capsule with strong ribs. Hypocotyl absent. Xanthones possibly
absent. – Tristichoideae are sister-group to
[Weddellinoideae+Podostemoideae]. Some species of
Dalzellia have a cupule at pedicel base, developed from leafy shoots,
the outer and inner integuments are developing together, and the megasporangium
is coenocytic. Tristicha has an adaxial stamen and the median carpel
may be abaxial. Cussetia is probably closely allied to
Tristicha and Terniopsis.
[Weddellinoideae+Podostemoideae]
Pistil composed of two connate carpels.
Ovary with apical septum.
Weddellinoideae (C.
Cusset et G. Cusset) Engl. in Engler et Prantl, Nat. Pflanzenfam., ed. 2, 18a:
28. 3 Mai 1930
1/1. Weddellina (1; W.
squamulosa; northern South America). – Plant scaly. Flowers terminal,
solitary. Tepals (four or) five (or six), single-veined. Stamens five to c. 25.
Pollen grains smooth. Stigmas spherical. Integuments developing simultaneously.
Megagametophyte development very variable. Capsule without ribs. Tegmic cells
thick-walled. Hypocotyl present.
Podostemoideae Wedd.
in A. P. de Candolle et A. L. P. P. de Candolle, Prodr. 17: 39, 43. 16 Oct 1873
[‘Podostemoneae’]
50/278–285. Diamantina (1;
D. lombardii; Brazil); ‘Mourera’ (7; M.
alcicornis, M. aspera, M. elegans, M.
fluviatilis, M. glazioviana, M. schwackeana, M.
weddelliana; northern South America; paraphyletic), Monostylis
(1; M. capillacea; Brazil), Castelnavia (11; Brazil),
Rhyncholacis (c 25; northern tropical South America),
Apinagia (≤50; tropical South America), Jenmaniella (7;
J. ceratophylla, J. fimbriata, J. guianensis, J.
isoetifolia, J. jenmanii, J. tridactylitifolia, J.
varians; northeastern South America), Noveloa (2; N.
coulteriana, N. longifolia; Mexico, Central America),
Wettsteiniola (3; W. accorsii, W. apipensis, W.
pinnata; southern Brazil, northern Argentina), Marathrum (≤25;
southern Mexico, Central America, the West Indies, northwestern South America),
Autana (1; A. andersonii; Venezuela), Ceratolacis
(2; C. erythrolichen, C. pedunculatum; Brazil),
Cipoia (2; C. inserta, C. ramosa; Brazil),
Lonchostephus (1; L. elegans; Amazonian Brazil),
Lophogyne (1; L. lacunosa; eastern central Brazil),
Macarenia (1; M. clavigera; Colombia), Oserya (7;
O. biceps, O. coulteriana, O. flabellifera, O.
longifolia, O. minima, O. perpusilla, O.
sphaerocarpa; southern Mexico, Central America, northern South America),
Tulasneantha (1; T. monadelpha; western Brazil),
Vanroyenella (1; V. plumosa; southwestern Mexico);
Podostemum (17; eastern and southern United States, Mexico, Central
America, the West Indies, tropical South America); Inversodicraea (4;
I. congolana, I. garrettii, I. tenax, I.
warmingiana; tropical Africa), Monandriella (1; M.
linearifolia; Cameroon), Saxicolella (1; S. flabellata;
tropical West and Central Africa), ’Ledermanniella’ (45–50;
tropical and southern Africa; non-monophyletic), Letestuella (1;
L. tisserantii; Kunene River in Namibia), Stonesia (2; S.
fascicularis, S. gracilis; tropical West and Central Africa),
Macropodiella (4–6; M. garrettii, M. hallaei,
M. macrothyrsa, M. mildbraedii, M. pellucida, M.
uoroensis; tropical West and Central Africa), Leiothylax (2;
L. quangensis, L. warmingii; tropical Africa),
Winklerella (1; W. dichotoma; tropical West Africa),
Dicraeanthus (4; D. africanus, D. ramosus, D.
taylorii, D. zehnderi; tropical West and Central Africa),
Djinga (1; D. felicis; Cameroon), Endocaulos (1;
E. mangorense; Madagascar), Thelethylax (2; T.
isalensis, T. minutiflora; Madagascar), Angolaea (1;
A. fluitans; Angola), Paleodicraeia (1; P.
imbricata; Madagascar), Sphaerothylax (2; S. abyssinica,
S. algiformis; tropical and southern Africa, Madagascar),
Maferria (1; M. indica; southwestern India),
Zehnderia (1; Z. microgyna; Cameroon); Cladopus (10;
southern China, southern Japan, Southeast Asia, Malesia to New Guinea and
northeastern Queensland), Paracladopus (2; P.
chantaburiensis, P. chiangmaiensis; Thailand);
Hydrodiscus (1; H. koyamae; Laos), Hydrobryum
(5–10; H. floribundum, H. griffithii, H.
japonicum, H. koribanum, H. puncticulatum; southern
India, eastern Nepal, Assam, China, southern Japan), Hanseniella (2;
H. heterophylla, H. smitinandii; Thailand),
Thawatchaia (1; T. trilobata; Thailand); Willisia
(1–2; W. arekaliana, W. selaginoides; Kerala in southern
India), ‘Zeylanidium’ (5; Z. barberi, Z.
maheshwarii, Z. olivaceum, Z. sessile, Z.
subulatum; India, Sri Lanka; non-monophyletic), Griffithella (1;
G. pierrei; Western Ghats in India), Farmeria (1; F.
metzgerioides; Sri Lanka), Polypleurum (4; P.
dichotomum, P. elongatum, P. stylosum, P.
wallichii; India, Sri Lanka, northeastern Thailand), Diplobryum
(4; D. koyamae, D. minutale, D. ramosum, D.
vientianense; Laos, southern Vietnam). – Tropical and subtropical
regions on both hemispheres, few species in warm-temperate regions. Shoots
usually without apical meristem. Root apical meristems usually on lower side of
thallus (sometimes on both upper and lower side). Roots sometimes exogenous,
sometimes without root cap. Leaves ensiform, bifacial, often distichous,
without normal epidermis, endogenously developed. Stipules sometimes also
abaxial relative to leaf (leaves dithecal: one leaf sheath apically directed,
additional leaf sheath basally directed). Stomata absent? Flowers or floral
fascicles enclosed by non-vascularized spathella (possibly formed by fusion of
two foliar structures). Tepals two to c. 25, with narrow lobes, sometimes
replaced by stamens. Stamens one to three (to numerous). Microsporogenesis
sometimes successive, with tetragonal tetrads. Pollen grains, 3–5-colpate,
shed in monads or dyads, usually calymmate. Pistil composed of three (to seven)
connate carpels. Ovary sometimes unilocular. Gynophore usually present. Style
short, simple or branched, with long branches. Outer integument first
developing. Megasporangium amoeboid-periplasmodial prior to fertilization.
Megagametophyte sometimes disporous (Polypleurum and
Podostemon types). Polar nucleus degenerating. Double fertilization
not occurring. Capsule with ribs. Hypocotyl and radicula usually absent
(hypocotyl present in Zeylanidium olivaceum). – Diamantina
is sister to the remaining Podostemoideae (Kato & al. 2009; Ruhfel
& al. 2011; Koi & al. 2012). In Old World Podostemoideae
(“the Old World clades”) pollen grains are usually shed in dyads, whereas
monads dominate in New World Podostemoideae (“the New World
clade”).
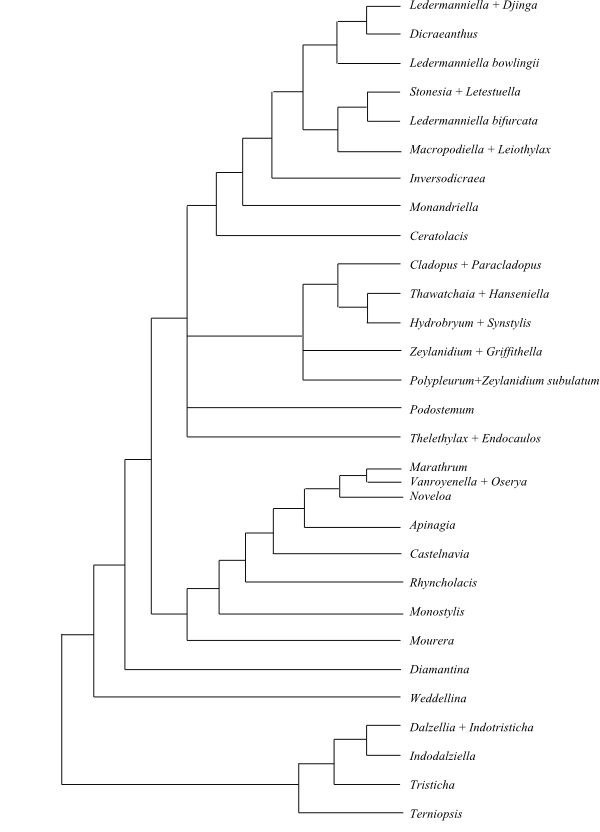 |
Cladogram of Old World Podostemaceae based
on DNA sequence data (Kato & al. 2009; Ruhfel & al. 2011).
|
Meisner, Plant. Vasc. Gen., Tab. Diagn. 345,
Comm.: 258. 13-15 Feb 1842 [’Putranjiveae’]
Genera/species 1–2/c 225
Distribution Pantropical,
southeastern Africa, Madagascar, subtropical East Asia, northern and eastern
Australia, New Caledonia.
Fossils Uncertain. Fossil
pollen grains attributed to Putranjivaceae have been
reported from the Paleocene (Muller 1981).
Habit Usually dioecious (some
species of Drypetes monoecious or polygamodioecious), evergreen trees
or shrubs.
Vegetative anatomy Phellogen?
Vessel elements with simple or scalariform perforation plates; lateral pits
alternate, simple pits. Bordered intervessel pits abundant. Imperforate
tracheary xylem elements libriform fibres with simple pits, non-septate. Wood
rays uniseriate or multiseriate, heterocellular. Axial parenchyma apotracheal
diffuse or diffuse-in-aggregates, or paratracheal reticulate, scalariform, or
banded. Sieve tube plastids S type? Nodes? Latex and laticifers absent. Silica
bodies present in parenchyma cells in some species. Wood parenchyma and/or wood
ray cells with prismatic calciumoxalate crystals and silica bodies.
Trichomes Hairs unicellular,
simple, or absent.
Leaves Alternate (distichous),
simple, entire, often coriaceous, with ? ptyxis. Stipules present; leaf sheath
absent. Petiole vascular bundle transection elliptic. Leaf base usually
asymmetric. Venation pinnate; veins sometimes proceeding into transparent
caducous teeth or spines. Stomata brachyparacytic with subsidiary cells
overlying guard cells Cuticular wax crystalloids? Secretory cavities absent.
Leaf margin serrate (sometimes spinose-serrate) or entire.
Inflorescence Axillary,
fascicle (female inflorescences in Putranjiva one- to
three-flowered).
Flowers Actinomorphic, small?
Hypogyny. Sepals four or five (to seven), usually with imbricate (in female
flowers of some species of Drypetes open) aestivation, usually free
(in Putranjiva connate in lower part), in female flowers usually
caducous. Petals absent. Nectary usually present (absent in
Putranjiva). Disc annular (rarely absent), sometimes lobate (in
Drypetes madagascariensis trilobate), with alternisepalous lobes, or
cup-shaped, in male flowers central and encircling rudimentary gynoecium.
Androecium Stamens (two to)
four to 20 (to more than 50 in Drypetes longifolia), diplostemonous in
some species of Drypetes. Filaments usually free from each other and
from tepals. Anthers usually basifixed (sometimes slightly dorsifixed), usually
non-versatile, tetrasporangiate, usually extrorse or latrorse (rarely
introrse), longicidal (dehiscing by longitudinal slits). Tapetum secretory,
with binucleate cells. Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains tricolporate, shed as monads,
bicellular at dispersal. Exine tectate or semitectate, with columellate?
infratectum, perforate or reticulate, usually smooth.
Gynoecium Pistil composed of
two or three (to seven) connate carpels (sometimes seemingly one carpel;
probably pseudomonomery). Ovary superior, synascidiate, unilocular to
quadrilocular. Style usually single or absent (styles in Putranjiva
two or three, connate at base), usually simple, expanded, usually short.
Stigmas usually wide and flat (flap-shaped), simple or bifurcate (rarely
multilobate, sometimes capitate), non-papillate (Drypetes) or
papillate (Putranjiva), type? Pistillodium, very small, usually
present (rarely absent) in male flowers.
Ovules Placentation axile.
Ovules two per carpel, anatropous, pendulous, epitropous, usually bitegmic (in
Drypetes macrostigma unitegmic with inner integument lost), weakly
crassinucellar (with two parietal cell layers) or incompletely tenuinucellar.
Micropyle endostomal or bistomal (sometimes exostomal; variation extensive).
Outer integument three to seven cell layers thick, with few discrete vascular
bundles. Inner integument five to nine (or more) cell layers thick,
multiplicative. Single integument in Drypetes macrostigma six to nine
cell layers thick. Obturator placental, stout, formed from funicular tissue
near micropyle. Hypostase weakly differentiated in older ovules. Endothelium
present. Parietal tissue approx. two cell layers thick. Archespore usually
bicellular or tricellular (rarely quadricellular or quinquecellular).
Megasporangium approx. two cell layers thick, early disintegrating. Nucellar
beak absent. Nucellar cap absent. Megagametophyte monosporous,
Polygonum type. Antipodal cells early degenerating. Endosperm
development ab initio nuclear. Endosperm haustoria absent? Embryogenesis?
Fruit Usually a unilocular to
quadrilocular drupe, often with persistent stigmas.
Seeds Aril absent. Carunculus
absent. Testa vascularized. Sarcotesta thin or absent. Exomesotesta sclereidal.
Endotesta? Tegmen multiplicative (six to more than 24 cell layers thick).
Exotegmen sometimes fibrous. Exotegmic cells usually isodiametric, cuboidal,
sometimes longitudinally elongate. Mesotegmen and endotegmen collapsing.
Perisperm not developed. Endosperm copious, oily. Embryo straight, chlorophyll?
Cotyledons two. Germination phanerocotylar?
Cytology n = (19) 20 (21); x =
10
DNA
Phytochemistry Insufficiently
known. Biflavonoyls, cucurbitacins and other triterpenes, and glucosinolates
(mustard oil glycosides) present. Flavonols? Ellagic acid? Saponins?
Calciumoxalate present at least in floral organs.
Use Timber.
Systematics Drypetes
(c 220; sub-Saharan Africa, southern and eastern Asia, Australasia to northern
and eastern Australia, New Caledonia, tropical America; incl.
Putranjiva?), Putranjiva (4; P. formosana, P.
matsumurae, P. roxburghii, P. zeylanica; Pakistan,
India, Sri Lanka, the Himalayas, southern China, Southeast Asia, Malesia to New
Guinea, Taiwan, Japan, Ryukyu Islands; in Drypetes?).
Putranjivaceae appear to be
sister-group to Lophopyxis (Lophopyxidaceae).
Putranjivaceae share with
Erythroxylaceae,
Rhizophoraceae,
Linaceae, and Chrysobalanaceae the
combination of fibrous exotegmen, endothelium, and thick, multiplicative inner
integument.
Engler in von Martius, Fl. Bras. 12(1): 475, 476.
1 Apr 1888, nom. cons.
Genera/species 4/c 50
Distribution Central America,
the West Indies, tropical South America, with their largest diversity in
Amazonia.
Fossils Unknown.
Habit Usually bisexual or
androdioecious (sometimes polygamomonoecious, dioecious, or polygamodioecious),
evergreen trees, shrubs or lianas.
Vegetative anatomy Mycorrhiza
absent. Phellogen? Cortical vascular bundles absent. Vessel elements usually
with simple (sometimes scalariform) perforation plates; lateral pits alternate,
bordered pits. Vestured pits present. Imperforate tracheary xylem elements
tracheids and fibres with bordered pits, non-septate (also vasicentric
tracheids). Wood rays uniseriate or multiseriate, heterocellular. Axial
parenchyma apotracheal diffuse, or paratracheal scanty, aliform,
lozenge-aliform, winged-aliform, or vasicentric, or absent. Sieve tube plastids
S type. Nodes 3:3, trilacunar with three leaf traces. Cortex with cristarque
cells. Parenchyma with secretory cells and druses. Heartwood in some species
with resinous substance. Silica bodies or prismatic calciumoxalate crystals
present in some species.
Trichomes Hairs unicellular,
simple.
Leaves Opposite or
verticillate, usually simple (in Froesia and Touroulia
pinnately compound), entire or pinnately lobed, with ? ptyxis. Stipules usually
interpetiolar, large, rigid or foliaceous (in Froesia deeply split),
persistent; leaf sheath absent. Petiole vascular bundle transection annular,
often complex. Venation (impari-)pinnate; secondary veins coarse, densely
spaced; tertiary veins paxillate, parallel to plumose-reticulate, densely
spaced; leaf veins surrounded by very thick-walled fibres. Stomata anisocytic
or paracytic. Cuticular wax crystalloids? Lamina with lysigenous secretory
mucilage cavities. Mesophyll with calciumoxalate as druses and single prismatic
crystals. Leaf margin serrate or crenate.
Inflorescence Terminal or
axillary, basically thyrsoid (determinate thyrse).
Flowers Usually actinomorphic
(rarely somewhat zygomorphic). Hypogyny. Sepals four or five, with imbricate
aestivation, unequal, free. Petals four or five (to eight), usually with
imbricate (sometimes contorted) aestivation, free. Nectary absent. Disc
absent.
Androecium Stamens twelve to
more than 170. Filaments free or connate at base, free from or adnate at base
to petals. Anthers dorsifixed, non-versatile, tetrasporangiate, introrse
(thecae separate) or latrorse (thecae arranged side by side, in
Froesia), longicidal (dehiscing by longitudinal slits). Tapetum
secretory? Staminodia absent.
Pollen grains
Microsporogenesis simultaneous? Pollen grains tricolporate, shed as monads,
?-cellular at dispersal. Exine tectate, with columellate infratectum,
microperforate.
Gynoecium Pistil composed of
two, three or seven to 14 (in Lacunaria four to 14) carpels usually
paracarpously connate below (in Froesia three, free, apocarpy). Ovary
superior, bilocular, trilocular or septa to 14-locular (in Lacunaria
quadrilocular to 14-locular). Stylodia two, three or seven to 14, free. Stigmas
obliquely peltate, type? Pistillodium often present in male flowers.
Ovules Placentation basal to
axile. Ovules two per carpel, anatropous, ascending, bitegmic, tenuinucellar.
Micropyle ?-stomal. Outer integument ? cell layers thick. Inner integument ?
cell layers thick. ‘False endothelium’ present on surface of
megasporangium. Megagametophyte monosporous, Polygonum type? Endosperm
development nuclear? Endosperm haustoria? Embryogenesis?
Fruit A berry or berry-like
single- to four-seeded valvate capsule (in Froesia follicles) with
persistent calyx. Exocarp with lacunae.
Seeds Aril absent. Seeds
usually densely tomentose (in Froesia glabrous or almost glabrous).
Testa? Tegmen? Perisperm not developed. Endosperm absent. Embryo straight, well
differentiated, chlorophyll? Cotyledons two, thick. Germination
cryptocotylar.
Cytology n = ?
DNA
Phytochemistry Virtually
unknown. Cyanogenic compounds not found.
Use Timber.
Systematics Froesia
(5; F. crassiflora, F. diffusa, F. gereauana, F.
tricarpa, F. venezuelensis; northern tropical South America);
Touroulia (1; T. guianensis; northern tropical South
America), Quiina (c 35; Belize, tropical South America),
Lacunaria (8; L. crenata, L. grandifolia, L.
jenmanii, L. macrostachya, L. oppositifolia, L.
panamensis, L. sampaioi, L. umbonata; Central America,
tropical South America).
Quiinaceae are part of a
trichotomy also including Ochnaceae and Medusagyne
(Medusagynaceae), or
Medusagyne is sister to Quiinaceae (Schneider & al.
2014).
Froesia is sister to the remaining
Quiinaceae (Schneider
& al. 2006; Schneider & Zizka 2017).
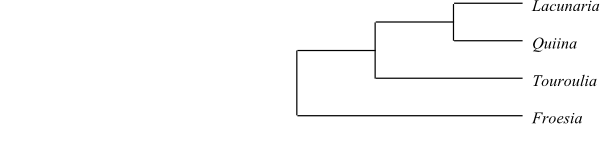 |
Cladogram of Quiinaceae based on
morphology and DNA sequence data (Schneider & al. 2006).
|
Dumortier, Anal. Fam. Plant.: 13, 14. 1829, nom.
cons.
Rafflesiales R. Br.
in C. F. P. von Martius, Consp. Regn. Veg.: 18. Sep-Oct 1835 [‘Rafflesiaceae’];
Rafflesianae Thorne ex Reveal in Phytologia 79: 71.
29 Apr 1996
Genera/species 2–3/c 35
Distribution Assam, Bhutan,
southernmost China, Burma, Thailand, Indochina, West Malesia to the
Philippines.
Fossils Unknown.
Habit Usually dioecious (in
Rhizanthes lowii and R. zippelii also bisexual),
achlorophyllous perennial herbaceous endophytic stem and root holoparasites
without rhizome or ordinary roots, parasitizing species of Tetrastigma
(Vitaceae). Flowers
evil-smelling.
Vegetative anatomy Mycorrhiza
absent. Vegetative tissue consisting of mostly uniseriate parenchymatous
filaments probably capable of intrusive intercellular growth and transfer of
nutrient and water (possibly derived from laticifers with similar type of
growth and frequently present in Euphorbiaceae). Hypha-like
cellular threads invading host plant and forming endophytic system inside roots
and/or stem. Phellogen absent. Secondary lateral growth absent. Vessel elements
probably absent. Imperforate tracheary xylem elements libriform fibres? (or
absent?) Wood rays absent. Axial parenchyma absent? Sieve tube plastids
S0 type, without starch or protein inclusions. Nodes? Crystals?
Trichomes Hairs absent?
Leaves Usually verticillate
(sometimes opposite or alternate spiral), reduced and scale-like, or absent,
with ? ptyxis. Stipules and leaf sheath absent. Venation? Stomata anomalous,
with three or more guard cells (Rafflesia), or absent. Cuticular waxes
absent. Leaf margin entire.
Inflorescence Flowers
solitary. Floral shoots endogenous, bursting out through cortex of host
plant.
Flowers Actinomorphic,
medium-sized to very large (Rafflesia arnoldii with largest flower of
all angiosperms, reaching almost 1 m across). Flower in Rafflesia and
Rhizanthes surrounded by three whorls of scale-like bracts with five
bracts in each. Epigyny. Tepals (sepals?) four to ten (to 16), usually with
imbricate (in Rhizanthes valvate) aestivation, in one whorl
(uniseriate; in Sapria biseriate?), with incurved margins, usually
connate in lower part, in Rafflesia and Sapria inserted
around thin annular horizontal central tissue, diaphragm (homologous to corona
or corolla?), on apex of perianth tube; apex of central column in
Rafflesia enlarged and disc-shaped; apices of 16 tepals in
Rhizanthes inserted inside cavity in central column (diaphragm
absent). Base of perianth tube in Rafflesia covered by special
outgrowths, ramenta, on adaxial side; diaphragm in Sapria covered by
ramenta. Nectary present at stylar base or absent. Androgynophore present?
Androecium Stamens twelve to
more than 50, inserted on and adnate to ring immediately below (disc-shaped)
apex of central column. Filaments absent. Anthers basifixed, non-versatile,
disporangiate (monothecal) to polysporangiate, connate into annular synandrium,
extrorse, poricidal (dehiscing by apical pore). Tapetum secretory? Female
flowers with staminodia (rudimentary anthers).
Pollen grains
Microsporogenesis successive. Pollen grains inaperturate, shed as monads,
bicellular at dispersal. Exine intectate; pollen surface smooth?
Gynoecium Pistil composed of
three to ten connate carpels; carpellary margins occluded through postgenital
fusion and secretion. Ovary inferior, unilocular. Style single, simple, very
short. Stigma in Rafflesia and Rhizanthes as annular
structure on outer margin or lower side of apex of (disc-shaped) central
column, papillate, type? Male flowers in Rhizanthes with pistillodium
(rudimentary ovary).
Ovules Placentation
laminar-parietal. Ovules numerous per ovary, anatropous, unitegmic or bitegmic,
tenuinucellar. Micropyle usually endostomal (rarely exostomal). Outer
integument one cell layer thick or absent. Inner integument ? cell layers
thick. When unitegmic, then swelling present on chalaza (representing second
integument?). Nucellar cap present. Megasporangial epidermis persistent.
Megagametophyte monosporous, Polygonum type. Endosperm development ab
initio nuclear. Endosperm haustoria? Embryogenesis caryophyllad or solanad?
Fruit A many-seeded berry.
Seeds Aril absent. Seed
consisting of two different parts; part covered by testa not enclosing embryo.
Exotesta as basal chalazal appendage. Endotesta hard. Exotegmic cell walls with
U-shaped thickenings. Endotegmic cell walls with thickenings. Perisperm not
developed. Endosperm single-layered, sparse. Embryo rudimentary,
undifferentiated (with few cells in few tiers), without chlorophyll? Cotyledons
two. Germination phanerocotylar?
Cytology n = 11
(Rhizanthes), 12 (Rafflesia)
DNA
Phytochemistry Virtually
unknown. Tannins present.
Use Unknown.
Systematics Rafflesia
(c 28; Thailand, the Malay Peninsula, West Malesia to the Philippines; incl.
Rhizanthes?), Rhizanthes (4; R. deceptor, R.
infanticida, R. lowii, R. zippelii; peninsular Thailand,
the Malay Peninsula, western Java, Sumatra, Borneo; in Rafflesia?),
Sapria (3; S. himalayana, S. poilanei, S.
ram; northeastern India, Burma, southern China, Southeast Asia).
Sapria is sister to [Rafflesia+Rhizanthes].
Rafflesiaceae are sister to
Euphorbiaceae. This is
supported by mitochondrial, plastid (matK) and nuclear (LSU and SSU
rDNA) gene sequences. The mitochondrial genes nad1B-c in Vitaceae and Rafflesiaceae have very
similar sequences, a fact interpreted as horizontal gene transfer from the host
plant Tetrastigma (Vitaceae) to the parasite
(Davis & Wurdack 2004).
The shoot apex is formed secondarily through
schizogeny (internal cell separation) at the distal interface boundary between
the tissues of the parasite and its host plant. Moreover, the carpels are not
initiated from the apex of the flower. Instead, the radially directed gynoecial
ovarial clefts are likewise formed schizogenously. This secondarily derived
inner surface of the gynoecium may represent a synapomorphy of Rafflesiaceae (Nikolov &
al. 2014).
Pollination in Rafflesia
is carried out at least partially by carrion flies (e.g. Chrysomya and
Lucilia; Beaman & al. 1988). The fly enters the grooves on the
central male floral column. It is directed by hairs on the ridges, thereby
placing the viscous pollen matrix on the back of the fly. The translucent
“windows” on the perigone diaphragm probably orient the fly within the
flower. The fly, loaded by pollen, then visits the female flower and supposedly
enters through the infradiscoidal sulcus. This tissue consists of the annular
stigma and the base of the pistillar central column. The fly, by crawling into
the sulcus, deposits the pollen on to the stigmatic surface.
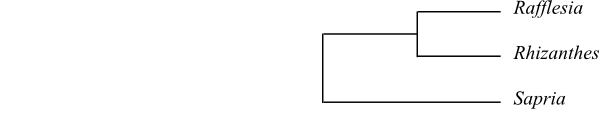 |
Cladogram of Rafflesiaceae based
on DNA sequence data (Davis & al. 2007).
|
Persoon, Syn. Plant. 2: 2. Nov 1806
[’Rhizophorea’, ’Rhizophoreae’], nom. cons.
Rhizophorales Pers.
ex Bercht. et J. Presl, Přir. Rostlin: 257. Jan-Apr 1820
[‘Rhizophoreae’]; Mangiaceae Raf., Fl.
Tellur. 3: 73. Jan-Mar 1837 [‘Mangidia’];
Legnotidaceae (Endl.) Blume, Mus. Bot. Lugd.-Bat.:
126. Oct 1849 [‘Legnotideae’], nom. illeg.;
Cassipoureaceae J. Agardh, Theoria Syst. Plant.: 246.
Apr-Sep 1858 [‘Cassipoureae’];
Macarisiaceae J. Agardh, Theoria Syst. Plant.: 295.
Apr-Sep 1858 [‘Macharisieae’];
Rhizophoranae (Pers.) Takht. ex Reveal et Doweld in
Novon 9: 550. 30 Dec 1999
Genera/species 14/112–114
Distribution Atlantic and
Indian Ocean coasts of Africa, islands in the Indian Ocean and the Pacific,
Atlantic and Pacific coasts of Central and South America, coasts in the West
Indies, with their highest diversity in Madagascar and tropical Asia.
Fossils Pollen grains
(resembling those in Bruguiera, Kandelia and
Rhizophora) have been found in Eocene layers in France. Fossil Rhizophoraceae pollen is
also known from Caribbean layers of Late Eocene age, and have been recorded
from Oligocene and younger strata in Mexico, Venezuela, the Caribbean, Nigeria
and Malesia (extant distribution in this area is possibly less than 11 My old).
Eocene macrofossils (Ceriops cantiensis, Palaeobruguiera
elongata, etc.) are known from Europe.
Habits Usually bisexual
(rarely polygamomonoecious), evergreen trees or shrubs. Many species are
mangrove trees. Pneumatophores (in Gynotrocheae and
Rhizophoreae) and stilt roots often present.
Vegetative anatomy Root hairs
absent in Rhizophoreae. Phellogen ab initio at least sometimes
superficial. Vessel elements with simple and/or scalariform perforation plates;
lateral pits scalariform, opposite or alternate, simple or bordered pits.
Vestured pits present. Imperforate tracheary xylem elements tracheids with
simple or bordered pits, septate or non-septate. Wood rays uniseriate or
multiseriate, homocellular or heterocellular. Axial parenchyma apotracheal
diffuse or diffuse-in-aggregates, or paratracheal scanty, aliform,
lozenge-aliform, confluent, reticulate, scalariform, vasicentric, unilateral,
or banded. Tyloses often frequent. Sieve tube plastids Pc (PV) type, with 20 or
more square or polygonal protein crystalloids. Nodes ≥3:≥3, trilacunar or
multilacunar with three or more leaf traces, often with split lateral vascular
bundles. Heartwood often with ethereal substances. Prismatic calciumoxalate
crystals frequent; acicular crystals, styloids, crystal sand and other types of
crystals (in Paradrypetes raphides) often present.
Trichomes Hairs unicellular or
sometimes multicellular, uniseriate, simple or stellate; glandular hairs often
present.
Leaves Usually opposite
(rarely verticillate), simple, entire, coriaceous, usually with involute (in
Rhizophoreae supervolute) ptyxis. Stipules interpetiolar, large,
sheathing, caducous, with basal adaxial gum-excreting colleters; leaf sheath
absent. Petiole vascular bundle transection? Venation pinnate. Stomata
paracytic, cyclocytic (Rhizophoreae) or anomocytic (rarely
anisocytic). Cuticular wax crystalloids? Epidermis with or without mucilaginous
idioblasts. Mesophyll with or without sclerenchymatous idioblasts (with
H-shaped and other sclereids). Leaf margin serrate, crenate or entire (in
Paradrypetes spinose-serrate); leaf teeth theoid.
Inflorescence Usually
axillary, fasciculate, raceme- or spike-like (flowers in some species of
Bruguiera solitary axillary; inflorescence in Paradrypetes
epipetiolar).
Flowers Actinomorphic. Pedicel
articulated. Hypanthium-like structure usually present. Hypogyny
(Macarisieae), epigyny or half epigyny. Sepals (three or) four or five
(to 16), with valvate aestivation, usually carnose or coriaceous, persistent,
free. Petals (three or) four or five (to 16), with contorted or inflexed
aestivation, small, alternisepalous, usually hairy and carnose, often bilobate,
usually with apiculate arista and fimbriate or with filiform appendages, often
clawed, free. Subepidermal laticifers present. Nectariferous disc
intrastaminal, inserted on ovary or “hypanthium”, or absent. Sepals and
ovary with layer of hypodermal laticiferous cells (in Gynotroches and
Pellacalyx as numerous secretory idioblasts).
Androecium Stamens eight to
numerous, usually at least twice as many as petals (sometimes as many as
petals), diplostemonous or obdiplostemonous. Filaments usually free (rarely
connate at base), antepetalous, single or in fascicles of two to five stamens,
free from tepals, usually inserted on abaxial side of nectariferous disc; each
stamen or staminal fascicle usually enclosed by petal. Anthers dorsifixed,
versatile, tetrasporangiate (in Rhizophora multilocellate due to
transverse septa), introrse or latrorse, longicidal (dehiscing by longitudinal
valve). Tapetum secretory. Staminodia absent. Rhizophoreae with
secondary pollen display.
Pollen grains
Microsporogenesis simultaneous or successive (Gynotrocheae). Pollen
grains 3(–4)-colporate to 3(–4)-colporoidate, shed as monads, bicellular at
dispersal. Exine tectate or semitectate, with columellate or
granular-columellate infratectum, punctate-rugulate, punctate, reticulate or
smooth (in Paradrypetes echinate).
Gynoecium Pistil composed of
(two or) three to five (to 20) connate antesepalous carpels; when two carpels,
then lateral (in Rhizophora transverse). Ovary superior, inferior or
semi-inferior, unilocular or multilocular, synascidiate. Style single, usually
simple (in Gynotroches branched; absent in Paradrypetes).
Stigma punctate, capitate or lobate, usually papillate, type? Pistillodium
absent.
Ovules Placentation apical to
axile. Ovules two to numerous per carpel, anatropous to hemianatropous (in
Bruguiera and Carallia campylotropous), pendulous,
epitropous?, bitegmic, usually crassinucellar (in Gynotroches and
Pellacalyx tenuinucellar). Micropyle usually bistomal, Z-shaped
(zig-zag) (sometimes endostomal). Outer integument usually three to six (in
Carallia approx. ten) cell layers thick, in Rhizophoreae
vascularized. Inner integument four to eight (to 20) cell layers thick.
Obturator present (Macarisieae) or absent (Gynotrocheae,
Rhizophoreae). Endothelium usually present. Parietal tissue one to
three cell layers thick. Megasporocytes often several. Megagametophyte
monosporous, Polygonum type. Antipodal cells ephemeral. Endosperm
development ab initio nuclear. Endosperm haustorium micropylar and chalazal
(Ceriops). Embryogenesis?
Fruit A single- or many-seeded
berry (Gynotrocheae), a drupe with one seed per locule, a capsule
(Macarisieae, Crossostylis), or with hard pericarp and
indehiscent (Rhizophoreae; seedling in Bruguiera dispersed
together with fruit).
Seeds Seeds sometimes winged.
Aril/carunculus exostomal or absent. Seed coat usually exotegmic. Testa
sometimes multiplicative, indistinct (Rhizophoreae), or (in
Macarisieae and Gynotrocheae) exotestal cells more or less
enlarged, thick-walled. Endotesta sometimes crystalliferous. Tegmen usually
thickened (absent in ripe seeds ofRhizophoreae), with invaginations.
Exotegmen in Macarisieae and Crossostylis fibrous. Endotegmen
crushed (Macarisieae) Perisperm not developed. Endosperm sparse or
copious, oily. Embryo long or short, straight, well differentiated, with
chlorophyll. Cotyledons usually two (rarely three or four), with unilacunar
node. Germination phanerocotylar or viviparous. In mangrove trees
(Rhizophoreae) vivipary, with much enlarged hypocotyl.
Cytology n = (13) 14, 16, 18,
21, 32 (Anopyxis)
DNA
Phytochemistry Flavonols
(kaempferol, quercetin), cyanidin, afzelechin, myricetin, ethereal oils (in
wood), ellagic acid, methylated ellagic acids, non-hydrolyzable tannins,
proanthocyanidins (procyanidin, prodelphinidins), necin and tropane (hygrolin)
alkaloids (oxytropanes, brugine, tropin, etc.), and pyrrolizidine alkaloids as
1-aminopyrrolizidine derivatives present. Saponins and cyanogenic compounds not
found.
Use Timber.
Systematics Rhizophoraceae are sister to
Erythroxylaceae.
Macarisieae Baill.,
Hist. Plant. 6: 295, 302. Jan-Mai 1876
6/c 63. Paradrypetes (2; P.
ilicifolia, P. subintegrifolia; western Amazonas, coastal
Brazil); Sterigmapetalum (c 9; tropical South America),
Cassipourea (c 40; tropical, eastern and southern Africa, Madagascar,
the Mascarene Islands, southern India, Sri Lanka, tropical America),
Macarisia (8; Madagascar), Anopyxis (3; A.
ealaensis, A. klaineana, A. occidentalis; tropical
Africa), Blepharistemma (1; B. serratum; southwestern India),
Comiphyton (1; C. gabonense; Gabon to eastern Congo). –
Tropical and southern Africa, Madagascar, southwestern India, Sri Lanka,
tropical South America. Calcium oxalate crystals solitary. Stipules valvate.
Leaf teeth often theoid. Hypanthium-like structure sometimes present. Sepals
with open aestivation. Filaments often of two different lengths. Anthers
latrorse; connectives often enlarged. Stigma capitate or punctate. Aril often
present. Fruit in Cassipourea a capsule. Seeds sometimes winged at
micropylar end. Exotegmen fibrous. – Paradrypetes may be sister to
the remaining Macarisieae. Brazil. Dioecious trees. Raphides present.
Lamina with long, Z-shaped intersecondary veins. Flowers small. Tepals in male
flowers three or four. Nectary absent. Exine spinulate. Style absent. Placental
obturator present. Fruit a drupe. Testa and tegmen vascularized. Endosperm
copious, starchy. Cotyledons wide, plicate.
[Gynotrocheae+Rhizophoreae]
Stilt roots or pneumatophores present.
Rootlets without root hairs. Leaves bijugate. Stipules imbricate.
Hypanthium-like structure present. Epigyny. Obturator absent. Testa
vascularized.
Gynotrocheae Engl.,
Syllabus, ed. 1: 147. Apr 1892
4/30–32. Carallia (11;
Madagascar, tropical Asia to tropical Australia and Solomon Islands),
Crossostylis (10–12; Melanesia, southwestern Polynesia),
Gynotroches (1; G. axillaris; Burma, Southeast Asia, Malesia
to New Guinea, Solomon Islands, the Caroline Islands and northern Queensland),
Pellacalyx (c 8; tropical Asia). – Madagascar, tropical Asia to
tropical Australia, islands in western Pacific. Stilt roots absent in
Pellacalyx. Carpels often more numerous than sepals. Ovules sometimes
up to eight per carpel, tenuinucellar. Outer integument two or three cell
layers thick. Inner integument two to four cell layers thick. Archespore
sometimes one. Fruit usually a berry (fruit in Crossostylis a
capsule). Aril usually absent (present in Crossostylis). Exotesta
mucilaginous, tanniniferous. Meso- and endotestal cells crystalliferous. Tegmen
absent or fibrous to palisade. Exotegmen usually well developed (absent in
Carallia); exotegmen in Crossostylis fibrous. Mesotegmen and
endotegmen persistent. Cotyledons in Carallia and Pellacalyx
involute.
Rhizophoreae Bartl.,
Ord. Nat. Plant.: 320. Sep 1830 [‘Rhizophorea genuina’]
4/19. Bruguiera
(6; B. cylindrica, B. exaristata, B. gymnorhiza,
B. hainesii, B. parviflora, B. sexangula; coasts of
tropical East Africa and east to Samoa), Rhizophora
(6; R. apiculata, R. mangle, R. mucronata, R.
racemosa, R. samoensis, R. stylosa; tropical coasts on
both hemispheres), Kandelia (2; K. candel, K.
obovata; coasts of India and Bangladesh to Borneo, China and Kyushu in
southern Japan), Ceriops (5; C. australis, C.
decandra, C. pseudodecandra, C. tagal, C.
zippeliana; coasts of East Africa and India to Queensland, Melanesia,
Micronesia and northern to southeastern China). – Tropical coasts on both
hemispheres. Stomata cyclocytic. Abaxial hypodermis present. Sclerenchymatous
sheath of midvein absent or poorly developed. Leaf margin entire. Petals
sometimes postgenitally fused above base. Anthers in Rhizophora
locellate. Endothelium absent. Fruit single-seeded, indehiscent. Seed coat
undifferentiated, vascularized. Tegmen not persistent. Endosperm usually
overflowing and opening micropyle (not in Bruguiera).
Embryo usually large. Seeds germinating on tree (viviparous). Cotyledons in Bruguiera
and Rhizophora
convolute; cotyledonary node trilacunar or multilacunar. Hypocotyl finally
straightening.
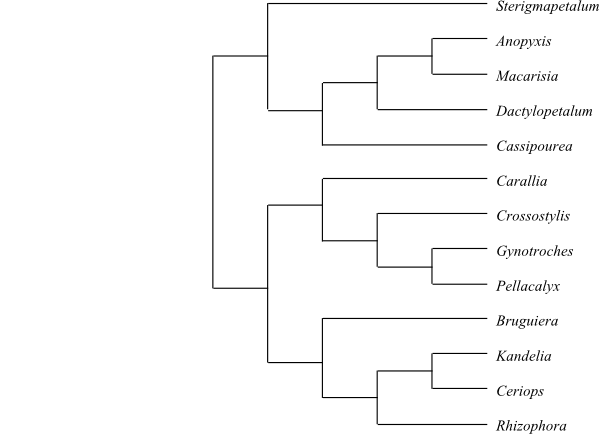 |
Cladogram of Rhizophoraceae based
on DNA sequence data and morphology (Schwarzbach & Ricklefs 2000).
Paradrypetes is sister to Cassipourea (Wurdack &
Davis 2009).
|
Mirbel, Elém. Physiol. Vég. Bot. 2: 905. 24-30
Jun 1815 [’Salicineae’], nom. cons.
Prockiaceae Bertuch,
Taf. Allg. Naturgesch. Gewächs-Reich: Enum. 5, Syn. Tab. 3. 1801
[’Prockiae’]; Samydaceae Vent. in Mém.
Cl. Sci. Math. Inst. Natl. France 1807(2): 149. 1808 [’Samydeae’],
nom. cons.; Homaliaceae R. Br. in J. H. Tuckey, Narr.
Exped. Zaire: 438. 5 Mar 1818 [’Homalinae’];
Samydales Vent. ex Bercht. et J. Presl, Přir.
Rostlin: 227. Jan-Apr 1820 [‘Samydeae’];
Flacourtiaceae Rich. ex DC., Prodr. 1: 255. med Jan
1824 [‘Flacourtianeae’], nom. cons.;
Blakwelliaceae T. Lestib., Botanogr. Élem.: 519. Jun
1826 [‘Blakwelliées’], nom. illeg.;
Salicopsida Bartl., Ord. Nat. Plant: 92, 118. Sep
1830 [’Salicinae’]; Salicales Lindl.,
Nix. Plant.: 17. 17 Sep 1833 [‘Salicinales’];
Flacourtiales Rich. in C. F. P. von Martius, Consp.
Regn. Veg.: 58. Sep-Oct 1835 [‘Flacourtianeae’];
Homaliales R. Br. in C. F. P. von Martius, Consp.
Regn. Veg.: 63. Sep-Oct 1835 [‘Homalineae’];
Flacourtiineae Engl., Syllabus, ed. 2: 154. Mai 1898;
Scyphostegiaceae Hutch., Fam. Fl. Pl. 1: 229. 15 Jan
1926, nom. cons.; Scyphostegiales Croizat, Kirkia 15:
137. Sep 1994; Bembiciaceae R. C. Keating et Takht.
in Bot. Žurn. 81(2): 85. Mai-Jun 1996;
Poliothyrsidaceae (G. S. Fan) Doweld, Tent. Syst.
Plant. Vasc.: xxxi. 23 Dec 2001; Caseariaceae Doweld,
New Syllabus Pl. Fam.: 693. 2008
Genera/species
56/1.225–1.240
Distribution Mainly tropical
and subtropical regions, some genera in temperate regions, few in Australia,
absent from New Zealand; Salix and Populus mainly in
temperate regions and some species of Salix only in arctic-alpine
regions on the Northern Hemisphere.
Fossils Pseudosalix
from the Eocene of Utah and Colorado is morphologically intermediate between
Salix and genera formerly included in ‘Flacourtiaceae’
(e.g. Bennettiodendron, Idesia, and Polyothyris).
Fossil pollen grains assigned to Casearia have been described from
Oligocene layers.
Habit Usually bisexual
(sometimes dioecious, e.g., Salix, Populus,
Chosenia, and Scyphostegia), evergreen or deciduous trees or
shrubs (some genera climbing; few species of Salix dwarf shrubs).
Casearia sometimes with phyllanthoid branching: orthotropic branches
with reduced spiral leaves, and plagiotropic branches sylleptic with normal
leaves distichous.
Vegetative anatomy
Ectomycorrhiza present in at least Salix, Populus and
Chosenia. Phellogen ab initio superfical. Primary medullary rays
narrow. Primary vascular tissue cylinder, without separate bundles. Vessel
elements with simple or scalariform perforation plates; lateral pits alternate,
usually bordered (sometimes simple) pits. Imperforate tracheary xylem elements
fibre tracheids or libriform fibres with simple or bordered pits, septate or
non-septate (also vasicentric tracheids). Wood rays uniseriate or multiseriate,
homocellular or heterocellular. Axial parenchyma absent or very rare. Tyloses
often abundant. Sieve tube plastids S type; sieve tubes with non-dispersive
protein bodies? Nodes usually 3:3, trilacunar with three leaf traces (in
Xylosma and some Casearia 1:1, unilacunar with one trace, in
some species of Azara 2:2, bilacunar with two traces). Secondary
phloem sometimes stratified into hard fibrous and soft parenchymatous layers
(Salix, Populus, Chosenia?), in, e.g.,
Flacourtia and Xylosma with groups of large sclereids.
Secretory cavities absent. Heartwood often with ethereal? substances. Prismatic
calciumoxalate crystals abundant; acicular crystals, druses, styloids, crystal
sand and other types of crystals often present.
Trichomes Hairs unicellular or
multicellular, uniseriate or multiseriate, simple, furcate, stellate, peltate,
lepidote, or vesicular, or absent; glandular hairs often present (transparent
in, e.g., Abatia, Casearia, Ryania, and
Zuelania).
Leaves Usually alternate
(spiral or distichous; in Abatia opposite), simple, usually entire
(sometimes lobed), sometimes coriaceous, with supervolute-curved or involute
ptyxis. Stipules small to foliaceous, intrapetiolar, usually caducous
(sometimes persistent; rarely absent); leaf sheath absent. Petiole vascular
bundle transection arcuate or annular; petiole with wing bundles (petiole in
Salix, Populus and Chosenia with unique arrangement
of vascular strands as one or several closed cylinders of xylem and floem, in
Populus often superimposed). Venation usually pinnate (rarely
palmate), brochidodromous, campylodromous or actinodromous. Stomata anomocytic,
paracytic or anisocytic. Cuticular wax crystalloids as rosettes of platelets
(Fabales type) or absent. Domatia as pits or hair tufts. Epidermis
with or without mucilaginous idioblasts, with or without crystalliferous
idioblasts. Cystoliths present (Homalium) or absent. Mesophyll with
calciumoxalate as druses and single prismatic crystals, with or without
sclerenchymatous idioblasts. Lamina sometimes gland-dotted. Leaf margin usually
serrate (rarely entire, crenate or crispate), often with salicoid teeth (one
vein running into tooth, tooth apex expanding into spheroid glandular
structure). Extrafloral nectaries often present on petiole and/or lamina
(usually pairwise on junction of petiole and lamina).
Inflorescence Terminal or
axillary (in Bembicia epiphyllous and cone-shaped; in
Phyllobotryon and Phylloclinium epiphyllous and solitary or
few-flowered), usually cymose (sometimes racemose) of various shape (spike-,
head- or catkin-like, fasciculate, panicle, spike, or catkin; flowers rarely
solitary). Floral prophylls (bracteoles) present or absent. Telescoping floral
bracts present in Scyphostegia.
Flowers Actinomorphic, often
small. Hypanthium present in some genera. Usually hypogyny (rarely epigyny or
half epigyny). Sepals three to eight (to c. 15), with imbricate or valvate
aestivation, often persistent, usually free (sometimes connate at base; rarely
petaloid; sometimes absent). Petals three to eight (to c. 15; in, e.g., some
Scolopieae outnumbering sepals), with valvate or imbricate
aestivation, usually free (rarely connate at base; sometimes absent); tepals
rarely (in, i.a., Oncoba) spiral and hardly subdivided into calyx and
corolla; tepals in Salix connate and modified into one or several
nectaries (tepals sometimes absent). Corona in some genera present as scales,
hairs or lobes on petal bases. Nectariferous disc extrastaminal or
intrastaminal, as glands, scales or lobate annular disc (sometimes absent).
Androecium Stamens one to c.
40 (to more than 100), usually antesepalous (sometimes alternisepalous), in one
or several whorls (sometimes in three to eight antepetalous fascicles).
Filaments filiform, free or connate at least at base (in Scyphostegia
entirely connate), free from tepals. Anthers dorsifixed or basifixed,
non-versatile, tetrasporangiate, usually introrse (rarely extrorse), longicidal
(dehiscing by longitudinal slits); connective sometimes apically prolonged.
Tapetum secretory. Staminodia in Scyphostegia three, extrastaminal.
Female flowers often with staminodia.
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually tricolporate or
tricolpate to tricolporoidate (Salix; in Populus
inaperturate), shed as monads, usually bicellular (rarely tricellular) at
dispersal. Exine tectate or semitectate, with columellate infratectum,
perforate or reticulate, verrucate, spinulate, or echinate.
Gynoecium Pistil composed of
two to five (to 13) usually connate carpels (in Oncoba secondarily
free); gynophore present in some species. Ovary usually superior (rarely
inferior or semi-inferior), usually unilocular (in Scyphostegia
unilocular in lower part, multilocular in upper part; rarely entirely
multilocular). Style single, simple, persistent, or stylodia several, free or
connate in lower parts (sometimes absent). Stigmas one or several, lobate,
capitate or attenuate, commissural or carinal, non-papillate, Dry type. Male
flowers often with pistillodium.
Ovules Placentation usually
parietal or free central (sometimes intrusively parietal; rarely axile or
basal; in Scyphostegia basal). Ovules usually two to more than 100
(rarely one) per ovary, usually orthotropous (sometimes anatropous or
hemianatropous), ascending, usually bitegmic (sometimes unitegmic, with inner
integument rudimentary), crassinucellar. Micropyle usually bistomal (rarely
exostomal or endostomal), sometimes Z-shaped (zig-zag). Outer integument two to
five cell layers thick. Inner integument two to five cell layers thick.
Hypostase usually absent (present in Casearia). Parietal tissue five
to seven cell layers thick, finally resorbed. Nucellar cap usually present,
finally resorbed. Megagametophyte usually monosporous, Polygonum type
(rarely disporous, Allium type), often protruding through micropyle.
Synergids sometimes with a filiform apparatus. Endosperm development ab initio
nuclear. Endosperm haustoria? Embryogenesis onagrad or asterad.
Fruit Usually a berry or
loculical capsule (rarely a drupe or samara).
Seeds Seeds often with aril
(in Salix, Populus and Chosenia with arillar hair
tuft at base: aril modified into hair tuft). Testa multiplicative, carnose
(otherwise indistinct), or exotesta or endotesta (Oncoba) palisade.
Exotegmen usually fibrous, lignified. Exotegmic cells is Dovyalis
ribbon type. Endotegmen persistent? Perisperm usually not developed (in
Scyphostegia thin). Endosperm usually copious (sometimes sparse,
absent in Salix), oily. Embryo large, usually straight, well
differentiated, with or without chlorophyll. Cotyledons two, plano-convex or
flat. Germination phanerocotylar or cryptocotylar.
Cytology n = 9–12, 19
DNA Gene duplication (“the
salicoid duplication”) present in Populus and Salix.
Plastid gene rps16 lost in Populus (P. deltoides)
and Salix (S. amygdaloides).
Phytochemistry Flavonols
(kaempferol, quercetin), biflavonoids, biflavanoids, cyanidin, ellagic acid,
tannins with proanthocyanidins and catechin, proanthocyanidins
(prodelphinidins), phenolic glycosides (salicin, populin, etc.), gynocardins,
saponins, acetophenones, arbutin, myo-inisitol, nigracin, and
co-carcinogenic substances present. Cyclopentenoid cyanogenic glycosides not
found. Aluminium accumulated in some species.
Use Ornamental plants, matches
(Populus), baskets (withies from Salix), bioenergy
(Salix), timber, protective plantations (against wind and erosion),
fruits (Dovyalis), medicinal plants.
Systematics Salicaceae are probably sister
to Lacistemataceae.
Salicaceae are under
construction and the subdivision below is highly provisional.
Samydeae Vent. in B.
C. J. Dumortier, Anal. Fam. Plant.: 18. 1829
14/240–245. Casearia (c 180;
tropical regions on both hemispheres), Euceraea (3; E.
nitida, E. rheophytica, E. sleumeriana; Venezuela,
Guyana, Suriname, Brazil), Hecatostemon (1; H. completus;
Venezuela, Guyana, Brazil), Laetia (9; Central America, tropical South
America), Lunania (15; southern Mexico, Central America, Cuba,
Jamaica, northern South America), Neoptychocarpus (3; N.
apodanthus, N. chocoensis, N. killipii; tropical South
America), Ophiobotrys (1; O. zenkeri; tropical West and
Central Africa), Osmelia (4; O. gardneri, O.
grandistipulata, O. maingayi, O. philippina; Sri Lanka,
Malesia), Pseudosmelia (1; P. moluccana; the Moluccas),
Ryania (9; tropical America), Samyda (11; Central America,
the West Indies), Tetrathylacium (2; T. johansenii, T.
macrophyllum; tropical America), Zuelania (1; Z.
guidonia; southern Mexico, Central America, Venezuela);
Trichostephanus (2; T. acuminatus, T. gabonensis;
tropical West and Central Africa). – Tropical regions on both hemispheres.
Bisexual (in Trichostephanus monoecious) trees or shrubs. Stipules
usually caducous. Lamina often with pellucid glandular dots and lines. Leaf
margin serrate or entire; leaf teeth theoid (e.g. Casearia) or
salicoid. Hypanthium present. Sepals three to seven, connate at base (absent in
Trichostephanus). Petals absent. Nectariferous disc present at adaxial
side of sepal bases (calyx tube), lobate. Stamens three to twelve (sometimes
more). Ovary superior to semi-inferior, unilocular. Style simple or distally
trifid. Stigma trilobate or stigmas capitate. Ovules several per carpel. Fruit
a carnose or coriaceous capsule (often berry-like), usually with persistent
sepals, disc, filaments and style. Aril vascularized, often fimbriate.
Exotegmic cells laterally flattened, crystalliferous. – Casearia is
sometimes identified as sister-group with low support to the remaining Salicaceae. The position of
Trichostephanus is enigmatic and provisional.
[Scyphostegia+[Prockieae+Saliceae+Homalieae+Scolopieae+Oncobeae]]
Lamina with small vein proceeding into
tooth and expanding. Salicoid teeth present (tooth apex spherical and variously
coloured gland or stout hair).
Scyphostegieae
(Hutch.) Zmarzty in M. W. Chase et al. in Kew Bull. 57: 170. 2002
2/2. Scyphostegia (1; S.
borneensis; northern and central Borneo), Dianyuea (1; D.
turbinata; Yunnan). – Dioecious trees. Phellogen superficial. Vessel
elements in radial multiples, with usually simple (rarely scalariform)
perforation plates; lateral pits alternate. Fibres with thick walls and large
bordered pits. Wood rays usually uniseriate, heterocellular. Axial parenchyma
almost absent. Leaves distichous. Petiole vascular bundle transection annular;
adaxial xylem and phloem developing into inverted adaxial plate of vascular
tissue. Stipules small, caducous. Stomata paracytic. Inflorescence terminal,
panicle. Bracts tubular, overlapping. Pedicel not articulated. Hypogyny.
Perianth possibly consisting of three sepals alternating with three petals.
Nectariferous lobes opposite three “antepetalous” stamens. Filaments
connate. Anthers extrorse. Pollen grains tricolpate? Pistil composed of eight
to 13 connate carpels. Ovary unilocular, septated near apex. Style absent.
Stigma discoid, with eight to 13 rays and central orifice. Placentation basal.
Ovules numerous per carpel. Micropyle bistomal (or exostomal?). Outer
integument two or three cell layers thick. Inner integument three or four cell
layers thick. Nucellar cap present, persistent. Fruit a fleshy capsule with
lignified commissural valves. Aril formed from funicle and outer integument.
Exotegmen fibrous. Perisperm very thin. Endosperm sparse. x = 9.
[Prockieae+Saliceae+Homalieae+Scolopieae+Oncobeae]
Stamens centrifugally developing. Pistil
composed of two to five (to 13) connate carpels. Megagametophyte protruding
through micropyle. Benzoylated glycosides present. Cyanogenic glycosides
usually absent.
Prockieae Endl., Gen.
Plant.: 918. Nov 1839
9/67. Banara (34; tropical
America), Hasseltia (3; H. allenii, H. floribunda,
H. guatemalensis; southern Mexico to Brazil and Bolivia),
Hasseltiopsis (1; H. dioica; Central America), Azara (9;
temperate and subtropical South America), Neosprucea (9; Panamá,
northern South America), Pineda (2; P. incana, P.
ovata; the Andes in Ecuador and Peru), Prockia (5; P.
costaricensis, P. crucis, P. flava, P. krusei,
P. pentamera; southern Mexico to northern Argentina), Abatia
(10; mountain regions in tropical America), Aphaerema (1; A.
spicata; southern Brazil). – Tropical America, temperate and subtropical
South America. Nodes in some species of Azara
bilacunar with two leaf traces. Opposite leaves present in Abatia.
Tepals in Abatia with valvate aestivation, connate at base. Nectary
absent in Abatia. Cyanogenic glycosides present in Banara.
Saliceae Rchb., Fl.
Germ. Excurs. 1(2): 165. Jan-Apr 1831 [‘Salicinae’]
12/550–555. Populus
(35–40; temperate regions on the Northern Hemisphere, on species, P.
ilicifolia, in East Africa), Salix (c
450; temperate and arctic-alpine regions on the Northern Hemisphere, few
species on the Southern Hemisphere), Bennettiodendron (7; B.
brevipes, B. cordatum, B. lanceolatum, B.
leprosipes, B. longipes, B. macrophyllum, B.
subracemosum; southern China, tropical Asia), Olmediella (1;
O. betschleriana; Central America), Idesia
(1; I. polycarpa; China, Japan), Macrohasseltia (1; M.
macroterantha; Central America), Carrierea (4; C.
calycina, C. dunniana, C. rehderiana, C.
vieillardii; southern and southwestern China, Southeast Asia),
Itoa (1; I. orientalis; southern China, tropical Asia),
Lasiochlamys (11; New Caledonia), Ludia (27; tropical East
Africa, Madagascar, the Mascarene Islands), Poliothyrsis (1; P.
sinensis; China), Tisonia (14; Madagascar). – Temperate and
arctic-alpine regions on the Northern Hemisphere, tropical East Africa,
Madagascar, the Mascarene Islands, East and tropical Asia, New Caledonia,
Central America, few species on the Southern Hemisphere. Cuticular wax
crystalloids as rosettes of platelets (Fabales type) or cuticular
waxes absent. Inflorescence often catkin. Perianth highly reduced (petals often
absent). Pollen grains in Populus
inaperturate. Pistil composed of two collateral connate carpels. Ovules usually
unitegmic. Micropyle in Idesia
exostomal. Long placental hairs present at seed base. Tegmen impermanent.
Endosperm absent in Salix.
Embryo with chlorophyll. – The Idesia
clade and the Salix
clade appear to be very closely allied. They have salicoid leaf teeth, similar
or identical phenolic compounds (e.g. salicin), and similar or identical
parasites (rusts and insect larvae). Populus,
Salix,
Banara and Idesia
with foliar extrafloral nectaries. Chosenia is nested in Salix,
according to Leskinen & Alström-Rapaport (1999).
Homalieae Dumort.,
Anal. Fam. Plant.: 18. 1829 [‘Homalineae’]
8/c 200. Bartholomaea (3;
B. mollis, B. paniculata, B. sessiliflora; Central
America), Bivinia (1; B. jalbertii; tropical East Africa,
Madagascar), Byrsanthus (1; B. brownii; tropical West
Africa), Calantica (10; Madagascar), Dissomeria (2; D.
crenata, D. glanduligera; tropical Africa), Homalium (c
180; tropical and subtropical regions on both hemispheres),
Neopringlea (3; N. integrifolia, N. trinervia,
N. viscosa; southern Mexico, Guatemala), Bembicia (1; B.
axillaris; Madagascar). – Tropical and subtropical regions on both
hemispheres. Epigyny present in Homalium.
Scolopieae Warb. in
Engler et Prantl, Nat. Pflanzenfam. III, 6a: 13. 28 Dec 1893
5/43. Hemiscolopia (1; H.
trimera; tropical Asia), Mocquerysia (2; M.
coerulipennis, M. multiflora; tropical Africa),
Phyllobotryon (2; P. bracteatum, P. paradoxum;
tropical Africa), Pseudoscolopia (1; P. polyantha; South
Africa), Scolopia (37; tropical regions in the Old World east to
northeastern Queensland). – Tropical regions in the Old World.
Oncobeae Benth. in
Proc. Linn. Soc., Bot. 5(Suppl. 2): 77. 1861
6/115–120. Oncoba (c 30;
tropical and southern Africa, the Arabian Peninsula), Flacourtia (22;
tropical and southern Africa, Madagascar, Southeast Asia, Malesia to Fiji), Dovyalis
(17; tropical and southern Africa, Madagascar, tropical Asia from Sri Lanka to
New Guinea), Trimeria (2; T. grandifolia, T.
trinervis; tropical East Africa to southern Africa),
Pleuranthodendron (1; P. lindenii; southern Mexico to
Brazil), Xylosma (c 45?; East and Southeast Asia, Malesia to New
Guinea, eastern Queensland, New Caledonia, Vanuatu, Polynesia, Guam, Central
America, the West Indies, northern South America). – Pantropical. Micropyle
in Oncoba endostomal. Testa in Oncoba sarcotesta. Endotesta
in Oncoba palisade. – Oncoba resembles some species in
Achariaceae-Lindackerieae,
although they differ in, i.a., leaf teeth type, stamen initiation and
phytochemistry.
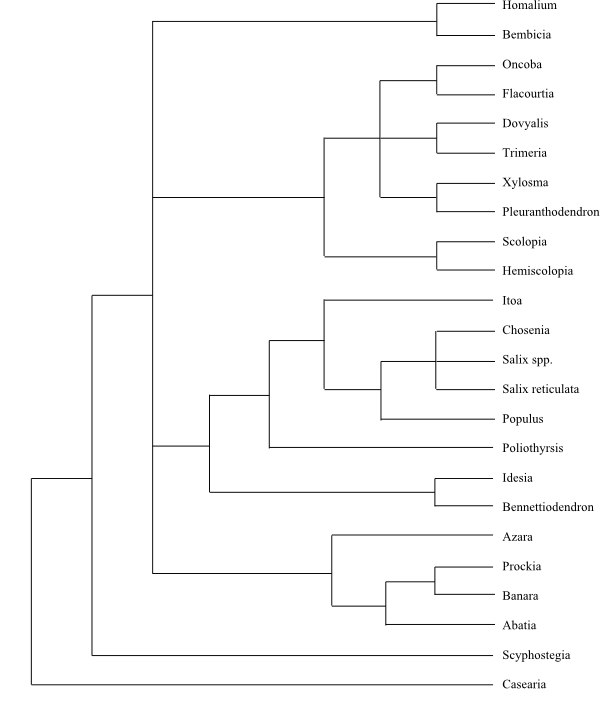 |
Cladogram of Salicaceae (one of 709
equally most parsimonious trees of Malpighiales) based on
DNA sequence data (Sosa & al. 2003). Lunania is sister to
Casearia, Laetia and Samyda, according to
Shang & al. (2017), and Hasseltia is sister to a clade
comprising, e.g., Homalium, Xylosma,
Scolopia, Oncoba, and Flacourtia.
|
de Jussieu in V. V. D. d’Orbigny, Dict. Univ.
Hist. Nat. 12: 670. 7 Jul 1849, nom. cons.
Genera/species 5/28
Distribution Madagascar, West
Malesia, Central America, tropical South America.
Fossils Unknown.
Habit Bisexual, evergreen
trees, shrubs or lianas.
Vegetative anatomy Phellogen
ab initio superficial. Vessel elements usually with simple (rarely also
scalariform) perforation plates; lateral pits almost absent, alternate,
bordered pits. Vestured pits present. Imperforate tracheary xylem elements
tracheids and fibre tracheids (libriform fibres and intraxylary phloem absent)
with bordered pits, septate or non-septate (also vasicentric tracheids). Wood
rays uniseriate or multiseriate, heterocellular. Axial parenchyma apotracheal
diffuse, diffuse-in-aggregates, or banded, or paratracheal scanty, aliform,
vasicentric, or banded (wood ray and axial parenchyma cell walls with helical
thickenings). Wood in at least one species fluorescent. Intraxylary phloem
absent. Sieve tube plastids Ss type. Nodes 1:1, unilacunar with one leaf trace;
lateral bundles split. Branched sclereids present. Silica bodies absent.
Rhomboid or irregularly shaped calciumoxalate crystals frequent.
Trichomes Hairs unicellular or
multicellular, simple or T-shaped; glands stalked or unstalked.
Leaves Usually opposite (in
Trigoniastrum alternate, spiral or distichous), simple, entire, with ?
ptyxis. Stipules interpetiolar (when leaves opposite), caducous, often connate;
leaf sheath absent. Petiole articulated. Petiole vascular bundle transection
arcuate. Venation pinnate. Stomata paracytic. Inconspicuous pilose domatia
present on leaves in Isidodendron. Cuticular wax crystalloids?
Epidermis and hypodermis with or without mucilaginous idioblasts. Leaf margin
entire. Leaf margin and abaxial side of bracts in Trigoniastrum with
impressed glands.
Inflorescence Terminal or
axillary, panicle or thyrse (in Isidodendron raceme). Floral prophylls
(bracteoles) two or three.
Flowers Obliquely zygomorphic.
Hypanthium-like structure (“floral cup”) short or absent. Half epigyny.
Sepals five, with imbricate quincuncial aestivation, unequal in size, connate
below; median sepal adaxial. Petals three or five, usually with imbricate
quincuncial or contorted (rarely valvate) aestivation, free; adaxial inner
petal forming spurred or at base saccate velum together with one or two sepals;
plicae of abaxial outer and abaxial lateral two petals forming often saccate
carina or abaxial petals saccate; two lateral petals forming spatulate alae.
One to three nectariferous glands, each up to trilobate, present at base of
adaxial side of velum (large adaxial inner petal) and usually adjacent to slit
on staminal tube, adnate to stamens and staminodia (nectariferous disc absent
in Isidodendron, nectariferous glands here present at staminodial
bases).
Androecium Five to eight (to
13) anterior four to nine stamens fertile and one to six lateral and/or four to
six rudimentary posterior stamens staminodial (often scale-like),
haplostemonous or diplostemonous. Filaments more or less connate into tube,
slit on one side, free from tepals. Anthers dorsifixed (almost basifixed),
somewhat versatile, tetrasporangiate, introrse, longicidal (dehiscing by
longitudinal slits); connective often dorsally thickened? Tapetum secretory?
Staminodia (one to) three to six (intrastaminal?; sometimes absent).
Pollen grains
Microsporogenesis simultaneous? Pollen grains tri- to pentaporate, shed as
monads, ?-cellular at dispersal. Exine tectate, with columellate? infratectum,
verrucate to smooth.
Gynoecium Pistil composed of
three (or four) connate carpels; median carpel adaxial; carpel partially
synascidiate. Ovary semi-inferior, unilocular or trilocular (or quadrilocular).
Style single, simple. Stigma single, trilobate with reflexed lobes, papillate,
Dry type. Pistillodium absent.
Ovules Placentation usually
axile (parietal when ovary unilocular, with deeply intrusive placentae). Ovules
one to ten (to c. 20) per carpel, anatropous, pendulous to ascending,
epitropous (antitropous) to apotropous (syntropous), bitegmic, usually finally
tenuinucellar by absorption of megasporangium (rarely crassinucellar).
Micropyle bistomal, Z-shaped (zig-zag), or endostomal. Outer integument two or
three cell layers thick. Inner integument four to six cell layers thick,
entirely endothelial. Obturator consisting of unicellular funicular hairs or
papillae. Megagametophyte monosporous, Polygonum type. Endosperm
development ab initio nuclear. Endosperm haustoria? Embryogenesis?
Fruit Usually a septicidal
capsule with carpels internally dehiscing and with persistent central fibre
strands (in Humbertiodendron and Trigoniastrum a three-winged
samaroid).
Seeds Aril absent. Testa
sometimes winged, often with long lignified hairs. Exotestal cells with
thickened outer walls. Endotesta? Tegmen often multiplicative. Exotegmen?
Endotegmic cells with tannins and somewhat thickened walls. Perisperm not
developed. Endosperm absent. Embryo straight, well differentiated, in
Trigonia with chlorophyll. Cotyledons two, large, thin, flat.
Hypocotyl elongate. Germination phanerocotylar.
Cytology n = c. 10
DNA
Phytochemistry Virtually
unknown. Tannins present. Aluminium not accumulated.
Use Timber.
Systematics Trigonia
(24; Central America, tropical South America), Humbertiodendron (1;
H. saboureaui; eastern Madagascar), Trigoniastrum (1; T.
hypoleucum; West Malesia), Trigoniodendron (1; T.
spiritusanctense; coastal rainforest in Espírito Santo state in Brazil),
Isidodendron (1; I. tripterocarpum; the Río Magdalena valley
in central Colombia).
Trigoniaceae are sister-group
to Dichapetalaceae.
There is no available phylogeny of Trigoniaceae.
de Candolle, Prodr. 3: 345. med Mar 1828, nom.
cons.
Piriquetaceae
Martinov, Tekhno-Bot. Slovar: 484. 3 Aug 1820 [’Piriquettae’];
Turnerales Link, Handbuch 2: 47. 4-11 Jul 1829
[’Turneraceae’]
Genera/species 12/210–220
Distribution Tropical,
subtropical and warm-temperate regions in Mexico, Central and South America,
the West Indies and Africa south of Sahara, Madagascar, Rodriguez.
Fossils Unknown.
Habit Bisexual, usually
perennial herbs or evergreen shrubs (rarely trees). Often evil-smelling.
Vegetative anatomy Phellogen
ab initio superficial. Cortical vascular bundles abundant. Medulla usually
parenchymatous, sometimes with sclereids. Vessel elements usually with simple
(sometimes scalariform) perforation plates; lateral pits? Imperforate tracheary
xylem elements libriform fibres with thick walls and small bordered pits,
non-septate? (also vasicentric tracheids). Wood rays uniseriate or
multiseriate?, heterocellular. Axial parenchyma apotracheal diffuse? Sieve tube
plastids Ss type; sieve tubes with non-dispersive protein bodies? Nodes?
Crystalliferous and tanniniferous cells frequent; parenchyma cells sometimes
with calciumoxalate crystals and starch grains.
Trichomes Hairs unicellular or
multicellular, usually uniseriate, simple, sometimes multi-armed or stellate;
glandular hairs excreting nasty-smelling substance often present.
Leaves Alternate (spiral),
usually simple (simetimes pinnately compound), entire, sometimes ericoid,
usually with conduplicate (sometimes revolute) ptyxis. Stipules usually small
or absent (present in Erblichia and some species of Turnera);
leaf sheath absent. Stipules and/or prophylls and leaf primordia with
colleters. Petiole vascular bundle transection? Distal part of petiole or leaf
base often with extrafloral nectaries. Venation pinnate, with coarse veins;
vein proceeding into transparent and caducous tooth apex? Stomata anomocytic,
paracytic or anisocytic. Cuticular wax crystalloids? Lamina sometimes
gland-dotted. Epidermis with or without mucilaginous idioblasts. Mesophyll with
or without sclerenchymatous idioblasts with calciumoxalate druses. Leaf margin
serrate or crenate (teeth sometimes glanduliferous).
Inflorescence Flowers usually
solitary axillary, sometimes in terminal or axillary raceme (flowers in
Piriqueta and several species of Turnera epiphyllous). Floral
prophylls (bracteoles) often large.
Flowers Actinomorphic.
Hypogyny or half epigyny. Sepals five, with imbricate quincuncial aestivation,
usually connate into caducous tube (in Erblichia and
Mathurina almost free). Petals five, with contorted aestivation,
clawed, early withering and caducous, usually with claws adnate to calyx and
together with this forming tubular, campanulate or infundibuliform structure
(hypanthium; in Erblichia and Mathurina free); in orifice
sometimes five nectariferous glands, fringed corona or five lobes between
corona and stamens (nectaries sometimes present in perianth tube, in
Mathurina and Stapfiella on sepals, in some species of
Turnera on stamens or as five nectariferous pockets). Disc
extrastaminal, annular, or absent. Heterostyly sometimes present.
Androecium Stamens five,
haplostemonous, antesepalous, alternipetalous. Filaments usually free from each
other, usually free from tepals (sometimes adnate at base to petals; filaments
in Erblichia and Hyalocalyx adnate at base to calyx). Anthers
usually dorsifixed (in Erblichia and some species of Turnera
almost basifixed), sometimes versatile, tetrasporangiate, introrse, longicidal
(dehiscing by longitudinal slits). Tapetum secretory. Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains tricolpor(oid)ate, shed as
monads, bicellular at dispersal. Exine semitectate, with columellate
infratectum, reticulate.
Gynoecium Pistil composed of
(two or) three connate carpels. Ovary superior to semi-inferior, unilocular.
Stylodia (two or) three, filiform, often bifid, free. Stigmas concave,
commissural, fringed and bristle-like, non-papillate, Dry type. Pistillodium
absent.
Ovules Placentation usually
parietal (in Stapfiella basal). Ovules one (Stapfiella) to
more than 100 per ovary (Stapfiella with one basal ovule), anatropous,
ascending, bitegmic, crassinucellar. Micropyle bistomal, Z-shaped (zig-zag).
Outer integument ? cell layers thick. Inner integument ? cell layers thick.
Hypostase present. Megagametophyte monosporous, Polygonum type.
Endosperm development ab initio nuclear. Endosperm haustoria? Embryogenesis
onagrad.
Fruit A loculicidal capsule,
usually dehiscing from apex (rarely laterally), sometimes with persistent
perianth tube.
Seeds Aril or elaiosome (in
Mathurina fimbriate) often present. Operculum present. Testa hard,
non-multiplicative. Exotestal cells arranged in rows. Exotegmen palisade, with
sclereids. Endotegmen persistent? Perisperm not developed. Endosperm copious,
fleshy, oily. Embryo straight, well differentiated, chlorophyll? Cotyledons
two, plano-convex. Germination phanerocotylar.
Cytology x = 5, 7, 10 (13) –
Polyploidy occurring.
DNA Plastid gene
rps16 lost in at least Turnera.
Phytochemistry Insufficiently
known. Luteolin, ethereal oils, tannins, alkaloids, and cyclopentenoid
cyanogenic glycosides and/or cyclopentenylic fatty acids present. Flavonols,
ellagic acid, and proanthocyanidins not found.
Use Ornamental plants,
medicinal plants.
Systematics Erblichia
(1; E. odorata; Central America), Mathurina (1; M.
penduliflora; Rodriguez), Arboa (2; A. berneriana,
A. integrifolia; Madagascar), Stapfiella (4; S.
claoxyloides, S. lucida, S. ulugurica, S.
usambarica; tropical Africa), Hyalocalyx (1; H. setifer;
southeast tropical Africa, Madagascar), Tricliceras (11; tropical and
subtropical Africa), Loewia (1; L. glutinosa; northeastern
tropical Africa), Afroqueta (1; A. capensis; South Africa,
Swaziland), Streptopetalum (3–6; S. arenarium, S.
graminifolium, S. hildebrandtii, S. luteoglandulosum,
S. serratum, S. wittei; tropical and southern Africa),
Adenoa (1; A. cubensis; eastern Cuba), Piriqueta (c
45; tropical America), Turnera (140–145; tropical and subtropical
America, one species, T. oculata, in northwesternmost Namibia and
southwesternmost Angola, one species, T. thomasii, in Northeast
Africa).
Turneraceae are sister to
Passifloraceae.
A clade consisting of Adenoa,
Piriqueta and Turnera
is sister-group to the remaining Turneraceae, according to
Thulin & al. (2012). Erblichia was recovered as sister to the
remaining Turneraceae by
Arbo & al. (2015).
 |
Bayesian majority-rule consensus tree
(simplified) of Turneraceae based on
DNA sequence data (Thulin & al. 2012). A similar topology was
recovered by Tokuoka (2012).
|
 |
Phylogeny of Turneraceae
based on DNA sequence data (Arbo & al. 2015)
|
Batsch, Tab. Affin. Regni Veg.: 57. 2 Mai 1802
[‘Violariae’], nom. cons.
Violales Vent. ex
Bercht. et J. Presl, Přir. Rostlin: 220. Jan-Apr 1820 [‘Violaceae’];
Ionidiaceae Mert. et W. J. Koch in J. C. Röhlings,
Deutschl. Fl., ed. 3, 1(1): 244, 260. Jan-Mai 1823 [’Jonidien’,
’Jonidiae’], nom. illeg.; Ionidiales
Vent. in C. F. P. von Martius, Consp. Regn. Veg.: 50. Sep-Oct 1835
[‘Jonidieae’], nom. illeg.; Violopsida
Brongn., Enum. Plant. Mus. Paris: xxiv, 88. 12 Aug 1843
[’Violineae’]; Leoniaceae A. DC. in A.
P. de Candolle et A. L. P. P. de Candolle, Prodr. 8: 668. med Mar 1844;
Alsodeiaceae J. Agardh, Theoria Syst. Plant.: 197.
Apr-Sep 1858 [‘Alsodineae’]; Violanae R.
Dahlgren ex Reveal in Novon 2: 237. 13 Oct 1992
Genera/species c
31/900–1.000
Distribution Cosmopolitan
except polar areas.
Fossils Unknown.
Habit Usually bisexual (in
Melicytus dioecious), perennial or annual herbs, evergreen or
deciduous shrubs or trees (rarely lianas).
Vegetative anatomy Phellogen
superficial. Primary vascular tissue cylinder without separate vascular
bundles, or cylinder of bundles. Cambium in Viola storied. Vessel
elements with simple or scalariform perforation plates; lateral pits alternate,
opposite or scalariform, usually simple (sometimes bordered) pits. Imperforate
tracheary xylem elements libriform fibres with simple or bordered pits, septate
or non-septate (also vasicentric tracheids). Wood rays uniseriate or
multiseriate, homocellular or heterocellular (sometimes absent). Axial
parenchyma rare (paratracheal scanty) or absent. Sieve tube plastids S type;
sieve tubes with non-dispersive protein bodies? Endodermis in Viola
cunninghamii consisting of large cells with suberised walls. Nodes usually
3:3, trilacunar with three leaf traces (in Fusispermum 5:5,
quinquelacunar with five traces). Prismatic calciumoxalate crystals and druses
frequent.
Trichomes Hairs unicellular or
multicellular, uniseriate, simple; glandular hairs sometimes present.
Leaves Usually alternate
(spiral or distichous; in some species of Hybanthus and
Rinorea opposite), simple, entire or lobed, with often involute
ptyxis. Stipules sometimes petiolar, foliaceous, often lobed; leaf sheath
absent. Colleters usually present. Petiole vascular bundle transection usually
arcuate. Venation pinnate or palmate. Stomata paracytic or anomocytic
(anisocytic?), often on adaxial side of lamina only. Cuticular wax
crystalloids? Domatia as pits or hair tufts. Epidermis often with mucilaginous
idioblasts. Secretory cavities absent. Leaf margin serrate or entire.
Inflorescence Terminal or
axillary, panicle, raceme-like or capitate, or flowers solitary axillary.
Flowers Zygomorphic (in, i.a.,
Melicytus almost actinomorphic). Pedicel articulated. Hypogyny. Sepals
five, with median sepal adaxial, with imbricate quincuncial or open
aestivation, often persistent, free or connate at base, sometimes with basal
appendages. Petals five, usually with apotact (in Fusispermum
convolute, in Leonia and some species of Gloeospermum
imbricate quincuncial) aestivation, free, abaxial (anterior) petal often with
nectariferous spur directed backwards. Nectaries present on lower part of
stamens or absent. Disc usually absent (in Fusispermum fleshy,
annular, quinquelobate).
Androecium Stamens usually
five (in Leonia triandra three), antesepalous, alternipetalous (in
Fusispermum with very small fringed apical scales). Filaments usually
free (sometimes connate at base into tube; in Fusispermum adnate with
indentations to inner surface of petals), free from tepals; all or two abaxial
stamens often with abaxial nectariferous appendage in lower part, or with
prolonged connective (anthers of Leonia without dorsal connective
scales). Anthers basifixed to laterofixed, usually connivent forming ring,
non-versatile, tetrasporangiate, introrse, longicidal (dehiscing by
longitudinal slits; thecae in Fusispermum cordate/trapezoid);
connective often prolonged into membranous scale-like appendage. Tapetum
secretory. Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains 3(–5)-colporate, shed as
monads, bicellular at dispersal. Exine tectate or semitectate, with columellate
infratectum, perforate, reticulate or microreticulate.
Gynoecium Pistil composed of
(two or) three (in Leonia five, antesepalous) connate carpels; median
carpel abaxial. Ovary superior, unilocular. Style single, simple, straight or
curved, or stylodia (two or) three (in Leonia five), free (absent in
Melicytus). Stigma subcapitate or lobate, often asymmetrical (stigmas
in Melicytus three to five), papillate, Dry type. Pistillodium
absent.
Ovules Placentation parietal.
Ovules one to numerous per ovary, anatropous, bitegmic, crassinucellar.
Micropyle usually bistomal, Z-shaped (zig-zag; rarely endostomal). Outer
integument two to four cell layers thick. Inner integument approx. three cell
layers thick. Hypostase present. Parietal tissue two or three cell layers
thick. Nucellar cap approx. two cell layers thick. Megagametophyte monosporous,
Polygonum type. Synergids rarely with a filiform apparatus. Antipodal
cells usually ephemeral. Endosperm development ab initio nuclear. Endosperm
haustoria? Embryogenesis asterad.
Fruit Usually an explosive
loculicidal capsule (in Leonia a nut; in Melicytus berry),
often with persistent calyx. Maturing seeds in Anchietea and
Decorsella exposed on open carpels.
Seeds Seeds often with aril
and/or carunculus (in Viola sometimes with elaiosome). Seed coat
endotestal-exotegmic, in Gloeospermum and Leonia
mucilaginous, subglobose. Testa in Agatea, Anchietea and
Corynostylis winged. Exotesta subpalisade to tabular, sometimes with
thickened cell walls. Mesotesta sometimes sclerenchymatous. Endotesta cells
usually with calciumoxalate crystals. Fibre layer one to three cell layers
thick. Exotegmen fibrous; exotegmic cells tracheidal, with thickened lignified
walls. Endotegmen persistent, usually crystalliferous. Perisperm not developed.
Endosperm usually copious, oily (in Gloeospermum absent). Embryo
usually large, straight, with chlorophyll (at least in Viola).
Cotyledons two, flat. Germination phanerocotylar.
Cytology n = 6–13, 17, 21,
23
DNA Plastid gene
rps16 absent in at least two species of Viola. Mitochondrial
coxI intron present.
Phytochemistry Insufficiently
known. Flavonols (kaempferol, quercetin), alkaloids, saponins, and cyclotide
proteins present. Ellagic acid, tannins, proanthocyanidins and cyanogenic
compounds not found. Aluminium accumulated in some species.
Use Ornamental plants,
medicinal plants, perfumes, flavouring and confection (Viola odorata),
timber.
Systematics Violaceae are sister-group to the
clade [Malesherbiaceae+[Passifloraceae+Turneraceae]].
Fusispermum is sister to the remaining
Violaceae.
Fusispermoideae
Hekking in Proc. Kon. Ned. Akad. Wetensch., ser. C, 7: 128. 18 Jun 1984
1/3. Fusispermum (3; F.
laxiflorum, F. minutiflorum, F. rubrolignosum; Panamá,
Colombia, Peru). – Medullary cells thin-walled. Nodes 5:5, quinquelacunar
with five leaf traces. Petiole with elliptical medullary bundle. Phloem
internal. Petals with contorted aestivation. Disc annular, quinquelobate,
carnose, with lobes alternating with stamens. Filaments adnate to inner surface
at indentations. Thecae cordate/trapezoid, confluent at apex?; connective as
short paired fimbriate ventral apical scales. Fruit a loculicidal capsule.
Seeds elongate, longitudinally winged. Exotegmen moderately developed?, with
slightly elongate cells. n = ?
Violoideae Beilschm.
in Flora 16(Beibl. 7): 88. 14 Jun 1833 [‘Violieae’]
c 30/900–1.000. ‘Rinorea’
crenata group (3; R. apiculata, R. crenata; southern
Central America, tropical South America); Rinorea (225–275; tropical
regions on both hemispheres); Viola
(580–620; temperate and alpine regions on both hemispheres, the Andes,
tropical mountains), Allexis (3; A. cauliflora, A.
obanensis, A. zygomorpha; Ghana, Nigeria, Cameroon),
Noisettia (1; N. orchidiflora; Guyana, Brazil, Peru),
Schweiggeria (1; S. fruticosa; eastern Brazil);
Rinoreocarpus (1; R. ulei; Amazonia); Decorsella (2;
D. arborea, D. paradoxa; tropical tropical West Africa),
Paypayrola (8; southern Central America, tropical South America; incl.
Hekkingia?), Hekkingia (1; H. bordenavei; French
Guiana, northern Brazil; in Paypayrola?); Leonia (4; L.
crassa, L. cymosa, L. glycycarpa, L. triandra;
southern Central America, tropical South America; non-monophyletic?),
Gloeospermum (c 12; Central America, northern South America),
Amphirrhox (1; A. longifolia; Central America, northern South
America), Pigea (c 10; southern Australia, New Caledonia, islands in
southern Pacific), Hybanthus
(3; H. concolor, H. havanensis, H. yucatanensis;
eastern North America, Mexico, the West Indies), Mayanaea (1; M.
caudata; Guatemala), Orthion (5–6; O. guatemalense,
O. malpighiifolium, O. montanum, O. oblanceolatum,
O. subsessile, O. veracruzense; Central America, northern
South America); ‘Rinorea’ virgata group (1; R.
virgata; southern India, Sri Lanka, the Andaman Islands, Southeast Asia),
‘Hybanthus’
enneaspermus group (c 25; Africa, the Arabian Peninsula, India,
northern Australia), ‘Hybanthus’
fruticulosus group (3; H. fruticulosus, H.
serrulatus; Mexico, Central America), ‘Hybanthus’
thiemei group (4–6; H. galeotii, H. nanus, H.
thiemei; Central America, the West Indies, northern South America),
Ixchelia (2; I. mexicanus, I. uxpanapana; Mexico,
Central America), Isodendrion
(4; I. hosakae, I. laurifolium, I. longifolium,
I. pyrifolium; the Hawaiian Islands), Pombalia (c 65;
southwestern United States, Central America, the West Indies, tropical South
America), Melicytus
(c 10; southeastern South Australia, Victoria, eastern New South Wales,
Tasmania, Solomon Islands, Norfolk Island, New Zealand, Fiji, the Hawaiian
Islands), ‘Hybanthus’
guanacastensis group (2; H. denticulatus, H.
guanacastensis; Mexico, Central America), Anchietea (9–10;
tropical South America), Hybanthopsis (1; H. bahiensis;
eastern Brazil), Calyptrion (4; C. arboreum, C.
carthagenense, C. pubescens, C. volubile; Central
America, tropical South America), Agatea (c 8; New Guinea, New
Caledonia, islands in southern Pacific). – Cosmopolitan. Petals often with
quincuncial aestivation. Nectary lobes opposite stamens; nectaries usually
separately adnate to filament bases. Anther thecae sometimes horizontal;
connective lobate, as long as and wider than anther, free, with apex not or
very slightly bilobate and margin entire or erose, usually covering thecae from
abaxial side. Stigmatic head subcapitate; receptive small. – Rinorea
apiculata and R. crenata form a sister-group to the remaining
Violoideae (Wahlert 2014). Rinorea has nectary shaped as
thick semicircular lobes at filament bases, thecae visible from abaxial side,
subapical anther connective, style widening subapically, and one ovule per
carpel.
 |
Cladogram (simplified) of Violaceae based on DNA
sequence data (Tokuoka 2008). Ixchelia is sister to
[Isodendrion+Pombalia], according to Wahlert &
al. (2015).
|
Literature
Abdul-Salim K. 2002. Systematics and biology
of Symphonia L. f. (Clusiaceae). – Ph.D. diss., Harvard
University, Cambridge, Massachusetts.
Abou-Shoer M, Suwqanborirux K, Habib AAM,
Chang CJ, Cassady JM. 1993. Xanthones and vismiones from Psorospermum
febrifugum. – Phytochemistry Nov 1993. v.34 (5).
Abreu V, Bove C, Philbrick C, Mendonça C,
Gonçalves-Esteves V. 2012. Pollen morphology of the aquatic Brazilian endemic
genus Castelnavia Tul. & Wedd. (Podostemaceae). – Plant Syst.
Evol. 298: 1455-1461.
Achoundong G. 2003. Novitates Gabonenses 45.
Une nouvelle espèce de Rinorea (Violaceae) du Gabon. – Adansonia,
sér. III, 25: 211-214.
Achoundong G, Bakker FT. 2006. Deux nouvelles
espèces de Rinorea, série Ilicifoliae (Violaceae) du Cameroun. – Adansonia,
sér. III, 28: 129-136.
Achoundong G, Bos JJ. 2001. Deux espèces
nouvelles de Rinorea (Violaceae) du Congo et du Gabon. –
Adansonia, sér. III, 23: 155-159.
Achoundong G, Cheek M. 2003. Two new species
of Rinorea (Violaceae)
from Western Cameroon. – Kew Bull. 58: 957-964.
Achoundong G, Cheek M. 2006. Two further new
species of Rinorea (Violaceae) from Cameroon. – Kew
Bull. 60: 581-586.
Achoundong G, Onana J-M. 1998. Allexis
zygomorpha (Violaceae): a new
species from the littoral forest of Cameroon. – Kew Bull. 53: 1009-1010.
Adams LG, George AS. 1982. Violaceae. – In: George AS (ed),
Flora of Australia 8, Australian Government Publ. Service, Canberra, pp.
91-110.
Agar JTH, Evans WC. 1975. A new tropane
heterodiester from Erythroxylum monogynum. – J. Pharm. Pharmacol. 27
(Suppl.): 85P.
Agar JTH, Evans WC. 1976. Alkaloids of the
genus Erythroxylum 1. E. monogynum Roxb. roots. – J. Chem.
Soc., Perkin Transact. I: 1550-1553.
Agar JTH, Evans WC, Treagust PG. 1974.
Alkaloids of the roots of Erythroxylum monogynum Roxb. – J. Pharm.
Pharmacol. 26 (Suppl.): 111P-112P.
Agostini G. 1973. El genero Lozania
Mutis (Lacistemaceae). – Acta Bot. Venezuel. 8: 167-175.
Airy Shaw HK. 1960. Notes on Malaysian Euphorbiaceae. – Kew Bull. 14:
353-397.
Airy Shaw HK. 1963. Notes on Malaysian and
other Asiatic Euphorbiaceae.
– Kew Bull. 16: 341-372.
Airy Shaw HK. 1965. Notes on Malaysian and
other Asiatic Euphorbiaceae.
– Kew Bull. 19: 299-328.
Airy Shaw HK. 1967a. Notes on Malaysian and
other Asiatic Euphorbiaceae.
– Kew Bull. 20: 379-415.
Airy Shaw HK. 1967b. Notes on the genus
Bischofia Bl. (Bischofiaceae). – Kew Bull. 21: 327-329.
Airy Shaw HK. 1968. Notes on Malaysian and
other Asiatic Euphorbiaceae.
– Kew Bull. 21: 353-418.
Airy Shaw HK. 1969. Notes on Malesian and
other Asiatic Euphorbiaceae.
– Kew Bull. 23: 1-131.
Airy Shaw HK. 1970. The genus
Androstachys Prain in Madagascar. – Adansonia, sér. II, 10:
519-524.
Airy Shaw HK. 1971a. Notes on Malesian and
other Asiatic Euphorbiaceae.
– Kew Bull. 25: 473-553.
Airy Shaw HK. 1971b. Tapoides
villamilii (Merr.) Airy Shaw. – Hooker’s Icon. Plant. 37: t. 3632.
Airy Shaw HK. 1972a. The Euphorbiaceae of Siam. – Kew
Bull. 26: 191-363.
Airy Shaw HK. 1972b. A second species of the
genus Aristogeitonia Prain (Euphorbiaceae) from East Africa.
– Kew Bull. 26: 495-498.
Airy Shaw HK. 1972c. Notes on Malesian and
other Asiatic Euphorbiaceae
CLXI. Note on Blumeodendron bullatum. – Kew Bull. 27: 3-93.
Airy Shaw HK. 1974a. Noteworthy Euphorbiaceae from Tropical Asia
(Burma to New Guinea). – Hooker’s Icon. Plant. 38(1): tt. 3701-3732.
Airy Shaw HK. 1974b. Notes on Malesian and
other Asiatic Euphorbiaceae.
– Kew Bull. 29: 281-331.
Airy Shaw HK. 1975. The Euphorbiaceae of Borneo. – Kew
Bull. Add. Ser. IV: 1-245.
Airy Shaw HK. 1976. New or noteworthy
Australian Euphorbiaceae. –
Kew Bull. 31: 341-398.
Airy Shaw HK. 1977. Additions and corrections
to the Euphorbiaceae of Siam.
– Kew Bull. 32: 69-83.
Airy Shaw HK. 1978a. Notes on Malesian and
other Asiatic Euphorbiaceae.
– Kew Bull. 32: 361-418.
Airy Shaw HK. 1978b. Notes on Malesian and
other Asiatic Euphorbiaceae
CCXIV. Croton L. – Kew Bull. 33: 25-77.
Airy Shaw HK. 1979. Three interesting plants
from the Northern Territory of Australia (Thymelaeaceae,
Flacourtiaceae and Hanguanaceae).
– Kew Bull. 33: 1-5.
Airy Shaw HK. 1980a. The Euphorbiaceae of New Guinea. –
Kew Bull. Add. Ser. VIII: 1-253.
Airy Shaw HK. 1980b. New Euphorbiaceae from New Guinea. –
Kew Bull. 34: 591-598.
Airy Shaw HK. 1980c. A partial synopsis of
the Euphorbiaceae-Platylobeae
of Australia (excluding Phyllanthus, Euphorbia, and
Calycopeplus). – Kew Bull. 35: 577-700.
Airy Shaw HK. 1981a. The Euphorbiaceae of Sumatra. – Kew
Bull. 36: 239-374.
Airy Shaw HK. 1981b. Notes on Asiatic,
Malesian and Melanesian Euphorbiaceae. – Kew Bull. 36:
599-612.
Airy Shaw HK. 1981c. New species of
Antidesma (Stilaginaceae) from Malesia and Australia. – Kew Bull.
36: 635-637.
Airy Shaw HK. 1982a. The Euphorbiaceae of Central Malesia
(Celebes, Moluccas, Lesser Sunda Is.). – Kew Bull. 37: 1-40.
Airy Shaw HK. 1982b. New Euphorbiaceae from Sumatra, New
Guinea, Australia and New Caledonia. – Kew Bull. 37: 377-381.
Airy Shaw HK. 1983a. New combinations in
Philippine Euphorbiaceae. –
Kew Bull. 38: 68.
Airy Shaw HK. 1983b. Euphorbiaceae. – In: Morley BD,
Tolken HR (eds), Flowering plants in Australia, Rigby Publ., Adelaide, pp.
129-135.
Airy Shaw HK. 1983c. An alphabetical
enumeration of the Euphorbiaceae of the Philippine
Islands. – Royal Botanic Gardens, Kew, United Kingdom.
Akhani H. 2004. A new spiny, cushion-like
Euphorbia (Euphorbiaceae) from South-West
Iran with special reference to the phytogeographic importance of local endemic
species. – Bot. J. Linn. Soc. 146: 107-121.
Alfarhan AH. 2000. An account of the genus
Croton L. in Saudi Arabia with a new record of C.
bonplandianus Baill. – Saudi J. Biol. Sci. 7: 39-45.
Alford MH. 2003. Claves para los géneros de
Flacourtiaceae de Perú y del Nuevo Mundo. – Arnaldoa 10: 19-38.
Alford MH. 2005. Systematic studies in
Flacourtiaceae. – Ph.D. diss., Cornell University, Ithaca, New York.
Alford MH. 2006. A new species of
Hasseltia (Salicaceae)
from Costa Rica and Panama. – Brittonia 58: 277-284.
Alford MH. 2008. Revision of
Neosprucea (Salicaceae).
– Syst. Bot. Monogr. 85: 1-62.
Allaby RG, Peterson GW, Merriwether DA, Fu
YB. 2005. Evidence of the domestication history of flax (Linum
usitatissimum) from genetic diversity of the sad2 locus. –
Theor. Appl. Gen. 112: 58-65.
Al-Nowaihi AS, Khalifa SF. 1973. Studies on
some taxa of the Geraniales II. Floral
morphology of certain Linaceae, Rutaceae and Geraniaceae with a
reference to the consistency of some characters. – J. Indian Bot. Soc. 52:
198-206.
Alston AHG. 1925. Revision of
Cassipourea. – Kew Bull 1925: 241-276.
Alvin KL. 1987. Leaf anatomy of
Androstachys johnsonii Prain and its functional significance. – Ann.
Bot., N. S., 59: 579-591.
Alvin KL, Rao TA. 1987. On the unusual
distribution pattern of leaf sclereids in Androstachys johnsonii Prain
(Euphorbiaceae). – Bot. J.
Linn. Soc. 95: 55-60.
Amaral MCE. 1991. Phylogenetische Systematik
der Ochnaceae. – Bot. Jahrb.
Syst. 113: 105-195.
Amaral MCE, Bittrich V. 1998. Ontogenia
inicial do androceu de espécias de Ochnaceae subfam. Sauvagesioideae
através sa análise em microscopia electrônica de varredura – Rev. Brasil.
Bot. 21: 269-273.
Amaral MCE, Bittrich V, Endress PK, Stevens
PF. 2017. The unique morphology of resin-producing multilocellate anthers and
their evolution in Clusia (Clusiaceae). – Bot. J. Linn. Soc.
184: 79-93.
Ameka KG, Pfeifer E, Rutishauser R. 2002.
Developmental morphology of Saxicolella amicorum and S.
submersa (Podostemaceae:
Podostemoideae). – Bot. J. Linn. Soc. 139: 255-273.
Ameka KG, Clerk CG, Pfeifer E, Rutishauser R.
2003. Developmental morphology of Ledermanniella bowlingii (Podostemaceae) from Ghana. –
Plant Syst. Evol. 237: 165-183.
Amonkar A, Chang C-J, Cassady JM. 1981.
6-gernyloxy-3-methyl-1,8-dihydroxyanthrone, a novel anti-leukemic agent from
Psorospermum febrifugum Sprach var. ferugineum (Hook. fil.).
– Experienta 37: 1138-1139.
Amorim AM. 2001. Two new species of
Heteropterys (Malpighiaceae) from southeastern
Brazil. – Contr. Univ. Michigan Herb. 23: 29-34.
Amorim AM. 2003a. Five new species of
Heteropterys (Malpighiaceae) from Central and
South America. – Brittonia 54: 217-232.
Amorim AM. 2003b. The anomalous-stemmed
species of Heteropterys subsect. Aptychia (Malpighiaceae). – Brittonia 55:
127-145.
Amorim AM. 2004. A new species of
Heteropterys (Malpighiaceae) from the
semideciduous forests of Bahia, Brazil. – Brittonia 56: 143-146.
Ancibor E. 1990. Anatomía de las especies
Argentinas de Podostemum Michaux (Podostemaceae). – Parodiana 6:
31-47.
Anderson C. 1986. Novelties in
Stigmaphyllon (Malpighiaceae). – Syst. Bot. 11:
120-130.
Anderson C. 1995. Revision of
Thryallis (Malpighiaceae). – Contr. Univ.
Michigan Herb. 20: 3-14.
Anderson C. 1997a. Revision of
Pterandra (Malpighiaceae). – Contr. Univ.
Michigan Herb. 21: 1-27.
Anderson C. 1997b. Monograph of
Stigmaphyllon (Malpighiaceae). – Syst. Bot.
Monogr. 51: 1-313.
Anderson C. 2001a. The identity of two
water-dispersed species of Heteropterys (Malpighiaceae): H. leona
and H. platyptera. – Contr. Univ. Michigan Herb. 23: 35-47.
Anderson C. 2001b. Novelties in
Mascagnia (Malpighiaceae). – Brittonia 53:
405-415.
Anderson C. 2003. Resolution of the
Galphimia langlassei complex (Malpighiaceae) from the Pacific
slope of Mexico. – Syst. Bot. 28: 714-722.
Anderson C. 2007. Revision of
Galphimia (Malpighiaceae). – Contr. Univ.
Michigan Herb. 25: 1-82.
Anderson C. 2011. Revision of
Ryssopterys and transfer to Stigmaphyllon (Malpighiaceae). – Blumea 56:
73-104.
Anderson WR. 1975. The taxonomy of
Acmanthera (Malpighiaceae). – Contr. Univ.
Michigan Herb. 11: 41-50.
Anderson WR. 1977 [1978]. Byrsonimoideae, a
new subfamily of the Malpighiaceae. – Leandra 7:
5-18.
Anderson WR. 1979a. Mcvaughia, a new
genus of Malpighiaceae from
Brazil. – Taxon 28: 157-161.
Anderson WR. 1979b. Floral conservatism in
Neotropical Malpighiaceae. –
Biotropica 11: 219-223.
Anderson WR. 1980a. Ectopopterys, a
new genus of Malpighiaceae from
Colombia and Peru. – Contr. Univ. Michigan Herb. 14: 11-15.
Anderson WR. 1980b. Cryptic
self-fertilization in the Malpighiaceae. – Science 207:
892-893.
Anderson WR. 1980c. A new species of
Acmanthera (Malpighiaceae). – Syst. Bot. 5:
438-441.
Anderson WR. 1981. Malpighiaceae. – In: The botany
of the Guayana Highlands XI, Mem. New York Bot. Gard. 32: 21-305.
Anderson WR. 1982. Notes on Neotropical Malpighiaceae – I. – Contr.
Univ. Michigan Herb. 15: 93-136.
Anderson WR. 1983. Lophanthera, a
genus of Malpighiaceae new to
Central America. – Brittonia 35: 37-41.
Anderson WR. 1985. Peregrina, a new
genus of Malpighiaceae from
Brazil and Paraguay. – Syst. Bot. 10: 303-307.
Anderson WR. 1987. Notes on Neotropical Malpighiaceae – II. – Contr.
Univ. Michigan Herb. 16: 55-108.
Anderson WR. 1990a. The origin of the Malpighiaceae – the evidence
from morphology. – Mem. New York Bot. Gard. 64: 210-224.
Anderson WR. 1990b. The taxonomy of
Jubelina (Malpighiaceae). – Contr. Univ.
Michigan Herb. 17: 21-37.
Anderson WR. 1990c. Notes on Neotropical Malpighiaceae – III. – Contr.
Univ. Michigan Herb. 17: 39-54.
Anderson WR. 1993a. Chromosome numbers of
Neotropical Malpighiaceae. –
Contr. Univ. Michigan Herb. 19: 341-354.
Anderson WR. 1993b. Notes on Neotropical Malpighiaceae – IV. – Contr.
Univ. Michigan Herb. 19: 355-392.
Anderson WR. 1995. Notes on Neotropical Malpighiaceae – V. – Contr.
Univ. Michigan Herb. 22: 15-36.
Anderson WR. 1997a. Excentradenia, a
new genus of Malpighiaceae from
South America. – Contr. Univ. Michigan Herb. 21: 29-36.
Anderson WR. 1997b. Notes on Neotropical Malpighiaceae – VI. – Contr.
Univ. Michigan Herb. 21: 37-84.
Anderson WR. 1999. Notes on Neotropical Malpighiaceae – VII. – Contr.
Univ. Michigan Herb. 22: 1-19.
Anderson WR. 2001a. Observations on the
Malagasy genus Rhynchophora (Malpighiaceae). – Contr. Univ.
Michigan Herb. 23: 53-58.
Anderson WR. 2001b. Notes on Neotropical Malpighiaceae – VIII. – Contr.
Univ. Michigan Herb. 23: 63-81.
Anderson WR. 2006. Eight segregates from the
Neotropical genus Mascagnia (Malpighiaceae). – Novon 16:
168-204.
Anderson WR. 2007. Notes on Neotropical Malpighiaceae – IX. – Contr.
Univ. Michigan Herb. 25: 95-111.
Anderson WR, Corso S. 2007.
Psychopterys, a new genus of Malpighiaceae from Mexico and
Central America. – Contr. Univ. Michigan Herb. 25: 113-135.
Anderson WR, Davis CC. 2001. Monograph of
Lophopterys (Malpighiaceae). – Contr. Univ.
Michigan Herb. 23: 83-105.
Anderson WR, Davis CC. 2005a. The
Mascagnia cordifolia group (Malpighiaceae). – Contr. Univ.
Michigan Herb. 24: 33-44.
Anderson WR, Davis CC. 2005b. Transfer of
Mascagnia leticiana to Malpighia (Malpighiaceae). – Contr. Univ.
Michigan Herb. 24: 45-49.
Anderson WR, Davis CC. 2006. Expansion of
Diplopterys at the expense of Banisteriopsis (Malpighiaceae). – Harvard Pap.
Bot. 11: 1-16.
Anderson WR, Davis CC. 2007. Generic
adjustments in neotropical Malpighiaceae. – Contr. Univ.
Michigan Herb. 25: 137-166.
Anderson WR, Gates B. 1981.
Barnebya, a new genus of Malpighiaceae from Brazil. –
Brittonia 33: 275-284.
Anton R. 1974. Étude chimiotaxonomique sur
le genre Euphorbia (Euphorbiacées). – Thèse, l’Université Louis
Pasteur, Strasbourg, France.
Applequist WL. 2016a. A revision of the
Malagasy species of Homalium Sect. Eumyriantheia Warb. (Salicaceae). – Candollea 71:
33-60.
Applequist WL. 2016b. A reconsideration of
the infrageneric classification of Homalium Jacq. (Salicaceae). – Candollea 71:
231-256.
Applequist WL. 2018. A revision of
Homalium sect. Odontolobus (Salicaceae) endemic to Madagascar.
– Candollea 73: 27-48.
Applequist WL, Schatz GE. 2016. A synoptic
revision of the Malagasy species of Scolopia Schreb. (Salicaceae, Scolopieae). –
Adansonia 38: 99-115.
Applequist WL, Phillipson PB, Schatz GE.
2014. A synoptic revision of the Malagasy endemic genus Calantica
Jaub. ex Tul. (Salicaceae). –
Adansonia, sér. 3, 36: 83-102.
Araujo PAM, Mattos Filho A. 1984. Estrutura
das madeiras brasileiras de dicotiledôneas XXVI. Euphorbiaceae. – Rodriguésia
36: 25-40.
Arber A. 1920. Water plants. –
Cambridge.
Arbo MM. 1977. Adenoa, nuevo género
Americano de Turneraceae. –
Hickenia 1: 87-91.
Arbo MM. 1979. Revisión del género
Erblichia (Turneraceae).
– Adansonia, sér. II, 18: 459-482.
Arbo MM. 1985. Notas taxonómicas sobre
Turneráceas Sudamericanas. – Candollea 40: 175-191.
Arbo MM. 1986. Paraguay, centro importante de
especiación en las Turneráceas. – Candollea 41: 211-218.
Arbo MM. 1993. Two new species of
Piriqueta (Turneraceae)
from Pico das Almas, Brazil. – Kew Bull. 48: 9-11.
Arbo MM. 1995. Flora Neotropica. Monograph
67. Turneraceae I.
Piriqueta. – New York Botanical Garden, Bronx, New York.
Arbo MM. 1997. Estudios sistemáticos en
Turnera (Turneraceae) I.
Series Salicifoliae y Stenodictyae. – Bonplandia 9:
151-208.
Arbo MM. 1999. Two new species of
Piriqueta (Turneraceae)
from Bahia, Brazil. – Kew Bull. 54: 459-464.
Arbo MM. 2000. Estudios sistemáticos en
Turnera (Turneraceae)
II. Series Annulares, Capitatae, Microphyllae y
Papilliferae. – Bonplandia 10: 1-82.
Arbo MM. 2004. Revisión taxonómica de las
especies de Turnera de las series Anomalae y
Turnera. – Ph.D diss., Universidad Nacional del Nordeste,
Corrientes, Argentina.
Arbo MM. 2005. Estudios sistemáticos en
Turnera (Turneraceae)
III. Series Anomalae y Turnera. – Bonplandia 14:
115-318.
Arbo MM. 2006. Turneraceae. – In: Kubitzki K
(ed), The families and genera of vascular plants IX. Flowering plants.
Eudicots. Berberidopsidales,
Buxales, Crossosomatales, Fabales p. p., Geraniales, Gunnerales, Myrtales p. p., Proteales, Saxifragales, Vitales, Zygophyllales, Clusiaceae Alliance, Passifloraceae Alliance, Dilleniaceae, Huaceae, Picramniaceae, Sabiaceae, Springer, Berlin,
Heidelberg, New York, pp. 458-466.
Arbo MM. 2008. Estudios sistemáticos en
Turnera (Turneraceae)
IV. Series Leiocarpae, Conciliatae, y Sessiliflorae.
– Bonplandia 17: 107-334.
Arbo MM, Espert SM. 2009. Morphology,
phylogeny and biogeography of Turnera L. (Turneraceae). – Taxon 58:
457-467.
Arbo MM, Mazza SM. 2011. The major diversity
centre for Neotropical Turneraceae. – Syst. Biodiv. 9:
203-210.
Arbo MM, Gonzalez AM, Sede SM. 2015.
Phylogenetic relationships within Turneraceae based on morphological
characters with emphasis on seed micromorphology. – Plant Syst. Evol. 301:
1907-1926.
Arekal GD, Nagendran CR. 1974. Additional
notes on Farmeria indica Willis (Podostemaceae). – Proc. Indian
Acad. Sci., Sect. B, 80: 226-228.
Arekal GD, Nagendran CR. 1975. Is there a
Podostemum type of embryo sac in the genus Farmeria? –
Caryologia 28: 229-235.
Arekal GD, Nagendran CR. 1975 [1976]. Embryo
sac of Hydrobryopsis sessilis (Podostemaceae) – origin,
organization, and significance. – Bot. Not. 128: 332-338.
Arekal GD, Nagendran CR. 1977a. Female
gametophyte of Zeylanidium (Podostemaceae): a clarification.
– Phytomorphology 27: 123-129.
Arekal GD, Nagendran CR. 1977b. The female
gametophyte in two Indian genera of Tristichoideae (Podostemaceae) – a
reinvestigation. – Proc. Indian Acad. Sci., Sect. B, 86: 287-294.
Arènes J. 1947. Monographie du genre
Tristellateia. – Mém. Mus. Natl. Hist. Nat. Paris 21: 275-330.
Arènes J. 1950. Famille 108. Malpighiacées.
– In: Humbert H (ed), Flore de Madagascar et des Comores, Muséum National
d’Histoire Naturelle, Paris.
Arènes J. 1957. Répartition géographique
des Malpighiacées vivantes et fossile 1. – Compte Rendue Séances Somm. Soc.
Biog. 290: 81-108.
Argus GW. 1973. The genus Salix in
Alaska and Yukon. – Natl. Mus. Nat. Sci. Canada Publ. Bot. 1.
Argus GW. 1974. An experimental study of
hybridization and pollination in Salix (willow). – Can. J. Bot. 52:
1613-1619.
Argus GW. 1997. Infrageneric classification
of Salix (Salicaceae) in
the New World. – Syst. Bot. Monogr. 52: 1-121.
Arista M, Oliveira PE, Gibbs PE, Talavera S.
1997. Pollination and breeding system of two co-occurring Hirtella
species (Chrysobalanaceae)
in Central Brazil. – Bot. Acta 110: 496-502.
Armbruster WS. 1982. Seed production and
dispersal in Dalechampia (Euphorbiaceae): divergent patterns
and ecological consequences. – Amer. J. Bot. 69: 1429-1440.
Armbruster WS. 1984. Two new species of
Dalechampia (Euphorbiaceae) from Mesoamerica.
– Syst. Bot. 9: 272-278.
Armbruster WS. 1988a. Multilevel comparative
analysis of the morphology, function, and evolution of Dalechampia (Euphorbiaceae) blossoms. –
Ecology 69: 1746-1761.
Armbruster WS. 1988b. A new species, section,
and synopsis of Dalechampia (Euphorbiaceae) from Costa Rica.
– Syst. Bot. 13: 303-312.
Armbruster WS. 1993. Evolution of plant
pollination systems: hypotheses and tests with the neotropical vine
Dalechampia. – Evolution 47: 1480-1505.
Armbruster WS. 1994. Early evolution of
Dalechampia (Euphorbiaceae): insights from
phylogeny, biogeography, and comparative ecology. – Ann. Missouri Bot. Gard.
81: 302-316.
Armbruster WS. 1996a. Evolution of floral
morphology and function: an integrative approach to adaptation, constraint, and
compromise in Dalechampia (Euphorbiaceae). – In: Lloyd DG,
Barrett SCH (eds), Floral biology: studies in floral evolution in
animal-pollinated plants, Chapman & Hall, New York, pp. 241-272.
Armbruster WS. 1996b. Cladistic analysis and
revision of Dalechampia Sections Rhopalostylis and
Brevicolumnae (Euphorbiaceae). – Syst. Bot. 21:
209-235.
Armbruster WS, Herzig AL. 1984. Partitioning
and sharing of pollinators by four sympatric species of Dalechampia
(Euphorbiaceae) in Panama. –
Ann. Missouri Bot. Gard. 71: 1-16.
Armbruster WS, Webster GL. 1979. Pollination
of two species of Dalechampia (Euphorbiaceae) in Mexico by
euglossine bees. – Biotropica 11: 278-283.
Armbruster WS, Pérez-Barrales R, Arroyo J,
Edwards ME, Vargas P. 2006. Three-dimensional reciprocity of floral morphs in
wild flax (Linum suffruticosum): a new twist on heterostyly. – New
Phytol. 171: 581-590.
Armbruster WS, Lee J, Baldwin BG. 2009.
Macroevolutionary patterns of defense and pollination in Dalechampia
vines: adaptation, exaption, and evolutionary novelty. – Proc. Natl. Acad.
Sci. U.S.A. 106: 18085-18090.
Arnal C. 1945. Récherches morphologiques et
physiologiques sur la fleur des Violacées. – Ph.D. diss. University of
Dijon, Dijon, France.
Arreguín-Sánchez M 1985. Una nueva especie
de Linum (Linaceae) del
valle de México. – Phytologia 57: 262-263.
Arumugasamy K, Inamdar JA, Subramanian RB.
1988. Structure, ontogeny and secretion of oil secreting glands in Hiptage
acuminata Wall. – Curr. Sci. 58: 260-261.
Ascari J, Takahashi JA, Boaventura MAD. 2010.
Phytochemical and biological investigations of Caryocar brasiliense Camb. –
Bol. Latinoamer. Caribe Plantas Medic. Arom. 9: 20-28.
Assailly A. 1954. Contribution à la
determination des Euphorbiacées par la méthode anatomique. – Bull. Soc.
Hist. Nat. Toulouse 89: 157-194.
Aston HI. 1990. Podostemaceae. – In: George AS
(ed), Flora of Australia 18, Australian Government Publ. Service, Canberra, pp.
1-5.
Athiê-Souza SM, Melo AL de, Silva MJ da,
Oliveira L dos SD de, Sales MF de. 2015. Gradyana (Euphorbiaceae): a new genus from
northestern Brazil. – Syst. Bot. 40: 527-533.
Ayensu ES, Stern WL. 1964. Systematic anatomy
and ontogeny of the stem in Passifloraceae. – Contr. U.S.
Natl. Herb. 34: 43-72.
Azevedo MAM de. 2008. Three new species of
Passiflora subgenus Decaloba (Passifloraceae) from Brazil. –
Brittonia 60: 310-317.
Azuma T, Kajita T, Yokoyama J, Ohashi H.
2000. Phylogenetic relationships of Salix (Salicaceae) based on rbcL
sequence data. – Amer. J. Bot. 87: 67-75.
Baas P. 1970. Floral and vegetative anatomy
of Eliaea from Madagascar and Cratoxylum from Indomalesia
(Guttiferae). – Blumea 18: 369-391.
Backer CA. 1951. Elatinaceae. – In: Steenis CGGJ
van (ed), Flora Malesiana I, 4(3), Noordhoff-Kolff N. V., Groningen, pp.
203-206.
Badré F. 1973a. Nectaropetalaceae. – In:
Aubréville A, Leroy J-F (eds), Flore du Gabon 21, Muséum National
d’Histoire Naturelle, Paris, pp. 19-22.
Badré F. 1973b. Erythroxylaceae. – In:
Aubréville A, Leroy J-F (eds), Flore du Gabon 21, Muséum National
d’Histoire Naturelle, Paris, pp. 49-54.
Badré F. 1973b. Linaceae. – In: Aubréville A, Leroy
J-F (eds), Flore du Gabon 21, Muséum National d’Histoire Naturelle, Paris,
pp. 23-39.
Badré F. 1973c. Erythroxylaceae. – In:
Aubréville A, Leroy J-F (eds), Flore du Gabon 21, Muséum National
d’Histoire Naturelle, Paris, pp. 49-54.
Bagavathi R, Sorg B, Hecker E. 1988.
Tigliane-type diterpene esters from Synadenium grantii. – Planta
Medica 54: 506-510.
Bahadur B, Reddy NP, Rao MM, Farooqui SM.
1984. Corolla handedness in Oxalidaceae, Linaceae and Plumbaginaceae.
– J. Indian Bot. Soc. 63: 408-411.
Bahadur B, Reddy NP, Ramaswamy N. 1996.
Heterostyly and pollen dimorphism in Reinwardtia indica Dum. (Linaceae). – J. Palyn. 32: 67-77.
Baillon H. 1868. Sur le genre
Thelyra de Dupetit Thouars. – Adansonia 8: 159-161.
Baillon H. 1873a. Sur la symétrie florale
des Trigoniées. – Adansonia 11: 23-24.
Baillon H. 1873b. Nouvelles observations sur
les Euphorbiacées. – Adansonia 11: 72-138.
Bajaj R, Chang CJ, McLaughlin JL, Powell RG,
Smith CR Jr. 1986. Tiliroside from the seeds of Eremocarpus setigerus.
– J. Nat. Prod. (Lloydia) 49: 1174-1175.
Bakker FT, Gemerden BS van, Achoundong G.
2006. Molecular systematics of African Rinorea Aubl. (Violaceae). – In: Ghazanfar SA,
Beentje H (eds), Taxonomy and ecology of African plants, their conservation and
sustainable use, Royal Botanic Gardens, Kew, pp. 33-44.
Bakker HJ, Ghisalberti EL, Jefferies PR.
1972. Biosynthesis of diterpenes in Beyeria leschenaultii. –
Phytochemistry 11: 2221-2231.
Balaji K, Subramanian RB, Inamdar JA. 1996.
Occurrence of laticifers in Kirganelia reticulata (Poir.) Baill. (Euphorbiaceae). –
Phytomorphology 46: 81-84.
Balakrishnan NP, Chakrabarty T. 1993. The
genus Paracroton (Euphorbiaceae) in the Indian
subcontinent. – Kew Bull. 48: 715-726.
Balakrishnan NP, Chakrabarty T. 2007. The
family Euphorbiaceae in India,
a synopsis of its profile, taxonomy and bibliography. – Bishen Singh and
Mahendra Pal Singh, Dehradun, India.
Baldwin J, Schultes R. 1947. A conspectus of
the genus Cunuria. – Bot. Mus. Leafl. Harvard Univ. 12: 325-351.
Ballard Jr HE. 1996. Phylogenetic
relationships and infrageneric groups in Viola (Violaceae) based on morphology,
chromosome numbers, natural hybridization and internal transcribed spacer (ITS)
sequences. – Ph.D. diss., University of Wisconsin, Madison, Wisconsin.
Ballard Jr HE, Sytsma KJ. 2000. Evolution and
biogeography of the woody Hawaiian violets (Viola, Violaceae): arctic origins, herbaceous
ancestry and bird dispersal. – Evolution 54: 1521-1532.
Ballard Jr HE, Sytsma KJ, Kowal RR. 1999.
Shrinking the violets: phylogenetic relationships of infrageneric groups in
Viola (Violaceae) based on
internal transcribed spacer DNA sequences. – Syst. Bot. 23: 439-458.
Ballment ER, Smith TJ III, Stoddart JA. 1988.
Sibling species in the mangrove genus Ceriops (Rhizophoraceae), detected using
biochemical genetics. – Aust. Syst. Bot. 1: 391-397.
Bally PRO. 1959. Some new species, varieties
and forms of Monadenium (Euphorbiaceae). – Candollea 17:
25-36.
Bally PRO. 1961. The genus
Monadenium. – Benteli Publ., Berne.
Bally PRO, Carter S. 1976. Succulent
euphorbias of Somalia I. – Cact. Succ. J. (U.S.) 48: 120-129.
Bamber RK. 1974. Fibre types in the wood of
Euphorbiaceae. – Aust. J.
Bot. 22: 629-634.
Bamps P. 1966. Notes sur les Guttiferae
d’Afrique tropicale. – Bull. Jard. Bot. État Bruxelles 36: 425-459.
Bamps P, Robson N, Verdcourt B. 1978.
Guttiferae. – In: Polhill RM (eds), Flora of tropical East Africa, Crown
Agents for Oversea Governments and Administrations, London, pp. 1-34.
Bancilhon L. 1971. Contribution à l’étude
taxonomique du genre Phyllanthus (Euhorbiacées). – Boissiera 18:
1-81.
Banerji I. 1949. A contribution to the life
history of Acalypha fallax Muell. Arg. – Bull. Bot. Soc. Bengal 3:
29-32.
Banerji I, Dutt MK. 1944. The development of
the female gametophyte in some members of the Euphorbiaceae. – Proc. Indian
Acad. Sci., Sect. B, 20: 51-60.
Bänfer G, Fiala B, Weisin K. 2004. AFLP
analysis of phylogenetic relationships among myrmecophytic species of
Macaranga (Euphorbiaceae) and their allies.
– Plant Syst. Evol. 249: 213-231.
Bänfer G, Moog U, Fiala B, Mohamed M,
Weising K, Blattner FR. 2006. A chloroplast genealogy of myrmecophytic
Macaranga species (Euphorbiaceae) in Southeast Asia
reveals hybridization, vicariance and long-distance dispersals. – Mol. Ecol.
15: 4409-4424.
Bänziger H. 1995. Ecological, morphological
and taxonomic studies on Thailand's fifth species of Rafflesiaceae: Rhizanthes
zippelli (Blume) Spach. – Nat. Hist. Bull. Siam Soc. 43: 337-365.
Bänziger H, Hansen B. 1997. Unmasking the
identity of Sapria poilanei Gagnepain emend., and description of
Sapria ram sp. n. (Rafflesiaceae). – Nat. Hist.
Bull. Siam Soc. 45: 149-170.
Bänziger H, Hansen B. 2000. A new taxonomic
revision of a deceptive flower, Rhizanthes Dumortier (Rafflesiaceae). – Nat. Hist.
Bull. Siam Soc. 48: 117-143.
Bänziger H, Lamb A, Kocyan A. 2007. Bisexual
Rhizanthes lowii (Beccari) Harms (Rafflesiaceae) from Borneo: first
description of flowers, fruits and seeds. – Nat. Hist. Bull. Siam Soc. 55:
341-352.
Baranova MA, Jeffrey C. 2006. Leaf anatomy
and the systematics of the Rhizophoraceae sensu lato. –
Bot. Žurn. 91: 1787-1815.
Barberá P, Velayos M, Aedo C. 2013.
Annotated checklist and identification keys of the Acalyphoideae (Euphorbiaceae) of Equatorial
Guinea (Annobón, Bioko and Río Muni). – Phytotaxa 140: 1-25.
Barberá P, Velayos M, Aedo C. 2014.
Taxonomic revision of Grossera (Crotonoideae, Euphorbiaceae): a Central African
genus. – Syst. Bot. 39: 490-509.
Barcelona JF, Fernando ES. 2002. A new
species of Rafflesia (Rafflesiaceae) from Panay Island,
Philippines. – Kew Bull. 57: 647-651.
Barcelona JF, Cajano MAO, Hadsall AS. 2006.
Rafflesia baletei, another new Rafflesia (Rafflesiaceae) from the
Philippines. – Kew Bull. 61: 231-237.
Barcelona JF, Pelser PB, Cajano MAO. 2008.
Rafflesia banahaw (Rafflesiaceae), a new species from
Luzon, Philippines. – Blumea 52: 345-350.
Barcelona JF, Pelser PB, Cabutaje E,
Bartolome NA. 2008. Another new species of Rafflesia (Rafflesiaceae) from Luzon,
Philippines: R. leonardi. – Blumea 53: 223-228.
Barcelona JF, Pelser PB, Balete DS, Co LL.
2009. Taxonomy, ecology, and conservation status of Philippine
Rafflesia (Rafflesiaceae). – Blumea 54:
77-93.
Bardon L, Chamagne J, Dexter KG, Sothers CA,
Prance GT, Chave J. 2013. Origin and evolution of Chrysobalanaceae: insights into
the evolution of plants in the Neotropics. – Bot. J. Linn. Soc. 171:
19-37.
Bardon L, Sothers C, Prance GT, Malé P-JG,
Xi Z, Davis CC, Muriene J, García-Villacorta R, Coissac E, Lavergne S, Chave
J. 2016. Unraveling the biogeographical history of Chrysobalanaceae from plastid
genomes. – Amer. J. Bot. 103: 1089-1102.
Baretta-Kuipers T. 1976. Comparative wood
anatomy of Bonnetiaceae, Theaceae, and
Guttiferae. – In: Baas P, Bolton AJ, Catling DM (eds), Wood structure in
biological and technological research, Leiden, Bot., Ser. 3, Leiden University
Press, Leiden, pp. 76-101.
Barkman TJ, Lim S-H, Salleh KM, Nais K. 2004.
Mitochondrial DNA sequences reveal the photosynthetic relatives of
Rafflesia, the world’s largest flower. – Proc. Natl. Acad. Sci.
U.S.A. 101: 787-792.
Barkman TJ, Bendiksby M, Lim S-H, Salleh KM,
Nais J, Madulid D, Schumacher T. 2008. Accelerated rates of floral evolution at
the upper size limit for flowers. – Curr. Biol. 18: 1508-1513.
Barradas MM. 1973. Morfologia do fruto e da
semente de Caryocar brasiliense (piqui), em váraias fases de
desenvolvimento. – Rev. Biol. 9: 69-84.
Barres L, Vilatersana R, Molero J, Susanna A,
Galbany-Casals M. 2011. Molecular phylogeny of Euphorbia subg.
Esula sect. Aphyllis (Euphorbiaceae) inferred from nrDNA
and cpDNA markers with biogeographic insights. – Taxon 60: 705-720.
Barres L, Galbany-Casals M, Hipp AL, Molero
J, Vilatersana R. 2017. Phylogeography and character evolution of
Euphorbia sect. Aphyllis subsect. Macaronesicae (Euphorbiaceae). – Taxon 66:
324-342.
Barrett SCH. 1978. Heterostyly in a tropical
weed: the reproductive biology of the Turnera ulmifolia complex (Turneraceae). – Can. J. Bot. 56:
1713-1725.
Barth F. 1896. Anatomie comparée de la tige
et de la feuille des Trigoniacées et des Chailletiacées (Dichapétalées).
– Bull. Herb. Boissier 4: 481-520.
Barth OM. 1963. Catálogo sistemático dos
pólens das plantas arbóreas do Brasil Meridional III: Theaceae, Marcgraviaceae, Ochnaceae, Guttiferae e Quiinaceae. – Instituto Oswaldo
Cruz, Rio de Janeiro, pp. 97-104.
Barth OM. 1980. Morfologia do pólen e
palinotaxinomia do género Kielmeyera (Guttiferae). – Rodriguesia
32(55): 105-133.
Barth OP. 1965. Elektronenmikroskopische
Beobachtungen am Sporoderm der Caryocaraceen. – Grana Palynol., n. s., 6:
7-25.
Battaglia E. 1971. The embryo sac of Podostemaceae: an interpretation.
– Caryologia 24: 403-420.
Battaglia E. 1986. Embryological questions 8.
Euphorbia dulcis type versus Fritillaria type. – Ann. Bot.
(Roma) 44: 97-136.
Battaglia E. 1987. Embryological questions
11. Has the debated case of Podostemaceae been resolved? –
Ann. Bot. (Roma) 45: 37-64.
Baum H. 1951. Die Frucht von Ochna
multiflora, ein Fall ökologischer Apokarpie. – Österr. Bot. Zeitschr.
98: 383-394.
Beaman JH, Adam J. 1983 [1984]. Observations
on Rafflesia in Sabah. – Sabah Soc. J. 7: 208-212.
Beaman RS, Decker PJ, Beaman JH. 1988.
Pollination of Rafflesia (Rafflesiaceae). – Amer. J. Bot.
75: 1148-1162.
Beattie AJ. 1974. Floral evolution in
Viola. – Ann. Missouri Bot. Gard. 61: 781-793.
Beattie AJ, Lyon N. 1975. Seed dispersal in
Viola (Violaceae):
adaptations and strategies. – Amer. J. Bot. 62: 714-722.
Becker W. 1902. Ergebnisse einier Revision
der Violae des Herbarium Barbey-Boissier. – Bull. Herb. Boissier 1902:
852-856.
Becker W. 1909. Violenstudien 1. – Beih.
Bot. Zentralbl. 26: 1-44.
Becker W. 1910. Violae Europaeae. – C.
Heinrich, Dresden-N.
Becker W. 1916. Violae asiaticae et
australienses I. – Beih. Bot. Centralbl. 34: 208-266.
Becker W. 1918. Violae asiaticae et
australienses III. – Beih. Bot. Centralbl. 36: 15-59.
Becker W. 1923. Violae asiaticae et
australienses V. – Beih. Bot. Centrlbl. 40: 67-118.
Becker W. 1925. Viola. – In:
Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien, Wilhelm Engelmann,
Leipzig, 21, pp. 363-376.
Becker W. 1928. New Chinese species of
Viola. – Kew Bull. 6: 247-252.
Behnke H-D. 1982. Sieve-element plastids of
Cyrillaceae, Erythroxylaceae, and Rhizophoraceae: description and
significance of subtype PV plastids. – Plant Syst. Evol. 141: 31-39.
Behnke H-D. 1988. Sieve-element plastids and
systematic relationships of Rhizophoraceae, Anisophylleaceae,
and allied groups. – Ann. Missouri Bot. Gard. 75: 1387-1409.
Behnke H-D, Richter K. 1990. Primary phloem
development in the shoot apex of Rhizophora mangle L. (Rhizophoraceae). – Bot. Acta
103: 296-304.
Beille L. 1908. Euphorbiaceae. – In: Chevalier A
(ed), Novitates florae africanae, Bull. Soc. Bot. France 55 (Mém. 2), 8:
54-85.
Belgrano MJ, Pozner R. 2005. Stillingia
yungasensis (Euphorbiaceae): a new species from
northwestern Argentina and southern Bolivia. – Syst. Bot. 30: 134-138.
Belin-Depoux M. 1977. Introduction à
l’étude des glandes foliaires de l’Alchornea cordata (Juss.)
Muell. Arg. (Euphorbiaceae).
– Rev. Gén. Bot. 84: 127-136.
Belin-Depoux M, Clair-Maczulaitys D. 1974.
Introduction à l’étude des glande foliaires de l’Aleurites
moluccana Willd. (Euphorbiacée) I. La glande et son ontogenèse. – Rev.
Gén. Bot. 81: 335-351.
Belin-Depoux M, Clair-Maczulaitys D. 1975.
Introduction à l’étude des glandes foliaires de l’Aleurites
moluccana Willd. (Euphorbiacée) II. Aspects histologiques et cytologiques
de la glande pétiolaire fonctionelle. – Rev. Gén. Bot. 82: 119-155.
Belliard J, Besse J. 1980. Étude du rôle
des feuilles dans un phénomène d’incompatibilité entre deux taxons de
Phyllanthus odontadenius Muell. Arg. – Bull. Soc. Bot. France, 127,
Lettres Bot. (1): 5-21.
Bendiksby M, Schumacher T, Gussarova G, Nais
J, Mat-Salleh K, Sofiyanti N, Madulid D, Smith SA, Barkman T. 2010. Elucidating
the evolutionary history of the Southeast Asian, holoparasitic, giant-flowered
Rafflesiaceae: Pliocene
vicariance, morphological convergence and character displacement. – Mol.
Phylogen. Evol. 57: 620-633.
Benitez-Vieyra S, Hempel de Ibarra N, Wertien
AM, Cocucci AA. 2007. How to look like a mallow: evidence of floral mimicry
between Turneraceae and Malvaceae. – Proc. Roy. Soc.
London, B, 274: 2239-2248.
Bennett EM. 1972. A revision of the
Australian species of Hybanthus Jacquin (Violaceae). – Nuytsia 1: 218-241.
Bennett GJ, Lee H-H. 1989. Xanthones from
Guttiferae. – Phytochemistry 28: 967-998.
Bennett GJ, Lee H-H, Lowrey TK. 1990. Novel
metabolites from Ploiarium alternifolium: a bixanthone and two
anthraquinolyxanthones. – Tetrahedron Lett. 31: 751-754.
Benson WW, Brown KS Jr, Gilbert LE. 1976.
Coevolution of plants and herbivores: passion flower butterflies. – Evolution
29: 659-680.
Bentham G. 1878. Notes on Euphorbiaceae. – Bot. J. Linn.
Soc. 17: 185-267.
Berazaín Iturralde R. 2003. A new species of
Ouratea (Ochnaceae) from
Cuba. Novitiae florae cubenses 13. – Willdenowia 33: 183-186.
Berazaín Iturralde R. 2006. Notes on the
taxonomy and distribution of the Ochnaceae in the Greater Antilles. –
Willdenowia 36: 455-461.
Berg RY. 1975. Fruit, seed, and
myrmecochorous dispersal in Micrantheum (Euphorbiaceae). – Norw. J. Bot.
22: 173-194.
Berger A. 1907. Sukkulente Euphorbien. –
Eugen Ulmer, Stuttgart.
Berger MG. 1919. Étude organographique,
anatomique et pharmacologique de la famille des Turneracées. – Thèse,
Faculté de Médécine et de Pharmacie, Université de Lille, France.
Bernhard A. 1999a. Floral structure and
development of Ceratiosicyos laevis (Achariaceae) and its systematic
position. – Bot. J. Linn. Soc. 131: 103-113.
Bernhard A. 1999b. Floral structure,
development, and systematics in Passifloraceae and in
Abatia (Flacourtiaceae). – Intern. J. Plant Sci. 160: 135-150.
Bernhard A, Endress P. 1999. Androecial
development and systematics in Flacourtiaceae s.l. – Plant Syst. Evol. 215:
141-155.
Bernhard F. 1966. Contribution à l’étude
des glandes foliaire chez les Crotonoïdées (Euphorbiaceae). – Mém. Inst.
Fond. Afrique Noire 75: 71-156.
Berry EW. 1922. Saccoglottis, recent
and fossil. – Amer. J. Sci. 20: 127-130.
Berry EW. 1924. New Tertiary species of
Anacardium and Vantanea from Colombia. – Pan Amer. Geol.
42: 259-263.
Berry PE, Gaskin JF. 1998. A new
Croton (Euphorbiaceae)
from the western Guayana Shield and its anomalous sectional placement. –
Syst. Bot. 23: 171-175.
Berry PE, Wiedenhoeft AC. 2004. Micrandra
inundata (Euphorbiaceae),
a new species with unusual wood anatomy from Black-water river banks in
southern Venezuela. – Syst. Bot. 29: 125-133.
Berry PE, Tobe H, Gómez JA. 1991.
Agamospermy and the loss of distyly in Erythroxylum undulatum (Erythroxylaceae) from northern
Venezela. – Amer. J. Bot. 78: 595-600.
Berry PE, Hipp A, Wurdack KJ, Ee B van, Riina
R. 2005. Molecular phylogenetics of the giant genus Croton and tribe
Crotoneae (Euphorbiaceae sensu
stricto) using ITS and trnL-trnF sequence data. – Amer. J.
Bot. 92: 1520-1534.
Berry PE, Cordeiro I, Wiedenhoeft AC,
Vittorino-Cruz MA, Lima LR. 2005. Brasiliocroton, a new crotonoid
genus of Euphorbiaceae s.s.
from eastern Brazil. – Syst. Bot. 30: 357-365.
Beutler JA, Alvarado-Lindner AB, McCloud TG,
Cragg GM. 1989. Distribution of phorbol ester bioactivity in the Euphorbiaceae. – Phytotherapy
Res. 3: 188-192.
Beutler JA, Alvarado-Lindner AB, McCloud TG,
Cragg GM. 1996. Further studies on phorbol ester bioactivity in the Euphorbiaceae. – Ann. Missouri
Bot. Gard. 83: 530-533.
Bezuidenhout A. 1964. The pollen of the
African Podostemaceae. –
Pollen Spores 6: 463-478.
Bhatnagar AK, Kapil RN. 1973. Bischofia
javanica – its relationship with Euphorbiaceae. – Phytomorphology
23: 264-267.
Bhatnagar AK, Kapil RN. 1979. Ontogeny and
taxonomic significance of anther in Bischofia javanica. –
Phytomorphology 29: 298-306.
Bharucha KE, Gunstone FD. 1956. Vegetable
oils V. Component acids of Cephalocroton cordofanus seed oil. – J.
Sci. Food Agric. 7: 606-609.
Bicchi C, Rubiolo P, Camargo EES, Vilegas W,
Gracioso J de S, Brito ARMS. 2003. Components of Turnera diffusa
Willd. var. afrodisiaca (Ward) Urb. essential oil. – Flavours
Fragrances J. 18: 59-61.
Biesboer DD. Mahlberg PG. 1981. Laticifer
starch grain morphology and laticifer evolution in Euphorbia (Euphorbiaceae). – Nord. J. Bot.
1: 447-457.
Bigio NC, Secco RS. 2012. As espécies de
Pera (Euphorbiaceae
s.s.) in Brazilian Amazon. – Rodriguésia 63: 163-207.
Bilia AR, Yusuf AW, Braca A, Keita A, Morelli
I. 2000. New prenylated anthraquinones and xanthones from Vismia
guineensis. – J. Nat. Prod. 63: 16-21.
Binns WW, Blumden G, Woods DL. 1968.
Distribution of leucoanthocyanidins, phenolic glycosides, and imino-acids in
leaves of Salix species. – Phytochemistry 7: 1577-1581.
Binojkumar MS, Balakrishnan NP. 1993. Two new
species of Euphorbia L. (Euphorbiaceae) from Burma. – Kew
Bull. 48: 795-798.
Bissiengou P, Chatrou LW, Wieringa JJ, Sosef
MSM. 2013. Taxonomic novelties in the genus Campylospermum (Ochnaceae). – Blumea 58: 1-7.
Bittrich V, Amaral M do CE. 1996. Flower
morphology and pollination biology of some Clusia species from the
Gran Sabana (Venezuela). – Kew Bull. 51: 681-694.
Bittrich V, Amaral M do CE. 1997. Flower
biology of some Clusia species from Central Amazonia. – Kew Bull.
52: 617-635.
Bittrich V, Amaral MCE, Machado SMF,
Marsaioli AJ. 2003. Floral resin of Tovomitopsis saldanhae
(Guttiferae) and 7-Epi-nemorosone: structural revision. – Zeitschr.
Naturforsch. 58c: 643-648.
Blank F. 1939. Beitrag zur Morphologie von
Caryocar nuciferum L. – Ber. Schweiz. Bot. Ges. 49: 437-494.
Blattner FR, Weising K, Banfer G, Maschwitz
U, Fiala B. 2001. Molecular analysis of phylogenetic relationships among
myrmecophytic Macaranga species (Euphorbiaceae). – Mol. Phylogen.
Evol. 19: 331-344.
Blaxland K. 2004. A new species of
Viola (Violaceae) from
South-West Turkey. – Bot. J. Linn. Soc. 2004: 505-509.
Boblioff W. 1923. Anatomy of Hevea
brasiliensis. – Zürich.
Boeckler CA, Gershenzon J, Unsicker SP. 2011.
Phenolic glycosides of the Salicaceae and their role as
anti-herbivore defenses. – Phytochemistry 72: 1497-1509.
Boes TK, Strauss SH. 1994. Floral phenology
and morphology of black cottonwood, Populus trichocarpa (Salicaceae). – Amer. J. Bot. 81:
562-567.
Boesewinkel FD. 1980a. Development of ovule
and testa of Linum usitatissimum L. – Acta Bot. Neerl. 29: 17-32.
Boesewinkel FD. 1980b. A comparative study of
ovules and seed-coats in the genus Dichapetalum. – Acta Bot. Neerl.
29: 212.
Boesewinkel FD. 1985. The ovule and seed of
Humiria balsamifera (Aubl.) St. Hil. – Acta Bot. Neerl. 34:
183-191.
Boesewinkel FD. 1987. Ovules and seeds of Trigoniaceae. – Acta Bot. Neerl.
36: 81-91.
Boesewinkel FD. 1994. Ovules and seed
characters of Balanites aegyptiaca and the classification of the
Linales-Geraniales-Polygalales assembly.
– Acta Bot. Neerl. 43: 15-25.
Boesewinkel FD, Bouman F. 1980. Development
of ovule and seed coat of Dichapetalum mombuttense Engl. with notes on
other species. – Acta Bot. Neerl. 29: 103-115.
Boesewinkel FD, Geenen J. 1980. Development
of ovule and seed coat of Erythroxylum coca Lamk. – Acta Bot. Neerl.
29: 231-241.
Bonne G. 1926a. Sur la constitution du
gynécée chez les Chrysobalanées. – Compt. Rend. Acad. Sci. Paris 182:
1404-1406.
Bonne G. 1926b. La nature de la coupe florale
chez les Chrysobalanées. – Compt. Rend. Acad. Sci. Paris 183. 73-75.
Bor J, Bouman F. 1975. Development of ovule
and integuments in Euphorbia milii and Codiaeum variegatum.
– Phytomorpology 4: 280-296.
Bor J, Kapil RN. 1975. Euphorbia
geniculata – ovule to seed. – Acta Bot. Neerl. 24: 257-268.
Bor J, Kapil RN. 1976. Anatropy and ontogeny
of the bitegmic ovule in Chrozophora A. H. L. Jussieu (Euphorbiaceae). – Acta Bot.
Neerl. 25: 385-400.
Borges RM, Somnathan H, Mall S. 1997.
Alternations of sexes in a deciduous tree: temporal dioecy in Bridelia
retusa. – Curr. Sci. 72: 940-944.
Borhidi A. 1972. La taxonomía del género
Platygyne Merc. – Ann. Hist.-Nat. Mus. Natl. Hung. 64: 89-94.
Bosser J. 1976. Voatamalo, nouveau
genre d’Euphorbiacées de Madagascar. – Adansonia, sér. II, 15:
333-340.
Botta B, Delle Monache F, Delle Monache G,
MariniBettolo GB, Oguakwa JU. 1983.
3-Geranyloxy-6-methyl-1,8-dihydroxyanthraquinone and vismiones C, D and E from
Psorospermum febrifugum. – Phytochemistry 22: 539-542.
Bouchat A, Léonard J. 1986. Révision du
genre Necepsia Prain (Euphorbiacée africano-malgache). – Bull.
Jard. Bot. Belg. 56: 179-194.
Boucher LD, Manchester SR, Judd WS. 2003. An
extinct genus of Salicaceae based
on twigs with attached flowers, fruits, and foliage from the Eocene Green River
formation of Utah and Colorado, USA. – Amer. J. Bot. 90: 1389-1399.
Bouharmont J. 1962. Fécondation de l’ovule
et développement de la graine après croisement et autopollination chez
Hevea brasiliensis. – Cellule 62: 119-130.
Bouman F, Meijer W. 1986. Comparative seed
morphology in Rafflesiaceae.
– Acta Bot. Neerl. 35: 521.
Bouman F, Meijer W. 1994. Comparative
structure of ovules and seeds in Rafflesiaceae. – Plant Syst.
Evol. 193: 187-212.
Bove CP. 1997. Phylogenetic analysis of Humiriaceae with notes on the
monophyly of Ixonanthaceae. –
J. Comp. Biol. 2: 19-24.
Bove CP, Melhem TS. 2000. Humiriaceae Juss. – World Pollen
and Spore Flora 22: 1-35.
Bove CP, Philbrick CT, Novelo RA. 2006. A new
species of Cipoia (Podostemaceae) from Minas Gerais,
Brazil. – Syst. Bot. 31: 822-825.
Bove CP, Philbrick CT, Costa WJEM. 2011.
Taxonomy, distribution and emended description of the Neotropical genus
Lophogyne (Podostemaceae). – Brittonia 63:
156-160.
Brandza G. 1908. Recherches anatomiques sur
la germination des Hypéricacées et des Guttifères. – Ann. Sci. Nat. IX,
Bot. 8: 221-300.
Breckon G. 1975. Cnidoscolus,
Section Calytosolen (Euphorbiaceae) in Mexico and
Central America. – Ph.D. diss., University of California, Davis,
California.
Breedlove DE, McClintock E. 1976.
Thornea (Hypericaceae),
a new genus from Mexico and Guatemala. – Madroño 23: 368-373.
Breteler FJ. 1973. The African Dichapetalaceae. A taxonomical
revision. – Meded. Landbouwh. Wageningen 73(13): 1-123.
Breteler FJ. 1978. The African Dichapetalaceae IV. A
taxonomical revision. Species c-f. – Meded. Landbouwh. Wageningen 78(10).
Breteler FJ. 1979. The African Dichapetalaceae V. A taxonomical
revision. Species g-l. – Meded. Landbouwh. Wageningen 79(16).
Breteler FJ. 1981. The African Dichapetalaceae VII. A
taxonomical revision. Species m-q. – Meded. Landbouwh. Wageningen 81(10).
Breteler FJ. 1982. The African Dichapetalaceae VIII. A
taxonomical revision. Species r-z. – Meded. Landbouwh. Wageningen 82(8).
Breteler FJ. 1986. The African Dichapetalaceae IX. A
taxonomical revision. – Agric. Univ. Wageningen Pap. 86(3).
Breteler FJ. 1988. Dichapetalaceae. – In:
Milne-Redhead E, Polhill RM (eds), Flora of tropical East Africa, Crown Agents
for Oversea Governments and Administrations, London, pp. 1-19.
Breteler FJ. 1991. Dichapetalaceae. – In:
Aubréville A, Leroy J-F (eds), Flore du Gabon, vol. 32, Muséum National
d’Histoire Naturelle, Paris, pp. 1-221.
Breteler FJ. 1999. Novitates Gabonenses 34.
Dactyladenia ndjoleensis (Chrysobalanaceae), a new
species from Gabon. – Syst. Geogr. Plants 69: 111-114.
Breteler FJ. 2002. A new species of
Tapura (Dichapetalaceae) from Cameroun.
– Adansonia, sér. III, 24: 267-269.
Breteler FJ. 2003a. Novitates Gabonenses 47.
Another new Dichapetalum (Dichapetalaceae) from Gabon. –
Adansonia, sér. III, 25: 223-227.
Breteler FJ. 2003b. Novitates Gabonenses 48.
A new species of Paropsia (Passifloraceae) from Gabon. –
Adansonia, sér. III, 25: 247-249.
Breteler FJ. 2004. Novitates Gabonenses 49.
Aristogeitonia (formerly Euphorbiaceae, now Picrodendraceae) present in
Gabon by a new species A. gabonica. – Adansonia, sér. III, 26:
167-170.
Breteler FJ. 2005. Novitates Gabonenses 53. A
curious new species of Dichapetalum (Dichapetalaceae) from Gabon. –
Adansonia, sér. III, 27: 231-234.
Breteler FJ. 2006. Novitates Gabonenses 62.
Dichapetalum neglectum (Dichapetalaceae), a second new
species from Gabon with 4-5-locular ovaries, with an adapted key to the Central
African species. – Adansonia, sér. III, 28: 299-309.
Breteler FJ. 2008a. A synopsis of
Casearia Jacq. (Samydeae-Salicaceae) in West and Central
Africa with a description of a new species from Eastern Congo (Kinshasa). –
Kew Bull. 63: 101-112.
Breteler FJ. 2008b. Novitates Gabonenses 68.
The genus Cassipourea (Rhizophoraceae) in continental
tropical Africa with emphasis on Gabon: subgeneric division, identification
keys, and description of two new species. – Edinburgh J. Bot. 765:
407-424.
Breteler FJ. 2014. Protomegabaria
Hutch. (Phyllanthaceae): some
observations concerning its morphology, taxonomy and geography. – Adansonia,
sér. 3, 36: 103-112.
Breteler FJ, Mennega AMW. 1994. Novitates
Gabonenses 17. Conceveiba leptostachys, a new Euphorbiacea from Gabon
and Cameroun. – Bull. Jard. Bot. Belg. 63: 209-217.
Brown WH. 1912. The relation of Rafflesia
manillana to its host. – Philipp. J. Sci. 7: 209-224.
Brunel JF. 1987. Sur le genre
Phyllanthus L. et quelques genres voisins de la tribu des Phyllantheae
Dumort. (Euphorbiaceae,
Phyllantheae) en Afrique intertropicale et à Madagascar. – Ph.D. diss.,
Université Louis Pasteur Strasbourg I, Strasbourg, France.
Brunel JF, Roux J. 1981a.
Phyllanthus subsect. Odontadenii (Euphorbiaceae) au nord du fleuve
Congo (Afrique de l’Ouest). – Willdenowia 11: 69-89.
Brunel JF, Roux J. 1981b. Phyllantheae de
Madagascar I. À propos de deux Phyllanthus de la sous-section
Swartziani Webster. – Adansonia, sér. II, 20: 393-403.
Brunel JF, Roux J. 1984. South-East Asian
Phyllantheae II. Some Phyllanthus of subsect. Swartziani. –
Nord. J. Bot. 4: 469-473.
Brunsfeld SJ, Soltis DE, Soltis PS. 1992.
Evolutionary patterns and processes in Salix Sect.
Longifoliae: evidence from chloroplast DNA. – Syst. Bot. 17:
239-256.
Brunsfeld SJ, Miller TR, Carstens BC. 2007.
Insights into the biogeography of the Pacific Northwest of North America:
evidence from the phylogeography of Salix melanopsis. – Syst. Bot.
32: 129-139.
Bruyns PV, Mapaya RJ, Hedderson T. 2006. A
new subgeneric classification for Euphorbia (Euphorbiaceae) in southern Africa
based on ITS and psbA-trnH sequence data. – Taxon 52:
397-420.
Bruyns PV, Klak C, Hanàček P. 2011. Age and
diversity in Old World succulent species of Euphorbia (Euphorbiaceae). – Taxon 60:
1717-1733.
Buchler W. 1986. Neue Chromosomenzählungen
in der Gattung Salix. 2. Teil. – Bot. Helv. 96: 135-143.
Buck W, Huft M. 1977. Two new species of
Euphorbia subgenus Agaloma from Mexico. – J. Arnold Arbor.
58: 343-348.
Bullock SH. 1982. Componentes fenológicos
del sistema de cruzamiento monóico de Cnidoscolus spinosus (Euphorbiaceae) en Jalisco. –
Bol. Soc. Bot. Mexic. 42: 1-9.
Burke BA, Chan WR, Prince EC, Manchand PS,
Eickman N, Clardy J. 1976. The structure of corylifuran, a clerodane-type
diterpene from Croton corylifolius Lam. – Tetrahedron 32:
1881-1884.
Burkhardt G, Schild W, Becker H, Grubert M.
1992. Biphenyls and xanthones from the Podostemaceae. – Phytochemistry
31: 543-548.
Burkhardt G, Becker H, Grubert M, Thomas J,
Eicher T. 1994. Bioactive chromenes from Rhyncholacis penicillata. –
Phytochemistry 37: 1593-1597.
Burman R, Gruber CW, Rizzardi K, Herrmann A,
Craik DJ, Gupta MP, Göransson U. 2010. Cyclotide proteins and precursors from
the genus Gloeospermum: filling in a blank spot in the cyclotide map
of Violaceae. – Phytochemistry
71: 13-20.
Buske A, Schmidt J, Hoffmann P. 2002.
Chemotaxonomy of the tribe Antidesmeae (Euphorbiaceae): antidesmone and
related compounds. – Phytochemistry 60: 489-496.
Byng JW. 2015. Ixonanthaceae. – In: Sosef M
(ed), Flore d’Afrique Centrale, Meise Botanic Garden, Belgium, pp. 1-9.
Byng JW, Bernardini B, Christenhusz MJM,
Chase MW. 2016. Systematics of Irvingiaceae and Ixonanthaceae (Malpighiales): phylogenetic
analysis based on three plastid DNA loci. – Phytotaxa 260. DOI:
http://dx.doi.org/10.11646/phytotaxa.260.2.5
Cabral FN, Bittrich V, Amaral M do CE do.
2018. Five new species of Caraipa (Calophyllaceae) from the
Venezuelan Guayana. – Syst. Bot. 43: 240-249.
Cacho NI, Berry PE, Olson ME, Steinmann VW,
Baum DA. 2010. Are spurred cyathia a key innovation? Molecular systematics and
trait evolution in the slipper spurges (Pedilanthus clade:
Euphorbia, Euphorbiaceae). – Amer. J. Bot.
97: 493-510.
Calixto JB, Santos ARS, Filho VC, Yunes RA.
1998. A review of the plants of the genus Phyllanthus: their
chemistry, pharmacology and therapeutic potential. – Med. Res. Rev. 18:
225-258.
Callmander MW, Phillipson PB. 2012. Notes on
the genus Ochna L. (Ochnaceae) in Madagascar. –
Candollea 67: 142-144.
Camacho MR, Mata R, Castaneda P, Kirby GC,
Warhurst DC, Croft SL, Phillipson JD. 2000. Bioactive compounds from
Celaenodendron mexicanum. – Planta Med. 66: 463-468.
Cameron KM, Chase MW, Anderson WR, Hills HG.
2001. Molecular systematics of Malpighiaceae: evidence from
plastid rbcL and matK sequences. – Amer. J. Bot. 88:
1847-1862.
Cammerloher H. 1920. Der
Spaltöffnungsapparat von Brugmansia und Rafflesia. –
Österr. Bot. Zeitschr. 69: 153-164.
Capers RS, Les DH. 2001. An unusual
population of Podostemum ceratophyllum (Podostemaceae) in a tidal
Connecticut river. – Rhodora 103: 219-223.
Carano E. 1914. Embriologia delle Podostemaceae. – Ann. Bot.
(Roma) 12: 163-164.
Carano E. 1926. Ulteriori osservazioni su
Euphorbia dulcis L. in rapporto col suo comportamento apomittico. –
Ann. Bot. (Roma) 17: 50-79.
Cardiel JM, Rodríguez PM. 2015. Synopsis of
Acalypha (Euphorbiaceae) of Argentina,
Paraguay, and Uruguay. – Ann. Missouri Bot. Gard. 101: 384-405.
Cardinal-McTeague WM, Gillespie LJ. 2016.
Molecular phylogeny and pollen evolution of Euphorbiaceae tribe Plukenetieae.
– Syst. Bot. 41: 329-347.
Cario R. 1881. Anatomische Untersuchung von
Tristicha hypnoides Spreng. – Bot. Zeitung 39: 25-33, 41-48, 57-64,
73-82.
Carlquist SJ. 1970. Wood anatomy of Hawaiian,
Macaronesian, and other species of Euphorbia. – In: Robson NKB,
Cutler DF, Gregory M (eds), New research in plant anatomy, Bot. J. Linn. Soc.
63, Suppl. 1, Academic Press, New York, London, pp. 181-193.
Carlquist SJ. 1980. Anatomy and systematics
of Balanopaceae. – Allertonia
2: 191-246.
Carlquist SJ. 1984a. Wood and stem anatomy of
Bergia suffruticosa: relationships of Elatinaceae and broader significance
of vascular tracheids, vasicentric tracheids, and fibriform vessel elements.
– Ann. Missouri Bot. Gard. 71: 232-242.
Carlquist SJ. 1984b. Wood anatomy of Malesherbiaceae. –
Phytomorphology 34: 180-190.
Carlquist SJ. 1989. Balanopaceae. – In: George AS
(ed), Flora of Australia 3, Australian Government Publ. Service, Canberra, pp.
93-95.
Carmo RM, Franceschinelli EV. 2002.
Pollinação e biologia floral de Clusia arrudea Planchon & Triana
(Clusiaceae) na Serra da Calçada,
município de Brumadinho, MG. – Rev. Brasil. Bot. 25: 351-360.
Cartellieri E. 1926. Das Absorptionssystem
der Rafflesiacee Brugmansia. – Bot. Arch. 14: 284-311.
Carter S. 1984. New taxa and notes on
herbaceous species of Euphorbia from East and Northeast Africa. –
Kew Bull. 39: 643-652.
Carter S. 1985. New species and taxonomic
changes in Euphorbia from East and Northeast tropical Africa and a new
species from Oman. – Kew Bull. 40: 809-825.
Carter S. 1987a. New taxa in
Euphorbia subgen. Euphorbia from eastern tropical Africa. –
Kew Bull. 42: 371-383.
Carter S. 1987b. Taxonomic changes in
Synadenium (Euphorbiaceae) from East Africa.
– Kew Bull. 42: 667-671.
Carter S. 1987c. New species and taxonomic
changes amongst the succulent trees and shrubs of Euphorbia subgen.
Euphorbia from East Africa. – Kew Bull. 42: 673-682.
Carter S. 1987d. New taxa and observations in
Monadenium (Euphorbiaceae) in East Africa. –
Kew Bull. 42: 903-918.
Carter S. 1988a. Euphorbieae. – In: Polhill
RM (ed), Flora of tropical East Africa, Euphorbiaceae, A. A. Balkema,
Rotterdam, pp. 409-564.
Carter S. 1988b. Euphorbia section
Somalica in Somalia. – Euphorbia J. 5: 26-38.
Carter S. 1990a. New taxa and taxonomic
changes amongst herbaceous Euphorbia species from southern tropical
Africa. – Kew Bull. 45: 327-337.
Carter S. 1990b. New Euphorbia
species related to E. longituberculosa Boiss. – Kew Bull. 45:
653-659.
Carter S. 1992a. New pair-spined species of
Euphorbia (Euphorbiaceae) from Somalia. –
Nord. J. Bot. 12: 403-422.
Carter S. 1992b. New species of
Euphorbia subgenus Tirucalli (Euphorbiaceae) from Somalia and
Oman. – Nord. J. Bot. 12: 675-679.
Carter S. 1992c. New herbaceous and woody
species of Euphorbia (Euphorbiaceae) from Somalia. –
Nord. J. Bot. 12: 681-688.
Carter S. 1993. Two new species of
Monadenium (Euphorbiaceae) from Somalia. –
Nord. J. Bot. 13: 541-543.
Carter S. 1994. A preliminary classification
of Euphorbia subgenus Euphorbia. – Ann. Missouri Bot. Gard.
81: 368-379.
Carter S. 1999. New Euphorbia taxa
in the Flora Zambesiaca area. – Kew Bull. 54: 959-965.
Carter S. 2000. Taxonomic changes in
Monadenium and Synadenium (Euphorbiaceae) for Flora
Zambesiaca. – Kew Bull. 55: 435-442.
Carter S, Gilbert MG. 1987. Euphorbia
heterochroma, E. stapfii and related taxa. – Kew Bull. 42:
385-394.
Carter S, Radcliffe-Smith A. 1988. Euphorbiaceae (Part 2). – In:
Polhill RM (ed), Flora of Tropical East Africa, A. A. Balkema, Rotterdam, pp.
409-597.
Carter S, Wood JRI. 1982. Two new succulent
Euphorbia species from Southwest Arabia. – Kew Bull. 37: 73-76.
Caruzo MBR, Cordeiro I. 2013. Taxonomic
revision of Croton section Cleodora (Euphorbiaceae). – Phytotaxa 121:
1-41.
Caruzo MBR, Ee BW van, Cordeiro I, Berry PE,
Riina R. 2011. Molecular phylogenetics and character evolution of the
“sacaca” clade: novel relationships of Croton section
Cleodora (Euphorbiaceae). – Mol. Phylogen.
Evol. 60: 193-206.
Cassinelli G, Geroni C, Botta B, Monache G
delle, Monache F delle. 1986. Cytotoxic and antitumor activity of vismiones
isolated from Vismieae. – J. Nat. Prod. 49: 929-931.
Castillo G, Marquez-Guzman J, Collazo-Ortega
M. 2013. Seed germination and early development in seedlings of Noveloa
coulteriana (Podostemaceae). – Aquatic Bot.
109: 25-30.
Cervantes A, Flores Olvera H. 2005. Six new
Mexican species of Bernardia (Euphorbiaceae). – Bot. J. Linn.
Soc. 149: 241-256.
Cervantes A, Steinmann VW, Olvera HF. 2003.
Adelia cinerea (Euphorbiaceae), formerly in
Bernardia. – Brittonia 55: 4-9.
Cervantes A, Terrazas T, Hernández HM. 2009.
Foliar architecture and anatomy of Bernardia and other genera of
Acalyphoideae (Euphorbiaceae).
– Brittonia 61: 375-391.
Cervantes-Alcayde M-A, Olson ME, Olsen KM,
Eguiarte LE. 2015. Apparent similarity, underlying homoplasy: morphology and
molecular phylogeny of the North American clade of Manihot. – Amer. J. Bot.
102: 520-532.
Cervi AC. 1997. Passifloraceae do Brasil. Estudo
do gênero Passiflora L., subgênero Passiflora. –
Fontqueria 45: 1-92.
Cervi AC. 2002. A new species of
Passiflora (Passifloraceae) from Amazonian
Brazil. – Brittonia 54: 54-56.
Cervi AC. 2006. A new species of
Passiflora (Passifloraceae) from Minas
Gerais, Brazil. – Brittonia 58: 385-387.
Cesca G. 1961. Ricerche cariologiche ed
embriologiche sulle Euphorbiaceae. I – su alcuni
biotipi di Euphorbia dulcis L. della Toscana. – Caryologia 14:
79-96.
Cesca G. 1969. Cytological and embryological
studies in the genus Euphorbia: Euphorbia epithymoides L. –
Proc. Indian Natl. Inst. Sci., Sect. B, 35: 139-152.
Chacon J, Madrinan S, Debouck D, Rodriguez F,
Tohme J. 2008. Phylogenetic patterns in the genus Manihot (Euphorbiaceae) inferred from
analyses of nuclear and chloroplast DNA regions. – Mol. Phylogen. Evol. 49:
260-267.
Chafe PDJ. 2011. Molecular phylogenetics and
breeding system evolution of the Turneraceae. – Ph.D. diss., York
University, Canada.
Chai X-Y, Ren H-Y, Xu Z-R, Bai C-C, Zhou F-R,
Ling S-K, Pu X-P, Li F-F, Tu P-F. 2009. Investigation of two flacourtiaceae
plants: Bennettiodendron leprosipes and Flacourtia ramontchi.
– Planta Med. 75:1246-1252.
Chakrabarty T, Balakrishnan NP. 1985 [1987].
A note on the genus Ostodes (Euphorbiaceae). – Bull. Bot.
Surv. India 27: 259-260.
Chakrabarty T, Balakrishnan NP. 1997. A
revision of Croton L. (Euphorbiaceae) for the Indian
subcontinent. – Bull. Bot. Surv. India 34: 1-88.
Chakrabarty T, Gangopadhyay M, Balakrishnan
NP. 1997. The genus Drypetes (Euphorbiaceae) in the Indian
subcontinent. – J. Econ. Taxon. Bot. 21: 251-280.
Chalet J. 1998. Rafflesia, une
Stapelia geante? – Succulentes 21: 24-25.
Champault A. 1970. Étude caryosystématique
et écologique de quelques Euphorbiacées herbacées et arbustives africaines.
– Bull. Soc. Bot. France 117: 137-168.
Chao H-C. 1948. Discovery of Podostemonaceae
in China. – Contr. Inst. Bot., Natl. Acad. Peiping 6: 1-16.
Chao H-C. 1980. A new genus
(Terniopsis gen. nov.) of Podostemonaceae from Fujian, China. – Acta
Bot. Yunnan. 2: 298-299.
Chappill JA. 1992. Cladistics and the Chrysobalanaceae. –
Taxon 41: 211-223.
Chase MW. 1981. A revision of
Dicella (Malpighiaceae). – Syst. Bot. 6:
159-171.
Chase MW, Zmartzty S, Lledó MD, Wurdack KJ,
Swensen SM, Fay MF. 2002. When in doubt, put it in Flacourtiaceae: a molecular
phylogenetic analysis based on plastid rbcL DNA sequences. – Kew
Bull. 57: 141-181.
Chattopadhyay D, Sharma AK. 1988. Sex
difference and chromosomes in Putranjiva roxburghii Wall. – Curr.
Sci. 57: 1017-1019.
Chaudhary A, Khanduri P, Tandon R, Uniyal PL,
Mohan Ram HY. 2014. Central cell degeneration leads to three-celled female
gametophyte in Zeylanidium lichenoides Engl. (Podostemaceae). – South Afr. J.
Bot. 91: 99-106.
Chauveaud LG. 1891. Recherches embryogénique
sur l’appareil laticifère des Euphorbiacées, Urticacées, Apocynées et
Asclepiadées. – Ann. Sci. Nat. Bot. VII, 14: 1-161.
Cheek M. 2003. A new species of
Ledermanniella (Podostemaceae) from western
Cameroon. – Kew Bull. 58: 733-739.
Cheek M, Ameka G. 2008. Ledermanniella
pollardiana sp. nov. (Podostemaceae) from western
Cameroon. – Nord. J. Bot. 26: 214-217.
Cheek M, Haba P. 2016.
Inversodicraea Engl. resurrected and I. pepehabai sp. nov.
(Podostemaceae), a submontane
forest species from the Republic of Guinea. – Kew Bull. 71: 55 DOI
10.1007/S12225-016-9673-2
Cheek M, Luke Q. 2016. Calophyllum
(Clusiaceae – Guttiferae) in
Africa. – Kew Bull. 71: 20 DOI 10.1007/S12225-016-9637-6
Chen J-H, Sun H, Yang Y-P. 2008. Comparative
morphology of leaf epidermis of Salix (Salicaceae) with special emphasis on
sections Lindleyanae and Retusae. – Bot. J. Linn. Soc. 157:
311-322.
Chen J-H, Sun H, Wen J, Yang Y-P. 2010.
Molecular phylogeny of Salix L. (Salicaceae) inferred from three
chloroplast datasets and its systematic implications. – Taxon 59: 29-37.
Chen P, Chen L, Wen J. 2011. The first
phylogenetic analysis of Tetrastigma (Miq.) Planch., the host of Rafflesiaceae. – Taxon 60:
499-512.
Chen Y-J, Chen S-H, Huang T-C, Wu M-J. 2009.
Pollen morphology of the Philippine species of Phyllanthus (Phyllanthaceae, Euphorbiaceae s.l.). – Blumea
54: 47-58.
Chen Y-S, Yang Q-E. 2006a. A distinctive new
species of Viola (Violaceae) from Yunnan, China. –
Bot. J. Linn. Soc. 149: 115-119.
Chen Y-S, Yang Q-E. 2006b. A new species of
Viola L. (Violaceae) from
Sichuan, China. – Bot. J. Linn. Soc. 149: 365-368.
Chen Y-S, Yang Q-E. 2009a. A new species of
Viola (Violaceae) from
southern China. – Bot. J. Linn. Soc. 158: 755-761.
Chen Y-S, Yang Q-E. 2009b. Two new
stoloniferous species of Viola (Violaceae) from China. – Bot. J.
Linn. Soc. 159: 349-356.
Chennaveeraiah MS, Radzan MK. 1980.
Karyomorphological and phytochemical studies in evaluating species
relationships in Garcinia L. and systematic position of the G.
xanthochymus complex. – J. Indian Bot. Soc. 59: 251-262.
Chiarugi A. 1933. Lo sviluppo del gametofito
femineo della Weddellina squamulosa. – Compt. Rend. Acad. Naz.
Lincei, Roma, 17: 1095-1099.
Chiarugi A, Francini E. 1930. Apomissia in
”Ochna serratula” Walp. – Nuovo Giorn. Bot. Ital., n. s., 37:
1-250.
Chikkannaiah PS, Mahalingappa MS. 1974.
Embryological studies in Ochna squarrosa Linn. – J. Karnatak Univ.
Sci. 19: 247-249.
Chirtoiü M. 1918. Lacistemacées et
Symplocacées. – Bull. Soc. Bot. Genève XI(10): 350-361.
Chodat R. 1895. Sur la place à attribuer au
genre Trigoniastrum. – Bull. Herb. Boissier 3: 136-140.
Chong DKX, Zsuffa L, Aravanopoulos FA. 1995.
Genetic relationship between Salix exigua and other North American
willows (Salix L.): evidence from allozyme variation. – Biochem.
Syst. Ecol. 23: 767-771.
Chopra RN, Mukkada AJ. 1966. Gametogenesis
and pseudo-embryosac in Indotristicha ramosissima (Wight) van Royen.
– Phytomorphology 16: 182-188.
Chopra S. 1970. Development of female
gametophytes in Sapium sebiferum Roxb. – Curr. Sci. 39: 17-18.
Chopra S, Singh RP. 1969. Structure and
development of seed in Phyllanthus urinaria L. – J. Indian Bot. Soc.
48: 212-216.
Cicarelli D, Andreucci AC, Pagni AM. 2001a.
Translucent glands and secretory canals in Hypericum perforatum L. (Hypericaceae): morphological,
anatomical and histochemical studies during the course of ontogenesis. – Ann.
Bot. 88: 637-644.
Cicarelli D, Andreucci AC, Pagni AM. 2001b.
The black nodules of Hypericum perforatum L. ssp. perforatum:
anatomical and histochemical studies during the course of ontogenesis. –
Israel J. Plant Sci. 49: 33-40.
Clausen J. 1926. Genetical and cytological
investigations on Viola tricolor L. and Viola arvensis Murr.
– Hereditas 8: 1-156.
Clausen J. 1927. Chromosome number and
relationship of species in the genus Viola. – Ann. Bot. 41:
677-714.
Clausen J. 1964. Cytotaxonomy and
distributional ecology of western North American violets. – Madroño 17:
173-204.
Clausen V, Frydenvang K, Koopmann R,
Jørgensen LB, Abbiw DK, Ekpe P, Jaroszewski JW. 2002. Plant analysis by
butterflies: occurrence of cyclopentenylglycines in Passifloraceae, Flacourtiaceae
and Turneraceae and discovery of
the novel nonproteinogenic amino acid 2-(3’-cyclopentenyl)glycine in
Rinorea. – J. Nat. Prod. 65: 542-547.
Coates D, Cullis CA. 1987. Chloroplast DNA
variability among Linum species. – Amer. J. Bot. 74: 260-268.
Collinson ME. 1992. The early fossil history
of Salicaceae: a brief review. –
Proc. Roy. Soc. Edinb. 98B: 155-167.
Commock T, Campbell KC St E, Meikle J,
Francisco-Ortega J, Jestrow B. 2015. Conservation and taxonomic updates for the
Jamaican endemic genus Dendrocousinsia (Euphorbiaceae). – Brittonia 67:
87-95.
Connelly WJ, Orth DJ, Smith RK. 1999. Habitat
of the riverweed darter, Etheostoma podostemonae Jordan, and the
decline of riverweed, Podostemum ceratophyllum, in the tributaries of
the Roanoke River, Virginia. – J. Freshwater Ecol. 14: 93-102.
Contreras VR, Scogin R, Philbrick CT. 1993. A
phytochemical study of selected Podostemaceae: systematic
implications. – Aliso 13: 513-520.
Coode MJE. 1976. Typification of
Macaranga Du Petit-Thouars. – Taxon 25: 184.
Cook CDK, Rutishauser R. 2001. Name changes
in the Podostemaceae. – Taxon
50: 1163-1167.
Cook CDK, Rutishauser R. 2006. Podostemaceae. – In: Kubitzki K
(ed), The families and genera of vascular plants IX. Flowering plants.
Eudicots. Berberidopsidales,
Buxales, Crossosomatales, Fabales p. p., Geraniales, Gunnerales, Myrtales p. p., Proteales, Saxifragales, Vitales, Zygophyllales, Clusiaceae Alliance, Passifloraceae Alliance, Dilleniaceae, Huaceae, Picramniaceae, Sabiaceae, Springer, Berlin,
Heidelberg, New York, pp. 304-344.
Cook MT. 1907. The embryology of
Rhizophora mangle. – Bull. Torrey Bot. Club 34: 271-277.
Coradin L, Giannasi DE, Prance GT. 1985.
Chemosystematic studies of the Chrysobalanaceae I. Flavonoids
in Parinari. – Brittonia 37: 169-178.
Cordeiro I. 1992. Flora da Serra do Cipó,
Minas Gerais: Euphorbiaceae.
– Bol. Bot. Univ. São Paulo 13: 169-217.
Cordeiro I, Carneiro-Torres DS. 2004. A new
species of Phyllanthus (Phyllanthaceae) from Chapada
Diamantina, Bahia, Brazil. – Bot. J. Linn. Soc. 146: 247-250.
Cordeiro I, Berry PE, Caruzo MBR, Ee BW van.
2008. Croton laceratoglandulosus (Euphorbiaceae s.s.), a new
glandular-stipulate species from Brazil and Bolivia, and its systematic
position based on molecular analysis. – Bot. J. Linn. Soc. 158: 493-498.
Correia MCR, Ormond WT, Pinheiro MCB, Lima HA
de. 1993. Estudo de biologia floral de Clusia criuva Camb. Um caso de
mimetismo. – Bradea 6: 209-219.
Craven LA. 1979. Eight new species of
Homalium (Flacourtiaceae) from Papuasia. – Brunonia 2: 107-124.
Cremers G. 1977. Architecture végétative de
quelques espèces malgaches du genre Euphorbia L. – Bull. Jard. Bot.
Natl. Belg. 47: 55-81.
Crepet WL, Daglian CP. 1982. Euphorbioid
inflorescences from the Middle Eocene Claiborne formation. – Amer. J. Bot.
69: 258-266.
Crepet WL, Nixon KC. 1998. Fossil Clusiaceae from the Late Cretaceous
(Turonian) of New Jersey and implications regarding the history of bee
pollination. – Amer. J. Bot. 85: 1122-1133.
Crété P. 1937. Développement et structure
du tegument seminal chez le Radiola linoides Roth. – Bull. Soc. Bot.
France 84: 655-659.
Croizat LC. 1936. On the classification of
Euphorbia I. How important is the cyathium? – Bull. Torrey Bot. Club
63: 525-532.
Croizat LC. 1937. On the classification of
Euphorbia II. How should the cyathium be interpreted? – Bull. Torrey
Bot. Club 64: 523-537.
Croizat LC. 1938. Notes of Euphorbiaceae, with a new genus
and a new subtribe of the Euphorbieae. – Philipp. J. Sci. (Bot.) 64:
397-412.
Croizat L. 1940a. On the phylogeny of the Euphorbiaceae and some of their
presumed allies. – Rev. Univ. (Universidad Católica de Chile, Santiago) 25:
205-220.
Croizat L. 1940b. A significant new species
from New Guinea: Euphorbia euonymoclada Croiz., sp. nov. – Bull.
Jard. Bot. Buitenzorg, sér. III, 16: 351-357.
Croizat L. 1941a. The tribe Plukenetiinae of
the Euphorbiaceae in eastern
tropical Asia. – J. Arnold Arbor. 22: 417-431.
Croizat L. 1941b. Notes on the Euphorbiaceae II. 1. The
systematic position of the Omphalea. – Bull. Jard. Bot. Buitenzorg,
ser. III, 17: 204-208.
Croizat L. 1942a. On certain Euphorbiaceae from the tropical
Far East. – J. Arnold Arbor. 23: 29-54.
Croizat L. 1942b. New and critical Euphorbiaceae chiefly from the
southeastern United States. – Bull. Torrey Bot. Club 69: 445-460.
Croizat L. 1942c. Peculiarities of the
inflorescence in the Euphorbiaceae. – Bot. Gaz. 103:
771-779.
Croizat L. 1943a. New or critical Euphorbiaceae of Brazil. – Trop.
Woods 76: 11-14.
Croizat L. 1943b. Novelties in American Euphorbiaceae. – J. Arnold
Arbor. 24: 165-189.
Croizat L. 1945. New or critical Euphorbiaceae from the Americas.
– J. Arnold Arbor. 26: 181-196.
Croizat L. 1965. An introduction to the
subgeneric classification of Euphorbia L., with stress on the South
African and Malagasy species I. – Webbia 20: 573-706.
Croizat L. 1972. An introduction to the
subgeneric classification of Euphorbia L. with stress on the South
African and Malagasy species III. – Webbia 27: 1-221.
Croizat L. 1973. Les Euphorbiacées vue en
elles-mêmes, et dans leurs rapports envers l’angiospermie en général. –
Mem. Soc. Brot. 23:. 5-206.
Cruz ND de, Sellito Boaventura VM, Sellito
YM. 1990. Cytological studies on some species of the genus Clusia L.
(Guttiferae). – Rev. Brasil. Genet. 13: 335-345.
Cu J, Perineau QF, Gaset A. 1992. Volatile
components of violet leaves. – Phytochemistry 31: 571-573.
Cuatrecasas J. 1950. Studies in South
American plants II. – Fieldiana, Bot. 27: 55-113.
Cuatrecasas J. 1958. Prima Flora Colombiana
2. Malpighiaceae. – Webbia
13: 343-664.
Cuatrecasas J. 1961. A taxonomic revision of
the Humiriaceae. – Contr. U. S.
Natl. Herb. 35: 25-214.
Cuautle M, Rico-Gray V. 2003. The effects of
wasps and ants on the reproductive success of the floral nectaried plant
Turnera ulmifolia (Turneraceae). – Funct. Ecol. 17:
417-423.
Culwell DE. 1970. A taxonomic study of the
section Hypericum in the eastern United States. – Ph.D. diss.,
University of North Carolina, Chapel Hill, North Carolina.
Cusset C. 1972 [1973]. Les Podostemaceae de Madagascar. –
Adansonia, sér. II, 12: 557-568.
Cusset C. 1978. Contribution à l’étude
des Podostemaceae 5. Le genre
Macropodiella Engl. – Bull. Mus. Natl. Hist. Nat. Paris, sér. V,
sect. B, Adansonia, sér. II, 17: 293-303.
Cusset C. 1980. Contribution à l’étude
des Podostemaceae 6. Les genres
Leiothylax, et Letestuella. – Bull. Mus. Natl. Hist. Nat.
Paris, sér. V, sect. B, Adansonia, sér. II, 20: 199-207.
Cusset C. 1983 [1984]. Contribution à
l’étude des Podostemaceae 7.
Ledermanniella Engl. sous-genre Phyllosoma C. Cusset. –
Bull. Mus. Natl. Hist. Nat. Paris, sér. V, sect. B, Adansonia 4: 361-390.
Cusset C. 1984. Contribution à l’étude
des Podostemaceae 8.
Ledermanniella Engl. sous-grenre Ledermanniella. – Bull.
Mus. Natl. Hist. Nat. Paris, sér. VI, sect. B, Adansonia 3: 249-278.
Cusset C. 1992. Contribution à l’étude
des Podostemaceae 12. Les
genres asiatique. – Bull. Mus. Natl. Hist. Nat. Paris, sér. IV, sect. B,
Adansonia 14: 13-54.
Cusset C, Cusset G. 1988a. Étude sur les
Podostemales 9. Délimitations taxinomiques dans les Tristichaceae. – Bull.
Mus. Natl. Hist. Nat. Paris, sér. IV, sect. B, Adansonia 10: 149-177.
Cusset C, Cusset G. 1988b. Étude sur les
Podostemales 10. Structures florales et végétatives des Tristichaceae. –
Bull. Mus. Natl. Hist. Nat. Paris, sér. IV, sect. B, Adansonia 10: 179-218.
Cusset C, Cusset G. 1988c. Étude sur les
Podostemales 11. Répartition et évolution des Tristichaceae. – Bull. Mus.
Natl. Hist. Nat. Paris, sér. IV, sect. B, Adansonia 10: 223-262.
Cusset G. 1968. Les vrilles des
Passifloracées. – Bull. Soc. Bot. France 115: 45-61.
Cusset G. 1974. Quelques traits remarquables
de l’organisation du Thelethylax minutiflora C. Cusset
(Podostémacée). – In: Actes 99ème Congrès National des
Sociétés Savantes, Besançon, 1974, Sciences, fasc. 2, pp. 177-188.
Cusset G, Cusset C. 1989. Biogéographie
évolutive de Tristicha trifaria (Bory ex Willd.) Sprengel. – Bull.
Mus. Natl. Hist. Nat. Paris, sér. IV, sect. B, Adansonia 11: 39-70.
Dahl AO. 1955. The pollen morphology of
several genera excluded from the family Icacinaceae. –
J. Arnold Arbor. 35: 159-163.
Dahlgren RMT. 1988. Rhizophoraceae and Anisophylleaceae:
summary statement, relationships. – Ann. Missouri Bot. Gard. 75:
1259-1277.
Dahlgren R, Wyk AE van. 1988. Structures and
relationships of families endemic to or centered in southern Africa. –
Monogr. Syst. Bot. Missouri Bot. Gard. 25: 1-94.
D’Alascio Deschamps R. 1973. Organisation
du sac embryonnaire du Linum catharticum L., espèce récoltée en
station naturelle; étude ultrastructurale. – Bull. Soc. Bot. France 120:
189-200.
D’Alascio Deschamps R. 1981. Embryologie du
Linum catharticum L. Le zygote: étude ultrastructurale. – Bull.
Soc. Bot. France 128: 269-278.
Dalziel JM. 1931. The hairs lining the loculi
of fruits of species of Parinarium. – Proc. Linn. Soc. London 143:
99.
Dang-Van-Liem. 1962. Recherches sur
l’embryogénie des Tricoques. – Ph.D. diss., l’Université de Paris,
France.
D’Arcy WG. 1978. Dystovomita, a
new genus of neotropical Guttiferae. – Ann. Missouri Bot. Gard. 65:
694-697.
Da Silva MJ, De Sales MF. 2008. Reinstatement
of Phyllanthus retroflexus Brade (Phyllanthaceae). – Bot. J.
Linn. Soc. 158: 78-81.
Dathan ASR, Singh D. 1971. Embryology and
seed-development in Bergia L. – J. Indian Bot. Soc. 50: 362-370.
Dathan ASR, Singh D. 1973a. Structure and
development of ovule and seed in Flacourtia indica (Burn. F.) Merr.
– Proc. Indian Acad. Sci., Sect. B, 39: 172-179.
Dathan ASR, Singh D. 1973b. Structure and
development of seed coat in Viola spp. – J. Indian Bot. Soc. 52:
119-126.
Dathan ASR, Singh D. 1973c. Development and
structure of seed in Tacsonia Juss. and Passiflora L. –
Proc. Indian Acad. Sci., Sect. B, 77: 5-18.
Dathan ASR, Singh D. 1979. Structure and
development of female gametophyte and seed in Hydnocarpus laurifolia
(Dennst.) Sleumer. – J. Indian Bot. Soc. 59: 256-263.
Daumann E. 1972. Zur Blütenmorphologie und
Bestäubungsökologie von Mercurialis L. – Preslia 44: 308-315.
Davis CC. 2002. Madagasikaria (Malpighiaceae): a new genus from
Madagascar with implications for floral evolution in Malpighiaceae. – Amer. J. Bot.
89: 699-706.
Davis CC. 2008. Floral evolution: dramatic
size change was recent and rapid in the world’s largest flowers. – Curr.
Biol. 18: R1102-R1104.
Davis CC, Anderson WR. 2010. A complete
generic phylogeny of Malpighiaceae inferred from
nucleotide sequence data and morphology. – Amer. J. Bot. 97: 2031-2048.
Davis CC, Chase MW. 2004. Elatinaceae are sister to Malpighiaceae; Peridiscaceae
belong to Saxifragales. –
Amer. J. Bot. 91: 262-273.
Davis CC, Wurdack KJ. 2004. Host-to-parasite
gene transfer in flowering plants: phylogenetic evidence from Malpighiales. – Science 305:
676-678.
Davis CC, Anderson WR, Donoghue MJ. 2001.
Phylogeny of Malpighiaceae:
evidence from chloroplast ndhF and trnL-F nucleotide
sequences. – Amer. J. Bot. 88: 1830-1846.
Davis CC, Bell CD, Fritsch PW, Mathews S.
2002. Phylogeny of Acridocarpus-Brachylophon (Malpighiaceae): implications for
Tertiary tropical floras and Afroasian biogeography. – Evolution 56:
2395-2405.
Davis CC, Bell CD, Mathews S, Donoghue MJ.
2002. Laurasian migration explains Gondwanan disjunctions: evidence from Malpighiaceae. – Proc. Natl.
Acad. Sci. U.S.A. 99: 6833-6837.
Davis CC, Fritsch PW, Bell CD, Mathews S.
2004. High-latitude Tertiary migrations of an exclusively tropical clade:
evidence from Malpighiaceae.
– Intern. J. Plant Sci. 165(Suppl.): S107-S121.
Davis CC, Anderson WR, Wurdack KJ. 2005. Gene
transfer from a parasitic flowering plant to a fern. – Proc. Roy. Soc.
London, Sect. B, Biol. Sci. 272: 2237-2242.
Davis CC, Webb CO, Wurdack KJ, Jaramillo CA,
Donoghue MJ. 2005. Explosive radiation of Malpighiales supports a
mid-Cretaceous origin of modern tropical rain forests. – Amer. Natur. 165:
E36-E65.
Davis CC, Latvis M, Nickrent DL, Wurdack KJ,
Baum DA. 2007. Floral gigantism in Rafflesiaceae. – Science 315:
1812.
Davis CC, Endress PK, Baum DA. 2008. The
evolution of floral gigantism. – Curr. Opin. Plant Biol. 11: 49-57.
Dechamps R, Mosango M, Robbecht E. 1985.
Études systématiques sur les Hymenocardiaceae d’Afrique: la morphologie du
pollen et l’anatomie du bois. – Bull. Jard. Bot. État Bruxelles 55:
473-485.
Deginani NB. 2001. Las especies argentinas
del género Passiflora (Passifloraceae). – Darwiniana
39: 43-129.
Deginani NB, Escobar A. 2002. Números
cromosómicos de especies de Passiflora (Passifloraceae). – Hickenia 3:
143-144.
Dehay C. 1935. L’Appareil libéro-ligneux
foliaire des Euphorbiacées. – Ann. Sci. Nat. Bot., sér. X, 17: 147-296.
Dehgan B. 1980. Application of epidermal
morphology to taxonomic delimitations in the genus Jatropha L. (Euphorbiaceae). – Bot. J. Linn.
Soc. 80: 257-278.
Dehgan B. 1982. Comparative anatomy of the
petiole and infrageneric relationships in Jatropha (Euphorbiaceae). – Amer. J. Bot.
69: 1283-1295.
Dehgan B. 1984. Phylogenetic significance of
interspecific hybridization in Jatropha (Euphorbiaceae). – Syst. Bot. 9:
467-478.
Dehgan B, Craig ME. 1978. Types of laticifers
and crystals in Jatropha and their taxonomic implications. – Amer.
J. Bot. 65: 345-352.
Dehgan B, Schutzman B. 1994. Contributions
toward a monograph of neotropical Jatropha: phenetic and phylogenetic
analyses. – Ann. Missouri Bot. Gard. 81: 349-367.
Dehgan B, Webster GL. 1978. Three new species
of Jatropha from western Mexico. – Madroño 25. 30-39.
Dehgan B, Webster GL. 1979. Morphology and
infrageneric relationships of the genus Jatropha (Euphorbiaceae). – Univ. Calif.
Publ. Bot. 74: 1-73.
Dejax J. 1987. Sur la presence de grains de
pollen à sculpture crotonoide dans le Crétacé inférieur de Congo. – Mém.
Trav. Inst. Montpellier, École Prat. Haut. Études 17: 253-271.
Delay C, Mangenot G. 1960. Le développement
de la graine chez Allanblackia floribunda Oliv. – Ann. Sci. Nat.
XII, Bot. 1: 387-440.
Delevoryas T. 1964. Two petrified angiosperms
from the Upper Cretaceous of South Dakota. – J. Paleontology 38: 584-586.
Delle Monache F, Delle Monache G, Gáes-Baitz
E. 1991. Chemistry of the Clusia genus 6. Prenylated benzophenones
from Clusia sandiensis. – Phytochemistry 30: 2003-2005.
Demchenko NI. 1973. On the morphology of
pollen of the family Chrysobalanaceae. – In: Trudy
3rd International Palynological Conference, USSR, Novosibirsk, 1971,
Leningrad, pp. 69-73. [In Russian]
De Melo NE, Guerra M. 2003. Variability of
the 5S and 45S rDNA sites in Passiflora L. (Passifloraceae). – Ann. Bot.
92: 309-316.
De Melo NE, Cervi AC, Guerra M. 2001.
Karyology and cytotaxonomy of the genus Passiflora L. (Passifloraceae). – Plant Syst.
Evol. 226: 69-84.
Denis M. 1921. Les Euphorbiées des Îles
Australes d’Afrique. – In: Imprimerie Nemourienne, Nemours, pp. 66-69.
De-Nova JA, Sosa V. 2007. Phylogeny and
generic delimitation of Adelia (Euphorbiaceae) inferred from
molecular and morphological data. – Taxon 56: 1027-1036.
De-Nova JA, Sosa V, Wurdack KJ. 2006.
Phylogenetic relationships and the description of a new species of
Enriquebeltrania (Euphorbiaceae s.s.): an enigmatic
genus endemic to Mexico. – Syst. Bot. 31: 533-546.
De-Nova JA, Sosa V, Steinmann VW. 2007. A
synopsis of Adelia (Euphorbiaceae s.s.). – Syst.
Bot. 32: 583-595.
De-Paula OC, Sajo M das G. 2011. Morphology
and development of the anthers and ovules in Croton and
Astraea (Euphorbiaceae). – Nord. J. Bot.
29: 505-511.
De-Paula OC, Sajo MG, Prenner G, Cordeiro I,
Rudall PJ. 2011. Morphology, development and homologies of the perianth and
floral nectaries in Croton and Astraea (Euphorbiaceae-Malpighiales). – Plant Syst.
Evol. 292: 1-14.
Descoings B. 1960. Révision de
Dichapetalum de Madagascar. – Mém. Inst. Sci. Madag. B9: 63-120.
Dess B, McComb AJ. 1974. Resin production and
glandular hairs in Beyeria viscosa (Labill.) Miq. Euphorbiaceae). – Aust. J. Bot.
25: 195-210.
Dettmann ME, Clifford HT. 2002.
Spondylostrobus F. Mueller: operculate fruits of an extinct dicotyledon from
the mid-Tertiary of Australia. – Rev. Palaeobot. Palynol. 122: 219-237.
De Wilde WJJO. 1971a. The systematic position
of the tribe Paropsieae, in particular the genus Ancistrothyrsis, and
a key to the genera of Passifloraceae. – Blumea 19:
99-104.
De Wilde WJJO. 1971b. A monograph of the
genus Adenia Forsk. (Passifloraceae). – Meded.
Landbouwh. Wageningen 71(18): 1-281.
De Wilde WJJO. 1974. The genera of tribe
Passifloreae (Passifloraceae)
with special reference to flower morphology. – Blumea 22: 31-35.
De Wilde WJJO. 1975. Passifloraceae. – In: Polhill
RM (ed), Flora of tropical East Africa, Crown Agents for Oversea Governments
and Aministrations, London, pp. 1-70.
De Wildeman E. 1919. Notes sur les espèces
Africaines du genre Dichapetalum Thonn. – Rév. Zool. Afr. 4(2),
Suppl. Bot. 1-75.
Diaz DMV. 2013. Multivariate analysis of
morphological and anatomical characters of Calophyllum (Calophyllaceae) in South America.
– Bot. J. Linn. Soc. 171: 587-626.
Dick CW, Abdul-Salim K, Bermingham E. 2003.
Molecular systematics reveals cryptic Tertiary diversification of a widespread
tropical rainforest tree. – Amer. Natur. 160: 691-703.
Dickison WC. 1990a. A study of floral
morphology of the Caryocaraceae. – Bull. Torrey
Bot. Club 117: 123-137.
Dickison WC. 1990b. The morphology and
relationships of Medusagyne (Medusagynaceae). – Plant Syst.
Evol. 171: 27-55.
Dickison WC. 1990c. An additional note on the
floral morphology and affinities of Medusagyne oppositifolia (Medusagynaceae). – Brittonia
42: 191-196.
Dickison WC, Weitzman AL. 1996. Comparative
anatomy of the young stem, node, and leaf of Bonnetiaceae, including
observations on a foliar endodermis. – Amer. J. Bot. 83: 405-418.
Dickison WC, Weitzman AL. 1998. Floral
morphology and anatomy of Bonnetiaceae. – J. Torrey Bot.
Soc. 125: 268-286.
Diederichsen A, Richards K. 2003. Cultivated
flax and the genus Linum. – In: Muir AD, Westcott ND (eds), Flax:
The genus Linum, Routledge, London, New York, pp. 22-54.
Dilcher DL, Manchester SR. 1988.
Investigations of angiosperms from the Eocene of North America: a fruit
belonging to the Euphorbiaceae.
– Tertiary Res. 9: 45-58.
Dįnç M, Yildirimli Ş. 2002. A new species
of Viola (Violaceae) from
Turkey. – Bot. J. Linn. Soc. 138: 483-487.
Dįnç M, Bağci Y, Yildirimli Ş. 2003. A
new species of Viola L. (Violaceae) from South Anatolia. –
Bot. J. Linn. Soc. 141: 477-482.
Ding Hou 1957. A conspectus of the genus
Bruguiera (Rhizophoraceae). – Nova Guinea,
n.s. 8: 163-171.
Ding Hou. 1958a. Rhizophoraceae. – In: Steenis
CGGJ van (ed), Flora Malesiana I, 5(4), Noordhoff-Kolff N. V., Djakarta, pp.
429-493.
Ding Hou. 1958b. A conspectus of the genus
Bhesa (Celastraceae). –
Blumea 4: 149-153.
Ding Hou 1960. A review of the genus
Rhizophora with special reference to the Pacific species. – Blumea
10: 625-634.
Dingler H. 1885. Die Flachsprosse der
Phanerogamen. Erstes Heft: Phyllanthus sect. Xylophylla. –
T. Ackermann, München.
Dissanayake D. 1999. Phylogenetic research on
the family Chrysobalanaceae.
– Ph.D. thesis, Department of Botany, University of Reading, United
Kingdom.
Djarwaningsih T. 2004. Revision of
Pimelodendron (Euphorbiaceae) in Malesia. –
Blumea 49: 407-423.
Dobson III FH. 1983. Novelties in
Bunchosia (Malpighiaceae). – Syst. Bot. 8:
269-276.
Dominguez CA, Bullock SH. 1989. La
reproducción de Croton suberosus (Euphorbiaceae) en luz y sombre.
– Rev. Biol. Trop. 37: 1-10.
Dominguez CA, Dirzo R, Bullock SH. 1989. On
the function of floral nectar in Croton suberosus (Euphorbiaceae). – Oikos 56:
109-114.
Dorn RD. 1976. A synopsis of American
Salix. – Can. J. Bot. 54: 2769-2789.
Dorn RD. 1998. A taxonomic study of
Salix section Longifoliae (Salicaceae). – Brittonia 50:
193-210.
Dorn RD. 2000. A taxonomic study of
Salix sections Mexicanae and Viminella subsection
Sitchenses (Salicaceae)
in North America. – Brittonia 52: 1-19.
Dorr LJ. 1999. A new combination in
Croizatia (Euphorbiaceae). – Sida 18:
831-836.
Dorsey BL, Haevermans T, Aubriot X, Morawetz
JJ, Riina R, Steinmann VW, Berry PE. 2013. Phylogenetics, morphological
evolution, and classification of Euphorbia subgenus
Euphorbia. – Taxon 62: 291-315.
D’Ovidio R. 1992. Nucleotide sequence of a
5.8S rDNA gene and the internal transcribed spacers from Populus
deltoides. – Plant Mol. Biol. 19: 1069-1072.
Doweld AB. 1998. On the phylogenetic
relationships of Medusagyne (Medusagynaceae) as evidenced by
the structure of its fruits and seeds. – Bot. Žurn. 83: 54-68. [In
Russian]
Dransfield J, Whitmore TC. 1970. A
Podostemacea new to Malaya: Indotristicha malayana. – Blumea 18:
152-155.
Dressler RL. 1954. The genus
Tetracoccus (Euphorbiaceae). – Rhodora 56:
45-61.
Dressler RL. 1957. The genus
Pedilanthus (Euphorbiaceae). – Contr. Gray
Herb. 182: 1-188.
Dressler RL. 1962. A synopsis of
Poinsettia. – Ann. Missouri Bot. Gard. 48: 329-341.
Dressler S. 1996a. Proposal to conserve the
name Bridelia (Euphorbiaceae) with a conserved
spelling. – Taxon 45: 337-338.
Dressler S. 1996b. The genus
Bridelia in Malesia and Indochina. – Blumea 41: 263-331.
Dressler S. 1996c. Bridelia (Euphorbiaceae) in New Guinea with
a description of a new species. – Kew Bull. 51: 601-607.
Ducke A. 1934. Anomalocalyx. –
Notizbl. Bot. Mus. Gart. Berlin-Dahlem 11: 344-345.
Duke NC. 2010. Overlap of eastern and western
mangroves in the south-western Pacific: hybridization of all three
Rhizophora (Rhizophoraceae) combinations in
New Caledonia. – Blumea 55: 171-188.
Dulberger R. 1973. Distyly in Linum
pubescens and L. mucronatum. – Bot. J. Linn. Soc. 66:
117-126.
Dulberger R. 1974. Structural dimorphism of
stigmatic papillae in distylous Linum species. – Amer. J. Bot. 61:
238-243.
Dulberger R. 1981. Dimorphic exine
sculpturing in three distylous species of Linum (Linaceae). – Plant Syst. Evol. 139:
113-119.
Dunthorn MS. 2002. Anatomy and palynology of
Mammea L. (Clusiaceae).
– M.Sc. thesis, University of Missouri, St. Louis, Missouri.
Dunthorn MS. 2004. Cryptic dioecy in
Mammea (Clusiaceae). –
Plant Syst. Evol. 249: 191-196.
Dunthorn MS. 2009. Foliar anatomy and fiber
motifs in Mammea (Clusiaceae, Kielmeyeroideae). –
Plant Syst. Evol. 280: 153-166.
Dupuy P, Guédès M. 1975. Placentation and
possible partial stachyospory in Hypericum sect. Eremanthe.
– Flora 164: 37-49.
Durand B. 1957. L’organisation de
l’androecée des Mercurialis. – Compt. Rend. Acad. Sci. Paris 244:
650-653.
Durkee LT, Gaal DJ, Reisner H. 1981. The
floral and extrafloral nectaries of Passiflora I. The floral nectary.
– Amer. J. Bot. 68: 453-462.
Durkee LT, Baird CW, Cohen PF. 1984. Light
and electron microscopy of the resin glands of Passiflora foetida (Passifloraceae). – Amer. J.
Bot. 71: 596-602.
Dutt MK. 1942. Development of the microspores
and the nuclear behaviour in the tapetal cells of Putranjiva
roxburghii, Wall. – Sci. & Cult. 8: 309-310.
Dwyer JD. 1943. The taxonomy of the
monogeneric tribe Elvasieae (Ochnaceae). – Bull. Torrey Bot. Club
70: 42-49.
Dwyer JD. 1944a. The taxonomy of the Mexican,
Central American and West Indian species of Ouratea (Ochnaceae). – Lloydia 7: 121-145.
Dwyer JD. 1944b. Philacra, a new
genus of the Ochnaceae. –
Brittonia 5: 124-127.
Dwyer JD. 1944c. A discussion of the
ochnaceous genus Fleurydora A. Chev. and the allied genera of the
Luxemburgieae. – Bull. Torrey Bot. Club 71: 175-178.
Dwyer JD. 1945. The taxonomy of the genus
Sauvagesia (Ochnaceae).
– Bull. Torrey Bot. Club 72: 521-540.
Dwyer JD. 1946. The taxonomy of
Godoya R. & P., Rhytidanthera van Tieghem, and
Cespedezia Goudot (Ochnaceae). – Lloydia 9: 45-61.
Dwyer JD. 1951. The genus
Luxemburgia (Ochnaceae).
– Lloydia 14: 82-97.
Dwyer JD. 1964. The taxonomy of
Lavradia Vell. (Ochnaceae). – Bull. Jard. Bot. Ètat
Bruxelles 34: 507-518.
Dwyer JD. 1965. The Amazonian genus
Wallacea Spruce ex Hook. f. (Ochnaceae). – Bull. Jard. Bot. État
Bruxelles 35: 85-90.
Eckardt NA, Baum D. 2010. The podostemad
puzzle: the evolution of unusual morphology in the Podostemaceae. – The Plant Cell
22: 2104.
Eckenwalder JE. 1977. North American
cottonwoods (Populus, Salicaceae) of sections
Abaso and Aigeiros. – J. Arnold Arbor. 58: 193-207.
Eckenwalder JE. 1980. Foliar heteromorphism
in Populus (Salicaceae),
a source of confusion in the taxonomy of Tertiary leaf remains. – Syst. Bot.
5: 366-383.
Eckenwalder JE. 1996. Salix. – In:
Stettler RF (ed), Biology of Populus and its implications for
management and conservation, Natl. Res. Council Canada Res. Press, Ottawa, pp.
7-32.
Ee BW van, Berry PE. 2009. A phylogenetic and
taxonomic review of Croton (Euphorbiaceae s.s.) on Jamaica
including the description of Croton jamaicensis, a new species of
Section Eluteria. – Syst. Bot. 34: 129-140.
Ee BW van, Berry PE. 2010. Taxonomy and
phylogeny of Croton section Heptallon (Euphorbiaceae). – Syst. Bot. 35:
151-167.
Ee BW van, Berry PE. 2011. Croton
section Pedicellati (Euphorbiaceae), a novel New World
group, and a new subsectional classification of Croton section
Lamprocroton. – Syst. Bot. 36: 88-98.
Ee BW van, Berry PE, Riina R, Amaro JEG.
2008. Molecular phylogenetics and biogeography of the Caribbean-centered
Croton subgenus Moacroton (Euphorbiaceae s.s.). – Bot. Rev.
74: 132-165.
Ee BW van, Riina R, Berry PE. 2011. A revised
infrageneric classification and molecular phylogeny of New World
Croton (Euphorbiaceae). – Taxon 60:
791-823.
Ee BW van, Forster PI, Berry PE. 2015.
Phylogenetic relationships and a new sectional classification of
Croton (Euphorbiaceae)
in Australia. – Aust. Syst. Bot. 28: 219-233.
Ehrendorfer F, Morawetz W, Dawe J. 1984. The
Neotropical angiosperm families Brunelliaceae and Caryocaraceae: first
karyosystematical data and affinities. – Plant Syst. Evol. 145: 183-191.
Ehrenfeld J. 1976. Reproductive biology of
three species of Euphorbia subgenus Chamaesyce (Euphorbiaceae). – Amer. J. Bot.
63: 406-413.
Elffers J, Taylor P. 1956. Cavacoa
aurea (Cavaco) Léonard. – Hooker’s Icon. Plant 36: t. 3561.
El-Ghazaly G. 1989. Pollen and orbicule
morphology of some Euphorbia species. – Grana 28: 243-259.
El-Ghazaly GA, Chaudhary R. 1993. Pollen
morphology of some species of the genus Euphorbia L. – Rev.
Palaeobot. Palynol. 78: 293-319.
El-Ghazaly G, Raj B. 1986. A contribution to
the pollen morphology of Andrachne (Euphorbiaceae). – Pollen Spores
28: 297-310.
Elias MG. 2006. El género Alchornea
(Euphorbiaceae) en
Mesoamérica. – Tesis de Licenciatura, Facultad de Ciencias, UNAM,
México.
Elias MG, Martínez M, Espinosa-Matias S.
2008. Caracteres foliares del género Alchornea Sw. (Euphorbiaceae) en Mesoamérica.
– Candollea 63: 39-55.
Elias TS, Rozich W, Newcombe L. 1975. The
foliar and floral nectaries of Turnera ulmifolia L. – Amer. J. Bot.
62: 570-576.
Elmer ADE. 1910. Euphorbiaceae collected on Sibuyan
Island. – Leafl. Philipp. Bot. 3: 903-931.
Elo Manga SS, Tih AE, Ghogomu RT, Blond A,
Bodo B. 2009. Biflavonoid constituents of Campylospermum mannii. –
Biochem. Syst. Ecol. 37: 402-404.
Emmons LH, Nias J, Brium A. 1991. The fruit
and consumers of Rafflesia keithii (Rafflesiaceae). – Biotropica 23:
197-199.
Endress PK, Voser P. 1975. Zur
Androeciumanlage und Antherenentwicklung bei Caloncoba echinata
(Flacourtiaceae). – Plant Syst. Evol. 123: 241-253.
Endress PK, Davis CC, Matthews ML. 2013.
Advances in the floral structural characterization of the major subclades of Malpighiales, one of the largest
orders of flowering plants. – Ann. Bot. 111: 969-985.
Engler A. 1889. Lacistemaceae. – In: Engler
A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(1), W. Engelmann,
Leipzig, pp. 14-15.
Engler A. 1895a. Quiinaceae. – In: Engler A, Prantl
K (eds), Die natürlichen Pflanzenfamilien III(6), W. Engelmann, Leipzig, pp.
165-167.
Engler A. 1895b. Guttiferae. – In: Engler
A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(6), W. Engelmann,
Leipzig, pp. 195-242; Engler A. 1897. Nachträge zu III(6), pp. 247-250.
Engler A. 1896a. Dichapetalaceae. – In: Engler
A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(4), W. Engelmann,
Leipzig, pp. 345-351.
Engler A. 1896b. Icacinaceae.
– In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(5), W.
Engelmann, Leipzig, pp. 233-257.
Engler A. 1897. Balanopsidaceae. – In:
Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien, Nachträge zu
III(1), pp. 114-116.
Engler A. 1903. Linaceae africanae. – Engl. Bot.
Jahrb. Syst. 23: 104-110.
Engler A. 1912. Panda oleosa, ein
Ölsamenbaum Westafrikas. – Notizbl. Königl. bot. Gart. Mus. Berlin 5:
274-276.
Engler A. 1925. Quiinaceae. – In: Engler A, Gilg E
(eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 21, W. Engelmann,
Leipzig, pp. 106-108.
Engler A. 1926. Beiträge zur Flora von
Afrika LII. Podostemonaceae Africanae IV. – Engl. Bot. Jahrb. Syst. 60:
451-467.
Engler A. 1930. Podostemonaceae. – In:
Engler A, Harms H (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 18a,
W. Engelmann, Leipzig, pp. 3-68, 483-484.
Engler A (†). 1931. Simarubaceae. – In:
Engler A (†), Harms H, Pax F (eds), Die natürlichen Pflanzenfamilien, 2.
Aufl., Bd. 19a, W. Engelmann, Leipzig, pp. 359-405.
Engler A, Keller R. 1925. Guttiferae. – In:
Engler A, Gilg E (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 21, W.
Engelmann, Leipzig, pp. 154-237.
Engler A (†), Krause K. 1931. Dichapetalaceae. – In: Engler
A (†), Harms H, Pax F (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd.
19c, W. Engelmann, Leipzig, pp. 1-11.
Engler A, Melchior H. 1925. Medusagynaceae. – In: Engler A,
Gilg E (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 21, W.
Engelmann, Leipzig, pp. 50-52.
English S, Greenaway W, Whatley FR. 1991.
Analysis of phenolics of Populus trichocarpa bud extract by GC-MS. –
Phytochemistry 30: 531-533.
Erben M. 1985. Cytotaxonomische
Untersuchungen an südosteuropäischen Viola-Arten der Sektion
Melanium. – Mitt. Bot. Staatssamml. München 21: 339-740.
Erben M. 1996. The significance of
hybridization on the forming of species in the genus Viola. –
Bocconea 5: 113-118.
Erdelská O. 1967. Type of endosperm of the
species Linum austriacum L. – Biologia (Bratislava) 22: 172-176.
Erdtman G. 1955. Pollen grains of
Ctenolophon from tertiary deposits in India. – Bot. Not. 108:
138-145.
Erlanson EW, Hermann FJ. 1928. The morphology
and cytology of perfect flowers of Populus tremuloides. – Mich.
Acad. Sci. 8: 97-110.
Ernst A, Schmid E. 1913. Über Blüthe und
Frucht von Rafflesia. – Ann. Jard. Bot. Buitenzorg, sér. II, 12:
1-58.
Ernst E (ed). 2003. Hypericum. The
genus Hypericum. Medicinal and aromatic plants – Industrial
Profiles, 31. – Taylor & Francis, London, New York.
Escobar LK. 1986. New species and varieties
of Passiflora (Passifloraceae) from the Andes of
South America. – Syst. Bot. 11: 88-97.
Escobar LK. 1994. Two new species and a key
to Passiflora subg. Astrophea. – Syst. Bot. 19: 203-210.
Espinoza de Pernía N, Welle BJH ter. 1998.
Flora of the Guianas. Ser. A: Phanerogams. Fasc. 21. Wood and timber. Euphroniaceae. – Royal Botanic
gardens, Kew, pp. 81-83.
Esser H-J. 1994. Systematische Studien an den
Hippomaneae Adr. Jussieu ex Bartling (Euphorbiaceae), insbesondere den
Mabeinae Pax & K. Hoffm. – Ph.D. diss., Universität Hamburg, Germany.
Esser H-J. 1996a. Proposal to conserve the
name Homalanthus (Euphorbiaceae) with a conserved
spelling. – Taxon 45: 555-556.
Esser H-J. 1996b. Glyphostylus (Euphorbiaceae, Hippomaneae)
re-examined. – Nord. J. Bot. 16: 579-581.
Esser H-J. 1999. A partial revision of the
Hippomaneae (Euphorbiaceae) in
Malesia. – Blumea 44: 149-215.
Esser H-J. 2001a. Tribes Hippomaneae,
Pachystromateae & Hureae. – In: Radcliffe-Smith A, Genera
Euphorbiacearum, Royal Botanic Gardens, Kew.
Esser H-J. 2001b. New combinations in African
Shirakiopsis (Euphorbiaceae). – Kew Bull. 56:
1017-1018.
Esser H-J. 2003a. Variation in fruit
characters of Euphorbiaceae –
is there another subfamily? – Palm. Hortus Francofurtensis 7: 149.
Esser H-J. 2003b. Fruit characters in
Malesian Euphorbiaceae. –
Telopea 10: 169-177.
Esser H-J. 2012. The tribe Hippomaneae (Euphorbiaceae) in Brazil. –
Rodriguésia 63: 209-225.
Esser H-J. 2017. New species of
Gymnanthes (Euphorbiaceae) from Bolivia and
Colombia, and taxonomic notes on the genus in Venezuela. – Willdenowia 47:
217-224.
Esser H-J, Taylor SE. 1983. Pro-inflammatory,
tumour-promoting and anti-tumour diterpenes of the families Euphorbiaceae and Thymelaeaceae. – Prog.
Chem. Org. Nat. Prod. 44: 1-99.
Esser H-J, Welzen PC van. 2001.
Colobocarpos, a new genus of South-East Asian Euphorbiaceae. – Kew Bull. 56:
657-659.
Esser H-J, Welzen PC van, Djarwaningsih T.
1997. A phylogenetic classification of the Malesian Hippomaneae (Euphorbiaceae). – Syst. Bot. 22:
617-628.
Esser H-J, Berry PE, Riina R. 2009.
EuphORBia: a global inventory of the spurges. – Blumea 54: 11-12.
Estabrook GF, Gates B. 1984. Character
analysis in the Banisteriopsis campestris complex (Malpighiaceae), using spatial
autocorrelation. – Taxon 33: 13-25.
Estrada-Chavarria A. 2000.
Chiangiodendrum mexicanum Wendt (Flacourtiaceae), un nuevo registro
para la flora arborescente de Costa Rica. – Brenesia 54: 77-80.
Evans FJ. 1986. Macrocyclic diterpenes of the
family Euphorbiaceae. – In:
Evans FJ (ed), Naturally occurring phorbol esters, CRC Press, Boca Raton,
Florida, pp. 139-170.
Evans FJ, Kinghorn AD. 1977. A comparative
phytochemical study of the diterpenes of some species of the genera
Euphorbia and Elaeophorbia (Euphorbiaceae). – Bot. J. Linn.
Soc. 74: 23-35.
Evans FJ, Taylor SE. 1983. Pro-inflammatory,
tumour-promoting and anti-tumour diterpenes of the families Euphorbiaceae and Thymelaeaceae. – Progr.
Chem. Org. Nat. Prod. 44: 1-99.
Evans M, Aubriot X, Hearn D, Lanciaux M,
Lavergne S, Cruaud C, Lowry PP, Haevermans T. 2014. Insights on the evolution
of plant succulence from a remarkable radiation in Madagascar (Euphorbia). –
Syst. Biol. 63: 697-711.
Exell AW. 1963. 42. Irvingiaceae. – In: Exell AW,
Fernandes A, Wild H (eds), Flora Zambesiaca 2 (Part 1), Crown Agents for
Oversea Governments and Administrations, London, p. 220.
Exell AW, Robson NKB. 1960. New species of
Polygala and Garcinia from tropical Africa. – Bol. Soc.
Brot., ser. II, 34: 93-97.
Fabijan DM, Packer JG, Denford KE. 1987. The
taxonomy of the Viola nuttallii complex. – Can. J. Bot. 65:
2562-2580.
Faivre-Rampant P. 1992. Réconnaissance
d’espèces, de clones et d’hybrides de peupliers grace au polymorphisme des
genes nucléaires codant pur les ARN ribosomiques: hypotheses sur
l’évolution du genre Populus. – Ph.D. diss., l’Université
Blaise Pascal, Clermont-Ferrand II, France.
Fan GS. 1990. A preliminary study on
Flacourtiaceae from China. – J. Wuhan Bot. Res. 8: 131-141.
Farnsworth NR, Blomster RN, Meser WM, King
JC, Persinos GJ, Wilkes JD. 1969. A phytochemical and biological review of the
genus Croton. – Lloydia 32: 1-28.
Farron C. 1957. Contribution à la cytologie
des Ouratea d’Afrique occidentale française. – Ber. Schweiz. Bot.
Ges. 676: 26-32.
Farron C. 1963. Contribution à la taxinomie
des Ourateeae Engl. – Bull. Soc. Bot. Suisse 73: 196-217.
Farron C. 1985. Les Ouratinae (Ochnaceae) d’Afrique continentale.
Cartes de distribution et clés de determination de tous les genres et
espèces. – Bot. Helv. 95: 59-72.
Fatemi M, Gross CL, Bruhl JJ. 2007. The first
phenetic analysis of species limits in Bertya (Euphorbiaceae). – Aust. Syst.
Bot. 20: 448-463.
Fay MF, Swensen SM, Chase MW. 1997. Taxonomic
affinities of Medusagyne oppositifolia (Medusagynaceae). – Kew Bull.
52: 111-120.
Feio AC, Riina R, Meira RMSA. 2016. Secretory
structures in leaves and flowers of two dragon’s blood Croton (Euphorbiaceae): new evidence and
interpretations. – Intern. J. Plant Sci. 177: 511-522.
Feio AC, Ore-Rengifo MI, Berry PE, Riina R.
2018. Four new species of dragon’s blood Croton (Euphorbiaceae) from South America.
– Syst. Bot. 43: 212-220.
Feng M. 2005. Floral morphogenesis and
molecular systematics of the family Violaceae. – PhD diss., Ohio
University.
Feres F. 2001. O gênero Luxemburgia
A. St.-Hil. (Ochnaceae) –
revisão taxonômica e estudo cladístico. – Dissertação de Mestrado,
Universidade de Campinas, Brazil.
Fernandes A, Fernandes R. 1978. 84. Passifloraceae. – In: Launert E
(ed), Flora Zambesiaca 4, Flora Zambesiaca Managing Committee, London, pp.
368-411.
Fernandes R. 1975. Turneraceae africanae: notulae
systematicae et taxa nova. – Bol. Soc. Brot. 49: 13-27.
Fernandes R. 1978. 83. Turneraceae. – In: Launert E (ed),
Flora Zambesiaca 4, Flora Zambesiaca Managing Committee, London, pp.
348-368.
Fernandes VF, Thadeo M, Dalvi VC, Marquete R,
Meira RMSA. 2016. Colleters in Casearia (Salicaceae): a new interpretation for
the theoid teeth. – Bot. J. Linn. Soc. 181: 682-691.
Fernández A. 1987. Estudios cromosómicos en
Turnera y Piriqueta (Turneraceae). – Bonplandia 6:
1-21.
Fernández C. 1984. Estudios sobre el género
Viola L. en la Península Ibérica I. – Cariologia Fontqueria 5:
23-32.
Fernández-Alonso JL, Pérez-Zabala JA,
Idarraga-Piedrahita A. 2000. Isidodendron, un nuevo género
neotropical de árboles de la familia Trigoniaceae. – Rev. Acad.
Colomb. Ci. 24: 347-357.
Fernando ES, Ong PS. 2005. The genus
Rafflesia R. Br. (Rafflesiaceae) in the Philippines.
– Asia Life Sci. 14: 263-270.
Fernando ES, Gadek PA, Quinn CJ. 1995. Simaroubaceae, an
artificial construct: evidence from rbcL sequence variation. – Amer.
J. Bot. 82: 92-103.
Feuillet C. 2002. A new series and three new
species of Passiflora subgenus Astrophea from the Guianas.
– Brittonia 54: 18-29.
Feuillet C, MacDougal JM. 2004. A new
infrageneric classification of Passiflora L. (Passifloraceae). – Passiflora
13: 34-35, 37-38.
Feuillet C, MacDougal JM. 2006. Passifloraceae. – In: Kubitzki
K (ed), The families and genera of vascular plants IX. Flowering plants.
Eudicots. Berberidopsidales,
Buxales, Crossosomatales, Fabales p. p., Geraniales, Gunnerales, Myrtales p. p., Proteales, Saxifragales, Vitales, Zygophyllales, Clusiaceae Alliance, Passifloraceae Alliance, Dilleniaceae, Huaceae, Picramniaceae, Sabiaceae, Springer, Berlin,
Heidelberg, New York, pp. 270-281.
Fiala B, Meyer U, Hashim R, Maschwitz U.
2011. Pollination systems in pioneer trees of the genus Macaranga (Euphorbiaceae) in Malaysian
rainforests. – Biol. J. Linn. Soc. 103: 935-953.
Fiaschi P, Cordeiro I. 2005. Discocarpus
pedicellatus, a new species of Phyllanthaceae (Euphorbiaceae s.l.) from southern
Bahia, Brazil. – Brittonia 57: 248-251.
Fiaschi P, Groppo M. 2008.
Kuhlmanniodendron Fiaschi & Groppo, a new eastern Brazilian genus
of Achariaceae sensu lato
segregated from Carpotroche Endl. (formerly included in
Flacourtiaceae). – Bot. J. Linn. Soc. 157: 103-109.
Figier J. 1968. Étude infrastructurale et
cytochimique des glandes pétiolaires de Mercurialis annua L. Essai
d’interpretation en rapport avec la secretion. – Compt Rend. Acad. Sci.
Paris D267: 491-494.
Filho WW, Da Rocha AI, Yoshida M, Gottlieb
OR. 1985. Ellagic acid derivatives from Rhabdophyllum macrophyllum.
– Phytochemistry 24: 1991-1997.
Fišer Pečnikar Ž, Kulju KKJ, Sierra SEC,
Baas P, Welzen PC van. 2012. Leaf anatomy of Mallotus and the related
genera Blumeodendron and Hancea (Euphorbiaceae sensu stricto). –
Bot. J. Linn. Soc. 169: 645-676.
Fisher MJ. 1928. The morphology and anatomy
of the flowers of the Salicaceae.
– Amer. J. Bot. 15: 307-326, 372-394.
Fitzgerald JS. 1965. (+)-hygroline, the major
alkaloid of Carallia brachiata (Rhizophoraceae). – Aust. J.
Chem. 18: 589-591.
Floret J-J. 1974. Comiphyton genre
nouveau Gabonais, Rhizophoraceae-Macarisieae. –
Adansonia, sér. II, 14: 499-506.
Floret J-J. 1976. À propos de Comiphyton
gabonense (Rhizophoraceae-Macarisieae). –
Adansonia, sér. II, 16: 39-49.
Floret J-J. 1988. Cassipourea Aublet
(Rhizophoraceae-Macarisieae):
organisation florale et divisions subgénériques. – Adansonia, sér. IV, 10:
25-45.
Focke WO. 1894. Rosaceae. – In: Engler A, Prantl
K (eds), Die natürlichen Pflanzenfamilien III(3), W. Engelmann, Leipzig, pp.
1-61.
Focke WO. 1895. Eucryphiaceae. – In: Engler
A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(6), W. Engelmann,
Leipzig, pp. 129-131.
Forman LL. 1965. A new genus of Ixonanthaceae with notes on the
family. – Kew Bull. 19: 517-526.
Forman LL. 1966. The reinstatement of
Galearia Zoll. & Mor. and Microdesmis Hook. f. in the Pandaceae. With appendices by C. R.
Metcalfe and N. Parameswaran. – Kew Bull. 20: 309-321.
Forman LL. 1968. The systematic position of
Panda Pierre. – Proc. Linn. Soc. London 179: 269-270.
Forman LL. 1971. A synopsis of
Galearia Zoll. & Mor. (Pandaceae). – Kew Bull. 26:
153-165.
Forster PI. 1994. A taxonomic revision of
Tragia (Euphorbiaceae)
in Australia. – Aust. Syst. Bot. 7: 377-383.
Forster PI. 1995. Sankowskya, a new
genus of Euphorbiaceae
(Dissiliariinae) from the Australian Wet Tropics. – Austrobaileya 4:
329-335.
Forster PI. 1996. A taxonomic revision of
Aleurites J. R. Forst. & G. Forst. (Euphorbiaceae) in Australia and
New Guinea. – Muelleria 9: 5-13.
Forster PI. 1997a. A taxonomic revision of
Austrobuxus Miq. (Euphorbiaceae: Dissiliariinae) in
Australia. – Austrobaileya 4: 619-626.
Forster PI. 1997b. A taxonomic revision of
Drypetes Vahl (Euphorbiaceae) in Australia. –
Austrobaileya 4: 477-494.
Forster PI. 2003. A taxonomic revision of
Croton L. (Euphorbiaceae) in Australia. –
Austrobaileya 6: 349-436.
Forster PI. 2005. A taxonomic revision of
Actephila Blume (Euphorbiaceae/Phyllanthaceae) in Australia. –
Austrobaileya 7: 57-98.
Fosberg FR. 1977. A fossil Garcinia
fruit from the New Hebrides, Melanesia. – Pacific Sci. 31: 293-297.
Foster AS. 1950a. Morphology and venation of
the leaf in Quiina acutangula. – Amer. J. Bot. 37: 159-171.
Foster AS. 1950b. Venation and histology of
the leaflets in Touroulia guianensis Aubl. and Froesia
tricarpa Pires. – Amer. J. Bot. 37: 848-862.
Foster AS. 1951. Heterophylly and foliar
venation in Lacunaria. – Bull. Torrey Bot. Club 78: 382-400.
Foster PI. 1994. Revision of Euphorbia
plumerioides Teijsm. ex Hassk. (Euphorbiaceae) and allies. –
Austrobaileya 4: 227-246.
Fox J. 1992. Pollen limitation of
reproductive effort in willows. – Oecologia 90: 283-287.
Fraga CN, Saavedra MM. 2006. Three new
species of Elvasia (Ochnaceae) from the Brazilian Atlantic
Forest, with an emended key for Subgenus Hostmannia. – Novon 16:
483-489.
Frajman B, Schönswetter P. 2011. Giants and
dwarfs: molecular phylogenies reveal multiple origins of annual spurges within
Euphorbia subg. Esula. – Mol. Phylogen. Evol. 61:
413-424.
Franco RP. 1990. The genus Hyeronima
in South America. – Bot. Jahrb. Syst. 111: 297-346.
Fregene MA, Vargas J, Ikea J, Angel F, Tohme
J, Asiedu RA, Akorda MO, Roca WM. 1994. Variability of chloroplast DNA and
nuclear ribosomal DNA in cassava (Manihot esculenta Crantz) and its
wild relatives. – Theor. Appl. Gen. 89: 719-727.
Freitas L, Bernardello G, Galetto L, Paoli
AAS. 2001. Nectaries and reproductive biology of Croton sarcopetalus
(Euphorbiaceae). – Bot. J.
Linn. Soc. 136: 267-277.
Friedrich WL, Koch BE. 1970. Comparison of
fruits and seeds of fossil Spirematospermum with those of living
Ctenolophon. – Bull. Geol. Soc. Denmark 20: 192-195.
Friis I, Vollesen K. 1980. The identity of
the Ethiopian monotypic genus Tzellemtinia Chiov. – Bot. Not. 133:
347-349.
Frisendahl A. 1927. Über die Entwicklung
chasmogamer und kleistgamer Blüten bei der Gattung Elatine. – Acta
Horti Gothoburg. 3: 99-142.
Fritsch C. 1888. Über die Gattungen der
Chrysobalanaceen. –Verh. Zool.-Bot. Ges. Wien 38: 93-95.
Froembling W. 1896. Anatomisch-systematische
Untersuchung von Blatt und Axe der Crotoneen und Euphyllantheen. – Bot.
Centralbl. 65: 129-139, 177-192, 241-249, 289-297, 321-329, 369-378, 403-411,
433-442.
Fryns-Claessens E, Cotthem W van. 1966.
L’appareil stomatique des Pandacées et Davidsoniacées. – Rev. Gén. Bot.
63: 783-789.
Fu Y-B, Peterson G, Diederichsen A, Richards
KW. 2002. RAPD analysis of genetic relationships of seven flax species in the
genus Linum L. – Genet. Res. Crop Evol. 49: 253-259.
Fujinami R, Imaichi R. 2009. Developmental
anatomy of Terniopsis malayana (Podostemaceae, subfamily
Tristichoideae), with implications for body plan evolution. – J. Plant Res.
122: 551-558.
Fujinami R, Ghogue JP, Imaichi R. 2013.
Developmental morphology of the controversial ramulus organ of Tristicha
trifaria (Subfamily Tristichoideae, Podostemaceae): implications for
evolution of a unique body plan in Podostemaceae. – Intern. J.
Plant Sci. 147: 609-618.
Furness CA. 2011. Comparative structure and
development of pollen and tapetum in Malpighiales, with a focus on the
parietal clade. – Intern. J. Plant Sci. 172: 980-1011.
Furness CA. 2013. Evolution of pollen and
tapetal characters in Ochnaceae (Malpighiales). – Intern. J. Plant
Sci. 174: 1134-1152.
Gage AT. 1922. Euphorbiaceae novae e Peninsula
Malayana. – Records Bot. Surv. India 9: 219-249.
Gagliardi K, Souza L, Albiero A. 2014.
Comparative fruit development in some Euphorbiaceae and Phyllanthaceae. – Plant Syst.
Evol. 300: 775-782.
Gagnepain F. 1924 [1925]. Quelques genres
nouveaux d’Euphorbiacées. – Bull. Soc. Bot. France 71: 864-879.
Galang R, Madulid DA. 2006. A second new
species of Rafflesia (Rafflesiaceae) from Panay Island,
Philippines. – Folia Malaysiana 7: 1-8.
Galimberti Z. 1963. Embriologia di
Codiaeum variegatum Blume var. pictum (Lodd.) Mull. – Nuovo
Giorn. Bot. Ital. 70: 21-32.
Gama TSS, Cordeiro I, Demarco D. 2016. Floral
structure and development reveal presence of petals in Phyllanthus L.
(Phyllanthaceae). – Intern.
J. Plant Sci. 177: 749-759.
Ganders FR. 1979. Heterostyly in
Erythroxylum coca (Erythroxylaceae). – Bot. J.
Linn. Soc. 78: 11-20.
García MTA, Gottsberger G. 2009. Composition
of the floral nectar of different subgenera of Argentinian Passiflora
species. – Plant Syst. Evol. 283: 133-147.
García MTA, Galati BG, Anton AM. 2002.
Microsporogenesis, microgametogenesis and pollen morphology of
Passiflora species (Passifloraceae). – Bot. J.
Linn. Soc. 139: 383-394.
García MTA, Galati BG, Anton AM. 2003.
Development and ultrastructure of the megagametophyte in Passiflora
caerulea L. (Passifloraceae). – Bot. J.
Linn. Soc. 142: 73-81.
Garcia-Villacorta R, Hammel BE. 2004. A
noteworthy new species of Tovomita (Clusiaceae) from Amazonian white sand
forests of Peru and Colombia. – Brittonia 56: 132-135.
Gates B. 1977. A monograph of the Central
Brazilian species of Banisteriopsis (Malpighiaceae). – Ph.D. diss.,
University of Michigan, Ann Arbor, Michigan.
Gates B. 1982. Flora Neotropica. Monograph
30. Banisteriopsis and Diplopterys (Malpighiaceae). – New York
Botanical Garden, Bronx, New York.
Gaucher L. 1902. Recherches anatomiques sur
les Euphorbiacées. – Ann. Sci. Nat. Bot., sér. VIII, 15: 161-309.
Gavrilova OA. 1993. Types of pollen grain
sculpture and their significance for systematics of the family Flacourtiaceae.
– Bot. Žurn. 12: 45-52. [In Russian]
Gavrilova OA. 1998. Palynomorphology of the
family Kiggelariaceae. – Bot. Žurn. 83: 20-27. [In Russian]
Gehrmann K. 1908. Vorarbeiten zu einer
Monographie der Gattung Bridelia mit besonderer Berücksichtigung der
afrikanischen Arten. – Engl. Bot. Jahrb. Syst. 41, Beibl. 95: 1-42.
Gehrmann K. 1911. Zur Blütenbiologie der Rhizophoraceae. – Ber. Deutsch.
Bot. Ges. 29: 303-318.
Geltman DV. 2013. Revision of
Euphorbia sect. Chylogala (Euphorbiaceae). – Willdenowia
43: 5-12.
Gengler-Novak KM. 2002a. Reconstruction of
the biogeographical history of Malesherbiaceae. – Bot. Rev.
68: 171-188.
Gengler-Novak KM. 2002b. Phenetic analysis of
morphological traits in the Malesherbia humilis complex (Malesherbiaceae). – Taxon 51:
281-293.
Gengler-Novak KM. 2003. Molecular phylogeny
and taxonomy of Malesherbiaceae. – Syst. Bot.
28: 333-344.
Gengler-Novak KM, Crawford DJ. 2000. Genetic
diversities of four little-known species of Malesherbia (Malesherbiaceae) endemic to the
arid inter-Andean valleys of Peru. – Brittonia 52: 303-310.
Gentry AH. 1975. Humiriaceae: flora of Panama. –
Ann. Missouri Bot. Gard. 62: 35-44.
Gershoy A. 1932. Descriptive notes for
Viola exhibits. The Nominum and Chamaemelanium
sections. – In: IV Intern. Congr. Genetics, Publ. Vermont Agric. Exper. Sta.
1932: 1-27.
Gershoy A. 1934. Studies in North American
violets III. Chromosome numbers and species characters. – Vermont Agric.
Exper. Sta. Bull. 367: 1-92.
Gessner F, Hammer L. 1962.
Ökologisch-physiologische Untersuchungen an den Podostemonaceen des Caroni.
– Intl. Rev. Gesellsch. Hydrobiol. 47: 497-541.
Ghahremaninejad F, Khalili Z, Maassoumi AA,
Mirzaie-Nodoushan H, Riahi M. 2012. Leaf epidermal features of Salix
species (Salicaceae) and their
systematic significance. – Amer. J. Bot. 99: 769-777.
Ghisalberti EL, Jeffries PR, Sefton MA. 1978.
Secobeyerane. Diterpenes from Beyeria calycina. – Phytochemistry 17:
1961-1965.
Ghislain B, Nicolini E-A, Romain R, Ruelle J,
Yoshinaga A, Alford MH, Clair B. 2016. Multilayered structure of tension wood
cell walls in Salicaceae sensu
lato and its taxonomic significance. – Bot. J. Linn. Soc. 182: 744-756.
Ghogue J-P, Ameka GK, Grob V, Huber KA,
Pfeifer E, Rutishauser R. 2009. Enigmatic morphology of Djinga felicis
(Podostemaceae-Podostemoideae),
a badly known endemic from northwestern Cameroon. – Bot. J. Linn. Soc. 160:
64-81.
Gibson AC. 1980. Wood anatomy of
Thornea, including some comparisons with other Hypericaceae. – IAWA Bull., N.
S., 1: 87-92.
Giese AC. 1980. Hypericism. – Photochem.
Photobiol. Rev. 5: 229-255.
Gil-Ad NL. 1995. Systematics and evolution of
Viola L. subsection Boreali-Americanae (W. Becker) Brizicky.
– Ph.D. diss., University of Michigan, Ann Arbor, Michigan.
Gil-Ad NL. 1998. The micromorphologies of
seed coat and petal trichomes of the taxa of Viola subsect.
Boreali-Americanae (Violaceae) and their utility in
discerning orthospecies from hybrids. – Brittonia 50: 91-121.
Gilbert LE. 1971. Butterfly-plant
coevolution: has Passiflora adenopoda won the selectional race with
heliconiine butterflies? – Science 172: 585-586.
Gilbert LE. 1975. Ecological consequences of
a coevolved mutualism between butterflies and plants. – In: Gilbert LE, Raven
PH (eds), Coevolution of animals and plants, University of Texas Press, Austin,
Texas, pp. 210-240.
Gilbert MG. 1987a. Two new geophytic species
of Euphorbia with comments on the subgeneric groupings of its African
members. – Kew Bull. 42: 231-244.
Gilbert MG. 1987b. New and interesting
species of Euphorbiaceae from
Ethiopia. – Kew Bull. 42: 351-368.
Gilbert MG. 1990. Six new species of
Euphorbia (subgenus Esula) from Ethiopia. – Kew Bull. 45:
265-276.
Gilbert MG. 1992a. Notes on Tragia,
Dalechampia and Clutia (Euphorbiaceae) in Ethiopia and
Simalia. – Nord. J. Bot. 12: 389-401.
Gilbert MG. 1992b. Notes on Violaceae from Ethiopia. – Nord. J.
Bot. 12: 689-693.
Gilbert MG. 1993. Notes on Euphorbia
subgen. Chamaesyce in Ethiopia. – Kew Bull. 48: 125-126.
Gilbert MG. 1994. The relationships of the
Euphorbieae (Euphorbiaceae).
– Ann. Missouri Bot. Gard. 81: 283-288.
Gilbert MG, Thulin M. 1991. Synopsis of
Jatropha sect. Robecchiana s. lat. (Euphorbiaceae). – Nord. J. Bot.
11: 413-419.
Gilg E. 1894. Turneraceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien III(6a), W. Engelmann,
Leipzig, pp. 57-64.
Gilg E. 1895. Ochnaceae. – In: Engler A, Prantl K
(eds), Die natürlichen Pflanzenfamilien III(6), W. Engelmann, Leipzig, pp.
131-153.
Gilg E. 1908. Flacourtiaceae africanae. –
Engl. Bot. Jahrb. Syst. 40: 444-518.
Gilg E. 1918. Die bis jetzt aus Neu-Guinea
bekannt gewordenen Flacourtiaceen. – Engl. Bot. Jahrb. 55: 273-294.
Gilg E. 1925a. Ochnaceae. – In: Engler A, Gilg E
(eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 21, W. Engelmann,
Leipzig, pp. 53-87.
Gilg E. 1925b. Flacourtiaceae. – In: Engler
A, Gilg E (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 21, W.
Engelmann, Leipzig, pp. 377-457.
Gilg E. 1925c. Turneraceae. – In: Engler A, Gilg
E (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 21, W. Engelmann,
Leipzig, pp. 459-466.
Gill AM, Tomlinson PB. 1969. Studies on the
growth of red mangrove (Rhizophora mangle L.) I. Habit and general
morphology. – Biotropica 1: 1-9.
Gill BS, Bir SS, Bedi YS. 1981. Cytological
studies on woody Euphorbiaceae
from north and central India. – New Botanist 8: 35-44.
Gillespie LJ. 1988. A revision and
phylogenetic analysis of Omphalea (Euphorbiaceae). – Ph.D. diss.,
University of California, Davis, California.
Gillespie LJ. 1993. A synopsis of Neotropical
Plukenetia (Euphorbiaceae) including two new
species. – Syst. Bot. 18: 575-592.
Gillespie LJ. 1994a. Pollen morphology and
phylogeny of the tribe Plukenetieae (Euphorbiaceae). – Ann. Missouri
Bot. Gard. 81: 317-348.
Gillespie LJ. 1994b. A new section and two
new species of Tragia (Euphorbiaceae) from the Venezuelan
Guayana and French Guiana. – Novon 4: 330-338.
Gillespie LJ. 1997. Omphalea (Euphorbiaceae) in Madagascar: a
new species and a new combination. – Novon 7: 127-136.
Gillespie LJ. 2007. A revision of
paleotropical Plukenetia (Euphorbiaceae) including two new
species from Madagascar. – Syst. Bot. 32: 780-802.
Giraud B. 1983. Les cellules perforées des
rayons ligneux chez les Euphorbiacées. – Bull. Mus. Natl. Hist. Nat. Paris,
sér. IV, sect. B, Adansonia 5: 213-221.
Gnecco S, Bartulin J, Becerra J, Marticorena
C. 1989. n-Alkanes from Chilean Euphorbiaceae and Compositae
species. – Phytochemistry 28: 1254-1256.
Gogelein AJF. 1967. A revision of the genus
Cratoxylum Bl. (Guttiferae). – Blumea 15: 453-475.
Golysheva MD. 1975. Leaf anatomy of
Idesia polycarpa Maxim. and other Flacourtiaceae in relation to the
question of relationships between the families Salicaceae and Flacourtiaceae. –
Bot. Žurn. 60: 787-799.
Gonçalves-Esteves V, Mendonça CBF. 2001.
Estudo polínico em plantas de restinga do Estado do Rio de Janeiro – Clusiaceae Lindl. – Rev. Brasil.
Bot. 24: 527-536.
González AM. 1993. Anatomía y
vascularización floral de Piriqueta racemosa, Turnera
hassleriana y Turnera joelii (Turneraceae). – Bonplandia 7:
143-184.
González AM. 1996. Nectarios extraflorales
en Turnera, series Canaligerae y Leiocarpae. –
Bonplandia 9: 129-143.
González AM. 1998. Colleters in
Turnera and Piriqueta. – Bot. J. Linn. Soc. 128:
215-228.
González AM. 2000. Estudios anatómicos en
los géneros Piriqueta y Turnera (Turneraceae). – Ph.D. diss.,
Universidad Nacional de Córdoba, Argentina.
González AM. 2001. Nectarios y
vascularización floral de Piriqueta y Turnera (Turneraceae). – Bol. Soc. Argent.
Bot. 36: 47-68.
González AM, Arbo MM. 2004. Trichome
complement of Turnera and Piriqueta (Turneraceae). – Bot. J. Linn. Soc.
144: 85-97.
González AM, Arbo MM. 2005. Anatomía de
algunas especies de Turneráceas. – Acta Bot. Venezuelica 28: 369-394.
González AM, Arbo MM. 2013. Anatomía y
ontogenia de las semillas en Turnera y Piriqueta. – Bot.
Sci. 91: 399-416.
González AM, Salgado CR, Fernández A, Arbo
MM. 2012. Anatomy, pollen, and chromosomes of Adenoa (Turneraceae), a monotypic genus
endemic to Cuba. – Brittonia 64: 208-225.
Gottlieb OR, Kaplan MAC, Kubitzki K, Toledo
Barros JR. 1989. Chemical dichotomies in the Magnolialean comlex. – Nord. J.
Bot. 8: 437-444.
Gottwald H, Parameswaran N. 1967. Beiträge
zur Anatomie und Systematik der Quiinaceae. – Bot. Jahrb. Syst. 87:
361-381.
Govaerts R, Frodin DG, Radcliffe-Smith A.
2000. World checklist and bibliography of Euphorbiaceae (with Pandaceae) 1-4. – Redwood,
Trowbridge Wiltshire and The Royal Botanic Gardens, Kew.
Gracioso J de S, Vilegas W, Hiruma-Lima CA,
Souza Brito ARM. 2002. Effects of tea from Turnera ulmifolia L. on
mouse gastric mucosa support the Turneraceae as a new source of
antiulcerogenic drugs. – Biol. Pharmaceut. Bull. 25: 487-491.
Graham A. 2006. Paleobotanical evidence and
molecular data in reconstructing the historical phytogeography of Rhizophoraceae. – Ann. Missouri
Bot. Gard. 93: 325-334.
Green PS. 1986. New combinations in
Baloghia and Codiaeum (Euphorbiaceae). – Kew Bull. 41:
1026.
Grey-Wilson C. 1981. Notes on African Violaceae. – Kew Bull. 36:
103-126.
Grey-Wilson C. 1984. Further notes on East
African Violaceae. – Kew Bull.
39: 771-773.
Grey-Wilson C. 1986. Violaceae. – In: Polhill RM (ed),
Flora of tropical East Africa, A. A. Balkema, Rotterdam, pp. 1-38.
Grigorieva VV. 1990. The pollen grain
morphology in members of the Linaceae family. – Bot. Žurn. 75:
1345-1352. [In Russian with English summary]
Grob V, Pfeifer E, Rutishauser R. 2007.
Morphology, development and regeneration of Thelethylax minutiflora, a
Madagascan river-weed (Podostemaceae). – Phyton –
Ann. Rei Bot. 47: 205-229.
Groppo M, Fiaschi P, Salatino MLF,
Cecchantini GCT, Assis Ribeiro dos Santos F de, Verola CF, Antonelli A. 2010.
Placement of Kuhlmanniodendron Fiaschi & Groppo in Lindackerieae
(Acharaceae, Malpighiales)
confirmed by analyses of rbcL sequences, with notes on pollen
morphology and wood anatomy. – Plant Syst. Evol. 286: 27-37.
Groppo M, Favaretto BSG, Silva CI da, Jardim
JG, Fiaschi P. 2013. A new species of Kuhlmanniodendron
(Lindackerieae, Achariaceae) from
eastern Brazil and the systematic position of the genus in Achariaceae. – Syst. Bot. 38:
162-171.
Gros J-P. 1983. Irvingiaceoxylon
dechampsii n.g. et n.sp. du Cénozoïque d’Éthiopie, et
Simaroubaceoxylon (Irvingioxylon) taibaense n.g. et
n.comb. de l’Yprésien du Sénégal. – Rev. Gén. Bot. 90: 153-171.
Gross CL, Whalen MA. 1996. A revision of
Adriana (Euphorbiaceae). – Aust. Syst.
Bot. 9: 749-771.
Gruas-Cavagnetto C, Köhler E. 1992. Pollens
fossils d’Euphorbiacées de l’Éocène français. – Grana 31: 291-304.
Grubert M. 1970. Untersuchungen über die
Verankerung der Samen von Podostemonaceen. – Intl. Rev. Gesellsch. Hydrobiol.
55: 83-114.
Grubert M. 1974. Podostemaceen-Studien I. Zur
Ökologie einiger venezolanischer Podostemaceen. – Beitr. Biol. Pflanzen 50:
321-391.
Grubert M. 1976. Podostemaceen-Studien II.
Untersuchungen über die Keimung. – Bot. Jahrb. Syst. 95: 455-477.
Guèdès M, Sastre C. 1981. Morphology of the
gynoecium and systematic position of the Ochnaceae. – Bot. J. Linn. Soc. 82:
121-138.
Guillaumin A. 1925. Recherches sur
l’anatomie et la classification des Balanopsidacées. – Rev. Gén. Bot. 37:
433-449.
Guillaumin A. 1942. Matériaux pour la Flore
de la Nouvelle Calédonie LXX. Remarques sur les Violacées. – Bull. Soc.
Bot. France 89: 19-22.
Guimarães EF, Pereira JMC. 1966. Ochnaceae no Estado da Guanabara. –
Rodriguésia 25: 59-65.
Gustafsson MHG. 2000. Floral morphology and
relationships of Clusia gundlachii with a discussion of floral organ
identity and diversity on the genus Clusia. – Intern. J. Plant Sci.
161: 43-53.
Gustafsson MHG, Bittrich V. 2002. Evolution
of morphological diversity and resin secretion in flowers of Clusia
(Clusiaceae): insights from ITS
sequence variation. – Nord. J. Bot. 22: 183-202.
Gustafsson MHG, Bittrich V, Stevens PF. 2002.
Phylogeny of Clusiaceae based on
rbcL sequences. – Intern. J. Plant Sci. 163: 1045-1054.
Gustafsson MHG, Winter K, Bittrich V. 2007.
Diversity, phylogeny and classification of Clusia. – In: Lüttge U
(ed), Clusia: a woody neotropical genus of remarkable plasticity and
diversity, Ecol. Stud. 194, Springer, Berlin.
Haber JM. 1925. The anatomy and the
morphology of the flower of Euphorbia. – Ann. Bot. 39: 657-707.
Habib AM, Reddy KS, McCloud TG, Chang C-J,
Cassady JM. 1987. New xanthones from Psorospermum febrifugum. – J.
Nat. Prod. 50: 141-145.
Haegens R. 2000. Taxonomy, phylogeny and
biogeography of Baccaurea, Distichirhops, and
Nothobaccaurea (Euphorbiaceae). – Blumea
(Suppl.) 12: 1-218.
Haevermans T, Labat J-N. 2004. A synoptic
revision of the Malagasy endemic Euphorbia pervilleana group. –
Syst. Bot. 29: 118-124.
Haevermans T, Rouhan G, Hetterscheid W,
Teissier M, Belarbi K, Aubriot X, Labat J-N. 2009. Chaos revisited:
nomenclature and typification of the Malagasy endemic Euphorbia
subgenus Lacanthis (Raf.) M. G. Gilbert. – Adansonia, sér. III, 31:
279-299.
Hagemann I. 1989. Wuchsformen einiger
Hypericum-Arten, ein Beitrag zum morphologischen und zum ökologischen
Anliegen der Wuchsformen-Forschung. – Flora 183: 225-309.
Hagemann I, Meusel H. 1984. Hypericum
triquetrifolium Turra, ein Wurzelspross-geophyt: Wuchsform und
Verbreitung. – Flora 175: 385-405.
Hagerup O. 1930. Vergleichende morphologische
und systematische Studien über die Ranken und andere vegetative Organe der
Cucurbitaceen und Passifloraceen. – Dansk Bot. Ark. 6: 1-103.
Haïcour R. 1983. La variabilité de
compatibilité entre divers taxons de Phyllanthus urinaria L.
(Euphorbacées): mise en évidence et perspectives ouvetes pour son analyse.
– Bull. Soc. Bot. France 130, Lettres Bot. (3): 207-226.
Haïcour R. 1984a. Eléments d’analyse de
la structure des populations et de l’évolution d’une espèce rudérale
pantropicale, Phyllanthus urinaria L. (Euphorbiaceae) 1. Étude du
polymorphisme de l’entité P. urinaria et premier essai de
classification. – Bull. Mus. Natl. Hist. Nat. Paris, IV. 6, sect. B,
Adansonia 1: 63-74.
Haïcour R. 1984b. Eléments d’analyse de
la structure des populations et de l’évolution d’une espèce rudérale
pantropical, Phyllanthus urinaria L. (Euphorbiaceae) 2. Étude de la
variation génétique et de la traduction phénotypique. – Bull. Mus. Natl.
Hist. Nat. Paris, IV. 6, sect. B, Adansonia 2: 157-191.
Haïcour R, Rossignol L, Rossignol M, Gaisne
C. 1994. Patterns of diversification and evolution in Phyllanthus
odontadenius (Euphorbiaceae). – Ann. Missouri
Bot. Gard. 81: 289-301.
Håkansson A. 1955. Chromosome numbers and
meiosis in certain Salices. – Hereditas 41: 454-498.
Hakki MI. 1985. Studies on West Indian plants
3. On floral morphology, anatomy, and relationship of Picrodendron
baccatum (L.) Krug & Urban (Euphorbiaceae). – Bot. Jahrb.
Syst. 107: 379-394.
Halford DA, Henderson RJF. 2003. Studies in
Euphorbiaceae sens. lat. 5. A
revision of Pseudanthus Sieber ex Spreng. and Stachystemon
Planch. (Oldfieldioideae Köhler & Webster, Caletieae Müll. Arg.). –
Austrobaileya 6: 497-532.
Halford DA, Henderson RJF. 2005. Studies in
Euphorbiaceae s. lat. 6. A
revision of the genus Poranthera Rudge (Antidesmeae, Porantherinae) in
Australia. – Austrobaileya 7: 1-27.
Hall JB. 1971. New Podostemaceae from Ghana with
notes on related species. – Kew Bull. 26: 125-136.
Halle F. 1971. Architecture and growth of
tropical trees exemplified by the Euphorbiaceae. – Biotropica 3:
56-62.
Hallé N, Heine H. 1967. Deux nouvelles
espèces africaines du genre Tapura Aubl. (Dichapetalaceae). – Adansonia,
sér. II, 7: 43-51.
Hallé N, Wilde JJFE de. 1978.
Trichostephanus acuminatus Gilg (Flacourtiacées), une approche
biosystématique. – Adansonia 18: 167-182.
Hallier H. 1912. Sur le Philbornea, genre
nouveau de la famille des Linacées, avec quelques remarques sur les affinités
de cette famille. – Arch. Néerland. Sci. Exact. Nat., sér. IIIb (Sci. Nat.)
1: 104-111.
Hallier H. 1921. Beiträge zur Kenntnis der
Linaceen (DC. 1819) Dumort. – Beih. Bot. Centralbl., Abt. 2, 39: 1-178.
Ham RWJM van der. 1989. New observations on
the pollen of Ctenolophon Oliver (Ctenolophonaceae), with remarks
on the evolutionary history of the genus. – Rev. Palaeobot. Palynol. 59:
153-160.
Hammel BE. 1999a. Two new species of
Clusiella (Clusiaceae)
with a synopsis of the genus. – Novon 9: 349-359.
Hammel BE. 1999b. Synopsis of
Chrysochlamys (Clusiaceae: Clusioideae: Clusieae) in
Mesoamerica. – Novon 9: 360-374.
Hammond BL. 1936. Regeneration of
Podostemon ceratophyllum Michx. – Bot. Gaz. 97: 834-845.
Hammond BL. 1937. Development of
Podostemon ceratophyllum. – Bull.Torrey Bot. Club 64: 17-36.
Hamzeh M, Dayanandan S. 2004. Phylogeny of
Populus (Salicaceae)
based on nucleotide sequences of chloroplast trnT-trnF region
and nuclear rDNA. – Amer. J. Bot. 91: 1398-1408.
Handa T. 1940. Anomalous secondary growth in
the axis of Lophopyxis pentaptera (K. Schum.) Engler. – Bot. Mag.
Tokyo 54: 41-47.
Hans AS. 1973. Chromosomal conspectus of the
Euphorbiaceae. – Taxon 22:
591-636.
Hansen AK. Gilbert LE, Simpson BB, Downie SR,
Cervi AC, Jansen RK. 2006. Phylogenetic relationships and chromosome number
evolution in Passiflora. – Syst. Bot. 31: 138-150.
Hansen AK, Escobar LK, Gilbert LE, Jansen RK.
2007. Paternal, maternal, and biparental inheritance of the chloroplast genome
in Passiflora (Passifloraceae): implications for
phylogenetic studies. – Amer. J. Bot. 94: 42-46.
Hansmann P, Kleinig H. 1982. Violaxanthin
esters from Viola tricolor flowers. – Phytochemistry 21: 238-239.
Hao K-S. 1936. Synopsis of Chinese
Salix. – Dahlem, Berlin.
Harley RM, Giulietti AM, Leite KRB. 2006. Two
new species and a new record of Sauvagesia (Ochnaceae) in the Chapada Diamantina
of Bahia, Brazil. – Kew Bull. 60: 571-580.
Harms H. 1893. Über die Verwertung des
anatomischen Baues für die Umgrenzung und Einteilung der Passifloraceae. – Engl. Bot.
Jahrb. Syst. 15: 548-632.
Harms H. 1894a. Malesherbiaceae. – In: Engler
A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(6a), W. Engelmann,
Leipzig, pp. 65-68.
Harms H. 1894b. Passifloraceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien III(6a), W. Engelmann,
Leipzig, pp. 69-94; Harms H. 1897. Nachträge zu III(6a), pp. 253-256.
Harms H. 1897. Achariaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien, Nachträge zu III(6a), W.
Engelmann, Leipzig, pp. 256-257.
Harms H. 1922. Neue Arten der Gattung
Passiflora L. – Feddes Repert. 18: 294-299.
Harms H. 1923a. Beiträge zur Kenntnis der
amerikanischen Passifloraceae
I. – Feddes Repert. 19: 25-32.
Harms H. 1923b. Beiträge zur Kenntnis der
amerikanischen Passifloraceae
II. – Feddes Repert. 19: 56-60.
Harms H. 1925a. Malesherbiaceae. – In: Engler
A, Gilg E (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 21, W.
Engelmann, Leipzig, pp. 467-470.
Harms H. 1925b. Passifloraceae. – In: Engler A,
Gilg E (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 21, W.
Engelmann, Leipzig, pp. 470-507.
Harms H. 1925c. Achariaceae. – In: Engler A, Gilg
E (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 21, W. Engelmann,
Leipzig, pp. 507-510.
Harms H. 1929. Passifloraceae americanae novae.
– Notizbl. Bot. Gart. Berlin-Dahlem 10: 808-816.
Harms H. 1935. Rafflesiaceae. – In: Engler A
(†), Harms H (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 16b, W.
Engelmann, Leipzig, pp. 243-281.
Harris BD. 1968. Chromosome numbers and
evolution in North American species of Linum. – Amer. J. Bot. 55:
1197-1204.
Harris BJ. 1996. A revision of the Irvingiaceae of Africa. – Bull.
Jard. Bot. Nat. Belg. 65: 143-196.
Hasma H, Subramaniam A 1978. The occurrence
of a furanoid fatty acid in Hevea brasiliensis latex. – Lipids 13:
905-907.
Hassall DC. 1976. Numerical and cytotaxonomic
evidence for generic delimitation in Australian Euphorbieae. – Aust. J. Bot.
24: 633-640.
Hassall DC. 1977. The genus
Euphorbia in Australia. – Aust. J. Bot. 25: 429-453.
Hatada A, Isiguro S, Itioka T, Kawano S.
2007. Myrmecosymbiosis in the Bornean Macaranga species with special
reference to food bodies (Beccarian bodies) and extrafloral nectaries. –
Plant Species Biol. 16: 241-246.
Hauman L. 1951. Contribution à l’étude
des Chrysobalanoïdes africaines. – Bull. Jard. Bot. État Bruxelles 21:
167-198.
Hayden SM, Hayden WJ. 1996. A revision of
Discocarpus (Euphorbiaceae). – Ann. Missouri
Bot. Gard. 83: 153-167.
Hayden WJ. 1977. Comparative anatomy and
systematics of Picrodendron, genus incertae sedis. – J. Arnold
Arbor. 58: 257-279.
Hayden WJ. 1980. Systematic anatomy of
Oldfieldioideae (Euphorbiaceae). – Ph.D. diss.,
University of Maryland, College Park, Maryland.
Hayden WJ. 1987. The identity of the genus
Neowawraea (Euphorbiaceae). – Brittonia 39:
268-277.
Hayden WJ. 1988. Ontogeny of the cotyledonary
region of Chamaesyce maculata (Euphorbiaceae). – Amer. J. Bot.
75: 1701-1713.
Hayden WJ. 1990. Notes on neotropical
Amanoa (Euphorbiaceae). – Brittonia 42:
260-270.
Hayden WJ. 1994. Systematic anatomy of Euphorbiaceae subfamily
Oldfieldioideae. I. Overview. – Ann. Missouri Bot. Gard. 81: 180-202.
Hayden WJ, Brandt DS. 1984. Wood anatomy and
relationships of Neowawraea (Euphorbiaceae). – Syst. Bot. 9:
458-466.
Hayden WJ, Hayden SM. 2000. Wood anatomy of
Acalyphoideae (Euphorbiaceae).
– IAWA J. 21: 213-235.
Hayden WJ, Gillis WT, Stone DE, Broome CR,
Webster GL. 1984. Systematics and phylogeny of Picrodendron. Further
evidence for relationship with the Oldfieldioideae (Euphorbiaceae). – J. Arnold
Arbor. 65: 105-127.
Hayden WJ, Simmons MP, Swanson LJ. 1993. Wood
anatomy of Amanoa (Euphorbiaceae). – IAWA J. 14:
205-213.
Hearn DJ. 2006. Adenia (Passifloraceae) and its adaptive
radiation: phylogeny and growth form diversification. – Syst. Bot. 31:
805-821.
Hearn DJ. 2007. Novelties in Adenia
(Passifloraceae): four new
species, a new combination, a vegetative key, and diagnostic characters for
known Madagascan species. – Brittonia 59: 308-327.
Hearn DJ. 2009a. Developmental patterns in
anatomy are shared among separate evolutionary origins of stem succulent and
storage root-bearing growth habits in Adenia (Passifloraceae). – Amer. J.
Bot. 96: 1941-1956.
Hearn DJ. 2009b. Descriptive anatomy and
evolutionary patterns of anatomical diversification in Adenia (Passifloraceae). – Aliso 27:
13-38.
Hecker E. 1977. New toxic, irritant and
cocarcinogenic diterpene esters from Euphorbiaceae and Thymelaeaceae. – Pure
Appl. Chem. 49: 1423-1431.
Hecker E, Schmidt R. 1974. Phorbolesters –
the irritants and cocarcinogens of Croton tiglium. – Fortschr. Chem.
Organischer Naturst. 31: 378-467.
Heel WA van. 1967. Anatomical and ontogenetic
investigations on the morphology of the flowers and the fruit of
Scyphostegia borneensis Stapf (Schyphostegiaceae). – Blumea 15:
107-125.
Heel WA van. 1973. Flowers and fruits in
Flacourtiaceae I. – Blumea 21: 259-279.
Heel WA van. 1974. Flowers and fruits in
Flacourtiaceae II. – Blumea 22: 15-19.
Heel WA van. 1977. Flowers and fruits in
Flacourtiaceae III. Some Oncobieae. – Blumea 23: 349-369.
Heel WA van. 1979. Flowers and fruits in
Flacourtiaceae IV. Hydnocarpus, Kiggelaria africana L.,
Casearia, Berberidopsis corallina Hook. f. – Blumea 25:
513-529.
Hegnauer R. 1981. Chemotaxonomy of Erythroxylaceae (including some
ethnobotanical notes on Old World species). – J. Ethnopharm. 3: 279-292.
Heijkoop M, Vanwelzen PC. 2017. A revision of
the genus Actephila (Phyllanthaceae) in the Malesian
region. – Blumea 62: 7-25.
Heilborn O. 1926. Bidrag till violaceernas
cytologi. – Svensk Bot. Tidskr. 20: 414-419.
Heimermann WH, Holman RT. 1972. Highly
optically active triglycerides of Sebastiana ligustrina. –
Phytochemistry 11: 799-802.
Heimsch C, Tschabold EE. 1972. Xylem studies
in the Linaceae. – Bot. Gaz.
(Crawfordsville) 133: 242-253.
Heinricher E. 1905 Beiträge zur Kenntnis der
Rafflesiaceae 1. – Denkschr.
K. Akad. Wiss. Wien, Math.-Naturw. Kl 78: 1-25.
Heitz B, Jean R, Prensier G. 1971.
Observation de la surface du stigmate et des grains de pollen de Linum
austriacum L. hétérostyle. – Compt. Rend. Hébd. Séances Acad. Sci.,
D 273(25): 2493-2495.
Hekking WHA. 1984. Studies on neotropical Violaceae: the genus
Fusispermum. – Proc. K. Nederl. Akad. Wetensch. Bot, Ser. C, 87:
121-130.
Hekking WHA. 1988a. Flora Neotropica.
Monograph 46. Violaceae I.
Rinorea and Rinoreocarpus. – New York Botanical Garden,
Bronx, New York.
Hekking WHA. 1988b. Studies on Neotropical Violaceae 2: arrangement of leaves,
inflorescences and branchlets in Neotropical Rinorea. – Flora 180:
345-376.
Hemingway CA, Christensen AR, malcomber ST.
2011. B- and C-class gene expression during corona development of the blue
passionflower (Passiflora caerulea, Passifloraceae). – Amer. J.
Bot. 98: 923-934.
Hemming CF, Radcliffe-Smith A. 1987. A
revision of the Somali species of Jatropha (Euphorbiaceae). – Kew Bull. 42:
103-122.
Henderson RJF. 1992. Studies in Euphorbiaceae A. L. Juss., sens.
lat. I. A revision of Amperea Adr. Juss. (Acalyphoideae Ascherson,
Ampereae Muell. Arg.). – Aust. Syst. Bot. 5: 1-27.
Herbert H. 1897. Anatomische Untersuchung von
Blatt und Axe der Hippomaneen. – Ph.D. diss., Universität München,
Germany.
Herrera F, Manchester SR, Jaramillo C,
MacFadden B, da Silva-Caminha SA. 2010. Phytogeographic history and phylogeny
of the Humiriaceae. – Intern.
J. Plant Sci. 171: 392-408.
Herrera F, Manchester SR, Vélez-Juarbe J,
Jaramillo C. 2014. Phytogeographic history of the Humiriaceae. – Intern. J. Plant
Sci. 175: 828-840.
Hewson HJ. 1984. Dichapetalaceae. – In: George
AS (ed), Flora of Australia 22, Australian Government Publ. Service, Canberra,
pp. 218-219.
Hidayati SN. 1993. The biology of Rafflesiaceae: review and
synthesis. – University of Kentucky, Lexington, Kentucky.
Hisahi Y et al. 1984. Karyomorphological
studies in five species of mangrove genera in the Rhizophoraceae. – La Kromosomo
II, 35/36: 1115-1116.
Hiyama Y, Tsukamoto I, Imaichi R, Kato M.
2002. Developmental anatomy and branching of roots of four Zeylanidium
species (Podostemaceae), with
implications for evolution of foliose roots. – Ann. Bot. 90: 735-744.
Hochreutiner BPG. 1918. La formation
lodiculaire des corpuscles hypogynes chez les Guttifères. – Compt. Rend.
Soc. Phys. Hist. Nat. Genève 35: 82-85.
Hochwallner H, Weber A. 2006. Flower
development and anatomy of Clusia valerioi, a Central American species
of Clusiaceae offering floral
resin. – Flora 201: 407-418.
Hoffmann MH, Paula-Souza J, Flaschendräger
A, Röser M. 2010. The gynoecium of male Anchietea pyrifolia (Violaceae): preserved structure with a
new function. – Flora (Jena) 205: 429-433.
Hoffmann P. 1994. A contribution to the
systematics of Andrachne sect. Phyllanthopsis and sect.
Pseudophyllanthus compared with Savia s.l. (Euphorbiaceae) with special
reference to floral morphology. – Bot. Jahrb. Syst. 116: 321-331.
Hoffmann P. 1999a. New taxa and new
combinations in Asian Antidesma (Euphorbiaceae). – Kew Bull. 54:
347-362.
Hoffmann P. 1999b. The genus
Antidesma (Euphorbiaceae) in Madagascar and
the Comoro Islands. – Kew Bull. 54: 877-885.
Hoffmann P. 2000. Revision of
Andrachne sect. Pseudophyllanthus (Euphorbiaceae), with the
description of two new species from Madagascar. – Adansonia, sér. III, 22:
123-133.
Hoffmann P. 2005. Taxonomic revision of
Antidesma (Phyllanthaceae) in Malesia and
Thailand. – Royal Botanic Gardens, Kew.
Hoffmann P. 2008. Revision of
Heterosavia, stat. nov., with notes on Gonatogyne and
Savia (Phyllanthaceae). – Brittonia
60: 136-166.
Hoffmann P, Cheek M. 2003. Two new species of
Phyllanthus (Euphorbiaceae) from Southwest
Cameroon. – Kew Bull. 58: 437-446.
Hoffmann P, McPherson G. 2007. Revision of
Wielandia including Blotia and Petalodiscus (Phyllanthaceae, Euphorbiaceae s.l.). – Ann.
Missouri Bot. Gard. 94: 519-553.
Hoffmann P, Kathriarachchi H, Wurdack KJ.
2006. A phylogenetic classification of Phyllanthaceae (Malpighiales; Euphorbiaceae sensu lato). – Kew
Bull. 61: 37-53.
Holden R. 1912. Reduction and reversion in
North American Salicales. – Ann. Bot. 26: 165-173.
Holm L. 1969. An uredinological approach to
some problems in angiosperm taxonomy. – Nytt Mag. Bot. 16: 147-150.
Holm-Nielsen LB. 1974. Notes on Central
Andean Passifloraceae. –
Bot. Not. 127: 338-351.
Holm-Nielsen LB, Jørgensen PM. 1986.
Passiflora tryphostemmatoides and its allies. – Phytologia 60:
119-124.
Holm-Nielsen LB, Jørgensen PM, Lawesson JE.
1988. 126. Passifloraceae. –
In: Harling G, Andersson L (eds), Flora of Ecuador 31, Nord. J. Bot.,
Copenhagen, pp. 1-129.
Hong T, Zou-Li M. 1987. Floral morphology of
Populus lasiocarpa Oliv. and its phylogenetic position in
Populus. – Acta Bot. Sin. 29: 236-241.
Hooren AMN van, Nooteboom HP. 1984. A
taxonomic revision of the Malesian Linaceae and Ctenolophonaceae, especially of
Malesia, with notes on their demarcation and the relationships with Ixonanthaceae. – Blumea 29:
547-563.
Hooren AMN van, Nooteboom HP. 1986a. Linaceae. – In: Steenis CGGJ van
(†), Wilde WJJO de (eds), Flora Malesiana I, 10(3), Kluwer Academic Publ.,
Dordrecht, Boston, London, pp. 607-619.
Hooren AMN van, Nooteboom HP. 1986b. Ctenolophonaceae. – In:
Steenis CGGJ van (†), Wilde WJJO de (eds), Flora Malesiana I, 10(3), Kluwer
Academic Publ., Dordrecht, Boston, London, pp. 629-634.
Hoppe JR. 1985. Die Morphogenese der
Cyathiendrüsen und ihrer Anhänge, ihre blattypologische Deutung und
Bedeutung. – Bot. Jahrb. Syst. 105: 497-581.
Hoppe JR, Uhlarz H. 1981. Morphogenese und
typologische Interpretation des Cyathiums von Euphorbia-Arten. –
Beitr. Biol. Pfl. 56: 63-98.
Horn JW, Ee BW van, Morawetz JJ, Riina R,
Steinmann VW, Berry PE, Wurdack K. 2012. Phylogenetics and the evolution of
major structural charactes in the giant genus Euphorbia L. (Euphorbiaceae). – Mol. Phylogen.
Evol. 63: 305-326.
Hosseus CC. 1907. Eine neue
Rafflesiaceengattung aus Siam. – Engl. Bot. Jahrb. 41: 55-61.
Hou D. 1972. Germination, seedling, and
chromosome number of Schyphostegia borneensis Stapf
(Scyphostegiaceae). – Blumea 20: 88-92.
Hoyle AC, Brummitt RK. 1999. Three new
species of Brachystegia Benth. (Leguminosae-Caesalpinioideae). – Kew
Bull. 54: 155-161.
Hoyos-Gómez SE. 2011. Towards an
understanding of the basal evolution of Violaceae from an anatomical and
morphological perspective. – M.Sc. thesis, University of Missouri-St.Louis,
St Louis, Missouri.
Hoyos-Gómez SE. 2015. The evolution of Violaceae from an anatomical and
morphological perspective. – Ann. Missouri Bot. Gard. 100: 393-406.
Hu C-C, Crovello TJ, Sokal RR. 1985. The
numerical taxonomy of some species of Populus based only on vegetative
characters. – Taxon 34: 197-206.
Huft MJ. 1979. A monograph of
Euphorbia section Tithymalopsis. – Ph.D. diss., University
of Michigan, Ann Arbor, Michigan.
Huft MJ. 1984. A review of Euphorbia
(Euphorbiaceae) in Baja
California. – Ann. Missouri Bot. Gard. 71: 1021-1027.
Huft MJ. 1989. New and critical taxa of Euphorbiaceae from South America.
– Ann. Missouri Bot. Gard. 76: 1077-1086.
Hul S. 1991. Révision des Flacourtiaceae:
Phyllobotryoneae d’Afrique. – Bull. Mus. Natl. Hist. Nat. Paris, B,
Adansonia 13: 155-165.
Hul S, Breteler FJ. 1997. Réductions
génériques dans les Oncobeae (Flacourtiaceae). – Adansonia 19: 253-262.
Hunter JT, Bruhl JJ. 1997. Two new species of
Phyllanthus and notes on Phyllanthus and Sauropus
(Euphorbiaceae: Phyllantheae)
in New South Wales. – Telopea 7: 149-165.
Huntley B, Huntley JP. 1992. Willows in
prehistory. – Proc. Roy. Soc. Edinb. 98B: 149-154.
Hunziker AT. 1969. Parodiodendron
gen. nov.: un nuevo genero de Euphorbiaceae (Oldfieldioideae)
del noroeste Argentino. – Kurtziana 5: 329-341.
Hunziker AT, Espinar LA. 1967. Nota
aclaratoria sobre las Malesherbiaceae argentinas y una
clave para su identificación. – Kurtziana 4: 83-86.
Hunziker J. 1926. Beiträge zur Anatomie von
Rafflesia patma Bl. – Ph.D. diss, Universität Zürich.
Hurusawa I. 1954. Eine nochmalige Durchsicht
des herkömmlichen Systems der Euphorbiaceen im weiteren Sinne. – J. Fac.
Sci. Univ. Tokyo, Sect. III, Bot. 6: 209-342.
Hutchens JJ, Wallace JB, Romaniszyn ED. 2004.
Role of Podostemum ceratophyllum Michx. in structuring benthic
macroinvertebrate assemblages in a southern Appalachian river. – J. N. Amer.
Benthol. Soc. 23: 713-727.
Hutchinson J. 1922. XVII. The genus
Heywoodia. – Kew Bull. 1922: 114-116.
Hutchinson J. 1969. Tribalism in the family
Euphorbiaceae. – Amer. J.
Bot. 56: 738-758.
Huynh K. 1972. Étude de l’arrangement du
pollen dans la tétrade chez les angiospermes sur la base de données
cytologiques IV. Le genre Passiflora. – Pollen Spores 14: 51-60.
Ianishevskii DE. 1941. The extrafloral
nectar-glands of Salix. – Trudy Bot. Inst. Akad. Nauk SSR, Ser. IV,
Eksp. Bot. 5: 258-294. [In Russian]
Ilker R, Currier HB. 1975. Histochemical
studies of an inclusion body and P-protein in phloem of Xylosma
congestum. – Protoplasma 85: 127-132.
Imaichi R, Ichiba T, Kato M. 1999.
Developmental morphology and anatomy of the vegetative organs in
Malaccotristicha malayana (Podostemaceae). – Intern. J.
Plant Sci. 160: 253-259.
Imaichi R, Maeda R, Suzuki K, Kato M. 2004.
Developmental morphology of foliose shoots and seedlings of Dalzellia
zeylanica (Podostemaceae)
with special reference to their meristems. – Bot. J. Linn. Soc. 144:
289-302.
Imaichi R, Hiyama Y, Kato M. 2005. Leaf
development in absence of a shoot apical meristem in Zeylanidium
subulatum (Podostemaceae):
evolutionary implications. – Ann. Bot. 96: 51-58.
Inamdar JA, Gangadhara M. 1977. Studies on
the trichomes of some Euphorbiaceae. – Feddes Repert.
88: 103-111.
Ingram J. 1967. A revisional study of
Argythamnia subgenus Argythamnia (Euphorbiaceae). – Gentes Herb.
10: 1-38.
Ingram J. 1980a. The generic limits of
Argythamnia (Euphorbiaceae) defined. – Gentes
Herb. 11: 427-436.
Ingram J. 1980b. A revision of
Argythamnia subgenus Chiropetalum (A. Juss.) Ingram. –
Gentes Herb. 11: 437-468.
Jablonski E. 1967. Euphorbiaceae. – In: Maguire B
(ed), Botany of Guayana Highland VII, Mem. New York Bot. Gard. 17: 80-190.
Jacobs M. 1956. Malpighiaceae. – In: Steenis
CGGJ van (ed), Flora Malesiana I, 5(2), Noordhoff-Kolff N. V., Djakarta, pp.
125-145.
Jacobs M, Moore DM. 1971. Violaceae. – In: Steenis CGGJ van
(ed), Flora Malesiana, Ser. I, 7(1), Noordhoff International Publ., pp.
179-212.
Jäger-Zürn I. 1967. Embryologische
Untersuchungen an vier Podostemaceen. – Österr. Bot. Zeitschr. 114:
20-45.
Jäger-Zürn I. 1970. Morphologie der Podostemaceae I. Tristicha
trifaria (Bory ex Willd.) Spreng. – Beitr. Biol. Pflanzen 47: 11-52.
Jäger-Zürn I. 1992. Morphologie der Podostemaceae II.
Indotristicha ramosissima (Wight) van Royen (Tristichoideae). –
Trop. Subtrop. Pflanzenwelt 80: 1-48.
Jäger-Zürn I. 1994. Morphologie der Podostemaceae IV. Zur Kenntnis der
dithekischen Blätter bei Podostemum subulatum Gard. (Podostemoideae).
– Beitr. Biol. Pflanzen 68: 391-419.
Jäger-Zürn I. 1995. Morphologie der Podostemaceae III. Dalzellia
ceylanica (Gard.) Wight (Tristichoideae). – Trop. Subtrop. Pflanzenwelt
92: 1-77.
Jäger-Zürn I. 1997a. Comparative morphology
of the vegetative structures of Tristicha trifaria, Indotristicha
ramosissima and Dalzellia ceylanica. – Aquatic Bot. 57:
71-96.
Jäger-Zürn I. 1997b. Embryological and
floral studies in Weddellina squamulosa Tul. (Podostemaceae, Tristichoideae).
– Aquatic Bot. 57: 151-182.
Jäger-Zürn I. 1999. Developmental
morphology of the shoot system of Podostemum subulatum (Podostemaceae-Podostemoideae). –
Beitr. Biol. Pflanzen 71: 281-334.
Jäger-Zürn I. 2000a. Crustose root and
root-borne shoots of Zeylanidium olivaceum (Podostemaceae-Podostemoideae). –
Flora 195: 61-82.
Jäger-Zürn I. 2000b. Developmental
morphology of roots and root-born shoots of Podostemum subulatum as
compared with Zeylanidium olivaceum (Podostemaceae-Podostemoideae). –
Plant Syst. Evol. 220: 55-67.
Jäger-Zürn I. 2000c. The unusual
ramification mode of Sphaerothylax abyssinica (Wedd.) Warm. (Podostemaceae-Podostemoideae). –
Flora 195: 200-227.
Jäger-Zürn I. 2002a. Morphology and
morphogenesis of ensiform leaves in Apinagia multibranchiata and
Mourera fluviatilis (Podostemaceae-Podostemoideae). –
Flora 197: 394-407.
Jäger-Zürn I. 2002b. Comparative studies in
the morphology of Crenias weddelliana and Maferria indica
with reference to Sphaerothylax abyssinica (Podostemaceae: Podostemoideae).
– Bot. J. Linn. Soc. 138: 63-84.
Jäger-Zürn I. 2003a. The occurrence of
apical septum in the ovary of Rhyncholacis, Apinagia,
Marathrum, and Mourera (Podostemoideae-Podostemaceae): taxonomic
implications. – Bot. Jahrb. Syst. 124: 303-324.
Jäger-Zürn I. 2003b. The architecture of
Zeylanidium olivaceum (Podostemaceae) inferred from the
structure of the primary shoots. – Plant Syst. Evol. 241: 103-114.
Jäger-Zürn I. 2005a. Morphology and
morphogenesis of ensiform leaves, syndesmy of shoots and an understanding of
the thalloid plant body in species of Apinagia, Mourera and
Marathrum (Podostemaceae). – Bot. J. Linn.
Soc. 147: 47-71.
Jäger-Zürn I. 2005b. Shoot apex and
spathella: two problematical structures in Podostemaceae-Podostemoideae. –
Plant Syst. Evol. 253: 209-218.
Jäger-Zürn I. 2005c. Structural analysis of
the dissected ensiform leaves and shoot morphology of Marathrum
foeniculaceum (Podostemaceae). – Flora 200:
229-244.
Jäger-Zürn I. 2005d. Morphology of
Thelethylax isalensis (Perr.) C. Cusset (Podostemaceae-Podostemoideae). –
Bot. Jahrb. Syst. 127: 273-282.
Jäger-Zürn I. 2007. The shoot apex of Podostemaceae: de novo structure
or reduction of the conventional type? – Flora 202: 383-394.
Jäger-Zürn I. 2008. Morphological analysis
of shoots and roots in Thelethylax minutiflora and T.
insolata (Podostemaceae-Podostemoideae):
taxonomic and evolutionary implications. – Bot. Jahrb. Syst. 127: 245-272.
Jäger-Zürn I. 2009a. What is the dithecous
leaf? An investigation of the neotropical Podostemon rutifolium subsp.
riciiforme (Podostemaceae-Podostemoideae). –
Edinburgh J. Bot. 66: 469-481.
Jäger-Zürn I. 2009b. The ramification of
Apinagia riedelii: a key to the understanding of the plant
architecture of Podostemaceae,
subfamily Podostemoideae. – Flora 204: 358-370.
Jäger-Zürn I. 2011. Neglected features of
probable taxonomic value in Podostemaceae: the case of
Polypleurum. – Flora 206: 38-46.
Jäger-Zürn I, Grubert M. 2000. Podostemaceae depend on sticky
biofilms with respect to attachment to rocks in waterfalls. – Intern. J.
Plant Sci. 161: 599-607.
Jäger-Zürn I, Mathew CJ. 2002. Cupula
structure of Dalzellia ceylanica and Indotristicha
ramosissima (Podostemaceae). – Aquatic Bot.
72: 79-91.
Jäger-Zürn I, Novelo RA, Philbrick CT. 2005
[2006]. Microspore development in Podostemaceae-Podostemoideae, with
implications on the characterization of the subfamilies. – Plant Syst. Evol.
256: 209-216.
Jäger-Zürn I, Novelo RA, Philbrick CT,
Piepenbring M. 2007. Pinnately ramified ensiform leaves in the genus
Marathrum (Podostemaceae-Podostemoideae). –
Plant Syst. Evol. 268: 97-117.
Jangid PP, Gupta S. 2015. Comparative wood
anatomy of Indian Drypetes and Putranjiva (Putranjivaceae): systematic
implications, identification and comments on the synonymy of D.
sumatrana. – Nord. J. Bot. 33: 684-695.
Janssonius HH. 1929. A contribution to the
natural classification of the Euphorbiaceae. – Trop. Woods 19:
8-11.
Janzen DH. 1968. Reproductive behavior in the
Passifloraceae and some of its
pollinators in Central America. – Behaviour 32: 33-48.
Jardim A. 1999. A revision of
Roucheria Planch. and Hebepetalum Benth. (Hugoniaceae). –
M.Sc. Thesis, University of Missouri, St. Louis, Missouri.
Jarszewski JW, Olafsdottir ES. 1987.
Monohydroxylated cyclopentenoid cyanohydrin glucosides of Flacourtiaceae. –
Phytochemistry 26: 3348-3349.
Jedrzejczyk M, Rostanski A, Malkowski E.
2002. Accumulation of zinc and lead in selected taxa of the genus
Viola L. – Acta Biol. Cracov. Bot. 44: 49-55.
Jensen U, Vogel-Bauer I, Nitschke M. 1994.
Leguminlike proteins and the systematics of the Euphorbiaceae. – Ann. Missouri
Bot. Gard. 81: 160-179.
Jessup LW. 1982. Flacourtiaceae. – In:
George AS (ed), Flora of Australia 8, Australian Government Publ. Service,
Canberra, pp. 66-84.
Jessup SL. 2002. Six new species and
taxonomic revisions in Mexican Gaudichaudia (Malpighiaceae). – Madroño 49:
237-255.
Jestrow B, Rodríguez FJ, Francisco-Ortega J.
2010. Generic delimitation in the Antillean Adelieae (Euphorbiaceae) with description of
the Hispaniolan endemic genus Garciadelia. – Taxon 59: 1801-1814.
Johns SR, Lamberton JA. 1967a. New imidazole
alkaloids from a Glochidion species (Family Euphorbiaceae). – Aust. J. Chem.
20: 555-560.
Johns SR, Lamberton JA. 1967b. Meteloidine
from Erythroxylum australe F. Muell. – Aust. J. Chem. 20:
1301-1302.
Johns SR, Lamberton JA, Sioumis AA. 1967. The
occurrence of (+)-hygroline in Gynotroches axillaris Bl. (Rhizophoraceae). – Aust. J.
Chem. 20: 1303-1304.
Johns SR, Lamberton JA, Sioumis AA. 1970a.
Nβ-methyltetrahydroharman from Spathiostemon
javensis. – Aust. J. Chem. 23: 213.
Johns SR, Lamberton JA, Sioumis AA. 1970b.
Tropine-3,4,5-trimethoxycinnamate, a new alkaloid from Erythroxylum
ellipticum (Erythroxylaceae). – Aust. J.
Chem. 23: 421-422.
Johnson DM. 1986. Revision of the Neotropical
genus Callaeum (Malpighiaceae). – Syst. Bot. 11:
335-353.
Johnson SD, Griffiths ME, Peter CI, Lawes MJ.
2009. Pollinators, “mustard oil” volatiles, and fruit production in flowers
of the dioecious tree Drypetes natalensis (Putranjivaceae). – Amer. J.
Bot. 96: 2080-2086.
Johnston MC. 1975. Studies of the
Euphorbia species of the Chihuahuan desert region and adjacent areas.
– Wrightia 5: 120-143.
Johri BM, Kapil RN. 1953. Contribution to the
morphology and life history of Acalypha indica L. – Phytomorphology
3: 137-151.
Jolad SD, Hoffmann JJ, Schram KH, Cole JR,
Tempesta MS, Bates RB. 1982. Constituents of Eremocarpus setigerus.
– J. Organic Chem. 47: 1356-1358.
Jones K, Smith JB. 1969. The chromosome
identity of Monadenium Pax and Synadenium Pax (Euphorbiaceae). – Kew Bull. 23:
491-493.
Jones SJ. 1980. Morphology and major taxonomy
of Garcinia (Guttiferae). – Ph.D. diss., University of Leicester,
England.
Jongkind CCH. 2017. Decorsella
arborea, a second species in Decorsella (Violaceae), and
Decorsella versus Rinorea. – Willdenowia 47: 43-47.
Jordan MS, Hayden WJ. 1992. A survey of
mucilaginous testa in Chamaesyce. – Collect. Bot. (Barcelona) 21:
79-89.
Jørgensen PM, Holm-Nielsen LB, Lawesson JE.
1987. New species of Passiflora subg. Plectostemma and subg.
Tacsonia (Passifloraceae). – Nord. J.
Bot. 7: 127-133.
Jose T, Inamdar JA. 1989. Structure, ontogeny
and biology of nectaries in Croton bonplandianus Baill. – Beitr.
Biol. Pflanzen 64: 157-165.
Jud NA, Nelson CW, Herrera F. 2016. Fruits
and wood of Parinari from the arly Micene of Panama and the fossil
record of Chrysobalanaceae.
– Amer. J. bot. 103: 277-289.
Juel HO. 1915. Über den Bau des Gynöceums
bei Parinarium. – Ark. f. Bot. 14: 1-12.
Juncosa AM. 1982. Developmental morphology of
the embryo and seedling of Rhizophora mangle L. (Rhizophoraceae). – Amer. J.
Bot. 69: 1599-1611.
Juncosa AM. 1984a. Embryogenesis and seedling
development in Cassipourea elliptica (Sw.) Poit. (Rhizophoraceae). – Amer. J.
Bot. 71: 170-179.
Juncosa AM. 1984b. Embryogenesis and
developmental morphology of the seedling in Bruguiera exaristata Ding
Hou (Rhizophoraceae). –
Amer. J. Bot. 71: 180-191.
Juncosa AM. 1986. Systematic summary and
developmental floral anatomy and morphology of Rhizophoraceae and Anisophylleaceae.
– Amer. J. Bot. 73: 746-747.
Juncosa AM. 1988. Floral development and
character evolution in Rhizophoraceae. – In: Leins P,
Tucker SC, Endress PK (eds), Aspects of floral development, Berlin, pp.
83-101.
Juncosa AM, Tobe H. 1988. Embryology of tribe
Gynotrocheae (Rhizophoraceae)
and its developmental and systematic implications. – Ann. Missouri Bot. Gard.
75: 1410-1424.
Juncosa AM, Tomlinson PB. 1987. Floral
development in mangrove Rhizophoraceae. – Amer. J. Bot.
74: 1263-1279.
Juncosa AM, Tomlinson PB. 1988a. A historical
and taxonomic synopsis of Rhizophoraceae and Anisophylleaceae.
– Ann. Missouri Bot. Gard. 75: 1278-1295.
Juncosa AM, Tomlinson PB. 1988b. Systematic
comparison and some biological characteristics of Rhizophoraceae and Anisophylleaceae.
– Ann. Missouri Bot. Gard. 75: 1296-1318.
Jury SL, Reynolds T, Cutler DF, Evans FJ
(eds). 1987. The Euphorbiales: chemistry, taxonomy & economic botany. –
Bot. J. Linn. Soc. 94: 1-326.
Justesen PT. 1922. Morphological and
biological notes on Rafflesia flowers, observed in the highlands of
mid-Sumatra (Padangsche Bovenlanden). –Ann. Jard. Bot. Buitenzorg 32:
64-87.
Kabouw P, Welzen PC van, Baas P, Heuven BJ
van. 2008. Styloid crystals in Claoxylon (Euphorbiaceae) and allies
(Claoxylinae) with notes on leaf anatomy. – Bot. J. Linn. Soc. 156:
445-457.
Kajale LB. 1939. A contribution to the life
history of Bergia ammanioides. – J. Indian Bot. Soc. 18: 157-167.
Kakkar L, Paliwal GS. 1974. Studies on the
leaf anatomy of Euphorbia L. Epidermis. – Proc. Indian Nat. Acad.
Sci., Sect. B., 40: 55-67.
Kanis A. 1968. A revision of the Ochnaceae of the Indo-Pacific area.
– Blumea 16: 1-82.
Kanis A. 1971. Ochnaceae. – In: Steenis CGGJ van
(ed), Flora Malesiana I 7, Wolters-Noordhoff, Wageningen, pp. 97-119.
Kapil RN. 1956a. Development of embryo sac
and endosperm in Chrozophora rottleri A. Juss.: a reinvestigation. –
Bot. Gaz. 117: 242-257.
Kapil RN. 1956b. A further contribution to
the morphology and life hstory of Chrozophora Neck. –
Phytomorphology 6: 278-288.
Kapil RN. 1960. Embryology of
Acalypha Linn. – Phytomorphology 10: 174-184.
Kapil RN. 1961. Some embryological aspects of
Euphorbia dulcis L. – Phytomorphology 11: 24-36.
Kapil RN. 1970. Podostemaceae. – In: Proc. Symp.
Comparative embryology of angiosperms, Bull. Indian Natl. Sci. Acad., Sect. B,
41: 104-109.
Kapil RN, Bhatnagar AK. 1972. Endosperm in
Euphorbaceae – a critical appraisal. – In: Murty YS, Johri BM, Mohan Ram
HY, Verghese TM (eds), Advances in plant morphology, Rustogi Publ., Meerut, pp
376-393.
Kapil RN, Bhatnagar AK. 1994. The
contribution of embryology to the systematics of the Euphorbiaceae. – Ann. Missouri
Bot. Gard. 81: 145-159.
Karrenberg S, Kollmann J, Edwards PJ. 2002.
Pollen vectors and inflorescence morphology in four species of Salix.
– Plant Syst. Evol. 235: 181-188.
Katayama N, Koi S, Kato M. 2008.
Developmental anatomy of the reproductive shoot of Hydrobryum
japonicum (Podostemaceae).
– J. Plant Res. 121: 417-424.
Katayama N, Koi S, Kato M. 2010. Expression
of SHOOT MERISTEMLESS, WUSCHEL, and ASYMMETRIC
LEAVES1 homologs in the shoots of Podostemaceae: implications for
the evolution of novel shoot organogenesis. – The Plant Cell 22:
2131-2140.
Kathriarachchi H, Hoffmann P, Samuel R,
Wurdack KJ, Chase MW. 2005. Molecular phylogenetics of Phyllanthaceae inferred from five
genes (plastid atpB, matK, 3’ndhF, rbcL,
and nuclear PHYC). – Mol. Phylogen. Evol. 36: 112-134.
Kathriarachchi H, Samuel R, Hoffmann P,
Mlinarec J, Wardack KJ, Ralimanana H, Stuessy TF, Chase MW. 2006. Phylogenetics
of tribe Phyllantheae (Phyllanthaceae; Euphorbiaceae sensu lato) based on
nrITS and plastid matK sequence data. – Amer. J. Bot. 93:
637-655.
Kato L, Oliveira CMA de, Bittrich V, Amaral M
do CE. 2005. Xanthones from Weddellina squamulosa Tul. (Podostemaceae). – Biochem. Syst.
Ecol. 33: 331-334.
Kato M. 2004. Taxonomic study of Podostemaceae of Thailand 1.
Hydrobryum and related genera with crustaceous roots (subfamily
Podostemoideae). – Acta Phytotaxon. Geobot. 55: 133-165.
Kato M. 2006a. Taxonomic studies of Podostemaceae of Thailand 2.
Subfamily Tristichoideae and subfamily Podostemoideae with ribbon-like roots.
– Acta Phytotaxon. Geobot. 57: 1-54.
Kato M. 2006b. Distribution and biogeography
of Podostemaceae in Asia. –
Bull. Natl. Sci. Mus. Tokyo, B, 32: 19-27.
Kato M. 2008. A taxonomic study of Podostemaceae of Japan. – Bull.
Natl. Mus. Nat. Sci., Ser. B, 34: 63-73.
Kato M. 2009. Podostemaceae of Malesia:
taxonomy, phylogeny and biogeography. – Blumea 54: 198-202.
Kato M, Kita Y. 2003. Taxonomic study of Podostemaceae of China. – Acta
Phytotaxon. Geobot. 54: 87-97.
Kato M, Kita Y, Koi S. 2003. Molecular
phylogeny, taxonomy and biogeography of Malaccotristicha australis
comb. nov. (syn. Tristicha australis) (Podostemaceae). – Aust. Syst.
Bot. 16: 177-183.
Kato M, Koi S. 2009. Taxonomic studies of Podostemaceae of Thailand 3. Six
new and a rediscovered species. – Gard. Bull. Singapore 61: 55-72.
Kato M, Kita Y, Koi S. 2003. Molecular
phylogeny, taxonomy and biogeography of Malaccotristicha australis
comb. nov. (syn. Tristicha australis) (Podostemaceae). – Aust. Syst.
Bot. 16: 177-183.
Kato M, Koi S, Kita Y. 2004. A new
foliose-rooted genus of Podostemaceae from Thailand with a
note on root evolution. – Acta Phytotaxon. Geobot. 55: 65-73.
Kato M, Takimura A, Kawakita A. 2003. An
obligate pollination mutualism and reciprocal diversification in the tree genus
Glochidion (Euphorbiaceae). – Proc. Natl.
Acad. Sci. U.S.A. 100: 5264-5267.
Kato T. 1985. Taxonomical studies on the
Hypericum pseudopetiolatum complex I. Geographical differentiation in
the Japan Archipelago. – Bot. Mag. (Tokyo) 98: 359-370.
Kato T. 1986. Taxonomical studies on the
Hypericum pseudopetiolatum complex II. Natural hybridizations in
Kyushu. – Bull. Natl. Sci. Mus. Tokyo, Ser. B, 12: 139-149.
Kato T. 1987. Taxonomical studies on the
Hypericum pseudopetiolatum complex III. Taxonomy. – Bull. Natl. Sci.
Mus. Tokyo, Ser. B, 13: 69-80.
Kato T. 1990. Taxonomical studies on the
Hypericum pseudopetiolatum complex IV. Allozyme divergence and
phylogenetic inference. – J. Fac. Sci. Univ. Tokyo, Ser. III, Botany 14:
341-368.
Kaul RB. 1995. Reproductive structure and
organogenesis in a cottonwood, Populus deltoides (Salicaceae). – Intern. J. Plant
Sci. 156: 172-180.
Kaur D. 1970. Turneraceae. – In: Proceedings of
the symposium on comparative embryology of angiosperms, Indian National Science
Academy, New Delhi, pp. 194-198.
Kawakita A. 2010. Evolution of obligate
pollination mutualism in the tribe Phyllantheae (Phyllanthaceae). – Plant
Species Biol. 25: 3-19.
Kawakita A, Kato M. 2004a. Evolution of
obligate pollination mutualism in New Caledonia Phyllanthus (Euphorbiaceae). – Amer. J. Bot.
91: 410-415.
Kawakita A, Kato M. 2004b. Obligate
pollination mutualism in Breynia (Phyllanthaceae): further
documentation of pollination mutualism involving Epicephala
(Gracillariidae). – Amer. J. Bot. 91: 1319-1325.
Kawakita A, Kato M. 2006. Assessment of the
diversity and species specificity of the mutualistic association between
Epicephala moths and Glochidion trees. – Mol. Ecol. 15:
3567-3581.
Kawakita A, Kato M. 2009. Repeated
independent evolution of obligate pollination mutualism in the
Phyllantheae-Epicephala association. – Proc. Roy. Soc., Sect. B,
276: 417-426.
Kawakita A, Takimura A, Terachi T, Sota T,
Kato M. 2004. Cospeciation analysis of an obligate pollination mutualism: have
Glochidion trees (Euphorbiaceae) and pollinating
Epicephala moths (Gracillariidae) diversified in parallel? –
Evolution 58: 2201-2214.
Kawano S. 1965. Anatomical studies on the
androecia of some members of the Guttiferae-Moronoboideae. – Bot. Mag.
(Tokyo) 78: 97-108.
Kawashima T, Nakatsu T, Fukazawa Y, Ito S.
1976. Diterpenic lactones of Mallotus repandus. – Heerocycles 5:
227-232.
Keating RC. 1973. Pollen morphology and
relationships of the Flacourtiaceae. – Ann. Missouri Bot. Gard. 60:
273-305.
Keating RC. 1975 [1976]. Trends of
specialization in pollen of Flacourtiaceae with comparative observations of Cochlospermaceae and Bixaceae. – Grana 15: 29-49.
Keating RC, Randrianasolo V. 1988. The
contribution of leaf architecture and wood anatomy to classification of the Rhizophoraceae and Anisophylleaceae.
– Ann. Missouri Bot. Gard. 75: 1343-1368.
Kellogg EA, Weitzman AL. 1985. A note on the
Oceanic species of Melicytus (Violaceae). – J. Arnold Arbor. 66:
491-502.
Kelly LJ, Ameka GK, Chase MW. 2010. DNA
barcoding of African Podostemaceae (river-weeds): a
test of proposed barcode regions. – Taxon 59: 251-260.
Kenfack D, Gereau RE, Thomas DW, Sainge MN.
2015. The tropical African genus Crotonogynopsis (Euphorbiaceae), with two new
species. – Novon 24: 246-255.
Kenoyer LA. 1919. Dimorphic carpellate flower
of Acalypha indica L. – J. Indian Bot. 1: 3-7.
Khan MI, Ikram M, Hussain SF. 1983.
Bisbenzylisoquinoline alkaloids from Andrachne cordifolia. – Planta
Medica 47: 191.
Khanduri P, Tandon R, Uniyal PL, Bhat V,
Pandey AK. 2015. Comparative morphology and molecular systematics of Indian Podostemaceae. – Plant Syst.
Evol. 301: 861-882.
Khosla C, Mohan Ram HY. 1993. Morphology of
flower, fruit and seed in Polypleurum stylosum. – Aquatic Bot. 46:
255-262.
Khosla C, Shivanna KR, Mohan Ram HY. 2000.
Reproductive biology of Polypleurum stylosum (Podostemaceae). – Aquatic Bot.
67: 143-154.
Khosla C, Shivanna KR, Mohan Ram HY. 2001.
Cleistogamy in Griffithella hookeriana (Podostemaceae). – South Afr. J.
Bot. 67: 320-324.
Kiger RW. 1972. The genus Samyda
(Flacourtiaceae). – Ph.D. diss., University of Maryland, College Park,
Maryland.
Kiger RW. 1984. Exclusions from
Samyda Jacq. (Flacourtiaceae). – Taxon 33: 445-468.
Killip EP. 1938. The American species of Passifloraceae. – Publ. Field
Mus. Nat. Hist., Bot., 19: 1-613.
Killip EP. 1960. Supplemental notes on the
American species of Passifloraceae with descriptions
of new species. – Contr. U.S. Natl. Herb. 35: 1-23.
Kim KS, Sun BY, Whang SS, Chung GH. 1991.
Biosystematic study on the genus Viola in Korea. Comparative study of
the Viola albida complex. – Korean J.Bot. 34: 229-238.
Kimura A. 1928. Über Toisusu, eine
neue Salicaceen-Gattung und die systematische Stellung derselben. – Bot. Mag.
(Tokyo) 42: 287-290.
Kimura A. 1938. Symbolae Iteologicae VI. –
Sci. Rep. Tôhoku Univ., Ser. IV (Biology) 13: 381-394.
Kimura A. 1988. De salicis subgenere
Pleuradenia commentatio. – Sci. Rep. Tôhoku Univ., Ser. IV
(Biology) 39: 143-147.
Kimura C. 1963. On the embryo sac in some
members of the Salicaceae. –
Sci. Rep. Tôhoku Univ., Ser. IV (Biology) 29: 393-398.
Kimura Y. 1937. Conspectus omnium specierum
generis Hyperici (excl. sect. Ascyron) in Yezo, Sachalin et
Kuriles I-II. – Bot. Mag. (Tokyo) 51: 700-708, 730-738.
Kimura Y. 1938. Hypericorum Japonicarum
descriptio I-II. – Bot. Mag. (Tokyo) 52: 188-195, 403-408.
Kimura Y. 1939. Sur la groupe
d’Hypericum pseudopetiolatum. – J. Jap. Bot. 15: 292-301.
Kinghorn ad. 1979. Cocarcinogeic irritant Euphorbiaceae. – In: Kinghorn AD
(ed), Toxic plants, Columbia University Press, New York, pp. 137-159.
Kirchheimer F. 1951. Über das Vorkommen
einer Gattung der Humiriaceen im Europäischen Tertiär. – Planta 39:
75-90.
Kita Y. 2002. Molecular phylogeny,
morphological evolution, and biogeography of Podostemaceae. – Bunrui 2:
19-26. [In Japanese]
Kita Y, Kato M. 2001. Infrafamilial phylogeny
of the aquatic angiosperm Podostemaceae inferred from the
nucleotide sequences of the matK gene. – Plant Biol. 3: 156-163.
Kita Y, Kato M. 2004a. Phylogenetic
relationships between disjunctly occurring groups of Tristicha
trifaria (Podostemaceae).
– J. Biogeogr. 31: 1605-1612.
Kita Y, Kato M. 2004b. Molecular phylogeny of
Cladopus and Hydrobryum (Podostemaceae, Podostemoideae)
with implications for their biogeography in East Asia. – Syst. Bot. 29:
921-932.
Kita Y, Kato M. 2005. Seedling developmental
anatomy of an undescribed Malaccotristicha species (Podostemaceae, subfamily
Tristichoideae) with implications for body plan evolution. – Plant Syst.
Evol. 254: 221-232.
Kitanov GM, Nedialkov PT. 1998. Mangiferin
and isomangiferin in some Hypericum species. – Biochem. Syst. Ecol.
26: 647-653.
Kite GC, Fellows LE, Fleet GWJ, Liu PS,
Scofield AM, Smith NG. 1988. α-Homonojirimycin
[2,6-dideoxy-2,6-imino-D-glycero-L-gulohepitol] from Omphalea diandra
L.: isolation and glucosidase inhibition. – Tet. Let. 29: 6483-6486.
Kleiman R, Plattner RD, Specer GF. 977.
Alchornea cordifolia seed oil: a rich source of a new C20
epoxide, (+)-cis-14,15-epoxy-cis-11-eicosenoic acid. –
Lipids 12: 610-612.
Kleiman R, Smith CR Jr, Yates SG, Jones Q.
1965. Search for new industrial oils XII. Fifty-eight Euphorbiaeae oils,
including one rich in vernolic acid. – J. Amer. Oil Chem. Soc. 42:
169-172.
Kloos A, Bouman F. 1980. Case studies in aril
development of Passiflora suberosa L. and Turnera ulmifolia
L. – Beitr. Biol. Pflanzen 55: 49-66.
Klucking EP. 1992. Leaf venation patterns 6.
Flacourtiaceae. – J. Cramer, Berlin.
Klucking EP. 1998. Vol. 8, Euphorbiaceae, part 1.
Phyllanthoideae and Oldfieldioideae. – J. Cramer, Berlin, pp. 1-93.
Knapp S, Mallet J. 1984. Two new species of
Passiflora (Passifloraceae), with comments on
their natural history. – Ann. Missouri Bot. Gard. 71: 1068-1074.
Knoll F. 1905. Die Brennhaare der
Euphorbiaceen-Gattungen Dalechampia und Tragia. –
Sitzungsber. K. Akad. Wiss. Math.-Nat. Kl. 114: 29-48.
Kobuski CE. 1948. Studies in the Theaceae XVII. A
review of the genus Bonnetia. – J. Arnold Arbor. 29: 393-413.
Kobuski CE. 1950. Studies in the Theaceae XIX. The
genera Archytaea and Ploiarium. – J. Arnold Arbor. 31:
196-207.
Koch BE. 1972. Fossil Picrodendron
fruit from the Upper Danian of Núgssuaq, West Greenland. – Medd. Grønland
193: 1-32.
Kogi M. 1984. A karyomorphological study of
the genus Hypericum (Hypericaceae) in Japan. – Bot.
Mag. (Tokyo) 97: 333-343.
Köhler E. 1965. Die Pollenmorphologie der
biovulaten Euphorbiaceae und
ihre Bedeutung für die Taxonomie. – Grana Palynol. 6: 26-120.
Koi S, Kato M. 2003. Comparative
developmental anatomy of the root in three species of Cladopus (Podostemaceae). – Ann. Bot. 91:
927-933.
Koi S, Kato M. 2007. Developmental morphology
of the shoot in Weddellina squamulosa and implications for shoot
evolution in the Podostemaceae.
– Ann. Bot. 99: 1121-1130.
Koi S, Kato M. 2010a. Developmental
morphology of seedling and shoot and phylogenetic relationship of
Diplobryum koyamae (Podostemaceae). – Amer. J. Bot.
97: 373-387.
Koi S, Kato M. 2010b. Developmental anatomy
of seedcling of Indodalzellia gracilis (Podostemaceae). – Plant Biol.
12: 794-799.
Koi S, Kato M. 2012. A taxonomic study of Podostemaceae subfamily
Podostemoideae of Laos with phylogenetic analyses of Cladopus,
Paracladopus and Polypleurum. – Kew Bull. 67: 331-365.
Koi S, Imaichi R, Kato M. 2005. Endogenous
leaf initiation in the apical-meristemless shoot of Cladopus
queenslandicus (Podostemaceae) and implications
for evolution of shoot morphology. – Intern. J. Plant Sci. 166: 199-206.
Koi S, Fujinami R, Kubo N, Tsukamoto I,
Inagawa R, Imaichi R, Kato M. 2006. Comparative anatomy of root meristem and
root cap in some species of Podostemaceae and the evolution of
root dorsiventrality. – Amer. J. Bot. 93: 682-692.
Koi S, Kita Y, Kato M. 2008. Paracladopus
chanthaburiensis, a new species of Podostemaceae from Thailand, with
notes on its morphology, phylogeny and distribution. – Taxon 57: 201-210.
Koi S, Rutishauser R, Kato M. 2009.
Phylogenetic relationship and morphology of Dalzellia gracilis (Podostemaceae, subfamily
Tristichoideae) with proposal of a new genus. – Intern. J. Plant Sci. 170:
237-246.
Koi S, Kita Y, Hirayama Y, Rutishauser R,
Huber KA, Kato M. 2012. Molecular phylogenetic analysis of Podostemaceae: implications for
taxonomy of major groups. – Bot. J. Linn. Soc. 169: 461-492.
Kolbe K-B, John J. 1979. Serologische
Untersuchungen zur Systematik der Violales. – Bot. Jahrb. Syst. 101: 3-15.
Kollmann R. 1960a. Untersuchungen über das
Protoplasma der Siebröhren von Passiflora coerulea I. Mitteilung:
lichtoptische Untersuchungen. – Planta 54: 611-640.
Kollmann R. 1960b. Untersuchungen über das
Protoplasma der Siebröhren von Passiflora coerulea II. Mitteilung:
elektronenoptische Untersuchungen. – Planta 55: 67-107.
Kolterman DA, Breckon GJ. 1982.
Chemotaxonomic studies in Cnidoscolus (Euphorbiaceae) I. Flavonol
glycosides of the C. tubulosus complex. – Syst. Bot. 7: 178-185.
Kolterman DA, Breckon GJ, Kowal RR. 1984.
Chemotaxonomic studies in Cnidoscolus (Euphorbiaceae) II. Flavonoids of
C. aconitifolius, C. souzae, and C. spinosus. –
Syst. Bot. 9: 22-32.
Kool R. 1980. A taxonomic revision of the
genus Ixonanthes. – Blumea 26: 191-204.
Kool R. 1986. Ixonanthaceae. – In: Steenis
CGGJ van (†), Wilde WJJO de (eds), Flora Malesiana I, 10(3), Kluwer Academic
Publ., Dordrecht, Boston, London, pp. 621-627.
Koorders SH. 1918. Botanisch overzicht der Rafflesiaceae van
Nederlandsch-Indië. – G. Kolff, Batavia.
Korotkova N, Schneider JV, Quandt D, Worberg
A, Zizka G, Borsch T. 2009. Phylogeny of the eudicot order Malpighiales: analysis of a
recalcitrant clade with sequences of the petD group II intron. –
Plant Syst. Evol. 282: 201-228.
Kostermans AJGH. 1965. The genus
Acioa Aublet (Rosaceae-Chrysobalanoideae) in
Malesia. – Reinwardtia 7: 9-18.
Koutnik DL. 1984. Chamaesyce (Euphorbiaceae) – a newly
recognized genus in southern Africa. – South Afr. J. Bot. 3: 262-264.
Koutnik DL. 1987. A taxonomic revision of the
Hawaiian species of the genus Chamaesyce (Euphorbiaceae). – Allertonia 4:
331-388.
Krahenbühl M, Yuan Y-M, Küpfer P. 2002.
Chromosome and breeding system evolution of the genus Mercurialis (Euphorbiaceae): implications of
ITS molecular phylogeny. – Plant Syst. Evol. 234: 155-169.
Krause K. 1908. Linaceae andinae. – Engl. Bot. Jahrb.
Syst. 40: 277-279.
Krause K. 1925. Lacistemaceae. – In: Engler
A, Gilg E (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 21, W.
Engelmann, Leipzig, pp. 321-323.
Krishnan N. 1977. Cytotaxonomical studies on
Bixaceae and Samydadeae from
South India with collected evidences from palynology, anatomy and biochemistry.
– Ph.D. diss., Annamalai University, India.
Krishnan N. 1981. Pollen morphology of some
Flacourtiaceae. – Proc. Indian Acad. Sci., Sect. B, 90: 163-168.
Krosnick SE, Freudenstein JV. 2005. Monophyly
and floral character homology of Old World Passiflora (subgenus
Decaloba: supersection Disemma). – Syst. Bot. 30:
139-152.
Krosnick SE, Harris EM, Freudenstein JV.
2006. Patterns of anomalous floral development in the Asian Passiflora
(subgenus Decaloba: supersection Disemma). – Amer. J. Bot.
93: 620-636.
Krosnick SE, Ford AJ, Freudenstein JV. 2009.
Taxonomic revision of Passiflora subgenus Tetrapathea
including the monotypic genera Hollrungia and Tetrapathea (Passifloraceae), and a new
species of Passiflora. – Syst. Bot. 34: 375-385.
Krosnick SE, Porter-Utley KE, MacDougal JM,
Jørgensen PM, McDade LA. 2013. New insights into the evolution of
Passiflora subgenus Decaloba (Passifloraceae): phylogenetic
relationships and morphological synapomorphies. – Syst. Bot. 38: 692-713.
Kruijt RC. 1996. A taxonomic monograph of
Sapium Jacq., Anomostachys (Baill.) Hurus,
Duvigneaudia J. Léonard and Sclerocroton Hochst. (Euphorbiaceae tribe Hippomaneae).
– Bibl. Bot. 146: 1-109.
Kruijt RC, Zijlstra G. 1989. Proposal to
conserve 4483 Sapium Jacq., 1760 against P. Browne, 1756 (Euphorbiaceae). – Taxon 38:
320-325.
Kubitzki K. 1978. The botany of Guyana
Highlands X. Caraipa and Mahurea (Bonnetiaceae). – Mem. New York
Bot. Gard. 29: 82-128.
Kubitzki K. 2006. Malesherbiaceae. – In:
Kubitzki K (ed), The families and genera of vascular plants IX. Flowering
plants. Eudicots. Berberidopsidales,
Buxales, Crossosomatales, Fabales p. p., Geraniales, Gunnerales, Myrtales p. p., Proteales, Saxifragales, Vitales, Zygophyllales, Clusiaceae Alliance, Passifloraceae Alliance, Dilleniaceae, Huaceae, Picramniaceae, Sabiaceae. – Springer,
Berlin, Heidelberg, New York, pp. 247-249.
Kubitzki K (ed). 2014. The families and
genera of vascular plants XI. Flowering plants – eudicots – Malpighiales. – Springer, Berlin,
Heidelberg, New York, 331 pp.
Kubitzki K, Mesquita AAL, Gottlieb OR. 1978.
Chemosystematic implications of xanthones in Bonnetia and
Archytaea. – Biochem. Syst. Ecol. 6: 185-187.
Kuhlmann JG. 1935. Novas especies da Hylea.
– Arq. Inst. Biol. Veg. 2: 83-89.
Kuhlmann JG. 1940. Especies novas
equatoriais. – Anais Reunião Sul.-Amer. Bot. 3: 78-86.
Kulju KKM, Sierra SEC, Draisma SGA, Samuel R,
Welzen PC van. 2007. Molecular phylogeny of Macaranga,
Mallotus, and related genera (Euphorbiaceae s.s.): insights from
plastid and nuclear DNA sequence data. – Amer. J. Bot. 94: 1726-1743.
Kulju KKM, Sierra SEC, Welzen PC van. 2007.
Reshaping Mallotus II: inclusion of Neotrewia,
Octospermum and Trewia in Mallotus s. str. (Euphorbiaceae s. str.). – Blumea
52: 115-136.
Kulju KKM, Ham RWJM van der, Breteler FJ.
2008. Rediscovery and phylogenetic position of the incertae sedis genus
Afrotrewia (Euphorbiaceae): morphological,
pollen and molecular evidence. – Taxon 57: 137-143.
Kulshreshtha K, Ahmad KJ. 1992. Cuticular
ornamentations in some genera of Euphorbiaceae. – Feddes Repert.
103: 317-326.
Kumar PV, Bahadur B. 1978. Structure of the
epidermis and ontogeny of stomata in Reinwardtia indica Dum. (Linaceae). – Indian J. Bot. 1:
127-131.
Kusari S, Lamshöft M, Zühlke S, Spiteller
M. 2008. An endophytic fungus from Hypericum perforatum that produces
hypericin. – J. Nat. Prod. 71: 159-162.
Küster E. 1897. Die anatomischen Charaktere
der Chrysobalaneen, insbesondere ihre Kieseleinlagerungen. – Bot. Centralbl.
69: 46-54, 97-106, 129-139, 161-169, 193-202, 225-234.
Kwon DJ, Bae YS. 2009. Phenolic glucosides
from bark of Populus alba x glandulosa (Salicaceae). – Biochem. Syst. Ecol.
37: 130-132.
Lack A. 1978. The ecology of the flowers of
the savanna tree Maranthes polyandra and their visitors, with
particular reference to bats. – J. Ecol. 66: 287-295.
Lacoste JF, Alexandre DY. 1991. Le goupi
(Goupia glabra Aubl), essence forestière d’avenir en Guyane:
analyse bibliographique. – Ann. Sci. For. 48: 429-441.
La Fon R (ed). 1980-1996. Euphorbia
Journal 1-10. – Strawberry Press, Mill Valley, California.
Lægaard S. 2008. Elatine
rotundifolia sp. nov. (Elatinaceae) from Ecuador. – Nord.
J. Bot. 26: 235-236.
Lakshmanan KK, Poornima S. 1988.
Microsporogenesis in Rhizophora lamarckii Montr. – Curr. Sci. 57:
1084. 1085.
Landes M. 1946. Seed development in
Acalypha rhomboidea and some other Euphorbiaceae. – Amer. J. Bot.
33: 562-568.
Larsson G, Bremer B. 1991. Korgviden –
nyttoväxter förr och nu. – Svensk Bot. Tidskr. 85: 185-200.
Launert E. 1963. 35. Malpighiaceae. – In: Exell AW,
Fernandes A, Wild H (eds), Flora Zambesiaca 2 (Part 1), Crown Agents for
Oversea Governments and Administrations, London, pp. 109-125.
Launert E. 1968. Malpighiaceae. – In:
Milne-Redhead E, Polhill RM (eds), Flora of tropical East Africa, Crown Agents
for Oversea Governments and Administrations, London, pp. 1-27.
Lauter WM, Foote PA. 1955. Toxic principles
of Hippomane mancinella II. Isolation of a toxic principle. – J.
Amer. Pharm. Assoc. 44: 3361-363.
Lauterbach C. 1922. Beiträge zur Flora von
Papuasien IX. Die Guttiferen Papuasiens. – Engl. Bot. Jahrb. Syst. 58:
1-49.
Leach GJ. 1989. Taxonomic revision of
Bergia (Elatinaceae) in
Australia. – J. Adelaide Bot. Gard. 11: 75-100.
Leach LC. 1973. New and interesting taxa of
the tribe Euphorbieae (Euphorbiaceae) from Portuguese
Africa. – Garcia de Orta, Ser. Bot. 1: 31-42.
Leach LC. 1976. Distributional and
morphological studies of the tribe Euphorbieae (Euphorbiaceae) and their relevance
to its classification and possible evolution. – Excelsa 6: 3-19.
Leal DO, Malafaia C, Cesar R, Pimentel RR,
Santiago-Fernandes LDR, Lima HA, Sá-Haiad B. 2012. Floral structure of
Garcinia brasiliensis in relation to flower biology and evolution. –
Intern. J. Plant Sci. 173: 172-183.
Léandri J. 1938. Euphorbiacées malgaches
nouvelles récoltées par M. H.Perrier de la Bâthie. – Bull. Soc. Bot.
France 85: 523-533.
Léandri J. 1938 [1939]. Le genre
Tragia (Euphorbiacées) à Madagascar. – Bull. Trim. Acad. Malgache,
n.s. 21: 65-68.
Léandri J. 1940. Sur un genre malgache
nouveau d’Euphorbiacées. – Bull. Soc. Bot. France 87: 279-285.
Léandri J. 1949. Sur la présence d’une
Trigoniacée dans la flore malgache. – Compt. Rend. Acad. Sci. Paris 229:
846-848.
Léandri J. 1957. Euphorbia
mandravioky, nom. nov., et un nom nouveau pour une sous-section du genre
Euphorbe. – Bull. Soc. Bot. France 104: 499-501.
Léandri J. 1958. Euphorbiacées. – In:
Humbert H (ed), Flore de Madagascar 111, Firmin-Didot et Cie,
Paris.
Léandri J. 1971. Un sous-genre malgache
nouveau de Tragia (Euphorbiaceae). – Adansonia,
sér. II, 13: 437-439.
Lebreton P, Bouchez M-P. 1967. Recherches
chimiotaxinomiques sur les plantes vasculaires V. Distribution des composés
polyphénoliques chez les Pariétales. – Phytochemistry 6: 1601-1608.
Lee DE, Bannister JM, Raine JI, Conran JG.
2010. Euphorbiaceae:
Acalyphoideae fossils from early Miocene New Zealand:
Mallotus-Macaranga leaves, fruits, and inflorescence with in situ
Nyssapollenites endobalteus pollen. – Rev. Palaeobot. Palynol. 163:
127-138.
Leenhouts PW. 1956. Some notes on the genus
Dichapetalum (Dichapetalaceae) in Asia,
Australia, and Melanesia. – Reinwardtia 4: 75-87.
Leenhouts PW. 1957. Dichapetalaceae. – In: Steenis
CGGJ van (ed), Flora Malesiana I(5), Noordhoff-Kolff N. V., Groningen, pp.
305-316.
Leinfellner W. 1954. Beiträge zur
Kronblattmorphologie I. Erythroxylum novogranatense. – Österr. Bot.
Zeitschr. 101: 428-434.
Leins P. 1964. Die frühe Blütenentwicklung
von Hypericum hookerianum Wight et Arn. und H. aegyptiacum L.
– Ber. Deutsch. Bot. Ges. 77: 112-123.
Leins P, Erbar C. 1991. Fascicled androecia
in Dilleniidae and some remarks on the Garcinia androecium. – Bot.
Acta 104: 336-344.
Lemke DE. 1983. Taxonomy of
Neopringlea (Flacourtiaceae). – Syst. Bot. 8: 430-435.
Lemke DE. 1987a. Tribal relationships of
Bartholomaea (Flacourtiaceae). – Brittonia 39: 436-439.
Lemke DE. 1987b. Morphology, wood anatomy,
and relationships of Neopringlea (Flacourtiaceae). – Syst. Bot. 12:
609-616.
Lemke DE. 1988. A synopsis of Flacourtiaceae.
– Aliso 12: 29-43.
Léonard J. 1956. Notulae systematicae XXI.
Observations sur les genres Oldfieldia, Paivaeusa et
Cecchia (Euphorbiaceae
Africanae). – Bull. Jard. Bot. État Bruxelles 26: 335-343.
Léonard J. 1959a. Observations sur les
genres Pycnocoma et Argomuellera (Euhorbiacées africaines).
– Bull. Soc. Roy. Bot. Belg. 91: 267-281.
Léonard J. 1959b. Notulae systematicae XXVI.
Notes sur les espèces africaines continentales des genres Sapium P.
Br. et Excoecaria L. – Bull. Jard. Bot. État Bruxelles 29:
133-146.
Léonard J. 1962a. Notulae systematicae 33.
Sur les limites entre les genres Drypetes Vahl et
Lingelsheimia Pax (Euphorbiaceae). – Bull. J. Bot.
État Bruxelles 32: 513-516.
Léonard J. 1962b. Euphorbiaceae II. Crotoneae. –
In: Flore du Congo Belge et du Rwanda-Burundi 8, 1, Institut national pour
l’étude agronomique du Congo, Bruxelles, pp. 50-84.
Léonard J. 1989. Révision du genre africain
Martretia Beille (Euphorbiaceae) et la nouvelle
tribu des Marthretieae. – Bull. Jard. Bot. État. Bruxelles 59: 319-332.
Léonard J. 1995. Révision des espèces
zaïroises des genres Thecacoris A. Juss. et Cyathogyne
Müll. Arg. (Euphorbiaceae).
– Bull. Jard. Bot. Belg. 64: 13-52.
Léonard J. 1996. Euphorbiaceae. – In: Flore
d’Afrique Centrale 8(3): 1-74.
Léonard J, Dessart P. 1994. Avis de
recherché: Torridincolidés (Coleoptera) vivant en symbiose avec
Podostémacées (Podostémales). – Bull. Ann. Soc. Roy. Belg. Entomol. 130:
71-76.
Léonard J, Mosango M. 1985.
Hymenocardiaceae. – In: Flore d’Afrique Centrale: Spermatophytes, Bull.
Jard. Bot. Natl. Belg., Bruxelles, pp. 1-16.
Léonard J, Nkounkou J. 1989. Révision du
genre Spondianthus Engl. (Euphorbiacée africaine). – Bull. Jard.
Bot. Natl. Belg. 59: 133-149.
Léon Enriquez BL, Vester HFM Vester, Hallé
F. 2008. The architecture of Phyllanthus acuminatus Vahl: a prelude to
understanding the architectural evolution in the Phyllanthaceae. – Adansonia,
sér. III, 30: 137-149.
Leroy C, Jauneau A, Quilichini A, Dejean A,
Orivel J. 2008. Comparison between the anatomical and morphological structure
of leaf blades and foliar domatia in the ant-plant Hirtella physophora
(Chrysobalanaceae). – Ann.
Bot. 101: 501-507.
Leroy J-F. 1976. Recherches sur la nature et
l’origine de la fleur angiospermienne: interpretation des structures dans un
groupe singulier d’Euphorbiaceae. – Compt. Rend.
Acad. Sci. Paris, sér. D, 283: 147.
Lersten NP, Curtis JD. 1974. Colleter anatomy
in red mangrove, Rhizophora mangle (Rhizophoraceae). – Can. J. Bot.
52: 2277-2278.
Les DH, Philbrick CT, Novelo RA. 1997. The
phylogenetic position of river-weeds (Podostemaceae): insights from
rbcL sequence data. – Aquatic Bot. 57: 5-27.
Leskinen E, Alström-Rapaport C. 1999.
Molecular phylogeny of Salicaceae
and closely related Flacourtiaceae: evidence from 5.8S, ITS1 and ITS2 of the
rDNA. – Plant Syst. Evol. 215: 209-227.
Letouzey R. 1961. Notes sur les
Scytopétalacées (Révision des Scytopétalacées de l’herbier de Paris).
– Adansonia, sér. II, 1: 106-142.
Levin GA. 1986a. Systematic foliar morphology
of Phyllanthoideae (Euphorbiaceae) I. Conspectus. –
Ann. Missouri Bot. Gard. 73: 29-85.
Levin GA. 1986b. Systematic foliar morphology
of Phyllanthoideae (Euphorbiaceae) II. Phenetic
analysis. – Ann. Missouri Bot. Gard. 73: 86-98.
Levin GA. 1986c. Systematic foliar morphology
of Phyllanthoideae (Euphorbiaceae) III. Cladistic
analysis. – Syst. Bot. 11: 515-530.
Levin GA. 1992. Systematics of
Paradrypetes (Euphorbiaceae). – Syst. Bot. 17:
74-83.
Levin GA. 1998. Evolution in the Acalypha
gracilens/monococca complex (Euphorbiaceae): morphological
analysis. – Syst. Bot. 23: 269-287.
Levin GA, Simpson MG. 1994a. Phylogenetic
implications of pollen ultrastructure in the Oldfieldioideae (Euphorbiaceae). – Ann. Missouri
Bot. Gard. 81: 203-238.
Levin GA, Simpson MG. 1994b. Phylogenetic
relationships of Didymocistus and Hymenocardia (Euphorbiaceae). – Ann. Missouri
Bot. Gard. 81: 239-244.
Levin GA, Morton JK, Robbrecht E. 2007. Two
new species of Acalypha (Euphorbiaceae) from tropical
Africa, and a review of some Robyns names for cupricolous plants from the
Democratic Republic of the Congo. – Syst. Bot. 32: 576-582.
Lewis J. 1954. Turneraceae. – In: Turrill WB,
Milne-Redhead E (eds), Flora of tropical East Africa, The Crown Agents for the
Colonies, London, pp. 1-20.
Lewis J. 1956. Rhizophoraceae. – In: Turrill
WB, Milne-Redhead E (eds), Flora of tropical East Africa, Crown Agents for
Oversea governments and Administrations, London, pp. 1-20.
Lewis WH. 1964. A hexaploid Linum
(Linaceae) from eastern Ethiopia.
– Sida 1: 383-384.
Li A, Wang KQ, G S. 2000. Genetic diversity
within and among populations of Viola tenuicornis with reference to
sampling strategies. – Acta Bot. Sin. 42: 1069-1074.
Li H. 1975. The Rafflesiaceae in China. – Acta
Phytotax. Sin. 13: 79-82. [in Chinese]
Li PT. 1991. A new genus of Euphorbiaceae and some new
nomenclatural combination of the asclepiadaceous plants. – J. S. China Agric.
Univ. 12: 38-42.
Li Y, Dressler S, Zhang D, Renner SS. 2009.
More Miocene dispersal between Africa and Asia – the case of
Bridelia (Phyllanthaceae). – Syst. Bot.
34: 521-529.
Lieberei R. Selmar D, Biehl B. 1985.
Metabolization of cyanogenic glycosides in Hevea brasiliensis. –
Plant Syst. Evol. 150: 49-63.
Lim AL. 1984. The embryology of Garcinia
mangostana L. (Clusiaceae).
– Gard. Bull. (Singapore) 37: 93-103.
Lima LR, Pirani JR. 2003. O gênero
Croton L. (Euphorbiaceae) na Cadeia do
Espinhaço, Minas Gerais, Brasil. – Bol. Bot. Univ. São Paulo 21:
299-344.
Lin Q, Duan L-D. 2009. Two new species and a
new series of Elatostema (Urticaceae) from China. – Bot.
J. Linn. Soc. 158: 674-680.
Lindman CAM. 1906. Zur Kenntnis der Corona
einiger Passifloren. – In: Sernander R, Svedelius N, Norén CO (eds),
Botaniska studier tillägnade F. R. Kjellman, Almqvist & Wiksell, Uppsala,
pp. 55-79.
Link DA. 1992a. The floral nectaries of the
Geraniales and their
systematic implications IV. Ctenolophonaceae Badre. –
Flora 187: 103-107.
Link DA. 1992b. The floral nectaries in the
Irvingiaceae. – Plant Syst.
Evol. 180: 235-242.
Link DA. 1992c. The floral nectaries of the
Geraniales and their
systematic implications VI. Ixonanthaceae Exell and Mendonça.
– Bot. Jahrb. Syst. 114: 81-90.
Link DA. 1992d. The nectaries of the Geraniales and their
systematic implications VII. Humiriaceae Cuatr. – Bot. Jahrb.
Syst. 114: 211-241.
Liogier AH. 1952. Estudios en Euforbiáceas
Cubanas. – Contr. Ocas. Mus. Hist. Nat. Colegio La Salle 11: 1-12.
Liogier AH. 1971. Novitates Antillanae IV. Euphorbiaceae. – Mem. New York
Bot. Gard. 21: 123-133.
Litt A, Chase MW. 1999. The systematic
position of Euphronia, with comments on the position of
Balanops: an analysis based on rbcL sequence data. – Syst.
Bot. 23: 401-409.
Livaniou-Tiniakou A. 1991. Biosistimatiki
meleti tou genous Viola section Viola (Violaceae) stin Ellada. – Ph.D.
diss., University of Patras, Greece.
Lleras E. 1972. Review of the genus
Haplochlathra (Bonnetiaceae). – Mem. New York
Bot. Gard. 22: 129-136.
Lleras E. 1976. Revision and taxonomic
position of the genus Euphronia Martius ex Martius & Zuccarini (Vochysiaceae). – Acta
Amazonica 6: 43-47.
Lleras E. 1978. Flora Neotropica. Monograph
19. Trigoniaceae. – New York
Botanical Garden, Bronx, New York.
Lloyd DG, Webb CJ, Dulberger R. 1990.
Heterostyly in species of Narcissus (Amaryllidaceae),
Hugonia (Linaceae) and
other disputed cases. – Plant Syst. Evol. 172: 215-227.
Lo EYY, Duke NC, Sun M. 2014. Phylogeographic
pattern of Rhizophora (Rhizophoraceae) reveals the
importance of both vicariance and long-distance oceanic dispersal to modern
mangrove distribution. – BMC Evol. Biol. 2014, 14:83.
Lobreau D. 1967. Contribution à l’étude
du pollen des Malpighiaceae
d’Afrique. – Pollen Spores 9: 241-277.
Lobreau D. 1968. Le pollen des Malpighiacées
d’Afrique et de Madagascar. – Bull. Inst. Fond. Afr. Noire, sér. A, Sci.
Nat. 30: 59-83.
Lobreau D. 1969. Les limites de
l’”ordre” des Célastrales d’après le pollen. – Pollen Spores 11:
499-555.
Lobreau-Callen D. 1983. Analyse de la
répartition géographique des Malpighiaceae d’après les
caractères du pollen et de la pollinisation. – Bothalia 14: 871-881.
Lobreau-Callen D. 1984. Pollen et
paléobotanique des Malpighiaceae. – Rev.
Paleobiol., Spec. Vol., pp. 131-138.
Lobreau-Callen D, Suarez-Cervera M. 1989. Le
pollen de Martretia Beille (Euphorbiacée africaine). – Bull. Jard.
Bot. Natl. Belg. 59: 333-349.
Lobreau-Callen D, Suarez-Cervera M. 1994.
Pollen ultrastructure of Hymenocardia Wallich ex Lindley and
comparison with other Euphorbiaceae. – Rev. Paleobot.
Palyn. 81: 257-278.
Lobreau-Callen D, LeThomas A, Suarez-Cuervera
M. 1998. Caractères ultrastructuraux du pollen de quelques Podostémales.
Affinités avec les Rosidae évoluées. – Comt. Rend. Acad. Sci. Paris, sér.
III, 321: 335-345.
Lobreau-Callen D, Malécot V, Suarez-Cervera
M. 2000. Comparative study of pollen from apetalous Crotonoideae and some other
uniovulate Euphorbiaceae: exine
ultrastructure at the aperture. – In: Harley MM, Morton CM, Blackmore S
(eds), Pollen and spores: morphology and biology, Royal Botanic Gardens, Kew,
pp. 301-324.
Loder JW, Russell GB. 1966.
(+)-tropine-1,2-dithiolane-3-carboxylate, a new alkaloid from Bruguiera
sexangula. – Tetr. Letters 6327-6329.
Loder JW, Russell GB. 1969. Tumour inhibitory
plants. The alkaloids of Bruguiera sexangula and Bruguiera
exaristata (Rhizophoraceae). – Aust. J.
Chem. 22: 1271-1275.
Loesener T. 1942. Celastraceae. – In:
Engler A (†), Harms H, Mattfeld J (eds), Die natürlichen Pflanzenfamilien,
2. Aufl., Bd. 20b, W. Engelmann, Leipzig, pp. 87-197.
Lombello RA, Forni-Martins ER. 2002.
Cytogenetics of twelve species of Malpighiaceae A. Juss. from
southeastern Brazil. – Caryologia 55: 241-250.
Lombello RA, Forni-Martins ER. 2003. Malpighiaceae: correlations
between habit, fruit type and basic chromosome number. – Acta Bot. Brasil.
17: 171-178.
Lopes AV, Machado IC. 1998. Floral biology
and reproductive ecology of Clusia nemorosa (Clusiaceae) in northeastern Brazil.
– Plant Syst. Evol. 213: 71-90.
Lorente MA. 1986. Palynology and palynofacies
of the upper Tertiary in Venezuela. – Diss. Bot. 99: 1-222.
Lotocka B, Osinska E. 2010. Shoot anatomy and
secretory structures in Hypericum species (Hypericaceae). – Bot. J. Linn.
Soc. 163: 70-86.
Lourteig A, O’Donnell CA. 1941. Tragiae
Argentinae (Euphorbiaceae). –
Lilloa 6: 347-380.
Lowrie SR. 1982. The palynology of the Malpighiaceae and its contribution
to family systematics. – Ph.D. diss., University of Michigan, Ann Arbor,
Michigan.
Lü H-F, Hu Z-H. 2001. Comparative anatomy of
secretory structures of leaves in Hypericum. – Acta Phytotaxon. Sin.
39: 393-404. [In Chinese]
Lü H-F, Chu Q-G, Hu Z-H. 2001. Comparative
study on the epidermal micromorphology of Hypericum and
Triadenum. – Acta Bot. Bor.-Occ. Sin. 21: 693-699. [In Chinese]
Lundell CL. 1945. The genus Garcia
Vahl, a potential source of superior hard quick-drying oil. – Wrightia 1:
1-12.
Lundell CL. 1985. Goupia
guatemalensis (Celastraceae), a genus
and species new to Mesoamerica. – Phytologia 57: 238-239.
Luo S, Zhang D, Renner SS. 2007. Duodichogamy
and androdioecy in the Chinese Phyllanthaceae Bridelia
tomentosa. – Amer. J. Bot. 94: 260-265.
Lüttge U. 1999. One morphotype, three
physiotypes: sympatric species of Clusia with obligate C3
photosynthesis, obligate CAM, and C3-CAM intermediate behavior. – Plant Biol.
1: 138-148.
Lüttge U. 2002. The genus Clusia
L.: molecular evidence for independent evolution of photosynthetic flexibility.
– Plant Biol. 4: 86-93.
Lüttge U (ed). 2007. Clusia: a
woody neotropical genus of remarkable plasticity and diversity. – Ecol. Stud.
194, Springer, Berlin.
Machado SR, Paleari LM, Paiva ÉAS, Rodrigues
TM. 2015. Colleters on the inflorescence axis of Croton glandulosus
(Euphorbiaceae): structural and
functional characterization. – Intern. J. Plant Sci. 176: 86-93.
McCann LP. 1945. Embryology of the tung tree.
– J. Agric. Res. 71: 215-229.
McCusker A. 1984. Rhizophoraceae. – In: George AS
(ed), Flora of Australia 22, Australian Government Publ. Service, Canberra, pp.
1-10.
McDill JR, Simpson BB. 2011. Molecular
phylogenetics of Linaceae with
complete generic sampling and data from two plastid genes. – Bot. J. Linn.
Soc. 165: 64-83.
McDill JR, Repplinger M, Simpson BB, Kadereit
JW. 2009. The phylogeny of Linum and Linaceae subfamily Linoideae, with
implications for their systematics, biogeography, and evolution of heterostyly.
– Syst. Bot. 34: 386-405.
MacDougal JM. 1994. Revision of
Passiflora subgenus Decaloba section Pseudodysosmia
(Passifloraceae). – Syst.
Bot. Monogr. 41: 1-146.
McKee AC, Covington CD, Fuller RW, Bokesch
HR, Young S, Cardellina JH II, Kadushin MR, Soejarto DD, Stevens PF, Cragg GM,
Boyd MR. 1996. Pyranocoumarins from tropical species of the genus
Calophyllum: a chemotaxonomic study of extracts in the National Cancer
Institute collection. – J. Nat. Prod. 61: 1252-1256.
McLaughlin Decker J. 1966. Wood anatomy and
phylogeny of Luxemburgieae (Ochnaceae). – Phytomorphology 16:
39-55.
McLaughlin Decker J. 1967. Petiole
vascularization of Luxemburgieae (Ochnaceae). – Amer. J. Bot. 54:
1175-1181.
McPherson G. 1985. Scagea, a new
genus of Euphorbiaceae from New
Caledonia. – Bull. Mus. Natl. Hist. Nat., sér. IV, sect. B, Adansonia 7:
247-250.
McPherson G. 2000. Drypetes (Euphorbiaceae) in Madagascar and
the Comoro Islands. – Adansonia, sér. III, 22: 205-209.
McPherson G. 2017. Six new species of
Argomuellera (Euphorbiaceae) from Madagascar.
– Novon 25: 286-297.
McPherson G, Tirel C. 1987. Euphorbiacées I.
Euphorbioideae, Crotonoideae, Acalyphoideae, Oldfieldioideae. – In: Morat P,
Mackee HS (eds), Flore de la Nouvelle-Calédonie et Dépendances 14, Muséum
National d’Histoire Naturelle, Paris.
McVaugh R. 1944. The genus
Cnidoscolus: generic limits and intrageneric groups. – Bull. Torrey
Bot. Club 71: 457-474.
McVaugh R. 1945. The genus Jatropha
in America: principal intergeneric groups. – Bull. Torrey Bot. Club 72:
271-294.
McVaugh R. 1961. Euphorbiaceae novae
Novo-Galicianae. – Brittonia 13: 145-205.
Madulid DA, Buot IE, Agoo EMG. 2007.
Rafflesia panchoana (Rafflesiaceae), a new species from
Luzon Island, Philippines. – Acta Manilana 55: 43-47.
Madulid DA, Tandang DN, Agoo EMG. 2005.
Rafflesia magnifica (Rafflesiaceae), a new species from
Mindanao, Philippines. – Acta Manilana 53: 1-6.
Magnus W. 1913. Die atypische
Embryosackentwicklung der Podostemaceen. – Flora 105: 275-336.
Maguire B. 1972. The botany of Guyana
Highland IX. Bonnetiaceae. –
Mem. New York Bot. Gard. 23: 131-165.
Maguire B. 1976. Apomixis in the genus
Clusia (Clusiaceae) – a
preliminary report. – Taxon 25: 241-244.
Maguire B. 1977a. A revision of
Clusia L. section Cochlanthera (Choisy) Engler. – Caldasia
11: 129-146.
Maguire B. 1977b. Notes on the Clusiaceae – chiefly of Panama I.
– Phytologia 36: 391-407.
Maguire B. 1977c. Notes on the Clusiaceae – chiefly of Panama II.
– Phytologia 38: 203-214.
Maguire B, Steyermark J. 1989. Botany of
Guayana Highland XIII. Ouratea (Ochnaceae) in Guayana and adjacent
Amazonian hylea. – Mem. New York Bot. Gard. 51: 56-102.
Maheshwari JK. 1964. Taxonomic studies on
Indian Guttiferae III. The genus Garcinia L. – Bull. Bot. Surv.
India 6: 107-135.
Maheshwari P. 1942. The embryo-sac of
Euphorbia heterophylla L. – a reinvestigation. – Proc. Indian
Acad. Sci., Sect. B, 15: 158-166.
Maheshwari P. 1945. The place of angiosperm
embryology in research and teaching. – J. Indian Bot. Soc. 24: 25-41.
Mahlberg PG. 1975. Evolution of the laticifer
in Euphorbia as interpreted from starch grain morphology. – Amer. J.
Bot. 62: 577-583.
Mahlberg PG, Sabharwal PS. 1968. Origin and
early development of non-articulated laticifers in embryos of Euphorbia
marginata. – Amer. J. Bot. 55: 375-381.
Mahlberg PG, Davis DG, Galitz DS, Manners GD.
1987. Laticifers and the classification of Euphorbia: the
chemotaxonomy of Euphorbia esula L. – Bot. J. Linn. Soc. 94:
165-180.
Maiquetía M, Cáceres A, Herrera A. 2009.
Mycorrhization and phosphorus nutrition affect water relations and CAM
induction by drought in seedlings of Clusia minor. – Ann. Bot. 103:
525-532.
Mahlberg PG, Pleszczynska J, Furr M. 1988.
Latex triterpenoid profiles of normal and cristata taxa of Euphorbia
and their application as chemotaxonomic characters. – Monogr. Syst. Bot.
Missouri Bot. Gard. 25: 623-629.
Malé PJG, Bardon L, Besnard G, Coissac E,
Delsuc F, Engel J, Lhuillier E, Scotti-Saintagne C, Tinaut A, Chave J. 2014.
Genome skimming by shotgun sequencing helps resolve the phylogeny of a
pantropical tree family. – Mol. Ecol. Res. 14: 966-976.
Mamede MCH, Mayo SJ. 1992. A cladistic
analysis of the genus Camarea (Malpighiaceae). – Kew Bull. 47:
491-501.
Manchester SR, Hottenrott M. 2009.
Large-fruited Salicaceae s.l. from
the Miocene tuff of the Eichelskopf, northern Hessen, Germany. – Feddes
Repert. 120: 373-378.
Manchester SR, Dilcher DL, Tidwell WD. 1986.
Interconnected reproductive and vegetative remains of Populus (Salicaceae) from the Middle Eocene
Green River Formation, northeastern Utah. – Amer. J. Bot. 73: 156-160.
Marcano-Berti L. 1989. Euphroniaceae: una nueva familia.
– Pittieria 18: 15-19.
Marco HF. 1935. Systematic anatomy of the
woods of the Rhizophoraceae.
– Trop. Woods 44: 1-20.
Marcussen T. 2003a. A new violet species (Violaceae) from the South-West Alps.
– Bot. J. Linn. Soc. 142: 119-123.
Marcussen T. 2003b. Evolution,
phylogeography, and taxonomy within the Viola alba complex (Violaceae). – Plant Syst. Evol. 237:
51-74. – Erratum: 239: 169.
Marcussen T, Borgen L. 2000. Allozymic
variation and relationships within Viola subsection Viola (Violaceae). – Plant Syst. Evol. 223:
29-57.
Marcussen T, Borgen L, Nordal I. 2001.
Viola hirta (Violaceae)
and its relatives in Norway. – Nord. J. Bot. 21: 5-17.
Marcussen T, Borgen L, Nordal I. 2005. New
distributional and molecular information call into question the systematic
position of the West Asian Viola sintenisii (Violaceae). – Bot. J. Linn. Soc.
147: 91-98.
Marcussen T, Jakobsen KS, Danihelka J,
Ballard HE, Blaxland K, Brysting AK, Oxelman B. 2012. Inferring species
networks from gene trees in high-polyploid North American and Hawaiian violets
(Viola, Violaceae). –
Syst. Biol. 61: 107-126.
Marinho LC, Fiaschi P, Amorim AM, Santos F de
AR dos. 2015. The taxonomic significance of pollen morphology in
Tovomita Aubl. (Clusiaceae: Clusieae) and related
genera. – Plant Syst. Evol. 301: 1759-1766.
Mark PJL, Wortley AH, Furness CA. 2012. Not a
shrinking violet: pollen morphology of Violaceae (Malpighiales). – Grana 51:
181-193.
Martin HA. 1974. The identification of some
Tertiary pollen belonging to the family Euphorbiaceae. – Aust. J. Bot.
22: 271-291.
Martinez C. 2017. Passifloraceae seeds from the
late Eocene of Colombia. – Amer. J. Bot. 104: 1857-1866.
Martínez MG, Espinosa SM. 2005. Tricomas
foliares de Croton sección Barhamia (Euphorbiaceae). – Acta Bot. Mex.
72: 39-51.
Martzenitzina KK. 1927. The chromosomes of
some species of the genus Linum L. – Bull. Appl. Bot. Genet. Plant
Breeding 17: 253-264.
Masters MT. 1871. Contributions to the
natural history of the Passifloraceae. – Trans. Linn.
Soc. London 27: 593-645.
Matamoro-Vidal A, Furness CA, Gouyun P-H,
Wurdack KJ, Albert B. 2012. Evolutionary stasis in Euphorbiaceae pollen: selection
and constraints. – J. Evol. Biol. 25: 1077-1096.
Mathew CJ, Satheesh VK. 1997. Taxonomy and
distribution of the Podostemaceae in Kerala, India.
– Aquatic Bot. 57: 243-274.
Mathew CJ, Jäger-Zürn I, Nileena CB. 2001.
Dalzellia gracilis: a new species of Podostemaceae (Tristichoideae)
from Kerala, India. – Intern. J. Plant Sci. 162: 899-909.
Mathew CJ, Nileena CB, Jäger-Zürn I. 2003.
Morphology and ecology of two new species of Polypleurum (Podostemaceae) from Kerala, India.
– Plant Syst. Evol. 237: 209-217.
Mathis C, Ourisson G. 1963. Étude
chimiotaxonomique du genre Hypericum 1. Répartition de
l’Hypéricine. – Phytochemistry 2: 157-171.
Matos Araujo PA de, Mattos Filho A de. 1984.
Estrutura das madeiras brasileiras de Dicotiledôneas XXVI. Euphorbiaceae. – Rodriguésia
36: 25-40.
Matt Salleh K, Latiff A. 1988. A new species
of Rafflesia and notes on other species from Trus Madi Range, Sabah
(Borneo). – Blumea 34: 111-116.
Matt Salleh K, Latiff A. 1995. On the
morphology of the female flower of Rafflesia tengku-adlinii and notes
on the status of R. borneensis (Rafflesiaceae). – Flora Males.
Bull. 11: 425-428.
Matthews ML, Endress PK. 2008. Comparative
floral structure and systematics in Chrysobalanaceae s.l. (Chrysobalanaceae, Dichapetalaceae, Euphroniaceae, Trigoniaceae; Malpighiales). – Bot. J. Linn.
Soc. 157: 249-309.
Matthews ML, Endress PK. 2011. Comparative
floral structure and systematics in Rhizophoraceae, Erythroxylaceae, and the
potentially related Ctenolophonaceae, Linaceae, Irvingiaceae, and Caryocaraceae (Malpighiales). – Bot. J. Linn.
Soc. 166: 331-416.
Matthews ML, Endress PK. 2013. Comparative
floral structure and systematics of the clade of Lophopyxidaceae and Putranjivaceae (Malpighialaes).
– Bot. J. Linn. Soc. 172: 404-448.
Matthews ML, Amaral M do, Carmo E, Endress
PK. 2012. Comparative floral structure and systematics in Ochnaceae s.l. (Ochnaceae, Quiinaceae and Medusagynaceae; Malpighiales). – Bot. J. Linn.
Soc. 170: 299-392.
Matthiesen F. 1908. Beiträge zur Kenntnis
der Podostemaceen. – Bibl. Bot. 1908: 1-55.
Mauritzon J. 1933a. Über die systematische
Stellung der Familien Hydrostachyaceae
und Podostemonaceae. – Bot. Not. 1933: 172-180.
Mauritzon J. 1933b. Über die Embryologie der
Turneraceae und Frankeniaceae.
– Bot. Not. 86: 543-554.
Mauritzon J. 1934. Etwas über die
Embryologie der Zygophyllaceen sowie einige Fragmente über die der
Humiriaceen. – Bot. Not. 87: 407-422.
Mauritzon J. 1936. Zur Embryologie einiger
Parietales-Familien. – Svensk Bot. Tidskr. 30: 79-113.
Mayfield M. 1991. Euphorbia
johnstonii (Euphorbiaceae), a new species from
Tamaulipas, Mexico with notes on Euphorbia subsection Acutae.
– Sida 14: 573-579.
Mayfield M. 1997. A systematic treatment of
Euphorbia subgenus Poinsettia (Euphorbiaceae). – Ph.D. diss.,
University of Texas, Austin, Texas.
Mazer SJ, Tiffney BH. 1982. Fruits of
Wetherellia and Palaeowetherellia (?Euphorbiaceae) from Eocene
sediments in Virginia and Maryland. – Brittonia 34: 300-333.
Mbing JN, Bassomo MY, Pegnyemb DE, Tih RG,
Sondemgam BL, Blond A, Bodo B. 2003. Constituents of Ouratea flava.
– Biochem. Syst. Ecol. 31: 215-217.
Medeiros D, Senna Valle L de, Valka Alves RJ.
2013. Revalidation of the genera Bia and Zuckertia (Euphorbiaceae) with B.
capivarensis sp. nov. from Serra da Capivara, Brazil. – Nord. J. Bot.
31: 595-602.
Meeuse ADJ. 1975. Taxonomic relationships of
Salicaceae and Flacourtiaceae:
their bearing on interpretative floral morphology and dilleniid phylogeny. –
Acta Bot. Neerl. 24: 437-457.
Meeuse ADJ. 1990. The Euphorbiaceae auct. plur.: an
unnatural taxon. – Eburon, Delft, The Netherlands.
Meijer W. 1958. A contribution to the
taxonomy and biology of Rafflesia arnoldii in West Sumatra. – Ann.
Bogor. 3: 33-44.
Meijer W. 1983. Rafflesia hasseltii.
– Malay. Nat. J., 36: suppl. 22-27.
Meijer W. 1984. New species of
Rafflesia (Rafflesiaceae). – Blumea 30:
209-215.
Meijer W. 1990. Species diversity and
conservation status of Rafflesia in Malesia. – Malay. Nat. J. 45:
213-218.
Meijer W. 1993. Rafflesiaceae. – In: Kubitzki K,
Rohwer JG, Bittrich V (eds), The families and genera of vascular plants II.
Flowering plants. Dicotyledons. Magnoliid, hamamelid and caryophyllid families,
Springer, Berlin, Heidelberg, New York, pp. 557-563.
Meijer W. 1996. Rafflesia
gadutensis, eine in Sumatra endemische Art der tropisch-subtropischen
Parasitenfamilie Rafflesiaceae.
– Palmengarten 60: 38-41.
Meijer W. 1997. Rafflesiaceae. – In: Kalkman C,
Kirkup DW, Nooteboom HP, Stevens PF, de Wilde WJJO (eds), Flora Malesiana, I,
13, Flora Malesiana Foundation, Rijksherbarium/Hortus Botanicus, Leiden, pp.
1-42.
Meijer W, Elliot S. 1990. Taxonomy, ecology
and conservation of Rafflesia kerrii Meijer in southern Thailand. –
Nat. Hist. Bull. Siam Soc. 38: 117-133.
Meijer W, Veldkamp JF. 1988. A revision of
Rhizanthes (Rafflesiaceae). – Blumea 33:
327-342.
Meijer W, Wong, M. 1993. Rafflesia
cantleyi and R. hasseltii compared. – Malay. Nat. J. 47:
10-12.
Meikle RD. 1984. Willows and poplars of Great
Britain and Ireland. – Botanical Society of the British Isles, London.
Melchior H. 1925a. Die phylogenetische
Entwicklung der Violaceen und die natürlichen Verwandtschaftsverhältnisse
ihrer Gattungen. – Feddes Repert. Beih. 36: 83-125.
Melchior H. 1925b. Theaceae. – In:
Engler A, Gilg E (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 21, W.
Engelmann, Leipzig, pp. 109-154.
Melchior H. 1925c. Violaceae. – In: Engler A, Gilg E
(ed), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 21, W. Engelmann,
Leipzig, pp. 329-377.
Melchior H. 1930. Decaphalangium,
eine neue Gattung der Guttiferen aus Peru. – Notizbl. Bot. Gart. Mus.
Berlin-Dahlem 10: 946-950.
Melhem TS, Moura CAF, Lieu J. 1971. Pollen
grains of plants of the “Cerrado” – Styracaceae and Turneraceae. – Hoehnea 1:
153-178.
Melikian AP, Dildarian BI. 1977. Comparative
anatomical and palynological study of representatives of Elatinaceae family. –
Biologicheskii Žurnal Armenii 30: 44-49.
Melo AL. 2006. Revisão de
Sebastiania Spreng. sensu stricto (Euphorbiaceae – Hippomaneae).
– Ph.D. diss., Universidade Federal Rural de Pernambuco, Recife, Brazil.
Melo NF de, Guerra M. 2003. Variability of
the 5S and 45S rDNA sites in Passiflora L. species with distinct base
chromosome numbers. – Ann. Bot. (Oxford) 92: 309-316.
Melo NF de, Cervi AC, Guerra M. 2001.
Karyology and cytotaxonomy of the genus Passiflora L. (Passifloraceae). – Plant Syst.
Evol. 226: 69-84.
Mennega AMW. 1984. Wood structure of
Jablonskia congesta (Euphorbiaceae). – Syst. Bot. 9:
236-239.
Mennega AMW. 1987. Wood anatomy of the Euphorbiaceae, in particular of
the subfamily Phyllanthoideae. – Bot. J. Linn. Soc. 94: 111-126.
Mennega AMW. 2005. Wood anatomy of the
subfamily Euphorbioideae. A comparison with subfamilies Crotonoideae and
Acalyphoideae and the implications for the circumscription of the Euphorbiaceae. – IAWA J. 26:
1-68.
Merino Sutter D, Endress PK. 1995. Aspects of
gynoecial structure and macrosystematics in Euphorbiaceae. – Bot. Jahrb.
Syst. 116: 517-536.
Merino Sutter D, Endress PK. 2003. Structure
of the female flowers and cupules in Balanopaceae, an enigmatic rosid
family. – Ann. Bot. 92: 459-469.
Merino Sutter D, Forster PI, Endress PK.
2006. Female flowers and systematic position of Picrodendraceae (Euphorbiaceae s.l., Malpighiales). – Plant Syst.
Evol. 261: 187-215.
Merrill ED. 1912. Notes on Philippine Euphorbiaceae. – Philipp. J.
Sci. 7: 379-410.
Merrill ED. 1920. Notes on Philippine Euphorbiaceae III. – Philipp. J.
Sci. 16: 539-579.
Merxmüller H, Lippert W. 1977.
Veilchenstudien V-VII. – Mitt. Bot. Staatssamml. München 13: 503-534.
Meseguer AS, Aldasoro JJ, Sanmartín I. 2013.
Bayesian inference of phylogeny, morphology and range evolution revels a
complex evolutionary history in St. John’s wort (Hypericum). –
Mol. Phylogen. Evol. 67: 379-403.
Mesquita R de CG, Franciscon CH. 1995. Flower
visitors of Clusia nemorosa G. F. W. Meyer (Clusiaceae) in an Amazonian
white-sand campina. – Biotropica 27: 254-257.
Metcalfe CR. 1956. Scyphostegia
borneensis Stapf, anatomy of stem och leaf in relation to the taxonomic
position. – Reinwardtia 4: 99-104.
Mezzonato-Pires AC, Milward-de-Azevedo MA,
Mendonça CBF, Gonçalves-Esteves V. 2015. Pollen morphology and detailed
sexine of Passiflora subgenus Astrophea (Passifloraceae). – Plant Syst.
Evol. 301: 2189-2202.
Michaelis P. 1924. Blütenmorphologische
Untersuchungen an den Euphorbiaceen, unter besonderer Berücksichtigung der
Phylogenie der Angiospermenblüte. – Goebel Bot. Abhandl. (Jena) 3: 1-150.
Milanez FR. 1935. Anatomie de
Paradrypetes ilicifolia. – Arq. Inst. Biol. Veg. 2: 133-156.
Mildbraed J. 1931. Pandaceae. – In: Engler A (†),
Harms H, Pax F (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 19a, W.
Engelmann, Leipzig, pp. 1-3.
Mildner RA, Rogers CM. 1978. Revision of the
native South American species of Linum (Linaceae). – Phytologia 39:
343-390.
Miller KI, Webster GL. 1962. Systematic
position of Cnidoscolus and Jatropha. – Brittonia 14:
174-180.
Miller KI, Webster GL. 1966. Chromosome
numbers in the Euphorbiaceae.
– Brittonia 18: 372-377.
Miller KI, Webster GL. 1967. A preliminary
revision of Tragia (Euphorbiaceae) in the United
States. – Rhodora 69: 241-305.
Miller RB. 1975. Systematic anatomy of the
xylem and comments on the relationships of Flacourtiaceae. – J. Arnold Arbor.
56: 20-102.
Millspaugh CF. 1889. Contributions to North
American Euphorbiaceae. –
Proc. Calif. Acad. Sci., Ser. II, 2: 217-230.
Millspaugh CF. 1913. The genera
Pedilanthus and Cubanthus, and other American Euphorbiaceae. – Publ. Field
Mus. Nat. Hist., Bot. Ser. 2: 353-377.
Millspaugh CF. 1914. Contributions to North
American Euphorbiaceae V. –
Publ. Field Columbian Mus. Nat. Hist., Bot. Ser. 2: 383-397.
Millspaugh CF. 1916. Contributions to North
American Euphorbiaceae VI. –
Publ. Field Columbian Mus. Nat. Hist., Bot. Ser. 2: 401-420.
Millspaugh CF. 1917. Trichosterigma
benedictum. – Addisonia 2: 3-4.
Milne R. 1994. New species of, and notes on,
Bornean Trigonostemon, Cleistanthus & Macaranga
(Euphorbiaceae). – Kew Bull.
49: 445-454.
Milne R. 1995. Notes on Bornean and other
West Malesian Trigonostemon (Euphorbiaceae). – Kew Bull. 50:
25-49.
Milne-Redhead E. 1953. Hypericaceae. – In: Turrill WB,
Milne-Redhead E (eds), Flora of tropical East Africa, Crown Agents for the
Colonies, London, pp. 1-23.
Milne-Redhead E. 1957. Heywoodia
lucens Sim. – a tree new to tropical Africa. – Bull. Jard. Bot. État.
Bruxelles 27: 327-333.
Milward-de-Azevedo MA, Baumgratz JFA,
Gonçalves-Esteves V. 2012. A taxonomic revision of Passiflora
subgenus Decaloba (Passifloraceae) in Brazil. –
Phytotaxa 53: 1-68.
Mitchell AD, Heenan PB, Murray BG, Molloy
BPJ, Lange PJ de. 2009. Evolution of the south-western Pacific genus
Melicytus (Violaceae):
evidence from DNA sequence data, cytology and sex expression. – Aust. Syst.
Bot. 22: 143-157.
Miyama M, Simizu H, Sugiyama M, Hanagata N.
2006. Sequencing and analysis of 14,842 expressed sequence tags of burma
mangrove, Bruguiera gymnorrhiza. – Plant Sci. 171: 234-241.
Miyoshi N, Kato H. 1982. Pollen morphology by
means of scanning electron microscope 5. Angiospermae (Piperales, Podostemonales).
– Jap. J. Palynol. 28: 7-11.
Mkpong OE, Yan H, Chism G, Sayre RT. 1990.
Purification, characterization and localization of linamarase in Cassava. –
Plant Physiol. (Lancaster) 93: 176-181.
Modilewski J. 1910. Weitere Beiträge zur
Embryobildung einiger Euphorbiaceen. – Ber. Deutsch. Bot. Ges. 28:
413-418.
Modilewski J. 1911. Über die abnormale
Embryosackentwicklung bei Euphorbia palustris L. und anderen
Euphorbiacen. – Ber. Deutsch. Bot. Ges. 29: 430-436.
Mohan Ram HY, Sehgal A. 1992. Podostemaceae – the strange
family of aquatic angiosperms. – Palaeobotanist 41: 192-197.
Mohan Ram HY, Sehgal A. 1997. In vitro
studies on developmental morphology of Indian Podostemaceae. – Aquatic Bot.
57: 97-132.
Mohan Ram HY, Sehgal A. 2001. Biology of
Indian Podostemaceae. – In:
Rangaswamy NS (ed), Phytomorphology (Recent trends in plant sciences) Golden
Jubilee Issue 2001, International Society of Plant Morphologists, Delhi, pp.
365-391.
Molau U. 1983. 130. Elatinaceae. – In: Harling G,
Sparre B (eds), Flora of Ecuador 20, Swedish Natural Science Research Council,
Stockholm, pp. 19-23.
Molero J, Rovira AM. 1998. A note on the
taxonomy of the Macaronesian Euphorbia obtusifolia complex (Euphorbiaceae). – Taxon 47:
321-332.
Molero J, Garnatje T, Rovira A, Garcia-Jacas
N, Susanna A. 2002. Karyological evolution and molecular phylogeny in
Macaronesian dendroid spurges (Euphorbia subsect.
Pachycladae). – Plant Syst. Evol. 231: 109-132.
Moline P, Les D, Philbrick CD, Novelo AR,
Pfeifer E,Rutishauser R. 2006. Comparative morphology and molecular systematics
of Podostemum (including Crenias) – American river-weeds
(Podostemonaceae). – Bot. Jahrb. Syst. 126: 427-476.
Moline P, Thiv M, Ameka GK, Chogue J-P,
Pfeifer E, Rutishauser R. 2007. Comparative morphology and molecular
systematics of African Podostemaceae-Podostemoideae, with
emphasis on Dicraeanthus and Ledermanniella from Cameroon.
– Intern. J. Plant Sci. 168: 159-180.
Moore DM, Harvey MJ. 1961. Cytogenetic
relationship of Viola lactea Sm. and other West European arosulate
violets. – New Phytol. 60: 85-95.
Morawetz W. 1981. Zur systematischen Stellung
der Gattung Prockia: Karyologie und Epidermisstruktur im Vergleich zu
Flacourtia (Flacourtiaceae), Grewia (Tiliaceae) und
verwandten Gattungen. – Plant Syst. Evol. 139: 57-76.
Morton CV. 1944. Taxonomic studies of
tropical American plants: the genus Hybanthus in continental North
America. – Contr. U.S. Natl. Herb. 29: 74-82.
Morton CV. 1968. A typification of some
subfamily, sectional, and subsectional names in the family Malpighiaceae. – Taxon 17:
314-324.
Mosquin T. 1971. Biosystematic studies on
North American species of Linum, section Adenolinum (Linaceae). – Can. J. Bot. 49:
1379-1388.
Mosseler A. 1990. Hybrid performance and
species crossability relationships in willows (Salix). – Can. J.
Bot. 68: 2329-2338.
Motta LB, Salatino A, Salatino MLF. 2009.
Foliar cuticular alkanes of Camarea (Malpighiaceae) and their taxonomic
significance. – Biochem. Syst. Ecol. 37: 35-39.
Motta LB, Furlan CM, Salatino A, Salatino
MLF. 2009. Flavonoids and the taxonomy of Camarea (Malpighiaceae). – Biochem. Syst.
Ecol. 37: 201-205.
Mourão KSM, Beltrati CM. 1996a. Morfologia
dos frutos, sementes e plântulas de Platonia insignis Mart. (Clusiaceae) I. Aspectos anatômicos
dos frutos e sementes em desenvolvimento. – Acta Amazonia 25: 11-31.
Mourão KSM, Beltrati CM. 1996b. Morfologia
dos frutos, sementes e plântulas de Platonia insignis Mart. (Clusiaceae) II. Morfo-anatomia dos
frutos e sementes maduros. – Acta Amazonia 25: 33-46.
Mourão KSM, Beltrati CM. 2000. Morphology
and anatomy of developing fruits and seeds of Mammea americana L. (Clusiaceae). – Revista Brasil.
Biol. 60: 701-711.
Mourão KSM, Beltrati CM. 2001. Morphology
and anatomy of developing fruits and seeds of Vismia guianensis
(Aubl.) Choisy (Clusiaceae). –
Rev. Brasil. Biol. 61: 147-158.
Muir AD, Westcott ND (eds). 2003. Flax: the
genus Linum. – Taylor and Francis, London.
Mukherjee PK. 1957. Studies in the embryology
of Euphorbia hypericifolia Linn. – Bull. Bot. Soc. Univ. Saugar 9:
7-18.
Mukherjee PK. 1958. The female gametophyte of
Acalypha malabarica Muell. with a brief discussion on the
Penaea type of embryo sac. – J. Indian Bot. Soc. 37: 504-508.
Mukherjee PK. 1962. Gametophytes of three Euphorbiaceae. – Bull. Bot.
Soc., Coll. Sci., Nagpur 3: 10-14.
Mukherjee PK. 1964. Further contribution to
the embryology of the genus Acalypha Linn. –Proc. Indian Natl. Acad.
Sci., Sect. B, 34: 129-141.
Mukherjee PK. 1965. Contribution to the
embryology of Euphorbia peltata Roxb. – Proc. Indian Natl. Acad.
Sci., Sect. B, 35: 327-337.
Mukherjee PK. 1968. Trends of specialization
in the family Euphorbiaceae 1.
Microsporangium and microsporogenesis. – Maharashtra Vidnyan Mandir Patrika
3: 10-16.
Mukherjee PK, Padhye MD. 1964. Contribution
to the embryology of the genus Phyllanthus Linn. – Proc. Indian
Natl. Acad. Sci., Sect. B, 3: 117-128.
Mukkada AJ. 1962. Some observations on the
embryology of Dicraea stylosa Wight. – In: Maheshwari P (ed), Plant
embryology. A symposium, New Delhi: C.S.I.R., pp. 139-145.
Mukkada AJ. 1964. An addition to the bisporic
embryo sacs – the Dicraea type. – New Phytol. 63: 289-292.
Mukkada AJ. 1969. Some aspects of the
morphology, embryology, and biology of Terniola zeylanica (Gardner)
Tulasne. – New Phytol. 68: 1145-1158.
Mukkada AJ, Chopra RN. 1973.
Post-fertilization development in Indotristicha ramosissima (Wight)
van Royen. – New Phytol. 72: 639-646.
Mulgura de Romero ME, Gutierrez de
Sanguinetti MM. 1989. Actualización taxonómica de Tragia (Euphorbiaceae) para Argentina y
regiones limítrofes. – Darwiniana 29: 77-138.
Muller J. 1969. Pollen morphological notes on
Ochnaceae. – Rev. Palaeobot.
Palynol. 9: 149-173.
Muller J, Caratini C. 1977. Pollen of
Rhizophora (Rhizophoraceae) as a guide
fossil. – Pollen Spores 19: 361-389.
Müller-Doblies U, Albert G, Müller-Doblies
D. 1975. Der Blütenstand von Euphorbia fulgens Karw. ex Klotzsch und
seine variablen Grössen. – Bot. Jahrb. Syst. 96: 290-323.
Müller-Stoll WR, Mädel-Angeliewa E. 1986.
Ein neues Guttiferenholz aus dem Tertiär von Java, Calophylloxylon
intermedium sp. nov. – Feddes Repert. 97: 225-233.
Munzinger JK. 2001. Two new species of
Agatea (Violaceae) endemic
to New Caledonia, with some taxonomic notes and a key to New Caledonian
species. – Bot. J. Linn. Soc. 137: 91-97.
Munzinger JK, Ballard HE Jr. 2003.
Hekkingia (Violaceae), a
new arborescent violet genus from French Guiana, with a key to genera in the
family. – Syst. Bot. 28: 345-351.
Murguía-Sánchez G, Novelo RA, Philbrick CT,
Márquez Guzmán GJ. 2001. Desarrollo de los verticilos sexuales de
Vanroyenella plumosa Novelo & Philbrick. – Acta Bot. Mexic. 57:
37-50.
Murguía-Sánchez G, Novelo RA, Philbrick CT,
Márquez-Guzmán GJ. 2002. Embryo sac development in Vanroyenella
plumosa, Podostemaceae.
– Aquatic Bot. 73: 201-210.
Muschner VC, Lorenz AP, Cervi AC, Bonatto SL,
Souza-Chies TT, Salzano FM, Freitas LB. 2003. A first molecular phylogenetic
analysis of Passiflora (Passifloraceae). – Amer. J.
Bot. 90: 1229-1238.
Muzik TJ. 1954. Development of fruit, seed,
embryo, and seedling of Hevea brasiliensis. – Amer. J. Bot. 41:
39-43.
Nadot S, Ballard HE Jr, Creach JB, Dajoz I.
2000. The evolution of pollen heteromorphism in Viola: a phylogenetic
approach. – Plant Syst. Evol. 223: 155-171.
Nagao S. 1941. Cytogenetics in the genus
Linum. – Jap. J. Genet. 17: 109-116.
Nagendran CR. 1974. Is the embryo sac of Podostemaceae bisporic? – Curr.
Sci. 43: 259-260.
Nagendran CR. 1975. Studies on Podostemaceae. – Ph.D. diss.,
University of Mysore, India.
Nagendran CR, Arekal GD. 1976. Embryo sac of
Griffithella hookeriana: a reinvestigation. – Phytomorphology 26:
359-363.
Nagendran CR, Subramanyam K, Arekal GD. 1976.
Development of the female gametophyte in Hydrobryum griffithii (Podostemaceae). – Ann. Bot., N.
S., 40: 511-513.
Nagendran CR, Arekal GD, Subramanyam K. 1977.
Embryo sac studies in three Indian species of Polypleurum (Podostemaceae). – Plant Syst.
Evol. 128: 215-226.
Nair N, Abraham V. 1962. Floral morphology of
a few species of Euphorbiaceae.
– Proc. Indian Acad. Sci., Sect. B, 56: 1-12.
Nair N, Abraham V. 1963. A contribution to
the morphology and embryology of Micrococca mercurialis Benth. – J.
Indian Bot. Soc. 42: 583-593.
Nair N, Abraham V. 1980. Floral morphology
and embryology of Tragia involucrata L. var. angustifolia
Hook. f. – In: Periasamy K (ed), Symposium on histochemistry, development and
structural anatomy of angiosperms, Autonomous P. G. Centre, University of
Madras, Tiruchirapalli, pp. 95-101.
Nair N, Maitreyi M. 1962. Morphology and
embryology of Sebastiania chamaelea. – Bot. Gaz. 124: 58-68.
Nais J. 2001. Rafflesia of the
world. – Sabah Parks and Natural History Publ., Kota Kinabalu, Sabah,
Malaysia.
Nakai T. 1920. Chosenia, a new genus
of Salicaceae. – Bot. Mag.
(Tokyo) 34: 66-69.
Narayana LL. 1960. Studies in Erythroxylaceae I. – Proc.
Indian Acad. Sci, Ser. B, 51: 270-275.
Narayana LL. 1964a. A contribution to the
floral anatomy and embryology of Linaceae. – J. Indian Bot. Soc. 43:
343-357.
Narayana LL. 1964b. Embryology of a few
species of Erythroxylum. – Curr. Sci. 33: 441-442.
Narayana LL. 1970a. Linaceae. – Bull. Indian Natl. Sci.
Acad. 41: 127-132.
Narayana LL. 1970b. Erythroxylaceae. – Bull.
Indian Natl. Sci. Acad. 41: 133-135.
Narayana LL. 1975. Contribution to the floral
anatomy and embryology of Ochnaceae. – J. Jap. Bot. 50:
329-336.
Narayana LL, Rao D. 1966. Floral morphology
of Linaceae. – J. Jap. Bot. 41:
1-10.
Narayana LL, Rao D. 1969-1977. Contributions
to the floral anatomy of Humiriaceae 1-6. – J. Jap. Bot.
44: 328-335 (1969), 48: 143-146, 242-276 (1973), 51: 12-15, 42-44 (1976), 52:
145-153 (1977).
Narayana LL, Rao D. 1969-1978. Contributions
to the floral anatomy of the Linaceae 1-14. – J. Jap. Bot. 44:
289-294 (1969), 48: 205-208 (1973), 51: 92-96, 349-352 (1976), 52: 56-59,
231-234, 315-317 (1977), 53: 12-14, 161-163, 213-218, 300-312 (1978);
Phytomorphology 21: 64-67 (1971 [1972]); Curr Sci. 43: 226-227, 391-393
(1974).
Narayana LL, Rao D. 1978. Systematic position
of Humiriaceae, Linaceae, and Erythroxylaceae in the light of
their comparative floral morphology and embryology: a discussion. – J. Indian
Bot. Soc. 57: 258-266.
Narayanaswami S, Sawhney S. 1959.
Microsporogenesis and embryo sac development in Casearia tomentosa
Roxb. – Phyton (Buenos Aires) 13: 133-144.
Nauenburg JD. 1988. Zur Karyologie und
Taxonomie der heimischen Schwermetallsippen der Gattung Viola, Sekt.
Melanium. – Decheniana 14: 96-102.
Nauenburg JD. 1990. Eine neue Viola
arvensis-Sippe aus Mitteleuropa (mit einem Bestimmungs-Schlüssel für die
Artengruppen Viola tricolor/V. lutea). – Bauhinia 9:
233-244.
Nazma BS, Vijendrarao R. 1981. Occurrence of
perforated ray cells in the wood of Drypetes roxburghii (Wall.)
Hurusawa. – IAWA Bull., N. S., 2: 384-421.
Ngulube MR, Hall JB, Maghembe JA. 1998.
Reproductive ecology of Uapaca kirkiana (Euphorbiaceae) in Malawi, southern
Africa. – J. Trop. Ecol. 14: 743-760.
Nicholls MS. 1985. The evolutionary breakdown
of distyly in Linum tenuifolium (Linaceae). – Plant Syst. Evol. 150:
291-302.
Nickrent DL, Duff RJ. 1996. Molecular studies
of parasitic plants using ribosomal RNA. – In: Moreno MT, Cubero JI, Berner
D, Joel D, Musselman LJ, Parker C (eds), Advances in parasitic plant re-search,
Cordoba, Spain: Junta de Andalucia, Direccion General de Investigacion Agraria,
pp. 28-52.
Nickrent DL, Duff RJ, Colwell AE, Wolfe AD,
Yung ND, Steinem KE, dePamphilis CW. 1997. Molecular phylogenetic and
evolutionary studies of parasitic plants. – In: Soltis D, Soltis P, Doyle J
(eds), Molecular systematics of plants 2: DNA sequencing, Chapter 8, pp.
211-241, Kluwer Academic, Boston.
Nickrent DL, Blarer A, Qiu Y-L, Vidal-Russel
V, Anderson FE. 2004. Phylogenetic inference in Rafflesiales: the influence of
rate heterogeneity and horizontal gene transfer. – BMC Evol. Biol. 4:
40-56.
Niedenzu F. 1890. Über eine neue Eintheilung
der Malpighiaceae. – Ber.
Deutsch. Bot. Ges. 8: 190-194.
Niedenzu F. 1895. Elatinaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien III(6), W. Engelmann,
Leipzig, pp. 277-283.
Niedenzu F. 1896. Malpighiaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien III(4), W. Engelmann,
Leipzig, pp. 41-74.
Niedenzu F. 1912a. Malpighiaceae americanae 1. –
Arbeiten aus dem botanischen Institut des Kgl. Lyceum hosianum in Braunsberg,
pp. 1-34.
Niedenzu F. 1912b. Malpighiaceae americanae 2. –
Verzeichnis der Vorlesungen am Königlichen Lyceum hosianum zu Braunsberg, pp.
1-62.
Niedenzu F. 1914. Malpighiaceae americanae 3. –
Arbeiten aus dem botanischen Institut des Kg. Lyceum hosianum in Braunsberg,
pp. 1-61.
Niedenzu F. 1915. Malpighiaceae paleotropicae 1. –
Arbeiten aus dem botanischen Institut der Kgl. Akademie (vorm. Kgl. Lyceum
hosianum) in Braunsberg, pp. 1-63.
Niedenzu F. 1918. Anatomie der Laubblätter
der amerikanischen Malpighiaceen. – Verzeichnis der Vorlesungen an der Kgl.
Akademie zu Braunsberg im Winter-Halbjahr, pp. 1-23.
Niedenzu F. 1921. Anatomie der Laubblätter
der paläotropischen Malpighiaceen. – Verzeichnis der Vorlesungen an der Kgl.
Akademie zu Brunsberg, pp. 1-10.
Niedenzu F. 1924. Malpighiaceae paleotropicae 2. –
Verzeichnis der Vorlesungen an der Akademie zu Braunsberg im Sommer 1924, pp.
1-19.
Niedenzu F. 1926. Malpighiaceae novae. – Arbeiten
aus dem botanischen Institut der Staatlichen Akademie (vorm. Kgl. Lyceum
hosianum) in Braunsberg, pp. 59-64.
Niedenzu F. 1925. Elatinaceae. – In: Engler A, Gilg
E (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 21, W. Engelmann,
Leipzig, pp. 270-276.
Nikitin VV. 1998. The system of the genus
Viola (Violaceae) of
eastern European and Caucasian flora. – Bot. Žurn. 83: 123-137. [In
Russian]
Nikolov LA, Davis CC. 2017. The big, the bad,
and the beautiful: biology of the world’s largest flowers. – J. Syst. Evol.
55: 516-524.
Nikolov LA, Staedler YM, Manickam S,
Schönenberger J, Endress PK. Kramer EM, Davis CC. 2014. Floral structure and
development in Rafflesiaceae
with emphasis on their exceptional gynoecia. – Amer. J. Bot. 101: 225-243.
Noa IC. 2008. Ouratea neuridesii (Ochnaceae), a new species from central
Cuba. – Willdenowia 38: 173-176.
Noack KL. 1939. Fortpflanzungsverhältnisse
und Bastarde von Hypericum perforatum L. – Zeitschr. Indukt.
Abstamm. Vererb. 76: 569-601.
Nogueira PC de L, Marsaioli AJ, Amaral M do
CE, Bittrich V. 1998. The fragrant oils of Tovomita species. –
Phytochemistry 49: 1009-1112.
Nooteboom HP. 1967. The taxonomic position of
Irvingioideae, Allantospermum Forman, and Cyrillopsis Kuhlm.
– Adansonia, sér. II, 7: 161-168.
Noro T, Suzuki H, Kanayama T. 1994a.
Phenology and distribution of Hydrobryum japonicum Imamura (Podostemaceae) in the Kaminkawa
River, Kagoshima, Japan. – J. Jap. Bot. 69: 162-166. [In Japanese]
Noro T, Suzuki H, Kanayama T. 1994b. Water
quality at the habitat of Hydrobryum japonicum Imamura (Podostemaceae) in Japan. – J.
Jap. Bot. 69: 167-175. [In Japanese]
Notis C. 2004. Phylogeny and character
evolution of Kielmeyeroideae (Clusiaceae) based on molecular and
morphological data. – M.Sc. thesis, University of Florida, Gainesville,
Florida.
Novelo RA, Philbrick CT. 1993.
Vanroyenella: a new genus of Podostemaceae from Jalisco,
Mexico. – Syst. Bot. 18: 64-67.
Novelo RA, Philbrick CT. 1995. A new species
of Oserya (Podostemaceae) from Jalisco,
Mexico. – Novon 5: 54-56.
Novelo RA, Philbrick CT. 1997a. Taxonomy of
Mexican Podostemaceae. –
Aquatic Bot. 57: 275-303.
Novelo RA, Philbrick CT. 1997b.
Podostemum ricciformi (Podostemaceae) rediscovered and
redescribed. – Taxon 46: 451-455.
Nowicke JW. 1984. A palynological study of
the Pandaceae. – Pollen Spores
26: 31-42.
Nowicke JW. 1994. A palynological study of
Crotonoideae (Euphorbiaceae).
– Ann. Missouri Bot. Gard. 81: 245-269.
Nowicke JW. 1994. A palynological study of
Crotonoideae (Euphorbiaceae).
– Ann. Missouri Bot. Gard. 81: 245-269.
Nowicke JW, Takahashi M. 2002. Pollen
morphology, exine structure and systematics of Acalyphoideae (Euphorbiaceae), part 4: tribes
Acalypheae pro parte (Erythrococca, Claoxylon,
Claoxylopsis, Mareya, Mareyopsis,
Discoclaoxylon, Micrococca, Amyrea,
Lobanilia, Mallotus, Deuteromallotus,
Cordemoya, Cococceras, Trewia, Neotrewia,
Rockinghamia, Octospermum, Acalypha,
Lasiococca, Spathiostemon, Homonoia), Plukenetieae
(Haematostemon, Astrococcus, Angostyles,
Romanoa, Eleutherostigma, Plukenetia,
Vigia, Cnesmone, Megistostigma,
Sphaerostylis, Tragiella, Platygyna,
Tragia, Acidoton, Pachystylidium,
Dalechampia), Omphaleae (Omphalea), and discussion and
summary of the complete subfamily. – Rev. Paleobot. Palynol. 121:
231–336.
Nowicke JW, Takahashi M, Webster GL. 1998.
Pollen morphology and the exine structure of Acalyphoideae (Euphorbiaceae), part 1. tribes
Clutieae (Clutia), Pogonophoreae (Pogonophora), Chaetocarpeae
(Chaetocarpus, Trigonopleura), Pereae (Pera),
Cheiloseae (Cheilosa), Diocoelieae (Diocoelia), Galearieae
(Galearia, Microdesmis, Panda) and Ampereeae
(Amperea, Monotaxis). – Rev. Palaeobot. Palyn. 102:
115-152.
Nowicke JW, Takahashi M, Webster GL. 1999.
Pollen morphology, exine structure and systematics of Acalyphoideae (Euphorbiaceae), part 2: tribes
Agrostistachydeae, Chrozophoreae, Caryodendreae, Bernardieae and Pycnocomeae.
– Rev. Palaeobot. Palyn. 105: 1-62.
Nozeran R. 1953. Sur quelques fleurs males
d’Euphorbiacées. – Rec. Trav. Lab. Bot. Univ. Montpellier Bot. 6:
99-114.
Nürk NM, Blattner FR. 2010. Cladistic
analysis of morphological characters in Hypericum (Hypericaceae). – Taxon 59:
1495-1507.
Nürk NM, Madriñán S, Carine MA, Chase MW,
Blattner FR. 2013. Molecular phylogenetics and morphological evolution of St.
John’s wort (Hypericum; Hypericaceae). – Mol. Phylogen.
Evol. 66: 1-16.
Nürk NM, Scheriau C, Madriñán S. 2013.
Explosive radiation in high Andean Hypericum – rates of
diversification among New World lineages. – Frontiers in Genetics 4: 17.
Obama C, Breteler FJ. 2004. A synopsis of
Dasylepis Oliv. (Achariaceae) with a description of a
new species from Lower Guinea. – Kew Bull. 59: 585-591.
Oberhäuser R, Kollmann R. 1977.
Cytochemische Charakterisierung des sogenannten Freien Nucleolus als
Proteinkörper in den Siebelementen von Passiflora coerulea. –
Zeitschr. Pflanzenphysiol. 84: 61-74.
Ockendon DJ. 1968. Biosystematic studies in
the Linum perenne group. – New Phytol. 67: 787-813.
Odinetz-Collart O, Tavares AS, Enriconi A.
2001. Response of Podostemaceae
aquatic biocenosis to environmental perturbations in central Amazonian
waterfalls. – Verh. Intl. Verein. Limnol. 27: 4063-4068.
O’Donell CA, Lourteig A. 1943. Malpighiaceae argentinae. –
Lilloa 9: 221-316.
Oh TJ, Gorman M, Cullis CA. 2000. RFLP and
RAPD mapping in flax (Linum usitatissimum). – Theor. Appl. Gen. 101:
590-593.
Okada H, Kato M. 2002. Pollination systems
inferred from pollen-ovule ratio of some species of Podostemaceae. – Acta
Phytotaxon. Geobot. 53: 51-61.
Okamoto M. 1984. A new species of
Viola collected in East Kalimantan, with notes of the seed dispersal
of the allied species concerned. – Bull. Osaka Mus. Nat. Hist. 37: 9-15.
Okamoto M. 1987. On the violet group subsect.
Serpentes in Southeast Asia. – Acta Phytotaxon. Geobot. 38: 113-122.
[In Japanese with English summary]
Okamoto M, Ueda K. 1986. A new species of
violet from Seram (Moluccas). – Acta Phytotaxon. Geobot. 37: 1-8.
Okamoto M, Okada H, Ueda K. 1993. Morphology
and chromosome number of Viola pilosa, and its systematic position.
– Taxon 42: 781-787.
Okuda T, Yoshida T, Shiola N, Nobuhara J.
1975. A new amino acid from seeds of Aleurites fordii. –
Phytochemistry 14: 2304-2305.
Olafsdottir ES, Jaroszewski JW, Arbo MM.
1990. Cyanohydrin glucosides of Turneraceae. – Biochem. Syst.
Ecol. 18: 435-438.
Olah LV. 1960. Cytological and morphological
investigations in Rafflesia arnoldi R. Br. – Bull. Torrey Bot. Club
87: 406-416.
Oliveira CMA, Porto ALM, Bittrich V, Vencato
I, Marsaioli AJ. 1996. Floral resins of Clusia spp.: chemical
composition and biological function. – Tetrahedron Lett. 37: 6427-6430.
Oliveira PEAM de, Sazima K. 1990. Pollination
biology of two species of Kielmeyera (Guttiferae) from Brazilian
cerrado vegetation. – Plant Syst.Evol. 172: 35-49.
Oliveira LDSD de, Silva MJ da, Sales MF de.
2013. Synopsis of the tribe Hureae (Euphorbioideae, Euphorbiaceae). – Brittonia 65:
310-329.
Olowokudejo JD. 1993. Comparative epidermal
morphology of West African species of Jatropha L. (Euphorbiaceae). – Bot. J. Linn.
Soc. 111: 139-154.
Oltmann O. 1968 [1969]. Die Pollenmorphologie
der Erythroxylaceae und ihre
systematische Bedeutung. – Ber. Deutsch. Bot. Ges. 81: 505-511.
Oltmann O. 1971.
Pollenmorphologisch-systematische Untersuchungen innerhalb der Geraniales. – Diss. Bot.
11: 1-163.
Omer S, Quaiser M. 1985a. A new species of
Viola from Pakistan. – Pakistan J. Bot. 17: 137-139.
Omer S, Quaiser M. 1985b. Violaceae. – In: Nasir E, Ali SI
(eds), Flora of Pakistan 166, Karachi University, Pakistan.
O’Neill SP, Osborn JM, Philbrick CT. 1997.
Comparative pollen morphology of five New World genera of Podostemaceae. – Aquatic Bot.
57: 133-150.
Onelli E, Rivetta A, Giorgi A, Bignami M,
Cocucci M, Patrignani G. 2002. Ultrastructural studies on the developing
secretory nodules of Hypericum perforatum. – Flora 197: 92-102.
Oostermeijer JGB. 1989. Myrmecochory in
Polygala vulgaris L., Luzula campestris (L.) DC., and
Viola curtisii Forster in a Dutch dune area. – Oecologia 78:
302-311.
Oropeza N, Mercado-Ruaro P, Novelo RA,
Philbrick CT. 1998. Karyomorphological studies of Mexican species of
Marathrum (Podostemaceae). – Aquatic Bot.
62: 207-211.
Oropeza N, Palomino G, Novelo RA, Philbrick
CT. 2002. Karyomorphological studies in Oserya, Vanroyenella
and Tristicha (Podostemaceae sensu lato). –
Aquatic Bot. 73: 163-171.
Osborn JM, O’Neill SP, El-Ghazaly G. 2000.
Pollen morphology and ultrastructure of Marathrum schiedeanum (Podostemaceae). – Grana 39:
221-225.
Ota M, Imaichi R, Kato M. 2001. Developmental
morphology of the thalloid Hydrobryum japonicum (Podostemaceae). – Amer. J. Bot.
88: 382-390.
Ottens-Treurniet MAD, Welzen PC van. 2016. A
revision of the Malesian genus Blumeodendron (Euphorbiaceae). – Blumea 61:
64-82.
Oudejans RCHM. 1990. World catalogue of
species names published in the tribe Euphorbieae (Euphorbiaceae) with their
geographical distribution. – Utrecht.
Owen PT, Scheinmann F. 1974. Extractives from
Guttiferae XXVI. Isolation and extraction of six xanthones, a biflavanoid, and
triterpenoids from the heartwood of Pentaphalangium
solomon[en]se. – J. Chem. Soc. Perkins Trans. 1, 1974:
1018-1021.
Pais M, Marchand J, Monseur X, Jarreau F,
Goutarel R. 1967.Chemie organique. – Alcaloïdes peptidiques. Structure de
l’hymenocardine, alcaloïde de l’Hymenocardia acida Tul.
(Euphorbiacées). – Compt. Rend. Acad. Sci. Paris 264: 1409-1411.
Paiva EAS, Machado SR. 2006. Ontogênese,
ultra-estrutura e secreção dos coléteres de Caryocar brasiliense
Camb. (Caryocaraceae). –
Braz. J. Biol. 66: 301-308.
Paiva J. 1972. Rafflesiaceae. – An. Soc. Brot.
38: 141-143.
Pang X, Song J, Zhu Y, Xie C, Chen S. 2010.
Using DNA barcoding to identify species within Euphorbiaceae. – Planta Medica
76: 1784-1786.
Pannier F. 1960. Physiological responses of
Podostemaceae in their natural
habitat. – Intl. Rev. Gesellsch. Hydrobiol. 45: 347-354.
Park K-R. 1996. Phylogeny of New World
subtribe Euphorbiinae (Euphorbiaceae). – Korean J.
Plant Taxon. 26: 235-256.
Park K-R. 1998. Monograph of
Euphorbia sect. Tithymalopsis (Euphorbiaceae). – Edinburgh J.
Bot. 55: 161-208.
Park K-R, Backlund A. 2002. Origin of the
cyathium-bearing Euphorbieae (Euphorbiaceae): phylogenetic study
based on morphological characters. – Bot. Bull. Acad. Sin. 43: 57-72.
Park K-R, Elisens WS. 2000. A phylogenetic
study of tribe Euphorbieae (Euphorbiaceae). – Intern. J.
Plant Sci. 161: 425-434.
Park K-R, Jansen RK. 2007. A phylogeny of
Euphorbieae subtribe Euphorbiinae (Euphorbiaceae) based on molecular
data. – J. Plant Biol. 50: 644-649.
Pascarella JB. 1992. Notes on flowering
phenology, nectar robbing, and pollination of Symphonia globulifera L.
f. (Clusiaceae) in a lowland rain
forest in Costa Rica. – Brenesia 38: 83-96.
Pasqua G, Monacelli B, Cuteri A, Spuntarelli
F, Rascio N, Botta B, Delle Monache G, Scurria R. 1995. Accumulation of
vismione A in regenerated plants of Vismia guianensis DC. –
Protoplasma 189: 9-16.
Passarelli LM, Girarde SB, Tur NM. 2002.
Palynology of South American Podostemaceae 1. Apinagia
Tul. – Grana 41: 10-15.
Passos L, Oliveira PS. 2002. Ants affect the
distribution and performance of seedlings of Clusia criuva, a
primarily bird-dispersed rain forest tree. – J. Ecol. 90: 517-528.
Patel VC, Skvarla JJ, Raven PH. 1983. Pollen
ultrastructure of Chrysobalanaceae. – Vidya 26:
1-10.
Patino S, Aalto T, Edwards AA, Grace J. 2002.
Is Rafflesia an endothermic flower? – New Phytologist 154:
429-437.
Patino S, Grace J, Banziger H. 2000.
Endothermy by flowers of Rhizanthes lowii (Rafflesiaceae). – Oecologia 124:
149-155.
Paula JE de. 1976. Anatomia de Lorostemon
coelhoi Paula, Caraipa valioli Paula e Clusia aff.
macropoda Klotzsch (Guttiferae da Amazônica). – Acta Amazonica 6:
273-291.
Paula-Souza J de, Ballard HE Jr. 2014.
Re-establishment of the name Pombalia, and new combinations from the
polyphyletic Hybanthus (Violaceae). – Phytotaxa 183:
1-15.
Paula-Souza J de, Pirani JR. 2014.
Reestablishment of Calyptrion (Violaceae). – Taxon 63:
1335-1339.
Paula-Souza J de, Souza VC. 2002. A new
combination in Hybanthus (Violaceae) from South America. –
Brittonia 54: 92-93.
Paula-Souza J de, Souza VC. 2003a.
Hybanthopsis, a new genus of Violaceae from eastern Brazil. –
Brittonia 55: 206-210.
Paula-Souza J de, Souza VC. 2003b. A new
species of Hybanthus (Violaceae) from north-eastern Brazil.
– Bot. J. Linn. Soc. 141: 503-506.
Paula-Souza J de, Pirani JR, Feliciano CD.
2011. Taxonomic and geographic notes on the Hybanthus lanatus (A.
St.Hil.) Baill. complex (Violaceae). – Candollea 66:
367-375.
Pauzé F, Sattler R. 1978. L’androecée
centripète d’Ochna atropurpurea. – Can. J. Bot. 56: 2500-2511.
Pauzé F, Sattler R. 1979. La placentation
axillaire chez Ochna atropurpurea. – Can. J. Bot. 57: 100-107.
Pax F. 1884. Die Anatomie der Euphorbiaceen
in ihrer Beziehung zum System derselben. – Engl. Bot. Jahrb. Syst. 5:
384-421.
Pax F. 1889. Salicaceae. – In: Engler A, Prantl
K (eds), Die natürlichen Pflanzenfamilien III(1), W. Engelmann, Leipzig, pp.
29-37.
Pax F. 1893. Euphorbiaceae africanae I. –
Engl. Bot. Jahrb. Syst. 15: 522-535.
Pax F. 1894a. Euphorbiaceae africanae II. –
Engl. Bot. Jahrb. Syst. 19: 76-127.
Pax F. 1894b. Euphorbiaceae, Plantae
Gürichianae. – Engl. Bot. Jahrb. Syst. 19: 142-143.
Pax F. 1896. Euphorbiaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien III(5), W. Engelmann,
Leipzig, pp. 1-119; Pax F. 1897. Nachträge zu III(5), pp. 210-213.
Pax F. 1904. Monographische übersicht über
die afrikanische Arten aus der Sektion Diacanthium der Gattung
Euphorbia. – Engl. Bot. Jahrb. Syst. 34: 61-85.
Pax F. 1910. Euphorbiaceae africanae XI. –
Engl. Bot. Jahrb. Syst. 45: 234-241.
Pax F. 1924. Die Phylogenie der Euphorbiaceae. – Engl. Bot.
Jahrb. Syst. 59: 129-182.
Pax F, Hoffmann K. 1931. Euphorbiaceae. – In: Engler A
(†), Harms H, Pax F (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd.
19c, W. Engelmann, Leipzig, pp. 11-233.
Pax F, Hoffmann K. 1933. Über die Stellung
der Gattung Gonatogyne innerhalb der Euphorbiaceae. – Feddes Repert.
31: 190-191.
Payens JPDW. 1958. Erythroxylaceae. – In: Steenis
CGGJ van (ed), Flora Malesiana I, 5(4), Noordhoff-Kolff N. V., Groningen, pp.
543-552.
Pearcy RW, Troughton J. 1975. C4
photosynthesis in tree form Euphorbia species from Hawaiian rainforest
sites. – Plant Physiol. 55: 1054-1056.
Peccoud J, Piatscheck F, Yockteng R, Garcia
M, Sauve M, Djiéto-Lordon C, Harris DJ, Wieringa JJ, Breteler FJ, Born C,
McKey D, Blatrix R. 2013. Multi-locus phylogenies of the genus
Barteria (Passifloraceae) portray complex
patterns in the evolution of myrmecophytism. – Mol. Phylogen. Evol. 66:
824-832.
Pegel KH, Piacenza LPL, Phillips L, Waight
ES. 1971. ent-3β-Hydroxybeyer-15(16)-ene-2,12-dione from
Androstachys johnsonii Prain. (Euphorbiaceae). – Chem.
Communications 1346-1347.
Peirson JA, Riina R, Mayfield MH, Ferguson
CJ, Urbatsch LE. Berry PE. 2014. Phylogenetics and taxonomy of the New World
leafy spurges, Euphorbia section Tithymalus (Euphorbiaceae). – Bot. J. Linn.
Soc. 175: 191-228.
Pellegrin F. 1952. Les Flacourtiacées du
Gabon. – Mém. Soc. Bot. France 1952: 105-121.
Pelser PB, Nickrent DL, Barcelona JF. 2016.
Untangling a vine and its parasite: host specificity of Philippine
Rafflesia (Rafflesiaceae). – Taxon 65:
739-758.
Pérez JO, d’Eeckenbrugge GC. 2017.
Morphological characterization in the genus Passiflora L.: an approach
to understanding its complex variability. – Plant Syst. Evol. 303:
531-558.
Perkins G, Estes JR, Thorp RW. 1975.
Pollination of Cnidoscolus texanus (Euphorbiaceae) in south-central
Oklahoma. – Southw. Natur. 20: 391-396.
Perrier de la Bâthie H. 1945. Fam. 143.
Passifloracées. – In: Humbert H (ed), Flore de Madagascar et des Comores,
Firmin-Didot et Cie, Paris, pp. 1-50.
Perrier de la Bâthie H. 1946. Fam. 140.
Flacourtiaceae. – In: Humbert H (ed), Flore de Madagascar et des Comores,
Firmin-Didot et Cie, Paris.
Perrier de la Bâthie H. 1951. Fam. 136.
Guttifères (Guttiferae). – In: Humbert H (ed), Flore de Madagascar et des
Comores, Firmin-Didot et Cie, Paris.
Perry B. 1943. Chromosome number and
phylogenetic relationships in the Euphorbiaceae. – Amer. J. Bot.
30: 527-543.
Petersen OG. 1896. Trigoniaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien III(4), W. Engelmann,
Leipzig, pp. 309-311.
Pfeifer E, Grob V, Thiv, M, Rutishauser R.
2009. Stonesia ghoguei, peculiar morphology of a new Cameroonian
species (Podostemaceae,
Podostemoideae). – Novon 19: 102-116.
Pfeiffer HH. 1951. Lophopyxis als
Typus einer eigenen Familie. – Rev. Sudamer. Bot. 10: 3-6.
Philbrick CT. 1984. Aspects of floral
biology, breeding system, and seed and seedling biology in Podostemum
ceratophyllum (Podostemaceae). – Syst. Bot. 9:
166-174.
Philbrick CT, Bogle AL. 1988. A survey of
floral variation in five populations of Podostemum ceratophyllum
Michx. (Podostemaceae). –
Rhodora 90: 113-121.
Philbrick CT, Crow GE. 1992. Isozyme
variation and population structure in Podostemum ceratophyllum Michx.
(Podostemaceae): implications
for colonization of glaciated North America. – Aquatic Bot. 43: 311-325.
Philbrick CT, Novelo RA. 1993. A fascinating
family of aquatic flowering plants. – Aquaphyte 13: 1-7.
Philbrick CT, Novelo RA. 1995. New World Podostemaceae: ecological and
evolutionary enigmas. – Brittonia 47: 210-222.
Philbrick CT, Novelo RA. 1997. Ovule number,
seed number and seed size in Mexican and North American species of Podostemaceae. – Aquatic Bot.
57: 183-200.
Philbrick CT, Novelo RA. 1998. Flowering
phenology, pollen flow, and seed production in Marathrum rubrum (Podostemaceae). – Aquatic Bot.
62: 199-206.
Philbrick CT, Novelo RA. 2004. Monograph of
Podostemum (Podostemaceae). – Syst. Bot.
Monogr. 70: 1-106.
Philbrick CT, Novelo RA, Irgang BE. 2004a.
Two new genera of Podostemaceae
from the state of Minas Gerais, Brazil. – Syst. Bot. 29: 109-117.
Philbrick CT, Novelo RA, Irgang BE. 2004b. A
new species of Ceratolacis (Podostemaceae) from the state of
Minas gerais, Brazil. – Novon 14: 108-113.
Philbrick CT, Vomela M, Novelo AR. 2006.
Preanthesis cleistogamy in the genus Podostemum (Podostemaceae). – Rhodora 108:
195-202.
Philbrick CT, Bove CP, Edson Jr TC. 2009.
Monograph of Castelnavia (Podostemaceae). – Syst. Bot. 34:
715-729.
Philbrick CT, Bove CP, Stevens HI. 2010.
Endemism in Neotropical Podostemaceae. – Ann. Missouri
Bot. Gard. 97: 425-456.
Philbrick CT, Malecki J, Tippery NP, Stevens
HI. 2011. A new genus of Podostemaceae from Venezuela. –
Novon 21: 475-480.
Philcox D. 1995. Two new Euphorbiaceae from Sri Lanka. –
Kew Bull. 50: 119-123.
Phillips EP. 1935. The genera
Erythroxylon L. and Nectaropetalum Engl. – South African J.
Sci. 32: 305-312.
Pierre L. 1896. Plantes du Gabon:
Dichostemma. – Bull. Mens. Soc. Linn. Paris 1(159): 1259-1260.
Pierre L. 1897. Sur quelques Phytocrénacées
du Gabon et de l’Indo-Chine. – Bull. Mens. Soc. Linn. Paris 167:
1315-1320.
Pijl L van der. 1952. The stamens of
Ricinus. – Phytomorphology 2: 130-132.
Pilger R. 1925. Caryocaraceae. – In: Engler A,
Gilg E (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 21, W.
Engelmann, Leipzig, pp. 90-93.
Plowman T. 1982. Three new species of
Erythroxylum (Erythroxylaceae) from Venezuela.
– Brittonia 34: 442-447.
Plowman T. 1989. 93. Erythroxylaceae. – In: Harling
G, Andersson L (eds), Flora of Ecuador 36, Nord. J. Bot., Copenhagen, pp.
1-31.
Plowman T, Hensold N. 2004. Names, types, and
distribution of neotropical species of Erythroxylum (Erythroxylaceae). – Brittonia
56: 1-53.
Pojarkova AI. 1960. Incrementa ad
monographiam generum Andrachne L. et Leptopus Decne. – Bot.
Mater. Gerb. Bot. Inst. Komarova Akad. Nauk S.S.S.R. 20: 251-274.
Poncy O, Offroy B. 2006. Une nouvelle espèce
de Tovomita Aubl. (Clusiaceae) de Guyane française. –
Adansonia, sér. III, 28: 113-117.
Ponsinet G, Ourisson G. 1965. Études
chimio-taxonomiques dans la famille des euphorbiacées I. Introduction
générale et séparation et identification des triterpènes tetracyliques
monohydroxylés naturels. – Phytochemistry 4: 799-811.
Poole MM. 1981. Pollen diversity in
Zimmermannia (Euphorbiaceae). – Kew Bull. 36:
129-138.
Porto AM, Machado SMF, Oliveira CMA, Bittrich
V, Amaral M do CE, Marsaioli AJ. 2000. Polyisoprenylated benzophenones from
Clusia floral resins. – Phytochemistry 55: 755-768.
Powell RG, Weisleder D, Smith CR Jr. 1981.
Novel maytansinoid tumor inhibitors from Trewia nudiflora: trewiasine,
dehydrotrewiasine, and dimethyltrewiasine. – J. Organic Chem. 46:
4398-4403.
Powell RG, Smith CR Jr, Plattner RD, Jones
BE. 1983. Additional new maytansinoids from Trewia nudiflora:
10-epitrewiasine and nortrewiasine. – J. Nat. Prod. (Lloydia) 46: 660-666.
Prain D. 1911. A review of the genera
Erythrococca and Micrococca. – Ann. Bot. 25: 575-638.
Prain D. 1913. The Mercurialineae and
Adenoclineae of South Africa. – Ann. Bot. 27: 371-410.
Prain D. 1918. The genus
Chrozophora. – Kew Bull. 1918: 49-120.
Prain D, Hutchinson J. 1913. Notes on some
species of Acalypha. – Kew Bull. 1913: 1-28.
Prakash N, Lau YY. 1976. Morphology of
Ploiarium alternifolium and the taxonomic position of
Ploiarium. – Bot. Not. 129: 279-285.
Prakash U, Bande MB, Lalitha V. 1986. The
genus Phyllanthus from the Tertiary of India with critical remarks on
the nomenclature of fossil woods of Euphorbiaceae. – Palaeobotanist
35: 106-115.
Prance GT. 1963. A taximetric study of the Chrysobalanaceae. – Ph.D.
diss., The University of Oxford, England.
Prance GT. 1972a. Flora Neotropica. Monograph
9. Chrysobalanaceae. – New
York Botanical Garden, Bronx, New York.
Prance GT. 1972b. Flora Neotropica. Monograph
10. Dichapetalaceae. – New
York Botanical Garden, Bronx, New York.
Prance GT. 1974a. A new Peruvian species of
chiropterophilous Couepia (Chrysobalanaceae). –
Brittonia 26: 302-304.
Prance GT. 1974b. Phytogeographic support for
the theory of Pleistocene forest refuges in the Amazon basin, based on evidence
from distribution patterns in Caryocaraceae, Chrysobalanaceae, Dichapetalaceae and Lecythidaceae.
– Acta Amazonica 3: 5-28.
Prance GT. 1977. A new Colombian species of
Dichapetalaceae. – Mutisia
42: 1-3.
Prance GT. 1979a. New genera and species of
Chrysobalanaceae from
Malesia and Oceania. – Brittonia 31: 79-95.
Prance GT. 1979b. 80. Chrysobalanaceae. – In:
Harling G, Sparre B (eds), Flora of Ecuador 10, Swedish Natural Science
Research Council, Stockholm, pp. 1-23.
Prance GT. 1980. 121. Dichapetalaceae. – In: Harling
G, Sparre B (eds), Flora of Ecuador 12, Swedish Natural Science Research
Council, Stockholm, pp. 1-13.
Prance GT. 1987. An update on the taxonomy
and distribution of the Caryocaraceae. – Opera Bot. 92:
179-183.
Prance GT. 1989a. Flora Neotropica. Monograph
9, Suppl. Chrysobalanaceae.
– New York Botanical Garden, Bronx, New York.
Prance GT. 1989b. Chrysobalanaceae. – In:
Steenis CGGJ van (†), Wilde WJJO de (eds), Flora Malesiana I, 10(4), Kluwer
Academic Publ., Dordrecht, Boston, London, pp. 635-678.
Prance GT. 1991. Two new species of
Neotropical Chrysobalanaceae. – Kew Bull.
46: 105-109.
Prance GT. 1992a. Five new species of
Neotropical Chrysobalanaceae. – Kew Bull.
47: 247-256.
Prance GT. 1992b. New species and new records
of Neotropical Chrysobalanaceae. – Kew Bull.
47: 633-646.
Prance GT. 1993. Four new species of
Neotropical Dichapetalaceae.
– Kew Bull. 49: 129-136.
Prance GT. 1994. Two new species of
Neotropical Chrysobalanaceae. – Kew Bull.
49: 359-363.
Prance GT. 1995a. Two new species of
Licania (Chrysobalanaceae). – Kew
Bull. 50: 141-145.
Prance GT. 1995b. A synopsis of
Stephanopodium (Dichapetalaceae). – Kew Bull.
50: 295-305.
Prance GT. 1995c. New taxa and notes on
Neotropical Chrysobalanaceae. – Kew Bull.
50: 707-721.
Prance GT. 1997. Additions to Neotropical
Dichapetalum. – Kew Bull. 52: 213-219.
Prance GT. 1999a. New species and notes on
Neotropical Chrysobalanaceae. – Kew Bull.
54: 103-115.
Prance GT. 1999b. A new species of
Hirtella L. (Chrysobalanaceae) from Ecuador.
– Kew Bull. 54: 995-997.
Prance GT. 2002. New combinations in African
Chrysobalanaceae. – Kew
Bull. 57: 993-995.
Prance GT, Silva MF da. 1973. Flora
Neotropica. Monograph 12. Caryocaraceae. – New York
Botanical Garden, Bronx, New York.
Prance GT, Mori SA. 1983. Dispersal and
distribution of Lecythidaceae
and Chrysobalanaceae. –
Sonderb. Naturw. Ver. Hamburg 7: 163-186.
Prance GT, Sothers CA. 2003a. Species
plantarum. Flora of the world 9. Chrysobalanaceae 1:
Chrysobalanus to Parinari. – Australian Biological
Resources, Canberra.
Prance GT, Sothers CA. 2003b. Species
plantarum. Flora of the world 10. Chrysobalanaceae 2:
Acioa to Magnistipula. – Australian Biological Resources,
Canberra.
Prance GT, White F. 1979. Resurrection of the
genus Dactyladenia (Chrysobalanaceae). –
Brittonia 31: 483-487.
Prance GT, White F. 1988. The genera of Chrysobalanaceae: a study in
practical and theoretical taxonomy and its relevance to evolutionary biology.
– Phil. Trans. Roy. Soc., London, Ser. B, 320: 1-184.
Prance GT, Rogers DJ, White F. 1969. A
taxonomic study of an angiosperm family: generic delimitation in the Chrysobalanaceae. – New
Phytol. 68: 1203-1234.
Prance GT, da Silva MF, Albuquerque BW,
Araújo de J da SI, Carreira LMM, Braga MMN, Macedo M, da Conceição PN,
Lisbõa PLB, Braga PI, Lisbõa RCL, Vilhena RCQ. 1975. Revisão taxonômica das
espécies amazônicas de Rhizophoraceae. – Acta
Amazonica 5: 5-22.
Prenner G, Rudall PJ. 2007. Comparative
ontogeny of the cyathium in Euphorbia (Euphorbiaceae) and its allies:
exploring the organ-flower-inflorescence boundary. – Amer. J. Bot. 94:
1612-1629.
Prenner G, Hopper SD, Rudall PJ. 2008.
Pseudanthium development in Calycopeplus paucifolius, with particular
reference to the evolution of the cyathium in Euphorbieae (Euphorbiaceae-Malpighiales). – Aust. Syst. Bot.
21: 153-161.
Prenner G, Box MS, Cunniff J, Rudall PJ.
2008. The branching stamens of Ricinus and the homologies of the
angiosperm stamen fascicle. – Intern. J. Plant Sci. 169: 735-744.
Prenner G, Cacho NI, Baum D, Rudall PJ. 2011.
Is LEAFY a useful marker for the flower-inflorescence boundary in the
Euphorbia cyathium? – J. Experim. Bot. 62: 345-350.
Presting D. 1964 [1965]. Die Systematik der
Passifloraceen aus pollenmorphologischer Sicht. – Ber. Deutsch. Bot. Ges. 77:
40-44.
Presting D. 1965. Zur Morphologie der
Pollenkörner der Passifloraceen. – Pollen Spores 7: 193-247.
Prieto RO. 2003. Novelties in
Erythroxylum (Erythroxylaceae) of the Greater
Antilles. – Willdenowia 33: 187-195.
Pritzel E. 1897. Der systematische Wert der
Samenanatomie, insbesondere des Endosperms, bei den Parietales. – Engl. Bot.
Jahrb. Syst. 24: 348-394.
Prokhanov JI. 1933. Conspectus systematicus
Tithymalorum Asiae Mediae. – Trans. Rubber and Guttapercha Inst., Moscow. [In
Russian]
Pruesapan K, Telford IRH, Bruhl JJ, Draisma
SGA, Welzen PC van. 2008. Delimitation of Sauropus (Phyllanthaceae) based on plastid
matK and nuclear ribosomal ITS DNA sequence data. – Ann. Bot. 102:
1007-1018.
Pruesapan K, Telford IRH, Bruhl JJ, Welzen PC
van. 2012. Phylogeny and proposed circumscription of Breynia,
Sauropus and Synostemon (Phyllanthaceae), based on
chloroplast and nuclear DNA sequences. – Aust. Syst. Bot. 25: 313-330.
Pulle AA. 1909. Rafflesiaceae. – Rec. Trav. Bot.
Néerl. 6: 259-261.
Punt W. 1962. Pollen morphology of the Euphorbiaceae with special
reference to taxonomy. – Wentia 7: 1-116.
Punt W. 1975. Pollen morphology of the Dichapetalaceae with special
reference to evolutionary trends and mutual relationships of pollen types. –
Rev. Palaeobot. Palyn. 19: 1-97.
Punt W. 1976. Evolutionary trends in the
pollen grains of Dichapetalaceae. – In:
Ferguson IK, Muller J (eds), The evolutionary significance of the exine, Linn.
Soc. Symposium, No.1, London and New York, pp.139-146.
Punt W. 1980. Pollen morphology of the
Phyllanthus species (Euphorbiaceae) occurring in New
Guinea. – Rev. Palaeobot. Palynol. 31: 155-177.
Punt W. 1986. Convergence in some interesting
pollen types of Phyllanthus (Euphorbiaceae). – Can. J. Bot.
64: 3127-3129.
Punt W. 1987. A survey of pollen morphology
in Euphorbiaceae with special
reference to Phyllanthus. – Bot. J. Linn. Soc. 94: 127-142.
Puri V. 1947. Studies in floral anatomy IV.
Vascular anatomy of the flower of certain species of the Passifloraceae. – Amer. J. Bot.
34: 562-573.
Puri V. 1948. Studies in floral anatomy V. On
the structure and nature of the corona in certain species of the Passifloraceae. – J. Indian
Bot. Soc. 17: 130-149.
Quiroz F, Novelo RA, Philbrick CT. 1997.
Water chemistry and the distribution of Mexican Podostemaceae: a preliminary
evaluation. – Aquatic Bot. 57: 201-212.
Qin X-S, Chen H-F, Xing F-W. 2007. A new
species of Drypetes (Putranjivaceae) from China. –
Nord. J. Bot. 25: 38-40.
Radcliffe-Smith AR. 1968. An account of the
genus Givotia Griff. (Euphorbiaceae). – Kew Bull. 22:
493-505.
Radcliffe-Smith AR. 1973a. An account of the
genus Cephalocroton Hochst. (Euphorbiaceae). – Kew Bull. 28:
123-132.
Radcliffe-Smith AR. 1973b. Allomorphic female
flowers in the genus Acalypha (Euphorbiaceae). – Kew Bull. 28:
5t25-529.
Radcliffe-Smith AR. 1974. The taxonomic
position of Euphorbia petiolata and the reduction of E.
postii (Euphorbiaceae).
– Kew Bull. 29: 503-505.
Radcliffe-Smith AR. 1975. Notes on African Euphorbiaceae VI. – Kew Bull.
30: 675-687.
Radcliffe-Smith AR. 1978. Notes on African Euphorbiaceae VII. – Kew Bull.
32: 475-481.
Radcliffe-Smith AR. 1980. A note on
Romanoa (Euphorbiaceae). – Kew Bull. 34:
591-592.
Radcliffe-Smith AR. 1981a. Notes on African
Euphorbiaceae X.
Zimmermannia. – Kew Bull. 36: 127-128.
Radcliffe-Smith AR. 1981b. New combinations
in the genus Euphorbia III. – Kew Bull. 36: 216.
Radcliffe-Smith AR. 1981c. On the identity of
the Arabian Bridelia (Euphorbiaceae). – Kew Bull. 36:
222.
Radcliffe-Smith AR. 1981d. Notes on African
Euphorbiaceae IX. – Kew Bull.
35: 777.
Radcliffe-Smith AR. 1982. Notes on African Euphorbiaceae XII. – Kew Bull.
37: 421-428.
Radcliffe-Smith AR. 1983. Notes on African Euphorbiaceae XIII.
Tragia, Tragiella &c. – Kew Bull. 37: 683-691.
Radcliffe-Smith AR. 1984. Notes on African Euphorbiaceae XIV. – Kew Bull.
39: 785-796.
Radcliffe-Smith AR. 1985a. Notes on African
Euphorbiaceae XV.
Tragia. – Kew Bull. 40: 231-234.
Radcliffe-Smith AR. 1985. Notes on African Euphorbiaceae XVI. – Kew Bull.
40: 657-658.
Radcliffe-Smith AR. 1986a. A review of the
family Euphorbiaceae. – In:
Evans FJ (ed), Naturally occurring phorbol esters, CRC Press, Boca Raton,
Florida, pp. 63-85.
Radcliffe-Smith AR. 1986b. Notes on African
Euphorbiaceae XVIII. – Kew
Bull. 41: 963-964.
Radcliffe-Smith AR. 1987a. Segregate families
from the Euphorbiaceae. –
Bot. J. Linn. Soc. 94: 47-66.
Radcliffe-Smith AR. 1987b. Euphorbiaceae (Part 1). – In:
Polhill RM (ed), Flora of Tropical East Africa, A. A. Balkema, Rotterdam, pp.
1-407.
Radcliffe-Smith AR. 1987c. Notes on African
Euphorbiaceae XIX.
Tragia (III) and notes on Croton & Erythrococca.
– Kew Bull. 42: 395-399.
Radcliffe-Smith AR. 1988. Notes on Madagascan
Euphorbiaceae I. On the
identity of Paragelonium and on the affinities of Benoistia
and Claoxylopsis (Euphorbiaceae). – Kew Bull. 43:
625-647.
Radcliffe-Smith AR. 1989. Notes on African Euphorbiaceae XX.
Acalypha (II), etc. – Kew Bull. 44: 439-454.
Radcliffe-Smith AR. 1990a. Notes on African
Euphorbiaceae XXII. The genus
Schinziophyton. – Kew Bull. 45: 157-160.
Radcliffe-Smith AR. 1990b. Notes on African
Euphorbiaceae XXIII.
Croton (II). – Kew Bull. 45: 555-560.
Radcliffe-Smith AR. 1990c. Notes on
Madagascan Euphorbiaceae III.
Stachyandra. – Kew Bull. 45: 561-568.
Radcliffe-Smith AR. 1990d. Notes on African
Euphorbiaceae XXIV.
Drypetes (II). – Kew Bull. 45: 671-675.
Radcliffe-Smith AR. 1991a. Notes on African
Euphorbiaceae XXV.
Jatropha (VI). – Kew Bull. 46: 141-157.
Radcliffe-Smith AR. 1991b. Notes on African
Euphorbiaceae XXVI.
Erythrococca (V). – Kew Bull. 46: 331-333.
Radcliffe-Smith AR. 1991c. Notes on
Madagascan Euphorbiaceae IV.
The genus Suregada in Madagascar and the Comoro Is. – Kew Bull. 46:
711-726.
Radcliffe-Smith AR. 1992a. Notes on African
Euphorbiaceae XXVII.
Clutia. – Kew Bull. 47: 111-119.
Radcliffe-Smith AR. 1992b. Notes on African
Euphorbiaceae XXVIII. – Kew
Bull. 47: 677-683.
Radcliffe-Smith AR. 1993a. Notes on
Australian Euphorbiaceae II.
Pseudanthus and Stachystemon. – Kew Bull. 48: 165-168.
Radcliffe-Smith AR. 1993b. Notes on African
Euphorbiaceae XXIX:
Uapaca. – Kew Bull. 48: 611-617.
Radcliffe-Smith AR. 1995. Additions and
corrections to ‘Euphorbiaceae’ for ‘Flora of
tropical East Africa’. – Kew Bull. 50: 809-816.
Radcliffe-Smith AR. 1996a. Notes on African
Euphorbiaceae XXX:
Phyllanthus (V) & c. – Kew Bull. 51: 301-331.
Radcliffe-Smith AR. 1996b. A new
Piranhea from Brazil, and the subsumption of the genus
Celaenodendron (Euphorbiaceae: Oldfieldioideae).
– Kew Bull. 51: 543-548.
Radcliffe-Smith AR. 1996c. Proposal to
conserve the name Angostylis (Euphorbiaceae) with a conserved
spelling. – Taxon 45: 705.
Radcliffe-Smith AR. 1997a. Notes on African
and Madagascan Euphorbiaceae.
– Kew Bull. 52: 171-176.
Radcliffe-Smith AR. 1997b. Notes on
Madagascan Euphorbiaceae V.
Jatropha. – Kew Bull. 52: 177-181.
Radcliffe-Smith AR. 1997c. Euphorbiaceae 1. – In: Polhill
RM (ed), Flora of tropical East Africa, A. A. Balkema, Rotterdam.
Radcliffe-Smith AR. 1998a. Notes on
Madagascan Euphorbiaceae VI. A
synopsis of Tannodia Baill. (Crotonoideae-Aleuritideae-Grosserinae)
with especial reference to Madagascar, and the subsumption of
Domohinea Leandri. – Kew Bull. 53: 173-186.
Radcliffe-Smith AR. 1998b. Notes on
Madagascan Euphorbiaceae VII. A
synopsis of the genus Amyrea Leandri (Euphorbiacae-Acalyphoideae). –
Kew Bull. 53: 437-451.
Radcliffe-Smith AR. 1998c. Notes on
Madagascan Euphorbiaceae VIII.
A third species of Aristogeitonia (Euphorbiaceae) for Madagascar. –
Kew Bull. 53: 977-980.
Radcliffe-Smith AR (ed). 2001. Genera
Euphorbiacearum. – Royal Botanic Gardens, Kew.
Radcliffe-Smith AR, Govaerts R. 1997a. New
names and combinations in the Crotonoideae. – Kew Bull. 52: 183-189.
Radcliffe-Smith AR, Govaerts R. 1997b. New
names and new combinations in the Euphorbiaceae-Acalyphoideae. –
Kew Bull. 52: 477-481.
Radcliffe-Smith AR, Harley MM. 1990. Notes on
African Euphorbiaceae XXI:
Aerisilvaea and Zimmermanniopsis, two new phyllanthoid genera
for the flora of Tanzania. – Kew Bull. 45: 147-156.
Radcliffe-Smith AR, Ratter JA. 1996. A new
Piranhea from Brazil, and the subsumption of the genus
Celaenodendron (Euphorbacee-Oldfieldioideae). – Kew Bull. 51:
54-548.
Radlkofer L. 1870. Über Pausandra,
ein neues Euphorbiaceen-Genus. – Flora 53: 81-95.
Raghavan TS, Srinivasan VK. 1940. A
contribution to the life history of Bergia capensis Linn. – J.
Indian Bot. Soc. 19: 283-291.
Rajkumar S, Janarthanam MK. 2007.
Agasthiyamalaia (Clusiaceae), a new genus for
Poeciloneuron pauciflorum, an endemic and endangered tree of Western
Ghats, India. – J. Bot. Res. Inst. Texas 1: 129-133.
Raju MVS. 1954. Pollination mechanism in
Passiflora foetida Linn. – Proc. Indian Natl. Acad. Sci., Sect. B,
20: 431-436.
Raju MVS. 1956a. Embryology of the Passifloraceae I. Gametogenesis
and seed development of Passiflora calcarata Mast. – J. Indian Bot.
Soc. 35: 126-138.
Raju MVS. 1956b. Development of embryo and
seed coat in Turnera ulmifolia L. var. angustifolia Willd.
– Bot. Not. 109: 308-312.
Raju MVS, Rao AN. 1953. The development of
the male and female gametophytes in Mallotus albus Muell. – J.
Mysore Univ. 13: 5-8.
Raju VS. 1984. Notes on Mischodon
zeylanicus Thwaites: a little-known euphorbiaceous plant from Sri Lanka
and southern India. – J. Econ. Taxon. Bot. 5: 165-167.
Raju VS, Rao PN. 1977. Variation in the
structure and development of foliar stomata in the Euphorbiaceae. – Bot. J. Linn.
Soc. 75: 69-97.
Raju VS, Rao PN. 1987. The taxonomic use of
the basal stomatal type in the generic delimitation of Chamaesyce (Euphorbiaceae). – Feddes Repert.
98: 137-141.
Ralimanana H. Hoffmann P, Rajeriarison C.
2013. Taxonomic revision of Phyllanthus (Phyllanthaceae) in Madagascar and
the Comoro Islands III: subgenera Swartziani, Afroswartziani
and Emblica. – Kew Bull. 68: 1-24.
Ralimanana H, Hoffmann P. 2014. Taxonomic
revision of Phyllanthus L. (Phyllanthaceae) in Madagascar and
the Comoro Islands II: subgenera Anisonemoides (Jean F. Brunel) Ralim.
& Petra Hoffm., stat. nov. and Menarda (Müll. Arg.) Ralim. &
Petra Hoffm., stat. nov. – Adansonia, sér. 3, 36: 265-301.
Ramaiah PA, Row LR, Reddy DS, Anjaneyulu ASR,
Ward RS, Pelter A. 1979. Isolation and characterization of bergenin derivatives
from Macaranga peltata. – J. Chem. Soc., Perkin Trans. I:
2313-2316.
Ramírez BW, Gómez PLD. 1978. Production of
nectar and gums by flowers of Monstera deliciosa (Araceae) and of
some species of Clusia (Guttiferae) collected by New World
Trigona bees. – Brenesia 14-15: 407-412.
Ramji MV. 1967. Morphology and ontogeny of
the foliar venation of Calophyllum inophyllum L. – Aust. J. Bot. 15:
437-443.
Rao AMS. 1940. Studies in the Malpighiaceae 1. Embryo-sac
development and embryogeny in the genera Hiptage, Banisteria
and Stigmaphyllum. – J. Indian Bot. Soc. 18: 145-156.
Rao AMS. 1941. Studies in the Malpighiaceae 2. Structure and
development of the ovules and embryo-sacs of Malpighia coccifera Linn.
and Tristellateia australis Linn. – Proc. Natl. Inst. Sci. India,
Sect. B, 7: 393-404.
Rao AN. 1957. The embryology of Hypericum
patulum Thunb. and H. mysorense Heyne. – Phytomorphology 7:
36-45.
Rao AN. 1964. Notes on embryology of
Hevea brasiliensis. – Curr. Sci. 33: 739-740.
Rao AR. 1970. Comparative embryology of
angiosperms: Euphorbiaceae. –
In: Proceedings of the symposium on comparative embryology of the angiosperms,
Bull. Indian Natl. Acad. Sci., Sect. B, 41: 136-141.
Rao AR, Malaviya M. 1964. On the latex-cells
and latex of Jatropha. – Proc. Indian Acad. Sci., Sect. B, 60:
95-106.
Rao AR, Tewari JP. 1960. On the morphology
and ontogeny of the foliar sclereids of Codiaeum variegatum Blume. –
Proc. Indian Natl. Acad. Sci., Sect. B, 26: 1-6.
Rao D. 1965. Floral anatomy of Erythroxylaceae. – Proc. Natl.
Inst. Sci. India, Ser. B, 35: 156-162.
Rao D. 1968. A contribution to the embryology
of Erythroxylaceae. – Proc.
Natl. Acad. Sci. India, Ser. B, 38: 53-65.
Rao D, Narayana LL. 1965. Embryology of Linaceae. – Curr. Sci. 34: 92-93.
Rao PN. 1962. A note on the embryology of
Micrococca mercurialis Benth. – Curr. Sci. 31: 426-427.
Rao PN. 1970. Euphorbiaceae. – Bull. Indian
Natl. Acad. Sci. 41: 136-141.
Rao PN. 1975. Embryological studies in
Meineckia parvifolia (Wight) Webster. – Curr. Sci. 44: 283-284.
Rao PN, Rao DS. 1976. Embryology of cassava
(Manihot). – Proc. Indian Natl. Acad. Sci., Sect. B, 42: 111-121.
Rao PR, Raju VS. 1985. Foliar trichomes in
the family Euphorbiaceae. –
In: Singh B, Singh MP (eds), Trends in plant research, Govil & Kumar, Dehra
Dun, pp. 128-136.
Rao PSP, Devi KRR. 1977. A note on the
occurrence of intracarpellary pollen sacs in Securinega virosa. – J.
jap. Bot. 52: 60-62.
Rao SRS, Sarma V. 1992. Morphology of 2-armed
trichomes in relation to taxonomy: Malpighiales. – Feddes Repert.
103: 559-565.
Rao TA, Bhattacharya J. 1975. On foliar
terminal sclereids in Goupia glabra Aubl. – Curr. Sci. 44:
132-133.
Rao VS. 1949. The morphology of the
calyx-tube and the origin of perigyny in Turneraceae. – J. Indian Bot. Soc.
28: 198-201.
Ray C. 1944. Cytological studies in the flax
genus, Linum. – Amer. J. Bot. 31: 241-248.
Razi BA. 1949. Embryological studies of the
members of Podostemonaceae. – Bot. Gaz. 111: 211-218.
Razi BA. 1955. Some aspects of the embryology
of Zeylanidium olivaceum (Tul.) Engl. and Lawia zeylanica
Tul. – Bull. Bot. Soc. Bengal 9: 36-41.
Razifard H, Rosman AJ, Tucker GC, Les DH.
2017. Systematics of the cosmopolitan aquatic genus Elatine. – Syst.
Bot. 42: 73-86.
Razifard H, Les DH, Tucker GC. 2017.
Reticulate evolution in Elatine L. (Elatinaceae), a predominantly
autogamous genus of aquatic plants. – Syst. Bot. 42: 87-95.
Rechinger KH. 1992. Salix taxonomy
in Europe – problems, interpretations, observations. – Proc. Roy. Soc.
Edinb. 98B: 1-12.
Record SJ. 1938. The American woods of the
family Euphorbiaceae. – Trop.
Woods 54: 7-40.
Record SJ. 1941. American woods of the family
Flacourtiaceae. – Trop. Woods 68: 40-57.
Reddi EUB, Reddi CS. 1983. Pollination
ecology of Jatropha gossypiifolia (Euphorbiaceae). – Proc. Indian
Acad. Sci., Sect. B, 92: 215-231.
Reddy KC, Kumar KA, Srimannarayana G. 1983.
5-Methoxyfurano(2’’,3’’,7,8)flavone from the stems of Ochna
squarrosa. – Phytochemistry 22: 800-801.
Reiche K. 1896a. Linaceae. – In: Engler A, Prantl K
(eds), Die natürlichen Pflanzenfamilien III(4), W. Engelmann, Leipzig, pp.
27-35.
Reiche K. 1896b. Humiriaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien III(4), W. Engelmann,
Leipzig, pp. 35-37.
Reiche K. 1896c. Erythroxylaceae. – In: Engler
A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(4), W. Engelmann,
Leipzig, pp. 37-40.
Reiche K, Taubert P. 1895. Violaceae. – In: Engler A, Prantl K
(eds), Die natürlichen Pflanzenfamilien III(6), W. Engelmann, Leipzig, pp.
322-336.
Reis MG, Faria AD de, Alves dos Santos I,
Amaral M do CE, Marsaioli AJ. 2007. Byrsonic acid – the clue to floral
mimicry involving oil-producing flowers and oil-collecting bees. – J. Chem.
Ecol. 33: 1421-1429.
Retana AN, Philbrick CT. 1997. Podostemum
ricciiforme (Podostemaceae) rediscovered and
redescribed. – Taxon 46: 451-455.
Reuschel C, Walther H. 2006. Studien über
oligozäne Populus-Arten aus der Weisselstersenke südlich von
Leipzig, Sachsen (Deutschland). – Feddes Repert. 117: 1-33.
Reveal JL, Hoffmann P, Doweld A, Wurdack KJ.
2007. Proposal to conserve the name Phyllanthaceae. – Taxon 56:
266.
Reyes-Ortega I, Sanchez-Coronado ME,
Orozco-Segovia A. 2009. Seed germination in Marathrum shiedeanum and
M. rubrum (Podostemaceae). – Aquatic Bot.
90: 13-17.
Rial A. 2002. Une nouvelle espèce de
Macropodiella (Podostemaceae) de Guinée
Équatoriale. – Adansonia, sér. III, 24: 295-297.
Ribes de Lima L, Rubens Pirani J. 2008. Three
new species of Croton (Euphorbiaceae) from Brazil. –
Kew Bull. 63: 121-129.
Ribes de Lima L, Cruz-Barros MAV da, Rubens
Pirani J, Silva CAM da. 2007. Pollen morphology of Croton sect.
Lamprocroton (Müll. Arg.) Pax (Euphorbiaceae) and its taxonomic
implications. – Nord. J. Bot. 25: 206-216.
Ricardi SM. 1967. Revisión taxonómica de
las Malesherbiáceas. – Gayana Bot. 16: 1-139.
Richards AJ. 1990a. Studies in
Garcinia, dioecious tropical forest trees: agamospermy. – Bot. J.
Linn. Soc. 103: 233-250.
Richards AJ. 1990b. Studies in
Garcinia, dioecious tropical forest trees: the phenology, pollination
biology and fertilization of G. hombroniana Pierre. – Bot. J. Linn.
Soc. 103: 251-261.
Richards AJ. 1990c. Studies in
Garcinia, dioecious tropical forest trees: the origin of the
mangosteen (G. mangostana L.). – Bot. J. Linn. Soc. 103: 301-308.
Ridola F. 1903. Interpretazione morphologica
del ciazio di Pedilanthus. – Boll. Orto Bot. Napoli 1: 415-418ö
Riina R, Berry PE, Cornejo X. 2007. A new
species of “sangre de drago” (Croton section Cyclostigma,
Euphorbiaceae) from coastal
Ecuador. – Brittonia 59: 97-101.
Riina R, Berry PE, Ee BW van. 2009. Molecular
phylogenetics of the dragon’s blood Croton Section
Cyclostigma (Euphorbiaceae): a polyphyletic
assemblage unravelled. – Syst. Bot. 34: 360-374.
Riina R, Ee B van, Wiedenhoeft AC, Cardozo A,
Berry PE. 2010. Sectional rearrangement of arborescent clades of
Croton (Euphorbiaceae)
in South America: evolution of arillate seeds and a new species, Croton
domatifer. – Taxon 59: 1147-1160.
Riina R, Peirson JA, Geltman DV, Molero J,
Frajman B, Pahlevani A, Barres L, Morawetz JJ, Salmaki Y, Zarre S, Kryukov A,
Bruyns PV, Berry PE. 2013. A worldwide molecular phylogeny and classification
of the leafy spurges, Euphorbia subgenus Esula (Euphorbiaceae). – Taxon 62:
316-342.
Risch C. 1960. Die Pollenkörner der
Salicaceen. – Willdenowia 2: 413-430.
Rittershausen P. 1892.
Anatomisch-systematische Untersuchung von Blatt und Axe der Acalypheen. –
Ph.D. diss., Universität München, Germany.
Rizk AM. 1987. The chemical constituents and
economic plants of the Euphorbiaceae. – In: Jury SL,
Reynolds T, Cutler DF, Evans FJ (eds), The Euphorbiales. Chemistry, taxonomy
and economic botany, Bot. J. Linn. Soc. 94: 293-326.
Rizk AM, El-Missiry MM. 1986. Non-diterpenoid
constituents of Euphorbiaceae
and Thymelaeaceae. –
In: Evans FJ (ed), Naturally occurring phorbol esters, CRC Press, Boca Raton,
Florida, pp. 107-138.
Robert EMR, Koedam N, Beeckman H, Schmitz N.
2009. A safe hydraulic architecture as wood anatomical explanation for the
difference in distribution of the mangroves Avicennia and
Rhizophora. – Funct. Ecol. 23: 649-657.
Roberts KD, Weiss E, Reichstein T. 1966. Die
Cardenolide der Samen von Mallotus paniculatus Muell. Arg. (Euphorbiaceae). – Helv. Chim.
Acta 49: 316-329.
Robertson A, Wise R, White F. 1989. 138.
Medusagyne oppositifolia. Medusagynaceae. – Kew Mag. 6:
166-171.
Robson NKB. 1960. 16. Violaceae. – In: Exell AW, Wild H
(eds), Flora Zambesiaca 1 (Part 1), Crown Agents for Oversea Governments and
Administrations, London, pp. 246-260.
Robson NKB. 1961. 25. Guttiferae (incl. Hypericaceae). – In: Exell AW,
Wild H (eds), Flora Zambesiaca 1 (Part 2), Crown Agents for Oversea Governments
and Administrations, London, pp. 378-404.
Robson NKB. 1963a. 32. Linaceae. – In: Exell AW, Fernandes
A, Wild H (eds), Flora Zambesiaca 2 (Part 1), Crown Agents for Oversea
Governments and Administrations, London, pp. 91-99.
Robson NKB. 1963b. 33. Ixonanthaceae. – In: Exell AW,
Fernandes A, Wild H (eds), Flora Zambesiaca 2 (Part 1), Crown Agents for
Oversea Governments and Administrations, London, pp. 100-102.
Robson NKB. 1963c. 34. Erythroxylaceae. – In: Exell
AW, Fernandes A, Wild H (eds), Flora Zambesiaca 2 (Part 1), Crown Agents for
Oversea Governments and Administrations, London, pp. 102-109.
Robson NKB. 1963d. 44. Ochnaceae. – In: Exell AW, Fernandes
A, Wild H (eds), Flora Zambesiaca 2 (Part 1), Crown Agents for Oversea
Governments and Administrations, London, pp. 224-262.
Robson NKB. 1972. Evolutionary recall in
Hypericum. – Trans. Bot. Soc. Edinb. 41: 365-383.
Robson NKB. 1974. Hypericaceae. – In: Steenis CGGJ
van (ed), Flora Malesiana, I, 8(1), Sijthoff & Noordhoff International
Publ., Alphen aan den Rijn, The Netherlands, pp. 1-29.
Robson NKB. 1977. Studies in the genus
Hypericum L. (Guttiferae) 1. Infrageneric classification. – Bull.
Brit. Mus. (Nat. Hist.) Bot. 5: 293-355.
Robson NKB. 1981. Studies in the genus
Hypericum L. (Guttiferae) 2. Characters of the genus. – Bull. Brit.
Mus. (Nat. Hist.) Bot. 8: 55-226.
Robson NKB. 1985. Studies in the genus
Hypericum L. (Guttiferae) 3. Sections 1. Campylosporus to 6a.
Umbraculoides. – Bull. Brit. Mus. (Nat. Hist.) Bot. 12: 163-325.
Robseon NKB. 1986. Studies in the genus
Hypericum L. (Guttiferae) 6. Sections 20. Myriandra to 28.
Elodes. – Bull. Brit. Mus. (Nat. Hist.) Bot. 26: 75-217.
Robson NKB. 1987. Studies in the genus
Hypericum L. Guttiferae 7. Section 29. Brathys (part 1). –
Bull. Brit. Mus. (Nat. Hist.) Bot. 16: 1-106.
Robson NKB. 1990. Studies in the genus
Hypericum L. (Guttiferae) 8. Sections 29. Brathys (part 2)
and 30. Trigynobrathys. – Bull. Brit. Mus. (Nat. Hist.) Bot. 20:
1-151.
Robson NKB. 1996. Studies in the genus
Hypericum L. (Guttiferae) 6. Sections 20. Myriandra to 28.
Elodes. – Bull. Brit. Mus. (Nat. Hist.) Bot. 26: 75-217.
Robson NKB. 2001. Studies in the genus
Hypericum L. (Guttiferae) 4(1). Sections 7. Roscyna to 9.
Hypericum sensu lato (part 1). – Bull. Brit. Mus. (Nat. Hist.) Bot.
31: 37-88.
Robson NKB. 2002. Studies in the genus
Hypericum L. (Guttiferae) 4(2). Section 9. Hypericum sensu
lato (part 2): subsection 1. Hypericum series 1. Hypericum.
– Bull. Brit. Mus. (Nat. Hist.) Bot. 32: 61-123.
Robson NKB. 2006. Studies in the genus
Hypericum L. (Clusiaceae)
4(3). Section 9. Hypericum sensu lato (part 3): subsection 1.
Hypericum series 2. Senanensia, subsection 2. Erecta
and section 9b. Graveolentia. – Syst. Biodiv. 4: 19-98.
Robson NKB. 2010a. Studies in the genus
Hypericum L. (Hypericaceae) 5(1). Sections 10.
Olympia to 15/16. Crossophyllum. – Phytotaxa 4: 5-126.
Robson NKB. 2010b. Studies in the genus
Hypericum L. (Hypericaceae) 5(2).
Sections 17. Hirtella to 19. Coridium. – Phytotaxa
4: 127-258.
Robson NKB. 2012. Studies in the genus
Hypericum L. (Hypericaceae) 9. Addenda,
corrigenda, keys, lists and general discussion. – Phytotaxa 72: 1-111.
Robson NKB, Adams WP. 1968. Chromosome
numbers in Hypericum and related genera. – Brittonia 20: 95-106.
Robson NKB, Airy HK. 1962. A note on the
taxonomic position of the genus Cyrillopsis Kuhlmann. – Kew Bull.
15: 387-388.
Rocha AES, Secco RS. 2004. Uma sinopse de
Lacunaria Ducke. – Acta Amazonica 34: 425-433.
Rodrigues CMC, Teixeira Osmond W, Pinheiro
MCB, Lima HA de. 1999. Biologia de reprodução de Clusia lanceolata
Camb. – Hoehnea 26: 61-73.
Rogers CM. 1963. Yellow flowered species of
Linum in eastern North America. – Brittonia 15: 97-122.
Rogers CM. 1966. Sclerolinon, a new
genus in the Linaceae. – Madroño
23: 153-159.
Rogers CM. 1968. Yellow-flowered species of
Linum in Central America and western North America. – Brittonia 20:
107-135.
Rogers CM. 1969. Relationships of the North
American species of Linum (flax). – Bull. Torrey Bot. Club 96:
176-190.
Rogers CM. 1972. The taxonomic significance
of the fatty acid content of seeds of Linum. – Brittonia 24:
415-419.
Rogers CM. 1975. Relationships of
Hesperolinon and Linum (Linaceae). – Madroño 23: 153-159.
Rogers CM. 1979. A new species of
Linum from southern Texas and adjacent Mexico. – Sida 8: 181-187.
Rogers CM. 1980. Pollen dimorphism in
distylous species of Linum sect. Linastrum (Linaceae). – Grana 19: 19-20.
Rogers CM. 1981. A revision of the genus
Linum in southern Africa. – Nord. J. Bot. 1: 711-722.
Rogers CM. 1982. The systematics of
Linum sect. Linopsis (Linaceae). – Plant Systematics and
Evolution 140: 225-234.
Rogers CM. 1984. A further note on the
relationships of the Euopean Linum hologynum and the Australian
species of Linum (Linaceae). – Plant Syst. Evol. 147:
327-328.
Rogers CM. 1985. Pollen morphology of the
monotypic genus Cliococca (Linaceae). – Grana 24: 121-123.
Rogers CM, Mildner R. 1971. The reevaluation
of the genus Cliococca (Linaceae) of South America. – Rhodora
73: 560-565.
Rogers CM, Xavier KS. 1971. Pollen morphology
as an aid in determining relationships among some widely separated Old World
species of Linum. – Grana 11: 55-57.
Rogers DJ. 1951. A revision of
Stillingia in the New World. – Ann. Missouri Bot. Gard. 38:
207-259.
Rogers DJ, Appan SG. 1973. Flora Neotropica.
Monograph 13. Manihot, Manihotoides (Euphorbaceae). – New
York Botanical Garden, Bronx, New York.
Rogers ZS, Sweeney PW. 2007. Two distinctive
new species of Malagasy Garcinia (Clusiaceae). – Syst. Bot. 32:
772-779.
Rojo JP. 1968. The wood anatomy of
Allantospermum borneense Forman and Allantospermum multicaule
(Capuron) Nooteboom. – Adansonia, sér. II, 8: 73-83.
Romano GR, Dwyer JD. 1971. A demonstration of
phloem in the Podostemaceae.
– Bull. Torrey Bot. Club 98: 46-53.
Romo Contreras V, Scogin R, Philbrick CT,
Novelo RA. 1993. A phytochemical study of selected Podostemaceae: systematic
implications. – Aliso 13: 513-520.
Ronse De Craene LP. 2017. Floral development
of the endangered genus Medusagyne (Medusagynaceae-Malpighiales): spatial constraints
of stamen and carpel increase. – Intern. J. Plant Sci. 178: 639-649.
Ronse De Craene LP, Smets E. 1991. Androecium
and floral nectaries of Harungana madagascariensis (Clusiaceae). – Plant Syst. Evol.
178: 179-194.
Ross ES. 2003. Rafflesia: the super
flower. – California Wild 56: 8-11.
Rossignol L, Rossignol M. 1985. Architecture
et tendances évolutives dans le genre Phyllanthus (Euphorbiaceae). – Bull. Brit.
Mus. (Nat. Hist.) Bot. 7: 67-80.
Rossignol L, Rossignol M, Haïcour R. 1987. A
systematic revision of Phyllanthus subsection Urinaria (Euphorbiaceae). – Amer. J. Bot.
74: 1853-1862.
Rössler L. 1943. Vergleichende Morphologie
der Samen europäischer Euphorbia-Arten. – Beih. Bot. Centralbl. 62:
97-174.
Rothdauscher H. 1896. Ueber die anatomischen
Verhältnisse von Blatt und Axe der Phyllantheen (mit Ausschluß der
Euphyllantheen). – Bot. Centralbl. 68: 65-79, 97-108, 129-136, 161-169,
193-203, 248-253, 280-285, 305-315, 338-346, 385-393.
Rouffiac R, Parello J. 1969. Étude chimique
des alcaloïdes du Phyllanthus niruri L. (Euphorbiacées). Présence
de l’antipode optique de la norsécurinine. – Plant. Med. Phytothér. 3:
220-223.
Rowley JR, Erdtman G. 1967. Sporoderm in
Populus and Salix. – Grana Palynol. 7: 517-567.
Roy SK, Ghosh PK. 1982. Fossil wood of Euphorbiaceae from the Tertiary of
West Bengal, India. – Feddes Repert. 93: 363-367.
Royen P van. 1951. The Podostemaceae of the New World I.
– Meded. Bot. Mus. Herb. Rijksuniv. Utrecht 107: 1-151.
Royen P van. 1953. The Podostemaceae of the New World II.
– Meded. Bot. Mus. Herb. Rijksuniv. Utrecht 115: 2-21.
Royen P van. 1955. The Podostemaceae of the New World
III. – Meded. Bot. Mus. Herb. Rijksuniv. Utrecht 119: 215-263.
Rudall PJ. 1987. Laticifers in Euphorbiaceae. A conspectus. –
Bot. J. Linn. Soc. 94: 143-163.
Rudall PJ. 1989. Laticifers in vascular
cambium and wood of Croton spp. (Euphorbiaceae). – IAWA Bull., N.
S., 10: 379-383.
Rudall PJ. 1994. Laticifers in Crotonoideae
(Euphorbiaceae): homology and
evolution. – Ann. Missouri Bot. Gard. 81: 270-282.
Ruhfel BRT. 2011. Systematics and
biogeography of the clusioid clade (Malpighiales). – Ph.D. diss.,
Harvard University, Cambridge, Massachusetts.
Ruhfel BRT, Bittrich V, Bove CP. Gustafsson
MHG, Philbrick CT, Rutishauser R, Xi Z, Davis CC. 2011. Phylogeny of the
clusioid clade (Malpighiales):
evidence from the plastid and mitochondrial genomes. – Amer. J. Bot. 98:
306-325.
Ruhfel BR, Stevens PF, Davis CC. 2013.
Combined morphological and molecular phylogeny of the clusioid clade (Malpighiales) and the placement of
the ancient rosid macrofossil Paleoclusia. – Intern. J. Plant Sci.
174: 910-936.
Ruhfel BR, Bove CP, Philbrick CT, Davis CC.
2016. Dispersal largely explains the Gondwanan distribution of the ancient
tropical clusioid plant clade. – Amer. J. Bot. 103: 1117-1128.
Rury PM. 1981. Systematic anatomy of
Erythroxylum P. Browne: practical and evolutionary implications for
the cultivated cocas. – J. Ethnopharm. 3: 229-263.
Rury PM. 1982. Systematic anatomy of the Erythroxylaceae. – Ph.D.
diss., University of North Carolina, Chapel Hill, North Carolina.
Rury PM. 1985. Systematic and ecological wood
anatomy of Erythroxylaceae.
– IAWA Bull. 6: 365-397.
Rutishauser R. 1995. Developmental patterns
of leaves in Podostemaceae
compared with more typical flowering plants: saltational evolution and fuzzy
morphology. – Can. J. Bot. 73: 1305-1317.
Rutishauser R. 1997. Structural and
developmental diversity in Podostemaceae (river-weeds). –
Aquatic Bot. 57: 29-70.
Rutishauser R, Grubert M. 1994. The
architecture of Mourera fluviatilis (Podostemaceae). – Bot. Helvetica
104: 179-194.
Rutishauser R, Grubert M. 1999. The
architecture of Mourera fluviatilis (Podostemaceae). Developmental
morphology of inflorescences, flowers, and seedlings. – Amer. J. Bot. 86:
907-922.
Rutishauser R, Grubert M. 2000. Developmental
morphology of Apinagia multibranchiata (Podostemaceae) from the Venezuelan
Guyanas. – Bot. J. Linn. Soc. 132: 299-323.
Rutishauser R, Huber KA. 1991. The
developmental morphology of Indotristicha ramosissima (Podostemaceae, Tristichoideae).
– Plant Syst. Evol. 178: 195-223.
Rutishauser R, Pfeifer E. 2002. Comparative
morphology of Cladopus (including Torrenticola, Podostemaceae) from East Asia to
northeastern Australia. – Aust. J. Bot. 50: 725-739.
Rutishauser R, Novelo RA, Philbrick CT. 1999.
Developmental morphology of New World Podostemaceae: Marathrum
and Vanroyenella. – Intern. J. Plant Sci. 160: 29-45.
Rutishauser R, Pfeifer E, Moline P, Philbrick
CT. 2003. Developmental morphology of roots and shoots of Podostemum
ceratophyllum (Podostemaceae-Podostemoideae). –
Rhodora 105: 337-353.
Rutishauser R, Pfeifer E, Bernhard A. 2004.
Podostemaceae of Africa and
Madagascar: keys to genera and species, including genera descriptions,
illustrations to all species known, synonyms, and literature list, version
15-09-04. http://www.systbot.unizh.ch/Podostemaceae
Rutishauser R, Pfeifer E, Novelo RA,
Philbrick CT. 2005. Diamantina lombardii – an odd Brazilian member
of the Podostemaceae. – Flora
200: 245-255.
Saad SI. 1961. Pollen morphology and
sporoderm stratification in Linum. – Grana Palynol. 3: 109-129.
Saad SI. 1962a. Pollen morphology of
Ctenolophon. – Bot. Not. 115: 49-57.
Saad SI. 1962b. Palynological studies in the
Linaceae. – Pollen Spores 4:
65-82.
Sabatier B. 1974. Contribution de la
palynologie à l’étude des Irvingiacées d’Afrique tropicale. –
Adansonia 14: 277-289.
Sabatier D. 2003. Vantanea ovicarpa
(Humiriaceae), a new species from
French Guiana. – Brittonia 54: 233-235.
Saddi N. 1984. Some new taxa in
Kielmeyera (Guttiferae). – Kew Bull. 39: 729-740.
Saddi N. 1987. New species of
Kielmeyera (Guttiferae) from Brazil. – Kew Bull. 42: 221-230.
Saddi N. 1989. Comparative external
morphological study in the genus Kielmeyera Martius (Guttiferae). –
Publ. Avuls. Herb. Central 2, Cuiabá.
Sagun VG, Ham RWJM van der. 2003. Pollen
morphology of the Flueggeinae (Euphorbiaceae, Phyllanthoideae).
– Grana 42: 193-219.
Sagun VG, Levin GA, Ham RWJM van der. 2006.
Pollen morphology and ultrastructure of Acalypha (Euphorbiaceae). – Rev.
Palaeobot. Palynol. 140: 123-143.
Sá-Haiad B de, Serpa-Ribeiro ACC, Barbosa
CN, Pizzini D, Leal D O, Senna-Valle L, Santiago-Fernandes LDR. 2009. Leaf
structure of species from three closely related genera from tribe Crotoneae
Dumort. (Euphorbiaceae s.l., Malpighiales). – Plant Syst.
Evol. 283: 179-202.
Sá-Haiad B de Torres CA, Abreu VHR de,
Gonçalves MR, Mendonça CBF, Santiago-Fernandes LDR de, Bove CP,
Gonçalves-Esteves V. 2010. Floral structure and palynology of Podostemum
weddellianum (Podostemaceae: Malpighiales). – Plant Syst.
Evol. 290: 141-149.
Sainty D, Bailleul F, Delaveau P, Jacquemin
H. 1981. Malpighiacées: nouvelle famille à iridoïdes étude du
Stigmaphyllon sagittatum. – J. Nat. Prod. 44: 576-578.
Salvosa FM. 1936. Rhizophora. –
Nat. Appl. Sci.Bull. Univ. Philipp. 5: 179-237.
Samuel R, Kathriarachchi H, Hoffman P,
Barfuss MHJ, Wurdack KJ, Davis CC, Chase MW. 2005. Molecular phylogenetics of
Phyllanthaceae. Evidence from
plastid matK and nuclear PHYC sequences. – Amer. J. Bot.
92: 132-141.
Santiago LJM, Louro RP, Emmerich M, Barth OM.
2004. The pollen morphology of Phyllanthus (Euphorbiaceae) section
Choretropsis. – Bot. J. Linn. Soc. 144: 243-250.
Santiago LJM, Louro RP, Emmerich M. 2006.
Phyllanthus section Choretropsis (Euphorbiaceae) in Brazil. – Bot.
J. Linn. Soc. 150: 131-164.
Santiago LJM, Louro RP, Emmerich M. 2008.
Phylloclade anatomy in Phyllanthus section Choretropsis (Phyllanthaceae). – Bot. J.
Linn. Soc. 157: 91-102.
Sanz JMC, Rodríguez PM. 2012. Synopsis of
Acalypha (Euphorbiaceae) of continental
Ecuador. – PhytoKeys 17: 1-17.
Sarkar AK, Datta N. 1980. Cytological
assessment of the family Euphorbiaceae II. Tribe
Phyllantheae. – Proc. Indian Sci. Congr. Assoc. (III, C) 67: 48-49.
Sastre C. 1970. Recherches sur les Ochnacées
II. Les espèces de Sauvagesia L. à placenta basale. – Caldasia 10:
497-516.
Sastre C. 1971. Recherches sur les Ochnacées
V. Essai de taxonomie numérique et schéma évolutif du genre
Sauvagesia L. – Sellowia 23: 9-44.
Sastre C. 1975a. Étude du genre
Cespedesia Goudot (Ochnacées). – Cespedesia 4: 191-214.
Sastre C. 1975b. L’importance des
caractères anatomiques dans la systématique des Ochnacées. – C. R. 100ème
Congrès National Soc. Sav. 2: 185-196.
Sastre C. 1981. Ochnacées nouvelles du
Brésil. – Bull. Jard. Bot. Natl. Belg. 51: 347-413.
Sastre C. 1988. Studies on the Flora of the
Guianas 34. Synopsis generis Ouratea Aublet (Ochnaceae). – Bull. Mus. Natl. Hist.
Nat. Paris, sér. IV, 10, sect. B, Adansonia 1: 47-67.
Sastre C. 1992. Vicariance et distribution
géographique de quelques Ochnacées des Guyanes. – C. R. Soc. Biogéogr. 68:
35-45.
Sastre C. 1995. Novelties in the neotropical
genus Ouratea Aublet (Ochnaceae). – Novon 5: 193-200.
Sastre C. 2001. New Ouratea species
(Ochnaceae) from Venezuela and
adjacent countries. – Novon 11: 105-118.
Sastre C. 2004. Une nouvelle espèce
d’Ouratea (Ochnaceae) du
Venezuela. – Adansonia, sér. III, 26: 129-131.
Sastre C. 2005. Une nouvelle espèce
d’Ouratea (Ochnaceae) de
l’Amazonie brésilienne. – Adansonia, sér. III, 27: 85-88.
Sastre C. 2006. Deux nouvelles espèces
d’Ouratea (Ochnaceae)
des Guyanes. – Adansonia, sér. III, 28: 119-127.
Sastre C. 2007. Six nouvelles espèces
d’Ouratea (Ochnaceae)
des Guyanes. – Adansonia, sér. III, 29: 77-91.
Sastre C, Offroy B. 2009. Description de
trois nouveaux Ouratea L. (Ochnaceae) du Paraguay, de Bolivie et
d’Équateur. Considérations taxonomiques, nomenclaturales et
biogéographiques sur les espèces affines d’O. superba Engl. –
Adansonia, sér. III, 31: 89-101.
Sastre C, Offroy B. 2016. Révision
nomenclaturale des binômes du genre néotropical Ouratea Aublet (Ochnaceae) décrits par Van Tieghem.
– Adansonia 38: 55-98.
Sastre C, Whitefoord C, Knapp S. 1999. A new
species of Elvasia (Ochnaceae) from Mesoamerica with
discussion of subgeneric classification and phytogeography. – Novon 9:
252-256.
Sastry SD, Waller GR. 1972. Biosynthesis of
N-methyl-5-carboxamid-2-pyridone from Trewia nudiflora. –
Phytochemistry 11: 2241-2245.
Satabié B. 1974. Contribution de la
palynologie à l’étude des Irvingiacées d’Afrique tropicale. –
Adansonia, sér. II, 14: 277-289.
Sato A, Ogiso A, Kuwano H. 1980. Acyclic
diterpenes from Croton kerrii. – Phytochemistry 19: 2207-2209.
Satterthwait DR. 1982. Passifloraceae. – In: George AS
(ed), Flora of Australia 8, Australian Government Publ. Service, Canberra, pp.
147-158.
Sauer H. 1933. Blüte und Frucht der
Oxalidaceen, Linaceen, Geraniaceen, Tropaeolaceen und Balsaminaceen.
Vergleichend-entwicklungsgeschichtliche Untersuchungen. – Planta 19:
417-481.
Saxena M, Srivastava SK, Rusia K. 1986. A new
flavone from Sapium insigna. – Planta Medica 52: 502.
Sazima M, Sazima I. 1978. Bat pollination of
the passion flower, Passiflora mucronata, in southeastern Brazil. –
Biotropica 10: 100-109.
Schaar F. 1898. Über den Bau des Thallus von
Rafflesia rochussenii Teysm. & Binnend. – Sitzungsber. K. Akad.
Wiss. Math.-Naturwiss. Cl., Abt. I, 107: 1039-1056.
Schaeffer J. 1971. A revision of
Endospermum Benth. (Euphorbiaceae). – Blumea 19:
171-192.
Schappert PJ, Shore JS. 1995. Cyanogenesis in
Turnera ulmifolia L. (Turneraceae) I. Phenotypic
distribution and genetic variation for cyanogenesis on Jamaica. – Heredity
74: 392-404.
Schappert PJ, Shore JS. 1999. Effects of
cyanogenesis polymorphism in Turnera ulmifolia on Euptoieta
hegesia and potential Anolis predators. – J. Chem. Ecol. 25:
1455-1479.
Schappert PJ, Shore JS. 2000. Cyanogenesis in
Turnera ulmifolia L. (Turneraceae) II. Developmental
expression, heritability and cost of cyanogenesis. – Evol. Ecol. Res. 2:
337-352.
Schatz GE, Lowry II PP. 2003. Two new species
of Prockiopsis Baill. (Achariaceae) from Madagascar. –
Adansonia, sér. III, 25: 45-51.
Schenk JJ, Thomas DW. 2004. A new species of
Ledermanniella (Podostemaceae) from Cameroon. –
Novon 14: 227-232.
Schewe LC, Sawhney VK, Davis AR. 2011.
Ontogeny of floral organs in flax (Linum usitatissimum; Linaceae). – Amer. J. Bot. 98:
1077-1085.
Schimper AFW. 1893. Rhizophoraceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien III(7), W. Engelmann,
Leipzig, pp. 42-56.
Schmidt A. 1961. Zytotaxonomische
Untersuchungen an europäischen Viola-Arten der Sektion
Nomimium. – Österr. Bot. Zeitschr. 108: 20-88.
Schmidt A. 1962. Eine neue Grundzahl in der
Gattung Viola. – Ber. Deutsch. Bot. Ges. 75: 78-83.
Schmidt H. 1907. Über die Entwicklung der
Blüten und Blütenstände von Euphorbia L. und Diplocyathium
n. g. – Beih. Bot. Centralbl. 12: 21-69.
Schmidt RJ. 1986. Biosynthetic and
chemosystematic aspects of the Euphorbiaceae and Thymelaeaceae. – Evans FJ
(ed), Naturally occurring phorbol esters, CRC Press, Boca Raton, Florida, pp.
87-106.
Schmidt TJ, Hemmati S, Klaes M, Konuklugil B,
Mohagheghzadeh A, Ionkova I, Fuss E, Alfermann AW. 2010. Lignans in flowering
aerial parts of Linum species – chemodiversity in the light of
systematics and phylogeny. – Phytochemistry 71: 1714-1728.
Schneider JV. 1998. El género
Quiina (Quiinaceae), con
especial referencia a las especies de Venezuela. – Acta Bot. Venez. 21:
1-74.
Schneider JV, Zizka G. 1997. Two new species
of Quiinaceae (Quiina,
Froesia) from the Venezuelan Guayana and some remarks on the genus
Froesia Pires. – Novon 7: 406-412.
Schneider JV, Zizka G. 2003. Taxonomic
novelties in the neotropical genus Quiina Aubl. (Quiinaceae). – Candollea 58:
461-471.
Schneider JV, Zizka G. 2012. Taxonomic
revision of the Neotropical genus Lacunaria (Quiinaceae/Ochnaceae s. l.). – Syst. Bot. 37:
165-188.
Schneider JV, Zizka G. 2017. Phylogeny,
taxonomy and biogeography of Neotropical Quiinoideae (Ochnaceae s.l.). – Taxon 66:
855-867.
Schneider JV, Swenson U, Zizka G. 2002.
Phylogenetic reconstruction of the neotropical family Quiinaceae (Malpighiales) based on morphology
with remarks on the evolution of an androdioecious sex distribution. – Ann.
Missouri Bot. Gard. 89: 64-76.
Schneider JV, Swenson U, Samuel R, Stuessy T,
Zizka G. 2006. Phylogenetics of Quiinaceae (Malpighiales): evidence from
trnL-trnF sequence data and morphology. – Plant Syst. Evol.
257: 189-203.
Schneider JV, Bissiengou P, Carmo E Amaral M
do, Tahir A, Fay MF, Thines M, Sosef MSM, Zizka G, Chatrou LW. 2014.
Phylogenetics, ancestral state reconstruction, and a new infrafamilial
classification of the pantropical Ochnaceae (Medusagynaceae, Ochnaceae s.str., Quiinaceae) based on five DNA
regions. – Molec. Phylogen. Evol. 78: 199-214.
Schnell RAA. 1967. Études sur l’anatomie
et la morphologie des Podostémacées. – Candollea 22: 157-225.
Schnell RAA. 1969. Contribution à l’étude
des Podostémacées de Guyane. – Adansonia, sér. II, 9: 249-271.
Schnell RAA. 1998. III. Anatomie des
Podostémacées. – In: Landolt E, Jäger-Zürn I, Schnell RAA (eds), Extreme
adaptations in angiospermous hydrophytes, Bornträger, Berlin, pp. 197-283.
[Handbuch der Pflanzenanatomie, Vol. 13(4)]
Schnell RAA, Cusset G. 1963. Remarques sur la
structure des plantules des Podostémonacées. – Adansonia, sér. II, 3:
358-369.
Schöfer G. 1954. Untersuchung über die
Polymorphie einheimischer Veilchen. – Planta 43: 537-565.
Schofield EK. 1968. Petiole anatomy of the
Guttiferae and related families. – Mem. New York Bot. Gard. 18: 1-55.
Schot AM. 1995. A synopsis of taxonomic
changes in Aporosa Bl. (Euphorbiaceae). – Blume 40:
449-460.
Schot AM. 2005. Systematics of
Aporosa Blume (Euphorbiaceae). – Blumea
(Suppl.) 17: 1-1382.
Schoute JC. 1937. On the aestivation in the
cyathium of Euphorbia fulgens, with some remarks on the morphological
interpretation of the cyathium in general. – Rec. Trav. Bot. Néerl. 34:
168-181.
Schultes RE. 1952. Studies in the genus
Micrandra I: the relationship of the genus Cunuria to
Micrandra. – Bot. Mus. Leafl. Harvard Univ. 15: 201-222.
Schultes RE. 1970. The history of taxonomic
studies in Hevea. – Bot. Rev. (Lancaster) 36: 197-276.
Schultes RE. 1987. A ne generic concept in
the Euphorbiaceae. – Bot.
Mus. Leafl. 17: 27-36.
Schultes RE. 1990. A brief taxonomic view of
the genus Hevea. – Malaysian Rubber Research and Development Board,
Monograph 14: 1-57.
Schulz OE. 1931. Erythroxylaceae. – In: Engler
A (†), Harms H, Pax F (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd.
19a, W. Engelmann, Leipzig, pp. 130-143.
Schulze GK. 1934. Neue Arten der Gattung
Hybanthus. – Notizbl. Bot. Gart. Berlin-Dahlem 12: 108-114.
Schulze-Menz GK. 1936.
Morphologisch-systematische Studien über die Gattung Hybanthus. –
Bot. Jahrb. Syst. 67: 437-492.
Schürhoff PN. 1924. Zytologische
Untersuchungen in der Reihe der Geraniales. – Jahrb.
Wissensch. Bot. 63: 707-759.
Schwarzbach AE, Ricklefs RE. 2000. Systematic
affinities of Rhizophoraceae
and Anisophylleaceae,
and intergeneric relationships within Rhizophoraceae, based on
chloroplast DNA, nuclear ribosomal DNA, and morphology. – Amer. J. Bot. 87:
547-564.
Schweiger J. 1905. Beiträge zur Kenntnis der
Samenentwicklung der Euphorbiaceen. – Flora 94: 339-379.
Scott DH. 1884. On the laticiferous tissue of
Manihot glaziovii (the Ceara rubber). – Quart. J. Microsp. Sci. 24:
193-203.
Seberg O. 1984. Taxonomy and phylogeny of the
genus Acalypha (Euphorbiaceae) in the Galápagos
Archipelago. – Nord. J. Bot. 4: 159-190.
Secco RSS. 1985. Notas sobre o novo conceito
de Sagotia racemosa em relação ás suas variedades. – Acta
Amazonica 15 [Suppl.]: 81-85.
Secco RSS. 1988. Dialissepalia do gênero
Sandwithia Lanj. (Euphorbiaceae): uma novidade
botânica do alto Rio Negro e da Venezuela. – Bol. Mus. Paraense Emilio
Goeldi, N. S., Bot. 4: 177-185.
Secco RSS. 1990. Revisão dos gêneros
Anomalocalyx Ducke, Dodecastigma Ducke, Pausandra
Radlk., Pogonophora Miers ex Benth. e Sagotia Baill. (Euphorbiaceae-Crononoideae) para a
América do Sul. – Bol. Mus. Paraense Emilio Goeldi, Belem.
Secco RSS. 1997. Revisão taxonômica das
espécies neotropicais da tribo Alchorneae (Hurusawa) Hutchinson (Euphorbiaceae). – Ph.D. diss.,
Universidad de São Paulo, Brazil.
Secco RSS. 2004. Croton
dissectistipulatus, a new species of Euphorbiaceae from Amazonian
Brazil. – Brittonia 56: 353-356.
Secco RSS, Rosa NA. 1992. Croton
ascendens (Euphorbiaceae),
a new liana from eastern Amazonia. – Novon 2: 252-254.
Secco RSS, Webster GL. 1990. Materiais para a
flora amazonica IX. Ensaio sobre a sistemática de gênero Richeria
Vahl (Euphorbiaceae). – Bol.
Mus. Paraense Emilio Goeldi, N. S., Bot. 6: 141-158.
Seemen OV. 1903. Salices Japonicae. –
Berlin.
Seetharam YN. 1985. Clusiaceae: palynology and
systematics. – Inst. Franç. Pondichéry Trav. Sect. Sci. Techn., Tom.
XXI.
Seetharam YN. 1989. Diversity of androecium
and pollen grains in the genus Garcinia L. and its bearing on
geographical distribution and evolution. – Proc. Indian Acad. Sci. Plant Sci.
99: 107-115.
Seetharam YN, Maheshwari JK. 1986. Scanning
electron microscopic studies on the pollen of some Clusiaceae. – Proc. Indian Acad.
Sci., Sect. B, 96:217-226.
Seetharam YN, Pocock SAJ. 1978. Taxonomy and
pollen morphology of Poeciloneuron (Guttiferae). – Bull. Jard. Bot.
Nat. Belg. 48: 359-365.
Sehgal A, Mohan Ram HY, Bhatt JR. 1993. In
vitro germination, growth, morphogenesis and flowering of an aquatic
angiosperm, Polypleurum stylosum (Podostemaceae). – Aquatic Bot.
45: 269-283.
Sehgal A, Sethi M, Mohan Ram HY. 2002.
Origin, structure, and interpretation of the thallus in Hydrobryopsis
sessilis (Podostemaceae).
– Intern. J. Plant Sci. 163: 891-905.
Sehgal A, Khurana JP, Sethi M, Ara H, Jain M.
2007. Organ identity of the thalloid plant body of Griffithella
hookeriana and Polypleurum stylosum – Podostemoideae (Podostemaceae). – Plant Syst.
Evol. 267: 93-104.
Sehgal A, Sethi M, Mohan Ram HY. 2009.
Development of the floral shoot and pre-anthesis cleistogamy in
Hydrobryopsis sessilis (Podostemaceae). – Bot. J. Linn.
Soc. 159: 222-236.
Sehgal A, Khurana JP, Sethi M, Ara H. 2011.
Occurrence of unique three-celled megagametophyte and single fertilization in
an aquatic angiosperm – Dalzellia zeylanica (Podostemaceae-Tristichoideae). –
Sex. Plant Repr. 24: 199-210.
Sehgal L, Paliwal GS. 1974. Studies on the
leaf anatomy of Euphorbia. – VIII. General conclusions and
systematic considerations. – Phytomorphology 24: 141-151.
Seigler DS. 1994. Phytochemistry and
systematics of the Euphorbiaceae. – Ann. Missouri
Bot. Gard. 81: 380-401.
Selling O. 1945. Fossil remains of the genus
Humiria. – Svensk Bot. Tidskr. 39: 257-269.
Selmar D. 1993. Transport of cyanogenic
glucosides: linustatin uptake by Hevea cotyledons. – Planta 191:
191-199.
Seo MN. 2008. Estudios sistemáticos y
evolutivos en especies argentinas del género Hybanthus Jacq. –
Ph.D. diss., Universidad de Buenos Aires, Argentina.
Setoguchi H, Ohba H. 1995. Phylogenetic
relationships in Crossostylis (Rhizophoraceae) inferred from
restriction site variation of chloroplast DNA. – J. Plant Res. 108: 87-92.
Setoguchi H, Tobe H, Ohba H. 1992. Seed coat
anatomy of Crossostylis (Rhizophoraceae): its evolutionary
and systematic implications. – Bot. Mag. (Tokyo) 105: 625-638.
Setoguchi H, Ohba H, Tobe H. 1996. Floral
morphology and phylogenetic analysis in Crossostylis (Rhizophoraceae). – J. Plant
Res. 109: 7-19.
Setoguchi H, Ohba H, Tobe H. 1998. Evolution
in Crossostylis (Rhizophoraceae) on the South
Pacific Islands. – In: Stuessy TF, Ono M (eds), Evolution and speciation of
island plants, Cambridge University Press, Cambridge, pp. 203-229.
Setoguchi H, Kosuge K, Tobe H. 1999.
Molecular phylogeny of Rhizophoraceae based on
rbcL gene sequences. – J. Plant Res. 112: 443-455.
Seymour FC. 1979. Acalypha,
Croton and Sapium in Nicaragua. – Phytologia 43:
133-195.
Shang C, Liao S, Guo Y-J, Zhag Z-X. 2017.
Dianyuea gen. nov. (Salicaceae: Scyphostegioideae) from
southwestern China. – Nord. J. Bot. 35: 499-505
Sharma BD, Karthikeyan S, Shetty BV. 1974.
Indotristicha tirunelveliana Sharma, Karthikeyan & Shetty – a
new species of Podostemaceae
from South India. – Bull. Bot. Survey India 6: 157-161.
Sharma KD. 1955. Cyto-embryological studies
in some Indian Euphorbiaceae.
– Sci. & Cult. 21: 270-271.
Sharsmith HK. 1961. The genus
Hesperolinon. – Univ. Calif. Publ. Bot. 32: 235-314.
Sheng-ye L. 1992. Bhesa sinica. –
Li-kuo F, Jian-ming J (eds), China plant red data book – rare and endangered
plants 1, Science Press, Beijing, pp. 206-207.
Sheue C-R, Liu H-Y, Yong JWH. 2003.
Kandelia obovata (Rhizophoraceae), a new mangrove
species from eastern Asia. – Taxon 52: 287-294.
Sheue C-R, Chesson P, Chen Y-J, Wu S-Y, Wu
Y-H, Yong JWH, Guu T-Y, Lim C-L, Randrianasolo RMA, Razanajatovo MH, Yang Y-P.
2013. Comparative systematic study of colleters and stipules of Rhizophoraceae with implications
for adaptation to challenging environments. – Bot. J. Linn. Soc. 172:
449-464.
Shi S, Zhong Y, Huang Y, Du Y, Qiu X, Chang
H. 2002. Phylogenetic relationships of the Rhizophoraceae in China based on
sequences of the chloroplast gene matK and the internal transcribed
spacer regions of nuclear ribosomal DNA and combined data set. – Biochem.
Syst. Ecol. 30: 309-319.
Shi S, Huang Y, Zeng K, Tan F, He H, Huang J,
Fu Y. 2005. Molecular phylogenetic analysis of mangroves: independent
evolutionary origins of vivipary and salt secretion. – Mol. Phylogen. Evol.
34: 159-166.
Shivamurthy GR, Sadanand KB. 1997. A new
species of Willisia Warm. (Podostemaceae) from the Silent
Valley, Kerala, India. – Kew Bull. 52: 243-245.
Shivanna KR, Ciampolini F, Cresti M. 1989.
The structure and cytochemistry of the pistil of Hypericum calycinum:
the stigma. – Ann. Bot., N. S., 63: 613-620.
Shore JS, Barrett SCH. 1985. Morphological
differentiation and crossability among populations of the Turnera
ulmifolia complex (Turneraceae). – Syst. Bot. 10:
308-321.
Shore JS, Obrist CM. 1992. Variation in
cyanogenesis within and among populations and species of Turnera
series Canaligerae (Turneraceae). – Biochem. Syst.
Ecol. 20: 9-18.
Shore JS, McQueen KL, Little SL. 1994.
Inheritance of plastid DNA in the Turnera ulmifolia complex. – Amer.
J. Bot. 81: 1636-1639.
Shore JS, Arbo MM, Fernández A. 2006.
Breeding system variation, genetics and evolution in the Turneraceae. – New Phytol. 171:
539-551.
Si C-L, Wu L, Zhu Z-Y. 2009. Phenolic
glycosides from Populus davidiana bark. – Biochem. Syst. Ecol. 37:
221-224.
Sibanda S, Nyanyira C, Nicoletti M, Galeffi
C. 1993. Vismiones L and M from Ochna pulchra. – Phytochemistry 34:
1650-1652.
Sierra SEC, Aparicio M, Kulju KKM, Fišer Ž,
Welzen P van, Ham RWJM van der. 2006. Reshaping Mallotus I: expanded
circumscription and revision of the genus Cordemoya. – Blumea 51:
519-540.
Sierra SEC, Kulju KKM, Veldkamp JF, Welzen P
van. 2007. Resurrection of Hancea and lectotypification of
Adisca (Euphorbiaceae). – Blumea 52:
361-366.
Sierra SEC, Kulju KKM, Fišer Ž, Aparicio M,
Welzen PC van. 2010. The phylogeny of Mallotus s.str. (Euphorbiaceae) inferred from DNA
sequence and morphological data. – Taxon 59: 101-116.
Sigrist MR, Sazima M. 2004. Pollination and
reproductive biology of twelve species of neotropical Malpighiaceae: stigma morphology
and its implications for the breeding system. – Ann. Bot. 94: 33-41.
Silva Souto L, Trombert Oliveira DM. 2005.
Morfo-anatomia e ontogênese do fruto e semente de Byrsonima
intermedia A. Juss. (Malpighiaceae). – Rev. Brasil.
Bot. 28: 697-712.
Simmonds MSJ, Blaney WM, Monache F delle,
Marquina Mac-Quhae M, Marini Bettolo GB. 1985. Insect antifeedant properties of
anthranoids from the genus Vismia. – J. Chem. Ecol. 11:
1593-1599.
Simon J, Vicens J. 1999. Estudis
biosistemátics en Euphorbia L. a la Mediterránia occidental. –
Inst. Estud. Catalans, Arx Secc. Ci. 122.
Simpson BB. 1989. Pollination biology and
taxonomy of Dinemandra and Dinemagonum (Malpighiaceae). – Syst. Bot. 14:
408-426.
Simpson MG, Levin GA. 1994. Pollen
ultrastructure of the biovulate Euphorbiaceae. – Intern. J.
Plant Sci. 155: 313-341.
Singh B. 1959. Studies in the family Malpighiaceae I. Morphology of
Thryallis glauca Kuntze. – Horticult. Advance 3: 1-19.
Singh B. 1961a. Studies in the family Malpighiaceae II. Morphology of
Malpighia glabra Linn. – Horticult. Advance 5: 83-96.
Singh B. 1961b. Studies in the family Malpighiaceae III. Development and
structure of seed and fruit of Malpighia glabra Linn. – Horticult.
Advance 5: 145-155.
Singh D. 1963. Structure and development of
ovule and seed of Viola tricolor L. and Ionidium
suffruticosum Ging. – J. Indian Bot. Soc. 42: 448-462.
Singh D. 1970a. Violaceae. – In: Proceedings of the
symposium on comparative embryology of angiosperms, Indian National Science
Academy, New Delhi, pp. 188-193.
Singh D. 1970b. Passifloraceae. – In:
Proceedings of the symposium on comparative embryology of angiosperms, Indian
National Science Academy, New Delhi, pp. 199-204.
Singh RP. 1954. Structure and development of
seeds in Euphorbiaceae.
Ricinus communis L. – Phytomorphology 4: 118-123.
Singh RP. 1956. Development of endosperm and
embryo in Phyllanthus niruri L. – Agra Univ. J. Res. (Sci.) 5:
163-167.
Singh RP. 1962. Forms of ovules in Euphorbiaceae. – In: Plant
Embryology, A symposium, New Delhi, pp. 124-128.
Singh RP. 1965a. Structure and development of
seeds in Codiaeum variegatum Blume. – J. Indian Bot. Soc. 44:
205-210.
Singh RP. 1965b. Structure and development of
seeds in Euphorbiaceae:
Antidesma menasu Miquel. – Balwant Vidyapeeth J. Agric. Sci. Res. 7:
96-99.
Singh RP. 1968. Structure and development of
seeds in Euphorbiaceae:
Melanthesa rhamnoides Wt. – Beitr. Biol. Pflanzen 45: 127-133.
Singh RP. 1969. Structure and development of
seeds in Euphorbia helioscopia. – Bot. Mag. (Tokyo) 82: 287-293.
Singh RP. 1970a. Structure and development of
seeds in Euphorbiaceae:
Jatropha species. – Beitr. Biol. Pflanzen 47: 79-90.
Singh RP. 1970b. Structure and development of
seeds in Putranjiva roxburghii Wall. – J. Indian Bot. Soc. 49A:
99-105.
Singh RP. 1972. Structure and development of
seeds in Phyllanthus niruri L. – J. Indian Bot. Soc. 1: 73-77.
Singh RP, Chopra S. 1970. Structure and
development of seeds in Croton bonplandianum Baill. –
Phytomorphology 20: 83-87.
Singh RP, Jain JL. 1965. Development of
female gametophyte in Euphorbia pilosa L. – Curr. Sci. 34:
611-612.
Singh RP, Pal A. 1968. Structure and
development of seeds in Euphorbiaceae: Dalechampia
roezeliana Muell.-Arg. – I: Techn. Commun. Natl. Bot. Gard., pp.
65-74.
Singh V, Singh A. 1975. Placentation in Euphorbiaceae. – Ann. Bot., N.
S., 39: 1137-1140.
Sirirugsa P. 1987. Three new species of
Hiptage (Malpighiaceae) from Thailand. –
Nord. J. Bot. 7: 277-280.
Skottsberg C. 1940. Observations on Hawaiian
violets. – Acta Horti Gothoburg. 13: 451-528.
Skvortsov AK. 1968. Willows of the USSR. A
taxonomic and geographic survey. – Nauka, Moscow. [In Russian with English
summary]
Sleumer H. 1934. Beiträge zur Kenntnis der
Flacourtiaceen südamericanas 1. – Notizbl. Bot. Gart. Berlin-Dahlem 11:
951-960.
Sleumer H. 1942. Icacinaceae.
– In: Engler A (†), Harms H, Mattfeld J (eds), Die natürlichen
Pflanzenfamilien, 2. Aufl., Bd. 20b, W. Engelmann, Leipzig, pp. 322-396.
Sleumer H. 1955. Flacourtiaceae. – In:
Steenis CGGJ van (ed), Flora Malesiana I, 5(1), Noordhoff-Kolff N. V.,
Djakarta, pp. 1-106.
Sleumer H. 1956. Note on the genus
Guidonia Plumier. – Taxon 5: 192-194.
Sleumer H. 1968. The genus
Lophopyxis Hook. f. (Lophopyxidaceae). – Blumea 16:
321-323.
Sleumer H. 1970. Le genre Paropsia
Noronha ex Thouars (Passifloraceae). – Bull. Jard.
Bot. Natl. Belg. 40: 49-75.
Sleumer H. 1971a. Lophopyxidaceae. – In: Steenis
CGGJ van (ed), Flora Malesiana, I, 7, Wolters-Noordhoff, Groningen, pp.
89-91.
Sleumer H. 1971b. Le genre Casearia
Jacq. (Flacourtiaceae) en Afrique, à Madagascar et aux Mascareignes. – Bull.
Jard. Bot. Natl. Belg. 41: 397-426.
Sleumer H. 1974. Die afrikanischen Arten der
Gattung Lindackeria Presl (Flacourtiaceae). – Bot. Jahrb. Syst. 94:
311-326.
Sleumer H. 1975. Flacourtiaceae. – In:
Polhill RM (ed), Flora of tropical East Africa, Crown Agents for Oversea
Government and Administrations, London, pp. 1-68.
Sleumer H. 1980. Flora Neotropica. Monograph
22. Flacourtiaceae. – New York Botanical Garden, Bronx, New York.
Slik JWF, Welzen PC van. 2001. A phylogeny of
Mallotus (Euphorbiaceae) based on
morphology: indications for a pioneer origin of Macaranga. – Syst.
Bot. 26: 786-796.
Small JK. 1917. The Jamaica walnut. – J.
New York Bot. Gard. 18: 180-186.
Smith CR Jr, Weisleder D, Miller RW. 1980.
Linustatin and neolinustatin: cyanogenic glucosides of lineseed meal that
protect animals against selenium toxicity. – J. Organic Chem. 45: 507-510.
Smith DL. 1966. Linaceae. – In: Hubbard CE,
Milne-Redhead E (eds), Flora of tropical East Africa, Crown Agents for Oversea
Governments and Administrations, London, pp. 1-12.
Smith LB, Downs RJ. 1964.
Kleinodendron, novo genero de Euforbiáceas. – Sellowia 16:
175-178.
Smith RL, Sytsma KJ. 1990. Evolution of
Populus nigra (sect. Aegiros): introgressive hybridization
and chloroplast contribution of Populus alba (sect. Populus).
– Amer. J. Bot. 77: 1176-1187.
Snow DW. 1981. Tropical frugivorous birds and
their food plants: a world survey. – Biotropica 13: 1-14.
Snow N, MacDougal JM. 1993. New chromosome
reports in Passiflora (Passifloraceae). – Syst. Bot.
18: 261-273.
Sofiyanti N, Mat-Salleh K, Purwanto D,
Syahputra E. 2007. The note on morphology of Rafflesia hasseltii
Surigar from Bukit Tiga Puluh National Park, Riau. – Biodiversitas 9:
257-261.
Sohma K. 1993. Pollen diversity in
Salix (Salicaceae). –
Sci. Rep. Tôhoku Univ., Ser. IV (Biology) 40: 77-178.
Solís Neffa VG, Fernández A. 2000.
Chromosome studies in Turnera (Turneraceae). – Gen. Mol. Biol.
23: 925-930.
Solís Neffa VG, Fernandez A. 2001.
Cytogeography of the South American Turnera sidoides L. complex (Turneraceae, Leiocarpae). – Bot.
J. Linn. Soc. 137: 189-196.
Solís Neffa VG, Faloci MM, Seijo JG. 2003.
Cyanogenesis variation in the Turnera sidoides L. polyploid complex
(Turneraceae). – Bot. J. Linn.
Soc. 141: 85-94.
Solms-Laubach H zu. 1874. Über den Bau des
Samens in den Familien der Rafflesiaceae und Hydnoraceae. – Bot.
Zeit. 32: 337-342, 353-358, 369-374, 385-389.
Solms-Laubach H zu. 1875. Das Haustorium der
Loranthaceen und der Thallus der Rafflesiaceen und Balanophoraceen. – Abh.
Naturf. Ges. Halle 13: 1-40, 238-276.
Solms-Laubach H zu. 1898. Die Entwicklung des
Ovulums und des Samens bei Rafflesia und Brugmansia. – Ann.
Jard. Bot. Buitenzorg Suppl. 2: 11-21.
Soltis DE, Mort ME, Soltis PS, Hibsch-Jetter
C, Zimmer EA, Morgan D. 1999. Phylogenetic relationships of the enigmatic
angiosperm family Podostemaceae
inferred from 18S rDNA and rbcL sequence data. – Mol. Phylogen.
Evol. 11: 261-272.
Soontornchainaksaeng P, Chaiyasut K. 1999.
Cytogenetic investigation of some Euphorbiaceae in Thailand. –
Cytologia 64: 229-234.
Soontornchainaksaeng P, Chantaranothai P,
Senakun C. 2003. Genetic diversity of Croton L. (Euphorbiaceae) in Thailand. –
Cytologia 68: 379-382.
Sosa V, Chase MW, Barcenas C. 2003.
Chiangiodendron (Achariaceae): an example of the
Laurasian flora of tropical forests of Central America. – Taxon 52:
519-524.
Sosef MSM. 2008. Révision du genre africain
Rhabdophyllum Tiegh. (Ochnaceae), avec sa distribution au
Cameroun et au Gabon. – Adansonia, sér. III, 30: 119-135.
Sosef MSM, Dauby G. 2012. Contribution to the
taxonomy of Garcinia (Clusiaceae) in Africa, including two
new species from Gabon and a key to the Lower Guinean species. – PhytoKeys
17: 41-62.
Sothers CA, Prance GT. 2014. Resurrection of
Angelesia, a Southeast Asian genus of Chrysobalanaceae. – Blumea
59: 103-105.
Sothers CA, Prance GT, Buerki S, De Kok R,
Chase MW. 2014. Taxonomic novelties in Neotropical Chrysobalanaceae: towards a
monophyletic Couepia. – Phytotaxa 172(3). DOI:
http://dx.doi.org/10.11646/phytotaxa.172.3.2
Sothers CA, Prance GT, Chase MW. 2016.
Towards a monophyletic Licania: a new generic classification of the
polyphyletic Neotropical genus Licania (Chrysobalanaceae). – Kew
Bull. 71: 58 DOI 10.1007/S12225-016-9664-3
Souèges R. 1936. Les relations
embryogéniques des Crassulacées, Saxifragacées et Hypéricacées. – Bull.
Soc. Bot. France 83: 317-329.
Souèges R. 1937. Développement de
l’embryon chez le Radiola linoides Roth. – Bull. Soc. Bot. France
84: 297-306.
Soukup J. 1965. Opiliaceae, Balanophoraceae, Aristolochiaceae, Rafflesiaceae, Polygonaceae
of Peru. – Biota 5: 315-339.
Soukup J. 1970. Las Passifloraceas del Perú,
sus generos y lista de especies. – Biota 8: 106-110.
Souto LS, Oliveira DMT. 2008. Morfoanatomia e
ontogênese das sementes de espécies de Banisteriopsis C. B. Robinson
e Diplopterys A. Juss. (Malpighiaceae). – Acta Bot.
Brasil. 22: 733-740.
Souto LS, Oliveira DMT. 2013. Evaluation of
the floral vasculature of the Janusia, Mascagnia and
Tetrapterys species as a tool to explain the decrease of floral organs
in Malpighiaceae. – Flora
208: 351-359.
Souza JP. 2002. Levantamento da espécies de
Hybanthus Jacq. (Violaceae) do Brasil. – M.Sc.
thesis, Universidade do São Paulo, Brasil.
Souza S de. 1979. Chrysobalanus
icaco Linn. (Chrysobalanacées): ses sous-espèces et variétés en
République Populaire de Benin. – Ann. Univ. Abidjan, sér. C, 15: 97-105.
Sparre B. 1950. Estudio sobre las Violáceas
argentinas I. Los géneros de Hybanthus y Anchietea. –
Lilloa 23: 515-574.
Spencer KC. 1988. Chemical mediation of
coevolution in the Passiflora-Heliconius interaction. – In:
Spencer KC (ed), Chemical mediation of coevolution, Academic Press, San Diego,
pp. 167-240.
Spencer KC, Seigler DS. 1978. Cyanogenic
glycosides of Malesherbia. – Biochem. Syst. Ecol. 13: 23-24.
Spencer KC, Seigler DS. 1985b. Cyanogenic
glycosides and the systematics of the Flacourtiaceae. – Biochem. Syst. Ecol.
13: 421-431.
Spencer KC, Seigler DS, Fraly SW. 1985.
Cyanogenic glycosides of the Turneraceae. – Biochem. Syst.
Ecol. 13: 433-435.
Speranza PR, Seijo JG, Grela IA, Solís Neffa
VG. 2007. Chloroplast DNA variation in the Turnera sidoides L. complex
(Turneraceae). – J. Biogeogr.
34: 427-436.
Spirlet M-L. 1959. Étude taxonomique des
epidermis foliaires des Hypéricacées et des Guttiféracées du basin du
fleuve Congo. – Bull. Inst. Franç. Afr. Noire 29: 5-91.
Spirlet M-L. 1965. Utilisation taxonomique
des grains de pollen des Passifloracées I. – Pollen Spores 7: 249-301.
Sprague TA, Boodle LA. 1909. Kokoti
(Anopyxis ealaensis Sprague). – Bull. Misc. Inform. (Kew Bull.)
1909: 309-312.
Stajsic V, Walsh NG, Douglas R, Messina A,
Molloy BPJ. 2015. A revision of Melicytus (Violaceae) in mainland Australia and
Tasmania. – Aust. Syst. Bot. 27: 305-323.
Standley PC. 1927. Celaenodendron.
– In: Ferris RS (ed), Preliminary report on the flora of the Tres Marías
Islands, Contr. Dudley Herb. 1: 3-88, pp. 76-77.
Steenis CGGJ van. 1949. Trigoniaceae. – In: Steenis CGGJ
van (ed), Flora Malesiana I, 4(2), Noordhoff-Kolff N. V., Batavia, pp.
58-60.
Steenis CGGJ van. 1957a. Scyphostegiaceae.
– In: Steenis CGGJ van (ed), Flora Malesiana I, 5(3), Noordhoff-Kolff N. V.,
Djakarta, pp. 297-299.
Steenis CGGJ van. 1957b. Dichapetalaceae. – In: Steenis
CGGJ van (ed), Flora Malesiana I, 5(3), Noordhoff-Kolff N. V., Djakarta, pp.
305-316.
Steenis CGGJ van. 1964. The identity of the
genus Austrobuxus Miq. (Euphorbiaceae). – Blumea 12:
362.
Steenis CGGJ van. 1972a. Addenda, corrigenda
et emenanda: Scyphostegiaceae. – Flora Malesiana Bull. 6: 967-968.
Steenis CGGJ van. 1972b. Podostemaceae. – In: Steenis
CGGJ van (ed), Flora Malesiana, I, 6, Wolters-Noordhoff, Groningen, pp.
963-964.
Steiner KE. 1982. Mistake pollination of
Hura crepitans (Euphorbiaceae) by frugivorous
bats. – Ph.D. diss., University of California, Davis, California.
Steiner KE. 1983. Pollination of Mabea
occidentalis (Euphorbiaceae) in Panama. –
Syst. Bot. 8: 105-117.
Steiner KE, Whitehead VB. 1991. Resin
collection and the pollination of Dalechampia capensis (Euphorbiaceae) by
Pachyanthidium (Megachilidae) in South Africa. – J. Entomol. Soc.
South Afr. 54: 67-72.
Steinmann VW. 2001. The evolution of
succulence in the New World species of Euphorbia (Euphorbiaceae). – Ph.D. diss.,
Claremont Graduate University, Claremont, California.
Steinmann VW. 2003. The submersion of
Pedilanthus into Euphorbia (Euphorbiaceae). – Acta Bot.
Mexicana 65: 45-50.
Steinmann VW. 2005. New Euphorbiaceae from Mexico II. –
Contr. Univ. Michigan Herb. 24: 173-187.
Steinmann VW, Gordillo MM. 2007. Croton
balsensis (Euphorbiaceae),
a new species from the Basas Depression, Mexico. – Brittonia 59: 380-384.
Steinmann VW, Porter JM. 2002. Phylogenetic
relationships in Euphorbieae (Euphorbiaceae) based on ITS and
ndhF sequence data. – Ann. Missouri Bot. Gard. 89: 453-490.
Steinmann VW, Ramírez-Amezcua Y. 2013.
Bia manuelii (Euphorbiaceae: Acalyphoideae), a
new species from Sierra de Coalcomán, Michoacán, Mexico. – Rev. Mexicana
Biodiv. 84: 746-750.
Steinmann VW, Van Ee B, Berry PE, Gutiérrez
J. 2007. The systematic position of Cubanthus and other shrubby
endemic species of Euphorbia (Euphorbiaceae) in Cuba. – An.
Jard. Bot. Madrid 64: 123-133.
Stenar H. 1937. Zur Embryosackentwicklung
einiger Malpighiaceen. – Bot. Not. 1937: 110-118.
Stern WL. 1967. Kleinodendron and
xylem anatomy of Clutieae (Euphorbiaceae). – Amer. J. Bot.
54: 663-676.
Steude H. 1935. Beiträge zur Morphologie und
Anatomie von Mourera aspera. – Beih. Bot. Centralbl. 53A:
627-650.
Stevens PF. 1974. A review of
Calophyllum L. (Guttiferae) in Papuasia. – Aust. J. Bot. 22:
349-411.
Stevens PF. 1976. The Old World species of
Calophyllum (Guttiferae) I. The Mascarene species. – J. Arnold
Arbor. 57: 167-184.
Stevens PF. 1980. A revision of the Old World
species of Calophyllum (Guttiferae). – J. Arnold Arbor. 61:
117-699.
Stevens PF. 2006a. Clusiaceae-Guttiferae. – In:
Kubitzki K (ed), The families and genera of vascular plants IX. Flowering
plants. Eudicots. Berberidopsidales,
Buxales, Crossosomatales, Fabales p.p., Geraniales, Gunnerales, Myrtales p.p., Proteales, Saxifragales, Vitales, Zygophyllales, Clusiaceae Alliance, Passifloraceae Alliance, Dilleniaceae, Huaceae, Picramniaceae, Sabiaceae, Springer, Berlin,
Heidelberg, New York, pp. 48-66.
Stevens PF. 2006b. Hypericaceae. – In: Kubitzki K
(ed), The families and genera of vascular plants IX. Flowering plants.
Eudicots. Berberidopsidales,
Buxales, Crossosomatales, Fabales p.p., Geraniales, Gunnerales, Myrtales p.p., Proteales, Saxifragales, Vitales, Zygophyllales, Clusiaceae Alliance, Passifloraceae Alliance, Dilleniaceae, Huaceae, Picramniaceae, Sabiaceae, Springer, Berlin,
Heidelberg, New York, pp. 194-201.
Steyermark JA. 1984. Theaceae (Bonnetiaceae). – In: Flora of the
Venezuelan Guayana I, Ann. Missouri Bot. Gard. 71: 323-330.
Steyermark JA, Bunting GS. 1975. Revision of
the genus Froesia (Quiinaceae). – Brittonia 27:
172-178.
Steyermark JA, Liesner R. 1983. Revision of
the genus Sterigmapetalum (Rhizophoraceae). – Ann.
Missouri Bot. Gard. 70: 179-193.
Steyermark JA, Luteyn JL. 1980. Revision of
the genus Ochthocosmus (Linaceae). – Brittonia 32:
128-143.
Steyn EMA, Wyk AE van, Smith GF. 2002. Ovule,
seed and seedling characters in Acharia (Achariaceae) with evidence of
myrmecochory in the family. – South Afr. J. Bot. 68: 143-156.
Steyn EMA, Wyk AE van, Smith GF. 2002. A
study of ovule-to-seed development in Ceratosicyos (Achariaceae) and the systematic
position of the genus. – Bothalia 32: 201-210.
Steyn EMA, Wyk AE van, Smith GF. 2003.
Embryology and systematic relationships of Kiggelaria
(Flacour-tiaceae). – Bothalia 33: 199-206.
Steyn EMA, Wyk AE van, Smith GF. 2004.
Functional and taxonomic significance of seed structure in Salix
mucronata (Salicaceae). –
Bothalia 34: 53-59.
Steyn EMA, Wyk AE van, Smith GF. 2005a.
Ovule-to-seed development in Dovyalis caffra (Salicaceae: Flacourtieae) with notes
on the taxonomic significance of the extranucellar embryo sac. – Bothalia 35:
101-108.
Steyn EMA, Wyk AE van, Smith GF. 2005b. Ovule
and seed structure in Scolopia zeyheri (Scolopieae), with notes on the
embryology of Salicaceae. –
Bothalia 35: 175-183.
St. John H. 2004. Omalanthus (Euphorbiaceae) in southeastern
Polynesia – Pacific plant studies 40. – Nord. J. Bot. 4: 53-56.
Stoops E, Welzen PC van. 2013. A revision of
Ptychopyxis (Euphorbiaceae) in southeast Asia.
– Nord. J. Bot. 31: 94-112.
Stuppy W. 1996. Systematische Morphologie und
Anatomie der Samen der biovulaten Euphorbiaceen. – Ph.D. diss., Fachbereich
Biologie, Universität Kaiserslautern, Germany.
Stuppy W, Welzen PC van, Klinratana P, Posa
MCT. 1999. Revision of the genera Aleurites, Reutealis and
Vernicia (Euphorbiaceae). – Blumea 44:
73-98.
Suárez-Cervera M, Gillespie L, Arcalís E,
Le Thomas A, Lobreau-Callen D, Seoane-Camba J. 2001. Taxonomic significance of
sporoderm structure in pollen of Euphorbiaceae: tribes Plukenetieae
and Euphorbieae. – Grana 40: 78-104.
Subramanyam K. 1962. Embryology in relation
to systematic botany, with particular reference to the Crassulaceae. –
In: Plant embryology: a symposium, C.S.I.R., New Delhi, pp. 94-112.
Subramanyam K, Sreemadhavan CP. 1969. A
conspectus of the families Podostemaceae and Tristichaceae.
– Bull. Bot. Surv. India 11: 161-168.
Subramanian RB, Arumugasamy K, Inamdar JA.
1990. Studies in the secretory glands of Hiptage sericea (Malpighiaceae). – Nord. J. Bot.
10: 57-62.
Subra Rao AM. 1940. Studies in the Malpighiaceae 1. Embryo sac
development and embryogeny in the genera Hiptage, Banistera,
and Stigmatophyllum. – J. Indian Bot. Soc. 18: 145-156.
Subra Rao AM. 1941. Studies in the Malpighiaceae 2. Structure and
development of the ovules and embryo sacs of Malpighia coccifera Linn.
and Tristellateia australis Linn. – Proc. Indian Acad. Sci., Sect.
B, 7: 393-404.
Suda Y. 1963. The chromosome numbers of
salicaceous plants in relation to their taxonomy. – Sci. Rep. Tôhoku Imp.
Univ., Ser. IV (Biology), 29: 413-430.
Suda Y, Argus GW. 1969. Chromosome numbers of
some North American arctic and boreal Salix. – Can. J. Bot. 47:
859-862.
Sugawara T, Tanaka N, Murata J, Zaw KM. 2002.
Dimorphism of pollen grains and stigmas in the heterostylous subshrub
Reinwardtia indica (Linaceae) in Myanmar. – Acta
Phytotaxon. Geobot. 53: 173-180.
Suksathan P, Larsen K. 2006. A new species of
Tirpitzia (Linaceae) from
Thailand. – Thai Forest Bull. 34: 201-205.
Suryakanta. 1974. Pollen morphological
studies in the Humiriaceae. –
J. Jap. Bot. 49: 112-122.
Susila Rani SRM, Balakrishnan NP. 1995. A
revision of the genus Claoxylon Adr. Jussieu (Euphorbiaceae) in India. –
Rheedea 5: 113-141.
Sussex IM. 1975. Growth and metabolism of the
embryo and attached seedling of the viviparous mangrove, Rhizophora
mangle. – Amer. J. Bot. 62: 948-953.
Sutter DM, Endress PK. 1995. Aspects of
gynoecial structure and macrosystematics in Euphorbiaceae. – Bot. Jahrb.
Syst. 116: 517-536.
Sutter DM, Endress PK. 2003. Female flower
and cupule structure in Balanopaceae, an enigmatic rosid
family. – Ann. Bot. 92: 459-469.
Sutter DM, Forster PI, Endress PK. 2006.
Female flowers and systematic position of Picrodendraceae (Euphorbiaceae s.l., Malpighiales). – Plant Syst.
Evol. 261: 187-215.
Suzuki K, Kita Y, Kato M. 2002. Comparative
developmental anatomy of seedlings in nine species of Podostemaceae (subfamily
Podostemoideae). – Ann. Bot. 89: 755-765.
Swaine MD, Beer T. 1976. Explosive seed
dispersal in Hura crepitans L. (Euphorbiaceae). – New Phytol.
78: 695-708.
Swamy BGL. 1953. On the floral structure of
Scyphostegia. – Proc. Indian Acad. Sci., Sect. B, 19: 127-142.
Swamy BGL, Ganapathy PM. 1957. A new type of
endosperm haustorium in Nothapodytes foetida. – Phytomorphology 7:
331-336.
Swanepoel W. 2009. Euphorbia ohiva
(Euphorbiaceae), a new species
from Namibia and Angola. – South Afr. J. Bot. 75: 249-255.
Sweeney PW. 2008. Phylogeny and floral
diversity in the genus Garcinia (Clusiaceae) and relatives. –
Intern. J. Plant Sci. 169: 1288-1303.
Sweeney PW. 2010. Floral anatomy in
Garcinia nervosa and G. xanthochymus (Clusiaceae): a first step toward
understanding the nature of nectaries in Garcinia. – Bull. Peabody
Mus. Nat. Hist. 51: 157-168.
Szyszyłowicz I von. 1895a. Caryocaraceae (Rhizoboleae). –
In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(6), W.
Engelmann, Leipzig, pp. 153-157.
Szyszyłoicz I von. 1895b. Theaceae (Ternstroemiaceae).
– In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(6), W.
Engelmann, Leipzig, pp. 175-192.
Tabor RJ. 1911. The leaf buds of
Archytaea alternifolia. – Ann. Bot. 25: 1015-1021.
Takahashi M, Nowicke JW, Webster GL, Orli SS,
Yankowski S. 2000. Pollen morphology, exine structure, and systematics of
Acalyphoideae (Euphorbiaceae),
part 3: tribes Epiprineae, Adelieae, Alchorneae, Acalypheae pro parte. – Rev.
Palaeobot. Palynol. 110: 1-66.
Takayama K, Tamura M, Tateishi Y, Webb EL,
Kajita T. 2013. Strong genetic structure over the American continents and
transoceanic dispersal in the mangrove genus Rhizophora (Rhizophoraceae) revealed by
broad-scale nuclear and chloroplast DNA analysis. – Amer. J. Bot. 100:
1191-1201.
Takhtajan A, Meyer NR, Kosenko R, Kosenko VN.
1985. Pollen morphology and classification in Rafflesiaceae s. l. – Bot.
Žurn. 70: 153-162. [In Russian]
Tammaro F, Pogliani M. 1977. Andrachne
telephioides L. nella Valle dell’Aterno, nuovo reperto per la Flora
Abruzzese. – Webbia 32: 135-145.
Tateishi S. 1927. On the development of the
embryo sac and fertilization of Acalypha australis L. – Bot. Mag.
(Tokyo) 41: 477-485.
Taylor DW, Crepet WL. 1990. Fossil floral
evidence of Malpighiaceae and
an early plant-pollinator relationship. – Amer. J. Bot. 74: 274-286.
Taylor FH. 1972. The secondary xylem of the
Violaceae: a comparative study. –
Bot. Gaz. 133: 230-242.
Taylor G. 1953. Notes on Podostemaceae for the revision of
the Flora of West Tropical Africa. – Bull. Brit. Mus. (Nat. Hist.) Bot. 1:
53-79.
Taylor HL, Brooker RM. 1969. Isolation of
uliginosin A and uliginosin B from Hypericum uliginosum. – Lloydia
32: 217-219.
Telford IRH, Pruesapan K, Welzen PC van,
Bruhl JJ. 2015. Molecular data consistently recover a ‘Queensland clade’ of
Synostemon (Phyllanthaceae, Phyllantheae)
with distinctive floral morphology. – Aust. Syst. Bot. 27: 450-461.
Tennant JR. 1963. Notes on tropical African
Violaceae. – Kew Bull. 16:
409-435.
Thadeo M, Cassino MF, Vitarelli NC, Azevedo
AA, Araújo JM, Valente VMM, Meira RMSA. 2008. Anatomical and histochemical
characterization of extrafloral nectaries of Prockia crucis (Salicaceae). – Amer. J. Bot. 95:
1515-1522.
Thadeo M, Azevedo A, Meira R. 2014. Foliar
anatomy of neotropical Salicaceae:
potentially useful characters for taxonomy. – Plant Syst. Evol. 300:
2073-2089.
Thakur HA, Patil DA. 2002. Nodal organisation
in some Euphorbiaceae. – J.
Swamy Bot. Club 19: 59-62.
Thanikaimoni G, Caratini C, Nilsson S,
Grafström E. 1984. Omniaperturate Euphorbiaceae pollen with striate
spines. – Bull. Jard. Bot. Belg. 54: 105-125.
Thathachar T. 1952. Morphological studies in
the Euphorbiaceae: I.
Acalypha lanceolata Willd. – Phytomorphology 2: 197-201.
Thathachar T. 1953a. Morphological studies in
the Euphorbiaceae II.
Mallotus philippensis M. Arg. – Proc. Indian Acad. Sci., Sect. B,
19: 469-474.
Thathachar T. 1953b. Morphological studies in
the Euphorbiaceae. – J.
Mysore Univ., N. S., 13: 363-388.
Thiebaut LF, Hoffmann P. 2005. Occurrence of
colleters in Erythroxylaceae.
– Kew Bull. 60: 455-459.
Thiele KR, Prober SM. 2003. Two new species
and a new hybrid in the Viola hederacea species complex. – Muelleria
18: 7-25.
Thieme H, Benecke R. 1966. Isolierung eines
neuen Phenolglykosides aus Populus nigra L. – Pharmazie 21:
59-60.
Thin NN. 1984. Tribus Alchornieae (Euphorbiaceae) of Vietnamese
flora. – Tap Chi Sinh Hoc 6: 26-29.
Thin NN. 1988. Tribe Epiprineae (Müll. Arg.)
Hurusawa (Euphorbiaceae) in
Vietnam. – Tap Chi Sinh Hoc 10: 30-33.
Thin NN. 1989. Tribus Codiaeae (Pax) Hutch.
in Vietnam. – Tap Chi Sinh Hoc 11: 14-17.
Thiv M, Ghogue J-P, Grob V, Huber K, Pfeifer
E, Rutishauser R. 2009. How to get off the mismatch at the generic rank in
African Podostemaceae? –
Plant Syst. Evol. 283: 57-77.
Thomas DW. 1990. Conceveiba Aublet
(Euphorbiaceae) new to Africa.
– Ann. Missouri Bot. Gard. 77: 856-858.
Thompson JD, Shivanna KR, Kenrick J, Knox RB.
1989. Sex expression, breeding system, and pollen biology of Ricinocarpos
pinifolius: a case of androdioecy. – Amer. J. Bot. 76: 1048-1059.
Thompson JD, Pailler T, Strasberg D,
Manicacci D. 1996. Tristyly in the endangered Mascarene island endemic
Hugonia serrata (Linaceae).
– Amer. J. Bot. 83: 1160-1167.
Thulin M. 1991. Four new species of
Jatropha (Euphorbiaceae) from Somalia. –
Nord. J. Bot. 11: 527-533.
Thulin M. 1996. Erythroxylon (Erythroxylaceae) on Socotra. –
Nord. J. Bot. 16: 301-302.
Thulin M. 2009. New species of
Euphorbia (Euphorbiaceae) from eastern
Ethiopia. – Kew Bull. 64: 469-476.
Thulin M, Al-Gifri AN. 1995. Euphorbia
applanata sp. nov. (Euphorbiaceae) from Yemen, with a
note on E. quaitensis. – Nord. J. Bot. 15: 193-195.
Thulin M, Razafimandimbison SG, Chafe P,
Heidari N, Kool A, Shore JS. 2012. Phylogeny of the Turneraceae clade (Passifloraceae s.l.):
trans-atlantic disjunctions and two new genera in Africa. – Taxon 61:
308-323.
Thurston EL. 1976. Morphology, fine structure
and ontogeny of the stinging emergences of Tragia ramosa and T.
saxicola (Euphorbiaceae).
– Amer. J. Bot. 63: 710-718.
Thury M. 1897. Observations sur la
morphologie et l’organogénie florales des Passiflores. – Bull. Herb.
Boissier 5: 494-503.
Tieghem P van. 1901. Sur le genre
Lophira considéré comme type d’une famille distincte, les
Lophiracées. – J. Bot. (Paris) 15: 169-194.
Tieghem P van. 1902a. L’embryon des
Ochnacées et son emploi dans la définition des genres. – Bull. Mus. Natl.
Hist. Nat. Paris 8: 208-218.
Tieghem P van. 1902b. Le cristarque dans la
tige et la famille des Ochnacées. – Bull. Mus. Natl. Hist. Nat. Paris 8:
266-273.
Tieghem P van. 1902c. Sur les Ochnacées. –
Ann. Sci. Nat. Bot., sér. 8e, 16: 161-416.
Tieghem P van. 1903. Structure de l’ovule
des Dichopetalacées et place de cette famille dans la classification. – J.
Bot. (Paris) 17: 229-233.
Tieghem P van. 1904. Sur les
Luxembourgiacées. – Ann. Sci. Nat. Bot. 8, 19:1-96.
Tieghem P van. 1905. Sur les Irvingiacées.
– Ann. Sci Nat. Bot., sér. 9, 1: 247-320.
Tillett SS. 1988. Passionis passifloris II.
Terminología. – Ernstia 48: 1-40.
Tiniakou A. 1991. Cytogeographical studies on
some species of Viola sect. Viola (Violaceae) from Greece. –
Willdenowia 20: 153-158.
Tippery NP, Philbrick CT, Bove CP, Les DH.
2011. Systematics and phylogeny of neotropical riverweeds (Podostemaceae: Podostemoideae).
– Syst. Bot. 36: 105-118.
Tobe H. 1987. The Rhizophoraceae: circumscription
and relationships. – Acta Phytotaxon. Geobot. 38: 275-282.
Tobe H, Raven PH. 1984. An embryological
contribution to systematics of the Chrysobalanaceae I. Tribe
Chrysobalaneae. – Bot. Mag. (Tokyo) 97: 397-411.
Tobe H, Raven PH. 1987. The embryology and
relationships of Cassipourea and Sterigmapetalum (Rhizophoraceae-Macarisieae). –
Opera Bot. 92: 253-264.
Tobe H, Raven PH. 1988a. Seed morphology and
anatomy of Rhizophoraceae:
inter- and intrafamilial relationships. – Ann. Missouri Bot. Gard. 75:
1319-1342.
Tobe H, Raven PH. 2011. Embryology of the Irvingiaceae, a family with
uncertain relationships among the Malpighiales. – J. Plant Res.
124: 577-591.
Tokuoka T. 2007. Molecular phylogenetic
analysis of Euphorbiaceae sensu
stricto based on plastid and nuclear DNA sequences and ovule and seed character
evolution. – J. Plant Res. 120: 511-522.
Tokuoka T. 2008. Molecular phylogenetic
analysis of Violaceae (Malpighiales) based on plastid and
nuclear DNA sequences. – J. Plant Res. 121: 253-260.
Tokuoka T. 2012. Molecular phylogenetic
analysis of Passifloraceae
sensu lato (Malpighiales) based
on plastid and nuclear DNA sequences. – J. Plant Res. 125: 489-497.
Tokuoka T, Peng C-I. 1997. Floral morphology
and its systematic implications in Drypetes integerrima (Koidz.) Hook.
(Euphorbiaceae, tribe
Drypeteae) from Bonin Islands, Japan. – Acta Phytotaxon. Geobot. 48:
159-166.
Tokuoka T, Tobe H. 1995. Embryology and
systematics of Euphorbiaceae
sens. lat.: a review and perspective. – J. Plant Res. 108: 97-106.
Tokuoka T, Tobe H. 1998. Ovules and seeds in
Crotonoideae (Euphorbiaceae):
structure and systematic implications. – Bot. Jahrb. Syst. 120: 165-186.
Tokuoka T, Tobe H. 1999. Embryology of tribe
Drypeteae, an enigmatic taxon of Euphorbiaceae. – Plant Syst.
Evol. 215: 189-208.
Tokuoka T, Tobe H. 2001. Ovules and seeds in
subfamily Phyllanthoideae (Euphorbiaceae): structure and
systematic implications. – J. Plant Res. 114: 75-92.
Tokuoka T, Tobe H. 2002. Ovules and seeds in
Euphorbioideae (Euphorbiaceae):
structure and systematic implications. – J. Plant Res. 115: 361-374.
Tokuoka T, Tobe H. 2003. Ovules and seeds in
Acalyphoideae (Euphorbiaceae):
structure and systematic implications. – J. Plant Res. 116: 355-380.
Tokuoka T, Tobe H. 2006. Phylogenetic
analyses of Malpighiales using
plastid and nuclear DNA sequences, with particular reference to the embryology
of Euphorbiaceae sens. str. –
J. Plant Res. 119: 599-616.
Tomar DPS, Desmukh PS, Sinha SK. 1979.
Importance of sepals in fruit and seed development in linseed (Linum
usitiatissimum L.). – Euphytica 28: 739-745.
Tomlinson PB. 1986. The botany of mangrove.
– Cambridge.
Tomlinson PB. 1988. Systematic comparison and
some biological characters of Rhizophoraceae and Anisophylleaceae.
– Ann. Missouri Bot. Gard. 75: 1297-1318.
Tomlinson PB, Cox PA. 2000. Systematic and
functional anatomy of seedlings in mangrove Rhizophoraceae: vivipary
explained? – Bot. J. Linn. Soc. 134: 215-231.
Tomlinson PB, Wheat DW. 1979. Bijugate
phyllotaxis in Rhizophoreae (Rhizophoraceae). – Bot. J.
Linn. Soc. 78: 317-321.
Tomlinson PB, Primack RB, Bunt JS. 1979.
Preliminary observations on floral biology in mangrove Rhizophoraceae. – Biotropica
11: 256-277.
Torre AR. 1963. 47. Dichapetalaceae. – In: Exell
AW, Fernandes A, Wild H (eds), Flora Zambesiaca 2 (Part 1), Crown Agents for
Oversea Governments and Administrations, London, pp. 319-328.
Torre AR, Gonçalves AE. 1978. 72. Rhizophoraceae. – In: Launert E
(ed), Flora Zambesiaca 4, Flora Zambesiaca Managing Committee, London, pp.
81-99.
Tran Ha NM, Bancilhon-Rossignol L. 1976.
Premières données sur le mode de reproduction des Phyllanthus
(Euphorbiacées) découlant d’une étude cytogénétique comparée de six
taxons de P. odontadenius Muell. Arg. à garnitures chromosomiques
différentes (2n = 12, 24, 28, 56). – Rev. Cyt. Biol. Végét. 39:
201-234.
Tran Ha NM, Belliard J. 1976. Analyse
morphologique et biométrique comparée de six taxons de Phyllanthus
odontadenius Muell. Arg. à garnitures chromosomiques différentes (2n =
12, 24, 28, 56) et de leur descendance. – Rev. Gen. Bot. 83: 269-310.
Truyens S, Arbo MM, Shore JS. 2005.
Phylogenetic relationships, chromosome and breeding system evolution in
Turnera (Turneraceae):
inferences from ITS sequence data. – Amer. J. Bot. 92: 1749-1758.
Tschapka M, Dressler S, Helversen O von.
2006. Bat visits to Marcgravia pittieri and notes on the inflorescence
diversity within the genus Marcgravia (Marcgraviaceae).
– Flora 201: 383-388.
Tunmann O, Jenzer R. 1910. Zur Anatomie der
Blüten von Pilocarpus pennatifolius Lem. und Erythroxylon
coca Lam. – Arch. d. Pharmaz. 248: 514-519.
Tur NM. 1975. Nueva especie de Podostemaceae para Argentina:
Wettsteiniola apipensis. – Bull. Bot. Soc. Argentina 16: 320-324.
Tur NM. 1997. Taxonomy of Podostemaceae in Argentina. –
Aquatic Bot. 57: 213-241.
Tuskan GA, DiFazio S, Jansson S, Bohlmann J,
Grigoriev I, Hellsten U, Putnam M, Ralph S, Rombauts S, Salamov A, Schein J,
Sterck L, Aerts A, Bhalerao RR, Bhalerao RP, Blaudez D, Boerjan W, Brun A,
Brunner A, Busov V, Campbell M, Carlson J, Chalot M, Chapman J, Chen G-L,
Cooper D, Coutinho PM, Couturier J, Covet S, Cronk Q, Cunningham R, Davis J,
Degroeve S, Déjardin A, dePamphilis C, Detter J, Dirks B, Dubchak I, Duplessis
S, Ehlting J, Ellis B, Gendler K, Goodstein D, Gribskov M, Grimwood J, Groover
A, Gunter L, Hamberger B, Heinze B, Helariutta Y, Henrissat B, Holligan D, Holt
R, Huang W, Islam-Faridi, N., Jones, S., Jones-Rhoades, M., Jorgensen, R.,
Joshi, C., Kangasjärvi, J., Karlsson, J., Kelleher C, Kirkpatrick R, Kirst M,
Kohler A, Kalluri U, Larimer F, Leebens-Mack J, Leplé J-C, Locascio P, Lou Y,
Lucas S, Martin F, Montanini M, Napoli C, Nelson DR, Nelson C, Nieminen K,
Nilsson O, Pereda V, Peter G, Philippe R, Pilate G, Poliakov A, Razumovskaya J,
Richardson P, Rinaldi C, Ritland K, Rouzé P, Ryaboy D, Schmutz J, Schrader J,
Segerman B, Shin H, Siddiqui A, Sterky F, Terry A, Tsai C-J, Uberbacher E,
Unneberg P, Vahala J, Wall K, Wessler S, Yang G, Yin T, Douglas C, Marra M,
Sandberg G, Peer Y van de, Rokhsar D. 2006. The genome of black cottonwood
Populus tricocarpa (Torr. & Gray). – Science 313: 1596-1604.
Ueda K, Hanyuda T, Nakano A, Shiuchi T, Seo
A, Okubo H, Hotta M. 1997. Molecular phylogenetic position of Podostemaceae, a marvellous
aquatic flowering plant family. – J. Plant Res. 110: 87-92.
Uhlarz H. 1978. Über die Stipularorgane der
Euphorbiaceae, unter besonderer
Berücksichtigung ihrer Rudimentation. – Trop. Subtrop. Pflanzenwelt 23:
1-65.
Ulmer T, MacDougal JM. 2004.
Passiflora: passionflowers of the world. – Timber Press, Portland,
Oregon.
Ulubelen A, Kerr RR, Mabry TJ. 1982. Two new
neoflavonoids and C-glycosylflavones from Passiflora serratodigitata.
– Phytochemistry 21: 1145-1147.
Uniyal PL. 1999. Studies on Indotristicha
tirunelveliana Sharma, Karthik. & Shetty (Podostemaceae): an endemic, rare
and enigmatic taxon. – Flora 194: 169-178.
Uniyal PL, Mohan Ram HY. 1994. Karyological
studies in some members of Podostemaceae. – Aquatic Bot.
47: 85-90.
Uniyal PL, Mohan Ram HY. 1996. In vitro
germination and seedling morphology of Dalzellia zeylanica (Gardner)
Wight (Podostemaceae). –
Aquatic Bot. 54: 59-71.
Uno GE. 1984. The role of persistent sepals
in the reproductive biology in Linum pratense (Linaceae). – Southw. Natur. 29:
429-434.
Urban I. 1883. Monographie der Familie der
Turneraceen. – Jahrb. Königl. Bot. Gart. Berlin 2: 1-152.
Urban I. 1898. Plantae novae americanae
imprimis Glaziovianae II. Turneraceae adjectis specierum
nonnullarum africanarum descriptionibus. – Engl. Bot. Jahrb. Syst. 25, Beibl.
60: 2-12.
Urbatsch LE, Bacon JD, Hartman RL, Johnston
MC, Watson TJ Jr, Webster GL. 1975. Chromosome numbers for North American Euphorbiaceae. – Amer. J. Bot.
62: 494-500.
Valen F van. 1978. Contribution to the
knowledge of cyanogenesis in angiosperms 10. Communication. Cyanogenesis in Euphorbiaceae. – Planta Medica
34: 408-413.
Valentine DH. 1962. Variation and evolution
in the genus Viola. – Preslia 34: 190-206.
Vanderplank RJR. 2006 [2007]. Plant/insect
mimicry in Passiflora. – Passiflora 16: 26-27.
Vartak VD, Kumbhojkar MS. 1984. Palynological
study of the family Podostemaceae from Western India.
– Biovigyanam 10: 89-92.
Vasconcellos NC, Carvalho MJC, Andrade TAP,
Berg MEVD. 1972. O pólen em plantas da Amazônia: Família Guttiferae. –
Bol. Mus. Paraense Emilio Goeldi 44: 1-12.
Vasudeva Rao MK, Chakrabarty T. 1985.
Drypetes longifolia (Euphorbiaceae) in Andamans. – J.
Econ. Taxon. Bot. 6: 445.
Vaughan JG, Rest JA. 1969. Note on the testa
structure of Panda Pierre, Galearia Zoll. et Mor. and
Microdesmis Hook. f. (Pandaceae). – Kew Bull. 23:
215-218.
Vazart B, Vazart J. 1965. Infrastructure de
l’ovule de lin, Linum usitiatissimum L. L’assise jaquette ou
endothélium. – Compt. Rend. Hébd. Séances Acad. Sci. Paris 261:
2927-2930.
Vazart B, Vazart J. 1966. Infrastructure du
sac embryonnaire du lin (Linum usitatissimum L.). – Rév. Cytol.
Biol. Vég. 29: 251-266.
Vazart J. 1969. Organisation et
ultrastructure du sac embryonnaire du lin (Linum usitatissimum L.).
– Rév. Cytol. Biol. Vég. 32: 227-240.
Vazart J. 1971. Dégénérescence d’une
synergide et penetration du tube pollinique dans le sac embryonnaire de
Linum usitatissimum L. –Ann. Univ. A.R.E.R.S. 9: 89-97.
Vega AS, Castro MA, Anderson WR. 2002.
Occurrence and phylogenetic significance of latex in the Malpighiaceae. – Amer. J. Bot.
89: 1725-1729.
Vela D. 2010. Multivariate analysis of
morphological and anatomical characters of Calophyllum L. (Calophyllaceae) in South America.
– M.Sc. thesis, University of Missouri, St. Louis, Missouri.
Velzen R van, Wahlert GA, Sosef MSM, Onstein
RE, Bakker FT. 2015. Phylogenetics of African Rinorea (Violaceae): elucidating infrageneric
relationships using plastid and nuclear DNA sequences. – Syst. Bot. 40:
174-184.
Venkata Rao C. 1971. Anatomy of the
inflorescence of some Euphorbiaceae. With a discussion
on the phylogeny and evolution of the inflorescence including the cyathium. –
Bot. Not. 124: 39-64.
Venkata Rao C. 1972. Floral anatomy of
Ricinocarpus pinifolius with some observations on the phylogeny and
centre of origin of Euphorbiaceae. – Adv. Plant
Morph. 1972: 85-91.
Venkata Rao C, Ramalakshmi T. 1968. Floral
anatomy of the Euphorbiaceae I.
Some non-cyathium taxa. – J. Indian Bot. Soc. 47: 278-300.
Venkateswarlu J, Rao PN. 1963. Endosperm in
Euphorbiaceae and occurrence of
endosperm haustoria in two species of Croton Linn. – Curr. Sci. 32:
514-516.
Venkateswarlu J, Rao PN, Rao DS. 1973.
Occurrence of stylar obturator in two Euphorbiaceae. – Curr. Sci. 43:
128-129.
Ventura M. 1934. Sulla poliembrionia di
Mallotus japonicus Muell. Arg. – Ann. Bot. (Roma) 20: 568-578.
Verdcourt B. 1954. Notes from the East
African Herbarium II. – Kew Bull. 9: 35-402.
Verdcourt B. 1968. Elatinaceae. – In: Milne-Redhead
E, Polhill RM (eds), Flora of tropical East Africa, Crown Agents for Oversea
Governments and Administrations, London, pp. 1-5.
Verdcourt B. 1980. A new species of
Nectaropetalum (Erythroxylaceae) from Tanzania.
– Kew Bull. 36: 43-45.
Verdcourt B. 1984a. Ixonanthaceae. – In: Polhill RM
(ed), Flora of tropical East Africa, A. A. Balkema, Rotterdam, pp. 1-11.
Verdcourt B. 1984b. Erythroxylaceae. – In: Polhill
RM (ed), Flora of tropical East Africa, A. A. Balkema, Rotterdam, pp. 1-11.
Verdus MC. 1976. L’évolution
pseudocyclique des plantules des Euphorbiaceae. – Taxon 25:
99-107.
Vesque J. 1889. Epharmosis, sive materiae ad
instruendam anatomiam systematis naturalis 2. Genitalia foliaque Garcinearum et
Calophyllearum. – Delapierre, Vincennes.
Vesque J. 1893. Guttiferae. – In: Candolle
ACP de (ed), Monographiae Phanerogamarum 8, Masson, Paris.
Vestal PA. 1937. The significance of
comparative anatomy in establishing the relationship of the Hypericaceae to the Guttiferae and
their allies. – Philipp. J. Sci. 64: 199-256.
Vester H. 1999. Architectural diversification
within the genus Vismia (Clusiaceae) in the Amazonian
rainforest (Ararucuara, Colombia). – In: Kurmann MH, Hemsley AR (eds), The
evolution of plant architecture, Royal Botanic Gardens, Kew, pp. 147-158.
Vezey EL, Shah VP, Skvarla JJ, Raven PH.
1988. Morphology and phenetics of Rhizophoraceae pollen. – Ann.
Missouri Bot. Gard. 75: 1369-1386.
Vidyashankar B. 1988a. Seed germination and
seedling morphology in Indotristicha ramosissima (Podostemaceae) grown in vitro. –
Curr. Sci. 57: 369-373.
Vidyashankari B. 1988b. Developmental biology
of Griffithella hookeriana. – Ph.D. diss., University of Delhi,
India.
Vidyashankari B, Mohan Ram HY. 1987. In vitro
germination and origin of thallus in Griffithella hookeriana (Podostemaceae). – Aquatic Bot.
28: 161-169.
Vijayaraghavan MR, Dlar U. 1976.
Scytopetalum tieghemii: embryologically unexplored taxon and
affinities of the family. – Phytomorphology 26: 16-22.
Vijayaraghavan MR, Kaur D. 1967. Morphology
and embryology of Turnera ulmifolia L. and affinities of the family Turneraceae. – Phytomorphology 16:
539-553.
Vindt J. 1960. Monographie des Euphorbiacées
du Maroc. – Trav. Inst. Sci. Chérifien, Sér. Bot. 19: 219-533.
Vitarelli NC, Riina R, Caruzo MBR, Cordeiro
I, Fuertes-Aguilar J, Meira RMSA. 2015. Foliar secretory structures in
Crotoneae (Euphorbiaceae):
diversity, anatomy, and evolutionary significance. – Amer. J. Bot. 102:
833-847.
Vitta FA, Bernacci LC. 2004. A new species of
Passiflora in section Tetrastylis (Passifloraceae) and two
overlooked species of Passiflora from Brazil. – Brittonia 56:
89-95.
Vliet GTCM van 1976. Wood anatomy of the Rhizophoraceae. – Leiden Bot.
Ser. III: 20-75.
Vogel C. 1986. Phytoserologische
Untersuchungen zur Systematik der Euphorbiaceae. Beiträge zur
infrafamiliaren Gliederung und zu Beziehungen im extrafamiliaren Bereich. –
Diss. Bot. 98: 1-124.
Vogel S. 1990. History of the Malpighiaceae in the light of
pollination ecology. – Mem. New York Bot. Gard. 55: 130-142.
Vorontsova MS, Hoffmann P. 2008. A
phylogenetic classification of tribe Poranthereae (Phyllanthaceae, Euphorbiaceae sensu lato). – Kew
Bull. 63: 41-59.
Vorontsova MS, Hoffmann P. 2009. Revision of
the genus Leptopus (Phyllanthaceae, Euphorbiaceae sensu lato). – Kew
Bull. 64: 627-644.
Vorontsova MS, Hoffmann P, Maurin O, Chase
MW. 2007. Molecular phylogeny of tribe Poranthereae (Phyllanthaceae; Euphorbiaceae sensu lato). –
Amer. J. Bot. 94: 2026-2040.
Vorontsova MS, Hoffmann P, Kathriarachchi H,
Kolterman DA, Chase MW. 2007. Andrachne cuneifolia (Phyllanthaceae; Euphorbiaceae s.l.) is a
Phyllanthus. – Bot. J. Linn. Soc. 155: 519-525.
Vos JM de, Breteler FJ. 2009. A revision of
the African genera Paropsiopsis and Smeathmannia (Passifloraceae – Paropsieae),
including a new species of Paropsiopsis from Cameroon. – Edinburgh
J. Bot. 66: 27-49.
Wagner WL, Lorence DH. 2011. A nomenclator of
Pacific oceanic island Phyllanthus (Phyllanthaceae), including
Glochidion. – PhytoKeys 4: 67-94.
Wahlert GA, Ballard HE Jr. 2012. A phylogeny
of Rinorea (Violaceae)
inferred from plastid DNA sequences with an emphasis on the African and
Malagasy species. – Syst. Bot. 37: 964-973.
Wahlert GA, Marcussen T, Paula-Souza J de,
Feng M, Ballard HE Jr. 2014. A phylogeny of the Violaceae (Malpighiales) inferred from plastid
DNA sequences: implications for generic diversity and intrafamilial
classification. – Syst. Bot. 39: 239-252.
Wahlert GA, Ballard Jr HE, Paula-Souza J de.
2015. Ixchelia, a new genus of Violaceae from Mexico and Mesoamerica.
– Brittonia 67: 273-283.
Wahlert GA, Hoyos-Goméz SE, Ballard Jr HE.
2018. Systematic studies in Neotropical Rinorea (Violaceae): two new sections and a new
generic segregate. – Brittonia 70: 140-147.
Wallnöfer B. 1991. Beschreibung der zweiten
Art in der neotropischen Gattung Nealchornea Huber (Euphorbiaceae). – Linzer Biol.
Beitr. 23: 775-785.
Wallnöfer B. 1998. A revision of
Perissocarpa Steyerm. & Maguire (Ochnaceae). – Ann. Naturhist. Mus.
Wien 100 B: 683-707.
Warburg O. 1894. Flacourtiaceae. – In:
Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(6a), W.
Engelmann, Leipzig, pp. 1-56.
Ward PFN, Hall RJ, Peters RA. 1964. Fluoro
fatty acids in the seeds of Dichapetalum toxicarium. – Nature 201:
611-612.
Warming E. 1881. Familien Podostemaceae I. – Kong. Danske
Vidensk. Selsk. Nat. Math. Afd. 2: 1-34.
Warming E. 1882. Familien Podostemaceae II. – Kong. Danske
Vidensk. Selsk. Nat. Math. Afd. 2: 77-130.
Warming E. 1888. Familien Podostemaceae III. – Kong.
Danske Vidensk Selsk. Nat. Math. Afd. 4: 443-514.
Warming E. 1891a. Familien Podostemaceae IV. – Kong. Danske
Vidensk. Selsk. Nat. Math. Afd. 7: 135-179.
Warming E. 1891b. Podostemaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien III(2a), W. Engelmann,
Leipzig, pp. 1-22.
Warming E. 1899. Familien Podostemaceae V. – Kong. Danske
Vidensk. Selsk. Nat. Math. Afd. 9: 105-154.
Warming E. 1901. Familien Podostemaceae VI. – Kong. Danske
Vidensk. Selsk. Nat. Math. Afd. 11: 1-67.
Warmke HE. 1952. Studies on natural
pollination of Hevea brasiliensis in Brazil. – Science 116:
474-475.
Warnock BH, Johnston MC. 1960. The genus
Savia (Euphorbiaceae)
in extreme western Texas. – Southw. Natur. 5: 1-6.
Webber BL, Miller RE. 2008. Gynocardin from
Baileyoxylon lanceolatum and a revision of cyanogenic glycosides in Achariaceae. – Biochem. Syst.
Ecol. 36: 545-553.
Webber BL, Woodrow IE. 2006. Morphological
analysis and a resolution of the Ryparosa javanica species complex (Achariaceae) from Malesian and
Australian tropical rainforests. – Aust. Syst. Bot. 19: 541-569.
Webber BL, Moog J, Curtis ASO, Woodrow IE.
2007. The diversity of ant-plant interactions in the rainforest understorey
tree, Ryparosa (Achariaceae): food bodies, domatia,
prostomata, and hemipteran trophobionts. – Bot. J. Linn. Soc. 154:
353-371.
Weber-El Ghobary MO. 1985. Pollen morphology
of four succulent species of Euphorbia (Euphorbiaceae). – An. Asoc.
Palin. Lengua Esp. 2: 75-86.
Weberling F, Lörcher H, Böhnke E. 1980. Die
Stipeln der Irvingioideae und Recchioideae und ihre systematische Wertung nebst
Bemerkungen zur Holzanatomie und Palynologie. – Plant Syst. Evol. 133:
261-283.
Webster GL. 1956. A monographic study of the
West Indian species of Phyllanthus. – J. Arnold Arbor. 37: 91-122,
217-268, 340-359.
Webster GL. 1957. A monographic study of the
West Indian species of Phyllanthus. – J. Arnold Arbor. 38: 51-80,
170-198, 295-373.
Webster GL. 1958. Amonographic study of the
West Indian species of Phyllanthus. – J. Arnold Arbor. 39: 49-100,
111-212.
Webster GL. 1965. A revision of the genus
Meineckia (Euphorbiaceae). – Acta Bot.
Neerl. 14: 323-365.
Webster GL. 1967. Acidoton (Euphorbiaceae) in Central America.
– Ann. Missouri Bot. Gard. 54: 191.
Webster GL. 1970a. A revision of
Phyllanthus (Euphorbiaceae) in the continental
United States. – Brittonia 22: 44-76.
Webster GL. 1970b. Notes on Galápagos Euphorbiaceae. – Madroño 20:
257-263.
Webster GL. 1975. Conspectus of a new
classification of the Euphorbiaceae. – Taxon 24:
593-601.
Webster GL. 1979. A revision of
Margaritaria (Euphorbiaceae). – J. Arnold
Arbor. 60: 403-444.
Webster GL. 1982. Systematic status of the
genus Kleinodendron (Euphorbiaceae). – Taxon 31:
535-539.
Webster GL. 1983. A botanical Gordian knot:
the case of Ateramnus and Gymnanthes (Euphorbiaceae). – Taxon 32:
304-305.
Webster GL. 1984a. Jablonskia, a new
genus of Euphorbiaceae from
South America. – Syst. Bot. 9: 229-235.
Webster GL. 1984b. A revision of
Flueggea (Euphorbiaceae). – Allertonia 3:
259-312.
Webster GL. 1986. A revision of
Phyllanthus (Euphorbiaceae) in Eastern
Melanesia. – Pacific Sci. 40: 88-105.
Webster GL. 1987a. The saga of the spurges: a
review of classification and relationships in the Euphorbiales. – Bot. J.
Linn. Soc. 94: 3-46.
Webster GL. 1987b. A new species of
Jatropha (Euphorbiaceae) from Nicaragua. –
Ann. Missouri Bot. Gard. 74: 117-120.
Webster GL. 1989. Revised conspectus of the
Euphorbiaceae. – Euphorbiaceae Newsletter 2.
Webster GL. 1992a. Realignments in American
Croton (Euphorbiaceae). – Novon 2:
269-273.
Webster GL. 1992b. Revision of
Astrocasia (Euphorbiaceae). – Syst. Bot. 17:
311-323.
Webster GL. 1993. A provisional synopsis of
the sections of the genus Croton (Euphorbiaceae). – Taxon 42:
793-823.
Webster GL. 1994a. Classification of the Euphorbiaceae. – Ann. Missouri
Bot. Gard. 81: 3-32.
Webster GL. 1994b. Synopsis of the genera and
suprageneric taxa of Euphorbiaceae. – Ann. Missouri
Bot. Gard. 81: 33-144.
Webster GL. 2001. Synopsis of Croton
and Phyllanthus (Euphorbiaceae) in western tropical
Mexico. – Contr. Univ. Michigan Herb. 23: 353-388.
Webster GL. 2002. A synopsis of the Brazilian
taxa of Phyllanthus section Phyllanthus (Euphorbiaceae). – Lundellia 5:
1-26.
Webster GL. 2003. A synopsis of
Phyllanthus section Nothoclema (Euphorbiaceae). – Lundellia 6:
19-36.
Webster GL. 2007. Taxonomic and nomenclatural
changes in American Euphorbiaceae sensu lato. –
Contr. Univ. Michigan Herb. 25: 235-239.
Webster GL, Airy Shaw HK. 1971. A provisional
synopsis of the New Guinea taxa of Phyllanthus (Euphorbiaceae). – Kew Bull. 26:
85-109.
Webster GL, Armbruster WS. 1982. An unusual
new species of Dalechampia (Euphorbiaceae) from Surinam. –
Syst. Bot. 7: 484-488.
Webster GL, Armbruster WS. 1991. A synopsis
of the neotropical species of Dalechampia (Euphorbiaceae). – Bot. J. Linn.
Soc. 105: 137-177.
Webster GL, Carpenter KJ. 2002. Pollen
morphology and phylogenetic relationships in neotropical Phyllanthus
(Euphorbiaceae). – Bot. J.
Linn. Soc. 138: 325-338.
Webster GL, Carpenter KJ. 2008. Pollen
morphology and systematics of palaeotropical Phyllanthus and related
genera of subtribe Phyllanthinae (Euphorbiaceae). – Bot. J. Linn.
Soc. 157: 591-608.
Webster GL, Huft MJ. 1988. Revised synopsis
of Panamanian Euphorbiaceae.
– Ann. Missouri Bot. Gard. 75: 1087-1144.
Webster GL, Miller K. 1963. The genus
Reverchonia (Euphorbiaceae). – Rhodora 65:
193-207.
Webster GL, Poveda LJ. 1978. A
phytogeographically significant new species of Jatropha (Euphorbiaceae) from Costa Rica.
– Brittonia 30: 265-270.
Webster GL, Rupert EA. 1973. Phylogenetic
significance of pollen nuclear number in the Euphorbiaceae. – Evolution 27:
524-531.
Webster GL, Webster BD. 1972. The morphology
and relationships of Dalechampia scandens (Euphorbiaceae). – Amer. J. Bot.
59: 573-586.
Webster GL, Brown WV, Smith BN. 1975.
Systematics of photosynthetic carbon fixation pathways in Euphorbia.
– Taxon 24: 27-33.
Webster GL, Rupert E, Koutnik D. 1982.
Systematic significance of pollen nuclear number in Euphorbiaceae, tribe Euphorbieae.
– Amer. J. Bot. 69: 407-415.
Webster GL, Gillespie L, Steyermark J. 1987.
Systematics of Croizatia (Euphorbiaceae). – Syst. Bot. 12:
1-8.
Webster GL, Del-Arco-Aquilar MJ, Smith BA.
1996. Systematic distribution of foliar trichome types in Croton (Euphorbiaceae). – Bot. J. Linn.
Soc. 121: 41-57.
Weidenhoeft AC. 2008. Tracking the phylogeny
of Crotoneae with comparative wood anatomy of Croton. – University
of Wisconsin, Madison, Wisconsin.
Weitzman AL. 2005. Bonnetiaceae. – In: Steyermark
JA, Berry PE, Holst BK (eds.), Flora of the Venezuelan Guayana, Missouri
Botanical Garden Press, St. Louis, Missouri, pp. 313-324.
Weitzman AL, Stevens PF. 1997. Notes on the
circumscription of Bonnetiaceae
and Clusiaceae, with taxa and new
combinations. – BioLlania Ed. Espec. 6: 551-564.
Weitzman AL, Kubitzki K, Stevens PF. 2006. Bonnetiaceae. – In: Kubitzki K
(ed), The families and genera of vascular plants IX. Flowering plants.
Eudicots. Berberidopsidales,
Buxales, Crossosomatales, Fabales p. p., Geraniales, Gunnerales, Myrtales p. p., Proteales, Saxifragales, Vitales, Zygophyllales, Clusiaceae Alliance, Passifloraceae Alliance, Dilleniaceae, Huaceae, Picramniaceae, Sabiaceae, Springer, Berlin,
Heidelberg, New York, pp. 36-39.
Wellendorph P, Clausen V, Jørgensen LB,
Jaroszewski JW. 2001. Cyclopentanoids of Maturina penduliflora. –
Biochem. Syst. Ecol. 29: 649-651.
Welzen PC van. 1994a. Taxonomy, phylogeny and
geography of Neoscortechinia Hook. f. ex Pax (Euphorbiaceae). – Blumea 39:
301-320.
Welzen PC van. 1994b. A taxonomic revision of
S.E. Asian Chaetocarpus Thwaites (Euphorbiaceae). – Rheedea 4:
93-101.
Welzen PC van. 1995. Taxonomy and phylogeny
of the Euphorbiaceae tribe
Erismantheae G. L. Webster. – Blumea 40: 375-396.
Welzen PC van. 2011. Revision of
Dicoelia (Phyllanthaceae; Euphorbiaceae s.l.). – Blumea
56: 209-213.
Welzen PC van. 2015. A revision of the
Malesian species of Blachia (Euphorbiaceae). – Blumea
59: 163-166.
Welzen PC van. 2016. Bischofia and
Hymenocardia (Phyllanthaceae) in Malesia.
– Blumea 61: 272-279.
Welzen PC van. 2017. Reduction of
Breynia subgenus Hemisauropus to B. section
Cryptogynium and discussion of the B. quadrangularis complex
(Phyllanthaceae). –
Blumea 62: 90-91.
Welzen PC van, Baas P. 1984. A
leaf-anatomical contribution to the classification of the Linaceae complex. – Blumea 29:
453-479.
Welzen PC van, Bulalacao LJ. 2007. The genus
Alchornea (Euphorbiaceae) in the Malay
Archipelago and Thailand. – Syst. Bot. 32: 803-818.
Welzen PC van, Chayamarit K. 2001. Two new
Mallotus and two new Sauropus species (Euphorbiaceae) endemic to
Thailand. – Kew Bull. 56: 649-656.
Welzen PC van, Forster PI. 2010. A revision
of Malesian Austrobuxus (Picrodendraceae/Euphorbiaceae s.l. subfam.
Oldfieldioideae). – Nord. J. Bot. 28: 189-195.
Welzen PC van, Oostrum AF van. 2015. Revision
of the Malesian species of Dimorphocalyx (Euphorbiaceae). – Blumea 59:
191-201.
Welzen PC van, Stuppy W. 1999. Phylogenetic
considerations of Euphorbiaceae
tribe Aleuritideae. – Ann. Missouri Bot. Gard. 86: 894-903.
Welzen PC van, Winkel E. 2015. A revision of
Ostodes (Euphorbiaceae) in Malesia. –
Blumea 59: 185-190.
Welzen PC van, Bulalacao LJ, Ôn T van. 1995.
A taxonomic revision of the Malesian genus Trigonopleura Hook. f. (Euphorbiaceae). – Blumea 40:
363-374.
Welzen PC van, Pruesapan K, Telford IRH,
Esser H-J, Bruhl JJ. 2014. Phylogenetic reconstruction prompts taxonomic
changes in Sauropus, Synostemon and Breynia (Phyllanthaceae tribe
Phyllantheae). – Blumea 59: 77-94.
Welzen PC van, Pruesapan K, Telford IRH,
Bruhl JJ. 2015. Historical biogeography of Breynia (Phyllanthaceae): what caused
speciation? – J. Biogeogr. 42: 1493-1502.
Welzen PC van, Sweet FST, Fernández-Casas
FJ. 2017. A revision of Jatropha (Euphorbiaceae) in Malesia. –
Blumea 62: 58-74.
Wendt T. 1988. Chiangiodendron
(Flacourtiaceae: Pangieae): a new genus from southeastern Mexico representing a
new tribe for the New World flora. – Syst. Bot. 13: 435-441.
Went FAFC. 1909. The development of the
ovule, embryo sac, and egg of Podostemaceae. – Rec. Bot.
Néerl. 5: 1-16.
Went FAFC. 1910. Untersuchungen über
Podostemaceen I. – Verh. Kon. Akad. Wetesch. Amsterdam 2(16): 1-88.
Went FAFC. 1912. Untersuchungen über
Podostemaceen II. – Verh. Kon. Akad. Wetesch. Amsterdam 17: 5-18.
Went FAFC. 1926. Untersuchungen über
Podostemaceen III. – Verh. Kon. Akad. Wetesch. Amsterdam 25: 3-58.
Went FAFC. 1929. Morphological and
histological peculiarities of the Podostemaceae. – Proc. Intern.
Congr. Plant Sci. Ithaca 1: 351-358.
Westra LYT, Koek-Noorman JK. 2004. Wood atlas
of the Euphorbiaceae s.l. –
Nationaal Herbarium Nederland. [IAWA J., Suppl. 4]
Wetterwald X. 1889. Blatt- und Sprossbildung
bei Euphorbien und Cacteen. – Nova Acta Acad. Caes. Leop.-Carol. German. Nat.
Cur. 53: 379-440.
Weyland H. 1938. Die fossielen
Sacoglottis: Früchte und eine neue Art der Gattung, Sacoglottis
germanica n. sp. – Decheniana 98: 153-162.
Wheat DW. 1981. Sylleptic branching in the
Rhizophoreae (Rhizophoraceae).
– Bot. Gaz. 142: 115-123.
Wheeler LC. 1939. A miscellany of New World
Euphorbiaceae II. – Contr.
Gray Herb. 127: 48-78.
Wheeler LC. 1941. Euphorbia Subgenus
Chamaesyce in Canada and the United States exclusive of Southern
Florida. – Rhodora 43: 97-154, 168-205, 223-286.
Wheeler LC. 1943 The genera of living
Euphorbieae. – Amer. Midl. Natur. 30: 456-503.
Wheeler LC. 1975. Euphorbiaceous genera
lectotypified. – Taxon 24: 534-538.
White A, Dyer RA, Sloane BL. 1941. The
succulent Euphorbieae (Southern Africa) 1-2. – Abbey Garden Press, Pasadena,
California.
White F. 1976. Chrysobalanaceae.
Distributiones plantarum africanarum 10, maps 281-334.
White F. 1978. 63. Chrysobalanaceae. – In:
Launert E (ed), Flora Zambesiaca 4, Flora Zambesiaca Managing Committee,
London, pp. 33-48.
Whitmore TC. 1973. Tree flora of Malaya 2. Euphorbiaceae. – Longman,
London, pp. 34-136.
Whitmore TC. 1984. Studies in
Macaranga XII. New species and corrections in Macaranga (Euphorbiaceae) of Malesia. – Kew
Bull. 39: 607-610.
Whitmore TC. 2008. The genus
Macaranga, a prodromus. – Kew Publ., Royal Botanic Gardens, Kew,
England.
Wiedenhoft AC, Riina R, Berry PE. 2009.
“Ray intrusive” laticifers in species of Croton section
Cyclostigma (Euphorbiaceae). – IAWA J. 30:
135-148.
Wiehr E. 1930. Beiträge zur Kenntnis der
Anatomie der wichtigsten Euphorbiaceen-Samen under besonderer Berücksichtigung
ihrer Erkennungsmerkmale in Futtermitteln. – Landw. Versuchsstationen 110:
313-398.
Wieringa JJ, Schoonhoven JGA. 2002. Proposal
to conserve the name Acridocarpus against Anomalopteris (Malpighiaceae). – Taxon 51:
387-388.
Wilbu RL. 1954. A synopsis of
Jatropha, subsection Eucurcas, with the description of two
new species from Mexico. – J. Elisha Mitchell Sc.Soc. 70: 92-101.
Wild H. 1960. Flacourtiaceae (incl.
Samydaceae). – In: Exell AW, Wild H (eds), Flora Zambesiaca 1 (Part 1), Crown
Agents for Oversea Governments and Administrations, London, pp. 261-298.
Wild H. 1961. 24. Elatinaceae. – In: Exell AW, Wild
H (eds), Flora Zambesiaca 1 (Part 2), Crown Agents for Oversea Governments and
Administrations, London, pp. 373-378.
Wilde WJJO de. 1971a. A monograph of the
genus Adenia Forssk. (Passifloraceae). – Meded.
Landbouwh. Wageningen 71(18): 1-281.
Wilde WJJO de. 1971b. The systematic position
of tribe Paropsieae, in particular the genus Ancistrothyrsus, and a
key of the genera of Passifloraceae. – Blumea 19:
99-104.
Wilde WJJO de. 1972. The indigenous Old World
passifloras. – Blumea 20: 227-250.
Wilde WJJO de. 1973. Revision of
Basananthe, formerly Tryphostemma (Passifloraceae). – Blumea 21:
327-356.
Wilde WJJO de. 1974. The genera of tribe
Passifloreae (Passifloraceae),
with special reference to flower morphology. – Blumea 22: 37-50.
Wilkinson HP. 1981. The anatomy of the
hypocotyls of Ceriops. – Bot. J. Linn. Soc. 82: 139-164.
Wilkinson HP. 2007. Leaf teeth in certain Salicaceae and ‘Flacourtiaceae’.
– Bot. J. Linn. Soc. 155: 241-256.
Willemstein SC. 1987. An evolutionary basis
for pollination ecology. – Leiden Bot. Ser. 10: 1-425.
Willis JC. 1902a. A revision of the Podostemaceae of India and Ceylon.
– Ann. Roy. Bot. Gard. (Peradeniya) 1: 181-250.
Willis JC. 1902b. Studies in the morphology
and ecology of the Podostemaceae of Ceylon and India.
– Ann. Roy. Bot. Gard. (Peradeniya) 1: 267-465.
Willis JC. 1915. A new natural family of
flowering plants – Tristichaceae. – Bot. J. Linn. Soc. 43: 49-54.
Willis JC. 1926. The evolution of the
Tristichaceae and Podostemaceae. – Ann. Bot. 40:
349-367.
Wilmot-Dear CM. 1985. Salicaceae. – In: Polhill RM (ed),
Flora of tropical East Africa, A. A. Balkema, Rotterdam, pp. 1-8.
Winkler H. 1927. Über eine
Rafflesia aus Zentralborneo. – Planta 4: 1-97.
Winkler H. 1931. Linaceae. – In: Engler A (†), Harms
H, Pax F (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 19a, W.
Engelmann, Leipzig, pp. 82-130.
Wolfes O, Hromatka O. 1933. Über ein neues
Tropanderivat aus Cocablättern. – E. Merck’s Jahresber. 47: 45-53.
Wollenweber E, Weber W. 1973. Coloring
chalcones of Populus bud oils. – Zeitschr. Pflanzenphys. 69:
125-128.
Wong M. 1990. Rafflesia hasseltii.
– Nat. Malays. 15: 56-59.
Wong M. 2002. Rafflesias of Malaysia. –
Malay. Nat. 55: 20–27.
Wong M, Latiff, A. 1994. Rafflesias of
Peninsular Malaysia. – Nat. Malays. 19: 84-88.
Wong M, Latiff A. 2001. A fragrant
Rafflesia cantleyi discovered! – Fol. Malays. 2: 211-218.
Woodson RE, Schery RW. 1958. Passifloraceae. – In: Flora of
Panama 7: 1-22.
Woodworth RH. 1935. Fibriform vessel members
in the Passifloraceae. –
Trop. Woods 41: 8-16.
Wurdack KJ. 2002. Molecular systematics and
evolution of Euphorbiaceae
sensu lato. – Ph.D. diss., University of North Carolina, Chapel Hill, North
Carolina.
Wurdack KJ, Davis CC. 2009. Malpighiales phylogenetics: gaining
ground on one of the most recalcitrant clades in the angiosperm tree of life.
– Amer. J. Bot. 96: 1551-1570.
Wurdack KJ, Farfan-Rios W. 2017.
Incadendron: a new genus of Euphorbiaceae tribe Hippomaneae
from the sub-Andean cordilleras of Ecuador and Peru. – PhytoKeys 85:
69-86.
Wurdack KJ, Hoffmann P, Samuel R, Bruijn A
de, Bank M van der, Chase MW. 2004. Molecular phylogenetic analysis of Phyllanthaceae (Phyllanthoideae
pro parte, Euphorbiaceae sensu
lato) using plastid rbcL sequences. – Amer. J. Bot. 91:
1882-1900.
Wurdack KJ, Hoffmann P, Chase MW. 2005.
Molecular phylogenetic analysis of uniovulate Euphorbiaceae (Euphorbiaceae sensu stricto) using
plastid rbcL and trnL-F DNA sequences. – Amer. J. Bot. 92:
1397-1420.
Xavier KS, Mildner RA, Rogers CM. 1980.
Pollen morphology of Linum sect. Linastrum (Linaceae). – Grana 19: 183-188.
Xi Y, Zhou S. 1992. A contribution to the
pollen morphology of Tetraena and Malphgiaceae, with discussion of the
affinity and taxonomic position of Tetraena. – Chinese J. Bot. 4:
6-12.
Xi Z-X, Ruhfel BR, Schaefer H, Amorim AM,
Sugumaran M, Wurdack KJ, Endress PK, Matthews ML, Stevens PF, Mathews S, Davis
CC. 2012. Phylogenomics and a posteriori partitioning resolve the Cretaceous
angiosperm radiation of Malpighiales. – Proc. Natl. Acad.
Sci. U. S. A. 109: 17519-17524.
Xinying Z, Baas P, Mennega AMW. 1990. Wood
anatomy of Bhesa sinica (Celastraceae). – IAWA
Bull. 11: 57-60.
Yakandawala D, Morton CM, Prance GT. 2010.
Phylogenetic relationships of the Chrysobalanaceae inferred from
chloroplast, nuclear, and morphological data. – Ann. Missouri Bot. Gard. 97:
259-281.
Yang X-D, Chen W, Zhao J-F, Yang L-J, Zhang
H-B, Li L. 2009. Ent-kaurane diterpenes and phenolic compounds from Croton
kongensis. – Biochem. Syst. Ecol. 37: 237-240.
Yang Y, Berry PE. 2011. Phylogenetics of the
Chamaesyce clade (Euphorbia, Euphorbiaceae): reticulate
evolution and long-distance dispersal in a prominent C4 lineage. –
Amer. J. Bot. 98: 1486-1503.
Yang Y, Riina R, Morawetz JJ, Haevermans T,
Aubriot X, Berry PE. 2012. Molecular phylogenetics and classification of
Euphorbia subgenus Chamaesyce (Euphorbiaceae). – Taxon 61:
764-789.
Yao G, Zhang D-X. 2016. Pollen morphology of
Chinese Glochidion (Phyllanthaceae) and its taxonomic
implications. – Nord. J. Bot. 34: 102-110.
Yi X-Z. 1979. Pollen morphology of Guttiferae
in China. – Acta Bot. Sin. 21: 36-41. [In Chinese]
Yilmaz O, Kaynak G. 2008. A new species of
Linum (Linaceae) from West
Anatolia, Turkey. – Bot. J. Linn. Soc. 156: 459-462.
Yin GS, Keng H. 1974. Morphological studies
on some inland Rhizophoraceae.
– Gard. Bull. Straits Settlem. 27: 183-220.
Yockteng R, Nadot S. 2004a. Phylogenetic
relationships among Passiflora species based on the glutamine
synthetase nuclear gene expressed in chloroplast (ncpGS). – Mol.
Phylogen. Evol. 31: 379-396.
Yockteng R, Nadot S. 2004b. Infrageneric
phylogenies: a comparison of chloroplast-expressed glutamine synthetase,
cytosol-expressed glutamine synthetase and cpDNA maturase K in
Passiflora. – Mol. Phylogen. Evol. 31: 397-402.
Yokhioka H, Kondo K, Legrand M, Nehira K,
Maxeda S. 1984. Karyomorphological studies in five species of mangrove genera
in Rhizophoraceae. – La
Kromosomo, ser. II, 35-36: 1111-1116.
Young FE. 2008 onwards. Lacistemataceae Holistic
Database at https://urlproxy.sunet.se/canit/urlproxy.php?_q=aHR0cDovL3d3dy5sYWNpc3RlbWF0YWNlYWUub3Jn&_s=ZGVmYXVsdA%3D%3D&_c=1eb8c273&_r=c3Utc2U%3D.
Yousefi N, Hehrvarz SS, Marcussen T. 2012.
Anatomical studies on selected species of Viola (Violaceae). – Nord. J. Bot. 30:
461-469.
Yu R-Y, Welzen PC van. 2018. A taxonomic
revision of Trigonostemon (Euphorbiaceae) in Malesia. –
Blumea 62: 179-229.
Yunus D. 1990. Studies in the pollen
morphology of Malpighiaceae.
– Phytomorphology 40: 21-25.
Zhang W, Steinmann VW, Nikolov L, Kramer EM,
Davis CC. 2013. Divergent genetic mechanisms underlie reversals to radial
floral symmetry from diverse zygomorphic flowered ancestors. – Front Plant
Sci. 2013; 4: 302. publ. online
Zhang X, Baas P, Mennega AMW. 1990. Wood
anatomy of Bhesa sinica (Celastraceae). – IAWA
Bull., N.S., 11: 57-60.
Zhang Z-G, Meng A-P, Li J-Q, Ye Q-G, Wang
H-C, Endress PK. 2012. Floral development of Phyllanthus chekiangensis
(Phyllanthaceae), with special
reference to androecium and gynoecium. – Plant Syst. Evol. 298: 1229-1238.
Zhou J-S, Xing F-W. 2007. Viola
changii sp. nov. (Violaceae)
from Guangdong, southern China. – Nord. J. Bot. 25: 303-305.
Zhou J-S, Gong Q, Xing F-W. 2007. Two new
synonyms of Viola Linn. (Violaceae). – J. Trop. Subtrop. Bot.
15: 366-368. [In Chinese]
Zhou Z, Gu B-J, Sun H, Zhu H, Tan Y-H. 2017.
Molecular phylogenetic analyses of Euphorbiaceae tribe Epiprineae,
with the description of a new genus, Tsaiodendron gen. nov., from
south-western China. – Bot. J. Linn. Soc. 184: 167-184.
Zimmermann NFA, Ritz CM, Hellwig FH. 2010.
Further support for the phylogenetic relationships within Euphorbia L.
(Euphorbiaceae) from nrITS and
trnL-trnF IGS sequence data. – Plant Syst. Evol. 286:
39-58.
Zizka G, Schneider JV. 1999. The genus
Touroulia Aubl. (Quiinaceae). – Willdenowia 29:
227-234.