“ERICIDAE”
[Ericales+Gentianidae]
ERICALES Bercht. et J. Presl
Berchtold et Presl, Přir. Rostlin: 251. Jan-Apr
1820 [‘Ericeae’]
Ericopsida Bartl.,
Ord. Nat. Plant.: 120, 152. Sep 1830 [’Ericineae’];
Ericanae Takht., Sist. Filog. Cvetk. Rast. [Syst.
Phylog. Magnolioph.]: 221. 4 Feb 1967; Theanae R.
Dahlgren ex Reveal in Phytologia 74: 179. 25 Mar 1993;
Primulanae R. Dahlgren ex Reveal in Phytologia 79:
71. 29 Apr 1996; Theidae Doweld, Tent. Syst. Plant.
Vasc.: xliii. 23 Dec 2001; Ericidae C. Y. Wu in Acta
Phytotaxon. Sin. 40: 308. 2002
Fossils Actinocalyx
bohrii from the Late Santonian to the Early Campanian of Sweden comprises
hypogynous flowers with a pentamerous biserial perianth, five haplostemonous
stamens and three connate carpels. The five petals are basally connate and the
filaments are adnate to the corolla. The pollen grains are tricolpate with a
finely foveolate exine. The ovary is trilocular, the placentation is central,
the ovules are anatropous and the fruits are capsular. Paradinandra
suecica from the Late Santonian to the Early Campanian of Sweden resembles
Actinocalyx, although it has basally connate stamens which are
arranged in an outer whorl of five and an inner whorl of ten stamens. The
paired stamens were probably antepetalous and solitary stamens were possibly
likewise antepetalous. There is an intrastaminal nectariferous disc. The
anthers probably dehisced by apical pores or slits, and the pollen grains were
tricolpate. The placentation is intrusively parietal and the ovules are
campylotropous.
Paleoenkianthus sayrevillensis, from
the Turonian of New Jersey, is represented by hypogynous pentamerous flowers
and fruits. The petals are basally connate and have a valvate aestivation.
There is one series of eight stamens with inverted anthers bearing two or three
appendages at their base, and the pollen grains are probably tricolporate with
reticulate exine. The four connate carpels form a quadrilocular ovary with four
free styles, and developed into a capsule with septicidal-loculicidal
dehiscence.
Pentapetalum trifasciculandricum, from
the Turonian of New Jersey, includes pentamerous hypogynous flowers with a
large number of stamens in three fascicles, and three carpels forming free
styles and a trilocular ovary containing numerous ovules.
‘Pentaphylax’ protogaea, fossil fruits from the
Maastrichtian of Germany, has been assigned to the extant Pentaphylacaceae, although
there are several important differences. The fossils have a thick lignified
pericarp and the persistent calyx lobes have unequal length. Moreover, the
seeds are anatropous.
Habit Bisexual, monoecious,
polygamomonoecious, dioecious, polygamodioecious or functionally monoecious,
dioecious or gynodioecious, evergreen or deciduous trees, shrubs, suffrutices
or lianas, or perennial, biennial or annual herbs.
Vegetative anatomy Usually
with arbuscular mycorrhiza with Glomales. Phellogen ab initio
superficially or deeply seated. Secondary lateral growth normal or absent.
Vessel elements with scalariform or simple (sometimes reticulate) perforation
plates; lateral pits usually alternate, scalariform or opposite, simple or
bordered pits (vessels occasionally absent). Pit membrane remnants may occur in
some species. Imperforate tracheary xylem elements tracheids or fibre tracheids
with bordered pits, non-septate (sometimes also vasicentric tracheids). Wood
rays uniseriate or multiseriate, usually heterocellular (sometimes
homocellular), or absent. Axial parenchyma apotracheal diffuse or
diffuse-in-aggregates, or paratracheal scanty vasicentric, reticulate,
unilateral or banded (rarely aliform, confluent or scalariform), or absent.
Sieve tube plastids usually Ss type (sometimes Pcf type). Nodes usually 1:1,
unilacunar with one trace (rarely 3:3, trilacunar with three traces).
Idioblasts with mucilage often present. Laticifers with milky latex
occasionally present; ducts with gutta-percha sometimes present. Parenchyma
often with idioblasts containing calciumoxalate raphides. Prismatic or acicular
crystals, crystal sand, styloids or druses sometimes present.
Trichomes Hairs unicellular or
multicellular, uniseriate or multiseriate, simple or branched, bristle-like,
flattened, furcate, stellate or dendritic (sometimes fasciculate, T-shaped,
candelabra-, funnel- or cup-shaped, rarely arachnoid, peltate or lepidote), or
absent; multicellular glandular hairs with capitate head (sometimes thick and
colleter-like, rarely peltate-lepidote) sometimes present.
Leaves Usually alternate
(spiral or sometimes distichous, rarely tetrastichous; rarely opposite or
verticillate), simple, entire, sometimes coriaceous or ericoid (rarely
scale-like), with conduplicate, supervolute, involute, convolute or revolute
(rarely circinnate or plicate) ptyxis. Stipules very small and caducous or
absent; leaf sheath absent. Colleters often abundant. Petiole vascular bundle
transection arcuate or annular (sometimes D-shaped or complex, sometimes with
one or two medullary bundles). Venation pinnate, eucamptodromous,
brochidodromous, craspedodromous or semicraspedodromous (rarely
reticulodromous, acrodromous to palinactinodromous or actinodromous), or leaves
single-veined; vascular bundles often collateral; some veins proceeding into
teeth. Stomata usually anomocytic (sometimes paracytic, anisocytic, cyclocytic
or actinocytic, rarely stephanocytic, staurocytic or parallelocytic). Cuticular
wax crystalloids as platelets or rodlets (sometimes amorphous or as tubuli
dominated by diketones), or absent. Epidermis with or without mucilage cells.
Mesophyll often with calciumoxalate raphides. Mesophyll with or without
sclerenchymatous idioblasts containing brachysclereids, osteosclereids and
other kinds of sclereids. Secretory oil cavities present or absent. Ducts with
gutta-percha sometimes present. Leaf margin usually serrate or entire
(sometimes crenate). Leaf tooth apex often widened, translucent, persistent,
sometimes gland-tipped (rarely with nectariferous glands); teeth often theoid,
with single vein and opaque deciduous cap (sometimes with single multicellular
hair replacing cap).
Inflorescence Usually axillary
(sometimes terminal), panicle, thyrsopaniculate, thyrsoid, botryoid,
fasciculate, corymbose, head-, umbel-, raceme- or spike-like, cymose or
racemose (flowers sometimes solitary, axillary or terminal). Floral prophylls
(bracteoles) present or absent.
Flowers Actinomorphic or
zygomorphic. Usually hypogyny (sometimes epigyny or half epigyny). Sepals (two
to) five (to twelve), with imbricate or valvate (sometimes open, rarely
contorted) aestivation, usually persistent, free or more or less connate.
Petals (three to) five (to 18), with imbricate, valvate or contorted (rarely
conduplicate or subinduplicate) aestivation, usually caducous, free or more or
less connate into hypocrateromorphous, discoid, campanulate, infundibuliform,
tubular or urceolate corolla (sometimes nectariferous at base). Nectary and
disc usually absent (nectariferous disc sometimes annular or consisting of
separate parts, intrastaminal or occasionally extrastaminal, or petals
sometimes nectariferous).
Androecium Stamens (two to)
five to more than 1.200, in one or more whorls (sometimes in fascicles).
Filaments usually free (rarely connate at base), usually free from tepals
(sometimes epipetalous). Anthers dorsifixed, ventrifixed or subbasifixed,
versatile or non-versatile, often inverting and finally resupinate, usually
tetrasporangiate (rarely disporangiate), extrorse or introrse (rarely
latrorse), longicidal (dehiscing by longitudinal or transverse slits) or
poricidal (dehiscing by seemingly apical to subapical pores). Tapetum usually
secretory (rarely amoeboid-periplasmodial). Staminodia sometimes present.
Pollen grains
Microsporogenesis simultaneous. Pollen grains (2–)3(–6)-colpor(oid)ate,
(2–)3(–5)-colpate or 3(–5)-porate (rarely pantocolporate, pantoporate or
stephanocolpate), usually shed as monads (sometimes tetrads, rarely dyads,
triads or polyads), usually bicellular (rarely tricellular) at dispersal. Exine
tectate or semitectate (rarely intectate), usually with granular (sometimes
columellate) infratectum, pertectate, reticulate, foveolate, scabrate,
fossulate, areolate, rugulate, psilate, gemmate, verrucate, verruculate,
echinate, scrobiculate, granulate or microgranulate.
Gynoecium Pistil composed of
(one to) five to c. 20 (to c. 30) more or less connate carpels. Ovary usually
superior (sometimes inferior or semi-inferior), (unilocular to) quinquelocular
to c. 20(–30)-locular (sometimes incompletely septate). Style single, simple,
or stylodia (three to) five to c. 20, short, free or partially connate,
sometimes persistent. Stigma capitate, punctate, peltate or lobate, papillate
or non-papillate, Dry or Wet type. Male flowers sometimes with pistillodium.
Ovules Placentation usually
axile (sometimes apical or parietal, rarely basal; placentae often diffuse).
Ovules one to numerous per carpel, usually anatropous (sometimes hemianatropous
or campylotropous, rarely orthotropous), pendulous to ascending, apotropous or
epitropous, usually unitegmic (sometimes bitegmic), tenuinucellar. Micropyle
endostomal or bistomal (when bitegmic usually endostomal). Megagametophyte
usually monosporous, Polygonum type (sometimes disporous,
Allium type, rarely tetrasporous, Adoxa type). Synergids
sometimes with a filiform apparatus. Antipodal cells sometimes persistent and
proliferating. Endosperm development usually cellular (sometimes nuclear).
Endosperm haustoria chalazal and/or micropylar or absent. Embryogenesis
solanad, onagrad or asterad (sometimes caryophyllad or chenopodiad).
Fruit A loculicidal and/or
septifragal capsule (rarely a pyxidium or a denticidal capsule), a berry, a
drupe or a samara (rarely a nut enclosed by perianth or a drupaceous
schizocarp), often with persistent and sometimes accrescent calyx.
Seeds Aril usually absent.
Exotesta with inner cell walls of outer epidermis often thickened, with theoid
exotestal thickenings. Perisperm not developed. Endosperm copious to sparse
(rarely ruminate), oily or proteinaceous. Embryo large to small, usually
straight (sometimes curved), well differentiated, usually without chlorophyll.
Cotyledons two. Germination usually phanerocotylar (rarely cryptocotylar).
Cytology x =
(3–)7–13(–20)
DNA Nuclear gene PI
duplicated. I copy of nuclear gene RPB2 present.
Mitochondrial intron coxII.i3 often lost.
Phytochemistry Flavonols
(kaempferol, quercetin, myricetin, gossypetin), catechins, cyanidin,
davidigenin, Route I secoiridoids, Group I carbocyclic iridoids (aucubin,
daphylloside, monotropein), Group II decarboxylated iridoids (e.g. cornine),
Group IV carbocyclic iridoids (rarely unedoside, a C8 iridoid glycoside), Group
VI secoiridoids (morroniside), Group X secoiridoids (nepetalactones,
iridoidpyridine alkaloids), sarracenin, oleanolic acid derivatives,
monoterpenes, diterpenes (e.g. andromedotoxin), sesquiterpenes, triterpenes,
free triterpenic acids (betulinic acid, ursolic acid), dammaranes, arjunolic
acid derivatives, ellagic acid (frequent to absent), gallic acid, ellagitannins
(geraniin, pedunculagin, tellimagrandin II), non-hydrolyzable tannins,
proanthocyanidins (prodelphinidins), caffeic acid, chlorogenic acid, phenolic
heterosides, 3-galactoside, triterpene saponins, benzoquinones (e.g. arbutin,
rapanone) and naphthoquinones and their derivatives (e.g. naphthoquinone
derivatives of 7-methyljugone, lawsone, plumbagin, droserone), anthraquinones,
polyacetate-derived arthroquinones, benzopyrones, cucurbitacins,
dihydrosterculic acid, lignans (pinoresinol), myo-inositol, and
ethereal oils present. Alkaloids (e.g. pyrrolizidine alkaloids, indole
alkaloids and their glycosides) and cyanogenic compounds rare. Carbohydrates
sometimes stored as oligosaccharides with kestose or isokestose bonds (inulin).
Aluminium accumulated in some groups.
Systematics Ericales are sister-group to
Asteridae except Loasales.
The clade [Balsaminaceae+[Marcgraviaceae+Tetrameristaceae]] is
sister-group to all other Ericales. Potential synapomorphies
are, according to Stevens (2001 onwards): vessel elements with usually simple
perforation plates; vessels in radial multiples; presence of raphid sacs in
stem; presence of branched sclereids; absence of druses; leaf with supervolute
ptyxis; lamina elongating in bud and with obscure abaxial lines; leaf margin
serrate; inflorescence racemose; abaxial petal surface with stomata; filiform
appendages present along stomium; stamens as many as sepals, antesepalous, free
from petals; anthers subbasifixed; ovary with mucilaginous secretions; absence
of gynoecial nectary; style short, stigma little expanded; ovules bitegmic;
micropyle endostomal; presence of micropylar endosperm haustorium; presence of
myricetin; and absence of ellagic acid. Balsaminaceae and Tetrameristaceae share the
synapomorphies: sepals petaloid, nectariferous; filaments postgenitally
connate; and stylar canal present, quinqueradiate.
The leaves in this basal clade are often
glabrous, simple and usually lack stipules (some Balsaminaceae have stipules).
Extrafloral nectaries or glands are often present on the adaxial side of leaf
(including petiole), calyx or corolla. The pollen grains are tricolporate and
bicellular at dispersal, and the ovules are bitegmic and tenuinucellar. The
endosperm development is cellular, endosperm haustorium is present, and the
endosperm is sparse. The nodes are unilacunar in Balsaminaceae and Marcgraviaceae, and there is a
caducous calyptra, developed from androecium (Balsaminaceae) or petals (Marcgraviaceae). Marcgraviaceae and Tetrameristaceae have
indehiscent fruit, and Pelliciera and Marcgraviaceae have large
cortical air cavities.
The Ericales main clade may be
characterized by connate corolla with well developed tube, and long style. The
clade [Polemoniaceae+Fouquieriaceae] is part of a
trichotomy forming the base of the remaining Ericales. Fouquieria and
Polemoniaceae share the
potential synapomorphies (Stevens 2001 onwards): phellogen outer-cortical;
vessel elements with simple perforation plates; inflorescence terminal, cymose;
hypogyny; sepals free, with membranous margins and abaxial stomata; petals
largely connate; presence of nectaries; filaments adnate to corolla; anther
thecae largely separate, without septum; pistil composed of three connate
carpels; presence of gynoecial nectary; style hollow, strongly lobate; ovules
arranged in two ranks, apotropous; fruit a loculicidal capsule, with persistent
calyx and seeds inserted on central columella; seed coat winged; endosperm
sparse; and loss of mitochondrial intron coxII.i3. Lecythidaceae are the second
lineage of the trichotomy, whereas the third monophyletic group comprises the
main clade of Ericales.
The first of three clades in a basal trichotomy
forming the remainder of Ericales has the topology [Sladeniaceae+[Pentaphylacaceae+Ternstroemiaceae]]. This clade
has the following potential synapomorphies (Stevens 2001 onwards): vessel
elements with scalariform perforation plates; bordered vessel-fibre pits; nodes
1:1; presence of mucilage cells; unicellular hairs; petiole vascular bundle
transection arcuate; campanulate corolla; petals less than one centimetre long
and connate only at base; absence of nectary; basifixed anthers; pollen grains
14–30 μm long; exine usually only little ornamented; placentae becoming
swollen; bitegmic ovules; endostomal micropyle; inner integument three or four
cell layers thick; fruit a capsule with columella and calyx persistent;
presence of endosperm; and long embryo. Pentaphylax and Ternstroemiaceae share the
following synapomorphies: parenchyma apotracheal; intervessel pitting
opposite-scalariform; leaves with supervolute ptyxis; flowers solitary axillary
or inflorescence fasciculate; corolla usually greenish to yellowish; style
hollow; ovules campylotropous to hemitropous; mesotesta well developed; embryo
U-shaped; and accumulation of aluminium.
The second clade in this trichotomy has the
following topology: [Sapotaceae+ [Ebenaceae+[Maesaceae+[[Theophrastaceae+Samolaceae]+[Myrsinaceae+Primulaceae]]]]]. Morphological and
phytochemical characters supporting this clade are few: connate petals;
apotropous ovules; and absence of ellagic acid. Furthermore, bitegmic ovules
and inner integument thicker than outer are two potential synapomorphies
uniting Ebenaceae with the
following clade. [Maesaceae+[[Theophrastaceae+Samolaceae]+[Myrsinaceae+Primulaceae]]] is a very strongly
supported clade. Synapomorphies are, e.g., the following (Stevens 2001
onwards): schizogenous secretory canals with yellow, red or brown substances
(e.g. tannins) sometimes present; nodes 3:3?; stomata sometimes anisocytic;
presence of small immersed and often peltate glandular hairs; inflorescence
racemose; petals and stamens arising from common primordia; presence of
nectaries; stamens as many as petals, antepetalous; staminodia antesepalous,
represented by at least a vascular trace; carpels five, antepetalous; style
short, hollow; stigma capitate; placentation free central, with large placenta;
ovules at least partially immersed in swollen placenta, apotropous, bitegmic;
micropyle bistomal; endosperm development nuclear; tanniniferous endothelium
present; seeds angular; endotesta crystalliferous; endosperm copious, sometimes
with amyloid (xyloglucans) or hemicellulose, and sometimes with thick and
pitted cell walls.
The clade [[Theophrastaceae+Samolaceae]+[Myrsinaceae+Primulaceae]] is characterized by
wood rays at least 5-seriate; absence of uniseriate rays in woody species;
presence of small immersed often peltate or glandular hairs; absence of floral
prophylls (bracteoles); corolla subrotate, with imbricate aestivation; corolla
tube fairly short; petals arising abaxially from common primordium; and
staminal primordium larger than petal primordium. Theophrastaceae and
Samolus share the characters bracts displaced up pedicels and presence
of petaloid staminodia, whereas Myrsinaceae och Primulaceae share two identical
deletions in plastid genendhF.
The third clade of the trichotomy comprises (1)
Theaceae, (2) the clade [Symplocaceae+ [Styracaceae+Diapensiaceae]] and (3) the clade
[Sarraceniaceae+[Actinidiaceae+Roridulaceae]]+ [Clethraceae+[Cyrillaceae+Ericaceae]] and may be characterized
by the synapomorphies: vessel elements with scalariform perforation plates, and
serrate leaves. An additional lineage is the mycotrophical holoparasitic
Mitrastemma although with a highly uncertain position.
The clade [Symplocaceae+[Styracaceae+Diapensiaceae]] is characterized
by the features: woody habit; racemose inflorescence; hollow style; and copious
endosperm. The lineage [Styracaceae+Diapensiaceae] has pericyclic
phellogen; nodes 1:1; absence of glandular hairs; spiral leaves with usually
serrate margin; nectariferous disc absent or poorly developed and annular;
stamens usually twice as many as petals; filaments connate at base or adnate to
corolla tube; anthers basifixed, introrse, longicidal; endothecium well
developed and with fibrous thickenings; pollen grains tricolporate, shed as
monads and usually bicellular at dispersal; pistil composed of two to four
carpels; hollow style continuing without interruption into locules; ovules
numerous per carpel; unitegmic, tenuinucellar; megagametophyte usually with
large amount of starch; endosperm development cellular; and fruit usually a
loculicidal capsule. Furthermore, calciumoxalate crystals are often present as
aggregations in idioblasts in the parenchyma.
The [[Sarraceniaceae+[Actinidiaceae+Roridulaceae]]+[Clethraceae+[Cyrillaceae+Ericaceae]]] clade has the potential
synapomorphies (Stevens 2001 onwards): inflorescence racemose; anthers
extrorse, inverting during development, and dehiscing by apical pores or short
slits; pollen grains rugulate; tectum and foot layer solid; infratectum
granular; pistil composed of three carpels, with median carpel adaxial, or five
antepetalous carpels; style impressed into ovary apex; ovules numerous per
carpel; endothelium present; fruit a capsule; exotesta with strongly thickened
inner cell walls; endosperm copious; and absence of mitochondrial intron
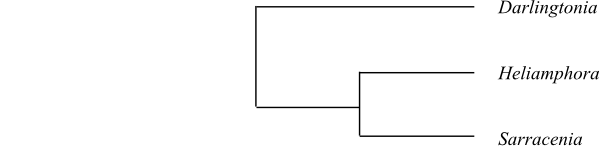
coxII.i3. The clade [Sarraceniaceae+[Actinidiaceae+Roridulaceae]] is characterized by
the features: absence of nectary; stigma Dry type; presence of hypostase; and
presence of Route I secoiridoids. The lineage [Clethraceae+[Cyrillaceae+Ericaceae]] has the synapomorphies:
phellogen pericyclic; absence of pericyclic fibres; leaves spiral;
inflorescence racemose; absence of floral prophylls; flowers pendulous; basal
part of ovary wall nectariferous; stamens twice as many as sepals; style
hollow; endosperm with micropylar and chalazal haustoria; testa reduced; embryo
terete; and presence of ellagic acid. Finally, Cyrillaceae and Ericaceae share the synapomorphies:
presence of colleters; connate petals; stigma Wet type; and presence of
myricetin.
The strongly supported clade [[Sarraceniaceae+[Actinidiaceae+Roridulaceae]]+[Clethraceae+[Cyrillaceae+Ericaceae]]] has the potential
synapomorphies (Stevens 2001 onwards; Löfstrand & Schönenberger 2015):
inflorescence racemose; anthers attached adaxially, extrorse, inverting during
development, and dehiscing by apical pores or short slits; pollen grains
rugulate; tectum and foot layer solid; infratectum granular; depression at
ovary-to-style transition; pistil composed of three carpels, with median carpel
adaxial, or five antepetalous carpels; style impressed into ovary apex; ovules
numerous per carpel; endothelium present; fruit a capsule; exotesta with
strongly thickened inner cell walls; endosperm copious; and absence of
mitochondrial intron coxII.i3. The clade [Sarraceniaceae+[Actinidiaceae+Roridulaceae]] is characterized by
features such as: petals thick to massive; nectary absent; polystemony; stigma
Dry type; hypostase present; and Route I secoiridoids present. The lineage
[Actinidiaceae+Roridulaceae] is characterized by,
e.g.: presence of calcium oxalate raphides; mucilage cells; inner gynoecium
surface secretory; and synlateral vasculature absent from ovary. The clade
[Clethraceae+[Cyrillaceae+Ericaceae]] has the synapomorphies:
phellogen pericyclic; absence of pericyclic fibres; leaves spiral;
inflorescence racemose; absence of floral prophylls; flowers pendulous;
androecium two-worled; basal part of ovary wall nectariferous; stamens twice as
many as sepals; style hollow; endosperm with micropylar and chalazal haustoria;
testa reduced; embryo terete; and presence of ellagic acid. Finally, Cyrillaceae and Ericaceae share the synapomorphies:
presence of colleters; connate petals; stigma Wet type; and presence of
myricetin.
ACTINIDIACEAE Gilg et
Werderm.
|
( Back to Ericales )
|
Gilg et Werdermann in Engler et Prantl, Nat.
Pflanzenfam., ed. 2, 21: 36. Dec 1925, nom. cons.
Saurauiaceae Griseb.,
Grundr. Syst. Bot.: 98. 1-2 Jun 1854 [’Sauraujeae’], nom. cons.;
Actinidiales Takht. ex Reveal in Phytologia 74: 174.
25 Mar 1993
Genera/species 3/360
Distribution Tropical and
subtropical regions of East and Southeast Asia, the Himalayas, Malesia to New
Guinea, northeastern Queensland, Solomon Islands, Fiji, Mexico, Central
America, the Andes to Chile.
Fossils Late Cretaceous
records of Actinidiaceae
are known from North America and Europe and they are found at many Cenozoic
sites in the Northern Hemisphere. Saurauia alenae and S.
antiqua, anatropous seeds with reticulate surface, have been found in the
Late Turonian to the Maastrichtian of Central Europe, and fossils similar to
Actinidia are recorded from the Late Eocene onwards.
Actinidiophyllum has been described from Cenozoic strata in Japan.
Parasaurauia allonensis and Glandulocalyx, represented by
flowers with free pentamerous perianth, ten stamens in two series and three
carpels with free styles, was described from the Late Cretaceous (Late
Santonian/Early Campanian) of Georgia in the United States as sister-group to
[Actinidia+Saurauia] (Keller & al. 1996). Leaf
impressions resembling Saurauia are known from mid-Eocene layers of
North America.
Habit Bisexual,
morphologically dioecious (Clematoclethra), functionally dioecious or
monoecious, evergreen or deciduous trees, shrubs or lianas.
Vegetative anatomy Phellogen
ab initio superficially or deeply seated. Medulla often septate by diaphragms.
Endodermis prominent. Pericycle with continuous ring of sclerenchyma. Vessel
elements dimorphic, with scalariform or simple (sometimes reticulate)
perforation plates; lateral pits usually opposite (sometimes scalariform or
alternate), simple or bordered pits. Imperforate tracheary xylem elements
tracheids or fibre tracheids with bordered pits, non-septate. Wood rays
uniseriate or multiseriate, heterocellular. Axial parenchyma apotracheal
diffuse or diffuse-in-aggregates, or paratracheal scanty vasicentric or banded.
Sieve tube plastids S type. Nodes usually 1:1, unilacunar with one leaf trace
(rarely 3:3, trilacunar with three traces). Parenchyma with idioblasts (raphide
cells) containing raphides; calciumoxalate as crystal sand present in at least
Clematoclethra.
Trichomes Hairs multicellular,
uniseriate, often bristle-like or flattened, or branched, stellate or
dendritic; glandular hairs, short and long, sometimes present.
Leaves Usually alternate
(spiral; rarely opposite), simple, entire, with conduplicate ptyxis. Stipules
very small and caducous or absent; leaf sheath absent. Petiole vascular bundle
transection deeply arcuate with wing bundles (Actinidia), or annular
(sometimes with medullary bundles). Venation usually pinnate, camptodromous
(sometimes subpalmate); some veins proceeding into teeth. Stomata usually
anomocytic (sometimes paracytic). Cuticular wax crystalloids as platelets or
transversely ridged rodlets. Mesophyll with calciumoxalate raphides. Leaf
margin usually serrate (sometimes entire); tooth apices widened, translucent,
persistent.
Inflorescence Axillary,
usually thyrsopaniculate etc. (flowers sometimes solitary, axillary). Floral
prophylls (bracteoles) present or absent.
Flowers Actinomorphic.
Hypogyny. Sepals (three to) five (to eight), with imbricate quincuncial
aestivation, usually persistent, free. Petals (three to) five (to nine), with
imbricate quincuncial aestivation, caducous, free or connate at base, sometimes
nectariferous at base. Nectary usually absent. Disc absent.
Androecium Stamens c. 20 to c.
240 (in Clematoclethra ten), in two or more whorls (primarily
diplostemonous), usually inflexed in bud (not in Saurauia), sometimes
in five antepetalous fascicles. Filaments usually free (rarely connate at
base), usually free from petals (sometimes epipetalous). Anthers dorsifixed,
versatile, late inverted (finally resupinate), tetrasporangiate, extrorse,
longicidal (dehiscing by longitudinal or short slits widening at apex) or
poricidal (dehiscing by seemingly subapical pores). Tapetum secretory, with
multinucleate cells (sometimes with fused nuclei). Female flowers sometimes
with staminodia?
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually tricolporate (sometimes
tetracolporate), usually shed as monads (rarely tetrads), bicellular at
dispersal. Exine tectate, with granular infratectum, imperforate, psilate,
microgranulate or rugulate, often transversely striate.
Gynoecium Pistil composed of
(three to) five to c. 20 incompletely connate synascidiate carpels. Ovary
superior, (trilocular to) quinquelocular to 20-locular (sometimes incompletely
septate). Stylodia (three to) five to c. 20, short, free or partially connate,
sometimes impressed, sometimes persistent, or style single, simple and hollow
(Clematoclethra; in Actinidia furrowed). Stigmas capitate,
peltate or lobate, papillate, Dry type. Male flowers sometimes with
pistillodia?
Ovules Placentation axile.
Ovules usually numerous (in Clematoclethra approx. ten) per carpel,
anatropous, unitegmic, tenuinucellar. Integument six to nine cell layers thick.
Hypostase well developed. Parietal tissue approx. three cell layers thick.
Nucellar cap approx. three cell layers thick. Megasporocytes usually one
(occasionally several, archespore multicellular). Megagametophyte monosporous,
Polygonum type. Endosperm development cellular. Endosperm haustoria?
Embryogenesis solanad. Polyembryony often occurring.
Fruit Usually a berry
(sometimes a loculicidal capsule), often with persistent calyx; seeds embedded
in pulp formed by placentae.
Seeds Aril usually absent
(present in Actinidia). Testa multiplicative. Exotesta with inner cell
walls of outer epidermis thick, with theoid exotestal thickenings. Endotesta?
Perisperm not developed. Endosperm copious, oily or proteinaceous. Embryo
large, usually straight (sometimes curved), well differentiated, without
chlorophyll. Cotyledons two. Germination phanerocotylar.
Cytology n = 20, 24, 29, 30,
39 (x = 12 in Clematoclethra; x = 13 in Saurauia; x = 29 in
Actinidia) – Polyploidy frequently occurring.
DNA Horizontal transfer of
mitochondrial gene rps2 in Actinidia. Mitochondrial intron
coxII.i3 lost.
Phytochemistry Flavonols
(kaempferol, quercetin, myricetin?), catechins, Route I secoiridoids, Group X
secoiridoids (nepetalactones, iridoidpyridine alkaloids), gallic acid,
proanthocyanidins (cyanidin, prodelphinidin), and polyacetate-derived
arthroquinones present. Actinidin (a proteinase) present in Actinidia.
Alkaloids usually absent (matatabi lactone and actinidine present). Ellagic
acid, saponins, and cyanogenic compounds not found.
Use Ornamental plants,
fruits.
Systematics
Clematoclethra (1; C. scandens; western and central China),
Actinidia
(c 60; southern and eastern China, the Korean Peninsula, Japan, Siberia,
Southeast Asia), Saurauia (c 300; tropical Asia, the Himalayas,
tropical and subtropical regions of East and Southeast Asia, Malesia to New
Guinea, northeastern Queensland, Solomon Islands, Fiji, Mexico and Central
America, northern Andes to Chile).
Actinidiaceae are sister-group to
Roridula
(Roridulaceae).
Clematoclethra differs from Actinidia
and Saurauia, e.g., in having ten (instead of numerous) stamens and a
single hollow style, and in being morphologically dioecious. The topology
[Clematoclethra+[Actinidia+Saurauia]]
was suggested by Friis & al. (2011). Two geographical lineages were
identified by Löfstrand & Schönenberger (2015) in Saurauia. One
lineage is characterized by choripetaly and free styles, whereas the second
lineage has sympetaly and partially united styles. Gynoecium merism and base
chromosome numbers are additional characters.
BALSAMINACEAE A.
Rich.
|
( Back to Ericales )
|
Richard in J. B. G. M. Bory de Saint-Vincent,
Dict. Class. Hist. Nat. 2: 173. 31 Dec 1822 [’Balsamineae’], nom.
cons.
Balsaminales Bercht.
et J. Presl, Přir. Rostlin: 221. Jan-Apr 1820 [‘Balsamineae’];
Hydroceraceae Wilbrand, Nat. Pflanzenfam.: 26.
Mai-Dec 1834 [’Hydrocereae’], nom. illeg.;
Impatientaceae Lem., Ill. Hort. 1: ad t. 9. Mar 1854
[’Impatientiaceae’]; Balsaminineae
Engl., Syllabus, ed. 2: 146. Mai 1898; Balsaminanae
Doweld, Tent. Syst. Plant. Vasc.: xliv. 23 Dec 2001
Genera/species 2/>1.000
Distribution Tropical and
subtropical Africa, Madagascar, tropical Asia to New Guinea, with their largest
diversity in Madagascar and Southeast Asia, few species in temperate parts of
Eurasia, Africa and North America.
Fossils Unknown.
Habit Bisexual, although
usually functionally monoecious due to extreme protandry, usually perennial or
annual herbs (rarely partially woody). Stem more or less succulent, often
almost translucent. Impatiens tuberosa and the epiphytic I.
etindensis with large stem tubers. Numerous species are helophytes.
Vegetative anatomy Phellogen?
Primary vascular tissue a cylinder of vascular bundles. Secondary lateral
growth usually absent. Wood paedomorphic. Vessels solitary. Vessel elements
with simple perforation plates, lateral pits? Imperforate tracheary xylem
elements? Wood rays absent. Axial parenchyma? Sieve tube plastids S type. Nodes
1:1, unilacunar with one leaf trace. Sclerenchyma and sclereids absent.
Idioblasts with mucilage present. Cortex with calciumoxalate raphides in raphid
sacs.
Trichomes Hairs usually
absent.
Leaves Usually alternate
(spiral; rarely opposite or verticillate), simple, entire, with involute
ptyxis. Stipules and leaf sheath absent. Petiole often with glands (rarely with
extrafloral nectaries or foliaceous outgrowths at leaf base, petiole or stem).
Petiole vascular bundle transection arcuate. Venation pinnate. Stomata usually
anomocytic (sometimes almost anisocytic). Cuticular wax crystalloids?
Hydathodes abundant. Mesophyll with calciumoxalate raphides. Leaf margin
usually serrate (sometimes crenate or entire); teeth apiculate, basal teeth
often with nectariferous glands.
Inflorescence Axillary, raceme
or umbel-like. Floral prophylls (bracteoles) sometimes absent.
Flowers Zygomorphic, usually
resupinate (inverting during growth). Hypogyny. Sepals usually three (sometimes
five), with imbricate aestivation, caducous, free, two lateral abaxial sepals
reduced and small or absent, median adaxial sepal petaloid, usually with
nectariferous spur (absent in some Malagasy species) with secretory cells on
inner side, two abaxial/upper sepals very small or absent. Petals five, unequal
in size, with imbricate aestivation, dorsal petal free, flat or cucullate, four
lower/lateral petals usually pairwise connate (in Hydrocera free);
when three sepals, then adaxial petal often with sepaloid keel. Disc absent.
Androecium Stamens five,
haplostemonous, antesepalous, alternipetalous. Filaments short, stout, connate
at apex and forming ring around gynoecium, upper filaments slightly longer than
lower ones, free from tepals. Anthers ab initio connate into cucullate calyptra
above stigma, finally coming loose at base and uplifted by accrescent
gynoecium, dorsifixed to subbasifixed, non-versatile, usually dithecal
(sometimes polythecal and septate by transverse trabeculae), tetrasporangiate,
introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory.
Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains 3(–5)-colpate or
3(–5)-porate, starchy, shed as monads, usually bicellular (rarely
tricellular) at dispersal. Exine semitectate, with granular infratectum,
reticulate; with raphides and with cellulose threads adhering pollen grains to
anther.
Gynoecium Pistil composed of
(four or) five connate antepetalous carpels; when carpels four, then adaxial
carpel larger. Ovary superior, (quadrilocular or) quinquelocular. Style single,
very short, or absent. Stigmas usually one or (four or) five, relatively wide,
non-papillate, Wet type. Pistillodium absent.
Ovules Placentation axile.
Ovules usually numerous (in Hydrocera one to three) per carpel,
usually anatropous (rarely hemitropous), pendulous, apotropous, usually
(incompletely) bitegmic (sometimes unitegmic), incompletely tenuinucellar.
Micropyle endostomal. Outer integument four to ten cell layers thick (in
Hydrocera six to eight cell layers thick). Inner integument three to
six cell layers thick. Micropylar epistase present. Megagametophyte usually
monosporous, Polygonum type (sometimes disporous, 8-nucleate,
Allium type), finally strongly prolonged. Endosperm development
helobial (cellular?). Endosperm haustorium chalazal (in Hydrocera also
micropylar). Embryogenesis onagrad.
Fruit Usually a loculicidal
and septifragal carnose explosion capsule, dehiscing by capsule walls rolling
in from base due to tensions in fleshy convex fruit wall; tensions liberated at
slightest touch by bursting of wall along septa (in Hydrocera a
drupaceous schizocarp with hard endocarp splitting into five mericarps each
with one seed and two air chambers).
Seeds Seed pachychalazal. Seed
mucilaginous hairs sclerotized, with spiral thickenings. Aril? Calciumoxalate
raphide bundles abundant. Exotesta lignified, thickened. Mesotesta crushed.
Endotesta? Tegmen? Testa in Hydrocera sclerotized, consisting of six
to eight layers of thickened cells and five layers of unthickened cells.
Perisperm not developed. Endosperm thin, rich in lipids, poor in starch. Embryo
large, straight, well differentiated, without chlorophyll. Cotyledons two,
large, planoconvex. Germination phanerocotylar.
Cytology n = (3–)7–10 (or
more, up to 33; Impatiens), n = 8 (Hydrocera)
DNA Intron present in
mitochondrial gene coxII.i3. Duplication of class B DEF
genes.
Phytochemistry Flavonols
(kaempferol, quercetin, myricetin?), cyanidin, non-hydrolyzable tannins,
proanthocyanidins (prodelphinidins), and naphthoquinones and their derivatives
(e.g. lawsone) present. Ellagic acid, alkaloids, saponins, and cyanogenic
compounds not found. Seed oils with glycerides of acetic acid and parinaric
acid. Xyloclucans sometimes present in endosperm.
Use Ornamental plants.
Systematics Hydrocera
(1; H. triflora; India, Indochina); Impatiens
(>1.000; tropical and subtropical Africa, Madagascar, tropical Asia to
southern China and New Guinea, with their largest diversity in Madagascar and
mountain regions in India and Southeast Asia, few species in temperate parts of
Eurasia, Africa and North America).
Balsaminaceae are probably sister
to Tetrameristaceae.
CLETHRACEAE
Klotzsch
|
( Back to Ericales )
|
Klotzsch in Linnaea 24: 12. Mai 1851, nom.
cons.
Genera/species 2/90–95
Distribution Madeira, East
Asia to the Korean Peninsula, Japan and Taiwan, Hainan, Southeast Asia, Malesia
to New Guinea, eastern and southeastern United States, Mexico, tropical
America.
Fossils Two species of
Clethra are known from the Miocene of Europe. In addition several
fossilized capsules from Maastrichtian sediments in Europe have been assigned
to Clethraceae, although
their systematic affiliation may be questioned, such as Disoclethra
with three species, Purdiaeopsis campanulatus and
Valvaecarpus with four species. A fossil flower, Glandulocalyx
upatoiensis, from the Santonian of Georgia has been attributed to Actinidiaceae or Clethraceae by Schönenberger &
al. (2012).
Habit Bisexual (rarely
functionally gynodioecious), evergreen or deciduous trees or shrubs.
Vegetative anatomy Arbuscular
mycorrhiza present. Phellogen ab initio pericyclic. Stem in Clethra
with prominent endodermis and heterogeneous medulla. Vessel elements with
scalariform perforation plates (often with numerous cross-bars); lateral pits
opposite or alternate, bordered pits. Vestured pits sometimes present.
Imperforate tracheary xylem elements tracheids or fibre tracheids with bordered
pits, non-septate. Wood rays uniseriate or multiseriate, heterocellular. Axial
parenchyma apotracheal diffuse or diffuse-in-aggregates, or paratracheal
scanty. Sieve tube plastids S type. Nodes 1:1, unilacunar with one leaf trace.
Raphide cells absent.
Trichomes Hairs multicellular,
uniseriate or tufted and/or stellate.
Leaves Alternate (spiral),
simple, entire, sometimes coriaceous, with conduplicate-subplicate ptyxis.
Stipules and leaf sheath absent. Petiole vascular bundle transection arcuate or
annular; petiole with medullary bundle. Venation pinnate, eucamptodromous,
brochidodromous or semicraspedodromous (in Purdiaea acrodromous to
palinactinodromous). Stomata anomocytic, anisocytic, paracytic or actinocytic.
Cuticular wax crystalloids? Domatia as hair tufts present in some species.
Petiole and phloem with secretory cells. Calciumoxalate druses abundant. Leaf
margins usually serrate or glandular serrate (sometimes entire).
Inflorescence Terminal or
axillary, simple racemes, or paniculate, fasciculate or umbel-like cymose.
Bracts often caducous (in Purdiaea large). Floral prophylls
(bracteoles) absent.
Flowers Actinomorphic or
slightly zygomorphic, small. Pedicel articulated. Hypogyny. Sepals five (or
six), with imbricate quincuncial aestivation, persistent, free or partially
connate, sometimes unequal in length. Petals five (or six), with imbricate
quincuncial aestivation, caducous, usually free (rarely connate below). Ovary
base often nectariferous. Disc absent.
Androecium Stamens usually 5+5
(rarely 6+6; in Purdiaea with outer stamens alternipetalous),
diplostemonous (often secondarily obdiplostemonous). Filaments free from each
other, usually free from tepals (sometimes adnate to petal bases). Anthers
bilobate-sagittate to caudate (seemingly apically prolonged), ventrifixed
(dorsifixed?), versatile, late inverted (finally resupinated),
tetrasporangiate, at anthesis inverted from extrorse to introrse, poricidal
(dehiscing by seemingly apical pores or short slits). Tapetum secretory, with
binucleate cells. Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually tricolporate, shed as
monads, bicellular at dispersal. Exine tectate, with granular infratectum,
rugulate or psilate.
Gynoecium Pistil composed of
three (to five) connate carpels. Ovary superior, usually trilocular (rarely
quadri- or quinquelocular). Style single, simple. Stigma simple or usually
trilobate (rarely quadri- or quinquelobate), papillate, Dry type. Pistillodium
absent.
Ovules Placentation usually
axile (in Purdiaea apical). Ovules few to numerous (in
Purdiaea one) per carpel, usually anatropous (in Purdiaea
orthotropous, pendulous), unitegmic, tenuinucellar. Integument ? cell layers
thick (in Purdiaea gradually degenerating). Megagametophyte
monosporous, Polygonum type. Endosperm development cellular. Endosperm
haustoria chalazal and micropylar. Embryogenesis asterad.
Fruit Usually a loculicidal
capsule, sometimes with persistent calyx (in Purdiaea a nut).
Seeds Aril absent. Seed coat
often winged. Testa consisting of thin exotesta, with theoid exotestal
thickenings (testa in Purdiaea indistinct, disappearing). Perisperm
not developed. Endosperm copious, fleshy, oily and with hemicellulose. Embryo
short, straight, well differentiated, chlorophyll? Cotyledons two, short.
Germination phanerocotylar.
Cytology n = 8 – Polyploidy
occurring.
DNA Mitochondrial intron
coxII.i3 lost.
Phytochemistry Flavonols
(quercetin), cyanidin, triterpenes, ellagic and gallic acids, and
proanthocyanidins (prodelphinidin) present. Iridoids? Alkaloids and cyanogenic
compounds not found. Carbohydrates stored as oligosaccharides with kestose or
isokestose bonds.
Use Ornamental plants, timber,
carpentries.
Systematics Clethra
(80–85; China, the Korean Peninsula, Japan, Taiwan, Southeast Asia, Malesia
to New Guinea, eastern and southeastern United States [C. alnifolia],
Mexico, Central America, the West Indies, tropical South America, one species,
C. arborea, on Madeira), Purdiaea (12; southeastern United
States, Central America, Cuba, Venezuela, Ecuador, Peru, with their highest
diversity on Cuba).
Clethraceae are sister-group to
[Cyrillaceae+Ericaceae].
Species delimitation in Clethra
is difficult. Clethra arborea (Madeira) may be sister to C.
alnifolia of eastern North America.
CYRILLACEAE (Torr. et
A. Gray) Lindl.
|
( Back to Ericales )
|
Lindley, Veg. Kingd.: 445. 14-28 Mar 1846, nom.
cons.
Cyrillales Doweld,
Tent. Syst. Plant. Vasc.: xliv. 23 Dec 2001
Genera/species 2/2
Distribution Coastal plains in
southeastern United States, the West Indies, Central America, northern South
America.
Fossils Fossil wood and pollen
grains assigned to Cyrilla are found in Oligocene layers in North
America. Cenozoic brown coal in western Europe contains fossil wood similar to
that in Cyrilla. The fossil fruits of Epacridicarpum from the
Late Cretaceous and the Cenozoic of Europe have also been assigned to Cyrillaceae, but this systematic
placement is doubtful.
Habit Bisexual, evergreen or
deciduous shrubs or small trees.
Vegetative anatomy Phellogen
ab initio pericyclic. Vessel elements with scalariform perforation plates;
lateral pits scalariform or opposite, bordered pits. Imperforate tracheary
xylem elements tracheids or fibre tracheids with bordered pits, non-septate.
Wood rays uniseriate or multiseriate, heterocellular. Axial parenchyma
apotracheal diffuse. Sieve tube plastids Pcf type (with protein crystals and
fibrils, but no starch). Nodes 1:1, unilacunar with one leaf trace. Raphid
cells absent. Wood ray cells sometimes with prismatic calciumoxalate
crystals.
Trichomes Hairs absent.
Leaves Alternate (spiral),
simple, entire, sometimes coriaceous, with supervolute ptyxis. Stipules and
leaf sheath absent; lateral or sublateral red or blackish ligulate? glandular
structures – colleters – at buds and bracts may be reduced stipules.
Petiole vascular bundle transection annular, complex or deeply concave.
Venation pinnate. Stomata anomocytic. Cuticular wax crystalloids? Epidermis
with or without mucilage cells. Mesophyll with calciumoxalate as druses and
solitary prismatic crystals. Leaf margin entire.
Inflorescence Terminal or
axillary, racemes. Floral prophylls (bracteoles) absent?
Flowers Actinomorphic
(occasionally slightly zygomorphic). Hypogyny. Sepals five (to seven), with
open (or imbricate quincuncial) aestivation, unequal in size, persistent,
connate at base. Petals five (to seven), with imbricate quincuncial
aestivation, free or connate at base, in Cyrilla nectariferous. Disc
intrastaminal (in Cliftonia with nectaries?).
Androecium Stamens five (to
seven), haplostemonous, antesepalous (inner whorl absent; Cyrilla), or
(5+5) 6+6 or 7+7, diplostemonous (Cliftonia). Filaments free from each
other and from tepals. Anthers dorsifixed, not inverted at anthesis, versatile,
tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits).
Tapetum secretory, with binucleate cells. Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains 3(–6)-colporate, shed as
monads, bicellular at dispersal. Exine tectate, with granular infratectum,
microperforate, smooth.
Gynoecium Pistil composed of
(two to) five connate carpels. Ovary superior, (bilocular to) quinquelocular.
Basal part of ovary wall nectariferous? Style single, simple, very short, or
absent. Stigma punctate or (bilobate to) quinquelobate, non-papillate, Dry
type. Pistillodium absent.
Ovules Placentation axile or
apical. Ovules one to three per carpel, anatropous, pendulous, usually
apotropous, unitegmic, tenuinucellar. Integument four to seven cell layers
thick, sometimes vascularized, gradually degenerating. Megagametophyte
monosporous, Polygonum type (in at least Cliftonia sometimes
also aposporous Hieracium type). Antipodal cells persistent. Endosperm
development cellular. Endosperm haustoria chalazal and micropylar.
Embryogenesis?
Fruit A one- to four-seeded
dry drupe or a one-seeded two- to five-winged samara, often with persistent and
accrescent calyx. Pericarp with tannins and druses.
Seeds Aril absent. Testa
indistinct, early disappearing, with theoid exotestal thickenings? Perisperm
not developed. Endosperm moderately developed, fleshy. Embryo large, straight,
well differentiated, chlorophyll? Cotyledons two, small. Germination
phanerocotylar?
Cytology n = 20
DNA
Phytochemistry Insufficiently
known. Flavonols (kaempferol, quercetin, myricetin), cyanidin, and ellagic acid
(Cyrilla) present. Alkaloids and cyanogenic compounds not found.
Condensed and hydrolyzable tannins? Iridoids?
Use Ornamental plants.
Systematics Cyrilla
(1; C. racemiflora; southeastern United States to northern South
America), Cliftonia (1; C. monophylla; southeastern United
States).
Cyrillaceae are sister to Ericaceae.
DIAPENSIACEAE (Link)
Lindl.
|
( Back to Ericales )
|
Lindley, Intr. Nat. Syst. Bot., ed. 2: 233. 13
Jun 1836, nom. cons.
Galacinaceae D. Don
in Edinburgh New Philos. J. 6: 53. Oct-Dec 1828 [’Galacinae’];
Diapensiineae Link, Handbuch 1: 595. Jan-Aug 1829
[‘Diapensiaceae’];
Diapensiales Engl. et Gilg in Engler, Syllabus, ed.
9-10: 314. Nov-Dec 1924; Diapensianae Doweld, Tent.
Syst. Plant. Vasc.: xlv. 23 Dec 2001
Genera/species 5/12
Distribution Cold-temperate
and arctic-alpine regions on the Northern Hemisphere, the Himalayas, eastern
Tibet, western China, northern Burma, Japan, Taiwan, southeastern United
States.
Fossils Uncertain.
Actinocalyx bohrii was described from the Late Cretaceous of southern
Sweden. It had separate stylodia and the pollen grains were smaller than in
extant Diapensiaceae.
However, the systematic affiliation is questioned (Martínez-Millán 2010).
Habit Bisexual, evergreen
dwarf shrubs or perennial herbs.
Vegetative anatomy Mycorrhiza
ectotrophic and endotrophic. Phellogen ab initio usually pericyclic (sometimes
superficial). Pericyclic fibres usually absent (present in Shortia).
Vessel elements usually with simple (sometimes scalariform) perforation plates;
lateral pits? Imperforate tracheary xylem elements ? with bordered pits. Wood
rays absent. Axial parenchyma? Sieve tube plastids S type. Nodes usually 3:3,
trilacunar with three leaf traces (in Pyxidanthera 1:1, unilacunar
with one trace). Calciumoxalate crystals (single or compound) sometimes
present.
Trichomes Hairs absent
(always?); glandular hairs absent.
Leaves Alternate (spiral),
simple, entire, coriaceous, with ? ptyxis. Stipules and leaf sheath absent.
Petiole vascular bundle transection arcuate to annular; medullary bundles
sometimes present. Venation pinnate to palmate or leaves uninerved, in
Diapensia and Pyxidanthera inverted eucamptodromous (lateral
veins curved at base, narrowing without fusing with main vein or other lateral
veins), in Galax and Shortia actinodromous or camptodromous,
in Berneuxia brochidodromous; secondary veins subpinnate to palmate.
Stomata usually anomocytic, without subsidiary cells (rarely anisocytic).
Cuticular wax crystalloids? Calciumoxalate as groups of crystals or single
crystals (Galax). Leaf margin serrate or entire.
Inflorescence Terminal,
raceme-like cymose, or flowers solitary terminal (sometimes axillary).
Flowers Actinomorphic.
Hypogyny. Sepals five, with imbricate aestivation, persistent, free or connate
into tube. Petals five, usually with imbricate (rarely contorted) aestivation,
caducous, usually connate below (in Galax free or almost free),
sometimes dentate at apex. Basal part of ovary in some species
nectar-producing. Disc absent or strongly reduced.
Androecium Stamens five,
haplostemonous, antesepalous, alternipetalous. Filaments flattened, usually
free (in Galax connate at base), at least outer staminal whorl
epipetalous. Anthers usually basifixed (or transverse with horizontal thecae),
incurved (inverted during development, base becoming directed upwards),
non-versatile, in Galax and Pyxidanthera with basal
appendages, usually tetrasporangiate (in Galax disporangiate),
introrse, longicidal (dehiscing by longitudinal [Berneuxia,
Diapensia, Shortia] or transverse [Galax,
Pyxidanthera] slits). Tapetum secretory, with binucleate cells.
Staminodia absent in Diapensia and Pyxidanthera; in
Berneuxia, Galax and Shortia five epipetalous
antepetalous inner staminodia alternating with five fertile outer
(alternipetalous) stamens.
Pollen grains
Microsporogenesis simultaneous. Pollen grains tri- or hexacolp(oroid)ate, shed
as monads, tricellular (bicellular?) at dispersal. Exine semitectate, with
columellate infratectum, reticulate, or intectate.
Gynoecium Pistil composed of
three connate carpels; median carpel adaxial. Ovary superior, trilocular,
sometimes nectariferous. Style single, usually simple (in Diapensia
trilobate), hollow (with stylar canal). Stigma usually single, shortly trifid
(stigmas in Diapensia capitate), non-papillate, Wet type. Pistillodium
absent.
Ovules Placentation usually
axile (sometimes parietal). Ovules several to numerous per carpel, usually
anatropous (sometimes hemianatropous or campylotropous), unitegmic,
tenuinucellar. Integument five to seven cell layers thick. Megagametophyte
monosporous, Polygonum type (in at least Pyxidanthera with
large amount of starch). Synergids in Diapensia with a filiform
apparatus. Antipodal cells sometimes proliferating (in Shortia up to
c. 40 cells), persistent. Endosperm development cellular. Endosperm haustoria
absent. Embryogenesis solanad?
Fruit A loculicidal
capsule.
Seeds Aril absent? Exotesta
two or three cell layers thick, with thick inner cell walls, with theoid
exotestal thickenings. Endotesta? Perisperm not developed. Endosperm copious,
fleshy, containing lipids. Embryo relatively large, straight to somewhat
curved, well differentiated, chlorophyll? Cotyledons two. Germination?
Cytology n = 6 (tetraploidy
occurring in Galax urceolata)
DNA
Phytochemistry Flavonols
(kaempferol, quercetin, gossypetin), ellagic and gallic acids present. Ursolic
acid, proanthocyanidins, alkaloids, and cyanogenic compounds not found.
Iridoids? Aluminium accumulated.
Use Ornamental plants.
Systematics Galax (1;
G. urceolata; in and near the Appalachian Mountains in eastern United
States), Pyxidanthera (1; P. barbulata; coastal regions in
eastern United States), Diapensia
(4; D. lapponica: arctic-alpine Europe; D. himalaica: the
Himalayas, Burma, Yunnan, Xizang; D. purpurea: Yunnan, Sichuan; D.
wardii: southeastern Xizang), Berneuxia
(1; B. thibetica; eastern Himalayas, western China), Shortia
(5; S. exappendiculata: Taiwan; S. galacifolia: the
Appalachian Mountains in southeastern United States; S. sinensis:
China; S. soldanelloides, S. uniflora: to Japan,
Yakushima).
Diapensiaceae may be sister to
Styracaceae, although the
support is sometimes fairly weak.
Galax and Pyxidanthera are
successive sister-groups to the remaining Diapensiaceae.
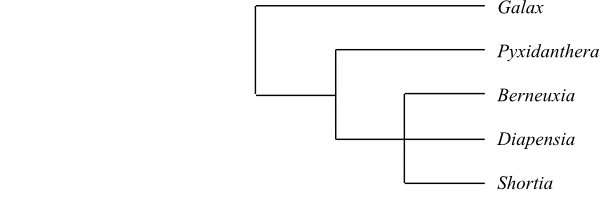 |
Cladogram of Diapensiaceae based on
DNA sequence data and morphology (Rönblom & Anderberg 2002).
|
Gürcke in Engler et Prantl, Nat. Pflanzenfam.,
IV, 1: 153. Dec 1891, nom. cons.
Guaiacanaceae Juss.,
Gen. Plant.: 155. 4 Aug 1789 [’Guiacanae’];
Diospyraceae Vest, Anleit. Stud. Bot.: 271, 294. 1818
[’Diospyroideae’]; Diospyropsida
Brongn., Enum. Plant. Mus. Paris: xxi, 72. 12 Aug 1843
[’Diospyroideae’]; Diospyrales Prantl,
Lehrb. Bot.: 216. 20 Apr 1874 [’Diospyrinae’];
Ebenales Engl., Syllabus: 155. Apr 1892;
Ebenineae Bessey in C. K. Adams, Johnson’s
Universal Cyclop. 8: 463. 15 Nov 1895 [‘Ebenales’];
Diospyrineae Engl., Syllabus, ed. 2: 170. Mai 1898;
Lissocarpaceae Gilg in Engler et Gilg, Syllabus, ed.
9-10: 324. 6 Nov 1924, nom. cons.
Genera/species 2/550–600
Distribution Temperate to
tropical regions on both hemispheres, with their highest diversity in
Madagascar and Malesia; some species in temperate parts of eastern North
America, India, eastern China, and Japan.
Fossils Leaves, flowers and
fruits attributed to Ebenaceae
are known from the Eocene onwards, also from Europe. Austrodiospyros
cryptostoma is represented by male flowers and leaves from the Eocene of
southeastern Australia. Diospyros pollen have been found in Eocene
strata of North America.
Habit Usually dioecious
(rarely monoecious, polygamomonoecious or bisexual), usually evergreen (rarely
deciduous) trees or shrubs. Bark, roots and heartwood often black or, in air,
blackening.
Vegetative anatomy Phellogen
ab initio usually superficial (sometimes pericyclic). Primary medullary rays
narrow. Vessel elements usually with simple (rarely scalariform) perforation
plates; lateral pits alternate or opposite, bordered pits. Imperforate
tracheary xylem elements fibre tracheids or libriform fibres with simple pits,
non-septate. Wood rays uniseriate or multiseriate. heterocellular. Axial
parenchyma apotracheal diffuse or diffuse-in-aggregates, or paratracheal scanty
vasicentric, reticulate, or banded. Wood elements often storied. Sieve tube
plastids S type. Nodes usually 1:1, unilacunar with one leaf trace (sometimes
1:3 or 3:3, unilacunar or trilacunar with three traces). Heartwood with
gum-like substances. Sclereids present. Prismatic calciumoxalate crystals
frequent.
Trichomes Hairs unicellular,
simple or furcate, often malpighiaceous, with branches of unequal lengths, or
multicellular, uniseriate or stellate (in Lissocarpa absent);
glandular hairs, sometimes peltate-lepidote.
Leaves Usually alternate
(spiral or distichous; rarely opposite or verticillate), simple, entire,
coriaceous, with conduplicate ptyxis. Stipules and leaf sheath absent. Petiole
vascular bundle transection arcuate. Abaxial side of lamina usually with
flattened glands, extrafloral nectaries. Venation pinnate. Stomata usually
anomocytic (sometimes cyclocytic or actinocytic). Cuticular wax crystalloids
absent (in Lissocarpa?). Mesophyll often with sclerenchymatous
idioblasts containing brachysclereids, osteosclereids or other types of
sclereids. Extrafloral nectaries often present on abaxial side. Secretory
cavities absent. Leaf margin usually entire.
Inflorescence Axillary
fasciculate, raceme-like or paniculate cymose (flowers sometimes solitary,
terminal).
Flowers Actinomorphic. Pedicel
articulated. Usually hypogyny (in Lissocarpa epigyny). Sepals three to
five (to eight), with valvate or imbricate aestivation, persistent, connate.
Petals three to five (to eight), with contorted aestivation, connate (corolla
in Lissocarpa usually with eight-lobed corona). Nectariferous disc
usually absent.
Androecium Stamens (three to)
twelve to 20 (to c. 100), as many as or twice or four (or more) times the
number of petals, usually unequal in length, in two (to four) whorls. Filaments
flattened, free or connate two, three or more together (sometimes all filaments
connate into a tube), usually adnate at base to corolla tube. Anthers
basifixed, non-versatile, tetrasporangiate, external anthers introrse,
mid-anthers latrorse and internal anthers extrorse, usually longicidal
(dehiscing by longitudinal slits; sometimes poricidal, with apical pores);
connective often slightly prolonged. Tapetum secretory, with multinucleate
cells. Female flowers usually with a single staminodial whorl.
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually tricolporate (rarely
tetracolporate; in Lissocarpa triporate), usually shed as monads
(rarely tetrads), bicellular at dispersal. Exine tectate, with granular
infratectum (Diospyros), rugulate, microrugulate, striate, gemmate or
granulate (in Lissocarpa fossulate to rugulate).
Gynoecium Pistil composed of
two to eight connate carpels. Ovary usually superior (in Lissocarpa
inferior), usually bilocular to 16-locular; each locule usually divided by
secondary septa (ovary rarely unilocular; ovary in Lissocarpa
quadrilocular, not divided by secondary septa). Stylodia two to eight, free or
partially connate (style in Lissocarpa single, simple, clavate).
Stigmas capitate or bilobate (stigma in Lissocarpa often slightly
quadrilobate), non-papillate, Dry type. Male flowers usually with
pistillodium.
Ovules Placentation usually
apical (in Lissocarpa axile). Ovules one or two per carpel,
anatropous, pendulous, apotropous, bitegmic, tenuinucellar. Micropyle bistomal
or endostomal. Outer integument three to seven cell layers thick. Inner
integument five to ten cell layers thick. Megagametophyte monosporous,
Polygonum type. Endosperm development cellular (nuclear?). Endosperm
haustoria? Embryogenesis probably chenopodiad.
Fruit Usually a berry (rarely
capsule-like) with persistent and usually accrescent calyx. Pericarp with
tanniniferous idioblasts.
Seeds Aril absent. Testa
vascularized, with circumperipheral vascular loop surrounding seed. Tegmen?
Perisperm not developed. Endosperm copious, hard, oily, with mannose-rich
polysaccharides, sometimes ruminate. Embryo straight or curved, well
differentiated (Lissocarpa), without chlorophyll. Cotyledons two,
foliaceous. Radicula long. Germination phanerocotylar or cryptocotylar.
Cytology n = 15, 30, 45 (or
more) – Polyploidy occurring.
DNA Mitochondrial
coxI intron present.
Phytochemistry Flavonols
(kaempferol, quercetin, myricetin), cyanidin, C-30-oxidized triterpenes,
oleanolic acid derivatives, ellagic and gallic acids, methylated ellagic acids,
condensed tannins, proanthocyanidins (prodelphinidins), saponins,
naphthoquinone derivatives of 7-methyljugone and plumbagin (droserone; bark and
wood blackening when substances oxidized, cf. ebony), anthraquinones, and
benzopyrones present. Alkaloids and cyanogenic compounds rare. Aluminium
accumulated in some species.
Use Ornamental plants, fruits,
timber, medicinal plants, fish poison.
Systematics Ebenaceae may be sister-group to the
clade [Maesa+[Samolus+Theophrastaceae]+ [Primulaceae+Myrsinaceae]] (Schönenberger &
al. 2005).
Ebenaceae and Sapotaceae have similar
malpighiaceous hairs (T-shaped with branches of unequal lengths).
Lissocarpa is sister to the remaining
Ebenaceae.
Lissocarpoideae (Gilg)
B. Walln. in Ann. Naturhist. Mus. Wien, Ser. B, 105: 523. 22 Apr 2004
1/8. Lissocarpa (8;
northwestern South America). – Vessel elements sometimes with scalariform
perforation plates. Hairs absent. Petiole sometimes with wing bundles and
recurved edges. Stomata anomocytic or cyclocytic. Inflorescence subfasciculate,
or flowers solitary axillary. Floral prophylls (bracteoles) large, apical.
Epigyny. Sepals four (or five). Petals four (or five). Corolla with an
eight-lobed corona. Stamens eight. Filaments connate. Connective prolonged.
Pollen grains triporate. Ovary in Lissocarpa quadrilocular, not
divided by secondary septa. Exine psilate. Pistil composed of four connate
carpels. Ovary quadrilocular, without secondary septa, inferior. Style single,
simple. Stigma clavate, often slightly quadrilobate, hairy at apex.
Placentation axile. Seeds one or two. Endosperm bony. Embryo well
differentiated. Cotyledons foliaceous. n = ? Iridoids, naphthoquinones etc.?
Ebenoideae (Juss.)
Thorne et Reveal in Bot. Rev. (Lancaster) 73: 121. 29 Jun 2007
1/550–600. Diospyros
(550–600; temperate to tropical regions on both hemispheres, with their
highest diversity in Madagascar and Malesia). – Phellogen usually superficial
(sometimes pericyclic). Cambium storied. Nodes sometimes 1:3, unilacunar with
three leaf traces. Silica bodies sometimes (‘Euclea’,
‘Royena’) present. Secretory cells abundant. Hairs usually
unicellular, sometimes T-shaped. Leaves usually distichous (sometimes spiral;
rarely opposite), with conduplicate ptyxis. Stomata usually paracytic.
Cuticular wax crystalloids absent. Inflorescence cymose, with short axis.
Floral buds acute, with depressed brown hairs. Sepals three to seven. Petals
three to seven, sometimes with valvate aestivation. Nectary absent. Stamens
(three to) twelve to 20 (to c. 100). Anthers extrorse, often hairy. Female
flowers usually with staminodia. Pollen grains usually tricolporate. Pistil
composed of two to eight connate antesepalous or antepetalous carpels. Ovary
locules usually divided by secondary septa. Stylodia two to eight, free or
partially connate. Stigmas capitate or lobate, little expanded, Dry type. Male
flowers with pistillodium. Placentation apical. Ovule often one per carpel,
bitegmic. Micropyle bistomal or endostomal. Endothelium present. Fruit often
with accrescent calyx. Seeds pachychalazal, often ruminate. Testa
multiplicative, usually vascularized, often parenchymatous. Exotesta often
fibriform or mucilaginous; exotestal cells cuboid to palisade. Endotesta often
crystalliferous; endotestal cell walls often thickened. Endosperm hard, with
thick cell walls. Radicula in the ‘Euclea’ and
‘Royena’ clades surrounded by ingrowth of seed coat. x = 15.
Flavonols (myricetin etc.), C-30-oxidized triterpenes, proanthocyanidins
(prodelphinidins), saponins, and naphthoquinone derivatives of 7-methyljugone
and plumbagin present. Ellagic acid not found. – Euclea (Africa) and
Royena (Africa) have sometimes been identified as the sister-group to
Diospyros
sensu stricto.
de Jussieu, Gen. Plant.: 159. 4 Aug 1789
[’Ericae’], nom. cons.
Kalmiaceae Durande,
Notions Elém. Bot.: 271. 1782 [’Kalmiae’], nom. illeg.;
Rhododendraceae Juss., Gen. Plant.: 158. 4 Aug 1789
[’Rhododendra’]; Rhodoraceae Vent.,
Tabl. Règne Vég. 2: 449. 5 Mai 1799; Ledaceae J. F.
Gmel., Allg. Gesch. Pflanzengifte, ed. 2: 404. Jun 1803 [’Leda’];
Epacridaceae R. Br., Prodr. Fl. Nov.-Holl.: 535. 27
Mar 1810 [’Epacrideae’], nom. cons.;
Azaleaceae Vest, Anleit. Stud. Bot.: 272, 294. 1818
[’Azaleoideae’]; Monotropaceae Nutt.,
Gen. N. Amer. Pl. 1: 272. 14 Jul 1818 [’Monotropeae’], nom. cons.;
Vacciniaceae DC. ex Perleb, Vers. Artzneikr. Pfl.:
228. Mai 1818 [’Vaccinieae’], nom.cons.;
Empetrales Bercht. et J. Presl, Přir. Rostlin: 251.
Jan-Apr 1820 [‘Empetreae’]; Epacridales
R. Br. ex Bercht. et J. Presl, Přir. Rostlin: 251. Jan-Apr 1820
[‘Epacrideae’]; Monotropales Bercht. et
J. Presl, Přir. Rostlin: 252. Jan-Apr 1820 [‘Monotropeae’];
Empetraceae Hook. et Lindl. in W. J. Hooker, Fl.
Scot.: 297. 10 Mai 1821 [’Empetreae’], nom. cons.;
Pyrolaceae Lindl., Syn. Brit. Fl.: 175. 16 Mar 1829
[’Pyroleae’], nom. cons.; Empetrineae
Link, Handbuch 1: 617. 4-11 Jul 1829 [‘Empetreae‘];
Epacridineae Link, Handbuch 1: 601. 4-11 Jul 1829
[‘Epacrideae‘]; Ericineae Link, Handbuch
1: 601. 4-11 Jul 1829 [‘Ericeae genuinae‘];
Vacciniales Dumort., Anal. Fam. Plant.: 28. 1829
[‘Vaccinarieae’]; Stypheliaceae Horan.,
Prim. Lin. Syst. Nat.: 72. 2 Nov 1834 [’Stypheliaceae
(Epacrideae)’]; Arbutaceae (Meisn.) Miers
in Bromhead in Mag. Nat. Hist., n.s., 4: 337, 338. Jul 1840
[’Arbuteae’]; Andromedaceae Döll,
Rhein. Fl.: 428. 24-27 Mai 1843 [’Andromedeae’];
Arbutineae J. Presl in Nowočeská Bibl. [Wšobecný
Rostl.]: 987. 1846; Pyrolineae J. Presl in
Nowočeská Bibl. [Wšobecný Rostl.]: 987, 989. 1846
[‘Pyroleae‘]; Rhododendrineae J. Presl
in Nowočeská Bibl. [Wšobecný Rostl.]: 987, 996. 1846
[‘Rhododendreae‘]; Rhodorales Horan.,
Char. Ess. Fam.: 105. 30 Jun 1847 [‘Rhodorastra’];
Hypopityaceae Klotzsch in Linnaea 24: 11. Mai 1851
[’Hypopithieae’]; Menziesiaceae Klotzsch
in Linnaea 24: 11. Mai 1851; Siphonandraceae Klotzsch
in Linnaea 24: 11. Mai 1851, nom. illeg.;
Arctostaphylaceae J. Agardh, Theoria Syst. Plant.:
106. Apr-Sep 1858 [’Arctostaphyleae’];
Salaxidaceae J. Agardh, Theoria Syst. Plant.: 104.
Apr-Sep 1858 [’Salaxideae’];
Diplarchaceae Klotzsch in Monatsber. Königl. Preuss.
Akad. Wiss. Berlin 1859: 15. 1857 [’Diplarchaceen’], nom. illeg.;
Oxycoccaceae A. Kern., Pflanzenleben 2: 713, 714. 8
Aug 1891; Prionotaceae Hutch., Evol. Phylog. Fl. Pl.:
306. 28 Aug 1969
Genera/species c
117/3.900–3.950
Distribution Cosmopolitan,
although few species in tropical lowland regions, with their largest diversity
in the Himalayas to southwestern China, New Guinea, South Africa, Australia,
and New Zealand.
Fossils Seeds and fruits from
several genera with only extra-European recent distribution in the Northern
Hemisphere, e.g. Eubotrys, Leucothoe, Lyonia and
Zenobia, have been found in Late Cretaceous and Early Palaeogene
strata in Europe. Diplycosiopsis walbeckensis and Viticocarpum
minimum from the Maastrichtian of Germany is represented by drupes with
five many-seeded and one-seeded locules, respectively. It is, however,
uncertain whether they emanate from species of Ericaceae. Fossil leaves and pollen
grains from supposed Styphelioideae are known from (possibly the Late
Cretaceous to) the Early Palaeogene onwards in, above all, Australia.
Habit Usually bisexual (rarely
monoecious or dioecious), usually evergreen (sometimes deciduous) shrubs or
suffrutices (rarely trees, lianas or perennial herbs). Some genera consist of
achlorophyllous and mycotrophic plants. Numerous species are xerophytic. Some
species are helophytes. A starchy lignotuber is present especially in many
species in mediterranean climates.
Vegetative anatomy
Ectomycorrhiza usually with Basidiomycotina (i.a.
Sebacinales) or often with certain Ascomycotina (particularly
Pezizales, e.g. Hymenoscyphus) as intracellular hyphal
complexes in root epidermis (monotropoid, ericoid or arbutoid mycorrhiza);
ericoid mycorrhiza penetrates only epidermal and outer cortical cells of hair
roots (consisting of endodermis, exodermis, tracheids, sieve tubes and
companion cell) covered with hyphae (Hartig net and fungal mantle not formed);
arbutoid mycorrhiza penetrates epidermal and outer cortical cells of hair
roots, these being covered by Hartig net with hyphae following cell walls or
forming complete envelope (Hartig net and fungal mantle formed); in monotropoid
mycorrhiza root covered in the same way, although hyphae form peg-shaped
invaginations into exodermal hair root cells (Hartig net and fungal mantle
formed); arbuscular mycorrhiza present in Enkianthus; standard
ectomycorrhiza not known in Ericaceae. Endophytic fungi
frequently present. Phellogen ab initio usually pericyclic (sometimes
superficial; absent in Monotropoideae). Pericyclic fibres little
developed. Secondary lateral growth usually normal (absent in most
Monotropoideae). Vessel elements usually with simple or scalariform
(rarely reticulate) perforation plates; lateral pits scalariform, opposite or
alternate (vessels absent in most Monotropoideae), simple and/or
bordered pits. Vestured pits sometimes present. Imperforate tracheary xylem
elements tracheids or fibre tracheids or libriform fibres with simple and/or
bordered pits, usually non-septate (also vasicentric tracheids). Wood rays
uniseriate or multiseriate, usually heterocellular. Axial parenchyma
apotracheal diffuse (rarely diffuse-in-aggregates), or paratracheal scanty
vasicentric or unilateral, or absent. Sieve tube plastids usually S type
(sometimes S0 type). Endodermis rarely with suberised cell walls.
Nodes usually 1:1, unilacunar with one leaf trace (rarely ≥3:≥3, trilacunar
or multilacunar with three or more traces). Cortex with sclereids. Raphid cells
absent. Calciumoxalate druses or single prismatic crystals often frequent.
Trichomes Hairs unicellular or
multicellular, uniseriate or branched, dendritic, stellate, lepidote,
bristle-like, sometimes candelabra-, funnel- or cup-shaped; glandular hairs
present (sometimes peltate-lepidote), often thick and colleter-like.
Leaves Alternate (sometimes
distichous or tetrastichous), opposite or verticillate, simple, entire, usually
coriaceous, often ericoid (in Monotropoideae usually scale-like), with
convolute, involute or revolute ptyxis. Stipules and leaf sheath absent.
Colleter-like thick glandular hairs often present on adaxial side of petiole
base. Petiole vascular bundle transection usually arcuate (sometimes annular).
Extrafloral nectaries sometimes present on petiole or base of lamina. Venation
usually pinnate (rarely palmate or parallelodromous) or leaves one-veined.
Stomata usually anomocytic or paracytic (rarely parallelocytic, cyclocytic or
stephanocytic). Cuticular wax crystalloids usually amorphous or as platelets
(sometimes as tubuli, dominated by β-diketones, or as coiled or triangular
rodlets). Epidermis with or without mucilage cells. Secretory oil cavities
present or absent. Leaf margin serrate, crenate or entire; teeth united via
multicellular hairs.
Inflorescence Terminal or
axillary, racemes, spikes, panicles or fascicles (sometimes umbels, rarely
heads or flowers solitary).
Flowers Actinomorphic or
zygomorphic. Hypogyny to epigyny. Sepals (two to) four or five (to seven), with
imbricate quincuncial aestivation, persistent, usually connate at base. Petals
(three or) four or five (to seven), with valvate or imbricate (rarely
contorted) aestivation, persistent or caducous, usually connate into
campanulate, tubular or urceolate corolla (rarely free; in Richea
connate into calyptra). Nectary or nectariferous disc (annular or of separate
parts, intrastaminal) usually at base (sometimes at apex) of ovary (sometimes
absent).
Androecium Stamens usually
four or five (rarely two or three), antesepalous, alternipetalous, or 4+4,
5+5(–16), usually obdiplostemonous. Filament usually free from each other and
from tepals (in, e.g., Styphelioideae often epipetalous). Anthers
basifixed or dorsifixed, late inverted, finally resupinate (in
Enkianthoideae, Monotropoideae and Arbutoideae
sometimes not inverted), often versatile, usually free (rarely connate), often
with two (rarely four) small usually apical horn-shaped outgrowths (sometimes
dorsal spurs; in Vaccinioideae sometimes tubular outgrowths), usually
tetrasporangiate (in Styphelioideae disporangiate), subintrorse and
poricidal (dehiscing by apical pores), or introrse (rarely latrorse or
extrorse) and longicidal (dehiscing by longitudinal slits; in
Styphelioideae usually with a single slit). Tapetum secretory, with
uni-, bi- or multinucleate cells. Staminodia absent. Secondary pollen display
present in Styphelioideae.
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually 3(–5)-colporate
(sometimes 3(–5)-colpate, 3(–5)-colporoidate or porate), usually shed as
tetrahedral tetrads (sometimes as monads, rarely as dyads, triads or polyads;
in some Styphelioideae three pollen cells of tetrad degenerate),
usually bicellular (sometimes tricellular) at dispersal. Exine tectate, with
granular infratectum, scabrate, verrucate, rugulate, microrugulate or psilate.
Viscinous threads present on pollen surface in many groups.
Gynoecium Pistil composed of
(one to) four or five (to twelve) connate, usually antepetalous (in, e.g.,
Monotropa, Dracophyllum and Vaccinium antesepalous)
carpels. Ovary superior to inferior, (unilocular to) quadri- or quinquelocular,
nectariferous at base. Style single, simple, usually hollow (with stylar canal;
rarely widened at apex). Stigma capitate or punctate to somewhat lobate or
peltate, usually papillate, Dry or Wet type. Male flowers rarely with
pistillodium.
Ovules Placentation usually
axile to intrusively parietal (parietal placentation present in, e.g.,
Monotropoideae; rarely apical or basal). Ovules usually numerous
(sometimes several, rarely single) per carpel, anatropous or hemianatropous to
(almost) campylotropous, pendulous to ascending, apotropous to epitropous,
unitegmic, tenuinucellar. Integument two to five cell layers thick.
Megagametophyte usually monosporous, Polygonum type (in
Arbutoideae sometimes disporous, Allium type). Synergids
sometimes with a filiform apparatus. Antipodal cells often persistent,
sometimes proliferating. Endosperm development cellular or nuclear. Endosperm
haustoria chalazal and/or micropylar (rarely absent). Embryogenesis usually
solanad (in Pyroleae caryophyllad).
Fruit A loculicidal or
septicidal (rarely septifragal) capsule, a berry, or a drupe (with one or more
pyrenes; rarely a nut surrounded by fleshy perianth), with persistent calyx.
Seeds Aril absent. Seed coat
winged or unwinged. Testa usually one-layered, consisting of exotesta, with
inner and radial but no outer cell walls thickened, with theoid exotestal
thickenings (sometimes mucilaginous). Perisperm not developed. Endosperm
copious, fleshy, often oily. Embryo straight, usually well differentiated (in
Monotropoideae usually undifferentiated; in Monotropa
uniflora two-celled, in M. hypopitys nine-celled, in
Allotropa and Pleuricospora two- or three-celled, in
Monotropsis four-celled, in Sarcodes several-celled with a
globular structure, in Chimaphila undifferentiated), with or without
chlorophyll. Cotyledons two, short. Germination phanerocotylar.
Cytology n = 11
(Enkianthoideae); n = 8, 13, 19, 23 etc. (Monotropoideae); n
= 13 (Arbutoideae); n = 13 (Cassiopoideae); n = 12, 13 (26)
(Ericoideae); n = 16 (Harrimanelloideae); n = 12 (17, 18, 24)
(Vaccinioideae); n = 4, 6–13 (Styphelioideae) –
Polyploidy occurring (12x in Rhododendron manipurense). Protein
crystals present in nucleus in some species.
DNA Plastid gene infA
lost/defunct (Andromeda, Chamaedaphne, Pyrola).
Mitochondrial intron coxII.i3 lost. Mitochondrial coxI intron
present in Pyrola.
Phytochemistry Flavonols
(kaempferol, quercetin, myricetin, gossypetin), cyanidin, catechins, Route I
secoiridoids (also decarboxylated iridoids?), Group I carbocyclic iridoids
(aucubin, daphylloside, monotropein), Group IV carbocyclic iridoids (unedoside,
a C8 iridoid glycoside, in Arbutus and Arctostaphylos),
diterpenes (e.g. andromedotoxin in Arbutoideae and
Ericoideae), triterpene acids (ursolic acid), ellagic acid (rare),
gallic acid, ellagitannins (geraniin), proanthocyanidins (prodelphinidins),
alkaloids, cyanogenic compounds, chlorogenic acid, 3-galactoside, benzo- and
naphthoquinones, myo-inisitol, and ethereal oils present. Arbutin
present in Arbutoideae, Monotropoideae and
Vaccinioideae. Monotropeoside present in Monotropoideae.
Saponins not found. Polysaccharides stored as oligosaccharides with kestose and
isokestose bonds.
Use Ornamental plants, fruits
(of Vaccinium, Oxycoccus, Myrtillus,
Gaylussacia, Arctostaphylos, etc.), honey (from, e.g.,
Calluna vulgaris), timber, carpentries, smoking-pipes (from Erica
arborea and E. scoparia), medicinal plants.
Systematics Ericaceae are sister to Cyrillaceae.
A probable topology is the following:
[Enkianthoideae+[Monotropoideae+[Arbutoideae+[[Cassiopoideae+Ericoideae]+[Harrimanelloideae+[Styphelioideae+Vaccinioideae]]]]]]
(Kron & al. 2002).
According to the ICBN, the name Ericaceae Juss. should be preferred
before Vacciniaceae Adans.
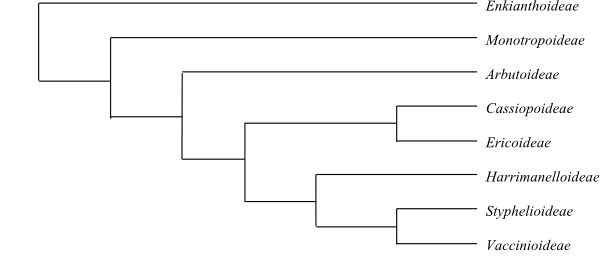 |
Cladogram (simplified) of Ericaceae based on morphology
and DNA sequence data (Kron & al. 2002). Monotropoideae
are sister-group to Arbutoideae and Pyroloideae are
sister to the remaining Ericaceae except
Enkianthoideae, Monotropoideae and
Arbutoideae (Rose & al. 2018).
|
Enkianthoideae (P. F.
Stevens) Kron, Judd & Anderb. in K. A. Kron et al., Bot. Rev. (Lancaster)
68: 401. 23 Oct 2002
1/17. Enkianthus
(15–17; Japan, southern China, northern Burma, Indochina). – Mycorrhiza
arbuscular, endomycorrhizal, Paris type; hair roots absent. Medulla
heterocellular, with small thick-walled lignified larger and thin-walled cells
mixed. Vestured pits present. Hairs with distal end dying off long before
proximal end. Leaves pseudoverticillate. Buds perulate (axillary branches
developing exclusively in axils of perulae; also present in some
Rhodoreae). Anthers with paired bristles and fibrous endothecium.
Pollen grains shed as monads, tricellular at dispersal. Tectum with granulate
surface. Ventral carpel bundles present in septal plane. Megagametophyte
inserted on megasporangial pedestal. Megagametophyte with ear-like appendages.
Seed raphe with vascular bundles. n = 11. – Enkianthus
has the most well preserved remnants of pit membranes in Ericaceae.
[Monotropoideae+[Arbutoideae+[[Cassiopoideae+Ericoideae]+[Harrimanelloideae+[Styphelioideae+Vaccinioideae]]]]]
Mycorrhiza types of ectendomycorrhiza.
Hair roots present, consisting of endodermis, exodermis, tracheid, sieve tube
and companion cell. Fungal hyphae with complex coiled intrusions into hair root
exodermal cells (only pegs present in Monotropeae and
Pterosporeae). Anthers poricidal, with exothecium. Pollen grains shed
in tetrahedral tetrads. Seed without chalazal vascular bundle. Insertions
present in plastid gene matK.
Monotropoideae Arn.,
Botany: 118. 9 Mar 1832 [‘Monotropeae’]
14/c 50.
Pyroleae Dumort., Anal. Fam. Plant.: 47. 1829. Pyrola (c
35; temperate regions on the Northern Hemisphere to mountains on Taiwan and
northern Sumatra, temperate South America), Orthilia
(1; O. secunda; temperate and arctic-alpine regions on the Northern
Hemisphere), Chimaphila
(5; C. japonica, C. maculata, C. menziesii, C.
monticola, C. umbellata; Europe, temperate Asia, North and
tropical America), Moneses
(1; M. uniflora; temperate regions on the Northern Hemisphere). –
Monotropeae Dumort., Anal. Fam. Plant.: 47. 1829.
Allotropa (1; A. virgata; western United States),
Cheilotheca (3; C. khasiana, C. malayana, C.
sleumerana; Assam to West Malesia), Hemitomes (1; H.
congestum; western United States), Monotropa
(2; M. hypopitys, M. uniflora; temperate regions on the
Northern Hemisphere, mountains in Central America), Monotropastrum (2;
M. humile, M. sciaphilum; East Asia, Sumatra),
Monotropsis (1; M. odorata; southeastern United States),
Pityopus (1; P. californica; western United States),
Pleuricospora (1; P. fimbriolata; southwestern Canada,
western United States). – Pterosporeae Baill.,
Hist. Plant. 11: 161, 206. Jun-Jul 1891. Pterospora (1; P.
andromeda; southern Canada, western and northeastern United States,
northern Mexico), Sarcodes (1; S. sanguinea; western United
States, Mexico). – Temperate and arctic-alpine regions on the Northern
Hemisphere, mountains in Central America and mountains on Taiwan and northern
Sumatra. Many species achlorophyllous and hyperparasitic mycoheterotrophs.
Herbs with ericoid or arbutoid mycorrhiza (Pyroleae) or monotropoid
mycorrhiza (with fungal hyphae forming peg-shaped invaginations into exodermal
hair root cells; Monotropeae, Pterosporeae). Association
between achlorophyllous Monotropoideae and mycorrhiza-forming
basidiomycetes often specific. Hartig net frequently present. Sieve tube
plastids at least in some achlorophyllous species S0 type.
Multicellular hairs usually absent (present in Pterosporeae). Leaves
in Monotropeae and Pterosporeae scale-like, in
Pyroleae foliaceous. Inflorescence racemose. Floral prophylls
(bracteoles) usually absent (present in Pterospora and
Sarcodes). Sepals and petals three to eight, often free (sometimes
absent). Anthers with various types of dehiscence, usually longicidal
(dehiscing by slits). Tapetum cells binucleate. Pollen grains usually shed as
monads (in Pyroleae as tetrahedral tetrads). Placentation axile to
intrusively parietal. Seed coat often winged. Testa with thickened or
unthickened cell walls. Embryo reduced, small to minute and undifferentiated
(Monotropa uniflora has bicellular embryo). n = 8, 11, 13, 16, 19, 23
(26). Cell nuclei with protein crystals. Polysaccharides stored as kestose and
isokestose oligosaccharides. – Pyroleae (Pyroloideae
Beilschm. in Flora 16(Beibl. 7): 72, 109. 14 Jun 1833) are sister-group to
[Monotropeae+Pterosporeae]. Pyroleae are herbaceous,
rhizomatous and have photosynthesizing foliaceous leaves. The mycorrhiza is
ericoid or arbutoid. The anthers have very short or no tubules and no
outgrowths. The pollen grains are usually shed as tetrahedral tetrads
(sometimes as monads). Nectary is usually absent. The placentation is
intrusively parietal. The testal walls are thin. A possible although unstable
topology is [[Orthilia+Pyrola]+[Moneses+Chimaphila]]
(Freudenstein & al. 1999; Liu & al. 2011). An alternative topology was
recovered by Freudenstein & al. (2016), where Pyroleae
(Pyroloideae) were sister-group to either
[Arbutoideae+[Monotropeae+Pterosporeae]] or to core
Ericaceae (with
Arbutoideae sister to [Monotropeae+Pterosporeae]).
The latter hypothesis was supported by Rose & al. (2018).
[Arbutoideae+[[Cassiopoideae+Ericoideae]+[Harrimanelloideae+[Styphelioideae+Vaccinioideae]]]]
Petiole vascular bundle transection
annular. Floral prophylls (bracteoles) present. Anthers with poricidal
dehiscence. Endothecium absent. Pollen grains shed as tetrahedral tetrads.
Arbutoideae (Benth.)
Nied. in Engl. Bot. Jahrb. Syst. 11: 135. 18 Jun 1889
3–5/c 85. Arbutus
(12; western Europe, the Canary Islands, the Mediterranean, southwestern
Canada, western United States, northwestern Mexico; possibly paraphyletic and
incl. Arctostaphylos,
Comarostaphylis,
Ornithostaphylos?), Arctostaphylos
(c 60; southwestern Canada and western United States, Mexico, mountains in
Central America; in Arbutus?),
Arctous
(3; A. alpina, A. microphyllus, A. ruber; temperate
and arctic-alpine regions on the Northern Hemisphere), Comarostaphylis
(10; southern California, northwestern Mexico; in Arbutus?),
Ornithostaphylos (1; O. oppositifolia; southern California,
northwestern Mexico; in Arbutus?).
– Temperate regions on the Northern Hemisphere southwards to mountains in
Central America, with their highest diversity in California. Mycorrhiza
arbutoid. Hartig net frequently present. Corolla urceolate, with unicellular
hairs on adaxial side. Filaments distinctly asymmetrically widened. Anthers
with paired bristles. Endothecium absent. Style continuous. Ovules ten or fewer
per carpel. Fruit a berry or a drupe (sometimes covered with large fleshy
multicellular papillae), with bony or fibrous endocarp. Testal cell walls
fairly thick. x = 13. C-8 iridoid glucosides and ellagic acid present.
[[Cassiopoideae+Ericoideae]+[Harrimanelloideae+[Styphelioideae+Vaccinioideae]]]
Mycorrhiza ericoid, usually with
ascomycetes. Hartig net absent. Mycorrhiza-forming fungi usually ascomycetes.
Stem with well developed pericyclic fibres connected to foliar vascular
bundles. Anthers early inverted. Endothecium absent. Toxic andromedane
diterpenes sometimes present.
[Cassiopoideae+Ericoideae]
Leaves opposite, ericoid (usually
needle-like), with revolute ptyxis.
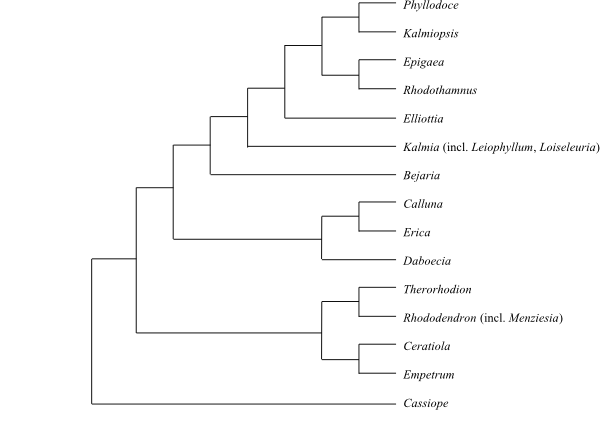 |
Cladogram of Cassiopoideae and
Ericoideae based on DNA sequence data (Kron 1997).
|
Cassiopoideae (H. T.
Cox ex P. F. Stevens) Kron et Judd in K. A. Kron et al., Bot. Rev. (Lancaster)
68: 404. 23 Oct 2002
1/12. Cassiope
(12; arctic-alpine regions on the Northern Hemisphere, the Himalayas). –
Mycorrhiza ericoid. Medulla Calluna
type, with large thin-walled cells surrounded by smaller thick-walled lignified
cells. Special fasciculate hairs present. Leaves decussate, linear, peltate,
hypoascidiate etc., with flat to recurved ptyxis, without or with poorly
developed associated fibres in mid vein. Inflorescence axillary, reduced. Bud
scales absent. Anthers with bristles. Megagametophyte disporous.
Ericoideae Arn.,
Botany: 118. 9 Mar 1832 [’Ericeae’]
16/1.770–1.780.
Phyllodoceae Drude in Engler et Prantl, Nat.
Pflanzenfam. IV, 1: 31, 38. Oct 1889.Bejaria (15; southern United
States, Mexico, Central America, tropical South America), Elliottia
(4; E. bracteata, E. paniculata: Japan; E.
pyroliflora: Alaska, western Canada, northwestern United States; E.
racemosa: southeastern United States), Kalmia (10;
Canada, United States, one species, K. ericoides, on Cuba, one
species, K. procumbens, in arctic-alpine regions on the Northern
Hemisphere), Epigaea (3; E. gaultherioides: eastern Turkey,
the Caucasus; E. asiatica: Japan; E. repens: eastern United
States), Rhodothamnus (2; R. chamaecistus: eastern Alps;
R. sessilifolius: northeastern Turkey), Kalmiopsis
(1; K. leachiana; Oregon), Phyllodoce
(7; P. aleutica, P. breweri, P. caerulea, P.
deflexa, P. empetriformis, P. glanduliflora, P.
nipponica; cold-temperate and arctic-alpine regions on the Northern
Hemisphere). – Bryantheae Gillespie et Kron in
Brittonia 64(1): 75. 1 Mar 2012. Bryanthus (1; B. gmelinii;
Kamchatka, Japan), Ledothamnus (7; L. atroadenus, L.
decumbens, L. guyanensis, L. jauaensis, L.
luteus, L. parviflorus, L. sessiliflorus; the Guayana
Highlands). – Ericeae DC. ex Duby, Bot. Gall. 1:
316. 12-14 Apr 1828 [‘Ericaceae’]. Daboecia
(2; D. cantabrica: Ireland to Spain; D. azorica: the Azores),
Calluna
(1; C. vulgaris; Europe, Turkey), Erica (c
860; Europe, Macaronesia, the Mediterranean, tropical and southern Africa,
Madagascar, the Comoros, with their highest diversity in the Cape Provinces).
– Empetreae Horan., Char. Ess. Fam.: 109. 17 Jun
1847. Ceratiola (1; C. ericoides; southeastern United
States), Corema (2; C. album: Portugal, western Spain, the
Azores; C. conradii: eastern Canada, northeastern United States), Empetrum
(3; E. eamesii, E. nigrum, E. rubrum; temperate and
arctic-alpine regions on the Northern Hemisphere, southern Andes in Chile from
35°S to 55°S, the Falkland Islands, Tristan da Cunha). –
Rhodoreae DC. ex Duby, Bot. Gall. 1: 318. 12-14 Apr
1828 [‘Rhodoraceae’].Rhododendron
(c 850; temperate and alpine regions on the Northern Hemisphere and south to
mountains in Southeast Asia, Malesia to New Guinea and eastern Australia from
northeastern Queensland to eastern Victoria, with their largest diversity in
the Himalayas, western China, northern Burma and alpine New Guinea). –
Temperate, arctic-alpine and montane regions in the Northern Hemisphere,
mountain areas in the Southern Hemisphere, the Falkland Islands, Tristan da
Cunha, with their highest diversity in South Africa, mountains in Southeast
Asia and New Guinea. Usually bisexual (in Empetreae usually monoecious
or dioecious). Mycorrhiza ericoid. Leaves alternate (spiral) or opposite (in
Empetreae and Ericeae often verticillate), with usually flat
(sometimes convolute, in Empetreae and Ericeae strongly
revolute) ptyxis. Pedicel sometimes articulated. Petals usually four or five
(in Rhodoreae often more than five; in Empetreae four, free).
Anthers usually without appendages (in Ericeae often spurred). Pollen
grains in Rhodoreae often with viscin threads. Stigma in
Empetreae expanded. Ovule in Empetreae one per carpel, basal,
apotropous. Fruit usually a septicidal capsule (in Erica also
loculicidal; in Empetreae a drupe). Gossypetin present in
Rhodoreae and Empetreae. – Phyllodoceae are sister
to the remaining Ericoideae, according to Gillespie & Kron (2012),
whereas Bryantheae are sister-group to the clade
[Ericeae+[Empetreae+Rhodoreae]].
[Harrimanelloideae+[Styphelioideae+Vaccinioideae]]
Fruit with persistent and non-withering
calyx.
Harrimanelloideae Kron
et Judd in K. A. Kron et al., Bot. Rev. (Lancaster) 68: 409. 23 Oct 2002
1/2. Harrimanella
(2; H. hypnoides: arctic-alpine regions in northern Europe to northern
Ural, arctic Canada to Quebec and northeastern United States; H.
stelleriana: high mountains in Japan, the Kurile Islands, Kamchatka,
Alaska, northwestern Canada, northwestern United States). – Mycorrhiza
ericoid? Leaves acicular, sublinear. Leaf margin entire. Flowers solitary,
axillary. Floral prophylls (bracteoles) absent. Anthers spurred, poricidal.
[Styphelioideae+Vaccinioideae]
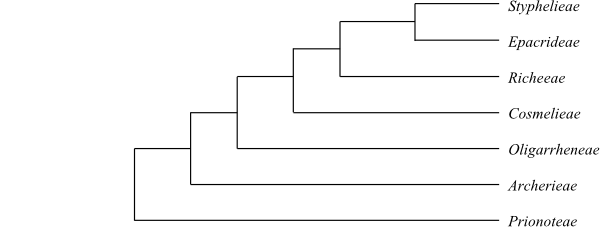 |
Cladogram (simplified) of
Styphelioideae based on DNA sequence data (Crayn & Quinn
2000).
|
Styphelioideae Sweet,
Fl. Australas.: ad t. 47. 1 Mai 1828 [‘Stypheliae’]
36/510–525.
Prionoteae Drude in Engler et Prantl, Nat.
Pflanzenfam. IV, 1: 72. Dec 1889. Lebetanthus (1; L.
myrsinites; southernmost Andes, Patagonia, Tierra del Fuego),
Prionotes (1; P. cerinthoides; Tasmania). –
Archerieae Crayn et Quinn in Austral. Syst. Bot. 11:
23. 31 Mar 1998. Archeria (6; A. comberi, A.
eriocarpa, A. hirtella, A. serpyllifolia: Tasmania;
A. racemosa, A. traversii: New Zealand). –
Oligarrheneae Crayn et Quinn in K. A. Kron et al.,
Bot. Rev. (Lancaster) 68: 412. 23 Oct 2002. Needhamiella (1; N.
pumilio; southwestern Western Australia), Oligarrhena (1; O.
micrantha; southwestern Western Australia), Dielsiodoxa (3;
D. leucantha, D. oligarrhenoides, D. tamariscina;
southwestern Western Australia). – Cosmelieae Crayn
et Quinn in K. A. Kron et al., Bot. Rev. (Lancaster) 68: 413. 23 Oct 2002.Andersonia
(c 35; southwestern Western Australia), Cosmelia (1; C.
rubra; southwestern Western Australia), Sprengelia (4; S.
distichophylla, S. incarnata, S. monticola, S.
sprengelioides; eastern South Australia to southeastern Queensland,
Tasmania). – Richeeae Crayn et Quinn in K. A. Kron
et al., Bot. Rev. (Lancaster) 68: 412. 23 Oct 2002. Dracophyllum
(50–55; eastern Queensland, eastern New South Wales, Tasmania, New Caledonia,
New Zealand), Richea (11; southeastern New South Wales, Victoria,
Tasmania, with their highest diversity in Tasmania), Sphenotoma
(6; S. capitatum, S. dracophylloides, S. drummondii,
S. gracilis, S. parviflorum, S. squarrosa;
southwestern Western Australia). – Epacrideae
Dumort., Anal. Fam. Plant.: 28. 1829 [‘Epacreae’].
Epacris (55–60; southeastern South Australia to southeastern
Queensland, Tasmania, New Caledonia, New Zealand), Lysinema
(6; L. ciliatum, L. conspicuum, L. elegans, L.
fimbriatum, L. lasianthum, L. pentapetalum; southwestern
Western Australia), Woollsia (1; W. pungens; southeastern
Queensland, eastern New South Wales). – Styphelieae
Bartl., Ord. Nat. Plant.: 158. Sep 1830 [‘Stypheliae’].
Pentachondra (4; P. dehiscens, P. ericifolia, P.
involucrata, P. pumila; eastern New South Wales, eastern
Victoria, Tasmania, New Zealand), Acrothamnus (6; A.
colensoi, A. hookeri, A. maccraei, A. montanus,
A. spathaceus, A. suaveolens; northeastern Queensland,
eastern New South Wales, eastern Victoria, Tasmania, New Zealand),
Acrotriche (16; southwestern Western Australia, southeastern South
Australia to eastern Queensland, Tasmania), Androstoma (2; A.
verticillata: Tasmania; A. empetrifolia: New Zealand), ’Astroloma’
(28; southwestern Western Australia, southeastern South Australia, Victoria,
eastern New South Wales, Tasmania; non-monophyletic), Stenanthera (5;
S. brachyloma, S. ciliata, S. conostephioides,
S. pinifolia, S. squamuligera; eastern New South Wales,
Victoria, Tasmania), Brachyloma (8; southwestern Western Australia,
southeastern South Australia to southeastern Queensland, Tasmania),
Coleanthera (3; C. coelophylla, C. myrtoides, C.
virgata; southwestern Western Australia), Conostephium (10–12;
southwestern Western Australia), Croninia (1; C. kingiana;
southwestern Western Australia), Cyathodes (5; C. dealbata,
C. glauca, C. petiolaris, C. platystoma, C.
straminea; Tasmania), Cyathopsis (1; C. floribunda; New
Caledonia), Decatoca (1; D. spenceri; New Guinea), Leptecophylla
(13; New Guinea, Tasmania, New Zealand to the Hawaiian Islands), ’Leucopogon’
(155–165; Malesia to New Guinea, Australia, Tasmania, New Caledonia, New
Zealand; non-monophyletic), ’Lissanthe’ (7; L.
brevistyla, L. pluriloculata, L. powelliae, L.
rubicunda, L. sapida, L. scabra, L. strigosa;
southwestern Western Australia, southeastern South Australia to southeastern
Queensland, Tasmania; non-monophyletic), Melichrus (13?; M.
adpressus, M. erubescens, M. hirsutus, M.
procumbens, M. urceolatus; continental southwestern Western
Australia, eastern Queensland to Victoria), ’Monotoca’ (18;
southwestern Western Australia, southeastern South Australia to eastern
Queensland, Tasmania; non-monophyletic), Planocarpa (3; P.
nitida, P. petiolaris, P. sulcata; Tasmania), Styphelia
(15; southwestern Western Australia, southeastern South Australia to
southeastern Queensland, Tasmania), Trochocarpa (12; Malesia to New
Guinea, northeastern Queensland, eastern New South Wales, eastern Victoria,
Tasmania). – Chile, southern Indochina, Malesia to New Guinea, Australia,
Tasmania, Melanesia, New Zealand, the Hawaiian Islands and other islands in the
Pacific. Ericoid mycorrhiza? Nodes in Richeeae ≥3:≥3. Leaves
xeromorphic, ericoid, not recurved, often with apical spine, in
Cosmelieae and Richeeae with sheathing base. Venation
parallel or palmate; mid-vein usually indistinct (in Prionoteae
distinct, with three veins from leaf base). Foliar vascular bundles with well
developed abaxial fibrous envelopes. Adaxial cap absent. Leaf epidermis
lignified (also in Lyonieae in Vaccinioideae); epidermal
cells rectangular, arranged in longitudinally parallel rows; anticlinal
epidermal cells walls sinuous (at least on abaxial side of leaf); abaxial
epidermis and associated hypodermis detach from mesophyll in
Archerieae. Stomata in Richeeae brachyparacytic; in
Styphelieae parallel to foliar longitudinal axis. Abaxial foliar
surface in Oligarrheneae without ribbon wax and papillae; adaxial
cuticular wax crystalloids absent in Richeeae. Multicellular hairs and
leaf teeth usually absent (present in Prionoteae). Inflorescence
terminal or axillary, usually spicate; flowers often solitary axillary with
numerous floral prophylls (absent in Richeeae). Inflorescence bracts
membranous. Petals in Oligarrheneae with valvate or induplicate
valvate aestivation. Stamens usually as many as sepals, antesepalous (in
Oligarrheneae two to five). Filaments usually adnate to corolla tube
(epipetalous). Anthers disporangiate (monothecal), longicidal (usually
dehiscing by a single slit, in Prionoteae by two slits), without
appendages. Tapetum with uninucleate cells. Pollen grains in
Styphelieae shed as pseudomonads (only one out of four meiospores
developing). Pistil in Oligarrheneae composed of two carpels. Ovule in
Oligarrheneae one per carpel. Ovules usually epitropous (in Lysinema
in Epacrideae apotropous). Fruit in Oligarrheneae a nut, in
Styphelieae a drupe; corolla persistent in fruit. – A probable
topology is (Crayn & Quinn 2000):
[Prionoteae+[Archerieae+[Oligarrheneae+[Cosmelieae+[Richeeae+[Epacrideae+Styphelieae]]]]]].
 |
Cladogram (simplified) of
Vaccinioideae based on morphology and DNA sequence data (Kron,
Judd & Crayn 1999).
|
Vaccinioideae Arn.,
Botany: 118. 9 Mar 1832 [‘Vaccinieae’] (under construction)
45/1.450–1.480.
Vaccinieae Rchb., Fl. Germ. Excurs. 1(3): 203.
Jul-Dec 1831. ’Vaccinium’
(c 150?; temperate and arctic-alpine regions on the Northern Hemisphere,
mountains in Central and southeastern Africa and Madagascar, the Hawaiian
Islands, mountains in tropical America; polyphyletic), Gaylussacia
(>50; eastern Canada, eastern United States, the Andes in South America;
incl. Vaccinium
pro parte), Oxycoccus
(4; O. erythrocarpus, O. macrocarpus, O.
microcarpus, O. palustris; temperate and arctic-alpine regions on
the Northern Hemisphere), Symphysia (15; Central America, the West
Indies), Agapetes
(c 400; tropical Asia from India to New Guinea; incl. Vaccinium
pro parte), Didonica (4; D. crassiflora, D.
panamensis, D. pendula, D. subsessilis; Costa Rica,
Panamá), Notopora (5; N. auyantepuiensis, N.
cardonae, N. chimantensis, N. schomburgkii, N.
smithiana; eastern Venezuela), Orthaea (34; Mexico, Central
America, the Andes south to Bolivia), Gonocalyx (9; Central America),
Dimorphanthera (75–80; Malesia, with their highest diversity on New
Guinea), Paphia (c 20; eastern New Guinea, eastern Queensland, New
Caledonia, Fiji; incl. Dimorphanthera keysseri), Cavendishia
(100–110; southern Mexico, Central America, tropical South America; incl.
Thibaudia pro parte), Macleania (35–40; southern Mexico,
Central America, western tropical America), Psammisia (c 60; tropical
America), Satyria (c 25; southern Mexico, Central America, tropical
South America), Mycerinus (3; M. chimantensis, M.
sclerophyllus, M. viridiflorus; the Guayana Highlands),
Polyclita (1; P. turbinata; Bolivia), Anthopteropsis
(1; A. insignis; central Panamá), Ceratostema (23–32;
mountain regions in South America, with their highest diversity in the Andes in
Ecuador), Semiramisia (3; S. karsteniana, S.
pulcherrima, S. speciosa; northern Andes), Oreanthes (7;
O. buxifolius, O. ecuadorensis, O. fragilis, O.
glanduliferus, O. hypogaeus, O. rotundifolius, O.
sperlingii; the Andes in Ecuador), Siphonandra (5; S.
boliviana, S. elliptica, S. magnifica, S.
nervosa, S. santa-barbarensis; the Andes), Pellegrinia
(6; P. coccinea, P. dichogama, P. grandiflora,
P. guascensis, P. harmsiana, P. hirsuta; the Andes),
’Disterigma’ (c 35; northern and central Andes; polyphyletic),
Utleya (1; U. costaricensis; Costa Rica),
Sphyrospermum (>22; mountains in tropical America; incl.
Disterigma pro parte), Rusbya (1; R. taxifolia;
northern Bolivia), Themistoclesia (c 25; northern Andes),
Plutarchia (11; Colombia, Ecuador), Anthopterus (12; the
Andes; incl. Thibaudia pro parte), ’Thibaudia’ (c 60;
Central America, northwestern tropical South America; polyphyletic),
Demosthenesia (11; the Andes), ’Diogenesia’ (13; the
Andes; polyphyletic). – Andromedeae Klotzsch,
Pfl.-Abbild. Beschr.: 16. 1838. Andromeda
(2; A. glaucophylla, A. polifolia; temperate regions
on the Northern Hemisphere), Zenobia (1; Z. pulverulenta;
southeastern United States). – Gaultherieae Nied.
in Engl. Bot. Jahrb. Syst. 11: 145. 18 Jun 1889. Chamaedaphne
(1; C. calyculata; temperate regions on the Northern Hemisphere),
Eubotrys (2; E. racemosa, E. recurva; southeastern
United States), Gaultheria
(c 135; East Asia to Malesia, Australia, New Zealand, Alaska, Canada, United
States, Mexico, Central America, South America), ’Leucothoe’
(5; L. axillaris, L. davisiae, L. fontanesiana,
L. griffithiana, L. keiskei; the Himalayas, Japan, North
America; polyphyletic), Tepuia (7; T. cardonae, T.
intermedia, T. multiglandulosa, T. speciosa, T.
tatei, T. vareschii, T. venusta; the Guayana Highlands).
– Lyonieae Kron et Judd in K. A. Kron et al., Amer.
J. Bot. 86: 1298. 15 Sep 1999. Agarista (c 30; tropical Africa,
Madagascar, the Mascarene Islands, southeastern United States, Mexico, Central
America, tropical South America), Craibiodendron (5; C.
henryi, C. scleranthum, C. stellatum, C.
vietnamense, C. yunnanense; southern China, Southeast Asia), Lyonia (c
35; East and Southeast Asia, eastern United States, Mexico, the West Indies),
Pieris
(7; P. cubensis, P. floribunda, P. formosa, P.
japonica, P. nana, P. phillyreifolia, P.
swinhoei; the Himalayas to East Asia and eastern Siberia, southeastern
United States, Cuba). – Oxydendreae Cox ex Reveal
in Phytoneuron 2012-37: 218. 23 Apr 2012.Oxydendrum
(1; O. arboreum; eastern United States). – Cosmopolitan. Often
epiphytic. Mycorrhiza ericoid. Phellogen in ‘Vaccinium’
and Agapetes
superficial. Secondary phloem in Lyonieae often with bands of fibres.
Apical bud aborted. Epidermis in Lyonieae lignified. Stomata often
paracytic (in Lyonieae often anomocytic). Inflorescence usually
axillary. Pedicel often articulated. Hypogyny frequent. Anthers spurred, with
two or four bristles or without appendages; abaxial side of anthers with
disintegration tissue. Tapetum cells in Vaccinium
binucleate. Ovary often inferior, in ‘Vaccinium’
and Agapetes
appearing decemlocular. Stigma truncate. x = 12.
 |
Cladogram of Vaccinieae based on DNA
sequence data (Kron, Powell & Luteyn 2002). – Agapetes
clade A: Vaccinium caudatifolium, V. filiforme,
V. lancifolium. – Agapetes
clade B: Agapetes,
V. gaultheriifolium, V. nummularia. –
Bracteata-Oarianthe clade: V. cercidifolium, V.
leptospermoides, V. summifaucis, V. finisterrae,
V. horizontale. – Myrtillus clade: Costera
endertii, V. hirtum, V. smallii, V.
parvifolium, V. myrtillus, V. dentatum, V.
reticulatum. – Orthaea-Notopera clade: V.
crenatum, Notopora schomburgkii, Orthaea
apophysata, Orthaea venamensis. – Vaccinium
clade: V. macrocarpon, V. vitis-idaea. – East
Malesian clade: Paphia, Dimorphanthera. – Andean
clade A: Thibaudia floribunda, Anthopterus,
Themistoclesia, Diogenesia, Demosthenesia;
Satyria, Orthaea fimbriata, Cavendishia
(incl. Thibaudia jahnii). – Andean clade B:
Ceratostema, Oreanthes, Sphyrospermum (incl.
Disterigma pro parte), Macleania, Psammisia,
Plutarchia, Polyclita (incl. Thibaudia
macrocalyx), Satyria boliviana, Thibaudia
densiflora, Semiramisia, Thibaudia parvifolia,
Siphonandra, Thibaudia pachyantha. –
Meso-American/Caribbean clade: Symphysia, Disterigma
trimerum, Utleya, V. poasanum,
Gonocalyx, Macleania megabracteolata. – Vaccinium
Section Hemimyrtillus may be sister-group to the remaining
Vaccinieae, yet with weak support (Powell & Kron 2002).
|
de Candolle, Prodr. 3: 349. med Mar 1828
[’Fouquieraceae’], nom. cons.
Fouquieriales DC. in
C. F. P. von Martius, Consp. Regn. Veg.: 50. Sep-Oct 1835 [’Fouquieriaceae’];
Fouquierineae Engl., Syllabus, ed. 2: 153. Mai
1898
Genera/species 1/11
Distribution Arid regions in
Mexico and southwestern United States.
Fossils Unknown.
Habit Bisexual, deciduous
shrubs or small trees. Xerophytic, often stem succulents, spiny, with little
branched long and short shoots. Periderm transparent, often exfoliating.
Vegetative anatomy Phellogen
ab initio superficially (in root) or deeply seated (often outer-cortical).
Layers of fibre cells alternating with layers of cork cells in stem phelloderm.
Stem cortex reticulum consisting of groups of superficial sclereids and
assimilating tissue, and inside this partly water-storing and partly
starch-storing tissue. Medulla septated by diaphragms. Vessel elements with
usually simple (rarely scalariform) perforation plates; lateral pits alternate
or opposite, simple and/or bordered pits. Imperforate tracheary xylem elements
fibre tracheids with bordered pits, non-septate (also vasicentric tracheids).
Wood rays multiseriate, homocellular or heterocellular. Axial parenchyma
apotracheal diffuse or diffuse-in-aggregates, or paratracheal scanty. Xylem
parenchyma well developed and water-storing. Sieve tube plastids S type. Nodes
1:1, unilacunar with one leaf trace. Prismatic calciumoxalate crystals
abundant.
Trichomes Hairs unicellular or
absent.
Leaves Alternate (spiral),
simple, entire, heteromorphic, with ? ptyxis. Stipules and leaf sheath absent.
Petioles of long shoots modified into long cortical spines, with base
proceeding down stem as coarse ridges. Axillary short shoots with normal
leaves. Petiole vascular bundles? Venation pinnate. Stomata anomocytic.
Cuticular wax crystalloids absent. Epidermis with mucilage cells (with
mucilaginous inner walls). Leaf margin entire.
Inflorescence Terminal or
axillary, spike- or raceme-like, panicle or corymbo-paniculate.
Flowers Actinomorphic.
Hypogyny. Sepals five, with imbricate quincuncial aestivation, spiral, free,
with membranous margin, persistent. Petals five, with imbricate quincuncial
aestivation, connate into tube. Base of ovary nectariferous. Disc small,
annular.
Androecium Stamens usually ten
(rarely up to 23), diplostemonous (antepetalous stamens sometimes duplicated).
Filaments free, free from or slightly adnate to corolla tube, sometimes with
ligulate spur at base. Anthers dorsifixed, versatile?, tetrasporangiate,
introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory.
Staminodia absent.
Pollen grains
Microsporogenesis simultaneous? Pollen grains tricolporate, shed as monads,
bicellular at dispersal. Exine semitectate, with columellate infratectum,
reticulate, often striate.
Gynoecium Pistil composed of
three connate carpels. Ovary superior, unilocular, with nectariferous
vascularized tissue at base. Stylodia three, connate in lower part. Stigmas
punctate, type? Pistillodium absent.
Ovules Placentation at
anthesis parietal with deeply intrusive placentae (axile at base), finally free
central. Ovules six to c. 20 per carpel, anatropous, ascending, apotropous,
bitegmic, tenuinucellar. Micropyle endostomal. Outer integument three or four
cell layers thick. Inner integument (four to) seven or eight cell layers thick.
Megagametophyte monosporous, Polygonum type. Endosperm development
cellular. Endosperm haustoria chalazal and micropylar. Embryogenesis
asterad.
Fruit A loculicidal
capsule.
Seeds Aril? Seed coat winged.
Testa multiplicative, gradually crushed; hypodermis with band-shaped
thickenings. Tegmen multiplicative, crushed. Perisperm not developed. Endosperm
thin, oily and proteinaceous, or absent. Embryo small, straight, well
differentiated, chlorophyll? Cotyledons two, flat. Germination
phanerocotylar.
Cytology x = 12 – Polyploidy
occurring.
DNA Mitochondrial intron
coxII.i3 lost.
Phytochemistry Flavonols,
cyanidin, Route I secoiridoids, dammarane, ellagic acid, pelargonidin (in
red-flowered species), caffeic acid, indole alkaloids and their glycosides, and
saponins present. Myricetin and cyanogenic compounds not found.
Use Ornamental plants,
ocotillo-wax.
LECYTHIDACEAE A.
Rich.
|
( Back to Ericales )
|
Richard in J. B. G. M. Bory de Saint-Vincent,
Dict. Class. Hist. Nat. 9: 269. 25 Feb 1825 [‘Lecythideae’], nom.
cons.
Belvisiaceae R. Br.
in Trans. Linn. Soc. London 13: 222. Mai-Jun 1821 [‘Belviseae’],
nom. illeg.; Napoleonaeaceae A. Rich. in J. B. G. M.
Bory de Saint-Vincent, Dict. Class. Hist. Nat. 11: 432. 10 Feb 1827
[‘Napoleoneae’]; Barringtoniaceae (DC.)
F. Rudolphi, Syst. Orb. Veg.: 56. 5-12 Jul 1830
[‘Barringtonieae’], nom. cons.;
Barringtoniales DC. in C. F. P. von Martius, Consp.
Regn. Veg.: 65. Sep-Oct 1835 [‘Barringtonieae’];
Belvisiales R. Br. in C. F. P. von Martius, Consp.
Regn. Veg.: 63. Sep-Oct 1835 [’Belvisieae’], nom. illeg.;
Gustaviaceae Burnett, Outl. Bot.: 717, 1092, 1135.
Feb 1835; Lecythidales Poit. in C. F. P. von Martius,
Consp. Regn. Veg.: 65. Sep-Oct 1835 [’Lecythideae’];
Barringtoniineae J. Presl in Nowočeská Bibl.
[Wšobecný Rostl.] 7: 583, 602. 1846;
Scytopetalaceae Engl. in Engler et Prantl, Nat.
Pflanzenfam. Nachtr. 1: 242. Oct 1897, nom. cons.;
Scytopetalineae Engl., Syllabus, ed. 2: 150. Mai
1898; Rhaptopetalaceae Tiegh. ex Solereder, Syst.
Anat. Dicot. Ergänz.: 53. Mai 1908; Asteranthaceae
R. Knuth in Engler, Pflanzenr., IV, 219b (Heft 105): 1. 22 Aug 1939, nom.
cons.; Foetidiaceae (Engl.) Airy Shaw in Kew Bull.
18: 258. 8 Dec 1965; Lecythidanae Takht. ex Reveal in
Novon 2: 236. 13 Oct 1992
Genera/species 24/355–360
Distribution Pantropical,
subtropical regions of South and East Asia.
Fossils The fossil flower
Lecythidoanthus kugleri has been described from the Miocene of
Trinidad. Fossil wood, fruits and pollen grains assigned to Lecythidaceae were recorded from
Late Cretaceous (Maastrichtian) and Cenozoic sediments in India and Europe.
Habit Bisexual, usually
evergreen trees (sometimes shrubs or lianas; in Scytopetaloideae
rarely perennial herbs). Foetidia has evil-smelling wood. Bark often
fibrous.
Vegetative anatomy Phellogen
ab initio superficial. Stem cortex with vascular bundles. Primary medullary
rays narrow or wide. Vessel elements usually with simple (sometimes
scalariform, rarely reticulate) perforation plates; lateral pits alternate or
opposite, simple and/or bordered pits. Imperforate tracheary xylem elements
tracheids (in Allantoma), fibre tracheids or libriform fibres usually
with simple (sometimes bordered) pits, septate or non-septate (also vasicentric
tracheids). Wood rays uniseriate to multiseriate, homocellular or
heterocellular. Axial parenchyma apotracheal diffuse or diffuse-in-aggregates,
or paratracheal scanty vasicentric, reticulate or banded (rarely aliform,
confluent or scalariform). Tyloses often abundant (sometimes sclerotic).
Secondary phloem stratified into hard fibrous and soft parenchymatous layers,
often with unilaterally thickened cells with crystals (sometimes with cuneate
rays). Sieve tube plastids S type. Nodes 1:1, unilacunar with one leaf trace,
or ≥3:≥3, trilacunar to multilacunar with three or more traces. Cristarque
cells sometimes present. Cells with calciumoxalate crystals or silica bodies.
Prismatic crystals abundant.
Trichomes Hairs unicellular or
multicellular, usually uniseriate (sometimes stellate) or absent; glandular
hairs sometimes present.
Leaves Usually alternate
(sometimes distichous; rarely opposite), simple, entire, often coriaceous, with
supervolute or involute ptyxis. Stipules usually absent (in
Asteranthos and Scytopetalum small, paired, cauline,
caducous); leaf sheath absent. Colleters present. Petiole vascular bundle
transection arcuate or annular; petiole with numerous vascular bundles.
Venation pinnate, sometimes brochidodromous. Stomata usually anisocytic
(sometimes paracytic or anomocytic). Cuticular wax crystalloids as polygonal
platelets. Domatia as pits (Combretodendron) or absent. Secretory
cavities absent. Mesophyll in Napoleonaeoideae with idioblasts
containing ethereal oils and sclerenchymatous idioblasts containing prismatic
calciumoxalate crystals. Leaf margin usually entire (sometimes serrate or
crenate).
Inflorescence Terminal or
axillary, spike- or raceme-like, panicle, fasciculate, thyrsoid, botryoid
cymose, or racemose, or flowers solitary axillary (each flower in
Napoleonaeoideae subtended by four to seven often glanduliferous
bracts, extrafloral nectaries).
Flowers Actinomorphic or
zygomorphic (especially androecium), often large. Pedicel articulated.
Hypanthium present or absent. Epigyny or half epigyny (in
Scytopetaloideae usually hypogyny). Sepals (two or) four to six (to
twelve), usually with imbricate (sometimes valvate) aestivation, persistent or
caducous (in Asteranthos accrescent in fruit), usually more or less
connate. Petals three to six (to eight, ten, twelve, 16, or 18), with imbricate
aestivation, caducous, usually connate (absent in Foetidioideae and
probably in Napoleonaeoideae and Scytopetaloideae). Nectaries
often present at base of staminodia on androphore. Nectariferous disc annular,
intrastaminal or extrastaminal, present in Napoleonaeoideae,
Barringtonioideae and Foetidia (in Asteranthos
formed by fused staminodia).
Androecium Stamens ten to more
than 1.200, with various positions, often whorled, usually centrifugally
(sometimes centripetally) developing from annular primordium, often (some
Lecythidoideae) on annular or asymmetrical androphore (consisting of
staminodia and/or fertile stamens), sometimes curved and hood-like above ovary.
Filaments usually more or less connate (in Foetidioideae and
Napoleonaeoideae free), free from tepals (in Napoleonaeoideae
and Scytopetaloideae adnate to base of pseudocorolla probably
consisting of staminodial tissue). Anthers basifixed, often versatile, usually
tetrasporangiate (in Napoleonaeoideae disporangiate), extrorse,
latrorse or introrse, usually longicidal (dehiscing by longitudinal slits; in
Scytopetaloideae sometimes poricidal, with apical short slits or
pores). Tapetum secretory or amoeboid-periplasmodial. Staminodia (sometimes
with fodder pollen in anthers) often several to numerous on androphore or
pseudocorolla, extrastaminal (outer staminodial whorl in Napoleonaea
forming pseudocorolla, and two inner staminodial whorls forming corona;
staminodial whorl in Scytopetaloideae forming pseudocorolla).
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually tricolpate or
tricolpor(oid)ate or syntricolpate (Barringtonioideae), shed as
monads, bicellular or tricellular at dispersal. Exine tectate, with granular
infratectum, smooth, perforate to microreticulate, foveolate or
scabrate-verrucate.
Gynoecium Pistil composed of
two to six (to eight) connate usually antesepalous (sometimes antepetalous)
carpels. Ovary inferior to superior, unilocular to sexalocular (to
octalocular). Style single, simple, or absent. Stigma one, capitate, punctate
or trilobate to octalobate, papillate or non-papillate, Dry or Wet type.
Pistillodium absent.
Ovules Placentation apical,
apical-axile to basal-axile (in Eschweilera basal; in
Foetidia axile, with peltate placentae). Ovules (one or) two to c. 115
per carpel, usually anatropous (rarely campylotropous), pendulous, horizontal
or ascending, bitegmic, tenuinucellar. Micropyle usually endostomal (in, e.g.,
Couroupita exostomal). Outer integument six to 18 cell layers thick.
Inner integument three to seven cell layers thick. Archespore multicellular.
Megagametophyte monosporous, Polygonum type. Antipodal cells
ephemeral. Endosperm development ab initio nuclear. Endosperm haustoria?
Embryogenesis?
Fruit A drupe (in
Foetidioideae and Barringtonioideae, sometimes winged, in
Napoleonaeoideae with persistent calyx and style), large to very
large, indehiscent and juicy inside, or a woody pyxidium (rarely a loculicidal
capsule or a berry-like fruit).
Seeds Aril present in many
species of Lecythis. Seed coat mesotestal, in Cariniana and
Couroupita winged. Testa vascularized, multiplicative, often strongly
lignified. Exotestal cells of various thickness, palisade, or with sinuous
anticlinal walls. Mesotesta often sclerotized. Endotesta? Tegmen? Perisperm not
developed. Endosperm usually very sparse or absent (in
Scytopetaloideae copious, with amyloidiferous cell walls and usually
ruminate). Embryo large, straight or curved, undifferentiated or well
differentiated, chlorophyll? Cotyledons two, fleshy planoconvex or foliaceous,
or undifferentiated. Hypocotyl often strongly developed. Germination
phanerocotylar or cryptocotylar.
Cytology n = 13 (26)
(Barringtonioideae); n = 16 (Napoleonaeoideae); n = 11, 18,
21 (Scytopetaloideae); n = 17 (18) (34) (Lecythidoideae); n =
? (Foetidioideae)
DNA Mitochondrial intron
coxII.i3 lost (Bertholletia). Mitochondrial coxI
intron present (Barringtonia).
Phytochemistry Flavonols
(quercetin etc.), cyanidin, oleanolic acid derivatives, ellagic and gallic
acids, methylated ellagic acids, proanthocyanidins (prodelphinidins),
triterpene saponins, and cyanogenic compounds present. Kaempferol and alkaloids
not found. Selenium accumulated in some species. Aluminium accumulated in some
Scytopetaloideae.
Use Ornamental plants, edible
seeds (Bertholletia excelsa, Lecythis zabucajo), timber.
Systematics The sister-group
relationships of Lecythidaceae are unresolved.
They are part of a trichotomy also comprising [Fouquieriaceae+Polemoniaceae] and the majority
of Ericales.
Lecythidaceae and Sapotaceae have seeds with
multiplicative lignified testa.
A plausible topology of the Lecythidaceae subclades is the
following:
[Napoleonaeoideae+[Scytopetaloideae+[Lecythidoideae+[Barringtonioideae+Foetidioideae]]]].
Napoleonaeoideae (R.
Br.) Benth. in W. J. Hooker, Niger Fl.: 360. Nov-Dec 1849
[‘Napoleoneae’]
2/c 13. Napoleonaea (c 10;
tropical West and Central Africa to Angola and Zambia), Crateranthus
(3; C. congolensis, C. le-testui, C. talbotii;
southern Nigeria, Gabon, Congo). – Tropical West and Central Africa.
Secondary xylem containing crystal chains. Leaves distichous, with supervolute
ptyxis. Stipules present or absent. Lamina often with glands on abaxial side at
base. Leaf margin serrate. Sepals three (Crateranthus) or five
(Napoleonaea). Petals with valvate aestivation, connate, plicate.
Nectaries present on abaxial side of sepals. Filaments adnate to corolla.
Anthers long (in Napoleonaea ten, pairwise, antepetalous, extrorse,
monothecal). Corona formed by two inner staminodial whorls. Carpels
antepetalous, initiated prior to stamens. Style absent. Stigma wide,
quinquangular, flat. Ovules four per carpel, in Napoleonaea collateral
in pairs. Endothelium absent. Fruit a drupe with one or several seeds. Embryo
curved. n = 16. – Napoleonaeoideae have mixed simple and scalariform
vessel perforation plates, scalariform vessel-ray pitting, and high
multiseriate wood rays, features also present in Scytopetaloideae. The
wood structure of Napoleonaea is distinct, but its hypothetical
sister-group Crateranthus strongly resembles
Scytopetaloideae.
[Scytopetaloideae+[Lecythidoideae+[Barringtonioideae+Foetidioideae]]]
Scytopetaloideae O.
Appel in Bot. J. Linn. Soc. 121: 225. 17 Sep 1996
6/22–23. Asteranthos (1;
A. brasiliensis; Venezuela, northern Brazil), Oubanguia (3;
O. africana, O. alata, O. laurifolia; tropical West
and Central Africa), Scytopetalum (3; S. klaineanum, S.
pierreanum, S. tieghemii; tropical West and Central Africa),
Rhaptopetalum (11–12; tropical West and Central Africa),
Pierrina (1; P. zenkeri; Cameroon, Gabon, Equatorial Guinea),
Brazzeia (3; B. congoensis, B. longipedicellata,
B. soyauxii; Central Africa). – Tropical West and Central Africa,
northern Brazil. Leaf traces passing two internodes prior to entering leaves.
Sclereids present. Leaves distichous. Stipules minute. Pedicel articulated.
Usually hypogyny (sometimes half hypogyny). Sepals connate. Petals with valvate
aestivation, calyptrate, caducous, sometimes connate at base (sometimes
absent). Nectariferous disc usually present (nectary sometimes absent).
Filaments connate at base, adnate to corolla and to staminodia. Tapetum
secretory. Staminodia six to 28, petalous, connate. Pollen grains tricolpate or
sometimes tricolporoidate. Staminodia six to 16 or 24 to 28
(Asteranthos), connate, petaloid. Style long, thin. Stigma punctate.
Ovules two or four per carpel. Micropyle prolonged. Endothelium present. Fruit
a capsule or indehiscent, often one-seeded. Seed coat hairy, usually ruminate
(not in Oubanguia). Endosperm with hemicellulose. Embryo J-shaped. n =
11, 18, 21. Aluminium accumulated in some species. – Asteranthos
resembles Napoleonaeoideae in its morphology and phytochemistry, but
is placed in Scytopetaloideae by DNA sequence analyses.
[Lecythidoideae+[Barringtonioideae+Foetidioideae]]
Embryo usually with starch.
Lecythidoideae
Beilschm. in Flora 16(Beibl. 7): 98, 108. 14 Jun 1833
[‘Lecythideae’]
10/c 220. Gustavia (c 40;
Central America, tropical South America), Grias (12; Panamá to Peru),
Allantoma (8; northeastern tropical South America), Cariniana
(9; tropical South America), Couroupita (3; C. guianensis,
C. nicaraguarensis, C. subsessilis; Central America, tropical
South America), Corythophora (4; C. alta, C.
amapaensis, C. labriculata, C. rimosa; French Guiana,
Suriname, Brazil), Bertholletia (1; B. excelsa; tropical
South America), Couratari (c 20; tropical South America),
’Eschweilera’ (c 95; southern Mexico, Central America, tropical
South America; non-monophyletic), ’Lecythis’ (27; Central America,
tropical South America; non-monophyletic). – Southern Mexico, Central
America, northern and central South America to Uruguay. Bark fibrous. Cortical
vascular bundles in Gustavia inverted. Secondary xylem with crystal
chains. Leaves spiral or distichous, with involute ptyxis. Extraflora nectaries
present in Gustavia. Flowers usually (secondarily?) zygomorphic
(sometimes actinomorphic). Nectariferous disc present or absent. Filaments
contracted at apex, connate at base forming basal ring. Pollen grains
tricolp(oroid)ate, sometimes heteromorphic. Fodder pollen produced in many
species. Style usually short (sometimes long). Endothelium absent. Fruit
usually a pyxidium (rarely indehiscent). Wing-shaped aril or swollen funicle
present or absent. Embryo often curved, with hypocotyl or long radicula and
foliaceous (sometimes folded) or thick cotyledons. n = 17 (18) (34).
[Barringtonioideae+Foetidioideae]
Cortical vascular bundles inverted.
Leaves with supervolute ptyxis. Fruit indehiscent.
Barringtonioideae
Beilschm. in Flora 16(Beibl. 7): 98, 104. 14 Jun 1833
[‘Barringtonieae’]
5/c 84. Petersianthus (2;
P. macrocarpus: tropical West and Central Africa to Angola and Congo;
P. quadrialatus: the Philippines), Barringtonia (c 70; East
Africa, Madagascar, tropical Asia to the Ryukyu Islands and Micronesia),
Chydenanthus (1; C. excelsus; Burma, the Andaman and Nicobar
Islands, Malesia to New Guinea), Abdulmajidia (2; the Malay
Peninsula), Careya (3; C. arborea: Afghanistan to the Malay
Peninsula; C. herbacea: the Himalayas; C. valida: the Andaman
Islands), Planchonia (8; the Andaman and Nicobar Islands, the Malay
Peninsula to New Guinea, Solomon Islands and tropical Australia). – Tropical
East Africa, Madagascar, South and Southeast Asia, Malesia to New Guinea,
tropical Australia, Melanesia to the Ryukyu Islands and Micronesia. Secondary
xylem without crystal chains. Nodes 1:1. Leaves spiral. Glands often replacing
stipules. Nectariferous disc annular. Filaments connate at base forming basal
ring. Pollen grains syn(tri)colpate, with strong margin ridge at colpus. Style
long. Endothelium usually absent. Fruit usually one-seeded. Embryo with
hypocotyl or long radicula and foliaceous cotyledons. n = 13 (26). –
Barringtonioideae and Lecythidoideae both have vessel
elements with exclusively simple perforations and two types of vessel ray
pitting. However, they may be distinguished from each other by the size of
intervessel pitting, the shape of body ray cells arranged in multiseriate rays,
and the type of crystalliferous axial parenchyma cells.
Foetidioideae Engl.,
Syllabus, ed. 1: 146. Apr 1892
1/18. Foetidia (18; Madagascar,
the Comoros, the Mascarene Islands, one species, F. africana, in
tropical East Africa). – Secondary xylem with crystal chains. Nodes 1:1.
Leaves elongating in bud. Sepals with valvate aestivation, lignified. Petals
absent. Nectariferous disc indistinct. Filaments free. Anthers introrse. Style
trilobate or quadrilobate. Placentae peltate, with ovules inserted in two rows.
Endothelium present. n = ? – The isolated position of Foetidia
(Foetidioideae) may be supported by a unique type of vessel ray
pitting similar in shape and size to intervessel pitting (distinctly bordered,
<5 mm).
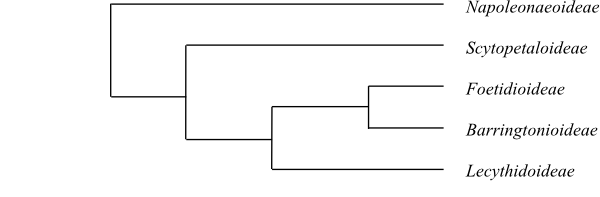 |
Cladogram of Lecythidaceae based on
morphology, anatomy and DNA sequence data (Morton, Mori & al.
1997).
|
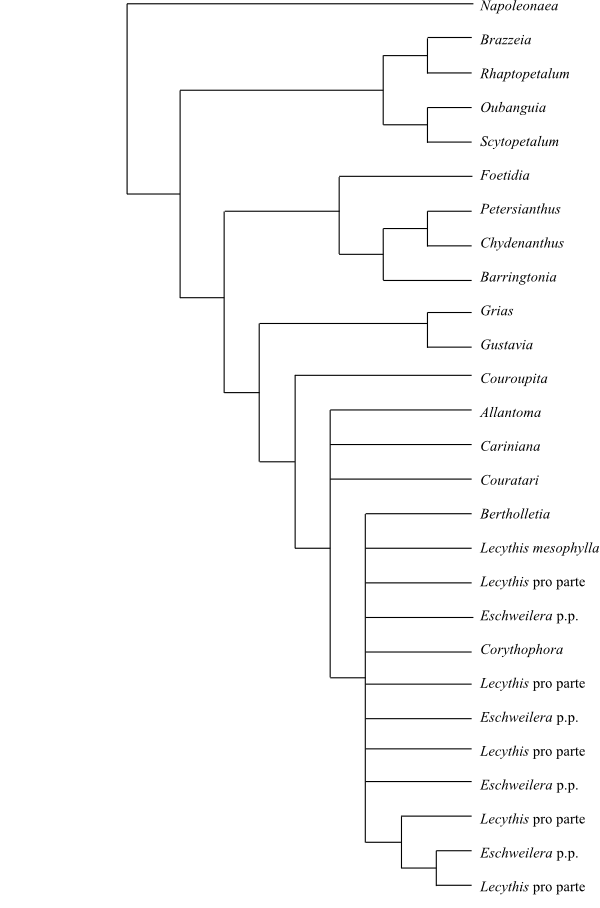 |
Cladogram of Lecythidaceae based on
DNA sequence data (Mori & al. 2007).
|
MAESACEAE (A. DC.)
Anderb., B. Ståhl et Källersjö
|
( Back to Ericales )
|
Anderberg, Ståhl et Källersjö in Taxon 49:
185. 16 Mai 2000
Genera/species 1/100–110
Distribution Tropical Africa,
Southeast Africa, Madagascar, Yemen, Sri Lanka, East Asia to Japan, Southeast
Asia, Malesia to New Guinea, Melanesia, Queensland, islands in the Pacific.
Fossils Uncertain.
Habit Usually bisexual (often
functionally polygamous, or functionally or cryptically dioecious?), evergreen
trees, shrubs or lianas.
Vegetative anatomy Phellogen
ab initio superficial? Vessel arranged in radial multiples. Vessel elements
with simple and/or scalariform (sometimes reticulate) perforation plates;
lateral pits alternate, bordered pits. Imperforate tracheary xylem elements
libriform fibres? with simple pits, septate. Wood rays uniseriate or
multiseriate, heterocellular. Axial parenchyma apotracheal diffuse, or
paratracheal scanty. Sieve tube plastids S type? Nodes unilacunar? with ? leaf
trace; lateral vascular bundles arising approx. halfway down internode below
supported leaf. Schizogenous secretory canals with tannins etc. Parenchyma
cells with calciumoxalate druses.
Trichomes Hairs uniseriate or
scale-like; glandular hairs sunken?
Leaves Alternate (spiral or
distichous), simple, entire, with induplicate ptyxis. Stipules and leaf sheath
absent. Petiole vascular bundle transection annular. Venation pinnate, often
indistinct. Stomata anisocytic? Cuticular wax crystalloids? Lamina with well
developed schizogenous secretory canals. Abaxial epidermis often with groups of
prismatic calciumoxalate druses. Leaf margin serrate or entire.
Inflorescence Axillary simple
or compound raceme. Floral prophylles (bracteoles) two.
Flowers Actinomorphic. Half
epigyny (perigyny). Sepals four or five (or six), with quincuncial aestivation,
persistent, free or connate. Petals four or five (or six), with
induplicate-valvate aestivation, connate into campanulate corolla. Petals and
stamens developing from common primordia; stamen primordium smaller than petal
primordium. Tepals with well developed and distinct canals. Nectaries present
on ovary wall. Disc absent.
Androecium Stamens usually
four or five (sometimes six), haplostemonous, antepetalous, alternisepalous.
Filaments connate at base, adnate to corolla tube (epipetalous). Anthers
dorsifixed, versatile?, tetrasporangiate, introrse, longicidal (dehiscing by
longitudinal slits). Tapetum secretory. Female flowers with staminodia?
Pollen grains
Microsporogenesis simultaneous? Pollen grains colpate or colpor(oid)ate, shed
as monads, bicellular at dispersal. Exine semitectate, with columellate
infratectum, subreticulate.
Gynoecium Pistil composed of
usually three or four connate antepetalous carpels. Ovary semi-inferior,
unilocular, nectariferous. Style single, simple (hollow?). Stigma truncate,
capitate or bilobate to quinquelobate, type? Male flowers with pistillodium?
Ovules Placentation free
central. Ovules numerous per ovary, anatropous, apotropous, bitegmic,
tenuinucellar. Micropyle endostomal? Outer integument approx. two cell layers
thick. Inner integument three or four cell layers thick. Megagametophyte
monosporous, Polygonum type. Endosperm development ab initio nuclear.
Endosperm haustoria? Embryogenesis onagrad.
Fruit A many-seeded drupaceous
berry with persistent calyx. Mesocarp often thin. Endocarp somewhat
lignified.
Seeds Aril absent. Exotesta?
Endotesta with rhomboidal crystals. Tegmen? Perisperm not developed. Endosperm
copious, amyloidiferous and lipidiferous. Embryo small, straight, chlorophyll?
Cotyledons two, small. Germination?
Cytology n = 10
DNA Intron present in
mitochondrial gene coxII.i3.
Phytochemistry Insufficiently
known. Flavonols (kaempferol, quercetin, myricetin?), alkaloids, triterpene
saponins, and maesaquinone and other quinones present. Cyanidin? Ellagic acid?
Proanthocyanidins? Glycosides? Cyanogenic compounds?
Use Medicinal plants, seed
oil, spices (Maesa indica), fish poisons, insecticides, timber.
Systematics Maesa
(100–110; tropical Africa, Southeast Africa, Madagascar, Yemen, Sri Lanka,
East Asia to Japan, Southeast Asia, Malesia to New Guinea, Queensland,
Melanesia, islands in the Pacific).
Maesa is sister to the clade [[Samolaceae+Theophrastaceae]+[Myrsinaceae+Primulaceae]].
MARCGRAVIACEAE Bercht.
et J. Presl
|
( Back to Ericales )
|
Berchtold et Presl., Přir. Rostlin: 218. Jan-Apr
1820 [’Marcgraviae’], nom. cons.
Marcgraviales Juss.
in C. F. P. von Martius, Consp. Regn. Veg.: 61. Sep-Oct 1835 [’Marcgraviaceae’];
Noranteaceae (Choisy) DC. ex Mart., Consp. Regn.
Veg.: 61. Sep-Oct 1835
Genera/species 7/170–175
Distribution Southern Mexico,
Central America, the West Indies, northern to central South America.
Fossils Fossil pollen
sometimes attributed to Marcgravia and Norantea s.lat. have
been reported from Early Oligocene sediments in Puerto Rico. Fossil wood from
Cenozoic layers on Sumatra has been described under the name of
Ruyschioxylon.
Habit Bisexual evergreen
shrubs, often epiphytic or semi-epiphytic, or lianas (rarely small trees).
Vegetative anatomy Phellogen
ab initio superficial. Primary medullary rays alternating wide and narrow.
Primary vascular tissue a cylinder, without separate vascular bundles. Vessel
elements with simple and/or scalariform perforation plates; lateral pits
alternate or opposite, simple or bordered pits. Imperforate tracheary xylem
elements fibre tracheids or libriform fibres with simple and/or bordered pits,
usually septate. Wood rays uniseriate to multiseriate, homocellular or
heterocellular. Axial parenchyma apotracheal diffuse or diffuse-in-aggregates,
or paratracheal scanty vasicentric. Sieve tube plastids Ss type. Nodes 1:1,
unilacunar with one leaf trace. Cortical parenchyma with idioblasts containing
sclereids or calciumoxalate raphides (in raphid sacs).
Trichomes Hairs usually
unicellular (rarely multicellular); glands frequent.
Leaves Alternate (spiral or in
Marcgravia distichous), simple, entire, often coriaceous, with
supervolute ptyxis; in Marcgravia often heterophyllous, with small
cordate leaves on sterile shoots with climbing roots and ordinary leaves on
fertile root-less shoots. Stipules and leaf sheath absent. Petiole vascular
bundles? Extrafloral nectaries often present on basal parts of abaxial surface
of lamina. Venation pinnate, brochidodromous, indistinct. Stomata staurocytic.
Cuticular wax crystalloids as platelets. Mesophyll with sclerenchymatous
idioblasts containing calciumoxalate raphides. Abaxial side of lamina often
with nectariferous glands (in Marcgravia also along margin). Domatia
as pits. Leaf margin usually entire (sometimes serrate). Leaf apex with often
caducous mucro.
Inflorescence Terminal raceme,
umbel or spike. Flower in some species with petaloid bract adnate to pedicel
and modified into leaf-, spur-, sac-, cup- or pitcher-like extrafloral nectary
(nectaries in Marcgravia inserted on central aborted flowers).
Flowers Actinomorphic.
Hypogyny. Sepals usually five (in Marcgravia four), with imbricate
quincuncial aestivation, unequal in size, persistent, free or somewhat connate
at base. Petals three to five, with imbricate aestivation, free or more or less
connate (in Marcgravia four, in the form of a calyptra falling off at
beginning of anthesis). Nectariferous disc absent.
Androecium Stamens (three to)
five to more than 100, in one or two whorls. Filaments filiform to flattened,
free or connate at base, usually free from tepals (rarely adnate to petal
bases). Anthers basifixed or subbasifixed, non-versatile?, tetrasporangiate,
introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory, with
uninucleate cells. Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually tricolporate (sometimes
tetracolporate), shed as monads, bicellular at dispersal. Exine tectate to
semitectate, with usually columellate infratectum, imperforate, perforate or
reticulate.
Gynoecium Pistil composed of
two to eight connate carpels. Ovary superior, unilocular at first, gradually
entirely or incompletely bilocular to 15-(to 20-)locular. Style single, simple,
short, or absent. Stigma simple or lobed, Dry type. Pistillodium absent.
Ovules Placentation strongly
intrusively parietal (seemingly axile). Ovules five to c. 20 (in
Marcgraviastrum much more than 20) per carpel, anatropous,
(incompletely) bitegmic, incompletely tenuinucellar. Micropyle endostomal.
Outer integument ? cell layers thick. Inner integument ? cell layers thick.
Megagametophyte monosporous, Polygonum type. Endosperm development
cellular. Endosperm haustorium micropylar. Embryogenesis?
Fruit A loculicidal and
septifragal capsule (or irregularly dehiscing) with persistent calyx, style and
stigma. Pericarp leathery. Placentae carnose.
Seeds Aril often present. Seed
coat exotestal? Exotestal cells enlarged, with inner walls strongly thickened,
with theoid exotestal thickenings. Endotesta? Tegmen? Perisperm not developed.
Endosperm sparse, starchy, or absent. Embryo large, straight or somewhat
curved, well differentiated, chlorophyll? Cotyledons two, small to large.
Germination phanerocotylar.
Cytology n = 18?
(Marcgravia evenia ssp. calcicola)
DNA
Phytochemistry Flavonols
(myricetin), terpenes, tannins, proanthocyanidins, leucopelargonidin,
alkaloids, and triterpene saponins present. Iridoids, ellagic acid and
cyanogenic compounds not found. Carbohydrates sometimes stored as
oligosaccharides with kestose or isokestose bonds (inulin).
Use Medicinal plants.
Systematics
Marcgravia (c 100; southern Mexico, Central America, the West Indies,
tropical South America); ‘Sarcopera’ (7; S. anomala,
S. cordachida, S. flammifera, S. oxystylis, S.
rosulata, S. sessiliflora, S. tepuiensis; Honduras, the
Guayana Highlands, northern Andes; non-monophyletic), ’Souroubea’
(19; southern Mexico to Bolivia; non-monophyletic), Norantea (1;
N. guianensis; the West Indies, tropical South America),
Ruyschia (10; tropical America), Schwartzia (18–20; Central
America, northern Andes, southeastern Brazil), Marcgraviastrum (15;
Central America, tropical South America).
Marcgraviaceae are probably
sister to [Balsaminaceae+Tetrameristaceae].
Marcgravia is sister to the remaining
Marcgraviaceae. The
leaves in Marcgravia are distichous. The inflorescence is umbel-like,
racemose, with sterile flowers. The flowers have four sepals, four petals
connate into a calyptra, and nectaries adnate to the sterile aborted flowers.
The remaining Marcgraviaceae have spiral
leaves, inflorescence with exclusively fertile flowers, three or five sepals,
five entirely or partially free petals, and free (not adnate) nectaries.
Marcgraviastrum has an umbel-like racemose inflorescence.
’Sarcopera’ (non-monophyletic) has a spicate inflorescence.
MITRASTEMONACEAE
Makino
|
( Back to Ericales )
|
Makino in Bot. Mag. (Tokyo) 25: 252. Dec 1911,
nom.cons.
Mitrastemonales Makino in Bot.
Mag. (Tokyo) 25: 252. Dec 1911
Genera/species 1/2
Distribution Northeastern
India, Japan, the Ryukyu Islands, Taiwan, Indochina, Malesia to New Guinea,
southern Mexico, Guatemala, northwestern Colombia.
Fossils Unknown.
Habit Bisexual,
achlorophyllous, herbaceous endophytes without rhizome or normal roots.
Holoendoparasites on roots of Fagaceae
(Castanopsis, Lithocarpus, Quercus, and
Trigonobalanus).
Vegetative anatomy Mycorrhiza
absent. Hypha-like threads of cells invading host plant and anastomosing
forming reticulum in host root phloem. Phellogen absent. Secondary lateral
growth absent. Vessels absent. Imperforate tracheary xylem elements? Wood rays
absent. Axial parenchyma absent. Sieve tube plastids? Nodes absent.
Crystals?
Trichomes Hairs absent.
Leaves Opposite, reduced,
scale-like (uppermost scales storing nectar), or absent. Stipules and leaf
sheath absent. Venation? Stomata abnormal. Cuticular wax crystalloids cushion-
or granule-shaped. Leaf margin entire.
Inflorescence Flowers
terminal, solitary (flowers sometimes forming ‘fairy rings’ on ground).
Flowers Actinomorphic.
Hypogyny. Tepals usually four (rarely five or six), with decussate-imbricate
aestivation, somewhat petaloid, connate in lower part. Nectar-secreting stomata
present on ovary and on base of androecium and upper scales. Disc?
Androecium Stamens numerous,
entirely connate into cone-shaped tubular synandrous structure surrounding
gynoecium, except for apical pore. Anthers arranged in several successive
vertical whorls on different heights each whorl with approx. ten anthers,
dorsifixed, non-versatile, polysporangiate (polythecate), extrorse, longicidal
(anther whorls dehiscing circumscissely, splitting when pushed up by expanding
style). Tapetum secretory? Staminodia absent.
Pollen grains
Microsporogenesis simultaneous? Pollen grains usually diporate or triporate
(rarely tetraporate or possibly dicolpate?), shed as monads, bicellular at
dispersal. Exine intectate?; ectexine reduced to small tubercles.
Gynoecium Pistil composed of
eight to 15 connate carpels. Ovary superior, unilocular. Style single, simple,
stout. Stigma hemispherical, sub-bilobate, type? Pistillodium absent.
Ovules Placentation parietal,
with eight to 20 intrusive placentae. Ovules numerous per ovary, anatropous,
unitegmic, tenuinucellar. Integument approx. two cell layers thick. Funicular
obturator present. Megagametophyte monosporous, Polygonum type.
Endosperm development ab initio cellular. Endosperm haustoria? Embryogenesis
solanad?
Fruit A many-seeded baccate
pyxidium (dehiscing by horizontal slit along groove between style and
ovary).
Seeds Aril absent. Funicle
short, viscid. Seed coat endotestal. Exotestal cells with massive U-shaped
thickenings. Endotesta? Perisperm not developed. Endosperm one-layered, oily,
enclosing embryo. Embryo undifferentiated, quadricellular, without chlorophyll?
Cotyledons two. Germination?
Cytology n = 20
DNA
Phytochemistry Unknown.
Use Unknown.
Systematics
Mitrastemon (2; M. matudae: southern Mexico,
Guatemala, northwestern Colombia; M. yamamotoi: northeastern
India, Japan, the Ryukyu Islands, Taiwan, Indochina, Malesia to New Guinea).
Mitochondrial DNA indicates a position within
the Ericales (perhaps close to
Ericaceae). However, Rose
& al. recovered the clade [Mitrastemonaceae+Lecythidaceae] with weak
support.
Reveal (in Taxon 59: 299–300) proposed to
conserve the generic name Mitrastemon.
Brown, Prodr. Fl. Nov.-Holl.: 532. 27 Mar 1810
[’Myrsineae’], nom. cons.
Lysimachiaceae Juss.,
Gen. Plant.: 95. 4 Aug 1789 [’Lysimachiae’];
Anagallidaceae Batsch ex Borkh., Bot. Wörterb. 1:
19. 1797 [’Anagallides’]; Ardisiaceae
Juss. in Ann. Mus. Natl. Hist. Nat. 15: 350. 1810;
Myrsinales R. Br. ex Bercht. et J. Presl, Přir.
Rostlin: 251. Jan-Apr 1820 [‘Myrsineae’];
Myrsinopsida Bartl., Ord. Nat. Plant.: 121, 158. Sep
1830 [’Myrsineae’]; Aegicerataceae
Blume, Nov. Plant. Expos.: 17. Aug-Dec 1833 [’Aegicereae’];
Lysimachiales Döll, Rhein. Fl.: 385. 24-27 Mai 1843
[‘Lysimachiae’]; Ardisiales J. Presl in
Nowočeská Bibl. [Wšobecný Rostl.] 7: 1025. 1846
[’Ardisiaceae’]; Coridaceae J. Agardh,
Theoria Syst. Plant.: 332. Apr-Sep 1858 [’Corideae’];
Embeliaceae J. Agardh, Theoria Syst. Plant.: 140.
Apr-Sep 1858 [’Embelieae’]
Genera/species
34/1.525–2.350
Distribution Cosmopolitan
except polar areas.
Fossils Lysimachia
and Myrsine are reported from the Neogene of Europe.
Habit Usually bisexual (rarely
monoecious, polygamomonoecious or dioecious), evergreen or deciduous trees (in
Aegiceras mangrove trees), shrubs, lianas or suffrutices, perennial or
annual herbs. Cyclamen has a tuber formed by the hypocotyl.
Vegetative anatomy Phellogen
ab initio superficially or deeply seated. Endodermis sometimes prominent?
Vessel elements usually with simple (sometimes scalariform) perforation plates;
lateral pits alternate, usually bordered pits. Imperforate tracheary xylem
elements fibre tracheids or libriform fibres usually with simple pits, usually
septate. Wood rays usually multiseriate, homocellular or heterocellular. Axial
parenchyma paratracheal scanty vasicentric. Wood elements sometimes partially
storied. Sieve tube plastids S type. Nodes usually 1:1, unilacunar with one
leaf trace (sometimes 1:3 or 3:3, unilacunar or trilacunar with three traces).
Secretory cells and schizogenous resinous canals with often reddish or
blackish-red substance. Styloids, crystal sand and acicular calciumoxalate
crystals present. Prismatic crystals often abundant.
Trichomes Hairs unicellular or
multicellular, uniseriate or branched (sometimes articulated, stellate,
dendritic, peltate or lepidote); glandular hairs multicellular, often sunken
and with unicellular or multicellular stalk and multicellular head; secretory
hairs (colleters?) present in some species of Ardisia.
Leaves Usually alternate
(spiral; sometimes opposite or verticillate), simple, entire, often coriaceous
(in Coris ericoid), usually with involute, supervolute or conduplicate
(rarely revolute) ptyxis. Stipules and leaf sheath absent. Petiole vascular
bundles? Venation usually pinnate (rarely palmate). Stomata anomocytic or
anisocytic (rarely helicocytic). Cuticular wax crystalloids as irregular
granules (Aegiceras). Lamina gland-dotted or glandular hairy,
sometimes with extrafloral nectaries. Epidermis often with mucilage cells.
Schizogenous secretory cavities with reddish-brown, red or yellow resin usually
present. Mesophyll sometimes with sclerenchymatous idioblasts. Calciumoxalate
as druses and solitary crystals present in most tropical woody representatives.
Domatia as pockets or hair tufts, or absent. Leaf margin usually entire
(sometimes serrate or crenate).
Inflorescence Terminal or
axillary, spike-, raceme- or umbel-like, corymbose, fasciculate etc., or
flowers solitary. Floral prophylls (bracteoles) absent.
Flowers Usually actinomorphic
(in Coris zygomorphic; calyx in Aegiceras asymmetrical).
Epicalyx (consisting of ten to 15 spine-shaped acute outgrowths below calyx)
present in Coris. Hypogyny. Sepals (three or) four or five (to nine),
usually with imbricate (rarely contorted or valvate) aestivation, free or
connate (median sepal in Coris abaxial). Petals (three or) four or
five (to nine), usually with imbricate or contorted (rarely valvate or
conduplicate) aestivation, usually connate into campanulate or urceolate
corolla (sometimes only at base; corolla lobes in Cyclamen strongly
recurved; in Embelia and Heberdenia free; absent in some
species of Lysimachia [’Glaux’]). Petals and stamens
developing from common primordia (stamens sometimes initiated as adaxial
appendages). Nectariferous hairs often present on petals and carpels; base of
ovary in Coris with nectary. Disc?
Androecium Stamens (three or)
four or five (to nine), obhaplostemonous, alternisepalous, antepetalous.
Filaments free or connate; usually adnate to corolla tube (epipetalous).
Anthers dorsifixed or basifixed, sometimes versatile, tetrasporangiate (in
Aegiceras transversely septate, locellate), introrse, usually
longicidal (dehiscing by longitudinal slits; rarely poricidal, dehiscing by
apical pore-like slits), usually free (often connivent into cone-shaped
structure around style; rarely connate). Tapetum secretory, with uninucleate
cells. Female flowers often with staminodia.
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually 3(–5)-colporate or
3(–5)-colpate (rarely pantocolporate), shed as monads, bicellular at
dispersal. Exine tectate or semitectate, with columellate infratectum,
reticulate, scabrate or psilate.
Gynoecium Pistil composed of
three to five (or six) connate antepetalous carpels. Ovary superior,
unilocular, nectariferous. Style usually single, simple (sometimes absent).
Stigma punctate, capitate or truncate (rarely discoid or fimbriate), papillate,
Dry or Wet type. Male flowers often with pistillodium?
Ovules Placentation free
central to basal. Ovules several (in Coris five or six) to numerous
per ovary, usually anatropous (in Cyclamen campylotropous, in
Coris hemianatropous), ascending to horizontal, usually bitegmic (in
Aegiceras and Cyclamen unitegmic), usually tenuinucellar (in
Aegiceras crassinucellar?). Micropyle usually bistomal (in
Coris endostomal). Outer integument two or three cell layers thick.
Inner integument two or three (to seven) cell layers thick. Integument in
Aegiceras four or five cell layers thick, in Cyclamen approx.
two cell layers thick. Parietal tissue approx. four cell layers thick
(Cyclamen). Archespore multicellular. Megagametophyte monosporous,
Polygonum type. Endosperm development ab initio nuclear. Endosperm
haustoria usually absent (in Aegiceras developed from secondary
endosperm tissue and invading funicle and integument). Embryogenesis usually
caryophyllad (rarely onagrad). Polyembryony sometimes present.
Fruit A drupe, berry or
capsule, often dehiscing irregularly (in some species of Lysimachia a
pyxidium). Placentae juicy.
Seeds Aril absent. Funicle in
Aegiceras not vascularized. Testa multiplicative. Exotesta? Endotestal
cells in Cyclamen with crystals and U-shaped wall thickenings. Tegmen
at first thick, finally crushed. Endotegmen sometimes with crystals. Perisperm
not developed. Endosperm usually copious (often ruminate; absent in
Aegiceras), oily and amyloidiferous (in Cyclamen with thick
and pitted cell walls). Embryo usually straight (sometimes curved; in
Cyclamen diagonal; in Aegiceras very large), without
chlorophyll. Cotyledons usually two (in Cyclamen one), short (in
Aegiceras connate into tube around plumule). Germination
phanerocotylar or cryptocotylar (vivipary present in Aegiceras).
Cytology n = 10–13, 15, 17,
23 (48) – Polyploidy occurring.
DNA Two deletions present in
the plastid gene ndhF.
Phytochemistry Flavonols
(kaempferol, quercetin, myricetin) and their glycosides, cyanidin, delphinidin,
alkaloids, triterpene saponins, and benzoquinones present. Ellagic acid and
cyanogenic compounds not found.
Use Ornamental plants,
medicinal plants.
Systematics (under
construction) Coris (1; C. monspeliensis; western and central
Mediterranean, Somalia), Ardisiandra
(3; A. primuloides, A. sibthorpioides, A.
wettsteinii; mountains in tropical Africa), Lysimachia
(c 175; temperate and subtropical regions on both hemispheres, with their
highest diversity in East Asia), Cyclamen
(22; southern Europe, the Mediterranean to the Caucasus and Iran, northeastern
Somalia), Embelia (c 130; tropical and subtropical regions in the Old
World), Grenacheria (12; Malesia), Heberdenia
(1; H. bahamensis; Madeira, the Canary Islands), Pleiomeris
(1; P. canariensis; the Canary Islands), Myrsine
(c 300; tropical to warm-temperate regions on both hemispheres), Ardisia
(250–1.050; tropical to warm-temperate regions of Asia and America, few
species in Australia), Hymenandra (17; tropical Asia, Nicaragua to
Colombia), Solonia (1; S. reflexa; eastern Cuba),
Geissanthus (50–55; western tropical South America),
Emblemantha (1; E. urnulata; Sumatra), Sadiria (4;
S. aberrans, S. boweri, S. erecta, S.
subsessilifolia; Assam, eastern Himalayas), Stylogyne (c 60;
Mexico, Central America, the West Indies, tropical South America),
Ctenardisia (5; C. amplifolia, C. ovandensis, C.
purpusii, C. speciosa, C. stenobotrys; Central Amerca,
tropical South America), Antistrophe (6; A. caudata, A.
curtisii, A. glabra, A. oxyantha, A.
serratifolia, A. solanoides; tropical Asia from northern India to
Southeast Asia and Malesia), Parathesis (70–80; tropical America),
Aegiceras (1; A. corniculatum; coastal areas in Southeast
Asia to islands in the Pacific), Amblyanthus (3; A.
glandulosus, A. multiflorus, A. obovatus; eastern
Himalayas to Southeast Asia, Malesia to New Guinea), Amblyanthopsis
(3; A. bhotanica, A. membranacea, A. philippinensis;
the Himalayas to Southeast Asia and West Malesia), Elingamita (1;
E. johnsonii; Three Kings Islands in New Zealand), Loheria
(10; the Philippines, New Guinea), Cybianthus (150–160; Mexico,
Central America, the West Indies, tropical South America), Vegaea (1;
V. pungens; Hispaniola), Oncostemum (c 90; Madagascar, the
Mascarene Islands), Badula
(17; Madagascar, Mauritius, Réunion), Discocalyx
(c 105; Central Malesia to tropical Australia, Melanesia and Polynesia, with
their largest diversity in the Philippines and on New Caledonia),
Labisia (17; Southeast Asia, Malesia), Systellantha (3;
S. brookeae, S. fruticosa, S. serratifolia; Borneo),
Monoporus (9; Madagascar), Fittingia (7; F.
carnosifolia, F. conferta, F. grandiflora, F.
mariae, F. tuberculata, F. tubiflora, F.
urceolata; New Guinea), Conandrium (2; C. polyanthum,
C. rhynchocarpum; East Malesia to New Guinea and the Bismarck
Archipelago).
Myrsinaceae are sister to Primulaceae.
Herbaceous lineages such as Coris, Ardisiandra,
Lysimachia
and Cyclamen
are probably basal in Myrsinaceae. Phylogenetic analyses
of the entire Myrsinaceae
are in demand.
PENTAPHYLACACEAE
Engler
|
( Back to Ericales )
|
Engler in Engler et Prantl, Nat. Pflanzenfam.,
Nachtr. 1: 214. Oct 1897, nom. cons.
Genera/species 1/1
Distribution Southeastern
China, northern Indochina, the Malay Peninsula, Sumatra.
Fossils Uncertain. Fossil
fruits assigned to Pentaphylax are described from the Maastrichtian of
Central Europe, yet their affinity is questioned.
Habit Bisexual or unisexual,
evergreen tree or shrub.
Vegetative anatomy Phellogen?
Pericyclic envelope consisting of fibres alternating with lignified parenchyma
cells. Vessel elements with scalariform perforation plates; lateral pits
opposite to scalariform, simple and/or bordered pits. Imperforate tracheary
xylem elements tracheids usually with bordered (sometimes simple) pits,
non-septate. Wood rays uniseriate or multiseriate, heterocellular? Axial
parenchyma apotracheal diffuse, diffuse-in-aggregates or in short tangential
lines. Articulated laticifers abundant. Sieve tube plastids S type? Nodes?
Druses absent. Crystals?
Trichomes Hairs simple.
Leaves Leaves alternate
(spiral or distichous), simple, entire or lobed, with supervolute ptyxis.
Stipules and leaf sheath absent. Buds perulate (perulae present). Petiole
vascular bundle transection annular. Venation pinnate, often indistinct.
Stomata usually paracytic. Cuticular wax crystalloids? Lamina with blackish,
caducous and probably glanduliferous apex. Leaf margin serrate or entire.
Inflorescence Up to c. 15
flowers in terminal or axillary racemose inflorescences, with flowers
principally solitary in axils of reduced leaves; when floral shoot strongly
reduced, then inflorescence becoming fasciculate. Bracts with black, caducous
and probably glanduliferous apex. Floral prophylls (bracteoles) two.
Flowers Actinomorphic.
Hypogyny. Sepals five, with imbricate quincuncial aestivation, free or more or
less connate. Some sepals with black, caducous and probably glanduliferous
apex. Petals five, with imbricate quincuncial aestivation, free or more or less
connate. Tepals with well developed and distinct canals. Nectary present on
ovary wall. Disc absent.
Androecium Stamens five,
haplostemonous, alternisepalous, antepetalous. Filaments very wide and
relatively long, narrowing distally and incurved at apex, free, tightly pressed
against petals and forming tube. Anthers basifixed, non-versatile?,
tetrasporangiate, introrse, valvicidal (dehiscing by uplifted subapical valve).
Tapetum secretory. Female flowers with staminodia?
Pollen grains
Microsporogenesis simultaneous? Pollen grains tricolporate, shed as monads,
bicellular at dispersal. Exine tectate, with intermediate infratectum
(columellae poorly developed), smooth. Tectum thin. Endexine thick.
Gynoecium Pistil composed of
five connate antepetalous carpels. Ovary superior, unilocular. Style single,
simple, hollow. Stigmatic areas shortly radiate, type? Male flowers with
pistillodium?
Ovules Placentation
apical-axile. Ovules two per carpel, campylotropous to hemitropous, pendulous,
bitegmic, crassinucellar. Micropyle ?-stomal. Outer integument approx. three?
cell layers thick. Inner integument ? cell layers thick. Megagametophyte
monosporous, Polygonum type. Endosperm development? Endosperm
haustoria? Embryogenesis?
Fruit A loculicidal capsule,
with midrib separating from rest of valves. Endocarp cells transversely
elongate.
Seeds Seeds flattened Aril
absent. Seed coat winged. Exotestal cells somewhat thickened, with theoid
exotestal thickenings, elongate. Mesotestal cells large, thin-walled.
Endotesta? Tegmen? Perisperm not developed. Endosperm sparse. Embryo straight?,
chlorophyll? Cotyledons longer than radicula. Germination phanerocotylar.
Cytology n = ?
DNA
Phytochemistry Virtually
unknown. Aluminium accumulated.
Use Unknown.
Systematics
Pentaphylax (1; P. euryoides; Guangdong, Hainan, northern
Indochina, the Malay Peninsula, Sumatra).
Pentaphylax is sister to Ternstroemiaceae.
POLEMONIACEAE Juss.
|
( Back to Ericales )
|
de Jussieu, Gen. Plant.: 136. 4 Aug 1789
[’Polemonia’], nom. cons.
Polemoniales Juss. ex
Bercht. et J. Presl, Přir. Rostlin: 248. Jan-Apr 1820
[‘Polemoniae’]; Cobaeaceae D. Don in
Edinburgh Philos. J. 10: 111. Jan 1824 [’Cobeaceae’];
Cobaeineae Link, Handbuch 1: 822. 4-11 Jul 1829
[‘Cobaeaceae’]; Polemoniineae Bessey in
C. K. Adams, Johnson’s Universal Cyclop. 8: 463. 15 Nov 1895
[‘Polemoniales’]
Genera/species 23/375–380
Distribution Temperate and
arctic Eurasia, the Himalayas, North America including arctic areas, Mexico,
Central America, northern and western South America to southern Chile and
southern Argentina, with their largest diversity in western North America.
Fossils The herbaceous fossil
Gilisenium hueberi from the mid-Eocene of Utah has been assigned to
Polemoniaceae.
Habit Bisexual, usually
perennial, biennial or annual herbs (sometimes shrubs or suffrutices; rarely
lianas; in Cantua trees). Often evil-smelling.
Vegetative anatomy Phellogen
ab initio usually superficial (sometimes pericyclic). Cambium usually not
storied. Endodermis sometimes prominent. Vessel elements usually with simple
(rarely also scalariform) perforation plates; lateral pits alternate, bordered
pits. Imperforate tracheary xylem elements tracheids, fibre tracheids or
libriform fibres with simple or bordered pits, septate or non-septate. Wood
rays uniseriate or multiseriate, heterocellular, or absent. Axial parenchyma
paratracheal scanty vasicentric. Wood elements often storied. Sieve tube
plastids S type. Nodes 1:1, unilacunar with one leaf trace. Phloem in
Cobaea with secretory cavities containing yellow secretion.
Crystals?
Trichomes Hairs multicellular,
usually uniseriate (in, e.g., Langloisia branched), sometimes
arachnoid, bristle-shaped or dendritic (sometimes calcified/silicified);
glandular hairs with unicellular or multicellular head often present.
Leaves Usually alternate
(spiral; in Linanthus and Phlox opposite; in
Gymnosteris verticillate), simple or pinnately or palmately compound,
entire or lobed (in Cobaea with terminal leaflet modified into
tendril; in Acanthogilia dimorphic, with young leaves pinnately
compound and older leaves simple and entire), with conduplicate ptyxis.
Stipules usually absent (in Cobaea intrapetiolar, foliaceous); leaf
sheath absent. Colleter-like glandular hairs often present on adaxial side of
petiole base. Petiole vascular bundles? Venation usually pinnate (sometimes
palmate). Stomata usually anomocytic (sometimes almost paracytic). Cuticular
wax crystalloids? Epidermis with or without mucilage cells. Leaf margin lobed,
serrate or entire. Multicellular bristles present in Langloisia and
some species of Loeselia.
Inflorescence Usually terminal
(in Acanthogilia axillary), umbel-like, capitate, corymbose, panicle,
thyrsopaniculate or botryoid (flowers rarely solitary). Inflorescences
sometimes pseudanthia with involucral bracts. Floral prophylls (bracteoles)
absent.
Flowers Usually actinomorphic
(sometimes zygomorphic). Hypogyny. Sepals (four or) five (or six), with
imbricate, valvate or open aestivation, persistent, connate (rarely almost
free). Petals (four or) five (or six), usually with convolute or
contortuplicate (rarely imbricate) aestivation, connate into campanulate,
infundibuliform or discoid corolla; median petal adaxial or abaxial; corolla
sometimes bilabiate (e.g. 2:3, 3:2 or 5:0). Nectariferous disc intrastaminal,
annular, entire or lobed (rarely absent).
Androecium Stamens (three to)
five (or six), usually as many as sepals, haplostemonous, antesepalous,
alternipetalous, often unequal in length. Filaments free from each other,
usually adnate to corolla tube (epipetalous; sometimes free from petals).
Anthers usually ventrifixed (rarely dorsifixed), in Cobaea versatile,
tetrasporangiate, introrse?, longicidal (dehiscing by longitudinal slits).
Tapetum usually secretory (in Cobaea amoeboid-periplasmodial?).
Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually (tetra- to) hexa- to c.
50-pantoporate (rarely zonotreme, bizonotreme, anomotreme/porate or
[stephano]colp[or]ate; in Acanthogilia zonotreme), shed as monads,
bicellular at dispersal. Exine tectate to semitectate, with columellate
infratectum, reticulate, striate or verrucate.
Gynoecium Pistil composed of
(two or) three (or four) connate carpels, median carpel adaxial. Ovary
superior, (bilocular or) trilocular (or quadrilocular). Style single, filiform,
usually trilobate. Stigmas decurrent along entire lobes, papillate, Dry type.
Pistillodium absent.
Ovules Placentation axile.
Ovules one to numerous per carpel, anatropous to hemitropous, apotropous,
unitegmic, usually tenuinucellar (in Cobaea pseudocrassinucellar).
Integument three to 20 cell layers thick. Nucellar cap present at least in
Cobaeoideae. Megagametophyte monosporous, Polygonum type.
Endosperm development ab initio nuclear (endosperm finally cellular). Endosperm
haustoria absent. Embryogenesis chenopodiad.
Fruit Usually a loculicidal
(in Acanthogilia and Cobaea septicidal) capsule (rarely a
nut).
Seeds Aril?Seed coat sometimes
winged, usually mucilaginous when wet. Exotesta of various thickness, with
thickened radial cell walls. Epidermal cells often cylindrical with distinct
spiral wall thickenings and mucilage. Endotesta reduced to pigmentary layer.
Perisperm not developed. Endosperm usually copious (sometimes sparse), oily
(absent in Cobaea). Embryo usually straight (sometimes curved), well
differentiated (Cobaea), with or without chlorophyll. Cotyledons two.
Germination usually phanerocotylar (in Cobaea cryptocotylar).
Cytology n = 6–12, with
large chromosomes (Polemonioideae); n = (9, Acanthogilia) 15,
26, 27, with small chromosomes (Cobaeoideae) – Polyploidy and
aneuploidy occurring.
DNA Mitochondrial intron
coxII.i3 lost.
Phytochemistry Flavonols
(kaempferol, quercetin), 6-metoxyflavonols, flavone-C-glycosides etc.,
cyanidin, oleanolic acid derivatives, triterpene saponins, and cucurbitacins
present. Iridoids, ellagic acid, alkaloids, and cyanogenic compounds not found.
Carbohydrates stored as oligosaccharides with kestose and isokestose bonds
(inulin).
Use Ornamental plants.
Systematics Polemoniaceae are sister to
Fouquieriaceae.
Cobaeoideae Arn.,
Botany: 121. 9 Mar 1832 [‘Cobaeeae’]
3/31–33. Cantua (12; the
Andes in Ecuador, Peru and Bolivia), Cobaea
(18–20; Mexico, Central America, tropical South America), Bonplandia
(1; B. geminiflora; Mexico, Guatemala). – Baja California in
northwestern Mexico to tropical South America. Twining herbs, lianas, trees.
Stipules in Cobaea
intrapetiolar, foliaceous. Flowers large, in Cobaea
somewhat zygomorphic. Sepals largely connate (sometimes only at base), with
green midrib. Petal veins united at base of lobe (sometimes also upper lobe).
Staminal traces present in two whorls. Filaments often superficially adnate to
corolla. Pollen grains sometimes 100–220 μm, with verrucate exine. Nucellar
cap present. Fruit a septicidal and/or loculicidal capsule. Seed coat narrowly
or broadly winged. Mesotestal cell walls thinly lignified. n = 15, 26, 27. –
Cobaeoideae may be sister-group to
[Acanthogilioideae+Polemonioideae] (Johnson & al.
2008).
Acanthogilioideae J.
M. Porter et L. A. Johnson in Aliso 19: 60. 28 Jul 2000
1/1. Acanthogilia (1; A.
gloriosa; Baja California in northwestern Mexico). – Shrub. Leaves very
dimorphic, deciduous on short shoots, modified into branched spines on long
shoots. Sepals with green midrib. Pollen grains stephanocolporate. Exine
corsely verrucate. Seeds few. n = 9. – Acanthogilia is sister to
either the clade [Cobaeoideae+Polemonioideae],
Cobaeoideae or Polemonioideae. Johnson & al. (2008)
recovered Acanthogilia as sister to Polemonioideae.
Polemonioideae Arn.,
Botany: 121. 9 Mar 1832 [‘Polemonieae’]
19/345–350. Western North America,
northern temperate regions, southern South America. Usually herbs (sometimes
suffrutices). Flowers sometimes zygomorphic. Petal veins usually free or united
high above base. Filaments usually adnate to corolla tube (free from petals in,
e.g., Collomia and
Loeselia.
Hypostase present. Seed coat usually not winged (in Loeselia narrowly
winged). Testa sometimes multiplicative. n = (6) 7 (8) 9 (or more). – A
plausible topology is
[[Polemonieae+Phlocideae]+[Gilieae+Loeselieae]]
with high posterior probability but low bootstrap support (Johnson & al.
2008).
[Polemonieae+Phlogieae]
Polemonieae Dumort.,
Anal. Fam. Plant.: 25. 1829 [‘Polemoneae’].
1/c 30. Polemonium
(c 30; temperate regions on the Northern Hemisphere south to Mexico, Chile).
– Usually perennial (P. micranthum annual) herbs. Leaves alternate
(spiral), pinnate. Calyx accrescent. Seeds dark, shiny. – Polemonium
has sometimes been recovered as sister to the remaining
Polemonioideae, to Phlocideae, or to
[Gilieae+Phlocideae].
Phlocideae J. M.
Porter et L. A. Johnson in Aliso 17: 84. 8 Jun 1998 (Phlocideae
Dumort., Anal. Fam. Plant.: 25. 1829).
3/120–125. Linanthus
(c 55; southwestern Canada, western United States, Mexico),
Gymnosteris (2; G. nudicaulis, G. parvula; western
United States), Phlox
(65–70; northeastern Asia, Canada, United States, northern Mexico, one
species in Siberia). – Northeastern Asia, North America to northern Mexico.
Leaves usually opposite. Corolla usually hypocraterimorphous. – Linanthus
is sister to the remainder.
[Loeselieae+Gilieae]
Loeselieae J. M.
Porter et L. A. Johnson in Aliso 17: 84. 8 Jun 1998.
9/c 97. Aliciella (21; western
United States, northwestern Mexico), Giliastrum (10; western United
States, Mexico, Central America), Loeselia (14; California),
Dayia (5; D. glutinosa, D. grantii, D.
havardii, D. scabra, D. sonorae; southern United States,
northern and northwestern Mexico, northern Chile), Bryantiella (1;
B. palmeri; southern California; in Ipomopsis?),
Ipomopsis
(c 30; southwestern Canada, United States, Mexico, Chile, Argentina;
non-monophyletic?), Microgilia (1; M. minutiflora; western
United States), Eriastrum (14; southwestern United States), Langloisia
(1; L. setosissima; southwestern United States, northern Mexico). –
Style usually persistent in fruit. – Aliciella is sister-group to
the remaining Loeselieae.
Gilieae (Rchb.) V. E.
Grant, Nat. Hist. Phlox Fam.: 120. 1959.
6/c 100. Saltugilia (4; S.
australis, S. caruifolia, S. latimeri, S.
splendens; California, Baja California); Lathrocasis (1; L.
tenerrima; western United States, northwestern Mexico), ’Gilia’
(c 40; southwestern Canada, western United States, Mexico to northern Chile;
non-monophyletic), Allophyllum (5; A. divaricatum, A.
gilioides, A. glutinosum, A. integrifolium, A.
nemophilophyllum; western United States), Collomia
(15; southwestern Canada, western United States, Mexico, Bolivia to temperate
Chile and Argentina), Navarretia
(35; southwestern Canada, western United States, northern Mexico, Chile,
Argentina). – Saltugilia is sister to the rest.
 |
Cladogram of Polemoniaceae based on
DNA sequence data (Johnson & al. 2008). Acanthogilia is
sometimes recovered as sister to Cobaeoideae and/or
Polemonioideae.
|
PRIMULACEAE Batsch ex
Borkh.
|
( Back to Ericales )
|
Borkhausen, Bot. Wörterbuch 2: 240. 1797
[‘Primulae’], nom. cons.
Primulales Juss. ex
Bercht. et J. Presl, Přir. Rostlin: 241. Jan-Apr 1820 [‘Primulaceae’];
Primulineae Burnett, Outlines Bot.: 901, 1101. Feb
1835 [‘Primulosae’]; Primulopsida
Brongn., Enum. Plant. Mus. Paris: xxi, 69. 12 Aug 1843
[’Primulineae’]; Hottoniaceae Döll, Fl.
Baden 2: 641. med 1858 [’Hottonieae’]
Genera/species 6/590–600
Distribution Temperate and
arctic-alpine regions on the Northern Hemisphere, tropical mountains in East
Africa, the Arabian Peninsula and Malesia, southernmost South America.
Fossils Fossil long-stalked
ebracteate hypogynous to almost hypogynous pentamerous flowers of primuloid
origin were described from the Campanian to the Maastrichtian of Portugal. The
ovary is unilocular and the placentation is free central, with numerous
anatropous ovules. Most of the outer tissues are beset with schizogenous
secretory cavities.
Habit Bisexual, perennial or
annual herbs (sometimes cushion-shaped and woody at base), usually with a basal
leaf rosette. Some species are xerophytes. Hottonia is aquatic.
Vegetative anatomy Phellogen?
Primary vascular tissue a cylinder without separate vascular bundles, or a
cylinder of bundles or scattered bundles. Secondary lateral growth anomalous or
absent. Endodermis usually prominent. Vessel elements with simple perforation
plates; lateral pits alternate. Imperforate tracheary xylem elements libriform
fibres with simple or bordered pits. Wood rays multiseriate, heterocellular?
Axial parenchyma? Sieve tube plastids S type. Nodes 1:1, unilacunar with one
leaf trace. Secretory cells and schizogenous resinous canals present.
Crystals?
Trichomes Hairs multicellular,
uniseriate, articulated, often long, stellate hairs present in some species of
Androsace and Primula; glandular hairs short; immersed
glandular hairs absent.
Leaves Usually alternate
(sometimes opposite or verticillate), usually simple (rarely pinnately
compound), usually entire (rarely pinnately lobed), with involute or
conduplicate (sometimes revolute) ptyxis. Stipules or leaf sheath absent.
Petiole vascular bundles? Venation pinnate or palmate; marginal veins ending
with a hydathode. Stomata usually anomocytic. Cuticular wax crystalloids as
threads, chemically dominated by flavonoids. Lamina in Omphalogramma,
Bryocarpum and some species of Primula with black flattened
glandular dots. Sclereids present in some species. Schizogenous or lysigenous
secretory cavities? Leaf margin serrate, crenate or entire.
Inflorescence Terminal or
axillary, verticels, a single umbel, raceme, spike, or head, or cymose and
usually long-stalked (scapose), or flowers solitary. Floral prophylls
(bracteoles) absent.
Flowers Usually actinomorphic
(rarely somewhat zygomorphic). Hypogyny. Sepals five (to eight), usually with
imbricate aestivation, usually persistent, often connate. Petals five (to
eight), usually with imbricate aestivation, connate into campanulate or discoid
(hypocraterimorphous) corolla (corolla lobes in Soldanella fimbriate,
in some species of Primula [’Dodecatheon’] strongly
recurved). Petals and stamens developing from common primordia. Nectaries
present on ovary. Disc absent. Diheterostyly frequent.
Androecium Stamens five (to
eight), haplostemonous, antepetalous, alternisepalous. Filaments free, adnate
to corolla tube (epipetalous). Anthers sometimes connivent and strongly
protruding, dorsifixed or basifixed, non-versatile?, tetrasporangiate,
introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory, with
uninucleate cells. Staminodia usually absent (in Soldanella
extrastaminal, antesepalous, scale-like).
Pollen grains
Microsporogenesis simultaneous. Pollen grains (2–)3(–10)-colporate,
-colporoidate or -colpate (in many species of Primula syncolpate or
stephanocolpate), shed as monads, bicellular at dispersal. Exine tectate, with
columellate infratectum, perforate, often granulate.
Gynoecium Pistil composed of
five connate antepetalous (in Primula antesepalous?) carpels. Ovary
superior, unilocular (rarely with rudimentary septa at base), nectariferous.
Style single, simple, with stylar canal. Stigma truncate to capitate, papillate
or non-papillate, Dry type. Pistillodium absent.
Ovules Placentation free
central. Ovules five to more than 100 per ovary, anatropous or hemitropous,
ascending, usually bitegmic (in Androsace [‘Douglasia’]
unitegmic), tenuinucellar. Micropyle bistomal. Outer integument approx. two
cell layers thick. Inner integument three or four cell layers thick. Integument
in ‘Douglasia’ six to ten cell layers thick. Archespore
multicellular. Megagametophyte monosporous, Polygonum type Endosperm
development ab initio nuclear. Endosperm haustoria absent. Embryogenesis
caryophyllad. Polyembryony present in some species.
Fruit A denticidal or
irregularly dehiscing capsule (in one? species of Androsace
[’Pomatosace’] a pyxidium).
Seeds Aril absent. Exotesta
persistent; exotestal cell walls often thick. Endotesta in Primula
with thick inner cell walls. Exotegmen? Endotegmen often with rhomboidal
crystals. Perisperm not developed. Endosperm usually copious, usually with
thick and pitted cell walls, oily and amyloidiferous (absent in
Hottonia and Soldanella). Embryo short, straight, without
chlorophyll. Cotyledons two, short. Germination phanerocotylar.
Cytology x = 8–12 –
Polyploidy frequently occurring.
DNA Plastid gene infA
lost/defunct (Primula). Two deletions in plastid gene
ndhF.
Phytochemistry Flavonols
(kaempferol, quercetin, myricetin), cyanidin, oleanolic acid derivatives,
ellagic acid (at least in Hottonia), proanthocyanidins
(prodelphinidins), alkaloids, triterpene saponins, benzoquinones (rapanone),
and cucurbitacins (absent from Soldanella) present. Cyanogenic
compounds not found.
Use Ornamental plants,
medicinal plants.
Systematics Androsace
(c 110; temperate regions on the Northern Hemisphere, with their largest
diversity in alpine regions), Primula
(c 450; temperate and alpine regions on the Northern Hemisphere, tropical
mountains in Ethiopia and tropical Asia to high mountains on Java and New
Guinea, southern Andes, with their largest diversity in eastern Himalayas to
western China), Omphalogramma (15; the Himalayas, western China), Hottonia
(2; H. palustris: Europe, western Asia; H. inflata:
southeastern Canada, eastern United States), Soldanella
(16; mountain regions in central and southern Europe), Bryocarpum (1;
B. himalaicum; eastern Himalayas).
Primulaceae are sister-group to
Myrsinaceae. Androsace
is sister to the clade [Primula+[Omphalogramma+[Hottonia+Soldanella]]],
according to Trift & al. (2002).
 |
Cladogram of Primulaceae based on DNA
sequence data (Trift & al. 2002).
|
RORIDULACEAE Augier ex
Martinov
|
( Back to Ericales )
|
Martinov, Tekhno-Bot. Slovar: 549. 3 Aug 1820
[’Roriduleae’], nom. cons.
Roridulales Bercht.
et J. Presl, Přir. Rostlin: 217. Jan-Apr 1820 [‘Roridulae’]
Genera/species 1/2
Distribution Mountains in
Western Cape in southwestern South Africa.
Fossils Unknown.
Habit Bisexual, evergreen
shrubs. Semicarnivorous (producing resins without digesting enzymes); living in
mutualistic relationship with two species of carnivorous hemipterans,
Pameridea marlothii (on Roridula dentata) and P.
roridulae (on both species of Roridula), which feed on insects
trapped by the sticky resins from the glandular hairs. The urea excreted by the
bugs is absorbed by the Roridula plant through the leaf surface. The
Pameridea species have not been found in nature outside
Roridula.
Vegetative anatomy Mycorrhiza
absent? Phellogen? Stem endodermis continuous and starchy; inside this a
sclerenchymatous ring. Primary medullary rays narrow. Pericyclic fibres absent.
Vessel elements with scalariform or reticulate perforation plates; lateral pits
usually alternate, scalariform or opposite, bordered pits. Imperforate
tracheary xylem elements tracheids with bordered pits. Wood rays usually
uniseriate (rarely biseriate or multiseriate), homocellular. Axial parenchyma
apotracheal diffuse, or paratracheal scanty. Sieve tube plastids Ss type.
Nodes? Wood ray cells sometimes with gum-like deposits. Calciumoxalate druses
sometimes present.
Trichomes Foliar hairs
unicellular bristle-like and multicellular glandular hairs; glandular hairs
stalked, capitate, resin-producing; gland stalk uniseriate to quinqueseriate;
head sexacellular to multicellular, in larger glandular hairs with central
column bearing resin-producing cells.
Leaves Alternate (spiral),
simple, entire to narrowly pinnately lobed (lamina linear, narrowing towards
apex), with ? ptyxis. Stipules and leaf sheath absent. Petiole vascular
bundles? Venation brochidodromous. Stomata anomocytic. Cuticular wax
crystalloids? Epidermis with calciumoxalate druses. Leaf margin entire or
narrowly lobate. Resin-producing glandular hairs numerous along margin and
along midvein on abaxial side, also elsewhere.
Inflorescence Terminal,
botryoid or raceme-like cymose, or flowers solitary axillary.
Flowers Actinomorphic.
Hypogyny. Sepals five, with quincuncial imbricate aestivation, persistent, free
(connate at base?). Petals five, with quincuncial imbricate aestivation, free
(slightly connate at base?). Nectaries present on staminal bases. Disc
absent.
Androecium Stamens five,
haplostemonous, antesepalous, alternipetalous. Filaments filiform, thicker at
base, free, adnate to petal base, nectariferous. Anthers subbasifixed, in bud
inflexed, early inverted (as mature resupinated), versatile?, tetrasporangiate,
seemingly extrorse, poricidal (dehiscing by apical pores or short slits);
connective swollen at anther base. Fibrous endothecium absent. Tapetum
secretory. Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually tricolpor(oid)ate (rarely
tetracolporate), shed as monads, bicellular at dispersal. Exine tectate, with
granular infratectum, perforate, spinulate (Roridula dentata) or
insular with angular to rounded segments (Roridula gorgonias).
Gynoecium Pistil composed of
three connate carpels. Ovary superior, trilocular. Style single, simple. Stigma
capitate or reversed conical, papillate, type? Pistillodium absent.
Ovules Placentation axile to
apical. Ovules one to four per carpel, anatropous, pendulous, unitegmic,
tenuinucellar. Integument ? cell layers thick. Hypostase? Megagametophyte
monosporous, Polygonum type. Endosperm development cellular. Endosperm
haustorium micropylar. Embryogenesis solanad.
Fruit A loculicidal (and
septicidal?) capsule, with central columella persistent after dehiscence.
Seeds Aril? Exotesta
mucilaginous when moistened; outer epidermal cells of exotesta with thickened
inner tangential and radial walls. Endotesta? Perisperm not developed.
Endosperm copious, fleshy. Embryo small, straight, well differentiated,
chlorophyll? Cotyledons two. Germination phanerocotylar.
Cytology n = 6
DNA
Phytochemistry Insufficiently
known. Route I iridoids (also secoiridoids?) present. Tannins present in
endosperm and integument. Ellagic acid? Proanthocyanidins, cyanogenic compounds
and naphthoquinones not found.
Use Ornamental plants.
Systematics Roridula
(2; mountains in Western Cape; R. dentata: Pakhuis to Elandskloof
Mountains; R. gorgonias: Hottentots Holland to Kleinrivier and
Riviersonderend Mountains).
Roridula
is sister to Actinidiaceae.
The stamens of Roridula
are tigmotactic and the anthers revert instantaneously when touched releasing
their pollen on the pollinator, which may be a specimen of
Pameridea.
Rafinesque in Ann. Gén. Sci. Phys. Bruxelles 5:
349. Jul-Sep 1820 [’Samolia’]
Samolales Dumort.,
Anal. Fam. Plant.: 29. 1829 [‘Samolinarieae’]
Genera/species 1/c 12
Distribution Europe,
Macaronesia, Africa, southwestern Asia to southern China, East Asia to Japan,
southern Australia, Tasmania, New Caledonia, New Zealand, eastern and southern
North America, southern and central South America.
Fossils Unknown.
Habit Bisexual, usually
perennial or annual herbs (sometimes woody at base).
Vegetative anatomy Phellogen?
Vessel elements with simple? perforation plates; lateral pits alternate?
Imperforate tracheary xylem elements libriform fibres? Wood rays absent? Axial
parenchyma? Sieve tube plastids S type? Nodes 1:1?, unilacunar with one leaf
trace? System of secretory canals present especially in inflorescence.
Crystals?
Trichomes Hairs usually
absent; sessile or stalked glandular hairs with one or few stalk cells and
unicellular or multicellular head sometimes present.
Leaves Alternate (spiral),
simple, entire (rarely scale-like), with ? ptyxis. Stipules and leaf sheath
absent. Petiole vascular bundle transection arcuate; bundles probably diverging
very soon after leaf trace departs from central stele. Venation pinnate.
Stomata anomocytic. Cuticular wax crystalloids? Leaf margin entire.
Inflorescence Terminal, simple
or compound raceme or corymbose. Floral prophylls (bracteoles) absent.
Flowers Actinomorphic. Half
epigyny (perigyny). Sepals five, with open aestivation, persistent, connate,
adnate to ovary. Petals five, with imbricate aestivation, connate into
campanulate or urceolate corolla. Petals and stamens developing from common
primordia; stamen primordia initially larger. Nectaries present on ovary. Disc
absent.
Androecium Stamens five,
haplostemonous, antepetalous, alternisepalous. Filaments free, adnate to
corolla tube (epipetalous). Anthers basifixed, non-versatile, tetrasporangiate,
introrse, longicidal (dehiscing by longitudinal slits); connective sometimes
prolonged. Tapetum secretory? Staminodia five, extrastaminal, antesepalous,
alternipetalous, triangular or linear, epipetalous, or absent.
Pollen grains
Microsporogenesis simultaneous? Pollen grains tricolporoidate, shed as monads,
bicellular at dispersal. Exine tectate, with columellate? infratectum, finely
reticulate.
Gynoecium Pistil composed of
five connate antepetalous carpels. Ovary semi-inferior, unilocular,
nectariferous. Style single, simple, impressed. Stigma truncate to capitate,
type? Pistillodium absent.
Ovules Placentation free
central to basal. Ovules numerous per ovary, hemitropous, bitegmic,
tenuinucellar. Micropyle bistomal? Outer integument approx. two cell layers
thick. Inner integument approx. two cell layers thick. Megagametophyte
monosporous, Polygonum type. Endosperm development ab initio nuclear.
Endosperm haustoria absent? Embryogenesis caryophyllad?
Fruit A many-seeded,
loculicidal-denticidal, quinquedentate capsule with five antesepalous
valves.
Seeds Aril absent? Seed coat
indistinct. Exotestal cells tanniniferous. Endotesta? Exotegmen? Endotegmen
tanniniferous, with rhomboidal crystals. Perisperm not developed. Endosperm
copious, with thin to slightly thickened cell walls, amyloidiferous. Embryo
small, straight, chlorophyll? Cotyledons two, narrow. Germination
phanerocotylar?
Cytology n = (12) 13
DNA
Phytochemistry Insufficiently
known. Flavonols (kaempferol, quercetin, myricetin)? and other polyphenols
present. Cyanidin? Delphinidin? Glycosides? Triterpene saponins? Ellagic acid,
alkaloids and cyanogenic compounds not found?
Use Aquarium plants.
Systematics Samolus
(c 12; Europe, Macaronesia, Africa, Southwest Asia to southern China, East Asia
to Japan, southern Australia, Tasmania, New Caledonia, New Zealand, eastern and
southern North America, central to southern South America, with their largest
diversity on the Southern Hemisphere).
Samolus
is sister to Theophrastaceae.
de Jussieu, Gen. Plant.: 151. 4 Aug 1789
[’Sapotae’], nom. cons.
Achradaceae Vest,
Anleit. Stud. Bot.: 273, 299. 1818 [’Achratoideae’];
Sapotales Juss. ex Bercht. et J. Presl, Přir.
Rostlin: 250. Jan-Apr 1820 [‘Sapoteae’];
Bumeliaceae Barnhart in Bull. Torrey Bot. Club 22:
21. 15 Jan 1895; Sapotineae Engl., Syllabus, ed. 2:
169. Mai 1898; Boerlagellaceae H. J. Lam in Bull.
Jard. Bot. Buitenzorg, sér. 3, 7: 250. Feb 1925;
Sarcospermataceae H. J. Lam in Bull. Jard. Bot.
Buitenzorg, sér. 3, 7: 248. Feb 1925 [’Sarcospermaceae’], nom.
cons.
Genera/species
66/1.200–1.225
Distribution Mainly
pantropical; some species in subtropical regions (southeastern United States,
central South America, southeastern Africa, South Asia).
Fossils Pollen resembling
those in Sapotaceae have been
reported from Eocene and younger layers. Late Oligocene branches and fruits of
putative sapotacean origin are known from Germany.
Habit Usually bisexual
(sometimes monoecious or dioecious, rarely gynomonoecious), evergreen trees or
shrubs (rarely lianas).
Vegetative anatomy Phellogen
ab initio superficial. Medullary vascular bundles sometimes present. Vessel
elements usually with simple (rarely scalariform) perforation plates; lateral
pits alternate, usually simple pits. Imperforate tracheary xylem elements fibre
tracheids or libriform fibres usually with simple (sometimes bordered) pits,
usually non-septate (in Sarcosperma also septate; vasicentric
tracheids abundant). Wood rays uniseriate or multiseriate, homocellular or
heterocellular. Axial parenchyma apotracheal diffuse or diffuse-in-aggregates,
or paratracheal reticulate, scalariform or banded. Tyloses often frequent
(sometimes sclerotic). Sieve tube plastids S type. Nodes usually 3:3,
trilacunar with three leaf traces (rarely 1:1, unilacunar with one trace).
Laticifers articulated, usually with white (rarely yellow or bluish-green)
latex. Wood elements often silicified and/or with silica bodies. Calciumoxalate
crystals as druses, styloids, acicular and prismatic crystals and/or crystal
sand sometimes present.
Trichomes Hairs usually
unicellular (in Delpydora multicellular), uniseriate or T-shaped,
often malpighiaceous, with branches of unequal lengths, often brownish.
Leaves Usually alternate
(spiral or distichous; sometimes opposite or verticillate), simple, entire,
coriaceous, with conduplicate ptyxis. Stipules cauline (intrapetiolar) or
absent; leaf sheath absent. Petiole often swollen at base (in some species of
Sarcosperma and Pradosia with two stipule-like appendages,
stipulules, at apex). Petiole vascular bundle transection arcuate, D-shaped or
annular; petiole sometimes with wing bundles. Venation pinnate, usually
brochidodromous (sometimes eucamptodromous or craspedodromous); secondary veins
often parallel and densely spaced. Stomata usually anomocytic (sometimes
paracytic). Cuticular wax crystalloids? Epidermis with or without mucilage
cells. Mesophyll usually with sclerenchymatous idioblasts; mesophyll fibres
sometimes with spiral thickenings. Special laticifers and cells secreting
gutta-percha. Sclereids present. Domatia as pits. Leaf margin usually entire
(sometimes serrate, rarely serrate-dentate). Two-branched hairs frequent.
Inflorescence Axillary,
usually fasciculate, raceme-like or panicle (sometimes solitary axillary).
Flowers Actinomorphic. Pedicel
not articulated. Hypogyny. Sepals four to six, in one whorl, usually with
imbricate quincuncial aestivation, free or connate in lower part; or six to
eleven, spiral, with imbricate aestivation, free; or 2+2, 3+3 or 4+4, outer
sepals with valvate, inner sepals with imbricate aestivation, free or connate
at base. Petals three to six, 4+4, 5+5 or up to 18, entire, lobed or divided
above, or divided down to base into three segments with mid-segment entire and
two lateral (dorsal) segments entire, lobed or connate. Nectariferous disc
annular or cushion-shaped, or absent.
Androecium Stamens four to c.
35(–43), as many as petals, alternisepalous, antepetalous; or (when more
numerous than petals) some stamens antepetalous and others alternipetalous; or
many stamens in antepetalous fascicles; or numerous stamens (rarely up to six
times as many as petals) in two or three whorls. Filaments usually free (rarely
connate into a tube), usually adnate to corolla tube or corolla lobes. Anthers
basifixed or dorsifixed, sometimes versatile?, tetrasporangiate (in
Magodendron locellate, with septa), introrse to extrorse (in
Sarcosperma sometimes latrorse), longicidal (dehiscing by longitudinal
slits). Tapetum secretory, with binucleate to quadrinucleate cells. Staminodia
up to eight (to twelve), extrastaminal, in one whorl, antesepalous, alternating
with stamens, often adnate to corolla tube, often lobed or divided (sometimes
petaloid), or absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains (2–)3–4(–6)-colporate, shed
as monads, bicellular or tricellular at dispersal. Exine tectate, with
columellate or granular infratectum, punctate, perforate, microreticulate,
reticulate, psilate, scabrate, verruculate, striate or rugulate (rarely
spinulate).
Gynoecium Pistil composed of
(one to) four to 14 (to 30) connate antesepalous carpels. Ovary superior
(unilocular to) quadrilocular to 14-locular (to 30-locular), with hairs inside.
Style single, intire. Stigma punctate or somewhat lobate, papillate, Dry type.
Pistillodium?
Ovules Placentation usually
axile or axile-basal (when ovary unilocular, then basal-parietal). Ovules one
(to five) per carpel, anatropous to hemianatropous, ascending, apotropous,
unitegmic, tenuinucellar. Integument ? cell layers thick. Hypostase absent (not
organized). Megagametophyte monosporous, Polygonum type. Synergids at
least sometimes with a filiform apparatus. Antipodal cells ephemeral. Endosperm
development ab initio nuclear. Endosperm haustorium absent. Embryogenesis?
Fruit Usually a berry (rarely
a drupe or a loculicidal capsule) with persistent calyx.
Seeds Aril absent. Testa
multiplicative. Exotestal cells isodiametric and strongly lignified and
sclerotized (tanniniferous). Endotesta finally crushed. Perisperm not
developed. Endosperm copious to sparse, oily, with or without amyloid?, or
absent. Embryo straight, well differentiated, without chlorophyll. Cotyledons
two, foliaceous. Germination phanerocotylar or cryptocotylar.
Cytology n = (10–)12–14
DNA Mitochondrial intron
coxII.i3 lost (Chrysophyllum).
Phytochemistry Flavonols
(kaempferol, quercetin, myricetin), cyanidin, oleanolic acid derivatives,
C-30-oxidized triterpenes, pentacyclic triterpenes, latex of trans- and/or
cis-polyisoprenes, dammaranes, gallic acid, condensed and hydrolyzable tannins,
proanthocyanidins (prodelphinidins), pyrrolizidine alkaloids as aliphatic
monocarboxylic esters and esters of arylic and aralkylic acids, saponins,
tyrosine- or phenylalanine-derived cyanogenic compounds naphthoquinones,
dihydrosterculic acid, myo-inisitol, and resins present. Ellagic acids
not found.
Use Fruits
(Manilkara, Chrysophyllum), latex (gutta-percha, balata,
chicle, tewing gums), seed oil (Butyrospermum), timber.
Systematics Sapotaceae may be sister to the
clade [Ebenaceae+[Maesa+[Samolus+
Theophrastaceae]+[Myrsinaceae+Primulaceae]]].
Sapotaceae and Ebenaceae have similar malpighiaceous
hairs. Sapotaceae and Lecythidaceae have seeds with
lignified multiplicative testal cells.
A plausible topology is
[Sarcosperma+[Eberhardtia+[Xantolis+[‘Sapotoideae’+’Chrysophylloideae’]]]].
 |
Bayesian majority rule consensus tree of
Sapotaceae based on
DNA sequence data and morphology (Smedmark & al. 2006).
|
Sarcospermatoideae (H.
J. Lam) Swenson et Anderb. in Cladistics 21: 120. Apr 2005
1/11. Sarcosperma (11; eastern
Himalayas, southern China, Southeast Asia, Malesia). – Laticifers and latex
sometimes absent? Leaves opposite. Stipules cauline. Some species with paired
stipulules on petiole apex. Inflorescence axis well developed (in reality
consisting of a reduced branch). Staminodia scale-like, short and relatively
wide. Pistil composed of one or two connate carpels. Style stout. Hilar scar
distinct. Endosperm absent. – Sarcosperma is sister-group to the
remaining Sapotaceae.
[Eberhardtia+[Xantolis+[‘Sapotoideae’+’Chrysophylloideae’]]]
Stipules cauline or absent. Stamens as
many as corolla lobes, antepetalous, or twice (to six times) as many as corolla
lobes. Staminodia present, often petaloid, antesepalous (sometimes absent).
Pistil composed of one to 14 (to 30) connate antesepalous carpels. Seed in
Omphalocarpum with amyloid (xyloglucans).
Eberhardtia
1/3. Eberhardtia (3; E.
aurata, E. krempfii, E. tonkinensis; southern China,
Laos, Vietnam).
[Chrysophylloideae+[Neohemsleya+[[Xantolis+Spiniluma
oxyacantha]+Sapotoideae]]]
Chrysophylloideae
Leurss., Handb. Syst. Bot. 2: 946. Jul 1882 [‘Chrysophylleae’]
36/660–680. Vanroyena (1;
V. castanosperma; northeastern Queensland), Sersalisia (2;
S. sericea, S. sessiliflora; tropical Australia),
Pichonia (10; the Moluccas, New Guinea, the Bismarck Archipelago,
Solomon Islands, New Caledonia), Magodendron (2; M. mennyae,
M. venefici; Papua New Guinea), Niemeyera (3; N.
chartacea, N. prunifera, N. whitei; Queensland, New
South Wales), Pycnandra (c 60; New Caledonia),‘Planchonella’
(c 60; East Malesia to islands in western Pacific; polyphyletic),
Amorphospermum (1; A. antilogum; northeastern and eastern
Queensland, northeastern New South Wales), Micropholis (c 40?; Central
America, tropical South America), Ragala (5; R. balata,
R. guianensis, R. sanguinolenta, R. spuria, R.
ulei; northeastern South America), Ecclinusa (11; Trinidad,
tropical South America), Elaeoluma (4; E. crispa, E.
glabrescens, E. nuda, E. schomburgkiana; Panamá to
central Brazil), Nemaluma (2; N. anomala, N.
engleri; northeastern South America), Pleioluma (>30; Malesia
to tropical Australia, New Caledonia), Prieurella (7; tropical South
America), Diploon (1; D. cuspidatum; southeastern Brazil),
Martiusella (2?; M. gonocarpa, M. imperialis;
eastern Brazil), Cornuella (1; C. venezuelanensis;
northern South America), Achrouteria (2; A. durifructa,
A. pomifera; northeasternmost South America?; in
Chloroluma?), Chloroluma (1; C. gonocarpum; tropical
South America; incl. Achrouteria?), Chrysophyllum (15–20?;
Africa, Madagascar, India, Southeast Asia, Malesia to tropical Australia,
tropical America; incl. Villocuspis?), Villocuspis (6?;
tropical South America; in Chrysophyllum?), Donella (17;
tropical Africa, Madagascar), Gambeya (2–5; G. africana,
G. korupensis, G. lacourtiana, G. madagascariensis,
G. subnuda; Central Africa), Lucuma (20–25; tropical South
America), Sarcaulus (5; S. brasiliensis, S.
inflexus, S. oblatus, S. vestitus, S.
wurdackii; tropical South America; in Pouteria?),
Chromolucuma (8; southern Central America, tropical South America; in
Pouteria?), Pradosia (23; tropical South America; in
Pouteria?), Pouteria (c 300?; tropical Africa, tropical Asia
to the Pacific, tropical America). – Unplaced
Chrysophylloideae Aubregrinia (1; A.
taiensis; Ivory Coast, Ghana), Breviea (1; B. sericea;
Ivory Coast, Ghana, Congo?), Delpydora (2; D. gracilis,
D. macrophylla; Gabon), Diploon (1; D. cuspidatum;
southeastern Brazil), Englerophytum (10–15; tropical Africa),
Omphalocarpum (27; tropical West and Central Africa),
Sarcaulus (5; tropical South America), Synsepalum (c 20?;
tropical Africa), Tridesmostemon (4; T. bequaertii, T.
congoense, T. mortehani, T. omphalocarpoides; Central
Africa).
 |
Simplified phylogeny (maximum clade
credibility tree) of Chrysophylloideae based on nuclear DNA
sequence data (Swenson & al. 2013).
|
[Neohemsleya+[[Xantolis+Spiniluma
oxyacantha]+Sapotoideae]]
Neohemsleya
1/1. Neohemsleya (1; N.
usambarensis; Tanzania). – Neohemsleya usambarensis was sister
to Sapotoideae in Gautier & al. (2013).
[[Xantolis+Spiniluma
oxyacantha]+Sapotoideae]
[Xantolis+Spiniluma
oxyacantha]
2?/c 16. Xantolis (c 15; South
Asia, southern China, Indochina, the Philippines), Spiniluma (1;
S. oxyacantha; Ethiopia, Erithrea, southern Arabian Peninsula). –
The sister-group relationship between Xantolis and Spiniluma
is uncertain. The only species of Spiniluma investigated by Gautier
& al. (2013) was S. oxyacantha (S. discolor is the second
species in Spiniluma). They found this clade to be sister-group to
Sapotoideae.
Sapotoideae Eaton,
Bot. Dict., ed. 4: 35. Apr-Mai 1836
c 23?/515–520.
Sideroxyleae Small, Man. S.E. Fl.: 1031. 30 Nov 1933.
Sideroxylon
(75–80; tropical and subtropical regions on both hemispheres). –
Lecomtedoxa (6; L. biraudii, L. heitzana, L.
klaineana, L. nogo, L. plumosa, L.
saint-aubinii; Gabon), Neolemonniera (3; N. batesii,
N. clitandrifolia, N. ogouensis; tropical West Africa). The
clade [Lecomtedoxa+Neolemonniera] was sister-group to the
remaining Sapotoideae in Gautier & al. (2013). –
Tseboneae L. Gaut. Et Naciri in Taxon 62: 979. 22 Oct
2013. Tsebona (1; T. macrantha; Madagascar),
Bemangidia (1; B. lowryi; southeastern Madagascar),
Capurodendron (23; Madagascar). –
Isonandreae T. D. Penn., Gen. Sapotac.: 147. 1991.
Burckella (14; East Malesia to Tonga), ’Madhuca’ (c 100;
India, Sri Lanka, Southeast Asia, Malesia to New Guinea, tropical Australia;
polyphyletic), Diploknema (7; D. butyracea, D.
butyraceoides, D. oligomera, D. ramiflora, D.
sebifera, D. siamensis, D. yunnanensis; India to Yunnan,
Southeast Asia and Borneo), Payena (c 20; West Malesia, Mindanao,
with their highest diversity on Borneo; non-monophyletic?), Isonandra
(c 10; southern India, Sri Lanka), Palaquium (c 110; India, Sri Lanka,
southern China, Southeast Asia, Taiwan, Malesia to Samoa), Northia (1;
N. seychellana; the Seychelles). –
Sapoteae Reichenb., Handb. Nat. Pflanz.-Syst.: 214.
1–7 Oct 1837. Inhambanella (2; I. guereensis, I.
henriquezii; western and southeastern tropical Africa),
Vitellariopsis (5; V. cuneata, V. dispar, V.
ferruginea, V. kirkii, V. marginata; East Africa),
Vitellaria (1; V. paradoxa; tropical West and Central Africa;
in Vitellariopsis?), Tieghemella (2; T. africana,
T. heckelii; tropical West and Central Africa), Mimusops (c
40; tropical Africa, Madagascar, the Mascarene Islands, the Seychelles, one
species, M. elengi, to tropical Asia), Autranella (1; A.
congolensis; tropical West and Central Africa), Labramia (9;
Madagascar), Labourdonnaisia (7; L. calophylloides, L.
glauca, L. lecomtei, L. madagascariensis, L.
revoluta, L. richardiana, L. thouarsii; Madagascar,
Mauritius, Réunion), Faucherea (11; Madagascar), Manilkara
(c 65; tropical regions on both hemispheres).
 |
Bayesian majority rule consensus tree of
Sapotoideae based on DNA sequence data (Gautier & al.
2013).
|
Unplaced Sapotaceae
Baillonella (1; B.
toxisperma; Central Africa to Angola), Gluema (1; G.
ivorensis; tropical West and Central Africa).
SARRACENIACEAE
Dumort.
|
( Back to Ericales )
|
Dumortier, Anal. Fam. Plant.: 53. 1829, nom.
cons.
Sarraceniales Lindl.
in C. F. P. von Martius, Consp. Regn. Veg.: 61. Sep-Oct 1835
[’Sarracenieae’]; Heliamphoraceae
Chrtek, Slavíková et Studnička in Preslia 64: 8. 10 Feb 1992;
Sarracenianae Thorne ex Reveal in Phytologia 79: 71.
29 Apr 1996; Sarraceniineae Reveal in Kew Bull. 66:
48. Mar 2011
Genera/species 3/33–34
Distribution Western United
States, eastern North America, the Guayana Highlands.
Fossils Uncertain.
Archaeamphora longiservia from the Lower Cretaceous of northeastern
China may be a conifer.
Habit Bisexual, perennial
rhizomatous herbs. Carnivorous. Helophytes.
Vegetative anatomy Mycorrhiza
absent. Phellogen absent? Primary vascular tissue in scattered separate
bundles. Secondary lateral growth absent. Vessel elements (in rhizome) with
scalariform perforation plates; lateral pits scalariform, opposite or
alternate, simple and/or bordered pits. Imperforate tracheary xylem elements
tracheids with bordered pits, non-septate. Wood rays uniseriate or
multiseriate, heterocellular?, or absent. Axial parenchyma apotracheal diffuse.
Sieve tube plastids S type. Nodes? Crystals?
Trichomes Colleter-like
glandular hairs (on petioles).
Leaves Alternate spiral,
simple, entire, usually in basal rosette (in some species of
Heliamphora on upright, usually unbranched stem), with ? ptyxis.
Leaves ascidiate, infundibuliform or tubular, usually directed inwards (in
Darlingtonia twisted 180° hence directed outwards), modified into
water-holding (not in Heliamphora?) insect-trapping organs, usually
with lid- or hood-like dorsal terminal lobe. Funnel-shaped zone of lamina in
Heliamphora and Sarracenia consisting of adaxially fused
margins (in Sarracenia with adaxial keel) and in uppermost part
operculum (in Heliamphora small). In Darlingtonia a winged
sheathing lower zone, a keeled tubular zone above and, in uppermost part, a
stout dome-shaped almost closed zone. Distally, especially around entrance of
pitcher, a nectar-secreting zone with nectariferous glands. Inner side of trap
with a zone covered by downwardly directed hairs or bristles (in
Darlingtonia scattered waxy platelets). In lower part of lamina an
absorption zone. Proteolytic enzymes absent from pitcher leaf, nutrients
instead becoming available to plant by bacterial decomposition of animal bodies
in pitcher liquid. Lepidote or ensiform leaves sometimes forming late in
season. Stipules and leaf sheath absent. Colleter-like glandular hairs present
on adaxial side of petiole base. Petiole vascular bundle transection
hippocrateromorphous (Ω-shaped), collateral, each bundle surrounded by
sclerenchyma and starchy tissue. Venation pinnate? Stomata anomocytic.
Cuticular wax crystalloids as platelets (in Darlingtonia, detachable,
on inner side of pitcher). Leaf margin crenate to entire.
Inflorescence Flowers
Sarracenia and Darlingtonia solitary, terminal; in
Heliamphora usually terminal few-flowered raceme-like inflorescences.
Floral prophylls (bracteoles) three.
Flowers Actinomorphic, often
large. Hypogyny. Sepals (three or) four to six, with imbricate aestivation,
persistent, free (in Heliamphora petaloid). Petals (three or) four to
six, with imbricate aestivation, caducous, free (absent in
Heliamphora). Ovary wall in Sarracenia with ten nectaries on
stylar base above staminal groups; flowers in Darlingtonia and
Heliamphora without nectaries. Disc absent.
Androecium Stamens eight to
ten (Heliamphora) or numerous (Darlingtonia,
Sarracenia), often in fascicles (in Sarracenia usually
originating from ten staminal primordia). Filaments short, free or connate in
ten fascicles (in Sarracenia), free from tepals. Anthers basifixed
(Darlingtonia, Heliamphora) or dorsifixed
(Sarracenia), non-versatile (Darlingtonia,
Heliamphora) or versatile (Sarracenia), tetrasporangiate,
introrse, usually longicidal (dehiscing by longitudinal slits; in
Heliamphora poricidal, with short pore-like slits on caudate
appendages). Tapetum secretory. Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains (3–)4–5-colpor(oid)ate
(Heliamphora), 4–6-colpor(oid)ate (Darlingtonia) or
6–9-colpor(oid)ate (Sarracenia), shed as monads, bicellular at
dispersal. Exine tectate, with granular infratectum, verruculate
(Heliamphora) or scrobiculate (Darlingtonia,
Sarracenia).
Gynoecium Pistil composed of
three (Heliamphora) or five (Darlingtonia,
Sarracenia) connate carpels. Ovary superior, trilocular
(Heliamphora) or quinquelocular (often incompletely divided in upper
part). Style trilobate and apically truncate (Heliamphora),
quinquelobate (Darlingtonia) or widened like an umbrella
(Sarracenia), hollow?, in Sarracenia nectariferous. Stigmas
three or five, small (in Sarracenia present on lower sides of five
lobe apices), papillate, Dry type. Pistillodium absent.
Ovules Placentation axile in
lower part, often intrusively parietal in upper part. Ovules numerous per
carpel, anatropous, horizontal, usually unitegmic (in Darlingtonia
bitegmic?), tenuinucellar. Integument approx. four cell layers thick.
Hypostase? Megagametophyte monosporous, Polygonum type. Antipodal
cells sometimes persistent. Endosperm development cellular. Endosperm
haustoria? Embryogenesis caryophyllad.
Fruit A loculicidal
capsule.
Seeds Aril? Seed coat with
wings or hairs. Exotesta with thick radial and inner cell walls, with theoid
exotestal thickenings. Inner testal layers crushed. Perisperm not developed.
Endosperm copious, oily and proteinaceous. Embryo straight, well
differentiated, chlorophyll? Cotyledons two. Germination phanerocotylar.
Cytology n = 13
(Sarracenia), 15 (Darlingtonia), 21 (Heliamphora)
DNA Mitochondrial intron
coxII.i3 lost.
Phytochemistry Flavonols
(kaempferol, quercetin), O-methyl flavonols; cyanidin Route I iridoids
(also decarboxylated iridoids), Group VI secoiridoids (morroniside), sarracenin
(enoldiacetal monoterpene), and alkaloids present. sometimes present. Ellagic
acid, saponins and cyanogenic compounds not found. Tannins?
Use Ornamental plants.
SLADENIACEAE (Gilg et
Werderm.) Airy Shaw
|
( Back to Ericales )
|
Airy Shaw in Kew Bull. 18: 267. 8 Dec 1965
Genera/species 2/3
Distribution East Africa,
Southeast Asia.
Fossils Uncertain. Fossil wood
(Sladenioxylum africanum), resembling the wood in extant
Sladenia, has been found in Albian-Cenomanian layers in eastern
Sudan.
Habit Bisexual, evergreen
trees.
Vegetative anatomy Phellogen
ab initio pericyclic. Vessel elements in Sladenia arranged in radial
groups. Vessel elements with scalariform perforation plates; lateral pits
scalariform (Ficalhoa), opposite to alternate, simple or bordered
pits. Imperforate tracheary xylem elements fibre tracheids with bordered pits,
non-septate. Wood rays uniseriate or multiseriate, heterocellular. Axial
parenchyma apotracheal diffuse or diffuse-in-aggregates, or paratracheal scanty
vasicentric. Sieve tube plastids S type? Nodes 1:1?, unilacunar with one? leaf
trace. Exudate in Ficalhoa white, latex-like. Chambered crystals
present or absent. Calciumoxalate prismatic crystals or druses.
Trichomes Hairs unicellular or
absent.
Leaves Alternate (spiral or
distichous), simple, entire, with ? ptyxis. Stipules and leaf sheath absent.
Petiole vascular bundle transection arcuate; petiole in Sladenia also
with wing bundles (associated fibres absent in Ficalhoa). Venation
pinnate. Stomata anomocytic. Cuticular wax crystalloids? Epidermis in
Sladenia with mucilage cells. Mesophyll with calciumoxalate druses.
Sclereids absent. Leaf margin usually serrate (sometimes entire).
Inflorescence Axillary,
few-flowered cymose, or flowers solitary axillary.
Flowers Actinomorphic, small.
Hypogyny. Sepals four to seven, with imbricate aestivation, persistent, free
(Sladenia) or connate (Ficalhoa). Petals five (or six),
spiral or whorled, with imbricate aestivation, free (Sladenia) or
connate at base (Ficalhoa). Spiral scale-like leaves present below
perianth. Nectaries present on lower part of ovary (Sladenia). Disc
absent.
Androecium Stamens (eight to)
ten to 15, in one whorl. Filaments free, swollen (Sladenia) or connate
in five fascicles, narrow (Ficalhoa), in Sladenia free from
tepals, in Ficalhoa adnate to petals. Anthers basifixed,
non-versatile, tetrasporangiate, introrse (Sladenia), poricidal (in
Sladenia dehiscing by apical pores; in Ficalhoa by apical
transverse slits). Tapetum secretory. Staminodia absent.
Pollen grains
Microsporogenesis successive. Pollen grains tricolp(or)ate (Sladenia),
shed as monads, bicellular at dispersal. Exine tectate, with columellate?
infratectum, verruculate or smooth.
Gynoecium Pistil composed of
(two or) three (Sladenia) or five (or six) antepetalous
(Ficalhoa) connate carpels. Ovary superior, in Sladenia
usually trilocular (rarely bilocular), in Ficalhoa? usually
quinquelocular (sometimes sexalocular). Style single, usually simple, short
(sometimes with short acute lobes). Stigmas (two or) three (Sladenia),
punctate, type? Pistillodium absent.
Ovules Placentation
apical-axile (Sladenia) or axile (Ficalhoa). Ovules two
collateral anatropous pendulous and epitropous (Sladenia) or numerous
(Ficalhoa) per carpel, bitegmic, tenuinucellar. Micropyle endostomal
(Sladenia). Outer integument three or four cell layers thick
(Sladenia). Inner integument three or four cell layers thick
(Sladenia). Obturator, hypostase, nucellar beak, and nucellar cap
absent (Sladenia). Megagametophyte tetrasporous, 8-nucleate,
Adoxa type. Endosperm development nuclear? Endosperm haustoria?
Embryogenesis?
Fruit A loculicidal capsule
with persistent calyx and central columella (Ficalhoa) or a
schizocarp? with thin exocarp, crustaceous endocarp and three mericarps
(Sladenia).
Seeds Aril absent. Seed coat
in Sladenia winged. Testa crustaceous, with polygonal cells, little
thickened (Ficalhoa). Perisperm not developed.. Endosperm copious.
Embryo straight, well differentiated, chlorophyll? Cotyledons two, short.
Germination?
Cytology n = 24
(Sladenia) – Sladenia has cell wall development of
monocotyledonous type (also in Ficalhoa?).
DNA
Phytochemistry Unknown.
Use Timber.
Systematics Sladenia
(2; S. celastrifolia, S. integrifolia; Burma, Yunnan,
northern Thailand), Ficalhoa (1; F. laurifolia; tropical
mountains in East Africa).
Sladeniaceae are sister to
[Pentaphylax+Ternstroemiaceae] and resemble
this clade, e.g., in their wood anatomy and pollen morphology.
Some analyses do not place Sladenia as
sister to Ficalhoa. Ficalhoa is sister to
Euryodendron (Ternstroemiaceae) in an
analysis by Luna & Ochoterena (2004).
STYRACACEAE DC. et
Spreng.
|
( Back to Ericales )
|
de Candolle et Sprengel (transl. W. Jameson),
Elem. Philos. Pl.: 140. Jul 1821 [’Styraceae’], nom. cons.
Halesiaceae D. Don in
Edinburgh New Philos. J. 6: 49. Oct-Dec 1828;
Halesiales Link, Handbuch 1: 667. 4-11 Jul 1829;
Styracopsida Bartl., Ord. Nat. Plant.: 121, 158. Sep
1830 [’Styracinae’]; Styracales Rich. in
C. F. P. von Martius, Consp. Regn. Veg.: 26. Sep-Oct 1835
[’Styraceae’]
Genera/species 13/160–165
Distribution The United
States, Mexico, the West Indies, Central America, South America south to
Argentina, the northern and eastern Mediterranean, eastern Himalayas (Nepal to
Arunachal-Pradesh), Assam, eastern India, East Asia to the Korean Peninsula and
Japan, Southeast Asia, Malesia, New Guinea and Solomon Islands.
Fossils Fossils assigned to
Rehderodendron have been found in Eocene to Miocene layers of
Europe.
Habit Usually bisexual (in
Bruinsmia gynodioecious), evergreen or deciduous trees or shrubs.
Vegetative anatomy Phellogen
ab initio pericyclic. Vessel elements usually with scalariform perforation
plates (with numerous cross-bars; vessel elements rarely with simple
perforation plates); lateral pits opposite or alternate (sometimes
scalariform), bordered pits. Imperforate tracheary xylem elements fibre
tracheids or libriform? fibres usually with bordered (sometimes also simple)
pits, non-septate. Wood rays uniseriate or multiseriate, heterocellular. Axial
parenchyma apotracheal diffuse or diffuse-in-aggregates, or paratracheal
scanty. Sieve tube plastids S type. Nodes 1:1, unilacunar with one leaf trace.
Secretory cavities with resinous canals often present. Silica present in wood
at least in many species of Styrax. Prismatic calciumoxalate crystals
present in some species.
Trichomes Hairs multicellular,
uniseriate, stellate, peltate or lepidote (often brown to reddish-brown).
Leaves Alternate (spiral),
simple, entire, with conduplicate-plicate or supervolute ptyxis. Stipules and
leaf sheath absent. Petiole vascular bundle transection arcuate or D-shaped;
medullary bundles and/or wing bundles sometimes present (in Parastyrax
complex). Venation pinnate, camptodromous. Stomata usually anomocytic.
Cuticular wax crystalloids? Calciumoxalate as druses and/or solitary prismatic
crystals. Leaf margin serrate, crenate or entire; leaf teeth gland-tipped.
Inflorescence Terminal or
axillary, often raceme-like or paniculate (rarely one- or two-flowered) cymose.
Floral prophylls (bracteoles) often absent.
Flowers Actinomorphic. Pedicel
often articulated. Hypanthium present or absent. Half epigyny or sometimes
(Halesia) epigyny. Sepals four or five (to nine), with open or valvate
aestivation, persistent, connate. Petals (four or) five (to eight), with
imbricate or subinduplicate-valvate aestivation, connate (in Halesia
largely connate). Nectary absent. Disc absent.
Androecium Stamens (five to)
eight to ten (to 20), usually twice as many as (rarely the same number or three
or four times as many as) petals, usually obdiplostemonous (rarely
haplostemonous, alternipetalous). Filaments stout (not distinctly delimited
from anthers), usually flattened, usually connate below, usually adnate to
petals (epipetalous). Anthers basifixed, non-versatile, tetrasporangiate,
introrse or latrorse, longicidal (dehiscing by longitudinal slits); connective
sometimes apically prolonged. Tapetum secretory. Female flowers with five
staminodia.
Pollen grains
Microsporogenesis simultaneous. Pollen grains tricolpor(oid)ate or
tetracolpor(oid)ate, shed as monads, bicellular at dispersal. Exine tectate,
with columellate infratectum, perforate, spinulate, verruculate or smooth.
Gynoecium Pistil composed of
usually two to four (sometimes five) connate, alternisepalous, antepetalous
carpels, often hairy inside; median carpel abaxial. Ovary inferior or
semi-inferior, usually bilocular to quadrilocular in lower part (sometimes
quinquelocular), usually unilocular in upper part (in Parastyrax
multilocular). Style usually single, simple, filiform (occasionally lobed),
hollow (with stylar canal). Stigma punctate, capitate or somewhat lobate, Dry
type. Pistillodium absent.
Ovules Placentation usually
axile (rarely subbasal). Ovules usually four to nine (rarely one or up to c.
30) per carpel, anatropous or hemianatropous, pendulous to ascending, usually
apotropous (sometimes epitropous), unitegmic or bitegmic (Styrax),
tenuinucellar. Micropyle endostomal. Outer integument in Styrax ? cell
layers thick. Inner integument ? cell layers thick. Placental obturator present
in some species. Megagametophyte monosporous, Polygonum type.
Endosperm development cellular. Endosperm haustoria? Embryogenesis solanad.
Fruit A loculicidal capsule
(Alniphyllum, Huodendron, Styrax), a drupe
(Parastyrax, Styrax), a samara (Halesia,
Pterostyrax), or a berry-like (Bruinsmia) or nutlike
(Pterostyrax, Melliodendron, Rehderodendron,
Sinojackia, Styrax) fruit with persistent hypanthium and
calyx.
Seeds Aril? Seed coat in
Alniphyllum winged. Testa thin, vascularized, crushed. Tegmen in
Styrax? Perisperm not developed. Endosperm copious, oily. Embryo
straight or somewhat curved, well differentiated, without chlorophyll.
Cotyledons two, flat or almost terete. Germination phanerocotylar.
Cytology n = 8 (12)
DNA Intron present in
mitochondrial gene coxII.i3.
Phytochemistry Flavonols
(kaempferol, quercetin), cyanidin, oleanolic acid derivatives, ellagic acid,
tannins, triterpene saponins, caffeic acid, and hydroxycinnamic acids
(p-coumaric acid, ferulic acid) present. Myricetin, alkaloids and
cyanogenic compounds not found.
Use Ornamental plants,
medicinal plants, balsam resins (benyamin-gum, benzoin etc. from
Styrax) for pharmaceutical, confectionery and perfume industries,
incense.
Systematics Styrax (c
130; the Mediterranean, Southeast Asia, Malesia, southern and southeastern
United States, Mexico, Central America, Puerto Rico, tropical South America),
Huodendron (4; H. biaristatum, H. parviflorum,
H. tibeticum, H. tomentosum; southern China, Southeast Asia);
Bruinsmia (2; B. polysperma, B. styracoides; eastern
Himalayas, Burma, southern China, Indochina, Malesia to New Guinea),
Alniphyllum (3; A. eberhardtii, A. fortunei, A.
pterospermum; eastern Himalayas, southwestern and central China, Taiwan,
Southeast Asia), Halesia (1; H. carolina; southeastern United
States), 'Halesia' monticola (Arkansas, North Carolina),
Melliodendron (1; M. xylocarpum; southern China),
Changiostyrax (1; C. dolichocarpus; southeastern China),
Perkinsiodendron (1; P. macgregorii; eastern China),
Rehderodendron (5; R. gongshanense, R. indochinense,
R. kwangtungense, R. kweichowense, R. macrocarpum;
southern and western China, Burma, Vietnam), 'Pterostyrax' (4; P.
burmanicus: Burma; P. corymbosus, P. hispidus, P.
psilophyllus: China, Japan; polyphyletic), Sinojackia (8;
southern China); unplaced: Parastyrax (2; P. lacei, P.
macrophyllus; Yunnan, Burma).
Styracaceae are probably sister to
Diapensiaceae.
Styrax or the clade [Styrax+Huodendron]
is sister to the remainder (Fritsch & al. 2001, Yan & al. 2018). The
East North American Halesia carolina is nested in a clade together
with Pterostyrax, whereas the Chinese Perkinsiodendron
macgregorii is closely allied to Rehderodendron, according to Yan
& al. (2018).
 |
One of three equally parsimonious cladograms
of Styracaceae based
on morphology and DNA sequence data (Fritsch & al. 2001).
‘Halesia’ macgregorii is recombined into
Perkinsiodendron (Fritsch & al. 2016). Styrax was
sister to the remaining Styracaceae in the analyses
by Yan & al. (2018). Moreover, according to their study,
Halesia carolina was closely allied to Pterostyrax
hispidus.
|
Desfontaines in Mém. Mus. Natl. Hist. Nat. 6: 9.
1820 [’Symplocearum’, ’Symploceae’], nom. cons.
Genera/species 1/300–320
Distribution Tropical and
subtropical regions in southern, eastern and southeastern Asia, Malesia to New
Guinea, eastern Australia, Melanesia, southeastern United States, southern
Mexico, Central America, the West Indies, tropical South America; some species
in temperate regions in East Asia and eastern North America.
Fossils Fossil
Triporopollenites pollen from the Maastrichtian of California has been
assigned to Symplocos. Fossil endocarps, pollen grains and leaves of
Symplocaceae have been
found in sediments from the Early Eocene to Miocene of Europe.
Symplocos Subgenus Hopea as well as the extinct
Pallioporia and Sphenotheca occur as fossil endocarps in
European Eocene and later layers and Sphenotheca is very similar to
some extant species of Symplocos in East Asia. During the Palaeogene
Symplocos seems to have been widely distributed and largely dominating
on the North American and Eurasiatic continents.
Habit Usually bisexual (rarely
polygamomonoecious or dioecious), usually evergreen trees or shrubs
(Symplocos paniculata is deciduous).
Vegetative anatomy Phellogen
ab initio superficial. Vessel elements with scalariform perforation plates
(often with numerous cross-bars); lateral pits scalariform, alternate or
opposite, simple and/or bordered pits. Vestured pits sometimes present.
Imperforate tracheary xylem elements tracheids with bordered pits, non-septate.
Wood rays uniseriate or multiseriate, heterocellular. Axial parenchyma
apotracheal diffuse or diffuse-in-aggregates, or paratracheal scanty, or
absent. Sieve tube plastids S type. Nodes 1:1, unilacunar with one leaf trace.
Calciumoxalate as crystal sand and lumps of prismatic crystals.
Trichomes Hairs unicellular or
multicellular, uniseriate, septate (rarely with swollen base), or absent.
Leaves Alternate (usually
spiral, sometimes distichous), simple, entire, often coriaceous, with
supervolute ptyxis. Stipules and leaf sheath absent. Petiole vascular bundles?
Venation pinnate. Stomata usually paracytic and as sparsely occurring large
water stomata (protruding unicellular water-secreting, bladder-like on outer
part, constricted internally where they run into lamina via rosette of
epidermal cells). Cuticular wax crystalloids? Leaf margin serrate, glandular
serrate (usually with caducous glands) or entire.
Inflorescence Usually axillary
(rarely terminal), spike, raceme, panicle, fasciculate or head-like cymose
(flowers sometimes solitary axillary).
Flowers Actinomorphic. Pedicel
usually articulated (in Cordyloblaste clade not articulated). Usually
epigyny (rarely half epigyny). Sepals (three to) five, with valvate or
imbricate aestivation, persistent, connate at base. Petals (three to) five or
ten (or eleven), with imbricate aestivation, deeply bifid, entirely fused into
tube or connate only at base. Nectariferous disc annular, cylindrical or
quinquelobate, with five glands.
Androecium Stamens usually
numerous (sometimes three to five antesepalous, or ten), obdiplostemonous.
Filaments fused into tube or connate only at base all or in five antesepalous
alternipetalous fascicles (sometimes? free), usually adnate to corolla tube
(epipetalous). Anthers almost spherical, basifixed to dorsifixed, versatile,
tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits).
Tapetum secretory. Female flowers with staminodia.
Pollen grains
Microsporogenesis simultaneous. Pollen grains (2–)3(–4)-colporate, shed as
monads, bicellular at dispersal. Exine tectate to semitectate, with granular or
columellate infratectum, punctate, perforate to reticulate, psilate, scabrate,
rugulate, areolate, verrucate, spinulate or echinate.
Gynoecium Pistil composed of
two to five connate carpels; median carpel abaxial. Ovary usually inferior
(rarely semi-inferior), usually trilocular (sometimes bi-, quadri- or
quinquelocular), in upper part with incomplete septa, nectariferous. Style
single, simple, narrow or wide, usually hollow (with stylar canal). Stigma
small, capitate or bilobate to quinquelobate, Dry type or Wet type. Male
flowers with pistillodium.
Ovules Placentation mostly
axile. Ovules (two to) four per carpel, anatropous, pendulous, epitropous,
unitegmic, tenuinucellar. Integument ? cell layers thick. Megagametophyte
monosporous, Polygonum type. Endosperm development cellular. Endosperm
haustoria? Embryogenesis?
Fruit Usually a one-seeded
drupe with persistent calyx. Pyrene with as many germination pores as fertile
carpels.
Seeds Aril absent. Testa
vascularized. Inner walls of exotestal cells usually thick (theoid exotestal
thickenings) or thin. Endotesta? Perisperm not developed. Endosperm copious,
oily and often starchy. Embryo large, straight or curved (sometimes U-shaped),
chlorophyll? Cotyledons two, very short. Germination phanerocotylar.
Cytology n = 11 (12) –
Polyploidy occurring.
DNA Mitochondrial intron
coxII.i3 lost. Mitochondrial coxI intron present.
Phytochemistry Flavonols
(kaempferol), O-methyl flavonols, cyanidin, catechins, davidigenin,
Group II decarboxylated iridoids (e.g. cornine), oleanolic acid derivatives,
arjunolic acid derivatives, ellagic and gallic acids, hydrolyzable tannins,
proanthocyanidins (prodelphinidins), saponins, and lignans (pinoresinol)
present. Indole alkaloids rare. Myricetin and cyanogenic compounds not found.
Aluminium accumulated in many species.
Use Beverages (maté), dyeing
sources, carpentries, carvings.
Systematics Symplocos
(300–320; tropical and subtropical regions in southern, eastern and
southeastern Asia, Malesia to New Guinea, eastern Australia and Melanesia,
southeastern United States, southern Mexico, Central America, the West Indies,
tropical South America; few species in temperate regions in East Asia and
eastern North America).
Symplocos
may be sister to [Diapensiaceae+Styracaceae].
The clade Cordyloblaste appears to be
sister-group to the remaining species of Symplocos.
TERNSTROEMIACEAE Mirb.
ex DC.
|
( Back to Ericales )
|
de Candolle, Essai Propr. Méd. Pl., ed. 2: 203.
11 Mai 1816, nom. cons. prop.
Ternstroemiales Mirb.
in C. F. P. von Martius, Consp. Regn. Veg.: 52. Sep-Oct 1835 [‘Ternstroemiaceae’]
Genera/species 11/330–340
Distribution Tropical and
subtropical regions, the Canary Islands, tropical West and Central Africa, the
Himalayas, East Asia, Southeast Asia, Malesia to New Guinea and Queensland,
Melanesia, islands in southwestern Pacific, the Hawaiian Islands, Mexico,
Central America, the West Indies, tropical South America.
Fossils Campylotropous seeds
assigned to Eurya (e.g. Eurya crassitesta) and
Visnea have been described from the Santonian onwards of Central
Europe and from the Maastrichtian of Germany, and fossil pentamerous capsules
and seeds of Protovisnea from the Late Turonian to the Maastrichtian.
Seeds of Eurya having thick sclerotic testa are frequently recorded
from Cenozoic sediments.
Habit Bisexual or dioecious,
evergreen trees and shrubs.
Vegetative anatomy Phellogen
ab initio superficial. Medulla often septated by diaphragms consisting of
sclereids. Narrow primary medullary rays alternating with wide ones. Vessel
elements usually with scalariform (rarely reticulate) perforation plates;
lateral pits scalariform or opposite (rarely alternate), simple and/or bordered
pits. Vestured pits sometimes present. Imperforate tracheary xylem elements
(tracheids? or) fibre tracheids with bordered pits, non-septate. Wood rays
uniseriate or multiseriate, heterocellular. Axial parenchyma apotracheal
diffuse, or paratracheal scanty, or absent. Tyloses often abundant. Sieve tube
plastids S type. Nodes usually 1:1, unilacunar with one leaf trace (rarely 3:3,
trilacunar with three traces). Calciumoxalate as prismatic crystals.
Trichomes Hairs unicellular,
simple.
Leaves Alternate (spiral or
distichous), simple, entire, usually coriaceous, with conduplicate-involute or
supervolute ptyxis. Stipules and leaf sheath absent. Colleters sometimes
present (in, e.g., Freziera). Perulae absent. Petiole vascular bundles
one to five, arcuately arranged. Venation usually brochidodromous (sometimes
reticulodromous). Stomata usually anomocytic (sometimes cyclocytic). Cuticular
waxes? Domatia in Eurya as pits. Epidermis in some species with
mucilage cells. Mesophyll and petiolar cortex with sclerenchymatous idioblasts
containing simple or branched sclereids. Leaf margin usually serrate (sometimes
entire), with blackish caducous gland at leaf apex and tooth apices.
Inflorescence Flowers usually
solitary axillary (sometimes in few-flowered axillary racemose inflorescence).
Bracts with blackish gland at apex.
Flowers Actinomorphic. Usually
hypogyny (rarely epigyny). Sepals (four or) five (to seven), with imbricate
quincuncial aestivation, persistent, usually free (sometimes connate at base),
usually with small gland at apex. Petals (four or) five (to seven; in
Archboldiodendron usually 5+5), with imbricate quincuncial
aestivation, usually free (sometimes connate at base). Nectaries present or
absent.
Androecium Stamens (five to)
numerous, often inflexed at apex. Filaments wide, flat or thick, often
narrowing against apex, shorter than to at least twice as long as anthers, free
or connate at base in five fascicles (in Visnea in antepetalous
pairs), often adnate to petals at base. Anthers basifixed, non-versatile,
tetrasporangiate, introrse, usually longicidal (dehiscing by longitudinal
slits; sometimes poricidal, dehiscing with short pore-like slits; anthers in
Eurya septate); connective usually prolonged, crystalliferous. Tapetum
secretory, with binucleate to quadrinucleate cells. Female flowers usually with
staminodia.
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually tricolporate, shed as
monads, bicellular at dispersal. Exine tectate, with columellate? infratectum,
psilate to scabrate, rugulate or foveolate.
Gynoecium Pistil composed of
(two or) three to five (or six) connate carpels. Ovary usually superior (rarely
inferior), (bilocular or) trilocular to quinquelocular (or sexalocular). Style
single, hollow, simple or branched at apex, or stylodia several, free. Stigmas
usually capitate?, papillate, Wet? type. Male flowers usually with
pistillodium.
Ovules Placentation usually
axile (rarely apical or parietal). Ovules (one to) numerous per carpel,
anatropous (amphitropous?) or campylotropous, apotropous or epitropous,
bitegmic, usually tenuinucellar. Micropyle endostomal. Outer integument three
to nine cell layers thick. Inner integument approx. three cell layers thick.
Megagametophyte monosporous, Polygonum type. Endosperm development ab
initio nuclear. Endosperm haustoria? Embryogenesis solanad.
Fruit In Freziereae
usually a berry; in Ternstroemia an irregularly dehiscing capsule; in
some species of Freziera a drupe; in Visnea a nut surrounded
by fleshy accrescent calyx.
Seeds Aril absent. Seed coat
mesotestal. Sarcotesta present in Anneslea and Ternstroemia.
Exotestal cells often enlarged, sometimes with thickened walls, with theoid
exotestal thickenings. Mesotesta of various thickness, often with lignified
cell walls, sometimes with sclereids, often crystalliferous. Endotesta? Tegmen?
Perisperm not developed. Endosperm copious or sparse, oily. Embryo large,
U-shaped to almost straight, well differentiated, without chlorophyll.
Cotyledons two, incumbent, shorter than radicula. Germination
phanerocotylar?
Cytology n = 20, 25
(Ternstroemieae); n = 12, 15, 18, 21–23 or more (21 most frequent)
(Freziereae)
DNA
Phytochemistry Flavonols
(kaempferol, quercetin, myricetin), cyanidin, triterpene acids (betulinic
acid), ellagic acid, proanthocyanidins (prodelphinidins), and saponins present.
Iridoids not found. Aluminium accumulated in many species.
Use Ornamental plants,
timber.
Systematics Ternstroemiaceae are
sister-group to Pentaphylax (Pentaphylacaceae). The clade
[Sladeniaceae+[Pentaphylax+Ternstroemiaceae]] may be
sister to Primulaceae clade.
An updated phylogenetic analysis of Ternstroemiaceae is badly
needed.
Ternstroemieae DC.,
Prodr. 1: 523. Jan (med.) 1824
2/90–100. Anneslea (4; A.
donnaiensis, A. fragrans, A. paradoxa, A.
steenisii; tropical Asia, southern China), Ternstroemia
(90–100; tropical and subtropical regions on both hemispheres, with their
largest diversity in Malesia and in Central and South America, few species in
tropical West and Central Africa). – Pantropical. Sclereids richly branched.
Leaves pseudoverticillate, often with black spots. Leaf margin usually entire
(sometimes crenulate). Flower solitary, axillary, subtended by reduced leaf.
Usually hypogyny (in Anneslea epigyny). Sepals antepetalous. Filament
shorter than anther. Pistil composed of two or three connate carpels.
Placentation apical. Ovules four to twelve per carpel. Outer integument six to
nine cell layers thick. Fruit a few-seeded irregularly dehiscing capsule.
Sarcotesta exotestal or consisting of pockets of fleshy cells on either side of
seed. Exotesta ten or more cell layers thick. Mesotesta sclerified, seven to 15
cell layers thick. n = 20, 25. – Anneslea, Ternstroemia and
Freziereae form a trichotomy in the analyses by Wu & al.
(2007).
Freziereae DC., Prodr.
1: 524. Jan (med.) 1824
9/c 240. Visnea
(1; V. mocanera; Madeira, the Canary Islands), Adinandra (c
85; India, Sri Lanka, Burma, China, southern Japan, Southeast Asia, Malesia to
New Guinea, tropical Africa), Archboldiodendron (1; A.
calosericeum; mountains on New Guinea), Cleyera (18; Mexico,
Central America, the West Indies, one species, C. japonica, in the
Himalayas and China to the Korean Peninsula and Japan); Euryodendron
(1; E. excelsum; southern China), Symplococarpon (5; S.
airy-shawianum, S. australe, S. flavifolium, S.
guatemalense, S. purpusii; tropical America), Freziera
(57; tropical America, with their largest diversity in mountain areas),
Eurya (c 70; tropical and subtropical regions in Asia, islands in the
western Pacific, the Hawaiian Islands), Balthasaria (1; B.
schliebenii; tropical Africa). – Macaronesia, tropical Africa, tropical
and subtropical Asia to Japan, islands in western Pacific and the Hawaiian
Islands, Central America, northern South America, with their highest diversity
in Southeast Asia and Malesia. Bisexual or dioecious. Nodes sometimes 1:3 or
3:3 (some species of Freziera). Sclereids usually little branched.
Leaves usually distichous (sometimes spiral). Leaf margin serrate or entire.
Inflorescence fasciculate or flowers solitary, at least some axillary (in axils
of expanded leaves). Usually hypogyny (in Symplococarpon epigyny).
Sepals sometimes connate. Petals in Freziera urceolate. Stamens five
to c. 30 (to c. 60), in a single whorl, arising from annular primordium.
Connective usually prolonged. Pistil composed of (one to) three (to ten)
connate carpels. Ovary base in Cleyera and Eurya nectar
secreting. Style single or stylodia separate. Placentation usually axile
(sometimes parietal). Ovules four to numerous per carpel. Outer integument
three or four cell layers thick. Fruit usually a many-seeded berry (sometimes a
one-seeded drupe). Inner exotestal cell walls often thickened and lignified.
Mesotesta sclerified, one to five cell layers thick. Embryo usually straight
(sometimes curved). n = 12, 13?, 15, 18, 21(most frequent) to 23 or more. –
Visnea was found to be sister-group to
[Adinandra+Cleyera],
[Euryodendron+Symplococarpon], Freziera, and
Eurya (Tsou & al. 2016).
 |
Phylogeny (simplified) of
Ternstroemiaceae
based on DNA sequence data (Wu & al. 2007).
|
TETRAMERISTACEAE (H.
Hallier) Hutch.
|
( Back to Ericales )
|
Hutchinson, Fam. Fl. Pl. ed. 2, 1: 277, Fig. 140.
4 Jun 1959
Pellicieraceae
(Triana et Planch.) L. Beauvis. ex Bullock in Taxon 8: 192. 24 Jul 1959
Genera/species 3/3
Distribution West Malesia,
southern Venezuela, mangroves along the Pacific coast and islands in Central
America, Colombia and northern Ecuador, and the Atlantic coast in Colombia.
Fossils Fossil pollen grains
(Lanagiopollis crassa) assigned to Pelliciera from the Eocene
onwards indicate that during the Paleogene this group was widely distributed
along the Caribbian and Central American coasts, and also along the Nigerian
coast.
Habit Bisexual, evergreen
trees or shrubs (Pelliciera consists of mangrove trees with
buttresses). Aerial roots frequent along stem base in Pelliciera.
Vegetative anatomy Phellogen
ab initio inner-cortical (Pentamerista, Tetramerista) or
superficial (Pelliciera). Vessel elements usually with simple
(sometimes scalariform) perforation plates; lateral pits usually alternate
(rarely opposite), bordered pits. Imperforate tracheary xylem elements fibre
tracheids or libriform fibres with simple or bordered pits, septate or
non-septate. Wood rays uniseriate or multiseriate, homocellular or
heterocellular. Axial parenchyma apotracheal diffuse or diffuse-in-aggregates,
or paratracheal scanty (in Tetramerista also ray-adjacent). Sieve tube
plastids S type? Nodes 3:3, trilacunar (in Pelliciera unilacunar?)
with three leaf traces. Cortex and medulla in Pelliciera with branched
sclerenchymatous idioblasts and thick-walled fibres. Heartwood in
Tetramerista with gum-like substances. Parenchyma in
Pelliciera with calciumoxalate raphides in raphid sacs. Crystal sand
present or absent.
Trichomes Hairs absent; glands
present.
Leaves Alternate (spiral),
simple, entire, coriaceous (in Pelliciera somewhat asymmetrical), with
supervolute (Tetramerista, Pentamerista) or involute
(Pelliciera) ptyxis. Stipules and leaf sheath absent. Petiole in
Pelliciera with one pair of extrafloral nectaries. Petiole vascular
bundle transection in Pelliciera annular. Venation pinnate. Stomata
usually anomocytic (sometimes paracytic; in Pelliciera cyclocytic).
Cuticular wax crystalloids? Abaxial side of lamina in Tetramerista
with black glandular dots, in Pentamerista with domatia along veins.
Mesophyll in Tetramerista with sclerenchymatous idioblasts with
calciumoxalate raphides and often crystal sand. Spongy tissue in
Pelliciera with stone cells. Margin of young leaves glandular-serrate,
with gland-like structures (in Pelliciera salt-excreting?).
Inflorescence In
Pentamerista and Tetramerista axillary, umbel-like, corymbose
or capitate cymose; flowers in Pelliciera solitary, axillary. Bracts
in Pelliciera large and petaloid. Bud scales absent in
Pelliciera. Floral prophylls (bracteoles) often large, caducous or
persistent.
Flowers Actinomorphic, usually
small (in Pelliciera large). Hypogyny. Sepals four or five, with
imbricate aestivation, petaloid, unequal in size, with shining glandular dots
on adaxial side near middle (Tetramerista) or close to base?
(Pelliciera; absent in Pentamerista), persistent or caducous,
free. Petals four or five, with imbricate aestivation, in Tetramerista
and Pelliciera with adaxial glandular dots, persistent or caducous,
free. Nectaries as bottle-shaped glands at calyx base. Disc absent.
Androecium Stamens four or
five, haplostemonous, antesepalous, alternipetalous. Filaments flattened,
inflexed in bud (finally reversed), at base usually somewhat connate (in
Pelliciera free), free from tepals. Anthers in Pentamerista
and Tetramerista dorsifixed, in Pelliciera basifixed and
sagittate with apical appendage, non-versatile, tetrasporangiate, usually
introrse (finally extrorse; in Pelliciera extrorse to latrorse),
longicidal (dehiscing by longitudinal slits); connective in Pelliciera
apically prolonged. Tapetum secretory? Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually tricolporate
(constricticolporate), shed as monads, bicellular at dispersal. Exine
semitectate, with granular infratectum, reticulate (in Pelliciera also
scabrate).
Gynoecium Pistil composed of
usually four or five (in Pelliciera two) connate carpels. Ovary
superior, quadrilocular or quinquelocular (in Pelliciera bilocular,
one locule sterile or aborted, although basically quinquecarpellate). Style
single, simple (in Pelliciera persistent in fruit). Stigma punctate or
somewhat lobate (in Pelliciera bifid), type? Pistillodium absent.
Ovules Placentation subapical
(Pelliciera) to basal (Tetramerista, Pentamerista).
Ovule one per carpel, in Pentamerista and Tetramerista
anatropous and ascending (epitropous?), in Pelliciera campylotropous
and pendulous, (incompletely) bitegmic, incompletely tenuinucellar. Micropyle
endostomal? Outer integument ? cell layers thick. Inner integument ? cell
layers thick. Megagametophyte monosporous, Polygonum type? Endosperm
development nuclear? Endosperm haustorium micropylar? Embryogenesis?
Fruit A four- or five-seeded
coriaceous berry (in Pelliciera up to 13 cm in diameter, spongy inside
and with persistent style).
Seeds Aril absent. Testa in
Tetramerista and Pentamerista multi-layered; testa in
Pelliciera absent, with mature seed consisting of large naked embryo,
hemispherical cotyledons surrounding red plumule, and radicula at maturation
penetrating stylar beak of fruit. Tegmen? Perisperm not developed. Endosperm
usually copious (absent? in Pelliciera). Embryo large, straight, well
differentiated, chlorophyll? Cotyledons two, usually much shorter than
hypocotyl (in Pelliciera large, fleshy). Germination
phanerocotylar.
Cytology n = ?
DNA
Phytochemistry Unknown.
Tannins? Polyphenols? Aluminium not accumulated.
Use Timber.
Systematics Tetrameristaceae are probably
sister to Balsaminaceae.
Pelliciera is sister to
[Pentamerista+Tetramerista].
Tetrameristeae Hallier
in Beih. Bot. Centralbl. 34: 37. 1917
2/2. Tetramerista (1; T.
glabra; the Malay Peninsula, Sumatra, Borneo), Pentamerista (1;
P. neotropica; the Guayana Highlands in southern Venezuela). –
Malesia, the Guayana Highlands. Shrubs or small trees. Phellogen
inner-cortical. Wood fluorescent. Stone cells present in stem. Branched
sclereids absent? Nodes trilacunar. Leaves spiral, simple, entire. Venation
indistinct. Leaf margin glandular. Flowers in umbel-like, corymbose or capitate
cymose inflorescences, small. Floral prophylls (bracteoles) sometimes
persistent. Sepals and petals similar, petaloid, with lobes spreading. Adaxial
surface of sepals glandular. Filaments slightly connate at base. Anthers
dorsifixed. Pistil composed of four or five connate carpels. Stigma punctate to
slightly lobate. Placentation basal. Ovule anatropous, ascending (epitropous?).
Fruit a berry. Testa multi-layered, with thickened cell walls. Endosperm
copious. Cotyledons small.
Pelliciereae Triana et
Planch. in Ann. Sci. Nat. Bot., sér. 4, 17: 380. Jun 1862
[‘Pellicerieae’]
1/1. Pelliciera (1; P.
rhizophorae; mangroves along the Pacific coast and islands in
Nicaragua, Costa Rica, Panamá, Colombia and northern Ecuador, and the Atlantic
coast in Colombia). – Mangrove trees. Buttresses consisting of vertical rows
of adventitious roots and conically surrounding stem base. Phellogen
superficial. Vessels present in multiples. Nodes unilacunar? Leaves
pseudoverticillate, with involute ptyxis; leaf base asymmetrical. Petiole
vascular bundle transection annular; petiole bundle almost cylindrical, flat
where joining stem. Stomata cyclocytic. Leaf margin dentate. Terminal buds
long, pointed. Bud scales absent. Flowers solitary, axillary, large. Floral
prophylls (bracteoles) petaloid. Sepals caducous, with adaxial glands. Petals
caducous. Filaments free. Anthers basifixed, extrorse, very long; connective
prolonged and pointed. Exine reticulate, scabrate. Pistil composed of two
connate carpels; carpels oblique or floral prophylls not in lateral position
and carpels transverse. Ovary bilocular, with one locule reduced. Style long,
persistent in fruit. Stigma bifid, punctate. Placentation subapical. Ovule
campylotropous, pendulous. Fruit one-seeded, large, acute, dry, indehiscent,
berry-like. Testa absent. Endosperm absent. Cotyledons large, fleshy.
Germination phanerocotylar.
THEACEAE Mirb. ex Ker
Gawl.
|
( Back to Ericales )
|
Ker Gawler in Bot. Reg. 2: ad t. 112. 1 Mai 1816,
nom. cons.
Camelliaceae Mirb. ex
A. P. de Candolle, Essai Propr. Méd. Pl., ed. 2: 97. 11 Mai 1816
[‘Camellieae’]; Theales Bercht. et J.
Presl, Přir. Rostlin: 219. Jan-Apr 1820 [‘Theaceae’];
Gordoniaceae (DC.) Spreng., Syst. Veg. 3: 12. Jan-Mar
1826; Camelliales Link, Handbuch 2: 346. 4-11 Jul
1829 [’Camelliaceae’]; Gordoniales J.
Presl in Nowočeská Bibl. [Wšobecný Rostl.] 7: 180. 1846
[‘Gordoniaceae’]; Malachodendraceae J.
Agardh, Theoria Syst. Plant.: 130. Apr-Sep 1858
[’Malachodendreae’], nom. illeg.;
Theineae Engl., Syllabus, ed. 2: 150. Mai 1898
Genera/species c
9/250–450
Distribution Southeastern
United States, the West Indies, Central America, tropical South America, East
Asia to the Korean Peninsula and Japan, Southeast Asia, Malesia, New Guinea.
Fossils A large number of
seeds from the Late Cretaceous and especially from the Cenozoic have been
assigned to Theaceae, although
it is sometimes doubtful whether these should be attributed to Theaceae. Palaeoschima
consists of fruits and seeds that has been described from the Late Cretaceous,
but the systematic affiliation is problematic. Camellia is represented
by a number of Cenozoic fossils and was formerly distributed also in North
America. Fossil flowers of Theaceae have been found in amber from
the Baltic area, and several extant genera are known from the Cenozoic of
Europe.
Habit Bisexual, usually
evergreen (rarely deciduous) trees and shrubs.
Vegetative anatomy Phellogen
ab initio usually close to pericycle (rarely subepidermal). Pericyclic envelope
collenchymatous, lignified or with groups of fibres. Vessel elements with
scalariform perforation plates; lateral pits scalariform, alternate or
opposite, simple pits. Vestured pits sometimes present. Imperforate tracheary
xylem elements tracheids or fibre tracheids with bordered pits, non-septate.
Wood rays uniseriate or multiseriate, homocellular or heterocellular. Axial
parenchyma apotracheal diffuse or diffuse-in-aggregates, or paratracheal
scanty, or absent. Sieve tube plastids S type. Nodes 1:1, unilacunar with one
leaf trace. Sclereids abundant. Mucilage cells frequently present.
Calciumoxalate as prismatic crystals.
Trichomes Hairs unicellular or
multicellular, usually uniseriate (sometimes stellate, rarely fasciculate), or
absent.
Leaves Alternate (usually
spiral, sometimes distichous), simple, entire, usually coriaceous, usually with
involute or supervolute (sometimes conduplicate) ptyxis. Stipules and leaf
sheath absent. Petiole vascular bundle transection arcuate (U- or V-shaped),
sometimes also with lateral bundles. Venation pinnate, craspedodromous. Stomata
usually paracytic, anisocytic to cyclocytic, gordoniaceous type, with guard
cells surrounded by two to four narrow subsidiary cells (rarely anomocytic).
Cuticular wax crystalloids as rodlets. Epidermal cells sometimes mucilaginous.
Mesophyll and petiolar cortex with or without sclerenchymatous idioblasts
containing numerous branched thickened sclereids (often visible as points or
dots). Leaf margin usually serrate (rarely entire), with small caducous gland
associated with each tooth (theoid teeth).
Inflorescence Flowers
axillary, solitary. Buds usually perulate. Floral prophylls (bracteoles) two or
more, alternate (spiral) and grading into usually whorled (sometimes spiral)
sepals.
Flowers Actinomorphic, often
large. Usually hypogyny (rarely half epigyny). Sepals (four or) five to seven,
with imbricate aestivation, often persistent, usually free or connate at base.
Petals usually five (sometimes 5+5, rarely numerous), with imbricate
aestivation, free or connate at base, sometimes spiral. Nectary absent or
nectar produced at base of filaments or ovary. Disc absent.
Androecium Stamens (ten to) 20
to c. 40 (to much more than 100), in two to five whorls, produced from five
antepetalous primordia or an annular primordium. Filaments usually free
(sometimes in five antepetalous fascicles; rarely connate into a tube), often
adnate to petal bases. Anthers articulated, usually dorsifixed (rarely
basifixed), often versatile, tetrasporangiate, introrse, longicidal (dehiscing
by longitudinal slits); connective sometimes prolonged. Tapetum secretory.
Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains tricolpor(oid)ate, shed as
monads, bicellular at dispersal. Exine tectate, with columellate infratectum,
rugulate or perforate to finely reticulate, scabrate. Pseudopollen formed in
cells of connective.
Gynoecium Pistil composed of
(three to) five (to ten) connate, usually antepetalous carpels. Ovary usually
superior (rarely semi-inferior), (trilocular to) quinquelocular (to
decemlocular), often nectariferous. Style single, simple or branched, or
stylodia several, free. Stigma capitate or slightly lobate or stigmas punctate,
papillate, Wet type. Pistillodium absent.
Ovules Placentation usually
axile (sometimes basal). Ovules two or more per carpel, usually anatropous (in
Schima campylotropous), usually pendulous, apotropous or
pleurotropous, bitegmic, tenuinucellar. Micropyle endostomal. Outer integument
four to ten cell layers thick. Inner integument four to eleven cell layers
thick. Hypostase present. Megagametophyte usually monosporous,
Polygonum type (in ‘Camellia’ disporous, Allium
type). Endosperm development ab initio nuclear. Endosperm haustoria?
Embryogenesis solanad. Polyembryony occurring.
Fruit Usually a loculicidal
(in Franklinia also septicidal) capsule often with persistent
columella and calyx (in some species of Pyrenaria a drupe; a capsule
in some species of ‘Camellia’ irregularly dehiscing).
Seeds Aril absent. Seed coat
mesotestal, in Gordonia, Schima and Stewartia
winged. Testa and tegmen vascularized. Exotesta with thickenings on radial and
inner tangential cell walls (not on outer tangential walls), with theoid
exotestal thickenings, often lignified. Mesotesta lignified (sometimes with
fibres, sometimes with sclereids). Endotesta often lignified. Tegmen? Perisperm
not developed. Endosperm usually sparse, oily, or absent. Embryo usually
straight (rarely curved), without chlorophyll. Cotyledons two, large (longer
than radicula). Germination phanerocotylar or cryptocotylar.
Cytology n = 15, 18 –
Polyploidy occurring.
DNA Plastid gene
rpl22 absent in Stewartia (present in nuclear genome?).
Mitochondrial intron coxII.i3 lost.
Phytochemistry Flavonols
(kaempferol, quercetin, myricetin), biflavonoids, catechins (e.g.
epigallocatechin-3-gallate), cyanidin, oleanolic acid derivatives, triterpenes,
gallic acid (in cultivated species of ‘Camellia’ Section
Thea), ellagic acid, methylated ellagic acids, ellagitannins
(pedunculagin, tellimagrandin II), proanthocyanidins (prodelphinidins),
chlorogenic acid, alkaloids (purine bases such as theobromine, theofylline and
caffeine in cultivated species of ‘Camellia’ Section
Thea), and triterpene saponins present. Cyanogenic compounds not
found. Aluminium and fluorides accumulated.
Use Ornamental plants,
stimulants (Camellia sinensis), seed-oils (Camellia
sasanqua), timber.
Systematics The sister-group
relationships of Theaceae are
unresolved. Theaceae are
sometimes recovered as sister to Symplocaceae and these two
lineages in turn forming a sister-group to [Styracaceae+Diapensiaceae]. On the other
hand, Symplocaceae is more
probably sister to the [Styracaceae+Diapensiaceae] clade.
A probable topology is the following:
[Theeae+[Gordonieae+Stewartieae]] (Li & al.
2013). Species delimitations, particularly in ‘Camellia’,
Gordonia, Schima and Stewartia,
are notorious.
Theeae Szyszyl. in
Engler et Prantl, Nat. Pflanzenfam. III, 6: 180, 181. Mai 1893
c 5/c 260. ’Camellia’
(c 125?; East Asia to Japan and Taiwan, tropical Asia; paraphyletic),
Laplacea (57; tropical America), Polyspora (c 40; southern
China, Southeast Asia), Pyrenaria (42; southern China, Southeast Asia,
West Malesia), Apterosperma (1; A. oblatum; China). – East
and Southeast Asia, Malesia, tropical America. Pedicel with numerous spirally
arranged floral prophylls (bracteoles). Prophylls successively modified into
sepals intergrading into petals. Stamens arranged in two whorls. Filament bases
nectariferous. Pseudopollen provided with ribs. Outer integument vascularized.
Megagametophyte in ‘Camellia’
sometimes disporous (with chalazal megaspores), octacellular, Allium
type. Fruit a capsule with persistent central columella. Seed coat often
winged. n = 15. – Theeae form a polytomy.
[Gordonieae+Stewartieae]
Gordonieae DC., Prodr.
1: 527. Jan (med.) 1824
3/3–60. Gordonia (1–40;
southern China, Taiwan, Southeast Asia, southeastern United States),
Schima (1–20; northeastern India, southern Himalayas, China, the
Ryukyu Islands, Southeast Asia, Malesia), Franklinia (1; F.
alatamaha; southeastern Georgia, probably extinct in the wild). –
Southeast Asia, West Malesia, Georgia (probably extinct). Pedicel with two
floral prophylls (bracteoles). Sepals five, different from petals. Stamens in
three to five whorls. Anther connective with stomata. Pseudopollen provided
with pores. Ovules in Schima campylotropous. Inner integument
vascularized. Fruit a capsule with persistent central columella (in
Franklinia with septicidal-loculicidal dehiscence). Seed coat in
Gordonia and Schima apically winged. Testa in Schima
proliferating. n = (15) 18. – Franklinia is sister to
Schima with high support, according to Li & al. (2013).
Stewartieae Choisy in
D. F. L. von Schlechtendal in Bot. Zeitung 31: 773. 31 Oct 1856
1/9–30. Stewartia
(9–30; central China, the Korean Peninsula, Japan, southeastern United
States). – Pedicel with two floral prophylls (bracteoles). Sepals five,
different from petals. Stamens numerous, initiated in fascicles and arranged in
radii of innermost petals. Fruit a capsule without columella. Seed coat often
narrowly winged. n = 15, 17, 18. – Stewartia
is paraphyletic, according to Li & al. (2013).
 |
Cladogram of Theaceae based on DNA sequence
data (Prince & Parks 2001).
|
THEOPHRASTACEAE
(Bartl.) G. Don
|
( Back to Ericales )
|
Don in Edwards’s Bot. Reg. 21: ad t. 1764. 1835
[’Theophrasteae’], nom. cons.
Genera/species 7/95–100
Distribution Southern Florida,
Mexico, Central America, the West Indies, South America south to northern
Paraguay.
Fossils Uncertain.
Habit Usually bisexual (in
Clavija usually dioecious, gynodioecious? or polygamodioecious),
usually evergreen (rarely deciduous) trees or shrubs, often pachycaul.
Vegetative anatomy Phellogen?
Primary medullary rays wide. Vessel elements with simple perforation plates;
lateral pits alternate, bordered pits. Imperforate tracheary xylem elements
libriform fibres with simple pits, septate or non-septate. Wood rays usually
multiseriate, heterocellular. Axial parenchyma paratracheal scanty vasicentric,
or absent. Sieve tube plastids S type? Nodes 1:1, unilacunar with one leaf
trace, 3:3, trilacunar with three traces, or 5:5, pentalacunar with five
traces. Subepidermal fibres sometimes non-lignified. Resins absent. Wood ray
cells often with silica bodies and/or prismatic calciumoxalate crystals.
Styloids present in some species.
Trichomes Hairs simple or
branched; glandular hairs (on leaves) consisting of unicellular stalk and
multicellular head; glandular hairs usually sunken.
Leaves Alternate (usually
pseudoverticillate on branch tips), simple, entire, coriaceous, gland-dotted
(often scale-like; in Jacquinia sometimes needle-like, in
Theophrasta sometimes very large), with conduplicate (or involute?)
ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle transection
deeply arcuate or annular; petiole with small adaxial inverted bundles.
Venation pinnate. Stomata? Cuticular wax crystalloids? Lamina on both sides
usually striated by subepidermal fibres. Mesophyll with calciumoxalate druses.
Leaf margin serrate (often serrate-dentate) or entire.
Inflorescence Usually terminal
(in Clavija and Neomezia lateral), racemes, corymbs or
panicles (flowers rarely solitary). Bracts inserted on pedicels. Floral
prophylls (bracteoles) absent?
Flowers Actinomorphic.
Hypogyny to half epigyny. Sepals (four or) five, with imbricate aestivation,
persistent, free or somewhat connate at base. Petals (four or) five, with
imbricate aestivation, usually slightly unequal in size, usually connate into a
campanulate or infundibuliform corolla. Petals and stamens developing from
common primordia. Nectary? Disc absent.
Androecium Stamens (four or)
five, antepetalous, alternisepalous. Filaments flattened, connate usually only
at base (in Clavija often entirely connate into tube), adnate to lower
part of corolla tube. Anthers forming cone in centre of young flower, later
spreading, basifixed to dorsifixed, sometimes versatile?, tetrasporangiate,
extrorse or introrse, longicidal (dehiscing by longitudinal slits); connective
in Neomezia and Theophrasta prolonged at apex, ab initio
incurved above stigma. Nectariferous hairs sometimes present. Tapetum
secretory, with uninucleate cells. Staminodia (four or) five, antesepalous,
alternipetalous, extrastaminal, epipetalous (inserted above fertile stamens),
petaloid, gibbous, narrow or triangular, often glanduliferous. Upper and lower
parts of thecae filled with druses and single crystals of calciumoxalate.
Pollen grains
Microsporogenesis simultaneous. Pollen grains tricolporate (or tetracolporate),
shed as monads, bicellular at dispersal. Exine tectate or semitectate, with
columellate infratectum, reticulate, foveolate, fossulate or rugulate.
Gynoecium Pistil composed of
(four or) five connate antepetalous carpels. Ovary superior or semi-inferior,
unilocular, mucilaginous. Style single, simple. Stigma capitate, punctate,
discoid, truncate or somewhat lobate, non-papillate Dry type, or papillate Wet
type. Pistillodium?
Ovules Placentation free
central to basal (central column sometimes reduced). Ovules numerous per ovary,
anatropous, hemitropous or campylotropous, ascending, bitegmic, tenuinucellar.
Micropyle bistomal. Outer integument two to four cell layers thick. Inner
integument ? cell layers thick. Megagametophyte monosporous, Polygonum
type. Endosperm development ab initio nuclear. Endosperm haustoria?
Embryogenesis?
Fruit Usually a one- or
several-seeded berry with dry (sometimes woody) pericarp and juicy placentae
(rarely a one-seeded drupe).
Seeds Aril absent. Seed coat
exotestal. Testa multiplicative. Exotestal cells flattened, with thick walls.
Hypodermal cells sometimes with thick anticlinal walls. Mesotestal cells often
with crystals. Endotesta? Tegmen? Perisperm not developed. Endosperm copious,
hard, oily (in many species with thick cell walls with pores), amyloid? Embryo
large, straight, well differentiated, chlorophyll? Cotyledons two, usually
foliaceous (sometimes small). Germination phanerocotylar.
Cytology n = 14, 20, (24)
DNA
Phytochemistry Insufficiently
known. Saponins present. Ellagic acid, proanthocyanidins, alkaloids, and
cyanogenic compounds not found. Polyphenols? Glycosides?
Use Ornamental plants, fruits
(Clavija), fish poison, soap (bark and cortex of
Jacquinia).
Systematics Clavija
(c 55; southern Nicaragua to Brazil, Hispaniola), Theophrasta
(2; T. americana, T. jussieui; Hispaniola), Neomezia
(1; N. cubensis; Cuba), Jacquinia
(13; the West Indies), Bonellia (22; tropical America), Deherainia
(3; D. lageniformis, D. matudai, D. smaragdina;
Central America), Votschia (1; V. nemophila; Panamá).
Theophrastaceae are sister to
Samolus
(Samolaceae).
 |
Cladogram of three of the genera in Theophrastaceae based
on DNA sequence data (Ståhl 2010).
|
Literature
Abbott CL. 1936. The phylogeny of the Ericales. – Trillia 9: 62-69.
Abe JP. 2005. An arbuscular mycorrhizal genus
in the Ericaceae. – Inoculum 56(4):
6.
Abou-El-Enain MM. 2006. Chromosomal
variability in the genus Primula (Primulaceae). – Bot. J. Linn. Soc.
150: 211-219.
Ackerman WI. 1973. Species compatibility
relationships within the genus Camellia. – J. Hered. 64: 356-358.
Adams JE. 1940. A systematic study of the
genus Arctostaphylos Adans. – J. Elisha Mitchell Sci. Soc. 56:
1-62.
Adlassnig W, Peroutka M, Lendl T. 2011. Traps
of carnivorous pitcher plants as a habitat: composition of the fluid,
biodiversity and mutualistic activities. – Ann. Bot. 107: 181-194.
Affre L, Thompson JD. 1998. Floral trait
variation in four Cyclamen (Primulaceae) species. – Plant Syst.
Evol. 212: 279-293.
Agostini G. 1980. Una nueva clasificación
del género Cybianthus (Myrsinaceae). – Acta Biol. Venez. 10:
129-185.
Airy Shaw HK. 1936. Notes on the genus
Schima and on the classification of the Theaceae-Camellioïdeae. – Kew Bull.
1936: 496-499.
Airy Shaw HK. 1948. Studies in the Ericales VIII. A new section of
Vaccinium from the eastern Himalayas. – Kew Bull. 1948: 244-247.
Airy Shaw HK. 1951. Coris (Primulaceae?) in Somaliland. – Kew
Bull. 1951: 29-31.
Airy Shaw HK. 1964. Studies in the Ericales XIV. The systematic position of
the genus Diplarche. – Kew Bull. 17: 507-509.
Akiyama S, Wakabayashi M, Ohba H. 1992.
Chromosome evolution in Himalayan Impatiens (Balsaminaceae). – Bot. J. Linn. Soc.
109: 247-257.
Akuzawa E. 1985. Problems concerning the
period of occurrence of Kinabalu Mitrastemon. – Parasitic Plant Mag.
13: 23-27.
Akuzawa E. 1985. Confirmation of the
inflorescence of Mitrastemon in Mt. Kinabalu, North Borneo. –
Parasitic Plant Mag. 14: 33-37.
Albert VA, Williams SE, Chase MW. 1992.
Carnivorous plants: phylogeny and structural evolution. – Science 257:
1491-1495.
Albrecht DE, Hislop M. 2011. A revision of
Dielsiodoxa (Ericaceae:
Styphelioideae: Oligarrheneae). – Nuytsia 21: 107-126.
Albrecht DE, Owens CT, Weiller CM, Quinn CJ.
2010. Generic concepts in Ericaceae-Styphelioideae-the
Monotoca group. – Aust. Syst. Bot. 23: 320-332.
Allaway WG, Ashford AE. 1996. Structure of
the hair roots in Lysinema ciliatum R. Br. and its implications for
their water relations. – Ann. Bot., N. S., 77: 383-388.
Almeida Jr E, Araújo J, Santos-Filho F,
Zickel C. 2013. Leaf morphology and antomy of Manilkara Adans. (Sapotaceae) from northeastern Brazil. –
Plant Syst. Evol. 299: 1-9.
Alvarado JL, Ludlow-Wiechers B. 1982.
Catálogo palinológico para la flora de Veracruz 10. Familia Clethraceae. – Biotica 8: 619-629.
Alves-Araújo A, Alves M. 2011. Two new
species of Pouteria (Sapotaceae) from the Atlantic Forest in
Brazil. – Syst. Bot. 36: 1004-1007.
Alves-Araújo A, Alves M. 2012a. Two new
species and a new combination of Neotropical Sapotaceae. – Brittonia 64: 23-29.
Alves-Araújo A, Alves M. 2012b. Pouteria
ciliata, P. confusa, P. nordestinensis and P.
velutinicarpa spp. nov. (Sapotaceae) from Brazil. – Nord. J.
Bot. 30: 399-406.
Al Wadi H, Richards AJ. 1992. Palynological
variation in Primula L. subgenus Sphondylia (Duby) Rupr., and
its relationship with Dionysia Fenzl. – New Phytol. 121: 303-310.
Amaral MCE. 1991. Phylogenetische Systematik
der Ochnaceae. –
Bot. Jahrb. Syst. 113: 105-196.
An H-X, Cai D-R, Wang J-R, Quan N-F. 1983.
Investigations on early embryogenesis of Actinidia chinensis Planch.
var. chinensis. – Acta Bot. Sin. 25: 99-104.
Anderberg AA. 1992. The circumscription of
the Ericales, and their cladistic
relationships to other families of “higher” dicotyledons. – Syst. Bot.
17: 660-675.
Anderberg AA. 1993. Cladistic
interrelationships and major clades of the Ericales. – Plant Syst. Evol. 184:
207-231.
Anderberg AA. 1994a. Cladistic analysis of
Enkianthus with notes on the early diversification of the Ericaceae. – Nord. J. Bot. 14:
385-401.
Anderberg AA. 1994b. Phylogeny of the
Empetraceae, with special emphasis on character evolution in the genus
Empetrum. – Syst. Bot. 19: 35-46.
Anderberg AA. 1994c. Phylogeny and subgeneric
classification of Cyclamen L. (Primulaceae). – Kew Bull. 49:
455-467.
Anderberg AA. 2000. Maesaceae, a new primuloid family in the
order Ericales s.l. – Taxon 49:
183-187.
Anderberg AA. 2004. Primulaceae. – In: Kubitzki K (ed),
The families and genera of vascular plants VI. Flowering plants. Dicotyledons.
Celastrales,
Oxalidales, Rosales, Cornales, Ericales, Springer, Berlin, Heidelberg, New
York, pp. 313-319.
Anderberg AA, El-Ghazaly G. 2000. Pollen
morphology in Primula sect. Carolinella (Primulaceae) and its taxonomic
implications. – Nord. J. Bot. 20: 5-14.
Anderberg AA, Kelso S. 1996. Phylogenetic
implications of endosperm cell wall morphology in Douglasia,
Androsace and Vitaliana (Primulaceae). – Nord. J. Bot. 16:
481-486.
Anderberg AA, Ståhl B. 1995. Phylogenetic
interrelationships in the order Primulales, with special emphasis on the family
circumscriptions. – Can. J. Bot. 73: 1699-1730.
Anderberg AA, Swenson U. 2003. Evolutionary
lineages in Sapotaceae (Ericales): a cladistic analysis based on
ndhF sequence data. – Intern. J. Plant Sci. 164: 763-773.
Anderberg AA, Zhang X-P. 2002. Phylogenetic
relationships of Cyrillaceae and Clethraceae (Ericales) with special emphasis on the
genus Purdiaea Planch. – Organisms Divers. Evol. 2: 127-137.
Anderberg AA, Ståhl B, Källersjö M. 1998.
Phylogenetic relationships in the Primulales inferred from rbcL
sequence data. – Plant Syst. Evol. 211: 93-102.
Anderberg AA, Trift I, Källersjö M. 1998.
On the systematic position of the genus Coris (Primulaceae). – Nord. J. Bot. 18:
203-207.
Anderberg AA, Ståhl B, Källersjö M. 2000.
Maesaceae, a new primuloid family in
the order Ericales s.l. – Taxon 49:
183-187.
Anderberg AA, Trift I, Källersjö M. 2000.
Phylogeny of Cyclamen L. (Primulaceae): evidence from morphology
and sequence data from the internal transcribed spacers of nuclear ribosomal
DNA. – Plant Syst. Evol. 220: 147-160.
Anderberg AA, Peng C-I, Trift I, Källersjö
M. 2001. The Stimpsonia problem: evidence from DNA sequences of
plastid genes atpB, ndhF and rbcL. – Bot. Jahrb.
Syst. 123: 369-376.
Anderberg AA, Rydin C, Källersjö M. 2002.
Phylogenetic relationships in the order Ericales s.l.: analyses of molecular data
from five genes from the plastid and mitochondrial genomes. – Amer. J. Bot.
89: 677-687.
Anderberg AA, Manns U, Källersjö M. 2007.
Phylogeny and floral evolution of the Lysimachieae (Ericales, Myrsinaceae): evidence from
ndhF sequence data. – Willdenowia 37: 407-421.
Anderson AJ. 1988. Mycorrhizae-host
specificity and recognition. – Phytopathology 78: 375-378.
Anderson B, Midgley JJ, Stewart BB. 2003.
Facilitated selfing offers reproductive assurance: a mutualism between a
hemipteran and carnivorous plant. – Amer. J. Bot. 90: 1009-1015.
Anderson GJ, Bernardello G, Lopez P, Stuessy
TF, Crawford DJ. 2000. Dioecy and wind pollination in Pernettya rigida
(Ericaceae) of the Juan Fernandez
Islands. – Bot. J. Linn. Soc. 132: 121-141.
Anderson LC. 1983. Trichomes and stomata of
Gordonia lasianthus (Theaceae).
– Sida 10: 103-113.
Anderson RC, Beare MH. 1983. Breeding system
and pollination ecology of Trientalis borealis (Primulaceae). – Amer. J. Bot. 70:
408-415.
Anderson RC, Loucks OL. 1973. Aspects of the
biology of Trientalis borealis Raf. – Ecology 54: 798-808.
Andres H. 1914a. Studien zur speziellen
Systematik der Pirolaceae. – Österr. Bot. Zeitschr. 64: 45-50, 232-254.
Andres H. 1914b. Piroleen-Studien. –
Verhandl. Bot. Ver. Prov. Brandenburg 56: 1-76.
Andres H. 1936. Beiträge zur speziellen
Systematik der Pirolaceae. – Feddes Repert. 40: 233-236.
Antlfinger AE. 1986. Field germination and
seedling growth of chasmogamous and cleistogamous progeny of Impatiens
capensis (Balsaminaceae). –
Amer. J. Bot. 73: 1267-1273.
Appel O. 1996. Morphology and systematics of
the Scytopetalaceae. – Bot. J. Linn. Soc. 121: 207-227.
Appel O. 2004. Scytopetalaceae. – In:
Kubitzki K (ed), The families and genera of vascular plants VI. Flowering
plants. Dicotyledons. Celastrales,
Oxalidales, Rosales, Cornales, Ericales, Springer, Berlin, Heidelberg, New
York, pp. 426-429.
Aranha Filho, JLM, Fritsch PW, Almeda F,
Martins AB. 2007. Symplocos saxatilis (Symplocaceae), a new dioecious species
from Serra do Cipó, Minas Gerais, Brazil. – Brittonia 59: 233-237.
Aranha Filho JLM, Fritsch PW, Almeda F,
Martins AB. 2009. Cryptic dioecy is widespread in South American species of
Symplocos section Barberina (Symplocaceae). – Plant Syst. Evol.
244: 99-104.
Arber A. 1941. On the morphology of the
pitcher-leaves in Heliamphora, Sarracenia,
Darlingtonia, Cephalotus, and Nepenthes. – Ann.
Bot., N. S., 5: 563-578.
Arends JC. 1976. Somatic chromosome numbers
of some African Sapotaceae. – Acta
Bot. Neerl. 25: 449-457.
Argent G. 2006. Rhododendrons of Subgenus
Vireya. – Royal Horticultural Society.
Arisawa M, Pezzuto J, Kinghorn A, Douglas A,
Cordell G, Farnsworth N. 1984. Plant anticancer agents 30. Cucurbitacins from
Ipomopsis aggregata (Polemoniaceae). – J. Pharmaceut.
Sci. 73: 411-413.
Arisumi T. 1974. Chromosome numbers and
breeding behavior of hybrids among Celebes, Java and New Guinea species of
Impatiens L. – Hort. Sci. 9: 478-479.
Arisumi T. 1980. Chromosome numbers and
comparative breeding behavior of certain Impatiens from Africa, India,
and New Guinea. – J. Amer. Soc. Hort. Sci. 105: 99-102.
Arroyo S. 1975. Lebetanthus, género
austroamericano de Epacridaceae y sus differencias con el género
Prionotes de Tasmania. – Darwiniana 19: 312-330.
Artopoeus A. 1903. Über den Bau und die
Öffnungsweise der Antheren und die Entwickelung der Samen der Erikaceen. –
Flora 92: 309-345.
Ashford AE, Allaway WG, Reed ML. 1996. A
possible role for thick-walled epidermal cells in the mycorrhizal hair roots of
Lysinema ciliatum R. Br. and other Epacridaceae. – Ann. Bot., 77:
375-381.
Assem J van den. 1953. Revision of the Sapotaceae of the Malaysian area in a
wider sense 4. Ganua Pierre ex Dubard. – Blumea 7: 364-400.
Assi LA. 2001. Observations sur la diversité
des Sapotaceae de la flore naturelle
de la Côte d’Ivoire. – Syst. Geogr. Plants 71: 187-195.
Atkinson RG, Cipriani G, Whittaker DJ,
Gardner RC. 1994. The allopolyploid origin of kiwifruit, Actinidia
deliciosa (Actinidiaceae). –
Plant Syst. Evol. 205: 111-124.
Atkinson R, Jong K, Argent G. 1995.
Cytotaxonomic observations in tropical Vaccinieae (Ericaceae). – Bot. J. Linn. Soc. 117:
135-145.
Auamcharoen W, Kijjoa A, Chandrapatya A,
Pinto MM, Silva AMS, Naengchomnong W, Herz W. 2009. A new tetralone from
Diospyros cauliflora. – Biochem. Syst. Ecol. 37: 690-692.
Aubréville A. 1961a. Notes sur les
Sapotacées de l’Afrique equatoriale. – Notulae Syst. (Paris) 16:
223-279.
Aubréville A. 1961b. Notes sur les
Pouteriées africaines et sud américaines. – Adansonia, sér II, 1: 6-38.
Aubréville A. 1962a. Notes sur des
Pouteriées américaines. – Adansonia, sér II, 1: 150-191.
Aubréville A. 1962b. Notes sur les
Sapotacées de la Nouvelle Calédonie. – Adansonia, sér II, 2: 172-199.
Aubréville A. 1963a. Notes sur les
Sapotacées. – Adansonia, sér. II, 3: 19-42.
Aubréville A. 1963b. Notes sur les
Poutériées océaniennes (Sapotacées). – Adansonia, sér. II, 3:
327-335.
Aubréville A. 1964a. Les Sapotacées:
taxonomie et phytogéographie. – Adansonia, Mém. 1: 1-157.
Aubréville A. 1964b. Notes sur des
Sapotacées III. 1. Réhabilitation des genres américains Ragala
Pierre et Prieurella Pierre. – Adansonia, sér. II, 4: 367-391.
Aubréville A. 1964c. Système de
classification des Sapotacées. – Adansonia, sér. II, 4. 38-42.
Aubréville A. 1965. Notes sur des
Sapotacées australiennes. – Adansonia, sér. II, 5: 21-26.
Aubréville A. 1967. Sapotacées. – In:
Aubréville A, Leroy J-F (eds), Flore de la Nouvelle Calédonie et Dépendances
1, Muséum National d’Histoire Naturelle, Paris, pp. 1-168.
Aubréville A. 1971. Éssais de
géophylétique des Sapotacées 2. – Adansonia, sér. II, 11: 425-436.
Aubréville A. 1974. 164e famille.
Sapotacées. – In: Humbert H (ed), Flore de Madagascar et des Comores,
Muséum National d’Histoire Naturelle, Paris, pp. 3-125.
Aubréville A, Pellegrin F. 1934. De quelques
Sapotacées de la Côte d’Ivoire. – Bull. Soc. Bot. France 81: 792-800.
Aurada E, Jurenitsch J, Kubelka W. 1982.
Structure of triterpene sapogenins from Polemonium caeruleum L. –
Sci. Phar. 50: 331-350.
Ayala-Nieto ML, Ludlow-Wiechers B. 1983.
Catálogo Palinológico para la Flora de Veracruz 13. Familia Ebenaceae. – Biótica 8: 215-226.
Ayensu EA. 1972. Morphology and anatomy of
Synsepalum dulcificum (Sapotaceae). – Bot. J. Linn. Soc. 65:
179-187.
Baas P. 1985. Comparative leaf anatomy of
Pernettya Gaud. (Ericaceae).
– Bot. Jahrb. Syst. 105: 481-495.
Bačić T, Lawrence TJ, Cutler DF. 1992. Leaf
anatomy of an Arbutus taxon from Yugoslavia. – Kew Bull. 47:
535-543.
Baehni C. 1938. Mémoires sur les Sapotacées
I. Système de classification. – Candollea 7: 394-507.
Baehni C. 1942. Mémoires sur les Sapotacées
II. Le genre Pouteria. – Candollea 9: 147-475.
Baehni C. 1964. Genres nouveaux de
Sapotacées. – Arch. Sci. 17: 77-79.
Baehni C. 1965. Mémoires sur les Sapotacées
III. Inventaire des genres. – Boissiera 11: 1-261.
Bailey IW. 1922. The pollination of
Marcgravia: a classical case of ornithophily? – Amer. J. Bot. 9:
370-384.
Baillon HE. 1890. Observations sur les
Sapotacées de la Nouvelle-Calédonie. – Bull. Mens. Soc. Linn. Paris 2:
889-896.
Baillon HE. 1891. Remarques sur les
Ternstroemiacées. – Bull. Mens. Soc. Linn. Paris, sér. II, 121: 965-966.
Baker HA, Oliver EGH. 1967. Ericas in
southern Africa. – Cape Town, Johannesburg.
Bakhuizen van den Brink RC. 1936-1955.
Revisio Ebenacearum Malayensium. – Bull. Jard. Bot. Buitenzorg III, 15: 1-49
(1936), 50-368 (1938), 369-515 (1941); i-xx, 1-92 (1955).
Baldwin JT. 1939. Chromosomes of the Diapensiaceae: a cytological approach
to a phylogenetic problem. – J. Hered. 30: 169-171.
Baldwin JT. 1941. Galax: The genus
and its chromosomes. – J. Hered. 32: 249-254.
Balthazar M von, Schönenberger J. 2013.
Comparative floral structure and systematics in the balsaminoid clade including
Balsaminaceae, Marcgraviaceae and Tetrameristaceae (Ericales). – Bot. J. Linn. Soc. 173:
325-386.
Banik D, Sanjappa M. 2008. Notes on identity
of some Indian species of Agapetes (Ericaceae). – Nord. J. Bot. 26: 4-9.
Baretta-Kuipers T. 1976. Comparative wood
anatomy of Bonnetiaceae,
Theaceae, and Guttiferae. – In: Baas
P, Bolton AJ, Catling DM (eds), Wood structure in biological and technological
research, Leiden, Bot., Ser. III, Leiden University Press, Leiden, Netherlands,
pp. 76-101.
Barker WR. 1980. Taxonomic revisions in Theaceae in Papuasia 1. Gordonia,
Ternstroemia, Adinandra and Archboldiodendron. –
Brunonia 3: 1-60.
Barrett RL. 2006. A review of
Planchonia (Lecythidaceae)
in Australia. – Aust. Syst. Bot. 19: 147-153.
Barrett SCH, Harder LD, Worley AC. 1996. The
comparative biology of pollination and mating in flowering plants. – Phil.
Trans. Roy. Soc. London, Sect. B, 351: 1271-1280.
Barros MA, Barth OM. 1994. Catálogo
sistemático do pólen das plantas arbóreas do Brasil meridional 28. Burseraceae e Clethraceae. – Revista Brasil. Biol.
54: 317-322.
Barry AR, Perez de Paz J. 1979. Estudio
anatómico/palinológico Myrsinaceae
y Sapotaceae en la region
Macaronésica. – Bot. Macaron. 5: 21-39.
Barth OM. 1973. ‘Caracteres
palinológicos’. – In: Rizzini CT. 1973. Sobre Pouteria venosa
(Mart.) Baehni e espécies afins (Sapotaceae). – Rev. Brasil. Biol. 33:
595-606.
Barth OM. 1979. Pollen morphology of
Brazilian Symplocos. – Grana 18: 99-107.
Barth OM. 1982. The sporoderm of Brazilian
Symplocos pollen types (Symplocaceae). – Grana 21: 65-69.
Bartish IV, Swenson U, Munzinger J, Anderberg
AA. 2005. Phylogenetic relationships among New Caledonian Sapotaceae (Ericales): molecular evidence for generic
polyphyly and repeated dispersal. – Amer. J. Bot. 92: 667-673.
Bartish IV, Antonelli A, Richardson JE,
Swenson U. 2011. Vicariance or long-distance dispersal: historical biogeography
of the pantropical subfamily Chrysophylloideae (Sapotaceae). – J. Biogeogr. 38:
177-190.
Barua PK, Wight W. 1959. Leaf sclereids in
the taxonomy of the camellias I. Wilson’s and related camellias. –
Phytomorphology 8: 257-264.
Basinger JF, Christophel DC. 1985. Fossil
flowers and leaves of the Ebenaceae
from the Eocene of southern Australia. – Can. J. Bot. 63: 1825-1843.
Bate-Smith EC. 1964. Chemistry and taxonomy
of Fouquieria splendens Engelm.: a new member of the asperuloside
group. – Phytochemistry 3: 623-625.
Bawa B. 1970. Theaceae. – Bull. Indian Natl. Sci. Acad.
41: 75-77.
Bayer RJ, Hufford L, Soltis DE. 1996.
Phylogenetic relationships in Sarraceniaceae based on rbcL
and ITS sequences. – Syst. Bot. 21: 121-134.
Baylis GTS. 1975. The magnolioid mycorrhiza
and mycotrophy in root systems derived from it. – In: Sanders FE, Mosse B,
Tinker PB (eds), Endomycorrhizas. Proceedings of a symposium held at the
University of Leeds, 22-25 July 1974, Academic Press, London, pp. 373-389.
Beauvisage L. 1920. Contribution à
l’étude anatomique de la famille des Ternstroemiacées. – E. Arrault,
Tours, France.
Beck AR, Weigle JL, Kruger EW. 1974. Breeding
behavior and chromosome numbers among New Guinea and Java Impatiens
species, cultivated varieties, and their interspecific hybrids. – Can. J.
Bot. 52: 923-925.
Bedell HG. 1981. Leaf architecture and foliar
sclereids in Marcgraviaceae
(Theales). – Publ. Bot. Soc. Amer., Misc. Ser. 160: 62.
Bedell HG. 1985. A generic revision of Marcgraviaceae I. The
Norantea complex. – Ph.D. diss., University of Maryland, College
Park, Maryland.
Beentje HJ. 1990. Name changes in East
African Ericaceae. – Utafiti 3(1):
13.
Behnke H-D. 1976. Sieve-element plastids of
Fouquieria, Frankenia (Tamaricales), and
Rhabdodendron (Rutaceae), taxa
sometimes allied with Centrospermae (Caryophyllales).
– Taxon 25: 265-268.
Behnke H-D. 1982. Sieve-element plastids of
Cyrillaceae, Erythroxylaceae,
and Rhizophoraceae:
description and significance of subtype PV plastids. – Plant Syst. Evol. 141:
31-39.
Bell CD, Patterson RW. 2000. Molecular
phylogeny and biogeography of Linanthus (Polemoniaceae). – Amer. J. Bot. 87:
1857-1870.
Bell CD, Patterson R, Hamilton LA. 2000.
Sectional integrity in Linanthus (Polemoniaceae): a molecular phylogeny
of Section Dianthoides. – Syst. Bot. 24: 632-644.
Bell CR. 1949. A cytotaxonomic study of the
Sarraceniaceae of North America.
– J. Elisha Mitchell Sci. Soc. 65: 137-166.
Bell CR. 1952. Natural hybrids in the genus
Sarracenia I. History, distribution, and taxonomy. – J. Elisha
Mitchell Sci. Soc. 68: 55-80.
Bell TL. 1995. Biology of Australian
Epacridaceae: with special reference to growth, fire response and mycorrhizal
nutrition. – Ph.D. diss., Department of Botany, University of Western
Australia.
Bell TL, Ojeda F. 1999. Underground storage
in Erica species of the Cape floristic region – differences between
seeders and resprouters. – New Phytol. 144: 143-152.
Bell TL, Pate JS. 1996a. Growth and fire
response of selected Epacridaceae of south-western Australia. – Aust. J. Bot.
44: 509-526.
Bell TL, Pate JS. 1996b. Nitrogen and
phosphorus nitrution in mycorrhizal Epacridaceae of South-west Australia. –
Ann. Bot., 77: 389-397.
Bell TL, Pate JS, Dixon KW. 1996.
Relationships between fire response, morphology, root anatomy and starch
distribution in south-west Australian Epacridaceae. – Ann. Bot., 77:
357-364.
Bennell AP, Hu C-M. 1983. The pollen
morphology and taxonomy of Lysimachia. – Notes Roy. Bot. Gard.
Edinb. 40: 425-458.
Bennett ST, Grimshaw JM. 1991. Cytological
studies in Cyclamen subg. Cyclamen (Primulaceae). – Plant Syst. Evol. 176:
135-143.
Berazaín R, Rodríguez S. 1992. Novedades
taxonómicas en el género Purdiaea Planchon (Cyrillaceae) en Cuba. – Rev. Jard.
Bot. Nac. Univ. Habana 13: 21-25.
Berazaín IR Sorribes A BE. 1987. El género
Kalmia L. (Ericaceae) en Cuba.
– Rev. Jard. Bot. Nac. Univ. Habana 8: 3-17.
Bergman HF. 1920. Internal stomata in
ericaceous and other unrelated fruits. – Bull. Torrey Bot. Club 47:
213-221.
Berry EW. 1924. A fossil flower from the
Miocene of Trinidad. – Amer. J. Sci. 7: 103-108.
Berry PE. 1999. A synopsis of the family
Lissocarpaceae. – Brittonia 51: 214-216.
Berry PE. 2001. Lissocarpaceae. – In: Berry
PE, Yatskievych K, Holst BK (eds), Flora of the Venezuelan Guayana 6, Missouri
Botanical Garden Press, St. Louis, pp. 19-20.
Berry PE, Savolainen V, Sytsma KJ, Hall JC,
Chase MW. 2001. Lissocarpa is sister to Diospyros (Ebenaceae). – Kew Bull. 56: 725-729.
Bezbaruah HP. 1971. Cytological
investigations in the family Theaceae I.
Chromosome numbers in some Camellia species and allied genera. –
Caryologia 24: 423-426.
Bhatnagar SP, Gupta M. 1969. Structure and
ontogeny of stomata in Mimusops elengi. – J. Indian Bot. Soc. 48:
362-367.
Bhatnagar SP, Gupta M. 1970. Morphology and
embryology of Mimusops elengi L. – Bot. Not. 123: 455-473.
Bidartondo MI, Bruns TD. 2001. Extreme
specificity in epiparasitic Monotropoideae (Ericaceae): widespread phylogenetic and
geographical structure. – Mol. Ecol. 10: 2285-2295.
Bidartondo MI, Bruns TD. 2002. Fine-level
mycorrhizal specificity in the Monotropoideae (Ericaceae): specificity for fungal species
groups. – Mol. Ecol. 11: 557-569.
Bittrich V, Amaral MCE, Melo GAR. 1993.
Pollination biology of Ternstroemia laevigata and T. dentata
(Theaceae). – Plant Syst. Evol. 185:
1-6.
Björkman E. 1960. Monotropa
hypopithys L. – an epiparasite on tree roots. – Physiol. Plant. 13:
308-327.
Blasdale WC. 1945. Composition of the solid
secretion produced by Primula denticulata. – J. Amer. Chem. Soc. 67:
493-495.
Blasdale WC. 1948. The cultivated species of
Primula. – University of California Press, Berkeley, California.
Bloembergen S. 1952. A critical study in the
complex-polymorphous genus Schima (Theaceae). – Reinwardtia 2: 133-183.
Bobbit JM, Rao KV, Kiely DE. 1966. The
constituents of Monotropa uniflora. – Lloydia 29: 90-93.
Böcher TW. 1981. Evolutionary trends in
Ericalean leaf structure. – Kong. Danske Vidensk.-Selsk. Biol. Skr. 23(2):
1-64.
Boesewinkel FD, Bouman F. 1991. The
development of bi- and unitegmic ovules and seeds in Impatiens (Balsaminaceae). – Bot. Jahrb. Syst.
113: 87-104.
Bohm BA, Averett JE. 1989. Flavonoids in some
Monotropoideae. – Biochem. Syst. Ecol. 17: 399-401.
Bohm BA, Brim SW, Hebda RJ, Stevens PF. 1978.
Generic limits in the tribe Cladothamneae (Ericaceae) and its position in the
Rhododendroideae. – J. Arnold Arbor. 59: 311-341.
Bohm BA, Banek HM, Maze JR. 1984. Flavonoid
variation in North American Menziesia (Ericaceae). – Syst. Bot. 9: 324-345.
Bokdam J. 1977. Seedling morphology of some
African Sapotaceae and its taxonomical
significance. – Meded. Landbouwhogesch. Wageningen 77(20): 1-84.
Bokhari MH. 1988. Importance of foliar
anatomy in taxonomy and phylogeny of Dionysia. – Biologia (Lahore)
34: 367-395.
Bokhari MH, Wendelbo P. 1976. Anatomy of
Dionysia I. Foliar sclereids. – Notes Roy. Bot. Gard. Edinb. 35:
131-141.
Bokhari MH, Wendelbo P. 1985. Anatomy of
Dionysia II. Xeromorphic features. – Notes Roy. Bot. Gard. Edinb.
42: 327-345.
Bone RE, Strijk JS, Fritsch PW, Buerki S,
Strasberg D, Thébaud C, Hodkinson TR. 2012. Phylogenetic inference of
Badula (Primulaceae), a rare
and threatened genus endemic to the Mascarene Archipelago. – Bot. J. Linn.
Soc. 169: 284-296.
Bonfante-Fasolo P, Gianinazzi-Pearson V,
Martinengo L. 1984. Ultrastructural aspcts of endomycorrhiza in the Ericaceae IV. Comparison of infection by
Pezizella ericae in host and non-host plants. – New Phytologist 98:
329-333.
Bonnefille R. 1971. Atlas des pollens
d’Éthiopie. Principales espèces de forêts de montagne. – Pollen Spores
13: 15-72.
Boojh R, Ramakrishnan PS. 1982. Seed
germination and seedling establishment of two closely related Schima
species. – Proc. Indian Acad. Sci Plant Sci. 91: 397-407.
Boojh R, Ramakrishnan PS. 1983. The growth
pattern of two species of Schima. – Biotropica 15: 142-147.
Boratyński A, Puente MLV de la. 1995. The
Empetraceae on the Iberian Peninsula. – Willdenowia 25: 39-53.
Borhidi A, Muñiz O. 1978. El género
Jacquinia (Theophrastaceae) en Cuba. – Plant
Syst. Evol. 129: 1-11.
Boyd DW, Harris WM, Murry LE. 1982. Sclereid
development in Camellia petioles. – Amer. J. Bot. 69: 339-347.
Boykin LM, Vasey MC, Parker VT. 2005. Two
lineages of Arctostaphylos (Ericaceae) identified using the internal
transcribed spacer (ITS) region of the nuclear genome. – Madroño 52:
139-147.
Bradshaw WE, Creelman RA. 1984. Mutualism
between the carnivorous purple pitcher plant and its inhabitants. – Amer.
Natur. 112: 294-304.
Brand A. 1908. Polemoniacea peruviana. –
Engl. Bot. Jahrb. Syst. 42: 174-175.
Braukmann T, Stafanović S. 2012. Plastid
genome evolution in mycoheterotropich Ericaceae. – Plant Mol. Biol. 79:
5-20.
Breteler FJ. 2002. Scytopetalaceae are
stipulate. – Kew Bull. 57: 759-761.
Breuer B, Stuhlfauth T, Fock H, Huber H.
1987. Fatty acids of some Cornaceae, Hydrangeaceae, Aquifoliaceae, Hamamelidaceae
and Styracaceae. – Phytochemistry
26: 1441-1445.
Briggs CL, Ashford AE. 2001. Structure and
composition of the thick wall in hair root epidermal cells of Woolsia
pungens. – New Phytol. 149: 219-232.
Brough P. 1924. Studies in the Epacridaceae
I. The life history of Styphelia longifolia (R. Br.). – Proc. Linn.
Soc. New South Wales 49: 162-178.
Brown EA, Streiber N. 1999. Systematic
studies in Dracophyllum (Epacridaceae) 2. New species of
Dracophyllum in New South Wales. – Telopea 8: 393-401.
Brown EGS. 1935. The floral mechanism of
Saurauia subspinosa Anth. – Trans. Proc. Bot. Soc. Edinb. 31:
485-497.
Brown GK. 2003. Vireya
rhododendrons: an insight into their relationships. – In: Argent G, McFarlane
M (eds), Rhododendrons in horticulture and science, Royal Botanic Gardens,
Edinburgh, pp. 95-110.
Brown GK, Craven LA, Udovicic F, Ladiges PY.
2006a. Phylogeny of Rhododendron section Vireya (Ericaceae) based on two non-coding regions
of cpDNA. – Plant Syst. Evol. 257: 57-93.
Brown GK, Craven LA, Udovicic F, Ladiges PY.
2006b. Phylogenetic relationships of Rhododendron section
Vireya (Ericaceae) inferred
from the ITS nrDNA region. – Aust. Syst. Bot. 19: 329-342.
Brown GK, Nelson G, Ladiges PY. 2006.
Historical biogeography of Rhododendron section Vireya and
the Malesian archipelago. – J. Biogeogr. 33: 1929-1944.
Brown WL, Clark RB. 1940. The chromosome
complement of Bumelia lanuginosa and its phylogenetic significance.
– Amer. J. Bot. 27: 237-239.
Bruce AN. 1907. On the distribution,
structure, and function of the tentacles of Roridula. – Notes Roy.
Bot. Gard. Edinb. 17: 83-98.
Brummitt RK. 1972. Nomenclatural and
historical considerations concerning the genus Galax. – Taxon 21:
303-317.
Brundell DJ. 1975a. Flower development of the
Chinese gooseberry (Actinidia chinensis Planch.) I. Development of the
flowering shoot. – New Zealand J. Bot. 13: 473-483.
Brundell DJ. 1975b. Flower development of the
Chinese gooseberry (Actinidia chinensis Planch.) II. Development of
the flower bud. – New Zealand J. Bot. 13: 485-496.
Brunotte C. 1900. Recherches embryogéniques
et anatomiques sur quelques espèces des genres Impatiens (L.) et
Tropaeolum (L.). – Ph.D. diss., L’Université de Paris, France.
Bruun HG. 1930. The cytology of the genus
Primula. – Svensk Bot. Tidskr. 24: 468-475.
Bruun HG. 1932. Cytological studies in
Primula with special reference to the relation between the karyology
and taxonomy of the genus. – Symb. Bot. Upsal. 1: 1-239.
Buckmire RE, Francis FJ. 1976. Anthocyanins
and flavonols in miracle fruit Synsepalum dulcificum Schum. – J.
Food Sci. 41: 1363-1365.
Bullock AA. 1954. A new name for
Lagenocarpus Klotzsch (Ericaceae). – Kew Bull. 1953: 533.
Bunge A. 1871. Die Arten der Gattung
Dionysia Fenzl. – Bull. Acad. Imp. Sci. St. Petersburg 16:
547-563.
Burrows J. 1999. Myrsine. – Plant
Life (South Afr.) 20: 25-26.
Burton TL, Husband BC. 1999. Population
cytotype structure in the polyploid Galax urceolata (Diapensiaceae). – Heredity 82:
381-390.
Busch P. 1913. Anatomisch-systematische
Untersuchung der Gattung Diospyros. – Wilhelm Greven, Crefeld.
Bush CM, Kron KA. 2008. A phylogeny of
Bejaria (Ericaceae:
Ericoideae) based on molecular data. – J. Bot. Res. Inst. Texas 2:
1193-1205.
Bush CM, Wagstaff SJ, Fritsch PW, Kron KA.
2009. The phylogeny, biogeography and morphological evolution of
Gaultheria (Ericaceae) from
Australia and New Zealand. – Aust. Syst. Bot. 22: 229-242.
Bush CM, Lu L, Fritsch PW, Li DZ, Kron KA.
2009. Phylogeny of Gaultherieae (Ericaceae: Vaccinioideae) based on DNA
sequence data from matK, ndhF and ITS. – Intern. J. Plant
Sci. 170: 355-364.
Cador L. 1900. Blattstruktur der
matelieferenden Symplocos-Arten. – Bot Centralbl. II, 184: 248-251,
345, 369-371.
Cairney JWG, Ashford AE. 2002. Biology of
mycorrhizal associations of epacrids (Ericaceae). – New Phytol. 154:
305-326.
Callan HG. 1941. The cytology of
Gaultheria wisleyensis (Marchant) Rehder, a new mode of species
formation. – Ann. Bot., N. S., 5: 579-585.
Camp WH. 1939. Studies in the Ericales IV. Notes on Chimaphila,
Gaultheria and Pernettya in Mexico and adjacent regions. –
Bull. Torrey Bot. Club 66: 7-28.
Camp WH. 1940. Aphyllous forms in
Pyrola. – Bull. Torrey Bot. Club 67: 453-465.
Camp WH. 1941. Studies in the Ericales. A discussion of the genus
Befaria in North America. – Bull. Torrey Bot. Club 68: 100-111.
Cane JH, 1993. Role of sterile pollen in
Saurauia (Actinidiaceae),
a cryptically dioecious Neotropical tree. – Biotropica 25: 493-495.
Cane JH, Eickwort GC, Wesley FR, Spielholz J.
1985. Pollination ecology of Vaccinium stamineum (Ericaceae: Vaccinioideae). – Amer. J.
Bot. 72: 135-142.
Capuron R. 1962. Contributions à l’étude
de la flore forestière de Madagascar 4. Tsebona, genre nouveau de
Sapotacées de Madagascar. – Adansonia, sér. II, 2: 122.
Caratini C. 1974. Sapotaceae. – In: Caratini C, Guinet PC
(eds), Pollen et spores d’Afrique tropicale, A.P.L.E. Travaux et Documents de
Géographie Tropicale.
Carey G, Fraser L. 1932. The embryology and
seedling development of Aegiceras majus Gaertn. – Proc. Linn. Soc.
New South Wales 57: 341-360.
Caris P. 2013. Bloemontogenetische patronen
in de Ericales sensu lato. – Leuven
Groep Wetenschap & Technologie, Leuven, Belgium.
Caris P, Smets EF. 2004. A floral ontogenetic
study on the sister group relationship between the genus Samolus (Primulaceae) and the Theophrastaceae. – Amer. J. Bot.
91: 627-643.
Caris P, Ronse De Craene LP, Smets E,
Clinckemaillie D. 2000. Floral development of three Maesa species,
with special emphasis on the position of the genus within Primulales. – Ann.
Bot. 86: 87-97.
Caris P, Ronse De Craene LP, Smets E,
Clinckemaillie D. 2002. The uncertain systematic position of Symplocos
(Symplocaceae): evidence from a
floral ontogenetic study. – Intern. J. Plant Sci. 163: 67-74.
Caris P, Geuten KP, Janssens SB, Smets EF.
2006. Floral development in three species of Impatiens (Balsaminaceae). – Amer. J. Bot. 93:
1-14.
Carlquist SJ. 1976. Wood anatomy of Roridulaceae: ecological and
phylogenetic implications. – Amer. J. Bot. 63: 1003-1008.
Carlquist SJ. 1984 [1985]. Wood anatomy and
relationships of Pentaphylacaceae: significance of
vessel features. – Phytomorphology 34: 84-90.
Carlquist SJ. 1988. Wood anatomy of
Scytopetalaceae. – Aliso 12: 63-76.
Carlquist SJ. 1989. Wood and bark anatomy of
Empetraceae; comments on paedomorphosis in woods of certain small shrubs. –
Aliso 12: 497-515.
Carlquist SJ. 2001. Wood anatomy of Fouquieriaceae in relation to habit,
ecology, and systematics; nature of meristems in wood and bark. – Aliso 19:
137-163.
Carlquist SJ, Schneider EL. 2004. Perforation
plate pit membrane remanants and other vessel details of Clethraceae: primitive features in wood
of Ericales. – Intern. J. Plant Sci.
165: 369-375.
Carlquist SJ, Schneider EL. 2005. Vestigial
pit membrane remnants in perforation plates and helical thickening in vessels
of Ericaceae. – Nord. J. Bot. 23:
353-363.
Carlquist SJ, Eckhart VM, Michener DC. 1984.
Wood anatomy of Polemoniaceae. –
Aliso 10: 547-572.
Carrasquel N. 1970. Estudios
anátomo-morfológicos de las especies del género Jacquinia en
Venezuela para su interpretación taxonómica. – Acta Bot. Venezuel. 4:
303-357.
Carrijo TT, Freitas M de F, Peixoto AL. 2012.
The genus Stylogyne (Myrsinoideae-Primulaceae) in Brazil. – Syst. Bot.
37: 478-489.
Carrion JS, Delgado MJ, Garcia M. 1993.
Pollen grain morphology of Coris (Primulaceae). – Plant Syst. Evol. 184:
89-100.
Case FW, Case RB. 1974. Sarracenia
alabamensis, a newly recognized species from central Alabama. – Rhodora
76: 650-655.
Case FW, Case RB. 1976. The Sarracenia
rubra complex. – Rhodora 78: 270-325.
Chamberlain DF, Rae SJ. 1990. A revision of
Rhododendron IV. Subgenus Tsutsusi. – Edinburgh J. Bot. 47:
89-200.
Chambers KL. 1960. On the origin of an
unusual Dipholis from the Florida Keys. – Tropical Woods 112:
40-57.
Chandler B. 1911. Deherainia
smaragdina Dcne. – Notes Roy. Bot. Gard. Edinb. 5: 49-56.
Chang H-T. 1963. Euryodendron, a new
genus of Theaceae. – Acta Sci. Nat.
Univ. Sunyatseni 1963: 129-132. [In Chinese]
Chang H-T. 1976. Apterosperma –
genus novum theacearum. – Acta Sci. Nat. Univ. Sunyatseni 1976: 89-92. [In
Chinese]
Chang H-T. 1981. A taxonomy of the genus
Camellia. – Acta Sci. Nat. Univ. Sunyatseni, Monogr. Ser. 1: 1-180.
[In Chinese]
Chang H-T. 1996. Diagnosis on the systematic
development of Theaceae I. A review on
the sections Chrysantha and Archaecamellia of the genus
Camellia. – Acta Sci. Nat. Univ. Sunyatseni 35: 77-83.
Chang H-T, Bartholomew B. 1984. Camellias.
– BT Batsford, London, and Timber Press, Portland, Oregon.
Chantaranothai P. 1995a.
Barringtonia (Lecythidaceae) in Thailand. – Kew
Bull. 50: 677-694.
Chantaranothai P. 1995b. Six new species of
Barringtonia (Lecythidaceae). – Kew Bull. 50:
695-705.
Chat J, Jauregui B, Petit RJ, Nadot S. 2004.
Reticulate evolution in kiwifruit (Actinidia, Actinidiaceae) identified by comparing
their material and paternal phylogenies. – Amer. J. Bot. 91: 736-747.
Cheek M, Fischer E. 1999. A tuberous and
epiphytic new species of Impatiens (Balsaminaceae) from Southwest
Cameroon. – Kew Bull. 54: 471-475.
Chen F-H, Hu C-M. 1979. Taxonomic and
phytogeographic studies on Chinese species of Lysimachia. – Acta
Phytotaxon. Sin. 17: 21-53.
Chen C-T. 1995. Changiostyrax, a new
genus of Styracaceae from China. –
Guihaia 15: 289-292.
Chen C-T, Li G-Y. 1997. A new species of
Sinojackia Hu (Styracaceae)
from Zhejiang, east China. – Novon 7: 350-352.
Cherry W, Gadek PA, Brown EA, Heslewood MM,
Quinn CJ. 2001. Pentachondra dehiscens sp. nov. – an aberrant new
member of Styphelieae. – Aust. Syst. Bot. 14: 513-533.
Chesnais F. 1944. Étude anatomique du genre
Sarcosperma Hook. f. (Sarcospermacées). – Bull. Mus. Natl. Hist.
Nat. Paris, sér. II, 16: 514-518.
Chester E. 1966. A biosystematic study of the
genus Halesia, Ellis (Styracaceae). – Ph.D. diss.,
University of Tennessee, Knoxville, Tennessee.
Chirtoiü M. 1918. Lacistemacées et
Symplocacées. – Bull. Soc. Bot. Genève XI(10): 350-361.
Chou Y-L. 1952. Floral morphology of three
species of Gaultheria. – Bot. Gaz. 114: 198-221.
Christensen NL. 1977. The role of carnivory
in Sarracenia flava L. with regard to specific nutrient deficiencies.
– J. Elisha Mitchell Sci. Soc. 92: 144-147.
Christoph H. 1921. Untersuchungen über die
mycotrophen Verhältnisse der ‘Ericales’ und die Keimung von Pyrolaceen.
– Beih. Bot. Centralbl. 38: 115-157.
Christophel DC, Basinger JF. 1982. Earliest
floral evidence for the Ebenaceae in
Australia. – Nature 296: 439-441.
Chrtek J, Slavíková Z, Studnička M. 1992.
Beitrag zur Morphologie und Taxonomie der Familie Sarraceniaceae. – Preslia 64:
1-10.
Chuang T-I, Hsieh WC, Wilken DH. 1978.
Contribution of pollen morphology to systematics of Collomia (Polemoniaceae). – Amer. J. Bot. 65:
450-458.
Chun WY. 1925. Two new trees from Chekiang.
– J. Arnold Arbor. 6: 144-145.
Chun WY, Chun F. 1935. Notes on some Chinese
Styracaceae. – Sunyatsenia 3:
28-35.
Chung J-D, Lin T-P, Chen Y-L, Cheng Y-P,
Hwang S-Y. 2007. Phylogeographic study reveals the origin and evolutionary
history of a Rhododendron species complex in Taiwan. – Mol.
Phylogen. Evol. 42: 14-24.
Chung M-G, Kang S-S. 1996. Comparisons of
genetic diversity in a narrow endemic, Impatiens hypophylla, and in
its widespread congener, I. textori (Balsaminaceae). – Nord. J. Bot. 16:
359-365.
Clemants SE. 1995. Ericaceae subfamily Rhododendroideae 6.
Bejaria Mutis ex Linnaeus. – In: Luteyn JL (ed), Flora neotropica.
Monograph 66. Ericaceae, part II. The
superior-ovaried genera, New York Botanical Garden, New York, pp. 54-106.
Clennett JCB. 2002. An analysis and revision
of Cyclamen L. with emphasis on subgenus Gyrophoebe O.
Schwarz. – Bot. J. Linn. Soc. 138: 473-481.
Clinckemaillie D, Smets EF. 1992. Floral
similarities between Plumbaginaceae
and Primulaceae: systematic
significance. – Belg. J. Bot. 125: 151-153.
Cochrane S, Day A. 1994. A heterostylous
Gilia (Polemoniaceae) from
central Nevada. – Madroño 41: 120-127.
Collins JP, Berkelhamer RC, Mesler M. 1977.
Notes on the natural history of the mangrove Pelliciera rhizophorae
Tr. & Pl. (Theaceae). – Brenesia
10/11: 17-29.
Collinson ME, Crane PR. 1978.
Rhododendron seeds from the Palaeocene of England. – Bot. J. Linn.
Soc. 76: 195-205.
Conran JG. 2004. Roridulaceae. – In: Kubitzki K (ed),
The families and genera of vascular plants VI. Flowering plants. Dicotyledons.
Celastrales,
Oxalidales, Rosales, Cornales, Ericales, Springer, Berlin, Heidelberg, New
York, pp. 339-342.
Conran JG, Dowd JM. 1993. The phylogenetic
relationships of Byblis and Roridula (Byblidaceae-Roridulaceae) inferred from partial 18S
ribosomal RNA sequences. – Plant Syst. Ecol. 188: 73-86.
Constance L. 1938. A revision of the genus
Douglasia Lindl. – Amer. Midl. Natur. 19: 249-259.
Conti E, Suring E, Boyd D, Jorgensen J, Grant
J, Kelso S. 2000. Phylogenetic relationships and character evolution in
Primula L.: the usefulness of ITS sequence data. – Plant Biosystems
134: 385-392.
Contreras LS, Lersten NR. 1984. Extrafloral
nectaries in Ebenaceae: anatomy,
morphology, and distribution. – Amer. J. Bot. 71: 865-872.
Coode MJE. 1976. Notes on Pittosporaceae and Myrsinaceae of the Mascarenes. – Kew
Bull. 31: 221-225.
Copeland HF. 1932. Philippine Ericaceae III. A taxonomic revision. –
Philipp. J. Sci. 47: 57-63.
Copeland HF. 1933. The development of seeds
in certain Ericales. – Amer. J. Bot.
20: 513-517.
Copeland HF. 1935. On the genus
Pityopus. – Madroño 3: 154-168.
Copeland HF. 1937. The reproductive
structures of Pleuricospora. – Madroño 4: 1-40.
Copeland HF. 1938a. The structure of
Allotropa. – Madroño 4: 137-168.
Copeland HF. 1938b. The Styrax of
northern California and the relationships of the Styracaceae. – Amer. J. Bot. 25:
771-780.
Copeland HF. 1939. The structure and function
of Monotropsis and the classification of the Monotropoideae. –
Madroño 5: 105-136.
Copeland HF. 1941. Further studies on the
Monotropoideae. – Madroño 6: 97-119.
Copeland HF. 1943. A study, anatomical and
taxonomic, of the genera of Rhododendroideae. – Amer. Midl. Natur. 30:
533-625.
Copeland HF. 1947. Observations on the
structure and classification of the Pyroleae. – Madroño 9: 65-102.
Copeland HF. 1953. Observations on the Cyrillaceae, particularly on the
reproductive structures of the North American species. – Phytomorphology 3:
405-411.
Copeland HF. 1954. Observations on certain
Epacridaceae. – Amer. J. Bot. 41: 215-222.
Cox HT. 1948. Studies in the comparative
anatomy of the Ericales I. Ericaceae – subfamily Rhododendroideae.
– Amer. Midl. Natur. 39: 220-245.
Cox HT. 1949. Studies in the comparative
anatomy of the Ericales II. Ericaceae – subfamily Arbutoideae. –
Amer. Midl. Natur. 40: 493-516.
Craig T. 1934. A revision of the subgenus
Hugelia of the genus Gilia (Polemoniaceae). – Bull. Torrey Bot.
Club 61: 385-397, 411-428.
Crampton B. 1954. Morphological and
ecological considerations in the classification of Navarretia (Polemoniaceae). – Madroño 12:
225-256.
Craven LA. 2011. Diplarche and
Menziesia transferred to Rhododendron (Ericaceae). – Blumea 56: 33-35.
Craven LA, Goetsch LA, Hall BD, Brown GK.
2008. Classification of the Vireya group of Rhododendron (Ericaceae). – Blumea 53: 435-442.
Craven LA, Danet F, Veldkamp JF, Goetsch LA,
Hall BD. 2011. Vireya rhododendrons: their monophyly and classification (Ericaceae, Rhododendron section
Schistanthe). – Blumea 56: 153-158.
Crayn DM, Quinn CJ. 1998. Archerieae: a new
tribe in Epacridaceae. – Aust. Syst. Bot. 11: 23-24.
Crayn DM, Quinn CJ. 2000. The evolution of
the atpB-rbcL intergenic spacer in the epacrids (Ericales) and its systematic and
evolutionary implications. – Kew Bull. 55: 238-252.
Crayn DM, Kron KA, Gadek PA, Quinn CJ. 1996.
Delimitation of Epacridaceae: preliminary molecular evidence. – Ann. Bot. 77:
317-321.
Crayn DM, Kron KA, Gadek PA, Quinn CJ. 1998.
Phylogenetics and evolution of epacrids: a molecular analysis using the plastid
gene rbcL with a reappraisal of the position of Lebetanthus.
– Aust. J. Bot. 46: 187-200.
Crayn DM, Brown EA, Powell JM. 2003. A
revision of Lissanthe (Styphelioideae: Ericaceae). – Aust. Syst. Bot. 16:
595-619.
Crayn DM, Hislop M, Heslewood MM. 2005.
Additions to Lissanthe (Styphelioideae: Ericaceae) in Western Australia: L.
synandra sp. nov. and L. pleurandroides comb. nov. – Aust.
Syst. Bot. 18: 555-561.
Creech JL. 1955. An embryological study in
the Rhododendron subgenus Anthodendron Endl. – Bot. Gaz.
116: 234-243.
Crepet WL, Nixon KC, Gandolfo MA. 1996.
Turonian flowers of the Theales. – Amer. J. Bot. 83: 148-149.
Crepet WL, Nixon KC, Daghlian CP. 2013.
Fossil Ericales from the Upper
Cretaceous of New Jersey. – Intern. J. Plant Sci. 174: 572-584.
Cresswel JE, Galen C. 1991.
Frequency-dependent selection and adaptive surfaces for floral character
combinations: the pollination of Polemonium viscosum. – Amer. Natur.
138: 1342-1353.
Crété P. 1944a. Polyembryonie chez
l’Actinidia chinensis Planch. – Bull. Soc. Bot. France 91:
89-92.
Crété P. 1944b. Recherches anatomiques sur
la séminogenèse de l’Actinidia chinensis Planch. Affinités des
Actinidiacées. – Bull. Soc. Bot. France 91: 153-160.
Cristofolini G, Pignatti S. 1962. Revisione
delle forme italiane di Soldanella L. – Webbia 16: 443-475.
Cronquist A. 1945a. Studies in the Sapotaceae III. Dipholis and
Bumelia. – J. Arnold Arbor. 26: 435-471.
Cronquist A. 1945b. Studies in the Sapotaceae IV. The North American species
of Manilkara. – Bull. Torrey Bot. Club 72: 550-562.
Cronquist A. 1946a. Studies in the Sapotaceae II. Survey of the North
American genera. – Lloydia 9: 241-292.
Cronquist A. 1946b. Studies in the Sapotaceae V. The South American species
of Chrysophyllum. – Bull. Torrey Bot. Club 73: 286-311.
Cronquist A. 1946bc. Studies in the Sapotaceae VI. Miscellaneous notes. –
Bull. Torrey Bot. Club 73: 465-471.
Crowden RK, Menadue Y. 1990. Morphometric
analysis of variation in the ‘Epacris tasmanica complex’
(Epacridaceae). – Aust. Syst. Bot. 3: 253-264.
Crowhurst RN, Lints R, Atkinson RG, Gardner
RC. 1990. Restriction fragment length polymorphisms in the genus
Actinidia (Actinidiaceae).
– Plant Syst. Evol. 174: 193-203.
Crusio WE. 1981. Die Gattung
Samolus. – Inform. ZAG Wasserpfl. 11: 3-8.
Cullen J. 1980. A revision of
Rhododendron 1. Subgenus Rhododendron sections
Rhododendron & Pogonanthum. – Notes Roy. Bot. Gard.
Edinb. 39: 1-207.
Cullings KW. 1994. Molecular phylogeny of the
Monotropoideae (Ericaceae) with a note
on the placement of the Pyroloideae. – J. Evol. Biol. 7: 501-516.
Cullings KW. 1996. Single phylogenetic origin
of ericoid mycorrhizae within the Ericaceae. – Can. J. Bot. 74:
1896-1909.
Cullings KW. 2000. Reassessment of
phylogenetic relationships of some members of the Monotropoideae based on
partial 28S ribosomal RNA gene sequencing. – Can. J. Bot. 78: 1-2.
Cullings KW, Bruns TD. 1992. Phylogenetic
origin of the Monotropoideae inferred from partial 28S ribosomal RNA gene
sequences. – Can. J. Bot. 70: 1703-1708.
Cullings KW, Hileman L. 1997. The
Monotropoideae is a monophyletic sister group to the Arbutoideae (Ericaceae): a molecular test of
Copeland’s hypothesis. – Madroño 44: 297-299.
Cullings KW, Szaro TM, Bruns TD. 1996.
Evolution of extreme specialization within a lineage of ectomycorrhizal
epiparasites. – Nature 379: 63-66.
Cuong NM, Soejarto DD, Li J. 2007. A
taxonomic revision of Actinidiaceae
of Vietnam. – Blumea 52: 209-243.
Dahl AO, Rowley JR. 1991. Microspore
development in Calluna (Ericaceae). Exine formation. – Ann. Sci.
Nat. Bot., Paris, 13e sér., 11: 155-176.
Dahlgren KVO. 1916. Zytologische und
embryologische Studien über die reihen Primulales und Plumbaginales. –
Kungl. Sv. Vetensk.-Akad. Handl. 56(4): 1-80.
Dahlgren RMT, Wyk AE van. 1988. Structures
and relationships of families endemic to or centered in Southern Africa. –
Monogr. Syst. Bot. Missouri Bot. Gard. 25: 1-94.
Dahlgren RMT, Jensen SR, Nielsen BJ. 1976.
Iridoid compounds in Fouquieriaceae and notes on its
possible affinities. – Bot. Not. 129: 207-212.
Danet F. 2005. Trois nouvelles espèces et un
nouvel hybride naturel de Rhododendron (Ericaceae) de Nouvelle-Guinée. –
Adansonia, sér. III, 27: 267-280.
Danet F. 2007. Deux nouvelles espèces de
Rhododendron section Siphonovireya (Ericaceae) de Nouvelle-Guinée. –
Adansonia, sér. III, 29: 105-111.
D’Arcy WG. 1973. Corelliana (Myrsinaceae), a new palmoid genus of the
tropical rain forest. – Ann. Missouri Bot. Gard. 60: 442-448.
Dawson MI. 2000. Index of chromosome numbers
in the Epacridaceae. – Proc. Linn. Soc. New South Wales 73: 37-56.
Dawson ML. 1936. The floral morphology of the
Polemoniaceae. – Amer. J. Bot.
23: 501-511.
Day AG. 1965. The evolution of a pair of
sibling allotetraploid species of cobwebby gilias (Polemoniaceae). – Aliso 6: 25-75.
Day AG. 1993. New taxa and nomenclatural
changes in Allophyllum, Gilia, and Navarretia (Polemoniaceae). – Novon 3:
331-340.
Day AG, Moran R. 1986. Acanthogilia,
a new genus of Polemoniaceae from
Baja California, Mexico. – Proc. Calif. Acad. Sci. 44: 111-126.
Day RT, Scott PJ. 1981. Autecological aspects
of Diapensia lapponica L. in Newfoundland. – Rhodora 83: 101-109.
Day RT, Scott PJ. 1984. The biology of
Diapensia lapponica in Newfoundland. – Can. Field-Natur. 98:
425-439.
Dean FM, Costa AMBSRCS, Harborne JB, Smith
DM. 1978. Leptodactylone, a yellow coumarin from Leptodactylon and
Linanthus species. – Phytochemistry 17: 505-509.
DeBuhr LE. 1975. Phylogenetic relationships
of the Sarraceniaceae. – Taxon
24: 297-306.
DeBuhr LE. 1977. Wood anatomy of the Sarraceniaceae: ecological and
evolutionary implications. – Plant Syst. Evol. 128: 159-169.
Debussche M, Thompson JD. 2002. Morphological
differentiation among closely related species with disjunct distributions: a
case study of Mediterranean Cyclamen L. subgen. Psilanthum
Schwarz (Primulaceae). – Bot. J.
Linn. Soc. 139: 133-144.
Debussche M, Garnier E, Thompson JD. 2004.
Exploring the causes of variation in phenology and morphology in Mediterranean
geophytes: a genus-wide study of Cyclamen. – Bot. J. Linn. Soc. 145:
469-484.
Decaisne MJ. 1876. Note sur quelques plantes
du groupe des Théophrastées. – Ann. Sci. Nat. Bot., sér. VI, 3:
138-145.
Decrock E. 1901. Anatomie des Primulacées.
– Ann. Sci. Nat. Bot. Biol. Veg., sér. VIII, 13: 1-199.
De Faria AD, Pirani JR, Da Silva Ribeiro JEL,
Nylinder S, Terra-Araujo MH, Vieira PP, Swenson U. 2017. Towards a natural
classification of Sapotaceae subfamily
Chrysophylloideae in the Neotropics. – Bot. J. Linn. Soc. 185: 27-55.
De Groot S. 2011. Spatial analysis of
morphology in Eriastrum eremicum (Polemoniaceae). – Syst. Bot. 36:
449-464.
Dehay C. 1955. Caractères de la feuille chez
les Scytopétalacées. – Bull. Soc. Bot. Nord France 8: 76-81.
Delpino F. 1869. Rivista monografica della
famiglia delle Marcgraviaceae. –
Nuovo Giorn. Bot. Ital. 1: 257-290.
Denford KE. 1981. Chemical subdivisions
within the genus Arctostaphylos based on flavonoid profiles. –
Experientia 37: 1287-1288.
Deng L, Baas P. 1990. Wood anatomy of trees
and shrubs from China II. Theaceae. –
IAWA Bull., N. S., 11: 337-378.
Deng L, Baas P. 1991. The wood anatomy of the
Theaceae. – IAWA Bull., N. S., 12:
333-353.
De-Nova JA, Sánchez-Reyes LL, Eguiarte LE,
Magallón S. 2018. Recent radiation and dispersal of an ancient lineage: the
case of Fouquieria (Fouquieriaceae, Ericales) in North American deserts. –
Mol. Phylogen. Evol. 126: 92-104.
Dickison WC. 1972. Observations on the floral
morphology of some species of Saurauia, Actinidia, and
Clematoclethra. – J. Elisha Mitchell Scient. Soc. 88: 43-54.
Dickison WC. 1993. Floral anatomy of the Styracaceae, including observations on
intra-ovarian trichomes. – Bot. J. Linn. Soc. 112: 223-255.
Dickison WC, Phend KD. 1985. Wood anatomy of
the Styracaceae: evolutionary and
ecological considerations. – IAWA Bull., N.S., 6: 3-22.
Dickison WC, Nowicke JW, Skvarla JJ. 1982.
Pollen morphology of the Dilleniaceae and Actinidiaceae. – Amer. J. Bot. 69:
1055-1073.
Dickson J. 1936. Studies in floral anatomy
III. An interpretation of the gynaeceum in the Primulaceae. – Amer. J. Bot. 23:
385-393.
Diehl GA. 1935. A study of the Lecythidaceae. – Trop. Woods 43:
1-15.
Diels L. 1914. Diapensiaceen-Studien. –
Engl. Bot. Jahrb. Syst. [Suppl.] 50: 304-330.
Diels L. 1930. Roridulaceae. – In: Engler A, Harms H
(eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 18a, W. Engelmann,
Leipzig, pp. 346-348.
Diggs Jr GM. 1981. Systematic studies in the
Arbuteae (Ericaceae, Vaccinioideae)
including a revision of the genus Comarostaphylis. – Ph.D. diss.,
University of Wisconsin, Madison, Wisconsin.
Diggs Jr GM. 1987. Numerical systematics of
Comarostaphylis (Ericaceae:
Arbuteae). – Syst. Bot. 12: 586-600.
Diggs Jr GM. 1995. Comarostaphylis
Zuccarini. – In: Luteyn JL (ed), Flora neotropica. Monograph 66. Ericaceae, part II. The superior-ovaried
genera, New York Botanical Garden, Bronx, New York, pp. 146-193.
Diggs Jr GM, Breckon GJ. 1981. Generic
circumscription in the Arbuteae (Ericaceae). – In: Diggs GM Jr (ed),
Systematic studies in the Arbuteae (Ericaceae, Vaccinioideae) including a
revision of the genus Comarostaphylis, Ph.D. diss., University of
Wisconsin, Madison, Wisconsin, pp. 5-88.
Ditsch F, Barthlott W. 1994. Mikromorphologie
der Epicuticularwachse und die Systematik der Dilleniales,
Lecythidales, Malvales und Theales.
– Trop. Subtrop. Pflanzenwelt 88: 1-74.
Dixon CJ, Schönswetter P, Schneeweiss GM.
2008. Morphological and geographical evidence are misleading with repect to the
phylogenetic position and origin of the narrow endemic polyploid Androsace
cantabrica (Primulaceae). –
Syst. Bot. 33: 384-389.
Dobbins DR, Alden H, Marvel D. 1983.
Developmental anatomy of juvenile and adult shoots of Marcgravia
rectifolia L. – Amer. J. Bot. 70: 1263-1271.
Docters van Leeuwen WM. 1922. Über einige
von Aphiden an Styrax-Arten gebildete Gallen. – Bull. Jard. Bot.
Buitenzorg, sér. III, 4: 147-162.
Dome AP. 1999. xPhylliopsis –
Phyllodoce spp. x Kalmiopsis – ericaceous aristocrats. –
Rock Gard. Quart. 57: 35-45.
Dormer KJ. 1944. Morphology of the vegetative
shoot in Epacridaceae. – New Phytol. 44: 149-151.
Dorr LJ. 1980. The reproductive biology and
pollination ecology of Zenobia (Ericaceae). – Master’s thesis,
University of North Carolina, Chapel Hill, North Carolina.
Douglas GE. 1936. Studies in the vascular
anatomy of the Primulaceae. – Amer.
J. Bot. 23: 199-212.
Doyle BE, Goss LM. 1941. Some details of the
reproductive structures of Sarcodes. – Madroño 6: 1-7.
Dressler S. 1997. 321. Marcgravia
umbellata. – [Curtis’s] Bot. Mag., ser. VI, 14: 130-136.
Dressler S. 1999. Nektarkannen im Kronendach
– zur blütenökologischen Vielfalt der Marcgraviaceae. – In: Zizka G,
Schneckenburger S (eds), Blütenökologie – faszinierendes Miteinander von
Pflanzen und Tieren, pp. 141-148. [Kleine Senckenberg-Reihe 33; Palmengarten
Sonderheft 31]
Dressler S. 2000a. Marcgraviaceae – ein Portät der
neotropischen Familie. – In: Breckle S-W, Schweizer B, Arndt U (eds),
Ergebnisse weltweiter ökologischer Forschung / Results of worldwide ecological
studies, Stuttgart, pp. 413-424.
Dressler S. 2000b. A new species of
Marcgravia (Marcgraviaceae) from Amazonia with
some notes on the Galeatae group including a key. – Willdenowia 30:
369-374.
Dressler S. 2004. Marcgraviaceae. – In: Kubitzki K
(ed), The families and genera of vascular plants VI. Flowering plants.
Dicotyledons. Celastrales,
Oxalidales, Rosales, Cornales, Ericales, Springer, Berlin, Heidelberg, New
York, pp. 258-265.
Dressler S, Bayer C. 2004. Actinidiaceae. – In: Kubitzki K
(ed), The families and genera of vascular plants VI. Flowering plants.
Dicotyledons. Celastrales,
Oxalidales, Rosales, Cornales, Ericales, Springer, Berlin, Heidelberg, New
York, pp. 14-19.
Dressler S, Tschapka M. 2002. Bird versus bat
pollination in the genus Marcgravia and the description of a new
species. – [Curtis’s] Bot. Mag., ser. VI, 19: 104-114.
Drinkell C, Utteridge TMA. 2015. A revision
of the genus Systellantha B. C. Stone. Studies in Malaysian Myrsinaceae IV. – Kew Bull. 70: 50 DOI
10.1007/S12225-015-9603-8
Drude O. 1891a. Clethraceae. – In: Engler A, Prantl K
(eds), Die natürlichen Pflanzenfamilien IV(1), W. Engelmann, Leipzig, pp.
1-2.
Drude O. 1891b. Pirolaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien IV(1), W. Engelmann, Leipzig,
pp. 3-11.
Drude O. 1891c. Ericaceae (Haidegewächse). – In: Engler
A, Prantl K (eds), Die natürlichen Pflanzenfamilien IV(1), W. Engelmann,
Leipzig, pp. 15-65.
Drude O. 1891d. Epacridaceae. – In: Engler
A, Prantl K (eds), Die natürlichen Pflanzenfamilien IV(1), W. Engelmann,
Leipzig, pp. 66-79.
Drude O. 1891e. Diapensiaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien IV(1), W. Engelmann, Leipzig,
pp. 80-84.
Drude O. 1891f. Droseraceae.
– In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(2), W.
Engelmann, Leipzig, pp. 261-272.
Du XY, Zhang QL, Luo Z-R. 2009. Comparison of
four molecular markers for genetic analysis in Diospyros L. (Ebenaceae). – Plant Syst. Evol. 281:
171-181.
Duangjai S, Wallnoeffer B, Samuel R,
Munzinger F, Chase MW. 2006. Generic delimitation and relationships in Ebenaceae sensu lato: evidence from six
plastid DNA regions. – Amer. J. Bot. 93: 1808-1827.
Duangjai S, Samuel R, Munzinger J, Forest F,
Wallnöfer B, Barfuss MHJ, Fischer G, Chase MW. 2009. A multi-locus plastid
phylogenetic analysis of the pantropical genus Diospyros (Ebenaceae), with an emphasis on the
radiation and biogeographic origins of the New Caledonian endemic species. –
Mol. Phylogen. Evol. 52: 602-620.
Dubard M. 1908. Les Sapotacées du groupe des
Illipées. – Rev. Gén. Bot. 20: 193-206.
Dubard M. 1909. Les Sapotacées de groupe des
Isonandrées. – Rev. Gén. Bot. 21: 392-398.
Dubard M. 1911. Descriptions de quelques
espèces de Lucumées africaines d’aprés les documents de L. Pierre. –
Notul. Syst. (Paris) 2: 89-91.
Dubard M. 1912. Les Sapotacées de groupe des
Sideroxylinées. – Ann. Inst. Bot.-Géol. Colon. Marseille, sér II, 10:
1-98.
Dubard M. 1915. Les Sapotacées du groupe des
Sideroxylinées-Mimusopées. – Ann. Inst. Bot.-Géol. Colon. Marseille, sér.
III, 3: 1-62.
Dubéarnès A, Julius A, Utteridge TMA. 2015.
A synopsis of the genus Embelia in Peninsular Malaysia and Singapore.
Studies in Malaysian Myrsinaceae III.
– Kew Bull. 70: 25 DOI 10.1007/S12225-015-9570-0
Duddridge J, Read DJ. 1982. An
ultrastructural analysis of the development of mycorrhizas in Rhododendron
ponticum. – Can. J. Bot. 60: 2345-2356.
Dunbar A. 1970. A surface specialization on
the wall of pollen grains in Aegiceras corniculatum. – J. Cell Biol.
47: 53a-54a.
Dunbar A, Erdtman G. 1969. On the pollen
grains of Aegiceras corniculatum (Myrsinaceae). – Grana Palynol. 9:
63-71.
Dunn ST. 1911. A revision of the genus
Actinidia, Lindl. – Bot. J. Linn. Soc. 34: 394-411.
Eastwood A. 1934. A revision of the genera
formerly included in Arctostaphylos. – Leafl. Western Bot. 1:
97-100.
Ebinger JE. 1974. A systematic study of the
genus Kalmia (Ericaceae). –
Rhodora 76: 315-398.
Eck P. 1990. The American cranberry. –
Rutgers University Press, New Brunswick.
Elias TS, Gelband H. 1977. Morphology,
anatomy, and relationship of extrafloral nectaries and hydathodes in two
species of Impatiens (Balsaminaceae). – Bot. Gaz. 138:
206-212.
Ellis AG, Midgley JJ. 1996. A new
plant-animal mutualism involving a plant with sticky leaves and a resident
hemipteran insect. – Oecologia 106: 478-481.
Elmer ADE. 1908. Six new Myrsinaceae. – Leafl. Philipp. Bot.
2(22): 439-444.
Elmer ADE. 1910. Myrsinaceae from Mt. Apo. – Leafl.
Philipp. Bot. 2(38): 659-675.
Elsler E. 1907. Das extraflorale Nektarium
und die Papillen der Blattunterseite bei Diospyros discolor Willd. –
Sitzungsber. K. Akad. Wiss., Math.-Naturwiss. Cl., Abt. 1, 116: 1563-1590.
Englander L, Hull RJ. 1980. Reciprocal
transfer of nutrients between ericaceous plants and a Clavaria sp. –
New Phytologist 84: 661-667.
Engler A. 1891. Sapotaceae. – In: Engler A, Prantl K
(eds), Die natürlichen Pflanzenfamilien IV(1), W. Engelmann, Leipzig, pp.
126-153; Engler A. 1897. Nachträge zu IV(1), pp. 271-280.
Engler A. 1897a. Pentaphylacaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien, Nachträge zu III(5), W.
Engelmann, Leipzig, pp. 214-215.
Engler A. 1897b. Scytopetalaceae. – In:
Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien, Nachträge zu
III(6), W. Engelmann, Leipzig, pp. 242-245.
Engler A. 1897c. Theaceae. – In: Engler A, Prantl K (eds),
Die natürlichen Pflanzenfamilien, Nachträge zu III(6), W. Engelmann, Leipzig,
pp. 245-247.
Engler A. 1904. Monographien afrikanischer
Pflanzen-Familien und -Gattungen 8. Sapotaceae. – W. Engelmann, Leipzig.
Engler A. 1909. Scytopetalaceae Africanae II.
– Engl. Bot. Jahrb. Syst. 43: 373-377.
Epling C, Dobzhansky T. 1942. Genetics of
natural populations VI. Microgeographic races in Linanthus parryae.
– Genetics 27: 317-332.
Erbar C. 1986. Untersuchungen zur Entwicklung
der spiraligen Blüte von Stewartia pseudocamellia (Theaceae). – Bot. Jahrb. Syst. 106:
391-407.
Étienne P. 1919. Étude anatomique de la
famille des Épacridacées. – Thèse Doctorale, Faculté Pharmacologique,
Université de Toulouse.
Eugster CH, Hürlimann H, Leuenberger HJ.
1969. 90. Crocetindialdehyd und Crocetinhalbaldehyd als Blütenfarbstoffe von
Jacquinia angustifolia. – Helvetica Chem. Acta 52: 806-807.
Evans RC, Vander Kloet S. 2010. Comparative
analysis of hypocotyl development in epiphytic, lignotuber-forming, and
terrestrial Vaccinieae (Ericaceae). –
Botany 88: 556-564.
Evans WE. 1927. A revision of the genus
Diapensia with special reference to the Sino-Himalayan species. –
Notes Roy. Bot. Gard. Edinb. 15: 209-236.
Ewane-Nyambi G, Bois P, Raymond G. 1993. The
effects of Agauria salicifolia leaf extract on calcium current and
excitation-contraction coupling of isolated frog muscle cells. – J.
Ethnopharmacol. 38: 55-61.
Ewango CEN, Kenfack D, Sainge MN, Thomas DW,
van der Burgt XM. 2016. Gambeya korupensis (Sapotaceae: Chrysophylloideae), a new
rain forest tree species from the Southwest Region in Cameroon. – Kew Bull.
2016: 1–6. DOI 10.1007/S12225-016-9633-X
Eyma PJ. 1936. Notes on Guiana Sapotaceae. – Rec. Trav. Bot. Néerl.
33: 156-210.
Fajardo D, Senalik D, Ames M, Zhu H, Steffan
SA, Harbut R, Polashock J, Vorsa N, Gillespie E, Kron K, Zalapa JE. 2013.
Complete plastid genome sequence of Vaccinium macrocarpon: structure,
gene content, and rearrangements revealed by next generation sequencing. –
Tree Gene Genomes 9: 489-498.
Fan GS. 1996. Generic numerical taxonomy of
Styracaceae from Asia. – Guihaia
16: 305-307.
Fang M-Y. 1985. A new species of
Enkianthus Lour. from Hunan. – Bull. Bot. Res. North-East. Forest.
Inst. 5: 165-168.
Faure P. 1968. Contribution à l’étude
caryo-taxonomique des Myrsinacées et des Théophrastacées. – Mém. Mus.
Natl. Hist. Nat., sect. B, Bot. 18: 37-57.
Federov A. 1950. Sredinskya Fed.
Genus novum Primulacearum. – Not. Syst. (Leningrad) 13: 199-203.
Fenderson GK. 1986. A synoptic guide to the
genus Primula. – Allen, Lawrence, Kansas.
Ferguson AR. 1990. Botanical nomenclature:
Actinidia chinensis, Actinidia deliciosa and Actinidia
setosa. – In: Warrington IJ, Weston GC (eds), Kiwifruit: science and
management, Ray Richards, Auckland, pp. 36-56.
Ferguson AR, Bollard EG. 1990. Domestication
of the kiwifruit. – In: Warrington IJ, Weston GC (eds), Kiwifruit: science
and management, Ray Richards, Auckland, pp. 165-246.
Ferguson CJ, Jansen RK. 2002. A chloroplast
DNA phylogeny of eastern Phlox (Polmoniaceae): implications of
congruence and incongruence with the ITS phylogeny. – Amer. J. Bot. 89:
1324-1335.
Ferguson CJ, Krämer F, Jansen RK. 2000.
Relationships of eastern North American Phlox (Polemoniaceae) based on ITS sequence
data. – Syst. Bot. 24: 616-631.
Fernald ML. 1920. The American varieties of
Pyrola chlorantha. – Rhodora 22: 49-53.
Fernald ML, Wiegand KM. 1913. The genus
Empetrum in North America. – Rhodora 15: 211-217.
Fernandes A. 1978. 76. Barringtoniaceae. –
In: Launert E (ed), Flora Zambesiaca 4, Flora Zambesiaca Managing Committee,
London, pp. 216-219.
Fior S, Karis PO, Anderberg AA. 2003.
Phylogeny, taxonomy, and systematic position of Clethra (Clethraceae, Ericales) with notes on biogeography:
evidence from plastid and nuclear DNA sequences. – Intern. J. Plant Sci. 164:
997-1006.
Fischer E. 2004. Balsaminaceae. – In: Kubitzki K
(ed), The families and genera of vascular plants VI. Flowering plants.
Dicotyledons. Celastrales,
Oxalidales, Rosales, Cornales, Ericales, Springer, Berlin, Heidelberg, New
York, pp. 20-25.
Fischer E, Rahelivololona ME. 2002. New taxa
of Impatiens (Balsaminaceae) from Madagascar I. –
Adansonia, sér. III, 24: 271-294.
Fischer E, Rahelivololona ME. 2004. New taxa
of Impatiens (Balsaminaceae) from Madagascar III.
– Adansonia, sér. III, 26: 37-52.
Fischer E, Rahelivololona ME. 2007a. New taxa
of Impatiens (Balsaminaceae) from Madagascar IV. –
Adansonia, sér. III, 29: 296-315.
Fischer E, Rahelivololona ME. 2007b. New taxa
of Impatiens (Balsaminaceae) from Madagascar V. New
species of Impatiens from Masoala Peninsula. – Adansonia, sér. III,
29: 317-332.
Fischer E, Dhetchuvi J-B, Ntaganda C. 2003. A
new species of Impatiens (Balsaminaceae) from Nyungwe Forest,
Rwanda. – Syst. Geogr. Plants 73: 91-95.
Fischer E, Wohlhauser S, Rahelivololona ME.
2003. New taxa of Impatiens (Balsaminaceae) from Madagascar II. A
collection from Masoala Peninsula. – Adansonia, sér. III, 25: 17-31.
Fischer O. 1992. New knowledges about the
distribution of the genus Kalmia L. in the European Tertiary. –
Cour. Forsch.-Inst. Senck. 147: 383-391.
Fletcher HR. 1939. The genus
Omphalogramma. – Notes Roy. Bot. Gard. Edinb. 20: 125-159.
Floyd JW. 2002. Phylogenetic and
biogeographic patterns in Gaylussacia (Ericaceae) based on morphological, nuclear
DNA, and chloroplast DNA variation. – Syst. Bot. 27: 99-115.
Ford JH. 1971. Ultrastructural and chemical
studies of pollen wall development in the Epacridaceae. – In: Brooks J, Grant
PR, Muir M, Gijzel P van, Shaw G (eds), Sporopollenin, Academic Press, London,
New York, pp. 130-173.
Foss PJ, Doyle GJ. 1988. A palynological
study of the Irish Ericaceae and
Empetrum. – Pollen Spores 30: 151-178.
Foster AS. 1944. Structure and development of
sclereids in the petiole of Camellia japonica. – Bull. Torrey Bot.
Club 71: 302-326.
Foster AS. 1950. Venation and histology of
the leaflets in Touroulia guianensis Aubl. and Froesia
tricarpa Pires. – Amer. J. Bot. 37: 848-862.
Fourny AC da S, Carrijo TT, Mendonça CBF,
Gonçalves-Esteves V. 2018. Pollen morphology in delimiting subgenera and
species of the genus Cybianthus s.l. (Myrsinoideae-Primulaceae). – Plant Syst. Evol. 304:
535-548.
Frame D, Durou S. 2001. Morphology and
biology of Napoleonaea vogelii (Lecythidaceae) flowers in relation to
the natural history of insect visitors. – Biotropica 33: 458-471.
Franceschi G de. 1993. Phylogénie des
Ebénales: analyse de l’ordre et origine biogéographique des espèces
indiennes.. – Publications du Département d’Écologie, Inst. Franç.
Pondichéry, 33, pp. 1-153.
Francis SP. 1991. The relationships of the Sapotaceae within the Ebenales. – In:
Pennington TD (ed), The genera of Sapotaceae, Royal Botanic Gardens, Kew,
pp. 1-13.
Franks JW, Watson L. 1963. The pollen
morphology of some critical Ericales.
– Pollen Spores 5: 51-68.
Freudenstein JV. 1999. Relationships and
character transformation in Pyroloideae (Ericaceae) based on ITS sequences,
morphology, and development. – Syst. Bot. 24: 398-408.
Freudenstein JV, Broe MB, Feldenkris ER.
2016. Phylogenetic relationships at the base of Ericaceae: implications for vegetative and
mycorrhizal evolution. – Taxon 65: 794-804.
Frezet C, Raynaud J, Bouillant M-L. 1975. Sur
la présence de la di-C-glucosyl-6,8 apigénine chez Coris
monspeliensis (Primulaceae). –
Compt. Rend. Acad. Sci. Paris 280: 1079-1081.
Friedmann F. 1981. 116. Sapotacées. – In:
Bosser J, Cadet T, Guého J, Marais W (eds), Flore des Mascareignes, IRD
Éditions, Institut de recherche pour le développement, Paris, Mauritius Sugar
Industry Research Institute, Ile Maurice, and The Royal Botanic Gardens, Kew,
pp. 1-27.
Fries TCE. 1923. Die Anagallis-Arten
der afrikanischen Hochgebirge. – Notizbl. Bot. Gart. Berlin-Dahlem 8:
329-339.
Friis I. 1978. A reconsideration of the
genera Monotheca and Spiniluma (Sapotaceae). – Kew Bull. 33: 91-98.
Friis I. 1985. The identity of E.
Chiovenda’s Somalian Sapotaceae. –
Kew Bull. 40: 395-398.
Frimodt-Møller C, Grey-Wilson C. 1999. Two
new taxa of Impatiens (Balsaminaceae) from the Udzungwa
Mountains, Tanzania. – Kew Bull. 54: 179-184.
Fritsch PW. 1994. Systematic and
biogeographic studies in the genus Styrax. – Ph.D. diss., Claremont
Graduate School, Claremont, California.
Fritsch PW. 1996a. Isozyme analysis of
intercontinental disjuncts within Styrax (Styracaceae): implications for the
Madrean-Tethyan hypothesis. – Amer. J. Bot. 83: 342-355.
Fritsch PW. 1996b. Population structuring and
patterns of morphological variation in California Styrax (Styracaceae). – Aliso 14: 205-218.
Fritsch PW. 1997. A revision of
Styrax (Styracaceae) for
western Texas, Mexico, and Mesoamerica. – Ann. Missouri Bot. Gard. 84:
705-761.
Fritsch PW. 1999. Phylogeny of
Styrax based on morphological characters, with implications for
biogeography and infrageneric classification. – Syst. Bot. 24: 356-378.
Fritsch PW. 2001. Phylogeny and biogeography
of the flowering plant genus Styrax (Styracaceae) based on chloroplast DNA
restriction sites and DNA sequences of the Internal Transcribed Spacer region.
– Mol. Phylogen. Evol. 19: 387-408.
Fritsch PW. 2003. Multiple geographic origins
of Antillean Styrax. – Syst. Bot. 28: 421-430.
Fritsch PW. 2004. Styracaceae. – In: Kubitzki K (ed),
The families and genera of vascular plants VI. Flowering plants. Dicotyledons.
Celastrales,
Oxalidales, Rosales, Cornales, Ericales, Springer, Berlin, Heidelberg, New
York, pp. 434-442.
Fritsch PW, Lucas SD. 2000. Clinal variation
in the Halesia carolina complex (Styracaceae). – Syst. Bot. 25:
197-210.
Fritsch PW, Morton CM, Chen T, Meldrum C.
2001. Phylogeny and biogeography of the Styracaceae. – Intern. J. Plant Sci.
162(6 Suppl.): S95-S116.
Fritsch PW, Cruz BC, Almeida F, Wang Y, Shi
S. 2006. Phylogeny of Symplocos based on DNA sequences of the
chloroplast trnC-trnD intergenic region. – Syst. Bot. 31:
181-192.
Fritsch PW, Kelly LM, Wang Y, Almeda F,
Kriebel R. 2008. Revised infrafamilial classification of Symplocaceae based on phylogenetic data
from DNA sequences and morphology. – Taxon 57: 823-852.
Fritsch PW, Zhou L, Lu L, Bartholomew B.
2008. The flowering plant genus Gaultheria (Ericaceae) in the Gaoligong Shan, along
the border region of China and Myanmar. – Proc. Calif. Acad. Sci., ser. 4,
59: 147-214.
Fritsch PW, Bush CM, Cruz BC, Kron KA, Li
D-Z. 2011. Phylogenetic analysis of the wintergreen-group (Ericaceae) based on six genic regions. –
Syst. Bot. 36: 990-1003.
Fritsch PW, Cruz BC, Simison WB, Campbell AJ,
Harris JK. 2015. Early phylogenetic divergence of gynodioecious species
warrants the recognition of subseries in Styrax Series
Valvatae. – Syst. Bot. 40: 1081-1092.
Fritsch PW, Manchester SR, Stone RD, Cruz BC,
Almeda F. 2015. Northern Hemisphere origins of the amphi-Pacific tropical plant
family Symplocaceae. – J.
Biogeogr. 42: 891-901.
Fritsch PW, Lu L, Wang H, Li D-Z. 2015. New
species, taxonomic renovations, and typifications in Gaultheria series
Trichophyllae (Ericaceae). –
Phytotaxa 201: 1-26.
Fritsch PW, Yao X, Simison WB, Cruz BC, Chen
T. 2016. Perkinsiodendron, a new genus in the Styracaceae based on morphology and DNA
sequences. – J. Bot. Res. Inst. Texas 10: 109-117.
Frohne D, John J. 1978. The Primulales:
serological contributions to the problem of their systematic position. –
Biochem. Syst Ecol. 6: 315-322.
Frye EM. 1934. American Diapensiaceae. – New Flora and Silva
6: 192-193.
Fuchs HP. 1970. Ecological and palynological
notes on Pelliciera rhizophorae. – Acta Bot. Neerl. 19: 884-894.
Furness CA. 2009. Pollen evolution and
development in Ericaceae, with
particular reference to pseudomonads and variable pollen sterility in
Styphelioideae. – Intern. J. Plant Sci. 170: 476-495.
Fusconi A, Bonfante-Fasolo P. 1984.
Ultrastructural aspects of host-endophyte relationships in Arbutus
unedo L. mycorrhizas. – New Phytol. 96: 397-410.
Galen C. 1989. Measuring pollinator-mediated
selection on morphometric floral traits: bumblebees and the alpine skypilot
Polemonium viscosum. – Evolution 43: 882-890.
Galen C. 1992. Pollen dispersal dynamics in
an alpine wildflower, Polemonium viscosum. – Evolution 46:
1043-1051.
Galen C, Newport M. 1988. Pollination
quality, seed set, and flower traits in Polemonium viscosum:
complementary effects of variation in flower scent and size.- Amer. J. Bot. 75:
900-905.
Ganapathy PS, Palser BF. 1964. Studies of
floral morphology in the Ericales VII.
Embryology in the Phyllodoceae. – Bot. Gaz. 125: 280-297.
Gao L-M, Li D-Z, Zhang C-Q, Yang J-B. 2002.
Infrageneric and sectional relationships in the genus Rhododendron (Ericaceae) inferred from ITS sequence
data. – Acta Bot. Sin. 44: 1351-1356.
Gao M, Xu WB, Yang HL, Yu SX. 2011.
Characters of leaf epidermis and their taxonomic significance in
Impatiens (Balsaminaceae)
obligated to limestone region from China. – Guihaia 31: 730-734.
Gardner IC, Miller IM, Scott A. 1981. The
fine structure of the leaf nodules of Ardisia crispa (Thunb.) A. D. C.
(Myrsinaceae). – Bot. J. Linn. Soc.
83: 93-102.
Gautier L, Naciri Y. 2018.Three critically
endangered new species of Capurodendron (Sapotaceae) from Madagascar. –
Candollea 73: 121-129.
Gautier L, Naciri Y, Anderberg AA, Smedmark
JEE, Randrianaivo R, Swenson U. 2013. A new species, genus and tribe of Sapotaceae, endemic to Madagascar. –
Taxon 62: 972-983.
Geeraerts A, Raeymaekers JAM, Vinckier S,
Pletsers A, Smets E, Huysmans S. 2009. Systematic palynology in Ebenaceae with focus on Ebenoideae:
morphological diversity and character evolution. – Rev. Palaeobot. Palynol.
153: 336-353.
Geetha K, Umadevi I, Daniel M. 1993.
Primulales – a reassessment of the taxonomy and phylogeny of the group. –
Feddes Repert. 104: 67-71.
Geuten K, Smets E, Schols P, Yuan Y-M,
Janssens S, Küpfer P, Pyck N. 2004. Conflicting phylogenies of balsaminoid
families and the polytomy in Ericales:
combining data in a Bayesian framework. – Mol. Phylogen. Evol. 31:
711-729.
Geuten K, Becker A, Kaufmann K, Caris P,
Janssens S, Viaene T, Theißen G, Smets E. 2006. Petaloidy and petal identity
MADS-box genes in the balsaminoid genera Impatiens and
Marcgravia. – Plant J. 47: 501-518.
Gibbs PE, Talavera S. 2001. Breeding system
studies in three species of Anagallis (Primulaceae): self-incompatibility and
reduced female fertility in A. monelli L. – Ann. Bot. 88:
139-144.
Gibson DN. 1967. Flora of Peru, Polemoniaceae. – Field Mus. Nat.
Hist., Bot. Ser. 8: 112-131.
Giebel KP, Dickison WC. 1975. Wood anatomy of
Clethraceae. – J. Elisha Mitchell
Sci. Soc. 92: 17-26.
Gielly L, Debussche M, Thompson JD. 2001.
Geographic isolation and evolution of Mediterranean endemic Cyclamen:
insights from chloroplast trnL(UAA) intron sequence variation. –
Plant Syst. Evol. 230: 75-88.
Gilg E. 1896. Cyrillaceae. – In: Engler A, Prantl K
(eds), Die natürlichen Pflanzenfamilien III(5), W. Engelmann, Leipzig, pp.
179-182.
Gilg E, Werdermann E. 1925a. Actinidiaceae. – In: Engler A, Gilg
E (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 21, W. Engelmann,
Leipzig, pp. 36-47.
Gilg E, Werdermann E. 1925b. Marcgraviaceae. – In: Engler A,
Gilg E (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 21, W.
Engelmann, Leipzig, pp. 94-106.
Gillespie EL, Kron KA. 2010. Molecular
phylogenetic relationships and a revised classification of the subfamily
Ericoideae (Ericaceae). – Mol.
Phylogen. Evol. 56: 343-354.
Gillespie EL, Kron KA. 2012. A new tribe,
Bryantheae (Ericoideae: Ericaceae),
composed of disjunct genera from South America and Japan. – Brittonia 64:
73-75.
Gillespie EL, Kron KA. 2013. Molecular
phylogenetic relationships and morphological evolution within the tribe
Phyllodoceae (ericoideae, Ericaceae).
– Syst. Bot. 38: 752-763.
Gilly CL. 1943. Studies in the Sapotaceae 2. The
Sapodilla-Nispero complex. – Trop. Woods 73: 1-22.
Giovannetti M, Lioi L. 1990. The mycorrhizal
status of Arbutus unedo in relation to compatible and incompatible
fungi. – Can.. J. Bot. 68: 1239-1244.
Giraud B, Bussert R, Schrank E. 1992. A new
Theacean wood from the Cretaceous of northern Sudan. – Rev. Palaeobot.
Palynol. 75: 289-299.
Glasau F. 1939. Monographie der Gattung
Cyclamen auf morphologisch-cytologischer Grundlage. – Planta 30:
507-550.
Godley EJ. 1966. Breeding systems in New
Zealand plants 4. Self sterility in Pentachondra pumila. – New
Zeland J. Bot. 53: 324-355.
Goetsch L, Eckert AJ, Hall BD. 2005. The
molecular systematics of Rhododendron (Ericaceae): a phylogeny based on
RPB2 gene sequences. – Syst. Bot. 30: 616-626.
Goetsch L, Raven LA, Hall BD. 2011. Major
speciation accompanied the dispersal of vireya rhododendrons (Ericaceae, Rhododendron sect.
Schistanthe) through the Malayan archipelago: evidence from nuclear
gene sequences. – Taxon 60: 1015-1028.
Gonsoulin GJ. 1974. A revision of
Styrax (Styracaceae) in
North America, Central America, and the Carribbean. – Sida 5: 191-258.
González MV, Coque M, Herrero M. 1996.
Pollen-pistil interaction in kiwifruit (Actinidia decliciosa; Actinidiaceae). – Amer. J. Bot. 83:
148-154.
Good RDO. 1926. The genera
Phyllodoce and Cassiope. – J. Bot. 64: 1-10.
Good RDO. 1927. The genus Empetrum
L. – Bot. J. Linn. Soc. 47: 489-523.
Goodwillie C. 1999. Multiple origins of
self-compatibility in Linanthus section Leptosiphon (Polemoniaceae): phylogenetic evidence
from internal-transcribed-spacer sequence data. – Evolution 53: 1387-1395.
Govaerts R, Frodin DG, Pennington TD. 2001.
World checklist and bibliography of Sapotaceae. – Royal Botanic Gardens,
Kew.
Govindarajan T, Subramanian D. 1986.
Karyotaxonomy of South Indian Balsaminaceae. – Cytologia 51:
107-116.
Graham A. 1977. New records of
Pelliciera (Theaceae/Pellicieraceae) in the Tertiary of
the Caribbean. – Biotropica 9: 48-52.
Grant A, Grant V. 1955. The genus
Allophyllum (Polemoniaceae). – Aliso 3:
93-110.
Grant A, Grant V. 1956. Gentic and taxonomic
studies in Gilia VIII. The cobwebby gilias. – Aliso 3: 203-287.
Grant V. 1950. Genetic and taxonomic studies
in Gilia I. Gilia capitata. – Aliso 2: 239-316.
Grant V. 1952a. Genetic and taxonomic studies
in Gilia II. Gilia capitata abrotanifolia. – Aliso 2:
361-374.
Grant V. 1952b. Genetic and taxonomic studies
in Gilia III. Gilia tricolor. – Aliso 2: 375-388.
Grant V. 1953. The role of hybridization in
the evolution of the leafy-stemmed gilias. – Evolution 7: 51-64.
Grant V. 1954a. Genetic and taxonomic studies
in Gilia IV. Gilia achilleaefolia. – Aliso 3: 1-18.
Grant V. 1954b. Genetic and taxonomic studies
in Gilia V. Gilia clivorum. – Aliso 3 19-34.
Grant V. 1954c. Genetic and taxonomic studies
in Gilia VI. Interspecific relationships in the leafy-stemmed
Gilias. – Aliso 3: 35-50.
Grant V. 1956. A synopsis of
Ipomopsis. – Aliso 3: 351-362.
Grant V. 1959. Natural history of the phlox
family I. Systematic Botany. – Martinus Nijhoff, The Hague, Netherlands.
Grant V. 1964. Genetic and taxonomic studies
in Gilia XII. Fertility relationships of the polyploidy cobwebby
gilias. – Aliso 5: 479-507.
Grant V. 1966. The selective origin of
incompatibility barriers in the plant genus Gilia. – Amer. Natur.
100: 99-118.
Grant V. 1989. Taxonomy of the tufted alpine
and subalpine Polemoniums (Polemoniaceae). – Bot. Gaz.
(Crawfordsville) 150: 158-169.
Grant V. 1992. Systematics and phylogeny of
the Ipomopsis aggregata group (Polemoniaceae): traditional and
molecular approaches. – Syst. Bot. 17: 683-691.
Grant V. 1997 [1998]. Nomenclature of
subfamilies and tribes in the Polemoniaceae. – Phytologia 83:
385-389.
Grant V. 1998a. Primary classification and
phylogeny of the Polemoniaceae,
with comments on molecular cladistics. – Amer. J. Bot. 85: 741-752.
Grant V. 1998b [1999]. Classification of the
genus Gilia (Polemoniaceae). – Phytologia 84:
69-86.
Grant V. 2001. A guide to understanding
recent classifications of th family Polemoniaceae. – Lundellia 4:
12-24.
Grant V. 2003. Taxonomy of the Polemoniaceae: the subfamilies and
tribes. – SIDA 20: 1371-1385.
Grant V. 2004. Taxonomy of he Poleoniaceae:
Gilia and Lathrocasis. – SIDA 21: 531-546.
Grant V, Day AG. 1998. Transfer of some
species from Gilia to Allophyllum and Tintinabulum,
and the effects of the transfer on the generic definition of Gilia (Polemoniaceae). – Phytologia 84:
368-382.
Grant V, Grant A. 1954. Genetic and taxonomic
studies in Gilia VII. The woodland gilias. – El Aliso 3: 59-91.
Grant V, Grant A. 1956. Genetic and taxonomic
studies in Gilia IX. Conspectus of the subgenus Gilia. –
Aliso 3: 297-300.
Grant V, Grant A. 1960. Genetic and taxonomic
studies in Gilia XI. Fertility relationships of the diploid cobwebby
gilias. – Aliso 4: 435-481.
Grant V, Grant A. 1965. Flower pollination in
the Phlox family. – Columbia University Press, New York, London.
Gray A. 1870. Revision of the North American
Polemoniaceae. – Proc. Amer.
Acad. Arts 8: 247-282.
Gray A. 1873. Reconstruction of the order
Diapensiales. – Proc. Amer. Acad. Arts Sci. 8: 243-247.
Gray A. 1878. Note sur les Shortia
galacifolia et révision des Diapensiacées. – Ann. Sci. Nat. Bot. Biol.
Vég. 7: 173-179.
Greene EL. 1887. Some American Polemoniaceae I. – Pittonia 1:
120-139.
Greene EL. 1892. Some American Polemoniaceae II. – Pittonia 2:
251-260.
Greene EL. 1898a. A new genus of Polemoniaceae. – Pittonia 3:
29-30.
Greene EL. 1898b. Some western Polemoniaceae. – Pittonia 3:
299-305.
Greilhuber J. 1989. Karyotype structure and
evolution in Cyclamen L. subg. Psilanthus Schwz. (Primulaceae). – Flora 183: 103-113.
Greuter W. 2001. Proposal to conserve
Manilkara nom. cons. (Sapotaceae), against an additional name,
Sapota. – Taxon 50: 1215-1216.
Grey-Wilson C. 1976. The genus
Dionysia in cultivation and in the wild. – Alpine Garden Society,
Woking, England.
Grey-Wilson C. 1977. The genus
Dionysia in cultivation and in the wild. Supplement and key. –
Alpine Garden Society, Woking, England.
Grey-Wilson C. 1980a. Studies in Balsaminaceae II. Hybridization in
African Impatiens. – Kew Bull. 34: 689-722.
Grey-Wilson C. 1980b. Studies in Balsaminaceae V. Hydrocera
triflora, its floral anatomy and relationship with Impatiens. –
Kew Bull. 35: 213-219.
Grey-Wilson C. 1980c. Studies in Balsaminaceae VI. Some observations on
the floral vascular anatomy of Impatiens. – Kew Bull. 35:
221-227.
Grey-Wilson C. 1980d. Impatiens of
Africa. Morphology, pollination and pollinators, ecology, phytogeography,
hybridisation, keys and systematic treatment of all African species. – A. A.
Balkema, Rotterdam.
Grey-Wilson C. 1982. Balsaminaceae. – In: Polhill RM
(ed), Flora of tropical East Africa, A. A. Balkema, Rotterdam, The Netherlands,
pp. 1-76.
Grey-Wilson C. 1988. The genus
Cyclamen. – Royal Botanic Gardens, Kew, and Timber Press, Portland,
Oregon.
Grey-Wilson C. 1989a. The genus
Dionysia. – Alpine Garden Society, Woking, England.
Grey-Wilson C. 1989b. Studies in Balsaminaceae VII. Impatiens
bicornuta and ist allies. – Kew Bull. 44: 61-66.
Grey-Wilson C. 1989c. Studies in Balsaminaceae VIII. A revision of
Sumatran Impatiens. – Kew Bull. 44: 67-106.
Grey-Wilson C. 1989d. Studies in Balsaminaceae IX.
Semeiocardium Zoll.; is it a good genus? – Kew Bull. 44: 107-113.
Grey-Wilson C. 1989e. Studies in Balsaminaceae X. The Impatiens
jurpia complex. – Kew Bull. 44: 115-122.
Grey-Wilson C. 1989f. Studies in Balsaminaceae XI. Impatiens
cymbifera and ist allies. – Kew Bull. 44: 711-716.
Grey-Wilson C. 1997. Cyclamen. A
guide for gardeners, horticulturists and botanists. – B. T. Batsford,
London.
Griss A. 1959. Neue Primulaceen und
Chenopodiaceen aus Afghanistan. – Feddes Repert. 62: 22-26.
Gross J, Husband BC, Stewart SC. 1998.
Phenotypic selection in a natural population of Impatiens pallida
Nutt. (Balsaminaceae). – J. Evol.
Biol. 11: 589-609.
Grosse A. 1908. Anatomisch-systematische
Untersuchungen der Myrsinaceen. – Bot. Jahrb. Syst., Beibl. 95: 1-46.
Grote PJ, Dilcher DL. 1989. Investigations of
angiosperms from the Eocene of North America: a new genus of Theaceae based on fruit and seed remains.
– Bot. Gaz. 150: 190-206.
Grote PJ, Dilcher DL. 1992. Fruits and seeds
of tribe Gordonieae (Theaceae) from the
Eocene of North America. – Amer. J. Bot. 79: 744-753.
Grubert M, Hambch M. 1972. Untersuchungen
über die verschleimenden Samen von Collomia grandiflora Dougl. (Polemoniaceae). – Beitr. Biol.
Pflanzen 48: 187-206.
Guédès MS, Schmid R. 1978. The peltate
(ascidiate) carpel theory and carpel peltation in Actinidia chinensis
(Actinidiaceae). – Flora 167:
525-543.
Guillaumin A. 1944. Matériaux pour la flore
de la Nouvelle-Calédonie 82. Sapotacées nouvelles. – Bull. Soc. Bot. France
91: 68-72.
Gunasekera SP, Kumar V, Sultanbawa MUS,
Balasubramaniam S. 1977. Triterpenoids and steroids of some Sapotaceae and their chemotaxonomic
significance. – Phytochemistry 16: 923-926.
Gupta HP, Sharma C. 1977. Palynotaxonomy and
phylogeny of Indian Symplocaceae and
Sapotaceae. – Geophytology 7:
147-159.
Gürke M. 1891a. Ebenaceae. – In: Engler A, Prantl K
(eds), Die natürlichen Pflanzenfamilien IV(1), W. Engelmann, Leipzig, pp.
153-165.
Gürke M. 1891b. Symplocaceae. – In: Engler A, Prantl
K (eds), Die natürlichen Pflanzenfamilien IV(1), W. Engelmann, Leipzig, pp.
165-172.
Gürke M. 1891c. Styracaceae. – In: Engler A, Prantl K
(eds), Die natürlichen Pflanzenfamilien IV(1), W. Engelmann, Leipzig, pp.
172-180.
Gustafsson C. 1992. 146A. Clethraceae. – In: Harling G,
Andersson L (eds), Flora of Ecuador 45, Nord. J. Bot., Copenhagen, pp. 1-26.
Haan I de, Doorenbos J. 1951. The cytology of
the genus Cyclamen. – Meded. Landbouwh. Wageningen 51: 151-166.
Haber E. 1983. Morphological variability and
flavonol chemistry of the Pyrola asarifolia complex (Ericaceae) in North America. – Syst.
Bot. 8: 277-298.
Haber E. 1987. Variability, distribution, and
systematics of Pyrola picta s.l. (Ericaceae) in western North America. –
Syst. Bot. 12: 324-335.
Haber E, Cruise JE. 1974. Generic limits in
the Pyoloideae (Ericaceae). – Can. J.
Bot. 52: 877-883.
Haber WA, Bawa KS. 1984. Evolution of dioecy
in Saurauia (Dilleniaceae). –
Ann. Missouri Bot. Gard. 71: 289-293.
Hagerup E, Hagerup O. 1953. Thrips
pollination of Erica tetralix. – New Phytol. 52: 1-7.
Hagerup O. 1928. Morphological and
cytological studies of Bicornes. – Dansk Bot. Ark. 6(1): 1-26.
Hagerup O. 1941. Zytoökologische
Bicornes-Studien. – Planta 32: 6-14.
Hagerup O. 1946. Studies on the Empetraceae.
– Biol. Meddel. Kong. Danske Vidensk. Selsk. 20(5): 1-49.
Hagerup O. 1953. The morphology and
systematics of the leaves in Ericales.
– Phytomorphology 3: 459-464.
Hague SM. 1911. A morphological study of
Diospyros virginiana. – Bot. Gaz. (Crawfordsville) 52: 34-44.
Halda JJ. 1992. The genus Primula in
cultivation and in the wild. – Tethys Books, Denver, Colorado.
Hall JB. 1996. Vitellaria paradoxa:
a monograph. – University of Wales, Bangor.
Halliday P. 1984. Myrsinaceae. – In: Polhill RM (ed),
Flora of tropical East Africa, A. A. Balkema, Rotterdam, The Netherlands, pp.
1-21.
Hamilton MB, Braverman JM, Soria-Hernanz DF.
2003. Patterns and relative rate of nucleotide and insertion/deletion evolution
at six chloroplast intergenic regions in New World species of the Lecythidaceae. – Mol. Biol. Evol.
20: 1710-1721.
Handel-Mazzetti H. 1928. A revision of the
Chinese species of Lysimachia, with a new system of the whole genus.
– Notes Roy. Bot. Gard. Edinb. 16: 51-122.
Handel-Mazzetti H, Peter-Stibal E. 1943. Eine
Revision der chinesischen Arten der Gattung Symplocos Jacq. – Beih.
Bot. Centralbl. 62B: 1-42.
Hansen I. 1950. Die europäischen Arten der
Gattung Erica L. – Bot. Jahrb. Syst. 75: 1-81.
Hao G, Hu C-M. 2001. Phylogenetic
relationships in Lysimachia (Primulaceae): a cladistic analysis. –
J. Trop. Subtrop. Bot. 9: 93-100.
Hao G, Yuan Y-M, Hu C-M, Ge X-J, Zhao N-X.
2004. Molecular phylogeny of Lysimachia (Myrsinaceae) based on chloroplast
trnL-F and nuclear ribosomal ITS sequences. – Mol. Phylogen. Evol.
31: 323-339.
Hara N. 1958. Structure of the vegetative
shoot apex and development of the leaf in the Ericaceae and their allies. – J. Fac.
Sci. Univ. Tokyo, Sect. 3; Bot. 7: 367-450.
Harborne JB, Smith DM. 1978. Correlations
between anthocyanin chemistry and pollination ecology in Polemoniaceae. – Biochem. Syst.
Ecol. 6: 127-130.
Harborne JB, Williams CA. 1973. A
chemotaxonomic survey of flavonoids and simple phenols in leaves of the Ericaceae. – Bot. J. Linn. Soc. 66:
37-54.
Harder LD, Barclay RMR. 1994. The functional
significance of poricidal anthers and buzz pollination: controlled pollen
removal from Dodecatheon. – Funct. Ecol. 8: 509-517.
Hardin JW. 1966. An analysis of variation in
Symplocos tinctoria. – J. Elisha Mitchell Sci. Soc. 82: 6-12.
Harley MM. 1986a. Ditinguishing pollen
characters for the Sapotaceae. –
Can. J. Bot. 64: 3091-3100.
Harley MM. 1986b. The nature of the
endoaperture in the pollen of the Sapotaceae. – In: Blackmore S, Ferguson
IK (eds), Pollen and spores: form and function, Linn. Soc. Symp. Ser. 12,
Academic Press, London, pp. 417-419.
Harley MM. 1990. Pollen morphology of
neotropical Sapotaceae. – In:
Pennington TD (ed), Sapotaceae, Flora
Neotropica. Monograph 52, New York Botanical Garden, New York, pp. 11-29,
710-741.
Harley MM. 1991a. The pollen morphology of
the Sapotaceae. – Kew Bull. 46:
379-491.
Harley MM. 1991b. Pollen morphology of the Sapotaceae. – In: Pennington TD (ed),
The genera of Sapotaceae, Royal
Botanic Gardens, Kew, NewYork Botanical Garden, New York, pp. 23-50.
Harley MM, Kurmann MH, Ferguson IK. 1991.
Systematic implications of comparative morphology in selected Tertiary and
extant pollen from the Palmae and the Sapotaceae. – In: Blackmore S, Barnes
HS (eds), Pollen and spores: patterns of diversification, Clarendon Press,
Oxford, pp. 225-238.
Harms H. 1935. Rafflesiaceae.
– In: Engler A (†), Harms H (eds), Die natürlichen Pflanzenfamilien, 2.
Aufl., Bd. 16b, W. Engelmann, Leipzig, pp. 243-281.
Harrison HK. 1972. Contributions to the study
of the genus Eriastrum II. Notes concerning the type specimens and
descriptions of the species. – Brigham Young Univ. Sci. Bull. Biol. Ser. 16:
1-26.
Hartog MM. 1878. On the floral structure and
affinities of Sapotaceae. – J. Bot.
16: 65-72, 145.
Hartog MM. 1879. Notes on Sapotaceae II. – J. Bot. 17:
356-359.
Harvey CF, Fraser LG. 1988. Floral biology of
two species of Actinidia (Actinidiaceae) II. Early embryology.
– Bot. Gaz. 149: 37-44.
Harvey CF, Fraser LG, Pavis SE, Considine JA.
1987. Floral biology of two species of Actinidia (Actinidiaceae) I. The stigma,
pollination, and fertilization. – Bot. Gaz. 148: 426-432.
Hatusima S. 1936. Miscellaneous notes on the
Symplocaceae of Eastern Asia. – J.
Jap. Bot. 12: 279-283.
Hausen BM. 1978. On the occurrence of the
contact allergen primin and other quinoid compounds in species of the family Primulaceae. – Arch. Dermatol. Res.
261: 311-322.
Hayata B. 1913. Über die systematische
Stellung von Mitrastemon als einer neuen Gattung und besonderern
Tribus der Rafflesiaceen. – Bot. Jahrb. 51: 154-176.
Hayata B. 1913. On the systematic position of
Mitrastemon. – Icon. Plant. Form. 3: 199-213.
He Z-C, Li J-Q, Cai Q, Wang Q. 2005. The
cytology of Actinidia, Saurauia and Clematoclethra
(Actinidiaceae). – Bot. J. Linn.
Soc. 147: 369-374.
Heads M. 2003. Ericaceae in Malesia: vicariance
biogeography, terrane tectonics and ecology. – Telopea 10: 211-449.
Hebda RJ, Chinnappa CC. 1980. Cytological
evidence for generic limits in tribe Cladothamneae (Ericaceae). – Can. J. Bot. 58:
1119-1120.
Heel WA van. 1987. Androecium development in
Actinidia chinensis and A. melanandra (Actinidiaceae). – Bot. Jahrb. Syst.
109: 17-23.
Heine H. 1960. Notes on African Sapotaceae I. – Kew Bull. 14:
301-303.
Heine H. 1963. Sapotaceae in Flora of West tropical
Africa, 2nd ed., vol. II: 16-30.
Heine H, Hemsley JH. 1960. Notes on African
Sapotaceae II. The genus
Bequaertiodendron De Wild. – Kew Bull. 14: 304-309.
Hemsley JH. 1966. Notes on African Sapotaceae. – Kew Bull. 20: 461-510.
Hemsley JH. 1968. Sapotaceae. – In: Milne-Redhead E,
Polhill RM (eds), Flora of tropical East Africa, Crown Agents for Oversea
Governments and Administrations, London, pp. 1-78.
Henderson MW. 1919. A comparative study of
the structure and saprophytism of the Pyrolaceae and the Monotropaceae, with
reference to their derivation from the Ericaceae. – Contr. Bot. Lab. Morris
Arbor. Univ. Pennsylvania 5: 42-109.
Henderson RJ. 1982. Lecythidaceae. – In: George AS (ed),
Flora of Australia 8, Australian Government Publ. Service, Canberra, pp.
1-6.
Henrickson J. 1967. Pollen morphology of the
Fouquieriaceae. – Aliso 6:
137-160.
Henrickson J. 1968. Vegetative morphology of
the Fouquieriaceae. – Ph.D.
diss., Claremont Graduate School, Claremont, California.
Henrickson J. 1969a. An introduction to the
Fouquieriaceae. – Cactus Succ.
J. 41: 97-106.
Henrickson J. 1969b. Anatomy of periderm and
cortex of Fouquieriaceae. –
Aliso 7: 97-126.
Henrickson J. 1969c. The succulent
fouquierias. – Cactus Succ. J. 41: 178-184.
Henrickson J. 1972. A taxonomic revision of
the Fouquieriaceae. – Aliso 7:
439-537.
Henrickson J. 1973. Fouquieriaceae DC. – World Pollen
and Spore Flora 1, Almqvist & Wiksell, pp. 1-12.
Henrickson J. 1983. A revision of Samolus
ebracteatus (sensu lato) (Primulaceae). – SouthWestern Nat. 28:
303-314.
Henrickson J. 1987. New species,
combinations, and notes in Ipomopsis (Polemoniaceae). – Aliso 11:
589-598.
Hepburn JS, St. John EQ, Jones FM. 1920. The
absorption of nutrients and allied phenomena in the pitchers of the Sarraceniaceae. – J. Franklin Inst.
189: 147-184.
Hepburn JS, St. John EQ, Jones FM. 1927. The
biochemistry of the American pitcher plants. – Trans. Wagner Free Inst. Sci.
Philadelphia 11: 1-95.
Hermann PM, Cambi VN. 1992. Nuevas datos
sobre la sexualidad de Gaultheria caespitosa P. & E. (Ericaceae). – Parodiana 7: 83-90.
Hermann PM, Palser BF. 2000. Stamen
development in the Ericaceae I. Anther
wall, microsporogenesis, inversion, and appendages. – Amer. J. Bot. 87:
934-957.
Hermann-Erlee MPM, Royen P van. 1957.
Revision of the Sapotaceae of the
Malaysian area in a wider sense IX. Pouteria Aublet. – Blumea 8:
452-509.
Hersey RE, Vander Kloet SP. 1976. Taxonomy
and distribution of Gaultheria in the Caribbean. – Can. J. Bot. 54:
2465-2472.
Hess H. 1953. Anagallis kochii H.
Hess, n. sp., eine neue Wasserpflanze aus Süd-Angola. – Ber. Schweiz. Bot.
Ges. 63: 213-217.
Hess H. 1957. Anagallis hürneri H.
Hess, nov. spec., eine neue Wasserpflanze aus der Belgischen Kongo. – Ber.
Schweiz. Bot. Ges. 67: 80-82.
Hiern WP. 1873. A monograph of Ebenaceae. – Trans. Cambridge Philos.
Soc. 12: 27-300.
Hiern WP. 1898. A new genus of Ericaceae from Angola. – J. Bot. 36:
329-330.
Higashi H, Ikeda H, Setoguchi H. 2015.
Molecular phylogeny of Shortia sensu lato (Diapensiaceae) based on multiple
nuclear sequences. – Plant Syst. Evol. 301: 523-529.
Higham RK, McQuillan PB. 2000. Cyathodes
divaricata (Epacridaceae) – the first record of a bird-pollinated
dioecious plant in the Australian flora. – Aust. J. Bot. 48: 93-99.
Hildebrand F. 1898. Die Gattung
Cyclamen L., eine systematische und biologische Monographie. –
Gustav Fischer, Jena.
Hileman LC, Vasey MC, Parker VT. 2001.
Phylogeny and biogeography of the Arbutoideae (Ericaceae): implications for the
Madrean-Tethyan hypothesis. – Syst. Bot. 26: 131-143.
Hiller K, Paulick A, Doehnert H, Franke P.
1979. Saponins of Polemonium caeruleum L. – Pharmazie 34:
565-566.
Hillis WE, Soenardi P. 1994. Formation of
ebony and streaked woods. – I.A.W.A. J. 15: 425-437.
Hindakova M, Schwarzova T. 1980. Chromosome
number reports LXIX. – Taxon 29: 728.
Hislop M. 2011. New, locally endemic taxa in
Leucopogon (Ericaceae:
Styphelioideae: Styphelieae) from the Perth and midwest regions of Western
Australia. – Nuytsia 21: 75-89.
Hislop M. 2013. A taxonomic update of
Conostephium (Ericaceae:
Styphelioideae: Styphelieae). – Nuytsia 23: 313-335.
Hislop M, Puente-Lelievre C, Crayn D. 2012.
Leucopogon extremus (Styphelieae, Styphelioideae, Ericaceae), a remarkable new species that
expands the morphological circumscription of Leucopogon sens. str. –
Aust. Syst. Bot. 25: 202-209.
Hislop M, Wilson AJG, Puente-Lelièvre C.
2013. Four new species of Astroloma (Ericaceae: Styphelioideae: Styphelieae)
from Western Australia. – Nuytsia 23: 23-42.
Hitzemann C. 1886. Beiträge zur
vergleichende Anatomie der Terstroemiaceen, Dilleniaceen, Dipterocarpaceen und
Chlaenaceen. – von Giebel & Oehlschlägel, Osterode.
Hoerold R. 1909. Systematische Gliderung und
geographische Verbreitung der amerikansichen Thibaudieen. – Engl. Bot. Jahrb.
Syst. 42: 251-334.
Holle G. 1892. Über den anatomischen Bau des
Blattes in der Familie der Sapotaceae
und dessen Bedeutung für die Systematik. – Ph.D. diss., Universität
Erlangen.
Holm T. 1898. Pyrola aphylla: a
morphological study. – Bot. Gaz. 25: 246-254.
Hooker JD. 1899. Icones Plantarum, Ser. IV,
vol. 7(1): t. 2624.
Hopping ME. 1976. Structure and development
of fruit and seeds in Chinese gooseberry (Actinidia chinensis
Planch.). – New Zealand J. Bot. 14: 63-68.
Hopping ME. 1990. Floral biology,
pollination, and fruit set. – In: Warrington IJ, Weston GC (eds), Kiwifruit:
science and management, Ray Richards, Auckland, pp. 71-96.
Hopping ME, Jerram EM. 1979. Pollination of
kiwifruit (Actinidia chinensis Planch.): stigma-style structure and
pollen tube growth. – New Zealand J. Bot. 17: 233-240.
Hosaka EY. 1940. A revision of the Hawaiian
species of Myrsine (Suttonia, Rapanea), Myrsinaceae. – Occas. Pap. Bernice P.
Bishop Mus. 16: 25-76.
House HD. 1907. The genus Shortia.
– Torreya 7: 233-235.
Howard RA. 1970. The ecology of an elfin
forest in Puerto Rico X. Notes on two species of Marcgravia. – J.
Arnold Arbor. 51: 41-55.
Howpage D, Vithanage V, Spooner-Hart R. 1998.
Pollen tube distribution in the kiwifruit (Actinidia deliciosa A.
Chev. C. F. Liang) pistil in relation to its reproductive process. – Ann.
Bot., N. S., 81: 697-703.
Hsieh CF, Ling LK, Yang KC. 1996. Theaceae. – In: Huang TC (ed), Flora of
Taiwan, 2nd ed., Vol. 2, Editorial Committee of the Flora of Taiwan,
Taipei, pp. 662-693.
Hsu T-Z. 1982. Classification, distribution
and phylogeny of the genus Enkianthus. – Acta Bot. Yunnan. 4:
355-362.
Hsu T-Z. 1985. A new species of
Enkianthus from China. – Acta Bot. Yunnan. 7: 151-152.
Hu CM. 1983a. Two new Asiatic Primulas of the
Malacoides section. – Notes Roy.Bot. Gard. Edinb. 41: 327-331.
Hu CM. 1983b. Two new species of
Lysimachia (Primulaceae).
– Kew Bull. 38: 333-334.
Hu CM. 1990. A new species of
Primula from Thailand with critical notes on the section
Carolinella. – Nord. J. Bot. 10: 399-401.
Hu H-H. 1965. New species and varieties of
Camellia and Theopsis of China 1. – Acta Phytotaxon. Sin.
10: 131-142. [In Chinese]
Huang T-C, Hsiao A. 1998. Notes on the Flora
of Taiwan 31 – Shortia (Diapensiaceae). – Taiwania 43:
33-37.
Huang Y-Y, Scott AM, Prance GT. 2008. A
phylogeny of Cariniana (Lecythidaceae) based on morphological
and anatomical data. – Brittonia 60: 69-81.
Huang Y-Y, Mori SA, Kelly LM. 2011. A
morphological cladistic analysis of Lecythidoideae with emphasis on
Bertholletia, Corythophora, Eschweilera, and
Lecythis. – Brittonia 63: 396-417.
Huertas GG. 1969. Un nuevo genero y especie
fosiles de las lecitidaceas. – Caldasia 10(48): 365-369.
Humphrey RR. 1931. Thorn formation in
Fouquieria splendens and Idria columnaris. – Bull. Torrey
Bot. Club 58: 263-264.
Humphrey RR. 1935. A study of Idria
columnaris and Fouquieria splendens. – Amer. J. Bot. 22:
184-206.
Humphrey RR, Marx DB. 1980. Distribution of
the boojum tree (Idria columnaris) on the coast of Sonora, Mexico, as
influenced by climate. – Desert Plants 2: 183-187.
Hunter GE. 1966. Revision of Mexican and
Central American Saurauia (Dilleniaceae). –
Ann. Missouri Bot. Gard. 53: 47-89.
Hunter JR. 1981. Tendu (Diospyros
melanoxylon) leaves, bidi cigarettes, and resource management. – Econ.
Bot. 35: 450-459.
Hurlbert AH, Hosoi SA, Temeles EJ, Ewald PW.
1996. Mobility of Impatiens capensis flowers: effect on pollen
deposition and hummingbird foraging. – Oecologia 105: 243-246.
Hutton BJ. 1997. Biology and ecology of
endophytes of Australian native heaths (Epacridaceae). – Ph.D. diss.,
Department of Botany, University of Western Australia.
Hutton BJ, Dixon KW, Sivasithamparam K. 1994.
Ericoid endophytes of Western Australian heaths (Epacridaceae). – New Phytol.
127: 557-566.
Huynh K-L. 1968. Morphologie du pollen des
Tropaeolacées et des Balsaminacées. – Grana Palynol. 8: 88-184, 277-516.
Huynh K-L. 1970a. Quelques caractères
cytologiques, anatomiques, et embryologiques distinctifs du genre
Tropaeolum et du genre Impatiens, et position taxinomique de
la famille des Balsaminacées. – Bull. Soc. Neuchâtel. Sci. Nat. 93:
165-177.
Huynh K-L. 1970b. Le pollen et la
systématique chez le genre Lysimachia (Primulaceae) I. Morphologie générale
du pollen et palynotaxonomie. – Candollea 25: 267-296.
Huynh K-L. 1971. Le pollen et la
systématique chez le genre Lysimachia (Primulaceae) II. Considérations
générales. – Candollea 26: 279-295.
Hynson NA, Bruns TD. 2009. Evidence of a
myco-heterotroph in the plant family Ericaceae that lacks mycorrhizal
specificity. – Proc. Roy. Soc. B, Biol. Sci. 276: 4053-4059.
Hynson NA, Preiss K, Gebauer G, Bruns TD.
2009. Isotopic evidence of full and partial myco-heterotrophy in the plant
tribe Pyroleae (Ericaceae). – New
Phytol. 182: 719-726.
Ikuse M. 1954. The presence of the viscid
threads among pollen grains in Phyllodoceae, etc. of Ericaceae. – J. Jap. Bot. 29:
146-148.
Infantes Ver JG. 1962. Revision del género
Cantua (Polemoniaceae).
– Lilloa 31: 75-107.
Iturralde RB. 2009. New species of
Cyrilla (Cyrillaceae) from
Cuba. – Willdenowia 39: 121-140.
Jackes BR. 1968. Floral anatomy of the genus
Oligarrhena R. Br. (Epacridaceae). – Aust. J. Bot. 16: 451-454.
Jackes BR. 2005. Revision of Myrsine
(Myrsinaceae) in Australia. – Aust.
Syst. Bot. 18: 399-438.
Jackes BR, Powell JM. 1980. Additions to the
genus Acrotriche R. Br. (Epacridaceae). – Telopea 1: 421-428.
Jacobs SWL. 1980. A new species of
Samolus (Primulaceae). –
J. Adelaide Bot. Gard. 2: 187-189.
Jacques F. 1965. Morphologie de pollen et des
ovules de Couroupita guianensis Aubl. (Lécythidacées). – Pollen
Spores 7: 175-180.
Jaffé K, Michelangeli F, Gonzalez JM, Miras
B, Ruiz MC. 1992. Carnivory in pitcher plants of the genus Heliamphora
(Sarraceniaceae). – New Phytol.
122: 733-744.
Jamzad Z. 1996. The genus Dionysia
(Primulaceae) in Iran. – Iranian J.
Bot. 7: 15-30.
Janaki Ammal EK, Enoch IC, Bridgwater M.
1950. Chromosome numbers in species of Rhododendron. – Rhodod. Year
Book 5: 78-95.
Jansen S, Watanabe T, Caris P, Geuten K, Lens
F, Pyck N, Smets E. 2004. The distribution and phylogeny of aluminium
accumulating plants in the Ericales. –
Plant Biol. 6: 498-505.
Janssens SB, Lens F, Dressler S, Geuten K,
Smets E, Vinckler S. 2005. Palynological variation in balsaminoid Ericales II. Balsaminaceae, Tetrameristaceae, Pellicieraceae
and general conclusions. – Ann. Bot. 96: 1061-1073.
Janssens SB, Geuten K, Yuan YM, Song Y,
Küpfer P, Smets E. 2006. Phylogenetics of Impatiens and
Hydrocera (Balsaminaceae)
using chloroplast atpB-rbcL spacer sequences. – Syst. Bot.
31: 171-180.
Janssens SB, Geuten K, Viaene T, Yuan Y-M,
Song Y, Smets E. 2007. Phylogenetic utility of the AP3/DEF K-domain and its
molecular evolution in Impatiens (Balsaminaceae). – Mol. Phylogen.
Evol. 43: 225-239.
Janssens SB, Knox EB, Dessein S, Smets EF.
2009. Impatiens msisimwanensis (Balsaminaceae): description, pollen
morphology and phylogenetic position of a new East African species. – South
Afr. J. Bot. 75: 104-109.
Janssens SB, Knox EB, Huysmans S, Smets EF,
Merckx VSFT. 2009. Rapid radiation of Impatiens (Balsaminaceae) during Pliocene and
Pleistocene: result of a global climate change. – Mol. Phylogen. Evol. 52:
806-824.
Janssens SB, Fischer E, Stévart T. 2010. New
insights into the origin of two new epiphytic Impatiens species (Balsaminaceae) from West Central
Africa based on molecular phylogenetic analyses. – Taxon 59: 1508-1518.
Janssens SB, Wilson YS, Yuan Y-M, Nagels A,
Smets EF, Huysmans S. 2012. A total evidence approach using palynological
characters to infer the complex evolutionary history of the Asian
Impatiens (Balsaminaceae).
– Taxon 61: 355-367.
Janssens SB, Smets EF, Vrijdaghs A. 2012.
Floral development of Hydrocera and Impatiens reveals
evolutionary trends in the most early diverged lineages of the Balsaminaceae. – Ann. Bot. 109:
1285-1296.
Janzen DH. 1970. Jacquinia pungens,
a heliophile from the understory of tropical deciduous forest. – Biotropica
2: 112-119.
Jarman SJ. 1975. Experimental taxonomy in the
family Epacridaceae. – Ph.D. diss., Department of Botany, University of
Tasmania.
Jarman SJ, Crowden RK. 1974. Anthocyanins in
the Epacridaceae. – Phytochemistry 13: 743-750.
Jarman SJ, Crowden RK. 1977. The occurrence
of flavonol arabinosides in the Epacridaceae. – Phytochemistry 16:
929-930.
Jay M, Lebreton P. 1973. Recherches
chimiotaxinomiques sur les plantes vasculaires XXVI. Les flavanoides des
Sarracéniacées, Nepenthacées, Droséracées, et Céphalotacées; étude
critique de l’ordre des Sarracéniales. – Nat. Can. 91: 607-613.
Jaynes RA. 1968. Interspecific crosses of
Kalmia. – Amer. J. Bot. 55: 1120-1125.
Jaynes RA. 1969. Chromosome counts of
Kalmia species and reevaluation of K. polifolia var.
microphylla. – Rhodora 71: 280-284.
Jaynes RA. 1971. The kalmias and their
hybrids. – Quart. Bull. Amer. Rhododendron Soc. 25: 160-164.
Jensen LCW. 1961. Pollination studies with
native Minnesota Pyrola and Moneses species. – Proc.
Minnesota Acad. Sci. 29: 210-218.
Jensen SR, Nielsen BJ. 1982. Iridoid
glucosides in Fouquieriaceae. –
Phytochemistry 21: 1623-1629.
Jerling L. 1988. Population dynamics of
Glaux maritima L. along a distributional cline. – Vegetatio 74:
161-170.
Jessup LW. 2001. New combinations and a new
name in Australian Sapotaceae. –
Austrobaileya 6: 161-163.
Jessup LW. 2014. A taxonomic revision of
Diospyros L. (Ebenaceae) in
Australia. – Austrobeileya 9: 155-194.
Jiang B, Peng Q-F, Shen Z-G, Möller M, Pi
E-X, Lu H-F. 2010. Taxonomic treatments of Camellia (Theaceae) species with secretory structures
based on integrated leaf characters. – Plant Syst. Evol. 290: 1-20.
Jímenez JA. 1984. A hypothesis to explain
the reduced distribution of the mangrove Pelliciera rhizophorae Tr.
& Pl. – Biotropica 16: 304-308.
Jin X-F, Yang S-Z, Chen Z-H, Qian L. 2008.
Impatiens yilingiana sp. nov. and I. huangyanensis subsp.
attenuata subsp. nov. (Balsaminaceae) from Zhejiang, eastern
China. – Nord. J. Bot. 26: 207-213.
Jochems SCJ. 1928. Die Verbreitung der
Rafflesiaceengattung Mitrastemon. – Rec. Trav. Bot. Néerl. 25A:
203-207.
Johansen DA. 1936. Morphology and embryology
of Fouquieria. – Amer. J. Bot. 23: 95-99.
John J, Kolbe K-P. 1980. The systematic
position of the “Theales” from the view point of serology. – Biochem.
Syst. Ecol. 8: 241-248.
Johnson KA, Holland BR, Heslewood MM, Crayn
DM. 2012. Supermatrices, supertrees and serendipitous scaffolding: inferring a
well-resolved, genus-level phylogeny of Styphelioideae (Ericaceae) despite missing data. – Mol.
Phylogen. Evol. 62: 146-158.
Johnson LA. 1996. A molecular approach to
resolving phylogenetic relationships in Polemoniaceae. – Ph.D. diss.,
Washington State University, Pullman, Washington.
Johnson LA. 2007. Transfer of the western
north American species Gilia splendens to Saltugilia (Polemoniaceae), and the taxonomic
affinities of Gilia scopulorum, Gilia stellata, and Gilia
yorkii. – Novon 17: 193-197.
Johnson LA, Johnson RL. 2006. Morphological
delimitation and molecular evidence for allopolyploidy in Collomia
wilkenii (Polemoniaceae), a
new species from northern Nevada. – Syst. Bot. 31: 349-360.
Johnson LA, Porter M. 2017. Fates of
angiosperm species following long-distance dispersal: examples from American
amphitropical Polemoniaceae. –
Amer. J. Bot. 104: 1729-1744.
Johnson LA, Soltis DE. 1995. Phylogenetic
inference in Saxifragaceae
sensu stricto and Gilia (Polemoniaceae) using matK
sequences. – Ann. Missouri Bot. Gard. 82: 149-175.
Johnson LA, Soltis DE. 1998. Assessing
congruence: examples from molecular data. – In: Soltis PS, Soltis DE, Doyle
JJ (eds), Molecular systematics of plants II: DNA sequencing, Kluwer Academic
Publ., Boston, Massachusetts, pp. 297-348.
Johnson LA, Weese TL. 2000. Geographic
distribution, morphological and molecular characterization, and relationships
of Lathrocasis tenerrima (Polemoniaceae). – West. N. Amer.
Nat. 60: 355-373.
Johnson LA, Schultz JL, Soltis DE, Soltis PS.
1996. Monophyly and generic relationships of Polemoniaceae based on matK
sequences. – Amer. J. Bot. 83: 1207-1224.
Johnson LA, Soltis DE, Soltis PS. 1999.
Phylogenetic relationships of Polemoniaceae inferred from 18S
ribosomal DNA sequences. – Plant Syst. Evol. 214: 65-89.
Johnson LA, Huish KH, Porter JM. 2004. Seed
surface sculpturing and its systematic significance in Gilia (Polemoniaceae) and segregate genera.
– Int. J. Plant Sci. 165: 153-172.
Johnson LA, Chan LM, Weese TL, Busby LD,
McMurry S. 2008. Nuclear and cpDNA sequences combined provide strong inference
of higher phylogenetic relationships in the phlox family (Polemoniaceae). – Mol. Phylogen.
Evol. 48: 997-1012.
Johnson MAT. 1991. Cytology. – In:
Pennington TD (ed), The genera of Sapotaceae, chapter 2, Royal Botanic
Gardens, Kew, New York Botanical Garden, New York, pp. 15-22.
Jones K, Smith JB. 1966. The cytogeography of
Impatiens L. (Balsaminaceae). – Kew Bull. 20:
63-72.
Jones K, Anderberg AA, Ronse De Craene LP,
Wanntorp L. 2012. Origin, diversification, and evolution of Samolus
valerandi (Samolaceae, Ericales). – Plant Syst. Evol. 298:
1523-1531.
Jordan GJ, Hill RS. 1995. Oligocene leaves of
Epacridaceae from Little Rapid River, Tasmania, and the identification of
fossil Epacridaceae leaves. – Aust. Syst. Bot. 8: 71-83.
Jordan GJ, Hill RS. 1996. The fossil record
of the Epacridaceae. – Ann. Bot., II, 77: 341-346.
Jordan GJ, Bromfield KE, Sniderman JMK, Crayn
D. 2007. Diverse fossil epacrids (Styphelioideae; Ericaceae) from early Pleistocene
sediments at Stony Creek Basin, Victoria, Australia. – Int. J. Plant Sci.
168: 1359-1376.
Jordan GJ, Bannister JM, Mildenhall DC,
Zetter R, Lee FE. 2010. Fossil Ericaceae from New Zealand: deconstructing
the use of fossil evidence in historical biogeography. – Amer. J. Bot. 97:
59-70.
Judd WS. 1979. Generic relationships in the
Andromedeae (Ericaceae). – J. Arnold
Arbor. 60: 477-503.
Judd WS. 1981. A monograph of Lyonia
(Ericaceae). – J. Arnold Arbor. 62:
63-128, 129-209, 315-436.
Judd WS. 1982. A taxonomic revision of
Pieris (Ericaceae). – J.
Arnold Arbor. 63: 103-144.
Judd WS. 1983. Kalmia ericoides
revisited. – Rhodora 85: 45-54.
Judd WS. 1984. A taxonomic revision of the
American species of Agarista (Ericaceae). – J. Arnold Arbor. 65:
255-342.
Judd WS. 1986. A taxonomic revision of
Craibiodendron (Ericaceae).
– J. Arnold Arbor. 67: 441-469.
Judd WS. 1990. A new variety of
Lyonia (Ericaceae) from Puerto
Rico. – J. Arnold Arbor. 71: 129-133.
Judd WS. 1995a. 13. Lyonia Nuttall.
– In: Luteyn JL (ed), Flora Neotropica. Monograph 66. Ericaceae II – the superior-ovaried
genera, New York Botanical Garden, Bronx, New York, pp. 222-294.
Judd WS. 1995b. 14. Agarista D. Don
ex G. Don. – In: Luteyn JL (ed), Flora Neotropica. Monograph 66. Ericaceae II – the superior-ovaried
genera, New York Botanical Garden, Bronx, New York, pp. 295-344.
Judd WS. 1995c. 15. Pieris D. Don.
– In: Luteyn JL (ed), Flora Neotropica. Monograph 66. Ericaceae II – the superior-ovaried
genera, New York Botanical Garden, Bronx, New York, pp. 345-350.
Judd WS, Hermann PM. 1990. Circumscription of
Agarista boliviensis (Ericaceae). – Sida 14: 263-266.
Judd WS, Kron KA. 1993. Circumscription of Ericaceae (Ericales) as determined by preliminary
cladistic analyses based on morphological, anatomical, and embryological
features. – Brittonia 45: 99-114.
Judd WS, Melvin NC III, Waselkov K, Kron KA.
2012. Taxonomic revision of Eubotrys (Ericaceae, Gaultherieae). – Brittonia
64: 165-178.
Judd WS, Melvin NC III, Waselkov K, Kron KA.
2013. A taxonomic revision of Leucothoë (Ericaceae; tribe Gaultherieae). –
Brittonia 65: 417-438.
Juel HO. 1887. Beiträge zur Anatomie der Marcgraviaceae. – Kongl. Sv.
Vetensk.-Acad. Handl. 12, Afd. 3, Nr. 5, Bihang: 1-28.
Julius A, Utteridge TMA. Revision of
Ardisia subgenus Bladhia in Peninsular Malaysia; studies in
Malaysian Myrsinaceae I. – Kew
Bull. 67: 379-388.
Jurenitsch J, Haslinger E, Kubelka W. 1979.
Structure of sapogenins from Polemonium reptans. – Pharmazie 34:
445-446.
Källersjö M, Ståhl B. 2003. Phylogeny of
Theophrastaceae (Ericales s. lat.). – Int. J. Plant Sci.
164: 579-591.
Källersjö M, Bergqvist G, Anderberg AA.
2000. Generic realignment in primuloid families of the Ericales s. l.: a phylogenetic analysis
based on DNA sequences from three chloroplast genes and morphology. – Amer.
J. Bot. 87: 1325-1341.
Kamelina OP. 1997. Dopolneniya k embriologii
semeystv Lactoridaceae i Fouquieriaceae. – Bot. Žurn. 82:
25-29.
Kang N, Wang S, Huang R, Wu X. 1993. Studies
on the pollen morphology of nine species of genus Actinidia. – J.
Wuhan Bot. Res. 11: 111-116.
Kapil RN, Sethi S Bala. 1963. Development of
male and female gametophytes in Camellia sinensis (L.) O. Kuntze. –
Proc. Indian Natl. Inst. Sci., Sect. B, 29: 567-574.
Kapil RN, Rustagi PN, Venkataraman R. 1968. A
contribution to the embryology of the Polemoniaceae. – Phytomorphology 18:
403-412.
Karagatzides JD, Butler JL, Ellison AM. 2009.
The pitcher plant Sarracenia purpurea can directly acquire organic
nitrogen and short-circuit the inorganic nitrogen cycle. – PLoS ONE 4:
1-9.
Kartawinata EK. 1965. The genus
Planchonia Blume (Lecythidaceae). – Bull. Bot. Surv.
India 7: 162-187.
Kato E, Hiura T. 1999. Fruit set in
Styrax obassia (Styracaceae): the effect of light
availability, display size, and local floral density. – Amer. J. Bot. 86:
495-501.
Kato M. 1988. Bumblebee visits to
Impatiens spp. – pattern and efficiency. – Oecologia 76:
364-370.
Kavaljian LG. 1952. The floral morphology of
Clethra alnifolia with some notes on C. acuminata and C.
arborea. – Bot. Gaz. 113: 392-413.
Keeley JE, Parker VT, Vasey MC. 2017.
Characters in Arctostaphylos taxonomy. – Madroño 64: 138-153.
Keighery GJ. 1988. A new species of
Samolus (Primulaceae) from
Western Australia. – Nord. J. Bot. 8: 329-330.
Keighery GJ. 1996. Phytogeography, biology
and conservation of Western Australian Epacridaceae. – Ann. Bot., N. S., 77:
347-355.
Keller BT, Breedlove DE. 1981. Two new
species of Saurauia (Actinidiaceae) from Mexico. – Syst.
Bot. 6: 65-73.
Keller JA, Herendeen PS, Crane PR. 1996.
Fossil flowers and fruits of the Actinidiaceae from the Campanian (Late
Cretaceous) of Georgia. – Amer. J. Bot. 83: 528-541.
Kelly LM, Almeda F. 2006. Symplocos
pachycarpa (Symplocaceae), a
new species from southern Mexico. – Brittonia 53: 318-321.
Kelso S. 1991a. Conspectus of the genus
Douglasia (Primulaceae) with
comments on Douglasia alaskana, an Alaska-Yukon alpine endemic. –
Can. J. Bot. 70: 593-596.
Kelso S. 1991b. Taxonomy of Primula
sections Aleuritia and Armerina in North America. – Rhodora
93: 67-99.
Keng H. 1950. The Theaceae of Taiwan. – Taiwania 1:
223-268.
Keng H. 1962. Comparative morphological
studies in the Theaceae. – Univ.
Calif. Publ. Bot. 33: 269-384.
Keng H. 1980a. The genus Pyrenaria
(Theaceae) in Malesia (Flora Malesianae
Precursores LVIII, Part One). – Gard. Bull. Singapore 33: 264-289.
Keng H. 1980b. On the unification of
Laplacea and Gordonia (Theaceae). – Gard. Bull. (Singapore) 33:
303-311.
Kerdell-Vargas F. 1966. The depilatory and
cytotoxic action of Coco de Mono (Lecythis ollaria) and its
relationship to chronic seloniosis. – Econ. Bot. 20: 187-195.
Khan R. 1943. The ovule and embryo sac of
Fouquieria. – Proc. Natl. Inst. Sci. India, Sect. B, 9: 253-256.
Kim KH, Nilsson S, Praglowski J. 1988. A note
on the pollen morphology of the Empetraceae. – Grana 27: 283-290.
Kirchheimer F. 1950. Die Symplocaceae der erdgeschichtlichen
Vergangenheit. – Palaeontographica, Ser. B, 90: 1-52.
Kiredjian M, Smith DM. 1985. A
chemosystematic investigation on Ipomopsis. – Biochem. Syst. Ecol.
13: 141-143.
Kirkman WB, Ballington JR. 1990. Creeping
blueberries (Ericaceae:
Vaccinium sect. Herpothamnus) – a new look at V.
crassifolium including V. sempervirens. – Syst. Bot. 15:
679-699.
Kloet SP van der. 1985. On the generic status
of Symphysia. – Taxon 34: 440-447.
Kloet SP van der, Avery TS. 2007. The
taxonomic utility of staminal features in Vaccinieae (Ericaceae). – Taxon 56: 897-904.
Kloet SP van der, Baltzer JL, Appleby JH,
Evans RC, Stewart DT. 2004. A re-examination of the taxonomic boundaries of
Symphysia (Ericaceae). –
Taxon 53: 91-98.
Knaben G, Engelskjøn T. 1968. Studies in
Pyrolaceae, especially in the Pyrola rotundifolia complex. – Årb.
Univ. Bergen. Mat.-Naturv. Ser. 167: 1-71.
Knudsen J, Mori SA. 1996. Floral scents and
pollination in Lecythidaceae. –
Biotropica 28: 42-60.
Knudsen JT, Olesen JM. 1993. Buzz-pollination
and patterns in sexual traits in north European Pyrolaceae. – Amer. J. Bot.
80: 900-913.
Knudsen JT, Ståhl B. 1994. Floral odours in
the Theophrastaceae. – Biochem.
Ecol. Syst. 22: 259-268.
Knudsen JT, Tollsten L. 1991. Floral scent
and intrafloral scent differentiation in Moneses and Pyrola
(Pyrolaceae). – Plant Syst. Evol. 177: 81-91.
Knuth R. 1934. Über die Gattung
Asteranthos. – Notizbl. Bot. Gart. Berlin-Dahlem 11: 1034-1036.
Ko SC, Im IT, Kim YS. 1986. A cytotaxonomic
study on the genus Lysimachia in Korea. – Korean J. Plant Taxon. 16:
187-197.
Kobayashi N, Handa T, Yoshimyura K, Tsumura
Y, Arisumi K, Takayanagi K. 2000. Evidence for introgressive hybridization
based on chloroplast DNA polymorphisms and morphological variation in wild
evergreen azalea populations of the Kirishima mountains, Japan. – Edinburgh
J. Bot. 57: 209-219.
Kobuski CE. 1937. Studies in the theaceae II.
Cleyera. – J. Arnold Arbor. 18: 118-129.
Kobuski CE. 1940. Studies in the Theaceae V. The Theaceae of New Guinea. – J. Arnold
Arbor. 21: 134-162.
Kobuski CE. 1941a. Studies in the Theaceae VII. The American species of the
genus Cleyera. – J. Arnold Arbor. 22: 395-416.
Kobuski CE. 1941b. Studies in the Theaceae VIII. A synopsis of the genus
Freziera. – J. Arnold Arbor. 22: 457-496.
Kobuski CE. 1942a. Studies in the Theaceae XII. Notes on the South American
species of Ternstroemia. – J. Arnold Arbor. 23: 298-343.
Kobuski CE. 1942b. Studies in the Theaceae XIII. Notes on the Mexican and
Central American species of Ternstroemia. – J. Arnold Arbor. 23:
464-478.
Kobuski CE. 1943. Studies in the Theaceae XIV. Notes on the West Indian
species of Ternstroemia. – J. Arnold Arbor. 24: 60-76.
Kobuski CE. 1947. Studies in the Theaceae XV. A review of the genus
Adinandra. – J. Arnold Arbor. 28: 1-98.
Kobuski CE. 1949. Studies in the Theaceae XVIII. The West Indian species of
Laplacea. – J. Arnold Arbor. 30: 166-186.
Kobuski CE. 1950. Studies in the Theaceae XX. Notes on the South and Central
American species of Laplacea. – J. Arnold Arbor. 31: 405-429.
Kobuski CE. 1951a. Studies in the Theaceae XXIII. The genus
Pelliciera. – J. Arnold Arbor. 32: 256-262.
Kobuski CE. 1951b. Studies in the Theaceae XXIV. The genus Sladenia.
– J. Arnold Arbor. 32: 403-409.
Kobuski CE. 1952a. Studies in the Theaceae XXV. The genus Anneslea.
– J. Arnold Arbor. 33: 79-90.
Kobuski CE. 1952b. Studies in the Theaceae XXVI. The genus Visnea.
– J. Arnold Arbor. 33: 188-191.
Kobuski CE. 1956. Studies in the Theaceae XXVIII. Melchiora, a new
genus in Africa. – J. Arnold Arbor. 37: 153-159.
Kobuski CE. 1957. Studies in the Theaceae XXIX. Further studies in the genus
Melchiora. – J. Arnold Arbor. 38: 199-205.
Kobuski CE. 1961. Studies in the Theaceae XXXII. A review of the genus
Ternstroemia in the Philippine Islands. – J. Arnold Arbor. 42:
263-275.
Kobuski CE. 1963a. Studies in the Theaceae XXXIV. Some Asiatic taxa of
Ternstroemia. – J. Arnold Arbor. 44: 421-434.
Kobuski CE. 1963. Studies in the Theaceae XXXV. Two new species of
Ternstroemia from the Lesser Antilles. – J. Arnold Arbor. 44:
434-435.
Kodela PG. 2006. Lysimachia (Myrsinaceae) in New South Wales. –
Telopea 11: 147-154.
Kolbe K-P, John J. 1980. Serology and
systematics of the Ebenales and the Theales. – Biochem. Syst. Ecol. 8:
249-256.
Kollmann F, Feinbrun N. 1968. A
cyto-taxonomic study in Palestinian Anagallis arvensis L. – Notes
Roy Bot. Gard. Edinb. 28: 173-186.
Kondo K. 1977a. Chromosome numbers in the
genus Camellia. – Biotropica 9: 86-94.
Kondo K. 1977b. Cytological studies and
cultivated species of Camellia. – Japan. J. Breeding 27: 28-38.
Kong H-Z, Liu J-Q. 1999. Karyomorphology of
the genus Pomatosace Maxim. (Primulaceae). – Acta Phytotaxon. Sin.
37: 445-450.
Koske RE, Gemma JN, Englander L. 1990.
Vesicular-arbuscular mycorrhizae in Hawaiian Ericales. – Amer. J. Bot. 77: 64-68.
Kowal RR. 1989. Chromosome numbers of
Asteranthos and the putatively related Lecythidaceae. – Brittonia 41:
131-135.
Kowal RR, Mori SA, Kallunki JA. 1977.
Chromosome numbers of Panamanian Lecythidaceae and their use in
subfamilial classification. – Brittonia 29: 399-410.
Kraemer M. 2001. On the pollination of
Bejaria racemosa Mutis ex Linné f. (Ericaceae), an ornithophilous Andean
páramo shrub. – Flora 196: 59-62.
Kreß A. 1963a. Zytotaxonomische
Untersuchungen an den Primeln der Sektion Auricula Pax. – Österr.
Bot. Zeitschr. 110: 53-102.
Kreß A. 1963b. Zytotaxonomische
Untersuchungen an Primulaceen. – Phyton 10: 225-236.
Kreß A. 1969. Zytotaxonomische
Untersuchungen an Primulaceen. – Phyton 13: 211-225.
Kreß A. 1970. Zytotaxonomische
Untersuchungen an einigen Insektenfängern (Droseraceae, Byblidaceae, Cephalotaceae,
Roridulaceae, Sarraceniaceae). – Ber. Deutsch.
Bot. Ges. 83: 55-62.
Kreß A. 1981. Primulaceen-Studien 2.
Primula section Auricula subsection Erythrodrosum:
neue Unterarten und Hybriden. – Grobenzell bei München.
Kretzer AM, Bidartondo MI, Grubisha LC,
Spatafora JW, Szaro TM, Bruns TD. 2000. Regional specialization of Sarcodes
sanguinea on a single fungal symbiont from the Rhizopogon ellenae
(Rhizopogonaceae) complex. – Amer. J. Bot. 87: 1778-1782.
Křisa B. 1965. Beitrag zur Gliederung der
Gattung Pyrola L. in holoarktischen Gebieten. – Novit. Bot. Inst.
Bot. Univ. Carol. Pragensis 1965: 31-35.
Křisa B. 1966. Contribution to the taxonomy
of the genus Pyrola L. in North America. – Bot. Jahrb. Syst. 85:
612-637.
Křisa B. 1967. Two new species of
Pyrola from the Himalayan region of Asia. – Bot. Not. 120:
423-432.
Křísa B. 1971. Beiträge zur Taxonomie und
Chorologie der Gattung Pyrola L. – Bot. Jahrb. Syst. 90: 476-508.
Kron KA. 1996. Phylogenetic relationships of
Empetraceae, Epacridaceae, Ericaceae,
Monotropaceae, and Pyrolaceae: evidence from nuclear ribosomal 18S sequence
data. – Ann. Bot., N. S., 77: 293-303.
Kron KA. 1997. Phylogenetic relationships of
Rhododendroideae (Ericaceae). – Amer.
J. Bot. 84: 973-980.
Kron KA. 2003. Phylogenetic relationships and
major clades of Rhododendron (Rhodoreae, Ericoideae, Ericaceae). – In: Argent G, McFarlane M
(eds), Rhododendrons in horticulture and science, Royal Botanic Gardens,
Edinburgh, pp. 79-85.
Kron KA, Chase MW. 1993. Systematics of the
Ericaceae, Empetraceae, Epacridaceae
and related taxa based upon rbcL sequence data. – Ann. Missouri Bot.
Gard. 80: 735-741.
Kron KA, Judd WS. 1990. Phylogenetic
relationships within the Rhodoreae (Ericaceae) with specific comments on the
placement of Ledum. – Syst. Bot. 15: 57-68.
Kron KA, Judd WS. 1997. Systematics of the
Lyonia group (Andromedeae, Ericaceae) and the use of species as
terminals in higher-level cladistic analyses. – Syst. Bot. 22: 479-492.
Kron KA, King JM. 1996. Cladistic
relationships of Kalmia, Leiophyllum, and
Loiseleuria (Phyllodoceae, Ericaceae) based on rbcL and
nrITS data. – Syst. Bot. 21: 17-29.
Kron KA, Luteyn JL. 2005. Origin and
biogeographic patterns in Ericaceae:
new insights from recent phylogenetic analyses. – Biol. Skr. 55: 479-500.
[Friis I, Balslev H (eds), Proceedings of a symposium on plant diversity and
complexity patterns – Local, regional and global dimensions, Danish Academy
of Sciences and Letters, Copenhagen, pp. 479-500]
Kron KA, Fuller R, Crayn DM, Gadek PA, Quinn
CJ. 1999. Phylogenetic relationships of epacrids and vaccinioids (Ericaceae s. l.) based on matK
sequence data. – Plant Syst. Evol. 218: 55-65.
Kron KA, Judd WS, Crayn DM. 1999.
Phylogenetic analyses of Andromedeae (Ericaceae subfam. Vaccinioideae). –
Amer. J. Bot. 86: 1290-1300.
Kron KA, Powell EA., Luteyn JL. 2002.
Phylogenetic relationships within the blueberry tribe (Vaccinieae, Ericaceae) based on sequence data from
matK and nuclear ribosomal ITS regions, with comments on the placement
of Satyria. – Amer. J. Bot. 89: 327-336.
Kron KA, Judd WS, Stevens PF, Crayn DM,
Anderberg AA, Gadek PA, Quinn CJ, Luteyn JL. 2002. Phylogenetic classification
of Ericaceae: molecular and
morphological evidence. – Bot. Rev. 68: 335-423.
Kron KA, Judd WS, Anderberg AA. 2008.
Validation of Kalmia buxifolia (Bergius) Gift & Kron and
Kalmia procumbens (L.) Gift & Kron. – Nord. J. Bot. 26:
47-48.
Kubitzki K. 2004a. Cyrillaceae. – In: Kubitzki K (ed),
The families and genera of vascular plants VI. Flowering plants. Dicotyledons.
Celastrales,
Oxalidales, Rosales, Cornales, Ericales, Springer, Berlin, Heidelberg, New
York, pp. 114-116.
Kubitzki K. 2004b. Fouquieriaceae. – In: Kubitzki K
(ed), The families and genera of vascular plants VI. Flowering plants.
Dicotyledons. Celastrales,
Oxalidales, Rosales, Cornales, Ericales, Springer, Berlin, Heidelberg, New
York, pp. 195-198.
Kubitzki K. 2004c. Sarraceniaceae. – In: Kubitzki K
(ed), The families and genera of vascular plants VI. Flowering plants.
Dicotyledons. Celastrales,
Oxalidales, Rosales, Cornales, Ericales, Springer, Berlin, Heidelberg, New
York, pp. 422-425.
Kubitzki K. 2004d. Pellicieraceae. – In:
Kubitzki K (ed), The families and genera of vascular plants VI. Flowering
plants. Dicotyledons. Celastrales,
Oxalidales, Rosales, Cornales, Ericales, Springer, Berlin, Heidelberg, New
York, pp. 297-299.
Kubitzki K. 2004e. Tetrameristaceae. – In: Kubitzki
K (ed), The families and genera of vascular plants VI. Flowering plants.
Dicotyledons. Celastrales,
Oxalidales, Rosales, Cornales, Ericales, Springer, Berlin, Heidelberg, New
York, pp. 461-462.
Kubota M, McGonigle TP, Hyakumachi M. 2001.
Clethra barbinervis, a member of the order Ericales, forms arbuscular mycorrhizae. –
Can. J. Bot. 79: 300-306.
Kühdorf K, Muenzenberger B, Begerow D,
Karasch-Wittmann C, Gomez-Laurito J, Huettl RF. 2014. Sebacina sp. is
a mycorrhizal partner of Comarostaphylis arbutoides (Ericaceae). – Mycol. Progr. 13:
733-744.
Kühdorf K, Muenzenberger B, Begerow D,
Gomez-Laurito J, Huettl RF. 2015. Leotia cf. lubrica forms
arbutoid mycorrhiza with Comarostaphylis arbutoides (Ericaceae). – Mycorrhiza 25: 109-120.
Kukachka BF. 1978-1981. Wood anatomy of the
Neotropical Sapotaceae I-XXIII. –
U.S.D.A. Forest Service Res. Pap. Forest Products Laboratory, Madison, pp.
325-331 (1978), 349-354 (1979), 358-363 (1980).
Kümpers BMC, Richardson JE, Anderberg AA,
Wilkie P, Ronse de Craene LP. 2016. The significance of meristic changes in the
flowers of Sapotaceae. – Bot. J.
Linn. Soc. 180: 161-192.
Kupicha FK. 1978. Notes on East African Sapotaceae. – Candollea 33: 29-41.
Kupicha FK. 1983a. 104. Primulaceae. – In: Launert E (ed),
Flora Zambesiaca 7 (Part 1), Flora Zambesiaca Managing Committee, London, pp.
184-197.
Kupicha FK. 1983b. 105. Myrsinaceae. – In: Launert E (ed),
Flora Zambesiaca 7 (Part 1), Flora Zambesiaca Managing Committee, London, pp.
198-210.
Kupicha FK. 1983c. 106. Sapotaceae. – In: Launert E (ed), Flora
Zambesiaca 7 (Part 1), Flora Zambesiaca Managing Committee, London, pp.
210-247.
Kurashige Y, Etoh J-I, Handa T, Takayanagi K,
Yukawa T. 2001. Sectional relationships in the genus Rhododendron
(ERicaceae): evidence from matK and trnK intron sequences.
– Plant Syst. Evol. 228: 1-14.
Kurtto A. 1996. Impatiens
glandulifera (Balsaminaceae)
as an ornamental and escape in Finland, with notes on the other Nordic
countries. – Symb. Bot. Upsal. 31: 221-228.
Kvaček Z, Walther H. 1984a. Nachweis
tertiärer Theaceen Mitteleuropas nach blatt-epidermalen Untersuchungen. I.
Teil – Epidermale Merkmalskomplexe rezenter Theaceae. – Feddes Repert. 95:
209-227.
Kvaček Z, Walther H. 1984b. Nachweis
tertiärer Theaceen Mitteleuropas nach blatt-epidermalen Untersuchungen. II.
Teil – Bestimmung fossiler Theaceen-Sippen. – Feddes Repert. 95:
331-346.
Kwiatkowska D. 1992. The relationships
between the primary vascular system and phyllotactic patterns of Anagallis
arvensis (Primulaceae). –
Amer. J. Bot. 79: 904-913.
Lam HJ. 1925. The Sapotaceae, Sarcospermaceae, and
Boerlagellaceae of the Dutch East Indies and surrounding countries (Malay
Peninsula and Philippine Islands). – Bull. Jard. Bot. Buitenzorg, sér. III,
7: 1-289.
Lam HJ. 1927. Further studies on Malayan Sapotaceae 1. – Bull. Jard. Bot.
Buitenzorg, sér. III, 8: 381-493.
Lam HJ. 1938. Monograph of the genus
Nesoluma (Sapotaceae). –
Occas. Pap. Bernice Pauahi Bishop Mus. 14: 127-165.
Lam HJ. 1939. On the system of the Sapotaceae, with some remarks on
taxonomical methods. – Rec. Trav. Bot. Néerl. 36: 509-525.
Lam HJ. 1941. Note on the Sapotaceae-Mimusopoideae in general and
on the far-eastern Manilkara allies in particular. – Blumea 4:
323-358.
Lam HJ, Royen P van. 1952. Concise revision
of the Sarcospermataceae. – Blumea 7: 148-153.
Lam HJ, Varossieau WW. 1938. Revision of the
Sarcospermataceae. – Blumea 3: 183-200.
Langlet O. 1936. Några bidrag till
kännedomen om kromosomtalen inom Nymphaeaceae, Ranunculaceae, Polemoniaceae, och Compositae. –
Svensk Bot. Tidskr. 30: 288-294.
Largent DL, Sugihara N, Wishner C. 1980.
Occurrence of mycorrhizae on ericaceous and pyrolaceous plants in northern
California. – Can. J. Bot. 58: 2274-2279.
Larsen K. 1981. Chromosome numbers in
Impatiens from Thailand. – Nord. J. Bot. 1: 43-44.
Larsen K, Hu C-M. 1991. New taxa of Myrsinaceae from Thailand. – Nord. J.
Bot. 11: 61-78.
Larsen K, Hu C-M. 1992. Additional new taxa
of Ardisia (Myrsinaceae)
from Thailand. – Nord. J. Bot. 12: 311-313.
Larsen K, Hu C-M. 1995. Reduction of
Tetrardisia to Ardisia. – Nord. J. Bot. 15: 161-162.
Launert E. 1963. 39. Balsaminaceae. – In: Exell AW,
Fernandes A, Wild H (eds), Flora Zambesiaca 2 (Part 1), Crown Agents for
Oversea Governments and Administrations, London, pp. 162-180.
Lavier-George L. 1936. Recherches sur les
epidermis foliaires des Philippia de Madagascar, utilisation de leurs
caractères comme bases d’une classification. – Bull. Mus. Natl. Hist. Nat.
Paris 8: 173-199.
Lechner S. 1914. Anatomische Untersuchungen
über die Gattungen Actinidia, Saurauia, Clethra und
Clematoclethra mit besonderer Berücksichtigung ihrer Stellung im
System. – Beih. Bot. Zentralbl. 32: 431-467.
Lecocq O. 2000. Een bloemontogenetische
studie van de Ericaceae sensu APG. –
Thesis, Katholieke Universiteit Leuven, Leuven, Belgium.
Lee ST. 1987. A palynotaxonomic study on the
Korean Theaceae. – Korean J. Bot. 30:
215-223.
Leins P. 1964. Entwicklungsgeschichtliche
Studien an Ericales-Blüten. – Bot.
Jahrb. Syst. 83: 57-88.
Leins P. 1972. Das zentrifugale Androeceum
von Couroupita guianensis (Lecythidaceae). – Beitr. Biol.
Pflanzen 48: 313-319.
Leins P, Winhard W. 1973.
Entwicklungsgeschichtliche Studien an Loasaceenblüten. – Österr. Bot.
Zeitschr. 122: 145-165.
Leiser AT. 1968. A mucilaginous root sheath
in Ericaceae. – Amer. J. Bot. 55:
391-398.
Lems K. 1962a. Evolutionary studies in the Ericaceae I. Shoot development in the
Andromedeae. – Ecology 43: 524-528.
Lems K. 1962b. Evolutionary studies in the Ericaceae II. Leaf anatomy as a
phylogenetic index in the Andromedeae. – Bot. Gaz. 125: 178-186.
Lems K. 1964. Evolutionary studies in the Ericaceae II. Leaf anatomy as a
phylogenetic index in the Andromedeae. – Bot. Gaz. 125: 178-186.
Lemson KL. 1996. Current problems in the
taxonomy of Andersonia R. Br. – Ann. Bot., II, 77: 323-326.
Lemson KL. 2001. The phylogeny and taxonomy
of Andersonia R. Br. (Ericaceae/Epacridaceae). – Ph.D. diss.,
Department of Botany, University of Western Australia.
Lemson KL. 2011. Pollen development in
Cosmelieae (Ericaceae: Styphelioideae):
are monads in Andersonia macranthera unique? – Intern. J. Plant Sci.
172: 664-673.
Leins P. 1964. Entwicklungsgeschichtliche
Studien an Ericales-Blüten. – Bot.
Jahrb. Syst. 83: 57-88.
Lens F. 2005. Systematic significance of wood
anatomical characters in Ericales. –
Doctor in de Wetenschappen, K. U. Leuwen, Laboratory of Plant Systematics.
Lens F, Gasson P, Smets E, Jansen S. 2003.
Comparative wood anatomy of epacrids (Styphelioideae, Ericaceae s.l.). – Ann. Bot., II, 91:
835-856.
Lens F, Luteyn JL, Smets E, Jansen S. 2004.
Ecological trends in the wood anatomy of Vaccinioideae (Ericaceae s. l.). – Flora 199:
309-319.
Lens F, Smets E, Jansen S. 2004. Comparative
wood anatomy of Andromedeae s. s., Gaultherieae, Lyonieae and Oxydendreae
(Vaccinioideae, Ericaceae s. l.). –
Bot. J. Linn. Soc. 144: 161-179.
Lens F, Kron KA, Luteyn JL, Smets E, Jansen
S. 2004. Comparative wood anatomy of the blueberry tribe (Vaccinieae, Ericaceae s. l.). – Ann. Missouri Bot.
Gard. 91: 566-592.
Lens F, Jansen S, Caris P, Serlet L, Smets E.
2005. Comparative anatomy of the primuloid clade (Ericales s.l.). – Syst. Bot. 30:
163-183.
Lens F, Dressler S, Jansen S, Evelghem L van,
Smets E. 2005. Relationships with balsaminoid Ericales: a wood anatomical approach. –
Amer. J. Bot. 92: 941-953.
Lens F, Dressler S, Vinckier S, Janssens S,
Dessein S, Evelghem L van, Smets E. 2005. Palynological variation in
balsaminoid Ericales I. Marcgraviaceae. – Ann. Bot. 96:
1047-1060.
Lens F, Baas P, Jansen S, Smets E. 2007. A
search for phylogenetically informative wood characters within Lecythidaceae s. l. – Amer. J. Bot.
94: 483-502.
Lens F, Schönenberger J, Baas P, Jansen S,
Smets E. 2007. The role of wood anatomy in phylogeny reconstruction of Ericales. – Cladistics 23: 229-254.
Lens F, Baas P, Jansen S, Smets E. 2007. A
search for phylogenetically informative wood characters within Lecythidaceae s. l. – Amer. J. Bot.
94: 483-502.
Lens F, Eeckhout S, Zwartjes R, Smets E,
Janssens SB. 2012. The multiple fuzzy origins of woodiness within Balsaminaceae using an integrated
approach. Where do we draw the line? – Ann. Bot. 109: 783-799.
Lepper L. 1975. Nachtrag zur Cytologie von
Cyclamen L. – Wiss. Zeitschr. Friedrich-Schiller-Univ. Jena,
Math.-Naturwiss. Reihe 24: 429-436.
Lepper L. 1983a. Die Familie Theophrastaceae in Cuba. Überblick
und floristiche Mitteilungen. – Wiss. Zeitschr. Friedrich-Schiller-Univ.
Jena, Math.-Naturwiss. R. 32: 865-874.
Lepper L. 1983b. Theophrastaceae cubanae novae II.
Jacquinia lippoldii Lepper spec. nov. – Wiss. Zeitschr.
Friedrich-Schiller-Univ. Jena, Math.-Naturwiss. R. 32: 875-880.
Lepper L. 1985a. Theophrastaceae cubanae novae III.
Jacquinia bissei Lepper sp. nov. – Feddes Repert. 96: 517-521.
Lepper L. 1985b. Die Bedeutung einiger
morphologischer und anatomischer Merkmalskomplexe für die Gliederung der
Gattung Jacquinia L. (Theophrastaceae) in Cuba. – Feddes
Repert. 96: 677-678.
Lepper L. 1989. Infloreszenzstruktur,
Blütenbau und Blütenökologie von Deherainia smaragdina Decne. (Theophrastaceae). – Wiss.
Zeitschr. Friedrich-Schiller-Univ. Jena, Naturwiss. R. 38: 337-345.
Lersten NR. 1977. Trichome forms in
Ardisia (Myrsinaceae) in
relation to the bacterial leaf nodule symbiosis. – Bot. J. Linn. Soc. 75:
229-244.
Lersten NR, Carvey KA. 1974. Leaf anatomy of
ocotillo (Fouquieria splendens; Fouquieriaceae) especially
vein-endings and associated veinlet elements. – Can. J. Bot. 52:
2017-2021.
Lersten NR, Horner HT. 1976. Bacterial leaf
nodule symbiosis in angiosperms with emphasis on Rubiaceae and Myrsinaceae. – Bot. Rev. 42:
145-214.
Letouzey R. 1960. Notes sur les
Scytopétalacées: révision des Scytopétalacées de l’herbier de Paris. –
Adansonia, sér. II, 1: 106-142.
Letouzey R. 1977. Nouvelle espèces de
Rhaptopetalum Oliv. (Scytopétalacées) du Cameroun et du Gabon. –
Adansonia, sér. II, 17: 129-138.
Letouzey R. 1978a. Scytopétalacées. – In:
Aubreville A, Leroy J-F (eds), Flore du Cameroun 20, Muséum National
d’Histoire Naturelle, Paris, pp. 139-193.
Letouzey R. 1978b. Scytopétalacées. – In:
Aubreville A, Leroy J-F (eds), Flore du Gabon 24, Muséum National d’Histoire
Naturelle, Paris, pp. 139-193.
Levin DA. 1966. The Phlox pilosa
complex: crossing and chromosome relationships. – Brittonia 18: 142-162.
Levin DA, Smith DM. 1965. An enigmatic
Phlox from Illinois. – Brittonia 17:254-266.
Li H-L. 1943. On the Sino-Himalayan species
of Shortia and Berneuxia. – Rhodora 45: 333-337.
Li H-L. 1952. A taxonomic review of the genus
Actinidia. – J. Arnold Arbor. 23: 1-61.
Li J, Alexander III J, Ward T, Del Tredici P,
Nicolson R. 2002. Phylogenetic relationships of Empetraceae inferred from
sequences of chloroplast gene matK and nuclear ribosomal DNA ITS
region. – Mol. Phylogen. Evol. 25: 306-215.
Li J, Tredici P del, Yang S, Donoghue MJ.
2002. Phylogenetic relationships and biogeography of Stewartia
(Camellioideae, Theaceae) inferred from
nuclear ribosomal DNS ITS sequences. – Rhodora 104: 117-133.
Li J, Huang H, Sant T. 2002. Molecular
phylogeny and infrageneric classification of Actinidia (Actinidiaceae). – Syst. Bot. 27:
408-415.
Li J-W, Rui G, Liang M-Y, Pang C. 1989.
Studies on the pollen morphology of the Actinidia. – Guihaia 9:
335-339.
Li L. 2001. Chromosome number of Sladenia
celastrifolia. – Acta Bot. Yunnan. 23: 223-224.
Li L, Liang H-X, Peng H, Lei L-G. 2003.
Sporogenesis and gametogenesis in Sladenia and their systematic
implication. – Bot. J. Linn. Soc. 143: 305-314.
Li L, Liang H-X, Peng H. 2003. Karyotype of
Sladenia and its systematic insights. – Acta Bot. Yunnan. 25:
321-326. [In Chinese with English summary]
Li M-M, Li J-H, Del Tredici P, Corajod J, Fu
C-X. 2013. Phylogenetics and biogeography of Theaceae based on sequences of plastid
genes. – J. Syst. Evol. 51: 396-404.
Li R, Yang J-B, Yang S-X, Li D-Z. 2011
[2012]. Phylogeny and taxonomy of the Pyrenaria complex (Theaceae) based on nuclear ribosomal ITS
sequences. – Nord. J. Bot. 29: 780-787.
Li Y-H, Yu C-H. 1985. Pollen morphology of Styracaceae and its taxonomic
significance. – Acta Phytotaxon. Sin. 23: 81-90. [In Chinese with English
summary]
Liang C-F. 1983. On the distribution of
actinidias. – Guihaia 3: 229-248.
Liang C-F, Ferguson AR. 1986. The botanical
nomenclature of the kiwifruit and related taxa. – New Zealand J. Bot. 24:
183-184.
Liang D, Baas P. 1990. Wood anatomy of trees
and shrubs from China II. Theaceae. –
IAWA Bull., N. S., 11: 337-378.
Liang D, Baas P. 1991. The wood anatomy of
the Theaceae. – I.A.W.A. Bull., N. S.,
12: 333-353.
Liben L. 1971. Révision du genre africain
Napoleonaea P. Beauv. (Lecythidaceae). – Bull. Jard. Bot.
Natl. Belg. 41: 363-382.
Liben L. 1989. La véritable identité des
genres et espèces confondus sous le nom de Bequaertiodendron
magalismontanum (Sond.) Heine & Hemsley (Sapotaceae) en Afrique centrale et
occidentale. – Bull. Jard. Bot. Natl. Belg. 59: 151-169.
Lidén M. 2000. Notes on Dionysia,
Corydalis and Fumaria in Iran. – Iranian J. Bot. 8:
303-308.
Lidén M. 2007. The genus Dionysia
(Primulaceae), a synopsis and five
new species. – Willdenowia 37: 37-61.
Lignier O. 1890. Recherches sur l’anatomie
des organes végétatifs des Lécythidées, des Napoléonées et des
Barringtoniées (Lecythidacées). – Bull. Sci. France Belgique 21:
291-420.
Liu Y-J, Liu J, Hu C-M, Hao G. 2015.
Non-monophyly of Primula subgenera Auganthus and
Carolinella (Primulaceae) as
confirmed by the nuclear DNA sequence variation. – Plant Syst. Evol. 301:
2057-2071.
Liu Z-W, Zhou J, Liu E-D, Peng H. 2010. A
molecular phylogeny and a new classification of Pyrola (Pyroleae, Ericaceae). – Taxon 59: 1690-1700.
Liu Z-W, Wang Z-H, Zhou J, Peng H. 2011.
Phylogeny of Pyroleae (Ericaceae):
implications for character evolution. – J. Plant Res. 124: 325-337.
Liu Z-W, Jolles DD, Zhou J, Peng H, Milne RI.
2014. Multiple origins of circumboreal taxa in Pyrola (Ericaceae), a group with a Tertiary relict
distribution. – Ann. Bot. 114: 1701-1709.
Lloyd FM. 1934. Is Roridula a
carnivorous plant? – Can. J. Res. 10: 780-786.
Lloyd FM. 1942. The carnivorous plants.
2nd ed. – Chronica Botanica Co., Waltham, Massachusetts.
Loesener T. 1896. Beiträge zr Kenntnis der
Matéepflanzen. – Ber. Deutsch. Pharm. Ge. 6: 203.
Löfstrand SD, Schönenberger J. 2015a.
Molecular phylogenetics and floral evolution in the sarracenioid clade (Actinidiaceae, Roridulaceae and Sarraceniaceae) of Ericales. – Taxon 64: 1209-1224.
Löfstrand SD, Schönenberger J. 2015b.
Comparative floral structure and systematics in the sarracenioid clade (Actinidiaceae, Roridulaceae and Sarraceniaceae) of Ericales. – Bot. J. Linn. Soc. 178:
1-46.
Löfstrand SD, Balthazar M von,
Schönenberger J. 2016. Early floral development and androecium organization in
the sarracenioid clade (Actinidiaceae, Roridulaceae and Sarraceniaceae) of Ericales. – Bot. J. Linn. Soc. 180:
295-318.
Lott TA, Manchester SR, Dilcher DL. 1998. A
unique and complete polemoniaceous plant from the middle Eocene of Utah, USA.
– Rev. Palaeobot. Palynol. 104: 39-49.
Lourteig A. 1942. Primulaceae Argentinae. – Lilloa 8:
231-267.
Lu H-F, Jiang B, Shen Z-G, Shen J-B, Peng
Q-F, Cheng C-G. 2008. Comparative leaf anatomy, FTIR discrimination and
biogeographical analysis of Camellia section Tuberculata (Theaceae) with a discussion of its
taxonomic treatments. – Plant Syst. Evol. 274: 223-235.
Lu H-F, Shen J-B, Lin X-Y, Fu J-L. 2008.
Relevance of Fourier transform infrared spectroscopy and leaf anatomy for
species classification in Camellia (Theaceae). – Taxon 57: 1274-1288.
Lu L, Fritsch PW, Wang H, Li HT, Li DZ, Chen
JQ. 2009. Pollen morphology of Gaultheria L. and related genera of
subfamily Vaccinioideae: taxonomic and evolutionary significance. – Rev.
Palaeobot. Palynol. 154: 106-123.
Lu L, Fritsch PW, Bush CM, Dong L-N, Li H-T,
Li D-Z. 2010. Systematic implications of seed coat diversity in Gaultherieae
(Ericaceae). – Bot. J. Linn. Soc.
162: 477-495.
Lu L, Fritsch PW, Cruz BC, Wang H, Li D-Z.
2010. Reticulate evolution, cryptic species, and character convergence in the
core East Asian clade of Gaultheria (Ericaceae). – Mol. Phylogen. Evol. 57:
364-379.
Lu X, Chen L, Chen Y-P, Peng H. 2014.
Molecular phylogeography and conservation genetics of Sladenia
celastrifolia inferred from chloroplast DNA sequence variation. – J.
Syst. Evol. 52: 458-465.
Lu Y-Q. 1991. Pollen morphology of
Impatiens L. (Balsaminaceae) and its taxonomic
implications. – Acta Phytotaxon. Sin. 29: 352-357.
Lu Y-Q, Chen Y-L. 1991. Seed morphology of
Impatiens L. (Balsaminaceae) and its taxonomic
significance. – Acta Phytotaxon. Sin. 29: 252-257.
Luna BN de, Freitas M de F, Baas P, De Toni
KLG, Barros CF. 2017. Leaf anatomy of five Neotropical genera of Primulaceae. – Intern. J. Plant Sci.
178: 362-377.
Luna BN de, Freitas M de F, Barros CF. 2018.
Systematic and phylogenetic implications of the wood anatomy of six Neotropical
genera of Primulaceae. – Plant
Syst. Evol. 304: 775-791.
Luna I. 1997. Relaciones filogenéticas de
los géneros de la familia Theaceae D.
Don. – Ph.D. thesis. Facultad de Ciencias, UNAM, México.
Luna I, Contreras-Medina R. 2000.
Distribution of the genera of Theaceae
(Angiospermae: Theales): a panbiogeographic analysis. – Biogeographica 76:
79-88.
Luna I, Ochoterena H. 2004. Phylogenetic
relationships of the genera of Theaceae
based on morphology. – Cladistics 20: 223-270.
Luna I, Villaseñor JL. 1996 Géneros de Theaceae: aspectos taxonómicos y
nomenclaturales. – Bol. Soc. Bot. México 59: 81-95.
Lundell CL. 1963. Studies of American Myrsinaceae I. – Wrightia 3: 77-90.
Lundell CL. 1964. Studies of the American Myrsinaceae II. – Wrightia 3:
97-114.
Lundell CL. 1966. The genus
Parathesis of the Myrsinaceae. – Contr. Tex. Res. Found.
Bot. Stud. 5: 1-206.
Lundell CL. 1981a. Neotropical Myrsinaceae IV. – Phytologia 48:
137-142.
Lundell CL. 1981b. Neotropical Myrsinaceae VI. – Phytologia 49:
341-354.
Lundell CL. 1982. Neotropical Myrsinaceae VII. – Wrightia 7:
38-50.
Lundell CL. 1983. Neotropical Myrsinaceae VIII. – Wrightia 7:
245-250, 255-265.
Luteyn JL. 1976. A revision of the
Mexican-Central American species of Cavendishia (Vacciniaceae). –
Mem. New York Bot. Gard. 28: 1-138.
Luteyn JL. 1977. Notes on Neotropical
Vaccinieae (Ericaceae) IV. A review of
the genus Oreanthes. – Brittonia 29: 173-176.
Luteyn JL. 1983. Cavendishia. –
In: Luteyn JL (ed), Flora Neotropica. Monograph 35. Ericaceae I – in inferior-ovaried
genera, New York Botanical Garden, Bronx, New York, pp. 1-290.
Luteyn JL. 1984. Revision of
Semiramisia (Ericaceae:
Vaccinieae). – Syst. Bot. 9: 359-367.
Luteyn JL. 1987. Orthaea (Ericaceae-Vaccinieae): new species and
redefinition of the genus. – Nord. J. Bot. 7: 31-37.
Luteyn JL. 1989. Speciation and diversity of
Ericaceae in neotropical montane
vegetation. – In: Holm-Nielsen LB, Nielsen IC, Balslev H (eds), Tropical
forests: botanical dynamics, speciation and diversity, Academic Press, London,
pp. 297-310.
Luteyn JL. 1990. Gonocalyx
amplexicaulis (Ericaceae): a new
Panamanian blueberry. – Syst. Bot. 15: 745-747.
Luteyn JL. 1991a. A synopsis of the genus
Didonica (Ericaceae:
Vaccinieae) with two new species. – Syst. Bot. 16: 587-597.
Luteyn JL. 1991b. Key to the subfamilies and
genera of neotropical Ericaceae. –
Nord. J. Bot. 11: 623-627.
Luteyn JL. 1995a. 16. Tepuia. –
In: Luteyn JL (ed), Flora Neotropica. Monograph 66. Ericaceae II – the superior-ovaried
genera, New York Botanical Garden, Bronx, New York, pp. 351-364.
Luteyn JL. 1995b. 17. Pernettya. –
In: Luteyn JL (ed), Flora Neotropica. Monograph 66. Ericaceae II – the superior-ovaried
genera, New York Botanical Garden, Bronx, New York, pp. 365-383.
Luteyn JL. 1995c. 18. Gaultheria.
– In: Luteyn JL (ed), Flora Neotropica. Monograph 66. Ericaceae II – the superior-ovaried
genera, New York Botanical Garden, Bronx, New York, pp. 384-488.
Luteyn JL (ed). 1995. Flora Neotropica.
Monograph 66. Ericaceae II. The
superior-ovaried genera. – New York Botanical Garden, New York.
Luteyn JL. 1996. Ericaceae. – In: Harling G, Andersson L
(eds), Flora of Ecuador 54, Nord. J. Bot., Copenhagen, pp. 1-402.
Luteyn JL. 1996 [1997]. Redefinition of the
neotropical genus Anthopterus (Ericaceae: Vaccinieae). – Brittonia 48:
605-614.
Luteyn JL. 2001. Two new species and two new
combinations in Mesoamerican Ericaceae.
– Brittonia 53: 437-446.
Luteyn JL. 2002a. Diversity, adaptation and
endemism in neotropical Ericaceae:
biogeographical patterns in the Vaccinieae. – Bot. Rev. 68: 55-87.
Luteyn JL. 2002b. Key to the subfamilies and
genera of Neotropical Ericaceae. –
Nord. J. Bot. 11: 623-627.
Luteyn JL, Pedraza-Peñalosa P. 2013.
Nomenclature, taxonomy, and conservation of the neotropical genus
Sphyrospermum (Ericaceae:
Vaccinieae), including five new species for Colombia, Ecuador, and Peru. –
Phytotaxa 79: 1-29.
Luteyn JL, Sylva S DS. 1999. “Murrí”
(Antioquia Department, Colombia): hotspot for neotropical blueberries (Ericaceae: Vaccinieae). – Brittonia 51:
280-302.
Luteyn JL, Wilbur RL. 1977. New genera and
species of Ericaceae (Vaccinieae) from
Costa Rica and Panama. – Brittonia 29: 255-276.
Luteyn JL, Harborne JB, Williams CA. 1980.
Survey of the flavonoids and simple phenols in the leaves of
Cavendishia (Ericaceae). –
Brittonia 32: 1-16.
Lys J. 1955. Sur la nature et l’évolution
biochimique des glucides des Lysimaques. – Compt. Rend. Hebd. Séances Acad.
Sci. 241: 1842-1844.
Lys J. 1956. Les glucides de quelques
Primulacées. – Rev. Gén. Bot. 63: 95-100.
Ma OSW, Saunders RMK. 2003. Comparative
floral ontogeny of Maesa (Maesaceae), Aegiceras (Myrsinaceae) and Embelia (Myrsinaceae): taxonomic and phylogenetic
implications. – Plant Syst. Evol. 243: 39-58.
Mabberley DJ. 2002. A note on the
Macaronesian Sideroxylon (Sapotaceae). – Taxon 51: 179-180.
Machado IC, Lopes AV. 2000. Souroubea
guianensis Aubl.: quest for its legitimate pollinator and the first record
of tapetal oil in the Marcgraviaceae. – Ann. Bot., N. S.,
85: 705-711.
McAbee JM, Kuzoff RK, Gasser CS. 2005.
Mechanisms of derived unitegmy among Impatiens species. – Plant Cell
17: 1674-1684.
MacBride JF. 1944. Vaccinium and
relatives in the Andes of Peru. – Univ. Wyoming Publ. 11: 37-46.
MacBride JF. 1959. Flora of Peru. Ericaceae. – Field Mus. Nat. Hist., Bot.
Ser. 13(5, 1): 50-149.
McCloskey R. 1948. Blueberries for Sal. –
Viking, New York.
McConchie CA, Hough T, Singh MB, Knox RB.
1986. Pollen presentation on petal combs in the geoflorous heath Acrotriche
serrulata (Epacridaceae). – Ann. Bot., II, 57: 155-164.
McDaniel S. 1971. The genus
Sarracenia (Sarraceniaceae). – Bull. Tall
Timbers Res. Station 9: 1-36.
McDowall MA. 1970. Anionic proteinase from
Actinidia chinensis. Preparation and properties of the crystalline
enzyme. – Eur. J. Biochem. 14: 214-221.
MacDougal DT, Lloyd FT. 1900. The roots and
mycorrhizas of some of the Monotropaceae. – Bull. New York Bot. Gard. 1:
419-429.
McEwen M. 1894. The comparative anatomy of
Corema alba and Corema conradii. – Bull. Torrey Bot. Club
21: 277-285.
MacFarlane JM. 1889. Observations on
pitchered insectivorous plants I. Introduction. – Ann. Bot. 3: 253-265.
MacFarlane JM. 1893. Observations on
pitchered insectivorous plants II. Histology of Darlingtonia,
Sarracenia, and Heliamphora with remarks on adaptations for
insect-catching. – Ann. Bot. 7: 403-458.
MacKay M, Smith G, Gardiner SE. 2016.
Analysis of geographic and taxonomic groups informs conservation of
Rhododendron subgenus Vireya (Ericaceae). – Blumea 61: 170-180.
Mackinder B, Harris DJ, Gautier L. 2016. A
reinstatement, recircumscription and revision of the genus Donella (Sapotaceae). – Edinburgh J. Bot. 73:
297-339.
McLean CB. 1995. Mycorrhizae of the
Epacridaceae and its use in propagation. – Combined Proc. Int. Plant
Propagators 45: 108-111.
McLean CB. 1999. Investigation of mycorrhizas
of the Epacridaceae. – Ph.D. diss., Department of Applied Biology and
Biotechnology, RMIT University.
McLennan E. 1935. Non-symbiotic development
of seedlings of Epacris impressa Labill. – New Phytol. 34: 55-63.
McPherson S, Schnell DE. 2011. Sarraceniaceae of North America. –
Redfern Natural History Publ., Poole.
McPherson S, Wistuba A, Fleischmann A, Nerz
J. 2011. Sarraceniaceae of South
America. – Redfern Natural History Publ., Poole.
Maguire B. 1972. Tetrameristaceae. – In: Maguire B
& al. (eds), The botany of the Guayana Highlands IX, Mem. New York Bot.
Gard. 23: 165-192.
Maguire B. 1978. Sarraceniaceae. – In: Maguire B
& al. (eds), The botany of the Guayana Highland X, Mem. New York Bot. Gard.
29: 36-62.
Maguire B, Huang Y-C. 1978. Symplocaceae. – In: Maguire B &
al. (eds), The botany of the Guayana Highlands X, Mem. New York Bot. Gard. 29:
223-230.
Maguire B, Steyermark JA, Luteyn JL. 1978.
The botany of the Guayana Highland, Ericaceae. – Mem. New York Bot. Gard.
29: 139-203.
Maguire B, Zeeuw C de, Huang Y-C, Clare CC
Jr. 1972. Tetrameristaceae. –
In: Maguire B et al. (eds), The botany of Guayana Highland IX, Mem. New York
Bot. Gard. 23: 165-192.
Mai DH. 1971. Über fossile Lauraceae und Theaceae in Mitteleuropa. – Feddes
Repert. 82: 313-341.
Mai DH. 1988. Über antillanische Styracaceae. – Feddes Repert. 99:
173-181.
Majourhat K, Jabbar Y, Araneda L,
Zeinalabedini M, Hafidi A, Martinez-Gomez P. 2007. Karyotype characterization
of Argania spinosa (L.) Skeel (Sapotaceae). – South Afr. J. Bot. 73:
661-663.
Makino T. 1894. Revision of the Japanese
species of Andromeda, Pieris and Enkianthus. –
Bot. Mag. (Tokyo) 8: 212-216.
Makino T. 1909. Mitrastemma
yamamotoi. – Bot. Mag. (Tokyo) 23: 325-327.
Makino T. 1911. Observations on the flora of
Japan: Mitrastemon yamamotoi. Bot. Mag. (Tokyo) 25: 251-257.
Mallavadhani UV, Panda AK, Rao YR. 1998.
Pharmacology and chemotaxonomy of Diospyros. – Phytochemistry 49:
901-951.
Manchester SR, Fritsch PW. 2014. European
fossil fruits of Sphenotheca related to extant Asian species of
Symplocos. – J. Syst. Evol. 52: 68-74.
Mandossian AJ. 1965. Some aspects of the
ecological life history of Sarracenia purpurea. – Ph.D. diss.,
Michigan State University, East Lansing.
Manns U, Anderberg AA. 2005. Molecular
phylogeny of Anagallis (Myrsinaceae) based on ITS,
trnL-F, and ndhF sequence data. – Intern. J. Plant Sci.
166: 1019-1028.
Manns U, Anderberg AA. 2007a. Character
evolution in Anagallis (Myrsinaceae) inferred from morphological
and molecular data. – Syst. Bot. 32: 166-179.
Manns U, Anderberg AA. 2007b. Relationships
of Anagallis foemina and A. arvensis (Myrsinaceae): new insights inferred from
DNA sequence data. – Mol. Phylogen. Evol. 45: 971-980.
Manns U, Anderberg AA. 2009. New combinations
and names in Lysimachia (Myrsinaceae) for species of
Anagallis, Pelletiera, and Trientalis. –
Willdenowia 39: 49-54.
Manshard E. 1936. Embryologische
Untersuchungen an Styrax obassia Sieb. et Zucc. – Planta 25:
364-383.
Marie-Victorin [Frère]. 1948. Le genre
Purdiaea (Cyrillaceae), avec
description de quatre espèces et de trois variétés nouvelles. – Contr.
Inst. Bot. Univ. Montréal 63: 49-62.
Markgraf F. 1955. Über Laubblatt-Homologien
und verwandtschaftliche Zusammenhänge bei Sarraceniales. – Planta 46:
414-446.
Markos SE. 1995. Phylogeny of
Arctostaphylos hookeri (Ericaceae) based on ribosomal DNA sequence
from the ITS region. – M.A. thesis, San Francisco State University, San
Francisco, California.
Markos SE, Parker VT, Hileman LC, Vasey MC.
1999. Phylogeny of the Arctostaphylos hookeri complex (Ericaceae) based on nrDNA data. –
Madroño 45: 187-199.
Marloth R. 1903. Some recent observations on
the biology of Roridula. – Ann. Bot. 17: 151-157.
Marloth R. 1910. Further observations on the
biology of Roridula. – Trans. Roy. Soc. South Afr. 2: 59-61.
Marr KL, Bohm BA. 1997. A taxonomic revision
of the endemic Hawaiian Lysimachia (Primulaceae) including three new
species. – Pacific Sci. 51: 254-287.
Marr KL, Bohm BA. 2000. Allozyme variation in
endemic Hawaiian Lysimachia (Primulaceae). – Syst. Bot. 24:
545-557.
Marsden-Jones EM, Weiss FE. 1938. The
essential differences between Anagallis arvensis Linn. and
Anagallis foemina Mill. – Proc. Linn. Soc. London 150: 146-155.
Martínez-Millán M, Crepet WL, Nixon KC.
2009. Pentapetalum trifasciculandricus gen. et sp. nov., a thealean
fossil flower from the Raritan Formation, New Jersey, USA (Turonian, Late
Cretaceous). – Amer. J. Bot. 96: 933-949.
Martins L, Oberprieler C, Hellwig FH. 2003. A
phylogenetic analysis of Primulaceae
s.l. based on internal transcribed spacer (ITS) DNA sequence data. – Plant
Syst. Evol. 237: 75-85.
Mason HL. 1941. The taxonomic status of
Microsteris Greene. – Madroño 6: 122-127.
Mason HL. 1945. The genus Eriastrum
and the influence of Bentham and Gray upon the problem of generic confusion in
Polemoniaceae. – Madroño 8:
33-59.
Mason HL, Grant AD. 1948. Some problems in
the genus Gilia. – Madroño 9: 201-220.
Massicotte HB, Melville LH, Peterson RL.
2005a. Structural characteristics of root-fungal interactions for five
ericaceous species in eastern Canada. – Can. J. Bot. 83: 1057-1064.
Massicotte HB, Melville LH, Peterson RL.
2005b. Structural features of mycorrhizal associations in two members of the
Monotropoideae, Monotropa uniflora and Pterospora andromedea.
– Mycorrhiza 15: 101-110.
Massicotte HB, Melville LH, Tackab erry LE,
Peterson RL. 2007. Pityopus californicus: structural characteristics
of seed and seedling development in a myco-heterotrophic species. –
Mycorrhiza 17: 647-653.
Massicotte HB, Melville LH, Tackaberry LE,
Peterson RL. 2008. A comparative study of mycorrhizas in several genera of
Pyroleae (Ericaceae) from western
Canada. – Botany 86: 610-622.
Massicotte HB, Melville LH, Peterson RL,
Tackaberry LE, Luoma DL. 2010. Structural characteristics of root-fungus
associations in two mycoheterotrophic species, Allotropa virgata and
Pleuricospora fimbriolata (Monotropoideae), from southwest Oregon,
USA. – Mycorrhiza 20: 391-397.
Mast AR, Reveal JL. 2007. Transfer of
Dodecatheon to Primula (Primulaceae). – Brittonia 59:
79-82.
Mast AR, Kelso S, Richards AJ, Lang DJ,
Feller DMS, Conti E. 2001. Phylogenetic relationships in Primula L.
and related genera (Primulaceae)
based on noncoding chloroplast DNA. – Intern. J. Plant Sci. 162:
1381-1400.
Mast AR, Feller DMS, Kelso S, Conti E. 2004.
Buzz-pollinated Dodecatheon originated within Primula section
Auriculastrum: a seven gene cpDNA phylogeny and its implications for
floral evolution. – Amer. J. Bot. 91: 918-925.
Mast AR, Kelso S, Conti E. 2006. Are any
primroses (Primula) primitively monomorphic? – New Phytol. 171:
605-616.
Masters MT. 1869. On the structure of the
flower in the genus Napoleona. – Bot. J. Linn. Soc. 10: 492-504.
Mathew CJ. 1978. Development of male and
female gametophytes in Camellia susanqua. – Phytomorphology 28:
262-269.
Matsuura H. 1935. A karyological
investigation of Mitrastemon yamamotoi Mk. with special reference to
the so-called ‘diffuse’ stage in meiosis. – J. Fac. Sci. Hokkaido Imp.
Univ., Ser. V, Bot 3: 189-204.
Mattfeld J. 1942. Pentaphylacaceae. – In: Engler A
(†), Harms H, Mattfeld J (eds), Die natürlichen Pflanzenfamilien, 2. Aufl.,
Bd. 20b, W. Engelmann, Leipzig, pp. 13-21.
Matthews JR, Knox EM. 1926. The comparative
morphology of the stamen in the Ericaceae. – Trans. Proc. Bot. Soc.
Edinb. 29: 243-281.
Matthews JR, Taylor G. 1926. The structure
and development of the stamen in Erica hirtiflora. – Trans. Proc.
Bot. Soc. Edinb. 29: 235-242.
Mattick F. 1935. Übersicht der Cyrillaceae. – Notizbl. Bot. Gart.
Berlin-Dahlem 12: 668-677.
Matuda E. 1947. On the genus
Mitrastemon. – Bull. Torrey Bot. Club 74: 133-141.
Mauritzon J. 1936. Embryologische Angaben
über Theophrastaceen. – Ark. f. Bot. 28B(1): 1-4.
Mauritzon J. 1939. Über die Embryologie von
Marcgravia. – Bot. Not. 1939: 249-255.
Meeuse ADJ. 1960. Notes on the Sapotaceae of Southern Africa. –
Bothalia 7: 317-379.
Meijden R van der. 1970. A survey of the
pollen morphology of the Indo-Pacific species of Symplocos (Symplocaceae). – Pollen Spores 12:
513-551.
Meijer W. 1993. Rafflesiaceae.
– In: Kubitzki K, Rohwer JG, Bittrich V (eds), The families and genera of
vascular plants II. Flowering plants. Dicotyledons. Magnoliid, hamamelid and
caryophyllid families, Springer, Berlin, Heidelberg, New York, pp. 557-563.
Meijer W. 1997. Rafflesiaceae.
– In: Kalkman C et al. (eds), Flora Malesiana, I, 13, Flora Malesiana
Foundation, Rijksherbarium/Hortus Botanicus, Leiden, pp. 1-42.
Meijer W, Veldkamp JF. 1993. A revision of
Mitrastema (Rafflesiaceae).
– Blumea 38: 221-229.
Melchior H. 1924. Über das Vorkommen von
Inulin in den Blättern der Marcgraviaceen. – Ber. Deutsch. Bot. Ges. 42:
198-204.
Melchior H. 1925. Theaceae. – In: Engler A, Gilg E (eds),
Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 21, W. Engelmann, Leipzig, pp.
109-154.
Melchior H. 1943. Entwicklungsgeschichte der
Primulaceen – Gattung Dionysia. – Mitt. Thüring. Bot. Ver., N.
F., 50: 156-174.
Melhem TS, Moura CAF, Lieu J. 1971. Pollen
grains of plants of the “Cerrado” – Styracaceae and Turneraceae.
– Hoehnea 1: 153-178.
Melvin NC. 1980. A systematic investigation
of the genus Leucothoë (Ericaceae). – Ph.D. diss., Miami
University, Oxford, Ohio.
Menadue Y, Crowden RK. 2000. Taxonomic
revision of Richea R. Br. (Epacridaceae). – Aust. Syst. Bot. 13:
773-802.
Méndez M, Munzinger M. 2010.
Planchonella, first record of gynomoecy for the family Sapotaceae. – Plant Syst. Evol. 287:
65-73.
Mennechet ML-A. 1902. Sur le fruit du
Jacquinia ruscifolia Jacq. et sur les poils épidermiques des
Myrsinéacées. – J. Bot. (Paris) 16: 349-358.
Merlin CM, Grant WF. 1986. Hybridization
studies in the genus Impatiens. – Can. J. Bot. 64: 1069-1074.
Mez C. 1901. Theophrastaceae. – In: Urban I
(ed), Symbolae antillanae 2, pp. 434-451.
Middleton DJ. 1991a. Ecology, reproductive
biology and hybridization in Gaultheria L. – Edinburgh J. Bot. 48:
81-89.
Middleton DJ. 1991b. Taxonomic studies in the
Gaultheria group of genera of the tribe Andromedeae (Ericaceae). – Edinburgh J. Bot. 48:
283-306.
Middleton DJ. 1991c. Infrageneric
classification of the genus Gaultheria L. (Ericaceae). – Bot. J. Linn. Soc. 106:
229-258.
Middleton DJ. 1992. A chemotaxonomic survey
of flavonoids and simple phenols in the leaves of Gaultheria L. and
related genera. – Bot. J. Linn. Soc. 110: 313-324.
Middleton DJ. 1993. A systematic survey of
leaf and stem anatomical characters in the genus Gaultheria and
related genera (Ericaceae). – Bot. J.
Linn. Soc. 113: 199-215.
Middleton DJ, Wilcock CC. 1990a. A critical
examination of the status of Pernettya as a genus distinct from
Gaultheria. – Edinburgh J. Bot. 47: 291-301.
Middleton DJ, Wilcock CC. 1990b. Chromosome
counts in Gaultheria and related genera. – Edinburgh J. Bot. 47:
303-313.
Midgley JJ, Stock WD. 1998. Natural abundance
of Delta N-15 confirms insectivorous habit of Roridula gorgonias,
despite it having no proteolytic enzymes. – Ann. Bot. 82: 387-388.
Miers J. 1874. On the Lecythidaceae. – Trans. Linn. Soc.
London, Bot. 30: 157-318.
Miers J. 1875a. On Napoleona,
Omphalocarpum, and Asteranthos. – Trans. Linn. Soc. London,
Bot., ser. II, 1: 17-19.
Miers J. 1875b. On the Barringtoniaceae. –
Trans. Linn. Soc. London, Bot., ser. II, 1: 47-118.
Miers J. 1879. On the Symplocaceae. – J. Linn. Soc. 17:
283-306.
Mihaich CM. 1989. Leaf epicuticular waxes in
the taxonomy of the Epacridaceae. – Ph.D. diss., Department of Plant
Sciences, University of Tasmania.
Miki S. 1968. Paleodavidia, synonym
of Melliodendron and fossil remains in Japan. – Bull. Mukogawa Women
Univ. 16: 287-291.
Miller IM, Gardner IC, Scott A. 1984.
Structure and function of trichomes in the shoot tip of Ardisia crispa
(Thunb.) A. DC. (Myrsinaceae). –
Bot. J. Linn. Soc. 88: 223-236.
Milne RI. 2004. Phylogeny and biogeography of
Rhododendron subsection Pontica, a group with a Tertiary
relict distribution. – Mol. Phylogen. Evol. 33: 389-401.
Ming T-L. 2000. Monograph of the genus
Camellia. – Yunnan Sci. Techn. Press, Kunming. [In Chinese]
Mitra K, Mondal, M. 1982. A note on the
pollen morphology of Mitrastemon yamamotoi (Makino) Makino (Rafflesiaceae).
– Bangladesh J. Bot. 11: 179-181.
Mochizuki R. 1969. A new species of the genus
Enkianthus in central Japan. – Hokuriku J. Geobot. 17: 38-39.
Molau U. 1997. Age-related growth and
reproduction in Diapensia lapponica, an arctic-alpine cushion plant.
– Nord. J. Bot. 17: 225-234.
Molina R, Trappe JM. 1982. Lack of
mycorrhizal specificity by the ericaceous hosts Arbutus menziesii and
Arctostaphylos uva-ursi. – New Phytol. 90: 495-509.
Monfils AK, Prather LA. 2004. The conserved
nature and taxonomic utility of pollen morphology in Cantua. –Grana
43: 249-256.
Monteiro-Scanavacca WR. 1974.
Vascularização do gineceu em Lecythidaceae. – Bol. Bot. Univ.
São Paulo 2: 53-69.
Monteiro-Scanavacca WR. 1975a.
Vascularização e natureza de estruturas do androceu em Lecythidaceae. – Bol. Bot. Univ.
São Paulo 3: 61-74.
Monteiro-Scanavacca WR. 1975b. Estudo da
placentação em Lecythidaceae. –
Bol. Bot. Univ. São Paulo 3: 75-86.
Moore DM, Harborne JB, Williams CA. 1970.
Chemotaxonomy, variation, and geographical distribution of the Empetraceae. –
Bot. J. Linn. Soc. 63: 277-293.
Morales F. 2012. Nuevas especies de Sapotaceae para Costa Rica. –
Darwiniana 50: 107-113.
Morawetz W. 1991. The karyology of some
Neotropical Styracaceae. – Plant
Syst. Evol. 177: 111-115.
Mori SA. 1989. Diversity of Lecythidaceae in the Guianas. – In:
Holm-Nielsen LB, Nielsen IC, Balslev H (eds), Tropical forests: botanical
dynamics, speciation, and diversity, Academic Press, New York, pp. 319-331.
Mori SA. 1992. The Brazil nut industry –
past, present, and future. – In: Plotkin M, Famolare L (eds), Sustainable
harvest and marketing of rain forest products, Island Press, Washington, D.C.,
pp. 241-251.
Mori SA. 1995. Observações sobre as
espécies de Lecythidaceae do leste
do Brasil. – Bol. Bot. Dep. Bot. Inst. Bioci. Univ. São Paulo 14: 1-31.
Mori SA, Black D. 1987. Chapter VII. Stem and
leaf. – In: Mori SA et al. (eds), The Lecythidaceae of a lowland neotropical
forest: La Fumée Mountain, French Guiana, Mem. New York Bot. Gard. 44:
72-85.
Mori SA, Black D. 1987. Chapter VIII. Habit
and bark. – In: Mori SA et al. (eds), The Lecythidaceae of a lowland neotropical
forest: La Fumée Mountain, French Guiana, Mem. New York Bot. Gard. 44:
137-155.
Mori SA, Kallunki J. 1976. Phenology and
floral biology of Gustavia superba (Lecythidaceae) in Central Panama. –
Biotropica 8: 184-192.
Mori SA, Prance GT. 1981. Relações entre a
classificação genérica de Lecythidaceae do Novo Mundo seus
polinizadores e dispersadores. – Rev. Bras. Bot. 4: 31-37.
Mori SA, Prance GT. 1987. A guide to
collecting Lecythidaceae. – Ann.
Missouri Bot. Gard. 74: 321-330.
Mori SA, Prance GT. 1990a. Flora Neotropica.
Monograph 21(2). Lecythidaceae II.
The zygomorphic-flowered New World genera (Couroupita,
Corythophora, Bertholletia, Couratari,
Eschweilera, and Lecythis). With a study of secondary xylem
of neotropical Lecythidaceae by
Carl de Zeeuw. – New York Botanical Garden, Bronx, New York.
Mori SA, Prance GT. 1990b. Taxonomy, ecology,
and economic botany of the Brazil nut (Bertholletia excelsa Humb.
& Bonpl.: Lecythidaceae). –
Adv. Econ. Bot. 8: 130-150.
Mori SA, Prance GT, Bolten AB. 1978.
Additional notes on the floral biology of neotropical Lecythidaceae. – Brittonia 30:
113-130.
Mori SA, Orchard JE, Prance GT. 1980.
Intrafloral pollen differentiation in the New World Lecythidaceae, subfamily
Lecythidoideae. – Science 209: 400-403.
Mori SA, Black D, Zeeuw C de. 1987. Habit and
bark. – In: Mori SA et al. (eds), The Lecythidaceae of a lowland neotropical
forest: La Fumée Mountain, French Guiana VII, Mem. New York Bot. Gard. 44:
86-99.
Mori SA & al. (eds). 1987. The Lecythidaceae of a lowland neotropical
forest: La Fumée Mountain, French Guiana VII, Mem. New York Bot. Gard. 44:
1-190.
Mori SA, Tsou C-H, Wu C-C, Cronholm B,
Anderberg AA. 2007. Evolution of Lecythidaceae with an emphasis on the
circumscription of neotropical genera: information from combined ndhF
and trnL-F sequence data. – Amer. J. Bot. 94: 289-301.
Moritz A. 1984. Estudos biológicos da
floração e da frutificação da Castanha-do-brasil (Bertholletia
excelsa H.B.K.). – EMBRAPA-CPATU, Documentos (Bélem) 29: 1-82.
Morozowska M, Czarna A, Kujawa M, Jagodzinski
AM. 2011. Seed morphology and endosperm structure of selected species of Primulaceae, Myrsinaceae, and Theophrastaceae and their systematic
importance. – Plant Syst. Evol. 291: 159-172.
Morton CM. 1994. The use of pollen morphology
and wood anatomy in the study of the phylogeny of Ebenaceae. – Ph.D. diss., New York
Botanical Garden and City University of New York.
Morton CM, Dickison WC. 1992. Comparative
pollen morphology of the Styracaceae.
– Grana 31: 1-15.
Morton CM, Chase MW, Kron KA, Swensen SM.
1996. A molecular evaluation of the monophyly of the order Ebenales based upon
rbcL sequence data. – Syst. Bot. 21: 567-586.
Morton CM, Mori SA, Prance GT, Karol KG,
Chase MW. 1997. Phylogenetic relationships of Lecythidaceae: a cladistic analysis
using rbcL sequence and morphological data. – Amer. J. Bot. 84:
530-540.
Morton CM, Prance GT, Mori SA, Thorburn LG.
1998. Recircumscription of the Lecythidaceae. – Taxon 47:
817-827.
Muller J. 1972. Pollen morphological evidence
for subdivision and affinities of Lecythidaceae. – Blumea 20:
351-355.
Muller J. 1973. Pollen morphology of
Barringtonia calyptrocalyx K. Sch. (Lecythidaceae). – Grana 13:
29-44.
Muller J. 1979. Pollen. – In: Prance GT,
Mori SA (eds), Flora Neotropica. Monograph 21. Lecythidaceae I. The
actinomorphic-flowered New World Lecythidaceae (Asteranthos,
Gustavia, Grias, Allantoma, and Cariniana),
New York Botanical Garden, Bronx, New York, pp. 72-76.
Müller-Stoll WR, Mädel-Angeliewa E. 1969.
Actinidioxylon princeps (Ludwig) n. comb., ein Lianenholz aus dem
Pliozän von Dernbach im Westerwald. – Senckenb. Leth. 50: 103-115.
Munzinger JAA, Swenson U. 2009. Three new
species of Planchonella Pierre (Sapotaceae) with a dichotomous and an
online key to the genus in New Caledonia. – Adansonia, sér. III, 31:
175-189.
Munzinger JAA, Swenson U. 2015. Revision of
Pycnandra subgenus Leptostylis and description of subgenus
Wagapensia (Sapotaceae), a
genus endemic to New Caledonia. – Aust. Syst. Bot. 28: 91-110.
Murata J. 2006. Mitrastemonaceae. In: Iwatsuki K,
Boufford DE, Ohba H (eds), Flora of Japan IIa, Kodansha Ltd., Tokyo, p. 387.
Muravnik LE, Shavarda AL. 2012. Leaf
glandular trichomes in Empetrum nigrum:morphology, histochemistry,
ultrastructure and secondary metabolites. – Nord. J. Bot. 30: 470-481.
Murphy HT, Hardin JW. 1976. A new and unique
venation pattern in the Diapensiaceae. – Torreya 103:
177-179.
Murthy SN. 1941. Morphological studies in the
Sapotaceae I. Embryology of Bassia
latifolia Roxb. and related genera. – J. Mysore Univ. 2: 67-80.
Nagamasu H. 1989a. Pollen morphology and
relationship of Symplocos tinctoria (L. f.) L’Her. (Symplocaceae). – Bot. Gaz. 150:
314-318.
Nagamasu H. 1989b. Pollen morphology of
Japanese Symplocos (Symplocaceae). – Bot. Mag. (Tokyo)
102: 149-164.
Nagamasu H. 1993. The Symplocaceae of Japan. – Contr. Biol.
Lab. Kyoto Univ. 28: 173-260.
Nakai T. 1941. Mitrastemon
cochinchinensis Nakai. – Icon. Plant. As. Orient 306: 104.
Namikawa I, Sisa M, Asai K. 1932. On flower
types of Diospyros kaki L. f. – Jap. J. Bot. 6: 139-172.
Narayana LL. 1963. Contributions to the
embryology of Balsaminaceae 1. –
J. Indian Bot. Soc. 42: 102-109.
Narayana LL. 1965. Contributions to the
embryology of Balsaminaceae 2. –
J. Jap. Bot. 40: 104-116.
Narayana LL. 1970. Balsaminaceae. – In: Proceedings of
the symposium on comparative embryology of angiosperms, Indian National Science
Academy, New Delhi, pp. 158-162.
Narayana LL. 1974. A contribution to the
floral anatomy of Balsaminaceae.
– J. Jap. Bot. 49: 315-322.
Nash GV. 1903. A revision of the family Fouquieriaceae. – Bull. Torrey Bot.
Club 30: 449-459.
Nayar MP, Giri GS. 1986. Parardisia
– a new genus in the family Myrsinaceae with two species. – Bull.
Bot. Surv. India 28: 247-252.
Nelson BW, Absy ML, Barbosa EM, Prance GT.
1987. Observations on flower visitors to Bertholletia excelsa H.B.K.
and Couratari tenuicarpa A. C. Sm. (Lecythidaceae). – Acta Amazonica
15(Suppl.): 225-234.
Nesom GL. 1983. Galax (Diapensiaceae): geographic variation
in chromosome numbers. – Syst. Bot. 8: 1-14.
Newman T, Ibrahim S, Wheeler JW, McLaughlin
WB, Petersen RL, Duffield RM. 2000. Identification of sarracenin in four
species of Sarracenia (Sarraceniaceae). – Biochem. Syst.
Ecol. 28: 193-195.
Neyland R, Merchant M. 2006. Systematic
relationships of Sarraceniaceae
inferred from nuclear ribosomal DNA sequences. – Madroño 53: 223-232.
Ng FSP. 1971. A taxonomic study of the Ebenaceae with special reference to
Malesia. – Ph.D. diss., University of Oxford.
Ng FSP. 1991a. The relationships of the Sapotaceae within the Ebenales. – In:
Pennington TD (ed), The genera of Sapotaceae, chapter 1, Royal Botanic
Gardens, Kew, and New York Botanical Garden, New York, pp. 1-13.
Ng FSP. 1991b. Manual of forest fruits, seeds
and seedlings 1. – Forest Research Institute Malaysia, Kuala Lumpur, Malayan
Forest Record 34: 61-62, 319-327.
Niedenzu F. 1890. Über den anatomischen Bau
der Laubblätter der Arbutoideae und Vaccinioideae in Beziehung zu ihrer
systematischen Gruppierung und geographischen Verbreitung. – Engl. Bot.
Jahrb. 11: 134-263.
Niedenzu F. 1893. Lecythidaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien, III(7), W. Engelmann,
Leipzig, pp. 26-41.
Niedenzu F. 1895b. Tamaricaceae.
– In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(6), W.
Engelmann, Leipzig, pp. 289-298.
Nishino E. 1983. Corolla tube formation in
the Primulaceae and Ericales. – Bot. Mag. (Tokyo) 96:
319-342.
Nishino E. 1988. Early floral organogenesis
in Tripetaleia (Ericaceae).
– In. Leins P, Tucker SC, Endress PK (eds), Aspects of floral development,
Cramer, Berlin, pp. 181-190.
Nixon KC, Crepet WL. 1993. Late Cretaceous
fossil flowers of ericalean affinity. – Amer. J. Bot. 80: 616-623.
Nooteboom HP. 1975. Revision of the Symplocaceae of the Old World, New
Caledonia excepted. – Leiden Bot. Ser. 1: 1-335, Leiden University,
Leiden.
Nooteboom HP. 1977. Symplocaceae. – In: Steenis CGGJ van
(ed), Flora Malesiana I, 8(2), Sijthoff & Noordhoff International Publ.,
Alphen aan den Rijn, The Netherlands, pp. 205-274.
Nooteboom HP. 1981. A revision of the
Australian species of Symplocos (Symplocaceae). – Brunonia 4:
309-326.
Nooteboom HP. 1989. Symplocaceae of New Caledonia. –
Bull. Mus. Natl. Hist. Nat. Paris, sér. IV, sect. B, Adansonia 11: 295-306.
Nooteboom HP. 1991. Symplocos. –
In: Prosea. Plant resources of South-East Asia 3. Dye and tannin producing
plants, Pudoc, Wageningen, pp. 115-118.
Nooteboom HP. 2004. Symplocaceae. – In: Kubitzki K (ed),
The families and genera of vascular plants VI. Flowering plants. Dicotyledons.
Celastrales,
Oxalidales, Rosales, Cornales, Ericales, Springer, Berlin, Heidelberg, New
York, pp. 443-449.
Nooteboom HP. 2005. Additions to Symplocaceae of the old world including
New Caledonia. – Blumea 50: 407-411.
Noshiro S, Suzuki M, Ohba H. 1995. Ecological
wood anatomy of Nepalese Rhododendron 1. Interspecific variation. –
J. Plant Res. 108: 1-9.
Nowicke JW. 1966. Pollen morphology and
classification of the Pyrolaceae and Monotropaceae. – Ann. Missouri Bot.
Gard. 53: 213-219.
Nowicke JW, Skvarla JJ. 1977. Pollen
morphology and the relationship of the Plumbaginaceae,
Polygonaceae,
and Primulaceae to the order
Centrospermae. – Smithsonian Contr. Bot. 37: 1-64.
Obase K, Matsuda Y, Ito S-I. 2013.
Enkianthus campanulatus (Ericaceae) is commonly associated with
arbuscular mycorrhizal fungi. – Mycorrhiza 23: 199-208.
Odell EA, Vander Kloet SP. 1991. The utility
of stem characters in the classification of Vaccinium L. (Ericaceae). – Taxon 40: 273-283.
Odell EA, Vander Kloet SP, Newell RE. 1989.
Stem anatomy of Vaccinium section Cyanococcus and related
taxa. – Can. J. Bot 67: 2328-2334.
Oever L van den, Baas P, Zandee M. 1981.
Comparative wood anatomy of Symplocos and latitude and altitude of
provenance. – IAWA Bull., N. S., 2: 3-24.
Oginuma K, Tobe H. 1991. Karyomorphology of
two species of Impatiens from Kenya. – Acta Phytotaxon. Geobot. 42:
67-71.
Oh I-C, Anderberg A-L, Schönenberger J,
Anderberg AA. 2008. Comparative seed morphology and character evolution in the
genus Lysimachia (Myrsinaceae) and related taxa. – Plant
Syst. Evol. 271: 177-197.
Ohara K, Kondo Y, Kawamura M. 1936a.
Einteilung der Ericaceae und
Kalkoxalatkristalle 1. – Theor. Appl. Bot. Zool. 4: 831-844.
Ohara K, Kondo Y, Kawamura M. 1936b.
Einteilung der Ericaceae und
Kalkoxalatkristalle 2. – Theor. Appl. Bot. Zool. 4: 993-1004.
Oldfield F. 1959. The pollen morphology of
some of the West European Ericales. –
Pollen Spores 1: 19-48.
Olechnowicz-Stepien W, Rzadkowska-Bodalska H,
Lamer-Zarawska E. 1978. Flavonoids of Calluna vulgaris flowers (Ericaceae). – Pol. J. Chem. 52:
2167-2172.
Oliver D. 1888. Talisia princeps
Oliv. – Hooker’s Icon. Plant. 8: pl. 1769.
Oliver D. 1895. Lissocarpa benthamii
Gürke. – Hooker’s Icon. Plant. 25: pl. 2413.
Oliver EGH. 1987a. Studies in the Ericoideae
(Ericaceae) V. the genus
Coilostigma. – Bothalia 17: 163-170.
Oliver EGH. 1987b. Studies in the Ericoideae
VII. The placing of the genus Philippia into synonymy under
Erica; the southern African species. – South Afr. J. Bot. 53:
455-458.
Oliver EGH. 1988. Studies in the Ericoideae
(Ericaceae) VI. The generic
relationship between Erica and Philippia in southern Africa.
– Bothalia 18: 1-10.
Oliver EGH. 1989. The Ericoideae and the
southern African heathers. – Bot. J. Linn. Soc. 101: 319-327.
Oliver EGH. 1991. The Ericoideae (Ericaceae) – a review. – Contr. Bolus
Herb. 13: 158-208.
Oliver EGH. 1992. Studies in the Ericoideae
(Ericaceae) IX. New combinations for
Philippia are made in Erica for the Flora Zambesiaca region.
– Kew Bull. 47: 665-668.
Oliver EGH. 1993a. Studies in the Ericoideae
(Ericaceae) X. Nomenclatural changes
for the Flore des Mascareignes region. – Kew Bull. 48: 767-769.
Oliver EGH. 1993b. Studies in the Ericoideae
(Ericaceae) XI. The generic
relationship between Erica and Blaeria. – Kew Bull. 48:
771-780.
Oliver EGH. 1993c. Studies in the Ericoideae
(Ericaceae) XII. The placing of the
genus Blaeria into synonymy under Erica; nomenclatural and
taxonomic changes for the southern African region. – Bothalia 23: 1-7.
Oliver EGH. 1994. Studies in the Ericoideae
(Ericaceae) XV. The generic
relationship between Erica and Ericinella. – Bothalia 24:
121-126.
Oliver EGH. 2000. Systematics of Ericeae (Ericaceae: Ericoideae): species with
indehiscent and partially dehiscent fruits. – Contr. Bolus Herb. 19:
1-483.
Oliver EGH, Linder HP, Rourke JP. 1983.
Geographical distribution of present-day Cape taxa and their phytogeographical
significance. – Bothalia 14: 427-440.
Olson AR. 1980. Seed morphology of
Monotropa uniflora L. (Ericaceae). – Amer. J. Bot. 67:
986-974.
Olson AR. 1991. Postfertilization changes in
ovules of Monotropa uniflora L. (Monotropaceae). – Amer. J. Bot. 78:
99-107.
Osmundson TW, Halling RE, Bakker HC. 2007.
Morphological and molecular evidence supporting an arbutoid mycorrhizal
relationship in the Costa Rican páramo. – Mycorrhiza 17: 217-222.
Oswald WW, Doughty ED, Ne’eman G, Ne’eman
R, Ellison AM. 2011. Pollen morphology and its relationship to taxonomy of the
genus Sarracenia (Sarraceniaceae). – Rhodora 113:
235-251.
Otegui M. 1998. Sinopsis del género
Myrsine L. (Myrsinaceae) en
el cono sur de América del Sur. – Candollea 53: 133-157.
Otegui M, Cocucci A. 1999. Flower morphology
and biology of Myrsine laetevirens, structural and evolutionary
implications of anemophily in Myrsinaceae. – Nord. J. Bot. 19:
71-85.
Pacini E. 1969. Embryology of Arbutus
unedo L. – Giorn. Bot. Ital. 103: 623-624.
Paige KN, Whitham TG. 1985. Individual and
population shifts in flower color by scarlet gilia: a mechanism for pollinator
tracking. – Science 227: 315-317.
Palibin JV. 1899. Revisio generis
Enkianthus Lour. – Scr. Bot. Horti Univ. Imp. Petropolitanae 15:
1-18.
Palm BT. 1934. A Mitrastemon, Rafflesiaceae,
from Sumatra. – Acta Hort. Gothoburg. 147-152.
Palser BF. 1951. Studies of floral morphology
in the Ericales I. Organography and
vascular anatomy in the Andromedeae. – Bot. Gaz. 112: 447-485.
Palser BF. 1952. Studies of floral morphology
in the Ericales II. Megasporogenesis and
megagametophyte development in the Andromedeae. – Bot. Gaz. 114: 33-52.
Palser BF. 1954. Studies of floral morphology
in the Ericales III. Organography and
vascular anatomy in several species of the Arbuteae. – Phytomorphology 4:
335-354.
Palser BF. 1958. Studies of floral morphology
in the Ericales IV. Observations on
three members of the Gaultherieae. – Trans. Illinois Acad. Sci. 51: 24-34.
Palser BF. 1961a. Studies of floral
morphology in the Ericales V.
Organography and vascular anatomy in several United States species of the
Vacciniaceae. – Bot. Gaz. 123: 79-111.
Palser BF. 1961b. Additional observations on
the floral morphology of the Andromedeae. – Amer. J. Bot. 48: 535-536.
Palser BF. 1961c. Some aspects of embryology
in the Ericales. – In: Recent advances
in botany 1, (IX International Botanical Congress Montreal, 1959), University
of Toronto Press, Toronto, pp. 685-689.
Palser BF. 1963. Studies of floral morphology
in the Ericales VI. The Diapensiaceae. – Bot. Gaz. 124:
200-219.
Palser BF, Murty YS. 1967. Studies of floral
morphology in the Ericales VIII.
Organography and vascular anatomy in Erica. – Bull. Torrey Bot. Club
94: 243-320.
Palser BF, Philipson WR, Philipson MN. 1989.
Development of ovule, megagametophyte and early endosperm in representative
species of Rhododendron L. (Ericaceae). – Bot. J. Linn. Soc. 101:
363-393.
Pankow H. 1959. Histogenetische
Untersuchungen an der Plazenta der Primulaceen. – Ber. Deutsch. Bot. Ges. 72:
111-122.
Paoletti C, Holsinger KE. 1999. Spatial
patterns of polygenic variation in Impatiens capensis, a species with
an environmentally controlled mixed mating system. – J. Evol. Biol. 12:
689-696.
Parry CC. 1884. Arctostaphylos
Adans. Notes on the U.S. Pacific coast species. – Proc. Davenport Acad. Nat.
Sci. 4: 31-37.
Pascarella JB. 1997a. Breeding systems of
Ardisia Sw. (Myrsinaceae).
– Brittonia 49: 45-53.
Pascarella JB. 1997b. Pollination ecology of
Ardisia escallonioides (Myrsinaceae). – Castanea 62: 1-7.
Patel RC, Inamdar JA. 1971. Structure and
ontogeny of stomata in some Polemoniales. – Ann. Bot. 35: 389-409.
Paterson BR. 1961. Studies of floral
morphology in the Epacridaceae. – Bot. Gaz. 122: 259-279.
Patterson R. 1977. The generic status of
perennial species of Linanthus (Polemoniaceae). – Taxon 26:
507-511.
Patterson R. 1980a. The occurrence of B
chromosomes in Linanthus pachyphyllus. – Caryologia 33: 141-149.
Patterson R. 1980b. Crossing relationships in
perennial Linanthus taxa (Polemoniaceae). – Syst. Bot. 5:
384-390.
Patterson R. 1989. Taxonomic relationships of
Gilia maculata (Polemoniaceae). – Madroño 36:
15-27.
Patterson R, Yoder-Williams M. 1984.
Leptodactylon glabrum, a new intermountain species of Polemoniaceae. – Syst. Bot. 9:
261-262.
Pax F. 1889. Monographische Übersicht über
die Arten der Gattung Primula. – Engl. Bot. Jahrb. Syst. 18:
75-241.
Pax F. 1891a. Myrsinaceae. – In: Engler A, Prantl K,
(eds), Die natürlichen Pflanzenfamilien, IV(1), W. Engelmann, Leipzig, pp.
84-97.
Pax F. 1891b. Primulaceae. – In: Engler A, Prantl K
(eds), Die natürlichen Pflanzenfamilien IV(1), W. Engelmann, Leipzig, pp.
98-116.
Pax F. 1896. Empetraceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien III(5), W. Engelmann,
Leipzig, pp. 123-127.
Payens JPDW. 1967. A monograph of the genus
Barringtonia (Lecythidaceae). – Blumea 15:
157-263.
Pearson V, Read DJ. 1973. The biology of
mycorrhiza in the Ericaceae I. The
isolation of the endophyte and synthesis of mycorrhizas in aseptic culture. –
New Phytol. 72: 371-379.
Pedraza-Peñalosa P. 2008. Three new species
of Disterigma (Ericaceae:
Vaccinieae) from western Colombia, with comments on morphological terminology.
– Brittonia 60: 1-10.
Pedraza-Peñalosa P. 2009. Systematics of the
Neotropical blueberry genus Disterigma (Ericaceae). – Syst. Bot. 34: 406-413.
Pedraza-Peñalosa P. 2010. Insensitive
blueberries: a total-evidence analysis of Disterigma s.l. (Ericaceae) exploring transformation costs.
– Cladistics 26: 388-407.
Pedraza-Peñalosa P, Salinas NR, Wheeler WC.
2013. Venation patterns of neotropical blueberries (Vaccinieae: Ericaceae) and their phylogenetic utility.
– Phytotaxa 96: 1-53.
Pedraza-Peñalosa P, Salinas NR, Virnig AS,
Wheeler WC. 2015. Preliminary phylogenetic analysis of the Andean clade and the
placement of new Colombian blueberries (Ericaceae, Vaccinieae). – PhytoKeys 49:
13-31.
Pellegrin F. 1938. Sur un genre africain peu
connu: Tridesmostemon Engl. (Sapotacées). – Bull. Soc. Bot. France
85: 179-181.
Peltrisot C-N. 1904. Développement et
structure de la graine chez les Ericacées. – Diss., Université de Paris,
France.
Peng C-I, Goldblatt P. 1983. Confirmation of
the chromosome number in Cephalotaceae
and Roridulaceae. – Ann. Missouri
Bot. Gard. 70: 197-198.
Pennington TD. 1990. Flora Neotropica.
Monograph 52. Sapotaceae. – New York
Botanical Garden, Bronx, New York.
Pennington TD. 1991. The genera of Sapotaceae. – Royal Botanic Gardens,
Kew, and New York Botanical Garden, Bronx, New York.
Pennington TD. 2004. Sapotaceae. – In: Kubitzki K (ed), The
families and genera of vascular plants VI. Flowering plants. Dicotyledons. Celastrales,
Oxalidales, Rosales, Cornales, Ericales, Springer, Berlin, Heidelberg, New
York, pp. 390-421.
Pennington TD. 2007. 152. Sapotaceae. – In: Harling G, Persson C
(eds), Flora of Ecuador 80, Department of Plant and Environmental Sciences,
Göteborg University, pp. 1-193.
Pereira JT. 1997. Five new species of
Payena (Sapotaceae) and notes
on the genus in Borneo. – Kew Bull. 52: 903-922.
Perkins J. 1928. Übersicht über die
Gattungen der Styracaceae. – W.
Engelmann, Leipzig.
Perotto S, Perotto R, Faccio A, Schubert A,
Varma A, Bonfante P. 1995. Ericoid mycorrhizal fungi: cellular and molecular
bases of their interactions with the host plant. – Can. J. Bot. 73 (Suppl.):
S557-S568.
Perrier de la Bâthie H. 1953. Myrsinacées.
– In: Humbert H (ed), Flore de Madagascar et des Comores 161, Mus. Natl.
Hist. Nat., Paris.
Peter A. 1897. Polemoniaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien IV(3a), W. Engelmann,
Leipzig, pp. 40-54, 377.
Petersen HE. 1912. The structure and biology
of arctic flowering plants II. Diapensiaceae. Diapensia
lapponica L. – Meddel. Grønland 36: 141-154.
Philipson WR. 1985. Shoot morphology in
Rhododendron. – Notes Roy. Bot. Gard. Edinb. 43: 161-171.
Philipson WR, Philipson MN. 1968. Diverse
nodal types in Rhododendron. – J. Arnold Arbor. 49: 193-224.
Pichon M. 1945. Le genre
Combretodendron et les Lecythidacées. – Not. Syst. (Paris) 12:
192-197.
Pierre JBL. 1890-1891. Notes botaniques
Sapotacées. – Paris.
Pillon Y, Nooteboom HP. 2009. A new species
of Symplocos (Symplocaceae)
from Mont Panié (New Caledonia). – Adansonia, sér. III, 31: 191-196.
Pipoly JJ. 1983. Contributions toward a
monograph of Cybianthus (Myrsinaceae) III. A revision of subgenus
Laxiflorus. – Brittonia 35: 61-80.
Pipoly JJ. 1987. A systematic revision of the
genus Cybianthus subgenus Grammadenia (Myrsinaceae). – Mem. New York Bot.
Gard. 43: 1-76.
Pipoly JJ. 1992a. The genus
Cybianthus subgenus Conomorpha (Myrsinaceae) in Guayana. – Ann.
Missouri Bot. Gard. 79: 908-957.
Pipoly JJ. 1992b. Estudios en el género
Myrsine (Myrsinaceae) de
Colombia. – Caldasia 17: 3-10.
Pipoly JJ. 1993. The genus
Geissanthus (Myrsinaceae) in
the Chocó floristic province. – Novon 3: 463-474.
Pipoly JJ. 1999. Two new species of Myrsinaceae from French Guiana. –
Brittonia 51: 128-133.
Pipoly JJ, Ricketson JM. 1999. Discovery of
the Indo-Malesian genus Hymenandra (Myrsinaceae) in the Neotropics, and its
boreotropical implications. – Sida 18: 701-746.
Pirie MD, Oliver EGH, Bellstedt DU. 2011. A
densely sampled ITS phylogeny of the Cape flagship genus Erica L.
suggests numerous shifts in floral macro-morphology. – Mol. Phylogen. Evol.
61: 593-601.
Pitard J. 1930. Myrsinaceae. – In: Lecomte H, Humbert
H (eds), Flore générale de l’Indo-Chine 3, Masson, Paris, pp. 765-877.
Plitmann U, Levin DA. 1983. Pollen-pistil
relationships in the Polemoniaceae.
– Evolution 37: 957-967.
Plitmann U, Levin DA. 1990. Breeding systems
in the Polemoniaceae. – Plan
Syst. Evol. 170: 205-214.
Pohl F. 1931. Über sich öffnende
Kristallräume in den Antheren von Deherainia smaragdina. – Jahrb.
Wiss. Bot. 75: 481-493.
Porter JM. 1993. Phylogenetic systematics of
Gilia sect. Giliandra (Polemoniaceae). – Ph.D. diss.,
University of Arizona, Tucson, Arizona.
Porter JM. 1996 [1997]. Phylogeny of Polemoniaceae based on nuclear
ribosomal internal transcribed spacer DNA sequences. – Aliso 15: 57-77.
Porter JM. 1998a. Aliciella, a
recircumscribed genus of Polemoniaceae. – Aliso 17: 23-46.
Porter JM. 1998b. Nomenclatural changes in Polemoniaceae. – Aliso 17: 83-85.
Porter JM, Johnson LA. 1998. Phylogenetic
relationships of Polemoniaceae:
inferences from mitochondrial nad1B intron sequences. – Aliso 17:
157-188.
Porter JM, Johnson LA. 2000. A phylogenetic
classification of Polemoniaceae.
– Aliso 19: 55-91.
Porter JM, Patterson RW. 2015. A fistful of
Polemoniaceae: new names and
combinations. – Aliso 32: 55-88.
Porter JM, Steinmann VW. 2009. Two new
Loeselia (Polemoniaceae)
species from Michoacán, Mexico. – Syst. Bot. 34: 730-736.
Porter JM, Johnson LA, Wilken D. 2010.
Phylogenetic systematics of Ipomopsis (Polemoniaceae): relationships and
divergence times estimated from chloroplast and nuclear DNA sequences. –
Syst. Bot. 35: 181-200.
Powell EA, Kron KA. 2001. An analysis of the
phylogenetic relationships in the wintergreen group (Diplycosia,
Gaultheria, Pernettya, Tepuia; Ericaceae). – Syst. Bot. 26: 808-817.
Powell EA, Kron KA. 2002. Hawaiian
blueberries and their relatives – a phylogenetic analysis of
Vaccinium sections Macropelma, Myrtillus, and
Hemimyrtillus (Ericaceae). –
Syst. Bot. 27: 768-779.
Powell EA, Kron KA. 2003. Molecular
systematics of the northern Andean blueberries (Vaccinieae, Vaccinioideae, Ericaceae). – Int. J. Plant Sci. 164:
987-995.
Powell EA, Vander Kloet SP. 1997. Foliar
venation characteristics of Vaccinium sect. Macropelma and
sect. Myrtillus (Ericaceae): a
comparative analysis. – Can. J. Bot. 75: 2015-2025.
Powell JM, Chapman AR, Doust ANL. 1987.
Classification and generic status in the Epacridaceae – a preliminary
analysis. – Aust. Syst. Bot. Soc. Newsl. 53: 70-78.
Powell JM, Robertson G, Wiecek BM, Scott JA.
1992. Studies in Australian Epacridaceae: changes to Styphelia. –
Telopea 5: 207-227.
Powell JM, Crayn DM, Gadek PA, Quinn CJ,
Morrison DA, Chapman AR. 1996. A re-assessment of relationships within
Epacridaceae. – Ann. Bot., N. S., 77: 305-315.
Powell JM, Morrison DA, Gadek PA, Crayn DM,
Quinn CJ. 1997. Relationships and generic concepts within Styphelieae
(Epacridaceae). – Aust. Syst. Bot. 10: 15-29.
Prakash V, Dayal R. 1965.
Barringtonioxylon eopterocarpum sp. nov. A fossil wood of Lecythidaceae from Deccan
intertrappean beds of Mahurzari. – Paleobotanist 13: 25-29.
Prance GT. 1976. The pollination and
androphore structure of some Amazonian Lecythidaceae. – Biotropica 8:
235-241.
Prance GT. 2004. Napoleonaeaceae. – In:
Kubitzki K (ed), The families and genera of vascular plants VI. Flowering
plants. Dicotyledons. Celastrales,
Oxalidales, Rosales, Cornales, Ericales, Springer, Berlin, Heidelberg, New
York, pp. 282-284.
Prance GT. 2008. A revision of
Foetidia (Lecythidaceae
subfamily Foetidioideae). – Brittonia 60: 336-348.
Prance GT, Jongkind CCH. 2015. A revision of
African Lecythidaceae. – Kew
Bull. 70: 6 DOI 10.1007/S12225-014-9547-4
Prance GT, Mori SA. 1978. Observations on the
fruits and seeds of neotropical Lecythidaceae. – Brittonia 30:
21-33.
Prance GT, Mori SA. 1979. Flora Neotropica.
Monograph 21. Lecythidaceae I. The
actinomorphic-flowered New World genera (Asteranthos,
Gustavia, Grias, Allantoma, &
Cariniana). – New York Botanical Garden, Bronx, New York.
Prance GT, Mori SA. 1983. Dispersal and
distribution of Lecythidaceae and
Chrysobalanaceae.
– Sonderbd. Naturw. Ver. Hamburg 7: 163-186.
Prance GT, Mori SA. 2004. Lecythidaceae. – In: Kubitzki K
(ed), The families and genera of vascular plants VI. Flowering plants.
Dicotyledons. Celastrales,
Oxalidales, Rosales, Cornales, Ericales, Springer, Berlin, Heidelberg, New
York, pp. 221-232.
Prather LA. 1994. A new species of
Phlox (Polemoniaceae) from
northern Mexico with an expanded circumscription of subsection divaricatae. –
Plant Syst. Evol. 192: 61-66.
Prather LA. 1996. Three new species of
Cobaea (Polemoniaceae).
– Brittonia 48: 111-119.
Prather LA. 1999a. Systematics of
Cobaea (Polemoniaceae).
– Syst. Bot. Monogr. 57: 1-81.
Prather LA. 1999b. The relative lability of
floral vs non-floral characters and a morphological phylogenetic analysis of
Cobaea (Polemoniaceae).
– Bot. J. Linn. Soc. 131: 433-450.
Prather LA, Jansen RK. 1998. Phylogeny of
Cobaea (Polemoniaceae)
based on sequence data from the ITS region of nuclear ribosomal DNA. – Syst.
Bot. 23: 57-72.
Prather LA, Ferguson CJ, Jansen RK. 2000. Polemoniaceae phylogeny and
classification: implications of sequence data from the chloroplast gene
ndhF. – Amer. J. Bot. 87: 1300-1308.
Primack RB, Wyatt R. 1975. Variation and
taxonomy of Pyxidanthera (Diapensiaceae). – Brittonia 27:
115-118.
Prince LM. 1998. Systematics of the Theoideae
(Theaceae) and the resolution of
morphological, anatomical and molecular data. – Ph.D. diss., Department of
Biology, University of North Carolina at Chapel Hill.
Prince LM. 2002. Circumscription and
biogeographic patterns in the eastern North American-East Asian genus
Stewartia (Theaceae:
Stewartieae): insight from chloroplast and nuclear DNA sequence data. –
Castanea 67: 290-301.
Prince LM. 2007. A brief nomenclatural review
of genera and tribes in Theaceae. –
Aliso 24: 105-121.
Prince LM, Parks CR. 2001. Phylogenetic
relationships of Theaceae inferred from
chloroplast DNA sequence data. – Amer. J. Bot. 88: 2309-2320.
Puente-Lelièvre C. 2013. Systematics and
biogeography of Styphelieae (Epacridoideae, Ericaceae). – Ph.D. thesis, James Cook
University, Cairns, Australia.
Puente-Lelièvre C, Hislop M, Harrington M,
Brown EA, Kuzmina M, Crayn DM. 2016. A five-marker molecular phylogeny of the
Styphelieae (Epacridoideae, Ericaceae)
supports a broad concept of Styphelia. – Aust. Syst. Bot. 28:
368-387.
Punt W. 1971. Pollen morphology of the genera
Norantea, Souroubea, and Marcgravia (Marcgraviaceae). – Pollen Spores
13: 199-232.
Punt W, de Leeuw van Weenen JS, Oostrum AP
van. 1974. The Northwest European pollen flora 3. Primulaceae. – Rev. Palaeobot.
Palynol. 14: 31-70.
Pyykkö M. 1968. Embryological and anatomical
studies on Finnish species of the Pyrolaceae. – Ann. Bot. Fenn. 5:
153-165.
Pyykkö M. 1969. Placentation in the Ericales I. Pyrolaceae and Monotropaceae.
– Ann. Bot. Fenn. 6: 255-268.
Qi C-J. 1981. A new species of Styracaceae from Hunan. – Acta
Phytotaxon. Sin. 19: 526-528.
Quinn CJ, Crayn DM, Heslewood MM, Brown EA,
Gadek PA. 2003. A molecular estimate of the phylogeny of Styphelieae (Ericaceae). – Aust. Syst. Bot. 16:
581-594.
Quinn CJ, Brown EA, Heslewood MM, Crayn DM.
2005. Generic concepts in Styphelieae (Ericaceae): the Cyathodes group.
– Aust. Syst. Bot. 18: 439-454.
Quinn CJ, Crowden RK, Brown EA, Southam MJ,
Thornhill AH, Crayn DM. 2015. A reappraisal of the generic concepts of
Epacris, Rupicola and Budawangia (Ericaceae, Epacridoideae, Epacrideae)
based on phylogenetic analysis of morphological and molecular data. – Aust.
Syst. Bot. 28: 63-77.
Radhakrishnan N, Gnanamani A, Mandal AB.
2011. A potential antibacterial agent embelin, a natural benzoquinone extracted
from Embelia ribes. – Biol. Med. 3 (Spec. Issue): 1-7.
Raju MVS. 1952. Embryology of Anagallis
pumila Swartz. – Proc. Indian Acad., Sci. Sect. B, 36: 34-42.
Raju VS, Nair VJ, Narayana LL, Dutt BSM.
2002. Proposal to conserve the name Hydrocera against Tytonia
(Balsaminaceae). – Taxon 51:
383-384.
Rama Devi D. 1991. Floral anatomy of six
species of Impatiens. – Feddes Repert. 102: 395-398.
Rama Devi D, Narayana LL. 1989. Floral
anatomy of Balsaminaceae. – In:
Trivedi M, Gill B, Saini S (eds), Plant Science Research in India, pp.
407-413.
Randall JL, Hilu KW. 1990. Interference
through improper pollen transfer in mixed stands of Impatiens capensis
and I. pallida (Balsaminaceae). – Amer. J. Bot. 77:
939-944.
Rao A, Balakrishnan, NP. 1972.
Mitrastemon yamamotoi (Makino) Makino (Rafflesiaceae)
– a unique root parasite new to the Indian flora. – Indian Forester 98:
234-235.
Rao AN. 1953. Inverted polarity in the
embryo-sac of Saurauia napaulensis DC. – Curr. Sci. 22: 282.
Rao AN. 1971. Morphology and morphogenesis of
foliar sclereids in Aegiceras corniculatum. – Isr. J. Bot. 20:
124-132.
Rao RVS, Ayyangar KR, Sampathkumar R. 1986.
On the karyological characteristics of some members of Balsaminaceae. – Cytologia 51:
251-260.
Rao TA. 1951. Studies on foliar sclereids in
dicotyledons I. Structure and ontogeny of sclereids in the leaf of
Diospyros discolor. – Proc. Ind. Acad. Sci., Ser. B, 34: 92-96.
Rao TA. 1961. Studies in foliar sclereids in
dicotyledons VII. Origin and development of sclerenchymatous idioblasts in the
leaf of Manilkara hexandra (Roxb.) Dubard. – Proc. Indian Acad.
Sci., Sect. B, 52: 27-31.
Rao TA, Chakraborti S. 1985. The veinlet
syndrome in the tribe Andromedeae (Ericaceae). – Proc. Indian Acad. Sci.
94: 639-654.
Rathcke B, Real L. 1993. Autogamy and
inbreeding depression in mountain laurel, Kalmia latifolia (Ericaceae). – Amer. J. Bot. 80:
143-146.
Rathore JS. 1971. Studies in the root system
and regeneration of Diospyros melanoxylon Roxb. – Indian Forester
97: 379-386.
Raus T. 1987. Soldanella (Primulaceae) in Griechenland. –
Willdenowia 16: 335-342.
Ray JD Jr. 1956. The genus
Lysimachia in the New World. – Illinois Biol. Monogr. 24(3-4):
1-160.
Read DJ. 1983. The biology of mycorrhiza in
the Ericales. – Can. J. Bot. 61:
985-1004.
Read DJ. 1996. The structure and function of
the ericoid mycorrhizal root. – Ann. Bot., N. S., 77: 365-374.
Reader RJ. 1977. Bog ericad flowers: self
compatibility and relative attractiveness to bees. – Can. J. Bot. 55:
2279-2287.
Rebelo AG, Siegfried WR, Oliver EGH. 1985.
Pollination syndromes of Erica species in the south-western Cape. –
South Afr. J. Bot. 51: 270-280.
Record SJ. 1932. Woods of the Ericales, with particular reference to
Schizocardia. – Trop. Woods 32: 11-14.
Record SJ. 1939. American woods of the family
Sapotaceae. – Trop. Woods 59:
21-51.
Record SJ. 1942. American woods of the family
Theaceae. – Trop. Woods 70: 23-33.
Redgwell RJ. 1983. Composition of
Actinidia mucilage. – Phytochemistry 22: 951-956.
Renner SS. 1989. Floral biological
observations on Heliamphora tatei (Sarraceniaceae) and other plants from
Cerro de la Neblina in Venezuela. – Plant Syst. Evol. 163: 21-29.
Reveal JL. 2010. Proposal to conserve the
name Mitrastemon (Rafflesiaceae)
with that spelling. – Taxon 59: 299-300.
Reynolds JD. 1968. Morphological studies in
Diapensiaceae I. Chromosome number
and microspore development in Pyxidanthera brevifolia Wells. –
Torreya 95: 653-656.
Richards J. 1993. Primula. –
Timber Press, Portland, Oregon.
Richards J. 2003. Primula.
2nd ed. – Timber Press, Portland, Oregon.
Richardson JE, Bakar AM, Tosh J, Armstrong K,
Smedmark J, Anderberg AA, Slik F, Wilkie P. 2014. The influence of tectonics,
sea-level changes and dispersal on migration and diversification of Isonandreae
(Sapotaceae). – Bot. J. Linn. Soc.
174: 130-140.
Richter HG. 1982. The wood structure of
Couratari Aubl. and Couroupita Aubl. (Lecythidaceae). – IAWA Bull., N. S.,
3: 45-55.
Ricketson JM, Pipoly JJ. 1999. The genus
Myrsine (Myrsinaceae) in
Venezuela. – Sida 18: 1095-1144.
Ricketson JM, Pipoly JJ. 2003. Revision of
Ardisia subg. Auriculardisia (Myrsinaceae). – Ann. Missouri Bot.
Gard. 90: 179-317.
Rivera Nunez D, Ruiz Liminana JB. 1987.
Argania spinosa (L.) Skeels (Sapotaceae) subespontanea en la peninsula
Iberica. – An. Jard. Bot. Madrid 44(12): 173.
Robertson DC, Robertson JA. 1982.
Ultrastructure of Pterospora andromedea Nuttall and Sarcodes
sanguinea Torrey mycorrhizas. – New Phytol. 92: 539-551.
Robertson DC, Robertson JA. 1985.
Ultrastructural aspects of Pyrola mycorrhizae. – Can. J. Bot. 63:
1089-1098.
Robson NKB. 1961a. The taxonomic position of
Ficalhoa laurifolia Hiern. – Comptes Rendus de la IVe Réunion de
l’Association pour l’étude taxonomique de la flore d’Afrique tropicale,
Junta de Investigações do Ultramar, Lisboa, pp. 299-308.
Robson NKB. 1961b. 26. Theaceae (Ternstroemiaceae). – In: Exell
AW, Wild H (eds), Flora Zambesiaca 1 (Part 2), Crown Agents for Oversea
Governments and Administrations, London, pp. 405-407
Rodrígues S, Berazaín R. 1991.
Caracterización de la nervadura foliar en el género Purdiaea Planch.
(Cyrillaceae). – Revista Jard. Bot.
Nac. Univ. Habana 12: 69-73.
Rodrígues S, Berazaín R. 1992. Caracteres
diagnósticos en el género Purdiaea Planchon (Cyrillaceae) en Cuba. – Revista Jard.
Bot. Nac. Univ. Habana 13: 17-20.
Romeo JT, Bacon JD, Mabry TJ. 1977.
Ecological considerations of amino acids and flavonoids in Sarracenia
species. – Biochem. Syst. Ecol. 5: 117-120.
Rönblom K, Anderberg AA. 2002. Phylogeny of
Diapensiaceae based on molecular
data and morphology. – Syst. Bot. 27: 383-395.
Ronse De Craene L-P. 2011. Floral development
of Napoleonaea (Lecythidaceae), a deceptively complex
flower. – In: Wanntorp L, Ronse de Craene LP (eds), Flowers on the Tree of
Life, Syst. Assoc. Spec. Vol. 80, Cambridge University Press, Cambridge, pp.
279-295.
Ronse De Craene L-P, Smets E, Clinckemaillie
D. 1995. The floral development and floral anatomy of Coris
monspeliensis. – Can. J. Bot. 73: 1687-1698.
Roon AC de. 1967. Foliar sclereids in the Marcgraviaceae. – Acta Bot. Neerl.
15: 585-628.
Roon AC de. 1970. Flora of Panama VI. 121. Marcgraviaceae. – Ann. Missouri
Bot. Gard. 57: 29-50.
Roon AC de. 1975. Contributions towards a
monograph of the Marcgraviaceae.
– Ph.D. diss., University of Utrecht.
Roon AC de, Dressler S. 1997. New taxa of
Norantea Aubl. s. l. (Marcgraviaceae) from Central America
and adjacent South America. – Bot. Jahrb. Syst. 119: 327-335.
Rosatti TJ. 1981. A new chromosome number in
Arctostaphylos uva-ursi. – Can. J. Bot. 59: 272-273.
Rose JP, Kleist TJ, Löfstrand SD, Drew BT,
Schönenberger J, Sytsma KJ. 2018. Phylogeny, historical biogeography, and
diversification of angiosperm order Ericales suggest ancient Neotropical and
East Asian connections. – Mol. Phylogen. Evol. 122: 59-79.
Ross MN. 1936. Seed reproduction of
Shortia galacifolia. – J. New York Bot. Gard. 37: 208-211.
Ross R. 1983. 102. Ericaceae. – In: Launert E (ed), Flora
Zambesiaca 7 (Part 1), Flora Zambesiaca Managing Committee, London, pp.
157-181.
Røsvik A. 1966. On the taxonomic position of
the genera Dodecatheon L. and Cyclamen L. within Primulaceae. – Årb. Univ. Bergen,
Mat. Nat. Ser. 5: 1-16.
Røsvik A. 1968. Investigations on petal
epidermis and its bearing on taxonomy in Primulaceae. – Årb. Univ. Bergen,
Mat. Nat. Ser. 3: 1-32.
Røsvik A. 1969. On the taxonomic position of
the genera Ardisiandra Hook. and Stimpsonia Wright within Primulaceae. – Årb. Univ. Bergen,
Mat. Nat. Ser. 7: 1-15.
Roth I. 1959a. Histogenese und morphologische
Deutung der Kronblätter von Primula. – Bot. Jahrb. Syst. 79:
1-16.
Roth I. 1959b. Histogenese und morphologische
Deutung der Plazenta von Primula. – Flora 148: 129-152.
Roth I. 1969. Estructura anatómica de la
corteza de algunas especies arbóreas venezolanas de Lecythidaceae. – Act Bot. Venez. 4:
89-117.
Roth I, Carrasquel G. 1970. The peeling off
of the epidermis in fruits of Jacquinia loeflingii Carrasquel. –
Acta Biol. Venezuel. 6: 139-145.
Roy RP, Jha RP. 1965. Cytological studies in
Lecythidaceae II. – J. Indian
Bot. Soc. 44: 209-217.
Royen P van. 1957. Revision of the Sapotaceae of the Malaysian area in a
wider sense VII. Planchonella Pierre. – Blumea 8: 235-445.
Royen P van. 1958. Revision of the Sapotaceae of the Malaysian area in a
wider sense XIV. Diploknema Pierre. – Blumea 9: 75-88.
Royen P van. 1959. Revision of the Sapotaceae of the Malaysian area in a
wider sense XIX. Chelonespermum Hemsley. – Nova Guinea, n. s., 10:
137-142.
Royen P van. 1960. Revision of the Sapotaceae of the Malaysian area in a
wider sense XXII. Mastichodendron. – Blumea 10: 122-125.
Ruchisansakun S, Niet T van der, Janssens SB,
Triboun P, Techaprasan J, Jenjittikul T, Suksathan P. 2015. Phylogenetic
analyses of molecular data and reconstruction of morphological character
evolution in Asian Impatiens Section Semeiocardium (Balsaminaceae). – Syst. Bot. 40:
1063-1074.
Rust RW. 1977. Pollination in Impatiens
capensis and Impatiens pallida (Balsaminaceae). – Bull. Torrey Bot.
Club 104: 361-367.
Rust RW. 1979. Pollination of Impatiens
capensis: pollinators and nectar robbers. – J. Kansas Entomol. Soc. 52:
297-308.
Rzedowski J, Calderón de Rzedowski G,
Villarreal JA. 1995. Notas sobre algunas Polemoniaceae Mexicanas. – Acta Bot.
Mex. 31: 55-61.
Safijowska LD. 1960. Male gametophyte in
Enkianthus. – Bot. Gaz. 121: 190-195.
Salasoo I. 1983. Alkane distribution in
epicuticular waxes of Epacridaceae. – Phytochemistry 22: 937-942.
Salasoo I. 1985. Rimuene in Epacridaceae and
Ericaceae: ixistence of chemotypes? –
Aust. J. Bot. 33: 239-243.
Salasoo I. 1988. Epicuticular wax
hydrocarbons of Richea (Epacridaceae). – Aust. Syst. Bot. 1: 185-188.
Saleh NAM, Towers GHN. 1974. Flavonol
glycosides of Norantea guianensis flowers. – Phytochemistry 13:
2012.
Salter TM. 1953. A note on sex in Royena
glabra L. (Ebenaceae). – J.
South Afr. Bot. 19: 29-30.
Samuelson G. 1913. Studien über
Entwicklungsgeschichte der Blüten einiger Bicornes-typen: ein Beitrag zur
Kenntnis der systematischen Stellung der Diapensiaceen und Empetraceen. –
Svensk Bot. Tidskr. 7: 97-188.
Sangai GRW. Lecythidaceae. – In: Milne-Redhead
E, Polhill RM (eds), Flora of tropical East Africa, Crown Agents for Oversea
Governments and Administrations, London, pp. 1-6.
Sankara Rao K. 1972. Studies in Myrsinaceae I. A contribution to the
embryology of Maesa dubia Wall. – Proc. Indian Acad. Sci., Sect. B,
75: 160-166.
Saraiva L, Cesar O, Monteiro R. 1988.
Biologia da polinização e sistema de reprodução de Styrax camporum
Pohl e S. ferrugineus Nees et Mart. (Styracaceae). – Rev. Brasil. Bot. 11:
71-80.
Sarkar AK. 1988. Primulaceae – its evolution and
assessment in status as judged through cytotaxonomy. – Feddes Repert. 99:
113-132.
Sarwar AKMG, Takahashi H. 2006a. Pollen
morphology of Enkianthus (Ericaceae) and its taxonomic significance.
– Grana 45: 161-174.
Sarwar AKMG, Takahashi H. 2006b. The
taxonomic significance of pollen morphology in Andromedeae s.s., Gaultherieae,
Lyonieae and Oxydendreae (Ericaceae).
– Jap. J. Palyn. 52: 77-96.
Sarwar AKMG, Takahashi H. 2008. An overview
of pollen morphology and its systematic significance in tribe Vaccinieae
(Vaccinioideae: Ericaceae). – Jap. J.
Palyn. 53: 87-104.
Sarwar AKMG, Takahashi H. 2009. Pollen
morphology and systematics in two subfamilies of Ericaceae: Cassiopoideae and
Harrimanelloideae. – Bangladesh J. Plant Taxon. 16: 37-46.
Sarwar AKMG, Takahashi H. 2014. Pollen
morphology of Erica L. and related genera and its taxonomic
significance. – Grana 53: 221-231.
Sarwar AKMG, Ito T, Takahashi H. 2006a.
Pollen of Ceratostema (Ericaceae, Vaccinieae): tetrads without
septa. – J. Plant Res. 119: 685-688.
Sarwar AKMG, Ito T, Takahashi H. 2006b. An
overview of pollen morphology and its relevance to the sectional classification
of Vaccinium (Ericaceae). –
Jap. J. Palyn. 52: 15-34.
Sarwar AKMG, Ito T, Takahashi H. 2008. An
overview of pollen morphology in subfamily Arbutoideae (Ericaceae), and its systematic
significance. – Jap. J. Palyn. 54: 79-92.
Sattler R. 1962. Zur frühen Infloreszenz-
und Blütenentwicklung der Primulales sensu lato mit besonderer
Berücksichtigung der Stamen-Petalum-Entwicklung. – Bot. Jahrb. Syst. 81:
385-396.
Sauer H. 1933. Blüte und Frucht der
Oxalidaceen, Linaceen, Geraniaceen, Tropaeolaceen und Balsaminaceen.
Vergleichend-entwicklungsgeschichtliche Untersuchungen. – Planta 19:
417-481.
Saunders DE. 1975. Cyclamen, the
genus in the wild and in cultivation. Rev. by R. D. Meikle and C. Grey-Wilson.
– Alpine Garden Society, Woking.
Sax JH. 1960. Polyploidy in
Enkianthus (Ericaceae). – J.
Arnold Arbor. 41: 191-196.
Sazima M, Sazima I. 1980. Bat visits to
Marcgravia myriostigma Tr. et Pl. (Marcgraviaceae) in southeastern
Brazil. – Flora 169: 84-88.
Sazima I, Buzato S, Sazima M. 1993. The
bizarre inflorescence of Norantea brasiliensis (Marcgraviaceae): visits of hovering
and perching birds. – Bot. Acta 106: 507-513.
Schadel WE. 1978. Leaf anatomy and venation
patterns of the Styracaceae. –
Ph.D. diss., Master of Arts, University of North Carolina at Chapel Hill.
Schadel WE, Dickison WC. 1979. Leaf anatomy
and venation patterns of Styracaceae.
– J. Arnold Arbor. 60: 8-37.
Schaeppi H. 1934. Untersuchungen über die
Narben- und Antherenstellung in den Blüten der Primulaceen. – Arch. Julius
Klaus-Stift. Vererbungsforsch. Sozialanthropol. Rassenhyg. Zürich 9:
133-236.
Schaeppi H. 1937. Vergleichend-morphologische
Untersuchungen am Gynoeceum der Primulaceen. – Zeitschr. Gesamte Naturwiss.
(Brunswick) 3: 239-250.
Schemske DW. 1978. Evolution of reproductive
characteristics in Impatiens (Balsaminaceae): the significance of
cleistogamy and chasmogamy. – Ecology 59: 596-613.
Schemske DW. 1984. Population structure and
local selection in Impatiens pallida (Balsaminaceae), a selfing annual. –
Evolution 38: 817-832.
Schierenbeck KA, Stebbins GL, Patterson RW.
1992. Morphological and cytological evidence for polyphyletic allopolyploidy in
Arctostaphylos mewukka (Ericaceae). – Plant Syst. Evol. 179:
187-205.
Schlichting CD, Levin DA. 1986. Effects of
inbreeding on phenotypic plasticity in cultivated Phlox. – Theor.
Appl. Genet. 72: 114-119.
Schmid M. 2006. Contribution à la
connaissance des Myrsinaceae de
Nouvelle-Calédonie I. Le genre Maesa Forssk. – Adansonia, sér.
III, 28: 143-148.
Schmid M. 2009. Contribution à la
connaissance des Primulaceae (ex Myrsinaceae) de Nouvelle-Calédonie II.
Le genre Rapanea Aubl. – Adansonia, sér. III, 31: 341-395.
Schmid M. 2012. Contribution à la
connaissance des Primulaceae (ex Myrsinaceae) de Nouvelle-Calédonie III.
Les genres Tapeinosperma Hook. f. et Mangenotiella gen. nov.
– Adansonia 34: 279-341.
Schmid R. 1978a. Reproductive anatomy of
Actinidia chinensis (Actinidiaceae). – Bot. Jahrb. Syst.
100: 149-195.
Schmid R. 1978b. Actinidiaceae, Davidiaceae, and Paracryphiaceae:
systematic considerations. – Bot. Jahrb. Syst. 100: 196-204.
Schnarf K. 1924. Bemerkungen zur Stellung der
Gattung Saurauia im System. – Sitzungsber. K. Akad. Wiss. Wien,
Math.-Naturw. Kl., Abt. 1, 133: 17-28.
Schneeweiss GM, Schönswetter P, Kelso S,
Niklfeld H. 2004. Complex biogeographic patterns in Androsace (Primulaceae) and related genera:
evidence from phylogenetic analyses of nuclear internal transcribed spacer and
plastid trnL-F sequences. – Syst. Biol. 53: 856-876.
Schneider EL, Carlquist SJ. 2003. Unusual pit
membrane remnants in perforation plates of Cyrillaceae. – J. Torrey Bot. Club
130: 225-230.
Schneider EL, Carlquist SJ. 2004. Perforation
plate pit remnants in vessels of Sarraceniaceae: possible indicators
of relationship and ecology. – J. Torrey Bot. Club 131: 1-7.
Schneider JV, Bayer C. 2004. Clethraceae. – In: Kubitzki K (ed),
The families and genera of vascular plants VI. Flowering plants. Dicotyledons.
Celastrales,
Oxalidales, Rosales, Cornales, Ericales, Springer, Berlin, Heidelberg, New
York, pp. 69-73.
Schneider JV, Paule J, Gitaí J, Dressler S,
Gusmão CLS, Benko-Iseppon AM. 2015. Divergent genome sizes reflect the
infrafamilial subdivision of the neotropical woody Marcgraviaceae. – Bot. J. Linn.
Soc. 177: 1-14.
Schnell D. 1977. Infraspecific variations in
Sarracenia rubra Walt.: some observations. – Castanea 42:
149-170.
Schnell D. 1978. Sarracenia L. petal
extract chromatography. – Castanea 43: 107-115.
Schnell D. 1980. Notes on the biology of
Sarracenia oreophila (Kearney) Wherry. – Castanea 45: 166-170.
Schnell D. 1983. Notes on the pollination of
Sarracenia flava L. (Sarraceniaceae) in the Piedmont
Province of North Carolina. – Rhodora 85: 405-420.
Schnell D, Krider DM. 1976. Cluster analysis
of the genus Sarracenia L. in the southeastern United States. –
Castanea 41: 165-170.
Schnopf E, Deichgraber G. 1983. Structure and
formation of fibrillar mucilages in seed epidermal cells I. Collomia
grandiflora (Polemoniaceae).
– Protoplasma 114: 210-221.
Schoen DJ. 1982. The breeding system of
Gilia achilleifolia: variation in floral characteristics and
outcrossing rate. – Evolution 36: 352-360.
Schönenberger J. 2009. Comparative floral
structure and systematics of Fouquieriaceae and Polemoniaceae (Ericales). – Intern. J. Plant Sci. 170:
1132-1167.
Schönenberger J, Friis EM. 2001. Fossil
flowers of ericalean affinity from the Late Cretaceous of southern Sweden. –
Amer. J. Bot. 88: 467-480.
Schönenberger J, Grenhagen A. 2005. Early
floral development and androecium organization in Fouquieriaceae (Ericales). – Plant Syst. Evol. 254:
233-249.
Schönenberger J, Anderberg AA, Sytsma KJ.
2005. Molecular phylogenetics and patterns of floral evolution in the Ericales. – Intern. J. Plant Sci. 166:
265-288.
Schönenberger J, Balthazar M von, Sytsma KJ.
2010. Diversity and evolution of floral structure among early diverging
lineages in the Ericales. – Philos.
Trans. Roy. Soc. London, B, Biol. Sci. 365: 437-448.
Schönenberger J, Barthazar M von, Takahashi
M, Xioao X, Crane PR, Herendeen PS. 2012. Glandulocalyx upatoiensis, a
fossil flower of Ericales (Actinidiaceae/Clethraceae) from the Late Cretaceous
(Santonian) of Georgia, U.S.A. – Ann. Bot. 109: 921-936.
Schultheis LM, Baldwin BG. 1999. Molecular
phylogenetics of Fouquieriaceae:
evidence from nuclear rDNA ITS studies. – Amer. J. Bot. 86: 578-589.
Schulze W, Schulze ED, Pate JS, Gillison AN.
1997. The nitrogen supply from soils and insects during growth of the pitcher
plants Nepenthes mirabilis, Cephalotus follicularis and
Darlingtonia californica. – Oecologia 112: 464-471.
Schumann D, Kirsten G. 1992. Ericas of South
Africa. – Timber Press, Portland, Oregon.
Schürhoff PN. 1931. Die Haploidgeneration
der Balsaminaceae und ihre
Bedeutung für die Systematik. – Bot. Jahrb. Syst. 64: 324-356.
Schwarz O. 1938. Cyclamen-Studien.
– Gartenflora, N. F., pp. 11-38.
Schwarz O. 1955. Systematische Monographie
der Gattung Cyclamen L. 1. – Feddes Repert. 58: 234-283.
Schwarz O. 1963. Die Gattung
Vitaliana Sesl. und ihre Stellung innerhalb der Primulaceen. –
Feddes Repert. 67: 16-41.
Schwarz O. 1964. Systematische Monographie
der Gattung Cyclamen L. 2. – Feddes Repert. 69: 79-103.
Schwarz O. 1968. Beiträge zur Kenntnis der
Gattung Primula. – Wissensch. Zeitschr. Friedrich-Schiller-Univ.
Jena, Math.-Naturwiss. Reihe, Beitr. Phytotaxon. 17: 307-322.
Schweiger J. 1909. Vergleichende
Untersuchungen über Sarracenia und Cephalotus follicularis
betreffs ihrer etwaigen systematischen Verwandtschaft. – Beih. Bot.
Centralbl. 25: 490-539.
Scogin R. 1977. Anthocyanins of the Fouquieriaceae. – Biochem. Syst.
Ecol. 5: 265-267.
Scogin R. 1978. Leaf phenolics of the Fouquieriaceae. – Biochem. Syst.
Ecol. 6: 297-298.
Scott FM. 1932. Some features of the anatomy
of Fouquieria splendens. – Amer. J. Bot. 19: 673-678.
Scott PE, Buchmann SL, O’Rourke MK. 1993.
Evidence for mutualism between a flower-piercing carpenter bee and ocotillo:
use of pollen and nectar by nesting bees. – Ecol. Entomol. 18: 234-240.
Scott PJ. 2004. Diapensiaceae. – In: Kubitzki K
(ed), The families and genera of vascular plants VI. Flowering plants.
Dicotyledons. Celastrales,
Oxalidales, Rosales, Cornales, Ericales, Springer, Berlin, Heidelberg, New
York, pp. 117-121.
Scott PJ, Day RT. 1983. Diapensiaceae: a review of the
taxonomy. – Taxon 32: 417-423.
Sealy JR. 1958. A revision of the genus
Camellia. – Royal Horticultural Society, London.
Seithe A. 1980. Rhododendron hairs
and taxonomy. – In: Luteyn JL, O’Brien M (eds), Contributions toward a
classification of Rhododendron, New York Botanical Garden, New York,
pp. 89-115.
Selosse M-A, Setaro S, Glatard F, Richard F,
Urcelay C, Weiß M. 2007. Sebacinales are common mycorrhizal associates of Ericaceae. – New Phytol. 174:
864-878.
Sen S, Sen NK. 1954. Studies on the cytology
of Quisqualis indica and Mimusops elengi. – Sci. &
Culture 20: 244-245.
Setaro S, Weiß M, Oberwinkler F, Kottke I.
2006. Sebacinales form ectendomycorrhizas with Cavendishia nobilis, a
member of the Andean clade of Ericaceae, in the mountain rain forest of
southern Ecuador. – New Phytol. 169: 355-365.
Setaro S, Kottke I, Oberwinkler F. 2006.
Anatomy and ultrastructure of mycorrhizal associations of neotropical Ericaceae. – Mycol. Progr. 5:
243-254.
Sethi S Bala. 1965. Structure and development
of seed in Camellia sinensis (L.) O. Kuntze. – Proc. Indian Natl.
Inst. Sci., Sect. B, 31: 24-33.
Setoguchi H, Watanabe W, Maeda Y, Peng, C-I.
2008. Molecular phylogeny of the genus Pieris (Ericaceae) with special reference to
phylogenetic relationships of insular plants on the Ryukyu Islands. – Plant
Syst. Evol. 270: 217-230.
Shevock JR, Day AG. 1998. A new
Gilia (Polemoniaceae) from
limestone outcrops in the southern Sierra Nevada of California. – Madroño
45: 137-140.
Shimizu T. 1979. A comment on the limestone
flora of Thailand, with special reference to Impatiens. – Acta
Phytotaxon. Geobot. 30: 180-188.
Shimizu T, Utami N. 1997. Three new species
of Impatiens (Balsaminaceae) added to Flora
Malesiana. – Kew Bull. 52: 435-442.
Shreve F. 1906. The development and anatomy
of Sarracenia purpurea. – Bot. Gaz. 42: 107-126.
Shui Y-M, Zhang G-J, Zhou Z-K, Mo M-Z. 2002.
Sladenia integrifolia (Sladeniaceae), a new species from
China. – Novon 12: 539-542.
Shyamala R, Gulati N. 1981. Contribution to
the embryology of Symplocos cochinchinensis. – Indian J. Bot. 4:
229-235.
Shyamala R, Gulati N. 1986. Origin and
structure of integuments in Ebenales I. Symplocaceae. – Swamy Bot. Club 2:
113-117.
Simon L. 1975. Le gynécée de
l’Impatiens balsamina: Étude morphologique, ontogenique, et
tératologique. – Can. J. Bot. 53: 2361-2383.
Simpson BB, Neff JL, Seigler DL. 1983. Floral
biology and floral rewards of Lysimachia (Primulaceae). – Amer. Midl. Natur.
110: 249-256.
Simpson RB. 1994. Pitchers in trade: a
conservation review of the carnivorous genera of Sarracenia,
Darlingtonia, and Heliamphora. – Royal Botanic Gardens,
Kew.
Singh KG. 1974. Mycorrhiza in the Ericaceae with special reference to
Calluna vulgaris. – Svensk Bot. Tidskr. 68: 1-16.
Skallerup HR. 1953. The distribution of
Diospyros virginiana L. – Ann. Missouri Bot. Gard. 40: 211-225.
Sleumer H. 1934. Ericaceae americanae novae vel minus
cognitae I. – Notizbl. Bot. Gart. Berlin-Dahlem 12: 119-140.
Sleumer H. 1935. Revision der Gattung
Pernettya Gaud. – Notizbl. Bot. Gart. Berlin-Dahlem 12: 626-655.
Sleumer H. 1941. Vaccinioideen-Studien. –
Bot. Jahrb. Syst. 71: 375-510.
Sleumer H. 1959. Studien über die Gattung
Leucothoé D. Don. – Bot. Jahrb. Syst. 78: 435-480.
Sleumer H. 1964. Epacridaceae. – In:
Steenis CGGJ van (ed), Flora Malesiana I, 6, Noordhoff, Groningen.
Sleumer H. 1966-1967. Ericaceae. – In: Steenis CGGJ van (ed),
Flora Malesiana I, 6, Noordhoff, Leiden, pp. 469-914.
Sleumer H. 1967. Monographia Clethracearum
1-2. – Bot. Jahrb. Syst. 87: 36-175.
Sleumer H. 1971. Clethraceae. – In: Steenis CGGJ van
(ed), Flora Malesiana I, 7, Wolters-Noordhoff, Groningen, pp. 139-150.
Sleumer H. 1987. A revision of the genus
Maesa Forssk. (Myrsinaceae)
in New Guinea, the Moluccas, and the Solomon Islands. – Blumea 32: 39-65.
Sleumer H. 1988a. The genera
Discocalyx Mez, Fittingia Mez, Loheria Merr. and
Tapeinosperma Hook. f. (Myrsinaceae) in New Guinea. – Blumea
33: 81-107.
Sleumer H. 1988b. The genus
Conandrium Mez (Myrsinaceae). – Blumea 33: 109-113.
Sleumer H. 1988c. A revision of the genus
Ardisia Sw. (Myrsinaceae) in
New Guinea. – Blumea 33: 115-140.
Smedmark JEE, Anderberg AA. 2007.
Boreotropical migration explains hybridization between geographically distinct
lineages in the pantropical clade Sideroxyleae (Sapotaceae). – Amer. J. Bot. 94:
1491-1505.
Smedmark JEE, Swenson U, Anderberg AA. 2006.
Accounting for variation in substitution rates through time in Bayesian
phylogeny reconstruction of Sapotoideae (Sapotaceae). – Mol. Phylogen. Evol. 39:
706-721.
Smith AC. 1932. The American species of
Thibaudieae. – Contr. U.S. Natl. Herb. 28: 311-547.
Smith AC. 1933. The genera
Sphyrospermum and Disterigma. – Brittonia 1: 203-232.
Smith AC. 1936. Studies on South American
plants V. Additional notes on Thibaudieae. – Bull. Torrey Bot. Club 63:
307-316.
Smith AC. 1946. Studies of South American
plants XI. Noteworthy species of Hippocrateaceae and Vacciniaceae. – J.
Arnold Arbor. 27: 86-120.
Smith AC. 1950. Studies of South American
plants XII. – Contr. U.S. Natl. Herb. 29: 317-393.
Smith AC. 1952. Plants collected in Ecuador
by W. H. Camp – Vacciniaceae. – Mem. New York Bot. Gard. 8: 41-85.
Smith AC. 1973. Studies of Pacific island
plants XXV. The Myrsinaceae of the
Fijian region. – J. Arnold Arbor. 54: 1-41, 228-292.
Smith AC, Standley PC. 1932.
Schizocardia, a new genus of trees of the family Clethraceae. – Trop. Woods 32:
8-11.
Smith DM, Levin DA. 1967. Karyotypes of
eastern North American Phlox. – Amer. J. Bot. 54: 324-334.
Smith DM, Glennie CW, Harborne JB, Williams
CA. 1977. Flavonoid diversification in the Polemoniaceae. – Biochem. Syst.
Ecol. 5: 107-115.
Smith DM, Glennie CW, Harborne JB. 1982.
Flavonoid patterns in Leptodactylon and Linanthus. –
Biochem. Syst. Ecol. 10: 37-42.
Smith DW. 1969. A taximetric study of
Vaccinium in northeastern Ontario. – Can. J. Bot. 47: 1747-1759.
Smith GF, Lowe DB. 1977. Androsaces. –
Alpine Garden Society, Woking, England.
Smith WW, Fletcher HR. 1941. The genus
Primula: section Candelabra. – Trans. Roy. Soc. Edinb. 33:
122-181.
Smith WW, Fletcher HR. 1942a. The genus
Primula: sections Amethystina, Minutissima,
Bella, Muscarioides. – Trans. Roy. Soc. Edinb. 33:
209-294.
Smith WW, Fletcher HR. 1942b. The genus
Primula: section Nivales. – Trans. Roy. Soc. Edinb. 60:
563-627.
Smith WW, Fletcher HR. 1942c. The section
Soldanelloideae of the genus Primula. – Bot. J. Linn. Soc.
52: 321-335.
Smith WW, Fletcher HR. 1943a. The genus
Primula: sections Sikkimensis, Souliei and
Rotundifolia. – Trans. Roy. Soc. Edinb. 33: 431-487.
Smith WW, Fletcher HR. 1943b. The genus
Primula: section Farinosae. – Trans. Roy. Soc. Edinb. 61:
1-69.
Smith WW, Fletcher HR. 1944a. The genus
Primula: section Petiolares. – Trans. Roy. Soc. Edinb. 61:
271-314.
Smith WW, Fletcher HR. 1944b. The genus
Primula: sections Cortusoides, Malvacea,
Pycnoloba, Dryadifolia, Capitatae. – Trans. Roy.
Soc. Edinb. 34: 55-158.
Smith WW, Fletcher HR. 1946. The genus
Primula: sections Obconica, Sinensis,
Reinii, Pinnatae, Malacoides, Bullatae,
Carolinella, Grandis and Denticulata. – Trans.
Roy. Soc. Edinb. 61: 415-478.
Smith WW, Fletcher HR. 1948a. The genus
Primula: sections Cuneifolia, Floribundae,
Parryi, and Auricula. – Trans. Roy. Soc. Edinb. 61:
631-686.
Smith WW, Fletcher HR. 1948b. The genus
Primula: section Vernales. – Trans. Roy. Soc. Edinb. 34:
402-468.
Smith WW, Fletcher HR. 1950. Additions and
corrections to the genus Primula (up to and including 1949). –
Trans. Proc. Bot. Soc. Edinb. 35: 180-202.
Smith WW, Forrest G. 1928. The sections of
the genus Primula. – Notes Roy. Bot. Gard. Edinb. 16: 1-50.
Smith WW, Forrest G, Fletcher HR. 1977. The
genus Primula. – In: Cramer J, Swann HK (eds), Plant Monograph
Reprints No. 11, J. Cramer, Vaduz.
Smith-White S. 1948. A survey of chromosome
numbers in the Epacridaceae. – Proc. Linn. Soc. New South Wales 73: 37-56.
Smith-White S. 1955. Chromosome numbers and
pollen types in the Epacridaceae. – Aust. J. Bot. 3: 48-67.
Smith-White S. 1959. Pollen development
patterns in the Epacridaceae. A problem of cytoplasm-nucleus interaction. –
Proc. Linn. Soc. New South Wales 84: 8-35.
Soejarto DD. 1969. Aspects of reproduction in
Saurauia. – J. Arnold Arbor. 50: 180-196.
Soejarto DD. 1970. Saurauia species
and their chromosomes. – Rhodora 72(789): 81-93.
Soejarto DD. 1980. Revision of South American
Saurauia (Actinidiaceae).
– Fieldiana Botany, N. S., 2: 1-141.
Soejarto DD. 1982. 60. Actinidiaceae. – In: Harling G,
Sparre B (eds), Flora of Ecuador 17, Swedish Natural Science Research Council,
Stockholm, pp. 1-47.
Soejima A, Nagamasu H. 2004. Phylogenetic
analysis of Asian Symplocos (Symplocaceae) based on nuclear and
chloroplast DNA sequences. – J. Plant Res. 117: 199-207.
Soejima A, Nagamasu H, Ito M, Ono M. 1994.
Allozyme diversity and the evolution of Symplocos (Symplocaceae) on the Bonin (Ogasawara)
Islands. – J. Plant Res. 107: 221-228.
Soltis DE, Bohm BA, Nesom GL. 1983. Flavonoid
chemistry of cytotypes in Galax (Diapensiaceae). – Syst. Bot. 8:
15-23.
Song Y, Yuan Y-M, Kupfer P. 2003. Chromosomal
evolution in Balsaminaceae, with
cytological observations on 45 species from Southeast Asia. – Caryologia 56:
463-481.
Song Y, Yuan Y-M, Kupfer P. 2005. Seedcoat
micromorphology of Impatiens (Balsaminaceae) from China. – Bot. J.
Linn. Soc. 149: 195-208.
Sørensen PD. 1995. Arbutus
Linnaeus. – In: Luteyn JL (ed), Flora Neotropica. Monograph 66. Ericaceae II: the superior-ovaried genera,
New York Botanical Garden, New York, pp. 194-221.
Solthers CA. 2003. New species of
Diospyros (Ebenaceae) from
Brazil. – Kew Bull. 58: 473-477.
Souèges R. 1937. Embryogénie des
Primulacées: développement de l’embryon chez le Samolus Valerandi
L. – Compt. Rend. Hebd. Séances Acad. Sci. 204: 145-147.
Southall RM, Hardin JW. 1974. A taxonomic
revision of Kalmia (Ericaceae). – J. Elisha Mitchell Sci.
Soc. 90: 1-23.
Spanowsky W. 1962. Die Bedeutung der
Pollenmorphologie für die Taxonomie der Primulaceae-Primuloideae. – Feddes
Repert. 65: 149-214.
Spencer S, Porter J. 1997. Evolutionary
diversification and adaptation to novel environments in Navarretia (Polemoniaceae). – Syst. Bot. 22:
649-668.
Spongberg SA. 1974. A review of
deciduous-leaved species of Stewartia (Theaceae). – J. Arnold Arbor. 55:
182-214.
Spongberg SA. 1976. Styracaceae hardy in temperate North
America. – J. Arnold Arbor. 57: 54-73.
Stace HM, Fripp YJ. 1977. Raciation in
Epacris impressa III. Polymorphic populations. – Aust. J. Bot. 25:
325-336.
Stace HM, Chapman AR, Lemson KL, Powell JM.
1997. Cytoevolution, phylogeny and taxonomy in Epacridaceae. – Ann. Bot., N.
S., 79: 283-290.
Ståhl B. 1987. The genus
Theophrasta (Theophrastaceae). Foliar structures,
floral biology and taxonomy. – Nord. J. Bot. 7: 529-538.
Ståhl B. 1989. A synopsis of Central
American Theophrastaceae. –
Nord. J. Bot. 9: 15-30.
Ståhl B. 1990a. Taxonomic studies in the Theophrastaceae. – Ph.D. diss,
Department of Systematic Botany, University of Gothenburg, Sweden.
Ståhl B. 1990b. 149. Theophrastaceae, 150. Primulaceae. – In: Harling G,
Andersson L (eds), Flora of Ecuador 39, Nord. J. Bot., Copenhagen, pp. 1-35.
Ståhl B. 1991a. A revision of
Clavija (Theophrastaceae). – Opera Bot.
107: 1-77.
Ståhl B. 1991b. 155. Symplocaceae. – In: Harling G,
Andersson L (eds), Flora of Ecuador 43, Nord. J. Bot., Copenhagen, pp. 1-44.
Ståhl B. 1992a. On the identity of
Jacquinia armillaris (Theophrastaceae) and related
species. – Brittonia 44: 54-60.
Ståhl B. 1992b. 146B. Cyrillaceae. – In: Harling G,
Andersson L (eds), Flora of Ecuador 45, Nord. J. Bot., Copenhagen, pp.
29-34.
Ståhl B. 1993. Votschia, a new
genus of Theophrastaceae from
northeastern Panama. – Brittonia 45: 204-207.
Ståhl B. 1995a. A synopsis of
Jacquinia (Theophrastaceae) in the Antilles and
South America. – Nord. J. Bot. 15: 493-511.
Ståhl B. 1995b. Diversity and distribution
of Andean Symplocaceae. – In:
Churchill SP, Balslev H, Forero H, Luteyn JL (eds), Biodiversity and
conservation of neotropical montane forests, The New York Botanical Garden,
Bronx, New York, pp. 397-405.
Ståhl B. 1996. The relationships of
Heberdenia bahamensis and H. penduliflora (Myrsinaceae). – Bot. J. Linn. Soc.
122: 314-333.
Ståhl B. 2004a. Samolaceae. – In: Kubitzki K (ed), The
families and genera of vascular plants VI. Flowering plants. Dicotyledons. Celastrales,
Oxalidales, Rosales, Cornales, Ericales, Springer, Berlin, Heidelberg, New
York, pp. 387-389.
Ståhl B. 2004b. Theophrastaceae. – In: Kubitzki K
(ed), The families and genera of vascular plants VI. Flowering plants.
Dicotyledons. Celastrales,
Oxalidales, Rosales, Cornales, Ericales, Springer, Berlin, Heidelberg, New
York, pp. 472-478.
Ståhl B. 2010. Flora Neotropica 105. Theophrastaceae. – The New York
Botanical Garden, Bronx, New York.
Ståhl B, Anderberg AA. 2004a. Maesaceae. – In: Kubitzki K (ed), The
families and genera of vascular plants VI. Flowering plants. Dicotyledons. Celastrales,
Oxalidales, Rosales, Cornales, Ericales, Springer, Berlin, Heidelberg, New
York, pp. 255-257.
Ståhl B, Anderberg AA. 2004b. Myrsinaceae. – In: Kubitzki K (ed),
The families and genera of vascular plants VI. Flowering plants. Dicotyledons.
Celastrales,
Oxalidales, Rosales, Cornales, Ericales, Springer, Berlin, Heidelberg, New
York, pp. 266-281.
Standley PC. 1914. A revision of the genus
Cobaea. – Contr. U.S. Natl. Herb. 17: 448-458.
Stearn WT. 1968. Jamaican and other species
of Bumelia (Sapotaceae). –
J. Arnold Arbor. 49: 280-289.
Stearn WT. 1969. A synopsis of Jamaican Myrsinaceae. – Bull. Brit. Mus. (Nat.
Hist.) Bot. 4: 145-178.
Stearn WT. 1992. The genus Jacquinia
(Theophrastaceae) in Jamaica. –
Nord. J. Bot. 12: 231-238.
Steele KP, Vilgalys R. 1994. Phylogenetic
analyses of Polemoniaceae using
nucleotide sequences of the plastid gene matK. – Syst. Bot. 19:
126-142.
Steenis CGGJ van. 1932. The Styracaceae of Netherlands India. –
Bull. Jard. Bot. Buitenzorg, sér. III, 12: 212-272.
Steenis CGGJ van. 1949. Styracaceae. – In: Steenis CGGJ van
(ed), Flora Malesiana I, 4(2), Noordhoff-Kolff N. V., Batavia, pp. 49-56.
Steenis CGGJ van. 1956a. Pentaphylacaceae. – In: Steenis
CGGJ van (ed), Flora Malesiana I, 5(2), Noordhoff-Kolff N. V., Djakarta, pp.
121-124.
Steffen K. 1951. Zur Kenntnis des
Befruchtungsvorganges bei Impatiens glandulifera Lindl. – Planta 39:
175-244.
Stephens JD, Rogers WL, Heyduk K,
Cruse-Sanders JM, Determann RO, Glen TC, Malmberg RL. 2015. Resolving
phylogenetic relationships of the recently radiated carnivorous plant genus
Sarracenia using target enrichment. – Mol. Phylogen. Evol. 85:
76-87.
Stevens NE. 1911. Dioecism in trailing
arbutus, with notes on the morphology of the seed. – Bull. Torrey Bot. Club
38: 531-543.
Stevens NE. 1919. The development of the
endosperm in Vaccinium corymbosum. – Bull. Torrey Bot. Club 46:
465-468.
Stevens PF. 1969. Taxonomic studies in the Ericaceae. – Ph.D. diss., University of
Edinburgh, Scotland.
Stevens PF. 1970a. Agauria and
Agarista: an example of tropical transatlantic affinity. – Notes
Roy. Bot. Gard. Edinb. 30: 341-359.
Stevens PF. 1970b. Calluna,
Cassiope and Harrimanella: a taxonomic and evolutionary
problem. – New Phytol. 69: 1131-1148.
Stevens PF. 1971. A classification of the Ericaceae: subfamilies and tribes. –
Bot. J. Linn. Soc. 64: 1-53.
Stevens PF. 1972. Notes on the infrageneric
classification of Agapetes with four new taxa from New Guinea. –
Notes Roy. Bot. Gard. Edinb. 32: 13-28.
Stevens PF. 1974. Circumscription and
relationships of Dimorphanthera (Ericaceae) and notes on some Papuasian
species. – Contr. Herb. Austral. 8: 1-34.
Stevens PF. 1976. The altitudinal and
geographical distribution of flower types in Rhododendron section
Vireya, especially in the Papuasian species, and their significance.
– Bot. J. Linn. Soc. 72: 1-33.
Stevens PF. 1982. Phytogeography and
evolution of the Ericaceae of New
Guinea. – In: Gressitt JL (ed), Biogeography and ecology of New Guinea,
Monogr. Biol. 42: 331-354.
Stevens PF. 1985. Notes on Vaccinium
and Agapetes (Ericaceae) in
Southeast Asia. – J. Arnold Arbor. 66: 471-490.
Stevens PF. 1995. Familial and interfamilial
relationships. – In: Luteyn JL (ed), Flora Neotropica. Monograph 66. Ericaceae II – the superior-ovaried
genera, New York Botanical Garden, Bronx, New York, pp. 1-12.
Stevens PF, Weitzman AL. 2004. Sladeniaceae. – In: Kubitzki K (ed),
The families and genera of vascular plants VI. Flowering plants. Dicotyledons.
Celastrales,
Oxalidales, Rosales, Cornales, Ericales, Springer, Berlin, Heidelberg, New
York, pp. 431-433.
Stevens PF, Luteyn J, Oliver EGH, Bell TL,
Brown EA, Crowden RK, George AS, Jordan GJ, Ladd P, Lemson K, McLean CB,
Menadue Y, Pate JS, Stace HM,Weiller CM. 2004. Ericaceae. – In: Kubitzki K (ed), The
families and genera of vascular plants VI. Flowering plants. Dicotyledons. Celastrales,
Oxalidales, Rosales, Cornales, Ericales, Springer, Berlin, Heidelberg, New
York, pp. 145-194.
Stevens PF, Dressler S, Weitzman AL. 2004. Theaceae. – In: Kubitzki K (ed), The
families and genera of vascular plants VI. Flowering plants. Dicotyledons. Celastrales,
Oxalidales, Rosales, Cornales, Ericales, Springer, Berlin, Heidelberg, New
York, pp. 463-471.
Stierle DB, Stierle AA, Larsen RD. 1988.
Terpenoid and flavone constituents of Polemonium viscosum. –
Phytochemistry 27: 517-522.
Stone BC. 1982. New and noteworthy Malaysian
Myrsinaceae I. – Mal. For. 45:
101-121.
Stone BC. 1988a. Notes on the genus
Labisia Lindl. (Myrsinaceae). – Malayan Nat. J. 42:
43-51.
Stone BC. 1988b. New and noteworthy Malesian
Myrsinaceae II. Emblemantha,
a new genus from Sumatra. – Proc. Acad. Nat. Sci. Philadelphia 141:
263-306.
Stone BC. 1989. New and noteworthy Malesian
Myrsinaceae III. On the genus
Ardisia Sw. in Borneo. – Proc. Acad. Nat. Sci. Philadelphia 141:
263-306.
Stone BC. 1990. Studies in Malesian Myrsinaceae V. Additional new species of
Ardisia Sw. – Proc. Acad. Nat. Sci. Philadelphia 142: 21-58.
Stone BC. 1991a. The genus Loheria
Merrill (Myrsinaceae). –
Micronesica 24: 65-80.
Stone BC. 1991b. New and noteworthy Malesian
Myrsinaceae VI. Revision of the genus
Hymenandra A. DC. – Gard. Bull. (Singapore) 43: 1-17.
Stone BC. 1992. Systellantha, a new
genus of Myrsinaceae from Borneo. –
Malayan Nat. J. 46: 13-24.
Stone BC. 1993a. Reduction of the genus
Parardisia (Myrsinaceae).
– Nord. J. Bot. 13: 55-57.
Stone BC. 1993b. New and noteworthy Malesian
Myrsinaceae VII. Sclerantha,
a new subgenus of Ardisia. – Pac. Sci. 47: 276-294.
Stone BC, Whitmore TC. 1970. Notes on they
systematy of Solomon Islands’ plants and some of their New Guinea relatives
XI. Tapeinosperma (Myrsinaceae). – Reinwardtia 8:
3-11.
Strand AE, Wyatt R. 1991. Geographical
variation and biosystematics of sand myrtle, Leiophyllum buxifolium
(Ericaceae). – Syst. Bot. 16:
529-545.
Streiber N, Brown EA, Conn BJ, Quinn CJ.
1999. Systematic studies in Dracophyllum (Epacridaceae) 1.
Morphometric analyses of Dracophyllum secundum sensu lato. – Telopea
8: 381-391.
Stride G, Nylinder S, Swenson U. 2014.
Revisiting the biogeography of Sideroxylon (Sapotaceae) and an evaluation of the
taxonomic status of Argania and Spiniluma. – Aust. Syst.
Bot. 27: 104-118.
Stuchlik L. 1967a. Pollen morphology and
taxonomy of the family Polemoniaceae. – Rev. Palaeobot.
Palyn. 4: 325-333.
Stuchlik L. 1967b. Pollen morphology in the
Polemoniaceae. – Grana Palynol.
7: 146-240.
Stushnoff C, Palser BF. 1969 [1970].
Embryology of five Vaccinium taxa including diploid, tetraploid, and
hexaploid species or cultivars. – Phytomorphology 19: 312-331.
Su Y, Liao W, Wang T, Sun Y, Wei Q, Chang H.
2011. Phylogeny and evolutionary divergence times in Apterosperma and
Euryodendron: evidence of a Tertiary origin in southern China. –
Biochem. Syst. Ecol. 39: 769-777.
Subramanyam K, Narayana LL. 1968. Floral
anatomy and embryology of Primula floribunda Wall. – Phytomorphology
18: 105-113.
Subramanyam K, Narayana LL. 1976. A
contribution to the floral anatomy and embryology in certain members of Primulaceae. – J. Indian Bot. Soc. 55:
274-282.
Subramaniam SS, Nair AGR. 1973. Myricetin and
quercetin glycosides from the leaves of four sapotaceous species. – Curr.
Sci. (India) 42: 746-747.
Sugawara T, Akiyama S, Murata J, Yang Y-P.
1995. Karyology of ten species of Impatiens (Balsaminaceae) from SW Yunnan, China.
– Acta Phytotaxon. Geobot. 45: 119-125.
Sugawara T, Akiyama S, Yang Y-P, Murata J.
1997. Karyological characteristics of Impatiens (Balsaminaceae) in Yunnan, China. –
Acta Phytotaxon. Geobot. 48: 7-14.
Sugden EA. 1986. Anthecology and pollinator
efficacy of Styrax officinale subsp. redivivum (Styracaceae). – Amer. J. Bot. 73:
919-930.
Sugiyama M. 1991. Scanning electron
microscopy observation on early ontogeny of the flower of Camellia
japonica L. – J. Jap. Bot. 66: 295-299.
Sugiyama M. 1997. Floral anatomy of
Camellia japonica (Theaceae).
– J. Plant Res. 110: 45-54.
Sundberg MD. 1982a. Floral ontogeny in
Cyclamen persicum, F-1 Rosemunde Rose’ (Primulaceae). – Amer. J. Bot. 69:
380-388.
Sundberg MD. 1982b. Petal-stamen initiation
in the genus Cyclamen (Primulaceae). – Amer. J. Bot. 69:
1707-1709.
Svengsuksa BKK, Vidal JE. 1992. Styracaceae. – In: Morat P (ed), Flore
du Cambodge, du Laos et du Viêtnam 26, Mus. Natl. Hist. Nat. Paris, pp.
145-195.
Swamy BGL. 1948. A contribution to the
embryology of the Marcgraviaceae.
– Amer. J. Bot. 35: 628-633.
Swenson U, Anderberg AA. 2005. Phylogeny,
character evolution, and classification of Sapotaceae (Ericales). – Cladistics 21: 101-130.
Swenson U, Munzinger JAA. 2009. Revision of
Pycnandra subgenus Pycnandra (Sapotaceae), a genus endemic to New
Caledonia. – Aust. Syst. Bot. 22: 437-465.
Swenson U, Munzinger JAA. 2010a. Revision of
Pycnandra subgenus Achradotypus (Sapotaceae), with five new species from
New Caledonia. – Aust. Syst. Bot. 23: 185-216.
Swenson U, Munzinger JAA. 2010b. Taxonomic
revision of Pycnandra subgenus Trouettia (Sapotaceae), with six new species from
New Caledonia. – Aust. Syst. Bot. 23: 333-370.
Swenson U, Munzinger JAA. 2010c. Revision of
Pycnandra subgenus Sebertia (Sapotaceae) and a generic key to the
family in New Caledonia. Adansonia, sér. 3: 239-249.
Swenson U, Munzinger JAA. 2012. Revision of
Pichonia (Sapotaceae) in New
Caledonia. – Aust. Syst. Bot. 25: 31-48.
Swenson U, Munzinger JAA. 2016. Five new
species and a systematic synopsis of Pycnandra (Sapotaceae), the largest endemic genus in
New Caledonia. – Aust. Syst. Bot. 29: 1-40.
Swenson U, Bartisch IV, Munzinger JAA. 2007.
Phylogeny, diagnostic characters and generic limitation of Australasian
Chrysophylloideae (Sapotaceae, Ericales): evidence from ITS sequence data
and morphology. – Cladistics 23: 201-228.
Swenson U, Munzinger JAA, Bartisch IV. 2007.
Molecular phylogeny of Planchonella (Sapotaceae) and eight new species from
New Caledonia. – Taxon 56: 329-354.
Swenson U, Lowry II PP, Munzinger JAA, Rydin
C, Bartish IV. 2008. Phylogeny and generic limits in the Niemeyera
complex of New Caledonian Sapotaceae:
evidence of multiple origins of the anisomerous flower. – Mol. Phylogen.
Evol. 49: 909-929.
Swenson U, Richardson JE, Bartish IV. 2008.
Multi-gene phylogeny of the pantropical subfamily Chrysophylloideae (Sapotaceae): evidence of generic
polyphyly and extensive morphological homoplasy. – Cladistics 24:
1006-1031.
Swenson U, Nylinder S, Munzinger JAA. 2013.
Towards a natural classification of Sapotaceae subfamily Chrysophylloideae in
Oceania and Southeast Asia based on nuclear sequence data. – Taxon 62:
746-770.
Swenson U, Nylinder S, Munzinger JAA. 2014.
Sapotaceae biogeography supports New
Caledonia being an old Darwinian island. – J. Biogeogr. 41: 797-809.
Swenson U, Munzinger JAA, Lowry II PP,
Cronholm B, Nylinder S. 2015. Island life – classification, speciation and
cryptic species of Pycnandra (Sapotaceae) in New Caledonia. – Bot. J.
Linn. Soc. 179: 57-77.
Swenson U, Nylander JAA, Munzinger J. 2018.
Phylogeny, species delimitation and revision of Pleioluma (Sapotaceae) in New Caledonia, a
frequently gynodioecious genus. – Aust. Syst. Bot. 31: 120-165.
Szkudlarz P. 2009. Variation in seed
morphology in the genus Erica L. (Ericaceae). – Biodiv. Res. Conserv. 16:
1-105.
Szyszyłowicz I von. 1895a. Marcgraviaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien III(6), W. Engelmann,
Leipzig, pp. 157-164.
Szyszyłowicz I von. 1895b. Theaceae (Ternstroemiaceae). – In: Engler
A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(6), W. Engelmann,
Leipzig, pp. 175-192.
Taaffe G, Brown EA, Crayn DM, Gadek PA, Quinn
CJ. 2001. Generic concepts in Styphelieae: resolving the limits of
Leucopogon. – Aust. J. Bot. 49: 107-120.
Takahashi H. 1986a. Pollen morphology of
Pyrola and its taxonomic significance. – Bot. Mag. (Tokyo) 99:
137-154.
Takahashi H. 1986b. Pollen polyads and their
variation in Chimaphila (Pyrolaceae). – Grana 25: 161-169.
Takahashi H. 1987. Pollen morphology and its
taxonomic significance in the Monotropoideae (Ericaceae). – Bot. Mag. (Tokyo) 100:
385-405.
Takahashi H. 1988. Pollen morphology and
systematics in two subfamilies of the Ericaceae: Pyroloideae and Monotropoideae.
– Korean J. Plant Tax. 18: 9-17.
Takahashi H. 1993. Seed morphology and its
systematic implications in Pyroloideae (Ericaceae). – Int. J. Plant Sci. 154:
175-186.
Takahashi H, Sohma K. 1980. Pollen
development in Pyrola japonica Klenze. – Sci. Rep. Tohoku Univ.,
Ser. IV (Biology) 38: 57-71.
Takasi Y. 1972. Embryological studies in
Ebenales 4. – J. Jap. Bot. 47: 20-28.
Tam P-C. 1986. A new species of genus
Enkianthus. – Bull. Bot. Res. North-East. Forest. Inst. 6:
125-127.
Tamura S, Hiura T. 1998. Proximate factors
affecting fruit set and seed mass of Styrax obassia in a masting year.
– Ecoscience 5: 100-107.
Tanaka R, Oginuma K. 1980. Karyomorphologal
studies on Clethra barbinervis and two allied species. – J. Jap.
Bot. 55: 65-72.
Tanrisever N, Fronczek FR, Fischer NH,
Williamson GB. 1987. Ceratiolin and other flavonoids from Ceratiola
ericoides. – Phytochemistry 26: 175-179.
Taton A. 1979. Contribution à l’étude du
genre Ardisia Sw. (Myrsinaceae) en Afrique tropicale. –
Bull. Jard. Bot. Natl. Belg. 49: 81-120.
Taylor DW. 1989. Select palynomorphs from the
middle Eocene Claiborne formation. – Tennessee (U.S.A.), Rev. Palaeobot.
Palynol. 58: 111-128.
Taylor P. 1955. The genus Anagallis
in tropical and South Africa. – Kew Bull. 10: 321-350.
Taylor P. 1958a. Primulaceae. – In: Hubbard CE,
Milne-Redhead E (eds), Flora of tropical East Africa, Crown Agents for Oversea
Governments and Administrations, London, pp. 1-20.
Taylor P. 1958b. Tropical African Primulaceae. – Kew Bull. 13:
133-149.
Taylor TN, Levin DA. 1975. Pollen morphology
of Polemoniaceae in relation to
systematics and pollination systems: scanning electron microscopy. – Grana
15: 91-112.
Tedersoo L, Pellet P, Kõljalg U, Selosse
M-A. 2007. Parallel evolutionary paths to mycoheterotrophy in understorey Ericaceae and Orchidaceae:
ecological evidence for mixotrophy in Pyroleae. – Oecologia 151: 206-217.
Teillier S, Escobar F. 2013. Revisión del
género Gaultheria L. (Ericaceae) en Chile. – Gayana Bot. 70:
136-153.
Telford IR. 1992. Budawangia and
Rupicola, new and revised genera of Epacridaceae. – Telopea 5:
229-239.
Temple A. 1975. Ericaceae: étude architecturale de
quelques espèces. – Montpellier.
Terekhin ES. 1963. the development of the
ovule and the female gametophyte in Pyroleae and Monotropeae. – Bot. Žurn.
48: 406-414. [In Russian]
Terra-Araujo MH, Faria AD, Ribeiro JELS,
Swenson U. 2012. Flower biology and subspecies concepts in Micropholis
guyanensis (Sapotaceae): evidence
of ephemeral flowers in the family. – Aust. Syst. Bot. 25: 295-303.
Terra-Araujo MH, Faria AD de, Vicentini A,
Nylinder S, Swenson U. 2015. Species tree phylogeny and biogeography of the
Neotropical genus Pradosia (Sapotaceae, Chrysophylloideae). – Mol.
Phylogen. Evol. 87: 1-13.
Terra-Araujo MH, Faria AD de, Swenson U.
2016. A taxonomic update of Neotropical Pradosia (Sapotaceae, Chrysophylloideae). – Syst.
Bot. 41: 634-650.
Testolin R, Ferguson AR. 1997. Isozyme
polymorphism in the genus Actinidia and the origin of the kiwifruit
genome. – Syst. Bot. 22: 685-700.
Thanikaimoni G, Vasanthy G. 1972. Sarraceniaceae: palynology and
systematics. – Pollen Spores 14: 143-155.
Thenen S. 1911. Zur Phylogenie der
Primulaceenblüte. Studien über den Gefässbündelverlauf in Blütenachse und
Perianth. – Gustav Fischer, Jena.
Thomas JL. 1960. A monographic study of the
Cyrillaceae. – Contr. Gray Herb.
Harvard Univ. 186: 1-114.
Thomas JL. 1961. Schizocardia
belizensis: a species of Purdiaea (Cyrillaceae) from Central America. –
J. Arnold Arbor. 42: 110-111.
Thompson HJ. 1953. The biosystematics of
Dodecatheon. – Contr. Dudley Herb. 4: 73-154.
Thompson JM. 1921. Studies in floral
morphology II. The staminal zygomorphy of Couroupita guianensis Aubl.
– Trans. Roy Soc. Edinb. 53: 1-15.
Thompson WK. 1986. Effects of origin, time of
collection, auxins and planting media on rooting of cuttings of Epacris
impressa Labill. – Scientia Hortic. 30: 127-134.
Thulin M, Warfa AM. 1989. Cyclamen
(Primulaceae) in tropical Africa. –
Plant Syst. Evol. 166: 249-252.
Tieghem P van. 1899. Sur les genres
Actinidia et Saurauia, considérées comme types d’une
famille nouvelle, les Actinidiacées. – Ann. Sci Nat. Bot. Biol. Vég. 10:
137-140.
Tieghem P van. 1905. Sur les
Rhaptopetalacées. – Ann. Sci. Nat. Bot. 9: 321-388.
Tiffney WN Jr. 1972. Snow cover and the
Diapensia lapponica habitat in the White Mountains, New Hampshire. –
Rhodora 74: 358-377.
Timbrook S. 1986. Segregation of
Loeseliastrum from Langloisia (Polemoniaceae). – Madroño 33:
157-174.
Togashi M. 1974. Mitrastemon sp.
found in North Borneo. – J. Jap. Bot. 49: 214.
Towers GHN, Tse A, Maas WSG. 1966. Phenolic
acids and phenolic glycosides of Gaultheria species. –
Phytochemistry 5: 677-681.
Tralau H. 1965. Halesia cf.
carolina L. (Styracaceae) im
oberen Pliozän von Weilerswist in Westdeutschland. – Bot. Not. 118:
171-176.
Trift I. 2004. Cladistic studies in primuloid
Ericales. – Ph.D. diss., University of
Stockholm, Sweden.
Trift I, Anderberg AA. 2006. Foliar sclereids
in Dionysia (Primulaceae)
from a phylogenetic perspective. – Edinburgh J. Bot. 63: 21-48.
Trift I, Källersjö M, Anderberg AA. 2002.
The monophyly of Primula (Primulaceae) evaluated by analysis of
sequences from the chloroplast gene rbcL. – Syst. Bot. 27:
396-407.
Trift I, Lidén M, Anderberg AA. 2004.
Phylogeny and biogeography of Dionysia (Primulaceae). – Intern. J. Plant Sci.
165: 845-860.
Triono T, Brown AHD, West JG, Crisp MD. 2007.
A phylogeny of Pouteria (Sapotaceae) from Malesia and Australasia.
– Aust. Syst. Bot. 20: 107-118.
Tschapka M, Helversen O von. 1999.
Pollinators of syntopic Marcgravia species in Costa Rican lowland
rainforest: bats and opossums. – Plant Biol. 1: 382-388.
Tsou C-H. 1993. Applications of comparative
embryology to plant taxonomy: taking the Lecythidaceae as an example. – Rec.
Adv. Bot. 13: 293-302.
Tsou C-H. 1994a. The classification and
evolution of pollen types of Planchonioideae (Lecythidaceae). – Plant Syst. Evol.
189: 15-27.
Tsou C-H. 1994b. The embryology, reproductive
morphology and systematics of Lecythidaceae. – Mem. New York Bot.
Gard. 71: 1-110.
Tsou C-H. 1995. Embryology of Theaceae – anther and ovule development
of Adinandra, Cleyera and Eurya. – J. Plant Res.
108: 77-86.
Tsou C-H. 1997. Embryology of the Theaceae – anther and ovule development
of Camellia, Franklinia, and Schima. – Amer. J.
Bot. 84: 369-381.
Tsou C-H. 1998. Early floral development of
Camellioideae (Theaceae). – Amer. J.
Bot. 85: 1531-1547.
Tsou C-H, Mori SA. 2002. Seed coat anatomy
and its relationship to seed dispersal in subfamily Lecythidoideae of the Lecythidaceae (the Brazil nut family).
– Bot. Bull. Acad. Sin. 43: 37-56.
Tsou C-H, Mori SA. 2007. Floral organogenesis
and floral evolution of the Lecythidoideae (Lecythidaceae). – Amer. J. Bot. 94:
716-736.
Tsou C-H, Li L, Vijayan K. 2016. The
intra-familial relationships of Pentaphylacaceae s.l. as revealed
by DNA sequence analysis. – Biochem. Genet. 54: 270-282.
Turner B, Paun O, Munzinger JAA, Chase MW,
Samuel R. 2016. Sequencing of whole plastid genomes and nuclear ribosomal DNA
of Diospyros species (Ebenaceae) endemic to New Caledonia: many
species, little divergence. – Ann. Bot. 117: 1175-1185.
Turner BL. 1994a. Taxonomic overview of
Gilia section Giliastrum (Polemoniaceae) in Texas and Mexico.
– Phytologia 76: 52-68.
Turner BL. 1994b. Synopsis of the North
American species of Loeselia (Polemoniaceae). – Phytologia 77:
318-337.
Turner B, Munzinger JAA, Duangjai S, Temsch
EM, Stockenhuber R, Barfuss MHJ, Chase MW, Samuel R. 2013. Molecular
phylogenetics of New Caledonian Diospyros (Ebenaceae) using plastid and nuclear
markers. – Mol. Phylogen. Evol. 69: 740-763.
Turner B, Paun O, Munzinger JAA, Duangjai S,
Chase MW, Samuel R. 2013. Analyses of amplified fragment length polymorphisms
(AFLP) indicate rapid radiation of Diospyros species (Ebenaceae) endemic to New Caledonia. –
BMC Evol. Biol. 13: 269.
Ueno J. 1950. On Enkianthus. A
classification of the genus Enkianthus based upon the characters of
pollen grains and of crystals in the leaf. – J. Inst. Polytechn. Osaka City
Univ. 1: 55-62.
Ukers WH. 1935. All about tea 1-2. – The
Tea and Coffee Trade Journal Company, New York.
Uphof JCT. 1936. Sarraceniaceae. – In: Engler A
(†), Harms H (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 17b, W.
Engelmann, Leipzig, pp. 704-727.
Uphof JCT. 1942. Cyrillaceae. – In: Engler A (†),
Harms H, Mattfeld J (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd.
20b, W. Engelmann, Leipzig, pp. 1-12.
Urcelay C. 2002. Co-occurrence of three
fungal root symbionts in Gaultheria poeppiggi DC. in Central
Argentina. – Mycorrhiza 12: 89-92.
Utami N, Shimizu T. 2005. Seed morphology and
classification in Impatiens (Balsaminaceae). – Blumea 50:
447-456.
Utsunomiya N, Subhadrabandhu S, Yonemori K,
Oshida M, Kanzaki S, Nakatsubo F, Sugiura A. 1998. Diospyros species
in Thailand: their distribution, fruit morphology and uses. – Econ. Bot. 52:
343-351.
Utteridge TMA. 2000. Contributions tot he
flora of Mt Jaya I. Two new species of Maesa (Myrsinaceae) from Puncak Jaya, New
Guinea. – Kew Bull. 55: 443-449.
Utteridge TMA. 2001. Two new species of
Maesa (Maesaceae) from New
Guinea. – Kew Bull. 56: 677-683.
Utteridge TMA. 2012. Four new species of
Maesa Forssk. (Primulaceae)
from Malesia. – Kew Bull. 67: 367-378.
Utteridge TMA, Saunders RMK. 2001. Sexual
dimorphism and functional dioecy in Maesa perlarius and M.
japonica (Maesaceae/Myrsinaceae). – Biotropica 33:
368-374.
Utteridge TMA, Saunders RMK. 2004. The genus
Maesa (Maesaceae) in the
Philippines. – Bot. J. Linn. Soc. 145: 17-43.
Valdés B. 1980. A new species of
Pelletiera (Prtimulaceae) from Macaronesia. – Candollea 35:
641-648.
Valdés CP. 2003a. New species of
Ardisia and Myrsine (Myrsinaceae) from Cuba. – Willdenowia
33: 173-178.
Valdés CP. 2003b. Additions to
Wallenia subg. Homowallenia (Myrsinaceae) in Cuba. – Willdenowia
33: 179-182.
Valentin M, Reyes M, Berazaín R. 1992. La
nervadura foliar del género Cyrilla Garden ex L. en Cuba. – Revista
Jard. Bot. Nac. Univ. Habana 13: 27-31.
Valenzuela-Estrada LR, Vera-Caraballo V, Ruth
LE, Eissenstat DM. 2008. Root anatomy, morphology and longevity among root
orders in Vaccinium corymbosum (Ericacae). – Amer. J. Bot. 95:
1506-1514.
Vales MA, Moncada M, Machado S. 1988.
Anatomía comparada de Clethraceae en
Cub. – Revista Jard. Bot. Nac. Univ. Habana 9: 69-73.
Vander Kloet SP. 1978. The taxonomic status
of Vaccinium pallidum, the hillside blueberries including
Vaccinium vacillans. – Can. J. Bot. 56: 1559-1574.
Vander Kloet SP. 1980. The taxonomy of the
highbush blueberry, Vaccinium corymbosum. – Can. J. Bot. 58:
1187-1201.
Vander Kloet SP. 1983. The taxonomy of
Vaccinium sect. Cyanococcus: a summation. – Can. J. Bot.
61: 256-266.
Vander Kloet SP. 1985. On the generic status
of Symphysia. – Taxon 34: 440-447.
Vander Kloet SP. 1988. The genus
Vaccinium in North America. – Res. Branch Agricult. Can. Publ.
1828.
Vander Kloet SP. 1996. Taxonomy of
Vaccinium Section Macropelma (Ericaceae). – Syst. Bot. 21: 355-364.
Vander Kloet SP, Dickinson TA. 1992. The
taxonomy of Vaccinium section Hemimyrtillus. – Bot. Mag.
(Tokyo) 105: 601-614.
Vander Kloet SP, Dickinson TA. 1999. The
taxonomy of Vaccinium section Myrtillus (Ericaceae). – Brittonia 51: 231-254.
Vander Kloet SP, Paterson IG. 2000. RAPD
assessment of novelties resulting in a new species of Vaccinium L. (Ericaceae) from Vietnam. – Bot. J. Linn.
Soc. 134: 575-586.
Vander Kloet SP, Dickinson TA. 2005. RAPD
typification: phenetic analysis of Vaccinium inflorescences. – Bot.
J. Linn. Soc. 148: 445-457.
Vander Kloet SP, Dickinson TA, Strickland W.
2003. From Nepal to Formosa, a much larger foot print for Vaccinium
sect. Aëthopus. – Acta Bot. Yunnan. 25: 1-24.
Vani-Hardev S. 1972. Systematic embryology of
Roridula gorgonias Planch. – Beitr. Biol. Pflanzen 48: 339-351.
Vasanthy G, Pocock SA. 1981. A comparative
study of anomalous and normal pollen of Rapanea: morphology, elemental
analyses, sterility and fungal parasitism. – Pollen Spores 23: 349-379.
Vaudois-Miéja N. 1983. Extension
palaéogéographique en Europe de l’actuel genre asiatique
Rehderodendron Hu (Styracacées). – Compt. Rend. Acad. Sci. Paris,
sér. II, 296: 125-130.
Veillet-Bartoszewska M. 1959. Développement
de l’albumen chez le Rhododendron ferrugineum L. – Bull. Soc. Bot.
France 106: 17-20.
Veillet-Bartoszewska M. 1960a. Embryogénie
des Styracacées. Développement de l’embryon chez le Styrax
officinalis L. – Compt. Rend. Acad. Sci. Paris, sér. II, 250:
905-907.
Veillet-Bartoszewska M. 1960b. Embryogénie
des Clethracées. Développement de l’embryon chez le Clethra
alnifolia L. – Compt. Rend. Acad. Sci. Paris, sér. II, 251:
2572-2574.
Veillet-Bartoszewska M. 1961. Embryogénie
des Epacridacées. Développement de l’embryon chez le Dracophyllum
secundum R. Br. – Compt. Rend. Acad. Sci. Paris, sér. II, 253:
1000-1002.
Veillet-Bartoszewska M. 1963. Recherches
embryogéniques sur les Ericales:
comparaison avec les Primulales. – Rev. Gén. Bot. 70: 141-230.
Venkata Rao C. 1961. Pollen types in the
Epacridaceae. – J. Indian Bot.Soc. 40: 409-423.
Venkatasamy S, Khittoo G, Nowbuth P,
Vencatasamy DR. 2006. Phylogenetic relationships based on morphology among the
Diospyros (Ebenaceae) species
endemic to the Mascarene Islands. – Bot. J. Linn. Soc. 150: 307-313.
Venkateswarlu J. 1952. Embryological studies
in Lecythidaceae I. – J. Indian
Bot. Soc. 31: 103-116.
Venkateswarlu J, Lakshminarayana L. 1957. A
contribution to the embryology of Hydrocera triflora W. et A. –
Phytomorphology 7: 194-203.
Venter S, Munzinger J. 2007. Paphia
paniensis (Ericaceae), a new
species from New Caledonia critically compared with P. neocaledonica.
– New Zealand J. Bot. 45: 503-508.
Verdaguer D, Ojeda F. 2005. Evolutionary
transition from resprouter to seeder life history in two Erica (Ericaceae) species: insights from seedling
axillary buds. – Ann. Bot. 95: 593-599.
Verdcourt B. 1962. Theaceae. – In: Hubbard CE, Milne-Redhead
E (eds), Flora of tropical East Africa, Crown Agents for Overseas Governments,
London, pp. 1-8.
Verdcourt B. 1968. Scytopetalaceae. – In:
Milne-Redhead E, Polhill RM (eds), Flora of tropical East Africa, Crown Agents
for Oversea Governments and Administrations, London, pp. 1-3.
Vijayan K, Zhang W-J, Tsou C-H. 2009.
Molecular taxonomy of Camellia (Theaceae) inferred from nrITS sequences.
– Amer. J. Bot. 96: 1348-1360.
Vijayaraghavan MR. 1965. Morphology and
embryology of Actinidia polygama Franch. et Sav. and systematic
position of the family Actinidiaceae. – Phytomorphology 15:
224-235.
Vijayaraghavan MR. 1969. Studies in the
family Cyrillaceae I. Development of
male and female gametophytes in Cliftonia monophylla (Lam.) Britton ex
Sarg. – Bull. Torrey Bot. Club 96: 484-489.
Vijayaraghavan MR. 1970a. Actinidiaceae. – In: Johri BM
(convener), Proceedings of the symposium on comparative embryology of
angiosperms, Bull. Indian Natl. Acad. 41: 69-71.
Vijayaraghavan MR. 1970b. Comparative
embryology of angiosperms: Cyrillaceae. – Indian Nat. Sci. Acad.
Bull. 41: 163-167.
Vijayaraghavan MR, Dhar U. 1976.
Scytopetalum tieghemii – embryologically unexplored taxon and
affinities of the family Scytopetalaceae. – Phytomorphology 26: 16-22.
Vijayaraghavan MR, Dhar U. 1978. Embryology
of Cyrilla and Cliftonia (Cyrillaceae). – Bot. Not. 131:
127-138.
Villamil P, Palser B. 1981. Studies of floral
morphology in the Ericales IX.
Organography, vascular anatomy, and megagametophyte in three species of
Gaultherieae. – Phytomorphology 30: 250-265.
Vink W. 1957. Revision of the Sapotaceae of the Malaysian area in a
wider sense X. Leptostylis. XI. Pycnandra. – Nova Guinea,
N. S., 8: 87-124.
Vink W. 1958. Revision of the Sapotaceae of the Malaysian area in a
wider sense XIII. Chrysophyllum. – Blumea 9: 21-74.
Virot R. 1975. Epacridacées. – In: Flore
de la Nouvelle Calédonie et Dépendances 6, Muséum National d’Histoire
Naturelle, Paris.
Vishenskaya TD. 1980. Polymerous androecium
and its development in the flower of Thea sinensis L. (Theaceae). – Bot. Žurn. 65: 39-50. [In
Russian]
Vogel S. 1986. Ölblumen und ölsammelnde
Bienen 2. Lysimachia und Macropis. – Trop. Subtrop.
Pflanzenwelt 54: 1-168.
Vogel S, Cocucci AA. 1988. Pollen threads in
Impatiens: their nature and function. – Beitr. Biol. Pflanzen 63:
271-287.
Vohnik M, Sadowsky JJ, Kohout P, Lhotakova Z,
Nestby R, Kolarik M. 2012. Novel root-fungus symbiosis in Ericaceae: sheathed ericoid mycorrhiza
formed by a hitherto undescribed basidiomycete with affinities to
Trechisporales. – PLoS One 7: e39524.
Votsch W. 1904. Neue
systematische-anatomische Untersuchungen von Blatt und Achse der
Theophrastaceen. – Engl. Bot. Jahrb. Syst. 33: 502-546.
Wagstaff SJ, Dawson MI, Venter S, Munziger J,
Crayn DM, Steane DA, Lemson KL. 2010. Origin, diversification and
classification of the Australasian genus Dracophyllum (Richeeae, Ericaceae). – Ann. Missouri Bot. Gard.
97: 235-258.
Wahlert GA. 2005. A phylogeny of
Arctostaphylos (Ericaceae)
inferred from nuclear ribosomal ITS sequence data. – M.Sc. thesis, San
Francisco State University, San Francisco, California.
Wahlert GA, Parker VT, Vasey MC. 2009. A
phylogeny of Arctostaphylos (Ericaceae) inferred from nuclear ribosomal
ITS sequences. – J. Bot. Res. Inst. Texas 3: 673-682.
Walker EH. 1940. A revision of the eastern
Asiatic Myrsinaceae. – Philipp. J.
Sci. 73: 1-258.
Walker EH. 1954. Concerning the Myrsinaceae (’’Ardisiaceae’’) of
Japan IV. – Bot. Mag. (Tokyo) 67: 795-796.
Walker EH. 1959. A revision of the Myrsinaceae of Taiwan. – Quart. J.
Taiwan Mus. 12: 161-194.
Wallace GD. 1975a. Interrelationships of the
subfamilies of the Ericaceae and
derivation of the Monotropoideae. – Bot. Not. 128: 286-298.
Wallace GD. 1975b. Studies of the
Monotropoideae (Ericaceae): taxonomy
and distribution. – Wassmann J. Biol. 33: 1-88.
Wallace GD. 1976. Interrelationships of the
subfamilies of the Ericaceae and
derivation of the Monotropoideae. – Bot. Not. 128: 286-298.
Wallace GD. 1977. Studies of the
Monotropoideae (Ericaceae). Floral
nectaries: anatomy and function in pollination ecology. – Amer. J. Bot. 64:
199-206.
Wallace GD. 1987. Transfer of Eremotropa
sciaphila to Monotropastrum (Ericaceae). – Taxon 36: 128-130.
Wallace GD. 1995. Ericaceae subfamily Monotropoideae. –
In: Luteyn JL (ed), Flora Neotropica. Monograph 66. Ericaceae II. The superior-ovaried genera.
– New York Botanical Garden, New York, pp. 13-27.
Wallnöfer B. 1997. A revision of
Styrax L. section Pamphilia (Mart. ex A. DC.) B. Walln. (Styracaceae). – Ann. Naturhist. Mus.
Wien, B, 99: 681-720.
Wallnöfer B. 2001. The biology and
systematics of Ebenaceae: a review. –
Ann. Naturhist. Mus. Wien, B, 103: 485-512.
Wallnöfer B. 2004a. A revision of
Lissocarpa Benth. (Ebenaceae
subfam. Lissocarpoideae (Gilg in Engler) B. Walln.) – Ann. Naturhist. Mus.
Wien, B, 105: 515-564.
Wallnöfer B. 2004b. Ebenaceae. – In: Kubitzki K (ed), The
families and genera of vascular plants VI. Flowering plants. Dicotyledons. Celastrales,
Oxalidales, Rosales, Cornales, Ericales, Springer, Berlin, Heidelberg, New
York, pp. 125-130.
Wallnöfer B. 2004c. Lissocarpaceae. – In:
Kubitzki K (ed), The families and genera of vascular plants VI. Flowering
plants. Dicotyledons. Celastrales,
Oxalidales, Rosales, Cornales, Ericales, Springer, Berlin, Heidelberg, New
York, pp. 236-238.
Walton EF, Fowke PJ, Weis K, McLeay PL. 1997.
Shoot axillary bud morphogenesis in kiwifruit (Actinidia deliciosa).
– Ann. Bot. 80: 13-21.
Wang H-C, Meng A-P, Chu H-J, Li X-W, Li J-Q.
2010. Floral ontogeny of Sinojackia xylocarpa (Styracaceae), with special reference to
the development of the androecium. – Nord. J. Bot. 28: 371-375.
Wang M-K, Peng S-L, Ding L-S, Wu F-E, Chen
Y-Z. 1998. Triterpenoid saponins from Berneuxia thibetica. –
Phytochemistry 48: 1411-1414.
Wang Y-J, Li X-J, Hao G, Liu J-Q. 2004.
Molecular phylogeny and biogeography of Androsace (Primulaceae) and the convergent
evolution of cusion morphology. – Acta Phytotaxon. Sin. 42: 481-499. [In
Chinese]
Wang Y, Fritsch PW, Shi S, Almeda F, Cruz BC,
Kelly LM. 2004. Phylogeny and infrageneric classification of Symplocos
(Symplocaceae) inferred from DNA
sequence data. – Amer. J. Bot. 91: 1901-1914.
Wang Y-H, He H, Min T-L, Zhou LH, Fritsch PW.
2006. The phylogenetic position of Apterosperma (Theaceae) based on morphological and
karyotype characters. – Plant Syst. Evol. 260: 39-52.
Wang Y-H, Lu L, Fritsch PW, Wang H, Wang Y-H,
Li D-Z. 2015. Leaf epidermal character variation and evolution in Gaultherieae
(Ericaceae). – Bot. J. Linn. Soc.
178: 686-710.
Wang ZY, Gould KS, Patterson KJ. 1994.
Comparative root anatomy of five Actinidia species in relation to
root-stock effects on kiwifruit flowering. – Ann. Bot., N. S., 73:
403-413.
Wanntorp L, Anderberg AA. 2011. Evolution and
diversification of brook weeds (Samolus, Samolaceae, Ericales). – Intern. J. Plant Sci. 172:
250-266.
Wanntorp L, Ronse De Craene L, Peng C-I,
Anderberg A. 2012. Floral ontogeny and morphology of Stimpsonia and
Ardisiandra, two aberrant genera of the primuloid clade of Ericales. – Intern. J. Plant Sci. 173:
1023-1035.
Warburg O, Reiche K. 1896. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien III(5), W. Engelmann,
Leipzig, pp. 383-392.
Ward NM, Price RA. 2002. Phylogenetic
relationships of Marcgraviaceae:
insights from three chloroplast genes. – Syst. Bot. 27: 149-160.
Ward S. 1981. The cytology of the genus
Cyclamen. – In: Bailey RH (ed), Cyclamen 1980: Proc. Conf. Cyclamen
Soc., Cyclamen Society, Chigwell, pp. 17-25.
Warner BG, Chinnappa CC. 1986. Taxonomic
implications and evolutionary trends in pollen of Canadian Ericales. – Can. J. Bot. 64:
3113-3126.
Warrington IJ, Weston GC (eds). 1990.
Kiwifruit: science and management. – Ray Richards, Auckland.
Waselkov K, Judd WS. 2008. A phylogenetic
analysis of Leucothoé s.l. (Ericaceae; tribe Gaultherieae) based on
phenotypic characters. – Brittonia 60: 382-397.
Waser NM. 1979. Pollinator availability as a
determinant of flowering time in ocotillo (Fouquieria splendens). –
Oecologia 39: 107-121.
Waser NM, Price MV. 1989. Optimal outcrossing
in Ipomopsis aggregata: seed set and offspring fitness. – Evolution
43: 1097-1109.
Watanabe H. 1933. Ungeschlechtliche
Fortpflanzung von Mitrastemon yamamotoi. – Proc. Imp. Acad. Japan 9:
412-415.
Watanabe H. 1934a. Biologie von
Mitrastemon yamamotoi Makino (Rafflesiaceae)
II. Vegetative Fortpflanzung. – Bot. Mag. Tokyo 48: 467-472.
Watanabe H. 1934b. Geschlechtliche
Fortpflanzung von Mitrastemon yamamotoi. Proceedings of the Imperial
Academy of Japan 10: 421-423.
Watanabe H. 1934c. Über die
Flüssigkeitsabsonderung der Blüten von Mitrastemon yamamotoi. –
Proc. Imp. Acad. Japan 10: 507-508.
Watanabe H. 1934d. Abweichende Formen von
Mitrastemon yamamotoi. – Proc. Imp. Acad. Japan 10: 600-603.
Watanabe H. 1935a. Beziehung zwischen
Mitrastemon yamamotoi und M. kanehirai. – Proc. Imp. Acad.
Japan 11: 280-282.
Watanabe H. 1935b. Mitrastemon
kawa-sasakii aus Formosa. – Proc. Imp. Acad. Japan 11: 283-285.
Watanabe K. 1933. Biologie von
Mitrastemon yamamotoi Makino (Rafflesiaceae)
I. Früchte und Samen. – Bot. Mag. Tokyo 67: 798-805.
Watanabe K. 1936. Morphologische-biologische
Studien über die Gattung Mitrastemon I-IV. – J. Jap. Bot. 12:
603-618, 698-711, 759-773, 848-858.
Watanabe K. 1937. Morphologische-biologische
Studien über die Gattung Mitrastemon V-VII. – J. Jap. Bot. 13:
14-24, 75-86, 154-162.
Waterman PG, Mahmoud EN. 1991. Chemical
taxonomy of the Sapotaceae: patterns
in the distribution of some simple phenolic compounds. – In: Pennington TD
(ed), the genera of Sapotaceae,
chapter 4, Royal Botanic Gardens, Kew, New York Botanical Garden, New York, pp.
51-74.
Watson L. 1962. The taxonomic significance of
stomatal distribution and morphology in Epacridaceae. – New Phytol. 61:
36-40.
Watson L. 1964a. The taxonomic significance
of certain anatomical observations on Ericaceae – the Ericoideae,
Calluna, and Cassiope. – New Phytol. 63: 274-280.
Watson L. 1964b. Some remarkable
inflorescences in the Ericales and their
taxonomic significance. – Ann. Bot., N. S., 28: 311-318.
Watson L. 1965. The taxonomic significance of
certain anatomical variations among Ericaceae. – Bot. J. Linn. Soc. 59:
111-125.
Watson L. 1967. Taxonomic implications of a
comparative anatomical study of Epacridaceae. – New Phytol. 66: 495-504.
Watson L. 1976. Ericales revisited. – Taxon 35:
269-271.
Watson L, Williams WT, Lance GN. 1967. A
mixed-data approach to angiosperm taxonomy: the classification of Ericales. – Proc. Linn. Soc. London 178:
25-35.
Watt G. 1904. Observations on Indian
primulas. – J. Roy. Hortic. Soc. 19: 295-326.
Webby RF, Wilson RD, Ferguson AR. 1994. Leaf
flavonoids of Actinidia. – Biochem. Syst. Ecol. 22: 277-286.
Weber H. 1955. Haben die Marcgraviaceen
“Inulinblätter”? – Ber. Deutsch. Bot. Ges. 68: 408-412.
Weber H. 1956. Über die Blütenstände und
die Hochblätter von Norantea Aubl. (Marcgraviaceae). – Beitr. Biol.
Pflanzen 32: 313-329.
Weberling F. 1956. Weitere Untersuchungen zur
Morphologie des Unterblattes bei den Dikotylen I. Balsaminaceae; II. Plumbaginaceae.
– Beitr. Biol. Pfl. 33: 17-32.
Weberling F. 1958. Über das Vorkommen
rudimentärer Stipeln bei den Lecythidaceae (s. l.) und
Sonneratiaceae. – Flora 116: 72-77.
Weberling F. 1968. Bemerkungen über das
Vorkommen rudimentärer Stipeln I. Cyrillaceae und die Gattung
Cyrillopsis Kuhlm. II. Alzatea Ruiz & Pav. (Lythraceae) und
Tristania R. Br. – Acta Bot. Neerl. 17: 282-287.
Weese TL, Johnson LA. 2005. Utility of
NADP-dependent isocitrate dehydrogenase for species-level evolutionary
inference in angiosperm phylogeny: a case study in Saltugilia. –
Mol. Phylogen. Evol. 36: 24-41.
Wehnert A. 1906. Anatomisch-systematische
Untersuchungen der Gattung Symplocos. – Ph.D. diss., München.
Wei Z-X. 1997. Pollen ultrastructure of Theaceae and its systematic significance.
– Acta Bot. Yunnan. 19: 143-153. [In Chinese]
Wei Z-X, Li D-Z, Fan X-K, Zhang X-L. 1997.
Pollen ultrastructure of Pentaphylacaceae and Sladeniaceae and their relationships to
the family Theaceae. – Acta Bot.
Yunnan. 21: 202-206. [In Chinese with English summary]
Weiller CM. 1996a. Reassessment of
Cyathodes (Epacridaceae). – Aust. Syst. Bot. 9: 491-507.
Weiller CM. 1996b. Planocarpa
(Epacridaceae), a new generic name. – Aust. Syst. Bot. 9: 509-519.
Weiller CM. 1996c. Reinstatement of the genus
Androstoma Hook. f. (Epacridaceae). – New Zealand J. Bot. 34:
179-185.
Weiller CM. 1999. Leptecophylla, a
new genus for species formerly included in Cyathodes (Epacridaceae).
– Muelleria 12: 195-214.
Weiller CM, Crowden RK, Powell JM. 1994.
Morphology and taxonomic significance of leaf epicuticular waxes in the
Epacridaceae. – Aust. Syst. Bot. 7: 125-152.
Weiss M, Selosse MA, Rexer KH, Urban A,
Oberwinkler F. 2004. Sebacinales: a hitherto overlooked cosm of
heterobasidiomycetes with a broad mycorrhizal potential. – Mycol. Res. 108:
1003-1010.
Weitzman AL. 1987a. The systematics of
Freziera Willd. (Theaceae). –
Ph.D. diss., Department of Organismic and Evolutionary Biology, Harvard
University, Cambridge, Massachusetts.
Weitzman AL. 1987b. Taxonomic studies in
Freziera (Theaceae), with notes
on reproductive biology. – J. Arnold Arbor. 68: 323-334.
Weitzman AL, Dressler S, Stevens PF. 2004. Ternstroemiaceae. – In: Kubitzki
K (ed), The families and genera of vascular plants VI. Flowering plants.
Dicotyledons. Celastrales,
Oxalidales, Rosales, Cornales, Ericales, Springer, Berlin, Heidelberg, New
York, pp. 450-460.
Wels PV. 1969. The relation between mode of
reproduction and extent of speciation in woody genera of the California
chaparral. – Evolution 23: 264-267.
Wells PV. 1992. Subgenera and sections of
Arctostaphylos. – The Four Seasons 9: 64-69.
Wells PV. 2000. The manzanitas of California,
also of Mexico and the World. – Publ. by the author.
Wells TC, Bohm BA. 1994. Isozyme variation in
North American Menziesia (Ericaceae). – Syst. Bot. 19: 407-423.
Wendelbo P. 1959. Two new species of
Dionysia from Afghanistan. – Bot. Not. 1959: 495-501.
Wendelbo P. 1961a. Studies in Primulaceae I. A monograph of the genus
Dionysia. – Årb. Univ. Bergen, Mat. Nat., Ser. 1961(3): 1-83.
Wendelbo P. 1961b. Studies in Primulaceae II. An account of
Primula subgenus Sphondylia (syn. Sect. Floribundae)
with a review of the subdivision of the genus. – Årb. Univ. Bergen, Mat.
Nat., Ser. 1961(11): 1-49.
Wendelbo P. 1961c. Studies in Primulaceae III. On the genera related
to Primula with special reference to their pollen morphology. –
Årb. Univ. Bergen, Mat. Nat., Ser. 1961(19): 1-31.
Wendelbo P. 1964. Studies in Primulaceae IV. The genus
Dionysia in Afghanistan with descriptions of 6 new species. – Årb.
Univ. Bergen, Mat.-Nat., Ser. 1963(19): 1-28.
Wendelbo P. 1970. New and noteworthy species
of Primulaceae from the ‘Flora
Iranica’-Area. – Bot. Not. 123: 300-309.
Wendelbo P. 1971. On xeromorphic adaptations
in the genus Dionysia (Primulaceae). – Ann. Naturhist. Mus.
Wien 75: 249-254.
Wendelbo P. 1980. New species of
Dionysia, Kickxia and Onobrychis from Iran. –
Notes Roy. Bot. Gard. Edinb. 38: 105-110.
Weynants C. 1993. Studie van de systematische
verwantschappen binnen de Primulanae door middel van een cladistiche analyse.
– Unpubl. thesis, Katholieke Universiteit, Leuven, Belgium.
Wherry ET. 1933. The Appalachian relative of
Sarracenia flava. – Bartonia 15: 7-8.
Wherry ET. 1940. A provisional key to the Polemoniaceae. – Bartonia 20:
14-17.
Wherry ET. 1943. Microsteris,
Phlox, and an intermediate. – Brittonia 5: 60-63.
Wherry ET. 1944a. The minor genus
Polemoniella. – Amer. Midl. Natur. 31: 211-215.
Wherry ET. 1944b. Review of the genera
Collomia and Gymnosteris. – Amer. Midl. Natur. 31:
216-231.
Wherry ET. 1955. Morris Arboretum Monographs
III. The genus Phlox. – Morris Arboretum of the University of
Pennsylvania, Philadelphia.
Wherry ET. 1961. Remarks on the genus
Linanthus. – Aliso 5: 9-10.
White EB. 1956a. Notes on Ebenaceae I. – Bull. Jard. Bot.
L’État 26: 237-246.
White EB. 1956b. Notes on Ebenaceae II. – Bull. Jard. Bot.
L’État 26: 277-307.
White EB. 1957. Notes on Ebenaceae III. – Bull. Jard. Bot.
L’État 27: 515-531.
White EB. 1962. Notes on Ebenaceae IV. – Bol. Soc. Brot. 36:
97-100.
White EB. 1963. Notes on Ebenaceae V. – Bull. Jard. Bot.
L’État 33: 345-367.
White EB. 1981. Lissocarpaceae. – In:
Maguire B (ed), The botany of the Guayana Highland 11, Mem. New York Bot.
Gard.32: 329-330.
White F. 1962. Geographic variation and
speciation in Africa with particular reference to Diospyros. – Syst.
Assoc. Publ. 4: 71-103.
White F. 1980. Notes on the Ebenaceae VIII. The African sections of
Diospyros. – Bull. Jard. Bot. Belg. 50: 445-460.
White F. 1983. 107. Ebenaceae. – In: Launert E (ed), Flora
Zambesiaca 7 (Part 1), Flora Zambesiaca Managing Committee, London, pp.
248-300.
White F. 1988. The taxonomy, ecology and
chorology of African Ebenaceae II. The
non-Guineo-Congolian species of Diospyros (excluding section
Royena). – Bull. Jard. Bot. Natl. Belg. 58: 325-448.
White F. 1998. The vegetative structure of
African Ebenaceae and the evolution of
rheophytes and ring species. – In: Hopkins HCF, Huxley CR, Pannell CM, Prance
GT, White F (eds), The biological monograph, Royal Botanic Gardens, Kew, pp.
95-113.
White F, Barnes RD. 1958. Generic characters
in the Ebenaceae. – J. Oxford Univ.
Forest Soc. IV, 6: 31-34.
White F, Verdcourt B. 1996. Ebenaceae. – In: Polhill RM (ed), Flora
of tropical East Africa, A. A. Balkema, Rotterdam, The Netherlands, pp.
1-51.
White F, Vosa CG. 1980. The chromosome
cytology of African Ebenaceae with
special reference to polyploidy. – Bol. Soc. Brot. II, 53: 275-297.
White J. 1990. Pollen development in
Actinidia deliciosa var. deliciosa: histochemistry of the
microspore mother cell walls. – Ann. Bot. 65: 231-239.
Whitmore TC. 1974. Abdulmajidia, a
new genus of Lecythidaceae from
Malaysia. – Kew Bull. 29: 207-211.
Widmer E. 1891. Die europäischen Arten der
Gattung Primula. – Oldenburg, München.
Wilken DH. 2004. Polemoniaceae. – In: Kubitzki K
(ed), The families and genera of vascular plants VI. Flowering plants.
Dicotyledons. Celastrales,
Oxalidales, Rosales, Cornales, Ericales, Springer, Berlin, Heidelberg, New
York, pp. 300-312.
Wilken DH, Hartman RL. 1991. A revision of
the Ipomopsis spicata complex (Polemoniaceae). – Syst. Bot. 16:
143-161.
Wilken DH, Smith DM, Harborne JB, Glennie CW.
1982. Flavonoid and anthocyanin patterns and the systematic relationships in
Collomia. – Biochem. Syst. Ecol. 10: 239-243.
Willson MF, Miller LJ, Rathcke BJ. 1979.
Floral display in Phlox and Geranium: adaptative aspects. –
Evolution 33: 52-63.
Wilson P. 1995. Selection for pollination
success and the mechanical fit of Impatiens flowers around bumblebee
bodies. – Biol. J. Linn. Soc. 55: 355-383.
Wit HCD de. 1957. Some remarks on
Heberdenia A. DC., Pleiomeris A. DC. and Afrardisia
Mez (Myrs.). – Bull. Jard. Bot. État. Brux. 27: 233-242.
Wit HCD de. 1958. Revision of
Afrardisia Mez (Myrsinaceae). – Blumea 4 [Suppl.]:
242-262.
Wolf PG, Soltis PS. 1992. Estimates of gene
flow among populations, geographic races, and species in the Ipomopsis
aggregate complex. – Genetics 130: 639-647.
Wolf PG, Soltis PS, Soltis DE. 1993.
Phylogenetic significance of chloroplast DNA restriction site variation in the
Ipomopsis aggregata complex and related species (Polemoniaceae). – Syst. Bot. 18:
652-662.
Wollenweber E, Kohorst G. 1981. Epicuticular
leaf flavonoids from Eucalyptus species and from Kalmia
latifolia. – Zeitschr. Naturforsch. 36c: 913-915.
Wollenweber E, Kohorst G. 1984. Novel
epicuticular leaf flavonoids from Kalmia and Gaultheria (Ericaceae). – Zeitschr. Naturforsch.
39c: 710-713.
Wollenweber E, Schnepf E. 1970. Vergleichende
Untersuchungen über die flavonoiden Exkrete von “Mehl”– und
“Öl”-Drüsen bei Primeln und die Feinstruktur der Drüsenzellen. –
Zeitschr. Pflanzenphysiol. 62: 216-227.
Woodcock EF. 1933. Seed studies in
Cyclamen persicum Mill. – Ann. Rep. Michigan Acad. Sci. 17:
415-419.
Worley AC, Ghazvini H, Schemske DW. 2009. A
phylogeny of the genus Polemonium based on amplified fragment length
polymorphism (AFLP) markers. – Syst. Bot. 34: 149-161.
Wright H. 1904. The genus Diospyros
in Ceylon: its morphology, anatomy, and taxonomy. – Ann. Roy. Bot. Gard.
(Peradeniya) 2: 1-106, 133-210.
Wright S. 1943. An analysis of local
variability of flower color in Linanthus parryae. – Genetics 28:
139-156.
Wu C-C, Hsu Z-F, Tsou C-H. 2003. Studies of
Eurya (Ternstroemeriaceae) in Taiwan (1), a new endemic species,
Eurya septata. – Bot. Bull. Acad. Sin. 44: 67-72.
Wu C-C, Hsu Z-F, Tsou C-H. 2007. Phylogeny
and taxonomy of Eurya (Ternstroemiaceae) from Taiwan, as
inferred from ITS sequence data. – Bot. Stud. 48: 97-116.
Wunschmann E. 1891. Sarraceniaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien III(2), W. Engelmann,
Leipzig, pp. 244-252.
Xi Y-Z, Tang Y-C. 1990. Pollen morphology and
phylogenetic relationships in the Diapensiaceae. – Cathaya 2:
89-112.
Xiao G, Berch SM. 1995. The ability of known
ericoid mycorrhizal fungi to form mycorrhizae with Gaultheria shallon.
– Mycologia 87: 467-470.
Xiao T-J, Parks CR. 2003. Molecular analysis
of the genus Camellia. – Intern. Camellia J. 35: 57-65.
Xiong Z-T, Huang R-H. 1933. Chromosome
numbers of 10 species and 3 varieties in Actinidia Lindl. – Acta
Phytotaxon. Sin. 26: 245-247.
Xu HJ, Yang TB, Zeng B. 2012. Variation of
stomatal length and stomatal density in leaves of Rhododendron with
elevation. – Arid Zone Res. 29: 1054-1058.
Xu Y, Hu C-M, Hao G. 2016. Pollen morphology
of Androsace (Primulaceae)
and its systematic implications. – J. Syst. Evol. 54: 48-64.
Yamamoto Y. 1925. Species nova
Rafflesiacearum ex Formosa. – Bot. Mag. Tokyo 39: 142-145.
Yamamoto Y. 1926. Rafflesiaceae.
– Suppl. Icon. Plant Form. 2: 10-13.
Yamamoto Y. 1927. Notae ad plantas Japoniae
et Formosae VI. Mitrastemon kawa-sasakii Hayata ex Sumatra. – Bot.
Mag. Tokyo 41: 736-737.
Yamamoto Y. 1933. On Mitrastemon.
– Bot. & Zool. Tokyo 1: 1249-1258.
Yamamoto Y. 1936. Species nova
Mitrastemonacearum (Rafflesiacearum) ex Mexico. – Bot. Mag. Tokyo 50:
539-541.
Yamazaki T. 1966. The embryology of
Shortia uniflora with brief review of the systematic position of the
Diapensiaceae. – J. Jap. Bot. 41:
245-251.
Yamazaki T. 1970a. Embryological studies in
Ebenales 1. Styracaceae. – J. Jap.
Bot. 45: 267-273.
Yamazaki T. 1970b. Embryological studies in
Ebenales 2. Symplocaceae. – J.
Jap. Bot. 45: 353-358.
Yamazaki T. 1971. Embryological studies in
Ebenales 3. Sapotaceae. – J. Jap.
Bot. 46: 161-165.
Yamazaki T. 1972. Embryological studies in
Ebenales 4. Ebenaceae. – J. Jap. Bot.
47: 20-28.
Yamazaki T. 1975. Floral anatomy and
embryology of Tripetaleia paniculata and T. bracteata (Ericaceae). – Bot. Mag. (Tokyo) 88:
267-279.
Yan G, Yao J, Ferguson AR, McNeilage MA, Seal
AG, Murray BG. 1997. New reports of chromosome numbers in Actinidia
(Actinidiaceae). – New Zealand J.
Bot. 35: 181-186.
Yan H-F, He C-H, Peng C-I, Hu C-M, Hao G.
2010. Circumscription of Primula subgenus Auganthus (Primulaceae) based on chloroplast DNA
sequences. – J. Syst. Evol. 48: 123-132.
Yan M, Moore MJ, Meng A, Yao X, Wang H. 2017.
The first complete plastome sequence of the basal asteroid family Styracaceae (Ericales) reveals a large inversion. –
Plant Syst. Evol. 303: 61-70.
Yan M, Fritsch PW, Moore MJ, Feng T, Meng A,
Yang J, Deng T, Zhao C, Yao X, Sun H, Wang H. 2018. Plastid phylogenomics
resolves infrafamilial reationships of the Styracaceae and sheds light on the
backbone relationships of the Ericales.
– Mol. Phylogen. Evol. 121: 198-211.
Yang B-Y. 1952. Pollen grain morphology in
the Ericaceae. – Quart. J. Taiwan
Mus. 5: 1-24.
Yang J-B, Min TL. 1995a. Embryological
studies on genera Pyrenaria and Tutcheria of family Theaceae. – Acta Bot. Yunnan. 17: 67-71.
[In Chinese]
Yang J-B, Min TL. 1995b. Systematic position
of genera Pyrenaria, Tutcheria and Parapyrenaria of
family Theaceae. – Acta Bot. Yunnan.
17: 192-196. [In Chinese]
Yang J-B, Yang S-X, Li D-Z, Lei L-G, Ikeda T,
Yoshino H. 2006. Phylogenetic relationships of Theaceae inferred from mitochondrial
matR gene sequence data. – Acta Bot. Yunnan. 29: 29-36.
Yang S-X. 1988. Systematics, diversification,
and geographical distribution of Pyrenaria sensu lato (Theaceae). – Ph.D. diss., Kunming
Institute of Botany, Kunming, Yunnan. [In Chinese]
Yang S-X, Ming T-L. 1995a. Embryological
studies on genera Pyrenaria and Tutcheria of family Theaceae. – Acta Bot. Yunnan. 17: 67-71.
[In Chinese]
Yang S-X, Ming T-L. 1995b. Studies on the
systematic position of genera Pyrenaria, Tutcheria and
Parapyrenaria of family Theaceae. – Acta Bot. Yunnan. 17:
192-196. [In Chinese]
Yang S-X, Yang J-B, Lei L-G, Li D-Z, Yoshino
H, Ikeda T. 2004. Reassessing the relationships between Gordonia and
Polyspora (Theaceae) based on
the combined analysis of molecular data from the nuclear, plastid and
mitochondrial genomes. – Plant Syst. Evol. 248: 45-55.
Yang Y-P, Dwyer JD. 1989. Taxonomy of
subgenus Bladhia of Ardisia (Myrsinaceae). – Taiwania 34:
192-298.
Yao X, Ye Q, Fritsch PW, Cruz BC, Huang H.
2008. Phylogeny of Sinojackia (Styracaceae) based on DNA sequence and
microsatellite data: implications for taxonomy and conservation. – Ann. Bot.
101: 651-659.
Yao X, Tang P, Li Z, Li D, Liu Y, Huang H.
2015. The first complete chloroplast genome sequences in Actinidiaceae: genome structure and
comparative analysis. – PloS ONE 10: e0129347.
https://doi.org/10.1371/journal.pone.0129347
Yarsick S, Xena de Enrech N, Ramirez N,
Agostini G. 1986. Notes on the floral biology of Couroupita guianensis
Aubl. (Lecythidaceae). – Ann.
Missouri Bot. Gard. 73: 99-101.
Yasui K. 1915. Studies of Diospyros
kaki I. – Bot. Gaz. (Crawfordsville) 60: 362-373.
Ye C-X. 1988. The subdivisions of genus
Camellia with a discussion on their phylogenetic relationship. –
Acta Bot. Yunnan. 10: 61-67. [In Chinese]
Ye C-X. 1990a. The range of Gordonieae (Theaceae) and limitation of genera in the
tribe. – Guihaia 10: 99-103. [In Chinese]
Ye C-X. 1990b. A discussion on relationship
among the genera in Theoideae (Theaceae). – Acta Sci. Nat. Univ.
Sunyatseni 29: 74-81. [In Chinese]
Yonemori K, Sugiura A, Yamada M. 2000.
Persimmon genetics and breeding. – In: Janick J (ed), Plant breeding reviews,
vol. 19, Wiley, New York, pp. 191-225.
Young BW, Massicotte HB, Tackaberry LE,
Baldwin QF, Egger KN. 2002. Monotropa uniflora: morphological and
molecular assessment of mycorrhizae retrieved from sites in the Sub-Boreal
Spruce biogeoclimatic zone in central British Columbia. – Mycorrhiza 12:
75-82.
Yu S-X, Chen Y-L, Qin H-N. 2007.
Impatiens angulata (Balsaminaceae), a new species from
Guangxi, China. – Nord. J. Bot. 25: 27-30.
Yu S-X, Zhou X-R, Xu G-F, Meng L, Bi H-Y.
2010. Floral development in Impatiens chishuiensis (Balsaminaceae): formation of two
well-developed anterolateral sepals and four carpels. – Plant Syst. Evol.
286: 21-26.
Yu S-X, Janssens SB, Zhu X-Y, Lidén M, Gao
T-G, Wang W. 2016. Phylogeny of Impatiens (Balsaminaceae): integrating molecular
and morphological evidence into a new classification. – Cladistics 32:
179-197.
Yuan Y-M, Song Y, Geuten K, Rahelivololona E,
Wohlhauser S, Fischer E, Smets E, Küpfer P. 2004. Phylogeny and biogeography
of Balsaminaceae inferred from ITS
sequences. – Taxon 53: 391-403.
Zak B. 1974. Ectendomycorrhiza of Pacific
madrone (Arbutus menziesii). – Trans. Brit. Mycol. Soc. 62:
202-204.
Zakharov AM, Glyzin VI, Ban’Kovskii AI.
1971. Flavonoids of Primula turkestanica. – Khim. Prir. Soedin. 7:
814. [In Russian]
Zavada MS, Wei Z-X. 1993. A contribution to
the pollen morphology of Camellia (Theaceae). – Grana 32: 233-242.
Zeeuw CH de. 1987. Chapter IX. Wood anatomy.
– In: Mori SA et al. (eds), The Lecythidaceae of a lowland neotropical
forest: La Fumée Mountain, French Guiana, Mem. New York Bot. Gard. 44:
100-112.
Zeeuw CH de. 1990. Secondary xylem of
neotropical Lecythidaceae. – In:
Mori SA, Prance GT (eds), Flora Neotropica. Monograph 21. Lecythidaceae II. The
zygomorphic-flowered New World genera (Couroupita,
Corythophora, Bertholletia, Couratari,
Eschweilera, and Lecythis), pp. 49-59.
Zetter RM, Hesse M. 1996. The morphology of
pollen tetrads and viscin threads in some Tertiary, Rhododendron-like Ericaceae. – Grana 35: 285-294.
Zeybek N. 1970. Liefert Styrax
officinalis L. ein Harz? – Ber. Schweiz. Bot. Ges. 80: 189-193.
Zhang L-B, Kadereit JW. 2002. The systematics
of Soldanella (Primulaceae)
based on morphological and molecular (ITS, AFLPs) evidence. – Nord. J. Bot.
22: 129-169.
Zhang L-B, Kadereit JW. 2004a. Classification
of Primula sect. Auricula (Primulaceae) based on two molecular data
sets (ITS, AFLPs), morphology and geographical distribution. – Bot. J. Linn.
Soc. 146: 1-26.
Zhang L-B, Kadereit JW. 2004b. Nomenclature
of Soldanella L. (Primulaceae). – Taxon 53: 741-752.
Zhang L-B, Comes HP, Kadereit JW. 2001.
Phylogeny and quaternary history of the European montane/alpine endemic
Soldanella (Primulaceae)
based on ITS and AFLP variation. – Amer. J. Bot. 88: 2331-2345.
Zhang M-Y, Fritsch PW, Ma P-F, Wang H, Lu L,
Li D-Z. 2017. Plastid phylogenomics and adaptive evolution of
Gaultheria series Trichophyllae (Ericaceae), a clade from sky islands of
the Himalaya-Hengduan Mountains. – Mol. Phylogen. Evol. 110: 7-18.
Zhang R-J, Ma H-Y, Wang Y-H. 2007. Early
floral development of endangered Euryodendron excelsum
(Ternstroemioideae: Theaceae). – Acta
Bot. Yunnan. 29: 648-654.
Zhang R-J, Schönenberger J. 2014. Early
floral development of Pentaphylacaceae (Ericales) and its systematic implications.
– Plant Syst. Evol. 300: 1547-1560.
Zhang R-J, Ma H-Y, Wang Y-H. 2008. Early
development of Adinandra latifolia (Theaceae). – Guihaia 28: 160-163.
Zhang W, Hu Y, Li Z, Wang, P, Xu M. 2009.
Foliar sclereids in tea and its wild allies, with reference to their taxonomy.
– Aust. Syst. Bot. 22: 286-295.
Zhang X-P, Anderberg AA. 2002. Pollen
morphology in the ericoid clade of the order Ericales, with special emphasis on Cyrillaceae. – Grana 41: 201-215.
Zhang Z-Y. 1987. A study on the pollen
morphology of Actinidiaceae and its
systematic position. – Acta Phytotaxon. Sin. 25: 9-23. [In Chinese with
English summary]
Ziemer RR. 1973. Some field observations of
Darlingtonia and Pinguicula. – Carniv. Plant Newsl. 2:
25-27.
Zimmer K, Hynson NA, Gebauer G, Allen EB,
Allen MF, Read DJ. 2007. Wide geographic and ecological distribution of
nitrogen and carbon gains from fungi in pyroloids and monotropoids (Ericaceae) and in orchids. – New Phytol.
175: 166-175.
Zinderen Bakker EM van. 1969. Observations on
the distribution of Ericaceae in
Africa. – Argum. Geogr. 12: 89-97.
Zinoveva-Stahevitch AE, Grant WF. 1984.
Chromosome numbers in Impatiens (Balsaminaceae). – Can. J. Bot. 62:
2630-2635.
Zorzanelli JPF, Carrijo TT, Fiaschi P, Jardim
JG, Santamaría-Aguillar D, Amorim AM. 2015. A first record of
Freziera (Pentaphylacaceae) from the
Brazilian Atlantic Forest, with the description of a new species. – Syst.
Bot. 40: 1075-1080.
Zusi RL, Hamas MJ. 2001. Bats and birds as
potential pollinators of three species of Marcgravia lianas on
Dominica. – Caribbean J. Sci. 37: 274-278.