
|
Phylogeny of Plantaginales based on DNA sequence data (mainly according to Schäferhoff & al. 2010). Soltis & al. (2011) and Refulio-Rodriguez & Olmstead (2014) recovered the clade [Peltanthera+[Calceolariaceae+Gesneriaceae]] as sister-group to the remainder (i.e. except Plocosperma, Carlemanniaceae and Oleaceae), with a bootstrap support of 97%. Likewise, Andersson (2006) identified Calceolariaceae as sister to Gesneriaceae. Verbenaceae may be closely related to Thomandersia (Refulio-Rodriguez & Olmstead 2014). |
ACANTHACEAE Juss. |
( Back to Plantaginales ) |
Acanthales Juss. ex Bercht. et J. Presl, Přir. Rostlin: 246. Jan-Apr 1820 [‘Acanthaceae’]; Acanthineae Link, Handbuch 1: 500. 4-11 Jul 1829; Justiciaceae Raf., Fl. Tellur. 4: 60. med 1838 [’Justicoides’]; Avicenniaceae (Endl.) Miq. in J. G. C. Lehmann, Plant. Preiss. 1: 353. 14-16 Aug 1845 [’Avicennieae’], nom. cons.; Thunbergiaceae (Dumort.) Lilja, Skånes Fl., ed. 2: 979. Apr-Dec 1870; Mendonciaceae Bremek. in Proc. Kon. Ned. Akad. Wetensch., ser. C, 56: 540. 27 Apr 1954; Meyeniaceae Sreem. in Phytologia 37: 412. 22 Oct 1977; Nelsoniaceae (Nees) Sreem. in Phytologia 37: 412. 22 Oct 1977
Genera/species c 203/3.470–>3.570
Distribution Tropical regions, especially in South and Southeast Asia, Africa, Brazil and Central America, some species in warm-temperate regions, mangrove vegetation in tropical and subtropical regions on both hemispheres.
Fossils Fossil pollen grains similar to Acanthaceae have been reported from the Miocene in many places in Africa, South America and Southeast Asia. A fossilized seed from Late Eocene of England and assigned to Acanthus rugatus possibly belong in Acanthaceae. Fossils assigned to Ruellia have been found in Eocene layers in, e.g. Spain and California. Plausible acanthaceous pollen fossils (e.g. Areolipollis insularis) were found in Miocene layers of Nigeria and Mexico.
Habit Usually bisexual (rarely unisexual), usually perennial or annual herbs, evergreen shrubs or lianas (rarely evergreen trees; Avicennia consists of mangrove trees or mangrove shrubs with articulated branchlets). Some representatives are aquatic. Many species are xerophytic. Some species are epiphytic.
Vegetative anatomy Phellogen ab initio usually superficial (sometimes deeply seated). Medulla sometimes with inverted vascular bundles. Primary medullary rays narrow. Secondary lateral growth usually normal (sometimes anomalous, via concentric cambia or cylindrical cambium, occasionally via internal inverted cambium). Endodermis in Andrographis, Barleria and Thunbergia prominent. Vessel elements with simple perforation plates; lateral pits alternate, bordered pits. Imperforate tracheary xylem elements fibre tracheids or libriform fibres usually with simple (sometimes bordered) pits, septate or non-septate (also acicular fibres and vasicentric tracheids). Wood rays uniseriate or multiseriate, heterocellular. Axial parenchyma usually paratracheal scanty, sometimes vasicentric (in Avicennia apotracheal or paratracheal, also aliform, lozenge-aliform or confluent). Intraxylary (concentric) phloem sometimes present. Sieve tube plastids S type, Pc type or Pcs type. Nodes usually 1:1?, unilacunar with one? leaf trace (in Avicennia 3:3?, trilacunar with three? traces), often swollen. Cystoliths – outgrowths of epidermal cell wall, impregnated with calciumcarbonate – abundant as lines (absent in Nelsonioideae, Mendoncia and Anomacanthus). Heartwood in Avicennia with gum-like substances. Calciumoxalate as druses or prismatic crystals present in Avicennia.
Trichomes Hairs unicellular or multicellular, uniseriate or branched, stellate, candelabra-like, dendritic; glandular hairs, stalked to almost sessile, abundant.
Leaves Usually opposite, simple, entire or pinnately lobed, sometimes coriaceous, with ptyxis in Thunbergioideae strongly curved. Stipules and leaf sheath absent. Petiole vascular bundle transection arcuate or annular. Venation pinnate. Stomata diacytic or paracytic. Cuticular wax crystalloids? Mesophyll with or without calciumoxalate as druses or single prismatic crystals (rarely raphides). Leaf margin serrate, crenate or entire. Glandular hairs with ethereal oils. Salt glands present, i.a., in Avicennia. Extrafloral nectaries present on lamina in many species in, e.g. Ruellia.
Inflorescence Terminal or axillary, usually dichasial to monochasial (in Avicennia thyrsoid, spike- or umbel-like) cymose (sometimes spike or raceme, or flowers solitary axillary). Bracts and/or floral prophylls (bracteoles) often large and showy (prophylls absent in Nelsonia), petaloid. Inflorescence often with involucre of large bracts surrounding partial inflorescences. Extrafloral nectaries rarely present on floral prophylls and/or pedicels.
Flowers Zygomorphic (flowers sometimes partially or entirely inverted; in Avicennia almost actinomorphic). Hypogyny. Sepals (three to) five (to 16), with imbricate, contorted, valvate or open aestivation, often with acute apex, persistent, free or connate (in Thunbergioideae usually strongly reduced); when five, then median sepal adaxial (sepals in Neuracanthus connate 3+2). Petals usually five, usually with imbricate quincuncial, contorted or ascending-cochlear (rarely open; in Nelsonioideae descending-cochlear) aestivation, more or less connate into quinquelobate bilabiate corolla (upper lip bilobate, lower lip trilobate, with lobes sometimes narrow; upper lobes sometimes reduced; petals sometimes three or four). Nectariferous disc intrastaminal, annular or as glands (rarely absent).
Androecium Stamens usually two (in Nelsonioideae two adaxial-lateral) or two long and two short (didynamous; rarely two fertile and two staminodial; in Pentstemonacanthus five fertile), haplostemonous, antesepalous, alternipetalous. Filaments free from each other or connate in pairs, adnate to corolla tube (epipetalous). Anthers often connivent, dorsifixed, versatile, tetrasporangiate, introrse to extrorse, longicidal (dehiscing by longitudinal slits); connective sometimes prolonged into appendage; thecae often asymmetrically arranged. Tapetum secretory. Staminodia usually one (adaxial-median; when four fertile stamens) or three (one adaxial-median and two adaxial-lateral; when two fertile stamens); staminodia absent in, e.g., Nelsonioideae and Avicennia.
Pollen grains Microsporogenesis simultaneous. Pollen morphology very varying. Pollen grains 2–3(–9)-colpate, -porate, -colporate or -colporoidate (sometimes syncolpate, sometimes with few or numerous pseudocolpi; rarely pororate, pantoporate, inaperturate or spiraperturate), shed as monads, bicellular or tricellular at dispersal. Exine tectate or semitectate (rarely almost intectate), with columellate infratectum, reticulate, microreticulate, perforate, punctate, spinulate, echinate, gemmate, rugulate, foveolate, psilate, striate or smooth (sometimes with raised tectal areas or ridges/ribs).
Gynoecium Pistil composed of two connate carpels; carpels without septal vascular bundles. Ovary superior, usually bilocular (in Mendoncia unilocular due to pseudomonomery; primary locules in Avicennia divided at base by secondary septa). Style single, simple, narrow. Stigma bifid (adaxial lobe often smaller), trumpet-shaped etc., usually non-papillate, Dry type (sometimes papillate, Wet type). Pistillodium absent.
Ovules Placentation usually axile (rarely intrusively parietal; in Avicennia free central). Ovules usually two to more than ten (rarely one) per carpel, anatropous, hemianatropous, amphitropous or campylotropous, unitegmic, tenuinucellar. Funicle usually modified into hard hooked ejaculator (retinaculum). Integument ? cell layers thick. Micropyle absent in Avicennia. Megagametophyte monosporous, Polygonum type, long and curved (apex of quadrinucleate megagametophyte sometimes expanding and reaching outside micropyle into placenta where egg apparatus develops; an extremely long suspensor pushes back developing embryo into endosperm). Antipodal cells sometimes proliferating (up to four to 18 cells), sometimes persistent (not formed in Avicennia). Endosperm development cellular; endosperm asymmetrical, two haustoria becoming closely adjacent to each other (cf. Lamiaceae-Nepetoideae). Endosperm haustoria chalazal and micropylar. Embryogenesis onagrad or solanad. Polyembryony frequent in some genera.
Fruit Usually a loculicidal explosion capsule (in Mendoncia and closely allied genera a one- or two-seeded drupe) with usually cartilaginous walls and persistent calyx. Funicle usually provided by hook-shaped lignified ejaculators, retinacula, explosively throwing out seeds from dehiscing capsule (appendage in Nelsonioideae and Thunbergioideae as papillae or absent). Retinaculum consisting of persistent and during maturation lignified funicle, forming hook below each seed.
Seeds Aril usually absent. Seed coat exotestal. Exotesta usually palisade, often mucilaginous as wet (hygroscopical hairs). Endotesta? Perisperm not developed. Endosperm usually very sparse or absent (in, e.g., Avicennia and some Nelsonioideae copious, ruminate due to localized asymmetrical growth of endosperm cells, oily). Embryo small to large, straight or curved, well differentiated, usually without chlorophyll (in Avicennia with chlorophyll). Cotyledons two, planoconvex, plicate or crumpled (in Avicennia large), often with amyloid. Germination phanerocotylar. Avicennia viviparous.
Cytology n = 9 (Nelsonioideae); n = 9, 28 (Thunbergioideae); n = 18, 32 (Avicennia); n = very various (Acanthoideae; 13, 15, 30, 40 etc.)
DNA Mitochondrial coxI intron present. Deletion in plastid gene matK?
Phytochemistry Flavonols (kaempferol, quercetin), C8-iridoid glycosides (thunbergioside, stilbericoside; in Thunbergia), caffeic acid esters (verbascosides), alkaloids, saponins, cyanogenic compounds, shikimic acid derived arthroquinones (in Barleria), and quaternary methylammonium compounds present. Ellagic acid and proanthocyanidins not found. Iridoids?
Use Ornamental plants, timber, tanning of leather (Avicennia).
Systematics (under construction) The sister-group relationship of Acanthaceae is unresolved.
Either Elytraria or the entire Nelsonioideae may be sister-group to the remaining Acanthaceae. Avicennia is probably sister to Thunbergioideae (or Acanthoideae?).
An endosperm development similar to that in Acanthaceae occurs in Lamiaceae-Nepetoideae – a parallelism.
A probable topology is [Nelsonioideae+[Acanthoideae+[Thunbergioideae+Avicennia]]].
The systematics below mainly follows McDade & al. (2008).
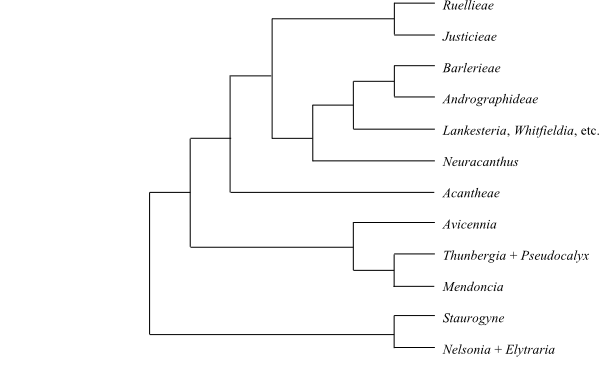 |
Phylogeny (simplified) of Acanthaceae based on DNA sequence data (McDade & Moody 1999; Schwarzbach & McDade 2002; McDade & al. 2008; Borg & al. 2008). Neuracanthus as sister to the clade [Whitfieldieae+[Barlerieae+Andrographideae]] is weakly supported. |
Nelsonioideae Lindl. ex Pfeiff., Nomencl. Bot. 1(1): 10. ante 8 Dec 1871 [‘Nelsonieae’]
7/105–110. Nelsonia (1; N. canescens; tropical Africa, Madagascar, tropical Asia to tropical Australia), Elytraria (17–21; tropical and subtropical regions on both hemispheres), Saintpauliopsis (1; S. lebrunii; tropical Africa), Anisosepalum (3; A. alboviolaceum, A. humbertii, A. lewallei; Central Africa), ‘Staurogyne’ (c 80; tropical regions on both hemispheres; paraphyletic), Gynocraterium (1; G. guianense; tropical South America; in Staurogyne?), Ophiorrhiziphyllon (2; O. diandrum, O. macrobotryum; Burma, southern China, Thailand, Indochina; in Staurogyne?). – Tropical and subtropical regions on both hemispheres. Herbs. Acicular fibres present. Cystoliths absent. Glandular hairs with bicellular head. Leaves opposite to alternate (spiral). Inflorescence usually a raceme (inflorescence branches in Saintpauliopsis cymose). Bracts spirally arranged. Nelsonia without floral prophylls (bracteoles). Corolla with descending cochlear aestivation (adaxial lobes overlapping abaxial lobes). Stamens two or four. Pollen grains usually tricolpate or tricolporate. Anthers of various shape. Stigma broadly (sometimes unequally) lobate, in Elytraria sensitive. Placentation in Elytraria parietal. Ovules usually six to numerous (rarely four or fewer) per carpel, campylotropous. Funicular obturator present. Endothelium present. Antipodal cells persistent. Endosperm development with cellular central area. Ejaculators (retinacula) rudimentary or absent. Seeds two to numerous, ruminate. Testa dissolved. Endosperm present, oily. n = 9. – It is uncertain whether Nelsonioideae have ejaculators (retinacula), but in that case they are non-functioning.
[Acanthoideae+[Thunbergioideae+Avicennia]]
Medulla sometimes with inverted vascular bundles. Acicular fibres present. Corolla sometimes with descending cochlear aestivation (adaxial corolla lobes outside the others). Ovules usually four (sometimes more) per carpel, collateral. Funicular obturator at least usually absent. Endothelium absent. Endosperm usually absent. Cotyledons often with amyloid (xyloglucans).
Acanthoideae Eaton, Bot. Dict., ed. 4: 33. Apr-Mai 1836 [‘Acanthaceae’] (under construction)
c 190/3.190–>3.280. Tropical regions, especially in South and Southeast Asia, Africa, Brazil and Central America, some species in warm-temperate regions. Usually herbs (rarely shrubs). Nodes often swollen; internode part immediately above node collapsing when dry. Cystoliths usually present (absent in Acantheae). Petiole vascular bundle transection usually arcuate, with bundles arranged in circle (rarely annular). Leaf margin sometimes spinose-serrate. Floral prophylls (bracteoles) often prominent. Calyx lobes often narrow. Corolla sometimes with contorted or descending cochlear aestivation (with abaxial lobes overlapping adaxial lobes); lobes sometimes narrow. Anthers sagittate or with thecae displaced, not opposite; anthers often monothecal (with one theca reduced). Pollen grains very variable, often porate. Stigma usually bilobate, Dry type. Fruit an explosive, almost woody capsule. Seeds several, flattened, sometimes hairy, inserted on ejaculators, retinacula, i.e. hook-shaped lignified modified funicles dispersing seeds when mature. Exotesta palisade, often mucilaginous as moistened (due to hygroscopic hairs). Hypodermal cells sometimes thickened. Cytologically very variable (n = 13, 15, 30, 40 etc.). – A possible topology of this “Retinaculate clade” is the following: [Acantheae+[[Neuracanthus+[Whitfieldia clade+[Barlerieae+Andrographideae]]]+[Ruellieae+Justicieae]]].
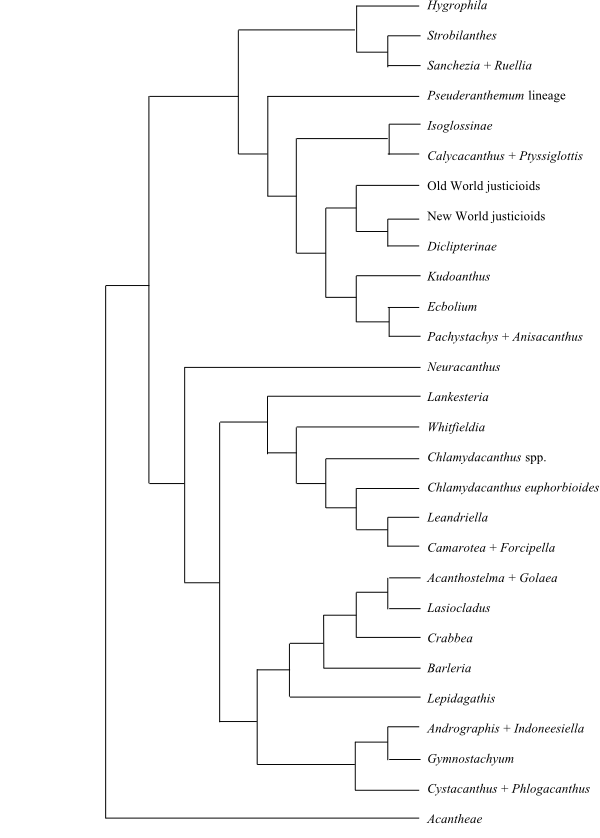 |
Phylogeny (simplified) of Acanthoideae based on DNA sequence data (McDade, Daniel & Kiel 2008). |
Acantheae Dumort., Anal. Fam. Plant.: 23. 1829
20/495–505. “Unilabiate Corolla Lineage” Crossandra (c 55; tropical Africa, Madagascar, the Arabian Peninsula, tropical Asia), Crossandrella (3; C. adamii, C. dusenii, C. laxispicata; tropical Africa), Sclerochiton (19; tropical and southern Africa), Streptosiphon (1; S. hirsutus; Tanzania), Cynarospermum (1; C. asperrimum; the West Indies), Blepharis (125–130; the Mediterranean, tropical regions in the Old World and southwards to South Africa), Acanthopsis (8; Namibia, South Africa), Acanthus (29; warm-temperate to tropical regions in the Old World). – “Bilabiate Corolla Lineage” Stenandrium (c 45; Africa, Madagascar, tropical and subtropical America), Salpixantha (1; S. coccinea; Jamaica), Holographis (16; Mexico), Neriacanthus (3; N. grandiflorus, N. harlingii, N. nitidus; tropical America), Rhombochlamys (1; R. elata; Colombia)?, Encephalosphaera (3; E. lasiandra, E. puberula, E. vitellina; tropical South America), Geissomeria (17; Mexico, Central America, tropical South America), ‘Aphelandra’ (170–175; tropical America; paraphyletic). – Unplaced Acantheae Cyphacanthus (1; C. atopus; Colombia), Orophochilus (1; O. stipulaceus; Peru), Strobilacanthus (1; S. lepidospermum; Panamá)?, Xantheranthemum (1; X. igneum; the Andes in Peru). – Pantropical, few species in warm-temperate regions. Cystoliths absent. Nodes not swollen. Corolla usually with imbricate quincuncial aestivation. Anthers monothecal. Pollen grains colpate or biporate girder pollen. Seeds usually without hygroscopic hairs (present in Blepharis). – Taxa with unilabiate (0:5) and bilabiate (2:3) corolla form different clades (McDade & al 2005).
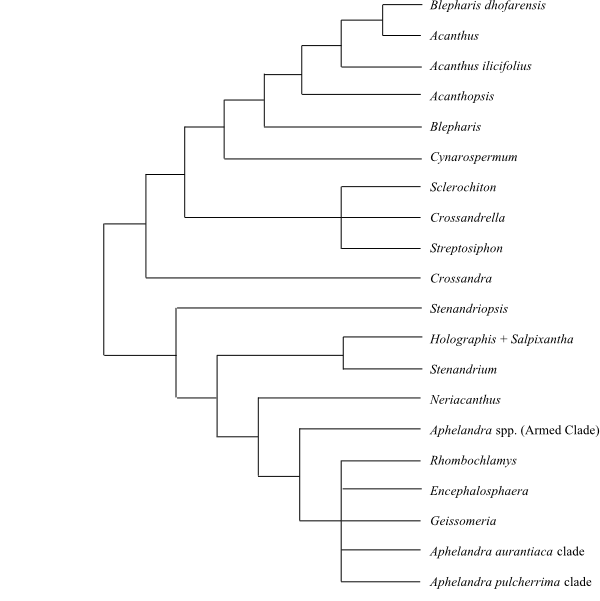 |
Phylogeny (simplified) of Acantheae based on DNA sequence data (McDade & al. 2005). |
[[Neuracanthus+[Whitfieldia clade+[Barlerieae+Andrographideae]]]+[Ruellieae+Justicieae]]
Cystoliths present. Nodes swollen. Anthers dithecal. Pollen grains porate. Endosperm development often unique, with a central area with free nuclear divisions, cell walls being established subsequently (sometimes with basal apparatus, i.e. area without cell wall development). Seeds usually with hygroscopic hairs.
[[Neuracanthus+[Whitfieldia clade+[Barlerieae+Andrographideae]]]
The “BAWN clade”.
Neuracanthus clade
1/13. Neuracanthus (13; tropical and subtropical East Africa, Madagascar, southern Arabian Peninsula, India to Indochina). – Inflorescence compact, with overlapping strongly-veined bracts (sometimes with spines consisting of modified partial inflorescences). Calyx two-lipped (upper lip trilobate, lower lib bilobate). Pollen grains tricolporate. Exine perforate, with interapertural regions of exine foveolate. Seeds with hygroscopic hairs.
[Whitfieldia clade+[Barlerieae+Andrographideae]]
Whitfieldia clade
8/c 38. Lankesteria (7; L. alba, L. barteri, L. brevior, L. elegans, L. glandulosa, L. hispida, L. thyrsoidea; tropical Africa, Madagascar), Whitfieldia (c 15; tropical Africa), ’Chlamydacanthus’ (4; C. dichrostachyus, C. euphorbioides, C. lindavianus, C. rupestris; tropical East Africa; non-monophyletic), Leandriella (2; L. oblonga, L. valvata; Madagascar), Camarotea (2; C. romiensis, C. souiensis; Madagascar), Zygoruellia (1; Z. benthamii; Madagascar), Forcipella (6; F. bosseri, F. cleistochlamys, F. involucrata, F. longistaminea, F. madagascariensis, F. repanda; Madagascar), Vindasia (1; V. virgata; Madagascar). – Tropical Africa, Madagascar. Corolla with contorted (Lankesteria, Whitfieldia) or ascending-cochlear aestivation. Vascular traces to corolla lobes trifurcating. Stamens four. Pollen grains lenticular, biporate, with typical girder-like band, and circular densely granular areas surrounding pores. Seeds usually covered with concentric rings of ridges of coarse scales, usually without hygroscopic hairs (present in Lankesteria).
[Barlerieae+Andrographideae]
Barlerieae Nees in Martius, Fl. Bras. 9: 7, 65. 1 Jun 1847
12/365–370. Barleria (250–255; tropical regions on both hemispheres), Barleriola (4; B. inermis, B. multiflora, B. satureioides, B. solanifolia; the West Indies), Borneacanthus (6; B. angustifolius, B. grandifolius, B. mesargyreus, B. paniculatus, B. parvus, B. stenothyrus; Borneo), Boutonia (1; B. cuspidata; Madagascar), Chroesthes (6; C. bracteata, C. lanceolata, C. longifolia, C. pubiflora, C. acemiflora, C. silvicola; southern China, Southeast Asia), Crabbea (14; tropical and southern Africa), Hulemacanthus (2; H. densiflorus, H. novoguineensis; New Guinea), Lasiocladus (2; L. anthospermifolius, L. rufopilus; Madagascar), Lepidagathis (c 70; tropical and subtropical regions on both hemispheres), Pericalypta (1; P. biflora; Madagascar), Podorungia (5; P. clandestina, P. decaryi, P. humblotii, P. lantzei, P. serotina; Madagascar), Pseudodicliptera (2; P. coursii, P. longifolia; Madagascar). – Tropical and subtropical regions. Corolla usually with imbricate quincuncial aestivation. Seeds usually with hygroscopic hairs.
Andrographideae Endl., Gen. Plant.: 707. Jan 1839
6/105–110. Andrographis (30–35; tropical Asia), Graphandra (1; G. procumbens; Thailand), Gymnostachyum (c 50; India to Central Malesia), Haplanthodes (4; H. neilgherrensis, H. plumosa, H. tentaculatus, H. verticillatus; India), Haplanthus (4; H. hygrophiloides, H. laxiflorus, H. ovatus, H. rosulatus; India, Bangladesh, eastern Himalayas, Burma, southern China, Southeast Asia, Malesia), Phlogacanthus (c 15; tropical Asia). – Tropical Asia. Corolla with ascending cochlear aestivation. Pollen grains tripororate or colporate. Exine thickened at aperture margins. Ovules usually six or more per ovary. Seeds without hygroscopic hairs.
[Ruellieae+Justicieae]
Ruellieae Dumort., Anal. Fam. Plant.: 23. 1829
c 45/995–1.030. Erantheminae Nees in A. P. de Candolle et A. L. P. P. de Candolle, Prodr. 11: 425. 27 Nov 1847. Brunoniella (6; B. acaulis, B. australis, B. linearifolia, B. neocaledonica, B. pumilio, B. spiciflora; New Guinea, northern and eastern Australia, New Caledonia), Leptosiphonium (11; New Guinea), Eranthemum (20–25; tropical Asia), Kosmosiphon (1; K. azureus; Cameroon), Pararuellia (3; P. alata, P. hainanensis, P. sumatrensis; southern China, Southeast Asia, Malesia). – Trichantherinae Benth. et Hook. f., Gen. Pl. 2: 1062, 1064. 1-16 Mai 1876. Louteridium (9; southern Mexico, Central America), Bravaisia (3; B. berlandieriana, B. grandiflora, B. integerrima; tropical America), Trichanthera (2; T. corymbosa, T. gigantea; northern South America), Trichosanchezia (1; T. chrysothrix; eastern Peru), Suessenguthia (4; S. cuscoenis, S. multisetosa, S. trochilophila, S. vargasii; eastern Andes in Peru and Bolivia), Sanchezia (c 55; tropical America, with their largest diversity in the Andes). – Hygrophilinae Nees in N. Wallich, Pl. Asiat. Rar. 3: 75. 15 Aug 1832. ‘Hygrophila’ (c 45; tropical regions on both hemispheres; paraphyletic; incl. Brillantaisia?), Brillantaisia (15–17; tropical Africa, Madagascar; in Hygrophila?). – Mimulopsinae E. Tripp in Int. J. Pl. Sci. 174(1): 111. Jan 2013. ‘Heteradelphia’ (2; H. paulojaegeria, H. paulowilhelmia; tropical West and Central Africa; paraphyletic; incl. Eremomastax?), Eremomastax (1; E. speciosa; tropical West and Central Africa, Madagascar?; in Heteradelphia?), ‘Mellera’ (5; M. lobulata, M. mentiodora, M. nyassana, M. parvifolia, M. submutica; tropical and subtropical Africa; paraphyletic; incl. Ionacanthus?), Ionacanthus (1; I. calcaratus; Madagascar; in Mellera?), Mimulopsis (15–30; tropical Africa, Madagascar). – Petalidiinae Benth. et Hook. f., Gen. Pl. 2: 1062, 1064. 1-16 Mai 1876. Ruelliopsis (2; R. damarensis, R. setosa; tropical and southern Africa), Phaulopsis (18; tropical Africa), Petalidium (c 35; tropical and southern Africa, western India, western Himalayas), Duosperma (26; tropical and southern Africa), Strobilanthopsis (1; S. linifolia; tropical Africa), Sautiera (1; S. tinctorum; Timor), Dyschoriste (c 100; tropical and subtropical regions on both hemispheres). – Strobilanthinae T. Anderson in J. Linn. Soc. London, Bot. 9: 443. 6 Apr 1866. Sinoacanthus (1–3; southern China, northern Vietnam), Hemigraphis (20–25; tropical Asia), ‘Strobilanthes’ (165–170; tropical Asia; paraphyletic). – Ruelliinae Nees in N. Wallich, Pl. Asiat. Rar. 3: 75. 15 Aug 1832. Dischistocalyx (13; tropical Africa), Satanocrater (2; S. paradoxa, S. ruspolii; tropical Africa), Acanthopale (9; tropical regions in the Old World), ‘Ruellia’ primuloides (tropical West and Central Africa), Benoicanthus (2; B. gruicollis, B. tachiadenus; Madagascar; in Ruellia?), Pseudoruellia (1; P. perrieri; Madagascar), ‘Ruellia’ (c 270; tropical and subtropical regions on both hemispheres, eastern United States, Argentina; paraphyletic), Eusiphon (3; E. geayi, E. longissimum, E. longistamineum; Madagascar; in Ruellia?), Polylychnis (1; P. fulgens; northeastern South America; in Ruellia?), Blechum (14; tropical America), Lychniothyrsus (1; L. mollis; Brazil). – Unplaced Ruellieae Calacanthus (1; C. grandiflorus; tropical Asia), Diceratotheca (1; D. bracteolata; northwestern Thailand), Echinacanthus (4; E. attenuatus, E. lofuensis, E. longipes, E. longzhouensis; the Himalayas, China), Physacanthus (3; P. batanganus, P. nematosiphon, P. talbotii; tropical Africa), Spirostigma (1; S. hirsutissima; Brazil)?, Stenothyrsus (1; S. ridleyi; Perak on the Malay Peninsula)? – Pantropical. Cystoliths present. Corolla with sinistrorsely contorted aestivation. Corolla tube usually obliquely-longitudinally divided by lamellar structure, “filament curtain”, formed from synstapetal part of corolla tube plus filaments (decurrent filament ridges and connate filament parts just above adnate portion). Anthers in Bravaisia with basal appendages. Ovules sometimes more than four per carpel. Seeds often with hygroscopic hairs.
Justicieae Dumort., Anal. Fam. Plant.: 23. 1829
c 92/1.170–>1.200. Pseuderanthemum clade: Spathacanthus (3; S. hahnianus, S. hoffmannii, S. parviflorus; Central America), Herpetacanthus (12; Panamá to Brazil), Asystasia (c 55; tropical regions in the Old World), Ruttya (6; R. bernieri, R. fragrans, R. fruticosa, R. ovata, R. speciosa, R. tricolor; tropical and southern Africa, Madagascar, Yemen), Ruspolia (5; R. decurrens, R. humbertii, R. hypocrateriformis, R. paniculata, R. seticalyx; tropical Africa, Madagascar), Pseuderanthemum (45–50; tropical regions on both hemispheres), Wuacanthus (1; W. microdontus; Sichuan, Yunnan), Oplonia (17; Madagascar, tropical America, with their highest diversity in the West Indies), Chileranthemum (3; C. lottiae, C. pyramidatum, C. trifidum; Mexico), Mackaya (2; M. bella, M. indica; Swaziland, northern and eastern South Africa, southern Asia); Isoglossinae: Ptyssiglottis (30–35?; tropical Asia), Isoglossa (c 50; tropical regions in the Old World, the Arabian Peninsula), Brachystephanus (22; tropical Africa, Madagascar), Razisea (5; R. citrina, R. ericae, R. spicata, R. villosa, R. wilburii; Central America), Stenostephanus (c 40; tropical South America); Chlamydocardia (3; C. buettneri, C. lanciformis, C. subrhomboidea; tropical West and Central Africa), Kudoacanthus (1; K. albonervosus; Taiwan); 'Justicia' pro parte, Clinacanthus (2; C. nutans, C. spirei; southern China, Southeast Asia, Malesia), Angkalanthus (1; A. oligophylla; Socotra), Chorisochora (3; C. minor, C. striata, C. transvaalensis; Mpumalanga and Northern Province in South Africa, Socotra), Ecbolium (22; tropical regions in the Old World; in Justicia?), Populina (2; P. perrieri, P. richardii; Madagascar), Megalochlamys (10; southwestern tropical Africa to Namibia and northern South Africa, the Arabian Peninsula), Trichaulax (1; T. mwasumbii; Kenya, Tanzania), Hoverdenia (1; H. speciosa; Mexico), 'Yeatesia' (3; Y. laetevirens, Y. platystegia, Y. viridiflora; southeastern United States to northeastern Mexico; polyphyletic), Streblacanthus (5; S. amoenus, S. cordatus, S. dubiosus, S. monospermus, S. roseus; Central America), 'Pachystachys' (12; tropical America; non-monophyletic), Fittonia (2; F. albivenis, F. gigantea; Peru), Ancistranthus (1; A. harpochiloides; Cuba), Schaueria (8; Brazil), Mirandea (6; M. andradenia, M. grisea, M. huastecensis, M. hyssopus, M. nutans, M. sylvatica; Mexico), Henrya (2; H. insularis, H. tuberculosperma; Central America), Aphanospermum (1; A. sinaloense; northwestern Mexico), Chalarothyrsus (1; C. amplexicaulis; Mexico), Gypsacanthus (1; G. nelsonii; Mexico), 'Anisacanthus' (18; southwestern United States, Mexico; polyphyletic), 'Carlowrightia' (c 25; southwestern United States, Mexico, Central America south to Costa Rica; polyphyletic), Tetramerium (30–35; Central America); Rungia (23; tropical regions in the Old World), Metarungia (3; M. galpinii, M. longistrobus, M. pubinervia; tropical and southern Africa; in Anisotes?); Anisotes (19; tropical Africa, Madagascar); Old World ‘Justicia’ (c 600; warm-temperate to tropical regions in the Old World; paraphyletic); Justicia betonica (tropical and southern Africa, tropical Asia); Diclipterinae: Rhinacanthus (10; tropical regions in the Old World), Peristrophe (c 20; tropical regions in the Old World), Hypoestes (100–105; tropical regions in the Old World; in Justicia?), Dicliptera (220–225; tropical and subtropical regions on both hemispheres; in Justicia?). – Unplaced Justicieae Afrofittonia (1; A. silvestris; tropical West and Central Africa), Ambongia (1; A. perrieri; Madagascar), Ascotheca (1; A. paucinervia; Gabon), Ballochia (3; B. amoena, B. atrovirgata, B. rotundifolia; Socotra), Calycacanthus (1; C. magnusianus; New Guinea), Celerina (1; C. seyrigii; Madagascar), Chameranthemum (4; C. beyrichii, C. durandii, C. tonduzii, C. venosum; tropical America), Chlamydostachya (1; C. spectabilis; tropical East Africa), Codonacanthus (3; C. pauciflorus, C. sanjappae, C. spicatus; northeastern India, southern China, Japan), Conocalyx (1; C. laxus; Madagascar), Cosmianthemum (14; western Borneo), Cyclacanthus (2; C. coccineus, C. poilanei; Southeast Asia), Danguya (1; D. pulchella; Madagascar), Dasytropis (1; D. fragilis; eastern Cuba), Dichazothece (1; D. cylindracea; eastern Brazil), Dicladanthera (2; D. forrestii, D. glabra; western Western Australia), Filetia (9; the Malay Peninsula, Sumatra), Glossochilus (2; G. burchellii, G. parviflorus; southern Africa), Graptophyllum (c 15; tropical regions in the Old World, northern and eastern Australia, Melanesia), Ichthyostoma (1; I. thulinii; southeastern Ethiopia, Somalia)?, Isotheca (1; I. alba; Trinidad), Jadunia (1; J. biroi; New Guinea), Juruasia (2; J. acuminata, J. rotundata; Brazil), Kalbreyeriella (3; K. gigas, K. rioquebradasiana, K. rostellata; Panamá, Colombia), Linariantha (1; L. bicolor; Borneo)?, Marcania (1; M. grandiflora; Thailand)?, Megalostoma (1; M. viridescens; Central America), Melittacanthus (1; M. divaricatus; Madagascar), Monothecium (3; M. aristatum, M. glandulosum, M. leucopterum; tropical Africa to southern India), Oreacanthus (3–4; O. coeruleus, O. mannii, O. sudanicus; Central Africa), Pelecostemon (1; P. trianae; Colombia), Phialacanthus (5; P. griffithii, P. major, P. minor, P. pauper, P. wrayi; the Himalayas to the Malay Peninsula)?, Pranceacanthus (1; P. coccineus; Amazonian Brazil), Psilanthele (1; P. eggersii; Ecuador), Pulchranthus (4; P. adenostachyus, P. congestus, P. surinamensis, P. variegatus; tropical South America), Ritonia (4; R. barbigera, R. humbertii, R. poisonii, R. rosea; Madagascar), Samuelssonia (1; S. verrucosa; Hispaniola)?, Sapphoa (2; S. ekmanii, S. rigidifolia; Cuba)?, Sebastiano-schaueria (1; S. oblongata; Brazil)?, Sphinctacanthus (1; S. griffithii; northeastern India to Burma)?, Tessmanniacanthus (1; T. chlamydocardioides; eastern Peru), Thysanostigma (2; T. odontites, T. siamense; southern Thailand, the Malay Peninsula)?, Trichocalyx (2; T. obovatus, T. orbiculatus; Socotra)?, Vavara (1; V. breviflora; Madagascar), Xerothamnella (2; X. herbacea, X. parvifolia; southern Queensland, northwestern New South Wales, northeastern South Australia)? – Pantropical. Corolla with imbricate quincuncial aestivation. Stamens two. Thecae with different height. Pollen grains usually hexapseudocolpate tricolporate. – A possible topology of Justicieae is [Pseuderanthemum clade+[Isoglossinae+[Tetramerium clade+[[Rungia+Metarungia]+[Duvernoia+[Anisotes+[Old World ‘Justicia’ spp.+[Justicia betonica+[Diclipterinae+New World ‘justicioids’]]]]]]]]] (McDade & al. 2000). Pollen grains in Isoglossinae often “girdle pollen”, i.e. lenticular biporate pollen grains with prominent circumferential band.
Unplaced Acanthoideae
Dolichostachys (1; D. elongata; Madagascar), Gymnophragma (1; G. simplex; Papua New Guinea), Morsacanthus (1; M. nemoralis; Brazil), Perenideboles (1; P. ciliatum; Nicaragua), Sericospora (1; S. crinita; the West Indies), Sphacanthus (2; S. brillantaisia, S. humbertii; Madagascar; Justicieae-Isoglossinae?).
[Thunbergioideae+Avicennia]
Ovules two per carpel. Cotyledons folded. – The support of this clade is not particularly strong (Schwarzbach & McDade 2002; McDade & al. 2008).
Thunbergioideae Kostel., Allg. Med.-Pharm. Fl. 3: 923. Apr-Dec 1834
5/165–170. Thunbergia (c 100; tropical and subtropical regions in the Old World), Meyenia (1; M. hawtayneana; India, Sri Lanka)?, Pseudocalyx (5; P. aurantiacus, P. heterochondros, P. macrophyllus, P. pasquierorum, P. saccatus; tropical Africa, Madagascar), Mendoncia (60–65; tropical Africa, Madagascar, tropical America), Anomacanthus (1; A. congolanus; Congo, Angola). – Pantropical. Usually twining-climbing herbs (sometimes upright). Medulla with inverted vascular bundles, or secondary lateral growth anomalous (inner and inverted cambium developing). Leaves with strongly curved ptyxis. Petiole vascular bundle transection arcuate or annular. Inflorescence with two or more flowers in median plane of leaf axil. Adaxial flowers first mature. Bracts absent. Floral prophylls (bracteoles) large, connate, surrounding flower as epicalyx. Calyx as a rim, or as up to 16 minute lobes; in Thunbergia with extrafloral nectaries. Corolla often with contorted (sometimes imbricate quincuncial) aestivation. Anthers poricidal, with lignified unicellular hairs (bristles), sagittate, sometimes slightly displaced; connective prolonged; basal staminal appendages sometimes present. Endothecium absent. Pollen grains octocolpate or spiraperturate. Connective elongate. Adaxial carpel in Mendoncia and Anomacanthus reduced and sterile. Stigma broadly and shortly bilobate (in Mendoncia and Anomacanthus) to trumpet-shaped (in Thunbergia, Pseudocalyx and Meyenia), with wide and often unequally sized lobes, papillate, Wet type. Ovules two per carpel. Fruit a one- or two-seeded drupe (Mendoncia and Anomacanthus) or a usually four-seeded septifragal capsule, without retinacula (Thunbergia, Pseudocalyx and Meyenia). Cotyledons sometimes (e.g. in Mendoncia) twice folded. n = 9, 28. Iridoids absent in Mendoncia. –– Mendoncia is sister to the clade [Thunbergia+Pseudocalyx] (Borg & al. 2008).
Avicennioideae Miers in London J. Bot. 7: 58. 1848 [‘Avicennieae’]
1/8. Avicennia (8; A. balanophora, A. bicolor, A. germinans, A. integra, A. marina, A. officinalis, A. schaueriana, A. tonduzii; tropical, subtropical and warm-temperate estuarine areas). – Mangrove trees or mangrove shrubs with pneumatophore roots. Wood with anomalous secondary lateral growth from successive cambia. Intraxylary phloem as islands in bands of conjunctive tissue? Nodes 3:3, trilacunar with three leaf traces, swollen. Leaves opposite, entire, somewhat fleshy, with abaxial side covered with clavate hairs, and with salt glands on both sides. Petiole vascular bundle transection annular. Sclereids and colleters present. Flowers in dense thyrsoid spike-like units. Flowers more or less zygomorphic (sometimes actinomorphic). Sepals four (to six), persistent, free or almost free. Petals four (to six). Corolla tube with nectar-secreting glands. Disc small (nectaries as secretory hair tufts?). Stamens four (to six), alternipetalous. Secondary septa sometimes present. Ovary with apically fused locules. Stigma bilobate, with obtuse lobes. Placentation free central to apical (ovules inserted on septa). Ovules sometimes orthotropous? Micropyle absent. Megagametophyte extra-ovular. Endosperm development cellular?, asymmetrical. Micropylar endosperm haustorium extra-ovular, highly branched, reaching placenta. Fruit one-seeded fleshy capsule-like, dehiscing when seed germinates, with persistent green calyx. Seed fairly large. n = 18, 32. Tannins and betaines present. – Avicennia is sister to Thunbergioideae, according to DNA data, although morphological features identify Acanthoideae as its sister-group (Schwarzbach & McDade 2002; McDade & al. 2008).
BIGNONIACEAE Juss. |
( Back to Plantaginales ) |
Bignoniales Juss. ex Bercht. et J. Presl, Přir. Rostlin: 246. Jan-Apr 1820 [‘Bignoniaceae’]; Bignoniopsida Nees in Flora 8: 142, 143. 7 Mar 1825 [’Bignoniaceae’]; Bignoniineae Link, Handbuch 1: 503. 4-11 Jul 1829 [‘Bignoniaceae‘]; Crescentiaceae Dumort., Anal. Fam. Plant.: 20, 24. 1829
Genera/species 77–78/870–885
Distribution Mainly tropical regions, with their largest diversity in tropical South America; some species in subtropical and warm-temperate Asia.
Fossils Winged seeds attributable to Bignoniaceae are reported from the Paleocene of North America and Japan.
Habit Bisexual, usually evergreen (rarely deciduous) trees, shrubs or lianas (Argylia, Incarvillea and Niedzwedzkia are perennial herbs, Tourrettia consists of twining perennial herbs). Often with leaf-tendrils. Lenticels often frequent on stems and branches.
Vegetative anatomy Phellogen ab initio superficial or cortical. Secondary lateral growth usually normal (in Bignonieae anomalous, via concentric cambia or cylindrical cambium; secondary xylem sometimes not formed). Cambium storied. Vessel elements with usually simple (rarely scalariform or reticulate) perforation plates; lateral pits alternate, usually bordered (rarely simple) pits. Vestured pits absent? Imperforate tracheary xylem elements often very long libriform fibres with simple or bordered pits, septate or non-septate (also vasicentric tracheids). Wood rays uniseriate or multiseriate, usually homocellular (sometimes heterocellular). Axial parenchyma usually paratracheal scanty, aliform, lozenge-aliform, winged-aliform, confluent, vasicentric, unilateral or banded. Wood element often entirely or partially storied. Tyloses often abundant. Secondary phloem often stratified into hard fibrous and soft parenchymatous layers. Sieve tube plastids S type. Nodes 1:1 or 1:≥3, unilacunar with one, three or more leaf traces. Sclerenchymatous fibres present in some species. Silica bodies or prismatic calciumoxalate crystals (sometimes styloids or acicular crystals) present in some species.
Trichomes Hairs multicellular, uniseriate or branched, furcate, stellate, dendritic, lepidote; sometimes glandular hairs.
Leaves Usually opposite (sometimes verticillate, rarely alternate, spiral), usually pinnately compound (sometimes bipinnate or more, or palmately compound, or simple/unifoliolate); terminal leaflet in lianas usually modified into tendril), when simple then usually pinnately lobed (sometimes palmately lobed or entire), usually with conduplicate (in, e.g., Pyrostegia involute) ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle transection annular; petiole sometimes also with edge bundles or adaxial bundles. Venation pinnate or palmate. Stomata usually anomocytic (sometimes paracytic, anisocytic, helicocytic, paracytic, cyclocytic, or diacytic). Cuticular wax crystalloids as clusters of terete rodlets. Domatia as pockets or hair tufts or absent. Leaf margin usually entire (sometimes serrate). Leaf apices, axillary buds and nodes often with extrafloral nectaries.
Inflorescence Terminal or axillary, dichasial, cincinnus or thyrse, raceme etc., or flowers solitary.
Flowers Zygomorphic (rarely almost actinomorphic), usually large. Hypogyny. Sepals five, with open or imbricate aestivation, connate into campanulate calyx (sometimes bilabiate or truncate; in some species of Lundia with calyptra), often with vascularized nectaries on abaxial side. Petals five, usually with imbricate quincuncial (rarely valvate) aestivation, connate into campanulate or infundibuliform corolla, quinquelobate or often bilabiate (upper lip bilobate, lower lip trilobate). Nectariferous disc intrastaminal, usually annular (sometimes cupular or absent).
Androecium Stamens usually two longer and two shorter (didynamous; in Catophractes, Oroxylum and ‘Rhigozum’ five), haplostemonous, antesepalous, alternipetalous; fifth stamen (adaxial, median) usually modified into staminodium or absent (in Catalpa and Paragonia two fertile stamens and three staminodia). Filaments free from each other, adnate to corolla tube (epipetalous). Anthers often head-to-head, usually connate, sometimes confluent, dorsifixed, often versatile, tetrasporangiate or disporangiate, introrse, longicidal (dehiscing by longitudinal slits). Tapetum amoeboid-periplasmodial. Staminodia usually one or three (rarely absent).
Pollen grains Microsporogenesis simultaneous. Pollen grains usually tricolpate or tricolporate (sometimes monocolpate, tetra- or pentacolpate, inaperturate, pericolpate, spiraperturate or syncolpate), usually shed as monads (sometimes as tetrads, rarely polyads), bicellular at dispersal. Exine tectate, semitectate or intectate, with columellate infratectum, reticulate, psilate, spinulate, or areolate.
Gynoecium Pistil composed of two connate carpels. Ovary superior, usually bilocular (sometimes unilocular or almost quadrilocular due to incomplete secondary septa); often with nectaries on abaxial side. Style single, simple. Stigma capitate or broadly bilobate, sensitive, papillate, Wet type. Pistillodium absent.
Ovules Placentation axile (when ovary bilocular) or intrusively parietal (when ovary unilocular); placentae usually two per locule, sometimes lobed. Ovules usually numerous (rarely few) per carpel (two series of ovules per carpel), usually anatropous or hemianatropous (rarely orthotropous), ascending, unitegmic, tenuinucellar. Integument ? cell layers thick. Megasporangial endothelium present. Hypostase usually present. Megagametophyte monosporous, Polygonum type. Antipodal cells sometimes persistent. Endosperm development cellular. Endosperm haustoria chalazal or micropylar and chalazal. Embryogenesis usually ? (in Catalpa onagrad).
Fruit Usually a loculicidal or septicidal capsule (often very large and lignified, often with replum; in Crescentieae and Colea clade usually indehiscent), often with extrafloral nectaries.
Seeds Seeds flat. Aril absent. Testa usually with membranous or suberous wings (in species with capsular fruit); wing cells with usually annular or helical (sometimes reticulate) thickenings. Exotestal cells? Endotesta? Perisperm not developed. Endosperm usually absent. Embryo straight, oily, without chlorophyll. Cotyledons two, usually large, lobate, foliaceous, obcordate, persistent. Germination usually phanerocotylar (sometimes cryptocotylar).
Cytology n = 11, 13–15, (18–)20, (21) (40) – Polyploidy occurring.
DNA Deletion present in plastid gene matK. Mitochondrial coxI intron present in Catalpa.
Phytochemistry Flavonols (quercetin), 6- or 8-hydroxyflavones or 6-methoxyflavones, Route II decarboxylated iridoids, Group I carbocyclic iridoids (catalpol, macfadienoside), Group X secoiridoids (iridoid pyridine alkaloids), iridoid glucosides, iridoid aldehydes, ursolic acid and caffeic acid esters (cornosides in Eccremocarpus, verbascosides), saponins, shikimic acid derived arthroquinones, and naphthoquinones present. Ellagic acid, proanthocyanidins and cyanogenic compounds not found. Carbohydrates stored as stachyose and other oligosaccharides.
Use Ornamental plants, medicinal plants, timber, ropes, calabashes and musical instruments (Crescentia), dyeing substances.
Systematics (according to Olmstead & al. 2009)
The sister-group relationship of Bignoniaceae is unresolved, although Refulio-Rodriguez & Olmstead (2014) recovered the following topology (with weak support): [Bignoniaceae+[[Schlegeliaceae+Lentibulariaceae]+[[Thomandersiaceae+Verbenaceae]+[Lamiaceae+[Mazaceae+[Phrymaceae+[Paulowniaceae+[Rehmanniaceae+Orobanchaceae]]]]]]]].
A probable topology of Bignoniaceae is the following: [Jacarandeae+[Tourrettieae+ [Tecomeae+[Delostoma+[Bignonieae+[[Catalpeae+Oroxyleae]+[Crescentieae+Coleeae]]]]]]]
Jacarandeae Seem. in Ann. Mag. Nat. Hist., ser. 3, 10: 31. Jul 1862
2/50–53. Jacaranda (c 50; Mexico, Central America, the West Indies, tropical South America), Digomphia (3; D. ceratophora, D. densicoma, D. laurifolia; the Guayana Highlands in southeastern Colombia, southern Venezuela, northwestern Brazil and Guyana). – Tropical and subtropical America. Evergreen trees or shrubs. Sepals free or almost free. Staminodium large, with beard-like indumentum. Placentation parietal. Fruit orbicular, angustiseptate. n = 18.
[Tourrettieae+[Tecomeae+[Delostoma+[Bignonieae+[[Catalpeae+Oroxyleae]+[Crescentieae+Coleeae]]]]]]
Tourrettieae G. Don, Gen. Hist. 4: 215, 231. 1837-8 Apr 1838 [‘Tourretieae’]
2/4. Eccremocarpus (3; E. longiflorus, E. scaber, E. vargasianus; Peru, Chile, western Argentina), Tourrettia (1; T. lappacea; Mexico to the Andes in tropical South America). – Mexico to Chile. Herbs twining with leaf-tendrils. Inflorescence a bracteate raceme. Staminodium absent in Tourrettia. Tourrettia with quadrilocular ovary having one row of ovules in each locule. Placentation in Eccremocarpus parietal. Cornosides present in Eccremocarpus.
[Tecomeae+[Delostoma+[Bignonieae+[[Catalpeae+Oroxyleae]+[Crescentieae+Coleeae]]]]]
Leaves once compound. Staminodia sometimes present, simple.
Tecomeae Endl., Gen. Plant.: 711. Jan 1839
11/71–72. Argylia (13; southern Peru, Chile, Argentina); Campsis (2; C. grandiflora: East Asia; C. radicans: southeastern United States), Tecoma (14; tropical and southern Africa, Arizona to northern Argentina, the West Indies), Incarvillea (c 16; Central Asia and the Himalayas to East Asia), Podranea (1; P. ricasoliana; tropical and southern Africa), Deplanchea (c 8; Malesia to New Guinea, northeastern Queensland and New Caledonia), Lamiodendron (1; L. magnificum; New Guinea), Tecomanthe (5–6; T. dendrophila, T. ternatensis: Malesia to New Guinea, New Britain, Solomon Islands; T. volubilis: New Guinea; T. hillii: eastern Queensland, northeastern New South Wales; T. speciosa: Three Kings Islands off New Zealand), ‘Pandorea’ (9; East Malesia to New Guinea, Australia, New Caledonia; polyphyletic), Campsidium (1; C. valdivianum; Chile, Argentina), Astianthus (1; A. viminalis; Mexico to Nicaragua). – Warm-temperate to tropical regions. Distinctive C-4 formyl iridoids present. – Argylia is sister-group to the remaining Tecomeae, with Campsis as successive sister to the rest. In Campsis and species of Tecoma the abaxial calyx nectaries have a cellular structure very similar to that in Thomandersia (Thomandersiaceae; Wortley & al. 2005).
[Delostoma+[Bignonieae+[[Catalpeae+Oroxyleae]+[Crescentieae+Coleeae]]]]
Delostoma clade
1/5. Delostoma (5; D. dentatum, D. gracile, D. hookeri, D. integrifolium, D. lobbii; the Andes).
[Bignonieae+[[Catalpeae+Oroxyleae]+[Crescentieae+Coleeae]]]
Bignonieae Dumort., Anal. Fam. Plant.: 23. 1829 [‘Bignoniaceae’]
20/415–420. Perianthomega (1; P. vellozoi; southeastern Brazil, southeastern Bolivia, northeastern Paraguay); Adenocalymma (84; southern Mexico, the Lesser Antilles, Central America to southeastern Brazil), Stizophyllum (c 20; southern Mexico, Central America, tropical South America); Manaosella (1; M. cordifolia; Venezuela, Brazil, Bolivia), Pleonotoma (16; northern Central America to southern Brazil), Dolichandra (8; southeastern United States, Mexico, the West Indies, Central America, tropical South America), Amphilophium (43; southern Mexico, the West Indies, Central America, tropical South America), Bignonia (28; southeastern United States, Mexico, Central America, tropical South America), Mansoa (12; southern Mexico, Central America, the West Indies, tropical South America), Anemopaegma (45; southern Mexico, Central America, tropical South America), Pyrostegia (1; P. venusta; Mexico, Central America, tropical South America); Callichlamys (1; C. latifolia; southern Mexico, Central America, tropical South America), Martinella (3; M. insignis, M. iquitoensis, M. obovata; southern Mexico, Central America, tropical South America), Pachyptera (5; P. aromatica, P. erythraea, P. incarnata, P. kerere, P. linearis; Central America, tropical South America), Tanaecium (16–18; southern Mexico, the West Indies, Central America, tropical South America), Cuspidaria (16–19; southern Mexico, Central America, tropical South America), Tynanthus (c 20; southern Mexico, Central America, tropical South America), Lundia (c 20; southern Mexico, Central America, tropical South America), Xylophragma (7; X. harleyi, X. heterocalyx, X. myrianthum, X. platyphyllum, X. pratense, X. seemannianum, X. unifoliolatum; southern Mexico, Central America, tropical South America), Fridericia (68; Mexico, Central America, tropical South America). – Southeastern United States to tropical South America. Lianas with leaf-tendrils. Secondary lateral growth anomalous, via concentric cambia or cylindrical cambium (xylem cylinder in principle quadrilobate, hypothetical basal condition), phloem discontinuous. Leaves usually ternate. Stamens in Paragonia two fertile stamens and three staminodia. Fruit usually a septifragal capsule (sometimes also loculicidal) with persistent septum and separate whip-like vascular strands of lignified tissue (vascular bundles opposite septum). – Perianthomega is sister to the remaining Bignonieae. It has biternate leaves, stout simple tendrils representing petioles and three small remnants of leaflets present at their apices. Adenocalymma is successive sister to the remainder.
[[Catalpeae+Oroxyleae]+[Crescentieae+Coleeae]]
[Catalpeae+Oroxyleae]
Catalpeae DC. ex Meisn., Plant. Vasc. Gen.: Tab. Diagn. 300, Comm. 208. 25-31 Oct 1840
1–2/11. Catalpa (10; East Asia, southeastern United States, the Greater Antilles; incl. Chilopsis?), Chilopsis (1; C. linearis; southwestern United States; in Catalpa?). – East Asia, southeastern United States, the Greater Antilles. Leaves opposite or verticillate (Catalpa) or alternate (spiral; Chilopsis), simple. Stamens in Catalpa two fertile stamens and three staminodia.
Oroxyleae A. H. Gentry ex Reveal et L. G. Lohmann in Phytoneuron 2012-37: 218. 23 Apr 2012
4/6–8. Hieris (1; H. curtisii; Penang in Malaysia), Millingtonia (1; M. hortensis; India to Burma and Southeast Asia, Malesia), Nyctocalos (3–5; N. brunfelsiiflorum, N. cuspidatum, N. jucundum, N. pinnatum, N. thomsonii; northeastern India to Burma, Yunnan and Southeast Asia, West Malesia), Oroxylum (1; O. indicum; Sri Lanka, India and the Himalayas to southern China, Southeast Asia, Malesia to Sulawesi and Timor). – Tropical Asia. Leaves sometimes bicompound. Flowers sometimes actinomorphic. Stamens in Oroxylum five. Fruit a septicidal capsule.
[Crescentieae+Coleeae]
Fruit more or less indehiscent, sometimes berry-like.
Coleeae Bojer ex Reveal in Phytoneuron 2012-37: 217. 23 Apr 20122012
18/140–145. ‘Rhigozum’ (7; R. brevispinosum, R. madagascariense, R. obovatum, R. somalense, R. trichotomum, R. virgatum, R. zambesiacum; northeast tropical and southern Africa, Madagascar; paraphyletic), Spathodea (1; S. campanulata; tropical West and Central Africa), Catophractes (1; C. alexandri; subtropical and southern Africa); Radermachera (c 17; southern China, Southeast Asia, West Malesia), Tecomella (1; T. undulata; the Arabian Peninsula to western India); Kigelia (1; K. africana; tropical and southern Africa), Stereospermum (20–25; tropical Africa, Madagascar, tropical Asia), Newbouldia (1; N. laevis; tropical West and Central Africa), Fernandoa (c 15; tropical Africa, Madagascar, southern China, Southeast Asia to Sumatra), Heterophragma (2; H. quadriloculare: India; H. sulfureum: Southeast Asia), Dolichandrone (10; East Africa, India to northern Australia, New Caledonia, islands in the Pacific), Markhamia (5–6; tropical and southern Africa, tropical Asia), Dinklageodoxa (1; D. scandens; Liberia), Perichlaena (1; P. richardi; Madagascar), Phylloctenium (2; P. bernieri, P. decaryanum; Madagascar), Phyllarthron (c 15; Madagascar, the Comoros), Rhodocolea (c 10; Madagascar), Colea (c 30; Madagascar, the Comoros, Mauritius, the Seychelles). – Tropical Africa, Madagascar, Indian Ocean islands, northwestern and western India, Southeast Asia, Malesia and eastwards to northern Queensland and Southwest Pacific Islands. Leaves sometimes phyllodinous, articulated. Inflorescence cauliflorous. Stamens in Catophractes and ‘Rhigozum’ five. Fruit indehiscent or almost indehiscent. Testa unwinged?
Crescentieae G. Don, Gen. Hist. 4: 216, 232. 1837-8 Apr 1838
12/c 160. Sparattosperma (4; S. catingae, S. leucanthum, S. rosea, S. vernicosum; tropical South America); Cybistax (1; C. antisyphilitica; Amazonian Brazil, eastern Peru), Godmania (5; G. aesculifolia, G. dardanoi, G. luteola, G. macrocarpa, G. uleana; southern Mexico, Central America, tropical America), Zeyheria (2; Z. montana, Z. tuberculosa; Brazil), Ekmanianthe (2; E. actinophylla, E. longiflora; Cuba, Hispaniola), ‘Tabebuia’ (c 75; Mexico, Central America, the West Indies, tropical South America; polyphyletic), Roseodendron (2; R. chryseum: northern Colombia, northwestrn Venezuela; R. donnell-smithii: southern Mexico, Central America), Handroanthus (c 30; Central America, the West Indies, tropical South America), Parmentiera (9; southern Mexico, Central America, northwestern Colombia), Spirotecoma (5–6; S. apiculata, S. guantanamense, S. holguinensis, S. rubriflora, S. spiralis; Cuba, Hispaniola), Amphitecna (19; southern Mexico, Central America, tropical South America), ‘Crescentia’ (6; C. alata, C. amazonica, C. cujete, C. linearifolia, C. mirabilis, C. portoricensis; southern United States, Mexico, Central America, the West Indies, tropical South America; paraphyletic). – Tropical America including Cuba and Hispaniola. Leaves palmately compound (sometimes unifoliolate); in, e.g., Amphitecna and ‘Crescentia’ spiral, simple, phyllodinous. Inflorescence sometimes cauliflorous. Fruit sometimes (e.g. Amphitecna and ‘Crescentia’) indehiscent. Testa sometimes unwinged. – Sparattosperma may be sister to the remaining Crescentieae.
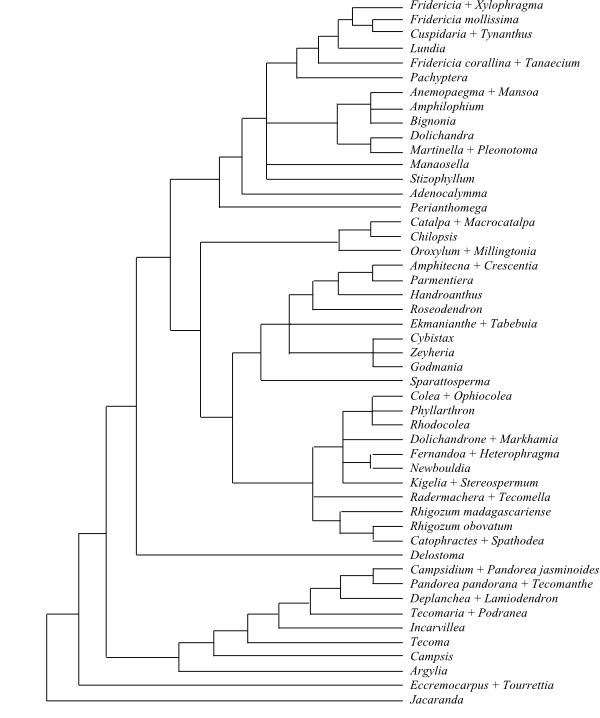 |
Parsimony strict consensus tree of Bignoniaceae based on DNA sequence data (Olmstead & al. 2009). |
Unplaced Bignoniaceae
Neosepicaea (5; N. aurantiaca, N. jucunda, N. leptophylla, N. superba, N. viticoides; the Moluccas, New Guinea, eastern Queensland), Pajanelia (1; P. longifolia; tropical Asia), Paratecoma (1; P. peroba; coastal regions in Brazil), Pauldopia (1; P. ghorta; northeastern India to Southeast Asia), Romeroa (1; R. verticillata; Colombia), Santisukia (2; S. kerrii, S. pagetii; Thailand).
BYBLIDACEAE (Engl. et Gilg) K. Domin |
( Back to Plantaginales ) |
Byblidales Nakai ex Reveal in Phytologia 74: 175. 25 Mar 1993
Genera/species 1/8
Distribution Northern and Western Australia, southern New Guinea.
Fossils A fossil seed of Byblis has been reported from mid-Eocene strata in the Golden Grove area in South Australia.
Habit Bisexual, evergreen suffrutices, perennial or annual herbs, often with woody rhizome. Probably not insectivorous (leaves apparently absorbing exudates/faeces from carnivorous mirids, cf. Roridula). Mycorrhiza absent.
Vegetative anatomy Mycorrhiza absent. Roots fibrous. Phellogen? Young stem with vascular tissue as separate bundles. Secondary lateral growth absent. Endodermis a starch sheath. Vessel elements usually with simple (sometimes scalariform) perforation plates; lateral pits alternate, bordered pits. Imperforate tracheary xylem elements tracheids with bordered pits, non-septate. Wood rays usually biseriate or triseriate (sometimes uniseriate), homocellular. Axial parenchyma apotracheal diffuse, or paratracheal scanty. Sieve tube plastids Ss type. Nodes 1:1 or 1:3, unilacunar with one or three leaf traces.
Trichomes Glandular hairs stalked or sessile, non-vascularized; glands with mucilaginous head usually consisting of a layer of (eight to) 32 cells radiating like an umbrella outwards from centre of head.
Leaves Alternate (spiral), linear to filiform, with flat or circinate, abaxially curved, ptyxis. Stipules and leaf sheath absent. Petiole vascular bundles? Veins parallelodromous (lamina reduced or absent?). Stomata paracytic. Cuticular wax crystalloids? Lamina covered with mucilage-secreting insect-trapping glandular hairs. Leaf margin entire, with vascularized apical hydathode. Foliar apex a knob-like swelling.
Inflorescence Flowers axillary, solitary. Bracts and floral prophylls (bracteoles) absent.
Flowers Slightly zygomorphic. Hypogyny. Sepals five, with imbricate aestivation, persistent, connate at base. Petals five, with contorted aestivation, connate at base, serrate to almost fimbriate at apex. Nectary absent. Disc absent.
Androecium Stamens five, haplostemonous, antesepalous, alternipetalous, displaced and often bent against one side of flower. Filaments short, subulate, free from each other, twisted, often slightly adnate to petals (epipetalous). Anthers connivent, basifixed, non-versatile, with cone-shaped apex and ephemeral epidermal cells, tetrasporangiate, introrse, poricidal (dehiscing by apical pores or short apical slits). Tapetum secretory, with binucleate cells. Staminodia absent.
Pollen grains Microsporogenesis simultaneous. Pollen grains 3(–4)-colpate (or tricolporate? or tetra- or hexarugate?), shed as monads, bicellular at dispersal. Exine tectate, with columellate? infratectum, punctitegillate.
Gynoecium Pistil composed of two connate carpels. Ovary superior, bilocular. Style single, simple, long, filiform. Stigma twisted, usually punctate to capitate (sometimes slightly bilobate), type? Pistillodium absent.
Ovules Placentation apical-axile. Ovules two to numerous per carpel, anatropous, unitegmic, tenuinucellar. Integument ? cell layers thick. Megagametophyte monosporous, Polygonum type. Antipodal cells persistent. Endosperm development ab initio cellular. Endosperm haustoria chalazal and micropylar. Embryogenesis onagrad.
Fruit A loculicidal capsule.
Seeds Aril absent. Exotestal cells tangentially elongate, with anticlinal walls not uniformly thickened. Mesotesta sclerenchymatous. Endotesta? Perisperm not developed. Endosperm copious, starchy and aleuroniferous. Embryo straight, elongate, chlorophyll? Cotyledons two, foliaceous. Germination phanerocotylar.
Cytology n = 8, (9) (12) 16 – Protein inclusions present in nucleus?
DNA Deletion in plastid gene matK?
Phytochemistry Insufficiently known. 6- and/or 8-hydroxylated flavone glycosides or 6-methoxy flavones present. Proanthocyanidins, cyanogenic compounds, and naphthoquinones not found. Iridoids? Carbohydrates stored as stachyose and other oligosaccharides.
Use Occasionally as ornamental plants.
Systematics Byblis (8; B. aquatica, B. filifolia, B. gigantea, B. guehoi, B. lamellata, B. liniflora, B. pilbarana, B. rorida; northern and western Australia, southern New Guinea).
The sister-group relationship of Byblis is unresolved. In Schäferhoff & al. (2010) it is part of trichotomy also including Linderniaceae and the remaining Plantaginales “above” Scrophulariaceae.
CALCEOLARIACEAE (D. Don) Olmstead |
( Back to Plantaginales ) |
Genera/species 2/390–395
Distribution Mexico to western South America, the Falkland Islands, New Zealand.
Fossils Unknown.
Habit Bisexual, perennial or annual herbs, suffrutices or shrubs.
Vegetative anatomy Phellogen? Vessel elements with simple? perforation plates; lateral pits? Vestured pits? Imperforate tracheary xylem elements? Wood rays? Axial parenchyma? Sieve tube plastids S type? Nodes? Crystals?
Trichomes Hairs unicellular or multicellular; glandular hairs often present.
Leaves Opposite, simple or pinnately compound, entire or pinnately lobes, sometimes connate at base, with ? ptyxis. Stipules and leaf sheath absent. Petiole vascular bundles? Venation pinnate or palmate. Stomata? Cuticular wax crystalloids? Leaf margin usually serrate or crenate (rarely entire).
Inflorescence Terminal, reduced thyrse with accessory paired flowers; thyrse consisting of one terminal flower and flower from its reduced prophyll (bracteole).
Flowers Zygomorphic. Usually hypogyny (sometimes half epigyny). Sepals four, with valvate aestivation, connate, orthogonally arranged/initiated. Petals four, with ascending cochlear aestivation? (adaxial corolla lobes of buds inserted outside the others), bilabiately connate, diagonally arranged/initiated; corolla bilabiate with large usually inflated saccate lower (abaxial) lip and small upper (adaxial) lip (fusion occurring late during development of each pair of petals; adaxial lip in Calceolaria triandra bipartite); inner side of lower (abaxial) corolla lip usually with elaiophores (oil glands attracting bees and bumblebees; absent in Jovellana) formed by hair cushions. Nectary usually absent (sometimes hair-like). Disc absent.
Androecium Stamens usually two, adaxial-lateral (in Calceolaria triandra three, i.e. two adaxial-lateral and one abaxial-median stamen). Filaments free from each other, adnate to corolla tube (epipetalous). Anthers basifixed, with thecae usually diverging, when dehiscing often confluent (sometimes parallel; sometimes with one theca), non-versatile, tetrasporangiate, dehiscing by apical pore-like slits. Placentoid? Tapetum secretory? Staminodia present or absent.
Pollen grains Microsporogenesis simultaneous? Pollen grains usually tricolpate?, shed as monads, bicellular at dispersal. Exine?, with ? infratectum, sculpturing?
Gynoecium Pistil composed of two connate carpels. Ovary usually superior (sometimes semi-inferior), bilocular. Style single, simple. Stigma small, capitate or slightly bilobate, type? Pistillodium absent.
Ovules Placentation axile. Ovules numerous per carpel, anatropous?, unitegmic, tenuinucellar. Integument three or four cell layers thick. Endothelium present; endothelial cells elongating (aulacospermous). Megagametophyte monosporous, Polygonum type. Endosperm development ab initio cellular. Endosperm haustoria? Embryogenesis onagrad.
Fruit A septicidal and loculicidal capsule.
Seeds Aril? Seed pedestals present. Testa with usually sinuate (sometimes straight) anticlinal cell walls. Exotesta? Endotesta? Perisperm not developed. Endosperm copious, prominent with longitudinal furrows. Embryo?, chlorophyll? Cotyledons two. Germination?
Cytology n = 8, 9, 15, 16 – Protein bodies in cell nuclei lamellar or absent.
DNA Mitochondrial coxI intron present. Deletion in plastid gene matK?
Phytochemistry Virtually unknown. Shikimic acid derived anthraquinones?
Use Ornamental plants.
Systematics Calceolaria (385–390; central Mexico, Central America, western South America southwards to Patagonia and the Falkland Islands, with their largest diversity in the Andes), Jovellana (8; J. albula, J. guentheri, J. petiolaris, J. punctata, J. sturmii, J. violacea: Chile; J. repens, J. sinclairii: New Zealand).
Calceolariaceae may be part of a clade [Calceolariaceae+Gesneriaceae+Peltanthera] or sister to Gesneriaceae. However, the support for any sister-group relationship among Calceolariaceae, Peltanthera and Gesneriaceae is not very high.
In spite of Calceolaria being one of the most frequently cultivated ornamental plants, the information on anatomy, pollen morphology, embryology, cytology, phytochemistry, etc. is extremely deficient.
CARLEMANNIACEAE Airy Shaw |
( Back to Plantaginales ) |
Carlemanniales Doweld, Tent. Syst. Plant. Vasc.: xlviii. 23 Dec 2001
Genera/species 2/5
Distribution Assam, eastern Himalayas, southern China, Southeast Asia, Sumatra.
Fossils Unknown.
Habit Bisexual, shrubs or perennial herbs.
Vegetative anatomy Phellogen ab initio cortical (Carlemannia). Vessel elements with simple perforation plates; lateral pits alternate? Vestured pits? Imperforate tracheary xylem elements? Wood rays heterocellular? Axial parenchyma? Sieve tube plastids S type. Nodes ?-lacunar with ? leaf traces, swollen. Calciumoxalate raphides absent. Palisade parenchyma with stellate (calciumoxalate?) crystals.
Trichomes Glandular hairs peltate, with unicellular stalk and multicellular head; cells in head with exclusively vertical walls.
Leaves Opposite, simple, entire, sometimes distinctly asymmetrical, with ? ptyxis. Stipules and leaf sheath absent. Petiole bases joined by line. Petiole vascular bundles? Venation pinnate, brochidodromous. Stomata anomocytic (Carlemannia) or diacytic (Silvianthus). Cuticular waxes absent. Leaf margin serrate. Extrafloral nectaries present in leaf axils in Carlemannia.
Inflorescence Terminal and axillary, paniculate, corymboid or head-like, cymose.
Flowers Somewhat obliquely zygomorphic to almost actinomorphic. Epigyny. Sepals four or five, with open aestivation, often unequal in size, persistent, connate. Petals four or five, with imbricate or induplicate-valvate aestivation, connate into campanulate or infundibuliform corolla. Nectaries present on ovary apex. Disc cylindrical or conical. Heterostyly present in Silvianthus.
Androecium Stamens two, antesepalous, alternipetalous. Filaments short, free from each other, adnate to corolla tube (epipetalous). Anthers dorsifixed, connivent around style, versatile?, tetrasporangiate, introrse (to latrorse?), longicidal (dehiscing by longitudinal slits). Placentoid? Tapetum secretory? Staminodia absent.
Pollen grains Microsporogenesis simultaneous? Pollen grains tricolpate (Silvianthus) or penta- or hexacolpate (Carlemannia), shed as monads, ?-cellular at dispersal. Exine semitectate, with columellate infratectum, reticulate.
Gynoecium Pistil composed of two connate carpels. Ovary inferior, bilocular. Style single, simple, elongate. Stigma clavate to fusiform, bifid, type? Pistillodium absent.
Ovules Placentation axile to subbasal. Ovules numerous per carpel, anatropous, unitegmic, tenuinucellar. Integument ? cell layers thick. Megagametophyte monosporous, Polygonum type. Endosperm development nuclear? Endosperm haustorium? Embryogenesis?
Fruit A dry bifid loculicidal capsule (Carlemannia) or a quadrilobate or quinquelobate fleshy capsule (Silvianthus) with persistent calyx (capsule valves same number as calyx lobes).
Seeds Aril? Exotestal cells palisade?, narrow, polygonal, with all walls thickened (Carlemannia) or with thickened radial walls and inside these cells with unthickened walls (Silvianthus). Endotesta? Perisperm not developed. Endosperm copious, fleshy, oily, ruminate (Silvianthus). Embryo small, chlorophyll? Cotyledons two. Germination?
Cytology n = 15 (Carlemannia), n = 19 (Silvianthus) – Protein bodies present in nucleus?
DNA
Phytochemistry Virtually unknown. Iridoids? Alkaloids not found.
Use Unknown.
Systematics Carlemannia (3; C. congesta, C. griffithii, C. tetragona; Assam, eastern Himalayas, southwestern China, mountains in Southeast Asia to Sumatra), Silvianthus (2; S. bracteatus, S. tonkinensis; Assam, southern China, Southeast Asia).
Carlemanniaceae are sister-group to Oleaceae.
Since Carlemanniaceae are basal in Plantaginales, it is probable that Route II decarboxylated iridoids are absent.
GESNERIACEAE Rich. et Juss. ex DC. |
( Back to Plantaginales ) |
Belloniaceae Martinov, Tekhno-Bot. Slovar: 67. 3 Aug 1820 [’Bellonides’]; Gesneriales Rich. ex Bercht. et J. Presl, Přir. Rostlin: 252. Jan-Apr 1820 [‘Gesneriae’]; Didymocarpaceae D. Don in Edinburgh Philos. J. 7: 83. 1822 [’Didymocarpeae’]; Cyrtandraceae Jack in Trans. Linn. Soc. London 14: 23. 1823; Gesneriineae Link, Handbuch 1: 505. 4-11 Jul 1829 [‘Gesneriaceae’]; Besleriaceae Raf., Sylva Tellur.: 70. Oct-Dec 1838 [’Beslerides’]; Cyrtandrineae J. Presl in Nowočeská Bibl. [Wšobecný Rostl.] 7: 981. 1846 [‘Cyrtandraceae’]; Ramondaceae Godr. in J. C. M. Grenier et D. A. Godron, Fl. France 2: 506. 1853
Genera/species 148/3.220–3.440
Distribution Tropical and subtropical regions in the Northern and Southern Hemispheres; some species in temperate regions.
Fossils Unknown.
Habit Usually bisexual (rarely monoecious), usually perennial herbs (sometimes lianas, rarely trees, shrubs or annual herbs). Many species are epiphytic. Stem and leaves often more or less succulent. Root fibrous.
Vegetative anatomy Phellogen ab initio superficially or deeply seated. Endodermis sometimes prominent. Vessel elements usually with simple (rarely scalariform) perforation plates; lateral pits alternate, bordered pits. Vestured pits? Imperforate tracheary xylem elements usually libriform fibres (in Coronanthera fibre tracheids) with simple or (vestigially) bordered pits, septate or non-septate. Wood rays uniseriate or multiseriate, homocellular or heterocellular, or absent. Axial parenchyma apotracheal diffuse, or paratracheal scanty vasicentric, or absent. Cambium sometimes storied. Wood elements (fibres) occasionally storied. Tyloses present. Sieve tube plastids S type. Nodes usually 1:1, unilacunar with one leaf trace, or 3:3, trilacunar with three traces (rarely 4:4, quadrilacunar, or 5:5, quinquelacunar), sometimes with lateral vascular bundles split at nodes. Schizogenous secretory canals with oils or resins present in some Epithematoideae. Sclereids of various ouline abundant. Calciumoxalate frequent (sometimes as rhomboidal crystals or raphides). Cortical cells often with druses and crystals.
Trichomes Hairs usually simple, uniseriate (rarely multiseriate, rarely branched), unicellular or multicellular, often with thickened terminal cells (sometimes calcified/silicified); stalked multicellular (sometimes lepidote) glandular hairs sometimes frequent. Extrafloral nectaries rarely present on petiole.
Leaves Usually opposite (rarely verticillate, spiral or distichous; sometimes seemingly alternate due to anisophylly, especially in Epithematoideae), usually simple (rarely pinnately compound), usually entire (rarely lobed; rarely with one cotyledon developing into solitary large leaf), often coriaceous, with involute ptyxis. Stipules and leaf sheath absent. Leaves connate pairwise at base. Petiole vascular bundle transection arcuate; bundles variously arranged. Venation pinnate. Stomata usually anomocytic (often large; sometimes anisocytic, helicocytic or paracytic). Cuticular wax crystalloids? Mesophyll with or without sclerenchymatous idioblasts. Secretory cavities with oils or resins present in some epithematoid genera. Leaf margin serrate, crenate, lobed or entire.
Inflorescence Usually axillary (sometimes terminal), thyrsoid; flowers often paired, or solitary axillary (in Chirita, Didymocarpus, and Streptocarpus sometimes epiphyllous). Accessory flowers often present.
Flowers Usually zygomorphic (rarely inverted 180o; rarely actinomorphic). Hypogyny, half epigyny or epigyny. Sepals (four or) five, usually with valvate (rarely imbricate) aestivation, usually connate into tube (calyx sometimes bilabiate or trilabiate; sepals sometimes free). Petals (four or) five, with usually descending cochlear aestivation, adaxial petals usually posterior, connate into infundibuliform, tubular, campanulate or hypocrateriform and usually bilabiate (two upper and three lower lobes) corolla (sometimes quinquelobate), sometimes with spur. Nectariferous disc intrastaminal, annular or cupular, sometimes unilateral or consisting of separate glands (disc sometimes absent); nectary vascularized from androecial trace.
Androecium Stamens usually two longer and two shorter (didynamous), fifth (adaxial) stamen staminodial or absent (sometimes two fertile stamens and three staminodia; rarely all five stamens fertile), haplostemonous, antesepalous, alternipetalous. Filaments usually free from each other, adnate to corolla tube (epipetalous). Anthers usually basifixed (rarely dorsifixed), usually connivent (sometimes connate, rarely separate) pairwise or all together, versatile?, tetrasporangiate, introrse?, longicidal (dehiscing by longitudinal slits) or poricidal (dehiscing by apical, rarely basal, pores). Tapetum secretory, with binucleate to quinquenucleate cells. Staminodia one to three or absent.
Pollen grains Microsporogenesis simultaneous. Pollen grains (2–)3(–6)-colpor(oid)ate or (2–)3(–6)-colpate, shed as monads, bicellular at dispersal. Exine tectate or semitectate, with columellate infratectum, usually perforate or reticulate, verrucate, scabrate, psilate or smooth.
Gynoecium Pistil composed of two connate carpels. Ovary superior, inferior or semi-inferior, usually unilocular (sometimes secondarily bilocular by ingrowth of placentae; rarely primarily bilocular or with one carpel sterile, pseudomonomerous). Style single, simple, narrow. Stigma capitate or broadly bifid to trumpet-shaped, papillate, Dry or Wet type. Pistillodium?
Ovules Placentation usually intrusively parietal (rarely axile and ovary thus primarily bilocular). Ovules numerous per carpel, usually anatropous (rarely orthotropous), unitegmic, tenuinucellar (reduced, with meiocyte semi-inferior). Integument three to five cell layers thick. Megagametophyte monosporous, Polygonum type. Synergids sometimes with long narrowing tips, entirely penetrating micropyle. Endosperm development cellular. Endosperm haustoria chalazal and micropylar. Embryogenesis onagrad.
Fruit Usually a loculicidal and/or septicidal capsule (sometimes fleshy, rarely irregularly dehiscing or a pyxidium; sometimes berry- or nut-like).
Seeds Aril present or absent. Exotestal cells elongate, with thickened walls. Endotestal cells degenerating or persistent. Perisperm not developed. Endosperm copious (multi-layered in neotropical Gesneriaceae) or sparse (uni-layered in paleotropical Gesneriaceae), oily, or absent. Embryo straight, without chlorophyll. Cotyledons two, often with non-uniform growth (in some species a single cotyledon, macrocotyledon, continuing its growth and being single leaf of plant); radicula sometimes with limited growth. Germination phanerocotylar.
Cytology n = 4, 7–18(–21, 24, 28, 30, 32, 36, 48, 64) – Polyploidy occurring. Protein bodies in cell nucleus lamellar?
DNA Deletion in plastid gene matK. Nuclear gene GCyc duplicated. Mitochondrial coxI intron present in Drymonia and Nematanthus.
Phytochemistry Flavones, 6- and/or 8-hydroxylated flavone glycosides, aurones and chalcones (paleotropical Gesneriaceae), 3-desoxyanthocyanins (neotropical Gesneriaceae), tannins, caffeic acid, caffeic acid esters (cornosides, verbascosides), naphthoquinones, and shikimic acid derived anthraquinones present. Flavonols, iridoids, ellagic acid, proanthocyanidins, alkaloids, saponins, and cyanogenic compounds not found. Carbohydrates stored as stachyose and other oligosaccharides.
Use Ornamental plants, medicinal plants.
Systematics The clade [Calceolariaceae+Gesneriaceae] is sister to the remaining Plantaginales “beyond” Tetrachondraceae. – For information on Peltanthera, see Plantaginales incertae sedis.
Sanangoideae A. Weber, J. L. Clark et Mich. Möller in Selbyana 31(2): 83. Dec 2013
1/1. Sanango (1; S. racemosum; eastern Peru) was sister to the remaining Gesneriaceae, according to Perret & al. (2013).
Analyses of DNA sequences identify Sanango racemosum outside (alternatively at the base of) Gesneriaceae (e.g. Perret & al. 2013). In ndhF analyses by Smith, Brown & al. (1997) it is recovered as sister to Gesneria. It is a small tree with opposite leaves. The hypogynous flowers are, often pairwise, arranged in terminal thyrses. The sepals are connate at base, and the petals have cochlear aestivation and are connate into a curved tube. The nectary is cup-shaped. The stamens are four, with filaments adnate to base of corolla tube. The anthers are versatile, introrse and dehisce along a hippocrepomorphic line. A single small staminodium is present. The ovary is incompletely septate in upper part and completely septate in lower part. The stigma is capitate, bilobate, and with lobes laterally adnate to style and directed downwards. The placentation is axile. The fruit is a capsule, at first septicidal, later loculicidal, with long persistent style. n = 16. Sananga has sanangoside, a caffeoyl phenylethanoid glycoside (also suggesting at least a position within Plantaginales), whereas iridoids have not been found.
Gesnerioideae Burnett, Outlines Bot.: 959, 1095, 1108. Feb 1835 [’Gesneridae’]
77/1.380–1.400. Mainly neotropical, some genera in East Asia and southwestern Pacific. Testa usually without surface ornamentation. Testal cells usually much elongated, usually spirally arranged. Endosperm copious. Nuclear gene GCyc2 absent (lost). 3-desoxyanthocyanins present. Chalcones and aurones not found. – Napeanthus, Titanotrichum, Besleria, Cremosperma, Anetanthus, Gasteranthus, and Reldia were identified as basal to the remaining Gesnerioideae in analyses by, e.g., Zimmer & al. (2002) and Perret & al. (2013).
Titanotricheae Yamaz. ex W. T. Wang, Fl. Reipubl. Popularis sin. 69: 577. 1990
1/1. Titanotrichum (1; T. oldhamii; southeastern China, southern Japan, the Ryukyu Islands, Taiwan). Stomata sometimes anomocytic. Vegetative propagules (‘bulbils’) formed after flowering from elongated inflorescence apex. Testa with striate-reticulate surface, and isocotylous cotyledons. n = 20. – Titanotrichum may be sister to Napeanthus (Perret & al. 2013).
Napeantheae Wiehler in Selbyana 6: 151. 31 Aug 1983
1/>20. Napeanthus (>20; Central America, tropical South America). Flowers almost actinomorphic, without nectaries. n = 16.
Beslerieae Bartl., Ord. Nat. Pl.: 175. Sep 1830 [’Besleriea’]
9/>275. Besleriinae G. Don 1837-1838 [‘Beslerieae’]. Besleria (>200; tropical and subtropical America, with their largest diversity in the northern Andes), Gasteranthus (c 35; southern Mexico, Central America, tropical South America), Reldia (5; R. alternifolia, R. calcarata, R. grandiflora, R. minutiflora, R. multiflora; Panamá to northern Peru), Cremosperma (25–30; Panamá to the Andes in Peru). – Anetanthinae A. Weber et J. L. Clark in Selbyana 31(2). Dec 2013. Anetanthus (3; A. gracilis, A. parviflorus, A. rubra; central Colombia, southeastern Brazil, Peru, Bolivia), Resia (3; R. ichthyoides, R. nimbicola, R. umbratica; Colombia, Venezuela), Shuaria (1; S. ecuadorica; Ecuador); Cremospermopsis (2; C. cestroides, C. parviflora; Colombia), Tylopsacas (1; T. cuneata; the Guayana Highlands). – Besleria has n = 16.
Coronanthereae Fritsch in Engler et Prantl, Nat. Pflanzenfam. IV, 3b: 143. Mai 1893
9/21–29. Coronantherinae Fritsch in Engler et Prantl, Nat. Pflanzenfam. IV, 3b: 143. May 1893. Coronanthera (13–20; New Caledonia, Solomon Islands, eastern Queensland; paraphyletic?), Rhabdothamnus (1; R. solandri; North Island in New Zealand). – Mitrariinae Hanst. in Linnaea 26: 198, 199. 1854 [‘Beslerieae subtr. Mitrarieae’]. Asteranthera (1; A. ovata; southern Chile, southwestern Argentina), Mitraria (1; M. coccinea; southern Chile, southwestern Argentina), Sarmienta (1; S. scandens; southern Chile), Fieldia (1; F. australis; southeastern Queensland, eastern New South Wales, eastern Victoria). – Negriinae V. L. Woo, J. F. Smith et Garn.-Jones in Int. J. Pl. Sci. 172(3): 454. 2011. Depanthus (1–2; D. glaber, D. pubescens; New Caledonia), Lenbrassia (1; L. australiana; northeastern Queensland), Negria (1; N. rhabdothamnoides; Lord Howe). – Eastern Australia, Solomon Islands, New Caledonia, Lord Howe, New Zealand, southern South America. Trees, shrubs or perennial herbs. Stomata usually anomocytic (sometimes paracytic). Flowers usually zygomorphic (occasionally actinomorphic). Corolla sometimes fringed. Nectaries embedded in ovary wall, vascularized from staminal traces. Stamens sometimes two (adaxial pair) or five. Capsule usually septicidal (sometimes loculicidal-septicidal or fruit a berry with fleshy placentae). n = 37(–45). Nuclear gene GCyc duplicated. – The Australian-southern South American clade.
Gesnerieae Dumort., Anal. Fam. Plant.: 30. 1829 [‘Gesnereae’]
57/1.065–>1.075. Gesneriinae Oerst., Centralamer. Gesner.: 10. 1858 [‘Gesnereae’]. Bellonia (2; B. aspera: Hispaniola; B. spinosa: Cuba), Gesneria (45–50; the West Indies), Pheidonocarpa (1; P. corymbosa; Cuba, Jamaica; in Gesneria?), Rhytidophyllum (c 20; the West Indies; in Gesneria?). – Gloxiniinae G. Don, Gen. Hist. 4: 644, 645. 1837– 8 Apr 1838 [‘Gloxinieae’]. Chautemsia (1; C. calcicola; Minas Gerais in Brazil), Gloxinia (3; G. erinoides, G. perennis, G. xanthophylla; Costa Rica, tropical South America), Seemannia (4; S. gymnostoma, S. nematanthodes, S. purpurascens, S. sylvatica; the Andes from Ecuador to northern Argentina), Gloxinella (1; G. lindeniana; Cajamarca in Peru), Monopyle (17; Guatemala to Bolivia), Diastema (>20; Mexico, Central America, the Andes in Venezuela to Bolivia), Nomopyle (2; N. dodsonii, N. peruviana; Ecuador, Peru), Kohleria (17; Mexico, Central America, Trinidad, tropical South America to Peru and the Guianas, with their largest diversity in Colombia), Moussonia (11; southern Mexico to Panamá; in Kohleria?), Capanea (2; C. humboldtii, C. oerstedii; tropical America; in Kohleria?), Gloxiniopsis (1; G. racemosa; the Andes in Colombia), Amalophyllon (c 15; southern Mexico, Central America, the Andes in Venezuela to Peru), Pearcea (17; the Andes from northern Colombia to northwestern Bolivia), Eucodonia (2; E. andrieuxii, E. verticillata; central and southern Mexico), Smithiantha (8; Mexico, Guatemala), Niphaea (2; N. mexicana, N. oblonga; southern Mexico, Guatemala), Solenophora (16; Central America), Phinaea (3; P. albolineata, P. multiflora, P. pulchella; Mexico, Central America, Cuba, Hispaniola, northwestern South America to Venezuela and Peru), Heppiella (4; H. repens, H. ulmifolia, H. verticillata, H. viscida; the Andes in western Venezuela and Peru), Mandirola (3; M. ichthyostoma, M. multiflora, M. rupestris; Brazil), Goyazia (2; G. petraea, G. rupicola; central Brazil), Achimenes (24; southern Mexico, Central America, the West Indies, Colombia). – Columneinae Hanst. in Linnaea 26: 198, 199. Apr 1854 [‘Columneae’]. The Guayana Shield clade: Pagothyra (1; P. maculata; Venezuela, Guyana), Rhoogeton (1–4; R. viviparus; Guyana), Lembocarpus (1; L. amoenus; Suriname, French Guiana), Cremersia (1; C. platula; French Guiana); ‘Episcia’ (9; Nicaragua to tropical South America; paraphyletic), Alsobia (2; A. dianthiflora, A. punctata; southern Mexico, Belize, Guatemala, Costa Rica; in Episcia?), Cobananthus (1; C. calochlamys; Central America), ‘Nematanthus’ (c 30; southern and southeastern Brazil; paraphyletic; incl. Codonanthe?), Codonanthe (>25; tropical America, with their highest diversity in Brazil; in Nematanthus?), Crantzia (2; C. cristata, C. tigrina; the West Indies), Drymonia (>140; tropical America, with their highest diversity in Colombia and Ecuador; paraphyletic; incl. Neomortonia?), Neomortonia (2; N. nummularia, N. rosea; Central America, western Colombia; in Drymonia?), Alloplectus (10; Guatemala, Costa Rica, Panamá, the West Indies, the Andes in Colombia, northern Ecuador and Peru), Glossoloma (>20; southern Mexico, Central America, tropical South America to Bolivia), Columnea (>250; Central America, the West Indies, northern tropical South America), Paradrymonia (c 10; Central America, northern tropical South America), Centrosolenia (15; the Guayana Highlands), Chrysothemis (9; southern Mexico, Central America, the Lesser Antilles, northern tropical South America), ‘Nautilocalyx’ (>30; tropical America; paraphyletic), Trichodrymonia (c 50; southern Mexico, Central America, the Andes, tropical South America); unplaced Columneinae: Christopheria (1; C. xantha; Guyana, French Guiana), Corytoplectus (15; Panamá to coastal Venezuela and Bolivia, the Guayana Highlands), Lampadaria (1; L. rupestris; Guyana), Lesia (1; L. savannarum; Colombia to Suriname and Peru), Oerstedina (2; O. cerricola, O. suffrutescens; Central America), Pachycaulos (1; P. nummularium; southern Mexico to Peru), Rufodorsia (4; R. congestiflora, R. intermedia, R. major, R. minor; Costa Rica, Panamá). – Sphaerorrhizinae A. Weber et J. L. Clark in Selbyana 31(2): 85. Dec 2013. Sphaerorrhiza (2; S. burchellii, S. sarmentiana; Brazil). – Ligeriinae Hanst. in Linnaea 26: 198, 199. Apr 1854 [‘Ligerieae’]. Sinningia (c 75; Central America, tropical South America, with their highest diversity in eastern and southern Brazil), Vanhouttea (8; southeastern Brazil; in Sinningia?), Paliavana (5; P. gracilis, P. plumerioides, P. prasinata, P. racemosa, P. schiffneri; eastern and southeastern Brazil). – Southern Mexico, Central America, the West Indies, South America. Lateral vascular bundles in Episcieae dividing at nodes. Nodes sometimes 3:3, trilacunar with three leaf traces. Calciumoxalate styloids and raphides sometimes present. Leaves rarely spiral (Gesneria etc.). Petiole vascular bundle transection sometimes deeply arcuate to annular; wing bundles sometimes present. Stomata sometimes present on raised mounds. Flowers rarely resupinate. Hypogyny or epigyny. Nectaries vascularized from large number of vascular bundles in ovary wall. Fruit sometimes with fleshy placentae or funicles; rarely a berry. n = (8) 9 (10) 11 (12) 13–14 (16). – The Neotropical clade. – As indicated above, Gesnerieae may be paraphyletic.
[Didymocarpoideae+Epithemateae]
Nectaries vascularized from staminal traces. Ovary wall not very vascularized. Endosperm inconspicuous. Cotyledons unequal (one of them accrescent). Chalcones and aurones present. 3-desoxyanthocyanins not found. – The Paleotropical clade.
Didymocarpoideae Arn., Botany: 121. 9 Mar 1832 [‘Didymocarpeae’] (Trichosporeae Nees in Flora 8: 143. 7 Mar 1825)
62/1.760–1.980. Jerdoniinae A. Weber et Mich. Möller in Selbyana 31(2): 86. Dec 2013. Jerdonia (1; J. indica; southwestern India). – Corallodiscinae A. Weber et Mich. Möller in Selbyana 31(2): 87. Dec 2013. Corallodiscus (6; C. bhutanicus, C. cooperi, C. forrestii, C. grandis, C. kingianus, C. lanuginosus; eastern Himalayas, northern and northeastern India, southern and southwestern China, northern Thailand). – Tetraphyllinae A. Weber et Mich. Möller in Selbyana 31(2): 87. Dec 2013. Tetraphyllum (>3; T. bengalense, T. confertiflorum, T. roseum; northeastern India, Bangladesh, Burma, Thailand). – Leptoboeinae C. B. Clarke in J. D. Hooker, Fl. Brit. India 4: 337. Jan 1884 [‘Leptoboeae’]. 5(6)/42(43). Boeica (c 12; eastern Himalayas to southern China, Burma, northern Vietnam and the Malay Peninsula), Rhynchotechum (13–15; southern Himalayas to southern China, Southeast Asia, Malesia to New Guinea), Leptobaea (2; L. glabra, L. multiflora; eastern Himalayas to Yunnan, Burma and Thailand), Beccarinda (c 8; Assam, Burma, southern China, Hainan, Vietnam, Sumatra), Platystemma (1; P. violoides; the Himalayas, southwestern China), Championia (1; C. reticulata; Sri Lanka)? – Ramondinae DC. ex Meisn., Pl. Vasc. Gen.: Tab. Diagn. 302, Comm. 212. 25–31 Oct 1840 [‘Ramondieae’]. Haberlea (1; H. rhodopensis; the Rhodopi Mountains on southern Balkan Peninsula), Ramonda (3; R. myconi: the Pyrenees, northeastern Spain; R. nathaliae, R. serbica: southern Balkan Peninsula; incl. Jancaea?), Jancaea (1; J. heldreichii; Mount Olimbos in central Greece; in Ramonda?). – Litostigminae A. Weber et Mich. Möller in Selbyana 31(2): 87. Dec 2013. Litostigma (2; L. coriaceifolium, L. crystallinum; southwestern China). – Streptocarpinae Ivanina in Bot. Žurn. (Moscow et Leningrad) 50: 33. 1 Feb 1965. Streptocarpus (c 150; tropical and southern Africa, Madagascar; incl. Acanthonema, Colpogyne, Hovanella, Linnaeopsis, Nodonema, Saintpaulia, Schizoboea and Trachystigma?). – Didissandrinae A. Weber et Mich. Möller in Selbyana 31(2): 87. Dec 2013. Didissandra (8; West Malesia), Tribounia (2; T. grandiflora, T. venosa; Thailand). – Loxocarpinae A. DC. in A. P. et A. L. P. P. de Candolle, Prodr. 9: 277. 1 Jan 1845 [‘Loxocarpeae’]. Boea (17; northeastern India, southern China, northern Thailand, Vietnam, Central and East Malesia to New Guinea, northeastern Queensland, the Bismarck Archipelago, Solomon Islands), ‘Streptocarpus’ pro parte (Asia), Rhabdothamnopsis (1; R. sinensis; southern China), Senyumia (1; S. minutiflora; Pahang on the Malay Peninsula), Emarhendia (1; E. bettiana; Pahang on the Malay Peninsula), Spelaeanthus (1; S. chinii; Pahang on the Malay Peninsula, Batu Luas), Paraboea (95–100; Burma, southern China, Thailand to New Guinea), Middletonia (4; M. evrardii, M. monticola, M. multiflora, M. regularis; India, Bhutan and Burma to southern China and Southeast Asia), Kaisupeea (3; K. cyanea, K. herbacea, K. orthocarpa; Burma, Thailand, southern Laos), Loxocarpus (20–25; peninsular Thailand, the Malay Peninsula, Sumatra, Borneo), Damrongia (c 10; southern Burma, Thailand, the Malay Peninsula, Sumatra), Somrania (3; S. albiflora, S. flavida, S. lineata; southern Thailand), Ornithoboea (11; southern China, eastern Burma, Thailand, Vietnam, northern Malay Peninsula), Orchadocarpa (1; O. lilacina; the Malay Peninsula). – Didymocarpinae G. Don, Gen. Hist. 4: 644, 658. 1837–8 Apr 1838 [‘Didymocarpeae’]. Codonoboea (80–85; Southeast Asia), Microchirita (c 35; India, Burma, southern China, Thailand, Indochina, West Malesia), Henckelia (c 55; southern India, Sri Lanka, southern and eastern China, peninsular Thailand, Laos, the Malay Peninsula, Malesia to New Guinea), Didymostigma (3; D. leiophyllum, D. obtusum, D. trichanthera; southeastern China including Hainan), Billolivia (9; Indochina), Conandron (1; C. ramondioides; eastern China, southern Japan, Taiwan), Ridleyandra (>20; the Malay Peninsula, Borneo), Hexatheca (3–4; H. dolichopoda, H. fulva, H. johannis-winkleri; Borneo), Cyrtandra (450–600; the Nicobar Islands and southern Thailand to Malesia, islands in southern Pacific to the Hawaiian Islands; paraphyletic?), Sepikea (1; S. cylindrocarpa; the Sepik area on New Guinea), Metapetrocosmea (1; M. peltata; Hainan), Aeschynanthus (c 185; northern and southern India, southern China, Malesia to New Guinea and Solomon Islands; paraphyletic?), Agalmyla (>95; Malesia to New Guinea), Allocheilos (2; A. cortusiflorus, A. guangxiensis; southern China, Vietnam), Liebigia (12; Sumatra, Java, Bali), Didymocarpus (>75; northern India, Nepal and southern China to the Malay Peninsula and northern Sumatra), Oreocharis (100–140; the Himalayas, central and southern China, Burma, Thailand, Vietnam), Cathayanthe (1; C. biflora; Hainan), Petrocodon (19; southern China, northeastern Thailand, northern Vietnam), Primulina (115–120; southern China), Deinostigma (1–7; D. poilanei; southern China, Vietnam), Loxostigma (>7; L. brevipetiolatum, L. cavaleriei, L. fimbrisepalum, L. glabrifolium, L. griffithii, L. mekongense, L. musetorum; northeastern India, the Himalayas, Burma, southern China, Indochina), Pseudochirita (1; P. guangxiensis; southern China, Vietnam), Allostigma (1; A. guangxiense; Guangxi in southern China), Petrocosmea (c 27; northeastern India, Burma, southern China, Southeast Asia), Chayamaritia (2; C. banksiae, C. smitinandii; Thailand, Laos), Briggsiopsis (1; B. delavayi; southern China), Glabrella (1; G. leiophylla; southern China), Hemiboea (c 27; central and southern China, the Ryukyu Islands, Taiwan, northern Vietnam), Anna (3; A. mollifolia, A. ophiorrhizoides, A. submontana; China, northern Vietnam), Lysionotus (c 30; eastern Himalayas, southern China, northern Thailand, northern Vietnam, southern Japan), ‘Raphiocarpus’ (c 15; southern China, Vietnam; polyphyletic). – Southern Europe, India, Sri Lanka and southern China to Japan, Taiwan, Malesia and tropical Australia, Solomon Islands and islands in the Pacific to the Hawaiian Islands, few species in tropical Africa and Madagascar. Nodes sometimes 1:1 or 3:3 with split laterals, or 5:5. Stamens sometimes two (abaxial pair). Ovary usually gradually narrowing into style. Placentae lamelliform-recurved. Ovules restricted to distal end. Fruit elongated, twisted, sometimes both loculicidal and septicidal (sometimes a pyxidium or a berry). Testal cells little elongated (sometimes ornamented, with extremely long hairs). n = (4, 8) 9–11 (12, 13) 14–17 or more. Polyploidy often occurring. Dihydrocaffeoyl ester present in at least Haberlea and Ramonda. – Jerdonia may be sister to the remaining Didymocarpoideae. Jerdonia has flattened filaments, pollen grains shed in tetrads, four separate parietal placentae, alveolate endosperm, isocotylous seedlings, and n = 14.
Epithemateae C. B. Clarke, Commelyn. et cyrtandr. Bengal. 67. 1874 [‘Epithemeae’]
7/>80. Loxotidinae G. Don, Gen. Hist. 4: 645, 664. 1837–8 Apr 1838 [’Loxotieae’]. Rhynchoglossum (c 10; India and southern China to Southeast Asia, Malesia to New Guinea, one to three species, R. azureum, in southern Mexico, Central America, Colombia and Venezuela). – Monophyllaeinae A. Weber et Mich. Möller in Selbyana 31(2): 86. Dec 2013. Whytockia (8; southern China, Taiwan), Monophyllaea (>30; peninsular Thailand, Malesia to New Guinea). – Loxoniinae A. DC. in A. P. et A. L. P. P. de Candolle, Prodr. 9: 274. 1 Jan 1845 [‘Loxonieae’]. Stauranthera (3; S. coerulea, S. grandifolia, S. umbrosa; northeast India and southern China to Southeast Asia, Malesia to New Guinea), Loxonia (3; L. burttiana, L. discolor, L. hirsuta; the Malay Peninsula, Sumatra, Java, Borneo), Gyrogyne (1; G. subaequifolia; Guangxi in southern China; possibly extinct). – Epithematinae DC. ex Meisn. Plant. Vasc. Gen.: Tab. Diagn. 303, Comm. 212. 25-31 Oct 1840 [‘Epithemeae’] 1/>20. Epithema (22; northeastern India and Nepal to southern China and Southeast Asia, Taiwan, Malesia to New Guinea, one species, E. tenue, in tropical West and Central Africa). – India, Southeast Asia, Malesia, one species in tropical West Africa, one species in tropical America. Secretory canals present. Medullary bundles present in Rhynchoglossum. Inflorescence (cymes) without floral prophylls (bracteoles). Abaxial corolla lobe sometimes inside others in bud. Nectaries sometimes vascularized. Stamens sometimes two (adaxial pair). Ovary short, abruptly narrowed into style. Placentation sometimes axile. Endosperm absent? n = (8–)10(–12). Dihydroxyphenols (i.a. acteoside) not found.
Unplaced Gesneriaceae
Hygea (1; H. barbigera; Chile).
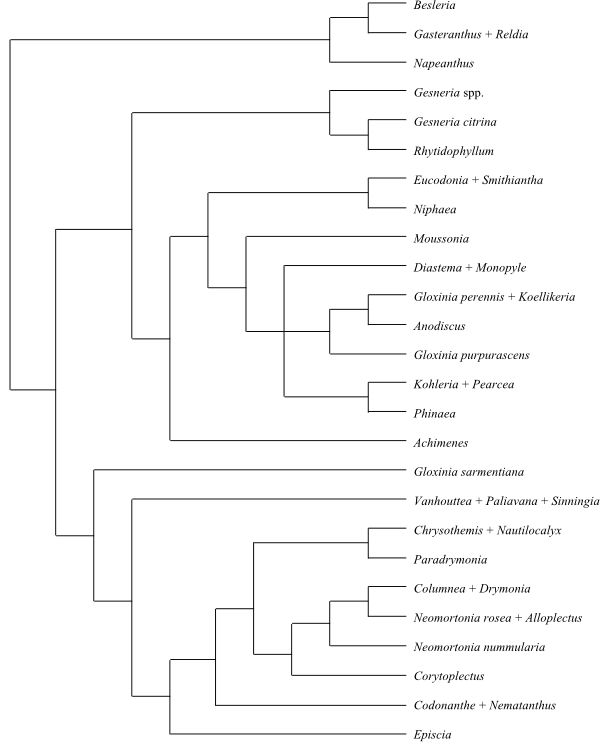 |
Cladogram of Gesnerieae based on DNA sequence data (Zimmer & al. 2002). |
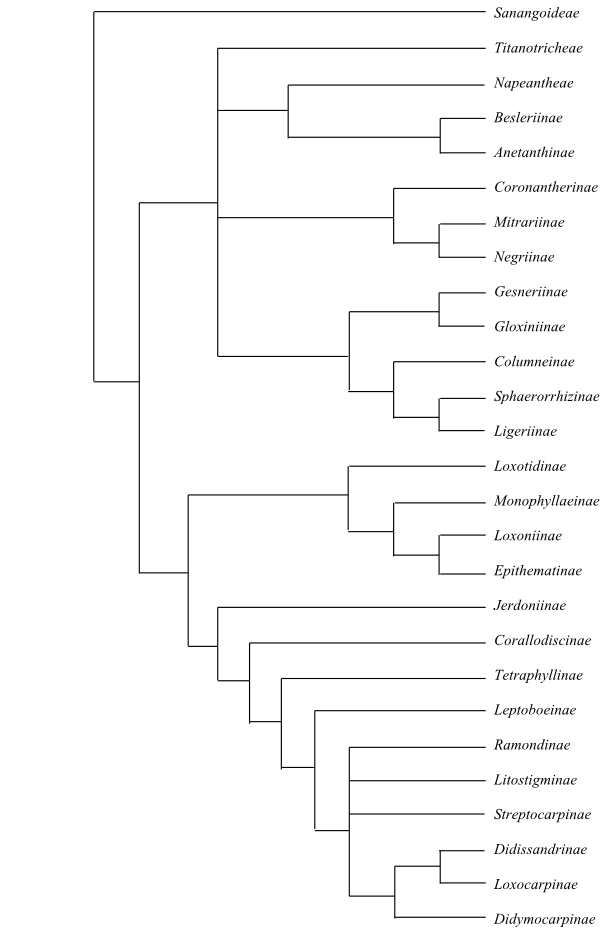 |
Phylogeny of Gesneriaceae, according to Weber & al. (2013). |
LAMIACEAE Martinov |
( Back to Plantaginales ) |
Labiatae Juss., Gen. Plant.: 110. 4 Aug 1789, nom. cons. et nom. alt.; Viticaceae Juss., Gen. Plant.: 106. 4 Aug 1789 [’Vitices’]; Glechomaceae Martinov, Tekhno-Bot. Slovar: 288. 3 Aug 1820 [‘Glecomeae’]; Melissaceae Bercht. et J. Presl, Přir. Rostlin: 245. Jan-Apr 1820 [’Melisseae’]; Melittidaceae Martinov, Tekhno-Bot. Slovar: 390. 3 Aug 1820 [‘Meliteae, Melytteae’]; Nepetaceae Bercht. et J. Presl, Přir. Rostlin: 245. Jan-Apr 1820 [‘Nepeteae’]; Salviaceae Bercht. et J. Presl, Přir. Rostlin: 245. Jan-Apr 1820; Viticales Link, Hort. Berol. 1: 446. 4-11 Jul 1829 [‘Viticeae’]; Menthaceae Burnett, Outl. Bot.: 969, 1095, 1106. Feb 1835; Menthales Burnett, Outl. Bot.: 1106. Jun 1835 [‘Menthinae’], nom. illeg.; Aegiphilaceae Raf., Sylva Tellur.: 161. Oct-Dec 1838 [‘Aegiphilia’]; Siphonanthaceae Raf., Fl. Tellur. 4: 87. med 1838 [‘Siphonanthia’]; Ajugaceae Döll, Rhein. Fl.: 375. 24-27 Mai 1843 [‘Ajugoideae’]; Scutellariaceae Döll, Rhein. Fl.: 373. 24-27 Mai 1843 [‘Scutellarineae’]; Stachydaceae Döll, Rhein. Fl.: 366. 24-27 Mai 1843 [’Stachydeae’]; Ajugineae J. Presl in Nowočeská Bibl. [Wšobecný Rostl.] 7: 1162, 1195. 1846; Menthineae J. Presl in Nowočeská Bibl. [Wšobecný Rostl.] 7: 1161, 1164. 1846; Nepetineae J. Presl in Nowočeská Bibl. [Wšobecný Rostl.] 7: 1162, 1179. 1846; Scutellariineae J. Presl in Nowočeská Bibl. [Wšobecný Rostl.] 7: 1162, 1193. 1846 [‘Scutellarineae’]; Stachydineae J. Presl in Nowočeská Bibl. [Wšobecný Rostl.] 7: 1162, 1193. 1846 [‘Stachydeae’]; Symphoremataceae Wight, Icon. Plant. Ind. Orient. 4(3): 13. Apr 1849 [’Symphoremeae’]; Dicrastylidaceae J. Drumm. ex Harv. in Hooker’s J. Bot. Kew Gard. Misc. 7: 56. 1855 [‘Dicrastyleae’], nom. nud.; Monardaceae Döll, Fl. Baden 2: 661. med 1858 [’Monardeae’]; Saturejaceae Döll, Fl. Baden 2: 664. med 1858 [‘Satureineae’]; Lamiineae Bessey in C. K. Adams, Johnson’s Universal Cyclop. 8: 464. 15 Nov 1895 [‘Lamiales’]; Chloanthaceae Hutch., Fam. Fl. Pl., ed. 2: 396. 4 Jun 1959; Salazariaceae F. A. Barkley in Phytologia 32: 304. 29 Oct 1975
Genera/species 215/6.860–7.450
Distribution Cosmopolitan except polar areas.
Fossils Various fossil leaves, flowers and fruits have been reported from the Eocene onwards of North America, India and Europe. Pollen grains of Lamiaceae have been found in Miocene layers of Central Europe and China.
Habit Usually bisexual (sometimes gynomonoecious or gynodioecious, rarely polygamomonoecious or dioecious), usually perennial, biennial or annual herbs (sometimes evergreen trees or shrubs, rarely lianas). Many species are xerophytic. Young stems and branches usually quadrangular in cross-section. Usually aromatic.
Vegetative anatomy Roots usually fibrous. Phellogen ab initio superficial or deeply seated. Primary medullary rays narrow or wide. Primary vascular tissue cylinder consisting of four bundles. Secondary lateral growth normal, anomalous (from concentric cambia) or absent. Endodermis often prominent. Vessel elements usually with simple (rarely scalariform) perforation plates; lateral pits alternate, simple and/or bordered pits. Vestured pits present. Imperforate tracheary xylem elements usually libriform fibres (sometimes very long; sometimes fibre tracheids) with simple pits, septate or non-septate (also vasicentric tracheids). Wood rays uniseriate or multiseriate, usually heterocellular (sometimes homocellular). Axial parenchyma usually paratracheal scanty, aliform, lozenge-aliform, winged-aliform, confluent, vasicentric or banded, or absent (rarely apotracheal diffuse or diffuse-in-aggregates). Wood elements sometimes storied. Tyloses sometimes abundant. Sieve tube plastids S type. Nodes usually 1:1 (sometimes 1:2 or 2:2), unilacunar with one or two leaf traces (sometimes bilacunar with two traces); nodes often swollen. Pericycle usually with sclerenchymatous cells. Medulla often with crystal inclusions. Heartwood sometimes with gum-like substances. Silica bodies present in wood ray cells in some representatives. Calciumoxalate as styloids, crystal sand and acicular or prismatic crystals sometimes frequent.
Trichomes Hairs unicellular or multicellular, uniseriate or branched, sometimes stellate or dendritic (rarely lepidote); often multicellular glandular hairs (above all in Nepetoideae), sometimes containing ethereal oils.
Leaves Usually opposite (sometimes verticillate, rarely alternate), usually simple (sometimes pinnately or palmately compound), entire or lobed, often coriaceous, often ericoid, with various ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle transection usually arcuate (sometimes annular). Venation pinnate or palmate, usually eucamptodromous to semicraspedodromous (sometimes brochidodromous), or leaves one-veined. Stomata usually diacytic or anomocytic (sometimes diallelocytic with three subsidiary cells, anisocytic or paracytic, rarely other types). Cuticular wax crystalloids? Crystal inclusions of different kinds often present. Leaf margin usually serrate, crenate or lobate (rarely entire). Glandular hairs with ethereal oils, etc. present. Extrafloral nectaries present in some genera on abaxial side of lamina and petiole.
Inflorescence Terminal or axillary, usually panicle, raceme-, spike- or head-like thyrse, often with whorls of dichasial or circinate partial inflorescences (flowers rarely solitary axillary). Bracts and floral prophylls (bracteoles) often large and petaloid.
Flowers Usually zygomorphic (rarely resupinate; rarely actinomorphic). Hypogyny. Sepals (four or) five (to nine), usually with imbricate or open (rarely valvate) aestivation, often unequal in size, usually persistent and often accrescent, connate (sometimes bilabiate); calciumoxalate crystals often frequent. Petals (four or) five (to 16), with imbricate aestivation, connate into usually bilabiate (usually two upper and three lower petals; in Ajugoideae five lower petals; sometimes unilabiate) corolla. Nectariferous disc intrastaminal, entire or lobed, often developed on abaxial side only (rarely absent).
Androecium Stamens usually two longer and two shorter (sometimes two, rarely five, six or up to 16), antesepalous, alternipetalous. Filaments usually free from each other (rarely connate), adnate to corolla tube (epipetalous). Anthers often connivent, basifixed or dorsifixed, often versatile, usually tetrasporangiate (in ‘Salvia’ disporangiate; in Ocimeae synthecal), usually extrorse (sometimes introrse?), usually longicidal (dehiscing by longitudinal slits; rarely poricidal, dehiscing by apical pores); connective often prolonged (in ‘Salvia’ modified into lever-shaped structure). Tapetum secretory, with multinucleate cells. Staminodia usually absent (sometimes one adaxial-median or two adaxial-lateral or abaxial-lateral staminodia).
Pollen grains Microsporogenesis simultaneous. Pollen grains usually tricolpate (rarely tetra- or pentacolpate, tricolporate? or triporate; in Nepetoideae hexacolpate, rarely octacolpate), shed as monads, usually bicellular (in Nepetoideae tricellular) at dispersal. Exine tectate or semitectate, with usually columellate (in some Ajugoideae granular) infratectum, microperforate to reticulate, rugulate, psilate-punctate, granulate or spinulate, or smooth.
Gynoecium Pistil composed of two (to five) connate carpels. Gynophore present in some genera. Ovary superior, bilocular (to quinquelocular; sometimes pseudomonomerous), during maturation with locules usually bipartite by anterio-posterior secondary septa through ingrowth of ovary wall (ovary secondarily quadrilocular; in Symphorematoideae incompletely quadrilocular). Style single, usually bifid at apex (sometimes unequally bilobate), usually gynobasic (sometimes terminal). Stigmas usually punctate (rarely one capitate or quadrilobate stigma), papillate, usually Dry (sometimes Wet) type. Pistillodium absent.
Ovules Placentation usually basal or basal to axile (rarely apical; in Symphorematoideae free central), attached to sides of inrolled carpellary walls (cf. Verbenaceae). Ovules two per carpel, usually anatropous (sometimes hemianatropous, rarely orthotropous), usually ascending (in Symphorematoideae pendulous), apotropous or epitropous, unitegmic, tenuinucellar. Integument five to nine cell layers thick. Funicular obturator present or absent. Megagametophyte monosporous, Polygonum type. Antipodal cells sometimes persistent. Endosperm development cellular. Endosperm haustoria chalazal and micropylar (sometimes absent). Embryogenesis usually onagrad (sometimes asterad).
Fruit A drupe (Symphorematoideae, Viticoideae) or a schizocarp with usually four one-seeded (sometimes two two-seeded) usually nutlike (in Prasium drupaceous) mericarps (rarely a capsule), with persistent and sometimes accrescent calyx. Exocarp in Nepetoideae usually with mucilaginous idioblasts.
Seeds Aril absent. Exotestal cells often elongate, usually with radial and often inner walls thickened. Hypodermal cells sometimes sclerenchymatous. Endotesta unspecialized? Perisperm not developed. Endosperm usually sparse (sometimes copious) or absent. Embryo usually straight (sometimes curved), oily (rich in linolic acid or linolenic acid), well differentiated, without chlorophyll. Cotyledons two, flat. Radicula usually directed downwards towards fruit base. Germination phanerocotylar or cryptocotylar.
Cytology n = 5–13, 15–18, 23–26 (up to 120) – Polyploidy frequently occurring.
DNA Deletion in plastid gene matK. Mitochondrial coxI intron present.
Phytochemistry Flavonols, 6- and/or 8-hydroxylated flavone glycosides, 6- or 8-hydroxyflavones or 6-methoxyflavones, apigenin- and luteolin-derived flavonoids, Route II iridoids (also C4-decarboxylated iridoids: iridoid glycosides, glycosides of monoterpenoid lactones; especially in Lamioideae, Viticoideae, Ajugoideae, and Scutellarioideae; harpagide and harpagioside in, e.g., Caryopteris), Route I carbocyclic iridoids (aucubin in Vitex, catalpol, melittoside), Route II carbocyclic iridoids (galiridoside, lamiide, caryoptoside, ipolamiide, lamalbide, ajugol, ajugoside, lamiol, lamioside), Group X secoiridoids (desoxyloganin, nepeta lactones), ethereal oils consisting of monoterpenoids, sesquiterpenoids, phenylpropanoids etc. (above all in Nepetoideae), diterpenoids (labdanes, neoclerodanes, abietanes, primaranes, ent-kaurans etc.; in Viticoideae, Ajugoideae, Prostantheroideae, Scutellarioideae, Lamioideae, Nepetoideae), triterpenoids, ursolic acid, caffeic acid esters (phenylethyl caffeoylic glucosides, e.g. verbascosides, acteoside, cornoside; in Ajugoideae, Lamioideae, Prostantheroideae, Scutellarioideae, and Viticoideae; rosmarinic acid in Nepetoideae), betaines (e.g. glycine betaine, particularly in Lamioideae, Ajugoideae and Nepetoideae), alkaloids, triterpene saponins, cyanogenic compounds, and shikimic acid derived arthroquinones (in Tectona) present. Ellagic acid, tannins, and proanthocyanidins not found. Carbohydrates stored as stachyose and other oligosaccharides. Cell walls containing arabinoxyloglucans and often galactoxyloglucan hemicelluloses.
Use Ornamental plants, spices, perfumes (Lavandula, Marrubium, Mentha, Pogostemon, Rosmarinus, Salvia), medicinal plants, honey (Phacelia etc.), timber (Viticoideae, Ajugoideae, Tectona grandis, etc.), seed-oils (Perilla etc.).
Systematics Lamiaceae are sister-group to a clade with the following topology: [Mazaceae+[Phrymaceae+[Paulowniaceae+[Rehmanniaceae+Orobanchaceae]]]].
Gynobasic style and schizocarp with four nutlike mericarps have evolved several times in Lamiaceae.
A probable topology is (Li & al. 2016) [[Callicarpa+Prostantheroideae]+[[Symphorematoideae+Viticoideae]+Nepetoideae+[Tectona+[Premnoideae+Ajugoideae+[Peronematoideae+[Scutellarioideae+[Cymarioideae+Lamioideae]]]]]]]].
Calliprostantherina B. Li, R. G. Olmstead et P. D. Cantino in Sci. Rep. 6:34343: 14. Oct 2016
Calliprostantherina consist of Callicarpa and Prostantheroideae.
Callicarpa clade
1/c 140. Callicarpa (c 140; tropical and subtropical regions on both hemispheres). – Callicarpa has branched/stellate hairs, and actinomorphic 4–5(–7)-merous flowers. – Bendiksby & al. (2011) found Callicarpa to be sister of all other Lamiaceae investigated.
Prostantheroideae Luerss., Handb. Syst. Bot. 2:1014, 1016. Sep 1882 [’Prostanthereae’]
14/310–320. Australia. Sometimes aromatic. Flowers usually actinomorphic (sometimes zygomorphic), tetramerous to octamerous. Nectariferous disc sometimes absent. Staminodia sometimes two. Endosperm present. n = ?
Westringieae Bartl., Ord. Nat. Plant.: 182. Sep 1830 [’Westringiea’]
5/210–215. Prostanthera (>100; Australia, Tasmania), Westringia (25–30; Australia, Tasmania, Lord Howe), Hemiandra (14; southwestern Western Australia), Microcorys (c 20; southwestern Western Australia), Hemigenia (c 50; Australia). – Australia, Tasmania, Lord Howe.
Chloantheae Benth. et Hook. f., Gen. Plant. 2: 1132. 1-16 Mai 1876
9/100–105. Brachysola (2; B. coerulea, B. halganiacea; southwestern Western Australia), Physopsis (5; P. chrysophylla, P. chrysotricha, P. lachnostachya, P. spicata, P. viscida; southwestern Western Australia), Newcastelia (10; Australia), Lachnostachys (6; L. albicans, L. bracteosa, L. coolgardiensis, L. eriobotrya, L. ferruginea, L. verbascifolia; Western Australia), ’Pityrodia’ (c 40; Western Australia, Northern Territory, eastern Queensland; polyphyletic), Dicrastylis (c 25; Australia), Cyanostegia (5; C. angustifolia, C. corifolia, C. cyanocalyx, C. lanceolata, C. microphylla; Western Australia, southern Northern Territory), Hemiphora (5; H. bartlingii, H. elderi, H. exserta, H. lanata, H. uncinata; southwestern Western Australia), Chloanthes (4; C. coccinea, C. glandulosa, C. parviflora, C. stoechadis; southwestern Western Australia, eastern Queensland, eastern New South Wales). – Australia. – Chloantheae have occasionally been identified as sister to Wenchengia (here in Scutellarioideae), and these as sister-group to Ajugoideae.
[[Symphorematoideae+Viticoideae]+Nepetoideae+[Tectona+[Premnoideae+Ajugoideae+[Peronematoideae+[Scutellarioideae+[Cymarioideae+Lamioideae]]]]]]
Viticisymphorina B. Li, R. G. Olmstead et P. D. Cantino in Sci. Rep. 6:34343: 13. Oct 2016
Fruit a drupe. – Viticisymphorina comprise Symphorematoideae and Viticoideae.
Symphorematoideae Briq. in Engler et Prantl, Nat. Pflanzenfam. IV, 3a: 144. 26 Feb 1895 [‘Symphoremoideae’]
3/c 30. Congea (11; northeastern India to Yunnan and Southeast Asia, West Malesia), Sphenodesme (c 15; northeastern India to southern China and Southeast Asia, West Malesia), Symphorema (3; S. involucratum, S. luzonicum, S. polyandrum; India, Sri Lanka, Yunnan, Burma, the Andaman Islands, Thailand, the Philippines, the Moluccas). – India to West Malesia. Lianas. Inflorescence three- to seven-flowered capitate cymose, with involucre consisting of more or less petaloid showy bracts. Flowers actinomorphic. Calyx 5–8-lobate. Corolla 5–16-lobate. Nectariferous disc absent. Stamens four to 18. Ovary incompletely bilocular. Placentation free central to apical. Ovules orthotropous, pendulous. Funicle absent. Megagametophyte present on surface of ovule. Endosperm absent? n = 12, 14, 17, 18. Diterpenoids absent? – Congea has occasionally been recovered as sister to the remaining Lamiaceae.
Viticoideae Briq. in Engler et Prantl, Nat. Pflanzenfam. IV, 3a: 144. 26 Feb 1895
4/c 285. ’Vitex’ (c 250; temperate to tropical regions on both hemispheres; non-monophyletic), Petitia (2; P. domingensis, P. urbanii; the West Indies), Pseudocarpidium (9; the West Indies, with their largest diversity on Cuba), Teijsmanniodendron (23; Southeast Asia, the Nicobar Islands, Malesia to New Guinea, the Bismarck Archipelago and Solomon Islands). – Temperate to tropical regions on both hemispheres.
Nepetoideae Burnett, Outlines Bot.: 971. Feb 1835 [’Nepetidae’]
105/3.540–3.840. Elsholtzieae Burnett, Outlines Bot.: 971. Feb 1835 [’Elscholzieae’]. Ombrocharis (1; O. dulcis; Hunan), Perillula (1; P. reptans; Japan); Elsholtzia (c 40; temperate regions and tropical mountains in the Old World), Collinsonia (11; China, Japan, Taiwan, southeastern Canada, eastern and southeastern United States), Mosla (c 15; the Caucasus, eastern India and the Himalayas to Burma, China, the Korean Peninsula Japan, the Ryukyu Islands, the Kuril Islands and the Russian Far East, Southeast Asia, Malesia to the Philippines), Perilla (1–4; P. frutescens; India and Bhutan to China, the Korean Peninaula and Japan, Taiwan, Southeast Asia, Java). – Mentheae Dumort., Fl. Belg.: 48. 1827. Salvia (900–1.000; temperate to tropical regions on both hemispheres, with their highest diversity in western North America, southwestern and Central Asia, the Himalayas and western China), Lepechinia (40–45; tropical and subtropical America); Lycopus (19; Europe, northwestern Asia, temperate North America, one species, L. australis, in southeastern South Australia to southeastern Queensland, Tasmania), Hyssopus (6; H. ambiguus, H. cuspidatus, H. latilabiatus, H. macranthus, H. officinalis, H. seravschanicus; southern Europe, the Mediterranean to Pakistan, Central Asia and Mongolia), Mentha (18–19; nearly cosmopolitan except South America), Thymbra (4; T. calostachya, T. capitata, T. sintenisii, T. spicata; the Mediterranean to Iran), Thymus (c 350; Europe, temperate Asia), Origanum (40–45; Europe, the Mediterranean, temperate Asia), Zataria (1; Z. multiflora; Iran, Afghanistan, Pakistan, Kashmir), Pentapleura (1; P. subulifera; Kurdistan), Satureja (c 45 or 200–210; nearly cosmopolitan, with their highest diversity in temperate regions in the Old World), Killickia (4; K. compacta, K. grandiflora, K. lutea, K. pilosa; Drakensberg, KwaZulu-Natal), Gontscharovia (1; G. popovii; Central Asia), Saccocalyx (1; S. saturejoides; Morocco, Algeria), Bystropogon (7; B. canariensis, B. maderensis, B. odoratissimus, B. origanifolius, B. plumosus, B. punctatus, B. wildpretii; Madeira, the Canary Islands), Cuminia (1; C. eriantha; Robinson Crusoe Island in the Juan Fernandez Islands), Minthostachys (17; the Andes from Venezuela to central Argentina), Cyclotrichium (9; southwestern Asia to Iran), Obtegomeria (1; O. caerulescens; Sierra Nevada de Santa Marta in northeastern Colombia), Kurzamra (1; K. pulchella; the Andes in Chile and northwestern Argentina), Ziziphora (17; the Mediterranean and the Caucasus to Russia, Central Asia, Afghanistan, Mongolia, Siberia and the Himalayas), Stachydeoma (1; S. graveolens; northwestern Florida), Hedeoma (c 40; southwestern Canada, United States, Mexico, Guatemala, subtropical South America), Rhododon (1; R. ciliatus; Texas), Pogogyne (7; P. abramsii, P. clareana, P. douglasii, P. floribunda, P. nudiuscula, P. serpylloides, P. ziziphoroides; southern Oregon to Baja California), Poliomintha (8; southwestern United States, northwestern Mexico), Hesperozygis (7; H. dimidiata, H. kleinii, H. myrtoides, H. nitida, H. rhododon, H. ringens, H. spathulata; southern Brazil), Glechon (7; G. ciliata, G. discolor, G. elliptica, G. hoehneana, G. marifolia, G. spathulata, G. thymoides; southern Brazil, Uruguay, Paraguay, northern Argentina), Hoehnea (4; H. epilobioides, H. minima, H. parvula, H. scutellarioides; southern Brazil, Paraguay), Rhabdocaulon (7; R. coccineum, R. denudatum, R. erythrostachys, R. gracile, R. lavanduloides, R. stenodontum, R. strictum; southern Brazil, Uruguay, Paraguay, northeastern Argentina), Eriothymus (1; E. rubiaceus; Minas Gerais in Brazil), Cunila (18; eastern and central United States, Mexico, Central America, tropical South America to Uruguay and Argentina), Piloblephis (1; P. rigida; Georgia, Florida, the Bahamas), Conradina (7; C. brevifolia, C. canescens, C. cygniflora, C. etonia, C. glabra, C. grandiflora, C. verticillata; southeastern United States), Dicerandra (6–11; D. densiflora, D. frutescens, D. fumella, D. linearifolia, D. odoratissima, D. radfordiana; southeastern United States), Monardella (c 35; southwestern Canada, western United States), Pycnanthemum (c 20; southeastern Canada, eastern and southeastern United States), Acanthomintha (4; A. duttonii, A. ilicifolia, A. lanceolata, A. obovata; California, northern Baja California), Monarda (c 20; southern Canada, United States, northern Mexico), Blephilia (3; B. ciliata, B. hirsuta, B. subnuda; southern Canada, eastern and central United States), Prunella (8; Europe, the Mediterranean and North Africa to the Caucasus, Russia and temperate Asia, one species, P. vulgaris, also in North America), Cleonia (1; C. lusitanica; western Mediterranean to Algeria), Horminum (1; H. pyrenaicum; the Alps, the Pyrenees); Nepeta (200–250; Europe, the Canary Islands, the Mediterranean, North Africa, mountains in tropical Africa, temperate Asia), Drepanocaryum (1; D. sewerzowii; Central Asia), Lophanthus (20–25; mountains in Iran, western Himalayas, Central Asia to Siberia and China), Hymenocrater (10–12; the Caucasus, Iran, Afghanistan), Marmoritis (5; M. complanata, M. decolorans, M. nivalis, M. pharica, M. rotundifolia; Pakistan and the Himalayas to Tibet and China), Agastache (22; mountains and arid regions in temperate Asia, Taiwan, southern Canada, United States and Mexico), Meehania (7; M. cordata, M. faberi, M. fargesii, M. henryi, M. montis-koyae, M. pinfaensis, M. urticifolia; China to Japan and the Russian Far East, eastern United States), Glechoma (7; G. biondiana, G. grandis, G. hederacea, G. hirsuta, G. longituba, G. sardoa, G. sinograndis; Europe, the Mediterranean, temperate Asia to the Russian Far East, Japan,Taiwan and Vietnam), Dracocephalum (60–70; Europe, the Mediterranean, North Africa, temperate Asia, North America), Lallemantia (5; L. baldshuanica, L. canescens, L. iberica, L. peltata, L. royleana; Turkey and the Caucasus to the Arabian Peninsula, Iran, Central Asia, the Himalayas and western Siberia), Cedronella (1; C. canariensis; Macaronesia except Cape Verde Islands), Schizonepeta (2; S. multifida, S. tenuifolia; southern Siberia, Mongolia, northern China), Melissa (4; M. axillaris, M. flava, M. officinalis, M. yunnanensis; Europe, the Mediterranean, North Africa, southwestern Asia to Iran, Central Asia, the Himalayas, Tibet, Yunnan, Southeast Asia and West Malesia, Taiwan). – Ocimeae Dumort., Anal. Fam. Plant.: 22. 1829 [’Ocymeae’]. Lavandula (45–50; Macaronesia, the Mediterranean, North Africa to Somalia, southwestern Asia to southeastern India); Isodon (c 105; tropical and subtropical regions in Africa and Asia)?, Siphocranion (2; S. macranthum, S. nudipes; China, Tibet, Burma, Southeast Asia; in Hanceola?)?, Hanceola (8; China; incl. Siphocranion?)?, Hyptidendron (17–18; the Guayana Highlands and northeastern Brazil to Peru and Bolivia), Eriope (>30; tropical South America, with their highest diversity in central and eastern Brazil), Hypenia (c 25; northeastern South America to Venezuela and Bolivia), Marsypianthes (5; M. burchellii, M. chamaedrys, M. foliolosa, M. hassleri, M. montana; southern Mexico, Central America, the West Indies, tropical South America to Paraguay and Argentina), Hyptis (300–400; southern United States, Mexico, Central America, the West Indies, tropical and subtropical South America to Peru and Argentina), Physominthe (2; P. longicaulis, P. vitifolia; eastern and northeastern Brazil), Mesosphaerum (c 25; southern Mexico, Central America, western tropical South America), Cantinoa (23; southeastern United States, Central America, the West Indies, tropical South America to Argentina), Oocephalus (14; Brazil, eastern Bolivia), Condea (26; western United States, Central America, the West Indies, tropical South America), Cyanocephalus (25; Cuba, tropical South America to eastern Paraguay and Bolivia), Eplingiella (3; E. brightoniae, E. cuniloides, E. fruticosa; northeastern Brazil), Eriopidion (1; E. strictum; southern Venezuela, northeastern Brazil), Gymneia (6; G. chapadensis, G. interrupta, G. malacophylla, G. moniliformis, G. platanifolia, G. virgata; northeastern and central Brazil), Leptohyptis (5; L. calida, L. leptostachys, L. macrostachys, L. pinheiroi, L. siphonantha; Brazil, especially northeastern parts), Martianthus (4; M. elongatus, M. leucocephalus, M. sancti-gabrielii, M. stachydifolius; northeastern Brazil, Huarochi in Peru), Medusantha (8; northeastern and central Brazil), Rhaphiodon (1; R. echinus; northeastern Brazil), Asterohyptis (4; A. mocinoana, A. nayarana, A. seemannii, A. stellulata; Mexico to Costa Rica), Ocimum (c 65; tropical and subtropical regions on both hemispheres, with their largest diversity in Africa), Syncolostemon (c 45; southern and southeastern Africa), Orthosiphon (40–45; tropical regions in the Old World; incl. Heterolamium?), Heterolamium (2; H. debile, H. flaviflorum; China; in Orthosiphon?), Fuerstia (9; tropical East and southern Africa), Benguellia (1; B. lanceolata; Angola), Catoferia (4; C. capitata, C. chiapensis, C. martinezii, C. spicata; southern Mexico, Central America, Colombia to Peru), Endostemon (20; tropical and southern Africa and Madagascar to the Arabian Peninsula and India), Haumaniastrum (c 35; tropical Africa, one species, H. villosum, on Madagascar), Platostoma (c 45; tropical Africa, Madagascar, tropical Asia to New Guinea), Dauphinea (1; D. brevilabra; Madagascar), Capitanopsis (3; C. albida, C. angustifolia, C. cloiselii; Madagascar), Madlabium (1; M. magenteum; northern Madagascar), Plectranthus (c 350; tropical and subtropical Africa, Madagascar, tropical Asia to tropical Australia and islands in the Pacific), Thorncroftia (6; T. greenii, T. longiflora, T. lotteri, T. media, T. succulenta, T. thorncroftii; Northern Province, Mpumalanga), Tetradenia (c 20; tropical and southern Africa, Madagascar), Anisochilus (17; tropical Asia from India and Sri Lanka to Indochina), Leocus (5; L. africanus, L. caillei, L. lyratus, L. membranaceus, L. pobeguinii; tropical Africa), Aeollanthus (40–45; tropical and subtropical Africa), Alvesia (3; A. clerodendroides, A. cylindricalyx, A. rosmarinifolia; Central Africa), Pycnostachys (c 35; tropical and southern Africa, one species, P. coerulea, on Madagascar). – Cosmopolitan, with their highest diversity in warm-temperate regions. Usually aromatic herbs. Prismatic calcium oxalate crystals present in calyx epidermis. Pollen grains hexacolpate, tricellular at dispersal. Style gynobasic. Endosperm development highly asymmetrical, two haustoria present adjacent to each other. Exocarp with mucilaginous cells (myxocarpy) producing hygroscopic spiral fibrils. Endosperm single-layered. Cotyledons enclosing embryo. Seeds with special fatty acids. n = 6 or more. Volatile terpenoids, rosmarinic acid, nepetoidin A and B (caffeic acid esters), and betaine (sparse) present. Iridoid glucosides and acteosides usually absent. – The polyphyletic ’Salvia’ is represented by at least three different clades in Mentheae. Lavandula is sister to the remaining Ocimeae (Li & al. 2016). The clade [Ombrocharis+Perillula] is sister-group to the rest of Elsholtzieae (Chen & al. 2016). Ombrocharis dulcis consists of aromatic tuberous perennial herbs with 11-veined two-lipped calyx, two lipped corolla with deeply bilobate upper lip and trilobate lower lip, and schizocarp with nutlike mericarps.
[Tectona+[Premnoideae+Ajugoideae+[Peronematoideae+[Scutellarioideae+[Cymarioideae+Lamioideae]]]]]
Tectona clade
1/3. Tectona (3; T. grandis, T. hamiltoniana, T. philippinensis; Pakistan, India, Burma, southern China, Southeast Asia, the Philippines). – Tropical Asia to southern China and the Philippines. Trees, shrubs or herbs. Hairs sometimes branched. Leaves sometimes compound. Nectariferous disc poorly developed or absent. Fruit a drupe. – Tectona has shikimic acid derived arthroquinones.
[Premnoideae+Ajugoideae+[Peronematoideae+[Scutellarioideae+[Cymarioideae+Lamioideae]]]]
Premnoideae B. Li, R. G. Olmstead et P. D. Cantino in Sci. Rep. 6:34343: 10. Oct 2016
3/170–180. Cornutia (8; southern Mexico, Central America, the West Indies, tropical South America); Premna (130–140; tropical Africa, Madagascar, Indian Ocean islands, tropical Asia to tropical Australia and islands in the Pacific), Gmelina (c 35; Pakistan, India, Sri Lanka, southern China, Southeast Asia, Malesia to New Guinea, tropical Australia, Solomon Islands, New Caledonia, Fiji). – Pantropical. Premnoideae have a drupe with a single four-seeded pyrene. – Cornutia is sister to [Premna+Gmelina].
Ajugoideae Luerss., Handb. Syst. Bot. 2: 1016. Sep 1882
23/740–770. Monochilus (2; M. gloxinifolius, M. obovatus; Brazil)?, Karomia (9; eastern and southern Africa, Madagascar, one species, K. fragrans, in Vietnam), Discretitheca (1; D. nepalensis; Nepal), Rotheca (35–60; tropical and southern Africa, tropical Asia), Glossocarya (10; Sri Lanka, Burma, Southeast Asia, New Guinea, eastern Queensland); Ajuga (35–40; temperate to tropical regions in the Old World to eastern Siberia, Japan, Australia and islands in western Pacific), Pseudocaryopteris (3; P. bicolor, P. foetida, P. paniculata; India, the Himalayas, Burma, southern China, Southeast Asia), Tripora (1; T. divaricata; China, the Korean Peninsula, Japan), Amethystea (1; A. caerulea; Central Asia, southern Siberia, Mongolia, China, the Korean Peninsula, Japan), Trichostema (17–19; southern Canada, United States, Mexico), Caryopteris (7; C. forrestii, C. glutinosa, C. incana, C. jinshajiangensis, C. mongholica, C. tangutica, C. trichosphaera; Central Asia, Tibet, Mongolia, China, the Korean Peninsula, Japan, Taiwan); Hosea (1; H. lobbii; northwestern Borneo), Oxera (24; Borneo to New Guinea, the Bismarck Archipelago, Solomon Islands, Queensland, New Caledonia, Vanuatu, Fiji, Samoa, Tonga), Ovieda (1; O. spinosa; Hispaniola), Aegiphila (c 150; Florida, southern Mexico, Central America, the West Indies, tropical South America), Clerodendrum (c 150; tropical and subtropical regions on both hemispheres), Tetraclea (1; T. coulteri; southern Arizona, western Texas, northern Mexico), Amasonia (6; A. angustifolia, A. arborea, A. calycina, A. campestris, A. hirta, A. obovata; Trinidad, tropical South America), Kalaharia (1; K. uncinata; tropical and southern Africa), Volkameria (c 30; tropical regions on both hemispheres); Schnabelia (5; S. aureoglandulosa, S. nepetifolia, S. oligophylla, S. terniflora, S. tetradonta; southwestern and southern China), Rubiteucris (2; R. palmata, R. siccanea; northeastern India, the Himalayas, Tibet, Burma, China, Taiwan), Teucrium (c 250; nearly cosmopolitan, with their highest diversity in the Mediterranean). – Subcosmopolitan (abundant in temperate areas), with their highest diversity in Southeast Asia to Australia. Sometimes aromatic. Flowers actinomorphic or zygomorphic (unilabiate, in Teucrium 0:5), in Aegiphila tetramerous. Nectariferous disc poorly developed or absent. Exine spinulate or verrucate, usually with branched (sometimes simple, granulate, etc.) columellae. Style often terminal. Antipodal cells sometimes numerous. Fruit a schizocarp with achene-like mericarps. Endosperm multilayered or absent. Cotyledons enclosing rest of embryo. n = 7, 10, 13, 14, 16 or more. Terpenoids sometimes absent. – The clade [Karomia+Rotheca] was recovered as sister-group to the remaining Ajugoideae (Li & al. 2016). However, Glossocarya and Discretitheca were not included in their analyses.
Perolamiina B. Li, R. G. Olmstead et P. D. Cantino in Sci. Rep. 6:34343: 13. Oct 2016
[Peronematoideae+[Scutellarioideae+[Cymarioideae+Lamioideae]]]
Peronematoideae B. Li, R. G. Olmstead et P. D. Cantino in Sci. Rep. 6:34343: 11. Oct 2016
4/17. Peronema (1; P. canescens; southern Burma to Sumatra and Borneo), Garrettia (1; G. siamensis; Yunnan, Thailand, Java), Hymenopyramis (7; H. acuminata, H. brachiata, H. cana, H. parvifolia, H. pubescens, H. siamensis, H. vesiculosa; Burma, Hainan, Thailand, Indochina), Petraeovitex (8; southern Burma and Thailand, the Malay Peninsula, Malesia to New Guinea, the Bismarck Archipelago, Solomon Islands and northeastern Queensland). – Shrubs, lianas or trees. Leaves simple or trilobate. Inflorescence in Garrettia axillary cyme with secondary monochasial branches. Flowers minute. Calyx quadridentate or quinquedentate. Corolla infundibuliform, unequally quadrilobate, or two-lipped, with lower lip trilobate and upper lip usually bilobate. Nectariferous disc absent or almost so. Stamens didynamous. Ovary bilocular when young, later secondarily quadrilocular, unlobed. Ovules one or two per locule. Stigma bilobate. Fruit a schizocarp with four single-seeded mericarps and inflated persistent calyx.
Scutelamiina B. Li, R. G. Olmstead et P. D. Cantino in Sci. Rep. 6:34343: 13. Oct 2016
[Scutellarioideae+[Cymarioideae+Lamioideae]]
Scutellarioideae Prantl, Handb. Bot.: 293. 1 Mar-15 Apr 1880 [’Scutellariae’]
5/325–385. Wenchengia (1; W. alternifolia; Hainan); Holmskioldia (3; H. gigas, H. speciosa, H. tettensis; the Himalayas), Renschia (1; R. heterotypica; northern Somalia), Tinnea (c 20; tropical and southern Africa), Scutellaria (300–360?; nearly cosmopolitan). – Subcosmopolitan. Inflorescence racemose. Calyx usually bilabiate (not in Holmskioldia), with rounded lobes and with numerous xylem fibres. Pericarps with tuberculate or elongate processes. Testa tuberculate. Endosperm various. n = 12 or more. Terpenoids sometimes absent. – Wenchengia is sister to the remaining Scutellarioideae. In Wenchengia the leaves are spirally arranged, the calyx bilobed and quinquedentate, and the style terminal.
Cymalamiina B. Li, R. G. Olmstead et P. D. Cantino in Sci. Rep. 6:34343: 12. Oct 2016
[Cymarioideae+Lamioideae]
Cymarioideae B. Li, R. G. Olmstead et P. D. Cantino in Sci. Rep. 6:34343: 12. Oct 2016
2/3. Cymaria (2; C. dichotoma, C. elongata; Hainan, Indochina, the Malay Peninsula to New Guinea), Acrymia (1; A. ajugiflora; Perak and Selangor on the Malay Peninsula). – Southeast Asia. Cymes axillary, with long peduncle and secondary monochasial branches. Ovary shallowly quadrilobate. Nutlets reticulately patterned.
Lamioideae Harley in Kew Bull. 58: 765. 26 Nov 2003
50/1.295–1.470. Pogostemoneae Briq. in H. G. A. Engler et K. A. E. Prantl, Nat. Pflanzenfam. IV, 3a: 208. Dec 1895. Holocheila (1; H. longipedunculata; Yunnan), Colebrookea (1; C. oppositifolia; the Himalayas to Burma, western China and Thailand); Craniotome (1; C. furcata; the Himalayas, Tibet and southern China to Laos and Vietnam), ’Microtoena’ (c 25; the Himalayas, Tibet, China, the Korean Peninsula, Southeast Asia to Java; paraphyletic), Anisomeles (5; A. candicans, A. heyneana, A. indica, A. malabarica, A. salviifolia; Madagascar, islands in the Indian Ocean, tropical Asia to Tibet, China and New Guinea, the Bismarck Archipelago, tropical Australia), Pogostemon (80–85; southern tropical Africa, East Asia to Japan, tropical Asia and Australia); Eurysolen (1; E. gracilis; northeastern India to Yunnan, Burma, Thailand, West Malesia), Leucosceptrum (1; L. canum; the Himalayas, Tibet, Burma and southern China to northern Thailand and northern Indochina), Comanthosphace (5; C. formosana, C. japonica, C. nanchuanensis, C. ningpoensis, C. stellipila; China, Japan, Taiwan), Achyrospermum (c 25; tropical regions in the Old World). Stamens four, of about equal length. Style gynobasic. Ovule in, e.g., with glandular hairs. Megagametophyte with micropylar lobe longer and wider than chalazal lobe. Endosperm multilayered. Embryo spatulate. n = 6 or more. Seed oils with laballenic fatty acids. – Colquhounia clade Colquhounia (5; C. coccinea, C. compta, C. elegans, C. seguinii, C. vestita; southern Himalayas, Tibet, Burma, southwestern China, Thailand, Indochina). – Gomphostemmateae Scheen et Lindqvist in A.-C. Scheen, M. Bendiksby, O. Ryding, C. Mathiesen, V. A. Albert et C. Lindquist, Ann. Missouri Bot. Gard. 97: 216. 9 Jul 2010. Gomphostemma (c 40; northeastern India, Burma, southern China, Southeast Asia, the Andaman Islands, the Malay Peninsula, Malesia to the Lesser Sunda Islands, Taiwan), Chelonopsis (17; Pakistan, Tibet, China, the Korean Peninsula, Japan, Taiwan). – Synandreae Raf., Fl. Tellur. 3: 84. Nov-Dec 1837 [’Synandrines’]. Synandra (1; S. hispidula; eastern United States), Macbridea (2; M. alba, M. caroliniana; southeastern United States), Physostegia (12; southern and southwestern Canada, United States, Mexico), Warnockia (1; W. scutellarioides; Oklahoma, Texas, northwestern Mexico), Brazoria (3; B. arenaria, B. enquistii, B. truncata; southern and central Texas). – Betonica-Galeopsis clade Betonica (6; B. alopecuros, B. graeca, B. haussknechtii, B. macrantha, B. monieri, B. officinalis; Europe, the Mediterranean, North Africa, western and southwestern Asia to the Caucasus and northern Iran), Galeopsis (9; Europe to the Caucasus and Siberia). – Stachydeae Dumort., Fl. Belg.: 44. 1827. Melittis (1; M. melissophyllum; Europe, Turkey), Stachys (300–450 or 450–600; temperate and subtropical regions on both hemispheres, tropical mountains, absent from Australia; incl. Sideritis: c 140; temperate regions in the Old World south to Macaronesia and North Africa). – Paraphlomideae Bendiksby in Taxon 60: 481. 8 Apr 2011. Paraphlomis (c 25; China, Southeast Asia to Central Malesia, Taiwan), Ajugoides (1; A. humilis; Japan), Matsumurella (5; M. chinensis, M. kwangtungensis, M. szechuanensis, M. tuberifera, M. yangsoensis; China, Japan, the Ryukyu Islands, Taiwan). – Phlomideae Mathiesen in A.-C. Scheen, M. Bendiksby, O. Ryding, C. Mathiesen, V. A. Albert et C. Lindquist, Ann. Missouri Bot. Gard. 97: 216. 9 Jul 2010. Phlomis (80–85; the Mediterranean to Central Asia and western China), Eremostachys (c 130; southeastern Russia and the Caucasus to West and Central Asia, India, the Himalayas, Mongolia, western and central China). – Leonureae Dumort., Fl. Belg.: 46. 1827. Lagochilus (c 45; the Caucasus, Iran, Afghanistan, Central Asia to northwestern China and Mongolia), Chaiturus (1; C. marrubiastrum; Europe, Russia, Turkey and the Caucasus to Central Asia), Loxocalyx (3; L. ambiguus, L. quinquenervius, L. urticifolius; China, Japan), Leonurus (c 25; Europe, the Mediterranean, the Caucasus, temperate Asia to the Himalayas, Japan and Taiwan), ’Lagopsis’ (5; L. darwiniana, L. eriostachya, L. flava, L. marrubiastrum, L. supina; Kashmir, Central Asia, Tibet, China and Mongolia to Siberia and Japan; polyphyletic?), Panzerina (2; P. canescens, P. lanata; western Siberia, Mongolia, northern China). – Marrubieae Vis., Fl. Dalmat. 2: 214. 1847. Roylea (1; R. cinerea; western Himalayas); Acanthoprasium (2; A. frutescens: the Alps; A. integrifolium: Cyprus); Moluccella (8; the Mediterranean, the Caucasus, the Middle East to northwestern India); ’Ballota’ (c 30; Europe, the Mediterranean, North Africa, the Arabian Peninsula, western Asia, one species, B. africana, in southern Namibia and South Africa; polyphyletic), Marrubium (c 45; southeastern Europe, the Mediterranean, the Caucasus, the Middle East to Iran and Kashmir). – Lamieae Coss. et Germ., Fl. Desc. Anal. Paris. 311, 324. 22 Feb 1845 [’Lamioideae’]. ’Lamium’ (40–50; Europe, the Mediterranean, North Africa, temperate Asia to Kamchatka, Japan and Taiwan; polyphyletic), Stachyopsis (4; S. lamiiflora, S. maleolens, S. marrubioides, S. oblongata; Central Asia, western China), Eriophyton (6; E. nepalense, E. rhomboideum, E. staintonii, E. sunhangii, E. tuberosum, E. wallichii; the Himalayas, Central Asia, Tibet, Yunnan). – Leucadeae Scheen et Ryding in A.-C. Scheen, M. Bendiksby, O. Ryding, C. Mathiesen, V. A. Albert et C. Lindquist, Ann. Missouri Bot. Gard. 97: 217. 9 Jul 2010. Rydingia (4; R. integrifolia, R. limbata, R. michauxii, R. persica; Ethiopia, southern Arabian Peninsula, Iran, Pakistan), ’Leucas’ (130–135; tropical and southern Africa, the Arabian Peninsula, southern and tropical Asia to Japan and Malesia to Queensland; polyphyletic), Isoleucas (2; I. somala: Somalia; I. arabica: southeastern Yemen), Otostegia (9; Cameroon, northeastern Africa, the Arabian Peninsula and Israel to Jordan, Iran to Pakistan),’Leonotis’ (1[–9]; L. leonurus; southern Africa, Angola [tropical Africa]; polyphyletic), ’Acrotome’ (8; southern and southeastern Africa; paraphyletic). – Unplaced Lamioideae Paralamium (1; P. griffithii; northeastern India and Yunnan to northern Burma and northern Vietnam; in Pogostemoneae?), Pseudomarrubium (1; P. eremostachydioides; Karatau Mountains in Kazakhstan), Metastachydium (1; M. sagittatum; Central Asia to western China). – Subcosmopolitan, with their highest diversity in temperate and subtropical regions in Europe and Asia. – Holocheila and Colebrookea form a sister-group to the rest of Pogostemoneae (Li & al. 2016). They have a two-lipped calyx with ten veins and the corolla lips are entire.
 |
|
50% majority rule consensus tree (simplified) partitioned Bayesian analysis of Lamiaceae based on DNA sequence data (Bendiksby & al. 2011). |
 |
|
Phylogeny of Lamiaceae based on DNA sequence data (Li & al. 2016). |
LENTIBULARIACEAE A. Rich. |
( Back to Plantaginales ) |
Utriculariaceae Hoffmanns. et Link, Fl. Portug. 1: 62. 1 Sep 1809 [’Utriculinae’]; Lentibulariales Rich. ex Bercht. et J. Presl, Přir. Rostlin: 242. Jan-Apr 1820 [‘Lentibulariae’]; Lentibulariineae Link, Handbuch 1: 511. 4-11 Jul 1829 [‘Lentibulariae’]; Pinguiculaceae Dumort., Anal. Fam. Plant.: 19, 23. 1829; Pinguiculales Dumort., Anal. Fam. Plant.: 19. 1829 [’Pinguicularieae’]; Utriculariales Döll, Rhein. Fl.: 283. 24-27 Mai 1843
Genera/species 3/320–330
Distribution Cosmopolitan except polar and arid regions.
Fossils Unknown.
Habit Bisexual, perennial or annual herbs. Roots present (Pinguicula) or absent (Genlisea, Utricularia). Mycorrhiza absent. Aquatic or helophytic; some species are epiphytic. Carnivorous. Stem in Genlisea and Utricularia photosynthesizing. Leaves in Utricularia with bladder-shaped suction traps (in terrestrial species with chemical attractants), in Genlisea modified into ‘eel trap’-like structures. Tubers or rhizomes present in many terrestrial species of Utricularia.
Vegetative anatomy Mycorrhiza absent. Radicula degenerating very soon after germination. Lateral roots at least usually without root cap. Phellogen absent. Stem vascular tissue in Genlisea and Utricularia not organized into vascular bundles (phloem and xylem independently distributed). Vascular bundles in Pinguicula cylindrical. Secondary lateral growth absent. Xylem weakly developed. Vessel elements with ? perforation plates; lateral pits? Imperforate tracheary xylem elements? Wood rays absent. Axial parenchyma? Sieve tube plastids S type. Nodes 1:?, unilacunar with ? leaf traces. Calciumoxalate crystals and crystalloids present in Pinguicula and Utricularia.
Trichomes Hairs unicellular or multicellular, uniseriate; glandular hairs stalked or sessile; each secretory gland attached to a single epidermal cell without contact with vessel elements.
Leaves Usually alternate (spiral, often arranged in rosette; in Utricularia often opposite or verticillate), simple or compound (in Utricularia extremely variable), entire or lobed (in Utricularia sometimes peltate leaf-like organs), with adaxial glands or with special traps secreting proteolytic enzymes, with ? ptyxis; leaf dimorphism present in Genlisea and Utricularia (leaves sometimes absent). Genlisea with tubular leaves arising from basal rhizophylls and modified into bipartite ‘eel traps’, spirally twisted structures trapping (mostly unicellular) organisms; inner sides of ‘eel traps’ beset with rows of inwardly directed stiff hairs. Utricularia with small bladder-shaped ‘suction traps’ with lid at orifice; stimulation of one of four tactile-sensitive hairs at corolla mouth causing lid rapidly spring open (change in concentration of cytochrome c oxidase may increase respiration rate); Pinguicula with ‘fly paper traps’ with stalked and sessile glandular hairs on adaxial foliar surface; glandular hairs secreting mucilage and proteolytic enzymes. Stipules and leaf sheath absent. Petiole vascular bundles? Venation pinnate. Stomata usually diacytic (sometimes anomocytic or anisocytic) or absent. Cuticular wax crystalloids absent? Epidermis with calciumoxalate crystals and crystalloids. Leaf margin serrate (numerous species of Utricularia) or entire.
Inflorescence Terminal or lateral, raceme or spike, or flowers solitary terminal.
Flowers Zygomorphic. Hypogyny. Sepals in Genlisea and Pinguicula five, equal or unequal in size, with imbricate or open aestivation, persistent, connate; in Utricularia usually connate into bilobate calyx (in subgenus Polypompholyx quadrilobate), often bilabiate, with open aestivation; median sepal adaxial. Petals five, with descending cochlear aestivation (abaxial lobe outside remaining lobes), connate into usually bilabiate (upper lip bilobate, lower lip trilobate) corolla, with abaxial nectar-secreting spur or bent inwards at base. Disc absent.
Androecium Stamens two (abaxial-lateral), antesepalous, alternipetalous. Filaments usually stout, free from each other, adnate to corolla tube (epipetalous). Anthers connivent (with thecae often confluent; sometimes superposed), dorsifixed, with ephemeral epidermal cells, versatile?, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory. Staminodia usually absent (sometimes two adaxial-lateral).
Pollen grains Microsporogenesis simultaneous. Pollen grains (3–)4–8-colporate (in Genlisea violacea spiraperturate; in some species of Utricularia stephanocolporate), usually shed as monads (rarely tetrads), tricellular at dispersal. Exine tectate to semitectate, with columellate infratectum, perforate, microreticulate or reticulate, often regulate.
Gynoecium Pistil composed of two connate carpels. Ovary superior, unilocular. Style, single, simple, hollow, short, or absent. Stigma non-uniformly broadly bilobate (bilabiate, with adaxial lobe reduced; sometimes sensitive), papillate, Wet type. Pistillodium absent.
Ovules Placentation free central or basal. Ovules usually numerous (in some species of Utricularia two) per carpel, anatropous, unitegmic, tenuinucellar (reduced, with meiocyte semi-inferior). Integument two to six cell layers thick. Megagametophyte monosporous, Polygonum type (sometimes protruding into micropyle). Antipodal cells sometimes persistent (uppermost cell sometimes strongly enlarged). Endosperm development cellular. Endosperm haustoria micropylar and (reduced) chalazal. Embryogenesis onagrad, asterad, or chenopodiad.
Fruit Usually a capsule, regularly dehiscing (with valves or pores, sometimes a pyxidium) or irregularly dehiscing (in some species of Utricularia a one-seeded nut).
Seeds Aril absent. Testa in some species of Utricularia winged or provided with hooks or hairs (in Genlisea possibly multiplicative). Exotestal cell walls thickened in different ways. Endotesta? Perisperm not developed. Endosperm absent (ab initio starchy). Embryo globular or ovoid, in Utricularia poorly differentiated when mature (two to 13 little differentiated cotyledon-like organs present), with chlorophyll. Cotyledons one or two (Pinguicula) or two often minute, undifferentiated. Germination phanerocotylar. Radicula ephemeral.
Cytology n = 7–12, 14, 15, 16, 21, 22, 24, 32. – Some species of Genlisea (e.g. G. margaretae) have the smallest known genome among angiosperms.
DNA Deletion in plastid gene matK. Mitochondrial coxI intron present. Mutation frequency in plastid gene matK in Utricularia and, above all, Genlisea among highest known in angiosperms; other genes in Lentibulariaceae also with high mutation frequency.
Phytochemistry Flavones (8-hydroxyapigenin, apigenin, luteolin, 6- and 8-hydroxyluteolin etc.), Route II decarboxylated iridoids, Group I carbocyclic iridoids (aucubin, catalpol), caffeic acid, iridoid glycosides, cyanogenic glycosides, and p-coumaride present. Alkaloids sometimes present. Flavonols, verbascosides, ellagic acid, proanthocyanidins, and saponins not found. Carbohydrates stored as stachyose and other oligosaccharides. Aluminium accumulated in some species of Utricularia.
Use Ornamental plants, nutrients (fermented viscous mild, using enzymes of Pinguicula vulgaris).
Systematics Pinguicula (c 80; temperate regions on the Northern Hemisphere, the Mediterranean, Central and South America), Genlisea (21; tropical and southern Africa, Madagascar, tropical America), Utricularia (220–230; cosmopolitan, with their highest diversity in Western Australia and tropical America).
The sister-group relationship of Lentibulariaceae is unresolved.
Pinguicula is sister to [Genlisea+Utricularia] (Jobson & al. 2003).
Insect-trapping in Byblidaceae, Lentibulariaceae and Plantaginaceae (Philcoxia) have evolved in parallel.
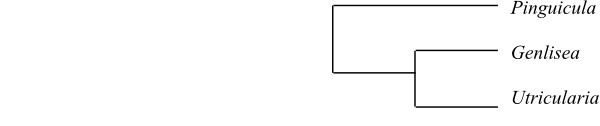 |
Cladogram of Lentibulariaceae based on DNA sequence data (Jobson & al. 2003). |
LINDERNIACEAE (Reichb.) Borsch, K. Müller et E. Fischer |
( Back to Plantaginales ) |
Genera/species 21/155–165
Ditribution Tropical to warm-temperate regions in the Northern and Southern Hemispheres, with their largest diversity in tropical Africa and Southeast Asia.
Fossils Unknown.
Habit Bisexual, annual or perennial herbs (sometimes lignified at base, rarely shrubs). Chamaegigas intrepidus is poikilohydric aquatic resurrection plant.
Vegetative anatomy Phellogen? Stem often tetragonal in cross-section, with four longitudinal ridges with thin strands of lignified tissue inside. Vessel elements with simple? perforation plates; lateral pits? Vestured pits? Imperforate tracheary xylem elements libriform fibres? Wood rays? Axial parenchyma? Sieve tube plastids S type. Nodes 1:3, unilacunar with three leaf traces. Crystals?
Trichomes Hairs simple, unicellular or multicellular, or absent; glandular hairs often present, heads of glandular hairs with vertically divided cells.
Leaves Opposite, usually simple (in Scolophyllum sometimes pinnately compound), usually entire (rarely lobed), sometimes pairwise connate at base, with ? ptyxis. Stipules and leaf sheath absent. Petiole vascular bundles?; petiole often with wing bundles. Venation pinnate or palmate. Stomata? Cuticular wax crystalloids? Leaf margin serrate, crenate or entire.
Inflorescence Terminal or axillary, raceme or head, or flowers solitary axillary. Floral prophylls (bracteoles) absent.
Flowers Zygomorphic (in Lindernia hypandra resupinate). Hypogyny. Sepals (four or) five, sometimes unequal in size, often winged, more or less connate (sometimes at base only). Petals four or five, connate into bilabiate to infundibuliform corolla, with glandular hairs on adaxial side (in ‘Lindernia’ with lobes covering anthers). Nectary? Disc?
Androecium Stamens usually four fertile or two fertile adaxial stamens and two abaxial staminodia (in Micranthemum two abaxial stamens). Filaments free from each other, adnate to corolla tube (epipetalous). Anthers parallel or head-to-head, dorsifixed, sometimes (in, e.g., Torenia) connate, non-versatile?, tetrasporangiate (in, e.g., Hemiarrhena and Torenia disporangiate), extrorse or introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory. Staminodia geniculate and Z-shaped or long and curved with clavate or spur-like appendage and blue and yellow glandular hairs, sometimes strongly reduced or absent.
Pollen grains Microsporogenesis simultaneous. Pollen grains 3(–5)-colpate, shed as monads, ?-cellular at dispersal. Exine tectate, with columellate infratectum, smooth (‘Lindernia’).
Gynoecium Pistil composed of two connate carpels. Ovary superior, bilocular. Style single, simple. Stigma bilobate, papillate, Wet type, usually sensitive. Pistillodium absent.
Ovules Placentation axile to basal. Ovules few to numerous per carpel, orthotropous, anatropous or hemitropous, unitegmic, tenuinucellar. Integument up to four cell layers thick. Megagametophyte monosporous, Polygonum type; often spathulate, in some species of ’Lindernia’ and Torenia bulging out of ovule. Endosperm development cellular? Endosperm haustoria? All endothelial cells curved inwards into endosperm; these may fuse making endosperm surface alveolated. Embryogenesis?
Fruit Usually a septicidal and septifragal capsule (in Torenia a septicidal and poricidal capsule with persistent and accrescent calyx).
Seeds Testa smooth or pitted. Aril absent? Exotesta? Endotesta? Perisperm not developed. Endosperm often ruminate (due to inpushings of endothelial cells), often alveolated, usually furrowed. Embryo?, chlorophyll? Cotyledons two. Germination phanerocotylar?
Cytology n = 7–9, 12, 14, 17, 20, 21 (28) – Protein bodies absent from cell nuclei.
DNA
Phytochemistry Virtually unknown. Iridoids not found.
Use Ornamental plants, aquarium plants (Micranthemum).
Systematics Stemodiopsis (5; S. buchananii, S. eylesii, S. glandulosa, S. humilis, S. rivae; tropical Africa), Micranthemum (17–18; eastern United States, Mexico, Central America, Cuba, tropical South America), Bryodes (1; B. micrantha; Madagascar, Mauritius, the Seychelles), Psammetes (1; P. madagascariensis; tropical Africa, Madagascar), Torenia (40–50; tropical and southern Africa, Indian Ocean islands, Southeast Asia; incl. Legazpia?), Legazpia (1; L. polygonoides; East Asia to islands in western Pacific; in Torenia?), Encopella (1; E. tenuifolia; Cuba), Dintera (1; D. pterocaulis; Waterberg Plateau in Namibia), Bythophyton (1; B. indicum; Southeast Asia, Malesia), Artanema (5; A. bantamense, A. evrardii, A. fimbriatum, A. finetianum, A. longifolium; tropical Africa, Southeast Asia, Malesia), Picria (1; P. felterrae; southern China, Southeast Asia, Malesia), Pierranthus (1; P. capitatus; Indochina), Chamaegigas (1; C. intrepidus; Namibia), ’Lindernia’ (c 30; warm-temperate to tropical regions on both hemispheres; non-monophyletic; incl. Hemiarrhenia, Schizotorenia, Scolophyllum?), Hemiarrhena (1; H. plantaginea; northernmost Western Australia; in Lindernia?), Schizotorenia (2; S. atropurpurea, S. finetiana; Southeast Asia; in Lindernia?), Scolophyllum (2; S. spinifidium, S. ubonensis; Southeast Asia; in Lindernia?), Craterostigma (9; tropical and southern Africa, Madagascar, Yemen, Socotra, India), Crepidorhopalon (28; tropical and southern Africa, Madagascar), Hartliella (4; H. bampsii, H. capitata, H. cupricola, H. suffruticosa; Katanga Province in Congo), Bampsia (2; B. lawalreana, B. symoensiana; Katanga Province in Congo).
The sister-group relationship of Linderniaceae is unresolved. In Schäferhoff & al. (2010) it is part of trichotomy also including Byblis (Byblidaceae) and the remaining Plantaginales “beyond” Scrophulariaceae.
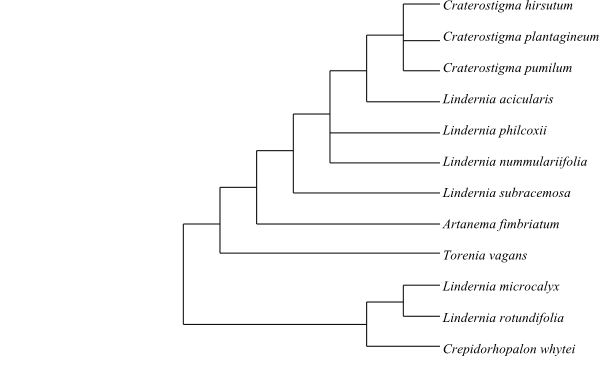 |
Cladogram of Linderniaceae based on DNA sequence data (Rahmanzadeh & al. 2005). |
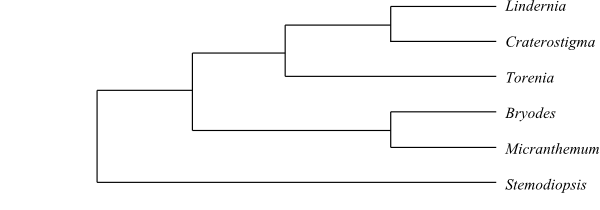 |
Phylogeny of Linderniaceae based on DNA sequence data (Schäferhoff & al. 2010). |
MARTYNIACEAE Horan. |
( Back to Plantaginales ) |
Genera/species 5/13
Distribution Warm and arid or semiarid regions from southern United States and Mexico to Argentina.
Fossils Unknown.
Habit Bisexual, usually annual (rarely perennial) herbs (rarely shrubs, in Holoregmia with fleshy young stems). Craniolaria annua has a large root tuber. With densely spaced glandular hairs and evil-smelling. Mycorrhiza probably absent. Some species (Proboscidea, Ibicella) are possibly carnivorous; the glands are similar to those in Byblis and Lentibulariaceae, although this may be a precursor to insect-trapping.
Vegetative anatomy Phellogen superficial. Vessel elements with simple perforation plates; lateral pits alternate, simple pits? Imperforate tracheary xylem elements libriform fibres with simple pits, non-septate. Wood rays uniseriate or multiseriate, heterocellular. Axial parenchyma paratracheal scanty vasicentric. Wood non-storied. Sieve tube plastids Ss type. Nodes unilacunar? with ? leaf traces. Crystals absent in parenchyma.
Trichomes Hairs unicellular or multicellular, uniseriate; mucilage glandular hairs consisting of unicellular or multicellular uniseriate stalk and multicellular apical head with flattened apex.
Leaves Usually opposite (rarely alternate, spiral), simple, entire or palmately lobed, with ? ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle transection strongly arcuate; petiole also with adaxial cortical and medullary bundles. Venation pinnate? or palmate. Stomata usually anomocytic or anisocytic (rarely paracytic or diacytic). Cuticular wax crystalloids? Leaf margin serrate or entire. Extrafloral nectaries?
Inflorescence Terminal raceme.
Flowers Zygomorphic, often large. Hypogyny. Sepals five, with imbricate quincuncial aestivation, free or connate; median sepal adaxial. Petals five, with descending imbricate aestivation, connate into quinquelobate, tubular and bilabiate (upper lip bilobate, lower lip trilobate) or somewhat non-uniform corolla. Nectariferous disc intrastaminal, annular.
Androecium Stamens usually two longer and two shorter (didynamous; sometimes two, equal in length). Median adaxial stamen staminodial (when four fertile stamens present); in Martynia one median adaxial and two adaxial lateral stamens staminodial (when two fertile stamens present), antesepalous, alternipetalous. Filaments free from each other, adnate to corolla tube (epipetalous). Anthers dorsifixed, connivent, with thecae separated 180°, versatile?, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits); connective with gland at apex. Tapetum secretory, with binucleate cells. Staminodia one or three.
Pollen grains Microsporogenesis simultaneous. Pollen grains inaperturate (or polyporate?), shed as monads, bicellular or tricellular at dispersal. Exine semitectate (sometimes intectate), subdivided into c. 20 to c. 40 platelets evenly distributed on tectal surface, with columellate infratectum, areolate (Ibicella, Proboscidea), annular (Craniolaria, sexine consisting of smooth mural rings supported by columellae encircling smooth to granulate aperturoid areas, sexine between rings consisting of rodlets, clavulae, pilulae, or cone-shaped elements) or reticulate (Martynia).
Gynoecium Pistil composed of two paracarp-connate carpels. Ovary superior, primarily unilocular, yet quadrilocular to multilocular due to secondary septa (often divided by gradually connate placentae). Style single, simple, filiform. Stigma bilobate, type? Pistillodium absent.
Ovules Placentation parietal with two T-shaped placentae. Ovules two to numerous per carpel, anatropous, pendulous, unitegmic, tenuinucellar. Integument ? cell layers thick. Megagametophyte monosporous, Polygonum type. Antipodal cells persistent. Endosperm development cellular. Endosperm haustoria micropylar and chalazal (Martynia). Embryogenesis onagrad (Catalpa variant).
Fruit An incompletely loculicidal capsule, usually with well developed beak-shaped apical part formed from sterile upper part of ovary. Exocarp and mesocarp fleshy, caducous when ripe. Endocarp lignified, with bristles at apex, sharp edges, and prickle-, comb-, horn- or spur-like outgrowths.
Seeds Aril? Testa subgelatinous or inner and radial testal cell walls with band of cellulose. Inner testal layers in Proboscidea lignified and tanniniferous; in Martynia radial and inner tangential cell walls of outer epidermis lignified, remaining layers crushed. Perisperm not developed. Endosperm thin or almost absent, oily and aleuroniferous. Embryo straight, chlorophyll? Cotyledons two. Germination phanerocotylar.
Cytology n = 15, 16
DNA Deletion in plastid gene matK?
Phytochemistry Flavonols (kaempferol), flavone glucosides, Group I carbocyclic iridoids (catalpol), Group II iridoids (harpagide, harpagioside and other 8β-8α-methylsubstituted iridoids, 10-hydroxylated carboxylic iridoids), and caffeic acid esters (verbascosides, cornoside, martynoside) present. Ellagic acid, proanthocyanidins, saponins, and cyanogenic compounds not found.
Use Ornamental plants.
Systematics Ibicella (3; I. lutea, I. nelsoniana, I. parodii; Brazil, Bolivia, Argentina), Martynia (1; M. annua; Mexico, Central America, the Greater Antilles), Craniolaria (3; C. annua, C. argentina, C. integrifolia; the Greater Antilles, South America), Holoregmia (1; H. viscida; Bahia in northeastern Brazil), Proboscidea (5; P. althaeifolia, P. louisianica, P. parviflora, P. sabulosa, P. spicata; southern United States, Mexico, Peru).
Martyniaceae are probably sister to Schlegeliaceae. Gutierrez 2008 suggested a sister-group relationship with Verbenaceae.
The heavily glandular Ibicella and Proboscidea have been reported to capture insects and other arthropods (Schäferhoff & al. 2010). However, protease activity has not been detected in the glands (Plachno & al. 2009). Yet there may be mutualistic relationships between predatory insects and the plants in a way similar to that in Byblis (Byblidaceae) and Roridula (Roridulaceae) (Rice 2008). In other words, the leaves may absorb exudates/faeces from carnivorous insects feeding on the trapped animals.
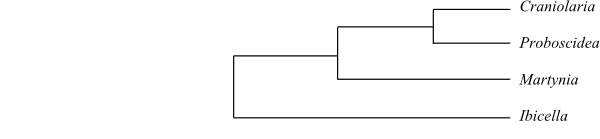 |
Cladogram of Martyniaceae based on DNA sequence data (Gutierrez 2008). |
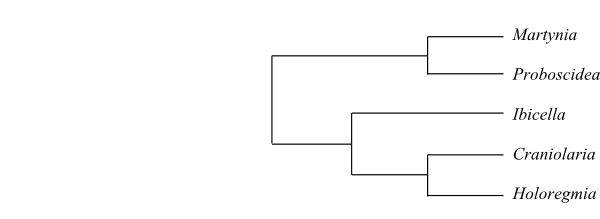 |
Cladogram of Martyniaceae based on DNA sequence data (Gormley & al. 2015). |
MAZACEAE Reveal |
( Back to Plantaginales ) |
Genera/species 2/c 22
Distribution Tibet, East and Southeast Asia, Malesia to New Guinea, southeastern Australia, Tasmania, New Zealand.
Fossils Unknown.
Habit Bisexual, usually perennial or annual herbs, often with rhizome. Young stem quadrangular in cross-section.
Vegetative anatomy Roots fibrous. Phellogen absent? Pericyclic envelope absent in Mazus. Primary vascular tissue cylinder of bundles. Vessel elements with simple? perforation plates; lateral pits? Imperforate tracheary xylem elements ? with simple pits, septate? Wood rays absent? Axial parenchyma paratracheal? Sieve tube plastids S type. Nodes 1:1 (Mazus), unilacunar with one leaf trace. Crystals?
Trichomes Hairs multicellular, uniseriate; glandular hairs often present.
Leaves Usually opposite (sometimes spiral), simple, entire or lobed, with ? ptyxis. Stipules and leaf sheath absent. Petiole vascular bundles? Venation pinnate. Stomata anomocytic. Cuticular wax crystalloids? Leaf margin serrate.
Inflorescence Terminal or axillary cyme or flowers solitary axillary.
Flowers Usually zygomorphic. Hypogyny. Sepals five, with valvate aestivation, persistent, connate into campanulate or bilabiate (3:2, three upper and two lower lobes) calyx. Petals (two to) four or five, connate into tubular or bilabiate (2:3, two upper and three lower lobes) corolla, sometimes with spur. Nectaries present. Disc absent.
Androecium Stamens usually two longer and two shorter (rarely two), haplostemonous, antesepalous, alternipetalous. Filaments free from each other, adnate to corolla tube (epipetalous). Anthers usually with divergent (sometimes confluent) thecae, dorsifixed, versatile?, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory. Staminodia absent.
Pollen grains Microsporogenesis simultaneous. Pollen grains tricolpate?, shed as monads, bicellular at dispersal. Exine tectate, with columellate infratectum, often microreticulate, granulate or spinulate?
Gynoecium Pistil composed of two connate carpels (abaxial carpel often degenerated). Ovary superior, usually unilocular (pseudomonomerous or bilocular). Style single, simple. Stigma bilobate to fan-shaped, sensitive, without asymmetrically swollen stigmatoid tissue, type? Pistillodium absent.
Ovules Placentation usually subbasal or axile. Ovules one or several per carpel, orthotropous or hemianatropous?, epitropous?, unitegmic, tenuinucellar. Integument five or six cell layers thick. Hypostase present? Megagametophyte monosporous, Polygonum type. Synergids with a filiform apparatus? Antipodal cells persistent? Endosperm development cellular. Endosperm haustorium chalazal. Embryogenesis solanad?
Fruit A berry-like more or less fleshy indehiscent fruit, enclosed by persistent and sometimes accrescent calyx.
Seeds Aril absent? Testa often thin. Exotesta? Endotesta? Perisperm not developed. Endosperm sparse or absent. Embryo straight, well differentiated, chlorophyll? Cotyledons two. Germination?
Cytology n = 19 (Mazus)
DNA Deletion in plastid gene matK?
Phytochemistry Very insufficiently known. Group I carbocyclic iridoids (aucubin, catalpol) present.
Use Ornamental plants.
Systematics Lancea (2; L. hirsuta, L. tibetica; Tibet, China), Mazus (c 20; East and Southeast Asia, Malesia to New Guinea, southeastern Queensland to southeastern South Australia, Tasmania, New Zealand).
Mazaceae are sister-group to a clade with the following topology: [Phrymaceae+[Paulowniaceae+[Rehmanniaceae+Orobanchaceae]]]. Other analyses have placed the two genera very close to Mimulus (Phrymaceae).
OLEACEAE Hoffmans. et Link |
( Back to Plantaginales ) |
Jasminaceae Juss., Gen. Plant.: 104. 4 Aug 1789 [’Jasmineae’]; Lilacaceae Vent., Tabl. Règne Vég. 2: 307. 5 Mai 1799 [‘Lilaceae’], nom. illeg.; Fraxinaceae Vest, Anleit. Stud. Bot.: 269, 288. 1818 [’Fraxinoideae’]; Fraxinales Bercht. et J. Presl, Přir. Rostlin: 224. Jan-Apr 1820 [’Fraxineae’]; Jasminales R. Br. ex Bercht. et J. Presl, Přir. Rostlin: 248. Jan-Apr 1820 [‘Jasmineae’]; Oleales R. Br. ex Bercht. et J. Presl, Přir. Rostlin: 248. Jan-Apr 1820 [‘Oleaceae’]; Ligustraceae G. Mey., Chloris Han.: 245, 254. Jul-Aug 1836 [’Ligustrinae’]; Bolivariaceae Griseb., Gen. Sp. Gent.: 20. Oct 1838; Ligustrales Bartl. ex Bisch., Lehrb. Bot.: 3(2): 529. 1840 [‘Ligustrinae’]; Ligustropsida Bartl. ex Meisn., Plant. Vasc. Gen.: Comm.: 164. 5-11 Apr 1840 [’Ligustrinae’]; Forestieraceae (Endl.) Meisn., Plant. Vasc. Gen.: Tab. Diagn.: 345, Comm. 257. 13-15 Feb 1842 [’Forestiereae’]; Oleineae J. Presl in Nowočeská Bibl. [Wšobecný Rostl.] 7: 1031. 1846 [‘Oleineae’]; Syringaceae Horan., Char. Ess. Fam.: 115. 30 Jun 1847 [’Syringaceae nob (s. Oleaceae)’]; Nyctanthaceae J. Agardh, Theoria Syst. Plant.: 284. Apr-Sep 1858 [’Nyctantheae’]; Schreberaceae (Wight) Schnizlein, Iconogr. Fam. Regni Veg. 2: ad t. 151*. 1857-1870; Oleanae Takht., Divers. Classif. Fl. Pl.: 449. 24 Apr 1997
Genera/species 23/560–570
Distribution Cosmopolitan except polar areas, with their largest diversity in Southeast Asia and Australia.
Fossils Fossil wood of Oleaceae has been found in the Late Cretaceous of India. Winged seeds assigned to Oleaceae (possibly Fraxinus) have been found in Eocene and younger layers in North America, Europe and Asia. Fossil pollen of Oleaceae have been recovered from Late Cretaceous (Early Campanian) and Eocene layers in North America, but also from younger strata in Europe.
Habit Usually bisexual (rarely polygamomonoecious or dioecious), evergreen or deciduous trees, shrubs or lianas (rarely suffrutices).
Vegetative anatomy Phellogen ab initio usually superficial (rarely deeply seated). Medulla in Forsythia and Jasminum sometimes septated by diaphragms? Vessel elements usually with simple (sometimes scalariform) perforation plates; lateral pits alternate, bordered pits. Vestured pits present. Imperforate tracheary xylem elements tracheids and fibre tracheids (sometimes libriform fibres) with simple and/or bordered pits, septate or non-septate (also vasicentric tracheids). Wood rays uniseriate or multiseriate, homocellular or heterocellular. Axial parenchyma usually paratracheal scanty vasicentric, aliform, confluent, or banded (sometimes apotracheal diffuse), or absent. Sieve tube plastids S type. Nodes 1:1?, unilacunar with one? leaf trace. Stem and leaves with calciumoxalate as druses, sphaerites, styloids, raphides, or acicular to prismatic (often very small) crystals and/or crystal sand; crystals often frequent in epidermal cells of trichome bases.
Trichomes Hairs unicellular or multicellular, often lepidote or peltate; glandular hairs present, also peltate-lepidote; cells in head with exclusively vertical walls; groups of multicellular secretory hairs sometimes forming extrafloral nectaries.
Leaves Usually opposite (in some species of Jasminum alternate), simple entire or imparipinnate or trifoliolate (sometimes unifoliolate), with conduplicate ptyxis (‘Chionanthus’). Stipules and leaf sheath absent. Petiole vascular bundle transection arcuate. Venation usually pinnate (rarely palmate). Stomata usually anomocytic (sometimes paracytic). Cuticle deeply furrowed. Cuticular wax crystalloids as rodlets or platelets. Domatia as pockets, pits or hair tufts, or absent. Mesophyll often with sclerenchymatous idioblasts with sclereids; calciumoxalte crystals frequent; nuclei of mesophyll parenchyma cells often with special crystalline enclosings. Leaf margin or leaflet margins serrate or entire. Hairs often peltate or as secretory glandular hairs – often as extrafloral nectaries – or sometimes sunken translucent points on abaxial side of lamina.
Inflorescence Terminal or axillary, usually thyrsoid, panicle, cymose or raceme (flowers sometimes solitary axillary).
Flowers Actinomorphic. Hypogyny. Sepals usually four (in Jasminum five to nine [to 15]), with usually valvate (sometimes open) aestivation, connate (rarely absent), orthogonally arranged/initiated. Petals usually four (in Jasminum five to nine [to twelve]), with imbricate, valvate, induplicate-valvate or convolute aestivation, usually more or less connate (rarely free or absent), diagonally arranged/initiated. Nectariferous disc intrastaminal, annular, or absent.
Androecium Stamens two (to five), antesepalous. Filaments free, usually adnate to corolla tube (epipetalous). Anthers basifixed or dorsifixed, with thecae arranged back-to-back, often with osmophores, usually non-versatile, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits). Placentoid? Tapetum secretory. Female flowers sometimes with staminodia.
Pollen grains Microsporogenesis simultaneous. Pollen grains (2–)3(–4)-colpate or (2–)3(–4)-colpor(oid)ate, shed as monads, usually bicellular (rarely tricellular) at dispersal. Exine semitectate, with columellate infratectum, reticulate, beset with rounded or elongate supratectal knobs (piloid excrescences), or psilate.
Gynoecium Pistil composed of two connate carpels, usually median (sometimes transverse or oblique). Ovary superior, bilocular (in Ligustrum nectar-secreting). Style single, simple, often short or absent. Stigma elongate-clavate, bilobate, papillate or non-papillate, Dry type. Male flowers sometimes with pistillodium.
Ovules Placentation axile, apical or basal. Ovules (one or) two (to four; in Forsythia numerous) per carpel, usually anatropous (rarely hemianatropous or amphitropous), pendulous or ascending, apotropous or epitropous, unitegmic, tenuinucellar. Integument usually approx. seven (rarely up to c. 20) cell layers thick, usually massive. Endothelium present. Megagametophyte usually monosporous, Polygonum type (rarely disporous, Allium type). Endosperm development usually cellular (rarely nuclear). Endosperm haustoria absent. Embryogenesis caryophyllad or solanad.
Fruit A loculicidal capsule (in ’Menodora’ clade of Jasminum a pyxidium), a samara (Fraxinus), a berry, a drupe (in Dimetra and Nyctanthes a bipartite schizocarp with samaroid mericarps).
Seeds Aril absent. Testa often vascularized, sometimes winged. Exotesta often palisade, moderately and evenly thickened. Endotesta sometimes fibrous. Endothelium usually persistent. Perisperm not developed. Endosperm copious or sparse, oily, or absent. Embryo straight, well differentiated to little differentiated or rudimentary, without chlorophyll. Cotyledons two, flattened, sometimes nutrient-storing. Germination phanerocotylar or cryptocotylar.
Cytology n = 14 (Forsythieae); n = 13 (Fontanesia); n = 11, 12 (Myxopyreae; in Nyctanthes 11, 18, 22, 23); n = 11–13 (Jasminum); n = 23 (Oleeae) – Polyploidy occurring. Globular crystalline protein bodies present in nucleus.
DNA Deletion of 9 bp in plastid gene ndhF. Plastid genome in Jasminum with two inversions (i.a. one 21 kb inversion). Plastid gene clpP lost from at least two species of Jasminum; plastid gene accD (ORF512, zpfA) lost in some species. Mitochondrial coxI intron present in Jasminum.
Phytochemistry Flavonols (kaempferol, quercetin, myricetin), flavones, flavone glycosides (in Oleeae), Route I iridoids (carbocyclic iridoids and secoiridoids), Group IV carbocyclic iridoids (forsythiide, kingiside), Group VII secoiridoids (swertiamarin, gentiopicroside), Group VIII secoiridoids (oleuropein), Group IX secoiridoids (indole alkaloids of corynantheane type), Group X secoiridoids (loganin, ketologanin), triterpenes, ursolic acid and caffeic acid esters (cornosides in Forsythieae; oleoside in Jasminum and Oleeae; verbascosides, orobanchin, etc.), saponins, syringin, coumarins, coniferin, lignans, and polyols present. Ellagic acid, tannins, proanthocyanidins and cyanogenic compounds not found. Carbohydrates usually stored as oligosaccharides (mannitol). Cell walls with arabinoxyloglucans and often galactoxyloglucan hemicelluloses.
Use Ornamental plants, fruits and fruit oils (Olea), perfumes (Jasminum, ‘Osmanthus’), timber.
Systematics Oleaceae are sister to Carlemanniaceae.
The basal branching is unresolved. Jasminum is sister to Oleeae.
Forsythieae H. Taylor ex L. A. S. Johnson in Contr. N. S. W. Natl. Herb. 2: 397. 18 Nov 1957
2/10. Forsythia (9; western Balkan Peninsula, East Asia), Abeliophyllum (1; A. distichum; the Korean Peninsula). – The Balkan Peninsula, East Asia. Medulla septated. Petals with valvate aestivation. Ovules one or several per carpel. Fruit a samara or a capsule. n = 14. Cornosides present. Iridoids sometimes absent. – In some analyses, Forsythia and Abeliophyllum form a sister-group to the remaining Oleaceae.
Fontanesieae H. Taylor ex L. A. S. Johnson in Contr. N. S. W. Natl. Herb. 2: 397. 18 Nov 1957
1/2. Fontanesia (2; F. phillyreoides: Sicily, Turkey, Syria, Lebanon; F. fortunei: east central China). – Vestured pits present. Petals free, with imbricate aestivation. Ovule one per carpel. Fruit a samara. n = 13. Secologanoside, loganic acid, and 5-hydroxylated derivatives (e.g. swertiamarin) present.
Myxopyreae Boerl., Handl. Fl. Nederl. Ind. 2: 324. 1 Jan 1899
3/7. Myxopyrum (4; M. nervosum, M. ovatum, M. pierrei, M. smilacifolium; India, Burma, Hainan, Southeast Asia, the Andaman and Nicobar Islands, Malesia to New Guinea and the Bismarck Archipelago), Dimetra (1; D. craibeana; northeastern Thailand), Nyctanthes (2; N. aculeata, N. arbo-tristis; the Himalayas, Thailand, Java, Sumatra). – Tropical Asia. Cortical vascular bundles present in corners of angled stem in Myxopyrum and Nyctanthes. Sepals diagonally initiated (Nyctanthes). Petals in Nyctanthes with contorted aestivation. Gynoecium in Nyctanthes transversely orientated. Placentation basal or subbasal. Ovule one (to three) per carpel, ascending (Nyctanthes). Integument in Nyctanthes c. 20 cell layers thick. Megasporocytes occasionally several and megagametophyte disporous 8-nucleate (Allium type). Fruit a berry or a schizocarp. Exotesta in Nyctanthes poorly developed. Mesotesta in Nyctanthes persistent. Endotestal cells in Nyctanthes sclerotic, tangentially elongate. n = 11, 12 (18, 22, 23). Myxopyroside iridoid pathway present. – Myxopyreae are sister (with very high bootstrap support) to the remaining Oleaceae in analyses by Lee & al. (2007).
[Jasmineae+Oleeae]
Ovules two (to four) per carpel. Fruit drupaceous or baccate. Oleoside present.
Jasmineae Lam. et DC., Syn. Plant. Fl. Gall.: 216. 30 Jun 1806
1/c 200. Jasminum (c 200; warm-temperate to tropical regions in the Old World). – Sepals and petals up to 14 or more. First four sepals diagonally initiated. Petals with imbricate-quincuncial aestivation. Ovules usually two (rarely four) per carpel. Endothelium absent. Megasporocytes and megagametophytes occasionally several. Fruit bilobate, a berry or a pyxidium. Seed coat multilayered. Mesotestal cells with completely thickened or band-thickened anticlinal walls. x = 11–13. Inversion of 21 kb present in plastid genome. Secoiridoids present.
Oleeae Hoffmanns. et Link ex Dumort., Fl. Belg.: 52. 1827 [’Oleaceae’]
16/340–345. Ligustrum (c 60; Europe, the Mediterranean, North Africa, East and Southeast Asia, Malesia to eastern Australia and Tasmania, with their highest diversity in East Asia); Comoranthus (3; C. madagascariensis: western Madagascar; C. minor: southwestern Madagascar; C. obconicus: Mayotte Island), Schrebera (8; S. alata, S. arborea, S. trichoclada: tropical to southern Africa; S. capuronii, S. orientalis: Madagascar; S. swietenioides: India, the Himalayas, Southeast Asia; S. kusnotoi: Borneo; S. americana: Peru); Fraxinus (40–45; temperate regions on the Northern Hemisphere, few species in tropical regions); ‘Olea’ (32–33; warm-temperate to tropical regions in the Old World; polyphyletic), Haenianthus (3; H. incrassatus: Jamaica; H. salicifolius: the Greater Antilles; H. variifolius: Cuba),‘Chionanthus’ (c 60; warm-temperate to tropical regions in Africa, Madagascar, East Asia and eastern United States; polyphyletic), Notelaea (12; tropical and eastern Australia, Tasmania), Picconia (1; P. excelsa; Madeira, the Canary Islands), Noronhia (c 45; Madagascar, the Comoros), Phillyrea (2; P. angustifolia, P. latifolia; Madeira, the Mediterranean to Syria and northern Iran),‘Osmanthus’ (32; southwestern Asia, China to West Malesia, southeastern United States, Mexico; polyphyletic), Nestegis (5; N. apetala: New Zealand, Norfolk Island; N. cunninghamii, N. lanceolata, N. montana: New Zealand; N. sandwicensis: the Hawaiian Islands), Forestiera (c 20; southern United States, Mexico, Central America, the West Indies, Ecuador), Hesperelaea (1; H. palmeri; Guadalupe Island off Baja California; probably extinct), Priogymnanthus (2; P. apertus, P. hasslerianus; tropical South America). – Warm-temperate to tropical regions on both hemispheres. Vessels in multiples. Vestured pits sometimes present. Libriform fibres usually present. Fibre tracheids usually absent. Marginal parenchyma often present. Peltate scales sometimes present. Sepals sometimes diagonally initiated. Petals with usually valvate (sometimes imbricate) aestivation, sometimes free or absent. Stamens sometimes four. Ovules usually two (rarely four) per carpel. x = (20) 23. Flavone glycosides and secoiridoids present. – Ligustrum (including Syringa) may be sister to the remaining Oleeae.
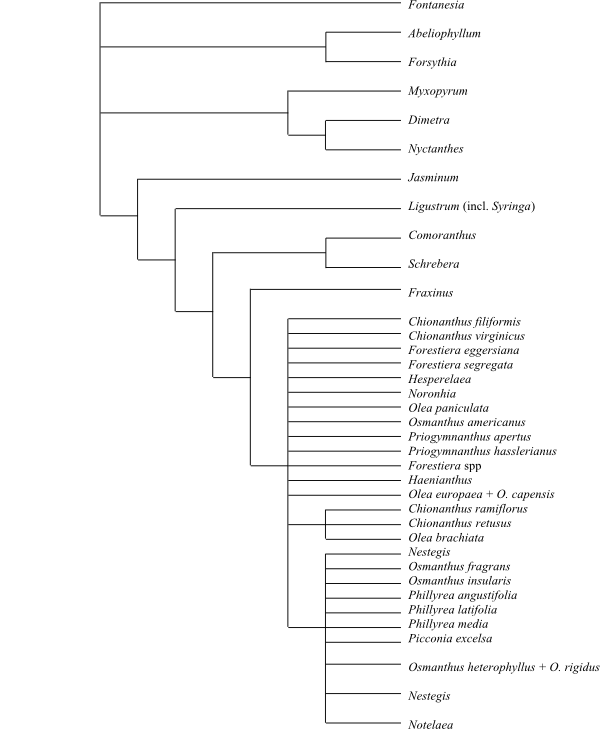 |
Cladogram of Oleaceae based on DNA sequence data (Wallander & Albert 2000). |
OROBANCHACEAE Vent. |
( Back to Plantaginales ) |
Pedicularidaceae Juss., Gen. Plant.: 99. 4 Aug 1789 [’Pediculares’]; Rhinanthaceae Vent., Tabl. Règne Vég. 2: 295. 5 Mai 1799 [’Rhinanthoideae’]; Euphrasiaceae Martinov, Tekhno-Bot. Slovar: 239. 3 Aug 1820 [‘Euphrasiae’]; Orobanchales Vent. ex Bercht. et J. Presl, Přir. Rostlin: 242. Jan-Apr 1820 [‘Orobanchoideae’]; Melampyraceae Rich. ex Hook. et Lindl. in W. J. Hooker, Fl. Scot. 2: 213. 11 Mar 1821; Rhinanthales Dumort., Anal. Fam. Plant.: 20. 1829 [’Rhinantharieae’]; Orobanchineae Link, Handbuch 1: 506. 4-11 Jul 1829 [‘Orobanchinae’]; Rhinanthineae Link, Handbuch 1: 513. 4-11 Jul 1829 [‘Rhinanthaceae’]; Phelypaeaceae Horan., Prim. Lin. Syst. Nat.: 73. 2 Nov 1834 [’Phelipaeaceae’]; Buchneraceae (Benth.) Lilja, Skånes Fl., ed. 2: 979. Apr-Dec 1870; Aeginetiaceae Livera in Ann. Roy. Bot. Gard. (Peradeniya) 10: 153. 31 Mai 1927; Cyclocheilaceae Marais in Kew Bull. 35: 805. 28 Apr 1981; Nesogenaceae Marais in Kew Bull. 35: 798. 28 Apr 1981; Lindenbergiaceae Doweld, Tent. Syst. Plant. Vasc.: xlix. 23 Dec 2001
Genera/species 98/1.850–2.150
Distribution Mainly temperate and alpine regions, with their highest diversity in temperate regions on the Northern Hemisphere and in Africa and Madagascar; some representatives in southern South America, South Asia, southern Australia, and New Zealand.
Fossils Unknown.
Habit Bisexual, usually perennial, biennial or annual herbs (rarely shrubs or suffrutices, e.g. Brandisia, Cyclocheilon, Asepalum). Nearly all species are either root hemiparasites having green assimilating leaves, or more or less succulent achlorophyllous root holoparasites with scale-like leaves. Often blackening when dry. Roots in Asepalum and Cyclocheilon at least usually red. Lindenbergia comprises autotrophic, photosynthesizing plants.
Vegetative anatomy Mycorrhiza absent. Roots with haustoria. Phellogen ab initio superficial? Medullary vascular bundles present or absent. Primary vascular tissue one or several cylinders of bundles. Secondary lateral growth normal or absent. Endodermis sometimes prominent (Euphrasia). Vessel elements with simple perforation plates; lateral pits alternate. Imperforate tracheary xylem elements libriform fibres? with simple pits, septate? Wood rays ?-seriate, usually heterocellular? Axial parenchyma paratracheal. Sieve tube plastids S type. Nodes usually 1:1?, unilacunar with one? leaf trace. Crystals?
Trichomes Hairs unicellular or multicellular, usually uniseriate (sometimes branched; in Brandisia also stellate); glandular hairs sometimes dendritic or lepidote; head of glandular hairs without vertical septa (not vertically divided).
Leaves Alternate (spiral) or opposite, simple, entire or pinnately lobed in hemiparasites, scale-like and membranous or absent in holoparasites, with ? ptyxis. Stipules and leaf sheath absent. Petiole vascular bundles? Venation pinnate. Stomata usually anomocytic?, not closing (not even in Lindenbergia). Cuticular wax crystalloids? Mesophyll with or without sclerenchymatous idioblasts. Leaf margin lobate, serrate or entire. Extrafloral nectaries present as glandular hairs on lamina in some species of Melampyrum.
Inflorescence Terminal or axillary, usually raceme (in Lindenbergia branched raceme) or spike (flowers occasionally solitary axillary). Floral prophylls (bracteoles) lateral (in Asepalum and Cyclocheilon large, free or connate, enclosing bud, persistent and accrescent in fruit), at pedicel base, shortly above foliaceous bract, or immediately below flower.
Flowers Zygomorphic. Hypogyny. Sepals four or five, with valvate or open aestivation, persistent, connate (in Asepalum and Cyclocheilon almost absent); median sepal adaxial? Petals five, with imbricate quincuncial or descending-cochlear aestivation (adaxial-lateral, posterior, corolla lobes usually covered in bud by one or two abaxial-lateral lobes; sometimes also abaxial lobe covering adaxial-lateral lobes; often ascending-cochlear aestivation in which abaxial lobe covers all other lobes; abaxial lobe often inserted outside of adaxial lobes), caducous or persistent (collar-like base of corolla tube persistent), connate into tubular or infundibuliform (often obliquely) usually bilabiate corolla. Nectariferous disc intrastaminal, annular, often fleshy (nectaries and/or disc sometimes absent).
Androecium Stamens usually two longer and two shorter (didynamous), haplostemonous, antesepalous, alternipetalous. Filaments free from each other, usually adnate to corolla tube (epipetalous). Anthers often connivent, with thecae parallel or confluent, often with one theca reduced, often hairy, sagittate to invertedly U-shaped (sometimes separate), dorsifixed, versatile?, tetrasporangiate, introrse, usually longicidal (dehiscing by longitudinal slits; in Bartsia and some species of Euphrasia poricidal, dehiscing by apical pores); connective sometimes prolonged at apex. Tapetum usually secretory (sometime amoeboid-periplasmodial). Staminodia usually absent (rarely one, adaxial-median).
Pollen grains Microsporogenesis simultaneous. Pollen grains usually 3(–4)-colpate or (2–)3(–4)-porate (rarely inaperturate; in, e.g., Lindenbergia tricolporate; in Pedicularis often syncolpate), shed as monads, bicellular at dispersal. Exine usually tectate (rarely semitectate), with columellate infratectum, usually perforate, microreticulate or finely rugulate, often with verrucoid or globular supratectal elements (retipilate; rarely reticulate).
Gynoecium Pistil composed of two (or three or five) connate carpels. Ovary superior, entirely or partially unilocular or bilocular. Style single, simple. Stigma clavate (e.g. Brandisia) to capitate or bilobate to quadrilobate (rarely lingulate or spatulate), papillate, Dry type. Pistillodium absent.
Ovules Placentation axile (when ovary bilocular) or parietal (when ovary unilocular; placentae sometimes two bilobate, four or six). Ovules usually numerous (rarely, e.g., in Nesogenes one) per carpel, usually anatropous, usually epitropous (rarely apotropous), unitegmic, tenuinucellar. Integument (two to) four to twelve cell layers thick. Megagametophyte monosporous, Polygonum type. Synergids sometimes with a filiform apparatus. Antipodal cells often persistent. Endosperm development cellular. Endosperm haustoria chalazal and often micropylar. Embryogenesis onagrad.
Fruit Usually a loculicidal or septicidal capsule (in Radamaea a berry; in Leucosalpa and Tozzia a drupe; in Nesogenes a two-seeded drupe with persistent calyx; in Asepalum a schizocarp with two mericarps).
Seeds Aril absent. Seed pedestals often present. Testa often winged; radial cell walls of wings with reticulate thickenings. Inner walls of exotestal cells variously thickened; cell walls of remaining layers thickened and lignified. Endotestal cell walls sometimes thickened and lignified. Perisperm not developed. Endosperm usually copious (sometimes sparse; absent in Monttea, Asepalum and Cyclocheilon), usually oily (often starchy). Embryo small to minute, often undifferentiated when mature (globular or ovoid, lacking histogens except dermatogen), usually straight, without chlorophyll. Cotyledons two (often reduced). Germination phanerocotylar or cryptocotylar, often through germination tube.
Cytology n = (6) 7 – 21 (or more?) – Polyploidy occurring; endomitotic polyploidization in inner tapetal cells (not in Pedicularis and Melampyrum); genome size decreasing after polyploidization. Protein bodies in cell nucleus lamellar.
DNA Deletion in plastid gene matK. Larger part of small single copy region of plastid DNA deleted in Epifegus. Plastid inverted repeat absent from Conopholis and one inversion present in its cpDNA. Plastid genome in Striga asiatica with three inversions. Numerous plastid genes (e.g. rps16 and infA) and introns lost in Epifegus and Conopholis.
Phytochemistry Group I carbocyclic iridoids (aucubin, catalpol, daphylloside), Group X secoiridoids (nepeta lactones, iridoidpyridine alkaloids), pyrrolizidine alkaloids as macrocyclic diesters (at least in Castilleja and Melampyrum), caffeic acid esters (bartsioside, orobanchin, verbascosides), and silicic acid present. Flavonols, 6- and/or 8-hydroxylated flavone glycosides, ellagic acid, tannins, proanthocyanidins, saponins, and cyanogenic compounds not found. Carbohydrates stored as hexites and other oligosaccharides (e.g. mannitol in some species). Many substances are transported from host to parasite, not manufactured by the parasite.
Use Ornamental plants?
Systematics Orobanchaceae are sister-group to Rehmanniaceae.
Lindenbergieae T. Yamaz. in Fl. Cambodge, Laos et Vietnam 21: 20. 25 Jan 1985
1/12. Lindenbergia (12; northeastern Africa, tropical Asia to the Philippines, with their highest diversity in India). – Bracts foliaceous. Floral prophylls (bracteoles) usually absent. Anther thecae inserted on connective arms. Pollen grains tricolporate. Testal surface usually with hook-shaped thickenings. n = 16.
Orobanchoideae Eaton, Bot. Dict., ed. 4: 32. Apr-Mai 1836 [‘Orobancheae’]
97/1.820–2.100. Cymbarieae D. Don in Edinburgh New Philos. j. 19: 112. Jul 1835. Schwalbea (1; S. americana; eastern United States), Siphonostegia (3; S. laeta: eastern Mediterranean; S. syriaca: eastern Greece, southern Turkey; S. chinensis: East Asia), Monochasma (2; M. savatieri, M. sheareri; East Asia), Bungea (2; B. trifida: southwestern Asia; B. vesiculifera: Tien Shan), Cymbaria (5; C. borysthenica, C. chaneti, C. daurica, C. linearifolia, C. mongolica; Ukraine and southern Russia to Central and East Asia). – Brandisia (11; Burma, China). – Orobancheae Lam. et DC., Syn. Pl. Fl. Gall.: 214. 30 Jun 1806. Kopsiopsis (2; K. hookeri, K. strobilacea; western North America), Xylanche (1; X. himalaica; Himalayas, China to Taiwan), Boschniakia (1; B. rossica; temperate Asia to Japan, northwestern North America), Conopholis (3; C. alpina, C. americana, C. panamensis; southeastern United States, Mexico to Panamá; in Boschniakia?), Epifagus (1; E. virginiana; North America; in Boschniakia?); ‘Orobanche’ (c 100; temperate and subtropical regions of both hemispheres; paraphyletic), Boulardia (1; B. latisquama; Spain, the Baleares), Diphelypaea (2–4; D. boissieri, D. coccinea, D. helenae, D. tournefortii; southeastern Europe, Turkey, the Caucasus), Phelipanche (c 50; Europe, the Mediterranean, Macaronesia, western and southwestern Asia), Aphyllon (21; southern Canada to Peru), Mannagettaea (2; M. hummelii, M. labiata; eastern Siberia to western China), Cistanche (16; the Mediterranean, Ethiopia to western India and northwestern China). – Rhinantheae Lam. et DC., Syn. Pl. Fl. Gall.: 208. 30 Jun 1806. Pterygiella (5; P. bartschioides, P. cylindrica, P. duclouxii, P. nigrescens, P. suffruticosa; southern China); Melampyrum (c 35; temperate regions on the Northern Hemisphere), Rhinanthus (c 45?; temperate regions on the Northern Hemisphere), Lathraea (4; L. clandestina, L. japonica, L. rhodopaea, L. squamaria; Europe, temperate Asia), Bartsia (1; B. alpina; cold-temperate and alpine regions in Europe and North America), Rhynchocorys (8; southern Balkan Peninsula, central Mediterranean to Iran), Euphrasia (170–350; temperate regions on the Northern Hemisphere, alpine regions in New Guinea, eastern Australia and New Zealand), Tozzia (1; T. alpina; the Alps, the Carpathians), Odontitella (1; O. virgata; the Iberian Peninsula), Nothobartsia (2; N. aspera, N. spicata; western Mediterranean), Hedbergia (1; H. abyssinica; mountains in tropical Africa), ‘Bartsia’ (c 60; Europe, the Mediterranean, mountains in tropical Africa, temperate Asia, North America to southern South America, with their largest diversity in the Andes; paraphyletic), Parentucellia (3; P. floribunda, P. latifolia, P. viscosa; the Mediterranean to southwestern Asia), Macrosyringion (2; M. glutinosum, M. longiflorum; the Mediterranean), Odontites (c 30; Europe, the Mediterranean to the Himalayas). – Buchnereae Benth. in Edwards’s Bot. Reg. 21: ad t. 1770. 1 Feb. 1835. Cyclocheilon (3; C. kelleri, C. physocalyx, C. somaliense; Somalia, Ethiopia, the Arabian Peninsula), Asepalum (1; A. eriantherum; Ethiopia, Kenya, Tanzania, Yemen), Sopubia (41; tropical and southern Africa, the Himalayas to Indochina and Taiwan), Graderia (4; G. fruticosa, G. linearifolia, G. scabra, G. subintegra; Africa, Socotra), Nesogenes (8; Tanzania, Madagascar, islands in the Indian and Pacific oceans), Radamaea (5; R. latifolia, R. montana, R. perrieri, R. prostrata, R. rupestris; Madagascar), Bardotia (1; B. ankaranensis; northern Madagascar), Rhamphicarpa (5; R. aquatica, R. brevipedicellata, R. capillacea, R. fistulosa, R. medwedewii; Russia, tropical and southern Africa, Turkey, India, tropical Australia), Sieversandreas (1; S. madagascarianus; southern Madagascar), Xylocalyx (5; X. aculeolatus, X. asper, X. carterae, X. hispidus, X. recurvus; Somalia, Socotra), Striga (40–45; tropical, subtropical and southern Africa, southern Asia, northern Australia), Cycnium (18; tropical East Africa to South Africa), Buchnera (c 100; tropical and subtropical regions on both hemispheres), Centranthera (c 13; China and south to northern and northeastern Australia), Alectra (c 40; tropical and southern Africa, tropical Asia), Melasma (25–35; southern Africa, tropical and subtropical America), Escobedia (c 10; Mexico, Central America, tropical South America), Hyobanche (9; southern Africa), Harveya (c 30; tropical and southern Africa, the Mascarene Islands), Aeginetia (8; India, Sri Lanka and Burma to China, Japan and Southeast Asia, Malesia), Christisonia (16–20; southwestern China, Southeast Asia, Malesia). – Pedicularideae Duby, Bot. Gall. 1: 351. 12-14 Apr 1828. Pedicularis (500–600; temperate and arctic-alpine regions on the Northern Hemisphere and south to the Andes in Colombia, with their largest diversity in Central Asia to western China), Phtheirospermum (5; P. glandulosum, P. japonicum, P. muliense, P. parishii, P. tenuisectum; East Asia), Lamourouxia (28; Mexico, Central America, tropical South America to Peru), Esterhazya (7; E. andina, E. caesarea, E. eitenorum, E. macrodonta, E. nanuzae, E. splendida, E. triflora; Brazil, Bolivia), Agalinis (c 70; United States, Mexico, Central America, the West Indies, tropical South America), Aureolaria (8; eastern United States, Mexico), Seymeria (c 25; southern United States, Mexico), Cordylanthus (19; western North America), Orthocarpus (8; southwestern Canada, western United States, northwestern Mexico), Castilleja (>200; temperate regions on the Northern Hemisphere to Central America and the Andes in northern South America), Triphysaria (6; T. chinensis, T. eriantha, T. floribunda, T. micrantha, T. pusilla, T. versicolor; British Columbia to California). – Unplaced Orobanchoideae Baumia (1; B. angolensis; Angola), Brachystigma (1; B. wrightii; Arizona, New Mexico, northwestern Mexico), Buttonia (3; B. hildebrandtii, B. natalensis, B. superba; tropical and southern Africa), Chloropyron (4; C. maritimum, C. molle, C. palmatum, C. tecopense; western United States, northwestern Mexico), Dasistoma (1; D. macrophylla; southeastern United States), Eremitilla (1; E. mexicana; Mexico), Gerardiina (2; G. angolensis, G. kundelungensis; tropical and southern Africa), Ghikaea (1; G. speciosa; northeastern Africa), Gleadovia (3; G. banerjiana, G. mupinensis, G. ruborum; western Himalayas, western China), Hiernia (1; H. angolensis; Angola, Namibia), Leptorhabdos (1; L. parviflora; the Caucasus, Iran to Central Asia and the Himalayas), Leucosalpa (3; L. grandiflora, L. madagascariensis, L. poissonii; Madagascar), Macranthera (2; M. flammea, M. fuchsioides; southeastern United States), Magdalenaea (1; M. limae; southeastern Brazil), Micrargeria (3; M. barteri, M. filiformis, M. wightii; tropical Africa, India), Micrargeriella (1; M. aphylla; Congo, Zambia), Nothochilus (1; N. coccineus; Brazil), Omphalotrix (1; O. longipes; northeastern Asia), Paraharveya (1; P. alba; Central and tropical East Africa), Parasopubia (3; P. bonatii, P. delphiniifolia, P. hofmannii; Southeast Asia), Petitmenginia (2; P. comosa, P. matsumurae; southern China, Southeast Asia), Phacellanthus (1; P. tubiflorus; China, the Korean Peninsula, Japan, the Russian Far East), Physocalyx (3; P. aurantiacus, P. major, P. scaberrimus; Brazil), Pseudomelasma (1; P. peduncularioides; Madagascar), Pseudosopubia (7; tropical Africa), Pseudostriga (1; P. cambodiana; Southeast Asia), Rhaphispermum (1; R. gerardioides; central Madagascar), Seymeriopsis (1; S. bissei; Cuba), Silviella (2; S. prostrata, S. serpyllifolia; Mexico), Tetraspidium (1; T. laxiflorum; Madagascar), Thunbergianthus (2; T. quintasii: São Tomé; T. ruwenzoriensis: Congo, tropical East Africa), Vellosiella (2; V. dracocephaloides, V. spathacea; Brazil). – Subcosmopolitan. Root hemi- or holoparasitic herbs (sometimes shrubs). Stomata not closing. Sepals sometimes free or almost free. Corolla tube development sometimes intermediate. Filaments in Eremitilla free from petals. Stamens in Castillejinae unithecal or unequally bithecal. Anthers often hairy and with tails or basal awns. Pollen grains often with starch. Cells of testal wings sometimes with reticulate thickenings on radial walls. Endosperm sometimes with thickened cell walls. Polysaccharides sometimes mannose-rich. – Schwalbea is probably basal in Orobanchoideae. It forms a clade – together with Bungea, Cymbaria, Monochasma and Siphonostegia – possibly being sister-group to the remainder. Cyclocheilaceae form an ingroup within Orobanchoideae. Nesogenes is sometimes identified as sister, with large support, to Radamaea.
The positions of Orobancheae and Rhinantheae relative to Buchnereae and Pedicularideae are ambiguous. Rhinantheae have been basal to Orobancheae in some earlier analyses, and Buchnereae are sometimes basal to both Rhinantheae and Pedicularideae.
Holoparasitism has evolved several times from hemiparasitism.
The position of Brandisia is uncertain. According to, Bennett & Mathews 2006 it may be sister to the core clade of hemiparasitic Orobanchoideae.Brandisia consists of shrubs, sometimes parasitic, with usually stellate hairs. The leaves are opposite, with entire or serrate margin. The flowers are axillary, solitary or pairwise, or arranged in racemes, and have two floral prophylls (bracteoles). The calyx is campanulate, two-lipped or almost actinomorphic, with two or five lobes. The corolla has a slightly curved tube and a two-lipped limb with bilobate upper lip and trilobate lower lip.The four stamens are didynamous, with filaments adnate to base of corolla tube. Staminodia are absent. The superior ovary is bilocular, the style is simple and the stigma capitate. The ovules are numerous per carpel. The fruit is a loculicidal capsule. The seed coat has reticulate pattern (cf. Paulownia in Paulowniaceae) and the testa is provided with membranous wings (cf. Paulowniaceae). Phenylethanoids are present.
 |
Phylogeny (simplified) of Orobanchaceae based on DNA sequence data (Wolfe & al. 2005; Morawetz & al. 2010; etc.). |
 |
Phylogeny (simplified) of Orobanchaceae based on DNA sequence data (McNeal & al. 2013). |
PAULOWNIACEAE Nakai |
( Back to Plantaginales ) |
Genera/species 1–2/7–18
Distribution Warm-temperate, subtropical and tropical regions in continental China to northern Indochina, the Korean Peninsula, Japan, Taiwan.
Fossils Uncertain. Fossils assigned to Paulownia columbiana have been described from the Miocene/Pliocene transition zone of Germany.
Habit Bisexual, deciduous trees.
Vegetative anatomy Phellogen ab initio outer-cortical. Medulla septated by diaphragms. Vessel elements with simple perforation plates; lateral pits alternate, simple or bordered pits. Vestured pits? Imperforate tracheary xylem elements ? with simple or bordered pits, non-septate. Wood rays uniseriate or multiseriate, homocellular. Axial parenchyma aliform, lozenge-aliform, winged-aliform, confluent or banded. Tyloses abundant. Sieve tube plastids S type? Nodes 1:1, unilacunar with one leaf trace (Paulownia). Crystals? C4 physiology present in Paulownia.
Trichomes Hairs multicellular, uniseriate or branched, or absent.
Leaves Opposite, simple, entire, with ? ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle transection annular. Venation pinnate. Stomata anomocytic? Cuticular wax crystalloids? Leaf margin entire. Extrafloral nectaries often present on lamina.
Inflorescence Terminal.
Flowers Zygomorphic, large. Hypogyny. Sepals five, with valvate aestivation, persistent, more or less connate (in Paulownia at base), campanulate, in Paulownia covered by long brown hairs; median sepal adaxial. Petals five, with ascending-cochlear aestivation, deciduous, connate inte bilabiate or hypocrateriform corolla (in Paulownia covered by simple hairs with tapering terminal cell). Nectaries vascularized. Disc intrastaminal.
Androecium Stamens two longer and two shorter (in terminal flowers often five), haptostemonous, antesepalous, alternipetalous. Filaments free from each other, adnate to corolla tube (epipetalous). Anthers with separate or confluent thecae (in Paulownia positioned head-to-head), basifixed, versatile, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits), in Paulownia with massive endothecium surmounting connective. Tapetum secretory? Placentoid present (Paulownia). Staminodia absent.
Pollen grains Microsporogenesis simultaneous? Pollen grains (2–)3(–4)-colporate (or –colpate), shed as monads, bicellular? at dispersal. Exine semitectate, with columellate? infratectum, foveolate-reticulate to microreticulate.
Gynoecium Pistil composed of two connate carpels. Ovary superior, bilocular. Style singe, simple, hollow, sometimes widened at apex. Stigma punctate, hollow, papillate?, type? Pistillodium absent.
Ovules Placentation axile, protruding. Ovules numerous per carpel, anatropous?, unitegmic, tenuinucellar. Integument ? cell layers thick. Megagametophyte monosporous, Polygonum type. Endosperm development cellular? Endothelial cells thickened laterally and at endosperm. Endosperm haustoria? Embryogenesis?
Fruit A loculicidal (Paulownia) or loculicidal-septicidal capsule with persistent woody calyx tube.
Seeds Aril absent? Seed pedestals present. Testa winged, with several membranous sinuous wings. Exotestal cells wide, with complex reticulate wall thickenings. Endotesta? Perisperm not developed. Endosperm smooth, uni- or multilayered, oily? Embryo straight?, well differentiated?, chlorophyll? Cotyledons two. Germination phanerocotylar?
Cytology n = (19) 20 (Paulownia)
DNA Mitochondrial coxI intron present.
Phytochemistry Insufficiently known. Flavones (luteolin), Group I iridoids (catalpol), iridoid glycosides (mussaenoside), sitosterolglycosides (daucosterol), sitosterols (gentiobioside), and verbascosides present.
Use Ornamental plants, medicinal plants, timber.
Systematics Paulownia (6–17; warm-temperate, subtropical and tropical regions in continental China to northern Indochina, the Korean Peninsula, Japan, Taiwan; incl. Shiuyinghua?), Shiuyinghua (1; S. silvestrii; central China; in Paulownia?).
Paulowniaceae are sister-group to the clade [Rehmanniaceae+Orobanchaceae]. However, there is medium support for a sister-group relationship between Paulownia and Lamiaceae (Olmstead & al. 2000), and a somewhat higher support for Paulownia being sister to Orobanchaceae.
PEDALIACEAE R. Br. |
( Back to Plantaginales ) |
Pedaliales R. Br. ex Bercht. et J. Presl, Přir. Rostlin: 246. Jan-Apr 1820 [‘Pedalinae’]; Sesamales Bercht. et J. Presl, Přir. Rostlin: 246. Jan-Apr 1820 [‘Sesamoideae’]; Sesamaceae Horan, Prim. Lin. Syst. Nat.: 74. 2 Nov 1834
Genera/species 14/77–80
Distribution Tropical and southern Africa, Madagascar, southwestern Asia, Malesia to New Guinea, northern and arid Australia.
Fossils Unknown.
Habit Bisexual, usually perennial or annual herbs (sometimes deciduous trees, shrubs or suffrutices; in Sesamothamnus and Uncarina with succulent stems; several genera with water-storing tuberous root; in Pterodiscus with caudex consisting of succulent sub- and supraterranean stem and root segments). Many species are xerophytic. Often with strong smell.
Vegetative anatomy Phellogen ab initio superficial. Cortical cells in some succulents with chloroplasts. Medulla in Pedalium sometimes septated by diaphragms? Cambium storied. Wood elements usually non-storied. Vessel elements with simple perforation plates; lateral pits alternate. Imperforate tracheary xylem elements usually libriform fibres (in Uncarina fibre tracheids) with simple pits, septate or non-septate. Wood rays uniseriate or multiseriate, heterocellular. Axial parenchyma apotracheal banded or usually paratracheal scanty vasicentric. Fibres sometimes few. Sieve tube plastids Ss type. Nodes? Pericycle also with sclereids; medulla and cortex in Sesamothamnus with sclereids.Wood ray cells sometimes with single rhomboidal calciumoxalate crystals.
Trichomes Hairs eglandular uniseriate (rarely branched), or glandular capitate-peltate/stellate mucilage hairs consisting of unicellular or multicellular uniseriate stalk and usually peltate (sometimes stellate) and quadricellular apical head; outer cell walls of glandular head extremely thickened; cell walls of mucilage glands when moistened dissolved and modified into mucilage.
Leaves Usually opposite (sometimes, e.g. in Sesamum alternate, spiral), simple, entire or pinnately lobed (sometimes palmately compound), with ? ptyxis. Stipules and leaf sheath absent. Petiole sometimes modified into spines. Petiole vascular bundle transection disrupted annular. Venation usually pinnate (sometimes palmate). Stomata usually anomocytic or anisocytic (rarely diacytic or paracytic) or absent. Cuticular wax crystalloids? Leaf margin serrate, lobate or entire; lobe apex in Uncarina with hydathode.
Inflorescence Flowers usually axillary solitary (probably corresponding to reduced cymes; sometimes in few-flowered axillary dichasial inflorescences; in Sesamothamnus raceme-like). In axils of floral prophylls (bracteoles) two (to five) aborted lateral floral buds modified into axillary vascularized nectariferous glands (not in Uncarina).
Flowers Zygomorphic, often large. Extrafloral nectaries often present on pedicel. Hypogyny. Sepals five, with imbricate aestivation, unequal in size, persistent, more or less connate; median sepal adaxial. Petals five, with imbricate aestivation, bilabiate (with two upper and three lower lobes) or somewhat non-uniformly quinquelobate connate into tubular or infundibuliform corolla (rarely with adaxial spur at base). Nectariferous disc intrastaminal?, annular, often asymmetrical.
Androecium Stamens usually four, two longer and two shorter (didynamous; often with staminodial adaxial additional stamen), haplostemonous, antesepalous, alternipetalous. Filaments free from each other, adnate to corolla tube (epipetalous). Anthers dorsifixed or basifixed, often pairwise connivent, versatile?, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits); thecae usually confluent, inserted at right angles to filaments; connective usually with apical gland. Tapetum secretory, with uninucleate to quadrinucleate cells. Staminodium one or absent.
Pollen grains Microsporogenesis simultaneous. Pollen grains (3–)5–13(–15)-stephanocolpate, usually shed as monads (in Sesamothamnus as tetrads), tricellular (bicellular?) at dispersal. Exine intectate?, with columellate? infratectum, pilate.
Gynoecium Pistil composed of usually two (in Josephinia four) connate carpels (rarely distinctly unequal in size). Ovary superior, usually bilocular (in Josephinia octalocular); locules often entirely or partially bipartite by secondary septa. Style single, simple, filiform. Stigma usually bilobate, often sensitive, papillate, Wet type. Pistillodium absent.
Ovules Placentation axile. Ovules two to numerous per carpel (in Josephinia one ovule per locule), anatropous, pendulous, horizontal or ascending, unitegmic, tenuinucellar. Integument seven to 20 cell layers thick. Archespore unicellular or bicellular. Hypostase present. Megagametophyte monosporous, Polygonum type. Endosperm development cellular. Endosperm haustoria chalazal and micropylar. Embryogenesis onagrad.
Fruit Usually a loculicidal (in Uncarina loculicidal-septicidal) capsule (sometimes a nut or schizocarp) with persistent calyx and hardening (indurating) stylar base, and with beak-shaped outgrowths from paracarp sterile upper part of ovary. Exocarp and mesocarp decaying during maturation. Endocarp sclerenchymatous, with bristles, sharp edges, prickles, hooks, wings or horn-shaped fibrous processes.
Seeds Aril? Testa multiplicative, often with wings. Outer epidermis tanniniferous. Exotestal cells palisade with lignified walls or with annular wall thickenings. Exotesta and mesotesta sometimes with stellate calciumoxalate crystals. Endotesta? Perisperm not developed. Endosperm very thin, oily, or absent. Embryo straight, well differentiated, chlorophyll? Cotyledons two, often rich in lipids and amyloid (xyloglucans). Germination phanerocotylar.
Cytology n = 8, 13, 16, 26, 32 – Polyploidy frequently occurring. Protein bodies present in cell nucleus?
DNA Mitochondrial coxI intron present. Deletion in plastid gene matK?
Phytochemistry Flavone-C-glycosides, Route II iridoids (also 10-hydroxylated carboxylic iridoids), Group II carbocyclic iridoids (harpagide, harpagioside, procumbide), iridoid glycosides, and verbascosides (i.a. orobanchin) present. Ellagic acid, proanthocyanidins, saponins, and cyanogenic compounds not found. Seed oils in, e.g., Sesamum containing up to 2,5% lignans (e.g. sesamin and sesamolin).
Use Ornamental plants, edible seeds, seed oils (Sesamum), medicinal plants (Harpagophytum).
Systematics Pedalieae Dumort., Anal. Fam. Plant.: 22. 1829. Uncarina (14; Madagascar), Rogeria (2; R. adenophylla, R. longiflora; tropical and southwestern Africa), Holubia (1; H. saccata; northeastern South Africa, Botswana, Zimbabwe), Harpagophytum (2; H. procumbens, H. zeyheri; Namibia, Botswana, northern South Africa, Madagascar), Pterodiscus (14; tropical and southern Africa), Pedaliodiscus (1; P. macrocarpus; Kenya, Tanzania), Pedalium (1; P. murex; northeastern Africa, probably introduced and naturalized in tropical and subtropical regions in the Old World). – Sesamothamneae Ihlenf. in Mitt. Staatsinst. Allg. Bot. Hamburg 12: 75. 1967. Sesamothamnus (6; S. benguellensis, S. busseanus, S. guerichii, S. leistneranus, S. lugardii, S. rivae; northeastern tropical Africa to southwestern and southeastern subtropical Africa). – Sesameae Dumort., Anal. Fam. Plant.: 22. 1829. ‘Sesamum’ (c 20; tropical and southern Africa, the Mascarene Islands, the Arabian Peninsula, southwestern Asia to India and Sri Lanka; non-monophyletic), Ceratotheca (5; C. integribracteata, C. reniformis, C. saxicola, C. sesamoides, C. triloba; tropical and Southern Africa), Dicerocaryum (4; D. eriocarpum, D. forbesii, D. senecioides, D. zanguebarium; eastern and southern Africa, Madagascar), Josephinia (3–6; J. africana, J. eugeniae, J. imperatricis; Kenya, Somalia, Malesia, tropical and arid regions in Australia). – Unplaced Pedaliaceae Linariopsis (2; L. chevalieri, L. prostrata; western and southwestern tropical Africa), Dewinteria (1; D. petrophila; Namibia).
Pedaliaceae may be sister to Acanthaceae, although their sister-group relationship is unresolved. According to Gormley & al. (2015), Pedaliaceae are possibly sister-group to Bignoniaceae.
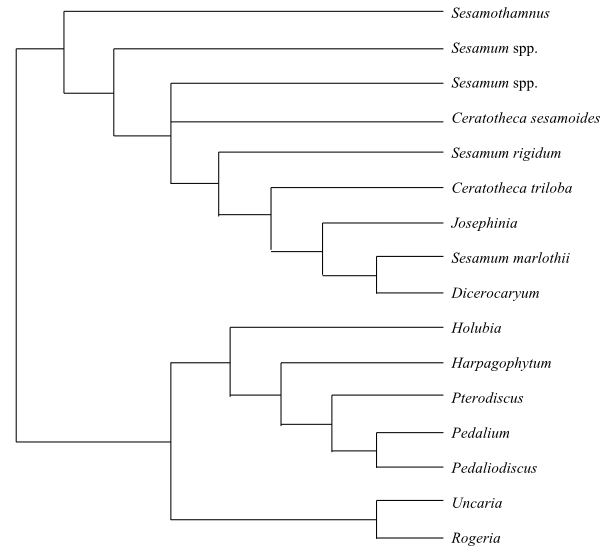 |
Maximum likelihood tree of Pedaliaceae based on DNA sequence data (Gormley & al. 2015). |
PHRYMACEAE Schauer |
( Back to Plantaginales ) |
Wightiaceae Bo Liu, Bing Liu, Su Liu & Y. H. Tan, J. Syst. Evol. 58: 12. https://doiorg/10.1111/jse.12513 17 May 2019.
Genera/species 2–15/c 135
Distribution Tropical and southern Africa, Madagascar, East Asia to Manchuria and Japan, South and Southeast Asia, Malesia, Australia, Tasmania, New Zealand, America.
Fossils Unknown.
Habit Bisexual, usually perennial or annual herbs, often with rhizome (rarely lignified in lower part). Wightia also contains evergreen trees, shrubs and lianas. Young stem quadrangular in cross-section. Some species are aquatic.
Vegetative anatomy Phellogen? Primary vascular tissue a cylinder of bundles (one bundle at each edge of young stem). Vessel elements with simple? perforation plates; lateral pits? Imperforate tracheary xylem elements ? with simple pits, septate? Wood rays absent? Axial parenchyma paratracheal? Sieve tube plastids S type. Nodes 1:3, unilacunar with three leaf traces (Phryma). Crystals?
Trichomes Hairs multicellular, uniseriate (in Wightia branched stellate); glandular hairs often present.
Leaves Alternate (spiral) or opposite, simple, entire or lobed, with ? ptyxis. Stipules and leaf sheath absent. Petiole vascular bundles? Venation pinnate. Stomata usually anomocytic (rarely anisocytic or diacytic). Cuticular wax crystalloids? Leaf margin usually serrate (in Wightia entire).
Inflorescence Terminal or axillary, cymose, raceme or spike (rarely capitate or flowers solitary axillary).
Flowers Usually zygomorphic (rarely almost actinomorphic). Hypogyny. Sepals (three to) five, with valvate aestivation (Mimulus), persistent, connate into tubular or bilabiate (3:2, three upper and two lower lobes) calyx. Petals (two to) four or five, connate into tubular or bilabiate (2:3, two upper and three lower lobes; Mimulus douglasii with two upper lobes only) corolla, sometimes with spur. Nectaries present or absent. Disc absent.
Androecium Stamens usually two longer and two shorter (rarely two), haplostemonous, antesepalous, alternipetalous. Filaments free from each other, adnate to corolla tube (epipetalous). Anthers often with confluent (in Mimulus sometimes connate) thecae, dorsifixed, versatile?, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory. Staminodia absent.
Pollen grains Microsporogenesis simultaneous. Pollen grains tricolpate or <10-colpodiorate (each colpus with two ora; rarely spiraperturate, penta- to heptastephanocolpate etc.), shed as monads, bicellular or tricellular at dispersal. Exine tectate, not thickest close to apertures, with columellate infratectum, often microreticulate, granulate or spinulate.
Gynoecium Pistil composed of two connate carpels (abaxial carpel often reduced and sterile). Ovary superior, usually unilocular (pseudomonomerous; sometimes bilocular). Style single, simple. Stigma usually broadly bilobate (sometimes capitate or faintly bilobate), without asymmetrically swollen stigmatoid tissue, in Mimulus sensitive, type? Pistillodium absent.
Ovules Placentation usually axile (sometimes subbasal, rarely parietal). Ovules one or several per carpel, orthotropous or hemitropous, ascending, apotropous or epitropous, unitegmic, tenuinucellar. Integument three to seven cell layers thick. Hypostase present. Megagametophyte monosporous, Polygonum type. Synergids with a filiform apparatus (Phryma). Antipodal cells persistent (Phryma), in Mimulus two, one of which binucleate. Endosperm development cellular. Endosperm haustorium chalazal. Embryogenesis solanad.
Fruit A usually loculicidal (rarely septicidal) capsule or a one-seeded nut, enclosed by persistent and sometimes accrescent calyx (dorsal calyx teeth often modified into prickles or hooks; rarely a fleshy fruit or a schizocarp).
Seeds Aril absent? Seed pedestals present. Exotesta? Endotesta? Perisperm not developed. Endosperm copious (Mimulus), sparse or absent. Embryo straight, well differentiated, chlorophyll? Cotyledons two, convolute, inrolled. Germination cryptocotylar. Radicula ephemeral (Phryma).
Cytology n = 7–10, 14, 22, 23, 27, 30, 32 – Polyploidy frequent. Protein bodies in cell nuclei lamellar.
DNA Deletion in plastid gene matK?
Phytochemistry Iridoids (Route I carbocyclic iridoids?) present (Phryma) or absent (Mimulus). Lignans (phrymarolin-I, phrymarolin-II, haedoxane A, leptostachyolacetate) present. Proanthocyanidins, alkaloids, saponins, and cyanogenic compounds not found.
Use Ornamental plants, insecticides.
Systematics Mimulus (c 120; tropical and southern Africa, Madagascar, India to Manchuria and Japan, Southeast Asia, Malesia to New Guinea, Australia, Tasmania, New Zealand, North America, Mexico, Central America, the Andes in South America); Wightia (2; W. speciosissima, W. borneensis; warm-temperate to tropical regions in the Himalayas, northeastern India, northern Burma, East and Southeast Asia and Malesia).
Phrymaceae are sister-group to the clade [Paulowniaceae+[Rehmanniaceae+Orobanchaceae]].
’Mimulus’ and ’Phryma’ in their traditional sense are paraphyletic. Moreover, ‘Mimulus’ is obviously paraphyletic relative to ‘Phryma’, and ‘Phryma’ is here included in Mimulus.
Phrymaceae often appear in the same clade as Paulowniaceae, the Rehmannia clade, Cyclocheilaceae and Orobanchaceae. Phrymaceae have a tubular dentate calyx, usually a loculicidal capsule (rarely a berry), and a bilamellate stigma, receptive only inside and closing when touched.
Cyrtandromoea was fairly highly supported as sister-group to the clade [Mimulus+Uvedalia+[Glossostigma+Peplidium]] in the analyses by Liu & al. (2020), whereas Wightia appeared as sister to all genera of Phrymaceae.
Cyrtandromoea (11–12; Southeast Asia, Malesia) was ambiguously placed in Gesneriaceae-Epithematoideae, identified as sister-group to the clade [Monophyllaea+Rhynchoglossum] in ndhF analyses by Smith, Brown & al. (1997). Cyrtandromoea has endosperm and exotesta with U-shaped laminated thickenings in cross-section. The cotyledons are isocotylous (Weber & al. 2013). Moreover, it has iridoids and other substances similar to the Plantaginaceae and “higher” Plantaginales.
Wightia consists of evergreen to deciduous trees, shrubs or lianas. The hairs are stellate. The leaves are opposite, sometimes with ‘glands’ (domatia?) at vein axils on abaxial side, and have entire margin. The flowers are arranged in lateral racemes or thyrses consisting of cymes. The calyx is campanulate, trilobate or quadrilobate or entire. The corolla limb is 2-lipped, with bilobate upper lip and trilobate lower lip. The four stamens are didynamous, with filaments adnate to the tube base. The anthers are basifixed and the thecae are parallel and fused at apex. Staminodia are absent. The pollen grains are usually tricolporate (sometimes di- or tetracolporate). The style is elongate and simple, and the stigma is punctate. The fruit is a septicidal capsule with persistent central columella. The ovules are numerous in each carpel and the testa has membranous wings. Wightiaceae, sister-group to Phrymaceae and comprising the genus Wightia, were described by Liu & al. (2019).
 |
Cladogram of Phrymaceae based on DNA sequence data (Beardsley & Olmstead 2002). |
PLANTAGINACEAE Juss. |
( Back to Plantaginales ) |
Globulariaceae DC. in de Lamarck et A. P. de Candolle, Fl. Franç., ed. 3, 3: 427. 17 Sep 1805 [’Globulariae’], nom. cons.; Antirrhinaceae Pers., Syn. Plant. 2: 154, 640. Sep 1807 [‘Antihirrhineae’, ’Antirrhineae’]; Veronicaceae Cassel, Lehrb. Nat. Pflanzenord.: 366. Apr-Mai 1817 [’Veronicae’]; Hippuridaceae Vest, Anleit. Stud. Bot.: 265, 278. 1818 [’Hippuroideae’], nom. cons.; Callitrichales Bercht. et J. Presl, Přir. Rostlin: 271. Jan-Apr 1820 [‘Callitricheae’]; Chelonaceae Augier ex Martinov, Tekhno-Bot. Slovar: 124. 3 Aug 1820 [’Cheloneae’]; Digitalidaceae Augier ex Martinov, Tekhno-Bot. Slovar: 202. 3 Aug 1820 [’Digitales’]; Gratiolaceae Martinov, Tekhno-Bot. Slovar: 294. 3 Aug 1820 [’Gratioleae’]; Linariaceae Bercht. et J. Presl, Přir. Rostlin: 243. Jan-Apr 1820 [‘Linariae’]; Callitrichaceae Link, Enum. Hort. Berol. Alt. 1: 7. 16 Mar-30 Jun 1821 [‘Callitrichinae’]), nom. cons.; Littorellaceae Gray, Nat. Arr. Brit. Pl. 2: 290, 294. 10 Jan 1822 [’Littorellideae’]; Globulariales Link, Handbuch 1: 675. 4-11 Jul 1829 [’Globulariaceae’]; Hippuridales Link, Handbuch 1: 288. 4-11 Jul 1829 [‘Hippurideae’]; Psylliaceae Horan., Prim. Lin. Syst. Nat.: 69. 2 Nov 1834; Aragoaceae D. Don in Edinburgh New Philos. J. 19: 109, 113. Jul 1835; Sibthorpiaceae D. Don in Edinburgh New Philos. J. 19: 114. Jul 1835; Plantaginopsida Meisn., Plant. Vasc. Gen.: Comm.: 226. 18-24 Jul 1841 [’Plantagoideae’]; Antirrhinineae J. Presl in Nowočeská Bibl. [Wšobecný Rostl.] 7: 1136, 1137. 1846 [‘Antirrhineae‘]; Veronicineae J. Presl in Nowočeská Bibl. [Wšobecný Rostl.] 7: 1136, 1140. 1846 [‘Veroniceae‘]; Antirrhinales Döll, Fl. Baden 2: 723. med. 1858 [’Antirrhineae’]; Oxycladaceae (Miers) Schnizlein, Iconogr. Fam. Regni Veg. 2: ad t. 151**. 1857-1870 [’Oxycladeae’]; Erinaceae Duvau ex L. K. G. Pfeiffer, Nomencl. Bot. 1(2): 1236. 16 Jan 1874; Stellariaceae C. MacMill., Metasp. Minnesota Valley: 344. 1892, nom. illeg. – non Stellariaceae Bercht. et J. Presl; Hippuridineae Engl., Syllabus, ed. 5: 175. 20-22 Jul 1907; Callitrichineae Engl. et Gilg in H. G. A. Engler, Syllabus, ed. 7: 240. Oct 1912-Mar 1913; Ellisiophyllaceae Honda in M. Honda et M. Sakisaka, Daikou Nippon Shokubutsu Bunruigaku [Syst. Plant. Japon.]: 373. 25 Apr 1930; Trapellaceae Honda et Sakisaka, Daikou Nippon Shokubutsu Bunruigaku [Syst. Pl. Japon.]: 378. 25 Apr 1930; Hemimeridaceae Doweld, Tent. Syst. Plant. Vasc.: xlix. 23 Dec 2001
Genera/species 92/1.785–1.850
Distribution Cosmopolitan except polar areas (mainly temperate regions).
Fossils Fruits of Trapella have been found in Miocene and Pliocene layers in Europe. Fossil pollen possibly originating from Plantago have been found in Miocene and younger layers in Europe.
Habit Usually bisexual (rarely monoecious, gynomonoecious, dioecious, or gynodioecious), usually perennial, biennial or annual herbs (rarely shrubs or suffrutices). Some genera are aquatic or semi-aquatic. Numerous representatives are xerophytes. At least one species of Philcoxia has carnivorous subterranean leaves.
Vegetative anatomy Phellogen ab initio epidermal to pericyclic; cork tissue usually not developed. Medullary vascular bundles present or absent. Secondary lateral growth normal or absent. Endodermis often prominent. Vessel elements with simple perforation plates; lateral pits alternate, simple and/or bordered pits. Vestured pits present. Imperforate tracheary xylem elements tracheids or libriform fibres with simple and/or bordered pits, septate or non-septate. Wood rays uniseriate or multiseriate, homocellular or heterocellular. Axial parenchyma usually paratracheal scanty vasicentric, or absent (rarely apotracheal diffuse). Cambium sometimes storied (in, e.g., Plantago, Penstemon). Wood elements sometimes storied. Sieve tube plastids S type. Nodes usually 1:1 or 3, unilacunar with one or three leaf traces (sometimes >3:>3, multilacunar with more than three traces). Sclereids sometimes present in stem and roots. Cystoliths present or absent. Calciumoxalate crystals as druses.
Trichomes Hairs unicellular or multicellular, uniseriate or branched, dendritic, sometimes peltate, lepidote or arachnoid (sometimes calcified or silicified); glandular hairs with unicellular stalk and multicellular head (sometimes peltate), sometimes with vertically dividing cells.
Leaves Alternate (spiral) or opposite (rarely verticillate), simple or compound, entire or lobed (in Trapella and closely allied genera anisophylly), rarely coriaceous (in, e.g., Globularieae), with conduplicate or ? ptyxis. Stipules absent; leaf sheath usually absent (petiole base rarely sheathing stem and decurrent). Petiole vascular bundles? Venation usually pinnate or palmate (rarely parallel; leaves sometimes uninerved). Stomata usually anomocytic (sometimes diacytic, anisocytic, paracytic, tetracytic, or cyclocytic), sometimes on adaxial side of lamina only. Cuticular wax crystalloids? Mesophyll with or without sclerenchymatous idioblasts. Leaf margin serrate, crenate or entire; teeth sometimes with hydathodes. Extrafloral nectaries rarely present on lamina.
Inflorescence Terminal or axillary, cymose or racemose of various shape (usually raceme or spike, rarely capitate; flowers sometimes solitary axillary; paired cymes present in, e.g., Penstemon), sometimes pseudanthia with involucre consisting of bracts. Floral prophylls (bracteoles) often absent in Antirrhinoideae.
Flowers Usually zygomorphic (rarely secondarily actinomorphic: Sibthorpia, Aragoa, Plantago). Usually hypogyny (in Hippuris, Trapella and closely allied genera epigyny). Sepals (two to) four or five (to eight in Sibthorpia), usually with imbricate or valvate aestivation, often persistent, more or less connate (in Trapella free?; absent in Callitriche); median sepal usually adaxial. Petals (two to) four or five (to eight; absent in Callitriche, Hippuris and sometimes in Synthyris and Veronica), usually with imbricate or valvate (rarely descending cochlear) aestivation, connate into hypocrateriform, infundibuliform, campanulate or bilabiate corolla (often with two upper and three lower lobes; sometimes with a spur). Nectariferous disc intrastaminal, annular, unilateral, glandular, or absent.
Androecium Stamens usually two longer and two shorter (rarely five to eight or two [in, e.g., Veronica, Gratiola and Trapella two adaxial-lateral stamens] or one [e.g. Callitriche and Hippuris]), haplostemonous, antesepalous, alternipetalous. Filaments free, usually adnate to corolla tube (epipetalous). Anthers usually with parallel thecae (thecae sometimes entirely fused) or sagittate, dorsifixed, sometimes versatile, tetrasporangiate, introrse, usually longicidal (dehiscing by longitudinal slits; in Globularioideae with short apical slit on fused thecae); connective in Trapella and closely allied genera large and peltate. Tapetum secretory, with binucleate or multinucleate cells. Staminodia one (sometimes in, e.g., Cheloneae and Antirrhinoideae), two (adaxial staminal pair; in Trapella and closely allied genera two abaxial-lateral stamens modified into staminodia) or absent.
Pollen grains Microsporogenesis simultaneous. Pollen grains usually (2–)3(–6)-colpate or (2–)3(–6)-colporate (in Plantago 3–15-polypantoporate; in Trapella and closely allied genera tricolpor[oid]ate; in Callitriche often inaperturate), usually shed as monads (in Anticharis as tetrads), usually bicellular at dispersal (sometimes tricellular; in Callitriche, Hippuris, and Trapella tricellular). Exine tectate or semitectate, with columellate? infratectum, perforate, reticulate, striate, rugulate or spinulate.
Gynoecium Pistil composed of usually two connate carpels (rarely three; in Hippuris one carpel). Ovary usually superior (rarely inferior), usually bilocular (rarely uni- or trilocular or pseudomonomerous; in Callitriche quadrilocular by secondary septa; abaxial locule degenerating in Trapella and allied genera). Stylodia two, partially free or entirely connate (in Callitriche two, completely free from each other, with stigmatic areas along their entire length). Stigma capitate or bilobate (in Trapella and allied genera non-uniformly bilabiate), papillate or non-papillate, usually Dry (sometimes Wet) type. Female flowers sometimes with pistillodium?
Ovules Placentation usually axile (sometimes axile to apical; rarely intrusively parietal; in Plantago basal to axile; in Globularioideae apical). Ovules one or two (in Trapella and closely allied genera in adaxial locule only) to numerous per carpel, usually anatropous or hemianatropous (rarely amphitropous or campylotropous), pendulous or ascending, apotropous or epitropous, unitegmic, tenuinucellar (reduced, with meiocyte semi-inferior). Integument three to 22 cell layers thick. Megagametophyte usually monosporous, Polygonum type. Synergids sometimes with a filiform apparatus. Antipodal cells sometimes persistent. Endosperm development cellular. Endosperm haustoria chalazal and micropylar. Endothelial cells often small, irregularly arranged and with evenly thick walls. Embryogenesis usually onagrad (in, e.g., Ellisiophyllum pinnatum solanad).
Fruit Usually a septicidal capsule (in, e.g., Gratiola and Veronica septicidal and/or loculicidal; in many Antirrhinoideae poricidal; rarely a pyxidium [in, e.g., Plantago], berry, nut or drupe, sometimes with persistent calyx; in Callitriche a schizocarp with four one-seeded drupaceous or nutlike mericarps; in Trapella and closely allied genera a nut with persistent calyx with five basal alternisepalous vascularized often long and hook-tipped appendages). Placenta sometimes with cushion-shaped scars of detached seeds.
Seeds Aril absent. Seed pedestals often present. Testa in Trapella and closely allied genera with thin cell walls. Exotestal cells with inner walls thickened (in Plantago large, mucilaginous). Endotestal cells in Plantago persistent. Perisperm not developed. Endosperm usually copious (sometimes sparse), oily. Embryo large or small, usually straight (rarely curved), usually well differentiated, usually without chlorophyll. Cotyledons two. Germination phanerocotylar. Radicula in Trapella and closely allied genera ephemeral.
Cytology n = 3–10, 12, 14, 16, 17, 19–21, 24, 26–28 (32) (34) (56) (n = c. 25 in Trapella and closely allied genera) – Polyploidy occurring. Protein bodies in cell nuclei in Gratioloideae lamellar or fibrillar; in Russelioideae lamellar or tubular; in Chelonoideae amorphous; in Antirrhinoideae amorphous, crystalline or tubular; in Globularioideae lamellar; in Digitalidoideae amorphous, crystalline or tubular, sometimes absent.
DNA Deletion in the plastid gene matK. Mitochondrial coxI intron present.
Phytochemistry Flavonols (kaempferol), 6- and/or 8-hydroxyflavones and their glycosides or 6-methoxyflavones, Route II decarboxylated iridoids, Group I carbocyclic iridoids (aucubin, catalpol, 6-O-esters of catalpol, daphylloside, monotropein), Group II carbocyclic iridoids (antirrhinoside, linarioside), Group X secoiridoids (loganin, antirrhide, gardoside, iridoid pyridine alkaloids), iridoid glucosides (e.g. ajugol; absent from some lineages, e.g. Digitalis with digitalis glucosides), terpenes, cardenolides (in, e.g., Digitalis), ursolic acid and caffeic acid esters (cornosides in, e.g., Digitalis and Veronica, verbascosides), saponins, polyols, and shikimic acid derived arthroquinones present. Ellagic acid, tannins, proanthocyanidins, and cyanogenic compounds not found. Carbohydrates stored as stachyose and other oligosaccharides (e.g. saccharose; sometimes also mannitol and sorbitol in Plantago).
Use Ornamental plants, medicinal plants (Digitalis, seeds of Plantago psyllium, etc.).
Systematics (under construction) Plantaginaceae are sister-group to the remaining Plantaginales “above” the Gesneriaceae clade.
Gratioloideae Luerss., Handb. Syst. Bot. 2: 993. Sep 1882 [‘Gratioleae’]
33/385–395. Gratioloideae are sister to the remaining Plantaginaceae, according to some analyses. In other studies (e.g. Rahmanzadeh & al. 2005) Gratioloideae are positioned outside of Plantaginaceae in a polytomy.
Angelonieae Pennell in Proc. Acad. Nat. Sci. Philadelphia 71: 227. 11 Mar 1920
6/c 75. Ourisia (18; Tasmania, New Zealand incl. Stewart Island, the Andes), Angelonia (c 45; Mexico, Central America, the West Indies, tropical South America), Basistemon (6; B. bogotensis, B. intermedius, B. klugii, B. peruvianus, B. silvaticum, B. spinosus; the Andes in Venezuela to Bolivia, western Brazil, Paraguay and northwestern Argentina), Monopera (2; M. micrantha, M. perennis; South America), Monttea (3; M. aphylla, M. chilensis, M. schickendantzii; Chile, western Argentina), Melosperma (1; M. andicola; Chile, western Argentina). – Tropical America. Usually shrubs. Integument five to twelve cell layers thick. Seeds few, with special oil attracting pollinators (at least in Angelonia, Basistemon and Monttea).
Gratioleae Benth. in Edwards’s Bot. Reg. 21: ad t. 1770. 1 Feb 1835
27/310–320. Bacopa (65–70; tropical and subtropical regions on both hemispheres), Maeviella (1; M. cochlearia; northeastern Brazil), Boelckea (1; B. beckii; Bolivia), Benjaminia (1; B. reflexa; Venezuela, Brazil), Braunblanquetia (1; B. litoralis; Venezuela to Argentina), Sophronanthe (1; S. hispida; southeastern United States), Trapella (1; T. sinensis; temperate to tropical regions in eastern Asia), Gratiola (c 25; temperate regions on the Northern Hemisphere, East Malesia to New Guinea, southern and eastern Australia, Tasmania, mountains in South America), Mecardonia (12; southeastern United States, Mexico, Central America, the West Indies, tropical and subtropical South America), Scoparia (10; pantropical), Deinostema (2; D. adenocaula, D. violacea; East Asia), Dopatrium (14; tropical and southern Africa, tropical Asia to New Guinea, tropical Australia), Hydrotriche (3; H. galiifolia, H. hottoniiflora, H. mayacoides; Madagascar), Limnophila (37; tropical and subtropical regions in the Old World), Philcoxia (3; P. bahiensis, P. goiasensis, P. minensis; Brazil), Adenosma (27; China, tropical Asia, northern Australia), Otacanthus (7; O. azureus, O. caeruleus, O. caparaoensis, O. crenatus, O. fernandesii, O. platychilus, O. villosus; Brazil), Achetaria (10; tropical South America), Tetraulacium (1; T. veroniciforme; Brazil), Dizygostemon (2; D. angustifolium, D. floribundum; Brazil), Stemodia (c 60; tropical and subtropical regions on both hemispheres), Darcya (3; D. costaricensis, D. mutisii, D. reliquiarum; Central America), Cheilophyllum (8; the West Indies), Schistophragma (4; S. intermedium, S. mexicanum, S. polystachyum, S. pusillum; western United States, Mexico, Central America), Conobea (16; tropical America), Leucospora (2; L. coahuilensis, L. multifida; eastern North America, Mexico), Schizosepala (1; S. glandulosa; Brazil). – Tropical and subtropical regions on both hemispheres, with their highest diversity in tropical and subtropical America. Integument three to six cell layers thick. Endothelial cells transversely elongate in six to eight vertical rows and thickened only near endosperm. Seeds with longitudinal ridges. Exotestal cells with hook-shaped thickenings. – Philcoxia comprises three Brazilian terrestrial species with subterranean stems and peltate leaves with circinate ptyxis. The adaxial side of their leaves are provided with stalked capitate glands. Protease activity and presence of phosphatases as well as digestion of nematodes and nutrient uptake have been detected. The rare case of circinate leaves in these carnivorous plants are remarkable, since the narrow leaves in both Byblis (Byblidaceae) and Drosophyllum (Drosophyllaceae) have circinate vernation.
Trapella sinensis is an aquatic rhizomatous and stoloniferous herb. The flowers are somewhat zygomorphic and epigynous with two fertile stamens (adaxial pair) and two staminodia. The anther has an apically peltate connective. The pollen grains are tricellular at dispersal. The abaxial ovary loculus degenerates. The stigma is bilobate, with unequal lobes. There are two ovules per carpel and the placentation is apical. The fruit is indehiscent and spiny, with persistent calyx and an appendage from below sepal apex. The testal cells are thin-walled. n = c. 25. Trapella is sister to Gratiola and Amphianthus, according to Gormley & al. (2015).
Russelieae Pennell in Proc. Acad. Nat. Sci. Philadelphia 71: 226. 11 Mar 1920
2/51–55. Tetranema (3; T. evolutum, T. megaphyllum, T. roseum; Central America), Russelia (48–52; Mexico, Central America, Cuba, Colombia). – Mexico, Central America, Cuba, Colombia. Heads of glandular hairs with vertically dividing cells. Fruit in Russelia a unique type of hard and dry capsule. – Russelieae are perhaps sister to Chelonoideae.
Chelonoideae Luerss., Handb. Syst. Bot. 2: 993. Sep 1882 [’Cheloneae’]
9/295–320. Collinsia (c 20; southern Canada, United States, Mexico, with their highest diversity in western United States), Tonella (2; T. floribunda, T. tenella; southwestern Canada, western United States), Keckiella (7; K. antirrhinoides, K. breviflora, K. cordifolia, K. corymbosa, K. lemmonii, K. rothrockii, K. ternata; western United States, northwestern Mexico), Chionophila (2; C. jamesii, C. tweedyi; Rocky Mountains), Uroskinnera (4; U. almedae, U. flavida, U. hirtiflora, U. spectabilis; Mexico, Central America), Brookea (4; B. albicans, B. auriculata, B. dasyantha, B. tomentosa; Borneo), Nothochelone (1; N. nemorosa; northwestern North America), Chelone (5; C. caeruleum, C. cuthbertii, C. glabra, C. lyonii, C. obliqua; southeastern Canada, eastern United States), Penstemon (250–275; North America, northern Mexico). – Borneo, North America, Mexico, Central America. Stem with conspicuous medulla. Hairs simple. Inflorescence cymose. Staminodia present. – Collinsia has papilionoid flowers resembling some species of Lupinus, with velum formed by two adaxial petals and carina consisting of median abaxial petal.Uroskinnera should probably be included in Chelonoideae or Russelieae; it is here tentatively placed in Chelonoideae.
Antirrhinoideae Kostel., Allg. Med.-Pharm. Fl. 3: 874. Apr-Dec 1834 (Benth. in Lindl., Veg. Kingd.: 684. Jan-Mai 1846 [‘Antirrhinideae’])
26/290–300. Antirrhinum (c 20; the Mediterranean, with their largest diversity in Spain), Chaenorhinum (26; the Mediterranean, southwestern Asia), Holzneria (2; southwestern Asia), Albraunia (3; southwestern Asia), Schweinfurthia (8; S. apterum, S. imbricata, S. latifolia, S. papilionacea, S. pedicellata, S. pterosperma, S. sphaerocarpa, S. spinosa; arid and semiarid regions in northeastern Africa to India), Acanthorrhinum (1; A. ramosissimum; northwestern Africa), Misopates (9; Europe, Macaronesia, the Mediterranean to Ethiopia and northwestern India), Linaria (95–100; Europe, the Mediterranean, northern Africa, temperate and subtropical Asia), Nuttallanthus (4; N. canadensis, N. floridanus, N. subandinus, N. texanus; southern Canada, United States, Mexico, western South America), Sairocarpus (12; southwestern United States, northwestern Mexico), Howelliella (1; H. ovata; eastern California), Neogaerrhinum (2; N. filipes, N. kelloggii; southwestern United States, northwestern Mexico), Pseudorontium (1; P. cyathiferum; southwestern United States, northwestern Mexico), Mohavea (2; M. breviflora, M. confertiflora; southwestern United States), Asarina (1; A. procumbens; southern France, northeastern Spain), Cymbalaria (10; Central Europe, the Mediterranean to Iran), Maurandya (8; southwestern United States, Mexico, Central America, the West Indies, tropical South America), Maurandella (1; M. antirrhiniflora; southwestern United States, northwestern Mexico), Epixiphium (1; E. wislizeni; southwestern United States, northwestern Mexico), Mabrya (6; M. acerifolia, M. coccinea, M. erecta, M. flaviflora, M. geniculata, M. rosei; southwestern United States, northwestern Mexico), Holmgrenanthe (1; H. petrophila; southwestern United States), Lophospermum (8; Mexico, Guatemala), Rhodochiton (3; R. atrosanguineus, R. hintonii, R. nubicola; Mexico, Central America), Galvezia (5; G. fruticosa, G. grandiflora, G. juncea, G. lanceolata, G. leucantha; Ecuador, the Galápagos Islands, coastal areas in Peru), Gambelia (4; G. glabrata, G. juncea, G. rupicola, G. speciosa; southwestern United States, northwestern Mexico), Kickxia (24; Europe, Macaronesia, the Mediterranean, northern and northeastern Africa, southwestern and southern Central Asia), Nanorrhinum (29; tropical and subtropical regions in the Old World), Anarrhinum (7; A. bellidfolium, A. corsicum, A. duriminium, A. forsskaolii, A. fruticosum, A. longipedicellatum, A. pedatum; Central and South Europe, the Mediterranean, Ethiopia). – Temperate regions on the Northern Hemisphere, northern and northeastern Africa, southwestern Asia, Mexico to western South America and the West Indies, few species in tropical regions of the Old World. Floral prophylls often absent. Flowers zygomorphic. Fruit a unique type of poricidal capsule. Antirrhinosides (unique group of iridoids) present. – Antirrhinoideae are sister to the clade [Globularioideae+Digitalidoideae].
Globularioideae Luerss., Handb. Syst. Bot. 2: 1038. Sep 1882 [‘Globulariaceae’]
3/52. Campylanthus (18; the Canary Islands, northeastern Africa, the Arabian Peninsula, Pakistan), Globularia (31; Europe, Madeira, the Canary Islands, Cape Verde Islands, the Mediterranean, Turkey, North and northeastern Africa), Poskea (3; P. africana, P. newbouldii, P. socotrana; Somalia, Socotra). – Europe, Macaronesia, the Mediterranean to Pakistan, northern and northeastern Africa, the Arabian Peninsula, Socotra. Phellogen epidermal to pericyclic. Tracheids present (fibre tracheids and libriform fibres are absent). Leaves with conduplicate ptyxis. Ovules one or two per carpel (one pendulous and one erect) or, alternatively, only abaxial carpel developed. Stigma Dry type. Fruit a nutlet with membranous pericarp. n = 8–10, 16 (19?). – Globularioideae are sister-group to Digitalidoideae.
Digitalidoideae Luerss., Handb. Syst. Bot. 2: 994. Sep 1882 [’Digitaleae’]
16/650–665
Digitalideae Dumort., Anal. Fam. Plant.: 24. 1829
3/c 28. Digitalis (c 25; Europe, Madeira, the Canary Islands, the Mediterranean to Central Asia), Erinus (2; E. alpinus: the Alps, the Pyrenees; E. thiabaudii: Morocco), Lafuentea (1; L. rotundifolia; southern Spain). – Europe, Macaronesia, the Mediterranean to Central Asia, Morocco. Corolla with descending-cochlear aestivation. Cornosides present (Digitalis).
Plantagineae Dumort., Anal. Fam. Plant.: 25. 1829
3/175–180. Aragoa (19; the Andes in Colombia and Venezuela), Plantago (155–160; cosmopolitan except polar regions), Littorella (1–3; L. uniflora: Europe, the Azores; L. americana: temperate North America; L. australis: temperate South America). – Almost cosmopolitan. Nodes 3:3. Leaves alternate (spiral). Leaf margin serrate or entire. Flowers actinomorphic, small. Sepals and petals usually four (occasionally three; in Aragoa five). Corolla with descending-cochlear aestivation. Pistil composed of two connate carpels. Ovary unilocular or bilocular. Style long-branched, stigmatic its entire length. Stigma Dry type. Placentation usually parietal (sometimes basal, with ovule single, epitropous). Fruit a pyxidium or a nut. Exotestal cells large, mucilaginous. Endotestal cells persistent. Endosperm present. Embryo usually straight (sometimes curved). Iridoid glucosides with 8,9 double bonds and sorbitol present. – Aragoa and Plantago are sister-groups. The substitution frequency in synonymous positions of the mitochondrial genome in Plantago is three to four thousand times higher than in nearly all other angiosperms (Mower & al. 2007). Three or more mitochondrial genes were shown to have been transferred from Cuscuta to the Plantago coronopus lineage (Mower & al. 2010).
Sibthorpieae Benth. in A. P. de Candolle et A. L. P. P. de Candolle, Prodr. 10: 189, 424. 8 Apr 1846
2/6. Ellisiophyllum (1; E. pinnatum; India, Southeast and East Asia to Japan and east to the Philippines, Taiwan and New Guinea), Sibthorpia (5; S. africana, S. conspicua, S. europaea, S. peregrina, S. repens; Europe, the Azores, Madeira, mountains in tropical East Africa, tropical America). – Europe, Macaronesia, East African mountains, India to Japan, the Philippines, Taiwan and New Guinea, tropical America. Flowers actinomorphic. Sepals in Sibthorpia up to eight. Megasporangium development unique type in Plantaginaceae. Embryogenesis in Ellisiophyllum solanad. – Sibthorpieae lack the iridoids typical of Plantaginales.
Veroniceae Duby, Bot. Gall. 1: 355. 12-14 Apr 1828
c 7/440–450. Kashmiria (1; K. himalaica; the Himalayas), Lagotis (c 20; eastern Europe, the Caucasus, northern and Central Asia, the Himalayas to western China), Picrorhiza (3; P. kurroa, P. minima, P. scrophulariiflora; the Himalayas), Scrofella (1; S. chinensis; northwestern China), Veronica (410–420; temperate and alpine regions on both hemispheres, tropical African mountains, New Zealand and surrounding islands, Tierra del Fuego, western Patagonia north to 45°53’ south latitude, the Falkland Islands), Wulfenia (4; W. baldacii, W. blechicii, W. carinthiaca, W. orientalis; southeastern Europe, Turkey), Wulfeniopsis (1; W. amherstiana; the Himalayas in eastern Afghanistan, Pakistan, northern India and Nepal). – Temperate and alpine regions on both hemispheres. Corolla with descending-cochlear aestivation. Stamens in Veronica two. Iridoid glucosides with 8,9 double bonds present.
Hemiphragmateae Rouy, Consp. Fl. France: 172. 15 Aug 1927 [‘Hemiphragmeae’]
1/1. Hemiphragma (1; H. heterophyllum; the Himalayas in northern India, Nepal, Sikkim, Assam and Bhutan to China, Taiwan, the Philippines, Sulawesi). – Perennial herb. Flowers zygomorphic. Corolla quinquelobate, campanulate. Thecae apically connate. Fruit a berry-like capsule.
Callitrichoideae Arn., Botany: 110. 9 Mar 1832 [‘Callitricheae’]
2/c 60. Callitriche (c 60; nearly cosmopolitan incl. the Falkland Islands, South Georgia Islands, Auckland Islands, Campbell Island, Antipodes and Antarctic islands), Hippuris (1; H. vulgaris; nearly cosmopolitan). – Subcosmopolitan. Aquatic, unisexual (monoecious). Flowers actinomorphic (zygomorphic by reduction), minute. Petals absent. Pollen grains tricellular at dispersal. Ovules two per carpel. Callitriche: Hairs stellate-peltate. Sepals absent. Stamens one (to three). Pollen grains often inaperturate. Exine intectate, reticulate or echinate (sometimes absent). Pistil composed of two transverse carpels. Ovary locules with secondary septa. Stylodia thin, separate. Integument thin. Fruit a schizocarp with four nutlike mericarps. Exotesta possibly persistent. n = 3–20. – Hippuris: Usually bisexual (sometimes unisexual). Hairs peltate, glandular. Leaves 4–16-verticillate. Epigyny. Calyx bilobate to quadrilobate or entire. Stamen single. Pollen grains colpoidate. Pistil composed of a single carpel. Style single, with stigmatic area extending its entire length. Stigma Dry type. Ovules pendulous, apotropous. Chalazogamy. Fruit a drupe or achene-like. Endosperm thin, starchy. n = 8, 15, 16. Flavones not found.
Trapelleae Stapf in Engl. et Prantl, Nat. Pflanzenfam. IV, 3b: 260, 265. Mai 1893
1/1–2. Trapella (1–2; temperate to tropical regions in eastern Asia). – Aquatic rhizomatous and stoloniferous herb. Flower somewhat zygomorphic. Epigyny. Stamens two (adaxial pair). Anthers with apically peltate connective. Staminodia two. Pollen grains tricellular at dispersal. Abaxial ovary loculus degenerating. Stigma bilobate, with unequal lobes. Placentation apical. Ovules two per carpel. Fruit indehiscent, spiny, with persistent calyx and an appendage from below sepal apex. Testal cells thin-walled. n = c. 25.
 |
Phylogeny of Plantaginaceae based on DNA sequence data (Schäferhoff & al. 2010). |
Genus incertae sedis
Cubitanthus (1; C. alatus; Bahia in Brazil) is a perennial herb with multicellular hairs, four-winged stem, opposite leaves with serrate margins, solitary axillary flowers without bracteoles, persistent calyx with free sepals, bilabiate corolla limb with trilobite lower lip, four didynamous stamens adnate to corolla base, annular nectariferous disc, superior ovary with parietal placentae, capitate stigma, septicidal bivalvular capsule, and striate seed surface. – It was placed in Gesneriaceae, although it is more similar to Plantaginaceae or Scrophulariaceae.
PLOCOSPERMATACEAE Hutch. |
( Back to Plantaginales ) |
Genera/species 1/1
Distribution Southern Mexico, Guatemala, Costa Rica.
Fossils Unknown.
Habit Functionally unisexual (cryptic dioecy), evergreen trees or shrubs.
Vegetative anatomy Phellogen? Prominent groups of fibres present in outer cortex, in places where leaf traces leave stem. Vessels present in radial multiples. Vessel elements with simple perforation plates; lateral pits alternate. Vestured pits? Imperforate tracheary xylem elements thick-walled fibre tracheids with bordered pits, septate or non-septate. Wood rays usually uniseriate, heterocellular. Axial parenchyma paratracheal scanty, lignified. Sieve tube plastids S type? Nodes 1:1?, unilacunar with one? leaf trace. Styloids present. Parenchyma with druses and single (prismatic?) crystals.
Trichomes Hairs unicellular, simple, filled with calciumcarbonate and accompanied by cystoliths in adjacent basal epidermal cells (or only cystoliths); glandular hairs bicellular, clavate; cells in head of glandular hair not entirely with vertical walls.
Leaves Opposite (often almost verticillate), simple, entire, coriaceous, with ? ptyxis. Stipules and leaf sheath absent. Colleters absent. Petiole articulated at base. Petiole vascular bundle transection annular. Venation pinnate. Stomata anomocytic. Cuticular wax crystalloids absent. Styloids present. Leaf margin entire or somewhat serrate. Hairs long, unicellular, simple.
Inflorescence Axillary, raceme-like (flowers sometimes solitary axillary). Floral prophylls (bracteoles) absent.
Flowers Somewhat zygomorphic. Hypogyny. Sepals five (or six), with imbricate or open aestivation, persistent, connate at base. Petals five (or six), with imbricate (contorted?) aestivation, equal or slightly unequal in size, connate into campanulate or infundibuliform corolla. Nectariferous disc at ovary base (hence only in female flowers).
Androecium Stamens five (or six), unequal in size, haplostemonous, antesepalous, alternipetalous. Filaments free, adnate to corolla tube (epipetalous). Anthers basifixed (dorsifixed?), versatile, tetrasporangiate, extrorse (to latrorse?), longicidal (dehiscing by longitudinal slits). Placentoid? Tapetum secretory? Female flowers with staminodia.
Pollen grains Microsporogenesis simultaneous? Pollen grains tricolporate, shed as monads, ?-cellular at dispersal. Exine semitectate, with columellate? infratectum, reticulate.
Gynoecium Pistil composed of two connate carpels. Ovary superior, unilocular, short-stalked (gynophore?). Stylodia two, bifid, caducous, without indusium, sometimes slightly connate at base. Stigmas clavate, not expanded, type? Male flowers with pistillodium.
Ovules Placentation basal to parietal and/or apical to parietal. Ovules two or four per ovary, ascending and/or pendulous, two parietal placentae each with one or two basal erect ovules or with two basal erect ovules on one placenta and two subapical pendulous ovules on the other, unitegmic, tenuinucellar? Integument ? cell layers thick. Megagametophyte monosporous, Polygonum type? Endosperm development? Endosperm haustoria? Embryogenesis?
Fruit A usually one-seeded (rarely two- to four-seeded) loculicidal capsule with two valves.
Seeds Aril? Chalazal end of seed with dense coma consisting of long multicellular hairs. Testa? Perisperm not developed. Endosperm sparse, thin, fleshy. Embryo straight, large, well differentiated, chlorophyll? Cotyledons two. Germination?
Cytology n = ?
DNA
Phytochemistry Insufficiently known. Caffeic acid and ursolic acid esters (caffeoyl phenylethanoid glucosides, e.g., echinacoside, compounds of the verbascoside group, cornoside, a quinole glucoside), and lugrandoside (a phenylpropanoid glucoside) present. Iridoids not found.
Use Unknown.
Systematics Plocosperma (1; P. buxifolium; southern Mexico, Guatemala, Costa Rica).
Plocosperma is sister to the remaining Plantaginales.
REHMANNIACEAE Kunkel ex Reveal |
( Back to Plantaginales ) |
Genera/species 2/8
Distribution China, the Korean Peninsula.
Fossils Unknown.
Habit Bisexual, perennial or annual herbs.
Vegetative anatomy Phellogen? Vessel elements with simple? perforation plates; lateral pits? Vestured pits? Imperforate tracheary xylem elements? with simple? pits. Wood rays absent. Axial parenchyma? Sieve tube plastids S type? Nodes in Rehmannia 1:3, unilacunar with three leaf traces. Crystals?
Trichomes Hairs unicellular or multicellular; brown to white glandular hairs present in Rehmannia; gead of glandular hairs without vertical septa (not dividing vertically).
Leaves Alternate (spiral), simple, entire or pinnately lobed, with ? ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle transection arcuate; petiole with wing bundles. Venation pinnate. Stomata anomocytic? Cuticular wax crystalloids? Leaf margin serrate, crenate, lobate or entire.
Inflorescence Axillary, few-flowered, cymose, or solitary (Rehmannia), or terminal raceme (Rehmannia, Triaenophora). Floral prophylls (bracteoles) one or two, lateral, present at pedicel base, immediately above foliaceous bract, or absent (aborted in most species of Rehmannia).
Flowers Zygomorphic. Hypogyny. Sepals five (to seven; calyx lobes in Rehmannia entire; in Triaenophora each calyx lobe trilobate), with ? aestivation, connate. Petals five, with imbricate quincuncial aestivation (posterior corolla lobes in bud covered by lateral lobes, abaxial lobe situated outside remaining lobes), connate into infundibular bilabiate corolla. Nectariferous disc intrastaminal, at base of ovary.
Androecium Stamens four, antesepalous, alternipetalous, initiated one-way from abaxial to adaxial side, didynamous (adaxial stamen absent). Filaments free from each other, adnate to corolla tube. Anthers dorsifixed, versatile?, tetrasporangiate, introrse?, longicidal (dehiscing by longitudinal lobes). Tapetum secretory? Staminodia usually absent (sometimes one).
Pollen grains Microsporogenesis simultaneous? Pollen grains tricolporate, shed as monads, ?-cellular at dispersal. Exine semitectate, with columellate infratectum, reticulate.
Gynoecium Pistil composed of two connate carpels. Ovary superior, in Rehmannia usually bilocular (rarely unilocular), in Triaenophora bilocular. Style single, simple. Stigma bilamellate, sensitive, type? Pistillodium absent.
Ovules Placentation axile. Ovules numerous per ovary, anatropous?, unitegmic, tenuinucellar. Integument ? cell layers thick. Megagametophyte monosporous, Polygonum type. Endosperm development? Endosperm haustoria micropylar and chalazal. Embryogenesis?
Fruit A loculicidal capsule with persistent calyx.
Seeds Aril absent. Exotesta cells with with complex reticulate wall thickenings. Endotesta? Perisperm not developed. Endosperm? Embryo?, chlorophyll? Cotyledons two. Germination phanerocotylar?
Cytology n = ? – Protein bodies in cell nuclei lamellar.
DNA Mitochondrial coxI intron present.
Phytochemistry Group I carbocyclic iridoids (catalpol, ajugol, 6-feruloylajugol, aucubin), iridoid glucosides, caffeoyl phenylethanoid glycosides, and ionone glucosides present. Harpagide and 6-rhamnopyranosyl-catalpol and their esters not found. Mannitol present. Sorbitol not found.
Use Ornamental plants, medicinal plants (Rehmannia).
Systematics Rehmannia (6; R. chingii, R. elata, R. glutinosa, R. henryi, R. piasezkii, R. solanifolia; China, the Korean Peninsula), Triaenophora (2; T. integra, T. rupestris; northeastern China).
Rehmanniaceae are sister-group to Orobanchaceae.
SCHLEGELIACEAE (A. H. Gentry) Reveal |
( Back to Plantaginales ) |
Genera/species 4/34–39
Distribution Tropical America.
Fossils Unknown.
Habit Bisexual, evergreen trees or shrubs (sometimes twining, often epiphytic). Bark often whitish.
Vegetative anatomy Phellogen? Vessel elements with simple perforation plates; lateral pits alternate, bordered pits. Vestured pits? Imperforate tracheary xylem elements libriform fibres? with simple or bordered pits, septate. Wood rays uniseriate or multiseriate, homocellular. Axial parenchyma paratracheal vasicentric. Sieve tube plastids S type? Nodes 1:3, unilacunar with three leaf traces. Pericycle with sclereids. Acicular crystals, styloids, crystal sand and other types of calciumoxalate crystals often present.
Trichomes Eglandular hairs?; glands sometimes present on lamina.
Leaves Opposite, simple, entire (in Gibsoniothamnus anisophyllous), often coriaceous, with ? ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle transection solid to almost annular; petiole with wing bundles, without pericyclic lignification. Venation pinnate. Stomata in Schlegelia anomocytic or paracytic, in Gibsoniothamnus anisocytic or cyclocytic. Cuticular wax crystalloids? Petiole in Schlegelia with sclereids. Leaf margin serrate (in Synapsis spinose-dentate) or almost entire. Lamina sometimes with small abaxial glandular hairs with radially arranged head cells.
Inflorescence Terminal or axillary, usually cymose (thyrse; in Exarata racemose).
Flowers Zygomorphic, often large. Hypogyny. Sepals (three to) five, with imbricate aestivation, connate, in Schlegelia with vascularized multicellular nectariferous glands inserted in groups and sunken into epidermis on abaxial surface of calyx. Petals five, with ascending-cochlear aestivation, connate into tubular bilabiate (upper lip bilobate, lower lip trilobate) corolla. Nectariferous disc present, vascularized from carpellary bundles, or absent.
Androecium Stamens two longer and two shorter (didynamous), haplostemonous, antesepalous, alternipetalous. Filaments free from each other, adnate to corolla tube (epipetalous). Anthers basifixed, non-versatile, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory? Staminodia present or absent.
Pollen grains Microsporogenesis simultaneous? Pollen grains tricolpate or triporate, shed as monads, ?-cellular at dispersal. Exine tectate to semitectate, with columellate infratectum, reticulate to finely reticulate or psilate-foveolate.
Gynoecium Pistil composed of two connate carpels. Ovary superior, bilocular? Style single, simple. Stigma capitate, bilobate or trilobate, papillate?, Wet type? Pistillodium absent.
Ovules Placentation axile; placentae sometimes widened. Ovules ? per carpel, anatropous?, unitegmic, tenuinucellar. Integument ? cell layers thick. Megagametophyte monosporous, Polygonum type? Endosperm development cellular? Endosperm haustoria? Embryogenesis?
Fruit A baccate fruit with lignified pericarp and persistent calyx.
Seeds Aril absent. Exotestal cells with scalariform thickenings on inner periclinal walls or mucilaginous without outer periclinal walls. Endotesta? Perisperm not developed. Endosperm usually present. Embryo stout, straight, well differentiated, chlorophyll? Cotyledons two, lobate. Germination phanerocotylar.
Cytology n = 20
DNA
Phytochemistry Unknown.
Use Timber?
Systematics Gibsoniothamnus (12; southern Mexico, Central America, northern Colombia), Schlegelia (20–25; southern Mexico, Central America, the West Indies, tropical South America), Synapsis (1; S. ilicifolia; eastern Cuba), Exarata (1; E. chocoensis; Colombia, Ecuador).
Schlegeliaceae are sister-group to Martyniaceae.
SCROPHULARIACEAE Juss. |
( Back to Plantaginales ) |
Myoporaceae R. Br., Prodr. Fl. Nov.-Holl.: 514. 27 Mar 1810 [‘Myoporinae’], nom. cons.; Caprariaceae Martinov, Tekhno-Bot. Slovar: 102. 3 Aug 1820 [‘Caprariae’]; Myoporales R. Br. ex Bercht. et J. Presl, Přir. Rostlin: 245. Jan-Apr 1820 [‘Myoporinae’]; Verbascaceae Bercht. et J. Presl, Přir. Rostlin: 243. Jan-Apr 1820 [’Verbaceae’]; Selaginaceae Choisy [Mém. Sélag. 19] in Mém. Soc. Phys. Genève 2: 89. 1823 [‘Selagineae’], nom. cons.; Scrophulariineae Link, Handbuch 1: 531. 4-11 Jul 1829 [’Scrofularinae’]; Scrophulariales Lindl., Nix. Plant.: 20. 17 Sep 1833 [’Scrophulales’]; Bontiaceae Horan., Prim. Lin. Syst. Nat.: 77. 2 Nov 1834 [’Bontiaceae (Myoporin.)’]; Hebenstretiaceae Horan., Prim. Lin. Syst. Nat.: 76. 2 Nov 1834 [‘Hebenstreitiaceae (Selagineae)’]; Selaginales Choisy in C. F. P. von Martius, Consp. Regn. Veg.: 19. Sep-Oct 1835 [‘Selagineae’]; Selaginopsida Brongn., Enum. Plant. Mus. Paris: xix, 64. 12 Aug 1843 [’Selaginoideae’]; Verbascineae J. Presl in Nowočeská Bibl. [‘Wšobecný Rostl.] 7: 1136, 1137. 1846 [‘Verbasceae‘]; Limosellaceae J. Agardh, Theoria Syst. Plant.: 340. Apr-Sep 1858 [‘Limoselleae’]; Spielmanniaceae J. Agardh, Theoria Syst. Plant.: 194. Apr-Sep 1858 [’Spielmannieae’], nom. illeg.; Verbascales Döll, Fl. Baden 2: 750. med. 1858 [’Verbasceae’]; Buddlejaceae K. Wilh., Samenpflanzen: 90. Oct 1910 [‘Buddleiaceae’], nom. cons.; Oftiaceae Takht. et Reveal in Phytologia 74: 284. 28 Apr 1993
Genera/species 53/1.830–1.850
Distribution Mainly subtropical and warm-temperate regions on the Northern and Southern Hemispheres, with their largest diversity in South Africa and Australia; some representatives in tropical East Africa, Madagascar, tropical Asia, New Guinea, tropical Australia and tropical America.
Fossils Unknown.
Habit Usually bisexual (rarely functionally dioecious), perennial or annual herbs, evergreen or deciduous shrubs (sometimes suffrutices or biennial herbs, rarely trees or lianas). Many species are xerophytes.
Vegetative anatomy Phellogen ab initio superficial, or in inner cortex or pericycle. Primary medullary rays narrow or alternately narrow and wide. Endodermis sometimes prominent (Scrophularia). Vessel elements with simple perforation plates; lateral pits alternate, simple and/or bordered pits. Vestured pits present. Imperforate tracheary xylem elements tracheids, fibre tracheids or libriform fibres usually with simple (rarely bordered) pits, septate or non-septate (also vasicentric tracheids). Wood rays uniseriate or multiseriate, heterocellular, or absent. Axial parenchyma paratracheal scanty vasicentric, confluent, or banded, or absent. Wood elements sometimes storied (at least in Myoporeae). Intraxylary phloem present in young stems of Oftia (and Teedia?). Sieve tube plastids S type. Nodes 1:1 or 1:3, unilacunar with one or three leaf traces, often girdling bundle. Primary cortex and medulla in Myoporeae usually with secretory cavities containing oils and resins. Prismatic and acicular calciumoxalate crystals, styloids and crystal sand present in some species.
Trichomes Hairs simple, unicellular or multicellular, or branched, sometimes dendritic, stellate, lepidote, peltate or with globular terminal cell (sometimes calcified/silicified); glandular hairs often present.
Leaves Alternate (spiral), opposite or verticillate, simple, entire or lobed, sometimes coriaceous, sometimes ericoid, often with flat ptyxis. Stipules usually absent (in Buddleja sometimes with interpetiolar stipule-like lobes at base, rarely foliaceous); leaf sheath absent. Petioles sometimes fused in pairs at base. Petiole vascular bundles?; petiole often with wing bundles. Venation pinnate or palmate. Stomata usually anomocytic (sometimes anisocytic or paracytic). Cuticular wax crystalloids? Secretory cavities containing oil or resin present in Myoporeae; lamina often with translucent or raised dots when secretory cavities large. Idioblasts (tanniniferous etc.) present in, e.g., Scrophularia and Verbascum. Leaf margin serrate, crenate or entire. Extrafloral nectaries often present.
Inflorescence Terminal or axillary, usually cymose (sometimes racemose), often panicle, thyrsoid, raceme-, spike- or headlike (flowers rarely solitary axillary).
Flowers Usually zygomorphic (sometimes actinomorphic). Hypogyny. Sepals (two to) five, often with open or imbricate aestivation, often unequal in size, often persistent, usually connate (in, e.g., Myoporeae often free); median sepal adaxial. Petals (four or) five, usually with imbricate (sometimes valvate) aestivation, connate into usually hypocrateriform, infundibuliform or tubular (sometimes campanulate or bilabiate) corolla (rarely with one or two spur-like appendages). Nectary small or absent. Disc intrastaminal. Many species with floral hairs secreting oils attracting pollinators.
Androecium Stamens usually two longer and two shorter (sometimes two or four stamens equal in length [e.g. Buddleja] or five stamens [e.g. Capraria and most species of Verbascum]), haplostemonous, antesepalous, alternipetalous. Filaments free from each other, usually adnate to corolla tube (epipetalous). Anthers with thecae often confluent at apex, head-to-head, U-shaped or parallel, dorsifixed, sometimes versatile, usually tetrasporangiate (rarely disporangiate), introrse, usually longicidal (dehiscing by longitudinal slits, in Myoporeae sometimes by transverse slits). Tapetum secretory. Staminodia one (adaxial-median), two (adaxial-lateral) or three (one adaxial-median and two adaxial-lateral) or absent; female flowers with staminodia.
Pollen grains Microsporogenesis simultaneous. Pollen grains (2–)3(–4)-colpate or (2–)3(–5)-colporate (colpi sometimes with two ora), shed as monads, bicellular at dispersal. Exine tectate, with columellate infratectum, smooth (Buddleja).
Gynoecium Pistil composed of usually two connate carpels (rarely one carpel). Ovary superior, usually bilocular (rarely unilocular, pseudomonomerous, or quadrilocular to decemlocular due to in-growing secondary septa). Style single, simple, sometimes persistent. Stigma usually capitate (sometimes bifid, rarely lingulate), papillate, Dry or Wet type. Male flowers with pistillodium.
Ovules Placentation usually axile (sometimes, e.g. in many Limoselleae apical; in Limosella free central in upper part). Ovules usually numerous (in many Limoselleae one; in Oftia and Tetraselago four; in Myoporeae sometimes one or two) per carpel, anatropous to hemianatropous, pendulous or ascending, apotropous, epitropous or pleurotropous, unitegmic, tenuinucellar. Integument five to twelve cell layers thick. Hypostase present in Buddleja. Megagametophyte monosporous, Polygonum type. Antipodal cells sometimes persistent. Endosperm development cellular. Endosperm haustoria chalazal and micropylar. Some endothelial cells, bothroblasts, protruding into endosperm (surface then becoming alveolated). Embryogenesis onagrad (Buddlejeae, Myoporeae) or solanad?
Fruit A usually septicidal and septifragal (sometimes also apically loculicidal) capsule (rarely a berry, drupe [Oftia, some Myoporeae and Buddleja], nutlet or schizocarp with one or two drupaceous mericarps [some Myoporeae and Limoselleae]). Placentae often with cushion-shaped scars of detached seeds.
Seeds Aril absent. Seed pedestals often present. Testa sometimes winged, sometimes multiplicative. Exotestal and endotestal cells usually with thickened inner walls (exotestal cells sometimes longitudinally prolonged, in Buddleja with thickened inner walls). Perisperm not developed. Endosperm copious, oily, sparse or absent (sometimes ruminate due to invaginations [unequal radial elongation] of endotestal cells). Embryo usually straight (sometimes curved), well differentiated, usually without chlorophyll. Cotyledons two. Germination phanerocotylar. Radicula in Limosella ephemeral.
Cytology n = 6–10, 12–20, 23–27 (n = 15, 18, 27, 34 in Myoporeae; n = 19 in Oftia) – Polyploidy occurring. Protein bodies in cell nuclei in Verbascum usually amorphous, in Teedieae and Scrophularieae usually lamellar, in Limoselleae and Hemimerideae lamellar, or absent.
DNA Deletion in plastid gene matK. Mitochondrial coxI intron present in Scrophularia and in Celsia clade of Verbascum.
Phytochemistry Flavonols (kaempferol), 6- and/or 8-hydroxylated flavone glycosides, 6- or 8-hydroxyflavones or 6-methoxyflavones, Group I carbocyclic iridoids (aucubin, catalpol, 6-O-rhamnopyranosylcatalpol), Group II carbocyclic iridoids (harpagide, harpagioside [8β-8α-methyl substituted iridoids]), Group X secoiridoids (nepeta lactones), sesquiterpenes, alkaloids, saponins, phenylalanine-derived cyanogenic compounds, shikimic acid derived arthroquinones, and caffeic acid esters of phenylethanoid glucosides (caffeoyl phenylethanoid glucosides: verbascosides, cornosides), and acetylenes (in Myoporeae) present. Ellagic acid, tannins and proanthocyanidins not found. Carbohydrates stored as stachyose and other oligosaccharides (not as mannitol).
Use Ornamental plants, medicinal plants, timber (Myoporum).
Systematics (under construction) Scrophulariaceae are sister-group to the remaining Plantaginales “above” Plantaginaceae.
The clade [Buddleja+Teedieae+Camptoloma+Phygelius] may be sister to [Scrophularieae+Limoselleae].
Colpias
1/1. Colpias (1; C. mollis; Northern Cape). – Small shrub. Leaves alternate (spiral). Leaf margin serrate. Flowers zygomorphic. Calyx quinquelobate. Corolla quinquelobate (2:3), with tube somewhat curved. Corolla at base with two pouches beset with oil-secreting glandular hairs. Fruit a septicidal capsule. n = 10. – Colpias may be sister to the remaining Scrophulariaceae.
[Hemimerideae+Myoporoideae+[[Buddleja+Teedieae+Camptoloma+Phygelius]+[Scrophularieae+Limoselleae]]]
Hemimerideae Benth. in Edwards’s Bot. Reg. 21: ad t. 1770. 1 Feb 1835
5/160–165. Diascia (c 70; southern Africa), Nemesia (60–65; tropical and southern Africa, especially Western Cape), Alonsoa (c 12; Western and Eastern Cape, Mexico to Chile), Hemimeris (7; H. centrodes, H. gracilis, H. montana, H. nana, H. pachyceras, H. racemosa, H. sabulosa; Northern, Western and Eastern Cape), Diclis (10; tropical and southern Africa, Madagascar). – Tropical and southern Africa, Madagascar, Mexico to Chile. Flowers in Hemimeris inverted. Corolla spurred (in Diascia with two spurs). Flowers with oil-secreting hairs. Protein bodies in cell nuclei lamellar.
Myoporoideae Arn., Botany: 123. 9 Mar 1832 [‘Myoporinae’]
10/295–300. Lamina with pellucid gland dots. Flowers usually zygomorphic (in Eremophila tubular).
[Aptosimeae+[Leucophylleae+Myoporeae]]
Aptosimeae Benth. et Hook. f., Gen. Plant. 2: 915, 916. 1-16 Mai 1876
3/41. Anticharis (14; Africa, the Arabian Peninsula to Malesia), Aptosimum (c 20; tropical and southern Africa), Peliostomum (7; P. calycinum, P. leucorrhizum, P. lugardiae, P. oppositifolium, P. origanoides, P. virgatum, P. viscosum; tropical and southern Africa). – Africa, the Arabian Peninsula to Malesia.Aptosimeae are sister to the [Leucophylleae+Myoporeae] clade.
[Leucophylleae+Myoporeae]
Pollen grains tricolpate. Colpi with two ora.
Leucophylleae Miers in Ann. Mag. Nat. Hist., ser. 2, 5: 252. Apr 1850
3/15. Eremogeton (1; E. grandiflorus; southern Mexico, Guatemala), Leucophyllum (12; southwestern United States, Mexico), Capraria (2; C. biflora, C. mexicana; Mexico, Central America). – Southwestern United States to South America. Leaves in Capraria with abundant pellucid glands, in Leucophyllum with one pellucid gland at apex, and in Eremogeton without pellucid glands. Stamens in Capraria five. – Leucophylleae are sister to Myoporeae.
Myoporeae Rchb., Handb. Nat. Pfl.-Syst.: 196. 1-7 Oct 1837 [‘Myoporinae’]
4/c 240. Androya (1; A. decaryi; southern Madagascar); Eremophila (210–215; Australia, one species, E. debilis, also in New Zealand, with their highest diversity in southwestern Western Australia), Bontia (1; B. daphnoides; the West Indies, tropical South America), Myoporum (c 30; Mauritius, Southeast Asia to China, East Malesia to New Guinea, Australia, New Zealand, Kermadec Islands, Polynesia including the Hawaiian Islands). – Mauritius, East and Southeast Asia, East Malesia to New Guinea, Australia, New Zealand to the Hawaiian Islands, the West Indies, tropical South America. Usually shrubs (sometimes herbs). Wood elements often storied. Primary cortex and medulla usually with secretory cavities containing oils and resins. Secretory oil- or resin-containing cavities present. Sepals often free. Anthers sometimes dehiscing by transverse slits. Ovules few (sometimes one or two) per carpel, epitropous. Fruit a drupe or a schizocarp. Endosperm sparse. n = 15, 18, 27, 34. Acetylenes present. – Myoporeae are sister to Leucophylleae. Androya is probably sister to the remaining Myoporeae.
[Teedieae+Buddleja+Camptoloma+Phygelius+[Limoselleae+Scrophularieae]]
Teedieae Benth. in Edwards’s Bot. Reg. 21: ad t. 1770. 1 Feb 1835
6/18–19. Ranopisoa (1; R. rakotosonii; Madagascar), Dermatobotrys (1; D. saundersii; Eastern Cape, KwaZulu-Natal), Freylinia (9; tropical and southern Africa), Teedia (2; T. lucida, T. pubescens; southern Africa), Oftia (3; O. africana, O. glabra, O. revoluta; Northern, Western and Eastern? Cape), Ameroglossum (1; A. pernambucense; Brazil)? – Tropical and southern Africa, Madagascar, Brazil? Young stems and branches in Oftia with intraxylary phloem. Inflorescence in Oftia raceme. Ovules in Oftia four per carpel. Fruit in Oftia a drupe. Endosperm in Oftia copious. n = 19 (Oftia). Protein bodies in cell nuclei usually lamellar. – Teedieae may be sister to Buddleja. The position of the Brazilian Ameroglossum is highly uncertain.
Buddlejeae Bartl., Ord. Nat. Plant.: 172. Sep 1830 [‘Buddlejea’]
1/c 140. Buddleja (c 140; warm-temperate to tropical regions in Africa, Madagascar, Asia and America, with their highest diversity in East Asia). – Shrubs or small treews. Interpetiolar stipule-like lobes (rarely foliaceous) sometimes present at base. Flowers often actinomorphic. Corolla sometimes quadrilobate. Stamens sometimes four. Fruit sometimes a drupe.
1/3. Camptoloma (3; C. canariense: Gran Canaria; C. lyperiiflorum: Somalia, southern Yemen, Socotra, Kuria Muria Islands; C. rotundifolium: western deserts in Angola, northern Namibia). – Small shrubs. Leaves opposite or spiral, glandular. Leaf margin serrate. Flowers zygomorphic. Calyx quinquelobate. Corolla quinquelobate, with tube curved. Stamens four. Ovules numerous per carpel. Fruit a bivalvular capsule.
1/2. Phygelius (2; P. aequalis, P. capensis; southern Africa). – Perennial herbs. Leaves opposite. Flowers zygomorphic. Corolla quinquelobular, narrowly tubular. Fruit a capsule.
Scrophularioideae Beilschm. in Flora 16(Beibl. 7): 67. 14 Jun 1833 [‘Scrofularomae’]
Limoselleae Dumort., Fl. Belg.: 32. 1827
25/640–650. Jamesbrittenia (c 85; Angola, Zambia to South Africa, one species also in Egypt, Sudan and southwestern Asia to India); Manuleopsis (1; M. dinteri; Namibia), Barthlottia (1; B. madagascariensis; Madagascar), Limosella (c 15; cosmopolitan), Manulea (74; southern Africa, India, with their highest diversity in Western Cape), Sutera (3; S. cooperi, S. griquensis, S. foetida; southern Africa from the Orange Free State and Transvaal to the Cape Provinces), Chaenostoma (46; Africa south of the Cunene and Zambezi rivers, with their highest diversity in the Cape Provinces, Natal and Transvaal), Lyperia (6; L. antirrhinoides, L. formosa, L. lychnidea, L. tenuiflora, L. tristis, L. violacea; Namibia to Western Cape), Glekia (1; G. krebsiana; Eastern Cape, Lesotho), Trieenea (10; Western and Eastern Cape), Hebenstretia (c 40; tropical and southern Africa north to Eritrea), Dischisma (11; Namibia, Western and Eastern Cape), Chenopodiopsis (3; C. chenopodioides, C. hirta, C. retrorsa; Northern and Western Cape), Pseudoselago (29; Northern, Western and Eastern Cape), Zaluzianskya (c 60; southern Africa, Mount Elgon at the Kenya/Uganda border), 'Phyllopodium' (c 23; Namibia, Northern, Western and Eastern Cape, KwaZulu-Natal; polyphyletic; incl. Polycarena?), Polycarena (17; Northern and Western Cape; in Phyllopodium?), Glumicalyx (6; G. apiculatus, G. flanaganii, G. goseloides, G. lesuticus, G. montanus, G. nutans; Drakensberg in Lesotho, Eastern Cape, KwaZulu-Natal and Free State), Strobilopsis (1; S. wrightii; Drakensberg in KwaZulu-Natal and Lesotho), Tetraselago (4; T. longituba, T. natalensis, T. nelsonii, T. wilmsii; KwaZulu-Natal, Northern Province, Mpumalanga, Swaziland), Melanospermum (1; M. transvaalense; northern South Africa to western Zimbabwe), Selago (c 190; tropical and southern Africa, Madagascar, with their highest diversity in Western and Eastern Cape), Cromidon (12; Namibia, Northern and Western Cape, Free State), Microdon (6; M. capitatus, M. dubius, M. nitidus, M. orbicularis, M. parviflorus, M. polygaloides; Northern and Western Cape), Globulariopsis (7; G. adpressa, G. montana, G. obtusiloba, G. pumila, G. stricta, G. tephrodes, G. wittebergensis; Western Cape), Gosela (1; G. eckloniana; Western Cape). – Cosmopolitan, with their highest diversity in the Cape Provinces in South Africa. Placentation often apical (in Limosella free central in apical part). Ovule often one per carpel. Radicula in Limosella ephemeral. Protein bodies in cell nuclei lamellar. – Limoselleae may be sister-group to Scrophularieae. Jamesbrittenia may be sister to the remaining Limoselleae.
Scrophularieae Dumort., Fl. Belg.: 35. 1827
6/c 570. Antherothamnus (1; A. pearsonii; southwestern Zimbabwe, southern Namibia, Botswana, northern South Africa); Verbascum (c 360; Europe, the Mediterranean, western Asia, mountains in Ethiopia and tropical East Africa), Rhabdotosperma (2; R. brevipedicellatum, R. scrophulariifolia; Cameroon, Congo, tropical East Africa, Ethiopia, the Arabian Peninsula), Nathaliella (1; N. alaica; Kyrgyzstan, western China), Scrophularia (c 200; Europe, Macaronesia, the Mediterranean, temperate Asia, the Himalayas and Tibet, North and tropical America). – Leaves in at least Scrophularia and Verbascum with (tanniniferous etc.) idioblasts. Stamens in Verbascum usually five. Protein bodies in cell nuclei usually lamellar (in Verbascum usually amorphous). – Scrophularieae are possibly sister-group to Limoselleae.
 |
Phylogeny (simplified) of Scrophulariaceae from Bayesian and Jacknife analyses based on DNA sequence data (Kornhall 2004; Kornhall & Bremer 2004). |
Genus incertae sedis
Peltanthera 1/1. Peltanthera (1; P. floribunda; Guatemala, Panamá, Ecuador, Peru, Bolivia). – Bisexual evergreen small tree or shrub. Phellogen? Vessel elements? Imperforate tracheary xylem elements? Wood rays? Axial parenchyma? Sieve tube plastids S type? Nodes 3:3, trilacunar with three leaf traces. Crystals? Hairs branched moniliform. Leaves opposite, simple, with involute ptyxis. Petiole vascular bundle transection flattened annular; petiole with mid-vein bundles (and sometimes medullary bundles). Stipules and leaf sheath absent. Venation pinnate. Stomata? Cuticular wax crystalloids? Leaf margin serrate. Inflorescence axillary, thyrsoid. Flowers somewhat zygomorphic. Hypogyny. Sepals five, with valvate aestivation, connate at base. Petals five, with valvate aestivation, connate. Nectariferous disc intrastaminal. Stamens five, haplostemonous, antesepalous, alternipetalous. Filaments free, adnate to corolla tube (epipetalous). Anthers basifixed, versatile?, tetrasporangiate, introrse?, longicidal, with confluent thecae. Tapetum? Staminodia absent. Pistil composed of two connate carpels. Ovary superior, bilocular. Style single, entire. Stigma bifid, type? Pistillodium absent. Placentation axile. Ovules numerous per carpel, anatropous, unitegmic, tenuinucellar. Integument ? cell layers thick. Megagametophyte monosporous, Polygonum type? Endosperm development? Endosperm haustoria? Embryogenesis? Fruit a loculicidal capsule. Aril absent. Exotestal cells? Endotestal cells? Perisperm not developed. Endosperm? Embryo? Cotyledons? Germination? n = ? DNA? Verbascosides and cornoside derivatives present. – Peltanthera is possibly allied to Buddleja, although this has to be investigated.
STILBACEAE Kunth |
( Back to Plantaginales ) |
Retziaceae (Bartl.) Choisy in Mém. Soc. Phys. Hist. Nat. Genève 6: 400. 11 Aug 1834; Stilbales Kunth in C. F. P. von Martius, Consp. Regn. Veg.: 19. Sep-Oct 1835 [’Stilbinae’]
Genera/species 12/c 38
Distribution Tropical and southern Africa, Madagascar, islands in the Indian Ocean, southwestern Arabian Peninsula, with their largest diversity in Western Cape in South Africa.
Fossils Unknown.
Habit Bisexual, evergreen trees or shrubs, or perennial herbs. Some species with lignotuber. Most species are xerophytes.
Vegetative anatomy Phellogen ab initio present immediately outside pericycle. Vessel elements usually with simple (sometimes scalariform or reticulate) perforation plates; lateral pits alternate, bordered pits. Vestured pits present. Imperforate tracheary xylem elements usually libriform fibres (sometimes fibre tracheids) with simple pits, usually septate (also vasicentric tracheids). Wood rays uniseriate or multiseriate, heterocellular. Axial parenchyma absent or very rare (paratracheal scanty vasicentric). Wood elements sometimes storied (Retzia). Sieve tube plastids S type? Nodes 1?:1?, unilacunar? with one? leaf trace. Silica absent. Calciumoxalate usually absent (rarely as acicular crystals, styloids or crystal sand).
Trichomes Hairs simple, sometimes capitate; glandular hairs sometimes present.
Leaves Usually verticillate (sometimes opposite, rarely alternate), simple, entire, coriaceous, often ericoid, often with revolute ptyxis. Stipules and leaf sheath absent. Petiole vascular bundles? Venation pinnate or leaves one-veined. Stomata anomocytic. Cuticular wax crystalloids as rodlets or threads. Leaf margin usually entire (rarely slightly serrate).
Inflorescence Usually terminal spike, head or axillary panicle, thyrsoid, spike- or head-like (flowers sometimes solitary axillary). Floral prophylls (bracteoles) as long as sepals.
Flowers Usually zygomorphic (sometimes actinomorphic). Hypogyny. Sepals four or five (to seven), usually with valvate (sometimes imbricate) aestivation, persistent or caducous, usually connate into tubular, campanulate or bilabiate calyx (petals sometimes free); median sepal adaxial. Petals four or five (to seven), with imbricate and/or valvate (sometimes induplicate-valvate) aestivation, equal or unequal in size, connate into tubular or infundibuliform corolla. Nectariferous disc small, intrastaminal, or absent. Oil-secreting glandular hairs often present.
Androecium Stamens four or five (to seven), as many as or one (adaxial median) fewer than sepals (adaxial median stamen rarely reduced and staminodial), haplostemonous, antesepalous, alternipetalous. Filaments filiform, free from each other, adnate to corolla tube (epipetalous). Anthers with thecae often confluent at apex or parallel (with seprate slits), dorsifixed, versatile?, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory? Staminodium one or absent.
Pollen grains Microsporogenesis simultaneous? Pollen grains tricolporate, shed as monads, ?-cellular at dispersal. Exine tectate, with columellate infratectum, perforate, with various sculpturing.
Gynoecium Pistil composed of two connate carpels. Ovary superior, bilocular, with one locule often sterile, or unilocular (septum absent) or bilocular in lower part (septum incomplete) and unilocular in apical part. Style single, filiform, simple or somewhat bifid at apex, sometimes persistent. Stigma capitate, punctate or slightly bilobate, type? Pistillodium absent.
Ovules Placentation usually axile or basal (in Nuxia axile-peltate; in Thesmophora apical, with two transversely arranged collateral carpels each containing one descending ovule, abaxial carpel with secondary septum?). Ovules one or two ascending-apotropous and/or pendulous per carpel (when ovary unilocular) or numerous per carpel (when ovary bilocular), anatropous, apotropous or epitropous, unitegmic, tenuinucellar. Integument several cell layers thick. Hypostase well developed at base of megagametophyte. Megagametophyte monosporous, Polygonum type. Two antipodal cells developing (at least in Retzia). Endosperm development probably cellular. Endosperm haustoria chalazal and strongly micropylar (Retzia). Embryogenesis?
Fruit A usually loculicidal (sometimes also septicidal) capsule, with calyx and corolla persistent (sometimes a nutlet). Placenta sometimes with cushion-shaped scars of detached seeds.
Seeds Aril? Seed pedestals present. Exotesta? Endotesta? Perisperm not developed. Endosperm copious, usually oily (sometimes starchy). Embryo straight, well differentiated, chlorophyll? Cotyledons two. Germination?
Cytology n = 10, 12, 19 – Protein bodies in cell nuclei crystalline (Halleria) or absent.
DNA
Phytochemistry Insufficiently known. Route II iridoids (decarboxylated and C8-iridoids), Group IV carbocyclic iridoids, C8-iridoid glycosides (e.g. unedoside, stilbericoside, capensioside, holmioside, s-deoxyholmioside, and thunbergioside; extremely rare in other angiosperms), and verbascoside (a C10-iridoid glycoside) present.
Use Ornamental plants.
Systematics Charadrophila (1; C. capensis; around Stellenbosch and on Hangklip Peninsula in Western Cape), Halleria (4; H. elliptica, H. ligustrifolia, H. lucida, H. ovata; Ethiopia to Angola and South Africa, Madagascar, Yemen); Ixianthes (1; I. retzioides; Western Cape), Anastrabe (1; A. integerrima; Mozambique, KwaZulu-Natal, Eastern Cape), Bowkeria (3; B. citrina, B. cymosa, B. verticillata; South Africa, Swaziland, Lesotho); Nuxia (c 15; tropical Africa, Madagascar, the Comoros, the Mascarene Islands, southwestern Arabian Peninsula), Euthystachys (1; E. abbreviata; central Western Cape), ‘Stilbe’ (7; S. albiflora, S. ericoides, S. gymnopharyngia, S. overbergensis, S. rupestris, S. serrulata, S. vestita; Western Cape; non-monophyletic; incl. Campylostachys?), Campylostachys (1; C. cernua; Western Cape; in Stilbe?), Retzia (1; R. capensis; mountains in Western Cape), Kogelbergia (2; K. phylicoides, K. verticillata; southwestern Western Cape), Thesmophora (1; T. scopulosa; Ceres Mountains in Western Cape).
Stilbaceae are sister-group to a clade comprising the two large subclades [Lamiaceae+[Mazaceae+[Phrymaceae+[Paulowniaceae+[Rehmanniaceae+Orobanchaceae]]]]] and [Thomandersia to Lentibulariaceae].
Most asterids have C10- or C11-iridoid glucosides, wheras C8-iridoid glucosides (C8-iridoids) are found in Nuxia, Retzia and Stilbe (Stilbaceae), Thunbergia (Acanthaceae), and Arbutus/Arctostaphylos (Ericaceae). Unedoside is present in some genera of Stilbaceae, as well as in Loasaceae and Hydrangeaceae (Loasales).
 |
Cladogram of Stilbaceae based on DNA sequence data (Kornhall 2004). |
TETRACHONDRACEAE Wettst. |
( Back to Plantaginales ) |
Polypremaceae Reveal in Kew Bull. 66: 47. Mar 2011
Genera/species 2/3
Distribution New Zealand, southeastern United States to northern South America, the West Indies, Paraguay?, southern Patagonia, Tierra del Fuego,
Fossils Unknown.
Habit Bisexual, perennial or annual herbs, prostrate to erect. Aquatic or helophytic. Succulent (Tetrachondra). Young stems in Polypremum quadrangular in cross-section.
Vegetative anatomy Phellogen ab initio superficial (Polypremum). Lateral vascular bundles split at nodes. Vessel elements with simple? perforation plattor; lateral pits? Imperforate tracheary xylem elements fibre tracheids (Polypremum) with simple? pits. Wood rays absent? Axial parenchyma? Sieve tube plastids S type? Nodes ?:?, split laterals. Etheral oils present in Tetrachondra. Crystals?
Trichomes Hairs unicellular or multicellular, in Polypremum moniliform; glandular hairs absent.
Leaves Opposite, simple, entire, coriaceous (Tetrachondra), with ? ptyxis. Stipules and leaf sheath absent. Petiole bases fused or connected by membranous interstipular sheathing structure. Petiole vascular bundles? Venation pinnate. Stomata anomocytic? Cuticular wax crystalloids? Lamina covered by sunken multicellular hairs consisting of basal cell, stalk cell and terminal head of at least four cells; lamina in Tetrachondra with numerous aromatic glands. Leaf margin serrate? or entire.
Inflorescence Terminal or axillary, few-flowered cymose (Polypremum), or flowers paired or solitary, axillary (Tetrachondra). Two or more pairs of floral prophylls (bracteoles) present.
Flowers Actinomorphic, small. Hypogyny (Tetrachondra) or partial epigyny (Polypremum). Sepals usually four (in Polypremum sometimes five), with valvate aestivation, persistent, connate in lower part into campanulate calyx; median sepal abaxial (Tetrachondra); sepals (Polypremum) diagonally arranged/initiated. Petals usually four (in Polypremum sometimes five), with imbricate aestivation, connate in lower part, orthogonally arranged/initiated (Polypremum). Nectary absent. Disc absent (at least in Polypremum).
Androecium Stamens usually four (in Polypremum sometimes five), haplostemonous, antesepalous, alternipetalous. Filaments free, adnate to corolla tube (epipetalous). Anthers dorsifixed, versatile?, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits). Placentoid? Tapetum secretory? Staminodia absent.
Pollen grains Microsporogenesis simultaneous? Pollen grains tricolporate (6-sulcate?), shed as tetrads, bicellular? at dispersal. Exine tectate, with columellate infratectum, microperforate, psilate.
Gynoecium Pistil composed of two connate carpels (in Tetrachondra fused only in lower parts), transversely orientated. Ovary superior or semi-inferior, bilocular (Polypremum), or secondarily quadrilocular by false septa due to ingrowth of ovary wall (Tetrachondra). Style single, simple, gynobasic (Tetrachondra) or absent to minute (Polypremum). Stigma capitate or sometimes slightly bilobate (Polypremum), or small and somewhat truncate (Tetrachondra), type? Pistillodium absent.
Ovules Placentation basal (Tetrachondra), or basal to axile with ovules on peltate placentae inserted at base of septum (Polypremum). Ovules two ascending (Tetrachondra) or numerous (Polypremum) per carpel, anatropous, unitegmic, tenuinucellar. Integument three or four cell layers thick (Polypremum). Endothelium present. Megagametophyte monosporous, Polygonum type. Micropylar end of megagametophyte penetrating megasporangial epidermis to ovule surface; micropylar end not surrounded by integument (Polypremum). Endosperm development cellular (Polypremum). Endosperm haustorium micropylar or chalazal (Polypremum). Embryogenesis onagrad (Polypremum).
Fruit A schizocarp divided by secondary septum and with four single-seeded nutlike mericarps (Tetrachondra), or a loculicidal (and sometimes septicidal) many-seeded capsule (Polypremum), with green persistent calyx.
Seeds Aril? Seed pedestals present. Testa thin. Endothelial cells with persistent thickened inner walls. Perisperm not developed. Endosperm copious, oily. Embryo straight (Polypremum), well differentiated, chlorophyll? Cotyledons two. Germination?
Cytology n = 10, 11 (Polypremum) – Protein bodies in nucleus?
DNA
Phytochemistry Insufficiently known. Caffeic acid esters (cornosides, verbascosides), scutellarin, essential oils, and sorbitol present. Iridoids not found. Shikimic acid derived anthraquinones?
Use Unknown.
Systematics Tetrachondra (2; T. hamiltonii: southern New Zealand; T. patagonica: southern Patagonia, Tierra del Fuego), Polypremum (1; P. procumbens; southeastern United States, Mexico, Central America, the West Indies, northern South America, Paraguay?).
Tetrachondraceae are sister to the remaining Plantaginales except Plocospermataceae and the [Oleaceae+Carlemanniaceae] clade.
THOMANDERSIACEAE Sreem. |
( Back to Plantaginales ) |
Genera/species 1/6
Distribution Tropical West and Central Africa.
Fossils Unknown.
Habit Bisexual, evergreen shrubs or small trees (sometimes lianas). Stem and branches not articulated.
Vegetative anatomy Phellogen? Secondary phloem stratified into hard fibrous and soft parenchymatous layers. Vessel elements with simple? perforation plates; lateral pits? Vestured pits? Imperforate tracheary xylem elements ? Wood rays? Axial parenchyma paratracheal? Pericyclic fibres short?, massively thickened. Sieve tube plastids S type? Nodes 1:3, unilacunar with three leaf traces. Cystoliths and acicular crystals absent.
Trichomes Hairs unicellular? or absent.
Leaves Opposite, anisophyllous, simple, usually entire (sometimes lobed), with ? ptyxis. Stipules and leaf sheath absent. Petiole swollen at base and apex. Petiole vascular bundle transection annular or incurved C-shaped. Venation pinnate, camptodromous. Stomata anisocytic. Cuticular wax crystalloids? Domatia in vein axils or absent. Leaf margin entire or serrate. Lamina with large and flat abaxial glands, blackening when dry. Extrafloral nectaries present.
Inflorescence Terminal or axillary, raceme-like cymose.
Flowers Zygomorphic. Hypogyny. Sepals five, persistent, connate, with imbricate? aestivation, on outer side between two abaxial calyx lobes with extranuptial vascularized multicellular nectaries (each one up to 3 mm wide and surrounded by swelling) consisting of layers or secretory cells. Petals five, connate into a bilabiate corolla (upper lip bilobate, lower lip trilobate), with irregular aestivation. Nectaries intrastaminal, vascularized by carpellary traces. Disc absent.
Androecium Stamens two longer and two shorter, haplostemonous, antesepalous, alternipetalous. Filaments free from each other, adnate to corolla tube (epipetalous). Anthers dorsifixed, versatile?, tetrasporangiate, extrorse, longicidal (dehiscing by longitudinal slits). Tapetum secretory? Staminodium one, adaxial-median.
Pollen grains Microsporogenesis simultaneous? Pollen grains penta- or hexacolpate, shed as monads, ?-cellular at dispersal. Exine tectate, with columellate? infratectum, punctate.
Gynoecium Pistil composed of two connate carpels. Gynoecial vascular tissue 8-shaped in cross-section. Ovary superior, bilocular, with nectariferous tissue at base. Style single, simple. Stigma cylindrical or bilobate, non-papillate?, Dry type? Pistillodium absent.
Ovules Placentation axile, with expanded placentae. Ovules one to three per carpel, hemianatropous, unitegmic, tenuinucellar. Integument ? cell layers thick. Hypostase present at chalazal end. Megagametophyte monosporous, Polygonum type. Endosperm development cellular? Endosperm haustoria? Embryogenesis?
Fruit A woody loculicidal, non-explosive, capsule with persistent and accrescent calyx. Each seed situated on flattened elongate hook-shaped lignified ejaculator, retinaculum, expanded modified funicle.
Seeds Aril absent. Hilum large. Seed coat with ascending-imbricate scales or spirally arranged warts (with up to six cell layers in warts). Exotesta palisade, non-lignified. Endotesta? Perisperm not developed. Endosperm absent. Embryo large?, strongly curved, without chlorophyll? Cotyledons two, thin-foliaceous, complexly folded. Germination phanerocotylar?
Cytology n = ?
DNA
Phytochemistry Insufficiently known. Terpenoids (e.g. thomandertriole) and 2-indolinone alkaloids (e.g. thomandersine and isothomandersine) present. Iridoids?
Use Unknown.
Systematics Thomandersia (6; T. anachoreta, T. butayei, T. congolana, T. hensii, T. laurentii, T. laurifolia; southern Liberia and Côte d’Ivoire, southeastern Nigeria, southern Cameroon, Gabon, Equatorial Guinea, Congo Brazzaville, Congo Kinshasa, southern Central African Republic).
The sister-group relationship of Thomandersia is unsolved. The lineage is sometimes placed, with weak support, as sister to Schlegeliaceae in DNA analyses. Other alternative sister-groups of Thomandersia are Bignoniaceae or Verbenaceae. On the other hand, the pollen morphology in Thomandersia resembles the one in Pedaliaceae.
Thomandersia has a large complex calyx nectary, extended placentae, special corolla aestivation, and scaly spherical seeds without endosperm. Like the majority of Acanthaceae (Acanthoideae), Thomandersia has lignified retinacula (this may be a parallelism), but contrary to Acanthaceae they lack an explosion capsule. Retinacula are wide and flat in Thomandersia, whereas in Acanthaceae they are narrower and U-shaped in cross-section (Wortley & al.2005). In Thomandersia the retinaculum is compressed between the seed and the carpellary wall; in Acanthaceae the seeds are usually discoid and the retinaculum, which is present along one of the edges of the seed, is formed like a channel. Whereas the retinacula in Acanthaceae probably have an important role in connection with seed dispersal from the explosion capsule, the role of the retinacula in Thomandersia rather seems to be keeping the seed inside the capsule after the dehiscence (Wortley & al.2005).
VERBENACEAE J. St.-Hil. |
( Back to Plantaginales ) |
Lantanaceae Martinov, Tekhno-Bot. Slovar: 357. 3 Aug 1820 [’Lantanae’]; Verbenales Juss. ex Bercht. et J. Presl, Přir. Rostlin: 245. Jan-Apr 1820 [‘Verbenaceae’]; Durantaceae J. Agardh, Theoria Syst. Plant.: 295. Apr-Sep 1858; Petreaceae J. Agardh, Theoria Syst. Plant.: 364. Apr-Sep 1858 [’Petraeaceae’]; Verbenineae Engl., Syllabus, ed. 2: 177. Mai 1898.
Genera/species 33–35/1.120–1.190
Distribution Southeastern North America, Mexico, the West Indies, southern Brazil to Argentina, Atlantic islands, Europe, northern and northeastern Africa, Southwest and Central Asia, Taiwan, the Korean Peninsula, Japan.
Fossils Pollen grains are reported from Neogene strata.
Habit Usually bisexual (some species of Citharexylum dioecious), evergreen or deciduous trees, shrubs or lianas, perennial or annual herbs (Pitraea tuberous perennial). Stem and branches often quadrangular in cross-section. Often aromatic.
Vegetative anatomy Phellogen ab initio superficial. Vessel elements usually with simple (sometimes scalariform) perforation plates; lateral pits alternate, bordered pits. Vestured pits sometimes present. Imperforate tracheary xylem elements often very long libriform fibres with simple pits, septate or non-septate (also vasicentric tracheids). Wood rays uniseriate or multiseriate, usually heterocellular (sometimes homocellular). Axial parenchyma paratracheal scanty vasicentric, aliform, lozenge-aliform, winged-aliform, confluent, or banded, or absent. Sieve tube plastids S type. Nodes 1:≥1, unilacunar with one or several leaf traces. Heartwood with resins. Calciumoxalate sometimes as styloids and acicular, stellate or cubical crystals.
Trichomes Eglandular hairs unicellular or multicellular, uniseriate or branched, often dendritic, sometimes capitate, hook-tipped, furcate or stellate (sometimes calcified/silicified); glandular hairs capitate, peltate or lepidote, often secreting resin.
Leaves Usually opposite (rarely verticillate or alternate), simple or pinnately compound, entire or lobed (sometimes scale-like), with imbricate? ptyxis. Stipules and leaf sheath absent. Petiole bases fused by line across node. Petiole vascular bundle transection arcuate; petiole sometimes also with medullary bundles, associated with median bundle. Venation pinnate. Stomata diacytic or anomocytic. Cuticular wax crystalloids? Mesophyll with or without sclerenchymatous idioblasts (sometimes with groups of brachysclereids). Acicular (sometimes stellate or cubical) crystals abundant. Leaf margin serrate, crenate, lobate or entire. Extrafloral nectaries present in many species on petiole and abaxial side of lamina.
Inflorescence Terminal or axillary, raceme-, spike- or head-like, simple or compound cymose; sometimes pseudanthium with involucre consisting of often large and sometimes petaloid bracts.
Flowers More or less zygomorphic. Hypogyny. Sepals (four or) five, with usually imbricate quincuncial aestivation, campanulate, persistent, connate; median sepal adaxial. Petals (four or) five, with usually imbricate quincuncial aestivation, connate into an infundibuliform or hypocrateriform corolla (sometimes bilabiate). Nectariferous disc intrastaminal, annular.
Androecium Stamens usually two longer and two shorter (didynamous; in Hierobotana and Stachytarpheta two fertile stamens; adaxial stamen sometimes staminodial; fertile stamens in Verbena five), haplostemonous, antesepalous, alternipetalous. Filaments free from each other, usually adnate to corolla tube (epipetalous). Anthers basifixed or dorsifixed, versatile?, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits); connective sometimes shrinked or expanded, occasionally with glandular appendage. Tapetum secretory, with binucleate to quadrinucleate cells. Staminodia usually absent (sometimes one or two).
Pollen grains Microsporogenesis simultaneous. Pollen grains usually 3(–5)-colporate (in Petrea and Stachytarpheta tricolpate; in Citharexylum sometimes 3(–4)-porate), shed as monads, bicellular or tricellular? at dispersal. Exine tectate to semitectate, with columellate infratectum, perforate to reticulate, rugulate-reticulate, psilate, spinulate or echinate (rarely verrucate).
Gynoecium Pistil composed of one or two connate, collateral, transverse carpels (often gradually bipartite by in-growing secondary septa; one carpel often aborted resulting in unicarpellate, pseudomonomerous, ovary; carpels in Duranta four). Ovary superior, bilocular or secondarily quadrilocular (by inflexion of carpellary margins; in Duranta secondarily octalocular; in Bouchea pseudomonomerous, with one carpel modified into carpophore). Style single, simple or bilobate, often persistent. Stigma usually capitate (rarely somewhat quadrangular), with asymmetrically swollen stigmatoid tissue and glandular surface, papillate, Wet type. Pistillodium absent.
Ovules Placentation usually basal or axile (rarely parietal), attached directly to margins of secondary carpellary septa (cf. Lamiaceae). Ovules usually two (ovule rarely one) per carpel, usually anatropous (rarely hemianatropous or orthotropous), usually ascending (rarely pendulous), apotropous, unitegmic, tenuinucellar. Integument five to nine cell layers thick. Obturator present. Megagametophyte monosporous, Polygonum type. Antipodal cells persistent, sometimes multinucleate. Endosperm development ab initio cellular. Endosperm haustorium chalazal and/or micropylar. Embryogenesis onagrad.
Fruit A dry or fleshy schizocarp with two or four one-seeded mericarps or two two-seeded mericarps, or a drupe with one, two or four pyrenes (bilocular mericarps or pyrenes may contain ovules from both carpels) with persistent calyx. Fruits occasionally winged.
Seeds Aril absent. Testa thin-walled. Exotesta present. Endotesta? Perisperm not developed. Endosperm usually absent (present in Lantaneae). Embryo straight, oily, without chlorophyll. Cotyledons two, spatulate. Germination phanerocotylar or cryptocotylar?
Cytology n = 5–12, 17–20 (sometimes more) – Polyploidy occurring.
DNA Deletion the plastid gene matK.
Phytochemistry 6- or 8-hydroxyflavones or 6-methoxyflavones and 6-methoxyflavone glycosides, Route-I-iridoids (some species of Verbena), Route II decarboxylated iridoids (4-carboxyiridoids), Group II carbocyclic iridoids (lamiide), Group III carbocyclic iridoids (cornine, hastatoside), iridoid glycosides, ursolic acid, caffeic acid esters (caffeoyl phenylethanoid glucosides, e.g. verbascoside, orobanchin; cornoside present in Phyla), naphthoquinones, toxic triterpene esters, and triterpene saponins present. Ethereal oils present in Lantaneae. Flavonols, ellagic acid, proanthocyanidins, and cyanogenic compounds not found. Carbohydrates usually stored as stachyose and other oligosaccharides.
Use Ornamental plants, medicinal plants, timber, carpentries (e.g. Citharexylum).
Systematics
The sister-group relationships of Verbenaceae are not clarified.
A probable topology is the following (Thode & al. 2013): [Petreeae+[Duranteae+[[Casselieae+Citherexyleae]+[Priveae+[Rhaphithamnus+[Neospartoneae+[Verbeneae+Lantaneae]]]]]]].
Petreeae Briq. in Engler et Prantl, Nat. Pflanzenfam. IV, 3a: 144, 157. 26 Feb 1895 [‘Petraeeae’]
2/12. Petrea (11; Mexico, Central America), Xolocotzia (1; X. asperifolia; Mexico). – Mexico to Amazonian Brazil. Petrea consists of lianas, whereas Xolocotzia is a shrub or small tree. Fruit a drupe consisting of two pyrenes derived from secondarily unicarpellate ovary (one carpel aborted). Calyx large, showy, exceeding corollas. – Petreeae are sister to the remaining Verbenaceae. Petrea has been identified as sister to Bignoniaceae in some previous analyses.
[Duranteae+[[Casselieae+Citherexyleae]+[Priveae+[Rhaphithamnus+[Neospartoneae+[Verbeneae+Lantaneae]]]]]]
Duranteae Benth. in Ann. Mag. Nat. Hist., ser. 1, 2: 448. Feb 1839
5/195–205. Duranta (c 20; the West Indies, South America), Bouchea (18; Texas, Mexico, Central America, tropical South America, one species, B. pterygocarpa, in Ethiopia), Chascanum (27–32; Africa, Madagascar, the Arabian Peninsula to western India), Recordia (1; R. boliviana; Bolivia), Stachytarpheta (120–125; Florida, Mexico, Central America, the West Indies, tropical South America). – Africa, Madagascar, southwestern Asia to India, southeastern United States to Argentina. Trees, shrubs or herbs. Eglandular hairs multicellular. Inflorescence terminal spike or compound raceme with terminal and axillary flowering shoots. Two floral prophylls (bracteoles) present in Bouchea, Recordia, species of Duranta and species of Chascanum. Two fertile stamens present in Stachytarpheta. Carpels in Duranta four. Ovary in Duranta secondarily octalocular, in Bouchea pseudomonomerous, with one carpel modified into carpophore. Calyx usually persistent and enclosing fruit (not in Bouchea). – Duranteae are sister to the remaining Verbenaceae except Petreeae.
[[Casselieae+Citherexyleae]+[Priveae+[Rhaphithamnus+[Neospartoneae+[Verbeneae+Lantaneae]]]]]
[Casselieae+Citherexyleae]
Casselieae (Schauer) Troncoso in Darwiniana 18: 385. Mar 1974
3/19. Casselia (11; tropical South America), Parodianthus (2; P. capillaris, P. ilicifolius; Argentina), Tamonea (6; T. boxiana, T. curassavica, T. euphrasiifolia, T. juncea, T. spicata, T. subbiflora; southern Mexico, Central America, tropical South America). – Mexico, Central America and the West Indies to Argentina. Inflorescence consisting of lateral racemes. Pistil composed of a single carpel. Secondarily bicarpellate ovaries with secondary septa fused to carpel walls only at base and apex. Ovule inserted in upper part of locule, attached to margins. Placental line broad; placental bundles entering ovules in upper part of locule. – Casselieae are sister to Citharexyleae.
Citharexyleae Briq. in Engler et Prantl, Nat. Pflanzenfam. IV, 3a: 144, 158. 26 Feb 1895
2–3/100–105. Citharexylum (100–105; Florida, Mexico, Central America, the West Indies, tropical South America to Argentina; incl. Verbenoxylum?), Verbenoxylum (1; V. retizii; Brazil; in Citharexylum?), Rehdera (2; R. penninervia, R. trinervis; Mexico, Central America). – Tropical America to Argentina. Staminodium single. Pollen grains in Citharexylum sometimes 3(–4)-porate. Bicarpellate ovary usually developing into a drupaceous to subdrupaceous fruit with two two-seeded mericarps, with minute often deciduous floral bracts and shortly pedicellate flowers.
[Priveae+[Rhaphithamnus+[Neospartoneae+[Verbeneae+Lantaneae]]]]
Priveae Briq. in Engler et Prantl, Nat. Pflanzenfam. IV, 3a: 144, 155. 26 Feb 1895
1–2/c 20. Priva (c 20; tropical and subtropical regions in Africa, southern Asia and southwestern United States to northern Argentina; incl. Pitraea?), Pitraea (1; P. cuneato-ovata; temperate South America; in Priva?). – Perennial herbs. Rhachis, stem, leaves and peduncles often with uncinate hairs. Fruit a dry schizocarp splitting into two two-seeded mericarps derived from a bicarpellate ovary.
[Rhaphithamnus+[Neospartoneae+[Lantaneae+ Verbeneae]]]
Rhaphithamnus clade
1/2. Rhaphithamnus (2; R. spinosus, R. venustus; Chile, Juan Fernandez, Argentina). – Spinescent shrubs. Inflorescence an axillary, one- to five-flowered raceme. Corolla tube long. Ovary bicarpellate. Fruit drupaceous.
[Neospartoneae+[Lantaneae+Verbeneae]]
Neospartoneae Olmstead et N. O’Leary in Amer. J. Bot. 97: 1653. 1 Oct 2010
3/10. Neosparton (4; N. aphyllum, N. darwinii, N. ephedroides, N. patagonicum; temperate regions in Chile and Argentina), Lampayo (5; L. aratae, L. castellani, L. hieronymi, L. officinalis, L. schickendantzii; arid regions in Bolivia, Chile and Argentina), Diostea (1; D. juncea; Chile, Argentina). – Bolivia, Chile, Argentina. Shrubs (sometimes Ephedra-like). Hairs absent. Inflorescence a usually terminal spike. Corolla tube much longer than calyx. Staminodium sometimes present. Carpel one. Fruit a drupe with two-seeded pyrene.
[Lantaneae+Verbeneae]
Staminodia absent.
Lantaneae Endl., Gen. Plant.: 635. Aug 1838
10/415–420. Coelocarpum (7; C. africanum, C. glandulosum, C. haggierense, C. humbertii, C. madagascariense, C. socotranum, C. swinglei; Somalia, Madagascar, Socotra); ‘Acantholippia’ (6; A. deserticola, A. riojana, A. salsoloides, A. seriphioides, A. tarapacana, A. trifida; arid regions in South America; polyphyletic), ‘Aloysia’ (30–35; United States, Mexico, Central America, South America; non-monophyletic), Xeroaloysia (1; X. ovalifolia; northwestern Argentina; in Aloysia),‘Lippia’ (c 200; tropical Africa, tropical America; paraphyletic), Phyla (c 11; tropical and subtropical regions on both hemispheres), Diphyllocalyx (7; D. armata, D. cayensis, D. galanus, D. myrtifolia, D. nipensis, D. urquiolae, D. variifolia; Cuba), Isidroa (1; I. spinifera; Hispaniola), Nashia (1; N. inaguensis; Puerto Rico, St Croix in the Virgin Islands),‘Lantana’ (c 150; tropical and southern Africa, southern Mexico, Central America, the West Indies, tropical South America; polyphyletic). – Tropical and subtropical Africa, Madagascar, Socotra, tropical and subtropical Asia, America. Stomata anisocytic. Inflorescence capitate. Ovary usually secondarily unicarpellate (one carpel aborted; in Coelocarpum bicarpellate). Ovule in Lantana one per carpel. Fruit a two-seeded drupe or a dry schizocarp splitting into two one-seeded mericarps. Fruit in Coelocarpum a fleshy drupe splitting into two two-seeded mericarps. Calyx persistent and more or less enclosing fruit. Endosperm present in Lantana. Ethereal oils present. – Lantaneae are sister to Verbeneae. Coelocarpum may be more allied to the Verbeneae or sister to [Lantaneae+Verbeneae] (Lu-Irving & Olmstead 2013). ‘Lantana’ is nested inside ‘Lippia’, according to Lu-Irving & Olmstead (2013).
Verbeneae Dumort., Anal. Fam. Plant.: 22. 1829
6/350–400. Dipyrena (1; D. glaberrima; Argentina); Mulguraea (11; arid regions in Peru, Chile and Argentina), ‘Verbena’ (200–250; temperate and subtropical regions in North and South America, 2–3 species in the Old World; non-monophyletic; incl. Hierobotana?), Hierobotana (1; H. inflata; Colombia, Ecuador, Peru; in Verbena?), ‘Junellia’ (c 40; South America; non-monophyletic), ‘Glandularia’ (c 95; temperate to tropical America; polyphyletic; in Junellia?). – North and South America, a few species in northern Africa, Europe and Asia. Fertile stamens in Verbena five, in Hierobotana two. Ovary bicarpellate. Fruit a dry schizocarp splitting into four single-seeded mericarps. – Dipyrena (leaves alternate; ovary bicarpellate and developing into a subdrupaceous fruit subdivided into two two-seeded mericarps) is sister to the remaining Verbeneae. Hierobotana may be included in Verbena. The following topology is thus possible: [Dipyrena+[Mulguraea+[Junellia+[Verbena+Glandularia]]]]. A number of species of ‘Glandularia’ are nested in Verbena, according to analyses using plastid DNA data (O’Leary & al. 2009).
 |
Cladogram (simplified) of Verbenaceae based on DNA sequence data (Marx & al. 2010). |
Literature
Aagard JA, Olmstead RG, Willis JH, Phillips PC. 2005. Duplication of floral regulatory genes in Lamiales. – Amer. J. Bot. 92: 1284-1293.
Abbiatti D. 1939. Las Martiniáceas Argentinas. – Notas Mus. La Plata Bot. 4: 443-473.
Abels J. 1975. Monographien der afrikanischen Pedaliaceae III-IV. Die Gattungen Ceratotheca Endl. und Dicerocaryum Boj. – Mem. Soc. Brot. 25: 1-358.
Abu-Asab MS. 1990. Phylogenetic implications of pollen morphology in subfamily Lamioideae (Labiatae) and related taxa. – Ph.D. diss., Ohio University, Athens, Ohio.
Abu-Asab MS, Cantino PD. 1987. Phylogenetic implications of leaf anatomy in subtribe Melittidinae (Labiatae) and related taxa. – J. Arnold Arbor. 68: 1-34.
Abu-Asab MS, Cantino PD. 1989. Pollen morphology of Trichostema (Labiatae) and its systematic implications. – Syst. Bot. 14: 359-369.
Abu-Asab MS, Cantino PD. 1992. Pollen morphology in subfamily Lamioideae (Labiatae) and its phylogenetic implications. – In: Harley RM, Reynolds T (eds), Advances in Labiate Science, Royal Botanic Gardens, Kew, pp. 97-112.
Abu-Asab MS, Cantino PD. 1993a. Phylogenetic implications of pollen morphology in tribe Ajugeae (Labiatae). – Syst. Bot. 18: 100-122.
Abu-Asab MS, Cantino PD. 1993b. Systematics implications of pollen morphology in tribe Prostanthereae (Labiatae). – Syst. Bot. 18: 563-574.
Abu-Asab MS, Cantino PD. 1994. Systematic implications of pollen morphology in subfamilies Lamioideae and Pogostemonoideae (Labiatae). – Ann. Missouri Bot. Gard. 81: 653-686.
Abu-Asab MS, Cantino PD, Nowicke JW, Sang T. 1993. Systematic implications of pollen morphology in Caryopteris (Labiatae). – Syst. Bot. 18: 502-515.
Abu Sbaih HA, Keith-Lucas DM, Jury SL. 1994. Pollen morphology of the genus Orobanche L. (Orobanchaceae). – Bot. J. Linn. Soc. 116: 305-313.
Ackermann M. 2011. Studies on systematics, morphology and taxonomy of Caiophora and reproductive biology of Loasaceae and Mimulus (Phrymaceae). – Ph.D. diss., Freie Universität Berlin.
Adamec L. 2011. Functional characteristics of traps of aquatic carnivorous Utricularia species. – Aquatic Bot. 95: 226-233.
Adatia RD, Sharma YB, Vijayaraghavan MR. 1971. Studies in the Gesneriaceae I. Morphology and embryology of Platystemma violoides Wall. – Bot. Not. 124: 25-38.
Adityachaudhury N, Das AK, Chaudhury A, Daskanungo PL. 1976. Aurentiacin, a new chalcone from Didymocarpus aurentiaca. – Phytochemistry 15: 229-230.
Adylov T, Kamelin R, Makhmedov A. 1986. Notae de Lamiaceis. – Novosti Sist. Vyssh. Rast. 23: 110-114.
Afanasiyeva NG. 1971. Endospermal haustoria and their significance for the elucidation of the phylogeny of the genus Veronica L. – Bot. Žurn. 56: 215-229. [In Russian]
Afanasiyeva NG, Meshkova L. 1961. Application of the karyogeographical study to the phylogeny of genus Veronica L. – Bot. Žurn. 46: 247-259. [In Russian]
Aguilar-Rodríguez S, Terrazas T. 2001. Anatomía de la madera de Buddleja L. (Buddlejaceae): análisis fenético. – Medera y Bosques 7: 63-85.
Ahedor AR, Elisens W. 2015. Seed morphology and its taxonomic significance in the subtribe Gratiolinae (Plantaginaceae). – Syst. Bot. 40: 845-852.
Ahmad KJ. 1972. Cuticular studies in some Acanthaceae and Solanaceae. – Ph.D. diss., Lucknow University, India.
Ahmad KJ. 1974a. Cuticular studies in some Nelsonioideae (Acanthaceae). – Bot. J. Linn. Soc. 68: 73-80.
Ahmad KJ. 1974b. Cuticular studies in some species of Mendoncia and Thunbergia (Acanthaceae). – Bot. J. Linn. Soc. 69: 53-63.
Ahmad KJ. 1974c. Cuticular and epidermal structures in some species of Eranthemum and Pseuderanthemum (Acanthaceae). – Bot. Not. 127: 256-266.
Ahmad KJ. 1974d. Cuticular studies in some species of Hemigraphis and Strobilanthes. – Proc. Indian Acad. Sci., Sect. B, 79: 29-40.
Ahmad KJ. 1978. Epidermal hairs of Acanthaceae. – Blumea 24: 101-117.
Airy Shaw HK. 1952. Notes on the taxonomic position of Nyctanthes L. and Dimetra Kerr. – Kew Bull. 1952: 271-272.
Airy Shaw HK. 1965. On a new species of the genus Silvianthus Hook. f., and on the family Carlemanniaceae. – Kew Bull. 19: 507-512.
Airy Shaw HK. 1981. Metastachydium validated. – Kew Bull. 36: 638.
Aitzetmüller K, Tsevegüren N. 1998. Phlomic acid in Lamioideae seed oils. – Lamiales Newslett. 6: 13-16.
Akçin OE, Özyurt MS, Şenel G. 2011. Petiole anatomy of some Lamiaceae taxa. – Pakistan J. Bot. 43: 1437-1443.
Albach DC. 2002. Biosystematics of Veronica. – Ph.D. diss., Universität Wien, Austria.
Albach DC. 2008. Further arguments for the rejection of paraphyletic taxa: Veronica subgen. Pseudolysimachium (Plantaginaceae). – Taxon 57: 1-6.
Albach DC, Briggs BG. 2012. Phylogenetic analysis of Australian species of Veronica (V. section Labiatoides; Plantaginaceae). – Aust. Syst. Bot. 25: 353-363.
Albach DC, Chase MW. 2001. Paraphyly of Veronica (Veroniceae; Scrophulariaceae): evidence from the internal transcribed spacer (ITS) sequences of nuclear ribosomal DNA. – J. Plant Res. 114: 9-18.
Albach DC, Chase MW. 2004. Incongruence in Veroniceae (Plantaginaceae): evidence from two plastid and a nuclear ribosomal DNA region. – Mol. Phylogen. Evol. 32: 183-197.
Albach DC, Meudt HM. 2010. Phylogeny of Veronica in the Southern and Northern hemispheres based on plastid, nuclear ribosomal and nuclear low-copy DNA. – Mol. Phylogen. Evol. 54: 457-471.
Albach DC, Martínez-Ortega MM, Fischer MA, Chase MW. 2004a. Evolution of Veroniceae: a phylogenetic perspective. – Ann. Missouri Bot. Gard. 91: 275-302.
Albach DC, Martínez-Ortega MM, Fischer MA, Chase MW. 2004b. A new classification of the tribe Veroniceae – problems and a possible solution. – Taxon 53: 429-452.
Albach DC, Martínez-Ortega MM, Chase MW. 2004. Veronica: parallel morphological evolution and phylogeography in the Mediterranean. – Plant Syst. Evol. 246: 177-194.
Albach DC, Gotfredsen CH, Jensen SR. 2004. Iridoid glucosides of Paederota lutea and the relationships between Paederota and Veronica. – Phytochemistry 65: 2129-2134.
Albach DC, Meudt HM, Oxelman B. 2005. Piecing together the ‘new’ Plantaginaceae. – Amer. J. Bot. 92: 297-315.
Albach DC, Utteridge T, Wagstaff SJ. 2005. Origin of Veroniceae (Plantaginaceae, formerly Scrophulariaceae) on New Guinea. – Syst. Bot. 30: 412-423.
Albach DC, Jensen SR, Özgökce F, Grayer RJ. 2005. Veronica: chemical characters for the support of phylogenetic relationships based on nuclear ribosomal and plastid DNA sequence data. – Biochem. Syst. Ecol. 33: 1087-1106.
Albach DC, Li H-Q, Zhao N, Jensen SR. 2007. Molecular systematics and phytochemistry of Rehmannia (Scrophulariaceae). – Biochem. Syst. Ecol. 35: 293-300.
Albach DC, Martínez-Ortega MM, Delgado L, Weiss-Schneeweiss H, Özgökce F, Fischer MA. 2008. Chromosome numbers in Veroniceae (Plantaginaceae): review and several new counts. – Ann. Missouri Bot. Gard. 95: 543-566.
Albach DC, Yan K, Rosendal Jensen SR, Li H-Q. 2009. Phylogenetic placement of Triaenophora (formerly Scrophulariaceae) with some implications for the phylogeny of Lamiales. – Taxon 58: 749-756.
Albaladejo RG, Aguilar JF, Aparicio A, Feliner GN. 2005. Contrasting nuclear-plastidial phylogenetic patterns in the recently diverged Iberian Phlomis crinita and P. lychnitis lineages (Lamiaceae). – Taxon 54: 987-998.
Albert VA, Williams SE, Chase MW. 1992. Carnivorous plants: phylogeny and structural evolution. – Science 257: 1491-1495.
Alberto CM, Sanso AM, Xifreda CC. 2003. Chromosomal studies in species of Salvia (Lamiaceae) from Argentina. – Bot. J. Linn. Soc. 141: 483-490.
Alcantara S, Lohmann LG. 2010. Evolution of floral morphology and pollination system in Bignonieae (Bignoniaceae). – Amer. J. Bot. 97: 782-796.
Alcorcés de Guerra N, Méndez Natera JR. 2007. Chromosome numbers of three Tabebuia species (Bignoniaceae). – Nord. J. Bot. 25: 359-360.
Alder LS, Wink M. 2001. Transfer of quinolizidine alkaloids from hosts to hemiparasites in two Castilleja-Lupinus associations: analysis of floral and vegetative tissues. – Biochem. Syst. Ecol. 29: 551-561.
Alimova GK, Yakovlev MS. 1982. A contribution to the ebryology of Streptocarpus rexii (Gesneriaceae). – Bot. Žurn. 67: 470-479. [In Russian]
Alkhalaf IA, Hübener T, Porembski S. 2009. Prey spectra of aquatic Utricularia species (Lentibulariaceae) in northeastern Germany: the role of planktonic algae. – Flora 204: 700-708.
Alkhalaf IA, Hübener T, Porembski S. 2011. Microalgae trapped by carnivorous bladderworts (Utricularia, Lentibulariaceae): analysis, attributes and structure of the microalgae trapped. – Plant Divers. Evol. 129: 125-138.
Allen AA. 1961. The foodplants of Phalonia degreyana McLach. (Lep., Phaloniidae) as further evidence of affinity between the Scrophulariaceae and Plantaginaceae. – Entomol. Monthly Mag. 96: 214.
Alonso R, Lence C, López MJ, Puente E, Penas A. 2003. A new species of Veronica L. (Scrophulariaceae) in the Cantabrian Range (Spain). – Bot. J. Linn. Soc. 141: 119-124.
Altamura L, Altamura Betti MM, Mazzolani G. 1987. Elements for the revision of the genus Olea (Tourn.) L. VII. The taxa present in Asia which can be ascribed to Olea and allied genera. – Ann. Bot. (Roma) 45: 119-134.
Altamura Betti MM, Pasqua G, Mazzolani G. 1982. Embryogenesis in Olea europaea L. – Ann. Bot. (Roma) 40: 141-152.
Altamura Betti MM, Altamura L, Mazzolani G. 1985. Elements for the revision of the genus Olea (Tourn.) L. VI. The taxa present in Oceania which can be ascribed to Olea and allied genera. – Ann. Bot. (Roma) 43: 45-52.
Alziar G. 1988-1993. Catalogue synonymique des Salvia L. du monde (Lamiaceae) I-VI. – Biocosme Mesogéen 5: 87-136 (1988); 6: 79-115, 163-204 (1989); 7: 59-109 (1990); 9: 413-497 (1992); 10: 33-117 (1993).
Amelunxen VF. 1964. Elektronenmikroskopische Untersuchungen an den Drüsenhaaren von Mentha piperita L. – Planta Medica 12: 121-139.
Amelunxen VF. 1965. Elektronenmikroskopische Untersuchungen an den Drüsenschuppen von Mentha piperita L. – Planta Medica 13: 457-473.
An BC, Hong SP. 2003. Systematic application of seed morphology in Korean Orobanchaceae. – Korean J. Plant Taxon. 33: 411-420.
Andary C, Wylde R, Laffite C, Privat G, Winternitz F. 1982. Structures of verbascoside and orobanchoside, caffeic acid sugar esters from Orobanche rapum-genistae. – Phytochemistry 21: 1123-1127.
Anders O. 1966. Entwicklung und Bau des Embryosacks von Streptocarpus rexii. – Flora, Abt. B, 156: 446-451.
Anderson F. 1922. The development of the flowers and embryogeny of Martynia louisiana. – Bull. Torrey Bot. Club 49: 141-157.
Anderson T. 1867. An enumeration of the Indian species of Acanthaceae. – J. Linn. Soc., Bot. 9: 425-526.
Andersson L, Molau U. 1980. The inflorescence of Calceolaria. – Bot. Not. 133: 21-32.
Andersson S. 2006. On the phylogeny of the genus Calceolaria (Calceolariaceae) as inferred from ITS and plastid matK sequences. – Taxon 55: 125-137.
Andrzejewska-Golec E. 2000. The hairs in species of Plantago L. section Palaeopsyllium Pilg. (Plantaginaceae). – Feddes Repert. 111: 9-14.
Andrzejewska-Golec E. 2003. The hairs of Plantago reniformis Beck, section Eremopsyllium Pilg. (Plantaginaceae). – Feddes Repert. 115: 204-207.
Andrzejewska-Golec E, Swietosławski J. 1987. The morphology of hairs in species of Plantago L. sectio Coronopus DC. – Acta Soc. Bot. Polon. 56: 367-379.
Andrzejewska-Golec E, Swietosławski J. 1993. Hair anatomy in Plantago subg. Psyllium (Plantaginaceae). – Plant Syst. Evol. 184: 113-123.
Antunes T, Sevinate-Pinto I. 1991. Glandular trichomes of Teucrium scorodonia L. Morphology and histochemistry. – Flora 185: 65-70.
Antunes T, Sevinate-Pinto I, Figueiredo AC, Barroso JG, Pedro LG, Fontinha SS, Scheffer JJC. 1997. Morphology and distribution of trichomes in two endemic Teucrium species of Macaronesia. – Acta Bot. Gallica 144: 363-369.
Araújo AO de, Souza VC, Perret M. 2010. Chautemsia calcicola: a new genus and species of Gloxinieae (Gesneriaceae) from Minas Gerais, Brazil. – Taxon 59: 203-208.
Araújo AO de, Chautems A, Cardoso-Gustavson P, Souza VC, Perret M. 2016. Taxonomic revision and phylogenetic position of the Brazilian endemic genus Sphaerorrhiza (Sphaerorrhizinae, Gesneriaceae) including two new species. – Syst. Bot. 41: 651-664.
Archibald JK, Mort ME, Wolfe AD. 2005. Phylogenetic relationships within Zaluzianskya (Scrophulariaceae s.s., tribe Manuleeae): classification based on DNA sequences from multiple genomes and implications for character evolution and biogeography. – Syst. Bot. 30: 196-215.
Archibald JK, Cook J, Anderson B, Johnson SD, Mort ME. 2017. A reassessment of the phylogeny and circumscription of Zaluzianskya (Scrophulariaceae). – Mol. Phylogen. Evol. 112: 194-208.
Arekal GD. 1961. Embryology of Klugia notoniana. – Bot. Gaz. 123: 144-150.
Arekal GD. 1963a. Contribution to the embryology of Chelone glabra L. – Phytomorphology 13: 376-388.
Arekal GD. 1963b. Embryological studies in Canadian representatives of the tribe Rhinantheae, Scrophulariaceae. – Can. J. Bot. 41: 267-302.
Arekal GD. 1963c. Contribution to the embryology of Chaenorrhinum minus (L.) Lange. – Proc. Indian Acad. Sci., Sect. B, 58: 375-385.
Arekal GD. 1964. Contribution to the embryology of Gerardia pedicularia L. (Scrophulariaceae). – J. Indian Bot. Soc. 43: 409-423.
Arekal GD. 1965. Embryology of Mimulus ringens. – Bot. Gaz. 126: 58-66.
Arekal GD. 1966. Embryology of Veronica serpyllifolia L. – Proc. Indian Acad. Sci., Sect. B, 64: 241-257.
Arekal GD, Raju D. 1964. The female gametophyte of Linaria ramosissima Wall. – Curr. Sci. 33: 591-592.
Arekal GD, Raju D. 1971. Contribution to the embryology of Calceolaria mexicana Benth. – J. Mysore Univ. 24: 120-126.
Arekal GD, Swamy SNR. 1974. The endosperm organization in Microcarpaea R. Br. (Scrophulariaceae). – Curr. Sci. 43: 87-88.
Arekal GD, Girijamma S, Swamy SNR. 1970. Contribution to the embryology of Lindernia hyssopioides (L.) Haines. – Proc. Indian Acad. Sci., Sect. B, 72: 221-235.
Arekal GD, Rajeshwari S, Swamy SNR. 1971. Contribution to the embryology of Scoparia dulcis L. – Bot. Not. 124: 237-248.
Argue CL. 1980. Pollen morphology in the genus Mimulus (Scrophulariaceae) and its taxonomic significance. – Amer. J. Bot. 67: 68-87.
Argue CL. 1981. The taxonomic implications of pollen morphology in some South American species of Mimulus (Scrophulariaceae) and its taxonomic significance. – Amer. J. Bot. 68: 200-205.
Argue CL. 1984. Pollen morphology in Dodartia, Lancea, Leucocarpus, and Mazus and an analysis of pollen morphotypes in the Mimuleae (Scrophulariaceae). – Can. J. Bot. 62: 1287-1297.
Argue CL. 1985. Pollen morphology in the genera Monttea and Melosperma (Scrophulariaceae). – Amer. J. Bot. 72: 1248-1255.
Argue CL. 1986. Pollen morphology of Amphianthus, Artanema, Curanga, Glossostigma, and Peplidium (Scrophulariaceae-Gratioleae). – Amer. J. Bot. 73: 1570-1576.
Argue CL. 1990. Pollen morphology of Deinostema, Geochorda, Gratiola, Ildefonsia, Sophronanthe, and Tragiola (Scrophulariaceae, Gratioleae, Gratiolinae). – Can. J. Bot. 68: 1651-1660.
Argue CL. 1993. Pollen morphology in the Selagineae, Manuleeae (Scrophulariaceae) and selected Globulariaceae, and its taxonomic significance. – Amer. J. Bot. 80: 723-733.
Argue CL. 1995. Pollen morphology of Lagotis (Scrophulariaceae). – Can. J. Bot. 73: 701-709.
Armstrong JE. 1985. The delimitation of Bignoniaceae and Scrophulariaceae based on floral anatomy, and the placement of problem genera. – Amer. J. Bot. 72: 755-766.
Armstrong JE. 1992. Lever action anthers and the forcible shedding of pollen in Torenia. – Amer. J. Bot. 79: 34-40.
Armstrong JE, Douglas AW. 1989. The ontogenetic basis for corolla aestivation in Scrophulariaceae. – Bull. Torrey Bot. Club 116: 378-389.
Arroyo MKT, Peñazola A. 1990. Genetic self-incompatibility in a South American species of Ourisia (Scrophulariaceae). – New Zealand J. Bot. 28: 467-476.
Aseyeva LA. 2002. Some morphological characters of corolla of Veronica (Scrophulariaceae) species and their taxonomic value. – Bot. Žurn. 87: 69-76. [In Russian]
Atkins H, Preston J, Cronk QCB. 2001. A molecular test of Huxley’s line: Cyrtandra (Gesneriaceae) in Borneo and the Philippines. – Biol. J. Linn. Soc. 72: 143-159.
Atkins S. 1997. Two new species of Callicarpa (Verbenaceae) from Brunei. – Kew Bull. 52: 227-230.
Atkins S. 2004. Verbenaceae. – In: Kubitzki K, Kadereit JW (eds), The families and genera of vascular plants VII. Flowering plants. Dicotyledons. Lamiales (except Acanthaceae including Avicenniaceae), Springer, Berlin, Heidelberg, New York, pp. 449-468.
Atkins S. 2005. The genus Stachytarpheta (Verbenaceae) in Brazil. – Kew Bull. 60: 161-272.
Atkinson R. 1998. A taxonomic revision of Hypenia (Mart. ex Benth.) Harley (Labiatae). – Ph.D. diss., University of St. Andrews, Scotland.
Atokple IDK, Singh BB, Emechebe AM. 1995. Genetics of resistance to Striga and Alectra in cowpea. – J. Heredity 86: 45-49.
Attar F, Hamzeh’ee B. 2006. Two new species of Scrophularia L. (Scrophulariaceae) from Iran. – Feddes Repert. 117: 508-511.
Attar F, Riahi M, Daemi F, Aghabeigi F. 2011. Preliminary molecular phylogeny of Eurasian Scrophularia (Scrophulariaceae) based on DNA sequence data from trnS-trnG and ITS regions. – Plant Biosystems, published online, DOI: 10.1080/11263504.2011.590826.
Attawi F. 1977. Morphologisch-anatomische Untersuchungen an den Haustorien einiger Orobanche-Arten. – Ber. Deutsch. Bot. Ges. 90: 173-182.
Ayensu ES et al. (eds). 1984. Striga: biology and control. – International Council of Scientific Unions, Paris.
Azizian D, Moore DM. 1982. Morphological and palynological studies in Phlomis L, Eremostachys Bunge and Paraphlomis Prain (Labiatae). – Bot. J. Linn. Soc. 85: 225-248.
Baas P, Esser PM, Westen MET van der, Zandee M. 1988. Wood anatomy of the Oleaceae. – IAWA Bull., N. S., 9: 103-182.
Bachmann ET. 1880. Die Entwicklungsgeschichte und der Bau der Samenschalen der Scrophularineen. – Nova Acta Leopold. 43: 1-179.
Bachmann ET. 1882. Darstellung der Entwicklungsgeschichte und des Baues der Samenschalen der Scrophularineen. – Nova Acta Acad. Caes. Leop.-Carol. German. Nat. Cur 43(1).
Backer CA. 1951. Pedaliaceae. – In: Steenis CGGJ van (ed), Flora Malesiana I, 4(3), Noordhoff-Kolff N. V., Batavia, pp. 216-221.
Backlund A, Hunde Asfaw, Långström E. 1993. A revision of Cycniopsis (Scrophulariaceae). – Nord. J. Bot. 13: 185-194.
Backlund M, Oxelman B, Bremer B. 2000. Phylogenetic relationships within the Gentianales based on ndhF and rbcL sequences, with particular reference to the Loganiaceae. – Amer. J. Bot. 37: 1029-1043.
Baden C. 1981a. New taxa in Anisotes (Acanthaceae). – Nord. J. Bot. 1: 35-36.
Baden C. 1981b. The genus Macrorungia (Acanthaceae), a taxonomic revision. – Nord. J. Bot. 1: 143-153.
Baden C. 1981c. The genus Anisotes (Acanthaceae), a taxonomic revision. – Nord. J. Bot. 1: 623-664.
Baden C. 1984. Metarungia, a valid name for Macrorungia auctt. (Acanthaceae). – Kew Bull. 39: 638.
Baden C. 1987. Biosystematic studies in the Nepeta sibthorpii group (Lamiaceae) in Greece. – Opera Bot. 93: 1-54.
Badr A, El-Kholy MA. 1987. Chromosomal studies in the Egyptian flora 2. Karyotype studies in the genus Plantago L. – Cytologia 52: 725-731.
Baillon H. 1858. Recherches sur l’organogénie du Callitriche et sur ses rapports naturels. – Bull. Soc. Bot. France 5: 337-341.
Baillon H. 1887. Notes sur les Crescentiées. – Bull. Linn. Soc. Paris 1: 678, 680, 683, 688, 690, 695.
Baillon H. 1888. Observations sur les Gesnériacées. – Bull. Mens. Soc. Linn. Paris 1: 731-732.
Bakker FT, Breman F, Merckx V. 2006. DNA sequence evolution in fast-evolving mitochondrial DNA nad1 exons in Geraniaceae and Plantaginaceae. – Taxon 55: 887-896.
Baldoni L, Guerrero C, Sossey-Aloui K, Abbott AG, Angioilillo A, Lumaret R. 2002. Phylogenetic relationships among Olea species, based on nucleotide variation at a non-coding chloroplast DNA region. – Plant Biol. 4: 346-351.
Baldwin BG, Kalisz S, Armbruster WS. 2011. Phylogenetic perspectives on diversification, biogeography, and floral evolution of Collinsia and Tonella (Plantaginaceae). – Amer. J. Bot. 98: 731-753.
Balkwill KF. 1985. Taxonomic studies in the tribe Justicieae of the family Acanthaceae. – Ph.D. diss., University of Natal, Pietermaritzburg, South Africa.
Balkwill K, Getliffe-Norris F. 1988. Classification of the Acanthaceae: a southern African perspective. – Monogr. Syst. Bot. Missouri Bot. Gard. 25: 503-516.
Balkwill K, Campbell-Young G. 1999. Taxonomic studies in Acanthaceae: testa microsculpturing in southern African species of Thunbergia. – Bot. J. Linn. Soc. 131: 301-325.
Balkwill K, Getliffe-Norris F, Balkwill M-J. 1996. Systematic studies in the Acanthaceae; Dicliptera in Southern Africa. – Kew Bull. 51: 1-61.
Balkwill K, Sebola RJ, Poriazis DL. 2017. Taxonomic revision of white-flowered Isoglossa Oerst. (Acanthaceae) in southern Africa. – South Afr. J. Bot. 108: 48-80.
Balkwill M-J, Balkwill K. 1996. Problems with generic delimitation and subdivision in a large genus, Barleria (Acanthaceae). – In: Maesen LJG van der, Burgt XM van der, Medenbach de Rooy JM van (eds), The biodiversity of African plants, Proceedings XIVth AETFAT Congress, Kluwer Academic Publ., Doordrecht, The Netherlands.
Balkwill M-J, Balkwill K. 1997. Delimitation and infra-generic classification of Barleria (Acanthaceae). – Kew Bull. 52: 535-573.
Balkwill M-J, Balkwill K. 1998. A preliminary analysis of distribution patterns in a large, pantropical genus, Barleria L. (Acanthaceae). – J. Biogeogr. 25: 95-110.
Baltisberger M. 1991. Chromosomenzahlen einiger Labiaten aus Albanien. – Ber. Geobot. Inst. ETH, Stiftung Rübel, Zürich 57: 165-181.
Banerji I. 1961. The endosperm in Scrophulariaceae. – J. Indian Bot. Soc. 40: 1-11.
Banka RA, Kiew R. 2009. Henckelia Section Loxocarpus (Gesneriaceae) in Peninsular Malaysia. – Edinb. J. Bot. 66: 239-261.
Barber JC, Ortega JF, Santos-Guerra A, Marrero A, Jansen RK. 2000. Evolution of endemic Sideritis (Lamiaceae) in Macaronesia: insights from a chloroplast DNA restriction site analysis. – Syst. Bot. 25: 633-647.
Barber JC, Francisco-Ortega J, Santos-Guerra A, Turner KG, Jansen RK. 2002. Origin of Macaronesian Sideritis L. (Lamioideae: Lamiaceae) inferred from nuclear and chloroplast sequence datasets. – Mol. Phylogen. Evol. 23: 293-306.
Barber JC, Finch CC, Francisco-Ortega J, Santos-Guerra A, Jansen RK. 2007. Hybridization in Macaronesian Sideritis (Lamiaceae): evidence from inconguence of multiple independent nuclear and chloroplast sequence. – Taxon 56: 74-88.
Barber SC. 1982. Taxonomic studies in the Verbena stricta complex (Verbenaceae). – Syst. Bot. 7: 433-456.
Barberan FAT. 1986. The flavonoid compounds from the Labiatae. – Fitoterapia 52: 67-95.
Barker RM. 1986. A taxonomic revision of Australian Acanthaceae. – J. Adelaide Bot. Gard. 9: 1-292.
Barker WR. 1982. Taxonomic studies in Euphrasia L. (Scrophulariaceae). a revised infrageneric classification, and a revision of the genus in Australia. – J. Adelaide Bot. Gard. 5: 1-304.
Barker WR. 1982. Evolution, adaptation and biogeography in arid Australian Scrophulariaceae. – In: Barker WR, Greenslade PJM (eds), Evolution of the flora and fauna of arid Australia, Peacock Publ., Adelaide, South Australia, pp. 341-350.
Barker WR. 1986. Biogeography and evolution in Euphrasia (Scrophulariaceae), particularly relating to Australia. – In: Barlow BA (ed), Flora and fauna of alpine Australasia, Ages and origins, CSIRO, Melbourne, pp. 489-510.
Barker WR, Kiehn M, Vitek E. 1988. Chromosome numbers in Australian Euphrasia (Scrophulariaceae). – Plant Syst. Evol. 158: 161-164.
Barlow BA. 1971. Cytogeography of the genus Eremophila. – Aust. J. Bot. 19: 295-310.
Barrabe L, Karnadi-Abdelkader G, Ounemoa J, De Kok RPJ, Robert N, Gateble G. 2015. Recircumscription of Oxera (Lamiaceae: Ajugoideae) to include Faradaya based on molecular and anatomical data. – Bot. J. Linn. Soc. 179: 693-711.
Barrett SCH, Strother JL. 1978. Taxonomy and natural history of Bacopa (Scrophulariaceae) in California. – Syst. Bot. 3: 408-419.
Barrett SCH, Wilken DH, Cole WW. 2000. Heterostyly in the Lamiaceae: the case of Salvia brandegeei. – Plant Syst. Evol. 223: 211-219.
Barrie FR, Skog LE, Clark JL. 2018. A new species of Alsobia (Gesneriaceae) from Belize, with a synopsis of the genus. – Novon 26: 1-8.
Barringer K. 1983. Monopera, a new genus of Scrophulariaceae from South America. – Brittonia 35: 111-114.
Barringer K. 1984. Cubitanthus, a new genus of Gesneriaceae from Brazil. – J. Arnold Arbor. 65: 145-147.
Barringer K. 1985a. Revision of the genus Basistemon (Scrophulariaceae). – Syst. Bot. 10: 125-133.
Barringer K. 1985b. Two new species of Esterhazya (Scrophulariaceae) from Brazil. – Brittonia 37: 195-198.
Barringer K. 1993. Five new tribes in the Scrophulariaceae. – Novon 3: 15-17.
Barringer K. 1999. A new species of Gibsoniothamnus (Schlegeliaceae) from Costa Rica and Panama. – Novon 9: 476-478.
Barringer K. 2004. A revision of Gibsoniothamnus L. O. Williams (Schlegeliaceae). – Brittonia 56: 213-237.
Bartels D. 2005. Desiccation tolerance studied in the resurrection plant Craterostigma plantagineum. – Integr. Comp. Biol. 45: 696-701.
Bartels D, Schneider K, Terstappen G, Piatkowski D, Salamini F. 1990. Molecular cloning of abscisic acid modulated genes which are induced during desiccation of the resurrection plant Craterostigma plantagineum. – Planta 181: 27-34.
Barthlott W. 1980. Morphogenese und Mikromorphologie komplexer Cuticularfaltungsmuster an Blüten-trichomen von Antirrhinum L. (Scrophulariaceae). – Ber. Deutsch. Bot. Ges. 93: 379-390.
Barthlott W, Porembski S, Fischer E, Gemmel B. 1998. First protozoa-trapping plant found. – Nature 392: 447.
Bassett IJ, Crompton CW. 1968. Pollen morphology and chromosome numbers of the family Plantaginaceae in North America. – Can. J. Bot. 46: 349-361.
Bassett IJ, Munro DB. 1986. Pollen morphology of the genus Stachys (Labiatae) in North America, with comparisons to some taxa from Mexico, Central and South America and Asia. – Pollen Spores 28: 279-295.
Baum VM. 1982. A revision of the genus Odontonema (Acanthaceae). – Master’s thesis, University of Maryland, College Park, Maryland.
Baum VM, Reveal JL, Nowicke JW. 1983. Pulchranthus (Acanthaceae), a new genus from northern South America. – Syst. Bot. 8: 211-220.
Beal EO, Quay TL. 1968. A review of Utricularia olivacea Wright ex Grisebach (Lentibulariaceae). – J. Elisha Mitchell Soc. 84: 462-466.
Beardsley PM. 1997. Colorado’s rare endemic plant, Mimulus gemmiparus, and its unique mode of reproduction. – Master’s thesis, Colorado State University, Fort Collins, Colorado.
Beardsley PM, Barker WR. 2005. Patterns of evolution in Australian Mimulus and related genera (Phrymaceae-Scrophulariaceae): a molecular phylogeny using chloroplast and nuclear sequence data. – Aust. Syst. Bot. 18: 61-73.
Beardsley PM, Olmstead RG. 2002. Redefining Phrymaceae: the placement of Mimulus, tribe Mimuleae, and Phryma. – Amer. J. Bot. 89: 1093-1102.
Beardsley PM, Schoenig S, Whittall JB, Olmstead RG. 2004. Patterns of evolution in western North American Mimulus (Phrymaceae). – Amer. J. Bot. 91: 474-489.
Beaufort-Murphy HT. 1983. The seed-surface morphology of the Gesneriaceae, utilizing the scanning electron microscope and a new system for diagnosing seed morphology. – Selbyana 6: 220-422.
Beck SG, Fleischmann A, Huaylla H, Müller KF, Borsch T. 2008. Pinguicula chuquisacensis (Lentibulariaceae), a new species from the Bolivian Andes, and first insights on phylogenetic relationships among South American Pinguicula. – Willdenowia 38: 201-212.
Beck von Mannagetta G. 1890. Monographie der Gattung Orobanche. – In: Luerssen C, Haenlein FH (eds), Bibliotheca botanica. Abhandlungen aus der Gesammtgebiete der Botanik, Theodor Fischer, Kassel, Germany.
Beck von Mannagetta G. 1895. Orobanchaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien IV(3b), W. Engelmann, Leipzig, pp. 123-132.
Bedigian D. 2011. Cultivated sesame and wild relatives in the genus Sesamum L. – In: Bedigian D (ed), Sesame: the genus Sesamum, CRC Press, Boca Raton, Florida, pp. 33-78.
Bedigian D. 2013. Ecogeography and taxonomy of Rogeria J. Gay ex Delile (Pedaliaceae). – Webbia 68: 103-126.
Bedigian D. 2015. Systematics and evolution of Sesamum L. (Pedaliaceae) 1: evidence regarding the origin of sesame and its closest relatives. – Webbia 70: 1-42.
Bedigian D, Harlan JR. 1986. Evidence of cultivation of sesame in the ancient world. – Econ. Bot. 40: 137-154.
Bedigian D, Seigler DS, Harlan JR. 1985. Sesamin, sesamol and the origin of sesame. – Biochem. Syst. Ecol. 13: 133-139.
Bedolla-García BY, Zamudio S. 2015. Four new species of Salvia (Lamiaceae) from central Mexico. – Phytotaxa 217: 035-052.
Beeks RM. 1962. Variation and hybridization in southern California populations of Diplacus (Scrophulariaceae). – Aliso 5: 83-122.
Behnke H-D. 1986. Contributions to the knowledge of P-type sieve-element plastids in dicotyledons IV. Acanthaceae. – Bot. Jahrb. Syst. 106: 499-510.
Bellini R. 1907. Criteri per una nuova classificazione delle Personatae (Scrophulariaceae et Rhinanthaceae). – Ann. Bot. (Roma) 6: 131-145.
Bello MA, Chase MW, Olmstead R, Rønsted N, Albach D. 2002. The páramo endemic Aragoa is the sister genus of Plantago (Plantaginaceae; Lamiales): evidence from plastid rbcL and nuclear ribosomal ITS sequence data. – Kew Bull. 57: 585-597.
Bello MA, Rudall PJ, González F, Fernández-Alonso JL. 2004. Floral morphology and development in Aragoa (Plantaginaceae) and related members of the order Lamiales. – Intern. J. Plant Sci. 165: 723-738.
Bendiksby M. 2011. Molecular phylogeny, taxonomy, and historical biogeography of Lamiaceae subfamily Lamioideae, including surveys of alloploid speciation in two Eurasian genera, Galeopsis and Lamium. – Ph.D. diss., University of Oslo, Norway.
Bendiksby M, Thorbek L, Scheen A-C, Lindqvist C, Ryding O. 2011. An updated phylogeny and classification of Lamiaceae subfamily Lamioideae. – Taxon 60: 471-484.
Bendiksby M, Brysting AK, Thorbek L, Gussarova G, Ryding O. 2011. Molecular phylogeny and taxonomy of the genus Lamium L. (Lamiaceae): disentangling origins of presumed allotetraploids. – Taxon 60: 986-1000.
Bendiksby M, Salmaki Y, Bräuchler C, Ryding
O. 2014. The generic position of Stachys tibetica Vatke and
amalgamation of the genera Eriophyton and Stachyopsis (Lamiaceae subfam. Lamioideae). –
Plant Syst. Evol. 300: 961-971.
Bendre AM. 1973. Studies in the family Loganiaceae I. Trichomes. – J. Indian Bot. Soc. 52: 225-234.
Bendre AM. 1975. Studies in the family Loganiaceae II. Embryology of Buddleia and Strychnos. – J. Indian Bot. Soc. 54: 272-279.
Benhura MAN, Marume M. 1993. The mucilaginous polysaccharide material isolated from ruredzo (Dicerocaryum zanguebarium). – Food Chem. 46: 7-11.
Bennell AP. 1983. A note on the pollen morphology of Dauphinea and Puntia (Labiatae). – Notes Roy. Bot. Gard. Edinb. 41: 123-125.
Bennett JR, Mathews S. 2006. Phylogeny of the parasitic plant family Orobanchaceae inferred from phytochrome A. – Amer. J. Bot. 93: 1039-1051.
Bennett JR, Scotland RW. 2003. A revision of Strobilanthes (Acanthaceae) in Java. – Kew Bull. 58: 1-82.
Benoist E. 1937. Recherches caryologiques sur quelques espèces du genre Salvia. – Rev. Cytol. Cytophysio. Végét. 2: 415-439.
Benoist R. 1911. Les genres Lepidagathis et Lophostachys sont-ils distincts? – Notul. Syst. (Paris) 2: 139-144.
Benoist R. 1925. Acanthacées de Madagascar. – Bull. Mus. Natl. Hist. Nat. Paris 31: 386-388.
Benoist R. 1927. Nouvelles Acanthacées d’Indo-Chine. – Bull. Mus. Natl. Hist. Nat. Paris 33: 106-109.
Benoist R. 1929. Descriptions d’espèces nouvelles d’Acanthacées de Madagascar. – Bull. Soc. Bot. France 76: 1031-1038.
Benoist R. 1934. Nouvelles Acanthacées d’Indo-Chine. – Bull. Soc. Bot. France 81: 600-605.
Benoist R. 1936. Acanthacées nouvelles d’Indochine. – Notul. Syst. (Paris) 5: 106-131.
Benoist R. 1940. Descriptions de nouvelles Acanthacées Malgaches. – Notul. Syst. (Paris) 9: 65-73.
Benoist R. 1942. Les Hypoestes Africains. – Notul. Syst. (Paris) 10: 241-248.
Benoist R. 1944. Contribution à la connaissance des Acanthacées africaines et malgaches. – Notul. Syst. (Paris) 11: 137-151.
Benoist R. 1945. Descriptions de nouvelles Acanthacées Malgaches. – Notul. Syst. (Paris) 12: 1-16.
Benoist R. 1946. Acanthacées nouvelles d’Indo-Chine. – Notul. Syst. (Paris) 5: 106-131.
Benoist R. 1950. Quelques Acanthacées des Colonies Portugaises africaines. – Bol. Soc. Brot., sér. II, 24: 5-39.
Benoist R. 1954. Les espèces du genre Eusiphon. – Notul. Syst. (Paris) 15: 5-65.
Benoist R. 1962. Nouvelles Acanthacées de Madagascar. – Bull. Soc. Bot. France 109: 129-137.
Benoist R. 1967a. Contribution à la connaissance des Acanthacées malgaches. – Bull. Soc. Bot. France 113: 531-536.
Benoist R. 1967b. 182. Acanthacées 1. – In: Humbert H (ed), Flore de Madagascar et des Comores, Musée National d’Histoire Naturel, Paris.
Bentham G. 1877. Notes on the gamopetalous orders belonging to the campanulaceous and oleaceous groups. – J. Linn. Soc. London 15: 1-16.
Bernacchia G, Salamini F, Bartels D. 1996. Molecular characterization of the rehydration process of the resurrection plant Craterostigma plantagineum. – Plant Physiol. 111: 1043-1050.
Besnard G, Bervillé A. 2002. On chloroplast DNA variations in the olive (Olea europaea L.) complex: comparison of RFLP and PCR polymorphisms. – Theor. Appl. Gen. 104: 1157-1162.
Besnard G, Green PS, Bervillé A. 2002. The genus Olea: molecular approaches of its structure and relationships to other Oleaceae. – Acta Bot. Gall. 149: 49-66.
Besnard G, García-Verdugo C, Rubio de Casas R, Treier UA, Galland N, Vargas P. 2008. Polyploidy in the olive complex (Olea europaea): evidence from flower cytometry and nuclear microsatellites analyses. – Ann. Bot. 101: 25-30.
Besnard G, Casas RR de, Christin P-A, Vargas P. 2009. Phylogenetics of Olea (Oleaceae) based on plastid and nuclear ribosomal DNA sequences: Tertiary climatic shifts and lineage differentiation times. – Ann. Bot. 104: 143-160.
Betsche I. 1984. Taxonomische Untersuchungen an Kickxia Dumortier (s.l.). Die neuen Gattungen Pogonorrhinum n. gen. und Nanorrhinum n. gen. (Phanerogamae: Scrophulariaceae). – Cour. Forsch. Inst. Senckenberg 71: 125-142.
Bhaduri S. 1944. A contribution to the morphology of pollen grains of Acanthaceae and its bearing on taxonomy. J. Dept. Sci. Calcutta Univ. 1: 25-38.
Bhandari NN, Natesh S. 1985. Development of endosperm in Scrophularia himalensis with a discussion on the variation in the endosperm of the tribe Scrophularieae (Scrophulariaceae). –Plant Syst. Evol. 148: 177-184.
Bhattacharjee R. 1980. Taxonomic studies in Stachys II. A new infrageneric classification of Stachys L. – Notes Roy. Bot. Gard. Edinb. 38: 65-96.
Bhatti GR, Ingrouille M. 1997. Systematics of Pogostemon (Labiatae). – Bull. Nat. Hist. Mus. Lond. (Bot.) 27: 77-147.
Bianco A, Lamesi S, Passacantilli P. 1984. Iridoid glucosides from Satureja vulgaris. – Phytochemistry 23: 121-123.
Bidgood S. 1992. The identity of Strigina Engl. (Scrophulariaceae). – Kew Bull. 47: 775-776.
Bidgood S. 1994. Synopsis of the continental African species of Fernandoa (Bignoniaceae). – Kew Bull. 49: 381-390.
Bidgood S, Brummitt RK. 1986. Acanthostelma, a new genus of Acanthaceae from Somalia. – Kew Bull. 40: 855-858.
Bidgood S, Brummitt RK. 1998. A revision of the genus Neuracanthus (Acanthaceae). – Kew Bull. 53: 1-76.
Bigazzi M. 1984. The occurrence of intranuclear inclusions in the Labiatae, Verbenaceae and Scrophulariaceae. – Caryologia 37: 269-292.
Bigazzi M. 1989a. Occurrence, ultrastructure and developmental features of nuclear inclusions in the tribe Antirrhineae (Scrophulariaceae) I. – Caryologia 42: 313-328.
Bigazzi M. 1989b. Occurrence, ultrastructure and developmental features of nuclear inclusions in the tribe Antirrhineae (Scrophulariaceae) II. Tubular inclusions. – Caryologia 42: 329-343.
Bigazzi M. 1989c. Ultrastructure of nuclear inclusions and the separation of Verbenaceae and Oleaceae (incl. Nyctanthes). – Plant Syst. Evol. 163: 1-12.
Bigazzi M. 1993. A survey on the intra[nu]clear inclusions in the Schrophulariaceae and their systematic significance. – Nord. J. Bot. 13: 19-31.
Bigazzi M. 1995. Investigation on occurrence and ultrastructure of the proteinaceous nuclear inclusions (PNIs) in the Bignoniaceae, with special reference to geographic distribution patterns. – Caryologia 48: 211-223.
Bigazzi M, Tardelli M. 1990. Pollen morphology and ultrastructure of the Old World Antirrhineae (Scrophulariaceae). – Grana 29: 257-275.
Bilimovitsch O. 1935. On the structure of the pericarp in the Labiatae from a systematic viewpoint. – Trudy Voronezhsk. Gosud. Univ. 7: 68-84. [In Russian]
Billings FH. 1909. The nutrition of the embryo sac and embryo in certain Labiatae. – Kansas Univ. Sci. Bull. 5: 67-83.
Bini Maleci L, Servettaz O. 1991. Morphology and distribution of trichomes in Italian species of Teucrium sect. Chamaedrys (Labiatae) – a taxonomical evaluation. – Plant Syst. Evol. 174: 83-91.
Bini Maleci L, Corsi G, Pagni AM. 1983. Trichomes tecteurs et secreteurs dans la sauge (Salvia officinalis L.). – Plantes Med. Phyt. 17: 4-17.
Bir SS, Saggoo MIS. 1981. Cytopalynology of certain Acanthaceae and Labiatae. – J. Palynol. 17: 93-102.
Bisse J, Lippold H, Casper SJ. 1975. Beiträge zur Kenntnis der westindischen Pinguicula-Arten. – Wiss. Zeitschr. Friedrich-Schiller-Univ. Jena, Math.-Naturwiss. Reihe 24: 377-385.
Bittencourt Jr NS, Mariath JEA. 2002a. Ovule ontogeny of Tabebuia pulcherrima Sandwith (Bignoniaceae): megasporogenesis and integument development. – Rev. Brasileira Bot. 25: 103-115.
Bittencourt Jr NS, Mariath JEA. 2002b. Ovule ontogeny of Tabebuia pulcherrima Sandwith (Bignoniaceae): embryo sac development. – Rev. Brasileira Bot. 25: 117-127.
Blanco-Pastor JL, Vargas P, Pfeil BE. 2012. Coalescent simulations reveal hybridization and incomplete lineage sorting in Mediterranean Linaria. – PloS One 7: e39089.
Blunden G, Yang M-H, Yuan Z-X, Smith BE, Patel AV, Cegarra JA, Máthé I Jr, Janicsák G. 1996. Betaine distribution in the Labiatae. – Biochem. Syst. Ecol. 24: 71-81.
Bocquillon H-T. 1861. Observations sur le genre Oftia Adans. – Adansonia 2: 5-12.
Boeshore I. 1920. The morphological continuity of Scrophulariaceae and Orobanchaceae. – Contr. Bot. Lab. Morris Arbor. Univ. Pennsylvania 5: 139-177.
Boggan JK. 1990. Lembocarpus amoenus. – Gloxinian 40: 20-22.
Boggan JK. 1991. A morphological study and cladistic analysis of Sinningia and associated genera, with particular reference to Lembocarpus, Lietzia, Paliavana, and Vanhouttea (Gesneriaceae: Gloxinieae). – M.Sc. thesis, Cornell University, Ithaca, New York.
Boggan JK. 1996. Fragrant flowers in Gesneriaceae. – Gloxinian 46: 17-27.
Bohlen C von. 1995a. El genero Mimulus L. (Scrophulariaceae) en Chile. – Gayana Bot. 52: 7-28.
Bohlen C von. 1995b. Mimulus crinitus A.L. Grant (Scrophulariaceae), transferido de la seccion Simiolus Green a la seccion Paradanthus A. L. Grant. – Gayana Bot. 52: 1-5.
Bokhari MH, Burtt BL. 1970. Studies in the Gesneriaceae of the Old World XXXII. Foliar sclereids in Cyrtandra. – Notes Roy. Bot. Gard. Edinb. 30: 11-22.
Bokhari MH, Hedge IC. 1971. Observations on the tribe Meriandreae of the Labiatae. – Notes Roy Bot. Gard. Edinb. 31: 53-67.
Bokhari MH, Hedge IC. 1976. Zhumeria (Labiatae): anatomy, taxonomy and affinities. – Iran. J. Bot. 1: 1-10.
Bolliger M. 1985. Die Drüsenhaare der Gattung Odontites Ludwig (Scrophulariaceae) und ihre systematische Bedeutung. – Bot. Jahrb. Syst. 107: 153-175.
Bolliger M. 1992. Monographie der Gattung Odontites (Scrophulariaceae) sowie der verwandten Gattungen Macrosyringion, Odontitella, Bornmuellerantha und Bartsiella. – Willdenowia 26: 37-168.
Bolliger M. 1993. Systematik und Chorologie der Gattung Odontites Ludwig s.l. (Scrophulariaceae). – Flora 188: 345-365.
Bolliger M. 1996. Monographie der Gattung Odontites (Scrophulariaceae) sowie der verwandten Gattungen Macrosyringion, Odontitella, Bornmuellerantha und Bartsiella. – Willdenowia 26: 37- 168.
Bolliger M, Molau U. 1992. Nothobartsia, a new genus of Scrophulariaceae from southwest Europe. – Plant Syst. Evol. 179: 59-71.
Bolliger M, Wick L. 1990. The pollen morphology of Odontites (Scrophulariaceae) and its taxonomic significance. – Plant Syst. Evol. 173: 159-178.
Borg AJ, Schönenberger J. 2011. Comparative floral development and structure of the black mangrove genus Avicennia and related taxa in the Acanthaceae. – Intern. J. Plant Sci. 172: 330-340.
Borg AJ, McDade LA, Schönenberger J. 2008. Molecular phylogenetics and morphological evolution of Thunbergioideae (Acanthaceae). – Taxon 57: 811-822.
Bornmüller J. 1927. Zur kenntnis einiger orientalischer Teucrium-Arten der Section Chamaedrys. – Mitt. Thüring. Bot. Ver., N. F., 37: 55-61.
Borsos O. 1967. Über einige Rorippa- und Veronica-Arten. – Acta Bot. Acad. Sci. Hung. 13: 1-10.
Borzova IA. 1960. The question of the origin of the six-grooved type of pollen of the mints. – Dokl. Akad. Nauk SSSR 133: 1465-1467. [In Russian]
Bosabalidis AM. 1990. Glandular trichomes in Satureja thymbra leaves. – Ann. Bot., N. S., 65: 71-78.
Bosabalidis AM, Tsekos I. 1982. Glandular scale development and essential oil secretion in Origanum dictamnus L. – Planta 156: 496-504.
Bosabalidis AM, Tsekos I. 1984. Glandular hair formation in Origanum species. – Ann. Bot., N. S., 53: 559-563.
Bose PC, Adityachaudhury N. 1978. Didymocarpin, a new flavanone from Didymocarpus pedicellata. – Phytochemistry 17: 587-588.
Bosser J. 1958. Sur deux nouvelles Lentibulariacées de Madagascar. – Natur. Malgache 10: 21-29.
Bosser J. 1973. Deux nouvelles espèces de Noronhia Stadm. ex Thouars (Oleaceae) de Madagascar. – Adansonia, sér. II, 13: 461-466.
Bothmer R von. 1969. Studies in the Aegean flora XIV. Studies in Scutellaria Section Vulgares Subsection Peregrinae from Greece and adjacent Turkey. – Bot. Not. 122: 38-56.
Bothmer R von. 1970. Studies in the Aegean flora XV. Chromosome numbers in Labiatae. – Bot. Not. 123: 52-60.
Bothmer R von. 1985. Differentiation patterns in the Scutellaria albida group (Lamiaceae) in the Aegean area. – Nord. J. Bot. 5: 421-439.
Bothmer R von. 1987. Differentiation patterns in the E Mediterranean Scutellaria rubicunda group (Lamiaceae). – Plant Syst. Evol. 155: 219-249.
Botta SM. 1979. Las especies del género Aloysia (Verbenaceae). – Darwiniana 22: 67-108.
Botta SM. 1980. Las especies del género Acantholippia (Verbenaceae). – Darwiniana 22: 511-532.
Botta SM. 1989. Studies in the South American genus Junellia (Verbenaceae Verbenoideae) I. Delimitation and infrageneric divisions. – Darwiniana 29: 371-396.
Botta SM. 1993. Notas en el género Glandularia (Verbenaceae-Verbenoideae) III. Estudio taxonómico de las especies patagónicas. – Parodiana 8: 9-36.
Botta SM, Brandham PE. 1993. The taxonomic significance of chromosome number in Junellia (Verbenaceae). – Kew Bull. 48: 143-150.
Bottega S, Corsi G. 2000. Structure, secretion and possible functions of calyx glandular hairs of Rosmarinus officinalis L. (Labiatae). – Bot. J. Linn. Soc. 132: 325-335.
Boumann F, Meeuse ADJ. 1992. Dispersal in Labiatae. – In: Harley RM, Reynolds T (eds), Advances in labiate science, Royal Botanic Gardens, Kew, pp. 193-202.
Bove CP. 1993. Pollen morphology of the Bignoniaceae from a south Brazilian Atlantic forest. – Grana 32: 330-337.
Bowden E. 1950. The flowering of Strobilanthes. – J. Bombay Nat. Hist. Soc. 49: 576.
Bradley D, Carpenter R, Copsey L, Vincent C, Rothstein S, Coen E. 1996. Control of inflorescence architecture in Antirrhinum. – Nature 379: 791-797.
Bradshaw HD Jr, Wilbert SM, Otto KG, Schemske DW. 1995. Genetic mapping of floral traits associated with reproductive isolation in monkeyflowers (Mimulus). – Nature 376: 762-765.
Bradshaw HD Jr, Otto KG, Frewen BE, McKay JK, Schemske DW. 1998. Quantitative trait loci affecting differences in floral morphology between two plant species of monkeyflower (Mimulus). – Genetics 149: 367-382.
Bramley GLC. 2006. Two new species of Pycnostachys (Lamiaceae) from tropical Africa. – Kew Bull. 60: 587-592.
Bramley GLC. 2013. The genus Callicarpa (Lamiaceae) in the Philippines. – Kew Bull. 68: 369-418.
Bramley GLC, Forest F, Kok RPJ de. 2009. Troublesome tropical mints: re-examining generic limits of Vitex and relations (Lamiaceae) in South East Asia. – Taxon 58: 500-510.
Brandegee TS. 1911. Lithophytum. – In: Plantae Mexicanae Purpusianae III, Univ. Calif. Publ. Bot. 4(11): 188-189.
Bräuchler C, Meimberg H, Heubl G. 2004. Molecular phylogeny of the genera Digitalis L. and Isoplexis (Lindley) Loudon (Veronicaceae) based on ITS- and trnL-F sequences. – Plant Syst. Evol. 248: 111-128.
Bräuchler C, Meimberg H, Abele T, Heubl G. 2005. Polyphyly of the genus Micromeria (Lamiaceae): evidence from cpDNA sequence data. – Taxon 54: 639-650.
Bräuchler C, Meimberg H, Heubl G. 2006. New names in Old World Clinopodium – the transfer of the species of Micromeria sect. Pseudomelissa to Clinopodium. – Taxon 55: 977-981.
Bräuchler C, Doroszenko A, Esser H-J, Heubl G. 2008. Killickia (Lamiaceae): a new genus from KwaZulu-Natal, South Africa. – Bot. J. Linn. Soc. 157: 575-586.
Bräuchler C, Ryding O, Heubi G. 2008. The genus Micromeria (Lamiaceae), a synoptical update. – Willdenowia 38: 363-410.
Bräuchler C, Meimberg H, Heubl G. 2010. Molecular phylogeny in Menthinae (Lamiaceae, Nepetoideae, Mentheae) – taxonomy, biogeography and conflicts. – Mol. Phylogen. Evol. 55: 501-523.
Braz DM, Monteiro R. 2011. O gênero sulamericano Gynocraterium Bremek. (Acanthaceae, Nelsonioideae). – Acta Amazonica 41. http://dx.doi.org/10.1590/S0044-59672011000400001
Braz DM, Carvalho-Okano RM, Kameyama C. 2002. Acanthaceae da reserva florestal Mata do Paraíso, Viçosa, Minas Gerais. – Revista Brasil. Bot. 25: 495-504.
Bremekamp CEB. 1926. On the opening mechanism of the acanthaceous fruit. – South Afr. J. Sci. 23: 488-490.
Bremekamp CEB. 1938a. Notes on the Acanthaceae of Surinam. – Meded. Bot. Mus. Herb. Rijksuniv. Utrecht 47: 130-176.
Bremekamp CEB. 1938b. Acanthaceae. – Rec. Trav. Bot. Néerl. 35: 160-164.
Bremekamp CEB. 1939. On the position of the genera Carlemannia Benth. and Silvianthus Hook. f. – Rec. Trav. Bot. Néerl. 36: 372.
Bremekamp CEB. 1942. The position of the genus Thomandersia Baill. – Rec. Trav. Bot. Néerl. 39: 166-175.
Bremekamp CEB. 1944a. Materials for a monograph of the Strobilanthinae (Acanthaceae). – Verh. Kon. Nederl. Akad. Wet., Afd. Natuurk., sect. II, 41: 1-305.
Bremekamp CEB. 1944b. Über Dischistocalyx T. And. ex Bth. und Acanthopale C. B. Clarke (Acanthaceae). – Bot. Jahrb. Syst. 73: 126-150.
Bremekamp CEB. 1948a. Notes on the Acanthaceae of Java. – Verh. Kon. Nederl. Akad. Wet., Afd. Natuurk., sect. II, 45: 1-78.
Bremekamp CEB. 1948b. List of the Acanthaceae collected in Celebes by Dr. W. Kaudern and Dr. G. Kjellberg. – Svensk Bot. Tidskr. 42: 372-403.
Bremekamp CEB. 1953. The delimitation of Acanthaceae. – Proc. Kon. Nederl. Akad. Wet., ser. C, 56: 533-546.
Bremekamp CEB. 1955a. A revision of the Malaysian Nelsonieae (Scrophulariaceae). – Reinwardtia 3: 157-261.
Bremekamp CEB. 1955b. Notes on some acanthaceous genera of controversial position. – Acta Bot. Neerl. 4: 644-655.
Bremekamp CEB. 1955c. The Thunbergia species of the Malesian area. – Verh. Kon. Akad. Wetensch., Afd. Natuurk., Sect. II, 50: 1-90.
Bremekamp CEB. 1960. New Bornean Acanthaceae. – Blumea 10: 151-175.
Bremekamp CEB. 1961. Studies in the flora of Thailand: Scrophulariaceae, Nelsonieae, Thunbergiaceae, Acanthaceae. – Dansk Bot. Ark. 20: 55-88.
Bremekamp CEB. 1964. On the systematic position of the Australian Nelsonias and Thunbergias and of the Ruellia species which by Domin were referred to Aporuellia Clarke. – Proc. Kon. Ned. Akad. Wetensch., Ser. C, Biol. Med. Sci. 67: 301-306.
Bremekamp CEB. 1965a. Delimitation and subdivision of the Acanthaceae. – Bull. Bot. Surv. India 7: 21-30.
Bremekamp CEB. 1965b. Scrophulariaceae, Nelsonieae, Thunbergiaceae, Acanthaceae. – Dansk Bot. Ark. 23: 195-224.
Bremekamp CEB. 1969. Scrophulariaceae-Nelsonieae, Acanthaceae, and Thunbergiaceae. – Dansk Bot. Ark. 27: 71-85.
Bremekamp CEB, Nannenga-Bremekamp NE. 1948. A preliminary survey of the Ruelliinae (Acanthaceae) of the Malay Archipelago and New Guinea. – Verh. Nederl. Akad. Wet., ser. II, 45: 1-39.
Bremer B, Olmstead RG, Struwe L, Sweere JA. 1994. rbcL sequences support exclusion of Retzia, Desfontainia, and Nicodemia from the Gentianales. – Plant Syst. Evol. 190: 213-230.
Brenan JPM. 1985. New species of Selago and Thesium from South Africa. – Kew Bull. 40: 81-83.
Breteler FJ. 1998. Novitates Gabonenses 33. A new species of Pseudocalyx (Acanthaceae) from Gabon with a synopsis of all species of this genus. – Adansonia, sér. III, 20: 271-280.
Breteler FJ. 2002. Novitates Gabonenses 42. The genus Jasminum (Oleaceae) in Gabon: identification key and description of a new species. – Adansonia, sér. III, 24: 49-54.
Breteler FJ. 2005. Novitates Gabonenses 51. A new Clerodendrum (Lamiaceae, formerly Verbenaceae) from Gabon. – Adansonia, sér. III, 27: 317-319.
Bretting PK. 1981. A systematic and ethnobotanical study of Proboscidea and allied genera of the Martyniaceae. – Ph.D. diss., Indiana University, Bloomington, Indiana.
Bretting PK. 1983. The taxonomic relationship between Proboscidea louisianica and Proboscidea fragrans (Martyniaceae). – Southw. Natur. 28: 445-449.
Bretting PK. 1985. Geographical intergradation in Proboscidea parviflora ssp. sinaloensis (Martyniaceae). – Southw. Natur. 30: 343-348.
Bretting PK, Nilsson S. 1988. Pollen morphology of the Martyniaceae and its systematic implications. – Syst. Bot. 13: 51-59.
Briggs BG. 1970. Some chromosome numbers in the Oleaceae. – Contr. New South Wales Natl. Herb. 4: 126-129.
Briggs BG. 1973. Chromosomal studies in Plantago in Australia. – Contr. New South Wales Natl. Herb. 4: 399-405.
Briggs BG. 1980. Plantago multiscapa, a new species from eremaean Australia, and notes on Plantago in Western Australia. – Telopea 2: 77-81.
Briggs BG, Ehrendorfer F. 1976. Chionohebe, a new name for Pygmea Hook. f. (Scrophulariaceae). – Contr. Herb. Australiense 25: 1-4.
Briggs BG, Ehrendorfer F. 1992. A revision of the Australian species of Parahebe and Derwentia (Scrophulariaceae). – Telopea 5: 241-287.
Briggs BG, Ehrendorfer F. 2006a. New Australian species and typifications in Veronica sens. lat. (PLantaginaceae). – Telopea 11: 276-292.
Briggs BG, Ehrendorfer F. 2006b. Chromosome numbers of Australian and New Guinean species of Veronica (Plantaginaceae). – Telopea 11: 294-298.
Briquet J. 1889. Notes sur quelques labiées américaines. – Bull. Soc. Bot. Genève 5: 108-121.
Briquet J. 1891. Les Labiées des Alpes Maritimes 1-3. – Georg & Co., Genève et Bâle.
Briquet J. 1894. Fragmenta amonographiae Labiatarum III. Sur un singulier Hyptis brésilien. – Bull Herb. Boissier 2: 715-719.
Briquet J. 1895. Phrymaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien IV(3b), W. Engelmann, Leipzig, pp. 361-362.
Briquet J. 1896. Fragmenta monographiae Labiatarum IV. Labiatae americanae Kuntzenae. – Bull. Herb. Boissier 4: 785-808.
Briquet J. 1897a. Verbenaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien IV(3a), W. Engelmann, Leipzig, pp. 132-182, 377-379.
Briquet J. 1897b. Labiatae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien IV(3a), W. Engelmann, Leipzig, pp. 183-375, 379-380.
Briquet J. 1897c. Contributions à la flore du Paraguay VII. Labiées. – Mem. Soc. Phys. Hist. Nat. Genève 32, 10: 3-45.
Briquet J. 1898. Fragmenta monographiae Labiatarum V. Observations sur quelques labiées intéressante ou nouvelles principalment de l’Herbier Delessert. – Ann. Cons. Jard. Bot. Genève 2: 102-251.
Bromley GLR. 1984. Notes on two Brtazilian species of Lippia (Verbenaceae). – Kew Bull. 39: 805-806.
Brooker SE. 1972. Pharmacognostical studies in the genus Antirrhinum. – Ph.D. diss., University of Bradford. England.
Brooker SE, Doaigey AR, Harkiss KJ. 1976. Comparative anatomy of the aerial parts of Antirrhinum molle and A. mollissimum. – Planta Medica 30: 219-327.
Bruce AN. 1905. On the activity of the glands of Byblis gigantea 1. – Notes Roy. Bot. Gard. Edinb. 16: 9-14.
Bruce AN. 1907. On the activity of the glands of Byblis gigantea 2. – Notes Roy. Bot. Gard. Edinb. 17: 83.
Bruce EA. 1953a. Notes on African Pedaliaceae. – Kew Bull. 1953: 417-429.
Bruce EA. 1953b. Pedaliaceae. – In: Turrill WB, Milne-Redhead E (eds), Flora of tropical East Africa, The Crown Agents for the Colonies, London, pp. 1-23.
Brugger J, Rutishauser R. 1989. Bau und Entwicklung landbewohnender Utricularia-Arten. – Bot. Helvetica 99: 91-146.
Brühl P. 1920. On the systematic position of Lindenbergia, Lehmann. – J. Dept. Sci. Univ. Calcutta, Bot. 2: 11-16.
Brullo S, Pavone P, Terrasi MC. 1985. Considerazioni cariologiche sul genere Plantago in Sicilia. – Candollea 40: 217-230.
Brummitt RK. 1979 [1980].Tab. 788. Fittonia albivenis (Acanthaceae). – Bot. Mag. 182: 156-168.
Brummitt RK. 1985. Additions to the tropical African species of Isoglossa (Acanthaceae). – Kew Bull. 40: 785-791.
Brummitt RK. 1989. Against separating Mendonciaceae from Acanthaceae. – Acanthus 5: 1-3.
Brummitt RK. 1990a. Two anisophyllous species of Justicia (Acanthaceae) from East Africa. – Kew Bull. 45: 281-285.
Brummitt RK. 1990b. Anomacanthus R. Good replaces Gilletiella De Wild. & T. Durand (Acanthaceae). – Kew Bull. 45: 710.
Brummitt RK. 1992. Santisukia, a new generic name in Bignoniaceae. – Kew Bull. 47: 436.
Brummitt RK, Seyani JH. 1987. A revision of the species related to Plectranthus stenophyllus (Labiatae) in Malawi. – Kew Bull. 42: 687-699.
Bruni A, Modenesi P. 1983. Development, oil storage and dehiscence of peltate trichomes in Thymus vulgaris (Lamiaceae). – Nord. J. Bot. 3: 245-251.
Budantsev AG. 1987. The system of the genus Dracocephalum (Lamiaceae). – Bot. Žurn. 72: 260-267.
Budantsev AG. 1993. A synopsis of the genus Nepeta (Lamiaceae). – Bot. Žurn. 78: 93-107.
Budantsev AG, Lobova TA. 1997. Fruit morphology, anatomy and taxonomy of tribe Nepeteae (Labiatae). – Edinburgh J. Bot. 54: 183-216.
Bueno OL, Leonhardt C. 2011. Distribuição e potencial paisagístico dos gêneros Citharexylum L. e Verbenoxylum Tronc. no Rio Grande do Sul, Brasil. – Iheringia. Sér. Bot. 66: 47-60.
Buhk N, Zhao L, Li H, Albach DC. 2015. Molecular systematics and morphometrics in Veronica subsect. Canae (Plantaginaceae). – Plant Syst. Evol. 301: 1967-1979.
Bunge A. 1873. Labiatae Persicae. – Mém. Acad. Sci. Petersb. Sér. 7(21): 40-53.
Bunsawat J, Elliott NE, Hertweck KL, Sproles E, Alice LA. 2004. Phylogenetics of Mentha (Lamiaceae): evidence from chloroplast DNA sequences. – Syst. Bot. 29: 959-964.
Bunting GS, Duke A. 1961. Sanango: new Amazonian genus of Loganiaceae. – Ann. Missouri Bot. Gard. 48: 269-274.
Burelo-Ramos CM, Lorea-Hernandez FG, Vovides AP. 2009. Palynological survey of subtribe Pithecocteniinae (Bignonieae, Bignoniaceae). – Bot. J. Linn. Soc. 159: 155-162.
Burger WC, BarringerK. 2000. Flora Costaricensis: 41. Schlegeliaceae Reveal. – Fieldiana Bot., N. S., pp. 69-77.
Burkhart A. 1939. El mecanismo floral de la Labiada Hyptis mutabilis (Rich.) Briq. y su convergencia hacia la flor papilionoidea. – Darwiniana 3: 425-427.
Burkill IH, Wright CH. 1899. On some African Labiatae with alternate leaves. – Bot. J. Linn. Soc. 34: 268-276.
Burtt BL. 1954. Studies in the Gesneriaceae of the Old World I. General introduction. – Notes Roy. Bot. Gard. Edinb. 21: 185-192.
Burtt BL. 1956a. Studies in the Gesneriaceae of the Old World X. The genus Beccarinda. – Notes Roy. Bot. Gard. Edinb. 22: 61-64.
Burtt BL. 1956b. An independent genus for Oreocharis primuloides. – Baileya 4: 160-162.
Burtt BL. 1958a. Studies in the Gesneriaceae of the Old World XI. The genus Ornithoboea. – Notes Roy. Bot. Gard. Edinb. 22: 287-299.
Burtt BL. 1958b. Studies in the Gesneriaceae of the Old World XIII. Miscellaneous transfers and reductions. – Notes Roy. Bot. Gard. Edinb. 22: 309-314.
Burtt BL. 1958c. Studies in the Gesneriaceae of the Old World XV. The genus Saintpaulia. – Notes Roy. Bot. Gard. Edinb. 22: 547-568.
Burtt BL. 1962. Studies in the Gesneriaceae of the Old World XXIII. Rhynchoglossum and Klugia. – Notes Roy. Bot. Gad. Edinb. 24: 167-171.
Burtt BL. 1963. Studies in the Gesneriaceae of the Old World XXIV. Tentative keys to the tribes and genera. – Notes Roy. Bot. Gard. Edinb. 24: 205-220.
Burtt BL. 1964. Studies in the Gesneriaceae of the Old World XXV. Additional notes on Saintpaulia. – Notes Roy. Bot. Gard. Edinb. 25: 191-195.
Burtt BL. 1965. The transfer of Cyrtandromoea from Gesneriaceae to Scrophulariaceae, with notes on the classification of that family. – Bull. Bot. Surv. India 7: 73-88.
Burtt BL. 1968. Studies in the Gesneriaceae of the Old World XXIX. A reconsideration of generic limits in tribe Trichosporeae. – Notes Roy. Bot. Gard. Edinb. 28: 219-225.
Burtt BL. 1970a. Studies in the Gesneriaceae of the Old World XXXI. Some aspects of functional evolution. – Notes Roy. Bot. Gard. Edinb. 30: 1-10.
Burtt BL. 1970b. Studies in the Gesneriaceae of the Old World XXXIII. Some species of Cyrtandra, chiefly Bornean. – Notes Roy. Bot. Gard. Edinb. 30: 23-42.
Burtt BL. 1971. Studies in the Gesneriaceae of the Old World XXXIV. A miscellany from South Eastern Asia. – Notes Roy. Bot. Gard. Edinb. 31: 35-52.
Burtt BL. 1974. Patterns of structural change in the flowering plants. – Trans. Bot. Soc. Edinb. 42: 133-142.
Burtt BL. 1975. Studies in the Gesneriaceae of the Old World XL. The genus Loxostigma. – Notes Roy. Bot. Gard. Edinb. 34: 101-105.
Burtt BL. 1976. Studies in the Gesneriaceae of the Old World XLI. Notes on Boeica and Didissandra. – Notes Roy. Bot. Gard. Edinb. 35: 369-374.
Burtt BL. 1977. Classification above the genus, as exemplified by Gesneriaceae, with parallels from other groups. – Plant Syst. Evol. [Suppl.] 1: 97-109.
Burtt BL. 1978. Studies in the Gesneriaceae of the Old World XLV. A preliminary revision of Monophyllaea. – Notes Roy. Bot. Gard. Edinb. 37: 1-59.
Burtt BL. 1984. Studies in the Gesneriaceae of the Old World XLVII. Revised generic concepts for Boea and its allies. – Notes Roy. Bot. Gard. Edinb. 41: 401-452.
Burtt BL. 1990. Gesneriaceae of the Old World I. New and little-known species of Cyrtandra from Malaysia. – Edinburgh J. Bot. 47: 201-233.
Burtt BL. 1997. Old World Gesneriaceae V. Suprageneric names. – Edinburgh J. Bot. 54: 85-90.
Burtt BL. 1998a. Taxonomic history of Didymocarpus and Henckelia (Gesneriaceae). – Beitr. Biol. Pflanzen 70: 365-375.
Burtt BL. 1998b. New species of phytogeographical interest in Beccarinda and Henckelia (Gesneriaceae). – Beitr. Biol. Pflanzen 70: 377-382.
Burtt BL. 1998c. Climatic accommodation and phytogeography of the Gesneriaceae of the Old World. – In: Mathew P, Sivadasan M (eds), Diversity and taxonomy of tropical flowering plants, Mentor Books, Kerala, Calicut, India, pp. 1-27.
Burtt BL. 1999. Old World Gesneriaceae VI. Six miscellaneous notes. – Edinburgh J. Bot. 56: 371-379.
Burtt BL. 2001a. Kaisupeea: a new genus of Gesneriaceae centred in Thailand. – Nord. J. Bot. 21: 115-119.
Burtt BL. 2001b. Flora of Thailand: annotated checklist of Gesneriaceae. – Thai Forest Bull. 29: 81-102.
Burtt BL. 2002. A survey of the genus Cyrtandra (Gesneriaceae). – Phytomorphology Golden Jubilee Issue 2001: 393-401.
Burtt BL, Bokhari M. 1973. Studies in the Gesneriaceae of the Old World XXXVI. Foliar sclereids in New Guinea and Pacific Cyrtandra. – Notes Roy. Bot. Gard. Edinb. 32: 397-402.
Burtt BL, Smith RM. 1965. Cosmianthemum: a Bornean genus of Acanthaceae. – Notes Roy. Bot. Gard. Edinb. 26: 365-381.
Burtt BL, Tan K. 1984. Studies in the Gesneriaceae of the Old World XLVIII: calcium accumulation and excretion in Paraboea. – Notes Roy. Bot. Gard. Edinb. 41: 453-456.
Burtt BL, Wiehler H. 1995. Classification of the family Gesneriaceae. – Gesneriana 1: 1-4.
Burtt BL, Woods PJB. 1975. Studies in the Gesneriaceae of the Old World XXXVIII: towards a revision of Aeschynanthus. – Notes Roy. Bot. Gard. Edinb. 33: 471-490.
Buurman J. 1978. Contribution to the pollen morphology of the Bignoniaceae, with special reference to the tricolpate type. – Pollen Spores 19: 447-519.
Byragi Reddy T, Subba Reddi C. 1994. Pollination ecology of Vitex negundo (Verbenaceae). – Proc. Indian Natl. Sci. Acad., Sect. B, 60: 57-66.
Call VB, Dilcher DL. 1992. Investigations of angiosperms from the Eocene of southwestern North America: samaras of Fraxinus wilcoxiana Berry. – Rev. Palaeobot. Palynol. 74: 249-266.
Callmander MW, Phillipson PB. 2011. Four new species of the endemic genus Rhodocolea Baill. (Bignoniaceae). – Adansonia, ser. 3, 33: 311-321.
Callmander MW, Phillipson PB. 2012. Two new species in the genus Colea Bojer ex Meisn. (Bignoniaceae) endemic to Madagascar. – Adansonia, sér. 3, 34: 115-122.
Callmander MW, Phillipson PB. 2015. The transfer of the Malagasy species of Strobilanthes Blume to Acanthopale C. B. Clarke (Acanthaceae). – Candollea 70: 253-256.
Callmander MW, Phillipson PB, Razanajatovo M, Nusbaumer L. 2011. The genus Ophiocolea (Bignoniaceae) in northern Madgascar with description of four new species and two lectotypifications. – Candollea 66: 133-142.
Callmander MW, Phillipson PB, Schatz GE. 2012. Two new species of the genus Stereospermum (Bignoniaceae) from Madagascar. – Novon 22: 141-147.
Callmander MW, Phillipson PB, Schatz GE. 2014. Towards a revision of the genus Vitex L. (Lamiaceae) in Madagascar I: a distinctive new species from North-eastern Madagascar. – Candollea 69: 141-147.
Callmander MW, Phillipson PB, Plunkett GM, Edwards MB, Buerki S. 2016. Generic delimitations, biogeography and evolution in the tribe Coleeae (Bignoniaceae), endemic to Madagascar and the smaller islands of the western Indian Ocean. – Mol. Phylogen. Evol. 96: 178-186.
Camp WH, Monachino J. 1939. Two new Linocieras and a review of the Antillean species. – Lloydia 2: 219-224.
Campbell DH. 1930. The relationships of Paulownia. – Bull. Torrey Bot. Club 57: 47-50.
Canne JH. 1979. A light and scanning electron microscope study of seed morphology in Agalinis (Scrtophulariaceae) and its taxonomic significance. – Syst. Bot. 4: 281-296.
Canne JH. 1980. Seed surface features in Aureolaria, Brachystigma, Tomanthera, and certain South American Agalinis (Scrophulariaceae). – Syst. Bot. 5: 241-252.
Canne-Hilliker JM. 1987. Patterns of floral development in Agalinis and allies (Scrophulariaceae) II. Floral development of Agalinis densiflora. – Amer. J. Bot. 74: 1419-1430.
Cantino PD. 1982a. Affinities of the Lamiales: a cladistic analysis. – Syst. Bot. 7: 237-248.
Cantino PD. 1982b. A monograph of the genus Physostegia (Labiatae). – Contr. Gray Herb. 211: 1-105.
Cantino PD. 1985a. Facultative autogamy in Synandra hispidula (Labiatae). – Castanea 50: 105-111.
Cantino PD. 1985b. Chromosome studies in subtribe Melittidinae (Labiatae) and systematic implications. – Syst. Bot. 10: 1-6.
Cantino PD. 1990. The phylogenetic significance of stomata and trichomes in the Labiatae and Verbenaceae. – J. Arnold Arbor. 71: 323-370.
Cantino PD. 1991. Conspecific status of Cardioteucris cordifolia (Labiatae) and Caryopteris siccanea (Verbenaceae). – Taxon 40: 441-443.
Cantino PD. 1992a. Toward a phylogenetic classification of the Labiatae. – In: Harley RM, Reynolds T (eds), Advances in labiate science, Royal Botanic Gardens, Kew, pp. 27-37.
Cantino PD. 1992b. Evidence for a polyphyletic origin of the Labiatae. – Ann. Missouri Bot. Gard. 79: 361-379.
Cantino PD. 1998. Caryopteris (Lamiaceae) and the conflict between phylogenetic and pragmatic considerations in botanical nomenclature. – Syst. Bot. 23: 369-386.
Cantino PD. 2004. Phrymaceae. – In: Kubitzki K, Kadereit JW (eds), The families and genera of vascular plants VII. Flowering plants. Dicotyledons. Lamiales (except Acanthaceae including Avicenniaceae), Springer, Berlin, Heidelberg, New York, pp. 323-326.
Cantino PD, Abu-Asab MS. 1993. A new look at the enigmatic genus Wenchengia (Labiatae). – Taxon 42: 339-344.
Cantino PD, Sanders RW. 1986. Subfamilial classification of Labiatae. – Syst. Bot. 11: 163-185.
Cantino PD, Doroszenko A. 1998. Obtegomeria (Lamiaceae), a new genus from South America. – Novon 8: 1-3.
Cantino PD, Wagstaff SJ. 1998. A re-examination of North American Satureja s.l. (Lamiaceae) in light of molecular evidence. – Brittonia 50: 63-70.
Cantino PD, Harley RM, Wagstaff SJ. 1992. Genera of Labiatae, status and classification. – In: Harley RM, Reynolds T (eds), Advances in labiate science, Royal Botanic Gardens, Kew, pp. 511-522.
Cantino PD, Olmstead RG, Wagstaff SJ. 1997. A comparison of phylogenetic nomenclature with the current system: a botanical case study. – Syst. Biol. 46: 313-331.
Cantino PD, Wagstaff SJ, Olmstead RG. 1998. Caryopteris (Lamiaceae) and the conflict between phylogenetic and pragmatic considerations in botanical nomenclature. – Syst. Bot. 23: 369-386.
Capuron R. 1970. Deux nouvelles Bignoniacées. – Adansonia, sér. 2, 10: 501-506.
Carine MA. 1999. A systematic study of the southern Indian and Sri Lankan Strobilanthinae (Acanthaceae). – Ph.D. diss., University of Oxford, United Kingdom.
Carine MA, Scotland RW. 1999. Pollen morphology of Strobilanthes Blume (Acanthaceae) from southern India and Sri Lanka. – Rev. Palaeobot. Palynol. 103: 143-165.
Carine MA, Scotland RW. 2000. The taxonomy and biology of Stenosiphonium Nees (Acanthaceae). – Bot. J. Linn. Soc. 133: 101-128.
Carine MA, Scotland RW. 2002. Classification of Strobilanthinae (Acanthaceae): trying to classify the unclassifiable? – Taxon 51: 259-279.
Carine MA, Jayasekara P, Scotland RW. 2000. A new species of Strobilanthes Blume (Acanthaceae) from Sri Lanka. – Kew Bull. 55: 971-976.
Carine MA, Alexander JM, Scotland RW. 2004. A revision of the Strobilanthes kunthiana-group (Phlebophyllum sensu Bremekamp) (Acanthaceae). – Kew Bull. 59: 1-25.
Carlquist SJ. 1970. Wood anatomy of insular species of Plantago and the problem of raylessness. – Bull. Torrey Bot. Club 97: 353-361.
Carlquist SJ. 1976. Wood anatomy of Byblidaceae. – Bot. Gaz. 137: 35-38.
Carlquist SJ. 1981. Wood anatomy of Chloanthaceae (Dicrastylidaceae). – Aliso 10: 19-34.
Carlquist SJ. 1986. Wood anatomy of Stilbaceae and Retziaceae: ecological and systematic implications. – Aliso 11: 299-316.
Carlquist SJ. 1987. Wood anatomy of Martyniaceae and Pedaliaceae. – Aliso 11: 473-483.
Carlquist SJ. 1992a. Wood anatomy of sympetalous dicotyledon families: a summary, with comments on systematic relationships and evolution of the wood habit. – Ann. Missouri Bot. Gard. 79: 303-332.
Carlquist SJ. 1992b. Wood anatomy of Lamiaceae. A survey with comments on vascular and vasicentric tracheids. – Aliso 13: 309-338.
Carlquist SJ. 1997. Wood anatomy of Buddlejaceae. – Aliso 15: 41-56.
Carlquist SJ, Hoekman DA. 1986a. Wood anatomy of Gesneriaceae. – Aliso 11: 279-297.
Carlquist SJ, Hoekman DA. 1986b. Wood anatomy of Myoporaceae: ecological and systematic considerations. – Aliso 11: 317-334.
Carlquist SJ, Webb A-A. 1964. Leaf anatomy as an indicator of Salvia apiana-mellifera introgression. – Aliso 5: 437-449.
Carlquist SJ, Zona S. 1988. Wood anatomy of Acanthaceae: a survey. – Aliso 12: 201-227.
Carlson EM, Stuart BC. 1936. Development of spores and gametophytes in certain New World species of Salvia. – New Phytol. 35: 68-91.
Carlson MC. 1957. Monograph of the genus Russelia (Scrophulariaceae). – Fieldiana Bot. 29(4), Natural History Museum, Chicago.
Carnicero P, Sáez L, Garcia-Jacas N, Galbany-Casals M. 2017. Different speciation types meet in a Mediterranean genus: the biogeographic history of Cymbalaria (Plantaginaceae). – Taxon 66: 393-407.
Caro JA. 1956. Las especies de Duranta (Verbenaceae) silvestres y cultivadas en la República Argentina. – Rev. Argentina Agron. 23: 1-28.
Caro JA. 1982. Sistematizacion del género Acantholippia Grisebach (Verbenaceae) y las especies de la Flora Argentina. – Dominguezia 3: 1-31.
Carr DE, Dudash MR. 1996. Inbreeding depression in two species of Mimulus (Scrophulariaceae) with contrasting mating systems. – Amer. J. Bot. 83: 586-593.
Carr DE, Fenster CB, Dudash MR. 1997. The relationship between mating-system characters and inbreeding depression in Mimulus guttatus. – Evolution 51: 363-372.
Carrick J. 1977. Studies in Australian Lamiaceae 2. Eichlerago, a new genus allied to Prostanthera. – J. Adelaide Bot. Gard. 1: 115-122.
Carter S. 1962. Revision of Xylocalyx Balf. f. (Scrophulariaceae). – Kew Bull. 16: 147-152.
Cartier D. 1965. Caryologie des plantains de la section Oreades Decne. – Compt. Rend. Acad. Sci. Paris 261: 4475-4478.
Cartier D. 1971. Étude biosystématique de quelques espèces du genre Plantago (Tourn.) L. (sections Coronopus DC. et Oreades Decne.) I. Historique, races chromosomiques du Plantago alpina L. et du Plantago serpentina All. – Rev. Gén. Bot. 78: 493-556.
Casper SJ. 1963. “Systematisch massgebende”: Merkmale für die Einordnung der Lentibulariaceen in das System. – Österr. Bot. Zeitschr. 110: 108-131.
Casper SJ. 1966. Monographie der Gattung Pinguicula L. – Bibl. Bot. 127/128: 1-209.
Casper SJ. 1987. On Pinguicula lignicola, an epiphytic heterophyllic member of the Lentibulariaceae in Cuba. – Plant Syst. Evol. 155: 349-354.
Casper SJ. 2003. Two new Pinguicula species (Lentibulariaceae) from east Cuba (Cuba oriental). – Haussknechtia 9: 141-155.
Casper SJ. 2004. Two new Pinguicula species (Lentibulariaceae; P. benedicta-group) from the eastern mountain range of Cuba (Greater Antilles) with reddish flowers. – Wulfenia 11: 1-13.
Casper SJ. 2007. Pinguicula lippoldii nova spec. and Pinguicula toldensis nova spec. – two endemic Pinguicula species (Lentibulariaceae) from East Cuba new to science. – Wulfenia 14: 75-96.
Casper SJ, Manitz H. 1975. Beiträge zur Taxonomie und Chorologie der mitteleuropäischen Utricularia-arten 2. – Feddes Repert. 86: 211-232.
Casper SJ, Stimper R. 2009. Chromosome numbers in Pinguicula (Lentibulariaceae): survey, atlas, and taxonomic conclusions. – Plant Syst. Evol. 277: 21-60.
Casper SJ, Urquiola Cruz AJ. 2003. Pinguicula cubensis (Lentibulariaceae), a new insectivorous species from western Cuba (Cuba occidental). – Willdenowia 33: 167-172.
Cavagnetto C, Anadón P. 1996. Preliminary
palynological data on floristic and climatic changes during the Middle
Eocene-Early Oligocene of the eastern Ebro Basin, northeast Spain. – Rev.
Palaeobot. Palyn. 92: 281-305.
Cedeño-Maldonado JA, O’Reilly RG. 1996. Nashia inaguensis Millsp. (Verbenaceae): new records for the floras of St. Croix and Puerto Rico. – Caribbean J. Sci. 32: 115-116.
Čelakovsky L. 1883. Über einige Arten der Gattung Teucrium. – Bot. Centralbl. 14: 187-190.
Celenk S, Tarimcilar G, Bicakci A, Kaynak G, Malyer H. 2008. A palynological study of the genus Mentha L. (Lamiaceae). – Bot. J. Linn. Soc. 157: 141-154.
Celenk S, Dirmenci T, Malyer H, Bicakci A. 2008. A palynological study of the genus Nepeta L. (Lamiaceae). – Plant Syst. Evol. 276: 105-123.
Chadwell TB, Wagstaff SJ, Cantino PD. 1992. Pollen morphology of Phryma and some putative relatives. – Syst. Bot. 17: 210-219.
Chambers HL. 1961. Chromosome numbers and breeding systems in Pycnanthemum (Labiatae). – Brittonia 13: 116-128.
Champluvier D. 1994. Brachystephanus myrmecophilus (Acanthaceae), espèce nouvelle du Zaïre oriental: un cas intéressant de myrmécophilie. – Belg. J. Bot. 127: 45-60.
Champluvier D. 1999. Un Pseudocalyx (Acanthaceae) nouveau du Kasai (Congo-Kinshasa). – Syst. Geogr. Plants 69: 199-204.
Champluvier D. 2000. A new species of Rungia (Acanthaceae) from Cameroon. – Syst. Geogr. Plants 70: 137-140.
Champluvier D. 2002a. Contribution à l’étude du genre Pseuderanthemum (Acanthaceae) en Afrique tropicale. – Syst. Geogr. Plants 72: 33-53.
Champluvier D. 2002b. A new and an unrecognized species of Justicia (Acanthaceae, Justiciinae) from Kwango and Katanga (R. D. Congo). – Syst. Geogr. Plants 72: 231-235.
Champluvier D, Senterre B. 2010. New taxa in Crossandrella, Dischistocalyx and Ascotheca (Acanthaceae) from Equatorial Guinea. – Plant Ecol. Evol. 143: 181-190.
Champluvier D, Parmentier I, Ngok L, Lejoly J. 2003. New taxa in Acanthaceae from Inselbergs and rainforest in Equatorial Guinea and Gabon. – Syst. Geogr. Plants 73: 209-214.
Chang H-T. 1951. A review of the Chinese species of Callicarpa. – Acta Phytotaxon. Sin. 1: 269-312. [In Chinese]
Chantaranothai P, Koomgratok S, Simpson DA. 2004. Taxonomic notes on some Southeast Asian species of Vitex (Lamiaceae). – Kew Bull. 59: 319-320.
Chatha GS, Bir SS. 1988. Cytology and distribution pattern of woody species of Verbenaceae in Palm Hills. – Proc. Indian Acad. Sci., Plant Sci. 98: 139-148.
Chau JH, O’Leary N, Sun W-B, Olmstead RG. 2017. Phylogenetic relationships in tribe Buddlejeae (Scrophulariaceae) based on multiple nuclear and plastid markers. – Bot. J. Linn. Soc. 184: 137-166.
Chaubal PD. 1966. Palynological studies in the family Acanthaceae of Western Ghats (India). – Poona.
Chautems A. 1984. Révision taxonomique d’un genre de Gesneriaceae endémique du Brésil: Nematanthus Schrader. – Candollea 39: 297-300.
Chautems A. 1988. Révision taxonomique et possibilités d’hybridations de Nematanthus Schrader (Gesneriaceae), genre endémique de la fôret côtière brésilienne. – Thesis, Botanique 112: 1-126.
Chautems A. 1990. Taxonomic revision of Sinningia Nees (Gesneriaceae): nomenclatural changes and new synonymies. – Candollea 45: 381-388.
Chautems A. 1991a. Taxonomic revision of Sinningia Nees (Gesneriaceae) II: new species from Brazil. – Candollea 46: 411-425.
Chautems A. 1991b. A familia Gesneriaceae na região cacaueira do Brasil. – Rev. Bras. Bot. 14: 51-59.
Chautems A. 1995. Taxonomic revision of Sinningia Nees (Gesneriaceae) III: new species from Brazil and new combinations. – Gesneriana 1: 8-14.
Chautems A. 1997. New Gesneriaceae from São Paulo, Brazil. – Candollea 52: 159-169.
Chautems A. 2000. Introducing Sinningia nordestina: an unusual annual new species from northeastern Brazil. – Gloxinian 50: 18-21.
Chautems A. 2002. New Gesneriaceae from Minas Gerais, Brazil. – Candollea 52: 261-279.
Chautems A, Weber A. 1999. Shoot and inflorescence architecture in the neotropical genus Sinningia (Gesneriaceae). – In: Kurmann MH, Hemsley AR (eds), The evolution of plant architecture, Royal Botanic Gardens, Kew, pp. 305-322.
Chautems A, Baracho GS, Sigueira Filho JA. 2000. A new species of Sinningia (Gesneriaceae) from northeastern Brazil. – Brittonia 52: 49-53.
Chen C, Cai M-Q, Xu B, Jin X-J, Wang R-H, Li P, Zhao Y-P, Fu C-X. 2017. Systematic position of Oreosolen (tribe Scrophularieae, Scrophulariaceae) based on nuclear and plastid sequences. – J. Syst. Evol. 55: 446-542.
Chen G, Sun W-B, Sun H. 2007. Ploidy variation in Buddleja L. (Buddlejaceae) in the Sino-Himalayan region and its biogeographical implications. – Bot. J. Linn. Soc. 154: 305-312.
Chen J-Y, Zhang Z-S, Hong D-Y. 2009. A taxonomic revision of the Syringa pubescens complex (Oleaceae). – Ann. Missouri Bot. Gard. 96: 237-250.
Chen S-T, Zhou Z-K, Guan K-Y, Nakata M. 2004. Karyomorphology of Incarvillea (Bignoniaceae) and its implications in distribution and taxonomy. – Bot. J. Linn. Soc. 144: 113-121.
Chen S-T, Guan K-Y, Zhou Z-K, Olmstead R, Cronk QCB. 2005. Molecular phylogeny of Incarvillea (Bignoniaceae) based on ITS and trnL-F sequences. – Amer. J. Bot. 92: 625-633.
Chen W-H, Moller M, Shui Y-M, Zhang M-D. 2009. A new species of Paraboea (Gesneriaceae) from a karst cave in Guangxi, China, and observations on variations in flower and inflorescence architecture. – Bot. J. Linn. Soc. 158: 681-688.
Chen W-H, Shui Y-M, Sima Y-K, Zhang R-M, Wei Z-D. 2009. Pararuellia glomerata (Acanthaceae), a new species from Yunnan, China. – Bot. Stud. 50: 261-267.
Chen W-H, Möller M, Shui Y-M, Wang H, Yang
J-B, Li G-Y. 2014. Three new species of Petrocodon (Gesneriaceae), endemic to the
limestone areas of Southwest China, and preliminary insights into the
diversification patterns of the genus. – Syst. Bot. 39: 316-330.
Chen Y-P, Li B, Olmstead RG, Cantino PD, Liu
E-D, Xiang C-L. 2014. Phylogenetic placement of the enigmatic genus
Holocheila (Lamiaceae)
inferred from plastid DNA sequences. – Taxon 63: 355-366.
Chen Y-P, Drew BT, Li B, Soltis DE, Soltis PS, Xiang C-L. 2016. Resolving the phylogenetic position of Ombrocharis (Lamiaceae), with reference to the molecular phylogeny of tribe Elsholtzieae. – Taxon 65: 123-136.
Chen Z. 1983. Preliminary study on the pollen morphology of Paulownia. – J. Wuhan Bot. Res. 1: 144-146. [In Chinese]
Chen Z. 1986. Suggestion on the classification of the genus Paulownia. – J. Huazhong Agricult. 5: 261-265. [In Chinese]
Chiang F, Frame D. 1987. The identity of Lithophytum (Loganiaceae, Plocospermeae). – Brittonia 39: 260-262.
Chinnock RJ. 2007. Eremophila and allied genera. A monograph of the plant family Myoporaceae. – Rosenberg Publ. Pty Ltd, Dural Delivery Centre, New South Wales.
Christenhusz MJM. 2012. On African violets and Cape primroses – towards a monophyletic Streptocarpus (Gesneriaceae). – Phytotaxa 46: 3-9.
Christie F, Mendum M. 2002. The ontogeny of Aeschynanthus seeds – a comparative study using scanning electron microscopy. – Bot. J. Linn. Soc. 138: 197-207.
Chu H. 1991. A revision of the Andrographis (Acanthaceae) of China. – Bull. Bot. Res. Harbin 11: 45-48.
Chuang T-I, Heckard LR. 1982. Chromosome numbers of Orthocarpus and related monotypic genera (Scrophulariaceae: subtribe Castillejinae). – Brittonia 34: 89-101.
Chuang T-I, Heckard LR. 1983. Systematic significance of seed-surface features in Orthocarpus (Scrophulariaceae-subtribe Castillejinae). – Amer. J. Bot. 70: 877-890.
Chuang T-I, Heckard LR. 1991. Generic realignment and synopsis of subtribe Castillejinae (Scrophulariaceae-tribe Pediculareae). – Syst. Bot. 16: 644-666.
Chuang T-I, Heckard LR. 1992a. Chromosome numbers of some North American Scrophulariaceae, mostly Californian. – Madroño 39: 137-149.
Chuang T-I, Heckard LR. 1992b. New species of bee-pollinated Castilleja from Peru, with a taxonomic revision of South American members of subg. Colacus. – Syst. Bot. 17: 417-431.
Chuang T-I, Heckard LR. 1992c. A taxonomic revision of Orthocarpus (Scrophulariaceae-tribe Pediculareae). – Syst. Bot. 17: 570-582.
Chumchim N, McDade LA, Fisher AE. 2015. Phylogeny of Dyschoriste (Acanthaceae). – Aliso 33: 77-89.
Cieslak T, Polepalli JS, White A, Müller K, Borsch T, Barthlott W, Steiger J, Marchant A, Legrende L. 2005. Phylogenetic analysis of Pinguicula (Lentibulariaceae): chloroplast DNA sequences and morphology support several geographically distinct radiations. – Amer. J. Bot. 92: 1723-1736.
Citerne H, Cronk QCB. 1999. The origin of the peloric Sinningia. – New Plantsman 6: 219-222.
Citerne H, Möller M, Cronk QCB. 2000. Diversity of cycloidea-like genes in Gesneriaceae in relation to floral symmetry. – Ann. Bot. 86: 167-176.
Clark DV. 1971. Speciation in Penstemon. – Ph.D. diss., University of Montana, Missoula, Montana.
Clark JL. 2009. Systematics of
Glossoloma (Gesneriaceae). – Syst. Bot.
Monogr. 88: 1-128.
Clark JL, Zimmer EA. 2003. A preliminary phylogeny of Alloplectus (Gesneriaceae): implications for the evolution of flower resupination. – Syst. Bot. 28: 365-375.
Clark JL, Herendeen PS, Skog LE, Zimmer EA. 2006. Phylogenetic relationships and generic boundaries in the Episceae (Gesneriaceae) inferred from nuclear, chloroplast, and morphological data. – Taxon 55: 313-336.
Clark JL, Neill DA, Weber A, Gruhn JA, Katan T. 2010. Shuaria (Gesneriaceae), an arborescent new genus from the Cordillera del Cóndor and Amazonian Ecuador. – Syst. Bot. 35: 662-674.
Clark JL, Roalson EH, Pritchard RA, Coleman CL, Teoh V-H, Matos J. 2011. Independent origin of radial floral symmetry in the Gloxinieae (Gesnerioideae: Gesneriaceae) is supported by the rediscovery of Phineas pulchella in Cuba. – Syst. Bot. 36: 757-767.
Clark JL, Funke MM, Duffy AM, Smith JF. 2012. Phylogeny of a Neotropical clade in the Gesneriaceae: more tales of convergent evolution. – Intern. J. Plant Sci. 173: 894-916.
Clark JR, Holst BK, Skog LE. 2003. An annotated checklist of Gesneriaceae type specimens in the Marie Selby Botanical Gardens herbarium (SEL). – Selbyana 24: 119-140.
Clark JR, Wagner WL, Roalson EH. 2009. Patterns of diversification and ancestral range reconstruction in the Southeast Asian-Pacific angiosperm lineage Cyrtandra (Gesneriaceae). – Mol. Phylogen. Evol. 53: 982-994.
Clarke B. 1865. On the structure and affinities of Callitrichaceae. – J. Bot. 3: 36-39.
Clarke CB. 1902. Acanthaceae in Flora of Koh Chang. – Bot. Tidsskr. 24: 348.
Clarke CB. 1908. Acanthaceae. – J. Asiat. Soc. Bengal II, Nat. Hist. 74, Extra No.: 628-698.
Claßen-Bockhoff R. 2007. Floral construction and pollination biology in the Lamiaceae. – Ann. Bot. 100: 359-360.
Claßen-Bockhoff R, Wester P, Tweraser E. 2003. The staminal lever arm mechanism in Salvia – a review. – Plant Biol. 5: 33-41.
Claßen-Bockhoff R, Crone M, Baikova E. 2004. Stamen development in Salvia L.: homology reinvestigated. – Intern. J. Plant Sci. 165: 475-498.
Claßen-Bockhoff R, Speck T, Tweraser E, Wester P, Thimm S, Reith M. 2004. The staminal lever mechanism in Salvia L. (Lamiaceae): a key innovation for adaptive radiation? – Organisms Divers. Evol. 4: 189-205.
Clayberg CD. 1967. Chromosome numbers in Sinningia and Rechteineria (Gesneriaceae). – Baileya 15: 33-35.
Clayberg CD. 1968. Biosystematic studies in Sinningia and Rechsteineria Gesneriaceae). –Amer. J. Bot. 55: 829-833.
Clayberg CD. 1970. Cytology of interspecific hybrids in Sinningia and Rechsteineria (Gesneriaceae). – Can. J. Genet. Cytol. 12: 759-768.
Clayberg CD. 1996. Interspecific hybridisation in Sinningia (Gesneriaceae). – Baileya 23: 184-194.
Clebsch B. 1997. A book of salvias. – Timber Press, Portland, Oregon.
Clebsch B. 2008. The new book of salvias: sages for every garden. – Timber Press, Cambridge, United Kingdom.
Clements T. 1988. African violets. – David & Charles, London.
Cockayne L, Allan HH. 1926. The present taxonomic status of the New Zealand species of Hebe. – Trans. & Proc. New Zealand Inst. 57: 11-47.
Codd LE. 1975. Plectranthus (Labiatae) and allied genera in Southern Africa. – Bothalia 11: 371-442.
Cohn F. 1875. Über die Funktion der Blasen von Aldrovanda und Utricularia. – Beitr. Biol. Pflanzen 1: 71-92.
Cole MD. 1992. The significance of the terpenoids in the Labiatae. – In: Harley RM, Reynolds T (eds), Advances in labiate science, Royal Botanic Gardens, Kew, pp. 315-324.
Collevatti RG, Dornelas MC. 2016. Clues to the evolution of genome size and chromosome number in Tabebuia alliance (Bignoniaceae). – Plant Syst. Evol. 302: 601-607.
Collins LT. 1973. Systematics of Orobanche section Myzorrhiza (Orobanchaceae), with emphasis on Orobanche ludoviciana. – Ph.D. diss., University of Wisconsin, Milwaukee, Wisconsin.
Colwell AEL. 1994. Genome evolution in a non-photosynthetic plant, Conopholis americana. – Ph.D. diss., Washington University, St. Louis, Missouri.
Combrinck S, Du Plooy GW, McCrindle RI, Botha BM. 2007. Morphology and histochemistry of the glandular trichomes of Lippia scaberrima (Verbenaceae). – Ann. Bot. 99: 1111-1119.
Conn BJ. 1984. A taxonomic revision of Prostanthera Labill. section Klanderia (F. v. Muell) Benth. (Labiatae). – J. Adelaide Bot. Gard. 6: 207-348.
Conn BJ. 1992a. Relationships within the tribe Prostanthereae (Labiatae). – In: Harley RM, Reynolds T (eds), Advances in labiate science, Royal Botanic Gardens, Kew, pp. 55-64.
Conn BJ. 1992b. New species of Plectranthus and Westringia (Labiatae) from New South Wales. – Telopea 4: 643-648.
Conn BJ. 1992c. Status of the genus Eichlerago (Labiatae). – Telopea 4: 649-651.
Conn BJ. 1997. Four rare and/or threatened new species of Prostanthera Section Prostanthera (Labiatae) from New South Wales. – Telopea 7: 231-244.
Conn BJ. 1998. Contributions to the systematics of Prostanthera (Labiatae) in south-eastern Australia. – Telopea 7: 319-332.
Conn BJ. 1999. The Prostanthera cryptandroides-P. euphrasioides-P. odoratissima complex (Labiatae). – Telopea 8: 265-272.
Conn BJ. 2014. Ocimum L. (Lamiaceae) in Australia and Papua New Guinea. – Telopea 17: 169-181.
Conn BJ, Streiber N, Brown EA, Henwood MJ, Olmstead RG. 2009. Infrageneric phylogeny of Chloantheae (Lamiaceae) based on chloroplast ndhF and nuclear ITS sequence data. – Aust. Syst. Bot. 22: 243-256.
Conn BJ, Henwood MJ, Streiber N. 2011. Synopsis of the tribe Chloantheae and new nomenclatural combinations in Pityrodia s.lat. (Lamiaceae). – Aust. Syst. Bot. 24: 1-9.
Conran JG. 1996. The embryology and relationship of the Byblidaceae. – Aust. Syst. Bot. 9: 243-254.
Conran JG, Carolin R. 2004. Byblidaceae. – In: Kubitzki K, Kadereit JW (eds), The families and genera of vascular plants VII. Flowering plants. Dicotyledons. Lamiales (except Acanthaceae including Avicenniaceae), Springer, Berlin, Heidelberg, New York, pp. 45-49.
Conran JG, Christophel DC. 2004. A fossil Byblidaceae seed from Eocene South Australia. – Intern. J. Plant Sci. 165: 691-694.
Conran JG, Dowd JM. 1993. The phylogenetic relationships of the Byblis-Roridula (Byblidaceae-Roridulaceae) complex inferred from 18S rRNA partial sequences. – Plant Syst. Evol. 188: 73-86.
Conran JG, Lowrie A. 1993. Byblis liniflora subsp. occidentalis (Byblidaceae). A new subspecies of Byblis from north-western Australia. – Aust. Syst. Bot. 6: 175-179.
Conran JG, Houben A, Lowrie A. 2002. Chromosome numbers in Byblidaceae. – Aust. J. Bot. 50: 583-586.
Conran JG, Lowrie A, Moyle-Croft J. 2002. A revision of Byblis (Byblidaceae) in south-western Australia. – Nuytsia 15:11-20.
Contandriopoulos J. 1978. Contribution à l’étude cytotaxinomique des Sideritis section Empedoclea (Labiatae). – Plant Syst. Evol. 129: 277-289.
Contandriopoulos J, Cauwet A-M. 1968. À propos du Globularia fuxeensis Gir. des Pyrénées. – Nat. Monspel., Sér. Bot. 19: 29-35.
Contandriopoulos J, Quezel P. 1967. À propos des phénomènes de reviviscence chez Ramonda nathaliae. – Compt. Rend. 91. Congrès National Soc. Savvantes, Rennes 1996.
Cook CDK. 1978. The Hippuris syndrome. – In: Street HE (ed), Essays in plant taxonomy, Academic Press, London, pp. 163-176.
Cook MT. 1907. The embryology of Rhytidophyllum. – Bull. Torrey Bot. Club 34: 179-184.
Cooke E, Schively AF. 1904. Observations on the structure and development of Epiphegus virginiana. – Contr. Bot. Lab. Univ. Pennsylvania 2: 352-398.
Cooper DC. 1941. Macrosporogenesis and the development of the seed of Phryma leptostachya. – Amer. J. Bot. 28: 755-761.
Cooper RL, Osborn JM, Philbrick CT. 2000. Comparative pollen morphology and ultrastructure of the Callitrichaceae. – Amer. J. Bot. 87: 161-175.
Cornejo X, Bonifaz C. 2006. Forestiera ecuadorensis: una nueva specie endémica de Oleaceae y un nuevo registro genérico para Ecuador. – Brittonia 58: 78-82.
Côrtes ALA, Rapini A. 2013. Justicieae (Acanthaceae) do Semiárido do Estado da Bahia, Brasil. – Hoehnea 40: 253-292.
Côrtes ALA, Rapini A, Daniel TF. 2015. The Tetramerium lineage (Acanthaceae: Justicieae) does not support the Pleistocene Arc hypothesis for South American seasonally dry forests. – Amer. J. Bot. 102: 992-1007.
Côrtes ALA, Daniel TF, Rapini A. 2016. Taxonomic revision of the genus Schaueria (Acanthaceae). – Plant Syst. Evol. 302: 819-851.
Cosacov A, Sérsic AN, Sosa V, De-Nova JA, Nylinder S, Cocucci AA. 2009. New insights into the phylogenetic relationships, character evolution, and phytogeographic patterns of Calceolaria (Calceolariaceae). – Amer. J. Bot. 96: 2240-2255.
Cousins DJ (ed). 1994. Medicinal, essential oil, culinary herb and pesticidal plants of the Labiatae 1973-1993. – CAB International, Wallingford, United Kingdom.
Craib WG. 1911. Contributions to the Flora of Siam Acanthaceae. – Kew Bull. 1911: 434-441.
Cramer LH. 1989. The Hygrophila complex (Acanthaceae) in India and Ceylon. – Nord. J. Bot. 9: 261-263.
Cramer LH. 1996. Notes on Sri Lankan Acanthaceae. – Kew Bull. 51: 553-556.
Crété P. 1942. Recherches histologiques et physiologiques sur l’embryologie des Labiatiflores. Contribution à l’étude des formations haustoriales. – D.Sc. thesis, l’Université de Paris, Jouve & Cie, Paris.
Crété P. 1943. Recherches histologiques et physiologiques sur l’embryologie des Labiatiflores. Embryologie du Globularia vulgaris L. – Bull. Soc. Bot. France 90: 29-32, 36-39.
Crété P. 1949. Recherches embryologiques sur les Gesnériacées. Développement de l’albumen et de l’embryon chez Chirita lavandulacea Stapf. – Bull. Soc. Bot. France 96: 234-235.
Crété P. 1950a. Embryologie des Scrofulariacées. L’albumen et l’embryon chez les Nemesia. – Bull. Soc. Bot. France 97: 177-179.
Crété P. 1950b. Embryologie des Scrofulariacées. Développement de l’albumen chez les Nemesia. – Compt. Rend. Acad. Sci. Paris 231: 711-713.
Crété P. 1953. Embryologie des Scrofulariacées. Développement de l’albumen et de l’embryon chez le Digitalis purpurea L. – Phytomorphology 3: 168-172.
Crété P. 1954. Embryologie de l’Erinus alpinus L. (Scrofulariacées). Les relations entre les genres Erinus L. et Digitalis L. – Phytomorphylogy 4: 325-328.
Crété P. 1955a. L’origine du sac embryonnaire et le développement de l’albumen chez l’Alloplectus sanguineus Mart. (Gesnériacées). – Bull. Soc. Bot. France 102: 205-208.
Crété P. 1955b. L’application de certaines données embryologique à la systématique des Orobanchacées et de quelques familles voisines. – Phytomorphology 5: 422-435.
Crocker CW. 1860. Notes on the germination of certain species of Cyrtandreae. – J. Proc. Linn. Soc. Bot. 5: 65-66.
Cronk QCB. 2001 [2002]. Phylogenetic studies in Streptocarpus (Gesneriaceae): reconstruction of biogeographic history and distribution patterns. – Syst. Geogr. Plants 71: 545-555.
Cronk QCB, Kiehn M, Wagner WL, Smith JF. 2005. Evolution of Cyrtandra (Gesneriaceae) in the Pacific Ocean: the origin of a supertramp clade. – Amer. J. Bot. 92: 1017-1024.
Crosswhite FS, Kawano S. 1970. Pennellianthus (Scrophulariaceae) – a new genus of Japan and USSR. – Amer. Midl. Natur. 83: 358-367.
Crowley AC. 1985. Columnea choices. – The Gloxinian 35: 19-24.
Cruden RW, Hermanutz L, Shuttleworth J. 1984. The pollination biology and breeding system of Monarda fistulosa (Labiatae). – Oecologia 64: 104-110.
Cruden RW, Baker KK, Cullinan TE, Disbrow KA, Douglas KL, Erb JD, Kirsten KJ, Malik ML, Turner EA, Weier JA, Wilmot SR. 1990. The mating systems and pollination biology of three species of Verbena (Verbenaceae). – J. Iowa Acad. Sci. 97: 178-183.
Curto MA, Puppo P, Ferreira D, Nogueira M, Meimberg H. 2012. Development of phylogenetic markers from single-copy nuclear genes for multi locus, species level analyses in the mint family (Lamiaceae). – Mol. Phylogen. Evol. 63: 758-767.
Curto M, Schachtler C, Puppo P, Meimberg H. 2018. Using a new RAD-sequencing approach to study the evolution of Micromeria in the Canary islands. – Mol. Phylogen. Evol. 119: 160-169.
Dahlgren RMT, Rao VS. 1971. The genus Oftia Adans. and its systematic position. – Bot. Not. 124: 451-472.
Dahlgren RMT, Wyk AE van. 1988. Structure and relationships of families endemic to or centered in Southern Africa. – In: Goldblatt P, Lowry PP (eds), Modern systematic studies in African botany, Monogr. Syst. Bot. Missouri Bot. Gard. 25: 1-94.
Dahlgren RMT, Nielsen BJ, Goldblatt P, Rourke JP. 1979. Further notes on Retziaceae – its chemical contents and affinities. – Ann. Missouri Bot. Gard. 66: 545-556.
Dalgaard V. 1979. Biosystematics of the Macaronesian species of Scrophularia. – Opera Bot. 51: 1-64.
Damtoft S, Jensen SR. 1993. Tomentoside and 7-hydroxytomentoside, two iridoid glucosides from Paulownia tomentosa. – Phytochemistry 34: 1636-1638.
Damtoft S, Franzyk H, Jensen SR, Nielsen BJ. 1993. Iridoids and verbascosides in Retzia. – Phytochemistry 34: 239-243.
Damtoft S, Franzyk H, Jensen SR. 1994. Biosynthesis of iridoids in Forsythia spp. – Phytochemistry 37: 173-178.
Damtoft S, Franzyk H, Jensen SR. 1995. Biosynthesis of secoiridoids in Fontanesia. – Phytochemistry 38: 615-621.
Daniel M, Sabnis SD. 1979. Chemotaxonomy of Loganiaceae. – Curr. Sci. 48: 383-385.
Daniel TF. 1978. A new Mirandea (Acanthaceae) from Nuevo Leon, Mexico. – Syst. Bot. 3: 428-433.
Daniel TF. 1981. Mexacanthus, a new genus of Acanthaceae from western Mexico. – Syst. Bot. 6: 288-293.
Daniel TF. 1982. The genus Mirandea (Acanthaceae). – Contr. Univ. Michigan Herb. 15: 171-175.
Daniel TF. 1983a. Flora Neotropica. Monograph 34. Carlowrightia (Acanthaceae). – New York Botanical Garden, Bronx, New York.
Daniel TF. 1983b. Systematics of Holographis (Acanthaceae). – J. Arnold Arbor. 64: 129-160.
Daniel TF. 1984. Artificial interspecific hybridization of three species of Anisacanthus (Acanthaceae). – J. Arizona-Nevada Acad. Sci. 19: 85-88.
Daniel TF. 1986. Systematics of Tetramerium (Acanthaceae). – Syst. Bot. Monogr. 12: 1-134.
Daniel TF. 1988a. Taxonomic, nomenclatural, and reproductive notes on Carlowrightia (Acanthaceae). –Brittonia 40: 245-255.
Daniel TF. 1988b. Aphanosperma, a new genus of Acanthaceae from Mexico with unusual diasporas. – Amer. J. Bot. 75: 545-550.
Daniel TF. 1988c. A systematic study of Bravaisia DC. (Acanthaceae). – Proc. Calif. Acad. Sci. 45: 111-132.
Daniel TF. 1988d. Three new species of Holographis (Acanthaceae) from Mexico. – Proc. Calif. Acad. Sci. 46: 73-81.
Daniel TF. 1990a. Systematics of Henrya (Acanthaceae). – Contr. Univ. Michigan Herb. 17: 99-131.
Daniel TF. 1990b. New and reconsidered Mexican Acanthaceae III. Justicia. – Contr. Univ. Michigan Herb. 17: 133-137.
Daniel TF. 1991. A synopsis of Poikilacanthus (Acanthaceae) in Mexico. – Bull. Torrey Bot. Club 118: 451-458.
Daniel TF. 1993a. A synopsis of Lophostachys (Acanthaceae) in Mexico and Central America. – Selbyana 14: 64-70.
Daniel TF. 1993b. New and reconsidered Mexican Acanthaceae V. – Contr. Univ. Michigan Herb. 19: 271-291.
Daniel TF. 1995a. Revision of Odontonema (Acanthaceae) in Mexico. – Contr. Univ. Michigan Herb. 20: 147-171.
Daniel TF. 1995b. New and reconsidered Mexican Acanthaceae VI. Chiapas. – Proc. Calif. Acad. Sci. 48: 253-284.
Daniel TF. 1997. The Acanthaceae of California and the Peninsula of Baja California. – Proc. Calif. Acad. Sci. 49: 309-403.
Daniel TF. 1998. Pollen morphology of Mexican Acanthaceae: diversity and systematic significance. – Proc. Calif. Acad. Sci. 50: 217-256.
Daniel TF. 1999a. Revision of Spathacanthus (Acanthaceae). – Contr. Univ. Michigan Herb. 22: 33-46.
Daniel TF. 1999b. Revision of Stenostephanus (Acanthaceae) in Mexico. – Contr. Univ. Michigan Herb. 22: 47-93.
Daniel TF. 1999c. Taxonomic notes on Mexican Ruellia (Acanthaceae). – Brittonia 51: 124-127.
Daniel TF. 2000. Additional chromosome numbers of American Acanthaceae. – Syst. Bot. 25: 15-25.
Daniel TF. 2001. Streblacanthus monospermus (Acanthaceae), a genus and species new to the flora of Mexico. – Contr. Univ. Michigan Herb. 23: 139-144.
Daniel TF. 2003a. New and reconsidered Mexican Acanthaceae X. Flora del Bajío Region. – Novon 13: 37-48.
Daniel TF.2003b. A new combination in Mirandea (Acanthaceae). – Acta Bot. Mexicana 62: 9-13.
Daniel TF. 2003c. A reconsideration of Megalostoma (Acanthaceae), a new species, and recognition of a new section of Justicia. – Proc. Calif. Acad. Sci. 54: 371-380.
Daniel TF. 2004a. A synopsis of Justicia section Mesoamericanae (Acanthaceae). – Proc. Calif. Acad. Sci. 55: 174-183.
Daniel TF. 2004b. Acanthaceae of Sonora: taxonomy and phytogeography. – Proc. Calif. Acad. Sci. 55: 690-805.
Daniel TF. 2007. Chromosome numbers of miscellaneous Malagasy Acanthaceae. – Brittonia 58: 291-300.
Daniel TF. 2009. Synopsis of Dicliptera (Acanthaceae) in the Nueva Galicia region of western Mexico with a new species, D. novogaliciana. – Proc. Calif. Acad. Sci. 60: 1-18.
Daniel TF. 2010. Catalogue of Guatemalan Acanthaceae: taxonomy, ecology, and conservation. – Proc. Calif. Acad. Sci. 61: 291-379.
Daniel TF. 2011. Justicia (Acanthaceae) in Texas. – J. Bot. Res. Inst. Texas 5: 595-618.
Daniel TF. 2014. Taxonomy of Anisotes Nees (Acanthaceae: Justicieae) in the Comoros Archipelago and a preliminary list of Acanthaceae in the islands. – Candollea 69: 45-54.
Daniel TF. 2016. Vascular plants of Arizona: Acanthaceae acanthus or shrimp-plant family. – Canotia 12: 22-54.
Daniel TF, Breedlove DE. 1992. A new species of Uroskinnera (Scrophulariaceae) from southern Mexico. – Madroño 39: 131-136.
Daniel TF, Chuang T-I. 1989. Chromosome numbers of some cultivated Acanthaceae. – Baileya 23: 86-93.
Daniel TF, Chuang T-I. 1993. Chromosome numbers of New World Acanthaceae. – Syst. Bot. 18: 283-289.
Daniel TF, Chuang T-I. 1998. Chromosome numbers of cultivated Acanthaceae and systematic implications. – In: Mathew P, Sivadasan M (eds), Diversity and taxonomy of tropical flowering plants, Mentor Books, Calicut, pp. 309-330.
Daniel TF, Chuang T-I. 2010. Catalog of Guatemalan Acanthaceae: taxonomy, ecology, and conservation. – Proc. Calif. Acad. Sci. 61: 289-377.
Daniel TF, Figueiredo E. 2009. The California Academy of Sciences Gulf of Guinea expeditions (2001, 2006, 2008) VII. Acanthaceae of São Tomé and Príncipe. – Proc. Calif. Acad. Sci. 60: 623-674.
Daniel TF, Wasshausen DC. 1993. A new Carlowrightia (Acanthaceae) from South America. – Nord. J. Bot. 13: 653-656.
Daniel TF, Parfitt BD, Baker MA. 1984. Chromosome numbers and their systematic implications in some North American Acanthaceae. – Syst. Bot. 9: 346-355.
Daniel TF, Chuang T-I, Baker MA. 1990. Chromosome numbers of American Acanthaceae. – Syst. Bot. 15: 13-25.
Daniel TF, Balkwill K, Balkwill M-J. 2000. Chromosome numbers of South African Acanthaceae. – Proc. Calif. Acad. Sci. 52: 143-158.
Daniel TF, McDade LA, Manktelow M, Kiel CA. 2008. The “Tetramerium lineage” (Acanthaceae: Acanthoideae: Justicieae): delimitation and intra-lineage relationships based on cp and nrITS sequence data. – Syst. Bot. 33: 416-436.
Daniel TF. Letsara R, Martín-Bravo S. 2013. Four new species of Anisotes (Acanthaceae) from Madagascar. – Novon 22: 396-408.
Danin A. 1977. Kickxia judaica sp. n. (Scrophulariaceae) and some related species from the deserts of Israel and Sinai. – Israel J. Bot. 26: 23-31.
Danin A. 1990. Two new species of Origanum (Labiatae) from Jordan. – Willdenowia 19: 401-404.
Danin A, Künne I. 1996. Origanum jordanicum (Labiatae), a new species from Jordan, and notes on the other species of O. sect. Campanulaticalyx. – Willdenowia 25: 601-611.
Darbyshire I. 2008a. New species in Barleria sect. Stellatohirta (Acanthaceae) from Africa. – Kew Bull. 63: 261-268.
Darbyshire I. 2008b. Notes on the genus Dicliptera (Acanthaceae) in Eastern Africa. – Kew Bull. 63: 361-383.
Darbyshire I. 2008c. A reassessment of the status of Barleria sect. Cavirostrata (Acanthaceae) in tropical Africa with a new species, B. richardsiae, described from the Tanzania-Zambia border region. – Kew Bull. 63: 601-611.
Darbyshire I. 2009a. Taxonomic notes and novelties in the genus Isoglossa (Acanthaceae) from east Africa. – Kew Bull. 64: 401-427.
Darbyshire I. 2009b. A reassessment of the status of Barleria sect. Cavirostrata (Acanthaceae) in tropical Africa, with a new species, B. richardsiae, described from the Tanzania-Zambia border region. – Kew Bull. 63: 601-611.
Darbyshire I. 2015. The genus Hypoestes (Acanthaceae) in Angola. – Kew Bull. 70: 44 DOI 10.1007/S12225-015-9595-4
Darbyshire I, Govaerts R. 2017. A synopsis of Chlamydocardia (Acanthaceae) including Linocalix. – Kew Bull. 72: 37. doi 10.1007/S12225-017-9711-8
Darbyshire I, Harris T. 2006. Notes on the genus Rhinacanthus (Acanthaceae) in Africa with a synopsis of the R. nasutus-R. gracilis complex and a key to the African members of the genus. – Kew Bull. 61: 401-418.
Darbyshire I, Vollesen K. 2007. The transfer of the genus Peristrophe to Dicliptera (Acanthaceae), with a new species described from eastern Africa. – Kew Bull. 62: 119-128.
Darbyshire I, Vollesen K, Chapman HM. 2009. A remarkable range disjunction recorded in Metarungia pubinervia (Acanthaceae). – Kew Bull. 63: 613-615.
Darbyshire I, Vollesen K, Kelbessa E. 2010. Acanthaceae 2. – In: Beentje H (ed), Flora of tropical East Africa, Royal Botanic Gardens, Kew, Richmond, United Kingdom.
Darbyshire I, Vollesen K, Kelbessa E. 2015. Acanthaceae 2. – In: Timberlake JR, Martins ES (eds), Flora Zambesiaca 8(6), Royal Botanic Gardens, Kew, Richmond, United Kingdom.
D’Arcy WG. 1997. A review of the genus Eccremocarpus (Bignoniaceae). – Ann. Missouri Bot. Gard. 84: 103-111.
D’Arcy WG, Keating RC. 1973. The affinities of Lithophytum: a transfer from Solanaceae to Verbenaceae. – Brittonia 25: 213-225.
Das VSR, Rao KN. 1966. Chemotaxonomic investigation of Nyctanthes. – Naturwissenschaften 54: 439.
Datson PM, Murray BG, Steiner KE. 2008. Climate and the evolution of annual/perennial life-histories in Nemesia (Scrophulariaceae). – Plant Syst. Evol. 270: 39-57.
Datwyler SL, Wolfe AD. 2004. Phylogenetic relationships and morphological evolution in Penstemon subg. Dasanthera (Veronicaceae). – Syst. Bot. 29: 165-176.
David E. 1938. Embryologische Untersuchungen an Myoporaceen, Salvadoraceen, Sapindaceen und Hippocrateaceen. – Planta 28: 680-703.
David RW. 1957. An introduction to the British species of Callitriche. – Proc. Bot. Soc. Brit. Isles 3: 28-32.
Davies B, Cartolano M, Schwarz-Sommer Z. 2006. Flower development: the Antirrhinum perspective. – Adv. Bot. Res. 44: 279-321.
De A. 1966. Cytological, anatomical and palynological studies as an aid in tracing affinity and phylogeny in the family Acanthaceae I. Cytological studies. – Trans. Bose Res. Inst. Calcutta 29: 139-175.
De A. 1967. Cytological, anatomical and palynological studies as an aid in tracing affinity and phylogeny in the family Acanthaceae II. Floral anatomy. – Trans. Bose Res. Inst. 30: 27-43.
Degtjareva GV, Casper J, Hellwig F, Sokoloff DD. 2004. Seed morphology in the genus Pinguicula (Lentibulariaceae) and its relation to taxonomy and phylogeny. – Bot. Jahrb. Syst. 125: 431-452.
Degtjareva GV, Casper J, Hellwig F, Schmidt AR, Steiger J, Sokoloff DD. 2006. Morphology and nrITS phylogeny of the genus Pinguicula L. (Lentibulariaceae), with special attention to embryo evolution. – Plant Biol. 8: 778-790.
De A. 1966. Cytological, anatomical, and palynological studies as an aid in tracing affinity and phylogeny in the family Acanthaceae I. Cytological studies. – Trans. Bose Res. Inst. Calcutta 29: 139-175.
De A. 1967a. Cytological, anatomical, and palynological studies as an aid in tracing affinity and phylogeny in the family Acanthaceae II. Floral anatomy. – Trans. Bose Res. Inst. Calcutta 30: 27-43.
De A. 1967b. Cytological, anatomical, and palynological studies as an aid in tracing affinity and phylogeny in the family Acanthaceae III. General anatomy. – Trans. Bose Res. Inst. Calcutta 30: 51-65.
De A. 1968. Cytological, anatomical, and palynological studies as an aid in tracing affinity and phylogeny in the family Acanthaceae IV. Palynology and final conclusion. – Trans. Bose Res. Inst. Calcutta 31: 17-29.
De Buhr LE. 1975. Observations on Byblis gigantea in Western Australia. – Carniv. Plants Newslett. 3: 60-61.
Degtjareva GV, Sokoloff DD. 2012. Inflorescence morphology and flower development in Pinguicula alpina and P. vulgaris (Lentibulariaceae: Lamiales): monosymmetric flowers are always lateral and occurrence of early sympetaly. – Organ. Div. Evol. 12: 99-111.
Degtjareva GV, Casper SJ, Hellwig FH, Schmidt AR, Steiger J, Sokoloff DD. 2006. Morphology and nrITS phylogeny of the genus Pinguicula L. (Lentibulariaceae), with special attention to embryo evolution. – Plant Biol. 8: 778-790.
Delestaing N. 1954. Contribution à l’étude cytologique du genre Salvia. – Rév. Cytol. Biol. Vég. 15: 195-236.
Dell B. 1975. Geographical differences in leaf resin components of Eremophila fraseri F. Muell. (Myoporaceae). – Aust. J. Bot. 23: 889-897.
Dell B, McComb J. 1975. Glandular hairs, resin production, and habitat of Newcastelia viscida E. Pritzel (Dicrastylidaceae). – Aust. J. Bot. 23: 373-390.
Dell B, McComb AJ. 1977. Glandular hair formation and resin secretion in Eremophila fraseri F. Muell. (Myoporaceae). – Protoplasma 92: 71-86.
Demissew S. 1989. Two new species of Chascanum (Verbenaceae) from NE Africa. – Nord. J. Bot. 8: 627-630.
Demissew S. 1990. The identity of Chascanum arabicum (Verbenaceae) and description of two new taxa of the genus. – Kew Bull. 45: 137-140.
Demissew S. 1993a. New species of Stachys (Labiatae) from tropical Africa. – Kew Bull. 48: 115-118.
Demissew S. 1993b. The genus Stachys (Labiatae) in Ethiopia and Somalia. – Kew Bull. 48: 327-341.
Demissew S. 2004. Cyclocheilaceae. – In: Kubitzki K, Kadereit JW (eds), The families and genera of vascular plants VII. Flowering plants. Dicotyledons. Lamiales (except Acanthaceae including Avicenniaceae), Springer, Berlin, Heidelberg, New York, pp. 60-62.
Demissew S, Harley RM. 1992. Trichome, seed-surface, and pollen characters in Stachys (Lamioideae: Labiatae) in tropical Africa. – In Harley RM, Reynolds T (eds), Advances in labiate science, Royal Botanic Gardens, Kew, pp. 149-166.
Demissew S, Puff C. 1989. Observations on Lippia carviodora, a dioecious Verbenaceae. – Beitr. Biol. Pflanzen 64: 221-230.
Demissew S, Verdcourt B. 1988. Svensonia Moldenke congeneric with Chascanum E. Mey. (Verbenaceae). – Kew Bull. 43: 519-521.
Den Y-F, Xia N-H. 2005. Proposal to conserve the name Leptostachya Nees (Acanthaceae) against Leptostachia Adans. (Phrymaceae) with a conserved type. – Taxon 54: 192-193.
Denduangboripant J, Cronk QCB. 2000. High intraindividual variation in internal transcribed spacer sequences in Aeschynanthus (Gesneriaceae): implications for phylogenetics. – Proc. Roy. Soc. London, Ser. B (Biol. Sci.), 267: 1407-1415.
Denduangboripant J, Cronk QCB. 2001. Evolution and alignment of the hypervariable arm 1 of Aeschynanthus (Gesneriaceae) ITS2 nuclear ribosomal DNA. – Mol. Phylogen. Evol. 20: 163-172.
Denduangboripant J, Mendum M, Cronk QCB. 2001. Evolution in Aeschynanthus (Gesneriaceae) inferred from ITS seqences. – Plant Syst. Evol. 228: 181-197.
Deng Y-F, Wood JRI, Scotland RW. 2006. New and reassessed species of Strobilanthes (Acanthaceae) in the flora of China. – Bot. J. Linn. Soc. 150: 369-390.
Deng Y-F, Wang H, Zhou S-S. 2007. Two newly recorded species of Strobilanthes (Acanthaceae) from China. – Acta Phytotaxon. Sin. 45: 849-854.
Deng Y-F, Gao C-M, Xia N-H, Peng H. 2016. Wuacanthus (Acanthaceae), a new Chinese endemic genus segregated from Justicia (Acanthaceae). – Plant Div. 38: 312-321.
Dengler NG, Sánchez-Burgos AA. 1988. Effect of light level on the expression of anisophylly in Paradrymonia ciliosa (Gesneriaceae). – Bot. Gaz. (Crawfordsville) 14: 158-165.
Devi HM. 1975. Embryology of Jasminum and its bearing on the position of Oleaceae. – Acta Bot. Indica 3: 52-61.
Deyrup M, Menges ES. 1997. Pollination ecology in the rare scrub mint Dicerandra frutescens (Lamiaceae). – Florida Scientist 60: 143-157.
Diaz A, Macnair MR. 1998. The effect of plant size on the expression of cleistogamy in Mimulus nasutus. – Funct. Ecol. 12: 92-98.
Dickison WC. 1994. A re-examination of Sanango racemosum 2. Vegetative and floral anatomy. – Taxon 43: 601-618.
Diels L. 1930. Byblidaceae. – In: Engler A, Harms H (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd 18a, W. Engelmann, Leipzig, pp. 286-288.
Dietrich H. 1969. Über die Aussagekraft pollenmorphologischer Fakten bei stenopalynen Verwandtschafts-bereichen am Beispiel der Plantaginaceae. – Feddes Repert. 79: 347-353.
Dietrich H. 1970. Bougueria nubicola Decne. – eine interessante Plantaginaceen-Gattung. – Wiss. Zeitschr. Friedrich-Schiller-Univ. Jena, Math.-Naturw. Reihe 19: 293-305.
Dietrich H. 1971. Blütenmorphologische und palynologische Untersuchungen an Littorella. – Feddes Repert. 82: 155-165.
Dietrich H. 1980. Cytologische Untersuchungen innerhalb der Familie der Plantaginaceae III. Cytotaxonomische Ergebnisse. – Wiss. Zeitschr. Friedrich-Schiller-Univ. Jena, Math.-Naturw. Reihe 29: 559-589.
Doaigey AR, Harkiss KJ. 1982. Comparative anatomy of the leaf and stem of Antirrhinum meonanthum Hoffmanns. and Lind and Antirrhinum braun-blanquetii Rothm. – J. Coll. Sci., King Saud Univ. 13: 249-257.
Doaigey AR, Harkiss KJ. 1991. Application of epidermal characters to the taxonomy of European species of Antirrhinum (Scrophulariaceae). – Nord. J. Bot. 11: 513-524.
Dobbins DR. 1971. Studies on the anomalous cambial activity in Doxantha unguis-cati (Bignoniaceae) II. A case of differential production of secondary tissues. – Amer. J. Bot. 58: 697-705.
Domin K. 1922. Byblidaceae: a new archichlamydous family. – Acta Bot. Bohemica 1: 3-4.
Domina G, Arrigoni PV. 2007. The genus Orobanche (Orobanchaceae) in Sardinia. – Flora Mediterr. 17: 115-136.
Domínguez XA, González H, Aragón R, Gutiérrez M, Marroquín JS, Watson W. 1976. Three new diterpene quinines from Salvia ballotaeflora. – Planta Medica 30: 237.
Dong L-N, Wang H, Wortley AH, Lu L, Li D-Z. 2013. Phylogenetic relationships in the Pterygiella complex (Orobanchaceae) inferred from molecular and morphological evidence. – Bot. J. Linn. Soc. 171: 491-507.
Dong L-N, Wang H, Wortley AH, Li D-Z, Lu L.
2015. Fruit and seed morphology in some representative genera of tribe
Rhinantheae sensu lato (Orobanchaceae) and related taxa.
– Plant Syst. Evol. 301: 479-500.
Dönmez AA. 2001. A new Turkish species of Salvia L. (Lamiaceae). – Bot. J. Linn. Soc. 137: 413-416.
Dönmez AA, Mutlu B. 2010. Bornmuellerantha alshehbaziana (Orobanchaceae), a new species from Turkey. – Novon 20: 265-267.
Dop P. 1927. Les glandes florales externes des Bignoniacées. – Bull. Soc. Hist. Nat. (Toulouse) 56: 189-198.
Doroszenko A. 1985. Taxonomic studies on the Satureja complex (Labiatae). – Ph.D. diss., University of Edinburgh, Scotland.
Dörr I. 1997. How Striga parasitizes its host: a TEM and SEM study. – Ann. Bot. 79: 463-472.
Dörr I, Kollmann R. 1975. Strukturelle Grundlagen des Parasitismus bei Orobanche II. Die Differenzierung der Assimilat-Leitungsbahn im Haustorialgewebe. – Protoplasma 83: 185-199.
Dörrstock S, Seine R, Porembski S, Barthlott W. 1996. First record of the American Utricularia juncea (Lentibulariaceae) from Africa. – Kew Bull. 51: 579-583.
Dos Santos EP. 1991. Genre Salvia L. sous-genre Calosphace (Benth.) Benth. section Nobiles (Benth.) Epl. (Labiatae). – Bradea 5: 436-453.
Dos Santos EP. 1995. Phylogenie et phytogéographie du genre Salvia L. section Rudes (Benth.) Epl. (Lamiacées). – Biogeographica 71: 15-32.
Dos Santos EP. 1996. Révision de la section Rudes (Benth.) Epling du genre Salvia L. sousgenre Calosphace (Benth.) Benth. (Labiatae). – Candollea 51: 19-57.
Dos Santos GMA. 1995. Wood anatomy, chloroplast DNA, and flavonoids of the tribe Bignonieae (Bignoniaceae). – Ph.D. diss., University of Reading, United Kingdom.
Dos Santos GMA, Miller RB. 1992. Wood anatomy of Tecomeae. – In: Gentry AH (ed), Bignoniaceae, Part II (Tribe Tecomeae), Flora Neotropica Monograph 25(2), The New York Botanical Garden Press, New York, pp. 336-358.
Dos Santos GMA, Miller RB. 1997. Wood anatomy of Jacaranda (Bignoniaceae): systematic relationships in sections Monolobos and Dilobos as suggested by twig and stem wood rays. – IAWA J. 18: 369-383.
Dowling RE. 1936. The structure of the ovary in the genus Plantago L. – Bot. J. Linn. Soc. 50: 323-336.
Drew BT. 2011. Phylogenetics and biogeography of Lepechinia (Lamiaceae), and evolutionary studies within the Mentheae tribe. – Ph.D. diss., University of Wisconsin, Madison, Wisconsin.
Drew BT, Sytsma KJ. 2011. Testing the monophyly and placement of Lepechinia in the tribe Mentheae (Lamiaceae). – Syst. Bot. 36: 1038-1049.
Drew BT, Sytsma KJ. 2012. Phylogenetics, biogeography, and staminal evolution in the tribe Mentheae (Lamiaceae). – Amer. J. Bot. 99: 933-953.
Drew BT, Sytsma KJ. 2013. The South American radiation of Lepechinia (Lamiaceae): phylogenetics, divergence times and evolution of dioecy. – Bot. J. Linn. Soc. 171: 171-190.
Drew BT, Cacho NI, Sytsma KJ. 2014. The
transfer of two rare monotypic genera, Neoeplingia and
Chaunostoma, to Lepechinia (Lamiaceae), and notes on their
conservation. – Taxon 63: 831-842.
Drew BT, González-Gallegos JG, Xiang C-L, Kriebel R, Drummond CP, Walker JB, Sytsma KJ. 2017. Salvia united: the greatest good for the greatest number. – Taxon 66: 133-145.
Drew BT, Liu S, Bonifacino JM, Sytsma KJ. 2017. Amphitropical disjunctions in New World Menthinae: three Pliocene dispersals to South America following late Miocene dispersal to North America from the Old World. – Amer. J. Bot. 104: 1695-1707.
Drewes SI, Martínez S. 1999. Morfologia de la inflorescencias en Verbenaceae-Verbenoideae II: Tribu Petreeae. – Darwiniana 37: 209-218.
Drude O. 1891. Droseraceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(2), W. Engelmann, Leipzig, pp. 261-272.
Dubuc-Lebreux M-A. 1976. Effets de quelques régulateurs de croissance sur le phyllomorphe cotylédonaire de Streptocarpus wendlandii Spreng. (Gesneriaceae). – Ann. Sci. Nat. Bot., sér. 12, 17: 259-276.
Dubuc-Lebreux M-A. 1978. Modification of unifoliate habit of Streptocarpus wendlandii and Streptocarpus michelmorei by some growth regulators. – Phytomorphology 28: 224-238.
Dubuc-Lebreux M-A, Vieth J. 1976. Effets de quelques régulateurs de croissance sur la morphologie florale et inflorescentielle de Streptocarpus rexii (Hook.) Lindl. – Bull. Soc. Bot. France 123: 273-291.
Dudash MR, Carr DE. 1998. Genetics underlying inbreeding depression in Mimulus with contrasting mating systems. – Nature 393: 682-684.
Duke NC. 1988. An endemic mangrove species, Avicennia integra sp. nov. (Avicenniaceae), in northern Australia. – Aust. Syst. Bot. 1: 177-180.
Duke NC. 1990. Morphological variation in the mangrove genus Avicennia in Australasia: systematic and ecological considerations. – Aust. Syst. Bot. 3: 221-239.
Duke NC. 1991. A systematic revision of the mangrove genus Avicennia (Avicenniaceae) in Australasia. – Aust. Syst. Bot. 4: 299-324.
Duman H. 2001. A new species of Globularia L. (Globulariaceae) from South Anatolia. – Bot. J. Linn. Soc. 137: 425-428.
Dunbar-Co S, Wieczorek AM, Morden CW. 2008. Molecular phylogeny and adaptive radiation of the endemic Hawaiian Plantago species (Plantaginaceae). – Amer. J. Bot. 95: 1177-1188.
Dunkić V, Kremer D, Grubešić RJ, Rodríguez JV, Ballian D, Bogunić F, Stešević D, Kosalec I, Bezić N, Stabentheiner E. 2017. Micromorphological and phytochemical traits of four Clinopodium L. species (Lamiaceae). – South Afr. J. Bot. 111: 232-241.
Dunn ST. 1913. Notes on Chinese Labiatae. – Notes Roy. Bot. Gard. Edinb. 8: 153-171.
Dute R, Rabaey D, Allison J, Jansen S. 2010. Torus-bearing pit membranes in species of Osmanthus. – IAWA J. 31: 217-226.
Dutta PC, Maithi RK. 1969. Study of floral anatomy of the tribe Justicieae (Acanthaceae) with an aim of taxonomic interpretation. – Acta Biol. Acad. Sci. Hung. 20: 311-317.
Eberle P. 1956. Cytologische Untersuchungen an Gesneriaceen I. Mitteilung. Die Struktur der Pachytän-chromosomen sowie eine Reihe neu bestimmter Chromosomenzahlen. – Chromosoma 8: 285-316.
Eberle P. 1957. Über das Vorkommen eines Viererringes und eines heteromorphen Bivalents in der Meiosis von Episcia tesselata Linden. – Zeitschr. Indukt. Abstammungs-Vererbungsl. 88: 184-188.
Edwards CE, Soltis DE, Soltis PS. 2006. Molecular phylogeny of Conradina and other scrub mints (Lamiaceae) from the southeastern USA: evidence for hybridization in Pleistocene refugia? – Syst. Bot. 31: 193-207.
Edwards TJ. 2006. Notes on the Lamiaceae: a new Tetradenia and a new Thorncroftia from South Africa. – South Afr. J. Bot. 72: 202-204.
Edwards T, Hughes M, Moller M, Bellstedt D. 2009. New Streptocarpus species (Gesneriaceae) from South Africa. – Bot. J. Linn. Soc. 158: 743-748.
Edwin G. 1970. New taxa and notes on the Scrophulariaceae of Peru. – Phytologia 19: 369-406.
Egger JM. 2002a. A new species of Castilleja (Orobanchaceae) from the central Sierra Madre Occidental, Mexico, and an annotated key to the Castilleja species of that region. – Brittonia 54: 6-12.
Egger JM. 2002b. A new species of Castilleja (Orobanchaceae) from central Hidalgo, Mexico. – Brittonia 54: 190-195.
Ehrendorfer F, Vitek E. 1984. Evolution alpiner Populationen von Euphrasia (Scrophulariaceae). Entdeckung kleinblütiger diploider Sippen. – Plant Syst. Evol. 144: 25-44.
Ehrhart C. 1997. Zur Cytologie chilenischer Calceolaria-Arten (Scrophulariaceae). – Sendtnera 4: 41-59.
Ehrhart C. 2000. Die Gattung Calceolaria (Scrophulariaceae) in Chile. – Bibl. Bot. 153: viii + 1-283.
Ehrhart C. 2005. The Chilean Calceolaria integrifolia s.l. species complex (Scrophulariaceae). – Syst. Bot. 30: 383-411.
Eiji S, Salmaki Y. 2016. Evolution of trichomes and its systematic significance in Salvia (Mentheae; Nepetoideae; Lamiaceae). – Bot. J. Linn. Soc. 180: 241-257.
Elenevsky AG. 1977. Sistema roda Veronica L. – Byull. Moskovsk.Obshch. Isp. Prir. Otd. Biol. 82: 149-160.
El-Gazzar A. 1969. A taxonomic study of Labiatae and related genera. – Ph.D. diss., Southampton University, Southampton, United Kingdom.
El-Gazzar A. 1974. Numerical taxonomy of the Verbenaceae: a reassessment. – Egypt. J. Bot. 17: 69-83.
El-Gazzar A, Watson L. 1968a. Labiatae: taxonomy and susceptibility to Puccinia menthae Pers. – New Phytol. 67: 739-743.
El-Gazzar A, Watson L. 1968b. The taxonomy of Salvia: a test of two radically different numerical methods. – Bot. J. Linn. Soc. 60: 237-250.
El-Gazzar A, Watson L. 1970a. A taxonomic study of Labiatae and related genera. – New Phytol. 69: 451-486.
El-Gazzar A, Watson L. 1970b. Some economic implications of the taxonomy of Labiatae: essential oils and rusts. – New Phytol. 69: 487-492.
El-Gazzar A, Watson L, Williams WT, Lance GN. 1968. The taxonomy of Salvia: a test of two radically different numerical methods. – J. Linn. Soc. (Bot.) 60: 237-250.
Elias TS, Gelband H. 1976. Morphology and anatomy of floral and extrafloral nectaries in Campsis (Bignoniaceae). – Amer. J. Bot. 63: 1349-1353.
Elias TS, Prance G. 1978. Nectaries on the fruit of Crescentia and other Bignoniaceae. – Brittonia 30: 175-181.
Elisens WJ. 1985a. Monograph of the Maurandyinae (Scrophulariaceae-Antirrhineae). – Syst. Bot. Monogr. 5.
Elisens WJ. 1985b. The systematic significance of seed coat anatomy among New World species of tribe Antirrhineae (Scrophulariaceae). – Syst. Bot. 10: 282-299.
Elisens WJ. 1985c. The systematic relationship of Asarina procumbens to New World species in tribe Antirrhineae (Scrophulariaceae). – Madroño 32: 168-178.
Elisens WJ. 1986. Pollen morphology and systematic relationships among New World species in the tribe Antirrhineae (Scrophulariaceae). – Amer. J. Bot. 73: 1298-1311.
Elisens WJ. 1989. Patterns of crossability and interfertility in subtribe Maurandyinae (Scrophulariaceae-Antirrhineae). – Syst. Bot. 14: 304-315.
Elisens WJ, Freeman CE. 1988. Floral sugar composition and pollinator type among New World genera in the tribe Antirrhineae. – Amer. J. Bot. 75: 971-978.
Elisens WJ, Nelson AD. 1993. Morphological and isozyme divergence in Gambelia (Scrophulariaceae): species delimitation and biogeographic relationships. – Syst. Bot. 18: 454-468.
Elisens WJ, Tomb AS. 1983. Seed morphology in New World Antirrhineae (Scrophulariaceae). Systematic and phylogenetic implications. – Plant Syst. Evol. 142: 23-47.
Ellis AG, Midgley JJ. 1996. A new plant-animal mutualism involving a plant with sticky leaves and a resident Hemipteran insect. – Oecologia 106: 478-481.
Ellis JL. 1962. Chromosome numbers in some members of Acanthaceae. – Sci. Cult. 28: 191-192.
El Oualidi J, Verneau O, Puech S, Dubuisson JY. 1999. Utility of rDNA, ITS sequences in the systematics of Teucrium section Polium (Lamiaceae). – Plant Syst. Evol. 215: 49-70.
Emboden WA. 1964. Pollen morphology of the genus Salvia section Audibertia. – Pollen Spores 6: 527-536.
Emboden WA. 1971. The role of introgressive hybridization in the development of Salvia section Audibertia. – Contr. Sci. 208: 1-15.
Emboden WA, Lewis H. 1967. Terpenes as taxonomic characters in Salvia section Audibertia. – Brittonia 19: 152-160.
Endress PK. 1992. Evolution and floral diversity: the phylogenetic surroundings of Arabidopsis and Antirrhinum. – Intern. J. Plant Sci. (Suppl.) 153: S106-S122.
Engell K. 1987. Embryology and taxonomic position of Retzia capensis (Retziaceae). – Nord. J. Bot. 7: 117-124.
Engler A. 1897. Scrophulariaceae africanae II. – Engl. Bot. Jahrb. Syst. 23: 497-517.
Engler A. 1905. Scrophulariaceae africanae III. Cycniopsis. – Engl. Bot. Jahrb. Syst. 36: 230-240.
Ensermu K, Brummitt RK, Furness CA. 1992. A reconsideration of Asystasiella Lindau (Acanthaceae). – Kew Bull. 47: 669-675.
Epling CC. 1925a. Monograph of the genus Monardella. – Ann. Missouri Bot. Gard. 12: 1-106.
Epling CC. 1925b. Studies on the South American Labiatae I. Synopses of the genera Teucrium, Rosmarinus, Marrubium, Prunella, Lamium, Leonurus, and Leonotis. – Ann. Missouri Bot. Gard. 12: 107-132.
Epling CC. 1926. Studies on the South American Labiatae II. Synopsis of the genus Sphacele. – Ann. Missouri Bot. Gard. 13: 35-70.
Epling CC. 1927. Studies on the South American Labiatae III. Synopsis of the genus Satureia. – Ann. Missouri Bot. Gard. 14: 47-86.
Epling CC. 1933a. Asterohyptis: a newly proposed genus of Mexico and Central America. – Bull. Torrey Bot. Club 60: 17-21.
Epling CC. 1933b. Synopsis of the genus Hyptis in North America. – Feddes Repert. 34: 73-130.
Epling CC. 1934a. Preliminary revision of American Stachys. – Feddes Repert. Beih. 80: 1-75.
Epling CC. 1934b. Synopsis of the genus Hyptis in North America. – Feddes Repert. 34: 73-130.
Epling CC. 1935a. Notes on Monarda: the subgenus Cheilyctis. – Madroño 3: 20-31.
Epling CC. 1935b. Synopsis of the South American Labiatae I. – Feddes Repert. Beih. 85: 1-96.
Epling CC. 1936a. Synopsis of the South American Labiatae II. – Feddes Repert. Beih. 85: 97-192.
Epling CC. 1936b. Synopsis of the South American Labiatae III. – Feddes Repert. Beih. 85: 193-288.
Epling CC. 1937. Synopsis of the South American Labiatae IV. – Feddes Repert. Beih. 85: 289-341.
Epling CC. 1937. The Labiatae of northern South America: Colombia, Ecuador and Venezuela. – Feddes Repert. Beih. 95: 1-144.
Epling CC. 1937. The Labiatae of Bolivia. – Rev. Sudamer. Bot. 4: 21-53, 86-124.
Epling CC. 1937. The Labiatae of Chile. – Rev. Univ. (Santiago) 22: 167-194.
Epling CC. 1938a. The Californian Salvias. A revision of Salvia, section Audibertia. – Ann. Missouri Bot. Gard. 25: 95-188.
Epling CC. 1938b. The Labiatae of Peru. – Feddes Repert. Beih. 105: 1-93.
Epling CC. 1938c. Las Labiadas de la Argentina, Paraguay y Uruguay. – Rev. Mus. La Plata, Secc. Bot. 2: 89-178.
Epling CC. 1938d. A synopsis of the Labiatae of the Guianas. – Kew Bull. 1938: 187-196.
Epling CC. 1939a. A revision of Salvia, subgenus Calosphace. – Feddes Repert. Beih. 100: 1-380.
Epling CC. 1939b. Notes on the Scutellariae of eastern North America. – Amer. J. Bot. 26: 17-24.
Epling CC. 1939c. Apuntes sobre el género Scutellaria de la America tropical y subtropical. – Lilloa 4: 229-275.
Epling CC. 1939d. Las Labiadas del noroeste de la Argentina. – Lilloa 4: 389-446.
Epling CC. 1939e. Two Mexican species of Hyptis. – Madroño 5: 15-16.
Epling CC. 1939f. Notes on the Scutellariae of western North America. – Madroño 5: 49-72.
Epling CC. 1940a. The Labiatae of the Yucatan Peninsula. – Publ. Carnegie Inst. Washington 522: 225-245.
Epling CC. 1940b. Supplementary notes on Salvia: Audibertia. – Ann. Missouri Bot. Gard. 27: 259-262.
Epling CC. 1940c. Supplementary notes on American Labiatae. – Bull. Torrey Bot. Club 67: 509-534.
Epling CC. 1941. Supplementary notes on American Labiatae II. – Bull. Torrey Bot. Club 68: 552-568.
Epling CC. 1942. The American species of Scutellaria. – Univ. Calif. Publ. Bot. 20: 1-146.
Epling CC. 1944. Supplementary notes on American Labiatae III. – Bull. Torrey Bot. Club 71: 484-497.
Epling CC. 1947. Supplementary notes on American Labiatae IV. – Bull. Torrey Bot. Club 74: 512-518.
Epling CC. 1948. A synopsis of the tribe Lepechinieae (Labiatae). – Brittonia 6: 352-364.
Epling CC. 1949. Revisión del género Hyptis (Labiatae). – Rev. Mus. La Plata, Secc. Bot., 7: 153-497.
Epling CC. 1951. Supplementary notes on American Labiatae V. – Brittonia 7: 129-142.
Epling CC. 1955. Harlanlewisia, a recently discovered genus of Labiatae. – Amer. J. Bot. 42: 436.
Epling CC. 1957. Labiatae. – In: Steyermark JA (ed), Botanical exploration in Venezuela IV, Fieldiana, Bot. 28: 1083-1085.
Epling CC, Játiva C. 1960. Supplementary notes on American Labiatae VII. – Brittonia 12: 140-150.
Epling CC, Játiva C. 1963. Supplementary notes on American Labiatae VIII. – Brittonia 15: 366-376.
Epling CC, Játiva C. 1964. Revisión del género Satureia en el América del Sur. – Brittonia 16: 393-416.
Epling CC, Játiva C. 1966a. A descriptive key to the species of Satureja indigenous to North America. – Brittonia 18: 244-248.
Epling CC, Játiva C. 1966b. Supplementary notes on American Labiatae IX. – Brittonia 18: 255-265.
Epling CC, Játiva C. 1968. Supplementary notes on American Labiatae X. – Brittonia 20: 295-313.
Epling CC, Mathias ME. 1957. Supplementary notes on American Labiatae VI. – Brittonia 8: 297-313.
Epling CC, Stewart WS. 1939. A revision of Hedeoma with a review of allied genera. – Feddes Repert. Beih. 115: 1-49.
Epling CC, Lewis H, Raven P. 1962. Chromosomes of Salvia: section Audibertia. – Aliso 5: 217-221.
Erbar C, Gülden C. 2011. Ontogeny of the flowers in Paulownia tomentosa – a contribution to the recognition of the resurrected monogeneric family Paulowniaceae. – Flora 206: 205-218.
Erbar C, Leins P. 2004. Callitrichaceae. – In: Kubitzki K, Kadereit JW (eds), The families and genera of vascular plants VII. Flowering plants. Dicotyledons. Lamiales (except Acanthaceae including Avicenniaceae), Springer, Berlin, Heidelberg, New York, pp. 50-56.
Erdtman G. 1945. Pollen morphology and plant taxonomy IV. Labiatae, Verbenaceae, and Avicenniaceae. – Svensk Bot. Tidskr. 39: 279-285.
Ernst A. 1961. Revision der Gattung Pinguicula. – Bot. Jahrb. Syst. 80: 145-194.
Ernst WR. 1972. Floral morphology and systematics of Lamourouxia (Scrophulariaceae: Rhinanthoideae). – Smiths. Contr. Bot. 6: 1-63.
Eseltine GP van. 1929. A preliminary study of the unicorn plants. – New York State Agricult. Exper. Station Techn. Bull. 149: 1-41.
Espejo-Serna A, Ramamoorthy TP. 1993. Revisión taxonómica de Salvia sección Sigmoideae (Lamiaceae). – Acta Bot. Mex. 23: 65-102.
Espinosa Matías S, Márquez Guzmán J, Zamudio S. 2005. Embriología de las estructuras reproductoras masculinas del género Pinguicula L. (Lentibulariaceae). – Bol. Soc. Bot. México 76: 43-52.
Esquivel B, Méndez A, Ortega A, Soriano-García M, Toscano A, Rodríguez-Hahn L. 1985. Two new clerodane-type diterpenoids from Salvia keerlii. – Phytochemistry 24: 1769.
Esquivel B, Cárdenas J, Ramamoorthy TP, Rodríguez-Hahn L. 1986. Clerodane diterpenoids of Salvia lineata. – Phytochemistry 25: 2381.
Esquivel B, Cárdenas J, Toscano A, Soriano-García M, Rodríguez-Hahn L. 1986. The diterpenoid constituents of Salvia fulgens Cav. and Salvia microphylla Kunth (Labiatae). – J. Nat. Prod. 50: 738-740.
Esquivel B, Hernández M, Cárdenas J, Ramamoorthy TP, Rodríguez-Hahn L. 1986. Semiatrin, a neo-clerodane diterpenoid from Salvia semiatratha. – Phytochemistry 25: 1484.
Estes D, Small RL. 2008. Phylogenetic relationships of the monotypic genus Amphianthus (Plantaginaceae tribe Gratioleae) inferred from chloroplast DNA sequences. – Syst. Bot. 33: 176-182.
Estes JR, Brown LS. 1973. Entomophilous, intrafloral pollination in Phyla incisa. – Amer. J. Bot. 60: 228-230.
Every AD. 1977. Biosystematics of Penstemon subgenus Dasanthera – a naturally hybridizing species complex. – Ph.D. diss., University of Washington.
Evrard C, DeMillecamps E. 1992. Étude systématique et palynologique du genre Whitfieldia (Acanthaceae-Whitfielieae) en Afrique Centrale. – Belg. J. Bot. 125: 89-100.
Ezcurra C. 1993. Systematics of Ruellia (Acanthaceae) in southern South America. – Ann. Missoui Bot. Gard. 80: 787-845.
Ezcurra C. 1994. Carlowrightia sulcata (Acanthaceae), una especie de Sudamérica austral tratada previamente en Siphonoglossa. – Novon 4: 221-223.
Ezcurra C, Azuke D de. 1989. Validation and genetic and morphological relationships of Ruellia macrosolen (Acanthaceae) from southern South America. – Syst. Bot. 14: 297-303.
Faden RB. 1988. Holmskioldia (Verbenaceae), a genus new to the Flora of tropical East Africa. – Kew Bull. 43: 659-662.
Fahn A, Shimony C. 1977. Development of glandular and nonglandular leaf hairs of Avicennia marina (Forsskål) Vierh. – Bot. J. Linn. Soc. 74: 37-46.
Falcão Ichaseo CL. 1978. Tipos de sementes encotradas nas Scrophulariaceae. – Ridriguesia 30: 335-344.
Fan R, Wu Q, Zou H. 1980. Embryological studies of Catalpa fargesii f. duclouxii (Dode) Gilmour. – J. Nanjing Techn. Coll. Forest Prod. 2: 67-73.
Farooq M. 1958. The development of embryo in Utricularia stellaris Linn. f. var. inflexa. – Sch. Cult. 23: 479-480.
Farooq M. 1964. Studies in the Lentibulariaceae 1. The embryology of Utricularia stellaris Linn. f. var. inflexa; II. Microsporangium, male gametophyte, fertilization, endosperm, embryo and seed. – Proc. Indian Natl. Inst. Sci. 30: 280-299.
Farooq M. 1965a. Studies in the Lentibulariaceae 2. The embryology of Utricularia arcuata Wight. – J. Indian Bot. Soc. 44: 326-346.
Farooq M. 1965b. Studies in the Lentibulariaceae 3. The embryology of Utricularia uliginosa Vahl. – Phytomorphology 15: 123-131.
Farooq M. 1966. Studies in the Lentibulariaceae 4. The embryology of Utricularia striatula Sm. – J. Indian Bot. Soc. 45: 1-13.
Farooq M, Bilquis S. 1966a. Studies in the Lentibulariaceae 7. The embryogeny in Utricularia scandens Benj. – Beitr. Biol. Pflanzen 42: 127-131.
Farooq M, Bilquis S. 1966b. Studies in the Lentibulariaceae 8. The life history of Utricularia scandens Benj. – Beitr. Biol. Pflanzen 42: 363-371.
Farooq M, Siddiqui A. 1966. Studies in the Lentibulariaceae 5. The development of endosperm in Utricularia vulgaris americana Gray (a reinvestigation). – New Phytiol. 65: 50-53.
Farooq M, Siddiqui A. 1967. Studies in the Lentibulariaceae 6. The embryology of Utricularia stellaris Linn. f. – J. Indian Bot. Soc. 46: 31-44.
Farrell BD, Mitter C. 1990. Phylogenesis of insect/plant interactions: have Phyllobrotica and the Lamiales diversified in parallel? – Evolution 44: 1389-1403.
Fassett NC. 1951. Callitriche in the New World. – Rhodora 53: 137-155, 161-182, 185-194, 209-222.
Fedotova TA. 1996. Morphology of the fruit and seed of Carlemannia species (Carlemanniaceae). – Bot. Žurn. 81: 24-34.
Fenster CB, Ritland K. 1992. Chloroplast DNA and isozyme diversity in two Mimulus species (Scrophulariaceae) with contrasting mating systems. – Amer. J. Bot. 79: 1440-1447.
Fernald ML. 1900. Contributions from the Gray Herbarium of Harvard University III. Some undescribed Mexican phanerogams, chiefly Labiatae and Solanaceae. – Proc. Amer.Acad.Arts Sci. 35: 562-573.
Ferguson IK. 1971. Notes on the genus Verbascum (Scrophulariaceae). – Bot. J. Linn. Soc. 64: 229-233.
Ferguson IK, Santisuk T. 1973. Notes on the pollen morphology of some Asiatic Bignoniaceae. – Kew Bull. 28: 187-194.
Fernald ML, Wiegand KM. 1915. The genus Euphrasia in North America. – Rhodora 17: 181-201.
Fernandes A, Queirós M, Fátima Santos M. 1977. Contribution à la connaissance cytotaxinomique des spermatophyte du Portugal XV. Scrophulariaceae. – Bol. Soc. Broteriana 51: 37-90.
Fernandes RB. 1985. Notes sur les Verbenaceae V. Identification des espèces d’Holmskioldia africaines et malgaches. – Garcia de Orta Ser. Bot. (Lisboa) 7: 33-46.
Fernandes RB, Verdcourt B. 2000. Rotheca (Labiatae) revived: more new combinations. – Kew Bull. 55: 147-154.
Fernández RJ, Pastor J. 1997. Morfología polínica de Veronica L. (Scrophulariaceae) en el suroeste de España. – Acta Bot. Malac. 22: 65-72.
Fernández-Alonso JL. 1995. Estudios en Labiatae de Colombia I. Novedades en los géneros Salvia e Hyptis. – Rev. Acad. Colombiana Ci. Ex. Fis. Nat. 19(74): 469-479.
Fernández-Alonso JL. 2006. Revisión taxonómica de Salvia sect. Siphonantha (Labiatae). – An. Jard. Bot. Madrid 63: 145-157.
Fernández-Alonso JL. 2012. Salvia guacana, una nueva Labiatae de Colombia con flores resupinadas y synopsis de Salvia sect. Tubiflorae. – Rev. Acad. Colomb. Ci. 36: 517-533.
Fernández-Mazuecos M, Vargas P. 2011. Historical isolation versus recent long-distance connections between Europe and Africa in bifid toadflaxes (Linaria sect. Versicolores). – PloS ONE 6: e22234.
Fernández-Mazuecos M, Vargas P. 2015. Quaternary radiation of bifid toadflaxes (Linaria sect. Versicolores) in the Iberian Peninsula: low taxonomic signal but high geographic structure of plastid DNA lineages. – Plant Syst. Evol. 301: 1411-1423.
Fernández-Mazuecos M, Blanco-Pastor JL, Vargas P. 2013. A phylogeny of toadflaxes (Linaria Mill.) based on nuclear internal transcribed spacer sequences: systematic and evolutionary consequences. – Intern. J. Plant Sci. 174: 234-249.
Fetscher AE, Kohn JR. 1999. Stigma behaviour in Mimulus aurantiacus. – Amer. J. Bot. 86: 1130-1135.
Feuillet C. 1979. Contribution à l’étude morphologique et architecturale des Gesneriacées. – Thèse, l’Université Pierre et Marie Curie, Paris, France.
Feuillet C, Skog LE. 2003a. Novae Gesneriaceae Neotropicarum XI. New genera and species from the Guianas. – Brittonia 54: 344-351.
Feuillet C, Skog LE. 2003b. Novae Gesneriaceae Neotropicarum XII. New species of Gesneriaceae from the Guianas. – Brittonia 54: 352-361.
Figueiredo E, Jury SL. 1996. Notes on Brachystephanus (Acanthaceae). – Kew Bull. 51: 753-763.
Figueiredo E, Keith-Lucas M. 1996. Pollen morphology of Brachystephanus (Acanthaceae-Justicieae). – Grana 35: 65-73.
Filonenko A, Bobrov AVFC, Melikian AP. 2009. On the systematic position of the genus Nyctanthes L. (Oleaceae/Verbenaceae/Nyctanthaceae). – Novit. Syst. Plant. Vasc. 41: 192-208. [In Russian]
Fineran BA, Lee MSL. 1975. Organization of quadrifid and bifid hairs in the trap of Utricularia monanthos. – Protoplasma 84: 43-70.
Finn V. 1930. On the history of development of male gametophyte in Labiatae. – J. Inst. Bot. Acad. Sci. Ukraine 20: 77-96. [In Russian]
Fischer E. 1989. Contributions for the flora of Central Africa II. Crepidorhopalon, a new genus within the relationship of Craterostigma, Torenia and Lindernia (Scrophulariaceae) with two new or noteworthy species from Central and South Central Africa (Zaire, Zambia). – Feddes Repert. 100: 439-450.
Fischer E. 1992. Systematik der afrikanischen Lindernieae (Scrophulariaceae). – Trop. Subtrop. Pflanzenwelt 82: 1-365.
Fischer E. 1995. Revision of the Lindernieae (Scrophulariaceae) in Madagascar 1. The genera Lindernia Allioni and Crepidorhopalon E. Fischer. – Bull. Mus. Natl. Hist. Nat. Paris, sér. IV, sect. B, Adansonia, 17: 227-257.
Fischer E. 1996a. A revision of the genus Alectra Thunberg (Scrophulariaceae) in Madagascar, with a description of Pseudomelasma, gen. nov. – Bull. Mus. Natl. Hist. Nat. B, Adansonia 18: 45-65.
Fischer E. 1996b. Barthlottia, a new monotypic genus of Scrophulariaceae-Manuleeae from Madagascar. – Bull. Mus. Natl. Hist. Nat., Adansonia 18: 351-356.
Fischer E. 1997a. Revision of the genus Stemodiopsis Engl. (Scrophulariaceae). – Bot. Jahrb. Syst. 119: 305-326.
Fischer E. 1997b. A revision of the genus Dopatrium Buch.-Ham. ex Benth. (Scrophulariaceae-Gratioloideae). – Nord. J. Bot. 17: 527-555.
Fischer E. 2004. Scrophulariaceae. – In: Kubitzki K, Kadereit JW (eds), The families and genera of vascular plants VII. Flowering plants. Dicotyledons. Lamiales (except Acanthaceae including Avicenniaceae), Springer, Berlin, Heidelberg, New York, pp. 333-432.
Fischer E, Hepper FN. 1991. Four new species of Lindernia (Scrophulariaceae) from East Africa. – Kew Bull. 46: 529-534.
Fischer E, Hepper FN. 1997. The genera Bryodes Benth. and Psammetes Hepper (Scrophulariaceae) in Madagascar and West Africa. – Kew Bull. 52: 749-752.
Fischer E, Lachenaud O. 2013. A new species of Torenia (Linderniaceae) from Gabon, remarks on Torenia mannii Skan, and a key to the African and Madagascan Torenia species. – Phytotaxa 125: 40-46.
Fischer E, Vogel S, Lopes AV. 1999. Ameroglossum, a new monotypic genus of Scrophulariaceae-Scrophularioideae from Brazil. – Feddes Repert. 110: 529-534.
Fischer E, Porembski S, Barthlott W. 2000. Revision of the genus Genlisea (Lentibulariaceae) in Africa and Madagascar with notes on ecology and phytogeography. – Nord. J. Bot. 20: 291-318.
Fischer E, Theisen I, Lohmann LG. 2004. Bignoniaceae. – In: Kubitzki K, Kadereit JW (eds), The families and genera of vascular plants VII. Flowering plants. Dicotyledons. Lamiales (except Acanthaceae including Avicenniaceae), Springer, Berlin, Heidelberg, New York, pp. 9-38.
Fischer E, Barthlott W, Seine R, Theisen I. 2004. Lentibulariaceae. – In: Kubitzki K, Kadereit JW (eds), The families and genera of vascular plants VII. Flowering plants. Dicotyledons. Lamiales (except Acanthaceae including Avicenniaceae), Springer, Berlin, Heidelberg, New York, pp. 276-282.
Fischer E, Schäferhoff B, Müller KF. 2012. The new monotypic genus Bardotia (Orobanchaceae) from Madagascar and remarks on the phylogenetic relationships of the African and Madagascan genera Parastriga, Radamaea, Rhamphicarpa and Sieversandreas. – Phytotaxa 46: 19-33.
Fischer J. 1920. Zur Entwicklungsgeschichte und Morphologie der Veronicablüte. – Zeitschr. Bot. 12: 113-171.
Fischer MA. 1969a. Einige Chromosomenzahlen aus den Gattungen Veronica, Pseudolysimachion, Paederota, Wulfenia und Lagotis (Scrophulariaceae-Veronicinae). – Österr. Bot. Zeitschr. 116:430-443.
Fischer MA. 1969b. Veronica caespitosa Boiss., V. bombycina Boiss. & Kotschy und V. thessalica Benth. gehören nicht in die Sektion Veronicastrum Benth. – Österr. Bot. Zeitschr. 117: 54-63.
Fischer MA. 1970. Zur Cytotaxonomie der Verwandtschaftsgruppe um Veronica orientalis Mill., emend. Ait. in der Türkei. – Österr. Bot. Zeitschr. 118: 131-161.
Fischer MA. 1974. Beitrag zu einer systematischen Neubearbeitung der Gruppe um Pseudolysimachion spicatum (L.) Opiz (= Veronica spicata L.). – Phyton (Horn) 16: 29-47.
Fisher AE, McDade LA, Kiel CA, Khoshravesh R, Johnson MA, Stata M, Sage TL, Sage RF. 2015. Evolutionary history of Blepharis (Acanthaceae) and the origin of C4 photosynthesis in section Acanthodium. – Intern. J. Plant Sci. 176: 770-790.
Fitzgerald MA, Orlovich DA, Allaway WG. 1992. Evidence that abaxial leaf glands are the sites of salt secretion in leaves of the mangrove Avicennia marina (Forsk.) Vierh. – New Phytol. 120: 1-7.
Fleischmann A, Rivadavia F, Gonella PM, Heubl G. 2011. A revision of Genlisea subgenus Tayloria (Lentibulariaceae). – Phytotaxa 33: 1-40.
Foley MJY. 1998a. Taxonomic problems in Euopean members of the genus Orobanche L. – University of Lancaster, England.
Foley MJY. 1998b. Two new Orobanche species from the Arabian Peninsula. – Edinburgh J. Bot. 55: 229-234.
Foley MJY. 2001. Orobanchaceae in the ‘Flora iberica’ area: new taxa, excluded taxa, and typification. – An. Jard. Bot. Madrid 58: 223-233.
Foley MJY. 2004. Orobanchaceae of the Arabian Peninsula. – Candollea 59: 231-254.
Fonseca LHM, Lohmann LG. 2015. Biogeography and evolution of Dolichandra (Bignonieae, Bignoniaceae). – Bot. J. Linn. Soc. 179: 403-420.
Fonseca LHM, Lohmann LG. 2018. Combining high-throughput sequencing and targeted loci data to infer the phylogeny of the “Adenocalymma-Neojobertia” clade (Bignonieae, Bignoniaceae). – Mol. Phylogen. Evol. 123: 1-15.
Fonseca LHM, Cabral SM, Agra M de F, Lohmann LG. 2015. Taxonomic updates in Dolichandra Cham. (Bignonieae, Bignoniaceae). – PhytoKeys 46: 35-43.
Ford CM, Johnson SD. 2008. Floral traits, pollinators and breeding systems in Syncolostemon (Lamiaceae). – Plant Syst. Evol. 275: 257-264.
Fosberg FR, Herbst D. 1983 [1984]. A Nesogenes (Chloanthaceae) from Micronesia. – Micronesica 19: 11-15.
Fraga BM, González AG, Herrera JR, Luis JG, Ravelo AG. 1986. Diterpenes from the roots of Salvia canariensis. – Phytochemistry 25: 269.
Franchet A. 1900. Les Scrofularinées de la Chine, dans l’Herbier du Paris. – Bull. Soc. Bot. France 67: 16-21.
Francisco JNC, Lohmann LG. 2018. Taxonomic revision of Pachyptera (Bignonieae, Bignoniaceae). – PhytoKeys 92: 89-131.
Franzyk H, Jensen SR, Olsen CE. 2001. Iridoid glucosides from Myxopyrum smilacifolium. – J. Nat. Prod. 64: 632-633.
Frederiksen NO, Carr DR, Lowe GD, Wosika EP.
1983. Middle Eocene palynomorphs from San Diego, California. – Amer. Assoc.
Stratigr. Palynol., Contr. Ser. No. 12.
Freeman CE, Scogin R. 1999. Potential utility of chloroplast trnL (UAA) gene intron sequences for inferring phylogeny in Scrophulariaceae. – Aliso 18: 141-159.
Freeman CE, Harrison JS, Janovec JP, Scogin R. 2003. Inferred phylogeny in Keckiella (Scrophulariaceae) based on noncoding chloroplast and nuclear ribosomal DNA sequences. – Syst. Bot. 28: 782-790.
Freiberg M. 1994. Phänomorphologie epiphytischer Gesneriaceen unter besonderer Berücksichtigung des Mikroklimas. – Diss. Fak. Naturwiss., Universität Ulm, Germany.
Freiberg M. 1996. Phenotype expression of epiphytic Gesneriaceae under different microclimatic conditions in Costa Rica. – Ecotropica 2: 49-57.
Fries RE. 1924. Zur Kenntnis der Scrophulariaceen des tropischen Ostafrika. – Acta Horti Berg. 8: 45-70.
Fritsch K. 1895. Gesneriaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien IV(3b), W. Engelmann, Leipzig, pp. 133-185.
Fritsch K. 1900. Gloxinia stolonifera. – Engl. Bot. Jahrb. Syst. 37: 493-494.
Fritsch K. 1904. Die Keimpflanzen der Gesneriaceen mit besonderer Berücksichtigung von Streptocarpus, nebst vergleichender Studien über die Morphologie dieser Familie. – Gustav Fischer, Jena.
Fritsch K. 1908. Über das Vorkommen von Cystolithen bei Klugia zeylanica. – In: Linsbauer L (ed), Wiesner Festschrift, Konegen, Wien, pp. 412-416.
Fritsch K. 1934. Zur Kenntnis der Gattung Besleria. – Notizbl. Bot. Gart. Berlin-Dahlem 11: 961-977.
Fritsch PW, Almeda F, Martins AB, Cruz BC, Estes D. 2007. Rediscovery and phylogenetic placement of Philcoxia minensis (Plantaginaceae), with a test of carnivory. – Proc. Calif. Acad. Sci. 58: 447-467.
Fritze KJ, Williams NH. 1988. The taxonomic significance of pollen morphology in the Columnea alliance (Gesneriaceae: Gesnerioideae). – Ann. Missouri Bot. Gard. 75: 168-191.
Froissart C. 2008. La connaissance des sauges. – Aix-en-Provence, France.
Fromm-Trinta E. 1979. Revisão das espécies do gênero Genlisea St.-Hil. (Lentibulariaceae) das regiões sudeste e sul do Brasil. – Rodriguésia 31: 17-139.
Fromm-Trinta E. 1981. Revisão do gênero Genlisea St.-Hil. no Brasil. – Bol. Mus. Nac. Rio de Janeiro, Bot. 61: 1-21.
Fromm-Trinta E. 1989. Genlisea lobata Fromm-Trinta – uma nova espécie para o gênero Genlisea St. Hil. sect. Tayloria. – Bradea 5: 152-155.
Frost LA, McAdams Tyson S, Lu-Irving P, O’Leary N, Olmstead RG. 2017. Origins of North American arid-land Verbenaceae: more than one way to skin a cat. – Amer. J. Bot. 104: 1070-1716.
Fujii N, Ueda K, Watano Y, Shimizu T. 1997. Intraspecific sequence variation of chloroplast DNA in Pedicularis chamissonis Steven (Scrophulariaceae) and geographic structuring of the Japanese “alpine” plants. – J. Plant Res. 110: 195-207.
Fujita Y. 1970. Evolution of chromosome numbers in the Lamiaceae, especially its relation to the classification and phylogeny of the genus Salvia based on the constituents of essential oils. – Acta Phytotaxon. Geobot. 24: 113-121.
Fujiwara I. 1956. Karyotype analysis in Plantago II. – Jap. J. Genet. 31: 184-191.
Furness CA. 1989. Pollen morphology of Ecbolium and Megalochlamys (Acanthaceae). – Kew Bull. 44: 681-693.
Furness CA. 1990. Pollen morphology of Crossandra Salisbury and Crossandrella C. B. Clarke (Acanthaceae: Acantheae). – Grana 29: 161-176.
Furness CA. 1991. Pollen morphology of Sclerochiton (Acanthaceae: Acantheae). – Kew Bull. 46: 51-59.
Furness CA. 1992. A note on the pollen of Trichaulax mwasumbii (Acanthaceae: Justicieae). – Kew Bull. 47: 619-624.
Furness CA. 1993. A pollen morphological survey of the Old World species of Stenandrium Nees (Acanthaceae: Acantheae). – Grana 32: 1-11.
Furness CA. 1994a. The pollen morphology of Hygrophila and Brillantaisia (Acanthaceae: Ruellieae). – Acta Bot. Gallica 141: 267-278.
Furness CA. 1994b. The caveate pollen of Streptosiphon hirsutus (Acanthaceae: Acantheae) and its taxonomic significance. – Kew Bull. 49: 409-414.
Furness CA. 1995a. A pollen morphological study of Dyschoriste Nees and Chaetacanthus Nees (Acanthaceae: Ruellieae). – Rev. Palaeobot. Palyn. 84: 331-345.
Furness CA. 1995b. Examination of the ultrastructure and function of caveate Acanthaceae pollen, using rehydrated herbarium material. – Grana 34: 1-9.
Furness CA. 1996. Pollen morphology of Acanthopsis Harvey, Acanthus L. and Blepharis Jussieu (Acanthaceae: Acantheae). – Rev. Palaeobot. Palyn. 92: 253-268.
Furness CA. 1997. Abnormal pollen in Blepharis and other genera in Acanthaceae. – In: Proceedings of the 4th EPPC 58, pp. 273-283.
Furness CA. 1998. Pollen morphology of Neuracanthus (Acanthaceae). – Kew Bull. 53: 77-81.
Furness CA, Grant MC. 1996. Pollen morphology of some Ruellia species (Acanthaceae) from Africa and Madagascar. – Grana 35: 231-239.
Furness CA, Vollesen K. 1991. The identity of Asystasia striata S. Moore (Acanthaceae). – Kew Bull. 46: 729-731.
Fussell CP. 1958. Chromosome numbers in the Gesneriaceae. – Baileya 6: 117-125.
Gadella TWJ. 1980. Cytology. – In: Leeuwenberg AJM (ed), Engler and Prantl’s Die natürlichen Pflanzenfamilien, Fam. Loganiaceae, vol. 28b(1), Duncker & Humblot, Berlin, pp. 211-237.
Gaisberg M von. 2000. A revision of Teucrium heterophyllum L’Hér. (Lamiaceae) with two new subspecies of the Canary Islands. – Willdenowia 30: 263-271.
Galati BG, Strittmatter LI. 1999. Ovule ontogeny and megasporogenesis in Jacaranda mimosifolia D. Don (Bignoniaceae). – Phytomorphology 49: 67-74.
Galetto L. 1995. Nectary structure and nectar characteristics in some Bignoniaceae. – Plant Syst. Evol. 196: 99-121.
Galicia MA, Esquivel B, Sánchez AA, Cárdenas J, Rodríguez-Hahn L, Ramamoorthy TP. 1988. Abietane diterpenoids from Salvia pubescens. – Phytochemistry 27: 217.
Gamble JD. 1888. The Nilgiri Strobilanthes. – Indian Forester 14: 153-158.
Gándara E, Sosa V. 2013. Testing the monophyly and position of the North American shrubby desert genus Leucophyllum (Scrophulariaceae: Leucophylleae). – Bot. J. Linn. Soc. 171: 508-518.
Garber ED. 1956. The genus Collinsia I. Chromosome number and chiasma frequency of species in two sections. – Bot. Gaz. 118: 71-73.
Garber ED. 1960. The genus Collinsia IX. Speciation and chromosome repatterning. – Cytologia 25: 233-243.
Garcia JG. 1942. Contribuição paea o estudo cario-sistematico do género Lavandula L. – Bot. Soc. Brot. 13: 183-193.
Garcia-Peña MR. 1989. A new species of Cunila (Lamiaceae) from southwestern Mexico. – Kew Bull. 44: 727-730.
Garnock-Jones PJ. 1976. Breeding systems and pollination in New Zealand Parahebe (Scrophulariaceae). – New Zealand J. Bot. 14: 291-298.
Garnock-Jones PJ. 1993a. Heliohebe (Scrophulariaceae-Veroniceae), a new genus segregated from Hebe. – New Zealand J. Bot. 31: 323-339.
Garnock-Jones PJ. 1993b. Phylogeny of the Hebe complex (Scrophulariaceae: Veroniceae). – Aust. Syst. Bot. 6: 457-479.
Garnock-Jones PJ, Albach D, Briggs B. 2007. Botanical names in Southern Hemisphere Veronica (Plantaginaceae): sect. Detzneria, sect. Hebe, and sect. Labiatoides. – Taxon 56: 571-582.
Gasparino EC, Da Cruz-Barros MAV, Chautems A. 2013. Pollen morphology in Brazilian species of Codonanthe (Mart.) Hanst. and Nematanthus Schrader (Gesneriaceae). – Grana 52: 258-274.
Gasson P, Dobbins DR. 1991. Wood anatomy of the Bignoniaceae, with a comparison of trees and lianas. – IAWA Bull., N.S., 12: 389-417.
Gâteblé G, lemay V, Ounémoa J. 2009. Advances in Oxera breeding. – Acta Horticult. 836: 85-90.
Gates RR, Latter J. 1927. Observations on the pollen development of two species of Lathraea. – J. Roy. Microscop. Soc. 1927: 209-225.
Gaudeul M, Véla E, Rouhan G. 2015. Eastward colonization of the Mediterranean Basin by two geographically structured clades: the case of Odontites Ludw. (Orobanchaceae). – Mol. Phylogen. Evol. 96: 140-149.
Gaudeul M, Siljak-Yakovlev S, Jang T-S, Rouhan G. 2018. Reconstructing species relationships within the recently diversified genus Odontites Ludw. (Orobanchaceae): evidence for extensive reticulate evolution. – Intern. J. Plant Sci. 179: 1-20.
Gavriliuk VA. 1965. A contribution to the biology of the parasitic plant Boschniakia rossica (Cham. et Schlecht.) B. Fedtsch. – Bot. Žurn. 50: 523-528.
Gentry AH. 1971. Note on Gibsoniothamnus. – Fieldiana, Bot. 34: 55.
Gentry AH. 1973. Generic delimitations of Central Bignoniaceae: a striking case of convergent evolution. – Plant Syst. Evol. 126: 255-266.
Gentry AH. 1974a. Studies of Bignoniaceae 11: a synopsis of the genus Distictis. – Ann. Missouri Bot. Gard. 61: 494-501.
Gentry AH. 1974b. Gibsoniothamnus (Scrophulariaceae) in Panama. – Ann. Missouri Bot. Gard. 61: 533-537.
Gentry AH. 1974c. Coevolutionary patterns in Central American Bignoniaceae. – Ann. Missouri Bot. Gard. 61: 728-759.
Gentry AH. 1974d. Flowering phenology and diversity in tropical Bignoniaceae. – Biotropica 6: 64-68.
Gentry AH. 1976a. Relationships of the Malagasy Bignoniaceae: a striking case of convergent evolution. – Plant Syst. Evol. 126: 255-266.
Gentry AH. 1976b. Amphitecna-Enallagma-Dendrosicus revisited. – Taxon 24: 108.
Gentry AH. 1976c. Studies in Bignoniaceae 19: generic mergers and new species of South American Bignoniaceae. – Ann. Missouri Bot. Gard. 63: 46-80.
Gentry AH. 1977a. 178. Bignoniaceae. – In: Harling G, Sparre B (eds), Flora of Ecuador 7, Swedish Natural Science Research Council, Stockholm, pp. 1-172.
Gentry AH. 1977b. Notes on Middle American Bignoniaceae. – Rhodora 79: 430-444.
Gentry AH. 1977c. Studies in Bignoniaceae 26: new taxa and combinations in northwestern South American Bignoniaceae. – Phytologia 34: 183-198.
Gentry AH. 1980. Flora Neotropica. Monograph 25(1). Bignoniaceae I: Crescentieae and Tourrettieae. – New York Botanical Garden, Bronx, New York.
Gentry AH. 1983. Dispersal and distribution in Bignoniaceae. – Sonderb. Naturwiss. Ver. Hamburg 7: 187-199.
Gentry AH. 1988. Distribution and evolution of the Madagascar Bignoniaceae. – In: Goldblatt P, Lowry PP II (eds), Modern systematic studies in African botany. – Monogr. Syst. Bot. Missouri Bot. Gard. 25: 175-185.
Gentry AH. 1990a. Evolutionary patterns in neotropical Bignoniaceae. – Mem. New York Bot. Gard. 55: 118-129.
Gentry AH. 1990b. Sphingiphila (Bignoniaceae), a new genus from the Paraguayan Chaco. – Syst. Bot. 15: 277-279.
Gentry AH. 1992a. Distributional patterns of Central American and West Indian Bignoniaceae. – Tulane Stud. Zool. Bot. Suppl. 1: 111-125.
Gentry AH. 1992b. Flora Neotropica. Monograph 25(2). Bignoniaceae II: Tribe Tecomeae. – New York Botanical Garden, Bronx, New York.
Gentry AH. 1992c. Exarata (Bignoniaceae), a new genus from the Chocó region of Ecuador and Colombia. – Syst. Bot. 17: 503-507.
Gentry AH. 1992d. A synopsis of Bignoniaceae ethnobotany and economic botany. – Ann. Missouri Bot. Gard. 79: 53-64.
Gentry AH. 1993. Six new species of Adenocalymna (Bignoniaceae) from Eastern South America. – Novon 3: 137-141.
Gentry AH. 1997. Bignoniaceae. – In: Steyermark JA (ed), Flora of Venezuelan Guayana, Timber Press, Portland, Oregon, pp. 403-491.
Gentry AH, Tomb AS. 1979. Taxonomic implications of Bignoniaceae palynology. – Ann. Missouri Bot. Gard. 66: 756-777.
George K, Geethamma S. 1983. Lactopropionic orcein as a suitable stain for chromosomes of Oleaceae. – Curr. Sci. 52: 733-734.
George K, Geethamma S. 1984. Cytological and other evidences for the taxonomic position of Nyctanthes arbor-tristis L. – Curr. Sci. 53: 439-441.
George K, Geethamma S, Ninan CA. 1989. Chromosome evolution in Oleaceae. – J. Cytol. Genet. 24: 71-77.
Georgiev MI, Ali K, Alipieva K, Verpoorte R, Choi YH. 2011. Metabolic differentiations and classification of Verbascum species by NMR-based metabolomics. – Phytochemistry 72: 2045-2051.
Gergis V, Argyriadou N, Poulos C. 1989. Composition of the essential oils of Sideritis clandestina ssp. cyllenea and Sideritis sipylea. – J. Sci. Food Agric. 47: 501-507.
Ghanasekaran G, Murthy GVS, Deng YF. 2016. Resurrection of the genus Haplanthus (Acanthaceae: Andrographinae). – Blumea 61: 165-169.
Ghebrehiwet M. 1999. Phylogenetic and taxonomic studies in the tribe Antirrhineae (Scrophulariaceae) with special reference to the genera Kickxia and Nanorrhinum. – Acta Univ. Ups. Comprehensive summaries of Uppsala dissertations from the Faculty of Science and Technology 489.
Ghebrehiwet M. 2000. Taxonomy, phylogeny and biogeography of Kickxia and Nanorrhinum (Scrophulariaceae). – Nord. J. Bot. 20: 655-689.
Ghebrehiwet M, Bremer B, Thulin M. 2000. Phylogeny of the tribe Antirrhineae (Scrophulariaceae) based on morphological and ndhF sequence data. – Plant Syst. Evol. 220: 223-239.
Ghisalberti EL. 1994. The phytochemistry of the Myoporaceae. – Phytochemistry 35: 7-33.
Ghisalberti EL, Jefferies PR, Vu HTN. 1990. Diterpenes from Eremophila species. – Phytochemistry 29: 316-318.
Gibbs PE, Bianchi M. 1993. Post-pollination events of Chorisia (Bombacaceae) and Tabebuia (Bignoniaceae) with late-acting self-incompatibility. – Bot. Acta 106: 64-71.
Gibson DN. 1970. Flora of Guatemala. Verbenaceae. – Fieldiana Bot. 24: 167-236.
Gill LS. 1980. A study of Stachys palustris L. complex (Labiatae) in northern North America. – Phytologia 46: 231-245.
Gill LS. 1981. Chromosomal evolution and incidence of polyploidy in the Canadian Labiatae. – Rev. Cytol. Biol. Vég. Bot. 4: 331-339.
Gill LS. 1984. The incidence of polyploidy in the Western Himalayan Labiatae. – Rev. Cytol. Biol. Vég. Bot. 7: 5-16.
Gill LS, Chinnappa CC. 1982. Pollen morphology of the West-Himalayan Labiatae. – Bangladesh J. Bot. 11: 107-122.
Gillett JB, Carter S. 1958. Xylocalyx in Somaliland. – Kew Bull. 13: 359-360.
Gilmer K, Hofacker A. 2001. Sinningia macrostachya (Lindl.) Chautems. – Kakteen Sukk. 52: 155-158.
Giuliani C, Maleci Bini L. 2008. Insight into the structure and chemistry of glandular trichomes of Labiatae, with emphasis on subfamily Lamioideae. – Plant Syst. Evol. 276: 199-208.
Gleisner G, Ricardi M. 1969. Revision del genero Argylia (Bignoniaceae). – Gayana 19: 1-62.
Glišić LM. 1924. Development of the female X-generation and embryo in Ramondia. – Belgrade.
Glišić LM. 1928. Development of the female gametophyte and endosperm in Haberlea rhodopensis Friv. – Bull. Inst. Jard. Bot. Univ. Beograd 1: 1-13.
Glišić LM. 1931-1932. Zur Entwicklungsgeschichte von Lathraea squamaria L. – Glasn. Bot. Zav. Baš. Univ. Beogr. 2: 20-56.
Glišić LM. 1934. Zur Kenntnis der Samenentwicklung der Gesneriaceen. Über die Endosperm- und Haustorienbildung von Roettlera. – Bull. Inst. Jard. Bot. Univ. Beograd 3: 94-111.
Glišić LM. 1936-1937. Ein Versuch der Verwertung der Endospermmerkmale für typologische und phylogenetische Zwecke innerhalb der Scrophulariaceen. – Bull. Inst. Jard. Bot. Univ. Beograd 4: 42-73.
Glück H. 1934. Limosella-Studien. Beiträge zur Systematik, Morphologie und Biologie der Gattung Limosella. – Bot. Jahrb. Syst. 66: 488-566.
Glück H. 1940 [1941]. Die Gattung Trapella. – Bot. Jahrb. Syst. 71: 267-336.
Gnanasekaran G. 2015. A systematic study on the genus Andrographis Wall. ex Nees (Acanthaceae) in India. – Ph.D. diss., Bharathiar University, Tamil Nadu, India.
Gnanasekaran G, Murthy GVS, Deng YF. 2016. Resurrection of the genus Haplanthus (Acanthaceae: Andrographinae). – Blumea 61: 165-169.
Goebel K. 1893. Zur Biologie von Genlisea. – Flora 77: 208-212.
Goldblatt P, Gentry AH. 1979. Cytology of Bignoniaceae. – Bot. Not. 132: 475-482.
Goldblatt P, Keating RC. 1976. Chromosome cytology, pollen structure, and relationships of Retzia capensis. – Ann. Missouri Bot. Gard. 63: 321-325.
Gomez JC jr. 1955. Contribução a sistematica das Bignoniaceae Brasileiras. – Arq. Serv. Florest. 9: 261-296.
Gómez JM. 2002. Self-pollination in Euphrasia willkommii Freyn (Scrophulariaceae), an endemic species from the alpine of the Sierra Nevada (Spain). – Plant Syst. Evol. 232: 63-71.
Gómez-Laurito J, Hammel B. 1994. New species in the Acanthaceae of Costa Rica. – Novon 4: 350-361.
González AM. 2011. Domacios y nectarios extraflorales en Bignoniáceas: componentes vegetales de una interacción mutualística. – Bol. Soc. Argentinas Bot. 46:271-281.
González-Gallegos JG. 2014. Revision of Salvia subg. Calosphace sect. Membranacea (Lamiaceae). – Telopea 16: 43-81.
González-Gallegos JG, Gama-Villanueva OJ. 2013. Resurrection of Salvia species (Lamiaceae) recently synonymized in Flora Mesoamericana. – Phytotaxa 151: 1-24.
Gonzalez Martin M, Cabrera Perez MA, Gonzalez Artiles FJ. 1994. Germination of Canarian species of Globularia L. – Inv. Agrar. Producc. Protecc. Veg. 9: 29-34.
Good RD. 1923. Anomacanthus: a new genus of Acanthaceae. – J. Bot. 61: 161-164.
Good TC, Zjhra ML, Kremen C. 2006. Radiation and risk. Dealing with data deficiency in classifying extinction risk: a case study of a radiation of Bignoniaceae from Madagascar. – Conserv. Biol. 20: 1099-1110.
Gormley IC, Bedigian D, Olmstead RG. 2015. Phylogeny of Pedaliaceae and Martyniaceae and the placement of Trapella in Plantaginaceae s. l. – Syst. Bot. 40: 259-268.
Govaerts R, Dransfield J, Zona SF, Hodel DR, Henderson A. 2013. World checklist of Lamiaceae and Verbenaceae. – The Royal Botanic Gardens, Kew, United Kingdom. http://apps.kew.org/wcsp/
Govindarajan T, Subramanian D. 1983. Karyomorphological studies in South Indian Acanthaceae. – Cytologia 48: 491-504.
Grabow-Seidensticker H. 1988. Der Sesamum calycinum-Komplex (Pedaliaceae R. Br.). – Mitt. Inst. Allg. Bot. Hamburg 22: 217-241.
Gracie C. 1991. Observation of dual function of nectaries in Ruellia radicans (Nees) Lindau (Acanthaceae). – Bull. Torrey Bot. Club 118: 188-190.
Graham RA. 1957. Orobanchaceae. – In: Turrill WB, Milne-Redhead E (eds), Flora of tropical East Africa, Crown Agents for Oversea Governments and Administrations, London, pp. 1-7.
Graham VAW 1988. Delimitation and infrageneric classification of Justicia (Acanthaceae). – Kew Bull. 43: 551-624.
Granger R, Verdier R. 1964. Le γ-terpinène precurseur de p-cymène dans Thymus vulgaris L. – Compt. Rend. Acad. Sci. Paris 258: 5539-5541.
Grant AL. 1924. A monograph of the genus Mimulus. – Ann. Missouri Bot. Gard. 11: 99-389.
Grant E, Epling CC. 1943. A study of Pycnanthemum (Labiatae). – Univ. Calif. Publ. Bot. 20: 195-240.
Grant WF. 1955. A cytogenetic study in the Acanthaceae. – Brittonia 8: 121-149.
Grau J. 1976. Die Cytologie südwestmediterraner Scrophularia-Arten. – Mitt. Bot. Staatssamml. München 12: 609-654.
Gray JT. 1872. Notes on Labiatae. – Proc. Amer. Acad. Arts Sci. 8: 365-372.
Gray RG. 1937. Cork formation in Veronica lyallii. – Trans. Bot. Soc. Edinb. 32: 362-367.
Grayer RJ, Kok RPJ de. 1998. Flavonoids and verbascoside as chemotaxonomic characters in the genera Oxera and Faradaya (Labiatae). – Biochem. Syst. Ecol. 26: 729-741.
Grayer RJ, Kite GC, Goldstone FJ, Bryan SE, Paton A, Putievsky E. 1996. Infraspecific taxonomy and essential oil chemotypes in sweet basil, Ocimum basilicum. – Phytochemistry 43: 1033-1039.
Grayer RJ, Veitch NC, Kite GC, Price AM, Kokubun T. 2001. Distribution of 8-oxygenated leaf-surface flavones in Ocimum. – Phytochemistry 56: 559-567.
Grayer RJ, Eckert MR, Veitch NC, Kite GC, Marin PD, Kokubun T, Simmonds MSJ, Paton AJ. 2003. The chemotaxonomic significance of two bioactive caffeic acid esters, nepetoidins A and B, in the Lamiaceae. – Phytochemistry 64: 519-528.
Grayer-Barkmeijer R. 1978. Flavonoids in Parahebe and Veronica: a chemosystematic study. – Biochem. Syst. Ecol. 6: 131-137.
Grayer-Barkmeijer R. 1979. Veronica. – Ph.D. diss., Universiteit Leiden, The Netherlands.
Grayum MH, Hammel BE. 1995. The genus Tetranema (Scrophulariaceae) in Costa Rica, with two new species. – Phytologia 79: 269-280.
Graze H. 1935. Weitere Chromosomenuntersuchungen bei Veronica-Arten der Sektion Pseudolysimachia Koch. – Jahrb. Wiss. Bot. 81: 609-662.
Green PS. 1958. A monographic revision of Osmanthus in Asia and America. – Notes Roy. Bot. Gard. Edinb. 22: 439-542.
Green PS. 1961. Studies in the genus Jasminum I, Section Alternifolia. – Notes Roy. Bot. Gard. Edinb. 23: 355-384.
Green PS. 1972. Osmanthus decorus and disjunct Asiatic-European distributions in the Oleaceae. – Kew Bull. 26: 487-490.
Green PS. 1973. Negria rhabdothamnoides. – Curtis’s Bot. Mag., n. s., t. 659.
Green PS. 1985. Studies in the genus Jasminum (Oleaceae) IX. Notes on two jasmines from South India & Ceylon. – Kew Bull. 40: 225-230.
Green PS. 1986. Studies in the genus Jasminum L. (Oleaceae) X. Jasminum in Arabia. – Kew Bull. 41: 413-418.
Green PS. 1990. Ligustrum (Oleaceae) in southern India. – Kew Bull. 45: 693-696.
Green PS. 1991. Notes on Oleaceae for ‘Flora Mesoamericana’. – Kew Bull. 46: 273-276.
Green PS. 1994. A revision of Chionanthus (Oleaceae) in S. America and the description of Priogymnanthus, gen. nov. – Kew Bull. 49: 261-286.
Green PS. 1995a. Taxonomic notes relating to Ligustrum (Oleaceae). – Kew Bull. 50: 379-386.
Green PS. 1995b. Studies in the genus Jasminum (Oleaceae) XIV. New species and combinations in Jasminum, especially from Thailand. – Kew Bull. 50: 567-580.
Green PS. 1996. Taxonomic notes on Asiatic Chionanthus (Oleaceae). – Kew Bull. 51: 765-770.
Green PS. 1997. Studies in the genus Jasminum (Oleaceae) XV. A revision of the pinnate-leaved species of Jasminum. – Kew Bull. 52: 933-947.
Green PS. 2001. Studies in the genus Jasminum XVII: sections Trifoliolata and Primulina. – Kew Bull. 56: 903-915.
Green PS. 2002. A revision of Olea L. (Oleaceae). – Kew Bull. 57: 91-140.
Green PS. 2003. Synopsis of the Oleaceae from the Indian sub-continent. – Kew Bull. 58: 257-295.
Green PS. 2004. Oleaceae. – In: Kubitzki K, Kadereit JW (eds), The families and genera of vascular plants VII. Flowering plants. Dicotyledons. Lamiales (except Acanthaceae including Avicenniaceae), Springer, Berlin, Heidelberg, New York, pp. 296-306.
Green PS, Wickens GE. 1989. The Olea europaea complex. – In: Kit Tan (ed), The Davis & Hedge Festschrift, Edinburg University Press, Edinburgh.
Greene EL. 1892. On certain Californian Labiatae. – Pittonia 2: 301-302.
Greilhuber J. 1971. Chromosomenzahlen von Verbascum austriacum, V. lanatum und einigen Rhinanthoideen (Scrophulariaceae). – Mitt. Bot. Arbeitsgem. Oberösterr. Landesmus. Linz 3: 31-35.
Greilhuber J. 1974. Uncommon caryological features in the anther tapetum of some Pedicularieae (Scrophulariaceae). – Caryologia 24: 169-182.
Greilhuber J, Borsch T, Müller K, Worberg A, Porembski S, Bartlott W. 2006. Smallest angiosperm genomes found in Lentibulariaceae, with chromosomes of bacterial size. – Plant Biol. 8: 770-777.
Greuter W. 1987. Some notes on Lesquereuxia (Scrophulariaceae). – Bot. Jahrb. Syst. 108: 251-257.
Greuter W, Rodríguez RR. 2010. Notes on some endemic Cuban species of Ruelliinae (Acanthaceae), on their seeds, pollen morphology and hygroscopic features. – Willdenowia 40: 285-304.
Greuter W, Rodríguez RR. 2016. Revision of the Caribbean endemics currently placed in Nashia (Verbenaceae). – Willdenowia 46: 5-22.
Grevenstuk T, Hooft JJJ van der, Vervoort J, Waard P de, Romano A. 2009. Iridoid and caffeoyl phenylethanoid glycosides of the endangered carnivorous plant Pinguicula lusitanica L. (Lentibulariaceae). – Biochem. Syst. Ecol. 37: 285-289.
Grose SW, Olmstead RG. 2007a. Evolution of a charismatic neotropical clade: molecular phylogeny of Tabebuia s.l., Crescentieae, and allied genera (Bignoniaceae). – Syst. Bot. 32: 650-659.
Grose SW, Olmstead RG. 2007b. Taxonomic revisions in the polyphyletic genus Tabebuia s.l. (Bignoniaceae). – Syst. Bot. 32: 660-670.
Gscheidle A. 1924. Über Haustorienbildung in der Gattung Veronica und ihre systematische Wertung. – Flora 117: 144-172.
Guédès M. 1974. Le gynécée de Paulownia et Schlegelia et le problème de la délimitation des Scrofulariacées et Bignoniacées. – Compt. Rend. Acad. Sci., Paris, sér. D, 278: 2629-2632.
Guerin GR. 2005a. Nutlet morphology in Hemigenia R. Br. and Microcorys R. Br. (Lamiaceae). – Plant Syst. Evol. 254: 49-68.
Guerin GR. 2005b. Floral biology of Hemigenia and Microcorys (Lamiaceae). – Aust. J. Bot. 53: 147-162.
Guerin GR. 2008a. Evidence for polyphyly in Hemigenia and Microcorys (Lamiaceae: Westringieae). – Aust. Syst. Bot. 21: 313-325.
Guerin GR. 2008b. A taxonomic revision of Hemigenia section Malleantha sect. nov. (Lamiaceae: Westringieae). – Aust. Syst. Bot. 21: 326-374.
Guerin GR. 2009. A revision of Westringia section Cephalowestringia (Lamiaceae: Westringieae). – Aust. Syst. Bot. 22: 121-136.
Guignard ML. 1893. Recherche sur le développement de la graine et en particulier du tégument séminal: Labiées. – J. Bot. 7: 241-250.
Guilford VB, Fisk EL. 1952. Megasporogenesis and seed development in Mimulus tigrinus and Torenia fournieri. – Bull. Torrey Bot. Club 79: 6-24.
Guillaumet J-L, Cornet A. 1976. Observations sur les variations morphologiques saisonnières de quelques Labiées malgaches. – Adansonia 15: 515-529.
Guo J, Yuan Y, Liu Z, Zhu J. 2013. Development and structure of internal glands and external glandular trichomes in Pogostemon cablin. – PLoS ONE 8, e77862.
Guo S-Q, Xiong M, Ji C-F, Zhang Z-R, Li D-Z, Zhang Z-Y. 2011. Molecular phylogenetic reconstruction of Osmanthus Lour. (Oleaceae) and related genera based on three chloroplast intergenic spacers. – Plant Syst. Evol. 294: 57-64.
Gupta KK, Taneja SC, Dhar KL, Atal CK. 1983. Flavonoids of Andrographis paniculata. – Phytochemistry 22: 314-315.
Gupta ML, Bhambie S. 1978. Studies in Lamiaceae IV. Foliar appendages in Ocimum L. and their taxonomic significance. – Proc. Indian Natl. Sci. Acad., Sect. B, 44: 154-160.
Gupta ML, Bhambie S. 1980. Studies in Lamiaceae VI. Foliar appendages in certain species of Salvia. – Folia Geobot. Phytotaxon. 15: 95-100.
Gürke M. 1895. Labiatae africanae III. – Engl. Bot. Jahrb. Syst. 22: 128-148.
Gürke M. 1905. Labiatae africanae VI. – Engl. Bot. Jahrb. Syst. 36: 120-136.
Gussarova G, Popp M, Vitek E, Brochmann C. 2008. Molecular phylogeny and biogeography of the bipolar Euphrasia (Orobanchaceae): recent radiations in an old genus. – Mol. Phylogen. Evol. 48: 444-460.
Gussman AB, Gottsberger G. 1996. Differences in floral morphology, floral nectar constituents, carotenoids, and flavonoids in petals of orange and yellow Pyrostegia venusta (Bignoniaceae) flowers. – Phyton (Austria) 36: 161-171.
Gusuleac M. 1937. Über die Orientierung des Ovulums bei den Boraginaceen und Labiaten, nebst Ausblicken auf das System dieser Familien. – Cernauti, publ. by the author.
Gutierrez R. 2002. A molecular phylogeny of the family Martyniaceae (Order Lamiales) based on nrDNA internal transcribed spacer sequences. – University of Texas at El Paso.
Gutierrez R. 2008. Preliminary chloroplast DNA studies on the flowering plant family Martyniaceae (order Lamiales). – J. Arizona-Nevada Acad. Sci. 40: 105-110.
Güvenalp Z, Özbek H, Ünsalar T, Kazaz C, Demirezer LÖ. 2006. Iridoid, flavonoid, and phenylethanoid glycosides from Wiedemannia orientalis. – Turk. J. Chem. 30: 391-400.
Haccius B, Hartl-Baude E. 1956. Embryologische und histogenetische Studien an ”monokotylen Dikotylen” II. Pinguicula vulgaris L. und Pinguicula alpina L. – Österr. Bot. Zeitschr. 103: 567-587.
Hadjikyriakou G, Hand R. 2008. Notes on Teucrium sect. Polium (Lamiaceae) in Cyprus. – Willdenowia 38: 111-125.
Hagemann JM, Earle FR, Wolff IA. 1967. Search for new industrial oils XIV. Seed oils of Labatae. – Lipids 2: 371-380.
Hagen SH. 1941. A revision of the North American species of the genus Anisacanthus. – Ann. Missouri Bot. Gard 28: 385-408.
Hair JB, Arroyo MTK. 1984. Contributions to a chromosome atlas of the New Zealand flora 28. Ourisia. – New Zealand J. Bot. 22: 357-359.
Hajra PK, Daniel P, Philcox D. 1985. Hoshiarpuria minutiflora (Scrophulariaceae): a new genus and species from Punjab, India. – Kew Bull. 40: 607-608.
Håkansson A. 1926. Zur Zytologie von Celsia und Verbascum. – Lunds Univ. Årsskr., N. F., Ser. II, 21(10): 1-47.
Hakki MI. 1977. Embryologische und morphologische Untersuchungen an Pflanzen aus Südafrika I. Über die Embryologie, Morphologie und systematische Zugehörigkeit von Dermatobotrys saundersii Bolus. – Bot. Jahrb. Syst. 98: 93-119.
Hakki MI. 1980. Embryology. – In: Leeuwenberg AJM (ed), Engler and Prantl’s Die natürlichen Pflanzenfamilien, Fam. Loganiaceae, vol. 28b(1), Duncker & Humblot, Berlin, pp. 211-237.
Hallé F, Delmotte A. 1973. Croissance et floraison de la Gésneriacée africaine Epithema tenue C. B. Clarke. – Adansonia, sér. II, 13: 273-287.
Hallier H. 1897. Indonesische Acanthaceen. – Nova Acta Acad. Caes. Leop.-Carol. German. Nat. Cur. 70(3): 193-240.
Hallier H. 1902. Über Tetrachondra Petrie, eine Scrophularieengattung mit Klausenbildung. – Ber. Deutsch. Bot. Ges. 20: 221-224.
Hallier H. 1903. Über die Abgrenzung und Verwandtschaft der einzelnen Sippen bei den Scrophularineen. – Bull. Herb. Boiss., sér. II, 3: 181-207.
Hamann U. 1958. Morphologische und histogenetische Studien an Veronica-Arten I. Zur Morphologie der Blütenstände im Hinblick auf einen allgemeinen Infloreszenzbegriff. – Bot. Jahrb. Syst. 78: 69-118.
Hamann U. 1960. Morphologische Beobachtungen an Hebe diosmifolia (Scrophulariaceae), besonders ihren Infloreszenzen. – Bot. Jahrb. Syst. 79: 405-427.
Hambler DJ. 1953. Prochromosomes and supernumerary chromosomes in Rhinanthus minor Ehrh. – Nature 172: 629-630.
Hambler DJ. 1954. Cytology of the Scrophulariaceae and Orobanchaceae. – Nature 174. 838.
Hambler DJ. 1956. Further chromosome counts in Orobanchaceae. – Nature 177: 438-439.
Hamdi SMM, Assadi M, Zarre S, Aghabeigi F. 2006. A new species of Linaria Mill. sect. Linaria Mill. (Scrophulariaceae-Antirrhineae) from Iran. – Feddes Repert. 117: 501-507.
Hamdi SMM, Assadi M, Maasoumi AA. 2009. Two new species of Linaria section Linaria (Scrophulariaceae-Antirrhineae) from Iran. – Bot. J. Linn. Soc. 158: 734-742.
Hamilton AG. 1903. Notes on Byblis gigantea. – Proc. Linn. Soc. New South Wales 28: 680-684.
Hanlidou E, Kokkini S, Bosabalidis AM, Bessiere J-M. 1991. Glandular trichomes and essential oil constituents of Calamintha menthifolia (Lamiaceae). – Plant Syst. Evol. 177: 17-26.
Hansen B. 1983. Notes on the genus Chroesthes (Acanthaceae). – Nord. J. Bot. 3: 207-211.
Hansen B. 1985a. Studies on the Acanthaceae of Thailand. – Flora Malesiana Bull. 38: 173-178.
Hansen B. 1985b. Taxonomic revision of the SE Asian species of Isoglossa (Acanthaceae). – Nord. J. Bot. 5: 1-13.
Hansen B. 1985c. Notes on the genus Sphinctacanthus (Acanthaceae). – Nord. J. Bot. 5: 225-228.
Hansen B. 1985d. Notes on Andrographis and Gymnostachyum (Acanthaceae). – Nord. J. Bot. 5: 353-356.
Hansen B. 1987. Justicia sect. Grossa sect. nov. (Acanthaceae). – Nord. J. Bot. 7: 505-509.
Hansen B. 1988. Revision of Thysanostigma (Acanthaceae). – Nord. J. Bot. 8: 227-230.
Hansen B. 1989. Notes on SE Asian Acanthaceae 1. – Nord. J. Bot. 9: 209-215.
Hansen B. 1992. The genus Ptyssiglottis (Acanthaceae). A taxonomic monograph. – Opera Bot. 116: 1-58.
Hansen B. 1995. Notes on SE Asian Acanthaceae 2. – Nord. J. Bot. 15: 583-590.
Hansen OJ. 1975. The East African species of Sopubia (Scrophulariaceae). – Kew Bull. 30: 543-558.
Hansen OJ. 1976. The genus Rhamphicarpa Benth. emend. Engl. (Scrophulariaceae). – Bot. Tidsskr. 70: 103-125.
Hansen OJ. 1978. The genus Cycnium Benth. emend. Engl. (Scrophulariaceae): a taxonomic revision. – Dansk Bot. Ark. 32(3): 11-72.
Happ GB. 1937. Monograph of Tetramerium and Henrya. – Ann. Missouri Bot. Gard. 24 51-582.
Harborne JB. 1966. Comparative biochemistry of the flavonoids II. 3-desoxyanthocyanins and their systematic distribution in ferns and gesnerads. – Phytochemistry 5: 589-600.
Harborne JB. 1967. Comparative biochemistry of the flavonoids VI. Flavonoid patterns in the Bignoniaceae and the Gesneriaceae. – Phytochemistry 6: 1643-1651.
Harborne JB, Green PS. 1980. A chemotaxonomic survey of flavonoids in leaves of the Oleaceae. – Bot. J. Linn. Soc. 81: 155-167.
Harborne JB, Tomás-Barberán FA, Williams CA, Gil MI. 1986. A chemotaxonomic study of flavonoids from European Teucrium species. – Phytochemistry 25: 2811-2816.
Harkiss KJ. 1970. Pharmacognostical studies in the genera Linaria and Antirrhinum. – Ph.D. diss., University of Bradford, England.
Harley MM. 1992. The potential value of pollen morphology as an additional taxonomic character in subtribe Ociminae (Ocimeae: Nepetoideae: Labiatae). – In: Harley RM, Reynolds T (eds), Advances in labiate science, Royal Botanic Gardens, Kew, pp. 125-138.
Harley MM, Banks HI. 1994. Pollen morphology of two new East African species of Fernandoa (Bignoniaceae). – Kew Bull. 49: 391-400.
Harley MM, Wilde V 2005. Hexacolpate Lamiaceae pollen from the Eocene of Messel. – In: Harley RM, Reynolds T (eds), Advances in Labiate science, Royal Botanic Gardens, Kew, pp. 125-138.
Harley MM, Paton A, Harley RM, Cade PG. 1992. Pollen morphological studies in tribe Ocimeae (Nepetoideae: Labiatae) I. Ocimum L. – Grana 31: 161-176.
Harley RM. 1971. An explosive pollination mechanism in Eriope crassipes, a Brazilian labiate. – Biol. J. Linn. Soc. 3: 183-186.
Harley RM. 1974. Notes on New World Labiatae III. New collections of Labiatae from Brazil. – Kew Bull. 29: 125-140.
Harley RM. 1976. A review of Eriope and Eriopidion (Labiatae). – Hooker’s Ic. Plant. 38(3): 1-107.
Harley RM. 1983a. Notes on New World Labiatae IV. A taxonomic revision of Hyptis recurvata and its allies. – Kew Bull. 37: 637-642.
Harley RM. 1983b. Becium Lindl., a genus of Labiatae new to India and Sri Lanka. – Kew Bull. 38: 56.
Harley RM. 1985a. Notes on New World Labiatae VI. New taxa in Hyptis sect. Polydesmia Benth. from Bahia, Brazil. – Kew Bull. 40: 609-625.
Harley RM. 1985b. Notes on New World Labiatae VII. New taxa in Hyptis sect. Cyanocephalus Benth. from Brazil. – Kew Bull. 40: 627-634.
Harley RM. 1986a. Notes on New World Labiatae VIII. New species of Hyptis (Labiatae) from South America. – Kew Bull. 41: 141-150.
Harley RM. 1986b. Notes on New World Labiatae IX. Hyptis sect. Pachyphyllae (Epling) Harley, sect. nov., in Brazil. – Kew Bull. 41: 995-1005.
Harley RM. 1986c. Cuminia eriantha (Benth.) Benth. – Kew Mag. 3: 151-156.
Harley RM. 1988a. Evolution and distribution of Eriope (Labiatae) and its relatives in Brazil. – In: Vanzolini PE, Heyer WR (eds), Proc. Workshop Neotropical Distribution Patterns, Acad. Bras. De Ciências, Rio de Janeiro, pp. 71-120.
Harley RM. 1988b. Revision of generic limits in Hyptis Jacq. (Labiatae) and its allies. – Bot. J. Linn. Soc. 98: 87-95.
Harley RM. 1992. New taxa of Labiatae from the Pico das Almas and the Chapada Diamantina. – Kew Bull. 47: 553-580.
Harley RM. 1998. Notes on New World Labiatae XI. Studies on Hyptis Jacq. (Labiatae) in the Venezuelan Guayana. – Kew Bull. 53: 973-976.
Harley RM. 1999. A revision of Hyptis sect. Polydesmia subsect. Malvastra (Labiatae) in the Neotropics. – Kew Bull. 54: 395-404.
Harley RM. 2003. Validation of the name Lamioideae (Labiatae). – Kew Bull. 58: 765-766.
Harley RM. 2004. Nesogenaceae. – In: Kubitzki K, Kadereit JW (eds), The families and genera of vascular plants VII. Flowering plants. Dicotyledons. Lamiales (except Acanthaceae including Avicenniaceae), Springer, Berlin, Heidelberg, New York, pp. 293-295.
Harley RM. 2014. Four new taxa of
Oocephalus (Hyptidinae: Lamiaceae) from Bahia, Brazil. –
Kew Bull. 69: 9539.
Harley RM. 2015. Physominthe
(Hyptidineae, Lamiaceae), endemic
to Brazil, with a new species, P. longicaulis, from Bahia. – Kew
Bull. 70: 1.
Harley RM, Brighton CA. 1977. Chromosome numbers in the genus Mentha L. – Bot. J. Linn. Soc. 74: 71-96.
Harley RM, Demissew S. 1989. Two new Endostemon (Labiatae) in Somalia and Ethiopia. – Kew Bull. 44: 703-707.
Harley RM, Granda Paucar A. 2000. List of species of tropical American Clinopodium (Labiatae), with new combinations. – Kew Bull. 54: 917-927.
Harley RM, Heywood CA. 1992. Chromosome numbers in tropical American Labiatae. – In: Harley RM, Reynolds T (eds), Advances in labiate science, Royal Botanic Gardens, Kew, pp. 211-246.
Harley RM, Pastore JFB. 2012. A generic revision and new combinations in the Hyptidinae (Lamiaceae), based on molecular and morphological evidence. – Phytotaxa 58: 1-55.
Harley RM, Paton A. 1999. Notes on New World Scutellaria. – Kew Bull. 54: 221-225.
Harley RM, Reynolds T (eds). 1992. Advances in labiate science, Royal Botanic Gardens, Kew, pp. 211-246.
Harley RM, Giulietti AM, Santos FR dos. 2003. Holoregmia Nees, a recently rediscovered genus of Martyniaceae from Bahia, Brazil. – Kew Bull. 58: 205-212.
Harley RM, Paton AJ, Ryding O. 2003. New synonymy and taxonomic changes in the Labiatae. – Kew Bull. 58: 485-489.
Harley RM, Atkins S, Budantsev AL, Cantino PD, Conn BJ, Grayer R, Harley MM, Kok R de, Krestovskaja T, Morales R, Paton AJ, Ryding O, Upson T. 2004. Labiatae. – In: Kubitzki K, Kadereit JW (eds), The families and genera of vascular plants VII. Flowering plants. Dicotyledons. Lamiales (except Acanthaceae including Avicenniaceae), Springer, Berlin, Heidelberg, New York, pp. 167-275.
Harms H, Reiche C. 1895. Plantaginaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien IV(3b), W. Engelmann, Leipzig, pp. 363-373.
Harms S. 1999. Prey selection in three species of the carnivorous aquatic plant Utricularia (bladderwort). – Arch. Hydrobiol. 146: 449-470.
Harpe AC de la, Grobbelaar N, Visser JH. 1980. The ultrastructure of the chloroplast and the chlorophyll content of various South African parasitic flowering plants. – Zeitschr. Pflanzenphys. 100: 85-90.
Harpe AC de la, Visser JH, Grobbelaar N. 1981. Photosynthetic characteristics of some South African parasitic flowering plants. – Zeitschr. Pflanzenphys. 103: 265-275.
Harrison CJ, Möller M, Cronk QCB. 1999. Evolution and development of floral diversity in Streptocarpus and Saintpaulia. – Ann. Bot. 84: 49-60.
Hart JA. 1983. Systematic and evolutionary studies in the genus Lepechinia (Lamiaceae). – Ph.D. diss., Harvard University, Cambridge, Massachusetts.
Hart JA. 1985a. Peripheral isolation and the origin of diversity in Lepechinia sect. Parviflorae (Lamiaceae). – Syst. Bot. 10: 134-146.
Hart JA. 1985b. Evolution of dioecism in Lepechinia Will. Sect. Parviflorae (Lamiaceae). – Syst Bot. 10: 147-154.
Hartl D. 1955. Das Vorkommen rhinanthoider Knospendeckung bei Lindenbergia Lehm., einer Gattung der Scrophulariaceae-Antirrhinoideae. – Österr. Bot. Zeitschr. 102: 80-83.
Hartl D. 1956a. Die Stellung von Lindenbergia Lehmann im System der Scrophulariaceen. – Beitr. Biol. Pflanzen 32: 265-277.
Hartl D. 1956b. Die Beziehungen zwischen den Plazenten der Lentibulariaceen und Scrophulariaceen nebst einem Exkurs über die Spezialisationsrichtungen der Plazentation. – Beitr. Biol. Pflanzen 32: 471-490.
Hartl D. 1956c. Morphologische Studien am Pistill der Scrophulariaceen. – Österr. Bot. Zeitschr. 103: 185-242.
Hartl D. 1959. Das alveolierte Endosperm bei Scrophulariaceen, seine Entstehung, Anatomie und taxonomische Bedeutung. – Beitr. Biol. Pflanzen 35: 95-110.
Hartl D. 1962. Die morphologische Natur und die Verbreitung des Apicalseptums. – Beitr. Biol. Pflanzen 37: 241-330.
Hartl D. 1963. Das Placentoid der Pollensäcke, ein Merkmal der Tubifloren. – Ber. Deutsch. Bot. Ges. 76: 71-72.
Hartl D. 1967. Interkalarblätter bei Pedicularis L. – Österr. Bot. Zeitschr. 114: 119-124.
Hartl D. 1977. Rhabdotosperma, eine neue, aus Gliedern von Verbascum L. und Celsia L. gebildete Gattung der Scrophulariaceen. – Beitr. Biol. Pflanzen 53: 55-60.
Hartley IH, Balkwill K. 1990. A taxonomic account of Agathelpis, Globulariopsis, and Gosela (Scrophulariaceae). – J. South Afr. Bot. 56: 471-481.
Hartmann A. 1923. Zur Entwicklungsgeschichte und Biologie der Acanthaceen. – Flora 116: 216-258.
Hartmeyer S. 1997. Carnivory in Byblis revisited I. A simple method for enzyme testing on carnivorous plants. –Carniv. Plants Newsl. 26: 39-45.
Hartmeyer S. 1998. Carnivory in Byblis revisited II. The phenomenon of symbiosis on insect trapping plants. – Carniv. Plants Newsl. 27: 110-113.
Hartvig P. 1987. A taxonomical revision of Thymus sect. Teucrioides (Lamiaceae). – Plant Syst. Evol. 155: 197-213.
Harvey YB. 1996. The Stachys aculeolata/aethiopica complex in tropical Africa. – Kew Bull. 51: 433-454.
Hasegawa T, Koike K, Takahashi S, Ariyoshi U. 1982. Constituents of leaves and roots of Kailei Jio (Rehmannia glutinosa Libsch. Forma hueichingensis Hsiao). – Shoyakugaku Zasshi 36: 1-5.
Hassan N, Osman AK, El Garf IA. 2009. Pollen types of the Egyptian species of the genus Salvia (Lamiaceae). – Feddes Repert. 120: 394-404.
Hasselberg GBE. 1937. Zur Morphologie des vegetativen Sprosses der Loganiaceen. – Symb. Bot. Upsal. 2(3): 1-170.
Haston E, Ronse de Craene LP. 2007. Inflorescence and floral development in Streptocarpus and Saintpaulia (Gesneriaceae) with particular reference to the impact of bracteole suppression. – Plant Syst. Evol. 265: 13-25.
Hauk WD. 1997. A review of the genus Cydista (Bignoniaceae). – Ann. Missouri Bot. Gard. 84: 815-840.
Hauk WD. 1998. A review of the genus Paragonia (Bignoniaceae). – Ann. Missouri Bot. Gard. 85: 460-474.
Hawa A, Nyandat E, Galeffi C, Messana I, Nicoletti M, Marini-Bettollo GB. 1986. Cyclohexanols of Halleria lucida. – Phytochemistry 25: 2821-2823.
Heads MJ. 1994. Biogeographic studies in New Zealand Scrophulariaceae: tribes Rhinantheae, Calceolarieae and Gratioleae. – Candollea 49: 55-80.
Heart KM, Theobald WL. 1979. Comparative studies of vegetative anatomy and morphology of the Gesneriaceae of Sri Lanka. – Bot. J. Linn. Soc. 78: 285-298.
Heart RM, Theobald WL. 1979. Comparative studies of vegetative anatomy and morphology of the Gesneriaceae of Sri Lanka. – Bot. J. Linn. Soc. 78: 285-298.
Heckard LR. 1968. Chromosome numbers and polyploidy in Castilleja (Scrophulariaceae). – Brittonia 20: 212-226.
Heckard LR, Chuang T-I. 1975. Chromosome numbers and polyploidy in Orobanche (Orobanchaceae). – Brittonia 27: 179-186.
Heckard LR, Chuang T-I. 1977. Chromosome numbers, polyploidy, and hybridisation in Castilleja (Scrophulariaceae) of the Great Basin and Rocky Mountains. – Brittonia 29: 159-172.
Heckel E. 1894. Étude monographique de la famille des Globulariacées au point de vue botanique, chimique et thérapeutique. – Ann. Fac. Sci. Marseille, Paris, Suppl. 3.
Hedberg I, Hedberg O. 2003. Callitrichaceae. – In: Beentje HJ, Ghazanfar SA (eds), Flora of tropical East Africa, A. A. Balkema Publ., Lisse, The Netherlands, pp. 1-4.
Hedberg O. 1955. A taxonomic revision of the genus Sibthorpia L. – Bot. Not. 108: 161-183.
Hedberg O. 1970. The genus Zaluzianskya F. W. Schmidt (Scrophulariaceae) found in tropical East Africa. – Bot. Not. 123: 512-518.
Hedberg O. 1975. A cytogenetic study of the genus Sibthorpia L. (Scrophulariaceae). – Caryologia 28: 251-260.
Hedberg O, Ericson B, Grill-Willén A, Hunde A, Källsten L, Löfgren O, Ruuth T, Ryding O. 1979. The yellow-flowered species of Bartsia (Scrophulariaceae) in Tropical Africa. – Norw. J. Bot. 26: 1-9.
Hedberg O, Holmlund P-E, Mahunnah RLA, Mhoro B, Mziray WR, Nordenhed A-C. 1980. The Bartsia abyssinica-group (Scrophulariaceae) in tropical Africa. – Bot. Not. 133: 205-213.
Hedge IC. 1957. Studies in East Mediterranean species of Salvia. – Notes roy. Bot. Gard. Edinb. 22: 173-188.
Hedge IC. 1959. Studies in East Mediterranean species of Salvia II. – Notes Roy. Bot. Gard. Edinb. 23: 47-69.
Hedge IC. 1961a. Ziziphora. – Notes Roy. Bot. Gard. Edinb. 23: 209-221.
Hedge IC. 1961b. Studies in East Mediterranean species of Salvia VI. – Notes Roy. Bot. Gard. Edinb. 23: 559-567.
Hedge IC. 1963. A new species of Lamium from Nepal. – Notes Roy. Bot. Gard. Edinb. 30: 49-50.
Hedge IC. 1966. Studies in the flora of Afghanistan III. An account of Salvia. – Notes Roy. Bot. Gard. Edinb. 26: 407-425.
Hedge IC. 1970. Observations on the mucilage of Salvia fruits. – Notes Roy. Bot. Gard. Edinb. 30: 79-95.
Hedge IC. 1972a. Salvia in Madagascar. – Notes Roy. Bot. Gard. Edinb. 32: 1-11.
Hedge IC. 1972b. The pollination mechanism of Aeollanthus njassae. – Notes Roy. Bot. Gard. Edinb. 32: 45-48.
Hedge IC. 1974. A revision of Salvia in Africa including Madagascar and the Canary Islands. – Notes Roy. Bot. Gard. Edinb. 35: 1-121.
Hedge IC. 1982. Studies in the flora of Arabia II: some new and interesting species of Labiatae. – Notes Roy. Bot. Gard. Edinb. 40: 63-73.
Hedge IC. 1983. Two new monotypic genera of Labiatae. – Notes roy. Bot. Gard. Edinb. 41: 115-121.
Hedge IC. 1986. Labiatae of South-West Asia: diversity, distribution and endemism. – Proc. Roy. Soc. Edinb., Ser. B, 89: 23-35.
Hedge IC. 1992. A global survey of the biogeography of the Labiatae. – In: Harley RM, Reynolds T (eds), Advances in labiate science, Royal Botanic Gardens, Kew, pp. 7-18.
Hedge IC. 2005. A new species of Plectranthus (Lamiaceae) from Madagascar. – Adansonia, sér. III, 27: 321-323.
Hedge IC, Lamond JM. 1968. Studies in the flora of Afghanistan VII. Labiatae: Lam.-end. – Notes Roy. Bot. Gard. Edinb. 28: 89-191.
Hedge IC, Clement RA, Paton AJ, Phillipson PB. 1998. Fam. 175. Labiatae. – In: Morat P (ed), Flore de Madagascar et des Comores, Muséum National d’Histoire Naturelle, Paris, pp. 1-430.
Hedrén M. 1986. Two new species of Justicia sect. Harnieria (Acanthaceae) fron Tanzania. – Nord. J. Bot. 6: 301-306.
Hedrén M. 1988a. The Justicia capensis species group (sect. Harnieria, Acanthaceae) in tropical Africa. – Kew Bull. 43: 349-359.
Hedrén M. 1988b. Two new species in Justicia sect. Harnieria (Acanthaceae) from tropical Africa. – Nord. J. Bot. 8: 161-165.
Hedrén M. 1988c. The taxonomy of the Justicia mollugo group (Justicia sect. Harnieria, Acanthaceae). – Bull. Jard. Bot. Natl. Belg. 58: 129-158.
Hedrén M. 1989a. Variation within Justicia diclipteroides s. lat. (Justicia sect. Harnieria, Acanthaceae). – Bull. Mus. Natl. Hist. Nat. Paris, sér. IV, sect. B, Adansonia 10: 345-367.
Hedrén M. 1989b. Justicia sect. Harnieria (Acanthaceae) in tropical Africa. – Symb. Bot. Ups. 29(1): 1-141.
Hedrén M. 1990a. The Justicia elegantula complex (Justicia sect. Harnieria, Acanthaceae) in tropical Africa. – Bot. J. Linn. Soc. 103: 263-280.
Hedrén M. 1990b. Justicia tetrasperma sp. nov. – a linking species between Justicia and Monechma (Acanthaceae). – Nord. J. Bot. 10: 149-153.
Hedrén M. 1990c. Three new species of Justicia sect. Justicia (Acanthaceae) from tropical Africa. – Nord. J. Bot. 10: 265-271.
Hedrén M. 1990d. The Justicia striata complex in tropical Africa (Justicia sect. Harnieria, Acanthaceae). – Nord. J. Bot. 10: 357-398.
Hedrén M. 1993a. Ruellia nocturna sp. nov. (Acanthaceae) from Central Somalia. – Nord. J. Bot. 13: 511-513.
Hedrén M. 1993b. A new species of Lepidagathis (Acanthaceae) from central Somalia and the demise of Lindauea. – Nord. J. Bot. 13: 515-518.
Hedrén M. 1993c. Justicia galeata and J. kuchari, two new species of Justicia (Acanthaceae) from tropical Africa. – Nord. J. Bot. 13: 646-651.
Hedrén M. 2006. New species and combinations in Acanthaceae from Somalia. – Willdenowia 36: 751-759.
Hedrén M, Vollesen K. 1996. Ichthyostoma – a new genus in the Acanthaceae from NE tropical Africa. – Nord. J. Bot. 16: 441-444.
Hedrén M, Chase MW, Olmstead RG. 1995. Relationships in the Acanthaceae and related families as suggested by cladistic analysis of rbcL nucleotide sequences. – Plant Syst. Evol. 194: 93-109.
Hegelmaier F. 1864. Monographie der Gattung Callitriche. – Ebner & Seubert, Stuttgart.
Hegnauer R. 1969. Chemical evidence for the classification of some plant taxa. – In: Harborne JB, Swin T (eds), Perspectives in phytochemistry, London, New York, pp. 121-138.
Hegnauer R, Kooiman P. 1978. Die systematische Bedeutung von iridoiden Inhaltsstoffen im Rahmen von Wettstein’s Tubiflorae. – Plant. Med. (Stuttgart) 33: 1-33.
Heide-Jørgensen HS. 2008. Parasitic flowering plants. – Koninklijke Brill, Leiden, The Netherlands.
Heide-Jørgensen HS, Kuijt J. 1995. The haustorium of the root parasite Triphysaria (Scrophulariaceae), with special reference to xylem bridge ultrastructure. – Amer. J. Bot. 82: 782-797.
Heil H. 1927. Vergleichend-anatomische Studien an Samen von Chamaegigas und verwandten Gattungen. – Ber. Deutsch. Bot. Ges. 45: 555-561.
Heilmeier H, Ratcliffe G, Hartung W. 2000. Urea: a nitrogen source for the aquatic resurrection plant Chamaegigas intrepidus Dinter. – Oecologia 123: 9-14.
Heine H. 1962. Tropical African plants XXVI: some West African Acanthaceae. – Kew Bull. 16: 161-183.
Heine H. 1963. Acanthaceae. – In: Hutchinson J, Dalziel JM (eds), Flora of West Tropical Africa, 2nd ed., Crown Agents for Overseas Governments and Administrations, London, pp. 391-432.
Heine H. 1966a Acanthacées. – In: Aubréville A (ed), Flore du Gabon 13, Muséum National d’Histoire Naturelle, Paris, pp. 1-250.
Heine H. 1966b. Révision du genre Thomandersia Baill. (Acanthaceae). – Bull. Jard. Bot. État Bruxelles 36: 207-248.
Heine H. 1971. Notes sur les Acanthacées africaines: Hygrophila R. Br. – Adansonia, sér. II, 11: 656-659.
Heine H, Raynal A. 1968. Benoicanthus Heine and A. Raynal (Acanthaceae), nouveau genre Malgachée. – Adansonia 8: 189-198.
Heinrich G. 1973a. Entwicklung, Feinbau und Ölgehalt der Drüsenschuppen von Monarda fistulosa. – Planta Medica 23: 154-166.
Heinrich G. 1973b. Die Feinstruktur der Trichom-Hydathoden von Monarda fistulosa. – Protoplasma 77: 271-278.
Heinrich G. 1977. Die Feinstruktur und das ätherische Öl eines Drüsenhaares von Monarda fistulosa. – Biochem. Physiol. Pflanzen 171: 17-24.
Heinrich M. 1992. Economic botany of American Labiatae. – In: Harley RM, Reynolds T (eds), Advances in labiate science, Royal Botanic Gardens, Kew, pp. 475-488.
Heinricher E. 1902. Die grünen Halbschmarotzer IV. Nachträge zu Euphrasia, Odontites und Alectorolophus. – Jahrb. Wiss. Bot. 37: 264-337.
Heinricher E. 1931. Monographie der Gattung Lathraea. – Jena.
Heisey RM, Delwiche CC. 1984. Phytotoxic volatiles from Trichostema lanceolatum (Labiatae). – Amer. J. Bot. 71: 821-828.
Hemsley WB. 1913. On the genera Radamaea Benth. and Nesogenes A. de Candolle. – J. Linn. Soc. Bot. 41: 311-316.
Henderson DM, Prentice H, Hedge IC. 1968. Pollen morphology of Salvia and some related genera. – Grana Palynol. 8: 70-85.
Henderson LB. 1926. Floral anatomy of several species of Plantago. – Amer. J. Bot. 13: 397-405.
Henrickson J. 1982. On the recognition of Trichostema mexicanum Epling (Lamiaceae). – Madroño 29: 104-108.
Henrickson J, Flyr LD. 1985. Systematics of Leucophyllum and Eremogeton (Scrophulariaceae). – Sida 11: 107-172.
Henrickson J, Hiriart P. 1988. New species and transfers into Justicia (Acanthaceae). – Aliso 12: 45-58.
Hepper FN. 1984. Notes on tropical African Scrophulariaceae. – Kew Bull. 38: 598.
Hepper FN. 1987a. Two new species of East African Craterostigma (Scrophulariaceae). – Kew Bull. 42: 945-946.
Hepper FN. 1987b. Transfer of three tropical African species of Craterostigma to Torenia (Scrophulariaceae). – Bol. Soc. Broteriana, Sér. II: 271-272.
Hepper FN. 1989. Two new species of parasitic Harveya (Scrophulariaceae) from East Africa. – Kew Bull. 44: 163-165.
Hepper FN. 1992. Another new species of Harveya (Scrophulariaceae) from East Africa. – Kew Bull. 47: 729-730.
Herl V, Albach DC, Müller-Uri F, Bräuchler C, Heubl G, Kreis W. 2008. Using progesterone 5β-reductase, a gene encoding a key enzyme in the cardenolide biosynthesis, to infer the phylogeny of the genus Digitalis. – Plant Syst. Evol. 271: 65-78.
Hernández M, Esquivel B, Cárdenas J, Rodríguez-Hahn L, Ramamoorthy TP. 1987. Diterpenoid abietane quinines isolated from Salvia regla. – Phytochemistry 26: 3297-3299.
Heslop-Harrison Y, Heslop-Harrison J. 1981. The digestive glands of Pinguicula: structure and cytochemistry. – Ann. Bot., N. S., 47: 293-319.
Heslop-Harrison Y, Knox RB. 1971. A cytochemical study of leaf-gland enzymes of insectivorous plants of the genus Pinguicula. – Planta 96: 183-211.
Hesse M, Morawetz W. 1980. Skulptur und systematischer Wert der Samenoberfläcke bei Jacaranda und anderen Bignoniaceae. – Plant Syst. Evol. 135: 1-10.
Hevly RH. 1969. Nomenclatural history and typification of Martynia and Proboscidea (Martyniaceae). – Taxon 18: 527-534.
Hickel B. 1967. Zur Kenntnis einer xerophilen Wasserpflanze: Chamaegigas intrepidus Dtr. aus Südwestafrika. – Intern. Rev. Ges. Hydrobiol. 52: 361-400.
Hidalgo P, Hesse M, Ubera J, Frosch-Radivo A. 1999 [2000]. Microsporogenesis in male sterile Rosmarinus officinalis L. (Lamiaceae), an ultrastructural study. – Grana 38: 343-355.
Hielscher T. 1883. Anatomie und Biologie der Gattung Streptocarpus. – Beitr. Biol. Pflanzen 3: 1-24.
Hiesey WM, NBS MA, Bjorkman O. 1971. Experimental studies in the nature of species V. Biosystematics, genetics, and physiological ecology of the Erythranthe section of Mimulus. – Carnegie Institution of Washington Publ. 628, Washington, D.C.
Higashiyama T, Inatsugi R, Sakamoto S, Sasaki N, Mori T, Kuroiwa H, Nakada T, Nozaki H, Kuroiwa T, Nakano A. 2006. Species preferentiality of the pollen tube attractant derived from the synergid cell of Torenia fournieri. – Plant Physiol. 142: 481-491.
Hill AW. 1938. The monocotylous seedlings of certain dicotyledons, with special reference to the Gesneriaceae. – Ann. Bot. (London) 2: 127-143.
Hilliard OM. 1990. A brief survey of Scrophulariaceae-Selagineae. – Edinburgh J. Bot. 47: 315-343.
Hilliard OM. 1992. Miscellaneous new names and combinations in Scrophulariaceae – Manuleae. – Edinburgh J. Bot. 49: 297.
Hilliard OM. 1994. The Manuleeae, a tribe of Scrophulariaceae. – Edinburgh University Press, Edinburgh.
Hilliard OM. 1995. Pseudoselago, a new segregate from Selago. – Edinburgh J. Bot. 52: 243-332.
Hilliard OM. 1999. The tribe Selagineae (Scrophulariaceae). – Royal Botanic Gardens, Edinburgh.
Hilliard OM. 2003. A revision of Chirita Sect. Liebigia (Gesneriaceae). – Edinburgh J. Bot. 60: 361-387.
Hilliard OM, Burtt BL. 1971. Streptocarpus, an African plant study. – University of Natal Press, Pietermaritzburg, Republic of South Africa.
Hilliard OM, Burtt BL. 1977. Notes on some plants of Southern Africa chiefly from Natal VI. – Notes Roy. Bot. Gard. Edinb. 35: 155-177.
Hilliard OM, Burtt BL. 1984. A revision of Diascia section Racemosae. – J. South Afr. Bot. 50: 2969-340.
Hilliard OM, Burtt BL. 2002. The genus Agalmyla (Gesneriaceae-Cyrtandroideae). – Edinburgh J. Bot. 59: 1-210.
Hillson CJ. 1959. Comparative studies of floral morphology of the Labiatae. – Amer. J. Bot. 46: 451-459.
Hilsenbeck RA. 1989. Taxonomy of Yeatesia (Acanthaceae). – Syst. Bot. 14: 427-438.
Hilsenbeck RA. 1990. Systematics of Justicia sect. Pentaloba (Acanthaceae). – Plant Syst. Evol. 169: 219-235.
Hilsenbeck RA, Marshall DL. 1983. Schaureria calycobractea (Acanthaceae), a new species from Veracruz, Mexico. – Brttonia 35: 362-366.
Hilton JL, Boyd RS. 1996. Microhabitat requirements and seed/microsite limitation of the rare granite outcrop endemic Amphianthus pusillus (Scrophulariaceae). – Bull. Torrey Bot. Club 123: 189-196.
Himmelbaur W, Stibal E. 1933-1935. Entwicklungsrichtungen in der Blütenregion der Gattung Salvia L. I-III. – Biol. Gener. 8: 449-474 (1933); 9: 129-150 (1934); 10: 17-48 (1935).
Hinsinger DD, Gaudeul M, Couloux A, Bousquet
J, Frascaria-Lacoste N. 2014. The phylogeography of Eurasian Fraxinus
species reveals ancient transcontinental reticulation. – Molec. Phylogen.
Evol. 77: 223-237.
Hjertson ML. 1995. Taxonomy, phylogeny and biogeography of Lindenbergia (Scrophulariaceae). – Bot. J. Linn. Soc. 119: 265-321.
Hjertson ML. 1997. Systematics of Lindenbergia and Campylanthus (Scrophulariaceae). – Acta Univ. Upsal. 331, Uppsala.
Hjertson ML, Henrot J, Thulin M. 2008. Campylanthus hajarensis sp. nov. and a new record of Campylanthus (Scrophulariaceae) from Oman. – Nord. J. Bot. 26: 35-37.
Hobein M. 1884. Über den systematischen Werth der Cystolithen bei den Acanthaceen. – Engl. Bot. Jahrb. Syst. 5: 422-440.
Hoehne FC. 1958. Novidades da familia das Gesneriaceae do Brasil. – Sellowia 9: 37-81.
Hofelich A. 1935. Die Sektion Alsinebe Griseb. der Gattung Veronica in ihren chromosomalen Grundlagen. – Jahrb. Wiss. Bot. 81: 541-572.
Hofmann H-P, Fischer E. 1998. Preliminary revision of the genus Sopubia (Scrophulariaceae) in Madagascar. – Bull. Mus. Natl. Hist. Nat., sect. B, Adansonia, sér. 4, 20: 299-312.
Hofmann H-P, Fischer E. 2004. Generic delimitation of Sopubia Buch.-Ham. (Scrophulariaceae), revision of Petitmenginia Bonati and description of the new Asian genus Parasopubia. – Bot. Jahrb. Syst. 125: 341-375.
Hoggard RK, Kores PJ, Molvray M, Hoggard GD, Broughton DA. 2003. Molecular systematics and biogeography of the amphibious genus Littorella (Plantaginaceae). – Amer. J. Bot. 90: 429-435.
Hollstein RTH. 1878. Ueber den Gefässbündelverlauf im Stammen der Gesneraceen. – Diss. Universität Halle, Germany.
Holm T. 1913. Phryma leptostachya L., a morphological study. – Bot. Gaz. 56: 306-318.
Holm T. 1924. Polypremum procumbens L. A morphological study. – Amer. J. Sci. 207: 210-218.
Holmgren NH. 1998. Two new species of Penstemon (Scrophulariaceae: sect. Saccanthera) from Nevada, U.S.A. – Brittonia 50: 159-164.
Holmgren NH, Molau U. 1984. 177. Scrophulariaceae. – In: Harling G, Sparre B (eds), Flora of Ecuador 21, Swedish Natural Science Research Council, Stockholm, pp. 1-188.
Holmqvist JP-H, Manktelow M, Daniel TF. 2005. Wing pollination by bees in Mexanthus (Acanthaceae)? – Acta Bot. Mexicana 71: 11-17.
Holub J. 1970. Lamiastrum versus Galeobdolon and comments on problems of unitary designations in Fabricius’s work “Enumeratio methodica plantarum horti medici helmstadiensis”. – Folia Geobot. Phytotaxon. 5: 61-88.
Holub J. 1990. Some taxonomic and nomenclatural changes within Orobanche s.l. (Orobanchaceae). – Preslia 62: 193-198.
Honda M, Sakisaka M. 1930. Trapellaceae. – Daikou Nippon Shokubutsu Bunraigaku: 378. [In Japanese]
Hong D-Y. 1980a. Wulfeniopsis Hong – a new genus of Scrophulariaceae from Himalaya. – Acta Phytotaxon. Sin. 18: 50-52.
Hong D-Y. 1980b. Kashmiria (Scrophulariaceae, Veroniceae), a new name for Falconeria Hook. fil. from the western Himalayas. – Bot. Not. 133: 565-567.
Hong D-Y. 1983. The distribution of Scrophulariaceae in the holarctic with special reference to the floristic relationships between eastern Asia and eastern North America. – Ann. Missouri Bot. Gard. 70: 701-712.
Hong D-Y. 1984. Taxonomy and evolution of the Veroniceae (Scrophulariaceae) with special reference to palynology. – Opera Bot. 75: 1-60.
Hong D-Y, Nilsson S. 1983. On the validity of the genus Cochlidiosperma Reichenb. (Scrophulariaceae) as supported by additional palynological evidence. – Acta Phytotaxon. Sin. 21: 146-150.
Hong-Wa C, Besnard G. 2013. Intricate patterns of phylogenetic relationships in the olive family as inferred from multi-locus plastid and nuclear DNA sequence analyses: a close-up on Chionanthus and Noronhia (Oleaceae). – Mol. Phylogen. Evol. 67: 367-378.
Hong-Wa C, Besnard G. 2014. Species limits and diversification in the Madagascar olive (Noronhia, Oleaceae). – Bot. J. Linn. Soc. 174: 141-161.
Hooghiemstra H. 1983. Pollen morphology of the Plantago species of the Colombian Andes and its application to fossil material. – Rev. Acad. Colomb. Ci. Exact. 15: 41-66.
Hosozawa S, Kato N, Munakata K. 1972. 5,6,7-Trimethoxy flavone from Callicarpa japonica. – Phytochemistry 11: 2362.
Hossain ABME. 1971. Studies in the classification and affinities of Acanthaceae. – Ph.D. diss., University of Edinburgh, Scotland.
Hossain ABME. 1973. Notes on Asiatic Acanthaceae. – Notes Roy. Bot. Gard. Edinb. 32: 405-410.
Hossain ABME, Emumwen T. 1981. Apropos of Nelsonia canescens and N. smithii (Acanthaceae). – Kew Bull. 36: 565-568.
Hosseus CC. 1907. Die aus Siam bekannten Acanthaceen. – Engl. Bot. Jahrb. Syst. 41: 61-73.
Houghton PJ, Mensah AY, Iessa N, Hong LY. 2003. Terpenoids in Buddleja: relevance to chemosystematics, chemical ecology and biological activity. – Phytochemistry 64: 385-393.
Howard RA. 1975. The genus Anetanthus (Gesneriaceae). – J. Arnold Arbor. 56: 364-368.
Howe GF. 1986. A note on the trigger pollination mechanism in the camphor weed (Trichostema lanceolatum) as related to pollinator weight and behaviour. – Bull. South Calif. Acad. Sci. 85: 177-179.
Howell JT. 1931. The genus Pogogyne. – Proc. Calif. Acad. Sci., Ser. IV, 20: 105-128.
Hrubý K. 1934. Zytologie und Anatomie der mitteleuropäischen Salbei-Arten. – Beih. Bot. Centralbl. A, 52: 298-380.
Hrubý K. 1962. Key to the supraspecific taxa of the genus Salvia. – Preslia 34: 368-373.
Hsieh TH, Huang TC. 1995. Notes on the flora of Taiwan (20). Scutellaria (Lamiaceae) in Taiwan. – Taiwania 40: 35-56.
Hu S-Y. 1958. A monograph of the genus Paulownia. – Quart. J. Taiwan Mus. 12: 1-54.
Hu S-Y. 1974. A contribution to our knowledge of Leonurus L. I-mu-ts’ao, the Chinese motherword. – J. Chin. Univ. Hong Kong 2: 365-387.
Huang M. 2002. Systematics of Trichostema L. (Lamiaceae) and phylogenetic relationships with its disjunct taxa in Asia. – Ph.D. diss., Ohio State University, Columbus, Ohio.
Huang M, Freudenstein JV, Crawford DJ. 2000. Phylogenetic relationships of the Caryopteris-Trichostema complex (Lamiaceae) based on ndhF sequence data. – Amer. J. Bot. 87: 174-175.
Huang M, Crawford DJ, Freudenstein JV, Cantino PD. 2008. Systematics of Trichostema (Lamiaceae): evidence from ITS, ndhF, and morphology. – Syst. Bot. 33: 437-446.
Huang S-Q, Fenster CB. 2007. Absence of long-proboscid pollinators for long-corolla-tubed Himalayan Pedicularis species: implications for the evolution of corolla length. – Intern. J. Plant Sci. 168: 325-331.
Huang TC, Wu JT. 1975. A revision of Formosan Salvia. – Taiwania 20: 213-228.
Huber E. 1953. Beitrag zur anatomischen Untersuchung der Antheren von Saintpaulia. – Sitzungsber. Akad. Wiss. Wien, Math.-Naturwiss. Kl., Abt. I, 162: 227-234.
Huber-Morath A. 1971. Die türkischen Verbasceen. – Denkschr. Schweiz. Naturf. Ges. 87: 1-166.
Huber-Morath A. 1973. Verbascum L. s.l. (incl. Celsia L. et Staurophragma Fisch. & Mey.). – Bauhinia 5: 7-16.
Huber-Morath A, Rechinger KH. 1960. Zur Kenntnis der Gattungen Verbascum und Celsia in Griechenland. – Mitt. Thüring. Bot. Ges. 2: 42-55.
Huck RB. 1987. Systematics and evolution of Dicerandra (Labiatae). – Phanerog. Monogr. 19: 1-343.
Huck RB. 1992. Overview of pollination biology in the Labiatae. – In: Harley RM, Reynolds T (eds), Advances in labiate science, Royal Botanic Gardens, Kew, pp. 167-181.
Huck RB, Judd WS, Whitten WM, Skean JD Jr, Wunderlin RP, Delaney KR. 1989. A new Dicerandra (Labiatae) from the Lake Wales Ridge of Florida, with a cladistic analysis and discussion of endemism. – Syst. Bot. 14: 197-213.
Hueso-Rodríguez JA, Jimeno ML, Rodríguez B, Savona G, Bruno M. 1983. Abietane diterpenoids from the root of Salvia phlomoides. – Phytochemistry 22: 2005.
Hufford L. 1992. Floral structure of Besseya and Synthyris (Scrophulariaceae). – Intern. J. Plant Sci. 153: 217-229.
Hufford L, McMahon M. 2004. Morphological evolution and systematics of Synthyris and Besseya (Veronicaceae): a phylogenetic analysis. – Syst. Bot. 29: 716-736.
Hunziker AT, Di Fulvio E. 1958. Observaciones morfológicas sobre Peltanthera (Loganiaceae), con referencia a su posicion systematica. – Bol. Acad. Nac. Ci. 40: 217-228.
Hussain H, Aziz S, Miana GA, Ahmad VU, Anwar S, Ahmed I. 2009. Minor chemical constituents of Verbascum thapsus. – Biochem. Syst. Ecol. 37: 124-126.
Huynh KL. 1968. Étude de la morphologie du pollen du genre Utricularia L. – Pollen Spores 10: 11-55.
Huynh KL. 1972. Le pollen et la systématique du genre Sideritis L. (Labiatae). – Bull. Mus. Natl. Hist. Nat. Paris, sér. III, Bot. 45: 1-26.
Ietswaart JH. 1980. A taxonomic revision of the genus Origanum (Labiatae). – Leiden Bot. Ser. 4: 1-153.
Ihlenfeldt H-D. 1967. Über die Abgrenzung und die natürliche Gliederung der Pedaliaceae R. Br. – Mitt. Staatsinst. Allg. Bot. Hamburg 12: 43-128.
Ihlenfeldt H-D. 1994. Phytogeography of Pedaliaceae R. Br. – In: Proc. 13th Plen. Meet. AETFAT, Malawi, vol. 2, pp. 1063-1075.
Ihlenfeldt H-D. 2001. Fruits in Pterodiscus Hook. and a key to the species. – Mitt. Inst. Allg. Bot. Hamburg 28-29: 5-21.
Ihlenfeldt H-D. 2004a. Martyniaceae. – In: Kubitzki K, Kadereit JW (eds), The families and genera of vascular plants VII. Flowering plants. Dicotyledons. Lamiales (except Acanthaceae including Avicenniaceae), Springer, Berlin, Heidelberg, New York, pp. 283-288.
Ihlenfeldt H-D. 2004b. Pedaliaceae. – In: Kubitzki K, Kadereit JW (eds), The families and genera of vascular plants VII. Flowering plants. Dicotyledons. Lamiales (except Acanthaceae including Avicenniaceae), Springer, Berlin, Heidelberg, New York, pp. 307-322.
Ihlenfeldt H-D. 2004c. Trapellaceae. – In: Kubitzki K, Kadereit JW (eds), The families and genera of vascular plants VII. Flowering plants. Dicotyledons. Lamiales (except Acanthaceae including Avicenniaceae), Springer, Berlin, Heidelberg, New York, pp. 445-448.
Ihlenfeldt H-D. 2010. Pedaliaceae – evolution and phylogeny of the succulent genera. – Schumannia 6: 151-182.
Ihlenfeldt H-D, Grabow-Seidensticker U. 1979. The genus Sesamum and the origin of cultivated sesame. – In: Kunkel K (ed), Taxonomic aspects of African economic botany, Proc. 9th Plen. Meet. AETFAT, Las Palmas de Gran Canaria 1978, pp. 53-60.
Ihlenfeldt H-D, Hartmann H. 1970. Monographien der afrikanischen Pedaliaceae II. Die Gattung Harpagophytum (Burch.) DC. ex Meissn. – Mitt. Staatsinst. Allg. Bot. Hamburg 13: 15-69.
Imaichi R, Inokuchi S, Kato M. 2001. Developmental morphology of the one-leaf plant Monophyllaea singularis (Gesneriaceae). – Plant Syst. Evol. 229: 171-185.
Imaichi R, Omura-Shimadate M, Ayano M, Kato M. 2007. Developmental morphology of the caulescent species Streptocarpus pallidiflorus (Gesneriaceae), with implications for evolution of monophylly. – Intern. J. Plant Sci. 168: 251-260.
Imlay JB. 1938. The taxonomy of the Siamese Acanthaceae. – Ph.D. diss., University of Aberdeen, Scotland.
Imlay JB. 1939. Contributions to the Flora of Siam. Additamentum 51. New and re-named Siamese Acanthaceae. – Kew Bull. 1939: 109-150.
Immelman KL. 1986. Notes on Southern African species of Justicia L. – Bothalia 16: 39-64.
Immelman KL. 1992. Studies in the Justicia and Siphonoglossa (Acanthaceae) species of southern Africa: final conclusions. – Bothalia 22: 171-175.
Inamdar JA. 1969a. Epidermal structure and ontogeny of stomata in some Verbenaceae. – Ann. Bot., N. S., 33: 55-66.
Inamdar JA. 1969b. Epidermal structure and stomatal ontogeny in vegetative and floral organs of Martynia annua L., Pedalium murex L.. and Sesamum indicum L. – Flora, Abt. B, 158 (1968/1969): 526-537.
Inamdar JA. 1969c. Structure and ontogeny of foliar nectaries and stomata in Bignonia chamberlaynii Sims. – Proc. Indian Natl. Acad. Sci., Sect. B, 70: 232-240.
Inamdar JA. 1970. Epidermal structure and ontogeny of caryophyllaceous stomata in some Acanthaceae. – Bot. Gaz. 131: 261-268.
Inamdar JA, Bhat DC. 1972. Structure and development of stomata in some Labiatae. – Ann. Bot., N. S., 36: 335-344.
Inamdar JA, Patel RC, Mohan JSS. 1986. Structure and ontogeny of stomata in some Oleaceae. – Feddes Repert. 97: 291-302.
İnceoğlu Ö. 1982. Pollen grains in some Turkish Rhinantheae (Scrophulariaceae). – Grana 21: 83-96.
Ingrouille M, Bhatti GR. 1998. Infrageneric relationships within Pogostemon Desf. (Labiatae). – Bot. J. Linn. Soc. 128: 159-183.
Inoue K, Ueda S, Nayeshira H, Moritome N, Inouye H. 1984. Biosynthesis of naphtaquinones and anthraquinones in Streptocarpus dunnii cell cultures. – Phytochemistry 23: 313-318.
Irmscher E. 1959. Über Blüten- und Blütenstandsvarianten bei Saintpaulia ionantha Wendl. (Gesneriac.) und die morphologische Valenz der Vorblüten. – Flora 148: 179-202.
Irving RS. 1972. A revision of the genus Poliomintha (Labiatae). – Sida 5: 8-22.
Irving RS. 1976. Chromosome numbers in Hedeoma (Labiatae) and related genera. – Syst. Bot. 1: 46-56.
Irving RS. 1980. The systematics of Hedeoma (Labiatae). – Sida 8: 218-295.
Ishikawa N, Yokoyama J, Tsukaya H. 2009. Molecular evidence of reticulate evolution in the subgenus Plantago (Plantaginaceae). – Amer. J. Bot. 96: 1627-1635.
Ising G. 1969. Cytogenetic studies in Cyrtanthus III. Aneuploidy and structural chromosome changes. – Hereditas 63: 213-256.
Ito Y, Tanaka N, Barfod AS, Kaul RB, Muasya AM, Garcia-Murillo P, De Vere N, Duyfjes BEE, Albach DC. 2017. From terrestrial to aquatic habitats and back again: molecular insights into the evolution and phylogeny of Callitriche (Plantaginaceae). – Bot. J. Linn. Soc. 184: 46-58.
Ivanina LI. 1965. Application of the carpological method to the taxonomy of Gesneriaceae. – Notes Roy. Bot. Gard. Edinb. 26: 383-402.
Ivanina LI. 1967. The family Gesneriaceae (the carpological review). – Komarov Bot. Inst., Leningrad.
Iwarsson M, Harvey Y. 2003. Monograph of the genus Leonotis (Pers.) R. Br. (Lamiaceae). – Kew Bull. 58: 597-645.
Iwatsubo Y, Mishima M, Naruhashi N. 1993. Chromosome numbers in four Japanese species of Strobilanthes (Acanthaceae). – Chrom. Inf. Serv. 54: 3-5.
Iwo GA, Husaini SWH, Olaniyan GO. 1993. Cytological observations and distribution of Striga species in central part of Nigeria. – Feddes Repert. 104: 497-501.
Jaarsveld EJ van, Wyk AE van. 2007. Dewinteria, a new semi-succulent, cliff-dwelling genus endemic to the Kaokoveld, Namibia. – Bothalia 37: 198-201.
Jackes BR. 2017. A revision of Dolichandrone (Bignoniaceae) in Australia. – Aust. Syst. Bot. 30: 26-37.
Jäggi M, Cook CDK. 1998. Reproductive biology of Callitriche cophocarpa Sendner (Callitrichaceae). – Candollea 53: 101-115.
Jaitly SC. 1966. Development of seeds and fruits in Anisomeles (Linn.) O.Kze. – Phytomorphology 16: 430-436.
Jalas J. 1971. Notes on Thymus (Labiatae) in Europe. – Bot. J. Linn. Soc. 64: 199-235, 247-271.
Jalas J. 1974. Notes on Thymus L. (Labiatae) in Europe III. – Ann. Bot. Fenn. 11: 262-266.
Jalas J. 1980. Turkish taxa of Thymus (Labiatae) described as new or revised. – Ann. Bot. Fenn. 17: 315-324.
Jalas J, Uotila M. 1976. Chromosome studies in Thymus L. (Labiatae) VI. Counts on Macedonian and Thracian taxa. – Ann. Bot. Fenn. 13: 61-64.
James EA, Knox RB. 1993. Reproductive biology of the Australian species of the genus Pandorea (Bignoniaceae). – Aust. J. Bot. 41: 611-626.
Jamzad Z, Harley MM, Ingrouille M, Simmonds M, Jalil A. 2000. Pollen exine and nutlet surface morphology of the annual species of Nepeta L. (Lamiaceae) in Iran. – In: Harley MM, Morton CM, Blackmore S (eds), Pollen and spores: morphology and biology, Royal Botanic Gardens, Kew, pp. 385-397.
Jamzad Z, Chase MW, Ingrouille M, Simmonds MSJ, Jalili A. 2003. Phylogenetic relationships in Nepeta L. (Lamiaceae) and related genera based on ITS sequence data. – Taxon 52: 21-32.
Jamzad Z, Ingrouille M, Simmonds MSJ. 2003. Three new species of Nepeta (Lamiaceae) from Iran. – Taxon 52: 93-98.
Jeandroz S, Roy A, Bousquet J. 1997. Phylogeny and phylogeography of the circumpolar genus Fraxinus (Oleaceae) based on internal transcibed spacer sequences of nuclear ribosomal DNA. – Mol. Phylogen. Evol. 7: 241-251.
Jenks A, Walker J, Kim S. 2013. Phylogeny of New World Salvia subgenus Calosphace (Lamiaceae) based on cpDNA (psbA-trnH) and nrDNA (ITS) sequence data. – J. Plant Res. 126: 483-496.
Jensen AU, Franzyk H, Wallander E. 2002. Chemotaxonomy of the Oleaceae; iridoids as taxonomic markers. – Phytochemistry 60:213-231.
Jensen HFV, Jensen SR, Nielsen BJ. 1988. Chemotaxonomy of the Acanthaceae. Iridoids and quaternary amines. – Phytochemistry 27: 2581-2589.
Jensen SR. 1992. Systematic implications of the distribution of iridoids and other chemical compounds in the Loganiaceae and other families of the Asteridae. – Ann. Missouri Bot. Gard. 79: 284-302.
Jensen SR. 1994. A re-examination of Sanango racemosum 3. Chemotaxonomy. – Taxon 43: 619-623.
Jensen SR. 1996. Caffeoyl phenylethanoid glycosides in Sanango racemosum and in the Gesneriaceae. – Phytochemistry 43: 777-783.
Jensen SR. 1999. Chemotaxonomy of the genus Nuxia (Buddlejaceae). – In: Yang C-R, Tanaka O (eds), Advances in plant glycosides, chemistry & biology, Studies in Plant Science 6, Elsevier, Amsterdam, pp. 379-382.
Jensen SR. 2000a. Chemistry of Buddlejaceae. – In: Norman E (ed), Flora Neotropica. Monograph 81. Buddlejaceae, New York Botanical Garden, Bronx, New York, pp. 42-61.
Jensen SR. 2000b. Chemical relationships of Polypremum procumbens, Tetrachondra hamiltonii and Peltanthera floribunda. – Biochem. Syst. Ecol. 28: 45-51.
Jensen SR, Nielsen BJ, Dahlgren R. 1975. Iridoid compounds, their occurrence and systematic importance in the angiosperms. – Bot. Not. 128: 148-173.
Jensen SR, Ravnkilde LR, Schripsema J. 1998. Unedoside derivatives in Nuxia and their biosynthesis. – Phytochemistry 47: 1007-1010.
Jensen SR, Franzyk H, Wallander E. 2002. Chemotaxonomy of the Oleaceae: iridoids as taxonomic markers. – Phytochemistry 60: 213-231.
Jensen SR, Albach DC, Ohno T, Grayer RJ. 2005. Veronica: iridoids and cornoside as chemosystematic markers. – Biochem. Syst. Ecol. 33: 1031-1047.
Jensen SR, Gotfredson CH, Pierce S. 2007. Iridoid glucosides of Paederota bonarota and the relationships between Paederota and Veronica. – Biochem. Syst. Ecol. 35: 501-505.
Jensen SR, Gotfredson CH, Grayer RJ. 2008. Unusual iridoid glycosides in Veronica sects. Hebe and Labiatoides. – Biochem. Syst. Ecol. 36: 207-215.
Jensen SR, Li H-Q, Albach DC, Gotfredsen CH. 2008. Phytocemistry and molecular systematics of Triaenophora rupestris and Oreosolen wattii (Scrophulariaceae). – Phytochemistry 69: 2162-2166.
Jensen SR, Gotfredsen CH, Zidorn C. 2009. Iridoids and phenylethanoids in Lagotis integrifolia and Wulfeniopsis amherstiana (Plantaginaceae). – Biochem. Syst. Ecol. 37: 421-425.
Jérémie J, Jeune B. 1985. Un cas probable de spéciation sympatrique chez Utricularia alpina Jacq. (Lentibulariaceae) aux Petites Antilles. – Bull. Mus. Hist. Nat., sect. B, Adansonia 7: 213-237.
Jiménez M, Portugal E, Lira-Rocha A, Soriano-García M, Toscano R. 1988. A new royleanone-type diterpene from Salvia sessei. – J. Nat. Prod. 51: 243-248.
Jobson RW. 2013. Five new species of Utricularia (Lentibulariaceae) from Australia. – Telopea 15: 127-142.
Jobson RW, Morris EC. 2001. Feeding ecology of a carnivorous bladderwort (Utricularia uliginosa, Lentibulariaceae). – Aust. J. Ecol. 26: 680-691.
Jobson RW, Baleeiro PC, Reut MS. 2017. Molecular phylogeny of subgenus Polypompholyx (Utricularia; Lentibulariaceae) based on three plastid markers: diversification and proposal for a new section. – Aust. Syst. Bot. 30: 259-278.
Jobson RW, Playford J, Cameron KM, Albert VA. 2003. Molecular phylogenetics of Lentibulariaceae inferred from plastid rps16 intron and trnL-F DNA sequences: implications for character evolution and biogeography. – Syst. Bot. 28: 157-171.
Jobson RW, Nielsen R, Laakkonen L, Wikström M, Albert VA. 2004. Adaptive evolution of cytochrome c oxidase: infrastructure for a carnivorous plant radiation. – Proc. Natl. Acad. Sci. U.S.A. 101: 18064-18068.
Joel DM. 2009. The new nomenclature of Orobanche and Phelipanche. – Weed Res. 49: 6-7.
Johnson LAS. 1957. A review of the family Oleaceae. – Contr. New South Wales Natl. Herb. 2: 395-418.
Johnson MA, Clark JR, Wagner WL, McDade LA. 2017. A molecular phylogeny of the Pacific clade of Cyrtandra (Gesneriaceae) reveals a Fijian origin, recent diversification, and the importance of founder events. – Mol. Phylogen. Evol. 116: 30-48.
Johnston MC. 1957. Synopsis of the United States species of Forestiera (Oleaceae). – Southw. Natur. 2: 140-151.
Johri BM, Singh H. 1970. The morphology, embryology and systematic position of Elytraria acaulis (Linn. f.) Lindau. – Bot. Not. 112: 227-251.
Jones CE, Rich PV. 1972. Ornithophily and extrafloral color patterns in Columnea florida Morton (Gesneriaceae). – Bull. South. Calif. Acad. Sci. 71: 113-116.
Jong K. 1970. Developmental aspects of vegetative morphology in Streptocarpus. – Ph.D. diss., University of Edinburgh, Scotland.
Jong K. 1973. Streptocarpus (Gesneriaceae) and the phyllomorph concept. – Acta Bot. Neerl. 22: 244-245.
Jong K. 1978. Phyllomorphic organisation in rosulate Streptocarpus. – Notes Roy. Bot. Gard. Edinb. 36: 369-396.
Jong K. 1993. Variation in chromosome number in the Manuleeae (Scrophulariaceae) and its cytotaxonomic implications. – Edinburgh J. Bot. 50: 365-379.
Jong K, Burtt BL. 1975. The evolution of morphological novelty exemplified in the growth patterns of some Gesneriaceae. – New Phytol. 75: 297-311.
Jong K, Möller M. 2000. New chromosome counts in Streptocarpus (Gesneriaceae) from Madagascar and the Comoro Islands and their taxonomic significance. – Plant Syst. Evol. 224: 173-182.
Jongkind CCH. 2002. A new species of Clerodendrum (Lamiaceae) from West Africa. – Syst. Geogr. Plants 72: 239-240.
Jordaan A. 2011. Seed coat development, anatomy and scanning electron microscopy of Harpagophytum procumbens (Devil’s Claw), Pedaliaceae. – South African J. Bot. 77: 404-414.
Jørgensen CA. 1923. Studies on Callitrichaceae. – Bot. Tidsskr. 38: 81-126.
Jørgensen CA. 1925. Frage der systematischen Stellung der Callitrichaceae. – Jahrb. Wiss. Bot. 64: 440-442.
Joshi AB, Hardas MW. 1956. Ploidy in two bignoniaceous garden climbers. – Indian J. Genet. Plant Breeding 16: 57-59.
Joshi AC. 1938. Evolution of the vegetative form in the Gesneriaceae. – Curr. Sci. 7: 234-236.
Joshi BS, Gawad DH. 1977. Flavones of Pajanelia multijuga P. DC. and Ligustrum neilgherense var. obovata C. B. Cl. – Proc. Indian Acad. Sci. 86A: 41-44.
Juan R, Fernandez I, Pastor J. 1997. Morphological and anatomical studies on fruits of Veronica from south-west Spain. – Bot. J. Linn. Soc. 123: 157-171.
Juel O. 1910. Cynomorium und Hippuris. – Svensk Bot. Tidskr. 4: 151-159.
Juel O. 1911. Studien über die Entwicklungsgeschichte von Hippuris vulgaris. – Nova Acta Reg. Soc. Sci. Upsal., IV, 2(11): 1-26.
Junell S. 1934. Zur Gynäceummorphologie und Systematik der Verbenaceen und Labiaten nebst Bemerkungen über ihre Samenentwicklung. – Symb. Bot. Upsal. 1(4): 1-219.
Junell S. 1937. Die Samenentwicklung bei einigen Labiaten. – Svensk Bot. Tidskr. 31: 67-110.
Junell S. 1961. Ovarian morphology and taxonomic position of Selagineae. – Svensk Bot. Tidskr. 55: 168-192.
Juzepczuk S. 1951. Scutellariarum novarum decades 1-4. – Not. Syst. Herb. Inst. Bot. Acad. Sci. URSS 14: 356-435.
Kadereit JW. 2004. Lamiales: introduction and conspectus. – In: Kadereit JW (ed), The families and genera of vascular plants VII. Flowering plants. Dicotyledons. Lamiales (except Acanthaceae including Avicenniaceae), Springer, Berlin, pp. 1-8.
Kadry AER, Tewfic H. 1956. Seed germination in Orobanche crenata Forssk. – Svensk Bot. Tidskr. 50: 270-286.
Kaehler M, Michelangeli FA, Lohmann LG. 2012. Phylogeny of Lundia (Bignoniaceae) based on ndhF and PepC sequences. – Taxon 61: 368-380.
Kahraman A, Celep F, Dogan M, Guerin GR, Bagherpour S. 2011. Mericarp morphology and its systematic implications for the genus Salvia L. section Hymenosphace Benth. (Lamiaceae) in Turkey. – Plant Syst. Evol. 292: 33-39.
Kalisz S, Vogler D, Fails B, Finer M, Shepard E, Herman T, Gonzales R. 1999. The mechanism of delayed selfing in Collinsia verna (Scrophulariaceae). – Amer. J. Bot. 88: 447-454.
Kameyama C. 2008. New species, nomenclatural changes and lectotypifications in Neotropical Lepidagathis Willd. (Acanthaceae). – Kew Bull. 63: 565-581.
Kamienski F. 1895. Lentibulariaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien IV(3b), W. Engelmann, Leipzig, pp. 108-123.
Kampany CM. 1995. Pollination and flower diversity in Scrophulariaceae. – Bot. Rev. 61: 350-366.
Kampany CM, Canne-Hilliker JM. 1987. Patterns of floral development in Agalinis and allies (Scrophulariaceae) I. Floral development of Agalinis fasciculata and A. tenuifolia. – Can. J. Bot. 65: 2255-2262.
Kampany CM, Canne-Hilliker JM. 1988. Aspects of floral development in Scrophulariaceae: striking early differences in three tribes. – In: Leins P, Tucker SC, Endress PK (eds), Aspects of floral development, Berlin, Stuttgart, pp. 147-157.
Kampany CM, Dengler NG. 1997. Evolution of flower shape in Veroniceae (Scrophulariaceae). – Plant Syst. Evol. 205: 1-25.
Kampany CM, Dickinson TA, Dengler NG. 1993. Quantitative comparison of floral development in Veronica chamaedrys and Veronicastrum virginicum (Scrophulariaceae). – Amer. J. Bot. 80: 449-460.
Kampany CM, Dickinson TA, Dengler NG. 1994. Quantitative floral development in Pseudolysimachion (Scrophulariaceae): intraspecific variation and comparison with Veronica and Veronicastrum. – Amer. J. Bot. 81: 1343-1353.
Kanchanapoom T, Kasai R, Yamasaki K. 2002. Phenolic glycosides from Markhamia stipulata. – Phytochemistry 59: 557-563.
Kao M-T, Devol CE. 1972. The Gesneriaceae of Taiwan. – Taiwania 17: 142-169.
Kapil RN, Vani RS. 1966. Nyctanthes arbor-tristis Linn.: embryology and relationships. – Phytomorphology 16: 553-563.
Kapoor T. 1976. Ontogeny, structure and distribution of trichomes on the floral parts of Celsia coromandeliana Vahl. – Curr. Sci. 44: 65-66.
Kapoor T, Parulekar NK, Vijayaraghavan MR. 1975. Contribution to the embryology of Celsia coromandeliana Vahl with a discussion on its affinities with Verbascum thapsus L. – Bot. Not. 128: 338-449.
Kar RK. 1996. On the Indian origin of Ocimum (Lamiaceae): a palynological approach. – Palaeobotanist 43: 45-50.
Karatela YY, Gill LS. 1984. Phytodermology and ontogeny of stomata in some Pedaliaceae. – J. Econ. Taxon. Bot. 5: 237-240.
Karavelioğulları FA, Aytaç Z. 2008. revision of the genus Verbascum L. (Group A) in Turkey. – Bot. Res. J. 1: 9-32.
Karavelioğulları FA, Duran A, Hamzaoğlu E. 2004. Verbascum tuna-ekimii (Scrophulariaceae), a new species from Turkey. – Ann. Bot. Fenn. 41: 227-231.
Karavelioğulları FA, Vural M, Polat H. 2006. Two new taxa from Central Anatolia, Turkey. – Israel J. Plant Sci. 54: 105-111.
Karlström P-O. 1972. Embryological studies in the Acanthaceae I. The genus Ruellia L. – Svensk Bot. Tidskr. 66: 303-313.
Karlström P-O. 1973. Embryological studies in the Acanthaceae II. The genera Pseuderanthemum Radlk., Ruspolia Lindau, Eranthemum L., and Lankesteria Lindl. – Svensk Bot. Tidskr. 67: 257-280.
Karlström P-O. 1974a. Embryological studies in the Acanthaceae III. The genera Barleria L. and Crabbea Harv. – Svensk Bot. Tidskr. 68: 121-135.
Karlström P-O. 1974b. Embryological studies in the Acanthaceae IV. The genera Asystasia Bl. and Chamaeranthemum Nees. – Svensk Bot. Tidskr. 68: 315-328.
Karlström P-O. 1978. Epidermal leaf structures in species of Strobilantheae and Petalidieae (Acanthaceae). – Bot. Not. 131: 423-433.
Karlström P-O. 1980. Epidermal leaf structures in species of Asystasieae, Pseuderanthemeae, Graptophylleae and Odontonemeae (Acanthaceae). – Bot. Not. 133: 1-16.
Karousou R. 1995. Taxonomic studies on the Cretan Labiatae. Distribution, morphology and essential oils. – Ph.D. diss., University of Thessaloniki, Greece. [In Greek with English summary]
Karousou R, Bosabalidis AM, Kokkini S. 1992. Sideritis syriaca ssp. syriaca: glandular trichome structure and development in relation to systematics. – Nord. J. Bot. 12: 31-37.
Karousou R, Kokkini S, Bessière J-M, Vokou D. 1996. Calamintha cretica (Lamiaceae), a Cretan endemic: distribution and essential oil composition. – Nord. J. Bot. 16: 247-252.
Karrfalt EE, Tomb AS. 1983. Air spaces, secretory cavities, and the relationship between Leucophylleae (Scrophulariaceae) and Myoporaceae. – Syst. Bot. 8: 29-32.
Karron JD, Jackson RT, Thumser NN, Schlicht SL. 1997. Outcrossing rates of individual Mimulus ringens genets are correlated with anther-stigma separation. – Heredity 79: 365-370.
Kästner A. 1978-1989. Beiträge zur Wuchsformenanalyse und systematischen Gliederung von Teucrium L. I-VI. – Flora 167: 485-514 (1978); 168: 431-467 (1979); 171: 466-519 (1981); 176: 73-93 (1985); 178: 111-138 (1986); 183: 189-224 (1989).
Kästner A. 1989. Übersicht zur systematischen Gliederung der Gattung Teucrium L. – Biocosme Mésogéen (Nice) 6: 63-77.
Kato M, Itino T, Nagamitsu T. 1993. Melittophily and ornithophily of long-tubed flowers in Zingiberaceae and Gesneriaceae in West Sumatra. – Tropics 2: 129-142.
Katzir N, Portnoy V, Tzuri G, Castejon-Munz M, Joel DM. 1996. Use of random amplified polymorphic DNA (RAPD) markers in the study of the parasitic weed Orobanche. – Theor. Appl. Gen. 93: 367-372.
Kaufmann M, Wink, M 1994. Molecular systematics of the Nepetoideae (family Labiatae): phylogenetic implications from rbcL gene sequences. – Zeitschr. Naturf., Ser. C, 49: 635-645.
Kaur J. 1970. Chromosome numbers in Acanthaceae V. – Sci. Cult. 36: 103-106.
Kausik SB, Raju MVS. 1955. A contribution to the floral morphology and embryology of Utricularia reticulata Smith. – Proc. Indian Acad. Sci., Sect. B, 41: 155-166.
Kausik SB, Raju MVS. 1956. Variation in the development of proembryo in Utricularia coerulea L. – Curr. Sci. 25: 296-297.
Kawakubo N. 1998. Evolution of cryptic dioecy in Callicarpa (Verbenaceae) on the Bonin Islands. – In: Stuessy TF, Ono M (eds), Evolution and speciation of island plants, Cambridge University Press, Cambridge, England, pp. 155-168.
Kaya A, Kutluk H. 2007. Pollen morphology of Acinos Miller species growing in Turkey. – J. Integr. Plant Biol. 49: 1386-1392.
Kaya A, Goger F, Baser KHC. 2007. Morphological, anatomical and playnological characteristics of Salvia halophila endemic to Turkey. – Nord. J. Bot. 25: 351-358.
Kaya A, Satil F, Dirmenci T, Selvi S. 2013. Trichome micromorphology in Turkish species of Ziziphora (Lamiaceae). – Nord. J. Bot. 31: 270-277.
Kaynak G, Daşkın R, Yılmaz Ö, Erdoğan E. 2006. Verbascum yurtkuranianum (Scrophulariaceae), a new species from northwest Anatolia, Turkey. – Ann. Bot. Fenn. 43: 456-459.
Keenan J. 1969a. Notes on Buddleja I. Polyploid pattern in B. colvilei. – Notes Roy. Bot. Gard. Edinburgh 29: 197-198.
Keenan J. 1969b. Notes on Buddleja II. Pollen. – Notes Roy. Bot. Gard. Edinb. 29: 199-202.
Kelbessa E. 1989. Two new species of Justicia sect. Ansellia (Acanthaceae) from E and NE tropical Africa. – Nord. J. Bot. 9: 399-404.
Kelbessa E. 1990. Justicia sect. Ansellia (Acanthaceae). – Symb. Bot. Ups. 29(2): 1-96.
Kelbessa E. 1998. Studies in the genus Asystasia (Acanthaceae) in tropical Africa II: further new species. – Kew Bull. 53: 929-935.
Kelbessa E. 2003. Two new species of Acanthaceae from NE tropical Africa and Arabia. – Kew Bull. 58: 703-712.
Kelbessa E. 2009. Three new species of Acanthaceae from Ethiopia. – Kew Bull. 64: 57-65.
Kelbessa E, Darbyshire I. 2018. Six new species of Barleria L. (Acanthaceae) from Northeast Tropical Africa. – Kew Bull. 73: 1. doi 10.1007/S12225-017-9725-2
Kelbessa E, Brummitt RK, Furness CA. 1992. A reconsideration of Asystasiella Lindau (Acanthaceae). – Kew Bull. 47: 669-675.
Kelchner SA. 2003. Phylogenetic structure, biogeography, and evolution of Myoporaceae. – Ph.D. diss., The Australian National University, Canberra, Australia.
Kemularia-Nathadze LM. 1953. Paederotella (Wulff) Kem.-Nath. genus novum Scrophulariacearum. – Not. Syst. Geogr. Inst. Bot. Thbilissiensis 17: 17-25.
Keng H. 1969. Flora Malesianae precursors XLVIII – a revision of Malesian Labiatae. – Gardens’ Bull. (Singapore) 24: 13-180.
Keng H. 1978. Labiatae. – In: Steenis CGGJ van (ed), Flora Malesiana I, 8(3), Sijthoff & Noordhoff International Publ., Alphen aan den Rijn, The Netherlands, pp. 301-394.
Kern JH, Steenis CGGJ van. 1951. Caprifoliaceae. – In: Steenis CGGJ van (ed), Flora Malesiana I, 4, Noordhoff-Kolff, Djakarta, pp. 175-194.
Khan R. 1954. A contribution to the embryology of Utricularia flexuosa Vahl. – Phytomorphology 4: 80-117.
Khan R. 1970. Comparative embryology of angiosperms: Lentibulariaceae. – Bull. Indian Natl. Sci. Acad. 41: 290-297.
Kiehn M. 2001. South Pacific and Hawaiian Cyrtandra: molecular and micromorphological studies. – Malayan Nature J. 55: 21-27.
Kiehn M, Weber A. 1998. Chromosome numbers of Malayan and other palaeotropical Gesneriaceae II. Tribes Trichosporeae, Cyrtandreae and Epithemateae. – Beitr. Biol. Pflanzen 70: 445-470.
Kiehn M, Weber A. 2001. Karyological differentiation and speciation in Malesian Gesneriaceae. – In: Saw LG, Chua LSL, Khoo KC (eds), Taxonomy: the cornerstone of biodiversity, Proc. 4th Intern. Flora Malesiana Symp. 1998, Forest Res. Inst. Malaysia (FRIM), Kuala Lumpur, pp. 139-142.
Kiehn M, Hellmayr E, Weber A. 1998. Chromosome numbers of Malayan and other palaeotropical Gesneriaceae I. Tribe Didymocarpeae. – Beitr. Biol. Pflanzen 70: 407-444.
Kiel CA, McDade LA. 2014. The
Mirandea clade (Acanthaceae, Justicieae,
Tetramerium lineage): phylogenetic signal from molecular data and
micromorphology makes sense of taxonomic confusion caused by remarkable
diversity of floral form. – Syst. Bot. 39: 950-964.
Kiel CA, McDade LA, Daniel TF, Champluvier D. 2006. Phylogenetic delimitation of Isoglossinae (Acanthaceae: Justicieae) and relationships among constituent genera. – Taxon 55: 683-694.
Kiel CA, Daniel TF, Darbyshire I, McDade LA. 2017. Unraveling relationships in the morphologically diverse and taxonomically challenging “justicioid” lineage (Acanthaceae: Justicieae). – Taxon 66: 645-674.
Kiel CA, Daniel TF, McDade LA. 2018. Phylogenetics of New World ‘justicioids’ (Justicieae: Acanthaceae): major lineages, morphological patterns, and widespread incongruence with classification. – Syst. Bot. 43: 459-484.
Kiew R. 1979. Florae Malesianae praecursores LX. The Oleaceae of Malesia II. The genus Olea. – Blumea 25: 305-313.
Kiew R. 1983. Two unusual Chionanthus species from Borneo and the position of Myxopyrum in the Oleaceae. – J. Arnold Arbor. 64: 619-626.
Kiew R. 1984a. Preliminary pollen study of the Oleaceae in Malesia. – Gard. Bull. (Singapore) 37: 225-230.
Kiew R. 1984b. The genus Myxopyrum L. (Oleaceae). – Blumea 29: 499-512.
Kiew R. 1990. Reassessment of the generic status of Codonoboea (Gesneriaceae) and its species. – Blumea 35: 167-176.
Kiew R. 1992. Five new species of Didymocarpus (Gesneriaceae) from Peninsular Malaysia. – Gardens’ Bull. (Singapore) 44: 23-42.
Kew R. 1995. A new species and section of Didymocarpus (Gesneriaceae) from Belum and Temengor, Hulu Perak, Peninsular Malaysia. – Mal. Nat. J. 48: 201-207.
Kiew R, Baas P. 1984. Nyctanthes is a member of the Oleaceae. – Proc. Indian Acad. Sci. (Plant Sci.) 93: 349-358.
Kiew R, Ibrahim CS. 1982. Comparative study of leaf anatomy of Malayan species of Chionanthus and Olea (Oleaceae) with special reference to foliar sclereids. – Bot. J. Linn. Soc. 84: 79-101.
Kiew R, Vollesen K. 1997. Asystasia (Acanthaceae) in Malaysia. – Kew Bull. 52: 965-971.
Kiew R, Weber A. 1988. Two new species (Didissandra porphyrantha and Didymocarpus nitidus) and a new combination (Didymocarpus breviflorus), Gesneriaceae, from Selangor, Malaysia. – Gard. Bull. (Singapore) 41: 1-9.
Kiew R, Weber A, Burtt L. 1998. Three new genera of Gesneriaceae from limestone of Peninsular Malaysia. – Beitr. Biol. Pflanzen 70: 383-403.
Kilian N, Hein P, Bahah SO. 2002. A new species of Campylanthus (Scrophulariaceae) from Ras Fartak, Al-Mahra, and notes on other species of the genus in Yemen. – Willdenowia 32: 271-279.
Kim D-K, Kim J-H. 2011. Molecular phylogeny of the tribe Forsythieae (Oleaceae) based on nuclear ribosomal DNA internal transcribed spacers and plastid DNA trnL-F and matK gene sequences. – J. Plant Res. 124: 339-347.
Kim K-J, Jansen RK. 1998. A chloroplast DNA phylogeny of lilacs (Syringa, Oleaceae): plastome groups show a strong correlation with crossing groups. – Amer. J. Bot. 85: 1338-1351.
Kimata M. 1978. Comparative studies on the reproductive systems of Mazus japonicus and M. miquelii (Scrophulariaceae). – Plant Syst. Evol. 129: 243-253.
Kitagawa I, Nishimura T, Furubayashi A, Yosioka I. 1971. Constituents of rhizome of Rehmannia glutinosa f. hueichingensis. – Yakugaku Zasshi 91: 593-596.
Kleinfeldt SE. 1978. Ant-gardens: the interaction of Codonanthe crassifolia (Gesneriaceae) and Crematogaster longispina (Formicidae). – Ecology 59: 449-456.
Knoblauch E. 1895. Oleaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien IV(2), W. Engelmann, Leipzig, pp. 1-16.
Kobayashi T, Shimamura T. 1952. Morphological and cytological studies on induced polyploidy in Sesamum indicum L. – Jap. J. Gen. 27: 157-171.
Kobuski CE. 1928. A new genus of the Acanthaceae. – Ann. Missouri Bot. Gard. 15: 1-8.
Koedam A. 1986. Volatile oil composition of Greek mountain tea (Sideritis spp.). – J. Sci. Food Agric. 36: 681-684.
Kohyama T, Hotta M. 1986. Growth analysis of Sumatran Monophyllaea, possessing only one leaf throughout perennial life. – Plant Spec. Biol. 1: 117-125.
Kok RPJ de. 1997. The biology and systematics of Oxera, Faradaya and Hosea (Labiatae). – Ph.D. diss., University of Oxford, United Kingdom.
Kok RPJ de. 1998. The systematics and pollination biology of Oxera Labill., Faradaya F. Muell. and Hosea Ridl. (Labiatae). – In: Saw LG, Chua LSL, Khoo KC (eds), 4th International Flora Malesiana Symposium, Forest Research Institute, Kuala Lumpur, Malaysia, pp. 87-91.
Kok RPJ de. 2008. The genus Vitex (Labiatae) in the Flora Malesiana region, excluding New Guinea. – Kew Bull. 63: 17-40.
Kok RPJ de 2012. A revision of the genus Gmelina (Lamiaceae). – Kew Bull. 67: 293-329.
Kok RPJ de. 2013. The genus Premna L. (Lamiaceae) in the Flora Malesiana area. – Kew Bull. 68: 55-84.
Kok RPJ de, Atkins S. 1997. The genus Archboldia E. Beer & H. J. Lam is put into the synonymy of Clerodendrum L. (Labiatae). – Kew Bull. 52: 503-504.
Kok RPJ de, Mabberley DJ. 1999a. Generic and intra-generic delimitation of Oxera Labill. (Labiatae). – Kew Bull. 54: 257-264.
Kok RPJ de, Mabberley DJ. 1999b. A synopsis of Oxera Labill. (Labiatae). – Kew Bull. 54: 265-300.
Kok RPJ de, Mabberley DJ. 1999c. The genus Faradaya F. Muell. (Labiatae). – Blumea 44: 321-342.
Kok RPJ de, Grayer RJ, Kite GC. 2000. Relationships of the endemic Australian genus Huxleya Ewart & Rees (Labiatae) based on fruit and flavonoid characters. – Aust. Syst. Bot. 13: 425-428.
Kok RPJ de, Rusea G, Latiff A. 2009. The genus Teijsmanniodendron Koord. (Lamiaceae). – Kew Bull. 64: 587-625.
Kokkalou E. 1987. Constituants entrainable à la vapeur d’eau de Sideritis scardica Gris. ssp. scardica. – Plants Med. Phyt. 21: 262-266.
Kokkini S, Vokou D, Karouscou R. 1989. Essential oil yield of Lamiaceae plants in Greece. – In: Bhattacharyya SC, Sen N, Sethi KL (eds), Proceedings of 11th International Congress of essential oils, fragrances and flavours 3, Oxford & IBH Publ., New Delhi, Bombay, Calcutta, pp. 5-12.
Kolberg H, Slageren M van. 2016. A synopsis of Aptosimum and Peliostomum (Scrophulariaceae) in Namibia, including the description of a new species, Aptosimum radiatum, and keys to all accepted species. – Kew Bull. 71: 16 DOI 10.1007/S12225-016-9628-7
Komaitis ME, Falirea A, Voudouris EC. 1985. Constituents of the essential oil of Sideritis cretica Boiss. – J. Sci. Food Agric. 3: 970-972.
Kondo K. 1972. Comparison of variability in Utricularia cornuta and Utricularia juncea. – Amer. J. Bot. 59: 23-37.
Kondo K. 1973a. The karyotypes of the species of Byblis. – Bull. Torrey Bot. Club 100: 367-369.
Kondo K. 1973b. The chromosome numbers of Striga asiatica and Triphyophyllum peltatum. – Fyton 31: 1-2.
Kondo K, Segawa M, Nehira K. 1978. Anatomical studies on seeds and seedlings of some Utricularia (Lentibulariaceae). – Brittonia 30: 89-95.
Kong LD, Wolfender JL, Cheng CH, Hostettmann K, Tan RX. 1999. Xanthine oxidase inhibitors from Brandisia hancei. – Planta Med. 65: 744-746.
Kooiman PT. 1970. The occurrence of iridoid glucosides in the Scrophulariaceae. – Acta Bot. Neerl. 19: 329-340.
Kooiman PT. 1972. The occurrence of iridoid glucosides in the Labiatae. – Acta Bot. Neerl. 21: 417-427.
Kooiman PT. 1975. The occurrence of iridoid glucosides in the Verbenaceae. – Acta Bot. Neerl. 24: 459-468.
Kopczynska K. 1964. Embryo sac development in Pinguicula vulgaris L. – Acta Soc. Bot. Pol. 33: 141-156.
Kornhall P. 2004. Phylogenetic studies in the Lamiales with special focus on Scrophulariaceae and Stilbaceae. – Ph.D. diss., University of Uppsala, Sweden.
Kornhall P, Bremer B. 2004. New circumscription of the tribe Limoselleae (Scrophulariaceae) that includes taxa of the tribe Manuleeae. – Bot. J. Linn. Soc. 146: 453-467. – Corrigendum (2005): Bot. J. Linn. Soc. 147: 385-386.
Kornhall P, Heidari N, Bremer B. 2001. Selagineae and Manuleeae, two tribes or one? Phylogenetic studies in the Scrophulariaceae. – Plant Syst. Evol. 228: 199-218.
Kosch M. 1992. Die Gattung Wulfenia Jacq. – ein Überblick. – Wulfenia 1: 27-33.
Krahulcová A, Jarolímová V. 1991. Relationship between Pinguicula bohemica Krajina and Pinguicula vulgaris L. (Lentibulariaceae) from the karyological point of view. – Preslia 63: 323-328.
Kränzlin F. 1907. Beiträge zur Kenntnis der Gattung Calceolaria. – Ann. K. K. Naturhist. Hofmus. Wien 22: 191-196.
Kränzlin F. 1916. Scrophulariaceae andinae. – Engl. Bot. Jahrb. Syst. 54, Beibl. 119: 18-21.
Kränzlin F. 1929a. Neue Arten von Calceolaria L. – Feddes Repert. 27: 1-26.
Kränzlin F. 1929b. Beiträge zur Kenntnis der Familie der Myoporaceae R. Br. – Feddes Repert., Beih. 54.
Krawczyk K, Głowacka K. 2015. Nutlet micromorphology and its taxonomic utility in Lamium L. (Lamiaceae). – Plant Syst. Evol. 301: 1863-1874.
Kress A. 1970. Zytotaxonomische Untersuchungen an einigen Insektenfängern (Droseraceae, Byblidaceae, Cephalotaceae, Roridulaceae, Sarraceniaceae). – Ber. Deutsch. Bot. Ges. 83: 55-62.
Krestovskaya TV. 2003. A new section of the genus Stachys (Lamiaceae). – Nov. Sist. Vyssh. Rast. 35: 147-148.
Krestovskaya TV. 2006. A new section of the genus Stachys (Lamiaceae) from South-West Africa. – Bot. Žurn. 91: 1584-1586.
Krestovskaya TV. 2007. A new section of the genus Stachys (Lamiaceae) from Africa. – Bot. Žurn. 92: 285-293.
Kriebel R. 2005. A new species of Columnea and range extension in the Gesneriaceae from Costa Rica. – Brittonia 57: 39-42.
Krishna Iyengar CV. 1937. Development of the embryo-sac and endosperm-haustoria in some members of Scrophularineae I. An account of Sopubia delphinifolia G. Don. and Alonsoa sp. – J. Indian Bot. Soc. 16: 99-109.
Krishna Iyengar CV. 1939. Development of the embryo-sac and endosperm-haustoria in some members of Scrophularineae II. Isoplexis canariensis Lindl. et Celsia coromandeliana Vahl. – J. Indian Bot. Soc. 18: 13-20.
Krishna Iyengar CV. 1940a. Development of embryo-sac and endosperm-haustoria in some members of Scrophularineae III. Ilysanthes hyssopioides Benth. and Bonnaya tenuifolia Spreng. – J. Indian Bot. Soc. 19: 5-18.
Krishna Iyengar CV. 1940b. Development of the embryo-sac and endosperm-haustoria in some members of Scrophularineae IV. Vandellia hirsuta Ham. and V. scabra Benth. – J. Indian Bot. Soc. 18: 179-189.
Krishna Iyengar CV. 1940c. Development of the embryo-sac and endosperm-haustoria in some members of Scrophularineae V. Ilysanthes hyssopioides Benth. and Bonnaya tenuifolia Spreng. – J. Indian Bot. Soc. 19: 5-17.
Krishna Iyengar CV. 1941. Development of the embryo sac and endosperm-haustoria in Torenia cordifolia Roxb. and T. hirsuta Benth. – Proc. Indian Natl. Inst. Sci. 7: 61-71.
Krishna Iyengar CV. 1942a. Development of embryo sac and endosperm-haustoria in Tetranema mexicana Benth. and Verbascum thapsus Linn. – Proc. Indian Natl. Inst. Sci. 8: 59-69.
Krishna Iyengar CV. 1942b. Development of seed and its nutritional mechanism in Scrophulariaceae I. Rhamphicarpa longiflora Benth., Centranthera hispida Br. and Pedicularis zeylanica Benth. – Proc. Indian Natl. Inst. Sci. 8: 249-261.
Krishna Iyengar CV. 1942c. Development of embryosac and endosperm haustoria in Rehmannia angulata Hemsl. – J. Indian Bot. Soc. 21: 51-57.
Kristen U. 1974. Feinstruktur und Entwicklung der äusseren Fangblasendrüsen von Utricularia minor L. – Cytobiologie 9: 321-330.
Kristen U, Lockhausen J. 1985. The leaf glands of Veronica beccabunga L.: ultrastructure and possible pathway of secretion. – Israel J. Bot. 34: 147-156.
Kshetrapal S, Tiagi YG. 1970. Structure, vascular anatomy, and evolution of the gynoecium in family Oleaceae and their bearing on the systematic position of the genus Nyctanthes L. – Acta Biol. Acad. Sci. Hungar. 16: 143-151.
Kvist LP, Skog LE. 1988. Columnea incredibilis and Cremosperma filicifolium – two remarkable new Gesneriaceae from western Colombia. – Nord. J. Bot. 8: 253-257.
Kubitzki K, Kadereit JW (eds). 2004. The families and genera of vascular plants VII. Flowering plants. Dicotyledons. Lamiales (except Acanthaceae including Avicenniaceae). – Springer, Berlin, Heidelberg, New York.
Kudô Y. 1929. Labiatarum sino-japonicarum prodromus. – Mem. Fac. Sci. Taihoku Imp. Univ. 2: 37-332.
Kudrjaschew S. 1939. Fragmenti k monografji roda Otostegia Benth. – Trudi Bot. Sect. Kom. Nauk USSR (Taschkent).
Kuijt J. 1969. The biology of parasitic flowering plants. – University of California Press, Berkeley, California.
Kuijt J, Toth R. 1985. Structure of the host-parasite interface of Boschniakia hookeri Walpers (Orobanchaceae). – Acta Bot. Neerl. 34: 257-270.
Kuijt J, Visser JH, Weber HC. 1978. Morphological observations on leaf haustoria and related organs of the South African genus Hyobanche (Scrophulariaceae). – Can. J. Bot. 56: 2981-2986.
Kuiper PJC, Bos M (eds). 1992. Plantago: a multidisciplinary study. – Springer, Berlin, Heidelberg, New York.
Kumar VS, Sharma BD. 1995. Two new taxa of Pogostemon (Lamiaceae) from India. – Nord. J. Bot. 15: 163-166.
Kundu BC, De A. 1968. Taxonomic position of the genus Nyctanthes. – Bull. Bot. Surv. India 10: 397-408.
Kupffender H. 1891. Beiträge zur Anatomie der Globulariaceen und Selaginaceen und zur Kenntnis des Blattcambiums. – Ph.D. diss., Universität Erlangen, Kiel, Germany.
Kupicha FK. 1983. 108. Oleaceae. – In: Launert E (ed), Flora Zambesiaca 7 (Part 1), Flora Zambesiaca Managing Committee, London, pp. 300-327.
Kuriachen PM, Dave YS. 1989. Structural studies in the fruits of Oleaceae with discussion on the systematic position of Nyctanthes L. – Phytomorphology 39: 51-60.
Kurz L. 1959. Anatomische und entwicklungsphysiologische Untersuchungen an Utricularia. – Beitr. Biol. Pflanzen 35: 111-135.
Kusano S. 1908. On the parasitism of Siphonostegia (Rhinantheae). – Bull. Coll. Agric. Tokyo Imp. Univ. 8: 51-71.
Kutney JP, Hanssen HW. 1971. 5,6,7-Trimethoxyflavone and 5,6,7,8-tetramethoxyflavone from Zeyheria tuberculosa. – Phytochemistry 10: 3298-3302.
Kutney JP, Warnock WDC, Gilbert B. 1970. Pinocembrin 7-beta-neohesperidoside, a flavanone glycoside from Sparattosperma vernicosum. – Phytochemistry 9: 1877-1878.
Kvist LP. 1984. Gesneriaceae’s taxonomi med specielt henblik på kemiske karakterer. – Ph.D. diss., Århus Universitet, Danmark.
Kvist LP. 1990. Revision of Heppiella (Gesneriaceae). – Syst. Bot. 15: 720-735.
Kvist LP, Pedersen JA. 1986. Distribution and taxonomic implications of some phenolics in the family Gesneriaceae determined by EPR spectroscopy. – Biochem. Syst. Ecol. 14: 385-405.
Kvist LP, Skog LE. 1988a. Columnea incredibilis and Cremosperma filicifolium, two remarkable new Gesneriaceae from western Colombia. – Nord. J. Bot. 8: 253-257.
Kvist LP, Skog LE. 1988b. The genus Cremosperma (Gesneriaceae) in Ecuador. – Nord. J. Bot. 8: 259-269.
Kvist LP, Skog LE. 1989. Revision of Reldia (Gesneriaceae). – Nord. J. Bot. 9: 601-611.
Kvist LP, Skog LE. 1992. Revision of Kohleria (Gesneriaceae). – Smithsonian Contr. Bot. 79: 1-83.
Kvist LP, Skog LE. 1993. The genus Columnea (Gesneriaceae) in Ecuador. – Allertonia 6: 327-400.
Kvist LP, Skog LE. 1996. Revision of Pearcea (Gesneriaceae). – Smithsonian Contr. Bot. 84: 1-47.
Laakkonen L, Jobson RW, Albert VA. 2006. A new model for the evolution of carnivory in the bladderwort plant (Utricularia): adaptive changes in cytochrome c oxidase (COX) provide respiratory power. – Plant Biol. 8: 758-764.
Labat J-N, Pignal M, Pascal O. 1999. Trois espèces nouvelles d’Oleaceae et note sur la présence d’Olea capensis dans l’archipel des Comores. – Novon 9: 66-72.
La Harpe AC de, Grobbelaar N, Visser JH. 1980. The ultrastructure of the chloroplast and the chlorophyll content of various South African parasitic flowering plants. – Zeitschr. Pflanzenphys. 100: 85-90.
La Harpe AC de, Visser JH, Grobbelaar N. 1981. Photosynthetic characteristics of some South African parasitic flowering plants. – Zeitschr. Pflanzenphys. 103: 265-275.
Lakshminarayana K. 1987. Embryology and systematics of Jasminum. – Beitr. Biol. Pflanzen 61: 373-380.
Lakshminarayana K, Devi HM. 1985. Embryology of Linociera intermedia (Oleaceae). – J. Jap. Bot. 95: 213-219.
Lakusic R. 1978. Die chorologisch-ökologische und morphologisch-zytologische differenzierung der europäischen Arten der Gattung Wulfenia Jacq. – Bot. Jahrb. Syst. 99: 443-461.
Lam HJ. 1919. The Verbenaceae of the Malayan Archipelago. – M. de Waal, Groningen.
Lamond M, Vieth J. 1972. L’androcée synanthéré du Rechsteineria cardinalis (Gesnériacées). Une contribution au problème des fusions. – Can. J. Bot. 50: 1633-1637.
Lang A. 1940. Untersuchungen über einige Verwandtschafts- und Abstammungsfragen in der Gattung Stachys L. auf cytogenetischer Grundlage. – Bibl. Bot. 118: 1-94.
Lang FX. 1901. Untersuchungen über Morphologie, Anatomie und Samenentwicklung von Polypompholyx und Byblis gigantea. – Flora 88: 149-206.
Laroche R. 1974. Anatomic considerations of the calyx of Adenocalymma comosum (Lam.) A. P. DC. – Ann. Missouri Bot. Gard. 61: 530-533
La-Serna I. 1984. Revisión del género Bystropogon. – J. Cramer, Vaduz, Liechtenstein.
Las Heras VJ, Gomez-Mercado F, Guil GJ-L, Rodriguez-Garcia I, Garcia-Maroto F. 1999. Genetic relationships and population structure within taxa of the endemic Sideritis pusilla (Lamiaceae) assessed using RAPDs. – Bot. J. Linn. Soc. 129: 345-358.
Laurent V. 1923. Zur Entwicklungsgeschichte von Corytoloma cyclophyllum Dus. n. sp. ined. – Svensk Bot. Tidskr. 7: 65-174.
Lawrence BM. 1992. Chemical components of Labiatae oils and their exploitation. – In: Harley RM, Reynolds T (eds), Advances in labiate science, Royal Botanic Gardens, Kew, pp. 399-436.
Lawrence TJ, Green PS. 1993. The anatomy of a dehiscent berry. – Kew Bull. 48: 53-57.
Lawrence WJC. 1957. Studies on Streptocarpus IV. Genetics of flower colour patterns. – Heredity 11: 337-357.
Lawrence WJC. 1958. Studies on Streptocarpus V. Speciation and gene systems. – Heredity 12: 333-356.
Lawrence WJC, Sturgess VC. 1957. Studies on Streptocarpus III. Genetis and chemistry of flower colour in the garden forms, species and hybrids. – Heredity 11: 303-336.
Lawrence WJC, Scott-Moncrieff R, Sturgess VC. 1939. Studies on Streptocarpus I. Genetics and chemistry of flower colour in the garden strains. – J. Genet. 38: 299-306.
Lawrence BM, Hogg JW, Terhune SJ, Morton JK, Gill LS. 1972. Terpenoid composition of some Canadian Labiatae. – Phytochemistry 11: 2636-2638.
Leclerc-Potin C, Ritland K. 1994. Modes of self-fertilization in Mimulus guttatus (Scrophulariaceae): a field experiment. – Amer. J. Bot. 81: 199-205.
Lee H-L, Jansen RK, Chumley TW, Kim KJ. 2007. Gene relocations within chloroplast genomes of Jasminum and Menodora (Oleaceae) are due to multiple, overlapping inversions. – Mol. Biol. Evol. 24: 1161-1180.
Lee RE. 1962. Chromosome counts in the Gesneriaceae. – Baileya 10: 33-45.
Lee RE. 1964. Additional chromosome counts in the Gesneriaceae. – Baileya 12: 159.
Lee RE. 1966. Additional chromosome numbers in the Gesneriaceae. – Baileya 14: 35-36.
Lee RE. 1967. Additional chromosome numbers in the Gesneriaceae. – Baileya 15: 118.
Lee RE, Grear JW. 1963. Additional chromosome numbers in the Gesneriaceae. – Baileya 11: 131.
Leeratiwong C, Chantaranothai P, Paton AJ. 2011. A synopsis of the genus Clerodendrum L. (Lamiaceae) in Thailand. – Trop. Nat. Hist. 11: 177-211.
Leerefeld H, Meeuse ADJ, Stelleman P. 1976. Anthecological relations between reputedly anemophilous flowers and syrphid flies II. Plantago media L. – Acta Bot. Neerl. 25: 205-211.
Leeuwenberg AJM. 1958. The Gesneriaceae of Guiana. – Acta Bot. Neerl. 7: 291-444.
Leeuwenberg AJM. 1964. The Loganiaceae of Africa VI. Retzia. – Acta Bot. Neerl. 13: 333-339.
Leeuwenberg AJM. 1967. Notes on American Loganiaceae I. Revision of Plocosperma Benth. – Acta Bot. Neerl. 16: 56-61.
Leeuwenberg AJM. 1971. Notes on American Loganiaceae V. Key to the genera represented in America. – Acta Bot. Neerl. 20: 539-542.
Leeuwenberg AJM 1975. The Loganiaceae of Africa XIV. A revision of Nuxia Lam. – Meded. Landbouwh. Wageningen 75: 1-80.
Leeuwenberg AJM. 1977. The Loganiaceae of Africa XVI. Gomphostigma Turcz. – Meded. Landbouwh. Wageningen 77: 15-30.
Leeuwenberg AJM. 1979. The Loganiaceae of Africa XVIII. Buddleja L. II. Revision of the African and Asiatic species. – Meded. Landbouwh. Wageningen 79: 1-163.
Leeuwenberg AJM (ed). 1980. Angiospermae: Ordnung Gentianales Fam. Loganiaceae. – In: Hiepko P, Melchior H (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Vol. 28bI, Duncker & Humblot, Berlin, pp. 1-255.
Leeuwenberg AJM. 1983b. 109. Loganiaceae. – In: Launert E (ed), Flora Zambesiaca 7 (Part 1), Flora Zambesiaca Managing Committee, London, pp. 327-374.
Leeuwenberg AJM, Leenhouts PW. 1980. Taxonomy of the Loganiaceae. – Hakki MI. 1980. Embryology. – In: Leeuwenberg AJM (ed), Engler and Prantl’s Die natürlichen Pflanzenfamilien, Fam. Loganiaceae, vol. 28b(1), Duncker & Humblot, Berlin, pp. 8-96.
Legendre L. 2000. The genus Pinguicula L. (Lentibulariaceae): an overview. – Acta Bot. Gall. 147: 77-95.
Lehmann E. 1918. Die Pentasepalie in der Gattung Veronica und die Vererbungsveise der pentasepalen Zwischenrassen. – Ber. Deutsch. Bot. Ges. 36: 28-46.
Lehmann E. 1940. Polyploidie und geographische Verbreitung der Arten der Gattung Veronica. – Jahrb. Wiss. Bot. 89: 461-542.
Leinfellner W. 1973. Das Gynözeum der Bignoniaceen II. Die U-förmige Plazenta von Schlegelia (Crescentieae). – Österr. Bot. Zeitschr. 121: 13-22.
Leins P, Erbar C. 1988. Einige Bemerkungen zur Blütenentwicklung und systematischen Stellung der Wasserpflanzen Callitriche, Hippuris und Hydrostachys. – Beitr. Biol. Pflanzen 63: 157-178.
Leins P, Erbar C. 2004. Hippuridaceae. – In: Kubitzki K, Kadereit JW (eds), The families and genera of vascular plants VII. Flowering plants. Dicotyledons. Lamiales (except Acanthaceae including Avicenniaceae), Springer, Berlin, Heidelberg, New York, pp. 163-166.
Leitner J. 1942. Ein Beitrag zur Kenntnis der Pollenkörner der Labiatae. – Österr. Bot. Zeitschr. 91: 29-40.
Lenski I. 1966. Chromosomenzahlen in der Gattung Sibthorpia (Scrophulariaceae). – Naturwissenschaften 53: 710-711.
Leonard EC. 1927. The North American species of Scutellaria. – Contr. U. S. Natl. Herb. 22: 703-748.
Leonard EC. 1950. Five new species of Acanthaceae from Honduras. – Ceiba 1: 103-115.
Leonard EC. 1951. The Acanthaceae of Colombia I. – Contr. U.S. Natl. Herb. 31: 1-118.
Leonard EC. 1953. The Acanthaceae of Colombia II. – Contr. U.S. Natl. Herb. 31: 119-332.
Leonard EC. 1958. The Acanthaceae of Colombia III. – Contr. U.S. Natl. Herb. 31: 323-781.
Leonard EC, Smith LB. 1964. Sanchezia and related American Acanthaceae. – Rhodora 66: 313-343.
Lepper L. 1964. Die Cytotaxonomie der Gattung Wulfenia Jacq. – ein Beitrag zum Kleistoploidieproblem. – Ph.D. diss., Friedrich-Schiller-Universität, Jena, Germany.
Lepper L. 1970. Die Evolution der Gattung Wulfenia Jacq. – ein Beitrag zum Wulfenia-Problem. – Wiss. Zeitschr. Friedrich-Schiller-Univ. Jena 19:345-361.
Leroy J-F. 1972. La genène d’un genre chez les Bignoniacées-Crescentiées de Madagascar. – Compt. rend. Hebd. Séances Acad. Sci., sér. D, t. 275: 2675-2678.
Lersten NR, Beaman JM. 1998. First report of oil cavities in Scrophulariaceae and reinvestigation of air spaces in leaves of Leucophyllum fructescens. – Amer. J. Bot. 85: 1646-1649.
Lersten NR, Curtis JD. 1997. Anatomy and distribution of foliar idioblasts in Scrophularia and Verbascum (Scrophulariaceae). – Amer. J. Bot. 84: 1638-1645.
Lersten NR, Curtis JD. 2001. Idioblasts and other unusual internal foliar secretory structures in Scrophulariaceae. – Plant Syst. Evol. 227: 63-73.
Lersten NR, Horner HT. 2009. Crystal diversity and macropatterns in leaves of Oleaceae. – Plant Syst. Evol. 282: 87-102.
Lersten NR, Krueger L, Curtis JD. 2002. Tracheoid variation among Bignoniaceae seed wings, with emphasis on Campsis radicans. – Intern. J. Plant Sci. 163: 369-378.
Les DH, Capers RS, Tippery NP. 2006. Introduction of Glossostigma (Phrymaceae) to North America: a taxonomic and ecological overview. – Amer. J. Bot. 93: 927-939.
Lewis DQ. 2000. A revision of the New World species of Lindernia (Scrophulariaceae). – Castanea 65: 93-122.
Lewis WH. 1945. A revision of the genus Trichostema. – Brittonia 5: 276-303.
Lewis WH. 1960. Chromosome numbers and phylogeny of Trichostema. – Brittonia 12: 93-97.
Lewis WH. 2006. Trichostema ruygtii (Lamiaceae) a new species from Napa County, California. – Madroño 53: 282-287.
Lewis WH, Oliver RL. 1961. Cytogeography and phylogeny of the North American species of Verbena. – Amer. J. Bot. 48: 638-643.
Lewis WH, Rzedowski J. 1978. The genus Trichostema (Labiatae) in Mexico. – Madroño 25: 151-154.
Li B, Xu W, Tu T, Wang Z, Olmstead RG, Peng H, Francisco-Ortega J, Cantino PD, Zhang D. 2012. Phylogenetic position of Wenchengia (Lamiaceae): a taxonomically enigmatic and critically endangered genus. – Taxon 61: 392-401.
Li B, Cantino PD, Olmstead RG, Bramley GLC, Xiang CL, Ma ZH, Tan YH, Zhang DX. 2016. A large-scale chloroplast phylogeny of the Lamiaceae sheds new light on its subfamilial classification. – Sci. Rep. 6: 34343 https://doi.org/10.1038/srep34343
Li G-D, Kim C, Zha H-G, Zhou Z, Nie Z-L, Sun
H. 2014. Molecular phylogeny and biogeography of the arctic-alpine genus
Lagotis (Plantaginaceae). – Taxon 63:
103-115.
Li H-L. 1947. Relationship and taxonomy of the genus Brandisia. – J. Arnold Arbor. 28: 127-136.
Li H-L. 1948. A revision of the genus Rehmannia. – Taiwania 1: 71-82.
Li H-L. 1953. Euphrasia in China. – Notulae Naturae 254: 1-6.
Li H-L. 1954. Trapellaceae: a familial segregate from the Asiatic flora. – J. Washington Acad. Sci. 44: 11-13.
Li H-W. 1983. A new genus of Acanthaceae from Yunnan. – Acta Phytotaxon. Sin. 21: 470-472.
Li H-W. 1988. Taxonomic review of Isodon (Labiatae). – J. Arnold Arbor. 69: 289-400.
Li J. 2008. Phylogeny of Catalpa (Bignoniaceae) inferred from sequences of chloroplast ndhF and nuclear ribosomal DNA. – J. Syst. Evol. 46: 341-348.
Li J, Alexander JH, Zhang D. 2002. Paraphyletic Syringa (Oleaceae): evidence from sequences of nuclear ribosomal DNA ITS and ETS regions. – Syst. Bot. 27: 592-597.
Li J-H. 2008. Phylogeny of Catalpa (Bignoniaceae) inferred from sequences of chloroplast ndhF and nuclear ribosomal DNA. – J. Syst. Evol. 46: 341-348.
Li J-M, Wang Y-Z. 2007. Phylogenetic reconstruction among species of Chiritopsis and Chirita Sect. Gibbosaccus (Gesneriaceae) based on nrDNA ITS and cpDNA trnI-F sequences. – Syst. Bot. 32: 888-898.
Li J-M, Sun W-J, Chang Y, Yang W-G. 2016. Systematic position of Gyrocheilos and some odd species of Didymocarpus (Gesneriaceae) inferred from molecular data, with reference to pollen and other morphological characters. – J. Syst. Evol. 54: 113-122.
Li Q-Q, Li M-H, Yuan Q-J, Cui Z-H, Huang L-Q, Xiao P-G. 2013. Phylogenetic relationships of Salvia (Lamiaceae) in China: evidence from DNA sequence datasets. – J. Syst. Evol. 51: 184-195.
Li X, Jang T-S, Temsch EM, Kato H, Takayama K, Schneeweiss GM. 2017. Molecular and karyological data confirm that the enigmatic genus Platypholis from Bonin-Islands (SE Japan) is phylogenetically nested within Orobanche (Orobanchaceae). – J. Plant Res. 130: 273-280.
Li X-D, Li J-Q. 2006. Morphology characters of leaf epidermis of the genera Rehmannia and Triaenophora. – J. Wuhan Bot. Res. 24: 559-564. [In Chinese with English abstract]
Li X-D, Li J-Q, Zan Y-Y. 2005. A new species of Triaenophora (Scrophulariaceae) from China. – Novon 15: 559-561.
Li X-D, Li J-Q, Zan Y-Y. 2007. Allozymic variability in Rehmannia and Triaenophora (Scrophulariaceae). – Acta Phytotaxon. Sin. 45: 561-569. [In Chinese with English abstract]
Li X-D, Zan Y-Y, Li J-Q, Yang S-Z. 2008. A numerical taxonomy of the genera Rehmannia and Triaenophora (Scrophulariaceae). – J. Syst. Evol. 46: 730-737.
Li X-D, Ge J-W, Zan Y-Y, Li J-Q. 2008. A new synonym of Triaenophora (Scrophulariaceae) from China. – J. Wuhan Bot. Res. 26: 362-365.
Li X-D, Zan Y-Y, Luo M-M, Liu H-T. 2011. Taxonomic revision of the genus Rehmannia. – Plant Sci. J. 1: 423-431.
Liang Y-S, Wang J-C. 2014. A systematic study
of Bonnaya section Bonnaya (Linderniaceae). – Aust. Syst.
Bot. 27: 180-198.
Lim CL. 2014. Revision of Codonoboea sect. Boeopsis and sect. Salicini (Gesneriaceae) in Peninsular Malaysia. – Ph.D. thesis, University of Malaya, Kuala Lumpur, Malaysia.
Lim CL, Kiew R. 2014. Codonoboea (Gesneriacee) sections in Peninsular Malaysia. – Reinwardtia 14: 1-248.
Lima AC, Pace MR, Angyalossy V. 2010. Seasonality and growth rings in lianas of Bignoniaceae. – Trees 24: 1045-1060.
Lindau G. 1893. Übersicht über die bisher bekannten Arten der Gattung Thunbergia L. f. – Engl. Bot. Jahrb. Syst. 41: 31-41.
Lindau G. 1894a. Beiträge zur Systematik der Acanthaceen. Tribus XI: Asystasieae. – Engl. Bot. Jahrb. Syst. 18: 36-64.
Lindau G. 1894b. Acanthaceae africanae 2. – Engl. Bot. Jahrb. Syst. 20: 1-78.
Lindau G. 1895. Acanthaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien, IV(3b), W. Engelmann, Leipzig, pp. 274-354; Lindau G. 1897. Nachträge zu IV(3b), pp. 304-309.
Lindau G. 1897a. Acanthaceae americanae et asiaticae novae vel minus cognitae. – Bull. Herb. Boissier 5: 643-681.
Lindau G. 1897b. Acanthaceae africanae III. – Engl. Bot. Jahrb. Syst. 22: 221-227.
Lindau G. 1899. Megalochlamys nov. gen. Acanthacearum. – Engl. Bot. Jahrb. Syst. 26: 345-346.
Lindau G. 1901. Acanthaceae africanae V. – Engl. Bot. Jahrb. Syst. 30: 111-114.
Lindau G. 1902. Acanthaceae africanae VI. – Engl. Bot. Jahrb. Syst. 33: 183-193.
Lindau G. 1904. Acanthaceae americanae III. – Bull. Herb. Boissier, sér. II, 4: 313-328.
Lindau G. 1912. Einige neue Acanthaceen. – Feddes Repert. 11: 122-124.
Lindau G. 1913. Acanthaceae africanae IX. – Engl. Bot. Jahrb. Syst. 49: 399-409.
Lindau G. 1920. Acanthaceae africanae X. – Engl. Bot. Jahrb. Syst. 57: 20-24.
Linder HP. 2004. Stilbaceae. – In: Kubitzki K, Kadereit JW (eds), The families and genera of vascular plants VII. Flowering plants. Dicotyledons. Lamiales (except Acanthaceae including Avicenniaceae), Springer, Berlin, Heidelberg, New York, pp. 433-440.
Lindqvist C, Albert VA. 1999. Phylogeny and conservation of African violets (Saintpaulia: Gesneriaceae): new findings based on nuclear ribosomal 5S non-transcribed spacer sequences. – Kew Bull. 54: 363-377.
Lindqvist C, Albert VA. 2001. A high elevation ancestry for the Usambara Mountains and lowland populations of African violets (Saintpaulia, Gesneriaceae). – Syst. Geogr. Plants 71: 37-44.
Lindqvist C, Albert VA. 2002. Origin of the Hawaiian endemic mints within North American Stachys (Lamiaceae). – Amer. J. Bot. 89: 1709-1724.
Lindqvist C, Motley TJ, Jeffery JJ, Albert VA. 2003. Cladogenesis and reticulation in the Hawaiian endemic mints (Lamiaceae). – Cladistics 19: 480-495.
Lint H, Epling CC. 1945. A revision of Agastache. – Amer. Midl. Natur. 33: 207-230.
Lippert W. 1979. Zur Kenntnis von Salvia Sektion Salvia im Westlichen Mittelmeergebiet. – Mitt. Bot. Staatssamml. München 15: 397-423.
Litvinenko VI, Popova TP, Simonian AV, Zoz IG, Sokolov VS. 1975. ”Gerbstoffe” und Oxyzimtsäure-abkömmlinge in Labiaten. – Planta Medica 27: 372-380.
Liu B, Tan Y-H, Liu S, Olmstead RG, Min D-Z, Chen Z-D, Joshee N, Vaidya BN, Chung RCK, Lo B. 2020. – J. Syst. Evol. 58: 1–17. doi: 10.1111/jse.12513.
Liu M-L, Yu W-B, Li D-Z, Mill R, Wang H. 2013. Seed morphological diversity of Pedicularis (Orobanchaceae) and its taxonomic significance. – Plant Syst. Evol. 299: 1645-1657.
Liu M-L, Yu W-B, Kuss P, Li D-Z, Wang H. 2015. Floral nectary morphology and evolution in Pedicularis (Orobanchaceae). – Bot. J. Linn. Soc. 178: 592-607.
Livera EJ. 1924. Plaesianthera. – Ann. Roy. Bot. Gard. Peradeniya 9: 95-197.
Lloyd FE. 1932. The range of structural and functional variety in the traps of Utricularia and Polypompholyx. – Flora 126: 303-328.
Lloyd FE. 1935. Utricularia. – Biol. Rev. Biol. Proc. Cambridge Philos. Soc. 10: 72-110.
Lloyd FE. 1942. The carnivorous plants. – Waltham, Massachusetts.
Lobin W, Düll B. 1996. Die Gattung Cistanche (Orobanchaceae) auf den Kapverdischen Inseln unter Berücksichtigung der Arten des westafrikanischen Kontinents. – Willdenowia 25: 583-594.
Lobin W, Porembski S. 1994. The genus Verbascum (Scrophulariaceae) on the Cape Verde Islands, W Africa. – Willdenowia 24: 65-81.
Lobreau-Callen D, Jérémie J, Suarez-Cervera M. 1999. Morphologie et ultrastructure du pollen dans le genre Utricularia L. (Lentibulariaceae). – Can. J. Bot. 77: 744-767.
Lohmann LG. 2003. Phylogeny, classification, morphological diversification and biogeography of Bignonieae (Bignoniaceae). – Ph.D. diss., University of Missouri, St. Louis, Missouri.
Lohmann LG. 2006. Untangling the phylogeny of neotropical lianas (Bignonieae, Bignoniaceae). – Amer. J. Bot. 93: 304-318.
Lohmann LG, Taylor CM. 2014. A new generic
classification of tribe Bignonieae (Bignoniaceae). – Ann. Missouri
Bot. Gard. 99: 348-489.
Lohmann LG, Bell CD, Calió MF, Winkworth RC. 2013. Pattern and timing of biogeographical history in the Neotropical tribe Bignonieae (Bignoniaceae). – Bot. J. Linn. Soc. 171: 154-170.
Long FX. 1901. Untersuchungen über Morphologie, Anatomie, und Samenentwicklung von Polypompholyx und Byblis gigantea. – Flora 88: 3-60.
Long RW. 1971. Floral polymorphy and amphimictic breeding systems in Ruellia caroliniensis (Acanthaceae). – Amer. J. Bot. 58: 525-531.
López González G. 1981. Conspectus saturejarum. – An. Jard. Bot. Madrid 38: 361-415.
López Guillén J. 1968. La sección Aposecos del subgénero Calceolaria Penn. en el Peru. Primera revisión de las especies endémicas Peruanas. – Raymondiana 1: 29-87.
López Guillén J. 1969. La sección Aposecos del subgénero Calceolaria Penn. en el Peru. Segunda revisión de las especies endémicas Peruanas. – Raymondiana 2: 5-44.
López-Palacios S. 1991. Aspectos críticos de la sistemática de Verbenaceae de Venezuela. Addenda delenda et corrigenda a Verbenaceae Flora de Venezuela (1977). – Pittieria 19: 40-52.
Lowrie A, Conran JG. 1998. A taxonomic revision of the genus Byblis (Byblidaceae) in northern Australia. – Nuytsia 12: 59-74.
Lowrie A, Conran JG. 2008. Byblis guehoi (Byblidaceae), a new species from the Kimberley, Western Australia. – Telopea 12: 23-29.
Lowrie A, Cowie ID, Conran JG. 2008. A new species and section of Utricularia (Lentibulariaceae) from northern Australia. – Telopea 12: 31-46.
Lowry JB. 1972. Anthocyains of some Malaysian members of the Gesneriaceae. – Phytochemistry 11: 3267-3269.
Lowry JB. 1973. Rhabdothamnus solandri: some phytochemical results. – New Zealand J. Bot. 11: 555-560.
Lu A-M. 1990. A preliminary cladistic study of the families of the superorder Lamiiflorae. – Bot. J. Linn. Soc. 103: 39-57.
Lu KL, Mesler MR. 1981. Ant dispersal in a neotropical forest floor gesneriad. – Biotropica 13: 159-160.
Lu L, Wang H, Blackmore S, Li D-Z, Dong L-N. 2007. Pollen morphology of the tribe Rhinantheae (Orobanchaceae) and its systematic significances. – Plant Syst. Evol. 268: 177-198.
Lu Y, Xu P-J, Chen Z-N, Liu GM. 1998a. The anthraquinones of Rhynchotechum vestitum. – Phytochemistry 47. 315-317.
Lu Y, Xu P-J, Chen Z-N, Liu GM. 1998b. Anthraquinone glycosides from Rhynchotechum vestitum. – Phytochemistry 49: 1135-1137.
Luegmayr E. 1993a. Pollen of Hawaiian Cyrtandra (Gesneriaceae), including notes on Southeast Asian taxa. – Blumea 38: 25-38.
Luegmayr E. 1993b. The generative cell and its close association with the endoplasmatic reticulum of the vegetative cell in pollen of Cyrtandra pendula (Gesneriaceae). – Protoplasma 177: 73-81.
Luegmayr E. 1993c. Pollen characters of Old World Gesneriaceae (Cyrtandroideae) with special reference to SE Asian taxa. – Grana 32: 221-232.
Luegmayr E. 1994. Pollenmorphologische und pollenontogenetische Untersuchungen an ausgewählten Gesneriaceae. – Diss., Formal- & Naturwiss. Fakultät, Universität Wien, Austria.
Luhan M. 1954. Über das Vorkommen von Sklerenchym-Idioblasten bei Globularia-Arten. – Ber. Deutsch. Bot. Ges. 67: 346-355.
Lu-Irving P, Olmstead RG. 2013. Investigating the evolution of Lantaneae (Verbenaceae) using multiple loci. – Bot. J. Linn. Soc. 171: 103-119.
Lu-Irving P, O’Leary N, O’Brien A,
Olmstead RG. 2014. Resolving the genera Aloysia and
Acantholippia within tribe Lantaneae (Verbenaceae), using chloroplast and
nuclear sequences. – Syst. Bot. 39: 644-655.
Lukhoba C, Paton A. 2000. Two new species of Plectranthus L’Hér. (Labiatae) from East Africa. – Kew Bull. 55: 957-964.
Lundgren L, Stenhagen G. 1982. Leaf volatiles from Thymus vulgaris, T. serpyllum, T. praecox, T. pulegioides and T. x citriodorus (Labiatae). – Nord. J. Bot. 2: 445-452.
Lunsford DE. 1939. Studies in the life cycle of Amphianthus pusillus Torrey. – M.Sc. thesis, Emory University, Atlanta, Georgia.
Luo D, Carpenter R, Vincent C, Copsey L, Coen E. 1996. Origin of floral asymmetry in Antirrhinum. – Nature 383: 794-799.
Luo Y, Feng C, Tian Y, Li B, Zhang G. 2003. Three novel nortriterpenoids from Notochaete hamosa Benth. (Labiatae). – Tetrahedron 59: 8227-8232.
Lüönd B, Lüönd R. 1981. Insect dispersal of pollen and fruits in Ajuga. – Candollea 36: 167-179.
Ma Z, Bramley GLC, Zhang D. 2016. Pollen morphology of Callicarpa L. (Lamiaceae) from China and its systematic implications. – Plant Syst. Evol. 302: 67-88.
Mabberley DJ. 1998. On Neorapinia (Vitex sensu lato, Labiatae-Viticoideae). – Telopea 7: 313-317.
McCallum DA, Balkwill K. 2004. A new species of Ocimum (Lamiaceae) from Swaziland. – Bot. J. Linn. Soc. 145: 379-383.
McClintock E, Epling CC. 1942. A review of the genus Monarda (Labiatae). – Univ. Calif. Publ. Bot. 20: 147-194.
McClintock E, Epling CC. 1946. A revision of Teucrium in the New World, with observations on its variation, geographical distribution and history. – Brittonia 5: 491-510.
McCullagh D. 1934. Chromosome and chromosome morphology in Plantaginaceae I. – Genetica 16: 1-44.
McDade LA. 1982. New species of Justicia and Razisea (Acanthaceae) from Costa Rica, with taxonomic notes. – Syst. Bot. 7: 489-497.
McDade LA. 1984. Systematics and reproductive biology of the Central American species of the Aphelandra pulcherrima complex (Acanthaceae). – Ann. Missouri Bot. Gard. 71: 104-165.
McDade LA. 1988. Recognition of Aphelandra glabrata (Acanthaceae) from western South America, with notes on phylogenetic relationships. – Syst. Bot. 13: 235-239.
McDade LA, Moody ML. 1999. Phylogenetic relationships among Acanthaceae: evidence from non-coding trnL-trnF chloroplast DNA sequences. – Amer. J. Bot. 86: 70-80.
McDade LA, Tripp EA. 2007. A synopsis of Costa Rican Ruellia (Acanthaceae), with descriptions of four new species. – Brittonia 59: 199-216.
McDade LA, Turner MD. 1997. Extrafloral nectaries in Aphelandra: anatomy, development and systematic implications. – Amer. J. Bot. 84: 1-15.
McDade LA, Masta SE, Moody ML, Waters E. 2000. Phylogenetic relationships among Acanthaceae: evidence from two genomes. – Syst. Bot. 25: 106-121.
McDade LA, Daniel TF, Masta SE, Riley KM. 2000. Phylogenetic relationships within the tribe Justicieae (Acanthaceae): evidence from molecular sequences, morphology, and cytology. – Ann. Missouri Bot. Gard. 87: 435-458.
McDade LA, Daniel TF, Kiel CA, Vollesen K. 2005. Phylogenetic relationships among Acantheae (Acanthaceae): major lineages present contrasting patterns of molecular evolution and morphological differentiation. – Syst. Bot. 30: 834-862.
McDade LA, Daniel TF, Kiel CA. 2008. Toward a comprehensive understanding of phylogenetic relationships among lineages of Acanthaceae s.l. (Lamiales). – Amer. J. Bot. 95: 1136-1152.
McDade LA, Kiel C, Daniel TF, Tripp EA. 2008. Biogeography of the Acanthaceae. – South Afr. J. Bot. 74: 358.
McDade LA, Daniel TF, Kiel CA, Borg AJ. 2012. Phylogenetic placement, delimitation, and relationships among genera of the enigmatic Nelsonioideae (Lamiales: Acanthaceae). – Taxon 61: 637-651.
McDade LA, Daniel TF, Kiel CA. 2018. The Tetramerium lineage (Acanthaceae, Justicieae) revisited: phylogenetic relationships reveal polyphyly of many New World genera accompanied by rampant evolution of floral morphology. – Syst. Bot. 43: 97-116.
Macior LW. 1983. The pollination dynamics of sympatric species of Pedicularis (Scrophulariaceae). – Amer. J. Bot. 70: 844-853.
MacMinn HE. 1951. Studies in the genus Diplacus (Scrophulariaceae). – Madroño 11: 1-32.
McNeal JR, Bennett JR, Wolfe AD, Mathews S. 2013. Phylogeny and origins of holoparasitism in Orobanchaceae. – Amer. J. Bot. 100: 971-983
Macukanovic-Jocic MP, Rancic DV, Dajic Stevanovic ZP. 2007. Floral nectaries of basil (Ocimum basilicum): morphology, anatomy and possible mode of secretion. – South Afr. J. Bot. 73: 636-641.
McVaugh R, Schmid R. 1967. Novelties in Satureja sect. Gardoquia (Labiatae). – Brittonia 19: 261-267.
Maffei M, Chialva F, Sacco T. 1989. Glandular trichomes and essential oils in developing peppermint leaves I. Variation of peltate trichome number and terpene distribution within leaves. – New Phytol. 111: 707-716.
Maggi A, Taskova R, Gotfredsen CH, Bianco A, Jensen SR. 2009. Chemical markers in Veronica sect. Hebe III. – Biochem. Syst. Ecol. 37: 731-736.
Magin N, Classen R, Gack C. 1989. The morphology of false anthers in Craterostigma plantagineum and Torenia polygonioides (Scrophulariaceae). – Can. J. Bot. 67: 1931-1937.
Maheshwari JK. 1954. Floral morphology and embryology of Lippia nodiflora Rich. – Phytomorphology 4: 217-230.
Maheshwari JK. 1961. The genus Wightia Wall. in India with a discussion on its systematic position. – Bull. Bot. Surv. India 3: 31-35.
Maheshwar P, Negi V. 1955. The embryology of Dipteracanthus patulus (Jacq.) Nees. – Phytomorphology 5: 456-472.
Majumdar S, Chanda S. 1978. Pollen morphology and taxonomy of Carlemannia and Silvianthus of the family Rubiaceae sensu lat. – Trans. Bose Res. Inst. Calcutta 41: 99-105.
Makarova ZI. 1967. On the significance of the anatomical structure of the pericarp for the taxonomy of the tribe Nepeteae. – Bot. Žurn. 52: 33-41. [In Russian]
Makholela TM, Balkwill K, Manning JC. 2008. Clarification of generic delimitation in Justicia and Siphonoglossa (Acanthaceae). – South Afr. J. Bot. 74: 371-372.
Makino T. 1915. Two new genera Matsumurella Makino and Ajugoides Makino. – Bot. Mag. (Tokyo) 29: 379-383.
Maksudov MS, Faskhutdinov MF, Umarova RU, Saatov Z. 1995. Iridoids of Incarvillea olgae and Dodartia orientalis. – Khim. Prir. Soedin. 5: 751-752.
Maldonado de Magnano S. 1986. Estudios embriológicos en Buddleja (Buddlejaceae) I. Endosperma y episperma. – Darwiniana 27: 225-236.
Maldonado de Magnano S. 1987. Estudios embriológicos en Buddleja (Buddlejaceae) II. Embriogénesis. – Darwiniana 28: 391-395.
Malo AC, Xifreda CC. 2006. El género Cunila (Lamiaceae, Mentheae) en Argentina. – Darwiniana 44: 298-308.
Manen J-F, Habashi C, Jeanmonod D, Park J-M, Schneeweiss GM. 2004. Phylogeny and intraspecific variability of holoparasitic Orobanche (Orobanchaceae) inferred from plastid rbcL sequences. – Mol. Phylogen. Evol. 33: 482-500.
Manktelow M. 1996. Phaulopsis (Acanthaceae) – a monograph. – Symb. Bot. Upsal. 31(2): 1-184.
Manktelow M. 2000. The filament curtain: a structure important to systematics and pollination biology in Acanthaceae. – Bot. J. Linn. Soc. 133: 129-160.
Manktelow M, McDade LA, Oxelman B, Furness CA, Balkwill M-J. 2001. The enigmatic tribe Whitfieldieae (Acanthaceae): delimitation and phylogenetic relationships based on molecular and morphological data. – Syst. Bot. 26: 104-119.
Manning JC, Goldblatt P. 2010. Scrophulariaceae. Two new species of Limoselleae from western South Africa: Trieenia occulta and Zaluzianskya regalis. – Bothalia 40: 84-90.
Manning JC, Goldblatt P. 2014. Reyemia included in Zaluzianskya (Scrophulariaceae: Limoselleae), with the new combination Zaluzianskya chasmanthiflora (Hilliard). – In: Goldblatt P, Manning JC (eds), Nomenclatural adjustments in African plants 1, Bothalia 44, 9 pp.
Mansfeld R. 1925. Neue andine Labiaten der Sammlung Weberbauer. – Notizbl. Bot. Gart. Mus. Berlin-Dahlem 9: 283-289.
Mantegazza R, Tononi P, Möller M, Spada A. 2009. WUS and STM homolgs are linked to the expression of lateral dominance in the acaulescent Streptocarpus rexii (Gesneriaceae). – Planta 230: 529-542.
Manzanares P, Gómez-Campo C, Tortosa ME. 1983. Estudios sobre el indumento de las especies ibéricas y baleáricas del género Teucrium L. (Lamiaceae). – An. Jard. Bot. Madrid 40: 93-106.
Marais W. 1979. A new combination in Nesogenes (Dicrastylidaceae). – Kew Bull. 33: 420.
Marais W. 1981. Two new gamopetalous families, Cyclocheilaceae and Nesogenaceae, for extra-Australian ‘Dicrastylidiaceae’. – Kew Bull. 35: 797-812.
Marais W. 1983. Madagascan Nesogenes (Nesogenaceae). – Kew Bull. 38: 37-39.
Marais W. 1984a. Cyclocheilaceae. – In: Polhill RM (ed), Flora of tropical East Africa, A. A. Balkema, Rotterdam, The Netherlands, pp. 1-5.
Marais W. 1984b. Nesogenaceae. – In: Polhill RM (ed), Flora of tropical East Africa, A. A. Balkema, Rotterdam, The Netherlands, pp. 1-3.
Marin PD, Sajdl V, Kapor S, Tatic B, Petkovic B. 1991. Fatty acids of the Saturejoideae, Ajugoideae and Scutellarioideae (Lamiaceae). – Phytochemistry 30: 2979-2982.
Marin PD, Petkovic B, Duletic S. 1994. Nutlet sculpturing of selected Teucrium species (Lamiaceae): a character of taxonomic significance. – Plant Syst. Evol. 192: 199-214.
Markova ML, Ivanova PS. 1982. Karyological study of the genus Salvia L. in Burgaria. – Filologija 20: 3-19.
Markovska YK, Kimenov GP, Tsonev TD. 1989. Presence of Crassulacean Acid Metabolism in higher poikilohydric plants Haberlea rhodopensis Friv. and Ramonda serbica Panc. – Photosynthetica 23: 364-367.
Markovska YK, Tsonev TD, Kimenov GP, Tutekov A. 1994. Physioloical changes in higher poikilohydric plants – Haberlea rhodopensis Friv. and Ramonda serbica Panc. during drought and rewatering at different light regimes. – J. Plant Physiol. 144: 100-108.
Markovska YK, Tsonev TD, Kimenov GP. 1997. Regulation of CA and respiratory recycling by water supply in higher poikilohydric plants Haberlea rhodopensis Friv. and Ramonda serbica Panc. at transition from biosis to anabiosis and vice versa. – Bot. Acta 110: 18-24.
Markowa M, Ivánová P. 1971. Karyologische Untersuchung der Vertreter der Fam. Boraginaceae, Labiatae und Scrophulariaceae in Bulgarien II. – Izvest. Bot. Inst. (Sofia) 21: 123-131.
Marloth R. 1898. Charadrophila Marloth nov. gen. – Engl. Bot. Jahrb. Syst. 26: 358-359.
Marrero A. 1992. Chromosomal evolutionary trends in the genus Sideritis subgenus Marrubiastrum. – In: Harley RM, Reynolds T (eds), Advances in labiate science, Royal Botanic Gardens, Kew, pp. 247-256.
Marsden MPF, Bailey IW. 1955. A fourth type of nodal anatomy in dicotyledons, illustrated by Clerodendron dichotomum Thunb. – J. Arnold Arbor. 36: 1-50.
Martínez S, Múlgura de Romera ME. 1997. Yemas axilares multiples, morfologia y tipologia de la inflorescencia en Duranta (Verbenaceae-Citharexyleae). – Bol. Soc. Argent. Bot. 33: 113-122.
Martínez S, Múlgura de Romera ME. 2003. The taxonomic position of Parodianthus (Verbenaceae): a morphological survey of the gynoecium and inflorescence. – Kew Bull. 58: 929-938.
Martínez S, Botta S, Múlgura de Romera ME. 1996. Morfologia de las inflorescencias en Verbenaceae-Verbenoideae I: Tribu Verbeneae. – Darwiniana 34: 1-17.
Martínez-Ortega MM, Rico E. 2001a. Seed morphology and its systematic significance in some Veronica species (Scrophulariaceae) mainly from the Western Mediterranean. – Plant Syst. Evol. 228: 15-32.
Martínez-Ortega MM, Rico E. 2001b. Taxonomy of Veronica subsect. Serpyllifoliae (Scrophulariaceae). – Bot. J. Linn. Soc. 135: 179-194.
Martínez-Ortega MM, Sánchez Sánchez J, Rico E. 2000. Palynological study of Veronica L. sect. Veronica and sect. Veronicastrum (Scrophulariaceae) and its taxonomic significance. – Grana 39: 21-31.
Martínez-Ortega MM, Delgado L, Albach DC, Elena-Rossello JA, Rico E. 2004. Species boundaries and phylogeographic patterns in cryptic taxa inferred from AFLP markers: Veronica subgen. Pentasepalae (Scrophulariaceae) in the western Mediterranean. – Syst. Bot. 29: 965-986.
Martín Mosquero A. 2002. Micromorfología y anatomía en núculas de Lamiaceae de Andalucia occidental. – Ph.D. diss., Universidad de Sevilla, Spain.
Martins AC, Scherz MD, Renner SS. 2014.
Several origins of floral oil in the Angelonieae, a southern hemisphere
disjunct clade of Plantaginaceae. – Amer. J.
Bot. 101: 2113-2120.
Martinsson K. 1991. Geographical variation in fruit morphology in Swedish Callitriche hermaphroditica (Callitrichaceae). – Nord. J. Bot. 11: 497-512.
Martinsson K. 1993. The pollen of Swedish Callitriche (Callitrichaceae) – trends towards submergence. – Grana 32: 198-209.
Marx HE, O’Leary N, Yuan Y-W, Lu-Irving P, Tank DC, Múlgara ME, Olmstead RG. 2010. A molecular phylogeny and classification of Verbenaceae. – Amer. J. Bot. 97: 1647-1663.
Mason HL, Bacigalupi R. 1954. A new Gratiola from Boggs Lake, Lake County, California. – Madroño 12: 150-152.
Mateo C, Sanz J, Calderón J. 1983. Essential oil of Sideritis hirsuta. – Phytochemistry 22: 171-173.
Mateo C, Sanz J, Calderón J. 1984. The essential oils of some eastern Spain Sideritis. – Phytochemistry 23: 319-322.
Mateo C, Calderón J, Sanz J. 1988. Essential oils of some Sideritis species from central and southern Spain. – Phytochemistry 27: 151-153.
Mateu-Andres I. 1999. Allozymic variation and divergence in three species of Antirrhinum L. (Scrophulariaceae-Antirrhineae). – Bot. J. Linn. Soc. 131: 187-199.
Mateu-Andres I. 2001. A new species of Misopates Raf. (Scrophulariaceae, Antirrhineae). – Bot. J. Linn. Soc. 137: 421-424.
Mathew L, Shah GL. 1984. Crystals and their taxonomic significance in some Verbenaceae. – Bot. J. Linn. Soc. 88: 279-289.
Mathew L, Shah GL. 1988. Pollen morphology and their taxonomic significance in some Verbenaceae. – Geophytology 18: 89-101.
Mathiesen C, Scheen A-C, Lindqvist C. 2011. Phylogeny and biogeography of the lamioid genus Phlomis (Lamiaceae). – Kew Bull. 66: 83-99.
Mathur G, Mohan Ram HY. 1986. Floral biology and pollination of Lantana camara. – Phytomorphology 36: 79-100.
Mattern G, Vogel S. 1994. Lamiaceen-Blüten duften mit dem Kelch – Prüfung einer Hypothese II. Olfactorischer und gaschromatographischer Vergleich des Laub- und kelchduftes. – Beitr. Biol. Pflanzen 68: 203-248.
Matthew KM. 1971. The flowering of the strobilanth (Acanthaceae). – J. Bombay Nat. Hist. Soc. 67: 502-506.
Mattos JR. 1970. Handroanthus, um novo gênero para os ”ipes” do Brasil. – Loefgrenia 50: 1-4.
Mauritzon J. 1934. Die Endosperme und Embryoentwicklung einiger Acanthaceen. – Lunds Univ. Årsskr. II, sekt. 2, 30(5): 1-41.
Mayberry ME. 1947. Martynia louisiana Mill.: an anatomical study. – Trans. Kansas. Acad. Sci. 50: 164-171.
Mayer E. 1972. Notulae ad floram Jugoslaviae V. Conspectus generis Odontites Ludw. – Glasn. Prir. Muz. Beogradu, Ser. B, Biol. Nauke 4: 5-14.
Mayer V, Möller M, Perret M, Weber A. 2003. Phylogenetic position and generic differentiation of Epithemateae (Gesneriaceae) inferred from plastid DNA sequence data. – Amer. J. Bot. 90: 321-329.
Mayr EM, Weber A. 2006. Calceolariaceae: floral development and systematic implications. – Amer. J. Bot. 93: 327-343.
Medeiros MCMP de, Lohmann LG. 2015. Phylogeny and biogeography of Tynanthus Miers (Bignonieae, Bignoniaceae). – Mol. Phylogen. Evol. 85: 32-40.
Mehra KR, Kulkarni AR. 1985. Embryological studies in Bignoniaceae. – Phytomorphology 35: 235-251.
Mehra PN, Atal CK. 1961. Studies in the mucilage yielding seeds I. Nutlet structure and mucilage formation in Lallemantia royellana Benth., Ocimum basilicum Linn. and Ocimum canum Sims. – Panjab Univ. Res. Bull. 12: 169-182.
Meimberg H, Abele T, Bräuchler C, McKay JK, Perez de Paz PL, Heubl G. 2006. Molecular evidence for adaptive radiation of Micromeria Benth. (Lamiaceae) on the Canary Islands as inferred from chloroplast and nuclear DNA sequences and ISSR fingerprint data. – Mol. Phylogen. Evol. 41: 566-578.
Melchior H. 1927. Der natürliche Formenkreis der Pithecocteniinae Innerhalb der Familie der Bignoniaceae. – Repert. Spec. Nov. regni Veg. Beih. 46: 71-82.
Melchior H. 1940. Beitrag zur Kenntnis der Gattung Melasma. – Notisbl. Bot. Gart. Berlin-Dahlem 15: 119-127.
Melchior H. 1941. Die Gattung Alectra Thunb. – Notizbl. Bot. Gart. Berlin-Dahlem 15: 423-447.
Melhelm T, Mauro C. 1973. Pollen morphological studies in Gesneriaceae. – Hoehnea 3: 13-27.
Méndez Larios I, Villaseñor Ríos JL. 2001. La familia Scrophulariaceae en México: diversidad y distribución. – Bol. Soc. Bot. México 69: 101-121.
Méndez Santos IE. 1993. La tribu Lantaneae Briq. (Verbenaceae) en Cuba. – Fontqueria 36: 245-251.
Méndez Santos IE. 1994. Nuevo tratamiento sistemático para la tribu Lantaneae (Verbenaceae, Verbenoideae) en Cuba. – Ph.D. diss., Jardín Botánico Nacional, Universidad de La Habana, Cuba.
Méndez Santos IE. 1998. Evaluación morfo-anatómica de los géneros cubanos de Lantaneae (Verbenaceae) en Cuba. – Rev. Jard. Bot. Nac. Univ. Habana 19: 17-40.
Méndez Santos IE. 2002. A taxonomic revision of Lantana sect. Lantana (Verbenaceae) in the Greater Antilles. – Willdenowia 22: 285-301.
Mendoza-Heuer I. 1977. Datos comparativos acerca de especies mediterráneas y macaronésicas del género Sideritis L. (Lamiaceae). – Bot. Macaronés. IV, Ci. 3: 61-71.
Mendum M, Lassnig P, Weber A, Christie F. 2001. Testa and seed appendage morphology in Aeschynanthus (Gesneriaceae): phytogeographical patterns and taxonomic implications. – Bot. J. Linn. Soc. 135: 195-213.
Menezes de Sequeira M, Capelo JH, Costa JC, Jardim R. 2008. Teucrium francoi M. Seq., Capelo, J. C. Costa & R. Jardim, a new species of Teucrium gr. scorodonia (Lamiaceae) from Madeira. – Bot. J. Linn. Soc. 156: 639-647.
Mennega AMW. 1980. Anatomy of the secondary xylem of the Loganiaceae. –In: Leeuwenberg AJM (ed), Engler and Prantl’s Die natürlichen Pflanzenfamilien, Fam. Loganiaceae, vol. 28b(1), Duncker & Humblot, Berlin, pp. 112-161.
Mennema J. 1989. A taxonomic revision of Lamium (Lamiaceae). – Leiden Bot. Ser. 11: 1-198.
Merl EM. 1915. Beiträge zur Kenntnis der Utricularien und Genliseen. – Flora 108: 127-200.
Merxmüller H. 1953. Eine neue Gattung der Acanthaceen. – Mitt. Bot. Staatssamml. München 1: 175-181.
Messana I, Sperandei M, Multari G, Galeffi C, Marini-Bettollo GB. 1984. A cyclohexadienone and a cyclohexenone from Halleria lucida. – Phytochemistry 23: 2617-2619.
Meudt HM. 2006. Monograph of Ourisia
(Plantaginaceae). – Syst.
Bot. Monogr. 77: 1-188.
Meudt HM. 2008. Taxonomic revision of Australasian snow hebes (Veronica, Plantaginaceae). – Aust. Syst. Bot. 21: 387-421.
Meudt HM, Bayly MJ. 2008. Phylogeographic patterns in the Australasian genus Chionohebe (Veronica s.l., Plantaginaceae) based on AFLP and chloroplast DNA sequences. – Mol. Phylogen. Evol. 47: 319-338.
Meudt HM, Simpson BB. 2007. Phylogenetic analysis of morphological characters in Ourisia (Plantaginaceae): taxonomic and evolutionary implications. – Ann. Missouri Bot. Gard. 94: 554-570.
Meunier A. 1897. Le développement séminal dans le genre Veronica. – La Cellule 12: 297-333.
Meyers DG, Strickler JR. 1979. Capture enhancement in a carnivorous aquatic plant: function of antennae and bristles in Utricularia vulgaris. – Science 203: 1022-1025.
Michener DC. 1986. Systematic and ecological wood anatomy of Californian Scrophulariaceae II. Penstemon subgenus Saccanthera. – Aliso 11: 365-375.
Middleton DJ, Möller M. 2012. Tribounia, a new genus of Gesneriaceae from Thailand. – Taxon 61: 1286-1295.
Middleton DJ, Triboun P. 2012. Somrania, a new genus of Gesneriaceae from Thailand. – Thai Forest Bull., Bot. 40: 9-13.
Middleton DJ, Weber A, Yao TL, Sontag S, Möller M. 2013. The current status of the species hitherto assigned to Henckelia (Gesneriaceae). – Edinburgh J. Bot. 70: 385-404.
Middleton DJ, Atkins H, Truong LH, Nishii K, Möller M. 2014. Billolivia, a new genus of Gesneriaceae from Vietnam with five new species. – Phytotaxa 161: 241-269.
Middleton DJ, Nishii K, Puglisi C, Forrest LL, Möller M. 2015. Chayamaritia (Gesneriaceae: Didymocarpoideae), a new genus from Southeast Asia. – Plant Syst. Evol. 301: 1947-1966.
Mielcarek R. 1996. Les Scrophulariaceae dans la flore d’Afrique centrale (excl. Lindernieae). – Fragm. Florist. Geobot. 41: 3-248.
Mildbraed J. 1932. Acanthaceae. – In: Mildbraed J (ed), Neue und seltene Arten aus dem südlichen Ostafrika (Tanganyika-Territ. Maandat) leg. H. J. Schlieben, Notizbl. Königl. Bot. Gart. Berlin-Dahlem 11: 393-417.
Mildbraed J. 1937. Neue und seltene Acanthacéen aus dem oestlichen belgischen Kongo. – Bull. Jard. Bot. Bruxelles 14: 353-361.
Mill RR. 2000. Notes relating to the flora of Bhutan XLII. Scrophulariaceae, excluding Pedicularis. – Edinburgh J. Bot. 57: 413-428.
Mill RR. 2001. Notes relating to the flora of Bhutan XLIII. Scrophulariaceae: Pedicularis. – Edinburgh J. Bot. 58: 57-98.
Miller AG. 1980. A revision of Campylanthus. – Notes Roy. Bot. Gard. Edinb. 38: 373-385.
Miller AG. 1982. Further notes on Campylanthus. – Notes Roy. Bot. Gard. Edinb. 40: 331-332.
Miller AG. 1985. The genus Lavandula in Arabia and tropical NE Africa. – Notes Roy. Bot. Gard. Edinb. 42: 503-528.
Miller AG. 1988. Two new species of Campylanthus. – Notes Roy. Bot. Gard. Edinb. 45: 73-76.
Miller AG, Short M, Sutton DA. 1982. A revision of Schweinfurthia. – Notes Roy. Bot. Gard. Edinb. 40: 23-40.
Milliard OM, Burtt BL. 2004. Bornea species of Cyrtandra (Gesneriaceae) closely allied to C. chrysea and C. eximia. – Kew Bull. 59: 251-259.
Milne-Redhead E. 1932. The genus Strobilanthopsis. – Kew Bull. 7: 344-347.
Minkin JP, Eshbaugh WH. 1989. Pollen morphology of the Orobanchaceae and rhinanthoid Scrophulariaceae. – Grana 28: 1-18.
Misra KC. 1939. A contribution to the embryology of the Verbenaceae. – Proc. Indian Acad. Sci., Sect. B, 9: 49-56.
Mitchell RS, Maenza Gmelch TE, Barbour JG. 1994. Utricularia inflata Walt. (Lentibulariaceae), new to New York State. – Bull. Torrey Bot. Club 121: 295-297.
Mitra K. 1968. Pollen morphology in Bignoniaceae in relation to taxonomy. – Bull. Bot. Surv. India 10: 319-326.
Mitterbusch A. 1976. Die Organopoiënese der Blüte von Calceolaria tripartita R. et P. (Scrophulariaceae). Phänomene der Morphogenese komplexer biologischer Systeme I. – Bot. Jahrb. Syst. 95: 267-320.
Miyase T, Ishino M, Akahori C, Ueno A, Ohkawa Y, Tanizawa H. 1991. Phenylethanoid glycosides from Plantago asiatica. – Phytochemistry 30: 2015-2018.
Mogensen HL. 1978. Synergids of Proboscidea louisianica (Martyniaceae) before fertilization. – Phytomorphology 28: 122-144.
Mohamed KI, Musselman LJ, Riches CR. 2001. The genus Striga (Scrophulariaceae) in Africa. – Ann. Missouri Bot. Gard. 88: 60-103.
Mohan JSS, Inamdar JA. 1983. Studies of the leaf architecture of the Oleaceae with a note on the systematic position of the genus Nyctanthes. – Feddes Repert. 94: 201-211.
Mohan Ram HY. 1960a. Post-fertilisation studies in the ovule of Ruellia tuberosa Linn. – Lloydia 23: 21-27.
Mohan Ram HY. 1960b. The development of seed of Andrographis serpyllifolia. – Amer. J. Bot. 47: 215-219.
Mohan Ram HY. 1962. Post-fertilisation development of the ovule in Barleria cristata Linn. – J. Indian Bot. Soc 41: 288-296.
Mohan Ram HY, Masand P. 1962. Endosperm and seed development in Andrographis echioides. – Curr. Sci. 31: 7-8.
Mohan Ram HY, Masand P. 1963a. The embryology of Nelsonia campestris R. Br. – Phytomorphology 13: 82-91.
Mohan Ram HY, Wadhi M. 1964. Endosperm in Acanthaceae. – Phytomorphology 14: 388-413.
Mohan Ram HY, Wadhi M. 1965. Embryology and delimitation of the Acanthaceae. – Phytomorphology 15: 201-205.
Mohrbutter C. 1936. Embryologische Studien an Loganiaceen. – Planta 26: 64-80.
Molau U. 1978a. The genus Calceolaria in NW South America I. Taxonomic characters and generic subdivision. Fasciculata, a new section. – Bot. Not. 131: 219-227.
Molau U. 1978b. The genus Calceolaria in NW South America II. The sections Chasmatochila, Thamnobia, Ericoides and Lehmannina. – Bot. Not. 131: 293-316.
Molau U. 1979a. The genus Calceolaria in NW South America III. The sections Symplocophylla and Dermatophylla. – Bot. Not. 132: 31-48.
Molau U. 1979b. New species of Calceolaria (Scrophulariaceae) from northern Peru. – Bot. Not. 132: 233-238.
Molau U. 1980a. The genus Calceolaria in NW South America IV. The sections Anacyrta, Polyclada and Phaeanthera. – Bot. Not. 133: 33-45.
Molau U. 1980b. The genus Calceolaria in NW South America V. New taxa in the sections Lehmannina and Dermatophylla. – Bot. Not. 133: 363-366.
Molau U. 1981a. The genus Calceolaria in NW South America VI. The sections Urticopsis, Lobatae and Micranthera. – Nord. J. Bot. 1: 165-185.
Molau U. 1981b. The genus Calceolaria in NW South America VII. The section Zygophylla. – Nord. J. Bot. 1: 493-519.
Molau U. 1981c. The genus Calceolaria in NW South America VIII. The section Calceolaria and appendices to parts I-VIII. – Nord. J. Bot. 1: 595-615.
Molau U. 1981d. The genus Calceolaria in NW South America. – Ph.D. diss., University of Gothenburg, Sweden.
Molau U. 1984. New taxa and combinations in Calceolaria (Scrophulariaceae) from Peru and Bolivia. – Nord. J. Bot. 4: 629-654.
Molau U. 1988a. Flora Neotropica. Monograph 47. Scrophulariaceae I: Calceolarieae. – New York Botanical Garden, Bronx, New York.
Molau U. 1988b. Hedbergia, a new genus of Scrophulariaceae from Africa. – Nord. J. Bot. 8: 193-195.
Molau U. 1990. The genus Bartsia (Scrophulariaceae-Rhinanthoideae). – Opera Bot. 102: 1-99.
Molau U. 1993. Reproductive ecology of the three Nordic Pinguicula species (Lentibulariaceae). – Nord. J. Bot. 13: 149-157.
Molau U, Eriksen B, Teilmann Knudsen J. 1989. Predispersal seed predation in Bartsia alpina. – Oecologia 81: 181-185.
Moldenke HN. 1939. The ”Verbenaceae” and ”Avicenniaceae” of Trinidad and Tobago. – Lilloa 4: 283-336.
Moldenke HN. 1940a. Some new species and varieties of Verbenaceae. – Caribb. For. 2: 13-17.
Moldenke HN. 1940b. Novelties in the Avicenniaceae and Verbenaceae. – Phytologia 1: 409-432.
Moldenke HN. 1958a. Materials toward a monograph of the genus Citharexylum III. – Phytologia 6: 332-368.
Moldenke HN. 1958b. Materials toward a monograph of the genus Citharexylum IV. – Phytologia 6: 383-432.
Moldenke HN. 1959. Materials towards a monograph of the genus Citharexylum VII. – Phytologia 7: 49-77.
Moldenke HN. 1960. Materials towards a monograph of the genus Avicennia I. – Phytologia 7: 123-168; II, 179-232; III, 259-293.
Moldenke HN. 1965. Materials toward a monograph of the genus Lippia III. – Phytologia 12: 130-180.
Moldenke HN. 1971. A fifth summary of the Verbenaceae, Avicenniaceae, Stilbaceae, Dicrastylidaceae, Symphoremaceae, Nyctanthaceae, and Eriocaulaceae of the world as to valid taxa, geographic distribution, and synonymy 1-2. – The William Paterson College of New Jersey, Wayne, New Jersey.
Moldenke HN. 1980a. A sixth summary of the Verbenaceae, Avicenniaceae, Stilbaceae, Chloanthaceae, Symphoremaceae, Nyctanthaceae, and Eriocaulaceae of the world as to valid taxa, geographic distribution, and synonymy. – Phytologia Mem. 2.
Moldenke HN. 1980b. Notes on the genus Nashia. – Phytologia 46: 172-180.
Moldenke HN. 1981. Notes on the genus Hoseanthus (Verbenaceae). – Phytologia 48: 116-121.
Moldenke HN. 1982a. Notes on the genus Faradaya. – Phytologia 51: 384-400.
Moldenke HN. 1982b. Additional notes on the genus Faradaya (Verbenaceae) I. – Phytologia 52: 20-45.
Möller M, Cronk QCB. 1997a. Origin and relationships of Saintpaulia H. Wendl. (Gesneriaceae) based on ribosomal DNA internal transcribed spacer (ITS) sequences. – Amer. J. Bot. 84: 956-965.
Möller M, Cronk QCB. 1997b. Phylogeny and disjunct distribution: evolution of Saintpaulia (Gesneriaceae). – Proc. Roy. Soc. London, Ser. B (Biol. Sci.), 264: 1827-1836.
Möller M, Cronk QCB. 1999. New approaches to the taxonomy of Saintpaulia and Streptocarpus. – In: Andrews S, Leslie AC, Alexander C (eds), Taxonomy of cultivated plants, Proc. 3rd Intern. Symp., Royal Botanical Gardens, Kew, pp. 253-264.
Möller M, Cronk QCB. 2001a. Phylogenetic studies in Streptocarpus (Gesneriaceae): reconstruction of biogeographic history and distribution patterns. – Syst. Geogr. Plants 71: 545-555.
Möller M, Cronk QCB. 2001b. Evolution of morphological novelty: a phylogenetic analysis of growth patterns in Streptocarpus (Gesneriaceae). – Evolution 55: 918-929.
Möller M, Kiehn M. 2004. A synopsis of cytological studies in Gesneriaceae. – Edinburgh J. Bot. 60: 425-457.
Möller M, Clokie M, Cubas P, Cronk QCB. 1999. Integrating molecular phylogenies and developmental genetics: a Gesneriaceae case study. – In: Hollingsworth PM, Bateman RM, Gornall RJ (eds), Molecular systematics and plant evolution, Taylor & Francis, London, pp. 375-402.
Möller M, Jong K, Kokubugata G. 2008. nrDNA inheritance in the African genus Streptocarpus and the phylogenetic implications. – South Afr. J. Bot. 74: 372.
Möller M, Pfosser M, Jang C-G, Mayer V, Clark A, Hollingsworth ML, Barfuss MHJ, Wang Y-Z, Kiehn M, Weber A. 2009. A preliminary phylogeny of the ‘didymocarpoid Gesneriaceae’ based on three molecular data sets: incongruence with available tribal classifications. – Amer. J. Bot. 967: 989-1010.
Möller M, Middleton D, Nishii K, Wei Y-G, Sontag S, Weber A. 2011. A new delineation for Oreocharis incorporating an additional ten genera of Chinese Gesneriaceae. – Phytotaxa 23: 1-36.
Möller M, Forrest A, Wei Y-G, Weber A. 2011. A molecular phylogenetic assessment of the advanced Asiatic and Malesian didymocarpoid Gesneriaceae with focus on non-monophyletic and monotypic genera. – Plant Syst. Evol. 292: 223-248.
Möller M, Chen WH, Shui YM, Atkins H, Middleton DJ. 2014. A new genus of Gesneriaceae in China and the transfer of Briggsia species to other genera. – Gard. Bull. (Singapore) 66: 195-205.
Möller M, Nampy Santhosh, Janeesha AP, Weber A. 2017. The Gesneriaceae of India: consequences of updated generic concepts and new family classification. – Rheedea 27: 23-41.
Monachino JV. 1949. A note on Schlegelia and Dermatocalyx. – Phytologia 3: 102-105.
Moncontié C. 1969. Les stomates des Plantaginacées. – Rev. Gen. Bot. 76: 491-529.
Monod T. 1986. Nectaires extra-floraux et fleurs avortées chez les Pédaliacées (Note préliminaire). – Bull. Mus. Natl. Hist. Nat. Paris, sér. IV, B. Adansonia 2: 103-115.
Montserrat-Recoder P. 1968. Orofitismo y endemismo en el género Veronica. – Publ. Centro Piren. Biol. Exp. 2: 39-89.
Moody A, Diggle PK, Steingraeber DA. 1999. Developmental analysis of the evolutionary origin of vegetative propagules in Mimulus gemmiparus (Scrophulariacee). – Amer. J. Bot. 86: 1512-1522.
Moon H-K. 2012. A new synonym of Lepechinia (Salviinae: Lamiaceae). – Phytotaxa 71: 52.
Moon H-K, Vinckier S, Walker JB, Smets EF, Huysmans S. 2008. A search for phylogenetically informative pollen characters in the subtribe Salviinae (Mentheae: Lamiaceae). – Intern. J. Plant Sci. 169: 455-471.
Moon H-K, Vinckier S, Smets E, Huysmans S. 2008. Palynological evolutionary trends within the tribe Mentheae with special emphasis on subtribe Menthinae (Nepetoideae: Lamiaceae). – Plant Syst. Evol. 275: 93-108.
Moon H-K, Hong S-P, Smets E, Huysmans S. 2009. Micromorphology and character evolution of nutlets in tribe Mentheae (Nepetoideae, Lamiaceae). – Syst. Bot. 34: 760-776.
Moon H-K, Hong S-P, Smets E, Huysmans S. 2009. Phylogenetic significance of leaf micromorphology and anatomy in the tribe Mentheae (Nepetoideae: Lamiaceae). – Bot. J. Linn. Soc. 160: 211-231.
Moon H-K, Smets E, Huysmans S. 2010. Phylogeny of tribe Mentheae (Lamiaceae): the story of molecules and micromorphological characters. – Taxon 59: 1065-1076.
Moore HE. 1957. African violets and their relatives. – Macmillan, New York.
Moore HE. 1973a. A synopsis of the genus Codonanthe (Gesneriaceae). – Baileya 19: 4-33.
Moore HE. 1973b. Comments on cultivated Gesneriaceae. – Baileya 19: 35-41.
Moore RJ. 1947. Cytotaxonomic studies in the Loganiaceae I. Chromosome numbers and phylogeny in the Loganiaceae. – Amer. J. Bot. 34: 527-538.
Moore RJ. 1948. Cytological studies in the Loganiaceae II. Embryology of Polypremum procumbens. – Amer. J. Bot. 35: 404-410.
Moore SLM. 1894. New Acanthaceae from Tropical Africa. – J. Bot. 32: 129-139.
Moore SLM. 1906. Alabastra diversa XIII. Acanthaceae. – J. Bot. 44: 150-151.
Moore SLM. 1907. New or rare Acanthaceae from German south-west Africa. – J. Bot. 45: 226.
Moore SLM. 1929. Some new or rare African Acanthaceae. – J. Bot. 67: 225-231.
Moore TE, Verboom GA, Cramer MD. 2008. The adaptive significance of leaf size and shape variation in Jamesbrittenia (Scrophulariaceae: Manuleeae). – South Afr. J. Bot. 74: 373.
Mora MM, Clark JL. 2016. Molecular phylogeny of the Neotropical genus Paradrymonia (Gesneriaceae), reexamination of generic concepts and the resurrection of Trichodrymonia and Centrosolenia. – Syst. Bot. 41: 82-104.
Morales R. 1987. El género Thymbra L. (Labiatae) – An. Jard. Bot. Madrid 44: 349-380.
Morales R. 1991. El género Micromeria Bentham (Labiatae) en la Península Ibérica e Islas Baleares. – An. Jard. Bot. Madrid 48: 131-156.
Mora-Olivo A, Daniel TF, Martínez M. 2008. Hygrophila polysperma (Acanthaceae), una maleza acuática registrada por primera vez para la flora Mexicana. – Rev. Mex. Biodiv. 79: 265-269.
Morawetz JJ. 2007. Systematics of Alectra (Orobanchaceae) and phylogenetic relationships among the tropical clade of Orobanchaceae. – Ph.D. diss., The Ohio State University, Columbus, Ohio.
Morawetz JJ, Wolfe AD. 2009. Assessing the monophyly of Alectra and its relationship to Melasma (Orobanchaceae). – Syst. Bot. 34: 561-569.
Morawetz JJ, Randle CP, Wolfe AD. 2010. Phylogenetic relationships within the tropical clade of Orobanchaceae. – Taxon 59: 416-426.
Morgan MT, Dengler NG. 1988. Vascular architecture in isophyllous and facultatively anisophyllous species of Pentadenia (Gesneriaceae). – Amer. J. Bot. 75: 1485-1494.
Morley BD. 1972. Some karyotype diversity in Columnea L. sensu lato (Gesneriaceae). – Bot. J. Linn. Soc. 65: 25-36.
Morley BD. 1974. Notes on some critical characters in Columnea classification. – Ann. Missouri Bot. Gard. 61: 514-525.
Morley BD. 1976. A key, typification and synonymy of the sections in the genus Columnea L. (Gesneriaceae). – Contr. Natl. Bot. Gard. Glasnevin 1: 1-11.
Moro FV, Pintno ACR, Dos Santos JM, Damiao Filho CF. 2001. A scanning electron microscopy study of the seed and post-seminal development in Angelonia salicariifolia Bonpl. (Scrophulariaceae).. – Ann. Bot. 88: 499-506.
Morton CV. 1935. The genus Cremosperma. – J. Washington Acad. Sci. 25: 283-291.
Morton CV. 1938. Notes on Cremosperma. – J. Washington Acad. Sci. 28: 348-349.
Morton CV. 1944. Studies of tropical American plants: a revision of Cremosperma. – Contr. U.S. Natl. Herb. 29: 31-35.
Morton CV. 1963. A revision of Trichantha (Gesneriaceae). – Contr. U.S. Natl. Herb. 38: 1-27.
Morton JK. 1939. A revision of Besleria. – Contr. U.S. Natl. Herb. 26: 395-474.
Morton JK. 1956. The chromosome numbers of British Mentae. – Watsonia 3: 244-252.
Morton JK. 1962. Cytotaxonomic studies in the West African Labiatae. – Bot. J. Linn. Soc. 58: 231-283.
Morton JK. 1973. A cytological study of British Labiatae. – Watsonia 9: 239-246.
Morton JK. 1978. A revision of the Justicia insularis-striata complex (Acanthaceae). – Kew Bull. 32: 433-448.
Morton JK. 1998. Two new species of Plectranthus from Northeast Africa. – Kew Bull. 53: 997-999.
Morucchio GB. 1970. Adumbratio florae aethiopicae 20. Globulariaceae. – Webbia 24: 619-635.
Mosyakin SL, Tsymbalyuk ZM. 2015a. Pollen morphology of the southern African tribe Teedieae, an early-branching lineage of crown Scrophulariaceae. – Willdenowia 45: 65-75.
Mosyakin SL, Tsymbalyuk ZM. 2015b. Pollen morphology of tribes Aptosimeae and Myoporeae supports the phylogenetic pattern in early-branching Scrophulariaceae revealed by molecular studies. – Willdenowia 45: 209-222.
Mosyakin SL, Tsymbalyuk ZM. 2017. Pollen morphology of the tribe Hemimerideae: possible evidence of ancestral pollen types and parallel evolution in the basalmost clade of Scrophulariaceae s.str. – Willdenowia 47: 15-27.
Mourad MM, Abdel-Hameed UK, Mariam IH, Tantawy ME. 2015. Diversity and evolutionary trends in the floral characters of some taxa of Scrophulariaceae sensu lato. – Adansonia 37: 149-159.
Mower JP, Touzet P, Gummow JS, Delph LF, Palmer JD. 2007. Extensive variation in synonymous substitution rates in mitochondrial genes of seed plants. – BMC Evol. Biol. 7: 135.
Mower JP, Sefanoviç S, Hao W, Gummow JS, Jain K, Ahmed D, Palmer JD. 2010. Horizontal acquisition of multiple mitochondrial genes from a parasitic plant followed by gene conversion with host mitochondrial genes. – BMC Biol. 8: 150.
Moya E, Brea M. 2018. First Pleistocene record of fossil wood of Bignoniaceae in the Americas and a comparison with the extant Tabebuia alliance and Tecomeae. – Bot. J. Linn. Soc. 187: 303-318.
Moylan EC. 1999. Hemigraphis (Nees) (Acanthaceae) from the Philippines: an example of species delimitation. – Oxford Plant Syst. 7: 9-11.
Moylan EC, Scotland RW. 2000. Hemigraphis neocaledonica Heine from New Caledonia is transferred to Brunoniella Bremek. – Kew Bull. 55: 477-481.
Moylan EC, Pennington RT, Scotland RW. 2002. Taxonomic account of Hemigraphis Nees (Strobilanthinae-Acanthaceae) from the Philippines. – Kew Bull. 57: 769-825.
Moylan EC, Bennett JR, Carine MC, Olmstead RG, Scotland RW. 2004. Phylogenetic relationships among Strobilanthes s.l. (Acanthaceae): evidence from ITS nrDNA, trnL-F cpDNA, and morphology. – Amer. J. Bot. 91: 724-735.
Moylan EC, Rudall PJ, Scotland RW. 2004. Comparative floral anatomy of Strobilanthinae (Acanthaceae), with particular reference to internal partitioning of the flower. – Plant Syst. Evol. 249: 77-98.
Mukerjee SK. 1940. A revision of the Labiatae of the Indian Empire. – Rec. Bot. Surv. India 14: 1-228.
Mukherjee J. 1972. Pollen morphological affinity of Teucridium (Verbenaceae) and Teucrium (Labiatae). – Sci. & Cult. 38: 143-144.
Mukherjee J. 1974. Role of palynology in the taxonomy and phylogeny of Myoporaceae. – Sci. Cult. 40: 331-332.
Mukherjee J. 1976. The use of pollen morphology in the taxonomy of the Chloanthoideae Briq. (Verbenaceae). – Trans. Bose Res. Inst. Calcutta 39: 37-46.
Mukherjee KS, Ghosh PK, Baduddoza S. 1983. Diterpenoid quinines of Salvia lanata. – Phytochemistry 22: 1296.
Mulert U von. 2001. Phylogenie der Verbenaceae: kladistische Untersuchungen mit morphologischen und chemischen Merkmalen. – Ph.D. diss., Die Albert-Ludwigs-Universität Freiburg im Breisgau, Germany.
Múlgura de Romera ME. 2000. Las especies de Lippia L. sect. Dioicolippia Tronc. (Verbenaceae). – Candollea 55: 227-254.
Múlgura de Romera ME. 2003. The taxonomic position of Parodianthus (Verbenaceae): a morphological survey of the gynoecium and the inflorescence. – Kew Bull. 58: 929-938.
Múlgura de Romera ME, Martínez S, Suyama A. 1998. Morfología de las inflorescencias en Lippia (Verbenaceae). – Darwiniana 36: 1-12.
Múlgura de Romera ME, Martínez S, Atkins S, Rotman AD. 2002. Morfología de las inflorescencias en Verbenaceae-Verbenoideae II: tribu Lantaneae p.p. – Darwiniana 40: 1-15.
Muller J, Schuller M, Straka H, Friedrich B. 1989. Palynologia Madagassica et Mascarenica. Fam. 182: Acanthaceae. – Trop. Subtrop. Pflanzenwelt 67: 138-187.
Müller K, Albach DC. 2010. Evolutionary rates in Veronica L. (Plantaginaceae): disentangling the influence of life history and breeding system. – J. Mol. Evol. 70: 44-56.
Müller K, Borsch T. 2005. Phylogenetics of Utricularia (Lentibulariaceae) and molecular evolution of the trnK intron in alineage with high substitution rates. – Plant Syst. Evol. 250: 39-67.
Müller K, Borsch T, Legrende L, Porembski S, Theisen I, Barthlott W. 2004. Evolution of carnivory in Lentibulariaceae and the Lamiales. – Plant Biol. 6: 477-490.
Müller K, Borsch T, Legrende L, Porembski S, Barthlott W. 2006. Recent progress in understanding the evolution of carnivorous Lentibulariaceae (Lamiales). – Plant Biol. 8: 748-757.
Mulligan GA, Munro DM. 1989. Taxonomy of species of North American Stachys (Labiatae) found North of Mexico. – Le Natur. Can. 11: 35-51.
Munday J. 1980. The genus Monechma Hochst. (Acanthaceae tribe Justicieae) in southern Africa. – M.Sc. thesis, University of the Witwatersrand, Johannesburg, South Africa.
Munir AA. 1976. A taxonomic revision of the genus Spartothamnella (Chloanthaceae). – J. Adelaide Bot. Gard. 1: 3-25.
Munir AA. 1977a. A taxonomic revision of the genus Chloanthes (Chloanthaceae). – J. Adelaide Bot. Gard. 1: 83-106.
Munir AA. 1977b. A taxonomic revision of the genus Cyanostegia (Chloanthaceae). – Brunonia 1: 45-67.
Munir AA. 1978a. Taxonomic revision of Chloanthaceae trib. Physopsideae. – Brunonia 1: 407-692.
Munir AA. 1978b. A taxonomic revision of the genus Hemiphora (Chloanthaceea). – J. Adelaide Bot. Gard. 1: 161-166.
Munir AA. 1979. A taxonomic revision of the Pityrodia (Chloanthaceae). – J. Adelaide Bot. Gard. 2: 1-138.
Munir AA. 1985. A taxonomic revision of the genus Viticipremna H. J. Lam (Verbenaceae). – J. Adelaide Bot. Gard. 7: 181-200.
Munir AA. 1987. A taxonomic revision of the genus Faradaya F. Muell. (Verbenaceae) in Australia. – J. Adelaide Bot. Gard. 10: 165-177.
Munir AA. 1990. A taxonomic revision of the genus Glossocarya Wallich ex Griffith (Verbenaceae) in Australia. – J. Adelaide Bot. Gard. 13: 17-34.
Munir AA. 1991. A taxonomic revision of the genus Oncinocalyx F. Muell. (Verbenaceae). – J. Adelaide Bot. Gard. 14: 77-84.
Munir AA. 1992. A taxonomic revision of the genus Stachytarpheta Vahl (Verbenaceae) in Australia. – J. Adelaide Bot. Gard. 14: 133-168.
Muñoz-Centeno LM, Albach DC, Sánchez-Agudo JA, Martínez-Ortega MM. 2006. Systematic significance of seed morphology in Veronica (Plantaginaceae): a phylogenetic perspective. – Ann. Bot. 98: 335-350.
Muñoz-Centeno LM, Delgado-Sanchez L, Santos-Vicente M, Martinez-Ortega MM. 2007. Taxonomy of Veronica L. subsect. Veronica (Plantaginaceae) in the western Mediterranean. – Bot. J. Linn. Soc. 155: 65-81.
Müntzing A. 1930. Outlines to a genetic monograph of the genus Galeopsis. – Hereditas 13: 185-341.
Munz PA. 1926. The Antirrhinoideae-Antirrhineae of the New World. – Proc. Calif. Acad. Sci., Ser. IV, 15: 323-397.
Munz PA. 1930. The North American species of Orobanche, section Myzorrhiza. – Bull. Torrey Bot. Club 57: 611-624.
Murbeck SV. 1921. Sur quelques espèces nouvelles ou critiques des genres Celsia et Onopordon. – Acta Univ. Lund., ser. II, 17: 1-18.
Murbeck SV. 1926. Monographie der Gattung Celsia. – Acta Univ. Lund., ser. II, 22: 1-239.
Murbeck SV. 1933. Monographie der Gattung Verbascum. – Acta Univ. Lund., ser. II, 29(2): 1-630.
Murthi SN. 1940. Studies in the Labiatae I. Embryology of Ocimum sanctum, O. canum and O. basilicum. – Half-Yearly J. Mysore Univ. 1(10).
Murthi SN. 1946. Studies of the Labiatae V. Contributions to the morphology of Anisomeles indica R. Br. and Anisomeles malabarica O. Kze. – J. Univ. Bombay 15: 1-14.
Murthi SN. 1947. Studies in the Labiatae IV. Contribution to the morphology of Orthosiphon stamineus Benth. – J. Indian Bot. Soc. 26: 87-94.
Murthy GSR, Aleykutty KM, Rao VS, Inamdar JA. 1978. Vessels of Oleaceae and Verbenaceae. – Feddes Repert. 89: 359-368.
Musselman LJ. 1980. The biology of Striga, Orobanche, and other root-parasitic weeds. – Ann. Rev. Phytopathol. 18: 463-489.
Musselman LJ, Dickison WC. 1975. The structure and development of the haustorium in parasitic Scrophulariaceae. – Bot. J. Linn. Soc. 70: 183-212.
Musselman LJ, Hepper FN. 1986. The witchweeds (Striga, Scrophulariaceae) of the Sudan Republic. – Kew Bull. 41: 205-221.
Musselman LJ, Hepper FN. 1988. Studies in the flora of Arabia XX: the genus Striga in Arabia. – Notes Roy. Bot. Gard. Edinb. 45: 43-50.
Musselman LJ, Mann WF. 1976. A survey of surface characteristics of seeds of Scrophulariaceae and Orobanchaceae using scanning electron microscopy. – Phytomorphology 26: 370-378.
Musselman LJ, Mann WF. 1977. Parasitism and haustorial structure of Schwalbea americana (Scrophulariaceae). – Beitr. Biol. Pflanzen 53: 309-315.
Musselman LJ, Parker C. 1982. Preliminary host ranges of some strains of economically important broomrapes (Orobanche). – Econ. Bot. 36: 270-273.
Musselman LJ, Press MC. 1995. Introduction to parasitic plants. – In: Press MC, Graves JD (eds), Parasitic plants, Chapman and Hall, London, pp. 1-13.
Naanaie SY, Tavassoli A. 2010. Taxonomy of the genus Nanorrhinum (Scrophulariaceae) in Iran. – Iran. J. Bot. 16: 114-124.
Nabli MA. 1971. Ultrastructure de l’endexine et de la tryphine chez quelques espèces du genre Teucrium L. – Compt. Rend. Acad. Sci. Paris 273: 2075-2078.
Nabli MA. 1976. Étude ultrastructurale comparée de l’exine chez quelques genres de Labiatae. – In: Ferguson IK, Muller J (eds), The evolutionary significance of the exine, Linnean Society Symposium Series 1, Academic Press, London, pp. 499-525.
Nafanailova II, Zakirova RO. 1991. Caryological research of some representative of Lamiaceae Lindl. – Izv. Akad. Nauk Kazahsk. SSR, Ser. Biol. 2: 71-72.
Nagao S. 1941. The number of chromosomes in some species and varieties of Mentha. – J. Sapporo Soc. Agric. For. 32: 28-36.
Naghiloo S, Dadpur MR, Gohari G, Endress PK. 2013. Comparative study of inflorescence development in Oleaceae. – Amer. J. Bot. 100: 647-663.
Naghiloo S, Khodaverdi M, Esmaillou Z,
Dadpour MR, Rudall PJ. 2014. Comparative floral development in the tribe
Mentheae (Nepetoideae: Lamiaceae)
and its bearing on the evolution of floral patterns in asterids. – J. Syst.
Evol. 52: 195-214.
Narayanan CR. 1951. Somatic chromosomes in the Acanthaceae. – J. Madras Univ. 21: 220-231.
Natarajan AT. 1957. Studies in the morphology of pollen grains – Tubiflorae. – Phyton (Buenos Aires) 8: 21-42.
Natarajan A, Ahuja M. 1957. Cytotaxonomical studies in the genus Lantana. – J. Indian Bot. Soc. 36: 35-45.
Navarro T. 1995. Revisión del género Teucrium L. sección Polium (Mill.) Schreb. (Lamiaceae) en la Peninsula Ibérica y Baleares. – Acta Bot. Malacitana 20: 173-265.
Navarro T, El Oualidi J. 1997. Synopsis of the genus Teucrium L. (Lamiaceae) in Morocco. – Acta Bot. Malac. 22: 187-203.
Navarro T, El Oualidi J. 1999. Flower and life strategy diversity in Teucrium L. (Lamiaceae). – Acta Bot. Malac. 24: 63-75.
Navarro T, El Oualidi J. 2000a. Trichome morphology in Teucrium L. (Labiatae). A taxonomic review. – An. Jard. Bot. Madrid 57: 277-297.
Navarro T, El Oualidi J. 2000b. Synopsis of Teucrium L. (Labiatae) in the Mediterranean region and surrounding areas. – Flora Medit. 10: 349-363.
Nayar NM. 1976. Sesame. – In: Simmonds NW (ed), Evolution of crop plants, Longman, London.
Neel MC, Cummings MP. 2004. Section-level relationships of North American Agalinis (Orobanchaceae) based on DNA sequence analysis of three chloroplast gene regions. – BioMed Central Evol. Biol. 4: 15.
Neissess KR. 1983. Evolutions, systematics and terpene relationships of Salvia section Audibertia. – Ph.D. diss., University of California, Riverside, California.
Neizgoda CJ, Tomb AS. 1975. Systematic palynology of the tribe Leucophylleae (Scrophulariaceae) and selected Myoporaceae. – Pollen Spores 17: 495-516.
Nelson AD, Elisens WJ. 1999. Polyploid evolution and biogeography in Chelone (Scrophulariaceae): morphological and isozyme evidence. – Amer. J. Bot. 86: 1487-1501.
Newberry PE. 1937. On some African species of the genus Olea and the original home of the cultivated olive-tree. – Proc. Linn. Soc. 150: 3-16.
Newsom VM. 1929. A revision of the genus Collinsia (Scrophulariaceae). – Bot. Gaz. 87: 260-301.
Ngadjui BT, Dongo E, Ayafor JF, Connolly JD. 1994. Thomandertriol, a triterpenoid from the twigs of Thomandersia laurifolia. – J. Nat. Products 57: 161-163.
Ngadjui BT, Tamboue H, Ayafor JF, Connolly JD. 1995. Thomandersine and isothomandersine, 2-indolinone alkaloids from Thomandersia laurifolia. – Phytochemistry 39: 1249-1251.
Nickrent DL, Duff RJ. 1996. Molecular studies of parasitic plants using ribosomal DNA. – In: Moreno MT, Cubero JI, Berner D, Joel D, Musselman LJ, Parker C (eds), Advances in parasitic plant research, Junta de Andalucia, Dirección General de Investigación Agraria, Cordoba, Spain, pp. 28-52.
Nickrent DL, Duff RJ, Colwell AE, Wolfe AD, Young ND, Steiner KE, dePamphilis CW. 1997. Molecular phylogenetic and evolutionary studies of parasitic plants. – In: Soltis DE, Soltis PS, Doyle JJ (eds), Molecular systematics of plants. 2nd ed., Kluwer Academic Publ., Boston, & Chapman and Hall, New York, pp. 211-241.
Nicoletti M, Sarafini N, Garbarino JA, Gambaro M. 1988. A chemosystematic study of Scrophulariaceae: iridoid glycosides. – Giorn. Bot. Italiano 122: 13-24.
Nicoletti M, Galeffi C, Messana I, Marini-Bettolo GB, Garbarino JA, Gambaro V. 1988. Phenylpropanoid glycosides from Calceolaria hypericina. – Phytochemistry 27: 639-641.
Nicolson DH. 1975. Diphelypaea (Orobanchaceae), nom. nov. and other cauterizations on a nomenclatural hydra. – Taxon 24: 651-657.
Nie Z-L, Sun H, Beardsley PM, Olmstead RG, Wen J. 2006. Evolution of biogeographic disjunction between eastern Asia and eastern North America in Phryma (Phrymaceae). – Amer. J. Bot. 93: 1343-1356.
Niezgoda CJ, Tomb AS. 1975. Systematic palynology of tribe Leucophylleae (Scrophulariaceae) and selected Myoporaceae. – Pollen Spores 17: 495-516.
Niketić M, Tomović G. 2008. Taxonomy and nomenclature of the Linaria genistifolia complex (Plantaginaceae-Antirrhineae) in S.E. Europe and Anatolia. – Taxon 57: 619-629.
Nilson S. 2000. Fragrance glands (osmophores) in the family Oleaceae. – In: Nordenstam B, El-Ghazaly G, Kassas M (eds), Plant systematics for the 21st Century, Portland Press, London, pp. 305-320.
Nilsson S. 1988. A survey of the pollen morphology of Olea with particular reference to O. europaea sensu lat. – Kew Bull. 43: 309-315.
Nilsson S, Hong D-Y. 1993. The taxonomic significance of Aragoa-pollen (Scrophulariaceae). – Opera Bot. 121: 275-278.
Nishii K, Hughes M, Briggs M, Haston E, Christie F, DeVilliers MJ, Hanekom T, Roos WG, Bellstedt DU, Möller M. 2015. Streptocarpus redefined to include all Afro-Malagasy Gesneriaceae: molecular phylogenies prove congruent with geographical distribution and basic chromosome numbers and uncover remarkable morphological homoplasies. – Taxon 64: 1243-1274.
Noel ARA, Staden J van. 1975. Phyllomorph senescence in Streptocarpus molweniensis. – Ann. Bot., N. S., 39: 921-929.
Noll F. 1883. Entwicklungsgeschichte der Veronica-Blüthe. – Diesterweg, Frankfurt.
Nonaka G, Nishioka I. 1977. Bitter phenylpropanoid glycosides from Conandron ramondioides. – Phytochemistry 16: 1265-1267.
Norman EM. 1982. 176. Buddlejaceae. – In: Harling G, Sparre B (eds), Flora of Ecuador 16, Swedish Natural Science Research Council, Stockholm, pp. 1-23.
Norman EM. 1994. A re-examination of Sanango racemosum 1. Taxonomy and distribution. – Taxon 43: 591-600.
Norman EM. 2000. Flora Neotropica. Monograph 81. Buddlejaceae. – New York Botanical Garden, Bronx, New York, pp. 1-225.
Novitskaya GV, Krishtopa VI. 1971. Composition of the fatty acids of Labiatae oils in connection with their taxonomic position. – Rast. Resur. 7: 32-40. [In Russian]
Nylinder S, Swenson U, Persson C, Janssens SB, Oxelman B. 2012. A dated species-tree approach to the trans-Pacific disjunction of the genus Jovellana (Calceolariaceae, Lamiales). – Taxon 61: 381-391.
Obermayer R, Greilhuber J. 2005 [2006]. Cryptopolyploidy revisited: the case of Vinca (Apocynaceae). – Plant Syst. Evol. 256: 201-208.
Obermeijer AA. 1936. The South African species of Petalidium. – Ann. Transvaal Mus. 18: 151-162.
Oehlkers F. 1923. Entwicklungsgeschichte von Monophyllaea Horsfieldii. – Beih. Bot. Centralbl., Abt. A, 39: 128-151.
Oehlkers F. 1962. Entwicklungsgeschichtliche Untersuchungen II. Die Blütenentwicklung von Streptocarpus wendlandii. – Zeitschr. Bot. 50: 217-236.
Oehm G. 1932. Beitrag zur Kenntnis der Blattanatomie und Behahrung von Plantago media L., Pl. major L. und Pl. lanceolata L. – Beih. Bot. Centralbl. 50: 20-43.
Ogutcen E, Vamosi JC. 2016. A phylogenetic study of the tribe Antirrhineae: genome duplications and long-distance dispersals from the Old World to the New World. – Amer. J. Bot. 103: 1071-1081.
O’Leary N, Múlgura de Romera ME. 2010. A taxonomic revision of Casselia (Verbenaceae), a genus endemic to the South American Cerrado and Mata Atlántica biogeographic provinces. – J. Torrey Bot. Soc. 137: 166-179.
O’Leary N, Múlgura de Romera ME. 2012. A taxonomic revision of the genus Phyla (Verbenaceae). – Ann. Missouri Bot. Gard. 98: 578-596.
O’Leary N, Peralta P. 2007. Nuevas combinaciones en el género Glandularia (Verbenaceae). – Darwiniana 45: 218-232.
O’Leary N, Thode V. 2016. The genus Glandularia (Verbenaceae) in Brazil. – Ann. Missouri Bot. Gard. 101: 699-749.
O’Leary N, Múlgura de Romera ME, Morrone O. 2007a. Revisión taxonómica de las especies del género Verbena (Verbenaceae): serie Pachystachyae. – Ann. Missouri Bot. Gard. 94: 571-621.
O’Leary N, Múlgura de Romera ME, Morrone O. 2007b. New combinations in South American Glandularia (Verbenaceae). – Novon 17: 503-511.
O’Leary N, Peralta P, Múlgura de Romera ME. 2008. A taxonomic revision of the genus Tamonea (Verbenaceae). – Bot. J. Linn. Soc. 157: 357-371.
O’Leary N, Yuan Y-W, Chemisquy A, Olmstead RG. 2009. Reassignment of species of paraphyletic Junellia s.l. to the new genus Mulguraea (Verbenaceae). – Syst. Bot. 34: 777-786.
O’Leary N, Múlgura de Romero ME, Morrone O. 2010. Revisión taxonómica de las especies del género Verbena L. (Verbenaceae) II: serie Verbena. – Ann. Missouri Bot. Gard. 97: 369-428.
O’Leary N, Calviño CI, Martínez S, Lu-Irving P, Olmstead RG, Múlgura de Romero ME. 2012. Evolution of morphological traits in Verbenaceae. – Amer. J. Bot. 99: 1778-1792.
O’Leary N, Lu-Irving P, Moroni P, Siedo S. 2016. Taxonomic revision of Aloysia (Verbenaceae, Lantaneae) in South America. – Ann. Missouri Bot. Gard. 101: 568-609.
Olesen JM, Forfang A-S, Báez M. 1998. Stress-induced male sterility and mixed mating in the island plant Cedronella canariensis (Lamiaceae). – Plant Syst. Evol. 212: 159-176.
Oliveira LO, Huck RB, Gitzendanner MA, Judd WS, Soltis DE, Soltis PS. 2007. Molecular phylogeny, biogeography, and systematics of Dicerandra (Lamiaceae), a genus endemic to the southeastern United States. – Amer. J. Bot. 94: 1017-1027.
Oliver D. 1887. Trapella sinensis, Oliv. – Hooker’s Icon. Plant. 16: t. 1595.
Oliver D. 1888. On the structure, development, and affinities of Trapella Oliv., a new genus of Pedalineae. – Ann. Bot. 2: 75-115.
Oliver D. 1892. Tetrachondra hamiltonii. – Hooker’s Icon. Plant. Vol. III, Part II, t. 2250.
Oliver D. 1895. Diagnoses africanae VII. – Kew Bull. 105: 211-230.
Oliver FW. 1888. On the structure, development and affinities of Trapella Oliv., a new genus of Pedalineae. – Ann. Bot. 2: 75-118.
Oliver WRB. 1944. The Veronica-like species of New Zealand. – Rec. Domin. Mus. 1: 228-231.
Olmstead RG. 1989. Phylogeny, phenotypic evolution, and biogeography of the Scutellaria angustifolia complex (Lamiaceae): inference from morphological and molecular data. – Syst. Bot. 14: 320-338.
Olmstead RG. 2002. Whatever happened to the Scrophulariaceae? – Fremontia 30: 13-22.
Olmstead RG. 2013. Phylogeny and biogeography in Solanaceae, Verbenaceae and Bignoniaceae: a comparison of continental and intercontinental diversification patterns. – Bot. J. Linn. Soc. 171: 80-102.
Olmstead RG, Reeves PA. 1995. Evidence for the polyphyly of the Scrophulariaceae based on chloroplast rbcL and ndhF sequences. – Ann. Missouri Bot. Gard. 82: 176-193.
Olmstead RG, Scott KM, Palmer JD. 1992. A chloroplast DNA phylogeny for the Asteridae: implications for the Lamiales. – In: Harley RM, Reynolds T (eds), Advances in labiate science, Royal Botanic Gardens, Kew, pp. 19-26.
Olmstead RG, Reeves PA, Lepschi BJ. 1998. Confirmation of a monophyletic Chloanthoideae (Lamiaceae) comprising tribes Chloantheae and Prostanthereae. – Lamiales Newslett. 6: 7-10.
Olmstead RG, dePamphilis CW, Wolfe AD, Young ND, Elisons WJ, Reeves PA. 2001. Disintegration of the Scrophulariaceae. – Amer. J. Bot. 88: 348-361.
Olmstead RG, Zjhra ML, Lohmann LG, Grose SO, Eckert AJ. 2009. A molecular phylogeny and classification of Bignoniaceae. – Amer. J. Bot. 96: 1731-1743.
Olsen RT, Kirkbride JH Jr. 2017. Taxonomic revision of the genus Catalpa (Bignoniaceae). – Brittonia 69: 387-421.
Olsen S, Olsen ID. 1980. The seed of Boschniakia hookeri (Orobanchaceae). – Bot. Tidsskr. 75: 161-174.
Olsen S, Olsen ID. 1981. Germination and development of the soma in Boschniakia hookeri (Orobanchaceae). – Nord. J. Bot. 1: 246-259.
Ortega A, Blount JF, Manchand PS. 1982. Salvinorin, a new trans-clerodane diterpene from Salvia divinorum (Labiatae). – J. Chem. Soc. Perkin Trans. 1: 2502.
Osborn JM, Philbrick CT. 1994. Comparative pollen structure and pollination biology in the Callitrichaceae. – Acta Bot. Gallica 141: 257-266.
Osborn JM, El-Ghazaly G, Cooper RL. 2001. Development of the exineless pollen wall in Callitriche truncata (Callitrichaceae) and the evolution of underwater pollination. – Plant Syst. Evol. 228: 81-87.
Oshi H, Inouye H. 1982. Iridoid glycosides of Rehmannia glutinosa. – Phytochemistry 21: 133-138.
Otieno DF, Balkwill K, Paton AJ, Savolainen V. 2006. A reassessment of Hemizygia and Syncolostemon (Ocimeae-Lamiaceae). – Taxon 55: 941-958.
Outer RW den, Veenendaal WLH van. 1983. Wood anatomy of Uncarina leandrii H. Humb. (Pedaliaceae) and its relation to Bignoniaceae. – IAWA Bull. 4: 53-59.
Owens SJ, Ubera-Jiménez JL. 1992. Breeding systems in Labiatae. – In: Harley RM, Reynolds T (eds), Advances in labiate science, Royal Botanic Gardens, Kew, pp. 257-280.
Oxelman B, Backlund M, Bremer B. 1999. Relationships of the Buddlejaceae s.l. investigated using parsimony jackknife and branch support analysis of chloroplast ndhF and rbcL sequence data. – Syst. Bot. 24: 164-182.
Oxelman B, Kornhall P, Norman EM. 2004. Buddlejaceae. – In: Kubitzki K, Kadereit JW (eds), The families and genera of vascular plants VII. Flowering plants. Dicotyledons. Lamiales (except Acanthaceae including Avicenniaceae), Springer, Berlin, Heidelberg, New York, pp. 39-44.
Oxelman B, Kornhall P, Olmstead RG, Bremer B. 2005. Further disintegration of Scrophulariaceae. – Taxon 54: 411-425.
Oyama RK, Baum DA. 2004. Phylogenetic relationships of North American Antirrhinum (Veronicaceae). – Amer. J. Bot. 91: 918-925.
Ozaki Y, Johne S, Hesse M. 1979. Natural organic substances 174. Iridoid glucosides from Leucocarpus perfoliatus G. Don. – Helv. Chim. Acta 672: 2708-2711.
Özcan T, Dirmenci T, Martin E, Altınordu F. 2015. Cytotaxonomical study in five taxa of the genus Teucrium L. (Lamiaceae). – Caryologia 68: 1-8.
Özhatay N. 1986. Two new Allium species from Turkey. – Notes Roy. Bot. Gard. Edinb. 44: 147-150.
Özhatay N, Mathew B. 1995. New taxa and notes on the genus Allium (Alliaceae) in Turkey and Arabia. – Kew Bull. 50: 723-731.
Pace MR, Angyalossy V. 2013. Wood anatomy and evolution: a case study in the Bignoniaceae. – Intern. J. Plant Sci. 174: 1014-1048.
Pace MR, Lohmann LG, Angyalossy V. 2009. The rise and evolution of the cambial variant in Bignonieae (Bignoniaceae). – Evol. Devel. 11: 465-479.
Pace MR, Lohmann LG, Angyalossy V. 2011. Evolution of disparity between the regular and variant phloem in Bignonieae (Bignoniaceae). – Amer. J. Bot. 98: 602-618.
Pace MR, Angyalossy V. 2013. Wood anatomy and evolution: a case study in the Bignoniaceae. – Intern. J. Plant Sci. 174: 1014-1048.
Pace MR, Alcantara S, Lohmann LG, Angyalossy V. 2015. Secondary phloem diversity and evolution in Bignonieae (Bignoniaceae). – Ann. Bot. 116: 333-358.
Pace MR, Lohmann LG, Olmstead RG, Angyalossy V. 2015. Wood anatomy of major Bignoniaceae clades. – Plant Syst. Evol. 301: 967-995.
Pace MR, Zuntini AR, Lohmann LG, Angyalossy V. 2016. Phylogenetic relationships of enigmatic Sphingiphila (Bignoniaceae) based on molecular and wood anatomical data. – Taxon 65: 1050-1063.
Paclt J. 1952. Synopsis of the genus Catalpa (Bignoniaceae) III. – Candollea 13: 241-285.
Padmanabhan D. 1960. The embryology of Avicennia officinalis I. Floral morphology and gametophytes. – Proc. Indian Acad. Sci., Sect. B, 52: 131-145.
Padmanabhan D. 1961. A contribution to the embryology of Epithema carnosum. – J. Madras Univ., Sect. B, 31: 37-46.
Padmanabhan D. 1966. The octant embryo of Epithema carnosum Benth. – Proc. Indian Acad. Sci., Sect. B, 64: 6.
Padmanabhan D. 1970. Comparative embryology of angiosperms: Verbenaceae. – Bull. Natl. Sci. Acad. India 41: 250-254.
Pal N. 1951. Studies in the embryology of some Verbenaceae. – J. Indian Bot. Soc. 30: 59-74.
Paliwal GS. 1966. Structure and ontogeny of stomata in some Acanthaceae. – Phytomorphology 16: 527-532.
Palomino G, Mercado P, Ramamoorthy TP. 1986. Chromosomes of Salvia subgenus Calosphace (Lamiaceae), a preliminary report. – Cytologia 51: 381-386.
dePamphilis CW, Palmer JD. 1990. Loss of photosynthetic and chlororespiratory genes from the plastid genome of a parasitic flowering plant. – Nature 348: 337-339.
dePamphilis CW, Young ND, Wolfe AD. 1997. Evolution of plastid gene rps2 in a lineage of hemiparasitic and holoparasitic plants: many losses of photosynthesis and complex patterns of rate variation. – Proc. Natl. Acad. Sci. U.S.A. 94: 7362-7372.
Pan KY, Li Z-Y, Wang Y-Z. 2002. Floral organogenesis of Titanotrichum oldhamii (Gesneriaceae). – Acta Bot. Sin. 44: 895-902.
Pan Y-Z, Fang L-Q, Hao G, Cai J, Gong X. 2009. Systematic positions of Lamiophlomis and Paraphlomis (Lamiaceae) based on nuclear and chloroplast sequences. – J. Syst. Evol. 47: 535-542.
Panigrahi G. 1975. Taxonomic notes on certain taxa of Asiatic angiosperms. – Phytologia 32: 473-479.
Panigrahi G, Das GC. 1981. A revision of Haplanthodes O. Kuntze (Acanthaceae). – Bull. Bot. Surv. India 23: 197-203.
Papageourgiou VP, Kokkini S, Argyriadou N. 1982. Chemotaxonomy of the Greek species of Sideritis I. Components of the volatile fraction of Sideritis raeseri ssp. raeseri. – In: Margaris N, Koedam A, Vokou D (eds), Aromatic plants: basic and applied aspects, Martinus Nijhoff Publ., The Hague, pp. 211-220.
Papanikolaou K, Kokkini S. 1982. A taxonomic revision of Sideritis L. section Empedoclia (Rafin.) Bentham (Labiatae) in Greece. – In: Margaris N, Koedam A, Vokou D (eds), Aromatic plants: basic and applied aspects, Martinus Nijhoff Publ., The Hague, pp. 101-128.
Papanikolaou K, Kokkini S. 1984. A thin-layer chromatographic study of some chemical leaf constituents in Sideritis L. sect. Empedoclia (Rafin.) Bentham (Labiatae) in Greece. – Feddes Repert. 95: 359-368.
Paran I, Gidoni D, Jacobsohn R. 1997. Variation between and within broomrape (Orobanche) species revealed by RAPD markers. – Heredity 78: 68-74.
Parija P, Samal K. 1936. Extra-floral nectaries in Tecoma capensis Lindl. – J. Indian Bot. Soc. 15: 241-246.
Park JM, Manen JF, Schneeweiss GM. 2007. Horizontal gene transfer of a plastid gene in the non-photosynthetic flowering plants Orobanche and Phelipanche (Orobanchaceae). – Mol. Phylogen. Evol. 43: 974-985.
Park JM, Schneeweiss GM, Weiss-Schneeweiss H. 2007. Diversity and evolution of Ty1-copia and Ty3-gypsy retroelements in the non-photosynthetic flowering plants Orobanche and Phelipanche (Orobanchaceae). – Gene 387: 75-86.
Park JM, Manen JF, Colwell AE, Schneeweiss GM. 2008. A plastid gene phylogeny of the non-photosynthetic parasitic Orobanche (Orobanchaceae) and related genera. – J. Plant Res. 121: 365-376.
Parolly G, Eren Ö. 2008. Verbascum haraldi-adnani (Scrophulariaceae), a new chasmophytic species from SW Anatolia, Turkey. – Willdenowia 38: 127-134.
Parolly G, Tan K. 2007. Verbascum lindae (Scrophulariaceae), a new species from SW Anatolia, Turkey. – Willdenowia 37: 277-282.
Parvati A, Narayana LL. 1978. Chemotaxonomy of a few taxa of Pedaliaceae. – Curr. Sci. 47: 282-283.
Pastore JFB, Harley RM, Forest F, Paton A, Berg C van den. 2011. Phylogeny of the subtribe Hyptidinae (Lamiaceae tribe Ocimeae) as inferred from nuclear and plastid DNA. – Taxon 60: 1317-1329.
Patel RC, Inamdar JA. 1974. Studies in the trichomes and nectaries of some Gentianales. – In: Puri V et al. (eds), Biology of land plants, S. Prakashan, Meerut.
Paterman H. 1935. Beiträge zur Zytologie der Verbenaceen. – Berlin.
Paton AJ. 1990. A global taxonomic investigation of Scutellaria (Labiatae). – Kew Bull. 45: 399-450.
Paton AJ. 1992a. The adaptive significance of calyx and nutlet morphology in Scutellaria. – In: Harley RM, Reynolds T (eds), Advances in labiate science, Royal Botanic Gardens, Kew, pp. 203-210.
Paton AJ. 1992b. The genus Scutellaria L. (Labiatae) in tropical Africa. – Kew Bull. 47: 41-48.
Paton AJ. 1992c. A synopsis of Ocimum L. in Africa. – Kew Bull. 47: 405-435.
Paton AJ. 1993. Notes on Fuerstia (Labiatae) in tropical Africa. – Kew Bull. 48: 129-137.
Paton AJ. 1995. The genus Becium (Labiatae) in East Africa. – Kew Bull. 50: 199-242.
Paton AJ. 1997a. Classification and species of Platostoma and its relationship with Haumaniastrum (Labiatae). – Kew Bull. 52: 257-292.
Paton AJ. 1997b. A revision of Haumaniastrum (Labiatae). – Kew Bull. 52: 293-378.
Paton AJ. 1998. New records and new combinations in Hemizygia and Syncolostemon (Labiatae). – Kew Bull. 53: 483-485.
Paton AJ, Ryding O. 1998. Hanceola, Siphocranion and Isodon and their position in the Ocimeae (Labiatae). – Kew Bull. 53: 723-731.
Paton AJ, Harley MM, Harley RM, Weeks S. 1994. A revision of Endostemon (Labiatae). – Kew Bull. 49: 673-716.
Paton AJ, Springate D, Suddee S, Otieno D, Grayer RJ, Harley MM, Willis F, Simmonds MSJ, Powell MP, Savolainen V. 2004. Phylogeny and evolution of basils and allies (Ocimeae, Labiatae) based on three plastid DNA regions. – Mol. Phylogen. Evol. 31: 277-299.
Patudin A, Romanowa A, Sokolow WS, Pribylowa G. 1974. Das Vorkommen von Phenanthrenchinonen in Arten der Gattung Salvia. – Planta Medica 26: 201.
Patudin AV, Yurtsev VN, Pakaln DA. 1975. Chromosome number in some species of Salvia L. (Lamiaceae). – Bot. Žurn. 60: 529-534. [In Russian]
Patzak A. 1959. Revision der Gattung Ballota Section Acanthoprasium und Section Beringeria. – Ann. Nat. Mus. Wien 83: 3-81.
Pax F. 1896. Callitrichaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(5), W. Engelmann, Leipzig, pp. 120-123.
Pax F, Hoffmann K. 1931. Callitrichaceae. – In: Engler A (†), Harms H, Pax F (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 19c, W. Engelmann, Leipzig, pp. 236-240.
Pazy B. 1998. Diploidization failure and apomixis in Orobanchaceae. – Bot. J. Linn. Soc. 128: 99-103.
Pedersen JA. 2000. Distribution and taxonomic implications of some phenolics in the family Lamiaceae determined by ESR spectroscopy. – Biochem. Syst. Ecol. 28: 229-253.
Pedersen P, Gotfredsen CH, Wagstaff SJ, Jensen SR. 2007. Chemical markers in Veronica sect. Hebe II. – Biochem. Syst. Ecol. 35: 777-784.
Peev DR. 1975. Genus Veronica in Bulgaria – some new taxa and chromosome numbers. – Fitologija 2: 42-56.
Peirson JA, Cantino PD, Ballard HE. 2006. A taxonomic revision of Collinsonia (Lamiaceae) based on phenetic analyses of morphological variation. – Syst. Bot. 31: 398-409.
Peng C-I, Kenton A. 1982. Chromosome numbers of Byblis liniflora Salisb. (Byblidaceae). – Ann. Missouri Bot. Gard. 69: 417-419.
Pennell FW. 1919. Scrophulariaceae of the southeastern United States. – Proc. Acad. Nat. Sci. Philadelphia 71: 224-291.
Pennell FW. 1920. Scrophulariaceae of Colombia I. – Proc. Acad. Nat. Sci. Philadelphia 72: 136-187.
Pennell FW. 1921a. “Veronica” in North and South America. – Rhodora 23: 1-41.
Pennell FW. 1921b. Scrophulariaceae of the West Gulf States. – Proc. Acad. Nat. Sci. Philadelphia 73: 459-536.
Pennell FW. 1923. Scrophulariaceae of Cuba. – Proc. Acad. Nat. Sci. Philadelphia 75: 1-21.
Pennell FW. 1925. The genus Allophyton of southern Mexico and Guatemala. – Proc. Acad. Nat. Sci. Philadelphia 77: 269-272.
Pennell FW. 1933. A revision of Synthyris and Besseya. – Proc. Acad. Nat. Sci. Philadelphia 85: 77-106.
Pennell FW. 1934. Castilleja in Alaska and northwestern Canada. – Proc. Acad. Nat. Sci. Philadelphia 86: 517-540.
Pennell FW. 1935. The Scrophulariaceae of eastern temperate North America. – Proc. Acad. Nat. Sci. Philadelphia, Monogr. 1, Wickersham, Lancaster, Pennsylvania, pp. 1-650.
Pennell FW. 1943. The Scrophulariaceae of the western Himalayas. – Proc. Acad. Nat. Sci. Philadelphia, Monogr. 5, Wickersham, Lancaster, Pennsylvania, pp. 1-163.
Pennell FW. 1945. The genus Calceolaria in southeastern Peru. – Proc. Acad. Nat. Sci. Philadelphia 97: 137-177.
Pennell FW. 1947. Some hitherto undescribed Scrophulariaceae of the Pacific states. – Proc. Acad. Nat. Sci. Philadelphia 99: 155-199.
Pennell FW. 1951. The genus Calceolaria in Ecuador, Colombia and Venezuela. – Proc. Acad. Nat. Sci. Philadelphia 103: 85-196.
Peralta P, Múlgura de Romero ME, Denham SS, Botta SM. 2008. Revisión del género Junellia (Verbenaceae). – Ann. Missouri Bot. Gard. 95: 338-390.
Pereira CG, Almenara DP, Winter CE, Fritsch PW, Lambers H, Oliveira RS. 2012. Underground leaves of Philcoxia trap and digest nematodes. – Proc. Natl. Acad. Sci. U.S.A. 109: 1154-1159.
Pereira Jr EJ, Bittencourt Jr NS. 2016. Comparative ovule ontogeny in some members of the Tabebuia alliance (Bignoniaceae). – Intern. J. Plant Sci. 177: 481-497.
Pérez de Paz PL. 1978. Revisión del género Micromeria Bentham (Lamiaceae-Stachyoideae) en la región macaronésica. – Inst. Univ. Estud. Canar. La Laguna (Tenerife), Monogr., secc. IV, 16.
Pérez DP, Negrìn P, Sosa L. 1992. Revisión taxonomica de Sideritis L. Subgenéro Marrubiastrum (Moench) Mend.-Heuer (endemismo macaronésico). – J. Cramer, Berlin.
Peroutka M, Adlassnig W, Volgger M, Lendl T, Url WG, Lichtscheidl IK. 2008. Utricularia: a vegetarian carnivorous plant? Algae as prey of bladderwort in oligotrophic bogs. – Plant Ecol. 199: 153-163.
Perret M, Chautems A, Spichiger R, Peixoto M, Savolainen V. 2001. Nectar sugar concentration in relation to pollination syndromes in Sinningieae (Gesneriaceae). – Ann. Bot. 87: 267-273.
Perret M, Chautems A, Spichiger R, Kite G, Savolainen V. 2003. Systematics and evolution of tribe Sinningieae (Gesneriaceae): evidence from phylogenetic analyses of six plastid DNA regions and nuclear ncpGS. – Amer. J. Bot. 90: 445-460.
Perret M, Chautems A, Spichiger R. 2006. Dispersal-vicariance analysis in the tribe Sinningieae (Gesneriaceae): a clue to understanding biogeographical history of the Brazilian Atlantic forest. – Ann. Missouri Bot. Gard. 93: 340-358.
Perret M, Chautems A, Spichiger R, Barraclugh TG, Savolainen V. 2007. The geographical pattern of speciation and floral diversification in the neotropics: the tribe Sinningieae (Gesneriaceae) as a case study. – Evolution 61: 1641-1660.
Perret M, Chautems A, Araujo AO de, Salamin N. 2013. Temporal and spatial origin of Gesneriaceae in the New World inferred from plastid DNA sequences. – Bot. J. Linn. Soc. 171: 61-79.
Perrier de la Bâthie H. 1938. Les Bignoniacées de la région Malgache (Madagascar, Mascareignes, Seychelles et Comores). – Ann. Inst. Bot.-Geol. Col. Marseille 46: 1-101.
Perrier de la Bâthie H. 1949. Révision des Oléacées de Madagascar et des Comores. – Mém. Inst. Sci. Madagascar. sér. B, Biol. Vég. 2: 275-310.
Perrier de la Bâthie H. 1952. Sur le genre Androya gen. nov. (Oleaceae) de Madagascar. – Bull. Mus. Natl. Hist. Nat. Paris, sér. II, 24: 400-401.
Persidsky D. 1934. On the development of endosperm and haustoria in Linaria genistaefolia L. – Visn. Kÿyiv. Bot. Sadu 17: 11-18.
Persson D. 1981. Biosystematics of Stachys swainsonii Benth. (Lamiaceae) and its relations to some other chasmophytic Stachys species. – Ph.D. diss., University of Lund, Sweden.
Perveen A, Qaiser M. 2007. Pollen flora of Pakistan LIII. Verbenaceae. – Pak. J. Bot. 39: 663-669.
Petanidou T. 1996. Labiatae: a key family for wild bees and the pollination ecology in Mediterranean phryganic communities. – In: Harley RM, Paton AJ (eds), Lamiales Newslett. 4: 4-6.
Peter E. 1936. Revision der indischen und tibetanischen Arten der Gattung Salvia L. – Feddes Repert. Spec. Nov. Regni Veget. 39: 173-186.
Peterson KM. 1978. Systematic studies of Salvia L. subgenus Calosphace (Benth.) Benth. in Benth. & Hook. section Farinaceae (Epling) Epling (Lamiaceae). – Ph.D. thesis, University of Maryland, Baltimore.
Petersen OG. 1893. Halorrhagidaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(7), W. Engelmann, Leipzig, pp. 226-237.
Phatak VG, Ambegaokar KB. 1961. Embryological studies in Acanthaceae IV. Development of embryo-sac and seed formation in Haplanthus tentaculatus Nees. – J. Indian Bot. Soc. 40: 525-534.
Philbrick CT. 1984. Pollen tube growth within vegetative tissues of Callitriche (Callitrichaceae). – Amer. J. Bot. 71: 882-886.
Philbrick CT. 1993. Underwater cross-pollination in Callitriche hermaphroditica (Callitrichaceae): evidence from random amplified polymorphic DNA markers. – Amer. J. Bot. 80: 391-394.
Philbrick CT. 1994. Chromosome counts for Callitriche (Callitrichaceae) in North America. – Rhodora 96: 383-386.
Philbrick CT, Anderson GJ. 1992. Pollination biology in the Callitrichaceae. – Syst. Bot. 17: 282-292.
Philbrick CT, Jansen RK. 1991. Phylogenetic studies of North American Callitriche (Callitrichaceae) using chloroplast DNA restriction fragment analysis. – Syst. Bot. 16: 478-491.
Philbrick CT, Les DH. 2000. Phylogenetic studies in Callitriche: implications for interpretation of ecological, karyological and pollination system evolution. – Aquatic Bot. 68: 123-141.
Philbrick CT, Osborn JM. 1994. Exine reduction in underwater flowering Callitriche (Callitrichaceae): implications for the evolution of hypohydrophily. – Rhodora 96: 370-381.
Philcox D. 1965. Contributions to the flora of tropical America LXXIV. Revision of the New World species of Buchnera L. (Scrophulariaceae). – Kew Bull. 18: 275-315.
Philcox D. 1970. A taxonomic revision of the genus Limnophila R. Br. (Scrophulariaceae). – Kew Bull. 24: 101-170.
Philcox D. 1985. New names in Scrophulariaceae. – Kew Bull. 40: 606.
Philcox D. 1986. Hoshiarpuria debunked. – Kew Bull. 41: 432.
Philcox D. 1987a. New species of Buchnera (Scrophulariaceae) from southern tropical Africa. – Kew Bull. 42: 208.
Philcox D. 1987b. Further new species of Buchnera (Scrophulariaceae) from southern tropical Africa. – Kew Bull. 42: 384.
Phillippi A, Tyrl RJ. 1979. The reproductive biology of Proboscidea louisianica (Martyniaceae). – Rhodora 81: 345-361.
Phillipson PB, Steyn CF. 2008. Tetradenia (Lamiaceae) in Africa: new species and new combinations. – Adansonia, sér. III, 30: 177-196.
Piazzano M. 1998. Numeros cromosomicos en Bignoniaceae de Argentina. – Kurtziana 26: 179-189.
Pichon M. 1945. Notes sur les Bignoniacées. – Bull. Soc. Bot. France 92: 222-229.
Piechura JE, Fairbrothers DE. 1979. Serological investigation of the Oleaceae and putative relatives. – Bot. Soc. Amer. Misc. Ser. Publ. 157: 65.
Piechura JE, Fairbrothers DE. 1983. The use of protein-serological characters in the systematics of the family Oleaceae. – Amer. J. Bot. 70: 780-789.
Pieper W. 1928. Vorarbeiten zu einer Revision der afrikanischen Vitex-Arten mit Berücksichtigung der übrigen. – Engl. Bot. Jahrb. Syst. 62, Beibl. 141: 1-91.
Pijl L van der. 1972. Functional considerations and observations on the flowers of some Labiatae. – Blumea 20: 93-103.
Pijnacker LP, Schotsman HD. 1988. Nuclear DNA amounts in European Callitriche species (Callitrichaceae). – Acta Bot. Neerl. 37: 129-135.
Pilger R. 1898. Vergleichende Anatomie der Gattung Plantago, mit Rücksicht auf die Existenzbedingungen. – Engl. Bot. Jahrb. Syst. 25: 296-351.
Pilger R. 1914. Über Plantago section Plantaginella Decne. – Engl. Bot. Jahrb. Syst. 50: 61-71.
Pilger R. 1928. Die Gattung Plantago in Zentral- und Südamerika. – Engl. Bot. Jahrb. Syst. 62: 1-112.
Pinar M. 1973. 5,6,7-Trimethoxyflavone and 5,6,7,4’-tetramethoxyflavone from Kickxia lanigera. – Phytochemistry 12: 3014-3015.
Pinto-Carrasco D, Scheunert A, Heubl G, Rico E, Martinez-Ortega MM. 2017. Unravelling the phylogeny of the root-hemiparasitic genus Odontites (tribe Rhinantheae, Orobanchaceae): evidence for five main lineages. – Taxon 66: 886-908.
Piovano MA, Bernardello LM. 1991. Chromosome numbers in Argentinean Acanthaceae. – Syst. Bot. 16: 89-97.
Pischinger F. 1903. Über Bau und Regeneration des Assimilationsapparates von Streptocarpus und Monophyllaea. – Sitzungsber. Akad. Wiss. Wien, Math.-Naturwiss. Kl., 111: 278-302.
Piwowarczyk R. 2015. Seed micromorphology of central European Orobanche and Phelipanche (Orobanchaceae) in relation to preferred hosts and systematic implications. – Aust. Syst. Bot. 28: 124-136.
Piwowarczyk R, Kasińska J. 2017. Petal epidermal micromorphology in holoparasitic Orobanchaceae and its significance for systematics and pollination ecology. – Aust. Syst. Bot. 30: 48-63.
Plachno BJ, Swiatek P. 2011. Unusual embryo structure in viviparous Utricularia nelumbifolia, with remarks on embryo evolution in genus Utricularia. – Protoplasma 239: 69-80.
Plachno BJ, Kozieradzka-Kiszkurno M, Swiatek P. 2007. Functional ultrastructure of Genlisea (Lentibulariaceae) digestive hairs. – Ann. Bot. 100: 195-203.
Plachno BJ, Adamec L, Huet H. 2009. Mineral nutrient uptake from prey and glandular phosphatase activity as a dual test of carnivory in semidesert plants with glandular leaves suspected of carnivory. – Ann. Bot. 104: 649-654.
Plaza L, Fernández I, Juan R, Pastor J, Pujadas A. 2004. Micromorphological sudies on seeds of Orobanche species fom the Iberian Peninsula and the Balearic Islands, and their systematic significance. – Ann. Bot. 94: 167-178.
Pocock SAJ, Vasanthy G. 1988. Cornetipollis reticulata, a new pollen with angiospermid features from Upper Triassic (Carnian) sediments of Arizona (U.S.A.), with notes on Equisetosporites. – Rev. Palaeobot. Palynol. 55: 337-356.
Pohlheim F. 2003. Vergleichende Untersuchungen zur Sprossvariation bei Plectranthus L’Herit. (Lamiaceae). – Feddes Repert. 114: 488-496.
Poinar Jr GO. 2016. The first fossil flowers of Bignoniaceae (Lamiales): Catalpa hispaniolae sp. nov. in Dominican Republic amber. – Novon 25: 57-63.
Pollard BJ, Paton A. 2009. The African Plectranthus (Lamiaceae) expansion continues. Vale Leocus! – Kew Bull. 64: 259-261.
Pollard BJ, Paton A. 2012. Further expansion of African Plectranthus (Lamiaceae): completing the subsumption of Isodictyophorus. – Kew Bull. 67: 49-50.
Pongrcic O. 1931. Beiträge zur Anatomie der Gesneriaceen. – Sitzungsber. Akad. Wiss. Wien, Math.-Naturwiss. Kl., Abt. I, 140: 183-219.
Pool A. 2007a. A revision of the genus Pithecoctenium (Bignoniaceae). – Ann. Missouri Bot. Gard. 94: 622-642.
Pool A. 2007b. A review of the genus Distictis (Bignoniaceae). – Ann. Missouri Bot. Gard. 94: 791-820.
Pool A. 2007c. New species of Stachys (Lamiaceae) from Mesoamerica. – Novon 17: 60-66.
Pool A. 2008. A review of the genus Pyrostegia (Bignoniaceae). – Ann. Missouri Bot. Gard. 95: 495-510.
Pool A. 2009. A review of the genus Distictella (Bignoniaceae). – Ann. Missouri Bot. Gard. 96: 286-323.
Poser GL von, Toffoli ME, Sobral M, Henriques AT. 1997. Iridoid glucosides substitution patterns in Verbenaceae and their taxonomic implication. – Plant Syst. Evol. 205: 265-287.
Poser GL von, Schripsema J, Henriques AT, Jensen SR. 2000. The distribution of iridoids in Bignoniaceae. – Biochem. Syst. Ecol. 28: 351-366.
Potgieter CJ, Edwards TJ. 2001 [2002]. The occurrence of long, narrow corolla tubes in southern African Lamiaceae. – Syst. Geogr. Plants 71: 493-502.
Potgieter CJ, Edwards TJ, Miller RM, Staden J van. 1999. Pollination of seven Plectranthus spp. (Lamiaceae in southern Natal, South Africa. – Plant Syst. Evol. 218: 99-112.
Pozhidaev AE. 1989. Exine structure in pollen grains of the Lamiaceae family. – Bot. Žurn. 74: 1410-1422. [In Russian with English summary]
Pozhidaev AE. 1992. The origin of three- and six-colpate pollen grains in the Lamiaceae. – Grana 31: 49-52.
Praglowski J, Gyllander K. 1970. Globulariaceae. – In: World Pollen and Spore Flora 4: 1-21.
Prain D. 1890. Novitiae Indicae III. Some additional species of Labiatae. – J. Asiat. Soc. Bengal, II, Nat. Hist. 59: 294-318.
Prain D. 1891. Some additional species of Labiatae. – J. Asiat. Soc. Bengal. 59: 311.
Prain D. 1897. Novitiae Indicae XVI. More additional species of Labiatae. – J. Asiat. Soc. Bengal, II, Nat. Hist. 66: 518-522.
Prain D. 1904. Some new plants from Eastern Asia. – J. Asiat. Soc. Bengal, II, Nat. Hist. 73: 20-21.
Prather LA, Monfils AK, Posto AL, Williams RA. 2002. Monophyly and phylogeny of Monarda (Lamiaceae): evidence from the internal transcribed spacer (ITS) region of nuclear ribosomal DNA. – Syst. Bot. 27: 127-137.
Press JR. 1982. Taxonomic studies in the Labiatae tribe Pogostemoneae. – Bull. Brit. Mus. (Nat. Hist.) Bot. 10: 1-89.
Press MC. 1989. Autotrophy and heterotrophy in root hemiparasites. – Trends Ecol. Evol. 4: 258-263.
Press MC, Graves JD (eds). 1995. Parasitic plants. – Chapman and Hall, London.
Press MC, Phoenix GK. 2005. Effects of climate change on parasitic plants: the root hemiparasitic Orobanchaceae. – Folia Geobot. 40: 205-216.
Preston JC, Martínez CC, Hileman LC. 2011. Gradual disintegration of the floral symmetry gene network is implicated in the evolution of a wind-pollination syndrome. – Proc. Natl. Acad. Sci. U.S.A. 108: 2343-2348.
Privat G. 1960. Recherches sur les phanérogames parasites (etude d’Orobanche hederae Duby). – Ann. Sci. Nat. Bot. 12e sér., 1: 721-871.
Profice SR. 1988. Mendoncia Vell. ex Vand. (Acanthaceae): espécies ocorrentes no Brasil. – Arq. Jard. Bot. Rio de Janeiro 29: 201-279.
Profice SR, Leitman P. 2013. Clistax bahiensis (Acanthaceae), a new epiphytic species from Bahia, Brazil. – Phytotaxa 132: 47-52.
Puech S. 1975. Contribution caryologique à l’étude des Teucrium de la section Polium du basin Méditerranéen occidentale. – In: Sharma A, Sarkar AK (eds), La Flore du basin Méditerranéen, Colloques Internationals, C.N.R.S., Paris, pp. 223-238.
Puech S. 1976. Recherches de biosystématique sur les Teucrium (Labiées) de la Section Polium au basin Méditerranéen occidental (Espagne et France). – Montpellier.
Puech S. 1978. Les Teucrium de la section Polium au Portugal. – Bol. Soc. Brot. sér. II, 52: 37-50.
Puech S. 1990. Contribution à l’étude biosystématique des Teucrium de la section Polium (Labiatae) de Tunisie III. – Bull. Soc. Bot. France 137: 63-76.
Puglisi C. 2014. Systematic studies in the Boea group. – Ph.D. diss., University of Edinburgh, Edinburgh.
Puglisi C, Middleton DJ. 2017a. A revision of Middletonia (Gesneriaceae) in Thailand. – Thai Forest Bull., Bot. 45: 35-41.
Puglisi C, Middleton DJ. 2017b. A revision of Damrongia (Gesneriaceae) in Thailand. – Thai Forest Bull., Bot. 45: 79-93.
Puglisi C, Middleton DJ, Triboun P, Möller M. 2011. New insights into the relationships between Paraboea, Trisepalum, and Phylloboea (Gesneriaceae) and their taxonomic consequences. – Taxon 60: 1693-1702.
Puglisi C, Yao TL, Milne R, Möller M, Middleton DJ. 2016. Generic recircumscription in the Loxocarpinae (Gesneriaceae), as inferred by phylogenetic and morphological data. – Taxon 65: 277-292.
Pujadas-Salva AJ, Arguimbau PFI. 2009. A new species of Orobanche (Orobanchaceae) from the Balearic Islands. – Bot. J. Linn. Soc. 158: 722-729.
Pujadas-Salva AJ, Crespo MB. 2004. A new species of Orobanche (Orobanchaceae) from south-eastern Spain. – Bot. J. Linn. Soc. 146: 97-102.
Pujadas-Salva AJ, Velasco L. 2000. Comparative studies on Orobanche cernua L. and O. cumana Wallr. (Orobanchaceae) in the Iberian Peninsula. – Bot. J. Linn. Soc. 134: 513-527.
Punt W. 1978. Evolutionary trends in the Buddleieae (Loganiaceae). – In: Proc. 4th Intern. Palynol. Conf., Lucknow 1976-1977, 1: 285-290.
Puppo P, Curto M, Gusmão-Guedes J, Cochofel J, Paz PLP de, Bräuchler C, Meimberg H. 2015. Molecular phylogenetics of Micromeria (Lamiaceae) in the Canary Islands, diversification and inter-island colonization patterns inferred from nuclear genes. – Mol. Phylogen. Evol. 89: 160-170.
Qin X-K. 1996. The use of peroxidases in the systematics of Oleaceae. – Acta Bot. Yunnan. 18: 159-166.
Radlkofer L. 1883. Über den systematischen Werth der Pollen-Beschaffenheit be den Acanthaceen. – Sitzungsber. Math.-Phys. Cl. Akad. Wiss. München 13: 256-314.
Raghavan TS, Srinivasan. 1940. Studies in the Scrophulariaceae I. The cytology of Angelonia grandiflora C. Morr. and some related genera. –Cytologia 11: 37-54.
Raghavan TS, Srinivasan VK. 1941. Morphological and cytological studies in the Scrophulariaceae IV. The development of embryosac and endosperm in Scoparia dulcis Linn. – Proc. Indian Acad. Sci., Sect. B, 13: 229-234.
Raghavan TS, Venkatusubban KR. 1940. Studies in the Bignoniaceae I. Chromosome number and epidermal hydathodes in Spathodea campanulata Beauv. – J. Indian Bot. Soc. 19: 293-298.
Rahmani A, Nejadsatari T, Mahdi Hamdi SM, Mehregan I, Assadi M. 2014. A phylogenetic analysis of Linaria (Plantaginaceae) species from Iran based on ITS sequence data. – Europ. J. Experim. Biol. 4: 127-134.
Rahmanzadeh R, Müller K, Fischer E, Bartels D, Borsch T. 2004. The Linderniaceae and Gratiolaceae are further lineages distinct from the Scrophulariaceae (Lamiales). – Plant Biol. 7: 67-78.
Rahn K. 1957. Chromosome numbers in Plantago. – Bot. Tidsskr. 53: 369-378.
Rahn K. 1974. Plantago section Virginica. – Dansk Bot. Ark. 30(2): 1-180.
Rahn K. 1975b. Plantaginaceae. – In: Harling G, Sparre B (eds), Flora of Ecuador 182, Swedish Natural Science Research Council, Stockholm, 4: 25-40.
Rahn K. 1978. Nomenclatorial changes within the genus Plantago, infraspecific taxa and subdivisions of the genus. – Bot. Tidsskr. 73: 106-111.
Rahn K. 1979a. Plantago ser. Gnaphaloides Rahn, a taxonomic revision. – Bot. Tidsskr. 73: 137-154.
Rahn K. 1979b. Plantago ser. Ovatae, a taxonomic revision. – Bot. Tidsskr. 74: 13-20.
Rahn K. 1981. Plantago ser. Sericeae, a taxonomic revision. – Nord. J. Bot. 1: 297-323.
Rahn K. 1982. Plantago ser. Hispidulae, a taxonomic revision. – Nord. J. Bot. 2: 29-39.
Rahn K. 1983a. Phenetic and phylogenetic studies based on measurements of Plantago ser. Brasilienses. – Nord. J. Bot. 3: 319-329.
Rahn K. 1983b. Plantago ser. Brasilienses, a taxonomic revision. – Nord. J. Bot. 3: 331-342.
Rahn K. 1984. Plantago sect. Oliganthos in southern South America, a taxonomic revision. – Nord. J. Bot. 4: 601-627.
Rahn K. 1985. Plantago sect. Carpophorae, a taxonomic study. – Nord. J. Bot. 5: 143-151.
Rahn K. 1992. Trichomes within Plantaginaceae. – Nord. J. Bot. 12: 3-12.
Rahn K. 1996. A phylogenetic study of the Plantaginaceae. – Bot. J. Linn. Soc. 120: 145-198.
Raj B. 1961. Pollen morphological studies in the Acanthaceae. – Grana Palynol. 3: 3-108.
Raj B. 1973. Further contribution to the pollen morphology of the Acanthaceae. – J. Palynol. 9: 91-141.
Raj B. 1974. Pollen morphology of Indian Labiatae 1. Tribes Ocimoideae and Satureineae. – J. Palynol. 10: 89-105.
Raj B. 1983. A contribution to the pollen morphology of Verbenaceae. –Rev. Palaeobot. Palynol. 39: 343-422.
Raj B. 1985. A contribution to the pollen morphology of Nesogenaceae and Cyclocheilaceae. – Pollen Spores 27: 295-306.
Raj B. 1987. Pollen morphology of three monotypic genera of Verbenaceae. – Pollen Spores 29: 353-358.
Raj B. 1993. A contribution to the pollen morphology of Stilbaceae Kunth. – Pollen Spores 25: 395-408.
Raj B, El-Ghazaly G. 1987. Morphology and taxonomic application of orbicules (Ubisch bodies) in Chloanthaceae. – Pollen Spores 29: 151-166.
Raj B, Grafström E. 1984. A contribution to the pollen morphology of Chloanthaceae. – Grana 23: 139-156.
Rajanna L, Shivamurthy GR, Niranjana R, Vijay CR. 2005. Occurrence of phloem in the haustorium of Aeginetia pedunculata Wall. – a root holoparasite of Orobanchaceae. – Taiwania 50: 109-116.
Ram M, Wadhi M. 1964. Endosperm in Acanthaceae. – Phytomorphology 14: 388-413.
Ramamoorthy TP. 1984. Notes on the genus Salvia (Lamiaceae) in Mexico with three new species. – J. Arnold Arbor. 65: 135-143.
Ramamoorthy TP. 1986. A revision of Catoferia (Labiatae). – Kew Bull. 41: 299-305.
Ramamoorthy TP. 1989. Poikilacanthus capitatus: a new combination in Mexican Acanthaceae. – Syst. Bot. 14: 150-151.
Ramamoorthy TP, Lorence DH. 1987. Species vicariance in the Mexican flora and description of a new species of Salvia. – Bull. Mus. Natl. Hist. Nat. Paris 4: 167-175.
Ramamoorthy TP, Esquivel B, Sánchez AA, Rodríguez-Hahn L. 1988. Phytogeographical significance of the occurrence of abietane-type diterpenoids in Salvia sect. Erythrostachys (Lamiaceae). – Taxon 37: 908-912.
Ramamurthy K. 1971. A new genus of Acanthaceae from Kerala State, South India. – Bull. Bot. Surv. India 13: 153-155.
Raman RV, Prakasa Rao PS, Narayana LL. 1983. A contribution to the floral anatomy of Phryma leptostachya L. – Curr. Sci. 52: 922-924.
Raman RV, Rao PSP, Dutt BSM, Narayana L. 2000. Embryology of Phryma leptostachya L. (Verbenaceae) with considerations of its systematic status and affinities. – Feddes Repert. 111: 231-248.
Raman S. 1987. A code proposed for the classification of trichomes as applied to the Scrophulariaceae. – Beitr. Biol. Pflanzen 62: 349-367.
Raman S. 1989a. The trichomes on the corolla of the Scrophulariaceae I. Tribes Aptosimeae and Scrophularieae. – Beitr. Biol. Pflanzen 64: 127-140.
Raman S. 1989b. The trichomes on the corolla of the Scrophulariaceae II. Tribes Hemimerideae and Calceolarieae. – Beitr. Biol. Pflanzen 64: 141-155.
Raman S. 1989c. The trichomes on the corolla of the Scrophulariaceae III. Tribe Digitalideae. – Beitr. Biol. Pflanzen 64: 199-212.
Raman S. 1989d. The trichomes on the corolla of the Scrophulariaceae V. Tribe Antirrhineae Chavannes. – Beitr. Biol. Pflanzen 64: 357-375.
Raman S. 1990a. The trichomes on the corolla of the Scrophulariaceae VI. Tribe Pedicularieae D. Don. 1825 (Rhinantheae Benth. 1835). – Beitr. Biol. Pflanzen 64: 377-390.
Raman S. 1990b. The trichomes on the corolla of the Scrophulariaceae VII. Tribe Cheloneae. – Beitr. Biol. Pflanzen 65: 223-234.
Raman S. 1991. The trichomes on the corolla of the Scrophulariaceae X. Taxa of uncertain relationship to, or within, the family. – Beitr. Biol. Pflanzen 66: 127-143.
Ramírez-Roa MA. 1987. Revision de Achimenes (Gesneriaceae). – Thesis, Universidad Nacional Autonoma de Mexico, D. F.
Ramírez-Roa MA, Chávez-Rendón C, Rodríguez-Flores YCI. 2009. Primer registro del género Corytoplectus (Gesneriaceae: Episcieae) en México, con descripción de una nueva especie. – Brittonia 61: 218-224.
Randle CP. 2006. Revision of Harveya
(Orobanchaceae) of Southern
Africa. – Syst. Bot. Monogr. 80: 1-74.
Randle CP, Wolfe AD. 2005. The evolution and expression of rbcL in holoparasitic sister-genera Harveya and Hyobanche (Orobanchaceae). – Amer. J. Bot. 92: 1575-1585.
Rangan TS, Rangaswamy NS. 1968. Morphogenic investigations on parasitic angiosperms I. Cistanche tubulosa (Orobanchaceae). – Can. J. Bot. 46: 263-266.
Rani RS. 1994. Floral anatomy and the affinities of Byblidaceae. – Rheedea 4: 144-150.
Rao L. 1926. A short note on the extrafloral nectaries in Spathodea stipulata. – J. Indian Bot. Soc. 5: 113-116.
Rao UN. 1936. Chromosome number in Millingtonia hortensis Linn. f. (Bignoniaceae). – Curr. Sci. 4: 654.
Rao VS. 1952. The floral anatomy of some Verbenaceae with special reference to the gynoecium. – J. Indian Bot. Sci. 31: 297-315.
Rao VS. 1953. The floral anatomy of some Bicarpellatae I. Acanthaceae. – J. Univ. Bombay, N. S., 21(5B): 1-34.
Rao VS. 1954. The floral anatomy of some Bicarpellatae II. Bignoniaceae. – J. Univ. Bombay, N. S., 22(5B): 55-70.
Rao VS. 1955. The floral anatomy of some Bicarpellatae III. Pedaliaceae. – J. Univ. Bombay, N. S., 23(5B): 18-26.
Rashid MH, Jong K, Mendum M. 2001. Cytotaxonomic observations in the genus Aeschynanthus (Gesneriaceae). – Edinburgh J. Bot. 58: 31-43.
Rathore JS, Garg SK, Gupta SR. 1981. A chalcone and flavanones from Didymocarpus pedicellata. – Phytochemistry 20: 1755-1756.
Ratter JA. 1963. Some chromosome numbers in the Gesneriaceae. – Notes Roy. Bot. Gard. Edinb. 25: 221-229.
Ratter JA. 1975. A survey of chromosome numbers in the Gesneriaceae of the Old World. – Notes Roy. Bot. Gard. Edinb. 33: 527-543.
Ratter JA, Prentice HT. 1964. Chromosome numbers in the Gesneriaceae II. – Notes Roy. Bot. Gard. Edinb. 25: 303-307.
Ratter JA, Prentice HT. 1967. Chromosome numbers in the Gesneriaceae III. – Notes Roy. Bot. Gard. Edinb. 27: 205-209.
Ratzel S, Uhlich H. 2004. Orobanche benkertii sp. nova (Orobanchaceae Vent.) und weitere Orobanche-Sippen aus dem Nordwest-Kaukasus. – Feddes Repert. 115: 189-211.
Ravenna P. 2008. Studies in Verbenaceae V: Dipyrena Hook. a valid genus antedating Diostea Miers. including a new species and transfers. – Onira 11: 40-45.
Ravid U, Putievsky E. 1983. Constituents of essential oils from Majorana syriaca, Coridothymus capitatus and Satureja thymbra. – Planta Medica 49: 248-249.
Ravi Kumar KV, Hanumantha Rao B. 1988. Wood anatomy of three species of Avicennia L. – Swamy Bot. Club 5: 83-88.
Rawat R, Awasthi DK, Kumar V. 1988. Floral ontogeny in Mazus pumilus (Scrophulariaceae). – Bot. Mag. (Tokyo) 101: 459-471.
Raynal-Roques A. 1979. Le genre Hydrotriche (Scrophulariaceae). – Adansonia 19: 145-173.
Raza Bhatti G, Ingrouille MJ. 1997. The sexual status of Colebrookea (Labiatae). – Telopea 7: 221-225.
Razifard H, Les DH, Tucker GC. 2016. Evidence for the transfer of Elatine rotundifolia to Linderniaceae. – Syst. Bot. 41: 665-671.
Reales A, Rivera D, Palazon JA, Obon C. 2004. Numerical taxonomy study of Salvia sect. Salvia (Labiatae). – Bot. J. Linn. Soc. 145: 353-371.
Rebernig CA, Weber A. 2007. Diversity, development and systematic significance of seed pedestals in Scrophulariaceae (s.l.). – Bot. Jahrb. Syst. 127: 133-150.
Rechinger K. 1899. Vergleichende Untersuchungen über die Trichome der Gesneriaceeen. – Österreich. Bot. Zeitschr. 49: 89-92, 142-146, 180-183, 207-213.
Rechinger KH. 1937. Revision des Formenkreises der Stachys cretica. – Ann. Naturhist. Mus. Wien 48: 165-178.
Rechinger KH. 1941a. Monographische Studie über Teucrium Sect. Chamaedrys. – Bot. Archiv 42: 335-420.
Rechinger KH. 1941b. Scutellaria Sect. Vulgares Subsect. Peregrinae im Mittelmeergebiet und Orient. – Bot. Arch. 43: 1-70.
Rechinger KH. 1941c. Neue uind kritische Labiaten aus dem Orient und Mittelmeergebiet. – Bot. Jahr. Syst. 71: 526-546.
Rechinger KH. 1962. Zur Kenntnis orientalischer Labiaten. – Kulturpflanze 3: 47-73.
Rechinger KH, Wendelbo P. 1967. Zhumeria, eine neue Labiaten-Gattung aus Süd-Iran. – Nytt Mag. Bot. 14: 39-43.
Record SJ, Hess RW. 1940. American timbers of the family Bignoniaceae. – Trop. Woods 63: 9-38.
Record SJ, Hess RW. 1941. American woods of the family Verbenaceae. – Trop. Woods 67: 19-33.
Reddy MI, Radhakrishnaiah M, Narayana LL, Ghosh RB. 1980. Floral anatomy of Nelsonieae (Acanthaceae) with a note on its taxonomic status. – Bot. Not. 133: 311-317.
Reddy MS, Vitaya Kumari C, Radhakrishnaiah M. 1993. Systematic position of Avicenniaceae. – Feddes Repert. 104: 237-239.
Ree RH. 2005. Phylogeny and the evolution of floral diversity in Pedicularis (Orobanchaceae). – Intern. J. Plant Sci. 166: 595-613.
Reeves PA, Olmstead RG. 1998. Evolution of novel morphological and reproductive traits in a clade containing Antirrhinum majus (Scrophulariaceae). – Amer. J. Bot. 85: 1047-1056.
Refulio-Rodriguez NF, Olmstead RG. 2014. Phylogeny of Lamiidae. – Amer. J. Bot. 101: 287-299.
Reifenrath K, Theisen I, Schnitzler J, Porembski S, Barthlott W. 2006. Trap architecture in carnivorous Utricularia (Lentibulariaceae). – Flora 201: 597-605.
Reisfield AS. 1987. Systematic studies in Salvia L. (Lamiaceae) with special emphasis on subgenus Calosphace (Benth.) Benth. section Dusenostachys Epl. – M.Sc. thesis, University of Wisconsin, Madison, Wisconsin.
Reith M, Baumann G, Claßen-Bockhoff R, Speck T. 2007. New insights into the functional morphology of the lever mechanism of Salvia pratensis (Lamiaceae). – Ann. Bot. 100: 393-400.
Rendle AB. 1896. Dr. Donaldson Smith’s Acanthaceae. – J. Bot. 34: 409-414.
Rendle AB. 1897. New and interesting Acanthaceae. – J. Bot. 35: 375-380.
Retief E, Reyneke WF. 1984. The genus Thunbergia in southern Africa. – Bothalia 15: 107-116.
Reut MS. 1993. Trap structure of the carnivorous plant Genlisea (Lentibulariaceae). – Bot. Helv. 103: 101-111.
Reut MS, Jobson RW. 2010. A phylogenetic study of subgenus Polypompholyx: a parallel radiation of Utricularia (Lentibulariaceae) throughout Australasia. – Aust. Syst. Bot. 23:152-161.
Reveal JL, Judd WS, Olmstead RG. 1999. Proposal to conserve the name Antirrhinaceae against Plantaginaceae (Magnoliophyta). – Taxon 48: 182.
Reveal JL, Olmstead R, Judd WS. 2008. Proposals to conserve the name Veronicaceae (Magnoliophyta), and to conserve it against Plantaginaceae, a “superconservation”. – Taxon 57: 643-644.
Rice B. 2008. Reassessing commensal-enabled carnivory in Proboscidea and Ibicella? – Carniv. Plants Newsletter 2008: 15-19.
Richardson A. 1972. Revision of Louteridium (Acanthaceae). – Tulane Stud. Zool. Bot. 17: 63-76.
Richardson PM. 1992. The chemistry of the Labiatae: an introduction and overview. – In: Harley RM, Reynolds T (eds), Advances in labiate science, Royal Botanic Gardens, Kew, pp. 291-297.
Richmond GS, Chinnock RJ. 1994. Seed germination of the Australian desert shrub Eremophila (Myoporaceae). – Bot. Rev. 60: 483-503.
Richmond GS, Ghisalberti EL. 1994. Seed dormancy and germination mechanisms in Eremophila (Myoporaceae). – Aust. J. Bot. 42: 705-715.
Richmond GS, Ghisalberti EL. 1996. Population and plant growth studies of six species of Eremophila (Myoporaceae) from central Western Australia. – J. Roy. Soc. West. Aust. 79: 175-181.
Rico E, Delgado L, Herrero A. 2009. Reassessing the Odontites purpureus group (Orobanchaceae) from South-East Spain and North-West Africa. – Bot. J. Linn. Soc. 158: 701-708.
Riek R. 1935. Systematische und pflanzengeographische Untersuchungen in der Veronica-Sektion Chamaedrys Griseb. – Feddes Repert. 79: 1-68.
Rimpler H. 1972. Iridoids from Stilbe species. – Phytochemistry 11: 3096-3097.
Rimpler H, Winterhalter C, Falk U. 1992. Cladistic analysis of the subfamily Caryopteridoideae Briq. and related taxa of Verbenaceae and Lamiaceae using morphological and chemical characters. – In: Harley RM, Reynolds T (eds), Advances in labiate science, Royal Botanic Gardens, Kew, pp. 39-54.
Rios S, Crespo MB, Rivera D. 2001. The West Mediterranean orophilous taxa of Sideritis L. (Lamiaceae): a new species of subsection Hyssopifolia from south-eastern Spain. – Bot. J. Linn. Soc. 136: 247-254.
Risch C. 1956. Die Pollenkörner der Labiaten. – Willdenowia 1: 617-641.
Ritland CR, Ritland K. 1989. Variation of sex allocation among eight taxa of the Mimulus guttatus species complex (Scrophulariaceae). – Amer. J. Bot. 76: 1731-1739.
Ritland CE, Ritland K, Straus NA. 1993. Variation in the ribosomal internal transcribed spacers (ITS1 and ITS2) among eight taxa of the Mimulus guttatus species complex. – Mol. Biol. Evol. 10: 1273-1288.
Rivera Nuñez D, Obón de Castro C. 1992. The ethnobotany of Old World Labiatae. – In: Harley RM, Reynolds T (eds), Advances in labiate science, Royal Botanic Gardens, Kew, pp. 455-473.
Rivera Nuñez D, Obón de Castro C, Alcaraz F, Llorach R. 1999. Systematics of the high mountain taxa of the genus Sideritis L. section Sideritis, subsection Fruticulosae Obon & D. Rivera (Lamiaceae). – Bot. J. Linn. Soc. 129: 249-265.
Rix M. 1987. The genus Rehmannia. – Plantsman 8: 193-195.
Roalson EH, Clark JL. 2006. Phylogenetic patterns of diversification in the Beslerieae (Gesneriaceae). – In: Sharma AK, Sharma A (eds), Plant genome biodiversity and evolution 1, C. Phanerogams (Angiosperms-Dicotyledons), Science Publ., Enfield, New Hampshire, pp. 251-268.
Roalson EH, Senters AE, Skog LE, Zimmer EA. 2002. A morphological cladistic analysis of the neotropical flowering plant genus Gasteranthus (Gesneriaceae). – Syst. Bot. 27: 573-591.
Roalson EH, Skog LE, Zimmer EA. 2003. Phylogenetic relationships and the diversification of floral form in Achimenes (Gesneriaceae). – Syst. Bot. 28: 593-608.
Roalson EH, Boggan JK, Skog LE, Zimmer EA. 2005. Untangling Gloxinieae (Gesneriaceae) I. Phylogenetic patterns and generic boundaries inferred from nuclear, chloroplast, and morphological cladistic datasets. – Taxon 54: 389-410.
Roalson EH, Boggan JK, Skog LE. 2005. Reorganization of tribal and generic boundaries in the Gloxinieae (Gesneriaceae: Gesnerioideae) and the description of a new tribe in the Gesnerioideae, Sphaerorhizeae. – Selbyana 25: 225-238.
Roalson EH, Skog LE, Zimmer EA. 2008. Untangling Gloxinieae (Gesneriaceae) II. Reconstructing biogeographic patterns and estimating divergence times among New World continental and island lineages. – Syst: Bot. 33: 159-175.
Robart BW. 2005. Morphological diversification and taxonomy among the varieties of Pedicularis bracteosa Benth. (Orobanchaceae). – Syst. Bot. 30: 644-656.
Robart BW, Gladys C, Frank T, Kilpatrick S. 2015. Phylogeny and biogeography of North American and Asian Pedicularis (Orobanchaceae). – Syst. Bot. 40: 229-258.
Robert EMR, Koedam N, Beeckman H, Schmitz N. 2009. A safe hydraulic architecture as wood anatomical explanation for the difference in distribution of the mangroves Avicennia and Rhizophora. – Funct. Ecol. 23: 649-657.
Roberts J. 1985. xGlokeria ‘Dragonsong’: a new intergeneric hybrid. – Crosswords 9: 6-8.
Robey MJ. 1988. African violets – gifts from nature. – Cornwall Books, London.
Robins RJ, Subramanyam K. 1980. Scanning Electron Microscope study of the seed surface morphology of some Utricularia (Lentibulariaceae) species from India. – Proc. Indian Natl. Sci. Acad., Sect. B, 46: 310-324.
Robyns FHEAW. 1943. Un nouveau genre de Labiatae-Ocimoideae de l’Afrique tropical central. – Bull. Jard. Bot. État 17: 27-30.
Robyns W. 1932. L’étude detaillée des formes florales et son importance pour la systématique (Solanacées, Labiatées). – Bull. Assoc. Franç.: 264-270.
Robyns W. 1966. On the status of Acrocephalus Benth. with some new species from Katanga (Congo Republic). – Bot. Not. 119: 185-195.
Robyns W, Lebrun J. 1928. Revision des espèces congolaise du genre Acrocephalus Benth. – Ann. Soc. Sci. Bruxelles, sect. B, 48: 169-203.
Rodondi G, Beretta M, Andreis C. 2010. Pollen morphology of alpine butterworts (Pinguicula L., Lentibulariaceae). – Rev. Palaeobot. Palynol. 162: 1-10.
Rodrigues A, Shaya Shana, Dickinson TA. Stefanović S. 2013. Morphometric analyses and taxonomic revision of the North American holoparasitic genus Conopholis (Orobanchaceae). – Syst. Bot. 38: 795-804.
Rodríguez B, Robledo A, Pascual-Villalobos MJ. 2009. Rearranged abietane diterpenoids from the root of Teucrium lanigerum. – Biochem. Syst. Ecol. 37: 76-79.
Rodríguez-Hahn L, Esquivel B, Sánchez C, Cárdenas J, Estebanez L, Soriano-García M, Toscano RA, Ramamoorthy TP. 1986. New highly oxidized diterpene quinines from Salvia fruticulosa (Labiatae). – Tetrahedron Lett. 27: 5459-5462.
Rodríguez-Hahn L, Esquivel B, Cárdenas J, Ramamoorthy TP. 1992. The distribution of diterpenoids in Salvia. – In: Harley RM, Reynolds T (eds), Advances in labiate science, Royal Botanic Gardens, Kew, pp. 335-347.
Roeder E, Borauel T. 1992. Pyrrolizidine alkaloids from Melampyrum pratense. – Nat. Toxins 1: 35-37.
Roelofs FM. 1980. The reproductive biology of Cyrtandra grandiflora (Gesneriaceae) on Oahu. – Pacific Sci 33: 223-231.
Roessler H. 1979. Revision der Gattungen Hebenstretia L. und Dischisma Choisy (Scrophulariaceae-Selagineae). – Mitt. Bot. München 15: 1-160.
Rogers OM. 1954. Chromosome counts in the Gesneriaceae. – Baileya 2: 14-18.
Rohwer JG. 1993. A preliminary survey of the fruits and seeds of the Oleaceae. – Bot. Jahrb. Syst. 115: 271-291.
Rohwer JG. 1994. Fruits and seeds of Nyctanthes arbor-tristis L. (Oleaceae): a comparison with some Verbenaceae. – Bot. Jahrb. Syst. 115: 461-473.
Rohwer JG. 1995. Fruit and seed structures in Menodora (Oleaceae): a comparison with Jasminum. – Bot. Acta 108: 163-168.
Rohwer JG. 1996. Die Frucht- und Samenstrukturen der Oleaceae – eine vergleichend-anatomische Untersuchung. – Bibl. Bot. 148: 1-177.
Rohwer JG. 1997a. The fruits of Nyctanthes aculeata (Oleaceae). – Bot. Jahrb. Syst. 119: 293-299.
Rohwer JG. 1997b. The fruits of Jasminum mesnyi (Oleaceae), and the distinction between Jasminum and Menodora. – Ann. Missouri Bot. Gard. 84: 848-856.
Romanova AC, Pribilova GF, Sajarov PI, Sheichenko UJ, Bankovski AI. 1971. New quinone from Salvia nemorosa. – Khim. Prirod. Soedinenii 7: 199.
Römpp H. 1928. Die Verwandtschaftsverhältnisse in der Gattung Veronica. – Feddes Repert. 50: 1-171.
Ronniger K. 1924. Beiträge zur Kenntnis der Thymus-Flora der Balkanhalbinsel I. – Feddes Repert. 20: 334-336.
Rønsted N, Göbel E, Franzyk H, Jensen SR, Olsen CE. 2000. Chemotaxonomy of Plantago. Iridoid glucosides and caffeoyl phenylethanoid glycosides. – Phytochemistry 55: 337-348.
Rønsted N, Chase MW, Albach DC, Bello MA. 2002. Phylogenetic relationships within Plantago (Plantaginaceae): evidence from nuclear ribosomal ITS and plastid trnL-F sequence data. – Bot. J. Linn. Soc. 139: 323-338.
Rønsted N, Bello MA, Jensen SR. 2003. Aragoside and iridoid glucosides from Aragoa cundinamarcensis. – Phytochemistry 64: 529-533.
Rosales M. 1997. Trichanthera gigantea (Humboldt & Bonpland) Nees: a review. – Livestock Res. Rural Developm. 9: 1-7.
Rosén W. 1940. Notes on the embryology of Globularia vulgaris L. – Bot. Not. 1940: 253-261.
Rosenbaumová R, Placková I, Suda J. 2004. Variation in Lamium subg. Galeobdolon (Lamiaceae) – insights from ploidy levels, morphology and isozymes. – Plant Syst. Evol. 244: 219-244.
Rosenblum IM. 1981. An approach toward understanding some of the morphogenetic bases of phylogeny of Streptocarpus (Gesneriaceae). – Ph.D. diss, City University of New York, New York.
Rosenblum IM, Basile DV. 1984. Hormonal regulation of morphogenesis in Streptocarpus and its relevance to evolutionary history of the Gesneriaceae. – Amer. J. Bot. 71: 52-64.
Ross H. 1902. Byblis gigantea. – Gartenflora 51: 337-339, pl. 1500.
Rosser EM, Burtt BL. 1969. Studies in the Gesneriaceae of the Old World XXX. Anatomical characters in the tribe Trichosporeae. – Notes Roy. Bot. Gard. Edinb. 29: 39-58.
Rossow RA. 1985. Melospermeae, nueva tribu de Scrophulariaceae. – Parodiana 3: 365-396.
Rosúa JL, Blanca G. 1986. Revisión del género Salvia L. (Lamiaceae) en el Mediterráneo Occidental: la sección Salvia. – Acta Bot. Mal. 11: 227-271.
Rosúa JL, Blanca G. 1988. Revisión del género Salvia L. sect. Aethiopis Benth. (Lamiaceae) en el Mediterráneo Occidental. – Collectanea Bot. 17: 205-236.
Rothmaler W. 1943a. Zur Gliederung der Antirrhineae. – Feddes Repert. 52: 16-39.
Rothmaler W. 1943b. Die Aufspaltung von Odontites Hall. ex Zinn. – Mitth. Thüring. Bot. Ver., Ser. II, 50: 224-230.
Rothmaler W. 1954. Notes on Western Antirrhineae. – Leafl. Bot. 7: 113-117.
Rothmaler W. 1956. Taxonomische Monographie der Gattung Antirrhinum. – Feddes Repert. 136: 1-124.
Rotman AD. 2009. El género Lantana L. (Verbenaceae-Verbenoideae) en Paraguay: sinopsis y novedades. – Candollea 64: 297-301.
Rouke JP. 1977. A revision of Xeroplana Briq. (Stilbaceae). – J. South Afr. Bot. 43: 1-8.
Rourke JP. 1993. Thesmophora, a new genus of Stilbaceae from South Africa. – Edinburgh J. Bot. 50: 89-95.
Rourke JP. 2000. A review of generic concepts in the Stilbaceae. – Bothalia 30: 9-15.
Rouy G. 1909. ‘Conspectus’ des tribus et des genres de la famille des Scrophulariacées. – Rev. Gén. Bot. 21: 194-207.
Roy T, Lindqvist C. 2015. New insights into evolutionary relationships within the subfamily Lamioideae (Lamiaceae) based on pentatricopeptide repeat (PPR) nuclear DNA sequences. – Amer. J. Bot. 102: 1721-1735.
Roy T, Chang TH, Lan T, Lindqvist C. 2013. Phylogeny and biogeography of New World Stachydeae (Lamiaceae) with emphasis on the origin and diversification of Hawaiian and South American taxa. – Mol. Phylogen. Evol. 69: 218-238.
Roy T, Cole LW, Chang T-H, Lindqvist C. 2015. Untangling reticulate evolutionary relationships among New World and Hawaiian mints (Stachydeae, Lamiaceae). – Mol. Phylogen. Evol. 89: 46-62.
Roy T, Catlin NS, Garner DMG, Cantino PD, Scheen A-C, Lindqvist C. 2016. Evolutionary relationships within the lamioid tribe Synandreae (Lamiaceae) based on multiple low-copy nuclear loci. – PeerJ PrePrints 4, e1725v1.
Royen P van. 1972. The Scrophulariaceae of the alpine regions of New Guinea. – Bot. Jahrb. Syst. 91: 383-437.
Rudall PJ. 1979. Leaf and twig anatomy of Eriope, a xeromorphic genus of Labiatae. – Bot. J. Linn. Soc. 78: 157-180.
Rudall PJ. 1980a. Leaf anatomy of the subtribe Hyptidinae (Labiatae). – Bot. J. Linn. Soc. 80: 319-340.
Rudall PJ. 1980b. Pollen morphology in the subtribe Hyptidinae (Labiatae). – Kew Bull. 35: 453-457.
Rudall PJ. 1981a. Wood anatomy in the Hyptidinae (Labiatae). – Kew Bull. 35: 735-741.
Rudall PJ. 1981b. Flower anatomy of the subtribe Hyptidinae (Labiatae). – Bot. J. Linn. Soc. 83: 251-262.
Rudall PJ. 1985. Perforated ray cells in Hyptis hagei – a new record for Labiatae. – IAWA Bull., N. S., 6: 161-162.
Rudall PJ. 1986. Leaf anatomy of Hyptis sect. Pachyphyllae (Labiatae) and related species. – Kew Bull. 41: 1017-1025.
Rudall PJ, Clark L. 1992. The megagametophyte in Labiatae. – In: Harley RM, Reynolds T (eds), Advances in labiate science, Royal Botanic Gardens, Kew, pp. 65-85.
Ruengsawang K, Chantaranothai P, Simpson DA. 2012. Contributions to the seed morphology and taxonomy of Justicia (Acanthaceae) from Thailand. – J. Syst. Evol. 50: 153-162.
Rueangsawang K, Chantaranothai P, Simpson DA. 2013a. Taxonomic notes on the genus Justicia (Acanthaceae) from Thailand. – Phytotaxa 130: 43-49.
Rueangsawang K, Chantaranothai P, Simpson DA. 2013b. Pollen morphology of Justicia L. (Acanthaceae) from Thailand and its taxonomic value. – Grana 52: 275-288.
Rueda RM. 1994. Systematics and evolution of the genus Petrea (Verbenaceae). – Ann. Missouri Bot. Gard. 81: 610-652.
Ruiz E, Marticorena C, Crawford D, Stuessy T, González F, Montoya R, Silva M, Becerra J. 2000. Morphological and ITS sequence divergence between taxa of Cuminia (Lamiaceae), an endemic genus of the Juan Fernandez Islands, Chile. – Brittonia 52: 341-350.
Runemark H. 1967. Studies in the Aegean flora X. Cytologic and morphologic notes on Plantago. – Bot. Not. 120: 9-16.
Russel GF, Olson KV. 1972. The volatile constituents of oil of thyme. – J. Food Sci. 37: 405-407.
Rustan ØH, Brochmann C. 1988. The genus Kickxia Dumortier (Scrophulariaceae) in the Cape Verde Islands, W Africa. – Cour. Forsch.-Inst. Senckenberg 105: 67-72.
Rutishauser R, Isler B. 2001. Developmental genetics and morphological evolution of flowering plants, especially bladderworts (Utricularia): fuzzy arberian morphology complements classical morphology. – Ann. Bot. 88: 1173-1202.
Rutishauser R, Sattler R. 1989. Complementarity and heuristic value of contrasting models in structural botany III. Case study on shoot-like “leaves” and leaf-like “shoots” in Utricularia macrorhiza and U. purpurea (Lentibulariaceae). – Bot. Jahrb. Syst. 111: 121-137.
Ruttle ML. 1931. Cytological and embryological studies on the genus Mentha. – Gartenbauwiss. 4: 428-468.
Ruwen F. 1981. A study on the embryological development of Catalpa ovata. – J. Nanjing Techn. Coll. Forest Prod. 4: 64-74.
Ryding O. 1980. Notes on Aeollanthus (Labiatae) in West Africa. – Bot. Not. 133: 229-233.
Ryding O. 1981. The Aeollanthus buchnerianus group (Labiatae). – Nord. J. Bot. 1: 154-164.
Ryding O. 1982. The Aeollanthus abyssinicus group (Labiatae). – Nord. J. Bot. 2: 219-229.
Ryding O. 1986. The genus Aeollanthus s. lat. (Labiatae). – Symb. Bot. Ups. 26(1): 1-152.
Ryding O. 1991. Notes on the genus Erythochlamys (Lamiaceae). – Nord. J. Bot. 11: 633-635.
Ryding O. 1992a. The distribution and evolution of myxocarpy in Lamiaceae. – In: Harley RM, Reynolds T (eds), Advances in labiate science, Royal Botanic Gardens, Kew, pp. 85-96.
Ryding O. 1992b. Pericarp structure and phylogeny within Lamiaceae subfamily Nepetoideae tribe Ocimeae. – Nord. J. Bot. 12: 273-298.
Ryding O. 1993a. Pericarp structure and systematic positions of five genera of Lamiaceae subg. Nepetoideae tribe Ocimeae. – Nord. J. Bot. 13: 631-635.
Ryding O. 1993b. Pericarp structure of Leucas and related genera (Lamiaceae subfam. Lamioideae). – Nord. J. Bot. 13: 637-646.
Ryding O. 1993c. A reconsideration of the genus Rabdosiella (Lamiaceae). – Plant Syst. Evol. 185: 91-97.
Ryding O. 1994a. Pericarp structure and phylogeny of Lamiaceae subfamily Pogostemonoideae. – Nord. J. Bot. 14: 59-63.
Ryding O. 1994b. Pericarp structure in the subtribe Melittidinae (Lamiaceae-Lamioideae) and its systematic implications. – Bot. Jahrb. Syst. 115: 547-555.
Ryding O. 1994c. Pericarp structure in the tribe Prasieae (Lamiaceae-Lamioideae) and its systematic implications. – Bot. Jahrb. Syst. 116: 391-399.
Ryding O. 1995. Pericarp structure and phylogeny of the Lamiaceae-Verbenaceae-complex. – Plant Syst. Evol. 198: 101-141.
Ryding O. 1998. Phylogeny of the Leucas group (Lamiaceae). – Syst. Bot. 23: 235-247.
Ryding O. 1999a. The identity of Bovonia diphylla (Lamiaceae). – Syst. Geogr. Plants 69: 195-197.
Ryding O. 1999b. Notes on Plectranthus (Lamiaceae) in Somalia. – Kew Bull. 54: 117-127.
Ryding O. 2001 [2002]. Myxocarpy in the Nepetoideae (Lamiaceae) with notes on myxodiaspory in general. – Syst. Geogr. Plants 71: 503-514.
Ryding O. 2003. Reconsideration of Wiedemannia and notes on the circumscription of Lamium (Lamiaceae). – Bot. Jahrb. Syst. 124: 325-335.
Ryding O. 2006. Revision of the Clinopodium abyssinicum group (Lamiaceae). – Bot. j. Linn. Soc. 150: 391-408.
Ryding O. 2007a. Revision of the Micromeria (Labiatae) in tropical to southern Africa and on the Arabian Peninsula. – Bot. J. Linn. Soc. 155: 427-446.
Ryding O. 2007b. Amount of calyx fibres in Lamiaceae, relation to calyx structure, phylogeny and ecology. – Plant Syst. Ecol. 268: 45-58.
Ryding O. 2008. Pericarp structure and phylogeny of the Phlomis group (Lamiaceae subfam. Lamioideae). – Bot. Jahrb. Syst. 127: 299-316.
Ryding O. 2009. Pericarp structure in Monarda (Lamiaceae). – Bot. Jahrb. Syst. 127: 453-458.
Ryding O. 2010a. Crystals in calyces of Lamiaceae and their phylogenetic and adaptive significance. – Plant Syst. Evol. 290: 201-215.
Ryding O. 2010b. Pericarp structure and phylogeny of tribe Mentheae (Lamiaceae). – Plant Syst. Evol. 285: 165-175.
Ryding O, Paton A, Thulin M, Springate D. 2003. Reconsideration of the genus Puntia and a new species of the genus Endostemon (Lamiaceae). – Kew Bull. 58: 919-927.
Rye BL. 2000. Proposal to conserve the name Dicrastylis against Lachnocephalus and Mallophora (Prostantheroideae, Lamiaceae). – Taxon 49: 815-816.
Sacchetti G, Bruni A, Dall’Olio G, Nicoletti M, Di Fabio A, Poli F. 1996. Development and morphology of secretory trichomes of Calceolaria volckmanni (Scrophulariaceae). – Nord. J. Bot. 16: 505-513.
Sachs M. 1915. Anatomisch-systematische Untersuchungen über die Blattstruktur bei den Gesnerioiden und einigen Triben der Cyrtandroideen. – Ph.D. diss., Universität Erlangen, Germany.
Sachse M. 2001. Oleaceous laurophyllous leaf fossils and pollen from the European Tertiary. – Rev. Palaeobot. Palynol. 115: 213-234.
Saeidi-Mehrvarz S, Zarre S. 2004. A cladistic analysis of the Iranian species of the genus Veronica (Scrophulariaceae) with emphasis on the patterns of homoplasy. – Feddes Repert. 115: 519-529.
Saez L, Crespo MB. 2005. A taxonomic revision of the Linaria verticillata group (Antirrhineae, Scrophulariaceae). – Bot. J. Linn. Soc. 148: 229-244.
Saez L, Sainz M, Crespo MB. 2004. Taxonomic notes on Linaria Mill. (Scrophulariaceae) for Flora Iberica. – Folia Geobot. 39: 293-318.
Saggoo MIS, Bir SS. 1982. Cytological studies on certain Acanthaceae from central India. – Proc. Indian Acad. Sci., Sect. B, 91: 479-486.
Saggoo MIS, Bir SS. 1986a. Meiotic studies on some east Himalayan members of family Labiatae. – J. Indian Bot. Soc. 65: 304-309.
Saggoo MIS, Bir SS. 1986b. Meiotic studies in certain members of family Acanthaceae from South India. – J. Indian Bot. Soc. 65: 310-315.
Sahasrabudhe S, Stace CA. 1974. Developmental and structural variation in the trichomes and stomata of some Gesneriaceae. – New Bot. 1: 46-62.
Sahay SK. 1980. Palynotaxonomy of Boraginaceae and some other families of Tubiflorae. – Biol. Mem. 4: 117-205.
Sales F. 2001. A synopsis of Vitex L. in the Flora Zambesiaca area. – Kew Bull. 56: 189-207.
Salinas MF, Arroyo MTK, Armesto JJ. 2010. Epiphytic growth habits of Chilean Gesneriaceae and the evolution of epiphytes within the tribe Coronanthereae. – Ann. Missouri Bot. Gard. 97: 117-127.
Salmaki Y, Zarre S, Jamzad Z. 2008. Nutlet micromorphology and its systematic implication in Stachys L. (Lamiaceae) in Iran. – Feddes Repert. 119: 607-621.
Salmaki Y, Jamzad Z, Zarre S, Bräuchler C. 2008. Pollen morphology of Stachys (Lamiaceae) in Iran and its systematic implication. – Flora 203: 627-639.
Salmaki Y, Zarre S, Jamzad Z, Bräuchler C. 2009. Trichome micromorphology of Iranian Stachys (Lamiaceae) with emphasis on its systematic implication. – Flora 204: 371-381.
Salmaki Y, Zarre S, Lindqvist C, Heubl G, Bräuchler C. 2011. Comparative leaf anatomy of Stachys (Lamiaceae: Lamioideae) in Iran with a discussion on its subgeneric classification. – Plant Syst. Evol. 294: 109-125.
Salmaki Y, Zarre S, Ryding O, Scheunert A, Bräuchler C, Heubl G. 2012. Phylogeny of the tribe Phlomideae (Lamioideae: Lamiaceae) with special focus on Eremostachys and Phlomoides: new insights from nuclear and chloroplast sequences. – Taxon 61: 161-197.
Salmaki Y, Zarre S, Govaerts R, Bräuchler C. 2012. A taxonomic revision of the genus Stachys (Lamiaceae: Lamioideae) in Iran. – Bot. J. Linn. Soc. 170: 573-617.
Salmaki Y, Zarre S, Ryding O, Lindqvist C, Bräuchler C, Heubl G, Barber G, Bendiksby M. 2013. Molecular phylogeny of tribe Stachydeae (Lamiaceae subfamily Lamioideae). – Mol. Phylogen. Evol. 69: 535-551.
Salmaki Y, Kattari S, Heubl G, Bräuchler C. 2016. Phylogeny of non-monophyletic Teucrium (Lamiaceae: Ajugoideae): implications for character evolution and taxonomy. – Taxon 65: 805-822.
Samuel R, Kiehn M, Pinsker W. 1997. Relationships among six genera of Gesneriaceae-Cyrtandroideae inferred from cpDNA atpB/rbcL spacer sequences. – In: Hollingsworth PM, Bateman RM, Gornall RJ (eds), Advances in plant molecular systematics, University of Glasgow.
Samuel R, Pinsker W, Kiehn M. 1997. Phylogeny of some species of Cyrtandra (Gesneriaceae) inferred from the atpB/rbcL cpDNA intergene region. – Bot. Acta 110: 503-510.
Samuelsen AB. 2000. The traditional uses, chemical constituents and biological activities of Plantago major L. A review. – J. Ethnopharmacol. 71: 1-21.
Sánchez AA, Esquivel B, Pera A, Cárdenas J, Soriano-García M, Toscano A, Rodríguez-Hahn L. 1987. Lasianthin, a neo-clerodane diterpenoid from Salvia lasiantha. – Phytochemistry 26: 479.
Sánchez-Burgos AA, Dengler NG. 1988. Leaf development in isophyllous and facultatively anisophyllous species of Pentadenia (Gesneriaceae). – Amer. J. Bot. 75: 1472-1484.
Sanders RW. 1981. Cladistic analysis of Agastache (Lamiaceae). – In: Funk VA, Brooks DR (eds), Advances in cladistics, New York Botanical Garden, Bronx, New York, pp. 95-114.
Sanders RW. 1984a. Taxonomic problems in Lantana sect. Camara (Verbenaceae) of Caribbean North America. – Amer. J. Bot. 71: 185.
Sanders RW. 1984b. Provisional synopsis of the species and natural hybrids in Duranta (Verbenaceae). – Sida 10: 308-318.
Sanders RW. 1987a. Taxonomic significance of chromosome observations of Caribbean species of Lantana (Verbenaceae). – Amer. J. Bot. 74: 914-920.
Sanders RW. 1987b. Taxonomy of Agastache section Brittonastrum (Lamiaceae-Nepeteae). – Syst. Bot. Monogr. 15: 1-92.
Sanders RW. 1987c. Identity of Lantana depressa and L. ovatifolia (Verbenaceae) of Florida and the Bahamas. – Syst. Bot. 12: 44-60.
Sanders RW, Cantino PD. 1984. Nomenclature of the subdivisions of the Lamiaceae. – Taxon 33: 64-72.
Sandwith NY. 1954. Contributions to the flora of tropical America LVI. Further studies in Bignoniaceae. – Kew Bull. 1953: 451-484.
Sandwith NY. 1968. Contributions to the flora of tropical America LXXVI. Notes on Bignoniaceae XXIX: Arrabidaea in Martius’s Flora Brasiliensis and subsequently. – Kew Bull. 22: 403-420.
San Martin-Gajardo I, Freitas L. 1999. Hummingbird pollination in Besleria longimucronata Hoehne (Gesneriaceae) in southeastern Brazil. – Biociéncias (Porto Alegre) 7: 13-24.
Santapau H. 1950. Notes on the Scrophulariaceae of Bombay. – J. Bombay Nat. Hist. Soc. 49: 25-26.
Santapau H. 1951. The Acanthaceae of Bombay. – Bot. Mem. Univ. Bombay 2: 1-104.
Santos EP. 2004. Notes on Salvia sect. Secundae (Lamiaceae) and two new species from Brazil. – Kew Bull. 59: 285-290.
Santos EP, Harley RM. 2004. Notes on Salvia section Nobiles (Lamiaceae) and two new species from Brazil. – Kew Bull. 59: 103-109.
Santos G dos, Miller RB. 1992. Flora Neotropica. Monograph 25(1). Wood anatomy of Tecomeae. – New York Botanical Garden, Bronx, New York,pp. 336-358.
Santos G dos, Miller RB. 1997. Wood anatomy o Jacaranda (Bignoniaceae): systematic relationships in sections Monolobos and Dilobos as suggested by twig and stem wood rays. – IAWA J. 18. 369-383.
Sattler R, Rutishauser R. 1990. Structural and dynamic descriptions of the development of Utricularia foliosa and U. australis. – Can. J. Bot. 68: 1989-2003.
Saueregger J, Weber A. 2004. Factors controlling initiation and orientation of the macrocotyl in anisocotylous Gesneriaceae. – Edinburgh J. Bot. 60: 467-482.
Saunders ER. 1934. A study of Veronica from the viewpoint of certain floral characters. – Bot. J. Linn. Soc. S49: 453-493.
Savidge JP. 1958. The experimental taxonomy of European Callitriche. – Proc. Linn. Soc. London 171: 128-130.
Savile DBO. 1968. The rusts of Cheloneae (Scrophulariaceae): a study in the co-evolution of hosts and parasites. – Nova Hedwigia 15: 369-392.
Savona G, Paternostro MP, Piozzi F, Hanson JR, Hitchcock PB, Thomas JA. 1978. Salviarina, a clerodane diterpenoid from Salvia splendens. – J. Chem. Soc. Perkin Trans. 1: 643.
Savona G, Bruno M, Paternostro MP, Marco JL, Rodríguez B. 1982. Salviacoccin, a new clerodane diterpenoid from Salvia coccinea. – Phytochemistry 21: 2563.
Savona G, Raffa D, Bruno M, Rodríguez B. 1983. Salvifarin and salvifaricin, neo-cleordane diterpenoids from Salvia farinacea. – Phytochemistry 22: 784.
Saxena MR. 1975. Pollen morphology of the Nyctanthoideae (Verbenaceae). – J. Indian Bot. Soc. 54: 71-74.
Schäferhoff B, Fleischmann A, Fischer E, Albach DC, Borsch T, Heubl G, Müller KF. 2010. Towards resolving Lamiales relationships: insights from rapidly evolving chloroplast sequences. – BMC Evol. Biol. 10: 352.
Schantz M von, Ivars L. 1964. Über die Zusammensetzung des ätherischen Öles von Thymus serpyllum ssp. tanaënsis (Hyl.) Jalas. – Ann. Univ. Turku (A II) 32: 301-307.
Scheen A-C, Albert VA. 2007. Nomenclatural and taxonomic changes within the Leucas clade (Lamioideae; Lamiaceae). – Syst. Geogr. Plants 77: 229-238.
Scheen A-C, Albert VA. 2009. Molecular phylogenetics of the Leucas group (Lamioideae; Lamiaceae). – Syst. Bot. 34: 173-181.
Scheen A-C, Lindqvist C, Fossdal CG, Albert VA. 2008. Molecular phylogenetics of tribe Synandreae, a North American lineage of lamioid mints (Lamiaceae). – Cladistics 24: 299-314.
Scheen A-C, Bendiksby M, Ryding O, Mathiesen C, Albert VA, Lindqvist C. 2010. Molecular phylogenetics, character evolution, and suprageneric classification of Lamioideae (Lamiaceae). – Ann. Missouri Bot. Gard. 97: 191-217.
Schemske DW, Bradshaw HD. 1999. Pollinator preference and evolution of floral traits in monkeyflowers (Mimulus). – Proc. Natl. Acad. Sci. U.S.A. 96: 11910-11915.
Schenk W. 1943. Morphologisch-anatomische Untersuchungen an der Gattung Streptocarpus Lindl. – Bot. Arch. 44: 217-284.
Scheunert A, Heubl G. 2011. Relationships of New World Scrophularia L. (Scrophulariaceae): new insights inferred from DNA sequence data. – Plant Syst. Evol. 291: 69-89.
Scheunert A, Heubl G. 2014. Diversification
of Scrophularia (Scrophulariaceae) in the
Western Mediterranean and Macaronesia – phylogenetic relationships,
reticulate evolution and biogeographic patterns. – Mol. Phylogen. Evol. 70:
296-313.
Scheunert A, Fleischmann A, Olano-Marín C, Bräuchler C, Heubl G. 2012. Phylogeny of tribe Rhinantheae (Orobanchaceae) with a focus on biogeography, cytology and re-examination of generic concepts. – Taxon 61: 1269-1285.
Schiller P, Heilmeier H, Hartung W. 1997. Abscisic acid (ABA) relations in the aquatic resurrection plant Chamaegigas intrepidus under naturally fluctuating environmental conditions. – New Phytol. 136: 603-611.
Schiller P, Heilmeier H, Hartung W. 1998. Uptake of amino acids by the aquatic resurrection plant Chamaegigas intrepidus and its implication for N nutrition. – Oecologia 117: 63-69.
Schiller P, Wolf R, Hartung W. 1999. A scanning microscopical study of hydrated and desiccated submerged leaves of the aquatic resurrection plant Chamaegigas intrepidus. – Flora 194: 97-102.
Schilling G, Huegel M, Mayer W. 1982. Verbascoside and isoverbascoside from Paulownia tomentosa Steud. – Zeitschr. Naturforsch., Abt. B, 37: 1633-1655.
Schindler AK. 1904. Die Abtrenning der Hippuridaceen von den Halorrhagaceen. – Engl. Bot. Jahrb. Syst. 34, Beibl. 77: 1-77.
Schlag-Edler B, Kiehn M. 2001. Palynology of South Pacific Cyrtandra (Gesneriaceae) with notes on some Hawaiian taxa. – Grana 40: 192-196.
Schlenker G. 1936. Systematische Untersuchungen über die Sektion Beccabunga der Gattung Veronica. – Feddes Repert. 90: 1-40.
Schmid E. 1906. Beiträge zur Entwicklungsgeschichte der Scrophulariaceen. – Beih. Bot. Zentralbl. 20: 175-299.
Schmidt-Lebuhn AN. 2003. A taxonomic revision of the genus Suessenguthia Merxm. (Acanthaceae). – Candollea 58: 101-128.
Schmidt-Lebuhn AN. 2007. Using amplified fragment length polymorphism (AFLP) to unravel species relationships and delimitations in Minthostachys (Labiatae). – Bot. J. Linn. Soc. 153: 9-19.
Schmidt-Lebuhn AN. 2008. Monophyly and phylogenetic relationships of Minthostachys (Labiatae, Nepetoideae) examined using morphological and nrITS data. – Plant Syst. Evol. 270: 25-38.
Schmidt-Lebuhn AN, Kessler M, Müller J. 2005. Evolution of Suessenguthia (Acanthaceae) inferred from morphology, AFLP data and ITS rDNA sequences. – Organisms Divers. Evol. 5: 1-13.
Schnack BJC, Covas G. 1978. Subgéneros de Glandularia. – Apuntes para la Flora de La Pampa 57: 225-226.
Schnarf K. 1917. Beiträge zur Kenntnis der Samenentwicklung der Labiaten. – Denkschr. Akad. Wien, Math.-Nat. Kl. 94: 211-274.
Schnarf K. 1921. Kleine beiträge zur Entwicklungsgeschichte der Angiospermen II. Klugia zeylanica (R. Brown) Gardn. – Österr. Bot. Zeitschr. 70: 255-261.
Schneeweiss GM. 2013. Phylogenetic relationships and evolutionary trends in Orobanchaceae. – In: Joel DM, Gressel J, Mussleman LJ (eds), Parasitic Orobanchaceae, Springer, Berlin, Heidelberg, pp. 243-265.
Schneeweiss GM, Weiss H. 2003. Polyploidy in Aeginetia indica L. (Orobanchaceae). – Cytologia 68: 15-17.
Schneeweiss GM, Colwell A, Park J-M, Jang C-G, Stuessy TF. 2004. Phylogeny of holoparasitic Orobanche (Orobanchaceae) inferred from nuclear ITS sequences. – Mol. Phylogen. Evol. 30: 465-478.
Schneeweiss GM, Palomeque T, Colwell AE, Weiss-Schneeweiss H. 2004. Chromosome numbers and karyotype evolution in holoparasitic Orobanche (Orobanchaceae) and related genera. – Amer. J. Bot. 91: 439-448.
Schneeweiss GM, Colwell AEL, Park J-M, Jang C-G, Stuessy TF. 2004. Phylogeny of holoparasitic Orobanche (Orobanchaceae) inferred from nuclear ITS sequences. – Mol. Phylogen. Evol. 30: 465-478.
Schneider AC. 2016. Resurrection of the genus Aphyllon for New World broomrapes (Orobanche s.l., Orobanchaceae). – PhytoKeys 75: 107-118.
Schönenberger J. 1999. Floral structure, development and diversity in Thunbergia (Acanthaceae). – Bot. J. Linn. Soc. 130: 1-36.
Schönenberger J, Endress PK. 1998. Structure and development of the flowers in Mendoncia, Pseudocalyx, and Thunbergia (Acanthaceae) and their systematic implications. – Intern. J. Plant Sci. 159: 446-465.
Schotsman HD. 1961. Les Callitriches. Espèces de France et taxa nouveaux d’Europe. – Paris.
Schotsman HD. 1977. Callitriches de la region Méditerranéenne, nouvelle observation. – Bull. Cent. Étud. Rech. Sci., Biarritz 11: 241-312.
Schotsman HD. 1982. Biologie florale des Callitriche: étude sur quelques espèces d’Espagne méridionale. – Adansonia, sér. II, 3-4: 111-160.
Schratz E, Schnelle FJ, Qédan S. 1968. Die Zusammensetzung der ätherischen Öles in der Sammelart Thymus serpyllum. 2. Mitteilung. – Sci. Pharm. 36: 13-21.
Schulte LJ, Clark JL, Novak SJ, Ooi M T-Y,
Smith JF. 2014. Paraphyly of Section Stygnanthe (Columnea, Gesneriaceae) and a revision of
the species of Section Angustiflorae, a new section inferred from ITS
and chloroplast DNA data. – Syst. Bot. 39: 613-636.
Schulte LJ, Clark JL, Novak SJ, Jeffries SK, Smith JF. 2015. Speciation within Columnea section Angustiflora (Gesneriaceae): islands, pollinators and climate. – Mol. Phylogen. Evol. 84: 125-144.
Schultes RE. 1941. A synopsis of the genus Uroskinnera. – Bot. Mus. Leafl. Harvard Univ. 9: 65-83.
Schultze W, Zänglein A, Hose S, Kubeczka KH, Czygan FC. 1992. Volatiles in flowers of balm (Melissa officinalis L.). – In: Harley RM, Reynolds T (eds), Advances in labiate science, Royal Botanic Gardens, Kew, pp. 357-366.
Schumann K. 1891. Rubiaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien IV(4), W. Engelmann, Leipzig, pp. 1-156.
Schumann K. 1895. Bignoniaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien IV(3b), W. Engelmann, Leipzig, pp. 189-252; Schumann K. 1897. Nachträge zu IV(3b), pp. 301-304.
Schwarz O. 1938. Die Gattung Globularia. – Bot. Jahrb. Syst. 69: 318-373.
Schwarzbach AE. 2004. Plantaginaceae. – In: Kubitzki K, Kadereit JW (eds), The families and genera of vascular plants VII. Flowering plants. Dicotyledons. Lamiales (except Acanthaceae including Avicenniaceae), Springer, Berlin, Heidelberg, New York, pp. 327-329.
Schwarzbach AE, McDade LA. 2002. Phylogenetic relationships of the mangrove family Avicenniaceae based on chloroplast and nuclear ribosomal DNA sequences. – Syst. Bot. 27: 84-98.
Schwinn K, Venail J, Shang Y, Macakay S, Alm V, Butelli E, Oyama R, Bailey P, Davies K, Martin C. 2006. The small family of MYB-regulatory genes controls floral pigmentation intensity and patterning in the genus Antirrhinum. – Plant Cell 18: 831-851.
Scog LE. 1976. A study of the tribe Gesnerieae, with a revision of Gesneria (Gesneriaceae: Gesnerioideae). – Smithsonian Contr. Bot. 29: 1-182.
Scogin R. 1980. Anthocyanins of the Bignoniaceae. – Biochem. Syst. Ecol. 8: 273-276.
Scogin R, Romo-Contreras V. 1992. Familial assignment of Polypremum: evidence from phenolic chemistry. – Biochem. Syst. Ecol. 20: 787-788.
Scora RW. 1967. Interspecific relationships in the genus Monarda (Labiatae). – Univ. Calif. Publ. Bot. 41: 1-59.
Scotland RW. 1990. Palynology and systematics of Acanthaceae. – Ph.D. diss., University of Reading, England.
Scotland RW. 1991. A systematic analysis of pollen morphology of Acanthaceae genera with contorted corollas. – In: Blackmore S, Barnes SH (eds), Pollen and spores: patterns of diversification, Clarendon Press, Oxford, pp. 269-289.
Scotland RW. 1992a. Pollen morphology of Andrographideae (Acanthaceae). – Rev. Palaeobot. Palyn. 72: 229-243.
Scotland RW. 1992b. Systematics, similarity and Acanthaceae pollen morphology. – Bot. J. Linn. Soc. 109: 529-541.
Scotland RW. 1992c. Pollen morphology and taxonomic characters in Acanthaceae. – Syst. Bot. 17: 337-340.
Scotland RW. 1993. Pollen morphology of Contortae (Acanthaceae). – Bot. J. Linn. Soc. 111: 471-504.
Scotland RW. 1998. One new and one rediscovered species of Strobilanthes Blume (Acanthaceae). – Bot. J. Linn. Soc. 128: 203-210.
Scotland RW, Vollesen K. 2000. Classification of Acanthaceae. – Kew Bull. 55: 513-589.
Scotland RW, Endress PK, Lawrence TJ. 1994. Corolla ontogeny and aestivation in the Acanthaceae. – Bot. J. Linn. Soc. 114: 49-65.
Scotland RW, Sweere JA, Reeves PA, Olmstead RG. 1995. Higher-level systematics of Acanthaceae determined by chloroplast DNA sequences. – Amer. J. Bot. 82: 266-275.
Scudeller VV. 2000. Proposal to conserve the name Adenocalymma Mart. ex Meisn. (Bignoniaceae) with a conserved spelling. – Taxon 49: 303-304.
Sebald O. 1973. Die Gattung Otostegia Bentham (Labiatae) in Afrika und auf der arabischen Halbinsel. – Stuttg. Beitr. Naturk., Ser. A (Biol.), 263: 1-84.
Sebald O. 1977. Studien an afrikanischen Leucas-Arten (Labiatae) I. Leucas tsavoensis Sebald spec. nov., ein Doppelgänger von Leucas bracteosa Gürke. – Stuttg. Beitr. Naturk., Ser. A (Biol.), 298: 1-12.
Sebald O. 1978. Studien an afrikanischen Leucas-Arten (Labiatae) III. Die Arten der Sektion Lasiocorys (Benth.) Gürke emend. Sebald. – Stuttg. Beitr. Naturk., Ser. A (Biol.), 308: 1-42.
Sebald O. 1980. Die Gattung Leucas R. Brown (Labiatae) in Afrika und auf der Arabischen Halbinsel. – Stuttgarter Beitr. Naturk., Ser. A (Biol.), 341: 1-200.
Seegeler CJP. 1989. Sesamum orientale (Pedaliaceae): Sesame’s correct name. – Taxon 38: 656-658.
Seemann BC. 1862. Ueber neue und verkannte Clerodendron-Arten. – Bonplandia 10: 249-250.
Segarra JG, Mateu I. 2001. Seed morphology of Linaria species from eastern Spain: identification of species and taxonomic implications. – Bot. J. Linn. Soc. 135: 375-389.
Sehr EM, Weber A. 2009. Floral ontogeny of Oleaceae and its systematic implications. – Intern. J. Plant Sci. 170: 845-859.
Seibert RJ. 1948. The use of glands in a taxonomic consideration of the family Bignoniaceae. – Ann. Missouri Bot. Gard. 35: 123-137.
Seine R, Fischer E, Barthlott W. 1995. Notes on the Scrophulariaceae in Zimbabwean inselbergs with description of Lindernia syncerus sp. nov. – Feddes Repert. 106: 7-12.
Seine R, Porembski S, Balduin M, Theisen I, Wilbert N, Barthlott W. 2002. Different prey strategies of terrestrial and aquatic species in the carnivorous genus Utricularia (Lentibulariaceae). – Bot. Jahrb. Syst. 124: 71-76.
Sell PD, Yeo PF. 1970. A revision of the North American species of Euphrasia L. – Bot. J. Linn. Soc. 63: 189-234.
Sell Y. 1969. Les complexes inflorescentiels de quelques Acanthacées. Étude particulière des phénomènes de condensation, de racémisation, d’homogénéisation et de troncature. – Ann. Sci. Nat. Bot. Biol. Vég. 10: 225-350.
Sen N, Sahni V. 1955. Triploid, tetraploid and pentaploid Lantana. – Sci. & Cult. 20: 558-559.
Sendra JM, Canat P. 1980. Volatile phenolic constituents of Spanish origanum (Coridothymus capitatus) essential oil. – Phytochemistry 19: 1513-1517.
Serpooshan F, Jamzad Z, Nejadsattari T, Mehregan I. 2018. Molecular phylogenetics of Hymenocrater and allies (Lamiaceae): new insights from nrITS, plastid trnL intron and trnL-F intergenic spacer DNA sequences. – Nord. J. Bot. 36: e01600
Sérsic AN. 2004. Pollination biology in the genus Calceolaria L. (Calceolariaceae). – Stapfia 82: 1-121.
Sérsic AN, Cocucci AA. 1999. An unusual kind of nectary in the oil flowers of Monttea: its structure and function. – Flora 194: 393-404.
Sérsic AN, Mascó M, Noy-Meir I. 2001. Natural hybridization between species of Calceolaria with different pollination syndromes in southern Patagonia, Argentina. – Plant Syst. Evol. 230: 111-124.
Servettaz O, Bini Maleci L, Pinetti A. 1992. Micromorphological and phytochemical characters of Teucrium marum and T. subspinosum (Labiatae) from Sardinia and Balearic Islands. – Plant Syst. Evol. 179: 129-139.
Seybold S. 1978. Revision der persischen Marrubium-Arten (Labiatae). Vorarbeiten zur Flora Iranica Nr. 20. – Stuttgarter Beitr. Naturk., Ser. A (Biologie) 310.
Seybold S. 1988. Die Arten der Gattung Satureja L. (Labiatae) in Äthiopien. – Stuttgarter Beitr. Naturk., Ser. A (Biologie), 421: 1-38.
Sharma BD, Vishnu-Mittre 1963. Contribution to the pollen morphology of the genera Eranthemum Linn. and Pseuderanthemum Radlkof. – Proc. Natl. Inst. Sci. India, Sect. B, 29: 520-526.
Sharma N, Koul P, Koul AK. 1993. Pollination biology of some species of genus Plantago L. – Bot. J. Linn. Soc. 111: 129-138.
Shashikumar, Paliwal GS. 1975. Foliar anatomy of the family Acanthaceae II. The tribes Thunbergieae and Nelsonieae. – Acta Bot. Ind. 3: 121-131.
Shet MH, Brack-Hanes SD. 1984. Development of spiraperturate pollen in Thunbergia alata. – Pollen Spore 26: 181-186.
Shi S-H, Du Y-Q, Boufford DE, Gong X, Huang Y-L, He H-H, Zong Y. 2003. Phylogenetic position of Schnabelia, a genus endemic to China: evidence from sequences of cpDNA matK gene and nrDNA ITS regions. – Chin. Sci. Bull. 48: 1576-1580.
Shimai H, Masuda Y, Panfet Valdés CM, Kondo K. 2007. Phylogenetic analysis of Cuban Pinguicula (Lentibulariaceae) based on internal transcribed spacer (ITS) region. – Chromosome Bot. 2: 151-158.
Shirke DR. 1976. Contributions to the life-history of Spathodea campanulata Beauv. – Indian Sci. Congr. Assoc. Proc. 63: 88.
Shoyama Y, Matsumoto M, Nishioka I. 1986. Four caffeoyl glycosides from callus tissue of Rehmannia glutinosa. – Phytochemistry 25: 1633-1636.
Siadati S, Salmaki Y, Mehrvarz SS, Heubl G, Weigend M. 2018. Untangling the generic boundaries in tribe Marrubieae (Lamiaceae: Lamioideae) using nuclear and plastid DNA sequences. – Taxon 67: 770-783.
Siddiqui SA. 1975. Studies in the Lentibulariaceae 7. The development of endosperm and embryo in Utricularia coerulea var. filicaulis Clarke. – Bot. Not. 128: 432-437.
Sidwell K. 1998. A revision of Brillantaisia (Acanthaceae). – Bull. Nat. Hist. Mus. London (Bot.) 28: 67-113.
Siegert A, Wagner F. 1994. Zum Bau einiger Bignoniaceen-Dornen unter besonderer Berücksichtigung der Blatt-Dornen von Parmentiera aculeata (H.B.K.) Seem. – Beitr. Biol. Pflanzen 67: 387-437.
Šilić E. 1979. Monografija rodova Satureja L., Calamintha Miller, Micromeria Bentham, Acinos Miller i Clinopodium L. u flori Jugoslavije. – Sarajevo.
Silva LMC, Borges RLB de. 2017. Pollen morphology and ultrastructure of representatives of the Thyrsacanthus clade (Acanthaceae). – Plant Syst. Evol. 303: 1341-1349.
Silva SR, Gibson R, Adamec L, Domínguez Y, Miranda VFO. 2018. Molecular phylogeny of bladderworts: a wide approach of Utricularia (Lentibulariaceae) species relationships based on six plastidial and nuclear DNA sequences. – Mol. Phylogen. Evol. 118: 244-264.
Silveira MA, Simpson MG. 2013. Phylogenetic systematics of the mesa mints: Pogogyne (Lamiaceae). – Syst. Bot. 38: 782-794.
Simmonds MSJ, Blaney WM. 1992. Labiate-insect interactions: effects of labiate-derived substances on insect behaviour. – In: Harley RM, Reynolds T (eds), Advances in labiate science, Royal Botanic Gardens, Kew, pp. 375-392.
Simmonds MSJ, Blaney WM, Esquivel B, Rodriguez-Hahn L. 1996. Effect of clerodane-type diterpenoids isolated from Salvia spp. on the feeding behaviour of Spodoptera littoralis. – Pest. Sci. 47: 17-23.
Simpson BB, Neff JL, Dieringer G. 1990. The production of floral oils by Monttea (Scrophulariaceae) and the function of tarsal pads in Centris bees. – Plant Syst. Evol. 173: 209-222.
Sinclair J. 1956. Two new Malayan species, Justicia johorensis and Petraeovitex wolfei. – Gard. Bull. Straits Settlem. (Singapore) 15: 18-19.
Singh RP, Singh CBP, Kumar J. 1993. Meiotic studies in Hygrophila serpyllum T. Anders. – Proc. Indian Sci. Congr. 80: 147.
Singh SP. 1958. Morphological studies in some members of the family Pedaliacae. – Thesis, Agra University, Agra, India.
Singh SP. 1960a. Morphological studies in some members of the family Pedaliaceae. – Agra Univ. J. Res., Sci. 9: 218-220.
Singh SP. 1960b. Morphological studies in some members of the family Pedaliaceae – Sesamum indicum D.C. – Phytomorphology 10: 65-82.
Singh SP. 1963. Morphological studies in some members of the family Pedaliaceae II. Pedalium murex L. – Agra Univ. J. Res., Sci. 12: 143-161.
Singh SP. 1970. Comparative embryology of angiosperms: Pedaliaceae, Myrtyniaceae. – Bull. Natl. Sci. Acad. India 41: 273-277, 278-281.
Singh TP. 1995. Alterations in the basic chromosome number as a means of speciation in Labiatae. – Feddes Repert. 106: 39-47.
Singh V. 2001. Monograph on Indian Leucas R. Br. (Dronapushpi) Lamiaceae. – Scientific Publ., Jodhpur.
Singh V, Jain DK. 1975a. Trichomes in Acanthaceae I. General structure. – J. Indian Bot. Soc. 54: 116-127.
Singh V, Jain DK. 1975b. Floral development of Justicia gendarussa (Acanthaceae). – Bot. J. Linn. Soc. 70: 243-253.
Singh V, Jain DK. 1978. Floral anatomy and systematic position of Cyrtandromoea – Proc. Indian Acad. Sci., Sect. B, 87: 71-74.
Sirová D, Borovec J, Cerná B, Rejmánková, Adamec L. 2009. Microbial community development in the traps of aquatic Utricularia species. – Aquatic Bot. 90: 129-136.
Sirová D, Borovec J, Santruçková H, Santruçek J, Vrba J, Adamec L. 2010. Utricularia carnivory revisited: plants supply photosynthetic carbon to traps. – J. Experim. Bot. 61: 99-103.
Skelton BW, Ghisalberti EL, White AH. 1997. A new class of tricyclic diterpenes from Eremophila georgei (Myoporaceae). – Aust. J. Chem. 50: 705.
Skog LE. 1974. New Peruvian Gesneriaceae. – Phytologia 28: 233-240.
Skog LE. 1976. A study of the tribe Gesnerieae, with a revision of Gesneria (Gesneriaceae: Gesnerioideae). – Smithsonian Contr. Bot. 29: 1-182.
Skog LE. 1978a. New Panamanian species of Gesneriaceae. – Brittonia 30: 319-326.
Skog LE. 1978b. A look a leaf surfaces of Reldia using scanning electron microscopy. – The Gloxinian 28: 9-12.
Skog LE. 1978 [1979]. Flora of Panama IX. Family 175. Gesneriaceae. – Ann. Missouri Bot. Gard. 65: 783-996.
Skog LE. 1982. New Gesneriaceae from Peru and Ecuador. – Selbyana 7: 94-99.
Skog LE. 1984. A review of the chromosome numbers in the Gesneriaceae. – Selbyana 7: 252-273.
Skog LE, Boggan JK. 2005. The world checklist of Gesneriaceae. – Dept. of Botany, Smithsonian Inst., Washington, DC. http://persoon.si.edu/gesneriaceae/checklist
Skog LE, Boggan JK. 2006. A new classification of the Western Hemisphere Gesneriaceae. – Gesneriads 56: 12-17.
Skog LE, Boggan JK. 2007. The bibliography of Gesneriaceae. – Dept. of Botany, Smithsonian Inst., Washington, DC. http://persoon.si.edu/gesneriaceae/bibliography
Skog LE, Jesus FF de. 1997. A review of Resia (Gesneriaceae). – BioLlania (ed. esp. 6): 515-525.
Skog LE, Kvist LP. 2000. Revision of Gasteranthus (Gesneriaceae). – Syst. Bot. Monogr. 59: 1-118.
Skottsberg C. 1912. Tetrachondra patagonica n. sp. und die systematische Stellung der Gattung. – Engl. Bot. Jahrb. Syst., Beibl. 107: 17-26.
Skutch AF. 1992. Tussacia friedrichsthaliana, a terrestrial herb with aquatic flowers. – Brenesia 37: 151-156.
Skutch F. 1928. The capture of prey by the bladderwort. – New Phytol. 27: 261-297.
Slavkovska V, Couladis M, Bojovic S, Tzakou O, Pavlovic M, Lakusic B, Jancic R. 2005. Essential oil and its systematic significance in species of Micromeria Bentham from Serbia & Montenegro. – Plant Syst. Evol. 255: 1-15.
Sleesen EHL. 1959. Revision of Malaysian Orthosiphon (Lab.). – Reinwardtia 5: 37-43.
Smiley CJ. 1961. A record of Paulownia in the Tertiary of North America. – Amer. J. Bot. 48: 175-179.
Smith JF. 1991. The evolution and systematics of Columnea sections Pentadenia and Stygnanthe (Gesneriaceae). – Ph.D. diss., University of Wisconsin, Madison, Wisconsin.
Smith JF. 1994. Systematics of Columnea section Pentadenia and section Stygnanthe (Gesneriaceae). – Syst. Bot. Monogr. 44: 1-89.
Smith JF. 1996. Tribal relationships within Gesneriaceae: a cladistic analysis of morphological data. – Syst. Bot. 21: 497-513.
Smith JF. 2000a. Phylogenetic resolution within the tribe Episcieae (Gesneriaceae): congruence of ITS and ndhF sequences from parsimony and maximum-likelihood analyses. – Amer. J. Bot. 87: 883-897.
Smith JF. 2000b. A phylogenetic analysis of tribes Beslerieae and Napeantheae (Gesneriaceae) and evolution of fruit types: parsimony and maximum likelihood analyses of ndhF sequences. – Syst. Bot. 25: 72-81.
Smith JF. 2000c. Phylogenetic signal common to three data sets: combining data which initially appear heterogeneous. – Plant Syst. Evol. 221: 179-198.
Smith JF. 2001. The phylogenetic relationships of Lembocarpus and Goyazia (Gesneriaceae) based on ndhF sequences. – Ann. Missouri Bot. Gard. 88: 135-143.
Smith JF, Atkinson S. 1998. Phylogenetic analysis of the tribes Gloxinieae and Gesnerieae (Gesneriaceae): data from ndhF sequences. – Selbyana 19: 122-131.
Smith JF, Carroll CL. 1997. A cladistic analysis of the tribe Episcieae (Gesneriaceae) based on ndhF sequences: origin of morphological characters. – Syst. Bot. 22: 713-725.
Smith JF, Clark JL. 2013. Molecular phylogenetic analyses reveal undiscovered monospecific genera in the tribe Episcieae (Gesneriaceae). – Syst. Bot. 38: 451-463.
Smith JF, Sytsma KJ. 1994a. Molecules and morphology: congruence of data in Columnea (Gesneriaceae). – Plant Syst. Evol. 193: 37-52.
Smith JF, Sytsma KJ. 1994b. Evolution in the Andean epiphytic genus Columnea (Gesneriaceae) I. Morphological variation. – Syst. Bot. 19: 220-235.
Smith JF, Sytsma KJ. 1994c. Evolution in the Andean epiphytic genus Columnea (Gesneriaceae) II. Chloroplast DNA restriction site variation. – Syst. Bot. 19: 317-336.
Smith JF, Sytsma KJ, Shoemaker JS, Smith RL. 1992. A qualitative comparison of total cellular DNA extraction protocols. – Phytochem. Bull. 23: 2-9.
Smith JF, Burke CC, Wagner WL. 1996. Interspecific hybridization in natural populations of Cyrtandra (Gesneriaceae) on the Hawaiian Islands: evidence from RAPD markers. – Plant Syst. Evol. 200: 61-77.
Smith JF, Wolfram JC, Brown KD, Carroll CL, Denton DS. 1997. Tribal relationships in the Gesneriaceae: evidence from DNA sequences of the chloroplast gene ndhF. – Ann. Missouri Bot. Gard. 84: 50-66.
Smith JF, Brown KD, Carroll CL, Denton DS. 1997. Familial placement of Cyrtandromoea, Titanotrichum and Sanango, three problematic genera of the Lamiales. – Taxon 46: 65-74.
Smith JF, Kresge ME, Möller M, Cronk QCB. 1998a. A cladistic analysis of ndhF sequences from representative species of Saintpaulia and Streptocarpus sections Streptocarpus and Streptocarpella (Gesneriaceae). – Edinburgh J. Bot. 55: 1-11.
Smith JF, Hileman LC, Powell MP, Baum DA. 2004. Evolution of GCYC, a Gesneriaceae homolog of CYCLOIDEA, within Gesnerioideae (Gesneriaceae). – Mol. Phylogen. Evol. 31: 765-779.
Smith JF, Draper SB, Hileman LC, Baum DA. 2004. A phylogenetic analysis within tribes Gloxinieae and Gesnerieae (Gesnerioideae: Gesneriaceae). – Syst. Bot. 29: 947-958.
Smith JF, Funke MM, Woo VL. 2006. A duplication of gcyc predates divergence within tribe Coronanthereae (Gesneriaceae): phylogenetic analysis and evolution. – Plant Syst. Evol. 261: 245-256.
Smith VA. 1973. A revision of the genus Kickxia with particular reference to the section Heterophyllae in the Canary Islands. – M.Sc. thesis, University of Reading, United Kingdom.
Snogerup B. 1977. Chromosome numbers of Scandinavian Odontites species. – Bot. Not. 130: 121-124.
Snogerup B. 1983. Northwest European taxa of Odontites (Scrophulariaceae). – Acta Bot. Fenn. 124.
Sohma K. 1975. Pollen morphology of the Japanese species of Utricularia L. and Pinguicula L. with notes on fossil pollen of Utricularia from Japan. – J. Jap. Bot. 50: 164-179, 193-210.
Sokolovskaya AP, Probatova NS, Rudyka EG. 1986. A contribution to the study of chromosome numbers and geographical distribution of some species of the family Lamiaceae in the Soviet Far East. – Bot. Žurn. 71: 195-200. [In Russian]
Solereder H. 1893. Ein Beitrag zur anatomischen Charakteristik und zur Systematik der Rubiaceae. – Bull. Herb. Boissier 1: 167-183.
Solereder H. 1895. Loganiaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien IV(2), W. Engelmann, Leipzig, pp. 19-50.
Solereder H. 1909. Über die Gattung Rehmannia. – Ber. Deutsch. Bot. Ges. 27: 390-404.
Solereder H. 1915. Über die Versetzung der Gattung Heteranthia von den Scrophulariaceen zu den Solanaceen. – Beih. Bot. Zentralbl. 23: 113-117.
Sontag S, Weber A. 1998. Seed coat structure of Didissandra, Ridleyandra and Raphiocarpus (Gesneriaceae). – Beitr. Biol. Pflanzen 70: 179-190.
Souèges R. 1921. Embryogénie des Scrofulariacées. Développement de l’embryon chez le Veronica arvensis L. – Compt. Rend. Acad. Sci. Paris 172: 703-705.
Sousa-Baena MS, Sinha NR, Lohmann LG. 2014.
Evolution and development of tendrils in Bignonieae (Lamiales, Bignoniaceae). – Ann. Missouri
Bot. Gard. 99: 323-347.
Souza VC, De Paula-Souza J. 2005. A new species of Achetaria (Plantaginaceae) from south-eastern Brazil. – Bot. J. Linn. Soc. 148: 73-75.
Spangler RE, Olmstead RG. 1999. Phylogenetic analysis of Bignoniaceae based on the cpDNA gene sequences rbcL and ndhF. – Ann. Missouri Bot. Gard. 86: 33-46.
Speta F. 1972. Über Eiweikörper in Zellkernen bei Scrophulariaceen; Vorkommen, Form und systematische Bindung. – Österr. Bot. Zeitschr. 120: 117-136.
Speta F. 1979. Weitere Untersuchungen über Proteinkörper in Zellkernen und ihre taxonomische Bedeutung. – Plant Syst. Evol. 132: 1-26.
Speta F. 1982. Drei neue Antirrhineen-Gattungen aus dem Orient: Holzneria, Huebelia und Albraunia (Scrophulariaceae). – Bot. Jahrb. Syst. 103: 9-45.
Speta F, Fuchs F. 1982. Neue Pinguicula-Arten (Lentibulariaceae) aus Mexiko. – Stapfia 10: 111-119.
Speta F, Geilhuber J. 1970. Über das gleichzeitige Vorkommen von zweierlei Eiweisskörpern in den Zellkernen von Pseudolysimachion spicatum und einigen anderen Scrophulariaceen. – Österr. Bot. Zeitschr. 118: 1-16.
Spies JJ. 1984. A cytotaxonomic study of Lantana camara (Verbenaceae) from South Africa. – South Afr. J. Bot. 3: 231-250.
Spies JJ, Stirton CH. 1982. Meiotic studies of some South African cultivars of Lantana camara (Verbenaceae). – Bothalia 14: 101-111.
Spira TP. 1980. Floral parameters, breeding system and pollinator type in Trichostema (Labiatae). – Amer. J. Bot. 67: 278-284.
Sreemadhavan CP. 1964. Bremekampia – a new generic name. – Bull. Bot. Surv. India 6: 323-324.
Sreemadhavan CP. 1967. Neesiella – a new genus of Acanthaceae. – Phytologia 15: 270-271.
Sreemadhavan CP. 1968. Indoneesiella – a substitute name in Acanthaceae. – Phytologia 16: 466.
Sreemadhavan CP. 1976. Leaf architecture and systematics of Acanthaceae and related families. – Ph.D. diss., University of South Florida, Tampa, Florida.
Sreemadhavan CP. 1977. Diagnosis of some new taxa and some new combinations in Bignoniales. – Phytologia 37: 413-416.
Srinath KV. 1939. Morphological and cytological studies in the genus Calceolaria 4. Somatic chromosomes. – Zeitschr. Indukt. Abstamm. Vererb. 79: 104-134.
Srinath KV. 1940a. Morphological studies in the genera Calceolaria and Herpestis. – Proc. Linn. Soc. London 152: 151-174.
Srinath KV. 1940b. Morphological and cytological studies in the genus Calceolaria 2. Meiosis in diploid and aneuploid Calceolarias. – Cytologia 10: 467-491.
Srinivasan VK. 1940. Morphological and cytological studies in the Scrophulariaceae II. Floral morphology and embryology of Angelonia grandiflora C. Morr. and related genera. – J. Indian Bot. Soc. 20: 197.
Staden J van. 1973. Changes in endogenous cytokinin levels during abscission and senescence of Streptocarpus leaves. – J. Exp. Bot. 24: 667-673.
Ståhl B. 1991. 156. Oleaceae. – In: Harling G, Andersson L (eds), Flora of Ecuador 43, Nord. J. Bot., Copenhagen, pp. 47-57.
Stant MY. 1952. Anatomical evidence for including Nyctanthes and Dimetra in the Verbenaceae. – Kew Bull. 7: 273-276.
Stapf O. 1895a. Pedaliaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien IV(3b), W. Engelmann, Leipzig, pp. 253-265.
Stapf O. 1895b. Martyniaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien IV(3b), W. Engelmann, Leipzig, pp. 265-269.
Stapf O. 1901. Plate 2685. Cyclocheilon minutibracteolatum. – In: Hooker WJ (ed), Icones plantarum, Longman, Rees, Orme, Brown, Green & Longman, London.
Stapf O. 1929. Selago serrata. – Curtis’s Bot. Mag. 155: t. 9265.
Steane DA, Mabberley DJ. 1998. Rotheca (Lamiaceae) revived. – Novon 8: 204-206.
Steane DA, Scotland RW, Mabberley DJ, Wagstaff SJ, Reeves PA, Olmstead RG. 1997. Phylogenetic relationships of Clerodendrum s. l. (Lamiaceae) inferred from chloroplast DNA. – Syst. Bot. 22: 229-243.
Steane DA, Scotland RW, Mabberley DJ, Olmstead RG. 1999. Molecular systematics of Clerodendrum (Lamiaceae): ITS sequences and total evidence. – Amer. J. Bot. 86: 98-107.
Steane DA, Kok RPJ de, Olmstead RG. 2004. Phylogenetic relationships between Clerodendron (Lamiaceae) and other ajugoid genera inferred from nuclear and chloroplast DNA sequence data. – Mol. Phylogen. Evol. 32: 39-45.
Stearn WT. 1969. The Jamaican species of Columnea and Alloplectus (Gesneriaceae). – Bull. Brit. Mus. (Nat. Hist.), Bot. 4: 179-236.
Stearn WT. 1971a. A survey of the tropical genera Oplonia and Psilanthele (Acanthaceae). – Bull. Brit. Mus. (Nat. Hist.), Bot. 4: 259-323.
Stearn WT. 1971b. Notes on Jamaican plants: Acanthaceae. – J. Arnold Arbor. 52: 636-647.
Stearn WT. 1977. Union of Chionanthus and Linociera (Oleaceae). – Ann. Missouri Bot. Gard. 63: 355-357.
Stearn WT. 1979. Three new West Indian species of Chionanthus (Oleaceae). – Bot. Not. 132: 57-60.
Stearn WT. 1980. African species of Chionanthus L. (Oleaceae) hitherto included in Linociera Swartz. – Bot. J. Linn. Soc. 80: 191-206.
Steenis CGGJ van. 1949. Notes on the genus Wightia (Scrophulariaceae). – Bull. Bot. Gard. Buitenzorg, sér. III, 18: 213-227.
Steenis CGGJ van. 1955. Reduction of the genera Schizopremna Baill. (Verb.), and Worcesterianthus Merr. (Olacac.). – Acta Bot. Neerl. 4: 477-480.
Steenis CGGJ van. 1971. Byblidaceae. – Flora Malesiana Bull. Suppl., ser. I, 7: 135-137.
Steenis CGGJ van. 1977. Bignoniaceae. – In: Steenis CGGJ van (ed), Flora Malesiana I, 8(2), Sijthoff & Noordhoff International Publ., Alphen aan den Rijn, The Netherlands, pp. 114-186.
Steiger J, Casper SJ. 2001. A new Pinguicula (Lentibulariaceae) from the pre-alpine region of northern Italy (Friul-Venezia Giulia): Pinguicula poldinii Steiger & Casper spec. nov. – Wulfenia 8: 27-37.
Steiner KE. 1985. The role of nectar and oil in the pollination of Drymonia serrulata by Epicharis bees (Anthophoridae) in Panama. – Biotropica 17: 217-229.
Steiner KE. 1990. The Diascia (Scrophulariaceae) window: an orientation cue for oil-collecting bees. – Bot. J. Linn. Soc. 102: 175-195.
Steiner KE. 1992. A new Diascia species from the Richtersweld, South Africa. – South Afr. J. Bot. 58: 36-38.
Steiner KE. 1996. Chromosome numbers and relationships in tribe Hemimerideae (Scrophulariaceae). – Syst. Bot. 21: 63-76.
Steiner KE, Whitehead VB. 1990. Pollinator adaptation to oil-secreting flowers – Rediviva and Diascia. – Evolution 44: 1701-1707.
Steiner KE, Whitehead VB. 2002. Oil secretion and the pollination of Colpias mollis (Scrophulariaceae). – Plant Syst. Evol. 235: 53-66.
Stenzel E, Heni J, Rimpler H, Vogellehner D. 1988. Phenetic relationships in Clerodendrum (Verbenaceae) and some phylogenetic considerations. – Plant Syst. Evol. 159: 257-271.
Stephenson AG, Thomas WW. 1977. Diurnal and nocturnal pollination of Catalpa speciosa (Bignoniaceae). – Syst. Bot. 2: 191-198.
Stevanovic B, Glisic O. 1997. Eco-anatomical differences between Balkan endemo-relict species of Gesneriaceae. – Bocconea 5: 661-665.
Steyermark JA. 1975. Novedades Venezolanas. Scrophulariaceae. – Acta Bot. Venezuelica 10: 243-246.
Steyermark JA. 1978. The Calceolaria palustris complex in Venezuela. – Pittieria 7: 25-30.
Stibal EP. 1934. Labiatae – Salvia L. (nebst Revision der chinesischen und ostbirmanischen Arten der Gattung). – Acta Horti Gothoburg. 9: 101-145.
Stibal EP. 1935a. Revision der indischen und tibetanischen Arten der Gattung Salvia. – Feddes Repert. 39: 173-186.
Stibal E. 1935b. Revision der Gruppe der Salvia japonica. – Acta Horti Gothoburg. 10: 55-69.
St. John H. 1966. Monograph of Cyrtandra (Gesneriaceae) on Oahu, Hawaiian Islands. – Bull. Bernice P. Bishop Mus. 229: 1-465.
Stöckigt J, Srocka U, Zenk MH. 1973. Structure and biosynthesis of a new anthraquinone from Streptocarpus dunnii. – Phytochemistry 12: 2389-2391.
Stopp K. 1958a. Die kongolesischen Arten der Labiaten-Gattung Aeolanthus mit zygomorph dehiszierenden Fruchtkelchen. – Beitr. Biol. Pflanzen 34: 395-399.
Stopp K. 1958b. Notiz über die Dehiszenzweise der Kapselfrüchte von Genlisea hispidula Stapf. – Beitr. Biol. Pflanzen 34: 401-403.
Stopp K. 1958c. Über einige kongolesische Aeolanthus-arten. – J. Bot. État Bruxelles 28: 307-314.
Storey WB. 1966. Chromosome numbers in Cyrtandra. – In: St. John H (ed), Monograph of Cyrtandra (Gesneriaceae) on Oahu, Hawaiian Islands, Bull. Bernice P. Bishop Mus. 229: 31-33.
Strachan JL. 1982. A revision of the Salvia dorrii complex (Lamiaceae). – Brittonia 34: 151-169.
Straka H, Ihlenfeldt H-D. 1965. Pollenmorphologie und Systematik der Pedaliaceae R. Br. – Beitr. Biol. Pflanzen 41: 175-207.
Straw RM. 1966. A redefinition of Penstemon (Scrophulariaceae). – Brittonia 18: 80-95.
Straw RM. 1967. Keckiella: new name for Keckia Straw (Scrophulariaceae). – Brittonia 19: 203-204.
Strid A. 1965. Studies in the Aegean flora VI. Notes on some genera of Labiatae. – Bot. Not. 118: 104-122.
Stroh G. 1942. Die Gattung Veronica L. Versuch einer systematischen Kodifizierung der Arten (mit Ausnahme der endemischen Arten von Neuseeland). – Beih. Bot. Centralbl., Abt. B, 61: 348-451.
Struwe L, Jensen SR. 2004. Plocospermataceae. – In: Kubitzki K, Kadereit JW (eds), The families and genera of vascular plants VII. Flowering plants. Dicotyledons. Lamiales (except Acanthaceae including Avicenniaceae), Springer, Berlin, Heidelberg, New York, pp. 330-332.
Studnička M. 1985. Pinguicula rotundiflora – new species from Mexico. – Folia Geobot. Phytotaxon. 20: 201-204.
Su B-N, Zhu Q-X, Gao K, Yuan C-S, Jia Z-J. 1999. Lignan and phenylpropanoid glycosides from Lancea tibetica and their antitumor activity. – Planta Medica 65: 558-561.
Su S-W. 1985. A study on the two types of flowers in Schnabelia oligophylla Handel-Mazzetti. – Bull. Bot. Res. 5: 109-120.
Subba Reddi C, Rama Das K, Raju AJS, Atluri JB. 1996. Sexual system and pollination ecology of Gmelina asiatica L. (Verbenaceae). – J. Palynol. 32: 41-50.
Subbramanian D, Pondmudi R. 1987. Cytotaxonomical studies of South Indian Scrophulariaceae. – Cytologia 52: 529-541.
Subramanyam K, Narayana LL. 1978. Studies in Indian Utricularia L.: floral anatomy of Utricularia aurea Lour., U. exoleta R. Br., U. stellaris Linn. f. and U. striatula Sm. – J. Indian Bot. Soc. 57: 244-252.
Subramanian RB, Inamdar JA. 1989. The structure, secretion and biology of nectaries in Tecomaria capensis Thunb. (Bignoniaceae). – Phytomorphology 39: 69-74.
Subramanian SS, Nair AGR. 1972. Flavonoids of the leaves of Oroxylum indicum and Pajanelia longifolia. – Phytochemistry 11: 439-440.
Subramanian SS, Nagarajan A, Sulochana N. 1972a. Chrysin-7-rutinoside from the leaves of Dolichandrone falcata. – Phytochemistry 11: 438-439.
Subramanian SS, Nagarajan A, Sulochana N. 1972b. Flavonoids of eight bignoniaceous plants. – Phytochemistry 11: 1499.
Suddee S. 2001. A taxonomic revision of tribe Ocimeae Dumort. (Labiatae) in continental South East Asia. – Ph.D. diss., Trinity College, Dublin, Ireland.
Suddee S, Paton AJ. 2009. A revision of Anisochilus Wall. ex Benth. (Lamiaceae). – Kew Bull. 64: 235-257.
Suddee S, Paton AJ, Parnell JAN. 2004a. A taxonomic revision of tribe Ocimeae Dumort. (Lamiaceae) in continental South East Asia I. Introduction, Hyptidinae & Hanceolinae. – Kew Bull. 59: 337-378.
Suddee S, Paton AJ, Parnell JAN. 2004b. A taxonomic revision of tribe Ocimeae Dumort. (Lamiaceae) in continental South East Asia II. Plectranthinae. – Kew Bull. 59: 379-414.
Suddee S, Paton AJ, Parnell JAN. 2005. Taxonomic revision of tribe Ocimeae Dumort. (Lamiaceae) in continental South East Asia III. Ociminae. – Kew Bull. 60: 3-75.
Suhua S, Yaqing D, Boufford DE, Xun G, Yelin H, Hanghang H, Yang Z. 2003. Phylogenetic position of Schnabelia, a genus endemic to China: evidence from sequences of cpDNA matK gene and nrDNA ITS regions. – Chin. Sci. Bull. 48: 1576-1580.
Sun BY, Stuessy TF, Humana AM, Riveros G, Crawford DJ. 1996. Evolution of Rhaphithamnus venustus (Verbenaceae), a gynodioecious hummingbird-pollinated endemic of the Juan Fernandez Islands, Chile. – Pacific Sci. 50: 55-65.
Surina B, Pfanzelt S, Einzmann HJR, Albach
DC. 2014. Bridging the Alps and the Middle East: evolution, phylogeny and
systematics of the genus Wulfenia (Plantaginaceae). – Taxon 63:
843-858.
Suryakanta. 1973. Pollen morphological studies in the Bignoniaceae. – J. Palynol. 9: 45-82.
Sutton DA. 1987. Peloric flowers in the tribe Antirrhineae (Scrophulariaceae). – Watsonia 16: 337-338.
Sutton DA. 1988. A revision of the tribe Antirrhineae. – British Museum (Natural History), London, Oxford University Press, Oxford.
Svensson H. 1928. Zur Entwicklungsgeschichte der Blüten und Samen von Limosella aquatica L. – Svensk Bot. Tidskr. 22: 465-476.
Svoma E, Kiehn M. 1993. Beiträge zur Embryologie der Gattung Cyrtandra (Gesneriaceae). – In: Heiselmayer P (ed), Österr. Botanikertreffen, Kurzf. Beitr., 50, Universität Salzburg.
Swamy BGL, Padmanabhan D. 1961. The quadrant proembryo of Epithema carnosum. – Proc. Indian Acad. Sci. B, 63: 4.
Takano A. 2017. Taxonomic study on Japanese Salvia (Lamiaceae): phylogenetic position of S. akiensis, and polyphyletic nature of S. lutescens var. intermedia. – PhytoKeys 80: 87-104.
Takano A, Okada H. 2011. Phylogenetic relationships among subgenera, species, and varieties of Japanese Salvia L. (Lamiaceae). – J. Plant Res. 124: 245-252.
Tammaro F, Pace L. 1987. Il genere Pinguicula L. (Lentibulariaceae) in Italia centrale ed instituzione di una nuova specie P. fiorii Tamm. et Pace. – Inf. Bot. Ital. 19: 429-436.
Tanaka H. 1973. Moth pollination of Clerodendron trichotomum. – J. Jap. Bot. 48: 209-214.
Tange C. 1998. Silvianthus (Carlemanniaceae) a genus and family new to Thailand. – Thai Forest Bull. 26: 59-65.
Tangmitcharven S, Owens JN. 1997. Floral biology, pollination, pistil receptivity and pollen tube growth of teak (Tectona grandis L. f.). – Ann. Bot. 79: 227-241.
Taniguchi E, Oshima Y. 1972a. Phrymarolin-I, a novel lignan from Phryma leptostachya L. – Agric. Biol. Chem. 36: 1013-1025.
Taniguchi E, Oshima Y. 1972b. New gmelinol-type lignan, leptostachyol acetate. – Tetrahedron Lett. 8: 653-656.
Taniguchi E, Imamura K, Ishibashi F, Matsui T, Nishio A. 1989. Structure of the novel insecticidal sesquilignan, Haedoxan A. – Agric. Biol. Chem. 53: 631-643.
Tank DC, Olmstead RG. 2008. From annuals to perennials: phylogeny of subtribe Castillejinae (Orobanchaceae). – Amer. J. Bot. 95: 608-625.
Tank DC, Olmstead RG. 2009. The evolutionary origin of a second radiation of annual Castilleja (Orobanchaceae) species in South America: the role of long distance dispersal and allopolyploidy. – Amer. J. Bot. 96: 1907-1921.
Tank DC, Beardsley PM, Kelchner SA, Olmstead RG. 2006. L. A. S. Johnson review 7. Review of the systematics of Scrophulariaceae s.l. and their current disposition. – Aust. Syst. Bot. 19: 289-307.
Tank DC, Egger JM, Olmstead RG. 2009. Phylogenetic classification of subtribe Castillejinae (Orobanchaceae). – Syst. Bot. 34: 182-197.
Taskova RM, Peev D, Handjieva N. 2002. Iridoid glucosides of the genus Veronica s. l. and their systematic significance. – Plant Syst. Evol. 231: 1-17.
Taskova RM, Evstatieva L, Handjieva N, Popov S. 2002. Iridoid patterns of genus Plantago L. and their systematic significance. – Zeitschr. Naturforsch. 57: 42-50.
Taskova RM, Albach DC, Grayer RJ. 2004. Phylogeny of Veronica: a combination of molecular and chemical evidence. – Plant Biol. 6: 673-682.
Taskova RM, Gotfredsen CH, Jensen SR. 2005. Chemotaxonomy markers in Digitalideae (Plantaginaceae). – Phytochemistry 66: 1440-1447.
Taskova RM, Gotfredsen CH, Jensen SR. 2006. Chemotaxonomy of Veroniceae and its allies in the Plantaginaceae. – Phytochemistry 67: 286-301.
Tatachar T. 1940. The development of the embryo sac and formation of haustoria in Lantana indica Roxb. and Stachytarpheta indica Vahl. – J. Indian Bot. Soc. 19: 45-52.
Tate P. 1925. On the anatomy of Orobanche hederae Duby, and its attachment to the host. – New Phytol. 24: 284-293.
Tay ML, Meudt HM, Garnock-Jones PJ, Ritchie PA. 2010. DNA sequences from three genomes reveal multiple long-distance dispersals and non-monophyly of sections of Australasian Plantago (Plantaginaceae). – Aust. Syst. Bot. 23: 47-68.
Taylor G. 1933. The genus Poskea Vatke. – J. Bot. 71: 310-312.
Taylor G. 1936. Notes on Labiatae III. Ceratanthus, a collection of certain species allied to Plectranthus. – J. Bot. 74: 33-41.
Taylor H. 1945. Cyto-taxonomy and phylogeny of the Oleaceae. – Brittonia 5: 337-367.
Taylor P. 1964. The genus Utricularia L. (Lentibulariaceae) in Africa (south of the Sahara) and Madagascar. – Kew Bull. 18: 1-245.
Taylor P. 1973. Lentibulariaceae. – In: Polhill RM (ed), Flora of tropical East Africa, Crown Agents for Oversea Governments and Administrations, London, pp. 1-27.
Taylor P. 1975. Lentibulariaceae. – In: Harling G, Sparre B (eds), Flora of Ecuador 182, Swedish Natural Science Research Council, Stockholm, pp. 9-21.
Taylor P. 1977. Lentibulariaceae. – In: Steenis CGGJ van (ed), Flora Malesiana I, 8(2), Sijthoff & Noordhoff International Publ., Alphen aan den Rijn, The Netherlands, pp. 275-300.
Taylor P. 1986. New taxa in Utricularia (Lentibulariaceae). – Kew Bull. 41: 1-18.
Taylor P. 1989. The genus Utricularia – a taxonomic monograph. – Kew Bull. Add. Ser. XIV: I-XI, 1-724.
Taylor P. 1991. The genus Genlisea St. Hil. – Carniv. Plant Newsl. 20: 20-35.
Taylor P, Souza VG, Giulietti AM, Harley RM. 2000. Philcoxia: a new genus of Scrophulariaceae with three new species from Eastern Brazil. – Kew Bull. 55: 155-163.
Terao H. 1983. Taxonomic study of the genus Strobilanthes Bl. (Acanthaceae). Generic delimitation and infrageneric classification. – Ph.D. diss., Kyoto University, Kyoto, Japan.
Terekhin ES, Nikiticheva ZI. 1981. The family Orobanchaceae: ontogeny and phylogeny. – NAUKA, Leningrad. [In Russian]
Těšitel J, Říha O, Svobodová Š, Malinová T, Štech M. 2010. Phylogeny, life history evolution and biogeography of the rhinanthoid Orobanchaceae. – Folia Geobot. 45: 347-367.
Thaler I. 1951. Morphologisches über Veronica filiformis und ihre Verwandten. – Phyton 3: 216-226.
Thalouarn P, Theodet C, Russo N, Delavault P. 1994. The reduced plastid genome of a non-photosynthetic angiosperm Orobanche hederae has retained the rbcL gene. – Plant Physiol. Biochem. 32: 233-242.
Thanikaimoni G. 1966. Pollen morphology of the genus Utricularia. – Pollen Spores 8: 265-284.
Thathachar T. 1942. Studies in Gesneriaceae. Gametogenesis and embryogeny of Didymocarpus tomentosa Wt. – J. Indian Bot. Soc. 21: 185-193.
Thathachar T. 1943. Studies in Gesneriaceae. Development of seed in Rhynchoglossum obliquum Bl. – J. Indian Bot. Soc. 22: 51-57.
Theisen I, Fischer E. 2004. Myoporaceae. – In: Kubitzki K, Kadereit JW (eds), The families and genera of vascular plants VII. Flowering plants. Dicotyledons. Lamiales (except Acanthaceae including Avicenniaceae), Springer, Berlin, Heidelberg, New York, pp. 289-292.
Thieret JW. 1954. The tribes and genera of Central American Scrophulariaceae. – Ceiba 4: 164-184.
Thieret JW. 1955. The seeds of Veronica and allied genera. – Lloydia 18: 37-45.
Thieret JW. 1967. Supraspecific classification in the Scrophulariaceae: a review. – Sida 3: 87-106.
Thieret JW. 1972. Synopsis of Hemichaena, including Berendtiella (Scrophulariaceae). – Fieldiana Bot. 34: 89-99.
Thieret JW. 1976. Floral biology of Proboscidea louisianica (Martyniaceae). – Rhodora 78: 169-179.
Thiv M. 2004. Carlemanniaceae. – In: Kubitzki K, Kadereit JW (eds), The families and genera of vascular plants VII. Flowering plants. Dicotyledons. Lamiales (except Acanthaceae including Avicenniaceae), Springer, Berlin, Heidelberg, New York, pp. 57-59.
Thiv M, Thulin M, Hjertson M, Kropf M, Linder HP. 2010. Evidence for a vicariant origin of Macaronesian-Eriteo/Arabian disjunctions in Campylanthus Roth (Plantaginaceae). – Mol. Phylogenet. Evol. 54: 607-616.
Thode VA, Mentz LA. 2010. O gênero Glandularia J. F. Gmel. (Verbenaceae) no Rio Grande do Sul, Brasil. – Acta Bot. Brasilica 24: 529-557.
Thode VA, O’Leary N, Olmstead RG, Freitas LB. 2013. Phylogenetic position of the monotypic genus Verbenoxylum (Verbenaceae) and new combination under Recordia. – Syst. Bot. 38: 805-817.
Thomas V, Dave Y. 1992. Structure and biology of nectaries in Tabebuia serratifolia Nichols (Bignoniaceae). – Bot. J. Linn. Soc. 109: 395-400.
Thompson DM. 1988. Systematics of Antirrhinum (Scrophulariaceae) in the New Wold. – Syst. Bot. Monogr. 22.
Thompson DM. 1997. The genus Mimulus. – In: O’Brien BC, Fuentes LC, Newcombe LF (ed), Rancho Santa Ana Bot. Gard. Occas. Publ. No. 1, Symposium Proceedings, Rancho Santa Ana Botanic Garden, Claremont, California, pp. 112-125.
Thompson DM. 2005. Systematics of Mimulus subg. Schizoplacus (Scrophulariaceae). – Syst. Bot. Monogr. 75.
Thor G. 1988. The genus Utricularia in the Nordic countries, with special emphasis on U. stygia and U. ochroleuca. – Nord. J. Bot. 8: 213-225.
Thulin M. 1987. A new species of Xylocalyx (Scrophulariaceae) from Somalia. – Nord. J. Bot. 7: 267-269.
Thulin M. 1993. Salvia (Labiatae) in the mountains of northern Somalia. – Opera Bot. 121: 145-148.
Thulin M. 1995. A new species of Campylanthus (Scrophulariaceae) from Yemen. – Nord. J. Bot. 15: 191-192.
Thulin M. 2007. Synopsis of Satanocrater (Acanthaceae). – Nord. J. Bot. 24: 385-388.
Tiagi B. 1956. A contribution to the embryology of Striga orobanchoides Benth. and S. euphrasiodes Benth. – Bull. Torrey Bot. Club 83: 154-170.
Tiagi B. 1963. Studies in the family Orobanchaceae IV. Embryology of Boschniakia himalaica Hook. and B. tuberosa (Hook.) Jepson, with remarks on the evolution of the family. – Bot. Not. 116: 81-93.
Tiagi B. 1965. Development of the seed and fruit in Melampyrum nemorosum L. and M. arvense L. – Can. J. Bot. 43: 1511-1521.
Tiagi B. 1966. Development of the seed and fruit in Rhinanthus major and R. serotinus. – Amer. J. Bot. 53: 645-651.
Tieghem P van. 1898. Avicenniacées et Symphorémacées: place de ces deux nouvelles familles dans la classification. – J. Bot. (Morot) 12: 345-352.
Tieghem P van. 1903. Structure de l’étamine chez les Scrofulariacées. – Ann. Sci. Nat. 17: 363-371.
Tieghem P van. 1907. Structure du pistil et du fruit des Labiées, des Boragacées et des familles voisines. – Ann. Sci. Nat., sér. IX, 5: 321-350.
Tieghem P van. 1908a. Structure du pistil et de l’ovule, du fruite et de la graine des Acanthacées. Dédoublement de cette famille. – Ann. Sci. Nat., sér. IX, Bot. 7: 1-24.
Tieghem P van. 1908b. Restauration du genre Hexacentris dans la famille nouvelle des Thunbergiacées. –Ann. Sci. Nat., sér. IX, Bot. 7: 111-116.
Tkach N, Ree RH, Kuss P, Röser M, Hoffmann
MH. 2014. High mountain origin, phylogenetics, evolution, and niche
conservatism of arctic lineages in the hemiparasitic genus Pedicularis
(Orobanchaceae). – Molec.
Phylogen. Evol. 76: 75-92.
Tolmatchew A. 1923. Labiatae Riedelianae. – Not. Syst. Herb. Horti Bot. Petropolitani 4: 62-64.
Tomás-Barberán FA, Gil MI. 1992. Chemistry and natural distribution of flavonoids in the Labiatae. – In: Harley RM, Reynolds T (eds), Advances in labiate science, Royal Botanic Gardens, Kew, pp. 299-305.
Tomás-Barberán FA, Grayer-Barkmeijer RJ, Gil MI, Harborne JB. 1988. Distribution of 6-hydroxy-, 6-methoxy- and 8-hydroxyflavone glycosides in the Labiatae, the Scrophulariaceae and related families. – Phytochemistry 27: 2631-2645.
Tomlinson PB. 1986. Avicenniaceae. – In: The botany of mangroves, Cambridge, pp. 186-207.
Tomlinson PB, Fawcett P. 1972. Dioecism in Citharexylum (Verbenaceae). – J. Arnold Arbor. 53: 386-389.
Tononi P, Möller M, Bencivenga S, Spada A. 2010. GRAMINIFOLIA homolog expression in Streptocarpus rexii is associated with the basal meristems in phyllomorphs, a morphological novelty in Gesneriaceae. – Evol. Devel. 12: 61-73.
Torke BM. 2000. A revision of Salvia sect. Ekmania (Lamiaceae). – Brittonia 52: 265-302.
Tournay R, Lawalrée A. 1952. Une classification nouvelle des familles appartenent aux ordres des Ligustrales et des Contortées. – Bull. Soc. Bot. France 99: 262-263.
Toyama S, Itow S. 1975. the distribution and ecology of a mangrove-like plant, Myoporum bontioides A. Gray (Myoporaceae), in western Kyushu, Japan. – Hikobia 7: 117-124.
Tralau H. 1964. The genus Trapella Oliver in the Tertiary of Europe. – Bot. Not. 117: 119-123.
Tralau H. 1965. New facts and new finds of fossil Trapella Oliver in Europe. – Bot. Not. 118: 21-24.
Traveset A. 1994. Reproductive biology of Phillyrea angustifolia L. (Oleaceae) and effect of galling-insects on its reproductive output. – Bot. J. Linn. Soc. 114: 153-166.
Trehan KB, chand H, Mehta SK, Baijal SK. 1974. Studies on floral biology in sesame (Sesamum indicum L.). – Oilseeds J. 4: 7-11.
Trejo-Torres JC. 2009. Tabebuia karsoana (Bignoniaceae), a new species from the northern karst of Puerto Rico. – Kew Bull. 64: 295-299.
Trinta EF. 1979. Revisão das espécies do gênero Genlisea St.-Hil. (Lentibulariaceae) das regiões sudeste e sul do Brasil. – Rodriguesia 31: 17-139.
Tripp EA. 2007. Evolutionary relationships within the species-rich genus Ruellia (Acanthaceae). – Syst. Bot. 32: 628-649.
Tripp EA. 2010. Taxonomic revision of Ruellia sect. Chiropterophila (Acanthaceae): a lineage of rare and endemic species from Mexico. – Syt.Bot. 35: 629-661.
Tripp EA, Darbyshire I. 2017. Phylogenetic relationships among Old World Ruellia L.: a new classification and reinstatement of the genus Dinteracanthus Schinz. – Syst. Bot. 42: 470-483.
Tripp EA, Fatimah S. 2012. Comparative anatomy, morphology, and molecular phylogenetics of the African genus Satanocrater (Acanthaceae). – Amer. J. Bot. 99: 967-982.
Tripp EA, McDade LA. 2012. New synonymies for Ruellia (Acanthaceae) of Costa Rica and notes on other Neotropical species. – Brittonia 64: 305-317.
Tripp EA, McDade LA. 2014. A rich fossil
record yields calibrated phylogeny for Acanthaceae (Lamiales) and evidence
for marked biases in timing and directionality of intercontinental
disjunctions. – Syst. Biol. 63: 660-684.
Tripp EA, Manos PS. 2008. Is floral specialization and evolutionary dead end? Pollination system transitions in Ruellia (Acanthaceae). – Evolution 62: 1712-1737.
Tripp EA, Daniel TF, Lendemer JC, McDade LA. 2009. New molecular and morphological insights prompt transfer of Blechum to Ruellia (Acanthaceae). – Taxon 58: 893-906.
Tripp EA, Daniel TF, Fatimah S, McDade LA. 2013. Phylogenetic relationships within Ruellieae (Acanthaceae) and a revised classification. – Intern. J. Plant Sci. 174: 97-137.
Troncoso NS. 1941. Un nuevo género de Verbenáceas de la Argentina: Parodianthus, nov. gen. – Darwiniana 5: 31-40.
Troncoso NS. 1961. Sobre dioecia en especies de Lippia. – Bol. Soc. Arg. Bot. 9: 181-185.
Troncoso NS. 1971. Verbenoxylum, nuevo género de Verbenáceas arbóreas de Brasil austral. – Darwiniana 16: 622-626.
Troncoso NS. 1974. Los géneros de Verbenacea de Sudamerica extratropical. – Darwiniana 18: 295-412.
Troncoso NS. 1980. Taxonomic novelties in the genera Lantana and Lippia (Verbenaceae). – Hickenia 1: 227-231.
Trudel MCG, Morton JK. 1992. Pollen morphology and taxonomy in North American Labiatae. – Can. J. Bot. 70: 975-995.
Trusty JL, Olmstead RG, Bogler DJ, Santos-Guerra A, Francisco-Ortega J. 2004. Using molecular data to test a biogeographic connection of the Macaronesian genus Bystropogon (Lamiaceae) to the New World: a case of conflicting phylogenies. – Syst. Bot. 29: 702-715.
Tseng AL. 1973. A phytochemical study of Phryma leptostachya L. and Echinocystis lobata Michx. – M.Sc. thesis, North Dakota State University, Fargo, North Dakota.
Tsoong PC, Chang KT. 1965. Palynological study of Pedicularis and its relation with the taxonomic systems of the genus. – Acta Phytotaxon. Sin. 10: 257-281, 357-374. [In Chinese]
Tsukaya H. 1997. Determination of the unequal fate of cotyledons of a one-leaf plant, Monophyllaea. – Development 124: 1275-1280.
Tsymbalyuk ZM, Mosyakin SL. 2009. Peculiarities of pollen grains in representatives of the families Hippuridaceae and Callitrichaceae. – Ukrayins’k. Bot. Žurn. 66: 529-540. [In Ukrainian]
Tsymbalyuk ZM, Mosyakin SL. 2010. Pollen morphology of the genus Digitalis L. (Scrophulariaceae s.l.). – Ukrayins’k. Bot. Žurn. 67: 79-92. [In Ukrainian]
Tsymbalyuk ZM, Mosykain SL. 2012. Palynomorphology of species of the genus Melampyrum L. (Orobanchaceae) in the flora of Ukraine. – Ukrayins’k. Bot. Žurn. 69: 818-831. [In Ukrainian]
Tsymbalyuk ZM, Mosyakin SL. 2013. Atlas of pollen grains of representatives of Plantaginaceae and Scrophulariaceae. – Nash Format Publ., Kyiv. [In Ukrainian]
Turgeon R, Beebe DU, Gowan E. 1993. The intermediary cell: minor-vine anatomy and raffinose oligosaccharide synthesis in the Scrophulariaceae. – Planta 191: 446-456.
Turner BL. 2009a. Recension of the Mexican species of Salvia (Lamiaceae), section Scorodonia. – Phytologia 91: 256-269.
Turner BL. 2009b. Recension of the Mexican species of section Uliginosae of Salvia (Lamiaceae). – Phytologia 91: 440-466.
Turner BL. 2011. Overview of the genus Asterohyptis (Lamiaceae) and description of a new species from Northern Mexico. – Phytoneuron 2011(2): 1-6.
Turner BL. 2013. Taxonomic overview of the Mexican species of Salvia sect. Flocculosae (Lamiaceae). – Phytoneuron 36: 1-11.
Turner MW. 1996. Systematic study of the genus Brazoria (Lamiaceae), and Warnockia (Lamiaceae), a new genus from Texas. – Plant Syst. Evol. 203: 65-82.
Turrill WB. 1919. A revision of the genus Mendoncia. –Kew Bull. 1919: 407-425.
Turrill WB. 1934. The correlation of morphological variation with distribution in some species of Ajuga. – New Phytol. 33: 218-230.
Turrill WB. 1952. Oleaceae. – In: Turrill WB, Milne-Redhead E (eds), Flora of tropical East Africa, The Crown Agents for the Colonies, London, pp. 1-31.
Tzvelev N. 1975. Nota de genere Callitriche L. in URSS. – Nov. Syst. Vysš. Rast. 12: 237-238.
Ubera-Jiménez JL, Hidalgo-Fernández PJ. 1992. Temporal gynodioecy in Rosmarinus officinalis. – In: Harley RM, Reynolds T (eds), Advances in labiate science, Royal Botanic Gardens, Kew, pp. 281-289.
Udulutsch RG, Assis MA de, Dias P. 2013. Four new species of Adenocalymma (Bignoniaceae) and a key to the species from southeastern Brazil. – Nord. J. Bot. 31: 176-185.
Uhlich H, Pusch J, Barthel K-J. 1995. Die Sommerwurzarten Europas. – Magdeburg.
Umber RE. 1979. The genus Glandularia (Verbenaceae) in North America. – Syst. Bot. 4: 72-102.
Upadhyay N, Trivedi BS, Verma CL. 1989 [1991]. Foliar epidermal studies in Indian Jasminum. – Bull. Bot. Surv. India 31: 136-148.
Upson TM. 1997. Systematics of the genus Lavandula (Lamiaceae). – Ph.D. diss., University of Reading, England.
Upson TM, Andrews S. 2003. A new species of Lavandula L. (Lamiaceae) from Gran Canaria, Canary Islands. – Kew Bull. 58: 903-907.
Upson TM, Andrews S. 2004. The genus Lavandula. A Botanical Magazine monograph. – Royal Botanic Gardens, Kew.
Upson TM, Jury SL. 2002. A revision of native Moroccan species of Lavandula L. section Pterostoechas Ging. (Lamiaceae). – Taxon 51: 309-327.
Upson M, Jury SL. 2003. The re-circumscription and lectotypification of Lavandula antineae Maire and description of a new species L. saharica Upson & Jury (Lamiaceae). – Kew Bull. 58: 889-901.
Upson TM, Grayer RJ, Greenham JR, Williams CA, Al-Ghamdi F, Chen F-H. 2000. Leaf flavonoids as systematic characters in the genera Lavandula and Sabaudia. – Biochem. Syst. Ecol. 28: 991-1007.
Urban I. 1884. Studien über die Scrophulariaceen-Gattungen Ilysanthes, Bonnaya, Vandelia und Lindernia. – Ber. Deutsch. Bot. Ges. 2: 429-442.
Urban I. 1916. Über Ranken und Pollen der Bignoniaceae. – Ber. Deutsch. Bot. Ges. 34: 723-758.
Uribe-Convers S, Tank DC. 2016. Phylogenetic revision of the genus Bartsia (Orobanchaceae): disjunct distributions correlate to independent lineages. – Syst. Bot. 41: 672-684.
Vaarama A. 1949. Some chromosome numbers in the genera Angelica, Ocimum, Satureja, Thymus and Cnicus. – Arch. Soc. Vanamo 2: 55-59.
Valdés B. 1970. Revisión de las especies europeas de Linaria con semillas aladas. – An. Univ. Hipalense 7: 1-288.
Valdés LJ III, Hatfield GM, Koreeda M, Paul AG. 1987. Studies of Salvia divinorum (Lamiaceae), an hallucinogenic mint from the Sierra Mazateca in Oaxaca, Central Mexico. – Econ. Bot. 41: 283-291.
Valdés LJ III, Mislankar SG, Paul AG. 1987. Coleus barbatus (C. forskohlii) (Lamiaceae) and the potential new drug forskolin (coleonol). – Econ. Bot. 41: 474-483.
Valdes-Bermejo E, Sanchez-Crespo A. 1978. Datos cariologicos y taxonomicos sobre el genero Teucrium L. (Labiatae) en la peninsula iberica. – Acta Bot. Malac. 4: 27-54.
Vargas P, Kadereit JW. 2001. Molecular fingerprinting evidence (ISSR, INTER-Simple Repeats) for a wild status of Olea europaea L. (Oleaceae) in the Eurosiberian North of the Iberian Peninsula. – Flora 196: 142-152.
Vargas P, Rosselló JA, Oyama R, Güemes J. 2004. Molecular evidence for naturalness of genera in the tribe Antirrhineae (Scrophulariaceae) and three independent evolutionary lineages from the New World and the Old. – Plant Syst. Evol. 249: 151-172.
Vargas P, Carrió E, Guzmán B, Amat E, Güemes J. 2009. A geographical pattern of Antirrhinum (Scrophulariaceae) speciation since the Pliocene based on plastid and nuclear DNA polymorphisms. – J. Biogr. 36: 1297-1312.
Vargas P, Valente LM, Blanco-Pastor JL, Liberal I, Guzman B, Cano E, Forrest A, Fernandez-Mazuecos M. 2014. Testing the biogeographical congruence of palaeofloras using molecular phylogenetics – snapdragons and the Madrean-Tethyan flora. – J. Biogeogr. 41: 932-943.
Varghese TM. 1968. Studies in the family Schrophulariaceae II. Pollen morphology. – J. Palynol. 4: 91-97.
Varghese TM. 1969. A contribution on the foliar venation of Scrophulariaceae. – In: Chowdhury KA (ed), Recent advances in the anatomy of tropical seed plants, Hindustan Publ., Delhi, pp. 253-266.
Varghese TM, Verma DPS. 1968. Pollen morphology of some Indian Labiatae. – J. Palynol. 4: 77-83.
Vasanthy G, Pocock SAJ. 1986. Radial through rotated symmetry of striate pollen of the Acanthaceae. – Can. J. Bot. 64: 3050-3058.
Vassilyev AE, Muravnik LE. 1988. The ultrastructure of the digestive glands in Pinguicula vulgaris L. (Lentibulariaceae) relative to their function I. The changes during maturation. – Ann. Bot., N. S., 62: 329-341.
Velasco L, Goffman FD, Pujadas-Salvà AJ. 2000. Fatty acids and tocochromanols in seeds of Orobanche. – Phytochemistry 54: 295-300.
Vencatachalam KV, Kjonaas R, Croteau R. 1984. Development and essential oil content of secretory glands of sage (Salvia officinalis). – Plant Physiol. 76: 148-150.
Venkata Ramana R, Prakasa Rao PS, Dutt BSM, Narayana LL. 2000. Embryology of Phryma leptostachya L. (Verbenaceae) with considerations of its systematic status and affinities. – Feddes Repert. 111: 231-248.
Venkatasubban KR. 1944. Cytological studies in Bignoniaceae. – Annamalai University, Annamalainagar.
Venkatasubban KR. 1945. Cytological studies in Bignoniaceae IV. The cytology of Dolichandrone rheedii Seem. and allied genera. – Proc. Indian Acad. Sci., Sect. B, 21: 77-92.
Venu P. 2007. Strobilanthes Blume (Acanthaceae) in peninsular India. – Botanical Survey of India, Kolkata.
Verdan MH, Cervi AC, Campos FR, Barison A, Stefanello MEA. 2009. Anthraquinones and ethylcyclohexane derivatives from Sinningia speciosa “Fyfiana”. – Biochem. Syst. Evol. 37: 40-42.
Verdcourt B. 1971. Plantaginaceae. – In: Milne-Redhead E, Polhill RM (eds), Flora of tropical East Africa, Crown Agents for Oversea Governments and Administrations, London, pp. 1-8.
Verdcourt B. 1992. Verbenaceae. – In: Polhill RM (ed), Flora of tropical East Africa, A. A. Balkema, Rotterdam, The Netherlands, pp. 1-155.
Varghese TM. 1963. Studies in the family Scrophulariaceae I. A contribution to the embryology of Veronica agrestis L. – Proc. Indian Acad. Sci., Sect. B, 48: 333-347.
Vestri Alvarenga SA, Gastmans JP, Rodrigues G do V, Moreno PRH, Emerenciano V de P. 2001. A computer-assisted approach for chemotaxonomic studies – diterpenes in Lamiaceae. – Phytochemistry 56: 583-595.
Viccini LF, Pierre PMO, Praça MM, Costa DC Souza da, Romanel E da Costa, Sousa SM de, Peixoto PH Perira, Salimena, FR Gonçalves. 2005 [2006]. Chromosome numbers in the genus Lippia (Verbenaceae). – Plant Syst. Evol. 256: 171-178.
Vickery Jr RK. 1969. Crossing barriers in Mimulus. – J. Jap. Gen. 44 (suppl. I): 325-336.
Vickery Jr RK. 1997. A systematic study of the Mimulus wiensii complex (Scrophulariaceae: Mimulus section Simiolus), including M. yeocoresis and M. minutiflorus, new species from western Mexico. – Madroño 44: 384-393.
Vickery Jr RK. 1978. Case studies in the evolution of species complexes in Mimulus. – Evol. Biol. 11: 405-507.
Vickery Jr RK, Wullstein BM. 1987. Comparison of six approaches to the classification of Mimulus sect. Erythranthe (Scrophulariaceae). – Syst. Bot. 12: 339-364.
Viemont J-D. 1976. Ontogenèse de l’appareil végétatif chez le Streptocarpus rexii Lindl. – Bull. Soc. Bot. France 123: 9.
Viemont J-D, Astie M. 1974. Quelques particularités du développement des deux Streptocarpus. – Bull. Soc. Bot. France 121: 11-22.
Vij SP, Kashyap SK. 1975. Pollen grain studies in some Labiatae. – J. Palynol. 11: 29-42.
Vij SP, Kashyap SK. 1976. Cytological studies in some north Indian Labiatae. – Cytologia 41: 713-719.
Vijayaraghavan MR, Ratnaparkhi S. 1972. Some aspects of embryology of Alectra thomsoni. – Phytomorphology 22: 1-8.
Villiers M de, Bellstet DU, Hughes M, Moeller M, Edwards TJ. 2008. Systematics, population genetics and phylogeography of the plant genus Streptocarpus in Southern Africa. – South Afr. J. Bot. 74: 365.
Vincent O, Weisskopf C, Poppinga S, Masselter T, Speck T, Joyeux M, Quilliet C, Marmottant P. 2011. Ultra-fast underwater suction traps. – Proc. Biol. Sci., Sect. B, 278: 2909-2914.
Vinckier S, Smets E. 2002. Morphology, ultrastructure and typology of orbicules in Loganiaceae s.l. and related genera, in relation to systematics. – Rev. Palaeobot. Palynol. 119: 161-189.
Vishnu-Mittre, Sharma BD. 1963. Contribution to the pollen morphology of the genera Eranthemum Linn. and Pseuderanthemum Radlkof. – Proc. Natl. Inst. Sci. India 29: 520-526.
Visser JH, Dörr I, Kollmann R. 1977. On the parasitism of Alectra vogelii Benth. (Scrophulariaceae) I. Early development of the primary haustorium and initiation of the stem. – Zeitschr. Pflanzenphys. 84: 213-222.
Viswanathan MB, Manikandan U. 2009. Teucrium ramaswamii sp. nov. (Lamiaceae) from India. – Nord. J. Bot. 27: 86-89.
Vitek E. 1985a. Evolution alpiner Populationen von Euphrasia (Scrophulariaceae): die mittel- bis kleinblütigen drüsenhaarigen Arten. – Plant Syst.Evol. 148: 215-237.
Vitek E. 1985b. Evolution alpiner Populationen von Euphrasia (Scrophulariaceae): E. alpina und E. christii. – Plant Syst. Evol. 149: 1-18.
Vitek E. 1986. Evolution alpiner Populationen von Euphrasia (Scrophulariaceae): die tetraploide E. minima. – Plant Syst. Evol. 151: 241-269.
Vogel S. 1966. Parfümsammelnde Bienen als Bestäuber von Orchideen und Gloxinia. – Österr. Bot. Zeitschr. 113: 302-361.
Vogel S. 1984. The Diascia flower and its bee – an oil-based symbiosis in southern Africa. – Acta Bot. Neerl. 33: 509-518.
Vogel S. 1988. Die Ölblumensymbiosen – Parallelismus und andere Aspekte ihrer Entwicklung in Raum und Zeit. – Zeitschr. Zool. Syst. Evol.-Forsch. 26: 341-362.
Vogel S, Cocucci A. 1995. Pollination of Basistemon (Scrophulariaceae) by oil-collecting bees in Argentina. – Flora 190: 353-363.
Vogel S, Machado IC. 1991. Pollination of four sympatric species of Angelonia (Scrophulariaceae) by oil-collecting bees in North East Brazil. – Plant Syst. Evol. 153-178.
Vogel S, Michener CD. 1985. Long bee legs and oil-producing floral spurs, and a new Rediviva (Hymenoptera, Melittidae; Scrophulariaceae). – J. Kansas Entomol. Soc. 58: 359-364.
Vogel S, Machado IC, Lopes AV. 2004. Harpochilus neesianus and other novel cases of chiropterophily in neotropical Acanthaceae. – Taxon 53: 55-60.
Vogelmann JE. 1985. Crossing relationships among North American and eastern Asian populations of Agastache sect. Agastache (Labiatae). – Syst. Bot. 10: 445-452.
Vollesen K. 1975. A taxonomic revision of the genera Tinnea and Renschia (Lamiaceae-Ajugoideae). – Bot. Tidsskr. 70: 1-63.
Vollesen K. 1989. A revision of Megalochlamys and Ecbolium (Acanthaceae: Justicieae). – Kew Bull. 44: 601-680.
Vollesen K. 1990a. Notes on Crossandra (Acanthaceae). – Kew Bull. 45: 121-135.
Vollesen K. 1990b. The genus Crossandra (Acanthaceae) in the African continent. – Kew Bull. 45: 503-534.
Vollesen K. 1991. A revision of the African genus Sclerochiton (Acanthaceae: Acantheae). – Kew Bull. 46: 1-50.
Vollesen K. 1992a. The Old World species of Stenandrium (Acanthaceae: Acantheae). – Kew Bull. 47: 169-202.
Vollesen K. 1992b. Trichaulax (Acanthaceae: Justicieae), a new genus from East Africa. – Kew Bull. 47: 613-618.
Vollesen K. 1994a. The genus Streptosiphon (Acanthaceae: Acantheae). – Kew Bull. 49: 401-407.
Vollesen K. 1994b. Delimitation of Angkalanthus (Acanthaceae: Justicieae) and the new genus Chorisochora. – Kew Bull. 49: 469-479.
Vollesen K. 1997. Synopsis of the genus Crossandra (Acanthaceae) in Madagscar. – Kew Bull. 52: 379-415.
Vollesen K. 1999. Cynarospermum, a new genus of Acanthaceae from India. – Kew Bull. 54: 171-177.
Vollesen K. 2000. Two new Tanzanian Acanthaceae. – Kew Bull. 55: 965-969.
Vollesen K. 2002. Three new species of Blepharis (Acanthaceae). – Kew Bull. 57: 451-457.
Vollesen K. 2006. A taxonomic revision of the genus Duosperma (Acanthaceae). – Kew Bull. 61: 289-351.
Vos MP de. 1947. Die ontwikkeling van die saadknop en saad by die Myoporaceae en die systematise posisie van Oftia Adans. – South Afr. J. Sci. 43: 171-187.
Vos WT. 1995. A systematic study of Leonotis (Pers.) R. Br. (Lamiaceae) in southern Africa. – Ph.D. diss., University of Natal, Pietermaritzburg, Republic of South Africa.
Vujicic R, Grubisic D, Konjevic R. 1993. Scanning electron microscopy of the seed coat in the genus Paulownia. – Bot. J. Linn. Soc. 111: 505-511.
Vural M, Aydoğdu M. 1993. A new species from Central Anatolia – Verbascum gypsicola (Scrophulariaceae). – Karaca Arbor. Mag. 2: 75-78.
Waayers GM. 1996. Hybridization, introgression, and selection in Mimulus aurantiacus ssp. australis and Mimulus puniceus. – Master’s thesis, San Diego State University, San Diego, California.
Wadhi M. 1970. Comparative embryology of angiosperms: Acanthaceae. – Bull. Indian Nat. Sci. Acad. 41: 267-272.
Wagenitz G. 2004. Globulariaceae. – In: Kubitzki K, Kadereit JW (eds), The families and genera of vascular plants VII. Flowering plants. Dicotyledons. Lamiales (except Acanthaceae including Avicenniaceae), Springer, Berlin, Heidelberg, New York, pp. 159-162.
Wagner S. 1914. Contribution à l’étude anatomique du fruit des labiées. – Thèse, Université de Paris, France.
Wagstaff SJ. 1992a. Phylogeny and character evolution in subfamily Nepetoideae (Labiatae). – Ph.D. diss., Ohio University, Athens, Ohio.
Wagstaff SJ. 1992b. A phylogenetic interpretation of pollen morphology in tribe Mentheae (Labiatae). – In: Harley RM, Reynolds T (eds), Advances in labiate science, Royal Botanic Gardens, Kew, pp. 113-124.
Wagstaff SJ, Hickerson L, Spangler R, Reeves PA. Olmstead RG. 1998. Phylogeny in Labiatae s.l. inferred from cpDNA sequences. – Plant Syst. Evol. 209: 265-274.
Wagstaff SJ. 2004. Tetrachondraceae. – In: Kubitzki K, Kadereit JW (eds), The families and genera of vascular plants VII. Flowering plants. Dicotyledons. Lamiales (except Acanthaceae including Avicenniaceae), Springer, Berlin, Heidelberg, New York, pp. 441-444.
Wagstaff SJ, Garnock-Jones PJ. 1998. Evolution and biogeography of the Hebe complex (Scrophulariaceae) inferred from ITS sequences. – New Zealand J. Bot. 36: 425-437.
Wagstaff SJ, Garnock-Jones PJ. 2000. Patterns of diversification in Chionohebe and Parahebe (Scrophulariaceae) inferred from ITS sequences. – New Zealand J. Bot. 38: 389-407.
Wagstaff SJ, Olmstead RG. 1997. Phylogeny of Labiatae and Verbenaceae inferred from rbcL sequences. – Syst. Bot. 22: 165-179.
Wagstaff SJ, Olmstead RG, Cantino PD. 1995. Parsimony analysis of cpDNA restriction site variation in subfamily Nepetoideae (Labiatae). – Amer. J. Bot. 82: 886-892.
Wagstaff SJ, Hickerson L, Spangler R, Reeves PA, Olmstead RG. 1998. Phylogeny in Labiatae s.l., inferred from cpDNA sequences. – Plant Syst. Evol. 209: 265-274.
Wagstaff SJ, Martinsson K, Swenson U. 2000. Divergence estimates of Tetrachondra hamiltonii and T. patagonica (Tetrachondraceae) and their implications for austral biogeography. – New Zealand J. Bot. 38: 587-596.
Wagstaff SJ, Bayly MJ, Garnock-Jones PJ, Albach DC. 2002. Classification, origin, and diversification of the New Zealand hebes (Scrophulariaceae). – Ann. Missouri Bot. Gard. 89: 38-63.
Walker JB. 2006. Systematics of the genus Salvia (Lamiaceae). – Ph.D. diss., University of Wisconsin, Madison, Wisconsin.
Walker JB, Elisens WJ. 2001. A revision of Salvia section Heterosphace in western North America. – Sida 19: 571-589.
Walker JB, Sytsma KJ. 2006. A molecular phylogenetic analysis of Salvia subgenus Audibertia. – In: Walker JB (ed), Systematics of the genus Salvia (Lamiaceae), University of Wisconsin, Madison, Wisconsin, pp. 133-194.
Walker JB, Sytsma KJ. 2007. Staminal evolution in the genus Salvia (Lamiaceae): molecular phylogenetic evidence for multiple origins of the staminal level. – Ann. Bot. 100: 375-391.
Walker JB, Sytsma KJ, Treutlein J, Wink M. 2004. Salvia (Lamiaceae) is not monophyletic: implications for the systematics, radiation, and ecological specializations of Salvia and tribe Mentheae. – Amer. J. Bot. 91: 1115-1125.
Walker JB, Drew BT, Sytsma KJ. 2015. Unravelling species relationships and diversification within the iconic California Floristic Province sages (Salvia Subgenus Audibertia, Lamiaceae). – Syst. Bot. 40: 826-844.
Wall B. 1990. African violets and related plants. – Cassell, London.
Wall WE, Long RW. 1965. Megasporogenesis and embryo sac development in Ruellia caroliniensis. – Bull. Torrey Bot. Club 92: 372-377.
Wallander E. 2008. Systematics of Fraxinus (Oleaceae) and evolution of dioecy. – Plant Syst. Evol. 273: 25-49.
Wallander E, Albert VA. 2000. Phylogeny and classification of Oleaceae based on rps16 and trnL-F sequence data. – Amer. J. Bot. 87: 1827-1841.
Walsh MA, Popovich TM. 1977. Some ultrastructural aspects of metaphloem sieve elements in the aerial stem of the holoparasitic angiosperm Epifagus virginiana (Orobanchaceae). – Amer. J. Bot. 64: 326-336.
Walther E, Walther K. 1957. Systematische Studien an Micromeria biflora Benth. aus Afrika. – Mitt. Thüring. Bot. Ges. 1: 1-12.
Wang C-N, Möller M, Cronk QCB. 2004. Phylogenetic position of Titanotrichum oldhamii (Gesneriaceae) inferred from four different gene regions. – Syst. Bot. 29: 407-418.
Wang H, Blackmore S. 2003. Pollen morphology in Strobilanthes Blume (Acanthaceae) in China and its taxonomic implications. – Grana 42: 82-87.
Wang H, Yu WB, Chen JQ, Blackmore S. 2009. Pollen morphology in relation to floral types and pollination syndromes in Pedicularis (Orobanchaceae). – Plant Syst. Evol. 277: 153-162.
Wang L, Wang Y-Z. 2005. Floral development of Triaenophora (Veronicaceae) and phylogenetic implications. – Plant Syst. Evol. 250: 69-79.
Wang M, Zhang L, Ding CB, Yang RW, Zhou YH, Yang ZJ, Yin ZQ. 2009. Meiotic observations of eight taxa in the genus Salvia. – Caryologia 62: 334-340.
Wang W-T. 1981. Quinque genera nova Gesneriacearum e Sina. – Bull. Bot. Res. Harbin 1: 21-51.
Wang W-T. 1985. The second revision of the genus Petrocosmea (Gesneriaceae). – Acta Bot. Yunnan. 7: 49-68.
Wang W-T, Pan K-Y, Li Z-Y. 1992. Keys to the Gesneriaceae of China. – Edinburgh J. Bot. 49: 5-74.
Wang W-Y, Pai RCP, Lai C-C, Lin T-P. 1994. Molecular evidence for the hybrid origin of Paulownia taiwaniana based on RAPD markers and RFLP of chloroplast DNA. – Theor. Appl. Genet. 89: 271-275.
Wang X-Q, Li Z-Y.1998. The application of sequence analysis of rDNA fragment to the systematic study of the subfamily Cyrtandroideae (Gesneriaceae). – Acta Phytotaxon. Sin. 36: 97-105. [In Chinese with English summary]
Wang Y-Z. 1995. Two new species of Whytockia (Gesneriaceae) from Yunnan. – Acta Phytotaxon. Sin. 33: 297-301.
Wang Y-Z. 2000. The growth pattern of shoots in Whytockia (Geseriaceae) with phylogenetic implications. – Acta Phytotaxon. Sin. 38: 231-235.
Wang Y-Z. 2003. Ovary structure of the genus Gyrogyne (Gesneriaceae, Epithemateae). – Aust. Syst. Bot. 16: 629-632.
Wang Y-Z, Li Z-Y. 1997. A new species of the genus Whytockia W. W. Smith (Gesneriaceae) from Guizhou, China. – Acta Phytotaxon. Sin. 35: 67-69.
Wang Y-Z, Li Z-Y. 2002. Inflorescence development of Whytockia (Epithemateae, Gesneriaceae) and phylogenetic implications within Gesneriaceae. – Plant Syst. Evol. 236: 45-54.
Wang Y-Z, Pan K-Y. 1996. Comparative floral anatomy of Whytockia (Gesneriaceae) endemic to China. – Proc. IFDC: 352-366.
Wang Y-Z, Gao Z, Liang H, Wu Z. 1997. Floral morphogenesis of Rhynchoglossum omeiense (Gesneriaceae) and its phylogenetic implication. – Acta Bot. Yunnan. 19: 265-270.
Wang Y-Z, Gu Z-J, Hong D-Y. 1998. Karyotypes of Whytockia (Gesneriaceae). – Acta Phytotaxon. Sin. 36: 28-35.
Wang Y-Z, Li Z-Y, Pan K-Y, Zou X-H. 2002. Pattern and significance of seedling development in Titanotrichum oldhamii (Gesneriaceae). – Acta Bot. Sin. 44: 903-907.
Wang Y-Z, Liang R-H, Wang B-H, Li J-M, Qiu Z-J, Li Z-Yu, Weber A. 2010. Origin and phylogenetic relationships of the Old World Gesneriaceae with actinomorphic flowers inferred from ITS and trnL-trnF sequences. – Taxon 59: 1044-1052.
Wang Y-Z, Mao R-B, Liu Y, Li J-M, Dong Y, Li Z-Y, Smith JF. 2011. Phylogenetic reconstruction of Chirita and its allies (Gesneriaceae) with taxonomic treatments. – J. Syst. Evol. 49: 50-64.
Wannan BS, Waterhouse JT. 1985. A taxonomic revision of the Australian species of Limnophila R. Br. (Scrophulariaceae). – Aust. J. Bot. 33: 367-380.
Ward HM, Dale E. 1898. On Craterostigma pumilum Hochst., a rare plant from Somali-Land. – Trans. Linn. Soc. Bot., Ser. II, 5: 343-355.
Wasshausen DC. 1970. A synopsis of the genus Suessenguthia (Acanthaceae). – Rhodora 72: 119-125.
Wasshausen DC. 1973. New combinations in cultivated Justicia (Acanthaceae). – Baileya 19: 1-3.
Wasshausen DC. 1975. The genus Aphelandra (Acanthaceae). – Smithsonian Contr. Bot. 18: 1-157.
Wasshausen DC. 1984. Pranceacanthus coccineus (Acanthaceae): a new genus and species from Amazonian Brazil. – Brittonia 36: 1-7.
Wasshausen DC. 1986. The systematics of the genus Pachystachys (Acanthaceae). – Proc. Biol. Soc. Washington 99: 160-185.
Wasshausen DC. 1990. New species of Stenandrium (Acanthaceae) from the Planalto Brazil. – Brittonia 42: 1-6.
Wasshausen DC. 1993. Notes on Acanthaceae from Pico das Almas, Bahia, Brazil. – Kew Bull. 48: 17-20.
Wasshausen DC. 1996a. New species, new variety and new combinations in Stenandrium (Acanthaceae) from Ecuador and Colombia. – Nord. J. Bot. 16: 383-388.
Wasshausen DC. 1996b. New species and new combinations in Aphelandra (Acanthaceae) from Ecuador and adjacent Peru. – Nord. J. Bot. 16: 389-407.
Wasshausen DC. 1999. The genus Stenostephanus (Acanthaceae) in Bolivia. – Harvard Pap. Bot. 4: 279-288.
Wasshausen DC. 2003. New species of Justicia (Acanthaceae) from the Guianas. – Brittonia 54: 286-297.
Wasshausen DC, Wood JRI. 2001. Further discoveries in the genus Stenostephanus (Acanthaceae). – Harvard Pap. Bot. 6: 449-454.
Wasshausen DC, Wood JRI. 2003a. The genus Dyschoriste (Acanthaceae) in Bolivia and Argentina. – Brittonia 55: 10-18.
Wasshausen DC, Wood JRI. 2003b. Notes on the genus Justicia in Bolivia. – Kew Bull. 58: 769-831.
Wasshausen DC, Wood JRI. 2004. Acanthaceae of Bolivia. – Contr. U.S. Natl. Herb. 49: 1-152.
Waterman AH. 1960. Pollen grain studies of the Labiatae of Michigan. – Webbia 15: 399-415.
Webb DA. 1971. Taxonomic notes on Antirrhinum L. – Bot. J. Linn. Soc. 64: 271-275.
Webber JM, Ball PW. 1980. Introgression in Canadian populations of Lycopus americanus Muhl. and L. europaeus L. (Labiatae). – Rhodora 82: 281-304.
Weber A. 1971. Zur Morphologie des Gynoeceums der Gesneriaceen. – Österr. Bot. Zeitschr. 119: 234-305.
Weber A. 1973a. Die Struktur der paarblütigen Partialfloreszenzen der Gesneriaceen und bestimmter Scrophulariaceen. – Beitr. Biol. Pflanzen 49: 429-460.
Weber A. 1973b. Über die “Stipeln” von Rhytidophyllum Mart. (Gesneriaceae). – Österr. Bot. Zeitschr. 121: 107-109.
Weber A. 1975a. Beiträge zur Morphologie und Systematik der Klugieae und Loxonieae (Gesneriaceae) I. Die Sproß- und Infloreszenzorganisation von Monophyllaea R. Br. – Bot. Jahrb. Syst. 95: 174-207.
Weber A. 1975b. The cristate inflorescence of Chirita sect. Microchirita (Gesneriaceae). – Notes Roy. Bot. Gard. Edinb. 34: 221-230.
Weber A. 1975c. Beiträge zur Morphologie und Systematik der Klugieae und Loxonieae (Gesneriaceae) II. Morphologie, Anatomie und Ontogenese der Blüte von Monophyllaea R. Br. – Bot. Jahrb. Syst. 95: 435-454.
Weber A. 1976a. Beiträge zur Morphologie und Systematik der Klugieae und Loxonieae (Gesneriaceae) III. Whytockia als morphologische und phylogenetische Ausgangsform von Monophyllaea. – Beitr. Biol. Pflanzen 52: 183-205.
Weber A. 1976b. Beiträge zur Morphologie und Systematik der Klugieae und Loxonieae (Gesneriaceae) IV. Wuchsform, Infloreszenz- und Blütenmorphologie von Epithema. – Plant Syst. Evol. 126: 287-322.
Weber A. 1977a. Beiträge zur Morphologie und Systematik der Klugieae und Loxonieae (Gesneriaceae) V. Revision der Gattung Loxonia (Gesneriaceae). – Plant Syst. Evol. 127: 201-216.
Weber A. 1977b. Beiträge zur Morphologie und Systematik der Klugieae und Loxonieae (Gesneriaceae) VI. Morphologie und Verwandtschaftsbeziehungen von Loxonia und Stauranthera. – Flora 166: 153-175.
Weber A. 1978a. Beiträge zur Morphologie und Systematik der Klugieae und Loxonieae (Gesneriaceae) VII. Sproß-, Infloreszenz- und Blütenbau von Rhynchoglossum. – Bot. Jahrb. Syst. 99: 1-47.
Weber A. 1978b. Beiträge zur Morphologie und Systematik der Klugieae und Loxonieae (Gesneriaceae) VIII. Ein typologischer Vergleich zwischen Rhynchoglossum klugioides und Loxonia. – Linzer Biol. Beitr. 10: 217-228.
Weber A. 1978c. Transitions from pair-flowered to normal cymes in Gesneriaceae. – Notes Roy. Bot. Gard. Edinb. 36: 355-368.
Weber A. 1979. Ornithoboea arachnoidea – an “orchid-flowered” gesneriad. – Gloxinian 29: 9-12.
Weber A. 1980. Die Gattungsmerkmale von Schizoboea (Gesneriaceae-Didymocarpeae). – Plant Syst. Evol. 134: 183-192.
Weber A. 1982a. Contributions to the morphology and systematics of Klugieae and Loxonieae (Gesneriaceae) IX. The genus Whytockia. – Notes Roy. Bot. Gard. Edinb. 40: 113-121.
Weber A. 1982b. Evolution and radiation of the pair-flowered cyme in Gesneriaceae. – Aust. Syst. Bot. Soc. Newslett. 30: 23-41.
Weber A. 1988. Beiträge zur Morphologie und Systematik der Klugieae und Loxonieae (Gesneriaceae) X. Development and interpretation of the inflorescence of Epithema. – Beitr. Biol. Pflanzen 63: 431-451.
Weber A. 1989a. Family position and conjectural affinities of Charadrophila capensis Marloth. – Bot. Jahrb. Syst. 111: 87-119.
Weber A. 1989b. Didymocarpus geitleri, a remarkable new species of Gesneriaceae with deceptive pollen flowers. – Plant Syst. Evol. 165: 95-100.
Weber A. 1995. Developmental aspects of the pair-flowered cyme of Gesneriaceae. – Gesneriana 1: 18-28.
Weber A. 2004a. Gesneriaceae and Scrophulariaceae: Robert Brown and now. – Telopea 10: 543-571.
Weber A. 2004b. Gesneriaceae. – In: Kubitzki K, Kadereit JW (eds), The families and genera of vascular plants VII. Flowering plants. Dicotyledons. Lamiales (except Acanthaceae including Avicenniaceae), Springer, Berlin, Heidelberg, New York, pp. 63-158.
Weber A. 2013. Pair-flowered cymes in the Lamiales: structure, distribution and origin. – Ann. Bot. 112: 1577-1595.
Weber A, Burtt BL. 1983. Didymocarpus corchorifolius and its allies (Gesneriaceae). – Blumea 28: 291-309.
Weber A, Burtt BL. 1985. Supplementary notes on “Didymocarpus corchorifolius and its allies (Gesneriaceae)”. – Blumea 31: 155-159.
Weber A, Burtt BL. 1998a. Didissandra: redefinition and partition of an artificial genus of Gesneriaceae. – Beitr. Biol. Pflanzen 70: 153-177.
Weber A, Burtt BL. 1998b. Revision of the genus Didissandra (Gesneriaceae). – Beitr. Biol. Pflanzen 70: 191-223.
Weber A, Burtt BL. 1998c.Revision of the genus Ridleyandra (Gesneriaceae). – Beitr. Biol. Pflanzen 70: 225-273.
Weber A, Burtt BL. 1998d. Remodelling of Didymocarpus and associated genera (Gesneriaceae). – Beitr. Biol. Pflanzen 70: 293-363.
Weber A, Kiew R. 1983. Gesneriads of Peninsular Malaysia. – Nature Malaysiana 8: 24-31.
Weber A, Vogel S. 1986. The pollination syndrome of Deplanchea tetraphylla (Bignoniaceae). – Plant Syst. Evol. 154: 237-250.
Weber A, Till S, Eberwein R. 1992. Komplexe Blütenstände bei Acanthaceen: Crabbea velutina und Dicliptera laxata. – Flora 187: 227-257.
Weber A, Burtt BL, Vitek E. 2000. Materials for a revision of Didymocarpus (Gesneriaceae). – Ann. Naturhist. Mus. Wien, B, 102: 441-475.
Weber A, Middleton DJ, Forrest A, Kiew R, Lim CL, Rafidah AR, Sontag S, Triboun P, Wei Y-G, Yao TL, Möller M. 2011. Molecular systematics and remodelling of Chirita and associated genera (Gesneriaceae). – Taxon 60: 767-790.
Weber A, Wei Y-G, Sontag S, Möller M. 2011. Inclusion of Metabriggsia into Hemiboea (Gesneriaceae). – Phytotaxa 23: 37-48.
Weber A, Wei Y-G, Puglisi C, Wen F, Mayer V, Möller M. 2011. A new definition of the genus Petrocodon (Gesneriaceae). – Phytotaxa 23: 49-67.
Weber A, Clark JL, Möller. 2013. A new formal classification of Gesneriaceae. – Selbyana 31: 68-94.
Weber H. 1936. Vergleichend-morphologische Studien über die sproßbürtige Bewurzelung. – Nova Acta Leopold. 4(21): 230-298.
Weber HC. 1975. Vergleichende Betrachtungen über die unterirdischen Organe von Lathraea squamaria L. und Tozzia alpina L. (Scrophulariaceae). – Beitr. Biol. Pflanzen 51: 1-15.
Weber HC. 1976a. Über Wirtspflanzen und Parasitismus einiger mitteleuropäischer Rhinanthoideae (Scrophulariaceae). – Plant Syst. Evol. 125: 97-107.
Weber HC. 1976b. Studies on new types of haustoria in some Central European Rhinanthoideae (Scrophulariaceae). – Plant Syst. Evol. 125: 223-232.
Weber HC. 1976c. Anatomische Studien an den Haustorien einiger parasitischer Scrophulariaceen Mitteleuropas. – Ber. Deutsch. Bot. Ges. 89: 57-84.
Weber HC. 1980. Zur Evolution des Parasitismus bei den Scrophulariaceae und Orobanchaceae. – Plant Syst. Evol. 136: 217-232.
Weber HC, Weberling F. 1975. Zur Morphologie der Haustorien von Rhinanthoideen (Scroph.). – Ber. Deutsch. Bot. Ges. 88: 329-345.
Wegener T. 1998. Die Teufelskralle (Harpagophytum procumbens DC.) in der Therapie rheumatischer Erkrankungen. – Zeitschr. Phytotherapie 19: 284-294.
Wei Y-G, Pan B, Tang W-X. 2007. Chirita guihaiensis sp. nov. (Gesneriaceae) from Guangxi, China. – Nord. J. Bot. 25: 296-298.
Wei Y-G, Wen F, Chen W-F, Shui Y-M, Möller M. 2010. Litostigma, a new genus from China: a morphological link between basal and derived didymocarpoid Gesneriaceae. – Edinburgh J. Bot. 67: 161-184.
Wei Z. 1989. Pollen morphology of Wightia and its taxonomic significance. – Acta Metall. Sin. 11: 1-3.
Weigend M, Förther H. 2002. A revision of the Central American genus Solenophora (Gesneriaceae). – Harvard Pap. Bot. 7: 37-78.
Weiß G. 1932. Weitere Beiträge zur Kenntnis der Endospermhaustorien in der Gattung Veronica. – Flora 126: 418-464.
Weiss-Schneeweiss H, Greilhuber J, Schneeweiss GM. 2005. Genome size evolution in holoparasitic Orobanche (Orobanchaceae) and related genera. – Amer. J. Bot. 93: 148-156.
Welch AJ, Collins K, Ratan A, Drautz-Moses DI, Schuster SC, Linqvist C. 2016. The quest to resolve recent radiations: plastid phylogenomics of extinct and endangered Hawaiian endemic mints (Lamiaceae). – Mol. Phylogen. Evol. 99: 16-33.
Wen F, Maciejewski S, He XQ, Han J, Wei YG. 2015. Briggsia leiophylla, a new species of Gesneriaceae from southern Guizhou, China. – Phytotaxa 202: 51-56.
Wen F, Wei Y-G, Möller M. 2015. Glabrella leiophylla (Gesneriaceae), a new combination for a former Briggsia species from Guizhou, China. – Phytotaxa 218: 193-194.
Wendelbo P. 1964. Two new species of Scrophularia from East Afghanistan. – Bot. Not. 117: 366-370.
Wendelbo P. 1980. New species of Dionysia, Kickxia and Onobrychis from Iran. – Notes Roy. Bot. Gard. Edinb. 38: 105-110.
Weng R-F, Zhang M-Z. 1992. Chromosome numbers in Chinese Oleaceae I. – Investigatio et Studium Naturae 12: 66-77.
Wenk RC, Daniel TF. 2009. Molecular phylogeny of Nelsonioideae (Acanthaceae) and phylogeny of Elytraria. – Proc. Calif. Acad. Sci., Ser. IV, 60: 53-68.
Werker E, Ravid U, Putievsky E. 1985a. Structure of glandular hairs and identification of the main components of their secreted material in some species of the Labiatae. – Israel J. Bot. 34: 31-45.
Werker E, Ravid U, Putievsky E. 1985b. Glandular hairs and their secretions in the vegetative and reproductive organs of Salvia sclarea and S. dominica. – Israel J. Bot. 34: 239-252.
Werker E, Putievsky E, Ravid U. 1985. The essential oils and glandular hairs in different chemotypes of Origanum vulgare L. – Ann. Bot., N. S., 55: 793-801.
Werner K. 1960. Zur Nomenklatur und Taxonomie von Digitalis L. – Bot. Jahrb. Syst. 79: 218-254.
Wessinger CA, Freeman CC, Mort ME, Rausher MD, Hileman LC. 2016. Multiplexed shotgun genotyping resolves species relationships within the North American genus Penstemon. – Amer. J. Bot. 103: 912-922.
Wester P, Claßen-Bockhoff R. 2006. Bird pollination in South African Salvia species. – Flora 201: 396-406.
Wester P, Claßen-Bockhoff R. 2007. Floral diversity and pollen transfer mechanisms in bird-pollinated Salvia species. – Ann. Bot. 100: 401-421.
Wester P, Claßen-Bockhoff R. 2011. Pollination syndromes of New World Salvia species with special reference to bird pollination. – Ann. Missouri Bot. Gard. 98: 101-155.
Westfall JJ. 1949. Cytological and embryological evidence for the reclassification of Paulownia. – Amer. J. Bot. 36: 805.
Wettstein R von. 1895a. Scrophulariaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien IV(3b), W. Engelmann, Leipzig, pp. 39-107; Wettstein R von. 1897. Nachträge zu IV(3b), pp. 293-299.
Wettstein R von. 1895b. Globulariaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien IV(3b), W. Engelmann, Leipzig, pp. 270-273.
Wettstein R von. 1895c. Myoporaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien IV(3b), W. Engelmann, Leipzig, pp. 354-360.
Wettstein R. von. 1896a. Monographie der Gattung Euphrasia. – W. Engelmann, Leipzig.
Wettstein R. von 1896b. Zur Systematik der europäischen Euphrasia-Arten. – Österr. Bot. Zeitschr. 46: 381-386.
Whipple HL. 1972. Structure and systematics of Phryma leptostachya L. – J. Elisha Mitchell Sci. Soc. 88: 1-17.
Whitehouse E. 1949. Revision of Salvia section Salviastrum. – Field and Laboratory 17: 151-165.
Whittall JB, Carlson ML, Beardsley PM, Meinke RJ, Liston A. 2006. The Mimulus moschatus alliance (Phrymaceae): molecular and morphological phylogenetics and their conservation implications. – Syst. Bot. 31: 380-397.
Whitten WM. 1981. Pollination ecology of Monarda didyma, M. clinopodia, and hybrids (Lamiaceae) in the southern Appalachian mountains. – Amer. J. Bot. 68: 435-442.
Wickett NJ, Honaas LA, Wafula EK, Das M, Huang K, Wu B, Landherr L, Timko MP, Yoder J, Westwood JH, dePamphilis CW. 2011. Transcriptomes of the parasitic plant family Orobanchaceae reveal surprising conservation of chlorophyll synthesis. – Curr. Biol. 21: 2098.-2104.
Wiehler H. 1968. xKoellikohleria, a new hybrid genus (Gesneriaceae). – Baileya 16: 29-34.
Wiehler H. 1973. One hundred transfers from Alloplectus and Columnea (Gesneriaceae). – Phytologia 27: 306-330.
Wiehler H. 1975a. Rufodorsia, a new Central-American genus in the Gesneriaceae. – Selbyana 1: 32-35.
Wiehler H. 1975b. Besleria L. and the re-establishment of Gasteranthus Benth. (Gesneriaceae). – Selbyana 1: 150-156.
Wiehler H. 1976. A report on the classification of Achimenes, Eucodonia, Gloxinia, Goyazia, and Anetanthus (Gesneriaceae). – Selbyana 1: 374-404.
Wiehler H. 1977. New genera and species of Gesneriaceae from the Neotropics. – Selbyana 2: 67-132.
Wiehler H. 1978a. Parakohleria, a new South American genus in the Gesneriaceae. – Selbyana 5: 4-10.
Wiehler H. 1978b. The genera Episcia, Alsobia, Nautilocalyx, and Paradrymonia (Gesneriaceae). – Selbyana 5: 11-60.
Wiehler H. 1978c. Miscellaneous transfers and new species in neotropical Gesneriaceae. – Selbyana 5: 61-93.
Wiehler H. 1983. A synopsis of the neotropical Gesneriaceae. – Selbyana 6: 1-219.
Wiehler H. 1984. Miscellaneous new species in the Gesneriaceae. – Selbyana 7: 328-347.
Wiehler H. 1992. New species of Gesneriaceae from the Neotropics. – Phytologia 73: 220-241.
Wiehler H. 1993. Gesneriad trees in Colombia. – Gesneriad Res. Found. Bull. 4: 2.
Wiehler H. 1994. A re-examination of Sanango racemosum 4. Its new systematic position in Gesneriaceae. – Taxon 43: 625-632.
Wiehler H. 1995a. New species of Gesneriaceae from the Neotropics (II). – Gesneriana 1: 29-97.
Wiehler H. 1995b. Medicinal gesneriads. 122 species of the rain forest plant family Gesneriaceae used medicinally in the neotropics. – Gesneriana 1: 98-120.
Wiehler H, Chautems A. 1995. A reduction of Lietzia to Sinningia. – Gesneriana 1: 5-7.
Wildeman E de. 1906. Thomandersia butayei. – Ann. Mus. Congo Bot 1: 312.
Wilkins DA. 1963. Plasticity and establishment in Euphrasia. – Ann. Bot. (Oxford) 27: 533-552.
Will M, Claßen-Bockhoff R. 2014. Why Africa
matters: evolution of Old World Salvia (Lamiaceae) in Africa. – Ann. Bot.
114: 61-83.
Will M, Claßen-Bockhoff R. 2017. Time to split Salvia s.l. (Lamiaceae) – new insights from Old World Salvia phylogeny. – Mol. Phylogen. Evol. 109: 33-58.
Will M, Schmalz N, Claßen-Bockhoff R. 2015. Towards a new classification of Salvia s.l. – (re)establishing genus Pleudia Raf. – Turk. J. Bot. 39: 693-707.
Willemse RH. 1985. Notes on East African Plectranthus species (Labiatae). – Kew Bull. 40: 93-96.
Willemse RH. 1991. New combinations and names for Macaronesian Satureja taxa (Labiatae). – Willdenowia 21: 81-85.
Williams JHL. 1938. The flowering of Strobilanthes. – J. Bombay Nat. Hist. Soc. 39: 877-879.
Williams LO. 1970. An overlooked genus of the Scrophulariaceae. – Fieldiana Bot. 32: 211-214.
Williams ML, Drinnan AN, Walsh NG. 2006. Variation within Prostanthera spinosa (Lamiaceae): evidence from morphological and molecular studies. – Aust. Syst. Bot. 19: 467-477.
Williams NH. 1978. Pollen structure and the systematics of the neotropical Gesneriaceae. – Selbyana 2: 310-322.
Williamson SD. 1995. Four new species of Becium Lindl. (Labiatae) from South Africa. – Kew Bull. 50: 739-751.
Willis JH. 1999. The role of genes of large effect on inbreeding depression in Mimulus guttatus. – Evolution 53: 1678-1691.
Wilson CL. 1974a. Floral anatomy in Gesneriaceae I. Cyrtandroideae. – Bot Gaz. (Crawfordsville) 135: 247-256.
Wilson CL. 1974b. Floral anatomy in Gesneriaceae II. Gesnerioideae. – Bot. Gaz. (Crawfordsville) 135: 256-268.
Wilson P, Castellanos MC, Wolfe AD, Thomson JD. 2006. Shifts between bee and bird pollination in penstemons. – In: Waser NM, Ollerton J (eds), Plant-pollinator interactions: from specialization to generalization, University of Chicago Press, Chicago, pp. 47-68.
Wilson TC, Conn BJ, Henwood MJ. 2012. Molecular phylogeny and systematics of Prostanthera (Lamiaceae). – Aust. Syst. Bot. 25: 341-352.
Wimpee CF, Wrobel RL, Garin DK. 1991. A divergent plastid genome in Conopholis americana, an achlorophyllous parasitic plant. – Plant Mol. Biol. 17: 161-166.
Wink M, Kaufmann M. 1996. Phylogenetic relationships between some members of the subfamily Lamioideae inferred from nucleotide sequences of the rbcL gene. – Bot. Acta 109: 139-148.
Witsch H. 1932. Chromosomenstudien an mitteleuropäischen Rhinantheen. – Österr. Bot. Zeitschr. 81: 108-141.
Witztum A. 1978. Mucilaginous plate cells in the nutlet epidermis of Coleus blumei Benth. (Labiatae). – Bot. Gaz. 139: 430-435.
Witztum A, Schulgasser K. 1995. The mechanics of seed expulsion in Acanthaceae. – J. Theor. Biol. 176: 531-542.
Wojciechowska B. 1958. Taxonomy, morphology and anatomy of seeds in the genus Salvia. – Monogr. Botan. 6: 1-56. [In Polish]
Wojciechowska B. 1961a. Fruits in the Middle European species of Prunella. – Monogr. Botan. 12: 49-87. [In Polish]
Wojciechowska B. 1961b. Fruits in the Middle European species of some genera of Stachyoideae. – Monogr. Botan. 12: 89-120. [In Polish]
Wojciechowska B. 1966. Morphology and anatomy of fruits and seeds in the family Labiatae with particular respect to medicinal species. – Monogr. Bot. 21: 3-243. [In Polish]
Wojciechowska B. 1972. Morphology and anatomy of fruits of Scutellaria, Chaiturus, Galeobdolon and Sideritis of the Labiatae. – Monogr. Botan. 37: 137-168. [In Polish]
Wolfe AD, Elisens WJ. 1995. Evidence of chloroplast capture and pollen-mediated gene flow in Penstemon sect. Peltanthera (Scrophulariaceae). – Syst. Bot. 20: 395-412.
Wolfe AD, dePamphilis CW. 1997. Alternate paths of evolution for the photosynthetic gene rbcL in four nonphotosynthetic species of Orobanche. – Plant Mol. Biol. 33: 965-977.
Wolfe AD, dePamphilis CW. 1998. The effect of relaxed functional constraints on the photosynthetic gene rbcL in parasitic plants of Scrophulariales. – Mol. Biol. Evol. 15: 1243-1258.
Wolfe AD, Randle CP. 2001. Relationships within and among species of the holoparasitic genus Hyobanche (Orobanchaceae) inferred from ISSR banding patterns and nucleotide sequences. – Syst. Bot. 26: 120-130.
Wolfe AD, Elisens WJ, Watson LE, dePamphilis CW. 1997. Using restriction-site variation of PCR-amplified cpDNA genes for phylogenetic analysis of tribe Cheloneae (Scrophulariaceae). – Amer. J. Bot. 84: 555-564.
Wolfe AD, Xiang Q-Y, Kephart SR. 1998a. Assessing hybridization in natural populations of Penstemon (Scrophulariaceae) using hypervariable intersimple sequence repeat markers. – Mol. Ecol. 7: 1107-1125.
Wolfe AD, Xiang Q-Y, Kephart SR. 1998b. Diploid hybrid speciation in Penstemon (Scrophulariaceae). – Proc. Natl. Acad. Sci. U.S.A. 95: 5112-5115.
Wolfe AD, Datwyler SL, Randle CP. 2002. A phylogenetic and biogeographic analysis of the Cheloneae (Scrophulariaceae) based on ITS and matK sequence data. – Syst. Bot. 27: 138-148.
Wolfe AD, Randle CP, Liu L, Steiner KE. 2005. Phylogeny and biogeography of Orobanchaceae. – Folia Geobot. 40: 115-134.
Wolfe AD, Randle CP, Datwyler SL, Morawetz JJ, Arguedas N, Diaz J. 2006. Phylogeny, taxonomic affinities, and biogeography of Penstemon (Plantaginaceae) based on ITS and cpDNA sequence data. – Amer. J. Bot. 93: 1699-1713.
Wolff K, Friso B, Damme JMM van. 1988. Outcrossing rates and male sterility in natural populations of Plantago coronopus. – Theor. Appl. Gen. 76: 190-196.
Wollenweber E, Rehse C, Dietz VH. 1981. The occurrence of aurentiacin and flavokawin B in Pityrogramma triangularis var. pallida and Didymocarpus species. – Phytochemistry 20: 1167-1168.
Wonisch F. 1909a. Über den Gefässbündelverlauf bei den Cyrtandroideen. – Sitzungsber. K. Akad. Wiss., Math.-Naturwiss. Cl., Abt. 1, 118: 453-486.
Wonisch F. 1909b. Die Sekretgänge von Monophyllaea, Klugia and Rhynchoglossum (Gesneriaceae). – Österr. Bot. Zeitschr. 59: 209-215.
Woo VL, Funke MM, Smith JF, Lockhart PJ, Garnock-Jones PJ. 2011. New World origins of southwest Pacific Gesneriaceae: multiple movements across and within the South Pacific. – Intern. J. Plant Sci. 172: 434-457.
Wood D. 1974. A revision of Chirita (Gesneriaceae). – Not. Roy. Bot. Gard. Edinb. 33: 123-205.
Wood DJ, Brummitt RK. 1989. Notes on Acanthus hirsutus Boiss. with a new key to Turkish taxa of Acanthus. – Notes Roy. Bot. Gard. Edinb. 46: 7-11.
Wood JRI. 1984. Eight new species and taxonomic notes on the flora of Yemen. – Kew Bull. 39: 123-139.
Wood JRI. 1988a. Colombian Acanthaceae: some new discoveries and some reconsiderations. – Kew Bull. 43: 1-51.
Wood JRI. 1988b. The genus Lepechinia (Labiatae) in Colombia. – Kew Bull. 43: 291-301.
Wood JRI. 1994. Notes relating to the flora of Bhutan XXIX. Acanthaceae, with special reference to Strobilanthes. – Edinburgh J. Bot. 51: 175-273.
Wood JRI. 1995. Notes on Strobilanthes (Acanthaceae) for the Flora of Ceylon. – Kew Bull. 50: 1-24.
Wood JRI. 2008. A revision of Tecoma Juss. (Bignoniaceae) in Bolivia. – Bot. J. Linn. Soc. 156: 143-172.
Wood JRI. 2014. New names and combinations in Indian Acanthaceae. – Novon 23: 385-395.
Wood JRI, Harley RM. 1989. The genus Salvia (Labiatae) in Colombia. – Kew Bull. 44: 211-278.
Wood JRI, Scotland RW. 2003a. The 2-lipped species of Strobilanthes (Acanthaceae). – Kew Bull. 58: 83-129.
Wood JRI, Scotland RW. 2003b. Strobilanthes: panicled species from East Asia. – Kew Bull. 58: 679-702.
Wood JRI, Scotland RW. 2009. New and little-known species of Strobilanthes (Acanthaceae) from India and South East Asia. – Kew Bull. 64: 3-47.
Wood JRI, Bennett JR, Scotland RW. 2003. Notes on Strobilanthes: the Sympagis group. – Kew Bull. 58: 131-173.
Wood JRI, Williams BP, Scotland RW. 2012. Diceratotheca, a new genus of Acanthaceae from Thailand. – Kew Bull. 67: 687-695.
Worley PJ. 1979. xNiphimenes (Niphaea x Achimenes), a new hybrid combination in the Gesneriaceae. – Selbyana 5: 214-215.
Wortley AH. 2004. Systematics of Thomandersia Baill. – Ph.D. diss., University of Oxford, England.
Wortley AH, Rudall PJ, Harris DJ, Scotland RW. 2005. How much data are needed to resolve a difficult phylogeny? Case study in Lamiales. – Syst. Biol. 54: 697-709.
Wortley AH, Scotland RW, Rudall PJ. 2005. Floral anatomy of Thomandersia (Lamiales), with particular reference to the nature of the retinaculum and extranuptial nectaries. – Bot. J. Linn. Soc. 149: 469-482.
Wortley AH, Harris DJ, Scotland RW. 2007. On the taxonomy and phylogenetic position of Thomandersia. – Syst. Bot. 32: 415-444.
Wu C-Y. 1959. Revisio Labiatarum Sinensium. – Acta Phytotaxon. Sin. 8: 1-66.
Wu C-Y, Chow S. 1962. Cardioteucris C. Y. Wu, Holocheila (Kudo) S. Chow – duo genera nova labiatarum ex Provencia Yunnan. – Acta Bot. Sin. 10: 247-252.
Wu C-Y, Chow S. 1965. Duo taxa nova labiatarum. – Acta Phytotaxon. Sin. 10: 249-255.
Wu C-Y, Li H. 1982. On the evolution and distribution in Labiatae. – Acta Bot. Yunnan. 4: 97-118.
Wu J-T, Huang T-C. 1975. Biosystematic studies of Formosan Salvia. – Taiwania 20: 77-98.
Wu M, Huang T, Huang S. 2009. Phylogenetic biogeography of Euphrasia section Malesianae (Orobanchaceae) in Taiwan and Malesia. – Blumea 54: 242-247.
Wunderlich R. 1963. The Pogostemoneae – a debatable group of Labiatae (some remarks on their taxonomic position with regard to their pollen grains). – J. Indian Bot. Soc., Sect. A, 42: 321-330.
Wunderlich R. 1967. Ein Vorschlag zu einer natürlichen Gliederung der Labiaten auf Grund der Pollenkörner, der Samenentwicklung und des reifen Samens. – Österr. Bot. Zeitschr. 114: 383-483.
Wunderlin U. 1992. Untersuchungen zur vergleichenden Entwicklungsgeschichte von Scrophulariaceen-Blüten. Die Entwicklung der Blüten von Digitalis lanata, Digitalis lutea, Digitalis isabelliana, Erinus alpinus, Wulfenia baldaccii, Alonsoa warscewiczii und Nemesia capensis. – Diss. Bot. 188: 1-313.
Xia Z, Wang Y-Z, Smith JF. 2009. Familial placement and relations of Rehmannia and Triaenophora (Scrophulariaceae s.l.) inferred from five gene regions. – Amer. J. Bot. 96: 519-530.
Xiang C-L, Liu E-D, Peng H. 2008. A key to the genus Chelonopsis (Lamiaceae) and two new combinations: C. rosea var. siccanea and C. souliei var. cashmerica comb. nov. – Nord. J. Bot. 26: 31-34.
Xiang C-L, Zhang Q, Scheen A-C, Cantino PD, Funamoto T, Peng H. 2013. Molecular phylogenetics of Chelonopsis (Lamiaceae: Gomphostemmateae) as inferred from nuclear and plastid DNA and morphology. – Taxon 62: 375-386.
Xiang C-L, Zhao F, Cantino PD, Drew BT, Li B, Liu E-D, Soltis DE, Soltis PS, Peng H. 2018. Molecular systematics of Caryopteris (Lamiaceae) and its allies with reference to the molecular phylogeny of subfamily Ajugoideae. – Taxon 67: 376-394.
Xu H, Li Z-Y, Jiang H. 2008. A new species of Chirita (Gesneriaceae) from Yunnan, China. – Bot. J. Linn. Soc. 158: 269-273.
Xu W-B, Zhang Q, Wen F, Liao W-B, Pan B, Chang H, Chung K-F. 2012. Nine new combinations and one new name of Primulina (Gesneriaceae) from South China. – Phytotaxa 64: 1-8.
Xu W-B, Meng T, Zhang Q, Wu W-H, Liu Y, Chung
K-F. 2014. Petrocodon (Gesneriaceae) in the limestone
karsts of Guangxi, China: three new species and a new combination based on
morphological and molecular evidence. – Syst. Bot. 39: 965-974.
Xu Z-R, Burtt BL, Skog LE, Middleton DJ. 2008. A revision of Paraboea (Gesneriaceae). – Edinburgh J. Bot. 65: 161-347.
Yakovleva SV. 1933. Caryological investigations of some Salvia species. – Bull. Appl. Bot. Genet. Plant Breeding II, 5: 207-213. [In Russian]
Yamazaki T. 1953a. On the floral structure, seed development, and affinities of Deinostema, a new genus of Scrophulariaceae. – J. Jap. Bot. 28: 129-133.
Yamazaki T. 1953b. On the floral structure, seed development, and affinities of Deinostema, a new genus of Scrophulariaceae II. – Bot. Mag. (Tokyo) 66: 141-149.
Yamazaki T. 1954. Notes on Lindernia, Vandellia, Torenia and their allied genera in Eastern Asia I. – J. Jap. Bot. 29: 299-306.
Yamazaki T. 1955. Notes on Lindernia, Vandellia, Torenia and their allied genera in Eastern Asia II. – Bot. Mag. (Tokyo) 68: 14-24.
Yamazaki T. 1957a. Taxonomical and phylogenetic studies of Scrophulariaceae-Veroniceae with special reference to Veronica and Veronicastrum in eastern Asia. – J. Fac. Sci. Univ. Tokyo, Sect. III, Bot. 7: 92-162.
Yamazaki T. 1957b. Seed formation of Ellisiophyllum pinnatum var. reptans. – Bot. Mag. (Tokyo) 70: 162-168.
Yamazaki T. 1963. Euphrasia of Eastern Asia 2. – Acta Phytotaxon. Geobot. 19: 164-172.
Yamazaki T. 1968. On the genus Pseudolysimachion. – J. Jap. Bot. 43: 405-412.
Yamazaki T. 1993. Two new species of Mimulus from the Himalayas. – J. Jap. Bot. 68: 23-26.
Yamazaki T. 1994. A new species of Brookea (Scrophulariaceae) from Borneo. – J. Jap. Bot. 69: 282-284.
Yan K, Zhao N, Li H-Q. 2007. Systematic relationships among Rehmannia (Scrophulariaceae) species. – Acta Bot. Boreal.-Occid. Sin. 27: 1112-1120. [In Chinese with English abstract]
Yang F-S, Wang X-Q. 2007. Extensive length variation in the cpDNA trnT-trnF region of hemiparasitic Pedicularis and its phylogenetic implications. – Plant Syst. Evol. 264: 251-264.
Yang X, Lu S-G, Peng H. 2007. First report of chromosome numbers of the Carlemanniaceae (Lamiales). – J. Plant Res. 120: 707-712.
Yang Z, Xun G, Yuezhi P. 2004. Cytological study of six Salvia species (Lamiaceae) from the Hengduanshan Mountains region of China. – Caryologia 57: 360-366.
Yang Z, Zhang L, Zhao H, Yang R, Ding C, Zhou Y, Wan D. 2009. Chromosome numbers of some species of Salvia (Lamiaceae) from Sichuan Province, China. – Nord. J. Bot. 27: 287-291.
Yang Z-J, Li Z-Y. 2003. Studies on pollen morphology of genus Didymocarpus (Gesneriaceae) in China. – Guizhou Sci. 21: 5-9.
Yao G, Deng YF, Ge XJ. 2015. A taxonomic revision of Pogostemon (Lamiaceae) from China. – Phytotaxon 200: 1-67.
Yao G, Drew BT, Yi T-S, Yan H-F, Yuan Y-M, Ge X-J. 2016. Phylogenetic relationships, character evolution and biogeographic diversification of Pogostemon s.l. (Lamiaceae). – Mol. Phylogen. Evol. 98: 184-200.
Yao TL. 2015. Three new species of Loxocarpus (Gesneriaceae) from Sarawak, Borneo. – Gardens’ Bull. (Singapore) 67: 289-296.
Yatskievych G, Jiménez JLC. 2009. A new genus of holoparasitic Orobanchaceae from Mexico. – Novon 19: 266-276.
Yeo PF. 1978. A taxonomic revision of Euphrasia in Europe. – Bot. J. Linn. Soc. 77: 223-334.
Yeo PF. 1990. A re-definition of Verbena brasiliensis. – Kew Bull. 45: 101-120.
Yetter TC. 1989. Systematic and phylogenetic relationships in the virginianum species complex of the genus Pycnanthemum (Labiatae). – Ph.D. diss., Miami University, Oxford, Ohio.
Yoshida S, Shirasu K. 2009. Multiple layers of incompatibility to the parasitic witchweed, Striga hermonthica. – New Phytol. 183: 180-189.
Yoshida S, Maruyama S, Nozaki H, Shirasu K. 2010. Horizontal gene transfer by the parasitic plant Striga hermonthica. – Science 328: 1128.
Young ND, de Pamphilis CW. 2000. Purifying selection detected in the plastid gene matK and flanking ribozyme regions within a group II intron of nonphotosynthetic plants. – Mol. Biol. Evol. 17: 1933-1941.
Young ND, dePamphilis CW. 2005. Rate variation in parasitic plants: correlated and uncorrelated patterns among plastid genes of different functions. – BioMed Central Evol. Biol. 5: 16.
Young ND, Steiner KE, dePamphilis CW. 1999. The evolution of parasitism in the Scrophulariaceae/Orobanchaceae: plastid gene sequences refute an evolutionary transition series. – Ann. Missouri Bot. Gard. 86: 876-893.
Yousefi N, Zarre S, Heubl G. 2016. Molecular phylogeny of the mainly Mediterranean genera Chaenorhinum, Kickxia and Nanorrhinum (Plantaginaceae, tribe Antirrhineae), with focus on taxa in the Flora Iranica region. – Nord. J. Bot. 34: 455-463.
Yu W-B. 2013. Nomenclatural clarifications for names in Boschniakia, Kopsiopsis and Xylanche (Orobanchaceae). – Phytotaxa 77: 40-42.
Yu X-Q, Maki M, Drew BT, Paton AJ, Li H-W,
Zhao J-L, Conran JG, Li J. 2014. Phylogeny and historical biogeography of
Isodon (Lamiaceae): rapid
radiation in south-west China and Miocene overland dispersal into Africa. –
Molec. Phylogen. Evol. 77: 183-194.
Yu Z, Li S, Hsu G, Hsu Y. 1987. A preliminary study on the number and morphology of chromosomes in Paulownia. – Acta Bot. Bor.-Occid. Sin. 7: 127-132. [In Chinese]
Yuan W-J, Zhang W-R, Han Y-J, Dong M-F, Shang F-D. 2010. Molecular phylogeny of Osmanthus (Oleaceae) based on non-coding chloroplast and nuclear ribosomal internal transcribed spacer regions. – J. Syst. Evol. 48: 482-489.
Yuan Y-W, Olmstead RG. 2008a. A species-level phylogenetic study of the Verbena complex (Verbenaceae) indicates two independent intergeneric chloroplast transfers. – Mol. Phylogen. Evol. 48: 23-33.
Yuan Y-W, Olmstead RG. 2008b. Evolution and phylogenetic utility of the PHOT gene duplicates in the Verbena complex (Verbenaceae): dramatic intron size variation and footprint of ancestral recombination. – Amer. J. Bot. 95: 1166-1176.
Yuan Y-W, Liu C, Marx HE, Olmstead RG. 2010. An empirical demonstration of using PPR (pentatricopeptide repeat) genes as phylogenetic tools: phylogeny of Verbenaceae and the Verbena complex. – Mol. Phylogen. Evol. 54: 23-35.
Yuan Y-W, Mabberley DJ, Steane DA, Olmstead RG. 2010. Further disintegration and redefinition of Clerodendrum (Lamiaceae): implications for the understanding of the evolution of an intriguing breeding strategy. – Taxon 59: 125-133.
Yuen CKKH, Dehgan B. 1982. Comparative morphology of the leaf epidermis in the genera Codonanthe (Martius) Hanstein and Nematanthus Schrader (Gesneriaceae). – Bot. J. Linn. Soc. 85: 283-296.
Yüzbaşıoğlu E, Dadandı MY. 2008. Phylogenetic relationships among species of the subsection Dendrophlomis Bentham. – Electr. J. Biotechn. ISSN: 0717-3458.
Zamora R, Jamilena M, Rejon MR, Blanca G. 1996. Two new species of the carnivorous genus Pinguicula (Lentibulariaceae) from Mediterranean habitats. – Plant Syst. Evol. 200: 41-60.
Zamski E. 1979. The mode of secondary growth and the three dimensional structure of the phloem in Avicennia. – Bot. Gaz. 140: 67-76.
Zamudio Ruiz S. 1988. Dos nuevas especies de Pinguicula (Lentibulariaceae) del centro y norte de México. – Acta Bot. Mexicana 3: 21-28.
Zamudio Ruiz S. 2001. Una especie nueva notable de Pinguicula (Lentibulariaceae) de los estados de Querétaro y San Luis Potosí, Mexico. – Bol. Soc. Bot. México 68: 85-88.
Zamudio Ruiz S. 2003. Pinguicula conazattii (Lentibulariaceae), unaespecie nueva del estado de Oaxaca, México. – Acta Bot. Mexic. 62: 15-20.
Zamudio Ruiz S. 2005. Dos especies nuevas de Pinguicula (Lentibulariaceae) de la sierra madre oriental México. – Acta Bot. Mexic. 70: 69-83.
Zamudio Ruiz S, Rzedowski J. 1991. Dos especies nuevas de Pinguicula (Lentibulariaceae) del estado de Oaxaca, Mexico. – Acta Bot. Mexicana 14: 23-32.
Zan Y. 2005. A new species of Triaenophora (Scrophulariaceae) from China. – Novon 15: 559-561.
Zhang R-X, Li M-X, Jia Z-P. 2008. Rehmannia glutinosa: review of botany, chemistry and pharmacology. – J. Ethnopharm. 117: 199-214.
Zhang Z-Y. 1990. Studies on the pollen morphology and seed coat of the genus Cistanche (Orobanchaceae) in China. – Aca Phytotaxon. Sin. 28: 294-298. [In Chinese]
Zhong JJ, Li L, Conran JG, Li H-W. 2010. Phylogeny of Isodon (Schrad. ex Benth.) Spach (Lamiaceae) and related genera inferred from nuclear ribosomal ITS, trnL-trnF region, and rps16 intron sequences and morphology. Syst. Bot. 35: 207-219.
Zhong JJ, Preston JC, Hileman LC, Kellogg EA. 2017. Repeated and diverse losses of corolla bilateral symmetry in the Lamiaceae. – Ann. Bot. 119: 1211-1223.
Zhongxin W. 1989. Pollen morphology of Wightia and its taxonomic significance. – Acta Metall. Sin. 11: 1-3.
Zhou Q-M, Jensen S, Liu G-L, Wang S, Li H-Q.
2014. Familial placement of Wightia (Lamiales). – Plant Syst. Evol.
300: 2009-2017.
Zhou S-L, Pan K-Y, Hong D-Y. 1997. Pollen and nutlet morphology in Mosla (Labiatae) and their systematic value. – Israel J. Plant Sci. 45: 343-350.
Zhou X-R, Wang Y-Z, Smith JF, Chen R. 2008. Altered expression patterns of TCP and MYB genes relating to the floral developmental transition from initial zygomorphy to actinomorphy in Bournea (Gesneriaceae). – New Phytol. 178: 532-543.
Zhu Z-Y, Min B-Q, Wang Q-L. 2011. Taxa nova Salviorum labiatarum. – Bull. Bot. Res. 31: 1-3.
Ziegler A. 1955. Die blau fluoreszierenden Idioblasten der Scrophulariaceen. – Sitzungsber. Österr. Akad. Wiss., Math.-Naturwiss. Kl., Abt.1, 164: 419-485.
Ziemer RR. 1973. Some field observations of Darlingtonia and Pinguicula. – Carniv. Plant Newsl. 2: 25-27.
Zimmer EA, Roalson EH, Skog LE, Boggan JK, Idnurm A. 2002. Phylogenetic relationships in the Gesnerioideae (Gesneriaceae) based on nrDNA ITS and cpDNA trnL-F and trnE-T spacer region sequences. – Amer. J. Bot. 89: 296-311.
Zjhra ML. 1988. Phylogenetics, biogeography, and pollination ecology of endemic Malagasy Coleeae (Bignoniaceae). – Ph.D. diss., University of Wisconsin, Madison, Wisconsin.
Zjhra ML. 2006. New taxa of Coleeae (Bignoniaceae) from Madagascar I. A collection from Masoala Peninsula. – Ann. Bot. Fenn. 43: 225-239.
Zjhra ML, Sytsma KJ, Olmstead RG. 2004. Delimitation of Malagasy tribe Coleeae and implications for fruit evolution in Bignoniaceae inferred from a chloroplast DNA phylogeny. – Plant Syst. Evol. 245: 55-67.
Zoz IG, Litvinenko VI. 1979. On the division of the family Lamiaceae Juss. into natural groups. – Bot. Žurn. 64: 989-997. [In Russian]
Zuntini AR, Lohmann LG. 2014. Synopsis of
Martinella Baill. (Bignonieae, Bignoniaceae), with the
description of a new species from the Atlantic forest of Brazil. – PhytoKeys
37: 15-24.