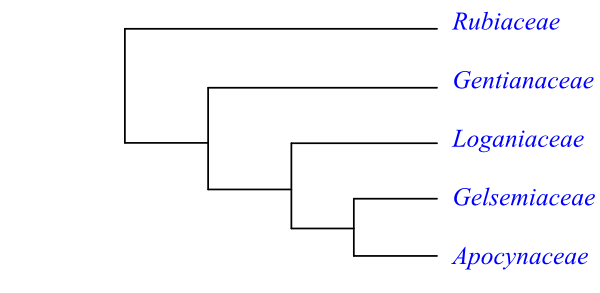
Cladogram of Rubiales based on DNA sequence data (Soltis & al. 2011; Frasier 2009; etc.).
[Rubiales+[Plantaginales+Solanales]]
Gentianales Juss. ex Bercht. et J. Presl, Přir. Rostlin: 248. Jan-Apr 1820 [‘Gentianeae’]; Gentiananae Thorne ex Reveal in Novon 2: 236. 13 Oct 1992
Habit Usually bisexual (rarely monoecious, gynomonoecious, polygamomonoecious, dioecious, gynodioecious, or functionally dioecious), evergreen trees, shrubs, suffrutices or lianas, perennial, biennial or annual herbs. Numerous species are xerophytes. Many are stem succulents. Some species are myrmecotrophic. Few species are epiphytic.
Vegetative anatomy Phellogen ab initio usually superficial (sometimes deeply seated). Secondary lateral growth normal or anomalous (from cylindrical cambium or concentric cambia). Endodermis often prominent. Vessel elements usually with simple (sometimes scalariform, rarely reticulate) perforation plates; lateral pits alternate, simple or bordered pits. Vestured pits present. Imperforate tracheary elements tracheids, fibre tracheids or libriform fibres with simple and/or bordered pits, usually non-septate (sometimes septate; also vasicentric tracheids). Wood rays uniseriate or multiseriate, homocellular or heterocellular, or absent. Axial parenchyma apotracheal diffuse or diffuse-in-aggregates, or paratracheal scanty vasicentric, reticulate, scalariform, unilateral, confluent or banded (sometimes absent). Cambium and wood elements sometimes storied. Intraxylary phloem usually present. Sieve tube plastids S0 or Ss type. Nodes usually 1:1, unilacunar with on leaf trace, or 3:3, trilacunar with three traces (sometimes multilacunar, with several traces). Non-articulated branched or unbranched laticifers with white or bluish latex sometimes present. Prismatic calciumoxalate crystals as raphids, crystal sand, acicular crystals, styloids or druses often present.
Trichomes Hairs unicellular or multicellular, uniseriate or multiseriate, simple or branched, stellate, T-shaped, spiral, band-like, dendritic, candelabra-shaped, moniliform, papillose or lepidote, or absent; glandular hairs sometimes present.
Leaves Usually opposite (sometimes verticillate, rarely alternate), simple, usually entire (rarely lobed or modified into spines), sometimes coriaceous (rarely scale-like), usually with conduplicate or flat ptyxis. Stipules interpetiolar, intrapetiolar, sheathing or absent; leaves sometimes united simply by line across stem/branch; leaf sheath absent. Colleters consisting of secretory palisade layer surrounding elongate central axis; usually present on adaxial side of stipules, in leaf axils, on nodes and/or petiole bases, also on leaves, calyx and corolla. Petiole vascular bundle transection arcuate or annular. Venation usually pinnate (sometimes palmate or leaves uninerved). Stomata anomocytic, paracytic, anisocytic, or cyclocytic (sometimes parallelocytic, rarely actinocytic). Cuticular wax crystalloids? Domatia as pits, pockets or hair tufts (acarodomatia or bacteriodomatia rarely present), or absent. Epidermis with or without crystal idioblasts. Secretory cavities (laticifers) sometimes with latex. Epidermis and/or mesophyll with or without mucilage cells. Mesophyll with or without sclerenchymatous idioblasts. Leaf margin usually entire (rarely serrate or crenate). Extrafloral nectaries present in some species on adaxial surface of lamina.
Inflorescence Terminal or axillary, panicle, thyrse, umbel-, spike- or head-like, dichasial, corymb, pseudoverticillate or fasciculate cymose, or flowers solitary terminal or axillary.
Flowers Usually actinomorphic (rarely zygomorphic). Epicalyx usually absent. Hypogyny, epigyny or partial epigyny. Sepals (three or) four or five (to 16), with imbricate, convolute or valvate (sometimes open or contorted) aestivation, often persistent (sometimes accrescent), usually more or less connate (rarely free), often with adaxial colleters at base. Petals (three or) four or five (to 16), usually with contorted, imbricate or valvate (rarely imbricate or cochleate) aestivation, more or less connate into hypocrateromorphous, infundibuliform, campanulate or tubular corolla, often with adaxial hairs. Nectaries usually present, sometimes on abaxial side of ovary, sometimes as nectariferous glands. Intrastaminal, annular disc often present.
Androecium Stamens (one, three or) four or five (to 25), haplostemonous, antesepalous, alternipetalous. Filaments free or connate into tube (rarely connate in fascicles), free from corolla tube or epipetalous (filaments sometimes with nectariferous appendages of various shape, forming parts of gynostegium). Anthers usually free (sometimes connate and/or adnate to style into gynostegium), basifixed or dorsifixed, versatile or non-versatile, usually tetrasporangiate (sometimes disporangiate), usually introrse (occasionally extrorse or latrorse), usually longicidal (dehiscing by longitudinal slits; sometimes poricidal, dehiscing by apical pores). Tapetum usually secretory (rarely amoeboid-periplasmodial). Staminodia usually absent (sometimes one to four, alternating with fertile stamens; female flowers often with staminodia). Pollinaria present in many Apocynaceae (especially in Asclepiadoideae), consisting of pollinia and translator.
Pollen grains Microsporogenesis usually simultaneous (sometimes successive). Pollen grains (2–)3(–6)-colporate or (1–)3(–6)-porate (rarely colpate, pororate, polyporate or syncolpate), usually shed as monads (sometimes tetrads, massulae or pollinia), bicellular or tricellular at dispersal. Exine tectate or semitectate (rarely intectate), usually with columellate (sometimes granular) infratectum, perforate, reticulate, microreticulate, striate, scabrate, psilate, fossulate, spinulate, verrucate, echinulate, granulate, or smooth.
Gynoecium Pistil composed of usually two (rarely three to eight or up to 16) connate carpels (carpels sometimes partially free, or secondarily free in ovary region). Ovary superior, inferior or semi-inferior, usually bilocular (sometimes unilocular, rarely trilocular to novemlocular, or divided by secondary septa). Style single, simple, or stylodia two (to five; sometimes connate above only), sometimes persistent (rarely absent). Stigma capitate, punctate, clavate, truncate, or bilobate (to quinquelobate; sometimes subpeltate, or swollen at apex and constricted in central part and in lower part ring-like), papillate or non-papillate, Dry or Wet type. Pistillodium usually absent (male flowers often with pistillodium).
Ovules Placentation axile or apical (when ovary bilocular or multilocular) or parietal (when ovary unilocular; sometimes intrusively parietal, rarely basal or free central). Ovules one to numerous per carpel, anatropous or amphitropous (rarely campylotropous, orthotropous, circinotropous or hypertropous), usually pendulous (sometimes horizontal or ascending), apotropous or epitropous, usually unitegmic (rarely ategmic), usually tenuinucellar (often reduced tenuinucellar; rarely crassinucellar or pseudocrassinucellar or megasporangium absent). Endothelium absent. Megagametophyte usually monosporous, Polygonum type (rarely disporous, Allium type, or tetrasporous, Drusa type). Synergids sometimes with a filiform apparatus. Antipodal cells sometimes persistent, occasionally proliferating and/or haustorial. Endosperm development usually ab initio nuclear (rarely cellular). Endosperm haustorium micropylar, chalazal (rarely lateral) or absent. Embryogenesis caryophyllad or solanad (rarely onagrad).
Fruit A loculicidal and/or septicidal (rarely denticidal) capsule, a berry, a drupe, or a schizocarp with usually two follicular, nutlike, drupaceous or baccate mericarps (rarely a follicle or a pyxidium, or a pseudofruit consisting of connate ovaries), calyx often persistent.
Seeds Aril sometimes present. Testa usually multiplicative (seed coat sometimes thin or absent). Exotestal cell walls usually thickened (theoidal exotestal thickenings). Mesotesta sometimes with flattened crystalliferous cells. Endotesta often crushed. Perisperm not developed. Endosperm copious to sparse, oily (sometimes with starch or hemicellulose), sometimes ruminate, or absent. Embryo large or small, straight or curved, well differentiated (rarely rudimentary, undifferentiated), with or without chlorophyll. Cotyledons two. Germination phanerocotylar or cryptocotylar.
Cytology x = (5–)8–15 – Protein crystalloids present in nucleus.
DNA Mitochondrial intron coxII.i3 present or absent.
Phytochemistry Flavonols (kaempferol, quercetin, myricetin [rare]), flavones, O-methylated flavones, cyanidin, delphinidin, Route I iridoids (also secoiridoids), Route II decarboxylated iridoids, Group I carbocyclic iridoids (theviridoside, daphylloside, plumieride, geniposide, scandoside, monotropein, gardenoside), Group II carbocyclic iridoids (shantziside), Group VI secoiridoids (secologanin), Group VII secoiridoids (sweroside, swertiamarin, gentiopicroside), Group IX secoiridoids (indole alkaloids of corynanthe type, aspidosperma and iboga type indole alkaloids, ipecacalkaloids), Group X secoiridoids (desoxyloganin, loganin, ketologanin, antirrhide, gardoside, iridoid pyridine alkaloids), iridoid coumarins (asperuloside), cardenolides, triterpenes (in latex), tannins, ursolic acid, caffeic acid, pyrrolizidine alkaloids as aliphatic monocarboxylic esters and esters of arylic and aralkylic acids, camptothecine and other C17 indole alkaloids, isoquinoline alkaloids, steroid alkaloids, tryptophane-derived alkaloids, toxic (cardiotonic) steroid glycosides, cyanogenic compounds (rare), saponins, anthraquinones (usually shikimic acid derived), xanthones, and arbutin present. Ellagic acid not found.
Systematics Rubiales may be sister to [Lamiales+Solanales], although the support is low.
Rubiaceae are sister to the [Gentianaceae+[Loganiaceae+[Gelsemiaceae+Apocynaceae]]] clade. Potential synapomorphies of this clade are presence of intraxylary phloem; late corolla tube formation; postgenital syncarpy; and presence of Route I secoiridoids (Stevens 2001 onwards). Gelsemiaceae and Apocynaceae share the potential morphological synapomorphies nodes 1:1 and flattened seeds.

|
Cladogram of Rubiales based on DNA sequence data (Soltis & al. 2011; Frasier 2009; etc.). |
APOCYNACEAE Juss. |
( Back to Rubiales ) |
Asclepiadaceae Borkh., Bot. Wörterb. 1: 37. 1797 [‘Asclepiadeae’], nom. cons.; Vincaceae Vest, Anleit. Stud. Bot.: 273, 299. 1818 [‘Vincoideae’]; Apocynales R. Br. ex Bercht. et J. Presl, Přir. Rostlin: 249. Jan-Apr 1820 [‘Apocineae’]; Asclepiadales R . Br. ex Bercht. et J. Presl, Přir. Rostlin: 249. Jan-Apr 1820 [‘Asclepiadeae’]; Cerberaceae Martinov, Tekhno-Bot. Slovar: 119. 3 Aug 1820 [‘Cerberides’]; Pacouriaceae Martinov, Tekhno-Bot. Slovar: 447. 3 Aug 1820 [‘Pacurides’]; Plumeriaceae Horan., Prim. Lin. Syst. Nat.: 70. 2 Nov 1834 [‘Plumeriaceae (Apoocyneae)’]; Stapeliaceae Horan., Prim. Lin. Syst. Nat.: 70. 2 Nov 1834 [‘Stapeliaceae (Asclepiadeae)’]; Ophioxylaceae Mart., Consp. Regn. Veg.: 24. Sep-Oct 1835 [‘Ophioxyleae’]; Cynanchaceae G. Mey., Chloris Han.: 245, 251. Jul-Aug 1836 [‘Cynancheae’]; Asclepiadopsida Brongn., Enum. Plant. Mus. Paris: xxiii, 51. 12 Aug 1843 [’Asclepiadineae’]; Vincales Horan., Char. Ess. Fam.: 111. 30 Jun 1847 [’Vincastra’]; Willughbeiaceae J. Agardh, Theoria Syst. Plant.: 256. Apr-Sep 1858 [‘Willughbejieae’]; Carissaceae Bertol. in Nuevo Giorn. Bot. Ital. 23: 212. 8 Jan 1891; Periplocaceae (Kostel.) Schltr. in K. Schum. et C. A. G. Lauterb., Nachtr. Fl. Schutzgeb. Südsee: 351. 16 Nov 1905, nom. cons.
Genera/species 311–313/5.100–5.230
Distribution Cosmopolitan except polar areas and cold-temperate regions.
Fossils Unknown.
Habit Usually bisexual (rarely functionally dioecious), evergreen trees, shrubs or lianas (sometimes perennial or annual herbs). Numerous species are xerophytic and many are stem succulents. Some species (e.g. Dischidia rafflesiana) are myrmecotrophic with ant symbiosis (ant colonies present in leaves).
Vegetative anatomy Endomycorrhiza Arum type, Paris type or intermediate types. Phellogen ab initio usually superficial (in, e.g., Rhazya deeply seated). Secondary lateral growth normal or anomalous (from a cylindrical cambium or concentric cambia). Cambium sometimes storied. Pericyclic fibres usually absent. Endodermis often prominent. Vessel elements with usually simple (sometimes scalariform) perforation plates; lateral pits alternate, bordered pits. Vestured pits present. Imperforate tracheary elements tracheids or fibre tracheids with simple or bordered pits, usually non-septate (also vasicentric tracheids). Wood rays uniseriate or multiseriate, homocellular or heterocellular. Axial parenchyma apotracheal diffuse or diffuse-in-aggregates, or paratracheal scanty vasicentric, reticulate, or banded, or absent. Wood elements sometimes storied. Intraxylary phloem (diffuse) usually present. Sieve tube plastids S type. Nodes usually 1:1, unilacunar with one leaf trace (rarely 3:3, trilacunar with three traces). Articulated or non-articulated branched or unbranched laticifers with white or bluish latex frequent (absent in, e.g., Nerium). Prismatic calciumoxalate crystals abundant; crystal sand or druses present in some representatives.
Trichomes Hairs unicellular or multicellular, simple or branched, sometimes dendritic, or absent.
Leaves Usually opposite (sometimes verticillate, rarely alternate), simple, usually entire (rarely lobed or transformed into spines), usually with conduplicate or flat ptyxis. Stipules absent (small, interpetiolar or cauline stipule-like processes sometimes present); leaf sheath absent. Axillary colleters often present (in association with stipules). Petiole vascular bundle transection arcuate?; petiole bundles sometimes adaxial. Venation usually pinnate (sometimes palmate or leaves one-veined). Stomata anomocytic, paracytic, anisocytic, cyclocytic, or parallelocytic (rarely actinocytic). Cuticular wax often as crust. Domatia as pits, pockets or hair tufts, or absent. Epidermis with or without crystal idioblasts. Secretory cavities (laticifers) with latex. Mesophyll with or without sclerenchymatous idioblasts. Leaf margin usually entire (rarely serrate). Extrafloral nectaries present on petiole and adaxial surface of lamina in numerous species.
Inflorescence Terminal or axillary, umbel-like, panicle, dichasial, etc. cymose, or flowers solitary axillary.
Flowers Actinomorphic. Hypogyny or partial epigyny. Sepals usually five (sometimes four), with imbricate quincuncial or valvate aestivation, more or less connate, with adaxial colleters at base. Petals usually five (sometimes four), usually with sinistrally contorted (rarely imbricate or valvate) aestivation, more or less connate; upper part of corolla tube (above staminal insertion point) formed by postgenital fusion; corona often present as scale-like appendages in corolla tube mouth. Nectaries usually present, sometimes on abaxial side of ovary. Intrastaminal disc often present.
Androecium Stamens (four or) five, haplostemonous, antesepalous, alternipetalous. Filaments short, free or connate into tube, free from or adnate to corolla tube; filaments in Secamonoideae? and Asclepiadoideae usually with nectariferous appendages of various shape, forming parts of gynostegium. Anthers free, often connivent, or more or less connate and sometimes adnate to upper part of style and stigma into gynostegium, with or without apical, lateral or dorsal appendages, basifixed or dorsifixed, non-versatile, disporangiate (in Asclepiadoideae) or tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits) or poricidal (dehiscing by apical pores); anthers often sterile below and prolonged into acute tip; connective often prolonged at apex, in Asclepiadoideae (and Secamonoideae?) with wing-like and membranous appendages forming part of corona complex. Tapetum secretory. Staminodia absent.
Pollen grains Microsporogenesis simultaneous or successive. Pollen grains (2–)3–6-colporate or (2–)3–6-porate (sometimes with pseudocolpi), shed as monads, tetrads (tetrahedral, rhomboidal or linear), massulae or pollinia, bicellular or tricellular at dispersal (usually transported within a foam-like substance). Exine tectate, with granular or columellate infratectum, perforate or psilate, fossulate, verrucate, granulate, or smooth (occasionally microreticulate or scabrate).
Gynoecium Pistil composed of usually two (rarely three to eight) connate carpels, or carpels secondarily free in ovary region, connate usually only in stylar part (often exclusively in upper[most] part of style); connation of carpels congenital or postgenital; carpels sometimes collateral; carpels sometimes transversely orientated. Ovary superior or semi-inferior, usually bilocular (sometimes unilocular); locules usually free. Stylodia two, connate entirely or only in upper(most) part; stylar apex usually swollen, pentagonal, with cells secreting pollen-adhering viscid substance consisting of terpenoids and polysaccharides. Stigma usually with terminal stigmatic head, swollen at apex, constricted in central part and with receptive ring, hair ring or membrane in lower part; pollen-receptive surfaces lateral, alternating with stamens; stigmatic surfaces papillate or non-papillate, Dry or Wet type. Gynostegium often present, developed through postgenital fusion of anther connective and stigmatic head. Pistillodium absent. Secondary pollen presentation often occurring (pollen grains deposited on apex of stigmatic head).
Ovules Placentation axile (ventral in each locule) or apical (when ovary bilocular or multilocular) or parietal (when ovary unilocular). Ovules two to numerous (rarely one) per carpel, anatropous, amphitropous or hemitropous (rarely orthotropous), usually pendulous, unitegmic, usually tenuinucellar (reduced tenuinucellar, with meiocyte semi-inferior; sometimes pseudocrassinucellar). Integument six to nine cell layers thick. Endothelium absent (always?). Megagametophyte monosporous, Polygonum type. Synergids sometimes with a filiform apparatus. Antipodal cells sometimes persistent. Endosperm development ab initio nuclear. Endosperm haustorium micropylar?; chalazal haustorium usually absent. Embryogenesis caryophyllad or solanad.
Fruit A capsule, a berry, a drupe, or a schizocarp with two follicular, nutlike, drupaceous or baccate (often divergent) mericarps.
Seeds. Aril sometimes present. Testa winged or unwinged, usually multiplicative, often with terminal coma consisting of long silky hairs (seed coat sometimes absent). All exotestal cell walls usually thickened (in, e.g., Periploca non-thickened). Mesotesta sometimes with flattened crystalliferous cells. Endotesta? Perisperm not developed. Endosperm copious to sparse, oily, sometimes ruminate, or absent. Embryo straight, well differentiated, with or without chlorophyll. Cotyledons two, flat, folded or in-rolled. Germination phanerocotylar or cryptocotylar. Seedling leaves sometimes alternate.
Cytology x = (6–)9–11(–14) (23) – Polyploidy frequently occurring. Protein crystalloids present in nucleus.
DNA Mitochondrial intron coxII.i3 lost in Catharantus. Mitochondrial coxI intron usually present.
Phytochemistry Flavonols (kaempferol, quercetin), O-methylated flavones, cyanidin, delphinidin, Route I carbocyclic iridoids (theviridoside, daphylloside, plumieride), Route II decarboxylated iridoids, Group VI secoiridoids (secologanin), Group VII secoiridoids (sweroside), Group IX secoiridoids (indole alkaloids of corynanthe-, aspidosperma- and iboga-type indole alkaloids), Group X secoiridoids (desoxyloganin, loganin, ketologanin, iridoid pyridine alkaloids), cardiac glycosides (cardenolides), triterpenes (in latex), tannins, ursolic acid, caffeic acid, pyrrolizidine alkaloids as aliphatic monocarboxylic esters and esters of arylic and aralkylic acids, steroidal pregnane pseudoalkaloids, toxic (cardiotonic) steroidal glycosides, tryptophane-derived alkaloids, and saponins present. Cyanogenic compounds very rare. Ellagic acid not found. Aluminium accumulated in some species.
Use Ornamental plants, medicinal plants (Cataranthus roseus, Rauvolfia, etc.), arrow poisons, perfumes (Plumeria etc.), timber, latex (gums from Carpodinus, Funtumia elastica, Hancornia speciosa, Landolphia, Mascarenhasia), fruits (Carissa carandas).
Systematics Apocynaceae are sister to Gelsemiaceae.
A plausible topology for the Apocynaceae phylogeny is the following (Livshultz & al. 2007; Lens & al. 2008).
[Aspidospermateae+[Alstonieae+[[Vinceae+[Willughbeeae+Tabernaemontaneae]]+[Diplorhynchus-Stephanostegia clade+Melodineae+Amsonia+[Condylocarpinae+[Chilocarpeae+[Alyxieae+[Plumerieae+[Carisseae+[Wrightieae+[Nerieae+[Malouetieae+[Rhabdadenia+[Periplocoideae+[Apocyneae+[New World clade+[Baisseeae+[Secamonoideae+Asclepiadoideae]]]]]]]]]]]]]]]]]]
Aspidospermateae Miers, Apocyn. S. Amer.: 7. Mai-Jun 1878 [‘Aspidospermeae’]
4/c 73. Aspidosperma (c 65; Central America, Hispaniola, tropical South America), Anechites (1; A. nerium; Nicaragua to Peru), Geissospermum (6; G. argenteum, G. fuscum, G. laeve, G. reticulatum, G. sericeum, G. urceolatum; Brazil), Haplophyton (1; H. cimicidum; Mexico, Guatemala). – Tropical America. Uniseriate wood rays absent. Seed in Haplophyton with coma. – Aspidospermateae are sister-group to the remaining Apocynaceae.
[Alstonieae+[[Vinceae+[Willughbeeae+Tabernaemontaneae]]+[Diplorhynchus-Stephanostegia clade+Melodineae+Amsonia+[Condylocarpinae+[Chilocarpeae+[Alyxieae+[Plumerieae+[Carisseae+[Wrightieae+[Nerieae+[Malouetieae+[Rhabdadenia+[Periplocoideae+[Apocyneae+[New World clade+[Baisseeae+[Secamonoideae+Asclepiadoideae]]]]]]]]]]]]]]]]]
Alstonieae G. Don, Gen. Hist. 4: 70, 86. 1837-8 Apr 1838
5/c 52. Alstonia (c 45; tropical Africa, Madagascar, southern China, tropical Asia, Melanesia, islands in the western Pacific, one species, A. longifolia, in Central America), Laxoplumeria (3; L. baehiana, L. macrophylla, L. tessmannii; eastern Peru, Brazil), Microplumeria (1; M. anomala; Amazonian Brazil), Strempeliopsis (2; S. arborea: Jamaica; S. strempelioides: Cuba), Dyera (2; D. costulata, D. polyphylla; West Malesia). – Almost pantropical. Uniseriate wood rays absent.
[[Vinceae+[Willughbeeae+Tabernaemontaneae]]+[Diplorhynchus-Stephanostegia clade+Melodineae+Amsonia+[Condylocarpinae+[Chilocarpeae+[Alyxieae+[Plumerieae+[Carisseae+[Wrightieae+[Nerieae+[Malouetieae+[Rhabdadenia+[Periplocoideae+[Apocyneae+[New World clade+[Baisseeae+[Secamonoideae+Asclepiadoideae]]]]]]]]]]]]]]]]
[Vinceae+[Willughbeeae+Tabernaemontaneae]]
Vinceae Duby, Bot. Gall. 1: 324. 12-14 Apr 1828
7/140–143. Petchia (8; Cameroon, Madagascar, the Comoros, Sri Lanka), Rauvolfia (76; tropical regions on both hemispheres), Catharanthus (8; Madagascar, India, Sri Lanka), Vinca (6; V. difformis, V. erecta, V. herbacea, V. major, V. minor, V. soneri; Europe, the Mediterranean, North Africa to Central Asia), Ochrosia (40–43; the Mascarene Islands, the Seychelles, Southeast Asia, Malesia to tropical Australia, islands in western Pacific to the Hawaiian Islands), Kamettia (1; K. caryophyllata; southern India), Rhazya (1; R. stricta; southeastern Europe to the Arabian Peninsula and Pakistan). – Tropical regions on both hemispheres. – Vinceae are sister to [Willughbeeae+Tabernaemontaneae]].
[Willughbeeae+Tabernaemontaneae]
Willughbeeae A. DC. in A. P. de Candolle et A. L. P. P. de Candolle, Prodr. 8: 318. Mar (med.) 1844 [‘Willughbeiae’]
18/c 157. Ancylobotrys (7; A. amoena, A. capensis, A. petersiana, A. pyriformis, A. robusta, A. scandens, A. tayloris; tropical and southern Africa, Madagascar), Bousigonia (3; B. angustifolia, B. mekongensis, B. tonkinensis; Southeast Asia), Chamaeclitandra (1; C. henriquesiana; tropical Africa), Clitandra (1; C. cymulosa; tropical Africa), Couma (5; C. catingae, C. guianensis, C. macrocarpa, C. rigida, C. utilis; northeastern South America), Cyclocotyla (1; C. congolensis; Central Africa), Cylindropsis (1; C. parvifolia; tropical West and Central Africa), Dictyophleba (6; D. leonensis, D. lucida, D. ochracea, D. rudens, D. setosa, D. stipulosa; tropical Africa), Hancornia (1; H. speciosa; Brazil), Lacmellea (24; tropical South America), Landolphia (c 65; tropical and southern Africa, the Mascarene Islands, tropical America), Leuconotis (4; L. anceps, L. bullata, L. eugenifolius, L. griffithii; Malesia), Orthopichonia (6; O. barteri, O. cirrhosa, O. indeniensis, O. schweinfurthii, O. seretii, O. visciflua; tropical West and Central Africa), Pacouria (3; P. boliviensis, P. guianensis, P. paraensis; tropical South America), Parahancornia (7; P. amara, P. fasciculata, P. krukovii, P. negroensis, P. oblonga, P. peruviana, P. surrogata; tropical South America), Saba (3; S. comorensis, S. senegalensis, S. thompsonii; tropical Africa, Madagascar), Vahadenia (2; V. caillei, V. laurentii; tropical West and Central Africa), Willughbeia (17; India and Sri Lanka to Borneo and Sulawesi). – Pantropical. – Willughbeeae may be sister to [Kopsia+Tabernaemontaneae].
Tabernaemontaneae G. Don, Gen. Hist. 4: 70, 87. 1837-8 Apr 1838
17/187–188. Kopsia (22; Southeast Asia, West Malesia, the Caroline Islands); Molongum (3; M. laxum, M. lucidum, M. zschokkeiforme; tropical South America), Schizozygia (1; S. coffeoides; tropical East Africa, the Comoros), Ambelania (3; A. acida, A. duckei, A. occidentalis; tropical South America), Callichilia (6; C. barteri, C. basileis, C. bequaertii, C. inaequalis, C. monopodialis, C. subsessilis; tropical Africa), Tabernaemontana (c 110; tropical regions on both hemispheres), Calocrater (1; C. preussii; tropical West and Central Africa), Carvalhoa (1; C. campanulata; eastern and southeastern Africa), Crioceras (1; C. dipladeniiflorus; Gabon to Angola), Macoubea (3; M. guianensis, M. mesoamericana, M. sprucei; Central America, tropical South America), Mucoa (2; M. duckei, M. pantchenkoana; tropical South America), Neocouma (2; N. parviflora, N. ternstroemiacea; tropical South America), Rhigospira (1; R. quadrangularis; tropical South America, the Andes), Spongiosperma (6; S. cataractarum, S. grandiflorum, S. longilobum, S. macrophyllum, S. oleifolium, S. riparium; tropical South America), Tabernanthe (2; T. elliptica, T. iboga; Central Africa), Voacanga (12; tropical and subtropical regions in the Old World), Woytkowskia (1–2; W. spermatochorda; tropical South America). – Pantropical. Calyx with colleters at base. Filaments absent. Anthers connivent, with lignified basal appendages (nectar guides). Nectaries paired and fused with stigmatic head complex. Pollen grains sometimes porate. Ovary often apocarpous (carpels more or less free). Stigma usually with stigmatic head complex consisting of apical quinquelobate stigmatic crest and thickened basal flange. Placentation axile or parietal. Fruit a berry, a drupe or a follicle. Seed sometimes arillate, sometimes ruminate. x = (9) 10, 11 (23). Indole alkaloids often present. – Kopsia may be sister to the remaining Tabernaemontaneae.
[Diplorhynchus-Stephanostegia clade+Melodineae+Amsonia+Hunterieae+[Condylocarpinae+[Chilocarpeae+[Alyxieae+[Plumerieae+[Carisseae+[Wrightieae+[Nerieae+[Malouetieae+[Rhabdadenia+[Periplocoideae+[Apocyneae+[New World clade+[Baisseeae+[Secamonoideae+Asclepiadoideae]]]]]]]]]]]]]]]
Diplorhynchus-Stephanostegia clade
3/4. Diplorhynchus (1; D. condylocarpon; tropical and southern Africa), Pycnobotrya (1; P. nitida; Central Africa), Stephanostegia (2; S. capuronii, S. hildebrandtii; Madagascar). – Tropical and southern Africa, Madagascar. The sister-group relationships of this clade are not resolved.
Melodineae G. Don, Gen. Hist. 4: 71, 101. 1837-8 Apr 1838
2/25. Craspidospermum (1; C. verticillatum; Madagascar), Melodinus (c 20; tropical Asia, islands in the Pacific). – Madagascar, tropical Asia, islands in the Pacific. The sister-group relationships of Melodineae are not resolved.
Amsonia clade
1/c 20. Amsonia (c 20; Greece, Turkey, China, the Korean Peninsula, Japan, the United States, Mexico). – The sister-group relationships of Amsonia are not resolved.
Hunterieae Miers, Apocyn. S. Amer.: 6. Mai-Jun 1878
4/21. Gonioma (2; G. kamassi: Western and Eastern Cape, KwaZulu-Natal, Swaziland; G. malagasy: southwestern Madagascar), Hunteria (12; tropical Africa), Picralima (1; P. nitida; tropical West and Central Africa), Pleiocarpa (6; P. bicarpellata, P. brevistyla, P. mutica, P. picralimoides, P. pycnantha, P. rostrata; tropical Africa). – Tropical and southern Africa, Madagascar. – The sister-group relationships of Hunterieae are not resolved.
[Condylocarpinae+[Chilocarpeae+[Alyxieae+[Plumerieae+[Carisseae+[Wrightieae+[Nerieae+[Malouetieae+[Rhabdadenia+[Periplocoideae+[Apocyneae+[New World clade+[Baisseeae+[Secamonoideae+Asclepiadoideae]]]]]]]]]]]]]]
Condylocarpinae Pichon ex Leeuwenb. in Wageningen Agric. Univ. Pap. 94(3): 56. 19 Aug 1994
1/7. Condylocarpon (7; C. amazonicum, C. glabrum, C. guyanense, C. intermedium, C. isthmicum, C. myrtifolium, C. pubiflorum; Nicaragua to Brazil).
[Chilocarpeae+[Alyxieae+[Plumerieae+[Carisseae+[Wrightieae+[Nerieae+[Malouetieae+[Rhabdadenia+[Periplocoideae+[Apocyneae+[New World clade+[Baisseeae+[Secamonoideae+Asclepiadoideae]]]]]]]]]]]]]
Chilocarpeae Pichon ex Leeuwenb. in Wageningen Agric. Univ. Pap. 94(3): 54. 19 Aug 1994
1/14. Chilocarpus (14; southern India and the Nicobar Islands to New Guinea).
[Alyxieae+[Plumerieae+[Carisseae+[Wrightieae+[Nerieae+[Malouetieae+[Rhabdadenia+[Periplocoideae+[Apocyneae+[New World clade+[Baisseeae+[Secamonoideae+Asclepiadoideae]]]]]]]]]]]]
Alyxieae G. Don, Gen. Hist. 4: 71, 96. 1837-8 Apr 1838
4–5/116–121. Alyxia (105–110; tropical Asia, islands in western Pacific, the Hawaiian Islands; incl. Pteralyxia?), Pteralyxia (2; P. kauaiensis, P. laurifolia; the Hawaiian Islands; in Alyxia?), Lepinia (4; L. solomonensis: New Guinea, Solomon Islands; L. ponapensis: Pohnpei in the Caroline Islands; L. marquisensis: Fatu Hiva in the Marquesas Islands; L. taitensis: Tahiti, Moorea), Lepiniopsis (2; L. ternatensis, L. trilocularis; Central and East Malesia to New Guinea, Micronesia), Plectaneia (3; P. longisepala, P. stenophylla, P. thouarsii; Madagascar). – Madagascar, tropical Asia to the Solomon Islands, Micronesia and Polynesia. Pollen grains irregularly-shaped, 2- or 3-porate. Ectoapertures with thickened margins.
[Plumerieae+[Carisseae+[Wrightieae+[Nerieae+[Malouetieae+[Rhabdadenia+[Periplocoideae+[Apocyneae+[New World clade+[Baisseeae+[Secamonoideae+Asclepiadoideae]]]]]]]]]]]
Plumerieae E. Mey., Comm. Plant. Afr. Austr. 2: 188. 1-8 Jan 1838
10/80–81. Allamanda (13–14; Mexico, Central America, the West Indies, tropical South America), Cameraria (7; C. angustifolia, C. latifolia, C. linearifolia, C. microphylla, C. obovalis, C. orientensis, C. retusa; the West Indies), Cerbera (6; C. dumicola, C. floribunda, C. inflata, C. laeta, C. manghas, C. odollam; coasts of the Indian and western Pacific Oceans), Cerberiopsis (3; C. candelabra, C. neriifolia, C. obtusifolia; New Caledonia), Himatanthus (13; tropical South America), Mortoniella (1; M. pittieri; Central America), Plumeria (c 20; southern Mexico, Central America, the West Indies, tropical South America), Skytanthus (3; S. acutus, S. hancorniifolius, S. martianus; Brazil, Chile), Thevetia (3; T. ahouai, T. amazonica, T. bicornuta; Mexico, Central America, Cuba, tropical South America), Vallesia (11; Florida, Mexico, Central America, the West Indies, tropical South America).
[Carisseae+[Wrightieae+[Nerieae+[Malouetieae+[Rhabdadenia+[Periplocoideae+[Apocyneae+[New World clade+[Baisseeae+[Secamonoideae+Asclepiadoideae]]]]]]]]]]
Carisseae Dumort., Anal. Fam. Plant.: 26. 1829
2/15. Acokanthera (7; A. laevigata, A. lycioides, A. oblongifolia, A. oppositifolia, A. pubescens, A. rotundata, A. schimperi; eastern and southern Africa, Yemen), Carissa (8; tropical and southern Africa, Madagascar, the Mascarene Islands, tropical Asia to tropical Australia and New Caledonia). – Tropical regions in the Old World to New Caledonia.
[Wrightieae+[Nerieae+[Malouetieae+[Rhabdadenia+[Periplocoideae+[Apocyneae+[New World clade+[Baisseeae+[Secamonoideae+Asclepiadoideae]]]]]]]]]
Corolla usually dextrorsely contorted. Anthers with lignified basal appendages, adnate to stylar head. Retinaculum present (staminal portion consisting of trichomes and attaching to stylar head). Pollen grains porate. Gynoecium apocarpous. Stylar head radially differentiated. Fruit a follicle. Coma (hair tuft) usually present on chalazal end of testa. Iridoids absent. – Gynostegium formed by postgenital close connection between androecial and gynoecial development. Special pollination mechanism consisting of gynostegium in combination with lignified nectar guides.
Wrightieae G. Don, Gen. Hist. 4: 70, 85. 1837-8 Apr 1838
3/c 40. Wrightia (34; tropical and subtropical regions in the Old World; non-monophyletic?); Stephanostema (1; S. stenocarpum; Tanzania), Pleioceras (5; P. afzelii, P. barteri, P. gilletii, P. orientale, P. zenkeri; tropical Africa). – Tropical and subtropical regions in the Old World. Corolla sinistrorsely contorted.
[Nerieae+[Malouetieae+[Rhabdadenia+[Periplocoideae+[Apocyneae+[New World clade+[Baisseeae+[Secamonoideae+Asclepiadoideae]]]]]]]]
Nerieae Baill., Hist. Plant. 10: 166, 198. Sep-Oct 1889
5–6/74. Nerium (1; N. oleander; the Mediterranean, southwestern Asia; geographic origin unknown), Adenium (5; A. boehmianum, A. multiflorum, A. obesum, A. oleifolium, A. swazicum; northeastern, tropical and southern Africa, southern Arabian Peninsula, Socotra); Strophanthus (38; tropical and southern Africa, Madagascar, tropical Asia), Isonema (3; I. buchholzii, I. infundibuliflorum, I. smeathmannii; tropical West and Central Africa), ‘Alafia’ (26; tropical Africa, Madagascar; paraphyletic), Farquharia (1; F. elliptica; southern Nigeria; in Alafia?). – The Mediterranean, tropical and southern Africa, Madagascar, the Arabian Peninsula, Socotra, southwestern and tropical Asia.
[Malouetieae+[Rhabdadenia+[Periplocoideae+[Apocyneae+[New World clade+[Baisseeae+[Secamonoideae+Asclepiadoideae]]]]]]]
Malouetieae Müll.-Arg. in C. F. P. von Martius, Fl. Bras. 6(1): 5, 89. 30 Jul 1866
11/c 105. Galactophora (6; G. angustifolia, G. colellana, G. crassifolia, G. pulchella, G. pumila, G. schomburgkiana; tropical South America); Pachypodium (24–25; southern and southeastern Africa, Madagascar), Neobracea (8; Cuba, the Bahamas), Spirolobium (1; S. cambodianum; Thailand, Indochina, the Malay Peninsula), Mascarenhasia (8; Madagascar, one species, M. arborescens, also in eastern and southern Africa), Holarrhena (5; H. congolensis, H. curtisii, H. floribunda, H. mitis, H. pubescens; tropical Africa, tropical Asia), Malouetia (c 30; tropical Africa, Central America, tropical South America), Funtumia (2; F. africana, F. elastica; tropical Africa), Kibatalia (c 15; Southeast Asia, Malesia to the Philippines), Allowoodsonia (1; A. whitmorei; the Solomon Islands), Carruthersia (4; C. glabra, C. latifolia, C. pilosa, C. scandens; the Philippines to the Solomon Islands, Fiji to Tonga). – Pantropical. Micropylar coma often absent. – Galactophora is sister to the remaining Malouetieae.
[Rhabdadenia+[Periplocoideae+[Apocyneae+[New World clade+[Baisseeae+[Secamonoideae+Asclepiadoideae]]]]]]
Rhabdadenia clade
1/3. Rhabdadenia (3; R. biflora, R. madida, R. ragonesei; the West Indies, tropical South America). – Vessels grouped in radial multiples. Tracheids absent. Wood fibres parenchymatous and very thin-walled. Retinaculum similar to that in Wrightieae, Nerieae and Malouetieae. – Rhabdadenia is recognized as basal to the Periplocoideae-Asclepiadoideae clade, according to analyses by Livshultz (2010), which is consistent with its morphology.
[Periplocoideae+[Apocyneae+[New World clade+[Baisseeae+[Secamonoideae+Asclepiadoideae]]]]]
Periplocoideae Endl., Gen. Plant.: 587. Aug 1838 [’Periploceae’]
37/166–167. Phyllanthera (1; P. grayi; tropical Asia to islands in the Pacific); Petopentia (1; P. natalensis; Eastern Cape, KwaZulu-Natal); Ischnolepis (1; I. graminifolia; Madagascar), Cryptostegia (2; C. grandiflora, C. madagascariensis; Madagascar), Camptocarpus (9; Madagascar, Mauritius), Baroniella (9; Madagascar), Pentopetia (21; Madagascar); Buckollia (2; B. tomentosa, B. volubilis; tropical East Africa), Epistemma (4; E. assianum, E. decurrens, E neuerburgii, E. rupestre; Ghana, Ivory Coast), ‘Finlaysonia’ (7; F. curtisii, F. insularum, F. khasiana, F. lanuginosa, F. obovata, F. pierrei, F. wallichii; tropical Asia to tropical Australia; paraphyletic), Streptocaulon (5; S. cumingii, S. juventas, S. kleinii, S. sylvestre, S. wallichii; tropical Asia), Atherandra (1; A. acutifolia; Southeast Asia, West Malesia), Gymnanthera (2; G. cunninghamii, G. oblonga; Malesia to northern Australia), Zygostelma (1; Z. benthamii; Thailand); ‘Decalepis’ (5; D. arayalpathra, D. hamiltonii, D. khasiana, D. nervosa, D. salicifolia; India; polyphyletic), Hemidesmus (1; H. indicus; southern India, Southeast Asia, Malesia), Sacleuxia (2; S. newii, S. tuberosa; tropical East Africa), Baseonema (1; B. gregorii; tropical East Africa), Schlechterella (2; S. abyssinica, S. africana; tropical East Africa), ‘Raphionacme’ (c 35; tropical and southern Africa, the Arabian Peninsula; non-monophyletic), Stomatostemma (1; S. monteiroae; Zambia, Mozambique, Zimbabwe), Tacazzea (5; T. apiculata, T. conferta, T. pedicellata, T. rosmarinifolia, T. venosa; tropical and southern Africa, southern Asia), Batesanthus (3; B. parviflorus, B. pseudopalpus, B. purpureus; tropical West and Central Africa), Mondia (2; M. ecornuta, M. whitei; tropical and southern Africa), Myriopteron (1; M. extensum; northeastern India, southern China, Southeast Asia, West Malesia); Ectadium (2; E. rotundifolium, E. virgatum; the Namib Desert in Namibia), Periploca (14; the Mediterranean, North and tropical Africa, East Asia), Cryptolepis (c 15; tropical and southern Africa, Madagascar, tropical Asia). – Unplaced Periplocoideae Atherolepis (2–3; A. pierrei, A. wallichii; Southeast Asia), Gongylosperma (1; G. lanuginosum; the Malay Peninsula), Maclaudia (1; M. felixii; Guinea), Meladerma (1; M. puberulum; Thailand), Pentanura (1; P. khasiana; Burma, Sumatra), Sarcorrhiza (1; S. epiphytica; tropical Africa), Stelmacrypton (1; S. khasianum; eastern India, southern China), Telectadium (3; T. dongnaiense, T. edule, T. linearicarpum; Southeast Asia), Zacateza (1; Z. pedicellata; tropical Africa). – Tropical, subtropical and arid temperate regions of the Old World to northern Australia. Roots often tuberous. Corolla sometimes with valvate aestivation. Nectariferous disc absent. Stamens inserted in corolla mouth. Staminal bases sometimes upright, connate and forming tube surrounding ovary. Nectaries present on margins of staminal bases and alternating with stamens. Anthers without lignified basal nectar guide appendages. Corpusculum basal. Pollen grains 4–16-porate, shed in calymmated (T-shaped tetragonal) tetrads, or in 20 pollinia (with inner walls reduced, free from translator apparatus), collected on translator (consisting of spoon-shaped structure + basal sticky viscidium). Exine smooth. Retinaculum formed through cellular fusion. Exotestal cells thickened or non-thickened. x = 11. – Phyllanthera is probably sister to the remaining Periplocoideae.
[Apocyneae+[New World clade+[Baisseeae+[Secamonoideae+Asclepiadoideae]]]]
Apocyneae Rchb., Fl. Germ. Excurs. 1(3): 410, 429. Jul-Dec 1831
22/125–126. Papuechites (1; P. aambe; the Moluccas, New Guinea), Ixodonerium (1; I. annamense; Southeast Asia), Anodendron (17; tropical Asia to Japan and Vanuatu), Streptoechites (1; S. chinensis; Hainan, Thailand, northern Vietnam), Sindechites (1; S. henryi; China), Amphineurion (1; A. marginatum; northeastern India, southern China, Southeast Asia, Malesia), Vallaris (3; V. glabra, V. indecora, V. solanacea; India, the Himalayas and Sri Lanka to southern China, Java and Flores), Beaumontia (9; India and China to Bali), Cleghornia (2; C. acuminata, C. malaccensis; Sri Lanka, southern China, Southeast Asia, West Malesia), Apocynum (5; A. androsaemifolium, A. cannabinum, A. pictum, A. sarmatiense, A. venetum; eastern Europe and southern Russia to China, temperate North America to northeastern Mexico), Urceola (18; tropical Asia from India to southern China and Malesia), Baharuia (1; B. gracilis; Sumatra, Borneo; in Urceola?), Chonemorpha (10; India, the Himalayas and Tibet to southern China, Java and the Philippines), Trachelospermum (c 20; India to Japan, southeastern United States), Micrechites (13; tropical Asia to southern China and New Guinea), Amalocalyx (1; A. microlobus; Burma, southern Yunnan, Southeast Asia), Pottsia (3; P. densiflora, P. grandiflora, P. laxiflora; tropical Asia to Java), Epigynum (5; E. auritum, E. cochinchinensis, E. graciliflorum, E. griffithianum, E. ridleyi; Kashmir, the Himalayas, southern China, tropical Asia to tropical Australia), Ichnocarpus (3; I. frutescens, I. fulvus, I. uliginosus; tropical Asia from India and Sri Lanka to southern China and Malesia, tropical Australia), Aganosma (9; Southeast Asia, West and Central Malesia). – Unplaced Apocyneae Eucorymbia (1; E. alba; Borneo), Parepigynum (1; P. funingense; Yunnan). – Russia to East and tropical Asia, Vanuatu, North America. Often lianas. Pollen sometimes shed in tetrats. Stylar head sometimes with strap-shaped bands of adhesive. – Apocyneae are sister-group to the “New World clade”, according to Livshultz (2010).
[New World clade+[Baisseeae+[Secamonoideae+Asclepiadoideae]]]
New World clade
c 29/440–490. The topology [Echiteae+Mesechiteae] is provisional and not very strongly supported.
Echiteae Bartl., Ord. Nat. Plant.: 204. Sep 1830 [‘Echitea’].
c 14/200–250. Laubertiinae J. F. Morales, M. E. Endress et Liede in Taxon 66(3): 637. Jun 2017. Laubertia (4; L. boissieri, L. brasiliensis, L. contorta, L. peninsularis; southern Mexico, Central America, tropical South America), Hylaea (2; H. arborescens, H. leptoloba; Amazonas in Venezuela and Brazil). – Peltastinae Pichon ex M. E. Endress in Phytotaxa 159: 182. 2014. Rhodocalyx (2; R. hypoleucus, R. rotundifolius; Brazil, Paraguay, Bolivia)?, Temnadenia (4; T. odorifera, T. ornata, T. stenantha, T. violacea; Colombia, Brazil, Peru, Bolivia), Macropharynx (c 15; tropical South America). – Echitinae Kitt., Taschenb. Fl. Deutschl., ed. 2: 449. 1843. Asketanthera (4; A. calycosa, A. dolichopetala, A. longiflora, A. picardae; tropical America), Echites (11; Florida, Central America, the West Indies), Thenardia (3; T. chiapensis, T. floribunda, T. galeottiana; southern Mexico, Central America), Thoreauea (3; T. aberrans, T. guerrerensis, T. paneroi; Mexico). – Parsonsiinae Benth. et Hook. f., Gen. Plant. 2: 687. 1876. Artia (5; A. amieuensis, A. balansae, A. brachycarpa, A. francii, A. lifuana; New Caledonia), Parsonsia (80–130?; East Asia to Taiwan, tropical Asia to northern Australia, New Caledonia, New Zealand, Fiji), Ecua (1; E. moluccensis; the Moluccas). – Prestoniinae Pichon ex M. E. Endress in Phytotaxa 159: 183. 2014. Prestonia (c 65; tropical America). – Unplaced Echiteae Bahiella (2; B. blancheti, B. infundibuliflora; Bahia in northeastern Brazil). – Tropical America, East and tropical Asia and eastwards to northern Australia, New Caledonia, New Zealand, Fiji. – Laubertia may be sister to the remaining Echiteae.
Mesechiteae Miers, Apocyn. S. Amer.: 10. Mai-Jun 1878.
15/c 240. Elytropus (1; E.
chilensis; central and southern Chile, Rio Negro in Argentina),
Allomarkgrafia (11; Nicaragua to Peru), Forsteronia (c 45;
southern Mexico, Central America, Jamaica, tropical South America),
Mesechites (12; Mexico, Central America, the West Indies, tropical
South America), Mandevilla (c 130; Texas, Mexico, Central America, the
West Indies, tropical South America), Tintinnabularia (3; T.
gratissima, T. mortonii, T. murallensis; southern
Mexico, Honduras), Cycladenia (1; C. humilis; southwestern
United States), Stipecoma (1; S. peltigera; Brazil),
Pinochia (4; P. corymbosa, P. floribunda, P.
monteverdensis, P. peninsularis; Mexico, Central America, the
Greater Antilles), Thyrsanthella (1; T. difformis;
southeastern United States), Secondatia (5; S. densiflora,
S. duckei, S. floribunda, S. macnabii,
S. schlimiana; Jamaica, tropical South America), Odontadenia
(c 20; southern Mexico, Central America, Hispaniola, tropical South America),
Salpinctes (1; S. kalmiifolius; the Guayana Highlands,
northern Brazil), Pentalinon (2; P. andrieuxii, P.
luteum; Florida, Central America, the West Indies), Angadenia (2;
A. berteroi, A. lindeniana; Florida, the West Indies). –
Southwestern United States, Florida, Mexico, Central America, the West Indies,
tropical South America.
[Baisseeae+[Secamonoideae+Asclepiadoideae]]
Baisseeae (Pichon ex De Kruif) M. E. Endress in Ann. Missouri Bot. Gard. 94(2): 266. Jul 2007
4/29. Dewevrella (1; D. cochliostema; tropical Africa); Baissea (18; tropical and southern Africa), Motandra (3; M. lujae, M. paniculata, M. poecilophylla; tropical West and Central Africa), Oncinotis (7; O. glabrata, O. gracilis, O. hirta, O. nitida, O. pontyi, O. tenuiloba, O. tomentella; tropical and southern Africa, Madagascar). – Tropical and southern Africa, Madagascar. Lianas. Hairs sometimes branched. Domatia sometimes present. Pollinia absent in Baissea. Stylar head sometimes with strap-shaped bands of adhesive. – Baisseeae are sister to [Secamonoideae+Asclepiadoideae]. Dewevrella is sister to the clade [Oncinotis+[Baissea+Motandra]].
[Secamonoideae+Asclepiadoideae]
Stamens inserted well below bases of corolla lobes. Corona usually formed from androecium. Nectariferous disc absent. Filaments absent. Gynostegium developed by postgenital fusion of androecial and gynoecial structures. Staminal feet erect, connate, forming tube around ovary. Anthers inserted on top of basal tube consisting of annular corona of staminal bases. Nectaries consisting of outgrowths of petaloid staminal bases (tissue from corolla and filaments) present behind nectar guides and alternating with stamens. Microsporogenesis successive. Pollen grains inaperturate, shed as T-shaped tetragonal tetrads. Pollen aggregated into pollinia in erect pollinaria. Pollinia of a pollinarium consisting of anther halves of adjacent stamens. Exine psilate. Translator – retinaculum – formed from hardened resinous mostly stigmatic secretion and apical adhesive corpusculum. Endosperm development usually nuclear (rarely cellular). Embryo with chlorophyll. Inulin and fructans sometimes present. Monoterpene indole alkaloids absent.
Secamonoideae Endl., Gen. Plant.: 589. Aug 1838 [‘Secamoneae’]
8/200–206. Calyptranthera (11; Madagascar), Genianthus (17; India, Bhutan and southern China to Malaysia and the Philippines), Goniostemma (2; G. acuminatum, G. punctatum; India, China; in Toxocarpus?), Pervillaea (5; P. brevirostris, P. decaryi, P. phillipsonii, P. tomentosa, P. venenata; Madagascar), Secamone (130–135; tropical and subtropical Africa, Madagascar, tropical and subtropical Asia to northern Australia), Secamonopsis (2; S. madagascariensis, S. microphylla; Madagascar), Toxocarpus (c 33; tropical regions in the Old World east to islands in the Pacific; incl. Goniostemma?), Trichosandra (1; T. borbonica; Mauritius). – Tropical and subtropical regions in the Old World to northern Australia and islands in the Pacific. Lianas. Corolla sinistrorsely or dextrorsely contorted. Pollinia 20, with outer walls reduced, connected to translator apparatus consisting of corpusculum (rarely also of one or two translator arms – caudicula). Pollen grains shed as tetrads. Exine with thick granular infratectal layer. x = 11.
Asclepiadoideae Burnett, Outlines Bot.: 1012, 1095, 1103. Feb 1835 [‘Stapelidae’, or ‘Asclepiadeae’]
112/3.000–3.050. Cosmopolitan except polar and cold-temperate regions, with their largest diversity in drier parts of Africa. Intraxylary phloem sometimes present. Leaves rarely alternate. Corolla often with valvate aestivation. Corona consisting of staminal parts. Anthers disporangiate (one sporangium from each theca absent). Nectaries present adjacent to or near base of nectar guides. Pollen tetrads linear during development. Pollinia ten (one pollinium from each theca fertile), with inner walls reduced, connected to translator apparatus made of corpusculum and (one or) two caudicula. Anther usually secreting wall material around pollinium. Compitum sometimes absent. Exine with thin granular infratectal layer. x = (9–)11(–14). - Unplaced Asclepiadoideae Vietnamia (1; V. inflexa; Vietnam).
[Fockeeae+[Marsdenieae+Ceropegieae+Asclepiadeae]]
Fockeeae H. Kunze, Meve et Liede in Taxon 43: 373. 31 Aug 1994
2/9. Fockea (6; F. angustifolia, F. capensis, F. comaru, F. edulis, F. multiflora, F. sinuata; tropical and southern Africa), Cibirhiza (3; C. albersiana, C. dhofarensis, C. spiculata; Tanzania, Zambia, Oman). – Tropical and southern Africa, Oman. Pollen grains shed as tetrads. Pollinia erect, without sterile wall. Caudicula absent. – Fockeeae are sister to the remaining Asclepiadoideae.
[Marsdenieae+Ceropegieae+Asclepiadeae]
Pollinia with caudicula. Pollen grains shed as monads.
Marsdenieae Benth., Fl. Austral. 4: 325, 333. 16 Dec 1868
23/c 585. Anatropanthus (1; A. borneensis; Borneo), Anisopus (2; A. efulensis, A. mannii; tropical West and Central Africa), Asterostemma (1; A. repandum; Java), Campestigma (1; C. purpureum; Southeast Asia), Clemensiella (1; C. mariae; the Philippines), Cosmostigma (3; C. cordatum: India, Sri Lanka; C. hainanense: Hainan; C. philippinense: the Philippines), Dischidia (c 120; northeastern India and southern China to Malesia, northeastern Queensland, New Caledonia), Dolichopetalum (1; D. kwangsiense; southern China), Gongronema (15; tropical regions in the Old World), Gunnessia (1; G. pepo; northernmost Queensland), Heynella (1; H. lactea; Java), Hoya (>200; tropical Asia, tropical and eastern Australia, islands in the Pacific to Polynesia), Lygisma (3; L. angustifolia, L. flava, L. inflexa; Southeast Asia), Marsdenia (>200; northern to southern Africa, Madagascar, tropical and subtropical Asia to Malesia, Australia, Melanesia, Central and South America, one species, M. erecta, in eastern Mediterranean), Oreosparte (1; O. celebica; Sulawesi), Pycnorhachis (1; P. maingayi; the Malay Peninsula), Rhyssolobium (1; R. dumosum; Namibia, Northern Cape), Sarcolobus (14; tropical Asia, tropical Australia, islands in western Pacific), Sphaerocodon (4; S. caffrum, S. melananthum, S. natalense, S. obtusifolium; Zambia to Namibia), Spirella (2; S. robinsonii, S. tylophoroides; Southeast Asia), Stigmatorhynchus (2; S. hereroensis, S. umbelliferus; eastern and southern Africa), Telosma (10; tropical and southern Africa, Madagascar, tropical Asia eastwards to the Malay Peninsula), Treutlera (1; T. insignis; eastern Himalayas). – Tropical and subtropical regions on both hemispheres. Latex watery (clear). Pollinia erect or pendent with two caudicula. Sterile wall sometimes present on external side of pollinium. Exotestal cells in Hoya with external walls unthickened.
Ceropegieae Orb., Dict. Univ. Hist. Nat. 3: 339. 1 Jul 1843 [‘Ceropegiae’]
12/780–785. Dittoceras (4; D. andersonii, D. garrettii, D. maculatum, D. stellaris; eastern Himalayas, Thailand), Heterostemma (12; tropical Asia, tropical Australia to eastern Queensland, islands in western Pacific), Pentasacme (4; P. caudatum, P. pulcherrima, P. shanense, P. wallichii; tropical Asia), Conomitra (1; C. linearis; Sudan), Leptadenia (5; L. arborea, L. lanceolata, L. madagascariensis, L. pyrotechnica, L. reticulata; tropical regions in the Old World), Orthanthera (6; O. albida, O. butayei, O. gossweileri, O. jasminiflora, O. stricta, O. viminea; southern tropical and southern Africa, India, Nepal), Neoschumannia (3; N. cardinea, N. gishwatiensis, N. kamerunensis; tropical West and Central Africa), Riocreuxia (10; tropical and southern Africa, India), Emplectanthus (3; E. cordatus, E. dalzellii, E. gerrardii; KwaZulu-Natal), Anisotoma (2; A. cordifolia, A. pedunculata; Eastern Cape, KwaZulu-Natal), Sisyranthus (12; tropical and southern Africa), Ceropegia (c 720; the Canary Islands, the Mediterranean, Africa, Madagascar, the Arabian Peninsula, Socotra, India, Sri Lanka to southern China, Malesia, New Guinea and Queensland). – The Canary Islands, the Mediterranean, tropical and subtropical regions in the Old World to eastern Queensland, islands in western Pacific, with their highest diversity in arid regions of eastern and southern Africa. Latex watery (clear). Pollinia erect with two caudicula, sterile wall present on apex or inner side of pollininium. Orbicules present in Riocreuxia.
Asclepiadeae Duby, Bot. Gall. 1: 323. 12-14 Apr 1828
c 74/1.630–1.680. Eustegia (5; E. filiformis, E. fraterna, E. macropetala, E. minuta, E. plicata; Northern and Western Cape); Oncinema (1; O. lineare; Western and Eastern Cape), Microloma (11; Namibia, Northern, Western and Eastern Cape, Free State), Astephanus (2; A. triflorus, A. zeyheri; southern Africa), Petalostelma (7; P. bracteoatum, P. calcaratum, P. cearense, P. dardanoi, P. martianum, P. roberti, P. sarcostemma; tropical South America), Minaria (21; tropical South America, with their highest diversity in the Espinhaçao Range in eastern Brazil), Barjonia (7; B. chlorifolia, B. cymosa, B. erecta, B. furlanii, B. glazioui, B. grazielae, B. laxa; Brazil), ‘Nephradenia’ (5–10; N. acerosa, N. asparagoides, N. filipes, N. laurifolium, N. linearis; tropical America; paraphyletic), ‘Hemipogon’ (8–10; South America; polyphyletic), Blepharodon (2; B. ampliflorum, B. lineare; southern South America), 'Blepharodon' (15–20?; Central America, tropical South America; non-monophyletic), Vailia (1; V. anomala; Mexico, Central America), Peplonia (9; Brazil), Ditassa (50–55?; tropical South America), Tassadia (c 30; tropical South America), Macroscepis (8–16; southern Mexico, Central America, tropical South America; in Matelea?), Schubertia (6–15; tropical South America; in Matelea?), Gonolobus (c 130; southern and southeastern United States, Mexico, Central America, the West Indies, tropical South America; Matelea?), Fischeria (16; tropical America), ‘Matelea’ (c 200; tropical South America; non-monophyletic; incl. Anemotrochus, Gonolobus, Ibatia, Macroscepis, Schubertia, Tylodontia?), Polystemma (5; P. canisferum, P. cordifolium, P. guatemalense, P. mirandae, P. viridiflorum; Mexico, Central America), Austrochthamalia (5; A. boliviana, A. teyucuarensis; tropical South America), Funastrum (c 20; southern United States, Mexico, Central America, tropical South America), Oxypetalum (c 125; Cuba, tropical South America), Pruskortizia (2; tropical South America), Rhytidostemma (8; Central America, tropical South America), Rotundanthus (1; R. fulvidus; Mexico, Central America), Tweedia (6; T. andina, T. aucaensis, T. birostrata, T. brunonis, T. echegarayi, T. stipitata; South America), Araujia (13; tropical South America), Philibertia (c 40; tropical South America), Pentacyphus (3; P. andinus, P. lehmannii; the Andes in northwestern South America), Diplolepis (14; Chile, Argentina), Monsanima (2; M. morrenioides, M. tinguaensis; tropical South America), Scyphostelma (10–15; tropical South America), Jobinia (c 25?; tropical South America), Orthosia (c 40?; tropical America), Cynanchum (c 315; tropical and subtropical regions on both hemispheres, southern Africa), Schizostephanus (2; S. alatus, S. gossweileri; southern Africa to Angola and Zimbabwe), Pentatropis (4; P. bentii, P. capensis, P. nivalis, P. pierrei; tropical and subtropical regions in the Old World to Australia), 'Tylophora' (c 50; tropical and subtropical regions of the Old World; paraphyletic), Vincetoxicum (c 70; Europe, temperate Asia, one species, V. carnosum, in Australia; incl. Tylophora?), Calciphila (2; C. galgalensis, C. gillettii; Somalia), Oxystelma (2; O. bornouense, O. esculentum; tropical regions in the Old World), Pergularia (2; P. daemia, P. tomentosa; Africa, Madagascar to India), Kanahia (2; K. carlsbergiana, K. laniflora; tropical East Africa, the Arabian Peninsula), Calotropis (3; C. acia, C. gigantea, C. procera; tropical and subtropical Africa to India), Asclepias (c 245-255; tropical and subtropical Africa and Asia, southern Africa, North America, Mexico, Central America). – Unplaced Asclepiadeae Aidomene (1; A. parvula; Angola), Amblystigma (7; Bolivia, Argentina), Anomotassa (1; A. macranthus; Ecuador), Biondia (13; China), Blyttia (2; B. fruticulosa, B. spiralis; East Africa, southern Arabian Peninsula), Cyathostelma (2; C. furcatum, C. latipes; Brazil), Diplostigma (1; D. canescens; East Africa), Emicocarpus (1; E. fissifolius; southeastern Africa), Goydera (1; G. somaliensis; Somalia), Lagoa (1; L. calcarata; Brazil), Mahawoa (1; M. montana; Sulawesi), Melinia (8; tropical South America), Merrillanthus (1; M. hainanensis; Hainan), Mitostigma (c 20; South America), Nautonia (1; N. nummularia; southern Brazil), Odontostelma (1; O. welwitschii; southern tropical Africa), Pentastelma (1; P. auritum; Hainan), Periglossum (4; P. angustifolium, P. kassneranum, P. mackenii, P. macrum; southeastern and southern Africa), Pherotrichis (4; P. leptogenia, P. mixtecana, P. schaffneri, P. villosa; Mexico), Prosthecidiscus (1; P. guatemalensis; Central America), Rhyncharrhena (1; R. linearis; Australia), Rhyssostelma (1; R. nigricans; Argentina), Stenomeria (3; S. decalepis, S. fosteri, S. pentalepis; Colombia, Venezuela, Peru), Trichosacme (1; T. lanata; Mexico), Vincetoxicopsis (1; V. harmandii; Southeast Asia), Widgrenia (1; W. corymbosa; Brazil). – Nearly cosmopolitan. Latex milky. Pollinia erect or pendant with two caudicula and with a sterile margin on top or on external side of the pollinium. – Eustegia minuta was recovered as sister to the remaining Asclepiadeae by Surveswaran & al. (2014).
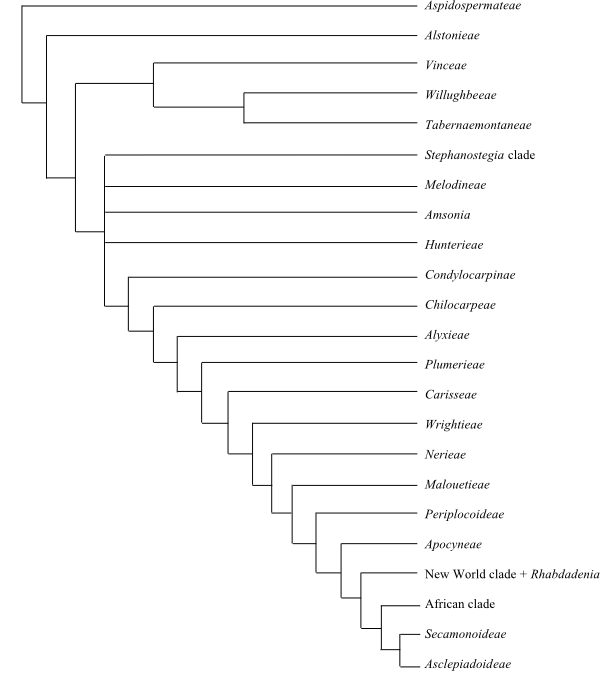 |
Cladogram (simplified) of Apocynaceae based on DNA sequence data (Livshultz & al. 2007; Lens & al. 2008). |
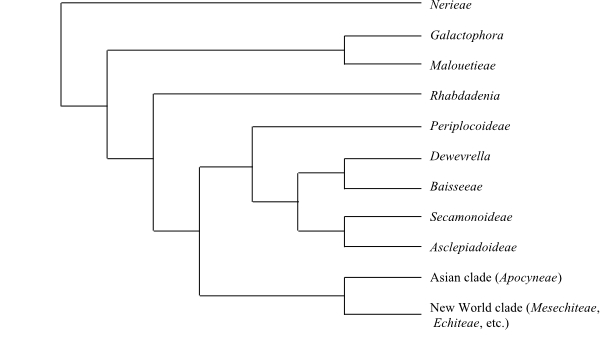 |
Stict consensus tree (simplified) of part of Apocynaceae based on DNA sequence data (Livshultz 2010). |
GELSEMIACEAE (G. Don) Struwe et V. A. Albert |
( Back to Rubiales ) |
Pteleocarpaceae R. K. Brummitt in Kew Bull. 66: 3. Mar? 2011
Genera/species 3/8
Distribution Tropical Africa, Madagascar, southern China, Southeast Asia, West Malesia, southeastern United States to northeastern South America.
Fossils Unknown.
Habit Bisexual, evergreen tree (Pteleocarpa), shrubs or lianas (Gelsemium, Mostuea).
Vegetative anatomy Phellogen? Primary medullary strands narrow or wide. Secondary lateral growth anomalous (via a cylindrical cambium). Vessel elements usually with simple (sometimes scalariform or reticulate) perforation plates (vessel elements in Pteleocarpa solitary); lateral pits alternate, bordered pits. Vestured pits present in Pteleocarpa. Imperforate tracheary xylem elements tracheids (in Gelsemium) or fibre tracheids with simple and/or bordered pits, septate or non-septate. Wood rays biseriate (Pteleocarpa) to multiseriate, homocellular or heterocellular. Axial parenchyma absent or rare (paratracheal scanty, unilateral or banded); in Pteleocarpa usually apotracheal diffuse-in-aggregates or unilateral banded. Intraxylary phloem present. Sieve tube plastids S type. Nodes 1:1, unilacunar with one leaf trace. Parenchyma in Pteleocarpa often with gum-like substances. Styloids, druses and elongate calciumoxalate crystals present.
Trichomes Hairs unicellular or absent.
Leaves Usually opposite (sometimes verticillate, in Pteleocarpa alternate, spiral), simple, entire, with convolute ptyxis (Pteleocarpa). Stipules two, interpetiolar to intrapetiolar or somewhat decurrent, or absent; leaf sheath absent. Axillary colleters present (especially in connection to stipules). Petiole bases sometimes connate via an ochrea or a line. Petiole vascular bundle transection arcuate? Venation pinnate, brochidodromous. Stomata paracytic or anomocytic. Cuticular waxes? Domatia as pockets or absent. Leaf margin faintly sinuate or entire.
Inflorescence Terminal or axillary, cymose (often panicle; flowers sometimes solitary).
Flowers Usually actinomorphic (sometimes slightly zygomorphic). Hypogyny. Sepals usually five (sometimes four), with imbricate quincuncial aestivation, sometimes unequal in length, persistent, free (Gelsemium) or connate in lower part (Mostuea, Pteleocarpa). Petals usually five (sometimes four), with imbricate quincuncial aestivation, sometimes unequal in length, connate into an infundibuliform or tubular perigone. Nectary? Disc absent. Heterostyly often present.
Androecium Stamens usually five (sometimes four), haplostemonous, antesepalous, alternipetalous. Filaments free, adnate to corolla tube (epipetalous). Anthers dorsifixed, versatile, tetrasporangiate, extrorse (Gelsemium, Pteleocarpa) or latrorse (Mostuea), longicidal (dehiscing by longitudinal splits). Tapetum secretory? Staminodia absent.
Pollen grains Microsporogenesis simultaneous? Pollen grains tricolporate, shed as monads, ?-cellular at dispersal. Exine tectate, with columellate infratectum, striate, finely reticulate or punctate.
Gynoecium Pistil composed of two connate carpels. Ovary superior, bilocular. Stylodia two, connate in lower part, stylar branches often bifid. Stigmas two or four, capitate, type? Pistillodium absent.
Ovules Placentation axile. Ovules two (Mostuea, Pteleocarpa) or two to eight (Gelsemium) per carpel (in Pteleocarpa one large pendulous epitropous and one small orthotropous and later aborted ovule), anatropous or amphitropous?, unitegmic, tenuinucellar. Integument ? cell layers thick. Megagametophyte monosporous, Polygonum type? Endosperm development nuclear? Endosperm haustoria? Embryogenesis?
Fruit In Gelsemium and Mostuea a loculicidal and/or septicidal capsule with persistent calyx; in Pteleocarpa a large flat suborbicular one-seeded samara with one wide membranous wing on each side, divided by suture leading to retuse apex.
Seeds Aril absent. Seed flattened. Testa thin, winged or hairy. Exotestal cells? Endotesta? Perisperm not developed. Endosperm copious, horny, starchy (Gelsemium). Embryo small, straight or curved, chlorophyll? Cotyledons two. Germination?
Cytology n = 8, 10
DNA
Phytochemistry Flavonols (kaempferol, quercetin), O-methylated flavones, Route I iridoids (also secoiridoids), Group IX secoiridoids (indole alkaloids of corynanthe type), and C-17 indole alkaloids present. Caffeic acid?
Use Ornamental plants, medicinal plants, timber (Pteleocarpa).
Systematics Mostuea (4; M. batesii, M. brunonis, M. hirsuta, M. surinamensis; tropical Africa, Madagascar, northeastern South America), Gelsemium (G. elegans: India, northern Burma, southern China, Southeast Asia, West Malesia, Taiwan; G. rankinii: southeastern United States; G. sempervirens: southern United States, eastern Mexico, Guatemala), Pteleocarpa (1; P. lamponga; West Malesia)
Gelsemiaceae are probably sister-group to Apocynaceae.
Nuclear ribosomal ETS analysis suggests Pteleocarpa being sister to [Gelsemium+Mostuea], whereas Mostuea is sister to [Gelsemium+Pteleocarpa] in analyses of plastid regions (Struwe & al. 2014).
The leaves are spirally arranged in Pteleocarpa, not opposite as in the majority of Gentianales. Gottwald (1982) suggested a possible affinity with Apocynaceae and Rubiaceae judging from wood anatomical similarities. However, Pteleocarpa is most closely related to Gelsemium and Mostuea, according to molecular analyses (Miller 2003; Struwe & al. 2014).
GENTIANACEAE Juss. |
( Back to Rubiales ) |
Coutoubeaceae Martinov, Tekhno-Bot. Slovar: 168. 3 Aug 1820 [‘Coutoubeae’]; Obolariaceae Martinov, Tekhno-Bot. Slovar: 427. 3 Aug 1820 [’Obolariae’]; Chironiaceae Bercht. et J. Presl, Přir. Rostlin 1(104-114): 1, 36. 1823 [‘Chironieae’]; Potaliaceae Mart., Nov. Gen. Sp. Plant. 2: 89, 91, 133. Jan-Jun 1827 [‘Potalieae’]; Chironiineae J. Presl in Nowočeská Bibl. [Wšobecný Rostl.] 7: 1071, 1078. 1846 [‘Chironieae’]; Gentianineae J. Presl in Nowočeská Bibl. [Wšobecný Rostl.] 7: 1071. 1846 [‘Gentianeae’]; Chironiales Griseb., Grundr. Syst. Bot.: 144. 1-2 Jun 1854 [‘Chironiflorae’]; Saccifoliaceae Maguire et Pires in Mem. New York Bot. Gard. 29: 242. 14 Jun 1978; Voyriaceae Doweld, New Syllabus Pl. Fam.: 857. Apr 2007
Genera/species 94–95/1.890–1.935
Distribution Cosmopolitan except polar and arid areas, with their largest diversity in temperate and subtropical regions and on tropical mountains.
Fossils Unknown.
Habit Usually bisexual (rarely polygamomonoecious), perennial, biennial or annual herbs (sometimes shrubs, rarely lianas or trees). Some genera are partially mycoheterotrophic. A few clades (Cotylanthera, Leiphaimos, Voyria, and Voyriella) consisting of achlorophyllous holoparasitic mycotrophs.
Vegetative anatomy Arbuscular mycorrhiza often Paris type. Phellogen ab initio superficial. Primary medullary strands narrow. Medulla often with vascular bundles. Endodermis often prominent. Parenchyma often septate. Secondary lateral growth normal or anomalous, from cylindrical cambium. Vessel elements with usually simple (in Saccifolium scalariform) perforation plates; lateral pits alternate, simple or bordered pits. Vestured pits present. Imperforate tracheary xylem elements libriform fibres with simple and/or bordered pits, usually non-septate. Wood rays usually uniseriate, homocellular or heterocellular, or absent. Axial parenchyma apotracheal diffuse?, or paratracheal scanty vasicentric, scalariform or banded (absent in Saccifolium). Intraxylary phloem present. Sieve tube plastids S type. Nodes ≥1:≥1, at least unilacunar with one or more leaf traces (trilacunar nodes common, sometimes multilacunar, with several traces; sometimes with split lateral traces). Medulla sometimes with sclereids. Acicular crystals, styloids, crystal sand and other types of calciumoxalate crystals present in some species.
Trichomes Hairs unicellular or multicellular, simple, uniseriate or multiseriate, or absent; glandular hairs sometimes present.
Leaves Usually opposite (rarely verticillate; in Saccifolium alternate, spiral), simple, entire, sometimes coriaceous (in mycotrophs scale-like, reduced), with varying ptyxis (in Saccifolium saccate-vaginate near apex). Stipules absent (interpetiolar or intrapetiolar sheath-like or auriculate structures sometimes present at nodes); leaf sheath absent. Axillary colleters present in some species (in association with stipules). Leaves often connate at base. Petiole vascular bundles? Venation usually palmate (sometimes pinnate; in Saccifolium appearing parallelodromous). Stomata usually anomocytic (sometimes anisocytic). Cuticular wax crystalloids? Epidermis and/or mesophyll with or without mucilage cells. Mesophyll sometimes with sclerenchymatous idioblasts. Leaf margin usually entire (sometimes crenate). Extrafloral nectaries sometimes present on leaves (e.g. in Anthocleista and Irlbachia).
Inflorescence Terminal or axillary, usually dichasial, sometimes panicle or fasciculate cymose (rarely racemose; flowers sometimes solitary).
Flowers Usually actinomorphic (rarely zygomorphic). Epicalyx present or absent. Hypogyny. Sepals usually four or five (rarely up to 16), usually with imbricate (sometimes valvate or open) aestivation, often persistent, usually connate (rarely free). Petals usually four or five (rarely up to 16), usually with contorted (rarely imbricate) aestivation, marcescent, more or less connate; scales or nectariferous pits often present inside corolla tube (corolla tube in Halenia with five spurs). Nectariferous glands often present. Disc intrastaminal, annular or composed of separate parts, or absent. Diheterostyly present in some species.
Androecium Stamens usually four or five (rarely up to 16), antesepalous, alternipetalous. Filaments usually free (sometimes connate in lower part into a tube), adnate to corolla tube (epipetalous). Anthers usually free (sometimes connate at base; rarely connate into a tube), usually dorsifixed and versatile (sometimes basifixed and non-versatile), tetrasporangiate, usually introrse (rarely extrorse or latrorse), usually longicidal (dehiscing by longitudinal slits; in, e.g., Exacum poricidal, with apical pores; anthers rarely septate); connective sometimes slightly prolonged (sometimes glanduliferous); placentoids sometimes present. Tapetum usually secretory (sometimes amoeboid-periplasmodial), with uninucleate cells. Staminodia one to four, alternating with fertile stamens, or absent.
Pollen grains Microsporogenesis simultaneous. Pollen grains usually (2–)3(–4)-colporate or (1–)2–3-porate, usually shed as monads (sometimes as tetrads), bicellular or tricellular at dispersal. Exine tectate or semitectate, with columellate infratectum, perforate, reticulate, microreticulate, striate, echinulate, verrucate, regulate, or psilate. Pollen tubes sometimes several.
Gynoecium Pistil composed of two connate carpels (carpels median or transverse). Ovary superior, usually unilocular (rarely bilocular; rarely divided by secondary septa), sometimes with a gynophore. Style usually single, simple (sometimes persistent; absent in Lomatogonium). Stigma usually widely bilobate (sometimes capitate or subpeltate; in Lomatogonium decurrent along sides of ovary), papillate, Wet type. Pistillodium absent.
Ovules Placentation usually intrusively parietal (rarely basal or axile; in Cotylanthera free central). Ovules several to numerous per carpel, usually anatropous (sometimes hemianatropous or orthotropous), horizontal, usually unitegmic (in some mycotrophic species [Exacum, Voyria] ategmic), tenuinucellar (reduced, with meiocyte semi-inferior). Integument two to 20 cell layers thick. Hypostase present. Megagametophyte monosporous, Polygonum type (in some mycoheterotrophic species at least seemingly inverted 180o). Antipodal cells sometimes proliferating (diploid to polyploid, sometimes multiplying, persistent; in Halenia multinucleate). Endosperm development usually nuclear (in Voyriella cellular). Endosperm haustoria? Embryogenesis solanad.
Fruit Usually a septicidal capsule (sometimes a berry), often with persistent calyx.
Seeds Seeds sometimes winged. Aril absent. Testa multiplicative, exotestal. Exotestal cells sometimes elongate, usually with thickened inner walls. Endotesta disintegrating. Perisperm not developed. Endosperm usually copious (sometimes sparse), oily (starch absent). Embryo small, straight, well differentiated or rudimentary (in Cotylanthera, Leiphaimos, Voyria and Voyriella 5- to 24-celled, undifferentiated), with or without chlorophyll. Cotyledons two (rarely much reduced). Germination phanerocotylar.
Cytology n = 5<, 9–15, 17 etc.
DNA Deletion of 100 bp present in plastid gene trnL.
Phytochemistry Flavonols, O-methylated flavones, flavone-O-glucosides (in two species of Exacum), flavone-C-glycosides (in Potalieae and Gentianeae), Route I iridoids, Group VI secoiridoids (secologanin), Group VII secoiridoids (sweroside, swertiamarin, gentiopicroside, gentianin), Group X secoiridoids (loganin, iridoid pyridine alkaloids), caffeic acid, alkaloids, saponins, cyanogenic compounds, and 6-substituted xanthones (norathyriol etc., in Chironieae and Gentianeae) present. Indole alkaloids? Ellagic acid, tannins and proanthocyanidins not found. Carbohydrates stored as oligosaccharides (not starch). Aluminium accumulated in a few species.
Use Ornamental plants, medicinal plants.
Systematics Gentianaceae are sister-group to the [Loganiaceae+[Gelsemiaceae+Apocynaceae]] clade. A plausible topology is the following (Merckx & al. 2013): [Saccifolieae+[Exaceae+[Voyrieae+[Chironieae+[Potalieae+[Helieae+Gentianeae]]]]]]
Saccifolieae (Maguire et Pires) Struwe, Thiv, V. A. Albert & Kadereit in L. Struwe et V. A. Albert, Gentianac.: Syst. Nat. Hast.: 48. 2002
5/19–21. Curtia (8–10; the Guayana Highlands to Uruguay), Hockinia (1; H. montana; eastern Brazil), Saccifolium (1; S. bandeirae; the Guayana Highlands), Tapeinostemon (8; northeastern South America), Voyriella (1; V. parviflora; northeastern South America). – Northeastern South America to Uruguay. Sometimes shrubs or achlorophyllous mycoheterotrophic herbs. Leaves sometimes alternate (spiral). Flowers usually pentamerous (sometimes tetra- or hexamerous). Heterostyly sometimes present. Placentation parietal. Endosperm development in Voyriella cellular. Cotyledons sometimes absent. n = 10–14.
[Exaceae+[Voyrieae+[Chironieae+[Potalieae+[Helieae+Gentianeae]]]]]
Exaceae Colla, Herb. Pedem. 4: 174. 15-31 Aug 1835
8/c 150. Lagenias (1; L. pusillus; Western Cape), Sebaea (c 40; tropical and subtropical Africa, Madagascar, Socotra, the Arabian Peninsula to India and Sri Lanka, southern Australia, Tasmania, New Zealand), Exochaenium (22; tropical and southeastern Africa), Exacum (c 70; tropical regions in the Old World, with their highest diversity in Madagascar), Klackenbergia (2; K. condensata, K. stricta; Madagascar), Ornichia (3; O. lancifolia, O. madagascariensis, O. trinervis; Madagascar), Gentianothamnus (1; G. madagascariensis; Madagascar), Tachiadenus (11; Madagascar). – Tropical regions in the Old World, southern Australia, Tasmania, New Zealand, with their highest diversity in Madagascar. Some species of Exacum and Sebaea achlorophyllous mycoheterotrophic herbs. Flowers usually actinomorphic (in Exacum and Orphium zygomorphic), usually pentamerous (sometimes tetramerous). Sepals free or connate. Sebaea with one pair of collateral secondary stigmas at stylar base. Ovules in some species of Exacum orthotropous, ategmic. Anticlinal exotestal cell walls sinuate. n = 9, 11, 15 or more. Flavone-O-glycosides present in few species of Exacum.
[Voyrieae+[Chironieae+[Potalieae+[Helieae+Gentianeae]]]]
Voyrieae Gilg in H. G. A. Engler et K. A. E. Prantl, Nat. Pflanzenfam. IV, 2: 62, 102. Jun 1895
1/20. Voyria (20; Florida, southern Mexico, Central America, tropical South America, one species, V. primuloides, in tropical Africa). – Achlorophyllous mycoheterotrophic herbs. Roots and shoots exogenous or endogenous. Rhizoids usually absent. Vascular bundles bicollateral, separate. Colleters present or absent. Pollen grains 1–6-porate. Exine scabrate to smooth. Sometimes with stamen-like appendages (staminodia or nectaries?) on each side of ovary. Stigma infundibular. Ovules orthotropous ategmic or anatropous unitegmic. Endothelium present. Nucellar cap present. Endosperm development cellular or nuclear. Fruit a septicidal capsule. Seed coat exotestal. Endosperm sparse to almost absent. Embryo undifferentiated. n = 16–20.
[Chironieae+[Potalieae+[Helieae+Gentianeae]]]
Placentation parietal. Xanthones and L-(+)-bornesitol present.
Chironieae Dumort., Fl. Belg.: 51. 1827
27/175–180. Bisgoeppertia (3; B. gracilis, B. robustior, B. scandens; the West Indies), Blackstonia (4; B. acuminata, B. grandiflora, B. imperfoliata, B. perfoliata; Europe, the Mediterranean), Centaurium (c 20; Europe, the Mediterranean, temperate Asia), Chironia (c 30; tropical and southern Africa, Madagascar), Cicendia (2; C. filiformis: Europe, the Mediterranean; C. quadrangularis: Oregon, California, western South America), Zeltnera (c 25; southwestern United States, northwestern Mexico), Gyrandra (3; Mexico, Central America), Schenkia (5; S. australis, S. clementii, S. japonica: Australia, Tasmania, islands in western Pacific; S. sebaeoides: the Hawaiian Islands; S. spicata: Europe, the Mediterranean, North Africa), Eustoma (2; E. exaltatum, E. russellianum; southern United States and Mexico to northern South America), Exaculum (1; E. pusillum; Europe), Geniostemon (5; G. atarjanum, G. coulteri, G. gypsophilum, G. rotundifolius, G. schaffneri; Mexico), Ixanthus (1; I. viscosus; the Canary Islands), Orphium (1; O. frutescens; Western Cape), Gyrandra (5; G. chironioides, G. pterocaulis, G. tenuifolia: Mexico; G. brachycalyx, G. pauciflora: Central America), Sabatia (21; eastern and central North America, Mexico, Central America, the West Indies), Zygostigma (1; Z. australe; Brazil, Argentina); Canscora (9–10; tropical regions in the Old World to tropical Australia), Cracosna (3; C. carinata, C. gacilis, C. xyridiformis; Southeast Asia), Duplipetala (2; D. hexagona, D. pentanthera; Thailand, the Malay Peninsula), Hoppea (2; H. dichotoma, H. fastigiata; India, Sri Lanka, Burma), Microrphium (1; M. pubescens; peninsular Thailand, the Malay Peninsula, Palawan), Phyllocyclus (5; P. helferianus, P. lucidissimus, P. minutiflorus, P. parishii, P. petelotii; Burma, southern China, Thailand), Schinziella (1; S. tetragona; tropical Africa); Coutoubea (5; C. humilis, C. minor, C. ramosa, C. reflexa, C. spicata; the West Indies, tropical South America), Deianira (7; D. chiquitana, D. cordifolia, D. cyathifolia, D. damazioi, D. erubescens, D. nervosa, D. pallescens; tropical America), Schultesia (11–15; tropical America), Symphyllophyton (2; S. campos-portoi, S. caprifolioides; Brazil), Xestaea (1; X. lisianthoides; Panamá, Venezuela). – Subcosmopolitan, with their highest diversity in tropical and subtropical regions. Usually chlorophyllous herbs (sometimes shrubs). Flowers (2–)4–5(–12)-merous. Sepals connate. Pollen grains sometimes shed as tetrads. n = 10, 13–15, 17 or more. Special 6-substituted xanthones.
[Potalieae+[Helieae+Gentianeae]]
Nectaries present.
Potalieae Endl., Gen. Plant.: 576. Aug 1838
13/180–190. Congolanthus (1; C. longidens; tropical Africa), Djaloniella (1; D. ypsilostyla; the Diaguissa Plateau in Guinea), Enicostema (3; E. axillare, E. elizabethae, E. littorale; tropical Africa and Madagascar to the Lesser Sunda Islands, Central America, the West Indies), Faroa (c 20; tropical Africa), Karina (1; K. tayloriana; Congo), Lisianthius (c 30; southern United States, Mexico, Central America, tropical South America), Neurotheca (3; N. congolana, N. corymbosa, N. loeselioides; tropical Africa, one species, N. loeselioides, also in tropical South America), Oreonesion (1; O. testui; Gabon), Pycnosphaera (1; P. buchananii; tropical Africa), Urogentias (1; U. ulugurensis; Uluguru and Nguru Mountains in Tanzania); Anthocleista (c 50; tropical Africa, Madagascar, the Mascarene Islands), Fagraea (60–70; southern India, Sri Lanka to China, Southeast Asia, Malesia and islands in western Pacific, with their highest diversity on Borneo), Potalia (9; Central America, tropical South America). – Pantropical, with their highest diversity in Africa, Madagascar and Malesia. Trees, shrubs, lianas, herbs. Nodes ≥5:≥5, multilacunar with at least five leaf traces. Epidermal and cortical sclereids often present. Interpetiolar or intrapetiolar sheath-like structures or auricles often present. Flowers 3–16(–24)-merous. Sepals connate at base. Carpellary fusion sometimes congenital. Fruit sometimes a berry. n = ? Flavone-C-glucosides present. – Potaliinae: nodes multilacunar, sclereids present, flowers up to 16-merous (Anthocleista, Potalia), corolla caducous, pollen grains porate, carpellary fusion congenital, fruit a berry. Anthocleista has more than two metres long seedling leaves.
[Helieae+Gentianeae]
Helieae Gilg in Engler et Prantl, Nat. Pflanzenfam. IV, 2: 62, 95. Jun 1895
19–20/210–215. Prepusa (5; P. alata, P. connata, P. hookeriana, P. montana, P. viridiflora; mountains in Brazil), Macrocarpaea (c 105; Central America, the Greater Antilles, tropical South America), Tachia (12; tropical South America), Chorisepalum (5; C. carnosum, C. ovatum, C. psychotrioides, C. rotundifolium, C. sipapoanum; the Guayana Highlands), Neblinantha (2; N. parvifolia; the Guayana Highlands), Irlbachia (14; Venezuela, Brazil), Roraimaea (2; R. aurantiaca, R. coccinea; roraimas in southern Venezuela and northern Brazil), Aripuana (1; A. cullmaniorum; Amazonian Brazil), Lehmanniella (5; L. acuminata, L. huanucensis, L. princeps, L. pulchra, L. splendens; Panamá, Colombia, Peru), Celiantha (3; C. bella, C. chimantensis, C. imthurnia; the Guayana Highlands), Calolisianthus (4; C. imthurnianus, C. pedunculatus, C. pendulus, C. speciosus; Brazil), Symbolanthus (c 30; Costa Rica to Peru), Sipapoantha (2; S. obtusisepala, S. ostrina; southern Venezuela, northern Brazil), Rogersonanthus (3; R. arboreus, R. coccineus, R. quelchii; northeastern South America, Trinidad), Tetrapollinia (1; T. caerulescens; Venezuela, Guianas), ‘Calolisianthus’ pro parte (4; Brazil; in Tetrapollinia?), Helia (c 12; Central America, tropical South America). – Unplaced Helieae: Senaea (2; S. coerulea, S. janeirensis; mountains in Brazil), Yanomamua (1; Y. araca; Amazonian Brazil), Zonanthus (1; Z. cubensis; Cuba). – Tropical America, with their largest diversity in northern tropical South America, especially the Guayana Highlands. Usually herbs (sometimes shrubs). Interpetiolar or intrapetiolar stipules or sheath-like structure sometimes present. Flowers usually pentamerous (sometimes tetra- or hexamerous). Pollen grains sometimes shed as tetrads or polyads. Style often long, spirally twisted and flattened as dry. n = ? – Prepusa is sister to the rest (Calió & al. 2017).
Gentianeae Dumort., Anal. Fam. Plant.: 25. 1829
21/1.135–1.155. Gentiana (c 350; temperate and arctic-alpine regions on both hemispheres, tropical mountains), Kuepferia (12; northern India, Sikkim, southern and eastern Tibet, northwestern Yunnan, southwestern Sichuan, Nepal, Bhutan, northern Burma), Crawfurdia (16; the Himalayas to northern Burma and China), Metagentiana (14; China, northern Burma, northern Thailand), Sinogentiana (2; S. souliei, S. striata; China), Tripterospermum (30–35; East Asia); Bartonia (3; B. paniculata, B. verna, B. virginica; eastern North America), Comastoma (c 25; temperate and arctic-alpine regions on the Northern Hemisphere, the Himalayas), Frasera (15; North America), ‘Gentianella’ (c 300; temperate regions on the Northern Hemisphere, eastern New South Wales, Victoria, Tasmania, the Andes, with their highest diversity in the South American Andes; polyphyletic), Gentianopsis (c 20; temperate regions on the Northern Hemisphere), Halenia (c 80; mountain regions in Europe, Asia and North America, the Andes), Jaeschkea (3; J. canaliculata, J. microsperma, J. oligosperma; the Himalayas), Latouchea (1; L. fokienensis; eastern China), ’Lomatogonium’ (21; temperate and arctic-alpine regions in Europe and Asia; polyphyletic), Megacodon (1; M. stylophorus; the Himalayas), Obolaria (1; O. virginica; southeastern Canada, eastern United States), Pterygocalyx (1; P. volubilis; East Asia), Lomatogoniopsis (3; L. alpina, L. galeiformis, L. ovatifolia; China), ’Swertia’ (135–150; Europe, temperate Asia, Madagascar, mountain regions in Africa and Malesia; polyphyletic), Veratrilla (2; V. baillonii, V. burkilliana; eastern Himalayas, western China). – Northern temperate, to Sulawesi (some species of Tripterospermum), and Africa and Madagascar (some species of Swertia). Corolla sometimes with nectaries. Style often short or absent. n = 5 or higher, very variable. Special xanthones, flavone-C-glucosides present. Fructans or inulin sometimes present.
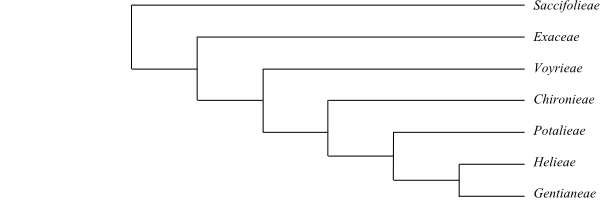 |
Cladogram (simplified) of Gentianaceae based on DNA sequence data (Merckx & al. 2013). |
LOGANIACEAE R. Br. ex Mart. |
( Back to Rubiales ) |
Strychnaceae DC. ex Perleb, Vers. Artzneikr. Pfl.: 244. Mai 1818 [’Strychneae’]; Spigeliaceae Bercht. et J. Presl, Přir. Rostlin 1: 40. 1823 [’Spigelieae’]; Strychnales Link, Handbuch 1: 439. 4-11 Jul 1829 [‘Strychnaceae’]; Loganiales Lindl., Nix. Plant.: 19. 17 Sep 1833; Gardneriaceae Wall. ex Perleb, Clav. Class.: 23. Jan-Mar 1838 [’Gardnereae’]; Spigeliineae J. Presl in Nowočeská Bibl. [Wšobecný Rostl.] 7: 1071, 1081. 1846 [‘Spiegelieae’]; Strychnineae J. Presl in Nowočeská Bibl. [Wšobecný Rostl.] 7: 1051, 1060. 1846 [‘Strychneae’]; Antoniaceae Hutch., Fam. Fl. Pl., ed. 2: 375. 4 Jun 1959; Geniostomaceae L. Struwe et V. A. Albert in Cladistics 10: 206. Jun 1995
Genera/species 16/395–415?
Distribution Tropical and subtropical regions in both hemispheres.
Fossils Unknown.
Habit Usually bisexual (rarely monoecious, gynomonoecious, dioecious, or gynodioecious), evergreen or deciduous trees, shrubs or lianas (with branch tendrils), perennial or annual herbs.
Vegetative anatomy Phellogen ab initio superficially or deeply seated. Primary medullary strands usually narrow (sometimes wide or alternately narrow and wide). Secondary lateral growth normal or anomalous (from cylindrical cambium). Vessel elements usually with simple (rarely scalariform or reticulate) perforation plates; lateral pits alternate, bordered pits. Vestured pits sometimes present (Geniostoma). Imperforate tracheary xylem elements fibre tracheids or libriform fibres with simple or bordered pits, septate or non-septate (also vasicentric tracheids). Wood rays uniseriate or multiseriate, homocellular or heterocellular. Axial parenchyma apotracheal diffuse, or paratracheal scanty vasicentric, confluent or banded, or absent. Intraxylary phloem (diffuse) present in, e.g., Strychnos. Tyloses sometimes frequent. Sieve tube plastids S type. Nodes 1:≥1, unilacunar with one or more leaf traces, 3:3, trilacunar with three traces, or multilacunar with several traces (sometimes with split lateral strands). Prismatic calciumoxalate crystals often abundant; styloids present in some species.
Trichomes Hairs usually unicellular simple (sometimes multicellular, multi-armed, stellate, candelabra-shaped or lepidote; sometimes glandular) or absent.
Leaves Usually opposite (rarely verticillate or pseudoverticillate), simple, entire, sometimes coriaceous (rarely scale-like), often with flat ptyxis. Stipules interpetiolar, intrapetiolar (sometimes sheathing) or absent; leaf sheath absent. Axillary colleters present in many species (especially in association with stipules). Petiole bases usually connate, often via a line or short ochrea. Petiole vascular bundles? Venation usually pinnate (sometimes palmate; leaves sometimes one-veined). Stomata anomocytic, paracytic, anisocytic or cyclocytic. Cuticular wax crystalloids? Domatia as pockets or hair tufts or absent. Epidermis with or without mucilage cells. Mesophyll with or without sclerenchymatous idioblasts (branched stone cells). Leaf margin serrate or entire. Extrafloral nectaries present or absent.
Inflorescence Terminal or axillary, dichasial, panicle, thyrsoid, spike-like, corymb or umbel-like cymose (in Mitreola and Spigelia sometimes spike-like cincinni; flowers sometimes solitary axillary). Some species of Mitrasacme with pseudanthia at fruit ripening surrounded by accrescent involucre consisting of bracts or possibly floral prophylls (bracteoles).
Flowers Usually actinomorphic (in Usteria zygomorphic). Hypogyny (rarely partial epigyny). Sepals (four or) five, with imbricate, valvate or open aestivation, uniform or non-uniform (rarely petaloid), often persistent, usually connate at base (median sepal in Logania abaxial), sometimes with adaxial colleters. Petals (four or) five, usually with imbricate or valvate (rarely contorted) aestivation, connate into an infundibuliform or tubular corolla, often with adaxial hairs at lobe bases. Nectariferous disc poorly developed, intrastaminal, or absent. Nectaries sometimes present on ovary walls. Diheterostyly present in some genera (e.g. Geniostoma).
Androecium Stamens usually five (sometimes four; in Usteria one, abaxial), haplostemonous, antesepalous, alternipetalous. Filaments free, adnate to corolla tube (epipetalous). Anthers usually dorsifixed (sometimes basifixed), versatile?, tetrasporangiate, usually introrse (sometimes latrorse or extrorse), longicidal (dehiscing by longitudinal slits); connective sometimes slightly prolonged at apex. Tapetum secretory. Staminodia one to three, extrastaminal, or absent; female flowers often with staminodia. Secondary pollen display present in some species.
Pollen grains Microsporogenesis simultaneous. Pollen grains usually (2–)3(–4)-colporate (rarely 2–4-colpate, tripororate, polypantoporate, polyzonoporate or syncolpate), shed as monads, tricellular at dispersal. Exine tectate or semitectate, with columellate infratectum, perforate to reticulate, psilate.
Gynoecium Pistil composed of two (or three) usually connate carpels (sometimes partially free; carpels sometimes congenitally syncarpous; sometimes postgenitally connate exclusively at apex). Ovary usually superior (rarely semi-inferior), usually bilocular (rarely unilocular or trilocular). Style single, simple, thick, or stylodia two (rarely three), free (stylodia rarely connate at apex only), often persistent, or absent. Stigma capitate, punctate, clavate, truncate, or bilobate (or trilobate), papillate, Wet type (Mitreola). Male flowers often with pistillodium.
Ovules Placentation usually axile (rarely apical). Ovules usually several to numerous (rarely one) per carpel, usually anatropous or hemianatropous (rarely amphitropous, campylotropous or orthotropous), unitegmic, tenuinucellar. Integument four to six cell layers thick. Endothelium present in Mitrasacme. Megagametophyte monosporous, Polygonum type. Endosperm development usually nuclear (rarely cellular). Endosperm haustoria lateral or absent. Embryogenesis usually solanad (in Strychnos and allied taxa onagrad).
Fruit A loculicidal and/or septicidal or denticidal capsule, a follicle (rarely a pyxidium) or a schizocarp with two follicle-like mericarps; in Strychnos, Neuburgia etc. a drupe or a berry.
Seeds Aril absent. Testa thin or thick, sometimes winged or (in Geniostoma and Labordia) embedded in juicy tissue or swollen placentae. Exotestal cells hairy or papillate, thick-walled and lignified (except outer wall). Endotesta? Perisperm not developed. Endosperm usually copious (rarely sparse), carnose or horny (rarely ruminate), with starch or hemicellulose. Embryo large or small, straight or curved, well differentiated, chlorophyll? Cotyledons two. Germination phanerocotylar.
Cytology n = 10–12, 16
DNA Mitochondrial coxI intron present in Strychnos.
Phytochemistry Flavonols (kaempferol, quercetin), O-methylated flavones, Route I iridoids, Group VI secoiridoids (secologanin), Group VII secoiridoids (sweroside, gentiopicroside), Group IX secoiridoids (indole alkaloids of corynanthe-type), Group X secoiridoids (desoxyloganin, loganin, ketologanin), tryptophane-derived alkaloids, indole alkaloids (in Strychneae), and saponins present. Ellagic acid, proanthocyanidins and cyanogenic compounds not found. Caffeic acid? Aluminium accumulated in some species of Strychnos and allied groups.
Use Ornamental plants, medicinal plants, poisons (Strychnos), fruits (Strychnos spp.), timber.
Systematics Usteria (1; U. guineensis; tropical West and Central Africa), Bonyunia (4; B. antoniifolia, B. aquatica, B. minor, B. superba; Colombia to Brazil, Peru and Bolivia), Antonia (1; A. ovata; tropical South America); Gardneria (5; G. angustifolia, G. lanceolata, G. multiflora, G. nutans, G. ovata; India to central Japan and Southeast Asia, Java), Neuburgia (10–12; the Philippines and Sulawesi to New Guinea, tropical Australia, New Caledonia, Vanuatu and Fiji), Spigelia (50–60; tropical and subtropical South America), Strychnos (c 190?; tropical and subtropical regions on both hemispheres); Adelphacme (1; A. minima; southwestern Western Australia), Mitrasacme (c 45; tropical Asia, China, Australia, New Caledonia), Phyllangium (5; P. distylis, P. divergens, P. palustre, P. paradoxum, P. sulcatum; southwestern and southeastern Australia, Tasmania), Schizacme (5; S. archeri, S. ciliata, S. helmsii, S. montana, S. novae-zelandiae; southeastern Australia, New Zealand), Logania (c 22; Australia, New Caledonia, New Zealand, with their highest diversity in southwestern Western Australia, South Australia and New South Wales), Geniostoma (c 40; the Mascarene Islands, Malesia to southern Japan, Bonin Islands and Taiwan, eastern Queensland, Vanuatu, Fiji, New Zealand, Samoa, Tonga, Tahiti, Henderson Island, the Hawaiian Islands), Mitreola (5; M. pedicellata, M. petiolata, M. petiolatoides, M. reticulata, M. turgida; tropical regions on both hemispheres), Orianthera (13; Australia, with their highest diversity in southwestern Western Australia); Norrisia (2; N. major, N. malaccensis; Southeast Asia, West Malesia).
Loganiaceae are sister to [Gelsemiaceae+Apocynaceae].
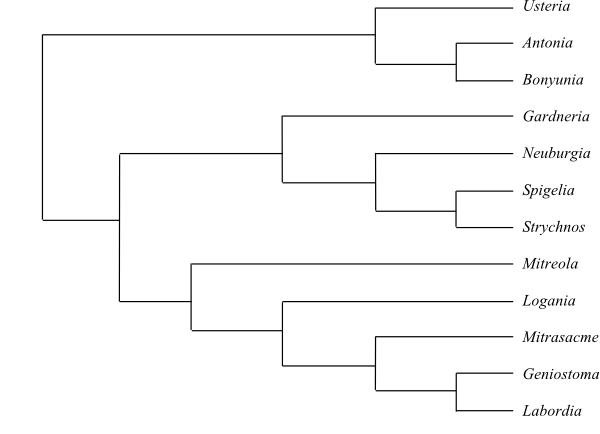 |
Consensus tree of Loganiaceae from successive weighting based on DNA sequence data (Backlund & al. 2000). |
RUBIACEAE Juss. |
( Back to Rubiales ) |
Cinchonaceae Batsch, Tab. Affin. Regni Veg.: 234. 2 Mai 1802 [’Cinchoneae’]; Coffeaceae Batsch, Tab. Affin. Regni Veg.: 233. 2 Mai 1802; Guettardaceae Batsch, Tab. Affin. Regni Veg.: 235. 2 Mai 1802 [’Guettardeae’]; Aparinaceae Hoffmanns. et Link, Fl. Portug. 2: 3, 38. 1813-1829 [’Aparines’]; Operculariaceae Juss. ex Perleb, Vers. Artzneikr. Pfl.: 207. Mai 1818 [’Operculariae’]; Spermacocaceae Bercht. et J. Presl, Přir. Rostlin: 256. Jan-Apr 1820 [’Spermacoceae’]; Catesbaeaceae Martinov, Tekhno-Bot. Slovar: 111. 3 Aug 1820 [’Catesbiaceae’]; Coutareaceae Martinov, Tekhno-Bot. Slovar: 168. 3 Aug 1820 [’Coutareae’]; Hydrophylacaceae Martinov, Tekhno-Bot. Slovar: 318. 3 Aug 1820 [’Hydrophylaceae’]; Nonateliaceae Martinov, Tekhno-Bot. Slovar: 420. 3 Aug 1820 [’Nonateliae’]; Pagameaceae Martinov, Tekhno-Bot. Slovar: 447. 3 Aug 1820 [’Pagameae’]; Randiaceae Martinov, Tekhno-Bot. Slovar: 534. 3 Aug 1820 [’Randiae’]; Sabiceaceae Martinov, Tekhno-Bot. Slovar: 555. 3 Aug 1820 [’Sabiceae’]; Cephalanthaceae Raf. in Ann. Gén. Sci. Phys. Bruxelles 6: 86. Oct-Dec 1820 [’Cephalantia’]; Rubiineae Raf. in Ann. Gén. Sci. Phys. Bruxelles 6: 83. Oct-Dec 1820 [‘Rubiacea‘]; Hedyotidaceae Dumort., Comment. Bot.: 57. Nov-Dec 1822 [’Hediotideae’]; Gardeniaceae Dumort., Anal. Fam. Plant.: 29, 32. 1829; Theligonaceae Dumort., Anal. Fam. Plant.: 15, 17. 1829 [’Theligoneae’], nom. cons.; Lygodisodeaceae Bartl., Ord. Nat. Plant.: 123, 207. Sep 1830 [’Lygodysodeaceae’]; Psychotriaceae F. Rudolphi, Syst. Orb. Veg.: 49. 5-12 Jul 1830 [’Psychotrieae’]; Rubiopsida Bartl., Ord. Nat. Plant.: 123, 206. Sep 1830 [’Rubiacinae’]; Cinchonales Lindl., Nix. Plant.: 18. 17 Sep 1833; Asperulaceae Cham. ex Spenn., Handb. Angew. Bot. 2: 492. 1835 [’Asperuleae’]; Hameliaceae Mart., Consp. Regn. Veg.: 31. Sep-Oct 1835 [‘Hamelieae’]; Lygodisodeales Bartl. in C. F. P. von Martius, Consp. Regn. Veg.: 31. Sep-Oct 1835 [‘Lygodyseaceae’]; Galiaceae Lindl., Intr. Nat. Syst. Bot., ed. 2: 249. 13 Jun 1836 [’Stellateae, or Galiaceae’]; Galiales Bromhead in Edinburgh New Philos. J. 24: 414. Apr 1838; Lippayaceae Meisn., Plant. Vasc. Gen.: Tab. Diagn. 156, Comm. 112. 16-22 Sept 1838 [’Lippayeae’]; Houstoniaceae Raf., Good Book: 21. Jan 1840 [’Houstonides’]; Cynocrambaceae Meisn., Plant. Vasc. Gen., Tab. Diagn. 345, Comm. 256. 13-15 Feb 1842 [’Cynocrambeae’], nom. illeg.; Coffeopsida Brongn., Enum. Plant. Mus. Paris: xvii, 48. 12 Aug 1843 [’Coffeineae’]; Cinchonineae J. Presl in Nowočeská Bibl. [Wšobecný Rostl.] 7: 790, 791. 1846 [‘Cinchoneae’]; Coffeineae J. Presl in Nowočeská Bibl. [Wšobecný Rostl.] 7: 791, 826. 1846 [‘Coffeaceae’]; Gardeniineae J. Presl in Nowočeská Bibl. [Wšobecný Rostl.] 7: 791, 804. 1846; Guettardineae J. Presl in Nowočeská Bibl. [Wšobecný Rostl.] 7: 791, 819. 1846; Hameliineae J. Presl in Nowočeská Bibl. [Wšobecný Rostl.] 7: 791, 817. 1846; Hedyotidineae J. Presl in Nowočeská Bibl. [Wšobecný Rostl.] 7: 791, 812. 1846; Spermacocineae J. Presl in Nowočeská Bibl. [Wšobecný Rostl.] 7: 791, 835. 1846 [‘Spermacoceae’]; Naucleaceae (DC.) Wernh. in New Phytol. 11: 225. 30 Jul 1912; Theligonales Nakai in J. Jap. Bot. 18: 92, 94. 10 Mar 1942 [’Thelygonales’]; Dialypetalanthaceae Rizzini et Occhioni in Lilloa 17: 253. 30 Dec 1948, nom. cons.; Henriqueziaceae Bremek. in Acta Bot. Neerl. 6 [Meded. Bot. Mus. Herb. Rijksuniv. Utrecht 141]: 371. Aug 1957
Genera/species 575/14.180–14.490
Distribution Cosmopolitan except polar areas, with their highest diversity in tropical and subtropical regions.
Fossils Fossil pollen grains of Rubiaceae have been found from the Late Eocene onwards. Fruits of Emmenopterys dilcheri are known from mid-Eocene layers in Oregon and Washington. Canthium and Guettarda are reported from the Late Eocene of Australia and Faramea from the Late Eocene of Panamá. Fossil leaves were described as Paleorubiaceophyllum from mid-Eocene layers in Tennessee and Kentucky and further genera are known from the Oligocene and, above all, the Miocene (Graham 2009). Cephalanthus is recorded from Cenozoic strata in Europe.
Habit Usually bisexual (rarely monoecious, polygamomonoecious or dioecious), evergreen or deciduous trees, shrubs, lianas or suffrutices, or perennial, biennial or annual herbs. Hydnophytum, Myrmecodia, Myrmeconauclea, Myrmephytum, etc. are epiphytic or (Myrmeconauclea) rheophytic myrmecophytes (ant plants), in the hollow swollen stems and branches – hypocotylar bases – of which ant colonies live. Young stems and branches often quadrangular in cross-section.
Vegetative anatomy Phellogen ab initio usually superficial (sometimes deeply seated). Primary medullary strands at least usually narrow. Secondary lateral growth usually normal (sometimes anomalous from concentric cambia). Endodermis sometimes significant. Vessel elements usually with simple (sometimes scalariform or reticulate) perforation plates; lateral pits alternate, bordered pits. Vestured pits present. Imperforate tracheary elements tracheids or fibre tracheids with simple and/or bordered pits, usually non-septate. Wood rays uniseriate or multiseriate, homocellular or heterocellular. Axial parenchyma apotracheal diffuse or diffuse-in-aggregates, or paratracheal scanty vasicentric, confluent, aliform-confluent, reticulate or banded, or absent. Tyloses sometimes abundant. Intraxylary phloem absent. Sieve tube plastids Ss type. Nodes 1(–≥3):1(–≥3), usually unilacunar (rarely trilacunar to multilacunar) with one leaf trace (rarely several traces), lateral bundles splitting off and forming vascular cylinder around stem and innervating stipules. Parenchyma often with secretory cells and cavities with gum-like substances. Colleters of different types often present. Prismatic crystals, crystal sand, druses, styloids, acicular and other (calciumoxalate?) crystal types often present; idioblasts (raphid cells) with calciumoxalate raphides present in, e.g., Rubioideae.
Trichomes Hairs unicellular or multicellular, simple or branched, cylindrical, papillose, moniliform, T-shaped, spiral or band-shaped (sometimes stellate or lepidote), in Rubioideae often septate; glandular hairs often present.
Leaves Usually opposite (sometimes verticillate), simple, usually entire (in Genipa and Posoqueria lobed), usually with flat ptyxis. Stipules usually interpetiolar (innervated from vascular cylinder), often serrate (sometimes intrapetiolar, interpetiolar-intrapetiolar or sheathing), often connate and adnate to petioles, caducous or persistent; leaf sheath absent. Axillary colleters abundant (especially in connection with stipules). Petiole vascular bundle transection arcuate or annular. Venation pinnate (leaves sometimes one-veined). Stomata usually paracytic (sometimes parallelocytic). Cuticular waxes? Domatia as pockets or hair tufts or absent (bacteriodomatia with Burkholderia strains present in some species of Pavetta, Psychotria and Sericanthe). Mesophyll with or without sclerenchymatous idioblasts. Calciumoxalate raphides present in Rubioideae; druses occurring in many species lacking raphides. Leaf margin serrate or entire. Extrafloral nectaries present on lamina in some species (e.g. in Tocoyena).
Inflorescence Terminal or axillary, panicle, thyrse, thyrsoid, pseudoverticillate, capitate, etc. (flowers sometimes solitary axillary). Some species with capitate pseudanthia bearing involucre consisting of (sometimes petaloid) bracts.
Flowers Usually actinomorphic (in Gleasonia, Henriquezia and Platycarpum zygomorphic). Epicalyx sometimes present. Usually epigyny (sometimes half epigyny; in Gaertnera and Pagamea secondary hypogyny; in Theligonum?). Sepals (three or) four or five, usually with open (sometimes imbricate, contorted or valvate) aestivation, often persistent (sometimes accrescent in fruit), usually connate (sometimes very small; rarely large and petaloid; in Dialypetalanthus 2+2, free; absent in Theligonum?). Petals (three or) four or five (to ten), with imbricate, valvate or contorted (rarely cochleate) aestivation, usually connate into hypocrateriform, tubular or infundibuliform corolla (in Dialypetalanthus 2+2, antesepalous, free; absent in Theligonum?). Nectariferous disc fleshy, annular (rarely absent). Diheterostyly often present.
Androecium Stamens (three or) four or five, antesepalous, alternipetalous (in Dialypetalanthus [eight to] 16 to 17 [to 25] in two whorls; in Theligonum [two to] seven to twelve [to 30], sometimes basally fused in fascicles of two, four or six stamens). Filaments usually free (in Dialypetalanthus connate at base), usually adnate to corolla tube (epipetalous; not in Dialypetalanthus). Anthers usually free (in, e.g., Argostemma connate), basifixed or dorsifixed, sometimes versatile, tetrasporangiate, usually introrse (sometimes extrorse), usually longicidal (dehiscing by longitudinal slits; in many genera poricidal, with apical pits); connective sometimes prolonged. Tapetum secretory. Staminodia absent. Secondary pollen display present in numerous Ixoroideae.
Pollen grains Microsporogenesis simultaneous. Pollen grains usually colporate (sometimes colpate, porate or inaperturate; in Theligonum 4–8-zonoporate), usually shed as monads (sometimes as tetrads), bicellular or tricellular at dispersal. Exine tectate or semitectate, with columellate infratectum, perforate, reticulate or microreticulate, spinulate, echinate, verrucate or with other types of supratectal processes, or psilate, or intectate.
Gynoecium Pistil composed of two (to five; rarely up to 16) connate carpels (in Theligonum seemingly? monocarpellate, with a pseudomonomerous? gynoecium). Ovary usually inferior (sometimes semi-inferior, rarely secondarily superior), usually bilocular (rarely unilocular, trilocular, quadrilocular, quinquelocular, or up to novemlocular). Style single and narrow, or stylodia two (to five) and free or partly connate (style in Theligonum gynobasic); often with apical or subapical thickening, on which pollen grains are deposited (secondary pollen display). Stigma capitate, or usually bilobate (rarely trilobate to quinquelobate), papillate or non-papillate, Dry or Wet type. Pistillodium absent.
Ovules Placentation usually axile (sometimes apical, rarely parietal [when unilocular] or basal). Ovule one (usually in Rubioideae) to numerous per carpel, usually anatropous or hemitropous or campylotropous? (rarely circinotropous or hypertropous; in Theligonum campylotropous or amphitropous), pendulous, horizontal or ascending, apotropous or epitropous, unitegmic, usually tenuinucellar (reduced, with meiocyte semi-inferior; rarely crassinucellar; megasporangium sometimes absent). Integument one to 14 cell layers thick. Funicular obturator often present. Megasporocytes often several. Parietal cells sometimes present. Megasporangial epidermal cells sometimes anticlinally elongate. Megagametophyte usually monosporous, Polygonum type (rarely tetrasporous, Drusa type, or disporous, Allium type; megagametophytes sometimes several, haustorial). Antipodal cell(s) sometimes persistent, occasionally proliferating and haustorial. Endosperm development usually nuclear (rarely cellular). Endosperm haustoria? Embryogenesis usually ? (sometimes solanad). Suspensor haustorium present in some species.
Fruit A loculicidal and/or septicidal capsule (rarely a pyxidium), a berry or a drupe (sometimes a schizocarp with two [to nine] nut-like mericarps, sometimes a pseudofruit consisting of fused ovaries; pericarp in Theligonum with elaiosome).
Seeds Seeds sometimes pachychalazal. Aril absent? Only exotesta persistent (testa sometimes absent); exotesta usually one-layered; tangential walls of exotestal cells often with thickenings or exotestal cells parenchyma-like (sometimes palisade-like). Mesotestal cell walls sometimes thickened. Endotesta usually parenchymatous and crushed. Perisperm not developed. Endosperm usually copious, horny or soft (sometimes ruminate), oily (often with hemicellulose, in Theligonum with starch; sometimes sparse). Embryo large or small, straight or curved, usually well differentiated, without chlorophyll. Cotyledons two, usually flat. Germination phanerocotylar or cryptocotylar.
Cytology n = 9–11(–17)
DNA atpB promoter usually lost in Rubioideae. Plastid gene infA lost/defunct (Galium, Pentas). Mitochondrial intron coxII.i3 lost (Coffea). Mitochondrial coxI intron present in many genera.
Phytochemistry Flavonols (kaempferol, quercetin), flavones, O-methylated flavones, Group I carbocyclic iridoids (geniposide, scandoside, daphylloside, monotropein, gardenoside), Group II carbocyclic iridoids (shantziside), Group IX secoiridoids (ipecacalkaloids, indole alkaloids of corynanthe type), Group X secoiridoids (loganin, antirrhide, gardoside), proanthocyanidins, quinoline alkaloids (e.g. quinine), iridoid coumarins (asperuloside), tannins, ursolic acid, caffeic acid, saponins, anthraquinones (usually shikimic acid derived, although in Asperula polyacetate derived, e.g. alizarin), and arbutin present. Cinchonoideae with corynantheane and complex indole alkaloids. Phenylalanine-derived cyanogenic compounds? Ellagic acid not found. Aluminium accumulated in, e.g., numerous species of Rubioideae.
Use Ornamental plants, medicinal plants, flavouring (quinine from Cinchona), perfumes (Gardenia etc.), stimulants (Coffea), beverages (Morinda), tanning, dyeing sources (Morinda, Rubia etc.), timber.
Systematics Luculia
may be sister to all other Rubiaceae, and
[Coptosapelta+Acranthera] successive sister to the remainder.
However, in some analyses Luculia is
recovered as sister to the clade [Coptosapelteae+Rubioideae]
(see, e.g., Manns 2012). Cinchonoideae are probably sister-group to
Ixoroideae. Rydin & al. (2017) found a different phylogeny using
mitochondrial DNA from 57 representatives of Rubiaceae.
Luculieae Rydin et B. Bremer in C. Rydin et al. in Plant Syst. Evol. 278: 120. Mar 2009
1/5. Luculia (5; L. grandifolia, L. gratissima, L. intermedia, L. pinceana, L. yunnanensis; the Himalayas, Yunnan, Burma, southern China, northern Thailand, northern Indochina). – Shrubs or small trees. Inflorescence umbel or corymb. Pollen grains tricolporate, acolumellate(?). – Luculia has often been recovered as a member of a basal polytomy also comprising Coptosapelteae, a third branch consisting of [Cinchonoideae+Ixoroideae] and a fourth branch consisting of Rubioideae. On the other hand, Luculia may be sister to [Coptosapelteae+Rubioideae] (with fairly weak support).
Coptosapelteae Bremek. ex S. P. Darwin in Taxon 25: 598. 26 Nov 1976
2/c 56. Coptosapelta (16; southeastern China, Southeast Asia, Malesia to New Guinea), Acranthera (c 40; India to central China, Southeast Asia, West Malesia to the Philippines, with their highest diversity on Borneo). – India, China, Southeast Asia, Western and Central Malesia. Raphides present. Hairs T-shaped. Corolla with dextrorsely contorted aestivation. Pollen grains 3–5-pororate. Exine with acolumellate infratectum, reticulate to psilate. Diheterostyly or secondary pollen presentation occurring. Aluminium accumulated. – It is possible that Coptosapelteae are sister-group to Rubioideae (see, e.g., Manns & al. 2012).
[Cinchonoideae+Ixoroideae]
Cinchonoideae Raf. in Ann. Gén. Sci. Phys. Bruxelles 6: 81. Oct-Dec 1820 [‘Cinchonaria’]
106/1.785–1.825. Cosmopolitan, but mainly Neotropical. Woody. Hairs usually cylindrical. Raphides present in Hamelieae and Hillieae. Calycophylly present in some species. Corolla contorted (usually sinisterally) or cochleate. Secondary pollen presentation frequently occurring. Stigma usually bilobate. Ovules often numerous per carpel. Fruit usually a capsule. Route II iridoids (carboxylated iridoids) and indole alkaloids present. Corynanthean and complex indole alkaloids present in e.g. Cinchoneae. Aluminium accumulated in some species. – A possible topology is the following (Manns & al. 2012): [[Cinchoneae+Isertieae]+[[Hymenodictyeae+Naucleeae]+[Guettardeae+Rondeletieae]+[Chiococceae+[[Chione+Colleteria]+[Hamelieae+Hillieae]]]]]
[Cinchoneae+Isertieae]
Cinchoneae DC. in Ann. Mus. Natl. Hist. Nat. 9: 217. 1807 [‘Cinchonaceae’]
9/c 130. Joosia (18; Panamá, western South America to Bolivia), Stilpnophyllum (4; S. grandifolium, S. lineatum, S. oellgaardii, S. revolutum; the Andes in Ecuador and Peru), Ciliosemina (2; C. pedunculata, C. purdieana; northwestern South America), Ladenbergia (c 35; Central America, tropical South America), Cinchonopsis (1; C. amazonica; central and western Amazonas), Cinchona (c 25; Costa Rica, Colombia and Venezuela to central Bolivia), Remijia (c 45; tropical South America), Maguireocharis (1; M. neblinae; the Guayana Highlands)?, Pimentelia (1; P. glomerata; Peru)? – Tropical America. – Cinchoneae are sister to Isertieae.
Isertieae A. Rich. ex DC., Prodr. 4: 342, 435. late Sep 1830
2/15. Isertia (14; Central America, tropical South America), Kerianthera (1; K. preclara; Amazonian Brazil). – Tropical America.
[[Hymenodictyeae+Naucleeae]+[Guettardeae+Rondeletieae]+[Chiococceae+[[Chione+Colleteria]+[Hamelieae+Hillieae]]]]
[Hymenodictyeae+Naucleeae]
Hymenodictyeae Razafim. et B. Bremer in Syst. Geogr. Plants 71: 535. 2002
2/25. Paracorynanthe (2; P. antankarana, P. uropetala; Madagascar), ‘Hymenodictyon’ (23; tropical regions in the Old World to Sulawesi; paraphyletic). – Tropical regions in the Old World to Sulawesi. Corolla with valvate aestivation. – Hymenodictyeae seem to be sister to Naucleeae.
Naucleeae Burnett, Outlines Bot.: 906. Feb 1835
17/183–>200. Cephalanthus (6; C. angustifolius, C. glabratus, C. natalensis, C. occidentalis, C. salicifolius, C. tetrandra; tropical regions on both hemispheres, southeastern United States), Mitragyna (7; M. diversifolia, M. hirsuta, M. inermis, M. parvifolia, M. rotundifolia, M. speciosa, M. tubulosa; tropical regions in the Old World), Sinoadina (1; S. racemosa; China, Japan, Burma, Thailand), Haldina (1; H. cordifolia; India, Sri Lanka, southern China, Southeast Asia), Adina (15; China, the Korean Peninsula, Japan, tropical Asia to New Guinea), Khasiaclunea (1; K. oligocephala; northeastern India; in Adina?), Neonauclea (75–80; southern China, tropical Asia to islands in western Pacific), Diyaminauclea (1; D. zeylanica; Sri Lanka; in Neonauclea?), Breonadia (1; B. salicina; tropical Africa, Madagascar), ‘Breonia’ (6–20; Madagascar; paraphyletic), Gyrostipula (3; G. comorensis, G. foveolata, G. obtusa; Madagascar, the Comoros), Janotia (1; J. macrostipula; Madagascar), Corynanthe (8?; tropical West and Central Africa), Pseudocinchona (3; P. africana, P. mayumbensis, P. pachyceras; tropical Africa), Uncaria (c 40; tropical regions on both hemispheres), Neolamarckia (2; N. cadamba, N. macrophylla; tropical Asia to tropical Australia), Nauclea (12; tropical regions in the Old World). – Tropical regions on both hemispheres, East Asia, one species in southeastern United States.
[Guettardeae+Rondeletieae]
Guettardeae DC. in Ann. Mus. Natl. Hist. Nat. 9: 217. 1807 [‘Guettardaceae’]
21/790–800. Rogiera (c 15; southern Mexico, Central America, the West Indies, northern South America), Allenanthus (2; A. erythrocarpus, A. hondurensis; Central America), Neoblakea (1; N. venezuelensis; Venezuela), ‘Antirhea’ (c 40; Madagascar, Malesia to Samoa; polyphyletic), Bobea (4; B. brevipes, B. gaudichaudii, B. sandwicensis, B. timonioides; the Hawaiian Islands), Hodgkinsonia (2; H. frutescens, H. ovatiflora; eastern Queensland, northeastern New South Wales; in Anthirhea?), Tinadendron (2; T. kajewskii: Vanuatu; T. noumeanum: New Caledonia; in Anthirhea?), ‘Guettarda’ (c 160; Vanuatu, New Caledonia, tropical America, one species, G. speciosa, along tropical coasts; polyphyletic), Timonius (c 170; Mauritius, the Seychelles, Sri Lanka, the Andaman Islands, Malesia to islands in the Pacific, with their largest diversity on New Guinea), ‘Chomelia’ (c 75; Mexico, Central America, the West Indies, tropical South America; polyphyletic), ‘Arachnothryx‘(107; Mexico, Central America, Trinidad, Colombia to Peru; paraphyletic), Javorkaea (1; J. hondurensis; Honduras; in Arachnothryx?), Cuatrecasasiodendron (2; C. colombianum, C. spectabile; Colombia; in Arachnothryx?), Gonzalagunia (c 40; southern Mexico, Central America, the West Indies, tropical South America), Malanea (c 40; tropical America), ‘Machaonia’ (32; southern Mexico, Central America, the West Indies, tropical South America; polyphyletic), Dichilanthe (2; D. zeylanica: Sri Lanka; D. borneensis: Borneo)?, Neolaugeria (35; the West Indies), Stenostomum (c 45; the West Indies, northern South America; incl. Resinanthus?), Resinanthus (16; the West Indies, one species, R. aromaticus, in Mexico, with their highest diversity on Cuba and Hispaniola; in Stenostomum?), Pittoniotis (2; P. protracta, P. trichantha; southern Mexico, Central America, tropical South America). – Madagascar, Mauritius, the Seychelles, Sri Lanka, the Andamans, Malesia to eastern Australia, Melanesia, Samoa and the Hawaiian Islands, tropical America. – Guettardeae are sister to Rondeletieae.
Rondeletieae Burnett, Outlines Bot.: 906. Feb 1835
13/c 210. Tainus (1; T. pitreana; southern Hispaniola), Acrosynanthus (7; A. jamaicensis, A. latifolius, A. minor, A. ovatus, A. parvifolius, A. revolutus, A. trachyphyllus; the West Indies), Mazaea (2; M. phialanthoides, M. shaferi; Cuba), Phyllomelia (1; P. coronata; western Cuba; in Mazaea?), Arcythophyllum (c 15; tropical America), Roigella (1; R. correifolia; western Cuba), ‘Rondeletia’ (c 170; Panamá, the West Indies, tropical South America; polyphyletic), Rovaeanthus (2; R. strigosus, R. suffrutescens; southern Mexico, Central America), Suberanthus (7; S. brachycarpus, S. canellifolius, S. hincheanus, S. neriifolius, S. pungens, S. stellatus, S. yumuriensis; Cuba, Hispaniola), Acunaeanthus (1; A. tinifolius; Cuba), Blepharidium (1; B. guatemalense; Central America), Habroneuron (1; H. radicans; Mexico), Lindenia (2–3; L. austrocaledonica: New Caledonia; L. vitiensis: Fiji; ?L. rivalis: Central America). – Melanesia, tropical America.
[Chiococceae+[[Chione+Colleteria]+[Hamelieae+Hillieae]]]]
Chiococceae Benth. et Hook. f., Gen. Plant. 2: 9, 21. 7-9 Apr 1873
28/230–235. Strumpfia (1; S. maritima; the West Indies); Exostema (c 45; southern Mexico, Central America, the West Indies, tropical South America, with their highest diversity in the West Indies), Siemensia (1; S. pendula; western Cuba), Bikkia (c 20; East Malesia to islands in western Pacific), Erithalis (8; Florida, the West Indies), Badusa (3; B. corymbifera, B. palauensis, B. palawanensis; Palawan, New Guinea, islands in western Pacific), Scolosanthus (27; the West Indies), Chiococca (c 25; southern Florida, tropical America), Schmidtottia (15; eastern Cuba), Ceuthocarpus (1; C. involucratus; eastern Cuba), Salzmannia (1; S. nitida; eastern Brazil), Eosanthe (1; E. cubensis; eastern Cuba), Phialanthus (22; the West Indies), Ceratopyxis (1; C. verbenacea; western Cuba), Coutarea (5; C. andrei, C. corymbosa, C. diervilloides, C. hexandra, C. mollis; Mexico, Central America, tropical South America), Isidorea (17; the West Indies), Portlandia (6; P. coccinea, P. grandiflora, P. harrisii, P. microsepala, P. platantha, P. proctorii; Jamaica), Cubanola (2; C. daphnoides, C. domingensis; Cuba, Hispaniola), Cigarrilla (1; C. mexicana; Mexico), Nernstia (1; N. mexicana; Mexico), Osa (1; O. pulchra; Costa Rica), Thogsennia (1; T. lindeniana; eastern Cuba, Hispaniola), Phyllacanthus (1; P. grisebachianus; western Cuba), Catesbaea (17–20; Florida Keys, the West Indies), Asemnantha (1; A. pubescens; Mexico), Coutaportla (2; C. ghiesbreghtiana, C. pailensis; Mexico), Morierina (2; M. montana, M. propinqua; New Caledonia), Shaferocharis (3; S. cubensis, S. multiflora, S. villosa; eastern Cuba). – East Malesia, Melanesia, tropical America, with their highest diversity in the West Indies.
[[Colleteria+Chione]+[Hillieae+Hamelieae]]
[Colleteria+Chione]
2/3. Chione (1; C. venosa; Central America, the West Indies), Colleteria (2; C. exserta, C. seminervis; the West Indies). – Central America, the West Indies.
[Hillieae+Hamelieae]
Raphides present.
Hillieae Bremek. ex S. P. Darwin in Taxon 25: 603. 26 Nov 1976
3/28–29. Cosmibuena (4; C. grandiflora, C. macrocarpa, C. matudae, C. valerii; southern Mexico, Central America, tropical South America), Balmea (1; B. stormae; Mexico), Hillia (23–24; tropical America). – Tropical America. – Hillieae sensu stricto (comprising Cosmibuena, Balmea and Hillia) are sister to Hamelieae.
Hamelieae A. Rich. ex DC., Prodr. 4: 342, 438. late Sep 1830
11/175–180. Hamelia (17; Florida, Mexico, Central America, the West Indies, tropical South America), Syringantha (1; S. coulteri; Mexico); Deppea (c 25; central and southern Mexico, Central America, southeastern Brazil); Pinarophyllon (2; P. bullatum, P. flavum; Central America), ‘Deppeopsis’ (3; D. foliosa, D. hernandezii, D. tubaeana; southern Mexico; diphyletic), Hoffmannia (115–120; Mexico, Central America, the West Indies, tropical South America), Pseudomiltemia (2; P. filisepala, P. longipes; southern Mexico), Plocaniophyllon (1; P. flavum; Chiapas in Mexico), Omiltemia (4; O. filisepala, O. guerrerensis, O. longipes, O. parvifolia; Mexico), Renistipula (3; R. costaricensis, R. galeottii, R. izabalensis; southern Mexico, Central America); unplaced: Cosmocalyx (1; C. spectabilis; Mexico). – Tropical America.
Ixoroideae Raf. in Ann. Gén. Sci. Phys. Bruxelles 6: 84. Oct-Dec 1820 [‘Ixorinia’]
236/4.065–4.155. Pantropical. Latex-like substance sometimes present. Corolla with contorted or cochleate aestivation. Stigma usually bilobated. Placentation usually axile (sometimes parietal). Fruit often a drupe or a berry. Embryo short. – A plausible topology is the following: [Posoquerieae+Sipaneeae]+Condamineeae+[[Mussaendeae+Sabiceeae]+[Steenisia+[Retiniphyllum+[“Vanguerieae alliance”+”Coffeeae alliance”]]]]
[Posoquerieae+Sipaneeae]
This clade may be sister-group to the remaining Ixoroideae.
Posoquerieae Delprete in P. G. Delprete et al., Fl. Ilustr. Catarin. Rubiaceas 1: 23. 27 Dec 2004
5/c 40. Gleasonia (5; G. cururuensis, G. duidana, G. macrocalyx, G. prance, G. uaupensis; tropical South America), Henriquezia (3; H. jenmanii, H. nitida, H. verticillata; Amazonian Brazil), Platycarpum (12; northern South America), Molopanthera (1; M. paniculata; eastern Brazil), Posoqueria (c 19; southern Mexico, Central America, the West Indies, tropical South America). – Tropical America. Flowers in Gleasonia, Henriquezia and Platycarpum zygomorphic. – Posoquerieae are sister to Sipaneeae.
Sipaneeae Bremek. in Rec. Trav. Bot. Néerl. 31: 252. 1934
10?/43. Maguireothamnus (2; M. speciosus, M. tatei; Venezuela), Sipanea (19; tropical South America), Neobertiera (1; N. gracilis; Guyana), Sipaneopsis (7; S. cururuensis, S. foldatsii, S. huberi, S. maguirei, S. morichensis, S. pacimonensis, S. rupicola; northwestern South America), Dendrosipanea (2; D. revoluta, D. spigelioides; northern South America), Limnosipanea (3; L. erythraeoides, L. palustris, L. spruceana; Panamá to tropical South America), Chalepophyllum (4; C. coriaceum, C. guianense, C. pungens, C. speciosum; Venezuela, Guyana), Steyermarkia (1; S. guatemalensis; Central America), Neblinathamnus (2; N. argyreus, N. brasiliensis; Venezuela, northwestern Brazil)?, Pteridocalyx (2; P. appunii, P. minor; Guyana)? – Central America, tropical South America.
Condamineeae Benth. et Hook. f., Gen. Plant. 2: 12. 7-9 Apr 1873
31/355–360. Dioicodendron (1; D. dioicum; northwestern tropical South America), Emmenopterys (2; E. henryi, E. rehderi; southwestern China, Burma, Thailand), Pinckneya (1; P. bracteata; southeastern United States), Ferdinandusa (c 25; southern Central America, tropical South America), Dolicholobium (c 30; the Philippines to Fiji), Mussaendopsis (3; M. beccariana, M. celebica, M. malayana; West Malesia), Mastixiodendron (7; M. flavidum, M. pachyclados, M. pilosum, M. plectocarpum, M. robustum, M. smithii, M. stoddardii; East Malesia to Fiji), Capirona (1; C. decorticans; northeastern South America), Parachimarrhis (1; P. breviloba; Amazonia), Simira (c 45; Central America, tropical South America), Calycophyllum (11; southern Mexico, Central America, the West Indies, tropical South America), Alseis (18; southern Mexico, Central America to Brazil), Dialypetalanthus (1; D. fuscescens; eastern Brazil), Bothriospora (1; B. corymbosa; ; northern tropical South America), Wittmackanthus (1; W. stanleyanus; Panamá, Colombia, Ecuador, Peru, Guyana), Dolichodelphys (1; D. chlorocrater; Colombia, Ecuador, Peru), Chimarrhis (13; Central America, the West Indies, tropical South America), Warszewiczia (8; southern Mexico, Central America, the West Indies, tropical South America), Bathysa (16; Amazonia in Brazil), Picardaea (1–2; P. cubensis; Cuba, Hispaniola), Pogonopus (3; P. exsertus, P. speciosus, P. tubulosus; Colombia, Venezuela), Condaminea (5; C. corymbosa, C. elegans, C. glabrata, C. microcarpa, C. venosa; the Andes), Macbrideina (1; M. peruviana; Ecuador, Peru), Elaeagia (c 25; southern Mexico, Central America, the West Indies, tropical South America), Macrocnemum (8; Central America, Colombia), Rustia (15; Central America, tropical South America), Hippotis (12; Central America, tropical South America), Pentagonia (c 35; Central America, northern tropical South America), Sommera (10–12; Central America, tropical South America), Tammsia (1; T. anomala; Colombia, Venezuela), Hintonia (3; H. latiflora, H. lumaeana, H. octomera; Central America). – Southeast Asia, Malesia, Melanesia, southeastern United States, tropical America. – Condamineeae may be sister-group to the remaining Ixoroideae. Dialypetalanthus of eastern Brazil is sister to the clade [Bothriospora+Wittmackanthus]. The phloem is stratified, and the perianth members are decussate and free from each other. Moreover, Dialypetalanthus has (8–)16–17(–25) basally connate stamens with poricidal anthers.
[[Mussaendeae+Sabiceeae]+[Steenisia+[Retiniphyllum+[“Vanguerieae alliance”+”Coffeeae alliance”]]]]
[Mussaendeae+Sabiceeae]
Mussaendeae Benth. et Hook. f., Gen. Plant. 2: 8, 15. 7-9 Apr 1873
6/225–235. Heinsia (5; H. bussei, H. crinita, H. gossweileri, H. myrmoecia, H. zanzibarica; tropical Africa), Bremeria (19; Madagascar, the Mascarene Islands), Pseudomussaenda (6; P. angustifolia, P. flava, P. gossweileri, P. monteiroi, P. mozambicensis, P. stenocarpa; tropical Africa), ’Mussaenda’ (190–200; tropical regions in the Old World; non-monophyletic), Landiopsis (1; L. capuronii; Madagascar), Neomussaenda (2; N. kostermansiana, N. xanthophytoides; Borneo). – Tropical regions in the Old World (absent from Australia and eastwards). – Mussaenda is characterized by reduplicate-valvate aestivation and glabrous style, whereas Bremeria is distinguished from the remaining Mussaendeae by having reduplicate-valvate as well as induplicate-valvate aestivation, and densely pubescent style.
Sabiceeae A. Stahl, Estud. Fl. Puerto-Rico 5: 32. 1887 [‘Sabicieae’]
4/c 160. Sabicea (c 150; tropical Africa, Madagascar, tropical America), Tamridaea (1; T. capsulifera; Socotra), Virectaria (8; tropical Africa), Hekistocarpa (1; H. minutiflora; Nigeria, Cameroon). – Tropical Africa, Madagascar, Socotra, tropical America. – Sabiceeae seem to be sister-group to Mussaendeae and [Mussaendeae+Sabiceeae] in turn sister to [Steenisia+[Retiniphyllum+[Crossopteryx+[Scyphiphora+[Vanguerieae+[Greeneeae+[Alesanthieae+Ixoreae]]]]]]].
[Steenisia+[Retiniphyllum+[“Vanguerieae alliance”+”Coffeeae alliance”]]]
Steenisieae Kainul. et B. Bremer in K. Kainulainen, S. G. Razafimandimbison et B. Bremer, Bot. J. Linn. Soc. 173: 397. [DOI: 10.1111/boj.12038]. 25 Apr 2013
1/5. Steenisia (5; S. borneensis, S. corollina, S. elata, S. pleurocarpa, S. pterosepala; Borneo, Natuna Islands). – Corolla with contorted aestivation. – Steenisia is sister to the remaining Ixoroideae below.
[Retiniphyllum+[Crossopteryx+[Scyphiphora+[Vanguerieae+[Greeneeae+[Alesanthieae+Ixoreae]]]]]]
Retiniphylleae Benth. et Hook. f., Gen. Plant. 2: 9, 20. 7-9 Apr 1873
1/c 20. Retiniphyllum (c 20; Colombia, Venezuela, Brazil, the eastern Andes). – Resiniferous. Corolla with contorted aestivation. Stamens with basal and apical sterile appendages. Ovary quinquelocular. Ovules two per locule, collateral, pendulous. Drupe one-seeded. – Retiniphylleae are sister-group to the [“Vanguerieae alliance”+”Coffeeae alliance”] clade.
“Vanguerieae alliance”
The “Vanguerieae alliance” comprises Crossopteryx, Jackiopsis, Scyphiphora, Trailliaedoxa, Glionnetia, Vanguerieae, Greeneeae, Aleisanthieae, and Ixoreae.
[Crossopteryx+[Jackiopsis+[Scyphiphora+[Trailliaedoxa+[Glionnetia+[Vanguerieae+[Greeneeae+[Aleisanthieae+Ixoreae]]]]]]]]
Crossopterygeae F. White ex Bridson, Fl. Zambes. 5(3): 389. 2003
1/1. Crossopteryx (1; C. febrifuga; tropical and southern Africa). – Crossopteryx may be sister to the remaining “Vanguerieae alliance”.
[Jackiopsis+[Scyphiphora+[Trailliaedoxa+[Glionnetia+[Vanguerieae+[Greeneeae+[Aleisanthieae+Ixoreae]]]]]]]
Jackieae Korth. in Ned. Kruidk. Arch. 2/2): 196. 1851 [‘Jackiae’]
1/1. Jackiopsis (1; J. ornata; the Malay Peninsula, Borneo). – Jackiopsis may be sister to the remaining “Vanguerieae alliance”.
[Scyphiphora+[Trailliaedoxa+[Glionnetia+[Vanguerieae+[Greeneeae+[Aleisanthieae+Ixoreae]]]]]]
Scyphiphoreae Kainul. et B. Bremer in K. Kainulainen, S. G. Razafimandimbison et B. Bremer, Bot. J. Linn. Soc. 173: 397. [DOI: 10.1111/boj.12038]. 25 Apr 2013
1/1. Scyphiphora (1; S. hydrophyllacea; coastal areas in Southeast Asia, West Malesia, tropical Australia and New Caledonia). – Mangrove shrub. – Scyphiphora may be sister to the remaining “Vanguerieae alliance”.
[Trailliaedoxa+[Glionnetia+[Vanguerieae+[Greeneeae+[Aleisanthieae+Ixoreae]]]]]
Trailliaedoxeae Kainul. et B. Bremer in K. Kainulainen, S. G. Razafimandimbison et B. Bremer, Bot. J. Linn. Soc. 173: 397. [DOI: 10.1111/boj.12038]. 25 Apr 2013
1/1. Trailliaedoxa (1; T. gracilis; southwestern China). – Trailliaedoxa may be sister to the remaining “Vanguerieae alliance”.
[Glionnetia+[Vanguerieae+[Greeneeae+[Aleisanthieae+Ixoreae]]]]
Glionnetia
1/1. Glionnetia (1; G. sericea; the Seychelles). – Glionnetia may be sister to the clade [Vanguerieae+[Greeneeae+[Aleisanthieae+Ixoreae]]], although the support for this position is weak (see Kainulainen & al. 2013, where it was part of a trichotomy).
[Vanguerieae+[Greeneeae+[Aleisanthieae+Ixoreae]]]
Vanguerieae (A. Rich.) Dumort., Anal. Fam. Plant.: 32. 1829
24/650–680. Psydrax (80–100; tropical regions in the Old World to tropical Australia and islands in the Pacific), 'Pyrostria' (c 60; tropical Africa, Madagascar, Mauritius, Rodrigues, the Seychelles; paraphyletic), Peponidium (c 45; Madagascar, the Comoros; in Pyrostria?), Afrocanthium (17; eastern and southern Africa), Keetia (c 32; tropical and southern Africa), Kanapia (2; K. monstrosa, K. wenzelii; the Philippines), Bullockia (6; B. dyscritos, B. fadenii, B. impressinervia, B. mombazensis, B. pseudosetiflora, B. setiflora; eastern and southern Africa, Madagascar; in Pyrostria?), Bridsonia (1; B. chamaedendrum; southern Africa), Canthium (105–110?; tropical Africa, Madagascar, India, Sri Lanka), Eriosemopsis (1; E. subanisophylla; KwaZulu-Natal, border of Eastern Cape), Rytigynia (c 125; tropical and and southern Africa), Cuviera (c 25; tropical West and Central Africa), Globulostylis (8; Central Africa), Vangueriella (18; tropical West and Central Africa), Pygmaeothamnus (2; P. chamaedendrum, P. zeyheri; tropical and southern Africa), Multidentia (9–11; tropical Africa), Robynsia (1; R. glabrata; tropical West Africa), Vangueriopsis (4; V. gossweileri, V. lanciflora, V. longiflora, V. rubiginosa; tropical Africa), ’Vangueria’ (55–60; tropical Africa, Madagascar; non-monophyletic). – Unplaced Vanguerieae Meyna (11; eastern tropical Africa, the Comoros to Southeast Asia), Ancylanthos (1; A. rubiginosus; tropical Africa), Hutchinsonia (2; H. barbata, H. glabrescens; tropical West Africa), Perakanthus (1; P. velutinus; the Malay Peninsula), Cyclophyllum (c 40; Malesia to tropical Australia, New Caledonia and Fiji). – Tropical regions in the Old World to tropical Australia, Melanesia and Polynesia.
[Greeneeae+[Aleisanthieae+Ixoreae]]
Greeneeae Mouly, J. Florence et B. Bremer in A. Mouly et al. in Ann. Missouri Bot. Gard. 96: 155. Apr 2009
2/10. Greenea (9; Southeast Asia, West Malesia), Spathichlamys (1; S. oblonga; Burma). – Southeast Asia, West Malesia.
[Aleisanthieae+Ixoreae]
Aleisanthieae Mouly, J. Florence et B. Bremer in A. Mouly et al. in Ann. Missouri Bot. Gard. 96: 155. Apr 2009
3/10. Aleisanthia (2; A. rupestris, A. sylvatica; the Malay Peninsula), Aleisanthiopsis (2; A. distantiflora, A. multiflora; Borneo), Greeniopsis (6; G. discolor, G. euphlebia, G. megalantha, G. multiflora, G. philippinensis, G. pubescens; the Philippines). – West Malesia. – Aleisanthieae are sister to Ixoreae.
Ixoreae Benth. et Hook. f., Gen. Plant. 2: 9, 22. 7-9 Apr 1873
1/c 550. Ixora (c 550; tropical regions on both hemispheres).
“Coffeeae alliance”
The “Coffeeae alliance” comprises Airospermeae, Augusteae, Alberteae, the [Nematostylis+Razafimandimbisonia] clade, the [Bertiera+Coffeeae] clade, the Octotropideae and the [Sherbournieae+Cordiereae+Pavetteae+Gardenieae] clade.
[Airospermeae+[Augusteae+[Alberteae+[[Nematostylis+Razafimandimbisonia]+[[Bertiera+Coffeeae]+[Octotropideae+[Sherbournieae+Cordiereae+Pavetteae+Gardenieae]]]]]]]
Airospermeae Kainul. et B. Bremer in K. Kainulainen, S. G. Razafimandimbison et B. Bremer, Bot. J. Linn. Soc. 173: 395. [DOI: 10.1111/boj.12038]. 25 Apr 2013.
2/7. Airosperma (6; A. fuscum, A. grandifolia, A. psychotrioides, A. ramuensis, A. trichotomum, A. vanuense; New Guinea, Fiji), Boholia (1; B. nematostylis; Sumatra, the Philippines, Flores). – Malesia to New Guinea, Fiji. – This clade is sister to the remaining “Coffeeae alliance”.
[Augusteae+[Alberteae+[[Nematostylis+Razafimandimbisonia]+[[Bertiera+Coffeeae]+[Octotropideae+[Sherbournieae+Cordiereae+Pavetteae+Gardenieae]]]]]]
Augusteae Kainul. et B. Bremer in K. Kainulainen, S. G. Razafimandimbison et B. Bremer, Bot. J. Linn. Soc. 173: 395. [DOI: 10.1111/boj.12038]. 25 Apr 2013
2/c 84. Augusta (4; A. austrocaledonica: New Caledonia; A. vitiensis: Fiji; A. rivalis: Mexico, Central America, Colombia; A. longifolia: northeastern and central Brazil), Wendlandia (c 80; northeastern Africa, Iraq to Malesia, tropical Australia). – Tropical and subtropical South Asia to Melanesia, tropical Australia, Mexico, Brazil. – This clade is sister to the remaining “Coffeeae alliance”.
[Alberteae+[[Nematostylis+Razafimandimbisonia]+[[Bertiera+Coffeeae]+[Octotropideae+[Sherbournieae+Cordiereae+Pavetteae+Gardenieae]]]]]
Alberteae Sond. in W. H. Harvey et O. W. Sonder, Fl. Cap. 3: 1. 24 Feb-30 Jun 1865
1/1. Alberta (1; A. magna; southern Africa). – Alberta is sister to the remaining “Coffeeae alliance”.
[[Nematostylis+Razafimandimbisonia]+[[Bertiera+Coffeeae]+[Octotropideae+[Sherbournieae+Cordiereae+Pavetteae+Gardenieae]]]]
[Nematostylis+Razafimandimbisonia]
2/6. Nematostylis (1; N. anthophylla; Madagascar), Razafimandimbisonia (5; R. humblotii, R. minor, R. orientalis, R. regalis, R. sambiranensis; Madagascar). – This clade is sister to the remaining “Coffeeae alliance”.
[[Bertiera+Coffeeae]+[Octotropideae+[Sherbournieae+Cordiereae+Pavetteae+Gardenieae]]]
[Bertiera clade+Coffeeae]
This clade is sister-group to the remaining “Coffeeae alliance”.
Bertiereae Bridson in Fl. Zambes. 5(3): 386. 2003
c 1/c 55? 'Bertiera' pro parte (c 55?; tropical Africa, Madagascar, the Mascarene Islands, tropical America). – The Bertiera clade is sister to Coffeeae.
Coffeeae DC. in Ann. Mus. Natl. Hist. Nat. 9: 217. 1807 [‘Coffeaceae’]
11/305–310. Tricalysia (c 110; tropical Africa, Madagascar), Coffea (c 125; tropical Africa, Madagascar, the Mascarene Islands), Argocoffeopsis (9; tropical Africa), Calycosiphonia (3; C. macrochlamys, C. pentamera, C. spathicalyx; tropical Africa), Belonophora (5; B. coffeoides, B. coriacea, B. ongensis, B. talbotii, B. wernhamii; tropical Africa), Sericanthe (17; tropical Africa), Diplospora (23; tropical Asia), Pubistylus (1; P. andamanensis; the Andaman Islands), Discospermum (7; D. abnorme, D. apiocarpum, D. beccarianum, D. javanicum, D. polyspermum, D. sphaerocarpum, D. whitfordii; tropical Asia), Xantonnea (2; X. parvifolia, X. quocensis; Southeast Asia), Nostolachma (6; N. crassifolia, N. densiflora, N. jenkinsii, N. khasiana, N. odorata, N. viridiflora; tropical Asia). – Tropical Africa, Madagascar, the Mascarene Islands, the Andaman Islands, tropical Asia.
[[Burchellia+Galiniera]+[Mantalania+[Didymosalpinx+Monosalpinx]+[Octotropideae+[Sherbournieae+Cordiereae+Pavetteae+Gardenieae]]]]
[Burchellia+Galiniera]
2/3. Burchellia (1; B. bubalina; South Africa, Swaziland), Galiniera (2; G. myrtoides, G. saxifraga; tropical Africa, Madagascar). – This clade is sister-group to the remaining “Coffeeae alliance”.
[Mantalania+[Didymosalpinx+Monosalpinx]+[Octotropideae+[Sherbournieae+Cordiereae+Pavetteae+Gardenieae]]]
Mantalania clade
2/4. Mantalania (3; M. capuronii, M. longipedunculata, M. sambiranensis; Madagascar), Pseudomantalania (1; P. macrophylla; Madagascar)?
[Didymosalpinx+Monosalpinx]
2/5.Monosalpinx (1; M. guillaumetii; tropical West Africa), Didymosalpinx (4; D. abbeokutae, D. konguensis, D. lanciloba, D. norae; tropical Africa).
[Octotropideae+[Sherbournieae+Cordiereae+Pavetteae+Gardenieae]]
Octotropideae Bedd., Fl. Sylv. S. India 28: cxxvi, cxxxiv-12. 1874
27/c 110. Cremaspora (2; C. thomsonii, C. triflora; tropical Africa, the Comoros); Feretia (3; F. aeruginescens, F. apodanthera, F. virgata; tropical Africa), Kraussia (4; K. floribunda, K. kirkii, K. socotrana, K. speciosa; tropical and southern Africa, Socotra), Octotropis (1; O. travancorica; southern India, Burma), Xantonneopsis (1; X. robinsonii; Southeast Asia), Petitiocodon (1; P. parviflorus; Nigeria, Cameroon)?, Canephora (5; C. angustifolia, C. goudotii, C. humblotii, C. madagascariensis, C. maroana; Madagascar), Chapelieria (2; C. lemyrioides, C. madagascariensis; Madagascar), Fernelia (4; F. buxifolia, F. decipiens, F. obovata, F. pedunculata; the Mascarene Islands), Flagenium (6; F. farafanganense, F. latifolium, F. pedunculatum, F. petrikense, F. setosum, F. triflorum; Madagascar), Nargedia (1; N. macrocarpa; Sri Lanka), Paragenipa (1; P. lancifolia; the Seychelles), Lamprothamnus (1; L. zanguebaricus; tropical East Africa), Jovetia (1; J. humilis; Madagascar), Lemyrea (4; L. ciliolata, L. krugii, L. marojejyensis, L. utilis; Madagascar)?, Polysphaeria (c 20; tropical Africa, Madagascar, the Comoros), Cowiea (2; C. borneensis: Borneo; C. philippinensis: the Philippines), Gallienia (1; G. sclerophylla; Madagascar), Hypobathrum (c 30; tropical Asia), Hyptianthera (1; H. stricta; northern India, Thailand), Morindopsis (1; M. capillaris; Southeast Asia), Pouchetia (4; P. africana, P. baumanniana, P. confertiflora, P. parviflora; tropical Africa), Ramosmania (2; R. heterophylla, R. rodriguesi; Rodrigues), Rhadinopus (2; R. kurivana, R. papuana; New Guinea), Scyphostachys (2; S. coffeoides, S. pedunculatus; Sri Lanka), Villaria (6; V. acutifolia, V. fasciculiflora, V. glomerata, V. littoralis, V. philippinensis, V. rolfei; the Philippines), Zuccarinia (1; Z. macrophylla; West Malesia). – Tropical Africa, Madagascar, the Mascarene Islands, the Seychelles, India, Sri Lanka, Southeast Asia, Malesia, with their highest diversity in tropical Africa and Madagascar. – Cremaspora is sister to the remaining Octotropideae. – This clade is sister-group to the clade [Sherbournieae+Cordiereae+Pavetteae+Gardenieae].
[Sherbournieae+Cordiereae+Pavetteae+Gardenieae]
Sherbournieae Mouly et B. Bremer in Taxon 63: 814. 28 Aug 2014
4/c 65. Atractogyne (2; A. bracteata, A. gabonii; tropical West and Central Africa), Mitriostigma (5; M. axillare, M. barteri, M. greenwayi, M. monocaule, M. usambarense; tropical and southern Africa), Oxyanthus (c 45; tropical and subtropical Africa), Sherbournia (c 13; tropical Africa). – Seeds with folded testa.
Cordiereae A. Rich. ex DC., Prodr. 4: 342, 445. Late Sep 1830
13?/125–130. Botryarrhena (2; B. pendula, B. venezuelensis; Venezuela, Brazil, Peru), Stachyarrhena (14; tropical America), Stenosepala (1; S. hirsuta; Panamá, Colombia), Kutchubaea (13; tropical South America), Glossostipula (3; G. blepharophylla, G. concinna, G. strigosa; southern Mexico, Guatemala), Duroia (35–40; Costa Rica to tropical South America), Amaioua (c 10; tropical South America), Riodocea (1; R. pulcherrima; Brazil), Agouticarpa (7; A. curviflora, A. grandistipula, A. hirsuta, A. isernii, A. spinosa, A. velutina, A. williamsii; Costa Rica to Bolivia), Melanopsidium (1; M. nigrum; Brazil), Cordiera (9; northern tropical South America), Alibertia (c 20; southern Mexico, Central America, the West Indies, tropical South America) – Unplaced Cordiereae Borojoa (c 10; Central America, tropical South America)? – Tropical America.
Pavetteae Dumort., Anal. Fam. Plant.: 32. 1829
c 20/645–655. Pavetta (c 360; tropical regions in the Old World), ‘Tarenna’ pro parte (190–200; tropical regions in the Old World except continental Africa; non-monophyletic), Cladoceras (1; C. subcapitatum; eastern Kenya, eastern Tanzania), Nichallea (1; N. soyauxii; tropical Africa), Rutidea (22; tropical Africa), Triflorensia (3; T. australis, T. cameronii, T. ixoroides; Queensland, New South Wales), Homollea (3; H. leandrii, H. longiflora, H. perrieri; Madagascar), Leptactina (27; tropical and southern Africa), Kindia (1; K. gangan; Republic of Guinea), ‘Tarenna’ pro parte, Robbrechtia (2; R. grandifolia, R. milleri; Madagascar), Schizenterospermum (4; S. analamerense, S. grevei, S. majungense, S. rotundifolium; Madagascar), ‘Coptosperma’ (c 20; tropical Africa with the largest diversity in Madagascar; polyphyletic), Tulearia (2; T. capsaintemariensis, T. splendida; southern Madagascar), Helictosperma (2; H. malacophylla, H. poissoniana; Madagascar), Exallosperma (1; E. longiflora; northern Madagascar), Pseudocoptosperma (1; P. menabense; Madagascar), Paracephaelis (4; P. cinerea, P. saxatilis, P. tiliacea, P. trichantha; Madagascar), Tennantia (1; T. sennii; tropical East Africa). – Unplaced Pavetteae Pachystylus (2; P. henningsianus, P. zippelianus; New Guinea)?
Gardenieae A. Rich. ex DC., Prodr. 4: 342, 367. late Sep 1830 [‘Gardeniaceae’]
c 54/580–605.
Schumanniophyton (3; S. hirsutum, S. magnificum,
S. problematicum; tropical Africa)?; Ceriscoides (11;
tropical Asia), Genipa (3;
G. americana, G. infundibuliformis, G. spruceana;
southern Mexico, Central America, the West Indies, tropical South America),
Brenania (2; B. brieyi, B. rhomboideifolia; Central
Africa), ’Gardenia’
(140–150; tropical and subtropical regions in the Old World to islands in the
Pacific; non-monophyletic), Larsenaikia (3; L. jardinei,
L. ochreata, L. suffruticosa; Northern Territory, northern
and eastern Queensland), Kailarsenia (6; K. campanula, K.
godefroyana, K. hygrophila, K. lineata, K.
stenosepala, K. tentaculata; tropical Asia, eastern Queensland),
Coddia (1; C. rudis; South Africa, Swaziland),
Aulacocalyx (11; tropical Africa), Heinsenia (1; H.
diervilleoides; tropical Africa), Rothmannia (40–45; tropical
and southern Africa to the Seychelles and the Malay Peninsula),
Phellocalyx (1; P. vollessenii; Tanzania, Malawi,
Mozambique), Kochummenia (2; K. parviflora, K.
stenopetala; the Malay Peninsula), Morelia (1; M.
senegalensis; tropical Africa), Hyperacanthus (11; southeastern
Africa, Madagascar), Aidia (50–55; tropical regions in the Old
World), Aoranthe (5; A. annulata, A. castaneofulva,
A. cladantha, A. nalaensis, A. penduliflora;
tropical Africa), Benkara (30–35; tropical and subtropical Asia),
Massularia (1; M. acuminata; tropical Africa),
Tocoyena (c 20; southern Central America, Cuba, tropical South
America), Sphinctanthus (8; tropical South America), Rosenbergiodendron
(3; R. densiflorum, R. formosum, R. longiflorum;
Panamá, the West Indies, northern tropical South America),
‘Casasia’ (11; Central America, the West Indies;
non-monophyletic), ’Randia’ (c 100; tropical and subtropical
America from Florida to Bolivia; non-monophyletic), Melanoxerus (1;
M. suavissimus; Madagascar), Preussiodora (1; P.
sulphurea; tropical West Africa), Pleiocoryne (1; P.
fernandensis; tropical West and Central Africa), Ridsdalea (1;
R. pulcherrima; the Malay Peninsula), Singaporandia (1;
S. macrophylla; the Malay Peninsula, Singapore, Sumatra),
Oligocodon (1; O. cunliffeae; tropical West and Central
Africa), Euclinia (3; E. longiflora, E. squamifera,
E. suavissima; tropical Africa), Macrosphyra (3; M.
brachysiphon, M. brachystylis, M. longistyla; tropical
Africa), Calochone (2; C. acuminata, C. redingii;
Central Africa), Brachytome (8; southern China, tropical Asia),
Dioecrescis (1; D. erythroclada; India), Tamilnadia
(1; T. uliginosa; South and Southeast Asia), Vidalasia (5;
V. fusca, V. morindifolia, V. murina, V.
pubescens, V. tonkinensis; Southeast Asia, the Philippines),
Rubovietnamia (2; R. aristata, R. nonggangensis;
Guangxi, Vietnam), Duperrea (1; D. pavettifolia; India,
southern China, Southeast Asia), Porterandia (c 20; West Malesia),
Pitardella (3; P. caudatifolia, P. poilanei, P.
sikkimensis; eastern Himalayas to Southeast Asia), Tarennoidea
(2; T. axillaris, T. wallichii; tropical Asia), Atractocarpus
(29; the Philippines, New Caledonia, Fiji, Micronesia, central Polynesia),
Deccania (1; D. pubescens; India), Catunaregam (12;
tropical Africa, tropical Asia), Bungarimba (4; B.
kahayanensis, B. papuana, B. ridsdalei, B.
sessiliflora; the Malay Peninsula, Sumatra, Borneo, New Guinea). –
Unplaced Gardenieae Adenorandia (1; A.
kalbreyeri; tropical Africa)?, Aidiopsis (1; A.
orophila; Malesia)?, Alleizettella (2; A. leucocarpa,
A. rubra; southeastern China, northern Vietnam)?, Fosbergia
(4; F. alleizettii, F. petelotii, F. shweliensis,
F. thailandica; Burma, Yunnan, Thailand, Vietnam)?, Ganguelia
(1; G. gossweileri; Angola)?, Gardeniopsis (1; G.
longifolia; West Malesia)?, Himalrandia (2; H.
lichiangensis, H. tetrasperma; the Himalayas)?,
Pseudaidia (1; P. speciosa; India)? – Pantropical.
Rubioideae Bremek. ex Verdc. in Bull. Jard. Bot. État Bruxelles 28: 280. 30 Sep 1958
193/8.270–8.460. Cosmopolitan. Usually herbs. Root phellogen usually deeply seated (in e.g. Paederia superficial). Raphides present. Hairs septate. Corolla with valvate aestivation. Heterostyly frequently occurring. Pollen grains usually bicellular (sometimes tricellular) at dispersal. Integument one to 14 cell layers thick (rarely absent). Antipodal cells usually not persistent. Suspensor haustorium sometimes present. The atpB promoter absent (lost). Shikimic acid-derived anthraquinones and cyclotide proteins present. Route II carboxylated iridoids and indole alkaloids absent. Aluminium often accumulated (particularly in woody species).
Colletoecemateae Rydin et B. Bremer in C. Rydin et al. in Plant Syst. Evol. 278: 120. Mar 2009
1/2. Colletoecema (2; C. dewevrei, C. magna; Central Africa). – Colletoecema is sister to the remaining Rubioideae, according to, e.g., Robbrecht & Manen (2006) and Rydin & al. (2008).
Ophiorrhizeae Bremek. ex Verdc. in Bull. Jard. Bot. État Bruxelles 28: 281. 30 Sep 1958
6/350–355. Lerchea (10; Sumatra, Java), Xanthophytum (32; Java, Borneo to Fiji), Kajewskiella (2; K. polyantha, K. trichantha; Solomon Islands), Keenania (4; K. capitata, K. microcephala, K. ophiorrhizoides, K. tonkinensis; Assam, Southeast Asia), Neurocalyx (5; N. bremeri, N. calycinus, N. championii, N. gardneri, N. zeylanicus; Sri Lanka, southern India), Ophiorrhiza (c 300; tropical Asia). – Tropical Asia to Melanesia. – Ophiorrhiza has the atpB promotor region which is absent from other Rubioideae.
Urophylleae Bremek. ex Verdc. in Bull. Jard. Bot. État Bruxelles 28: 281. 30 Sep 1958
14?/245–250. Temnopteryx (1; T. sericea; Central Africa), Raritebe (1; R. palicoureoides; tropical America), Amphidasya (13; Central America, northwestern tropical South America), ’Urophyllum’ (c 125; tropical regions in the Old World to China and Japan; polyphyletic), Pentaloncha (2; P. humilis, P. rubriflora; tropical West and Central Africa), Pauridiantha (c 45; tropical Africa), Praravinia (c 50; Borneo, the Philippines, Sulawesi), Antherostele (4; A. banahaensis, A. callophylla, A. grandistipula, A. luzoniensis; the Philippines)?, Crobylanthe (1; C. pellacalyx; Borneo)?, Didymopogon (1; D. sumatranum; Sumatra)?, Lepidostoma (1; L. polythyrsum; Sumatra)?, Rhaphidura (1; R. lowii; Borneo)?, Rhipidantha (1; R. chlorantha; Uluguru Mountains in Tanzania)?, Stichianthus (1; S. minutiflorus; Borneo)? – Temnopteryx may be sister to the remaining Urophylleae, although seven of the genera in Urophylleae are not yet analyzed. Raritebe is sister to Amphidasya and Pentaloncha sister to Pauridiantha.
[[Lasiantheae+Perameae]+[Coussareeae+[”Spermacoceae alliance”+”Psychotrieae alliance”]]]
[Lasiantheae+Perameae]
Lasiantheae B. Bremer et Manen in Plant Syst. Evol. 225: 57. 10 Jan 2001
4/260–270. Lasianthus (220–230; tropical Africa, tropical Asia to tropical Australia), Trichostachys (14; tropical Africa), Ronabea (3; R. emetica, R. isanae, R. latifolia; Central America, tropical South America to Bolivia), Saldinia (22; Madagascar). – Tropical regions in the Old World.
Perameae Bremek. ex S. P. Darwin in Taxon 25: 605. 26 Nov 1976
1/14. Perama (14; tropical South America). – Perama is sister to Lasiantheae.
[Coussareeae+[”Spermacoceae alliance”+”Psychotrieae alliance”]]
Coussareeae Benth. et Hook. f., Gen. Plant. 2: 9, 24. 7-9 Apr 1873
8/410–420. Coccocypselum (22; southern Mexico, Central America, the West Indies, tropical South America), Declieuxia (c 30; southern Mexico, Central America, tropical South America), Hindsia (11; Brazil), Coussarea (125–130; southern Mexico, Central America, the West Indies, tropical South America), Faramea (210–215; Mexico, Central America, the West Indies, tropical South America), Cruckshanksia (7; C. hymenodon, C. lithiophila, C. macrantha, C. montiana, C. palmae, C. pumila, C. verticillata; northern Chile, northern Argentina; incl. Oreopolus?), Oreopolus (1; O. glacialis; the Andes; in Cruckshanksia?), Heterophyllaea (3; H. fiebrigii, H. lycioides, H. pustulata; Bolivia, Argentina). – Tropical America.
The “Spermacoceae alliance”
[Danaideae+[[Knoxieae+Spermacoceae]+[Anthospermeae+[Argostemmateae+[Dunnieae+[Paederieae+[Plocama+[Theligonum+Rubieae]]]]]]]]
Danaideae B. Bremer et Manen in Plant Syst. Evol. 225: 60. 10 Jan 2001
3/c 60. Danais (c 30; Tanzania, Madagascar, the Mascarene Islands), Schismatoclada (c 20; Madagascar), Payera (10; Madagascar). – Tanzania, Madagascar, the Mascarene Islands, with their highest diversity on Madagascar.
[[Knoxieae+Spermacoceae]+[Anthospermeae+[Argostemmateae+[Dunnieae+[Paederieae+[Plocama+[Theligonum+Rubieae]]]]]]]
[Knoxieae+Spermacoceae]
Knoxieae Benth. et Hook. f., Gen. Plant. 2: 9, 21. 7-9 Apr 1873
15/c 130. Chamaepentas (6; C. graniticola, C. greenwayi, C. hindsioides, C. longituba, C. nobilis, C. pseudomagnifica; tropical East Africa), Carphalea (3; C. cloiselii, C. linearifolia, C. madagascariensis; Madagascar), Dirichletia (5; D. glaucescens, D. obovata, D. pubescens, D. somaliensis, D. virgata; Ethiopia, Somalia, tropical East Africa to Namibia, Socotra), Triainolepis (13; tropical East Africa, Madagascar), Paracarphalea (3; P. angulata, P. kirondron, P. pervilleana; Madagascar), Parapentas (3; P. battiscombei, P. setigera, P. silvatica; tropical Africa, Madagascar?), Dolichopentas (4; D. decora, D. liebrechtsiana, D. lindenioides, D. longiflora; tropical Africa), Phyllopentas (14; tropical Africa, Madagascar), Knoxia (13; tropical Africa, tropical Asia), Pentas (16; tropical Africa, Madagascar, the Arabian Peninsula), Rhodopentas (2; R. bussei, R. parvifolia; tropical East Africa), Pentanisia (19; tropical Africa, Madagascar), Batopedina (3; B. linearifolia, B. pulvinellata, B. tenuis; western and southern tropical Africa), Otomeria (8; tropical Africa, Madagascar), Otiophora (18; tropical Africa, Madagascar). – Knoxieae are sister to Spermacoceae.
Spermacoceae Cham. et Schltdl. ex DC., Prodr. 4: 343, 538. late Sep 1830
69/1.380–1.410. Hedyotis (c 130; tropical and subtropical Asia to New Guinea, island in northwestern Pacific), Mexotis (4; M. kingii, M. latifolia, M. lorencei, M. terrellii; Mexico), Martensianthus (6; M. albiflorus, M. breviflorus, M. galeottii, M. macdougallii, M. micranthus, M. viticellus; Mexico), Terrellianthus (1; T. serpyllacea; southern Mexico, Central America), Agathisanthemum (4; A. assimile, A. bojeri, A. chlorophyllum, A. globosum; tropical Africa, the Comoros), Kohautia (c 35; tropical and subtropical Africa, the Arabian Peninsula to India, Thailand, tropical Australia), Manostachya (3; M. juncoides, M. staelioides, M. ternifolia; tropical Africa), Lathraeocarpa (2; L. acicularis, L. decaryi; southern Madagascar), Gomphocalyx (1; G. hernarioides; Madagascar), Phylohydrax (2; P. carnosa, P. madagascariensis; tropical East Africa, Madagascar), Amphiasma (7; A. benguellense, A. divaricatum, A. luzuloides, A. merenskyanum, A. micranthum, A. redheadii, A. robijnsii; tropical and southwestern Africa), Pentanopsis (2; P. fragrans, P. gracilicaulis; northeastern tropical Africa), Conostomium (5; C. gazense, C. longitubum, C. natalense, C. quadrangulare, C. zoutpansbergense; tropical East Africa), Dentella (8; tropical Asia to tropical Australia and New Caledonia), Pentodon (2; P. laurentioides, P. pentandrus; tropical and subtropical regions in Africa, the Seychelles, the Arabian Peninsula, tropical and subtropical America), Edrastima (4; E. angolensis, E. goreensis, E. trinervia, E. uniflora; tropical and subtropical Africa, tropical Asia, North and South America), Dibrachionostylus (1; D. kaessneri; tropical East Africa), Neanotis (c 30; tropical Asia to tropical Australia, islands in the Pacific), Involucrella (2; I. chereevensis, I. coronaria; Southeast Asia), Debia (4; D. andamanica, D. krewanhensis, D. oligocephala, D. ovatifolia; Southeast Asia, the Andaman Islands, the Philippine Islands), Kadua (c 30; French Polynesia, the Hawaiian Islands), Leptopetalum (5; L. foetidum, L. grayi, L. kanehirae, L. mexicanum, L. pachyphyllum; tropical Asia to tropical Australia, islands in the Pacific), Scleromitrion (c 10?; tropical Asia to tropical Australia, islands in the Pacific), Exallage (15; tropical Asia to tropical Australia, islands in the Pacific), Dimetia (7; D. ampliflora, D. capitellata, D. dianxiensis, D. hedyotidea, D. obliquinervis, D. pitardiana, D. scandens; tropical Asia), Cordylostigma (9; tropical and southern Africa, Madagascar), ‘Oldenlandia’ (c 250?; tropical and subtropical regions on both hemispheres; polyphyletic), Phialiphora (2; P. bevazahensis, P. capitulata; northwestern Madagascar), Stenaria (5; S. butterwickiae, S. mullerae, S. nigricans, S. rupicola, S. umbratilis; southern Canada, United States), Thecorchus (1; T. wauensis; tropical Africa), Hedythyrsus (2; H. spermacocinus, H. thamnoideus; tropical Africa), Pseudonesohedyotis (1; P. bremekampii; Uuguru Mountains in Tanzania), Mitrasacmopsis (1; M. quadrivalvis; tropical East Africa, Madagascar), Astiella (1; A. delicatula; Madagascar), Amphistemon (2; A. humbertii, A. rakotonasolianus; Madagascar), Thamnoldenlandia (1; T. ambovombensis; Madagascar), Synaptantha (2; S. scleranthoides, S. tillaeacea; tropical and subtropical regions in Australia), Arcytophyllum (17; southern Mexico to Bolivia), Houstonia (c 30; southern Canada, United States, Mexico), Richardia (16; southern United States, Mexico, Central America, the West Indies, tropical South America, Fiji), ‘Spermacoce’ (300–320; tropical and subtropical regions on both hemispheres; paraphyletic), Carajasia (1; C. cangae; Pará in Brazil; in Spermacoce?), Hydrophylax (1; H. maritima; tropical and subtropical Africa, Madagascar, India, Thailand), Bouvardia (c 50; southwestern United States, Mexico, Central America), Diodia (25–30; tropical and subtropical regions in Africa and America), Galianthe (c 45; Central America, tropical South America), Ernodea (9; southeastern United States, the West Indies; in Spermacoce?), Mitracarpus (c 60; Central America, tropical South America; in Spermacoce?), Psyllocarpus (9; Brazil; in Spermacoce?), Manettia (120–125; Mexico, Central America, the West Indies, tropical South America), Nesohedyotis (1; N. arborea; St. Helena), Emmeorhiza (1; E. pohliana; tropical South America), Denscantia (4; D. andrei, D. cymosa, D. macrobracteata, D. monodon; Brazil), Anthospermopsis (1; A. catechosperma; Brazil), Staelia (16; northern South America), Pleiocraterium (4; P. gentianifolium, P. plantaginifolium, P. sumatranum, P. verticillare; southern India, Sri Lanka, Sumatra), Carterella (1; C. alexanderae; Baja California in northwestern Mexico), Dolichometra (1; D. leucantha; eastern Usambara Mountains in Tanzania), Leptoscela (1; L. ruellioides; eastern Brazil), Lucya (1; L. tetrandra; the West Indies), Phyllocrater (1; P. gibbsiae; Borneo), Polyura (1; P. geminata; Assam), Stephanococcus (1; S. crepinianus; tropical Africa), Oldenlandiopsis (1; O. callitrichoides; the West Indies), Micrasepalum (2; M. eritrichoides: Cuba; M. haitiense: Hispaniola), Spiradiclis (38; India, southwestern China, Southeast Asia, Java), Crusea (15; southwestern United States, Mexico, Central America), Leptomischus (7; L. erianthus, L. funingensis, L. guangxiensis, L. modestus, L. parviflorus, L. primuloides, L. wallichii; Southeast Asia), Merumea (2; M. coccocypseloides, M. plicata; the Guayana Highlands). – Tropical and subtropical regions on both hemispheres, North America.
[Anthospermeae+[Argostemmateae+[Dunnieae+[Paederieae+[Plocama+[Theligonum+Rubieae]]]]]]
Anthospermeae Cham. et Schltdl. in Linnaea 3: 309. Oct 1828
10/210–215. Anthospermum (c 40; Africa, Madagascar), Nenax (9–11; southern Namibia, South Africa), Galopina (4; G. aspera, G. circaeoides, G. crocyllioides, G. tomentosa; southern Africa to Malawi), Phyllis (2; P. nobla, P. viscosa; Madeira, the Canary Islands), Coprosma (c 125; Kerguelen Island, southern China, southeastern South Australia, eastern New South Wales, Victoria, Tasmania, New Zealand incl. Stewart Island, Macquarie Island, Campbell Island, Auckland Islands, Antipodes Islands, the Hawaii Islands, southern South America, Tristan da Cunha), Durringtonia (1; D. paludosa; southeastern Queensland, northeastern New South Wales), Pomax (1; P. umbellata; Australia), Leptostigma (6; L. arnottianum, L. longiflorum, L. pilosum, L. reptans, L. setulosum, L. weberbaueri; Southeast Asia, New Zealand, western South America), Carpacoce (7; C. burchellii, C. curvifolia, C. gigantea, C. heteromorpha, C. scabra, C. spermacocea, C. vaginellata; Western and Eastern Cape), Opercularia (17–18; southwestern Western Australia, southeastern South Australia to southeastern Queensland, Tasmania). – Macaronesia, Africa, Madagascar, Southeast Asia, Australia, Tasmania, New Zealand, the Hawaiian Islands, western and southern South America, Subantarctic islands.
[Argostemmateae+[Dunnieae+[Paederieae+[Plocama+[Theligonum+Rubieae]]]]]
Argostemmateae Bremek. ex Verdc. in Bull. Jard. Bot. État Bruxelles 28: 281. 30 Sep 1958
3/c 220. Mouretia (5; M. guangdongensis, M. inaequalis, M. larsenii, M. tonkinensis, M. vietnamensis; Southeast Asia), Mycetia (c 55; tropical Asia), Argostemma (c 160; the Himalayas, Burma, China, Southeast Asia, Malesia to New Guinea, Japan, two species in tropical West Africa). – Tropical regions in the Old World. Anthers in Argostemma connate.
[Dunnieae+[Paederieae+[Plocama+[Theligonum+Rubieae]]]]
Dunnieae Rydin et B. Bremer in C. Rydin et al. in Plant Syst. Evol. 278: 121. Mar 2009
3/7 or 10. Dunnia (1 or 4; D. sinensis; northern India to southeastern China), Cyanoneuron (5; C. cyaneum, C. depauperatum, C. grandiflorum, C. pedunculatum, C. pubescens; Borneo, Sulawesi), Foonchewia (1; F. coriacea; Guangdong). – Northern India, southern China, Borneo, Sulawesi. – Cyanoneuroneae and Foonchewieae are here included in Dunnieae (Wen & Wang 2012; Ginter & al. 2015).
[Paederieae+[Plocama+[Theligonum+Rubieae]]]
Paederieae DC., Prodr. 4: 342, 470. late Sep 1830
6/130–135. Saprosma (45–50; tropical Asia), Paederia (c 30; tropical regions on both hemispheres), Spermadictyon (1; S. suaveolens; India), Leptodermis (c 50; the Himalayas to Japan), Serissa (1; S. japonica; southern China), Pseudopyxis (3; P. depressa, P. heterophylla, P. monilirhizoma; Japan). – Tropical regions on both hemispheres.
[Plocama+[Theligonum+Rubieae]]
Putorieae Lange in H. M. Willkomm et J. M. C. Lange, Prodr. Fl. Hispan. 2: 299. 1870
1/c 35. Plocama (c 35; the Canary Islands, the Mediterranean, southwestern Asia to northwestern India). – Plocama is sister to [Theligonum+Rubieae].
[Theligoneae+Rubieae]
Theligoneae Baill., Hist. Plant. 4: 46, 55. 1872
1/4. Theligonum (4; T. cynocrambe, T. formosanum, T. japonicum, T. macranthum; Macaronesia, the Mediterranean, southwestern China, Japan, Taiwan). – Usually unisexual (monoecious), annual or perennial herbs. Calyx strongly reduced. Corolla bilobate to quinquelobate. Stamens (two to) seven to twelve (to 30), sometimes basally fused in fascicles of two, four or six. Pollen grains 4–8-zonoporate. Pistil composed of two connate carpels (one carpel fertile, the second carpel aborted: pseudomonomery). Style gynobasic. Placentation basal. Ovule solitary, campylotropous or amphitropous. Fruit a nutlike drupe. Pericarp with elaiosome. Endosperm starchy. Iridoids present. – Theligonum is sister to Rubieae.
Rubieae Baill., Hist. Plant. 7: 365, 390. Feb 1880
4/985–>990. Kelloggia (2;
K. chinensis: southern China, Bhutan; K. galioides:
southwestern United States, northwestern Mexico); Didymaea (8; Central
America), Rubia
(75–80; Macaronesia, the Mediterranean, Africa, temperate Asia, North
America), Galium
(>900; cosmopolitan). – Cosmopolitan. – Rubieae have usually
foliaceous stipules resembling verticillate leaves. Kelloggia may be
sister to the remaining Rubieae. Didymaea, Galium
(sensu lato) and Rubia form a
polytomy in the analysis by Ehrendorfer & Barfuss (2014).
The “Psychotrieae alliance”
[Craterispermeae+Schradereae+[Gaertnereae+[Mitchelleae+Morindeae]]+Prismatomerideae+Psychotrieae]
Craterispermeae Verdc. in Bull. Jard. Bot. Bruxelles 28: 281. 30 Sep 1958
1/16. Craterispermum (16; tropical Africa, Madagascar, the Seychelles).
Schradereae Bremek. in Rec. Trav. Bot. Néerl. 31: 253. 1934
3/c 60. Schradera (c 55; Malesia, tropical America), Lecananthus (3; L. erubescens, L. peduncularis, L. pentander; West Malesia), Leucocodon (1; L. reticulatum; Sri Lanka). – Sri Lanka, Malesia, tropical America.
[Gaertnereae+[Mitchelleae+Morindeae]]
Gaertnereae Endl., Gen. Plant.: 577. Aug 1838
2/c 85. Pagamea (c 25; tropical South America), Gaertnera (c 60; tropical regions in the Old World). – Pantropical. – Gaertnereae are sister-group to the clade [Mitchelleae+Morindeae].
[Mitchelleae+Morindeae]
Mitchelleae Razafim. et B. Bremer in S. G. Razafimandimbison, C. Rydin et B. Bremer, Mol. Phylogen. Evol. 48: 221. Jul 2008
2/14. Damnacanthus (11; China, Japan, Taiwan), Mitchella (3; M. undulata: southern Korea, Japan, Taiwan; M. repens: North America; M. ovata: Ecuador). – East Asia, North America, Ecuador. – Mitchelleae are sister to Morindeae.
Morindeae Burnett, Outlines Bot.: 906. Feb 1835
6/165–170. Appunia (6–8; southern Mexico, Central America, the West Indies, tropical South America), ‘Morinda’ (c 130; tropical and subtropical regions on both hemispheres; polyphyletic), Gynochthodes (>20; Southeast Asia, Malesia to tropical Australia and Melanesia; incl. Morinda pro parte), Coelopyrena (1; C. salicifolia; East Malesia), Coelospermum (9; Southeast Asia, Malesia to eastern Queensland and New Caledonia), Siphonandrium (1; S. intricatum; New Guinea). – Tropical and subtropical regions on both hemispheres.
Prismatomerideae Y. Z. Ruan in Acta Phytotaxon. Sin. 26: 446. Dec 1988 [‘Prismatomereae’]
4/26. Prismatomeris (16; Sri Lanka, Assam, Southeast Asia, Hainan, West Malesia), Gentingia (1; G. subsessilis; northwestern Malay Peninsula), Motleyia (1; M. borneensis; northwestern Borneo), Rennellia (8; the Malay Peninsula, Sumatra, Borneo). – Sri Lanka, Assam, Southeast Asia, Hainan, West Malesia.
Psychotrieae Cham. et Schltdl. in Linnaea 4: 4. Jan 1829 [‘Psychotriaceae’]
26/3.480–3.580. Schizocolea (2; S. linderi, S. ochreata; tropical West Africa); ’Psychotria’ (1.900–2.000; tropical regions on both hemispheres; non-monophyletic), Gillespiea (1; G. speciosa; Fiji; in Psychotria?), Hedstromia (1; H. latifolia; Fiji; in Psychotria?), Notopleura (c 100; Mexico, Central America, the West Indies, tropical South America; in Psychotria?), Cremocarpon (9; Madagascar, the Comoros), Pyragra (2; P. ankarensis, P. obtusifolia; Madagascar), Amaracarpus (c 30; the Seychelles, tropical Asia to tropical Australia and Solomon Islands), Dolianthus (13; mountains in Papua New Guinea), Myrmecodia (27; Malesia to Fiji), Hydnophytum (c 95; tropical Asia, with their highest diversity on New Guinea), Squamellaria (4; S. imberbis, S. major, S. thekii, S. wilsonii; Fiji), Myrmephytum (5; M. arfakianum, M. beccarii, M. moniliforme, M. naumannii, M. selebicum; the Philippines, Sulawesi, western New Guinea), Anthorrhiza (9; New Guinea), Calycosia (8; Polynesia), Palicourea (310–315; southern Mexico, Central America, the West Indies, tropical South America), Rudgea (c 130; southern Mexico, Central America, the West Indies, tropical South America), Carapichea (23; Central America, tropical South America), Eumachia (83; tropical regions on both hemispheres), Hymenocoleus (12; tropical Africa), ‘Geophila’ (c 30; tropical regions on both hemispheres; polyphyletic), ‘Chassalia’ (c 110; tropical regions in the Old World, with their largest diversity on Madagascar; polyphyletic), Puffia (1; P. gerrardii; Madagascar), Coccochondra (1; C. laevis; the Guayana Highlands), Peripeplus (1; P. bracteosus; Central Africa), Streblosa (c 25; West Malesia). – Pantropical. Anthorrhiza, Hydnophytum, Myrmecodia, Myrmephytum and Squamellaria are myrmecophilous with ant colonies living in the hollow stems. – Schizocolea is sister to the remaining Psychotrieae. – Palicoureeae Robbr. et Manen in Syst. & Geogr. Plants 76: 137. 7 Jul 2006 are often separated from Psychotrieae. Awaiting a comprehensive analysis of the entire Psychotrieae-Palicoureeae clade, I have chosen to keep the Palicoureeae genera in Psychotrieae.
Unplaced Rubiaceae Acrobotrys (1; A. discolor; Colombia), Aphanocarpus (1; A. steyermarkii; Venezuela), Benzonia (1; B. corymbosa; Guinea), Berghesia (1; B. coccinea; Mexico), Bradea (5; B. anomala, B. bicornuta, B. brasiliensis, B. kuhlmanni, B. montana; Brazil), Byrsophyllum (2; B. ellipticum, B. tetrandrum; India, Sri Lanka), Clarkella (1; C. nana; the Himalayas, Thailand), Coptophyllum (8; India, West Malesia), Coryphothamnus (1; C. auyantepuiensis; southeastern Venezuela), Diacrodon (1; D. compressus; Brazil), Didymochlamys (2; D. connellii, D. whitei; Panamá, Colombia, Venezuela), Duidania (1; D. montana; Venezuela), Eizia (1; E. mexicana; Mexico), Fergusonia (1; F. zeylanica; southern India, Sri Lanka), Flexanthera (1; F. subcordata; Colombia, Bolivia), Holstianthus (1; H. barbigularis; the Guayana Highlands), Klossia (1; K. montana; the Malay Peninsula), Koenania (1; K. flava; China?), Lecariocalyx (1; L. borneensis; Borneo), Lorencea (1; L. guatemalensis; southern Mexico, Guatemala), Maschalodesme (2; M. arborea, M. versteegii; New Guinea), Nodocarpaea (1; N. radicans; Cuba), Pagameopsis (2; P. garryoides, P. maguirei; Venezuela), Placocarpa (1; P. mexicana; Mexico), Pseudodiplospora (1; P. andamanica; the Andaman Islands), Pseudohamelia (1; P. hirsuta; the Andes), Riqueuria (1; R. avenia; Peru), Sacosperma (2; S. paniculatum, S. parviflorum; tropical Africa), Schwendenera (1; S. tetrapyxis; southeastern Brazil), Sinospiradiclis (1; S. microphylla; China?), Stachyococcus (1; S. adinanthus; Peru), Standleya (5; S. erecta, S. glomerulata, S. kuhlmannii, S. limae, S. prostrata; Brazil), Streblosiopsis (1; S. cupulata; Borneo), Stylosiphonia (1; S. glabra; Central America), Temnocalyx (1; T. nodulosa; Tanzania), Tobagoa (1; T. maleolens; Panamá, Tobago, Venezuela), Tortuella (1; T. abietifolia; Île Tortue near Hispaniola).
 |
Cladogram (simplified) of Rubiaceae based on DNA sequence data (Bremer & Eriksson 2009, with Prismatomerideae added). ’Gardenieae’ are non-monophyletic. According to more recent analyses covering a larger part of the Rubiaceae taxa, Condamineeae are sister to the remaining Ixoroideae, and Mussaendeae and Sabiceeae are sister-groups. |
Literature
Aaron P, Bridson D, Jarvis C, Govaerts R. 2001. The typification and characterization of the genus Psychotria L. (Rubiaceae). – Bot. J. Linn. Soc. 135: 35-42.
Abdel Khalik K, Abd El-Ghani MM, Elkordy A. 2007 [2008]. A palynological study of Galium L. (Rubiaceae) in Egypt and its systematic implication. – Feddes Repert. 118: 311-326.
Abdel Khalik K, Abd El-Ghani M, El Kordy A. 2008. Fruit and seed morphology in Galium (Rubiaceae) and its importance for taxonomic identification. – Acta Bot. Croatica 67: 1-20.
Achille F. 2006. Tinadendron, nouveau genre de Rubiaceae, Guettardeae de Mélanésie orientale. – Adansonia, sér. III, 28: 167-180.
Achille F, Motley TJ, Lowry PP II, Jérémie J. 2006. Polyphyly in Guettarda L. (Rubiaceae, Guettardeae) based on nrDNA ITS sequence data. – Ann. Missouri Bot. Gard. 93: 103-121.
Adams CD, Verdcourt B. 1993. The identity of Diodia angolensis S. Moore (Rubiaceae). – Kew Bull. 48: 278.
Adams LG. 1995. Chionogentias (Gentianaceae), a new generic name for the Australasian ‘snow gentians’, and a revision of the Australian species. – Aust. Syst. Bot. 8: 935-1011.
Adams LG. 1996. Gentianaceae. – In: Orchard AE (ed), Flora of Australia 28, CSIRO, Melbourne, Australia, pp. 72-104.
Adams LG, Bridson DM, Robbrecht E. 1987. The identity of Lasianthus graciliflorus Bailey (Rubiaceae). – Kew Bull. 42: 209-214.
Adebowale A, Lamb J, Nicholas A, Naidoo Y. 2009. Phylogenetic relationships in southern African Strychnos (Strychnaceae) inferred from plastid and ITS sequences. – South Afr. J. Bot. 75: 391-392.
Adebowale A, Lamb J, Nicholas A, Naidoo Y. 2016. Molecular systematics of southern African monkey orange Strychnos L. (Loganiaceae). – Kew Bull. 71: 17 DOI 10.1007/S12225-016-9630-0
Agrawal AA, Salminen J-P, Fishbein M. 2009. Phylogenetic trends in phenolic metabolism of milkweeds (Asclepias): evidence for escalation. – Evolution 63: 663-673.
Agrawal AA, Fishbein M, Jetter R, Salminen J-P, Goldstein JB, Freitag AE, Sparks JP. 2009. Phylogenetic ecology of leaf surface traits in the milkweeds (Asclepias spp.): chemistry, ecophysiology, and insect behavior. – New Phytol. 183: 848-867.
Aiello A. 1979. A re-examination of Portlandia (Rubiaceae) and associated taxa. – J. Arnold Arbor. 60: 38-126.
Aiello M, Brullo S, Piccione V. 1980 [1981]. Numerical analysis applied to the taxonomy of the genus Valantia L. (Rubiaceae). – An. Jard. Bot. Madrid 37: 577-586.
Airy Shaw HK. 1937. The genus Neurocalyx in Borneo. – Kew Bull. 1937: 281-290.
Albers F. 1983. Cytotaxonomic studies in African Asclepiadaceae. – Bothalia 14: 795-798.
Albers F, Meve U. 1991. Mixoploidy and cytotypes: a study of possible vegetative species differentiation in stapeliads (Asclepiadaceae). – Bothalia 21: 67-72.
Albers F, Meve U. 2001. A karyological survey of Asclepiadoideae, Periplocoideae and Secamonoideae, and evolutionary considerations within Apocynaceae s.l. – Ann. Missouri Bot. Gard. 88: 624-656.
Albers F, Meve U (eds). 2002. Illustrated handbook of succulent plants: Asclepiadaceae. – Springer Verlag, Heidelberg.
Albers F, Liede S, Meve U. 1992. Deviating chromosome numbers in Asclepiadaceae. – Nord. J. Bot. 13: 37-39.
Albert VA, Struwe L. 1997. Phylogeny and classification of Voyria (saprophytic Gentianaceae). – Brittonia 49: 466-479.
Alejandro GJD. 2007. The current status of the Philippine Rubiaceae. – Philipp. J. Syst. Biol. 1: 47-60.
Alejandro GJD, Razafimandimbison SG, Liede-Schumann S. 2005. Polyphyly of Mussaenda inferred from ITS and trnT-F data and its implications for generic limits in Mussaendeae (Rubiaceae). – Amer. J. Bot. 92: 544-557.
Alejandro GJD, Meve U, Liede-Schumann S. 2008. Two new species of Mussaenda (Rubiaceae) from Panay Island, Philippines. – Bot. J. Linn. Soc. 158: 87-92.
Alejandro GJD, Meve U, Uy M, Mouly A, Thiv M, Liede-Schumann S. 2010. Molecular support of the classification of Greeniopsis Merr. in Aleisanthieae (Rubiaceae), with a revision of the genus. – Taxon 59: 1547-1564.
Alejandro GJD, Meve U, Mouly A, Thiv M, Liede-Schumann S. 2011. Molecular phylogeny and taxonomic revision of the Philippine endemic Villaria Rolfe (Rubiaceae). – Plant Syst. Evol. 296: 1-20.
Alejandro GJD, Arenas EZ, Cremen CM, Arriola AH. 2013. A new record of Pyrostria (Vanguerieae-Rubiaceae) from the Philippines inferred from molecular and morphological data. – Philipp. J. Syst. Biol. 7: 1-12.
Alejandro GJD, Magdaleno CMM, Pacia JAT, Paraguison LD, Quiogue KKC, Wong AED, Yayen KMR, Arriola AH. 2014. Generic affiliation of Canthium species placed under Pyrostria group B sensu Bridson (Vanguerieae, Rubiaceae) inferred from morphology and molecular data. – Bot. Stud. 55: 1-8.
Alejandro GJD, Meve U, Liede-Schumann S. 2016. A taxonomic revision of Philippine Mussaenda (Rubiaceae, Mussaendeae). – Ann. Missouri Bot. Gard. 101: 457-524.
Ali SJ, Robbrecht E. 1991. Remarks on the tropical Asian and Australian taxa included in Diplospora or Tricalysia (Rubiaceae-Ixoroideae-Gardenieae). – Blumea 35: 279-305.
Allen CK. 1933. A monograph of the American species of the genus Halenia. – Ann. Missouri Bot. Gard. 20: 119-222.
Allorge L. 1985. Monographie des Apocynacées-Tabernaemontanoïdées Américaines. – Mém. Mus. Natl. Hist. Nat., sér. B, Bot. 30: 1-216.
Allorge L, Husson J-P, Sastre C. 1980. Morphologie et chimiotaxonomie des Apocynacées. Conclusions phylogénétiques et biogéographiques. – Compt. Rend. Soc. Biogéogr. 57: 112-126.
Al Nawaihi AS, Hassan SA, Elwan ZA. 2006. Exomorphic seed characters of some species of the Apocynaceae s.l. in Egypt. – Feddes Repert. 117: 437-452.
Alvarado-Cárdenas LO, Ochoterena H. 2007. A phylogenetic analysis of the Cascabela-Thevetia species complex (Plumerieae, Apocynaceae) based on morphology. – Ann. Missouri Bot. Gard. 94: 298-323.
Alves R, Figueiredo E, Davis AP. 2005. Taxonomy and conservation of the genus Psychotria (Rubiaceae) in S. Tomé e Principe (Gulf of Guinea). – Bot. J. Linn. Soc. 147: 469-481.
Anderson CL, Rova JHE, Andersson L. 2001. Molecular phylogeny of the tribe Anthospermeae (Rubiaceae): systematic and biogeographic implications. – Aust. Syst. Bot. 14: 231-244.
Anderson WR. 1972. A monograph of the genus Crusea (Rubiaceae). – Mem. New York Bot. Gard. 22: 1-128.
Anderson WR. 1973. A morphological hypothesis for the origin of heterostyly in the Rubiaceae. – Taxon 22: 537-542.
Andersson L. 1992. A provisional checklist of neotropical Rubiaceae. – Scripta Bot. Belg. 1: 1-199.
Andersson L. 1993a. 162. Rubiaceae: introduction. – In: Harling G, Andersson L (eds), Flora of Ecuador 47, Nord. J. Bot., Copenhagen, pp. 1-11.
Andersson L. 1993b. 162(22). Rubiaceae-Anthospermeae. – In: Harling G, Andersson L (eds), Flora of Ecuador 47, Nord. J. Bot., Copenhagen, pp. 11-17.
Andersson L. 1993c. Pollen characteristics of the tribes Calycophylleae, Cinchoneae and Hillieae (Rubiaceae). – Nord. J. Bot. 13: 405-417.
Andersson L. 1995. Tribes and genera of the Cinchoneae complex (Rubiaceae). – Ann. Missouri Bot. Gard. 82: 409-427.
Andersson L. 1996. Circumscription of the tribe Isertieae (Rubiaceae). – Opera Bot. Belg. 7: 139-164.
Andersson L. 1998. A revision of the genus Cinchona (Rubiaceae-Cinchoneae). – Mem. New York Bot. Gard. 80: 1-75.
Andersson L. 2000. Peratanthe is a synonym of Nertera (Rubiaceae, Anthospermeae). – Brittonia 52: 351-353.
Andersson L. 2001a. Margaritopsis (Rubiaceae, Psychotrieae) is a pantropical genus. – Syst. Geogr. Plants 71: 73-85.
Andersson L. 2001b. Peratanthe is a synonym of Nertera (Rubiaceae, Anthospermeae). – Brittonia 52: 351-353.
Andersson L. 2002a. Relationships and generic circumscriptions in the Psychotria complex (Rubiaceae, Psychotrieae). – Syst. Geogr. Plants 72: 167-202.
Andersson L. 2002b. Validation of three new combinations in Margaritopsis (Rubiaceae, Psychotrieae). – Syst. Geogr. Plants 72: 230.
Andersson L. 2002c. Re-establishment of Carapichea (Rubiaceae, Psychotrieae). – Kew Bull. 57: 363-374.
Andersson L, Antonelli A. 2005. Phylogeny of the tribe Cinchoneae (Rubiaceae), its position in Cinchonoideae, and description of a new genus, Ciliosemina. – Taxon 54: 17-28.
Andersson L, Guarin FA. 2002. Relationships, circumscription, and biogeography of Arcytophyllum (Rubiaceae) based on evidence from cpDNA. – Brittonia 54: 40-49.
Andersson L, Persson C. 1991. Circumscription of the tribe Cinchoneae (Rubiaceae) – a cladistic approach. – Plant Syst. Evol. 178: 65-94.
Andersson L, Rova JHE. 1999. The rps16 intron and the phylogeny of the Rubioideae (Rubiaceae). – Plant Syst. Evol. 214: 161-186.
Andersson L, Rova JHE. 2004. 162(9). Rubiaceae-Hippotideae. – In: Harling G, Andersson L (eds), Flora of Ecuador 74, Botanical Institute, Göteborg University, pp. 1-44.
Andersson L, Ståhl B. 1999. 162(8). Rubiaceae-Isertieae. – In: Harling G, Andersson L (eds), Flora of Ecuador 62, Nord. J. Bot., Copenhagen, pp. 57-129.
Andersson L, Taylor CM. 1994. 162(1-4). Rubiaceae-Cinchoneae-Coptosapelteae. – In: Harling G, Andersson L (eds), Flora of Ecuador 50, Nord. J. Bot., Copenhagen, pp. 1-112.
Andersson L, Rova JHE, Guarin FA. 2002. Relationships, circumscription, and biogeography of Arcytophyllum (Rubiaceae) based on evidence from cpDNA. – Brittonia 54: 40-49.
Andreasen K. 1997. Phylogeny of Ixoroideae. – Ph.D. diss., University of Uppsala, Sweden.
Andreasen K, Bremer B. 1996. Phylogeny of the subfamily Ixoroideae (Rubiaceae). – Opera Bot. Belg. 7: 119-138.
Andreasen K, Bremer B. 2000. Combined phylogenetic analysis in the Rubiaceae-Ixoroideae: morphology, nuclear and chloroplast DNA data. – Amer. J. Bot. 87: 1731-1748.
Andreasen K, Baldwin BG, Bremer B. 1999. Phylogenetic utility of the nuclear rDNA ITS region in subfamily Ixoroideae (Rubiaceae): comparisons with cpDNA rbcL sequence data. – Plant Syst. Evol. 217: 119-135.
Andronova NN. 1977. Structure of the ovule in Rubiaceae Jull. – Bot. Žurn. 62: 1461-1469. [In Russian]
Andronova NN. 1984. The structure of the anther and pollen development in the Rubiaceae. – Bot. Žurn. 69: 43-54. [In Russian]
Andronova NN. 1988. Comparative embryology of Loganiaceae and Rubiaceae. – Bot. Žurn. 73: 937-951. [In Russian with English summary]
Ansari MY, Hemadri K. 1971. Seshagiria Ansari et Hemadri – a new genus of Asclepiadaceae from Sahyadri Ranges, India. – Indian Forester 97: 126-127.
Arriola AH, Paraguison LD, Alejandro GJD. 2016. Kanapia (Vanguerieae): a new endemic genus of Philippine Rubiaceae. – Plant Syst. Evol. 302: 911-920.
Axelius B. 1987. The genus Lerchea (Rubiaceae). – Blumea 32: 91-114.
Axelius B. 1990. The genus Xanthophytum (Rubiaceae): taxonomy, phylogeny and biogeography. – Blumea 34: 425-497.
Aymard CG, Dorr LJ, Cuello N. 1999. Rudgea tayloriae (Rubiaceae), an unusual new species from the eastern slopes of the Venezuelan Andes. – Novon 9: 315-317.
Azambuja D. 1946. Retificação da diagnose genêrica de Secondatia e apresentação de espécie nova para o Brasil. – Rodriguezia 20: 9-14.
Bacigalupo NM, Cabral EL. 1999. Revisión de las especies americanas del género Diodia (Rubiaceae-Spermacoceae). – Darwiniana 37: 153-155.
Bacigalupo NM, Cabral EL. 2006. Nuevas combinaciones en el género Diodella (Rubiaceae, Spermacoceae). – Darwiniana 44: 98-104.
Backlund M. 2005. Phylogenetic studies in the Gentianales: approaches at different taxonomic levels. – Ph.D. diss., University of Uppsala, Sweden.
Backlund M, Thulin M. 2007. Revision of the Mediterranean species of Plocama (Rubiaceae). – Taxon 56: 516-520.
Backlund M, Oxelman B, Bremer B. 2000. Phylogenetic relationships within the Gentianales based on ndhF and rbcL sequences, with particular reference to the Loganiaceae. – Amer. J. Bot. 87: 1029-1043.
Backlund M, Bremer B, Thulin M. 2007. Paraphyly of Paederieae, recognition of Putorieae and expansion of Plocama (Rubiaceae-Rubioideae). – Taxon 56: 315-328.
Baillon H. 1878. Sur l’organisation et les affinitées du Jackia. – Bull. Mens. Soc. Linn. Paris 1: 185-188.
Baillon MH. 1888. Observations sur le Veratrilla. – Bull. Mens. Soc. Linn. Paris 1: 729-730.
Baker HG. 1956. Pollen dimorphism in the Rubiaceae. – Evolution 10: 23-31.
Baker HG. 1958. Studies in the reproductive biology of West African Rubiaceae. – J. West African Sci. Assoc. (Fr.) 4: 9-24.
Bakhuizen van den Brink Jr RC. 1952. ”Steenisia”. A new genus of Malaysian Rubiaceae. – Webbia 8: 381-382.
Bakhuizen van den Brink Jr RC. 1953. Florae malesianae praecursores V. Notes on Malaysian Rubiaceae. – Blumea 7: 329-338.
Balistrieri CA. 2007. Stapeliads of Southern Africa and Madagascar. – Brittonia 59: 298-299.
Bamps P. 1982. Une Nouvelle espèce de Faroa (Gentianaceae) au Zaïre. – Bull. Jard. Bot. Natl. Belg. 52: 486-487.
Bamps P. 1987. Un nouveau Faroa (Gentianaceae) du Zaïre. – Bull. Jard. Bot. Natl. Belg. 57: 479-480.
Bangoura D. 1992. Beiträge zur Klärung der Gattungsabgrenzungsprobleme und Verwandtschafts-verhältnisse in der Tribus Pauridiantheae (Rubiaceae). – Ph.D. diss., Universität Wien, Austria.
Barrabé L, Buerki S, Mouly A, Davis AP, Munzinger J, Maggia L. 2012. Delimitation of the genus Margaritopsis (Rubiaceae) in the Asian, Australasian and Pacific region, based on molecular phylogenetic inference and morphology. – Taxon 61: 1251-1268.
Barrabé L, Maggia L, Pillon Y, Rigault F,
Mouly A, Davis AP, Buerki S. 2014. New Caledonian lineages of
Psychotria (Rubiaceae) reveal
different evolutionary histories and the largest documented plant radiation for
the archipelago. – Molec. Phylogen. Evol. 71: 15-35.
Baum H. 1949. Die Stellung der Samenanlagen am Karpell bei Asclepias syriaca, Cynanchum vincetoxicum und Erythraea centaurium. – Österr. Bot. Zeitschr. 95: 251-256.
Bawa KS, Beach JH. 1983. Self-incompatibility systems in the Rubiaceae of a tropical lowland wet forest. – Amer. J. Bot. 70: 1281-1288.
Behnke H-D. 1975. Elektronenmikroskopische Untersuchungen zur Frage der verwandtschaftlichen Beziehungen zwischen Theligonum und Rubiaceae: Feinbau der Siebelement-Plastiden und Anmerkungen zur Struktur der Pollenexine. – Plant Syst. Evol. 123: 317-326.
Bendre AM. 1973. Studies in the family Loganiaceae I. Trichomes. – J. Indian Bot. Soc. 52: 225-234.
Bendre AM. 1975. Studies in the family Loganiaceae II. Embryology of Buddleia and Strychnos. – J. Indian Bot. Soc. 54: 272-279.
Berthou T, Matthieu C, Vedel F. 1983. Chloroplast and mitochondrial DNA variation as indicator of phylogenetic relationships in the genus Coffea L. – Theor. Appl. Gen. 65: 77-84.
Bhattacharjee B, Deb DE. 1985. A revision of Knoxia (Rubiaceae). – J. Econ. Taxon. Bot. 6: 73-95.
Bidartondo MI, Redecker D, Hijri I, Wiemken A, Bruns TD, Domínguez LS, Sérsic A, Leake JR, Read DJ. 2002. Epiparasitic plants specialized on arbuscular mycorrhizal fungi. – Nature 419: 389-392.
Bir Bahadur. 1963. Heterostylism in Oldenlandia umbellata L. – J. Genet. 58: 429-439.
Bir Bahadur. 1966. Heterostyly in Oldenlandia scopulorum Bull. – J. Genet. 59: 267-272.
Bir Bahadur. 1968a. Heterostyly in Rubiaceae. A review. – J. Sci. Osmania Univ. (Golden Jubilee Vol.) 4: 207-238.
Bir Bahadur. 1968b. Pollen dimorphism in three heterostyled Rubiaceae. – Rev. Palaeobot. Palynol. 7: 233-239.
Bir Bahadur. 1970a. Heterostyly in Hedyotis nigricans (Lam.) Fosb. – J. Genet. 60: 175-177.
Bir Bahadur. 1970b. Heterostyly and homostyly in Pentas lanceolata (Forsk.) Desf. – J. Genet. 60: 199-204.
Bisset NG. 1958. The occurrence of alkaloids in the Apocynaceae I. – Ann. Bogor. 3: 105-236.
Bisset NG. 1961. The occurrence of alkaloids in the Apocynaceae II. A review of recent developments. – Ann. Bogor. 4: 65-144.
Bisset NG. 1975. Chemical structures and biosynthesis of Loganiaceae alkaloids. – Pharm. Weekbl. 110: 425-441.
Bisset NG. 1980a. Alkaloids of the Loganiaceae. – In: Phillipson JD, Zenk MH (eds), Indole and biogenetically related alkaloids, Academic Press, London, pp. 27-61.
Bisset NG. 1980b. Phytochemistry. – In: Leeuwenberg AJM (ed), Engler and Prantl’s ‘Die natürlichen Pflanzenfamilien’, Fam. Loganiaceae, vol. 28b(1), Duncker & Humblot, Berlin, pp. 211-237.
Bisset NG, Choudhury AK. 1974. Alkaloids and iridoids from Strychnos nux-vomica fruits. – Phytochemistry 13: 265-269.
Bisset NG, Gadella TWJ, Leeuwenberg AJM, Mennega AMW, Punt W. 1980. General discussion of relationships between taxa inside and with taxa outside the family Loganiaceae. – In: Leeuwenberg AJM (ed), Engler and Prantl’s, ‘Die natürlichen Pflanzenfamilien’, Fam. Loganiaceae, vol. 28b(1), Duncker & Humblot, Berlin, pp. 3-7.
Blackwell WH jr. 1968. Revision of Bouvardia (Rubiaceae). – Ann. Missouri Bot. Gard. 55: 201-421.
Blake SF. 1915. Notes on the genus Sabatia. – Rhodora 17: 50-57.
Boiteau P, Allorge L. 1978. Morphologie et biologie florales des Apocynacées I. Différences essentielles entre les Plumérioïdées et les Tabernaemontanoïdées. – Adansonia 17: 305-326.
Boiteau P, Sastre C. 1975. Sur l’arille des Macoubeae et la classification de la sous-famille des Tabernaemontanoïdées (Apocynacées). – Adansonia 15: 239-250.
Boom BB. 1984. A revision of Isertia (Isertieae: Rubiaceae). – Brittonia 36: 425-454.
Boom BM. 2005. A new species of Remijia (Rubiaceae) from Amazonian Brazil. – Brittonia 57: 105-107.
Borhidi A. 1982. Studies in Rondeletieae (Rubiaceae) III. The genera Rogiera and Arachnothryx. – Acta Bot. Acad. Sci. Hung. 28: 65-71.
Borhidi A. 1989. Studies in Rondeletieae (Rubiaceae) XI. Critical notes on some Central American species of Rondeletia s.l. – Acta Bot. Hung. 35: 309-312.
Borhidi A. 1993-1994 [1994]. Studies in Rondeletieae XII. New combinations of Mexican and Central American taxa. – Acta Bot. Hung. 38: 139-142.
Borhidi A. 2002. Los generous Ceuthocarpus Aiello y Schmidtottia Urb. (Rubiaceae). – Acta Bot. Hung. 44: 49-65.
Borhidi A. 2006. Rubiáceas de México. – Akadémiai kiadó Budapest, Budapest.
Borhidi A. 2007. Resinanthus, género nuevo de Rubiaceae (Guettardeae). – Acta Bot. Hung. 49: 311-317.
Borhidi A. 2010. The inclusion of Stevensia Poit. (Rondeletieae, Rubiaceae) into Rondeletia L. – Acta Bot. Hung. 52: 247-249.
Borhidi A. 2012. Terrellianthus, a new genus (Spermacoceae, Rubiaceae) of Mexico and Meso-America. – Acta Bot. Hung. 54: 45-49.
Borhidi A. 2018. Coutaportla, género endémico de México, Lorencea (Rubiaceae) endémico en Mesoamérica. – Acta Bot. Hung. 60(1-2). doi.org/10.1556/034.60.2018.1-2
Borhidi A, Diego-Pérez N. 2002. Introducción a la taxonomía de la familia Rubiaceae en la flora de México. – Acta Bot. Hung. 44: 237-280.
Borhidi A, Fernández MZ. 1981a. Studies in Rondeletieae (Rubiaceae) I. A new genus: Roigella. – Acta Bot. Acad. Sci. Hung. 27: 309-312.
Borhidi A, Fernández MZ. 1981b. Studies in Rondeletieae (Rubiaceae) II. A new genus: Suberanthus. – Acta Bot. Acad. Sci. Hung. 27: 313-316.
Borhidi A, Fernández MZ. 1983. Studies in Rondeletieae (Rubiaceae) V. Los límites del género Suberanthus. – Acta Bot. Hung. 29: 29-34.
Borhidi A, Járai-Komlódi M. 1983. Studies in Rondeletieae (Rubiaceae) IV. A new genus: Javorkaea. – Acta Bot. Hung. 29: 13-27.
Borhidi A, Lozada-Pérez L. 2010. Neomartensia (Spermacoceae, Rubiaceae) género nuevo de México. – Acta Bot. Hung. 52: 251-264.
Borhidi A, Lozada-Pérez L. 2011. Martensianthus nomen novum to replace Neomartensia Borhidi et Lozada-Pérez 2010 (Rubiaceae) non Yoshida et Mikami 1996 (Delesseriaceae). – Acta Bot. Hung. 53: 25-30.
Borhidi A, Verdcourt B. 1990. New species of Rubiaceae-Psychotrieae from Tanzania. – Kew Bull. 45: 703-709.
Borhidi A, Járai-Komlódi M, Moncada M. 1980. Acunaeanthus, a new genus of Rubiaceae. – Acta Bot. Acad. Sci. Hung. 26: 277-287.
Borhidi A, Darók J, Kocsis M, Stranczinge Sz. 2004. Critical revision of the Omiltemia complex (Rubiaceae, Hamelieae). – Acta Bot. Hung. 46: 69-76.
Borhidi A, Darók J, Kocsis M, Stranczinger Sz, Kaposvári. 2004. Critical revision of the Deppea complex (Rubiaceae, Hamelieae). – Acta Bot. Hung. 46: 77-89.
Borhidi A, Martínez Salas E, Ramos Álvarez CH. 2015. Estudios sobre las Rubiáceas de México, XLIX. Una nueva especie del género Arachnothryx (Rubiaceae, Guettardeae) en la flora de Veracruz. – Acta Bot. Hung. 57: 17-22.
Bosser J. 1984. Sur le type du Cephalanthus chinensis Lam. Neolamarckia, un nouveau nom pour Anthocephalus auct. non A. Rich. (Rubiaceae). – Bull. Mus. Natl. Hist. Nat. Paris, sér. IV, sect. B, 6: 243-248.
Bosser J, Lobreau-Callen D. 1998. Landiopsis Capuron ex Bosser, genre nouveau de Rubiaceae de Madagascar. – Adansonia, sér. III, 20: 131-137.
Boulay A. 1925. Contribution à l’étude des Apocynacées toxiques. – Trav. Lab. Mat. Méd. Paris 16: 117.
Bouman F, Louis A. 1990. Seed structure in Voyria primuloides Baker (Gentianaceae): taxonomic and ecological implications. – In: Paré J, Bugnicourt M, Mortier J, Juguet M, Vignon F, Vignon J (eds), Some aspects and actual orientations in plant embryology, l’Université de Picardie, Amiens.
Bouman F, Schier S. 1979. Ovule ontogeny and seed coat development in Gentiana, with a discussion on the evolutionary origin of the single integument. – Acta Bot. Neerl. 28: 467-478.
Bouman F, Cobb L, Devente N, Goethals V, Maas PJM, Smets E. 2002. The seeds of Gentianaceae. – In: Struwe L, Albert VA (eds), Gentianaceae – systematics and natural history, Cambridge University Press, Cambridge, pp. 498-572.
Boutique R. 1971. Deux Gentianacées nouvelles du Congo-Kinshasa. – Bull. Jard. Bot. Natl. Belg. 41: 261-264.
Boutique R. 1972. Gentianaceae. – In: Bamps P (ed), Flore d’Afrique Centrale (Zaire-Ruanda-Burundi). – Jardin Botanique Ntional de Belgique, Bruxelles, pp. 1-56.
Brandt W. 1922. Monographie der Gattungen Corynanthe Welwitsch und Pausinystalia Pierre, Rubiaceae (Über die Stammpflanze der Yohimberinde und ihre Verwandten). – Archiv Pharm. 260: 49-94.
Brehm G, Hartmann T, Wilmott K. 2007. Pyrrolizidine alkaloids and pharmacophagous visitors of Prestonia amabilis (Apocynaceae) in a montane rainforest in Ecuador. – Ann. Missouri Bot. Gard. 94: 463-473.
Bremekamp CEB. 1934a. A monograph of the genus Pavetta L. – Feddes Repert. 37: 1-208.
Bremekamp CEB. 1934b. Notes on the Rubiaceae of Surinam. – Rec. Trav. Bot. Néerl. 31: 248-308.
Bremekamp CEB. 1937a. Notes on the Rubiaceae of tropical Asia. – Blumea, Suppl. 1: 112-122.
Bremekamp CEB. 1937b. On the systematic position of the genus Dolianthus C. H. Wright. – Kew Bull. 1936: 103-105.
Bremekamp CEB. 1940. On Urophyllum Wall. and its nearest allies. – Rec. Trav. Bot. Néerl. 37: 171-197.
Bremekamp CEB. 1941. Ist die Gattung Urophyllum Wall. in Afrika vertreten? – Bot. Jahrb. Syst. 71: 200-237.
Bremekamp CEB. 1947a. Siderobombyx Brem. nov. gen. Rubiacearum Hedyotidearum. – J. Arnold Arbor. 28: 204-206.
Bremekamp CEB. 1947b. A monograph of the genus Acranthera Arn. ex Meisn. (Rubiaceae). – J. Arnold Arbor. 28: 261-308.
Bremekamp CEB. 1952. The African species of Oldenlandia L. sensu Hiern et K. Schumann. – Verh. Kon. Nederl. Akad. Wetensch. Amsterdam, Sect. II, 48: 1-297.
Bremekamp CEB. 1954. Les sous-familles et les tribus des Rubiacées. – Huitième Congrès International de Botanique, Rapports et Communications, Paris, sect. II, 6: 113-114.
Bremekamp CEB. 1956. Monographie des Triainolépidées, tribu nouvelle des Rubioïdées (Rubiacées). – Proc. Kon. Ned. Akad. Wetensch., Ser. C, Biol. Med. Sci. 59: 1-21.
Bremekamp CEB. 1957a. On the position of Platycarpum Humb. & Bonpl., Henriquezia Spruce ex Bth., and Gleasonia Standl. – Acta Bot. Neerl. 6: 351-377.
Bremekamp CEB. 1957b. Les Lathraeocarpées. Tribu nouvelle des Rubioïdées (Rubiacées). – Bull. Jard. Bot. État Bruxelles 27: 159-166.
Bremekamp CEB. 1963. On the pollen dimorphism in heterostylous Psychotrieae, especially in the Mapouria Aubl. – Grana Palynol. 4: 53-63.
Bremekamp CEB. 1966. Remarks on the position, the delimitation, and the subdivision of the Rubiaceae. – Acta Bot. Neerl. 15: 1-33.
Bremer B. 1979. The genus Neurocalyx (Rubiaceae-Argostemmateae) in Ceylon. – Bot. Not. 132: 399-407.
Bremer B. 1984. The genus Steenisia (Rubiaceae) and its taxonomical position. – Nord. J. Bot. 4: 333-345.
Bremer B. 1987. The sister group of the paleotropical tribe Argostemmateae: a redefined neotropical tribe Hamelieae (Rubiaceae, Rubioideae). – Cladistics 3: 35-51.
Bremer B. 1989. The genus Argostemma (Rubiaceae-Argostemmateae) in Borneo. – Ann. Missouri Bot. Gard. 76: 7-49.
Bremer B. 1992. Phylogeny of the Rubiaceae (Chiococceae) based on molecular and morphological data: useful approaches for classification and comparative ecology. – Ann. Missouri Bot. Gard. 79: 380-387.
Bremer B. 1996. Phylogenetic studies within Rubiaceae and relationships to other families based on molecular data. – Opera Bot. Belg. 7: 33-50.
Bremer B. 1996 [1997]. Combined and separate analyses of morphological and molecular data in the plant family Rubiaceae. – Cladistics 12: 21-40.
Bremer B. 2009. A review of molecular phylogenetic studies of Rubiaceae. – Ann. Missouri Bot. Gard. 96: 4-26.
Bremer B, Eriksson O. 1992. Evolution of fruit characters and dispersal modes in the tropical family Rubiaceae. – Biol. J. Linn. Soc. 47: 79-95.
Bremer B, Eriksson T. 2009. Time tree of Rubiaceae: phylogeny and dating the family, subfamilies, and tribes. – Intern. J. Plant Sci. 170: 766-793.
Bremer B, Jansen RK. 1991. Comparative restriction site mapping of chloroplast DNA implies new phylogenetic relationships within Rubiaceae. – Amer. J. Bot. 78: 198-213.
Bremer B, Manen J-F. 2000. Phylogeny and classification of the subfamily Rubioideae (Rubiaceae). – Plant Syst. Evol. 225: 43-72.
Bremer B, Struwe L. 1992. Phylogeny of the Rubiaceae and the Loganiaceae: congruence or conflict between morphological and molecular data? – Amer. J. Bot. 79: 1171-1184.
Bremer B, Thulin M. 1998. Collapse of Isertieae, re-establishment of Mussaendeae, and a new genus of Sabiceeae (Rubiaceae); phylogenetic relationships based on rbcL data. – Plant Syst. Evol. 211: 71-92.
Bremer B, Olmstead RG, Struwe L, Sweere JA. 1994. rbcL sequences support exclusion of Retzia, Desfontainia, and Nicodemia from the Gentianales. – Plant Syst. Evol. 190: 213-230.
Bremer B, Andreasen K, Olsson D. 1995. Subfamilial and tribal relationships in the Rubiaceae based on rbcL sequence data. – Ann. Missouri Bot. Gard. 82: 383-397.
Bremer B, Jansen RK, Oxelman B, Backlund M, Lantz H, Kim K-J. 1999. More characters or more taxa for a robust phylogeny – a case study from the coffee family (Rubiaceae). – Syst. Biol. 48: 413-435.
Bridson DM. 1978a. A short revision of Rutidea (Rubiaceae). – Kew Bull. 33: 243-278.
Bridson DM. 1978b. Nichallea, a new tropical African genus in the Rubiaceae. – Kew Bull. 33: 287-293.
Bridson DM. 1979a. Studies in Oxyanthus and Mitriostigma (Rubiaceae subfam. Cinchonoideae) for part 2 of “Flora of Tropical East Africa: Rubiaceae”. – Kew Bull. 34: 113-130.
Bridson DM. 1979b. Studies in Tarenna sensu lato (Rubiaceae subfam. Cinchonoideae) for part 2 of “Flora of Tropical East Africa: Rubiaceae”. – Kew Bull. 34: 377-402.
Bridson DM. 1980. Phellocalyx, a new tropical African genus in Rubiaceae (Gardenieae). – Kew Bull. 35: 315-321.
Bridson DM. 1982. Studies in Coffea and Psilanthus (Rubiaceae subfam. Cinchonoideae) for part 2 of ‘Flora of tropical East Africa’: Rubiaceae. – Kew Bull. 36: 817-859.
Bridson DM. 1984. Notes on Rothmannia fischeri and allied species (Rubiaceae subfam. Cinchonoideae). – Kew Bull. 39: 67-72.
Bridson DM. 1985. The reinstatement of Psydrax (Rubiaceae, subfam. Cinchonoideae tribe Vanguerieae) and a revision of the African species. – Kew Bull. 40: 687-725.
Bridson DM. 1986a. Additional notes on Coffea (Rubiaceae) from East tropical Africa. – Kew Bull. 41: 307-311.
Bridson DM. 1986b. The reinstatement of the African genus Keetia (Rubiaceae subfam. Cinchonoideae, tribe Vanguerieae). – Kew Bull. 41: 965-994.
Bridson DM. 1987a. Additional taxa in Oxyanthus, Pavetta & Tarenna (Rubiaceae subfam. Cinchonoideae) from Tanzania. – Kew Bull. 42: 251-261.
Bridson DM. 1987b. Nomenclatural notes on Psilanthus, including Coffea sect. Paracoffea (Rubiaceae tribe Coffeeae). – Kew Bull. 42: 453-460.
Bridson DM. 1987c. Studies in African Rubiaceae: Vanguerieae: a new circumscription of Pyrostria and a new subgenus, Canthium subgen. Bullockia. – Kew Bull. 42: 611-639.
Bridson DM. 1987c. The recognition and recircumscription of the African genus Multidentia (Rubiaceae: Vanguerieae). – Kew Bull. 42: 641-654.
Bridson DM. 1988. Pavetteae. – In: Polhill RM (ed), Flora of tropical East Africa, Rubiaceae 2, A. A. Balkema, Rotterdam, pp. 581-693.
Bridson DM. 1992. The genus Canthium (Rubiaceae-Vanguerieae) in tropical Africa. – Kew Bull. 47: 353-401.
Bridson DM. 1994. Additional notes on Coffea (Rubiaceae) from tropical East Africa. – Kew Bull. 49: 331-342.
Bridson DM. 1996. The tropical African genus Ancylanthos (Rubiaceae-Vanguerieae) reconsidered. – Kew Bull. 51: 343-352.
Bridson DM. 2000. The identity of Tsiangia (Rubiaceae). – Kew Bull. 55: 1011-1012.
Bridson DM. 2001a. Une nouvelle espèce d’Aulacocalyx (Rubiaceae, Aulacocalyceae) du sud-ouest du Cameroun. – Syst. Geogr. Plants 71: 17-23.
Bridson DM. 2001b. Additional notes on Pavetta (Rubiaceae: Pavetteae) from tropical Eastern and Southern Africa. – Kew Bull. 56: 567-600.
Bridson DM, Puff C. 1989. The West African monotypic genus Paragophyton (Rubiaceae) identified as Spermacoce. – Kew Bull. 44: 138.
Bridson DM, Robbrecht E. 1985a. Further notes on the tribe Pavetteae (Rubiaceae). – Bull. Jard. Bot. Natl. Belg. 55: 83-115.
Bridson DM, Robbrecht E. 1985b. Validation of the African genus Hyperacanthus E. Mey. (Rubiaceae tribe Gardenieae). – Kew Bull. 40: 273-286.
Bridson DM, Verdcourt B. 1988. Rubiaceae 2. – In: Polhill RM (ed), Flora of tropical East Africa, A. A. Balkema, Rotterdam, pp. 415-747.
Bridson DM, Gasson P, Robbrecht E. 1980. Phellocalyx, a new tropical African genus in the Rubiaceae (Gardenieae). – Kew Bull. 35: 315-321.
Broome CR. 1973. Systematics of Centaurium (Gentianaceae) of Mexico and Central America. – Ph.D. diss., Duke University, Durham, North Carolina.
Broome CR. 1976. The Central American species of Centaurium (Gentianaceae). – Brittonia 28: 413-426.
Broome CR. 1977. Four new species of Centaurium from Mexico. – Madroño 24: 237-244.
Broome CR. 1978. Chromosome numbers and meiosis in North and Central American species of Centaurium (Gentianaceae). – Syst. Bot. 3: 299-312.
Broome CR. 1981. A new variety of Centaurium namophilum (Gentianaceae) from the Great Basin. – Great Basin Nat. 41: 192-197.
Browicz K. 1966. The genus Periploca L. A monograph. – Arbor. Kórnickie 11: 5-104.
Bruce EA. 1955. Notes on the African species of the genus Anthocleista Afzl. ex R. Br. – Kew Bull. 1955: 45-57.
Bruce EA, Lewis J. 1960. Loganiaceae. – In: Hubbard CE, Milne-Redhead E (eds), Flora of tropical East Africa, Crown Agents for Oversea Governments and Administrations, London, pp. 1-47.
Brullo S. 1979. Valantia calva, a new species from Linosa, Sicily. – Bot. Not. 132: 61-64.
Brullo S. 1980. Valantia deltoidea Brullo, sp. nov. from Sicily. – Bot. Not. 133: 63-66.
Brummitt RK. 2007. Pteleocarpaceae. – In: Heywood VH, Brummitt RK, Culham A, Seberg O (eds.), Flowering plant families of the world, Royal Botanic Gardens, Kew, p. 270.
Brummitt RK. 2011. Valid publication of the family name Pteleocarpaceae. – Kew Bull. 66: 1-3.
Bruyns PV. 1985. Notes on Ceropiegias of the Cape Province. – Bradleya 3: 1-47.
Bruyns PV. 1986. The genus Ceropegia on the Canary Islands (Asclepiadaceae-Ceropegieae). – Beitr. Biol. Pflanzen 60: 427-458.
Bruyns PV. 1987a. The genus Caralluma R. Br. in Israel. – Israel J. Bot. 36: 73-86.
Bruyns PV. 1987b. Miscellaneous notes on Stapelieae (Asclepiadaceae). – Bradleya 5: 77-90.
Bruyns PV. 1988a. A revision of the genus Echidnopsis Hook. f. (Asclepiadaceae). – Bradleya 6: 1-48.
Bruyns PV. 1988b. Studies in the flora of Arabia 21: Cibirhiza, a new genus of Asclepiadaceae from Oman. – Notes Roy. bot. Gard. Edinb. 45: 51-54.
Bruyns PV. 1988c. Studies in the flora of Arabia 24: the genus Ceropegia in Arabia. – Notes Roy. Bot. Gard. Edinb. 45: 287-326.
Bruyns PV. 1989a. Two new species of Ceropegia (Asclepiadaceae: Stapelieae). – Kew Bull. 44: 721-726.
Bruyns PV. 1989b. Studies in the Flora of Arabia XXIV: the genus Ceropegia in Arabia. – Notes Roy. Bot. Gard. Edinburgh 45: 287-326.
Bruyns PV. 1995. New records and new species of Asclepiadaceae from Namibia. – Bothalia 25: 155-172.
Bruyns PV. 1998. Proposal to conserve the name Orbea (Asclepiadaceae) with a conserved type. – Taxon 47: 483-484.
Bruyns PV. 1999a. A systematic assessment of Ophionella (Apocynaceae; Asclepiadoideae; Stapelieae). – Bot. J. Linn. Soc. 131: 383-398. – Erratum: 133 (2000): 547-548.
Bruyns PV. 1999b. Subtribes and genera of Asclepiadeae. A response to Liede. – Taxon 48: 23-26.
Bruyns PV. 1999c. A systematic analysis with notes on the taxonomy of Notechidnopsis (Apocynaceae: Asclepiadoideae). – Kew Bull. 54: 327-345.
Bruyns PV. 1999d. The relationships of Huerniopsis and Piaranthus (Apocynaceae-Asclepiadoideae). – Syst. Bot. 24: 379-397.
Bruyns PV. 2000. Phylogeny and biogeography of the stapeliads. – Plant Syst. Evol. 221: 199-226.
Bruyns PV. 2000. Baynesia, a new genus of stapeliad from the northwestern-most corner of Namibia (Apocynaceae). – Novon 10: 354-358.
Bruyns PV. 2002. Monograph of Orbea
and Ballyanthus (Apocynaceae-Asclepiadoideae-Ceropegieae).
– Syst. Bot. Monogr. 63: 1-196.
Bruyns PV. 2003a. Three new succulent species of Apocynaceae (Asclepiadoideae) from Southern Africa. – Kew Bull. 58: 427-435.
Bruyns PV. 2003b. A revision of Astephanus (Apocynaceae). – Kew Bull. 58: 867-887.
Bruyns PV. 2005. Stapeliads of southern Africa and Madagascar 1-2. – Umdaus Press, Hatfield, Republic of South Africa.
Bruyns PV. 2014. The Apocynaceae of Namibia. – Strelitzia 34: 1-158.
Bruyns PV, Forster PI. 1991. Recircumscription of the Stapelieae (Asclepiadaceae). – Taxon 40: 381-391.
Bruyns PV, Klak C. 2007. A systematic study of the Old World genus Fockea (Apocynaceae-Asclepiadoideae). – Ann. Missouri Bot. Gard. 93: 535-564.
Bruyns PV, Klak C. 2009. The rediscovery of Schizostephalus gossweileri and its phylogenetic position. – South Afr. J. Bot. 75: 532-536.
Bruyns PV, Linder HP. 1991. A revision of Microloma R. Br. (Asclepiadaceae-Asclepiadeae). – Bot. Jahrb. Syst. 112: 453-527.
Bruyns PV, Nowell TL, Hedderson TAJ. 2005. A revision and phylogenetic analysis of Stapeliopsis (Apocynaceae). – Bot. J. Linn. Soc. 148: 125-155.
Bruyns PV, Farsi A al, Hedderson T. 2010. Phylogenetic relationships of Caralluma R. Br. (Apocynaceae). – Taxon 59: 1031-1043.
Bruyns PV, Klak C, Hanáček P. 2014.
Evolution of the stapeliads (Apocynaceae-Asclepiadoideae) –
repeated major radiation across Africa in an Old World group. – Mol.
Phylogen. Evol. 77: 251-263.
Bruyns PV, Klak C, Hanáček P. 2015. Recent radiation of Brachystelma and Ceropegia (Apocynaceae) across the Old World against a background of climatic change. – Mol. Phylogen. Evol. 90: 49-66.
Bruyns PV, Klak C, Hanáček P. 2017. A revised, phylogenetically-based concept of Ceropegia (Apocynaceae). – South Afr. J. Bot. 112: 399-436.
Buchner R, Puff C. 1993. The genus complex Danais-Schismatoclada-Payera (Rubiaceae). Character states, generic delimitation and taxonomic position. – Bull. Mus. Natl. Hist. Nat., Paris, sér. III, Adansonia, 15: 23-74.
Bullock AA. 1952. Notes on African Asclepiadaceae I. – Kew Bull. 7: 405-426.
Bullock AA. 1953a. Notes on African Asclepiadaceae II. – Kew Bull. 8: 51-67.
Bullock AA. 1953b. Notes on African Asclepiadaceae III. – Kew Bull. 8: 329-362.
Bullock AA. 1954a. Notes on African Asclepiadaceae IV. – Kew Bull. 9: 349-373.
Bullock AA. 1954b. Notes on African Asclepiadaceae V. – Kew Bull. 9: 579-594.
Bullock AA. 1955. Notes on African Asclepiadaceae VI. – Kew Bull. 10: 265-292.
Bullock AA. 1956. Notes on African Asclepiadaceae VII. – Kew Bull. 1955: 503-507.
Bullock AA. 1957. Notes on African Asclepiadaceae VIII. – Kew Bull. 1956: 503-522.
Bullock AA. 1961. Notes on African Asclepiadaceae IX. – Kew Bull. 1961: 193-206.
Bullock AA. 1962. Pentagonanthus, Triodoglossum, Sarcorrhiza. – Hooker’s Icon. Plant: t. 3583-3585.
Bullock AA. 1963. Notes on African Asclepiadaceae X. – Kew Bull. 17: 183-196.
Burck MW. 1883. Sur l’organisation florale chez quelques Rubiacées. – Ann. Jard. Bot. Buitenzorg 3: 105-119.
Cabaña Fader AA, Salas RM, Dessein S, Cabral EL. 2016. Synopsis of Hexasepalum (Rubiaceae), the priority name for Diodella and a new species from Brazil. – Syst. Bot. 41: 408-422.
Cabral EL. 2004. Novelties in Borreria (Rubiaceae-Spermacoceae) from Brazil. – Kew Bull. 59: 277-284.
Cabral EL. 2005. Notes on Galianthe (Spermacoceae, Rubiaceae) in the Bolivian flora. – Brittonia 57: 141-149.
Cabral EL. 2009. Revisión sinóptica de Galianthe subgen. Galianthe (Rubiaceae: Spermacoceae), con una sección nueva. – Ann. Missouri Bot. Gard. 96: 27-60.
Cabral EL, Bacigalupo NM. 2001a. Scandentia, nuevo género de Rubiaceae-Spermacoceae. – Darwiniana 39: 29-41.
Cabral EL, Bacigalupo NM. 2001b. Denscantia, nuevo nombre en reemplazo de Scandentia (Rubiaceae-Spermacoceae). – Darwiniana 39: 353.
Cabral EL, Bacigalupo NM. 2005. Novelties in Spermacoceae (Rubiaceae) from Bolivia and Paraguay. – Brittonia 57: 129-140.
Cabral EL, Cabaña Fader AA. 2010. Nuevas combinaciones y nuevos sinónimos en especies de Brasil de Diodia s. l. (Spermacoceae-Rubiaceae). – Rodriguésia 61: 119-121.
Cabral EL, Salas RM. 2006. Una nueva especie y una nueva combinación en el género Staelia (Rubiaceae-Spermacoceae) de Bolivia. – Darwiniana 44: 500-503.
Cabral EL, Miguel LM, Salas RM. 2011. Dos especies nuevas de Borreria (Rubiaceae), synopsis y clave de las especies para Bahia, Brasil. – Acta Bot. Bras. 25: 255-276.
Calió MF, Pirani JR, Struwe L. 2008. Morphology-based phylogeny and revision of Prepusa and Senaea (Gentianaceae: Helieae) – rare endemics from eastern Brazil. – Kew Bull. 63: 169-191.
Calió MF, Lepis KB, Pirani JR, Struwe L. 2017. Phylogeny of Helieae (Gentianaceae): resolving taxonomic chaos in a Neotropical clade. – Mol. Phylogen. Evol. 106: 192-208.
Calviño CI, Fernandez M, Ezcurra C. 2015. Is
the southern South American genus Tweedia (Apocynaceae: Asclepiadoideae)
monophyletic? Molecular phylogenies, distribution and taxonomy. – Taxon 63:
1265-1274.
Cameron DD, Bolin JF. 2010. Isotopic evidence of partial mycoheterotrophy in the Gentianaceae: Bartonia virginica and Obolaria virginica as case studies. – Amer. J. Bot. 97: 1272-1277.
Camp WH. 1949. Cinchona at high altitudes in Ecuador. – Brittonia 6: 394-430.
Cantley JT, Swenson NG, Markey A, Keeley SC.
2014. Biogeographic insights on Pacific Coprosma (Rubiaceae) indicate two colonizations to
the Hawaiian Islands. – Bot. J. Linn. Soc. 174: 412-424.
Cantley JT, Sporck-Koehler M, Chau MM. 2016. New and resurrected Hawaiian species of pilo (Coprosma, Rubiaceae) from the island of Maui. – PhytoKeys 60: 33-48.
Capuron R. 1969. À propos des Rubiacées-Vanguériées de Madagascar. – Adansonia, sér. II, 9: 47-55.
Carbonnier J, Massias M, Molho D. 1977. Importance taxonomique du schéma de substitution des xanthones chez Gentiana l. – Bull. Mus. Hist. Nat. Paris, sér. III, 504, Sci. Phys.-Chim. 13: 23-40.
Card HH. 1931. A revision of the genus Frasera. – Ann. Missouri Bot. Gard. 18: 245-282.
Cardona MA. 1984. Caryosystématique et différenciation évolutive de quelques Rubia méditerranéennes. – Webbia 38: 513-529.
Carlquist SJ. 1984. Wood anatomy of some Gentianaceae: systematic and ecological conclusions. – Aliso 10: 573-582.
Carlquist SJ, Grant JR. 2005. Wood anatomy of Gentianaceae, tribe Helieae, in relation to ecology, habit, systematics, and sample diameter. – Brittonia 57: 276-291.
Cavaco A. 1965. Remarques sur les genres Alberta E. Mey. et Nematostylis Hook. f. (Rubiaceae). – Adansonia, sér. II, 5: 515-518.
Chassot P. 2003a. A new species of Swertia L. (Gentianaceae) from Nepal. – Bot. J. Linn. Soc. 141: 389-394.
Chassot P. 2003b. Molecular phylogenetic, karyological and palynological studies in subtribe Swertiinae (Gentianaceae). – Ph.D. diss., Université de Neuchâtel, Neuchâtel, Switzerland.
Chassot P, Hagen KB von. 2008. Pollen morphology of the Swertiinae (Gentianaceae): phylogenetic implications. – Bot. J. Linn. Soc. 157: 323-341.
Chassot P, Nemomissa S, Yuan Y-M, Küpfer P. 2001. High paraphyly of Swertia L. (Gentianaceae) in the Gentianella-lineage as revealed by nuclear and chloroplast DNA sequence variation. – Plant Syst. Evol. 229: 1-21.
Chauhan AKS, Raghuvanshi SS. 1977. Cytogenetical studies of some members of Apocynaceae. – Cytologia 42: 723-729.
Chauhan JS, Kumar S, Chaturvedi R. 1984. A flavone glycoside from the stem of Ixora arborea. – Phytochemistry 23: 2404-2405.
Chauveaud LG. 1891. Recherches embryogénique sur l’appareil laticifère des Euphorbiacées, Urticacées, Apocynées et Asclepiadées. – Ann. Sci. Nat. Bot., sér. VII, 14: 1-161.
Chaw SM, Darwin SP. 1992. A systematic study of the paleotropical genus Antirhea (Rubiaceae: Guettardeae). – Tulane Stud. Zool. Bot. 28: 25-118.
Cheek M. 2005. Two further new species of Psychotria (Rubiaceae) from Western Cameroon. – Kew Bull. 60: 293-300.
Cheek M, Bridson D. 2002. Two new species of Psychotria (Rubiaceae) from Western Cameroon. – Kew Bull. 57: 389-395.
Cheek M, Csiba L. 2002. A revision of the Psychotria chalconeura complex (Rubiaceae) in Guineo-Congolian Africa. – Kew Bull. 57: 375-387.
Cheek M, Dawson S. 2000. A synoptic revision of Belonophora (Rubiaceae). – Kew Bull. 55: 63-80.
Cheek M, Corcoran M, Horwath A. 2008. Four new submontane species of Psychotria (Rubiaceae) with bacterial nodules from Western Cameroon. – Kew Bull. 63: 405-418.
Cheek M, Magassouba S, Howes M-JR, Doré T, Doumbouya S, Molmou D, Grall A, Couch C, Larridon I. 2018. Kindia (Pavetteae, Rubiaceae), a new cliff-dwelling genus with chemically profiled colleter exudate from Mt Gangan, Republic of Guinea – PeerJ. 2018; 6: e4666. doi: 10.7717/peerj/4666
Chen S, Xia T, Wang Y, Liu J, Chen S. 2005. Molecular systematics and biogeography of Crawfurdia, Metagentiana and Tripterospermum (Gentianaceae) based on nuclear ribosomal and plastid DNA sequences. – Ann. Bot. 96: 413-424.
Chen S-L, He T-N, Liu J-Q, Hong D-Y. 2000a. Embryology of Tripterospermum cordatum (Gentianaceae). – Acta Bot. Yunnan. 22: 53-58.
Chen S-L, He T-N, Liu J-Q, Hong D-Y. 2000b. Embryology of Crawfurdia delavayi (Gentianaceae) and its systematic value. – Israel J. Plant Sci. 48: 113-119.
Chen T. 2007. Observations and a new species in the genus Pseudopyxis (Rubiaceae). – Edinburgh J. Bot. 64: 303-309.
Chennaveeralah MS, Shivakumar PM. 1983. Pollen bud formation and its role in Ophiorrhiza spp. – Ann. Bot., N. S., 51: 449-452.
Chevalier A. 1909. Pseudocinchona africana A. Chev. – Les végétaux utiles de l’Afrique tropicale Française 5: 229-230.
Chevalier A. 1926. Sur les Cinchonées de l’Afrique tropicale. – Compt. Rend. Acad. Sci. Paris 182: 1402-1404.
Chevalier A. 1947. Les Caféiers du Globe 3. – Encycl. Biol. 28: 5-352.
Chinnappa CC, Warner BG. 1981. Pollen morphology in the genus Coffea (Rubiaceae) and its taxonomic significance. – Bot. J. Linn. Soc. 83: 221-236.
Chinnappa CC, Warner BG. 1982. Pollen morphology in the genus Coffea (Rubiaceae) II. Pollen polymorphism. – Grana 21: 29-37.
Chuba D, Goyder D, Chase MW, Fishbein M. 2017. Phylogenetics of the African Asclepias complex (Apocynaceae) based on three plastid DNA regions. – Syst. Bot. 42: 148-159.
Church SA. 2003. Molecular phylogenetics of Houstonia (Rubiaceae): descending aneuploidy and breeding system evolution in the radiation of the lineage across North America. – Mol. Phylogen. Evol. 27: 223-238.
Church SA, Taylor DR. 2005. Speciation and hybridization among Houstonia (Rubiaceae) species: the influence of polyploidy on reticulate evolution. – Amer. J. Bot. 92: 1372-1380.
Civeyrel L. 1994. Variation and evolution of pollen types in the genus Secamone (Asclepiadaceae, Secamonoideae). – Compt. Rend. Acad. Sci. Paris 317: 1159-1165.
Civeyrel L. 1995. Pollen morphology and ultrastructure of the genus Secamone in Africa. – In: 2nd Symposium of African Palynology, Publ. Occas. CIFEG 31, pp. 207-216.
Civeyrel L. 1996. Phylogénie des Asclepiadaceae, approche palynologique et moléculaire. – Ph.D. diss., l’Université de Montpellier, France.
Civeyrel L, Klackenberg J. 1996. A second species of the genus Secamonopsis (Asclepiadaceae). – Novon 6: 144-146.
Civeyrel L, Rowe N. 2001. Relationships of Secamonoideae based on the plastid gene matK, morphology and biomechanics. – Ann. Missouri Bot. Gard. 88: 583-602.
Civeyrel L, Le Thomas A, Ferguson K, Chase MW. 1998. Critical reexamination of palynological characters used to delimit Asclepiadaceae in comparison to the molecular phylogeny obtained from plastid matK sequences. – Mol. Phylogen. Evol. 9: 517-527.
Clarke CB. 1875. Notes on Indian Gentianaceae. – Bot. J. Linn. Soc. 14: 423-457.
Claßen-Bockhoff R. 1996. A survey of flower-like inflorescences in the Rubiaceae. – Opera Bot. Belg. 7: 329-367.
Clifford MN, Williams T, Bridson DM. 1989. Chlorogenic acids and caffeine as possible taxonomic criteria in Coffea and Psilanthus. – Phytochemistry 28: 829-838.
Cobb L, Maas PJM. 1983. Seed coat micromorphology in Irlbachia (Gentianaceae). – Proc. Kon. Nederl. Akad. Wetensch., Ser. C, 86: 127-136.
Cobb L, Maas PJM. 1998. A new species of Tachia (Gentianaceae) from Suriname. – Brittonia 50: 11-18.
Colegate SM, Gardner DR, Betz JM, Fischer OW, Liede-Schumann S, Boppré M. 2016. Pro-toxic 1,2-dehydropyrrolizidine alkaloid esters, including unprecedented 10-membered macrocyclic diesters, in the medicinally-used Alafia cf. caudata and Amphineurion marginatum (Apocynaceae: Apocynoideae: Nerieae and Apocyneae). – Phytochem. Analysis 27: 257-276.
Conn BJ. 1979. Notes on Neuburgia Blume (Loganiaceae) in Papuasia. – Brunonia 2: 99-105.
Conn BJ. 1980. A taxonomic revision of Geniostoma subg. Geniostoma (Loganiaceae). – Blumea 26: 245-364.
Conn BJ. 1994. Revision of Logania R. Br. section Stomandra (R. Br.) DC. (Loganiaceae). – Telopea 5: 657-692.
Conn BJ. 1995a. Description of inflorescence axes in the genus Logania R. Br. (Loganiaceae). – Kew Bull. 50: 777-783.
Conn BJ. 1995b. Taxonomic revision of Logania section Logania (Loganiaceae). – Aust. Syst. Bot. 8: 585-665.
Conn BJ, Brown EA. 1993a. Notes on Strychnos L. (Loganiaceae) in Australia. – Aust. Syst. Bot. 6: 309-319.
Conn BJ, Brown EA. 1993b. Review of Fagraea gracilipes complex (Loganiaceae). – Telopea 5: 363-374.
Conn BJ, Brown EA, Dunlop CR. 1996. Loganiaceae. – In: Orchard AE (ed), Flora of Australia 28, CSIRO, Melbourne, Australia, pp. 1-72.
Cortés-B R, Delprete PG, Motley TJ. 2009. Phylogenetic placement of the tribe Retiniphylleae among the subfamily Ixoroideae (Rubiaceae). – Ann. Missouri Bot. Gard. 96: 61-67.
Costa CB, Mamede MCH. 2006. Lipostoma is a synonym of Coccocypselum (Rubiaceae). – Brittonia 58: 170-177.
Cowan JM. 1932. The genus Wendlandia. – Notes Roy. Bot. Gard. Edinb. 16: 233-314.
Cranfield R, Keighery G. 2006. Logania wendyae (Loganiaceae), a new species from south-west Western Australia. – Nuytsia 16: 11-14.
Crepet WL, Daghlian CP. 1981. Lower Eocene and Paleocene Gentianaceae: floral and palynological evidence. – Science 214: 75-77.
Cros J, Combes MC, Trouslot P, Anthony F, Hamon S, Charrier A, Lashermes P. 1998. Phylogenetic analysis of chloroplast DNA variation in Coffea L. – Mol. Phylogen. Evol. 9: 109-117.
Cuatrecasas J. 1953. Caracterizacion del genero Borojoa. – Acta Agronomica 3: 91-98.
Dalvi VC, Meira RMSA, Azevedo AA. 2013. Extrafloral nectaries in neotropical Gentianaceae: occurrence, distribution patterns, and anatomical characterization. – Amer. J. Bot. 100: 1779-1789.
Daniel M, Sabnis SD. 1979. Chemotaxonomy of Loganiaceae. – Curr. Sci. 48: 383-385.
Daniel M, Sabnis SD. 1990. The chemical phylogeny of the order Gentianales. – In: Bilgrami KS, Dogra VV (eds), Phytochemistry and plant taxonomy, Delhi, pp. 151-156.
Darwin SP. 1976a. The subfamilial, tribal, and subtribal nomenclature of the Rubiaceae. – Taxon 25: 595-610.
Darwin SP. 1976b. The pacific species of Ophiorrhiza L. (Rubiaceae). – Lyonia 1: 47-102.
Darwin SP. 1976c. The genus Lindenia (Rubiaceae). – J. Arnold Arbor. 57: 426-449.
Darwin SP. 1977. The genus Mastixiodendron (Rubiaceae). – J. Arnold Arbor. 58: 349-381.
Darwin SP. 1979. A synopsis of the indigenous genera of Pacific Rubiaceae. – Allertonia 2: 1-44.
Darwin SP. 1980a. Notes on Airosperma (Rubiaceae), with a new species from Fiji. – J. Arnold Arbor. 61: 95-105.
Darwin SP. 1980b. Habroneuron Standley, a little-known genus of Mexican Rubiaceae. – Brittonia 32: 343-347.
Darwin SP. 1993. A revision of Timonius subgenus Timonius (Rubiaceae: Guettardeae). – Allertonia 7: 1-39.
Darwin SP. 1994. Systematics of Timonius subgenus Abbottia (Rubiaceae-Guettardeae). – Syst. Bot. Monogr. 42: 1-86.
Darwin SP. 1997. New species of the Timonius flavescens alliance (Rubiaceae: Guettardeae) in Papuasia. – Syst. Bot. 22: 85-98.
Dave YS, Kuriachen PM. 1991. Comparative anatomical characters of Periplocaceae follicles and their taxonomic significance. – Feddes Repert. 102: 63-68.
Dave YS, Thomas V, Kuriachen PM. 1987. Structure and development of colleters in Aganosma caryophyllata G. Don. – Pak. J. Bot. 19: 243-248.
Dave YS, Kuriachen PM, Vinoth T. 1988. Development, structure and senescence of colleters in Gardenia lucida Roxb. (Rubiaceae). – Acta Soc. Bot. Polon. 57: 3-7.
Dávila N, Kinoshita L, Taylor CM. 2018. Platycarpum vriesendorpiae sp. nov. a second new species of tribe Henriquezieae (Rubiaceae) from nutrient-poor soils in the Peruvian Amazon. – Nord. J. Bot. 36. doi-org.ludwig.lub.lu.se/10.1111/njb.01654
Davis AP. 2001. Two new species of Coffea L. (Rubiaceae) from eastern Madagascar. – Kew Bull. 56: 479-489.
Davis AP. 2010. Six species of Psilanthus transferred to Coffea (Coffeeae, Rubiaceae). – Phytotaxa 10: 41-45.
Davis AP. 2011. Psilanthus mannii, the type species of Psilanthus, transferred to Coffea. – Nord. J. Bot. 29: 471-472.
Davis AP, Mvungi EF. 2004. Two new and endangered species of Coffea (Rubiaceae) from the Eastern Arc Mountains (Tanzania) and notes on associated conservation issues. – Bot. J. Linn. Soc. 146: 237-245.
Davis AP, Rakotonasolo F. 2000. Three new species of Coffea L. (Rubiaceae) from Madagascar. – Kew Bull. 55: 405-416.
Davis AP, Rakotonasolo F. 2001a. Three new species of Coffea L. (Rubiaceae) from NE Madagascar. – Adansonia, sér. III, 23: 137-146.
Davis AP, Rakotonasolo F. 2001b. Two new species of Coffea L. (Rubiaceae) from northern Madagascar. – Adansonia, sér. III, 23: 337-345.
Davis AP, Rakotonasolo F. 2003. New species of Coffea L. (Rubiaceae) from Madagascar. – Bot. J. Linn. Soc. 142: 111-118.
Davis AP, Rakotonasolo F. 2008. A taxonomic revision of the Paracoffea alliance: nine remarkable Coffea species from western Madagascar. – Bot. J. Linn. Soc. 158: 355-390.
Davis AP, Razafimandimbison SG. 2010. Three species of Madagascan Plectronia transferred to Peponidium (Vamguerieae, Rubiaceae). – Phytotaxa 10: 46-48.
Davis AP, Govaerts R, Bridson DM, Stoffelen P. 2006. An annotated taxonomic conspectus of the genus Coffea (Rubiaceae). – Bot. J. Linn. Soc. 152: 465-512.
Davis AP, Chester M, Maurin O, Fay MF. 2007. Searching for the relatives of Coffea (Rubiaceae, Ixoroideae): the circumscription and phylogeny of Coffeeae based on plastid sequence data and morphology. – Amer. J. Bot. 94: 313-329.
Davis AP, Govaerts R, Bridson DM, Ruhsam M, Moat J, Brummitt NA. 2009. A global assessment of distribution, diversity, endemism, and taxonomic effort in the Rubiaceae. – Ann. Missouri Bot. Gard. 96: 68-78.
Davis AP, Tosh J, Ruch N, Fay MF. 2011. Growing coffee: Psilanthus (Rubiaceae) subsumed on the basis of molecular and morphological data; implications for the size, morphology, distribution and evolutionary history of Coffea. – Bot. J. Linn. Soc. 167: 357-377.
Davitashvili N, Karrer G. 2006. Taxonomic relationships of the western Asian taxa of Gentiana sect. Pneumonanthe. – Bot. J. Linn. Soc. 152: 197-208.
Dawson SE. 2002. A new species of Stelechantha Bremek. (Rubiaceae, Urophylleae) from Cameroon. – Kew Bull. 57: 397-402.
Dawson SE. 2016. Characteristics of the Madagascan genus Canephora (Rubiaceae: Octotropideae), and the description of two new species. – Kew Bull. 71: 45 DOI 10.1007/S12225-016-9654-5
Dayang Awa AL. 1996. Pteleocarpa. – In: Soepadmo E, Wong KM, Saw LG (eds.), Tree flora of Sabah and Sarawak II, Sabah Forestry Department and Sarawak Forestry Department, Forest Research Institute Malaysia, Kuala Lumpur, Malaysia, pp. 103-105.
Deb DB. 1996. Taxonomic and nomenclatural status of Myrioneuron R. Br. ex Hook. f. (Rubiaceae). – J. Bombay Nat. Hist. Soc. 93: 30-33.
Deb DB, Gangopadhyay M. 1987. Taxonomic study of the genus Brachytome Hook. f. (Rubiaceae). – Candollea 42: 351-360.
Deb DB, Gangopadhyay M. 1989. Taxonomic revision of the genus Psychotria (Rubiaceae) in India. – J. Econ. Taxon. Bot., Add. Ser. 7: 1-166.
Deb DB, Mondal DC. 1982. Two new species of Ophiorrhiza (Rubiaceae) from the Khasi Hills, India. – Kew Bull. 37: 483-487.
Deb DB, Rout RC. 1990. A new species of Indopolysolenia (Rubiaceae) from Burna. – Kew Bull. 45: 339-341.
Deb DB, Rout RC. 1991. Jainia, a new synonym of Coptophyllum (Rubiaceae). – Taxon 40: 324-325.
Deb DB, Rout RC. 1992. Two new species of Ixora (Rubiaceae subfam. Ixoroideae) from India and Burma. – Kew Bull. 47: 295-300.
De Block P. 1997. Biosystematic studies in the tribe Pavetteae (Rubiaceae-Ixoroideae). – Ph.D. diss., Universiteit Instelling Antwerpen, Belgium.
De Block P. 1998. The African species of Ixora. – Opera Bot. Belg. 9: 1-218.
De Block P. 2003a. Robbrechtia, a new Rubiaceae genus from Madgascar. – Syst. Bot. 28: 145-156.
De Block P. 2003b. New combinations in the genus Paracephaelis (Pavetteae, Rubiaceae). – Syst. Geogr. Plants 73: 99-100.
De Block P. 2006. A new Mantalania species (Rubiaceae) from Madagascar. – Bot. J. Linn. Soc. 151: 421-424.
De Block P. 2007. Three new Madagascan Ixora species (Rubiaceae) with flowers up to 23 cm long. – Nord. J. Plants 25: 75-84.
De Block P. 2014. Eight new species of Ixora (Ixoreae-Rubiaceae) from Madagascar. – Plant Ecol. Evol. 147: 237-255.
De Block P. 2018. Revision of the Madagascan endemic Homollea (Rubiaceae-Pavetteae), with description of two new species. – Europ. J. Taxon. 423: 1-24.
De Block P, Davis AP. 2006. A new Mantalania species (Rubiaceae) from Madagascar. – Bot. J. Linn. Soc. 151: 421-424.
De Block P, Igersheim A. 2001. Stigma of the African genera Rutidea and Nichallea (Rubiaceae-Ixoroideae-Pavetteae): highly modified receptive surfaces. – Intern. J. Plant Sci. 162: 567-578.
De Block P, Robbrecht E. 1997. Duperrea, a most remarkable Rubiaceae from Indochina. – Scripta Bot. Belg. 15: 44.
De Block P, Robbrecht E. 1998. Pollen morphology of the Pavetteae (Rubiaceae, Ixoroideae) and its taxonomic significance. – Grana 37: 260-275.
De Block P, Degreef J, Robbrecht E. 2001 [2002]. Reinstatement of the Afro-Malagasy genus Coptosperma (Rubiaceae, Ixoroideae, Pavetteae). – Syst. Geogr. Plants 71: 455-492.
De Block P, Rakotonasolo F, Tosh J. 2006. Mantalania longipedunculata (Rubiaceae) revisited. – Syst. Geogr. Plants 76: 235-240.
De Block P, Razafimandimbison SG, Janssens S, Ochoterena H, Robbrecht E, Bremer B. 2015. Molecular phylogenetics and generic assessment in the tribe Pavetteae (Rubiaceae). – Taxon 64: 79-95.
De Block P, Rakotonasolo F, Ntore S, Razafimandimbison SG, Janssens S. 2018. Four new endemic genera of Rubiaceae (Pavetteae) from Madagascar represent multiple radiations into drylands. – PhytoKeys 99: 1-66.
Degreef J. 2002. Flower galls in African Rubiaceae. – Syst. Geogr. Plants 72: 203-210.
Degreef J, De Block P, Robbrecht E. 2001 [2002]. A survey of continental African Coptosperma (Rubiaceae, Pavetteae). – Syst. Geogr. Plants 71: 367-382.
De Kruif APM. 1983. Series of revisions of Apocynaceae II. A revision of Motandra Apocynaceae. – Meded. Landbouwhogeschool Wageningen 83: 1-20.
De Kruif APM. 1985. Series of revisions of Apocynaceae XVI. A revision of Oncinotis Benth. – Wageningen Agr. Univ. Pap. 85: 5-45.
De Laet J, Smets E. 1996. A commentary on the circumscription and evolution of the order Gentianales, with special emphasis on the position of the Rubiaceae. – Opera Bot. Belg. 7: 11-18.
Delgado MN, Azevedo AA, Silva LC, Valente GE. 2011. Comparative anatomy of Calolisianthus species (Gentianaceae – Helieae) from Brazil: taxonomic aspects. – Edinburgh J. Bot. 68: 139-155.
Delprete PG. 1996a. Evaluation of the tribes Chiococceae, Condamineeae and Catesbaeeae (Rubiaceae) based on morphological characters. – Opera Bot. Belg. 7: 165-192.
Delprete PG. 1996b. Notes on calycophyllous Rubiaceae 1. Morphological comparisons of the genera Chimarrhis, Bathysa and Calycophyllum, with new combinations and a new species, Chimarrhis gentryana. – Brittonia 48: 35-44.
Delprete PG. 1996c. Notes on the taxonomic position of the monotypic Brazilian genus Kerianthera (Rubiaceae). – Opera Bot. Belg. 7: 271-275.
Delprete PG. 1997a. Notes on calycophyllous Rubiaceae II. Morphological comparison of the genera Bathysa and Schizocalyx. – Brittonia 49: 480-486.
Delprete PG. 1997b. Revision and typification of Brazilian Augusta (Rubiaceae, Rondeletieae), with ecological observations on the riverine vegetation of the cerrado and Atlantic forests. – Brittonia 49: 487-497.
Delprete PG. 1998a. Notes on calycophyllous Rubiaceae III. Systematic position of the monotypic Mexican genus Cosmocalyx and notes on the calycophyll development. – Brittonia 50: 309-317.
Delprete PG. 1998b. Notes on calycophyllous Rubiaceae IV. The monotypic Brazilian genus Blandibractea Wernham is a Simira (Rondeletieae). – Brittonia 50: 318-323.
Delprete PG. 1999a. Flora Neotropica. Monograph 77. Rondeletieae (Rubiaceae) I. – New York Botanical Garden, Bronx, New York, pp. 1-226.
Delprete PG. 1999b. Riodocea (Rubiaceae, Gardenieae), a new genus from the Brazilian Atlantic forest. – Brittonia 51: 15-23.
Delprete PG. 1999c. The Cuban endemic genera Ariadne, Mazaea, Acunaeanthus, Phyllomelia (Rondeletieae), and Eosanthe (Rubiaceae). – Brittonia 51: 217-230.
Delprete PG. 1999d. 162(7). Rubiaceae-Condamineeae. – In: Harling G, Andersson L (eds), Flora of Ecuador 62, Nord. J. Bot., Copenhagen, pp. 5-53.
Delprete PG. 2000. Melanopsidium Colla (Rubiaceae, Gardenieae): a monospecific Brazilian genus with a complex nomenclatural history. – Brittonia 52: 325-336.
Delprete PG. 2001. Notes on some South American species of Psychotria subgenus Heteropsychotria (Rubiaceae), with observations on rubiaceous taxonomic characters. – Brittonia 53: 396-404.
Delprete PG. 2009. Taxonomic history, morphology, and reproductive biology of the tribe Posoquerieae (Rubiaceae, Ixoroideae). – Ann. Missouri Bot. Gard. 96: 79-89.
Delprete PG, Cortés-B R. 2002. Análisis filogenético de la tribu Sipaneeae (Rubiaceae, Ixoroideae). – In: Rangel-Ch JO, Aguirre-C J, Andrade-C MG (eds), Libro de Resúmenes Octavo Congreso Latino-americano de Botánica, Instituto de Ciencias Naturales, Univ. Nacional de Colombia, Bogotá.
Delprete PG, Cortés-B R. 2004. A phylogenetic study of the tribe Sipaneeae (Rubiaceae, Ixoroideae), using trnL-F and ITS sequence data. – Taxon 53: 347-356.
Delprete PG, Cortés-B R. 2006. A synopsis of the Rubiaceae of the states of Mato Grosso and Mato Grosso do Sul, central-western Brazil, with a key to genera, and a preliminary species list. – Rev. Biol. Neotrop. 3: 13-96.
Delprete PG, Motley TJ. 2003. Portlandia proctorii (Rubiaceae, Catesbaeeae), a new combination for a narrow endemic Jamaican species. – Brittonia 55: 233-239.
Delprete PG, Nee M. 1997. The enigmatic genus Wernhamia S. Moore is a synonym of Simira (Rubiaceae, Rondeletieae). – Brittonia 49: 303-308.
Demarco D, Kinoshita LS, Casrom M de M. 2006. Laticiferos articulados anastomosados – novos registros para Apocynaceae. – Rev. Brasileira Bot. 29: 133-144.
Demeter K. 1922. Vergleichende Asclepiadeenstudien. – Flora 115: 130-176.
Dempster LT. 1990. The genus Galium (Rubiaceae) in South America IV. – Allertonia 5: 283-345.
Dempster LT. 1993. 162(23). Rubiaceae-Rubieae. – In: Harling G, Andersson L (eds), Flora of Ecuador 47, Nord. J. Bot., Copenhagen, pp. 21-36.
Deng Y. 2007. Fleroya, a substitute name for Hallea J.-F. Leroy (Rubiaceae). – Taxon 56: 247-248.
Dessein S. 2003. Systematic studies in the Spermacoceae (Rubiaceae). – Ph.D. diss., Katolieke Universiteit Leuven, Belgium.
Dessein S, Scheltens A, Huysmans S, Robbrecht E, Smets E. 2000. Pollen morphological survey of Pentas (Rubiaceae-Rubioideae) and its closest allies. – Rev. Palaeobot. Palynol. 112: 189-205.
Dessein S, Jansen S, Huysmans S, Robbrecht E, Smets E. 2001. A morphological and anatomical survey of Virectaria (African Rubiaceae), with a discussion of its taxonomic position. – Bot. J. Linn. Soc. 137: 1-29.
Dessein S, Andersson L, Robbrecht E, Smets E. 2001. Hekistocarpa (Rubiaceae): a member of an emended tribe Virectarieae. – Plant Syst. Evol. 229: 59-78.
Dessein S, Huysmans S, Robbrecht E, Smets E. 2002. Pollen of African Spermacoce species (Rubiaceae). Morphology and evolutionary aspects. – Grana 41: 69-89.
Dessein S, Jansen S, Robbrecht E, Smits E. 2002 [2004]. A new species of Spermacoce (Rubiaceae) from the Manika high plateau (Katanga; R. D. Congo). – Nord. J. Bot. 22: 513-523.
Dessein S, Ntore S, Robbrecht E, Smets E. 2003. Pollen and seeds reveal that Spermacoce thymoidea s.l. (African Rubiaceae, Spermacoceae) represents three endemic or disjunct species from the Zambezian high plateaus. – Syst. Bot. 28: 130-144.
Dessein S, Andersson L, Geuten K, Smets E, Robbrecht E. 2005. Gomphocalyx and Phylohydrax (Rubiaceae): sister taxa excluded from Spermacoceae s.s., featuring a remarkable case of convergent evolution. – Taxon 54: 91-107.
Dessein S, Ochoterena H, De Block P, Lens F, Robbrecht E, Schols P, Smets E, Vinckier S, Huysmans S. 2005. Palynological characters and their phylogenetic signal in Rubiaceae. – Bot. Rev. 71: 354-414.
Dessein S, Harwood R, Smets E, Robbrecht E. 2005. Pollen of the Spermacoce (Rubiaceae) species from the Northern Territory of Australia: morphology and taxonomic significance. – Aust. Syst. Bot. 18: 367-382.
Dessein S, Ochoterena H, Block P de, Lens F, Robbrecht E, Schols P, Smets E, Vinckier S, Huysmans S. 2005. Palynological characters and their phylogenetic signal in Rubiaceae. – Bot. Rev. 71: 354-414.
Dessein S, Lachenaud O, Sonké B. 2011. Colletoecema gabonensis, a third species of an unusual African genus of Rubiaceae. – Brittonia 63: 215-219.
Devesa JA, Ortega-Olivencia A. 2003. A new species of Valantia (Rubiaceae) from Spain. – Bot. J. Linn. Soc. 143: 331-335.
Devi HM. 1962. Embryological studies in the Gentianaceae (Gentianoideae and Menyanthoideae). – Proc. Indian Acad. Sci., Sect. B, 56: 195-216.
Devi HM. 1964. Embryological studies in Asclepiadaceae. – Proc. Indian Acad. Sci., Sect. B, 60: 54-65.
Devi HM. 1971. Embryology of Apocynaceae I. Plumerieae. – J. Indian Bot. Soc. 50: 74-85.
Devi HM. 1974. Embryology of Apocynaceae II. Arduineae (Carissa spinarum Linn.). – Plant Sci. 6: 24-29.
Devi HM, Lakshminarayana K. 1977. Embryological studies in Gentianaceae. – J. Indian Bot. Soc. 56: 182-188.
De Wildeman E. 1922. Notes sur les genres Corynanthe Welw. et Pasuinystalia Pierre. – Ann. Soc. Sci. Bruxelles 2: 173-180.
D’Hondt C. 2002. Pollen- en orbiculemorfologie van Hillieae (Rubiaceae). – Ph.D. diss., Katolieke Universitet Leuwen, Belgium.
D’Hondt C, Schols P, Huysmans S, Smets E. 2004. Systematic relevance of pollen and orbicule characters in the tribe Hillieae (Rubiaceae). – Bot. J. Linn. Soc. 146: 303-321.
Die J van. 1955. A comparative study of the particle fractions from Apocynaceae latices. – Ann. Bogor. 2: 1-124.
Dilst FJH van. 1995. Series of revisions of Apocynaceae XXXIX. Baissea A. DC. – Bull. Jard. Bot. Natl. Belg. 64: 89-178.
Dilst FJH van. 1999. Series of revisions of Apocynaceae XLVI. The Madagascan species of Landolphia. – Syst. Geogr. Plants 69: 91-110.
Di Malo FR. 1996 [1998]. Revisão taxonomica de gênero Hindsia Bentham (Rubiaceae, Hedyotideae). – Arch. Jard. Bot. Rio de Janeiro 34: 51-92.
Dioli M. 2002. Two new species of Pseudolithos P. R. O. Bally (Apocynaceae-Asclepiadoideae) from the Horn of Africa. – Kew Bull. 57: 985-988.
Dold AP. 2006. Ceropegia macmasteri (Apocynaceae-Asclepiadoideae-Ceropegieae), a new species from Eastern Cape, South Africa. – South Afr. J. Bot. 72: 144-146.
Dong L. 2013. Phylogenetic studies of New World species in the plant genus Psychotria (Rubiaceae). – Ph.D. diss., University of Georgia, Athens, Georgia.
Drapalik DJ. 1969. A biosystematic study of the genus Matelea in the southeastern United States. – Ph.D. diss., University of North Carolina, Chapel Hill, North Carolina.
Duan T, Deng X, Chen S, Luo Z, Zhao Z, Tu T, Khang NS, Razafimandimbison SG, Zhang D. 2018. Evolution of sexual systems and growth habit in Mussaenda (Rubiaceae): insights into the evolutionary pathways of dioecy. – Mol. Phylogen. Evol. 123: 113-122.
Duncan WH, Brown CL. 1954. Connate anthers in Gentiana (Gentianaceae). – Rhodora 56: 133-136.
Duncan WH, Dejong OW. 1964. Taxonomy and heterostyly of North American Gelsemium (Loganiaceae). –Sida 1: 346-357.
Dunn RA. 1967. A revision of the genus Centaurium of continental United States. – Ph.D. diss., Catholic University of America, Washington, D.C.
Dwyer JD. 1966. Notes on Coussareeae (Rubiaceae), especially the Panamanian species. – Ann. Missouri Bot. Gard. 53: 368-374.
Dwyer JD. 1969. The genus Hoffmannia (Rubiaceae) in Panama. – Ann. Missouri Bot. Gard. 56: 269-286.
Dwyer JD. 1980. Flora of Panama, part IX. Family 179. Rubiaceae. – Ann. Missouri Bot. Gard. 67: 1-522.
Dyer RA. 1979. Notes on African plants. The Riocreuxia flanaganii complex. – Bothalia 12: 632.
Dyer RA. 1983. Ceropegia, Brachystelma and Riocreuxia in Southern Africa. – A. A. Balkema, Rotterdam.
Edgar JA, Culvenor CCJ. 1975. Pyrrolizidine alkaloids in Parsonsia species (family Apocynaceae) which attract danaid butterflies. – Experientia 31: 393-394.
Ehrendorfer F. 1948. Rubiaceae. – In: Rechinger KH (ed), Ergebnisse einer botanischen Reise nach dem Iran, 1937. V. Teil. – Ann. Naturhist. Mus. Wien 56: 212-224.
Ehrendorfer F. 1958. Critical notes on Turkish Rubiaceae. – Notes Roy. Bot. Gard. Edinb. 22: 323-401.
Ehrendorfer F. 1962. Notizen zur Systematik und Phylogenie von Cruciata Mill. und verwandten Gattungen der Rubiaceae. – Ann. Naturhist. Mus. Wien 65: 11-20.
Ehrendorfer F. 1971. Evolution and eco-geographical differentiation in some South-West Asiatic Rubiaceae. – In: Davis PH et al. (eds), Plant life in South-West Asia, Botanical Society, Edinburgh, pp. 195-215.
Ehrendorfer F, Barfuss MHJ. 2014. Paraphyly
and polyphyly in the worldwide tribe Rubieae (Rubiaceae): challenges for generic
delimitation. – Ann. Missouri Bot. Gard. 100: 79-88.
Ehrendorfer F, Krendl F. 1974. Notes on Rubiaceae in Europe. – Bot. J. Linn. Soc. 68: 268-272.
Ehrendorfer F, Manen J-F, Natali A. 1994. cpDNA intergene sequences corroborate restriction site data for reconstructing Rubiaceae phylogeny. – Plant Syst. Evol. 190: 245-248.
El-Gazzar A, Hamza MK. 1973. Morphology of twin pollinia of Asclepiadaceae. – Pollen Spores 15: 459-470.
El-Gazzar A, Hamza MK. 1980. The subdivision of Asclepiadaceae. – Phytologia 45: 1-16.
El-Ghazaly G. 1990. Development of pollen grains of Catharanthus roseus (Apocynaceae). – Rev. Palaeobot. palynol. 64: 165-174.
El-Ghazaly G, Huysmans S. 2001. Re-evaluation of a neglected layer in pollen wall development with comments on its evolution. – Grana 40: 3-16.
El-Ghazaly G, Huysmans S, Smets E. 2001. Pollen wall development of Rondeletia odorata (Rubiaceae). – Amer. J. Bot. 88: 14-30.
Elias TS. 1976. A monograph of the genus Hamelia (Rubiaceae). – Mem. New York Bot. Gard. 26: 81-144.
Elst D van, Nuyens S, Wyk AE van, Verstraete B , Dessein S, Prinsen E. 2013. Distribution of the cardiotoxin pavettamine in the coffee family (Rubiaceae) and its significance for gousiekte, a fatal poisoning of ruminants. – Plant Physiol. Biochem. 67: 15-19.
Elst D van, Wyk AE van, Schultz RA, Prinsen E. 2013. Production of toxic pavettamine and pavettamine conjugates in the gousiekte-causing Fadogia homblei plant and its relation to the bacterial endosymbiont. – Phytochemistry 85: 92-98.
Endress ME. 2004a. Apocynaceae: Brown and now. – Telopea 10: 525-541.
Endress ME. 2004b. New species of Malouetia (Apocynaceae): a trio from Amazonia. – Brittonia 56: 307-313.
Endress ME, Bruyns PV. 2000. A revised classification of the Apocynaceae s.l. – Bot. Rev. 66: 1-56.
Endress ME, Hansen BF. 2007. Pinochia, a new genus of Apocynaceae, Apocynoideae from the Greater Antilles, Mexico and Central America. – Edinburgh J. Bot. 64: 269-274.
Endress ME, Meve U, Middleton DJ, Liede-Schuymann S. 2018. Apocynaceae. – In: Kadereit JW, Bittrich V (eds). 2018. The families and genera of vascular plants XV. Flowering plants – eudicots – Apiales, Gentianales (except Rubiaceae). Springer, Berlin, pp. 207-411.
Endress ME, Stevens WD. 2001. The renaissance of the Apocynaceae s.l.: recent advances in systematics, phylogeny, and evolution: introduction. – Ann. Missouri Bot. Gard. 88: 517-522.
Endress ME, Hesse M, Nilsson S, Guggisberg A, Zhu J-P. 1990. The systematic position of the Holarrheninae (Apocynaceae). – Plant Syst. Evol. 171: 157-185.
Endress ME, Sennblad B, Nilsson S, Civeyrel L, Chase MW, Huysmans S, Grafström E, Bremer B. 1996. A phylogenetic analysis of the Apocynaceae s. str. and some related taxa in Gentianales: a multidisciplinary approach. – Opera Bot. Belg. 7: 59-102.
Endress ME, Lorence DH, Endress PK. 1997. Structure and development of the gynoecium in Lepinia marquisensis and its systematic position in the Apocynaceae. – Allertonia 7: 257-272.
Endress ME, Ham RWJM van der, Nilsson S, Civeyrel L, Chase MW, Sennblad B, Potgieter K, Joseph J, Powell M, Lorence D, Zimmermann Y-M, Albert VA. 2007. A phylogenetic analysis of Alyxieae (Apocynaceae) based on rbcL, matK, trnL intron, trnL-F spacer sequences, and morphological characters. – Ann. Missouri Bot. Gard. 94: 1-35.
Endress ME, Liede-Schumann S, Meve U. 2007. Advances in Apocynaceae: the enlightenment, an introduction. – Ann. Missouri Bot. Gard. 94: 259-267.
Endress ME, Liede-Schumann S, Meve U. 2014. An updated classification for Apocynaceae. – Phytotaxa 159: 175-194.
Endress PK, Jenny M, Fallen ME. 1983. Convergent elaboration of apocarpous gynoecia in higher advanced dicotyledons (Sapindales, Malvales, Gentianales). – Nord. J. Bot. 3: 293-300.
Erbar C, Leins P. 1996. The formation of corolla tube in Rubiaceae and presumably related families. – Opera Bot. Belg. 7: 103-112.
Eriksson O, Bremer B. 1991. Fruit characteristics, life forms, and species richness in the plant family Rubiaceae. – Amer. Natur. 138: 751-761.
Es K. 1999. Geophila (Psychotrieae, Rubiaceae): revisie van de neotropische taxa. – Lic. thesis, Katolieke Universiteit, Leuven, Belgium.
Espírito Santo F da S do, Sousa DJL de, Chagas DB das, Morais IL de, Ribeiro PL, Rapini A. 2018. Four new species of Marsdenia (Apocynaceae) from the Cerrado domain. – Syst. Bot. 43: 571-578.
Estabrook GF, Anderson WR. 1978. An estimate of phylogenetic srelationships within the genus Crusea (Rubiaceae) using character compatibility analysis. – Syst. Bot. 3: 179-196.
Ewan J. 1947. A revision of Chorisepalum, an endemic genus of Venezuelan Gentianaceae. – J. Washington Acad. Sci. 37: 392-396.
Ewan J. 1948a. A revision of Macrocarpaea, a neotropical genus of shrubby gentians. – Contr. U.S. Natl. Herb. 29: 209-251.
Ewan J. 1948b. A review of Purdieanthus and Lehmanniella, two endemic Colombian genera of Gentianaceae, and biographical notes on Purdie and Lehmann. – Caldasia 5: 85-98.
Ewan J. 1952. A review of the neotropical lisianthoid genus Lagenanthus (Gentianaceae). – Mutisia 4: 1-5.
Ezcurra C. 1979. Sobre la posición sistemática del género Grisebachiella Lorentz. – Darwiniana 22: 177-183.
Ezcurra C. 1981. Novedades en los géneros Temnadenia y Macrosiphonia (Apocynaceae). – Hickenia 1: 241-245.
Ezcurra C, Belgrano MJ. 2007. A new species and a new combination in Matelea (Apocynaceae, Asclepiadoideae) from southern South America. – Syst. Bot. 32: 856-861.
Fabris HA. 1953. Sinopsis preliminar de las Gencianaceas Argentinas. – Bol. Soc. Argent. Bot. 4: 233-259.
Fagerlind F. 1934. Beiträge zur Kenntnis der Zytologie der Rubiaceen. – Hereditas 19: 223-232.
Fagerlind F. 1937. Embryologische, zytologische und bestäubungsexperimentelle Studien in der Familie Rubiaceae nebst Bemerkungen über einige Polyploiditätsprobleme. – Acta Horti Berg. 11(9): 195-470.
Fagerlind F. 1939. Der Bestäubungsapparat bei Posoqueria. – Svensk Bot. Tidskr. 33: 261-276.
Fagerlind F. 1942. Die Sproßfolge in der Gattung Randia und ihre Bedeutung für die Revision der Gattung. – Ark. f. Bot. 30A: 1-57.
Fagerlind F. 1948. Rosenbergiodendron gen. nov., eine polymorphe Rubiaceen-Gattung mit gewöhnlichem Vorkommen von Mikrosporogenesestörungen. – Svensk Bot. Tidskr. 42: 143-152.
Fallen ME. 1983. A taxonomic revision of Condylocarpon (Apocynaceae). – Ann. Missouri Bot. Gard. 70: 149-169.
Fallen ME. 1985. The gynoecial development and systematic position of Allamanda (Apocynaceae). – Amer. J. Bot. 72: 572-579.
Fallen ME. 1986. Floral structure in the Apocynaceae: morphological, functional, and evolutionary aspects. – Bot. Jahrb. Syst. 106: 245-286.
Farooq M, Inamuddin M. 1969. The embryology of Oldenlandia nudicaulis Roth. – J. Indian Bot. Soc. 48: 166-173.
Farrell BD, Mitter C. 1998. The timing of insect/plant diversification: Might Tetraopes (Coleoptera: Cerambycidae) and Asclepias (Asclepiadaceae) have coevolved? – Biol. J. Linn. Soc. 63: 553-577.
Favarger C. 1949. Contribution à l’étude caryologique et biologique des Gentianacées. – Bull. Soc. Bot. Suisse 59: 62-86.
Favarger C. 1952. Contribution à l’étude caryologique et biologique des Gentianacées II. – Bull. Soc. Bot. Suisse 62: 244-257.
Favarger C. 1960. Étude cytologique du Cicendia filiformis et du Microcala pusilla (Gentianaceae). – Bull. Soc. Bot. France 197: 94-98.
Favarger C. 1985. Quelques aspects de l’évolution et de la phylogénie dans la famille des Gentianaceae. – Atti dei Seminari di Fitochemica sulle piante contenenti principi amari, pp. 21-55.
Favre A, Yuan Y-M, Küpfer P, Alvarez N. 2010. Phylogeny of subtribe Gentianinae (Gentianaceae): biogeographic inferences despite limitations in temporal calibration points. – Taxon 59: 1701-1711.
Favre A, Matuszak S, Sun H, Liu E, Yuan Y-M,
Muellner-Riehl AN. 2014. Two new genera of Gentianinae (Gentianaceae): Sinogentiana
and Kuepferia supported by molecular phylogenetic evidence. – Taxon
63: 342-354.
Favre A, Michalak I, Chen CH, Wang JC, Pringle, JS, Matuszak S, Sun H, Yuan YM, Struwe L, Muellner-Riehl AN. 2016. Out-of-Tibet: the spatio-temporal evolution of Gentiana (Gentianaceae). – J. Biogeogr. 43: 1967-1978.
Fay MF, Bremer B, Prance GT, Bank M van der, Bridson D, Chase MW. 2000. Plastid rbcL sequences show Dialypetalanthus to be a member of Rubiaceae. – Kew Bull. 55: 853-864.
Feng C-L, Gong M-F, Zeng Y-B, Dai H-F, Mei W-L. 2010. Scyphiphin C, a new iridoid from Scyphiphora hydrophyllacea. – Molecules 15: 2473-2477.
Ferm J, Kårehed J, Bremer B, Razafimandimbison SG. 2016. Paracarphalea, a new genus of the coffee family segregated from the Malagasy endemic genus Carphalea (Rubiaceae, Rubioideae, Knoxieae). – Phytotaxa 263: 98-112.
Fernandes R. 1965. Duas variedades novas de Centaurium spicatum (L.) Fritsch. – Bol. Soc. Brot. 31: 15-29.
Fernández MZ. 1993-1994 [1994]. Estudio taxonómico del género Rondeletia L. s.l. (Rubiaceae) en Cuba. – Acta Bot. Hung. 38: 47-138.
Field DV. 1981. Ceropegia distincta (Asclepiadaceae) and some related species in tropical East and Southern Africa. – Kew Bull. 36: 441-450.
Field DV. 1982a. Two new African species of Ceropegia (Asclepiadaceae) and a reconsideration of C. subaphylla. – Kew Bull. 37: 305-313.
Field DV. 1982b. The identity of Odontanthera Wight (Asclepiadaceae) with notes on Glossonema Decne. and Conomitra Fenzl. – Kew Bull. 37: 341-347.
Field DV, Hall JB. 1982. Epistemma, a new genus of Periplocaceae from West Africa. – Kew Bull. 37: 117-120.
Field DV, Wood JRI. 1983. A new name for Pentatropis spiralis auctt., and the resurrection of the genus Blyttia (Asclepiadaceae). – Kew Bull. 38: 215-220.
Field DV, Friis I, Gilbert MG. 1986. A new species of Kanahia (Asclepiadaceae) with a reconsideration of the genus. – Nord. J. Bot. 6: 787-792.
Figueiredo E. 1997. A revision of Aulacocalyx (Rubiaceae-Gardenieae). – Kew Bull. 52: 637-658.
Figueiredo E. 2005. The Rubiaceae of São Tomé e Principe (Gulf of Guinea): taxonomy and conservation. – Bot. J. Linn. Soc. 149: 85-114.
Figueiredo E. 2007. The Rubiaceae of Cabinda (Angola). – Bot. J. Linn. Soc. 154: 455-495.
Figueiredo E. 2008. The Rubiaceae of Angola. – Bot. J. Linn. Soc. 156: 537-638.
Fischer E, Killmann D, Meve U. 2013. Neoschumannia gishwatiensis (Apocynaceae, Asclepiadoideae-Ceropegieae) from Gushwati Forest, Rwanda – a third and new species from a disjunct African genus. – Phytotaxa 77: 19-26.
Fishbein M. 2001. Evolutionary innovation and diversification in the flowers of Asclepiadaceae. – Ann. Missouri Bot. Gard. 88: 603-623.
Fishbein M. 2017. Taxonomic adjustments in North American Apocynaceae. – Phytologia 99: 86-88.
Fishbein M, Stevens WD. 2005. Resurrection of Seutera Reichenbach (Apocynaceae, Asclepiadoideae). – Novon 15: 531-533.
Fishbein M, Chuba D, Ellison C, Mason-Gamer RJ, Lynch SP. 2011. Phylogenetic relationships of Asclepias (Apocynaceae) estimated from non-coding cpDNA sequences. – Syst. Bot. 36: 1008-1023.
Fishbein M, Livshultz T, Straub SCK, Simões AO, Boutte J, McDonnell A, Foote A. 2018. Evolution on the backbone: Apocynaceae phylogenomics and new perspectives on growth forms, flowers, and fruits. – Amer. J. Bot. 105: 495-513.
Fishbein M, Straub SCK, Boutte J, Hansen K, Cronn RC, Liston A. 2018. Evolution at the tips: Asclepias phylogenomics and new perspectives on leaf surfaces. – Amer. J. Bot. 105: 514-524.
Fjell I. 1983. Anatomy of the xeromorphic leaves of Allamanda neriifolia, Thevetia peruviana and Vinca minor (Apocynaceae). – Nord. J. Bot. 3: 383-392.
Florence J, Lorence DH. 2000. Sertum polynesicum VI. Rubiaceae nouvelles des Îles Marquises (Polynésie française) 2. Le genre Hedyotis. – Adansonia, sér. III, 22: 223-230.
Fontella Pereira J. 1965. Contribuição ao estudo das Asclepiadaceae Brasileiras I. – Arch. Jard. Bot. Rio de Janeiro 18: 179-182.
Fontella Pereira J, Britto de Goes M. 2004. A new species of Orthosia Decne. (Apocynaceae-Asclepiadoideae) from Paraná, and a key to the species of southern Brazil. – Revista Biol. Neotrop. 1: 1-3.
Fontella Pereira J, Schwarz EA. 1981a. Estudos em Asclepiadaceae XII. Considerações sobre os generos Roulinia Decne. et Rouliniella Vail. – Bol. Mus. Bot. Municipal 45: 1-12.
Fontella Pereira J, Schwarz EA. 1981b. Estudos em Asclepiadaceae XIII. Novos sinônimos e novas combinações. – Bol. Mus. Bot. Municipal 46: 1-10.
Fontella Pereira J, Schwarz EA. 1982. Estudos em Asclepiadaceae XXV. Chave para as espécies do gênero Jobinia Fournier ocorrentes no Brasil. – Bol. Mus. Bot. Munic. 51: 1-18.
Fontella Pereira J, Valente MD. 1969. Contribuição ao estudo das Asclepiadaceae do estado do Paraná I. – Bol. Univ. Parana 22: 1-6.
Fontella Pereira J, Santos RGP, Cáceras Moral SA. 2014. Notas taxonómicas en Asclepiadoideae (Apocynaceae). – Bol. Soc. Argent. Bot. 49: 401-404.
Forster PI. 1988. Studies on the Australasian Asclepiadaceae I. Brachystelma Sims in Australia. – Nuytsia 6: 285-294.
Forster PI. 1991a. A taxonomic revision of Gymnanthere R. Br. (Asclepiadaceae: Periplocoideae) in Australia. – Aust. Syst. Bot. 4: 563-569.
Forster PI. 1991b. A taxonomic revision of Cryptostegia R. Br. (Asclepiadaceae: Periplocoideae). – Aust. Syst. Bot. 4: 571-577.
Forster PI. 1992a. A taxonomic revision of Tylophora R. Br. (Asclepiadaceae: Marsdenieae) in Australia. – Aust. Syst. Bot. 5: 29-51.
Forster PI. 1992b. A taxonomic revision of Sarcostemma R. Br. subgenus Sarcostemma (Asclepiadaceae: Asclepiadeae) in Australia. – Aust. Syst. Bot. 5: 53-70.
Forster PI. 1992c. A taxonomic revision of Heterostemma Wight & Arn. (Asclepiadaceae: Stapelieae) in Australia and the Western Pacific. – Aust. Syst. Bot. 5: 71-80.
Forster PI. 1992d. A taxonomic revision of Melodinus (Apocynaceae) in Australia. – Aust. Syst. Bot. 5: 387-400.
Forster PI. 1992e. Circumscription of Tabernaemontana pandacaqui (Apocynaceae) in Australia. – Aust. Syst. Bot. 5: 521-531.
Forster PI. 1992f. A taxonomic revision of Ichnocarpus (Apocynaceae) in Australia and Papuasia. – Aust. Syst. Bot. 5: 533-545.
Forster PI. 1992g. A taxonomic revision of Alyxia (Apocynaceae) in Australia. – Aust. Syst. Bot. 5: 547-580.
Forster PI 1992h. A taxonomic revision of Carissa (Apocynaceae) in Australia. – Aust. Syst. Bot. 5: 581-591.
Forster PI. 1992i. A taxonomic revision of Sarcolobus (Asclepiadaceae: Marsdenieae) in Fiji. – Aust. Syst. Bot. 5: 593-596.
Forster PI. 1992j. A taxonomic revision of Alstonia (Apocynaceae) in Australia. – Aust. Syst. Bot. 5: 745-760.
Forster PI. 1993a. Conspectus of Cryptolepis R. Br. (Asclepiadaceae: Periplocoideae) in Malesia. – Austrobaileya 4: 57-66.
Forster PI. 1993b. Taxonomic relationships and status of the genus Dorystephania (Asclepiadaceae: Marsdenieae) from the Philippines and Borneo. – Aust. Syst. Bot. 6: 351-357.
Forster PI. 1994. A taxonomic revision of Tylophora (Asclepiadaceae: Marsdenieae) in Papuasia. – Aust. Syst. Bot. 7: 485-505.
Forster PI. 1995a. New names and combinations in Marsdenia (Asclepiadaceae: Marsdenieae) from Asia and Malesia (excluding Papuasia). – Aust. Syst. Bot. 8: 691-701.
Forster PI. 1995b. Circumscription of Marsdenia (Asclepiadaceae: Marsdenieae), with a revision of the genus in Australia and Papuasia. – Aust. Syst. Bot. 8: 703-933.
Forster PI, Liddle DJ. 1996. Asclepiadaceae. – In: Orchard AE (ed), Flora of Australia 28, CSIRO, Melbourne, Australia, pp. 197-283.
Forster PI, Williams JB. 1996. Apocynaceae. – In: Orchard AE (ed), Flora of Australia 28, CSIRO, Melbourne, Australia, pp. 104-196.
Fosberg FR. 1940. Notes on Micronesian Rubiaceae. – Occas. Pap. Bernice Pauahi Bishop Mus. 15: 213-226.
Fosberg FR. 1964. Studies in Pacific Rubiaceae V. – Brittonia 16: 255-271.
Fosberg FR. 1982. A preliminary conspectus of the genus Leptostigma (Rubiaceae). – Acta Phytotaxon. Geobot. 33: 73-83.
Fosberg FR. 1987. The genus Trukia Kanehira (Rubiaceae). – Phytologia 62: 171-176.
Fosberg FR, Sachet M-H. 1974. A new variety of Fagraea berteriana (Gentianaceae). – Phytologia 28: 470-472.
Fosberg FR, Sachet M-H. 1980. Systematic studies in Micronesian plants. – Smithsonian Contr. Bot. 45: 1-40.
Foster CSP, Conn BJ, Henwood MJ, Ho SYW.
2014. Molecular data support Orianthera: a new genus of Australian Loganiaceae. – Telopea 16: 149-158.
Foster CSP, Ho SYW, Conn BJ, Henwood MJ.
2014. Molecular systematics and biogeography of Logania R. Br. (Loganiaceae). – Molec. Phylogen. Evol.
78: 324-333.
Franchet MA. 1899. Les Swertia et quelques autres Gentianées de la Chine. – Bull. Soc. Bot. France 46: 302-324.
Frasier CL. 2009. Evolution and systematics of the angiosperm order Gentianales with an in-depth focus on Loganiaceae and its species-rich and toxic genus Strychnos. – Ph.D. diss., Graduate School-New Brunswick, Rutgers, The State University of New Jersey.
Frasier CL, Struwe L. In press. Phylogeny of Loganiaceae (Gentianales: Asteridae) with an emphasis on pantropical Strychnos using structural alignment of ITS sequences. – Mol. Phylogen. Evol.
Frasier CL, Albert VA, Struwe L. 2008. Amazonian lowland, white sand areas as ancestral regions for South American biodiversity: biogeographic and phylogenetic patterns in Potalia (Angiospermae: Gentianaceae). – Organisms Divers. Evol. 8: 44-57.
Fries TCE. 1923. Die Swertia-Arten der afrikanischen Hochgebirge. – Notizbl. Bot. Gart. Berlin-Dahlem 77: 505-534.
Frodin DG. 2004. A note on Psychotria (Rubiaceae) in Papuasia. – Kew Bull. 59: 165.
Fukuda Y. 1988. Phyllotaxis in two species of Rubia, R. akane and R. sikkimensis. – Bot. Mag. (Tokyo) 101: 25-38.
Gadella TWJ. 1962. Some cytological observations in the Loganiaceae. – Acta Bot. Neerl. 10: 51-55.
Gadella TWJ. 1980. Cytology. – In: Leeuwenberg AJM (ed), Engler and Prantl’s ‘Die natürlichen Pflanzenfamilien’, Fam. Loganiaceae, vol. 28b(1), Duncker & Humblot, Berlin, pp. 211-237.
Gagnepain F. 1929. Un genre nouveau de Gentianacées. – Bull. Soc. Bot. France 76: 776-777.
Gagnidze R, Küpfer P, Yuan Y-M. 1992. Chromosome numbers of some Gentianaceae from the Caucasus. – Bull. Soc. Neuchâtel. Sci. Nat. 115: 47-52.
Gamalei YV, Batashev DR, Pakhomova MV. 2008. Terminal phloem structure of Rubiaceae family in the context of its phylogeny. – Bot. Žurn. 93: 1846-1862. [In Russian]
Gangopadhyay M, Chakrabarty T. 1992. A note on the status of Litosanthes Bl. (Rubiaceae). – J. Econ. Taxon. Bot. 16: 337-338.
García Kirkbride MC. 1979. Review of the neotropical Isertieae (Rubiaceae). – Brittonia 31: 313-332.
García Kirkbride MC. 1981. Duas novos tribos de Rubiaceae neotropicais. – Revta. Brasil. Bot. 4: 119-123.
Garg S. 1987. Gentianaceae of the northwest Himalaya (a revision). – Today and Tomorrow’s Printers and Publ., New Delhi.
Gargiulo R, Guacchio ED, Caputo P. 2015. Phylogenetic reconstruction of Asperula sect. Cynanchicae (Rubiaceae) reveals a mosaic of evolutionary histories. – Taxon 64: 754-769.
Ge X-J, Chiang Y-C, Chou C-H, Chian T-Y. 2002. Nested clade analysis of Dunnia sinensis (Rubiaceae), a monotypic genus from China based on organelle DNA sequences. – Conserv. Genet. 3: 351-362.
Geesink R. 1973. A synopsis of the genus Swertia (Gent.) in Malesia. – Blumea 21: 179-183.
Gentry AH. 1974. Notes on Panamanian Apocynaceae. – Ann. Missouri Bot. Gard. 61: 891-900.
Gibbons KL, Henwood MJ, Conn BJ. 2012. Phylogenetic relationships in Loganieae (Loganiaceae) inferred from nuclear ribosomal and chloroplast DNA sequence data. – Aust. Syst. Bot. 25: 331-340.
Gibbons KL, Conn BJ, Henwood MJ. 2013. Adelphacme (Loganiaceae), a new genus from south-western Australia. – Telopea 15: 37-43.
Gibbons KL, Conn BJ, Henwood MJ. 2014. The
Australasian genus Schizacme (Loganiaceae): new combinations and new
species in the New Zealand flora. – Telopea 17: 363-381.
Gielly L, Taberlet P. 1996. A phylogeny of the European gentians inferred from chloroplast trnL (UAA) intron sequences. – Bot. J. Linn. Soc. 120: 57-75.
Gielly L, Yuan Y-M, Küpfer P, Taberlet P. 1996. Molecular evolution of noncoding regions in the genus Gentiana L.: chloroplast trnL (UAA) intron versus nuclear ribosomal internal transcribed spacer sequences. – Mol. Phylogen. Evol. 5: 460-466.
Gilbert MG. 1990. A review of Caralluma R. Br. and its segregates. – Bradleya 8: 1-32.
Gilbert MG, Stevens WD, Li PT. 1995. Notes on the Asclepiadaceae of China. – Novon 5: 1-16.
Gilg C. 1939. Beiträge zur Kenntnis der Gentianaceen-Gattung Curtia Cham. & Schlecht. – Notizbl. Bot. Gart. Berlin-Dahlem 121: 66-93.
Gilg E. 1895a. Gentianaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien, IV(2), W. Engelmann, Leipzig, pp. 50-108; Gilg E. 1897. Nachträge zu IV(2), pp. 282-283.
Gilg E. 1895b. Ueber die Blüthenverhältnisse der Gentianaceengattungen Hockinia Gardn. und Halenia Borckh. – Ber. Deutsch. Bot. Ges. 13: 114-126.
Gilg E. 1896. Beiträge zur Kenntnis der Gentianaceae I. – Engl. Bot. Jahrb. Syst. 22: 301-347.
Gilg E. 1898a. Gentianaceae africanae. Beiträge zur Kenntniss der Gentianaceae. – Engl. Bot. Jahrb. Syst. 26: 86-110.
Gilg E. 1898b. Symphyllophyton. – Engl. Bot. Jahrb. Syst. Beibl. 60(25): 43.
Gilg E. 1916. Gentianaceae andinae. – Engl. Bot. Jahrb. Syst. Beibl. 118: 4-122.
Gillett JM. 1957. A revision of the North American species of Gentianella Moench. – Ann. Missouri Bot. Gard. 44: 195-269.
Gillett JM. 1959. A revision of Bartonia and Obolaria (Gentianaceae). – Rhodora 61: 43-62.
Gillett JM. 1963. The gentians of Canada, Alaska and Greenland. – Research Branch, Canadian Department of Agriculture, Ottawa.
Gilmartin AJ. 1981. Morphological variation within five angiosperm families: Asclepiadaceae, Bromeliaceae, Melastomataceae, Piperaceae, and Rubiaceae. – Syst. Bot. 6: 331-345.
Ginter A, Razafimandimbison SG, Bremer B. 2015. Phylogenetic affinities of Myrioneuron and Cyanoneuron, generic limits of the tribe Argostemmateae and description of a new Asian tribe, Cyanoneuroneae (Rubiaceae). – Taxon 64: 286-298.
Gleason HA, Wodehouse RP. 1931. Chorisepalum. – In: Gleason HA (ed), Botanical results of the Tyler-Duida expedition, Bull. Torrey Bot. Club 58: 277-506.
Good R. 1923. New tropical African Rubiaceae. – J. Bot. 61: 86.
Good R. 1952. An atlas of the Asclepiadaceae. – New Phytol. 51: 198-209.
Gopal Krishna G, Puri V. 1962. Morphology of the flower of some Gentianaceae with special reference to placentation. – Bot. Gaz. 124: 42-57.
Gottwald H. 1982. First description of the wood anatomy of Antrophora, Lepidocordia and Pteleocarpa (Boraginaceae). – IAWA Bull. n.s. 3: 161-165.
Gould KR, Struwe L. 2004. Phylogeny and evolution of Symbolanthus and Wurdackanthus (Gentianaceae – Helieae) in the Guayana Higlands and Andes, based on ribosomal 5S-NTS sequences. – Ann. Missouri Bot. Gard. 91: 438-446.
Govaerts R, Andersson L, Robbrecht E, Bridson D, Davis AP, Schanzer I, Sonké B. 2006. World checklist of Rubiaceae. – The Board of Trustees of the Royal Botanic Gardens, Kew. http://www.kew.org/wcsp/rubiaceae
Goyder DJ. 1992. Secamone (Asclepiadaceae subfam. Secamonoideae) in Africa. – Kew Bull. 47: 437-474.
Goyder DJ. 1993. Two new species of Asclepiadaceae from NE Brazil. – Kew Bull. 48: 21-24.
Goyder DJ. 1994. A revision of Anisopus N. E. Br. (Asclepiadaceae: Marsdenieae). – Kew Bull. 49: 737-747.
Goyder DJ. 1995. Notes on the African genus Glossostelma (Asclepiadaceae). – Kew Bull. 50: 527-555.
Goyder DJ. 1998a. A revision of Pachycarpus E. Mey. (Asclepiadaceae: Asclepiadeae) in tropical Africa with notes on the genus in Southern Africa. – Kew Bull. 53: 335-374.
Goyder DJ. 1998b. A revision of the African genus Stathmostelma K. Schum. (Apocynaceae: Asclepiadeae). – Kew Bull. 53: 577-616.
Goyder DJ. 1998c. Additional notes on Pachycarpus E. mey. in tropical Africa. – Kew Bull. 53: 889.
Goyder DJ. 2001a. A revision of the tropical African genus Trachycalymma (K. Schum.) Bullock (Apocynaceae: Asclepiadoideae). – Kew Bull. 56: 129-161.
Goyder DJ. 2001b. The identity of Hickenia Lillo (Apocynaceae subfam. Asclepiadoideae). – Kew Bull. 56: 162.
Goyder DJ. 2001c. Gomphocarpus (Apocynaceae: Asclepiadeae) in an African and a global context – an outline of the problem. – Biol. Skrifter 54: 55-62.
Goyder DJ. 2003. A synopsis of Morrenia Lindl. (Apocynaceae subfam. Asclepiadoideae). – Kew Bull. 58: 713-721.
Goyder DJ. 2004a. The identities of Corollonema Schltr., Dactylostelma Schltr. and Metoxypetalum Morillo (Apocynaceae: Aslepiadoideae). – Kew Bull. 59: 301-303.
Goyder DJ. 2004b. Key to the species of Gomphocarpus (Apocynaceae: Asclepiadeae): a correction. – Kew Bull. 59: 304.
Goyder DJ. 2004c. An amplified concept of Philibertia Kunth (Apocynaceae: Asclepiadoideae), with a synopsis of the genus. – Kew Bull. 59: 415-451.
Goyder DJ. 2006. A revision of the genus Pergularia L. (Apocynaceae – Asclepiadoideae). – Kew Bull. 61: 245-256.
Goyder DJ. 2008 [2009]. Nomenclatural changes resulting from the transfer of tropical African Sarcostemma to Cynanchum (Apocynaceae: Asclepiadoideae). – Kew Bull. 63: 471-472.
Goyder DJ. 2009. A synopsis of Asclepias (Apocynaceae: Asclepiadoideae) in tropical Africa. – Kew Bull. 64: 369-399.
Goyder DJ, Fontella-Pereira J. 2005. Notes on Oxypetalum R. Br. (Apocynaceae: Asclepiadoideae) in Bolivia and Peru. – Kew Bull. 60: 95-101.
Goyder DJ, Liede-Schumann S. 2008. Notes on Cynanchum and Pentarrhinum (Apocynaceae: Asclepiadoideae) in tropical Africa. – Kew Bull. 63: 463-466.
Goyder DJ, Nicholas A. 2001. A revision of Gomphocarpus R. Br. (Apocynaceae: Asclepiadeae). – Kew Bull. 56: 769-836.
Goyder DJ, Nicholas A, Liede-Schumann S. 2007. Phylogenetic relationships in subtribe Asclepiadinae (Apocynaceae: Asclepiadoideae). – Ann. Missouri Bot. Gard. 94: 423-434.
Graham A. 1984. Lisianthius pollen from the Eocene of Panama. – Ann. Missouri Bot. Gard. 71: 987-993.
Graham A. 2009. Fossil record of the Rubiaceae. – Ann. Missouri Bot. Gard. 96: 90-108.
Grant JR. 2009. A revision of Neotropical Bonyunia (Loganiaceae: Antonieae). – Ann. Missouri Bot. Gard. 96: 541-563.
Grant JR, Struwe L. 2001. Macrocarpaeae Grisebach (Gentianaceae) Species Novae seu notabiles Neotropicae I: an introduction to the genus Macrocarpaea and three new species from Colombia, Ecuador, and Guyana. – Harvard Pap. Bot. 5: 521-530.
Grant JR, Weaver RE. 2003. De Macrocarpaeae Grisebach (ex Gentianaceis) speciebus novis IV. Eleven new species of Macrocarpaea (Gentianaceae: Helieae) from Central and South America, and the first report of stipules in the family. – Harvard Pap. Bot. 8: 83-109.
Grant JR, Maas PJM, Struwe L. 2006. Yanomamua araca (Gentianaceae), a new genus and species from Serra do Araça, an outlier of the Guayana Region in Amazonas State, Brazil. – Harvard Pap. Bot. 11: 29-37.
Gravel E, Poupon E. 2010. Biosynthesis and biomimetic synthesis of alkaloids isolated from plants of the Nitraria and Myrioneuron genera: an unusual lysine-based metabolism. – Nat. Prod. Rep. 27: 32-56.
Greimler J. 2008. Patterns of evolution and speciation in Gentianella (Gentianaceae). – South Afr. J. Bot. 74: 368.
Greimler J, Hermanowski B, Jang C-G. 2004. A re-evaluation of morphological characters in European Gentianella section Gentianella (Gentianaceae). – Plant Syst. Evol. 248: 143-169.
Greimler J, Park J-M, Schneeweiss H. 2011. Gentianella (Gentianaceae): a model taxon for evolution in the Alps. – Taxon 60: 427-435.
Greuter W, Rankin R. 2008. Bisgoeppertia (Gentianaceae) unravelled. Account of a small genus of the Greater Antilles. – Willdenowia 38: 177-185.
Grice AC. 1996. Seed production, dispersal and germination in Cryptostegia grandiflora and Ziziphus mauritiana, two invasive shrubs in tropical woodlands in northern Australia. – Aust. J. Ecol. 21: 324-331.
Groeninckx I. 2005. Zoektocht naar de taxonomische positie van Mitrasacmopsis (Rubiaceae) op basis van moleculaire en morfologische data. – Lic. thesis, Katolieke Universiteit Leuven, Belgium.
Groeninckx I. 2009. Integrated study of the herbaceous Spermacoceae (Rubiaceae): systematics and evolution. – Ph.D. diss., Kon. Universiteit Leuven, Belgium.
Groeninckx I, Vrijdaghs A, Huysmans S, Smets E, Dessein S. 2007. Floral ontogeny of the Afro-Madagascan genus Mitrasacmopsis with comments on the development of superior ovaries in Rubiaceae. – Ann. Bot. 100: 41-49.
Groeninckx I, Block P de, Rakotonasolo F, Smets E, Dessein S. 2009. Rediscovery of Malagasy Lathraeocarpa allows determination of its taxonomic position within Rubiaceae. – Taxon 58: 209-226.
Groeninckx I, Dessein S, Ochoterena H, Persson C, Motley TJ, Kårehed J, Bremer B, Huysmans S, Smets E. 2009. Phylogeny of the herbaceous tribe Spermacoceae (Rubiaceae) based on plastid DNA data. – Ann. Missouri Bot. Gard. 96: 109-132.
Groeninckx I, Ochoterena H, Smets E, Dessein S. 2010. Molecular phylogenetic and morphological study of Kohautia (Spermacoceae, Rubiaceae), with the recognition of the new genus Cordylostigma. – Taxon 59: 1457-1471.
Groeninckx I, Briggs M, Davis A, De Block P, Robbrecht E, Smets E, Dessein S. 2010. A new herbaceous genus endemic to Madagascar: Phialiphora (Spermacoceae, Rubiaceae). – Taxon 59: 1815-1829.
Groeninckx I, De Block P, Robbrect E, Smets EE, Dessein S. 2010. Amphistemon and Thamnoldenlandia, two new genera of Rubiaceae (Spermacoceae) endemic to Madagascar. – Bot. J. Linn. Soc. 163: 447-472.
Grothe E, Maas PJM. 1984. A scanning electron microscopy study of the seed coat structure of Curtia Chamisso and Schlechtendahl and Hockinia Gardner (Gentianaceae). – Proc. Kon. Nederl. Akad. Wetensch., ser. C, 87: 33-42.
Guédès M. 1972. Stipules et ligules vraies chez quelques Apocynacées et Asclepiadacées. – Compt. Rend. Acad. Sci. Paris, sér. D, Sci. Nat. 274: 3218-3221.
Guédès M. 1975a. Jovetia Guéd., a new genus in the Rubiaceae Ixoreae. – Phyton 17: 131-135.
Guédès M. 1975b. Intrusive hair sclereids in Jovetia (Rubiaceae). – Bot. J. Linn. Soc. 71: 141-144.
Guérin P. 1904. Recherches sur le développement et la structure anatomique du tegument séminal des Gentianacées. – J. Bot. (Morot) 18: 33-52, 83-88.
Guggisberg A, Bretagnolle F, Mansion G. 2006. Allopolyploid origin of the Mediterranean endemic, Centaurium bianoris (Gentianaceae), inferred by molecular markers. – Syst. Bot. 31: 368-379.
Guimarães EF. 1977. Revisão taxonômica do gênero Deianira Chamisso et Schlechtendal (Gentianaceae). – Arq. Jard. Bot. Rio de Janeiro 21: 46-123.
Guimarães EF, Klein VLG. 1985. Revisão taxonômica do gênero Coutoubea Aublet (Gentianaceae). – Rodriguésia (Rio de Janeiro) 37: 21-45.
Guo X, Wang R-J, Simmons MP, But PP-H, Yu J. 2013. Phylogeny of the Asian Hedyotis-Oldenlandia complex (Spermacoceae, Rubiaceae): evidence for high levels of polyphyly and the parallel evolution of diplophragmous capsules. – Mol. Phylogen. Evol. 67: 110-122.
Gustafsson CGR. 1998. The neotropical Rosenbergiodendron (Rubiaceae, Gardenieae). – Brittonia 50: 452-466.
Gustafsson CGR, Persson C. 2002. Phylogenetic relationships among species of the neotropical genus Randia (Rubiaceae, Gardenieae) inferred from molecular and morphological data. – Taxon 51: 661-674.
Haegens RMAP. 1994. Series of revisions of Apocynaceae XXXV. Revision of Cylindropsis Pierre and Vahadenia Stapf. – Bull. Jard. Bot. Nat. Belg. 63: 313-328.
Hagen KB von. 2007. Description of new taxa of Halenia Borkh. (Gentianaceae) from Colombia and Venezuela with significance for testing a key innovation hypothesis. – Organisms Divers. Evol. 7: 1-11.
Hagen KB von, Kadereit JW. 2000. Notes on the systematics and evolution of Gentiana sect. Ciminalis. – Bot. Jahrb. Syst. 122: 305-339.
Hagen KB von, Kadereit JW. 2001. The phylogeny of Gentianella (Gentianaceae) and its colonization of the southern hemisphere as revealed by nuclear and chloroplast DNA sequence variation. – Organisms Divers. Evol. 1: 61-79.
Hagen KB von, Kadereit JW. 2002. Phylogeny and flower evolution of the Swertiinae (Gentianaceae-Gentianeae): homoplasy and the principle of variable proportions. – Syst. Bot. 27: 548-572.
Hagen KB von, Kadereit JW. 2003. The diversification of Halenia (Gentianaceae): ecological opportunity versus key innovation. – Evolution 57: 2507-2518.
Hakki MI. 1980. Embryology. – In: Leeuwenberg AJM (ed), Engler and Prantl’s Die natürlichen Pflanzenfamilien, Fam. Loganiaceae, vol. 28b(1), Duncker & Humblot, Berlin, pp. 211-237.
Hakki MI. 1998. On the floral morphology and embryology of Usteria guineensis Willd. (Loganiaceae). – Bot. Jahrb. Syst. 120: 275-293.
Halford DA, Ford AJ. 2009a. Coelospermum purpureum Halford & A. J. Ford (Rubiaceae), a new species from north-east Queensland. – Austrobaileya 8: 69-76.
Halford DA, Ford AJ. 2009b. Two species of Morinda L. (Rubiaceae) from northeast Queensland. – Austrobaileya 8: 81-90.
Hallé N. 1961. Contribution à l’étude biologique et taxonomique des Mussaendeae (Rubiaceae) d’Afrique tropicale. – Adansonia, sér. II, 1: 266-298.
Hallé N. 1963. Délimitation des genres Sabicea Aubl. et Ecpoma K. Schum. en regard d’un genre nouveau: Pseudosabicea (Mussaendeae-Rubiaceae). – Adansonia, sér. II, 3: 168-177.
Hallé N. 1966. Famille des Rubiacées (1re partie). – In: Aubreville A (ed), Flore du Gabon 12, Muséum National d’Histoire Naturelle, Paris.
Hallé N. 1967. Étude biologique et morphologique de la tribu des Gardéniées (Rubiacées). – Mém. O.R.S.T.O.M. 22: 1-146.
Hallé N. 1970. Famille des Rubiacées (2e partie). – In: Aubreville A, Leroy JF (ed), Flore du Gabon 17, Muséum National d’Histoire Naturelle, Paris.
Hallé N. 1971. Rubiaceae gabonaises nouvelles du genre Pseudosabicea. – Adansonia, sér. 2, 11: 313-317.
Ham R van der, Zimmermann Y-M, Nilsson S, Igersheim A. 2001. Pollen morphology and phylogeny of the Alyxieae (Apocynaceae). – Grana 40: 169-191.
Hamilton CW. 1989a. A revision of Mesoamerican Psychotria subgenus Psychotria (Rubiaceae) I. Introduction and species 1-16. – Ann. Missouri Bot. Gard. 76: 67-111.
Hamilton CW. 1989b. A revision of Mesoamerican Psychotria subgenus Psychotria (Rubiaceae) II. Introduction and species 17-47. – Ann. Missouri Bot. Gard. 76: 386-429.
Hamilton CW. 1989c. A revision of Mesoamerican Psychotria subgenus Psychotria (Rubiaceae) III. Introduction and species 48-61. – Ann. Missouri Bot. Gard. 76: 336-916.
Hamilton CW. 1990. Variations on a distylous theme in Mesoamerican Psychotria subgenus Psychotria (Rubiaceae). – Mem. New York Bot. Gard. 55: 62-75.
Hamon P, Grover CE, Davis AP, Rakotomalala J-J, Raharimalala NE, Albert VA, Sreenath HL, Stoffelen P, Mitchell SE, Couturon E, Hamon S, Kochko A de, Crouzillat D, Rigoreau M, Sumirat U, Akaffou S, Guyot R. 2017. Genotyping-by-sequencing provides the first well-resolved phylogeny for coffee (Coffea) and insights into the evolution of caffeine content in its species: GBS coffee phylogeny and the evolution of caffeine content. – Mol. Phylogen. Evol. 109: 351-361.
Hancock BL. 1942. Cytological and ecological notes on some species of Galium L. em. Scop. – New Phytol. XLI: 70-78.
Hansson T, El-Ghazaly G. 2000. Development and cytochemistry of pollen and tapetum in Mitriostigma axillare (Rubiaceae). – Grana 39: 65-89.
Hara H. 1975. A new species of Cotylanthera (Gentianaceae) from Philippines, with a conspectus of the genus. – J. Jap. Bot. 50: 321-328.
Harwood R, Dessein S. 2005. Australian Spermacoce (Rubiaceae: Spermacoceae) I. Northern Territory. – Aust. Syst. Bot. 18: 297-365.
Hasselberg GBE. 1937. Zur Morphologie des vegetativen Sprosses der Loganiaceen. – Symb. Bot. Upsal. 2(3): 1-170.
Havard A, Verdcourt B. 1987. A pollen survey of Tapiphyllum (Rubiaceae: Vanguerieae). – Kew Bull. 42: 605-609.
Haviland GD. 1897. A revision of the tribe Naucleeae. – Bot. J. Linn. Soc. 33: 1-94.
Hayden SMV. 1968. Systematic morphological study of New World rubiaceous seeds: Rubioideae sensu Bremekamp. – Ph.D. diss., University of St. Louis, Missouri.
Hayden SMV, Dwyer JD. 1969. Seed morphology in the tribe Morindeae (Rubiaceae). – Bull. Torrey Bot. Club 96: 794-810.
Heads MJ. 1996. Biogeography, taxonomy and evolution in the Pacific genus Coprosma (Rubiaceae). – Candollea 51: 381-405.
Heads MJ. 2018. Metapopulation vicariance in the Pacific genus Coprosma (Rubiaceae) and its Gondwanan relatives. – Aust. Syst. Bot. 30: 422-438.
Hechem V, Calviño CI, Ezcurra C. 2011. Molecular phylogeny of Diplolepis (Apocynaceae-Asclepiadoideae) and allied genera, and taxonomic implications. – Taxon 60: 638-648.
Heenan PB, Dawson MI, Bicknell. 2002. Evidence for apomictic seed formation in Coprosma waima (Rubiaceae). – New Zealand J. Bot. 40: 347-355.
Hegnauer R, Kooiman P. 1978. Die systematische Bedeutung von iridoiden Inhaltsstoffen im Rahmen von Wettstein’s Tubiflorae. – Plant. Med. (Stuttgart) 33: 1-33.
Heine H, Hallé N. 1970. Une Rubiacée des Îles Mascareignes à feuilles ornamentals: Enterospermum borbonicum. – Adansonia, sér. II, 10: 315-327.
Henderson RFJ, Guymer GP. 1985. Durringtonia (Durringtonieae), a new genus and tribe of Rubiaceae from Australia. – Kew Bull. 40: 97-107.
Hendrian. 2004. Revision of Ochrosia (Apocynaceae) in Malesia. – Blumea 49: 101-128.
Hendrian, Middleton DJ. 1999. A revision of Rauvolfia L. in Malesia. – Blumea 44: 449-470.
Hentrich H, Kaiser R, Gottsberger G. 2010. The reproductive biology of Voyria (Gentianaceae) species in French Guiana. – Taxon 59: 867-880.
Hepper FN. 1958. Sabicea Aubl. and Stipularia Beauv. (Rubiaceae-Mussaendeae) in tropical Africa. – Kew Bull. 1958: 289-294.
Hepper FN. 1960. Notes on tropical African Rubiaceae I. – Kew Bull. 1960: 253-261.
Hepper FN, Keay RWJ. 1963. 137. Rubiaceae. – In: Hepper FN (ed), Flora of west tropical Africa II, 2nd ed, Crown Agents for Oversea Governments and Administrations, Millbank, London, pp. 104-223.
Herrera CM. 1989. Seed dispersal by animals: a role in angiosperm diversification? – Amer. Natur. 133: 309-322.
Heusden ECH van. 1986. Floral anatomy of some neotropical Gentianaceae. – Proc. Kon. Nederl. Akad. Wetensch., ser. C, 89: 45-59.
Hifny Saber GH, Mahran GH, Salah Ahmed M. 1969. Thevetia nereifolia Juss. growing in Egypt III. Macro- and micromorphology of the stems and leaves. – Bull. Fac. Pharm. Cairo Univ. 8: 145-159.
Hill AW. 1908. Notes on Sebaea and Exochaenium. – Kew Bull. 8: 317-341.
Hill AW. 1913. The floral morphology of the genus Sebaea. – Ann. Bot. 27: 479-489.
Hill KD. 1988. A revision of Hoya (Asclepiadaceae) in Australia. – Telopea 3: 241-255.
Ho T-N, Liu S-W. 1980. Lomatogoniopsis T. N. Ho et S. W. Liu – a new genus of Gentianaceae. – Acta Phytotaxon. Sin. 18: 466-468.
Ho T-N, Liu S-W. 1990. The infrageneric classification of Gentiana (Gentianaceae). – Bull. Brit. Mus. (Nat. Hist.) Bot. 20: 169-192.
Ho T-N, Liu S-W. 1993. New combinations, names and taxonomic notes on Gentianella (Gentianaceae) from South America and New Zealand. – Bull. Brit. Mus. (Nat. Hist.), Bot. 23: 61-65.
Ho T-N, Liu S-W. 2001. A worldwide monograph of Gentiana. – Science Press, Beijing, New York.
Ho T-N, Xue C-Y, Wang W. 1994. The origin, dispersal and formation of the distribution pattern of Swertia L. (Gentianaceae). – Acta Phytotaxon. Sin. 32: 525-537.
Ho T-N, Liu S-W, Xi Y-Z, Ning J-C. 1994. Pollen morphology and phylogenetic analysis of Gentiana. – Cathaya 6: 93-114.
Ho T-N, Liu S-W, Lu X-F. 1996. A phylogenetic analysis of Gentiana (Gentianaceae). – Acta Phytotaxon. Sin. 34: 505-530.
Hoc P, Bravo L. 1984. Estudio palinológico sobre las especies presentes en Argentina de Spigelia, Strychnos, y Desfontainia (Loganiaceae). – Kurtziana 17: 71-89.
Hochreutiner BPG. 1928. Note sur les Centaurium d’Australie. – Candollea 3: 467-471.
Hodge WH. 1950. Notes on Peruvian cinchonas 1. – Bot. Mus. Leafl. Harvard Univ. 14: 137-155.
Holm R. 1950. The American species of Sarcostemma R. Br. (Asclepiadaceae). – Ann. Missouri Bot. Gard. 37: 477-561.
Homolle A-M. 1937. Carphalea et Dirichletia (Rubiacées). Valeur des caractères génériques et description d’espèces nouvelles. – Bull. Soc. Bot. France 83: 613-620.
Homolle A-M. 1938. Le genre Breonia de Madagascar. – Bull. Soc. Bot. France 84: 457-462.
Huang W-P, Sun H, Deng T, Razafimandimbison SG, Nie Z-L, Wen J. 2013. Molecular phylogenetics and biogeography of the eastern Asian-eastern North American disjunct Mitchella and its close relative Damnacanthus (Rubiaceae, Mitchelleae). – Bot. J. Linn. Soc. 171: 395-412.
Huber H. 1957. Revision der Gattung Ceropegia. – Mem. Soc. Brot. 12: 1-203.
Hul S. 2002. Nouvelles espèces de Crawfurdia, Tripterospermum et Gentiana (Gentianaceae) du Viêtnam. – Adansonia, sér. III, 24: 27-41.
Humbert H. 1937a. Gentianothamnus, genre nouveau des Gentianacées de Madagascar. – Compt. Rend. Acad. Sci. Paris 204: 1747-1749.
Humbert H. 1937b. Un genre nouveau de Gentianacées-Chironiinées de Madagascar. – Bull. Soc. Bot. France 84: 386-390.
Humbert H. 1963. Les Gentianacées de Madagascar. – Adansonia, sér. II, 3: 343-351.
Hungerer BK, Kadereit JW. 1998. The phylogeny and biogeography of Gentiana L. sect. Ciminalis (Adans.) Dumort.: a historical interpretation of distribution ranges in the European high mountains. – Perspect. Plant Ecol. Evol. Syst. 1: 121-135.
Huxley CR. 1978. The ant-plants Myrmecodia and Hydnophytum (Rubiaceae), and the relationships between their morphology, ant occupants, physiology and ecology. – New Phytol. 80: 231-268.
Huxley CR, Jebb MHP. 1990. New taxa in the myrmecophilous Psychotrieae (Rubiaceae. – Bull. Jard. Bot. Nat. Belg. 60: 420-421.
Huxley CR, Jebb MHP. 1991a. The tuberous epiphytes of the Rubiaceae 1: a new subtribe – the Hydnophytinae. – Blumea 36: 1-20.
Huxley CR, Jebb MHP. 1991b. The tuberous epiphytes of the Rubiaceae 2: the new genus Anthorrhiza. – Blumea 36: 21-41.
Huxley CR, Jebb MHP. 1991c. The tuberous epiphytes of the Rubiaceae 3: a revision of Myrmephytum ot include Myrmedoma. – Blumea 36: 43-52.
Huxley CR, Jebb MHP. 1993. The tuberous epiphytes of the Rubiaceae 5: a revision of Myrmecodia. – Blumea 37: 271-334.
Huxley TH. 1888. The Gentians: notes and queries. – Bot. J. Linn. Soc. 24: 101-124.
Huysmans S. 1993. De pollenmorfologie van de Coptosapelteae (Rubiaceae-Cinchonoideae). – Lic. thesis, Katolieke Universiteit de Leuven, Belgium.
Huysmans S. 1998. Palynology of the Cinchonoideae (Rubiaceae): morphology and development of pollen and orbicules. – Ph.D. diss., Katolieke Universiteit de Leuven, Belgium.
Huysmans S, Robbrecht E, Smets E. 1994. Are the genera Hallea and Mitragyna (Rubiaceae-Coptosapelteae) pollen morphologically distinct? – Blumea 39: 321-340.
Huysmans S, El-Ghazaly G, Nilsson S, Smets E. 1997. Systematic value of tapetal orbicules: a preliminary survey of the Cinchonioideae (Rubiaceae). – Can. J. Bot. 75: 815-826.
Huysmans S, Robbrecht E, Smets E. 1998. A collapsed tribe revisited: pollen morphology of the Isertieae (Cinchonoideae-Rubiaceae). – Rev. Palaeobot. Palynol. 104: 85-113.
Huysmans S, Robbrecht E, Delprete P, Smets E. 1999 [2000]. Pollen morphological support for the Catesbaeeae-Chiococceae-Exostema-complex (Rubiaceae). – Grana 38: 325-338.
Huysmans S, Dessein S, Smets E, Robbrecht E. 2003. Pollen morphology of NW European representatives confirms monophyly of Rubieae (Rubiaceae). – Rev. Palaeobot. Palynol. 127: 219-240.
Igersheim A. 1989. Beiträge zur Klärung der Gattungsabgrenzungprobleme innerhalb der Rubiaceae-Vanguerieae. – Ph.D. diss., Botanisches Institut, Vienna, Austria.
Igersheim A. 1991. Palynological investigations of Paederia L. (Rubiaceae-Paederieae). – In: Puff C (ed), The genus Paederia L. (Rubiaceae-Paederieae): a multidisciplinary study, Opera Bot. Belg. 3: 135-149.
Igersheim A. 1992. The ovary, fruit and seed development of Craterispermum (Rubiaceae). – Belg. J. Bot. 125: 101-113.
Igersheim A. 1993a. The character states of the Caribbean monotypic endemic Strumpfia (Rubiaceae). – Nord. J. Bot. 13: 545-559.
Igersheim A. 1993b. Gynoecium development in Rubiaceae-Vanguerieae, with particular reference to the “stylar head”-complex and secondary pollen presentation. – Plant Syst. Evol. 187: 175-190.
Igersheim A. 1993c. The palynology of the genus Rondeletia L. (Rubiaceae-Cinchonoideae-Rondeletieae). – Grana 32: 321-326.
Igersheim A. 1993d. The character states of the Caribbean monotypic endemic Strumpfia (Rubiaceae). – Nord. J. Bot. 13: 545-559.
Igersheim A, Robbrecht E. 1993. The character states and relationships of the Prismatomerideae (Rubiaceae-Rubioideae). Comparisons with Morinda and comments on the circumscription of the Morindeae s. str. – In: Robbrecht E (ed), Advances in Rubiaceae macrosystematics, Opera Bot. Belg. 6: 61-79.
Igersheim A, Rohrhofer U. 1993. The tribal position of Otiophora (Rubiaceae): new evidence from gynoecium structure and development. – South Afr. J. Bot. 59: 431-441.
Igersheim A, Weber M. 1993 [1994]. “Pollen bud” formation in Ophiorrhiza (Rubiaceae. – In: Robbrecht E (ed), Advances in Rubiaceae macrosystematics, Opera Bot. Belg. 6: 51-59.
Igersheim A, Puff C, Leins P, Erbar C. 1994. Gynoecial development of Gaertnera Lam. and presumably allied taxa of Psychotrieae (Rubiaceae): secondarily “superior” vs. inferior ovaries. – Bot. Jahrb. Syst. 116: 401-414.
Iltis HH. 1965. The genus Gentianopsis (Gentianaceae): transfer and phytogeographic comments. – Sida 2: 129-154.
Imbert FM, Richards JH. 1993. Protandry, incompatibility, and secondary pollen presentation in Cephalanthus occidentalis (Rubiaceae). – Amer. J. Bot. 80: 395-404.
Imhof S. 1999. Root morphology, anatomy and mycotrophy of the achlorophyllous Voyria aphylla (Jacq.) Pers. (Gentianaceae). – Mycorrhiza 9: 33-39.
Ionta GM, Judd WS. 2007. Phylogenetic relationships in Periplocoideae (Apocynaceae s.l.) and insights into the origin of pollinia. – Ann. Missouri Bot. Gard. 94: 360-375.
Jalaluddin S, Bruhl JJ. 2008. Testing species limits in Rennellia (Prismatomerideae, Rubioideae, Rubiaceae). – Taxon 57: 43-52.
Jang C-G, Mullner AN, Greimler J. 2005. Conflicting patterns of genetic and morphological variation in European Gentianella section Gentianella. – Bot. J. Linn. Soc. 148: 175-187.
Jansen ME. 1979. A revision of the genus Kajewskiella (Rubiaceae). – Blumea 25: 283-294.
Jansen S. 1994. Een houtanatomische en palynologische studie van Psychotrieae (Rubiaceae-Rubioideae). – Lic. thesis, Katolieke Universiteit Leuven, Belgium.
Jansen S, Smets E. 1998. Vestured pits in some woody Gentianaceae. – IAWA J. 19: 35-42.
Jansen S, Smets E. 2000. Morphology, distribution and systematic importance of vestures in the Gentianales. – In: Nordenstam B, El-Ghazaly G, Kassas M (eds), Plant systematics for the 21st Century, Portland Press, London, pp. 277-296.
Jansen S, Block P de, Beeckman H, Smets E. 1999. Systematic wood anatomy of the Pavetteae (Rubiaceae, Ixoroideae). – Syst. Geogr. Plants 68: 113-133.
Jansen S, Lens F, Ntore S, Piesschaert F, Robbrecht E, Smets E. 2001. Contributions to the wood anatomy of the Rubioideae (Rubiaceae). – J. Plant Res. 114: 269-289.
Jansen S, Robbrecht E, Smets E. 1996. The systematic value of endexine ornamentation in some Psychotrieae pollen (Rubiaceae-Rubioideae). – Grana 35: 129-137.
Jansen S, Robbrecht E, Beeckman H, Smets E. 1996. Gaertnera and Pagamea: genera within the Psychotrieae or constituting the tribe Gaertnereae? A wood anatomical and palynological approach. – Bot. Acta 109: 466-476.
Jansen S, De Block P, Smets E. 1997. Wood anatomy of the Pavetteae (Rubiaceae-Ixoroideae). – Scripta Bot. Belg. 15: 86.
Jansen S, Dessein S, Piesschaert F, Robbrecht E, Smets E. 2000. Aluminum accumulation in leaves of Rubiaceae: systematic and phylogenetic implications. – Ann. Bot. (Oxford) 85: 91-101.
Jansen S, Robbrecht E, Beeckman H, Smets E. 2002a. Aluminium accumulation in Rubiaceae: an additional character for the delimitation of subfamily Rubioideae? – IAWA J. 21: 197-212.
Jansen S, Robbrecht E, Beeckman H, Smets E. 2002b. A survey of the systematic wood anatomy of the Rubiaceae. – IAWA J. 23: 1-67.
Jansen S, Watanabe T, Smets E, Robbrecht E. 2003. A comparative study of metal levels in leaves of some Al-accumulating Rubiaceae. – Ann. Bot. 91: 657-663.
Janssens SB, Groeninckx I, De Block PJ, Verstraete B, Smets EF, Dessein S. 2016. Dispersing towards Madagascar: biogeography and evolution of the Madagascan endemics of the Spermacoceae tribe (Rubiaceae). – Mol. Phylogen. Evol. 95: 58-66.
Jardim JG, Zappi DC. 2008. Studies of Faramea Aubl. (Rubiaceae) in Brazil: two new species for Eastern Bahia – F. nocturna and F. biflora. – Kew Bull. 63: 131-136.
Jeanmaire M, Struwe L. 2008. Revision of ring-gentians (Symbolanthus, Gentianaceae) from Bolivia, Ecuador and Peru, with a first assessment of conservation status. – Syst. Biodiv. 6: 477-501.
Jebb MHP. 1991a. The tuberous epiphytes of the Rubiaceae 4: a revision of Squamellaria. – Blumea 36: 53-61.
Jebb MHP. 1991. Cavity structure and function in the tuberous Rubiaceae. – In: Huxley C, Cutler DF (eds), Ant-plant interactions, Oxford University Press, Oxford, pp. 374-389.
Jebb MHP. 1993. Anthorrhiza camilla – a new species of rubiaceous ant-plant. – Blumea 37: 341-344.
Jensen SR. 1992. Systematic implications of the distribution of iridoids and other compounds in the Loganiaceae and other families of the Asteridae. – Ann. Missouri Bot. Gard. 79: 284-302.
Jensen SR, Schripsema J. 2002. Chemotaxonomy and pharmacology of Gentianaceae. – In: Struwe L, Albert VA (eds), Gentianaceae – systematics and natural history, Cambridge University Press, Cambridge, pp. 573-631.
Jérémie J, Hallé N. 1976. Le genre Bikkia (Rubiaceae-Condamineeae) en Nouvelle-Calédonie. – Adansonia 15: 341-355.
Jeune B. 1980. Croissance des feuilles et stipules du Galium palustre L. subsp. elongatum (Presl) Lange et valeur phylogénique de ces données de morphogénèse. – Adansonia 19: 451-465.
Jiao Z, Li J. 2007. Phylogeny of intercontinental disjunct Gelsemiaceae inferred from chloroplast and nuclear DNA sequences. – Syst. Bot. 32: 617-627.
Joffroy G. 2001 [2002]. Le genre Sabicea (Rubiaceae) à São Tomé (São Tomé et Príncipe). – Syst. Geogr. Plants 71: 383-390.
Johansson JT. 1987a. Motleyia, a new genus of the Rubiaceae from Borneo. – Blumea 32: 149-155.
Johansson JT. 1987b. Pollen morphology of the tribe Morindeae (Rubiaceae). – Grana 26: 134-150.
Johansson JT. 1987c. Revision of the genus Prismatomeris Thw. (Rubiaceae, Morindeae). – Opera Bot. 94: 1-62.
Johansson JT. 1987d. A new species of Coelospermum (Rubiaceae) from New Caledonia. – Blumea 32: 321-322.
Johansson JT. 1988a. Revision of the genus Caelospermum Blume (Rubiaceae, Rubioideae, Morindeae). – Blumea 33: 265-297.
Johansson JT. 1988b. A new species of Gynochtodes (Rubiaceae, Rubioideae) from Australia. – Aust. Syst. Bot. 1: 369-372.
Johansson JT. 1989. Revision of the genus Rennellia Korth. (Rubiaceae-Rubioideae). – Blumea 34: 3-19.
Johansson JT. 1992. Pollen morphology in Psychotria (Rubiaceae, Rubioideae, Psychotrieae) and its taxonomic significance: a preliminary survey. – Opera Bot. 115: 1-71.
Johansson JT. 1994. The genus Morinda (Morindeae, Rubioideae, Rubiaceae) in New Caledonia: taxonomy and phylogeny. – Opera Bot. 122: 1-67.
Johansson JT, Wong K-M. 1988. The identity of Prismatomeris subsessilis King & Gamble (Rubiaceae, Rubioideae). – Blumea 33: 351-356.
Jongkind CCH. 2002. Two new species of Keetia (Rubiaceae) from West Africa. – Kew Bull. 57: 989-992.
Jonker FP. 1948. Remarks on the genera Stahelia and Tapeinostemon (Gentianaceae). – Rec. Trav. Néerl. 41: 145-149.
Jonsson L. 1974. Pollen morphology in African species of Swertia L. (Gentianaceae). – Grana 13: 119-128.
Joubert L, Venter AM. 2008. Taxonomic value of floral morphology in the genus Cryptolepis (Apocynaceae, Periplocoideae) in southern Africa. – South Afr. J. Bot. 74: 369.
Joubert L, Verhoeven RL. 2009. Leaf micromorphology and anatomy of Cryptolepis (Apocynaceae, Periplocoideae) in southern Africa. – South Afr. J. Bot. 75: 434.
Joubert L, Venter HJT, Verhoeven RL. 2008. A new species of Cryptolepis (Apocynaceae: Periplocoideae) from Kenya and Tanzania, Africa. – Bot. J. Linn. Soc. 157: 343-346.
Joubert L, Klak C, Venter AM, Venter HJT, Bruyns PV. 2016. A widespread radiation in the Periplocoideae (Apocynaceae): the case of Cryptolepis. – Taxon 65: 487-501.
Jovet P. 1941. Aux confins des Rubiacées et des Loganiacées. – Notul. Syst. (Paris) 10: 39-53.
Juárez-Jaimes V, Alvarado-Cárdenas LO, Villaseñor JL. 2007. La familia Apocynaceae sensu lato en México: diversidad y distribución. – Rev. Mex. Biodiv. 78: 459-482.
Judkevich MD, Salas RM, Gonzalez AM. 2017. Colleters in American Spermacoceae genera (Rubiaceae): morphoanatomical and evolutionary aspects. – Intern. J. Plant Sci. 178: 378-397.
Julius A, Kamin I, Kiew R, Utteridge TMA. 2013. Gardneria and Spigelia (Loganiaceae), two genera new to the Flora of Peninsular Malaysia. – Phytotaxa 129: 39-46.
Jumelle H, Perrier de la Bathie H. 1907. Les Secamone du nord-ouest de Madagascar. – Compt. Rend. Acad. Sci. paris 147: 687-689.
Jung-Mendaçolli SL. 1984. Contribuição ao estudo palinológico das Rubiaceae. – Ph.D. diss., Universidad de São Paulo, Brazil.
Jung-Mendaçolli SL, Melhem TS. 1995. Grãos de polen de especies heterostilicas de Rubiaceae. – Rev. Brasil. Bot. 18: 61-93.
Jürgens A, Dötterl S, Leide-Schumann S, Meve U. 2009. Chemical diversity of floral volatiles in Asclepiadoideae-Asclepiadeae (Apocynaceae). – Biochem. Syst. Ecol. 36: 842-852.
Kadereit JW, Bittrich V (eds). 2018. The families and genera of vascular plants XV. Flowering plants – eudicots – Apiales, Gentianales (except Rubiaceae). Springer.
Kadereit JW, Hagen KB von. 2003. The evolution of flower morphology in Gentianaceae-Swertiinae and the roles of key innovations and niche width for the diversification of Gentianella and Halenia in South America. – Intern. J. Plant Sci. 164(Suppl.): S441-S452.
Kainulainen K, Bremer B. 2014. Phylogeny of
Euclinia and allied genera of Gardenieae (Rubiaceae), and description of
Melanoxerus, an endemic genus of Madagascar. – Taxon 63: 819-830.
Kainulainen K, Mouly A, Khodabandeh A, Bremer B. 2009. Molecular phylogenetic analysis of the tribe Alberteae (Rubiaceae), with description of a new genus, Razafimandimbisonia. – Taxon 58: 757-768.
Kainulainen K, Persson C, Eriksson T, Bremer B. 2010. Molecular systematics and morphological character evolution of the Condamineeae (Rubiaceae). – Amer. J. Bot. 97: 1961-1981.
Kainulainen K, Razafimandimbison SG, Bremer B. 2013. Phylogenetic relationships and new tribal delimitations in subfamily Ixoroideae (Rubiaceae). – Bot. J. Linn. Soc. 173: 387-406.
Kambale SS, Surveswaran S, Yadav SR. 2014.
Two new species of Brachystelma Sims (Apocynaceae:
Asclepiadoideae-Ceropegieae) from the Western Ghats of India. – Kew Bull. 69:
9493.
Kapadia GJ, ShuklaYN, Bose AK, Fujiwara H, Lloyd HA. 1979. Revised structure of paederoside, a novel monoterpene S-methyl thiocarabonate.. – Tetrahedron Lett. 20: 1937-1938.
Kapoor SL, Sharma PC, Chandra V, Kapoor LD. 1969. Epidermal and venation studies in Apocynaceae II. – Bull. Bot. Surv. India 11: 372-376.
Kårehed J, Bremer B. 2007. The systematics of Knoxieae (Rubiaceae) – molecular data and their taxonomic consequences. – Taxon 56: 1051-1076.
Kårehed J, Groeninckx I, Dessein S, Motley TJ, Bremer B. 2008. The phylogenetic utility of chloroplast and nuclear DNA markers and the phylogeny of the Rubiaceae tribe Spermacoceae. – Mol. Phylogen. Evol. 49: 843-866.
Karpaati Z. 1970. Eine kritische-taxonomische Übersicht der Gattung Swertia in Europa. – Fragm. Florist. Geobot. 16: 53-60.
Keay RWJ. 1958. Randia and Gardenia in West Africa. – Bull. Jard. Bot. État, Bruxelles 58: 15-72.
Keddam-Malplanche M. 1980. Étude palynologique comparative des espèces lianescentes dans les genres Sherbournia et Porterandia (Rubiacées-Gardéniées). – Adansonia, sér. II, 19: 429-434.
Keddam-Malplanche M. 1985. Le pollen et les stomates des Gardeniées (Rubiacée) du Gabon. Morphologie et tendances évolutives. – Mém. Mus. Natl. Hist. Nat. Paris, Nouv. Sér., sér. B, 29: 1-109.
Kern JH, Steenis CGGJ van. 1951. Caprifoliaceae. – In: Steenis CGGJ van (ed), Flora Malesiana I, 4(3), Noordhoff-Kolff N. V., Batavia, pp. 175-194.
Kers LE. 1969. New and little known stapeliads from Southern Angola. – Bot. Not. 122: 172-182.
Khan SA. 2007. New delimitations and phylogenetic relationships of Sabiceeae (Ixoroideae, Rubiaceae) and revision of the Neotropical species of Sabicea Aubl. – Ph.D. diss., Universität Bayreuth, Germany.
Khan SA, Razafimandimbison SG, Bremer B, Liede-Schumann S. 2008a. Sabiceeae and Virectarieae (Rubiaceae, Ixoroideae): one or two tribes? New tribal and generic circumscriptions of Sabiceeae and biogeography of Sabicea s.l. – Taxon 57: 7-23.
Khan SA, Razafimandimbison SG, Bremer B, Liede-Schumann S. 2008b. Phylogeny and biogeography of the African genus Virectaria Bremek. (Sabiceeae s.l., Ixoroideae, Rubiaceae). – Plant Syst. Evol. 275: 43-58.
Khanum R, Surveswaran S, Meve U, Liede-Schumann S. 2016. Cynanchum (Apocynaceae: Asclepiadoideae): a pantropical asclepiadoid genus revisited. – Taxon 65: 467-486.
Khoshoo TN, Tandon SR. 1963. Cytological, morphological and pollination studies on some Himalayan species of Swertia. – Caryologia (Pisa) 16: 445-477.
Kiehn M. 1985. Karyosystematische Untersuchungen an Rubiaceae: Chromosomenzählungen aus Afrika, Madagaskar und Mauritius. – Plant Syst. Evol. 149: 89-118.
Kiehn M. 1986a. Karyosystematic studies on Rubiaceae: chromosome counts from Sri Lanka. – Plant Syst. Evol. 154: 213-223.
Kiehn M. 1986b. Karyologische Untersuchungen und DNA-Messungen an Rubiaceae und ihre Bedeutung für die Systematik dieser Familie. – Ph.D. diss., Formal- und Naturwissenschaftliche Fakultät, Universität Wien, Austria.
Kiehn M. 1995. Chromosome survey of the Rubiaceae. – Ann. Missouri Bot. Gard. 82: 398-408.
Kiehn M. 1996. Chromosomes of Rubiaceae occurring in Malesia, the Philippines, New Guinea, and the Pacific. – Opera Bot. Belg. 7: 249-260.
Kiehn M. 2010. Chromosomes of neotropical Rubiaceae I: Rubioideae. – Ann. Missouri Bot. Gard. 97: 91-105.
Kirkbride Jr JH. 1969. A revision of the Panamanian species of Rondeletia (Rubiaceae). – Ann. Missouri Bot. Gard. 55: 372-391.
Kirkbride Jr JH. 1976. A revision of the genus Declieuxia (Rubiaceae). – Mem. New York Bot. Gard. 28: 1-87.
Kirkbride Jr JH. 1979a. Revision of the genus Psyllocarpus (Rubiaceae). – Smithsonian Contr. Bot. 41: 1-32.
Kirkbride Jr JH. 1979b. Raritebe, an overlooked genus of the Rubiaceae. – Brittonia 31: 299-312.
Kirkbride Jr JH. 1984a. Manipulus rubiacearum III. Deppeeae, a new tribe of Rubioideae (Rubiaceae). – Brittonia 36: 317-320.
Kirkbride Jr JH. 1984b. Review of Omiltemia (Rubiaceae). – Syst. Bot. 9: 410-414.
Kirkbride Jr JH. 1985. Manipulus rubiacearum IV. Kerianthera (Rubiaceae), a new genus from Amazonian Brazil. – Brittonia 37: 109-116.
Kirkbride Jr JH. 1997. Manipulus rubiacearum VI. – Brittonia 49: 354-379.
Kirkbride Jr JH, Delprete PG. 2015. New combinations in Hexasepalum (Rubiaceae: Spermacoceae). – J. Bot. Res. Inst. Texas 9: 103-106.
Kirkbride Jr JH, Robbrecht E. 1984. Documentation of two new generic names in the Rubiaceae. – Taxon 33: 102-105.
Kirkbride MCG. 1979. Review of the neotropical Isertieae (Rubiaceae). – Brittonia 31: 313-332.
Kirkbride MCG. 1982. A preliminary phylogeny for Neotropical Rubiaceae. – Plant Syst. Evol. 141: 115-121.
Kisakürek MV, Hesse M. 1980. Chemotaxonomic studies of the Apocynaceae, Loganiaceae, and Rubiaceae with reference to indole alkaloids. – In: Phillipson JD, Zenk MH (eds), Indole and biogenetically related alkaloids, Academic Press, London, pp. 11-26.
Kisakürek MV, Leeuwenberg AJM, Hesse M. 1983. A chemotaxonomic investigation of the plant families of Apocynaceae, Loganiaceae, and Rubiaceae by their indole alkaloid content. – In: Pelletier SW (ed), Alkaloids, chemical and biological perspectives 1, John Wiley and Sons, New York, pp. 211-376.
Kissling J. 2007. Phylogenetics of tribe Exaceae (Gentianaceae) based on molecular, morphological and karyological data, with special emphasis on the genus Sebaea. Taxonomic treatment of Exochaenium, Lagenias and the new genus Klackenbergia. – Ph.D. diss., Université de Neuchâtel, Neuchâtel, Switzerland.
Kissling J. 2012. Taxonomy of Exochaenium and Lagenias: two resurrected genera of tribe Exaceae (Gentianaceae). – Syst. Bot. 37: 238-253.
Kissling J, Zeltner L, Kupfer P, Mansion G. 2008. Cytogeography of Gentianaceae-Exaceae in Africa, with a special focus on Sebaea: the possible role of dysploidy and polyploidy in the evolution of the tribe. – Bot. J. Linn. Soc. 158: 556-566.
Kissling J, Yuan Y-M, Kupfer P, Mansion G. 2009. The polyphyletic genus Sebaea (Gentianaceae): a step forward in understanding the morphological and karyological evolution of the Exaceae. – Mol. Phylogen. Evol. 53: 734-748.
Kissling J, Buerki S, Mansion G. 2009. Klackenbergia (Gentianaceae-Exaceae), a new endemic genus from Madagascar. – Taxon 58: 907-912.
Kissling J, Endress PK, Bernasconi G. 2010. Ancestral and monophyletic presence of diplostigmaty in Sebaea (Gentianaceae) and its potential role as a morphological mixed mating strategy. – New Phytol. 184: 303-310.
Klackenberg J. 1983. A reevaluation of the genus Exacum (Gentianaceae) in Ceylon. – Nord. J. Bot. 3: 355-370.
Klackenberg J. 1985. The genus Exacum (Gentianaceae). – Opera Bot. 84: 1-144.
Klackenberg J. 1986. The new genus Ornichia (Gentianaceae) from Madagascar. – Bull. Mus. Natl. Hist. Nat. Paris, sér. IV, sect. B, Adansonia 8: 195-206.
Klackenberg J. 1987a. Revision of the genus Tachiadenus (Gentianaceae). – Bull. Mus. Natl. Hist. Nat. Paris, sér. IV, sect. B, Adansonia 9: 43-80.
Klackenberg J. 1987b. A new species of Sebaea (Gentianaceae) from Madgascar. – Bull. Mus. Natl. Hist. Nat. Paris, sér. IV, sect. B, Adansonia 9: 133-136.
Klackenberg J. 1990. Famille 168. Gentianacées. Famille 168 bis. Menyanthacées. – In: Morat P (ed), Flore de Madagascar et des Comores, Association de Botanique Tropicale, Paris, pp. 1-185.
Klackenberg J. 1992a. Taxonomy of Secamone (Asclepiadaceae) in Asia and Australia. – Kew Bull. 47: 595-612.
Klackenberg J. 1992b. Taxonomy of Secamone s. lat. (Asclepiadaceae) in the Madagascar Region. – Opera Bot. 112: 1-127.
Klackenberg J. 1995a. Malagasy Asclepiadaceae: reinstatement of the genus Pervillea and two new combinations. – Phytologia 78: 189-191.
Klackenberg J. 1995b. Taxonomy and phylogeny of the SE Asian genus Genianthus (Asclepiadaceae). – Bot. Jahrb. Syst. 117: 401-467.
Klackenberg J. 1996a. Revision of the Malagasy genus Pervillea (Asclepiadaceae) and its phylogenetic relationship to Calyptranthera. – Nord. J. Bot. 16: 165-184.
Klackenberg J. 1996b. The new genus Calyptranthera (Asclepiadaceae) from Madagascar. – Novon 6: 25-27.
Klackenberg J. 1997a. Revision of the Malagasy genus Calyptranthera (Asclepiadaceae). – Adansonia, sér. III, 19: 21-37.
Klackenberg J. 1997b. Revision of the genus Baroniella Costantin & Gallaud (Asclepiadaceae, Periplocoidea). – Candollea 52: 383-407.
Klackenberg J. 1998. Taxonomy and phylogeny of the genus Camptocarpus s.l. (Periplocoideae, Asclepiadaceae). – Bot. Jahrb. Syst. 120: 45-85.
Klackenberg J. 1999. Revision of the Malagasy genera Pentopetia and Ischnolepis (Apocynaceae s.l., Periplocoideae). – Candollea 54: 257-339.
Klackenberg J. 2000a. Secamone brevicoronata and S. pedicellaris (Apocynaceae), two new species from Madagascar. – Willdenowia 30: 45-51.
Klackenberg J. 2000b. Two new species of Secamone (Apocynaceae, Secamoneae) from Madgascar. – Novon 10: 215-219.
Klackenberg J. 2000c. Novelties in Secamone (Apocynaceae s.l., Secamonoideae) from western Madagascar. – Candollea 55: 75-86.
Klackenberg J. 2001a. Revision of the genus Cryptostegia R. Br. (Apocynaceae, Periplocoideae). – Adansonia, sér. III, 23: 205-218.
Klackenberg J. 2001b. Notes on Secamonoideae (Apocynaceae) in Africa. – Adansonia, sér. III, 23: 317-335.
Klackenberg J. 2002a. Secamone rubra (Apocynaceae, Secamonoideae), a new species from Madagascar. – Bot. Jahrb. Syst. 124: 49-54.
Klackenberg J. 2002b. Tribe Exaceae. – In: Struwe L, Albert VA (eds), Gentianaceae – systematics and natural history, Cambridge University Press, Cambridge, pp. 66-108.
Klackenberg J. 2003. Secamone galinae (Apocynaceae), a new species from Madagascar. – Bot. Jahrb. Syst. 124: 421-425.
Klackenberg J. 2005. Pervillaea brevirostris Klack. (Apocynaceae, Secamonoideae), a new species from Mauritius. – Bot. Jahrb. Syst. 126: 203-209.
Klackenberg J. 2006. Cotylanthera transferred to Exacum (Gentianaceae). – Bot. Jahrb. Syst. 126: 477-481.
Klackenberg J. 2007a. Three new species of Calyptranthera (Apocynaceae, Secamonoideae) from Madagascar. – Adansonia, sér. III, 29: 113-121.
Klackenberg J. 2007b. New species of Baroniella and Pentopetia (Apocynaceae) from Madagascar. – Candollea 62: 231-235.
Klein DE, Gomes VM, Silva-Neto SJ da, Cunha M da. 2004. The structure of colleters in several species of Simira (Rubiaceae). – Ann. Bot. 94: 733-740.
Klett W. 1924. Umfang und Inhalt der Familie der Loganiaceen. – Bot. Archiv 5: 312-338.
Kliphuis E. 1962. Cytotaxonomical studies in the genus Galium. – Proc. Kon. Nederl. Akad. Wetensch., Ser. C, 65: 279-285.
Kliphuis E. 1967. Cytotaxonomic notes on some Galium species. – Acta Bot. Neerl. 15: 535-538.
Kliphuis E. 1983. Cytotaxonomic notes on some species of the genus Galium L. (Rubiaceae) collected in the north-western parts of Spain. – Lagascalia 11: 229-244.
Kliphuis E. 1984. Cytotaxonomic studies on the genus Galium L. Notes on some species collected in Portugal. – Mem. Soc. Brot. 27: 77-78.
Kloppenburg D. 1990. New Philippine Hoya species. – Fraterna 3 [Suppl.]: I-IV.
Knoblauch E. 1894. Beiträge zur Kenntniss der Gentianaceae. – Bot. Centralbl. 60: 321-334, 353-363, 385-401.
Knoblauch E. 1895. Ueber die dimorphen Blüthen von Hockinia montana und die Variabilität der Blüthenmerkmale bei den Gentianaceen. – Ber. Deutsch. Bot. Ges. 13: 289-298.
Koch I, Bittrich V, Kinoshita LS. 2002. Reproductive biology and functional aspects of the floral morphology of Rauvolfia sellowii Müll. Arg. (Apocynaceae; Rauvolfioideae) – a report of dioecy in Apocynaceae. – Bot. Jahrb. Syst. 124: 83-104.
Kock D de, Meve U. 2007. Stapeliad Checklist. A guide to alternative names used in recent stapeliad classification (Apocynaceae: Asclepiadoideae: Ceropegieae). – International Asclepiad Society.
Kocsis M, Borhidi A. 2003. Petiole anatomy of some Rubiaceae genera. – Acta Bot. Hung. 45: 345-353.
Kocsis M, Darók J, Borhidi A. 2004. Comparative leaf anatomy and morphology of some neotropical Rondeletia (Rubiaceae) species. – Plant Syst. Evol. 248: 205-218.
Koek-Noorman J. 1969a. A contribution to the wood anatomy of South American (chiefly Suriname) Rubiaceae I. – Acta Bot. Neerl. 18: 108-123.
Koek-Noorman J. 1969b. A contribution to the wood anatomy of South American (chefly Suriname) Rubiaceae II. – Acta Bot. Neerl. 18: 377-395.
Koek-Noorman J. 1970. A contribution to the wood anatomy of the Cinchoneae, Coptosapelteae, and Naucleeae (Rubiaceae). – Acta Bot. Neerl. 19: 154-164.
Koek-Noorman J. 1972. The wood anatomy of Gardenieae, Ixoreae, and Mussaendeae (Rubiaceae). – Acta Bot. Neerl. 21: 301-320.
Koek-Noorman J. 1977. Systematische Holzanatomie einiger Rubiaceen. – Ber. Deutsch. Bot. Ges. 90: 183-190.
Koek-Noorman J, Hogeweg P. 1974. The wood anatomy of Vanguerieae, Cinchoneae, Condamineeae, and Rondeletieae (Rubiaceae). – Acta Bot. Neerl. 23: 627-653.
Koek-Noorman J, Puff C. 1983. The wood anatomy of Rubiaceae tribes Anthospermeae and Paederieae. – Plant Syst. Evol. 174: 17-45.
Köhlein F. 1991. Gentians. – Timber Press, Portland, Oregon.
Köhler A. 1905. Der systematische Wert der Pollenbeschaffenkeit bei den Gentianaceen. – Ph.D. diss., Mitt. Bot. Mus. Zürich 25.
Kokubugata G, Matsumoto S, Kadota Y. 1998. Karyomorphological study of Nertera yamashitae (Rubiaceae) from Amami Island. – Ann. Tsukuba Bot. Gard. 17: 133-137.
Konno TUP, Pereira JF. 2004. Some nomenclatural and taxonomic notes on Brazilian Ditassa (Apocynaceae: Asclepiadoideae). – Kew Bull. 59: 297-300.
Konno TUP, Rapini A, Goyder DJ, Chase MW. 2006. The new genus Minaria (Asclepiadoideae, Apocynaceae). – Taxon 55: 421-430.
Kooiman P. 1969. The occurrence of asperulosidic glycosides in the Rubiaceae. – Acta Bot. Neerl. 18: 124-137.
Koroma L, Ita BN. 2009. Phytochemical compounds and antimicrobial activity of three medicinal plants (Alchornea hirtella, Morinda geminata and Craterispermum laurinum) from Sierra Leone. – Afr. J. Biotechn. 8: 6397-6401.
Krendl F. 1988. Die Arten der Galium mollugo-Gruppe in Griechenland. – Bot. Chron. 6-7: 5-170.
Krings A. 2005. Notes on the Matelea bayatensis-correllii-tigrina complex (Apocynaceae-Asclepiadoideae-Gonolobinae) in the Greater Antilles and Bahamas. – Sida 21: 1525-1533.
Krings A. 2006. Four novelties and a lectotypification in Matelea (Apocynaceae, Asclepiadoideae) from Hispaniola. – Sida 22: 941-953.
Krings A. 2007. Novelties in Gonolobus (Apocynaceae: Asclepiadoideae) from the Lesser Antilles. – Syst. Bot. 32: 180-194.
Krings A. 2009. A new species in the Ibatia species complex in Matelea s.l. (Apocynaceae: Asclepiadoideae: Asclepiadeae) from Colombia. – Syst. Bot. 34: 429-433.
Krings A, Saville AC. 2007. Two new species and three lectotypifications in the Ibatia-Matelea complex (Apocynaceae: Asclepiadoideae) from the northern South America. – Syst. Bot. 32: 862-871.
Krings A, Xiang Q-Y. 2004. The Gonolobus complex (Apocynaceae-Asclepiadoideae) in the southeastern United States. – Sida 21: 103-116.
Krings A, Xiang Q-Y. 2005. Taxonomy of the Gonolobus complex (Apocynaceae-Asclepiadoideae) in the southeastern US: ISSR evidence and parsimony analysis. – Harvard Pap. Bot. 10: 147-159.
Krings A, Thomas DT, Xiang Q-Y. 2008. On the generic circumscription of Gonolobus (Apocynaceae, Asclepiadoideae): evidence from molecules and morphology. – Syst. Bot. 33: 403-415.
Krishna GG, Puri V. 1962. Morphology of the flower of some Gentianaceae with special reference to placentation. – Bot. Gaz. 124: 42-57.
Krüger Å, Razafimandimbison SG, Bremer B. 2012. Molecular phylogeny of the tribe Danaideae (Rubiaceae: Rubioideae): another example of out-of-Madagascar dispersal. – Taxon 61: 629-636.
Kuhlmann JG. 1942. Rubiaceae. Dialypetalanthus, Kuhlmann. – Rodriguésia 6: 25-26.
Kuijt J, Van Der Ham RWJM. 1997. Pollen morphology of Alstonia (Apocynaceae). – Grana 36: 96-104.
Kunze H. 1982. Morphogenese und Synorganisation des Bestäubungsapparates einiger Asclepiadaceen. – Beitr. Biol. Pflanzen 56: 133-170.
Kunze H. 1990. Morphology and evolution of the corona in Asclepiadaceae and related families. – Trop. Subtrop. Pflanzenwelt 76: 1-51.
Kunze H. 1991. Structure and function in asclepiad pollination. – Plant Syst. Evol. 176: 227-253.
Kunze H. 1993. Evolution of the translator in Periplocaceae and Asclepiadaceae. – Plant Syst. Evol. 185: 99-122.
Kunze H. 1995. Floral morphology of some Gonolobeae (Asclepiadaceae). – Bot. Jahrb. Syst. 117: 211-238.
Kunze H. 1996. Morphology of the stamen in the Asclepiadaceae and its systematic significance. – Bot. Jahrb. Syst. 118: 547-579.
Kunze H. 1997. Corona and nectar system in Asclepiadinae (Asclepiadaceae). – Flora 192: 175-183.
Kunze H. 2005. Morphology and evolution of the corolla and corona in the Apocynaceae s.l. – Bot. Jahrb. Syst. 126: 347-383.
Kunze H, Liede S. 1991. Observations on pollination in Sarcostemma (Asclepiadaceae). – Plant Syst. Evol. 178: 95-105.
Kunze H, Wanntorp L. 2008a. Corona and anther skirt in Hoya (Apocynaceae, Marsdenieae). – Plant Syst. Evol. 271: 9-17.
Kunze H, Wanntorp L. 2008b. The gynostegium of Hoya spartioides (Apocynaceae-Asclepiadoideae): a striking case of incongruence between molecular and phenotypic evolution. – Organisms Divers. Evol. 8: 346-357.
Kunze H, Meve U, Liede S. 1994. Cibirhiza albersiana, a new species of Asclepiadaceae, and establishment of the tribe Fockeeae. – Taxon 43: 367-376.
Kupicha FK. 1982. Studies on African Apocynaceae: the genus Acokanthera. – Kew Bull. 37: 41-67.
Kupicha FK. 1984. Studies on African Asclepiadaceae. – Kew Bull. 38: 599-672.
Kusnetzov NJ. 1898. Subgenus Eugentiana Kusnetzow generis Gentiana Tournef. – Acta Horti Petrop. 15: 1-507.
Laan FV van der, Arends JC. 1985. Cytotaxonomy of the Apocynaceae. – Genetica 68: 3-35.
Lachenaud O, Sonké B. 2015. Le genre Oxyanthus DC. (Rubiaceae) en Afrique de l’Ouest: description d’une nouvelle espèce. – Candollea 70: 241-247.
Lahaye R, Giveyrel L, Speck T, Rowe NP. 2005. Evolution of shrub-like growth forms in the lianoid subfamily Secamonoideae (Apocynaceae s.l.) of Madagascar: phylogeny, biomechanics, and development. – Amer. J. Bot. 92: 1381-1396.
Lahaye R, Klackenberg J, Källersjö M, Campo E van, Civeyrel L. 2007. Phylogenetic relationships between derived Apocynaceae s.l. and within Secamonoideae based on chloroplast sequences. – Ann. Missouri Bot. Gard. 94: 376-391.
Lakshminarayana K, Devi HM. 1985. Embryological studies in Gentianaceae. – Proc. Indian Acad. Sci., Sect. B, 95: 213-219.
Langley RW. 1980. Taxonomic studies in the Asclepiadeae with particular reference to Xysmalobium R. Br. in southern Africa. – M.Sc. thesis, University of Natal, Pietermaritzburg, South Africa.
Lantz H, Bremer B. 2004. Phylogeny inferred from morphology and DNA data: characterizing well-supported groups in Vanguerieae (Rubiaceae). – Bot. J. Linn. Soc. 146: 257-283.
Lantz H, Bremer B. 2005. Phylogeny of the complex Vanguerieae (Rubiaceae) genera Fadogia, Rytigynia, and Vangueria with close relatives and a new circumscription of Vangueria. – Plant Syst. Evol. 253: 159-183.
Lantz H, Andreasen K, Bremer B. 2002. Nuclear rDNA ITS sequence used to construct the first phylogeny of Vanguerieae (Rubiaceae). – Plant Syst. Evol. 230: 173-187.
Lantz H, Klackenberg J, Razafimandimbison S, Mouly A. 2007. Three new species of Vanguerieae (Rubiaceae) from Madagascar. – Adansonia, sér. III, 29: 129-136.
Lavranos JJ. 1993. Two new species of Echidnopsis (Asclepiadaceae-Stapelieae) from Socotra. – Cactus Succ. J. (U.S.) 65: 293-295.
Leach LC. 1976. A preliminary review of the prominently papillose Huernia species. – J. South Afr. Bot. 42: 439-487.
Leach LC. 1978a. A contribution towards a new classification of Stapelieae (Asclepiadaceae), with a preliminary review of Orbea Haw. and description of three new genera. – Excelsa, Taxon. Ser. 1: 1-75.
Leach LC. 1978b. On the classification of Stapelieae and the reinstatement of Tridentea Haw. (Asclepiadaceae). – Trans. Rhod. Sci. Ass. 59: 1-5.
Leach LC. 1980. A review of Tridentea Haw. (Asclepiadaceae). – Excelsa, Taxon. Ser. 2: 1-68.
Leach LC. 1985. A revision of Stapelia L. (Asclepiadaceae). – Excelsa, Taxon. Ser. 3: 1-157.
Leach LC. 1986. The identity of Huernia concinna N. E. Br. and two new species from the Somali Republic. – Excelsa 12: 93-98.
Leach LC. 1988. A revision of Huernia R. Br. (Asclepiadaceae). – Excelsa, Taxon. Ser. 4: 1-197.
Lebrun J-P. 1984. Three new combinations in Spermacoce (Rubiaceae). – Kew Bull. 39: 778.
Lee YS, Fairbrothers DE. 1978. Serological approach to the systematics of the Rubiaceae and related families. – Taxon 27: 159-185.
Leenhouts PW. 1962. Loganiaceae. – In: Steenis CGGJ van (ed), Flora Malesiana I, 6, Wolters-Noordhoff, Groningen, pp. 293-387.
Leenhouts PW. 1967. Pollen morphology and taxonomy in the Loganiaceae. – Grana Palynol. 7: 469-516.
Leenhouts P, Steenis C van. 1962. Reduction of the genus Nautophylla Guillaumin to Logania R. Br. – Bull. Jard. Bot. État Bruxelles 32: 439-440.
Leeuwenberg AJM. 1961a. The Loganiaceae of Africa I. Anthocleista. – Acta Bot. Neerl. 10: 1-53.
Leeuwenberg AJM. 1961b. The Loganiaceae of Africa II. A revision of Mostuea Didr. – Meded. Landbouwh. Wageningen 61: 1-31.
Leeuwenberg AJM. 1961c. The Loganiaceae of Africa III. Spigelia L. – Acta Bot. Neerl. 10: 460-465.
Leeuwenberg AJM. 1963. The Loganiaceae of Africa V. Usteria Willd. – Acta Bot. Neerl. 12: 112-118.
Leeuwenberg AJM. 1969. The Loganiaceae of Africa VIII. Strychnos III. Revision of the species with notes on the extra-African sections. – Meded. Landbouwh. Wageningen 69: 1-316.
Leeuwenberg AJM. 1971. Notes on American Loganiaceae V. Key to the genera represented in America. – Acta Bot. Neerl. 20: 539-542.
Leeuwenberg AJM. 1975. The Loganiaceae of Africa XII. Revision of Mitreola. – Meded. Landbouwh. Wageningen 74: 1-28.
Leeuwenberg AJM. 1977. The Loganiaceae of Africa XV. Geniostoma Forst. – Meded. Landbouwh. Wageningen 77: 1-14.
Leeuwenberg AJM (ed). 1980. Angiospermae: Ordnung Gentianales Fam. Loganiaceae. – In: Hiepko P, Melchior H (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Vol. 28bI, Duncker & Humblot, Berlin, pp. 1-255.
Leeuwenberg AJM. 1983a. Some remarks on the taxonomy of the Plumerioideae (Apocynaceae). – Bothalia 14: 799-801.
Leeuwenberg AJM. 1983b. 109. Loganiaceae. – In: Launert E (ed), Flora Zambesiaca 7 (Part 1), Flora Zambesiaca Managing Committee, London, pp. 327-374.
Leeuwenberg AJM. 1984. 167. Loganiacées. – In: Leroy J-F (ed), Flore de Madagascar et des Comores, Muséum national d’histoire naturelle, Paris, pp. 1-107.
Leeuwenberg AJM. 1991. A revision of Tabernaemontana 1. The Old World species. – Royal Botanic Gardens, Kew.
Leeuwenberg AJM. 1994a. A revision of Tabernaemontana 2. The New World species and Stemmadenia. – Royal Botanical Gardens, Kew.
Leeuwenberg AJM. 1994b. Series of revisions of Apocynaceae XXXVIII. Taxa of the Apocynaceae above the genus level. – Wageningen Agric. Univ. Papers 94: 45-60.
Leeuwenberg AJM. 1997. Series of revisions of Apocynaceae XLIII. Alafia Thouars. – Kew Bull. 52: 769-839.
Leeuwenberg AJM. 2002a. Series of revisions of Apocynaceae LI. Leuconotis. – Syst. Geogr. Plants 72: 111-126.
Leeuwenberg AJM. 2002b. Series of revisions of Apocynaceae LII. Chilocarpus. – Syst. Geogr. Plants 72: 127-166.
Leeuwenberg AJM. 2003. Series of revisions of Apocynaceae LIII. Melodinus. – Syst. Geogr. Plants 73: 3-62.
Leeuwenberg AJM, Dilst FJH van. 2001. Series of revieions of Apocynaceae XLIX. Carissa L. – Wageningen Univ. Pap. 2001(1).
Leeuwenberg AJM, Leenhouts PW. 1980. Taxonomy. – In: Leeuwenberg AJM (ed), Engler and Prantl’s Die natürlichen Pflanzenfamilien, Fam. Loganiaceae, vol. 28b(1), Duncker & Humblot, Berlin, pp. 8-92.
Leeuwenberg AJM, Kupicha FK et al. 1985. 111. Apocynaceae. – In: Launert E (ed), Flora Zambesiaca 7 (Part 2), Flora Zambesiaca Managing Committee, London.
Lemaire B, Robbrecht E, Wyk B van, Oevelen S van, Verstraete B, Prinsen E. Smets E, Dessein S. 2011. Identification origin and evolution of leaf nodulating symbionts of Sericanthe (Rubiaceae). – J. Microbiol. 49: 935-941.
Lemaire B, Oevelen S van, De Block P, Verstraete B, Smets E, Prinsen E. Dessein S. 2012. Identification of the bacterial endosymbiont in leaf nodules of Pavetta (Rubiaceae). – Intern. J. Syst. Evol. Microbiol. 62: 202-209.
Lens F, Jansen S, Huysmans S, Robbrecht E, Smets E. 2000. Pollen morphological variation in Vanguerieae (Ixoroideae-Rubiaceae). – Grana 39: 90-102.
Lens F, Jansen S, Robbrecht E, Smets E. 2000. Wood anatomy of the Vanguerieae (Ixoroideae-Rubiaceae), with special emphasis on some geofrutices. – IAWA J. 21: 443-455.
Lens F, Endress ME, Baas P, Jansen S, Smets E. 2008. Wood anatomy of Rauvolfioideae (Apocynaceae): a search for meaningful non-DNA characters at the tribal level. – Amer. J. Bot. 95: 1199-1215.
Lens F, Groeninckx I, Smets E, Dessein S. 2009. Woodiness within the Spermacoceae-Knoxieae alliance (Rubiaceae): retention of the basal woody condition in Rubiaceae or recent innovation? – Ann. Bot. 103: 1049-1064.
Lens F, Endress ME, Baas P, Jansen S, Smets E. 2009. Vessel grouping patterns in subfamilies Apocynoideae and Periplocoideae confirm phylogenetic value of wood structure within Apocynaceae. – Amer. J. Bot. 96: 2168-2183.
Léonard J. 1984. Contribution à la connaissance de la flore de l’Iran VI. Le “complexe Gaillionia A. Rich. ex DC.” (Rubiaceae). – Bull. Jard. Bot. Belg. 54: 493-497.
Leonhard M. 1891. Beiträge zur Anatomie der Apocynaceen. – Bot. Centralbl. 45: 5.
Lepis KB. 2009. Evolution and systematics of Chelonanthus (Gentianaceae). – Ph.D. diss., Rutgers University, New Brunswick, Canada.
Leppik EE. 1977. Calyx-borne semaphylls in tropical Rubiaceae. – Phytomorphology 27: 161-168.
Leroy J-F. 1961. Les faux Caféiers du genre Argocoffea (Pierre) Lebrun. – J. Agric. Trop. Bot. Appl. 8: 65-69.
Leroy J-F. 1967a. Diagnose différentielle du genre Paracoffea Leroy. – J. Agric. Trop. Bot. Appl. 14: 276.
Leroy J-F. 1967b. Recherches sur les Caféiers. Sur la classification biologique des Caféiers et sur l’origine et l’aire du genre Coffea. – Compt. Rend. Acad. Sci. Paris, sér. D, 265: 1043-1045.
Leroy J-F. 1974a. Recherches sur les Rubiacées de Madagascar: les genres Mantalania et Pseudomantalania (Gardeniées). – Adansonia, sér. II, 14: 29-52.
Leroy J-F. 1974b. Recherches sur la phylogenèse du développement. Mise en evidence d’une série de trois états dans le genre Bertiera (Rubiacées). – Adansonia, sér. II, 14: 53-59.
Leroy J-F. 1975a. Note préliminaire sur les Rubiacées-Nauclées malgaches. – Adansonia, sér. II, 14: 681-685.
Leroy J-F. 1975b. Taxogénétique dans le genre Hallea sur la sous-tribu des Mitragyninae (Rubiaceae-Naucleeae). – Adansonia, sér. II, 15: 65-88.
Leroy J-F. 1980. Evolution et taxogenèse chez les Caféiers (Coffea L., Psilanthus Hook. f. et Nostolachma Durand). Hypothèse sur leur origine. – Compt. Rend. Acad. Sci. Paris, sér. D, 291: 593-596.
Lersten NR. 1974a. Morphology and distribution of colleters and crystals in relation to the taxonomy and bacterial leaf nodule symbiosis of Psychotria (Rubiaceae). – Amer. J. Bot. 61: 973-981.
Lersten NR. 1974b. Colleter morphology in Pavetta, Neorosea and Tricalysia (Rubiaceae) and its relationship to the bacterial leaf nodule symbiosis. – Bot. J. Linn. Soc. 69: 125-136.
Lersten NR. 1975. Colleter types in Rubiaceae, especially in relation to the bacterial leaf nodule symbiosis. – Bot. J. Linn. Soc. 71: 311-319.
Lersten NR, Horner HT. 1976. Bacterial leaf nodule symbiosis in angiosperms with emphasis on Rubiaceae and Myrsinaceae. – Bot. Rev. 42: 145-214.
Lersten NR, Horner HT. 2011. Unique calcium oxalate “duplex” and “concretion” idioblasts in leaves of tribe Naucleeae (Rubiaceae). – Amer. J. Bot. 98: 1-11.
Lewis WH. 1965a. Pollen morphology and evolution in Hedyotis subgenus Edrisia (Rubiaceae). – Amer. J. Bot. 52: 257-264.
Lewis WH. 1965b. Cytopalynological study of African Hedyotideae (Rubiaceae). – Ann. Missouri Bot. Gard. 52: 182-211.
Lewis WH. 1966. The Asian genus Neanotis nomen novum (Anotis) and allied taxa in the Americas (Rubiaceae). – Ann. Missouri Bot. Gard. 53: 32-46.
Li, H, Dao, Z-L, Li, R. 2006. Reappraisal of Fosbergia shweliensis, a species endemic to the Gaoligong Mountains, Western Yunnan, China. – Acta Phytotaxon. Sin. 44: 707-71.
Li P-T. 1984. A revision of Cleghornia and three new varieties of Alyxia from China. Guihaia 4: 191-194.
Li P-T. 1991. A new genus of Euphorbiaceae and some new nomenclatural combination of the asclepiadaceous plants. – J. South China Agric. Univ. 12: 38-42.
Liede S. 1993. A taxonomic revision of the genus Cynanchum in southern Africa. – Bot. Jahrb. Syst. 114: 503-550.
Liede S. 1994. Myth and reality of the subtribe Astephaninae (Decne.) Schumann (Asclepiadaceae). – Bot. J. Linn. Soc. 114: 81-98.
Liede S. 1995. Another differentiation in the Asclepiadaceae: form and function. – In: d’Arcy WG, Keating RC (eds), The anther: form, function, and phylogeny, Cambridge University Press, Cambridge, pp. 221-235.
Liede S. 1996a. Cynanchum-Rhodostegiella-Vincetoxicum-Tylophora (Asclepiadaceae): new considerations on an old problem. – Taxon 45: 193-211.
Liede S. 1996b. Sarcostemma (Asclepiadaceae) – a controversial generic circumscription reconsidered: morphological evidence. – Syst. Bot. 21: 31-44.
Liede S. 1996c. A revision of Cynanchum (Asclepiadaceae) in Africa. – Ann. Missouri Bot. Gard. 83: 283-345.
Liede S. 1996d. On the position of the genus Pergularia (Asclepiadaceae). – In: Van der Maesen LJG, Van der Burgt KM, Van Medenbach de Roy JM (eds), The biodiversity of African plants, Kluwer Academic Press, Wageningen, Dordrecht, pp. 481-488.
Liede S. 1997a. Subtribes and genera of the tribe Asclepiadeae (Apocynaceae, Asclepiadoideae) – a synopsis. – Taxon 46: 233-247.
Liede S. 1997b. Phylogenetic study of the African members of Cynanchum (Apocynaceae-Asclepiadoideae). – Syst. Bot. 22: 347-372.
Liede S. 1997c. American Cynanchum (Asclepiadaceae). A preliminary infrageneric classification. – Novon 7: 172-181.
Liede S. 1999. Subtribes and genera of Asclepiadeae: reply to Bruyns’s response. – Taxon 48: 27-29.
Liede S. 2001. Subtribe Astephaninae (Apocynaceae-Asclepiadoideae) reconsidered: new evidence based on cpDNA spacers. – Ann. Missouri Bot. Gard. 88: 657-668.
Liede S. 2001. Molecular considerations on the subtribe Astephaninae Endl. ex Meisn. (Apocynaceae – Asclepiadoideae). – Ann. Missouri Bot. Gard. 88: 657-668.
Liede S, Albers F. 1994. Tribal disposition of genera in the Asclepiadaceae. – Taxon 43: 201-231.
Liede S, Kunze H. 1993. A descriptive system for corona analysis in Asclepiadaceae and Periplocaceae. – Plant Syst. Evol. 185: 275-284.
Liede S, Kunze H. 2002. Cynanchum and the Cynanchinae (Apocynaceae-Asclepiadoideae): a molecular, anatomical and latex triterpenoid study. – Organisms Divers. Evol. 2: 239-269.
Liede S, Meve U. 1997. Some clarifications, new species, and new combinations in American Cynanchinae. – Novon 7: 38-45.
Liede S, Meve U. 2001a. Taxonomic changes in American Metastelminae (Apocynaceae-Asclepiadoideae). – Novon 11: 171-182.
Liede S, Meve U. 2001b. New combinations and new names in Malagasy Asclepiadoideae (Apocynaceae). – Adansonia, sér. III, 23: 347-351.
Liede S, Meve U. 2002 [2004]. Dissolution of Cynanchum sect. Macbridea (Apocynaceae-Asclepiadoideae). – Nord. J. Bot. 22: 579-591.
Liede S, Meve U. 2004. Revision of Metastelma (Apocynaceae-Asclepiadoideae) in southwestern North America and Central America. – Ann. Missouri Bot. Gard. 91: 31-86.
Liede S, Nicholas A. 1992. A revision of the genus Pentarrhinum E. Meyer (Asclepiadaceae). – Kew Bull. 47: 475-490.
Liede S, Täuber A. 2000. Sarcostemma R. Br. (Apocynaceae-Asclepiadoideae) – a controversial generic circumscription reconsidered: evidence from trnL-F spacers. – Plant Syst. Evol. 225: 133-140.
Liede S, Täuber A. 2002. Circumscription of the genus Cynanchum (Apocynaceae-Asclepiadoideae). – Syst. Bot. 27: 789-800.
Liede S, Weberling F. 1995. On the inflorescence structure of Asclepiadaceae. – Plant Syst. Evol. 197: 99-109.
Liede S, Täuber A, Schneidt J. 2002. Molecular considerations in the Tylophorinae K. Schum. (Apocynaceae-Asclepiadoideae). – Edinburgh J. Bot. 59: 377-403.
Liede S, Meve U, Täuber A. 2002. What is the subtribe Glossonematinae (Apocynaceae: Asclepiadoideae)? A phylogenetic study based on cpDNA spacer. – Bot. J. Linn. Soc. 139: 145-158.
Liede-Schumann S, Meve U. 2005. Notes on succulent Cynanchum species in East Africa. – Novon 15: 320-323.
Liede-Schumann S, Meve U. 2006. Calciphila, a new genus in African Asclepiadeae (Apocynaceae, Asclepiadoideae), and taxonomic rectifications in Cynanchum. – Novon 16: 368-373.
Liede-Schumann S, Meve U. 2008. Nomenclatural novelties and one new species in Orthosia (Apocynaceae, Asclepiadoideae). – Novon 18: 202-210.
Liede-Schumann S, Meve U. 2013. The Orthosiinae revisited (Apocynaceae, Asclepiadoideae, Asclepiadeae). – Ann. Missouri Bot. Gard. 99: 44-81.
Liede-Schumann S, Rapini A, Goyder DJ, Chase MW. 2005. Phylogenetics of the New World subtribes of Asclepiadeae (Apocynaceae-Asclepiadoideae): Metastelmatinae, Oxypetalinae, and Gonolobinae. – Syst. Bot. 30: 184-195.
Liede-Schumann S, Kong H, Meve U, Thiv M. 2012. Vincetoxicum and Tylophora (Apocynaceae: Asclepiadoideae: Asclepiadeae) – two sides of the same medal: independent shifts from tropical to temperate habitats. – Taxon 61: 803-825.
Liede-Schumann S, Nikolaus M, Soares e Silva
UC, Rapini A, Mangelsdorff RD, Meve U. 2014. Phylogenetics and biogeography of
the genus Metastelma (Apocynaceae-Asclepiadoideae-Asclepiadeae:
Metastelmatinae). – Syst. Bot. 39: 594-612.
Liede-Schumann S, Khanum R, Mumtaz AS, Gherghel I, Pahlevani A. 2016. Going west – a subtropical lineage (Vincetoxicum, Apocynaceae: Asclepiadoideae) expanding into Europe. – Mol. Phylogen. Evol. 94: 436-446.
Lienau K, Straka H, Friedrich B. 1986. Fam. 168. Gentianaceae. – In: Straka H (ed), Palynologia Madagassica et Mascarenica, Familien 167 bis 181, Tropische und Subtropische Pflanzenwelt 55, Akademie der Wissenschaften und der Literatur, Franz Steiner, Wiesbaden, Mainz, pp. 9-15.
Linczevski I. 1973. De genere Gaillonia A. Rich. ex DC. (Rubiaceae). – Nov. Sist. Vyssh. Rast. 10: 224-236.
Lindsey AA. 1940. Floral anatomy in the Gentianaceae. – Amer. J. Bot. 27: 640-651.
Ling S-K, Komorita A, Tanaka T, Fujioka T, Mihashi K, Kouno I. 2002. Iridoids and anthraquinones from the Malaysian medicinal plant, Saprosma scortechinii (Rubiaceae). – Chem. Pharm. Bull. (Tokyo) 50: 1035-1040.
Liu S-W, Ho T-N. 1992. Systematic study on Lomatogonium A. Br. (Gentianaceae). – Acta Phytotaxon. Sin. 30: 289-319.
Livshultz T. 2003a. Systematics of Dischidia (Apocynaceae, Asclepiadoideae). – Ph.D. diss., Cornell University, Ithaca, New York.
Livshultz T. 2003b. Lectotypification of Dolichostegia Schlechter (Asclepiadoideae, Apocynaceae) and a new combination, Dischidia boholensis. – Taxon 52: 595-600.
Livshultz T. 2010. The phylogenetic position of milkweeds (Apocynaceae subfamilies Secamonoideae and Asclepiadoideae): evidence from the nucleus and chloroplast. – Taxon 59: 1016-1030.
Livshultz T, Middleton DJ, Endress ME, Williams JK. 2007. Phylogeny of Apocynoideae and the APSA clade (Apocynaceae s.l.). – Ann. Missouri Bot. Gard. 94: 324-359.
Livshultz T, Mead JV, Goyder DJ, Brannin M. 2011. Climate niches of milkweeds with plesiomorphic traits (Secamonoideae; Apocynaceae) and the milkweed sister group link ancient African climates and floral evolution. – Amer. J. Bot. 98: 1966-1977.
Livshultz T, Middleton DJ, Ham RWJM van der, Khew G. 2018. Generic delimitation in Apocyneae (Apocynaceae). – Taxon 67: 341-358.
Lo H-S. 1993. A revision of the genus Leptomischus Drake. – Acta Phytotaxon. Sin. 31: 273-276. [In Chinese]
Lobreau-Callen D. 1978. L’aperture composée des Rubiaceae. – Ann. Min. Belg. 2: 167-173.
Lobreau-Callen D, Leroy J-F. 1980. Quelques données palynologiques sur le genre Coffea et autres genres du cercle des cafétiers. – Assoc. Sci. Intern. Café, 9e coll. (Londres): 479-506.
Löfstrand SD, Krüger Å, Razafimandimbison
SG, Bremer B. 2014. Phylogeny and generic delimitations in the sister tribes
Hymenodictyeae and Naucleeae (Rubiaceae). – Syst. Bot. 39: 304-315.
Löhne C, MacHado IC, Porembski S, Erbar C, Leins P. 2004. Pollination biology of a Mandevilla species (Apocynaceae), characteristic of NE-Brazilian inselberg vegetation. – Bot. Jahrb. Syst. 125: 229-243.
Longhi-Wagner HM, Oliveira RP de. 2002. New grass records for Bahia State, Brazil. – Kew Bull. 57: 971-977.
Lorence DH. 1986a. Glossostipula (Rubiaceae), a new genus from Mexico and Guatemala. – Candollea 41: 453-461.
Lorence DH. 1986b. The identity of Portlandia guatemalensis (Rubiaceae). – Syst. Bot. 11: 209-213.
Lorence DH. 1991. New species and combinations in Mexican and Central American Rondeletia (Rubiaceae). – Novon 1: 135-157.
Lorence DH. 1999. A nomenclator of Mexican and Central American Rubiaceae. – Monogr. Missouri Bot. Gard. 73: 1-177.
Lorence DH, Wagner WL, Mouly A, Florence J. 2007. Revision of Ixora (Rubiaceae) in the Marquesas Islands (French Polynesia). – Bot. J. Linn. Soc. 155: 581-597.
Löve Á, Löve D. 1972. Favargera and Gentianodes, two new genera of alpine Gentianaceae. – Bot. Not. 125: 255-258.
Löve Á, Löve D. 1975. The Spanish gentians. – Anal. Inst. Bot. Cavanilles 32: 221-232.
Löve Á, Löve D. 1976. The natural genera of Gentianaceae. – In: Kachroo P (ed), Recent advances in botany, Prof. P. N. Mehra commemorative volume, Dehra Dun, India, pp. 205-222.
Löve Á, Löve D. 1978. Holubogentia, a new name in Gentianaceae. – Bot. Not. 131: 385.
Löve D. 1953. Cytotaxonomical remarks on the Gentianaceae. – Hereditas 39: 225-235.
Lu X-L, Cao X, Liu X-Y, Long C, Liu J-H, Xu Q-Z, Jiao B-H. 2010. Iridoid glycosides from Saprosma ternatum. – Planta Medica 75: 1746-1748.
Ly TD. 1986. Die Familie Apocynaceae Juss. in Vietnam 1. Allgemeiner Teil. – Feddes Repert. 97: 235-273.
Ma J, Zhao R. 1991. Study on pollen morphology of Gentianaceae from Gansu. – Acta Bot. Boreal.-Occid. Sin. 11: 251-257.
Ma Y-C. 1951. Gentianopsis – a new genus of Chinese Gentianaceae. – Acta Phytotaxon. Sin. 1: 5-19.
Maas PJM. 1981. On the true identity of Lagenanthus parviflorus Ewan (Gentianaceae). – Ann. Missouri Bot. Gard. 68: 685-688.
Maas PJM. 1985. Nomenclatural notes on neotropical Lisyantheae (Gentianaceae). – Proc. Kon. Nederl. Akad. Wetensch., ser. C, 88: 405-412.
Maas PJM, Ruyters P. 1986. Flora Neotropica. Monograph 41. Voyria and Voyriella (saprophytic Gentianaceae). – New York Botanical Garden, Bronx, New York.
Maas PJM, Nilsson S, Hollants AMC, Welle BJH ter, Persoon H, Heusden ECH van. 1983. Systematic studies in neotropical Gentianaceae – the Lisianthius complex. – Acta Bot. Neerl. 32: 371-374.
Mabry TJ, Eifert IJ, Chang C, Mabry H, Kidd C, Behnke H-D. 1975. Theligonaceae: pigment and ultrastructural evidence which exclude it from the order Centrospermae. – Biochem. Syst. Ecol. 3: 53-55.
McDonnell A, Parks M, Fishbein M. 2018. Multilocus phylogenetics of New World milkweed vines (Apocynaceae, Asclepiadoideae, Gonolobinae). – Syst. Bot. 43: 77-96.
McDowell T. 1996a. Exostema (Rubiaceae): taxonomic history, nomenclature, position and subgeneric classification. – Opera Bot. Belg. 7: 277-295.
McDowell T. 1996b. Syringantha coulteri (Hooker f.) T. McDowell, a new combination, and remarks on the relationships of the monotypic Mexican genus Syringantha Standley (Rubiaceae). – Novon 6: 273-279.
McDowell T, Bremer B. 1998. Phylogeny, diversity, and distribution in Exostema (Rubiaceae): implications of morphological and molecular analyses. – Plant Syst. Evol. 212: 215-246.
McDowell T, Volovsek M, Manos P. 2003. Biogeography of Exostema (Rubiaceae) in the Caribbean region in light of molecular phylogenetic analyses. – Syst. Bot. 28: 431-441.
Macfarlane JM. 1933. The evolution and distribution of flowering plants I. The Apocynaceae and Asclepiadaceae. – Nobel Printing Comp., Philadelphia, Pennsylvania.
McGillivray DJ. 1983. A revision of Galium (Rubiaceae) in Australia and New Zealand. – Telopea 2: 355-377.
Machado ICS, Sazima I, Sazima M. 1998. Bat pollination of the terrestrial herb Irlbachia alata (Gentianaceae) in northeastern Brazil. – Plant Syst. Evol. 209: 231-237.
Maguire B. 1981. Gentianaceae. – In: Maguire B et al. (eds), The botany of the Guayana Highland XI, Mem. New York Bot. Gard 32: 330-388.
Maguire B. 1985. Gentianaceae – part 2. – Phytologia 57: 311-312.
Maguire B, Boom BM. 1989. Gentianaceae (part 3). – In: Maguire B et al. (eds), The botany of the Guayana Highland XIII, Mem. New York Bot. Gard. 51: 2-56.
Maguire B, Pires JM. 1978. Saccifoliaceae – a new monotypic family of the Gentianales. – In: Maguire B et al. (eds), The botany of the Guyana Highland X, Mem. New York Bot. Gard. 29: 230-245.
Maguire B, Weaver RE Jr. 1975. The neotropical genus Tachia (Gentianaceae). – J. Arnold Arbor. 56: 103-125.
Mahlberg PG. 1961. Embryogeny and histogenesis in Nerium oleander II. Origin and development of the non-articulated laticifers. – Amer. J. Bot. 48: 90-99.
Mahlberg PG. 1962. Evolution of the non-articulated laticifers in seedling axis of Nerium oleander. – Bot. Gaz. 124: 224-231.
Majumdar GP, Pal PK. 1958. Interpetiolar stipules of Galium mollugo L. – Proc. Indian Acad. Sci., Sect. B, 48: 211-222.
Majumdar S, Pal P. 1958. The stipules of the Rubiaceae: A review. – Trans. Bose Res. Inst. Calcutta 22: 57-68.
Malcomber ST. 2002. Phylogeny of Gaertnera Lam. (Rubiaceae) based on multiple DNA markers: evidence of a rapid radiation in a widespread, morphologically diverse genus. – Evolution 56: 42-57.
Malcomber ST, Davis AP. 2005. Six new species of Gaertnera (Rubiaceae) from Madagascar and phylogenetic analyses that support Hymenocnemis as a synonym of Gaertnera. – Monogr. Syst. Bot. Missouri Bot. Gard. 104: 371-398.
Malcomber ST, Taylor CM. 2009. A systematic revision of Gaertnera (Rubiaceae, Gaertnereae). – Ann. Missouri Bot. Gard. 96: 575-671.
Maldonado S. 1989. Embryological studies in Spigelia humboldtiana (Loganiaceae). – Phytomorphology 39: 299-309.
Malme GOA. 1928. Über paarige extraaxilläre Infloreszenzen bei den Asklepiadaceen. – Svensk Bot. Tidskr. 22: 49-56.
Malme GOA. 1932. Asclepiadaceae austroamericanae praecipue andinae. – Ark. Bot. 25A: 1-26.
Malplance M. 1971. Étude palynologique de tris genres de Rubiacées-Gardéniées d’Afrique. – Adansonia, sér. II, 11: 343-355.
Manen JF, Natali A. 1995. Comparison of the evolution of ribulose-1,5-bisphosphate carboxylase (rbcL) and atpB-rbcL non-coding spacer sequences in a recent plant group, the tribe Rubieae (Rubiaceae). – J. Mol. Evol. 41: 920-927.
Manen J-F, Natali A. 1996. The chloroplast atpB-rbcL spacer in Rubiaceae. – Opera Bot. Belg. 7: 51-57.
Manen J-F, Natali A, Ehrendorfer, F. 1994. Phylogeny of Rubiaceae-Rubieae inferred from the sequence of a cpDNA intergene region. – Plant Syst. Evol. 190: 195-211.
Mangalan S, Kurien KP, John P, Nair GM. 1990. Development, structure and cytochemistry of resin-secreting colleters of Gardenia gummifera (Rubiaceae). – Ann. Bot., N. S., 66: 123-132.
Mangelsdorff RD, Meve U, Liede-Schumann S. 2016. Phylogeny and circumscription of Antillean Anemotrochus, gen. nov., and Tylodontia (Apocynaceae: Asclepiadoideae: Gonolobinae). – Willdenowia 46: 443-474.
Manns U, Bremer B. 2010. Towards a better understanding of intertribal relationships and stable tribal delimitations within Cinchonoideae s.s. (Rubiaceae). – Mol. Phylogen. Evol. 56: 21-39.
Manns U, Wikström N, Taylor CM, Bremer B. 2012. Historical biogeography of the predominantly Neotropical subfamily Cinchonoideae (Rubiaceae): into or out of America? – Intern. J. Plant Sci. 173: 261-289.
Mansion G. 2004. A new classification of the polyphyletic genus Centaurium Hill (Chironiinae, Gentianaceae): description of the New World endemic Zeltnera, and reinstatement of Gyrandra Griseb. and Schenkia Griseb. – Taxon 53: 719-740.
Mansion G, Struwe L. 2004. Generic delimitation and phylogenetic relationships within the subtribe Chironiinae (Chironieae: Gentianaceae), with special reference to Centaurium: evidence from nrDNA and cpDNA sequences. – Mol. Phylogen. Evol. 32: 951-977.
Mansion G, Zeltner L. 2004. Phylogenetic relationships within the New World endemic Zeltnera (Gentianaceae-Chironiinae) inferred from molecular and karyological data. – Amer. J. Bot. 91: 2069-2086.
Mansion G, Zeltner L, Bretagnolle F. 2005. Phylogenetic patterns and polyploid evolution within the Mediteranean genus Centaurium (Gentianaceae-Chironieae). – Taxon 54: 931-950.
Mantell DE. 1985. the Afro-South-west Asiatic genus Kohauatia Cham. & Schlechtd. (Rubiaceae-Rubioideae-Hedyotideae): morphology, anatomy, taxonomy, phytogeography, and evolution. – Ph.D. diss., Universität Wien, Austria.
Marais W. 1961. Belmontia and Exochaenium synonyms with Sebaea. – Bothalia 7: 464.
Marais W. 1985. Notes on Mascarene Asclepiadaceae. – Kew Bull. 40: 205-207.
Markgraf F. 1972. New Apocynaceae and Asclepiadaceae from Venezuela. – Acta Bot. Venez. 6: 65-76.
Marloth R. 1909. A diplostigmatic plant, Sebaea exacoides (L.) Schinz (Belmontia cordata L.). – Trans. Roy. Soc. South Afr. 1: 311-314.
Marohasy J, Forster PI. 1991. A taxonomic revision of Cryptostegia R. Br. (Asclepiadaceae: Periplocoideae). – Aust. Syst. Bot. 4: 571-577.
Marquand CVB. 1931. New Asiatic gentians II. – Kew Bull. 1931: 68-88.
Marquand CVB. 1937. The gentians of China. – Kew Bull. 1937: 134-180.
Marques-Souza A;, Absy ML, Miranda IP de A, Küchmeister HEC. 1993. Características de flores, néctar y visitantes de Kerianthera preclara (Rubiaceae). – Rev. Biol. Trop. 41: 483-489.
Martínez M. 1942. A new genus of Rubiaceae from Mexico. – Bull. Torrey Bot. Club 69: 438-441.
Martínez-Cabrera D, Terrazas T, Ochoterena H. 2007 [2008]. Leaf architecture of Hamelieae (Rubiaceae). – Feddes Repert. 118: 286-310.
Martínez-Cabrera D, Terrazas T, Flores H, Ochotorena H. 2008. Morphology, anatomy, and taxonomic position of Plocaniophyllon Brandegee (Rubiaceae), a monospecific genus endemic to Mesoamerica. – Taxon 57: 33-42.
Martínez-Cabrera D, Terrazas T, Ochoterena H. 2009. Foliar and petiole anatomy of tribe Hamelieae and other Rubiaceae. – Ann. Missouri Bot. Gard. 96: 133-145.
Martínez-Cabrera D, Terrazas T, Ochoterena
H. 2014. Morfología y anatomía floral de la tribu Hamelieae (Rubiaceae). – Brittonia 66: 89-106.
Masinde PS. 1999. A new Kenyan species of Ceropegia (Asclepiadaceae-Stapelieae) with notes on allied taxa. – Kew Bull. 54: 477-481.
Masinde PS. 2004. Two new Ceropegia (Apocynaceae: Asclepiadoideae-Ceropegieae) species from Kenya. – Kew Bull. 59: 241-245.
Masinde PS. 2005. A revision of the African genus Riocreuxia Decne. (Apocynaceae: Asclepiadoideae-Ceropegieae). – Kew Bull. 60: 401-434.
Masinde PS. 2007. A revision of Brachystelma Sims (Apocynaceae: Asclepiadoideae-Ceropegieae) in East Africa. – Kew Bull. 62: 37-84.
Masrahi YS. 2015. A new species of Leptadenia (Apocynaceae) and two other new records from southwestern Saudi Arabia. – Saudi J. Biol. Sci. 22: 631-636.
Massias M, Carbonnier J, Molho D. 1977. Implications chimiotaxonomiques de la répartition des substances osidiques dans le genre Gentiana L. – Bull. Mus. Natl. Hist. Nat. Paris, sér. III, 504, Sci. Phys.-Chim. 13: 41-54.
Massias M, Carbonnier J, Molho D. 1980a. Constituants de Gentiana montserratii Vivant (Gentianaceae). – Bull. Mus. Natl. Hist. Nat. Paris, sér. IV, Sect. 2, B 2: 221-222.
Massias M, Carbonnier J, Molho D. 1980b. Chemotaxonomy of Gentianopsis: xanthones, C-glycosyl flavonoids and carbohydrates. – Biochem. Syst. Ecol. 10: 319-327.
Mata R, Rios L, Rayo Camacho M del, Reguero MT, Lorence D. 1988. Triterpenes from Cigarrilla mexicana. – Phytochemistry 27: 1887-1889.
Mathew PM, Omana P. 1986. The distribution and systematic significance of pollen nuclear number in the Rubiaceae. – Cytologia 51: 117-124.
Mathew PM, Philip O. 1983. Studies in the pollen morphology of South Indian Rubiaceae. – In: Nair PKK (ed), Advances in pollen-spore research 10, Today and Tomorrow’s Printers, New Delhi, pp. 1-80.
Mathew PM, Philip O. 1987. Developmental and systematic significance of pollen bud formation in Ophiorrhiza Linn. – New Botanist 14: 47-54.
Mathews KG, Dunne N, York E, Struwe L. 2009. A phylogenetic analysis and taxonomic revision of Bartonia (Gentianaceae: Gentianeae), based on molecular and morphological evidence. – Syst. Bot. 34: 162-172.
Mathews KG, Ruigrok MS, Mansion G. 2015. Phylogeny and biogeography of the eastern North American rose gentians (Sabatia, Gentianaceae). – Syst. Bot. 40: 811-825.
Maurin O, Davis AP, Chester M, Mvungi EF, Jaufeerally-Fakim Y, Fay MF. 2007. Towards a phylogeny for Coffea (Rubiaceae): identifying well-supported lineages based on nuclear and plastid DNA sequences. – Ann. Bot. 100: 1565-1583.
Melderis A. 1931. Genetical and taxonomical studies in the genus Erythrea Rich. – Acta Hort. Bot. Univ. Latv. 6: 123-256.
Melderis A. 1972. Taxonomic studies on the European species oft he genus Centaurium Hill. – Bot. J. Linn. Soc. 65: 224-250.
Melhem TSA, Bianco Rossi CL del, Fernandes Silvestre MS. 1974. Pollen morphological studies in Rubiaceae. – Hoehnea 4: 49-70.
Mena VP. 1990. A revision of the genus Arcytophyllum (Rubiaceae: Hedyotideae). – Mem. New York Bot. Gard. 60: 1-26.
Mendoza-Heuer I. 1987. Makaronesische Endemiten: zur Blütenbiologie von Plocama pendula Ait. (Rubiaceae). – Bauhinia 8: 235-241.
Mennega AMW. 1980. Anatomy of the secondary xylem. –In: Leeuwenberg AJM (ed), Angiospermae: Ordnung Gentianales, Fam. Loganiaceae, Die naturlichen Pflanzenfamilien, 2nd ed., 28bI, Duncker & Humblot, Berlin, pp. 112-161.
Merckx VSFT, Kissling J, Hentrich H, Janssens SB, Mennes CB, Specht CD, Smets EF. 2013. Phylogenetic relationships of the mycoheterotrophic genus Voyria and the implications for the biogeographic history of Gentianaceae. – Amer. J. Bot. 100: 712-721.
Merrill ED. 1915. On the application of the generic name Nauclea of Linnaeus. – J. Washington Acad. Sci. 5: 530-542.
Merrill ED. 1920. Myrmeconauclea, a new genus of Rubiaceous plants from Palawan and Borneo. – Philipp. J. Sci 17: 375-376.
Merrill ED. 1928. Rubiaceae: genus Canthium Lamarck. – Philipp. J. Sci. 35: 7-9.
Merrill ED. 1937. Rubiaceae. – In: Irmscher E (ed), Beiträge zur Kenntnis der Flora von Borneo, Mitt. Inst. Allgem. Bot. Hamburg 7: 270-301.
Merrill ED, Metcalf C. 1946. Hedyotis L. versus Oldenlandia L. and the status of Hedyotis lancea Thunb. in relation to H. consanguinea Hance. – J. Arnold Arbor. 23: 226-230.
Merrill ED, Perry LM. 1947. Kajewskiella, a new genus from the Solomon Islands. – J. Arnold Arbor. 28: 331-332.
Mészáros S. 1994. Evolutionary significance of xanthones in Gentianaceae: a reappraisal. – Biochem. Syst. Ecol. 22: 85-94.
Mészáros S, De Laet J, Smets E. 1996. Phylogeny of temperate Gentianaceae: a morphological approach. – Syst. Bot. 21: 153-168.
Mészáros S, De Laet J, Goethals V, Smets E, Nilsson S. 2002. Cladistics of Gentianaceae: a morphological approach. – In: Struwe L, Albert VA (eds), Gentianaceae – systematics and natural history, Cambridge University Press, Cambridge, pp. 310-376.
Meve U. 1995a. Neoschumannia (including Swynnertonia), a primitive genus of the Asclepiadaceae-Stapelieae. – Plant Syst. Evol. 197: 233-242.
Meve U. 1995b. Cytological and morphological differentiation in Caralluma burchardii (Asclepiadaceae). – Nord. J. Bot. 15: 459-467.
Meve U. 1997a. The genus Duvalia (Stapelieae): stem-succulents between the Cape and Arabia. – Springer-Verlag, Wien, New York.
Meve U. 1997b. A new combination in Nepalese Asclepiadaceae – Stapelieae. – Kew Bull. 52: 1011-1013.
Meve U. 1998. Emplectanthus N. E. Br.: a close relative of Riocreuxia Decne. in the Asclepiadaceae-Stapelieae. – Bot. Jahrb. Syst. 120: 123-130.
Meve U. 1999. Tylophora anomala (Asclepiadaceae): a cytologically anomalous species. – Syst. Geogr. Plants 68: 255-263.
Meve U. 2002. Species numbers and progress in asclepiad taxonomy. – Kew Bull. 57: 459-464.
Meve U, Albers F. 1990. Die Stipularrudimente der Stapelieae (Asclepiadaceae). – Beitr. Biol. Pflanzen 65: 99-107.
Meve U, Heneidak S. 2005. A morphological, karyological and chemical study of the Apteranthes (Caralluma) europaea complex. – Bot. J. Linn. Soc. 149: 419-432.
Meve U, Kusch G. 1990. The outer epidermal wall structure of African Stapelieae (Asclepiadaceae). – Nord. J. Bot. 9: 519-523.
Meve U, Liede S. 1994. Floral biology and pollination in stapeliads – new results and a literature review. – Plant Syst. Evol. 192: 99-116.
Meve U, Liede S. 2001a. Reconsideration of the status of Lavrania, Larryleachia and Notechidnopsis (Asclepiadoideae-Ceropegieae). – South Afr. J. Bot. 67: 161-168.
Meve U, Liede S. 2001b. Inclusion of Tenaris and Macropetalum in Brachystelma (Apocynaceae-Asclepiadoideae-Ceropegieae) inferred from non-coding nuclear and chloroplast DNA sequences. – Plant Syst. Evol. 228: 89-105.
Meve U, Liede S. 2002a. Floristic exchange between mainland Africa and Madagascar: case studies in Apocynaceae-Asclepiadoideae. – J. Biogeogr. 29: 865-873.
Meve U, Liede S. 2002b. A molecular phylogeny and generic rearrangement of the stapelioid Ceropegieae (Apocynaceae-Asclepiadoideae). – Plant Syst. Evol. 234: 171-209.
Meve U, Liede S. 2004. Subtribal division of Ceropegieae (Apocynaceae-Asclepiadoideae). – Taxon 53: 61-72.
Meve U, Liede-Schumann S. 2007. Ceropegia (Apocynaceae, Ceropegieae, Stapeliinae): paraphyletic but still taxonomically sound. – Ann. Missouri Bot. Gard. 94: 392-406.
Meve U, Liede-Schumann S. 2010. The manifold evolution of succulence in Apocynaceae. – Schumannia 6: 183-206.
Meve U, Liede-Schumann S. 2012. Taxonomic dissolution of Sarcostemma (Apocynaceae: Asclepiadoideae). – Kew Bull. 67: 751-758.
Meve U, Liede-Schumann S. 2015. Taxonomy of the Andean genus Pentacyphus (Apocynaceae: Asclepiadeae-Pentacyphinae). – Plant Syst. Evol. 301: 997-1004.
Meve U, Mangelsdorff RM. 2001. A new Ceropegia species from Yemen, and reconsideration of the status of C. arabica, C. barbigera and C. powysii (Apocynaceae: Asclepiadoideae-Ceropegieae). – Bot. J. Linn. Soc. 137: 99-105.
Meve U, Omlor R. 2000. The identity of the African genus Oncostemma K. Schum. (Asclepiadaceae). – Bot. J. Linn. Soc. 133: 195-201.
Meve U, Porembski S. 1993. Brachystelma Sims in West Tropical Africa. – Bot. Jahrb. Syst. 115: 315-324.
Meve U, Albers F, Kusch G. 1990. The outer epidermal wall structure of African Stapelieae (Asclepiadaceae). – Nord. J. Bot. 9: 519-523.
Meve U, Omlor R, Liede S. 2002. A new combination in Tylophora (Apocynaceae, Asclepiadoideae) from the Philippines. – Syst. Geogr. Plants 72: 27-32.
Meve U, Laurente O, Alejandro GJ, Livshultz T. 2009. Systematics of Clemensiella (Apocynaceae-Asclepiadoideae). – Edinburgh J. Bot. 66: 447-457.
Meve U, Heiduk A, Liede-Schumann S. 2017. Origin and early evolution of Ceropegieae (Apocynaceae-Asclepiadoideae). – Syst. Biodiv. 15: 143-155.
Meve U, Gâteblé G, Liede-Schumann S. 2017. Taxonomic novelties in Apocynaceae subfam. Asclepiadoideae from New Caledonia. – Adansonia, sér. 3, 39: 55-70.
Meyer T. 1943. Género Oxypetalum (Asclepiadaceae). – Lilloa 9: 1-72.
Meylan BA, Butterfield BJ. 1978. The structure of New Zealand woods. – New Zealand Dept. Sci. Ind. Res. Bull. 222: 1-250.
Middleton DJ. 1993a. A taxonomic revision of Willughbeia Roxb. (Apocynaceae). – Blumea 38: 1-24.
Middleton DJ. 1993b. A new combination in Chonemorpha (Apocynaceae). – Novon 3: 455.
Middleton DJ. 1994a. A taxonomic revision of Ichnocarpus (Apocynaceae). – Blumea 39: 73-94.
Middleton DJ. 1994b. Three new combinations in Chinese Apocynaceae. – Novon 4: 152.
Middleton DJ. 1994c. A new combination in Epigynum (Apocynaceae). – Kew Bull. 49: 304.
Middleton DJ. 1995a. A revision of Papuechites (Apocynaceae). – Blumea 40: 439-442.
Middleton DJ. 1995b. Baharuia, a new genus of Apocynaceae from malesia. – Blumea 40: 443-447.
Middleton DJ. 1995c. A revision of the genus Urceola (Apocynaceae) in Thailand. – Kew Bull. 49: 757-767.
Middleton DJ. 1996a. Ecua, a new genus of Apocynaceae from Malesia. – Blumea 41: 33-35.
Middleton DJ. 1996b. A revision of Anodendron Roxb. (Apocynaceae). – Blumea 41: 37-68.
Middleton DJ. 1996c. A revision of Aganonerion Pierre ex Spire, Parameria Benth. and Urceola Roxb. (Apocynaceae). – Blumea 41: 69-122.
Middleton DJ. 1996d. A revision of Aganosma (Blume) G. Don (Apocynaceae). – Kew Bull. 51: 455-482.
Middleton DJ. 1997a. A revision of Carruthersia Seem. – Blumea 42: 489-498.
Middleton DJ. 1997b. A revision of Parsonsia R. Br. in Malesia. – Blumea 42: 191-248.
Middleton DJ. 2000. Revision of Alyxia (Apocynaceae). Part 1: Asia and Malesia. – Blumea 45: 1-146.
Middleton DJ. 2002. Revision of Alyxia (Apocynaceae). Part 2: Australia and Pacific Islands. – Blumea 47: 1-93.
Middleton DJ. 2003a. A revision of Dyera (Apocynaceae, Rauvolfioideae). – Gard. Bull. Singapore 55: 209-217.
Middleton DJ. 2003b. A new species and a new combination in Bornean Kopsia (Apocynaceae: Rauvolfioideae). – Gard. Bull. Singapore 55: 65-68.
Middleton DJ. 2004. A revision of Kopsia Blume (Apocynaceae: Rauvolfioideae). – Harvard Pap. Bot. 9: 91-144.
Middleton DJ. 2005a. A new species of Kopsia (Apocynaceae, Rauvolfioideae) from Vietnam. – Adansonia, sér. III, 27: 287-289.
Middleton DJ. 2005b. A revision of Epigynum (Apocynaceae: Apocynoideae). – Harvard Pap. Bot. 10: 67-81.
Middleton DJ. 2005c. A revision of Wrightia (Apocynaceae: Apocynoideae) in Malesia. – Harvard Pap. Bot. 10: 161-182.
Middleton DJ. 2006a. Three new species of Chilocarpus (Apocynaceae: Rauvolfioideae) from Malesia. – Edinburgh J. Bot. 63: 201-207.
Middleton DJ. 2006b. Nomenclatural notes on Asian Apocynaceae, subfamilies Rauvolfioideae and Apocynoideae. – Taxon 55: 502-506.
Middleton DJ. 2007a. Angiosperms: Apocynaceae of Papua. – In: Marshall AJ, Beehler BM (eds), The ecology of Papua 1, pp. 355-358.
Middleton DJ. 2007b. Apocynaceae (subfamilies Rauvolfioideae and Apocynoideae). – In: Nooteboom HP (ed), Flora Malesiana I, 18, Foundation Flora Malesiana, Nationaal Herbarium of Nederland, Leiden, The Netherlands, pp. 1-471.
Middleton DJ, Livshultz T. 2012. Streptoechites gen. nov., a new genus of Asian Apocynaceae. – Adansonia 34: 365-375.
Middleton DJ, Regalado Jr JC. 2005. Nomenclatural notes on species of Apocynaceae described from Cambodia, Laos and Vietnam. – Adansonia, sér. III, 27: 291-308.
Mikheyev AD. 1992. The synopsis of the family Rubiaceae of the Caucasian flora. – Bot. Žurn. 77: 68-74. [In Russian]
Miller JS. 2003. Classification of Boraginaceae subfam. Ehretioideae: resurrection of the genus Hilsenbergia Tausch ex Meisn. – Adansonia, sér. III, 25: 151-189.
Mohan JSS, Inamdar JA. 1986. Ultrastructure and secretion of the extra floral nectaries of Plumeria rubra. – Ann. Bot., N. S., 57: 389-401.
Mohrbutter C. 1936. Embryologische Studien an Loganiaceen. – Planta 26: 64-80.
Molina JE, Struwe L. 2004. Neuburgia novocaledonica, comb. nov. and the first record of domatia in the family Loganiaceae. – Aust. Syst. Bot. 17: 399-406.
Molina JE, Struwe L. 2008. Revision of ring-gentians (Symbolanthus, Gentianaceae) from Bolivia, Ecuador and Peru, with a first assessment of conservation status. – Syst. Biodivers. 6: 477-501.
Molina JE, Struwe L. 2009. Utility of secondary structure in phylogenetic reconstructions using nrDNA ITS sequences – an example from Potalieae (Gentianaceae: Asteridae). – Syst. Bot. 34: 414-428.
Molina LS, Zegueira MF, Oliver PH. 2002. Pollen morphology of some Cuban Guettarda species (Rubiaceae: Guettardeae). – Grana 41: 142-148.
Mongrand S, Badoc A, Patouille B, Lacomblez C, Chavent M, Bessoule JJ. 2005. Chemotaxonomy of the Rubiaceae family based on leaf fatty acid composition. – Phytochemistry 66: 549-559.
Moore RJ. 1947. Cytotaxonomic studies in the Loganiaceae 1. Chromosome numbers and phylogeny in the Loganiaceae. – Amer. J. Bot. 34: 527-538.
Morales JF. 1996. Novelties in Prestonia (Apocynaceae). – Novon 6: 285-287.
Morales JF. 1997a. A reevaluation of Echites and Prestonia sect. Coalitae (Apocynaceae). – Brittonia 49: 328-336.
Morales JF. 1997b. A synopsis of the genus Allomarkgrafia (Apocynaceae). – Brittonia 49: 337-345.
Morales JF. 1997c. A synopsis of the genus Prestonia (Apocynaceae) section Tomentosae in Mesoamerica. – Novon 7: 59-66.
Morales JF. 1998a. A synopsis of the genus Mandevilla (Apocynaceae) in Mexico and Central America. – Brittonia 50: 214-232.
Morales JF. 1998b. A synopsis of the genus Macropharynx (Apocynaceae). – Rhodora 99: 252-262.
Morales JF. 1999a. Series of revisions of Apocynaceae XLV (ed. by AJM Leeuwenberg). A synopsis of the genus Odontadenia (Apocynaceae). – Bull. Jard. Bot. Natl. Belg. 67: 381-477.
Morales JF. 1999b. Hylaea (Apocynaceae-Apocynoideae), a new genus of South America. – Novon 9: 83-85.
Morales JF. 1999c. Rhodocalyx (Apocynaceae), a new synonym of Prestonia. – Novon 9: 89-91.
Morales JF. 1999d. New species of Stemmadenia and Tabernaemontana (Apocynaceae) from Costa Rica, Panama and Colombia. – Novon 9: 236-239.
Morales JF. 1999e. Miscellaneous notes in Temnadenia y Laubertia (Apocynaceae). – Novon 9: 240.
Morales JF. 2002a. Studies in Neotropical Apocynaceae I: a revision of the genus Laubertia. – Rhodora 104: 170-185.
Morales JF. 2002b. Studies in Neotropical Apocynaceae II: a revision of the genus Fernaldia. – Rhodora 104: 186-200.
Morales JF. 2003. Studies in Neotropical Apocynaceae III: a revision of the genus Secondatia A. DC., with discussion of generic classification. – Candollea 58: 305-319.
Morales JF. 2004. Estudios en las Apocynaceae Neotropicales VII: novedades taxonómicas en Prestonia (Apocynaceae, Apocynoideae) para Colombia y Ecuador, con comentarios sobre el grado de lobulación del nectario. – Candollea 59: 159-165.
Morales JF. 2005a. Estudios en las Apocynaceae Neotropicales XII: tres nuevas especies de Mandevilla Lindl. (Apocynoideae, Mesechiteae) para Colombia. – Candollea 60: 51-58.
Morales JF. 2005b. Estudios en las Apocynaceae Neotropicales XIII: revisión del género Temnadenia (Apocynoideae, Echiteae). – Candollea 60: 207-231.
Morales JF. 2005c. Estudios en las Apocynaceae Neotropicales XV: sinopsis del género Thoreauea (Apocynoideae, Echiteae), con una nueva especie de Veracruz, México. – Brittonia 57: 258-263.
Morales JF. 2005d. Estudios en las Apocynaceae Neotropicales XIX: la familia Apocynaceae s. str. (Apocynoideae, Rauvolfioideae) de Costa Rica. – Darwiniana 43: 90-191.
Morales JF. 2005e. Estudios en las Apocynaceae Neotropicales XX: monografía del género Peltastes (Apocynoideae, Echiteae), con una sinopsis de Stipecoma (Apocynoideae, Echiteae). – Candollea 60: 289-334.
Morales JF. 2006a. Estudios en las Apocynaceae Neotropicales XXVI: una monografía del género Mesechites (Apocynoideae, Mesechiteae). – Candollea 61: 215-277.
Morales JF. 2006b. Estudios en las Apocynaceae Neotropicales XXVIII: la familia Apocynaceae (Apocynoideae, Rauvolfioideae) de El Salvador, Centroamérica. – Darwiniana 44: 453-489.
Morales JF. 2007. Dos nuevas especies de Mandevilla (Apocynoideae, Mesechiteae) endémicas de Perú. – Darwiniana 45: 77-82.
Morales JF. 2009. Estudios en las Apocynaceae Neotropicales XXXVII: Monografía del genero Rhabdadenia (Apocynoideae: Echiteae). – J. Bot. Res. Inst. Texas 3: 541-564.
Morales JF. 2018. Studies in Neotropical Apocynaceae LIV: a synopsis of Asketanthera. – Candollea 73: 7-17.
Morales JF, Fuentes A. 2004a. Estudios en las Apocynaceae Neotropicales V: una nueva especie, nuevos reportes y nueva sinonimia en las Apocynaceae de Bolivia. – Sida 21: 165-174.
Morales JF, Fuentes A. 2004b. Estudios en las Apocynaceae Neotropicales VIII: nuevas especies de Mandevilla (Apocynoideae, Mesechiteae) para Perú y Bolivia, con notas sobre la morfología floral en corolas infundibuliformes. – Candollea 59: 167-174.
Morales JF, Méndez M. 2005. Estudios en las Apocynaceae Neotropicales XXII: nuevos realineamientos taxonómicos en el género Stemmadenia (Apocynaceae, Rauvolfioideae, Tabernaemontaneae). – Candollea 60: 345-371.
Morales JF, Williams J. 2004. Allotoonia, a new neotropical genus of Apocynaceae based on a subgeneric segregate of Echites. – Sida 21: 133-158.
Morales JF, Endress ME, Liede-Schumann S. 2017a. Sex, drugs and pupusas: disentangling relationships in Echiteae (Apocynaceae). – Taxon 66: 623-644.
Morales JF, Endress ME, Liede-Schumann S. 2017b. Systematics of Prestonia (Apocynaceae: Apocynoids: Echiteae) 80 years after Woodson. – Ann. Missouri Bot. Gard. 102: 520-541.
Moreira AX. 1961. Sõbre o pôlen de Gentianaceae. – An. Acad. Brasileira Ciën. 33: 2-3.
Moreira VF, Oliveira RR, Mathias L, Braz-Filho R, Vieira IJC. 2010. New chemical constituents from Borreria verticillata (Rubiaceae). – Helv. Chim. Acta 93: 1751-1757.
Morillo G. 1976. A revision of Blepharodon (Asclepiadaceae). – Ph.D. thesis, Saint Louis University, Saint Louis, Missouri.
Morillo G. 1978. Estudio preliminar de las especies venezolanas de Prestonia (Apocynaceae). – Mem. Soc. Ci. Nat. La Salle 38: 195-226.
Morillo G. 1988. Nuevas especies de Asclepiadaceae para Venezuela. – Ernstia 34: 12-30.
Morillo G. 1989. Veinte Asclepiadaceae sudamericanas nuevas ara la ciencia y una nueva combinación. – An. Jard. Bot. Madrid 47: 349-359.
Morillo G. 1990. Cuatro Apocynaceae nuevas o interesantes del Norte de Sudamérica. – An. Jard. Bot. Madrid 48: 25-30.
Morillo G. 1992. New species and new combinations in the Andean Cynanchum and Matelea (Asclepiadaceae). – Ernstia 2: 59-72.
Morillo G. 1993. Asclepiadaceae andinas nuevas o interesantes. – Acta Bot. Venez. 16: 55-71.
Morillo G. 1994a. Two new species and a new name in Andean Asclepiadaceae. – Nord. J. Bot. 14: 283-286.
Morillo G. 1994b. Fontellaea, gen. nov., y otras novedades o aportaciones en Asclepiadaceae andinas. – An. Jard. Bot. Madrid 52: 33-41.
Morillo G. 1994c. Seis Asclepiadaceae sudamericanas nuevas para la cienca. – Ernstia 4: 3-10.
Morillo G. 1997. Revisión preliminar de Metalepis Griseb. (Asclepiadaceae). – Pittieria 26: 65-99.
Morillo G. 2012. Aportes al conocimiento de las Gonolobinae (Apocynaceae-Asclepiadoideae). – Pittieria 36: 13-57.
Morillo G. 2013. Aportes al conocimiento de las Gonolobinae II (Apocynaceae, Asclepiadoideae). – Pittieria 37: 115-154.
Morillo G. 2015. Aportes al conocimiento de las Gonolobinae Parte III (Apocynaceae, Asclepiadoideae). – Pittieria 39: 191-258.
Motley TJ, Wurdack KJ, Delprete PG. 2005. Molecular systematics of the Catesbaeeae-Chiococceae complex (Rubiaceae): flower and fruit evolution and biogeographic implications. – Amer. J. Bot. 92: 316-329.
Mouly A. 2006. Statut de Plectronia paradoxa Virot, Rubiaceae myrmécophile de Nouvelle-Calédonie. – Adansonia, sér. III, 28: 161-166.
Mouly A. 2009. Les Rubiaceae émergentes de la canopée endémiques de l’archipel des Comores: affinities floristiques dans l’océan Indien et taxonomie. – Adansonia, sér. III, 31: 197-206.
Mouly A, Achille F. 2007. The enigmatic Rhopalobrachium fragrans transferred to Cyclophyllum (Rubiaceae). – Syst. Bot. 32: 883-887.
Mouly A, Hoang N. 2007. Une nouvelle espèce d’Ixora (Rubiaceae) cauliflore de Nouvelle-Calédonie. – Adansonia, sér. III, 29: 123-128.
Mouly A, Pisivin C. 2007. Rare and threatened new endemic Ixora (Rubiaceae) from New Caledonia. – Nord. J. Bot. 25: 14-19.
Mouly A, Razafimandimbison SG, Achille F, Haevermans T, Bremer B. 2007. Phylogenetic placement of Rhopalobrachium fragrans (Rubiaceae): evidence from molecular (rps16 and trnT-F) and morphological data. – Syst. Bot. 32: 872-882.
Mouly A, Razafimandimbison SG, Khodabandeh A, Bremer B. 2009. Phylogeny and classification of the species-rich pantropical showy genus Ixora (Rubiaceae-Ixoreae) with indications of geographical monophyletic units and hybrids. – Amer. J. Bot. 96: 686-706.
Mouly A, Razafimandimbison SG, Florence J, Jérémie J, Bremer B. 2009. Paraphyly of Ixora and new tribal delimitation of Ixoreae (Rubiaceae): inference from combined chloroplast (rps16, rbcL, and trnT-F) sequence data. – Ann. Missouri Bot. Gard. 96: 146-160.
Mouly A, Kainulainen K, Persson C, Davis AP,
Wong KM, Razafimandimbison SG, Bremer B. 2014. Phylogenetic structure and clade
circumscriptions in the Gardenieae complex (Rubiaceae). – Taxon 63: 801-818.
Moynihan J. 1999. Molecular phylogeny of the Caribbean endemic Neolaugeria (Rubiaceae), based on the internal transcribed spacer regions of nuclear ribosomal DNA. – M.S. thesis, Dept. of Botany, Miami University, Florida.
Moynihan J, Watson LE. 2001. Phylogeography, generic allies, and nomenclature of Caribbean endemic genus Neolaugeria (Rubiaceae) based on internal transcribed spacer sequences. – Intern. J. Plant Sci. 162: 393-401.
Mukherjee KS, Mukhopadhyay B, Mondal S, Gorai D, Brahmachari G. 2004. Triterpenoid constituents of Borreria articularis. – J. Chinese Chem. Soc. 51: 229-231.
Mulay BN, Deshpande BD, Tolani U. 1965. Studies in Asclepiadaceae II. Floral morphology and gametogenesis in certain members of the Asclepiadaceae. – J. Indian Bot. Soc. 44: 95-104.
Müller G. 1982. Contribution à la cytotaxonomie de la section Cyclostigma Griseb. du genre Gentiana L. – Feddes Repert. 93: 625-722.
Murata J. 1989. A synopsis of Tripterospermum (Gentianaceae). – J. Fac. Sci., Univ. Tokyo, sect. 3, Bot. 14: 273-339.
Murphy H. 1986. A revision of the genus Fischeria (Asclepiadaceae). – Syst. Bot. 11: 229-241.
Mwanyambo ML. 1996. Infraspecific variation in the African genus Margaretta (Asclepiadaceae: Asclepiadoideae). – Kew Bull. 51: 717-728.
Naiki A, Nagamasu H. 2003. Distyly and pollen dimorphism in Damnacanthus (Rubiaceae). – J. Plant Res. 116: 105-113.
Nakamura K, Chung S-W, Kokubugata G, Denda T, Yokota M. 2006. Phylogenetic systematics of the monotypic genus Hayataella (Rubiaceae) endemic to Taiwan. – J. Plant Res. 119: 657-661.
Natali A, Manen J-F, Ehrendorfer F. 1995. Phylogeny of the Rubiaceae-Rubioideae, in particular the tribe Rubieae: evidence from a non-coding chloroplast DNA sequence. – Ann. Missouri Bot. Gard. 82: 428-439.
Natali A, Manen J-F, Kiehn M, Ehrendorfer F. 1996. Tribal, generic, and specific relationships in the Rubioideae-Rubieae (Rubiaceae) based on sequence data of the cpDNA intergene spacer. – Opera Bot. Belg. 7: 193-203.
Nazar N, Goyder DJ, Clarkson JJ, Mahmood T, Chase MW. 2013. The taxonomy and systematics of Apocynaceae: where we stand in 2012. – Bot. J. Linn. Soc. 171: 482-490.
Negrón-Ortiz V. 1996. Reproductive biology of Ernodea (Rubiaceae-Spermacoceae) in the Bahamas and Puerto Rico. – In: Robbrecht E, Puff C, Smets E (eds), Proceedings of the second international Rubiaceae conference, Opera Bot. Belg. 7: 403-412.
Negrón-Ortiz V, Hickey RJ. 1996a. The genus Ernodea (Rubiaceae) in the Caribbean basin I. Allozyme variation and mating systems. – Syst. Bot. 21: 433-443.
Negrón-Ortiz V, Hickey RJ. 1996b. The genus Ernodea (Rubiaceae) in the Caribbean basin II. Morphological analyses and systematics. – Syst. Bot. 21: 445-458.
Negrón-Ortiz V, Watson LE. 2002. Molecular phylogeny and biogeography of Erithalis (Rubiaceae), an endemic of the Caribbean Basin. – Plant Syst. Evol. 234: 71-83.
Negrón-Ortiz V, Watson LE. 2003. Hypotheses for the colonization of the Caribbean basin by two genera of the Rubiaceae: Erithalis and Ernodea. – Syst. Bot. 28: 442-451.
Nemomissa S. 1998. A synopsis of Swertia (Gentianaceae) in east and northeast tropical Africa. – Kew Bull. 53: 419-436.
Nemomissa S. 1999. Two new species of Sebaea R. Br. (Gentianaceae) from Tanzania. – Kew Bull. 54: 191-196.
Nemomissa S. 2000. Two new species of Sebaea R. Br. (Gentianaceae) from Tanzania and one new combination. – Kew Bull. 55: 213-218.
Nemomissa S. 2002. Gentianaceae. – In: Beentje HJ, Smith SAL (eds), Flora of tropical East Africa, A. A. Balkema, Rotterdam, The Netherlands, pp. 1-68.
Nepokroeff M, Bremer B, Sytsma KJ. 1999. Reorganization of the genus Psychotria and tribe Psychotrieae (Rubiaceae) inferred from ITS and rbcL sequence data. – Syst. Bot. 24: 5-27.
Nepokroeff M, Sytsma KJ, Wagner WL, Zimmer EA. 2003. Reconstructing ancestral patterns of colonization and dispersal in the Hawaiian understory tree genus Psychotria (Rubiaceae): a comparison of parsimony and likelihood approaches. – Syst. Biol. 52: 820-838.
Neuba DFR, Lejoly J, Sonké B. 2006. Un Leptactina (Rubiaceae, Pavetteae) nouveau de République Centrafricaine: Leptactina deblockiae. – Adansonia, sér. III, 28: 373-378.
Neuba DFR, Malan DF, Kouadio YL. 2014. Notes
sur le genre Africain Leptactina Hook. f. (Rubiaceae, Pavetteae). – Adansonia,
sér. 3, 36: 121-153.
Neubauer HF. 1981. Der Knotenbau einiger Rubiaceae. – Plant Syst. Evol. 139: 103-111.
Neupane S, Dessein S, Wikström N, Lewis PO, Long C, Bremer B, Motley TJ. 2015. The Hedyotis-Oldenlandia complex (Rubiaceae: Spermacoceae) in Asia and the Pacific: phylogeny revisited with new generic delimitations. – Taxon 64: 299-322.
Neupane S, Lewis PO, Dessein S, Shanks H, Paudyal S, Lens F. 2017. Evolution of woody life form on tropical mountains in the tribe Spermacoceae (Rubiaceae). – Amer. J. Bot. 104: 419-438.
Newton LE. 1984. Terminology of structures associated with pollinia of the Asclepiadaceae. – Taxon 33: 619-621.
Nguembou K C, Zapfack L, Lejoly J. 2003. Les espèces camerounaises du genre Bertiera (Rubiaceae). – Syst. Geogr. Plants 73: 237-280.
Nguembou K C, Ewedje E-EBK, Droissart V, Stévart T, Sonké B. 2009. Une espèce nouvelle de Bertiera (sous-genre Bertierella, Rubiaceae) d’Afrique central atlantique. – Adansonia, sér. III, 31: 397-406.
Nicholas A. 1982. Taxonomic studies in Asclepias (Asclepiadaceae) with particular reference to the narrow-leaved species in southern Africa. – M.Sc. thesis, University of Natal, Pietermaritzburg, South Africa.
Nicholas A, Baijnath H. 1994. A consensus classification for the order Gentianales with additional details on the suborder Apocynineae. – Bot. Rev. 60: 440-482.
Nicholas A, Goyder DJ. 1990. Asclepiadaceae. Corona lobe variation and the generic position of Asclepias macra. – Bothalia 20: 87-90.
Nicholas A, Goyder DJ. 1992. Aspidonepsis (Asclepiadaceae), a new southern African genus. – Bothalia 22: 23-35.
Nie Z-L, Wen J, Sun H, Bartholomew B. 2005. Monophyly of Kelloggia Torrey ex Benth. (Rubiaceae) and evolution of its intercontinental disjunction between western North America and eastern Asia. – Amer. J. Bot. 92: 642-652.
Nie Z-L, Deng T, Meng Y, Sun H, Wen J. 2013. Post-boreotropical dispersals explain the pantropical disjunction in Paederia (Rubiaceae). – Ann. Bot. 111: 873-886.
Nilsson LA, Rabakonandriania E, Pettersson B, Ranaivo J. 1990. ‘Ixoroid’ secondary pollen presentation and pollination by small moths in the Malagasy treelet Ixora platythyrsa. – Plant Syst. Evol. 170: 161-175.
Nilsson S. 1964. On the pollen morphology in Lomatogonium A. Br. – Grana Palynol. 5: 298-329.
Nilsson S. 1967a. Notes on pollen morphological variation in Gentianaceae-Gentianinae. – Pollen Spores 9: 49-58.
Nilsson S. 1967b. Pollen morphological studies in the Gentianaceae-Gentianinae. – Grana Palynol. 7: 46-145.
Nilsson S. 1968. Pollen morphology in the genus Macrocarpaea (Gentianaceae) and its taxonomical significance. – Svensk Bot. Tidskr. 62: 338-364.
Nilsson S. 1970. Pollen morphological contributions to the taxonomy of Lisianthus L. s. lat. (Gentianaceae). – Svensk Bot. Tidskr. 64: 1-43.
Nilsson S. 2002. Gentianaceae: a review of palynology. – In: Struwe L, Albert VA (eds), Gentianaceae – systematics and natural history, Cambridge University Press, Cambridge, pp. 377-497.
Nilsson S, Skvarla JJ. 1969. Pollen morphology of saprophytic taxa in the Gentianaceae. – Ann. Missouri Bot. Gard. 56: 420-438.
Nilsson S, Endress ME, Grafström E. 1993. On the relationship of the Apocynaceae and Periplocaceae. – Grana Suppl. 2: 3-20.
Nilsson S, Hellbom M, Smolenski W. 2002. A reappraisal of the significance of pollen in classifications of the Gentianaceae. – Grana 41: 90-106.
Nishino E. 1983. Corolla tube formation in the Tubiflorae and Gentianales. – Bot. Mag. (Tokyo) 96: 223-243.
Nowak MD, Davis AP, Yoder AD. 2012. Sequence dta from new plastid and nuclear COSII regions resolves early diverging lineages in Coffea (Rubiaceae). – Syst. Bot. 37: 995-1005.
Nowicke J. 1970. Apocynaceae. – In: Woodson RE, Schery R & al. (eds), Flora of Panama IV, Ann. Missouri Bot. Gard. 57: 59-130.
Ntore S. 2004. Contribution à la connaissance systématique du genre afrotropical Pauridiantha (Rubiaceae). – Ph.D. diss., Katolieke Universiteit Leuwen, Belgium.
Ntore S. 2008. Révision du genre Afrotropical Pauridiantha (Rubiaceae). – Opera Bot. Belg. 15: 1-227.
Ntore S, Block P de, Huysmans S, Robbrecht E, Dessein S. 2003. Two new species from Gabon show the need to reduce Commitheca to the synonymy of Pauridiantha (Rubiaceae, Pauridiantheae). – Bot. J. Linn. Soc. 141: 105-117.
Ntore S, Robbrecht E, Smets E. Dessein S. 2003. Révision de Pauridiantha paucinervis (Rubiaceae-Pauridiantheae) et des espèces voisines. – Belg. J. Bot. 136: 73-90.
Ntore S, Robbrecht E, Smets E, Dessein S. 2003. Deux nouvelles espèces paléo-endémiques de Pauridiantha (Rubiaceae) des Monts Udzungwa (sud de la Tanzanie). – Adansonia, sér. III, 25: 65-76.
Ntore S, Lachenaud O, Dessein S. 2010. Four new Pauridiantha species (Rubiaceae) reflect the richness of Gabon’s rainforests. – Belg. J. Bot. 142: 177-193.
Ochoterena-Booth H. 2000. Systematics of Hintonia Bullock and the Portlandia complex (Rubiaceae). – Ph.D. diss., Cornell University, Ithaca, New York.
Oehler E. 1927. Entwicklungsgeschichtlich-zytologische Untersuchungen an einigen saprophytischen Gentianaceen. – Planta 3: 641-733.
Oevelen S van, Prinsen E, Wachter R de, Robbrecht E. 2001 [2002]. The taxonomic value of bacterial symbiont identification in African Psychotria (Rubiaceae). – Syst. Geogr. Plants 71: 557-563.
Okorie DA. 1976. A new phthalide and xanthones from Anthocleista djalonensis and Anthocleista vogelii. – Phytochemistry 15: 1799-1800.
Oliver D. 1873. Descriptions of three new genera of plants in the Malayan herbarium of the late Dr A. C. Maingay. – Trans. Linn. Soc. London 28: 515-518.
Ollerton J, Liede S. 1997. Pollination systems in the Asclepiadaceae: a survey and preliminary analysis. – Biol. J. Linn. Soc. 62: 593-610.
Ollerton J, Masinde S, Meve U, Picker M, Whittington A. 2009. Fly pollination in Ceropegia (Apocynaceae: Asclepiadoideae): biogeographic and phylogenetic perspectives. – Ann. Bot. 103: 1501-1514.
Omer S. 1989. Quaisera Omer, a new genus of Gentianaceae. – Bot. Jahrb. Syst. 111: 205-212.
Omer S. 1995. Gentianaceae. – In: Ali SI, Qaiser M (eds), Flora of Pakistan 197, Department of Botany, University of Karachi, Karachi, Pakistan, pp. 1-172.
Omer S, Qaiser M. 1991. Aliopsis, a new genus of Gentianaceae from C Asia. – Willdenowia 21: 189-194.
Omer S, Qaiser M. 1992. Generic limits in Gentiana (Gentianaceae) and related genera in Pakistan and adjoining areas along with a new genus Kurramiana. – Pakistan J. Bot. 24: 95-106.
Omer S, Qaiser M, Ali SI. 1988. Studies in the family Gentianaceae. The genus Aloitis Rafin. from Pakistan and Kashmir. – Pakistan J. Bot. 20: 153-160.
Omino EA. 1996. A contribution to the leaf anatomy and taxonomy of Apocynaceae in Africa. – Belmontia 29: 1-178.
Omino EA. 2002. Apocynaceae (Part 1). – In: Beentje HJ, Ghazanfar SA (eds), Flora of tropical East Africa, A. A. Balkema Publ., Lisse, The Netherlands, pp. 1-114.
Omlor R. 1996. Do Menabea venenata and Secamonopsis madagascariensis represent missing links between Periplocaceae, Secamonoideae and Marsdenieae (Asclepiadaceae)? – Kew Bull. 51: 695-715.
Omlor R. 1998. Generische Revision der Marsdenieae (Asclepiadaceae). – Shaker, Aachen.
Onana JM. 2008. A new combination and key to the species of Cuviera subgenus Globulostylis (Rubiaceae-Vanguerieae) from Central Africa. – Kew Bull. 63: 401-403.
Orchard AE. 1986. A revision of the Coprosma pumila (Rubiaceae) complex in Australia, New Zealand and the Subantarctic Islands. – Brunonia 9: 119-138.
Ornduff R. 1970. Systematics and breeding system of Gelsemium (Loganiaceae). – J. Arnold Arbor. 51: 1-17.
Ortega-Olivencia A, Devesa JA. 2003. Two new species of Galium (Rubiaceae) from the Iberian Peninsula. – Bot. J. Linn. Soc. 143: 177-187.
Owens SJ, Jackson A, Maunder M, Rudall P, Johnson MAT. 1993. The breeding system of Ramosmania heterophylla – dioecy or heterostyly? – Bot. J. Linn. Soc. 113. 77-86.
Oxelman B, Bremer B. 2000. Discovery of paralogous nuclear gene sequences coding for the second-largest subunit of RNA polymerase II (RPB2) and their phylogenetic utility in Gentianales of the asterids. – Mol. Biol. Evol. 17: 1131-1145.
Pacheco-Trejo J, Terrazas T, Ochoterena H. 2009. Leaf architecture of the genus Didymaea Hook. f. (Rubiaceae). – Plant Syst. Evol. 281: 137-149.
Padmanabhan D. 1961. A contribution to the embryology of Gomphandra polymorpha. – Proc. Indian Natl. Inst. Sci., Sect. B, 27: 389-398.
Pailler T, Thompson JD. 1997. Distyly and variation in heteromorphic incompatibility in Gaertnera vaginata (Rubiaceae) endemic to La Réunion Island. – Amer. J. Bot. 84: 315-327.
Païs M, Jarreau FX, Fouché P, Goutarel R. 1971. À propos d’une falsification des graines du Strophanthus gratus Franchet. Un nouvel alcaloïde, l’alafine, isolé des graines d’un Alafia sp. et de l’Alafia multiflora Stapf (Apocynacées). – Ann. Pharm. France 29: 57-72.
Paiva J, Noguiera I. 1990a. Studies in African Gentianaceae. – An. Real Jard. Bot. Madrid 47: 87-103.
Paiva J, Noguiera I. 1990b. Gentianaceae. – In: Launert E, Pope GV (eds), Flora Zambesiaca 7I(4), Flora Zambesiaca Managing Committee, Kew, pp. 3-51.
Pardi P. 1933. Studi sulla cariologia delle Asclepiadaceae. – Nuovo Giorn. Bot. Ital. 40: 576-589.
Parès Y, Ruat L. 1953. Observations sur le trichome des Rubiacées et des Loganiacées. – Rec. Trav. Laborat. Bot. Geol. Zool. Montpellier, sér. Bot., 6: 127-133.
Parra-Delgado H, Toscano RA, Garcia-Argaez AN, Martinez-Vazquez M. 2005. Chemical constituents of Coutaportla ghiesbreghtiana: co-crystallization of two ent-nor-kaurene diterpenes. – Zeitschr. Naturf. B, 60: 548-554.
Patel RC, Inamdar JA. 1974. Studies in the trichomes and nectaries of some Gentianales. – In: Puri V et al. (eds), Biology of land plants, S. Prakashan, Meerut.
Paudyal SK, Delprete PG, Motley TJ. 2014.
Using molecular, morphological, and palynological evidence to transfer
Strumpfia maritima to the monotypic tribe Strumpfieae (Cinchonoideae,
Rubiaceae), and a re-delimitation of
the tribe Chiococceae. – Syst. Bot. 39: 1197-1203.
Paul JR, Morton C, Taylor CM, Tonsor SJ. 2009. Evolutionary time for dispersal limits the extent but not the occupancy of species‘ potential ranges in the tropical plant genus Psychotria (Rubiaceae). – Amer. Natur. 173: 188-199.
Peckover R. 1996. The inclusion of the genera Tenaris E. May. and Macropetalum Burch. ex Decne. into Brachystelma R. Br. – Aloe 33: 41-43.
Perkins J. 1902. Monographische Übersicht der Arten der Gattung Lisianthus. – Bot. Jahrb. Syst. 31: 489-494.
Perrot ME. 1897 [1899]. Anatomie comparée des Gentianacées. – Ann. Sci. Nat. Bot., sér. VIII, 7: 105-292.
Perrot ME. 1903. Le Menabea venenata H. Bn. Ses charactères et sa position systématique. – J. Bot. 17: 109-116.
Perry JD. 1971. Biosystematic studies on the North American genus Sabatia (Gentianaceae). – Rhodora 73: 309-369.
Persson C. 1993. Pollen morphology of the Gardenieae-Gardeniinae (Rubiaceae). – Nord. J. Bot. 13: 561-582.
Persson C. 1995. Exotesta morphology of Gardenieae-Gardeniinae (Rubiaceae). – Nord. J. Bot. 15: 285-300.
Persson C. 1996. Phylogeny of the Gardenieae (Rubiaceae). – Bot. J. Linn. Soc. 121: 91-109.
Persson C. 1998. Phylogeny of the tribe Gardenieae (Rubiaceae). – Ph.D. diss., University of Gothenburg, Sweden.
Persson C. 2000a. Phylogeny of Gardenieae (Rubiaceae) based on chloroplast DNA sequences from the rps16 intron and trnL(UAA)-F(GAA) intergenic spacer. – Nord. J. Bot. 20: 257-269.
Persson C. 2000b. Phylogeny of the neotropical Alibertia group (Rubiaceae), with emphasis on the genus Alibertia, inferred from ITS and 5S ribosomal DNA sequences. – Amer. J. Bot. 87: 1018-1028.
Persson C. 2000c. Stenosepala hirsuta, a new genus and species of Gardenieae (Rubiaceae) from Colombia and Panama. – Novon 10: 403-406.
Persson C. 2003. Agouticarpa, a new neotropical genus of tribe Gardenieae (Rubiaceae). – Brittonia 55: 176-201.
Persson C, Delprete PG. 2008. Agouticarpa spinosa sp. nov. – a thorny member of the Alibertia group (Rubiaceae-Gardenieae) from northern Peru. – Nord. J. Bot. 26: 57-60.
Persson C, Ljungstrand E. 2006. Proposal to conserve Duroia, nom. cons. (Rubiaceae, Gardenieae) against an additional name, Coupoui. – Taxon 55: 236-237.
Pertot M, Poldini L. 1979. Le Gentianae della sect. Cyclostigma Griseb. nelle Alpi Friulane e nel Carso Triestino. – Gorttania, Atti Museo Friul. Storia Nat. 1: 91-119.
Petit E. 1964. Les espèces africaines du genre Psychotria L. (Rubiaceae) I. – Bull. Jard. Bot. État 34: 1-229.
Petit E. 1966. Les espèces africaines du genre Psychotria L. (Rubiaceae) II. – Bull. Jard. Bot. État 36: 65-190.
Pfanzelt S, Hagen KB von. 2016. Morphological variation of Gentiana section Chondrophyllae in South America and taxonomic implications. – Plant Syst. Evol. 302: 155-172.
Philip O, Mathew PM. 1975. Cytology of exceptional development of the male gametophyte in Ophiorrhiza mungos. – Can. J. Bot. 53: 2032-2037.
Philip O, Mathew PM. 1987. Cytology of the south India Rubiaceae and its bearing on the evolution and systematics of the family. – Glimpses Plant Res. 8: 177-244.
Philipson WR. 1972. The generic status of the southern hemisphere gentians. – In: Murty YS, Johri BM, Mohan Ram HY, Varghese TM (eds), Advances in plant morphology. Professor V. Puri commemorative volume, Sarita Prakashan, Meerut, pp. 417-422.
Pichon M. 1948a. Classification des Apocynacées I. Carissées et Ambélaniées. – Mém. Mus. Natl. Hist. Nat. Paris, n. s., 24: 111-181.
Pichon M. 1948b. Classification des Apcynacées V. Cerberoïdées. – Notul. Syst. (Paris) 13: 212-229.
Pichon M. 1948c. Classification des Apocynacées IX. Rauvolfiées, Alstoniées, Allamandées, et Tabernémontanoïdées. – Mém. Mus. Natl. Hist. Nat. Paris, n. s., 27: 153-252.
Pichon M. 1948d. Classification des Apocynacées XIX. Le rétinacle des Echitoïdées. – Bull. Soc. Bot. France 95: 211-216.
Pichon M. 1949. Les glandes nodales des Apocynacées et leurs modification. – Bull. Mus. Natl. Hist. Nat. Paris 21: 467-473.
Pichon M. 1950. Classification des Apocynacées XXV. Échitoïdées. – Mém. Mus. Natl. Hist. Nat. Paris, sect. B, Bot. 1: 1-174.
Piesschaert F. 2001. Carpology and pollen morphology of the Psychotrieae (Rubiaceae-Rubioideae): towards a new tribal and generic delimitation. – Ph.D. diss., Katolieke Universiteit de Leuven, Belgium.
Piesschaert F, Robbrecht E, Smets E. 1997. Dialypetalanthus fuscescens Kuhlm. (Dialypetalanthaceae): the problematic taxonomic position of an Amazonian endemic. – Ann. Missouri Bot. Gard. 84: 201-223.
Piesschaert F, Robbrecht E, Smets E. 1999. Chassalia subcordatifolia, a new combination in African Rubiaceae (Rubioideae, Psychotrieae). – Syst. Geogr. Plants 69: 189-194.
Piesschaert F, Jansen S, Huysmans S, Smets E, Robbrecht E. 1999. Chassalia petitiana (Rubiaceae-Psychotrieae), an overlooked epiphytic species hidden in the African canopy. – Syst. Bot. 24: 315-322.
Piesschaert F, Robbrecht E, Poulsen AD, Smets E. 1999. Pyrene and pollen observations in the pantropical genus Geophila (Rubiaceae-Psychotrieae). – Nord. J. Bot. 19: 93-100.
Piesschaert F, Huysmans S, Jaimes I, Robbrecht E, Smets E. 2000. Morphological evidence for an extended tribe Coccocypseleae (Rubiaceae-Rubioideae). – Plant Biol. 2: 536-546.
Piesschaert F, Andersson L, Jansen S, Dessein S, Robbrecht E, Smets E. 2000. Searching for the taxonomic position of the African genus Colletoecema (Rubiaceae): morphology and anatomy compared to an rps16-intron analysis of the Rubioideae. – Can. J. Bot. 78: 288-304.
Piesschaert F, Jansen S, Jaimes I, Robbrecht E, Smets E. 2001. Morphology, anatomy, and taxonomic position of Pagameopsis (Rubiaceae-Rubioideae). – Brittonia 53: 490-504.
Pire SM. 1996. Palynological study of American species of Borreria (Rubiaceae-Spermacoceae). – In: Robbrecht E, Puff C, Smets E (eds), Proceedings of the second international Rubiaceae conference, Opera Bot Belg. 7: 416-423.
Pire SM. 1997a. The pollen of Brazilian species of Richardia L. (Rubiaceae-Spermacoceae). – Rev. Univ. Guarulhos, Geosiências II, Sp. Vol.: 184-191.
Pire SM. 1997b. Género Galianthe subg. Ebelia (Rubiaceae: Spermacoceae): estudio palinológico. – Ann. Missouri Bot. Gard. 84: 878-887.
Pire SM. 1998. El polen de especies brasileñas de Richardia L. (Rubiaceae, Spermacoceae). – Geociências 2: 184-191.
Pire SM, Cabral EL. 1992. El valor del polen en la revalidación de Galianthe (Spermacoceae-Rubiaceae). – Darwiniana 31: 1-10.
Pissjaukova Y. 1961. Notulae de genere Swertia L. – Bot. Mater. Gerb. Bot. Inst. Komarova Akad. Nauk SSSR 21: 292-313.
Pitard J. 1922-1924. Rubiaceae. – In: Lecomte H, Humbert H (eds), Flore générale de l’Indo-Chine, Muséum National d’Histoire Naturelle, Paris, pp. 20-442.
Ploeg J van der. 1985. Series of revisions of Apocynaceae XVIII. Revision of the genera Cyclocotyla Stapf, Dewevrella De Wild. and of the African species of the genus Malouetia A. DC. (Apocynaceae). – Wageningen Agr. Univ. Pap. 85: 57-84.
Plowes DCH. 1990. An introduction to stapeliad genera. – Cact. Succ. J. (Los Angeles) 62: 111-129.
Plowes DCH. 1993. A new account of Echidnopsis Hook. f. (Asclepiadaceae: Stapelieae). – Haseltonia 1: 65-85.
Plowes DCH. 1995. A reclassification of Caralluma R. Br. (Stapelieae: Asclepiadaceae). – Haseltonia 3: 49-70.
Post DM. 1958. Studies in Gentianaceae I. Nodal anatomy of Frasera and Swertia perennis. – Bot. Gaz. 120: 1-14.
Potgeiter K, Albert VA. 2001. Phylogenetic relationships within Apocynaceae s.l. based on trnL intron and trnL-F spacer sequences and propagule characters. – Ann. Missouri Bot. Gard. 88: 523-549.
Pötter U, Klopfer K. 1987. Untersuchungen zur Blatt- und Blütenentwicklung bei Galium aparine L. (Rubiaceae). – Flora 179: 305-314.
Poulsen VA. 1893. Cynocrambaceae (Thelygonaceae). – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(1a), W. Engelmann, Leipzig, pp. 121-124.
Pringle JS. 1977. Taxonomy and distribution of Gentiana (Gentianaceae) in Mexico and Central America: 1, sect. Pneumonanthe. – Sida 7: 174-217.
Pringle JS. 1978. Sectional and subgeneric names in Gentiana (Gentianaceae). – Sida 7: 232-247.
Pringle JS. 1979. Taxonomy and distribution of Gentiana (Gentianaceae) in Mexico and Central America: 2, sect. Chondrophyllae. – Sida 8: 14-33.
Pringle JS. 1990. Taxonomic notes on western American Gentianaceae. – Sida 14: 179-187.
Pringle JS. 1995. 159A. Gentianaceae. – In: Harling G, Andersson L (eds), Flora of Ecuador 53, Nord. J. Bot., Copenhagen, pp. 1-131.
Pringle JS. 2017. Four new species of Gentianella (Gentianaceae, Gentianeae, Swertiinae) from Peru, with a review of the taxonomy of the genus. – Novon 25: 451-466.
Prosser F, Bertolli A. 2008. A new species of Gentiana sect. Calathianae (Gentianaceae) from the Brenta Group, European Alps, Italy. – Willdenowia 38: 423-431.
Puff C. 1978. The genus Galium L. (Rubiaceae) in Southern Africa. – J. South Afr. Bot. 44: 203-279.
Puff C. 1981a. Studies in Otiophora Zucc. (Rubiaceae) 1. The tropical African and Madagascan taxa. – Bull. Jard. Bot. Belg. 51: 119-141.
Puff C. 1981b. Studies in Otiophora Zucc. (Rubiaceae) 2. A new classification of the southern African taxa. – J. South Afr. Bot. 47: 297-329.
Puff C. 1982. The delimitation of the tribe Anthospermeae and its affinities to the Paederieae (Rubiaceae). – Bot. J. Linn. Soc. 84: 355-377.
Puff C. 1983. Studies in Otiophora Zucc. (Rubiaceae) 4. The taxonomic position of the genus. – Bothalia 14: 185-188.
Puff C. 1986a. Phylohydrax (Rubiaceae-Spermacoceae) – a new genus to accommodate the African and Madagascan “Hydrophylax” species. – Plant Syst. Evol. 154: 343-366.
Puff C. 1986b. A biosystematic study of the African and Madagascan Rubiaceae-Anthospermeae. – Plant Syst. Evol. [Suppl.] 3: 1-535.
Puff C. 1988a. Observations on Carphalea Juss. (Rubiaceae, Hedyotideae), with particular reference to the Madagascan taxa and its taxonomic position. – Bull. Jard. Bot. Natl. Belg. 58: 271-323.
Puff C. 1988b. Two new Neogaillonia species (Rubiaceae, tribe Paederieae) from Somalia. – Nord. J. Bot. 8: 331-336.
Puff C. 1989a. Observations on the Japanese endemic Pseudopyxis (Rubiaceae-Paederieae). – Plant Spec. Biol. 4: 131-144.
Puff C. 1989b. The affinities and relationships of the Japanese endemic Pseudopyxis (Rubiaceae-Paederieae). – Plant Spec. Biol. 4: 145-155.
Puff C. 1990. Comments on the Rubiaceae of N. E. Africa (Flora of Ethiopia and Flora of Somalia area). – Mitt. Inst. Allg. Bot. Hamburg 23b: 803-822.
Puff C. 1991a. The genus Paederia L. (Rubiaceae-Paederieae): taxonomic history, revised generic description, and subgeneric division. – In: Puff C (ed), The genus Paederia L.: a multidisciplinary study, Opera Bot. Belg. 3: 195-204.
Puff C. 1991b. Revision of the genus Paederia L. (Rubiaceae-Paederieae) in Africa and Madagascar. – In: Puff C (ed), The genus Paederia L. (Rubiaceae-Paederieae): a multidisciplinary study, Opera Bot. Belg. 3: 293-322.
Puff C. 1993. Pollen nuclear numbers in the Rubiaceae. – Opera Bot. Belg. 6: 31-49.
Puff C, Buchner R. 1994. Revision of Danais Vent. (Rubiaceae) in Madagascar and the Comores. – Bull. Mus. Natl. Hist. Nat., Paris, sér. IV, sect. B, Adansonia, 1: 11-64.
Puff C, Buchner R. 1998. Lecananthus and Leucocodon, two genera to be added to the tribe Schradereae (Rubiaceae). – Blumea 43: 265-286.
Puff C, Igersheim A. 1991. The flowers of Paederia L. (Rubiaceae-Paederieae). – In: Puff C (ed), The genus Paederia L. (Rubiaceae-Paederieae): a multidisciplinary study, Opera Bot. Belg. 3: 55-75.
Puff C, Igersheim A. 1994a. The character states and taxonomic position of Metabolus Bl. (syn. Allaeophania Thw.) (Rubiaceae). – Bull. Jard. Bot. Natl. Belg. 63: 241-262.
Puff C, Igersheim A. 1994b. The character states of Mussaendopsis Baill. (Rubiaceae-Coptosapelteae). – Flora 189: 161-178.
Puff C, Igersheim A. 1994. Intra-ovarian trichomes in Jackiopsis ornata (Wallich) Ridsdale (Rubiaceae-Jackieae). – Bot. J. Linn. Soc. 115: 29-33.
Puff C, Mantell DE. 1982. The tribal position and affinities of Crocyllis (Rubiaceae). – Bot. Jahrb. Syst. 103: 89-106..
Puff C, Robbrecht E. 1988. The taxonomic position of the Australian endemic Durringtonia (Rubiaceae). – Aust. Syst. Bot. 1: 191-197.
Puff C, Robbrecht E. 1989. A survey of the Knoxieae (Rubiaceae-Antirheoideae). – Bot. Jahrb. Syst. 110: 511-558.
Puff C, Rohrhofer U. 1993 [1994]. The character states and taxonomic position of the monotypic mangrove genus Scyphiphora (Rubiaceae). – In: Robbrecht E (ed), Advances in Rubiaceae macrosystematics, Opera Bot. Belg. 6: 143-172.
Puff C, Robbrecht E, Randrianasolo V. 1984. Observations on the SE African-Madagascan genus Alberta and its ally Nematostylis (Rubiaceae, Alberteae), with a survey of the species and a discussion of the taxonomic position. – Bull. Jard. Bot. Natl. Belg. 54: 293-366.
Puff C, Andersson L, Rohrhofer U, Igersheim A. 1993. The tribe Schradereae (Rubiaceae) re-examined. – Bot. Jahrb. Syst. 114: 449-479.
Puff C, Igersheim A, Rohrhofer U. 1993. Pseudomussaenda and Schizomussaenda (Rubiaceae): close allies of Mussaenda. – Bull. Jard. Bot. Natl. Belg. 62: 35-68.
Puff C, Igersheim A, Buchner R, Rohrhofer U. 1995. The united stamens of Rubiaceae. Morphology, anatomy; their role in pollination ecology. – Ann. Missouri Bot. Gard. 82: 357-382.
Puff C, Robbrecht E, Buchner R, de Block P. 1996. A survey of secondary pollen presentation in the Rubiaceae. – Opera Bot. Belg. 7: 369-402.
Puff C, Buchner R, Igersheim A. 1996. Dichilanthe, an unusual Asiatic Rubiaceae with “Lonicera flowers” and “dipterocarp fruits”. – Nord. J. Bot. 16: 145-164.
Puff C, Buchner R, Greimler J. 1998. Revision of Lecananthus (Rubiaceae-Schradereae). – Blumea 43: 337-346.
Puff C, Greimler J, Buchner R. 1998. Revision of Schradera (Rubiaceae-Schradereae) in Malesia. – Blumea 43: 287-335.
Puff C, Igersheim A, Buchner R. 1998. Character states and taxonomic position of the monotypic Sri Lankan Schizostigma (Rubiceae-Isertieae). – In: Dransfield J, Coode MJE, Simpson DA (eds), Plant diversity in Malesia III, Royal Botanic Gardens, Kew, pp. 187-203.
Puff C, Chayamarit C, Chamchumroon V. 2007. Rubiaceae of Thailand: a pictorial guide to indigenous and cultivated genera. – The Forest Herbarium, Department of National Parks, Wildlife and Conservation, Bangkok.
Punt W. 1978. Evolutionary trends in the Potalieae (Loganiaceae). – Rev. Palaeobot. Palynol. 26: 313-335.
Punt W. 1980. Pollen morphology. – In: Leeuwenberg AJM (ed), Engler and Prantl’s ‘Die natürlichen Pflanzenfamilien’, Angiospermae: Ordnung Gentianales, Fam. Loganiaceae, vol. 28b(1), Duncker & Humblot, Berlin, pp. 162-191.
Punt W, Leenhouts PW. 1967. Pollen morphology and taxonomy in the Loganiaceae. – Grana Palynol. 7: 469-516.
Puttock CF. 1988. A revision of Gardenia Ellis (Rubiaceae) from North-Eastern Queensland. – Austrobaileya 2: 433-449.
Puttock CF. 1989. Kailarsenia Tirvengadum emend. Puttock (Rubiaceae: Gardenieae). – Austrobaileya 3: 51-62.
Puttock CF. 1992. Systematics of the Australian Gardenieae (Rubiaceae). – Ph.D. diss., University of New South Wales, Sydney, Australia.
Puttock CF. 1994. The morphology and generic position of Australian Kailarsenia (Rubiaceae: Gardenieae). – Nord. J. Bot. 14: 515-526.
Puttock CF. 1999. Revision of Atractocarpus (Rubiaceae: Gardenieae) in Australia and new combinations for some extra-Australian taxa. – Aust. Syst. Bot. 12: 271-309.
Puttock CF, Quinn CJ. 1999. Generic concepts in Australian Gardenieae (Rubiaceae): a cladistic approach. – Aust. Syst. Bot. 12: 181-199.
Qarar M. 1973. Die Gattung Gaillonia A. Rich. (Rubiaceae-Anthospermeae) und ihre Differenzierung im Saharo-Sindischen und Irano-Turanischen Raum.. – Ph.D. diss., Phil. Fak., Universität Wien, Austria.
Rachmilevitz T, Fahn A. 1973. Ultrastructure of nectaries of Vinca rosea, L., Vinca major, L. and Citrus sinensis Osbeck, c. v. Valencia and its relation to the mechanism of nectar secretion. – Ann. Bot., N. S., 37: 1-9.
Razafimandimbison SG, Taylor CM, Wikström N,
Pailler T, Khodabandeh A, Bremer B. 2014. Phylogeny and generic limits in the
sister tribes Psychotrieae and Palicoureeae (Rubiaceae): evolution of schizocarps in
Psychotria and origins of bacterial leaf nodules of the Malagasy
species. – Amer. J. Bot. 101: 1102-1126.
Rahman MA. 1990. Taxonomic studies in the family Asclepiadaceae. – Ph.D. diss., University of Aberdeen, Scotland, United Kingdom.
Rahman MA, Wilcock CC. 1989. Morphology of the pollinaria in the genus Calotropis R. Br. (Asclepiadaceae): LM and SEM studies. – Asklepios 48: 37-40.
Rahman MA, Wilcock CC. 1991a. A taxonomic revision of the Asiatic genus Pentasacme (Asclepiadaceae). – Blumea 36: 109-121.
Rahman MA, Wilcock CC. 1991b. A taxonomic revision of Calotropis (Asclepiadaceae). – Nord. J. Bot. 11: 301-308.
Rakotonasolo F, Davis AP. 2002. Notes on the genus Hyperacanthus (Rubiaceae) including the description of a new species from Madagascar: H. grevei. – Kew Bull. 57: 955-962.
Rakotonasolo F, Davis AP. 2006. Six species of Madagascan Genipa transferred to Hyperacanthus (Rubiaceae-Gardenieae) and new data on general morphology, placentation and ovary structure in Hyperacanthus. – Taxon 55: 387-396.
Rakotonirina N, Rakouth B, Davis AP. 2012. A taxonomic revision of Madagascan Gardenia (Rubiaceae, Gardenieae). – Nord. J. Bot. 30: 712-728.
Ramachandran A, Viswan MBG. 2009. A new species of Gymnema (Asclepiadaceae) from the Kollihills in Peninsular India. – Adansonia, sér. III, 31: 407-411.
Ramam SS. 1954. Gametogenesis and fertilization of Stephegyne parviflora Korth. – Agra Univ. J. Res., Sci. 3: 243-348.
Ranarivelo-Randriamboavonjy T, Robbrecht E, Rabakonandrianina E, Block P de. 2007. Revision of the Malagasy species of the genus Tricalysia (Rubiaceae). – Bot. J. Linn. Soc. 155: 83-126.
Rao KS, Chinnappa CC. 1983. Studies in Gentianaceae: microsporangium and pollen. – Can. J. Bot. 61: 324-336.
Rao KS, Nagaraj M. 1982. Studies in Gentianaceae: embryology of Swertia minor. – Can. J. Bot. 60: 141-151.
Rao KS, Chinnappa CC. 1983. Pericolporate pollen in Gentianaceae. – Can. J. Bot. 61: 174-178.
Rao RS, Hemadri K. 1973. The genus Hedyotis Linn. in Maharastra State. – Indian For. 99: 372-379.
Rao VS, Ganguli A. 1963a. The floral anatomy of some Asclepiadaceae. – Proc. Indian Acad. Sci., Sect. B, 57: 15-44.
Rao VS, Ganguli A. 1963b. Studies in the floral anatomy of the Apocynaceae. – J. Indian Bot. Soc. 42: 412-433.
Rao VS, Ramarethinam S, Iyer CNL. 1964. The vascular anatomy of the flowers of Rubiaceae with reference to the ovary. – J. Univ. Bombay 32: 163-231.
Rapanarivo SHJV, Lavranos JJ, Leeuwenberg AJM, Röösli W. 1999. Pachypodium (Apocynaceae). Taxonomy, habitats and cultivation. – A. A. Balkema Publ., Rotterdam.
Rapini A. 2002. Six new species of Ditassa R. Br. from the Espinhaço Range, Brazil, with notes on generic delimitation in Metastelmatinae (Apocynaceae-Asclepiadoideae). – Kew Bull. 57: 565-583.
Rapini A, Chase MW, Goyder DJ, Griffiths J. 2003. Asclepiadeae classification: evaluating the phylogenetic relationships of New World Asclepiadoideae (Apocynaceae). – Taxon 52: 33-50.
Rapini A, Fontella-Pereira J, Lamare EH de, Liede-Schumann S. 2004. Taxonomy of Peplonia (including Gonioanthela) and a reinterpretation of Orthosieae (Asclepiadoideae, Apocynaceae). – Kew Bull. 2004: 531-539.
Rapini A, Goyder DJ, Konno TUP, Farinaccio MA. 2005. Progress in asclepiad taxonomy: species numbers in Brazilian Asclepiadoideae (Apocynaceae) through time. – Kew Bull. 60: 111-115.
Rapini A, Chase MW, Konno TUP. 2006. Phylogenetics of South American Asclepiadoideae (Apocynaceae). – Taxon 55: 119-124.
Rapini A, Berg C van den, Liede-Schumann S. 2007. Diversification of Asclepiadoideae (Apocynaceae) in the New World. – Ann. Missouri Bot. Gard. 94: 407-422.
Rapini A, Pereira JF, Goyder DJ. 2011. Towards a stable generic circumscription in Oxypetalinae (Apocynaceae). – Phytotaxa 26: 9-16.
Raymond-Hamet M. 1941. La présence de corynanthine et l’absence de yohimbine dans l’écorce de Pseudocinchona africana A. Chevalier et du Pseudocinchona mayumbensis (R. D. Good) Raymond-Hamet paraissent justifier la séparation des genres Pseudocinchona et Corynanthe. – Compt. Rend. Acad. Sci. Paris 212: 205-206.
Raynal A. 1965. Un nouveau genre Africain Oreonesion A. Rayn. (Gentianaceae). – Adansonia, sér. nov., 5: 271-275.
Raynal A. 1967a. Étude critique des genres Voyria et Leiphaimos (Gentianaceae) et révision des Voyria d’Afrique. – Adansonia, sér. II, 7: 53-71.
Raynal A. 1967b. Sur un Sebaea Africain saprophyte (Gentianaceae). – Adansonia, sér. II, 7: 207-219.
Raynal-Roques A. 1967. Étude critiques des genres Voyria et Leiphaimos (Gentianaceae) et révision des Voyria d’Afrique. – Adansonia, sér. II, 7: 53-71.
Raynal A. 1968. Les genres Neurotheca Benth. et Hook. et Congolanthus A. Raynal, gen. nov. (Gentianaceae). – Adansonia, sér. II, 8: 45-68.
Raynal A. 1969. Révision du genre Enicostema Blume (Gentianaceae). – Adansonia, sér. II, 9: 57-83.
Razafimandimbison SG. 2002. A systematic revision of Breonia (Rubiaceae-Naucleeae). – Ann. Missouri Bot. Gard. 89: 1-37.
Razafimandimbison SG, Bremer B. 2001 [2002]. Tribal delimitation of Naucleeae (Cinchonoideae, Rubiaceae): inference from molecular and morphological data. – Syst. Geogr. Plants 71: 515-538.
Razafimandimbison SG, Bremer B. 2002. Phylogeny and classification of Naucleeae s.l. (Rubiaceae) inferred from molecular (ITS, rbcL, and trnT-F) and morphological data. – Amer. J. Bot. 89: 1027-1041.
Razafimandimbison SG, Bremer B. 2006. Taxonomic revision of the tribe Hymenodictyeae (Rubiaceae, Cinchonoideae). – Bot. J. Linn. Soc. 152: 331-386.
Razafimandimbison SG, Bremer B. 2011. Nomenclatural changes and taxonomic notes in the tribe Morindeae (Rubiaceae). – Adansonia, sér. 3, 33: 283-309.
Razafimandimbison SG, Miller JS. 1999. New taxa and nomenclatural notes on the flora of the Marojejy Massif, Madagascar III. Rubiaceae. A new species of Sabicea. – Adansonia, sér. III, 21: 41-45.
Razafimandimbison SG, Kellogg EA, Bremer B. 2004. Recent origin and phylogenetic utility of divergent ITS putative pseudogenes: a case study from Naucleeae (Rubiaceae). – Syst. Biol. 53: 177-192.
Razafimandimbison SG, Moog J, Lantz H, Maschwitz U, Bremer B. 2005. Re-assessment of monophyly, evolution of myrmecophytism, and rapid radiation in Neonauclea s.s. (Rubiaceae). – Mol. Phylogen. Evol. 34: 334-354. – Correction. 2005. Mol. Phylogen. Evol. 37: 938-939.
Razafimandimbison SG, Lantz H, Bremer B. 2007. New combinations and names in Peponidium and Pyrostria (Rubiaceae, Vanguerieae). – Novon 17: 516-521.
Razafimandimbison SG, Rydin C, Bremer B. 2008. Evolution and trends in the Psychotrieae alliance (Rubiaceae) – a rarely reported evolutionary change of many-seeded carpels from one-seeded carpels. – Mol. Phylogen. Evol. 48: 207-223. – Corrigendum: Mol. Phylogen. Evol. 50 (2009): 667-668.
Razafimandimbison SG, McDowell TD, Halford DA, Bremer B. 2009. Molecular phylogenetics and generic assessment in the tribe Morindeae (Rubiaceae-Rubioideae): how to circumscribe Morinda L. to be monophyletic? – Mol. Phylogen. Evol. 52: 879-886.
Razafimandimbison SG, Lantz H, Mouly A, Bremer B. 2009. Evolutionary trends, major lineages, and new generic limits in the dioecious group of the tribe Vanguerieae (Rubiaceae): insights into the evolution of functional dioecy. – Ann. Missouri Bot. Gard. 96: 161-181.
Razafimandimbison SG, McDowell TD, Halford DA, Bremer B. 2010. Origin of the pantropical and nutriceutical Morinda citrifolia L. (Rubiaceae): comments on its distribution range and circuscription. – J. Biogeogr. 37: 520-529.
Razafimandimbison SG, Kainulainen K, Wong KM, Beaver K, Bremer B. 2011. Molecular support for a basal grade of morphologically distinct, monotypic genera in the species-rich Vanguerieae alliance (Rubiaceae, Ixoroideae): its systematic and conservation implications. – Taxon 60: 941-952.
Razafimandimbison SG, Ekman S, McDowell TD, Bremer B. 2012. Evolution of growth habit, inflorescence architecture, flower size, and fruit type in Rubiaceae: its ecological and evolutionary implications. – PLoS ONE 7: 10.
Razafimandimbison SG, Taylor CM, Wikström N, Pailler T, Khodabandeh A, Bremer B. 2014. Phylogeny and generic limits in the sister tribes Psychotrieae and Palicoureeae (Rubiaceae): evolution of schizocarps in Psychotria and origins of bacterial leaf nodules of the Malagasy species. – Amer. J. Bot. 101: 1102-1126.
Razafimandimbison SG, Kainulainen K, Wikström N, Bremer B. 2017. Historical biogeography and phylogeny of the pantropical Psychotrieae alliance (Rubiaceae), with particular emphasis on the Western Indian Ocean Region. – Amer. J. Bot. 104: 1407-1423. – Erratum: Amer. J. Bot. 104: 1596.
Reddy AS, Rao YBN, Khanam A, Durga IS. 1999. A contribution to the embryology of Mitreola oldenlandioides Wall. – Phytomorphology 49: 283-287.
Reese G. 1971. Untersuchungen über die Chromosomenzahlen der Stapelieae II. – Port. Acta Biol. 12: 1-23.
Reese G. 1973. The structure of the highly specialised carrion-flowers of stapeliads. – Cact. Succ. J. 45: 18-29.
Regalado JC, Soejarto DD. 1997. The genus Microrphium (Gentianaceae) in the Philippines. – Novon 7: 77-80.
Renobales G, Diego E de, Urcelay B, Lopez-Quintana A. 2001. Secretory hairs in Gentiana and allied genera (Gentianaceae, subtribe Gentianinae) from the Iberian Peninsula. – Bot. J. Linn. Soc. 136: 119-129.
Reveal JL, Barrie FR. 1992. Matelea suberosa (L.) Shinners (Asclepiadaceae) – once again. – Bartonia 57: 36-38.
Ribeiro JC, Ferreira MJP, Demarco D. 2017. Colleters in Asclepiadoideae (Apocynaceae): protection of meristems against desiccation and new functions assigned. – Intern. J. Plant Sci. 178: 465-477.
Ribeiro PL, Rapini A, Soares e Silva UC, Berg C van den. 2012. Using multiple analytical methods to improve phylogenetic hypotheses in Minaria (Apocynaceae). – Mol. Phylogen. Evol. 65: 915-925.
Ribeiro PL, Rapini A, Damascena LS, Berg C
van den. 2015. Plant diversification in the Espinhaço Range: insights from the
biogeography of Minaria (Apocynaceae). – Taxon 63:
1253-1264.
Ridley HN. 1940. Notes on some Malayan Rubiaceae. – Kew Bull. 10: 593-597.
Ridsdale CE. 1975. A synopsis of African and Madagascan Rubiaceae-Naucleeae. – Blumea 22: 541-553.
Ridsdale CE. 1976. A revision of the tribe Cephalantheae (Rubiaceae). – Blumea 23: 177-188.
Ridsdale CE. 1978a. A revision of Mitragyna and Uncaria (Rubiaceae). – Blumea 24: 43-100.
Ridsdale CE. 1978b. A revision of the tribe Naucleeae s.s. (Rubiaceae). – Blumea 24: 307-366.
Ridsdale CE. 1979. Jackiopsis, a new name for Jackia Wall. (Rubiaceae-Jackieae). – Blumea 25: 295-296.
Ridsdale CE. 1982. A revision of Badusa (Rubiaceae, Condamineeae, Portlandiinae). – Blumea 28: 145-150.
Ridsdale CE. 1985. The genus Fagerlindia (Rubiaceae) in the Philippines. – Blumea 31: 239-244.
Ridsdale CE. 1989. A revision of Neonauclea (Rubiaceae). – Blumea 34: 177-275.
Ridsdale CE. 1996. New taxa and combination for the Rubiaceae of Sri Lanka. – Blumea 41: 455-462.
Ridsdale CE. 2008. Thorny problems in the Rubiaceae: Benkara, Fagerlindia and Oxyceros. – Reinwardtia 12: 289-300.
Ridsdale CE, Bakhuizen van den Brink RC, Koek-Noorman J. 1972. Notes on New Guinea Rubiaceae. – Blumea 20: 338-348.
Riesman A, Sumanasinghe VA, Craig R. 2006. Cytology, crossability, and pollen fertility of Sri Lankan Exacum (Gentianaceae) and their hybrids. – Intern. J. Plant Sci. 167: 191-199.
Rintz RE. 1978. The Peninsular Malaysian species of Hoya (Asclepiadaceae). – Malayan Nat. J. 30: 467-522.
Rintz RE. 1980. A revision of the genus Sarcolobus (Asclepiadaceae). – Blumea 26: 65-79.
Rizzini CT, Occhioni P. 1949. Dialypetalanthaceae. – Lilloa 17: 243-288.
Robbrecht E. 1977. The tropical African genus Hymenocoleus (Rubiaceae-Psychotrieae): additions. – Bull. Jard. Bot. Belg. 47: 3-29.
Robbrecht E. 1978a. Sericanthe, a new African genus of Rubiaceae (Coffeeae). – Bull. Jard. Bot. Natl. Belg. 48: 3-78.
Robbrecht E. 1978b. Some observations in Preussiodora Keay (African Rubiaceae, Gardenieae). – Bull. Soc. Roy. Bot. Belg. 111: 3-9.
Robbrecht E. 1979. The African genus Tricalysia A. Rich. (Rubiaceae-Coffeeae) 1. A revision of the species of subgenus Empogona. – Bull. Jard. Bot. Natl. Belg. 49: 239-360.
Robbrecht E. 1980a. Bijdragen tot de classificatie van de Ixoroideae en tot de revisie van Tricalysia s.l. (Rubiaceae). – Ph.D. diss., Universiteit Gent, Belgium.
Robbrecht E. 1980b. The Hypobathreae (Rubiaceae-Ixoroideae) 1. Delimitation and division of a new tribe. – Bull. Jard. Bot. Natl. Belg. 50: 69-77.
Robbrecht E. 1981a. Studies in tropical African Rubiaceae I. 1. A new species of Tricalysia subgenus Empogona from Gabon. 2. The position of “Tricalysia auriculata” and “Neorosea adamii”. 3. A new species of Batopedina (Hedyotideae) from Shaba. 4. The distribution of Batopedina and Parapentas. – Bull. Jard. Bot. Natl. Belg. 51: 165-189.
Robbrecht E. 1981b. Studies in tropical African Rubiaceae II. 5. A survey of Argocoffeopsis. 6. A revision of Calycosiphonia. – Bull. Jard. Bot. Natl. Belg. 51: 359-378.
Robbrecht E. 1982a. The African genus Tricalysia A. Rich. (Rubiaceae-Coffeeae) 2. Ephedranthera, a new section of subgenus Tricalysia. – Bull. Jard. Bot. Natl. Belg. 52: 311-339.
Robbrecht E. 1982b. Pollen morphology of the tribes Anthospermeae and Paederieae (Rubiaceae) in relation to taxonomy. – Bull. Jard. Bot. Natl. Belg. 52: 349-366.
Robbrecht E. 1983. The African genus Tricalysia A. Rich. (Rubiaceae) 3. Probletostemon revived as a section for subgenus Tricalysia. – Bull. Jard. Bot. Natl. Belg. 53: 299-320.
Robbrecht E. 1984. The delimitation and taxonomic position of the tropical African genera Leptactina and Dictyandra (Rubiaceae). – Plant Syst. Evol. 145: 105-118.
Robbrecht E. 1985. Further observations on the pollen morphology of the South African genus Carpacoce (Rubiaceae- Anthospermeae). – Rev. Palaeobot. Palyn. 45: 361-371.
Robbrecht E. 1986. Studies in tropical African Rubiaceae 10. Morphological observations on the fruits and seeds of Didymosalpinx (Gardenieae). – Bull. Jard. Bot. Natl. Belg. 56: 145-162.
Robbrecht E. 1987. The African genus Tricalysia A. Rich. (Rubiaceae) 4. A revision of the species of section Tricalysia. – Bull. Jard. Bot. Natl. Belg. 57: 39-208.
Robbrecht E. 1988a. Tropical woody Rubiaceae. Characteristic features and progressions. Contributions to a new subfamilial classification. – Opera Bot. Belg. 1: 1-271.
Robbrecht E. 1988b. Studies in tropical African Rubiaceae 13. Petitiocodon, a new genus to accommodate Didymosalpinx parviflora (Gardenieae-Diplosporinae). – Bull. Jard. Bot. Natl. Belg. 58: 109-120.
Robbrecht E. 1989. A remarkable new Chazaliella (African Psychotrieae), exemplifying the taxonomic value of pyrene characters in the Rubiaceae. – Bull. Mus. Natl. Hist. Nat., Paris , sér. IV, 11, sect. B, Adansonia 4: 341-349.
Robbrecht E. 1993a. Seventy years of systematics of tropical Rubiaceae at the National Botanic Garden. – Bull. Jard. Bot. Natl. Belg. 62: 7-34.
Robbrecht E. 1993b. Supplement to the 1988 outline of the classification of the Rubiaceae, index to genera. – In: Robbrecht (ed), Advances in Rubiaceae macrosystematics, Opera Bot. Belg. 6: 173-196.
Robbrecht E. 1994a. Introduction to advances in Rubiaceae macrosystematics. – In: Robbrecht E (ed), Advances in Rubiaceae macrosystematics, Opera Bot. Belg. 6: 7-18.
Robbrecht E. 1994b. On the delimitation of the Rubiaceae. A review. – In: Robbrecht E (ed), Advances in Rubiaceae macrosystematics, Opera Bot. Belg. 6: 19-30.
Robbrecht E. 1994c. Supplement to the 1988 outline of the classification of the Rubiaceae. Index to genera. – In: Robbrecht E (ed), Advances in Rubiaceae macrosystematics, Opera Bot. Belg. 6: 173-196.
Robbrecht E. 1996a. Geography of African Rubiaceae with reference to glacial rain forest refuges. – In: Maesen LJG van der, Burgt XM van der, Medenbach de Rooy JM, van der (eds), The biodiversity of African plants, Kluwer Academic Publ., Netherlands, pp. 564-581.
Robbrecht E. 1996b. Generic distribution patterns in subsaharan African Rubiaceae (Angiospermae). – J. Biogeogr. 23: 311-328.
Robbrecht E, Block P de. 1999. The geofrutescent Leptactina species (Rubiaceae, Pavetteae) of the ’Flore d’Afrique Centrale’ area. – Syst. Geogr. Plants 69: 125-133.
Robbrecht E, Bridson DM. 1984. The taxonomic position of the East African genus Cladoceras (Rubiaceae). – Bull. Soc. Roy. Bot. Belg. 117: 247-251.
Robbrecht E, Manen JF. 2006. The major evolutionary lineages of the coffee family (Rubiaceae, angiosperms). Combined analysis (nDNA and cpDNA) to infer the position of Coptosapelta and Luculia, and supertree construction based on rbcL, rps16, trnL-trnF and atpB-rbcL data. A new classification in two subfamilies, Cinchonoideae and Rubioideae. – Syst. Geogr. Plants 76: 85-146.
Robbrecht E, Puff C. 1981. Mericocalyx Bamps, synonymous with Otiophora Zucc. (Rubiaceae). – Bull. Jard. Bot. Natl. Belg. 51: 143-151.
Robbrecht E, Puff C. 1986. A survey of the Gardenieae and related tribes (Rubiaceae). – Bot. Jahrb. Syst. 108: 63-138.
Robbrecht E, Puff C, Igersheim A. 1991. The genera Mitchella and Damnacanthus, evidence for their close alliance; comments on the campylotropy in the Rubiaceae and the circumscription of the Morindeae. – Blumea 35: 307-345.
Robbrecht E, Bridson D, Deb DB. 1993 [1994]. The South Indian genus Octotropis (Rubiaceae): an investigation of its characters and reinstatement of the tribal name Octotropideae. – In: Robbrecht E (ed), Advances in Rubiaceae macrosystematics, Opera Bot. Belg. 6: 81-91.
Robbrecht E, Rohrhofer U, Puff C. 1993 [1994]. A survey of Bertiera (Rubiaceae), including a discussion of its taxonomic position. – In: Robbrecht E (ed), Advances in Rubiaceae macrosystematics, Opera Bot. Belg. 6: 101-141.
Robbrecht E, Puff C, Smets E (eds). 1996. Second International Rubiaceae Conference. Proceedings. – Opera Bot. Belg. 7: 1-432.
Robbrecht E, Huysmans S, Figueiredo E. 1996. The generic status of Oxyanthus gossweileri (Rubiaceae) in Angola. – South Afr. J. Bot. 62: 17-22.
Robinson BL. 1910. Spermatophyta, new or reclassified, chiefly Rubiaceae and Gentianaceae. – Proc. Amer. Acad. 45: 394-412.
Robyns A. 1954. Essai d’étude systématique et écologique des Centaurium de Belgique. – Bull. Jard. Bot. Bruxelles 24: 349-398.
Rodriguez P. 1976. Estudio sobre frutos carnosos y sus semillas de las Rubiaceae de Venezuela. – Acta Bot. Venezuelica 11: 283-383.
Rogers GK. 1981a. A new Platycarpum (Rubiaceae) from Brazil. – Syst. Bot. 6: 87-89.
Rogers GK. 1981b. The wood of Gleasonia, Henriquezia, and Platycarpum (Rubiaceae) and its bearing on their classification: some new considerations. – Brittonia 33: 461-465.
Rogers GK. 1984. Flora Neotropica. Monograph 39. Gleasonia, Henriquezia and Platycarpum (Rubiaceae). – New York Botanical Garden, Bronx, New York.
Ronniger K. 1916. Centaurium (Erythraea). – Mitt. Naturw. Ver. Steiermark 52: 312-321.
Ronse De Craene L-P, Smets E. 2000. Floral development of Galopina tomentosa with a discussion of sympetaly and placentation in the Rubiaceae. – Syst. Geogr. Plants 70: 155-170.
Rork CL. 1949. Cytological studies in the Gentianaceae. – Amer. J. Bot. 36: 687-701.
Roth I, Lindorf H. 1974. The morphological interpretation of the seed of the Rubiaceae and especially that of Coffea. – Acta Bot. Venez. 9: 141-147.
Roth LJ Jr, Dilcher DL. 1979. Investigations of angiosperms from the Eocene of North America: stipulate leaves of the Rubiaceae including a probable polyploidy population. – Amer. J. Bot. 66: 1194-1207.
Rothe W. 1915. Über die Gattung Marsdenia R. Br. und die Stammpflanze der Condurangorinde. – Engl. Bot. Jahrb. Syst. 52: 354-434.
Rova JHE. 1999. The Condamineeae Rondeletieae-Sipaneeae complex (Rubiaceae). – Ph.D. diss., University of Göteborg, Sweden.
Rova JHE, Andersson L. 1995. A reevaluation of the tribes Hippotideae and Tammsieae (Rubiaceae). – Nord. J. Bot. 15: 269-284.
Rova JHE, Delprete PG, Andersson L, Albert VA. 2002. A trnL-F cpDNA sequence study of the Condamineeae-Rondeletieae-Sipaneeae complex with implications on the phylogeny of the Rubiaceae. – Amer. J. Bot. 89: 145-159.
Rova JHE, Delprete PG, Bremer B. 2009. The Rondeletia complex (Rubiaceae): an attempt to use ITS, rps16, and trnL-F sequence data to delimit Guettardeae, Rondeletieae, and sections within Rondeletia. – Ann. Missouri Bot. Gard. 96: 182-193.
Royen P van. 1973. Two superfluous genera in the New Guinean flora. – Bot. Not. 126: 417-425.
Ruhsam M, Davis AP. 2007. A taxonomic revision of the genus Flagenium Baill. (Rubiaceae-Octotropideae). – Bot. J. Linn. Soc. 155: 557-570.
Ruhsam M, Govaerts R, Davis AP. 2008. Nomenclatural changes in preparation for a World Rubiaceae Checklist. – Bot. J. Linn. Soc. 157: 115-124.
Rusby HH. 1931. The genus Cinchona in Bolivia. – Bull. Torrey Bot. Club 58: 523-530.
Rutishauser R. 1984. Blattquirle, Stipeln und Kolleteren bei der Rubieae (Rubiaceae) im Vergleich mit anderen Angiospermen. – Beitr. Biol. Pflanzen 59: 375-424.
Rutishauser R, Ronse De Craene L-P, Smets EF, Mendoza-Heuer L. 1998. Theligonum cynocrambe: developmental morphology of a peculiar rubiaceous herb. – Plant Syst. Evol. 210: 1-24.
Rydin C, Razafimandimbison SG, Bremer B. 2008. Rare and enigmatic genera (Dunnia, Schizocolea, Colletoecema), sisters to species-rich clades: phylogeny and aspects of conservation biology in the coffee family. – Mol. Phylogen. Evol. 48: 74-83.
Rydin C, Kainulainen K, Razafimandimbison SG, Smedmark JEE, Bremer B. 2009. Deep divergences in the coffee family and the systematic position of Acranthera. – Plant Syst. Evol. 278: 101-123.
Rydin C, Razafimandimbison SG, Khodabandeh A, Bremer B. 2009. Evolutionary relationships in the Spermacoceae alliance (Rubiaceae) using information from sex molecular loci: insights into systematic affinities of Neohymenopogon and Mouretia. – Taxon 58: 793-810.
Rydin C, Wikström N, Bremer B. 2017. Conflicting results from mitochondrial genomic data challenge current views of Rubiaceae phylogeny. – Amer. J. Bot. 104: 1522-1532.
Safwat FM. 1962. The floral morphology of Secamone and the evolution of the pollinating apparatus in Asclepiadaceae. – Ann. Missouri Bot. Gard. 49: 95-129.
Sainty D, Bailleul F, Delaveau P, Jacquemin H. 1981. Iridoids of Borreria verticillata. – Planta Medica 42: 260-264.
Salas RM. 2012. Revisión de Staelia s. l. (Rubiaceae). – Ph.D. diss., Universidad Nacional del Nordeste, Corrientes, Argentina.
Salas RM, Cabral EL. 2010. Rehabilitación y lectotipificación del género Tessiera, su relación con Diphragmus y Staelia (Rubiaceae, Spermacoceae): una nueva combinación y un nuevo sinónimo. – J. Bot. Res. Inst. Texas 4: 183-194.
Salas RM, Viana PL, Cabral EL, Dessein S, Janssens S. 2015. Carajasia (Rubiaceae), a new and endangered genus from the Carajás mountain range, Pará, Brazil. – In: Delprete PG, Dessein S (eds), Festschrift volume dedicated to Timothy Motley (1966-2013), Phytotaxa 206: 1-132.
Sales MF. 1993. Estudos taxonômicos do genero Mandevilla Lindley subgênero Mandevilla (Apocynaceae) no Brasil. – Ph.D. diss., Universidad Estadual do Campinas, São Paulo, Brasil.
Samson N, Bausher MG, Lee S-B, Jansen RK, Daniell H. 2007. The complete nucleotide sequence of the coffee (Coffea arabica L.) chloroplast genome: organization and implications for biotechnology and phylogenetic relationships among angiosperms. – Plant Biotechn. J. 5: 339-353.
Santapau H, Irani NA. 1962. The Asclepiadaceae and Periplocaceae of Bombay. – Bot. Mem. Univ. Bombay 4: 1-118.
Satake Y. 1944. Species Swertiae nipponenses. – J. Jap. Bot. 20: 334-344.
Schanzer IA. 1989. Notulae de generis Galium L. (Rubiaceae) speciebus Caucasicis nonnulis sectionis Orientigalium Ehrend. – Nov. Sist. Vysš. Rast. 26: 151-157.
Schanzer IA, Ehrendorfer F. 2002. Multivariate analysis, systematics, and distribution of Galium sect. Orientigalium Ehrend. (Rubiaceae) in the Caucasus region. – Candollea 57: 329-357.
Scheltens A. 1998. Pollenmorfologische studie van de Afrikaanse Hedyotideae (Rubiaceae). – Lic. thesis, Katolieke Universiteit de Leuven, Belgium.
Schick B. 1980. Untersuchungen über die Biotechnik der Apocynaceenblüte I. Morphologie und Funktion des Narbenkopfes. – Flora 170: 394-432.
Schick B. 1982a. Zur Morphologie, Entwicklung, Feinstruktur, und Funktion des Translators von Periploca L. (Asclepiadaceae). – Trop. Subtrop. Pflanzenwelt 40: 513-553.
Schick B. 1982b. Untersuchungen über die Biotechnik der Apocynaceenblüte II. Bau und Funktion des Bestäubungsapparates. – Flora 172: 347-371.
Schill R, Dannenbaum C. 1984. Bau und Entwicklung der Pollinien von Hoya carnosa (L.) Br. (Asclepiadaceae). – Trop. Subtrop. Pflanzenwelt 48: 7-54.
Schill R, Jäkel U. 1978. Beitrag zur Kenntnis der Asclepiadaceen-Pollinarien. – Trop. Subtrop. Pflanzenwelt 22: 3-122.
Schilling N. 1976. Distribution of L-(+)-bornesitol in the Gentianaceae and Menyanthaceae. – Phytochemistry 15: 824-826.
Schinz H. 1891. Zur Kenntniss afrikanischer Gentianaceen. – Vierteljahrsschr. Naturforsch. Ges. Zürich 36: 306-339.
Schinz H. 1903. Versuch einer monographischen Übersicht der Gattung Sebaea R. Br. 1. Die Sektion Eusebaea Griseb. – Mitt. Geogr. Ges. Naturhist. Mus. Lübeck 17: 3-55.
Schinz H. 1906a. Beiträge zur Kenntniss der afrikanischen Flora. Gentianaceae. Versuch einer monographischen Übersicht 1. Der Gattung Sebaea R. Br. – Bull. Herb. Boissier, sér. II, 6: 714-746.
Schinz H. 1906b. Beiträge zur Kenntniss der afrikanischen Flora. Gentianaceae. Versuch einer monographischen Übersicht 2. Der Gattung Exochaenium Griseb. – Bull. Herb. Boissier, sér. II, 6: 801-823.
Schlechter R. 1893. Beiträge zur Kenntnis der Orchidaceen und Asclepiadaceen Süd-Afrikas. – Verh. Bot. Ver. Prov. Brandenbrg 35: 44-54.
Schlechter R. 1894a. Beiträge zur Kenntnis südafrikanischer Asclepiadaceen. – Engl. Bot. Jahrb. Syst. 18, Beibl. 45: 1-37.
Schlechter R. 1894b. Contributions to South African Asclepiadology. – J. Bot. 32: 257-263, 353-358.
Schlechter R. 1895a. Beiträge zur Kenntnis südafrikanischer Asclepiadaceen. – Engl. Bot. Jahrb. Syst. 20, Beibl. 51.
Schlechter R. 1895b. Contributions to South African Asclepiadology. – J. Bot. 33: 267-274, 353-359.
Schlechter R. 1895c. Two new genera of Asclepiadaceae. – J. Bot. 33: 321-322.
Schlechter R. 1895d. Asclepiadaceae Elliotinae. – J. Bot. 33: 333-339.
Schlechter R. 1896a. Revision of extra-tropical South African Asclepiadaceae. – J. Bot. 34: 311-315, 417-421, 449-458.
Schlechter R. 1896b. Pentasachme Wall. and Spiladocorys Ridl. – J. Bot. 34: 15-16.
Schlechter R. 1897. Revision of extra-tropical South African Asclepiadaceae. – J. Bot. 35: 290-295.
Schlechter R. 1898. Revision of extra-tropical South African Asclepiadaceae. – J. Bot. 36: 475-487.
Schlechter R. 1900. Eine neue Gattung der Asclepiadaceae. – Engl. Bot. Jahrb. Syst. 29, Beibl. 66: 21-22.
Schlechter R. 1906. Asclepiadaceae andinae. – Bot. Jahrb. Syst. 37: 603-627.
Schlechter R. 1908. Beiträge zur Kenntnis der Asclepiadaceen des Monsun-Gebietes. – Engl. Bot. Jahrb. Syst. 40, Beibl. 92: 1-19.
Schlechter R. 1913. Die Asclepiadaceen von Deutsch-Neu-Guinea. – In: Lauterbach C (ed), Beiträge zur Flora von Papuasien II, Engl. Bot. Jahrb. Syst. 50: 81-164.
Schlechter R. 1914. Philibertia H. B. & Kth. und Funastrum Fourn. – Feddes Repert. 13: 279-287.
Schlechter R. 1915a. Asclepiadaceae Philippinenses I. – Feddes Repert. 13: 537-544.
Schlechter R. 1915b. Asclepiadaceae Philippinenses II. – Feddes Repert. 13: 554-566.
Schlechter R. 1916a. Asclepiadaceae andinae. – Bot. Jahrb. Syst. 54, Beibl. 119: 1-2.
Schlechter R. 1916b. Neue Asclepiadaceen von Sumatra und Celebes. – Beih. Bot. Centralbl. 34, Abt. 2: 1-18.
Schlechter R. 1927. Periplocaceae. – In: Fries RE, Fries TCE (eds), Beiträge zur Kenntnis der Flora des Kenia, Mt. Aberdare und Mt. Elgon V. – Notizbl. Bot. Gart. Berlin-Dahlem 9: 23-24.
Schnepf E, Witzig F, Schill R. 1979. Über Bildung und Feinstruktur des Translators der Pollinarien von Asclepias curassavica und Gomphocarpus fruticosus (Asclepiadaceae). – Trop. Subtrop. Pflanzenwelt 25.
Schoch E. 1903. Monographie der Gattung Chironia L. – Beih. Bot. Centralbl. 14: 177-242.
Schönbeck-Temesy E. 1991. New taxa of Rubiaceae-Rubieae from Iran. – Plant Syst. Evol. 174: 197-200.
Schönbeck-Temesy E, Ehrendorfer F. 1979. Additional new taxa of Rubiaceae for the Flora of Turkey. – Plant Syst. Evol. 133: 109-112.
Schönbeck-Temesy E, Ehrendorfer F. 1985. Asperula garganica und A. semanensis, zwei neue Arten aus dem Orient, und die palaeo-mediterrane Section Thliphthisa (Griseb.) Ehrend. (Rubiaceae). – Bot. Jahrb. Syst. 107: 75-93.
Schumann K. 1888. Über einige verkannte oder wenig bekannte Geschlechter der Rubiaceen Südamerikas. – Bot. Jahrb. Syst. 10: 302-363.
Schumann K. 1891. Rubiaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien IV(4), W. Engelmann, Leipzig, pp. 1-156; Schumann K. 1894. Nachträge zu IV(4), pp. 309-316.
Schumann K. 1893. Asclepiadaceae africanae. – Engl. Bot. Jahrb. Syst. 33: 321-331.
Schumann K. 1895a. Apocynaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien IV(4), W. Engelmann, Leipzig, pp. 109-189; Schumann K. 1897. Nachträge zu IV(2), pp. 283-285.
Schumann K. 1895b. Asclepiadaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien IV(4), W. Engelmann, Leipzig, pp. 189-306; Schumann K. 1897. Nachträge zu IV(2), pp. 285-288.
Schumann K. 1896 [1897]. Beiträge zur Flora von Afrika XIII. Rubiaceae africanae. – Engl. Bot. Jahrb. Syst. 23: 412-470.
Scott Elliott GF. 1896. A revision of the genus Pentas. – Bot. J. Linn. Soc. 32: 431-438.
Selvaraj D, Sarma RK, Shanmughanandhan D, Srinivasan R, Ramalingam S. 2015. Evaluation of DNA barcode candidates for the discrimination of the large plant family Apocynaceae. – Plant Syst. Evol. 301: 1263-1273.
Sennblad B. 1997. Phylogeny of the Apocynaceae s.l. – Ph.D. diss., Acta Univ. Upsal. 295, University of Uppsala, Sweden.
Sennblad B, Bremer B. 1996. The familial and subfamilial relationships of Apocynaceae and Asclepiadaceae evaluated with rbcL data. – Plant Syst. Evol. 202: 153-175.
Sennblad B, Bremer B. 1999. Is there a justification for differential a priori weighting in coding sequences? A case study from rbcL and Apocynaceae s.l. – Syst. Biol. 49: 101-113.
Sennblad B, Bremer B. 2002. Classification of Apocynaceae s.l. according to a new approach combining Linnaean and phylogenetic taxonomy. – Syst. Biol. 51: 389-409.
Sennblad B, Endress ME, Bremer B. 1998. Morphology and molecular data in phylogenetic fraternity: the tribe Wrightieae (Apocynaceae) revisited. – Amer. J. Bot. 85: 1143-1158.
Setchell WA, Christophersen E. 1935. Preliminary notes on Sarcopygme, a new rubiaceous genus from Samoa. – Occas. Pap. Bernice P. Bishop Mus. 11: 3-5.
Shah J. 1990. Gentianaceae monograph 1. Taxonomic studies in the genus Swertia L. – Sci. Khyber 3: 17-114.
Shah J. 1992. Gentianaceae monograph 2. Taxonomic studies in the genus Swertia L. – Sci. Khyber 5: 117-231.
Shamrov II. 1996. Ovule development and significance of its features for Gentianaceae systematics. – Opera Bot. Belg. 7: 113-118.
Shamrov II, Gevorkyan MM. 2010a. Structural organization of gynoecium in the family Apocynaceae. – Bot. Žurn. 94: 938-961. [In Russian]
Shamrov II, Govorkyan MM. 2010b. Comparative characteristics of the gynoecium in Apocynaceae, Asclepiadaceae and Gentianaceae families. – Bot. Žurn. 95: 1673-1687. [In Russian]
Sharma BD. 1968. Contribution to the pollen morphology and taxonomy of the genus Randia Houst. ex Linn. – J. Palynol. 4: 113-128.
Sherff EE. 1939. Genus Labordia. – Field Mus. Nat. Hist., Bot. Ser. 17: 449-546.
Shinners LH. 1950. The species of Matelea (including Gonolobus) in North Central Texas (Asclepiadaceae). – Field & Laboratory 18: 73-78.
Shinners LH. 1957. Synopsis of the genus Eustoma (Gentianaceae). – Southwest. Natur. 2: 38-43.
Shinners LH. 1964. Texas Asclepiadaceae other than Asclepias. – Sida 1: 358-367.
Sidiyasa K. 1998. Taxonomy, phylogeny and wood anatomy of Alstonia (Apocynaceae). – Blumea (Suppl.) 11: 1-230.
Sileshi N. 1998. A synopsis of Swertia (Gentianaceae) in east and northeast tropical Africa. – Kew Bull. 53: 419-436.
Silva NMF da, Pereira JF, Conceição Valente M. 2007. Asclepiadoideae (Apocynaceae) from southeastern Brazil I. The genus Oxypetalum from Rio de Janeiro State, Brazil. – Ann. Missouri Bot. Gard. 94: 435-462.
Silva UCS, Rapini A, Liede-Schumann S, Ribeiro PL, Berg C van den. 2012. Taxonomic considerations on Metastelmatinae (Apocynaceae) based on plastid and nuclear DNA datasets. – Syst. Bot. 37: 795-806.
Simões AO, Kinoshita L. 2002. The Apocynaceae s. str. of the Carrancas region, Minas Gerais, Brazil. – Darwiniana 40: 127-169.
Simões AO, Endress ME, Niet T van der, Kinoshita LS, Conti E. 2004. Tribal and intergeneric relationships of Mesechiteae (Apocynaceae, Apocynoideae): evidence from three non-coding plastid DNA regions and morphology. – Amer. J. Bot. 91: 1409-1418.
Simões AO, Endress ME, Niet T van der, Kinoshita LS, Conti E. 2006. Is Mandevilla (Apocynaceae, Mesechiteae) monophyletic? Evidence from five plastid DNA loci and morphology. – Ann. Missouri Bot. Gard. 93: 565-591.
Simões AO, Castro M de M, Kinoshita LS. 2006. Calycine colleters of seven species of Apocynaceae (Apocynoideae) from Brazil. – Bot. J. Linn. Soc. 152: 387-398.
Simões AO, Livshultz T, Conti E, Endress ME. 2007. Phylogeny and systematics of the Rauvolfioideae (Apocynaceae) based on molecular and morphological evidence. – Ann. Missouri Bot. Gard. 94: 268-297.
Simões AO, Kinoshita LS, Endress ME. 2007. New combinations in Mandevilla Lindley (Apocynaceae). – Novon 17: 87-90.
Simões AO, Scatolin do Rio MC, Moraes Castroe M de, Kinoshita LS. 2007. Gynostegium morphology of Mesechiteae Miers (Apocynaceae, Apocynoideae) as it pertains to the classification of the tribe. – Intern. J. Plant Sci. 168: 999-1012.
Simões AO, Endress ME, Conti E. 2010. Systematics and character evolution of Tabernaemontaneae (Apocynaceae, Rauvolfioideae) based on molecular and morphological evidence. – Taxon 59: 772-790.
Sivarajan VV, Biju SD, Mathew P. 1992. Revision of the genus Psilanthus Hook. f. (Rubiaceae tribe Coffeeae) in India. – Bot. Bull. Acad. Sin. 33: 209-223.
Sivarajan VV, Biju SD, Mathew P. 1993. Taxonomy and nomenclature of some Indian species of Hedyotis (Rubiaceae). – Kew Bull. 48: 391-396.
Skottsberg C. 1944. On the flower dimorphism in Hawaiian Rubiaceae. – Ark. f. Bot. 31A: 1-28.
Sluis WG van der. 1985a. Secoiridoids and xanthones in the genus Centaurium Hill (Gentianaceae) – a pharmacognostical study. – Drukkerij Elinkwij bv., Utrecht.
Sluis WG van der. 1985b. Chemotaxonomical investigations of the genera Blackstonia and Centaurium (Gentianaceae). – Plant Syst. Evol. 149: 253-286.
Smedmark JEE, Bremer B. 2011. Molecular systematics and incongruent gene trees of Urophylleae (Rubiaceae). – Taxon 60: 1397-1406.
Smedmark JEE, Rydin C, Razafimandimbison SG, Khan SA, Liede-Schumann S, Bremer B. 2008. A phylogeny of Urophylleae (Rubiaceae) based on rps16 intron data. – Taxon 57: 24-32.
Smedmark JEE, Eriksson T, Bremer B. 2010. Divergence time uncertainty and historical biogeography reconstruction – an example from Urophylleae (Rubiaceae). – J. Biogeogr. 37: 2260-2274.
Smedmark JEE, Razafimandimbison SG, Wikström
N, Bremer B. 2014. Inferring geographic range evolution of a pantropical tribe
in the coffee family (Lasiantheae, Rubiaceae) in the face of topological
uncertainty. – Molec. Phylogen. Evol. 70: 182-194.
Smith AC, Darwin SP. 1988. Rubiaceae. – In: Smith AC (ed), Flora vitiensis nova: a new flora of Fiji 4, National Tropical Botanical Garden, Lawai, Kauai, Hawaii, pp. 143-376.
Smith AC, Stone BC. 1962. Studies of Pacific island plants XVII. The genus Geniostoma (Loganiaceae) in the New Hebrides, Fiji, Samoa, and Tonga. – Contr. U.S. Natl. Herb. 37: 1-42.
Smith H. 1965. Notes on Gentianaceae I. The status of Crawfurdia and Tripterospermum; II. New species of Gentianaceae. – Notes Roy. Bot. Gard. Edinb. 26: 237-258.
Smith H. 1967. Gentianinae. – In: Nilsson S (ed), Pollen morphological studies in the Gentianaceae, Grana Palynol. 7: 46-145.
Smith H. 1970. New or little known Himalayan species of Swertia and Veratrilla (Gentianaceae). – Bull. Brit. Mus. (Nat. Hist.), Bot. 4: 239-258.
Soares e Silva UC, Rapini A, Liede-Schumann S, Ribeiro PL, Berg C van den. 2012. Taxonomic considerations on Metastelmatinae (Apocynaceae) based on plastid and nuclear DNA. – Syst. Bot. 37: 795-806.
Sohmer SH. 1977a. Psychotria L. (Rubiaceae) in the Hawaiian Islands. – Lyonia 1: 103-186.
Sohmer SH. 1977b. New taxa and names in Psychotria (Rubiaceae) in Sri Lanka. – Bot. Not. 129: 377-381.
Sohmer SH. 1988. The nonclimbing species of the genus Psychotria (Rubiaceae) in New Guinea and the Bismarck Archipelago. – Bishop Mus. Bull. Bot. 1: 1-339.
Solereder H. 1893. Ein Beitrag zur anatomischen Charakteristik und zur Systematik der Rubiaceen. – Bull. Herb. Boiss. 1: 309-326.
Solereder H. 1895. Loganiaceae. – In: EnglerA, Prantl K (eds), Die natürlichen Pflanzenfamilien IV(2), W. Engelmann, Leipzig, pp. 19-50.
Somers C. 1988. Aoranthe (Rubiaceae), a new genus to accommodate the African species of Porterandia. – Bull. Jard. Bot. Natl. Belg. 58: 47-75.
Song Z-Q, Xu D-X. 2016. Foonchewia coriacea, a new combination to replace F. guangdongensis (Rubiaceae). – Nord. J. Bot. 34: 685-689.
Sonké B. 1999. Oxyanthus (Rubiaceae) en Afrique centrale. – Opera Bot. Belg. 8: 1-106.
Sonké B. 2000. Une nouvelle espèce de Rothmannia (Rubiaceae, Gardenieae) de Banyang Mbo (Cameroun). – Syst. Geogr. Plants 70: 149-153.
Sonké B, Esono P, Nguembou K C, Stévart T. 2005. Une nouvelle espèce de Bertiera Aubl. (Rubiaceae) du sous-genre Bertierella découverte en Guinée Équatoriale et au Cameroun. – Adansonia, sér. III, 27: 309-315.
Sonké B, Nguembou KC, Davis AP. 2006. A new dwarf Coffea (Rubiaceae) from southern Cameroon. – Bot. J. Linn. Soc. 151: 425-430.
Sonké B, Djuikouo KM-N, Robbrecht E. 2007. Calycosiphonia pentamera sp. nov. (afrotropical Rubiaceae) from the ‘Lower Guinea’ area. – Nord. J. Bot. 25: 275-280.
Sonké B, Neuba D, Kenfack D, Block P de. 2007. An extraordinary new rheophyte in the genus Leptactina (Rubiaceae, Pavetteae) from Rio Muni (Equatorial Guinea). – Bot. J. Linn. Soc. 153: 109-113.
Sonké B, Dessein S, Taedoumg H, Groeninckx I, Robbrecht E. 2008. A new species of Colletoecema (Rubiaceae) from southern Cameroon with a discussion of relationships among basal Rubioideae. – Blumea 53: 533-547.
Sotolongo Molina L, Fernández Zequeira M, Herrera Oliver P. 2002. Pollen morphology of some Cuban Guettarda species (Rubiaceae: Guettardeae). – Grana 41: 142-148.
Souza EB de, Sales MF de. 2004. O gênero Staelia Cham. & Schltdl. (Rubiaceae-Spermacoceae) no Estado de Pernambuco, Brasil. – Acta Bot. Bras. 18: 919-926.
Soza VL, Olmstead RG. 2010a. Molecular systematics of tribe Rubieae (Rubiaceae): evolution of major clades, development of leaf-like whorls, and biogeography. – Taxon 59: 755-771.
Soza VL, Olmstead RG. 2010b. Evolution of breeding systems and fruits in New World Galium and relatives (Rubiaceae). – Amer. J. Bot. 97: 1630-1646.
Spegazzini C. 1926. Un nuevo género de Asclepiadáceas. – Physis 8: 269-274.
Sreedevi P, Namboodiri AN. 1982. The germination of pollinium and the organization of germ furrow in some members of Asclepiadaceae. – Can. J. Bot. 60: 166-172.
Srivastava SD, Srivastava SK. 1986. New C-glycosyl flavones from Adina cordifolia. – Curr. Sci. 55: 1069-1071.
Ståhl B. 1999. Gonzalagunia. – In: Harling G, Andersson L (eds), Flora of Ecuador 62: 70-101.
Standley PC. 1919. The Panamanian species of Leiphaimos. – In: Studies of tropical American phanerogams 3, Contr. U. S. Natl. Herb. 20: 194-200.
Standley PC. 1930. The Rubiaceae of Colombia. – Publ. Field Mus. Nat. Hist., Bot. Ser. 7: 1-175.
Standley PC. 1931a. The Rubiaceae of Ecuador. – Publ. Field Mus. Nat. Hist., Bot. Ser. 7: 177-251.
Standley PC. 1931b. The Rubiaceae of Bolivia. – Publ. Field Mus. Nat. Hist., Bot. Ser. 7: 253-339.
Standley PC. 1931c. The Rubiaceae of Venezuela. – Publ. Field Mus. Nat. Hist., Bot. Ser. 7: 341-485.
Standley PC. 1936. Rubiaceae. – In: Macbride JF (ed), Flora of Peru 6, Publ. Field Mus. Nat. Hist., Bot. Ser. 13: 3-261.
Standley PC. 1938. Flora of Costa Rica: Loganiaceae, Gentianaceae. – Field Mus. Nat. Hist., Bot. Ser. 18: 919-929.
Standley PC. 1940. Steyermarkia. Studies on American plants. – Field Mus. Nat. Hist., Bot. Ser. 22: 216-218.
Stanton SL, Meyer JJ, Merwe CF van der. 2013. An evaluation of the endophytic colonies present in pathogenic and non-pathogenic Vanguerieae using electron microscopy. – South Afr. J. Bot. 86: 41-45.
Steck H-J, Weberling F. 1989. Infloreszenzenuntersuchungen an Apocynaceae. – Trop. Subtrop. Pflanzenwelt 71: 1-62.
Steenis CGGJ van. 1939. Ecological observations on the genus Pleiocraterium in Gajoland, Sumatra. – Rec. Trav. Bot. Néerl. 36: 446-448.
Stevens WD. 1988. New names and combinations in Apocyneae, Asclepiadoideae. – Phytologia 64: 333-335.
Stevens WD. 2000. New and interesting milkweeds (Apocynaceae, Asclepiadoideae). – Novon 10: 243-256.
Stevens WD. 2005a. Fourteen new species of Gonolobus (Apocynaceae, Asclepiadoideae) from Mexico and Central America. – Novon 15: 222-244.
Stevens WD. 2005b. New and interesting mildweeds (Apocynaceae, Asclepiadoideae). – Novon 15: 602-619.
Stevens WD. 2005c. Novelties in Cynanchum L. sensu Woodson, in Mesoamerica. – Novon 15: 620-641.
Stevens WD. 2018. Vailia anomala, a new name for Blepharodon mucronatum (Apocynaceae, Asclepiadoideae). – Phytoneuron 2018-3: 1-5.
Steyermark JA. 1951. The genus Tapeinostemon (Gentianaceae). – Lloydia 14: 58-64.
Steyermark JA. 1962. Pittierothamnus, new genus of Rubiaceae. – Bol. Soc. Venez. Ci. Nat.. 23: 92-95.
Steyermark JA. 1964. Rubiaceae. – In: Maguire B, Wurdack JJ et al. (eds), The botany of the Guyana Highland V, Mem. New York Bot. Gard. 10: 1-278.
Steyermark JA. 1965. Género Hippotis. – Acta Bot. Venezuelica 9, Instituto Botanico, Caracas.
Steyermark JA. 1967. Rondeletia and Arachnothryx. – In: Maguire B, Wurdack JJ et al. (eds), The botany of tahe Guyana Highland VII, Mem. New York Bot. Gard. 17: 241-261.
Steyermark JA. 1968. Novedades de la Cordillera Costanera: Apocynaceae. – Acta Bot. Venez. 3: 206-209.
Steyermark JA. 1972. Rubiaceae. – In: Maguire B, Wurdack JJ et al. (eds), The botany of Guayana Highland IX, Mem. New York Bot. Gard. 23: 227-832.
Steyermark JA. 1974. Rubiaceae. – In: Lasser T, Steyermark JA (eds), Flora de Venezuela IX(1-3), Edición Especial del Instituto Botánico, Ministeria de Agricultura y Cria, Carácas, pp. 1-2070.
Steyermark JA. 1983. The genus Botryarrhena in Venezuela. – Ann. Missouri Bot. Gard. 70: 207-208.
Steyermark JA. 1986. Holstianthus, a new genus of Rubiaceae from the Guayana Highland. – Ann. Missouri Bot. Gard. 73: 495-497.
Steyermark JA, Kirkbride Jr JH. 1975. The genus Wittmackanthus (Rubiaceae). – Ann. Missouri Bot. Gard. 62: 504-509.
Steyermark JA, Kirkbride Jr JH. 1977. Review of the genus Perama (Rubiaceae). – Brittonia 29: 191-198.
Steyermark JA et al. 1953. Gentianaceae. Contributions to the flora of Venezuela. – Fieldiana 28: 496-499.
St. John H. 1941. Revision of the genus Swertia (Gentianaceae) of the Americas and the reduction of Frasera. – Amer. Midl. Natur. 26: 1-29.
Stockey RA, Manchester SR. 1986. A fossil flower with in situ Pistillipollenites from the Eocene of British Columbia. – Can. J. Bot. 66: 313-318.
Stoffelen P, Robbrecht E, Smets E. 1996. A revision of Corynanthe and Pausinystalia (African Rubiaceae-Coptosapelteae). – Bot. J. Linn. Soc. 120: 287-326.
Stoffelen P, Robbrecht E, Smets E. 1997a. Pollen morphology of Coffea and Psilanthus (Rubiaceae-Coffeeae), mainly from Africa. – Grana 36: 313-327.
Stoffelen P, Robbrecht E, Smets E. 1997b. Adapted to the rain forest floor: a remarkable new dwarf Coffea (Rubiaceae) from Lower Guinea (tropical Africa). – Taxon 46: 37-47.
Stoffelen P, Robbrecht E, Smets E. 1999. A new species of Coffea (Rubiaceae) from Central Africa, with notes on tentative other taxa. – Syst. Geogr. Plants 69: 119-124.
Stoffelen P, Noirot M, Couturon E, Anthony F. 2008. A new caffeine-free coffee from Cameroon. – Bot. J. Linn. Soc. 158: 67-72.
Stolt KAH. 1921. Zur Embryologie der Gentianaceen und Menyanthaceen. – Kungl. Sv. Vetensk.-Akad. Handl. 61(14): 3-56.
Stranczinger Sz, Galambos A, Szenasy D. Szalontai B. 2014. Phylogenetic relationships in the Neotropical tribe Hamelieae (Rubiaceae, Cinchonoideae) and comments on its generic limits. – J. Syst. Evol. 52: 643-650.
Straub SCK, Cronn RC, Edwards C, Fishbein M, Liston A. 2013. Horizontal transfer of DNA from the mitochondrial to the plastid genome and its subsequent evolution in milkweeds (Apocynaceae). – Genome Biol. Evol. 5: 1872-1885.
Straub SCK, Moore MJ, Soltis PS, Soltis DE,
Liston A, Livshultz T. 2014. Phylogenetic signal detection from an ancient
rapid radiation: effects of noise reduction, long-branch attraction, and model
selection in crown clade Apocynaceae.
– Mol. Phylogen. Evol. 80: 169-185.
Struwe L. 2014. Classification and evolution of the family Gentianaceae. – In: Rybczynski JJ et al. (eds), The Gentianaceae – Characterization and Ecology, vol. 1, Springer-Verlag, Berlin, pp. 13-36.
Struwe, L. 2018. Gelsemiaceae. – In: Kadereit JW, Bittrich V (eds). 2018. The families and genera of vascular plants XV. Flowering plants – eudicots – Apiales, Gentianales (except Rubiaceae). Springer, Berlin, pp. 447-452.
Struwe L, Albert VA. 1996. Phylogenetics of Guayanan Gentianaceae. – Fl. Guianas Newslett. 11: 31-32.
Struwe L, Albert VA. 1997. Floristics, cladistics, and classification: three case studies in Gentianales. – In: Dransfield J, Coode MJE, Simpson DA (eds), Plant diversity in Malesia III, Royal Botanic Gardens, Kew, pp. 321-352.
Struwe L, Albert VA. 1998a. Lisianthius P. Br., its probable homonym Lisyanthus Aubl. (Gentianaceae), and the priority of Helia Mart. over Irlbachia Mart. as its substitute. – Harvard Pap. Bot. 3: 63-71.
Struwe L, Albert VA. 1998b. Six new species of Gentianaceae from the Guayana Shield. – Harvard Pap. Bot. 3: 181-197.
Struwe L, Albert VA (eds). 2002a. Gentianaceae – systematics and natural history. – Cambridge University Press, Cambridge.
Struwe L, Albert VA. 2004. A monograph of neotropical Potalia Aublet (Gentianaceae: Potalieae). – Syst. Bot. 29: 670-701.
Struwe L, Albert VA, Bremer B. 1994 [1995]. Cladistics and family level classification of the Gentianales. – Cladistics 10: 175-206.
Struwe L, Gibbons KL, Conn BJ, Motley TJ. 2018. Loganiaceae. – In: Kadereit JW, Bittrich V (eds). 2018. The families and genera of vascular plants XV. Flowering plants – eudicots – Apiales, Gentianales (except Rubiaceae). Springer, Berlin, pp. 511-526.
Struwe L, Maas PJM, Albert VA. 1997. Aripuana cullmaniorum, a new genus and species of Gentianaceae from White Sands of southeastern Amazonas, Brazil. – Harvard Pap. Bot. 2: 235-253.
Struwe L, Pringle JS. 2018. Gentianaceae. – In: Kadereit JW, Bittrich V (eds). 2018. The families and genera of vascular plants XV. Flowering plants – eudicots – Apiales, Gentianales (except Rubiaceae). Springer, Berlin, pp. 453-503.
Struwe L, Thiv M, Kadereit JW, Pepper AS-R, Motley TJ, White PJ, Rova JHE, Potgieter K, Albert VA. 1998. Saccifolium (Saccifoliaceae), an endemic of Sierra de la Neblina on the Brazilian-Venezuelan border, is related to a temperate-alpine lineage of Gentianaceae. – Harvard Pap. Bot. 3: 199-214.
Struwe L, Kadereit JW, Klackenberg J, Nilsson S, Thiv M, Hagen KB von, Albert VA. 2002. Systematics, character evolution, and biogeography of Gentianaceae, including a new tribal and subtribal classification. – In: Struwe L, Albert VA (eds), Gentianaceae – systematics and natural history, Cambridge University Press, Cambridge, pp. 21-309.
Struwe L, Kinkade MP, Maas PJM. 2005. Two new Brazilian species of Tachia (Gentianaceae: Helieae). – Blumea 50: 457-462.
Struwe L, Nilsson S, Albert VA. 2008. Roraimaea (Gentianaceae: Helieae) – a new gentian genus from white sand campinas and Cerro de la Neblina of Brazil and Venezuela. – Harv. Pap. Bot. 13: 35-45.
Struwe L, Haag S, Heiberg E, Grant JR. 2009. Andean speciation and vicariance in Neotropical Macrocarpaea (Gentianaceae-Helieae). – Ann. Missouri Bot. Gard. 96: 450-469.
Struwe L, Albert VA, Calió MF, Frasier C, Lepis KB, Mathews KG, Grant JR. 2009. Evolutionary patterns in Neotropical Helieae (Gentianaceae): evidence from morphology, chloroplast and nuclear DNA sequences. – Taxon 58: 479-499.
Struwe L, Soza VL, Manickam S, Olmstead RG.
2014. Gelsemiaceae (Gentianales)
expanded to include the enigmatic Asian genus Pteleocarpa. – Bot. J.
Linn. Soc. 175: 482-496.
Sundell E. 1980. The subfamilial, tribal, and subtribal nomenclature of the Asclepiadaceae. – Taxon 29: 257-265.
Sundell E. 1981. The New World species of Cynanchum subgenus Mellichampia (Asclepiadaceae). – Evol. Monogr. 5: 1-62.
Suratman. 2018. The genus Gynochthodes (Rubiaceae) in Sumatra. – Blumea 62: 230-239.
Surveswaran S, Kamble MY, Yadav SR, Sun M. 2009. Molecular phylogeny of Ceropegia (Asclepiadoideae, Apocynaceae) from Indian Western Ghats. – Plant Syst. Evol. 281: 51-63.
Surveswaran S, Sun M, Grimm GW,
Liede-Schumann S. 2014. On the systematic position of some Asian enigmatic
genera of Asclepiadoideae (Apocynaceae). – Bot. J. Linn. Soc.
174: 601-619.
Swarupanandan K. 1983. An illegitimate Pergularia and the tribal names Ceropegieae, Marsdenieae and Stapelieae (Asclepiadaceae). – Taxon 32: 468-471.
Swarupanandan K, Mangaly JK. 1992. A new species of Ceropegia (Asclepiadaceae) from India. – Nord. J. Bot. 12: 699-701.
Swarupanandan K, Sasidharan N, Mangaly JK. 1989. A reconsideration of the generic circumscription of Heterostemma Wight & Arn. (Asclepiadaceae) and a new species from India. – Bot. J. Linn. Soc. 101: 249-259.
Swarupanandan K, Mangaly JK, Sonny TK, Kishorekumar K, Chand Basha S. 1996. The subfamilial and tribal classification of the family Asclepiadaceae. – Bot. J. Linn. Soc. 120: 327-369.
Sýkorová Z, Wiemken A, Redecker D. 2007. Co-occurring Gentiana verna and Gentiana acaulis and their neighboring plants in two Swiss upper montane meadows harbour distinct arbuscular mycorrhizal fungal communities. – Appl. Envir. Microbiol. 73: 5426-5434.
Sylla T, Albers F. 1988. Samenentwicklung und Samenmorphologie kräutiger und sukkulenter Asclepiadaceae. – Bot. Jahrb. Syst. 110: 479-492.
Sytsma KJ. 1987. The shrubby gentian genus Macrocarpaea in Panama. – Ann. Missouri Bot. Gard. 78: 310-313.
Sytsma KJ. 1988. Taxonomic revision of the Central American Lisianthius skinneri species complex (Gentianaceae). – Ann. Missouri Bot. Gard. 75: 1587-1602.
Sytsma KJ, Schaal BA. 1985. Phylogenetics of the Lisianthius skinneri (Gentianaceae) species complex in Panama utilizing restriction fragment analysis. – Evolution 39: 594-608.
Sytsma KJ, Schaal BA. 1990. Ribosomal DNA variation within and among individuals of Lisianthius (Gentianaceae) populations. – Plant Syst. Evol. 170: 97-106.
Takeda H. 1916. Some points in the morphology of the stipules in the Stellatae, with special reference to Galium. – Ann. Bot. (Oxford) 30: 197-214.
Takeda Y, Puff C, Kondoh Y, Yoshioka Y. 1991. Analysis of iridoid glycosides of Paederia L. species (Rubiaceae-Paederieae) by gaschromatography mass spectrometry. – Opera Bot. Belg. 3: 153-158.
Tammaro F. 1986. Systematic and biometric study on Gentiana gr. orbicularis (Gentianaceae) from the Central Apennines (Italy). – Arch. Bot. Biol. Ital., Torino.
Tange C. 1994. Neomussaenda (Rubiaceae), a new genus from Borneo. – Nord. J. Bot. 14: 495-500.
Tange C. 1995. The identity of Siderobombyx and a new species of Xanthophytum (Rubiaceae). – Nord. J. Bot. 15: 575-581.
Tange C. 1996a. Studies in SE Asiatic Rondeletieae I: the West Malaysian endemic genus Aleisanthia (Rubiaceae). – Nord. J. Bot. 16: 563-570.
Tange C. 1996b. Studies in SE Asiatic Rondeletieae II: Aleisanthiopsis (Rubiaceae), a new genus from Borneo. – Nord. J. Bot. 16: 571-578.
Tange C. 1997. A revision of the genus Mouretia (Rubiaceae). – Nord. J. Bot. 17: 123-132.
Tange C. 1998. Cyanoneuron (Rubiaceae), a new genus from Borneo and Sulawesi. – Nord. J. Bot. 18: 147-158.
Tapadar N, Roy N. 1964. Cytotaxonomic studies in Apocynaceae and delineation of the different evolutionary tendencies operating within the family. – Caryologia 77: 103-138.
Taylor CM. 1984. Palicourea spathacea (Rubiaceae), an unusual new species from Costa Rica. – Syst. Bot. 9: 226-228.
Taylor CM. 1989. Revision of Hillia subg. Ravnia (Rubiaceae: Cinchonoideae). – Selbyana 11: 26-34.
Taylor CM. 1994. Revision of Hillia (Rubiaceae). – Ann. Missouri Bot. Gard. 81: 571-609.
Taylor CM. 1996a. Taxonomic revision of Cruckshanksia and Oreopolus (Rubiaceae: Hedyotideae). – Ann. Missouri Bot. Gard. 83: 461-479.
Taylor CM. 1996b. Overview of the Psychotrieae (Rubiaceae) in the Neotropics. – Opera Bot. Belg. 7: 261-270.
Taylor CM. 1997. Conspectus of the genus Palicourea (Rubiaceae: Psychotrieae) with the description of some new species from Ecuador and Colombia. – Ann. Missouri Bot. Gard. 84: 224-262.
Taylor CM. 1999. 162(20). Rubiaceae-Coussareeae. – In: Harling G, Andersson L (eds), Flora of Ecuador 62, Nord. J. Bot., Copenhagen, pp. 245-314.
Taylor CM. 2001. Overview of the Neotropical genus Notopleura (Rubiaceae: Psychotrieae), with the description of some new species. – Ann. Missouri Bot. Gard. 88: 478-515.
Taylor CM. 2002. Rubiacearum Americanarum Magna Hama Pars X: new species and a new subspecies of Faramea (Coussareeae) from Central and South America. – Novon 12: 563-570.
Taylor CM. 2004. The neotropical genus
Ronabea (Rubiaceae,
Lasiantheae). – Syst. Geogr. Plants 74: 35-42.
Taylor CM. 2005. Margaritopsis (Rubiaceae, Psychotrieae) in the Neotropics. – Syst. Geogr. Plants 75: 161-177.
Taylor CM. 2006. Rubiacearum Americanarum Magna Hama Pars XIX: new species of Palicourea (Psychotrieae) from Ecuador, Peru, and Venezuela. – Brittonia 58: 156-169.
Taylor CM. 2010. Rubiacearum Americanarum Magna Hama Pars XXIII: overview of the Guettardeae tribe in Central and South America, with five new species and three new combinations in Chomelia, Neoblakea, Pittoniotis, and Stenostomum. – Novon 20: 351-362.
Taylor CM. 2011. The genus Coccochondra (neotropical Rubiaceae) expanded. – Plant Ecol. Evol. 144: 115-118.
Taylor CM. 2015a. Rubiacearum Americanarum
Magna Hama XXXIII: the new group Palicourea sect.
Didymocarpae with four new species and two new subspecies
(Palicoureeae). – Novon 23: 452-478.
Taylor CM. 2015b. Rubiacearum Americanarum Magna Hama Pars XXXIV: the new group Palicourea sect. Tricephalium with eight new species and a new subspecies (Palicoureeae). – Novon 24: 55-95.
Taylor CM. 2016. Rubiacearum Americanarum Magna Hama Pars XXXI: more new Neotropical species and morphological notes for Psychotria (Psychotrieae). – Novon 24: 413-434.
Taylor CM. 2017. Rubiacearum Americanarum Magna Hama Pars XXXVII: the new group Palicourea sect. Chocoanae of the Chocó biogeographic region, with two new species (Palicoureeae). – Novon 25: 322-342.
Taylor CM. 2018. Rubiacearum americanarum magna hama pars XXXVIII: a new circumscription of Palicourea sect. Bracteiflorae, an Andean radiation with several new species (Palicoureeae). – Novon 26: 66-138.
Taylor CM, Andersson L. 1999. 162(18). Rubiaceae-Psychotrieae (1). – In: Harling G, Andersson L (eds), Flora of Ecuador 62, Nord. J. Bot., Copenhagen, pp. 133-241.
Taylor CM, Clark JL. 2001. Rubiacearum Americanarum Magna Hama V. Amphidasya in Mesoamerica and Western South America. – Novon 11: 489-497.
Taylor CM, Gereau RE. 2013. The genus Carapichea (Rubiaceae, Psychotrieae). – Ann. Missouri Bot. Gard. 99: 100-127.
Taylor CM, Hollowell VC. 2016. Rubiacearum Americanarum Magna Hama Pars XXXV: the new group Palicourea sect. Nonatelia, with five new species (Palicoureeae). – Novon 25: 69-110.
Taylor CM, Lorence D. 2010. Rubiacearum Americanum Magna Hama Pars XXII: notable new species of South American Coutarea, Morinda, Patima, and Rosenbergiodendron. – Novon 20: 95-105.
Taylor CM, Zappi D. 2006. 162. Rubiaceae (part 5). Tribe 18. Psychotrieae (2). – In: Harling G, Persson C (eds), Flora of Ecuador 79, Department of Plant and Environmental Sciences, Göteborg University, pp. 1-111.
Taylor CM, Hammel B, Gereau RE. 2011. Rubiacearum Americanarum Magna Hama Pars XXVII: six new species and a new taxonomic view of Posoqueria. – Novon 21: 118-132.
Taylor CM, Neill DA, Gereau RE. 2011. Rubiacearum Americanarum Magna Hama Pars XXIX: overview of the Neotropical genus Schizocalyx (Condamineeae) and description of two new species. – Novon 21: 496-507.
Taylor CM, Malcomber S, Schatz GE. 2014.
Updated taxonomy of Gaertnera (Rubiaceae, Gaertnereae) in Madagascar,
with sixteen new species. – Ann. Missouri Bot. Gard. 99: 688-729.
Taylor CM, Bruniera CP, Zappi DC. 2015. Taxonomic transfers in Neotropical Palicoureeae: new combinations in Rudgea and Palicourea. – Kew Bull. 70: 45 DOI 10.1007/S12225-015-9596-3
Taylor CM, Razafimandimbison SG, Barrabé L, Jardim JG, Barbosa MRV. 2017. Eumachia expanded, a pantropical genus distinct from Psychotria (Rubiaceae, Palicoureeae). – Candollea 72: 289-318.
Taylor DW. 2003a. A taxonomic revision of the genus Chione (Rubiaceae). – Syst. Geogr. Plants 73: 171-198.
Taylor DW. 2003b. Colleteria (Rubiaceae), a new genus from the Caribbean. – Syst. Geogr. Plants 73: 199-208.
Taylor P. 1973. A revision of the genus Faroa Welwitsch. – Garcia de Orta, Bot. 1: 69-82.
Terrell EE. 1987. Carterella (Rubiaceae), new genus from Baja California, Mexico. – Brittonia 39: 248-252.
Terrell EE. 1990. Synopsis of Oldenlandia (Rubiaceae) in the United States. – Phytologia 68: 125-133.
Terrell EE. 1991. Overview and annotated list of North American species of Hedyotis, Houstonia, Oldenlandia (Rubiaceae), and related genera. – Phytologia 71: 212-243.
Terrell EE. 1996. Revision of Houstonia (Rubiaceae-Hedyotideae). – Syst. Bot. Monogr. 48: 1-118.
Terrell EE. 2001a. Taxonomy of Stenaria (Rubiaceae; Hedyotideae), a new genus including Hedyotis nigricans. – Sida 19: 591-614.
Terrell EE. 2001b. Stenotis (Rubiaceae), a new segregate genus from Baja California, Mexico. – Sida 19: 899-911.
Terrell EE, Lewis WH. 1990. Oldenlandiopsis (Rubiaceae), a new genus from the Caribbean basin, based on Oldenlandia callitrichoides Grisebach. – Brittonia 42: 185-190.
Terrell EE, Robinson H. 2003. Survey of Asian and Pacific species of Hedyotis and Exallage (Rubiaceae) with nomenclatural notes on Hedyotis types. – Taxon 52: 775-782.
Terrell EE, Robinson H. 2009. A new genus, Mexotis, for five Mexican species of Hedyotideae (Rubiaceae). – J. Bot. Res. Inst. Texas 3: 59-70.
Terrell EE, Wunderlin RP. 2002. Seed and fruit characters in selected Spermacoceae and comparison with Hedyotideae (Rubiaceae). – Sida 20: 549-557.
Terrell EE, Lewis WH, Robinson H, Nowicke JW. 1986. Phylogenetic implications of diverse seed types, chromosome numbers and pollen morphology in Houstonia (Rubiaceae). – Amer. J. Bot. 73: 103-115.
Terrell EE, Lewis WH, Robinson H, Nowicke JW. 1986. Cyaneuron (Rubiaceae), a new genus from Borneo and Sulawesi. – Nord. J. Bot. 147-158.
Terrell EE, Robinson HE, Wagner WL, Lorence DH. 2005. Resurrection of genus Kadua for Hawaiian Hedyotidinae (Rubiaceae), with emphasis on seed and fruit characters and notes on South Pacific species. – Syst. Bot. 30: 818-833.
Ter Welle BJH, Loureiro AA, Lisboa PLB, Koek-Noorman J. 1983. Systematic wood anatomy of the tribe Guettardeae (Rubiaceae). – Bot. J. Linn. Soc. 87: 13-28.
Theisen I, Barthlott W. 1994. Mikromorphologie der Epicuticularwachse und die Systematik der Gentianales, Rubiales, Dipsacales und Calycerales. – Trop. Subtrop. Pflanzenwelt 89: 1-62.
Thiv M. 2000. Molekulare und morphologische Phylogenie und Biogeographie der Gentianaceae-Chironieae und eine Revision der Gattungen Canscora, Cracosna, Duplipetala, Hoppea, Microrphium, Phyllocyclus und Schinziella (Gentianaceae-Canscorinae). – Ph.D. diss., Universität Mainz, Marburg, Germany.
Thiv M. 2003. A taxonomic revision of Canscora, Cracosna, Duplipetala, Hoppea, Microrphium, Phyllocyclus and Schinziella (Gentianaceae-Canscorinae). – Blumea 48: 1-46.
Thiv M, Kadereit JW. 2002. A morphological cladistic analysis of Gentianaceae-Canscorinae and the evolution of anisomorphic androecia in the subtribe. – Syst. Bot. 27: 780-788.
Thiv M, Meve U. 2007. A phylogenetic study of Echidnopsis Hook. f. (Apocynaceae-Asclepiadoideae) – taxonomic implications and the colonization of the Socotran archipelago. – Plant Syst. Evol. 265: 71-86.
Thiv M, Struwe L, Albert VA, Kadereit JW. 1999. The phylogenetic relationships of Saccifolium bandeirae Maguire and Pires (Gentianaceae) reconsidered. – Harvard Pap. Bot. 4: 519-526.
Thiv M, Struwe L, Kadereit JW. 1999 [2000]. The phylogenetic relationships and evolution of the Canarian laurel forest endemic Ixanthus viscosus (Alt.) Griseb. (Gentianaceae): evidence from matK and ITS sequence variation, and floral morphology and anatomy. – Plant Syst. Evol. 218: 299-317.
Thomas V, Dave Y. 1991. Comparative and phylogenetic significance of the colleters in the family Apocynaceae. – Feddes Repert. 102: 177-182.
Thomas V, Dave Y, Menon ARS. 1989. Anatomy and histochemistry of colleters in Poupelia grata (Apocynaceae). – Nord. J. Bot. 8: 493-496.
Thulin M. 1997. Gaillonia (Rubiaceae-Paederieae) in Africa and Arabia. – Nord. J. Bot. 18: 31-38.
Thulin M. 2001. Exacum (Gentianaceae) on the Arabian peninsula and Socotra. – Nord. J. Bot. 21: 243-247.
Thulin M. 2009. New species of Caralluma and Ceropegia (Apocynaceae: Asclepiadoideae-Ceropegieae) from eastern Ethiopia. – Kew Bull. 64: 477-483.
Thulin M, Bremer B. 2004. Studies in the tribe Spermacoceae (Rubiaceae-Rubioideae): the circumscriptions of Amphiasma and Pentanopsis and the affinities of Phylohydrax. – Plant Syst. Evol. 247: 233-239.
Thulin M, Hjertson M. 1995. Echidnopsis globosa sp. nov. (Asclepiadaceae-Stapelieae) from Yemen. – Nord. J. Bot. 15: 261-262.
Tiagi YC, Kshetrapal S. 1974. Studies on the floral anatomy, evolution of the gynoecium, and relationships of the family Loganiaceae. – Adv. Plant Morph. 1972: 408-416.
Tilney PM. 1986. The taxonomic significance of anatomical and morphological characters in the southern African species of Canthium Lam. (Rubiaceae). – Ph.D. diss., University of Pretoria, Republic of South Africa.
Tilney PM, Wyk AE van. 1997. Pollen morphology of Canthium, Keetia and Psydrax (Rubiaceae: Vanguerieae) in southern Africa. – Grana 36: 249-260.
Tilney PM, Wyk AE van. 2009. Keetia (Rubiaceae subfam. Ixoroideae, tribe Vanguerieae) in southern Africa – an update on the classification and selected recently-studied structural features. – South Afr. J. Bot. 75: 423.
Tirvengadum DD. 1978a. A synopsis of the Rubiaceae-Gardenieae of Ceylon (Sri Lanka). – Bull. Mus. Natl. Hist. Nat. Paris, sér. III, Bot. 35: 3-33.
Tirvengadum DD. 1978b. Re-establishment of the genus Catunaregam Wolf (Rubiaceae) – a contribution towards the plants described in Rheede’s “Hortus Malabaricus”. – Taxon 27: 513-517.
Tirvengadum DD. 1982a. Ramosmania, a new monotypic genus of Mascarene Rubiaceae. – Nord. J. Bot. 2: 323-327.
Tirvengadum DD. 1982b. A study of tribe Gardenieae (Rubiaceae) of South and South East Asia. Generic delimitation and revision with enumeration of species. – Thesis Lic.Sc., Botanical Inst., University of Aarhus, Denmark.
Tirvengadum DD. 1983a. New taxa and name changes in tropical Asiatic Rubiaceae. – Nord. J. Bot. 3: 455-469.
Tirvengadum DD. 1983b. Re-establishment of the genus Benkara Adanson (Rubiaceae): second contribution towards the plants described in Rheede’s “Hortus Malabaricus”. – Taxon 32: 436-441.
Tirvengadum DD. 1984. Glionnetia, un nouveau genre de Rubiacées (Rondélétiées) des Seychelles. – Bull. Mus. Natl. Hist. Nat. Paris, Sect. B, Adansonia, sér. II, 6: 197-205.
Tirvengadum DD. 1998. Novelties in Rubiaceae from the limestone flora of southeast Asia. – Biogeographica 74: 163-175.
Tirvengadum DD. 1991. Remarques sur le genre Pelagodendron et typification du genre Rhopalobrachium (Rubiaceae). – Candollea 46: 251-254.
Tirvengadum DD. 1993. Larsenaikia, a new genus of the Rubiaceae from Australia. – Nord. J. Bot. 13: 175-184.
Tirvengadum DD. 1998. Novelties in Rubiaceae from the limestone flora of Southeast Asia. – Biogeographica (The Hague) 74: 163-175.
Tirvengadum DD, Robbrecht E. 1985. Remarks on three Hypobathreae (Rubiaceae) from Rodrigues, Seychelles and Sri Lanka. – Nord. J. Bot. 5: 455-461.
Tirvengadum DD, Sastre C. 1979. La signification taxonomique des modes de ramification de Randia et genres affinés. – Bull. Mauritius Inst. 8: 77-98.
Tirvengadum DD, Sastre C. 1986. Étude taxonomique et systèmes deramifications chez Aidia et genres asiatiques affins et chez Brachytome (Rubiaceae). – Bull. Mus. Natl. Hist. Nat. Paris, sér. IV, 8, sect. B, Adansonia 3: 257-296.
Toni KLG de, Mariath JEA. 2008. Ovule ontogeny in Rubiaceae (Juss.): Chomelia obtusa (Cinchonoideae-Guettardeae) and Ixora coccinea (Ixoroideae-Ixoreae). – Plant Syst. Evol. 272: 39-48.
Toni KLG de, Mariath JEA 2010. Ovule ontogeny of Relbunium species in the evolutionary context of Rubiaceae. – Aust. J. Bot. 58: 70-79.
Toni KLG de, Mariath JEA. 2011. Developmental anatomy and morphology of the flowers and fruits of species from Galium and Relbunium (Rubieae, Rubiaceae). – Ann. Missouri Bot. Gard. 98: 206-225.
Torres N, Saaez L, Mus M, Rosselloa JA. 2001. The taxonomy of Galium crespianum J. J. Rodr. (Rubiaceae), a Balearic Islands endemic revisited. – Bot. J. Linn. Soc. 136: 313-322.
Torres-Montúfar A, Borsch T, Fuentes S, Clase T, Peguero B, Ochoterena H. 2017. The new Hispaniolan genus Tainus (Rubiaceae) constitutes an isolated lineage in the Caribbean biodiversity hotspot. – Willdenowia 47: 259-270.
Tosh J, Block P de, Davis AP, Dessein S, Robbrecht E, Emets EF. 2008. The tribal placement of the monospecific tropical African genus Petitiocodon (Rubiaceae) based on molecular data and morphology. – Blumea 53: 549-565.
Tosh J, Davis AP, Dessein S, De Block P, Huysmans S, Fay MF, Smets E, Robbrecht E. 2009. Phylogeny of Tricalysia (Rubiaceae) and its relationships with allied genera based on plastid DNA data: resurrection of the genus Empogona. – Ann. Missouri Bot. Gard. 96: 194-213.
Tosh J, Dessein S, Buerki S, Groeninckx I, Mouly A, Bremer B, Smets EF, De Block P. 2013. Evolutionary history of the Afro-Madagascan Ixora species (Rubiaceae): species diversification and distribution of key morphological traits inferred from dated molecular phylogenetic trees. – Ann. Bot. 112: 1723-1742.
Toyokuni H. 1961. Séparation de Comastoma, genre nouveau, d’avec Gentianella. – Bot. Mag. (Tokyo) 74: 198.
Toyokuni H. 1963. Conspectus gentianacearum japonicarum, a general view of the Gentianaceae indigenous to Japan. – J. Fac. Sci. Hokkaido Univ., Ser.V, 7: 137-259.
Toyokuni H. 1965. Systema gentianinarium novissimum – facts and speculation relating to the phylogeny of Gentiana, sensu lato and related genera. – Symb. Asahikawensis 1: 147-158.
Trivedi BS, Upadhyay N. 1977. Morphological studies in Apocynaceae: epidermal structures. – Geophytology 7: 29-37.
Troll W. 1959. Neue Beiträge zur Kenntnis der Blütenstande und Blüten von Ceropegia-Arten. – Akad. Wiss. Lit. Abhandl. Math.-Naturw. Kl. 5: 227-263.
Tsiang Y. 1936. Notes on the Asiatic Apocynales III. – Suynyatsenia 3: 121-239.
Turner BL. 1993. The Texas species of Centaurium (Gentianaceae). – Phytologia 75: 259-275.
Turner BL. 1994. Taxonomic revision of Geniostemon (Gentianaceae). – Phytologia 76: 8-13.
Turner BL. 2014. Taxonomic overview of Eustoma (Gentianaceae). – Phytologia 96: 7-11.
Ubsdell RAE. 1979. Studies and evolution in Centaurium Erythraea Rafn and Centaurium littorale (D. Turner) Gilmour in the British Isles; breeding systems, floral biology and general discussion. – Watsonia 12: 225-232.
Uesato S, Miyauchi M, Itoh H, Inouye H. 1986. Biosynthesis of iridoid glucosides in Galium mollugo, G. spurium var. echinospermum and Deutzia crenata. Intermediacy of deoxyloganic acid, loganin and iridodial glucoside. – Phytochemistry 25: 2515-2521.
Ulbrich E. 1934. Thelygonaceae (Cynocrambaceae.). – In: Engler A (†), Pax F, Harms H (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 16c, W. Engelmann, Leipzig, pp. 368-378.
Utteridge TMA. 2002. Contributions to the flora of Mt Jaya VII. New species of Coprosma (Rubiaceae) from New Guinea. – Kew Bull. 57: 195-203.
Utzschneider R. 1947. Der Fruchtknotenbau der Rubiaceen mit besonderer Berücksichtigung der Cinchonoideen. – Ph.D. diss., Ludwig Maximiliansuniversität, München, Germany.
Utzschneider R. 1951. Zur Abstammung der Rubiaceae. – Mitt. Bot. Staatssamml. München 3: 96-98.
Vaes E, Vrijdaghs A, Smets EF, Dessein S. 2006. Elaborate petals in Australian Spermacoce (Rubiaceae) species: morphology, ontogeny and function. – Ann. Bot. 98: 1167-1178.
Valeton T. 1913. Drei neue Arten von Neurocalyx. – Feddes Repert. 12: 513-514.
Valeton T. 1927. Die Rubiaceen von papuasien. Zweiter Teil. Coffeoideae. – Engl. Bot. Jahrb. Syst. 61: 32-163.
Vanthournout S. 2002. Geophila. Revisie van de paleotropische taxa. – Lic. thesis, Katolieke Universiteit de Leuven, Belgium.
Veldkamp JF. 1968. A synopsis of the genus Enicostema Bl., nom. cons. (Gentianaceae). – Blumea 41: 133-136.
Veldkamp JF. 1988. Notes on Pteleocarpa, incertae sedis. – Flora Males. Bull. 10: 47-50.
Ven EA van de, Ham RWJM van der. 2006. Pollen of Melodinus (Apocynaceae): monads and tetrads. – Grana 45: 1-8.
Venter HJT. 2008. Taxonomy of Chlorocyathus (Apocynaceae: Periplocoideae). – South Afr. J. Bot. 74: 288-294.
Venter HJT. 2009a. A taxonomic revision of Raphionacme (Apocynaceae: Periplocoideae). – South Afr. J. Bot. 75: 292-350.
Venter HJT. 2009b. Nomenclature correction in Parquetina (Apocynaceae: Periplocoideae). – South Afr. J. Bot. 75: 557-559.
Venter HJT, Verhoeven RL. 1990. A taxonomic revision of Ectadium (Periplocaceae). – South Afr. J. Bot. 56: 113-124.
Venter HJT, Verhoeven RL. 1994. Maclaudia felixii, a new genus and species in the Periplocaceae from Guinea, West Africa. – Bot. J. Linn. Soc. 115: 57-63.
Venter HJT, Verhoeven RL. 1997. A tribal classification of the Periplocoideae (Apocynaceae). – Taxon 46: 705-720.
Venter HJT, Verhoeven RL. 1999. A new species of Cryptolepis (Periplocoideae, Apocynaceae) from Arabia. – Bot. J. Linn. Soc. 131: 417-422.
Venter HJT, Verhoeven RL. 2001. Diversity and relationships within the Periplocoideae (Apocynaceae). – Ann. Missouri Bot. Gard. 88: 550-568.
Venter HJT, Verhoeven RL. 2009. Morphology and taxonomy of Baseonema and Batesanthus (Apocynaceae: Periplocoideae). – South Afr. J. Bot. 75: 445-455.
Venter HJT, Verhoeven RL, Kotze JDS. 1990. A monograph of Tacazzea (Periplocaceae). – South Afr. J. Bot. 56: 93-112.
Venter HJT, Thulin M, Verhoeven RL. 2006. Two new species of Cryptolepis (Apocynaceae: Periplocoideae) from Somalia, North-East Africa. – South Afr. J. Bot. 72: 139-143.
Venter HJT, Dold AP, Verhoeven RL, Ionta G. 2006. Kappia lobulata (Apocynaceae, Periplocoideae), a new genus from South Africa. – South Afr. J. Bot. 72: 529-533.
Venter HJT, Winter PJD, Verhoeven RL, Archer RH. 2007. Raphionacme villicorona (Apocynaceae: Periplocoideae), a new species from the Sekhukhuneland centre of plant endemism, South Africa. – South Afr. J. Bot. 73: 97-101.
Verdcourt B. 1950. A revision of the genus Otiophora Zucc. (Rubiaceae). – Bot. J. Linn. Soc. 53: 383-412.
Verdcourt B. 1952a. The identity of Ophiorrhiza lancolata Forsk. – Kew Bull. 6: 377-380.
Verdcourt B. 1952b. A revision of certain African genera of the herbaceous Rubiaceae I. The genus Pentanisia Harvey. – Bull. Jard. Bot. État Bruxelles 22: 233-286.
Verdcourt B. 1953a. A revision of certain African genera of the herbaceous Rubiaceae II. The genus Otomeria Benth. and the new genus Batopedina Verdcourt. – Bull. Jard. Bot. État Bruxelles 23: 5-34.
Verdcourt B. 1953b. A revision of certain African genera of the herbaceous Rubiaceae IV. Notes on the genera Parapentas Brem., Tapinopentas Brem., and Otiophora Zucc. – Bull. Jard. Bot. État Bruxelles 23: 53-64.
Verdcourt B. 1953c. A revision of certain African genera of the herbaceous Rubiaceae V. A revision of the genus Pentas Bentham together with a key to related genera. – Bull. Jard. Bot. État Bruxelles 23: 237-371.
Verdcourt B. 1958. Remarks on the classification of the Rubiaceae. – Bull. Jard. Bot. État Bruxelles 28: 209-290.
Verdcourt B. 1966. New taxa and records of Rubiaceae from Eastern Africa. – Kirkia 5: 273-277.
Verdcourt B. 1974. A revision of the African species of Carphalea Juss. (=Dirichletia Klotzsch) (Rubiaceae). – Kew Bull. 28: 423-428.
Verdcourt B. 1975a. Studies in the Rubiaceae-Rubioideae for the ’Flora of Tropical East Africa’ I. – Kew Bull. 30: 247-326.
Verdcourt B. 1975b. New sectional names in Spermacoce and a new tribe Virectarieae. – Kew Bull. 30: 366.
Verdcourt B. 1976a. Notes on African Rubiaceae. – Kew Bull. 31: 181-186.
Verdcourt B. 1976b. Rubiaceae (Part 1). – In: Polhill RH (ed), Flora of tropical East Africa, Crown Agents for Oversea Governments and Administrations, London, pp. 1-414.
Verdcourt B. 1979a. Notes on African Rubiaceae: Leptactina and Cuviera. – Kew Bull. 33: 491-498.
Verdcourt B. 1979b. Notes on African Gardenia (Rubiaceae). – Kew Bull. 34: 345-360.
Verdcourt B. 1980. A conspectus of Polysphaeria (Rubiaceae). – Kew Bull. 35: 97-130.
Verdcourt B. 1981a. A conspectus of Polysphaeria (Rubiaceae): Addendum. – Kew Bull. 36: 227-228.
Verdcourt B. 1981b. Identity of Calanda K. Schum. and Otocephalus Chiov. (Rubiaceae, Knoxieae). – Kew Bull. 36: 238.
Verdcourt B. 1981b. Notes on African Rubiaceae. – Kew Bull. 36: 493-557.
Verdcourt B. 1983. Notes on Mascarene Rubiaceae. – Kew Bull. 37: 521-574.
Verdcourt B. 1985. New taxa of Rytigynia (Rubiaceae- Vanguerieae). – Kew Bull. 40: 656.
Verdcourt B. 1986. The identity of Lepipogon Bertol. f. (Rubiaceae). – Kew Bull. 41: 44.
Verdcourt B. 1987. Notes on African Rubiaceae: Vanguerieae. – Kew Bull. 42: 123-199.
Verdcourt B. 1988. Rubiaceae (Part 2). – In: Polhill RM (ed), Flora of tropical East Africa, A. A. Balkema, Rotterdam, pp. 415-747.
Verdcourt B. 1989. Rubiaceae (Rubioideae). – In: Launert E (ed), Flora Zambesiaca, Flora Zambesiaca Managing Committee, London.
Verdcourt B, Bridson D. 1991. Rubiaceae (Part 3). – In: Polhill RM (ed), Flora of tropical East Africa, A. A. Balkema, Rotterdam, pp. 749-956.
Verellen J. 2002. Palynologische studie en revisie van Coptosapelta (Rubiaceae). – Lic. thesis, Katolieke Universiteit de Leuven, Belgium.
Verellen J, Smets E, Huysmans S. 2004. The remarkable genus Coptosapelta (Rubiaceae): pollen and orbicule morphology and systematic implications. – J. Plant Res. 117: 57-68.
Verellen J, Dessein S, Razafimandimbison SG, Smets E, Huysmans S. 2007. Pollen morphology of the tribes Naucleeae and Hymenodictyeae (Rubiaceae-Cinchonoideae) and its phylogenetic significance. – Bot. J. Linn. Soc. 153: 329-341.
Verhoeven RL, Venter HJT. 1988. Pollen morphology of Raphionacme (Periplocaceae). – South Afr. J. Bot. 54: 123-132.
Verhoeven RL, Venter HJT. 1994. Pollen morphology of the Periplocaceae from Madagascar. – Grana 33: 295-308.
Verhoeven RL, Venter HJT. 2001. Pollen morphology of the Periplocoideae, Secamonoideae, and Asclepiadoideae (Apocynaceae). – Ann. Missouri Bot. Gard. 88: 569-582.
Verhoeven RL, Liede S, Endress ME. 2003. The tribal position of Fockea and Cibirhiza (Apocynaceae: Asclepiadoideae): evidence from pollinium structure and cpDNA sequence data. – Grana 42: 70-81.
Verstraete B, Groeninckx I, Smets E, Huysmans S. 2011. Phylogenetic signal of orbicules at family level: Rubiaceae as case study. – Taxon 60: 742-757.
Verstraete B, Lachenaud O, Smets E, Dessein S, Sonké B. 2013. Taxonomy and phylogenetics of Cuviera (Rubiaceae-Vanguerieae) and reinstatement of Globulostylis with the description of three new species. – Bot. J. Linn. Soc. 173: 407-441.
Verstraete B, Janssens S, Lemaire B, Smets E, Dessein S. 2013. Phylogenetic lineages in Vanguerieae (Rubiaceae) associated with Burkholderia bacteria in sub-Saharan Africa. – Amer. J. Bot. 100: 2380-2387.
Verstraete B, Janssens S, Smets E, Dessein S. 2013. Symbiotic β-Proteobacteria beyond legumes: Burkholderia in Rubiaceae. – PloS ONE 8: e55260.
Verstraete B, Janssens S, Rønsted N. 2017. Non-nodulated bacterial leaf symbiosis promotes the evolutionary success of its host plants in the coffee family (Rubiaceae). – Mol. Phylogen. Evol. 113: 161-168.
Verstraete B, Steyn HM, Van Wyk AE. 2018. Taxonomic status of some geofrutex members of Vanguerieae (Rubiaceae): notes on Eriosemopsis and Pygmaeothamnus and the description of a new genus Bridsonia. – Bot. J. Linn. Soc. 186: 47-65.
Vethacke MU. 1994. Systematische Untersuchungen an Gattungen der Tribus Gonolobeae (Asclepiadaceae). – Diplomarbeit, Westfälische Wilhelms-Universität, Münster, Germany.
Victor JE, Nicholas A. 1998. In defense of Tenaris and Macropetalum (Asclepiadaceae). – South Afr. J. Bot. 64: 205-208.
Vieira MF, Shepherd GJ. 2002. Oxypetalum banksii subsp. banksii: a taxon of Asclepiadaceae with an extragynoecial compitum. – Plant Syst. Evol. 233: 199-206.
Vijayaraghavan MR, Padmanabhan U. 1969. Morphology and embryology of Centaurium ramosissimum Druce and affinities of the family Gentianaceae. – Beitr. Biol. Pflanzen 46: 15-37.
Vijayaraghavan MR, Shukla AK. 1977. The nature of the covering around the aggregate of microspores in Pergularia daemia (Forssk.) McC. & Blat. – Ann. Bot., N. S., 40: 417-421.
Vinckier S, Smets E. 2002a. Morphological and ultrastructural diversity of orbicules in relation to evolutionary tendencies in Apocynaceae s.l. – Ann. Bot. 90: 647-662.
Vinckier S, Smets E. 2002b. Systematic importance of orbicule diversity in Gentianales. – Grana 41: 158-182.
Vinckier S, Smets E. 2002c. Morphology, ultrastructure and typology of orbicules in Loganiaceae s.l. and related genera, in relation to systematics. – Rev. Palaeobot. Palynol. 119: 161-189.
Vinckier S, Huysmans S, Smets E. 2000. Morphology and ultrastructure of orbicules in the subfamily Ixoroideae (Rubiaceae). – Rev. Palaeobot. Palynol. 108: 151-174.
Vogel S. 1961. Die Bestäubung der Kesselfallen-Blüten von Ceropegia. – Beitr. Biol. Pflanzen 36: 159-237.
Vollesen K. 1980. A review of Pleioseras (Apocynaceae), and P. orientale, sp. nov. from SE. Tanzania. – Bot. Tidsskr. 75: 55-62.
Vollesen K. 1981. Catunaregam pygmaea (Rubiaceae) – a new geoxylic suffrutex from the woodlands of SE Tanzania. – Nord. J. Bot. 1: 735-740.
Wagenitz G. 1959. Die systematische Stellung der Rubiaceae. Ein Beitrag zum System der Sympetalen. – Bot. Jahrb. Syst. 79: 17-35.
Wagner WL, Lorence DH. 2011. Revision of Coprosma (Rubiaceae, tribe Anthospermeae) in the Marquesas Islands. – PhytoKeys 4: 109-124
Walker DB. 1975a. Postgenital carpel fusion in Catharanthus roseus (Apocynaceae) I. Light and scanning electron microscopic study of gynoecial ontogeny. – Amer. J. Bot. 62: 457-467.
Walker DB. 1975b. Postgenital carpel fusion in Catharanthus roseus (Apocynaceae) III. Fine structure of the epidermis during and after fusion. – Protoplasma 86: 43-63.
Walker DB. 1978. Postgenital carpel fusion in Catharanthus roseus (Apocynaceae) IV. Significance of the fusion. – Amer. J. Bot. 65: 119-121.
Wang J-N, Hou C-Y, Liu Y-L, Lin L-Z, Gil RR, Cordell GA. 1994. Swertifrancheside, an HIV-reverse transcriptase inhibitor and the first flavone-xanthone dimmer, from Swertia franchetiana. – J. Natur. Prod. 57: 211-217.
Wang JP, Raung SL, Lin CN, Teng CM. 1994. Inhibitory effect of norathyriol, a xanthone from Tripterospermum lanceolatum, on cutaneous plasma extravasation. – Eur. J. Pharmacol. 251: 35-42.
Wang L, Chen G-Y, Han C-R, Yuan Y, Yang B, Zhang Y, Wang J, Zhong XQ, Huang X. 2011. Two novel alkaloids from the stem of Saprosma hainanense and their cytotoxic activities in vitro. – Chem. Pharm. Bull. (Tokyo) 59: 338-340.
Wang R. 2002. Two new species of Spiradiclis (Rubiaceae) from China. – Novon 12: 420-423.
Wanntorp H-E. 1988. The genus Microloma (Asclepiadaceae). – Opera Bot. 98: 1-69.
Wanntorp L. 2007. Pollinaria of Hoya (Marsdenieae, Apocynaceae) – shedding light on molecular phylogenetics. – Taxon 56: 465-478.
Wanntorp L, Forster PI. 2007. Phylogenetic relationships between Hoya and the monotypic genera Madangia, Absolmsia, and Micholitzia (Apocynaceae, Marsdenieae): insights from flower morphology. – Ann. Missouri Bot. Gard. 94: 36-55.
Wanntorp L, Kunze H. 2009. Identifying synapomorphies in the flowers of Hoya and Dischidia – towards phylogenetic understanding. – Intern. J. Plant Sci. 170: 331-342.
Wanntorp L, Kocyan A, Renner SS. 2006. Wax plants disentangled: a phylogeny of Hoya (Marsdenieae, Apocynaceae) inferred from nuclear and chloroplast DNA sequences. – Mol. Phylogen. Evol. 39: 722-733.
Wanntorp L, Kocyan A, Donkelaar R van, Renner SS. 2006. Towards a monophyletic Hoya (Marsdenieae, Apocynaceae): inferences from the chloroplast trnL region and the rbcL-atpB spacer. – Syst. Bot. 31: 586-596.
Wanntorp L, Gotthardt K, Muellner AN. 2011. Revisiting the wax plants (Hoya, Marsdenieae, Apocynaceae): phylogenetic tree using the matK gene and psbA-trnH intergenic spacer. – Taxon 60:4-14.
Wanntorp L, Grudinski M, Forster PI,
Muellner-Riehl AN, Grimm GW. 2014. Wax plants (Hoya, Apocynaceae) evolution: epiphytism
drives successful radiation. – Taxon 63: 89-102.
Weaver RE Jr. 1969. Cytotaxonomic notes on some neotropical Gentianaceae. – Ann. Missouri Bot. Gard. 56: 439-443.
Weaver RE Jr. 1972. A revision of the neotropical genus Lisianthius (Gentianaceae). – J. Arnold Arbor. 53: 76-100, 234-272, 273-311.
Weaver RE Jr. 1974. The reduction of Rusbyanthus and the tribe Rusbyantheae (Gentianaceae). – J. Arnold Arbor. 55. 300-302.
Weaver RE Jr, Rüdenberg L. 1975. Cytotaxonomic notes on some Gentianaceae. – J. Arnold Arbor. 56: 211-222.
Weber M, Igersheim A. 1994. ‘Pollen buds’ in Ophiorrhiza (Rubiaceae) and their role in pollenkitt release. – Bot. Acta 107: 257-262.
Weberling F. 1977. Beiträge zur Morphologie der Rubiaceen-Infloreszenzen. – Ber. Deutsch. Bot. Ges. 90: 191-209.
Weide JC van der, Ham RWJM van der. 2012. Pollen morphology and phylogeny of the tribe Tabernaemontaneae (Apocynaceae, subfamily Rauvolfioideae). – Taxon 61: 131-145.
Wen H-Z, Wang R-J. 2012. Foonchewia guangdongensis gen. et sp. nov. (Rubioideae: Rubiaceae) and its systematic position inferred from chloroplast sequences and morphology. – J. Syst. Evol. 50: 467-476.
Wernham HF. 1914. A monograph of the genus Sabicea. – British Museum (Natural History), London.
Wernham HF. 1916. Pseudomussaenda: a new genus of Rubiaceae. – J. Bot. 54: 297-301.
Wertel HP. 1976. Vergleichend-morphologische und entwicklungsgeschichtliche Untersuchungen über Bau und Stellung der Infloreszenzen bei einigen Stapelieen-Gattungen (Fam. Asclepiadaceae). – Trop. Subtrop. Pflanzenwelt 17: 1-70.
Wettstein R. 1896. Die Gattungszugehörigkeit und systematische Stellung der Gentiana tenella Rottb. und G. nana Wulf. – Österr. Bot. Zeitschr. 46: 121-128, 172-176.
Whistler WA. 1986. A revision of Psychotria (Rubiaceae) in Samoa. – J. Arnold Arbor. 67: 341-370.
White A, Sloane BL. 1937. The Stapelieae. 2nd ed., 1-3. – Pasadena, California.
Wikström N, Avino M, Razafimandimbison SG, Bremer B. 2010. Historical biogeography of the coffee family (Rubiaceae, Gentianales) in Madagascar: case studies from the tribes Knoxieae, Naucleeae, Paederieae and Vanguerieae. – J. Biogeogr. 37: 1094-1113.
Wikström N, Neupane S, Kårehed J, Motley TJ, Bremer B. 2013. Phylogeny of Hedyotis L. (Rubiaceae: Spermacoceae): redefining a complex Asian-Pacific assemblage. – Taxon 62: 357-374.
Wikström N, Kainulainen K, Razafimandimbison SG, Smedmark JEE, Bremer B. 2015. A revised time tree of the asterids: establishing a temporal framework for evolutionary studies of the coffee family (Rubiaceae). PLoS ONE 10(5): e0126690. doi:10.1371/journal.pone.0126690
Wilbur RL. 1955. A revision of the North American genus Sabatia (Gentianaceae) I. – Rhodora 57: 1-104.
Wilbur RL. 1984a. A synopsis of the genus Halenia (Gentianaceae) in Central America. – Bull. Torrey Bot. Club 111: 366-374.
Wilbur RL. 1984b. A synopsis of the genus Halenia (Gentianaceae) in Mexico. – Rhodora 86: 311-337.
Wilbur RL. 2005. Systematic notes on the Rubiaceae tribe Spermacoceae I. The author and the type of the genus Diodella, with comments on tribal delimitation. – Rhodora 107: 408-413.
Williams JK. 1998. A revision of Thenardia H.B.K. (Apocynaceae, Apocynoideae). – Lundellia 1: 78-94.
Williams JK. 2002. Thoreauea (Apocynaceae: Apocynoideae), a new genus from Oaxaca, Mexico. – Lundellia 5: 47-58.
Williams JK. 2003. A further evaluation of Echites sect. Yucatanense (Apocynaceae) with additional notes on the genus. – Brittonia 54: 310-317.
Williams JK. 2004. Polyphyly of the genus Echites (Apocynaceae: Apocynoideae: Echiteae): evidence based on a morphological cladistic analysis. – Sida 21: 117-131.
Williams L. 1972. Tropical American plants XII. – Fieldiana Bot. 34: 101-132.
Williams RF, Metcalf RA, Gust LW. 1982. The genesis of form in oleander (Nerium oleander L.). – Aust. J. Bot. 30: 677-687.
Willson MF, Price PW. 1977. The evolution of inflorescence size in Asclepias (Asclepiadaceae). – Evolution 31: 495-511.
Wilson KL. 1980. The genus Rhyncharrhena (Asclepiadaceae). – Telopea 2: 35-39.
Wolfe LM, Massinga PH, Johnson SD. 2009. A quantitative evaluation of the distylous syndrome in Sebaea grandis (Gentianaceae). – South Afr. J. Bot. 75: 785-790.
Wolff D, Liede-Schumann S. 2007. Evolution of flower morphology, pollen dimorphism, and nectar composition in Arcytophyllum, a distylous genus of Rubiaceae. – Organisms Divers. Evol. 7: 106-123.
Wong K-M. 1982. Notes on Gardenia and Acranthera (Rubiaceae) from Peninsular Malaysia. – Gard. Bull. (Singapore) 35: 21-32.
Wong K-M. 1984a. The genera of Peninsular Malaysian Rubiaceae formerly confused with Randia. – Malayan Nat. J. 38: 1-57.
Wong K-M. 1984b. A synopsis of Morinda (Rubiaceae) in the Malay Peninsula, with two new species. – Malayan Nat. J. 38: 89-98.
Wong K-M. 1984c. A revision of Rennellia (Rubiaceae) in the Malay Peninsula. – Gard. Bull. (Singapore) 37: 193-198.
Wong K-M. 1988. The Antirheoideae (Rubiaceae) of the Malay Peninsula. – Kew Bull. 43: 491-518.
Wong K-M, Pereira JT. 2016. A taxonomic treatment of the Asiatic allies of Rothmannia (Rubiaceae: Gardenieae), including the new genera Ridsdalea and Singaporandia. – Sandakania 21: 21-64.
Wong K-M, Sugau JB. 1996. A revision of Fagraea (Loganiaceae) in Borneo, with notes on related Malesian species and 21 new species. – Sandakania 8: 1-93.
Wood KR, Wagner WL, Motley TJ. 2007. Labordia lorenciana (Loganiaceae): a new critically endangered species from Kaua’i, Hawaiian Islands with comments on its conservation. – Syst. Bot. 32: 195-199.
Woodson RE. 1928. Studies in the Apocynaceae II. A revision of the genus Stemmadenia. – Ann. Missouri Bot. Gard. 15: 341-379.
Woodson RE. 1930. Studies in the Apocynaceae I. A critical study of the Apocynoideae (with special reference to the genus Apocynum). – Ann. Missouri Bot. Gard. 17: 1-212.
Woodson RE. 1932. New or otherwise noteworthy Apocynaceae of Tropical America III. – Ann. Missouri Bot. Gard. 19: 375-387.
Woodson RE. 1933. Studies in the Apocynaceae IV. The American genera of Echitoideae. – Ann. Missouri Bot. Gard. 20: 605-790.
Woodson RE. 1935a. Observations on the inflorescence of Apocynaceae (with special reference to the American genera of Echitoideae). – Ann. Missouri Bot. Gard. 22: 1-48.
Woodson RE. 1935b. Studies in the Apocynaceae IV. The American genera of Echitoideae. – Ann. Missouri Bot. Gard. 22: 153-306.
Woodson RE. 1936. Studies in the Apocynaceae IV. The American genera of Echitoideae. – Ann. Missouri Bot. Gard. 23: 169-438.
Woodson RE. 1938a. Observations on the floral fibres of certain Gentianaceae. – Ann. Bot. 50: 759-766.
Woodson RE. 1938b. Studies in the Apocynaceae VII. An evaluation of the genera Plumeria L. and Himatanthus Willd. – Ann. Missouri Bot. Gard. 25: 189-224.
Woodson RE. 1939. New or otherwise noteworthy Apocynaceae of Tropical America VI. – Ann. Missouri Bot. Gard. 26: 95-98.
Woodson RE. 1941a. The North American Asclepiadaceae. – Ann. Missouri Bot. Gard. 28: 193-244.
Woodson RE. 1941b. Miscellaneous new Asclepiadaceae and Apocynaceae from Tropical America. – Ann. Missouri Bot. Gard. 28: 271-277.
Woodson RE. 1948. Miscellaneous new Apocynaceae and Asclepiadaceae. – Ann. Missouri Bot. Gard. 35: 233-238.
Woodson RE. 1954. The North American species of Asclepias L. – Ann. Missouri Bot. Gard. 41: 1-261.
Woodson RE. 1960. Miscellanea taxonomica II. – Ann. Missouri Bot. Gard. 47: 73-80.
Woodson RE, Moore JA. 1938. The vascular anatomy and comparative morphology of apocynaceous flowers. – Bull. Torrey Bot. Club 65: 135-166.
Wunderlich R. 1971. Die systematische Stellung von Theligonum. – Österr. Bot. Zeitschr. 119: 329-394.
Wyatt R. 1978. Experimental evidence concerning the role of the corpuscle in Asclepias pollination. – Syst. Bot. 3: 313-321.
Wyatt R, Lipow SR. 2007. A new explanation for the evolution of pollinia and loss of carpel fusion in Asclepias and the Apocynaceae s.l. – Ann. Missouri Bot. Gard. 94: 474-484.
Wyatt R, Broyles SB, Hamrick JL, Stoneburner A. 1993. Systematic relationships within Gelsemium (Loganiaceae): evidence from isozymes and cladistics. – Syst. Bot. 18: 345-355.
Wyk AE van, Bers NL van, Merwe CF van der. 1990. Non-pathological bacterial symbiosis in Pachystigma and Fadogia (Rubiaceae): its evolutionary significance and possible involvement in the aetiology of gousiekte in domestic ruminants. – South Afr. J. Sci. 86: 93-96.
Xiao L-Q, Zhu H. 2007. Paraphyly and phylogenetic relationships in Lasianthus (Rubiaceae) inferred from chloroplast rps16 data. – Bot. Stud. 48: 225-232.
Xie P, Tu T, Razafimandimbison SG, Zhu C,
Zhang D. 2014. Phylogenetic position of Guihaiothamnus (Rubiaceae): its evolutionary and
ecological implications. – Molec. Phylogen. Evol. 78: 375-385.
Xifreda CC. 1984. Estudios sobre Apocynaceae Argentinas IV. El género Macropharynx y una nueva combinación. – Kurtziana 17: 163-167.
Xifreda CC. 1996. Proposal to conserve the name Macrosiphonia Müll. Arg. 1860 (Apocynaceae) against Macrosyphonia Duby 1844 (Primulaceae). – Taxon 45: 137-138.
Xu Z. 1988. Series of revisions of Apocynaceae XXVI. A revision of Cleghornia Wight, Sinechites Oliv., and Epigynum chinense Merr. (Apocynaceae). – Wageningen Agric. Univ. Pap. 88: 1-35.
Xue C-Y, Li D-Z. 2005. Embryology of Megacodon stylophorus and Veratrilla baillonii (Gentianaceae): descriptions and systematic implications. – Bot. J. Linn. Soc. 147: 317-331.
Xue C-Y, Ho T-N, Li D-Z. 2007. Embryology of Swertia (Gentianaceae) relative to taxonomy. – Bot. J. Linn. Soc. 155: 383-400.
Yamashiro T, Fukuda T, Yokoyama J, Maki M. 2004. Molecular phylogeny of Vincetoxicum (Apocynaceae-Asclepiadoideae) based on the nucleotide sequences of cpDNA and nrDNA. – Mol. Phylogen. Evol. 31: 689-700.
Yamazaki T. 1963. Embryology of Mitrasacme alsinoides var. indica. – Sci. Rep. Tohoku Imp. Univ., Ser. IV, Biol. 29: 201-205.
Yamazaki T. 1970. A revision of the genus Randia L. in Eastern Asia. – J. Jap. Bot. 45: 337-341.
Yang L-E, Sun H, Ehrendorfer F, Nie Z-L. 2016. Molecular phylogeny of Chinese Rubia (Rubiaceae: Rubieae) inferred from nuclear and plastid DNA sequences. – J. Syst. Evol. 54: 37-47.
Yang L-E, Meng Y, Peng D-L, Nie Z-L, Sun H. 2018. Molecular phylogeny of Galium L. of the tribe Rubieae (Rubiaceae) – emphasis on Chinese species and recognition of a new genus Pseudogalium. – Mol. Phylogen. Evol. 126: 221-232.
Yang L-L, Li H-L, Wei L, Yang T, Kuang D-Y, Li M-H, Liao Y-Y, Chen Z-D, Wu H, Zhang S-Z. 2016. A supermatrix approach provides a comprehensive genus-level phylogeny for Gentianales. – J. Syst. Evol. 54: 400-415.
Young MCM, Braga MR, Dietrich SMC, Bolzani VS, Trevisan LMV, Gottlieb OR. 1996. Chemosystematic markers in Rubiaceae. – Opera Bot. Belg. 7: 205-212.
Yuan Y-M. 1993a. Seed coat micromorphology and its systematic implications for Gentianaceae of western China. – Bot. Helvetica 103: 73-82.
Yuan Y-M. 1993b. Karyological studies on Gentiana sect. Cruciata (Gentianaceae) from China. – Caryologia 46: 99-114.
Yuan Y-M, Küpfer P. 1993a. Karyological studies of Gentianopsis Ma and some related genera of Gentianaceae from China. – Cytologia 58: 115-123.
Yuan Y-M, Küpfer P. 1993b. Karyological studies on Gentiana sect. Frigida s.l. and sect. Stenogyne (Gentianaceae) from China. – Bull. Soc. Neuchâtel. Sci. Nat. 116: 65-78.
Yuan Y-M, Küpfer P. 1995. Molecular phylogenetics of the subtribe Gentianinae (Gentianaceae) inferred from the sequences of internal transcribed spacers (ITS) of nuclear ribosomal DNA. – Plant Syst. Evol. 196: 207-226.
Yuan Y-M, Küpfer P. 1997. The monophyly and rapid evolution of Gentiana sect. Chondrophyllae s.l.: evidence from the sequences of internal transcribed spacers (ITS) of nuclear ribosomal DNA. – Bot. J. Linn. Soc. 123: 25-43.
Yuan Y-M, Küpfer P, Doyle JJ. 1996. Infrageneric phylogeny of the genus Gentiana (Gentianaceae) inferred from nucleotide sequences of the internal transcribed spacers (ITS) of nuclear ribosomal DNA. – Amer. J. Bot. 83: 641-652.
Yuan Y-M, Küpfer P, Zeltner L. 1998. Chromosomal evolution of Gentiana and Jaeschkea (Gentianaceae), with further documentation of chromosome data for 35 species from western China. – Plant Syst. Evol. 210: 231-247.
Yuan Y-M, Wohlhauser S, Möller M, Chassot P, Mansion G, Grant J, Kupfer P, Klackenberg J. 2003. Monophyly and relationships of the tribe Exaceae (Gentianaceae) inferred from nuclear ribosomal and chloroplast DNA sequences. – Mol. Phylogen. Evol. 28: 500-517.
Yuan Y-M, Wohlhauser S, Möller M, Klackenberg J, Callmander MW, Küpfer P. 2005. Phylogeny and biogeography of Exacum (Gentianaceae): a disjunctive distribution in the Indian Ocean Basin resulted from long distance dispersal and extensive radiation. – Syst. Biol. 54: 21-34.
Zahid MS, Wong KM. 2010. The circumscription, taxonomy and biogeography of Porterandia (Rubiaceae – Gardenieae). – Edinburgh J. Bot. 67: 265-342.
Zappi D. 2003. Revision of Rudgea (Rubiaceae) in Southeastern and Southern Brazil. – Kew Bull. 58: 513-596.
Zappi D, Nunes TS. 2000. Notes on the Rubiaceae of northeastern Brazil I. Erithalis, Psychotria and Rudgea. – Kew Bull. 55: 655-668.
Zeltner L. 1970. Recherches de biosystématique sur les genres Blackstonia Huds. et Centaurium Hill (Gentianacées). – Bull. Soc. Neuchâtel. Sci. Nat. 93: 1-164.
Zeltner L. 1978. Recherches sur le Centaurium bianoris (Sennen) Sennen. – Rev. Biol. Ecol. Médit. 5: 51-58.
Zemagho L, Liede-Schumann S, Sonké B, Janssens S, Lachenaud O, Verstraete B, Dessein S. 2016. Phylogenetics of tribe Sabiceeae (Ixoroideae, Rubiaceae) revisited, with a new subgeneric classification for Sabicea. – Bot. J. Linn. Soc. 182: 551-580.
Zhang X-L, Wang Y-J, Ge X-J, Yuan Y-M, Yang H-L, Liu J-Q. 2009. Molecular phylogeny and biogeography of Gentiana sect. Cruciata (Gentianaceae) based on four chloroplast DNA datasets. – Taxon 58: 862-870.
Zhen J, Jianhua L. 2007. Phylogeny of intercontinental disjunct Gelsemiaceae inferred from chloroplast and nuclear DNA sequences. – Syst. Bot. 32: 617-627.
Zhu H. 2002. A revision of the genus Lasianthus (Rubiaceae) from China. – Syst. Geogr. Plants 72: 63-110.
Zhu H, Roos MC, Ridsdale CE. 2012. A taxonomic revision of the Malesian species of Lasianthus (Rubiaceae). – Blumea 57: 1-102.
Zijlstra G, Leeuwenberg AJM, Middleton DJ. 1998. Proposal to conserve the name Trachelospermum (Apocynaceae) with a conserved type. – Taxon 47: 163-164.
Zuev VV. 1984. On the systematics of the representatives of the Siberian genus Gentiana s.l. (Gentianaceae). – Bot. Žurn. 70: 916-923.
Zuev VV. 1990. On the systematics of the Gentianaceae family in Siberia. – Bot. Žurn. 75: 1296-1305.
Zwetsloot HJC. 1981. A revision of Farquharia Stapf and Funtumia Stapf (Apocynaceae). – Meded. Landbouwh. Wageningen 81: 1-46.