MYRTANAE
Takht.
Takhtajan, Sist. Filog. Cvetk. Rast. [Syst.
Phylog. Magnolioph.]: 295. 4 Feb 1967
Myrtopsida Bartl.,
Ord. Nat. Plant.: 225, 326. Sep 1830 [’Myrtinae’]; Myrtidae J. H.
Schaffn., Ohio Naturalist [’Myrtiflorae’] 11: 416. Dec 1911;
Geranianae Thorne ex Reveal in Novon 2: 236. 13 Oct
1992
[Geraniales+Myrtales]
MYRTALES Juss. ex Bercht. et J.
Presl
Berchtold et Presl, Přir. Rostlin: 233. Jan-Apr
1820 [‘Myrtaceae’]
Fossils Esgueiria
includes fossilized reproductive organs which have been attributed to Myrtaceae. Esgueiria, from
the Campanian to the Maastrichtian of Portugal and Japan, is represented by
epigynous flowers with a persistent calyx of five sepals, five petals, 3+5
stamens, tricolpate pollen grains, an annular (nectariferous) disc, three
stylodia and up to six apical anatropous ovules in the unilocular ovary; the
calyx and the ovary possess abundant simple hairs and peltate multicellular
glands.
Habit Usually bisexual (rarely
andromonoecious, polygamomonoecious, dioecious or androdioecious,
polygamodioecious), usually evergreen (rarely deciduous) trees or shrubs, or
perennial or annual herbs (sometimes lianas). Young stems and branches often
quadrangular in transverse section.
Vegetative anatomy Phellogen
ab initio subepidermal, pericyclic or cortical. Polyderm often present.
Secondary lateral growth normal, anomalous (from cylindrical cambium) or
absent. Vessel elements usually with simple (rarely scalariform or reticulate)
perforation plates; lateral pits usually alternate (sometimes opposite), simple
or bordered pits. Vestured pits usually present. Imperforate tracheary xylem
elements tracheids, fibre tracheids or libriform fibres, with simple or
bordered pits, septate or non-septate (sometimes also vasicentric tracheids).
Wood rays uniseriate or multiseriate, homocellular or heterocellular. Axial
parenchyma apotracheal diffuse or diffuse-in-aggregates, often banded, or
paratracheal scanty, aliform, lozenge-aliform, winged-aliform, confluent,
reticulate, scalariform, unilateral, vasicentric or banded. Intraxylary phloem
usually present. Interxylary phloem sometimes present. Secondary phloem in
young stems usually stratified into hard fibrous and soft parenchymatous
layers. Sieve tube plastids Ss type. Nodes 1:1 or 1:3, unilacunar with one or
three leaf traces, or 3:3, trilacunar with three traces. Schizolysigenous
secretory cavities with ethereal oils. Heartwood often with gum-like
substances. Silica bodies sometimes present. Calciumoxalate as prismatic,
rhomboidal or acicular crystals, styloids, or crystal sand often frequent
(raphides usually absent, sometimes frequent).
Trichomes Hairs unicellular,
bicellular or multicellular, uniseriate or multiseriate, simple or branched,
furcate, stellate, dendritic, peltate, lepidote or vesicular, or absent; glands
or glandular hairs (bristle-like or lepidote) often present.
Leaves Usually opposite
(sometimes verticillate or alternate), simple, entire, often coriaceous, with
conduplicate, supervolute, revolute, involute or flat ptyxis. Stipules usually
rudimentary non-vascularized or absent (sometimes hair-like, colleter-like or
glandular, rarely paired or few and intrapetiolar); leaf sheath absent.
Colleters often frequent. Petiole vascular bundle transection arcuate or
annular. Venation pinnate or palmate, brochidodromous, eucamptodromous,
craspedodromous, parallelodromous, palmate-parallel or acrodromous (leaves
sometimes one-veined). Stomata usually anomocytic (sometimes anisocytic,
paracytic, polycytic, diacytic, tetracytic, cyclocytic or anomocyclocytic).
Cuticular wax crystalloids sometimes as rosettes, often absent. Domatia as
pits, pockets or hair tufts (acarodomatia or myrmecodomatia sometimes present).
Lamina with gland-dots and schizogenous secretory cavities with ethereal oils.
Epidermis with or without mucilaginous idioblasts. Mesophyll often with
sclerenchymatous idioblasts containing sclereids of different types and with
calciumoxalate as single prismatic crystals, styloids, druses or crystal sand.
Leaf margin usually entire (rarely serrate or dentate).
Inflorescence Terminal or
axillary, panicle or thyrse, simple or branched racemose, spicate, umbellate or
capitate, or corymb (flowers sometimes solitary). Floral prophylls (bracteoles)
sometimes absent.
Flowers Usually actinomorphic
(sometimes zygomorphic, rarely asymmetrical). Hypanthium present, usually
elongate. Usually epigyny or half epigyny (rarely hypogyny). Sepals (two to)
four to eight (to 16), with imbricate, valvate, contorted or open (rarely
cochlear) aestivation, usually free (sometimes connate into caducous calyptra).
Petals (two to) four to eight (to numerous), with imbricate, valvate, plicate
or contorted aestivation, often clawed, free or connate at base (sometimes
forming caducous calyptra or absent). Nectariferous disc annular or unilateral
at ovary tip, ovary base, along elongated hypanthium, or intrastaminal, or
nectaries on adaxial side of hypanthium and disc intrastaminal on top of ovary,
or nectary and disc absent.
Androecium Stamens (one to)
six to ten in (one or) two whorls, or c. 20 to more than 150 in one or several
whorls, alternisepalous or antesepalous, alternipetalous or antepetalous.
Filaments free or connate at base into four or five fascicles, or connate into
tube, usually free from tepals (rarely epipetalous), inserted on hypanthium,
often incurved in bud. Anthers basifixed or dorsifixed, usually versatile,
usually tetrasporangiate (rarely monosporangiate, disporangiate or
trisporangiate), introrse, latrorse or extrorse, longicidal (dehiscing by
longitudinal slits) or poricidal (dehiscing by pores). Tapetum secretory.
Staminodia usually absent (rarely four to ten extrastaminal, or four or five
alternating with fertile stamens; female flowers rarely with staminodia).
Pollen grains
Microsporogenesis simultaneous. Pollen grains (2–)3(–6)-colpor(oid)ate,
(2–)3(–6)-colpate or (2–)3(–6)-por(or)ate, often heterocolpate (with
pseudocolpi alternating with apertures), usually shed as monads (occasionally
as tetrads or polyads), bicellular at dispersal. Exine tectate or semitectate,
with columellate (rarely acolumellate) infratectum, perforate, punctate,
reticulate, striate, rugulate, verrucate, echinate, scabrate, granulate or
smooth.
Gynoecium Pistil composed of
two to five (to 16) connate carpels. Ovary usually inferior or semi-inferior
(rarely superior), unilocular to quinquelocular (rarely up to 16-locular).
Style single, sunken, usually elongate, persistent, entire or branched above.
Stigma usually single, simple, capitate, or tri- or quadrilobate (stigmas then
three or four, punctate), papillate, Dry or Wet type. Male flowers sometimes
with pistillodium.
Ovules Placentation usually
axile (sometimes basal, parietal or free central, rarely apical-axile). Ovules
(one or) two to c. 30 (to more than 150) per carpel, anatropous, hemianatropous
or campylotropous (rarely orthotropous), ascending or horizontal (rarely
pendulous), apotropous or epitropous, usually bitegmic (rarely unitegmic),
crassinucellar. Micropyle bistomal (often Z-shaped) or endostomal. Funicular
obturator sometimes present. Nucellar cap often present (nucellar beak rarely
present). Archespore sometimes multicellular. Megagametophyte usually
monosporous, Polygonum type (sometimes quadrinucleate,
Oenothera type, rarely disporous, Allium type, or
tetrasporous, 16-nucleate, Penaea type). Synergids often with a
filiform apparatus. Antipodal cells usually ephemeral, sometimes not developed
(nuclei early degenerating). Endosperm development ab initio nuclear. Endosperm
haustoria absent? Embryogenesis usually onagrad (sometimes asterad or
solanad).
Fruit Usually a loculicidal
(sometimes septicidal or irregularly dehiscing) capsule, a berry or nutlike
fruit (rarely a denticidal capsule, pyxidium, drupe, or schizocarp), often with
calyx persistent and accrescent.
Seeds Aril absent. Funicular
elaiosome rarely present. Testa sometimes winged. Operculum, formed by hilum,
often present. Seed coat testal. Testa often multilayered, sometimes
multiplicative, sometimes with crystalliferous layers. Exotesta sometimes
palisade to cuboidal and lignified. Mesotesta sometimes sclerotic. Endotesta
often crystalliferous, sometimes collapsed. Tegmen often crushed or exotegmen
fibrous or tracheidal. Endotegmen usually crushed (rarely fibrous). Perisperm
usually not developed. Endosperm usually absent (sometimes scarce, oily).
Embryo small, straight, curved or spirally twisted, well differentiated, with
or without chlorophyll. Cotyledons (one or) two (to five). Germination
phanerocotylar or cryptocotylar.
Cytology x = (5–)7–15
(rarely higher)
DNA Plastid gene infA
lost/defunct. Mitochondrial intron coxII.i3 lost.
Phytochemistry Flavonols
(kaempferol, quercetin, myricetin), acylated anthocyanins, flavonoid sulfates,
flavonoid mono- and diglycosides, cyanidin, delphinidin, ethereal oils
(triterpenes, etc.), ursolic acid, ellagic and gallic acids, methylated ellagic
acids, galloyl- and ellagitannins (e.g. cuphiin), non-hydrolyzable tannins,
polyhydroxyalkaloids (usually frequent), quinolizidine alkaloids, triterpene
saponins, phenylalanine-derived cyanogenic compounds (prunasin etc.),
anthraquinones, and polyacetate-derived arthroquinones present. Raffinose and
stachyose in phloem exudates. Aluminium or oxalate accumulated in some
species.
Systematics Myrtales are sister-group to Geraniales (Wang &
al. 2009).
A plausible topology is [Combretaceae+[Lythraceae+Onagraceae]+[[Vochysiaceae+ Myrtaceae]+[[Melastomataceae+Memecylaceae]+[Crypteroniaceae+[Alzateaceae+[Rhyncho-calycaceae+[Oliniaceae+Penaeaceae]]]]]]].
Lythraceae and Onagraceae share the following
potential synapomorphies (Stevens 2001 onwards): vessels present in groups in
wood; fibres with simple or at most minutely bordered pits; petiole vascular
bundle transection arcuate; pollen grains at anthesis sometimes starchy;
presence of hypostase; megasporocytes several; megasporangium with starch
grains; sepals persistent in fruit; exotegmen fibrous; x = 8; tannins often not
abundant; and soluble oxalate accumulated.
The clade [[Vochysiaceae+Myrtaceae]+[[Melastomataceae+Memecylaceae]+[Cryptero-niaceae+[Alzateaceae+[Rhynchocalycaceae+[Oliniaceae+Penaeaceae]]]]]] is characterized by
the potential synapomorphy: inflorescence with at least branches cymose.
Myrtaceae and Vochysiaceae share the characters
(Stevens 2001 onwards): hairs unicellular or bicellular, simple; sepals and
petals with imbricate aestivation; pollen grains syncolporate; style depressed
in apex of gynoecium; and fruit a capsule.
The clade [[Melastomataceae+Memecylaceae]+[Crypteroniaceae+[Alzateaceae+[Rhyncho-calycaceae+
[Oliniaceae+Penaeaceae]]]]] has the following
potential synapomorphies in common (Stevens 2001 onwards): branched or
unbranched sclereids present or absent within same major clade; absence of
nectary; connective abaxially much expanded; and endothecial thickening absent
or atypical.
Melastomataceae and Memecylaceae share numerous
potentially advanced features including (Stevens 2001 onwards): presence of
internal phloem; presence of two or four distinct lateral veins arising from
near base of lamina; sepals with imbricate quincuncial aestivation; petals with
contorted aestivation; anthers poricidal, inverted during development, with
branched vascular trace; expansion of connective below anther well developed,
with various appendages; pollen grains with pseudocolpi; carpels antepetalous;
stigma punctate; outer and inner integuments approx. two cell layers thick; and
radicula curved.
The clade [Crypteroniaceae+[Alzateaceae+[Rhynchocalycaceae+[Oliniaceae+Penaeaceae]]]] has the following
potential synapomorphies: vessel/ray and vessel/parenchyma pits half-bordered;
stipules minute; stamens as many as petals, antepetalous; endothecium
ephemeral; fruit a capsule; exotestal cells periclinally elongated; and
endotegmen not fibrous.
The clade [Alzateaceae+[Rhynchocalycaceae+[Penaeaceae+Oliniaceae]]] is characterized by
stout style. The [Rhynchocalycaceae+[Penaeaceae+Oliniaceae]] clade has: leaves with
glandular apex; and x = 10. Finally, Oliniaceae and Penaeaceae share the characters
(Stevens 2001 onwards): hypanthium well developed; pollen grains psilate; foot
layer and tectum thick; exotegmen fibrous; absence of embryo suspensor;
presence of non-hydrolyzable tannins; and aluminium not accumulated.

|
Cladogram of Myrtales based on DNA sequence
data (Conti & al. 1997). The major clades are well supported,
although the position of Combretaceae is uncertain;
they are sometimes recovered as sister-group either to the remainder or
to the clade [Lythraceae+Onagraceae] (e.g. Soltis
& al. 2011, with low support).
|
ALZATEACEAE S. A.
Graham
|
( Back to Myrtales )
|
Graham in Ann. Missouri Bot. Gard. 71: 775. 19
Apr 1985
Genera/species 1/1
Distribution The Andes from
Costa Rica to Bolivia.
Fossils Unknown.
Habit Bisexual, evergreen
trees or shrubs (sometimes semi-epiphytic). Young stems and branches at first
quadrangular in cross-section, later terete.
Vegetative anatomy Phellogen
ab initio deeply seated, close to perivascular sclerenchyma. Cortical vascular
bundles present. Primary vascular tissue cylinder, without separate vascular
bundles, bicollateral. Vessel elements diffuse, with simple perforation plates;
lateral pits alternate?, bordered pits. Vessel/ray and vessel/parenchyma pits
simple. Vestured pits present in vessels. Imperforate tracheary xylem elements
thin-walled libriform fibres with simple pits (almost without bordered pits),
septate. Wood rays uniseriate or multiseriate, heterocellular. Axial parenchyma
paratracheal scanty (apotracheal parenchyma absent). Intraxylary phloem
present. Sieve tube plastids Ss type? Nodes 3:3, trilacunar with three leaf
traces. Phloem parenchyma with druses. Sclereids present. Solitary crystals
absent.
Trichomes Hairs absent.
Leaves Opposite or
verticillate, simple, entire, coriaceous, with ? ptyxis. Stipules small, paired
or few together, intrapetiolar (axillary), or absent; leaf sheath absent.
Petiole vascular bundle transection annular; petiole with wing bundles.
Venation pinnate, brochidodromous. Stomata anomocytic, laterocytic or
cyclocytic. Cuticular wax crystalloids? Mesophyll with sclerenchymatous
idioblasts (containing sclereids) and calciumoxalate druses. Leaf margin
entire.
Inflorescence Axillary, thyrse
or panicle. Bracteoles absent.
Flowers Actinomorphic, small.
Hypanthium short. Hypogyny (to half epigyny). Sepals five (or six), with
valvate aestivation, thick, free, pointed in bud. Petals absent (sometimes
rudimentary). Nectariferous disc intrastaminal, lobate.
Androecium Stamens five,
alternisepalous. Filaments short, inserted at margin of nectariferous disc,
free from each other and from tepals. Anthers dorsifixed, with terminal
microsporangia (inserted along apex of connective), versatile?,
tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits);
connective wide, cordately expanded, with appendage directed backwards;
endothecium ephemeral. Tapetum secretory? Staminodia absent.
Pollen grains
Microsporogenesis simultaneous? Pollen grains tricolporate, shed as monads,
bicellular at dispersal. Not heterocolpate (pseudocolpi almost absent). Exine
tectate?, with columellate infratectum, psilate to verrucate-areolate.
Gynoecium Pistil composed of
two connate carpels. Ovary superior (to semi-inferior), bilocular, with
incomplete septum. Transseptal vascular bundles present. Style single, simple,
short. Stigma capitate, Wet type? Pistillodium absent.
Ovules Placentation
intrusively parietal. Ovules c. 40 to c. 60 per carpel, anatropous, horizontal,
bitegmic, crassinucellar. Micropyle endostomal. Outer integument ? cell layers
thick. Inner integument ? cell layers thick. Archespore multicellular.
Megagametophyte disporous, 8-nucleate, Allium type. Endosperm
development probably nuclear. Endosperm haustoria? Embryogenesis?
Fruit A flattened loculicidal
capsule, with persistent calyx.
Seeds Aril absent. Seeds
flattened. Testa winged, with hairpin-shaped vascular bundle. Seed coat
exotestal. Exotestal cells low, with irregularly sinuate anticlinal walls.
Endotesta and tegmen collapsed. Perisperm not developed. Endosperm absent.
Embryo straight, well differentiated, chlorophyll? Cotyledons two.
Germination?
Cytology n = 14
DNA
Phytochemistry Insufficiently
known. Flavonol mono- and diglycosides (quercetin-3-O-glycoside,
quercetin-3-O-diglycoside, etc.) and ellagic acid present. Aluminium
not accumulated. Myricetin- and flavone-C-glycosides not found.
Use Unknown.
Systematics: Alzatea
(1; A. verticillata; cloud forests of Costa Rica and Panamà, lower
Andean slopes of Peru and Bolivia).
Alzatea is sister to the clade
[Rhynchocalyx+[Olinia+Penaeaceae]].
COMBRETACEAE R. Br.
|
( Back to Myrtales )
|
Brown, Prodr. Fl. Nov.-Holl.: 351. 27 Mar 1810,
nom. cons.
Terminaliaceae J.
St.-Hil., Expos. Fam. Nat. 1: 178. Feb-Apr 1805;
Combretales R. Br. ex Bercht. et J. Presl, Přir.
Rostlin: 234. Jan-Apr 1820 [‘Combretaceae’];
Myrobalanaceae Juss. ex Martinov, Tekhno-Bot. Slovar:
396. 3 Aug 1820 [’Mirobolaneae’];
Bucidaceae Spreng., Syst. Veg. 2: 282. Jan-Mar 1825
[’Bucideae’]; Myrobalanales Link,
Handbook 2: 440. 4-11 Jul 1829 [‘Myrobalaneae’]
Genera/species 10/485–520
Distribution Pantropical.
Fossils
Terminalioxylon is fossil wood assigned to Combretaceae known from numerous
Cenozoic sites in Africa, South America and tropical Asia. Pollen grains have
been recorded from the Late Eocene onwards.
Habit Usually bisexual (rarely
andromonoecious or dioecious), evergreen or deciduous trees, shrubs,
suffrutices or lianas (Lumnitzera and Laguncularia are
mangrove trees with pneumatophores). Bark often exfoliating. Sometimes
xerophytic.
Vegetative anatomy Phellogen
ab initio usually cortical? or epidermal (sometimes deeper). Secondary lateral
growth normal or anomalous (from cylindrical cambium). Vessel elements with
simple perforation plates; lateral pits alternate, bordered pits. Vestured pits
present in vessels. Imperforate tracheary xylem elements fibre tracheids and
often libriform fibres with usually simple pits (sometimes very small bordered
pits; e.g. Strephonema), septate or non-septate (also vasicentric
tracheids). Wood rays uniseriate or multiseriate, homocellular or
heterocellular. Axial parenchyma apotracheal diffuse, or paratracheal scanty,
aliform, lozenge-aliform, winged-aliform, confluent, scalariform, reticulate,
unilateral, vasicentric or banded. Tyloses sometimes frequent. Intraxylary
and/or interxylary phloem present in some genera, in cross-section often
distributed as reticulated islands. Sieve tube plastids Ss type. Nodes 1:1,
unilacunar with one leaf trace. Sclereids present or absent. Mucilage ducts
present in Terminalia. Heartwood sometimes with gum-like substances.
Silica bodies present in some species. Calciumoxalate raphides absent.
Prismatic crystals often frequent. Styloids or druses sometimes present.
Trichomes Hairs usually
unicellular (sometimes multicellular), thick-walled, often pointed, with
distinct basal segment (‘combretaceous hairs’), simple or peltate-lepidote
(sometimes furcate or vesicular), often adpressed; glandular hairs often
present (sometimes lepidote).
Leaves Alternate (often in two
or four rows) or opposite (rarely verticillate), simple, entire, with
conduplicate or supervolute (in Laguncularia revolute) ptyxis.
Stipules rudimentary or absent; leaf sheath absent. Petiole vascular bundle
transection arcuate to annular; wing bundles present in some species. Venation
pinnate, brochidodromous, eucamptodromous or craspedodromous. Stomata usually
anomocytic (in Strephonema paracytic; in Laguncularia and
Lumnitzera cyclocytic). Cuticular wax crystalloids as rosettes of
platelets (Fabales type). Domatia as pits, pockets or hair tufts
numerous, as well as glands (lamina sometimes gland-dotted) and/or pellucid
dots. Large mesophyll cells with solitary calciumoxalate druse. Mucilaginous
idioblasts cells present or absent. Hydathodes sometimes present. Leaf margin
entire. Paired extrafloral nectaries often present on petiole; foliar nectaries
present in many species.
Inflorescence Terminal or
axillary, simple or branched spicate or raceme (sometimes congested, rarely
umbellate).
Flowers Usually actinomorphic
(sometimes weakly zygomorphic). Hypanthium elongate. Usually epigyny (in
Strephonema half epigyny). Sepals four or five (to eight), in one
whorl, with imbricate or valvate aestivation, connate (in Getonia
persistent and accrescent in fruit). Petals four or five, with imbricate or
valvate aestivation, sometimes clawed, connate (absent in some species).
Nectariferous disc present at base of upper part of hypanthium, or nectary and
disc absent.
Androecium Stamens usually 4+4
or 5+5 (rarely four, five or 8+8), obdiplostemonous, alternipetalous or
antepetalous. Filaments inserted on adaxial side of upper part of hypanthium
(usually at two levels; one staminal whorl rarely staminodial), usually free
from each other and from tepals (in some species of Terminalia adnate
to petals, epipetalous). Anthers dorsifixed, usually versatile,
tetrasporangiate, latrorse, longicidal (dehiscing by longitudinal slits).
Tapetum secretory, usually binucleate (rarely trinucleate or quadrinucleate).
Staminodia?
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually tricolpate or
tricolporate, heterocolpate (with pseudocolpi alternating with apertures), shed
as monads, usually bicellular at dispersal. Exine tectate or semitectate, with
columellate infratectum, reticulate, echinate or psilate.
Gynoecium Pistil composed of
two to five (to eight) connate carpels; carpels alternisepalous or odd carpel
abaxial (sometimes with nectary at apex). Ovary usually inferior (rarely
semi-inferior), unilocular (sometimes with nectary at apex). Style single,
simple. Stigma usually punctate (sometimes capitate), non-papillate, Wet type.
Pistillodia?
Ovules Placentation axile to
apical. Ovules (one or) two to seven (to 20) per ovary, anatropous, pendulous,
bitegmic, crassinucellar. Funicle long. Micropyle usually bistomal, Z-shaped
(zig-zag; in at least one species of Guiera endostomal). Outer
integument three to five cell layers thick. Inner integument two or three cell
layers thick. Funicle long. Funicular obturator usually present. Hypostase
often present. Parietal tissue eight to ten cell layers thick. Nucellar cap six
to eight cell layers thick. Megagametophyte usually monosporous,
Polygonum type (rarely tetrasporous, 16-nucleate, Penaea
type). Synergids often with a filiform apparatus. Endosperm development ab
initio nuclear. Endosperm haustoria absent. Embryogenesis asterad. Vivipary
occurring in mangrove species.
Fruit Usually a single-seeded,
often two- to five-winged nutlike fruit (sometimes drupaceous, rarely
dehiscent), often flattened.
Seeds Aril absent. Seed large.
Exotesta? Endotesta tracheidal or sclerotic, without crystals? Exotegmen often
fibrous. Endotegmen? Perisperm not developed. Endosperm absent. Embryo large,
well differentiated, often with chlorophyll. Cotyledons one (by fusion), two or
three (in some species of Terminalia up to five), usually convolute
(sometimes conduplicate or plicate, sometimes hemispherical or spirally
twisted). Germination phanerocotylar or cryptocotylar.
Cytology x = 7, 11–13 –
Polyploidy occurring.
DNA
Phytochemistry Flavonols
(kaempferol, quercetin, myricetin), flavone-C-glycosides,
5-deoxyflavonoids, flavonoid sulphates, cyanidin, ellagic acid, galloyl- and
ellagitannins, condensed tannins, alkaloids, triterpene saponins, cyanogenic
compounds, and polyacetate derived arthroquinones present. Raffinose and
stachyose present in phloem exudates.
Use Ornamental plants, timber,
dyeing substances, tanning, fruits (Terminalia catappa), medicinal
plants.
Systematics Combretaceae may be sister-group
to the remaining Myrtales,
although with fairly low support. Here, they are part of a basal trichotomy
also including [Lythraceae+Onagraceae] and a clade comprising
the remainder of Myrtales.
Strephonema is sister to the remaining
Combretaceae.
Strephonematoideae
Engl. et Diels in Engler, Monogr. Afr. Pflanzen-Fam. 3: 2. Nov 1899
1/3. Strephonema (3; S.
mannii, S. pseudocola, S. sericeum; tropical West and
Central Africa). – Imperforate tracheary xylem elements with bordered pits.
Intraxylary or interxylary phloem absent. Hairs furcate, adpressed. Leaves
seemingly alternate. Stomata paracytic. Pollen grains without pseudocolpi.
Exine semitectate, reticulate. Ovary semi-inferior (fruit largely superior).
Ovules two per ovary. Cotyledons hemispherical, large, conduplicate. n = ?
Combretoideae (Lindl.)
Beilschm. in Flora 16(Beibl. 7): 97. 14 Jun 1833 [‘Combreteae’]
9/485–520. Petiole sometimes
glanduliferous. Pedicel often absent. Ovary inferior. Megagametophyte sometimes
tetrasporous, 16-nucleate. Fruit flattened and/or winged (sometimes
drupaceous). Cotyledons flattened and variously folded.
Laguncularieae Engl.
et Diels in Engler, Monogr. Afr. Pflanzen-Fam. 3: 3. Nov 1899
4/10. Laguncularia (1; L.
racemosa; coasts in tropical West Africa and tropical America), Lumnitzera
(2; L. littorea, L. racemosa; coasts in tropical East Africa,
Madagascar and the Seychelles to islands in the Pacific),
Macropteranthes (5; M. fitzalanii, M. kekwickii,
M. leichhardtii, M. leiocaulis, M. montana; Northern
Territory, eastern Queensland), Dansiea (2; D. elliptica,
D. grandiflora; eastern Queensland). – Pantropical, often mangroves.
Lamina sometimes glanduliferous, in Laguncularia with revolute ptyxis.
Stomata often cyclocytic. Floral prophylls (bracteoles) adnate to ovary. Petals
sometimes clawed. Cotyledons spirally folded (convolute). n = 13. – The
mangrove tree genera Lumnitzera
and Laguncularia are sister-groups in the analyses by Maurin & al.
(2010). On the other hand, Dansiea and Macropteranthes were
not included in their analyses.
Combreteae DC., Prodr.
3: 18. Mar (med.) 1828
5/475–510. Terminalia
(c 230; tropical regions on both hemispheres), Conocarpus (2; C.
erectus, C. lancifolius; coasts of tropical Africa, tropical Asia
and tropical America); Getonia (1; G. floribunda; tropical
Asia), Guiera (1; G. senegalensis; northern tropical Africa),
Combretum
(240–275; tropical regions on both hemispheres). – Pantropical. Usually
trees (sometimes lianas). Vessel elements of two very different diametres,
uniseriate wood rays and special radial vessels with perforations in tangential
cell walls present in Combretum.
Intraxylary phloem present. Mucilage ducts present in Terminalia.
Stomata anomocytic. Petals sometimes small or absent. Micropyle in
Guiera endostomal. Integuments sometimes multiplicative. Archespore
sometimes multicellular. – Combretum
is sister to the clade [Getonia+Guiera] (Maurin & al.
2010).
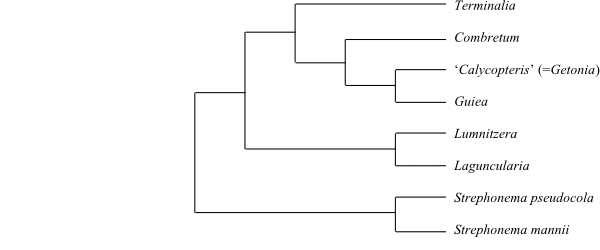 |
Cladogram of Combretaceae based on DNA
sequence data (Maurin & al. 2010).
|
CRYPTERONIACEAE A.
DC.
|
( Back to Myrtales )
|
A. de Candolle in A. P. de Candolle et A. L. P.
P. de Candolle, Prodr. 16(2): 677. Jul 1868, nom. cons.
Henslowiaceae Lindl.,
Key Bot.: 57. 15-30 Sep 1835 [’Hensloviaceae’];
Henslowiales Lindl. in C. F. P. von Martius, Consp.
Regn. Veg.: 14. Sep-Oct 1835 [‘Henslowiaceae’]
Genera/species 3/11
Distribution Sri Lanka,
northeastern India, southwestern China, the Andaman Islands, Indochina, Malesia
to New Guinea.
Fossils Uncertain. Fossil
pollen grains attributed to Dactylocladus have been found in Miocene
layers on Borneo.
Habit Bisexual or dioecious
(sometimes polygamodioecious), evergreen trees or shrubs. Young stems and
branches often quadrangular in cross-section.
Vegetative anatomy Phellogen
ab initio usually deeply seated (in Dactylocladus subepidermal).
Cortical vascular strands present or absent. Primary vascular tissue
bicollateral. Vessel elements diffuse, with simple perforation plates; lateral
pits alternate, simple or bordered pits. Vestured pits present in vessels.
Imperforate tracheary xylem elements fibre tracheids with bordered pits,
non-septate. Wood rays uniseriate or multiseriate, heterocellular. Axial
parenchyma paratracheal aliform, confluent, reticulate, or banded (apotracheal
parenchyma diffuse, diffuse-in-aggregates), rare or absent. Intraxylary phloem
present. Sieve tube plastids Ss type? Nodes 3:3, trilacunar with trifid/split
lateral vascular strands, or 1:3, unilacunar with three traces (with girdling
vascular bundles). Sclereids often present. Crystals absent. Calciumoxalate
raphides absent.
Trichomes Hairs usually absent
(sometimes unicellular, simple).
Leaves Opposite, simple,
entire, often coriaceous, with ? ptyxis. Stipules small lateral
(Crypteronia), or absent; leaf sheath absent. Petiole vascular bundle
transection arcuate or annular; petiole with wing bundles, sometimes with
medullary leaf trace. Venation usually pinnate (rarely palmate),
brochidodromous, with one continuous vein near leaf margin. Stomata usually
paracytic (in Dactylocladus laterocytic to anemocytic). Cuticular wax
crystalloids? Mesophyll usually with sclerenchymatous idioblasts (absent in
Axinandra) and calciumoxalate styloids (and druses?). Leaf margin
entire.
Inflorescence Terminal or
axillary, panicle with long racemose or spicate branches.
Flowers Actinomorphic, small.
Hypanthium tubular or cupular. Epigyny or half epigyny. Sepals four or five (or
six), very small, with valvate aestivation, persistent or caducous, free or
connate at base. Petals four or five (or six), very small, usually free,
modified to caducous calyptra (Axinandra) or individually abscised
(Crypteronia, Dactylocladus), or absent. Nectary? Disc
absent.
Androecium Stamens four or
five (or six; in Axinandra 5+5), haplostemonous or diplostemonous
(Axinandra). Filaments inflexed in bud, free from each other and
usually from tepals (in Axinandra epitepalous). Anthers basifixed,
non-versatile, tetrasporangiate (microsporangia lateral to terminal), introrse
to latrorse, longicidal (dehiscing by longitudinal slits); connective often
distally (abaxially) expanded; endothecium ephemeral. Tapetum secretory.
Staminodia absent?
Pollen grains
Microsporogenesis simultaneous? Pollen grains tri- or tetracolporate,
heterocolpate (in Axinandra, Dactylocladus with pseudocolpi
alternating with apertures, in Crypteronia disyncolporate), shed as
monads, bicellular at dispersal. Exine tectate, with columellate infratectum,
rugulate or smooth.
Gynoecium Pistil composed of
two to five (or six) connate carpels. Ovary inferior or semi-inferior, usually
unilocular (septa sometimes fused at base). Style single, simple, usually
filiform, often persistent. Stigma usually capitate (sometimes punctate), Wet
type? Pistillodium absent?
Ovules Placentation parietal
or basal-parietal. Ovules one to three (basal) to numerous (parietal) per
carpel (six to 15 per ovary), anatropous, horizontal or ascending, bitegmic,
crassinucellar. Micropyle bistomal. Outer integument ? cell layers thick. Inner
integument ? cell layers thick. Transseptal vascular bundles sometimes present.
Megasporangial tissue early degenerating. Megagametophyte monosporous,
Polygonum type. Endosperm development ab initio nuclear. Endosperm
haustoria? Embryogenesis?
Fruit A loculicidal capsule
(with two to five valves, usually adnate to style), sometimes flattened.
Seeds Aril absent. Testa often
winged. Exotestal cells tanniniferous. Endotesta multiplicative; endotestal
cells crystalliferous. Exotegmic and endotegmic cells tanniniferous. Remaining
layers more or less persistent. Perisperm not developed. Endosperm absent.
Embryo straight, chlorophyll? Cotyledons two. Germination?
Cytology n = 8
DNA
Phytochemistry Virtually
unknown. Aluminium accumulated.
Use Timber.
Systematics
Dactylocladus (1; D. stenostachys; Borneo), Crypteronia
(6; C. borneensis, C. elegans, C. glabriflora,
C. griffithii, C. macrophylla, C. paniculata; Assam,
the Benghal, southwestern China, Indochina, the Andaman Islands, Malesia to New
Guinea), Axinandra (4; A. zeylanica: Sri Lanka; A.
alata, A. beccariana, A. coriacea: the Malay Peninsula,
Borneo).
Crypteroniaceae are
sister-group to the clade [Alzateaceae+[Rhynchocalycaceae+[Oliniaceae+Penaeaceae]]].
Conti & al. (2002) recovered the topology
[Dactylocladus+[Crypteronia+Axinandra]]
for Crypteroniaceae.
LYTHRACEAE J.
St.-Hil.
|
( Back to Myrtales )
|
Saint-Hilaire, Expos. Fam. Nat. 2: 175. Feb-Apr
1805 [‘Lythrariae’], nom. cons.
Salicariaceae Juss.,
Gen. Pl.: 330. 4 Aug 1789 [‘Salicariae’], nom. illeg.;
Punicaceae Bercht. et J. Presl, Přir. Rostlin 2:
378. 1825 [‘Puniceae’], nom. cons.;
Trapaceae Dumort., Anal. Fam. Plant.: 36, 39. 1829,
nom. cons.; Ammanniaceae Horan., Prim. Lin. Syst.
Nat.: 86. 2 Nov 1834 [‘Ammanniaceae (Lythrariae)’];
Trapales J. Presl in Nowočeská Bibl. [Wšobecný
Rostl.] 7: 549. 1846 [‘Trapaceae’];
Lagerstroemiaceae J. Agardh, Theoria Syst. Plant.:
338. Apr-Sep 1858 [‘Lagerstroemieae’];
Lawsoniaceae J. Agardh, Theoria Syst. Plant.: 338.
Apr-Sep 1858 [‘Lawsonieae’]; Blattiaceae
Engl., Syllabus: 146. Apr 1892; Sonneratiaceae Engl.
in Engler et Prantl, Nat. Pflanzenfam. Nachtr. 1: 261. Oct 1897, nom. cons.;
Duabangaceae Takht., Florist. Reg. World: 332. 27 Apr
1986
Genera/species 27/580–595
Distribution Cosmopolitan
except polar areas, with their highest diversity in America.
Fossils Pollen grains assigned
to Lythrum elkensis have been found in Lower Campanian strata in
Wyoming. Seeds of Alatospermum, Microdiptera, Mneme
and the extant Decodon are frequent in Cenozoic layers in North
America and Europe. The palynogenus Florschuetzia from Cenozoic strata
in Europe and Asia may be a predecessor of Sonneratia (and perhaps of
Trapa). Lythrum and Trapa is known at least from the
mid-Cenozoic onwards and the fossil genus Trapago from the
Maastrichtian may possibly be attributed to Lythraceae.
Habit Usually bisexual (in
Capuronia dioecious; in Sonneratia and Duabanga
sometimes monoecious?), evergreen trees or shrubs, perennial or annual herbs
(rarely lianas). Many species are hygrophytes, some representatives are aquatic
(Sonneratia comprises mangrove trees with pneumatophores, producing
vertical anchor roots, and horizontal nutrition roots; Trapa is
aquatic). Bark often exfoliating. Young stems and branches usually quadrangular
in cross-section.
Vegetative anatomy Phellogen
ab initio usually deeply (in Sonneratia and Duabanga
superficially) seated. Polyderm present. Primary vascular tissue bicollateral.
Secondary lateral growth normal or absent. Vessel elements usually with simple
(in Sonneratia sometimes also scalariform and/or reticulate)
perforation plates; lateral pits alternate or opposite, simple and/or bordered
pits. Vestured pits present. Imperforate tracheary xylem elements usually fibre
tracheids (in, e.g., Punica and Sonneratia) libriform fibres
with simple or bordered pits (sometimes with crystals), septate or non-septate.
Wood rays usually uniseriate (rarely multiseriate), usually heterocellular
(rarely homocellular). Axial parenchyma apotracheal diffuse, or paratracheal
scanty (sometimes vasicentric, aliform, confluent, or banded, or absent, i.a.,
in Punica and Sonneratia). Tyloses sometimes frequent.
Intraxylary phloem present. Sieve tube plastids Ss type. Nodes usually 1:1 (or
1:3), unilacunar with one leaf trace (sometimes trilacunar).
Sonneratia has branched sclereids in pneumatophores. Punica
has secretory cells in cortex and medulla. Calciumoxalate as styloids, crystal
sand, or prismatic or rhomboidal crystals.
Trichomes Hairs uni-, bi-, or
multicellular, uniseriate or multiseriate, simple or branched (sometimes
furcate, dendritic or stellate), or absent; multicellular glandular hairs
present in some genera.
Leaves Usually opposite
(sometimes verticillate or alternate, spiral; in Trapa with floating
leaves in rosette and finely laciniate foliaceous submersed adventitious
roots), simple, entire, often coriaceous, with conduplicate to flat ptyxis.
Axillary glands usually present. Stipules small, early caducous, or absent;
leaf sheath absent. Petiole in Trapa inflated, aerenchymatous. Petiole
vascular bundle transection arcuate. Venation pinnate, usually brochidodromous.
Stomata usually anomocytic (sometimes anisocytic, rarely paracytic; in
Trapa adaxial). Cuticular wax crystalloids usually as platelets.
Lamina sometimes gland-dotted, in several genera with hydathodes,
salt-excreting glands, or nectaries. Epidermis often with large mucilaginous
idioblasts. Mesophyll usually without sclerenchymatous idioblasts (present in
Sonneratia and Duabanga), often with calciumoxalate as druses
or solitary prismatic crystals (in Punica with large prismatic
crystals). Sclereids present in Duabanga and asterosclereids in
Sonneratia. Leaf margin usually entire (sometimes serrate or dentate).
Extrafloral nectaries rarely (e.g. in Lafoensia) present on lamina.
Inflorescence Terminal or
axillary, cyme, thyrse, corymb, umbel- or head-like, spike or raceme (flowers
sometimes solitary axillary).
Flowers Actinomorphic or
zygomorphic. Hypanthium cupular or tubular (sometimes with spurs), often
distinctly ribbed, or absent. Usually hypogyny or half epigyny (rarely
epigyny). Androphore present in some genera. Sepals (three or) four to eight
(to 16), with valvate aestivation, often with alternate appendices (epicalyx),
often persistent and finally coriaceous, free. Petals (three or) four to eight
(to 16; in Decodon usually five), with plicate or imbricate
aestivation, often wrinkled in bud, often clawed, inserted at adaxial margin of
hypanthium, caducous, free (absent in some species). Nectariferous disc annular
or unilateral intrastaminal at ovary base, or absent (in Cuphea often
unilateral gland). Diheterostyly and triheterostyly occurring in some genera
(e.g. Lythrum).
Androecium Stamens one to more
than 100 (in Decodon usually 5+5; in Cuphea 11; in
Trapa four), in one, two or three whorls, sometimes as many as sepals,
antesepalous or antepetalous, usually obdiplostemonous (sometimes
diplostemonous or haplostemonous), centripetally or centrifugally developing.
Filaments free from each other and from tepals, inserted immediately below
petals to near ovary, with unequal lengths (when diplostemonous then
antesepalous filaments longer, inserted near or above base of perianth tube).
Anthers sometimes inflexed in bud, usually dorsifixed (rarely basifixed),
usually versatile, tetrasporangiate, introrse, longicidal (dehiscing by
longitudinal slits). Tapetum secretory, with binucleate to sexanucleate (in
Trapa multinucleate) cells. Staminodia usually absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually tricolpate or
tricolporate (in Duabanga triporate; in Trapa
trichotomocolpate; in Sonneratia?), usually heterocolpate (with three
or six pseudocolpi alternating with apertures), shed as monads, bicellular at
dispersal. Exine tectate, with columellate infratectum, psilate, scabrate or
finely verrucate or striate (in Trapa with three distinct ridges
running transversely across colpi and fusing at poles).
Gynoecium Pistil composed of
two (in, e.g., Trapa, transversely orientated) to six (to c. 15)
connate carpels; when carpels as many as sepals, then alternisepalous or
antesepalous; when carpels two, then often transverse or median; when carpels
three, then median carpel adaxial. Ovary superior to semi-inferior (rarely
inferior), usually bilocular to quadrilocular (rarely unilocular or
multilocular), with septa incomplete at apex or reduced to thin threads. Style
single, simple, thin (in Trapa hollow). Stigma punctate or capitate,
papillate, Dry or Wet type (Lagerstroemia, Punica).
Pistillodium?
Ovules Placentation usually
axile (almost free central at maturation; rarely parietal; in Trapa
apical-axile; carpels in Punica granatum in two or three superposed
layers, basal carpels with axile and remaining carpels with intrusively
parietal placentation). Ovules usually two to c. 50 per carpel, anatropous,
ascending or horizontal (in Trapa one, pendulous), bitegmic,
crassinucellar. Micropyle usually bistomal (in Punica exostomal?;
micropyle in Trapa formed by long and massive nucellar beak). Outer
integument two to seven (to nine) cell layers thick. Inner integument two or
three cell layers thick. Hypostase usually present. Parietal tissue approx.
seven cell layers thick. Megasporocytes several (archespore multicellular).
Megagametophyte monosporous, Polygonum type. Antipodal cells not
formed in Trapa. Endosperm development ab initio nuclear (endosperm
not developing in Trapa, suspensor haustorium formed). Endosperm
haustoria? Embryogenesis usually onagrad (in Trapa solanad).
Fruit Usually a loculicidal,
septicidal or irregularly dehiscing capsule (sometimes a pyxidium; rarely, e.g.
in Punica, a berry; in Trapa a one-seeded nutlike fruit with
persistent sepals modified into hard horn-like processes; fruit in
Cuphea dehiscing by placenta penetrating pericarp and hypanthium),
often more or less enclosed by persistent hypanthium and/or sepals. Placenta
sometimes expanded in fruit.
Seeds Aril absent. Seed
usually flat. Operculum often? present. Testa usually multiplicative,
multi-layered, sometimes winged, usually with epidermal internal finger-shaped
initially inverted invaginations of plasmalemma developing into mucilaginous
hairs when wet. Exotesta of various shape (seeds in Punica embedded in
pulpy tissue formed by enlarged epidermal exotestal cells). Endotestal cells
often sclerotic, often tracheidal (sometimes crystalliferous). Exotegmen and
endotegmen sometimes consisting of interwoven fibres (in Punica
undistinguished). Perisperm not developed. Endosperm usually absent (in
Trapa present, starchy). Spiral suspensor present in Trapa.
Embryo usually straight, well differentiated, usually without (in
Sonneratia and Duabanga with) chlorophyll. Cotyledons two,
oily, usually flat (in Lagerstroemia and Punica plicate; in
Trapa very unequal in size). Radicula absent in Trapa.
Germination phanerocotylar (in Trapa also cryptocotylar; large starchy
cotyledon persisting inside pericarp, whereas small cotyledon, plumule and
radicula penetrate apical pore arising when style detached).
Cytology n = (5–)8(–15,
24, 28, 32), n = 12 (Duabanga, Sonneratia); n = 20, 24
(Trapa); n = 6–c. 86 (Cuphea); x = 8 – Polyploidy and
aneuploidy frequently occurring.
DNA
Phytochemistry Flavonols
(kaempferol, quercetin), ellagic acid, tannins (e.g. cuphiin, ellagitannins),
quinolizidine alkaloids, and polyacetate derived arthroquinones present.
Proanthocyanidins, saponins and cyanogenic compounds not found. Raffinose and
stachyose present in phloem exudates. Dissolvable oxalate accumulated.
Use Ornamental plants, fruits
(Punica granatum, Trapa), vegetables (Trapa), dyeing
substances (Lawsonia inermis), medicinal plants, timber.
Systematics Lythraceae are sister-group to
Onagraceae.
Lythroideae are sister to the clade
[Lagerstroemioideae+Punicoideae] (Graham & al. 2011).
Lythroideae Juss. ex
Arn., Botany: 108. 9 Mar 1832 [‘Lythrarieae’]
4/c 87. Lythrum
(38; almost cosmopolitan), Decodon (1; D. verticillatus;
eastern United States); Rotala (c 45; temperate to tropical regions on
both hemispheres), Heimia (3; H. montana, H.
myrtifolia, H. salicifolia; southern Texas, southern New Mexico,
Mexico, Central America, the West Indies, South America to northern Argentina).
– Subcosmopolitan.
[Lagerstroemioideae+Punicoideae]
Testa with inverted mucilaginous
hairs.
Lagerstroemioideae
Beilschm. in Flora 16(Beibl. 7): 99, 108. 14 Jun 1833
[’Lagerstroemidae’]
9/115–125. Lagerstroemia
(50–55; tropical Asia, northern and eastern Australia), Duabanga (3;
D. grandiflora, D. moluccana, D. taylorii; tropical
Asia), Sonneratia (c 20; coasts of the Indian and Pacific Oceans), Trapa (1;
T. natans; warm-temperate regions in Europe, Africa and Asia);
Ammannia (25–30; nearly cosmopolitan), Crenea (2; C.
maritima, C. patentinervis; Trinidad, tropical South America),
Ginoria (14; Mexico, the West Indies), Tetrataxis (1; T.
salicifolia; Mauritius), Lawsonia (1; L. inermis; North
Africa, tropical regions in the Old World). – Nearly cosmopolitan.
Sonneratia consists of mangrove trees. – Lawsonia,
Ammannia, Crenea, Ginoria and Tetrataxis
may form a clade.
Punicoideae (Eichl.)
Luerss., Handb. Syst. Bot. 2: 822. Oct 1881 [‘Puniceae’]
14/380–385. Woodfordia (2;
W. uniflora: northeastern Africa, southern Arabian Peninsula; W.
fruticosa: Madagascar, tropical Asia to southern China and Timor),
Koehneria (1; K. madagascariensis; southern Madagascar), Cuphea (c
260; southeastern Canada, eastern United States, Mexico, Central America, the
West Indies, tropical and subtropical South America), Pleurophora (7;
P. anomala, P. patagonica, P. polyandra, P.
pulchra, P. pungens, P. pusilla, P. saccocarpa;
South America); Lafoensia (5; L. glyptocarpa, L.
nummulariifolia, L. pacari, L. punicifolia, L.
vandelliana; tropical America), Pemphis (1; P. acidula;
tropical regions in the Old World), Punica (2;
P. protopunica: Socotra; P. granatum: southwestern Asia),
Adenaria (1; A. floribunda; southern Mexico to Panamá,
eastern South America south to northern Argentina), Pehria (1; P.
compacta; Colombia, Venezuela), Capuronia (1; C.
madagascariensis; Madagascar), Galpinia (1; G.
transvaalica; eastern southern Africa north to Zimbabwe and Mozambique),
Diplusodon (100–105; Brazil), Lourtella (1; L.
resinosa; Peru), Physocalymma (1; P. scaberrimum;
tropical South America). – Pantropical, southeastern Africa, southwestern
Asia, warm-temperate and subtropical America. – Koehneria may be
sister to Woodfordia. Cuphea and
Pleurophora are sister-groups. Adenaria may be sister-group
to Pehria. Capuronia and Galpinia are
sister-groups.
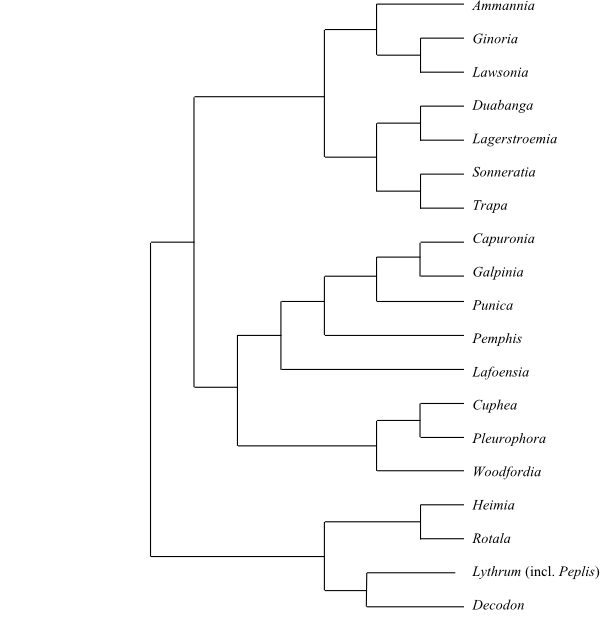 |
Maximum likelihood tree of Lythraceae based on DNA
sequence data (Graham & al. 2011).
|
MELASTOMATACEAE
Juss.
|
( Back to Myrtales )
|
de Jussieu, Gen. Plant.: 328. 4 Aug 1789
[’Melastomae’], nom. cons.
Melastomatales Juss.
ex Bercht. et J. Presl, Přir. Rostlin: 233. Jan-Apr 1820
[‘Melastomeae’]; Blakeaceae Reichb. ex
Barnhart in Bull. Torrey Bot. Club 22: 19. 15 Jan 1895;
Miconiaceae Mart., Consp. Regn. Veg.: 64. Sep-Oct
1835 [’Miconieae’]; Rhexiaceae Dumort.,
Comment. Bot.: 59. Nov-Dec 1822 [‘Rhexideae’]
Genera/species
170/4.635–4.980
Distribution Tropical and
subtropical regions on both hemispheres, with their largest diversity in
tropical South America.
Fossils Uncertain. Fossil
flowers, possibly attributable to Melastomataceae, have been
found in Turonian and younger layers (Crepet 2008).
Habit Usually bisexual (rarely
androdioecious), evergreen trees or shrubs, perennial herbs (sometimes aquatic)
or lianas. Many species are epiphytic. Young stems and branches often
quadrangular in cross-section.
Vegetative anatomy Phellogen
ab initio superficially or deeply seated. Cortical and medullary vascular
bundles present or absent. Primary vascular tissue bicollateral or centrifugal.
Secondary lateral growth normal, anomalous (via cylindrical cambium) or absent.
Vessel elements with usually simple (sometimes reticulate) perforation plates;
lateral pits alternate, usually simple. Vestured pits present. Imperforate
tracheary xylem elements usually libriform fibres (in, e.g.,
Pternandra fibre tracheids) with simple or bordered pits, septate or
non-septate (also vasicentric tracheids). Wood rays uniseriate or multiseriate,
homocellular or heterocellular. Axial parenchyma apotracheal diffuse, and/or
paratracheal scanty, reticulate, scalariform, vasicentric or banded.
Interxylary phloem usually absent (present in Pternandra). Sieve tube
plastids Ss type. Endodermis sometimes prominent (rarely with corky cell
walls). Nodes usually 1:1, unilacunar with one leaf trace (sometimes 1:3,
unilacunar with trifid/split lateral vascular strands). Cortex usually without
cristarque cells (present in Osbeckieae). Heartwood sometimes with
gum-like substances. Prismatic calciumoxalate crystals, druses and/or crystal
sand present or absent. Calciumoxalate raphides absent. Root tips with
anthocyanins.
Trichomes Hairs usually large,
multicellular, uniseriate or multiseriate, simple, stellate, peltate
(especially in Astronieae) or often lepidote or vesicular, often
dendritic; multicellular glandular hairs (sometimes lepidote) present
(Melastomateae with characteristic ’tibouchinoid’ hairs).
Leaves Usually opposite
(rarely verticillate), simple, entire, with conduplicate or supervolute ptyxis.
Stipules and leaf sheath absent. Petiole vascular bundle transection arcuate,
annular etc. Venation palmate-parallelodromous, acrodromous (rarely pinnate);
primary veins three to nine (to 19), arising arcuately at or near leaf base and
confluent at leaf tip; tertiary veins usually prominent, scalariform, running
at right angle against midvein. Stomata usually anomocytic (sometimes
anisocytic, paracytic, polycytic, diacytic, cyclocytic or anomocyclocytic).
Cuticular wax crystalloids? Domatia usually absent (in, e.g., Blakeeae
and Miconieae often acarodomatia or myrmecodomatia as pits or
pockets). Epidermis with or without mucilaginous idioblasts. Mesophyll usually
with sclerenchymatous idioblasts containing sclereids of various types and with
calciumoxalate as raphides, styloids, druses and crystal sand. Secretory
cavities absent. Leaf margin usually entire (sometimes serrate).
Inflorescence Terminal or
axillary, panicle, thyrse or other types of cymose inflorescences (flowers
rarely solitary terminal). Bracts and floral prophylls (bracteoles) often
showy.
Flowers Actinomorphic or
zygomorphic (especially by androecium). Hypanthium tubular or campanulate.
Epigyny or half epigyny. Sepals four or five (to seven), with imbricate,
valvate, contorted or open aestivation, on margin of hypanthium, often early
caducous, usually free (sometimes connate into caducous calyptra). Petals four
or five (to seven), with destrorsely-contorted aestivation, on margin of
hypanthium, free. Nectary usually absent (nectar in some genera secreted from
hypanthium, petal tips, filaments, anther connectives, ovary apex, or stigma).
Disc absent.
Androecium Stamens usually
3+3, 4+4, or 5+5 (rarely three or up to more than 100), usually diplostemonous
(rarely obdiplostemonous or haplostemonous); inserted on one side of flower.
Filaments often twisted, often geniculate at connective base, free from each
other and from tepals. Anthers sometimes inflexed in bud, inverted during
development, basifixed, non-versatile, tetrasporangiate, introrse, usually
poricidal (dehiscing by one, two or four apical pores, developing in
epidermis-free part of microsporangia by desiccation of exposed mesophyll;
fibre thickenings absent in endothecium; in Pternandra and
Astronieae introrse?, longicidal, with short or long longitudinal
slits); connective enlarged in characteristic way, often basally-ventrally
prolonged or with horn- or spur-like dorsal appendages, without glands. Tapetum
secretory, with ?-nucleate cells. Staminodia usually absent (sometimes four or
five, alternating with fertile stamens).
Pollen grains
Microsporogenesis simultaneous. Pollen grains (2–)3(–6)-colpor(oid)ate or
3(–6)-colpate, heterocolpate (usually with three pseudocolpi or intercolpate
depressions alternating with apertures), usually shed as monads (rarely as
tetrads or polyads), bicellular at dispersal. Exine tectate, with columellate
infratectum, perforate or punctate, striate, rugulate, rugulate-verrucate or
smooth.
Gynoecium Pistil composed of
(three or) four or five (to 14) connate usually antesepalous carpels. Ovary
inferior or semi-inferior, (unilocular or) quadri- or quinquelocular (to
14-locular), usually with antesepalous (in Pternandra and
Rhexia antepetalous) locules, often with interspace between ovary wall
and hypanthium. Style single, simple, sometimes hollow. Stigma punctate to
capitate, papillate, Wet type. Pistillodium?
Ovules Placentation usually
axile (in Astronieae basal; in Pternandra parietal). Ovules
usually numerous (sometimes one or few) per carpel, usually anatropous (in
Rhexia orthotropous), bitegmic, crassinucellar. Micropyle bistomal,
Z-shaped (zig-zag). Outer integument approx. two cell layers thick. Inner
integument two or three cell layers thick. Hypostase present. Parietal tissue
four to six cell layers thick. Nucellar cap present. Megagametophyte
monosporous, Polygonum type. Endosperm development ab initio nuclear.
Endosperm haustoria? Embryogenesis onagrad or solanad.
Fruit A loculicidal capsule,
with walls often degenerating during maturation and leaving vertical vascular
bundles (capsule sometimes dehiscing along epigynous part or irregularly), or a
berry.
Seeds Aril absent. Small seeds
with operculum formed by hilum. Exotesta palisade to cuboid and lignified.
Mesotesta sclerotic. Endotesta? Tegmen crushed, without fibrous exotegmen.
Perisperm not developed? Endosperm absent. Embryo small, well differentiated,
(with? or) without chlorophyll. Cotyledons two, small, often unequal in size.
Radicula curved, inserted in testal pouch. Germination phanerocotylar.
Cytology n = (8–)9–17 (23,
31) – Polyploidy frequently occurring.
DNA
Phytochemistry Flavonols
(kaempferol, quercetin, myricetin), cyanidin, delphinidin, acylated
anthocyanins, ellagic acid, tannins, and phenylalanine-derived cyanogenic
compounds present. Saponins not found. Aluminium accumulated at least in
Pternandra, Astronieae, Merianieae, and
Miconieae.
Use Ornamental plants, fruits,
timber, dyeing substances.
Systematics Melastomataceae are probably
sister to Memecylaceae.
Pternandra (Kibessioideae) is
sister to the remaining Melastomataceae.
Kibessioideae Naudin
in Ann. Sci. Nat. Bot., sér. 3, 12: 201. Oct 1849
[‘Kibessieae’]
1/c 15. Pternandra (c 15;
Indochina, Hainan, Malesia to northern Queensland). – Phellogen superficial.
Internal phloem present. Hairs uniseriate. Interpetiolar stipular flange
present below leaves. Petiole vascular bundle transection arcuate. Stomata
anomocyclocytic. Flowers tetramerous. Endothecium present only in internal wall
of inner microsporangium. Anther connectives with apical oil-glands. Carpels
antepetalous. Ovary divided by secondary septa. Placentation parietal. Capsule
carnose. Aluminium accumulated.
Melastomatoideae Ser.
ex DC., Prodr. 3: 100. Mar (med.) 1828 [‘Melastomeae’]
167/4.610–4.955. Internal phloem
absent. Petiole vascular bundle transection of various types. Leaf veins
without fibrous envelope. Inflorescence often terminal. Sepals sometimes
connate, calyptrate. Anthers poricidal (dehiscing by apical pores). Endothecium
ephemeral or absent. Carpels usually antesepalous, developing before
anthers.
Astronieae Triana in
Bull. Cong. Int. Bot. Hort. Amsterdam 1865: 457. 1866
3–4/c 150. Astrocalyx (2;
A. calycina, A. pleiosandra; the Philippines),
Astronia (c 60; tropical Asia to islands in the Pacific),
Astronidium (67; Borneo and the Philippines to New Guinea, Fiji,
Micronesia to the Society Islands; incl. Beccarianthus?),
Beccarianthus (>22; Borneo to New Guinea; in
Astronidium?). – Tropical Asia to the Society Islands. Phellogen
superficial. Petiole vascular bundles complex, open. Stomata usually
anomocytic. Hairs peltate-lepidote. Anthers sometimes longicidal (dehiscing by
slits). Carpels antepetalous. Placentation basal to basal-axile. Aluminium
accumulated. – Astronieae are sister-group to the remaining
Melastomatoideae.
[[Macrocentrum+[Merianieae+[Miconieae+Henrietteeae]]]+[Bertolonieae+[[Blakeeae+Sonerileae]+[[Microlicieae+Melastomateae]+[Rhexieae+Marcetieae]]]]]
Highest diversity in tropical and
subtropical South America. Trees or herbs (sometimes epiphytes, rarely lianas).
Phellogen sometimes superficial. Hair types diverse (including short-stalked
glands). Petiole vascular bundle transection arcuate or complex. Secondary
veins three or five from (near) base; tertiary veins perpendicular to mid-vein.
Stomata polycytic, cyclocytic, etc. Flowers (3–)4–5(–10)-merous. Calyx
sometimes with alternate lobes. Stamens sometimes as many as sepals,
antesepalous or antepetalous, numerous. Anthers poricidal. Connective often
with basal appendage, sometimes nectariferous. Carpels two to 15. Placentation
axile. Ovules sometimes one to few per carpel. Style sometimes hollow. Capsule
sometimes irregularly dehiscent (sometimes a berry). Aluminium accumulated in
Merianieae and Miconieae.
[Macrocentrum+[Merianieae+[Miconieae+Henrietteeae]]]
Macrocentrum clade
1/c 25. Macrocentrum (c 25;
Central America, northern tropical South America).
[Merianieae+[Miconieae+Henrietteeae]]
Merianieae Triana in
Bull. Cong. Int. Bot. Hort. Amsterdam 1865: 457. 1866
15/260–275. Adelobotrys (c
25; Central America), Axinaea (40–45; Central America, tropical
South America, with their highest diversity in the Andes; in
Meriania?), Behuria (14; southern Brazil),
Benevidesia (2; B. magdalenensis, B. organensis;
southeastern Brazil), Bisglaziovia (1; B. behurioides;
Brazil), Centronia (12–15; Central America, western tropical South
America, Guianas), Dolichoura (1; D. spiritusanctensis;
Brazil), Graffenrieda (c 45; southern Mexico, Central America, the
West Indies, tropical South America), Huberia (c 15; Ecuador, Peru,
southeastern Brazil), Meriania (90–95; southern Mexico, Central
America, the West Indies, tropical South America), Merianthera (3;
M. burlemarxii, M. pulchra, M. sipolisii; Brazil),
Neblinanthera (1; N. cumbrensis; Venezuela),
Ochthephilus (1; O. repentinus; Guyana), Phainantha
(5; P. laxiflora, P. maguirei, P. myrteoloides,
P. shuariorum, P. steyermarkii; tropical South America),
Tessmannianthus (6; T. calcaratus, T. carinatus,
T. cenepensis, T. cereifolius, T. gordonii, T.
heterostemon; Panamá, Colombia, Ecuador, Peru). – Tropical America.
[Miconieae+Henrietteeae]?
Miconieae DC., Prodr.
3: 152. Mar (med.) 1828
25/1.620–1.750. Allomaieta
(7; A. ebejicosana, A. grandiflora, A. hirsuta,
A. pancurana, A. strigosa, A. villosa, A.
zenufanasana; Colombia), Alloneuron (4; A. liron, A.
majus, A. ronliesneri, A. ulei; Colombia, Peru),
Quipuanthus (1; Q. epipetricus; the Andes in Ecuador and
Peru), Wurdastom (8; tropical South America); Anaectocalyx
(2; A. bracteosa, A. latifolia; Venezuela),
Boerlagea (1; B. grandifolia; Borneo), Calycogonium
(c 35; the West Indies), Catocoryne (1; C. linnaeoides;
Peru), Charianthus (6–13; the Lesser Antilles, tropical South
America), ’Clidemia’
(c 175; Mexico, Central America, the West Indies, tropical South America;
non-monophyletic), Conostegia (40–45; tropical America, with their
highest diversity in Central America), Creochiton (9; Central and East
Malesia), Eriocnema (1; E. fulva; Brazil),
Physeterostemon (3; P. fiaschii, P. jardimii, P.
thomasii; Bahia in Brazil), Killipia (1; K.
quadrangularis; Colombia), ’Leandra’ (c 250; tropical
America, with their highest diversity in Brazil; non-monophyletic?),
Maieta (8; southern Central America, tropical South America),
Mecranium (24; the West Indies), ’Miconia’ (900–1.000;
tropical America; non-monophyletic), Ossaea (c 90; southern Mexico,
Central America, the West Indies, tropical South America; polyphyletic?),
Pachyanthus (16; Cuba, Hispaniola, Colombia), Pleiochiton (5;
P. ebracteatum, P. glaziovianum, P. micranthum,
P. parvifolium, P. roseum; southern Brazil),
Pseudodissochaeta (3; P. raphioides, P. roseus,
P. spirei; northern India to Hainan), Tetrazygia (12;
Florida, the West Indies), ’Tococa’ (45–50; southern Mexico,
Central America, tropical South America; non-monophyletic). – Tropical Asia,
tropical America. – Allomaieta (including Cyphostyla),
Alloneuron and Wurdastom should possibly be transferred to
Cyphostyleae (Michelangeli & al. 2011). They have antesepalous
stamens, inferior ovary, and capsule-like fruit. Yet, will this render
Miconieae paraphyletic?
Henrietteeae Penneys,
Michelang., Judd et Almeda in Syst. Bot. 35(4): 795. 6 Dec 2010
3/89–94. Bellucia (17;
tropical America), Henriettea (60–65; tropical South America),
Kirkbridea (2; K. pentamera, K. tetramera;
Colombia). – Tropical America.
[Bertolonieae+[[Blakeeae+Sonerileae]+[[Microlicieae+Melastomateae]+[Rhexieae+Marcetieae]]]]
Bertolonieae Triana in
Bull. Cong. Int. Bot. Hort. Amsterdam 1865: 457. 1866
[‘Bertoloniceae’]
3/45–52. Bertolonia
(17; Venezuela, southeastern Brazil), Monolena
(8–10; tropical South America), Triolena (20–25; southern Mexico,
Central America, the West Indies, tropical South America). – Tropical
America.
[[Blakeeae+Sonerileae]+[[Microlicieae+Melastomateae]+[Rhexieae+Marcetieae]]]
[Blakeeae+Sonerileae]
Blakeeae Benth. et
Hook. f., Gen. Plant. 1: 727, 735. Sep 1867 (in Sonerileae?)
3/85–105. Blakea (80–100;
southern Mexico, Central America, the West Indies, tropical South America),
Chalybea (2; C. brevipedunculata, C. peruviana;
Colombia to Peru), Huilaea (3–4; H. calyptrata, H.
ecuadorensis, H. minor; Colombia). – Tropical America.
Sonerileae Triana in
Bull. Cong. Int. Bot. Hort. Amsterdam 1865: 457. 1866
44/800–890. Amphiblemma
(8–13; tropical West and Central Africa), Anerincleistus (30; India
and southern China to the Philippines), Aschistanthera (1; A.
cristanthera; Vietnam), Barthea (1; B. barthei; China,
Taiwan), Blastus (9–12; Assam to West Malesia), Boyania (1;
B. ayangannae; the Guianas), Bredia (12–30; East and
Southeast Asia), Calvoa (10–20; tropical Africa),
Catanthera (11–17; Sumatra, Borneo, New Guinea),
Centradeniastrum (2; C. album, C. roseum; western
tropical South America), Cincinnobotrys (4–7; C. acaulis,
C. felicis, C. pulchella, C. speciosa; tropical
Africa), Cyphotheca (1; C. montana; Yunnan),
Dicellandra (3; D. barteri, D. descoingsii, D.
glanduligera; tropical West and Central Africa), Diplarpea (1;
D. paleacea; Colombia), Diplectria (11; Southeast Asia,
Malesia), Dissochaeta (22; tropical Asia), Driessenia
(14–18; West and Central Malesia, with their largest diversity on Borneo),
Feliciadamia (1; F. stenocarpa; French Guinea),
Fordiophyton (9–14; southern China, Southeast Asia),
’Gravesia’ (c 110; tropical Africa, Madagascar; non-monophyletic),
Kendrickia (1; K. walkeri; southern India, Sri Lanka),
Kerriothyrsus (1; K. tetrandrus; Laos), Macrolenes
(15; Thailand, Malesia), Maguireanthus (1; M. ayangannae;
Guyana), ’Medinilla’
(c 200?; tropical Africa, Madagascar, tropical Asia to southern China, Taiwan
and New Guinea, northeastern Queensland, Fiji, Samoa; non-monophyletic),
Neodriessenia (9; Borneo), Ochthocharis (14; tropical Africa,
tropical Asia), Opisthocentra (1; O. clidemioides; northern
Brazil), Oxyspora (c 25; tropical Asia, southern China),
Pachycentria (10–15; Burma, Malesia), Phyllagathis
(30–55; southern China, Southeast Asia, West Malesia), Plethiandra
(9; West Malesia), Poikilogyne (21; Borneo, New Guinea),
Poilannammia (4; P. allomorphioidea, P. costata,
P. incisa, P. trimera; Vietnam), Preussiella (2;
P. gabonensis, P. kamerunensis; tropical West and Central
Africa), Salpinga (9; tropical South America), Sarcopyramis
(2; S. bodinieri, S. napalensis; tropical Asia),
Scorpiothyrsus (3–6; S. erythrotrichus, S.
shangszeensis, S. xanthostictus; Hainan, Southeast Asia), Sonerila
(c 175?; tropical Asia), Sporoxeia (4–6; S. clavicalcarata,
S. hirsuta, S. latifolia, S. sciadophila; Burma,
Southeast Asia), Stanmarkia (2; S. medialis, S.
spectabilis; Mexico, western Guatemala), Stussenia (1; S.
membranifolia; Vietnam), Tateanthus (1; T. duidae;
Venezuela), Tryssophyton (1; T. merumense; Guyana). –
Pantropical. Aluminium accumulated in some species. – The position of
Cambessedesia is not fully clarified.
[[Microlicieae+Melastomateae]+[Rhexieae+Marcetieae]]
Connective with basal appendage
(pedoconnective). Fruit a capsule. – Michelangeli & al. (2013) found that
Cambessedesia (21; eastern Brazil) is sister to a clade comprising
Microlicieae, Melastomateae and Rhexieae.
Furthermore, in their analyses Melastomateae were sister-group to
[Microlicieae+Rhexieae]. On the other hand,
Rupestrea (2; R. carvalhoana, R. johnwurdackiana;
Bahia in Brazil) was recovered as sister to the same large clade by Goldenberg
& al. (2015).
[Microlicieae+Melastomateae]
Microlicieae Naudin in
Ann. Sci. Nat. Bot., sér. 3, 12: 203. Oct 1849
[‘Microliciales’]
7/180–200. Rhynchanthera (c
20; Mexico to Bolivia, Paraguay and eastern Brazil); Trembleya
(9–11; southern and eastern Brazil), Chaetostoma (12; Brazil),
Lavoisiera (30–50; Brazil, with their largest diversity in eastern
Brazil), Lithobium (1; L. cordatum; Brazil)?,
Microlicia (c 110; tropical South America, with their highest
diversity in southern and eastern Brazil), Stenodon (2; S.
gracilis, S. suberosus; southern Brazil). – Tropical America,
with their highest diversity in the cerrado in southern and eastern Brazil. –
Microlicieae are sister-group to Melastomateae.
Melastomateae Bartl.,
Ord. Nat. Plant.: 329. Sep 1830 [‘Melastomea’]
39/670–720. ‘Pterolepis’
(7–16; tropical America, with their highest diversity in Brazil;
paraphyletic; incl. Pterogastra?), Pterogastra (2; P.
divaricata, P. minor; northern tropical South America; in
Pterolepis?), ‘Heterotis’ (7; H. amplexicaulis,
H. buettneriana, H. canescens, H. decumbens, H.
rotundifolia, H. rupicola, H. sylvestris; tropical
Africa; polyphyletic), Guyonia (14; tropical West and Central Africa;
in Heterotis?), Argyrella (6; A. amplexicaulis,
A. bambutorum, A. canescens, A. incana, A.
phaeotricha, A. richardsiae; tropical West, Central and
southeastern Africa), Melastomastrum (4; M. autranianum,
M. capitatum, M. segregatum, M. theifolium; tropical
Africa), Tristemma (11–16; tropical Africa, Madagascar, the
Mascarene Islands), Dichaetanthera (30–35; tropical Africa,
Madagascar), ‘Dissotis’ (c 110; tropical and southern Africa,
Madagascar; polyphyletic), Amphorocalyx (5; A. albus, A.
auratifolius, A. latifolius, A. multiflorus, A.
rupestris; Madagascar), Anaheterotis (1; A. pobeguinii;
Guinea, Sierra Leone), Dissotidendron (11; tropical West and Central
Africa), Dupineta (1–5; D. multiflora; tropical West and
Central Africa), Antherotoma (4; A. angustifolia, A.
debilis, A. naudinii, A. senegambiensis; tropical
Africa, Madagascar), Pseudosbeckia (1; P. swynnertonii;
tropical East Africa), Dionycha (3; D. boinensis, D.
bojerii, D. triangularis; Madagascar), Osbeckia (c 50;
Southeast Asia, Malesia to New Guinea, northern Australia), Melastoma
(20–25; Southeast Asia, Malesia, northern and eastern Australia),
Desmoscelis (2; D. calcarata, D. villosa; Brazil),
‘Tibouchina’ (230–240; Central America, the West Indies, South
America; non-monophyletic), Brachyotum (55–60; the Andes; in
Tibouchina?), Svitramia (7; S. canescens, S.
hatschbachii, S. integerrima, S. minor, S.
petiolata, S. pulchra, S. wurdackiana; southeastern
Brazil; in Tibouchina?), Tibouchinopsis (2; T.
glutinosa, T. mirabilis; northeastern Brazil; in
Tibouchina?), Heterocentron (17–28; southern Mexico,
Central America), Centradenia (4; C. floribunda, C.
grandifolia, C. inaequilateralis, C. paradoxa; southern
Mexico, Central America), Pilocosta (5; P. campanensis,
P. erythrophylla, P. nana, P. nubicola, P.
oerstedii; tropical America), Schwackaea (1; S.
cupheoides; southern Mexico, Central America), Bucquetia (3;
B. glutinosa, B. nigritella, B. vernicosa; the
Andes), Castratella (2; C. piloselloides, C. rosea;
the Andes), Chaetolepis (13; the Andes), Monochaetum (c 45;
Central America, the Andes), Nerophila (1; N. gentianoides;
tropical West Africa; in Chaetolepis?), Cailliella (1; C.
praerupticola; tropical West Africa), Dinophora (1; D.
spenneroides; tropical West and Central Africa), Dionychastrum
(1; D. schliebenii; Uluguru Mountains in Tanzania),
Loricalepis (1; L. duckei; Brazil), Mallophyton (1;
M. chimantense; Venezuela), Microlepis (2; M.
oleifolia, M. trianaei; southern Brazil), Poteranthera
(2; P. annectans, P. pusilla; tropical South America). –
Pantropical. Aluminium accumulated in some species.
[Rhexieae+Marcetieae]
Rhexieae DC., Prodr.
3: 114. Mar (med.) 1828
3/20–22. Arthrostemma (5; A.
alatum, A. ciliatum, A. lanceolatum, A.
parvifolium, A. primaevum; southern Mexico, Central America, the
West Indies, South America to Bolivia), Pachyloma (4; P.
coriaceum, P. huberioides, P. pusillum, P.
setosum; Amazonas), Rhexia (11–13; eastern North America,
southern and southeastern United States, the West Indies). – Tropical
America. – Rhexieae are sister-group to Marcetieae.
Marcetieae M. J. R.
Rocha, P. J. F. Guim. et Michelang. in Int. J. Pl. Sci. 179(1): 55. Jan 2018
c 20/665–670. Comoliopsis (1;
C. neblinae; the Guayana Highlands); Pseudoernestia (1;
P. cordifolia; northern tropical South America), Comolia (c
20; tropical South America), Rostranthera (1; R. tetraptera;
Suriname, Brazilian Amazonas), Dicrananthera (1; D.
hedyotidea; Guyana, Venezuela, northeastern Brazil), Noterophila
(3–6; N. beccabunga, N. inundata, N. tetramera;
Central America, the West Indies, tropical South America), Acisanthera
(c 20; Mexico, Central America, the West Indies, tropical South America),
Leiostegia (1; L. vernicosa; northern tropical South
America), Sandemania (1; S. hoehnei; Venezuela, Peru,
Amazonian savannas in Brazil), Siphanthera (16; tropical South
America), Macairea (c 25; tropical South America, with the largest
diversity in the Guayana Highlands), ‘Macairea’ pro parte (2?;
tropical South America; paraphyletic), Ernestia (6; E.
cataractae, E. cordifolia, E. maguirei, E.
pullei, E. quadriseta, E. tenella; tropical South
America), Brasilianthus (1; B. carajensis; southeastern Pará
in Brazil), Nepsera (1; N. aquatica; Central America, the
West Indies, tropical South America), Appendicularia (2; A.
entomophila, A. thymifolia; Venezuela, the Guianas, Brazil),
Acanthella (2; A. pulchra, A. sprucei; the Guayana
Highlands; + 7 spp. ‘Ernestia’ in tropical South America),
Marcetia (c 30; tropical South America), Fritzschia (9;
southeastern and central Brazil), Aciotis (16; southern Mexico,
Central America, the West Indies, tropical South America). – Tropical
America. – Comoliopsis is sister to the remaining
Marcetieae (Da Rocha & al. 2018).
Unplaced Melastomataceae
Dalenia (6; D.
beccariana, D. furfuracea, D. korthalsii, D.
pubescens, D. pulchra, D. speciosa; Borneo),
Vietsenia (4; V. laxiflora, V. poilanei, V.
rotundifolia, V. scaposa; Vietnam).
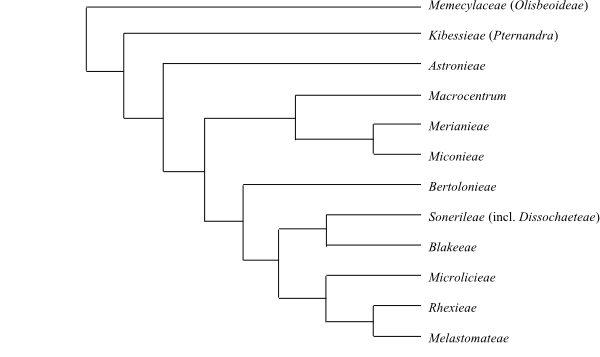 |
Cladogram (simplified) of Melastomataceae and
Memecylaceae based
on DNA sequence data (Clausing & al. 2000; Renner & al. 2001;
Fritsch & al. 2004; Renner & al., unpubl.).
|
de Candolle in D. F. L. von Schlechtendal in
Linnaea 2: 505. Aug-Oct 1827 [‘Memecyleae’]
Memecylales DC. in C.
F. P. von Martius, Consp. Regn. Veg.: 65. Sep-Oct 1835
[‘Memecyleae’]; Mouriraceae Gardner in
J. Bot. (Hooker) 2: 22. Feb 1840 [’Mouririaceae’]
Genera/species 6/345–355
Distribution Tropical Africa,
Madagascar, Mauritius, Malesia, Central America, the West Indies and tropical
South America.
Fossils Unknown.
Habit Usually bisexual (in
Lijndenia androdioecious), evergreen trees or shrubs, or perennial
herbs. Young stems and branches terete.
Vegetative anatomy Phellogen
ab initio superficially or deeply seated? Primary vascular tissue often
bicollateral. Secondary lateral growth normal or anomalous (via cylindrical
cambium). Vessel elements with simple perforation plates; lateral pits
alternate, usually (half-)bordered (rarely simple) pits. Vestured pits?
Imperforate tracheary xylem elements fibre tracheids with bordered pits,
non-septate. Wood rays uniseriate or multiseriate, heterocellular. Axial
parenchyma usually paratracheal scanty, reticulate, scalariform, aliform,
lozenge-aliform, winged-aliform, confluent, vasicentric, or narrowly banded
(sometimes apotracheal diffuse or diffuse-in-aggregates). Tyloses abundant.
Intraxylary phloem foraminate, diffuse. Interxylary phloem present in secondary
xylem. Sieve tube plastids Ss type. Nodes swollen, usually 1:1, unilacunar with
one leaf trace (sometimes 1:3, unilacunar with three traces). Heartwood often
with gum-like substances. Sclereids usually present. Calciumoxalate as acicular
crystals and/or styloids usually present (sometimes crystal sand).
Calciumoxalate raphides absent. Root tips without anthocyanins.
Trichomes Hairs usually absent
(uniseriate simple hairs sometimes present); glandular hairs absent.
Leaves Opposite, simple,
entire, with flat (Memecylon) or revolute (Mouriri) ptyxis.
Stipules usually absent (present in seedlings); leaf sheath absent. Petiole
vascular bundle transection arcuate or annular. Venation usually
palmate-parallelodromous (sometimes pinnate), acrodromous or (often seemingly
brochidodromous), with arcuate main-veins and indistinct lateral veins; usually
with fibrous envelope; in Memecylon and Mouriri with large
sclereids at vein tips. Stomata paracytic or anomocytic. Cuticular wax
crystalloids? Lamina sometimes pellucid-punctate. Mesophyll with or without
sclerenchymatous idioblasts. Secretory cavities absent. Leaf margin entire.
Inflorescence Axillary, often
fasciculate or umbel-like.
Flowers Actinomorphic or
zygomorphic, small. Pedicel articulated. Hypanthium tubular or campanulate.
Epigyny. Sepals (three or) four or five, with usually valvate (sometimes
imbricate quincuncial or open) aestivation, inserted on margin of hypanthium,
often caducous at beginning of anthesis, free. Petals (three or) four or five,
with dextrorsely contorted aestivation, inserted on margin of hypanthium, free.
Nectary absent. Disc absent.
Androecium Stamens usually
3+3, 4+4 or 5+5 (sometimes four or five). Filaments free, often bent in bud (in
Votomita straight), free from tepals. Anthers dorsifixed, versatile,
ab initio tetrasporangiate, as mature disporangiate, extrorse (inverted during
development), poricidal (dehiscing by apical pores) or longicidal (dehiscing by
longitudinal, sometimes short, slits, formed by irregular desiccation of
endothecial cells); connective fleshy, usually with depressed elliptic
terpenoid-secreting dorsal oil gland. Tapetum secretory, usually with
uninucleate (not in Mouriri) cells. Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually tri- or hexacolpate
and/or tri- or hexacolporate, heterocolpate (usually with three pseudocolpi or
intercolpate depressions alternating with apertures), shed as monads,
bicellular at dispersal. Exine tectate, with columellate infratectum,
perforate, punctate, rugulate or smooth.
Gynoecium Pistil composed of
(two to) four or five (to 14) connate carpels. Ovary inferior, unilocular to
quinquelocular, with antepetalous locules. Style single, simple. Stigma
punctate to capitate?, papillate?, Wet type. Pistillodium?
Ovules Placentation free
central (when ovary unilocular) or axile-basal (when ovary multilocular).
Ovules (one or) two to 18 (or more) per carpel, usually campylotropous,
ascending, apotropous, bitegmic, crassinucellar. Micropyle bistomal, Z-shaped
(zig-zag). Outer integument usually approx. two (in Mouriri four or
five) cell layers thick. Inner integument approx. two cell layers thick.
Parietal tissue approx. three cell layers thick. Megagametophyte monosporous,
Polygonum type. Endosperm development ab initio nuclear. Endosperm
haustoria? Embryogenesis?
Fruit A one- or several-seeded
berry.
Seeds Aril absent. Operculum?
Exotestal cells longitudinally elongated (in Memecylon few sclerotic
hypodermal exotestal cells). Endotesta? Exotegmen fibrous. Endotegmen? Subhilum
massively sclerotic. Perisperm developed, starchy? Endosperm absent. Embryo
usually large, well differentiated, with chlorophyll. Cotyledons two, thick,
straight or curved, lobate (in Memecylon crumpled). Radicula curved.
Hypocotyl often elongating during seed development. Germination cryptocotylar
or phanerocotylar.
Cytology n = 7
DNA
Phytochemistry Insufficiently
known. Non-acylated anthocyanins and cyanogenic compounds present. Saponins not
found. Aluminium accumulated.
Use Carpentries, dyeing
substances.
Systematics Memecylon
(105–110; tropical regions in the Old World), Warneckea (35;
tropical Africa, Madagascar, Mauritius), Lijndenia (12; tropical
regions in the Old World), Spathandra (1–6; S. barteri,
S. blakeoides, S. roborea; tropical Africa, Madagascar),
Mouriri (82; tropical America), Votomita (10; tropical
America).
Memecylaceae are sister to Melastomataceae.
Pternandra in Melastomataceae was sister to
[Mouriri+Memecylon],
according to some ndhF analyses, a fact that should render Memecylaceae an ingroup in Melastomataceae.
 |
Phylogeny (maximum-likelihood tree) of Memecylaceae based on DNA
sequence data (Stone 2006).
|
de Jussieu, Gen. Plant.: 322. 4 Aug 1789
[’Myrti’], nom. cons.
Melaleucaceae Vest, Anleit. Stud. Bot.: 268, 286.
1818 [’Melaleucoideae’]; Baeckeaceae
Bercht. et J. Presl, Přir. Rostlin: 233. Jan-Apr 1820 [‘Bekeae’];
Eugeniaceae Bercht. et J. Presl, Přir. Rostlin 2:
378. 1825; Leptospermaceae Bercht. et J. Presl,
Přir. Rostlin 2: 378. 1825; Lythrales Link, Handbuch
2: 47. 4-11 Jul 1829 [‘Lythrariae’];
Chamelauciaceae DC. ex F. Rudolphi, Syst. Orb. Veg.:
55. 5-12 Jul 1830 [’Chamaelaucieae’];
Myrtineae Burnett, Outl. Bot.: 616, 617, 1092, 1128.
Jun 1835; Myrrhiniaceae Arn. in Ann. Nat. Hist. 3:
154. 1839 [’Olinieae, or Myrrhinieae’];
Heteropyxidaceae Engl. et Gilg, Syllabus, ed. 8: 281.
Jan-Feb 1920, nom. cons.; Kaniaceae Nakai,
Chosakuronbun Mokuroku [Ord. Fam. Trib. Nov.]: 245. 20 Jul 1943;
Psiloxylaceae Croizat, Principia Bot.: 604, 1161,
1164. Jun 1961
Genera/species
133/5.535–5.570
Distribution Tropical,
subtropical and warm-temperate regions on both hemispheres, with their largest
diversity in Australia and South America.
Fossils Myrtaceae are often found as fossils
in the Cenozoic of North America and Europe and fossil leaves and pollen grains
are frequently recorded from the Australian Cenozoic. Myrtaceidites
comprises pollen grains, possibly of Myrtaceae, from the Maastrichtian of
Australia and the Paleocene of New Zealand. Paleomyrtinaea, a probably
quinquelocular fruit with parietal placentation and C-shaped embryos, has been
described from the Late Paleocene of North Dakota and the Early Eocene of
British Columbia. Fossils assigned to Eucalyptus have been found in
Early Eocene strata in Patagonia (Argentina).
Habit Usually bisexual (rarely
andromonoecious, polygamomonoecious, dioecious, or androdioecious), usually
evergreen (rarely deciduous) trees or shrubs. Often aromatic. Bark often
exfoliating. Numerous species are xerophytes. Osbornia consists of
mangrove trees.
Vegetative anatomy
Ectomycorrhiza usually present (sometimes also endomycorrhiza; in
Eucalyptus arbuscular mycorrhiza). Phellogen ab initio superficial or
pericyclic. Cork tissue usually stratified. Polyderm present. Primary vascular
tissue usually bicollateral. Vessel elements usually with simple (rarely
scalariform or reticulate) perforation plates; lateral pits alternate, simple
or bordered pits. Vestured pits often present. Imperforate tracheary xylem
elements tracheids or fibre tracheids, with simple or bordered pits, usually
non-septate (rarely septate; sometimes also vasicentric). Wood rays uniseriate
or multiseriate, heterocellular or homocellular. Axial parenchyma apotracheal
diffuse or diffuse-in-aggregates, often banded, or paratracheal scanty,
aliform, lozenge-aliform, winged-aliform, confluent, vasicentric, unilateral,
or banded. Tyloses sometimes frequent. Intraxylary phloem usually present.
Secondary phloem in young stems usually stratified into hard fibrous and soft
parenchymatous layers. Sieve tube plastids Ss type; sieve tubes usually with
non-dispersive protein bodies. Nodes 1:?, unilacunar with ? leaf traces.
Sclerenchymatous idioblasts abundant. Schizolysigenous secretory cavities (in,
e.g., medulla and/or cortex) with ethereal oils. Heartwood often with gum-like
substances. Wood rays with or without silica bodies. Calciumoxalate as
prismatic or acicular crystals, styloids, crystal sand or other types of
crystals often abundant.
Trichomes Hairs usually
unicellular, uniseriate, simple (sometimes bi- or multicellular, furcate);
bristle-like glands present or absent.
Leaves Usually opposite
(rarely alternate, spiral, or verticillate), simple, entire, often coriaceous,
with ? ptyxis. Stipules rudimentary, modified into colleters or hairs, or
absent; leaf sheath absent. Petiole vascular bundle transection arcuate to
U-shaped, occasionally with ends incurved. Venation pinnate or parallelodromous
(rarely palmate; leaves sometimes one-veined). Stomata usually anomocytic
(sometimes paracytic). Cuticular wax crystalloids as platelets, chemically
characterized by presence of primary alcohols and triterpenoids, or as tubuli
dominated by β-diketones, or as parallel grouped (usually non-entire)
platelets (Hypericum type). Domatia in Heteropyxis as
pockets. Lamina with gland-dots and schizogenous secretory cavities with
ethereal oils. Sclerenchymatous idioblasts abundant. Epidermis with or without
mucilaginous idioblasts. Leaf margin entire. Extrafloral nectaries rarely
present in leaf axils.
Inflorescence Terminal or
axillary, usually paniculate (rarely racemose, spicate, capitate or corymbose;
flowers rarely solitary, axillary). Inflorescence sometimes pseudanthium with
involucre of showy bracts.
Flowers Usually actinomorphic
(rarely zygomorphic, e.g. in Calothamnus). Hypanthium present. Usually
epigyny or half epigyny (ovary adnate to receptacle entirely or only in lower
part; rarely almost hypogyny; in Heteropyxis and Psiloxylon
hypogyny). Sepals (three or) four or five (to eight), with usually imbricate
quinquncial (sometimes valvate) aestivation, free (sometimes reduced or
modified into caducous circumscissile calyptra; sepals in Verticordia
fringed). Petals (three or) four or five (to twelve), with imbricate
aestivation, often early caducous, clawed, free or connate at base (sometimes
modified into caducous circumscissile calyptra or absent). Nectariferous disc
inserted on apex of ovary or on prolonged hypanthium. Secondary pollen display
sometimes present.
Androecium Stamens usually c.
20 to more than 150 (sometimes eight to ten, rarely four or five), in one or
several whorls, alternisepalous or antesepalous, alternipetalous or
antepetalous, basically antepetalous. Filaments usually inflexed in bud
(sometimes upright or twice folded), free or often connate at base into four or
five usually antepetalous fascicles, or connate into tube, free from tepals,
inserted on hypanthium, often very long; centripetally or centrifugally
developing. Anthers dorsifixed, versatile, usually tetrasporangiate (rarely
monosporangiate or trisporangiate), introrse, latrorse or extrorse, usually
longicidal (dehiscing by longitudinal slits; rarely poricidal, dehiscing by
pores); connective usually with one or several terpene-producing apical
oil-glands. Tapetum secretory. Staminodia usually absent (present in female
flowers of Psiloxylon).
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually markedly triangular in
polar view, (2–)3(–4)-colporate or (2–)3(–4)-porate (rarely
(2–)3(–4)-colpate), often (seemingly) syncolporate, not heterocolpate
(pseudocolpi absent), shed as monads, bicellular at dispersal. Exine tectate or
semitectate, with columellate infratectum, reticulate or scabrate to
psilate.
Gynoecium Pistil composed of
usually two to five (rarely up to 18) connate antesepalous or antepetalous
carpels, or odd carpel abaxial. Ovary usually inferior or semi-inferior,
bilocular to quinquelocular (rarely up to 18-locular or unilocular and
pseudomonomerous). Style single, sunken, usually elongate, persistent, simple
or branched in upper part. Stigma usually single, capitate, punctate, trilobate
or quadrilobate (rarely peltate; stigmas sometimes three or four), Dry or Wet
type. Male flowers often with pistillodium.
Ovules Placentation usually
axile (rarely parietal to basal, when ovary unilocular). Ovules usually c. 30
to more than 150 (rarely one to 15) per carpel, anatropous, hemianatropous or
campylotropous, ascending, usually bitegmic (rarely unitegmic), crassinucellar.
Micropyle bistomal or endostomal (sometimes exostomal?). Outer integument two
to six (to twelve) cell layers thick. Inner integument two to four cell layers
thick. Obturator sometimes present. Hypostase absent. Parietal tissue two to
twelve cell layers thick. Nucellar cap absent. Megagametophyte usually
monosporous, Polygonum type (in Heteropyxis and
Psiloxylon disporous, Allium type). Synergids often with a
filiform apparatus. Antipodal cells sometimes not developing (with nuclei early
degenerating). Endosperm development ab initio nuclear. Endosperm haustoria?
Embryogenesis onagrad (or adventitious). Polyembryony frequent in several
genera.
Fruit Usually a loculicidal
capsule or a berry (rarely a septicidal or denticidal capsule or a pyxidium,
drupe, schizocarp, or nutlike fruit). Hypanthium usually accrescent in
fruit.
Seeds Aril absent. Operculum
often? present. Seed coat testal. Testa sometimes winged, often with
crystalliferous layers, sometimes with endotestal micropylar plug, sometimes
multiplicative, often partially sclerotic. Exotesta and/or endotesta often
thickened. Exotegmen usually absent or collapsed. Endotegmen often collapsed.
Perisperm not developed. Endosperm usually absent or scarce (with oils and
starch). Embryo straight, curved or spirally twisted, well differentiated, with
or without chlorophyll. Cotyledons two, accumbent or incumbent, often plicate,
often connate. Germination phanerocotylar or cryptocotylar.
Cytology n = (5–)11, (12,
22)
DNA Plastid gene infA
lost/defunct (Callistemon).
Phytochemistry Flavonols
(kaempferol, quercetin, myricetin), cyanidin, delphinidin, ethereal oils
(triterpenes etc.), ursolic acid, ellagic and gallic acids, piperidine,
pyrrolidine and pyrrolizidine alkaloids (polyhydroxyalkaloids, usually
frequent), saponins, and phenylalanine-derived cyanogenic compounds present.
Many species produce gum. Raffinose and stachyose in phloem exudates. Aluminium
accumulated in some species.
Use Ornamental plants, fruits
(Psidium, Feijoa, Campomanesia, Eugenia,
etc.), spices (Eucalyptus, Pimenta, Syzygium), gums,
essential oils for perfumes, medicinal plants (Eucalyptus), timber,
paper pulp (Eucalyptus), drainage of swamps (Eucalyptus,
Metrosideros).
Systematics Myrtaceae are sister to Vochysiaceae.
The clade [Heteropyxis+Psiloxylon] is
sister to the remaining Myrtaceae.
Psiloxyloideae
(Croizat) Schmid in Taxon 29: 559. 14 Nov 1980
2/4. Central and southeastern Africa,
the Mascarene Islands. Dioecious trees or shrubs. Leaves alternate (spiral).
Petals clawed. Stamens erect in bud, not fasciculated, with separate leaf
traces. Anthers with thecae separately dehiscing. Female flowers with
staminodia. Pistil composed of (two or) three connate carpels. Ovary superior,
with narrow base. Male flowers with pistillodium. Megagametophyte disporous,
8-nucleate, Allium type. Endotestal cells periclinally elongate,
crystalliferous. x = 12. Tannins abundant or absent.
Heteropyxis
1/3. Heteropyxis (3; H.
canescens, H. dehniae, H. natalensis; central and
southeastern Africa). – Usually dioecious (sometimes monoecious), deciduous
aromatic trees or shrubs. Cork tissue not stratified. Pits bordered.
Vasicentric tracheids absent. Axial (apotracheal) xylem parenchyma absemt.
Fibres non-septate. Crystalloids as platelets; rhomboidal crystals only in wood
ray cells. Leaves simple, entire. Stipules minute. Cuticular wax crystalloids
as small platelets. Lamina with domatia and superepidermal secretory cavities
containing ethereal oils. Inflorescence terminal panicle. Flowers
actinomorphic, small. Hypanthium present. Hypogyny. Sepals (four or) five,
persistent. Petals (four or) five, gland-dotted, caducous, free,
alternisepalous, inserted on inner margin of hypanthium. Nectariferous disc
present in lower part of hypanthium. Stamens (four to) five to eight (to ten),
antesepalous (sometimes also one, two or three antepetalous), usually
obdiplostemonous, erect in bud. Filaments free from each other and from tepals,
not in fascicles. Anthers dorsifixed, versatile?, tetrasporangiate, usually
latrorse (rarely introrse), longicidal (dehiscing by longitudinal slits);
connective with one to three secretory cavities. Female flowers with (four or)
five to eight (to ten) staminodia. Pollen grains resembling those in
Myrtoideae, syncolporate, with depressions between colpi, not
heterocolpate (pseudocolpi absent). Pistil composed of (two or) three connate
carpels. Ovary bilocular (or trilocular). Style sunken into ovary. Stigma
capitate, persistent. Placenta axile. Ovules numerous, hemianatropous. Fruit a
loculicidal capsule (partially enclosed by persistent calyx). Testa with apical
wing (sometimes one at micropyle). Exotesta with tangentially elongate cells
with scalariform to reticulately thickened walls. Exotegmic cells elongate.
Endosperm absent. Embryo straight. Terpenes not found.
Psiloxylon
1/1. Psiloxylon (1; P.
mauritianum; Mauritius, Réunion). – Monoecious or dioecious (rarely
polygamodioecious), evergreen tree with white bark. Phellogen ab initio
pericyclic. Young stem with secretory canals. Intraxylary phloem present.
Secondary lateral growth from normal cylindrical cambium. Xylem without fibre
tracheids. Axial parenchyma usually scarce paratracheal. Wood fibres septated,
with rhomboidal crystals. Hairs absent. Leaves seemingly alternate, simple,
entire. Stipules modified into colleters; leaf sheath absent. Lamina
gland-dotted, with schizogenous secretory cavities not containing ethereal
oils. Inflorescence axillary panicle. Flowers actinomorphic. Pedicel
articulated. Hypanthium present. Hypogyny. Sepals four or five (or six), with
imbricate aestivation, coriaceous, persistent, free. Petals four or five (or
six), with imbricate aestivation, shortly clawed, coriaceous, caducous,
punctate, free. Stamens (4+4 or) 5+5 (or 6+6), twice as many as petals,
diplostemonous, erect in bud. Filaments inserted at perigynous nectariferous
disc, free from each other and from tepals. Anthers dorsifixed, versatile,
tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits).
Female flowers with staminodia. Pollen grains tricolporate, parasyncolporate,
?-cellular at dispersal. Exine rugulate. Pistil composed of three or four
connate carpels. Ovary trilocular (or quadrilocular), often stipitate. Style
very short, sunken into ovary, or absent. Stigma large, fleshy, usually
trilobate (sometimes bilobate or quadrilobate), persistent in fruit. Male
flowers with pistillodia. Placentation axile. Ovules c. 30 to c. 70 per carpel,
anatropous or hemicampylotropous. Fruit a many-seeded berry, gland-dotted.
Exotestal cells large. Exotegmen crushed. Endosperm absent. Embryo well
differentiated, fleshy, straight. Cotyledons two. Ethereal oils and
polyhydroxyalkaloids present.
Myrtoideae Sweet, Fl.
Australas.: ad t. 10. 1 Aug 1827 [‘Myrteae’]
131/5.530–5.565. Trees or shrubs.
Ectomycorrhiza often present (in species with dry fruits). Sieve tubes with
non-dispersive protein bodies. Hairs usually unicellular (sometimes
multicellular). Leaves usually opposite (sometimes alternate), with various
ptyxis. Stipules two or several, often modified into colleters, or absent.
Venation usually pinnate (rarely palmate). Stomata sometimes paracytic. Silica
sometimes present in leaf tissues. Hypanthium present (often operculate and
caducous). Usually epigyny (rarely hypogyny to half epigyny). Perianth
undifferentiated in some genera closely allied to Eucalyptus.
Calyx or entire perianth sometimes calyptrate, as caducous lid. Petals (absent
to) four or five (to twelve), often caducous. Stamens numerous, large, very
varying in number and shape (rarely five, as many as petals or in antepetalous
or antesepalous fascicles; rarely ten), principally antepetalous. Pollen grains
often syncolpate. Pistil composed of two (to 18) connate alternipetalous or
antepetalous carpels (sometimes with odd carpel abaxial). Ovary usually
inferior, with wide base, sometimes unilocular. Placentae thick. Transseptal
vascular bundles present. Style without small vascular bundles. Stigma punctate
to capitate (rarely peltate), Dry or Wet type. Placentation rarely basal.
Ovules one to four (to ten) per carpel, usually bitegmic (rarely unitegmic).
Usually a capsule (rarely a berry). Testa multiplicative, more or less
sclerotic or exotesta thickened (exotesta in species with capsule, sclerotised
testa in species with berry). Endotesta often thickened. Sclerotic palisade
cells sometimes present at micropyle. Embryo in Eugenia
undifferentiated. Polyhydroxy-alkaloids often abundant. Many species
gum-producing. – The phylogeny of Myrtoideae is largely unresolved
(Wilson & al. 2005). The clade
[Xanthostemoneae+Lophostemoneae] is sister group to the
remaining Myrtoideae. Syzygieae and Tristaneae form
a monophletic group. Lindsayomyrteae are probably sister to
[Chamelaucieae+Leptospermeae]. Myrteae and
Syzygieae have predominant baccate fruit and seeds with sclerotic
testa.
[Xanthostemoneae+Lophostemoneae]
Xanthostemoneae Peter
G. Wilson in P. G. Wilson et al., Plant Syst. Evol. 251: 14. 16 Mar 2005
3/c 50. Pleurocalyptus (2;
P. austrocaledonicus, P. pancheri; New Caledonia),
Purpureostemon (1; P. ciliatus; New Caledonia), Xanthostemon
(46–51; the Philippines, East Malesia to New Guinea, northern Australia to
eastern Queensland, Solomon Islands, New Caledonia). – Central and East
Malesia to New Guinea, northern Australia to eastern Queensland, Solomon
Islands, New Caledonia.
Lophostemoneae Peter
G. Wilson in P. G. Wilson et al., Plant Syst. Evol. 251: 14. 16 Mar 2005
4/7. Kjellbergiodendron (1;
K. celebicum; Sulawesi, New Guinea), Lophostemon
(4; L. confertus, L. grandiflorus, L. lactifluus,
L. suaveolens; southern New Guinea, northern and eastern Australia),
Welchiodendron (1; W. longivalve; New Guinea, northern
Queensland), Whiteodendron (1; W. moultonianum; Borneo). –
Borneo, Sulawesi, New Guinea, northern and eastern Australia.
The remaining
Myrtoideae
Osbornieae Peter G.
Wilson in P. G. Wilson et al., Plant Syst. Evol. 251: 14. 16 Mar 2005
1/1. Osbornia (1; O.
octodonta; coasts in the Philippines, East Malesia to New Guinea, northern
Australia to eastern Queensland, Palau). – Evergreen mangrove tree or shrub
without pneumatophores.
Melaleuceae Burnett,
Outlines Bot.: 712. Feb 1835
10/430–445. Beaufortia
(22; southwestern Western Australia), Callistemon
(c 50; Australia, Tasmania, New Caledonia), Calothamnus
(40–45; southwestern Western Australia), Conothamnus (3; C.
aureus, C. neglectus, C. trinervis; southwestern Western
Australia), Eremaea (16;
southwestern Western Australia), Lamarchea (2; L. hakeifolia,
L. sulcata; Western Australia, Northern Territory), Melaleuca
(290–300; Australia, Tasmania, New Caledonia, few species in tropical Asia to
islands in the Pacific), Petraeomyrtus (1; P. punicea;
northern Northern Territory), Phymatocarpus (3; P.
interioris, P. maxwellii, P. porphyrocephalus;
southwestern Western Australia), Regelia (5;
R. ciliata, R. cymbifolia, R. inops, R.
megacephala, R. velutina; southwestern Western Australia). –
Tropical Asia to islands in the Pacific, Australia, Tasmania, New Caledonia,
with their highest diversity in Australia.
Kanieae Peter G.
Wilson ex Reveal in Phytoneuron 2012-37: 217. 23 Apr
2012
9/c 60. Barongia (1; B.
lophandra; northeastern Queensland), Basisperma (1; B.
lanceolata; Papua New Guinea), Cloezia (6; C. aquarum,
C. artensis, C. buxifolia, C. deplanchei, C.
floribunda, C. glaberrima; New Caledonia), Kania (6;
K. microphylla, K. urdanetensis: the Philippines; K.
eugenioides, K. hirsutula, K. nettotensis, K.
platyphylla: New Guinea), Lysicarpus (1; L.
angustifolius; eastern Queensland), Mitrantia (1; M.
bilocularis; northeastern Queensland), Ristantia (3; R.
gouldii, R. pachysperma, R. waterhousei; northeastern
Queensland), Sphaerantia (2; S. chartacea, S.
discolor; northeastern Queensland), Tristaniopsis (c 40;
Southeast Asia, Malesia to New Guinea, eastern Australia). – Southeast Asia,
Malesia to New Guinea, eastern Australia.
Backhousieae Peter G.
Wilson in P. G. Wilson et al., Plant Syst. Evol. 251: 15. 16 Mar 2005
2/15. Backhousia (13; eastern
Queensland, eastern New South Wales), Choricarpia (2; C.
leptopetala, C. subargentea; eastern Queensland, eastern New
South Wales; in Backhousia?). – Eastern Queensland, eastern New
South Wales.
Metrosidereae Peter G.
Wilson in P. G. Wilson et al., Plant Syst. Evol. 251: 15. 16 Mar 2005.
1/55–60. Metrosideros
(55–60; East Malesia to New Guinea, northeastern Queensland, eastern New
South Wales, Solomon Islands, New Caledonia, Lord Howe, New Zealand, Fiji, the
Bonin Islands, the Tuamotus, the Hawaiian Islands, one species, M.
angustifolia, in Western Cape in South Africa, one species, M.
stipularis, in southern Chile and southern Argentina). – South Africa,
East Malesia, eastern Australia, Melanesia, New Zealand, Polynesia, temperate
South America.
[Syzygieae+Tristanieae]
Syzygieae Peter G.
Wilson in P. G. Wilson et al., Plant Syst. Evol. 251: 15. 16 Mar 2005
2/>500. Anetholea (1; A.
anisata; New South Wales), Syzygium
(>500; southeastern Africa, Madagascar, tropical and subtropical Asia,
northern and eastern Australia, Lord Howe, islands in the Pacific to Samoa).
– Tropical and subtropical regions in the Old World to the Pacific.
Tristanieae Peter G.
Wilson in P. G. Wilson et al., Plant Syst. Evol. 251: 15. 16 Mar 2005
3/26–28. Thaleropia (3;
T. hypargyrea, T. iteophylla, T. queenslandica; New
Guinea, northeastern Queensland), Tristania (1; T.
neriifolia; southeastern Queensland, eastern New South Wales),Xanthomyrtus
(22–24; Borneo, the Philippines, Sulawesi, the Moluccas, New Guinea, the
Bismarck Archipelago, one species, X. kanalaensis, in New Caledonia).
– Malesia to New Guinea, eastern Queensland, eastern New South Wales, New
Caledonia.
Myrteae (A. P. de
Candolle) DC. in D. F. L. von Schlechtendal in Linnaea 2: 504. Jul 1827
49/2.630–2.640. Myrtastrum
(1; M. rufopunctatum; New Caledonia); Gossia (37; New Guinea,
eastern Queensland, eastern New South Wales, New Caledonia), Uromyrtus
(23; Borneo to New Guinea, eastern Queensland, northeastern New South Wales,
New Caledonia), Rhodamnia (c 40; Southeast Asia, Hainan, Malesia to
New Guinea, northern and eastern Australia, Solomon Islands, New Caledonia),
Decaspermum (33; tropical Asia to New Guinea, eastern Queensland,
northeastern New South Wales and islands in western Pacific),
Austromyrtus (4; A. dulcis, A. glabra, A.
lotoides, A. tenufolia; southeastern Queensland, eastern New
South Wales), Rhodomyrtus (c 20; tropical Asia, Malesia to New Guinea,
eastern Queensland, eastern New South Wales, New Caledonia),
Octamyrtus (6; O. arfakensis, O. behrmannii, O.
glomerata, O. insignis, O. pleiopetala: Aru Islands to
New Guinea; O. halmaherensis: Halmahera), Kanakomyrtus (6;
K. dawsoniana, K. longipetiolata, K. mcphersonii,
K. myrtopsidoides, K. prominens, K. revoluta; New
Caledonia), Archirhodomyrtus (5; A. baladensis, A.
paitensis, A. turbinata, A. vieillardi: New Caledonia;
A. beckleri: eastern Queensland, northeastern New South Wales,),
Pilidiostigma (6; P. glabrum, P. papuanum, P.
rhytispermum, P. sessile, P. tetramerum, P.
tropicum; eastern Queensland, northeastern New South Wales);
Accara (1; A. elegans; Brazil), Calycolpus (15;
Central America, tropical South America), Chamguava (3; C.
gentlei, C. musarum, C. schippii; southern Mexico,
Central America), Myrtus (2; M. communis: the European
Mediterranean; M. nivellei: North Africa); Blepharocalyx (4;
B. cruckshanksii, B. eggersii, B. myriophyllus,
B. salicifolius; the West Indies to southern Chile); Mosiera
(c 30; Central America), Myrrhinium (1; M. atropurpureum;
tropical South America), Hottea (c 9; Cuba, Hispaniola),
Calyptrogenia (6; C. biflora, C. bracteosa, C.
cuspidata, C. ekmanii, C. grandiflora, C.
jeremiensis; Hispaniola, Jamaica; in Hottea?), Psidium
(c 100; southern United States, Mexico, Central America, the West Indies,
tropical South America); Algrizea (2; A. macrochlamys, A.
minor; Bahia in Brazil), Myrciaria (26; southern Mexico, Central
America, the West Indies, tropical South America), ‘Plinia’
(65–70; Central America, the West Indies, tropical South America;
paraphyletic), Neomitranthes (15; northeastern to southwestern Brazil;
in Plinia?), Siphoneugena (11; Central America, the West
Indies, tropical South America; in Plinia?); ‘Myrcia’ (c
770; Mexico, Central America, the West Indies, tropical South America, with
their largest diversity in southeastern Brazil; paraphyletic; incl.
Calyptranthes?, Marlierea?, Mitranthes?),
Calyptranthes (c 100; Florida, Mexico, Central America, the West
Indies, tropical South America; in Myrcia?), Marlierea (c 90;
Costa Rica, Puerto Rico, tropical South America; in Myrcia?),
Mitranthes (8; M. clarendonensis, M. glabra, M.
langsdorfii, M. macrophylla, M. maxonii, M.
nivea, M. ottonis, M. urbaniana; Jamaica, one species,
M. ottonis, on Cuba; in Myrcia?); Amomyrtus (2;
A. luma, A. meli; Chile, Argentina), Luma (2; L.
apiculata, L. chequen; Chile, Argentina), Myrceugenia (c
45; central and southeastern Brazil, Chile, Juan Fernandez, Argentina);
Ugni (4; U. candollei, U. molinae, U.
myricoides, U. selkirkii; Mexico, Central America, tropical South
America), Myrteola (3; M. acerosa, M. nummularia,
M. phylicoides; southern South America, tropical American mountains,
Juan Fernandez, Falkland Islands), Lenwebbia (2; L.
lasioclada, L. prominens; northeastern Queensland, eastern New
South Wales), Lophomyrtus (2; L. bullata, L.
obcordata; New Zealand; incl. Neomyrtus?), Neomyrtus (1;
N. pedunculata; New Zealand incl. Stewart Island; in
Lophomyrtus?); Curitiba (1; C. prismatica; Planalto in
southeastern Brazil), Legrandia (1; L. concinna; Chile),
Acca (3; A. lanuginosa, A. macrostema, A.
sellowiana; tropical South America), Campomanesia (c 40; tropical
South America), Pimenta (18; tropical America); Hexachlamys
(c 15; tropical South America), Myrcianthes (35–40; southern Mexico,
Central America, the West Indies, tropical South America), Eugenia (c
1000; tropical regions on both hemispheres, with their highest diversity in
tropical America). – Unplaced Myrteae
Amomyrtella (2; A. guili, A. irregularis; Ecuador,
Bolivia, northwestern Argentina), Lithomyrtus (11; New Guinea,
northern Australia), Myrtella (2; M. beccarii: New Guinea,
Solomon Islands; M. bennigseniana: New Guinea, the Caroline Islands,
Guam). – Pantropical, with their highest diversity in tropical America.
Eucalypteae (Benth. et
Hook. f.) Peter G. Wilson in P. G. Wilson et al., Plant Syst. Evol. 251: 16. 16
Mar 2005
7/>835. Allosyncarpia (1;
A. ternata; Arnhem Land in Northern Territory), Angophora
(14; Queensland, New South Wales, Victoria), Arillastrum (1; A.
gummiferum; southern New Caledonia), Corymbia (c
115; southern New Guinea, Australia, Tasmania), Eucalyptopsis (2;
E. alauda, E. papuana; Buru, New Guinea), Eucalyptus
(>800; Mindanao, Sulawesi, East Malesia to New Guinea, almost all species in
Australia incl. Tasmania), Stockwellia (1; S. quadrifida;
Atherton Tableland in northeastern Queensland). – The Philippines, Sulawesi,
the Moluccas, New Guinea, Australia, Tasmania, New Caledonia.
Syncarpieae Peter G.
Wilson in P. G. Wilson et al., Plant Syst. Evol. 251: 16. 16 Mar 2005
1/3. Syncarpia (3; S.
glomulifera, S. hillii, S. verecunda; eastern
Queensland, eastern New South Wales). – Trees.
[Lindsayomyrteae+[Leptospermeae+Chamelaucieae]]
Lindsayomyrteae Peter
G. Wilson in P. G. Wilson et al., Plant Syst. Evol. 251: 16. 16 Mar 2005
1/1. Lindsayomyrtus (1; L.
racemoides; the Moluccas, New Guinea, New Britain, northeastern
Queensland). – Tree.
[Leptospermeae+Chamelaucieae]
Leptospermeae (A. P.
de Candolle) DC. in D. F. L. von Schlechtendal in Linnaea 2: 504. Jul 1827
8/180–185. Agonis (5; A.
baxteri, A. flexuosa, A. fragrans, A.
theiformis, A. undulata; southwestern Western Australia),
Asteromyrtus (7; A. angustifolia, A. arnhemica,
A. brassii, A. lysicephala, A. magnifica, A.
symphyocarpa, A. tranganensis; southern New Guinea, northern
Australia), Homalospermum (1; H. firmum; coasts in
southwestern Western Australia), Kunzea (c 60;
southwestern Western Australia, southeastern Australia, Tasmania, one species,
K. ericoides, in New Zealand), ’Leptospermum’
(c 90; Southeast Asia, Malesia to New Guinea, Australia, Tasmania, New Zealand;
polyphyletic), Neofabricia (3; N. mjoebergii, N.
myrtifolia, N. sericisepala; northern Queensland),
Paragonis (1; P. grandiflora; southwestern Western
Australia), Pericalymma (4; P. crassipes, P.
ellipticum, P. megaphyllum, P. spongiocaule;
southwestern Western Australia), Taxandria (10–11; southwestern
Western Australia). – Southeast Asia, Malesia to New Guinea, Australia,
Tasmania, New Zealand.
Chamelaucieae (A. P.
de Candolle) DC. in D. F. L. von Schlechtendal in Linnaea 2: 504. Jul 1827
[‘Chamaelaucieae’]
30/>635. Actinodium
(1; A. cunninghamii; southwestern Western Australia; in
Darwinia?), Aluta (5; A. appressa, A.
aspera, A. maisonneuvei, A. quadrata, A. teres;
western and central Australia), Astartea (23; southwestern Western
Australia), Astus (4; A. duomilius, A. subroseus,
A. tetragonus, A. wittweri; southwestern Western Australia),
Babingtonia (12; Malesia to New Guinea, Western Australia,
southeastern South Australia, western Victoria, New Caledonia), Baeckea (22;
Australia, Tasmania, New Caledonia, one species, B. frutescens,
extending to Southeast Asia and China), Balaustion (1; B.
pulcherrimum; southwestern Western Australia), Calytrix (c
105; Australia, Tasmania, with their highest diversity in southwestern Western
Australia), Chamelaucium
(13; southwestern Western Australia), Corynanthera (1; C.
flava; southwestern Western Australia), Darwinia (c
70; southern Western Australia, South Australia, Victoria, southern New South
Wales), Enekbatus (11; southwestern Western Australia),
Euryomyrtus (7; E. denticulata, E. inflata, E.
leptospermoides, E. maidenii, E. patrickiae, E.
ramosissima, E. recurva; western and southern Australia,
Tasmania), Harmogia (1; H. densifolia; southeastern
Queensland, northeastern New South Wales), Homalocalyx (11; Western
Australia, Arnhem Land in Northern Territory, southern Queensland),
Homoranthus (31; eastern Queensland, eastern New South Wales, southern
South Australia), Hypocalymma
(27; southwestern Western Australia), Kardomia (6; K.
granitica, K. jucunda, K. odontocalyx, K.
prominens, K. silvestris, K. squarrulosa; southeastern
Queensland, northeastern New South Wales), Malleostemon (13;
southwestern Western Australia), Micromyrtus (c 50; Australia),
Ochrosperma (6; O. adpressum, O. citriodorum, O.
lineare, O. obovatum, O. oligomerum, O.
sulcatum; southeastern Queensland, eastern New South Wales, Northern
Territory), Pileanthus
(8; southwestern Western Australia), Rinzia (18; southwestern Western
Australia), Sannantha (16; eastern Queensland, eastern New South
Wales, eastern Victoria), Scholtzia
(c 15; western and southwestern Western Australia), Seorsus (4; S.
aequatorius, S. clavifolius, S. intratropicus, S.
taxifolius; Borneo, Northern Territory, southwestern Western Australia),
Stenostegia (1; S. congesta; northern Northern Territory; in
Baeckea?),
Thryptomene
(c 50; southern, central and northeastern Australia, Tasmania),
Triplarina (7; T. bancroftii, T. calophylla, T.
imbricata, T. nitchaga, T. nowraensis, T.
paludosa, T. volcanica; eastern Australia), Verticordia
(>100; Western Australia, northern Northern Territory, with their highest
diversity in southwestern Western Australia). – Southeast Asia, southern
China, Malesia to New Guinea, Australia, Tasmania, New Caledonia, with their
largest diversity in southwestern Western Australia.
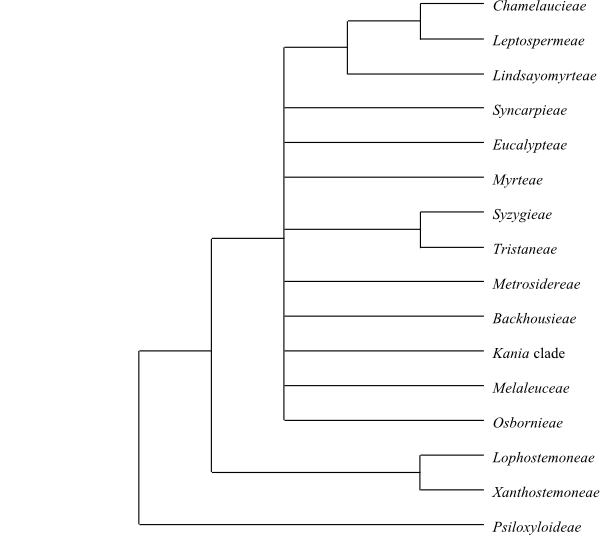 |
Cladogram (simplified) of Myrtaceae based on DNA
sequence data (Wilson & al. 2005). There is weak support for
Osbornieae being sister-group to Melaleuceae.
|
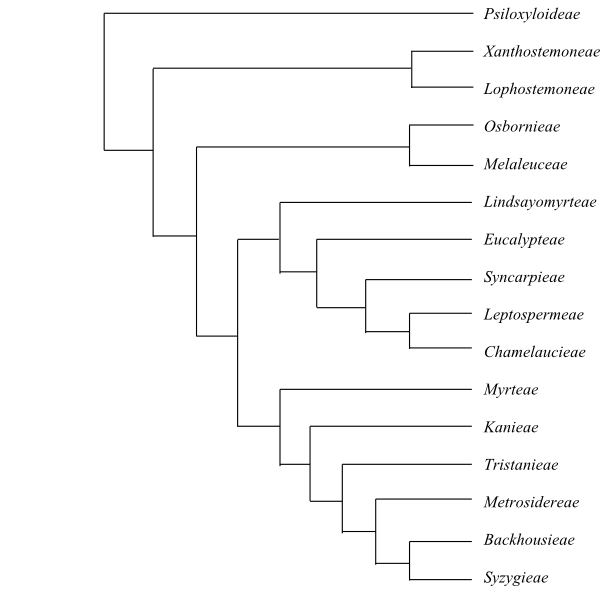 |
Phylogeny (simplified) of Myrtaceae
(Bayesian inference analysis) based on DNA sequence data (Thornhill & al. 2015)
|
Arnott in Ann. Nat. Hist. 3: 154. 1839
[’Olinieae or Myrrhinieae’], nom. cons.
Plectroniaceae Hiern,
Cat. Afr. Pl. Welw. 1(2): 369. Mar 1898
Genera/species 1/10
Distribution Southern and
eastern Africa.
Fossils Unknown.
Habit Bisexual, evergreen
shrubs. Young stems and branches quadrangular in cross-section or terete. Inner
bark usually almond-scented.
Vegetative anatomy Phellogen
ab initio subepidermal. Primary medullary rays narrow. Vessel elements diffuse,
with simple perforation plates; lateral pits alternate, bordered pits. Vestured
pits present in vessels. Imperforate tracheary xylem elements libriform fibres
with simple pits, septate. Wood rays uniseriate or multiseriate,
heterocellular. Axial parenchyma paratracheal scanty (apotracheal parenchyma
absent). Sieve tube plastids S type? Nodes 1:1, unilacunar with one leaf trace.
Intraxylary phloem present. Cortex with branched sclereids. Calciumoxalate
raphides absent.
Trichomes Hairs unicellular or
absent; glandular hairs present or absent.
Leaves Opposite or
verticillate, simple, entire, coriaceous, with ? ptyxis. Stipules very small,
rudimentary, cauline or inserted at leaf base, or absent; leaf sheath absent.
Petiole vascular bundle transection? Venation pinnate; midvein with apical
swelling or glandular tip. Stomata paracytic or anomocytic (or cyclocytic).
Cuticular wax crystalloids? Domatia as pits or absent. Terminal sclereids
present. Mesophyll with calciumoxalate as solitary prismatic crystals. Leaf
margin entire.
Inflorescence Terminal or
axillary, thyrsoid-paniculate.
Flowers Actinomorphic, small.
Hypanthium tubular, with (four or) five apical teeth (representing epicalyx? or
calyx?). Epigyny. Sepals (four or) five, with open (imbricate?) aestivation,
petaloid, spatulate, alternate with hypanthial teeth, caducous, free. Petals
(four or) five, with valvate aestivation, scale-like, concave, thick, hairy,
inserted on adaxial side of hypanthial margin, free. Nectariferous disc absent.
Idioblasts containing calciumoxalate druses abundant.
Androecium Stamens (four or)
five, obhaplostemonous, alternisepalous, antepetalous. Filaments short,
inserted below petals on adaxial side of hypanthial margin, inflexed in bud,
free from each other and from tepals. Anthers dorsifixed (basifixed?),
non-versatile, tetrasporangiate, introrse, longicidal (dehiscing by
longitudinal slits, microsporogenous tissue often divided into two packages by
band of tapetal cells); connective distally abaxially expanded; endothecium
ephemeral. Tapetum secretory, with binucleate cells. Staminodia four to ten, in
one or two whorls (one whorl sometimes rudimentary), extrastaminal, often
scale-like or petaloid.
Pollen grains
Microsporogenesis simultaneous. Pollen grains tricolporate, heteropolar (with
one pole colpate and one pole heterocolpate, with pseudocolpi limited to one of
two poles, half pseudocolpi), shed as monads, bicellular at dispersal; colpi
asymmetrical: each colpus consisting of one long segment on larger polar
surface and one short segment on smaller polar surface. Exine tectate, with
columellate infratectum, psilate.
Gynoecium Pistil composed of
(two to) five connate antesepalous carpels. Ovary inferior, usually
quinquelobate (rarely bilobate to quadrilobate). Transseptal vascular bundles
present. Style single, simple, subulate. Stigma capitate to clavate, papillate
(sometimes commissural), Wet type? Pistillodium absent?
Ovules Placentation axile.
Ovules two to ten per carpel, campylotropous, pendulous, apotropous, bitegmic,
crassinucellar. Micropyle bistomal. Outer integument approx. five cell layers
thick. Inner integument ? cell layers thick. Hypostase present. Parietal tissue
three or four cell layers thick. Chalaza strongly vascularized. Megagametophyte
monosporous, Polygonum type. Endosperm development ab initio nuclear.
Endosperm haustoria? Embryogenesis?
Fruit A single- or
several-seeded drupe.
Seeds Aril absent. Testa
thick, three- or four-layered, vascularized. Exotegmen fibrous. Endotegmen?
Perisperm not developed. Endosperm absent. Embryo straight, well
differentiated, without suspensor, chlorophyll? Cotyledons two, spirally
twisted or irregularly folded. Germination?
Cytology n = 12, c. 15, c. 20
– Polyploidy probably occurring.
DNA
Phytochemistry Insufficiently
known. Non-hydrolyzable tannins and phenylalanine-derived cyanogenic compounds
(prunasine, an almond-smelling cyanogenic glycoside) present. Aluminium not
accumulated.
Use Medicinal plants,
timber.
Systematics Olinia
(10; South Africa to Ethiopia and Angola).
Olinia is sister to Penaeaceae.
de Jussieu, Gen. Plant.: 317. 4 Aug 1789
[’Onagrae’], nom. cons.
Epilobiaceae Vent.,
Tabl. Règne Vég. 3: 307. 5 Mai 1799 [‘Epilobianae’];
Oenotheraceae C. C. Robin, Voy. Int. Louisiane 3:
489. 14-21 Dec 1807 [’Oenothera’];
Circaeaceae Bercht. et J. Presl, Přir. Rostlin: 232.
Jan-Apr 1820 [‘Circeaceae’];
Isnardiaceae Martinov, Tekhno-Bot. Slovar: 348. 3 Aug
1820 [’Isnardiae’]; Jussiaeaceae
Martinov, Tekhno-Bot. Slovar: 350. 3 Aug 1820 [’Jussieae’];
Onagrales Juss. ex Bercht. et J. Presl, Přir.
Rostlin: 232. Jan-Apr 1820 [‘Onagrae’];
Circaeales Lindl. in C. F. P. von Martius, Consp.
Regn. Veg.: 48. Sep-Oct 1835 [‘Circaeaceae’];
Epilobiales Vent. in C. F. P. von Martius, Consp.
Regn. Veg.: 48. Sep-Oct 1835 [’Epilobiaceae’];
Oenotherales Bromhead in Edinburgh New Philos. J. 24:
409. Apr 1838; Onagrineae Rchb., Deutsch. Bot.
Herb.-Buch: lxviii. Jul 1841; Oenotheropsida Brongn.,
Enum. Plant. Mus. Paris: xxx, 117. 12 Aug 1843 [’Oenotherineae’];
Fuchsiaceae (DC.) Lilja, Skånes Fl., ed. 2: 846,
980. Apr-Dec 1870; Lopeziaceae (Spach) Lilja, Skånes
Fl., ed. 2: 980. Apr-Dec 1870
Genera/species 22/725–750
Distribution: Cosmopolitan
except Antarctica, with their largest diversity in southwestern North
America.
Fossils Seeds of
Ludwigia have been found in Oligocene layers onwards in Europe and
Asia, and Fuchsia pollen is recorded from the Eocene of Argentina.
Seeds of Palaeeucharidium from the Eocene London Clay, southern
England, may be a representative of Onagraceae.
Habit Usually bisexual (rarely
unisexual), usually perennial, biennial or annual herbs (rarely trees or
shrubs, e.g. in Fuchsia). Some species are aquatic.
Vegetative anatomy Phellogen
ab initio deeply seated. Polyderm present. Endodermis sometimes prominent.
Primary vascular tissue usually bicollateral. Secondary lateral growth normal
or anomalous (from concentric cambia or from cylindrical cambium). Vessel
elements with simple perforation plates; lateral pits alternate, usually
bordered (sometimes simple) pits. Vestured pits present. Imperforate tracheary
xylem elements libriform fibres with simple or bordered pits, septate or
non-septate. Wood rays uniseriate or multiseriate, homocellular or
heterocellular. Axial parenchyma apotracheal banded, or paratracheal scanty
vasicentric, or absent. Tyloses sometimes frequent. Interxylary phloem often
present. Sieve tube plastids Ss type. Nodes 1:1, unilacunar with one leaf
trace. Epidermis often with cells containing ethereal oils. Calciumoxalate
raphides, prismatic crystals and styloids often present.
Trichomes Hairs unicellular or
multicellular simple (sometimes with widened base); glandular hairs often
frequent.
Leaves Usually opposite
(sometimes alternate, rarely verticillate), simple, usually entire (rarely
lobed), with involute to flat ptyxis. Stipules usually small, intrapetiolar,
caducous, or absent (in Ludwigia often conspicuous); leaf sheath
absent. Petiole vascular bundle transection arcuate. Venation pinnate. Stomata
with three or more subsidiary cells, anomocytic, anisocytic, tetracytic or
cyclocytic. Cuticular wax crystalloids as rosettes of platelets
(Fabales type) or transversely ridged rodlets, chemically dominated by
ketones. Epidermis with or without mucilaginous idioblasts. Mesophyll without
sclerenchymatous idioblasts. Calciumoxalate raphides abundant. Leaf margin
serrate or entire.
Inflorescence Usually terminal
(rarely axillary), panicle (rarely cyme), raceme or spike, or flowers solitary
axillary. Floral prophylls (bracteoles) sometimes absent.
Flowers Usually actinomorphic
(sometimes zygomorphic). Hypanthium usually present (absent in
Ludwigia). Epigyny or half epigyny. Sepals (two to) four (to seven),
with valvate aestivation, inserted on margin of hypanthium, free, usually
caducous. Petals (two to) four (to seven; in Circaea two; in
Lopezia two adaxial petals recurved, with pseudonectaries on claws),
with imbricate, valvate or contorted aestivation, inserted on margin of
hypanthium, often clawed (rarely absent), free, caducous. Nectaries present on
adaxial side of hypanthium. Disc intrastaminal, annular, on top of ovary, or
absent.
Androecium Stamens usually 4+4
or 6+6 (in Lopezia one fertile and one sterile stamen; in
Circaea two antesepalous stamens). Filaments free from each other and
from tepals, inserted on adaxial side of hypanthium or around epigynous
nectariferous disc. Anthers dorsifixed, versatile, tetrasporangiate or
polythecate, usually introrse (in Lopezia extrorse), longicidal
(dehiscing by longitudinal slits). Tapetum secretory. Staminodia one to four
(in Lopezia one petaloid), or absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually strongly triangular,
(2–)3(–6)-pororate, (2–)3(–6)-colpate or (2–)3(–6)-colporate, with
bulging apertures, starchy, usually covered by viscin threads of various types
(annular-vermiform, moniliform, smooth, etc.), not heterocolpate (pseudocolpi
absent), shed as monads, tetrads or polyads, bicellular at dispersal. Exine
tectate, with columellate or acolumellate infratectum, verrucate, granulate or
smooth. Ectexine with paracrystalline bodies.
Gynoecium Pistil composed of
(two to) four (to seven) connate alternisepalous carpels. Ovary inferior or
semi-inferior, (unilocular to) quadrilocular (to septalocular). Style single,
simple. Stigma single, capitate to quadrilobate (rarely commissural), papillate
or non-papillate, Dry or Wet type. Male flowers often with pistillodium.
Ovules Placentation usually
axile (rarely parietal). Ovules usually few to many (rarely one or two) per
carpel, anatropous, pendulous or ascending, bitegmic, crassinucellar. Micropyle
bistomal. Outer integument two to five cell layers thick. Inner integument two
or three cell layers thick. Hypostase usually present. Parietal tissue approx.
two (to c. 25) cell layers thick. Nucellar cap approx. two cell layers thick.
Archespore often multicellular. Megagametophyte quadrinucleate,
Oenothera type, with micropylar megaspore functional. Synergids with a
filiform apparatus. Antipodal cells not developed. Endosperm development ab
initio nuclear; endosperm diploid. Endosperm haustoria? Embryogenesis
onagrad.
Fruit Usually a loculicidal
(sometimes also septicidal) capsule dehiscing from apex downwards (in
Fuchsia a berry; in Circaea and species of Oenothera
a nutlet).
Seeds Aril absent. Seed coat
endotestal. Exotesta often densely hairy to papillate, with inner cell walls
thickened and lignified. Mesotestal cells sometimes thickened, sclerotic.
Endotestal cells stellate. Exotegmen often fibrous. Endotegmic cells sometimes
longitudinally elongate, tanniniferous, with inner walls thickened. Perisperm
not developed. Endosperm absent. Embryo straight or almost straight, well
differentiated, oily, without chlorophyll. Cotyledons two. Germination
phanerocotylar.
Cytology x = 7, 9–11, 18 –
Permanent reciprocal chromosomal translocations occurring in many
Onagreae (e.g. species of Oenothera, chromosomes forming ring
due to permanent translocations, entire genome inherited as one unit).
DNA Inversion of 45 kb present
in plastid genome in Oenothera sect. Oenothera. Plastid gene
clpP lost at least in Oenothera missouriensis. Plastid gene
infA lost in Oenothera.
Phytochemistry Flavonols
(kaempferol, quercetin, myricetin), flavonoid sulphates, delphinidin, ursolic
acid, ellagic acid, alkaloids, and cyanogenic compounds present. Saponins not
found. Raffinose and stachyose present in phloem exudates. Dissolved oxalates
accumulated.
Use Ornamental plants, seed
oils for medicinal purpose.
Systematics Onagraceae are sister-group to
Lythraceae.
Ludwigia
is sister to the remaining Onagraceae.
Jussiaeoideae
Beilschm. in Flora 16(Beibl. 7): 96, 108. 14 Jun 1833
[‘Jussiaceae’]
1/90–95. Ludwigia
(90–95; cosmopolitan, with their largest diversity in America). – Stipules
often prominent. Hypanthium absent. Flowers tetramerous or pentamerous. Pollen
grains usually shed as tetrads (rarely monads). Nectary present on apex of
ovary. Style short, with small vascular bundles. Stigma capitate. Hypostase
present. Parietal tissue three to six cell layers thick. Megasporocyte
single.
Onagroideae Beilschm.
in Flora 16(Beibl. 7): 96. 14 Jun 1833 [‘Onagrariae’]
21/635–655. Hauya (2; H.
elegans, H. heydeana; mountains in Central America); Circaea (7;
C. alpina, C. cordata, C. erubescens, C.
glabrescens, C. lutetiana, C. mollis, C.
repens; temperate regions on the Northern Hemisphere), Fuchsia
(110–115; New Zealand, Tahiti, Central and South America); Lopezia
(22; Mexico, Central America), Megacorax (1; M. gracielanus;
Durango in Mexico); Gongylocarpus (2; G. fruticulosus, G.
rubricaulis; Mexico, Central America); Epilobium
(210–215; temperate and arctic-alpine regions on both hemispheres, tropical
mountains, incl. Tasmania, New Zealand and adjacent islands, Tierra del Fuego,
with their highest diversity in western North America), Chamaenerion
(8; temperate regions on the Northern Hemisphere); Xylonagra (1;
X. arborea; Baja California in northwestern Mexico); Taraxia
(c 10; North America, Mexico), Clarkia
(40–45; western North America, Mexico, southern South America),
Gayophytum (9; western North America, western South America),
Chylismiella (1; C. pterosperma; southwestern United States);
Eulobus (4; E. angelorum, E. californicus, E.
crassifolius, E. sceptrostigma; California, Arizona, Baja
California in northwestern Mexico), Oenothera
(145–150; northwestern Europe, America, with their highest diversity in
western North America), Camissonia
(23; western North America to western South America), Eremothera (7;
E. boothii, E. chamaenerioides, E. gouldii, E.
minor, E. nevadensis, E. pygmaea, E. refracta;
western North America, western Mexico), Neoholmgrenia (2; N.
andina, N. hilgardii; western North America), Camissoniopsis
(14; southwestern United States, northwestern Mexico), Tetrapteron (2;
T. graciliflorum, T. palmeri; western United States,
northwestern Mexico). – Cosmopolitan, with their highest diversity in western
United States and northwestern Mexico. Intraxylary phloem present. Hypanthium
long, caducous. Flowers usually tetramerous (in Circaea
dimerous). Sepals reflexed, caducous. Stamens rarely one or two. Transseptal
vascular bundles present. Style sometimes short, often without vascular
bundles. Stigma usually entire (sometimes quadrilobate, Dry type). Parietal
tissue occasionally ten to c. 25 cell layers thick. Fruit sometimes a berry. n
= 5–11, 15, 18?
 |
Phylogeny of Onagraceae based on DNA
sequence data and morphology (Levin & al. 2003, 2004; Wagner &
Hoch 2005 onwards).
|
PENAEACEAE Sweet ex
Guill.
|
( Back to Myrtales )
|
Guillemin in J. B. G. M. Bory de Saint-Vincent,
Dict. Class. Hist. Nat. 13: 171. 1 Mar 1828, nom. cons.
Penaeales Lindl.,
Nix. Plant.: 15. 17 Sep 1833
Genera/species 7/23
Distribution Southwestern
South Africa, with their largest diversity in the southwestern parts of the
Western Cape Province.
Fossils Unknown.
Habit Bisexual, small,
evergreen, often procumbent shrubs with usually ericoid leaves (sometimes with
lignotubers). Many species are xerophytes.
Vegetative anatomy Phellogen
ab initio pericyclic. Cortical vascular bundles present or absent. Primary
medullary rays narrow. Polyderm present. Endodermis sometimes with large cells.
Vessel elements with simple perforation plates; lateral pits usually alternate
(rarely opposite), simple or bordered pits. Vestured pits present in vessels
and tracheids. Imperforate tracheary xylem elements usually tracheids (rarely
fibre tracheids) with bordered pits, non-septate. Wood rays uniseriate or
multiseriate, homocellular or heterocellular? Axial parenchyma paratracheal
scanty or vasicentric, sometimes diffuse (apotracheal parenchyma absent).
Intraxylary phloem present. Sieve tube plastids S type? Nodes 1:1, unilacunar
with one leaf trace. Calciumoxalate druses or crystal clusters often
present.
Trichomes Hairs usually absent
(unicellular simple hairs sometimes present).
Leaves Opposite, simple,
entire, coriaceous, often ericoid, with ? ptyxis. Stipules axillary,
rudimentary, colleter-like, or absent; leaf sheath absent. Petiole vascular
bundle transection? Venation pinnate, usually brochidodromous; midvein
sometimes with apical swelling or glandular apex. Stomata anomocytic. Cuticular
wax crystalloids?, sparse or absent. Mesophyll with fibres (sometimes with
spiral thickenings) and sclerenchymatous idioblasts with calciumoxalate druses.
Sclereids abundant. Leaf margin usually entire (in Sonderothamnus
serrate).
Inflorescence Axillary, cymose
or racemose, or flowers paired or solitary. Bracts often coloured, petaloid.
Floral prophylls (bracteoles) in one or several pairs.
Flowers Actinomorphic.
Hypanthium campanulate or cylindrical, persistent, often coloured. Half
epigyny. Sepals four, with simple-valvate or reduplicate-valvate (valvate with
recurved margins) aestivation, usually petaloid, persistent, free. Petals
absent. Nectar secreted from glands along lower part of adaxial side of
hypanthium. Disc absent.
Androecium Stamens four,
obhaplostemonous, alternisepalous. Filaments short, adnate to hypanthial
margin, sometimes inflexed in bud, free from each other and from tepals.
Anthers basifixed, sometimes versatile, tetrasporangiate, introrse, longicidal
(dehiscing by longitudinal slits); connective laminar, distally (abaxially)
expanded, often much longer than thecae; endothecium ephemeral. Tapetum
secretory, with finally binucleate cells. Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains 3–5(–10)-colporate,
heterocolpate (with pseudocolpi or intercolpate pits alternating with colpi),
shed as monads, bicellular at dispersal. Exine tectate, with columellate
infratectum, rugulate or psilate (sometimes punctate).
Gynoecium Pistil composed of
four connate antesepalous carpels. Ovary semi-inferior, quadrilocular. Style
single, simple, subulate or quadrangular in cross-section (in Penaea
and Stylapterus with four longitudinal commissural wings or ridges,
respectively, running from ovary to stylar apex). Stigma usually capitate or
quadrilobate, commissural (in Penaea and Stylapterus with
four subapical stigmatic areas in angles of wings or ridges, respectively), Wet
type? Pistillodium absent.
Ovules Placentation basal,
axile or basal-apical. Ovules two to four (to numerous) per carpel, anatropous,
ascending and/or pendulous, bitegmic, crassinucellar. Micropyle bistomal. Outer
integument ? cell layers thick. Inner integument two cell layers thick.
Parietal tissue three or four cell layers thick. Megagametophyte tetrasporous,
16-cellular, Penaea type. Endosperm development ab initio nuclear.
Chalazal endosperm haustorium present. Embryogenesis onagrad or asterad?
(Penaea).
Fruit A loculicidal capsule,
surrounded by persistent hypanthium.
Seeds Aril absent. Elaiosome
formed by funicle. Testa two-layered. Exotesta usually well developed
(sometimes crushed). Endotestal cells very prolonged; endotesta sometimes
collapsed and represented by crystalliferous layer. Exotegmen and endotegmen
fibrous or crushed. Perisperm not developed. Endosperm entirely absent or
almost absent. Embryo straight, well differentiated, without suspensor,
chlorophyll? Hypocotyl large. Cotyledons two, very small. Germination
phanerocotylar?
Cytology n = 10, c. 20
DNA
Phytochemistry Very
insufficiently known. Non-hydrolyzable tannins present. Aluminium not
accumulated.
Use Ornamental plants,
medicinal plants.
Systematics Endonema
(2; E. lateriflora, E. retzioides; southwestern Western
Cape); Glischrocolla (1; G. formosa; southwestern Western
Cape), Sonderothamnus
(2; S. petraeus, S. speciosus; southwestern Western Cape), Saltera (1;
S. sarcocolla; southwestern Western Cape), ’Brachysiphon’
(5; B. acutus, B. fucatus, B. microphyllus, B.
mundii, B. rupestris; southwestern Western Cape;
non-monophyletic), ’Stylapterus’ (8; S. barbatus, S.
candolleanus, S. dubius, S. ericifolius, S.
ericoides, S. fruticulosus, S. micranthus, S.
sulcatus; southwestern Western Cape; non-monophyletic), Penaea (4;
P. acutifolia, P. cneorum, P. dahlgrenii, P.
mucronata; southwestern and southern Western Cape, southern Eastern
Cape).
Penaeaceae are sister to
Oliniaceae.
Endonema is sister to the remaining
Penaeaceae and the clade
[Glischrocolla+Brachysiphon rupestris] is sister-group to the
remainder. Sonderothamnus,
Sarcocolla, and Brachysiphon mundii form a clade above these.
Penaea
appears to be monophyletic, although P. dahlgrenii is more closely
allied to Stylapterus ericoides (Schönenberger & Conti 2003).
 |
Cladogram of Penaeaceae based on DNA
sequence data (Schönenberger & Conti 2003).
|
RHYNCHOCALYCACEAE L. A.
S. Johnson et B. G. Briggs
|
( Back to Myrtales )
|
Johnson et Briggs in Ann. Missouri Bot. Gard.
71(1984): 732. 19 Apr 1985
Genera/species 1/1
Distribution Southeastern
South Africa.
Fossils Unknown.
Habit Bisexual, evergreen
trees. Young stems and branches terete or flattened in cross-section.
Vegetative anatomy Phellogen
ab initio cortical. Vessel elements with simple perforation plates; lateral
pits alternate, bordered pits. Vestured pits present in vessels. Imperforate
tracheary xylem elements libriform fibres usually with simple pits (almost
without bordered pits), septate. Wood rays multiseriate, heterocellular. Axial
parenchyma paratracheal scanty vasicentric (apotracheal parenchyma absent).
Phloem with scattered sclereids. Sieve tube plastids Ss type? Nodes 1:1,
unilacunar with one leaf trace. Calciumoxalate druses present. Fibres with
prismatic crystals.
Trichomes Hairs absent.
Leaves Opposite, simple,
entire, coriaceous, with ? ptyxis. Stipules rudimentary, colleter-like, on
petiole base, intrapetiolar (axillary) or absent; leaf sheath absent. Petiole
vascular bundle transection arcuate; petiole with branched sclereids. Venation
pinnate, eucamptodromous; midvein with apical gland. Stomata laterocytic,
intermediate between anomocytic and cyclocytic. Cuticular wax crystalloids?
Mesophyll without sclerenchymatous idioblasts. Leaf margin entire.
Inflorescence Usually terminal
(sometimes axillary), panicle.
Flowers Actinomorphic, small.
Hypanthium short. Hypogyny to almost half epigyny. Sepals (five or) six (or
seven), with valvate aestivation, pointed, persistent, free. Petals (five or)
six (or seven), with valvate? aestivation, alternisepalous, clawed, lobate,
caducous, free. Nectary absent. Disc absent.
Androecium Stamens (five or)
six (or seven), obhaplostemonous, alternisepalous, antepetalous. Filaments
long, flattened, inserted immediately below petals on adaxial margin of
hypanthium, inflexed in bud, free from each other and from tepals. Anthers
subbasifixed (basifixed to dorsifixed), versatile, tetrasporangiate
(microsporangia lateral), introrse, longicidal (dehiscing by longitudinal
slits); loculi opening separately; septum between two microsporangia of each
theca persistent also during anther dehiscence; connective little expanded
abaxially; endothecium ephemeral. Tapetum secretory, with finally binucleate
cells. Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains tricolporate, heterocolpate (with
pseudocolpi alternating with apertures), shed as monads, bicellular at
dispersal. Exine tectate, with columellate infratectum, perforate or
punctate.
Gynoecium Pistil composed of
two (or three) connate carpels. Ovary superior to semi-inferior, bilocular (or
trilocular), dorsiventrally compressed. Transseptal vascular bundles present.
Style single, simple, short, stout, with persistent lower part. Stigma capitate
to punctate, papillate, Wet type? Pistillodium absent.
Ovules Placentation parietal.
Ovules c. 15 to c. 20 (to c. 50?) per carpel, anatropous, horizontal, bitegmic,
crassinucellar. Micropyle endostomal. Outer integument ? cell layers thick.
Inner integument ? cell layers thick. Parietal tissue three or four cell layers
thick. Nucellar cap approx. three cell layers thick. Archespore multicellular.
Megagametophyte monosporous, Polygonum type. Endosperm development ab
initio nuclear. Endosperm haustoria? Embryogenesis onagrad.
Fruit An apically loculicidal
capsule, dorsiventrally compressed.
Seeds Aril absent. Seed coat
testal. Testa winged at micropylar end, membranous; all testal cells
persistent. Exotestal cells tanniniferous, with outer walls lignified.
Endotesta? Tegmen? Perisperm not developed. Endosperm absent. Embryo straight,
well differentiated, flattened, chlorophyll? Cotyledons two, folded.
Germination?
Cytology n = 10
DNA
Phytochemistry Very
insufficiently known. Myricetin not found. Aluminium accumulated.
Use Unknown.
VOCHYSIACEAE A.
St.-Hil.
|
( Back to Myrtales )
|
Saint-Hilaire, Mém. Mus. Natl. Hist. Nat. 6, 4:
265. 1820 [’Vochisieae’], nom. cons.
Vochysiales Link,
Handbuch 2: 60. 4-11 Jul 1829 [‘Vochysiaceae’]
Genera/species 7/200–210
Distribution Tropical West and
Central Africa, Central and tropical South America.
Fossils Unknown.
Habit Bisexual, usually
evergreen trees or shrubs (sometimes lianas, rarely herbs).
Vegetative anatomy Phellogen
ab initio superficial or pericyclic. Primary stem with bicollateral vascular
bundles. Medulla usually with sclerified vascular bundles (absent in
Callisthene and Qualea). Pericyclic fibres few or absent.
Secondary lateral growth normal or anomalous (via cylindrical cambium). Vessel
elements with simple perforation plates; lateral pits alternate, usually
bordered pits. Vestured pits present. Imperforate tracheary xylem elements
libriform fibres usually with simple pits (in Vochysia sometimes
bordered pits), septate or non-septate. Wood rays uniseriate or multiseriate,
homocellular or heterocellular. Axial parenchyma apotracheal banded, or
paratracheal scanty vasicentric, aliform, lozenge-aliform, winged-aliform,
confluent, scalariform, reticulate, unilateral, or banded. Tyloses sometimes
frequent. Premedullary diffuse interxylary phloem present in Erisma
and Erismadelphus. Sieve tube plastids S type. Nodes usually 1:1,
unilacunar with one leaf trace (in ‘Ruizterania’ clade of
Qualea 3:3, trilacunar with three traces); leaf traces proceeding
along stem before entering petiole. Medulla sometimes with secretory resinous
canals. Sclereids and mucilage cells present. Silica bodies often abundant.
Calciumoxalate crystals solitary prismatic, or in groups.
Trichomes Hairs usually
unicellular, uniseriate, simple or furcate (rarely multicellular, stellate),
often brown; glandular hairs?
Leaves Opposite or
verticillate, simple, entire, coriaceous, with conduplicate ptyxis. Stipules
small, cauline (in Qualea replaced by large colleter-like glands or
associated with extrafloral nectaries); leaf sheath absent. Petiole vascular
bundle transection? Venation pinnate, brochidodromous or eucamptodromous.
Stomata anomocytic or paracytic. Cuticular wax crystalloids as groups of
parallel platelets. Epidermis with or without mucilaginous idioblasts. Leaf
margin entire.
Inflorescence Terminal or
axillary, thyrse, panicle or raceme-like, composed of cincinni.
Flowers Transversely
zygomorphic or asymmetrical (sometimes appearing actinomorphic). Pedicel in
some species of Qualea with nectaries. Hypanthium absent. Epigyny
(Erismeae) or secondary hypogyny (ovary primarily epigynous). Sepals
five, usually with imbricate (sometimes cochlear) aestivation, unequal in size,
persistent, connate at base, one adaxial-lateral larger sepal with
nectariferous receptacular spur (three sepals in Korupodendron
petaloid), sometimes coiled. Petals usually one or five (rarely three or
absent), with contorted or imbricate aestivation, unequal in size, clawed,
caducous, free (Callisthene and Qualea with displaced petal).
Nectary present on sepal (see above). Disc absent.
Androecium Fertile stamen
single, straight, antepetalous (opposite abaxial lateral petal; in
Qualea and Callisthene offset and seemingly antesepalous),
principally obhaplostemonous. Filament free from tepal. Anther dorsifixed or
basifixed, non-versatile, tetrasporangiate, introrse, longicidal (dehiscing by
longitudinal slits). Tapetum secretory. Staminodia (one or) two (in
Erisma, most species of Vochysia and some species of
Qualea; in Salvertia and some species of Vochysia
four) or absent (in some species of Qualea).
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually tricolporate (rarely
syncolporate), not heterocolpate (pseudocolpi absent), shed as monads,
?-cellular at dispersal. Exine tectate or semitectate, with columellate
infratectum, reticulate or striate.
Gynoecium Pistil composed of
usually three (rarely four) connate carpels; median carpel adaxial. Ovary
inferior or secondarily superior (in mature flowers), unilocular
(Erismeae; in Erisma unilocular; at least in
Erismadelphus due to pseudomonomery: one or two carpels degenerating),
or trilocular. Style single, simple, sunken. Stigma terminal or lateral,
subcapitate to punctate, usually papillate (in Vochysia with
multicellular uniseriate hairs), Wet type. Pistillodium absent.
Ovules Placentation axile or
apical. Ovules one or two (Erismeae) to numerous per carpel,
hemianatropous, pendulous, epitropous, bitegmic, crassinucellar. Micropyle
bistomal (in Qualea exostomal?). Outer integument two or three cell
layers thick. Inner integument approx. two cell layers thick. Megagametophyte
monosporous, Polygonum type. Endosperm development ab initio nuclear.
Endosperm haustoria? Embryogenesis?
Fruit A loculicidal capsule or
one-seeded samaroid fruit (pseudosamara, Erismeae) with four
(Erisma) or five persistent calyx lobes modified into wings.
Seeds Aril absent. Testa
winged (wing in Qualea and Callisthene formed as extension of
testa; unilateral wing in Vochysia and Salvertia formed from
compressed hairs), thin, mesotesta sclerified?, endotestal cells thickened,
with pectine, mesotegmic cells fibrous, often with thickened walls, or testa
multiplicative, exotesta provided with thickened hairs. Some layers persistent.
Remaining testal layers and tegmen unorganized. Qualea and
Callisthene with endotesta containing crystalliferous layer, fibrous
exotegmen and tanniniferous endotegmen. In Vochysia inner integument
and inner crystalliferous layer of outer integument resorbed. Perisperm not
developed. Endosperm absent. Embryo straight, well differentiated, chlorophyll?
Cotyledons two, plicate or planoconvex (Erismeae). Germination?
Cytology n = 11, 12
DNA
Phytochemistry
5-deoxyflavonoids, ellagic acid and methylated ellagic acids, tannins, and
anthraquinones present. Aluminium accumulated in at least some species.
Use Timber, medicinal plants,
gums, seed oils (Erisma).
Systematics
Salvertieae Bill., Hist. Plant. 5: 100, 101. late
1873 vel prim. 1874. Salvertia (1; S. convallariodora;
southern Brazil). – Vochysieae Dumort., Anal. Fam.
Plant.: 41. 1829. Vochysia (105–110; Central America, tropical South
America). – ‘Qualeeae’
Qualea (c 60; Central America, tropical South America),
Callisthene (14; southern and central Brazil, northern Paraguay,
eastern Bolivia). – Erismeae Dumort., Anal. Fam.
Plant.: 41. 1829. Erisma (16–20; tropical South America),
Erismadelphus (1–2; E. exsul; tropical West and Central
Africa), Korupodendron (1; K. songweanum; Central Africa).
Vochysiaceae are sister to Myrtaceae.
There is no comprehensive phylogeny of Vochysiaceae available.
Erismeae are probably monophyletic. The phellogen is subepidermal to
cortical. The epigynous flower has a single carpel with one or two lateral to
apical ovules. The fruit is a pseudosamara (samaroid) with persistent and
accrescent calyx. The seed has an undifferentiated multilayered vascularized
testa.
Literature
Acosta Ramos Z. 2014. The genus
Plinia (Myrtaceae) in Cuba.
– Willdenowia 44: 269-277.
Adamson RS. 1910. Note on the roots of
Terminalia arjuna Bedd. – New Phytol. 9: 150-156.
Adjoud-Sadadou D, Halli-Hargas R. 2000.
Occurrence of arbuscular mycorrhiza on aged Eucalyptus. – Mycorrhiza
9: 287-290.
Ahmed B, Abbassi AM, Bano A, Khan KM. 1991.
Duabangoxylon pakistanicum sp. nov., a new taxon of Sonneratiaceae
from Ranikot Fort Area. – Pakistan J. Bot. 23: 55-61.
Ahmed M, Vardar Y. 1973. Distribution and
plasticity of Myrtus communis. – Phyton 15: 145-150.
Airy Shaw HK. 1949. Additions to the flora of
Borneo and other Malay Islands 20. The Myrtaceae of the Oxford University
Expedition to Sarawak, 1932. – Kew Bull. 4: 117-125.
Almeda F. 1977. Systematics of the
neotropical genus Centradenia (Melastomataceae). – J. Arnold
Arbor. 58: 73-108.
Almeda F. 1978. Systematics of the genus
Monochaetum (Melastomataceae) in Mexico and
Central America. – Univ. Calif. Publ. Bot. 75: 1-134.
Almeda F. 1989. Tessmannianthus, an
arborescent genus of Melastomataceae new to Panama. –
Ann. Missouri Bot. Gard. 76: 1-6.
Almeda F. 1990a. A third species of
Tessmannianthus (Melastomataceae: Merianieae) from
Panama. – Brittonia 42: 7-11.
Almeda F. 1990b. New species and new
combinations in Blakea and Topobea (Melastomataceae), with a historical
perspective on generic limits in the tribe Blakeeae. – Proc. Calif. Acad.
Sci. 46: 299-326.
Almeda F. 1993a. Stanmarkia, a new
genus of Melastomataceae from the
volcanic highlands of western Guatemala and adjacent Mexico. – Brittonia 45:
187-203.
Almeda F. 1993b. Pilocosta (Melastomataceae) revisited: a new
species, polyploidy, and the base chromosome number of the genus. – Novon 3:
311-316.
Almeda F. 1997a. Systematics of the Andean
genus Centradeniastrum (Melastomataceae). – BioLlania, Ed.
Espec. 6: 153-166.
Almeda F. 1997b. Chromosome numbers and their
evolutionary significance in some neotropical and paleotropical Melastomataceae. – BioLlania, Ed.
Espec. 6: 167-190.
Almeda F. 1997c. Chromosomal observations on
the Alzateaceae (Myrtales). – Ann. Missouri Bot. Garden
84: 305-308.
Almeda F. 1997d. Cytological and
nomenclatural notes on the Mesoamerican species of Aciotis (Melastomataceae). – Novon 7:
333-337.
Almeda F. 2000. The hexandrous species of
Topobea (Melastomataceae). – Proc. Calif.
Acad. Sci. 52: 97-109.
Almeda F, Chuang TI. 1992. Chromosome numbers
and their systematic significance in some Mexican Melastomataceae. – Syst. Bot. 17:
583-593.
Almeda F, Martins AB. 2001. New combinations
and new names in some Brazilian Microlicieae (Melastomataceae), with notes on the
delimitation of Lavoisiera, Microlicia, and
Trembleya. – Novon 11: 1-7.
Almeda F, Pacifico R. 2018. Neotropical
Poteranthera (Melastomataceae: Microlicieae)
revisited. – Syst. Bot. 43: 552-556.
Almeda F, Robinson OR. 2011. Systematics and
phylogeny of Siphanthera (Melastomataceae). – Syst. Bot.
Monogr. 93: 1-101.
Almeda F, Umaña Dodero G. 1991. A review of
the genus Triolena (Melastomataceae: Bertolonieae) in
Costa Rica. – Brittonia 43: 146-153.
Almeda F, Whiffin T. 1980.
Pilocosta, a new genus of tropical American Melastomataceae. – Syst. Bot. 5:
294-311.
Almeda F, Michelangeli FA, Viana PL. 2016.
Brasilianthus (Melastomataceae), a new monotypc
genus endemic to ironstone outcrops in the Brazilian Amazon. – Phytotaxa 273:
269-282.
Alwan A-RA. 1983. The taxonomy of
Terminalia (Combretaceae)
and related genera. – Ph.D. diss., University of Leicester, England.
Amarasinghe V, Graham SA, Graham A. 1991.
Trichome morphology in the genus Cuphea (Lythraceae). – Bot. Gaz. 152: 77-90.
Amorim AM, Goldenberg R, Michelangeli FA.
2009. A new species of Physeterostemon (Melastomataceae) from Bahia, Brazil,
with notes on the phylogeny of the genus. – Syst. Bot. 34: 324-329.
Amorim BS, Alves M. 2012. Myrtaceae from lowland Atlantic Forest
areas in the State of Pernambuco, Northeastern Brazil. – Phytotaxa 40:
33-54.
Anderson DMW, Bell PC. 1977. The composition
of the gum exudates from some Combretum species; the botanical
nomenclature and systematics of the Combretaceae. – Carbohydr. Res. 57:
215-221.
Andrade PM, Forni-Martins ER, Martins FR.
2007. Size structure and fertility in an Eriocnema fulva Naudin (Melastomataceae) population in
Southeastern Brazil. – Brazil. J. Biol. 67: 685-693.
Averett JE, Boufford DE. 1985. The flavonoids
and flavonoid systematics of Circaea (Circaeeae, Onagraceae). – Syst. Bot. 10:
363-373.
Averett JE, Graham SA. 1984. Flavonoids of Rhynchocalycaceae (Myrtales). – Ann. Missouri Bot. Gard. 71:
853-854.
Averett JE, Raven PH. 1984. Flavonoids of Onagraceae. – Ann. Missouri Bot. Gard.
71: 30-34.
Baas P. 1979. The anatomy of Alzatea
Ruiz et Pav. (Myrtales). – Acta Bot.
Neerl. 28: 156-158.
Baas P. 1981. A note on stomatal types and
crystals in the leaves of Melastomataceae. – Blumea 27:
475-479.
Baas P. 1986. Wood anatomy of Lythraceae: additional genera
(Capuronia, Haitia, Orias, and
Pleurophora). – Ann. Missouri Bot. Gard. 73: 810-819.
Baas P, Zweypfenning RCVJ. 1979. Wood anatomy
of the Lythraceae. – Acta Bot.
Neerl. 28: 117-155.
Backer CA, Steenis CGGJ van. 1951.
Sonneratiaceae. – In: Steenis CGGJ van (ed), Flora Malesiana I, 4(3),
Noordhoff-Kolff N. V., Batavia, pp. 280-289; 4(3), pp. 513-515.
Baker HG, Baker I. 1982. Starchy and
starchless pollen in the Onagraceae.
– Ann. Missouri Bot. Gard. 69: 748-754.
Bakhuizen van den Brink RC Jr. 1943. A
contribution to the knowledge of the Melastomataceae occurring in the
Malay Archipelago, especially in the Netherlands East Indies. – Rec. Trav.
Bot. Néerl. 40:1-391. [Reprinted in Meded. Bot. Mus. Herb. Rijksuniv. Utrecht
91(1947): 1-391.]
Balthazar M von, Schönenberger J. 2006. Oliniaceae. – In: Kubitzki K (ed), The
families and genera of vascular plants IX. Flowering plants. Eudicots. Berberidopsidales,
Buxales, Crossosomatales, Fabales p. p., Geraniales, Gunnerales, Myrtales p. p., Proteales, Saxifragales, Vitales, Zygophyllales, Clusiaceae Alliance, Passifloraceae
Alliance, Dilleniaceae, Huaceae, Picramniaceae, Sabiaceae, Springer, Berlin,
Heidelberg, New York, pp. 260-264.
Bamber RK. 1962. The anatomy of the barks of
Leptospermoideae. – Aust. J. Bot. 10:25-54.
Baranov PA. 1957. Coleorrhiza in Myrtaceae. – Phytomorphology 7:
237-243.
Barker WF. 1963. A new species of Penaeaceae. – J. South Afr. Bot. 29:
167-169.
Barlow BA. 1986. Contributions to a revision
of Melaleuca (Myrtaceae) 1-3.
– Brunonia 9: 163-177.
Barlow BA, Cowley KJ. 1988. Contributions to
a revision of Melaleuca (Myrtaceae) 4-6. – Aust. Syst. Bot. 1:
95-126.
Barlow BA, Forrester J. 1984. Variation in
indumentum morphology in the Melaleuca leucadendra complex (Myrtaceae). – Brunonia 7: 277-288.
Barrie FR. 2004. Synopsis of Plinia
(Myrtaceae) in Mesoamerica. – Novon
14: 380-400.
Barrie FR. 2005. Thirty-five new species of
Eugenia (Myrtaceae) from
Mesoamerica. – Novon 15: 4-49.
Barth OM, Barbosa AF. 1972. Catalogo
sistematico do polens das plantas arborea do Brasil meridional XV. Myrtaceae. – Mem. Inst. Oswaldo Cruz 70:
467-497.
Basinger JF, Greenwood DR, Wilson PG,
Christophel DC. 2007. Fossil flowers and fruits of capsular Myrtaceae from the Eocene of South
Australia. – Can. J. Bot. 85: 204-215.
Basso-Alves JP, Goldenberg R, Teixeira SP.
2017. Ontogeny elucidates the double calyx of Leandra melastomoides
(Miconieae, Melastomataceae). –
Intern. J. Plant Sci. 178: 740-752.
Baum DA, Sytsma KJ, Hoch PC. 1994. A
phylogenetic analysis of Epilobium (Onagraceae) based on nuclear ribosomal
DNA sequences. – Syst. Bot. 19: 363-388.
Baumgratz JFA. 1983-1985 [1988]. Morfología
dos frutos e sementes de Melastomatáceas brasileiras. – Arq. Jard. Bot. (Rio
de Janeiro) 27: 113-155.
Baumgratz JFA. 1989-1990. O gênero
Bertolonia Raddi (Melastomataceae): revisão
taxonômica e considerações anatômicas. – Arq. Jard. Bot. (Rio de Janeiro)
30: 69-213.
Baumgratz JFA. 2000. Two new species of
Huberia (Melastomataceae: Merianieae) from
Brazil. – Brittonia 52: 24-33.
Baumgratz JFA, D’El Rei Souza ML. 2007. A
new species of Leandra (Melastomataceae) from Brazil. –
Syst. Bot. 32: 743-747.
Baumgratz JFA, D’El Rei Souza ML, Woodgyer
EM, Nic Lughadha EM. 1996. Polysporangiate anthers: described for the first
time in Melastomataceae. – Kew
Bull. 51: 133-144.
Bayly MJ, Ladiges PY. 2007. Divergent
paralogues of ribosomal DNA in eucalypts (Myrtaceae). – Mol. Phylogen. Evol. 44:
346-356.
Bayly MJ, Udovicic F, Gibbs AK, Parra-O C,
Ladiges PY. 2008. Ribosomal DNA pseudogenes are widespread in the eucalypt
group (Myrtaceae): implications for
phylogentic analysis. – Cladistics 24: 131-146.
Bean AR. 1997a. Reinstatement of
Babingtonia Lindl. (Myrtaceae,
Leptospermoideae). – Austrobaileya 4: 627-646.
Bean AR. 1997b. A revision of
Baeckea (Myrtaceae) in eastern
Australia, Malesia and south-east Asia. – Telopea 7: 245-268.
Bean AR. 2004. Three new species of
Leptospermum (Myrtaceae) from
Queensland and northern New South Wales. – Telopea 10: 831-838.
Bean AR. 2008. Reinstatement of Ammannia
triflora Benth. (Lythraceae). –
Austrobaileya 74: 727-728.
Beardsell DV, Obrien SP, Williams EG, Knox
RB, Calder DM. 1993. Reproductive biology of Australian Myrtaceae. – Aust. J. Bot. 41:
511-526.
Bécquer-Granados ER. 2008. Taxonomía y
filogenia del género Pachyanthus (Melastomataceae: Miconieae). –
Ph.D. diss., Universidad de la Habana, Ciudad de La Habana.
Bécquer-Granados ER, Neubig KM, Judd WS,
Michelangeli FA, Abbott JR, Penneys DS. 2008. Preliminary molecular
phylogenetic studies in Pachyanthus (Miconieae, Melastomataceae). – Bot. Rev. 74:
37-52.
Behnke H-D. 1984 [1985]. Ultrastructure of
sieve-element plastids of Myrtales and
allied groups. – Ann. Missouri Bot. Gard. 71: 824-831.
Belsham SR. 2003. Floral development of
selected Myrtaceae with emphasis on the
fleshy-fruited taxa. – Ph.D. diss., University of Otago, Dunedin, New
Zealand.
Belsham SR, Orlovich DA. 2003. Development of
the hypanthium and androecium in Acmena smithii and Syzygium
australe (Acmena Alliance: Myrtaceae). – Aust. Syst. Bot. 16:
621-628.
Berger B. 2012. Myrtales: molecules, mangroves and
Metrosideros. – Ph.D. diss., University of Wisconsin, Madison,
Wisconsin.
Berger BA, Kriebel R, Spalink D, Sytsma KJ.
2016. Divergence times, historical biogeography, and shifts in speciation rates
of Myrtales. – Mol. Phylogen. Evol.
95: 116-136.
Bernardello L, Galetto L, Rodriguez IG. 1994.
Reproductive biology, variability of nectar features and pollination of
Combretum fruticosum (Combretaceae) in Argentina. – Bot. J.
Linn. Soc. 114: 293-308.
Berry EW. 1915. The origin and distribution
of the family Myrtaceae. – Bot. Gaz.
59: 484-490.
Berry PE. 1982. The systematics and evolution
of Fuchsia sect. Fuchsia (Onagraceae). – Ann. Missouri Bot. Gard.
69: 1-198.
Berry PE. 1987. Chromosome number reports
XCV. Vochysiaceae. – Taxon 36:
493.
Berry PE, Stein BA, Carlquist S, Nowicke J.
1988. Fuchsia pachyrrhiza (Onagraceae), a tuberous new species and
section of Fuchsia from western Peru. – Syst. Bot. 13: 483-492.
Berry PE, Skvarla JJ, Partridge A D, Macphail
MK. 1990. Fuchsia pollen from the Tertiary of Australia. – Aust.
Syst. Bot. 3: 739-744.
Berry PE, Hahn WJ, Sytsma KJ, Hall JC, Mast
A. 2004. Phylogenetic relationships and biogeography of Fuchsia (Onagraceae) based on noncoding nuclear
and chloroplast DNA data. – Amer. J. Bot. 94: 601-614.
Beusekom-Osinga RJ van. 1977. Crypteroniaceae. – In: Steenis
CGGJ van (ed), Flora Malesiana I, 8(2), Sijthoff & Noordhoff International
Publ., Alphen aan den Rijn, The Netherlands, pp. 187-204.
Beusekom-Osinga RJ van, Beusekom CF van.
1975. Delimitation and subdivision of the Crypteroniaceae (Myrtales). – Blumea 22: 255-266.
Biffin E, Craven LA, Crisp MD, Gadek PA.
2006. Molecular systematics of Syzygium and allied genera (Myrtaceae): evidence from the chloroplast
genome. – Taxon 55: 79-94.
Biffin E, Harrington MG, Crisp MD, Craven LA,
Gadek PA. 2007. Structural partitioning, paired-sites models and evolution of
the ITS transcript in Syzygium and Myrtaceae. – Mol. Phylogen. Evol. 43:
124-139.
Biffin E, Lucas EJ, Craven LA, Ribeiro da
Costa I, Harrington MG, Crisp MD. 2010. Evolution of exceptional species
richness among lineages of fleshy-fruited Myrtaceae. – Ann. Bot. 106: 79-93.
Bisse J. 1985 [1986]. El género
Mosiera Small (Myrtaceae-Myrtoideae) en Cuba I. – Rev.
Jard. Bot. Nac. Univ. Habana 6: 3-6.
Bisse J, Rankin R. 1984. Comparación
morfo-anatómica de los géneros Psidium L. y Myrtus L. (Myrtaceae) en Cuba. – Rev. Jard. Bot.
Nac. Univ. Habana 4: 11-26.
Blake ST. 1977. Allosyncarpia
ternata, a new genus and species of Myrtaceae subfamily Leptospermoideae from
northern Australia. – Austrobaileya 1: 43-46.
Boesewinkel FD, Venturelli M. 1987. Ovule and
seed structure in Vochysiaceae. –
Bot. Jahrb. Syst. 108: 547-566.
Bohte A. 2005. Flower and fruit development
in the tribe Eucalypteae (Myrtaceae).
– Ph.D. diss., University of Melbourne, Australia.
Bohte A, Drinnan A. 2005. Floral development
and systematic position of Arillastrum, Allosyncarpia,
Stockwellia and Eucalyptopsis (Myrtaceae). – Plant Syst. Evol. 251:
53-70.
Bohte A, Drinnan A. 2005. Ontogeny, anatomy
and systematic significance of ovular structures in the ‘eucalypt group’
(Eucalypteae, Myrtaceae). – Plant
Syst. Evol. 255: 17-39.
Bohte A, Drinnan A. 2012. Fruit development
in eucalypts (Myrtaceae: Eucalypteae).
– Aust. Syst. Bot. 24: 421-444.
Boissieu MH de. 1912. Une Mélastomacée
asiatique d’un genre africain. – Bull. Soc. Bot. France 59: 330-332.
Borhidi A. 1977. Tetrazygiopsis,
género nuevo de las Antillas y el género Tetrazygia L. C. Rich. (Melastomataceae) en Cuba. – Acta
Bot. Acad. Sci. Hung. 23: 33-39.
Boufford DE. 1982 [1983]. The systematics and
evolution of Circaea (Onagraceae). – Ann. Missouri Bot. Gard.
69: 804-994.
Brade AC. 1938. Melastomataceae Novae II. – Arch.
Inst. Biol. Veg. 4: 71-84.
Brambach F, Byng JW, Culmsee H. 2017. Five
new species of Syzygium (Myrtaceae) from Sulawesi, Indonesia. –
PhytoKeys 81: 47-78.
Brandis D. 1893. Combretaceae. – In: Engler A, Prantl
K (eds), Die natürlichen Pflanzenfamilien III(7), W. Engelmann, Leipzig, pp.
106-130.
Bremer K. 1979. Taxonomy of
Memecylon (Melastomataceae) in Ceylon. –
Opera Bot. 50: 1-32.
Bremer K. 1981. Seeds and embryos in Sri
Lanka (Ceylonese) species of Memecylon, with notes on
Spathandra (Melastomataceae). – Nord. J. Bot.
1: 62-65.
Bremer K. 1982. Lijndenia, a
re-established paleotropical genus of the Melastomataceae-Memecyleae. –
Nord. J. Bot. 2: 121-124.
Bremer K. 1983. Taxonomy of
Memecylon (Melastomataceae) in Borneo. –
Opera Bot. 69: 1-47.
Brenan JPM. 1946. Four new species of
Memecylon from East Tropical Africa. – Kew Bull. 1946: 89-96.
Brenan JPM. 1953a. Onagraceae. – In: Turrill WB,
Milne-Redhead E (eds), Flora of tropical East Africa, The Crown Agents for the
Colonies, London, pp. 1-23.
Brenan JPM. 1953b. Trapaceae. – In: Turrill
WB, Milne-Redhead E (eds), Flora of tropical East Africa, The Crown Agents for
the Colonies, London, pp. 1-3.
Brenan JPM. 1978. 82. Trapaceae. – In:
Launert E (ed), Flora Zambesiaca 4, Flora Zambesiaca Managing Committee,
London, pp. 346-348.
Bridgewater SD, Baas P. 1978. Wood anatomy of
the Punicaceae. – IAWA Bull. 1978: 3-6.
Briggs BG. 1962. The New South Wales species
of Darwinia. – Contr. New South Wales Natl. Herb. 3: 129-150.
Briggs BG, Johnson LAS. 1979. Evolution in
the Myrtaceae – evidence from
inflorescence structure. – Proc. Linn. Soc. New South Wales 102: 157-272.
Brooker MIH. 1979. A revision of the informal
series Foecundae Pryor and Johnson of the genus Eucalyptus
L’Herit. and notes on variation in the genus. – Brunonia 2: 125-170.
Brooker MIH. 1981. A new series,
Ovulares, of the genus Eucalyptus based on the subseries
Ovularinae Pryor & Johnson. – Brunonia 4: 1-26.
Brooker MIH. 2000. A new classification of
genus Eucalyptus L’Hér. (Myrtaceae). – Aust. Syst. Bot. 13:
79-148.
Brooker MIH, Bean AR. 1987. Two new ironbarks
and a new bloodwood (Eucalyptus, Myrtaceae) from Queensland. – Brunonia
10: 189-200.
Brooker MIH, Kleinig DA. 1999. Field guide to
eucalypts. 2nd ed. – Bloomings Books, Melbourne.
Brooker MIH, Slee AV, Briggs JD. 1995. A
taxonomic revision of Eucalyptus ser. Argyrophyllae. –
Aust. Syst. Bot. 8: 499-520.
Brophy JJ, Goldsack RJ, Forster PI. 1996. The
essential oils of the Australian species of Uromyrtus (Myrtaceae). – Flavour Fragrance J. 11:
133-138.
Brophy JJ, Goldsack RJ, Punruckvong A,
Forster PI. 1999. The leaf essential oils of Pilidiostigma (Myrtaceae). – Flavour Fragrance J. 14:
143-146.
Brown CA. 1967. Pollen morphology of the Onagraceae. – Rev. Palaeobot. Palynol.
3: 163-180.
Brown GK, Udovicic F, Ladiges PY. 2001.
Molecular phylogeny of Melaleuca, Callistemon and related
genera (Myrtaceae). – Aust. Syst.
Bot. 14: 565-585.
Buchmann SL, Buchmann MD. 1981. Anthecology
of Mouriri myrtilloides (Melastomataceae: Memecyleae), an oil
flower in Panama. – Biotropica 13(Suppl.): 7-24.
Bufford DE. 1982. The systematics and
evolution of Circaea (Onagraceae). – Ann. Missouri Bot. Gard.
69: 804-994.
Bult CJ, Zimmer EA. 1993. Nuclear ribosomal
RNA sequences for inferring tribal relationships within Onagraceae. – Syst. Bot. 18: 48-63.
Bünger MO. 2015. Revisão, filogenia e
biogeografia de Eugenia sect. Phyllocalyx (Myrtaceae). – Ph.D. thesis, Universidade
Federal de Minas Gerais, Brazil.
Bunninger L. 1972. Untersuchungen über die
morphologische Natur des Hypanthiums bei Myrtales- und Thymelaeales-Familien II. Myrtaceae; III. Vergleich mit den Thymelaeaceae. – Beitr.
Biol. Pflanzen 48: 79-156.
Bunninger L, Weberling F. 1968.
Untersuchungen über die morphologische Natur des Hypanthiums bei Myrtales-Familien I. Onagraceae. – Beitr. Biol. Pflanzen
421: 447-477.
Burret M. 1941. Myrtaceen-Studien. –
Notizbl. Bot. Gart. Mus. Berlin-Dahlem 15: 479-550.
Burrows GE. 2002. Epicormic strand structure
in Angophora, Eucalyptus and Lophostemon (Myrtaceae) – implications for fire
resistance and recovery. – New Phytol. 153: 111-131.
Byng JW, Wilson PG, Snow N. 2015.
Typifications and nomenclatural notes on Indian Myrtaceae. – Phytotaxa 217: 101-116.
Caetano APS. 2014. Contribuição da
embriologia na sistemática e na elucidação da apomixia em Melastomataceae Juss. –
Universidade Estadual de Campinas.
Caetano APS, Basso-Alves JP, Cortez PA, De
Brito VLG, Michelangeli FA, Reginato M, Goldenberg R, Carmello-Guerreiro SM,
Teixeira SP. 2018. Evolution of the outer ovule integument and its systematic
significance in Melastomataceae.
– Bot. J. Linn. Soc. 186: 224-246.
Cannon JR, Martin PF. 1977. The flavanones of
Agonis spathula (Myrtaceae).
– Aust. J. Chem. 30: 2099-2101.
Capuron R. 1967. Les Combretacées arbustives
ou arborescentes de Madagascar. – Centre Technique Forestier Tropicale,
Madagascar.
Cardoso CMV, Proença SL, Sajo MG. 2009.
Foliar anatomy of the subfamily Myrtoideae (Myrtaceae). – Aust. J. Bot. 57:
148-161.
Carlquist SJ. 1975. Wood anatomy of Onagraceae, with notes on alternative
modes of phytosynthate movement in dicotyledon woods. – Ann. Missouri Bot.
Gard. 62: 386-424.
Carlquist SJ. 1977. Wood anatomy of Onagraceae: additional species and
concepts. – Ann. Missouri Bot. Gard. 64: 627-637.
Carlquist SJ. 1982. Wood anatomy of Onagraceae: further species; root
anatomy; significance of vestured pits and allied structures in dicotyledons.
– Ann. Missouri Bot. Gard. 69: 755-769.
Carlquist SJ. 1987. Wood anatomy of
noteworthy species of Ludwigia (Onagraceae) with relation to ecology and
systematics. – Ann. Missouri Bot. Gard. 74: 889-896.
Carlquist S. 2017. What the Penaeaceae alliance (Myrtales) tells us about the nature of
vestured pits in xylem. – Brittonia 69: 276-294.
Carlquist SJ, DeBuhr L. 1977. Wood anatomy of
Penaeaceae (Myrtales): comparative, phylogenetic, and
ecological implications. – Bot. J. Linn. Soc. 75: 211-227.
Carlquist SJ, Raven PH. 1966. The systematics
and anatomy of Gongylocarpus (Onagraceae). – Amer. J. Bot. 53:
378-390.
Carmo-Oliveira R, de Morretes BL. 2009.
Stigmatic surface in the Vochysiaceae: reproductive and
taxonomical implications. – Acta Bot. Bras. 23: 780-785.
Carr BL, Gregory DP, Raven PH, Tai W. 1988.
Experimental hybridization, chromosomal diversity, and phylogeny within
Gaura sect. Pterogaura (Onagraceae). – Syst. Bot. 13:
324-335.
Carr BL, Crisci JV, Hoch PC. 1990. A
cladistic analysis of the genus Gaura (Onagraceae). – Syst. Bot. 15:
454-461.
Carr DJ, Carr SGM. 1959. Developmental
morphology of the floral organs of Eucalyptus I. The inflorescence.
– Aust. J. Bot. 7: 109-141.
Carr DJ, Carr SGM. 1981. Comments on
Schmid’s summary of characters in Myrtaceae. – Taxon 30: 820-821.
Carr DJ, Carr SGM, Hyland BPM, Wilson PG,
Ladiges PY. 2002. Stockwellia quadrifida (Myrtaceae), a new Australian genus and
species in the eucalypt group. – Bot. J. Linn. Soc. 139: 415-421.
Carr JD, Rogers CB. 1987. Chemosystematic
studies of the genus Combretum (Combretaceae) I. A convenient method of
identifying species of this genus by a comparison of the polar constituents
extracted from leaf material. – South Afr. J. Bot. 53: 173-176.
Carr SGM, Carr DJ. 1966. Cotyledonary
stipules in the Myrtaceae. – Nature
210: 185-186.
Carr SGM, Carr DJ. 1969. Oil glands and dots
in Eucalyptus L’Hérit. I. The phloem and the pith. – Aust. J.
Bot. 17: 471-513.
Carr SGM, Carr DJ. 1970. Oil glands and dots
in Eucalyptus L’Hérit. II. Development and structure of oil glands
in the embryo. – Aust. J. Bot. 18: 191-212.
Carr SGM, Carr DJ, Milkovits L. 1970. Oil
glands and ducts in Eucalyptus L’Hérit. III. The flowers of series
Corymbosae (Benth.) Miden. – Aust. J. Bot. 18: 313-333.
Carrucan AE, Drinnan AN. 2000. The
ontogenetic basis for floral diversity in the Baeckea sub-group (Myrtaceae). – Kew Bull. 55: 593-613.
Cavalcanti TB. 1991. New species of
Cuphea (Lythraceae) from
Brazil. – Kew Bull. 46: 253-268.
Cavalcanti TB. 2011. New taxa in
Diplusodon (Lythraceae) from
Brazil. – Phytotaxa 38: 29-35.
Cavalcanti TB, Rua GH. 2008. Inflorescence
patterns in the woody Brazilian genus Diplusodon (Lythraceae). – Flora 203: 261-271.
Cellinese N. 1997. Notes on the systematics
and biogeography of the Sonerila generic alliance (Melastomataceae) with special focus
on fruit characters. – Trop. Biodiv. 4: 83-93.
Cellinese N. 1999. Systematics of the Asian
Sonerileae (Melastomataceae). –
Ph.D. diss., University of Reading, England.
Cellinese N. 2007. Two new species in the
genus Poikilogyne (Melastomataceae) from Papua New
Guinea. – Novon 17: 20-23.
Cevallos-Ferriz SRS, Stockey R. 1988.
Permineralized fruits and seeds from the Princeton chert (Middle Eocene) of
British Columbia: Lythraceae. – Can.
J. Bot. 66: 303-312.
Chalson JM, Martin HA. 1995. The pollen
morphology of some co-occurring species of the family Myrtaceae from the Sydney region. –
Proc. Linn. Soc. New South Wales 115: 163-191.
Chantaranothai P, Parnell J. 1993. New taxa
and combinations in Cleistocalyx and Syzygium (Myrtaceae) in Thailand. – Kew Bull. 48:
589-610.
Chantaranothai P, Parnell J. 1994. A revision
of Acmena, Cleistocalyx, Eugenia s.s. and
Syzygium (Myrtaceae) in
Thailand. – Thai Forest Bull. 21: 1-123.
Chao A. 1958. A census of the Chinese species
of Combretaceae. – Acta
Phytotaxon. Sin. 7: 225-252.
Chappill JA, Ladiges PY. 1992. Geographical
variation in morphology of Eucalyptus mannifera and allied taxa. –
Aust. Syst. Bot. 5: 359-372.
Chappill JA, Ladiges PY. 1996. Phylogenetic
analysis of Eucalyptus informal subgenus Symphyomyrtus
section Maidenaria. – Aust. Syst. Bot. 9: 71-93.
Chattaway MM. 1959. The anatomy of bark VII.
Species of Eugenia (s.l.). – Trop. Woods 111: 1-14.
Chen C-J, Hoch PC, Raven PH. 1992.
Systematics of Epilobium (Onagraceae) in China. – Syst. Bot.
Monogr. 34: 1-209.
Chen L-G, Yen K-Y, Yang L-L, Hatano T, Okuda
T, Yoshida T. 1999. Macrocyclic ellagitannin dimers, cuphiins D1 and
D2, and accompanying tannins from Cuphea hyssopifolia. –
Phytochemistry 50: 307-312.
Cheung M, Sattler R. 1967. Early floral
development of Lythrum salicaria. – Can. J. Bot. 45: 1609-1618.
Chrtek J. 1969. Die Kronblattnervatur in der
Familie Lythraceae. – Preslia 55:
323-326.
Clarkson JR, Thompson J. 1989. A revision of
the genus Neofabricia (Myrtaceae). – Telopea 3: 291-300.
Clausing G. 1999. Die Systematik der
Dissochaeteae und ihre Stellung innerhalb der Melastomataceae. – Ph.D. diss.,
Johannes Gutenberg-Universität, Mainz, Germany.
Clausing G. 2000. Revision of the Southeast
Asian genus Pachycentria Blume (Melastomataceae). – Blumea 45:
341-375.
Clausing G, Renner SS. 2001a. Molecular
phylogenetics of Melastomataceae
and Memecylaceae: implications for
character evolution. – Amer. J. Bot. 88: 486-498.
Clausing G, Renner SS. 2001b. Evolution of
growth form in epiphytic Dissochaeteae (Melastomataceae). – Organisms
Divers. Evol. 1: 45-60.
Clausing G, Meyer K, Renner SS. 2000.
Correlations among fruit traits and evolution of different fruits within Melastomataceae. – Bot. J. Linn.
Soc. 133: 303-326.
Cleland RE. 1936. Some aspects of the
cytogenetics of Oenothera. – Bot. Rev. 2: 316-348.
Cleland RE. 1972. Oenothera:
cytogenetics and evolution. – Academic Press, London.
Coates DJ, Hnatiuk RJ. 1990. Systematic and
evolutionary inferences from isozyme studies in the genus Eremaea (Myrtaceae). – Aust. Syst. Bot. 3:
59-74.
Cogniaux CA. 1891. Melastomaceae. – In:
Candolle A de, Candolle C de (eds), Monographiae Phanerogamarum 7, G. Masson,
Paris, pp. 1-1256.
Cogniaux CA. 1909. Melastomataceae peruvianae. –
Engl. Bot. Jahrb. 42: 131-148.
Collinson ME, Pingen M. 1992. Seeds of the Melastomataceae from the Miocene of
Central Europe. – In: Kovar-Eder J (ed), Palaeovegetational development in
Europe, Naturhistorisches Museum, Wien, pp. 129-139.
Conti E. 1994. Phylogenetic relationships of
Onagraceae and Myrtales: evidence from rbcL
sequence data. – Ph.D. diss., University of Wisconsin, Madison, Wisconsin.
Conti E, Fischbach A, Sytsma KJ. 1993. Tribal
relationships in Onagraceae:
implications from rbcL sequence data. – Ann. Missouri Bot. Gard. 80:
672-685.
Conti E, Litt A, Sytsma KJ. 1996.
Circumscription of Myrtales and their
relationships to other rosids: evidence from rbcL sequence data. –
Amer. J. Bot. 83: 221-233.
Conti E, Litt A, Wilson PG, Graham SA, Briggs
BG, Johnson LAS, Sytsma KJ. 1997 [1998]. Interfamilial relationships in Myrtales: molecular phylogeny and patterns
of morphological evolution. – Syst. Bot. 22: 629-647.
Conti E, Eriksson T, Schönenberger J, Sytsma
KJ, Baum DA. 2002. Early Tertiary out-of-India dispersal of Crypteroniaceae: evidence from
phylogeny and molecular dating. – Evolution 56: 1931-1942.
Conti E, Rutschmann F, Eriksson T, Sytsma KJ,
Baum DA. 2004. Calibration of molecular clocks and the biogeographic history of
Crypteroniaceae: a reply to
Robert G. Moyle. – Evolution 58: 1874-1876.
Coode MJE. 1969. Manual of the forest trees
of Papua and New Guinea I (rev.). Combretaceae. – Department of
Forests, Lae.
Coode MJE. 1973. Notes on Terminalia
L. (Combretaceae) in Papuasia. –
Contr. Herb. Austral. 2: 1-33.
Cook CDK. 1979. A revision of the genus
Rotala (Lythraceae). –
Boissiera 29: 1-156.
Cook IO, Ladiges PY. 1991. Morphological
variation within Eucalyptus nitens s. lat. and recognition of a new
species, E. denticulata. – Aust. Syst. Bot. 4: 375-390.
Copeland LM, Bruhl JJ, Craven LA, Brubaker
CL. 2007. Phenetic analyses of Homoranthus (Myrtaceae: Chamelaucieae) on the basis of
morphology. – Aust. Syst. Bot. 20: 417-427.
Copeland LM, Bruhl JJ, Craven LA, Brubaker
CL. 2008. New chromosome numbers in Homoranthus (Myrtaceae: Chamelaucieae) and notes on
their taxonomic utility. – Aust. Syst. Bot. 21: 443-447.
Copeland LM, Craven LA, Bruhl JJ. 2012. A
taxonomic review of Homoranthus (Myrtaceae: Chamelaucieae). – Aust. Syst.
Bot. 24: 351-374.
Coppen JJW (ed). 2002. Eucalyptus.
The genus Eucalyptus. – Taylor and Francis, London.
Correns C. 1892. Über die Epidermis der
Samen von Cuphea viscosissima. – Ber. Deutsch. Bot. Ges. 10:
143-152.
Cortez PA, Carmello-Guerreiro SM. 2008.
Ontogeny and structure of the pericarp and the seed coat of Miconia
albicans (Sw.) Triana (Melastomataceae) from “cerrado”,
Brazil. – Rev. Brasileira Bot. 31: 71-79.
Costa IR, Dornelas MC, Forni-Martins ER.
2008. Nuclear genome size variation in fleshy-fruited Neotropical Myrtaceae. – Plant Syst. Evol. 276:
209-217.
Cotton E, Matezki S. 2003. Ecuadorian
novelties in Blakea and Topobea (Melastomataceae). – Brittonia 55:
73-81.
Cotton E, Meier W. 2003. Clidemia
intonsa and Miconia chapensis (Miconieae, Melastomataceae), two new species
endemic to cloud forest refuges in the Coastal Cordillera of Venezuela. –
Willdenowia 33: 197-203.
Couillault J. 1973. Organisation de
l’appareil conducteur de Trapa natans L. – Bull. Soc. Bot. France
119: 177-198.
Cowley KJ, Quinn FC, Barlow BA, Craven LA.
1990. Contributions to a revision of Melaleuca (Myrtaceae) 7-10. – Aust. Syst. Bot. 3:
165-202.
Craven LA. 1980. A review of the genus
Calytrix Labill. (Myrtaceae)
in Northern Australia. – Brunonia 3: 217-246.
Craven LA. 1987a. A taxonomic revision of
Calytrix Labill. (Myrtaceae).
– Brunonia 10: 1-138.
Craven LA. 1987b. A revision of
Homalocalyx, F. Muell. (Myrtaceae). – Brunonia 10: 139-158.
Craven LA. 1988. Reinstatement and revision
of Asteromyrtus (Myrtaceae).
– Aust. Syst. Bot. 1: 373-385.
Craven LA. 1990a. Three additional species in
Calytrix (including the reduction of Calythropsis), and notes
on Calytrix exstipulata (Myrtaceae). – Aust. Syst. Bot. 3:
719-725.
Craven LA. 1990b. One new species each in
Acmena and Eucalyptopsis and a new name in
Lindsayomyrtus (all Myrtaceae). – Aust. Syst. Bot. 3:
727-732.
Craven LA. 1991. A new species of
Calytrix (Myrtaceae,
Chamelaucieae) from northern Australia. – Aust. Syst. Bot. 4: 535-537.
Craven LA. 1998. Cleistocalyx
fullagarii transferred to Syzygium (Myrtaceae). – Muelleria 11: 95-96.
Craven LA. 1999. Generic position of
Melaleuca punicea: Petraeomyrtus, gen. nov. (Myrtaceae). – Aust. Syst. Bot. 12:
675-678.
Craven LA. 2001. Unravelling knots or
platting rope: what are the major taxonomic strands in Syzygium sens.
lat. (Myrtaceae) and what should be
done with them? – In: Saw LG, Chua LS, Khoo KC (eds), Taxonomy: the
cornerstone of biodiversity, Proceedings of the Fourth International Flora
Malesiana Symposium, 1998, Institute Penyelidikan, Kuala Lumpur, pp. 75-85.
Craven LA. 2003. Four new species of
Syzygium (Myrtaceae) from
Australia. – Blumea 8: 479-488.
Craven LA. 2006. New combinations in
Melaleuca for Australian species of Callistemon (Myrtaceae). – Novon 16: 468-475.
Craven LA, Biffen E. 2010. An infrageneric
classification of Syzygium (Myrtaceae). – Blumea 55: 94-99.
Craven LA, Jones SR. 1991. A taxonomic review
of Homoranthus and two new species of Darwinia (both Myrtaceae, Chamelaucieae). – Aust. Syst.
Bot. 4: 513-533.
Craven LA, Lepschi BJ. 2000. Enumeration of
the species and infraspecific taxa of Melaleuca (Myrtaceae) occurring in Australia and
Tasmania. – Aust. Syst. Bot. 12: 819-928.
Craven LA, Lepschi BJ, Broadhurst L, Byrne M.
2004. Taxonomic revision of the broombush complex in Western Australia (Myrtaceae, Melaleuca uncinata
s.l.). – Aust. Syst. Bot. 17: 255-271.
Craven LA, Biffin E, Ashton PS. 2006.
Acmena, Acmenosperma, Cleistocalyx,
Piliocalyx, and Waterhousea formally transferred to
Syzygium (Myrtaceae). –
Blumea 51: 131-142.
Cremers G. 1986. Architecture vegetative et
structure inflorescentielle de quelques Melastomaceae guyanaises. – Edition
ORSTOM, Paris, B 118, pp. 1-152.
Crété P. 1956. À propos de
l’embryogénie du Sonerila wallichii. – Bull. Soc. Bot. France
103: 599-603.
Crété P. 1957. Embryogénie des
Mélastomacées. Développement de l’embryon chez le Clidemia hirta
D. Don. – Compt. Rend. Acad. Sci. Paris 244: 374-376.
Crété P. 1960a. Embryogénie du Calvoa
orientalis Taub. – Compt. Rend. Acad. Sci. Paris 250: 3710-3712.
Crété P. 1960b. Embryogénie des
Mélastomacées. Développement de l’embryon chez le Bertolonia
maculata DC. – Compt. Rend. Acad. Sci. Paris 251: 1907-1909.
Crisci JV, Zimmer EA, Hoch PC, Johnson GB,
Mudd C, Pan NS. 1990. Phylogenetic implications of ribosomal DNA restriction
site variation in the plant family Onagraceae. – Ann. Missouri Bot. Gard.
77: 523-538.
Crisp MD, Cook LG, Steane DA. 2005. Molecular
dating and eucalypts: reply to Ladiges and Udovicic. – Aust. Syst. Bot. 18:
295-296.
Crisp MD, Burrows GE, Cook LG, Thornhill AH,
Bowman DM. 20aa. Flammable biomes dominated by eucalypts originated at the
Cretaceous-Palaeogene boundary. – Nature Commun. 2: 193.
doi:10.1038/ncomms1191.
Cronquist A. 1984 [1985]. A commentary on the
definition of the order Myrtales. –
Ann. Missouri Bot. Gard. 71: 780-782.
Cruz AJU, Ramos ZA. 2008. Cuban novelties in
the genus Mosiera (Myrtaceae).
– Willdenowia 38: 533-544.
Cruz F da, Turchetto-Zolet AC, Veto N, Mondin
CA, Sobral M, Almerão M, Margis R. 2013. Phylogenetic analysis of the genus
Hexachlamys (Myrtaceae) based
on plastid and nuclear DNA sequences and their taxonomic implications. – Bot.
J. Linn. Soc. 172: 532-543.
Cufodontis G. 1960. Die Identifizierung von
Tephea Delile und andere die Oliniaceae betreffende Feststellungen.
– Österr. Bot. Zeitschr. 107: 106-112.
Da Costa IR, Forni-Martins ER. 2007.
Karyotype analysis in South American species of Myrtaceae. – Bot. J. Linn. Soc. 155:
571-580.
Da Costa SM, Morim MP. 2008. A new species of
Eugenia (Myrtaceae) from
south-eastern Brazil. – Bot. J. Linn. Soc. 158: 306-308.
Dahlgren RMT. 1967a. Studies in Penaeaceae I. Systematics and gross
morphology of the genus Stylapterus A. Juss. – Opera Bot. 15:
1-40.
Dahlgren RMT. 1967b. Studies on Penaeaceae III. The genus
Glischrocolla. – Bot. Not. 120: 57-68.
Dahlgren RMT. 1967c. Studies on Penaeaceae IV. The genus
Endonema. – Bot. Not. 120: 69-83.
Dahlgren RMT. 1968. Studies on Penaeaceae II. The genera
Brachysiphon, Sonderothamnus and Saltera. – Opera
Bot. 18: 1-72.
Dahlgren RMT. 1971. Studies on Penaeaceae VI. The genus Penaea
L. – Opera Bot. 29: 1-58.
Dahlgren RMT, Thorne RF. 1984 [1985]. The
order Myrtales: circumscription,
variation, and relationships. – Ann. Missouri Bot. Gard. 71: 633-699.
Dahlgren RMT, Wyk AE van. 1988. Structures
and relationships of families endemic to or centered in Southern Africa. –
Monogr. Syst. Bot. Missouri Bot. Gard. 25: 1-94.
Da Rocha MJR, Batista JAN, Guimarães PJF,
Michelangeli FA. 2016. Phylogenetic relationships in the Marcetia
alliance (Melastomeae, Melastomataceae) and implications
for generic circumscription. – Bot. J. Linn. Soc. 181: 585-609.
Da Rocha MJR, Guimarães PJF, Michelangeli
FA, Romero R. 2016. Phylogenetic placement and a new circumscription of
Poteranthera (Microlicieae; Melastomataceae). – Phytotaxa 263:
219-232.
Da Rocha MJR, Guimarães PJF, Michelangeli
FA, Batista JAN. 2018. Taxonomy of Marcetieae: a new Neotropical tribe of Melastomataceae. – Intern. J.
Plant Sci. 179: 50-74.
Davis GL. 1966. Floral morphology and the
development of gametophytes in Eucalyptus melliodora A. Cunn. –
Aust. J. Bot. 16: 19-35.
Davis GL. 1969. Floral morphology and the
development of gametophytes in Eucalyptus stellulata Sieb. – Aust.
J. Bot. 17: 177-190.
Dawson JW. 1970. Pacific capsular Myrtaceae 1. Reproductive morphology of
Arillastrum gummiferum Panch. ex Baillon (New Caledonia). – Blumea
18: 441-445.
Dawson JW. 1972a. Pacific capsular Myrtaceae 4. The Metrosideros
complex: Xanthostemon, Nani, Pleurocalyptus,
Purpureostemon. – Blumea 20: 315-322.
Dawson JW 1972b. Pacific capsular Myrtaceae 5. The Metrosideros
complex: M. perforata and the M. operculata group. – Blumea
20: 327-329.
Dawson JW. 1972c. Pacific capsular Myrtaceae 7. Mooria. – Blumea
20: 331-334.
Dawson JW. 1972d. Pacific capsular Myrtaceae 8. Tepualia. – Blumea
20: 335-337.
Dawson JW. 1974. Pacific capsular Myrtaceae 9. The Metrosideros
complex: M. queenslandica group. – Blumea 22: 151-153.
Dawson JW. 1976. Pacific capsular Myrtaceae XI. Redefinition of
Metrosideros Banks ex Gaertn. and definition of infrageneric
categories. – Blumea 23: 7-11.
Dawson JW. 1984. New species and combinations
in New Caledonian Metrosideros and Carpolepis (Myrtaceae) with notes on other species.
– Adansonia 4: 465-489.
Dawson JW. 1992. Flore de la Nouvelle
Calédonie et Dépendances 18. Myrtaceae-Leptospermoideae. – Muséum
National d’Histoire Naturelle, Paris.
Dawson JW. 1999. Myrtaceae. Myrtoideae I:
Syzygium. – In: Morat P (ed), Flore de la Nouvelle-Calédonie 23,
Muséum de l’Histoire Naturelle, Paris.
De Cordemoy EJ. 1895. Bixacées, Trib. –
Psiloxylées. – In: Flore de l’Île de la Réunion, Klincksieck, Paris, pp.
351-355.
Dement WA, Rave PH. 1974. Pigments
responsible for UV patterns in flowers of Oenothera (Onagraceae). – Nature 252: 705-706.
Dias-Leme CL, Gasson P, Nic Lughada E. 1995.
Wood anatomy of four Myrtaceae genera
in the subtribe Myrciinae from South America. – IAWA J. 16: 87-95.
Dickie JB, Gasson PE. 1999. Comparative leaf
anatomy of the Penaeaceae and its
ecological implications. – Bot. J. Linn. Soc. 131: 327-351.
Diels FLE. 1921. Die Myrtaceen Mikronesiens.
– Bot. Jahrb. Syst. 56: 529-534.
Diels FLE. 1932. Beiträge zur Kenntnis der
Melastomataceen Ostasiens. Übersicht der Gattungen und Arten der Oxysporeae
und Sonerileae Ostasiens. – Bot. Jahrb. Syst. 65: 99-104.
Dietrich W, Raven PH, Wagner WL. 1985.
Revision of Oenothera sect. Oenothera subsect.
Emersonia (Onagraceae). –
Syst. Bot. 10: 29-48.
Drake DR. 1992. Seed dispersal of
Metrosideros polymorpha (Myrtaceae): a pioneer tree of Hawaiian
lava flows. – Amer. J. Bot. 79: 1224-1228.
Drinnan AN, Carrucan A. 2005. The ontogenetic
basis for floral diversity in Agonia, Leptospermum and
Kunzea (Myrtaceae). – Plant
Syst. Evol. 251: 71-88.
Drinnan AN, Ladiges PY. 1989. Corolla and
androecium development in some Eudesmia eucalypts (Myrtaceae). – Plant Syst. Evol. 165:
239-254.
Drinnan AN, Ladiges PY. 1991a. Floral
development and systematic position of Eucalyptus curtisii (Myrtaceae). – Aust. Syst. Bot. 4:
539-551.
Drinnan AN, Ladiges PY. 1991b. Floral
development in the ’Symphyomyrtus group’ of eucalypts
(Eucalyptus: Myrtaceae). –
Aust. Syst. Bot. 4: 553-562.
Duarte L. 1956. Melastomatáceas fósseis da
Bahia Tertiária de Fonseca, Minas Gerais. – Div. Geol. Minerol. Bol. 161:
8-32.
Dudareva N, Cseke L, Blanc VM, Pichersky E.
1996. Evolution of floral scent in Clarkia: novel patterns of
S-linalool synthase gene expression in the C. breweri flower. –
Plant Cell 8: 1137-1148.
Duke NC, Jackes BR. 1987. A systematic
revision of the mangrove genus Sonneratia (Sonneratiaceae) in
Australasia. – Blumea 32: 277-382.
Dulberger R. 1970. Tristyly in Lythrum
junceum. – New Phytol. 69: 751-759.
Eckert CG. 2002. Effect of geographical
variation in pollinator fauna on the mating system of Decodon
verticillatus (Lythraceae). –
Intern. J. Plant Sci. 163: 123-132.
Eckert CG, Barrett SCH. 1994. Tristyly,
self-compatibility and floral variation in Decodon verticillatus (Lythraceae). – Biol. J. Linn. Soc. 53:
1-30.
Edwards RD, Craven LA, Crisp, MD, Cook LG.
2010. Melaleuca revisited: cpDNA and morphological data confirm that
Melaleuca L. (Myrtaceae) is
not monophyletic. – Taxon 59: 744-754.
Egolf DR, Andrick AO. 1978. The
Lagerstroemia handbook/checklist. – Amer. Assoc. Bot. Gard.
Arboreta, Inc., Jamaica Plain, Maine.
Eichler AW. 1866. Thiloa und
Buchenavia zwei neue Gattungen der Combretaceen. – Flora 49:
145-152, 161-167.
El Ghazali GEB, Tsuji S, El Ghazaly GA,
Nilsson S. 1998. Combretaceae R.
Brown. – World Pollen and Spore Flora 21: 1-40.
Eliseu S, Dinis A. 2008. Ultrastructure and
cytochemistry of Eucalyptus globulus (Myrtaceae) pollen grain. – Grana 47:
39-51.
Engler A. 1897. Combretaceae. – In: Engler A, Prantl
K (eds), Die natürlichen Pflanzenfamilien, Nachträge zu III(6a), W.
Engelmann, Leipzig, pp. 262-263.
Engler A, Diels L. 1899. Monographien
afrikanischer Pflanzen-Familien und -Gattungen, III. Combretaceae africanae (I)
Combretum. – W. Engelmann, Leipzig.
Engler A, Diels L. 1900. Monographien
afrikanischer Pflanzen-Familien und -Gattungen, IV. Combretaceae africanae (II) excl.
Combretum. – W. Engelmann, Leipzig.
Erdtman G, Metcalfe CR. 1963. Affinities of
certain genera Incertae sedis suggested by pollen morphology and
vegetative anatomy I. The myrtaceous affinity of Kania eugenioides
Schltr. – Kew Bull. 17: 249-250.
Etheridge AL, Herr JM Jr. 1968. The
development of the ovule and megagametophyte in Rhexia mariana. –
Can. J. Bot. 46: 133-139.
Evans MEK, Hearn DJ, Hahn WJ, Spangle JM,
Venable DL. 2005. Climate and life-history evolution in evening primroses
(Oenothera, Onagraceae): a
phylogenetic comparative analysis. – Evolution 59: 1914-1927.
Eves DS. 1936. A revision of the genus
Axinaea (Melastomataceae). – Bull. Torrey
Bot. Club 63: 211-226.
Exell AW. 1931. The genera of Combretaceae. – J. Bot. 69:
113-128.
Exell AW. 1935. Species of
Terminalia from the Solomon Is. – J. Bot. 73: 131-134.
Exell AW. 1939. Some new species of
Dombeya, Grewia and Combretum from tropical Africa.
– J. Bot. 77: 172-173.
Exell AW. 1953. The Combretum
species of the New World. – Bot. J. Linn. Soc. 55: 103-141.
Exell AW. 1954. Combretaceae. – In: Steenis CGGJ van
(ed), Flora Malesiana I, 4(5), Noordhoff-Kolff N. V., Batavia, pp. 533-589.
Exell AW. 1962. Space problems arising from
the conflict between two evolutionary tendencies in the Combretaceae. – Bull. Soc. Roy. Bot.
Belg. 95: 41-49.
Exell AW. 1968. Notes on the Combretaceae of southern Africa. –
Bol. Soc. Broter., sér. II, 42: 5-35.
Exell AW. 1970. Summary of the Combretaceae of Flora Zambesiaca. –
Kirkia 7: 159-252.
Exell AW. 1978. 73. Combretaceae. – In: Launert E (ed),
Flora Zambesiaca 4, Flora Zambesiaca Managing Committee, London, pp.
100-183.
Exell AW, Stace CA. 1964. Reorganisation of
the genus Quisqualis. – Bol. Soc. Broter., sér. II, 38: 139-143.
Exell AW, Stace CA. 1966. Revision of the Combretaceae. – Bol. Soc. Broter.,
sér. II, 40: 5-25.
Exell AW, Stace CA. 1972. Patterns of
distribution in the Combretaceae.
– In: Valentine DH (ed), Taxonomy, phytogeography and evolution, Academic
Press, London, pp. 307-323.
Eyde RH. 1977. Reproductive structures and
evolution in Ludwigia (Onagraceae) I. Androecium, placentation,
merism. – Ann. Missouri Bot. Gard. 64: 644-655.
Eyde RH. 1978. Reproductive structures and
evolution in Ludwigia (Onagraceae) II. Fruit and seed. – Ann.
Missouri Bot. Gard. 65: 656-675.
Eyde RH. 1981. Reproductive structures and
evolution in Ludwigia (Onagraceae) III. Vasculature, nectaries,
conclusions. – Ann. Missouri Bot. Gard. 68: 470-503.
Eyde RH. 1982. Evolution and systematics of
Onagraceae: floral anatomy. – Ann.
Missouri Bot. Gard. 69: 735-747.
Eyde RH, Morgan JT. 1973. Floral structure
and evolution in Lopezieae (Onagraceae). – Amer. J. Bot. 60:
771-787.
Eyde RH, Teeri JA. 1967. Floral anatomy of
Rhexia virginica (Melastomataceae). – Rhodora 69:
163-178.
Fagerlind F. 1941. Der Bau der Samenanlage
und der Makrogametophyten bei Quisqualis indica. – Bot. Not. 1941:
217-222.
Faria JEQ. 2014. Revisão taxonomica e
filogenia de Eugenia sect. Pilothecium (Kiaersk.) D. Legrand
(Myrtaceae). – Ph.D. thesis,
Universidade de Brasilia, Brazil.
Farron C, Favarger C. 1983-1984. Contribution
à la cytotaxonomie des Melastomatacées africaines. – Garcia de Orta, Sér.
Bot. 6: 83-88.
Favarger C. 1952. Recherches sur quelques
Mélastomacées d’Afrique occidentale. – Ber. Schweiz. Bot. Ges. 62:
5-65.
Favarger C. 1962. Nouvelles recherches
cytologiques sur les Mélastomatacées. – Ber. Schweiz. Bot. Ges. 72:
290-305.
Feissly C. 1964. Sur l’ornementation du
tube calicinal de quelques Osbeckiées africaines. – Bull. Soc. Neuchâtel
Sci. Nat. 87: 137-170.
Fernandes A. 1971. Contribution à la
connaissance du genre Heteropyxis Harv. – Mitt. Bot. Staatssamml.
München 10: 207-234.
Fernandes A. 1975. Lythraceae africanae novae vel minus
cognitae I. – Bol. Soc. Brot., sér. II, 48: 115-169.
Fernandes A. 1978a. 75. Heteropyxidaceae. –
In: Launert E (ed), Flora Zambesiaca 4, Flora Zambesiaca Managing Committee,
London, pp. 212-215.
Fernandes A. 1978b. 78. Lythraceae. – In: Launert E (ed), Flora
Zambesiaca 4, Flora Zambesiaca Managing Committee, London, pp. 276-323.
Fernandes A. 1978c. 80. Sonneratiaceae. –
In: Launert E (ed), Flora Zambesiaca 4, Flora Zambesiaca Managing Committee,
London, pp. 327-329.
Fernandes A, Diniz MA. 1955. Lythraceae africanae novae. – Bot. Soc.
Brot., sér. II, 29: 87-99.
Fernandes A, Fernandes RB. 1954a.
Contribuição para o conhecimento das Melastomatáceas da Guiné Portuguesa.
– Garcia de Orta 2: 273-284.
Fernandes A, Fernandes RB. 1954b. Sur la
position systématique de la section Pseudodissotis Cogn. du genre
Osbeckia L. (Note préliminaire). – Bol. Soc. Brot., sér. 2, 28:
65-76.
Fernandes A, Fernandes RB. 1954c.
Contribution to the knowledge of the Melastomataceae of Moçambique. –
Bol. Soc. Brot., sér. 2, 28: 205-214.
Fernandes A, Fernandes RB. 1956a. Revisão
das Melastomatoideae do East African Herbarium e do Southern Rhodesia
Government Herbarium. – Mem. Soc. Brot. 11: 65-96.
Fernandes A, Fernandes RB. 1956b. Melastomataceae africanae novae vel
minus cognitae – III. – Bol. Soc. Brot., sér. 2, 30: 167-186.
Fernandes A, Fernandes RB. 1962. O gênero
Olinia Thunb. em Angola. – Mem. Junta Invest. Ultramar 38: 9-20.
Fernandes A, Fernandes RB. 1969. Melastomataceae africanae novae vel
minus cognitae – V. – Bol. Soc. Brot., sér. 2, 43: 285-306.
Fernandes RB, Fernandes A. 1978. 77. Melastomataceae. – In: Launert E
(ed), Flora Zambesiaca 4, Flora Zambesiaca Managing Committee, London, pp.
220-276.
Figueiredo E. 2001. A revision of
Calvoa Hook. f. (Melastomataceae). – Bot. J. Linn.
Soc. 136: 179-205.
Fikenscher LH, Hegnauer R. 1977. Über die
cyanogenen Verbindungen bei einigen Compositae, bei den Oliniaceae und in der Rutaceae-Gattung
Zieria. – Pharmaceut. Weekbl. 112: 11-20.
Fischer EA, Gordo M. 1993. Qualea
cordata, pollination by the territorial bee Centris tarsata in
the ”campos rupestres”, Brazil. – Ci. Cult. 45: 144-146.
Flores EM. 1993a. Vochysia
guatemalensis, chancho blanco – white yemeri. – Arboles y semillas del
Neotropico 2: 1-27.
Flores EM. 1993b. Vochysia
ferruginea, mayo colorado – red yemeri. – Arboles y semillas del
Neotropico 2: 29-52.
Ford VS, Gottlieb LD. 2003. Reassessment of
phylogenetic relationships in Clarkia sect. Sympherica. –
Amer. J. Bot. 90: 284-292.
Ford VS, Gottlieb LD. 2007. Tribal
relationships within Onagraceae
inferred from PgiC sequences. – Syst. Bot. 32: 348-356.
Forman LL. 1990. A synopsis of Thai Menispermaceae:
a correction. – Kew Bull. 45: 350.
Forster PI. 1994. Notes on Dansiea
Byrnes and Macropteranthes F. Muell. (Combretaceae). – Austrobaileya 4:
149-153.
Foster AS. 1946. Comparative morphology of
the foliar sclereids in the genus Mouriria Aubl. – J. Arnold Arbor.
27: 253-271.
Foster AS. 1947. Structure and ontogeny of
the terminal sclereids in the leaf of Mouriria huberi Cogn. – Amer.
J. Bot. 34: 501-514.
Fraga CN, Guimarães PJ. 2014. Two new
species of Pleroma (Melastomataceae) from Espírito
Santo, Brazil. – Phytotaxa 166: 77-84.
França F, Proença CEB. 2007. Vochysia
palmirana (Vochysiaceae), a new
species from Goiás and Tocantins, Brazil. – Brittonia 59: 374-376.
Freire-Fierro A. 2002. Monograph of
Aciotis (Melastomataceae). – Syst. Bot.
Monogr. 62: 1-99.
Friis EM, Pedersen KR, Crane PR. 1992.
Esgueiria gen. nov., fossil flowers with combretaceous features from
the Late Cretaceous of Portugal. – Kong. Danske Vidensk. Selsk., Biol. Skr.
41: 1-45.
Fritsch PW, Almeda F, Renner SS, Martins AB,
Cruz BC. 2004. Phylogeny and circumscription of the near-endemic Brazilian
tribe Microlicieae (Melastomataceae). – Amer. J. Bot.
91: 1105-1114.
Fukuoka N, Ito M, Iwatsuki K. 1986. Floral
anatomy of the mangrove genus Lumnitzera (Combretaceae). – Acta Phytotaxon.
Geobot. 37: 69-81.
Furtado CX, Srisuko M. 1969. A revision of
Lagerstroemia L. (Lythraceae). – Gard. Bull. (Singapore)
24: 185-334.
Gadek PA, Martin HA. 1981. Pollen morphology
in the subtribe Metrosiderinae of the Leptospermoideae (Myrtaceae) and its taxonomic significance.
– Aust. J. Bot. 29: 159-184.
Gadek PA, Martin HA. 1982. Exine
ultrastructure of myrtaceous pollen. – Aust. J. Bot. 30: 75-86.
Gadek PA, Wilson PG, Quinn CJ. 1996.
Phylogenetic reconstruction in Myrtaceae using matK, with
particular reference to the position of Psiloxylon and
Heteropyxis. – Aust. Syst. Bot. 9: 283-290.
Gandolfo MA, Hermsen EJ, Zamaloa MC, Nixon
KC, González CC, Wilf P, Cúneo NR, Johnson KR. 2011. Oldest known
Eucalyptus macrofossils are from South America. – PloS ONE 6(6):
e21084. https://urlproxy.sunet.se/canit/urlproxy.php?_q=aHR0cDovL2R4LmRvaToxMA%3D%3D&_s=ZGVmYXVsdA%3D%3D&_c=ee90496a&_r=c3Utc2U%3D.1371/journal.pone.0021084
George AS, Chippendale GM. 1988. Myrtaceae – Eucalyptus,
Angophora. – In: George AS (ed), Flora of Australia 19, Australian
Government Publ. Service, Canberra, pp. 1-510.
Giaretta A, Menezes LFT, Peixoto AL. 2015.
Diversity of Myrtaceae in the
southeastern Atlantic forest of Brazil as a tool for conservation. – Braz. J.
Bot. 38: 175-185.
Gibbs AK, Udovicic F, Drinnan AN, Ladiges PY.
2009. Phylogeny and classification of Eucalyptus subgenus
Eudesmia (Myrtaceae) based on
nuclear ribosomal DNA, chloroplast DNA and morphology. – Aust. Syst. Bot. 22:
158-179.
Gilg E. 1894a. Penaeaceae. – In: Engler A, Prantl K
(eds), Die natürlichen Pflanzenfamilien III(6a), W. Engelmann, Leipzig, pp.
208-213.
Gilg E. 1894b. Oliniaceae. – In: Engler A, Prantl K
(eds), Die natürlichen Pflanzenfamilien III(6), W. Engelmann, Leipzig, pp.
213-216.
Gilg E. 1897. Melastomataceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien, Nachträge zu III(7), W.
Engelmann, Leipzig, pp. 263-268.
Gilg E. 1898. Monographieen afrikanischer
Pflanzen-Familien und -Gattungen 2. Melastomataceae. – Engelmann,
Leipzig.
Gilmartin AJ. 1981. Morphological variation
within five angiosperm families: Asclepiadaceae, Bromeliaceae,
Melastomataceae, Piperaceae, and Rubiaceae. – Syst.
Bot. 6: 331-345.
Gleason HA. 1929a. Studies on the flora of
northern South America XII. Cyphostyleae – a new tribe of Melastomataceae. – Bull. Torrey
Bot. Club 56: 97-100.
Gleason HA. 1929b. The genus
Monochaetum in South America. – Amer. J. Bot. 16: 502-522.
Gleason HA. 1931a. The relationships of
certain myrmecophilous melastomes. – Bull. Torrey Bot. Club 58: 73-85.
Gleason HA. 1931b. Studies on the flora of
northern South America XV. Recent collections of Melastomataceae from Peru and
Amazonian Brazil. – Bull. Torrey Bot. Club 58: 215-262.
Gleason HA. 1932. A synopsis of the Melastomataceae of British Guiana.
– Brittonia 1: 127-184.
Gleason HA. 1933. Studies on the flora of
northern South America XVIII. Plantae lawranceanae Colombianae. – Phytologia
1: 25-38.
Gleason HA. 1934. The genus Macairea
DC. in northern South America. – Bull. Torrey Bot. Club 61: 35-40.
Gleason HA. 1939a. The genus
Clidemia in Mexico and Central America. – Brittonia 3: 97-130.
Gleason HA. 1939b. A new genus of Melastomataceae from Peru. – Kew
Bull. 8: 480-481.
Gleason HA. 1945. On Blakea and
Topobea. – Bull. Torrey Bot. Club 72: 385-393.
Gleason HA. 1950. The genus
Tibouchina in southern Venezuela. – Phytologia 3: 238-243.
Gleason HA. 1958. Flora of Panama: Melastomataceae. – Ann. Missouri
Bot. Gard. 45: 203-304.
Goldenberg R, Amorim M. 2006.
Physeterostemon (Melastomataceae): a new genus and
two new species from the Bahian Atlantic forest, Brazil. – Taxon 55:
965-972.
Goldenberg R, Anjos Mendes Tavares R dos.
2007. A new species of Dolichoura (Melastomataceae) and broadened
circumscription of the genus. – Brittonia 59: 226-232.
Goldenberg R, Caddah MK. 2013. Taxonomic
notes on South American Miconia (Melastomataceae) III. – Phytotaxa
94: 13-22.
Goldenberg R, Martins AB. 1999. Two new Melastomataceae from São Paulo,
Brazil. – Kew Bull. 54: 465-470.
Goldenberg R, Reginato M. 2007. Three new
species of Melastomataceae from
the Southeastern Atlantic Forest of Brazil. – Brittonia 59: 334-342.
Goldenberg R, Teixeira SP, Martins AB. 2003.
Anther dehiscence and circumscription of Miconia sect.
Hypoxanthus (Melastomataceae). – Kew Bull. 58:
195-203.
Goldenberg R, Penneys DS, Almeda F, Judd WS,
Michelangeli FA. 2008. Phylogeny of Miconia (Melastomataceae): patterns of stamen
diversification in a megadiverse Neotropical genus. – Intern. J. Plant Sci.
169: 963-979.
Goldenberg R, Fraga CN de, Fontana AP,
Nicolas AN, Michelangeli FA. 2012. Taxonomy and phylogeny of
Merianthera (Melastomataceae). – Taxon 61:
1040-1056.
Goldenberg R, Almeda F, Caddah MK, Martins
AB, Meirelles J, Michelangeli FA, Weiss M. 2013. Nomenclator botanicus for the
neotropical genus Miconia (Melastomataceae: Miconieae). –
Phytotaxa 106: 1-171.
Goldenberg R, Almeda F, Sosa K, Ribeiro RC,
Michelangeli FA. 2015. Rupestrea: a new Brazilian genus of Melastomataceae, with anomalous
seeds and dry indehiscent fruits. – Syst. Bot. 40: 561-571.
Goloneva LB. 1991. The new genus
Paleotrapa (Trapaceae?) and new species Quereuxia from the
Rarytkin Series (the Koryak upland, the Maastrichtian-Danian). – Bot. Žurn.
76: 601-610.
González Elizondo MS, López Enriquez IL,
Wagner WL. 2002. Megacorax gracielana (Onagraceae), a new genus and species from
Durango, México. – Novon 12: 360-365.
Gottlieb LD. 1986. Genetic differentiation,
speciation and phylogeny in Clarkia (Onagraceae). – In: Iwatsuki K, Raven
PH, Bock WJ (eds), Modern aspects of species, University of Tokyo Press, Tokyo,
pp. 145-160.
Gottlieb LD, Ford VS. 1996. Phylogenetic
relationships among the sections of Clarkia (Onagraceae) inferred from the nucleotide
sequences of PgiC. – Syst. Bot. 21: 45-62.
Gottschall M. 1900. Anatomisch-systematische
Untersuchung des Blattes der Melastomataeeen aus der Tribus Miconieae. –
Mém. Herb. Boiss. 19: 1-176.
Govaerts R, Sobral M, Ashton P, Barrie F,
Holst B, Landrum L, Matsumoto K, Mazine F, Nic Lughadha E, Proença C,
Soares-Silva L, Wilson P, Lucas E. 2008. World checklist of Myrtaceae. – Royal Botanical Gardens,
Kew.
Graham A. 1980. Morfología del polen de
Eugenia-Myrcia (Myrtaceae) y
Combretum-Terminalia (Combretaceae) en relacion a su alcance
estratigrafico en el Terciario del Caribe. – Biotica 5: 5-14.
Graham A, Graham SA. 1971. The geologic
history of the Lythraceae. –
Brittonia 23: 335-346.
Graham A, Nowicke JW, Skvarla JJ, Graham SA,
Patel V, Lee S. 1985. Palynology and systematics of the Lythraceae I. Introduction and genera
Adenaria through Ginoria. – Amer. J. Bot. 72: 1012-1031.
Graham A, Nowicke JW, Skvarla JJ, Graham SA,
Patel V, Lee S. 1987. Palynology and systematics of the Lythraceae II. Genera Haitia
through Peplis. – Amer. J. Bot. 74: 829-850.
Graham A, Graham SA, Nowicke JW, Patel V, Lee
S. 1990. Palynology and systematics of the Lythraceae III. Genera
Physocalymma through Woodfordia, addenda and conclusions. –
Amer. J. Bot. 77: 159-177.
Graham SA. 1977. The American species of
Nesaea and their relationship to Heimia and Decodon.
– Syst. Bot. 2: 61-71.
Graham SA. 1980. Cuphea inflata (Lythraceae), a new species from Mexico.
– Syst. Bot. 5: 322-325.
Graham SA. 1984 [1985]. Alzateaceae, a new family of Myrtales in the American tropics. – Ann.
Missouri Bot. Gard. 71: 757-779.
Graham SA. 1985. A revision of
Ammannia in the Western Hemisphere. – J. Arnold Arbor. 66:
395-420.
Graham SA. 1989a. Chromosome numbers in
Cuphea (Lythraceae): new
counts and a summary. – Amer. J. Bot. 76: 1530-1540.
Graham SA. 1989b. Cuphea: a new
plant source of medium-chain fatty acids. – CRC Crit. Rev. Food Sci.
Nutrition 28: 139-173.
Graham SA. 1989c. Revision of Cuphea
sect. Leptocalyx (Lythraceae). – Syst. Bot. 14: 43-76.
Graham SA. 1992. New chromosome counts in Lythraceae – systematic and
evolutionary implications. – Acta Bot. Mexicana 17: 45-51.
Graham SA. 1995a. Two new species in
Cuphea (Lythraceae), and a
note on Alzateaceae. – Novon 5:
272-277.
Graham SA. 1995b. Systematics of
Woodfordia (Lythraceae). –
Syst. Bot. 20: 482-502.
Graham SA. 2001. The problematic typification
of Cuphea (Lythraceae). –
Taxon 50: 487-490.
Graham SA. 2003. Biogeographic patterns of
Antillean Lythraceae. – Syst. Bot.
28: 410-420.
Graham SA. 2006a. Alzateaceae. – In: Kubitzki K (ed),
The families and genera of vascular plants IX. Flowering plants. Eudicots. Berberidopsidales,
Buxales, Crossosomatales, Fabales p. p., Geraniales, Gunnerales, Myrtales p. p., Proteales, Saxifragales, Vitales, Zygophyllales, Clusiaceae Alliance, Passifloraceae
Alliance, Dilleniaceae, Huaceae, Picramniaceae, Sabiaceae, Springer, Berlin,
Heidelberg, New York, pp. 26-28.
Graham SA. 2006b. Lythraceae. – In: Kubitzki K (ed), The
families and genera of vascular plants IX. Flowering plants. Eudicots. Berberidopsidales,
Buxales, Crossosomatales, Fabales p. p., Geraniales, Gunnerales, Myrtales p. p., Proteales, Saxifragales, Vitales, Zygophyllales, Clusiaceae Alliance, Passifloraceae
Alliance, Dilleniaceae, Huaceae, Picramniaceae, Sabiaceae, Springer, Berlin,
Heidelberg, New York, pp. 226-246.
Graham SA. 2013. Fossil records in the Lythraceae. – Bot. Rev. 28: 410-420.
Graham SA. 2017. A revision of
Cuphea Section Brachyandra s. s. (Lythraceae). – Syst. Bot. 42:
859-919.
Graham SA, Averett JE. 1984. Flavonoids of Alzateaceae (Myrtales). – Ann. Missouri Bot. Gard. 71:
855-857.
Graham SA, Cavalcanti TB. 1999a. The
yellow-flowered species of Cuphea (Lythraceae), including three new taxa.
– Brittonia 51: 24-30.
Graham SA, Cavalcanti TB. 1999b. A new
yellow-flowered species of Cuphea (Lythraceae) from Bolivia. – Brittonia
51: 303-306.
Graham SA, Cavalcanti TB. 2001. New
chromosome counts in the Lythraceae
and a review of chromosome numbers in the family. – Syst. Bot. 26:
445-458.
Graham SA, Cavalcanti TB. 2013. Taxonomic
revision of Cuphea sect. Euandra subsect. Oidemation
(Lythraceae). – Phytotaxa 113:
1-86.
Graham SA, Graham A. 2000. The evolution of
diaperturate pollen in the eurypalynous genus Cuphea (Lythraceae). – In: Harley MM, Morton
CM, Blackmore S (eds), Pollen and spores, morphology and biology, Royal Botanic
Gardens, Kew, pp. 349-364.
Graham SA, Graham A. 2014. Ovary, fruit, and
seed morphology of the Lythraceae. –
Intern. J. Plant Sci. 175: 202-240.
Graham SA, Kleiman R. 1987. Seed lipids of
the Lythraceae. – Biochem. Syst.
Ecol. 15: 433-439.
Graham SA, Lorence DH. 1978. The rediscovery
of Tetrataxis Hooker fil. (Lythraceae). – Bot. J. Linn. Soc. 76:
71-82.
Graham SA, Tobe H, Baas P. 1986.
Koehneria, a new genus of Lythraceae from Madagascar. – Ann.
Missouri Bot. Gard. 73: 788-809.
Graham SA, Baas P, Tobe H. 1987.
Lourtella, a new genus of Lythraceae from Peru. – Syst. Bot. 12:
519-533.
Graham SA, Crisci JV, Hoch PC. 1993.
Cladistic analysis of the Lythraceae
sensu lato based on morphological characters. – Bot. J. Linn. Soc.
113: 1-33.
Graham SA, Oginuma K, Raven PH, Tobe H. 1993.
Chromosome numbers in Sonneratia and Duabanga (Lythraceae s.l.) and their systematic
significance. – Taxon 42: 35-41.
Graham SA, Thorne RF, Reveal JL. 1998.
Validation of subfamily names in Lythraceae. – Taxon 47: 435-436.
Graham SA, Hall J, Sytsma K, Shi S-H. 2005.
Phylogenetic analysis of the Lythraceae based on four gene regions and
morphology. – Intern. J. Plant Sci. 166: 995-1017.
Graham SA, Freudenstein JV, Luker M. 2006. A
phylogenetic study of Cuphea (Lythraceae) based on morphology and
nuclear rDNA ITS sequences. – Syst. Bot. 31: 764-778.
Graham SA, Diazgranados M, Barber JC. 2011.
Relationships among the confounding genera Ammannia,
Hionanthera, Nesaea, and Rotala (Lythraceae). – Bot. J. Linn. Soc. 166:
1-19.
Granados ERB. 2012. Taxonomía de
Pachyanthus (Melastomataceae: Miconieae). –
Brittonia 64: 179-207.
Green JW. 1979. Corynanthera, a new
genus of Myrtaceae (Subfamily
Leptospermoideae, Tribe Chamelaucieae). – Nuytsia 2: 368-372.
Greenwood DR, Wilson PG, Christophel DC,
Basinger JF. 2007. Fossil flowers and fruits of capsular Myrtaceae from the Eocene of South
Australia. – Can. J. Bot. 85: 204-215.
Gregory DP, Klein WM. 1960. Investigations on
meiotic chromosomes of six genera in the Onagraceae. – Aliso 4: 505-521.
Griffiths ME. 1959. A revision of the African
species of Terminalia. – Bot. J. Linn. Soc. 55: 818-907.
Grifo FT. 1992. A revision of
Myrcianthes Berg (Myrtaceae).
– Ph.D. diss., Cornell University, Ithaca, New York.
Grimm D, Almeda F. 2013. Systematics,
phylogeny and biogeography of Chaetolepis (Melastomataceae). – J. Bot. Res.
Inst. Texas 7: 217-263.
Grímsson F, Zetter R, Hofmann C-C. 2011.
Lythrum and Peplis from the Late Cretaceous and Cenozoic of
North America and Eurasia: new evidence suggesting early diversification within
the Lythraceae. – Amer. J. Bot. 98:
1801-1815.
Grímsson F, Ferguson DK, Zetter R. 2012.
Morphological trends in the fossil pollen of Decodon and the
paleobiogeographic history of the genus. – Intern. J. Plant Scil 173:
297-317.
Grímsson F, Zetter R, Leng Q. 2012. Diverse
fossil Onagraceae pollen from a
Miocene palynoflora of north-east China: early steps in resolving the
phytogeographic history of the family. – Plant Syst. Evol. 298: 671-687.
Groenendijk JP, Bouman F, Cleef AM. 1996. An
exploratory study on the seed morphology of Miconia Ruíz and Pavon
(Melastomataceae), with taxonomic
and ecological implications. – Acta Bot. Neerl. 45: 323-344.
Gross CL. 1993. The breeding system and
pollinators of Melastoma affine (Melastomataceae); a pioneer shrub in
tropical Australia. – Biotropica 25: 468-474.
Grütter W. 1893. Ueber den Bau und die
Entwickelung der Samenschalen einiger Lythrarieen. – Bot. Zeit. 5, Abt. 1:
1-26.
Guimarães AR, Baumgratz JFA, Vieira RC.
2014. First report of reticulate perforation plates in the Melastomataceae. – IAWA J. 35:
12-18.
Guimarães PJF, Silva MFO da, Da Rocha MJR.
2017. Nomenclator botanicus for Acisanthera (Melastomataceae: Marcetia alliance).
– Brittonia 69: 231-240.
Guleria JS. 1991. On the occurrence of
carbonised woods resembling Terminalia and Sonneratia in
Palaeogene deposits of Gujarat, western India. – Palaeobotany 39: 1-8.
Hallam ND. 1970. Growth and regeneration of
waxes on the leaves of Eucalyptus. – Planta 93: 257-268.
Hallam ND, Chambers TC. 1970. The leaf waxes
of the genus Eucalyptus L’Heritier. – Aust. J. Bot. 18:
335-386.
Hansen C. 1977. The Asiatic species of
Osbeckia. – Ginkgoana 4: 1-150.
Hansen C. 1979. A revision of the genus
Sarcopyramis Wall. (Melastomataceae). – Bot. Tidsskr.
73: 177-184.
Hansen C. 1980a. Neodriessenia
membranifolia (Li) C. Hansen, comb. nov. (Melastomataceae). – Adansonia 20:
321-324.
Hansen C. 1980b. A revision of
Barthea. – Notes Roy. Bot. Gard. Edinb. 38: 489-493.
Hansen C. 1981. A revision of
Ochthocharis (Melastomataceae), including
Phaeoneuron of Africa. – Kew Bull. 36: 13-29.
Hansen C. 1982a. A revision of
Blastus Lour. (Melastomataceae). – Bull. Mus.
Natl. d’Hist. Nat. (Paris), B, Adansonia 44: 43-77.
Hansen C. 1982b. New species and combinations
in Anerincleistus, Driessenia, Oxyspora and
Phyllagathis (Melastomataceae) in Sarawak. –
Nord. J. Bot. 2: 557-559.
Hansen C. 1984. Vietsenia C. Hansen,
a new genus of the Melastomataceae from Vietnam. –
Bull. Mus. Natl. d’Hist. Nat. (Paris), sect. B, Adansonia 46: 147-156.
Hansen C. 1985a. Taxonomic revision of
Driessenia (Melastomataceae). – Nord. J. Bot.
5: 335-352.
Hansen C. 1985b. A revision of the genus
Neodriessenia Naya (Melastomaceae). – Bot. Jahrb. Syst. 106:
1-13.
Hansen C. 1985c. The genus Brittenia
(Melastomataceae). –
Willdenowia 15: 171-173.
Hansen C. 1985d. Stussenia, a new
genus in the Melastomataceae. –
Willdenowia 15: 175-176.
Hansen C. 1987a. Poilannammia C.
Hansen (Melastomataceae), a new
genus of four new species endemic to Vietnam. – Bull. Mus. Natl. Hist. Nat.,
sect. B, Adansonia 3: 263-271.
Hansen C. 1987b. Aschistanthera, a
monotypic new genus for Vietnam (Melastomataceae). – Nord. J. Bot.
7: 653-654.
Hansen C. 1988a. The genus Campimia
(Melastomataceae). –
Willdenowia 17: 147-151.
Hansen C. 1988b. Kerriothyrsus, a
new genus of Melastomataceae. –
Willdenowia 17: 153-157.
Hansen C. 1988c. A revision of the genus
Plagiopetalum Rehd. (Melastomataceae). – Bull. Mus.
Natl. Hist. Nat., sect. B, Adansonia 2: 127-136.
Hansen C. 1990a. Tylanthera (Melastomataceae), a new genus of two
species endemic to Thailand. – Nord. J. Bot. 9: 631-635.
Hansen C. 1990b. The monotypic genus
Cyphotheca (Melastomataceae). – Nord. J. Bot.
10: 21-23.
Hansen C. 1992. The genus
Phyllagathis (Melastomataceae): characteristics;
delimitation; the species in Indo-China and China. – Bull. Mus. Natl. Hist.
Nat., Sect. B, Adansonia, Bot. Phytochim. 14: 355-428.
Hansen C, Wickens GE. 1981. A revision of
Ochthocharis (Melastomataceae) including
Phaeoneuron in Africa. – Kew Bull. 36: 13-29.
Harrington MG, Gadek PA. 2004. Molecular
systematics of the Acmena alliance (Myrtaceae): phylogenetic analyses and
evolutionary implications with reference to Australian taxa. – Aust. Syst.
Bot. 17: 63-72.
Harrington MG, Jackes BR, Barrett MD, Craven
LA, Barrett RL. 2013. Phylogenetic revision of Backhousieae (Myrtaceae): Neogene divergence, a revised
circumscription of Backhousia and two new species. – Aust. Syst.
Bot. 25: 404-417.
Harte C. 1993. Oenothera:
contributions of a plant to biology. – Monogr. Theor. Appl. Genet. 20,
Springer, London.
Hartley TG, Craven LA. 1977. A revision of
the Papuasian species of Acmena (Myrtaceae). – J. Arnold Arbor. 58:
325-342.
Hartley TG, Perry LM. 1973. A provisional key
and enumeration of species of Syzygium (Myrtaceae) from Papuasia. – J. Arnold
Arbor. 54: 160-227.
Haussknecht C. 1884. Monographie der Gattung
Epilobium. – Gustav Fischer, Jena.
Heiden H. 1893. Anatomische Characteristik
der Combretaceen. – Bot. Centralbl. 55: 353-360, 385-391; 56: 1-12, 65-75,
129-136, 163-170, 193-200, 225-230.
Henderson MR. 1949. The genus
Eugenia (Myrtaceae) in Malaya.
– Gard. Bull. Straits Settlem. (Singapore) 12: 1-293.
Hermsen EJ, Gandolfo MA, Carmen Zamaloa M
del. 2012. The fossil record of Eucalyptus in Patagonia. – Amer. J.
Bot. 99: 1356-1374.
Heslop-Harrison Y. 1990. Stigma form and
surface in relation to self-incompatibility in the Onagraceae. – Nord. J. Bot. 10:
1-19.
Hewson HJ. 1990. Sonneratiaceae. – In:
George AS (ed), Flora of Australia 18, Australian Government Publ. Service,
Canberra, pp. 87-91.
Hewson HJ, Beesley PL. 1990. Lythraceae. – In: George AS (ed), Flora
of Australia 18, Australian Government Publ. Service, Canberra, pp. 91-113.
Hill KD. 1997a. New species in
Angophora and Eucalyptus (Myrtaceae) from New South Wales. –
Telopea 7: 97-109.
Hill KD. 1997b. New taxa in
Eucalyptus (Myrtaceae) from
New South Wales and Queensland. – Telopea 7: 187-198.
Hill KD. 1999. A taxonomic revision of the
white mahoganies, Eucalyptus series Acmenoideae (Myrtaceae). – Telopea 8: 219-247.
Hill KD, Johnson LAS. 1991a. Systematic
studies in the eucalypts 3. New taxa and combinations in Eucalyptus
(Myrtaceae). – Telopea 4: 223-267.
Hill KD, Johnson LAS. 1991b. Systematic
studies in the eucalypts 4. New taxa in Eucalyptus (Myrtaceae). – Telopea 4: 321-349.
Hill KD, Johnson LAS. 1992. Systematic
studies in the eucalypts 5. New taxa and combinations in Eucalyptus
(Myrtaceae) in Western Australia. –
Telopea 4: 561-634.
Hill KD, Johnson LAS. 1994. Systematic
studies in the eucalypts 6. A revision of the coolabahs, Eucalyptus
subgenus Symphyomyrtus section Adnataria series
Oliganthae subseries Microthecosae (Myrtaceae). – Telopea 5: 743-771.
Hill KD, Johnson LAS. 1995. Systematic
studies in the eucalypts 7. A revision of the bloodwoods, genus
Corymbia (Myrtaceae). –
Telopea 6: 185-504.
Hill KD, Johnson LAS. 1998. Systematic
studies in the eucalypts 8. A review of the eudesmioid eucalypts,
Eucalyptus subgenus Eudesmia. – Telopea 7: 375-414.
Hill KD, Johnson LAS. 2000. Systematic
studies in the eucalypts 10. New tropical and subtropical eucalypts from
Australia and New Guinea (Eucalyptus, Myrtaceae). – Telopea 8: 503-539.
Hill KD, Johnson LAS, Blaxell DF. 2001.
Systematic studies in the eucalypts 11. New taxa and combinations in
Eucalyptus Section Dumaria (Myrtaceae). – Telopea 9: 259-318.
Hnatiuk RJ. 1993. A revision of the genus
Eremaea (Myrtaceae). –
Nuytsia 9: 137-222.
Hoch PC, Raven PH. 1980. A new combination in
Epilobium (Onagraceae). –
Madroño 27: 146.
Hoch PC, Raven PH. 1992.
Boisduvalia, a coma-less Epilobium (Onagraceae). – Phytologia 73:
456-459.
Hoch PC, Crisci JV, Tobe H, Berry PE. 1993. A
cladistic analysis of the plant family Onagraceae. – Syst. Bot. 18: 31-47.
Hoch PC, Wagner WL, Raven P. 2015. The
correct name for a section of Ludwigia L. (Onagraceae). – PhytoKeys 50: 31-34.
Hoehne FC. 1922. Melastomáceas. – Mem.
Inst. Butantan, Sec. Bot. 1: 1-198.
Hofmeyr J, Phillips EP. 1922. The genus
Olinia. – Bothalia 1: 97-104.
Hoggard GD, Kores PJ, Molvray M, Hoggard RK.
2004. The phylogeny of Gaura (Onagraceae) based on ITS, ETS, and
trnL-F sequence data. – Amer. J. Bot. 91: 139-148.
Holsinger KE. 1985. A phonetic study of
Clarkia unguiculata Lindley (Onagraceae) and its relatives. – Syst.
Bot. 10: 155-165.
Holsinger KE, Gottlieb LD. 1988. Isozyme
variability in the tetraploid Clarkia gracilis (Onagraceae) and its diploid relatives.
– Syst. Bot. 13: 1-6.
Holst BK, Kawasaki ML. 2000. Two new species
of Eugenia (Myrtaceae) from
Central French Guiana. – Brittonia 52: 20-23.
Holub J. 1972. Taxonomic and nomenclatural
remarks on Chamaenerion. – Folia Geobot. Phytotaxon. 7: 81-90.
Hooker JD. 1860. On Fropiera, a new
Mauritian genus of calycifloral exogens, of doubtful affinity. – J. Proc.
Linn. Soc. (Bot.) 5: 1-2.
Hopper SD, McQuoid NK. 2009. Two new rare
species and a new hybrid in Eucalyptus series Tetrapterae (Myrtaceae) from southern coastal Western
Australia. – Aust. Syst. Bot. 22: 180-192.
Huang Y-L, Shi S-H. 2002. Phylogenetics of Lythraceae sensu lato: a preliminary
analysis based on chloroplast rbcL gene, psaA-ycf3
spacer, and nuclear rDNA internal transcribed spacer (ITS) sequences. –
Intern. J. Plant Sci. 163: 215-225.
Huertas G. 1977. Una Melastomatácea fósil
del Terciario carboneifero de Antioquia (Eoceno). – Caldasia 12: 35-39.
Hung K-H, Schaal BA, Hsu T-W, Chiang Y-C,
Peng C-I, Chiang T-Y. 2009. Phylogenetic relationships of diploid and polyploid
species in Ludwigia sect. Isnardia (Onagraceae) based on chloroplast and
nuclear DNAs. – Taxon 58: 1216-1225.
Hunter JT. 1998. Two new rare species of
Homoranthus (Myrtaceae:
Chamelaucieae) from the Northern Tablelands of New South Wales. – Telopea 8:
35-40.
Hunter JT, Bruhl JJ. 1999. Two new species of
Eucalyptus (Myrtaceae) from
northern New South Wales (series Viminales section
Maidenaria). – Telopea 8: 257-263.
Hupfer H, Swiatek M, Hornung S, Herrmann RG,
Maier RM, Chiu WL, Sears B. 2000. Complete nucleotide sequence of the
Oenothera elata plastid chromosome, representing plastome I of the
five distinguishable Euoenothera plastomes. – Mol. Gen. Genet. 263:
581-585.
Hyland BPM. 1983. A revision of
Syzygium and allied genera (Myrtaceae) in Australia. – Aust. J.
Bot., Suppl. Ser. 9: 1-164.
Hyland BPM, Steenis CGGJ van. 1973. The
generic identity of Xanthostemon brachyandrus C. T. White:
Lindsayomyrtus novum genus (Myrtaceae). – Blumea 21: 189-192.
Iconomidis J. 1958. La formation de l’ovule
et la séminogenèse chez le Clidemia hirta D. Don
(Mélastomatacées). – Bull. Soc. Bot. France 105: 230-234.
Immelman KL. 1991. Synopsis of the genera
Nesaea and Ammannia (Lythraceae) in southern Africa. –
Bothalia 21: 35-49.
Ingle HD, Dadswell HE. 1953. The anatomy of
the timbers of the south-west Pacific area III. Myrtaceae. – Aust. J. Bot. 1:
353-401.
Inglis PW, Cavalcanti TB. 2018. A molecular
phylogeny of the genus Diplusodon (Lythraceae), endemic to the campos
rupestres and cerrados of South America. – Taxon 67: 66-82.
Ionta GM, Judd WS, Williams NH, Whitten WM.
2007. Phylogenetic relationships in Rhexia (Melastomataceae): evidence from DNA
sequence data and morphology. – Intern. J. Plant Sci. 168: 1055-1066.
Jackson HD, Steane DA, Potts BM, Vaillancourt
RE. 1999. Chloroplast DNA evidence for reticulate evolution in
Eucalyptus (Myrtaceae). –
Mol. Ecol. 8: 739-751.
Jacono CC, Vandiver VV Jr. 2007. Rotala
rotundifolia, purple loosestrife of the south? – Aquatics 29: 4-9.
Jacques-Félix H. 1932. Sur quelques
melastomacées nouvelles ou peu connues de l’Afrique occidentale. – Bull.
Mus. Natl. Hist. Nat., sér. 2, 6: 678-687.
Jacques-Félix H. 1939. Sur quelques
Melastomacées africaines. – Bull. Mus. Natl. Hist. Nat., sér. 2, 10:
630-642.
Jacques-Félix H. 1951. Un nouveau genre
africain de Melastomaceae. – Bull. Mus. Natl. Hist. Nat. Paris 23:
448-452.
Jacques-Félix H. 1953. Sur quelques
Mélastomacées d’Afrique. – Bull. Inst. Franç. Afr. Noire 15:
972-1001.
Jacques-Félix H. 1973. Contribution à
l’étude du genre Rousseauxia (Melast.). – Adansonia, sér. II,
13: 177-193.
Jacques-Félix H. 1974a. Le genre
Amphiblemma Naud. (Mélatomacées). – Adansonia, sér. II, 13:
429-459.
Jacques-Félix H. 1974b. Le genre
Dicellandra Hook. f. (Mélastomacées). – Adansonia, sér. II, 14:
77-98.
Jacques-Félix H. 1974 [1975]. Le genre
Melastomastrum Naudin (Melastomataceae). – Bull. Mus.
Natl. Hist. Nat., sér. III, Bot. 17: 49-83.
Jacques-Félix H. 1975. Le genre
Cincinnobotrys Gilg (Mélastomatacées). – Adansonia, sér. II, 16:
355-377.
Jacques-Félix H. 1976. Le genre
Tristemma Jussieu (Melastomataceae). – Bull. Mus.
Natl. Hist. Nat., sér. III, Bot. 28: 137-206.
Jacques-Félix H. 1977a. Le genre
Preussiella Gilg (Mélastomatacées). – Adansonia, sér. II, 16:
405-414.
Jacques-Félix H. 1977b. La graine et
l’embryon chez les Memecylon (Mélastomatacées) africains. –
Adansonia, sér. II, 17: 193-203.
Jacques-Félix H. 1978a. Les subdivisions du
genre Memecylon (Melastomataceae) en Afrique. –
Adansonia, sèr. 17: 415-424.
Jacques-Félix H. 1978b. Les genres de
Memecyleae (Melastomataceae) en
Afrique, Madagascar et Mascareignes. – Adansonia, sér. II, 18: 221-235.
Jacques-Félix H. 1979. Espèces nouvelles et
peu connues du genre Memecylon (Melastomataceae) en Afrique. –
Adansonia, sér II, 18: 409-432.
Jacques-Félix H. 1981a. Révision du genre
Calvoa (Melastomataceae). – Bull. Mus.
Natl. Hist. Nat., sect. B, Adansonia 2: 123-143.
Jacques-Félix H. 1981b. Observations sur les
caractères staminaux et la classification des Osbeckieae (Melastomataceae) capsulaires
africaines. – Adansonia, sér. II, 20: 405-429.
Jacques-Félix H. 1984. Les Memecyleae (Melastomataceae) de Madagascar
(1re partie). – Bull. Mus. Natl. Hist. Nat., sect. B, Adansonia 6:
383-451.
Jacques-Félix H. 1985. Les Memecyleae (Melastomataceae) de Madagascar
(2ème partie). – Bull. Mus. Natl. Hist. Nat., sect. B, Adansonia
7: 3-58.
Jacques-Félix H. 1994 [1995]. Histoire des
Melastomataceae d’Afrique. –
Bull. Mus. Natl. Hist. Nat. Paris, sér. IV, 16, sect. B, Adansonia 2-4:
235-311.
Jacques-Félix H, Mouton JA. 1980.
Identification des Memecyleae (Melastomataceae) de
l’Ouest-Africain d’après leurs caractères végétatifs. – Bull. Mus.
Natl. Hist. Nat. (Paris), sér. IV, sect. B, 2: 13-19.
Jacques-Félix H, Mouton JA, Chalopin M.
1978. Nervation et types foliaires chez les Memecylon (Melastomataceae) africains. –
Adansonia, sér. II, 18: 67-81.
Jansen S, Watanabe T, Smets E. 2002.
Aluminium accumulation in leaves of 127 species in Melastomataceae, with comments on
the order Myrtales. – Ann. Bot. 90:
53-64.
Jansen S, Pletsers A, Rabaey D, Lens F. 2008.
Vestured pits: a diagnostic character in secondary xylem of Myrtales. – J. Trop. Forest Sci. 20:
147-155.
Jayaweera DMA. 1967. The genus
Duabanga. – J. Arnold Arbor. 48: 89-100.
Jeník J. 1970. Root system of tropical trees
5. The peg-roots and the pneumathodes of Laguncularia racemosa Gaertn.
– Preslia 42: 105-113.
Johansen DA. 1928. The hypostase: its
presence and function in the ovule of Onagraceae. – Proc. Natl. Acad. Sci.
U.S.A. 14: 710-714.
Johansen DA. 1931. Studies on the morphology
of Onagraceae IV. Stenosiphon
linifolium. – Bull. Torrey Bot. Club 57: 315-326.
Johansen DA. 1934. Studies on the morphology
of the Onagraceae VIII. Circaea
pacifica. – Amer. J. Bot. 21: 508-510.
Johnson CT. 1984. The wood anatomy of
Leptospermum Forst. (Myrtaceae). – Aust. J. Bot. 32:
323-335.
Johnson LAS. 1972. Evolution and
classification in Eucalyptus. – Proc. Linn. Soc. New South Wales 97:
11-29.
Johnson LAS. 1976. Problems of species and
genera in Eucalyptus (Myrtaceae). – Plant Syst. Evol. 125:
155-167.
Johnson LAS, Blaxell DF. 1980. New taxa and
combinations in Eucalyptus 4. – Telopea 1: 395-397.
Johnson LAS, Briggs BG. 1984 [1985]. Myrtales and Myrtaceae – a phylogenetic analysis. –
Ann. Missouri Bot. Gard. 71: 700-756.
Johnson LAS, Hill KD. 1990. New taxa and
combinations in Eucalyptus and Angophora (Myrtaceae). – Telopea 4: 37-108.
Johnson LAS, Hill KD. 1991. Systematic
studies in the eucalypts 2. A revision of the gimlets and related species:
Eucalyptus extracodical series Salubres and
Annulatae (Myrtaceae). –
Telopea 4: 201-222.
Johnson LAS, Hill KD. 1999. Systematic
studies in the eucalypts 9. A review of series Sociales
(Eucalyptus subgenus Symphyomyrtus, Section
Bisectaria, Myrtaceae). –
Telopea 8: 165-218.
Johnson MTJ, FitzJohn RG, Smith SD, Rauscher
MD, Otto SP. 2011. Loss of sexual recombination and segregation is associated
with increased diversification in evening primroses. – Evolution 65:
3230-3240.
Jongkind CCH. 1990. Novitates Gabonenses 6.
Some critical observations on Combretum versus Quisqualis (Combretaceae) and description of two
new species of Combretum. – Bull. Mus. Natl. Hist. Nat., sect. B,
Adansonia 12: 275-280.
Jongkind CCH. 1995a. Review of the genus
Strephonema (Combretaceae).
– Ann. Missouri Bot. Gard. 82: 535-541.
Jongkind CCH. 1995b. Prodromus for a revision
of Combretum (Combretaceae)
for Madagascar. – Bull. Mus. Natl. Hist. Nat., sect. B, Adansonia 17:
191-200.
Jongkind CCH. 2014. Notes of African
Combretum Loefl. species (Combretaceae). – Adansonia, sér. 3,
36: 315-327.
Jordaan M, Wyk AE van, Maurin O. 2011.
Generic status of Quisqualis (Combretaceae), with notes on the
taxonomy and distribution of Q. parviflora. – Bothalia 41:
161-169.
Joshi AC, Venkateswarlu J. 1935a.
Embryological studies in the Lythraceae I. Lawsonia inermis
Linn. – Proc. Indian Acad. Sci., Sect. B, 2: 481-493.
Joshi AC, Venkateswarlu J. 1935b.
Embryological studies in the Lythraceae II. Lagerstroemia
Linn. – Proc. Indian Acad. Sci., Sect. B, 2: 523-534.
Joshi AC, Venkateswarlu J. 1936.
Embryological studies in the Lythraceae III. – Proc. Indian Acad.
Sci., Sect. B, 3: 377-400.
Judd WS. 1986a. Taxonomic studies in the
Miconieae (Melastomataceae) I.
Variation in inflorescence position. – Brittonia 38: 150-161.
Judd WS. 1986b. Taxonomic placement of
Calycogonium squamulosum (Melastomataceae: Miconieae). –
Brittonia 38: 238-242.
Judd WS. 1989. Taxonomic studies in the
Miconieae (Melastomataceae) III.
Cladistic analysis of axillary-flowered taxa. – Ann. Missouri Bot. Gard. 76:
476-495.
Judd WS. 2007. Revision of Miconia
sect. Chaenopleura (Miconieae, Melastomataceae) in the Greater
Antilles. – Syst. Bot. Monogr. 81: 1-235.
Judd WS, Skean Jr JD. 1987. Two new species
of Meriania (Melastomataceae) from Hispaniola.
– Syst. Bot. 12: 374-380.
Judd WS, Skean Jr JD. 1991. Taxonomic studies
in the Miconieae (Melastomataceae) IV. Generic
realignments among terminal-flowered taxa. – Bull. Florida Mus. Nat. Hist.,
Biol. Sci. 36: 25-84.
Judd WS, Penneys DS, Skean Jr JD. 2004.
Rediscovery of Ossaea alloeotricha, an endemic of the high-elevation
Massif de la Hotte, Haiti, and its transfer to Miconia (Melastomataceae: Miconieae). –
Brittonia 56: 159-165.
Judd WS, Skean JD Jr, Penneys DS,
Michelangeli FA. 2008. A new species of Henriettea (Melastomataceae) from the Sierra de
Baoruco, the Dominican Republic. – Brittonia 60: 217-227.
Kadereit G. 2006. Revision of
Plethiandra Hook. f.: a polystaminate, East Asian genus of Melastomataceae. – Edinb. J. Bot.
62: 127-144.
Kadono Y, Schneider EL. 1986. Floral biology
of Trapa natans var. japonica. – Bot. Mag. (Tokyo) 99:
435-439.
Katinas L, Crisci J, Wagner WL, Hoch PC.
2004. Geographical diversification of tribes Epilobieae, Gongylocarpeae, and
Onagreae (Onagraceae) in North
America, based on parsimony analysis of endemicity and track compatibility
analysis. – Ann. Missouri Bot. Gard. 91: 159-185.
Kausel E. 1947 [1948]. Notas Mirtológicas.
– Lilloa 13: 125-149.
Kausel E. 1956. Beitrag zur Systematik der
Myrtaceen 1-2. – Ark. f. Bot. 3: 491-516, 607-611.
Kausel E. 1966. Lista de las Mirtáceas y
Leptospermáceas Argentinas. – Lilloa 32: 323-368.
Kawasaki ML. 1998. Systematics of
Erisma (Vochysiaceae). –
Mem. New York Bot. Gard. 81: 1-40.
Kawasaki ML. 2006. Vochysiaceae. – In: Kubitzki K (ed),
The families and genera of vascular plants IX. Flowering plants. Eudicots. Berberidopsidales,
Buxales, Crossosomatales, Fabales p. p., Geraniales, Gunnerales, Myrtales p. p., Proteales, Saxifragales, Vitales, Zygophyllales, Clusiaceae Alliance, Passifloraceae
Alliance, Dilleniaceae, Huaceae, Picramniaceae, Sabiaceae, Springer, Berlin,
Heidelberg, New York, pp. 480-487.
Kawasaki ML, Holst BK. 2002. Two new species
of Plinia (Myrtaceae) from
coastal forests of Brazil. – Brittonia 54: 94-98.
Keating RC. 1982 [1983]. The evolution and
systematics of Onagraceae: leaf
anatomy. – Ann. Missouri Bot. Gard. 69: 770-803.
Keating RC. 1984 [1985]. Leaf histology and
its contribution to relationships in the Myrtales. – Ann. Missouri Bot. Gard. 71:
801-823.
Keay RWJ. 1950. The systematic position of
suffrutescent species of Combretum Loefl. – Kew Bull. 5: 255-257.
Keay RWJ, Stafleu FA. 1953.
Erismadelphus. – Acta Bot. Neerl. 1: 594-599.
Keating RC, Hoch PC, Raven PH. 1982.
Perennation in Epilobium (Onagraceae) and its relation to
classification and ecology. – Syst. Bot. 7: 379-404.
Keighery GJ. 1975. Parallel evolution of
floral structures in Darwinia (Myrtaceae) and Pimelea (Thymelaeaceae). – West.
Aust. Natur. 13: 46-50.
Kessler-Rios MM, Kattan GH. 2012. Fruits of
Melastomataceae: phenology in
Andean forest and role as a food resource for birds. – J. Trop. Ecol. 28:
11-21.
Keszei A, Brubaker CL, Foley WJ. 2008. A
molecular perspective on terpene variation in Australian Myrtaceae. – Aust. J. Bot. 56:
197-213.
Keszei A, Brubaker CL, Carter R, Köllner T,
Degenhardt J, Foley WJ. 2010. Functional and evolutionary relationships between
terpene synthases from Australian Myrtaceae. – Phytochemistry 71:
844-852.
Kevan PG, Lack AJ. 1985. Pollination in a
cryptically dioecious plant, Decaspermum parviflorum (Lam.) A. J.
Scott (Myrtaceae), by pollen-collecting
bees in Sulawesi, Indonesia. – Biol. J. Linn. Soc. 25: 319-330.
Kim S-C, Graham SA, Graham A. 1994.
Palynology and pollen dimorphism in the genus Lagerstroemia (Lythraceae). – Grana 33: 1-20.
Klucking EP. 1988. Leaf venation patterns 3.
Myrtaceae. – J. Cramer, Berlin.
Klucking EP. 1989. Leaf venation patterns 4.
Melastomataceae. – J. Cramer,
Berlin.
Klucking EP. 1991. Leaf venation patterns 5.
Combretaceae. – J. Cramer,
Berlin.
Koehne E. 1880a. Lythraceae monographice describuntur.
Rotala. – Engl. Bot. Jahrb. Syst. 1: 142-178.
Koehne E. 1880b. Lythraceae monographice describuntur.
Ammannia. – Engl. Bot. Jahrb. Syst. 1: 240-262.
Koehne E. 1882. Lythraceae monographice describuntur.
Nesaea. – Engl. Bot. Jahrb. Syst. 3: 321-339.
Koehne E. 1884. Lythraceae monographice describuntur.
Morphologie der Vegetationsorgane. – Engl. Bot. Jahrb. Syst. 5: 95-132.
Koehne E. 1885. The Lythraceae of the United States. – Bot.
Gaz. 10: 269-277.
Koehne E. 1886. Die geographische Verbreitung
der Lythraceen. – Engl. Bot. Jahrb. Syst. 7: 1-61.
Koehne E. 1893. Lythraceae. – In: Engler A, Prantl K
(eds), Die natürlichen Pflanzenfamilien III(7), W. Engelmann, Leipzig, pp.
1-16.
Koek-Noorman J, Hogeweg P, Maanen WHM van,
Welle BJH ter. 1979. Wood anatomy of the Blakeeae (Melastomataceae). – Acta Bot.
Neerl. 28: 21-43.
Kopka S, Weberling F. 1984. Zur Morphologie
und Morphogenese der Blüte von Vochysia acuminata Bong. ssp.
laurifolia (Warm.) Stafleu (Vochysiaceae). – Beitr. Biol.
Pflanzen 59: 273-302.
Koschnitzke C, Martins AB. 2007.
Nomenclatural alterations in Microlicieae (Melastomataceae). – Novon 17:
472-475.
Kottek MH, Ladiges PY, Whiffin T. 1990. A new
species of stringybark, Eucalyptus mackintii, from eastern Victoria
and its phenetic and cladistic relationships. – Aust. Syst. Bot. 3:
671-687.
Krakos KN, Reece JS, Raven PH. 2014.
Molecular phylogenetics and reproductive biology of Oenothera Section
Kneiffia (Onagraceae). –
Syst. Bot. 39: 523-532.
Kral R, Bostick PE. 1969. The genus
Rhexia (Melastomataceae). – Sida 3:
387-440.
Krasser F. 1893. Melastomataceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien III(7), W. Engelmann,
Leipzig, pp. 130-199.
Kriebel R. 2008. Systematics and biogeography
of the Neotropical genus Acisanthera (Melastomataceae). – M.Sc. thesis,
San Francisco State University, San Francisco, California.
Kriebel R. 2012. A synopsis of the genus
Poteranthera (Melastomeae: Melastomataceae) with the
description of a new, apparently pollinator deceiving species. – Brittonia
64: 6-14.
Kriebel R. 2015. New insights into anatomical
and morphological adaptations to high elevations in the Melastomataceae: evidence from
Chaetolepis cufodontisii and Monochaetum amabile. – Flora
213: 12-19.
Kriebel R. 2016a. Phylogenetic placement of
the monotypic genus Schwackaea (Melastomeae: Melastomataceae) and the evolution
of its unique fruit. – Intern. J. Plant Sci. 177: 440-448.
Kriebel R. 2016b. A monograph of
Conostegia (Melastomataceae, Miconieae). –
PhytoKeys 67: 1-326.
Kriebel R, Almeda F. 2009. Three new species
in the neotropical genus Clidemia (Melastomataceae: Miconieae). –
Brittonia 61: 206-217.
Kriebel R, Michelangeli FA, Kelly LM. 2015.
Discovery of usunual anatomical and continuous characters in the evolutionary
history of Conostegia (Miconieae: Melastomataceae). – Mol. Phylogen.
Evol. 82: 289-313.
Kurabayashi M, Lewis H, Raven PH. 1962. A
comparative study of mitosis in the Onagraceae. – Amer. J. Bot. 49:
1003-1026.
Kuster RM, Arnold N, Wessjohann L. 2009.
Anti-fungal flavonoids from Tibouchina grandifolia. – Biochem. Syst.
Ecol. 37: 63-65.
Ladd PG, Parnell JAN, Thomson GJ. 1999.
Anther diversity and function in Verticordia DC. (Myrtaceae). – Plant Syst. Evol. 219:
79-97.
Ladiges PY. 1984. A comparative study of
trichomes in Angophora Cav. and Eucalyptus L’Hérit. – a
question of homology. – Aust. J. Bot. 32: 561-574.
Ladiges PY. 1997. Phylogenetic history and
classification of eucalypts. – In: Williams JE, Woinarski JCZ (eds), Eucalypt
ecology: individuals to ecosystems, Cambridge University Press, Cambridge.
Ladiges PY, Humphries CJ. 1983. A cladistic
study of Arillastrum, Angophora and Eucalyptus. –
Bot. J. Linn. Soc. 87: 105-134.
Ladiges PY, Udovicic F. 2000. Comment on a
new classification of the eucalypts. – Aust. Syst. Bot. 13: 149-152.
Ladiges PY, Udovicic F. 2005. Comment on
molecular dating of the age of eucalypts. – Aust. Syst. Bot. 18: 291-293.
Ladiges PY, Whiffin T. 1993. Taxonomic
revision of Eucalyptus alpina s.l. and recognition of three new
species, E. victoriana, E. serraensis and E.
verrucosa. – Aust. Syst. Bot. 6: 365-370.
Ladiges PY, Newnham MR, Humphries CJ. 1989.
Systematics and biogeography of the Australian “green ash” eucalypts
(Monocalyptus). – Cladistics 5: 345-364.
Ladiges PY, Udovicic F, Drinnan AN. 1995.
Eucalypt phylogeny – molecules and morphology. – Aust. Syst. Bot. 8:
483-497.
Ladiges PY, McFadden GI, Middleton N,
Orlovich DA, Treloar N, Udovicic F. 1999. Phylogeny of Melaleuca,
Callistemon, and related genera of the Beaufortia suballiance
(Myrtaceae) based on 5S and ITS-1
spacer regions of nrDNA. – Cladistics 15: 151-172.
Ladiges PY, Udovicic F, Nelson G. 2003.
Australian biogeographic connections and the phylogeny of large genera in the
plant family Myrtaceae. – J.
Biogeogr. 30: 989-998.
Ladiges PY, Bayly M, Nelson G. 2010.
East-west continental vicariance in Eucalyptus subgenus
Eucalyptus. – In: Williams D, Knapp S (eds), Beyond cladistics. The
branching of a paradigm, University of California Press, Berkeley, pp.
267-302.
Ladiges PY, Parra-O C, Gibbs A, Udovicic F,
Nelswon G, Bayly M. 2011. Historical biogeographical patterns in continental
Australia: congruence among areas of endemism of two major clades of eucalypts.
– Cladistics 27: 29-41.
Lam N, Wilson PG, Heslewood MM, Quinn CJ.
2002. A phylogenetic analysis of the Chamelaucium alliance (Myrtaceae). – Aust. Syst. Bot. 15:
535-543.
Landrum LR. 1981a. Flora Neotropica.
Monographs 29. The genus Myrceugenia (Myrtaceae). – New York Botanical Garden,
Bronx, New York.
Landrum LR. 1981b. The phylogeny and
geography of Myrceugenia (Myrtaceae). – Brittonia 33: 105-129.
Landrum LR. 1984. Taxonomic implications of
the discovery of calyptrate species of Myrceugenia (Myrtaceae). – Brittonia 36: 161-166.
Landrum LR. 1986. Flora Neotropica. Monograph
45. The genera Campomanesia, Pimenta, Blepharocalyx,
Legrandia, Acca, Myrrhinium, and Luma (Myrtaceae). – New York Botanical Garden,
Bronx, New York.
Landrum LR. 1988a. The myrtle family (Myrtaceae) in Chile. – Proc. Calif.
Acad. Sci. 45: 277-317.
Landrum LR. 1988b. Systematics of
Myrteola (Myrtaceae). –
Syst. Bot. 13: 120-132.
Landrum LR. 1990. Accara: a new
genus of Myrtaceae, Myrtinae from
Brazil. – Syst. Bot. 15: 221-225.
Landrum LR. 1991. Chamguava: a new
genus of Myrtaceae (Myrtinae) from
Mesoamerica. – Syst. Bot. 16: 21-29.
Landrum LR. 1992. Mosiera (Myrtaceae) in Mexico and Mesoamerica. –
Novon 2: 26-29.
Landrum LR. 2001. Two new species of
Campomanesia (Myrtaceae) from
Espírito Santo and Bahia, Brazil. – Brittonia 53: 534-538.
Landrum LR. 2008. Two new species of
Calycolpus (Myrtaceae) from
Brazil. – Brittonia 60: 252-256.
Landrum LR. 2010. A revision of
Calycolpus (Myrtaceae). –
Syst. Bot. 35: 368-389.
Landrum LR, Bonilla J. 1996. Anther
glandularity in the American Myrtinae (Myrtaceae). – Madroño 43: 58-68.
Landrum LR, Grifo T. 1988. The myrtle family
(Myrtaceae) in Chile. – Proc. Calif.
Acad. Sci. 45: 277-317.
Landrum LR, Kawasaki ML. 1997. The genera of
Myrtaceae in Brazil: an illustrated
synoptic treatment and identification keys. – Brittonia 49: 508-536.
Landrum LR, Sharp WP. 1989. Seed coat
characters of some American Myrtinae (Myrtaceae): Psidium and related
genera. – Syst. Bot. 14: 370-376.
Landrum LR, Stevenson D. 1986. Variability of
embryos in subtribe Myrtinae (Myrtaceae). – Syst. Bot. 11: 155-162.
Lange PJ de, Murray BG. 2004. Chromosome
numbers in Kunzea (Myrtaceae).
– Aust. J. Bot. 52: 609-617.
Lange PJ de, Smissen RD, Wagstaff SJ, Keeling
DJ, Murray BG, Toelken HR. 2010. A molecular phylogeny and infrageneric
classification of Kunzea (Myrtaceae) inferred from rDNA ITS and ETS
sequences. – Aust. Syst. Bot. 23: 309-319.
Larson BMH, Barrett SCH. 1999. The
pollination ecology of buzz-pollinated Rhexia virginica (Melastomataceae). – Amer. J. Bot.
86: 502-511.
Lawler IR, Foley WJ. 2002. Chemical ecology
of herbivory in eucalyptus: interactions between insect and mammalian
herbivores and plant essential oils. – In: Coppen JJW (ed), Eucalyptus: the
genus Eucalyptus, Taylor and Francis, London, pp. 324-344.
Le S, Nock C, Henson M, Shepherd M. 2009.
Genetic differentiation among and within three red mahoganies (series
Annulares), Eucalyptus pellita, E. resinifera and E.
scias (Myrtaceae). – Aust. Syst.
Bot. 22: 332-343.
Leach GJ. 1986. A revision of the genus
Angophora (Myrtaceae). –
Telopea 2: 749-779.
Lee DE, Conran JG, Bannister JM, Kaulfuss U,
Mildenhall DC. 2013. A fossil Fuchsia (Onagraceae) flower and an anther mass
with in situ pollen from the early Miocene of New Zealand. – Amer. J. Bot.
100: 2052-2065.
Lee S. 1979. Studies on the pollen morphology
in the Lythraceae. – Korean J. Bot.
22: 115-133.
Leeuwen BLJ. 1974. A preliminary revision of
the genus Rotala (Lythraceae)
in Malesia. – Blumea 19: 53-56.
Leinfellner W. 1958. Zur Morphologie des
Melastomataceen-Staubblattes. – Österr. Bot. Zeitschr. 105: 44-70.
Leins P. 1965. Die Infloreszenz und frühe
Blütenentwicklung von Melaleuca nesophila F. Muell. (Myrtaceae). – Planta 65: 195-204.
Leins P. 1988. Das zentripetale Androecium
von Punica. – Bot. Jahrb. Syst. 109: 555-561.
Lejoly J, Lisowski S. 1999. Novitates Guineae
Aequatorialis (6) Heterotis obamae (Melastomataceae) espèce nouvelle du
Rio Muni. – Syst. Geogr. Plants 69: 185-188.
Lempe J, Stevens KJ, Peterson RL. 2001. Shoot
responses of six Lythraceae species to
flooding. – Plant Biol. 3: 186-193.
Léon de Pinto G, Nava M, Martínez M, Rivas
C. 1993. Gum polysaccharides of nine specimens of Laguncularia
racemosa. – Biochem. Syst. Ecol. 21: 463-466.
Levin GM. 1980. Contributions to the study of
the family Punicaceae. – Bot. Žurn. 65: 427-430. [In Russian]
Levin RA, Wagner WL, Hoch PC, Nepokroeff M,
Pires JC, Zimmer EA, Sytsma KJ. 2003. Family-level relationships of Onagraceae based on chloroplast
rbcL and ndhF data. – Amer. J. Bot. 90: 107-115.
Levin RA, Wagner WL, Hoch PC, Hahn WJ,
Rodriguez A, Baum DA, Katinas L, Zimmer EA, Sytsma KJ. 2004. Paraphyly in tribe
Onagreae: insights into phylogenetic relationships of Onagraceae based on nuclear and
chloroplast sequence data. – Syst. Bot. 29: 147-164.
Lewis D, Rao AN. 1971. Evolution of
dimorphism and population polymorphism in Pemphis acidula Forst. –
Proc. Roy. Soc. London, Ser. B, Biol. Sci. 188: 247-256.
Lewis H, Lewis ME. 1955. The genus
Clarkia. – Univ. Calif. Publ. Bot. 20: 241-392.
Lewis H, Raven PH. 1992. New combinations in
the genus Clarkia (Onagraceae). – Madroño 39: 163-169.
Lewis H, Szweykowski J. 1964. The genus
Gayophytum (Onagraceae). –
Brittonia 16: 343-391.
Li H-L. 1944. Studies in the Melastomataceae of China. – J.
Arnold Arbor. 25: 1-42.
Lieben L. 1968. Combretaceae. – In: Bamps P (ed),
Flore du Congo, du Rwanda et du Burundi, Bruxelles.
Lieu J, Melhem TS. 1973. Palinologia em Myrtaceae. – Hoehnea 3: 1-11.
Litt AJ. 1999. Floral morphology and
phylogeny of Vochysiaceae. – Ph.D.
diss., City University of New York.
Litt AJ, Cheek M. 2002. Korupodendron
songweanum, a new genus and species of Vochysiaceae from West-Central Africa.
– Brittonia 54: 13-17.
Litt AJ, Stevenson DW. 2003a. Floral
development and morphology of Vochysiaceae I. The structure of the
gynoecium. – Amer. J. Bot. 90: 1533-1547.
Litt AJ, Stevenson DW. 2003b. Floral
development and morphology of Vochysiaceae II. The position of the
single fertile stamen. – Amer. J. Bot. 90: 1548-1559.
Little SA, Stockey RA. 2003. Vegetative
growth of Decodon allenbyensis (Lythraceae) from the Middle Eocene
Princeton chert with anatomical comparisons to Decodon verticillatus.
– Intern. J. Plant Sci. 164: 453-469.
Little SA, Stockey RA. 2006. Morphogenesis of
the specialized peridermal tissues in Decodon allenbyensis from the
Middle Eocene Princeton Chert. – IAWA J. 27: 73-87.
Little SA, Stockey RA, Keating RC. 2004.
Duabanga-like leaves from the Middle Eocene Princeton chert and
comparative leaf histology of Lythraceae sensu lato. – Amer. J. Bot.
91: 1126-1139.
Liu S-H, Hoch PC, Diazgranados M, Raven PH,
Barber JC. 2017. Multi-locus phylogeny of Ludwigia (Onagraceae): insights on infra-generic
relationships and the current classification of the genus. – Taxon 66:
1112-1127.
Liu Y-S, Zetter R, Ferguson D, Zou C. 2008.
Lagerstroemia (Lythraceae)
pollen from the Miocene of eastern China. – Grana 47: 262-271.
Lourteig A. 1965. On the systematic position
of Alzatea verticillata R. & P. – Ann. Missouri Bot. Gard. 52:
371-378.
Lourteig A. 1986. Revisión del género
Lafoensia Vandelli (Litraceas). – Mem. Soc. Ci. Nat. La Salle 45:
115-157.
Lourteig A. 1989. 135. Lythraceae. – In: Harling G, Andersson
L (eds), Flora of Ecuador 37, Nord. J. Bot., Copenhagen, pp. 1-46.
Louw N. 1996. A comparative study of the
reproduction, autoecology and genetic diversity of Brachysiphon
rupestris, B. acutus, and some other species of Penaeaceae. – Ph.D. diss., University
of Stellenbosch, Republic of South Africa.
Lowry JB. 1976. Anthocyanins of the Melastomataceae, Myrtaceae, and some allied families. –
Phytochemistry 15: 513-516.
Lozano G, Becerra de Lozano N. 1999. Los
géneros Allomaieta y Cyphostyla (Melastomataceae). – Rev. Acad.
Colomb. Ci. Exactas Fis. Nat. 23: 5-18.
Lucas EJ. 2007. Systematic studies in
Neotropical Myrtaceae with an emphasis
on Myrcia s. l. – The evolution and biogeography of a large South
America clade. – Ph.D. diss., Open University/Royal Botanic Gardens, Kew,
Richmond, United Kingdom.
Lucas EJ, Bünger MO. 2015. Myrtaceae in the Atlantic forest – their
role as a ‘model’ group. – Biodivers. Conserv. 24: 2165-2180.
Lucas EJ, Belsham SR, Nic Lughadha EM,
Orlovich DA, Sakuragui CM, Chase MW, Wilson PG. 2005. Phylogenetic patterns in
fleshy-fruited Myrtaceae –
preliminary molecular evidence. – Plant Syst. Evol. 251: 35-51.
Lucas EJ, Harris SA, Mazine FF, Belsham SR,
Nic Lughadha EM, Telford A, Gasson PE, Chase MW. 2007. Suprageneric
phylogenetics of Myrteae, the generically richest tribe in Myrtaceae (Myrtales). – Taxon 56: 1105-1128.
Lucas EJ, Matsumoto K, Harris SA, Nic
Lughadha EM, Bernadini B, Chase MW. 2011. Phylogenetics, morphology, and
evolution of the large genus Myrcia s.l. (Myrtaceae). – Intern. J. Plant Sci. 172:
915-934.
Lucas EJ, Wilson CE, Lima DF, Sobral M,
Matsumoto K. 2016. A conspectus of Myrcia sect. Aulomyrcia
(Myrtaceae). – Ann. Missouri Bot.
Gard. 101: 648-698.
Lucas EJ, Amorim BS, Lima DF, Lima-Lourenço
AR, Nic Lughadha EM, Proença CEB, Rosa PO, Rosário AS, Santos LL, Santos MF,
Souza MC, Staggemeier VG, Vasconcelos TNC, Sobral M. 2018. A new infra-generic
classification of the species-rich Neotropical genus Myrcia s.l. –
Kew Bull. 73: 9. doi 10.1007/S12225-017-9730-5
Lundin R. 1983. Taxonomy of Sonerila
(Melastomataceae) in Ceylon. –
Nord. J. Bot. 3: 633-656.
McAlpine D, Remfry JR. 1890. The transverse
sections of petioles of eucalypts as aids in the determination of species. –
Trans Roy. Soc. Victoria 2: 1-64.
Macbride JF. 1929. A new Miconia and
other large melastomes of Peru. – Trop. Woods 17: 12-14.
Macbride JF. 1941. Flora of Peru (Melastomataceae). – Fieldiana.
Botany 13: 249-521.
McDonald MW, Butcher PA, Bell JC, Larmour JS.
2000. Intra- and interspecific allozyme variation in eucalypts from the spotted
gum group, Corymbia, section ‘Politaria’ (Myrtaceae). – Aust. Syst. Bot. 13:
491-507.
McDonald MW, Brooker MIH, Butcher PA. 2009. A
taxonomic revision of Eucalyptus camaldulensis (Myrtaceae). – Aust. Syst. Bot. 22:
257-285.
McIntyre DJ. 1963. Pollen morphology of New
Zealand species of Myrtaceae. –
Trans. Roy. Soc. New Zealand 2: 83-107.
McKinnon GE, Vaillancourt RE, Steane DA,
Potts BM. 2008. An AFLP marker approach to lower-level systematics in
Eucalyptus (Myrtaceae). –
Amer. J. Bot. 95: 368-380.
McVaugh R. 1956. Nomenclatural notes on Myrtaceae and related families. – Taxon
5: 133-146, 162-167.
McVaugh R. 1958. Myrtaceae (Calyptranthes and
Marlierea). – Mem. New York Bot. Gard. 10: 61-91.
McVaugh R. 1963. Tropical American Myrtaceae: notes on generic concepts and
descriptions of previously unrecognized species. – Fieldiana Bot. 29:
145-228.
McVaugh R. 1968. The genera of American Myrtaceae – an interim report. – Taxon
17: 354-418.
McVaugh R. 1969. Botany of the Guayana
Highland III. Myrtaceae. – Mem. New
York Bot. Gard. 18: 55-286.
Mädel-Angeliewa E, Müller-Stoll WR. 1973.
Kritische Studien über fossile Combretaceen-Hölzer: über Hölzer vom Typus
Terminalioxylon G. Schönfeld mit einer Revision der bisher zu
Evodioxylon Chiarugi gestellten Arten. – Palaeontographica, Ser. B,
142: 117-136.
Majure LC, Neubig KM, Skean Jr JD, Bécquer
ER, Judd WS. 2015. Evolution of the sandpaper clade (Miconieae, Melastomataceae). – Intern. J.
Plant Sci. 176: 607-626.
Majure LC, Judd WS, Michelangeli FA. 2015.
Taxonomic revision of the Greater Antillean Pseudolima clade of
Miconia (Miconia sect. Krugiophytum: Miconieae: Melastomataceae). – Brittonia 67:
11-18.
Majure LC, Bécquer E, Judd W. 2016. Revision
of the Lima clade (Miconia sect. Lima, Miconieae, Melastomataceae) of the Greater
Antilles. – PhytoKeys 72: 1-99.
Malajczuk N, Molina R, Trappe JM. 1982.
Ectomycorrhiza formation in Eucalyptus I. Pure culture synthesis, host
specificity and mycorrhizal compatibility with Pinus radiata. – New
Phytol. 91: 467-482.
Malone MH, Rother A. 1994. Heimia
salicifolia: a phytochemical and phytopharmacologic review. – J.
Ethnopharmacol. 42: 135-159.
Manchester SR, O’Leary EL. 2010.
Phylogenetic distribution and identification of fin-winged fruits. – Bot.
Rev. 76: 1-82.
Manchester SR, Dilcher DL, Wing SL. 1998.
Attached leaves and fruits of Myrtaceous affinity from the middle Eocene of
Colorado. – Rev. Palaeobot. Palynol. 102: 153-163.
Mansfeld R. 1925. Die Melastomataceen von
Papuasien. – Engl. Bot. Jahrb. Syst. 60: 105-143.
Marcano-Berti L. 1969. Un nuevo género de
las Vochysiaceae. – Pittieria 2:
3-27.
Marcano-Berti L. 1989. Vochysiaceae: novidades y correcciones.
– Pittieria 18: 5-14.
Marcano-Berti L. 1998. Flora of the Guianas.
Ser. A: Phanerogams. Fasc. 21. 123 Vochysiaceae. – Royal Botanic
Gardens, Kew, pp. 1-48.
Marginson JC, Ladiges PY. 1988. Geographical
variation in Eucalyptus baxteri s.l. and the recognition of a new
species, E. arenacea. – Aust. Syst. Bot. 1: 151-170.
Martin CV, Michelangeli FA. 2009. Comparative
seed morphology of Leandra (Miconieae, Melastomataceae). – Brittonia 61:
175-188.
Martin CV, Little DP, Goldenberg R,
Michelangeli FA. 2008. A phylogenetic evaluation of Leandra
(Miconieae, Melastomataceae): a
polyphyletic genus where the seeds tell the story, not the petals. –
Cladistics 24: 315-327.
Martin PG, Dowd JM. 1986. Phylogenetic
studies using protein sequences within the order Myrtales. – Ann. Missouri Bot. Gard. 73:
442-448.
Martins AB. 1984. Revisão taxonômica do
gênero Cambessedesia DC. (Melastomataceae). – M.Sc. thesis,
Universidade Estadual de Campinas, Brazil.
Martins AB. 1989. Revisão taxonômica do
gênero Marcetia DC. (Melastomataceae). – Ph.D. diss.,
Universidade Estadual de Campinas, Brazil.
Martins AB. 1993. New species in Brazilian Melastomataceae. – Kew Bull. 48:
385-389.
Maschwitz U, Fiala B, Moog J, Saw LG. 1991.
Two new myrmecophytic associations from the Malay Peninsula: ants of the genus
Cladomyrma (Formicidae, Camponotinae) as partners of Saraca
thaipingensis (Caesalpiniaceae) and Crypteronia griffithii (Crypteroniaceae) 1. Colony
foundation and acquisition of trophobionts. – Insectes Sociaux 38: 27-35.
Maurin O, Chase MW, Jordaan M, Bank M van
der. 2010. Phylogenetic relationships of Combretaceae inferred from nuclear and
plastid DNA sequence data: implications for generic classification. – Bot. J.
Linn. Soc. 162: 453-476.
Maurin O, Gere J, van der Bank M, Boatwright
JS. 2017. The inclusion of Anogeissus, Buchenavia and
Pteleopsis in Terminalia (Combretaceae: Terminaliinae). – Bot.
J. Linn. Soc. 184: 312-325.
Mauritzon J. 1934. Zur Embryologie einiger Lythraceae. – Acta Horti Gothob. 9:
1-21.
Mauritzon J. 1939. Contribution to the
embryology of the orders Rosales
and Myrtales. – Acta Univ. Lund. 35:
3-121.
Maxwell JF. 1978. A revision of
Medinilla, Pachycentria, and Pogonanthera (Melastomataceae) from the Malay
Peninsula. – Gard. Bull. (Singapore) 31: 139-216.
Maxwell JF. 1980a. Revision of
Memecylon L. (Melastomataceae) from the Malay
Peninsula. – Gard. Bull. (Singapore) 33: 31-150.
Maxwell JF. 1980b. Taxonomic notes on the
tribe Dissochaeteae (Naudin) Triana (Melastomataceae). – Gard. Bull.
(Singapore) 33: 312-327.
Maxwell JF. 1981. A revision of the genus
Pternandra (Melastomataceae). – Gard. Bull.
(Singapore) 34: 1-90.
Maxwell JF. 1982. Taxonomic and nomenclatural
notes on Oxyspora DC., Anerincleistus Korth.,
Poikilogyne Baker f., and Allomorphia Bl. (Melastomataceae, tribe Oxysporeae).
– Gard. Bull. (Singapore) 35: 209-226.
Maxwell JF. 1983. Taxonomic studies of the Melastomataceae 1. A revision of
subtribes Diplectriinae Maxw. and Dissochaetinae (Naud.) Triana (genera
Diplectria (Bl.), Dissochaeta Bl., Macrolenes Naud.,
Creochiton Bl., and Pseudodissochaeta Nayar.). – Fed. Mus.
J. 29: 45-117.
Maxwell JF. 1989. The genus
Anerincleistus Korth. (Melastomataceae). – Proc. Acad.
Nat. Sci. Philadelphia 141: 29-72.
Maxwell JF, Veldkamp JF. 1990a. Notes on the
Astronieae (Melastomataceae) I.
Astrocalyx, Astronia. – Blumea 35: 71-114.
Maxwell JF, Veldkamp JF. 1990b. Notes on the
Astronieae (Melastomataceae) II.
Astronidium, Beccarianthus. – Blumea 35: 115-165.
Mayer R. 1990. Flavonoids from
Leptospermum scoparium. – Phytochemistry 29: 1340-1342.
Mayr B. 1969. Ontogenetische Studien an Myrtales-Blüten. – Bot. Jahrb. Syst. 89:
210-271.
Mazine FF, Souza VC. 2009a. New species of
Eugenia sect. Racemosae (Myrtaceae) from Brazilian Amazon
rainforest. – Kew Bull. 64: 147-153.
Mazine FF, Souza VC. 2009b. A new species of
Eugenia (Myrtaceae) from
north-eastern Brazil. – Bot. J. Linn. Soc. 158: 775-777.
Mazine FF, Souza VC, Sobral M, Forest F,
Lucas EJ. 2014. A preliminary phylogenetic analysis of Eugenia (Myrtaceae: Myrteae), with a focus on
neotropical species. – Kew Bull. 69: 1-14.
Mazine FF, Santos MF, Lucas EJ. 2014. New
combinations and new names in Myrcia (Myrtaceae) for Flora of São Paulo state,
Brazil. – Phytotaxa 173: 97-100.
Mazine FF, Faria JEQ, Giaretta A, Vasconcelos
T, Forest F, Lucas E. 2018. Phylogeny and biogeography of the hyperdiverse
genus Eugenia (Myrtaceae:
Myrteae), with emphasis on E. sect. Umbellatae, the most
unmanageable clade. – Taxon 67: 752-769.
Meijer W. 1972. The genus Axinandra
– Melastomataceae: a missing
link in Myrtales? – Ceylon J. Sci.
(Biol. Sci.) 10: 72-74.
Mendoza H, Ramirez B. 2006. Guía ilustrada
de géneros de Melastomataceae y
Memecylaceae de Colombia. –
Instituto de Investigación de Recursos Biológicos Alexander von Humboldt,
Universidad del Cauca, Bogotá.
Mendoza-Cifuentes H, Fernandez-Alonso JL.
2010. Evaluación de caracteres del cáliz y de los estambres en la tribu
Merianieae (Melastomataceae) y
definición de homologies. – Rev. Acad. Colomb. Ci. Exactas Fis. Nat. 34:
143-171.
Mendoza-Cifuentes H, Fernández-Alonso JL.
2012. Novedades em Centronia y Meriania (Merianieae, Melastomataceae) y revision
taxonômica de Meriania grupo brachycera. – An. Jard. Bot.
Madrid 69: 259-294.
Mentink H, Baas P. 1992. Leaf anatomy of the
Melastomataceae, Memecylaceae, and Crypteroniaceae. – Blumea 37:
189-225.
Merrill ED. 1950. Readjustments in the
nomenclature of Philippine Eugenia species. – Philipp. J. Sci. 79:
351-430.
Merrill ED, Perry LM. 1937. Reinstatement and
revision of Cleistocalyx Blume including Acicalyptus A. Gray,
a valid genus of the Myrtaceae. – J.
Arnold Arbor. 18: 322-343.
Merrill ED, Perry LM. 1938. A synopsis of
Acmena DC., a valid genus of the Myrtaceae. – J. Arnold Arbor. 19:
1-20.
Merrill ED, Perry LM. 1939. The myrtaceous
genus Syzygium Gaertner in Borneo. – Mem. Amer. Acad. Arts 18:
135-202.
Merwe MM van der, Wyk AE van, Botha AM. 2005.
Molecular phylogenetic analysis of Eugenia L. (Myrtaceae) with emphasis on southern
African taxa. – Plant Syst. Evol. 251: 21-34.
Meyer FS, Goldenberg R. 2014. Two new species
of Pleroma (Melastomataceae: Melastomeae) from
Brazil. – Kew Bull. 69: 9527.
Meyer K. 1999. Phylogenie und Systematik der
Gattung Melastoma (Melastomataceae) unter besonderer
Berücksichtigung des M. malabathricum-Komplexes. – Ph.D. diss.,
Johannes Gutenberg-Universität, Mainz, Germany.
Meyer K. 2001. Revision of the Southeast
Asian genus Melastoma (Melastomataceae). – Blumea 46:
351-398.
Michelangeli FA. 2000. A cladistic analysis
of the genus Tococa (Melastomataceae) based on
morphological data. – Syst. Bot. 25: 211-234.
Michelangeli FA. 2001. Tococa. –
In: Berry PE, Holst BK, Yatskievych K (eds), Flora of the Venezuelan Guayana 6,
Missouri Botanical Garden, St. Louis, Missouri.
Michelangeli FA. 2006. Two new species of
myrmecophytic Tococa (Melastomataceae) with unusually
large floral bracts. – Brittonia 58: 151-155.
Michelangeli FA, Penneys DS, Giza J, Soltis
D, Hils MH, Skean JD Jr. 2004. A preliminary phylogeny of the tribe Miconieae
(Melastomataceae) based on nrITS
sequence data and its implications on inflorescence position. – Taxon 53:
279-290.
Michelangeli FA, Judd WS, Penneys DS, Skean
JD Jr, Bécquer-Granados ER, Goldenberg R, Martin CV. 2008. Multiple events of
dispersal and radiation of the tribe Miconieae (Melastomataceae) in the Caribbean.
– Bot. Rev. 74: 53-77.
Michelangeli FA, Nicolas A, Morales-P ME,
David H. 2011. Phylogenetic relationships of Allomaieta,
Alloneuron, Cyphostyla, and Wurdastoma (Melastomataceae) and the
resurrection of the tribe Cyphostyleae. – Intern. J. Plant Sci. 172:
1165-1178.
Michelangeli FA, Guimarães PJF, Penneys DS,
Almeda F, Kriebel R. 2013. Phylogenetic relationships and distribution of New
World Melastomeae (Melastomataceae). – Bot. J. Linn.
Soc. 171: 38-60.
Michelangeli FA, Ulloa CU, Sosa K. 2014.
Quipuanthus, a new genus of Melastomataceae from the foothills
of the Andes in Ecuador and Peru. – Syst. Bot. 39: 533-540.
Michelangeli FA, Reyes WC, Sosa K. 2015. A
revision of Meriania (Melastomataceae) in the Greater
Antilles with emphasis on the status of the Cuban species. – Brittonia 67:
118-137.
Migliore J, Baumel A, Juin M, Medail F. 2012.
From Mediterranean shores to central Saharan mountains: key phylogeographical
insights from the genus Myrtus. – J. Biogeogr. 39: 942-956.
Miki S. 1959. Evolution of Trapa
from ancestral Lythrum through Hemitrapa. – Proc. Imp.
Acad. Japan 35(6): 289-294.
Mildbraed J. 1913. Erismadelphus
exsul Mildbr. n. gen. et spec. eine Vochysiacee aus Kamerun. – Engl.
Bot. Jahrb. Syst. 49: 547-551.
Miller JM. 2000. New taxa and nomenclatural
notes on the flora of the Marojejy Massif, Madagascar IV. Myrtaceae: new species of Eugenia
L. – Adansonia 22: 111-116.
Mistri PB, Kapgate DK. 1990. Report of a
winged fruit of the family Combretaceae from the Deccan
Intertrappean Beds of Mohgaonkalan (M.P.) India. – In: 3 IOPConference,
Melbourne, pp. 93-96.
Mohammed AMA, Coombes PH, Crouch NR,
Mulholland DA. 2009. Non-volatile isolates of two Heteropyxis species:
a first chemotaxonomic assessment of subfamily Psiloxyloideae (Myrtaceae). – Biochem. Syst. Ecol. 37:
241-243.
Moore BD, Wallis IR, Palá-Paúl J, Brophy
JJ, Willis RH, Foley WJ. 2004. Antiherbivore chemistry of Eucalyptus
– cues and deterrents for marsupial folivores. – J. Chem. Ecol. 30:
1743-1769.
Moore DM, Raven PH. 1970. Cytogenetics,
distribution and amphitropical affinities of South American Camissonia
(Onagraceae). – Evolution 24:
816-823.
Morales-P ME. 2010. Análisis filogenético
de Huilaea Wurdack (Melastomataceae) basado en datos
morfológicos y moleculares. – Ph.D. diss., Universidad Nacional de Colombia,
Bogotá, Colombia.
Morales-P ME, Penneys DS. 2010. New species
of Chalybea Naud. and Huilaea Wurd. (Melastomataceae). – Brittonia 62:
26-34.
Mora Osejo LE. 1966. Contribuciones al
conocimiento de la morfologia comparada de las inflorescencias y de las formas
de crecimiento de las Melastomataceae. – Caldasia 9:
303-312.
Morley T. 1953. The genus Mouriri
(Melastomataceae). A sectional
revision based on anatomy and morphology. – Univ. Calif. Publ. Bot. 26:
223-312.
Morley T. 1963. Votomita Aublet (Melastomataceae). – Bull. Torrey
Bot. Club 90: 1-16.
Morley T. 1976. Flora Neotropica. Monograph
15. Memecyleae (Melastomataceae).
– New York Botanical Garden, Bronx, New York.
Morley T. 1985. Five new taxa of a New World
Memecyleae (Melastomataceae). –
Ann. Missouri Bot. Gard. 72: 548-577.
Morley T. 1989. New species and other
taxonomic matters in the New World Memecyleae (Melastomataceae). – Ann. Missouri
Bot. Gard. 76: 430-433.
Morley RJ, Dick CW. 2003. Missing fossils,
molecular clocks, and the origin of the Melastomataceae. – Amer. J. Bot.
90: 1638-1644.
Morris JA. 2007. A molecular phylogeny of the
Lythraceae and inference of the
evolution of heterostyly. – Ph.D. diss., Kent State University, Kent,
Ohio.
Moyle RG. 2004. Calibration of molecular
clocks and the biogeographic history of Crypteroniaceae. – Evolution 58:
1871-1873.
Mújica MB, Cutler DF. 1974. Taxonomic
implications of anatomical studies on the Oliniaceae. – Kew Bull. 29: 93-123.
Muller J. 1969. A palynological study of the
genus Sonneratia (Sonneratiaceae). – Pollen Spores 11: 223-298.
Muller J. 1975. Note on the pollen morphology
of Crypteroniaceae s.l. –
Blumea 22: 275-294.
Muller J. 1978. New observations on pollen
morphology and fossil distribution of the genus Sonneratia
(Sonneratiaceae). – Rev. Palaeobot. Palynol. 26: 277-300.
Muller J. 1981. Exine architecture and
function in some Lythraceae and
Sonneratiaceae. – Rev. Palaeobot. Palynol. 35: 93-123.
Munz PA. 1935. Studies in the Onagraceae IX. The subgenus
Raimannia. – Amer. J. Bot. 22: 645-663.
Munz PA. 1943. A revision of the genus
Fuchsia (Onagraceae). –
Proc. Calif. Acad. Sci. 4: 1-138.
Munz PA. 1974. 141. Onagraceae. – In: Harling G, Sparre B
(eds), Flora of Ecuador 3, Swedish Natural Science Research Council, Stockholm,
pp. 1-46.
Murillo-A J, Ruiz-P E, Landrum LR, Stuessy
TF, Barfuss MHJ. 2012. Phylogenetic relationships in Myrceugenia (Myrtaceae) based on plastid and nuclear
DNA sequences. – Mol. Phylogen. Evol. 62: 764-776.
Murillo-A J, Stuessy TF, Ruiz E. 2013.
Phylogenetic relationships among Myrceugenia, Blepharocalyx,
and Luma (Myrtaceae) based on
paired-sites models and the secondary structures of ITS and ETS sequences. –
Plant Syst. Evol. 299: 713-729.
Murillo-A J, Stuessy TF, Ruiz E. 2016.
Explaining disjunct distributions in the flora of southern South America:
evolutionary history and biogeography of Myrceugenia (Myrtaceae). – J. Biogeogr. 43:
979-990.
Nagaraj M. 1954a. Contribution to the floral
morphology of Terminalia chebula Retz. – Curr. Sci. 23: 299-300.
Nagaraj M. 1954b. Megasporogenesis and
megagametophyte development in Combretum ovalifolium Roxb. – Curr.
Sci. 23: 370.
Nagaraj M. 1954c. Contribution to the floral
morphology of Terminalia catappa L. – Curr. Sci. 23: 408.
Nagaraj M. 1955. Floral morphology of
Terminalia bellerica Roxb. – Curr. Sci. 24: 89-90.
Nahrstedt A, Rockenbach J. 1993. Occurrence
of the cyanogenic glucoside prunasin and its corresponding mandelic acid amide
glucoside in Olinia species (Oliniaceae). – Phytochemistry 34:
433-436.
Narayanaswami S, Roy SK. 1960. Embryo sac
development and polyembryony in Syzygium cumini. – Bot. Not. 1960:
273-284.
Nayar MP. 1972. Centers of development and
patterns of distribution of the family Melastomataceae in Indo-Malesia. –
Bull. Bot. Surv. India 14: 1-12.
Nepi M, Guarnieri M, Pacini E. 2003.
“Real” and feed pollen of Lagerstroemia indica: ecophysiological
differences. – Plant Biol. (Stuttgart) 5: 311-314.
Nic Lughadha E. 1994. Notes on the Myrtaceae of the Pico das Almas, Bahia,
Brazil. – Kew Bull. 49: 321-329.
Nic Lughadha E, Proença CA. 1996. A survey
of the reproductive biology of the Myrtoideae (Myrtaceae). – Ann. Missouri Bot. Gard.
83: 480-503.
Nicolle D. 2005. A taxonomic revision and
morphological variation within Eucalyptus series Subulatae
subseries Decussatae and Decurrentes (Myrtaceae) of Australia. – Aust. Syst.
Bot. 18: 473-524.
Nicolle D, Conran JG. 1999. Variation in the
Eucalyptus flocktoniae complex (Myrtaceae) and the description of four new
taxa from southern Australia. – Aust. Syst. Bot. 12: 207-239.
Nicolle D, Whalen MA. 2006. A taxonomic
revision and morphological variation within Eucalyptus series
Subulatae subseries Spirales (Myrtaceae) of southern Australia. –
Aust. Syst. Bot. 19: 87-112.
Nicolle D, Byrne M, Whalen M. 2005. A
taxonomic revision and morphological variation within Eucalyptus
series Subulatae subseries Oleaginae (Myrtaceae), including the oil mallee
complex, of south-western Australia. – Aust. Syst. Bot. 18: 525-553.
Nicolle D, Whalen MA, Mackay DA. 2006.
Morphological variation and phylogenetic relationships within
Eucalyptus series Subulatae (Myrtaceae) of southern Australia. –
Aust. Syst. Bot. 19: 59-86.
Niedenzu F. 1893a. Blattiaceae. – In:
Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(7), W.
Engelmann, Leipzig, pp. 16-21.
Niedenzu F. 1893b. Punicaceae. – In: Engler
A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(7), W. Engelmann,
Leipzig, pp. 22-25.
Niedenzu F. 1893c. Myrtaceae. – In: Engler A, Prantl K
(eds), Die natürlichen Pflanzenfamilien III(7), W. Engelmann, Leipzig, pp.
57-105.
Noher de Halac I, Harte C. 1977. Different
patterns of callose wall formation during megasporogenesis in two species of
Oenothera (Onagraceae). –
Plant Syst. Evol. 127: 23-38.
O’Brien MM, Quinn CJ, Wilson PG. 2000.
Molecular systematics of the Leptospermum suballiance (Myrtaceae). – Aust. J. Bot. 48:
621-628.
Ocampo G, Almeda F. 2013. Seed diversity in
the Miconieae (Melastomataceae):
morphological characterization and phenetic relationships. – Phytotaxa 80:
1-129.
Ochieng JW, Henry RJ, Baverstock PR, Steane
DA, Shepherd M. 2007. Nuclear ribosomal pseudogenes resolve a corroborated
monophyly of the eucalypt genus Corymbia despite misleading hypotheses
at functional ITS paralogs. – Mol. Phylogen. Evol. 44: 752-764.
Odrzykoski IJ, Gottlieb LD. 1984.
Duplications of genes coding 6-phosphogluconate dehydrogenase in
Clarkia (Onagraceae) and
their phylogenetic implications. – Syst. Bot. 9: 479-489.
Ohri D. 1996. Genome size and polyploidy
variation in the tropical hardwood genus Terminalia (Combretaceae). – Plant Syst. Evol.
200: 225-232.
O’Kane SL Jr, Schaal BA. 1998.
Phylogenetics of Lopezia (Onagraceae): evidence from chloroplast
DNA restriction sites. – Syst. Bot. 23: 5-20.
Okuda T, Yoshida T, Hatano T, Yazaki K,
Ashida M. 1982. Ellagitannins of the Casuarinaceae, Stachyuraceae, and
Myrtaceae. – Phytochemistry 21:
2871-2874.
Oliveira PE. 1996. Reproductive biology,
evolution and taxonomy of the Vochysiaceae in Central Brazil. – In:
Owens S, Rudall P (eds), Reproductive biology: in systematics, conservation and
economic botany, Royal Botanical Gardens, Kew, Richmond, pp. 381-393.
Oliveira Bünger M de, Mazine FF, Forest F,
Bueno ML, Stehmann JR, Lucas EJ. 2016. The evolutionary history of
Eugenia sect. Phyllocalyx (Myrtaceae) corroborates historically
stable areas in the southern Atlantic forests. – Ann. Bot. 118: 1209-1223.
Oliveira Bünger M de, Mazine FF, Lucas EJ,
Stehmann JR. 2016. Circumscription and synopsis of Eugenia section
Speciosae Bünger & Mazine (Myrtaceae). – PhytoKeys 61: 73-80.
Oliver D. 1894. Rhynchocalyx
lawsonioides. – In: Hooker’s Ic. Pl. IV, 24: t. 2348.
Onyekwelu SSC. 1990. Germination, seedling
morphology and establishment of Combretum bauchiense Hutch. &
Dalz. (Combretaceae). – Bot. J.
Linn. Soc. 103: 133-138.
Orchard AE. 1979. Myriophyllum (Haloragaceae) in
Australasia 1. New Zealand: a revision of the genus and a synopsis of the
family. – Brunonia 2: 247-287.
Orlovich DA, Drinnan AN, Ladiges PY. 1996.
Floral development in the Metrosideros group (Myrtaceae) with special emphasis on the
androecium. – Telopea 6: 689-719.
Orlovich DA, Drinnan AN, Ladiges PY. 1999.
Floral development in Melaleuca and Callistemon (Myrtaceae). – Aust. Syst. Bot. 11:
689-710.
Ornduff R. 1979. The morphological nature of
distyly in Lythrum section Euhyssopifolia. – Bull. Torrey
Bot. Club 106: 4-8.
Oskolski AA, Feng XX, Jin JH. 2013.
Myrtineoxylon gen. nov.: the first fossil wood record of the tribe
Myrteae (Myrtaceae) in eastern Asia.
– Taxon 62: 771-778.
Outer RW den, Fundter JM. 1976. The secondary
phloem of some Combretaceae and the
systematic position of Strephonema pseudocola A. Chev. – Acta Bot.
Neerl. 25: 481-493.
Outer RW den, Veenendaal WLH van. 1995.
Development of included phloem in the stem of Combretum nigricans (Combretaceae). – IAWA J. 16:
151-158.
Pacini E, Bellani LM. 1986. Lagerstroemia
indica L. pollen: form and function. – In: Blackmore S, Ferguson IK
(eds), Pollen and spores: form and function, Academic Press, London, pp.
347-357.
Palézieu P de. 1899.
Anatomisch-systematische Untersuchung des Blattes der Melastomaceen mit
Ausschluss der Triben: Microlicieen, Tibouchineen, Miconieen. – Bull. Herb.
Boissier 7: 1-83.
Panigrahi SG. 1976. Studies on generic
delimitations on the four genera Rotala, Ammannia,
Nesaea and Hionanthera (Lythraceae) – a historical survey. –
Bull. Bot. Survey India 18: 178-193.
Panigrahi SG. 1980. Studies on the cuticle
and stomata of Ammannia, Rotala, Nesaea and
Hionanthera (Lythraceae). –
J. Orissa Bot. Soc. 2: 13-18.
Panigrahi SG. 1982. Contribution of anatomy
to the systematics of Ammannia. – Phytomorphology 30: 320-330.
Panigrahi SG. 1986. Seed morphology of
Rotala L., Ammannia L., Nesaea Kunth and
Hionanthera Fernandes & Diniz (Lythraceae). – Bot. J. Linn. Soc. 93:
389-403.
Panigrahi SG. 1988. Contribution of anatomy
to the systematics of Rotala L. (Lythraceae). – Bull. Bot. Survey India
30: 90-100.
Panigrahi S, Panigrahi G. 1977. Evolutionary
trends in the inflorescences of the family Lythraceae. – In: Padhi B (ed),
Frontiers of plant sciences, Utkal University, Bhubaneswar, pp. 401-410.
Parnell JAN. 1999. Numerical analysis of Thai
members of Eugenia-Syzygium group (Myrtaceae). – Blumea 44: 351-379.
Parnell JAN. 2003. Pollen of
Syzygium (Myrtaceae) from SE
Asia, especially Thailand. – Blumea 48: 303-317.
Parnell JAN, Nic Lughadha E. 1992. Notes on
Thai Myrtaceae. – Kew Bull. 47:
703-706.
Parnell JAN, Craven LA, Biffin E. 2007.
Matters of scale: dealing with one of the largest genera of angiosperms. –
In: Hodkinson TR, Parnell JAN (eds), Reconstructing the Tree of Life: taxonomy
and systematics of species rich taxa, Syst. Assoc. Spec. Vol. Ser. 72, CRC
Press, Boca Raton, Florida, pp. 251-273.
Parra-O C, Bayly M, Udovicic F, Ladiges P.
2006. ETS sequences support the monophyly of the eucalypt genus
Corymbia (Myrtaceae). –
Taxon 55: 653-663.
Parra-O C, Bayly MJ, Drinnan A, Udovicic F,
Ladiges P. 2009. Phylogeny, major clades and infrageneric classification of
Corymbia (Myrtaceae), based on
nuclear ribosomal DNA and morphology. – Aust. Syst. Bot. 22: 384-399. –
Correction: Aust. Syst Bot. 23 (2010): 141.
Passioura JA, Ash JE. 1993. Eucalyptus
saligna Smith subsp. botryoides (Smith) Passioura & Ash,
comb. nov.: a re-assessment of the Eucalyptus saligna-E. botryoides
complex. – Aust. Syst. Bot. 6: 181-183.
Patel RN. 1994. Wood anatomy of the
dicotyledons indigenous to New Zealand 23. Myrtaceae – subfam. Leptospermoideae
(part 1). – New Zealand J. Bot. 32: 95-112.
Patel RN. 1995. Wood anatomy of the
dicotyledons indigenous to New Zealand 25. Myrtaceae – subfam. Myrtoideae (part 1).
– New Zealand J. Bot. 33: 541-555.
Patel VC, Skvarla JJ, Raven PH. 1983. Half
pseudocolpi, a unique feature of Olinia (Oliniaceae) pollen. – Amer. J. Bot. 70:
469-473.
Patel VC, Skvarla JJ, Raven PH. 1984 [1985].
Pollen characters in relation to the delimitation of Myrtales. – Ann. Missouri Bot. Gard. 71:
858-969.
Pedley L. 1990. Combretaceae. – In: George AS (ed),
Flora of Australia 18, Australian Government Publ. Service, Canberra, pp.
255-293.
Peng C-I. 1983. Triploidy in
Ludwigia in Taiwan and the discovery of Ludwigia adscendens
(Onagraceae). – Bot. Bull. Acad.
Sin. 24: 129-134.
Peng C-I. 1988. The biosystematics of
Ludwigia sect. Microcarpium (Onagraceae). – Ann. Missouri Bot. Gard.
75: 970-1009.
Penneys DS. 2001. A systematic revision and
cladistic analysis of Charianthus (Miconieae: Melastomataceae) using morphological
and molecular characters. – M.Sc. thesis, University of Florida, Gainesville,
Florida.
Penneys DS. 2004 onwards. Melastomataceae of the World.
http://www.flmnh.ufl.edu/melastomes/melastome_databases_table.htm. (consulted
v.2012)
Penneys DS. 2007. Phylogeny and character
evolution in the Blakeeae (Melastomataceae). – Ph.D. diss.,
University of Florida, Gainesville, Florida.
Penneys DS, Judd WS. 2003. The resurrection
and lectotypification of Tetrazygia fadyenii (Melasomataceae:
Miconieae): a hummingbird pollinated treelet endemic to Jamaica. – Sida 20:
877-884.
Penneys DS, Judd WS. 2004. Two new species of
Charianthus (Melastomataceae: Miconieae) from the
Lesser Antilles. – Brittonia 56: 151-158.
Penneys DS, Judd WS. 2005. A systematic
revision and cladistic analysis of Charianthus (Melastomataceae) using morphological
and molecular characters. – Syst. Bot. 30: 559-584.
Penneys DS, Judd WS. 2011. Phylogenetics and
morphology in the Blakeeae (Melastomataceae). – Intern. J.
Plant Sci. 172: 78-106.
Penneys DS, Judd WS. 2013. Combined molecular
and morphological phylogenetic analyses of the Blakeee (Melastomataceae). – Intern. J.
Plant Sci. 174: 802-817.
Penneys DS, Michelangeli FA, Judd WS, Almeda
E. 2010. Henrietteeae (Melastomataceae): a new Neotropical
berry-fruited tribe. – Syst. Bot. 35: 783-800.
Pereira E. 1959. Contribuição ao
conhecimento das Melastomataceae
brasileiras. – Arq. Jard. Bot. Rio Janeiro 17: 125-160.
Pereira JT. 1996. Crypteroniaceae. – In: Soepadmo E,
Wong KM, Saw LG (eds), Tree flora of Sabah and Sarawak 2, Forest Research
Institute Malaysia, Sabah Forestry Department, and Sarawak Forestry Department,
Kuala Lumpur, Malaysia, pp. 135-149.
Pereira JT, Wong K-M. 1995. Three new species
of Crypteronia (Crypteroniaceae) from Borneo. –
Sandakania 6: 41-53.
Perrier de la Bâthie H. 1932. Les
Mélastomacées de Madagascar. – Mém. Acad. Malgache 12: 1-292.
Perrier de la Bâthie H. 1951. Famille 153.
Mélastomatacées. – In: Humbert H (ed), Flore de Madagascar et des Comores,
Muséum National d’Histoire Naturelle, Paris, pp. 1-326.
Perrier de la Bâthie H. 1953a. Les
Myrtacées de Madagascar et des Comores, révision, diagnoses et biologie. –
Mém. Inst. Sci. Madagascar, sér. B, IV(2): 161-202.
Perrier de la Bâthie H. 1953b. Fam. 152. Myrtaceae. – In: Humbert H (ed), Flore
de Madagascar et des Comores, Muséum National d’Histoire Naturelle, Paris,
pp. 1-80.
Petersen OG. 1896. Vochysiaceae. – In: Engler A, Prantl
K (eds), Die natürlichen Pflanzenfamilien III(4), W. Engelmann, Leipzig, pp.
312-319.
Pfeil BE, Henwood MJ, Edwards P. 2004.
Multivariate analysis of morphological variation in Eucalyptus series
Psathroxyla Blakely (Myrtaceae): taxonomic implications. –
Telopea 10: 711-724.
Pflaum F. 1897. Anatomisch-systematische
Untersuchung des Blattes der Melastomataceen aus den Triben Microlicieen und
Tibouchineen. – Ph.D. diss., Universität München, Germany.
Philcox D. 1986. Hoshiarpuria
debunked. – Kew Bull. 41: 432.
Pigg KB, Stockey RA, Maxwell SL. 1993.
Paleomyrtinaea, a new genus of permineralized myrtaceous fruits and
seeds from the Eocene of British Columbia and Paleocene of North Dakota. –
Can J. Bot. 71: 1-9.
Pijl L van der. 1934. Über die Polyembryonie
bei Eugenia. – Rec. Trav. Bot. Néerl. 31: 113-187.
Pike KM. 1956. Pollen morphology of Myrtaceae from the South-West Pacific
area. – Aust. J. Bot. 4: 13-53.
Pillon Y, Johansen JB, Sakishima T, Chamala
S, Barbazuk WB, Stacy EA. 2014. Primers for low-copy nuclear genes in
Metrosideros and cross-amplification in Myrtaceae. – Appl. Plant Sci. 2:
1400049.
Pillon Y, Lucas E, Johansen JB, Sakishima T,
Hall B, Geib SM, Stacy EA. 2015. An expanded Metrosideros (Myrtaceae) to include Carpolepis
and Tepualia based on nuclear genes. – Syst. Bot. 40: 782-790.
Pittier HF. 1947. Especies de
Pterolepis transferibles a Tibouchina y descripciones de dos
nuevas especies. – Bol. Soc. Ven. Ci. Nat. 11: 17-20.
Pizo MA. 2002. The seed-dispersers and fruit
syndromes of Myrtaceae in the Brazilian
Atlantic forest. – In: Levey DJ, Silva WR, Galetti M (eds), Seed dispersal
and frugivory: ecology, evolution, and conservation, CABI Publ., Wallingford,
Oxfordshire, United Kingdom, pp. 129-143.
Pizo MA, Morellato LP. 2002. A new
rain-operated seed dispersal mechanism in Bertolonia mosenii (Melastomataceae), a Neotropical
rainforest herb. – Amer. J. Bot. 89: 169-171.
Plitmann U, Raven PH, Breedlove PA. 1973. The
systematics of the Lopezieae (Onagraceae). – Ann. Missouri Bot. Gard.
60: 478-563.
Poke FS, Martin DP, Steane DA, Vaillancourt
RE, Reid JB. 2006. The impact of intragenic recombination on phylogenetic
reconstruction at the sectional level in Eucalyptus when using a
single copy nuclear gene (cinnamoyl CoA reductase). – Mol. Phylogen. Evol.
39: 160-170.
Porter EA, Nic Lughadha E, Simmonds MSJ.
2000. Taxonomic significance of polyhydroxyalkaloids in the Myrtaceae. – Kew Bull. 55: 615-632.
Prance GT. 1980. A note on the probable
pollination of Combretum by Cebus monkeys. – Biotropica 12:
239.
Praglowski J, Skvarla JJ, Raven PH, Nowicke
JW. 1983 Onagraceae Juss. Fuchsieae
L./Jussiaeeae L. – World Pollen and Spore Flora 12: 1-41.
Praglowski J, Nowicke JW, Raven PH, Skvarla
JJ, Wagner L. 1987. Onagraceae Juss.
Onagreae R. Raimann pro parte. – World Pollen and Spore Flora 15: 1-55.
Prakasa Rao PS. 1963. Some embryological
observations of Guiera senegalensis Lam. – Curr. Sci. 32: 30-31.
Proença CEB. 1990. A revision of
Siphoneugenia Berg. – Edinburgh J. Bot. 47: 239-271.
Proença CEB, Nic Lughadha EM, Lucas EJ,
Woodgyer EM. 2006. Algrizea (Myrteae, Myrtaceae): a new genus from the highlands
of Brazil. – Syst. Bot. 31: 320-326.
Pryor LD, Johnson LAS. 1971. A classification
of the eucalypts. – Australian National University Press, Canberra.
Pryor LD, Williams ER, Gunn BV. 1995. A
morphometric analysis of Eucalyptus urophylla and related taxa with
descriptions of two new species. – Aust. Syst. Bot. 8: 57-70.
Quirk JT. 1980. Wood anatomy of the Vochysiaceae. – IAWA Bull., N. S., 1:
172-179.
Ragonese AM. 1976. Consideraciones sobre el
problema de la clasificación de los elementos traqueales no perforados de las
diccótiledoneas y en especial de algunas Mirtáceas. – Darwiniana 20:
476-490.
Raimann R. 1893a. Onagraceae. – In: Engler A, Prantl K
(eds), Die natürlichen Pflanzenfamilien III(7), W. Engelmann, Leipzig, pp.
199-223.
Raimann R. 1893b. Hydrocaryaceae. – In:
Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(7), W.
Engelmann, Leipzig, pp. 223-226.
Ram M. 1956. Floral morphology and embryology
of Trapa bispinosa Roxb. with discussion on the systematic position of
the genus. – Phytomorphology 6: 312-323.
Rama Devi D, Satyavathi DVL, Narayana LL.
1991. Floral anatomy of some Melastomataceae. – Feddes Repert.
102: 595-599.
Ramamoorthy TP. 1979. A sectional revision of
Ludwigia sect. Myrtocarpus sensu lato (Onagraceae). – Ann. Missouri Bot. Gard.
66: 893-896.
Ramamoorthy TP, Zardini EM. 1987. The
systematics and evolution of Ludwigia sect. Myrtocarpus sensu
lato (Onagraceae). – Monogr. Syst.
Bot. Missouri Bot. Gard. 19: 1-120.
Ramos ZA, Cruz AJU. 2009. Novitiae florae
cubenses 32. A new species of Plinia (Myrtaceae, Eugeniinae) from quartzitic
sands of Pinar del Río, W Cuba. – Willdenowia 39: 141-144.
Rao RV, Sharma B, Chauhan L, Dayal R. 1987.
Reinvestigation of the wood anatomy of Duabanga and
Sonneratia with particular reference to their systematic position. –
IAWA Bull., N. S., 8: 337-345.
Rao TA. 1951. Studies on foliar sclereids in
dicotyledons V. Structure and development of the terminal sclereids in the leaf
of Memecylon heyneanum Benth. – Proc. Indian Acad. Sci., Sect. B,
34: 329-334.
Rao TA. 1957. Comparative morphology and
ontogeny of foliar sclereids in seed plants I. Memecylon L. –
Phytomorphology 7: 306-330.
Rao TA, Bhupal OP. 1974. The utility of
sclereid typology in solving problems of synonymy in a few taxa of the genus
Memecylon L. – Proc. Indian Acad. Sci., Sect. B, 80: 291-300.
Rao TA, Chakraborti S. 1982. A little looked
at attribute of the leaves of Sonneratia caseolaris (L.). – Curr.
Sci. 51: 303-305.
Rao TA, Chin SC. 1972. Branched and septate
root hairs in Melastoma malabathricum. – Cytologia 37: 111-118.
Rao TA, Dakshni KMM. 1963. Systematics of
Memecylon – a preliminary survey based on the sclereid morphology.
– Proc. Indian Acad. Sci., Sect. B, 58: 28-35.
Rao TA, Jacques-Félix H. 1978. Les types de
sclérites foliaires et la classifiction des Memecylon africains. –
Adansonia, sér. II, 18: 59-66.
Rao TA, Bremer K, Chakraborti S. 1980. Foliar
sclereids in Sri Lanka (Ceylonese) species of Memecylon (Melastomataceae). – Bot. Not. 133:
397-401.
Rao TA, Bremer K, Naidu TRB. 1983. Foliar
sclereids in Memecylon and Lijndenia (Melastomataceae) from Borneo, Java,
Malaya and Sumatra. – Nord. J. Bot. 3: 343-345.
Rao VS. 1965. On foliar sclereids in Penaeaceae. – Sci. Cult. 31: 380.
Rao VS, Dahlgren R. 1968. Studies on Penaeaceae V. The vascular anatomy of the
flower of Glischrocolla formosa. – Bot. Not. 121: 259-268.
Rao VS, Dahlgren R. 1969. The floral anatomy
and relationships of Oliniaceae. –
Bot. Not. 122: 160-171.
Raven PH. 1960. The genus Epilobium
in the Himalayan region. – Bull. Brit. Mus (Nat. Hist.) Bot. 2: 325-382.
Raven PH. 1962. The systematics of
Oenothera subgenus Chylismia. – Univ. Calif. Publ. Bot. 34:
1-122.
Raven PH. 1963. The Old World species of
Ludwigia (including Jussiaea), with a synopsis of the genus.
– Reinwardtia 6: 327-427.
Raven PH. 1964. The generic subdivision of Onagraceae, tribe Onagreae. – Brittonia
16: 276-288.
Raven PH. 1967. A revision of the African
species of Epilobium (Onagraceae). – Bothalia 9: 309-333.
Raven PH. 1969. A revision of the genus
Camissonia (Onagraceae). –
Contr. U.S. Natl. Herb. 37: 161-396.
Raven PH. 1976. Generic and sectional
delimitation in Onagraceae, tribe
Epilobieae. – Ann. Missouri Bot. Gard. 63: 326-340.
Raven PH. 1977. Onagraceae. – In: Steenis CGGJ van
(ed), Flora Malesiana I, 8(2), Sijthoff & Noordhoff International Publ.,
Alphen aan den Rijn, The Netherlands, pp. 98-113.
Raven PH. 1978. 81. Onagraceae. – In: Launert E (ed), Flora
Zambesiaca 4, Flora Zambesiaca Managing Committee, London, pp. 329-
Raven PH. 1979. A survey of reproductive
biology in Onagraceae. – New Zealand
J. Bot. 17: 575-593.
Raven PH. 1984. The order Myrtales: a symposium. – Ann. Missouri
Bot. Gard. 71: 631-632.
Raven PH. 1988. Onagraceae as a model of plant evolution.
– In: Gottlieb LD, Jain SK (eds), Plant evolutionary biology, Chapman and
Hall, New York, London, pp. 85-107.
Raven PH, Gregory DP. 1972. A revision of the
genus Gaura (Onagraceae). –
Mem. Torrey Bot. Club 23: 1-96.
Raven PH, Moore DM. 1965. A revision of
Boisduvalia (Onagraceae). –
Brittonia 17: 238-254.
Raven PH, Raven TE. 1976. The genus
Epilobium in Australasia: a systematic and evolutionary study. –
Bull. New Zealand Dept. Sci. Industr. Res. 216: 1-321.
Raven PH, Tai W. 1979. Observations of
chromosomes in Ludwigia (Onagraceae). – Ann. Missouri Bot. Gard.
66: 862-879.
Raven PH, Dietrich W, Stubbe W. 1979. An
outline of the systematics of Oenothera Subsect. Euoenothera
(Onagraceae). – Syst. Bot. 4:
242-252.
Regalado JC. Jr. 1990. Revision of
Medinilla (Melastomataceae) of Borneo. –
Blumea 35: 5-70.
Reginato M, Baumgratz JFA, Goldenberg R.
2013. A taxonomic revision of Pleiochiton (Melastomataceae, Miconieae). –
Brittonia 65: 16-41.
Reginato M, Michelangeli FA. 2016a.
Untangling the phylogeny of Leandra s.str. (Melastomataceae, Miconieae). –
Mol. Phylogen. Evol. 96: 17-32.
Reginato M, Michelangeli FA. 2016b. Diversity
and constraints in the floral morphological evolution of Leandra
s.str. (Melastomataceae). –
Ann. Bot. 118: 445-458.
Reginato M, Michelangeli FA, Goldenberg R.
2010. Phylogeny of Pleiochiton (Melastomataceae, Miconieae): total
evidence. – Bot. J. Linn. Soc. 162: 423-434.
Renner SS. 1986. The neotropical epiphytic Melastomataceae: phytogeographic
patterns, fruit types, and floral biology. – Selbyana 9: 104-111.
Renner SS. 1987. Sandemania hoehnei
(Tibouchineae: Melastomataceae):
taxonomy, distribution, and biology. – Brittonia 39: 441-446.
Renner SS. 1989a. A survey of reproductive
biology in neotropical Melastomataceae and Memecylaceae. – Ann. Missouri Bot.
Gard. 76: 496-518.
Renner SS. 1989b. Systematic studies in the
Melastomataceae:
Bellucia, Loreya and Macairea. – Mem. New York
Bot. Gard. 50: 1-111.
Renner SS. 1990a. Reproduction and evolution
in some genera of neotropical Melastomataceae. – Mem. New York
Bot. Gard. 55: 143-152.
Renner SS. 1990b. A revision of
Rhynchanthera (Melastomataceae). – Nord. J. Bot.
9: 601-630.
Renner SS. 1993. Phylogeny and classification
of the Melastomataceae and Memecylaceae. – Nord. J. Bot. 13:
519-540.
Renner SS. 1994a. Revisions of
Pterogastra and Schwackaea (Melastomataceae: Melastomeae). –
Nord. J. Bot. 14: 65-71.
Renner SS. 1994b. A revision of
Pterolepis (Melastomataceae: Melastomeae). –
Nord. J. Bot. 14: 73-104.
Renner SS. 2004a. Bayesian analysis of
combined chloroplast loci, using multiple calibrations, supports the recent
arrival of Melastomataceae in
Africa and Madagascar. – Amer. J. Bot. 91: 1427-1435.
Renner SS. 2004b. Multiple Miocene Melastomataceae dispersal between
Madagascar, Africa and India. – Phil. Trans. Roy. Soc. London, Ser. B, 359:
1485-1494.
Renner SS. 2005. Bayesian analysis of
combined chloroplast loci, using multiple calibrations, supports the recent
arrival of Melastomataceae in
Africa and Madagascar. – Amer. J. Bot. 91: 1427-1435.
Renner SS. 2004. Multiple Miocene Melastomataceae dispersal between
Madagascar, Africa and India. – Phil. Trans. Roy. Soc. London, Ser. B, 359:
1485-1494.
Renner SS. 2006. Crypteroniaceae. – In: Kubitzki K
(ed), The families and genera of vascular plants IX. Flowering plants.
Eudicots. Berberidopsidales,
Buxales, Crossosomatales, Fabales p. p., Geraniales, Gunnerales, Myrtales p. p., Proteales, Saxifragales, Vitales, Zygophyllales, Clusiaceae Alliance, Passifloraceae
Alliance, Dilleniaceae, Huaceae, Picramniaceae, Sabiaceae, Springer, Berlin,
Heidelberg, New York, pp. 123-126.
Renner SS, Meyer K. 2001. Melastomataceae come full circle:
biogeographic reconstruction and molecular clock dating. – Evolution 55:
1315-1324.
Renner SS, Clausing G, Meyer K. 2001.
Historical biogeography of Melastomataceae: the roles of
Tertiary migration and long-distance dispersal. – Amer. J. Bot. 88:
1290-1300.
Renner SS, Triebel D, Almeda F, Stone RD,
Ulloa C, Michaelangeli FA, Goldenberg R, Mendoza H (eds). 2007-. MEL names –
A database with names of Melastomataceae.
https://urlproxy.sunet.se/canit/urlproxy.php?_q=aHR0cDovL3d3dy5tZWxhc3RvbWF0YWNlYWUubmV0L01FTG5hbWVzLw%3D%3D&_s=ZGVmYXVsdA%3D%3D&_c=456b32aa&_r=c3Utc2U%3D
Romero R. 2013. Taxonomic notes in
Microlicia (Melastomataceae, Microlicieae). –
Phytotaxa 110: 48-54.
Romero R, Martins AB. 2003. Four new species
of Svitramia Cham. (Melastomataceae, Melastomeae) from
Minas Gerais, Brazil. – Kew Bull. 58: 403-413.
Romero R, Woodgyer EM. 2018. Six new species
of Microlicia (Melastomataceae) from Bahia, Brazil.
– Kew Bull. 73: 22. doi 10.1007/S12225-018-9747-4
Romero S, Versiane AFA. 2014. Taxonomic
novelty and typifications in Microlepis (Melastomataceae). – Novon 23:
217-233.
Ronse De Craene L-P, Smets E. 1991. The
impact of receptacular growth on polyandry in the Myrtales. – Bot. J. Linn. Soc. 105:
257-269.
Ross H, Suessenguth K. 1926. Das Apikalorgan
der Blätter von Lafoensia. – Flora 120: 1-18.
Rourke JP. 1995. A new species of
Brachysiphon (Penaeaceae)
from the Southern Cape, South Africa. – Nord. J. Bot. 15: 63-66.
Rourke JP, McDonald DJ. 1989. A new species
of Penaea (Penaeaceae), from
the Langeberg Range, southern Cape. – South Afr. J. Bot. 55: 400-404.
Rowley J, Skvarla J. 2007. Pollen development
in Epilobium (Onagraceae):
from microspore mitosis to formation of the intine. – Grana 46: 130-139.
Roy SK. 1960. Embryology of Eugenia
jambos. – Curr. Sci. 29: 189-190.
Roy SK. 1961. Embryology of Eugenia
fruticosa. – Proc. Indian Natl. Acad. Sci., Sect. B, 31: 80-87.
Roy SK. 1962. Embryology of Eugenia
myrtifolia. – Sci. Cult. 28: 376-378.
Rozefelds AC. 1996. Eucalyptus
phylogeny and history: a brief summary. – Tasforests 8: 15-26.
Ruiz E, Crawford DJ, Stuessy TF, Conzález F,
Samuel R, Becerra J, Silva M. 2004. Phylogenetic relationships and genetic
divergence among endemic species of Berberis, Gunnera,
Myrceugenia and Sophora of the Juan Fernández Islands
(Chile) and their continental progenitors based on isozymes and nrITS
sequences. – Taxon 53: 321-332.
Rutherford S, Wilson PG, Rossetto M, Bonser
SP. 2016. Phylogenomics of the green ash eucalypts (Myrtaceae): a tale of reticulate evolution
and misidentification. – Aust. Syst. Bot. 28: 326-354.
Rutschmann F, Eriksson T, Schönenberger J,
Conti E. 2004. Did Crypteroniaceae really disperse out
of India? Molecular dating evidence from rbcL, ndhF, and
rpl16 intron sequences. – Intern. J. Plant Sci. 165(Suppl.):
S69-S83.
Rutschmann F, Eriksson T, Abu Salim K, Conti
E. 2007. Assessing calibration uncertainty in molecular dating: the assignment
of fossils to alternative calibration points. – Syst. Biol. 56: 591-608.
Ruys JD. 1924. Contribution à l’histoire
de développement des Mélastomacées. – Ann. Jard. Bot. Buitenzorg 34:
1-23.
Rye BL. 1979. Chromosome number variation in
the Myrtaceae and its taxonomic
implications. – Aust. J. Bot. 27: 547-573.
Rye BL. 2009a. A reduced circumscription of
Balaustion and a description of the new genus Cheyniana (Myrtaceae: Chamaelaucieae) with three new
species. – Nuytsia 19: 129-148.
Rye BL. 2009b. Reinstatement of the Western
Australian genus Oxymyrrhine (Myrtaceae: Chamaelaucieae) with three new
species. – Nuytsia 19: 149-165.
Rye BL. 2013. A revision of the south-western
Australian genus Astartea (Myrtaceae: Chamelaucieae). – Nuytsia 23:
189-269.
Rye BL, James SH. 1992. The relationship
between dysploidy and reproductive capacity in Myrtaceae. – Aust. J. Bot. 40:
829-848.
Rye BL, Trudgen ME. 2005. A new
heterocarpidic fruit type for the Myrtaceae, with dehiscent and indehiscent
loculi, in two genera from Western Australia. – Nuytsia 15: 485-493.
Rye BL, Trudgen ME. 2008. Seorsus, a new
Gondwanan genus of Myrtaceae with a
disjunct distribution in Borneo and Australia. – Nuytsia 18: 235-257.
Sahni B. 1943. Indian silicified plants II.
Enigmocarpon Parijai, a silicified fruit from the Deccan, with a
review of the fossil history of the Lythraceae. – Proc. Indian Natl. Acad.
Sci, Sect. B, 17: 59-96.
Sajo MG, Rudall PJ. 2002. Leaf and stem
anatomy of Vochysiaceae in relation
to subfamilial and suprafamilial systematics. – Bot. J. Linn. Soc. 138:
339-364.
Salatino A, Faria Salatino M, Santos D dos,
Patricio M. 2000. Distribution and evolution of secondary metabolites in Eriocaulaceae,
Lythraceae and Velloziaceae
from “campos rupestres”. – Gen. Mol. Biol. 23: 931-940.
Sale MM, Potts BM, West AK, Reid JB. 1993.
Relationships within Eucalyptus using chloroplast DNA. – Aust. Syst.
Bot. 6: 127-138.
Sale MM, Potts BM, West AK, Reid JB. 1996.
Relationships within Eucalyptus using PCR-amplification and Southern
hybridization of chloroplast DNA. – Aust. Syst. Bot. 9: 273-282.
Salter TM. 1940. Some notes on the
nomenclature, generic definition and the species question in the genus
Sarcocolla, Kunth. – J. South Afr. Bot. 6: 41-43.
Salywon AM, Landrum LR. 2007.
Curitiba (Myrtaceae): a new
genus from the Planalto of southern Brazil. – Brittonia 59: 301-307.
Santos AK. 2009. Estudos filogenéticos e
biossistemáticos no gênero Marcetia DC. (Melastomataceae). – Ph.D. diss.,
Universidade Estadual de Feira de Santana, Brazil.
Santos APM dos, Fracasso CM, Luciene dos
Santos M, Romero R, Sazima M, Liveira PE. 2012. Reproductive biology and
species geographical distribution in the Melastomataceae: a survey based on
New World taxa. – Ann. Bot. 110: 667-679.
Santos MF, Sano PT, Forest F, Lucas E. 2016.
Phylogeny, morphology and circumscription of Myrcia sect.
Sympodiomyrcia (Myrcia s.l., Myrtaceae). – Taxon 65: 759-774.
Santos MF, Lucas E, Sano PT, Buerki S,
Staggemeier VG, Forest F. 2017. Biogeographical patterns of Myrcia
s.l. (Myrtaceae) and their correlation
with geological and climatic history in the Neotropics. – Mol. Phylogen.
Evol. 108: 34-48.
Sazima M, Sazima I. 1975. Quiropterofilia em
Lafoensia pacari St. Hil. (Lythraceae), na Serra do Cipó, Minas
Gerais. – Ciencia e Cultura 27: 405-416.
Sazima M, Vogel S, Prado AL do, Oliveira DM
de, Franz G, Sazima I. 2001. The sweet jelly of Combretum lanceolatum
flowers (Combretaceae): a cornucopia
resource for bird pollinators in the Pantanal, western Brazil. – Plant Syst.
Evol. 227: 195-208.
Schemske DW. 1980. Floral ecology and
hummingbird pollination of Combretum farinosum in Costa Rica. –
Biotropica 12: 169-181.
Schmid R. 1972a. Floral anatomy of Myrtaceae I. Syzygium s.l. –
Bot. Jahrb. Syst. 92: 433-489.
Schmid R. 1972b. Floral anatomy of Myrtaceae II. Eugenia. – J.
Arnold Arbor. 52: 336-363.
Schmid R. 1972c. A resolution of the
Eugenia-Syzygium controversy (Myrtaceae). – Amer. J. Bot. 59:
423-436.
Schmid R. 1980. Comparative anatomy and
morphology of Psiloxylon and Heteropyxis, and the subfamilial
and tribal classification of Myrtaceae.
– Taxon 29: 559-595.
Schmid R. 1984 [1985]. Reproductive anatomy
and morphology of Myrtales in relation
to systematics. – Ann. Missouri Bot. Gard. 71: 832-835.
Schmid R, Baas P. 1984. The occurrence of
scalariform perforation plates and helical vessel wall thickenings in wood of
Myrtaceae. – IAWA Bull., n. s., 5:
197-215.
Schnell CE. 1996. The genus
Conostegia (Melastomataceae). – Ph.D. diss.,
Harvard University, Cambridge, Massachusetts.
Schönenberger J. 2006. Rhynchocalycaceae. – In:
Kubitzki K (ed), The families and genera of vascular plants IX. Flowering
plants. Eudicots. Berberidopsidales,
Buxales, Crossosomatales, Fabales p.p., Geraniales, Gunnerales, Myrtales p.p., Proteales, Saxifragales, Vitales, Zygophyllales, Clusiaceae Alliance, Passifloraceae
Alliance, Dilleniaceae, Huaceae, Picramniaceae, Sabiaceae, Springer, Berlin,
Heidelberg, New York, pp. 409-412.
Schönenberger J, Conti E. 2003. Molecular
phylogeny and floral evolution of Penaeaceae, Oliniaceae, Rhynchocalycaceae, and Alzateaceae (Myrtales). – Amer. J. Bot. 90:
293-309.
Schönenberger J, Conti E, Rutschmann F.
2006. Penaeaceae. – In: Kubitzki K
(ed), The families and genera of vascular plants IX. Flowering plants.
Eudicots. Berberidopsidales,
Buxales, Crossosomatales, Fabales p.p., Geraniales, Gunnerales, Myrtales p.p., Proteales, Saxifragales, Vitales, Zygophyllales, Clusiaceae Alliance, Passifloraceae
Alliance, Dilleniaceae, Huaceae, Picramniaceae, Sabiaceae, Springer, Berlin,
Heidelberg, New York, pp. 282-291.
Schrenk H. 1889. On the floating tissue of
Nesaea verticillata (L.) H.B.K. – Bull. Torrey Bot. Club 16:
315-323.
Schuett BS, Abbadi A, Loddenkoetter B,
Brummel M, Spener F. 2002. Beta-ketoacyl-acyl carrier protein synthase IV: a
key enzyme for regulation of medium-chain fatty acid synthesis in Cuphea
lanceolata seeds. – Planta (Berlin) 215: 847-854.
Schulman L, Hyvönen J. 2003. A cladistic
analysis of Adelobotrys (Melastomataceae) based on
morphology, with notes on generic limits within the tribe Merianieae. – Syst.
Bot. 28: 738-756.
Scott AJ. 1978. A revision of
Rhodomyrtus (Myrtaceae). –
Kew Bull. 33: 311-329.
Scott AJ. 1979a. New species and combinations
in Myrtaceae from Malesia and
Australia. – Kew Bull. 33: 511-515.
Scott AJ. 1979b. A revision of
Anogeissus (Combretaceae).
– Kew Bull. 33: 555-566.
Scott AJ. 1979 [1980]. Notes on Myrtaceae in the Mascarenes with some
recombinations for taxa from Aldabra, Malaya, New Caledonia. – Kew Bull. 35:
473-498.
Scott AJ. 1983. Two new species of
Kania (Myrtaceae) from New
Guinea. – Kew Bull. 38: 309-310.
Scott AJ. 1984. Two new species of Myrtaceae from New Guinea. – Kew Bull.
39: 6569-660.
Scott AJ. 1985. Decaspermum (Myrtaceae) in New Guinea. – Kew Bull.
40: 149-165.
Scott AJ. 1986. A new combination in
Uromyrtus (Myrtaceae) from
Australia. – Kew Bull. 41: 286.
Scott AJ. 1990. Myrtacées. – In: Bosser J,
Cadet T, Guého J, Marais W (eds), Flore des Mascareignes 92, The Sugar
Industry Research Institute, Réduit, pp. 1-70.
Seavey SR. 1977. Segregation of translocated
chromosomes in Epilobium and Boisduvalia hybrids (Onagraceae). – Syst. Bot. 2:
109-121.
Seavey SR. 1992. Experimental hybridization
and chromosome homologies in Boisduvalia sect. Boisduvalia
(Onagraceae). – Syst. Bot. 17:
84-90.
Seavey SR, Boufford DE. 1983. Observations of
chromosomes in Circaea (Onagraceae). – Amer. J. Bot. 70:
1476-1481.
Seavey SR, Raven PH. 1977a. Chromosomal
evolution in Epilobium sect. Epilobium (Onagraceae). – Plant Syst. Evol. 127:
107-119.
Seavey SR, Raven PH. 1977b. Chromosomal
evolution in Epilobium sect. Epilobium (Onagraceae) II. – Plant Syst. Evol.
128: 195-200.
Seavey SR, Raven PH. 1977c. Experimental
hybrids in Epilobium (including Zauschneria) species with n =
15 (Onagraceae). – Amer. J. Bot. 64:
439-442.
Seavey SR, Raven PH. 1977d. Chromosomal
differentiation and the sources of the South American species of
Epilobium (Onagraceae). –
J. Biogeogr. 4: 55-59.
Seavey SR, Raven PH. 1978. Chromosomal
evolution in Epilobium sect. Epilobium (Onagraceae) III. – Plant Syst. Evol.
130: 79-83.
Seavey SR, Solomon JC. 1984. Cytogeography of
the South American species of Epilobium (Onagraceae). – Syst. Bot. 9: 17-21.
Seavey SR, Magill RE, Raven PH. 1977.
Evolution of seed size, shape, and surface architecture in the tribe Epilobieae
(Onagreae). – Ann. Missouri Bot. Gard. 64: 18-47.
Seavey SR, Wight P, Raven PH. 1977. A
comparison of Epilobium minutum and E. foliosum (Onagraceae). – Madroño 24: 6-12.
Sebola RJ, Balkwill K. 1999. Resurrection of
two previously confused species, Olinia capensis (Jacq.) Klotsch and
O. micrantha Decne. (Oliniaceae). – South Afr. J. Bot. 65:
97-103.
Sebola RJ, Balkwill K. 2013. A monographic
study of the Oliniaceae. – Kew Bull.
68: 419-456.
Seco RS 2006. Estudos taxonômicos no gênero
Comolia DC. (Melastomataceae-Melastomeae) no
Brasil. – M.Sc. thesis, Universidade Estadual de Campinas, Campinas,
Brazil.
Sell Y, Cremers G. 1987. The inflorescences
of Guyanese Melastomataceae:
their affiliation and taxonomic importance. – Can. J. Bot. 65: 999-1010.
Sennikov AN. 2011. Chamerion or
Chamaenerion (Onagraceae)?
The old story in new words. – Taxon 60: 1485-1488.
Sharma PN, Shoeb A, Kapil RS, Popli SP. 1982.
Arjunolone – a new flavone from stem bark of Terminalia arjuna. –
Indian J. Chem. 21B: 263-264.
Shepherd M, Kasem S, Ablett G, Ochieng J,
Crawford A. 2008. Genetic structuring in the spotted gum complex (genus
Corymbia, section Politaria). – Aust. Syst. Bot. 21:
15-25.
Shi S, Huang Y-L, Tan F-X, He X-J, Boufford
DE. 2000. Phylogenetic analysis of the Sonneratiaceae and its relationships to
Lythraceae based on ITS sequences of
nrDNA. – J. Plant Res. 113: 253-258.
Shukla VB. 1944. On Sahnianthus, a
new genus of petrified flowers from the Intertrappean Beds at Mohgaon Kalan in
the Deccan and its relation with the fruit Enigmocarpon Parjai Sahni
from the same locality. – Proc. Indian Natl. Acad. Sci., Sect. B, 14:
1-39.
Shukla VB. 1950. A fossil dicotyledonous leaf
(?Lythraceae). – J. Indian Bot. Soc.
29: 29.
Silverstone-Sopkin PA, Graham SA. 1986. Alzateaceae, a plant family new to
Colombia. – Brittonia 38: 340-343.
Sinha SC, Joshi BC. 1959. Vascular anatomy of
the flower of Punica granatum L. – J. Indian Bot. Soc. 38: 35-45.
Sinotô Y. 1928. Pollen development of
Jussiaea repens L. – Proc. Imp. Acad. Japan 4: 231-232.
Skean JD Jr. 1993. A monograph of
Mecranium (Melastomataceae-Miconieae). –
Syst. Bot. Monogr. 39: 1-116.
Skoczylas E, Swiezewska E, Chojnacki T,
Tanaka Y. 1994. Long-chain rubber-like polyisoprenoid alcohols in leaves of
Lumnitzera racemosa. – Plant Physiol. Biochem. (Montrouge) 32:
1-5.
Skvarla JJ, Raven PH, Praglowski J. 1975. The
evolution of pollen tetrads in Onagraceae. – Amer. J. Bot. 62:
6-35.
Skvarla JJ, Raven PH, Praglowski J. 1976.
Ultrastructural survey of Onagraceae
pollen. – In: Ferguson IK, Muller J (eds), The evolutionary significance of
the exine, Linnean Society Symposium Ser., vol. 1, Academic Press, London, New
York, pp. 447-479.
Skvarla JJ, Raven PH, Chissoe WF, Sharp M.
1978. An ultrastructural survey of viscin threads in Onagraceae pollen. – Pollen Spores 20:
5-143.
Skvarla JJ, Rowley JR, Hoch PC, Chissoe WF.
2005. Viscin threads on pollen of Circaea x intermedia Ehrh.
(Circaeeae: Onagraceae). – Taxon 54:
121-126.
Skvortsov AK, Rusanovitch II. 1974. Scanning
electron microscopy of the seed-coat surface in Epilobium species. –
Bot. Not. 127: 392-401.
Smith AC. 1973. Studies of Pacific island
plants XXVI. Metrosideros collina (Myrtaceae) and its relatives in the
southern Pacific. – Amer. J. Bot. 60: 479-490.
Smith BB, Herr JM. 1971. Ovule development,
megagametogenesis, and early embryogeny in Ammania coccinea Rothb. –
J. Elisha Mitchell Sci. Soc. 87: 192-199.
Smith JD, Rose JN. 1913. A monograph of the
Hauyeae and Gongylocarpeae, tribes of the Onagraceae. – Contr. U.S. Natl. Herb.
16: 287-298.
Smith-ramirez C, Armesto JJ, Figueroa J.
1998. Flowering, fruiting, and seed germination in Chilean rain forest Myrtaceae: ecological and phylogenetic
constraints. – Plant Ecol. 136: 119-131.
Smith-White S. 1954. Cytological studies in
the Myrtaceae IV. The subtribe
Euchamaelaucinae. – Proc. Linn. Soc. New South Wales 79: 21-28.
Snow N. 1999. Notes on generic concepts in
Rhodomyrtus, Archirhodomyrtus, Decaspermum, and
Pilidiostigma (Myrtaceae). –
Aust. Syst. Bot. Soc. Newsl. 99: 5-7.
Snow N. 2000. A conspectus of Australasian
Myrtinae (Myrtaceae). – Kew Bull. 55:
647-654.
Snow N. 2004. Systematics of
Pilidiostigma (Myrtaceae). –
Syst. Bot. 29: 393-406.
Snow N. 2007. Systematics of the Australian
species of Rhodamnia (Myrtaceae). – Syst. Bot. Monogr. 82:
1-69.
Snow N. 2008. Studies of Malagasy
Eugenia (Myrtaceae) I: two new
species from the Masoala Peninsula and generic transfers from
Monimiastrum. – Syst. Bot. 33: 343-348.
Snow N. 2009. Kanakomyrtus (Myrtaceae): a new endemic genus from New
Caledonia with linear stigma lobes and baccate fruits. – Syst. Bot. 34:
330-344.
Snow N. 2011. Studies of Malagasy
Eugenia (Myrtaceae) II: four
new species, including one eaten by black lemurs on Nosy Be. – Syst. Bot. 36:
677-689.
Snow N, Guymer GP. 1999. Systematic and
cladistic studies of Myrtella F. Muell. and Lithomyrtus F.
Muell. (Myrtaceae). – Austrobaileya
5: 173-207.
Snow N, Guymer GP. 2001. Revision of
Australian species of Uromyrtus (Myrtaceae) and two new combinations for
New Caledonia. – Syst. Bot. 26: 733-742.
Snow N, Plunkett GM. 2009.
Kanakomyrtus (Myrtaceae): a
new endemic genus from New Caledonia with linear stigma lobes and baccate
fruits. – Syst. Bot. 34: 330-344.
Snow N, Guymer GP, Sawvel G. 2003.
Systematics of Austromyrtus, Lenwebbia, and the Australian
species of Gossia (Myrtaceae).
– Syst. Bot. Monogr. 65: 1-95.
Snow N, McFadden J, Evans TM, Salywon AM,
Wojciechowski MF, Wilson PG. 2011. Morphological and molecular evidence of
polyphyly in Rhodomyrtus (Myrtaceae: Myrteae). – Syst. Bot. 36:
390-404.
Snow N, Rabenantoandro J, Randriatafika F,
Rabehevitra D, Razafimamonjy ND, Cable S. 2012. Studies of Malagasy
Eugenia (Myrtaceae) – III:
Seven new species of high conservation concern from the eastern littoral
forests. – Phytotaxa 48: 39-60.
Snow N, Callmander M, Phillipson PB. 2015.
Studies of Malagasy Eugenia – IV: Seventeen new endemic species, a
new combination, and three lectotypifications; with comments on distribution,
ecological and evolutionary patterns. – PhytoKeys 49: 59-121.
Snow N, Dawson JW, Callmander MW, Gandhi K,
Munzinger J. 2016. New species, new combinations, and lectotypifications in New
Caledonian Eugenia L. (Myrtaceae). – Candollea 71: 67-81.
Snow N, Byng JW, Munzinger J, Callmander MW,
Dawson JW. 2017. New Caledonian Piliocalyx transferred to
Syzygium (Myrtaceae) with an
updated conspectus of the species. – Candollea 72: 239-248.
Soares-Silva LH, Proença CEB. 2006. An old
species revisited and a new combination proposed in Psidium Myrtaceae. – Kew Bull. 61: 199-204.
Soares-Silva LH, Proenca CEB. 2008. A new
species of Psidium L. (Myrtaceae) from southern Brazil. – Bot.
J. Linn. Soc. 158: 51-54.
Sobral M. 1993. Sinopse de Myrciaria
(Myrtaceae). – Napea 9: 13-41.
Sobral M, Grippa CR, Souza MC, Aguiar OT,
Bertponcello R, Guimarães TB. 2012. Fourteen new species and two taxonomic
notes on Brazilian Myrtaceae. –
Phytotaxa 50: 19-50.
Soh W-K, Parnell J. 2011. Comparative leaf
anatomy and phylogeny of Syzygium Gaertn. – Plant Syst. Evol. 297:
1-32.
Soh W-K, Parnell J. 2015. A revision of
Syzygium Gaertn. (Myrtaceae)
in Indochina (Cambodia, Laos and Vietnam). – Adansonia 37: 179-275.
Solt ML, Wurdack JJ. 1980. Chromosome numbers
in the Melastomataceae. –
Phytologia 47: 199-220.
Srivastava PK. 1993. Pollination mechanisms
in genus Terminalia Linn. – Indian Forester 119: 147-150.
Srivastava PK, Raina SN, Thangalevu K. 2001.
Nuclear DNA and fruit (seed) variation in section Pentaptera of genus
Terminalia Linn. – Indian Forester 128: 289-295.
Stace CA. 1965. The significance of leaf
epidermis in the taxonomy of the Combretaceae I. A general review of
tribal, generic, and specific characters. – Bot. J. Linn. Soc. 59:
229-252.
Stace CA. 1968. A revision of the genus
Thiloa (Combretaceae). –
Bull. Torrey Bot. Club 95: 156-165.
Stace CA. 1969. The significance of the leaf
epidermis in the taxonomy of the Combretaceae II. The genus
Combretum subgenus Combretum in Africa. – Bot. J. Linn.
Soc. 62: 131-168.
Stace CA. 1980. The significance of the leaf
epidermis in the taxonomy of the Combretaceae V: the genus
Combretum subgenus Cacoucia in Africa. – Bot. J. Linn. Soc.
81: 185-203.
Stace CA. 1980 [1981]. The significance of
the leaf epidermis in the taxonomy of the Combretaceae: conclusions. – Bot. J.
Linn. Soc. 81: 327-339.
Stace CA. 2002. Proposal to conserve
Terminalia nom. cons. (Combretaceae) against an additional
name, Bucida. – Taxon 51: 193.
Stace CA. 2006. Combretaceae. – In: Kubitzki K (ed),
The families and genera of vascular plants IX. Flowering plants. Eudicots. Berberidopsidales,
Buxales, Crossosomatales, Fabales p.p., Geraniales, Gunnerales, Myrtales p.p., Proteales, Saxifragales, Vitales, Zygophyllales, Clusiaceae Alliance, Passifloraceae
Alliance, Dilleniaceae, Huaceae, Picramniaceae, Sabiaceae, Springer, Berlin,
Heidelberg, New York, pp. 67-82.
Stace CA. 2007. 140. Combretaceae. – In: Harling G,
Persson C (eds), Flora of Ecuador 81, Department of Plant and Environmental
Sciences, Göteborg University, pp. 1-61.
Stace CA, Alwan A-R. 2010. Flora Neotropica
monograph 107. Combretaceae –
Terminalia and Buchenavia. – New York Botanical Garden,
Bronx, New York.
Stacy EA, Johansen JB, Sakishima T, Price DK,
Pillon Y. 2014. Incipient radiation within the dominant Hawaiian tree
Metrosideros polymorpha. – Heredity 113: 334-342.
Stafleu FA. 1948. A monograph of the Vochysiaceae I. Salvertia and
Vochysia. – Rec. Trav. Bot. Néerl. 41: 397-540.
Stafleu FA. 1952. A monograph of the Vochysiaceae II. Callisthene.
– Acta Bot. Neerl. 1: 222-242.
Stafleu FA. 1953. A monograph of the Vochysiaceae III. Qualea. –
Acta Bot. Neerl. 2: 144-217.
Stafleu FA. 1954. A monograph of the Vochysiaceae IV. Erisma. –
Acta Bot. Neerl. 3: 459-480.
Staggemeier VG, Diniz-Filho JAF, Forest F,
Lucas E. 2015. Phylogenetic analysis in Myrcia section
Aulomyrcia and inferences on plant diversity in the Atlantic
rainforest. – Ann. Bot. 115: 747-761.
Standley PC, Williams LO. 1963. Flora of
Guatemala (Melastomaceae). – Fieldiana. Botany 24: 407-525.
Stapf O. 1892. On the Sonerileae of Asia. –
Ann. Bot. 6: 291-323.
Steane DA, Byrne M, Vaillancourt RE, Potts
BM. 1998. Chloroplast DNA polymorphism signals complex interspecific
interactions in Eucalyptus (Myrtaceae). – Aust. Syst. Bot. 11:
25-40.
Steane DA, McKinnon GE, Vaillancourt RE,
Potts BM. 1999. ITS sequence data resolve higher level relationshps among the
eucalypts. – Mol. Phylogen. Evol. 12: 215-223.
Steane DA, Nicolle D, McKinnon GE,
Vaillancourt RE, Potts BM. 2002. Higher-level relationships among the eucalypts
are resolved by ITS-sequence data. – Aust. Syst. Bot. 5: 49-62.
Steane DA, Nicolle D, Potts BM. 2007.
Phylogenetic positioning of anomalous eucalypts by using ITS sequence data. –
Aust. Syst. Bot. 20: 402-408.
Steane DA, Nicolle D, Sansaloni CP, Petroli
CD, Carling J, Kilian A, Myburg AA, Grattapaglia D, Vaillancourt RE. 2011.
Population genetic analysis and phylogeny reconstruction in Eucalyptus
(Myrtaceae) using high-throughput,
genome-wide genotyping. – Mol. Phylogen. Evol. 59: 206-224.
Steenis CGGJ van. 1952.
Kjellbergiodendron and Whiteodendron, Malaysian Myrtaceae – Leptospermoideae –
Metrosiderinae. – Acta Bot. Neerl. 1: 435-442.
Steenis CGGJ van. 1968. Reduction of the
genus Kania Schltr. to Metrosideros (Myrtaceae). – Blumea 16: 357-359.
Stein BA, Tobe H. 1989. Floral nectaries in
Melastomataceae and their
systematic and evolutionary implications. – Ann. Missouri Bot. Gard. 76:
519-531.
Stein J vom, Hachtel W. 1988.
Deletions/insertions, short inverted repeats, sequences resembling
att-lambda, and frame shift mutated open reading frames are involved
in chloroplast DNA differences in the genus Oenothera subsection
Munzia. – Mol. Gen. Genet. 213: 513-518.
Stephens EL. 1909. The embryo-sac and embryo
in certain Penaeaceae. – Ann. Bot.
23: 363-376.
Stern WL, Brizicky GK. 1958. The comparative
anatomy and taxonomy of Heteropyxis. – Bull. Torrey Bot. Club 85:
111-123.
Stevens KJ, Peterson RL, Reader RJ. 2002. The
aerenchymatous phellem of Lythrum salicaria (L.): a pathway for gas
transport and its role in flood tolerance. – Ann. Bot. 89: 621-625.
Stiles FG, Rosselli L. 1993. Consumption of
fruits of the Melastomataceae by
birds: how diffuse is coevolution? – Vegetatio 107-108: 57-73.
Stockey RA, Rothwell GW. 1997. The aquatic
angiosperm Trapago angulata from the Upper Cretaceous (Maastrichtian)
St. Mary River Formation of southern Alberta. – Intern. J. Plant Sci. 158:
83-94.
Stone BC, Weber A. 1988. A new species of
Phyllagathis (Melastomataceae) from the
Endau-Rompin proposed National Park, Malaysia. – Proc. Acad. Nat. Sci.
Philadelphia 139: 307-313.
Stone RD. 2004. Phylogenetic systematics of
Melastomataceae, subfamily
Olisbeoideae, a species-rich and geographically widespread group of tropical
forest understory trees. – Ph.D. diss., University of California,
Berkeley.
Stone RD. 2006a. Phylogeny of major lineages
in Melastomataceae, subfamily
Olisbeoideae: utility of nuclear glyceraldehyde 3-phosphate dehydrogenase
(GapC) gene sequences. – Syst. Bot. 31: 107-121.
Stone RD. 2006b. New species of
Memecylon L. and Warneckea Gilg (Melastomataceae) from Madagascar and
Mayotte. – Adansonia, sér. III, 28: 337-358.
Stone RD. 2014. The species-rich,
paleotropical genus Memecylon (Melastomataceae): molecular
phylogenetics and revised infrageneric classification of the African species.
– Taxon 63: 539-561.
Stone RD. 2015. Taxonomic treatment of
Memecylon L. section Felixiocyton R. D. Stone (Melastomataceae), with descriptions
of four new species from Cameroon, Gabon, and Equatorial Guinea (Bioko). –
Adansonia 37: 47-61.
Stone RD. 2017. Revised treatment of the
genus Lijndenia (Melastomataceae, Olisbeoideae) in
Madagascar. – Candollea 72: 67-86.
Stone RD, Andreasen K. 2010. The
Afro-Madagascan genus Warneckea (Melastomataceae): molecular
systematics and revised infrageneric classification. – Taxon 59: 83-92.
Stone RD, Ghogue J-P, Cheek M. 2008. Revised
treatment of Memecylon sect. Afzeliana (Melastomataceae: Olisbeoideae),
including three new species from Cameroon. – Kew Bull. 63: 227-241.
Stone RD, Cassimjee SF, Mncwabe NB. 2017.
Phylogenetic analysis of East and southern African Memecylon section
Buxifolia (Melastomataceae): insights on
patterns and processes of diversification. – South Afr. J. Bot. 113:
404-412.
Stout AB. 1925. Studies of Lythrum
salicaria II. A new form of flower in the species. – Bull. Torrey Bot.
Club 52: 81-85.
Strey RG, Leistner OA. 1968. The rediscovery
of Rhynchocalyx lawsonioides Oliv. – J. South Afr. Bot. 34: 9-13.
Stubbe W, Steiner E. 1999. Inactivation of
pollen and other effects of genome-plastome incompatibility in
Oenothera. – Plant Syst. Evol. 217:259-277.
Stubbs JM, Slabas AR. 1982. Ultrastructural
and biochemical characterization of the epidermal hairs of the seeds of
Cuphea procumbens. – Planta 155: 392-399.
Subramanyam K. 1942. Gametogenesis and
embryogeny in a few members of the Melastomataceae. – J. Indian Bot.
Soc. 21: 69-85.
Subramanyam K. 1944. A contribution to the
life-history of Sonerila wallachii Benn. – Proc. Indian Acad. Sci.,
Sect. B, 19: 115-120.
Subramanyam K. 1946. Further contributions to
the embryology of the genus Osbeckia Linn. – J. Mysore Univ., Sect.
B, 7: 1-11.
Subramanyam K. 1948. An embryological study
of Melastoma malabathricum L. – J. Indian Bot. Soc. 27: 11-19.
Subramanyam K. 1951a. Studies on the
relationships of the Melastomataceae. – J. Mysore
Univ., Sect. B, 12: 1-4.
Subramanyam K. 1951b. Embryology of
Oxyspora paniculata DC. – Phytomorphology 1: 205-212.
Subramanyam K, Narayana LL. 1969. A
contribution to the floral anatomy of some members of Melastomataceae. – J. Jap. Bot.
44: 6-16.
Supprian K. 1894. Beiträge zur Kenntnis der
Thymelaeaceae und Penaeaceae. – Engl. Bot. Jahrb. Syst.
18: 306-353.
Sytsma KJ, Gottlieb LD. 1986a. Chloroplast
DNA evidence for the origin of the genus Heterogaura from a species of
Clarkia (Onagraceae). –
Proc. Natl. Acad. Sci. U.S.A. 83: 5554-5557.
Sytsma KJ, Gottlieb LD. 1986b. Chloroplast
DNA evolution and phylogenetic relationships in Clarkia sect.
Peripetasma (Onagraceae). –
Evolution 40: 1248-1262.
Sytsma KJ, Smith JF. 1988. DNA and
morphology: comparisons in the Onagraceae. – Ann. Missouri Bot. Gard.
75: 1217-1237.
Sytsma KJ, Smith JF. 1992. Molecular
systematics of Onagraceae: examples of
Clarkia and Fuchsia. – In: Soltis PS, Soltis DE, Doyle JJ
(eds), Molecular systematics of plants, Chapman and Hall, New York, pp.
295-323.
Sytsma KJ, Smith JF, Gottlieb LD. 1990.
Phylogenetics in Clarkia (Onagraceae): restriction site mapping of
chloroplast DNA. – Syst. Bot. 15: 280-295.
Sytsma KJ, Smith JF, Gottlieb LD. 1991.
Biogeography and evolution of morphology, breeding systems, flavonoids, and
chloroplast DNA in the four old world species of Fuchsia (Onagraceae). – Syst. Bot. 16:
257-269.
Sytsma KJ, Smith JF, Berry PE. 1991. The use
of chloroplast DNA to assess biogeography and evolution of morphology, breeding
systems, and flavonoids in Fuchsia sect. Skinnera (Onagraceae). – Syst. Bot. 16:
257-269.
Sytsma KJ, Litt A, Zhjra ML, Pires JC,
Nepokroeff M, Conti E, Walker J, Wilson PG. 2004. Clades, clocks, and
continents: historical and biogeographical analysis of Myrtaceae, Vochysiaceae, and relatives in the
southern hemisphere. – Intern. J. Plant Sci. 165(Suppl.): S85-S105.
Takahashi M, Crane PR, Ando H. 1999.
Esgueiria futabensis sp. nov.; a new angiosperm flower from the Upper
Cretaceous (lower Coniacian) of northeastern Honshu, Japan. – Paleontol. Res.
3: 81-87.
Tan F-X, Shi S-H, Huang Y-L, Du Y-Q, Wang
Y-G, Gong X. 2001. Analysis of nrDNA ITS sequences in the subfamily
Combretoideae (Combretaceae) and its
systematic significance. – Acta Bot. Yunnan. 23: 239-242.
Tan F-X, Shi S-H, Zhong Y, Gong X, Wang Y-G.
2002. Phylogenetic relationships of Combretoideae (Combretaceae) inferred from plastid,
nuclear gene and spacer sequences. – J. Plant Res. 115: 475-481.
Tanaka R, Toko S, Oginuma K. 1982.
Karyomorphological studies on polyploidy series in Japanese Ludwigia.
– Chromosome Inform. Serv. 33: 30-32.
Tanaka R, Oginuma K, Toko S. 1988.
Karyomorphological studies on 26 species of ten genera of the Onagraceae. – La Kromosomo II-51-52:
1675-1696.
Tavares RDAM, Baumgratz JFA, Goldenberg R.
2008. A new species of Behuria Cham. (Melastomataceae: Merianieae) from
Brazil. – Bot. J. Linn. Soc. 158: 489-492.
Thanikaimoni G, Jayaweera DMA. 1966. Pollen
morphology of Sonneratiaceae. – Trav. Sect. Sci. Techn. Inst. Franç.
Pondichéry 5: 1-12.
Thiele K, Ladiges PY. 1988. A cladistic
analysis of Angophora Cav. (Myrtaceae). – Cladistics 4: 23-42.
Thompson J. 1989. A revision of the genus
Leptospermum (Myrtaceae). –
Telopea 3: 301-449.
Thompson J. 1990. Onagraceae. – In: George AS (ed), Flora
of Australia 18, Australian Government Publ. Service, Canberra, pp. 215-243.
Thornhill AH, Crisp MD. 2012. Phylogenetic
assessment of pollen characters in Myrtaceae. – Aust. Syst. Bot. 25:
171-187.
Thornhill AH, Macphail MK. 2012. Fossil
myrtaceous pollen as evidence for the evolutionary history of the Myrtaceae: a review of fossil
Myrtaceidites species. – Rev. Palaeobot. Palynol. 176-177: 1-23.
Thornhill AH, Popple LW, Carter RJ, Ho SYW,
Crisp MD. 2012. Are pollen fossils useful for calibrating relaxed molecular
clock dating of phylogenies? A comparative study using Myrtaceae. – Mol. Phylogen. Evol. 63:
15-27.
Thornhill AH, Hope GS, Craven LA, Crisp MC.
2012a. Pollen morphology of the Myrtaceae. Part 1: tribes Eucalypteae,
Lophostemoneae, Syncarpieae, Xanthostemoneae and subfamily Psiloxyloideae. –
Aust. J. Bot. 60: 165-199.
Thornhill AH, Hope GS, Craven LA, Crisp MC.
2012b. Pollen morphology of the Myrtaceae. Part 2: tribes Backhousieae,
Melaleuceae, Metrosidereae, Osbornieae and Syzygieae. – Aust. J. Bot. 60:
200-224.
Thornhill AH, Hope GS, Craven LA, Crisp MC.
2012c. Pollen morphology of the Myrtaceae. Part 3: tribes Chamelaucieae,
Leptospermeae and Lindsayomyrteae. – Aust. J. Bot. 60: 225-259.
Thornhill AH, Hope GS, Craven LA, Crisp MC.
2012d. Pollen morphology of the Myrtaceae. Part 4: tribes Kanieae, Myrteae
and Tristanieae. – Aust. J. Bot. 60: 260-289.
Thornhill AH, Ho SYW, Külheim C, Crisp MD.
2015. Interpreting the modern distribution of Myrtaceae using a dated molecular
phylogeny. – Mol. Phylogen. Evol. 93: 29-43.
Tiagi YD. 1969. Vascular anatomy of the
flower of certain species of Combretaceae. – Bot. Gaz. 130:
150-157.
Tieghem PEL van. 1891a. Classification
anatomique des Melastomacées. – Bull. Soc. Bot. France 38: 114-124.
Tieghem PEL van. 1891b. Sur la structure et
les affinités des Mémécylées. – Ann. Sci. Nat. Bot. VII, 13: 23-92.
Tieghem PEL van. 1892. Deuxième addition aux
recherches sur la structure et les affinitées des Mélastomacées. – Ann.
Sci. Nat. 7: 369-380.
Tieghem PEL van. 1893. Recherches sur la
structure et les affinités des Thyméléacées et des Pénéacées. – Ann.
Sci. Nat., Bot. VII, 17: 185-294.
Tiffney BH. 1981. Fruits and seeds of the
Brandon Lignite VI. Microdiptera (Lythraceae). – J. Arnold Arbor. 62:
487-516.
Tilney PM. 2002. A contribution to the leaf
and young stem anatomy of the Combretaceae. – Bot. J. Linn. Soc.
138: 163-196.
Tilney PM, Wyk AE van. 2004. Extrafloral
nectaries in Combretaceae:
morphology, anatomy and taxonomic significance. – Bothalia 34: 115-126.
Timonin AC. 1999. What is the protective
tissue replacing the stem epidermis in Trapa (Trapaceae, Myrtales). – Feddes Repert. 110:
99-101.
Tischler G. 1917. Über die Entwicklung und
phylogenetische Bedeutung des Embryosackes von Lythrum salicaria. –
Ber. Deutsch. Bot. Ges. 35: 233-246.
Tobe H, Raven PH. 1983a. An embryological
analysis of Myrtales: its definition and
characteristics. – Ann. Missouri Bot. Gard. 70: 71-94.
Tobe H, Raven PH. 1983b. The embryology of
Axinandra zeylanica (Crypteroniaceae, Myrtales) and the relationships of the
genus. – Bot. Gaz. 144: 426-432.
Tobe H, Raven PH. 1984a. The embryology and
relationships of Rhynchocalyx Oliv. (Rhynchocalycaceae, Myrtales). – Ann. Missouri Bot. Gard. 71:
836-843.
Tobe H, Raven PH. 1984b. The embryology and
relationships of Alzatea Ruiz & Pav. (Alzateaceae, Myrtales). – Ann. Missouri Bot. Gard. 71:
844-852.
Tobe H, Raven PH. 1984c. The number of cells
in the pollen of Melastomataceae.
– Bot. Mag. (Tokyo) 97: 131-136.
Tobe H, Raven PH. 1984d. The embryology and
relationships of Oliniaceae. – Plant
Syst. Evol. 146: 105-116.
Tobe H, Raven PH. 1984e. The embryology and
relationships of Penaeaceae (Myrtales). – Plant Syst. Evol. 146:
181-195.
Tobe H, Raven PH. 1985. The histogenesis and
evolution of integuments in Onagraceae. – Ann. Missouri Bot. Gard.
72: 451-468.
Tobe H, Raven PH. 1986a. Evolution of
polysporangiate anthers in Onagraceae.
– Amer. J. Bot. 73: 475-488.
Tobe H, Raven PH. 1986b. A comparative study
of the embryology of Ludwigia (Onagraceae): characteristics, variation,
and relationships. – Ann. Missouri Bot. Gard. 73: 768-787.
Tobe H, Raven PH. 1987a. The embryology and
relationships of Crypteronia (Crypteroniaceae). – Bot. Gaz. 148:
96-102.
Tobe H, Raven PH. 1987b. The embryology and
relationships of Dactylocladus (Crypteroniaceae) and a discussion of
the family. – Bot. Gaz. 148: 103-111.
Tobe H, Raven PH. 1987c. Embryology and
systematic position of Heteropyxis (Myrtales). – Amer. J. Bot. 74:
197-208.
Tobe H, Raven PH. 1990. Embryology and
systematic position of Psiloxylon (Myrtales). – Bot. Bull. Acad. Sin., N.
S., 31: 119-127.
Tobe H, Raven PH. 1996. Embryology of Onagraceae (Myrtales): characteristics, variation and
relationships. – Telopea 6: 667-688.
Tobe H, Raven PH, Graham S. 1986. Chromosome
counts for some Lythraceae sens. str.
(Myrtales), and base number of the
family. – Taxon 35: 13-20.
Tobe H, Wagner WL,Chin H-C.1987. A systematic
and evolutionary study of Oenothera (Onagraceae): seed coat anatomy. – Bot.
Gaz. 148: 235-257.
Tobe H, Hakki MI, Langhammer L. 1989. Floral
nectary in Medinilla magnifica, an Old World Melastomataceae. – Bot. Jahrb.
Syst. 111: 57-62.
Tobe H, Graham S, Raven PH. 1998. Floral
morphology andevolution in Lythraceae
sensu lato. – In: Owens SJ, Rudall PJ (eds), Reproductive biology, Royal
Botanic Gardens, Kew, pp. 329-344.
Todzia CA. 1999. Ten new species of
Tibouchina (Melastomataceae) from Mexico. –
Brittonia 51: 255-279.
Towner HF. 1977. The biosystematics of
Calylophus (Onagraceae). –
Ann. Missouri Bot. Gard. 64: 48-120.
Trela-Sawicka Z. 1978. Embryological studies
in Trapa natans L. – Acta Biol. Cracov., Ser. Bot., 22: 101-108.
Triana JJ. 1871. Les Mélastomacées. –
Trans. Linn. Soc. London 23: 1-188.
Trudgen ME. 1986. Reinstatement and revision
of Rinzia Schauer (Myrtaceae,
Leptospermeae, Baeckeinae). – Nuytsia 5: 415-440.
Trudgen ME. 1987. Ochrosperma, a new
genus of Myrtaceae (Leptospermeae,
Baeckeinae) from New South Wales and Queensland. – Nuytsia 6: 9-17.
Trudgen ME, Rye BL. 2014. An update to the
taxonomy of some Western Australian genera of Myrtaceae tribe Chamelaucieae. 2.
Cyathostemon. – Nuytsia 24: 7-16.
Tuler AC, Silva T da, Carrijo TT, Garbin ML,
Mendonça CBF, Peixoto AL, Gonçalves-Esteves V. 2017. Taxonomic significance
of pollen morphology for species delimitation in Psidium (Myrtaceae). – Plant Syst. Evol. 303:
317-327.
Turki ZA. 2007. The genus Ammannia
L. (Lythraceae) in Egypt. – Flora
Mediterr. 17: 97-114.
Turner GW, Lersten NR. 1983. Apical foliar
nectary of pomegranate: Punicaceae. – Amer. J. Bot. 70: 475-480.
Turner IM. 1996 [1997]. What should the kelat
trees of Malaya be called? – J. Singapore Natl. Acad. Sci. 22-24: 15-27.
Udovicic F, Ladiges PY. 2000. Informativeness
of nuclear and chloroplast DNA regions and the phylogeny of the eucalypts and
related genera (Myrtaceae). – Kew
Bull. 55: 633-645.
Udovicic F, McFadden GI, Ladiges PY. 1995.
Phylogeny of Eucalyptus and Angophora based on 5S rDNA spacer
sequence data. – Mol. Phylogen. Evol. 4: 247-256.
Valente M da C, Marquete Ferreira da Silva N,
Guimarães DJ. 1989. Morfologia e anatomia do fruto de Combretum
rotundifolium Rich. (Combretaceae). – Rodriguésia 67:
45-51.
Valente M da C, Marquete Ferreira da Silva N,
Guimarães DJ. 1994. Morfologia e anatomia do fruto de Laguncularia
racemosa (L.) Gaertn. f. (Combretaceae). – Arq. Jard. Bot. (Rio
de Janeiro) 32: 39-50.
Varassin IG, Penneys DS, Michelangeli FA.
2008. Comparative anatomy and morphology of nectar-producing Melastomataceae. – Ann. Bot. 102:
899-909.
Vasconcelos TNC, Proença CEB. 2015. Floral
cost vs. floral display: insights from the megadiverse Myrtales suggest that energetically
expensive floral parts are less phylogenetically constrained. – Amer. J. Bot.
102: 900-909.
Vasconcelos TNC, Prenner G, Bünger MO,
De-Carvalho PS, Wingler A, Lucas EJ. 2015. Systematic and evolutionary
implications of stamen position in Myrteae (Myrtaceae). – Bot. J. Linn. Soc. 179:
388-402.
Vasconcelos TNC, Proença CEB, Ahmad B,
Aguilar DS, Aguilar R, Amorim BS, Campbell K, Costa IR, De-Carvalho PS, Faria
JEQ, Giaretta A, Kooij PW, Lima DF, Mazine FF, Peguero B, Prenner G, Santos MF,
Soewarto J, Winger A, Lucas EJ. 2017. Myrteae phylogeny, calibration,
biogeography and diversification patterns: increased understanding in the most
species rich tribe of Myrtaceae. –
Mol. Phylogen. Evol. 109: 113-137.
Velasco L, Goffman FD. 1999. Tocopherol and
fatty acid composition of twenty-five species of Onagraceae Juss. – Bot. J. Linn. Soc.
129: 359-366.
Veldkamp JF. 1978. Notes on
Creochiton, Dissochaeta, and Macrolenes (Melastomataceae). – Blumea 24:
437-446.
Veldkamp JF, Franken NAP, Roos MC, Nayar MP.
1978. A revision of Diplectria (Melastomataceae). – Blumea 24:
405-430.
Venkatesh CS. 1955. The structure and
dehiscence of the anther in Memecylon and Mouriri. –
Phytomorphology 5: 435-440.
Venkateswarlu J. 1939. A contribution to the
embryology of Sonneratiaceae. – Proc. Indian Acad. Sci., Sect. B, 5:
206-223.
Venkateswarlu J. 1952. Contributions to the
embryology of Combretaceae I.
Poivrea coccinea DC. – Phytomorphology 2: 231-240.
Venkateswarlu J, Prakasa Rao PS. 1970. The
floral anatomy of Combretaceae. –
Proc. Indian Natl. Acad. Sci., Sect. B, 36: 1-20.
Venkateswarlu J, Prakasa Rao PS. 1971. Wood
anatomy and systematic position of Strephonema. – New Phytol. 70:
767-771.
Venkateswarlu J, Prakasa Rao PS. 1972.
Embryological studies in some Combretaceae. – Bot. Not. 125:
161-179.
Veranso-Libalah MC, Stone RD, Fongod AGN,
Couvreur TLP, Kadereit G. 2017. Phylogeny and systematics of African
Melastomateae (Melastomataceae).
– Taxon 66: 584-614.
Verdcourt B. 1975. Oliniaceae. – In: Polhill RM (ed),
Flora of tropical East Africa, Crown Agents for Overseas Governments and
Administrations, London, pp. 1-4.
Verdcourt B. 1978. 79. Oliniaceae. – In: Launert E (ed), Flora
Zambesiaca 4, Flora Zambesiaca Managing Committee, London, pp. 323-327.
Verdcourt B. 1994. Lythraceae. – In: Polhill RM (ed),
Flora of tropical East Africa, A. A. Balkema, Rotterdam, pp. 1-62.
Verdcourt B. 1998. FAS contribution 10:
Trapaceae. – Bothalia 28: 11-14.
Verdcourt B. 1999. The genus Eugenia
L. (Myrtaceae) in East Africa. – Kew
Bull. 54: 41-62.
Verdcourt B. 2001. Myrtaceae. – In: Beentje HJ, Smith SAL
(eds), Flora of tropical East Africa, A. A. Balkema, Rotterdam, pp. 1-89.
Verhoeven RL, Schijff HP van der. 1974.
Anatomical aspects of Combretaceae
in South Africa. – Phytomorphology 24: 158-164.
Verma JK. 1950. A fossil dicot wood from the
intertrappean cherts of Mohgaon Kalan. – J. Indian Bot. Soc. 29: 30.
Vickulin SV. 1999. Palaeogene leaf
compressions of myrtaceous affinity from Pasekovo, Middle Russian Upland,
southern European Russia. – Bot. J. Linn. Soc. 131: 65-98.
Viswan MBG, Manikandan U. 2008. A new species
of Syzygium (Myrtaceae) from
the Kalakkad-Mundanthurai Tiger Reserve in Peninsular India. – Adansonia,
sér. III, 30: 113-118.
Vliet GJCM van. 1975. Wood anatomy of Crypteroniaceae sensu lato. – J.
Microscopy 104: 65-82.
Vliet GJCM van. 1978. Vestured pits of Combretaceae and allied families. –
Acta Bot. Neerl. 27: 273-285.
Vliet GJCM van. 1979. Wood anatomy of the Combretaceae. – Blumea 25:
141-223.
Vliet GJCM van. 1981. Wood anatomy of the
palaeotropical Melastomataceae.
– Blumea 27: 395-462.
Vliet GJCM van, Baas P. 1975. Comparative
anatomy of the Crypteroniaceae
sensu lato. – Blumea 22: 175-195.
Vliet GJCM van, Baas P. 1984 [1985]. Wood
anatomy and classification of the Myrtales. – Ann. Missouri Bot. Gard. 71:
783-800.
Vliet GJCM van, Koek-Noorman J, Welle BJH
ter. 1981. Wood anatomy, classification, and phylogeny of the Melastomataceae. – Blumea 27:
463-473.
Vollesen K. 1981. Pteleopsis apetala
sp. nov. (Combretaceae) and the
delimitation of Pteleopsis and Terminalia. – Nord. J. Bot.
1: 329-332.
Wagner WL. 1981. Oenothera
acutissima (Onagraceae), a new
species from northwestern Colorado and adjacent Utah. – Syst. Bot. 6:
153-158.
Wagner WL. 2004. Resolving a nomenclatural
and taxonomic problem in Mexican Oenothera sect. Hartmannia
(Tribe Onagreae, Onagraceae). –
Novon 14: 124-133.
Wagner WL. 2005. Systematics of
Oenothera sections Contortae, Eremia, and
Ravenia (Onagraceae). –
Syst. Bot. 30: 332-355.
Wagner WL, Hoch PC. 2005 onwards. Onagraceae, The Evening Primrose Family
website. – http://botany.si.edu/onagraceae/index.cfm (May 2012)
Wagner WL, Hoch PC, Raven PH. 2007. Revised
classification of the Onagraceae. –
Syst. Bot. Monogr. 83, Amer. Soc. Plant Taxonomists.
Wallnöfer B. 1996. A revision of the genus
Alloneuron Pilg. and segregation of Wurdastom Gen. n. (Melastomataceae). – Ann.
Naturhist. Mus. Wien, Ser. B, Bot. Zool. 98: 447-462.
Wallnöfer B. 1999. Alloneuron Pilg.
(Melastomataceae): some
additions. – Ann. Naturhist. Mus. Wien, Ser. B, Bot. Zool. 101: 593-598.
Wanntorp L, Puglisi C, Penneys D, Ronse de
Craene LP. 2011. Multiplications of floral organs in flowers: a case study in
Conostegia (Melastomataceae, Myrtales). – In: Wanntorp L, Ronse de
Craene LP (eds), Flowers on the Tree of Life, Systematics Association Spec.
Vol. 80, Cambridge University Press, Cambridge, pp. 218-235.
Warburg O. 1894. Flacourtiaceae. – In:
Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(6a), W.
Engelmann, Leipzig, pp. 1-56.
Webb DA. 1967. Generic limits in European Lythraceae. – Feddes Repert. 74:
10-13.
Weber A. 1987. Two new species of
Phyllagathis related to P. tuberculata (Melastomataceae) from Peninsular
Malaysia. – Plant Syst. Evol. 157: 187-199.
Weberling F. 1956. Untersuchungen über
rudimentäre Stipeln bei den Myrtales.
– Flora 143: 201-218.
Weberling F. 1958. Über das Vorkommen
rudimentärer Stipeln bei den Lecythidaceae
(s.l.) und Sonneratiaceae. – Flora 116: 72-77.
Weberling F. 1960. Weitere Untersuchungen
über das Vorkommen rudimentärer Stipeln bei den Myrtales (Combretaceae, Melastomataceae). – Flora 149:
189-205.
Weberling F. 1963. Ein Beitrag zur
systematischen Stellung der Geissolomataceae,
Penaeaceae, und Oliniaceae sowie der Gattung
Heteropyxis (Myrtaceae). –
Bot. Jahrb. Syst. 82: 119-128.
Weberling F. 1966. Additional notes on the
Myrtalean affinity of Kania eugenioides Schltr. – Kew Bull. 20:
517-520.
Weberling F. 1968. Bemerkungen über das
Vorkommen rudimentärer Stipeln I. Cyrillaceae und
die Gattung Cyrillopsis Kuhlm. II. Alzatea Ruiz & Pav.
(Lythraceae) und Tristania R.
Br. – Acta Bot. Neerl. 17: 282-287.
Weberling F. 1984 [1985]. Stipular
structures. – In: Dahlgren RMT, Thorne R (eds), The order Myrtales: circumscription, variation, and
relationships, Ann. Missouri Bot. Gard. 71: 641-644.
Weberling F. 1988. The architecture of
inflorescences in the Myrtales. – Ann.
Missouri Bot. Gard. 75: 226-310.
Weberling F. 2000. Tepualia
stipularis (Myrtaceae), una planta
con apéndices estipuliformes y estípulas rudimentarias. – Kurtziana 28:
313-315.
Welch MB. 1921. The occurrence of oil ducts
in certain eucalypts and angophoras. – Proc. Linn. Soc. New South Wales 46:
475-486.
Welch MB. 1923. The occurrence of secretory
canals in certain Myrtaceous plants. – Proc. Linn. Soc. New South Wales 48:
660-673.
Welle BJH ter, Koek-Noorman J. 1978. On
fibres, parenchyma, and intermediate forms in the genus Miconia (Melastomataceae). – Acta Bot.
Neerl. 27: 1-9.
Welle BJH ter, Koek-Noorman J. 1981 Wood
anatomy of Neotropical Melastomataceae. – Blumea 27:
335-394.
Welle BJH ter, Mennega AMW. 1977. On the
presence of large styloids in the secondary xylem of the genus
Henriettea (Melastomataceae). – IAWA Bull. 2:
31-35.
Weyerstahl P, Christiansen C, Gundidza M,
Mavi S. 1992. Constituents of the essential oil of Heteropyxis
natalensis. – J. Essential Oil Res. 4: 439-445.
Weyerstahl P, Marschall H, Landrum LR. 1992.
Constituents of the leaf extract of Amomyrtus meli (R. A. Philippi)
Legrand et Kausel, Amomyrtus Luma (Molina) Legrand et Kausel and of
Amomyrtella guili (Speg.) Kausel. – Flavour Fragrance J. 7:
247-251.
Whiffin T. 1972. A systematic study of the
genus Heterocentron (Melastomataceae). – Ph.D. diss.,
University of Texas, Austin, Texas.
Whiffin T. 1990. Melastomataceae. – In: George AS
(ed), Flora of Australia 18, Australian Government Publ. Service, Canberra, pp.
243-255.
Whiffin T, Ladiges PY. 1992. Patterns of
variation and relationships in the Eucalyptus alpina-E. baxteri
complex (Myrtaceae) based on leaf
volatile oils. – Aust. Syst. Bot. 5: 695-709.
Whiffin T, Tomb AS. 1972. The systematic
significance of seed morphology in the neotropical capsular-fruited Melastomataceae. – Amer. J. Bot.
59: 411-422.
White CT. 1951. Some noteworthy Myrtaceae from the Moluccas, New Guinea
and the Solomon Islands. – J. Arnold Arbor. 32: 139-149.
White F. 1978. 74. Myrtaceae. – In: Launert E (ed), Flora
Zambesiaca 4, Flora Zambesiaca Managing Committee, London, pp.183-212.
Whittock S, Steane DA, Vaillancourt RE, Potts
BM. 2003. Molecular evidence shows that the tropical boxes (Eucalyptus
subgenus Minutifructus) are overranked. – Trans. Roy. Soc. South
Australia 127: 27-32.
Wickens GE. 1973. Combretaceae. – In: Polhill RM (ed),
Flora of tropical East Africa, Crown Agents for Oversea Governments and
Administrations, London, pp. 1-99.
Wickens GE. 1975. Melastomataceae. – In: Polhill RM
(ed), Flora of tropical East Africa, Crown Agents for Oversea Governments and
Administrations, London, pp. 1-95.
Williams LO. 1963. Tropical American plants
V. Melastomataceae. –
Fieldiana, Bot. 29: 549-586.
Williams Sangai GR. 1968. Sonneratiaceae. –
In: Milne-Redhead E, Polhill RM (eds), Flora of tropical East Africa, Crown
Agents for Oversea Governments and Administrations, London, pp. 1-3.
Wilson CE, Forest F, Devey DS, Lucas EJ.
2016. Phylogenetic relationships in Calyptranthes (Myrtaceae) with particular emphasis on its
monophyly relative to Myrcia s. l. – Syst. Bot. 41: 378-386.
Wilson CL. 1950. Vascularization of the
stamen in the Melastomataceae
with some phyletic implications. – Amer. J. Bot. 37: 431-444.
Wilson PG. 1982. Additions to the genus
Kania (Myrtaceae) in Malesia
with notes on Cloëzia. – Blumea 28: 177-180.
Wilson PG. 1990. A revision of the genus
Xanthostemon (Myrtaceae) in
Australia. – Telopea 3: 451-476.
Wilson PG. 1993. Thaleropia, a new
genus for Metrosideros queenslandica (Myrtaceae) and its allies. – Aust. Syst.
Bot. 6: 251-259.
Wilson PG. 1996. Myrtaceae in the Pacific, with special
reference to Metrosideros. – In: Keast A, Miller SE (eds), The
origin and evolution of Pacific island biota’s: New Guinea to eastern
Polynesia: patterns and processes, SPB Academic Publ., Amsterdam, pp.
233-245.
Wilson PG. 2009. Conspectus of the genus
Eugenia (Myrtaceae) in the
Philippines. – Gard. Bull. Singapore 60: 399-410.
Wilson PG. 2011. Myrtaceae. – In: Kubitzki K (ed),
Families and genera of vascular plants X. Flowering plants. Eudicots. Sapindales, Cucurbitales, Myrtaceae. Springer, Heidelberg,
Dordrecht, London, New York, pp. 212-271.
Wilson PG, Heslewood MM. 2014. An expanded
phylogenetic analysis of Sannantha (Myrtaceae) and description of a new
species. – Aust. Syst. Bot. 27: 78-84.
Wilson PG, Heslewood MM. 2016. Phylogenetic
position of Meteoromyrtus (Myrtaceae). – Telopea 19: 45-55.
Wilson PG, Hyland BPM. 1988. New taxa of
rainforest Myrtaceae from northern
Queensland. – Telopea 3: 257-271.
Wilson PG, Waterhouse JT. 1982. A review of
the genus Tristania R. Br. (Myrtaceae): a heterogeneous assemblage of
five genera. – Aust. J. Bot. 30: 413-446.
Wilson PG, O’Brien MM, Quinn CJ. 2000.
Anetholea (Myrtaceae), a new
genus for Backhousia anisata: a cryptic member of the Acmena
alliance. – Aust. Syst. Bot. 13: 429-435.
Wilson PG, O’Brien MM, Gadek PA, Quinn CJ.
2001. Myrtaceae: a reassessment of
infrafamilial groups. – Amer. J. Bot. 88: 2013-2025.
Wilson PG, O’Brien MM, Heslewood MM, Quinn
CJ. 2005. Relationships within Myrtaceae sensu lato based on a
matK phylogeny. – Plant Syst. Evol. 251: 3-19.
Wilson PG, Heslewood MM, Quinn CJ. 2007.
Re-evaluation of the genus Babingtonia (Myrtaceae) in eastern Australia and New
Caledonia. – Aust. Syst. Bot. 20: 302-318.
Wolfson R, Higgins KG, Sears BB. 1991.
Evidence for replication slippage in the evolution of Oenothera
chloroplast DNA. – Mol. Biol. Evol. 8: 709-720.
Wollenweber E, Kohorst G. 1981. Epicuticular
leaf flavonoids from Eucalyptus species and from Kalmia
latifolia. – Zeitschr. Naturforsch. 36c: 913-915.
Woodgyer EM. 2005. Multi-access key and
checklist to the species of Microlicia (Melastomataceae) in Bahia, Brazil.
– Kew Bull. 60: 441-448.
Woodgyer EM, Zappi DC. 2005. Two new species
of Microlicia D. Don (Melastomataceae) from Catolés,
Bahia, NE Brazil. – Kew Bull. 60: 435-440.
Woodgyer EM, Zappi DC. 2009. Two new species
of Microlicia D. Don (Melastomataceae) from Bahia, NE
Brazil. – Kew Bull. 64: 279-284.
World Checklist of Myrtaceae. 2006. The Board of Trustees of
the Royal Botanic Gardens, Kew. – http://www.kew.org/wcsp/myrtaceae.
Wright SD, Yong CG, Dawson JW, Whittaker DJ,
Gardner RC. 2000. Riding the ice age El Niño? Pacific biogeography and
evolution of Metrosideros subg. Metrosideros (Myrtaceae) inferred from nuclear ribosomal
DNA. – Proc. Natl. Acad. Sci. U.S.A. 97: 4118-4123.
Wright SD, Keeling DJ, Ashton FG, Dawson JW,
Gardner RC. 2000. Phylogenetic analyses of New Caledonian Metrosideros
and Carpolepis (Myrtaceae)
from nrDNA (ITS) sequences. – Aust. Syst. Bot. 13: 919-926.
Wright SD, Yong CG, Wichman SR, Dawson JW,
Gardner RC. 2001. Stepping stones to Hawaii: a trans-equatorial dispersal
pathway for Metrosideros (Myrtaceae) inferred from nrDNA (ITS +
ETS). – J. Biogeogr. 28: 769-774.
Wu S-B, Wen Y, Li X-W, Zhao Y, Zhao Z, Hu
J-F. 2009. Chemical constituents from the fruits of Sonneratia
caseolaris and Sonneratia ovata (Sonneratiaceae). – Biochem.
Syst. Ecol. 37: 1-5.
Wurdack JJ. 1953. A revision of the genus
Brachyotum (Tibouchineae-Melastomataceae). – Mem. New York
Bot. Gard. 8: 343-407.
Wurdack JJ. 1957a. Certamen Melastomataceis
IV. – Brittonia 9: 101-109.
Wurdack JJ. 1957b. Certamen Melastomataceis
V. – Phytologia 6: 1-11.
Wurdack JJ. 1962. Melastomataceae of Santa Catarina.
– Sellowia 14: 109-217.
Wurdack JJ. 1963. An evaluation of the genus
Poteranthera. – Fieldiana, Bot. 29: 535-541.
Wurdack JJ. 1964a. Melastomataceae. – In: Maguire B,
Wurdack JJ et al. (eds), The botany of the Guayana Highland 5, Mem. New York
Bot. Gard. 10: 135-186.
Wurdack JJ. 1964b. Certamen Melastomataceis
VIII. – Phytologia 9: 409-426.
Wurdack JJ. 1965. Certamen Melastomataceis
IX. – Phytologia 11: 377-400.
Wurdack JJ. 1966. Certamen Melastomataceis X.
– Phytologia 13: 65.
Wurdack JJ. 1970a. Erroneous data in Glaziou
collections of Melastomataceae.
– Taxon 19: 911-913.
Wurdack JJ. 1970b. Certamen Melastomataceis
XV. – Phytologia 20: 369-389.
Wurdack JJ. 1971. Certamen Melastomataceis
XVII. – Phytologia 21: 353-368.
Wurdack JJ. 1972a. Certamen Melastomataceis
XVIII. – Phytologia 22: 299-418.
Wurdack JJ. 1972b. Melastomataceae. – In: Maguire B
et al. (eds), The botany of the Guayana Highland 9, Mem. New York Bot. Gard.
23: 197-199.
Wurdack JJ. 1972c. Certamen Melastomataceis
XX. – Phytologia 24: 195-208.
Wurdack JJ. 1973. Melastomataceae. – In: Lasser T
(ed), Flora de Venezuela, Instituto Botánico, Caracas, pp. 1-819.
Wurdack JJ. 1974. Certamen Melastomataceis
XXIII. – Phytologia 29: 135-151.
Wurdack JJ. 1976. Endemic Melastomataceae of the Sierra Nevada
de Santa Marta, Colombia. – Brittonia 28: 138-143.
Wurdack JJ. 1977. Certamen Melastomataceis
XXVI. – Phytologia 35: 241-251.
Wurdack JJ. 1980. Melastomataceae. – In: Harling G,
Sparre B (eds), Flora of Ecuador 13, Swedish Natural Science Research Council,
Stockholm, pp. 1-405.
Wurdack JJ. 1981. Certamen Melastomataceis
XXXII. – Phytologia 48: 238-252.
Wurdack JJ. 1984. Certamen Melastomataceis
XXXVII. – Phytologia 55: 131-147.
Wurdack JJ. 1986. Atlas of hairs for
Neotropical Melastomataceae. –
Smithsonian Contr. Bot. 63: 1-80.
Wurdack JJ. 1988. Certamen Melastomataceis
XXXVIII. – Phytologia 64: 293-301.
Wurdack JJ. 1989. Una nueva especie
colombiana de Tessmannianthus. – Rev. Acad. Col. Ci. 17: 247-248.
Wurdack JJ. 1990. Certamen Melastomataceis
XXXIX. – Phytologia 69: 316-327.
Wurdack JJ. 1995. New species of Melastomataceae from Bahia, Brazil.
– Kew Bull. 50: 821-825.
Wurdack JJ, Renner SS. 1993.
Melastomatoideae. – In: Görts-van Rijn ARA (ed), Flora of the Guianas, ser.
A, Phanerogams, 13, Koeltz Scientific Books, Koenigstein, pp. 3-301.
Wyk AE van. 1984. A new species of
Combretum from Venda and taxonomic notes on the section
Angustimarginata (Combretaceae). – South Afr. J. Bot.
3: 125-134.
Wyk AE van, Botha R. 1984. The genus
Eugenia L. (Myrtaceae) in
southern Africa: ontogeny and taxonomic value of the seed. – South Afr. J.
Bot. 50: 63-80.
Wyk AE van, Dedekind I. 1985. The genus
Eugenia (Myrtaceae) in
southern Africa: morphology and taxonomic value of pollen. – South Afr. J.
Bot. 51: 371-378.
Wyk AE van, Botha DJ, Coetzee J. 1980. The
genus Eugenia L. (Myrtaceae)
in southern Africa 1. The nature and taxonomic value of the first-formed stem
periderm. – J. South Afr. Bot. 46: 67-88.
Wyk AE van, Robbertse PJ, Kok PDF. 1982. The
genus Eugenia L. (Myrtaceae)
in southern Africa: the structure and taxonomic value of stomata. – Bot. J.
Linn. Soc. 84: 41-56.
Xie L, Wagner WL, Ree RH, Berry PE, Wen J.
2009. Molecular phylogeny, divergence time estimates, and historical
biogeography of Circaea (Onagraceae) in the Northern Hemisphere.
– Mol. Phylogen. Evol. 53: 995-1009.
Zardini EM, Raven PH. 1992. A new section of
Ludwigia (Onagraceae) with a
key to the sections of the genus. – Syst. Bot. 17: 481-485.
Zardini EM, Gu H, Raven PH. 1991. On the
separation of two species within the Ludwigia uruguayensis complex (Onagraceae). – Syst. Bot. 16:
242-244.
Zardini EM, Peng C-I, Hoch PC. 1991.
Chromosome numbers in Ludwigia sect. Oligospermum and sect.
Oocarpon (Onagraceae). –
Taxon 40: 221-230.
Zetter R, Ferguson DK. 2001. Trapaceae pollen
in the Cenozoic. – Acta Palaeobot. 41: 321-339.
Ziegler A. 1925. Beiträge zur Kenntnis der
Androeceums und der Samenentwicklung einiger Melastomaceen. – Bot. Arch. 9:
398-467.








