
Cladogram of Staphyleales based on molecular data (Sosa & Chase 2003, with Guamatelaceae added). The two major clades are well supported (bootstrap support 100%).
[Staphyleales+[Picramniaceae+[Sapindales+[Huerteales+[Capparales+Malvales]]]]]
Habit Bisexual or dioecious (sometimes monoecious, polygamomonoecious, rarely andromonoecious or gynomonoecious), evergreen or deciduous trees or shrubs.
Vegetative anatomy Phellogen ab initio epidermal, subepidermal, outer-cortical or pericyclic. Vessel elements with scalariform or simple (sometimes reticulate) perforation plates; lateral pits alternate, scalariform or opposite, simple or bordered pits. Vestured pits sometimes present. Imperforate tracheary xylem elements tracheids or fibre tracheids with bordered pits, septate or non-septate (sometimes also vasicentric tracheids). Wood rays uniseriate or multiseriate, usually heterocellular (rarely homocellular). Axial parenchyma apotracheal diffuse, or paratracheal scanty or unilateral, sometimes vasicentric, or absent. Sieve tube plastids S type. Nodes 3:1–3, trilacunar with one to three leaf traces (sometimes 1:1, unilacunar with one trace, or 5:5, pentalacunar with five traces). Sclereids sometimes present. Calciumoxalate as acicular, rhomboidal or irregular crystals, or druses usually present.
Trichomes Hairs unicellular or multicellular, uniseriate, simple or T-shaped (sometimes malpighiaceous hairs), or absent; glandular hairs present or absent.
Leaves Usually alternate (spiral or distichous; sometimes opposite), usually simple (sometimes imparipinnate or trifoliolate), usually entire (rarely tridentate), often coriaceous, with involute ptyxis. Stipules small (sometimes bristle-like), sometimes fused with petiole base or leaf base, often intrapetiolar, or absent; leaf sheath absent. Petiole vascular bundle transection arcuate or annular. Venation usually pinnate, brochidodromous, eucamptodromous or semicraspedodromous (sometimes palmate). Stomata anomocytic or anisocytic. Cuticular wax crystalloids as scales, tubuli or platelets. Epidermis sometimes with mucilaginous idioblasts containing druses. Mesophyll with calciumoxalate crystals, druses or raphides. Leaf margin entire or serrate.
Inflorescence Terminal or axillary, few-flowered, fascicle, panicle, corymb, raceme-like, raceme or catkin, or flowers solitary.
Flowers Actinomorphic. Hypanthium present or absent. Hypogyny or half epigyny. Sepals (three or) four or five (or six), with imbricate or decussate aestivation, usually whorled (sometimes spiral), persistent, free or connate at base. Petals (three or) four or five (or six), uniform or non-uniform, with usually imbricate or imbricate-quincuncial (rarely cochlear or contorted) aestivation, sometimes clawed, persistent or caducous, usually free (sometimes partially or entirely connate; sometimes absent), usually with one trace. Nectariferous disc extrastaminal to intrastaminal, annular (rarely absent), or nectaries intrastaminal or inserted at carpel bases (then disc absent).
Androecium Stamens four to c. 50, in one to four whorls. Filaments free from each other, free from or adnate to tepals. Anthers basifixed or dorsifixed, versatile or non-versatile, tetrasporangiate, usually introrse (sometimes latrorse, rarely slightly extrorse), longicidal (dehiscing by longitudinal slits). Tapetum secretory. Staminodia usually absent (female flowers sometimes with staminodia).
Pollen grains Microsporogenesis simultaneous. Pollen grains usually tricolporate (sometimes di-, tetra- or pentacolporate, rarely tricolporoidate or hexapantoporate), shed as monads, usually bicellular (rarely tricellular) at dispersal. Exine tectate or semitectate, with columellate? infratectum, perforate, reticulate, microreticulate, striate, or smooth.
Gynoecium Pistil composed of one to five (to nine) usually secondarily free (rarely at base or in upper part connate) carpels (sometimes stipitate); carpel apices often postgenitally connate forming compitum. Ovary superior or semi-inferior, unilocular to quadrilocular (sometimes appearing partially quadrilocular due to intrusive placentae, rarely partially trilocular), often stipitate. Stylodia one to five (to nine), or style single, simple, or absent. Stigma usually apical, capitate or punctate (sometimes decurrent or lobate), papillate, Wet type. Pistillodium usually absent (male flowers sometimes with pistillodium).
Ovules Placentation axile, basal or intrusively parietal (sometimes apical, rarely lateral or marginal). Ovules (one or) two to more than 50 per carpel, anatropous, amphitropous or campylotropous, apotropous or epitropous, often pendulous, bitegmic, crassinucellar. Micropyle bistomal (sometimes Z-shaped), endostomal or exostomal. Funicular obturator sometimes present. Nucellar cap often present. Archespore often multicellular. Megagametophyte usually monosporous, Polygonum type (rarely disporous, Allium or Endymion type). Synergids often with a filiform apparatus. Endosperm development ab initio nuclear. Endosperm haustoria probably absent. Embryogenesis solanad.
Fruit A loculicidal capsule, a berry, a dry drupaceous fruit, or an assemblage of usually free follicles (rarely connate at base) with abaxial dehiscence (ventricidal; rarely a multifolliculus).
Seeds Funicular aril or caruncle present or absent. Seed coat testal. Testa multilayered, with (often strongly) lignified cell walls. Exotestal and endotestal cell walls often sclerotic, often thickened (especially outer walls). Exotegmen crushed. Endotegmen fibrous or crushed. Perisperm not developed. Endosperm copious or sparse, oily and often proteinaceous, or absent. Embryo straight or curved, well differentiated, at least sometimes with chlorophyll. Cotyledons two. Germination phanerocotylar.
Cytology x = 6, 11–14, 25
DNA Plastid gene infA lost/defunct. Mitochondrial intron coxII.i3 lost.
Phytochemistry Flavonols (kaempferol, quercetin), cyanidin, cyanidin-3-glycoside, oleanolic acid derivatives, ellagic and gallic acids, ellagitannins (casuaricitin, pedunculagin, stachyurin, tellimagrandin II, etc.), proanthocyanidins (prodelphinidins) and other non-hydrolyzable tannins, ursolic acid, acacetin-7-O-glycoside, acacetin-7-O-diglycoside, triterpene saponins, syringin, xanthones (mangiferin), and cyclic polyvalent alcohols present. Cyanogenic compounds not found.
Systematics Staphyleales are probably sister-group to the clade [Picramniaceae+[Sapindales+ [Huerteales+[Capparales+Malvales]]]].
A probable topology of Staphyleales is the following: [[Aphloiaceae+[Geissolomataceae+ [Ixerbaceae+Strasburgeriaceae]]]+[Staphyleaceae+[Guamatelaceae+[Stachyuraceae+Crosso-somataceae]]]].
According to Stevens (2001 onwards) the clade [Aphloiaceae+[Geissolomataceae+ [Ixerbaceae+Strasburgeriaceae]]] may have the potential synapomorphy pollen grain apertures with conspicuous protrusions. The clade [Geissolomataceae+[Ixerbaceae+Strasburgeriaceae]] has in turn the following potential synapomorphies: lignified, unicellular and T-shaped hairs; psilate pollen grains; non-stipitate synascidiate carpels alternating with tepals/seapals, and with abaxial (dorsal) ribs; punctate stigma formed from postgenitally fused twisted stylar apices; two collateral pendulous ovules per carpel; fruit a capsule with persistent style; and large hilum. Ixerba and Strasburgeria share the characters: cells (at least in flowers) with thickened mucilaginous inner tangential walls; presence of acicular crystals; leaf margins glandular-serrate; flowers large; sepals spirally arranged; petals clawed; filaments flat and broad; anthers more than 3 mm long; style long, persistent in fruit; and ovules sessile, epitropous.
The clade [Staphyleaceae+[Guamatelaceae+[Stachyuraceae+Crossosomataceae]]] has the potential synapomorphies (Stevens 2001 onwards): leaves or leaflets with involute ptyxis; inflorescence terminal; and stigma expanded. The clade [Guamatelaceae+[Stachyuraceae+Crosso-somataceae]] is characterized by presence of funicular aril. Finally, Stachyuraceae and Crossosomataceae share the following characters: flowers without crystals or druses; anthers X-shaped; and entire testa sclerotic.

|
Cladogram of Staphyleales based on molecular data (Sosa & Chase 2003, with Guamatelaceae added). The two major clades are well supported (bootstrap support 100%). |
APHLOIACEAE Takht. |
Genera/species 1/1
Distribution Tropical East and southeastern Africa, Madagascar, islands in western Indian Ocean.
Fossils Unknown.
Habit Bisexual, evergreen trees or shrubs.
Vegetative anatomy Phellogen ab initio pericyclic. Vessel elements with scalariform perforation plates; lateral pits alternate or opposite, bordered pits. Imperforate tracheary xylem elements fibre tracheids with bordered pits, septate or non-septate (also vasicentric tracheids). Tracheids dimorphic. Wood rays uniseriate or multiseriate, heterocellular. Axial parenchyma absent or very rare (apotracheal diffuse, or paratracheal scanty, vasicentric). Sieve tube plastids S type. Nodes 3:?, trilacunar with ? leaf traces. Brown to yellow staining amorphous inclusions present in axial parenchyma and ray cells. Crystals?
Trichomes Hairs absent.
Leaves Alternate (distichous), usually simple (rarely pinnately compound), entire, with ? ptyxis. Stipules small, caducous or persistent; leaf sheath absent. Petiole vascular bundle transection arcuate. Venation pinnate. Stomata anisocytic. Cuticular wax crystalloids? Leaf margin serrate.
Inflorescence Axillary, few-flowered, fascicle or raceme, or flowers solitary.
Flowers Actinomorphic. Hypanthium extended, wide, often with nectaries on surface. Hypogyny. Sepals four or five (or six), with imbricate aestivation, three inner ones thinner and petaloid, connate at base. Petals absent. Nectariferous disc intrastaminal.
Androecium Stamens numerous. Filaments filiform, persistent, free from each other and from tepals. Anthers basifixed, non-versatile?, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory. Staminodia absent.
Pollen grains Microsporogenesis simultaneous. Pollen grains tricolporate, shed as monads, bicellular at dispersal. Exine tectate, with ? infratectum, striate. Apertural membrane strongly bulging.
Gynoecium Pistil composed of (seemingly?) one carpel. Ovary superior, unilocular (monomerous?). Style absent. Stigma capitate-peltate to bilobate, slightly decurrent, papillate?, Wet type? Pistillodium absent.
Ovules Placentation lateral (marginal?). Ovules (two to) six to eight (to ten) per ovary, campylotropous, bitegmic, crassinucellar. Micropyle endostomal. Outer integument ? cell layers thick. Inner integument ? cell layers thick. Nucellar cap absent. Hypostase? Megagametophyte monosporous, Polygonum type? Endosperm development nuclear? Endosperm haustoria? Embryogenesis?
Fruit A berry with persistent calyx and stamens.
Seeds Aril absent. Seed coat testal. Testa multiplicative, multilayered, with lignified cell walls. Outer three to five testal layers (exotesta) strongly thickened; inside these layers small non-thickened cells and, finally, two or three innermost layers of elongate non-thickened cells. Tegmen crushed. Perisperm not developed. Endosperm sparse. Embryo hippocrepomorphic, terete, well differentiated, chlorophyll? Cotyledons two. Germination?
Cytology n = ?
DNA
Phytochemistry Very insufficiently known. Xanthones (mangiferin) present.
Use Unknown.
Systematics Aphloia (1; A. theiformis; tropical East and southeastern Africa, Madagascar, the Comoros, the Mascarene Islands, the Seychelles).
Aphloia is sister to the clade [Geissoloma+[Ixerba+Strasburgeria]].
The species limits are very diffuse in Aphloia and there may be a single variable species or up to eight different species.
CROSSOSOMATACEAE Engl. |
Crossosomatales Reveal in Phytologia 74: 174. 25 Mar 1993; Crossosomatanae Doweld, Tent. Syst. Plant. Vasc.: xxxiv. 23 Dec 2001; Crossosomatineae Reveal in Kew Bull. 66: 47. Mar 2011
Genera/species 4/9
Distribution Arid and semiarid regions in southwestern United States and northwestern and central Mexico.
Fossils Unknown.
Habit Usually bisexual (rarely andromonoecious, gynomonoecious or polygamomonoecious), usually deciduous shrubs (rarely trees), often spiny. Xerophytes.
Vegetative anatomy Phellogen ab initio superficial. Vessel elements with simple perforation plates; lateral pits alternate, bordered pits. Imperforate tracheary xylem elements tracheids with bordered pits, non-septate. Wood rays uniseriate or multiseriate, heterocellular. Axial parenchyma apotracheal diffuse, or paratracheal scanty. Sieve tube plastids S type. Nodes 3:1-3, trilacunar with one to three leaf traces, or 1:1, unilacunar with one trace. Single rhomboidal and yellow acicular and prismatic calciumoxalate crystals abundant (also in leaves). Kranz’ anatomy prevailing.
Trichomes Hairs translucent to black; glandular hairs present.
Leaves Usually alternate (spiral; in Apacheria opposite), simple, usually entire (rarely tridentate), small, with involute ptyxis. Stipules tiny (sometimes stipitate, sometimes fused with petiole base) or absent; leaf sheath absent. Petiole vascular bundle transection? Venation pinnate. Stomata anomocytic. Cuticular waxes absent? Mesophyll with calciumoxalate crystals as raphides. Leaf margin usually serrate (rarely entire).
Inflorescence Flowers terminal or axillary, solitary, in apex of short shoots.
Flowers Actinomorphic. Hypanthium usually short (in Velascoa long, tubular). Half epigyny. Sepals (three or) four or five (or six), equal or unequal, with imbricate aestivation, persistent, free. Petals (three or) four or five (or six), equal or unequal, with imbricate aestivation, often shortly clawed, persistent or caducous, free. Nectariferous disc extrastaminal to intrastaminal, annular (absent in Velascoa). Crystals and druses absent.
Androecium Stamens four to c. 50, in one to four series, sometimes unequal in length, antesepalous or developing from ten primordia? (ten trunk vascular bundles). Filaments free from each other and from tepals. Anthers basifixed (or slightly ventrifixed), versatile, tetrasporangiate, latrorse or slightly extrorse, longicidal (dehiscing by longitudinal slits), more or less X-shaped. Tapetum secretory, with multinucleate polyploid cells. Staminodia absent?
Pollen grains Microsporogenesis simultaneous. Pollen grains usually tricolporate (sometimes dicolporate), shed as monads, bicellular at dispersal. Exine semitectate, with columellate? infratectum, reticulate to microreticulate. Apertural membrane not very bulging.
Gynoecium Pistil composed of one to five (to nine) usually not (rarely at base) connate carpels; odd carpel adaxial. Ovary semi-inferior, usually unilocular (apocarpy). Stylodia one to five (to nine), short, or absent. Stigma usually apical, expanded (sometimes decurrent), Wet type? Pistillodium absent?
Ovules Placentation marginal. Ovules two to numerous (rarely one) per carpel, amphitropous or campylotropous, bitegmic, crassinucellar. Micropyle bistomal. Outer integument approx. seven cell layers thick. Inner integument three or four cell layers thick. Nucellar cap present? Obturator absent? Hypostase present. Archespore multicellular. Megagametophyte monosporous, Polygonum type. Endosperm development ab initio nuclear. Endosperm haustoria absent? Embryogenesis onagrad.
Fruit An assemblage of coriaceous, usually free follicles (rarely connate at base) with abaxial dehiscence.
Seeds Funicular aril entire or fimbriate, large or small. Seed coat testal. Testa multilayered, with lignified cell walls. Exotestal and endotestal cells sclerotic. Exotegmen and mesotegmen unspecialized. Endotegmen fibrous. Perisperm not developed. Endosperm copious or sparse, oily. Embryo narrowly elongate, curved, well differentiated, chlorophyll? Cotyledons two. Germination phanerocotylar.
Cytology n = 6
DNA
Phytochemistry Insufficiently known. Ellagic and gallic acid, proanthocyanidins, cyanidin-3-glycoside, acacetin-7-O-glycoside, diglycoside, and syringin present. Saponins and cyanogenic compounds not found. Flavonoids? Inulin as carbohydrate reserve.
Use Ornamental plants.
Systematics Apacheria (1; A. chiricahuensis; Arizona), Velascoa (1; V. recondita; Sierra Madre Oriental in Mexico), Glossopetalon (5; G. clokeyi, G. planitierum, G. pungens, G. spinescens, G. texense; southwestern United States, northern Mexico), Crossosoma (2; C. californicum: Palos Verdes Peninsula, San Clemente, Santa Catalina in California, Guadalupe Island in northwestern Mexico; C. bigelovii: arid regions in southern California, Arizona, Nevada, and Baja California in northwestern Mexico).
Stachyuraceae and Crossosomataceae are sister groups. Both clades possess X-shaped anthers.
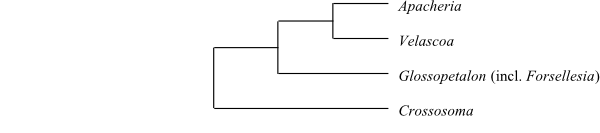 |
Cladogram of Crossosomataceae based on DNA sequence data (Sosa & Chase 2003). |
GEISSOLOMATACEAE Endl. ex A. DC. |
Geissolomatales Takht. ex Reveal in Novon 2: 238. 13 Oct 1992; Geissolomatineae Reveal in Kew Bull. 66: 47. Mar 2011
Genera/species 1/1
Distribution Western Cape Province in South Africa.
Fossils Unknown.
Habit Bisexual, evergreen shrub. Xerophyte. Branches and young stems with four longitudinal ridges.
Vegetative anatomy Phellogen ab initio outer-cortical. Vessel elements with scalariform perforation plates; lateral pits opposite or scalariform, simple and/or bordered pits. Imperforate tracheary xylem elements tracheids or fibre tracheids with bordered pits, non-septate (also vasicentric tracheids). Wood rays uniseriate or multiseriate, heterocellular. Axial parenchyma apotracheal diffuse. Sieve tube plastids S type? Nodes 1:1, unilacunar with one leaf trace. Stem cortex with scattered stone cells containing sclereids. Brown to yellow-staining amorphous inclusions present in axial parenchyma and ray cells. Wood ray cells with rhomboidal calciumoxalate crystals.
Trichomes Hairs on young branches and leaves unicellular, unevenly T-shaped (malpighiaceous hairs, with one long branch and one short branch).
Leaves Opposite, simple, entire, coriaceous, with ? ptyxis. Stipule-like appendages small, subulate, inserted at sides of petioloid leaf base; leaf sheath absent. Petiole vascular bundle transection? Venation palmate. Stomata anomocytic. Cuticular wax crystalloids as platelets; abaxial side of lamina with waxy scales or strings. Epidermis with mucilage cells. Epidermal and mesophyll cells with calciumoxalate druses. Leaf margin entire, thick, consisting of large epidermal cells and thick-walled mesenchyma cells; some marginal cells with calciumoxalate druses.
Inflorescence Flowers terminal, solitary, inserted on lateral short shoots, with three or four pairs of persistent basal opposite bracts, larger in upper part and almost petaloid.
Flowers Actinomorphic. Hypogyny. Sepals four, petaloid, with decussate aestivation, persistent, connate at base. Petals absent. Nectaries intrastaminal, consisting of four antetepalous nectariferous pockets. Disc absent.
Androecium Stamens 4+4, diplostemonous, antesepalous stamens longer than alternisepalous ones. Filaments thin, free from each other, adnate to sepal bases. Anthers dorsifixed, versatile, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory. Staminodia absent.
Pollen grains Microsporogenesis simultaneous? Pollen grains usually tricolporate (rarely hexapantoporate), shed as monads, ?-cellular at dispersal. Exine semitectate, with columellate infratectum, finely reticulate, psilate? Apertural membrane strongly bulging.
Gynoecium Pistil composed of four connate carpels. Ovary superior, quadrilocular. Stylodia four, narrow, apically connate at anthesis, spirally twisted and forming compitum. Stigmas four, punctate, non-papillate?, Wet type? Pistillodium absent.
Ovules Placentation axile to apical. Ovules two per carpel, anatropous, pendulous, bitegmic, crassinucellar. Micropyle exostomal (bistomal?). Outer integument at least four cell layers thick. Inner integument at least three cell layers thick. Nucellar cap absent. Hypostase present. Megagametophyte monosporous, 8-nucleate, probably Polygonum type. Endosperm development? Endosperm haustoria absent. Embryogenesis? Suspensor absent.
Fruit A loculicidal capsule, surrounded by persistent sepals.
Seeds Aril absent. Seed with caruncle consisting of swollen funicular part of seed. Seed coat testal. Testa multilayered, with thick lignified cell walls. Tegmen? Perisperm not developed. Endosperm copious, fleshy. Embryo small, straight, well differentiated, chlorophyll? Cotyledons two, thin, narrow. Germination?
Cytology n = ?
DNA
Phytochemistry Virtually unknown. Aluminium accumulated.
Use Unknown.
Systematics Geissoloma (1; G. marginatum; Langeberg Mountains in Swellendam and Riversdale Divisions in Western Cape).
Geissoloma is sister to [Ixerba+Strasburgeria].
GUAMATELACEAE S. Oh et D. Potter |
Genera/species 1/1
Distribution Central America.
Fossils Unknown.
Habit Bisexual, evergreen shrub (sometimes more or less prostrate). Most of the plant more or less hairy.
Vegetative anatomy Phellogen? Vessel elements with scalariform? perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids? Wood rays heterocellular? Axial parenchyma diffuse? Sieve tube plastids S type? Nodes 3:3, trilacunar with three leaf traces. Crystals?
Trichomes Hairs unicellular.
Leaves Opposite, simple, entire, densely tomentose on abaxial side, with involute? ptyxis. Stipules bristle-like; leaf sheath absent. Petiole vascular bundle transection annular. Leaf base cordate. Venation palmate. Stomata? Cuticular wax crystalloids? Leaf margin serrate.
Inflorescence Terminal, raceme. Bracts and floral prophylls (bracteoles) filiform.
Flowers Actinomorphic. Hypanthium present. Half epigyny. Sepals five, with imbricate aestivation, persistent, free. Petals five, with imbricate aestivation, persistent, usually shorter than sepals, free. Nectary? Disc?
Androecium Stamens ten, in one whorl. Filaments free from each other and from tepals. Anthers dorsifixed, versatile?, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory? Staminodia absent.
Pollen grains Microsporogenesis simultaneous? Pollen grains tricolporate, shed as monads, ?-cellular at dispersal. Exine tectate, with columellate? infratectum, perforate to microreticulate. Apertural membranes protruding.
Gynoecium Pistil composed of three carpels connate in uppermost part (stylar apices), otherwise free. Ovary semi-inferior, trilocular? above. Stylodia three, separating after anthesis. Stigmas capitate, type? Pistillodium absent.
Ovules Placentation? (placentae inserted in two rows along ventral suture). Ovules numerous per carpel, anatropous?, ascending, bitegmic?, crassinucellar? Micropyle ?-stomal. Outer integument ? cell layers thick. Inner integument ? cell layers thick. Nucellar cap present. Hypostase? Megagametophyte monosporous, Polygonum type? Endosperm development? Endosperm haustoria? Embryogenesis?
Fruit Usually three many-seeded ventricidal follicles almost enclosed by persistent sepals.
Seeds Funicular aril membranous. Seed coat testal. Testa hard, with a shining surface, multilayered, with lignified cell walls. Exotesta? Endotesta? Tegmen? Perisperm not developed. Endosperm very sparse or absent. Embryo?, chlorophyll? Cotyledons two. Germination?
Cytology n = ?
DNA
Phytochemistry Unknown.
Use Unknown.
Systematics Guamatela (1; G. tuerckheimii; Mexico, Honduras, Guatemala).
Guamatela is sister to [Stachyuraceae+Crossosomataceae].
IXERBACEAE Griseb. ex Doweld et Reveal |
Ixerbales Doweld et Reveal, New Syllabus Pl. Fam.: 731. Apr 2007
Genera/species 1/1
Distribution North Island of New Zealand.
Fossils Uncertain. Fossil Ixerba has been reported from the mid-Miocene onwards.
Habit Bisexual, evergreen small tree.
Vegetative anatomy Phellogen ab initio subepidermal. Vessel elements with scalariform perforation plates; lateral pits almost absent (scalariform or opposite), bordered pits. Vestured pits present. Imperforate tracheary xylem elements transitional between tracheids and fibre tracheids, with bordered pits, non-septate. Wood rays uniseriate and homocellular, or bi- to multiseriate and heterocellular. Axial parenchyma apotracheal diffuse (or diffuse-in-aggregates?), or paratracheal scanty (rarely vasicentric). Sieve tube plastids S type? Nodes 3:3, trilacunar with three leaf traces. Brown to yellow staining amorphous inclusions present in axial parenchyma and ray cells. Cortex with sclerenchymatous cells, and calciumoxalate as druses or solitary acicular crystals.
Trichomes Hairs unicellular, T-shaped, with lignified walls.
Leaves Alternate (spiral, pseudoverticillate) or opposite, simple, entire, coriaceous, with ? ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle transection arcuate. Venation pinnate-reticulate, semicraspedodromous. Stomata anomocytic. Cuticular wax crystalloids? Mesophyll cells with druses and solitary crystals. Leaf margin coarsely glandular-serrate, with gland-tipped teeth.
Inflorescence Terminal, few-flowered, umbel-like corymb or panicle.
Flowers Actinomorphic, relatively large. Pedicel articulated. Hypogyny to half epigyny. Sepals five, with imbricate aestivation, spiral, caducous, two outer sepals smaller than three inner ones, free. Petals five, with imbricate quincuncial, cochlear or contorted aestivation, clawed, free. Nectariferous disc intrastaminal, quinquelobate, with antepetalous lobes. Idioblasts with thickened striate mucilaginous inner tangential walls.
Androecium Stamens five, haplostemonous, antesepalous, alternipetalous, alternating with lobes of nectariferous disc. Filaments long, flattened, free from each other and from tepals? Anthers dorsifixed, versatile, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits); connective thick, prolonged. Tapetum secretory? Staminodia antepetalous.
Pollen grains Microsporogenesis simultaneous? Pollen grains tetracolporate or pentacolporate, shed as monads, bicellular at dispersal. Exine tectate, with columellate infratectum, smooth. Apertural membrane heavily bulging.
Gynoecium Pistil composed of five connate antepetalous carpels. Ovary superior to semi-inferior, quinquelocular, relatively large, gradually tapering into style, with five coarse longitudinal ridges alternating with five narrower ridges, with lobate nectary at base. Style coarsely ridged, spirally twisted at apex, hollow. Stigma punctate, non-papillate?, Dry type? Pistillodium absent.
Ovules Placentation axile. Ovules two per carpel, collateral, anatropous, pendulous, antitropous, bitegmic, crassinucellar. Micropyle bistomal? Outer integument at least four? cell layers thick. Inner integument at least four? cell layers thick. Nucellar cap absent. Obturator funicular. Hypostase? Parietal tissue approx. eight cell layers thick. Nucellar cap present. Megagametophyte monosporous, Polygonum type. Endosperm development nuclear? Endosperm haustoria? Embryogenesis?
Fruit A loculicidal capsule with adaxially splitting carpels. Valves lignified, reflexed.
Seeds Seeds with wide red arilloid structure containing oils. Hilum large; hilar scar elongate. Seed coat testal. Testa multilayered, with lignified cell walls; exotestal cells and adjacent cells with thickened walls. Endotesta? Tegmen crushed? Perisperm not developed. Endosperm sparse. Embryo large, chlorophyll? Cotyledons two, large. Germination? Seedlings with glands replacing cauline stipules.
Cytology n = 25
DNA
Phytochemistry Very insufficiently known. Proanthocyanidins and ursolic acid (free triterpenic acid) present. Non-hydrolyzable tannins not found.
Use Honey.
Systematics Ixerba (1; I. brexioides; lowland forests and lower mountain forests from lat. 35º30’S to south of 38ºS on North Island of New Zealand).
Ixerba is sister to Strasburgeria (Strasburgeriaceae).
STACHYURACEAE J. Agardh |
Genera/species 1/8
Distribution The Himalayas, southern Tibet, Assam, northern Burma, northern Indochina, China, Japan, Bonin Islands, Taiwan.
Fossils Seeds of Stachyurus are known from the Cenozoic of the Northern Hemisphere.
Habit Usually polygamomonoecious or functionally dioecious (rarely functionally bisexual), evergreen or deciduous shrubs or small trees (sometimes lianas).
Vegetative anatomy Phellogen ab initio epidermal. Vessel elements with scalariform (often almost simple) perforation plates; lateral pits scalariform or opposite, bordered pits. Imperforate tracheary xylem elements tracheids or fibre tracheids with bordered pits, non-septate. Wood rays uniseriate or multiseriate, heterocellular. Axial parenchyma usually apotracheal diffuse (or diffuse-in-aggregates?). Sieve tube plastids S type. Nodes 3:3, trilacunar with three leaf traces. Brown to yellow staining amorphous inclusions present in axial parenchyma and ray cells. Parenchyma with calciumoxalate crystals and tanniniferous cells.
Trichomes Hairs unicellular; glandular hairs absent.
Leaves Alternate (spiral), simple, entire, sometimes coriaceous, with involute ptyxis. Stipules early caducous; leaf sheath absent. Petiole vascular bundle transection arcuate. Venation pinnate-reticulate, brochidodromous or eucamptodromous; veins proceeding into transparent caducous leaf teeth. Stomata anomocytic. Cuticular wax crystalloids as scales or tubules. Mesophyll with calciumoxalate druses. Leaf margin serrate.
Inflorescence Terminal or axillary, pendulous raceme or spike (catkin). Floral prophyll (bracteoles) two, connate at base.
Flowers Actinomorphic. Hypanthium absent. Hypogyny. Sepals 2+2, decussate, with imbricate aestivation, outer two sepals smaller than inner two sepals, caducous, free. Petals four, in one whorl, with imbricate aestivation, free. Nectaries at carpellary bases. Disc absent. Crystals and druses absent.
Androecium Stamens 4+4, diplostemonous? Filaments subulate, free from each other and from tepals. Anthers dorsifixed, versatile, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits), more or less X-shaped. Tapetum secretory, usually with binucleate (sometimes uninucleate) cells. Female flowers with staminodia.
Pollen grains Microsporogenesis simultaneous. Pollen grains tricolporate or tricolporoidate, shed as monads, usually bicellular (rarely tricellular) at dispersal. Exine semitectate, with columellate? infratectum, finely reticulate, foveate to psilate. Apertural membrane somewhat bulging.
Gynoecium Pistil composed of four connate antesepalous carpels. Ovary superior, quadrilocular at base (incompletely divided above base) due to intrusive parietal placentae (ovary in reality unilocular), shortly stipitate, with necataries at base. Style single, simple, short, with compitum (running down style). Stigma capitate to shallowly quadrilobate, papillate, Wet type. Male flowers with pistillodium.
Ovules Placentation intrusively parietal (in lower part of ovary) to apical-axile. Ovules c. 30 to c. 50 per carpel, biseriate, anatropous, bitegmic, crassinucellar. Micropyle bistomal, Z-shaped (zig-zag). Outer integument four or five cell layers thick. Inner integument four or five cell layers thick. Nucellar cap present. Obturator absent. Hypostase present. Parietal tissue approx. three cell layers thick. Megasporangium massive. Archespore multicellular. Megagametophyte monosporous, Polygonum type, or disporous, Allium or Endymion type. Antipodal cells ephemeral. Endosperm development ab initio nuclear. Endosperm haustoria absent. Embryogenesis solanad. Polyembryony occurs.
Fruit A many-seeded, relatively dry, quadrilocular, berry.
Seeds Funicular aril fleshy. Seed coat exotestal. Testa multiplicative, multilayered, with lignified cell walls. Exotestal, mesotestal and endotestal cells sclerotic. Mesotesta thin. Tegmen degenerating. Mesotegmen and endotegmen crushed. Perisperm not developed. Endosperm copious, oily and proteinaceous. Embryo medium-sized, straight, well differentiated, without chlorophyll. Cotyledons two, large. Germination?
Cytology n = 12
DNA
Phytochemistry Flavonols (kaempferol, quercetin), cyanidin, methylated and non-methylated ellagic acids and ellagitannins (casuaricitin, pedunculagin, stachyurin, tellimagrandin II), proanthocyanidins (prodelphinidins), stachsterol, and ecdysteroids present. Cyanogenic compounds not found.
Use Ornamental plants.
Systematics Stachyurus (8; S. chinensis, S. cordatulus, S. himalaicus, S. obovatus, S. praecox, S. retusus, S. salicifolius, S. yunnanensis; the Himalayas, southern Tibet, Assam, northern Burma, northern Indochina, China, Japan, Bonin Islands, Taiwan).
Stachyurus and Crossosomataceae are sister-groups, both sharing the character of X-shaped anthers.
STAPHYLEACEAE Martinov |
Ochranthaceae A. Juss. in V. V. D. d’Orbigny, Dict. Univ. Hist. Nat. 8: 713. 1846; Staphyleineae J. Presl in Nowočeská Bibl. [Wšobecný Rostl.] 7: 295. 1846 [‘Staphylleaceae’]
Genera/species 3/40–50
Distribution Temperate regions on the Northern Hemisphere, tropical Asia, tropical America.
Fossils Seeds of Staphylea have been reported from Japan, Europe and North America from Eocene onwards. Staphyleoxylon kickapooense, fossilized wood resembling Staphylea, is described from Cenozoic layers in Mississippi.
Habit Usually bisexual (sometimes monoecious, polygamomonoecious or dioecious), evergreen or deciduous trees or shrubs, sometimes stoloniferous.
Vegetative anatomy Phellogen ab initio superficial. Vessel elements with scalariform or reticulate perforation plates; lateral pits scalariform, alternate or opposite, simple and/or bordered pits. Imperforate tracheary xylem elements tracheids or fibre tracheids with bordered pits, non-septate. Wood rays uniseriate or multiseriate, heterocellular. Axial parenchyma apotracheal diffuse, or paratracheal scanty or unilateral (sometimes vasicentric or ray-adjacent), or absent. Wood often fluorescent. Tyloses sometimes frequent. Sieve tube plastids S type. Nodes 3:3, trilacunar with three leaf traces, or 5:5, pentalacunar with five traces (usually with thin accessory leaf traces). Brown- to yellow-staining amorphous inclusions usually present in axial parenchyma and ray cells. Xylem cells in some species with rhomboidal calciumoxalate crystals.
Trichomes Hairs usually unicellular, uniseriate, or absent.
Leaves Opposite, compound (imparipinnate, trifoliolate or rarely unifoliolate) with entire leaflets, with involute? ptyxis. Stipules cauline, usually caducous (in Dalrympelea sometimes interpetiolar, often with colleters); leaf sheath absent. Stipulules usually present (sometimes modified into glands or absent). Petiole vascular bundle transection annular. Venation pinnate or palmate. Stomata anisocytic. Cuticular wax crystalloids as platelets (often parallel), crust-shaped or smooth. Epidermis often with mucilaginous idioblasts. Mesophyll with calciumoxalate druses. Leaflet articulations with extrafloral nectaries as small gland-like appendages. Leaflet margins glandular-serrate.
Inflorescence Terminal or axillary, thyrsoid, panicle or raceme-like.
Flowers Actinomorphic. Hypanthium absent. Hypogyny or half epigyny. Sepals (four or) five, with imbricate aestivation, almost petaloid, unequal in size, often persistent, free or more or less connate. Petals (four or) five, with imbricate-quincuncial aestivation, unequal in size, free or partially or entirely connate. Nectariferous disc intrastaminal, annular, often lobate.
Androecium Stamens (four or) five, haplostemonous, antesepalous, alternipetalous, inserted outside or alternating with lobes of nectariferous disc. Filaments complanate, free from each other and from tepals. Anthers dorsifixed, versatile, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory, with binucleate cells. Staminodia absent?
Pollen grains Microsporogenesis simultaneous. Pollen grains tricolporate, shed as monads, usually bicellular (rarely tricellular) at dispersal. Exine semitectate, with columellate infratectum, foveolate, reticulate or microreticulate. Apertural membrane not bulging.
Gynoecium Carpels two or three (or four), usually more or less connate in lower part (rarely free but inserted at surrounding nectariferous disc); median carpel adaxial. Ovary superior or semi-inferior, usually bilocular or trilocular (rarely quadrilocular or unilocular, if so then apocarpy). Stylodia two or three (or four), at least largely free, connate at apex. Stigma capitate or lobate, papillate, Wet type. Pistillodium absent?
Ovules Placentation basal to axile. Ovules two to twelve (rarely one) per carpel, anatropous, usually ascending, bitegmic, crassinucellar. Micropyle bistomal or endostomal (Z-shaped?). Outer integument four to six cell layers thick. Inner integument three or four cell layers thick. Nucellar cap present. Obturator absent. Hypostase present. Megagametophyte monosporous, Polygonum type. Antipodal cells ephemeral. Synergids with a filiform apparatus. Endosperm development ab initio nuclear. Endosperm haustoria absent. Embryogenesis?
Fruit A berry with fleshy to lignified exocarp (Dalrympelea) or a membranous dry vesicular capsule (rarely a schizocarp? consisting of ab initio fused follicles, a multifolliculus), often with persistent sepals.
Seeds Aril absent. Seed coat mesotestal. Testa multiplicative, sometimes vascularized, multilayered, with lignified cell walls. Exotesta usually palisade, cuboidal, tanniniferous. Mesotesta thick-walled, sclerotic. Endotesta multiplicative, somewhat thick-walled, non-lignified, parenchymatous. Tegmen multiplicative, five to 14 cell layers thick, crushed. Perisperm not developed. Endosperm usually copious (rarely sparse), fleshy, oily. Embryo straight, well differentiated, without chlorophyll. Cotyledons two, large. Germination phanerocotylar or cryptocotylar.
Cytology n = 11–14, 26, 39 – Polyploidy occurring.
DNA
Phytochemistry Flavonols (kaempferol, quercetin), oleanolic acid derivatives, cyanidin, proanthocyanidins, alkaloids, triterpene saponins, and cyclic polyvalent alcohols present. Ellagic acid and cyanogenic compounds not found.
Use Ornamental plants, timber.
Systematics Euscaphis (1; E. japonica; temperate China, the Korean Peninsula, Japan, northern Vietnam), Staphylea (11; Central and southeastern Europe, Turkey, the Caucasus, temperate Asia to East Asia, North America), Turpinia (30–40; China to Japan, southern India, Sri Lanka, the Himalayas, Southeast Asia, Malesia to New Guinea, northwestern South America to Bolivia).
Staphyleaceae are sister to the clade [Guamatela+[Stachyurus+Crossosomataceae]]. The topology [Euscaphis+[Staphylea+Turpinia]] was recovered by Harris & al. (2017).
STRASBURGERIACEAE Tiegh. |
Genera/species 1/1
Distribution New Caledonia.
Fossils Fossil pollen grains named Bluffopollis scabratus similar to those in Strasburgeria have been found in Paleocene to Pliocene layers in western and southern Australia, Tasmania, and New Zealand.
Habit Bisexual, evergreen tree.
Vegetative anatomy Phellogen? Stem with cortical vascular bundles. Wood cells very large and thick-walled. Vessel elements with scalariform perforation plates; lateral pits opposite, simple pits. Imperforate tracheary xylem elements tracheids or fibre tracheids with bordered pits, non-septate? Wood rays usually multiseriate (sometimes uniseriate), heterocellular. Axial parenchyma apotracheal diffuse (or diffuse-in-aggregates?), or paratracheal scanty. Sieve tube plastids S type. Nodes usually 3:3, trilacunar with three leaf traces (sometimes 5:5, pentalacunar with five traces). Cortex and medulla with secretory cavities. Brown to yellow staining amorphous inclusions usually present in axial parenchyma and ray cells. Cristarque cells absent. Calciumoxalate as druses, irregular crystal groups and acicular crystals in stem and leaves.
Trichomes Hairs unicellular, simple.
Leaves Alternate (spiral), simple, entire, coriaceous, with ? ptyxis. Stipules intrapetiolar, adaxially connate at base; leaf sheath absent. Petiole vascular bundle transection? Venation pinnate, brochidodromous; main veines surrounded by fibres. Stomata anomocytic. Cuticular wax crystalloids as almost thread-like scales. Mesophyll with mucilaginous idioblasts containing reddish sap. Lamina glandular-serrate (leaf margin losely serrate; leaf teeth with one vein and transparent caducous hood).
Inflorescence Flowers axillary, solitary.
Flowers Actinomorphic, large, campanulate. Hypanthium absent. Hypogyny. Sepals eight to ten, with imbricate aestivation, spiral, coriaceous, increasing in size from base upwards, free. Petals five (or sex), in one whorl, with imbricate aestivation, thick, clawed, non-uniform, free. Nectariferous disc intrastaminal (extrastaminal?), inserted at carpel bases, annular, usually decemlobate. Idioblasts with thickened striate mucilaginous inner tangential walls.
Androecium Stamens ten, in one whorl, haplostemonous, antepetalous and alternipetalous. Filaments wide, thick, flattened, free from each other and from tepals. Anthers dorsifixed, adnate to connective their whole length, versatile, tetrasporangiate, latrorse (to introrse?), longicidal (dehiscing by longitudinal slits); connective thick. Tapetum secretory?, one-layered. Staminodia absent.
Pollen grains Microsporogenesis simultaneous? Pollen grains usually tricolporate (sometimes tetracolporate), shed as monads, ?-cellular at dispersal. Exine tectate, with columellate? infratectum, psilate to psilate-granulate. Apertural membrane heavily bulging.
Gynoecium Pistil composed of four to seven laterally paracarp? connate carpels, adnate to central columella. Gynoecial vascular tissue abundant and well developed. Ovary superior, quadrilocular to septemlocular, nectariferous. Style single, long, twisted, hollow. Stigma capitate to lobate, Wet type? Pistillodium absent.
Ovules Placentation axile. Ovule usually one (rarely two) per carpel, sessile, anatropous, pendulous, epitropous, bitegmic, crassinucellar. Micropyle bistomal, Z-shaped (zig-zag). Outer integument seven or eight cell layers thick. Inner integument three or four cell layers thick. Nucellar cap absent. Obturator? Hypostase? Megagametophyte monosporous, Polygonum type? Endosperm development? Endosperm haustoria? Embryogenesis?
Fruit A drupaceous, dry, fibrous, lignified fruit with persistent calyx and style.
Seeds Funicular aril rudimentary. Hilum wide, wing-like, as long as seed. Seed coat exotestal. Testa multilayered, with lignified cell walls. Exotesta hard, consisting of five to twelve layers of crystalliferous sclereids. Endotesta? Tegmen crushed? Perisperm not developed. Endosperm thin (fleshy?), oily. Embryo straight, well differentiated, chlorophyll? Cotyledons two. Germination?
Cytology n = c. 250; x = 25 – Paleopolyploidy occurring.
DNA
Phytochemistry Triterpene saponins (24-hydroxytormentic acid ester glucoside, niga-ichigoside F1, niga-ichigoside F2, in the bark) present.
Use Unknown.
Systematics Strasburgeria (1; S. calliantha; New Caledonia, on ultrabasic soils).
Strasburgeria is sister to Ixerba (Ixerbaceae). The morphology of the testa is similar.
Literature
Amaral MCE. 1991. Phylogenetische Systematik der Ochnaceae. – Bot. Jahrb. Syst. 113: 105-196.
Bensel CR, Palser BF. 1975. Floral anatomy in the Saxifragaceae sensu lato I. Introduction, Parnassioideae and Brexioideae. – Amer. J. Bot. 62: 176-185.
Calderón G, Rzedowski J. 1997. Velascoa (Crossosomataceae), un género nuevo de la Sierra Madre Oriental de México. – Acta Bot. Mex. 39: 53-59.
Cameron KM. 2003. On the phylogenetic position of the New Caledonian endemic families Paracryphiaceae, Oncothecaceae, and Strasburgeriaceae: a comparison of molecules and morphology. – Bot. Rev. 68: 428-443.
Carlquist SJ. 1975. Wood anatomy and relationships of the Geissolomataceae. – Bull. Torrey Bot. Club 102: 128-134.
Carlquist SJ. 1990. Leaf anatomy of Geissolomataceae and Myrothamnaceae as a possible indicator of relationship to Bruniaceae. – Bull. Torrey Bot. Club 117: 420-428.
Carlquist SJ. 2007. Wood anatomy of Crossosomatales: patterns of wood evolution with relation to phylogeny and ecology. – Aliso 24: 1-18.
Carlquist SJ, Hoekman DA. 1985. Wood anatomy of Staphyleaceae: ecology, statistical correlations, and systematics. – Flora 177: 195-216.
Chen S-K. 1981. A study on the Stachyuraceae from China. – Acta Bot. Yunnan. 3: 125-137. [In Chinese]
Dahlgren R, Rao VS. 1969. A study of the Geissolomataceae. – Bot. Not. 122: 207-227.
Dahlgren R, Wyk AE van. 1988. Structures and relationships of families endemic to or centered in southern Africa. – Monogr. Syst. Bot. Missouri Bot. Gard. 25: 1-94.
DeBuhr LE. 1978. Wood anatomy of Forsellesia (Glossopetalon) and Crossosoma (Crossosomataceae, Rosales). – Aliso 9: 179-184.
Dickison WC. 1981. Contributions to the morphology and anatomy of Strasburgeria and discussion on the taxonomic position of the Strasburgeriaceae. – Brittonia 33: 564-580.
Dickison WC. 1986. Floral morphology and anatomy of Staphyleaceae. – Bot. Gaz. (Crawfordsville) 147: 312-326.
Dickison WC. 1987a. Leaf and nodal anatomy and systematics of Staphyleaceae. – Bot. Gaz. (Crawfordsville) 148: 475-489.
Dickison WC. 1987b. A palynological study of the Staphyleaceae. – Grana 26: 11-24.
Dickison WC. 2006. Strasburgeriaceae. – In: Kubitzki K (ed), The families and genera of vascular plants IX. Flowering plants. Eudicots. Berberidopsidales, Buxales, Crossosomatales, Fabales p. p., Geraniales, Gunnerales, Myrtales p. p., Proteales, Saxifragales, Vitales, Zygophyllales, Clusiaceae Alliance, Passifloraceae Alliance, Dilleniaceae, Huaceae, Picramniaceae, Sabiaceae, Springer, Berlin, Heidelberg, New York, pp. 446-448.
Dore WG. 1962. The bladdernut shrub at Ottowa. – Can. Field-Naturalist 76: 100-103.
Dravitzky PV. 1967. A comparative study of the wood anatomy and floral vascular systems of the New Zealand genera of the Escalloniaceae, Carpodetus, Ixerba and Quintinia. – M.Sc. thesis, University of Canterbury, Christchurch, New Zealand.
Engler A. 1891. Saxifragaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(2a), W. Engelmann, Leipzig, pp. 41-93.
Engler A. 1897. Crossosomataceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(3), W. Engelmann, Leipzig, pp. 185-186.
Engler A. 1925. Strasburgeriaceae. – In: Engler A, Gilg E (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 21, W. Engelmann, Leipzig, pp. 87-89.
Engler A. 1930. Saxifragaceae. – In: Engler A, Harms H (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 18a, W. Engelmann, Leipzig, pp. 74-226.
Ensign M. 1942. A revision of the celastraceous genus Forsellesia (Glossopetalon). – Amer. Midl. Natur. 27: 501-511.
Fagerlind F, Dunbar A. 1973. Some electron microscopical methods for solving wood anatomical problems. – Bot. Not. 126: 519-533.
Forest F. 2006. Geissolomataceae. – In: Kubitzki K (ed), The families and genera of vascular plants IX. Flowering plants. Eudicots. Berberidopsidales, Buxales, Crossosomatales, Fabales p. p., Geraniales, Gunnerales, Myrtales p. p., Proteales, Saxifragales, Vitales, Zygophyllales, Clusiaceae Alliance, Passifloraceae Alliance, Dilleniaceae, Huaceae, Picramniaceae, Sabiaceae, Springer, Berlin, Heidelberg, New York, pp. 155-156.
Foster RC. 1933. Chromosome number in Acer and Staphylea. – J. Arnold Arbor. 14: 386-393.
Gagnepain PF. 1948. Deux espèces nouvelles d’Euscaphis. – Not. Syst. Herb. Mus. Paris 13: 191-192.
Garwood NC, Horvitz CC. 1985. Factors limiting fruit and seed production of a temperate shrub, Staphylea trifolia L. (Staphyleaceae). – Amer. J. Bot. 72: 453-466.
Gilg E. 1894. Geissolomaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(6a), W. Engelmann, Leipzig, pp. 205-207.
Gilg E. 1895. Stachyuraceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(6), W. Engelmann, Leipzig, pp. 192-193.
Gilg E. 1925. Stachyuraceae. – In: Engler A, Gilg E (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 21, W. Engelmann, Leipzig, pp. 457-459.
Greene EL. 1893. Corrections in nomenclature III. (Forsellesia). – Erythea 1: 206.
Guérin P. 1901. Développement de la graine et en particulier du tégument séminal de quelques Sapindacées. – J. Bot. (Morot) 15: 336-362.
Han L, Hatano T, Okuda T, Yoshida T. 1995. Tannins of Stachyurus species: 3. Stachyuranins A, B and C, three new complex tannins from Stachyurus praecox leaves. – Chem. Pharmacol. Bull. (Tokyo) 43: 2109-2114.
Hannon DP. 2002. Crossosomataceae: a family primer. – Crossosoma 27: 29-34.
Harris AJ, Chen P-T, Xu X-W, Zhang J-Q, Yang X, Wen J. 2017. A molecular phylogeny of Staphyleaceae: implications for generic delimitation and classical biogeographic disjunctions in the family. – J. Syst. Evol. 55: 124-141.
Heads M. 2010. The endemic plant families and the palms of New Caledonia: a biogeographical analysis. – J. Biogeogr. 37: 1239-1250.
Hils MH. 1985. Comparative anatomy and systematics of twelve woody Australasian genera of the Saxifragaceae. – Ph.D. diss., University of Florida.
Holmgren NH. 1988. Glossopetalon (Crossosomataceae) and a new variety of G. spinescens from the Great Basin, U.S.A. – Brittonia 40: 269-274.
Holmgren NH. 1997. Crossosomataceae. – In: Cronquist A, Holmgren NH, Holmgren PK (eds) Intermountain flora 3A, The New York Botanical Garden, Bronx, New York, pp. 158-163.
Huang Y-J, Liu Y-S, Wen J, Quan C. 2015. First fossil record of Staphylea L. (Staphyleaceae) from North America, and its biogeographic implications. – Plant Syst. Evol. 301: 2203-2218.
Ishida T, Tokuoka T. 2017. Embryology and its character evolution in Staphyleaceae. – Plant Syst. Evol. 303: 1317-1329.
Jarzen DM, Pocknall DT. 1993. Tertiary Bluffopollis scabratus (Couper) Pocknall & Mildenhall, 1984, and modern Strasburgeria pollen: a botanical comparison. – New Zealand J. Bot. 31: 185-192.
Jin QJ, Wei ZX. 2002. Studies on pollen morphology of Stachyuraceae and Staphyleaceae. – Acta Bot. Yunnan. 24: 57-63. [In Chinese]
Jotani Y. 1977. On Stachyurus sigeyosii Masamune. – J. Jap. Bot. 52: 376-377.
Kamelina OP. 1992. On the embryology of the genus Ixerba in relation to its systematic position. – Bot. Žurn. 77: 112-117. [In Russian]
Kapil RN. 1970. Comparative embryology of angiosperms: Crossosomataceae. – Bull. Natl. Sci. Acad. India 41: 63-68.
Kapil RN, Vani RS. 1983. Embryology and systematic position of Crossosoma californicum Nutt. – Curr. Sci. 32: 493-495.
Keating RC. 1973. Pollen morphology and relationships of the Flacourtiaceae. – Ann. Missouri Bot. Gard. 60: 273-305.
Kimoto Y, Tokuoka T. 1999. Embryology and relationships of Stachyurus (Stachyuraceae). – Acta Phytotaxon. Geobot. 50: 187-200.
Koontz JA, Soltis DE. 1999. DNA sequence data reveal polyphyly of Brexioideae (Brexiaceae; Saxifragaceae sensu lato). – Plant Syst. Evol. 219: 199-208.
Krach JE. 1976. Samenanatomie der Rosifloren I. Die Samen der Saxifragaceae. – Bot. Jahrb. Syst. 97: 1-60.
Krause J. 1942. Staphyleaceae. – In: Engler A (†), Harms H, Mattfeld J (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 20b, W. Engelmann, Leipzig, pp. 255-321.
Krishnan N. 1981. Pollen morphology of some Flacourtiaceae. – Proc. Ind. Acad. Sci., Plant Sci., 90: 163-168.
Kubitzki K. 2006. Aphloiaceae. – In: Kubitzki K (ed), The families and genera of vascular plants IX. Flowering plants. Eudicots. Berberidopsidales, Buxales, Crossosomatales, Fabales p. p., Geraniales, Gunnerales, Myrtales p. p., Proteales, Saxifragales, Vitales, Zygophyllales, Clusiaceae Alliance, Passifloraceae Alliance, Dilleniaceae, Huaceae, Picramniaceae, Sabiaceae, Springer, Berlin, Heidelberg, New York, pp. 31-32.
Lemèsle R. 1948. Position phylogénétique de l’Hydrastis canadensis L. et du Crossosoma californicum Nutt., d’après les particularités histologiques du xylème. – Compt. Rend. Acad. Sci. Paris 227: 221-223.
Li H-L. 1943. The genus Stachyurus. – Bull. Torrey Bot. Club 70: 615-628.
Li H-L. 1992. On the origin of Stachyuraceae. – Acta Bot. Yunnan., Suppl., 5: 59-64.
Li H-L. 1993. Staphyleaceae. – In: Woody flora of Taiwan, Livingston Publ. Narbeth, Pennsylvania.
Linden BL van der. 1960. Staphyleaceae. – In: Steenis CGGJ van (ed), Flora Malesiana I, 6, Wolters-Noordhoff, Groningen, pp. 49-59.
Lobreau D. 1969. Les limites de l’”ordre” des Célastrales d’après le pollen. – Pollen Spores 11: 499-555.
McDonald DJ. 1998. The enigma of the Geissolomataceae. – Veld & Flora 84: 122-123.
Mason CT. 1975. Apacheria chiracahuensis: a new genus and species from Arizona. – Madroño 23: 105-108.
Mason CT. 1992. Crossosomataceae: Crossosoma family. – J. Arizona Nevada Acad. Sci. 26: 7-9.
Mathew CJ, Chaphekar M. 1977. Development of female gametophyte and embryogeny in Stachyurus chinensis. – Phytomorphology 27: 68-78.
Matthews ML, Endress PK. 2005. Comparative floral structure and systematics in Crossosomatales (Crossosomataceae, Stachyuraceae, Staphyleaceae, Aphloiaceae, Geissolomataceae, Ixerbaceae, Strasburgeriaceae). – Bot. J. Linn. Soc. 147: 1-46.
Mauritzon J. 1936. Zur Embryologie einiger Parietales-Familien. – Svensk Bot. Tidskr. 30: 79-113.
Miller RB. 1975. Systematic anatomy of the xylem and comments on the relationships of Flacourtiaceae. – J. Arnold Arb. 56: 20-102.
Narayana LL. 1960. Embryology of Staphyleaceae. – Curr. Sci. 10: 403-404.
Nepia RE, Clarkson BD. 2018. Biological flora of New Zealand (15): Ixerba brexioides, tāwari. – New Zealand J. Bot. 56: 2-25.
Oginuma K, Munzinger J, Tobe H. 2006. Exceedingly high chromosome number in Strasburgeriaceae, a monotypic family endemic to New Caledonia. – Plant Syst. Evol. 262: 97-101.
Oh S-H. 2010. Phylogeny and systematics of Crossosomatales as inferred from chloroplast atpB, matK, and rbcL sequences. – Korean J. Plant Taxon. 40: 208-217.
Oh S-H, Potter D. 2006. Description and phylogenetic position of a new angiosperm family, Guamatelaceae, inferred from chloroplast rbcL, atpB, and matK sequences. – Syst. Bot. 31: 730-738.
Ohi T, Wakabayashi M, Wu S-G, Murata J. 2003. Phylogeography of Stachyurus praecox (Stachyuraceae) in the Japanese archipelago based on chloroplast DNA haplotypes. – J. Jap. Bot. 78: 1-14.
Okuda T, Yoshida T, Hatano T, Yazaki K, Ashida M. 1982. Ellagitannins of the Casuarinaceae, Stachyuraceae, and Myrtaceae. – Phytochemistry 21: 2871-2874.
Okuda T, Yoshida T, Ashida M, Yazaki K. 1982. Casuariin, stachyurin and strictinin, new ellagitannins from Casuarina stricta and Stachyurus praecox. – Chem. Pharm. Bull. Feb 1982. v. 30: 766-769.
Pastre A, Pons A. 1973. Quelques aspects de la systématique des Saxifragacées à la lumière des données de la palynologie. – Pollen Spores 15: 117-133.
Patel RN. 1973. Wood anatomy of the dicotyledons indigenous to New Zealand 2. Escalloniaceae. – New Zealand J. Bot. 11: 421-434.
Pax F. 1896. Staphyleaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(5), W. Engelmann, Leipzig, pp. 258-262.
Pereira JT. 1995. Staphyleaceae. – In: Soepadmo E, Wong K-M (eds), Tree flora of Sabah and Sarawak 1, Forest Research Institute of Malaysia, Sabah.
Ramp E. 1987. Funktionelle Anatomie des Gynoeciums bei Staphylea. – Bot. Helvetica 97: 89-98.
Richardson PE. 1968. The comparative morphology of the Crossosomataceae. – Ph.D. diss., Graduate School, University of Cincinnati, Ohio.
Richardson PE. 1970. The morphology of the Crossosomataceae I. Leaf, stem, and node. – Bull. Torrey Bot. Club 97: 34-39.
Rzedowski GC de, Rzedowski J. 1997. Velascoa (Crossosomataceae), un genero nuevo de la Sierra Madre Oriental de Mexico. – Acta Bot. Mexicana 39: 53-59.
Satô Y. 1976. Embryological studies on Stachyurus praecox and its variety. – Sci. Rep. Tohoku Univ. IV, Biol. 37: 131-138.
Schneider JV. 2006. Ixerbaceae. – In: Kubitzki K (ed), The families and genera of vascular plants IX. Flowering plants. Eudicots. Berberidopsidales, Buxales, Crossosomatales, Fabales p. p., Geraniales, Gunnerales, Myrtales p. p., Proteales, Saxifragales, Vitales, Zygophyllales, Clusiaceae Alliance, Passifloraceae Alliance, Dilleniaceae, Huaceae, Picramniaceae, Sabiaceae, Springer, Berlin, Heidelberg, New York, pp. 205-207.
Schneider JV. 2006. Stachyuraceae. – In: Kubitzki K (ed), The families and genera of vascular plants IX. Flowering plants. Eudicots. Berberidopsidales, Buxales, Crossosomatales, Fabales p. p., Geraniales, Gunnerales, Myrtales p. p., Proteales, Saxifragales, Vitales, Zygophyllales, Clusiaceae Alliance, Passifloraceae Alliance, Dilleniaceae, Huaceae, Picramniaceae, Sabiaceae, Springer, Berlin, Heidelberg, New York, pp. 436-439.
Simmons SL. 2002. A molecular phylogenetic investigation of the Staphyleaceae (DC.) Lindl.: with implications for its taxonomy and biogeography. – Ph.D. Diss., University of Texas at Austin, Texas.
Simmons SL. 2006. Staphyleaceae. – In: Kubitzki K (ed), The families and genera of vascular plants IX. Flowering plants. Eudicots. Berberidopsidales, Buxales, Crossosomatales, Fabales p. p., Geraniales, Gunnerales, Myrtales p. p., Proteales, Saxifragales, Vitales, Zygophyllales, Clusiaceae Alliance, Passifloraceae Alliance, Dilleniaceae, Huaceae, Picramniaceae, Sabiaceae, Springer, Berlin, Heidelberg, New York, pp. 440-445.
Sosa V. 2006. Crossosomataceae. – In: Kubitzki K (ed), The families and genera of vascular plants IX. Flowering plants. Eudicots. Berberidopsidales, Buxales, Crossosomatales, Fabales p. p., Geraniales, Gunnerales, Myrtales p. p., Proteales, Saxifragales, Vitales, Zygophyllales, Clusiaceae Alliance, Passifloraceae Alliance, Dilleniaceae, Huaceae, Picramniaceae, Sabiaceae, Springer, Berlin, Heidelberg, New York, pp. 119-122.
Sosa V, Chase MW. 2003. Phylogenetics of Crossosomataceae based on rbcL sequence data. – Syst. Bot. 28: 96-105.
Stephens EL. 1910. The embryo-sac and embryo of Geissoloma marginata. – New Phytol. 8: 345-348.
Tang Y-C, Cao Y-L, Xi Y-Z, He J. 1983. Systematic studies on Chinese Stachyuraceae I. Phytogeography, cytology, and palynology. – Acta Phytotaxon. Sin. 21: 236-253. [In Chinese]
Tatsuno A, Scogin R. 1978. Biochemical profile of Crossosomataceae. – Aliso 9: 185-188.
Thorne RF, Scogin R. 1978. Forsellesia Greene (Glossopetalon Gray), a third genus in the Crossosomataceae, Rosineae, Rosales. – Aliso 9: 171-178.
Thouvenin M. 1890. Recherches sur la structure des Saxifragacées. – Ann. Sci. Nat., sér. VII (Bot.), 12: 1-174.
Tieghem P van. 1899. Sur le genre Neumannia considéré comme type d’une famille nouvelle, les Neumanniacées. – J. Bot. (Morot) 13: 361-367.
Tieghem P van. 1900. Sur les Stachyuracées et les Koeberliniacées. – J. Bot. (Morot) 14: 1-12.
Tieghem P van. 1903. Sur le genre Strasburgeria considéré comme type d’une famille nouvelle, les Strasburgeriacées. – J. Bot. (Morot) 17: 198-204.
Tiffney BH. 1979. Fruits and seeds of the Brandon Lignite III. Turpinia (Staphyleaceae). – Brittonia 31: 39-51.
Trifonova WI. 1992. Aphloiaceae. – In: Takhtajan A (ed), Anatomia seminum comparativa 4, Nauka, S:t Petersburg, p. 80. [In Russian]
Um B-H, Anton R. 2002. Détermination structurale de substances naturelles issues de la biodiversité végétale tropicale. – Ph.D. diss., Université Louis Pasteur, Strasbourg, France.
Um B-H,Pouplin T, Lobstein A, Weniger B, Litaudon M, Anton R. 2001. Saponins from Strasburgeria robusta. – Fitoterapia 72: 591-593.
Umadevi I, Daniel M, Sabnis SD. 1986. Interrelationships among the families Aceraceae, Hippocastanaceae, Melianthaceae, Staphyleaceae. – J. Plant Anat. Morph. 3: 169-172.
Wang Y-S, Yang J-H, Luo S-D, Zhang H-B, Li L. 2007. New cytotoxic steroid from Stachyurus himalaicus var. himalaicus. – Molecules 12: 536-542.
Watari S. 1939. Anatomical studies on the leaves of some saxifragaceous plants, with special reference to the vascular system. – J. Fac. Sci. Imp. Univ. Tokyo, Sect. III, Bot. 5: 195-316.
Weaver RE. 1980. The bladdernuts. – Arnoldia 40: 76-93.
Weberling F. 1963. Ein Beitrag zur systematischen Stellung der Geissolomataceae, Penaeaceae, und Oliniaceae sowie der Gattung Heteropyxis (Myrtaceae). – Bot. Jahrb. Syst. 82: 119-128.
Wei Z-X, Yang Z-H. 2001. Growth, development and some biological phenomena of Stachyurus himalaicus under different environmental condition. – Chin. J. Appl. Environm. Biol. 7: 315-320.
Wei Z-X, Jin Q-J, Hong W, Tian X, Chen S-K. 2002. Pollen morphology of Stachyuraceae and related taxa. – Acta Bot. Yunnan. 24: 483-496.
Wei Z-X, Wang F, Jin Q-J, Wang H. 2002. A cladistic analysis of Stachyuraceae and related taxa. – Acta Bot. Yunnan. 24: 591-599.
Wild H. 1960. Flacourtiaceae. – In: Exell AW, Wild H (eds), Flora Zambesiaca 1(1), Crown Agents, London, pp. 261-298.
Zhu Y-P, Wen J, Zhang Z-Y, Chen Z-D. 2006. Evolutionary relationships and diversification of Stachyuraceae based on sequences of four chloroplast markers and the nuclear ribosomal ITS region. – Taxon 55: 931-940.