
Cladogram of Juglandales based on DNA sequence data. The sister-group relationships between Myricaceae, Juglandaceae and Rhoiptelea are uncertain. Nothofagus and Fagaceae are – as usually found – successive sisters to the remaining Juglandales.
[Polygalales+[Rosales+[Cucurbitales+Juglandales]]]
Fagales Engl., Syllabus, ed. 1: 94. Apr 1892; Juglandanae Takht. ex Reveal in Novon 2: 236. 13 Oct 1992; Faganae Takht., Divers. Classif. Fl. Pl.: 146. 24 Apr 1997
Fossils Records from the mid-Cenomanian represent the oldest known fossils of presumed Juglandales. The group seems to have reached its peak of diversity during the Santonian to the Campanian, according to the wide range of Normapolles fossils, and inflorescences, flowers and pollen grains of Juglandales are fairly abundant from the Turonian onwards. Antiquacupula sulcata from the Late Santonian of Georgia (USA) includes hexamerous epigynous bisexual and male flowers and fruits resembling Fagaceae and Nothofagus. The tepals are inserted in two series with three tepals in each and the androecium is arranged in two whorls with six stamens each andAntiquacupula probably had nectaries at the filament bases. The pollen grains are tricolporate and the three carpels are biovular. Up to six flowers are surrounded by a pedunculate quadrilobate cupule. Archaefagacea futabensis from the Early Coniacian of Japan is represented by bisexual epigynous hexamerous flowers and fruits consisting of three carpels. The pollen grains are tricolpate with a striate exine and the carpels are biovular. The trilocular fruits are three-seeded.
The Normapolles complex comprises fossil tricolporate (rarely tripororate) oblate or suboblate pollen grains with granular infratectum. The apertures are strongly thickened and often formed through infratectal extensions, and the polar areas may be intectate. The exine surface is smooth to rugulate or finely spinulate. They are known from the mid-Cenomanian to the Oligocene, being most diverse in the Santonian to the Campanian and in the Eocene from eastern North America eastwards through Europe to western Siberia. Normapolles pollen is associated with usually bisexual hexamerous flowers or fruits of Antiquocarya, Bedellia, Budvaricarpus, Calathiocarpus, Caryanthus, Dahlgrenianthus, Endressianthus, Manningia and Normanthus in Juglandales. Of these, Dahlgrenianthus is hypogynous and Endressianthus and perhaps Bedellia are unisexual. The perianth and androecium are uniseriate. The stamens are alternitepalous in Endressianthus and Normanthus, otherwise antetepalous. The fruit is a nut. Caryanthus, from Late Cretaceous strata in Europe and perhaps eastern North America, are bisymmetrical epigynous quadritepalous flowers with six to eight stamens producing tricolporate pollen grains. The bicarpellate gynoecium has a unilocular ovary and two free styles. Caryanthus and Budvaricarpus are similar to extant Rhoiptelea chiliantha.
Endressianthus, described from the Campanian to the Maastrichtian of Portugal, comprises male and female compound cymes, epigynous flowers with bicarpellate gynoecium, nuts with single-seeded locules and other reproductive structures. The perianth is reduced and the number of stamens is four or less. The pollen grains have three endopores each grain with six exopores arranged in pairs, and the apertural areas are interconnected by crests. The Late Cretaceous Normanthus, likewise from Portugal, comprises bisexual pentapetalous flowers, alternipetalous stamens, two collateral carpels with separate and relatively long styles, and possibly parietal placentation.
Habit Usually monoecious or dioecious (rarely bisexual, andromonoecious, polygamodioecious or polygamous), evergreen or deciduous trees or shrubs. Buds with spiral scales.
Vegetative anatomy Ectomycorrhiza abundant. Root nodules with nitrogen fixing actinobacteria (at least two different Frankia clades responsible for nitrogen fixation in Juglandales; also Frankia species restricted to Juglandales). Frankia infection via root hairs. Phellogen ab initio superficially or deeply seated (sometimes outer-cortical). Primary vascular tissue cylinder, without separate vascular bundles. Vessel elements with simple or scalariform (rarely reticulate) perforation plates; lateral pits scalariform, opposite, intermediary or alternate, simple or bordered pits. Vestured pits often present. Imperforate tracheary xylem elements tracheids or fibre tracheids (sometimes libriform fibres) with simple or bordered pits, usually non-septate (also vasicentric tracheids). Wood rays uniseriate or multiseriate, homocellular or heterocellular. Axial parenchyma apotracheal diffuse or diffuse-in-aggregates or banded, or paratracheal scanty, vasicentric, aliform, confluent, reticulate, scalariform, unilateral or banded, or absent. Sieve tube plastids S type; sieve tubes with non-dispersive P-protein bodies. Nodes usually 3:3, trilacunar with three leaf traces (sometimes 1:1, unilacunar with one trace, rarely 5:5, pentalacunar with five traces). Bark cells with sclereids and rhomboid crystals. Bark often rich in tannins. Secretory cells present or absent. Heartwood sometimes with gum-like substances. Silica bodies rarely present. Prismatic or rhomboid calciumoxalate crystals often frequent in parenchyma cells.
Trichomes Hairs unicellular or multicellular, simple or branched (sometimes dendritic, fasciculate, stellate, peltate or lepidote, rarely vesicular or T-shaped); glandular hairs, also peltate-lepidote, often present (sometimes secreting ethereal oils and resins).
Leaves Alternate (spiral or distichous, sometimes verticillate), usually simple (sometimes pinnately compound), entire (sometimes scale-like), usually with conduplicate to plicate, involute or curved ptyxis. Stipules intrapetiolar, scale-like, caducous (sometimes absent); leaf sheath absent. Petiole vascular bundle transection arcuate or annular. Venation pinnate, craspedodromous, semicraspedodromous or camptodromous. Stomata anomocytic, cyclocytic or paracytic. Cuticular wax crystalloids? Domatia as pockets or hair tufts, or absent. Epidermis with or without mucilaginous idioblasts. Sometimes enlarged epidermal cells containing ethereal oils. Lamina sometimes with resinous glands (colleters). Leaf margin sinuous, dentate, serrate, biserrate or entire; leaf teeth sometimes cunonioid or modified urticoid (secondary veins proceeding to non-glandular teeth, higher-order veins converging on urticoid teeth).
Inflorescence Terminal or lateral, usually compound cymose spicate, catkin-like or capitate inflorescence, consisting of dichasia (flowers sometimes solitary). Bracts more or less connate into scales and/or reduced, often persistent and sometimes accrescent in fruit. One or several female dichasia often enclosed by lignified cupule, modified shoot consisting of sterile inflorescence parts.
Flowers Actinomorphic, minute. Epigyny (very rarely hypogyny). Tepals in male flowers one to seven (to nine), with imbricate aestivation, scale-like, sepaloid, free or more or less connate, in one or two whorls, or absent; in female flowers indistinct or absent. Nectary absent. Disc usually absent. Flowers often fused into pseudanthia.
Androecium Stamens one or four to eight (to more than 100), in one (antetepalous) or several whorls. Filaments free or more or less connate, free from or adnate at base to tepals, sometimes partially or entirely divided. Anthers basifixed or dorsifixed, usually non-versatile, tetrasporangiate, extrorse (sometimes introrse?), longicidal (dehiscing by longitudinal slits). Tapetum secretory. Staminodia usually absent (sometimes six to twelve).
Pollen grains Microsporogenesis simultaneous. Pollen grains (2–)3(–7)-por(or)ate, polypantopor(or)ate or 3(–4)-colpor(oid)ate (sometimes stephanocolpate, rarely di- or hexacolpate; with cavity, vestibulum, between outer and inner pore), shed as monads, bicellular at dispersal. Exine tectate, with columellate or granular infratectum, microperforate, striate, scabrate, rugulate, verrucate, spinulate, microechinate or psilate. Arci (band-shaped arches) of thick sexine running between pores. Pollen tube growth often interrupted (irregularly or periodically).
Gynoecium Pistil composed of two to six (to 15) more or less connate carpels. Ovary inferior, unilocular to trilocular (to 15-locular). Stylodia usually two to six (to nine), free or connate at base. Stigmas two (or three), capitate, punctate or adaxially decurrent, linear, papillate or non-papillate, Dry type. Pistillodium usually absent (male flowers sometimes with pistillodium).
Ovules Placentation axile, apical or basal. Ovules one or two per carpel (or locule), usually anatropous or orthotropous (sometimes campylotropous, rarely hemianatropous), ascending or pendulous, epitropous, bitegmic or unitegmic, crassinucellar, immature at pollination. Micropyle endostomal. Archespore sometimes multicellular. Megagametophyte monosporous, Polygonum type. Synergids sometimes with a filiform apparatus. Antipodal cells sometimes multiplicative. Fertilization usually delayed; chalazogamy frequently occurring. Endosperm development ab initio nuclear. Endosperm haustoria? Embryogenesis asterad or onagrad.
Fruit A one-seeded nut, often a samara, often densely surrounded by more or less connate and sometimes lignified bracts and/or floral prophylls, or a unilocular to trilocular calybium.
Seeds Aril absent. Testa vascularized, sometimes adnate to pericarp. Exotesta often enlarged and persistent. Tegmen? Perisperm not developed. Endosperm thin or absent. Embryo large, straight, well differentiated, oily, without chlorophyll. Cotyledons two, often large. Germination phanerocotylar or cryptocotylar.
Cytology x = (7) 8–16
DNA Plastid gene infA lost/defunct. Mitochondrial intron coxII.i3 lost.
Phytochemistry Flavonols (kaempferol, quercetin, myricetin), dihydroflavonols, biflavonoids, flavones, flavanones, flavononols, lipofilic flavonoids, cyanidin, delphinidin, ethereal oils, resins, and balsam (with O- and C-methylated flavones), catechin, tetracyclic and pentacyclic triterpenes (of taraxerane, ursane and lupane type), ellagic and gallic acids, hydrolyzable tannins (ellagotannins and galloyltannins), non-hydrolyzable tannins, proanthocyanidins (prodelphinidins), naphthoquinones, stilbenes, citrullin, and often shikimic and quinic acids present. Alkaloids and cyanogenic compounds rare. Saponins not found.
Systematics Juglandales are sister-group to Cucurbitales.
Nothofagus (Nothofagaceae) is sister to the remaining Juglandales, characterized by the common features: spiral leaves; and dorsifixed anthers, according to Stevens (2001 onwards). A plausible topology is the following: [Fagaceae+[[Myricaceae+[Juglandaceae+Rhoipte-leaceae]]+[Casuarinaceae+[Ticodendraceae+Betulaceae]]]]. Moreover, the clade [[Myri-caceae+[Juglandaceae+Rhoipteleaceae]]+[Casuarinaceae+[Betulaceae+Ticodendraceae]]] (descendants of the fossil Normapolles complex) has the following potential synapomorphies: chambered crystals in axial parenchyma; pollen grains pororate; exine consisting of two layers separated by alveolar zone and expanded around apertures; pistil composed of two connate carpels; and chalazogamy.
The clade [Myricaceae+[Juglandaceae+Rhoipteleaceae]] is characterized by the features (Stevens 2001 onwards): chains of crystal-containing cells present in wood; absence of P-protein bodies in sieve tubes; presence of aromatic peltate glandular hairs; absence of stipules; one flower per bract; stigma lamellular/laciniate; ovule one per ovary, orthotropous. Furthermore, the leaf teeth in Juglandaceae, Rhoipteleaceae and Myricaceae are intermediary, with apex extended (outwards/inwards) and non-glandular, and leaf tooth vein united with vein branches running below or above leaf tooth; tepals sometimes with three traces; and varying number of integuments. Juglandaceae and Rhoipteleaceae share the characteristics: imparipinnate leaves; four tepals; endosperm absent; and x = 16.
The clade [Casuarinaceae+[Ticodendraceae+Betulaceae]] has the following potential synapomorphies (Stevens 2001 onwards): pollen grains with rows of spinulae; Pollen tubes branched; stigmas elongate; and presence of dihydroflavonols (?). Betulaceae and Ticodendraceae share the features: sclereid nests in bark, cells with rhomboidal crystals; presence of mucilage cells; leaves distichous; and anther thecae separate or almost separate.

|
Cladogram of Juglandales based on DNA sequence data. The sister-group relationships between Myricaceae, Juglandaceae and Rhoiptelea are uncertain. Nothofagus and Fagaceae are – as usually found – successive sisters to the remaining Juglandales. |
BETULACEAE S. F. Gray |
( Back to Juglandales ) |
Corylaceae Mirb., Elém. Phys. Vég. Bot. 2: 906. 24-30 Jun 1815 [‘Corylacées (Corylaeae)’], nom. cons.; Carpinaceae Vest, Anleit. Stud. Bot.: 265, 280. 1818 [‘Carpinoideae’]; Corylales Dumort., Anal. Fam. Plant.: 11. 1829 [‘Corylarieae’]; Betulales Rich. in C. F. P. von Martius, Consp. Regn. Veg.: 17. Sep-Oct 1835 [‘Betulineae’]; Carpinales Döll, Fl. Baden 2: 536. med. 1858 [’Carpineae’]
Genera/species 6/c 140
Distribution Temperate and polar regions on the Northern Hemisphere and southwards to northern Argentina, North Africa, Himalaya, Indochina and Sumatra.
Fossils The oldest fossil representative of Betulaceae is Palaeocarpinus from the Paleocene and Eocene of Europe and North America. It comprises leaves, male flowers and fruits surrounded by spiny involucral bracts. Numerous Cenozoic Betulaceae are known, including Alnus, Betula, Corylus and Ostrya, and the fossil Asterocarpinus and Cranea.
Habit Monoecious, usually deciduous (rarely evergreen) trees or shrubs. Horizontal lenticels often abundant.
Vegetative anatomy Alnus has root nodules with nitrogen fixing actinobacteria (Frankia); cluster roots often present. Phellogen ab initio superficially or deeply seated (sometimes outer-cortical). Primary vascular tissue cylinder, without separate vascular bundles. Vessel elements with usually scalariform (sometimes simple) perforation plates; lateral pits scalariform, opposite or alternate, usually bordered (rarely simple) pits. Imperforate tracheary xylem elements tracheids and/or fibre tracheids with usually simple pits, non-septate (also vasicentric tracheids). Wood rays usually uniseriate, usually homocellular, often aggregated. Axial parenchyma usually apotracheal diffuse, diffuse-in-aggregates or banded (sometimes paratracheal scanty, reticulate or banded, or absent). Secondary phloem usually stratified into hard fibrous and soft parenchymatous layers. Sieve tube plastids S type. Nodes 3:3, trilacunar with three leaf traces. Heartwood sometimes with resinous? substances. Bark cells with sclereid and rhomboid calciumoxalate crystals. Prismatic crystals abundant.
Trichomes Hairs unicellular or multicellular, uniseriate, simple; glandular hairs present, also peltate-lepidote.
Leaves Alternate (spiral or distichous), simple, entire, usually with conduplicate (sometimes plicate) ptyxis. Stipules intrapetiolar, scale-like, caducous; leaf sheath absent. Colleters present. Petiole vascular bundle transection arcuate. Venation pinnate, craspedodromous. Stomata anomocytic. Cuticular wax crystalloids? Epidermis with or without mucilage cells. Domatia as pockets or hair tufts. Lamina sometimes with resinous glands (colleters). Leaf margin biserrate; leaf teeth modified urticoid.
Inflorescence Terminal or lateral, compound catkin-like or multi-flowered capitate groups, consisting of dichasia (female flowers in Corylus solitary). Up to five bracts (sometimes peltate) more or less connate into scales and/or reduced, often persistent (in Alnus lignified) in fruit (female flowers in Betula three per bract). Pseudanthium in Ostrya consisting of three flowers with approx. 15 pairs of divided stamens in total. Prophyll in Alnus adaxial.
Flowers Actinomorphic, small. Epigyny (sometimes very distinct). Tepals in male flowers one to six, sepaloid, or absent (perianth in Corylus reduced to ridge-like structure); tepals in female flowers indistinct or absent. Nectary absent. Disc absent.
Androecium Stamens (one to) four (to six to twelve?), in one whorl, antesepalous. Filaments filiform, sometimes partially or entirely split, free or more or less connate, free from or adnate at base to tepals. Anthers basifixed? or dorsifixed, non-versatile, tetrasporangiate, extrorse, longicidal (dehiscing by longitudinal slits); connective shorter than anther. Tapetum secretory. Staminodia usually absent.
Pollen grains Microsporogenesis simultaneous. Pollen grains 3(–7)-por(or)ate, starchy, shed as monads, bicellular at dispersal. Exine tectate, with granular infratectum, microperforate, scabrate to somewhat rugulate. Pollen tube branching.
Gynoecium Pistil composed of two (or three) connate carpels. Ovary inferior, unilocular in upper part, bilocular (or trilocular) in lower part. Stylodia two (or three), rarely connate at base. Stigmas papillate or non-papillate, Dry type. Pistillodium absent.
Ovules Placentation apical-axile. Ovules one or two (to four) per carpel, usually collateral (sometimes superposed), usually anatropous (in Corylus campylotropous), pendulous, usually unitegmic (in Carpinus bitegmic), crassinucellar. Micropyle endostomal (Carpinus). Outer integument ? cell layers thick. Inner integument ? cell layers thick. Lower part of integument vascularized. Parietal tissue one or two, or four to eight cell layers thick. Nucellar cap one or two cell layers thick or absent. Archespore usually multicellular (sometimes unicellular). Megagametophytes several. Megagametophyte monosporous, Polygonum type. Chalazogamy. Endosperm development nuclear. Endosperm haustoria? Embryogenesis asterad.
Fruit A single-seeded nut, often a two-winged samara (in Alnus in lignified cone-like infructescence); nut often densely surrounded by more or less connate bracts and floral prophylls (bracteoles).
Seeds Aril absent. Testa? Tegmen? Perisperm not developed. Endosperm thin or absent. Embryo large, straight, well differentiated, without chlorophyll. Cotyledons two, small, oily. Germination phanerocotyl or cryptocotyl.
Cytology x = (7) 8, 11, 14
DNA Horizontal transfer of mitochondrial gene rps11.
Phytochemistry Flavonols (kaempferol, quercetin, myricetin), flavones, lipofilic flavonoids, cyanidin, delphinidin, ellagic acid, and triterpenes (pentacyclic triterpenes in bark, pentacyclic and tetracyclic triterpenes in leaves) present. Alkaloids and cyanogenic compounds not found. Nitrogen transported as citrullin.
Use Ornamental plants, fruits (nuts from Corylus), timber, carpentry, charcoal, brushes and besoms, birch bark (Betula).
Systematics Betulaceae are sister-group to Ticodendron (Ticodendraceae).
Betuloideae Rich. ex Arn., Botany: 131. 9 Mar 1832 [‘Betulineae’]
2/c 70. Betula (c 35; temperate, boreal and polar regions on the Northern Hemisphere south to Southeast Asia), Alnus (c 35; temperate, boreal and polar regions on the Northern Hemisphere, mountains in tropical America). – Temperate and cold regions on the Northern Hemisphere, southwards to mountain areas in South America. Vessel elements without spiral thickenings. Peltate glandular hairs present. Female flowers usually without tepals (tepals sometimes two). Parietal tissue one or two cell layers thick. Nucellar cap approx. two cell layers thick. Infructescence with lignified or scale-like bracts separate from fruit. Nutlet samaroid, flat.
Coryloideae Hook. f., Stud. Fl. Brit. Isl.: 343. 1870 [‘Coryleae’]
4/c 70. Corylus (18; temperate regions on the Northern Hemisphere), Ostryopsis (3; O. davidiana, O. intermedia, O. nobilis; eastern Mongolia, northern to southwestern China), Carpinus (c 40; temperate regions on the Northern Hemisphere, Southeast Asia, mountains in Central America), Ostrya (9; temperate regions on the Northern Hemisphere south to Central America). – Temperate regions on the Northern Hemisphere, Southeast Asia, Central America. Vessel elements with spiral thickenings. Tracheids present. Dichasium (cymule) with one or two flowers. Male flowers without tepals. Female flowers with indistinct tepals. Filaments hairy. Integuments approx. six cell layers thick. Suprachalazal tissue massive. Parietal tissue four to eight cell layers thick. Nucellar cap one or two cell layers thick or absent. Megagametophyte sometimes with chalazal caecum. Infructescence with foliaceous bracts and accrescent foliaceous floral prophylls (bracteoles). Nuts large, not or little flattened.
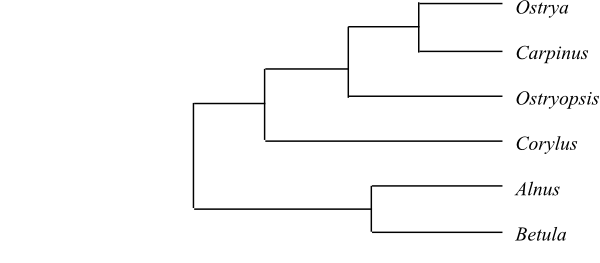 |
Cladogram of Betulaceae based on DNA sequence data and morphology (Chen & al. 1999). |
CASUARINACEAE R. Br. |
( Back to Juglandales ) |
Casuarinales Bercht. et J. Presl, Přir. Rostlin: 261. Jan-Apr 1820 [‘Casuarineae’]; Casuarinanae (Lindl.) Takht. ex Reveal et Doweld in Novon 9: 549. 30 Dec 1999
Genera/species 4/95–100
Distribution Madagascar, Malesia to New Guinea, Melanesia, Australia, with their largest diversity in Australia.
Fossils Pollen grains from the Early Paleocene have been found in New Zealand, and Paleocene to Miocene pollen fossils of Casuarinaceae are known from Australia, South Africa, Argentina and the Ninetyeast Ridge in the Indian Ocean. Wood, inflorescences and infructescences are recorded from Neogene layers onwards.
Habit Monoecious or dioecious, evergreen trees or shrubs with narrow furrowed Equisetum-like photosynthesizing branches. Most species are xerophytes.
Vegetative anatomy Root nodules containing nitrogen fixing actinobacteria (Frankia). Higher order rootlets clustered, with limited growth (proteoid roots). Branch furrows usually deep and closed (in Gymnostoma shallow and open). Phellogen ab initio deeply seated. Vessel elements with simple or scalariform perforation plates; lateral pits alternate or opposite, bordered pits. Imperforate tracheary xylem elements fibre tracheids with simple or bordered pits, non-septate (also vasicentric tracheids). Wood rays uniseriate or multiseriate, heterocellular (often compound; sometimes absent). Axial parenchyma apotracheal diffuse or diffuse-in-aggregates, or banded, or paratracheal scanty, vasicentric, scalariform. Tyloses sometimes abundant. Sieve tube plastids S type, with starch grains and non-dispersive P-protein bodies. Nodes 1:1, unilacunar with one leaf trace. Stomata tetracytic or paracytic, usually hidden inside closed longitudinal branch furrows perpendicular to length axis of branch (in Gymnostoma exposed). Cuticle thick, with crystalline bodies resembling inverted mushrooms. Heartwood often with resinous substances. Prismatic calciumoxalate crystals abundant.
Trichomes Hairs unicellular or multicellular, simple or branched (often dendritic).
Leaves Verticillate (four to c. 20 per whorl), simple, entire, minute and scale-like, membranous, fused with nearest internode and forming longitudinal ridges, with ? ptyxis. Stipules and leaf sheath absent. Venation reduced. Stomata sparse, tetracytic or paracytic, or absent, often hidden. Cuticular wax crystalloids? Leaf margin entire.
Inflorescence Male flowers in one or several spicate inflorescences; female flowers in axillary? verticillate capitate inflorescences, cone-like in fruit. Bracts more or less developed, scale-like, connate at base; each bract subtending one flower and two scale-like floral prophylls (bracteoles), in fruit strongly accrescent and lignified.
Flowers Actinomorphic, very small. Tepals in male flowers one or two, scale-like, caducous at anthesis; tepals absent in female flowers. Nectary absent. Disc absent.
Androecium Stamen one. Filament inflexed in bud, free from tepals. Anther basifixed, non-versatile, tetrasporangiate, introrse?, longicidal (dehiscing by longitudinal slits); connective shorter than anther. Tapetum secretory. Staminodia absent.
Pollen grains Microsporogenesis simultaneous? Pollen grains (2–)3(–5)-por(or)ate, shed as monads, bicellular at dispersal. Exine tectate, with granular infratectum, microperforate, scabrate, spinulate or rugulate. Pollen tube branching.
Gynoecium Pistil composed of two carpels connate in lower part; usually only abaxial carpel fertile (in Gymnostoma both carpels fertile). Ovary usually unilocular (pseudomonomerous; rarely bilocular). Stylodia two, long, filiform, connate at base, winged in lower part in fruit. Stigmas two, long, decurrent, collateral, non-papillate, Wet type. Pistillodium absent.
Ovules Placentation axile (basal at maturation). Ovules two per carpel, orthotropous (or anatropous?), ascending, epitropous, bitegmic, crassinucellar. Micropyle endostomal. Outer integument three or four cell layers thick. Inner integument two or three cell layers thick. Suprachalazal tissue massive. Parietal tissue five to seven cell layers thick. Megasporangium sometimes with tracheids (vascular bundle branched in chalaza). Archespore multicellular. Several to numerous megasporocytes formed at cell division resulting in two to more than 20 megagametophytes. Megagametophyte monosporous, Polygonum type, with chalazal caecum. Chalazogamy. Endosperm development ab initio nuclear. Endosperm haustoria? Embryogenesis?
Fruit A single-seeded samara surrounded by two lignified floral prophylls (bracteoles); adjacent ovaries connate into syncarps?; fruit liberated when enlarged floral prophylls separate from each other. Infructescence cone-like, with lignified bracts.
Seeds Aril absent. Testa adnate to pericarp, vascularized? Tegmen? Perisperm not developed. Endosperm absent. Embryo one or often several (polyembryony), straight, well differentiated, oily, chlorophyll? Cotyledons two, large, oily and proteinaceous. Germination phanerocotylar.
Cytology n = 8 (Gymnostoma) to 14 – Agamospermy sometimes occurring.
DNA
Phytochemistry Flavonols (kaempferol, quercetin), biflavonoids, flavones, cyanidin, catechin, pentacyclic triterpenes, ellagic acid, ellagitannins, and proanthocyanidins (prodelphinidins) present. Myricetin, alkaloids, saponins, and cyanogenic compounds not found.
Use Ornamental plants, timber (very hard wood), stabilization of sandy areas (Casuarina equisetifolia, etc.).
Systematics Gymnostoma (18; Malesia to islands in western Pacific), Ceuthostoma (2; C. palawanense: Palawan; C. terminale: Borneo, Halmahera, New Guinea), Casuarina (17; Southeast Asia and eastwards to islands in the western Pacific), Allocasuarina (c 60; Australia).
Casuarinaceae are sister to [Ticodendraceae+Betulaceae].
Gymnostoma is sister to the remaining Casuarinaceae.
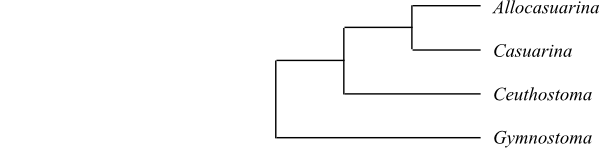 |
Cladogram (simplified) of Casuarinaceae based on DNA sequence data (Sogo & al. 2001). |
FAGACEAE Dumort. |
( Back to Juglandales ) |
Quercaceae Martinov, Tekhno-Bot. Slovar: 525. 3 Aug 1820 [’Quercoides’]; Quercales Bercht. et J. Presl, Přir. Rostlin: 228. Jan-Apr 1820 [‘Quercinae’]
Genera/species 8–10/590–875
Distribution Southern Canada to northwestern South America and Cuba, temperate parts of Europe and Southwest Asia, the Mediterranean, the Himalayas, East Asia to the Russian Far East and Japan, Southeast Asia, Malesia to New Guinea.
Fossils The oldest known flowers of Fagaceae are from the Santonian and fossilized wood is recorded from the Maastrichtian of Mexico. Protofagacea allonensis from the Late Santonian of Georgia in the United States is represented by inflorescences, pollen, fruits and epigynous flowers with six tepals and twelve stamens or three carpels. The cupule is quadrilobate. Fossils from the Cenozoic include a large number of wood, leaves, flowers, pollen and fruits in the Northern Hemisphere. Trigonobalanus is known from the Paleocene to the Oligocene of North America.
Habit Usually monoecious (rarely dioecious), evergreen or deciduous trees or shrubs.
Vegetative anatomy Phellogen ab initio superficial. Vessel elements with simple or scalariform perforation plates; lateral pits opposite, intermediary or alternate, usually simple (sometimes bordered) pits. Vestured pits present. Imperforate tracheary xylem elements libriform fibres? (fibre tracheids?) with simple or bordered pits, usually non-septate (also vasicentric tracheids). Wood rays usually uniseriate (sometimes multiseriate), homocellular or somewhat heterocellular. Axial parenchyma apotracheal diffuse, diffuse-in-aggregates, or banded, or paratracheal scanty, vasicentric, aliform, confluent, reticulate, or unilateral, or absent. Wood elements sometimes storied. Tyloses abundant. Tile cells present in some species. Secondary phloem often? stratified into hard fibrous and soft parenchymatous layers. Sieve tube plastids S type, with non-dispersive P-protein bodies. Nodes 3:3, trilacunar with three leaf traces. Sclereid nests with rhomboid crystals present in bark. Parenchyma sometimes with oil cells and/or mucilage cells. Parenchyma cells sometimes with prismatic calciumoxalate crystals.
Trichomes Hairs unicellular or multicellular, uniseriate or multiseriate, simple or branched, capitate, fasciculate or stellate; glandular hairs?
Leaves Usually alternate (spiral or distichous, rarely verticillate), simple, entire or lobed, often coriaceous, with conduplicate-plicate ptyxis. Stipules usually caducous; leaf sheath absent. Petiole vascular bundle transection annular. Venation pinnate, craspedodromous? (camptodromous when leaf margin entire). Stomata anomocytic or cyclocytic. Cuticular wax crystalloids? Domatia as hair tufts (comata) or absent. Lamina sometimes gland-dotted? Epidermis with or without mucilaginous idioblasts. Hydathodes sometimes present. Leaf margin sinuate, serrate, biserrate or entire.
Inflorescence Axillary, with male flowers solitary or two to c. 30 together in compound, often branched spicate, catkin-like or capitate inflorescence. Female flowers in distichous one- to three-flowered (to seven-flowered) dichasia, each one or several together surrounded by entire or bilobate to more than octalobate cupule, modified shoot consisting of sterile inflorescence parts, on outer side scaly, prickly or with other types of processes, lignified and sometimes stipitate. Cupule with sclereids, crystals and tannins; cupule lobes supernumerary (one more than number of fruits). Female flowers inserted at base of male inflorescence or in specialized axillary inflorescences. Flowers with numerous stamens interpreted as pseudanthia, formed by fusion of dichasia. Presence of extrafloral nectaries reported from leaf buds in species of Quercus.
Flowers Actinomorphic, small. Epigyny. Tepals (sepals?) (four to) six (to nine; sometimes absent), with imbricate aestivation, scale-like, usually more or less connate, in one or two whorls. Nectary absent. Disc absent.
Androecium Stamens four to c. 20 (to more than 90), antesepalous and/or alternisepalous. Filaments filiform, usually free from each other, free from tepals. Anthers dorsifixed or subbasifixed, versatile?, tetrasporangiate, extrorse?, longicidal (dehiscing by longitudinal slits); connective rarely prolonged. Tapetum secretory. Staminodia six to twelve or absent.
Pollen grains Microsporogenesis simultaneous. Pollen grains tricolporate or tricolporoidate (rarely tetracolporate), shed as monads, bicellular at dispersal. Exine tectate, with columellate agranular or granular infratectum, striate, anastomosingly striate, scabrate, verrucate, verruculate, rugulate, microrugulate or smooth. Pollen tubes usually branched.
Gynoecium Pistil composed of (two or) three to six (in Lithocarpus up to 15) connate carpels; carpels alternitepalous or median carpel abaxial. Ovary inferior, (bilocular or) trilocular to sexalocular (to 15-locular), at least in lower part. Stylodia two to six (to nine), entirely or almost entirely free. Stigmas two to six (to nine), capitate, punctate or adaxially decurrent, often with apical pore, non-papillate, Dry type. Male flowers often with pistillodium (in Trigonobalanus as hair tuft, coma).
Ovules Placentation apical to axile. Ovules two per carpel (or locule), anatropous to hemianatropous, pendulous, usually bitegmic (rarely unitegmic), crassinucellar. Micropyle bistomal (Fagus) or endostomal? Outer integument ? cell layers thick, vascularized. Inner integument ? cell layers thick. Suprachalazal tissue massive. Parietal tissue one, four or five cell layers thick. Nucellar cap approx. two cell layers thick. Megasporangium sometimes with tracheids. Megagametophyte monosporous, Polygonum type, with lateral or basal chalazal caecum, into which secondary megagametophyte nucleus migrates and becomes fertilized. Synergids sometimes with a filiform apparatus. Antipodal cells in Castanea multiplicative. Porogamy or chalazogamy. Endosperm development usually nuclear (rarely cellular). Endosperm haustoria? Embryogenesis onagrad. Fertilization very delayed.
Fruit A unilocular to trilocular nutlet, calybium (sometimes with several secondary septa), rounded, two- or three-angular or winged; fruits surrounded by bivalvular to quadrivalvular, often spiny cupule (possibly modified inflorescence; valves possibly modified partial inflorescences). Pericarp tanniniferous, with lignified outer layer. Endocarp usually hairy on inner side.
Seeds Seed pachychalazal. Aril absent. Testa? Tegmen? Perisperm not developed. Endosperm absent. Embryo large, without chlorophyll. Cotyledons two, usually not plicate, starchy (in Fagus plicate, fatty). Germination phanerocotylar (in Fagus and Trigonobalanus s. lat.) or cryptocotylar.
Cytology x = (11, n = 22 in Trigonobalanus) 12 (13, 21) – Polyploidy rarely occurring.
DNA Plastid gene rpl22 transferred from plastid genome to nuclear genome (at least in Quercus present as pseudogene in plastid genome). Plastid gene rps16 absent (lost) in Fagus.
Phytochemistry Dihydroflavonols (kaempferol, quercetin, myricetin), cyanidin, catechins, pentacyclic triterpenes, ellagic and gallic acids, hydrolyzable ellagi- and gallitannins, and condensed tannins (quercite, pentacid alcohol, in Quercus), and proanthocyanidins (prodelphinidins) present. Alkaloids rare. Cyanogenic compounds not found.
Use Ornamental plants, fruits (Castanea sativa), timber, carpentries, barrels, cork (Quercus suber), tanning (tannin from oak galls).
Systematics Fagaceae are sister-group to the remaining Fagales except Nothofagus.
Fagus is sister to all other Fagaceae.
Fagoideae K. Koch, Dendrologie 2(2): 16. Nov 1873 [’Fageae’]
1/10. Fagus (10; temperate regions on the Northern Hemisphere). – Male inflorescence capitate. Pollen grains with finely scabrate exine. Stigma capitate. Micropyle bistomal, elongate. Inner integument thinner than outer. Nucellar cap approx. 13 cell layers thick. Cotyledons plicate. Germination phanerocotylar. Ellagic acid absent.
Quercoideae Irvine, London Fl.: 45. Sep-Dec 1838 [‘Quercineae’]
7–9/580–865. Trigonobalanus (1; T. verticillata; Indochina, Fraser’s Hill on the Malay Peninsula, Mt. Kinabalu on Borneo; incl. Colombobalanus? and Formanodendron?), Formanodendron (1; F. doichangensis; southern Yunnan, northern Thailand; in Trigonobalanus?), Colombobalanus (1; C. excelsa; Colombia; in Trigonobalanus?), Castanea (8; temperate regions on the Northern Hemisphere, eastern Mediterranean to northern Iran), Castanopsis (c 120; tropical and subtropical regions in Asia, with their highest diversity on Borneo), Lithocarpus (100–335; India, Sri Lanka, Southeast Asia, Malesia), Chrysolepis (2; C. chrysophylla, C. sempervirens; western North America), Notholithocarpus (1; N. densiflorus; southwestern Oregon, California), Quercus (350–400; temperate regions on the Northern Hemisphere south to mountains in Malesia and Colombia). – Temperate to tropical regions on the Northern Hemisphere. Inflorescence spicate or catkin-like. Exine with granular infratectum, scabrate, verrucate, verruculate, rugulate or smooth, anastomosingly striate. Stigmas capitate, decurrent, or punctate, with apical pore. Pistillodium sometimes nectar-secreting. Micropyle endostomal? Outer and inner integuments of approx. same length. Parietal tissue one cell layer thick. Nucellar cap approx. two cell layers thick. Cupule often cup-shaped, scaly. Fruit often rounded. Germination cryptocotylar or phanerocotylar.
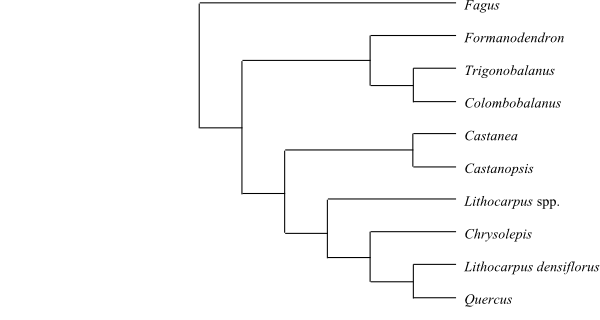 |
Cladogram of Fagaceae based on DNA sequence data (Manos & al. 2001). ‘Lithocarpus’ densiflorus has been transferred to the monospecific Notholithocarpus (as N. densiflorus). |
JUGLANDACEAE DC. ex Perleb |
( Back to Juglandales ) |
Engelhardtiaceae Reveal et Doweld in Novon 9: 552. 30 Dec 1999; Platycaryaceae Nakai ex Doweld in Byull. Mosk. Obshch. Ispyt. Prir., Biol. 105(5): 59. 9 Oct 2000
Genera/species 8/66
Distribution Southeast Europe to the Himalayas and Assam, East Asia to the Russian Far East and northern Vietnam, Taiwan, Southeast Asia to New Guinea, North America, the West Indies, Mexico to Colombia, the Andes, eastern Brazil.
Fossils Fossil wood, leaves, inflorescences, flowers, pollen grains and fruits of Juglandaceae are abundant in Cenozoic layers from the Paleocene onwards in the Northern Hemisphere. Fossil genera from the Palaeogene are, e.g., Alfaropsis, Casholdia, Cruciptera, Hooleya, Paleocarya, Paleooreomunnea, Paleoplatycarya, Paraengelhardia, Polyptera, and Sphaerocarya. Eocene fruit fossils, Alatonucula, similar to Juglandaceae, were found in Patagonia in Argentina (Hermsen & Gandolfo 2016).
Habit Usually monoecious (sometimes dioecious; in Platycarya occasionally bisexual), usually evergreen or deciduous trees (rarely shrubs). Sometimes aromatic. Buds covered by brown hairs, often scaly.
Vegetative anatomy Phellogen ab initio superficial. Medulla usually entire (in species of Cyclocarya, Juglans and Pterocarya septated by diaphragms). Vessel elements with simple or scalariform perforation plates; lateral pits alternate, simple or bordered pits. Imperforate tracheary xylem elements tracheids or fibre tracheids with simple or bordered pits, non-septate (also vasicentric tracheids). Wood rays uniseriate or multiseriate, heterocellular. Axial parenchyma apotracheal diffuse or diffuse-in-aggregates, or paratracheal scanty, aliform, confluent, vasicentric, scalariform, reticulate, unilateral, or banded (rarely absent). Tyloses abundant, thin-walled. Sieve tube plastids S type (without P-protein bodies). Nodes usually 3:3, trilacunar with three leaf traces (sometimes 5:5, pentalacunar with five traces). Secretory cells present or absent. Wood with chains of crystalliferous cells. Calciumoxalate as druses or prismatic or rhomboid crystals.
Trichomes Hairs unicellular or multicellular, uniseriate or multiseriate, simple or branched, fasciculate, multiradiate, stellate, peltate or lepidote; glandular hairs often as resin-producing peltate glandular scales.
Leaves Usually alternate (spiral; in Oreomunnia and Alfaroa opposite), paripinnate or imparipinnate, leaflets entire, with conduplicate or involute ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle transection? Venation pinnate, semicraspedodromous or camptodromous. Stomata anomocytic. Cuticular wax crystalloids? Domatia as pockets or hair tufts, or absent. Mesophyll with idioblasts containing calciumoxalate as druses or single rhomboid crystals. Glands peltate, aromatic, with resin secretions and ethereal oils. Leaflet margins serrate or entire; leaf teeth cunonioid, with splayed, usually glandular apex, main vein of tooth being joined by branches leaving below or one branch proceeding above tooth.
Inflorescence Terminal or lateral, cymes in spicate or catkin-like synflorescence. Male and female inflorescences usually separate (in Platycarya bisexual paniculate inflorescences with central cone-like female inflorescence with male flowers at apex and surrounded by male inflorescences). Male inflorescences solitary or three to eight together; floral prophylls (bracteoles) two, adnate to receptacle (absent in Platycarya). Female inflorescences many-flowered and catkin-like, or two- to many-flowered and spicate; floral prophylls (bracteoles) usually two or three (absent in Alfaroa). Bracts entire or trilobate.
Flowers Actinomorphic, small. Epigyny. Tepals in male flowers one to five (absent in Carya; in Platycarya reduced), whorled, free; in female flowers four, whorled, connate (absent in Carya). Nectary absent. Disc absent.
Androecium Stamens threeto more than 100, in one, antetepalous, or several whorls. Filaments filiform, short, free from each other and from tepals. Anthers basifixed, non-versatile, tetrasporangiate, introrse?, longicidal (dehiscing by longitudinal slits). Tapetum secretory. Staminodia absent.
Pollen grains Microsporogenesis simultaneous. Pollen grains usually monoporate to 37-por(or)ate (in Platycarya with two pseudocolpi on each half), shed as monads, bicellular at dispersal. Exine tectate, with granular infratectum, spinulate.
Gynoecium Pistil composed of two (to four) connate carpels; median carpel adaxial. Ovary inferior, unilocular in upper part, incompletely septate in lower part; primary septum always present, secondary and tertiary septa sometimes present (ovary then bilocular, quadrilocular or octalocular at base). Styles single, simple, or stylodia two, free or slightly connate. Stigma bilobate to quadrilobate, usually with decurrent stigmatic surface, carinal (opposite centre of carpel) or commissural (opposite margins of two connate carpels; in Carya adnate to sepals and forming stigmatic disc), non-papillate, Dry type. Male flowers sometimes with pistillodium.
Ovules Placentation modified axile (on top of incomplete primary septum) to basal. Ovule one per ovary (unilocular in upper part), orthotropous, ascending, unitegmic, crassinucellar. Integument six to ten cell layers thick. Parietal tissue three to eleven cell layers thick. Nucellar cap approx. two cell layers thick. Archespore multicellular. Megagametophyte monosporous, Polygonum type. Chalazogamy or porogamy. Endosperm development ab initio nuclear. Endosperm haustoria? Embryogenesis asterad.
Fruit A single-seeded nut, often with persistent bracts (in Pterocarya with two wings formed by floral prophylls; in Platycarya with two small processes each formed by one floral prophyll and one tepal; in Engelhardia and Oreomunnea with large trilobate wing formed by bract; in Cyclocarya with large circular wing formed by floral prophylls) or drupaceous (nut shell in Alfaroa formed by sepals; in Carya by involucral bract; in Juglans by involucral bract and sepals). Pericarp (endocarp) intrusive.
Seeds Seed pachychalazal, large, lobed. Aril absent. Testa? Perisperm not developed. Endosperm sparse or absent. Embryo large, well differentiated, oily, without chlorophyll. Cotyledons two, large, quadrilobate, strongly plicate, often fleshy, oily. Germination phanerocotylar or cryptocotylar.
Cytology n = 14, 15, 16, 28, 32 (x = 16)
DNA Plastid gene rpl22 absent (lost).
Phytochemistry Flavonols (kaempferol, quercetin, myricetin), flavones, cyanidin, delphinidin, ethereal oils, pentacyclic triterpenes, ellagic acid, galloyl tannins (especially in the bark), cyanogenic compounds, naphthoquinones, and citrullin present. Alkaloids not found. Aluminium accumulated in Carya and Engelhardia. Raffinose and stachyose present in phloem exudates
Use Ornamental plants, seeds and seed oils (Juglans, Carya), medicinal plants, timber, carpentries.
Systematics Juglandaceae are sister to Rhoiptelea (Rhoipteleaceae).
Engelhardioideae are sister-group to the remaining Juglandaceae.
Engelhardioideae Iljinsk. in Bot. Žurn. 75: 793. 11-30 Jun 1990
3/19. ’Engelhardia’ (8; E. apoensis, E. cathayensis, E. hainanensis, E. nudiflora, E. rigida, E. serrata, E. spicata, E. ursina; the Himalayas to China, Taiwan, Southeast Asia to New Guinea; paraphyletic), Oreomunnea (2; O. mexicana, O. pterocarpa; Mexico, Central America), Alfaroa (9; A. columbiana, A. costaricensis, A. guanacastensis, A. guatemalensis, A. hondurensis, A. manningii, A. mexicana, A. roxburghiana, A. williamsii; Mexico, Central America, Colombia). – Eastern Himalayas, China, Taiwan, Southeast Asia to New Guinea, Mexico to Colombia. Buds without bud scales. Foliar parenchyma with druses. Leaves sometimes opposite, pinnate, leaflets usually with entire margin. Bracts trilobate. Floral prophylls (bracteoles) one, two, adnate to lower part of ovary, or absent. Nut with layer of fibrous cells. – Platycarya has male and female flowers (sometimes bisexual flowers) in common spike-like inflorescence, strongly scented flowers, sticky pollen grains, cone-like infructescence, and bracts not forming parts of fruit.
Juglandoideae Eaton, Bot. Dict., ed. 4: 46. Apr-Mai 1836 [‘Juglandeae’]
5/47. Juglans (21; the Mediterranean through temperate Asia to Japan, southeastern Canada and the United States to the Andes in Argentina), Cyclocarya (1; C. paliurus; eastern China), Pterocarya (6; P. fraxinifolia: the Caucasus; P. hupehensis, P. macroptera, P. rhoifolia, P. stenoptera, P. tonkinensis: East and Southeast Asia), Carya (18; East Asia south to northern Vietnam, eastern North America to Central America), Platycarya (1; P. strobilacea; central and eastern China, the Korean Peninsula, Japan, northern Vietnam). – Southeast Europe, northern Turkey to the Himalayas, Assam, East Asia south to northern Vietnam and north to the Russian Far East, Central America, the West Indies, the Andes, eastern Brazil. Buds usually with bud scales. Vessel elements with simple perforation plates. Male flowers with unilobate bracts. Female flowers with entire bracts and floral prophylls (bracteoles) usually lateral and adnate to ovary. Pericarp with sclereids. Endocarp sometimes with lacunae. Outer part of fruit in Carya caducous.
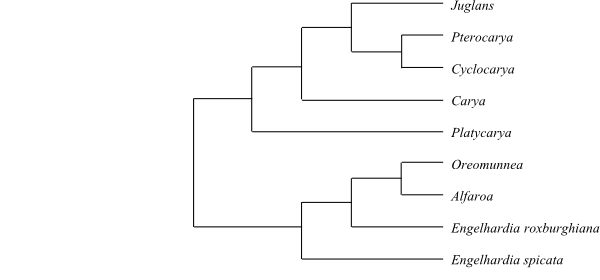 |
Cladogram of Juglandaceae based on DNA sequence data and morphology (Smith & Doyle 1995; Manos & Stone 2001). |
MYRICACEAE A. Rich. ex Kunth |
( Back to Juglandales ) |
Myricales A. Rich. in C. F. P. von Martius, Consp. Regn. Veg.: 12. Sep-Oct 1835 [’Myriceae’]; Canacomyricaceae Baum.-Bod. ex Doweld in Byull. Mosk. Obshch. Ispyt. Prir., Biol. 105(5): 59. 9 Oct 2000
Genera/species 4/c 50
Distribution Western and northern Europe, Macaronesia, tropical and southern Africa, Sri Lanka, the Himalayas, East Asia to Kamchatka and Japan, Southeast Asia, Malesia to New Guinea, New Caledonia, North America, Mexico, Central America, the West Indies, the Andes south to Bolivia and Argentina.
Fossils Flowers, pollen grains and fruits of Myricaceae are known from the Santonian and the Cenomanian (Cretaceous) and abundant in Cenozoic strata. Fossil Comptonia and Myrica have been found in the Neogene of Europe and Asia. Fossil pollen grains of Canacomyrica have been described from Eocene to Miocene layers in New Zealand.
Habit Monoecious, andromonoecious or dioecious, evergreen or deciduous trees or shrubs. Usually aromatic.
Vegetative anatomy Mycorrhiza probably absent. Root nodules containing nitrogen fixing actinobacteria (Frankia) present in most Myricaceae (not found in Canacomyrica). Higher order rootlets clustered, with limited growth (proteoid roots). Phellogen ab initio superficial. Vessel elements with scalariform or simple perforation plates; lateral pits alternate, scalariform or opposite, bordered pits. Imperforate tracheary xylem elements usually tracheids (in Myrica gale fibre tracheids) with simple or bordered pits, non-septate. Wood rays uniseriate or multiseriate, heterocellular. Axial parenchyma usually apotracheal diffuse, diffuse-in-aggregates, or banded (rarely paratracheal scanty). Sieve tube plastids S type (without P-protein bodies). Nodes usually 3:3, trilacunar with three leaf traces (rarely 1:1, unilacunar with one trace). Wood with chains of crystalliferous cells. Prismatic calciumoxalate crystals abundant.
Trichomes Hairs unicellular or multicellular, uniseriate, simple, often vesicular; peltate-lepidote glandular hairs usually secreting waxy aromatic substances; sometimes also multicellular uniseriate hairs with oils.
Leaves Alternate (spiral), simple, usually entire (rarely pinnately lobed), with conduplicate to curved ptyxis. Stipules usually absent (in Comptonia laciniate and foliaceous); leaf sheath absent. Petiole vascular bundle transection arcuate. Venation pinnate, semicraspedodromous or camptodromous. Stomata anomocytic. Cuticular wax crystalloids? Epidermal cells with ethereal oils. Leaf margin serrate or irregularly dentate; leaf teeth cunonioid, with splayed, usually glandular apex, main vein of tooth being joined by branches leaving below or one branch proceeding above tooth.
Inflorescence Axillary, in Canacomyrica simple spicate, in Comptonia, Morella and Myrica branched spicate or catkin-like, or flowers solitary. Floral prophylls (bracteoles) in female flowers two to four, often accrescent and enclosing fruit, or absent.
Flowers Actinomorphic, small. In Canacomyrica epigyny (in Comptonia hypogyny?). Tepals usually absent (in Canacomyrica six, very small, connate in lower part, gradually accrescent and enclosing fruit). Nectary absent. Disc annular, present in male flowers.
Androecium Stamens (one to) four to eight (to c. 20; in Canacomyrica six, antetepalous), in one whorl. Filaments usually free (sometimes connate), free from tepals. Anthers basifixed, non-versatile, tetrasporangiate, extrorse, longicidal (dehiscing by longitudinal slits). Tapetum secretory. Staminodia usually absent (female flowers in Canacomyrica with six epigynous staminodia).
Pollen grains Microsporogenesis simultaneous. Pollen grains usually tripor(or)ate (rarely tetraporate or di- or hexacolpate), shed as monads, bicellular at dispersal. Exine tectate, with granular infratectum, microperforate, coarsely scabrate (Canacomyrica), psilate or microechinate.
Gynoecium Pistil composed of two (or three) connate carpels. Ovary inferior (in Canacomyrica), unilocular. Stylodia two (or three), free or connate at base. Stigmas capitate?, non-papillate, Dry type. Male flowers often with pistillodium (most significant in Canacomyrica).
Ovules Placentation basal. Ovule one per ovary, orthotropous, ascending, usually unitegmic (in Canacomyrica bitegmic, with curved and prolonged micropyle), crassinucellar. Integument three to seven cell layers thick, vascularized. Parietal tissue six to nine cell layers thick. Megagametophyte monosporous, Polygonum type. Pseudoporogamy (pollen tube growth temporarily ceasing on surface of megasporangium). Endosperm development ab initio nuclear. Endosperm haustoria? Embryogenesis? Fertilization delayed.
Fruit Usually a drupe (in Comptonia a nutlet), often covered with outgrowths and sometimes fatty or waxy secretions, sometimes enclosed by floral prophylls (in Comptonia cupular; in Myrica gale modified into two buoyant structures).
Seeds Seed pachychalazal? Aril absent. Testa thickened. Perisperm not developed. Endosperm poorly developed or absent. Embryo straight, well differentiated, oily, chlorophyll? Cotyledons two, plano-convex. Germination phanerocotylar.
Cytology x = 8, 12 – Polyploidy frequently occurring (Myrica gale is a hexaploid in Europe and a dodecaploid in North America).
DNA
Phytochemistry Dihydroflavonols (kaempferol, quercetin, myricetin), ethereal oils, resins, and balsam (with O- and C-methylated flavones; in glandular hairs and epidermal cells), cyanidin, delphinidin, pentacyclic triterpenes (of taraxerane, ursane and lupane type), ellagic acid, and hydrolyzable and condensed tannins present. Alkaloids and cyanogenic compounds not found.
Use Spices (Myrica gale), aromatic waxes, tanninic acid, medicinal plants.
Systematics Myrica (2; M. gale: western and northern Europe, northeastern Siberia, Canada, northern United States; M. hartwegii: Sierra Nevada in California), Comptonia (1; C. peregrina; eastern Canada, eastern United States), Morella (c 45; Macaronesia, tropical and southern Africa, Sri Lanka, the Himalayas, East Asia to Kamchatka and Japan, Southeast Asia, Malesia to New Guinea, southern United States, Mexico, Central America, the West Indies, the Andes south to Bolivia and Argentina), Canacomyrica (1; C. monticola; New Caledonia).
Myricaceae are sister to [Rhoiptelea+Juglandaceae].
Canacomyrica is sister to the remaining Myricaceae, a probable topology being [Canacomyrica+[Morella+[Myrica+Comptonia]]].
The [Comptonia+Myrica] clade is nodulated by the Alnus-infective Frankia strains, whereas the Morella clade is nodulated by the Elaeagnaceae-infective Frankia strains (Huguet & al. 2005).
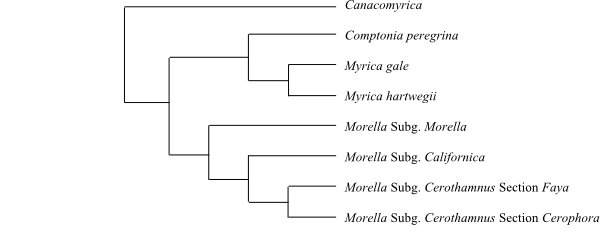 |
Cladogram of Myricaceae based on DNA sequence data (Huguet & al. 2005; Herbert & al. 2006). |
NOTHOFAGACEAE Kuprian. |
( Back to Juglandales ) |
Nothofagales Doweld, Tent. Syst. Plant. Vasc.: xl. 23 Dec 2001
Genera/species 1/37–38
Distribution New Guinea, the D’Entrecasteaux Islands (Goodenough, Normandie), New Britain, eastern and southeastern Australia, Tasmania, New Caledonia, New Zealand, southern South America (including Staten Island) south of 33ºS.
Fossils Nothofagus, mainly pollen grains, has been recorded in Antarctica and subantarctic areas, in southern South America, Australia, New Zealand and Melanesia in strata from the Maastrichtian onwards, and pollen grains of the fossil Nothofagidites, are known from the Early Campanian of South Australia. Nothofagoxylon, wood of probable nothofagaceous origin, has been found in Antarctica.
Habit Monoecious, evergreen or deciduous trees or shrubs. Horizontal lenticels often abundant. Bud scales decussate.
Vegetative anatomy Phellogen ab initio superficial. Vessel elements with simple or scalariform (sometimes reticulate) perforation plates; lateral pits scalariform, opposite or alternate, simple pits. Imperforate tracheary xylem elements libriform fibres with simple pits, septate or non-septate (also vasicentric tracheids). Wood rays uniseriate or multiseriate, homocellular or somewhat heterocellular. Axial parenchyma apotracheal diffuse or narrowly banded, or absent. Wood elements partially storied. Tyloses abundant. Sieve tube plastids S type, with non-dispersive P-protein bodies. Nodes usually 3:3, trilacunar with three leaf traces (in Nothofagus obliqua probably unilacunar). Heartwood with resinous substances? Sclereid nests? Silica bodies present in some species. Prismatic calciumoxalate crystals often frequent.
Trichomes Hairs multicellular, simple, branched or stellate?; glandular hairs peltate-lepidote.
Leaves Alternate (usually distichous, sometimes spiral), simple, entire, often with plicate ptyxis. Stipules usually peltate, caducous, at base surrounding narrowly elongate colleters; leaf sheath absent. Petiole vascular bundle transection? Venation pinnate. Stomata anomocytic. Cuticular wax crystalloids? Domatia? Epidermis with or without mucilage cells? Leaf margin serrate (often biserrate) or entire; leaf teeth compound. Glands large, multicellular (rarely absent).
Inflorescence Axillary, cymose catkin-like, usually composed of one- to three-flowered dichasia; each female inflorescence usually surrounded by (bilobate to) quadrilobate cupule, shoot consisting of sterile inflorescence parts, scaly on abaxial side (cupule in some species of Nothofagus as two small lobes or absent). Flowers with numerous stamens interpreted as pseudanthia, formed as connate dichasia.
Flowers Actinomorphic, small. Epigyny. Tepals in male flowers four to seven, with imbricate aestivation, scale-like, in one whorl, connate; tepals absent in female flowers. Nectary absent. Disc absent.
Androecium Stamens eight to c. 40. Filaments filiform, free from each other and from tepals; point of connection to anther markedly narrowed. Anthers basifixed, non-versatile, tetrasporangiate, extrorse?, longicidal (dehiscing by longitudinal slits); connective usually prolonged. Tapetum secretory. Female flowers sometimes with staminodia.
Pollen grains Microsporogenesis simultaneous. Pollen grains shortly (3–)6–7(–10)-stephanocolpate, shed as monads, bicellular at dispersal. Exine tectate, with granular acolumellate infratectum, loosely spinulate or verrucate.
Gynoecium Pistil composed of two or three connate carpels; median carpel abaxial; terminal flower often with two carpels, lateral flowers often with three carpels. Ovary inferior, bilocular or trilocular. Style single, simple, sometimes short. Stigma lobate, lobes decurrent, non-papillate, Dry type. Male flowers with or without pistillodium?
Ovules Placentation apical to axile. Ovules two per carpel, anatropous, pendulous, unitegmic, crassinucellar. Integument four to seven cell layers thick. Suprachalazal tissue massive. Parietal tissue probably one or two cell layers thick. Nucellar cap absent. Megagametophyte monosporous, Polygonum type, with lateral caecum at base? Porogamy or chalazogamy? Endosperm development ab initio nuclear. Endosperm haustoria? Embryogenesis onagrad?
Fruit A nutlet, calybium, sometimes winged; (one to) three (to seven) fruits surrounded by bivalvular to quadrivalvular usually lamellate cupule. Pericarp partially sclereidal. Endocarp glabrous inside.
Seeds Aril absent. Testa? Perisperm not developed. Endosperm absent. Embryo large, without chlorophyll. Cotyledons two, plicate, oily. Germination phanerocotylar?
Cytology n = 13
DNA
Phytochemistry Flavonols (kaempferol, quercetin, myricetin?), flavanones, flavanonols, catechin, ellagic acid, tannins, proanthocyanidins, and stilbenes present. Alkaloids? Cyanogenic compounds not found.
Use Ornamental plants, timber, carpentries.
Systematics Nothofagus (37–38; New Guinea, the D’Entrecasteaux Islands of Goodenough and Normandie, New Britain, New Caledonia, eastern Queensland, eastern New South Wales, Victoria, Tasmania, New Zealand, temperate Chile and Argentina).
Nothofagaceae are sister-group to the remaining Juglandales.
RHOIPTELEACEAE Hand.-Mazz. |
( Back to Juglandales ) |
Rhoipteleales Novák ex Reveal in Novon 2: 239. 13 Oct 1992
Genera/species 1/1
Distribution Southwestern China to northern Vietnam.
Fossils Campanian to Maastrichtian fossil fruits assigned to Rhoiptelea pontwallensis are known from Central Europe. Pollen grains resembling those in Rhoiptelea have been found in the Maastrichtian of eastern North America, and possibly in Late Cretaceous and Paleocene layers in Europe. Pollen grains, Plicapollis, which are associated with the Late Cretaceous Budvaricarpus and Caryanthus flowers, are similar to the Rhoiptelea pollen. Budvaricarpus and Caryanthus resemble to some degree Rhoiptelea flowers in, e.g., the quadritepalous perianth, the hexamerous androecium and the bicarpellate uniovular gynoecium.
Habit Bisexual or gynomonoecious, deciduous trees. Aromatic. Lenticels abundant. Bud scales absent.
Vegetative anatomy Phellogen? Wood diffusely porose (pits usually solitary). Vessel elements with scalariform perforation plates; lateral pits alternate, bordered pits. Imperforate tracheary xylem elements fibre tracheids with simple pits, non-septate. Wood rays uniseriate or multiseriate, heterocellular. Axial parenchyma apotracheal diffuse or diffuse-in-aggregates, or paratracheal vasicentric or banded. Tyloses abundant. Sieve tube plastids S type (without P-protein bodies?). Nodes 3:3, trilacunar with three leaf traces. Wood with chains of crystalliferous cells? Crystals?
Trichomes Hairs multicellular, uniseriate, simple, brown; glandular hairs aromatic, peltate or cupular, resinous.
Leaves Alternate (distichous), imparipinnate, with ? ptyxis. Stipules asymmetrically caudate, thin, caducous; leaf sheath absent. Petiole vascular bundle transection? Venation pinnate. Stomata anomocytic. Cuticular wax crystalloids? Leaflet margins serrate; leaf teeth with splayed, usually glandular apex, main vein of tooth being joined by branches leaving below or one branch proceeding above tooth.
Inflorescence Axillary, horsetail-like branched panicle with six to eight catkin-like branches. Partial inflorescences composed of dichasia, with one bisexual terminal flower and female or sterile lateral flowers.
Flowers Actinomorphic, small. Hypogyny (probably secondarily). Tepals in bisexual flowers 2+2, persistent in fruit. Nectary absent. Disc absent.
Androecium Stamens six. Filaments short, free from each other and from tepals. Anthers basifixed, non-versatile, tetrasporangiate, introrse?, longicidal (dehiscing by longitudinal slits). Tapetum secretory? Staminodia?
Pollen grains Microsporogenesis simultaneous? Pollen grains tricolporate, shed as monads, bicellular at dispersal. Exine tectate, with columellate to granular infratectum, scabrate, microperforate; exine plicate, without vestibulum between ectexine and endexine.
Gynoecium Pistil composed of two connate carpels; adaxial carpel usually sterile and degenerated. Ovary superior (probably secondarily), bilocular in lower part, unilocular in upper part. Stylodia two, free. Stigmas two, flattened, commissural, recurved, type? Pistillodium?
Ovules Placentation modified axile (on top of incomplete primary septum in one locule). Ovule one per ovary, campylotropous, bitegmic, crassinucellar. Micropyle ?-stomal. Outer integument ? cell layers thick. Inner integument ? cell layers thick. Megagametophyte monosporous, Polygonum type. Chalazogamy or porogamy? Endosperm development ab initio nuclear. Endosperm haustoria? Embryogenesis?
Fruit A one-seeded samaroid nut with persistent perianth. Exocarp covered with brown glands and provided with two wing-like processes.
Seeds Aril absent. Testa hard. Tegmen? Perisperm not developed. Endosperm absent. Embryo straight, oily, chlorophyll? Cotyledons two, thick. Germination?
Cytology n = 16
DNA
Phytochemistry Very insufficiently known. Tannins present (especially in bark).
Use Timber.
Systematics Rhoiptelea (1; R. chiliantha; southwestern China to northern Vietnam).
Rhoiptelea is sister to Juglandaceae.
TICODENDRACEAE Gómez-Laur. et L. D. Gómez |
( Back to Juglandales ) |
Genera/species 1/1
Distribution Mountains in southern Mexico and Central America.
Fossils Fossilized fruits, Ferrignocarpus bivalvis, and pollen grains possibly assignable to Ticodendron, have been found in Eocene layers in Oregon and in the Early Eocene London Clay in England.
Habit Dioecious or polygamodioecious, evergreen tree. Bud scales?
Vegetative anatomy Phellogen? Vessel elements with scalariform or reticulate perforation plates; lateral pits usually scalariform (sometimes opposite or intermediate), simple or bordered pits. Imperforate tracheary xylem elements tracheids with bordered pits, non-septate. Wood rays uniseriate or multiseriate, heterocellular. Axial parenchyma apotracheal diffuse or diffuse-in-aggregates. Secondary phloem not stratified. Sieve tube plastids S type (sieve tubes with non-dispersive P-protein bodies?). Nodes probably 3:3, trilacunar with three leaf traces. Prismatic calciumoxalate crystals abundant. Bark cells with sclereids and rhomboidal crystals.
Trichomes Hairs unicellular, T-shaped; glandular hairs absent.
Leaves Alternate (spiral), simple, entire, coriaceous, with ? ptyxis. Stipules narrowly elongate, intrapetiolar, sheathing, caducous; leaf sheath absent. Petiole vascular bundle transection? Venation pinnate, craspedodromous; almost all leaf teeth directly vascularized by secondary veins. Stomata anomocytic. Cuticular wax crystalloids? Idioblasts (sometimes with druses) present in hypodermis and below midvein. Mesophyll cells with druses of aberrant type. Epidermis with mucilage cells? Leaf margin biserrate; secondary veins proceeding directly into leaf teeth.
Inflorescence Male flowers in terminal or axillary spicate or catkin-like inflorescences, simple or branched (sometimes with terminal female flower), consisting of verticillate dichasia with one to three flowers; each dichasium subtended by one bract. Female flowers solitary (reduced cymes); each female flower surrounded by one bract and subtended by two floral prophylls (bracteoles); floral prophylls of female flowers with axillary groups of vascularized scales.
Flowers Actinomorphic, small. Epigyny. Male flowers without tepals; tepals of female flowers very small and reduced, connate. Nectary absent. Disc absent.
Androecium Stamens eight or ten or more, in two to four whorls surrounded by three caducous bracts. Filaments filiform, free. Anthers basifixed, non-versatile, tetrasporangiate, extrorse?, longicidal (dehiscing by longitudinal slits); connective prolonged at apex. Tapetum secretory. Staminodia usually absent (sometimes present in female flowers).
Pollen grains Microsporogenesis simultaneous? Pollen grains tripor(or)ate, shed as monads, bicellular at dispersal. Exine tectate, with columellate to granular infratectum, microperforate, spinulate.
Gynoecium Pistil composed of two (or three) connate (tangentially orientated?) carpels. Ovary inferior, quadrilocular, with septate locules. Stylodia two (or three), entirely covered by stigmatic areas. Stigmas long, non-papillate, Dry type? Pistillodium usually absent (rarely present in male flowers).
Ovules Placentation apical-axile. Ovule one per carpel, hemitropous, pendulous, unitegmic, crassinucellar. Integument c. 20 to c. 30 cell layers thick, vascularized. Parietal tissue approx. six cell layers thick. Nucellar cap absent. Megagametophyte monosporous, Polygonum type. Chalazogamy. Endosperm development nuclear? Endosperm haustoria? Embryogenesis? Fertilization delayed.
Fruit A single-seeded drupe.
Seeds Aril absent. Testa vascularized. Exotestal cells ab initio radially elongate, all cells thick-walled and tanniniferous. Endotesta? Perisperm not developed. Endosperm thin, two-layered. Embryo large, straight, well differentiated, oily, chlorophyll? Cotyledons two. Germination cryptocotylar.
Cytology n = 13
DNA
Phytochemistry Very insufficiently known. Dihydroflavonols (myricetin) and tannins present. Pentacyclic triterpenes? Ellagic acid?
Use Unknown.
Systematics Ticodendron (1; T. incognitum; southern and southeastern Mexico, Central America, especially eastern mountain slopes in eastern Mexico to central Panamá).
Ticodendron is sister to Betulaceae.
Literature
Abbe EC. 1935. Studies in the phylogeny of the Betulaceae I. Floral and inflorescence anatomy and morphology. – Bot. Gaz. 97: 1-67.
Abbe EC. 1938. Studies in the phylogeny of the Betulaceae II. Extremes in the range of variation of floral and inflorescence morphology. – Bot. Gaz. 99: 431-469.
Abbe EC. 1963. The male flowers and inflorescence of the Myricaceae. – Amer. J. Bot. 50: 632.
Abbe EC. 1972. The inflorescence and flower in male Myrica esculenta var. farquhariana. – Bot. Gaz. 133: 206-213.
Abbe EC. 1974. Flowers and inflorescences of the Amentiferae. – Bot. Rev. 40: 159-261.
Abbe LB, Abbe EC. 1971. The vessel member of Myrica esculenta Buch. Ham. – J. Minnesota Acad. Sci. 37: 72-76.
Acosta MC, Premoli AC. 2010. Evidence of chloroplast capture in South American Nothofagus (subgenus Nothofagus, Nothofagaceae). – Mol. Phylogen. Evol. 54: 235-242.
Akkermans ADL, Roelofsen W, Blom J, Huss-Danell K, Harkink R. 1983. Utilization of carbon and nitrogen compounds by Frankia in synthetic media and in root nodules of Alnus glutinosa, Hippophae rhamnoides, and Datisca cannabina. – Can. J. Bot. 61: 2793-2800.
Akkermans ADL, Hafeez F, Roelofsen W, Chaudhary AH, Baas R. 1983. Ultrastructure and nitrogenase activity of Frankia grown in pure culture and in actinorrhizae of Alnus, Colletia and Datisca. – In: Veeger C, Newton WE (eds), Advances in nitrogen fixation research, Nijhoff/Dr. W. Junk, The Hague, The Netherlands, pp. 311-319.
Aradhya MK, Potter D, Gao F, Simon CJ. 2007. Molecular phylogeny of Juglans (Juglandaceae): a biogeographic perspective. – Tree Gen. Genomes 3 363-378.
Axelrod DI. 1983. Biogeography of oaks in the Arcto-Tertiary province. – Ann. Missouri Bot. Gard. 70: 629-657.
Baas P. 1982. Comparative leaf anatomy of Trigonobalanus Forman (Fagaceae). – Blumea 28: 171-175.
Backer CA. 1951. Myricaceae. – In: Steenis CGGJ van (ed), Flora Malesiana I, 4(3), Noordhoff-Kolff N. V., Batavia, pp. 276-279.
Baird JR. 1969. A taxonomic revision of the plant family Myricaceae of North America, North of Mexico. – Ph.D. diss., University of North Carolina, Chapel Hill, North Carolina.
Barlow BA. 1958. Heteroploid twins and apomixis in Casuarina nana Sieb. – Aust. J. Bot. 6: 204-219.
Barlow BA. 1959a. Chromosome numbers in the Casuarinaceae. – Aust. J. Bot. 7: 230-237.
Barlow BA. 1959b. Polyploidy and apomixis in the Casuarina distyla species group. – Aust. J. Bot. 7: 238-251.
Barlow BA. 1983. Casuarina – a taxonomic and biogeographic review. – In: Midgley SJ, Turnbull JW, Johnston RD (eds), Casuarina ecology, management and utilization, CSIRO, Melbourne, Victoria, pp. 10-18.
Barnett EC. 1944. Keys to the species groups of Quercus, Lithocarpus and Castanopsis of eastern Asia with notes on their distribution. – Trans. Bot. Soc. Edinb. 34: 159-204.
Baumann-Bodenheim MG. 1953. Fagacées de la Nouvelle Calédonie. – Bull. Mus. Natl. Hist. Nat., sér. II, 25: 419-421.
Baumann-Bodenheim MG. 1992. Trisyngyne. Systematik der Flora von Neu-Caledonien (Melanesien-Südpazifik). – Mrs A. L. Baumann, Herrliberg.
Belahbib N, Pemonge M-H, Ouassu A, Sbay H, Kremer A, Petit RJ. 2001. Frequent cytoplasmic exchange between oak species that are not closely related: Quercus suber and Q. ilex in Morocco. – Mol. Ecol. 10: 2003-2012.
Behnke H-D. 1991. Sieve-element characters of Ticodendron. – Ann. Missouri Bot. Gard. 78: 131-134.
Bellarosa R, Delre V, Schirone B, Maggini F. 1990. Ribosomal RNA genes in Quercus spp. (Fagaceae). – Plant Syst. Evol. 172: 127-139.
Benoît LF, Berry AM. 1997. Flavonoid-like compounds from seeds of red alder (Alnus rubra) influence host nodulation by Frankia (Actinomycetales). – Physiol. Plant. 99: 588-593.
Bergero R, Perotto S, Girlanda M, Vidano G, Luppi AM. 2000. Ericoid mycorrhizal fungi are common root associates of a Mediterranean ectomycorrhizal plant (Quercus ilex). – Mol. Ecol. 9: 1639-1649.
Berquam DL. 1975. Floral morphology and anatomy of staminate Juglandaceae. – Ph.D. diss., University of Minnesota, St. Paul, Minnesota.
Berridge EM. 1914. The structure of the flower of the Fagaceae and its bearing on the affinities of the group. – Ann. Bot. 28: 509-526.
Berry AM, McIntyre L, McCully ME. 1986. Fine structure of root hair infection leading to nodulation in the Frankia-Alnus symbiosis. – Can. J. Bot. 64: 292-305.
Blokhina NI. 2004. On some aspects of systematics and evolution of the Engelhardioideae (Juglandaceae) by wood anatomy. – Acta Palaeont. Rom. 4: 13-21.
Bloom RA, Mullin BC, Tate L III. 1989. DNA restriction patterns and DNA-DNA solution hybridization studies of Frankia isolates from Myrica pensylvanica (Bayberry). – Appl. Environm. Microbiol. 55: 2155-2160.
Bloom RA, Lechevalier MP, Tate RL. 1989. Physiological, chemical, morphological, and plant infectivity characteristics of Frankia isolates from Myrica pensylvanica: correlation to DNA restriction patterns. – Appl. Environm. Microbiol. 5: 2161-2166.
Bond G. 1951. The fixation of nitrogen associated with the root nodules of Myrica Gale L., with special reference to its pH relation and ecological significance. – Ann. Bot., N. S., 15: 447-459.
Bond G. 1952. Some features of root growth in nodulated plants of Myrica gale L. – Ann. Bot., N. S., 16: 467-475.
Boodle LA, Worsdell WC. 1894. On the comparative anatomy of the Casuarinaceae, with special reference to the Gnetaceae and Cupuliferae. – Ann. Bot. 8: 231-264.
Bordács S, Popescu F, Slade D, Csaikl UM, Lesur I, Borovcs A, Kézdy P, König AO, Gömoröy D, Brewer S, Burg K, Petit RJ. 2002. Chloroplast DNA variation of white oaks in northern Balkans and in the Carpathian Basin. – For. Ecol. Manag. 156: 197-209.
Bos JAA, Punt W. 1991. Juglandaceae. – Rev. Palaeobot. Palynol. 69: 79-95.
Bousquet J, Lalonde M. 1990. The genetics of actinorhizal Betulaceae. – In: Schwintzer CR, Tjepkema JD (eds), The biology of Frankia and actinorhizal plants, Academic Press, Orlando, pp. 239-261.
Bousquet J, Cheliak WM, Lalonde M. 1987a. Genetic differentiation among 22 mature populations of green alder (Alnus crispa) in central Québec. – Can. J. For. Res. 17: 219-227.
Bousquet J, Cheliak WM, Lalonde M. 1987b. Allozyme variability in natural populations of green alder (Alnus crispa). – Genome 29: 345-352.
Bousquet J, Cheliak WM, Lalonde M. 1987c. Genetic diversity within and among 11 juvenile populations of green alder (Alnus crispa) in Canada. – Physiol. Plant. 70: 311-318.
Bousquet J, Cheliak WM, Lalonde M. 1988. Allozyme variation within and among mature populations of speckled alder (Alnus rugosa) and relationships with green alder (A. crispa). – Amer. J. Bot. 75: 1678-1686.
Bousquet J, Girouard E, Strobeck C, Dancik BP, Lalonde M. 1989. Restriction fragment polymorphisms in the rDNA region among seven species of Alnus and Betula papyrifera. – Plant and Soil 118: 231-240.
Bousquet J, Cheliak MW, Yang J, Lalonde M. 1990. Genetic divergence and introgressive hybridization between Alnus sinuata and A. crispa (Betulaceae). – Plant Syst. Evol. 170: 107-124.
Bousquet J, Strauss SH, Li P. 1992. Complete congruence between morphological and rbcL-based molecular phylogenies in birches and related species (Betulaceae). – Mol. Biol. Evol. 9: 1076-1088.
Brett DW. 1964. The inflorescence of Fagus and Castanea and the evolution of the cupules of the Fagaceae. – New Phytol. 63: 96-118.
Brown RW. 1946. Walnuts from the Late Tertiary of Ecuador. – Amer. J. Sci. 243: 554-556.
Brunner F, Fairbrothers DE. 1979. Serological investigation of the Corylaceae. – Bull. Torrey Bot. Club 106: 97-103.
Burger WC. 1975. The species concept in Quercus. – Taxon 24: 45-50.
Callaham D, Newcomb W, Torrey JG, Peterson RL. 1979. Root hair infection in actinomycete-induced root nodule initiation in Casuarina, Myrica, and Comptonia. – Bot. Gaz. (Suppl.) 140: S1-S9.
Campbell JD. 1985. Casuarinaceae, Fagaceae, and other plant megafossils from Kaikorai Leaf Beds (Miocene) Kaikorai Valley, Dunedin, New Zealand. – New Zealand J. Bot. 23: 311-320.
Campbell JD, Holden AM. 1984. Miocene casuarinacean fossils from Southland and Central Otago, New Zealand. – New Zealand J. Bot. 22: 159-167.
Camus A. 1929. Les chataigniers: monographie des Castanea et Castanopsis. – In: Encyclopédie économique de sylviculture 3, Académie des Sciences, Paris.
Camus A. 1936-1954. Les chênes. Monographie du genre Quercus et du genre Lithocarpus. – In: Encyclopédie économique de sylviculture 6-8, Académie de Sciences, Lechevalier, Paris.
Cannon CH, Manos PS. 2000. The Bornean Lithocarpus Bl. section Synaedrys (Lindley) Barnett (Fagaceae): discussion of its circumscription and description of a new species. – Bot. J. Linn. Soc. 133: 343-357.
Cannon CH, Manos PS. 2001. Combining and comparing morphometric shape descriptors with a molecular phylogeny: the case of fruit type evolution in Bornean Lithocarpus (Fagaceae). – Syst. Biol. 50: 860-880.
Cannon CH, Manos PS. 2003. Phylogeography of the Southeast Asian stone oaks (Lithocarpus). – J. Biogeogr. 30: 211-226.
Carlquist SJ. 1987. Pliocene Nothofagus wood from the Transantarctic Mountains. – Aliso 11: 571-583.
Carlquist SJ. 1991. Wood and bark anatomy of Ticodendron: comments on relationships. – Ann. Missouri Bot. Gard. 78: 96-104.
Carlquist SJ. 2002 [2004]. Wood and bark anatomy of Myricaceae: relationships, generic definitions, and ecological interpretations. – Aliso 21: 7-29.
Carpenter RJ, Bannister JM, Lee DE, Jordan
GJ. 2014. Nothofagus subgenus Brassospora (Nothofagaceae) leaf fossils from
New Zealand: a link to Australia and New Guinea? – Bot. J. Linn. Soc. 174:
503-515.
Chanda S. 1969. A contribution to the palynotaxonomy of Casuarinaceae. – J. Sen Mem. Vol., Bot. Soc. Bengal, Calcutta, pp. 191-208.
Chang C-Y. 1981. Morphology of the family Rhoipteleaceae in relation to its systematic position. – Acta Phytotaxon. Sin. 19: 168-178. [In Chinese with English summary]
Chen G, Sun W-B, Han C-Y, Coombes A. 2007. Karyomorphology of the endangered Trigonobalanus doichangensis (A. Camus) Forman (Fagaceae) and its taxonomic and biogeographical implications. – Bot. J. Linn. Soc. 154: 321-330.
Chen Y, Manchester SR, Song Z, Wang H. 2014. Oligocene fossil winged fruits of tribe Engelhardieae (Juglandaceae) from the Ningming Basin of Guangxi Province, south China. – Intern. J. Latest Res. Sci. Technol. 3: 13-17.
Chen Z-D. 1991. Pollen morphology of the Betulaceae. – Acta Phytotaxon. Sin. 29: 464-475. [In Chinese with English summary]
Chen Z-D. 1994. Phylogeny and phytogeography of the Betulaceae. – Acta Phytotaxon. Sin. 32: 1-32, 101-153. [In Chinese with English summary]
Chen Z-D, Li J-H. 2004. Phylogenetics and biogeography of Alnus (Betulaceae) inferred from sequences of nuclear ribosomal DNA ITS region. – Intern. J. Plant Sci. 165: 325-335.
Chen Z-D, Zhang Z-Y. 1991. A study on foliar epidermis in the Betulaceae. – Acta Phytotaxon. Sin. 29: 156-165. [In Chinese with English summary]
Chen Z-D, Lu A-M, Pan K-Y.1990. The embryology of the genus Ostryopsis (Betulaceae). – Cathaya 2: 53-62.
Chen Z-D, Wang X-Q, Sun H-Y, Han Y, Zhang Z-X, Zou Y-P, Lu A-M. 1998. Systematic position of the Rhoipteleaceae: evidence from nucleotide sequences of rbcL gene. – Acta Phytotaxon. Sin. 36: 1-7.
Chen Z-D, Manchester SR, Sun H-Y. 1999. Phylogeny and evolution of the Betulaceae as inferred from DNA sequences, morphology, and paleobotany. – Amer. J. Bot. 86: 1168-1181.
Chevalier A. 1901. Monographie des Myricacées: anatomie et histologie, organographies, classification et description des espèces, distribution géographique. – Mém. Soc. Sci. Nat. Cherbourg 32: 85-340.
Chevalier A. 1941. Variabilité et hybridité chez les noyers. Notes sur des Juglans peu connus, sur l’Annamocarya et un Carya d’Indochine. – Rev. Bot. Appl. Agric. Trop. 21: 477-509.
Chourey MS. 1974. A study of the Myricaceae from Eocene sediments of southeastern North America. – Palaeontographica, B 146: 88-153.
Christophel DC. 1980. Occurrence of Casuarina megafossils in the Tertiary of south-eastern Australia. – Aust. J. Bot. 28: 249-259.
Christopher RA. 1979. Normapolles and triporate pollen assemblages from the Raritan and Magothy Formations (Upper Cretaceous) of New Jersey. – Palynology 3: 73-121.
Clawson ML, Benson DR. 1999. Natural diversity of Frankia in actinorhizal root nodules from promiscuous hosts in the Myricaceae. – Appl. Environm. Microbiol. 65: 4521-4527.
Clawson ML, Bourret A, Benson DR. 2004. Assessing the phylogeny of Frankia-actinorhizal plant nitrogen-fixing root nodule symbioses with Frankia 16SrRNA and glutamine synthetase gene sequences. – Mol. Phylogen. Evol. 31: 131-138.
Cockayne L. 1926. Monograph on the New Zealand beech forests 1. The ecology of the forests and taxonomy of the beeches. – New Zeland State Forest Bull. 4, Government Printer, Wellington.
Cockayne L, Atkinson E. 1926. On the New Zealand wild hybrids of Nothofagus. – Genetica 8: 1-43.
Codaccioni M. 1962. Recherches morphologiques et ontogénétiques sur quelques cupulifères. – Rev. Cytol. Biol. Vég. 25: 1-208.
Coetzee JA, Praglowski J. 1984. Pollen evidence for the occurrence of Casuarina and Myrica in the Tertiary of South Africa. – Grana 23: 23-41.
Collins RP, Halim AF. 1973. Chemotaxonomy of the Myricaceae II. Essential oil analysis of three Central American species of Myrica. – Lloydia 36: 320-325.
Conde LF, Stone DE. 1970. Seedling morphology in the Juglandaceae: the cotyledonary node. – J. Arnold Arbor. 51: 463-477.
Cook LG, Crisp MD. 2005. Not so ancient: the extant crown group of Nothofagus represents a post-Gondwanan radiation. – Proc. Roy. Soc., Sect. B, 272: 2535-2544.
Cookson IC. 1952. Identification of Tertiary pollen grains with those of New Guinea and New Caledonian beeches. – Nature 170: 127.
Cookson IC, Pike KM. 1955. The pollen morphology of Nothofagus Bl. sub-section Bipartitae Steen. – Aust. J. Bot. 3: 197-206.
Corner EJH. 1990. On Trigonobalanus (Fagaceae). – Bot. J. Linn. Soc. 102: 219-223.
Côte B, Carlson RW, Dawson JO. 1988. Leaf photosynthetic characteristics of seedlings of actinorhizal Alnus spp. and Elaeagnus spp. – Photosynth. Res. 16: 211-218.
Coyne PD. 1983. Specificity between Casuarina species and root nodule organisms. – In: Midgley SJ, Turnbull JW, Johnston RD (eds), Casuarina ecology, management and utilization, CSIRO Publ., Melbourne, pp. 205-210.
Crane PR. 1981. Betulaceous leaves and fruits from the British upper Palaeocene. – Bot. J. Linn. Soc. 83: 103-136.
Crane PR. 1989. Early fossil history and evolution of the Betulaceae. – In: Crane PR, Blackmore S (eds), Evolution, systematics, and fossil history of the Hamamelidae 2: ‘Higher’ Hamamelidae, Syst. Assoc. Special Vol. 40B, Clarendon Press, Oxford, pp. 87-116.
Crane PR, Manchester SR. 1982. An extinct juglandaceous fruit from the Upper Paleocene of southern England. – Bot. J. Linn. Soc. 85: 89-101.
Crane PR, Stockey RA. 1987. Betula leaves and reproductive structures from the middle Eocene of British Columbia. – Can. J. Bot. 65: 2490-2500.
Cranwell LM. 1939. Southern beech pollens. – Rec. Auckland Inst., New Zealand 2: 175-196.
Crepet WL. 1989. History and implications of the early North American fossil record of Fagaceae. – In: Crane PR, Blackmore S (eds), Evolution, systematics, and fossil history of the Hamamelidae 2: ‘Higher’ Hamamelidae, Syst. Assoc. Special Vol. 40B, Clarendon Press, Oxford, pp. 45-66.
Crepet WL, Daghlian CP. 1980. Castaneoid inflorescences from the Middle Eocene of Tennessee and the diagnostic value of pollen (at the subfamily level) in the Fagaceae. – Amer. J. Bot. 67: 739-757.
Crepet WL, Nixon KC. 1989a. Earliest megafossil evidence of Fagaceae: phylogenetic and biogeographic implications. – Amer. J. Bot. 76: 842-855.
Crepet WL, Nixon KC. 1989b. Extinct transitional Fagaceae from the Oligocene and their phylogenetic implications. – Amer. J. Bot. 76: 1493-1505.
Crepet WL, Dilcher DL, Potter FW. 1975. Investigations of angiosperms from the Eocene of North America: a catkin with juglandaceous affinities. – Amer. J. Bot. 62: 813-823.
Crepet WL, Nixon KC, Gandolfo MA. 2004. Fossil evidence and phylogeny: the age of major angiosperm clades based on mesofossil and macrofossil evidence from Cretaceous deposits. – Amer. J. Bot. 91: 1666-1682.
Cutler DF. 1964. Anatomy of vegetative organs of Trigonobalanus Forman (Fagaceae). – Kew Bull. 17: 401-409.
Dadswell HE, Ingle HD. 1954. The wood anatomy of the New Guinea Nothofagus Bl. – Aust. J. Bot. 70: 639-649.
Daghlian CP, Crepet WL. 1983. Oak catkins, leaves and fruits from the Oligocene Catahoula Formation and their evolutionary significance. – Amer. J. Bot. 70: 639-649.
Davey AJ, Gibson CM. 1917. Note on the distribution of sexes in Myrica gale. – New Phytol. 16: 147-151.
Dawson JO, Gordon JC. 1979. Nitrogen fixation in relation to photosynthesis in Alnus glutinosa. – Bot. Gaz. (Chicago) (Suppl.) 140: 270-275.
Del Tredici P. 1977. The buried seeds of Comptonia peregrina, the Sweet Fern. –Bull. Torrey Bot. Club 104: 270-275.
Deng M, Zhou Z-K, chen Y-Q, Sun W-B. 2008. Systematic significance of the development and anatomy of flowers and fruit of Quercus schottkyana (subgenus Cyclobalanopsis: Fagaceae). – Intern. J. Plant Sci. 169: 1261-1277.
Deng M, Li Q, Yang S, Liu Y-C, Xu J. 2013. Comparative morphology of leaf epidermis in the genus Lithocarpus and its implication in leaf epidermal feature evolution in Fagaceae. – Plant Syst. Evol. 299: 659-681.
Dengler NG, MacKay LB. 1975. The leaf anatomy of beech, Fagus grandifolia. – Can. J. Bot. 53: 2202-2211.
Denk T. 2002. Revision of Tertiary Fagus cupules from Russia, Transcaucasia and western Siberia. – Feddes Repert. 113: 193-210.
Denk T. 2003. Phylogeny of Fagus L. (Fagaceae) based on morphological data. – Plant Syst. Evol. 240: 55-81.
Denk T, Beller B. 2001. Systematic significance of the cupule/nut complex in living and fossil Fagus. – Intern. J. Plant Sci. 162: 869-897.
Denk T, Grimm GW. 2009. Significance of pollen characteristics for infrageneric classification and phylogeny of Quercus (Fagaceae). – Intern. J. Plant Sci. 170: 926-940.
Denk T, Grimm GW. 2010. The oaks of western Eurasia: traditional classifications and evidence from two nuclear markers. – Taxon 59: 351-366.
Denk T, Grimm GW, Stogerer K, Langer M, Hemleben V. 2002. The evolutionary history of Fagus in western Eurasia: evidence from genes, morphology and the fossil record. – Plant Syst. Evol. 232: 213-236.
Denk T, Grímsson F, Zetter R. 2012. Fagaceae from the early Oligocene of Central Europe: persisting new world and emerging old world biogeographic links. – Rev. Palaeobot. Palynol. 169: 7-20.
Dettmann ME, Pocknall DT, Romero EJ, Zamaloa MC. 1990. Nothofagites Erdtman ex Potonié, 1960; a catalogue of species with notes on the paleogeographic distribution of Nothofagus Bl. (southern beech). – New Zealand Geol. Surv. Paleontol. Bull. 60: 1-79.
Diem HG, Gauthier D, Dommerges YR. 1983. An effective strain of Frankia from Casuarina sp. – Can. J. Bot. 61: 2815-2821.
Dilcher DL, Manchester SR. 1986. Investigations of angiosperms from the Eocene of North America: leaves of the Engelhardieae (Juglandaceae). – Bot. Gaz. 147: 189-199.
Dilcher DL, Potter FW Jr, Crepet WL. 1976. Investigations of angiosperms from the Eocene of North America: Juglandaceous winged fruits. – Amer. J. Bot. 63: 532-544.
Dilcher DL, Christophel DC, Bhagwandin Jr HO, Scriven LJ. 1990. Evolution of the Casuarinaceae: morphological comparisons of some extant species. – Amer. J. Bot. 77: 338-355.
Donoghue MJ, Doyle JA. 1989. Phylogenetic analysis of angiosperms and the relationships of the Hamamelidae. – In: Crane PR, Blackmore S (eds), Evolution, systematics, and fossil history of the Hamamelidae 1: Introduction and ‘lower’ Hamamelidae, Syst. Assoc. Spec. Vol. 40A, Clarendon Press, Oxford, pp. 17-45.
Doyle JA, Manchester SR, Sauquet H. 2008. A seed related to Myristicaceae in the Early Eocene of Southern England. – Syst. Bot. 33: 636-646.
Dumolin-Lapegue S, Demesure B, Fineschi S, Lecorre V, Petit RJ. 1997. Phylogeographic structure of white oaks throughout the European continent. – Genetics 146: 1475-1487.
Dumolin-Lapègue S, Pemonge MH, Petit RJ. 1998. Association between chloroplast and mitochondrial lineages in oaks. – Mol. Biol. Evol. 15: 1321-1331.
Dumolin-Lapègue S, Kremer A, Petit RJ. 1999. Are chloroplast and mitochondrial DNA variation species independent in oaks? – Evolution 53: 1406-1413.
Dunbar A, Rowley JR. 1984. Betula pollen development before and after dormancy: exine and intine. – Pollen Spores 26: 299-338.
Elliott LL, Mindell RA, Stockey RA. 2006. Beardia vancouverensis gen. et spec. nov. (Juglandaceae): permineralized fruits from the Eocene of British Columbia. – Amer. J. Bot. 93: 557-565.
Endress PK. 1967. Systematische Studie über die verwandtschaftlichen Beziehungen zwischen den Hamamelidaceen und Betulaceen. – Bot. Jahrb. Syst. 87: 431-525.
Endress PK. 1977. Evolutionary trends in the Hamamelidales-Fagales group. – In: Kubitzki K (ed), Flowering plants: evolution and classification of higher categories, Plant Syst. Evol. [Suppl.] 1: 321-347.
Endress PK. 1986. An entomophily syndrome in Juglandaceae: Platycarya strobilacea. – Veröff. Geobot. Inst. ETH Stiftung Rübel, Zürich 87: 100-111.
Engler A. 1889a. Casuarinaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(1), W. Engelmann, Leipzig, pp. 16-19.
Engler A. 1889b. Juglandaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(1), W. Engelmann, Leipzig, pp. 19-25.
Engler A. 1889c. Myricaceae. – In: Engler A, Prantl K (eds),Die natürlichen Pflanzenfamilien III(1), W. Engelmann, Leipzig, pp. 26-28.
Engler A. 1889d. Leitneriaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(1), W. Engelmann, Leipzig, pp. 28-29.
Erdogan V, Mehlenbacher SA. 2000. Phylogenetic relationships of Corylus species (Betulaceae) based on nuclear ribosomal DNA ITS region and chloroplast matK gene sequences. – Syst. Bot. 25: 727-737.
Erdtman G. 1967. On the pollen morphology of Trigonobalanus (Fagaceae). – Bot. Not. 120: 324-333.
Espinosa MR. 1926. Nota preliminar sobre dos especies nuevas chilenas del género Nothofagus Blume. – Rev. Chilena Hist. Nat. 30: 268.
Espinosa MR. 1928. Dos especies nuevas de Nothofagus. – Rev. Chilena Hist. Nat. 32: 171-197.
Ferguson DK. 1998. The contribution of micromorphology to the taxonomy and fossil record of the Myricaceae. – Taxon 47: 333-335.
Ferris C, Oliver RP, Davy AJ, Hewitt GM. 1993. Native oak chloroplasts reveal an ancient divide across Europe. – Mol. Ecol. 2: 337-344.
Ferris C, Oliver RP, Davy AJ, Hewitt GM. 1995. Using chloroplast DNA to trace post-glacial migration routes of oaks into Britain. – Mol. Ecol. 4: 731-738.
Feuer S. 1991. Pollen morphology and the systematic relationships of Ticodendron incognitum. – Ann. Missouri Bot. Gard. 78: 143-151.
Fey BS. 1981. Untersuchungen über Bau und Ontogenese der Cupula, Infloreszenzen, und Blüten sowie zur Embryologie bei Vertretern der Fagaceae und ihre Bedeutung für die Systematik. – Ph.D. diss., Universität Zürich, Switzerland.
Fey BS, Endress PK. 1983. Development and morphological interpretation of the cupule in Fagaceae. – Flora 173: 451-468.
Fineschi S, Taurchini D, Grossoni P, Petit RJ, Vendramin GG. 2002. Chloroplast DNA variation of white oaks in Italy. – For. Ecol. Manag. 156:103-114.
Fjellstrom RG, Parfitt DE. 1994. Walnut (Juglans spp.) genetic diversity determined by restriction fragment length polymorphisms. – Genome 37: 690-700.
Fjellstrom RG, Parfitt DE. 1995. Phylogenetic analysis and evolution of the genus Juglans (Juglandaceae) as determined from nuclear genome RFLPs. – Plant Syst. Evol. 197: 19-32.
Fletcher WW. 1955. The development and structure of the root-nodules of Myrica gale with special reference to the nature of the endophyte. – Ann. Bot., N. S., 19: 501-573.
Flores EM. 1980. Shoot vascular system and phyllotaxis of Casuarina (Casuarinaceae). – Amer. J. Bot. 67: 131-140.
Flores EM, Moseley MF. 1982. The anatomy of the pistillate inflorescence and flower of Casuarina verticillata Lamarck (Casuarinaceae). – Amer. J. Bot. 69: 1673-1684.
Forest F, Savolainen V, Chase MW, Lupia R, Bruneau A, Crane PR. 2005. Teasing apart molecular- versus fossil-based error estimates when dating phylogenetic trees: a case study in the birch family (Betulaceae). – Syst. Bot. 30: 118-133.
Forman LL. 1962. A new genus in the Fagaceae. – Taxon 11: 139-140.
Forman LL. 1964a. Trigonobalanus, a new genus of Fagaceae, with notes on the classification of the family. – Kew Bull. 17: 381-396.
Forman LL. 1964b. Trigonobalanus and its importance in the taxonomy of the Fagaceae. – Proc. Roy. Soc. London, Ser. B, Biol. Sci. 161: 48-49.
Forman LL. 1966a. On the evolution of cupules in the Fagaceae. – Kew Bull. 18: 385-419.
Forman LL. 1966b. Generic delimitation in the Castaneoideae. – Kew Bull. 18: 421-426.
Frascaria N, Maggia L Michaud M, Bousquet J. 1993. The rbcL gene sequence from chestnut indicates a slow rate of evolution in the Fagaceae. – Genome 36: 668-671.
Frenguelli J. 1943. Restos de Casuarina en el Mioceno de el Mirador Patagonia Central. – Notas Mus. La Plata 8 (Palaeontol. 56): 349-354.
Friis EM. 1983. Upper Cretaceous (Senonian) floral structures of juglandalean affinity containing Normapolles pollen. – Rev. Palaeobot. Palynol. 39: 161-188.
Friis EM, Pedersen KR, Schönenberger J. 2003. Endressianthus, a new Normapolles-producing plant genus of fagalean affinity from the Late Cretaceous of Portugal. – Intern. J. Plant Sci. 164(Suppl.): S201-S223.
Froggatt WW. 1933. The Coccidae of the Casuarinas. – Proc. Linn. Soc. New South Wales 58: 363-374.
Fujii N, Tomaru N, Okuyama K, Koike T, Mikami T, Ueda K. 2002. Chloroplast DNA phylogeography of Fagus crenata (Fagaceae) in Japan. – Plant Syst. Evol. 232: 21-33.
Funk DT. 1979. Black walnuts for nuts and timber. – In: Jaynes RA (ed), Nut tree culture in North America, Northern Nut Growers Assoc., Hamden, Connectitut, pp. 51-73.
Furlow JJ. 1979. The systematics of the American species of Alnus (Betulaceae). – Rhodora 81: 1-121, 151-248.
Furlow JJ. 1987a. The Carpinus caroliniana complex in North America I. A multivariate analysis of geographical variation. – Syst. Bot. 12: 21-40.
Furlow JJ. 1987b. The Carpinus caroliniana complex in North America II. Systematics. – Syst. Bot. 12: 416-434.
Gauthier G, Diem HG, Dommergues Y. 1983. Preliminary results of research on Frankia and endomycorrhizae associated with Casuarina equisetifolia. – In: Midgley SJ, Turnbull JW, Johnston RD (eds), Casuarina ecology, management and utilization, CSIRO Publ., Melbourne, pp. 211-217.
Germain E. 1994. The reproduction of hazelnut (Corylus avellana L.): a review. – Acta Horticult. 351: 195-209.
Gladkova VN. 1962. Fragments of the history of the Myricaceae family. – Pollen Spores 4: 345.
Gleeson SK. 1982. Heterodichogamy in walnuts: inheritance and stable ratios. – Evolution 36: 892-903.
Góczán F, Groot JJ, Krutzsch W, Pacltová B. 1967. Die Gattungen des “Stemma Normapolles Pflug 1953b” (Angiospermae) – Neubeschreibungen und Revision europäischer Formen (Oberkreide bis Eozän). – Paläontol. Abhandl. B, 2: 427-633.
Gómez-Laurito J, Gómez PL. 1989. Ticodendron: a new tree from Central America. – Ann. Missouri Bot. Gard. 76: 1148-1151.
Gomory D, Paule L, Vysny J. 2007. Patterns of allozyme variation in western Eurasian Fagus. – Bot. J. Linn. Soc. 154: 165-174.
Gonzalez-Villarreal LM. 2003a. Quercus tuitensis (Fagaceae, Quercus sect. Lobatae), a new deciduous oak from western Jalisco, Mexico. – Brittonia 55: 42-48.
Gonzalez-Villarreal LM. 2003b. Two new species of oak (Fagaceae, Quercus sect. Lobatae) from the Sierra Madre del Sur, Mexico. – Brittonia 55: 49-60.
Govaerts R, Frodin DG. 1998. World checklist and bibliography of Fagales (Betulaceae, Corylaceae, Fagaceae and Ticodendraceae). – Royal Botanic Gardens, Kew.
Grauke LJ, Wood B, Payne J. 1991. Genetic resources of Carya in Vietnam and China. – Ann. Rep. North. Nut Growers’ Assoc. 82: 80-87.
Grimsson F, Zetter R, Grimm G, Pedersen G,
Pedersen A, Denk T. 2015. Fagaceae
pollen from the early Cenozoic of West Greenland: revisiting Engler’s and
Chaney’s Arcto-Tertiary hypotheses. – Plant Syst. Evol. 301: 809-832.
Guillaumin A. 1939. La presence inattendue d’une Myricacée en Nouvelle-Calédonie. – Compt. Rend. Acad. Sci. Paris, 2ème sem. T209 no 4: 233-234.
Guillaumin A. 1940. Matériaux pour la flore de la Nouvelle-Calédonie LVII. La presence d’une Myricacée. – Bull. Soc. Bot. France 87: 299-300.
Gunter LE, Kochert G, Giannasi DE. 1994. Phylogenetic relationships of the Juglandaceae. – Plant Syst. Evol. 192: 11-29.
Haase P. Genetic relationships and inferred evolutionary divergence in the New Zealand taxa of Nothofagus – results from isozyme analysis. – Aust. Syst. Bot. 6: 47-55.
Håkansson A. 1955. Endosperm formation in Myrica gale L. – Bot. Not. 108: 6-16.
Halim AF, Collins RP. 1973. Essential oil analysis of the Myricaceae of the eastern United States. – Phytochemistry 12: 1077-1083.
Hall JW. 1952. The comparative anatomy and phylogeny of the Betulaceae. – Bot. Gaz. (Crawfordsville) 113: 235-270.
Hammel BE, Burger WG. 1991. Neither oak nor alder, but nearly: the history of Ticodendraceae. – Ann. Missouri Bot. Gard. 78: 89-95.
Handel-Mazzetti H. 1932. Rhoipteleaceae: eine neue Familie der Monochlamydeen. – Feddes Repert. 30: 75-80.
Hanks SL, Fairbrothers DE. 1976. Palynotaxonomic investigation of Fagus L. and Nothofagus Bl.: light microscopy, scanning electron microscopy, and computer analysis. – In: Heywood VH (ed), Botanical Systematics 1, Academic Press, London, pp. 1-142.
Hans AS. 1970. Chromosome numbers in the Juglandaceae. – J. Arnold Arbor. 51: 534-539.
Hardin JW. 1976. Terminology and classification of Quercus trichomes. – J. Elisha Mitchell Sci. Soc. 92: 151-161.
Hardin JW, Bell JM. 1986. Atlas of foliar surface features in woody plants IX. Betulaceae of eastern United States. – Brittonia 38: 133-144.
Hardin JW, Stone DE. 1984. Atlas of foliar surface features in woody plants VI. Carya (Juglandaceae) of North America. – Brittonia 36: 140-153.
Heenan PB, Smissen RD. 2013. Revised circumscription of Nothofagus and recognition of the segregate genera Fuscospora, Lophozonia, and Trisyngyne (Nothofagaceae). – Phytotaxa 146: 1-31.
Heimsch C, Wetmore RH. 1939. The significance of wood anatomy in the taxonomy of the Juglandaceae. – Amer. J. Bot. 26: 651-660.
Herbert J, Chase MW, Möller M, Abbott RJ. 2006. Nuclear and plastid DNA sequences confirm the placement of the enigmatic Canacomyrica monticola in Myricaceae. – Taxon 55: 349-357.
Herendeen PS, Crane PR, Drinnan AN. 1995. Fagaceous flowers, fruits and cupules from the Campanian (Late Cretaceous) of Central Georgia, U.S.A. – Intern. J. Plant Sci. 156: 93-116.
Heřmanová Z, Kvaček J, Friis EM. 2011. Budvaricarpus serialis Knobloch & Mai, an unusual new member of Normapolles complex from the Late Cretaceous of the Czech Republic. – Intern. J. Plant Sci. 172: 285-293.
Hermsen EJ, Gandolfo MA. 2016. Fruits of Juglandaceae from the Eocene of South America. – Syst. Bot. 41: 316-328.
Herrera F, Manchester SR, Koll R, Jaramillo C. 2014. Fruits of Oreomunnea (Juglandaceae) in the early Miocene of Panama. – In: Stevens WD, Montiel OM, Raven PH (eds), Paleobotany and biogeography, a Festschrift for Alan Graham in his 80th year, Missouri Botanical Garden Press, St. Louis, Missouri, pp. 124-133.
Hess WJ, Stoynoff NA. 1998. Taxonomic status of Quercus acerifolia (Fagaceae) and a morphological comparison of four members of the Quercus shumardii complex. – Syst. Bot. 23: 89-100.
Hewson HJ. 1989a. Betulaceae. – In: George AS (ed), Flora of Australia 3, Australian Government Publ. Service, Canberra, pp. 96.
Hewson HJ. 1989b. Fagaceae. – In: George AS (ed), Flora of Australia 3, Australian Government Publ. Service, Canberra, pp. 97-100.
Hickey LJ, Taylor DW. 1991. The leaf architecture of Ticodendron and the application of foliar characters in discerning its relationships. – Ann. Missouri Bot. Gard. 78: 105-130.
Hill RS. 1991. Tertiary Nothofagus (Fagaceae) macrofossils from Tasmania and Antarctica and their bearing on the evolution of the genus. – Bot. J. Linn. Soc. 105: 73-112.
Hill RS. 1992. Nothofagus: evolution from a southern perspective. – Trends Ecol. Evol. 7: 190-194.
Hill RS. 1994. Nothofagus smithtonensis (Nothofagaceae), a new macrofossil species from Oligocene sediments in Northwest Tasmania, Australia, and its phylogenetic significance. – Rev. Paleobot. Palynol. 80: 115-121.
Hill RS. 1996. The riddle of unique Southern Hemisphere Nothofagus on south-west Pacific islands: its challenge to biogeographers. – In: Keast A, Miller SE (eds), The origin and evolution of Pacific island biotas, New Guinea to eastern Polynesia: patterns and processes, SPB Academic Publ., Amsterdam, pp. 247-260.
Hill RS. 2001. Biogeography, evolution and palaeoecology of Nothofagus (Nothofagaceae): the contribution of the fossil record. – Aust. J. Bot. 49: 321-332.
Hill RS, Dettman ME. 1996. Origin and diversification of the genus Nothofagus. – In: Veblen TT, Hill RS, Read J (eds), The ecology and biodiversity of Nothofagus forest, Yale University Press, New Haven, Connecticut, pp. 11-23.
Hill RS, Jordan GJ. 1993. The evolutionary history of Nothofagus (Nothofagaceae). – Aust. Syst. Bot. 6: 111-126.
Hill RS, Read J. 1991. A revised infrageneric classification of Nothofagus (Fagaceae). – Bot. J. Linn. Soc. 105: 37-72.
Hill RS, Jordan GJ, Macphail MK. 2015. Why we should retain Nothofagus sensu lato. – Aust. Syst. Bot. 28: 190-193.
Hjelmquist H. 1948. Studies on the floral morphology and phylogeny of the Amentiferae. – Bot. Not. Suppl. 2(1): 1-171.
Hjelmquist H. 1957. Some notes on the endosperm and embryo development in Fagales and related orders. – Bot. Not. 110: 173-195.
Hjelmquist H. 1963. Some notes on Nothofagus from New Guinea and New Caledonia. – Bot. Not. 116: 225-237.
Hocher V, Alloisio N, Auguy F, Fournier P, Doumas P, Pujic P, Gherbi H, Queiroux C, Da Silva C, Wincker P, Normand P, Bogusz D. 2011. Transcriptomics of actinorhizal symbioses reveals homologs of the whole common symbiotic signaling cascade. – Plant Physiol. 156: 700-711.
Hope GS. 1996. History of Nothofagus in New Guinea and New Caledonia. – In: Veblen TT, Hill RS, Read J (eds), The ecology and biogeography of Nothofagus forest, Yale University Press, New Haven, Connecticut, pp. 257-270.
Hou D. 1971. Chromosome numbers of Trigonobalanus verticillata Forman (Fagaceae). – Acta Bot. Neerl. 20: 543-549.
Huang Y-L, Tsujita T, Tanaka T, Matsuo Y, Kouno I, Li D-P, Nonaka G-I. 2011. Triterpene hexahydroxydiphenoyl esters and a quinic acid purpurogallin carbonyl ester from the leaves of Castanopsis fissa. – Phytochemistry 72: 2006-2014.
Hubert F, Grimm GW, Jousselin E, Berry V,
Franc A, Kremer A. 2014. Multiple nuclear genes stabilize the phylogenetic
backbone of the genus Quercus. – Syst. Biodivers. 12: 405-423.
Huguet V, McCray Batzli J, Zimpfer JF; Normand P, Dawson JO, Fernandez MP. 2001. Diversity and specificity of Frankia strains in nodules of sympatric Myrica gale, Alnus incana, and Shepherdia canadensis determined by rrs gene polymorphism. – Appl. Enironm. Microbiol. 67: 2116-2122.
Huguet V, Gouy M, Normand P, Zimpfer JF, Fernandez MP. 2005. Molecular phylogeny of Myricaceae: a re-examination of host-symbiont specificity. – Mol. Phylogen. Evol. 34: 557-568.
Humphries CJ. 1981. Biogeographical methods and the southern beeches (Fagaceae: Nothofagus). – In: Funk VA, Brooks DR (eds), Biogeographical methods and the southern beeches (Fagaceae: Nothofagus), New York Botanical Garden, Bronx, New York, pp. 177-207.
Humphries CJ, Cox JM, Nielsen ES. 1986. Nothofagus and its parasites: a cladistic approach to co-evolution. – In: Stone AR, Hawksworth DL (eds), Coevolution and systematics, Clarendon Press, Oxford, pp. 53-86.
Hurd TM, Schwintzer CR. 1997. Formation of cluster roots and mycorrhizal status of Comptonia peregrina and Myrica pensylvanica in Maine, USA. – Physiol. Plant. 99: 680-689.
Hwang R, Conran JG. 2000. Seedling characteristics in the Casuarinaceae. – Telopea 8: 429-439.
Iljinskaya IA. 1953. Monograph of the genus Pterocarya Kunth. – Trudy Bot. Inst. Akad. Nauk SSSR, Ser. 1, Fl. Sist. Vysš. Rast. 10: 1-127. [In Russian]
Iljinskaja IA. 1990. On the taxonomy and phylogeny of the family Juglandaceae. – Bot. Žurn. 75: 792-803. [In Russian with English summary]
Iljinskaja IA. 1993. Alfaropsis, a new genus of the Juglandaceae. – Bot. Žurn. 78: 79-83. [In Russian with English summary]
Jacobs M. 1960. Juglandaceae. – In: Steenis CGGJ van (ed), Flora Malesiana, I, 6, Noordhoff, Leyden, pp. 143-154.
Jäger EJ. 1980. Progressionen im Synfloreszenzbau und in der Verbreitung bei den Betulaceen. – Flora 170: 91-113.
Jähnichen H, Mai DH, Walther H. 1977. Blätter und Früchte von Engelhardia Lesch. ex Bl. (Juglandaceae) aus dem europäischen Tertiär. – Feddes Repert. 88: 323-363.
Jähnichen H, Friedrich WL, Takáč M. 1984. Engelhardioid leaves and fruits from the European Tertiary, part II. – Tertiary Res. 6: 109-134.
Jaynes RA. 1979. Nut tree culture in North America. – Northern Nut Growers Association, Hamden, Connecticut.
Jenkins R. 1993. The origin of the fagaceous cupule. – Bot. Rev. 59: 81-111.
Jeong SC, Ritchie NJ, Myrold DD. 1999. Molecular phylogenies of plants and Frankia support multiple origins of actinorhizal symbioses. – Mol. Phylogen. Evol. 13: 493-503.
Jiang Z-H, Zhou R-H. 1990. An analysis of fatty acids in the seeds of Rhoipteleaceae and the implication for its systematic position. – J. Chin. Folk Mater. Med. 1990: 33-35. [In Chinese]
Jin J. 2009. Two Eocene fossil fruits from the Changchang Basin of Hainan Island, China. – Rev. Palaeobot. Palyn. 153: 150-152.
Johnson LAS. 1980. Notes on Casuarinaceae. – Telopea 2: 83-84.
Johnson LAS. 1982. Notes on Casuarinaceae II. – J. Adelaide Bot. Gard. 6: 73-87.
Johnson LAS. 1988. Notes on Casuarinaceae III: the new genus Ceuthostoma. – Telopea 3: 133-137.
Johnson LAS, Morris DI. 1994. Allocasuarina duncanii, a new species in Allocasuarina section Cylindropitys (Casuarinaceae). – Telopea 5: 793-794.
Johnson LAS, Wilson KL. 1989. Casuarinaceae: a synopsis. – In: Crane PR, Blackmore S (eds), Evolution, systematics, and fossil history of the Hamamelidae 2, ‘Higher’ Hamamelidae, Syst. Assoc. Special Vol. 40B, Clarendon Press, Oxford, pp. 167-188.
Johnson LAS, Wilson KL. 1993. Casuarinaceae. – In: Kubitzki K, Rohwer JG, Bittrich V (eds), The families and genera of vascular plants II. Flowering plants. Dicotyledons. Magnoliid, hamamelid and caryophyllid families, Springer, Berlin, Heidelberg, New York, pp. 237-242.
Jones JH. 1986. Evolution of the Fagaceae: the implications of foliar features. – Ann. Missouri Bot. Gard. 73: 228-275.
Jordan GJ. 2000. A new early Pleistocene species of Nothofagus and the climatic implications of co-occurring Nothofagus fossils. – Aust. Syst. Bot. 12: 757-765.
Jordan GJ, Hill RS. 1999. The phylogenetic affinities of Nothofagus (Nothofagaceae) leaf fossils based on combined molecular and morphological data. – Intern. J. Plant Sci. 160: 1177-1188.
Kamiya K, Harada K, Clyde MM, Mohamed AL. 2002. Genetic variation of Trigonobalanus verticillata, a primitive species of Fagaceae, from Malaysia revealed by chloroplast sequences and AFLP markers. – Genes & Genet. Syst. 77: 177-186.
Kato H, Oginuma K, Gu Z, Hammel B, Tobe H. 1998. Phylogenetic relationships of Betulaceae based on matK sequences with particular reference to the position of Ostryopsis. – Acta Phytotax. Geobot. 49: 89-97.
Kaul RB. 1985. Reproductive morphology of Quercus (Fagaceae). – Amer. J. Bot. 72: 1962-1977.
Kaul RB. 1986. Evolution and reproductive biology of inflorescence in Lithocarpus, Castanopsis, Castanea, and Quercus (Fagaceae). – Ann. Missouri Bot. Gard. 73: 284-296.
Kaul RB. 1987. Reproductive structure of Lithocarpus sensu lato (Fagaceae): cymules and fruits. – J. Arnold Arbor. 68: 73-104.
Kaul RB. 1988. Cupular structure in paleotropical Castanopsis (Fagaceae). – Ann. Missouri Bot. Gard. 75: 1480-1498.
Kaul RB. 1989. Fruit structure and ecology in paleotropical Lithocarpus (Fagaceae). – In: Crane PR, Blackmore S (eds), Evolution, systematics, and fossil history of the Hamamelidae 2, ‘Higher’ Hamamelidae, Syst. Assoc. Special Vol. 40B, Clarendon Press, Oxford, pp. 67-86.
Kaul RB, Abbe EC. 1984. Inflorescence architecture and evolution in the Fagaceae. – J. Arnold Arbor. 65: 375-401.
Kedves M, Pittau P. 1979. Contribution à la connaissance des pollens des Normapolles du Crétacé Supérieur du Portugal. – Pollen Spores 21: 169-209.
Kedves M, Uri-Kiss I. 1968. Études comparatives sur les pollens du genre Alnus de Tertiaire de Hongrie. – Acta Bot. Acad. Sci. Hungaricae 14: 315-321.
Kershaw AP. 1970. Pollen morphological variation within the Casuarinaceae. – Pollen Spores 12: 145-161.
Kershaw EM. 1909. The structure and development of the ovule of Myrica gale. – Ann. Bot. 23: 353-362.
Kikuzawa K. 1982. Leaf survival and evolution in Betulaceae. – Ann. Bot., N. S., 50: 345-354.
Killick DJB. 1969. The South African species of Myrica. – Bothalia 10: 5-17.
Killick DJB, Polill RM, Verdcourt B. 1998. New combinations in African Myricaceae. – Kew Bull. 53: 993-995.
Knapp M, Stöckler K, Havell D, Delsuc F, Sebastiani F, Lockhart PJ. 2005. Relaxed molecular clock provides evidence for long-distance dispersal of Nothofagus (southern beech). – PLoS Biol. 3: 38-43.
Kodrul T, Krassilov V. 2005. New juglandaceous fruit morphotype from the Palaeocene of Amur Province, Russian Far East. – Acta Palaeobot. 45: 139-144.
Koidzumi G. 1937. On the classification of the Juglandaceae. – Acta Phytotaxon. Geobot. 6: 1-17. [In Japanese and Latin]
Komanich IG. 1982. Kariologicheskoe issledovanie vidov roda Juglans L. – Izv. Glavn. Bot. Sada 125: 73-79.
Kotlaba F. 1961. Taxonomic-nomenclatural notes on the fossil Comptonia difformis (Sternb.) Berry and the recent Comptonia aspleniifolia (L.) Aiton. – Preslia 33: 130-140. [In Czech with English summary]
Krasser F. 1896. Bemerkungen zur Systematik der Buchen. – Ann. Naturhist. Hofmus. Wien 11: 155-163.
Kribs DA. 1927. Comparative anatomy of the woods of the Juglandaceae. – Trop. Woods 12: 16-21.
Kuang K-Z. 1941. Genus novum Juglandacearum ex Austro-Orientali Yunnan. – Iconogr. Fl. Sin. 1: 1-3.
Kuang K-Z. 1960. De familiis monotypica Rhoipteleaceae. – Acta Bot. Sin. 9: 43-47.
Kubitzki K. 1993a. Betulaceae. – In: Kubitzki K, Rohwer JG, Bittrich V (eds), The families and genera of vascular plants II. Flowering plants. Dicotyledons. Magnoliid, hamamelid and caryophyllid families, Springer, Berlin, Heidelberg, New York, pp. 152-157.
Kubitzki K. 1993b. Fagaceae. – In: Kubitzki K, Rohwer JG, Bittrich V (eds), The families and genera of vascular plants II. Flowering plants. Dicotyledons. Magnoliid, hamamelid and caryophyllid families, Springer, Berlin, Heidelberg, New York, pp. 301-309.
Kubitzki K. 1993c. Myricaceae. – In: Kubitzki K, Rohwer JG, Bittrich V (eds), The families and genera of vascular plants II. Flowering plants. Dicotyledons. Magnoliid, hamamelid and caryophyllid families, Springer, Berlin, Heidelberg, New York, pp. 453-457.
Kubitzki K. 1993d. Ticodendraceae. – In: Kubitzki K, Rohwer JG, Bittrich V (eds), The families and genera of vascular plants II. Flowering plants. Dicotyledons. Magnoliid, hamamelid and caryophyllid families, Springer, Berlin, Heidelberg, New York, pp. 594-596.
Kuprianova LA. 1962. Palynological data for the systematics of the orders Fagales and Urticales. – In: Proceedings of the First International Conference on palynology 1962, Tucson, Arizona, pp. 17-25. [In Russian]
Kuprianova LA. 1963. On a hitherto undescribed family belonging to the Amentiferae. – Taxon 12: 12-13.
Kuprianova LA. 1965. The palynology of the Amentiferae. – Acad. Sci. USSR, The Komarov Botanical Institute, Leningrad. [In Russian]
Kvaček Z, Walther H. 1989. Paleobotanical studies in Fagaceae of the European Tertiary. – Plant Syst. Evol. 162: 213-229.
Ladiges PY, Nelson G, Grimes J. 1997. Subtree analysis, Nothofagus, and Pacific biogeography. – Cladistics 13: 125-129.
Lalonde M. 1979. Immunological and ultrastructural demonstration of nodulation of the European Alnus glutinosa (L.) Gaertn. host plant by an actinomycetal isolate from North American Comptonia peregrina (L.) Coult. root nodule. – Bot. Gaz. (Chicago) (Suppl.) 140: S35-S43.
Lang P, Dane F, Kubisiak TL, Huang H. 2007. Molecular evidence for an Asian origin and a unique westward migration of species in the genus Castanea via Europe to North America. – Mol. Phylogen. Evol. 43: 49-59.
Langdon LDM. 1934. Embryogeny of Carya and Juglans, a comparative study. – Bot. Gaz. (Crawfordsville) 96: 93-117.
Langdon LDM. 1939. Ontogenetic and anatomical studies of the flower and fruit of the Fagaceae and Juglandaceae. – Bot. Gaz. (Crawfordsville) 101: 301-327.
Langdon LDM. 1947. The comparative morphology of the Fagaceae I. The genus Nothofagus. – Bot. Gaz. (Crawfordsville) 108: 350-371.
La Porte J. 1966. Numeros cromosómicos y algunas observaciones biológicas sibre tres especies americanas del género Juglans. – Darwiniana 14: 156-160.
Larson-Johnson K. 2016. Phylogenetic investigation of the complex evolutionary history of dispersal mode and diversification rates across living and fossil Fagales. – The New Phytologist 209: 418-435.
Lau-Cam CA, Chan HH. 1973. Flavonoids from Comptonia peregrina. – Phytochemistry 12: 1829.
Lawrie AC. 1982. Field nodulation in nine species of Casuarina in Victoria. – Aust. J. Bot. 30: 47-60.
Lechevalier MP, Baker D, Horrière F. 1983. Physiology, chemistry, serology, and infectivity of two Frankia isolates from Alnus incana subsp. rugosa. – Can. J. Bot. 61: 2826-2833.
Lee DE, Lee WG, Mortimer N. 2001. Where and why have all the flowers gone? Depletion and turnover in the New Zealand Cenozoic angiosperm flora in relation to palaeogeography and climate. – Aust. J. Bot. 49: 341-356.
Lehman JW. 1958. Products from hickory bolts. – In: Hickory Task Force Rep. 6, SE Forest Exp. Sta., Asheville, North Carolina, pp. 1-20.
Leroy J-F. 1921. Une Juglandacée du genre Carya en Indochine. – Bull. Mus. Natl. Hist. Nat. Paris 27: 437-440.
Leroy J-F. 1949. De la morphologie florale et de la classification des Myricaceae. – Compt. Rend. Acad. Sci. Paris 229: 1162-1163.
Leroy J-F. 1951. La théorie généralisée des carpelles-sporophylles et la fleur des Juglandales III. Discussion et conclusions. – Compt. Rend. Acad. Sci. Paris 233: 1214-1216.
Leroy J-F. 1953. La structure du bois d’Annamocarya. Notes sur le bois des noyers et autres Juglandacées. – Rev. Intern. Bot. Appl. Agric. Trop. 33: 216-220.
Leroy J-F. 1955. Étude sur les Juglandaceae. – Mém. Mus. Natl. Hist. Nat. Paris, sér. B, Bot. 6: 1-246.
Leroy J-F. 1957. Sur deux amentifères remarquables de la flore asiatico-pacifique et pacifique. – In: Proceedings of the 8th Pacific Science Congress 4: 459-464.
Lersten NR, Horner HT. 2008a. Crystal macropatterns in leaves of Fagaceae and Nothofagaceae: a comparative study. – Plant Syst. Evol. 271: 239-253.
Lersten NR, Horner HT. 2008b. Subepidermal idioblasts and crystal macropattern in leaves of Ticodendron (Ticodendraceae). – Plant Syst. Evol. 276: 255-260.
Li C, Li J-Q, Wang H-C, Li X-W, Peng Y-S. 2009. Lithocarpus longzhouicus comb. nov. (Fagaceae) from China: based on morphological and molecular data. – Nord. J. Bot. 27: 90-96.
Li H-M, Chen Y-F, Chen G-J, Kuang G-D, Huang Z-T. 2003. Tertiary fossil winged fruits of Palaeocarya from Ningming of Guangxi, S. China. – Acta Palaeontol. Sin. 42: 537-547.
Li J, Shoup S, Chen Z. 2005. Phylogenetics of Betula (Betulaceae) inferred from sequences of nuclear ribosomal DNA. – Rhodora 107: 69-86.
Li J, Shoup S, Chen Z. 2007. Phylogenetic relationships of diploid species of Betula (Betulaceae) inferred from DNA sequences of nuclear nitrate reductase. – Syst. Bot. 32: 357-365.
Li J-Q. 1996. On the phylogeny of the Fagaceae. – Acta Phytotaxon. Sin. 34: 597-609.
Li R, Sun B, Wang Q, Ma F, Xu X, Wang Y, Jia
H. 2015. Two new Castanopsis (Fagaceae) species based on cupule and
foliage from the upper Miocene of eastern Zhejiang, China. – Plant Syst.
Evol. 301: 25-39.
Li R-Q, Chen Z-D, Lu A-M, Soltis DE, Soltis PS, Manos PS. 2004. Phylogenetic relationships in Fagales based on DNA sequences from three genomes. – Intern. J. Plant Sci. 165: 311-324.
Li R-Q, Chen Z-D, Soltis DE. 2005. Organogenesis of the inflorescence and flowers in Platycarya strobilacea (Juglandaceae). – Intern. J. Plant Sci. 166: 449-457.
Liang X-Q, Wilde V, Ferguson DK, Kvacek Z, Ablaev AG, Wang Y-F, Li C-S. 2010. Comptonia naumannii (Myricaceae) from the early Miocene of Weichang, China, and the palaeobiogeographical implication of the genus. – Rev. Palaeobot. Palynol. 1623: 52-63.
Liao J-C. 1969. Morphological studies on the flowers and fruits of the genus Lithocarpus in Taiwan. – Natl. Taiwan Univ. Mem. Agric. 10: 1-113.
Lin R-Z, Zeng J, Chen Z-D. 2010. Organogenesis of reproductive structures in Betula alnoides (Betulaceae). – Intern. J. Plant Sci. 171: 586-594.
Linder PH, Crisp MD. 1995. Nothofagus and Pacific biogeography. – Cladistics 11: 5-32.
Liu M-Q, Zhou Z-K. 2006. Modern and geological distribution of Castanopsis (Fagaceae). – Acta Bot. Yunnan. 28: 223-235.
Liu M-Q, Deng M, Zhou Z-K. 2009. Taxonomic and ecological implications of leaf cuticular morphology in Castanopsis, Castanea, and Chrysolepis. – Plant Syst. Evol. 283: 111-123.
Lloyd DG. 1981. The distribution of sex in Myrica gale. – Plant Syst. Evol. 138: 29-45.
Lozano CF, Hernandez-Camacho J, Henao JE. 1979. Hallazgo del género Trigonobalanus Forman 1962 (Fagaceae), en el Neotropica I. – Caldasia 12: 517-537.
Lu A-M. 1982. On the geographical distribution of the Juglandaceae. – Acta Phytotaxon. Sin. 20: 257-274. [In Chinese with English summary]
Luo Y, Zhou Z-K. 2002. Leaf architecture in Quercus subgenus Cyclobalanopsis (Fagaceae) from China. – Bot. J. Linn. Soc. 140: 283-295.
Luza JG, Polito VS. 1991. Porogamy and chalazogamy in walnut (Juglans regia L.). – Bot. Gaz. 152: 100-106.
McCarthy BC, Quinn JA. 1990. Reproductive ecology of Carya (Juglandaceae): phenology, pollination, and breeding system of two sympatric tree species. – Amer. J. Bot. 77: 261-273.
Macdonald AD. 1974. Floral development of Comptonia peregrina (Myricaceae). – Can. J. Bot. 52: 2165-2169.
Macdonald AD. 1977. Myricaceae: floral hypothesis for Gale and Comptonia. – Can. J. Bot. 55: 2636-2651.
Macdonald AD. 1978. Organogenesis of the male inflorescences and flowers of Myrica esculenta. – Can. J. Bot. 56: 2415-2423.
Macdonald AD. 1979. Development of the female flower and gynecandrous partial inflorescence of Myrica californica. – Can. J. Bot. 57: 141-151.
Macdonald AD. 1980a. Inception of the cupule of Quercus macrocarpa and Fagus grandifolia. – Can. J. Bot. 58: 1777-1782.
Macdonald AD. 1980b. Organogenesis of the female reproductive structure of Myrica pensylvanica. – Can. J. Bot. 58: 2001-2006.
Macdonald AD. 1989. The morphology and relationships of the Myricaceae. – In: Crane PR, Blackmore S (eds), Evolution, systematics, and fossil history of the Hamamelidae 2, ‘Higher’ Hamamelidae, Syst. Assoc. Special Vol. 40B, Clarendon Press, Oxford, pp. 147-165.
Macdonald AD, Sattler R. 1973. Floral development of Myrica gale L. and the controversy over floral concepts. – Can. J. Bot. 51: 1965-1975.
Macklin ED. 1927. A revision of the “Distyla complex” of the genus Casuarina. – Trans. Proc. Roy. Soc. South Australia 51: 257-286.
McVean DN. 1953. Biological flora of the British Isles: Alnus Mill. – J. Ecol. 41: 447-466.
McVean DN. 1956. Ecology of Alnus glutinosa (L.) Gaertn. IV. Root system. – J. Ecol. 44: 219-225.
Maggia L, Bousquet J. 1994. Molecular phylogeny of actinorhizal Hamamelidae and relationships with host promiscuity towards Frankia. – Mol. Ecol. 3: 459-467.
Mai DH. 1970. Die tertiären Arten von Trigonobalanus Forman (Fagaceae) in Europa. – Jahrb. Geol. 3: 381-409.
Malterud KE, Anthonsen T, Lorentzen GB. 1977. Two new C-methylated flavonoids from Myrica gale. – Phytochemistry 16: 1805-1809.
Manchester SR. 1983. Fossil wood of the Engelhardieae (Juglandaceae) from the Eocene of North America: Engelhardioxylon gen. nov. – Bot. Gaz. 144: 157-163.
Manchester SR. 1987. The fossil history of the Juglandaceae. – Monogr. Syst. Bot. Missouri Bot. Gard. 21: 1-137.
Manchester SR. 1989. Early history of the Juglandaceae. – Plant Syst. Evol. 162: 231-250.
Manchester SR. 1991. Cruciptera, a new juglandaceous winged fruit from the Eocene and Oligocene of western North America. – Syst. Bot. 16: 715-725.
Manchester SR, Chen Z-D. 1996. Palaeocarpinus aspinosa sp. nov. (Betulaceae) from the Paleocene of Wyoming, USA. – Intern. J. Plant Sci. 157: 644-655.
Manchester SR, Chen Z-D. 1998. A new genus of Coryloideae (Betulaceae) from the Paleocene of North America. – Intern. J. Plant Sci. 159: 522-532.
Manchester SR, Crane PR. 1987. A new genus of Betulaceae from the Oligocene of western North America. – Bot. Gaz. 148: 263-273.
Manchester SR, Dilcher DL. 1982. Pterocaryoid fruits (Juglandaceae) in the Paleogene of North America and their evolutionary and biogeographic significance. – Amer. J. Bot. 69: 275-286.
Manchester SR, Dilcher DL. 1997. Reproductive and vegetative morphology of Polyptera (Juglandaceae) from the Paleocene of Wyoming and Montana. – Amer. J. Bot. 84: 649-663.
Manchester SR, Dillhoff RM. 2004. Fagus (Fagaceae) fruits, foliage, and pollen from the Middle Eocene of Pacific Northwestern North America. – Can. J. Bot. 82: 1509-1517.
Manchester SR, Guo S-X. 1996. Palaeocarpinus (extinct Betulaceae) from northwestern China: new evidence for Paleocene floristic continuity between Asia, North America and Europe. – Intern. J. Plant Sci. 157: 240-246.
Manchester SR, Collinson ME, Goth K. 1994. Fruits of the Juglandaceae from the Eocene of Messel, Germany, and implications for early Tertiary phytogeographic exchange between Europe and western North America – Intern. J. Plant Sci. 155: 388-394.
Manning WE. 1926. The morphology and anatomy of the flowers of the Juglandaceae. – Ph.D. diss., Cornell University, Ithaca, New York.
Manning WE. 1938. The morphology of the flowers of the Juglandaceae I. The inflorescence. – Amer. J. Bot. 25: 407-419.
Manning WE. 1940. The morphology of the flowers of the Juglandaceae II. The pistillate flowers and fruits. – Amer. J. Bot. 27: 839-852.
Manning WE. 1948. The morphology of the flowers of the Juglandaceae III. The staminate flowers. – Amer. J. Bot. 35: 606-621.
Manning WE. 1949. The genus Alfaroa. – Bull. Torrey Bot. Club 76: 196-209.
Manning WE. 1959. Alfaroa and Engelhardtia in the New World. – Bull. Torrey Bot. Club 86: 190-198.
Manning WE. 1960. The genus Juglans in South America and the West Indies. – Brittonia 12: 1-26.
Manning WE. 1966. New combinations and notes on Engelhardia (Juglandaceae) of the Old World. – Bull. Torrey Bot. Club 93: 34-52.
Manning WE. 1975. An analysis of the genus Cyclocarya Iljinskaya (Juglandaceae). – Bull. Torrey Bot. Club 102: 157-166.
Manning WE. 1978 [1979]. The classification within the Juglandaceae. – Ann. Missouri Bot. Gard. 65: 1058-1087.
Manning WE, Hjelmqvist H. 1951. Annamocarya, Rhamphocarya, and Carya sinensis. – Bot. Not. 1951: 319-330.
Manos PS. 1997. Systematics of Nothofagus (Nothofagaceae) based on rDNA spacer sequences (ITS): taxonomic congruence with morphology and plastid sequences. – Amer. J. Bot. 84: 1137-1155.
Manos PS, Fairbrothers DE. 1987. Allozyme variation in populations of six northeastern American red oaks (Fagaceae: Quercus Subg. Erythrobalanus). – Syst. Bot. 12: 365-373.
Manos PS, Stanford AM. 2001. The historical biogeography of Fagaceae: Tracking the Tertiary history of temperate and subtropical forests of the Northern Hemisphere. – Intern. J. Plant Sci. 162 (Suppl.): S77-S93.
Manos PS, Steele KP. 1997. Phylogenetic analyses of ‘higher’ Hamamelididae based on plastid sequence data. – Amer. J. Bot. 84: 1407-1419.
Manos PS, Stone DE. 2001. Evolution, phylogeny, and systematics of the Juglandaceae. – Ann. Missouri Bot. Gard. 88: 231-269.
Manos PS, Nixon KC, Doyle JJ. 1993. Cladistic analysis of restriction site variation within the chloroplast DNA inverted repeat region of selected Hamamelididae. – Syst. Bot. 18: 551-562.
Manos PS, Doyle JJ, Nixon KC. 1999. Phylogeny, biogeography, and processes of molecular differentiation in Quercus subgenus Quercus (Fagaceae). – Mol. Phylogen. Evol. 12: 333-349.
Manos PS, Zhou Z-K, Cannon CH. 2001. Systematics of Fagaceae: phylogenetic tests of reproductive trait evolution. – Intern. J. Plant Sci. 162: 1361-1379.
Manos PS, Soltis PS, Soltis DE, Manchester SR, Oh S-H, Bell CD, Dilcher DL, Stone DE. 2007. Phylogeny of extant and fossil Juglandaceae inferred from the integration of molecular and morphological data sets. – Syst. Biol. 56: 412-430.
Manos PS, Cannon CH, Oh S-H. 2008. Phylogenetic relationships and taxonomic status of the paleoendemic Fagaceae of western North America: recognition of a new genus, Notholithocarpus. – Madroño 55: 181-190.
Marquard RD. 1988. Outcrossing rates in pecan and the potential for increased yields. – J. Amer. Soc. Hortic. Sci. 113: 84-88.
Martin PG, Dowd JM. 1984. The study of plant phylogeny using amino acid sequences of ribulose-1,5-biphosphate carboxylase IV. Proteaceae and Fagaceae and the rate of evolution of the small subunit. – Aust. J. Bot. 32: 291-299.
Martin PG, Dowd JM. 1993. Using sequences of rbcL to study phylogeny and biogeography of Nothofagus species. – Aust. Syst. Bot. 6: 441-447.
Mayol M, Rosselló JA. 2001. Why nuclear ribosomal DNA spacers (ITS) tell different stories in Quercus. – Mol. Phylogen. Evol. 19: 167-176.
Mears JA. 1973. Chemical constituents and systematics of Amentiferae. – Brittonia 25: 385-394.
Medus J. 1981. Pollens Normapolles de coupes stratotypiques du Crétacé supérieur des Charentes et du Sénonien du Portugal. – Comun. Serv. Geol. Portugal 67: 19-28.
Melville R. 1982. The geography of Nothofagus and Trigonobalanus and the origin of the Fagaceae. – Bot. J. Linn. Soc. 85: 75-88.
Meng H-H, Su T, Huang Y-J, Zhu H, Zhou Z-K. 2015. Late Miocene Palaeocarya (Engelhardieae: Juglandaceae) from southwest China and ist biogeographic implications. – J. Syst. Evol. 53: 499-511.
Meurer B, Wiemann R, Strack D. 1988. Phenylpropanoid patterns in Fagales pollen and their phylogenetic relevance. – Phytochemistry 27: 823-828.
Middleton TM. 1988. Intervessel pits in the stem wood of New Zealand Nothofagus (Fagaceae). – IAWA Bull., n. s., 9: 327-331.
Midgley SJ,Turnbull JW, Johnston RD (eds). 1983. Casuarina ecology, management and utilization. – CSIRO Publ., Melbourne.
Miki S. 1955. Nut remains of Juglandaceae in Japan. – J. Inst. Polytechnics, Osaka City Univ., Ser. D, 6: 131-144.
Miller RB. 1976. Wood anatomy and identification of species of Juglans. – Bot. Gaz. (Crawfordsville) 137: 368-377.
Mindell RA, Stockey RA, Beard G. 2007. Cascadiacarpa spinosa gen. et sp. nov. (Fagaceae): castaneoid fruits from the Eocene of Vancouver Island, Canada. – Amer. J. Bot. 94: 351-361.
Mogensen HL. 1972. Fine structure and composition of the egg apparatus before and after fertilization in Quercus gambelii: the functional ovule. – Amer. J. Bot. 59: 931-941.
Mogensen HL. 1975. Ovule abortion in Quercus (Fagaceae). – Amer. J. Bot. 62: 160-165.
Molina A. 1968. Two Nicaraguan Juglandaceae. – Fieldiana, Bot. 31: 357-359.
Molina R. 1981. Ectomycorrhizal specificity in the genus Alnus. – Can. J. Bot. 9: 325-334.
Moseley MF. 1948. Comparative anatomy and phylogeny of the Casuarinaceae. – Bot. Gaz. (Crawfordsville) 110: 231-280.
Moseley MF. 1973. Vegetative anatomy and morphology of Amentiferae. – Brittonia 25: 356-370.
Muir G, Fleming CC, Schotterer C. 2001. Three divergent rDNA clusters predate the species divergence in Quercus petraea (Matt.) Liebl. and Quercus robur L. – Mol. Biol. Evol. 18: 112-119.
Murai S. 1964. Phytotaxonomical and geobotanical studies on genus Alnus in Japan III. Taxonomy of whole world species and distribution of each section. – Bull. For. Exper. Sta. Jap. 171: 1-107.
Murry MA, Zhang Z, Torrey JG. 1985. Effect of oxygen on vesicle formation, acetylene reduction and oxygen-uptake kinetics in Frankia sp. HFPCcI3 isolated from Casuarina cunninghamiana. – Can. J. Microbiol. 31: 804-809.
Nagel K. 1914. Studien über die Familie der Juglandaceen. – Engl. Bot. Jahrb. Syst. 50: 459-530.
Nast CG. 1935. Morphological development of the fruit of Juglans regia. – Hilgardia 9: 345-381.
Nast CG. 1941. The embryology and seedling morphology of Juglans regia L. – Lilloa 6: 163-205.
Natarajan S, Murty VVS, Seshadri TR. 1971. Chemotaxonomical studies of some Casuarina species. – Phytochemistry 10: 1083-1085.
National Research Council. 1984. Casuarinas: nitrogen-fixing trees for adverse sites. – National Academy Press, Washington.
Navarro E, Nalin R, Gauthier D, Normand P. 1997. The nodular microsymbionts of Gymnostoma spp. are Elaeagnus-infective Frankia strains. – Appl. Environm. Microbiol. 63: 1610-1616.
Navarro E, Bousquet J, Moiroud A, Munive A, Piou D, Normand P. 2003. Molecular phylogenyof Alnus (Betulaceae) inferred from nuclear ribosomal DNA ITS sequences. – Plant and Soil 254: 207-217.
Neal JL Jr, Trappe JM, Lu KC, Bollen WB. 1968. Some ectotrophic mycorrhizae of Alnus rubra. – In: Trappe JM, Franklin JF, Tarrant RF, Hansen GM (eds), Biology of alder, Portland, pp. 179-185.
Nichols DJ. 1973. North American and European species of Momipites (“Engelhardtia”) and related genera. – Geoscience and Man 7: 103-117.
Nicoloff MT. 1904-1905. Sur le type floral et le développement du fruit des Juglandées. – J. Bot. 18: 134-140, 141-152, 380-385 (1904); 19: 63-68 (1905).
Nixon KC. 1982. In support of the Nothofagaceae Kuprianova. – Bot. Soc. Amer. Misc. Ser. 162: 102.
Nixon KC. 1985. A biosystematic study of Quercus section Virentes (the live oaks) with phylogenetic analyses of Fagales, Fagaceae and Quercus. – Ph.D. diss., University of Texas, Austin, Texas.
Nixon KC. 1989. Origins of Fagaceae. – In: Crane PR, Blackmore S (eds), Evolution, systematics, and fossil history of the Hamamelidae 2: ‘Higher’ Hamamelidae, Syst. Assoc. Special Vol. 40B, Clarendon Press, Oxford, pp. 23-43.
Nixon KC. 1993. Infrageneric classification of Quercus (Fagaceae) and typification of sectional names. – Ann. Sci. For. (Suppl.) 50: 25S-34S.
Nixon KC, Barrie FR. 2017. Three previously undescribed species of Quercus (Fagaceae) from Mesoamerica and the designation of a lectotype for Q. acutifolia. – Novon 25: 444-450.
Nixon KC, Crepet WL. 1989. Trigonobalanus (Fagaceae): taxonomic status and phylogenetic relationships. – Amer. J. Bot. 76: 828-841.
Oersted AS. 1870. Notice sur les Juglandées. – Vidensk. Meddel. Dansk Naturh. For. Kjøbenhavn 1870: 1-3.
Oginuma K. 1999. Karyomorphology and evolution in Juglandales: a review. – Acta Phytotaxon. Geobot. 50: 229-241. [In Japanese with English summary]
Oginuma K, Tobe H. 1992. Karyomorphology of Juglandaceae. – In: Tanaka R (ed), Plant chromosome research, Proc. 2nd Sino-Japanese Symp. Plant Chromosomes, pp. 171-179.
Oginuma K, Gu Z, Yue Z-S. 1995. Karyomorphology of Rhoiptelea (Rhoipteleaceae). – Acta Phytotaxon. Geobot. 46: 147-151.
Oh S-H, Manos PS. 2008. Molecular phylogenetics and cupule evolution in Fagaceae as inferred from nuclear CRABS CLAW sequences. – Taxon 57: 434-451.
Okamoto M. 1980. A note on the seed and the seedling of Castanopsis fissa. – Bull. Osaka Mus. 33: 55-59.
Okamoto M. 1989a. New interpretation of the inflorescence of Fagus drawn from the developmental study of Fagus crenata, with description of an extremely monstrous cupule. – Amer. J. Bot. 76: 14-22.
Okamoto M. 1989b. A comparative study of the ontogenetic development of the cupules in Castanea and Lithocarpus (Fagaceae). – Plant Syst. Evol. 168: 7-18.
Okuda T, Yoshida T, Hatano T, Yazaki K, Ashida M. 1982. Ellagitannins of the Casuarinaceae, Stachyuraceae, and Myrtaceae. – Phytochemistry 21: 2871-2874.
Olsson U. 1975. The structure of stellate trichomes and their taxonomic implication in some Quercus species (Fagaceae). – Bot. Not. 128: 412-424.
Ørsted AS. 1870. Bidrag til kundskab om valnødplanterne. – Vidensk. Medd. Nat. For. Kjøbenhavn 1870: 159-173.
Pacltová B. 1981. The evolution and distribution of Normapolles pollen during the Cenophytic. – Rev. Palaeobot. Palyn. 35: 175-208.
Palibin JW. 1935. Sur la morphologie florale des Fagacées. – Bull. Acad. Sci. URSS. Cl. Sci. Math. et Nat. 1935: 349-381.
Palo RT, Sunnerheim K, Theander O. 1985. Seasonal variation of phenols, crude protein and cell wall content of birch (Betula pendula Roth) in relation to ruminant in vitro digestibility. – Oecologia 65:314-318.
Pant DD, Nautiyal DD, Singh S. 1974. The cuticle, epidermis, and stomatal ontogeny of Casuarina equisetifolia Forst. – Ann. Bot., N. S., 39: 1117-1123.
Parra-O C. 2000. A new species of Morella (Myricaceae) from Bolivia and Argentina. – Brittonia 52: 320-324.
Parra-O C. 2001. Lectotypification and epitypification of Morella cerifera (L.) Small (Myricaceae). – Caldasia 23: 135-137.
Parra-O C. 2003. New combinations in South American Myricaceae. – Brittonia 54: 322-326.
Paschke MW, Dawson JO. 1992. Frankia abundance in soils beneath Betula nigra and other non-actinorhizal woody plants. – Acta Oecol. 13: 407-415.
Paull R, Hill RS. 2003. Nothofagus kiandrensis (Nothofagaceae subgenus Brassospora), a new macrofossil leaf species from Miocene sediments at Kiandra, New South Wales. – Aust. Syst. Bot. 16: 549-559.
Peterson K, Bell CD, Pfister DH. 2010. Cophylogeny and biogeography of the fungal parasite Cyttaria and its host Nothofagus, the southern beech. – Mycologia 102: 1417-1425.
Petit RJ, Pineau E, Demesure B, Bacilieri R, Ducousso A, Kremer A. 1997. Chloroplast DNA footprints of postglacial recolonization by oaks. – Proc. Natl. Acad. Sci. U.S.A. 94: 9996-10001.
Petit RJ, Csaikl UM, Bordács S, Burg K, Coart E, Cottrell J. Dam BC van, Deans JD, Fineschi S, Dumolin-Lepègue S, Finkeldey R, Gillies A, Glaz I, Goicoechea PG, Jensen JS, König AO, Lowe AJ, Madsen SF, Mátiás G, Munro RC, Pomonge M-H, Popescu F, Slade D, Tabbener H, Taurchini D, Vries SMG de, Ziegenhagen B, Kremer A. 2002. Chloroplast DNA variation in European white oaks: phylogeography and patterns of diversity based on data from over 2600 populations. – For. Ecol. Manag. 156: 5-26.
Petit RJ, Bodenes C, Ducousso A, Roussel G, Kremer A. 2004. Hybridization as a mechanism of invasion in oaks. – New Phytol. 161: 151-164.
Philipson WR. 1988. Seedling and shoot morphology of the New Zealand species of Nothofagus (Fagaceae). – New Zealand J. Bot. 26: 401-407.
Philipson WR, Philipson MN. 1979. Leaf venation in Nothofagus. – New Zealand J. Bot. 17: 417-421.
Philipson WR, Philipson MN. 1988. A classification of the genus Nothofagus (Fagaceae). – Bot. J. Linn. Soc. 98: 27-36.
Pike KM. 1953. Fossil fruiting cones of Casuarina and Banksia from Tertiary deposits in Victoria. – Proc. Roy. Soc. Victoria, Ser. II, 65: 1-8.
Poisson J. 1874. Recherches sur les Casuarina et en particulier sur ceux de la Nouvelle-Calédonie. – Nouv. Arch. Mus. Natl. Hist. Nat. Paris 10: 59-111.
Polechko MA, Clarkson RB. 1984. A serological study of the systematics of the Juglandaceae. – Biochem. Syst. Ecol. 14: 33-39.
Polhill RM, Verdcourt B. 2000. Myricaceae. – In: Beentje HJ, Smith SAL (eds), Flora of tropical East Africa, A. A. Balkema, Rotterdam, The Netherlands, pp. 1-12.
Poole AL. 1950a. Studies of New Zealand Nothofagus species 1. Taxonomy and floral morphology. – Trans. Proc. Roy. Soc. New Zealand 78: 363-380.
Poole AL. 1950b. Studies of New Zealand Nothofagus species 2. Nut and cupule development. – Trans. Proc. Roy. Soc. New Zealand 78: 502-508.
Poole AL. 1952. The development of Nothofagus seed (including a preliminary account of embryogeny, etc.). – Trans. Roy. Soc. New Zealand 80: 207-212.
Poole AL. 1958. Studies of New Zealand Nothofagus species 3. The entire-leaved species. – Trans. Proc. roy. Soc. New Zealand 85: 551-564.
Poole I. 2002. Systematics of Cretaceous and Tertiary Nothofagoxylon: implications for Southern Hemisphere biogeography and evolution of the Nothofagaceae. – Aust. Syst. Bot. 15: 247-276.
Porsch O. 1950. Geschichtliche Lebenswertung der Kastanienblüte. – Österr. Bot. Zeitschr. 97: 269-321.
Praglowski J. 1982. Fagaceae L. (Fagoideae). – World Pollen and Spore Flora 11, Almqvist & Wiksell, Stockholm.
Praglowski J. 1984. Fagaceae L. (Castaneoideae). – World Pollen and Spore Flora 13, Almqvist & Wiksell, Stockholm.
Prantl K. 1889a. Betulaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(1), W. Engelmann, Leipzig, pp. 38-46.
Prantl K. 1889b. Fagaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(1), W. Engelmann, Leipzig, pp. 47-58.
Prat D. 1989. Effect of some pure and mixed Frankia strains on seedling growth in different Alnus species. – Plant and Soil 113: 31-38.
Premoli AC, Mathiasen P, Acosta MC, Ramos VA. 2011. Phylogeographically concordant chloroplast DNA divergence in sympatric Nothofagus s.s. How deep can it be? – New Phytologist 193: 261-275.
Puntieri J, Raffaele E, Martinez P, Barthelemy D, Brion C. 1999. Morphological and architectural features of young Nothofagus pumilio (Poepp. & Endl.) Krasser (Fagaceae). – Bot. J. Linn. Soc. 130: 395-410.
Qiu Y-L, Chase MW, Hoot SB, Conti E, Crane PR, Sytsma KJ, Parks CR. 1998. Phylogenetics of the Hamamelidae and their allies: parsimony analyses of nucleotide sequences of the plastid gene rbcL. – Intern. J. Plant Sci. 159: 891-905.
Racette S, Torrey JG. 1989a. The isolation, culture and ineffectivity of a Frankia strain from Gymnostoma papuanum (Casuarinaceae). – Plant and Soil 118: 165-170.
Racette S, Torrey JG. 1989b. Root nodule initiation in Gymnostoma (Casuarinaceae) and Shepherdia (Elaeagnaceae) induced by Frankia strain HFPGpI1. – Can. J. Bot. 67: 2873-2879.
Redell P, Bowen GD, Robson AD. 1986. Nodulation of Casuarinaceae in relation to host species and soil properties. – Aust. J. Bot. 34: 435-444.
Rodriques-Barrueco C, Bond G. 1969. Nodule endophytes in the genus Alnus. – In: Trappe JM, Franklin JF, Tarrant RF, Hansen GM (eds), Biology of alder, Portland, pp. 185-192.
Romero EJ. 1986. Fossil evidence regarding the evolution of Nothofagus Blume. – Ann. Missouri Bot. Gard. 73: 276-283.
Rosbrook PA, Bowen GD. 1987. The abilities of three Frankia isolates to nodulate and fix nitrogen with four species of Casuarina. – Physiol. Plant. 70: 373-377.
Rozefelds AC. 1998. Stamen morphology in Nothofagus (Nothofagaceae). – Intern. J. Plant Sci. 159: 655-667.
Rozefelds AC, Drinnan AN. 1998. Ontogeny and diversity in staminate flowers of Nothofagus (Nothofagaceae). – Intern. J. Plant Sci. 159: 906-922.
Rozefelds AC, Drinnan AN. 2002. Ontogeny of pistillate flowers and inflorescences in Nothofagus subgenus Lophozonia (Nothofagaceae). – Plant Syst. Evol. 233: 105-126.
Rüffle L. 1980. Wachstums-Modus und Blattmorphologie bei altertümlichen Fagales und Hamamelidales der Kreide und der Gegenwart. – In: 100 Jahre Arboretum Berlin, Akademie-Verlag, Berlin, pp. 329-341.
Rzedowski J, Palacios-Chávez R. 1977. El bosque de Engelhardtia (Oreomunnea) mexicana en la region de la Chinantla (Oaxaca, Mexico) – una reliquia del Cenozoico. – Bol. Soc. Bot. México 36: 93-123.
Saleh NAM, El-Lakany MH. 1979. A quantitative variation in the flavonoids and phenolics of some Casuarina species. – Biochem. Syst. Ecol. 7: 13-15.
Samuel R, Bachmair A, Jobst J, Ehrendorfer F. 1998. ITS sequences from nuclear rDNA suggest unexpected phylogenetic relationships between Euro-Mediterranean, East Asiatic and North American taxa of Quercus (Fagaceae). – Plant Syst. Evol. 211: 129-139.
Sauquet H, Ho SYW, Gandolfo MA, Jordan GJ, Wilf P, Cantrill DJ, Bayly MJ, Bromham L, Brown GK, Carpenter RJ, Lee DM, Murphy DJ, Sniderman JMK, Udovicic F. 2012. Testing the impact of calibration on molecular divergence times using a fossil-rich group: the case of Nothofagus (Fagales). – Syst. Biol. 61: 289-313.
Savard L, Michaud M, Bousquet J. 1993. Genetic diversity and phylogenetic relationships between birches and alders using ITS, 18S rRNA, and rbcL gene sequences. – Mol. Phylogen. Evol. 2: 112-118.
Scareli-Santos C, Herrera-Arroyo ML, Sánchez-Mondragón ML, González-Rodríguez A, Bacon J, Oyama K. 2007. Comparative analysis of micromorphological characters in two distantly related Mexican oaks, Quercus conzattii and Q. eduardii (Fagaceae), and their hybrids. – Brittonia 59: 37-48.
Schaarschmidt H. 1985. Zur Verwandtschaft von Carya Nutt. und Pterocarya Sieb. et Zucc. (Juglandaceae) und zur natürlichen Gliederung der Familie. – Feddes Repert. 96: 345-361.
Schaarschmidt H. 1987. Zur Position der Juglandaceae A. Rich. ex Kunth im aktuellen System der Magnoliatae. – Folia Geobot. Phytotaxon. 22: 271-286.
Schirone B, Schirone A, Romagnoli M, Angelaccio C, Bellarosa R. 1990. Preliminary considerations on the taxonomy of Quercus crenata Lamk. – In: Lorenzoni GG, Ruggiero L, Valenziano S (eds), Approcci metodologici per la definizione dell’ambiente fisico e biologico Mediterraneo, pp. 423-452. [In Italian]
Schönenberger J, Pedersen KR, Friis EM. 2001. Normapolles flowers of fagalean affinity from the Late Cretaceous of Portugal. – Plant Syst. Evol. 226: 205-230.
Schwarz O. 1936. Entwurf zu einem natürlichen System der Cupuliferen und der Gattung Quercus. – Notizbl. Bot. Garten Mus. Berlin-Dahlem 13: 1-22.
Schwarz O. 1936-1939. Monographie der Eichen Europas, besonders des Mittelmeergebietes. – Feddes Repert., Sonderbeih. D: 1-200.
Schwintzer CR. 1984. Production, decomposition, and nitrogen dynamics of Myrica gale litter. – Plant and Soil 78: 245-258.
Scriven LJ, Hill RS. 1995. Macrofossil Casuarinaceae: their identification and the oldest macrofossil record, Gymnostoma antiquum sp. nov., from the Late Paleocene of New South Wales, Australia. – Aust. Syst. Bot. 8: 1035-1053.
Scriven LJ, Hill RS. 1996. Relationships among Tasmanian Tertiary Nothofagus (Nothofagaceae) populations. – Bot. J. Linn. Soc. 121: 345-364.
Scriven LJ, McLoughlin S, Hill RS. 1995. Nothofagus plicata (Nothofagaceae), a new deciduous Eocene macrofossil species from southern continental Australia. – Rev. Palaeobot. Palynol. 86: 199-209.
Setoguchi H, Ono M, Doi Y, Koyama H, Tsuda M. 1997. Molecular phylogeny of Nothofagus (Nothofagaceae) based on the atpB-rbcL intergenic spacer of the chloroplast DNA. – J. Plant Res. 110: 469-484.
Shimaji K. 1962. Anatomical studies on the phylogenetic interrelationships of the genera in the Fagaceae. – Bull. Tokyo Univ. For. 57: 1-64.
Simon L, Stein A, Côte S, Lalonde M. 1985. Performance of in vitro propagated Alnus glutinosa (L.) Gaertn: clones inoculated with Frankia. – Plant and Soil 87: 125-133.
Sims HP, Herendeen PS, Crane PR. 1998. A new genus of fossil Fagaceae from the Santonian (Late Cretaceous) of central Georgia, USA. – Intern. J. Plant Sci. 159: 391-404.
Sims HP, Herendeen PS, Lupia R, Christopher RA, Crane PR. 1999. Fossil flowers with Normapolles pollen from the Upper Cretaceous of southeastern North America. – Rev. Palaeobot. Palynol. 106: 131-151.
Skarby A. 1986. Normapolles anthers from the Upper Cretaceous of southern Sweden. – Rev. Palaeobot. Palynol. 46: 235-256.
Skarby A, Rowley JR, Nilsson L. 1990. Exine structure of Upper Cretaceous Normapolles grains from anthers (northeastern Scania, Sweden). – Palynology 14: 145-173.
Smiley CJ, Huggins LM. 1981. Pseudofagus idahoensis, n. gen. et sp. (Fagaceae) from the Miocene Clarkia Flora of Idaho. – Amer. J. Bot. 68: 741-761.
Smith JF, Doyle JJ. 1995. A cladistic analysis of chloroplast DNA restriction site variation and morphology for the genera of the Juglandaceae. – Amer. J. Bot. 82: 1163-1172.
Smith ME, Pfister DH. 2009. Tuberculate ectomycorrhizae of angiosperms: the interaction between Boletus rubropunctus (Boletacee) and Quercus species (Fagaceae) in the United States and Mexico. – Amer. J. Bot. 96: 1665-1675.
Smolander A. 1990. Frankia population in soils under Betula pendula. – Plant and Soil 121: 1-10.
Soepadmo E. 1968. A revision of the genus Quercus L. subgen. Cyclobalanopsis (Oersted) Schneider in Malesia. – Gard. Bull. (Singapore) 22: 355-427.
Soepadmo E. 1970. Florae Malesianae precursores XLIX. Malesian species of Lithocarpus Bl. (Fagaceae). – Reinwardtia 8: 197-308.
Soepadmo E. 1972. Fagaceae. – In: Steenis CGGJ van (ed), Flora Malesiana I, 2(7), Sijthoff & Noordhoff International Publ., Alphen aan den Rijn, The Netherlands, pp. 265-403.
Sogo A, Tobe H. 2005. Intermittent pollen-tube growth in pistils of alders (Alnus). – Proc. Natl. Acad. Sci. U.S.A. 102: 8770-8775.
Sogo A, Tobe H. 2006a. Mode of pollen-tube growth in pistils of Myrica rubra (Myricaceae): a comparison with related families. – Ann. Bot. 97: 71-77.
Sogo A, Tobe H. 2006b. The evolution of fertilization modes independent of the micropyle in Fagales and ‘pseudoporogamy’. – Plant Syst. Evol. 259: 73-80.
Sogo A, Tobe H. 2006c. Delayed fertilization and pollen-tube growth in pistils of Fagus japonica (Fagaceae). – Amer. J. Bot. 93: 1748-1756.
Sogo A, Tobe H. 2008. Mode of pollen tube growth in pistils of Ticodendron incognitum (Ticodendraceae, Fagales) and the evolution of chalazogamy. – Bot. J. Linn. Soc. 157: 621-631.
Sogo A, Setoguchi H, Noguchi J, Jaffré T, Tobe H. 2001. Molecular phylogeny of Casuarinaceae based on rbcL and matK gene sequences. – J. Plant Res. 114: 459-464.
Solomon AM. 1983a. Pollen morphology and plant taxonomy of white oaks in eastern North America. – Amer. J. Bot. 70: 481-491.
Solomon AM. 1983b. Pollen morphology and plant taxonomy of red oaks in eastern North America. – Amer. J. Bot. 70: 495-507.
Sougoufara B. 1983. Méthodologie impliquée dans l’étude de la symbiose d’une non-légumineuse forestière tropicale (Casuarina) avec Frankia. – Thèse de DEA, l’Université de Nancy, France.
Sougoufara B. 1990. La fixation de N2 par les Casuarinas: amélioration par sélection clonale et quantification par différentes méthodes. – Ph.D. diss., l’Université de Nancy, France.
Spellenberg R, Bacon JR. 1996. Taxonomy and distribution of a natural group of black oaks of Mexico (Quercus, Section Lobatae, Subsection Racemiflorae). – Syst. Bot. 21: 85-99.
Stachurska A. 1961. Morphology of pollen grains of the Juglandaceae. – Monogr. Bot. 7: 121-143.
Stairs GR. 1964. Microsporogenesis and embryogenesis in Quercus. – Bot. Gaz. 125: 115-121.
Stanford AM, Harden R, Parks CR. 2000. Phylogeny and biogeography of Juglans (Juglandaceae) based on matK and ITS sequence data. – Amer. J. Bot. 87: 872-882.
Steane DA, Wilson KL, Hill RS. 2003. Using matK sequence data to unravel the phylogeny of Casuarinaceae. – Mol. Phylogen. Evol. 28: 47-59.
Steenis CGGJ van. 1952. Preliminary account of Papuan Nothofagus. – Blumea 7: 146-147. – Errata. – Blumea 7: 306.
Steenis CGGJ van. 1953. Results of the Archbold expeditions. Papuan Nothofagus. – J. Arnold Arbor. 34: 301-373.
Steenis CGGJ van. 1954. Additional note on Nothofagus. – J. Arnold Arbor. 35: 266-267.
Steenis CGGJ van. 1971a. Nothofagus, key genus of plant geography, in time and space, living and fossil, ecology and phylogeny. – Blumea 19: 65-98.
Steenis CGGJ van. 1971b. Revision of Nothofagus in New Caledonia. – Adansonia, sér. II, 11: 615-624.
Steenis CGGJ van. 1972. Nothofagus. – In: Steenis CGGJ van (ed), Flora Malesiana, series 1, 7(2), Noordhoff-Kolff, Djakarta.
St-Laurent L, Lalonde M. 1987. Isolation and characterization of Frankia strains isolated from Myrica gale. – Can. J. Bot. 65: 1356-1363.
St-Laurent L, Bousquet J, Simon L, Lalonde M. 1987. Separation of various Frankia strains in the Alnus and Elaeagnus host specificity groups using sugar analysis. – Can. J. Microbiol. 33: 764-772.
Stokes J. 1937. Cytological studies in the Myricaceae. – Bot. Gaz. (Crawfordsville) 99: 387-399.
Stone DE. 1961. Ploidal level and stomatal size in the American hickories. – Brittonia 13: 293-302.
Stone DE. 1962. Affinities of a Mexican endemic, Carya palmeri, with American and Asian hickories. – Amer. J. Bot. 49: 199-212.
Stone DE. 1963. Pollen size in hickories (Carya). – Brittonia 15: 208-215.
Stone DE. 1964. New chromosome counts for two species of hickories (Carya). – Brittonia 16: 230.
Stone DE. 1968. New World Juglandaceae: a new species of Alfaroa from Mexico. – Amer. J. Bot. 55: 477-484.
Stone DE. 1969. Alfaroa costaricensis. – In: Documented plant chromosome numbers 1969: 2, Sida 3: 352-355.
Stone DE. 1970. Evolution of cotyledonary and nodal vasculature in the Juglandaceae. – Amer. J. Bot. 57: 1219-1225.
Stone DE. 1972. New World Juglandaceae III. A new perspective of the tropical members with winged fruits. – Ann. Missouri Bot. Gard. 59: 297-321.
Stone DE. 1973. Patterns in the evolution of amentiferous fruits. – Brittonia 25: 371-384.
Stone DE. 1989. Biology and evolution of temperate and tropical Juglandaceae. – In: Crane PR, Blackmore S (eds), Evolution, systematics, and fossil history of the Hamamelidae 2, ‘Higher’ Hamamelidae, Syst. Assoc. Special Vol. 40B, Clarendon Press, Oxford, pp. 117-145.
Stone DE. 1993. Juglandaceae. – In: Kubitzki K, Rohwer JG, Bittrich V (eds), The families and genera of vascular plants II. Flowering plants. Dicotyledons. Magnoliid, hamamelid and caryophyllid families, Springer, Berlin, Heidelberg, New York, pp. 348-359.
Stone DE. 2010. Review of New World Alfaroa and Old World Alfaropsis (Juglandaceae). – Novon 20: 215-224.
Stone DE, Broome CR. 1971. Pollen ultrastructure: evidence for relationship of the Juglandaceae and the Rhoipteleaceae. – Pollen Spores 13: 5-14.
Stone DE, Broome CR. 1975. Juglandaceae A. Rich. ex Kunth. – In: Nilsson S (ed), World pollen and spore flora 4, Almqvist & Wiksell, Stockholm.
Stone DE, Reich J, Whitfield S. 1964. Fine structure of the walls of Juglans and Carya pollen. – Pollen Spores 6: 379-392.
Stone DE, Adrouny GA, Adrouny S. 1965. Morphological and chemical evidence on the hybrid nature of bitter pecan, Carya x lecontei. – Brittonia 17: 97-106.
Stone DE, Adrouny GA, Flake RH. 1969. New World Juglandaceae II. Hicory nut oils, phenetic similarities, and evolutionary implications in the genus Carya. – Amer. J. Bot. 56: 928-935.
Stone DE, Reich J, Whitfield S. 1964. Fine structure of the walls of Juglans and Carya pollen. – Pollen Spores 6: 379-392.
Stone DE, Oh S-H, Tripp EA, Ríos G LE, Manos PS. 2009. Natural history, distribution, phylogenetic relationships, and conservation of Central American black walnuts (Juglans sect. Rhysocaryon). – J. Torrey Bot. Soc. 136: 1-25.
Su S-W, He J-Q. 1984. Discovery of bisexual flowers in Pterocarya stenoptera C. DC. – Acta Phytotaxon. Sin. 22: 256-258. [In Chinese with English summary]
Subbarao NS, Rodríguez-Barrueco C. 1995. Casuarinas. – Science Publ., Lebanon, New Hampshire.
Sun F, Stockey RA. 1992. A new species of Palaeocarpinus (Betulaceae) based on infructescences, fruits, and associated staminate inflorescences and leaves from the Paleocene of Alberta, Canada. – Intern. J. Plant Sci. 153: 136-146.
Sun S-G, Lu Y, Huang S-Q. 2006. Floral phenology and sex expression in functionally monoecious Rhoiptelea chiliantha (Rhoipteleaceae). – Bot. J. Linn. Soc. 152: 145-151.
Sundberg MD. 1985. Pollen of the Myricaceae. – Pollen Spores 27: 15-28.
Süss H. 1986. Untersuchungen über fossile Buchenhölzer. – Feddes Repert. 97: 161-183.
Swamy BGL. 1948. A contribution to the life history of Casuarina. – Proc. Amer. Acad. Arts Sci. 77: 1-32.
Swenson U, Hill RS, McLoughlin S. 2000. Ancestral area analysis of Nothofagus (Nothofagaceae) and its congruence with the fossil record. – Aust. Syst. Bot. 13: 469-478.
Swenson U, Hill RS, McLoughlin S. 2001. Biogeography of Nothofagus supports the sequence of Gondwana break-up. – Taxon 50: 1025-1041.
Swenson U, Backlund A, McLoughlin S, Hill RS. 2001. Nothofagus biogeography revisited with special emphasis on the enigmatic distribution of subgenus Brassospora in New Caledonia. – Cladistics 17: 28-47.
Takahashi M, Friis EM, Herendeen PS, Crane PR. 2008. Fossil flowers of Fagales from the Kamikitaba Locality (Early Coniacian; Late Cretaceous) of northeastern Japan. – Intern. J. Plant Sci. 169: 899-907.
Tanai T. 1986. Phytogeographic and phylogenetic history of the genus Nothofagus Bl. (Fagaceae) in the southern hemisphere. – J. Fac. Sci. Hokkaido Univ., ser. 4, 21: 505-582.
Tang Y. 1932. Timber studies of Chinese trees 1. Timber anatomy of Rhoipteleaceae. – Bull. Fan Inst. Biol. Peking 3: 127-131.
Taylor DW, Hu S, Tiffney BH. 2012. Fossil floral and fruit evidence for the evolution of unusual developmental characters in Fagales. – Bot. J. Linn. Soc. 168: 353-376.
Tjepkema JD. 1978. The role of oxygen diffusion from the shoots and nodule roots in nitrogen fixation by root nodules of Myrica gale L. – Can. J. Bot. 61: 2898-2909.
Tobe H. 1991. Reproductive morphology, anatomy, and relationships of Ticodendron. – Ann. Missouri Bot. Gard. 78: 135-142.
Torrey JG. 1983. Root development and root nodulation in Casuarina. – In: Midgley SJ, Turnbull JW, Johnston RD (eds), Casuarina ecology, management, and utilization, CSIRO, Melbourne, pp. 180-192.
Torrey JG, Berg RH. 1988. Some morphological features for generic characterization among the Casuarinaceae. – Amer. J. Bot. 75: 864-874.
Torrey JG, Racette S. 1989. Specificity among the Casuarinaceae in root nodulation by Frankia. – Plant and Soil 118: 157-164.
TorreyJG, Baker DD, Callaham D, Del Tredici P, Newcomb W, Peterson RL, Tjepkema JD. 1980. On the nature of the endophyte causing root nodulation in Comptonia. – In: Newton WE, Orme-Johnson WH (eds), Nitrogen fixation II, pp. 217-227.
Toumi L, Lumaret R. 2001. Allozyme characterisation of four Mediterranean evergreen oak species. – Biochem. Syst. Ecol. 29: 799-817.
Trelease W. 1924. The American oaks. – Mem. Natl. Acad. Sci. 20: 1-255.
Treub M. 1891. Sur les Casuarinées et leur place dans le système naturel. – Ann. Jard. Bot. Buitenz. 10: 145-231.
Tschan GF, Denk T. 2012. Trichome types, foliar indumentum and epicuticular wax in the Mediterranean gall oaks, Quercus subsection Galliferae (Fagaceae): implictions for taxonomy, ecology and evolution. – Bot. J. Linn. Soc. 169: 611-644.
Tschudy RH. 1981. Geographic distribution and dispersal of Normapolles genera in North America. – Rev. Palaeobot. Palynol. 35: 283-314.
Turnbull JW, Martensz PN. 1982. Aspects of seed collection, storage and germination in Casuarinaceae. – Aust. For. Res. 12: 281-294.
Ueno J. 1963. On the fine structure of the pollen walls of Angiospermae III. Casuarina. – Grana Palynol. 4: 189-193.
Uyar T, Malterud KE, Anthonsen T. 1978. Two new dihydrochalcones from Myrica gale. – Phytochemistry 17: 2011-2013.
Valen L van. 1976. Ecological species, multispecies and oaks. – Taxon 25: 233-239.
Valencia SA, Delgado AS. 2003. Los tricomas foliares en la caracterización de un grupo de especies del género Quercus, sección Lobatae (Fagaceae). – An. Inst. Biol. Univ. Nac. Autón. México, Bot. 74: 5-15.
Vales MA, Borhidi A, Del-Risco E. 1982. Anatomia de la madera de Myricaceae en Cuba: consideraciones ecologicas. – Acta Bot. Acad. Scient. Hung. 28: 241-253.
Vandenbosch KA, Torrey JG. 183. Host-endophyte interactions in effective and ineffective nodules induced by the endophyte of Myrica gale. – Can. J. Bot. 61: 2898-2909.
Vanstraten J, Akkermans ADL, Roelofsen W. 1977. Nitrogenase activity of endophyte suspensions derived from root-nodules of Alnus, Hippophae, Shepherdia and Myrica spp. – Nature 266: 257-258.
Vazquez ML, Valencia AS, Nixon KC. 2004. Notes on red oaks (Quercus sect. Lobatae) in eastern Mexico, with description of a new species, Quercus hirtifolia. – Brittonia 56: 136-142.
Vazquez FM, Rodriguez RA. 1999. A new subspecies and two new combinations of Nothofagus Blume (Nothofagaceae) from Chile. – Bot. J. Linn. Soc. 129: 75-83.
Verdcourt B, Polhill R. 1997. Proposals to conserve the names Myrica and Gale (Myricaceae) with conserved types. – Taxon 46: 347-348.
Wang N, McAllister HA, Bartlett POR, Buggs RJA. 2016. Molecular phylogeny and genome size evolution of the genus Betula (Betulaceae). – Ann. Bot. 117: 1023-1035.
Wang P-L, Chang K-T. 1991. The pollen morphology in relation to the taxonomy and phylogeny of Fagaceae. – Acta Phytotaxon. Sin. 29: 60-66.
Wang P-L, Pu F-D. 2004. Pollen morphology and biogeography of Fagaceae. – Guangdong Science and Technology Press, Guangzhou.
Wardle J. 1984. The New Zealand beeches. Ecology, utilization and management. – New Zealand Forest Service, Christchurch.
Webb PN, Harwood DM. 1993. Pliocene fossil Nothofagus (southern beech) from Antarctica: phytogeography, dispersal strategies, and survival in high latitude glacial-deglacial environments. – In: Alden J, Mastrantonio JL, Odum S (eds), Forest development in cold climates, Plenum Press, New York, pp. 135-166.
Whang SS, Hill RS. 1995. Phytolith analysis in leaves of extant and fossil populations of Nothofagus subgenus Lophozonia. – Aust. Syst. Bot. 8: 1055-1065.
Whitcher IN, Wen J. 2001. Phylogeny and biogeography of Corylus (Betulaceae): inferences from ITS sequences. – Syst. Bot. 26: 283-298.
White F. 1993. African Myricaceae and the history of the afromontane flora. – Opera Bot. 121: 173-188.
Whitehead DR. 1963. Pollen morphology in the Juglandaceae I. Pollen size and pore number variation. J. Arnold Arbor. 44: 101-110.
Whitehead DR. 1965. Pollen morphology in the Juglandaceae II. Survey of the family. – J. Arnold Arbor. 46: 369-410.
Whittemore TC, Schaal BA. 1991. Interspecific gene flow in sympatric oaks. – Proc. Natl. Acad. Sci. U.S.A. 88: 2540-2544.
Wigston DL. 1979. Nothofagus (Blume) in Britain. – Watsonia 12: 344-345.
Wilbur RL. 1994. The Myricaceae of the United States and Canada: genera, subgenera, and series. – Sida 16: 93-107.
Wilbur RL. 2001. Five new combinations in the genus Morella (Myricaceae) for neotropical species. – Rhodora 103: 120-122.
Wilde V, Frankenhäuser H. 1995. Flügelfrüchte engelhardioider Juglandaceen aus dem Mitteleozän von Eckfeld bei Manderscheid (Eifel). – Mainzer Naturwiss. Archiv 33: 47-52.
Wilmot-Dear CM. 1985. Casuarinaceae. – In: Polhill RM (ed), Flora of tropical East Africa, A. A. Balkema, Rotterdam, The Netherlands, pp. 1-8.
Wilson KL, Johnson LAS. 1989. Casuarinaceae. – In: George AS (ed), Flora of Australia 3, Australian Government Publ. Service, Canberra, pp. 100-174.
Wing SL, Hickey LJ. 1984. The Platycarya perplex and the evolution of the Juglandaceae. – Amer. J. Bot. 71: 388-411.
Withner CL. 1941. Stem anatomy and phylogeny of the Rhoipteleaceae. – Amer. J. Bot. 28: 872-878.
Wolfe JA. 1959. Tertiary Juglandaceae of western North America. – M.A. thesis, University of California, Berkeley, California.
Wolfe JA. 1973. Fossil forms of Amentiferae. – Brittonia 25: 334-355.
Wollenweber E. 1975. Flavonoidmuster im Knospenexkret der Betulaceen. – Biochem. Syst. Ecol. 3: 47-52.
Wollenweber E, Stevens JF, Dörr M, Rozefelds AC. 2003. Taxonomic significance of flavonoid variation in temperate species of Nothofagus. – Phytochemistry 62: 1125-1131.
Woodroof JG. 1930. Studies of the staminate inflorescence and pollen of Hicoria pecan. – J. Agric. Res. 40: 1059-1104.
Woodroof NC. 1928. Development of the embryo sac and young embryo of Hicoria pecan. – Amer. J. Bot. 15: 416-421.
Woodworth RH. 1930. Meiosis of microsporogenesis in the Juglandaceae. – Amer. J. Bot. 17: 863-869.
Wu C-Y, Kubitzki K. 1993. Rhoipteleaceae. – In: Kubitzki K, Rohwer JG, Bittrich V (eds), The families and genera of vascular plants II. Flowering plants. Dicotyledons. Magnoliid, hamamelid and caryophyllid families, Springer, Berlin, Heidelberg, New York, pp. 584-585.
Wu J-Y, Wilf P, Ding S-T, An P-C, Dai J. 2017. Late Miocene Cyclocarya (Juglandaceae) from Southwest China and its biogeographic implications. – Intern. J. Plant Sci. 178: 580-591.
Xiang X-G, Wang W, Li R-Q, Lin L, Liu Y, Zhou
Z-K, Li Z-Y, Chen Z-D. 2014. Large-scale phylogenetic analyses reveal fagalean
diversification promoted by the interplay of diaspores and environments in the
Paleogene. – Persp. Plant Ecol. Evol. Syst. 16: 101-110.
Xie S, Sun B, Dilcher DL, Yan D, Wu J, Lin Z. 2010. Numerical taxonomy of Palaeocarya (Juglandaceae) from the Mangbang Formation of West Yunnan, China. – Rev. Palaeobot. Palyn. 162: 193-202.
Xing S-P, Chen Z-D, Lee A-M. 1998. Development of ovules and embryo sacs in Ostrya virginica (Betulaceae) and its systematic significance. – Acta Phytotaxon. Sin. 36: 428-435.
Yamazaki T, Takeoka M. 1959. Electron microscope investigation on the surface structure of the pollen membrane, based on the replica method V. On the pollen of genus Quercus. – J. Jap. For. Soc. 41: 125-129.
Yen TK. 1950. Structure and development of the flower and the fruit of Myrica rubra. – Peking Nat. Hist. Bull. 19: 1-20.
Yoo K-O, Wen J. 2002. Phylogeny and biogeography of Carpinus and subfamily Coryloideae (Betulaceae). – Intern. J. Plant Sci. 163: 641-650.
Yoo K-O, Wen J. 2007. Phylogeny of Carpinus and subfamily Coryloideae (Betulaceae) based on chloroplast and nuclear ribosomal sequence data. – Plant Syst. Evol. 267: 25-35.
Yoo K-O, Wen J. 2008. Phylogenetic relationships of Coryloideae based on waxy and atpB-rbcL sequences. – Korean J. Plant Taxon. 38: 371-388.
Youngken HW. 1919. The comparative morphology, taxonomy and distribution of the Myricaceae of the eastern United States. – Contr. Bot. Lab. Morris Arbor. Univ. Pennsylvania 4: 339-400.
Zaklinskaya ED. 1981. Phylogeny and classification of the Normapolles. – Rev. Palaeobot. Palynol. 35: 139-147.
Zamaloa M del C, Gandolfo MA, González CC, Romero EJ, Cúneo NR, Wilf P. 2006. Casuarinaceae from the Eocene of Patagonia, Argentina. – Intern. J. Plant Sci. 167: 1279-1289.
Zeng J, Li J-H, Chen Z-D. 2008. A new species of Betula section Betulaster (Betulaceae) from China. – Bot. J. Linn. Soc. 156: 523-528.
Zhang J-B, Li R-Q, Xiang X-G, Manchester SR, Lin L, Wang W, Wen J, Chen Z-D. 2013. Integrated fossil and molecular data reveal the biogeographic diversification of the eastern Asian-eastern North American disjunct hickory genus (Carya Nutt.). – PLoS ONE 8: e70449, doi: 10.1371/journal.pone.0070449.
Zhang Z, Torrey JG. 1985. Studies of an effective strain of Frankia from Allocasuarina lehmanniana of the Casuarinaceae. – Plant and Soil 87: 1-16.
Zhang Z, Lopez M, Torrey JG. 1984. A comparison of the cultural characteristics and infectivity of Frankia isolates from root nodules of Casuarina species. – Plant and Soil 78: 79-90.
Zhang Z-Y, Chen Z-D. 1994. Embryology of Carpinus L. and its systematic significance. – Cathaya 5: 59-68.
Zhang Z-Y, Lu A-M, Wen J. 1994. Embryology of Rhoiptelea chiliantha (Rhoipteleaceae) and its systematic relationship. – Cathaya 6: 57-66.
Zheng Z-H, Wang P-L, Pu F-D. 1999. A comparative study on pollen exine ultrastructure of Nothofagus and the other genera of Fagaceae. – Acta Phytotaxon. Sin. 37: 253-258.
Zhou W, Xia N-H. 2012. Leaf epidermal features of Lithocarpus (Fagaceae) from China and their systematic significance. – Bot. J. Linn. Soc. 168: 216-228.
Zhou Z, Wilkinson H, Zheng-Yi W. 1995. Taxonomical and evolutionary implications of the leaf anatomy and architecture of Quercus L. subgenus Quercus from China. – Cathaya 7: 1-34.
Zhu J-Y, Zhang L-F, Shen P, Ren B-Q, Yu L, Chen Z-D. 2014. The morphogenesis of inflorescence and flower in Corylus (Betulaceae). – Plant Div. Res. 36: 433-442.
Zimpfer JF, Kennedy GJ, Smyth CA, Hamelin J, Navarro E, Dawson JO. 1999. Localization of Casuarina-infective Frankia near Casuarina cunninghamiana trees in Jamaica. – Can. J. Bot. 77: 1248-1256.
Zoldos V, Siljak-Yakovlev S, Papes D, Sarr A, Panaud O. 2001. Representational difference analysis reveals genomic differences between Q. robur and Q. suber: implications for the study of genome evolution in the genus Quercus. – Mol. Genet. Genom. 265: 234-241.