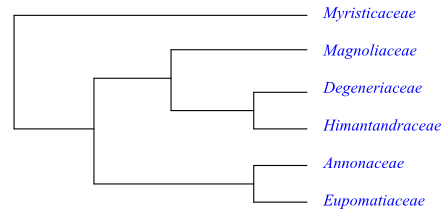
Phylogeny of Magnoliales based on morphological and DNA sequence data (Qiu & al. 1999; Sauquet & al. 2003).
[[Magnoliales+Laurales]+[Canellales+Piperales]]
Fossils Fossil flowers, which may be assigned to Magnoliales or closely related lineages, are comparatively frequent in some Turonian layers (the Sayreville Turonian angiosperm flora). A cupular receptaculum is present in several of these fossil remnants. Endressinia brasiliana, from the Upper Aptian to the Lower Albian of Brazil, consists of leafy shoots with pedicellate apocarpous flowers. The shallow receptacle have tepals along their margin, a spiral of presumed glandular staminodia inside these and central spirally arranged plicate carpels.
Habit Usually bisexual (sometimes monoecious or dioecious), evergreen or deciduous trees, shrubs or lianas (rarely dwarf-shrubs or suffrutices). Often aromatic.
Vegetative anatomy Phellogen ab initio usually superficial. Medulla septate, with sclerenchymatous diaphragmata. Primary stem with eustele or (pseudo)siphonostele with separate vascular bundles or with continuous vascular cylinder. Vessel elements with usually scalariform or simple (rarely reticulate) perforation plates; lateral pits alternate, scalariform or opposite, usually simple or reduced bordered pits. Imperforate tracheary xylem elements tracheids, fibre tracheids or libriform fibres with simple or bordered pits, septate or non-septate, or absent. Wood rays uniseriate or multiseriate, homocellular or heterocellular. Axial parenchyma apotracheal diffuse, diffuse-in-aggregates or banded, or paratracheal scanty vasicentric, scalariform, reticulate, banded, or absent. Secondary phloem stratified. Sieve tube plastids Ss or Psc type (rarely Pcs or Pcsf type). Nodes 3–≥5(–11):3–≥5(–11), trilacunar, pentalacunar or multilacunar with three, five or up to eleven leaf traces (sometimes 1:1, unilacunar with one trace). Secretory cavities with resins present or absent. Wood rays sometimes with oil cells or crystals. Idioblasts with ethereal oils often present. Asterosclereids often present. Silica bodies often abundant in xylem walls. Calciumcarbonate as prismatic or acicular crystals, styloids, druses or crystal sand often present.
Trichomes Hairs unicellular or multicellular, usually uniseriate, simple or branched (sometimes stellate or dendritic, rarely peltate-lepidote, candelabra-shaped, T-shaped or fimbriate), or absent.
Leaves Alternate (spiral or distichous), simple, usually entire (rarely lobed), with conduplicate or convolute ptyxis. Stipules usually absent (sometimes early caducous, ocreate, open just opposite petiole, enclosing young leaf); leaf sheath absent. Petiole vascular bundle transection arcuate (with an adaxial plate consisting of vascular tissue) or annular (sometimes bicollateral). Palisade parenchyma present. Venation usually pinnate, eucraspedodromous or brochidodromous (rarely palmate). Stomata usually paracytic (rarely anomocytic or actinocytic). Cuticular wax crystalloids as platelets or rodlets (often transversely ridged Aristolochia type crystalloids), chemically characterized by presence of palmitone (hentriacontan-16-one) and absence of nonacosan-10-ol. Lamina often gland-dotted. Mesophyll usually with idioblasts (secretory cavities) containing ethereal oils, resin or mucilage. Sclerenchymatous idioblasts with branched sclereids of various kinds (also asterosclereids) or fibres often frequent. Leaf margin entire.
Inflorescence Terminal, axillary or supra-axillary, cymose, paniculate or fasciculate, or flowers solitary axillary, terminal or pseudo-axillary (terminal on small axillary short shoots). Floral prophylls (bracteoles) usually single (rarely paired).
Flowers Actinomorphic. Usually hypogyny (rarely half epigyny, with urceolate, campanulate or infundibuliform receptacle and without tepals). Outer tepals (two or) three (or four), with valvate or imbricate aestivation, spiral or whorled, sepaloid or petaloid, free or connate at base; inner tepals three to numerous, with valvate or imbricate aestivation, petaloid, spiral or whorled, usually free (rarely connate at base). Nectary usually absent. Disc absent.
Androecium Stamens c. 20 to more than 200, laminar (foliaceous), spiral, not differentiated into filament and anther, with separate microsporangia embedded in distal part, or differentiated into filament and anther. Filaments when present usually free from each other (rarely connate at base), free from tepals; filaments often with three vascular bundles. Anthers when present basifixed, non-versatile, tetrasporangiate, sometimes with transversely septate thecae, usually extrorse (rarely latrorse or introrse), longicidal (dehiscing by longitudinal slits) or valvicidal (dehiscing by valves), sometimes connate into synandrium; or microsporangia four, usually adaxial or lateral (sometimes abaxial), usually introrse or latrorse (sometimes extrorse), longicidal (dehiscing by longitudinal slits) or valvicidal (dehiscing by valves). Tapetum secretory. Staminodia extrastaminal, intrastaminal, or absent.
Pollen grains Microsporogenesis simultaneous, or intermediate between simultaneous and successive (cytoplasm partially divided after first meiotic nuclear division by callose furrow, developing centripetally and orthogonally relative to spindle; growth of callose furrow subsequently ceasing and later completed after second nuclear division; cell plate not formed), or sometimes probably successive. Pollen grains usually monosulc(ul)ate (anasulcate) or inaperturate (rarely trichotomosulcate, zonizonasulc[ul]ate or ulcerate), boat-shaped, shed as monads, bicellular at dispersal. Exine tectate or intectate, with granular or intermediary infratectum, microperforate, rugulate or foveolate, gemmate, clavate, echinate or psilate.
Gynoecium Carpels usually ten to numerous (rarely one to three), spiral or whorled, usually free (sometimes connate or paracarpous); carpel plicate to conduplicate (sometimes basally ascidiate and not differentiated into ovary and style), usually postgenitally partially or entirely occluded by fusion and secretion, with secretory canal often open and filled by secretions (sometimes without canal). Ovary usually superior (rarely semi-inferior), unilocular to 15-locular or more. Stylodium single, simple. Stigma capitate, often decurrent, papillate, Dry or Wet type. Nectar sometimes secreted from exposed carpel surfaces. Pistillodium absent.
Ovules Placentation laminar, marginal, parietal, basal, subbasal or lateral. Ovules one to numerous per carpel, usually anatropous (rarely orthotropous or hemiorthotropous), ascending or pendulous, apotropous, usually bitegmic (rarely tritegmic), crassinucellar. Micropyle bistomal or endostomal (rarely exostomal). Funicular obturator sometimes present. Nucellar cap absent. Megagametophyte monosporous, Polygonum type. Antipodal cells and/or synergids ephemeral or persistent. Endosperm development usually cellular (sometimes nuclear). Chalazal endosperm haustoria sometimes present. Embryogenesis onagrad (Myosurus type) or irregular.
Fruit A usually fleshy (sometimes leathery or more or less woody), dehiscent or indehiscent, apocarpous follicular fruit or a cluster of follicles (multifolliculus), or a dry or fleshy pseudosyncarp, as ripe usually dehiscing and with sarcotestal seeds pendant in their funiculi, a loculicidal capsule or an assemblage of samaras (rarely berries or dry follicles).
Seeds Aril usually absent. Seed coat mesotestal or endotestal. Testa often vascularized. Exotesta and mesotesta sometimes as a multiplicative arilloid oily sarcotesta. Endotesta multi-layered, often with crystals. Tegmen unspecialized, usually crushed (often irregularly ruminate). Perisperm usually not developed. Endosperm usually copious, oily, often irregularly ruminate. Embryo small, straight, usually undifferentiated, without chlorophyll. Cotyledons two. Germination phanerocotylar.
Cytology x = 7–10, 12, 19
DNA A deletion of 30 bp (corresponding to 10 amino acids) present in PI-derived motif in nuclear gene AP3 in most Magnoliales (not in Myristicaceae).
Phytochemistry Flavonols (kaempferol, quercetin), flavones (velutin, 5-desoxyflavone, 5-desoxyisoflavone), flavanonols (taxifolin), diarylpropanes (1,3-diarylpropanes, 1,3-diaryl-2-hydroxypropanes) and other flavonoids, cyanidin, diterpenes (kauranes, clerodanes etc.), triterpenoids (tetracyclic etc.), oleanolic acid derivatives, sesquiterpenes, sesquiterpene lactones, oxyphenols and other aromatic substances, proanthocyanidins, caffeic acid, tannins, aporphine alkaloids (aporphines, oxoaporphines, etc.), benzylisoquinoline alkaloids (morphine etc.), protoberberine alkaloids (berberines etc.), C-methylated alkaloids, indole alkaloids (tryptamine), polyketide alkaloids (e.g. hallucinogenic pyridine alkaloids), quercetin glycosides, cyanogenic glycosides (triglochinin etc.), myristicin, lignans (veraguensin, dihydrocubebin), neolignans (aryltetralin, diaryltetrahydrofurans, etc.), polyketides (alkylsalicylate, acylfloroglucinol, arylalkylbutyrolactones, antibacterial acetogenins), nitrophenyl ethan, myo-inisitol, galbacin, licarin A, and amides present. Ellagic acid, gallo- and ellagitannins not found.
Systematics Myristicaceae are sister to the remaining Magnoliales. Features interpreted by Stevens (2001 onwards) and others as potential synapomorphies of Magnoliales except Myristicaceae, are, e.g., the following: primary stem containing eustele with often separate vascular bundles; wood rays wide; flowers solitary and large; floral receptacle sometimes well developed, usually with cortical vascular bundles; androecium consisting of a large number of spirally arranged stamens; filaments (when present) three-veined; thecae separate and embedded in broad foliaceous filaments; connective often prolonged; gynoecium often consisting of spirally arranged carpels; funicular obturator sometimes present; testa sometimes multiplicative; and a 30 bp (corresponding to ten amino acids) in the PI-derived motif of the nuclear AP3 gene.
Degeneria (Degeneriaceae) and Galbulimima (Himantandraceae) share the characters: axillary flowers; annular outer integument; indehiscent fruit; and x=12. Moreover, Annonaceae and Eupomatia (Eupomatiaceae) have the following characters in common: 8- to 15-seriate wood rays; stem leaves spirally arranged; arcuate petiole bundle transection; presence of inflorescence; baccate fruit; and fibrous mesotesta.
These four clades have usually: valvate anthers and intrastaminal staminodia; intectate pollen grains with psilate sexine and granular infratectum; differentiated pollen tube transmission tissue; and indehiscent fruit. However, molecular data etc. recover Magnoliaceae as sister-group to [Degeneria+Galbulimima] and this clade as sister to [Annonaceae+Eupomatia].

|
Phylogeny of Magnoliales based on morphological and DNA sequence data (Qiu & al. 1999; Sauquet & al. 2003). |
ANNONACEAE Juss. |
( Back to Magnoliales ) |
Annonales Juss. ex Bercht. et J. Presl, Přir. Rostlin: 223. Jan-Apr 1820 [‘Anoneae’]; Hornschuchiaceae J. Agardh, Theoria Syst. Plant.: 65. Apr-Sep 1858 [’Hornschuchieae’]; Monodoraceae J. Agardh, Theoria Syst. Plant.: 126. Apr-Sep 1858 [’Monodoreae’]; Annonanae Doweld, Tent. Syst. Plant. Vasc.: xxiv. 23 Dec 2001
Genera/species 105–106/2.345–2.360
Distribution Pantropical, with their highest diversity in tropical parts of the Old World; Asimina also in temperate eastern North America.
Fossils Fossilized seeds (e.g. Anonaspermum) are fairly abundant in Early Cenozoic layers of Europe and the Middle East (e.g. the Early Eocene London Clay of England). Foveomorphomonocolpites is a Maastrichtian to Paleocene pollen fossil of presumed annonacean affinity. Likewise, the fossil flower of Futabanthus asamigawaensis from the Early Coniacian of Japan possibly represents an early Annonaceae.
Habit Usually bisexual (sometimes dioecious, rarely monoecious), evergreen or deciduous trees, shrubs or lianas. Often aromatic.
Vegetative anatomy Phellogen ab initio usually superficial. Primary stem usually with eustele or (pseudo)siphonostele with continuous vascular cylinder. Cortical vascular tissue present. Medulla and cambium usually stratified. Medulla often septated by diaphragmata. Vessel elements usually with simple (sometimes scalariform) perforation plates; lateral pits usually alternate (rarely opposite?), bordered pits. Imperforate tracheary xylem elements fibre tracheids with simple and/or bordered pits, non-septate (also vasicentric tracheids). Wood rays usually multiseriate (rarely uniseriate), homocellular or somewhat heterocellular. Axial parenchyma apotracheal, banded, or paratracheal scanty vasicentric, scalariform, reticulate, or banded. Secondary phloem usually stratified into hard fibrous and soft parenchymatous layers. Sieve tube plastids usually Pcs type (in some species of Xylopia Pcsf type), with large protein crystalloids, often with protein fibrils. Nodes usually 3:3, trilacunar with three leaf-traces (median trace split; lateral traces often originating deep inside internode; sometimes 1:1, unilacunar with a single trace). Parenchyma often storied. Wood in some species fluorescent. Medulla and cortex usually with sclerenchyma cells. Wood rays sometimes with oil cells or crystals. Secretory cavities with resins present or absent. Prismatic crystals and druses sometimes abundant (in some species also styloids, crystal sand and/or acicular crystals).
Trichomes Hairs uniseriate, multicellular, simple or branched, with usually pointed terminal cell, stellate (peltate-lepidote in, e.g., Duguetia), or absent.
Leaves Alternate (usually distichous), simple, entire, with ? ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle transection arcuate or annular. Venation pinnate, eucraspedodromous or brochidodromous. Stomata usually paracytic (sometimes allelocytic etc.). Cuticular wax crystalloids as platelets or rodlets? Abaxial domatia (as pockets or hair tufts) present in some genera. Lamina often gland-dotted. Mesophyll usually with idioblasts (secretory cavities) with ethereal oils, resin or mucilage. Sclerenchymatous idioblasts with branched sclereids (also asterosclereids) or fibres abundant, or absent. Calciumoxalate crystals present. Leaf margin entire.
Inflorescence Terminal, axillary or supra-axillary (inserted between two nodes), cymose, or flowers solitary axillary. Floral prophyll (bracteole) usually single, median, adaxial (bracteoles rarely paired, lateral).
Flowers Actinomorphic. Hypogyny. Outer tepals (two or) three (or four), with open, valvate or imbricate aestivation, sepaloid, often thick and fleshy, in one whorl, free or connate at base; inner tepals three to six (to twelve), with open, valvate or imbricate aestivation, petaloid, often thick and fleshy, usually in one or two (rarely three) series, usually free (rarely connate at base). Adaxial side of inner tepals often with osmophores or perfume glands, often in collateral pairs, sometimes with nectaries in bands at base. Disc present.
Androecium Stamens c. 25 to several hundred, spiral, or three to six (to 15), whorled, with separate thecae embedded in distal part. Filaments usually free (rarely connate at base), free from tepals. Anthers basifixed, non-versatile, tetrasporangiate, sometimes with transversally septate thecae, usually extrorse (rarely latrorse or introrse), longicidal (dehiscing by longitudinal slits) or valvate (dehiscing by valves); connective usually peltate-truncate, often widened. Tapetum secretory, with usually binucleate (sometimes tri- or quadrinucleate) cells. Extrastaminal staminodia present in some species.
Pollen grains Microsporogenesis at least sometimes intermediate between simultaneous and successive (similar to Magnoliaceae). Pollen grains inaperturate or monosulc(ul)ate (rarely zonizonasulculate, with two circular sulculi, or ulcerate), usually shed as monads (rarely tetrads or polyads), bicellular at dispersal. Exine tectate or intectate, with granular infratectum, microperforate, perforate, microreticulate, foveolate, gemmate, clavate, echinate or psilate; endexine sometimes lamellate.
Gynoecium Carpels usually ten to c. 100 (rarely one to three), spiral or whorled (when three, then antesepalous), usually free (sometimes partially or entirely connate or paracarpous; in some species fused when mature); carpel plicate, usually postgenitally partially closed, with secretory canal open and filled by secretions; extragynoecial compitum present (internal and partial compitum rarely present). Ovary superior, unilocular to multilocular (bilocular to 15-locular or more). Stylodia single, simple, short, thick. Stigma capitate to U-shaped, papillate, Wet type. Pistillodium absent.
Ovules Placentation parietal, (sub)basal or lateral. Ovules one to numerous per carpel (or rarely per ovary), anatropous, ascending, apotropous, usually bitegmic (rarely tritegmic, with a multiplicative seedcoat-forming integument between inner and outer integuments), crassinucellar (outer integument in Monodora relatively thin). Micropyle endostomal. Outer integument at least four cell layers thick, vascularized. Inner integument two or three cell layers thick. Nucellar cap absent. Hypostase present or absent. Chalaza perichalazal. Obturator? Megagametophyte monosporous, Polygonum type. Antipodal cells ephemeral. Endosperm development ab initio cellular. Endosperm haustoria? Embryogenesis onagrad.
Fruit A usually fleshy (sometimes partially lignified), dehiscent or indehiscent, apocarpous follicular fruit or a cluster of follicles, multifolliculus etc. (rarely berries; in Anaxagorea dry follicles).
Seeds Seeds often relatively large, usually without wings (in ‘Richella’ winged). Aril absent or present (e.g. Anaxagorea, Annona, Canangium and Xylopia with a micropylar aril). Seed coat mesotestal. Testa multiplicative, ruminate (vascularized?). Exotesta unspecialized. Mesotesta a fibrous (consisting of crushed longitudinal and/or transverse fibres) sarcotesta. Endotesta with crystals (with thin-walled, elongate fibres); endotestal plug often present. Tegmen crushed, usually regularly (in some species irregularly) ruminate. Perisperm usually not developed. Endosperm copious, ruminate with usually regularly spiniform or lamelliform transverse ruminations, hard, oily (with amyloid?). Embryo small, straight, without chlorophyll. Cotyledons two. Germination phanerocotylar or cryptocotylar.
Cytology x = 7, 8, 9 (up to n = 32) – Polyploidy occurring.
DNA Plastid gene infA lost/defunct (Annona). Mitochondrial coxI intron present in Asimina. 30 bp deletion in PI-derived motif in nuclear gene AP3?
Phytochemistry Flavonols (quercetin, myricetin), cyanidin, diterpenes (kauranes, clerodanes etc.), triterpenoids (tetracyclic etc.), tannins, proanthocyanidins, aporphine alkaloids (aporphines, oxoaporphines, etc.), benzylisoquinoline alkaloids (morphine etc.), protoberberine alkaloids (berberine), azaphenanthrene alkaloids, C-methylated alkaloids, cyanogenic compounds, polyketides (antibacterial acetogenins), and nitrophenyl ethan present. Sesquiterpenes? Lignans? Neolignans? Ellagic acid not found.
Use Ornamental plants, fruits (Annona atemaya, cherimoya, ilarma, Artabotrys, Rollinia), spices, perfumes (Cananga odorata, Mkilua fragrans, Artabotrys odoratissima), medicinal plants, timber.
Systematics The sister-group of Annonaceae is Eupomatia (Eupomatiaceae).
Anaxagoreoideae Chatrou, Pirie, Erkens et Couvreur in Bot. J. Linn. Soc. 169(1): 32. 18 Apr 2012
1/c 30. Anaxagorea (c 30; Sri Lanka, Indochina, West Malesia, the Moluccas, tropical Central and South America). – Shrubs or small trees. Hairs uniseriate, with rounded terminal cell. Petiole vascular bundle transection annular, with three separate bundles often present in petiole and with adaxial bicollateral xylem plate in midvein. Stomata often allelocytic. Receptacle without vascular bundles. Stamens spirally arranged. Connective not peltate. Inner staminodia sometimes present. Pollen grains with lamellate endexine. Intine with massive inner layer having a channelled structure. Ovules two basal. Fruit a follicle, explosively dehiscing. Aril absent or rudimentary. Endotesta aerenchymatous (with only the tegmen being involved in the ruminations) and possessing idioblastic oil globules. x = 8. – Anaxagorea is sister to the remaining Annonaceae based on combined morphological and molecular data. Scharaschkin & Doyle (2005) suggested a West Gondwanan Eocene origin for Anaxagorea.
[Ambavioideae+[Annonoideae+Malmeoideae]
Hairs stellate. Internal staminodia absent. Endosperm with amyloid staining brownish violet.
Ambavioideae Chatrou, Pirie, Erkens et Couvreur in Bot. J. Linn. Soc. 169(1): 32. 18 Apr 2012
9/54. Meiocarpidium (1; M. lepidotum; tropical West Africa); Tetrameranthus (7; T. duckei, T. globuliferus, T. guianensis, T. laomae, T. macrocarpus, T. pachycarpus, T. umbellatus; tropical South America), Mezzettia (5; M. curtisii, M. herveyana, M. leptopoda, M. parviflora, M. umbellata; the Malay Peninsula to the Moluccas), Ambavia (2; A. capuronii, A. gerrardii; Madagascar), Cleistopholis (4; C. glauca, C. myristiciflora, C. patens, C. staudtii; tropical Africa), Lettowianthus (1; L. stellatus; Kenya, Tanzania), Drepananthus (26; tropical Asia to Fiji), Cananga (2; C. brandisia, C. odorata; tropical Asia to tropical Australia), Cyathocalyx (7; C. annamensis, C. globosus, C. harmandii, C. magnifructus, C. martabanicus, C. sumatranus, C. zeylanicus; tropical Asia to islands in southwestern Pacific). – Pantropical. Anthers with tongue-shaped connective. Pollen grains heteropolar-sulcate, with granular infratectum. Carpellary stipe articulated. Ovules usually two per carpel. A third integument present between the ordinary two integuments. x = 7.
[Annonoideae+Malmeoideae]
Annonoideae Raf., Anal. Nat.: 175. Apr-Jul 1815 [‘Annonidia’]
Bocageeae Endl., Gen. Pl.: 830. Jun 1839. 8/65. Mkilua (1; M. fragrans; Kenya, Tanzania), Porcelia (7; P. macrocarpa, P. magnifructa, P. mediocris, P. nitidifolia, P. ponderosa, P. stenbachii, P. venezuelensis; Central America, tropical South America), Cymbopetalum (27; Mexico to tropical South America), Cardiopetalum (3; C. calophyllum, C. plicatum, C. surinamense; tropical South America), Hornschuchia (10; eastern Brazil), Trigynaea (c 10; northern tropical South America), Bocagea (2–3; B. longipedunculata, B. viridis; eastern Brazil, possibly extinct), Froesiodendron (3; F. amazonicum, F. longicuspe, F. urceocalyx; Amazonia in Brazil). – Xylopieae Endl., Gen. Pl.: 831. Jun 1839. 2/c 260. Artabotrys (102; tropical regions in the Old World to tropical Australia), Xylopia (c 160; pantropical). – Duguetieae Chatrou, Pirie, Erkens et Couvreur in Bot. J. Linn. Soc. 169(1): 33. 18 Apr 2012. 5/100. Pseudartabotrys (1; P. letestui; tropical West Africa), Letestudoxa (3; L. bella, L. glabrifolia, L. lanuginosa; tropical West and Central Africa), Fusaea (3; F. decurrens, F. longifolia, F. peruviana; tropical South America), Duguetia (c 90; Central America, tropical South America, tropical West Africa), Duckeanthus (1; D. grandiflorus; tropical South America). – Guatterieae Hook. f. et Thomson, Fl. Ind. 1: 92, 126. Jul 1855. 1/c 210. Guatteria (c 210; southern Mexico, Central America, the West Indies, tropical South America). – Annoneae Endl., Gen. Pl.: 833. Jun 1839. 7–8/c 340. Anonidium (5; A. brieyi, A. floribundum, A. letestui, A. mannii, A. usambarense; tropical Africa), Neostenanthera (4; N. gabonensis, N. hamata, N. myristicifolia, N. robsonii; tropical Africa, tropical South America), Goniothalamus (c 135; India, Sri Lanka, Southeast Asia, West Malesia, New Caledonia), Annona (c 165; tropical Africa, tropical America), Disepalum (9; Southeast Asia, West Malesia), Asimina (17; eastern United States; incl. Deeringothamnus?), Deeringothamnus (2; D. pulchellus, D. rugelii; Florida; in Asimina?), Diclinanona (3; D. calycina, D. matogrossensis, D. tessmannii; eastern Peru, Brazil). – Monodoreae Baill., Hist. Pl. 1: 263, 288. Aug-Dec 1868. 11/c 90. Ophrypetalum (1; O. odoratum; tropical East Africa), Sanrafaelia (1; S. ruffonammari; Tanzania), Mischogyne (2; M. elliotianum, M. michelioides; tropical Africa), Uvariodendron (15; tropical Africa), Monocyclanthus (1; M. vignei; tropical West and Central Africa), Uvariopsis (18; tropical Africa), Isolona (20; tropical Africa, Madagascar), Monodora (14–16; tropical Africa), Asteranthe (3; A. asterias, A. lutea, A. trollii; tropical East Africa), Hexalobus (5; H. bussei, H. crispiflorus, H. monopetalus, H. mossambicensis, H. salicifolius; tropical and southern Africa, Madagascar), Uvariastrum (9; tropical Africa). – Uvarieae Hook. f. et Thomson, Fl. Ind. 1: 91, 92. 1-19 Jul 1855. 14/465–470. Dielsiothamnus (1; D. divaricatus; tropical East Africa), Mitrella (9; Malesia to tropical Australia), Fissistigma (c 65; tropical regions in the Old World), Uvaria (185–190; tropical Africa, Madagascar, tropical Asia to Japan and tropical Australia), Toussaintia (4; T. congolensis, T. hallei, T. orientalis, T. patriciae; tropical Africa), Sphaerocoryne (4; S. affinis, S. blanfordiana, S. diospyrifolia, S. gracilis; tropical Africa), Monanthotaxis (c 95; tropical and subtropical Africa, Madagascar), Friesodielsia (c 38; tropical and subtropical Asia), Dasymaschalon (c 27; tropical and subtropical Asia), Desmos (25; India, Southeast Asia to tropical Australia and islands in western Pacific), Afroguatteria (2; A. bequaertii, A. globosa; tropical Africa), Cleistochlamys (1; C. kirkii; tropical East Africa), Melodorum (12; Southeast Asia, Malesia to tropical Australia), Pyramidanthe (1; P. prismatica; West Malesia).
Malmeoideae Chatrou, Pirie, Erkens et Couvreur in Bot. J. Linn. Soc. 169(1): 34. 18 Apr 2012
Piptostigmateae Chatrou, Pirie, Erkens et Couvreur in Bot. J. Linn. Soc. 169(1): 34. 18 Apr 2012. 6/35. Annickia (8; tropical Africa), Greenwayodendron (2; G. oliveri, G. suaveolens; tropical Africa), Mwasumbia (1; M. alba; coastal Tanzania), Sirdavidia (1; S. solannona; Gabon), 'Piptostigma' (14; tropical Africa; non-monophyletic), Polyceratocarpus (9; tropical Africa). – Malmeeae Chatrou, Pirie, Erkens et Couvreur in Bot. J. Linn. Soc. 169(1): 34. 18 Apr 2012. 13/180. Unonopsis (48; Central America, tropical South America), Bocageopsis (4; B. canescens, B. mattogrossensis, B. multiflora, B. pleiosperma; Central America), Onychopetalum (2; O. amazonicum, O. periquino; Peru, Brazil), Malmea (7; M. depressa, M. dielsiana, M. dimera, M. guianensis, M. manausensis, M. obovata, M. surinamensis; southern Mexico, Central America, tropical South America), Pseudoxandra (23; tropical South America), Cremastosperma (29; tropical South America), Mosannona (14; Central America, tropical South America), Ruizodendron (1; R. ovale; Colombia, Ecuador, Peru, Amazonian Brazil, Bolivia), Ephedranthus (7; E. amazonicus, E. boliviensis, E. columbianus, E. dimerus, E. guianensis, E. parviflorus, E. pisocarpus; tropical South America), ‘Oxandra’ (27; Mexico, Central America, the West Indies, tropical South America; polyphyletic), Pseudomalmea (4; P. boyacana, P. darienensis, P. diclina, P. wingfieldii; Central America, northern tropical South America), 'Klarobelia' (12; Central America, tropical South America; non-monophyletic), Pseudephedranthus (2; P. enigmaticus: Guyana, Suriname, Pará in northern Brazil, P. fragrans; southern Venezuela, northeastern Brazil). – Maasieae Chatrou, Pirie, Erkens et Couvreur in Bot. J. Linn. Soc. 169(1): 34. 18 Apr 2012. 1/6. Maasia (6; M. discolor, M. glauca, M. hypoleuca, M. multinervis, M. ovalifolia, M. sumatrana; Southeast Asia, Malesia). – Fenerivieae Chatrou, Pirie, Erkens et Couvreur in Bot. J. Linn. Soc. 169(1): 35. 18 Apr 2012. 1/9–10. Fenerivia (9–10; Madagascar). – Phoenicantheae X. Guo et R. M. K. Saunders in Sci. Rep. 7: 7323. Aug 2017. 1/2. Phoenicanthus (2; P. coriacea, P. obliqua; Sri Lanka). – Dendrokingstonieae Chatrou, Pirie, Erkens et Couvreur in Bot. J. Linn. Soc. 169(1): 35. 18 Apr 2012. 1/3. Dendrokingstonia (3; D. acuminata, D. gardneri, D. nervosa; peninsular Thailand, the Malay Peninsula, Sumatra). – Monocarpieae Chatrou, Pirie, Erkens et Couvreur in Bot. J. Linn. Soc. 169(1): 35. 18 Apr 2012. 1/6. Monocarpia (6; M. blancoi, M. euneura, M. kalimantanensis, M. maingayi, M. marginalis, M. siamensis; Southeast Asia, West Malesia). – Miliuseae Hook. f. et Thomson, Fl. Ind. 1: 147. 1-19 Jul 1855. 23/490–495. Mitrephora (47; Southeast Asia, West Malesia), Platymitra (2; P. arborea, P. macrocarpa; West Malesia), Orophea (c 50; southern India, Hainan, Southeast Asia, Malesia to Moluccas), Trivalvaria (6; T. argentea, T. casseabriae, T. costata, T. macrophylla, T. nervosa, T. rubra; Assam to West Malesia), Pseuduvaria (57; India, Sri Lanka, Southeast Asia, Malesia to New Guinea and tropical Australia), Popovia (26; tropical Asia to tropical Australia), Huberanthus (27; tropical East Africa, Madagascar, the Comoros, southern India, Sri Lanka, Southeast Asia to New Guinea, Queensland, New Caledonia, Fiji), Alphonsea (25; tropical Asia to southern China and Malesia), Miliusa (c 50; Hainan and Malesia to Queensland), Wangia (1; W. sacopetaloides; Yunnan), Phaeanthus (9; India, Sri Lanka, Southeast Asia, Malesia), Sageraea (9; India, Sri Lanka, Southeast Asia to the Philippines), Stelechocarpus (1; S. burahol; Java), Winitia (2; W. cauliflora, W. expansa; Vietnam, peninsular Thailand, the Malay Peninsula, Borneo), ’Polyalthia’ (45–50; tropical regions in the Old World to Queensland; polyphyletic), Marsypopetalum (6; M. crassum, M. littorale, M. lucidum, M. modestum, M. pallidum, M. triste; Hainan, Southeast Asia, West Malesia), Neouvaria (7; N. acuminatissima, N. foetida, N. merrillii, N. parallelivenia, N. sparsistellata, N. telopea, N. viridifolia; West Malesia), Monoon (56; India, Sri Lanka, Ryukyu Islands, Hainan, Southeast Asia, West Malesia, New Guinea, northern Australia), Meiogyne (24; southwestern India, Hainan, Southeast Asia, Malesia to New Guinea, Queensland, New Caledonia, Micronesia, Polynesia), Wuodendron (1; W. praecox; Assam and Yunnan to Vietnam and Cambodia), Tridimeris (2; T. hahniana, T. tuxtlensis; Mexico), Sapranthus (7; S. campechianus, S. isae, S. microcarpus, S. nicaraguensis, S. palanga, S. violaceus, S. viridiflorus; Central America), Desmopsis (28; Mexico, Central America, Cuba, northwestern South America). – Piptostigmateae are sister-group to the rest and Malmeeae successive sister to the remaining Malmeoideae. The clade [Desmopsis+[Tridimeris+Sapranthus]] is proposed to form the Neotropical Sapranthinae (Ortiz-Rodriguez & al. 2016).
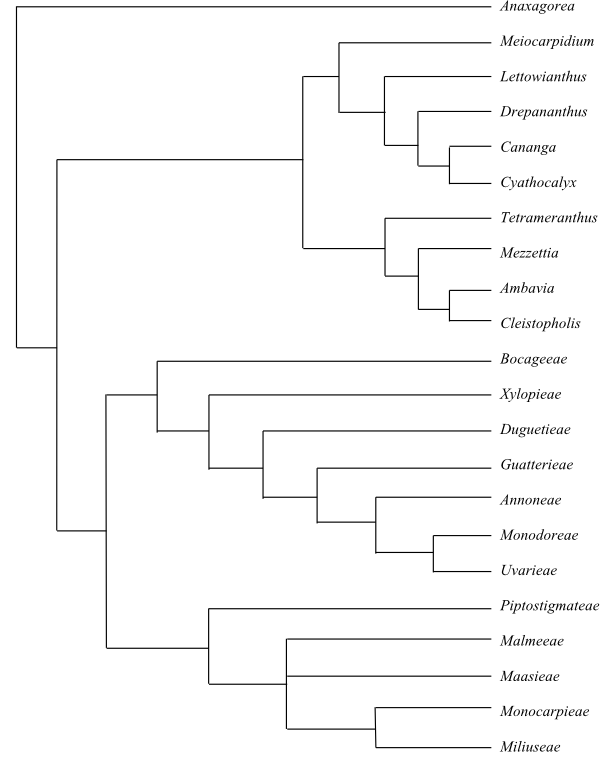 |
One of numerous most parsimonious cladograms of Annonaceae based on DNA data (Chatrou & al. 2012). |
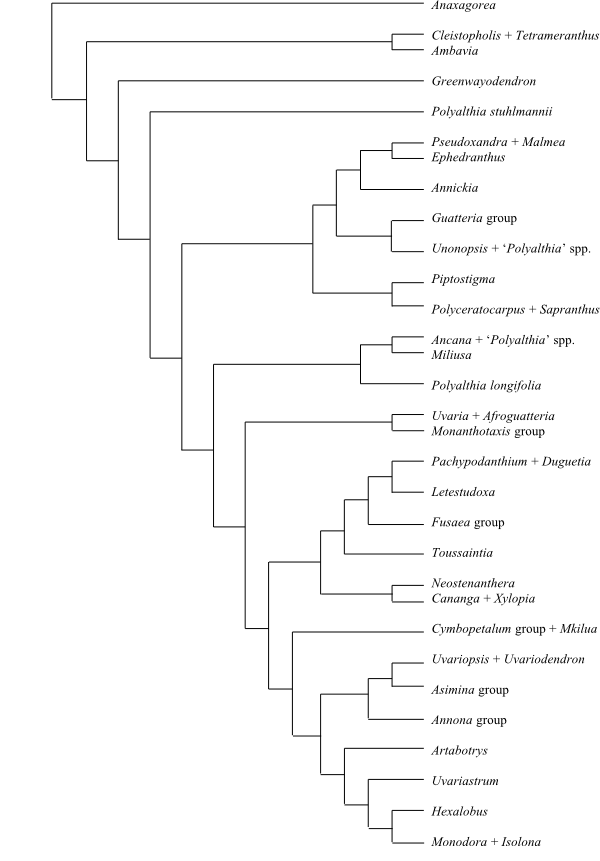 |
Cladogram of Annonaceae based on DNA sequence data (Doyle & Le Thomas 1997b). |
DEGENERIACEAE I. W. Bailey et A. C. Sm. |
( Back to Magnoliales ) |
Degeneriales C. Y. Wu in Acta Phytotaxon. Sin. 40: 291. 2002
Genera/species 1/2
Distribution Fiji.
Fossils Unknown.
Habit Bisexual, evergreen trees. Aromatic.
Vegetative anatomy Phellogen ab initio superficial. Primary stem containing eustele with separate vascular bundles. Cortical vascular tissue? Medulla septated by diaphragmata (with sclereids). Vessels usually in groups. Vessel elements with scalariform perforation plates; lateral pits scalariform. Imperforate tracheary xylem elements fibre tracheids with bordered pits, non-septate. Wood rays uniseriate or multiseriate, heterocellular. Axial parenchyma usually apotracheal diffuse, diffuse-in-aggregates or banded (paratracheal parenchyma rare or absent). Wood non-storied. Tyloses sometimes present in vessels. Secondary phloem stratified into hard fibrous and/or sclerified layers and soft parenchymatous layers. Sieve tube plastids Psc type (PI type), with a small protein crystalloid and approx. 15 starch grains. Nodes 5:5, pentalacunar with five leaf traces. Cells containing ethereal oils present in wood rays. Calciumoxalate druses frequent in phloem ray cells.
Trichomes Hairs absent.
Leaves Alternate (spiral), simple, entire, with ? ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle transection arcuate?, with medullary bundles as well. Venation pinnate. Stomata paracytic. Cuticular wax crystalloids as platelets. Lamina gland-dotted. Mesophyll with idioblasts containing ethereal oils. Secretory cells absent. Leaf margin entire.
Inflorescence Flowers supra-axillary, solitary (flower inserted between two nodes). Floral prophyll (bracteole) single, median.
Flowers Actinomorphic, large. Hypogyny. Outer tepals usually three (sometimes four), with imbricate aestivation, sepaloid, in a single whorl, fleshy, caducous, free; inner tepals twelve to c. 25, with imbricate aestivation, petaloid, spiral or whorled, fleshy, caducous, free. Nectary absent. Disc absent.
Androecium Stamens c. 20 to c. 30, laminar (foliaceous), spiral, free from each other and from tepals, not differentiated into filament and anther, with separate thecae embedded in distal part. Microsporangia four, abaxial, extrorse, longicidal (dehiscing by longitudinal slits) or valvate (dehiscing by valves); connective strongly prolonged and extended. Tapetum secretory. Staminodia three to approx. ten?, intrastaminal, foliaceous, fewer than fertile stamens, distinctly cucullate (domed above carpel), glanduliferous.
Pollen grains Microsporogenesis simultaneous. Pollen grains monosulcate (anasulcate, with often very elongate sulcus), shed as monads, bicellular at dispersal. Exine tectate, with granular infratectum, psilate.
Gynoecium Pistil composed of one foliaceous carpel (monocarpellate); carpel basally ascidiate, otherwise plicate, not differentiated into ovary and style, not completely closed in bud and early anthesis, postgenitally entirely occluded by fusion alone (without canal). Carpel superior, unilocular. Stigma adaxially deeply decurrent, papillate-hairy, type? Pistillodium absent.
Ovules Placentation laminar-lateral to marginal. Ovules c. 20 to 32 per carpel, anatropous, bitegmic (with lobed integuments), crassinucellar. Micropyle endostomal. Outer integument at least four cell layers thick, vascularized, annular. Inner integument two or three cell layers thick. Funicle long or absent. Funicular obturator distinct. Megagametophyte monosporous, Polygonum type. Endosperm development ab initio cellular. Endosperm haustoria? Embryogenesis?
Fruit A many-seeded, fleshy, ventricidal follicle with hard exocarp.
Seeds Aril absent. Seed coat endotestal. Testa multiplicative (and vascularized?). Exotesta palisade (cells radially elongate), thin-walled. Mesotesta an orange to red and oily sarcotesta. Endotesta multi-layered; endotestal cells with lignified fibrillar endoreticulum. Tegmen degenerating. Perisperm not developed. Endosperm copious, oily, ruminate. Multiseriate massive suspensor present. Embryo small, well differentiated, chlorophyll? Cotyledons usually three (sometimes four). Germination phanerocotylar.
Cytology x = 12 – Isozyme duplication not observed.
DNA Probably a 30 bp deletion in the PI-derived motif in the gene AP3?
Phytochemistry Very insufficiently known. Quercetin glycosides and cyanogenic compounds present. Sesquiterpenes? Alkaloids and proanthocyanidins not found.
Use Unknown.
Systematics Degeneria (2; D. roseiflora: Viti Levu in Fiji; D. vitiensis: Vanua Levu and Taveuni in Fiji).
Degeneria is sister to Galbulimima (Himantandraceae).
EUPOMATIACEAE Orb. |
( Back to Magnoliales ) |
Eupomatiales Takht. ex Reveal in Novon 2: 238. 13 Oct 1992
Genera/species 1/3
Distribution New Guinea, eastern and southeastern Australia.
Fossils Ambiguous.
Habit Bisexual, evergreen small trees, shrubs or dwarf-shrubs, with starch-storing tuberous roots (sometimes rhizomatous suffrutices). Often aromatic.
Vegetative anatomy Phellogen? Primary stem with eustele with separate vascular bundles. Cortical vascular tissue? Medulla non-septated, in young stems with secretory cells. Vessel elements with usually scalariform (sometimes reticulate) perforation plates; lateral pits scalariform to opposite, bordered pits. Vestured pits probably absent. Imperforate tracheary xylem elements fibre tracheids with simple and/or (reduced) bordered pits, septate. Wood rays uniseriate or multiseriate, heterocellular. Axial parenchyma absent or very rare (apotracheal diffuse, or paratracheal scanty). Secondary phloem stratified? Sieve tube plastids Pcs type (PIb type), with rod-shaped and polygonal protein crystalloids and few starch grains. Sieve tube nuclei with non-dispersive protein bodies? Nodes (6–)7(–11):(6–)7(–11), multilacunar with six to eleven leaf traces. Secretory idioblasts with ethereal oils relatively abundant in medulla and wood rays. Pith parenchyma cells often with single druses.
Trichomes Hairs (on young leaves) simple, ferrugineous, or absent.
Leaves Alternate (distichous), simple, entire, with ? ptyxis. Stipules and leaf sheath absent. Petiole on young stem with wings often extending to next lower node; petiole parenchyma with small lumps of crystals. Petiole vascular bundle transection arcuate. Venation pinnate, brochidodromous or eucamptodromous. Stomata usually paracytic (occasionally actinocytic). Cuticular waxes? Lamina often gland-dotted. Mesophyll usually with numerous idioblasts containing ethereal oils. Leaf margin entire.
Inflorescence Terminal or axillary, few-flowered, fasciculate, or flowers solitary axillary. Floral prophyll (bracteole) single, median.
Flowers Actinomorphic, large. Pedicel with approx. three bracts in two rows. Half epigyny. Receptacle urceolate. Tepals absent. Bud surrounded by calyptra, consisting of one or two perfoliate bracts with sclereids, dehiscing like an operculum at anthesis. Nectary absent. Disc absent.
Androecium Stamens c. 20 to more than 100, spiral, with separate thecae embedded in distal part. Filaments short, wide, connate at base, free from tepals. Anthers basifixed, non-versatile, tetrasporangiate, extrorse, longicidal (dehiscing by longitudinal slits); connective somewhat prolonged. Tapetum secretory, parietal. Staminodia c. 40 to c. 80, intrastaminal, petaloid, glandular, curved above gynoecium, connate at base and fused with fertile stamens to a synandrium; inner staminodia with mucilaginous unicellular hairs and covered by an oily secretion.
Pollen grains Microsporogenesis simultaneous. Pollen grains zonizonasulcate (with band-shaped aperture encircling equator), shed as monads, bicellular at dispersal. Exine intectate, with granular ’infratectum’, psilate.
Gynoecium Pistil composed of 13 to c. 70 largely connate, spiral carpels, enclosed in urceolate receptacle (postgenitally fused carpels); carpel (secondarily?) ascidiate, possibly primarily plicate; partial compitum present. Ovary semi-inferior, unilocular (apocarpy). Style absent. Stigma flattened, hairy, decurrent at base, papillate, type? Pistillodium absent.
Ovules Placentation sublaminal (ventral) to marginal. Ovules two to eleven per carpel, anatropous, apotropous, bitegmic, crassinucellar. Micropyle endostomal. Outer integument at least four cell layers thick. Inner integument two or three cell layers thick. Obturator? Megagametophyte monosporous, Polygonum type. Endosperm development cellular? Endosperm haustoria? Embryogenesis?
Fruit A many-seeded berry-like multifolliculus (secondarily syncarpous, sunken into receptacle). Exocarp with groups of stone cells.
Seeds Aril absent. Seed coat exotestal-mesotestal. Testa multiplicative, ruminate, non-vascularized? Exotestal cells with thickened, non-lignified walls. Mesotesta fibrous. Endotesta unspecialized, non-lignified. Tegmen unspecialized. Exotegmic cells cuboid, somewhat lignified. Endotegmic cells enlarged, crushed. Perisperm not developed. Endosperm copious, oily, ruminate. Embryo small, straight, well differentiated, chlorophyll? Cotyledons two. Germination phanerocotylar.
Cytology x = 10 – Isozyme duplication not observed.
DNA Probably a 30 bp deletion in the PI-derived motif in the gene AP3?
Phytochemistry Insufficiently known. Flavones (e.g. velutin), cyanidin and lignans present. Alkaloids? Sesquiterpenes? Proanthocyanidins? Flavonols, ellagic acid and cyanogenic compounds not found.
Use Ornamental plants, carpentries.
Systematics Eupomatia (3; E. barbata: northeastern Queensland, E. bennettii: northeastern New South Wales, eastern Queensland, E. laurina: eastern New Guinea, temperate parts of Victoria to subtropical regions of New South Wales and tropical Queensland).
Eupomatia is sister to Annonaceae. Staminodia and fertile stamens in Eupomatia surround the gynoecium in a perianth-like fashion, whereas the perianth itself is absent. This pattern and the calyptrate bracts surrounding the bud are similar to Galbulimima (Himantandraceae), yet is due to parallel evolution. The urceolate receptacle resembles that present in many Laurales.
HIMANTANDRACEAE Diels |
( Back to Magnoliales ) |
Himantandrales Doweld et Shevyryova in Ann. Bot. (London) 81: 345. Feb 1998
Genera/species 1/2
Distribution Sulawesi to Queensland.
Fossils Unknown.
Habit Bisexual, evergreen trees. Aromatic.
Vegetative anatomy Phellogen ab initio superficial. Primary stem containing eustele with separate vascular bundles. Cortical vascular tissue? Medulla septated by diaphragmata with sclereids. Vessel elements with usually simple (sometimes scalariform) perforation plates; lateral pits alternate, bordered pits. Imperforate tracheary xylem elements fibre tracheids with simple or bordered pits, non-septate. Wood rays uniseriate or multiseriate, homocellular. Axial parenchyma apotracheal diffuse, diffuse-in-aggregates, or banded. Secondary phloem usually stratified into hard fibrous and/or sclereidal layers, and soft parenchymatous layers. Sieve tube plastids S type, with approx. ten starch grains. Sieve elements with non-dispersive fusiform protein bodies. Nodes 3:3, trilacunar with three leaf traces. Heartwood with gum-like substances. Prismatic crystals abundant.
Trichomes Hairs multicellular, stellate, peltate, lepidote or fimbriate, copper-brown.
Leaves Alternate (distichous), simple, entire, with ? ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle transection arcuate? Venation pinnate, brochidodromous. Stomata paracytic. Cuticular waxes absent. Mesophyll cells often with sclereids and with idioblasts containing ethereal oils. Secretory cells abundant. Abaxial epidermis with pairs or lumps of crystals. Leaf margin entire.
Inflorescence Flowers usually axillary, solitary (rarely paired). Floral prophyll (bracteole) single, median.
Flowers Actinomorphic, large. Hypogyny. Tepals probably absent. Bud covered by two calyptrae, each consisting of a closed bract (possibly tepal?), dehiscing like an operculum at anthesis. Gynoecium surrounded by androecial receptacle. Nectary absent. Disc absent.
Androecium Fertile stamens 13 to c. 130, band-shaped, spiral, free from each other and from tepals, not differentiated into filament and anther, with separate thecae embedded in distal part. Microsporangia four, adaxial, sunken, valvate (dehiscing by longitudinal valves); connective much prolonged and widened. Tapetum secretory, with binucleate cells. Outer staminodia three to 23, extrastaminal, petaloid; inner staminodia 13 to c. 20, intrastaminal; inner staminodia and some fertile stamens glanduliferous.
Pollen grains Microsporogenesis successive. Pollen grains monosulcate (anasulcate), shed as monads, bicellular at dispersal. Exine intectate, with granular ’infratectum’, psilate.
Gynoecium Carpels six to ten (to 28), spiral, closed, ab initio almost free, in fruit more or less connate; carpel ascidiate, postgenitally fused in style, ‘ovary’ open and filled by secretions; extragynoecial compitum present. ’Ovary’ superior, multilocular. Stylodium single, simple, short. Stigma papillate, Wet type. Gynoecial apex at anthesis covered by mucilage layer (mucilaginous compitum?). Pistillodium absent.
Ovules Placentation apical to laminar-marginal. Ovule usually one (rarely two) per carpel, anatropous, pendulous, apotropous, bitegmic, crassinucellar. Micropyle endostomal. Integuments lobed? Outer integument at least four cell layers thick, vascularized, annular. Inner integument two or three cell layers thick. Obturator? Megagametophyte monosporous, Polygonum type. Antipodal nuclei ephemeral (rarely forming complete cells). Endosperm development cellular? Endosperm haustoria? Embryogenesis?
Fruit A drupaceous secondary syncarp with several usually one-seeded pyrenes.
Seeds Seeds laterally flattened, without long funicle. Seed coat mesotestal. Testa non-multiplicative. Exotesta unspecialized. Mesotesta partly fibrous, partly sarcotestal. Endotesta aerenchymatous, unspecialized. Tegmen unspecialized. Perisperm not developed. Endosperm copious, oily, not ruminate. Embryo small, straight, chlorophyll? Cotyledons two. Germination phanerocotylar.
Cytology x = 12
DNA Probably a 30 bp deletion in the PI-derived motif in the gene AP3?
Phytochemistry Insufficiently known. Sesquiterpenes, polyketide alkaloids (hallucinogenic pyridine alkaloids etc.), and neolignans (aryltetralin and diaryltetrahydrofurans) present. Flavonoids? Proanthocyanidins?
Use Medicinal plants.
Systematics Galbulimima (2; G. baccata: northeastern Queensland, G. belgraveana: Sulawesi, the Moluccas, New Guinea, New Britain, northeastern Queensland).
Galbulimima is sister to Degeneria (Degeneriaceae). Staminodia and fertile stamens in Galbulimima surround the gynoecium in a perianth-like fashion, whereas the perianth itself is either absent or transformed into bract-like calyptrae covering the bud. Hence, the flower resembles that in Eupomatia (Eupomatiaceae), although the similar patterns have evolved in parallel.
MAGNOLIACEAE Juss. |
( Back to Magnoliales ) |
Liriodendraceae F. A. Barkley in Phytologia 32: 304. 29 Oct 1975
Genera/species 2/210–220
Distribution Southern India, Sri Lanka, eastern Himalayas, Assam, East Asia to the Korean Peninsula and Japan, Southeast Asia, Malesia to New Guinea, New Britain, southeastern North America, Mexico, Central America, the West Indies, scattered areas in northern and central South America from Colombia to Uruguay.
Fossils Fossil wood, leaves and seeds of Magnoliaceae are known from the mid-Cretaceous onwards and abundant in Cenozoic layers in many places in North America, Greenland, Svalbard, Europe and Asia. Fossil pollen grains are rarely found. Fossilized wood is known as Liriodendroxylon, Magnolioxylon and Magnoliaceoxylon, and fossil seeds as Liriodendroidea (winged) and Magnoliaespermum. Fossil leaves (Liriodendrites, Liriodendropsis, Liriophyllum, etc.) are known from Late Cretaceous layers of North America and Europe. Archaeanthus – bilobate leaves, flowers and many-seeded spirally arranged stipitate follicles – from the Albian to the Cenomanian of North America may be assigned to Magnoliaceae, as well as Litocarpon (fruits and seeds) from the Santonian to the Campanian of Canada.
Habit Usually bisexual (in Magnolia Section Kmeria monoecious), evergreen or deciduous trees or shrubs.
Vegetative anatomy Phellogen ab initio superficial. Primary stem with eustele with separate vascular bundles. Cortical vascular tissue present. Medulla often septated by diaphragmata (sometimes with sclereids). Vessel elements with scalariform and/or simple perforation plates; lateral pits scalariform or opposite, usually simple (rarely bordered) pits. Imperforate tracheary xylem elements tracheids or fibre tracheids with simple and/or bordered pits, non-septate, or absent. Wood rays uniseriate or multiseriate, homocellular or heterocellular. Axial parenchyma apotracheal diffuse, or paratracheal scanty or banded, or absent. Wood fluorescent. Tyloses sometimes abundant. Secondary phloem often stratified into hard fibrous and/or sclereidal layers, and soft parenchymatous layers. Sieve tube plastids S type, with approx. ten starch grains, or Psc type, with a single small protein crystalloid and few starch grains. Nodes ≥5:≥5, pentalacunar or multilacunar with five or more leaf traces. Secretory cells absent. Idioblasts with ethereal oils often present. Silica often abundant in xylem walls. Calciumcarbonate as, e.g., acicular crystals present in many species.
Trichomes Hairs unicellular or multicellular, usually uniseriate, simple (sometimes stellate), or absent.
Leaves Alternate (spiral), simple, usually entire (in Liriodendron lobate), with ? ptyxis. Stipules early caducous, often ochreate, open just opposite petiole, enclosing young leaf; leaf sheath absent. Petiole vascular bundle transection arcuate?, usually with medullary bundles as well (in Liriodendron only as a cylinder). Venation usually pinnate (in Liriodendron palmate). Stomata usually paracytic (rarely anomocytic). Cuticular wax crystalloids as platelets or rodlets (or tubuli?). Lamina sometimes gland-dotted. Mesophyll often with mucilage cells, often with idioblasts with ethereal oils. Sclereids of various types (also asterosclereids) present in leaf cells of some species. Leaf margin entire.
Inflorescence Flowers terminal or pseudo-axillary (in reality terminal on small axillary short shoots), usually solitary (sometimes few together). Bud surrounded by caducous bracts, falling off at anthesis.
Flowers Actinomorphic, large. Hypogyny. Outer tepals three, with imbricate aestivation, sepaloid or petaloid, spiral or whorled, free; inner tepals three to numerous, with imbricate aestivation, petaloid, spiral or whorled, free. Sclerenchymatous diaphragms well developed in Magnolia Section Michelia. Nectary usually absent. Disc absent.
Androecium Stamens c. 50 to more than 200, spiral, laminar (foliaceous), not differentiated into filament and anther, with separate thecae embedded in distal part. Microsporangia four, usually adaxial or lateral (in Liriodendron abaxial), usually introrse or latrorse (in Liriodendron extrorse), longicidal (dehiscing by longitudinal slits) or valvate (dehiscing by valves); connective somewhat prolonged. Tapetum secretory, with multinucleate cells. Staminodia absent.
Pollen grains Microsporogenesis intermediate between simultaneous and successive: cytoplasm after first nuclear division partly dividing by callose furrow, developing centripetally and orthogonally relative to meiotic spindle; growth of callose furrow ceasing and later completed after second nuclear division; cell plate not formed. Pollen grains usually monosulcate (anasulcate; rarely trichotomosulcate), boat-shaped, shed as monads, bicellular at dispersal. Exine tectate or intectate, with intermediary to granular infratectum, microperforate or often rugulate.
Gynoecium Carpels usually numerous (in one species of Magnolia Section Michelia a single carpel, monocarpellary), ab initio free, in some species finally more or less connate, spiral; carpel ascidiate or plicate, postgenitally completely fused, with secretory canal, often stalked (on gynophore). Ovary superior, unilocular or multilocular. Style single, simple. Stigma terminal, often elongated, papillate, Dry type. Nectar in some species of Magnolia secreted from exposed surface of carpels. Pistillodium absent.
Ovules Placentation laminar (ventral, marginal). Ovules (one or) two to twelve (to 16) per carpel, anatropous, pendulous, bitegmic, crassinucellar, with secretory canal. Micropyle usually bistomal (in Magnolia Section Michelia exostomal). Integuments lobed. Outer integument at least four cell layers thick, vascularized. Inner integument two or three cell layers thick. Funicular obturator present in at least Liriodendron. Megagametophyte monosporous, Polygonum type. Antipodal cells and synergids ephemeral. Endosperm development cellular. Endosperm haustoria usually absent (chalazal endosperm haustoria present in, e.g., Magnolia obovata). Embryogenesis onagrad (Myosurus type) or irregular.
Fruit A dry or fleshy pseudosyncarp, often almost cone-shaped, as ripe usually dehiscing abaxially (sometimes both abaxially and adaxially or transversally dehiscent, rarely indehiscent) with fleshy sarcotestal seeds pendant in funiculi by extended annular thickenings on protoxylem, a loculicidal capsule (in Magnolia Section Pachylarnax) or an assemblage of samaras (Liriodendron).
Seeds Aril absent. Seeds in dehiscing fruits pendant in spirally twisted strongly elongated funicular vascular strand. Seed coat endotestal. Testa in Magnolia with simple or tubular pore (indicating passage of vascular strand through sclerotesta). Exotesta and mesotesta usually a multiplicative arilloid oily sarcotesta (absent in Liriodendron), and situated inside scleroendotesta (sclerotic endotesta) with crystals and lignified fibrils in cells. Endotesta multilayered, sclerenchymatous. Tegmen unspecialized, mostly crushed. Perisperm not developed. Endosperm usually copious (in Liriodendron scarce), usually not ruminate. Multiseriate massive suspensor present. Embryo small, usually undifferentiated, without chlorophyll. Cotyledons two. Germination phanerocotylar.
Cytology x = 19 – Polyploidy and aneuploidy occurring. Isozyme duplication indicating paleopolyploidy.
DNA The PI-derived motif of the nuclear gene AP3 has a deletion corresponding to ten amino acids.
Phytochemistry Flavonols (kaempferol, quercetin, myricetin), flavanonols (taxifolin), cyanidin, sesquiterpene lactones, proanthocyanidin, caffeic acid, isoquinoline alkaloids (benzylisoquinoline alkaloids, liriodenine), triglochinin (a cyanogenic glycoside), acuminatin (a bis-phenylpropide), lignans (calopiptin, galgravin, veraguensin, etc.), and neolignans present. Ellagic acid, gallo- and ellagitannins not found. Aluminium possibly accumulated in some species.
Use Ornamental plants, medicinal plants, timber, carpentries.
Systematics Magnolia (210–220; the Himalayas to Japan and West Malesia, eastern United States to tropical South America), Liriodendron (2; L. tulipifera: eastern North America; L. chinense: China).
Magnolia Section Talauma is sister to the remaining species of Magnolia.
Magnoliaceae are sister-group to [[Degeneria+Galbulimima]+[Eupomatia+Annonaceae]].
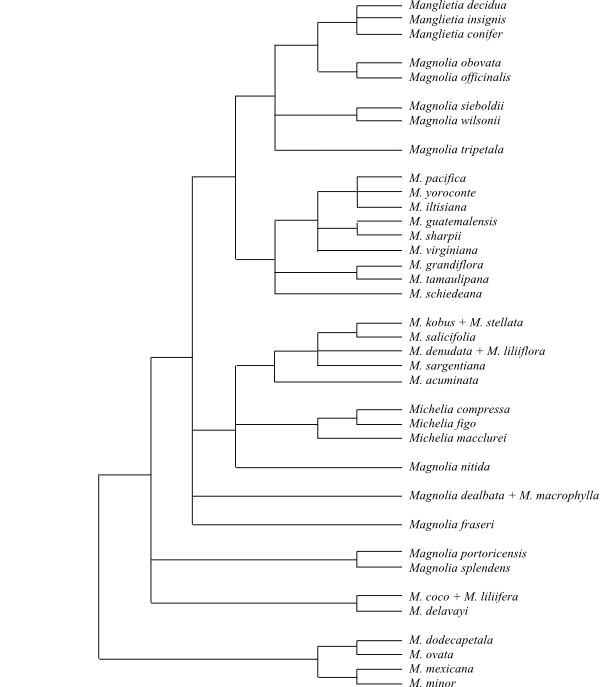 |
Maximum likelihood tree of Magnolia s.lat. based on DNA sequence data (Azuma & al. 2001). The neotropical clade Section Talauma is sister-group to the remainder. The former genera Elmerrillia, Kmeria, Manglietia, Michelia and Pachylarnax are nested inside Magnolia. |
MYRISTICACEAE R. Br. |
( Back to Magnoliales ) |
Myristicales R. Br. ex Bercht. et J. Presl, Přir. Rostlin: 235. Jan-Apr 1820 [’Myristiceae’]
Genera/species 20/475–485
Distribution Pantropical, with their largest diversity in tropical Asia.
Fossils Fossil wood from Paleocene layers of Sahara has been described under the name of Myristicoxylon, although it cannot unambiguously be assigned to Myristicaceae. An Early Eocene ruminate seed is known from the London Clay in England and several other Cenozoic fossil Myristicaceae have been found.
Habit Usually dioecious (in Endocomia and some Iryanthera monoecious), usually evergreen (rarely deciduous) trees (rarely shrubs or lianas). Often aromatic.
Vegetative anatomy Phellogen ab initio superficial (also in outer cortex). Primary stem with (pseudo)siphonostele with continuous vascular cylinder. Medulla often septated by diaphragmata. Vessel elements with simple and/or scalariform(-reticulate) perforation plates; lateral pits scalariform, opposite or alternate. Imperforate tracheary xylem elements libriform fibres with usually simple (sometimes bordered) pits, septate or non-septate. Wood rays uniseriate or multiseriate, usually heterocellular. Axial parenchyma apotracheal diffuse, or paratracheal scanty vasicentric, banded, or absent. Tyloses often abundant (sometimes sclerotic). Secondary phloem usually stratified into hard fibrous and/or sclereidal layers, and soft parenchymatous layers. Sieve tube plastids Psc type, with small protein crystalloids and ten or fewer starch grains, or Ss type, with c. 20 starch grains. Sieve element nuclei with non-dispersive protein bodies. Nodes 3:3, trilacunar with three leaf traces. Parenchyma, wood rays and phloem with tanniniferous canals containing reddish to yellowish resinous secretions. Idioblasts with ethereal oils present in many organs. Wood cells sometimes with silica bodies. Nucleus of young sieve element with a single protein crystal (rarely two crystals), which are released into cell lumen during nuclear degeneration and persist in enucleate mature sieve element (such crystals being unique among Magnoliidae).
Trichomes Hairs uniseriate, multicellular, dendritic, stellate (sometimes candelabrous), or T-shaped.
Leaves Alternate (usually distichous), simple, entire, with conduplicate or convolute ptyxis. Stipules and leaf sheath absent. Petiole vascular bundles bicollateral. Venation pinnate. Stomata paracytic. Cuticular wax crystalloids as platelets or rodlets. Lamina sometimes gland-dotted. Mesophyll cells and sometimes epidermal cells with acicular crystals or druses. Branched asterosclereids or fibres present in some genera. Mesophyll with idioblasts containing ethereal oils. Leaf margin entire.
Inflorescence Usually axillary (rarely terminal), panicle or fasciculate. Floral prophyll (bracteole) usually single (occasionally two).
Flowers Actinomorphic, usually small with little developed receptacle. Hypogyny. Tepals (two or) three (to five), with valvate aestivation, in one whorl, petaloid, often fleshy, connate at base and infundibuliform, campanulate or urceolate. Nectary absent. Disc absent.
Androecium Stamens two to c. 40, usually whorled (sometimes spiral). Filaments entirely or partially connate into a tubular, infundibuliform, cupular or globular structure; free from tepals. Anthers longifixed or basifixed, non-versatile, tetrasporangiate, usually extrorse (rarely latrorse), longicidal (dehiscing by longitudinal slits), usually connate into a synandrium (in Myristica with a sterile axial or staminal apex). Tapetum secretory. Staminodia absent.
Pollen grains Microsporogenesis probably successive. Pollen grains monosulcate (sulcoidate, spiraperturate or ulcerate) to almost inaperturate, shed as monads, bicellular at dispersal. Exine usually tectate (rarely intectate), with intermediary infratectum, microperforate (rarely psilate).
Gynoecium Pistil composed of one carpel; carpel ascoplicate (intermediate between ascidiate and plicate), postgenitally completely fused by secretory canal, sometimes shortly stipitate. Ovary superior, unilocular. Style single, simple, usually short (in Brochoneura long), or absent. Stigma simple or bifid, papillate, Dry type? Pistillodium absent.
Ovules Placentation basal or subbasal. Ovule one per ovary, usually anatropous (rarely orthotropous or hemiorthotropous), usually bitegmic (rarely tritegmic with a rudimentary intervening integument), crassinucellar. Micropyle endostomal. Outer integument at least four cell layers thick. Inner integument three to ten cell layers thick. Chalaza pachychalazal. Obturator? Megagametophyte monosporous, Polygonum type. Endosperm development ab initio nuclear. Endosperm haustoria? Embryogenesis?
Fruit A fleshy, leathery or woody follicle, usually both adaxially and abaxially dehiscent (rarely indehiscent).
Seeds Seed large, usually with a funicular-exostomal aril (absent in Haematodendron); aril usually large, entire or lobed, hard or fleshy, red, orange or yellow, partially enclosing seed. Seed coat endotestal. Testa vascularized, usually multiplicative. Inner epidermis of outer integument transformed into lignified palisade layer with crystals. Mesotesta stout, unspecialized. Endotesta palisade, with thick-walled, prismatic cells; parts of inner integument transformed into vascularized, massive and ruminate tegmen. Exotegmen with long wood fibres and sclerotic or tracheidal cells. Chalazal end with thick and lignified counter palisade. Perisperm not developed. Endosperm copious, oily, proteinaceous and starchy, ruminate. Embryo small, straight, well differentiated, without chlorophyll. Cotyledons two, often entirely or partially connate. Hypocotyl not developed. Germination cryptocotylar.
Cytology n = 19, 21, 22, 25, 26, 50, 51, c. 140 – Isozyme duplication in Myristica indicates paleopolyploidy.
DNA Mitochondrial coxI intron present.
Phytochemistry Flavonols (kaempferol, quercetin), flavones (5-desoxyflavone, 5-desoxyisoflavone), cyanidin, diarylpropanes (1,3-diarylpropanes, 1,3-diaryl-2-hydroxypropanes), oleanolic acid derivatives, sesquiterpenes, oxyphenols and other aromatic substances, proanthocyanidin, benzylisoquinoline alkaloids, protoberberine alkaloids, indole alkaloids (tryptamine), myristicin, lignans (veraguensin, dihydrocubebin), neolignans, polyketides (alkylsalicylate, acylfloroglucinol, arylalkylbutyrolactones), myo-inisitol, galbacin, licarin A, and amides present. Gallo- and ellagitannins not found.
Use Spices (Myristica fragrans: nutmeg from seeds, mace from arils), narcotics, medicinal plants, perfumes, carpentries.
Systematics Otoba (9; Central America, tropical South America), Coelocaryon (4; C. botryoides, C. oxycarpum, C. preussii, C. sphaerocarpum; tropical Africa), Pycnanthus (4; P. angolensis, P. dinklagei, P. marchalianus, P. microcephalus; tropical Africa), Staudtia (2; S. kamarunensis, S. pterocarpa; tropical West Africa to western Uganda), Cephalosphaera (1; C. usambarensis; mountains in tropical East Africa), Doyleanthus (1; D. arillata; Madagascar), Brochoneura (5; B. acuminata, B. chapelieri, B. dardainii, B. humblotii, B. rarabe; eastern Madagascar), Mauloutchia (10; eastern Madagascar); Compsoneura (17; southern Mexico, Central America, tropical South America), Endocomia (4; E. canarioides, E. macrocoma, E. rufirachis, E. virella; India, Sri Lanka, Southeast Asia, Malesia), Virola (c 75; tropical South America), Bicuiba (1; B. oleifera; Brazil), Haematodendron (1; H. glabrum; Madagascar), Iryanthera (c 20; Panamá, tropical South America), Osteophloeum (1; O. platyspermum; Amazonia in Brazil), Gymnacranthera (7; G. bancana, G. canarica, G. contracta, G. farquhariana, G. forbesii, G. maliliensis, G. ocellata; India, Sri Lanka, Southeast Asia, Malesia to New Guinea and the Bismarck Archipelago), Horsfieldia (c 100; tropical Asia to tropical Australia), Knema (c 60; India, Sri Lanka, Southeast Asia, Malesia to New Guinea), Scyphocephalium (2; S. mannii, S. ochocoa; Cameroon, Equatorial Guinea, Gabon), Myristica (150–160; tropical Asia to tropical Australia).
The flowers are unisexual. Parenchyma, wood rays and phloem contain canals with red, orange or yellow resins. The nucleus of the immature sieve element has one or rarely two protein crystals which are released inte the cytoplasm as the nucleus degenerates and persist within the mature nucleus-free sieve element. The androecium is very variable, although usually the stamens are borne in a single whorl and connate into a synandrium surrounding a central sterile column. Mauloutchia has (secondarily) free stamens, in some species spirally arranged and numerous, a small aril and distinctly granular exine. However, it does not hold a basal position in Myristicaceae. Instead, a clade comprising Otoba, Coelocaryon, Pycnanthus, Staudtia, Cephalosphaera, Doyleanthus, Brochoneura, and Mauloutchia (the “mauloutchioids” sensu Sauquet 2003) is sister-group to the remaining Myristicaceae.
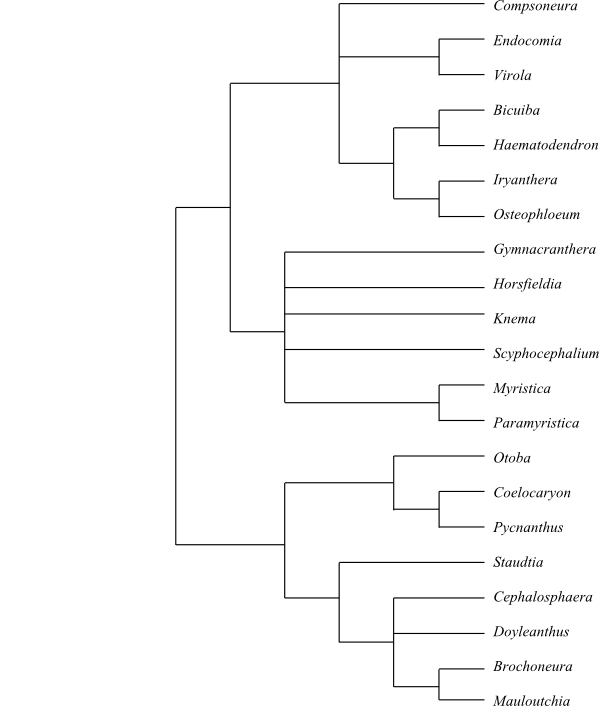 |
Cladogram of Myristicaceae based on morphology and DNA sequence data (Sauquet 2003). |
Literature
Abdelgaleil SAM, Hashinaga F. 2007. Allelopathic potential of two sesquiterpene lactones from Magnolia grandiflora L. – Biochem. Syst. Ecol. 35: 757-742.
Achenbach H, Hemrich H. 1991. Alkaloids, flavonoids and phenylpropanoids of the West African plant Oxymitra velutina. – Phytochemistry 30: 1265-1267.
Agababian VS. 1972. Pollen morphology of the family Magnoliaceae. – Grana 12: 166-176.
Airy-Shaw HK. 1939. Additions to the flora of Borneo and other Malay Islands XII. The Annonaceae of the Oxford Univ. Expedition to Sarawak, 1932. – Kew Bull. 1939: 275-290.
Almeida MEL de, Braz F R, Bülow MV von, Coreo JJL, Gottlieb OR, Maia JGS, Silva MS da. 1979. Diarylpropanoids from Iryanthera polyneura. – Phytochemistry 18: 1015-1016.
Andrade BM, Oliveira-Filho AT, Soares AR. 1995. Pollination ecology and breeding system of Xylopia brasiliensis Sprengel (Annonaceae) in southeastern Brazil. – J. Trop. Ecol. 11: 1-7.
Armstrong JE, Drummon III BA. 1986. Floral biology of Myristica fragrans Houtt.: the nutmeg of commerce. – Biotropica 18: 32-38.
Armstrong JE, Tucker SC. 1986. Floral development in Myristica (Myristicaceae). – Amer. J. Bot. 73: 1131-1141.
Armstrong JE, Wilson TK. 1978. Floral morphology of Horsfieldia (Myristicaceae). – Amer. J. Bot. 65: 441-449.
Azuma H, Thien LB, Kawano S. 1999a. Floral scents, leaf volatiles and thermogenic flowers in Magnoliaceae. – Plant Species Biol. 14: 121-127.
Azuma H, Thien LB, Kawano S. 1999b. Molecular phylogeny of Magnolia (Magnoliaceae) inferred from cpDNA sequences and evolutionary divergence of the floral scents. – J. Plant Res. 112: 291-306.
Azuma H, Thien LB, Kawano S. 2000. Molecular phylogeny of Magnolia based on chloroplast DNA sequence data (trnK intron, psbA-trnH and atpB-rbcL intergenic spacer regions) and floral scent chemistry. – In: Liu Y-H, Fan H-M, Chen Z-Y, Wu Q-G, Zeng Q-W (eds), Proceedings of the International Symposium on the Family Magnoliaceae, May 18-22, 1998, Guangzhou, China. Science Press, Beijing. pp. 219-227.
Azuma H, García-Franco JG, Rico-Gray V, Thien LB. 2001. Molecular phylogeny of the Magnoliaceae: the biogeography of tropical and temperate disjunctions. – Amer. J. Bot. 88: 2275-2285.
Bailey IW, Smith AC. 1942. Degeneriaceae, a new family of flowering plants from Fiji. – J. Arnold Arbor. 23: 356-365.
Bailey IW, Nast CG, Smith AC 1943. The family Himantandraceae. – J. Arnold Arbor. 24: 190-206.
Balgooy MMJ van. 1986. Himantandraceae. – Flora Malesiana Bull. 9: 298-299.
Bân NT. 1974a. Critical notes on the genera Melodorum Lour., Mitrella Miq. and Rauwenhoffia Scheff. (Annonaceae Juss.). – Bot. Žurn. 59: 237-245. [In Russian]
Bân NT. 1974b. On the taxonomy of the genus Goniothalamus (Blume) J. D. Hook. & Thomson (Annonaceae) 1. – Bot. Žurn. 59: 547-555. [In Russian]
Bân NT. 1975. A new genus of the Annonaceae Juss. – Enicosanthellum Bân. – Bot. Žurn. 60: 808-812. [In Russian]
Baranova M. 1969. Leaf anatomy of Magnoliaceae. – Bot. Žurn. 54: 19-52. [In Russian]
Baranova M. 1972. Systematic anatomy of the leaf epidermis in the Magnoliaceae and some related families. – Taxon 21: 447-469.
Baranova MA. 2004. The stomatal apparatus of Takhtajania perrieri (Capuron) M. Baranova & J.-F. Leroy (Winteraceae). – Kew Bull. 59: 141-144.
Barkley FA. 1975. Liriodendraceae fam. n., order Magnoliales. – Phytologia 32: 304.
Behnke H-D. 1971a. Über den Feinbau verdickter (nacré) Wände und der Plastiden in den Siebröhren von Annona und Myristica. – Protoplasma 72: 69-78.
Behnke H-D. 1971b. Sieve-tube plastids of Magnoliidae and Ranunculidae in relation to systematics. – Taxon 20: 723-730.
Behnke H-D. 1988. Sieve-element plastids, phloem protein, and evolution of flowering plants III. Magnoliidae. – Taxon 37: 699-732.
Behnke H-D. 1991. Sieve-element characters of Myristicaceae: nuclear crystals, S- and P-type plastids, nacreous walls. – Nord. J. Bot. 11: 333-344.
Bergström G, Groth I, Pellmyr O, Endress PK, Thien LB, Hübener A, Francke W. 1991. Chemical basis of a highly specific mutualism: chiral esters attract pollinating beetles in Eupomatiaceae. – Phytochemistry 30: 3221-3225.
Bernardi L, Spichiger R. 1980. Las Miristicáceas del Arboretum Jenaro Herrera. – Candollea 35: 133-182.
Berry PE, Miller RB, Wiedenhoeft AC. 2000. A new lightweight-wooded species of Anaxagorea (Annonaceae) from flooded black-water shrublands in southern Venezuela. – Syst. Bot. 24: 506-511.
Bhandari NN. 1971. Embryology of the Magnoliales and comments on their relationships. – J. Arnold Arbor. 52: 1-39, 285-304.
Biswas BK, Sharma AK. 1984. Chromosome studies in the family Magnoliaceae. – Cytologia 49: 193-200.
Blunden G, Kyi A, Jewers K. 1974. The comparative stem and root anatomy of Goniothalamus andersonii, G. macrophyllus, G. malayanus and G. velutinus (Annonaceae) from the peat swamps of Sarawak. – Bot. J. Linn. Soc. 68: 209-225.
Bobrov AVF, Romanov MS, Zdravchev NS, Endress PK. 2017. Fruit structure and development in Eupomatiaceae and comparison of fruit histology with other Magnoliales and with Laurales. – Bot. J. Linn. Soc. 185: 129-146.
Boerlage JG. 1899. Notes sur les Annonacées du jardin botanique de Buitenzorg. – Icon. Bogor. 1: 6-156, tab. XXVI-L.
Bos WJ van den, Koek-Noorman J, Berendsen W. 1939. Studies in Annonaceae XII. Domatia in Annona and Rollinia: occurrence, SEM structure, and taxonomic significance. – Proc. Kon. Ned. Akad. Wet., ser. C, 92: 325-330.
Bouman F. 1977. Integumentary studies in the Polycarpicae IV. Liriodendron tulipifera L. – Acta Bot. Neerl. 26: 213-223.
Boureau E. 1950. Étude paléoxylologique du Sahara IX. Sur un Myristicoxylon princeps n. gen., n. sp., du Danien d’Asselar. – Bull. Mus. Natl. Hist. Nat. Paris, sér. 2, 22: 523-528.
Boutique R. 1951. Annonacées nouvelles de la flore du Congo Belge et du Ruanda-Urundi. – Bull. Jard. Bot. État Bruxelles 21: 95-126.
Bowden WM. 1948. Chromosome numbers in the Annonaceae. – Amer. J. Bot. 35: 377-380.
Briechle-Mäck MH. 1994. Beiträge zur Histogenese des Blüten und Früchte pseudosynkarper Annonaceen-Arten. – Egelsbach, Frankfurt.
Buchheim G. 1962. Beobachtungen über den Bau der Frucht der Familie Himantandraceae. – Sitzungsber. Ges. Naturf. Freunde Berlin, N. F., 2: 78-92.
Burck W. 1911. Anonaceae [résultats de l’expédition scientifique Néerlandaise à la Nouvelle-Guinée en 1907 et 1909 sous les auspices Dr. H. A. Lorentz, 1er part, Botanique]. – Nova Guinea 8: 427-433.
Bygrave PC. 2000. Molecular systematics of Annonaceae Juss. – Ph.D. diss., University of Reading, England.
Cai Z, Penaflor C, Kuehl JV, Leebens-Mack J, Carlson JE, dePamphilis CW, Boore JL, Jansen RK. 2006. Complete chloroplast genome sequences of Drimys, Liriodendron, and Piper: implication for the phylogeny of magnoliids. – BMC Evol. Biol. 6: 77.
Canright JE. 1952. The comparative morphology and relationships of the Magnoliaceae I. Trends of specialization in the stamens. – Amer. J. Bot. 39: 484-497.
Canright JE. 1953. The comparative morphology and relationships of the Magnoliaceae II. Significance of the pollen. – Phytomorphology 3: 355-365.
Canright JE. 1955. The comparative morphology and relationships of Magnoliaceae IV. Wood and nodal anatomy. – J. Arnold Arbor. 36: 119-140.
Canright JE. 1960. The comparative morphology and relationships of the Magnoliaceae III. Carpels. – Amer. J. Bot. 47: 145-155.
Capuron R. 1972. Contribution à l’étude de la flore forestière de Madagascar A. Haematodendron, genre nouveau de Myristicaceae. – Adansonia, sér. II, 12: 375-379.
Capuron R. 1973. Observations sur les Myristicacées de Madagascar: les genres Brochoneura Warb. et Mauloutchia Warb. – Adansonia, sér. II, 13: 203-221.
Carlquist SJ. 1989 [1990]. Wood and bark anatomy of Degeneria. – Aliso 12: 485-495.
Carlquist SJ. 1992. Vegetative anatomy and relationships of Eupomatiaceae. – Bull. Torrey Bot. Club 119: 167-180.
Carvalho R, Webber AC. 2000. Biologia floral de Unonopsis guatterioides (A. DC.) R. E. Fr., uma Annonaceae polinizada por Euglossini. – Rev. Brasil. Bot. 23: 419-423.
Cavaco A, Keraudren M. 1957. Notes systématiques et biogéographiques sur les Annonacées de Madagascar et des Comores. – Bull. Jard. Bot. État Bruxelles 27: 59-93.
Cave A. 1989. Chemical research in Annonaceae. – Annonaceae Newsl. no. 6: 24-36.
Cevallos-Ferriz SRS, Stockey RA. 1990. Vegetative remains of the Magnoliaceae from the Princeton chert (Middle Eocene) of British Columbia. – Can. J. Bot. 68: 1327-1339.
Chaowasku T, Ham RWJM van der. 2013. Integrative systematics supports the establishment of Winitia, a new genus of Annonaceae (Malmeoideae, Miliuseae) allied to Stelechocarpus and Sageraea. – Syst. Biodiv. 11: 195-207.
Chaowasku T, Mols J, Ham RWJM van der. 2008. Pollen morphology of Miliusa and relatives (Annonaceae). – Grana 47: 175-184.
Chaowasku T, Zijlstra G, Chatrou LW. 2011. (2029) Proposal to conserve the name Meiogyne against Fitazalania (Annonaceae). – Taxon 60: 1522-1523.
Chaowasku T, Keßler PJA, Punnadee S, Ham RWJM van der. 2011. Taxonomic novelties and pollen morphological study in the genus Neo-uvaria (Annonaceae). – Phytotaxa 32: 27-42.
Chaowasku T, Johnson DM, van der Ham RWJM, Chatrou LW. 2012. Characterization of Hubera (Annonaceae), a new genus segregated from Polyalthia and allied to Miliusa. – Phytotaxa 69: 33-56.
Chaowasku T, Keßler PJA, Ham RWJM van der. 2012. A taxonomic revision and pollen morphology of the genus Dendrokingstonia (Annonaceae). – Bot. J. Linn. Soc. 168: 76-90.
Chaowasku T, Ham RWJM van der, Chatrou LW. 2013. Integrative systematics supports the establishment of Winitia, a new genus of Annonaceae (Malmeoideae, Miliuseae) allied to Stelechocarpus and Sageraea. – Syst. Biodivers. 11: 195-207.
Chaowasku T, Thomas DC, Ham RWJM van der,
Smets EF, Mols JB, Chatrou LW. 2014. A plastid DNA phylogeny of tribe
Miliuseae: insights into relationships and character evolution in one of the
most recalcitrant major clades of Annonaceae. – Amer. J. Bot. 101:
691-709.
Chaowasku T, Johnson DM, Ham RWJM van der, Chatrou LW. 2015. Huberantha, a replacement name for Hubera (Annonaceae: Malmeoideae: Miliuseae). – Kew Bull. 70: 23 DOI 10.1007/S12225-015-9571-Z
Chatrou LW. 1998. Revision of the Malmea alliance: Malmea and three new, Neotropical genera: Klarobelia, Mosannona, and Pseudomalmea. – In: Chatrou LW (ed), Changing genera: systematics studies in Neotropical and West African Annonaceae, Ph.D. diss., University of Utrecht, The Netherlands, pp. 105-192.
Chatrou LW. 2003. Myristicineae, a new suborder within Magnoliales. – Taxon 52: 277-279.
Chatrou LW, He P. 1999. Studies in Annonaceae XXXIII. A revision of Fusaea (Baill.) Saff. – Brittonia 52: 181-203.
Chatrou LW, Koek-Noorman J, Maas PJM. 2000. Studies in Annonaceae XXXVI. The Duguetia alliance: where the ways part. – Ann. Missouri Bot. Gard. 87: 234-245.
Chatrou LW, Pirie MD, Erkens RHJ, Couvreur TLP, Neubig KM, Abbott JR, Mols JB, Maas JW, Saunders RMK, Chase MW. 2012. A new subfamilial and tribal classification of the pantropical flowering plant family Annonaceae informed by molecular phylogenetics. – Bot. J. Linn. Soc. 169: 5-40.
Chen BL, Nooteboom HP. 1993. Notes on Magnoliaceae III. The Magnoliaceae of China. – Ann. Missouri Bot. Gard. 80: 999-1104.
Chen Z, Huang X, Wang R, Chen S. 2000. Chromosome data of Magnoliaceae. – In: Liu Y-H, Fan H-M, Chen Z-Y, Wu Q-G, Zeng Q-W (eds), Proceedings of the International Symposium on the family Magnoliaceae, May 18-22, 1998, Guangzhou, China, Science Press, Beijing, pp. 192-201.
Chevalier A. 1918. Magnoliacées. – Bull. Econ. Indochine 20: 790-792.
Chopin J, Hauteville M, Joshi BS, Gawad DH. 1978. A novel example of a natural 2,5-dihydroxyflavanone from Unona lawii. – Phytochemistry 17: 332-334.
Christmann M. 1986. Beiträge zur Histologie der Annonaceen-Samen. – Bot. Jahrb. Syst. 106: 379-390.
Christmann M. 1987. Systematische Anatomie der Annonaceen-Samen. – Thesis, Kaiserslautern, Germany.
Christmann M. 1989a. Genera and species of Annonaceae with tritegmic seeds. – Annonaceae Newsl. 6: 11-13.
Christmann M. 1989b. Die tritegmischen Annonaceen-Samen. – Bot. Jahrb. Syst. 110: 433-439.
Chun W-Y. 1963. Genus speciesque novae Magnoliacearum sinensium. – Acta Phytotaxon. Sin. 8: 281-288.
Coleman MA. 1966. Floral anatomy and morphology of Rollinia St.-Hil. – Arq. Bot. Estado São Paulo 4: 45-51.
Corner EJH. 1949. The annonaceous seed and its four integuments. – New Phytol. 48: 332-364.
Costa EV, Pinheiro MLB, Marques FA, Braga RM, Maia, BHLNS. 2009. First report of alkaloids in the genus Guatteriopsis (Annonaceae). – Biochem. Syst. Ecol. 37: 43-45.
Couvreur TLP. 2008. Revealing the secrets of African Annonaceae: systematics, evolution and biogeography of the syncarpous genera Isolona and Monodora. – Ph.D. diss., Universiteit Wageningen.
Couvreur TLP. 2009. Monograph of the syncarpous African genera Isolona and Monodora (Annonaceae). – Syst. Bot. Monogr. 87: 1-150.
Couvreur TLP. 2014. Revision of the African genus Uvariastrum (Annonaceae). – PhytoKeys 33: 1-40.
Courvreur TLP, Niangadouma R. 2016. New species of Uvariopsis (Annonaceae) and Laccosperma (Arecaceae/Palmae) from Monts de Cristal, Gabon. – PhytoKeys 68: 1-8.
Couvreur TLP, Gereau RE, Wieringa JJ, Richardson JE. 2006. Description of four new species of Monodora and Isolona (Annonaceae) from Tanzania and an overview of Tanzanian Annonaceae diversity. – Adansonia, sér. III, 28: 243-266.
Couvreur TLP, Richardson JE, Sosef MSM, Erkens RHJ, Chatrou LW. 2008. Evolution of syncarpy and other morphological characters in African Annonaceae: a posterior mapping approach. – Mol. Phylogen. Evol. 47: 302-318.
Couvreur TLP, Botermans M, Heuven BJ van, Ham R van der. 2008. Pollen morphology within the Monodora clade, a diverse group of five African Annonaceae genera. – Grana 47: 185-210.
Couvreur TLP, Ham RWJM van der, Mbele YM, Mbago FM, Johnson DM. 2009. Molecular and morphological characterization of a new monotypic genus of Annonaceae, Mwasumbia, from Tanzania. – Syst. Bot. 34: 266-276.
Couvreur TLP, Pirie MD, Chatrou LW, Saunders RMK, Su YCF, Richardson JE, Erkens RHJ. 2011. Early evolutionary history of the flowering plant family Annonaceae: steady diversification and boreotropical geodispersal. – J. Biogeography 38: 664-680.
Couvreur TLP, Maas PJM, Meinke SJA, Johnson DM, Keßler PJA. 2012. Keys to the genera of Annonaceae. – Bot. J. Linn. Soc. 169: 74-83.
Couvreur TLP, Niangadouma R, Sonke B, Sauquet H. 2015. Sirdavidia, an extraordinary new genus of Annonaceae from Gabon. – PhytoKeys 46: 1-19.
Crane PR. 1998. The phylogenetic position and fossil history of the Magnoliaceae. – In: Hunt D (ed), Magnolias and their allies, David Hunt, Milborne Port, pp. 21-36.
Crepet WL, Nixon KC. 1998. Two new fossil flowers of magnoliid affinity from the Late Cretaceous of New Jersey. – Amer. J. Bot. 85: 1273-1288.
Cruz Durán R, Jiménez Ramírez J, Vega Flores K, Vázquez García JA. 2007. Magnolia guerrerensis (Magnoliaceae), una especie nueva del bosque mesófilo de montaña del estado de Guerrero, México. – Bol. Soc. Bot. México 80: 73-76.
Dahl A, Rowley JR. 1965. Pollen of Degeneria vitiensis. – J. Arnold Arbor. 46: 308-323.
Dandy JE. 1927. The genera of Magnolieae. – Kew Bull. 7: 257-264.
Dandy JE. 1928. New or noteworthy Chinese Magnolieae. – Notes Roy. Bot. Gard. Edinb. 16: 123-133.
Dandy JE. 1930. New Magnolieae from China and Indochina. – J. Bot. 68: 204-213.
Dandy JE. 1950. A survey of the genus Magnolia together with Manglietia and Michelia. – In: Synge PM (ed), Camellias and Magnolias: report of the conference held by the Royal Horticultural Society, Royal Horticultural Society, London, pp. 64-81.
Dandy JE. 1971. The classification of the Magnoliaceae. – Newsl. Amer. Magnolia Soc. 8: 3-6.
Dandy JE. 1978. A revised survey of the genus Magnolia together with Manglietia and Michelia. – In: Treseder NG (ed), Magnolias, Faber & Faber, London, pp. 29-37.
De Boer R, Bouman F. 1972. Integument studies in the Polycarpicae II. Magnolia stellata and Magnolia virginiana. – Acta Bot. Neerl. 21: 617-629.
Delevoryas T, Mickle JE. 1995. Upper Cretaceous magnoliaceous fruit from British Columbia. – Amer. J. Bot. 82: 763-768.
Deroin T. 1985. Contribution à la morphologie comparée du gynécée des Annonaceae-Monodoroideae. – Bull. Mus. Natn. Hist. Nat. Paris, sér. IV, 7, sect. B, Adansonia 2N: 167-176.
Deroin T. 1989a. Définition et signification phylogénique des systèmes corticaux floraux: l’exemple des Annonacées. – Compt. Rend. Acad. Sci. Paris, 3e sér., 308: 71-75.
Deroin T. 1989b. Quelques aspects de la biologie florale d’une Annonacée savanicole: Annona senegalensis Pers. – Mém. Soc. Biogéogr. 3: 42-53.
Deroin T. 1991a. La vascularisation florale des Magnoliales: première approche expérimentale de son role au cours de la pollinisation. – Compt. Rend. Acad. Sci. Paris, 3e sér., 312: 355-360.
Deroin T. 1991b. La répartition des modèles de plateaux stigmatiques et l’évolution des Annonacées. – Compt. Rend. Acad. Sci. Paris, sér. III, 312: 561-566.
Deroin T. 1999. Functional impact of the vascular architecture of flowers in Annonaceae and Magnoliaceae, and its bearing on the interpretation of the magnoliaceous gynoecium. – Syst. Geogr. Plants 68: 213-224.
Deroin T. 2007. Floral vascular pattern of the endemic Malagasy genus Fenerivia Diels (Annonaceae). – Adansonia, sér. III, 29: 7-12.
Deroin T, Gautier L. 2006. Deux espèces nouvelles d’Uvaria (Annonaceae) du Sambirano, Madagascar. – Candollea 61: 51-60.
Deroin T, Gautier L. 2008. Artabotrys darainensis Deroin & L. Gaut. (Annonaceae), a new species from Madagascar. – Candollea 63: 93-99.
De Wilde WJJO. 1984. Endocomia, a new genus of Myristicaceae. – Blumea 30: 173-196.
De Wilde WJJO. 1991. The genera of Myristicaceae as distinguished by their inflorescences, and the description of a new genus, Bicuiba. – Beitr. Biol. Pflanzen 66: 95-125.
De Wilde WJJO. 1994. Paramyristica, a new genus of Myristicaceae. – Blumea 39: 341-350.
De Wilde WJJO. 2000. Myristicaceae. – In: Stevens PF (ed), Flora Malesiana I, 14, National Herbarium Netherlands, Leiden, pp. 1-632.
Diels L. 1912a. Die Anonaceen von Papuasien. – Bot. Jahrb. Syst. 49: 113-167.
Diels L. 1912b. Anonaceae [résultats de l’expédition scientifique Néerlandaise à la Nouvelle-Guinée en 1907 et 1909 sous les auspices Dr. H. A. Lorentz, 1er part, Botanique]. – Nova Guinea 8: 871-873.
Diels L. 1915. Neue Anonaceen von Papuasien. – Bot. Jahrb. Syst. 52: 177-186.
Diels L. 1917. Über die Gattung Himantandra, ihre Verbreitung und ihre systematische Stellung. – Engl. Bot. Jahrb. Syst. 55: 126-134.
Diels L. 1925. Revisio Anonacearum madagascariensium. – Notizbl. Bot. Gart. Berlin-Dahlem 9: 334-357.
Diels L. 1927. Anonaceae. – Mitt. Inst. Bot. Hamburg 7: 77-80.
Diels L. 1932. Die Gliederung der Anonaceae und ihre Phylogenie. – Sitzungsber. Preuss. Akad. Wiss., Phys.-Math. Kl. 1932: 77-85.
Dinis AM, Mesquita JF. 1993. The F-actin distribution during microsporogenesis in Magnolia soulangeana Soul. (Magnoliaceae). – Sexual Plant Repr. 6: 57-63.
Dorofeev PI. 1983. Dva novych vida Liriodendron iz treticnych otlozenii SSSR. – Bot. Žurn. 68: 1401-1408.
Doskotch RW, Flom MS. 1972. Acuminatin, a new bis-phenylpropide from Magnolia acuminata L. – Tetrahedron 28: 4711-4717.
Doweld AB, Shevyryova NA. 1997. Carpology, anatomy and taxonomic relationships of Galbulimima (Himan-tandraceae). – Ann. Bot. 81: 337-347.
Doyle, JA, Le Thomas A. 1994. Cladistic analysis and pollen evolution in Annonaceae. – Acta Bot. Gall. 141: 149-170.
Doyle JA, Le Thomas A. 1995. Evolution of pollen characters and relationships of African Annonaceae: implications of a cladistic analysis. – In: Le Thomas A, Roche E (eds), 2e symposium de palynologie africaine, Tervuren, Belgium, 1995, Centre International pour la Formation et les Échanges Géologiques, Orléans, pp. 241-254.
Doyle JA, Le Thomas A. 1996. Phylogenetic analysis and character evolution in Annonaceae. – Bull. Mus. Natl. Hist. Nat. Paris, sér. IV, sect. B, Adansonia 18: 279-334.
Doyle JA, Le Thomas A. 1997a. Significance of palynology for phylogeny of Annonaceae: experiments with removal of pollen characters. – Plant Syst. Evol. 206: 133-160.
Doyle JA, Le Thomas A. 1997b. Phylogeny and geographic history of Annonaceae. – Géogr. Phys. Quartern. 51: 252-361.
Doyle JA, Le Thomas A. 2012. Evolution and phylogenetic significance of pollen in Annonaceae. – Bot. J. Linn. Soc. 169: 190-221.
Doyle JA, Bygrave P, Le Thomas A. 2000. Implications of molecular data for pollen evolution in Annonaceae. – In: Harley MM, Morton CM, Blackmore S (eds), Pollen and spores: morphology and biology, Royal Botanic Gardens, Kew, pp. 259-284.
Doyle JA, Sauquet H, Scharaschkin T, Le Thomas A. 2004. Phylogeny, molecular and fossil dating, and biogeographic history of Annonaceae and Myristicaceae (Magnoliales). – Intern. J. Plant Sci. 165(Suppl.): S55-S67.
Doyle JA, Manchester SR, Sauquet H. 2008. A seed related to Myristicaceae in the Early Eocene of southern England. – Syst. Bot. 33: 636-646.
El-Sohly HN, Lassell Jr WL, Hufford CD. 1979. Two new C-benzylated flavanones from Uvaria chamae and C-13 NMR analysis of flavanone methyl ethers. – J. Nat. Prod. 42: 264-270.
Endress PK. 1977. Über Blütenbau und Verwandtschaft der Eupomatiaceae und Himantandraceae (Magnoliales). – Ber. Deutsch. Bot. Ges. 90: 83-103.
Endress PK. 1983. Dispersal and distribution in some small archaic relic angiosperm families (Austrobaileyaceae, Eupomatiaceae, Himantandraceae, Idiospermoideae-Calycanthaceae). – Sonderb. Naturwiss. Ver. Hamburg 7: 201-217.
Endress PK. 1984a. The role of inner staminodes in the floral display of some relic Magnoliales. – Plant Syst. Evol. 146: 269-282.
Endress PK. 1984b. The flowering process in the Eupomatiaceae (Magnoliales). – Bot. Jahrb. Syst. 104: 297-319.
Endress PK. 1985. Stamenabzission und Pollenpräsentation bei Annonaceae. – Flora 176: 95-98.
Endress PK. 1993a. Eupomatiaceae. – In: Kubitzki K, Rohwer JG, Bittrich V (eds), The families and genera of vascular plants II. Flowering plants. Dicotyledons. Magnoliid, hamamelid and caryophyllid families, Springer, Berlin, Heidelberg, New York, pp. 296-298.
Endress PK. 1993b. Himantandraceae. – In: Kubitzki K, Rohwer JG, Bittrich V (eds), The families and genera of vascular plants II. Flowering plants. Dicotyledons. Magnoliid, hamamelid and caryophyllid families, Springer, Berlin, Heidelberg, New York, pp. 338-341.
Endress PK. 2003. Early floral development and nature of the calyptra in Eupomatiaceae (Magnoliales). – Intern. J. Plant Sci. 164: 489-503.
Endress PK, Armstrong JE. 2011.Floral development and floral phyllotaxis in Anaxagorea (Annonaceae). – Ann. Bot. 108: 835-845.
Endress PK, Hufford LD. 1989. The diversity of stamen structures and dehiscence patterns among Magnoliales. – Bot. J. Linn. Soc. 100: 45-85.
Engler A. 1897. Anonaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien, Nachträge zu III(2), W. Engelmann, Leipzig, pp. 159-161.
Engler A, Diels L. 1901. Anonaceae. – In: Engler A (ed), Monographien afrikanischer Pflanzenfamilien und -gattungen 6, W. Engelmann, Leipzig.
Erbar C, Leins P. 1981. Zur Spirale in Magnolien-Blüten. – Beitr. Biol. Pflanzen 56: 225-241.
Erkens RHJ. 2007. From morphological nightmare to molecular conundrum: phylogenetic, evolutionary and taxonomic studies on Guatteria. – Gildeprint, Enschede.
Erkens RHJ, Maas PJM. 2008. The Guatteria group disentangled: sinking Guatteriopsis, Guatteriella, and Heteropetalum into Guatteria. – Rodriguésia 59: 401-406.
Erkens RHJ, Chatrou LW, Maas JW, Niet T van der, Savolainen V. 2007. The rapid diversification of rainforest trees (Guatteria; Annonaceae) following dispersal from Central into South America. – Mol. Phylogen. Evol. 44: 399-411.
Erkens RHJ, Chatrou LW, Koek-Noorman J, Maas JW, Maas PJM. 2007. Classification of a large and widespread genus of neotropical trees, Guatteria (Annonaceae), and its three segregate genera Guatteriella, Guatteriopsis and Heteropetalum. – Taxon 56: 757-774.
Erkens RHJ, Maas JW, Couvreur TLP. 2009. From Africa via Europe to South America: migrational route of a species-rich genus of Neotropical lowland rain forest trees (Guatteria; Annonaceae). – J. Biogeogr. 36: 2338-2352.
Erkens RHJ, Chatrou LW, Couvreur TLP. 2012. Radiations and key innovations in an early braching angiosperm lineage (Annonaceae; Magnoliales). – Bot. J. Linn. Soc. 169: 117-134.
Erkens RHJ, Chatrou LW, Chaowasku T, Westra
LYT, Maas JW, Maas PJM. 2015. A decade of uncertainty: resolving the
phylogenetic position of Diclinanona (Annonaceae), including taxonomic notes
and a key to the species. – Taxon 63: 1244-1252.
Erkens RHJ, Oosterhof J, Westra LYT, Maas PJM. 2017. Revisions of Ruizodendron and Pseudephedranthus (Annonaceae) including a new species and an overview of most up-to-date revisions of Neotropical Annonaceae genera. – PhytoKeys 86: 75-96.
Esposti MD, Ghelli A, Ratta M, Cortes D, Estornell E. 1994. Natural substances (acetogenins) from the family Annonaceae are powerful inhibitors of mitochondrial NADH dehydrogenase (Complex I). – Biochem. J. 301: 161-167.
Farr CH. 1918. Cell division by furrowing in Magnolia. – Amer. J. Bot. 5: 379-395.
Feild TS, Chatelet DS, Brodribb TJ. 2009. Giant flowers of southern magnolia are hydrated by the xylem. – Plant Physiol. 150: 1587-1597.
Fero M, Aedo C, Cabezas F, Velayos M. 2014.
Taxonomic revision of Neostenanthera (Annonaceae). – Syst. Bot. 39:
17-30.
Figlar RB. 1993. Stone Magnolias. – Arnoldia 53: 2-9.
Figlar RB. 2000. Proleptic branch initiation in Michelia and Magnolia subgenus Yulania provides basis for combinations in subfamily Magnolioideae. – In: Liu Y-H, Fan H-M, Chen Z-Y, Wu Q-G, Zeng Q-W (eds), Proceedings of the International Symposium on the Family Magnoliaceae, May 18-22, 1998, Guangzhou, China, Science Press, Beijing, pp. 14-25.
Figlar RB, Nooteboom HP. 2004. Notes on Magnoliaceae. – Blumea 49: 87-100.
Finet A, Gagnepain F. 1906. Contribution à l’étude de la flore de l’Asie orientale, fam. 5 – Anonacées. – Bull. Soc. Bot. France, Mém. 4: 55-170.
Franceschini MC. 2004. An unusual case of epigeal cryptocotylar germination in Rollinia salicifolia (Annonaceae). – Bot. J. Linn. Soc. 146: 53-56.
Fries RE. 1919. Studien über die Blütenstandsverhältnisse bei der Familie Anonaceae. – Acta Horti Berg. 6: 3-48.
Fries RE. 1930. Revision der Arten einiger Anonaceen-Gattungen I. – Acta Horti Berg. 10: 1-128.
Fries RE. 1931. Revision der Arten einiger Anonaceen-Gattungen II. – Acta Horti Berg. 10: 129-341.
Fries RE. 1934. Revision der Arten einiger Anonaceen-Gattungen III. – Acta Horti Berg. 12: 1-220.
Fries RE. 1937. Revision der Arten einiger Annonaceen-Gattungen IV. – Acta Horti Berg. 12: 221-288.
Fries RE. 1939. Revision der Arten einiger Annonaceen-Gattungen V. – Acta Horti Berg. 12: 289-577.
Fries RE. 1949a. Contributions to the knowledge of the Annonaceae in northern South America. – Ark. f. Bot. 1: 329-347.
Fries RE. 1949b. Sobre la caulifloría en la familia de las Anonáceas. – Lilloa 16: 251-261.
Fries RE. 1955. Verstreute Beobachtungen hinsichtlich der Familie Annonaceae. – Ark. Bot., n. s. 3, 2: 35-42.
Fries RE. 1959. Annonaceae. – In: Melchior H (ed), A. Engler und K. Prantl’s Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 17a, Duncker & Humblot,Berlin, pp. 1-171.
Friis EM, Pedersen KR. 2011. Canrightia resinifera, a new Early Cretaceous fruit of magnolialean affinity from Portugal. – Grana 50: 3-29.
Frodin DG, Govaerts R. 1996. World checklist and bibliography of Magnoliaceae. – Royal Botanic Gardens, Kew.
Frumin S, Friis EM. 1996. Liriodendroid seeds from the Late Cretaceous of Kazakhstan and North Carolina, USA. – Rev. Palaeobot. Palynol. 94: 39-55.
Frumin S, Friis EM. 1999. Magnoliid reproductive organs from the Cenomanian-Turonian of north-western Kazakhstan: Magnoliaceae and Illiciaceae. – Plant Syst. Evol. 216: 265-288.
Gabarayeva NI. 1986. The development of the exine in Michelia fuscata (Magnoliaceae) in connection with the changes in cytoplasmic organelles of microspore and tapetum. – Bot. Žurn. 71: 311-322. [In Russian with English summary]
Gabarayeva NI. 1987. Ultrastructure and development of sporoderm in Manglietia tenuipes (Magnoliaceae) during tetrad period: the primexine formation in connection with cytoplasmic organelle activity. – Bot. Žurn. 72: 281-290. [In Russian with English summary]
Gabarayeva NI. 1992. Sporoderm development in Asimina triloba I. The development events before callose dissolution. – Grana 31: 213-222.
Gabarayeva NI. 1993. Sporoderm development in Asimina triloba II. The development events after callose dissolution. – Grana 32: 210-220.
Gabarayeva NI. 1995. Pollen wall and tapetum development in Anaxagorea brevipes (Annonaceae): sporoderm substructure, cytoskeleton, sporopollenin precursor particles, and the endexine problem. – Rev. Palaeobot. Palynol. 85: 123-152.
Gabarayeva NI, Grigorjeva V. 2012. Sporoderm development and substructure in Magnolia sieboldii and other Magnoliaceae: an interpretation. – Grana 51: 119-147.
Gagnepain F. 1939. Magnoliacées nouvelle ou litigieuses. – Notul. Syst. 8: 63-66.
Garrett GA. 1933a. Bearing of wood anatomy on the relationships of the Myristicaceae. – Trop. Woods 36: 20-44.
Garrett GA. 1933b. Systematic anatomy of the woods of the Myristicaceae. – Trop. Woods 35: 6-48.
Gereau RE, Kenfack D. 2000. Le genre Uvariopsis (Annonaceae) en Afrique tropicale, avec la description d’une espèce nouvelle du Cameroun. – Adansonia, sér. III, 22: 39-43.
Ghogue J-P, Sonké B, Couvreur TLP. 2017. Taxonomic revision of the African genera Brieya and Piptostigma (Annonaceae). – Plant Ecol. Evol. 150: 173-216.
Gibbs PE, Semir J, Cruz ND da. 1977. Floral biology of Talauma ovata St.-Hil. (Magnoliaceae). – Ciência e Cultura 29: 1436-1441.
Goggin FL, Medville R, Turgeon R. 2001. Phloem loading in the tulip tree. Mechanisms and evolutionary implications. – Plant Physiol. 124: 891-899.
Goldblatt P. 1974. A contribution to the knowledge of cytology in Magnoliales. – J. Arnold Arbor. 55: 453-457.
Golenberg EM, Giannasi DE, Clegg MT, Smiley CJ, Durbin M, Henderson D, Zurawski G. 1990. Chloroplast DNA sequence from a Miocene Magnolia species. – Nature 344: 656-658.
Gottlieb OR. 1979. Chemical studies on medicinal Myristicaceae from Amazonia. – J. Ethnopharmac. 1: 309-323.
Gottlieb OR, Kaplan MAC, Kubitzki K, Toledo Barros JR. 1988 [1989]. Chemical dichotomies in the Magnolialean complex. – Nord. J. Bot. 8: 437-444.
Gottsberger G. 1970. Beiträge zur Biologie der Annonaceen-Blüten. – Österr. Bot. Zeitschr. 118: 237-279.
Gottsberger G. 1989. Beetle pollination and flowering rhythm of Annona spp. (Annonaceae) in Brazil. – Plant Syst. Evol. 167: 165-187.
Gottsberger G. 1993. Flower biological differentiation in Neotropical Annonaceae. – Annonaceae Newsl. 9: 29-33.
Gottsberger G. 1994. As Anonáceas do cerrado e a sua polinizacão. – Rev. Brasil. Biol. 54: 391-402.
Gottsberger G. 1999. Pollination and evolution in neotropical Annonaceae. – Plant Species Biol. 14: 143-152.
Gottsberger G, Gottsberger I. 1985. Pollen units, pollen shape, and apertural position in the Annonaceae: a reassessment. – Beitr. Biol. Pflanzen 59: 465-473.
Gottsberger G, Silberbauer-Gottsberger I. 1988. Pollination strategies of Annona species from the cerrado vegetation in Brazil. – Lagascalia 15: 665-672.
Gottsberger G, Webber AC, Hildenbrand M. 1998. Nutritious tissues in Annonaceae flowers. – Annonaceae Newsl. 12: 25-26.
Guédès M. 1968. Le carpelle du tulipier (Liriodendron tulipifera). – Österr. Bot. Zeitschr. 115: 372-378.
Guédès M, Le Thomas A. 1981. Le gynécée syncarpe de Monodora. – Compt. Rend. Acad. Sci. Paris, sér. III, 292: 1025-1028.
Gülz PG, Müller E, Schmitz K, Marner F-J, Güth S. 1992. Chemical composition and surface structures of epicuticular waxes of Ginkgo biloba, Magnolia grandiflora and Liriodendron tulipifera. – Zeitschr. Naturforsch. 47: 516-526.
Guo X, Wang J, Xue B, Thomas DC, Su YCF, Tan
Y-H, Saunders RMK. 2014. Reassessing the taxonomic status of two enigmatic
Desmos species (Annonaceae):
morphological and molecular phylogenetic support for a new genus, Wangia. –
J. Syst. Evol. 52: 1-15.
Guo X, Hoekstra PH, Tang CC, Thomas DC, Wieringa JJ, Chatrou LW, Saunders RMK. 2017. Cutting up the climbers: evidence for extensive polyphyly in Friesodielsia (Annonaceae) necessitates generic realighment across the tribe Uvarieae. – Taxon 66: 3-19.
Guo X, Tang CC, Thomas DC. Couvreur TLP, Saunders RMK. 2017. A mega-phylogeny of the Annonaceae: taxonomic placement of five enigmatic genera and support for a new tribe, Phoenicantheae. – Sci. Rep. 7: 7323.
Guo X, Thomas DC, Saunders RMK. 2018. Gene tree discordance and coalescent methods support ancient intergeneric hybridization between Dasymaschalon and Friesodielsia (Annonaceae). – Mol. Phylogen. Evol. 127: 14-29.
Guzzo F, Baldan B, Bracco F, Mariani P. 1994. Pollen development in Liriodendron tulipifera. Some unusual features. – Can. J. Bot. 72: 352-358.
Hamilton AG. 1898. On the fertilisation of Eupomatia laurina R. Br. – Proc. Linn. Soc. New South Wales 22: 48-55.
Hasegawa T, Fukuyama Y, Yamada T, Nakagawa K. 1988. Isolation and structure of magnolioside A, a new phenylpropanoid glycoside from Magnolia obovata Thunb. – Chem. Lett. 1988: 163-166.
Hayashi Y. 1960. On the microsporogenesis and pollen morphology in the family Magnoliaceae. – Sci. Rep. Tohôku Univ., Ser. IV (Biology) 26: 45-52.
Hayashi Y. 1963. The embryology of the Magnoliaceae sensu lato I. Megasporogenesis, female gametophyte and embryology. – Sci. Rep. Tohôku Univ., ser. IV (Biology) 29: 27-33.
Hayashi Y. 1964. The embryology of the Magnoliaceae sensu lato III. Magnolia liliiflora and Michelia fuscata. – Sci. Rep. Tohôku Univ., ser. IV (Biology) 30: 89-98.
Hayashi Y. 1965. The comparative embryology of the Magnoliaceae s. l. in relation to the systematic consideration of the family. – Sci. Rep. Tohôku Univ., ser. IV (Biology) 31: 29-44.
Hayashi Y. 1966. The embryology of the Magnoliaceae sensu lato IV. Microsporogenesis and development of the male gametophyte in Michelia figo Spreng. – Sci. Rep. Tohôku Univ., ser. IV (Biology) 32: 111-118.
Hayashi Y. 1984. Embryology of Magnolia salicifolia. – J. Jap. Bot. 59: 289-307.
Heel WA van. 1982. Note on the structure of developing seeds of Knema and Horsfieldia (Myristicaceae). – Blumea 28: 53-60.
Heijden E van der, Keßler PJA. 1990. Studies on the tribe Saccopetaleae (Annonaceae) III. Revision of the genus Mezzettia Beccari. – Blumea 35: 217-228.
Hesse M, Waha M. 1984. Sporoderm characters of Tetrameranthus duckei (Annonaceae) and their systematic implications. – Plant Syst. Evol. 147: 323-326.
Hesse M, Morawetz W, Ehrendorfer F. 1985. Pollen ultrastructure and systematic affinities of Anaxagorea (Annonaceae). – Plant Syst. Evol. 148: 253-285.
Hesse M, Halbritter H, Weber M. 2009. Beschorneria yuccoides and Asimina triloba (L.) Dun.: examples for proximal polar germinating pollen in angiosperms. – Grana 48: 151-159.
Heusden ECH van. 1992. Flowers of Annonaceae: morphology, classification, and evolution. – Blumea [Suppl.] 7: 1-218.
Heusden ECH van. 1994a. Revision of Meiogyne (Annonaceae). – Blumea 38: 487-511.
Heusden ECH van. 1994b. Revision of Haplostichanthus (Annonaceae). – Blumea 39: 215-234.
Heusden ECH van. 1996a. Revision of the Southeast Asian genus Trivalvaria (Annonaceae). – Nord. J. Bot. 17: 169-180.
Heusden ECH van. 1996b. The genus Meiogyne (Annonaceae) in New Caledonia: four new combinations. – Bull. Mus. Natl. Hist. Nat. Paris, sér. B, Adansonia 18: 75-83.
Heusden ECH van. 1997. Revision of the southeast Asian genus Sageraea (Annonaceae). – Nord. J. Bot. 17: 39-54.
Hiepko P. 1965. Vergleichend morphologische Untersuchungen über das Perianth der Magnoliales. – Bot. Jahrb. Syst. 84: 452-457.
Hiroshi A, Thien LB, Kawano S. 1999. Molecular phylogeny of Magnolia (Magnoliaceae) inferred from cpDNA sequences and evolutionary divergence of floral scents. – J. Plant Res. 112: 291-306.
Hoekstra PH, Wieringa JJ, Chatrou LW. 2016. A nonet of novel species of Monanthotaxis (Annonaceae) from around Africa. – PhytoKeys 69: 71-103.
Hoekstra PH, Wieringa JJ, Smets E, Brandão RD, Carvalho Lopes J de, Erkens RHJ, Chatrou LW. 2017. Correlated evolutionary rates across genomic compartments in Annonaceae. – Mol. Phylogen. Evol. 114: 63-72.
Holmstedt B, Lindgren JE, Plowman T, River L, Schultes RE, Tovar O. 1980. Indole alkaloids in Amazonian Myristicaceae. – Bot. Mus. Leafl. Harv. Univ. 28: 215-234.
Hotchkiss AT. 1955. Geographical distribution of the Eupomatiaceae. – J. Arnold Arbor. 36: 385-396.
Hotchkiss AT. 1959. Pollen and pollination in the Eupomatiaceae. – Proc. Linn. Soc. New South Wales 83: 86-91.
Howard RA. 1948. The morphology and systematics of the West Indian Magnoliaceae. – Bull. Torrey Bot. Club 75: 335-357.
Howe HF, Vande Kerckhove GA. 1980. Nutmeg dispersal by tropical birds. – Science 210: 925-927.
Hu H-H. 1940. A new genus of Magnoliaceae. – Sunyatsenia 4: 142-145.
Hu H-H, Cheng W-Y. 1951. Parakmeria, a new genus of Magnoliaceae of southwestern China. – Acta Phytotaxon. Sin. 1: 1-2.
Hufford CD, Oguntimein BO. 1980. Dihydrochalcones from Uvaria angolensis. – Phytochemistry 19: 2036-2038.
Hussin KH, Samah NA, Mat-Salleh K. 2000. Comparative leaf anatomy of Uvaria Linn., Cyathostemma Griff. and Ellipeia Hook. f. and Thomson (Annonaceae) from Malaysia. – J. Trop. Subtrop. Bot. 8: 215-224.
Hutchinson J. 1923. Contributions towards a phylogenic classification of flowering plants II. The genera of Annonaceae. – Kew Bull. 1923: 241-261.
Huysmans S, Verstraete B, Smets E, Chatrou LW. 2010. Distribution of orbicules in Annonaceae mirrors evolutionary trend in angiosperms. – Plant Ecol. Evol. 143: 199-211.
Igersheim A, Endress PK. 1997. Gynoecium diversity and systematics of the Magnoliales and winteroids. – Bot. J. Linn. Soc. 124: 213-271.
Imkhanitskaya NN. 1991. Rod Magnolia L. (Magnoliaceae) vo flore Kuby. – Nov. Sist. Vyssh. Rast. 28: 58-77.
Janovec JP. 2000. A systematic study of Compsoneura, a neotropical member of the nutmeg family (Myristicaceae). – Ph.D. diss., Dept. of Biology, Texas A & M University, College Station, Texas.
Janovec JP, Harrison JS. 2002. A morphological analysis of the Compsoneura sprucei complex (Myristicaceae), with a new combination for the Central American species Compsoneura mexicana. – Syst. Bot. 27: 662-673.
Janovec JP, Neill AK. 2003. Studies of the Myristicaceae: an overview of the Compsoneura atopa complex, with descriptions of new species from Colombia. – Brittonia 54: 251-261.
Jaramillo TS, Muriel P, Balslev H. 2004. 48. Myristicaceae– In: Harling G, Andersson L (eds), Flora of Ecuador 72, Botanical Institute, Göteborg University, pp. 1-99.
Jessup LW. 1986. The genus Goniothalamus (Blume) J. D. Hook. & Thomson (Annonaceae) in Australia. – Austrobaileya 2: 224-226.
Jessup LW. 1990. Habitat preferences and distribution of Australian Annonaceae. – Annonaceae Newslett. 8: 55-65.
Jessup LW. 2002. A new species of Eupomatia R. Br. (Eupomatiaceae) from Queensland. – Austrobaileya 6: 333-335.
Jessup LW. 2007. Annonaceae. – In: Wilson JG (ed), Flora of Australia 2, CSIRO Publ., Canberra, pp. 18-57.
Jiménez-Rojas EM, Londoño-Vega AC, Vester HFM. 2002. Descripción de la arquitectura de Iryanthera tricornis, Osteophloeum platyspermum y Virola pavonis (Myristicaceae). – Caldasia 24: 65-94.
Johnson DM. 1989. Revision of Disepalum (Annonaceae). – Brittonia 41: 356-378.
Johnson DM. 2003. Phylogenetic significance of spiral and distichous architecture in the Annonaceae. – Syst. Bot. 28: 503-511.
Johnson DM, Murray NA. 1995. Synopsis of the tribe Bocageeae (Annonaceae), with revisions of Cardiopetalum, Froesiodendron, Trigynaea, Bocagea, and Hornschuchia. – Brittonia 47: 248-319.
Johnson DM, Murray NA. 1999. Four new species of Polyalthia (Annonaceae) from Borneo and their relationship to Polyalthia insignis. – Contr. Univ. Michigan Herb. 22: 95-104.
Johnson DM, Murray NA. 2018. A revision of Xylopia L. (Annonaceae): the species of Tropical Africa. – PhytoKeys 97: 1-252.
Johnson DM, Mwasumbi LB, Mbago FM. 1999. New species of Xylopia and Uvaria (Annonaceae) from Tanzania. – Novon 9: 55-60.
Johnson MA, Fairbrothers DE. 1965. Comparison and interpretation of serological data in the Magnoliaceae. – Bot. Gaz. 126: 260-269.
Johnstone GH. 1950. The eastern magnolias, a gardener’s key. – Camellias and Magnolias, Rep. Conf. Roy. Horticult. Soc., pp. 44-63.
Joshi AC. 1946. A note on the development of pollen of Myristica fragrans Von Houtten of the family Myristicaceae. – J. Indian Bot. Soc. 25: 139-143.
Jovet-Ast S. 1942. Recherches sur les Annonacées d’Indochine: anatomie foliaire – répartition géographique. – Mém. Mus. Natl. Hist. Nat. Paris, n. s., 16: 125-308.
Juliano JB. 1935. Morphological contribution on the genus Annona Linnaeus. – Philipp. Agric. 24: 528-541.
Junikka L, Koek-Noorman J. 2007. Anatomical structure of barks in neotropical genera of Annonaceae. – Ann. Bot. Fenn. 44: 79-132.
Junikka L, Maas PJM, Maas-van de Kamer H, Westra LYT. 2016. Revision of Oxandra (Annonaceae). – Blumea 61: 215-266.
Kamelina OP. 1981. On the embryology of the non-investigated taxons I. Some data on the embryology of Eupomatiaceae. – Bot. Žurn. 66: 854-859. [In Russian]
Keng H. 1978. The delimitation of the genus Magnolia. – Gard. Bull. (Singapore) 31: 127-131.
Kerster J, Baas P. 1981. Comparative anatomy of the Asiatic Myristicaceae. – Blumea 27: 115-173.
Keßler PJA. 1988. Revision der Gattung Orophea Blume (Annonaceae). – Blumea 33: 1-80.
Keßler PJA. 1993. Annonaceae. – In: Kubitzki K, Rohwer JG, Bittrich V (eds), The families and genera of vascular plants II. Flowering plants. Dicotyledons. Magnoliid, hamamelid and caryophyllid families, Springer, Berlin, Heidelberg, New York, pp. 93-129.
Kim S, Park C-W, Kim Y-D, Suh Y. 2001. Phylogenetic relationships in family Magnoliaceae inferred from ndhF sequences. – Amer. J. Bot. 88: 717-728.
Kim S, Koh J, Ma H, Hu Y, Endress PK, Hauser BA, Buzgo M, Soltis PS, Soltis DE. 2005. Sequence and expression studies of A-, B-, and E-class MADS-box homologues in Eupomatia (Eupomatiaceae): support for the bracteate origin of the calyptra. – Intern. J. Plant Sci. 166: 185-198.
King G. 1893. The Anonaceae of British India. – Ann. Roy. Bot. Gard. Calcutta 4: 1-169.
Klucking EP. 1986. Leaf venation pattern 1. Annonaceae. – Berlin, Stuttgart.
Koek-Noorman J. 1989. Multidisciplinary approach to the systematics of Neotropical Annonaceae. – Annonaceae Newsl. 6: 3-10.
Koek-Noorman J, Westra LYT. 2012. Macrophotographic wood atlas of Annonaceae. – Bot. J. Linn. Soc. 169: 135-189.
Koek-Noorman J, Zandee M, Westra LYT. 1988. Studies in Annonaceae VIII. A cladistic analysis of Tetrameranthus. – Taxon 37: 346-353.
Koek-Noorman J, Westra LYT, Maas PJM. 1990. Studies in Annonaceae XIII. The role of morphological characters in subsequent classifications of Annonaceae: a comparative survey. – Taxon 39: 16-32.
Koek-Noorman J, Setten AK van, Zuilen CM van. 1997. Studies in Annonaceae XXVI. Flower and fruit morphology in Annonaceae. Their contribution to patterns in cluster analysis. – Bot. Jahrb. Syst. 119: 213-230.
Koster J, Baas P. 1981. Comparative leaf anatomy of the Asiatic Myristicaceae. – Blumea 27: 115-173.
Kral R. 1960. A revision of Asimina and Deeringothamnus. – Brittonia 12: 233-278.
Kubitzki K. 1993. Degeneriaceae. – In: Kubitzki K, Rohwer JG, Bittrich V (eds), The families and genera of vascular plants II. Flowering plants. Dicotyledons. Magnoliid, hamamelid and caryophyllid families, Springer, Berlin, Heidelberg, New York, pp. 290-291.
Kühn U, Kubitzki K. 1993. Myristicaceae. – In: Kubitzki K, Rohwer JG, Bittrich V (eds), The families and genera of vascular plants II. Flowering plants. Dicotyledons. Magnoliid, hamamelid and caryophyllid families, Springer, Berlin, Heidelberg, New York, pp. 457-467.
Law Y-W. 1979. A new genus of Magnoliaceae from China. – Acta Phytotaxon. Sin. 17: 72-74.
Law Y-W. 1984. A preliminary study on the taxonomy of the family Magnoliaceae. – Acta Phytotaxon. Sin. 22: 80-109. [In Chinese with English summary]
Leboeuf M, Cave A, Bhaumik PK, Bhaumik B, Mukherjee B, Mukherjee R. 1982. The phytochemistry of the Annonaceae. – Phytochemistry 21: 2783-2813.
Leinfellner W. 1966. Über die Karpelle verschiedener Magnoliales I. – Österr. Bot. Zeitschr. 113: 383-389.
Leinfellner W. 1967. Über die Karpelle verschiedener Magnoliales IV: Magnolia und Michelia. – Österr. Bot. Zeitschr. 114: 73-83.
Leinfellner W. 1969. Über die Karpelle verschiedener Magnoliales VIII. Überblick über alle Familien der Ordnung. – Österr. Bot. Zeitschr. 117: 107-127.
Leins P, Erbar C. 1979. Zur Entwicklung der Blüten von Monodora crispata (Annonaceae). – Beitr. Biol. Pflanzen 55: 11-22.
Leins P, Erbar C. 1982. Das monokarpellate Gynoeceum von Monodora crispata (Annonaceae). – Beitr. Biol. Pflanzen 57: 1-13.
Leins P, Erbar C. 1996. Early floral developmental studies in Annonaceae. – In: Morawetz W, Winkler H (eds), Reproductive morphology in Annonaceae, Österreichische Akademie der Wissenschaften, Wien, pp. 1-27.
Lemèsle R. 1937. Étude microchimique des divers tannoïdes de l’Eupomatia. – Bull. Soc. Bot. France 84: 535-538.
Lemèsle R. 1938. Contribution à l’étude du genre Eupomatia. – Rev. Gen. Bot. 50: 693-712.
Leonardia AAP, Keßler PJA. 2001. Additions to Orophea subgenus Sphaerocarpon (Annonaceae): revision and transfer of Mezzettiopsis. – Blumea 46: 141-163.
Leppik EE. 1975. Morphogenic stagnation in the evolution of Magnolia flowers. – Phytomorphology 25: 451-464.
Le Thomas A. 1963. Notes systématiques sur les Annonacées africaines et malgaches. – Adansonia 3: 287-293.
Le Thomas A. 1965. Un nouveau genre africain d’Annonacées: Boutiquea Le Thomas. – Adansonia 5: 531-535.
Le Thomas A. 1969. Flore du Gabon 16. Annonacées. – Muséum National d’Histoire Naturelle, Paris.
Le Thomas A. 1980. Ultrastructural characters of the pollen grains of African Annonaceae and their significance for the phylogeny of primitive angiosperms I. – Pollen Spores 22: 267-342.
Le Thomas A. 1981. Ultrastructural characters of the pollen grains of African Annonaceae and their significance for the phylogeny of primitive angiosperms II. – Pollen Spores 23: 5-36.
Le Thomas A. 1983. Morphologie et palynologie des Annonacées africains: interrelations phylogéniques. – Bothalia 14: 825-831.
Le Thomas A. 1988. Variation de la région aperturale dans le pollen des Annonacées. – Taxon 37: 644-650.
Le Thomas A, Doyle JA. 1996. Geographic relationships of Malagasy Annonaceae. – In: Lourenço WR (ed), Biogéographie de Madagascar, Éditions de l’ORSTOM, Paris, pp. 85-94.
Le Thomas A, Lugardon B. 1975. Ultrastructure d’un pollen original parmi les Annonacées. – Bull. Soc. Bot. France 122: 109-111.
Le Thomas A, Lugardon B. 1976. De la structure grenue à la structure columellaire dans le pollen des Annonacées. – Adansonia, sér. II, 15: 543-572.
Le Thomas A, Morawetz W, Waha M. 1986. Pollen of palaeo- and neotropical Annonaceae: definition of the aperture by morphological and functional characters. – In: Blackmore S, Ferguson IK (eds), Pollen and spores, form and function, Academic Press, London, pp. 375-388.
Le Thomas A, Lugardon B, Doyle JA. 1994. Pollen ultrastructure and relationships of Fusaea (Baillon) Safford and Duguetia A. Saint-Hilaire (Annonaceae). – Rev. Palaeobot. Palyn. 83: 55-64.
Li J, Conran JG. 2003. Phylogenetic relationships in Magnoliaceae subfam. Magnolioideae: a morphological cladistic analysis. – Plant Syst. Evol. 242: 33-47.
Li PS, Thomas DC, Saunders RMK. 2015. Phylogenetic reconstruction, morphological diversification and generic delimitation of Disepalum (Annonaceae). – PLoS ONE 10: e0143481.
Li P-T. 1976. Some notes on the Annonaceae of China. – Acta Phytotax. Sin. 14: 96-113.
Liao W-F, Xia N-H. 2007. Phyllotaxis of vegetative shoots, lamina rotation and their systematic implication in Magnoliaceae. – Nord. J. Bot. 25: 199-205.
Liao W-F, Xia N-H. 2009. Manglietia lawii sp. nov. (Magnoliaceae) from Yunnan, China. – Nord. J. Bot. 27: 1-3.
Litaudon M, Bousserouel H, Awang K, Nosjean O, Martin M-T, Dau METH, Hadi HA, Boutin JA, Sévenet T, Guéritte F. 2009. A dimeric sesquiterpenoid from a Malaysian Meiogyne as a new inhibitor of Bcl-xL/BakBH3 domain peptide interaction. – J. Nat. Prod. 72: 480-483.
Liu D-M, Zhou R-Z, Zeng Q-W, Xing F-W. 2009. Magnolia bawangensis sp. nov. (Magnoliaceae) from Hainan, China. – Nord. J. Bot. 27: 4-6.
Liu Y-H. 1984. A preliminary study on the taxonomy of the family Magnoliaceae. – Acta Phytotaxon. Sin. 22: 89-109. [In Chinese]
Lopes J de C, Chatrou LW, Mello-Silva R. 2014. Ephedranthus dimerus (Annonaceae), a new species from the Atlantic Forest of Brazil, with a key to the species of Ephedranthus. – Brittonia 66: 70-74.
Lopes JC, Chatrou LW, Mello-Silva R, Rudall PJ, Sajo MG. 2018. Phylogenomics and evolution of floral traits in the Neotropical tribe Malmeeae (Annonaceae). – Mol. Phylogen. Evol. 118: 379-391.
Lora J, Testillano PS, Risueño MC, Hormaza JI, Herrero M. 2009. Pollen development in Annona cherimola Mill. (Annonaceae). Implications for the evolution of aggregated pollen. – BMC Plant Biol. 9: 129.
Lora J, Herrero M, Hormaza JI. 2009. The coexistence of bicellular and tricellular pollen in Annona cherimola (Annonaceae). Implications for pollen evolution. – Amer. J. Bot. 96: 802-808.
Lozano-Contreras G. 1975. Contribucion a las Magnoliaceae de Colombia III. Dugandiodendron. – Caldasia 11: 27-50.
Lozano-Contreras G. 1983. Flora de Colombia 1. Magnoliaceae. – Universidad Nacional de Colombia, Colciencias, Bogotá, Colombia.
Lozana-Contreras G. 1994. Dugandiodendron y Talauma (Magnoliaceae) en el neotropico. – Muséo de Historia Natural, Universidad Nacional, Santafe de Bogotá.
Lúcio ASSC, Da Silva Almeida JRG, Barbosa-Filho JM, Pita JCLR, Branco MVSC, Diniz MDFM, Agra MD, Da-Cunha EVL, Da Silva MS, Tavares JF. 2011. Azaphenanthrene alkaloids with antitumoral activity from Anaxagorea dolichocarpa Sprague & Sandwith (Annonaceae). – Molecules 16: 7125-7131.
Maas PJM. 1983. Project Systematics of Annonaceae. – Taxon 32: 528-529.
Maas PJM. 1984. The Annonaceae Project. – Taxon 33: 800-801.
Maas PJM, Westra LYT. 1984. Studies in Annonaceae II. A monograph of the genus Anaxagorea A. St. Hil. 1. – Bot. Jahrb. Syst. 105: 73-134.
Maas PJM, Westra LYT. 1985. Studies in Annonaceae II. A monograph of the genus Anaxagorea A. St. Hil. 2. – Bot. Jahrb. Syst. 105: 145-204.
Maas PJM, Westra LYT. 2003. Revision of the Neotropical genus Pseudoxandra (Annonaceae). – Blumea 48: 201-259.
Maas PJM, Westra LYT. 2010. New species of Annonaceae from the Neotropics and miscellaneous notes. – Blumea 55: 259-275.
Maas PJM, Westra LYT. 2011. A taxonomic survey of Guatteria section Mecocarpus including the genera Guatteriopsis and Guatteriella p.p. (Annonaceae). – Blumea 56: 113-145.
Maas PJM, Mennega EA, Westra LYT. 1994. Studies in Annonaceae XXI. Index to species and infraspecific taxa of neotropical Annonaceae. – Candollea 49: 389-481.
Maas PJM, Westra LYT, Chatrou LW. 2003. Flora Neotropica monograph 88. Duguetia. – The New York Botanical Garden, Bronx, New York.
Maas PJM, Westra LYT, Vermeer M. 2007. Revision of the Neotropical genera Bocageopsis, Onychopetalum, and Unonopsis (Annonaceae). – Blumea 52: 413-554.
Maas PJM, Westra LYT, Guerrero SA, Lobão AQ, Scharf U, Zamora NA, Erkens RHJ. 2015. Confronting a morphological nightmare: revision of the Neotropical genus Guatteria (Annonaceae). – Blumea 60: 1-219.
Mackie A, Ghatge N. 1958. Chemical investigation of the leaves of Annona senegalensis II. – Carbohydrates, glycosides, proteins, amino-acids, sterols. – J. Sci. Food Agric. 9: 88-92.
McLaughlin RP. 1933. Systematic anatomy of the woods of the Magnoliales. – Trop. Woods 34: 3-39.
Mai DH. 1971. Fossile funde von Manglietia Blume (Magnoliaceae). – Feddes Repert. 82: 441-448.
Mai DH. 1975. Beiträge zur Bestimmung und Nomenklatur fossiler Magnolien. – Feddes Repert. 86: 559-578.
Maneval WE. 1914. The development of Magnolia and Liriodendron, including a discussion of the primitiveness of the Magnoliaceae. – Bot. Gaz. (Chicago) 57: 1-31.
Manilal KS. 1983. Morphology and anatomy of Myristica flowers. – Bull. Pure Appl. Sci. 2B: 7-13.
Martin PG, Dowd JM. 1984. The study of plant phylogeny using amino acid sequences of ribulose-1,5-biphosphate carboxylase V. Magnoliaceae, Polygonaceae and the concept of primitiveness. – Aust. J. Bot. 32: 301-309.
Massoni J, Forest F, Sauquet H. 2014. Increased sampling of both genes and taxa improves resolution of phylogenetic relationships within Magnoliidae, a large and early-diverging clade of angiosperms. – Mol. Phylogen. Evol. 70: 84-93.
Mat-Salleh K. 2001. New and noteworthy species of Bornean Goniothalamus (Annonaceae). – Folia Malaysiana 2: 75-116.
Meade CV. 2000. A systematic revision of the Uvaria L. group (Annonaceae) in continental Asia. – Ph.D. diss., University of Dublin, Trinity College, Dublin, Ireland.
Meade CV. 2005. A new species of Uvaria (Annonaceae) from Southeast Asia. – Adansonia, sér. III, 27: 17-20.
Meade CV, Parnell JAN. 2003. Multivariate analysis of leaf shape patterns in Asian species of the Uvaria group (Annonaceae). – Bot. J. Linn. Soc. 143: 231-242.
Meade CV, Hodkingins T, Chalermglin P, Chase MW, Parnell JAN. 2002. Revision of Uvaria L. in continental Southeast Asia 2. Floral character evolution in the Uvaria L. group. – Annonaceae Newsl. 13: 29-32.
Melville R. 1969. Studies in floral structure and evolution I. The Magnoliales. – Kew Bull. 23: 133-180.
Merrill ED. 1915. Studies on Philippine Annonaceae I. – Philipp. J. Sci., Sect. C, 10: 227-264.
Merrill ED. 1919. On the application of the generic name Melodorum of Loureiro. – Philipp. J. Sci., Sect. C, 15: 125-137.
Miller JM. 1988. A new species of Degeneria (Degeneriaceae) from the Fiji Archipelago. – J. Arnold Arbor. 69: 275-280.
Miller JM. 1989. The archaic flowering plant family Degeneriaceae: its bearing on an old enigma. – Natl. Geogr. Res. 5: 218-231.
Miquel FAW. 1865. Anonaceae Archipelagi Indici. – Ann. Mus. Bot. Lugd.-Bat. 2: 1-45.
Moeljono S. 2009. A taxonomic revision of the genus Popowia Endlicher (Annonaceae) in Malesia. – PhD diss., Bogor Agricultyural University, Bogor, Indonesia.
Mohana Rao PR. 1975. Seed anatomy of Artabotrys odoratissimus with discussion on chalaza, integumentary bundles, and ruminate endosperm. – Phytomorphology 25: 215-228.
Mohana Rao PR. 1982. Seed and fruit anatomy in Asimina triloba, with a discussion of the affinities of Annonaceae. – Bot. Jahrb. Syst. 103: 47-57.
Mohana Rao PR. 1983. Seed and fruit anatomy in Eupomatia laurina with a discussion of the affinities of Eupomatiaceae. – Flora, Ser. B, 173: 311-319.
Mohr BAR, Bernardes-de-Oliveira MEC. 2004. Endressinia brasiliana, a magnolialean angiosperm from the Lower Cretaceous Crato Formation (Brazil). – Intern. J. Plant Sci. 165: 1121-1133.
Mols JB, Keßler PJA. 2000. Revision of the genus Phaeanthus (Annonaceae). – Blumea 45: 205-233.
Mols JB, Keßler PJA. 2003a. Studies in the Miliuseae V. Review of the taxonomic history of a polyphyletic ’tribe’. – Telopea 10: 113-124.
Mols JB, Keßler PJA. 2003b. The genus Miliusa (Annonaceae) in the Austro-Malesian area. – Blumea 48: 421-462.
Mols JB, Gravendeel B, Chatrou LW, Pirie MD, Bygrave PC, Chase MW, Keßler PJA. 2004. Identifying clades in Asian Annonaceae: monophyletic genera in the polyphyletic Miliuseae. – Amer. J. Bot. 91: 590-600.
Mols JB, Co DLV, Gravendeel B, Chatrou LW, Pirie MD, Ham RWJM van der, Marle EJ van, Keßler PJA. 2004. Morphological character evolution in the miliusoid clade (Annonaceae). – In: Mols JB (ed), From Miliusa to Miliuseae to Miliusoid: Identifying clades in Asian Annonaceae, Nationaal herbarium Nederland, Universiteit Leiden branch, Leiden, pp. 37-75.
Mols JB, Keßler PJA, Rogstad SH, Saunders RMK. 2008. Reassignment of six Polyalthia species to the new genus Maasia (Annonaceae): molecular and morphological congruence. – Syst. Bot. 33: 490-494.
Momose K, Nagamitsu T, Inoue T. 1998. Thrips cross-pollination of Popowia pisocarpa (Annonaceae) in a lowland dipterocarp forest in Sarawak. – Biotropica 30: 444-448.
Morawetz W. 1984a. Karyological races and ecology of the Brazilian Duguetia furfuracea as compared with Xylopia aromatica (Annonaceae). – Flora 175: 195-209.
Morawetz W. 1984b. How stable are genomes of tropical woody plants? Porcelia, Annona, Drimys. – Plant Syst. Evol. 145: 29-39.
Morawetz W. 1986. Systematics and karyoevolution in Magnoliidae: Tetrameranthus as compared with other Annonaceae genera of the same chromosome number. – Plant Syst. Evol. 154: 147-173.
Morawetz W. 1988. Karyosystematics and evolution of Australian Annonaceae as compared with Eupomatiaceae, Himantandraceae, and Austrobaileyaceae. – Plant Syst. Evol. 159: 49-79.
Morawetz W, Le Thomas A. 1988. Karyology and systematics of the genus Ambavia and other Annonaceae from Madagascar. – Plant Syst. Evol. 158: 155-160.
Morawetz W, Waha M. 1985. A new pollen type, C-banded and fluorescent counterstained chromosomes and evolution in Guatteria and related genera (Annonaceae). – Plant Syst. Evol. 150: 119-141.
Muhammad I, Waterman PG. 1985. Chemistry of the Annonaceae 18. Benzylated indoles and dihydrochalcones in Uvaria angolensis from Tanzania. – J. Nat. Prod. 48: 571-580.
Murray NA. 1993. Revision of Cymbopetalum and Porcelia (Annonaceae). – Syst. Bot. Monogr. 40: 1-121.
Nagamitsu T, Inoue T. 1997. Cockroach pollination and breeding system of Uvaria elmeri (Annonaceae) in a lowland mixed-dipterocarp forest in Sarawak. – Amer. J. Bot. 84: 208-213.
Nair NC. 1972 [1975]. Floral morphology of Myristica malabarica Lamk. with a discussion of certain aspects of the systematics of Myristica. – In: Murty YS, Johri BM, Mohan Ram HY, Varghese TM (eds.), Advances in plant morphology, pp. 264-277.
Nakkuntod M, Su YCF, Seelanan T, Saunders RMK. 2009. Molecular phylogenetic and morphological evidence for the congeneric status of Goniothalamus and Richella (Annonaceae). – Taxon 58: 127-132.
Neubig KM, Abbott JR. 2010. Primer development for the plastid region ycf1 in Annonaceae and other magnoliids. – Amer. J. Bot. 97: e52-e55.
Nie Z-L, Wen J, Azuma H, Qiu Y-L, Sun H, Meng Y, Sun W-B, Zimmer EA. 2008. Phylogenetic and biogeographic complexity of Magnoliaceae in the Northern Hemisphere inferred from three nuclear data sets. – Mol. Phylogen. Evol. 48: 1027-1040.
Nishida H. 1985. A structurally preserved magnolialean fructification from the mid-Cretaceous of Japan. – Nature 318: 58-59.
Nishida M, Ohsawa T, Nishida H, Yoshida A, Kanie Y. 1996. A permineralized magnolialean fructification from the Upper Cretaceous of Hookaido, Japan. – Sci. Rep. Res. Inst. Evol. Biol. 8: 19-30.
Nkunya MHH, Achenbach H, Renner C, Waibel R, Weenen H. 1990. Schefflerin and isoschefflerin: prenylated chalcones and other constituents of Uvaria scheffleri. – Phytochemistry 29: 1261-1264.
Nong Van Tiep. 1980. Beiträge zur Sippenstruktur der Gattung Manglietia. – Feddes Repert. 91: 497-576.
Nooteboom HP. 1985. Notes on Magnoliaceae, with a revision of Pachylarnax and Elmerrillia and the Malesian species of Manglietia and Michelia. – Blumea 31: 65-121.
Nooteboom HP. 1986. Magnoliaceae. – In: Steenis CGGJ van (†), Wilde WJJO de (eds), Flora Malesiana I, 10(3), Kluwer Academic Publ., Dordrecht, Boston, London, pp. 561-605.
Nooteboom HP. 1987. Notes on Magnoliaceae II, revision of Magnolia sections Maingola (Malesian species), Aromadendron, and Blumiana. – Blumea 32: 343-382.
Nooteboom HP. 1993. Magnoliaceae. – In: Kubitzki K, Rohwer JG, Bittrich V (eds), The families and genera of vascular plants II. Flowering plants. Dicotyledons. Magnoliid, hamamelid and caryophyllid families, Springer, Berlin, Heidelberg, New York, pp. 391-401.
Nooteboom HP. 1998. The tropical Magnoliaceae and their classification. – In: Hunt D (ed), Magnolias and their allies, David Hunt, Milborne Port, United Kingdom, pp. 71-80.
Nooteboom HP. 2000. Different looks at the classification of Magnoliaceae. – In: Liu Y-H, Fan H-M, Chen Z-Y, Wu Q-G, Zeng Q-W (eds), Proceedings of the international symposium on the family Magnoliaceae, May 18-22, 1998, Guangzhou, China, Science Press, Beijing, pp. 26-37.
Norman EM. 2003. Reproductive biology of Deeringothamnus rugelii and D. pulchellus (Annonaceae). – Taxon 52: 547-555.
Norman EM, Clayton D. 1986. Reproductive biology of two Florida pawpaws: Asimina obovata and A. pygmaea (Annonaceae). – Bull. Torrey Bot. Club 113: 16-22.
Okada H. 1990. Reproductive biology of Polyalthia littoralis (Annonaceae). – Plant Syst. Evol. 170: 237-245.
Okada H. 1996. New genus and new species of the Annonaceae from the Malesian wet tropics. – Acta Phytotaxon. Geobot. 47: 1-9.
Okada H, Ueda K. 1984. Cytotaxonomical studies on Asian Annonaceae. – Plant Syst. Evol. 144: 165-177.
Okorie DA. 1977. New benzyldihydrochalcones from Uvaria chamae. – Phytochemistry 16: 1591-1594.
Olesen JM. 1992. Flower mining by moth larvae vs. pollination by beetles and bees in the cauliflorous Sapranthus palanga (Annonaceae) in Costa Rica. – Flora 187: 9-15.
Ortiz-Rodriguez AE, Ruiz-Sanchez E, Ornelas JF. 2016. Phylogenetic relationships among members of the Neotropical clade of Miliuseae (Annonaceae): generic non-monophyly of Desmopsis and Stenanona. – Syst. Bot. 41: 815-822.
Ortiz-Rodriguez AE, Escobar-Castellanos MA, Pérez-Farrera MA. 2016. Phylogenetic analyses and morphological characteristics support the description of a second species of Tridimeris (Annonaceae). – PhytoKeys 74: 79-96.
Ortiz-Rodriguez AE, Ornelas JF, Ruiz-Sanchez E. 2018. A jungle tale: molecular phylogeny and divergence time estimates of the Desmopsis-Stenanona clade (Annonaceae) in Mesoamerica. – Mol. Phylogen. Evol. 122: 80-94.
Ozenda P. 1943. Anatomie végétale. Sur la vascularisation des carpelles et du pistil chez les Magnolia. – Compt. Rend. Acad. Sci. Paris 217: 31-33.
Ozenda P. 1947. Structure du noeud foliaire des Magnoliacées et des Annonacées. – Compt. Rend. Acad. Sci. Paris 224: 1521-1523.
Padmanabhan D. 1960. A contribution to the embryology of Michelia champaca. – J. Madras Univ., Sect. B, 30: 155-165.
Paiva JAR. 1970. Notes on Annonaceae. – Bol. Soc. Brot., ser. II, 44: 369-373.
Pan Y-Z, Gong X, Liang H-X. 2003. A study on the embryology of the endangered plant Manglietia aromatica. – J. Wuhan Bot. Res. 21: 1-8. [In Chinese]
Panichpol K, Waterman PG. 1978. Novel flavonoids from the stem of Popowia cauliflora. – Phytochemistry 17: 1363-1367.
Parks CR, Wendel JF. 1990. Molecular divergence between Asian and North American species of Liriodendron (Magnoliaceae) with implications for interpretation of fossil floras. – Amer. J. Bot. 77: 1243-1256.
Parks CR, Miller NG, Wendel JF, McDougal KM. 1983. Genetic divergence within the genus Liriodendron (Magnoliaceae). – Ann. Missouri Bot. Gard. 70: 658-666.
Parks CR, Wendel JF, Sewell MM, Qiu Y-L. 1994. The significance of allozyme variation and introgression in the Liriodendron tulipifera complex (Magnoliaceae). – Amer. J. Bot. 81: 878-889.
Peigler RS. 1989. Fossil Magnoliaceae: a review of literature. – Magnolia (Journal of the Magnolia Society) 25: 1-11.
Pellegrin F. 1949. Popowia (Annonacées) d’Afrique. – Bull. Soc. Bot. France 96: 212-213.
Periasamy K. 1961. Studies on seeds with ruminate endosperm I. Morphology of ruminating tissue in Myristica fragrans. – J. Madras Univ. 31B: 53-58.
Periasamy K, Swamy BGL. 1959 [1960]. Studies in the Annonaceae I. Microsporogenesis in Cananga odorata och Miliusa wightiana. – Phytomorphology 9: 251-263.
Periasamy K, Swamy BGL. 1961. Studies in the Annonaceae II. The development of ovule and seed in Cananga odorata and Miliusa wightiana. – J. Indian Bot. Soc. 40: 206-216.
Pirie MD, Doyle JA. 2012. Dating clades with fossils and molecules: the case of Annonaceae. – Bot. J. Linn. Soc. 169: 84-116.
Pirie MD, Chatrou LW, Erkens RHJ, Maas JW, Niet T van der, Mols JB, Richardson JE. 2005. Phylogeny reconstruction and molecular dating in four Neotropical genera of Annonaceae: the effect of taxon sampling on age estimations. – In: Bakker FT, Chatrou LW, Gravendeel B, Pelser PB (eds), Plant species-level systematics: new perspectives on pattern and process, Regnum Vegetabile 143, A. R. G. Gantner, Koeltz, Koenigstein, pp. 149-174.
Pirie MD, Chatrou LW, Mols JB, Erkens RHJ, Oosterhof J. 2006. ‘Andean-centred’ genera in the short-branch clade of Annonaceae: testing biogeographic hypotheses using phylogeny reconstruction and molecular dating. – J. Biogeogr. 33: 31-46.
Pirie MD, Vargas MPB, Botermans M, Bakker FT, Chatrou LW. 2007. Ancient paralogy in the cpDNA trnL-F region in Annonaceae: implications for plant molecular systematics. – Amer. J. Bot. 94: 1003-1016.
Posluszny U, Fisher JB. 2000. Thorn and hook ontogeny in Artabotrys hexapetalus (Annonaceae). – Amer. J. Bot. 87: 1561-1570.
Praglowski J, Dandy JE. 1974. Magnoliaceae Juss. – In: Nilsson S (ed), World Pollen and Spore Flora 3, Almqvist & Wiksell, Stockholm, pp. 1-45.
Prakash N, Foreman DB, Griffith SJ. 1984. Gametogenesis in Galbulimima belgraveana (Himantandraceae). – Aust. J. Bot. 32: 605-612.
Prantl K. 1891a. Magnoliaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(2), W. Engelmann, Leipzig, pp. 12-19.
Prantl K. 1891b. Anonaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(2), W. Engelmann, Leipzig, pp. 23-39.
Prantl K. 1891c. Myristicaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(2), W. Engelmann, Leipzig, pp. 40-42.
Qiu Y-L, Parks CR. 1994. Disparity of allozyme variation levels in three Magnolia (Magnoliaceae) species from the southeastern United States. – Amer. J. Bot. 81: 1300-1308.
Qiu Y-L, Chase MW, Parks CR. 1995. A chloroplast DNA phylogenetic study of the eastern Asia-eastern North America disjunct section Rhytidospermum of Magnolia (Magnoliaceae). – Amer. J. Bot. 82: 1582-1588.
Qiu Y-L, Parks CR, Chase MW. 1995. Molecular divergence in the eastern Asia-eastern North America disjunct section Rhytidospermum of Magnolia (Magnoliaceae). – Amer. J. Bot. 82: 1589-1598.
Qiu Y-L, Lee J, Bernasconi-Quadroni F, Soltis DE, Soltis PS, Zanis M, Zimmer EA, Chen Z, Savolainen V, Chase MW. 1999. The earliest angiosperms: evidence from mitochondrial, plastid and nuclear genomes. – Nature 402: 404-407.
Rainer H. 2002. A new species of Annona (Annonaceae) from the northeastern Guayana Shield. – Brittonia 54: 136-140.
Rainer H. 2007. Monographic studies in the genus Annona L. (Annonaceae): inclusion of the genus Rollinia A. St.-Hil. – Ann. Naturhist. Mus. Wien, Ser. B, 108: 191-205.
Rainer H, Chatrou LW. 2006. AnnonBase: world species list of Annonaceae – version 1.1, 12 Oct 2006. https://mailfilter.sunet.se/canit/urlproxy.php?_q=aHR0cDovL3d3dy5zcDIwMDAub3JnYW5k&_s=ZGVmYXVsdA%3D%3D&_c=c5039b97&_r=c3Utc2U%3D https://mailfilter.sunet.se/canit/urlproxy.php?_q=aHR0cDovL3d3dy5hbm5vbmFjZWFlLm9yZw%3D%3D&_s=ZGVmYXVsdA%3D%3D&_c=1111612f&_r=c3Utc2U%3D
Rao HS. 1939. Cuticular studies of Magnoliales. – Proc. Natl. Sci. Acad. India, Sect. B, 9: 9-16.
Rao PRM- 1983. Seed and fruit anatomy of Eupomatia laurina with a discussion of the affinities of Eupomatiaceae. – Flora 173: 311-319.
Ratnayake RMCS, Gunatilleke IAUN, Wijesundara DSA, Saunders RMK. 2006. Reproductive biology of two sympatric species of Polyalthia (Annonaceae) in Sri Lanka I. Pollination by curculionid beetles. – Intern. J. Plant Sci. 167: 483-493.
Ratnayake RMCS, Su YCF, Gunatilleke IAUN, Wijesundara DSA, Saunders RMK. 2006. Reproductive biology of two sympatric species of Polyalthia (Annonaceae) in Sri Lanka II. Breeding systems and population genetic structure. – Intern. J. Plant Sci. 167: 495-502.
Read RW, Taylor WC. 1979. Constituents of Eupomatia species V. The isolation of eupomatenoid-13 (a new neolignan), (±)-trans-dehydrodiisoeugenol, and other extractives from the bark of Eupomatia laurina. – Aust. J. Chem.. 32: 2317-2321.
Richardson JE, Pennington RT, Pennington TD, Hollingsworth PM. 2001. Rapid diversification of a species-rich genus of neotropical rain forest trees. – Science 293: 2242-2245.
Richardson JE, Chatrou LW, Mols JB, Erkens RHJ, Pirie MD. 2004. Historical biogeography of two cosmopolitan families of flowering plants: Annonaceae and Rhamnaceae. – Philos. Trans. Roy. Soc. London, Ser. B, 359: 1495-1508.
Ritchie E, Taylor WC. 1967. The Galbulimima alkaloids. – In: Manske RHF (ed), The alkaloids 9, Academic Press, New York, pp. 529-543.
Rix M, Endress PK. 2007. 591. Eupomatia laurina (Eupomatiaceae). – Curtis’s Bot. Mag. 24: 230-234.
Robson NKB. 1960. 6. Annonaceae. – In: Exell AW, Wild H (eds), Flora Zambesiaca 1 (Part 1), Crown Agents for Oversea Governments and Administrations, London, pp. 104-149.
Rodrigues WA. 1980. Revisão taxonómica das espécies de Virola Aublet (Myristicaceae) do Brasil. – Acta Amazonica 10 [Suppl.]: 1-127.
Rogstad SH. 1989. The biosystematics and evolution of the Polyalthia hypoleuca complex (Annonaceae) of Malesia I. Systematic treatment. – J. Arnold Arbor. 70: 153-246.
Rogstad SH. 1990. The biosystematics and evolution of the Polyalthia hypoleuca species complex (Annonaceae) of Malesia II. Comparative distributional ecology. – J. Trop. Ecol. 6: 387-408.
Rogstad SH. 1994. The biosystematics and evolution of the Polyalthia hypoleuca species complex (Annonaceae) of Malesia III. Floral ontogeny and breeding systems. – Amer. J. Bot. 81: 145-154.
Rogstad SH, Le Thomas A. 1989. Pollen characters of the Polyalthia hypoleuca complex (Annonaceae): their significance in establishing monophyly and candidate outgroups. – Bull. Mus. Natl. Hist. Nat. Paris, Sect. B, Adansonia 11: 257-278.
Romanov MS, Dilcher DL. 2013. Fruit structure in Magnoliaceae s.l. and Archaeanthus and their relationships. – Amer. J. Bot. 100: 1494-1508.
Ronse De Craene LP, Smets E. 1990. The floral development of Popowia whitei (Annonaceae). – Nord. J. Bot. 10: 411-420.
Roth J. 1970. Estructura anatómica de la corteza de algunas especies de arbóreas de Myristicaceae. – Acta Bot. Venezuelica 4: 214-257.
Royen P van, Heel WA van. 1962. Sertulum Papuanum 6. Himantandraceae. – Nova Guinea, Bot., 8-10: 127-135.
Safford WE. 1912. Desmos the proper generic name for the so-called unonas of the Old World. – Bull. Torrey Bot. Club 39: 501-508.
Safford WE. 1916. Desmopsis, a new genus of Annonaceae. – Bull. Torrey Bot. Club 43: 183-193.
Santamour FS Jr. 1965. Biochemical studies in Magnolia I. Floral anthocyanins. – Morris Arbor. Bull. 16: 43-48.
Santos JK. 1929. Histological and microchemical studies on the bark and leaf of Artabotrys suaveolens Blume from the Philippines. – Philipp. J. Sci. 38: 269-282.
Sastri RLN. 1957. On the division of pollen mother cells in some Annonaceae. – Sci. Cult. 22: 633-634.
Sauer W, Ehrendorfer F. 1970. Chromosomen, Verwandtschaft und Evolution tropischer Holzpflanzen II. Himantandraceae. – Österr. Bot. Zeitschr. 118: 38-54.
Sauer W, Ehrendorfer F. 1984. Notes on the karyosystematics of Annonaceae. – Plant Syst. Evol. 146: 47-55.
Saunders RMK. 2002. The genus Goniothalamus (Annonaceae) in Sumatra. – Bot. J. Linn. Soc. 139: 225-254.
Saunders RMK. 2003. A synopsis of Goniothalamus species (Annonaceae) in Peninsular Malaysia, with a description of a new species. – Bot. J. Linn. Soc. 142: 321-339.
Saunders RMK. 2010. Floral evolution in the Annonaceae: hypotheses of homeotic mutations and functional convergence. – Biol. Rev. Cambridge Philos. Soc. 85: 571-591.
Saunders RMK, Chalermglin P. 2008. A synopsis of Goniothalamus species (Annonaceae) in Thailand, with descriptions of three new species. – Bot. J. Linn. Soc. 156: 355-384.
Saunders RMK, Munzinger J. 2007. A new species of Goniothalamus (Annonaceae) from New Caledonia, representing a significant range extension for the genus. – Bot. J. Linn. Soc. 155: 497-503.
Saunders RMK, Xue B. 2011. (1992) Proposal to conserve the name Enicosanthum against Monoon (Annonaceae). – Taxon 60: 236-237.
Saunders RMK, Su YCF, Chalermglin P. 2004. Craibella phuyensis (Annonaceae): a new genus and species from Thailand. – Syst. Bot. 29: 42-49.
Saunders RMK, Su YCF, Xue B. 2011. Phylogenetic affinities of Polyalthia species (Annonaceae) with columellar-sulcate pollen: enlarging the Madagascan endemic genus Fenerivia. – Taxon 60: 1407-1416.
Sauquet H. 1999. Phylogénie des Myristicaceae à partir de caractères morphologiques et de données de sequence du gene ndhF. – DEA (M.Sc.) thesis, Université Pierre et Marie Curie, Paris.
Sauquet H. 2003. Androecium diversity and evolution in Myristicaceae (Magnoliales), with the description of a new Malagasy genus, Doyleanthus gen. nov. – Amer. J. Bot. 90: 1293-1305.
Sauquet H. 2004. Systematic revision of Myristicaceae (Magnoliales) in Madagascar, with four new species of Mauloutchia. – Bot. J. Linn. Soc. 146: 351-368.
Sauquet H, Le Thomas A. 2003. Pollen diversity and evolution in Myristicaceae (Magnoliales). – Intern. J. Plant Sci. 164: 613-628.
Sauquet H, Doyle JA, Scharaschkin T, Borsch T, Hilu KW, Chatrou LW, Le Thomas A. 2003. Phylogenetic analysis of Magnoliaceae and Myristicaceae based on multiple data sets: implications for character evolution. – Bot. J. Linn. Soc. 142: 125-186.
Savage PJ. 1989. Magnolias in Michigan IV. – J. Magnolia Soc. 24: 10.
Scharaschkin T, Doyle JA. 2005. Phylogeny and historical biogeography of Anaxagorea (Annonaceae) using morphology and non-coding chloroplast sequence data. – Syst. Bot. 30: 712-735.
Scharaschkin T, Doyle JA. 2006. Character evolution in Anaxagorea (Annonaceae). – Amer. J. Bot. 93: 36-54.
Schatz GE. 1987. Systematic and ecological studies of Central American Annonaceae. – Ph.D. diss., University of Wisconsin, Madison, Wisconsin.
Schatz GE, Le Thomas A. 1990. The genus Polyalthia Blume (Annonaceae) in Madagascar. – Bull. Mus. Natl. Hist. Nat. Paris, Sect. B, Adansonia 12: 113-130.
Schatz GE, Maas PJM. 2010. Synoptic revision of Stenanona. – Blumea 55: 205-223.
Schatz GE, Wendt T. 2004. A new flagelliflorous species of Stenanona (Annonaceae) from Mexico, with a review of the phenomenon of flagelliflory. – Lundellia 7: 28-38.
Scheffer RHCC. 1885. Sur quelques plantes nouvelles ou peu connues de l’Archipel Indien (Annonaceae). – Ann. Jard. Bot. Buitenzorg 2: 1-31.
Serna M, Velásquez C, Cogollo Á. 2009. Novedades taxonómicas y un nuevo registro de Magnoliaceae para Colombia. – Brittonia 61: 35-40.
Setten AK van, Koek-Noorman J. 1986. Studies in Annonaceae VI. A leaf anatomical survey of genera of Annonaceae in the Neotropics. – Bot. Jahrb. Syst. 108: 17-50.
Setten AK van, Koek-Noorman J. 1992. Studies in Annonaceae XVII. Fruits and seeds of Annonaceae: morphology and its significance for classification and identification. – Bibl. Bot. 142: 1-101.
Sewell MM, Qiu Y, Parks CR, Chase MW. 1993. Genetic evidence for trace paternal transmission of plastids in Liriodendron and Magnolia (Magnoliaceae). – Amer. J. Bot. 80: 854-858.
Sewell MM, Parks CR, Chase MW. 1996. Intraspecific chloroplast DNA variation and biogeography of North American Liriodendron L. (Magnoliaceae). – Evolution 50: 1147-1154.
Siddiqi MR, Wilson TK. 1974. Wood anatomy of the genus Knema (Myristicaceae). – Bull. Torrey Bot. Club 101: 354-362.
Siddiqi MR, Wilson TK. 1975a. Leaf anatomy of the genus Knema. – Biologia (Pakistan) 21: 167-175.
Siddiqi MR, Wilson TK. 1975b. Pollen of the genus Knema (Myristicaceae). – Pakistan J. Bot. 7: 197-200.
Siddiqi MR, Wilson TK. 1976a. Floral anatomy of the genus Knema (Myristicaceae). – Biologia (Lahore) 22: 127-141.
Siddiqi MR, Wilson TK. 1976b. Comparative study of the genus Knema (Myristicaceae). – Biologia (Lahore) 22: 305-308.
Silberbauer-Gottsberger I, Gottsberger RA, Gottsberger G. 1997. Flower rhythm and pollination in a hybrid population of Annona in a small cerrado area in Mato Grosso, Brazil. – Annonaceae Newsl. 11: 55-60.
Silberbauer-Gottsberger I, Gottsberger G, Webber AC. 2003. Morphological and functional flower characteristics of New and Old World Annonaceae with respect to their mode of pollination. – Taxon 52: 701-718.
Sinclair J. 1951. Notes on Bornean Annonaceae. – Sarawak Mus. J. 5: 597-609.
Sinclair J. 1953. Notes on Siamese Annonaceae. – Gard. Bull. Straits Settlem. (Singapore) 14: 40-44.
Sinclair J. 1955. A revision of the Malayan Annonaceae. – Gard. Bull. Straits Settlem. (Singapore) 14: 149-516.
Sinclair J. 1958a. A revision of the Malayan Myristicaceae. – Gard. Bull. Straits Settlem. (Singapore) 16: 205-472.
Sinclair J. 1958b. Ararocarpus – a monstrosity. – Gard. Bull. Straits Settlem. (Singapore) 17: 93-95.
Sinclair J. 1961. Florae Malesianae Precursores 31. The genus Knema in Malaysia and outside Malaysia. – Gard. Bull. Straits Settlem. (Singaproe) 18: 102-327.
Sinnott EW. 1914. Investigations on the phylogeny of angiosperms I. The anatomy of the node as an aid in the classification of angiosperms. – Amer. J. Bot. 1: 303-322.
Skipworth JP. 1970. Development of floral vasculature in the Magnoliaceae. – Phytomorphology 20: 228-235.
Skipworth JP, Philipson WR. 1966. The cortical vascular system and the interpretation of the Magnolia flower. – Phytomorphology 16: 463-469.
Smith AC. 1949. Additional notes on Degeneria vitiensis. – J. Arnold Arbor. 30: 1-38.
Smith AC, Wodehouse RP. 1937. The American species of Myristicaceae. – Brittonia 2: 393-510.
Smith GH. 1928. Vascular anatomy of the Ranalian flowers II. Ranunculaceae, Menispermaceae, Calycanthaceae, Annonaceae. – Bot. Gaz. (Chicago) 85: 152-177.
Söderberg E. 1936. Verkieselung bei Magnoliales. – Svensk Bot. Tidskr. 30: 537-540.
Sprague TA,. Hutchinson J. 1916. XXVII. African Annonaceae. – Bull. Misc. Inform. Kew 1916: 145-161.
Steenis CGGJ van. 1964. An account of the genera Richella A. Gray and Oxymitra (Bl.) Hooker f. & Th. (Annonaceae). – Blumea 12: 353-361.
Stull GW, Johnson DM, Murray NA, Couvreur TLP, Reeger JE, Roy CM. 2017. Plastid and seed morphology data support a revised infrageneric classification and an African origin of the pantropical genus Xylopia (Annonaceae). – Syst. Bot. 42: 211-225.
Su YCF. 2002. Systematics and phylogeny of Pseuduvaria (Annonaceae). – PhD diss., University of Hong Kong.
Su YCF, Saunders RMK. 2003. Pollen structure, tetrad cohesion and pollen-connecting threads in Pseuduvaria (Annonaceae). – Bot. J. Linn. Soc. 143: 69-78.
Su YCF, Saunders RMK. 2006. Monograph of Pseuduvaria (Annonaceae). – Syst. Bot. Monogr. 79: 1-204.
Su YCF, Saunders RMK. 2009. Evolutionary divergence times in the Annonaceae: evidence of a late Miocene origin of Pseuduvaria in Sundaland with subsequent diversification in New Guinea. – BMC Evol. Biol. 9: 153.
Su YCF, Mols JB, Takeuchi W, Kessler PJA, Saunders RMK. 2005. Reassessing the generic status of Petalolophus (Annonaceae): evidence for the evolution of a distinct sapromyophilous lineage within Pseuduvaria. – Syst. Bot. 30: 494-502.
Su YCF, Smith GJD, Saunders RMK. 2008. Phylogeny of the basal angiosperm genus Pseuduvaria (Annonaceae) inferred from five chloroplast DNA regions, with interpretation of morphological character evolution. – Mol. Phylogen. Evol. 48: 188-206.
Su YCF, Chaowasku T, Saunders RMK. 2010. An extended phylogeny of Pseuduvaria (Annonaceae) with descriptions of three new species and a reassessment of the generic status of Oreomitra. – Syst. Bot. 35: 30-39.
Sugiyama M. 1976a. Comparative studies of the vascular system of node-leaf continuum in woody Ranales I. Diversity in successive nodes of first-year plants of Magnolia virginiana L. – Bot. Mag. (Tokyo) 89: 33-43.
Sugiyama M. 1976b. Comparative studies of the vascular system of node-leaf continuum in woody Ranales II. Node-leaf vascular system of Eupomatia laurina R. Br. – J. Jap. Bot. 51: 169-174.
Sugiyama M. 1979. A comparative study of nodal anatomy in the Magnoliales based on the vascular system in the node-leaf continuum. – J. Fac. Sci. Univ. Tokyo, Sect. III, Botany 12: 199-279.
Sun T-X, Wu H, Li P-T. 2008. Comparative anatomy on leaves of Annonaceae. – Acta Bot. Yunnan. 30: 19-37. [In Chinese]
Surveswaran S, Wang RJ, Su YCF, Saunders RMK. 2010. Generic delimitation and historical biogeography in the early-divergent ‘ambavioid’ lineage of Annonaceae: Cananga, Cyathocalyx and Drepananthus. – Taxon 59: 1721-1734.
Svoma E. 1997. Seed development and function in Artabotrys hexapetalus (Annonaceae). – Plant Syst. Evol. 207: 205-223.
Svoma E. 1998a. Seed morphology and anatomy in some Annonaceae. – Plant Syst. Evol. 209: 177-204.
Svoma E. 1998b. Studies on the embryology and gynoecium structures in Drimys winteri (Winteraceae) and some Annonaceae. – Plant Syst. Evol. 209: 205-229.
Swamy BGL. 1949. Further contributions to the morphology of the Degeneriaceae. – J. Arnold Arbor. 30: 10-38.
Takahashi A. 1985. Wood anatomical studies of Polycarpicae I. Magnoliales. – Sci. Rep. Osaka Univ. 34: 29-83.
Takahashi M, Friis EM, Uesugi K, Suzuki Y, Crane PR. 2008. Floral evidence of Annonaceae from the Late Cretaceous of Japan. – Intern. J. Plant Sci. 169: 890-898.
Takhtajan AL, Meyer NR. 1976. Some additional data on the morphology of pollen grains of Degeneria vitiensis (Degeneriaceae). – Bot. Žurn. 61: 1531-1535.
Talapatra B, Deb T, Talapatra SK. 1985. Isolation of pinocembrin from Goniothalamus grifithii and its biomimetic synthesis. – Indian J. Chem. 24B: 561.
Tanaka R, Okada H. 1972. Karyological studies in four species of Annonaceae, a primitive angiosperm. – J. Sci. Hiroshima Univ., Ser. B, Div. 2, Bot. 14: 85-105.
Tang CC, Thomas DC, Saunders RMK. 2015a. Molecular phylogenetics of the species-rich angiosperm genus Goniothalamus (Annonaceae) inferred from nine chloroplast DNA regions: synapomorphies and putative correlated evolutionary changes in fruit and seed morphology. – Mol. Phylogen. Evol. 92: 124-139.
Tang CC, Thomas DC, Saunders RMK. 2015b. Molecular and morphological data supporting phylogenetic reconstruction of the genus Goniothalamus (Annonaceae), including interpretations of previous infrageneric classification. – Data Brief 4: 410-421.
Taylor WC. 1985. Eupomatia alkaloids. – Alkaloids 24: 1-23.
Taylor WI. 1961. The structure and synthesis of liriodenine, a new type of isoquinoline alkaloid. – Tetrahedron 14: 42-45.
Teichert H, Dötterl S, Zimma B, Ayasse M, Gottsberger G. 2009. Perfume-collecting male euglossine bees as pollinators of a basal angiosperm: the case of Unonopsis stipitata (Annonaceae). – Plant Biol. 11: 29-37.
Thien LB. 1974. Floral biology of Magnolia. – Amer. J. Bot. 61: 1037-1045.
Thomas DC, Surveswaran S, Xue B, Sankowsky G, Mols JB, Keßler PJA, Saunders RMK. 2012. Molecular phylogenetics and historical biogeography of the Meiogyne-Fitzalania clade (Annonaceae): generic paraphyly and late Miocene-Pliocene diversification in Australasia and the Pacific. – Taxon 61: 559-575.
Thongpairoj U. 2008. Taxonomy and molecular phylogeny of Artabotrys R. Brown and palynology of tribe Unoneae (Annonaceae) in Thailand. – PhD diss., Chiang Mai University.
Thorne RF. 1974. A phylogenetic classification of the Annoniflorae. – Aliso 8: 147-209.
Tiffney BH. 1977. Fruits and seeds of the Brandon Lignite: Magnoliaceae. – Bot. J. Linn. Soc. 75: 299-323.
Tiffney BH, McClammer JUA. 1988. Seed of the Anonaceae from the Palaeocene of Pakistan. – Tertiary Res. 9: 13-20.
Treseder NG. 1978. Magnolias. – Faber & Faber, London.
Tsiang Y, Li P-T. 1964. Diagnoses of new annonaceous plants from Hainan. – Acta Phytotax. Sin. 9: 374-382.
Tsou C-H, Fu Y-L. 2002. Tetrad pollen formation in Annona (Annonaceae): proexine formation and binding mechanism. – Amer. J. Bot. 89: 734-747.
Tsou C-H, Fu Y-L. 2007. Octad pollen formation in Cymbopetalum (Annonaceae): the binding mechanism. – Plant Syst. Evol. 263: 13-23.
Tsou C-H, Johnson DM. 2003. Comparative development of aseptate and septate anthers of Annonaceae. – Amer. J. Bot. 90: 832-848.
Tuchinda P, Udchachon J, Reutrakul V, Santisuk T, Taylor WC, Farnsworth NR, Pezzuto JM, Kinghorn DA. 1991. Bioactive butenolides from Melodorum fruticosum. – Phytochemistry 30: 2685-2689.
Tucker SC. 1960. Ontogeny of the floral apex of Michelia fuscata. – Amer. J. Bot. 47: 266-277.
Tucker SC. 1961. Phyllotaxis and vascular organization of the carpels in Michelia fuscata. – Amer. J. Bot. 48: 60-71.
Tucker SC. 1974. Different guard cells in Magnoliaceae. – Science 185: 445-447.
Tucker SC. 1977. Foliar sclereids in the Magnoliaceae. – Bot. J. Linn. Soc. 75: 325-356.
Turner IM. 2009a. New species and new records for Polyalthia (Annonaceae) in Borneo. – Fol. Malays. 9: 77-98.
Turner IM. 2009b. New species and nomenclatural combinations in Polyalthia, Meiogyne and Mitrella (Annonaceae) from Borneo. – Malayan Nat. J. 61: 267-276.
Turner IM. 2010. A consideration of Cleistopetalum and a new combination in Polyalthia (Annonaceae). – Phytotaxa 8: 41-45.
Turner IM. 2011. A catalogue of the Annonaceae of Borneo. – Phytotaxa 36: 1-120.
Turner IM. 2012. Annonaceae of Borneo: a review of the climbing species. – Gard. Bull. (Singapore) 64: 371-479.
Turner IM. 2015. A conspectus of Indo-Burmese Annonaceae. – Nord. J. Bot. 33: 257-299.
Turner IM, Saunders RMK. 2009. Four new species of Goniothalamus (Annonaceae) from Borneo. – Nord. J. Bot. 26: 329-337.
Turner IM, Utteridge TMA. 2015. Contributions to the Flora of Mt Jaya XXI. A new species and a new combination in Meiogyne (Annonaceae) of New Guinea. – Kew Bull. 70: 1-5.
Ueda K. 1984. Vascular systems in Magnoliaceae. – Fac. Sci., Kyoto University, Kyoto.
Ueda K. 1986. Vascular systems in the Magnoliaceae. – Bot. Mag. (Tokyo) 99: 333-349.
Ueda K, Yamashita J, Tamura MN. 2000. Molecular phylogeny of the Magnoliaceae. – In: Liu Y-H, Fan H-M, Chen Z-Y, Wu Q-G, Zeng Q-W (eds), Proceedings of the International Symposium on the family Magnoliaceae, May 18-22, 1998, Guangzhou, China, Science Press, Beijing, pp. 205-209.
Umeda A, Imaichi R, Kato M. 1994. Ovular development and morphology of the outer integument of Magnolia grandiflora (Magnoliaceae). – Amer. J. Bot. 81: 361-367.
Utteridge TMA. 2000. Revision of the genus Cyathostemma (Annonaceae). – Blumea 45: 377-396.
Vázquez-García JA. 1994. Magnolia (Magnoliaceae) in Mexico and Central America: a synopsis. – Brittonia 46: 1-23.
Verdcourt B. 1969. The status of the genus Polyalthia Blume (Annonaceae) in Africa. – Adansonia, sér. II, 9: 87-94.
Verdcourt B. 1971a. Notes on East African Annonaceae. – Kew Bull. 25: 1-34.
Verdcourt B. 1971b. Annonaceae. – In: Milne-Redhead E, Polhill RM (eds), Flora of tropical East Africa, Crown Agents for Oversea Governments and Administration, London, pp. 1-131.
Verdcourt B. 1986. New taxa of East African Annonaceae. – Kew Bull. 41: 287-297.
Verdcourt B. 1997. Myristicaceae. – In: Polhill RM (ed), Flora of tropical East Africa, A. A. Balkema, Rotterdam, The Netherlands, pp. 1-11.
Vollesen K. 1980a. Notes on Annonaceae from Tanzania. – Bot. Not. 133: 53-62.
Vollesen K. 1980b. A new species of Polyalthia (Annonaceae) from Mozambique. – Bot. Not. 133: 403-404.
Wagner R. 1906. Über den Aufbau des Disepalum anomalum Hook. fil. – Sitzungsber. Kaiserl. Akad. Wiss., Math.-Naturwiss. Cl., Abt. I, 105: 881-894.
Waha M. 1987a. Sporoderm development of pollen tetrads in Asimina triloba (Annonaceae). – Pollen Spores 29: 31-44.
Waha M. 1987b. Different origins of fragile exines within the Annonaceae. – Plant Syst. Evol. 158: 23-27.
Waha M, Hesse M. 1988. Aperture types within Sparanthus and Polyalthia (Annonaceae). – Plant Syst. Evol. 161: 135-146.
Waha M, Morawetz W. 1988. Pollen evolution and systematics in Annonaceae with special reference to the disulcate Australian endemic genera. – Plant Syst. Evol. 161: 1-12.
Walker JW. 1971a. Pollen morphology, phytogeography, and phylogeny of the Annonaceae. – Contr. Gray Herb. 202: 1-132.
Walker JW. 1971b. Unique type of angiosperm pollen from the family Annonaceae. – Science 172: 565-567.
Walker JW. 1971c. Contributions to the pollen morphology and phylogeny of the Annonaceae I. – Grana 11: 45-54.
Walker JW. 1972a. Contributions to the pollen morphology and phylogeny of the Annonaceae II. – Bot. J. Linn. Soc. 65: 173-178.
Walker JW. 1972b. Chromosome numbers, phylogeny, phytogeography of the Annonaceae and their bearing on the (original) basic chromosome number of angiosperms. – Taxon 21: 57-65.
Walker JW. 1974. Aperture evolution in the pollen of primitive angiosperms. – Amer. J. Bot. 61: 1112-1136.
Walker JW, Walker AG. 1979. Comparative pollen morphology of the American myristicaceous genera Compsoneura and Virola. – Ann. Missouri Bot. Gard. 66: 731-755.
Walker JW, Walker AG. 1980. Comparative pollen morphology of the mainland African genera of Myristicaceae (Cephalosphaera, Coelocaryon, Pycnanthus, and Scyphocephalium). – Amer. J. Bot. 67: 603-611.
Walker JW, Walker AG. 1981. Comparative pollen morphology of the Madagascan genera of Myristicaceae (Mauloutchia, Brochoneura, and Haematodendron). – Grana 20: 1-17.
Walker JW, Walker AG. 1983. Comparative pollen morphology of the American myristicaceous genera Otoba, Iryanthera, and Osteophloeum. – Amer. J. Bot. 70: 315-326.
Wang J. 2009. Systematics and phylogeny of Dasymaschalon (Annonaceae). – PhD diss., The University of Hong Kong.
Wang J, Chalermglin P, Saunders RMK. 2009. The genus Dasymaschalon (Annonaceae) in Thailand. – Syst. Bot. 34: 252-265.
Wang J, Thomas DC, Su YCF, Meinke S, Chatrou LW, Saunders RMK. 2012. A plastid DNA phylogeny of Dasymaschalon (Annonaceae) and allied genera: evidence for generic non-monophyly and the parallel evolutionary loss of inner petals. – Taxon 61: 545-558.
Wang RJ, Saunders RMK. 2006a. A synopsis of Cyathocalyx species (Annonaceae) in Peninsular Malaysia, Sumatra, and Borneo, with descriptions of two new species. – Bot. J. Linn. Soc. 152: 513-532.
Wang RJ, Saunders RMK. 2006b. The genus Cyathocalyx (Annonaceae) in the Philippines. – Syst. Bot. 31: 285-297.
Wang Y-L, Li Y, Zhang S-Z, Yo X-S. 2006. The utility of matK gene in the phylogenetic analysis of the genus Magnolia. – Acta Phytotaxon. Sin. 44: 135-147. [In Chinese]
Warburg O. 1897a. Monographie der Myristicaceen. – Nova Acta Acad. Caes. Leop.-Carol. 68: 1-680.
Warburg O. 1897b. Myristicaceae. – In: Engler A, Prantl (eds), Die natürlichen Pflanzenfamilien, Nachträge zu III(2), W. Engelmann, Leipzig, pp. 161-167.
Warburg O. 1904. Myristicaceae africanae. – Bot. Jahrb. Syst. 33: 382-386.
Warburg O. 1905. Compsoneura costaricensis Warb. – Feddes Repert. 1: 71.
Waterman PG, Pootakahm K. 1979. Chemical studies of the Annonaceae V. Flavonoids of the fruit of Popowia cauliflora. – Planta Medica 35: 366-369.
Webber AC. 1981a. Biologia floral de algumas Annonaceae na região de Manaus AM. – M.Sc. thesis, Instituto Nacional de Pesquisas da Amazônia Fundação Universidade do Amazonas, Manaus.
Webber AC. 1981b. Algunos aspectos da biologia floral de Annona sericea Dun. (Annonaceae). – Acta Amazonica 11: 61-65.
Webber AC. 1996. Biologia floral, polinização e aspectos fenológicos de algunas Annonaceae na Amazônia Central. – Ph.D. diss., Instituto Nacional de Pesquisas da Amazônia, Fundação Universidade do Amazonas, Manaus, Brazil.
Webber AC, Gottsberger G. 1993. Floral biology and pollination of Cymbopetalum euneurum (Annonaceae) in Manaus, Amazonia. – Annonaceae Newsl. 9: 25-28.
Webber AC, Gottsberger G. 1995. Floral biology and pollination of Bocageopsis multiflora and Oxandra euneura in Central Amazonia, with remarks on the evolution of stamens in Annonaceae. – Feddes Repert. 106: 515-524.
Webber AC, Gottsberger G. 1997. Flowering biology and pollination of Bocageopsis multiflora and Oxandra euneura in Central Amazonia. – Annonaceae Newsl. 11: 61-66.
Weerasooriya AD. 2001. Systematics, phylogeny and reproductive biology of Mitrephora (Annonaceae). – Ph.D. diss., University of Hong Kong.
Weerasooriya AD, Saunders RMK. 2001. Three new species of Mitrephora (Annonaceae) from Sabah, Malaysia. – Bot. J. Linn. Soc. 135: 305-314.
Weerasooriya AD, Saunders RMK. 2005. The genus Mitrephora (Annonaceae) in Cambodia, Laos, and Vietnam. – Syst. Bot. 30: 248-262.
Weerasooriya AD, Saunders RMK. 2010. Monograph of Mitrephora (Annonaceae). – Syst. Bot. Monogr. 90: 1-167.
Wei Z-X, Wu Z-Y. 1993. Pollen ultrastructure of Liriodendron and its systematic implications. – Acta Bot. Yunnan. 15: 163-166. [In Chinese, with English summary]
Welle BJH ter, Rooden J van. 1982. Systematic wood anatomy of Desmopsis, Sapranthus, and Stenanona. – IAWA Bull. 3: 15-23.
Westra LYT. 1985. Studies in Annonaceae IV. A taxonomic revision of Tetrameranthus R. E. Fries. – Proc. Kon. Ned. Akad. Wetensch., Ser. C, 88: 449-482.
Westra LYT, Maas PJM. 2012. Tetrameranthus (Annonaceae) revisited including a new species. – Phytokeys 12: 1-21.
Whitaker TW. 1933. Chromosome number and relationship in the Magnoliales. – J. Arnold Arbor. 14: 376-384.
Wilson TK, Maculans LM. 1967. The morphology of the Myristicaceae I. Flowers of Myristica fragrans and M. malabarica. – Amer. J. Bot. 54: 214-220.
Woodland PS. 1982. Studies in the genus Eupomatia (Eupomatiaceae). Morphology, anatomy, embryology. – Ph.D. diss., University of New England, Armidale, New South Wales, Australia.
Woodland PS, Garlick PR. 1982. The fine structure of the pollen of Eupomatiaceae. – Aust. J. Bot. 30: 297-301.
Worsdell WC. 1908. Internal phloem in Myristica. – Ann. Bot. 22: 526-527.
Wyk RW van der, Canright JE. 1956. The anatomy and relationships of the Annonaceae. – Trop. Woods 104: 1-24.
Xu F-X. 2003. Sclerotesta morphology and its systematic implications in magnoliaceous seeds. – Bot. J. Linn. Soc. 142: 407-424.
Xu F-X, Kirchoff BK. 2008. Pollen morphology and ultrastructure of selected species of Magnoliaceae. – Rev. Palaeobot. Palynol. 150: 140-153.
Xu F-X, De Craene LR. 2010. Floral ontogeny of Annonaceae: evidence for high variability in floral form. – Ann. Bot. 106: 591-605.
Xu F-X, Rudall PJ. 2006. Comparative floral anatomy and ontogeny in Magnoliaceae. – Plant Syst. Evol. 258: 1-15.
Xu F-X, Chen D-Q, Specht C. 2013. Comparative microsporogenesis and anther development of selected species from Magnoliaceae. – Nord. J. Bot. 31: 291-300.
Xue B, Saunders RMK. 2013. Reassessing morphological homologies in the early-divergent angiosperm Fenerivia (Annonaceae) based on floral vascular anatomy: significance for interpreting putative homeotic mutations. – PLoS ONE 8: e81925.
Xue B, Su YCF, Mols JB, Keßler PJA, Saunders RMK. 2011. Further fragmentation of the polyphyletic genus Polyalthia (Annonaceae): molecular phylogenetic support for a broader delimitation of Marsypopetalum. – Syst. Biodiv. 9: 17-26.
Xue B, Su YCF, Thomas DC, Saunders RMK. 2012. Pruning the polyphyletic genus Polyalthia (Annonaceae) and resurrecting the genus Monoon. – Taxon 61: 1021-1039.
Xue B, Thomas DC, Chaowasku T, Johnson DM,
Saunders RMK. 2014. Molecular phylogenetic support for the taxonomic merger of
Fitzalania and Meiogyne (Annonaceae): new nomenclatural
combinations under the conserved name Meiogyne. – Syst. Bot. 39:
396-404.
Xue B, Tan Y-H, Thomas DC, Chaowasku T, Hou X-L, Saunders RMK. 2018. A new Annonaceae genus, Wuodendron, provides support for a post-boreotropical origin of the Asian-Neotropical disjunction in the tribe Miliuseae. – Taxon 67: 250-266.
Yamada T, Imaichi R, Kato M. 2003. The outer integument and funicular outgrowth complex in the ovule of Magnolia grandiflora (Magnoliaceae). – J. Plant Res. 116: 189-198.
Yi Q-F, Zhou R-Z, Zeng Q-W, Xing F-W. 2009. Michelia viridipetala sp. nov. (Magnoliaceae) from Yunnan, China. – Nord. J. Bot. 26: 338-343.
Young DA. 1983. Leaf flavonoids of Eupomatiaceae. – Biochem. Syst. Ecol. 11: 209-210.
Young DA, Sterner RW. 1981. Leaf flavonoids of primitive dicotyledonous angiosperms: Degeneria vitiensis and Idiospermum australense. – Biochem. Syst. Ecol. 9: 185-187.
Zagórska-Marek B. 1994. Phyllotaxic diversity in Magnolia flowers. – Acta Soc. Bot. Pol. 63: 117-137.
Zhang R-J, Zhou R-Z, Xing F-W, Chen H-F. 2006. A new species of Magnolia sect. Tulipastrum (Magnoliaceae) from Fujian, China. – Bot. J. Linn. Soc. 151: 289-292.
Zhou L, Su YCF, Saunders RMK. 2009. Molecular phylogenetic support for a broader delimitation of Uvaria (Annonaceae), inclusive of Anomianthus, Cyathostemma, Ellipeia, Ellipeiopsis and Rauwenhoffia. – Syst. Biodivers. 7: 249-258.
Zhou L, Su YCF, Chalermglin P, Saunders RMK. 2010. Molecular phylogenetics of Uvaria (Annonaceae): relationships with Balonga, Dasoclema and Australian species of Melodorum. – Bot. J. Linn. Soc. 163: 33-43.
Zhou L, Su YCF, Thomas DC, Saunders RMK. 2012. ‘Out-of-Africa’ dispersal of tropical floras during the Miocene climatic optimum: evidence from Uvaria (Annonaceae). – J. Biogeogr. 39: 322-335.
Zollinger H. 1858. Über die Annonaceen des ostindischen Archipels. – Linnaea 29: 297-325.
Zuilen CM van. 19976. Patterns and affinities in the Duguetia alliance (Annonaceae): molecular and morphological studies. – PhD diss., Utrecht University, Utrecht, Netherlands.