TRICOLPATAE M. J. Donoghue, J. A.
Doyle et P. D. Cantino
Donoghue, Doyle et Cantino in Taxon 56: E26. Aug
2007
[Ranunculales+[Sabiaceae+Proteales+[Trochodendrales+[Didymelales+Gunneridae]]]]
RANUNCULALES Juss. ex Bercht. et
J. Presl
Berchtold et Presl, Přir. Rostlin: 215. Jan-Apr
1820 [‘Ranunculaceae’]
Berberidopsida
Brongn., Enum. Plant. Mus. Paris: xxv, 94. 12 Aug 1843
[’Berberineae’]; Ranunculopsida Brongn.,
Enum. Plant. Mus. Paris: xxvi, 96. 12 Aug 1843 [’Ranunculineae’];
Ranunculanae Takht. ex Reveal in Novon 2: 236. 13 Oct
1992; Ranunculidae Takht. ex Reveal in Novon 2: 235.
13 Oct 1992; Berberidanae Doweld, Tent. Syst. Plant.
Vasc.: xxv, xxvi. 23 Dec 2001; Papaveranae Doweld,
Tent. Syst. Plant. Vasc.: xxv. 23 Dec 2001
Fossils Teixeiraea
lusitanica has been assigned to Ranunculales (and possibly to
Menispermaceae). It
is a fossilized male flower from the Late Aptian to the Early Albian of
Portugal. The spirally arranged floral parts are free, the outermost ones being
bracteate and grading into tepaloid parts. The 20 stamens have basifixed
anthers and the tectate-perforate pollen grains have a columellate
infratectum.
Habit Usually bisexual
(sometimes monoecious or dioecious, rarely polygamomonoecious), shrubs, lianas,
suffrutices, perennial, biennial or annual herbs (rarely trees).
Vegetative anatomy Phellogen
ab initio superficially or deeply seated, or absent. Primary vascular tissue
consisting of one or several cylinders of vascular bundles, or of scattered
bundles. Secondary lateral growth normal, from cylindrical cambium, anomalous,
from normal successive cambia, or absent. Fusiform cambial initials storied
(not in Glaucidiaceae). Vessels
confined to central portions of fascicular areas (vessel restriction patterns,
with very few or no vessels touching rays). Vessel elements with simple or
scalariform (sometimes reticulate) perforation plates; lateral pits alternate,
opposite or scalariform, bordered pits. Imperforate tracheary xylem elements
fibre tracheids or libriform fibres (sometimes tracheids) with simple or
bordered pits, septate or non-septate (often also vasicentric tracheids). Wood
rays usually multiseriate (sometimes uniseriate), homocellular or
heterocellular, or absent. Axial parenchyma apotracheal, diffuse or
diffuse-in-aggregates or banded, or paratracheal, intervascular or scanty
vasicentric, banded, or absent. Sieve tube plastids S or Ss type. Nuclei
sometimes with dispersive P-protein. Nodes usually ≥3:≥3, trilacunar or
multilacunar with three or more leaf traces (rarely 1:1, 1:4 or 1:5–11,
unilacunar with one to many traces, or 2:2, bilacunar with two traces). Latex
cells and laticifers with white or coloured latex sometimes present. Idioblasts
with raphides sometimes present. Prismatic or rhomboidal calciumoxalate
crystals often frequent; crystal sand sometimes present. Calcium oxalate
crystals usually present in wood rays (not in Glaucidiaceae and Ranunculaceae).
Trichomes Hairs unicellular
och multicellular, usually uniseriate (sometimes multiseriate, rarely
branched), or absent; glandular hairs often present.
LeavesUsually alternate
(spiral or distichous; sometimes opposite), simple or compound, entire or
pinnately or palmately lobed, with supervolute, involute, conduplicate,
plicate, subplicate, reclinate, curved or equitant ptyxis. Stipules
intrapetiolar or absent; petiole base sometimes sheathing. Petiole vascular
bundle transection arcuate or annular. Venation pinnate or palmate,
actinodromous or acrodromous (rarely parallelodromous or flabellate). Stomata
usually anomocytic (sometimes paracytic, staurocytic or cyclocytic) or absent.
Cuticular wax crystalloids usually as irregularly shaped platelets or clustered
tubuli (Berberistype), dominated by nonacosan-10-ol (without
triterpenoids), or absent. Mesophyll sometimes with sclerenchymatous idioblasts
and mucilage cells. Idioblasts with ethereal oils absent. Leaf margin serrate,
serrate-dentate, spinose-serrate or glandular-serrate, with chloranthoid or
platanoid teeth, crenate or entire.
Inflorescence Terminal or
axillary, simple or compound, cymose, raceme-, head- or umbel-like, fascicle,
panicle, thyrsoid, spike, raceme, or flowers solitary. Floral prophylls
(bracteoles) single or pairwise (sometimes absent).
Flowers Usually actinomorphic
(sometimes zygomorphic or bisymmetric). Hypogyny. Perianth sometimes trimerous.
Tepals (three or) four to 15, spiral or whorled (rarely absent). Outer tepals
two or more, with imbricate or valvate (rarely open) aestivation, sepaloid or
petaloid, persistent or caducous, usually free. Inner tepals (staminodial in
origin?) one to 13, with imbricate or valvate (sometimes crumpled), free, often
with one or several nectaries at base (nectary sometimes absent). Disc
absent.
Androecium Stamens (one to)
three to more than 500, spiral or whorled. Filaments free or more or less
connate, free from tepals. Anthers basifixed to somewhat dorsifixed, usually
non-versatile, usually tetrasporangiate (rarely disporangiate), extrorse,
latrorse or introrse, usually longicidal (dehiscing by longitudinal slits) or
valvate (dehiscing by longitudinal valves). Tapetum usually secretory (rarely
amoeboid-periplasmodial). Staminodia extrastaminal, petaloid and/or
nectariferous, or absent; female flowers sometimes with staminodia.
Pollen grains
Microsporogenesis usually simultaneous (rarely successive). Pollen grains di-
or tricolpate to polycolpate or -porate, di- or tricolporate, zono- or
pantoaperturate (sometimes syncolpate, rarely spiraperturate, clypeate or
inaperturate), usually shed as monads (rarely dyads or tetrads), usually
bicellular (sometimes tricellular) at dispersal. Exine tectate or semitectate,
with columellate infratectum, perforate, microreticulate, reticulate or
punctate, foveolate, striate, scabrate, spinulate, echinate, microechinate,
verrucate or psilate.
Gynoecium Carpels one
(monocarpellate or pseudomonomerous) to more than 100, spiral or whorled,
usually free (sometimes more or less connate), or pistil composed of one to
numerous carpels; carpel ascidiate, conduplicate or plicate, postgenitally
usually entirely occluded, without canal. Ovary superior, unilocular (apocarpy
or gynoecium monomerous) or trilocular to quinquelocular. Stylodia or style
single, simple, or absent. Stigma of various types, lobate to capitate
(sometimes decurrent), papillate or non-papillate, Dry or Wet type. Male
flowers sometimes with pistillodium.
Ovules Placentation marginal
or basal (when ovule single, or when ovary unilocular), laminal-lateral or
marginal-lateral (when ovules several), or axile (when ovary plurilocular).
Ovules one to numerous per carpel, usually anatropous (sometimes
hemianatropous, rarely amphitropous or campylotropous), ascending, horizontal
or pendulous, usually apotropous (rarely epitropous), usually bitegmic
(sometimes unitegmic), usually crassinucellar (sometimes pseudocrassinucellar).
Micropyle endostomal or bistomal, sometimes Z-shaped (zig-zag). Archespore
unicellular to at least 15-celled. Nucellar cap usually present.
Megagametophyte usually monosporous, Polygonum type (rarely disporous,
Allium type, or tetrasporous, Adoxa type,
Fritillaria type or Peperomia type). Synergids sometimes with
a filiform apparatus. Antipodal cells usually persistent, proliferating or
non-proliferating. Endosperm development ab initio usually nuclear (rarely
cellular). Endosperm haustoria chalazal or absent. Embryogenesis usually
onagrad (sometimes solanad, rarely caryophyllad or chenopodiad).
Fruit An assemblage of
follicles or achenes, a berry, or a loculicidal, septicidal or poricidal
capsule with basipetalous or acropetalous dehiscence (sometimes a
multifolliculus or a nut, rarely a lomentum with nut-like mericarps).
Seeds Aril usually absent.
Seed coat usually exotestal (rarely endotegmic). Testa often multiplicative
(sometimes thin). Exotesta palisade, with often thickened non-lignified cell
walls, or seeds more or less pachychalazal, with thin testa. Mesotesta,
endotesta and tegmen usually unspecialized. Exotegmen sometimes multiplicative.
Perisperm not developed. Endosperm scarce to copious (rarely absent), oily,
proteinaceous or starchy. Embryo small to large, undifferentiated to well
differentiated at dispersal, without chlorophyll. Cotyledons (one or) two.
Germination phanerocotylar or cryptocotylar.
Cytology n = 6–9, 11–13,
15, 19, 21
DNA Nuclear gene AP3
present and usually triplicated.
Phytochemistry Flavonols
(kaempferol, quercetin, myricetin, rhamnocitrin; frequently additional
oxygenation at carbons 6 or 8 of ring A in contrast to magnoliid clades),
O-methylated flavonoids, flavones, isoprenylated flavonoids, cyanidin,
delphinidin, dihydrochalcones, diterpenoids, tannins, cardioactive
bufadienolides, Digitalis cardenolides, poisonous sesquiterpene
lactones, caffeic acid, benzylisoquinoline and aporphine alkaloids
(benzyltetrahydroisoquinoline and aporphine derivatives in dimeric form,
bulbocapnine and other aporphines, morphinanes, pavines, isopavines,
dehydrogenated benzophene anthridines, reduced benzophene anthridines,
tyrosine-derived berberine, tetrahydroberberines, berberidine, protopines,
rhoeadines, narceines, spirobenzyl isoquinolines, hydrastine, protopine,
quaternary magnoflorine and its precursor corytuberine), hasubanane alkaloids
(protostephanines, erythrinanes, cocculolidines, morphines, quettamine-morphine
dimers, hasubanonines, acutumines, etc.), azafluoranthene alkaloids,
tropoloisoquinoline alkaloids, protoberberine alkaloids, diterpene alkaloids
(aconitine, methyl lycaconitine etc.), quinolizidine alkaloids (e.g.
darvasamine), pyrrolizidine alkaloids as macrocyclic diesters, tyrosine-derived
cyanogenic glycosides, lignan-β-glycosides, ranunculins (glucosides),
triterpene saponins, steroidal saponins, meconic acid, chelidonic acid, fumaric
acid, nitrophenyl ethan, phenylic cinnamide, furofuran lignans, mannitol, and
glaupalol (a furanocoumarin) present. Ethereal oils, prodelphinidin, and
flavonoids and tannins containing a trihydroxylated B-ring (gallic and ellagic
acid derivatives) not found.
Systematics Ranunculales are sister-group
to the remaining Tricolpatae.
Euptelea is sister to all other Ranunculales, according to
most molecular analyses. The majority of Ranunculales are characterized
by wide and tall multiseriate wood rays little altered during ontogeny, and
relatively intact extensions of primary rays. These contrast with multiseriate
rays dominating in other Tricolpatae, in which the large primary rays
are rapidly broken into smaller segments during stem and root growth. In
general, multiseriate rays in Ranunculales are composed of
procumbent cells with the exception of one or two layers of upright sheathing
cells.
The clade [[[Lardizabalaceae+Circaeasteraceae]+[Menispermaceae+[Berberidaceae+ [Hydrastidaceae+Glaucidiaceae]+Ranunculaceae]]]+[Papaveraceae s.lat.]] is
characterized by the following potential synapomorphies (Stevens 2001 onwards):
vessel elements with simple perforation plates, present in diagonal groups;
presence of nucleated libriform fibres and vasicentric tracheids; leaves with
palmate secondary venation; and Wet type stigma. Herbaceous growth and woody
habit have evolved several times in the two main clades above
Euptelea. Likewise, petaloid tepals have originated several times from
staminodial stamens (e.g. in Berberidaceae, Lardizabalaceae, Ranunculaceae; Drinnan &
al. 1994, etc.). In Ranunculaceae, the petaloid
tepals have a single vascular strand, share parastichs with the stamens, are
similar to stamens in their early development, and are often peltate.
Pteridophyllum and Papaveraceae have among others
the following potential synapomorphies in common (Stevens 2001 onwards):
herbaceous growth; roots diarch, i.e. lateral roots tetrastichous; perianth
differentiated into outer sepaloid and inner petaloid tepal whorls; sepaloid
tepals two, median; petaloid tepals four; carpels at least two collateral,
occluded by secretion; placentation parietal; capsule septicidal, with
persistent woody placenta; and endotesta well developed.
The second main clade has the topology [[Lardizabalaceae+Circaeasteraceae]+ [Menispermaceae+[Berberidaceae+Ranunculaceae]]] and the
potential synapomorphies: wide wood rays; trimerous flowers; outer and inner
tepals and stamens opposite each other; outer tepals with three or more
vascular traces; and triplication of nuclear gene
AP3. Both Lardizabalaceae and Circaeasteraceae have
extrorse anthers and cellular endosperm development.
The clade [Menispermaceae+[Berberidaceae+[[Hydrastis+Glaucidium]+Ranunculaceae]]] has the
benzylisoquinoline berberine and nuclear endosperm development. According to
Stevens (2001 onwards), the clade [Berberidaceae+[[Hydrastis+Glaucidium]+Ranunculaceae]] has the
potential synapomorphies: usually herbs with non-tuberous rhizome; bright
yellow roots and rhizome due to presence of berberine; roots diarch; lateral
roots tetrastichous; nodes often multilacunar; vascular bundles V-shaped, in
herbaceous species often closed, scattered or arranged in concentric cylinders;
broad leaf base; presence of petaloid staminodial nectaries; outer integument
at least four cell layers thick; presence of nucellar cap; endosperm with
reserves other than oils or proteins; and loss of mitochondrial intron
coxII.i3. Hydrastis, Glaucidium and Ranunculaceae share the
synapomorphies: uniseriate perianth; and numerous spiral stamens and carpels.
Hydrastis and Glaucidium have vessel elements with simple and
scalariform perforation plates, medullary vascular bundles, flattened vascular
bundles; petiole vascular bundles annular in cross-section; medullary petiole
vascular bundles; no palisade mesophyll; distichous leaves; flowers solitary
and terminal; no nectaries; bilobate stigma; outer integument four to 13 and
inner integument two to five cell layers thick; unmodified antipodal cells; and
also abaxial dehiscence of follicle.

|
Cladogram of Ranunculales based on
DNA sequence data (Soltis & al. 2011). Euptelea is usually
sister to the remainder with high bootstrap support and, likewise, the
clade [Papaveraceae+Pteridophyllum]
is sister to the remaining Ranunculales, often
with a bootstrap support of 100%. The other clades show high support
varying between 75% and 100%. Circaeasteraceae
are sister to Lardizabalaceae
with a bootstrap support of 78%. According to Wang & al. (2009),
Pteridophyllum (Pteridophyllaceae)
is nested inside Papaveraceae as sister
to Hypecoum. Until this hypothesis has been further confirmed,
Pteridophyllum is treated as sister to Papaveraceae,
according to, e.g., Kadereit & al. (1994, 1995).
|
de Jussieu, Gen. Plant.: 286. 4 Aug 1789
[’Berberides’], nom. cons.
Podophyllaceae DC.,
Syst. Nat. 1: 126. 1-15 Nov 1817 [’Podophylleae’], nom. cons.;
Berberidales Bercht. et J. Presl, Přir. Rostlin:
226. Jan-Apr 1820 [‘Berberideae’];
Leonticales Bercht. et J. Presl, Přir. Rostlin: 217.
Jan-Apr 1820 [‘Leonticinae’];
Podophyllales Dumort., Anal. Fam. Plant.: 45. 1829
[’Podophyllarieae’]; Diphylleiaceae
Schultz Sch., Nat. Syst. Pflanzenr.: 328. 30 Jan-10 Feb 1832;
Nandinaceae Horan., Prim. Lin. Syst. Nat.: 90. 2 Nov
1834 [’Nandinaceae (Berberideae)’];
Epimediaceae Menge, Cat. Plant. Grudent. Gedan.: 122.
1839 [’Epimedineae’]; Leonticaceae Airy
Shaw in Kew Bull. 18:263. 8 Dec 1965; Ranzaniaceae
(Kumaz. et Terab.) Takht. in Bot. Žurn. 79(1): 96. Jan 1994;
Nandinales Doweld in Byull. Mosk. Obshch. Ispyt.
Prir., Biol. 105(5): 60. 9 Oct 2000
Genera/species
12–14/>600
Distribution Eurasia from
Western Europe to Malesia, North Africa, East African mountains, North and
Central America, mountains in South America.
Fossils Fossil representatives
of Berberis are known from the Oligocene onwards in the Northern
Hemisphere.
Habit Bisexual, evergreen or
deciduous shrubs or biennial to perennial rhizomatous or tuberous herbs (rarely
trees). Roots and rhizome often bright yellow inside due to presence of
berberine.
Vegetative anatomy Phellogen
ab initio superficially or deeply sited (pericyclic). Primary medullary rays
wide in Nandina. Primary vascular tissue consisting of one or more
cylinders of bundles or scattered bundles. Secondary lateral growth absent or
from normal cylindrical cambium. Xylem V-shaped. Vessel elements with usually
simple (in Berberis sometimes also scalariform) perforation plates;
lateral pits usually opposite or alternate (in Vancouveria scalariform
or pseudoscalariform), bordered pits. Imperforate tracheary xylem elements
usually libriform fibres (in Nandina also fibre tracheids; in
Jeffersonia tracheids) with usually simple pits (in Nandina
bordered pits also), septate or non-septate (also vasicentric tracheids). Wood
rays multiseriate, homocellular or heterocellular. Axial parenchyma usually
absent (rarely vasicentric scanty). Wood elements partially storied. Sieve tube
elements Ss type. Nodes ≥3:≥3, trilacunar or multilacunar with three or
more leaf traces. Secondary tissue often yellow due to presence of berberine.
Prismatic calciumoxalate crystals often abundant. Wood rays often with
rhomboidal crystals.
Trichomes Hairs unicellular or
multicellular, uniseriate, or absent.
Leaves Alternate (sometimes in
a basal rosette), simple or compound (pinnately or palmately compound; in
Nandina and sometimes Leontice bipinnate; in
Berberis trifoliolate or unifoliolate), entire or lobate, with
conduplicate, reclinate, equitant or curved ptyxis; leaves of long shoots in
Berberis usually modified into spines. Stipules rudimentary,
intrapetiolar and caducous, or absent; leaf sheath absent. Petiole vascular
bundle transection annular or arcuate. Venation pinnate or palmate. Stomata
anomocytic. Cuticular wax crystalloids usually as clustered tubuli
(Berberis type), chemically dominated by nonacosan-10-ol (in
‘Podophyllum’ as solid rodlets sometimes aggregated to larger
units). Idioblasts with ethereal oils absent. Leaf margin serrate (sometimes
serrate-dentate or spinose-serrate), with chloranthoid teeth, or entire.
Inflorescence Terminal or
axillary, spike, raceme or fasciculate etc. (in Nandina panicle), or
flowers solitary.
Flowers Actinomorphic.
Hypogyny. Tepals (eight to) twelve (to 15) (3+3+3, 3+3, 2+2+2, or 2+2; in
Epimedium 4+4+4 or 4+5+5; absent in Achlys) in three to six
(rarely seven) series (in Nandina slightly spiral), with imbricate
aestivation, free, all tepals in Nandina sepaloid; outer tepals three
to nine (in Nandina c. 20 to c. 50), in two series, sepaloid or
petaloid (or bracts?), caducous; inner tepals six to twelve, in two to four
series, outer series petaloid sepals?, inner two or three series petaloid
staminodia? with nectaries (in Nandina nectar secreting staminodia;
absent inAchlys, Diphylleia and ‘Podophyllum’).
Four inner tepals in Epimedium with four nectariferous spurs. Disc
absent.
Androecium Stamens (three to)
six (to 19), usually as many (sometimes twice as many) as inner petals (in
Achlys seven to 15; in Epimedium 2+2), in one whorl,
alternisepalous, antepetalous, or in two or more whorls. Filaments free from
each other, free? from or adnate? at base to tepals. Anthers basifixed,
non-versatile, tetrasporangiate, usually extrorse (often with valves directed
ab initio backwards, later usually inverted and turned up-side-down, with
pollen grains against floral centre; in Dysosma and Nandina
introrse), usually valvicidal (dehiscing by valves; in Dysosma,
Nandina and ‘Podophyllum’ longicidal, dehiscing by
longitudinal slits); connective sometimes slightly prolonged. Tapetum secretory
or amoeboid-periplasmodial, with multinucleate cells. Staminodia three to nine,
petaloid, extrastaminal (or absent?).
Pollen grains
Microsporogenesis usually simultaneous (in Berberis and
Ranzania? at least sometimes successive). Pollen grains usually
tricolpate (rarely hexa- to dodecacolpate or spiraperturate; in
Berberis sometimes irregularly polysyncolpate, spiraperturate or
clypeate [with surface divided into platelets]; in Ranzania
hexacolpate), usually shed as monads (in Ranzania etc. dyads; in some
species of ‘Podophyllum’ tetrads), bicellular at dispersal. Exine
tectate or semitectate, with columellate infratectum, usually perforate,
microreticulate, striate-reticulate, psilate-punctate, punctate-striate,
echinate, gemmate (in Diphylleia spinulate), in Achlys,
Epimedium, Jeffersonia, and Vancouveria striate
(with compound layer of striae).
Gynoecium Pistil composed of
(seemingly?) one carpel (pseudomonomerous gynoecium developed from two or three
connate carpels?); carpel secondarily ascidiate (primarily plicate, rarely
ascoplicate), seemingly occluded postgenitally by secretion; closure of carpels
sometimes delayed. Ovary superior, unilocular. Style short or absent. Stigma
wide, often trilobate (rarely quadrilobate), papillate or non-papillate, Dry or
Wet type. Carpellary walls in Caulophyllum etc. not enclosing ripening
blue seeds. Pistillodium absent.
Ovules Placentation basal
(when single ovule) or marginal-lateral (when several ovules). Ovules one to
numerous per ovary (in Achlys one ovule; in Nandina two
ovules, one of which degenerating), usually anatropous (sometimes
hemianatropous or campylotropous), ascending, horizontal or pendulous,
bitegmic, crassinucellar to pseudocrassinucellar. Micropyle bistomal, often
Z-type (zig-zag). Outer integument five to eleven cell layers thick. Inner
integument two to five cell layers thick. Parietal tissue one or two cell
layers thick or absent. Nucellar cap six to eight cell layers thick, formed by
periclinal divisions from apical cells of megasporangial epidermis.
Megagametophyte usually monosporous, Polygonum type (in
Caulophyllum at least sometimes tetrasporous, Peperomia
type). Synergids sometimes with a filiform apparatus. Antipodal cells
persistent, endopolyploid, with large nuclei, non-proliferating. Endosperm
development ab initio usually nuclear (sometimes cellular). Endosperm haustoria
usually chalazal (absent in some genera, e.g. in ‘Podophyllum’).
Embryogenesis onagrad (or variations of this type).
Fruit Usually a berry or a
follicle (in Achlys a nut; in Jeffersonia a capsule; in
Gymnospermium a papery not completely closed envelope; fruit in
Caulophyllum entirely strongly reduced, with fleshy seeds growing out
through carpellary wall).
Seeds Elaiosome and aril
usually absent (more or less rudimentary aril present in, e.g.,
Nandina, Epimedium, Jeffersonia and certain species
of Berberis). Seed coat usually exotestal (in Nandina
endotegmic). Testa often multiplicative. Exotesta palisade, usually well
developed, in Berberis with thick-walled lignified cuboid cells.
Mesotesta and endotesta unspecialized. Tegmen usually unspecialized (endotegmen
in Nandina sclerotic). Perisperm not developed. Endosperm copious,
oily or with hemicellulose. Embryo very small, straight, rudimentary, without
chlorophyll. Cotyledons two. Germination phanerocotylar or cryptocotylar.
Cytology n = 6
(Podophylloideae); n = 7 (Berberidoideae); n = 8, 10
(Nandinoideae)
DNA Plastid inverted repeat
expanded by 11.5 kb into large single copy region in Berberis. Plastid
gene rps7 lost in Podophyllum. Mitochondrial intron
coxII.i3 lost. Triplication of nuclear gene
AP3.
Phytochemistry Flavonols
(kaempferol, quercetin, myricetin), isoprenylated flavonoids, cyanidin,
delphinidin, tannin, frequent caffeic acid, benzylisoquinoline alkaloids
(berberine, magnoflorine, protopine etc.), aporphine alkaloids, quinolizidine
alkaloids (in Caulophyllum, Leontice and
Gymnospermium; in Gymnospermium also darvasamine), cyanogenic
compounds (in Nandina tyrosine-derived), and saponins present.
Lignan-β-glycosides and similar compounds accumulated in Epimedium,
‘Podophyllum’ and Diphylleia (Dysosma?,
Vancouveria?). Ellagic acid not found.
Use Ornamental plants, fruits
(Berberis), medicinal plants, dyeing (yellow) substances.
Systematics Berberidaceae are
sister-group to the clade [Ranunculaceae+[Hydrastis+Glaucidium]].
Nandina was
sister to the remaining Berberidaceae in several
molecular analyses (e.g. Kim & Jansen 1998). However, Wang & al. (2007)
identified the Podophylloideae clade as sister-group to
Berberidoideae in a strict sense, in which Nandina were
nested in a clade sister to [Berberis+Ranzania].
The subdivision below follows the latter alternative, although the support for
this topology is at most moderate.
Podophylloideae Eaton,
Bot. Dict., ed. 4: 38. Apr-Mai 1836 [‘Podophylleae’]
6–8/c 90. Epimedium
(55–60; the Mediterranean and North Africa to southwestern Asia, western
Himalayas, northeastern Asia, Japan), Jeffersonia
(2; J. dubia: Manchuria, northern China, the Korean Peninsula; J.
diphylla: eastern United States), Achlys (2–3;
A. californica, A. triphylla: southwestern Canada, western
United States; A. japonica: Japan), Bongardia (1; B.
chrysogonum; Greece to Afghanistan), ‘Podophyllum’
(14; the Himalayas to East Asia, southeastern Canada, eastern United States;
paraphyletic; incl. Diphylleia
and Dysosma?), Diphylleia
(3; D. sinensis: western China, D. grayi: central and
northern Japan, Sakhalin; D. cymosa: eastern United States; in Podophyllum?),
Dysosma (10–12; Tibet, China, Vietnam; in Podophyllum?),
Vancouveria
(3; V. chrysantha, V. hexandra, V. planipetala;
western United States). – Temperate regions on the Northern Hemisphere.
Lowermost inflorescence branch arising from axil of reduced leaf. Outer tepals
four to 18 (absent in Achlys). Inner
tepals four to nine (absent in Achlys), with
or without nectariferous spurs. Stamens in Achlys seven to
15, in ‘Podophyllum’
up to 19. Anthers longicidal (dehiscing by longitudinal slits).
Microsporogenesis successive. Pollen grains sometimes shed as dyads or tetrads.
Pollen wall development by centripetal furrowing. Exine usually striate
(sometimes spinulate). Gynoecium arising from two carpellary primordia. Ovules
one to numerous per carpel. Micropyle sometimes bistomal. Integuments lobate.
Inner integument two or three cell layers thick. Megasporocytes in Diphylleia
several. Parietal tissue one or two cell layers thick (sometimes absent). Fruit
an achene, a berry or a follicle (also with transverse dehiscence). Aril
rudimentary or absent. Testa multiplicative. n = 6. Lignan-β-glycosides and
similar compounds often accumulated. – Podophylloideae may be
sister-group to [Nandinoideae+Berberidoideae].
[Nandinoideae+Berberidoideae]
Tepals with a single trace. Gynoecium
arising from two or three carpellary primordia. Stigma usually Wet type.
Nandinoideae Heintze,
Cormofyt. Fylog.: 101. 1 Jun 1927
4/15. Nandina (1;
Nandina domestica; central China?); Caulophyllum
(3; C. robustum: northeastern Asia; C. giganteum, C.
thalictroides: southeastern Canada, eastern United States),
Gymnospermium (12; southern Balkan Peninsula, Iran, Central Asia,
China, the Korean Peninsula), Leontice (3; L. leontopetalum:
southeastern Europe to North Africa; L. armeniacum: Turkey to Iran;
L. incerta: Central Asia). – East Europe to East Asia. Primary
medullary rays wide. In Nandina also
fibre tracheids present. Petiole concave at base. Inflorescence paniculate,
with lowermost branch arising from axil of expanded leaf. Tepals sepaloid,
usually with three traces. Outer tepals in Nandina c.
20–50, spiral. Inner tepals (’petals’) six. Nectary in Nandina
absent. Petaloid staminodium and stamen developing from single common
primordium. Anthers in Nandina
longicidal (dehiscing by longitudinal slits). Pollen grains in Nandina with
massive endexine. Ovules one or two (to four) per carpel, (in Nandina two,
one of which degenerating). Megasporangium in Nandina early
absorbed. Fruit a berry or with evanescent or bladder-like pericarp. Funicle
often swollen. Seed coat in Nandina
endotegmic, with thin-walled testal cells. Exotesta and mesotesta crushed (Nandina).
Endotestal cells in Nandina
crystalliferous. Endotegmic cells in Nandina
enlarged, lignified, thickened particularly on inner side, crystalliferous.
Embryo minute. n = 8, 10. Protopine (a benzylisoquinoline alkaloid) and in Nandina
tyrosine-derived cyanogenic compounds present.
Berberidoideae Eaton,
Bot. Dict., ed. 4: 41. Apr-Mai 1836
2/>500. Berberis
(>500; Europe, the Mediterranean, the Atlas Mountains, East African
mountains, temperate Asia, North to South America), Ranzania (1;
R. japonica; Japan). – Temperate and mountainous regions, North
Africa, South America, with their highest diversity in East Asia and eastern
North America. Usually shrubs. Phellogen also pericyclic. Vessel elements
usually with simple (in Berberis
sometimes scalariform) perforation plates. Leaves imparipinnate or
unifoliolate, with conduplicate to curved ptyxis. Stipules numerous. Petiole
vascular bundles in Berberis
arcuate. Venation sometimes pinnate. Leaf margin usually glandular- or
spinose-dentate (sometimes entire). Lowermost inflorescence bransch arising
from axil of reduced leaf. Outer tepals three to twelve. Nectar often secreted
from paired nectaries at base of four or six inner petaloid staminodia. Stamens
(four to) six (to more than 20). Anthers usually dehiscing by valves (rarely
slits), often sensitive. Tapetum amoeboid-periplasmodial. Pollen grains
6–12-colpate or spiraperturate etc. Exine striate-reticulate, often
undifferentiated. Placentation basal-lateral; placentae sometimes
protruding-diffuse and vascularized. Ovules one to numerous per carpel.
Megasporocytes sometimes several. Parietal tissue approx. two cell layers
thick. Endosperm development nuclear or cellular. Fruit a berry. Embryo
elongate, with relatively long radicula. n = 7.
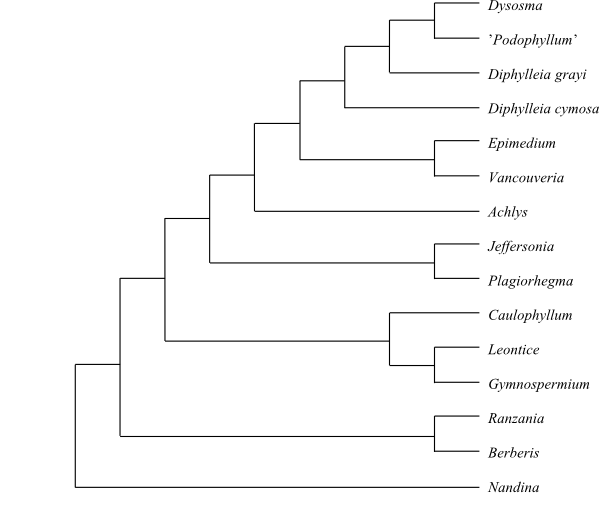 |
Cladogram of Berberidaceae based
on DNA sequence data (Kim & Jansen 1998).
|
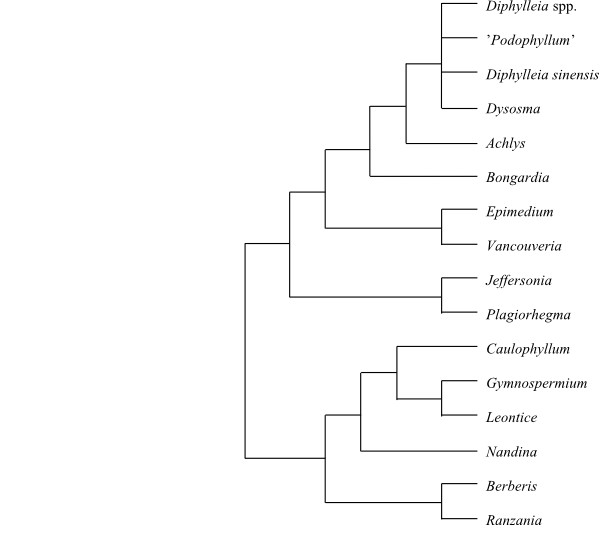 |
Cladogram of Berberidaceae based
on DNA sequence data (Wang & al. 2007)
|
Hutchinson, Fam. Fl. Pl. 1: 98. 15 Jan 1926, nom.
cons.
Kingdoniaceae A. S.
Foster ex Airy Shaw in Kew Bull. 18: 262. 8 Dec 1965;
Circaeasterales Takht., Divers. Classif. Fl. Pl.: 97.
24 Apr 1997
Genera/species 2/2
Distribution The Himalayas,
southwestern, western and northwestern China.
Fossils Unknown.
Habit Bisexual, annual or
perennial (Kingdonia) or annual (Circaeaster) herbs.
Vegetative anatomy Mycorrhiza
endotrophic (Kingdonia). Phellogen absent. Vessel elements usually
with simple (in Kingdonia sometimes scalariform) perforation plates,
bordered pits absent? Imperforate tracheary xylem elements?, non-septate? Wood
rays absent. Axial parenchyma absent? Sieve tube plastids? Nodes 1:1
(Circaeaster) or 1:1–4 (Kingdonia), unilacunar with one or
four leaf traces, respectively. Crystals?
Trichomes Hairs unicellular or
multicellular, uniseriate, uncinate (on surface of achenes in
Circaeaster), or absent.
Leaves Alternate (in
Circaeaster in basal rosette; in Kingdonia usually only a
single basal leaf, sometimes distichous), simple, entire or palmately lobed,
with ? ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle
transection arcuate? Venation flabellate (open-dichotomously branched), with
branches running into leaf teeth. Stomata anomocytic. Cuticular wax
crystalloids as clustered tubuli of Berberis type, dominated by
nonacosan-10-ol (Circaeaster). Leaf margin serrate.
Inflorescence Flowers
inCircaeaster in terminal, compound thyrsoid inflorescences; flowers
in Kingdonia terminal, solitary, long-pedunculate (scapose). Floral
prophyll (bracteole) in Kingdonia adaxial, in Circaeaster
absent.
FlowersAlmost actinomorphic,
small. Hypogyny. Tepals with valvate aestivation, spiral, persistent, in
Circaeastertwo or three scale-like sepaloid, in Kingdonia
(four or) five to seven petaloid. Kingdoniawith eight to 13
intratepalous clavate glistening glands (nectar-secreting staminodia?). Nectary
absent in Circaeaster. Disc absent.
Androecium Stamens
inCircaeaster usually two (sometimes one or three), alternitepalous,
in Kingdonia three to eight. Filaments free from each other and from
tepals. Anthers in Circaeaster basifixed, non-versatile,
disporangiate, introrse to latrorse, in Kingdonia tetrasporangiate,
extrorse, valvicidal (dehiscing by longitudinal valves). Tapetum secretory.
Staminodia in Circaeaster one sepaloid or absent, in
Kingdonia eight to 13 extrastaminal, apically nectariferous and
petaloid.
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually tricolpate (sometimes
tricolporate?), shed as monads, bicellular at dispersal. Exine (tectate to)
semitectate, with columellate infratectum, striate-reticulate (with compound
layer of fine striae).
Gynoecium Carpels usually two
(sometimes one or three; Circaeaster) or three to nine
(Kingdonia), short- to long-stalked, free; carpel ascidiate, occluded
by secretion? Ovary superior, unilocular (monomerous or apocarpous). Stylodia
short (Circaeaster) or subulate and recurved (Kingdonia).
Stigma somewhat oblique, papillate (Circaeaster), Wet? type.
Pistillodium absent.
Ovules Placentation
subapical-marginal. Ovules two submarginal (Circaeaster, upper one
degenerating) or one (Kingdonia) per carpel, orthotropous
(Circaeaster) or hemianatropous (Kingdonia), pendulous,
unitegmic, incompletely tenuinucellar, with meiocyte hypodermal at apex of
megasporangium. Integument in Circaeaster approx. two cell layers
thick (degenerating following fertilization), in Kingdonia two to five
cell layers thick. Megagametophyte in Kingdonia monosporous,
Polygonum type, in Circaeaster tetrasporous, Adoxa
type (tetranucleate or octanucleate). Endosperm formation at least in
Circaeaster cellular (in Kingdonia helobial?). Endosperm
haustorium chalazal (Circaeaster). Embryogenesis chenopodiad.
Fruit An achene or assemblage
of achenes.
Seeds Aril absent. Testa thin
or absent (degenerating; in Circaeaster replaced by inner epidermis of
pericarp and outer layer of endosperm). Perisperm not developed. Endosperm
copious. Embryo small (Circaeaster) or fairly large
(Kingdonia), straight, chlorophyll? Hypocotyl in Circaeaster
strongly elongated. Cotyledons two, linear, persistent (Circaeaster).
Germination phanerocotylar (Circaeaster). Leaves in
Circaeaster at apex of elongated hypocotyl.
Cytology n = 9
(Kingdonia), n = 15 (Circaeaster)
DNA
Phytochemistry Virtually
unknown. Flavonols? Cyanogenic compounds not found.
Use Unknown.
Systematics Circaeaster
(1; C. agrestis; northwestern Himalayas from Kumaun in India through
Nepal to southeastern Tibet and northwestern Yunnan, and the Kansu and Shensi
mountains in northwestern China), Kingdonia (1; K. uniflora;
western and northwestern China).
Circaeasteraceae are
sister group to Lardizabalaceae.
Wilhelm, Samenpflanzen: 17. Oct 1910, nom.
cons.
Eupteleales Hu ex
Reveal in Phytologia 74: 174. 25 Mar 1993;
Eupteleanae Doweld, Tent. Syst. Plant. Vasc.: xxv. 23
Dec 2001; Eupteleineae Shipunov in A. Shipunov &
J. L. Reveal, Phytotaxa 16: 63. 4 Feb 2011
Genera/species 1/2
Distribution Eastern
Himalayas, Assam, southwestern and central China, Japan.
Fossils Fossilized leaves,
pollen grains and fruits have been found in Paleocene to Miocene layers in
several places in the Northern Hemisphere.
Habit Usually bisexual
(sometimes unisexual male), deciduous trees.
Vegetative anatomy Phellogen
ab initio cortical. Vessel elements with scalariform or partially reticulate
perforation plates; lateral pits usually opposite to intermediary (sometimes
alternate or scalariform), bordered pits. Vessel restriction patterns absent.
Imperforate tracheary xylem elements fibre tracheids with usually bordered
(sometimes simple) pits, non-septate. Wood rays uniseriate or multiseriate,
heterocellular. Axial parenchyma apotracheal diffuse, diffuse-in-aggregates, or
in tangential bands. Multiseriate phloem rays strongly sclerified. Sieve tube
plastids S type, with approx. ten globular starch grains. Nodes 1:5–11,
unilacunar with five to eleven leaf traces. Medulla and petioles with secretory
cells, tanniniferous cells and cells with cluster crystals.
Trichomes Hairs present on
young leaves, uniseriate, caducous, or absent.
Leaves Alternate (spiral),
simple, entire, with subplicate-conduplicate ptyxis. Stipules and leaf sheath
absent. Petiole base hollow; petiole vascular bundles? Venation pinnate,
craspedodromous, with lateral veins ascending and running almost to leaf teeth,
or palmate. Stomata anomocytic. Cuticular wax crystalloids absent or as
clustered tubuli (Berberis type), chemically dominated by
nonacosan-10-ol. Mesophyll without sclerenchymatous idioblasts. Calciumoxalate
druses present. Idioblasts with ethereal oils absent. Leaf margin
glandular-serrate, with platanoid teeth (each gland with an apical cavity).
Inflorescence Axillary, with
flowers one or few together in raceme- or umbel-like fasciculate inflorescence.
Lowermost flowers often with one or two floral prophylls (bracteoles).
Flowers Bisymmetric, small,
bent downwards. Hypogyny? Tepals absent. Nectary absent. Disc absent.
Androecium Stamens six to c.
20, in one whorl. Filaments short or somewhat elongated, filiform, free.
Anthers basifixed, non-versatile, tetrasporangiate, latrorse, valvicidal
(dehiscing by longitudinal valves), horizontally somewhat widened; connective
prolonged. Tapetum secretory, with uninucleate to quadrinucleate cells.
Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains tricolpate or penta- to
heptacolpate (rarely tetracolpate), shed as monads, bicellular at dispersal.
Exine tectate or semitectate, with columellate infratectum, perforate to
microreticulate, verrucate.
Gynoecium Carpels six to 18
(to 31), whorled, free (apocarpous); carpel plicate and ascidiate
(intermediary), postgenitally closed, without canal, stipitate. Ovary
superior?, unilocular. Style absent. Stigma decurrent, not reaching carpellary
apex (due to asymmetrical growth of carpel), brush-like, papillate (with long
unicellular papillae), Dry or slightly Wet type. Pistillodium absent.
Ovules Placentation lateral
(submarginal). Ovules usually one to three (rarely four) per carpel,
anatropous, pendulous, apotropous or epitropous, bitegmic, crassinucellar.
Micropyle bistomal. Outer integument two to five cell layers thick. Inner
integument (except its inner epidermis) crushed, two or three cell layers
thick. Megagametophyte monosporous, Polygonum type. Endosperm
development cellular. Endosperm haustoria? Embryogenesis caryophyllad or
solanad.
Fruit A stalked discoid samara
with the brush-like stigma persistent on one side.
Seeds Aril absent. Seed coat
testal. Epidermis tanniniferous. Exotestal cells enlarged (wider than endo- and
mesotestal cells). Mesotesta often sclerotic. Endotesta subpalisade, with
lignified cell walls. Tegmen unspecialized. Perisperm not developed. Endosperm
copious, oily and proteinaceous. Embryo small, straight, little differentiated,
chlorophyll? Cotyledons two. Germination phanerocotylar.
Cytology n = 14
DNA The plastid gene
rpl22 is perhaps absent from Euptelea. Nuclear gene
AP3 triplicated?
Phytochemistry Flavonols
(kaempferol, quercetin), cyanidin, chalcones, dihydrochalcones, and triterpene
saponins present. Myricetin, ellagic acid and cyanogenic compounds not
found.
Use Medicinal plants,
timber.
Systematics Euptelea (2;
E. pleiosperma: eastern Himalayas, Assam, southwestern and central
China; E. polyandra: Japan).
Euptelea is
sister to the remaining Ranunculales.
Tamura in Bot. Mag. (Tokyo) 85: 40. Mar 1972
Glaucidiales Takht.
ex Reveal in Novon 2: 238. 13 Oct 1992
Genera/species 1/1
Distribution Japan.
Fossils Unknown.
Habit Bisexual, perennial
herb.
Vegetative anatomyPhellogen?
Primary vascular tissue consisting of two irregularly concentric cylinders of
vascular bundles: one outer cylinder with smaller bundles and one inner
cylinder with larger bundles. Endodermis absent. Palisade mesophyll absent.
Rhizome with normal secondary lateral growth. Xylem not V-shaped in
cross-section. Vessel elements usually with simple (sometimes scalariform with
few bars or reticulate) perforation plates; lateral pits scalariform or
pseudoscalariform (in reality alternate). Vessel restriction patterns
occurring. Imperforate tracheary xylem elements usually libriform fibres
(sometimes tracheids) with bordered pits. Wood rays multiseriate,
heterocellular? (with non-lignified cell walls). Axial parenchyma paratracheal,
pervasive or intervascular. Sieve tube plastids S type. Nodes multilacunar with
several? leaf traces. Crystals absent?
Trichomes Hairs
unicellular?
Leaves Alternate (distichous),
simple, palmately lobed, with conduplicate, supervolute to curved and plicate
ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle transection
annular and medullary. Venation palmate. Stomata anomocytic. Cuticle wax
crystalloids as few small irregular platelets, dominated by nonacosan-10-ol.
Leaf margin serrate.
Inflorescence Flowers
terminal, usually solitary (rarely pairwise).
Flowers Actinomorphic.
Hypogyny. Tepals four, petaloid, decussate, caducous, free. Nectary absent.
Disc absent.
Androecium Stamens c. 350 to
more than 500, spiral, fasciculate. Filaments filiform, free from each other
and from tepals. Anthers basifixed, non-versatile, tetrasporangiate, extrorse?,
longicidal (dehiscing by longitudinal slits). Tapetum secretory? Staminodia
absent.
Pollen grains
Microsporogenesis simultaneous? Pollen grains tricolpate, shed as monads,
bicellular at dispersal. Exine tectate, with columellate infratectum (with
reduced columellae), spinulate, punctate-perforate.
Gynoecium Carpels (one or) two
(to four), antesepalous, conduplicate-plicate, somewhat connate at base. Ovary
superior, unilocular (apocarpy; bilocular at base). Stylodia very short. Stigma
bifid, decurrent?, type? Pistillodium absent.
Ovules Placentation marginal.
Ovules c. 15 to c. 20 (to more than 30) per carpel, anatropous, bitegmic,
usually tenuinucellar (rarely pseudocrassinucellar). Micropyle endostomal.
Outer integument seven to ten (to 13) cell layers thick, vascularized. Inner
integument three to five cell layers thick. Archespore usually ten- to
15-celled (megasporocytes). Primary parietal cell not formed. Nucellar cap c.
15 to c. 20 cell layers thick, massive, formed by periclinal divisions from
apical cells of megasporangial epidermis. Megagametophyte monosporous,
Polygonum type. Antipodal cells ephemeral or persistent (degenerating
immediately after fertilization or earlier), non-proliferating. Endosperm
development nuclear. Endosperm haustoria? Embryogenesis unclassified type
(resembles onagrad). Polyembryony frequent.
Fruit Ventricidal and
dorsicidal follicles. Adaxial side of carpels expanding more than abaxial side
during fruit development, causing stigma to become inserted on ‘lower’
(abaxial) side at fruit maturation.
Seeds Aril absent. Seeds
flattened. Seed coat testal. Outer integument vascularized, developing into a
wing-like structure on testa. Inner integument degenerating. Tegmen collapsed?
Perisperm not developed. Endosperm copious, starchy? Embryo small, elongate,
well differentiated, chlorophyll? Cotyledons two, foliaceous, with more or less
connate petioles. Germination?
Cytology n = 10 –
Chromosomes 1,5–2,5 µm long, reniform, T type (Thalictrum type).
DNA Mitochondrial intron
coxII.i3 lost?
Phytochemistry Insufficiently
known. Flavonols (kaempferol, quercetin, rhamnocitrin) and glaupalol (a
furanocoumarin) present. Berberine and other alkaloids, and cyanogenic
compounds not found.
Use Ornamental plants.
Martinov, Tekhno-Bot. Slovar: 318. 3 Aug 1820
[’Hydrasteae’]
Hydrastidales Takht.,
Divers. Classif. Fl. Pl.: 98. 24 Apr 1997
Genera/species 1/1
Distribution Central and
eastern parts of temperate North America.
Fossils Unknown.
Habit Bisexual, perennial
herb. Roots and rhizome inside bright yellow due to presence of berberine.
Erect stem with swollen nodes.
Vegetative anatomyPhellogen?
Upright stem (not rhizome) multilacunar, with cortical vascular bundles.
Medullary bundles present. Primary vascular tissue amphicribral, consisting of
two irregularly concentric cylinders of vascular bundles: an outer cylinder
with smaller bundles and an inner cylinder with larger bundles. Endodermis
absent. Palisade mesophyll absent. Secondary lateral growth absent. Xylem not
V-shaped in cross-section. Vessel elements usually with simple (rarely
scalariform with a single bar, or reticulate) perforation plates; lateral pits
scalariform, bordered pits? Imperforate tracheary xylem elements libriform
fibres with bordered pits. Wood rays multiseriate, heterocellular? Axial
parenchyma paratracheal? Sieve tube plastids Ss type. Nodes multilacunar with
several leaf traces; lower leaves usually with 9-lacunar nodes, upper leaves
with 3–5-lacunar nodes. Crystals?
Trichomes Hairs absent?
Leaves Alternate (distichous,
usually one basal leaf and two stem leaves), simple, entire or usually
palmately lobed, with plicate ptyxis. Stipules and leaf sheath absent. Petiole
base enclosing rhizome. Petiole vascular bundles eight to 24, radially arranged
(transection annular and medullary: medullary petiole bundles present).
Venation palmate. Palisade mesophyll absent. Stomata anomocytic. Cuticular wax
crystalloids as few small irregular platelets, dominated by nonacosan-10-ol.
Leaf margin serrate to biserrate.
Inflorescence Flowers
terminal, solitary.
Flowers Actinomorphic.
Hypogyny. Tepals (two or) three (or four), petaloid, small, with imbricate
aestivation, early caducous. Nectary absent. Disc absent.
Androecium Stamens c. 40 to c.
75, spiral, free from each other and from tepals. Anthers basifixed,
non-versatile, tetrasporangiate, extrorse?, longicidal (dehiscing by
longitudinal slits). Tapetum secretory, with binucleate cells. Staminodia
absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains tricolpate, shed as monads,
bicellular at dispersal. Exine tectate or semitectate, with columellate
infratectum, striate-reticulate or striate (with complex layer of striae).
Gynoecium Carpels five to c.
15, spiral, conduplicate, ab initio free, later connate at base. Ovary
superior, unilocular (apocarpy). Stylodium very short. Stigma bifid, with
multicellular processes, type? Pistillodium absent.
Ovules Placentation marginal.
Ovules one or two (sometimes three or four) per carpel, anatropous, bitegmic,
crassinucellar. Micropyle bistomal, Z-shaped (zig-zag). Outer integument four
to eight (to 13) cell layers thick. Inner integument two to four (or five) cell
layers thick. Integument two or more cell layers thick, non-vascularized.
Archespore probably unicellular. Hypostase absent. Nucellar cap approx. eight
to ten cell layers thick, formed by periclinal divisions from apical cells of
megasporangial epidermis. Megagametophyte monosporous, Polygonum type.
Antipodal cells non-modified, proliferating (dividing into five to nine
long-lived cells). Endosperm development nuclear. Endosperm haustoria?
Embryogenesis?
Fruit A multifolliculus
consisting of five to c. 15 usually one-seeded fleshy berry-like (drupaceous?)
follicles, dehiscing adaxially and abaxially (cf. Glaucidium with
follicle both dorsicidal and ventricidal).
Seeds Aril absent. Seed coat
exotestal-exotegmic. Exotesta palisade, with very elongated cells,
multiplicative, accumulating dark-brown substance. Exotegmen lignified,
multiplicative. Remaining testal and tegmic cell layers parenchymatous and
collapsed. Perisperm not developed. Endosperm copious, starchy? Embryo very
small, straight, without chlorophyll. Cotyledons two. Germination?
Cytology n = 13 –
Chromosomes small, reniform, T type (Thalictrum type).
DNA Mitochondrial intron
coxII.i3 lost?
Phytochemistry Flavonols and
isoquinoline alkaloids (berberine, hydrastine etc.) present. Rhizome containing
a ribitole-like substance and benzylisoquinoline alkaloid compounds with
D-galactose. Cyanogenic compounds not found.
Use Medicinal plant.
Systematics Hydrastis
(1; H. canadensis; northeastern United States, southeastern
Canada).
Hydrastis
may be sister to Glaucidium
(Glaucidiaceae), the
two species together forming a sister-group of Ranunculaceae. The cuticular
wax crystalloids in Hydrastis
are very similar to those occurring in Glaucidium.
Hydrastis has a special organization of those vascular strands that supply the
stamens (more or less fascicled, supplied from vascular bundles of the central
cylinder) and the carpels (each carpel supplied by four vascular bundles).
Brown in Trans. Linn. Soc. London 13: 212. 23
Mai-21 Jun 1821 [‘Lardizabaleae’], nom. cons.
Sargentodoxaceae
Stapf ex Hutch., Fam. Fl. Pl. 1: 100. 15 Jan 1926
[‘Sargentadoxaceae’], nom. cons.;
Decaisneaceae (Takht. ex H. N. Qin) Loconte in H.
Loconte, L. M. Campbell et D. W. Stevenson, Plant Syst. Evol. 9: 105. Dec 1995;
Lardizabalales Loconte in D. W. Taylor et L. J.
Hickey, Fl. Pl. Orig. Evol. Phylog.: 274. 21 Dec 1995;
Sinofranchetiaceae Doweld in Byull. Mosk. Obshch.
Ispyt. Prir., Biol. 105(5): 60. 9 Oct 2000;
Lardizabalineae Shipunov in A. Shipunov et J. L.
Reveal, Phytotaxa 16: 63. 4 Feb 2011
Genera/species 7/29–34
Distribution Southern and
eastern Himalayas, East Asia, Indochina, southern South America.
Fossils Seeds of
Sargentodoxa are known from the Miocene of North America, and seeds of
Akebia and Decaisnea have been found in Miocene layers in
Germany. Kajanthus lusitanicus was described from a late Aptian to
early Albian layer in western Portugal. It is a trimerous, radially
symmetrical, bisexual flower with several perianth whorls, six stamens in two
whorls, and three free carpels.
Habit Usually monoecious or
dioecious (in Decaisnea polygamomonoecious), usually climbing or
scrambling evergreen or deciduous shrubs or lianas (Decaisnea an
upright shrub).
Vegetative anatomy Phellogen
ab initio usually superficial (in Sargentodoxa in innermost layer of
pericycle). Primary vascular tissue consisting of a cylinder of vascular
bundles. Primary medullary strands wide, usually lignified. Vessel elements
usually with simple (sometimes scalariform) perforation plates; lateral pits
usually alternate (in Decaisnea scalariform or transitional), usually
with bordered (in Decaisnea simple) pits. Imperforate tracheary
elements tracheids or fibre tracheids, usually with bordered pits (in
Holboellia and often also in Decaisnea simple pits), septate
or non-septate (also vasicentric tracheids). Wood rays usually multiseriate (in
Decaisnea usually uniseriate), homocellular or heterocellular. Axial
parenchyma apotracheal diffuse, paratracheal scanty vasicentric or banded, or
absent. Wood elements (particularly in secondary phloem) partially storied
(sometimes irregularly). Sieve tube plastids Ss type, very large. Phloem in
Sargentodoxa with tanniniferous secretory cells. Nodes 3:3, trilacunar
with three leaf traces. Medulla with sclerenchyma cells (not in
Decaisnea). Prismatic calciumoxalate crystals sometimes abundant. Wood
rays sometimes with rhomboidal crystals (Stauntonia).
Trichomes Hairs multicellular,
uniseriate, or absent.
Leaves Alternate (spiral),
usually palmately compound (in Decaisnea pinnately compound; in
Sargentodoxa sometimes simple), entire or lobate, with conduplicate
ptyxis; petiolules usually pulvinate at base. Stipules usually absent (present
in Lardizabala); leaf sheath absent. Petiole vascular bundle
transection arcuate. Venation usually palmate (in Decaisnea pinnate).
Stomata usually anomocytic (in some species of Stauntonia cyclocytic).
Cuticular wax crystalloids as clustered tubuli (Berberis type),
chemically dominated by nonacosan-10-ol. Mesophyll with calciumoxalate druses
or single prismatic crystals. Idioblasts with ethereal oils absent. Leaflet
margins entire or serrate with chloranthoid teeth.
Inflorescence Axillary,
panicle or raceme. Floral prophylls (bracteoles) present or absent.
Flowers Actinomorphic.
Hypogyny. Outer tepals (three to) 3+3 (to eight), with imbricate – or
outermost ones with valvate – aestivation, whorled, petaloid, free. Inner
tepals (nectariferous staminodia?) usually 3+3, whorled, petaloid (sometimes
absent). Nectaries present on apex of inner tepals (nectariferous staminodia?)
or stamens, or absent. Disc absent.
Androecium Stamens (three to)
3+3 (to eight), antepetalous, alternisepalous, occasionally somewhat
foliaceous. Filaments free or connate into tube, free from tepals. Anthers
basifixed, non-versatile, tetrasporangiate, extrorse or latrorse, longicidal
(dehiscing by longitudinal slits); connective often elongated into prolonged
appendage; microsporangia often sunken into almost foliaceous wide connective.
Tapetum secretory, with usually binucleate (sometimes trinucleate or
quadrinucleate) cells. Female flowers usually with six staminodia.
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually tricolpate to
tricolporate (sometimes dicolpate to dicolporate, rarely syncolpate), shed as
monads, bicellular at dispersal. Exine tectate, with sparsely columellate or
almost acolumellate infratectum, reticulate, microreticulate, (micro)perforate,
punctate, foveolate, psilate, striate, or smooth.
Gynoecium Carpels usually
three or six to nine (to twelve) in one to five whorls of three (in
Sargentodoxa c. 50 to c. 90, spiral), free; carpel plicate,
postgenitally partially occluded, with open secretory canal; extragynoecial
compitum sometimes present. Ovary superior, unilocular (apocarpy). Stylodia
very short or absent. Stigma peltate, often oblique, hairy, non-papillate,
usually Wet type. Male flowers sometimes with pistillodium.
Ovules Placentation usually
laminal or laminal-lateral (in Decaisnea submarginal, with ovules
along ventral line of fusion). Ovules usually numerous (sometimes few) per
carpel (in Sargentodoxa a single subapical pendulous ovule), usually
anatropous (in Boquila and Lardizabala hemitropous),
horizontal, bitegmic, crassinucellar. Micropyle endostomal. Outer integument
three to five cell layers thick. Inner integument two or three cell layers
thick. Parietal tissue approx. three cell layers thick. Megagametophyte
monosporous, Polygonum type. Antipodal cells usually not persistent.
Endosperm development ab initio cellular. Endosperm haustoria?
Embryogenesis?
Fruit An assemblage of fleshy
(with fleshy placenta) follicles (Akebia, species of
Decaisnea) or a berry with leathery pericarp. Fruit in
Decaisnea with laticifers.
Seeds Aril usually absent
(present in Akebia). Seed coat exotestal. Testa usually
multiplicative. Exotesta palisade. Mesotesta and endotesta unspecialized.
Tegmen unspecialized. Perisperm not developed. Endosperm copious, oily
(sometimes, e.g. in Sargentodoxa, also starchy or with hemicellulose).
Embryo small, straight, well differentiated, chlorophyll? Cotyledons two.
Germination phanerocotylar.
Cytology n = 11
(Sargentodoxa), n = 14 (Lardizabala), n = 15
(Decaisnea), n = 16 (Akebia)
DNA The nuclear gene
AP3 is triplicated.
Phytochemistry Flavonols
(kaempferol, quercetin), cyanidin, phenols, and triterpene saponins present.
Ellagic acid, alkaloids and cyanogenic compounds not found. Aluminium
accumulated in some species of Holboellia and Stauntonia.
Use Ornamental plants,
medicinal plants, fruits (Boquila, Lardizabala).
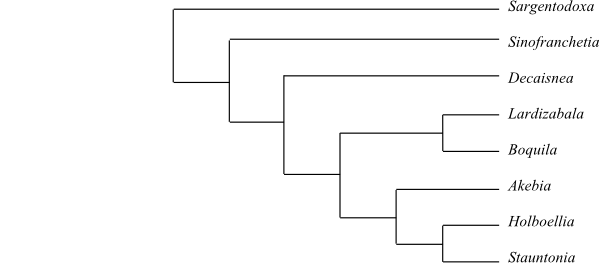
Systematics Lardizabalaceae are
sister-group to Circaeasteraceae.
Sargentodoxoideae
(Stapf ex Hutch.) Thorne et Reveal in Bot. Rev. (Lancaster) 73: 89. 29 Jun
2007
1/1. Sargentodoxa (1; S.
cuneata; China, Laos, Vietnam). – Usually dioecious (with few bisexual
flowers). Phellogen ab initio inner-pericyclic. Tanniniferous cells present.
Leaves trifoliolate. Outer tepals four to nine. Inner tepals five to seven.
Staminodia petaloid. Carpels c. 50 to c. 90, spiral, ascidiate. Ovule one per
carpel, pendulous. Outer integument approx. four cell layers thick. Placenta
fleshy in fruit. Testal surface unspecialized. n = 11. Triterpenoid saponins
not found.
Lardizabaloideae
Burnett, Outlines Bot.: 830. Feb 1835 [‘Lardizabalidae’]
6/28–33. Decaisnea
(1; D. insignis; eastern Himalayas to central China), Sinofranchetia
(1; S. chinensis; western and central China), Lardizabala (1;
L. funaria; central and southern Chile between the Andes and the
Pacific, southern Argentina), Boquila (1; B. trifoliolata;
central and southern Chile between the Andes and the Pacific, southern
Argentina), Akebia (4;
A. chingshuiensis, A. longeracemosa, A. quinata,
A. trifoliata; China, the Korean Peninsula, Japan, Taiwan),
Stauntonia (20–25; northeastern India, the Himalayas, China, the
Korean Peninsula, Japan, Taiwan). – Southern and eastern Himalayas, East
Asia, southern South America. Usually monoecious (rarely dioecious or
bisexual). Phellogen ab initio superficial. Vessel elements sometimes with
scalariform perforation plates. Stomata sometimes cyclocytic. Leaves sometimes
palmately compound or imparipinnate, with basal tooth or lobe. Venation
sometimes pinnate. Outer tepals sometimes three. Inner tepals sometimes absent.
Stamens sometimes three or eight. Filaments connate. Tapetum with up to
quadrinucleate cells. Pollen grains sometimes colporoidate, sometimes
tricellular at dispersal. Female flowers with staminodia. Carpels sometimes up
to twelve. Stigma sometimes peltate. Placentation sometimes laminar. Ovules
(few to) numerous per carpel, sometimes hemitropous. Outer integument three to
five cell layers thick. Inner integument two or three cell layers thick.
Parietal tissue approx. three cell layers thick. Antipodal cells in Decaisnea
persistent. Endosperm development usually cellular (in Decaisnea
nuclear). Fruit a berry or a fleshy follicle, often with fleshy placenta. Testa
multiplicative. Exotestal cells lignified, elongated (in Decaisnea),
or non-lignified and fibrous (in Akebia and
Stauntonia). Hypodermal cells thickened. Endosperm starchy or with
hemicellulose. n = 14–16, 17?, 18. Oleanone triterpenoid saponins present.
Aluminium accumulation occurs in Stauntonia, a synapomorphy of this
clade.
de Jussieu, Gen. Plant.: 284. 4 Aug 1789
[’Menisperma’], nom. cons.
Menispermales Juss.
ex Bercht. et J. Presl, Přir. Rostlin: 225. Jan-Apr 1820
[‘Menispermeae’]; Pseliaceae Raf., New
Fl. N. Amer. 4: 8. med 1838 [’Pselides’]
Genera/species 74/535–540
Distribution Tropical and
subtropical regions in the Northern and Southern Hemispheres, and a few species
in temperate eastern North America and temperate East Asia.
Fossils The oldest fossil
consists of keeled endocarps of Prototinomiscium from the Turonian to
the Maastrichtian of Central Europe. Anamirta pfeifferi is fossilized
wood (probably of a liana) from the Maastrichtian Deccan Intertrappean Beds in
India. A large number of endocarps, which can be assigned to Menispermaceae, are known
from Cenozoic layers in Europe and North America.
Habit Dioecious, usually
evergreen lianas, or scrambling and climbing perennial herbs (rarely shrubs or
trees [Burasaia, Penianthus, Sphenocentrum]; one
species of Stephania an erect herb).
Vegetative anatomy Phellogen
ab initio superficially or deeply seated. Secondary lateral growth usually
anomalous (particularly in lianas), from successive cambia. Vessel elements
with simple perforation plates; lateral pits alternate or scalariform, simple
and/or bordered pits. Imperforate tracheary xylem elements tracheids or
libriform fibres with simple and/or bordered pits, non-septate (also
vasicentric tracheids). Wood rays uniseriate, interfascicular, usually wide and
very tall (rarely narrow), homocellular or heterocellular, often lignified.
Axial parenchyma apotracheal diffuse or diffuse-in-aggregates, or paratracheal
scanty vasicentric, scalariform or in short bands connecting successive layers
of vascular bundles. Wood elements often storied, especially in secondary
phloem. Intraxylary (concentric) phloem usually present. Sieve tube plastids Ss
type, very large. Nodes 3:3, trilacunar with three leaf traces. Stem and leaves
with rows of secretory cells. Parenchyma with numerous asterosclereids and
osteosclereids. Crystal sand present or absent. Raphid idioblasts (raphid
cells) present or absent. Prismatic calciumoxalate crystals abundant. Wood rays
and axial parenchyma often with rhomboidal crystals.
Trichomes Hairs unicellular or
multicellular, uniseriate.
Leaves Alternate (spiral),
usually simple (in Burasaia trifoliolate), entire or lobed (one
species of Cocculus with phyllocladia), often peltate, with ? ptyxis.
Stipules usually absent; leaf sheath absent. Petiole often with proximal and
distal pulvinus. Petiole vascular bundle transection annular. Venation palmate,
actinodromous or acrodromous, or pinnate. Stomata anomocytic, paracytic,
staurocytic or often more or less cyclocytic (sometimes actinocytic), often
surrounded by a rosette of subsidiary cells. Cuticular wax crystalloids as
clustered tubuli (Berberis type), chemically dominated by
nonacosan-10-ol. Domatia usually as pockets (rarely hair tufts). Epidermal
cells often with calciumoxalate crystals, sometimes with silica or trichome
hydathodes. Mesophyll with sclerenchymatous idioblasts and mucilage cells.
Idioblasts with ethereal oils absent. Laminar surface sometimes with ridges of
laticifers. Leaf margin usually entire (sometimes serrate or lobed).
Extrafloral nectaries present or absent.
Inflorescence Terminal or
axillary, raceme- or headlike, or compound panicle (flowers rarely solitary or
paired).
Flowers Usually actinomorphic
(female flowers in Antizoma, Cyclea, Cissampelos and
some species of Stephania slightly zygomorphic). Hypogyny. Tepals
usually whorled (sometimes spiral); in female flowers sometimes fewer than in
male flowers. Outer tepals (one to) six (to more than twelve), with imbricate
or valvate aestivation, sepaloid, usually in whorls each one with usually three
(rarely one), usually free (in Cyclea and Synclisia slightly
connate). Inner tepals (absent or one to) six (to eight), with usually
imbricate aestivation, petaloid, usually free (in Cyclea and some
species of Disciphania connate; rarely enclosing stamens). Nectary
absent. Disc absent.
Androecium Stamens three, six,
twelve or more (in one species of Odontocarya one; in
Hypserpa up to c. 40), majority antepetalous, spiral or whorled.
Filaments free or more or less connate into synandrium, free from tepals.
Anthers basifixed, non-versatile, usually tetrasporangiate (rarely
disporangiate), with superposed thecae, usually introrse (rarely extrorse),
usually longicidal (dehiscing by usually longitudinal [sometimes transversal]
slits). Tapetum secretory. Female flowers often with staminodia.
Pollen grains
Microsporogenesis simultaneous. Pollen grains (2–)3(–4)-colpate,
(2–)3(–4)-colporate (sometimes syncolporate), (2–)3(–4)-porate or
(2–)3(–4)-pororate (rarely cryptoporate or inaperturate), shed as monads,
bicellular at dispersal. Exine semitectate, with columellate infratectum,
reticulate or microreticulate.
Gynoecium Carpels usually
three, six or more (rarely one or two; in one species of Tiliacora and
one species of Triclisia c. 20 to 32), free or slightly connate at
base, often stipitate (on gynophore); carpel plicate (ascidiate?),
postgenitally partially fused, with open secretory canal. Ovary superior,
unilocular (apocarpy). Style very short or absent. Stigma entire, bifid or
trifid, expanded above, hairy to non-papillate, usually Dry (occasionally Wet)
type. Male flowers often with pistillodium.
Ovules Placentation
ventral-marginal. Ovules usually two per carpel (one of which degenerating),
anatropous or campylotropous (often amphitropous after fertilization),
apotropous or epitropous, pendulous to horizontal (ascending?), unitegmic
(derived from integumentary shifting) or bitegmic, crassinucellar. Micropyle
endostomal or bistomal, Z-shaped (zig-zag). Outer integument two to five cell
layers thick. Inner integument two cell layers thick (when one integument, then
three or four cell layers thick). Megagametophyte monosporous,
Polygonum type. Synergids sometimes with a filiform apparatus.
Antipodal cells multinucleate, often proliferating. Endosperm development ab
initio nuclear (finally cellular). Endosperm haustoria? Embryogenesis
onagrad.
Fruit An assemblage of
single-seeded stipitate drupes on a globose, discoid, columnar or branched
carpophore, often flattened or strongly curved (cf. the name
Menispermum = ’moon-seed’). Exocarp thin or leathery. Mesocarp
fleshy to fibrous, sometimes sclerified. Endocarp sclerified, hard, usually
sculptured in different ways, usually with a condyle (placentary outgrowth or
invagination; sometimes absent; condyle produced during ovary development due
to intruding ovary wall on placenta, causing seed to curve).
Seeds Aril absent. Seed
usually curved. Testa little differentiated (sometimes absent). Exotesta
sometimes tabular, lignified. Mesotesta and endotesta unspecialized. Tegmen
unspecialized. Perisperm not developed. Endosperm usually copious (sometimes
partially or entirely ruminate; absent in most genera in cladogram 2), oily.
Embryo small to large, straight or curved, chlorophyll? Cotyledons usually two
(rarely one), flat or terete, often fleshy (genera in cladogram 2). Germination
phanerocotylar or cryptocotylar.
Cytology n = (9-)11-13 –
Polyploidy (tetraploids, hexaploids) occurring.
DNA The nuclear gene
AP3 is triplicated.
Phytochemistry Flavonols
(kaempferol), flavones, diterpenoids, sesquiterpenoids, tannins,
benzylisoquinoline and aporphine alkaloids (benzyltetrahydroisoquinoline and
aporphine derivatives in dimeric form, e.g. berberine, morphinane), hasubanane
alkaloids (protostephanines, erythrinanes, cocculolidines, morphines,
quettamine-morphine dimers, hasubanonines, acutumines, etc.), azafluoranthene
alkaloids, tropoloisoquinoline alkaloids, tubocurarine chloride (a curare
mixture of alkaloids), toxic sesquiterpene lactones, phenylic cinnamide,
furofuran lignans, and frequent cyanogenic compounds present. Tyrosine-derived
cyanogenic glycosides and caffeic acid rare. Ellagic acid and proanthocyanidins
not found.
Use Ornamental plants,
medicinal plants (Anamirta cocculus, Chondrodendron
tomentosum, Jateorhiza palmata etc), fish- and arrow poisons
(curare from Chondrodendron, Curarea, Sciadotenia
etc., coccel kernels and picrotoxine from Anamirta), timber.
Systematics Menispermaceae are
sister-group to the clade [Berberidaceae+[Ranunculaceae+ [Hydrastis+Glaucidium]]].
Burasaia was sister to the remaining
Menispermaceae,
according to analyses of morphological data by Jacques & Bertolino (2008).
Ortiz & al. (2007), using ndhF sequence data, recovered
Tinomiscium sister to the remainder. Hoot & al. (2009), using
atpB and rbcL sequence data, identified a clade comprising
Menispermum and Sinomenium as sister to the rest with low to
moderate support, and Burasaia was nested deep inside Menispermaceae. On the other
hand, adding ndhF data rendered Tinomiscium again sister to
the rest and Menispermum was nested deep within Menispermaceae.
Chasmantheroideae are sister-group to Menispermoideae (Ortiz
& al. 2016). The taxonomy below follows Ortiz & al. (2016).
Chasmantheroideae
Luerss., Handb. Syst. Bot. 2: 574. Nov 1880
29/>156. Pantropical. Tangential cell
walls of wood rays in Tinomiscium oblique to ray axis in
cross-section. Leaf surface sometimes (e.g. in Fibraurea and
Tinomiscium) with laticifers as fine ridges. Seed subglobose to
reniform, ruminate. Embryo spathuliform. Cotyledons foliaceous, more or less
divaricate.
Coscinieae Hook. f. et
Thomson, Flora Ind.: 177. 1855
3/6. Coscinium (2; C.
blumeanum, C. fenestratum; India, Sri Lanka, Southeast Asia, West
Malesia to Borneo), Anamirta (1; A. cocculus; India,
Southeast Asia, Malesia to Timor), Arcangelisia (3; A. flava,
A. gusanlung, A. tympanopoda; Southeast Asia, Malesia to New
Guinea). – Tropical Asia. Sepals in three worls. Petals absent. Filaments
more or less connate. Drupelet with remnant of style/stigma subapical-adaxial.
Endocarp and seed subglobose.
Burasaieae Endl., Gen.
Plant. Suppl. 5: 25. 1850
26/>150. Calycocarpum (1;
C. lyonii; eastern North America); Parabaena (6; P.
denudata, P. echinocarpa, P. elmeri, P.
megalocarpa, P. sagittata, P. tuberculata; Southeast
Asia, Malesia), Aspidocarya (1; A. uvifera; northeastern
India to southwestern China), Disciphania (c 25; Mexico, Central
America, tropical South America); Tinomiscium (1; T.
petiolare; Southeast Asia, Malesia), Fibraurea (3; F.
darshanii: India; F. recisa: southern China, Indochina; F.
tinctoria: India, Assam, Southeast Asia, the Philippines, Borneo,
Sulawesi), Borismene (1; B. japurensis; tropical South
America), Paratinospora (2; P. dentata: Taiwan; P.
sagittata: China), ‘Penianthus’ (4; P.
camerounensis, P. longifolius, P. patulinervis, P.
zenkeri; tropical West and Central Africa; non-monophyletic),
Sphenocentrum (1; S. jollyanum; tropical West Africa),
Burasaia (4; B. australis, B. congesta, B.
gracilis, B. madagascariensis; Madagascar), Orthogynium
(1; O. gomphloides; Madagascar?), Dioscoreophyllum (3; D.
cumminsii, D. gossweileri, D. volkensii; tropical
Africa), Jateorhiza (2; J. macrantha, J. palmata;
tropical Africa), ’Tinospora’ (36; tropical Asia, tropical
Australia; polyphyletic), Kolobopetalum (4; K. auriculatum,
K. chevalieri, K. leonense, K. ovatum; tropical
Africa), Rhigiocarya (2; R. peltata, R. racemifera;
tropical West and Central Africa), Hyalosepalum (>10; tropical
Africa), Sarcolophium (1; S. tuberosum; tropical Africa),
Chasmanthera (2; C. dependens: tropical Africa; C.
welwithschii: Congo), Leptoterantha (1; L. mayumbense;
tropical Africa), Syntriandrium (1; S. preussii; tropical
West and Central Africa), Dialytheca (1; D. gossweileri;
Angola), Odontocarya (36; southern Mexico, Central America, tropical
South America). – Unplaced Burasaieae
Chlaenandra (1; C. ovata; New Guinea),
Platytinospora (1; P. buchholzii; tropical West and Central
Africa). – Pantropical, one genus, Calycocarpum, in eastern North America.
Ovules anatropous. Endocarp and seed straight. Seed abaxially-adaxially
compressed, naviculiform. Calycocarpum is sister to the remaining Burasaieae
(e.g. Wang & al. 2017).
Menispermoideae Arn.
in R. Wight et G. A. W. Arnott, Prodr. Fl. Ind. Orient.: 11. 10 Oct 1834
[’Menispermeae’]
45/380–385. Pantropical, few species
in temperate regions. Style lateral to basal. Drupelet with remnant of
style/stigma subbasal to basal. Endocarp laterally compressed, usually curved
(in Orthomene straight), often sculptured. Seed curved, usually not ruminate.
Endosperm sometimes absent. Embryo curved, strap-shaped. Cotyledons fleshy,
cylindrical, adpressed. – Menispermeae are sister-group to the
remaining Menispermoideae and Anomospermeae successive
sister-group to the rest. – Ortiz & al. (2016) found the following
topology:
[Menispermeae+[Anomospermeae+[Limacieae+[Tiliacoreae+[Pachygoneae+[Spirospermeae+Cissampelideae]]]]]]
Menispermeae DC.,
Syst. Nat. 1: 510, 511. 1-15 Nov 1817 [’Menispermeae verae’]
2/3. Menispermum (2; M.
dauricum: East Asia; M. canadense: southeastern Canada, eastern
United States), Sinomenium (1; S. acutum; central China,
Japan). – East Asia, eastern North America. Stamens free, numerous. Endocarp
longitudinally and transversally ridged. Seed semiannular-crescentic.
Anomospermeae Miers in
Ann. Mag. Nat. Hist., ser. 2, 7: 36. Jan 1851
13/c 80. Diploclisia (2; D.
affinis: China; D. glaucescens: India, Sri Lanka, Burma, southern
China, Southeast Asia, Malesia to New Guinea), Sarcopetalum (1; S.
harveyanum; southern New Guinea, eastern Queensland, eastern New South
Wales, eastern Victoria), Legnephora (5; L. acuta, L.
microcarpa, L. minutiflora, L. moorei, L.
philippinensis; New Guinea, eastern Queensland), Parapachygone
(1; P. longifolia; northeastern Queensland), Hypserpa (10;
Southeast Asia, Malesia to Polynesia), Pericampylus (3; P.
glaucus, P. incanus, P. macrophyllus; China, Taiwan,
tropical Asia from India to Malesia), Echinostephia (1; E.
aculeata; southeastern Queensland, northeastern New South Wales);
’Anomospermum’ (8; tropical America; polyphyletic),
Caryomene (5; C. foveolata, C. glaucescens, C.
grandifolia, C. olivascens, C. prumnoides; tropical
America), ’Orthomene’ (4; O. hirsuta, O.
prancei, O. schomburgkii, O. verruculosa; tropical
America; non-monophyletic), Elephantomene (1; E. eburnea;
northeastern South America), Telitoxicum (8; tropical South America),
Abuta (31; tropical South America). – Tropical Asia to eastern
Australia and Polynesia, tropical America. Seed hippocrepiform. Embryo
hippocrepiform, strap-shaped. Cotyledons shorter than radicle. Neotropical
species with ruminate endosperm and cotyledons longer than radicle;
paleotropical species with continuous endosperm.
Limacieae Prantl in
Engler et Prantl, Nat. Pflanzenfam. 3(2): 88. 1888
1/3. Limacia (3; L.
blumei, L. oblonga, L. scandens; Burma to West Malesia).
– Burma to West Malesia. Endocarp with raised longitudinal band along axis
and with weakly convex lateral sides; external apertures large.
Tiliacoreae Miers in
Ann. Mag. Nat. Hist., ser. 2, 7: 36. Jan 1851
16/111. Chondrodendron (3;
C. microphyllum, C. platiphyllum, C. tomentosum;
Central America, tropical South America), Sciadotenia (19; tropical
America), Curarea (5; C. candicans, C. crassa,
C. cuatrecasasii, C. tecunarum, C. toxicofera;
tropical South America), Syrrheonema (3; S. fasciculatum,
S. hexastamineum, S. welwitschii; tropical West and Central
Africa), Carronia (4; C. multisepalea, C.
pedicellata, C. protensa, C. thyrsiflora; New Guinea,
eastern Queensland, eastern New South Wales), Pycnarrhena (9;
Southeast Asia, Malesia to tropical Australia), Beirnaertia (1; B.
cabindensis; tropical Africa), Triclisia (15; tropical Africa,
Madagascar), ‘Albertisia’ (19; tropical and subtropical Africa;
paraphyletic; incl. Anisocycla?), Anisocycla (5; A.
blepharosepala, A. cymosa, A. grandidieri, A.
jollyana, A. linearis; tropical Africa, Madagascar; in
Albertisia?), Tiliacora (22; tropical regions in the Old
World); unplaced Tiliacoreae: Eleutharrhena (1; E.
macrocarpa; Assam, Yunnan), Macrococculus (1; M.
pomiferus; New Guinea), Pleogyne (1; P. australis;
eastern Queensland), Synclisia (1; S. scabrida; Central
Africa), Ungulipetalum (1; U. filipendulum; Brazil). –
Pantropical. Male flower often with at least 4-tetracyclic calyx. Endocarp with
longitudinal grooves, ribs or rugose ornamentation abaxially. Seed
hippocrepiform. Endosperm usually absent. Embryo subcylindric.
Pachygoneae Miers ex
Hook. f et Thomson, Flora Ind. 1: 176, 202. 1-19 Jul 1855
4/45. Haematocarpus (2; H.
subpeltatus, H. validus; eastern Himalayas, Southeast Asia to
Sulawesi), ’Hyperbaena’ (22; Central America, tropical South
America; paraphyletic), Cocculus (9; tropical and southern Africa,
Madagascar, Socotra, tropical and subtropical Asia to northern Australia,
subtropical to temperate North America), Pachygone (12; southern
China, Southeast Asia, Malesia to islands in western Pacific). – Pantropical,
subtropical to temperate North America. Endosperm usually absent. Embryo
usually subcylindric (in Cocculus strap-shaped), dehiscence usually
transversal.
Spirospermeae R. Ortiz
et Wei Wang in Taxon 65(6): 1306. Dec 2016
4/10. Limaciopsis (1; L.
loangensis; tropical Africa), Rhaptonema (6; R.
bakeriana, R. cancellata, R. densiflora, R.
glabrifolium, R. latifolia, R. swinglei; Madagascar),
Spirospermum (1; S. penduliflorum; Madagascar),
Strychnopsis (1; S. thouarsii; Madagascar). – Tropical
Africa (one species), Madagascar. Usually trees. Stamens three. Carpels at
least six. Seed cochleate or spiral. Embryo subcylindric. Cotyledons laterally
adpressed.
Cissampelideae Hook.
f. et Thomson, Flora Ind. 1: 176, 194. 1-19 Jul 1855
5/c 130. ‘Stephania’ (c 70;
tropical regions in the Old World; paraphyletic), Perichasma (2;
P. laetificata, P. miersii; Central Africa, Angola),
Antizoma (3; A. angolensis, A. angustifolia, A.
miersiana; arid regions in southern Africa), ‘Cissampelos’
(23; tropical regions on both hemispheres; paraphyletic; incl.
Cyclea?), Cyclea (32; China to the Philippines; in
Cissampelos?). – Pantropical, southern Africa. Male flower with
1-cyclic corolla. Synandria present, with anthers horizontally arranged on
peltiform connective. Anthers dehiscing transversally. Carpel one. Embryo
strap-shaped. Cotyledons shorter than radicle.
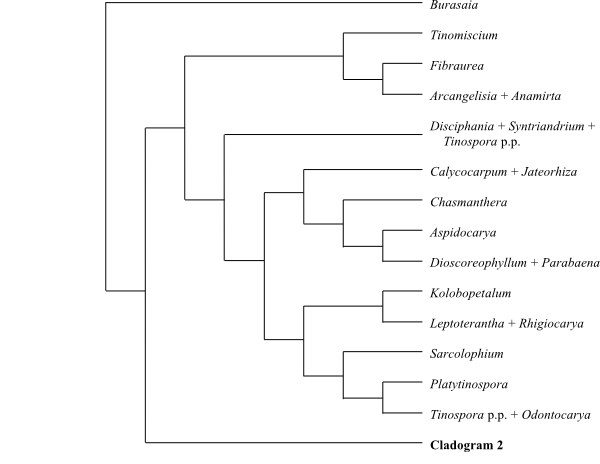 |
Cladogram 1 of Menispermaceae based
on morphological data (Jacques & Bertolino 2008).
|
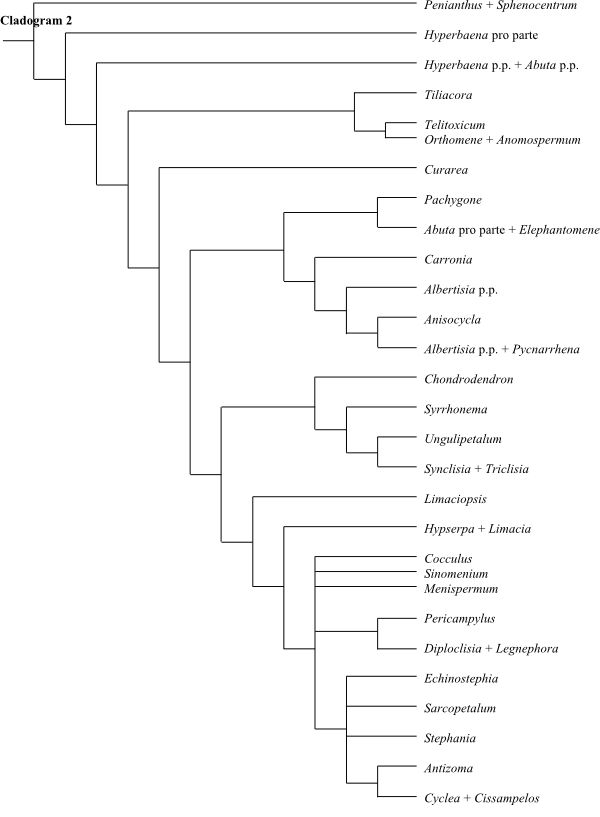 |
Cladogram 2 of Menispermaceae based
on morphological data (Jacques & Bertolino 2008).
|
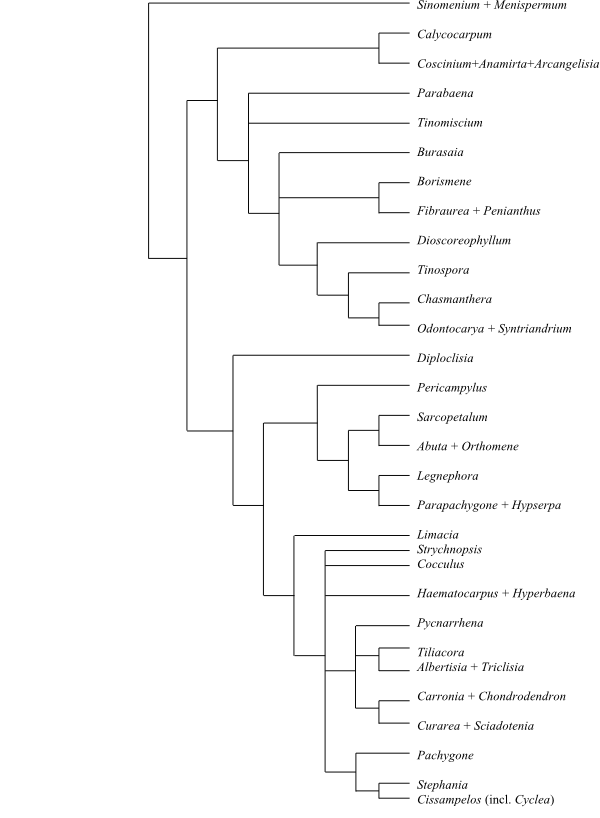 |
Bayesian inference tree of Menispermaceae based
on atpB/rbcL sequence data (Hoot & al. 2009).
|
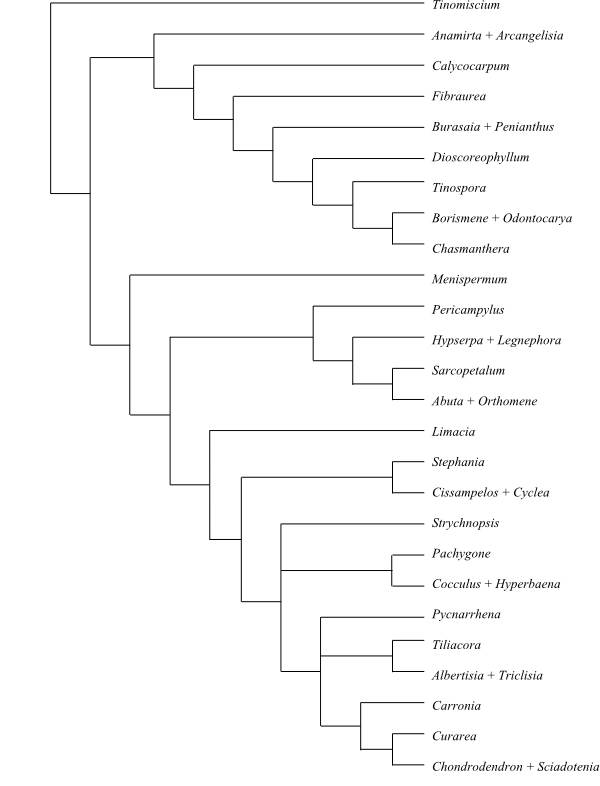 |
Bayesian inference tree of Menispermaceae based
on atpB, rbcL and ndhF sequence data (Hoot
& al. 2009).
|
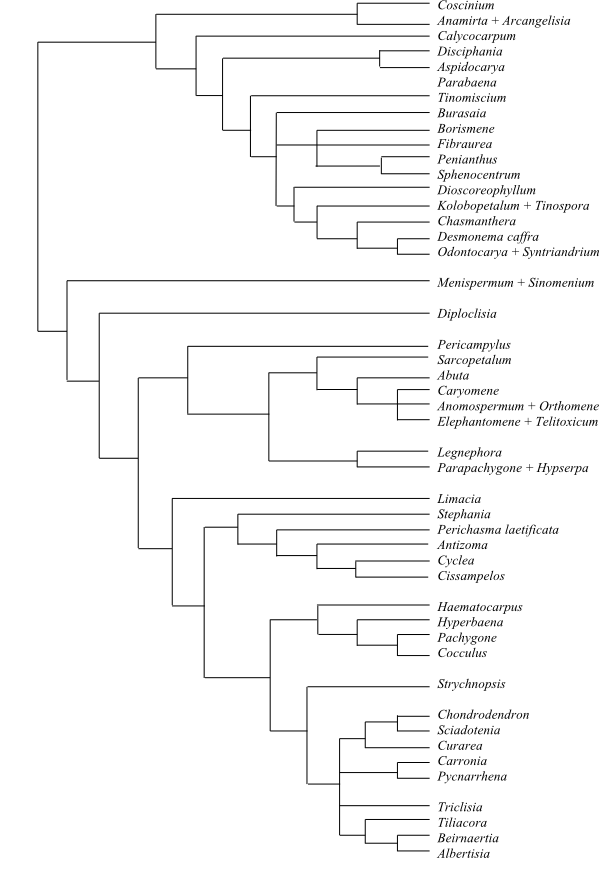 |
Bayesian inference tree of Menispermaceae based
on DNA sequence data (Wefferling & al. 2013; Sciadotenia added).
|
de Jussieu, Gen. Plant.: 235. 4 Aug 1789, nom.
cons.
Corydalaceae Vest,
Anleit. Stud. Bot.: 266, 283. 1818 [‘Corydaloidea’,
‘Corydaloideae’], nom. illeg.;
Chelidoniaceae Martinov, Tekhno-Bot. Slovar: 124. 3
Aug 1820 [’Chelidoneae’]; Fumariaceae
Marquis, Esq. Règne Vég.: 50. 15-22 Jul 1820 [‘Fumarieae’], nom.
cons.; Fumariales Bercht. et J. Presl, Přir.
Rostlin: 216. Jan-Apr 1820 [‘Fumariae’];
Papaverales Juss. ex Bercht. et J. Presl, Přir.
Rostlin: 216. Jan-Apr 1820 [‘Papaveraceae’];
Papaveropsida Brongn., Enum. Plant. Mus. Paris: xxv,
93. 12 Aug 1843 [’Papaverineae’];
Eschscholziaceae Ser., Fl. Jard. 2: 106. Apr 1847;
Platystemonaceae (Spach) Lilja, Skånes Fl., ed. 2:
862, 979. Apr-Dec 1870 [’Platystemoneae’];
Hypecoaceae (Dumort.) Willkomm et Lange, Prodr. Fl.
Hispan. 3: 875. Apr-Mai 1880 [’Hypecoëae’];
Papaverineae Thorne et Reveal in Bot. Rev.
(Lancaster) 73: 91. 29 Jun 2007
Genera/species 43/c 755
Distribution Temperate and
subtropical parts if the Northern Hemisphere, Central America, northern South
America, East African mountains, southern Africa, Macaronesia, with their
largest diversity in the Mediterranean, West, Central and East Asia, and the
southwestern United States.
Fossils Uncertain. No entirely
convincing reports, although some Miocene seeds are similar to
Corydalis.
Habit Bisexual, usually
perennial, biennial or annual herbs (sometimes climbing; some species of
Argemone, Bocconia, Dendromecon,
Hunnemannia, and Romneya evergreen shrubs or small trees).
Some species have tuberous rhizome or tuberous roots. Stem sometimes
pachycaul.
Vegetative anatomy Mycorrhiza
at least usually absent. Roots diarch and lateral roots tetrastichous.
Phellogen? Primary vascular tissue consisting of one or several cylinders of
vascular bundles; cross section of stem usually with a ring of loose collateral
vascular bundles separated by wide multiseriate medullary rays. Cortex and
medulla without vascular bundles. Secondary lateral growth usually absent
(sometimes from normal cylindrical cambium). Vessel elements with simple
perforation plates; lateral pits alternate, simple or bordered pits.
Imperforate tracheary xylem elements libriform fibres with simple pits,
non-septate (also vasicentric tracheids). Wood rays multiseriate,
heterocellular. Axial parenchyma paratracheal scanty, vasicentric. Wood
elements often storied. Sieve tube plastids Ss type. Nodes 1:1 or ≥3:≥3,
unilacunar or trilacunar (rarely multilacunar) with one or more leaf traces.
Latex cells and laticifers often articulated, often anastomosing, usually with
white, yellow, orange or red latex, numerous in most genera of
Papaveroideae. Idioblasts with watery secretions present in
Fumarioideae. Crystals absent.
Trichomes Hairs unicellular or
multicellular, uniseriate or multiseriate (in Bocconia and
Macleaya sometimes branched), or absent (multicellular glandular hairs
present in Dicranostigma and Glaucium).
Leaves Usually alternate
(spiral; rarely opposite or verticillate), simple or compound (usually once or
twice pinnately compound), entire or lobed (usually pinnately lobed), with
ptyxis of various types. Stipules and leaf sheath absent (petiole base
occasionally somewhat sheathing). Petiole vascular bundle transection arcuate.
Venation usually pinnate (sometimes palmate). Stomata anomocytic. Cuticular wax
crystalloids as clustered tubuli (Berberis type), chemically dominated
by nonacosan-10-ol. Hydathodes present in some species. Idioblasts with
ethereal oils absent. Leaf margin serrate (with chloranthoid teeth, often
incised), lobed or entire.
Inflorescence Terminal or
axillary, simple or compound, cymose or racemose of different shapes, or
flowers solitary terminal.
Flowers Actinomorphic,
bisymmetric or zygomorphic (transversely zygomorphic in most
Fumarioideae). Hypanthium usually absent (present in
Platystemon). Usually hypogyny (in Platystemon half epigyny).
Outer (sepaloid) tepals usually two or three (rarely four), with imbricate or
open aestivation, median, in one whorl, usually caducous, usually free (in
Eomecon and Eschscholzia connate either at base or entirely
into operculum), with or without basal spur or other type of appendage. Inner
(petaloid) tepals usually 2+2 or 3+3, crumpled or with imbricate aestivation,
whorled (in Sanguinaria six to twelve, often in several whorls; absent
in Bocconia and Macleaya), usually free (in
Fumarioideae more or less connate and adnate to stamens), usually
caducous, without spur, in Fumarioideae one or two petals usually
spurred. Nectaries in Papaveroideae absent, in Fumarioideae
present at staminal bases. Disc absent.
Androecium Stamens usually 16
up to more than 200 (in Meconella four to six or 6+6; in
Canbya six to twelve; in Hypecoum 2+2; in Romneya up
to c. 700) in three to 15 whorls; androphore present or absent. Filaments
filiform, clavate or wide (in some species of Dicentra petaloid),
usually free (in Fumarioideae two free stamens and two groups of
connate stamens opposite outer petaloid tepals, each group consisting of one
median entire and two lateral half anthers), free from tepals. Anthers
basifixed, non-versatile, usually tetrasporangiate (in Hypecoum
sometimes disporangiate; in Fumarioideae lateral anther halves in each
staminal group disporangiate and remaining anthers tetrasporangiate), usually
extrorse (rarely latrorse), longicidal (dehiscing by longitudinal slits).
Tapetum secretory or amoeboid-periplasmodial. Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually tricolpate to
hexacolpate, syncolpate, polyporate or polyforate (sometimes polycolpate,
pantoaperturate; in some species of Meconopsis inaperturate; in
Dicentra, Hypecoum, and Platystemon dicolpate; in
Fumarieae often polypantoporate, colliculate, with intine bulging from
pores), shed as monads, usually bicellular at dispersal (in Papaver
sometimes tricellular). Exine tectate or semitectate, with columellate
infratectum (columellae in Fumarioideae small), reticulate,
microreticulate, perforate, microperforate or punctate, psilate, verrucate or
echinulate (sometimes spinulate).
Gynoecium Pistil composed of
two (up to c. 25) paracarp and connate carpels (carpels in Platystemon
etc. partially free; carpels in Fumarioideae two transverse); carpel
plicate, seemingly occluded by secretion; closure of carpels sometimes delayed.
Ovary usually superior (in Platystemon semi-inferior), usually
unilocular (sometimes seemingly bilocular to 20-locular by intrusion of
placentae), stipitate or sessile (gynophore present or absent). Style single,
simple, short, with stylar canal (sometimes closed by hairs), or absent
(stylodia in Hypecoum two, partially connate). Stigma carinal and/or
commissural, capitate, or stigmas often connate and forming a disc-like roof on
top of ovary, papillate, Dry type. Pistillodium absent.
Ovules Placentation usually
parietal (when ovary unilocular) or axile (when ovary multilocular) (placentae
in Bocconia basal; in Romneya plurilocular ovary with
parietal placentation; placentae sometimes protruding to diffuse). Ovules one
to c. 100 per ovary (in Bocconia and one speces of Macleaya
one ovule), anatropous, hemicampylotropous to campylotropous or
hemiamphitropous, pendulous?, horizontal or ascending (with ventral raphe,
collateral or superposed or above carpellary surface or biseriate), bitegmic,
usually crassinucellar (in Papaver and Platystemon
pseudocrassinucellar). Micropyle bistomal (sometimes Z-shaped, zig-zag). Outer
integument (two to) four to ten cell layers thick. Inner integument two to four
cell layers thick. Parietal tissue two to four cell layers thick. Nucellar cap
approx. three cell layers thick. Megagametophyte usually monosporous,
Polygonum type (in Platystemon tetrasporous,
Fritillaria type). Synergids usually with a filiform apparatus.
Antipodal cells usually three (in Papaver sometimes five),
non-proliferating, persistent, endopolyploid (sometimes binucleate). Endosperm
development ab initio nuclear. Suspensor haustorium present in
Fumarioideae. Endosperm haustoria absent? Embryogenesis irregularly
solanad (Papaveroideae) or caryophyllad (Fumarioideae).
Fruit A poricidal, septicidal
(placenticidal) or loculicidal capsule with basipetalous or acropetalous
dehiscence, sometimes siliquiform with replum (in Fumaria and one
species of Macleaya a nut; in Platystemon and species of
Hypecoum lomentum-like with two to c. 30 one-seeded nut-like
mericarps).
Seeds Aril present or absent.
Elaiosome present in numerous species (in, e.g., Chelidonium,
Corydalis, Dendromecon); strophiole (tiny swellings of raphe)
sometimes (in, e.g., Chelidonium) present. Seed coat testal-tegmic.
Exotesta usually well developed, palisade. Endotesta often well developed, with
calciumoxalate crystals and coarse fibrillar endoreticulum. Exotegmen often
fibrous. Endotegmic cell walls thickened. Perisperm not developed. Endosperm
copious, oily (rarely granular). Embryo small, straight or curved, well
developed or poorly developed (seed in many Fumarioideae containing
only proembryo, further growth being intraseminal), without chlorophyll.
Cotyledons usually two (in some species of Dicentra and in tuberous
species of Corydalis one). Germination phanerocotylar or
cryptocotylar.
Cytology n = 6–11, (14, 19,
21) – Polyploidy frequently occurring (up to 2n = c. 140).
DNA Duplication of nuclear
CYCLOIDEA genes
Phytochemistry Flavonols
(kaempferol, quercetin), benzylisoquinoline alkaloids (e.g.
1-benzyltetrahydroisoquinoline alkaloids) and aporphine alkaloids
(dehydrogenated benzophene anthridines, reduced benzophene anthridines in
Chelidonieae, berberines, tetrahydroberberines, stylopine,
chelidonine, protopines, rhoeadines, narceines, spirobenzylisoquinolines, etc.
in Fumarioideae, aporphines, morphinanes, pavines, isopavines,
reticuline, scoulerine, narcotine), meconic acid (in Meconopsis,
Papaver and Roemeria), chelidonic acid (at least in
Chelidonium and Stylophorum), fumaric acid (in
Glaucium, Papaver and Fumarioideae), and nitrophenyl
ethan present. Tyrosine-derived cyanogenic glycosides sometimes present.
Ellagic acid, tannins and proanthocyanidins not found. Caffeic acid? The free
amino acid δ-acetylornithine is probably the principal nitrogen transport
compound in Fumarioideae.
Use Ornamental plants, baking
(seeds from Papaver somniferum), seed oils for soap
(Argemone, Glaucium, Papaver), medicinal plants,
narcotics (opium from Papaver somniferum and P.
bracteatum).
Systematics Papaveraceae are probably
sister to Pteridophyllum
(Pteridophyllaceae).
Papaveroideae Eaton,
Bot. Dict., ed. 4: 38. Apr-Mai 1836 [‘Papaveraceae’]
23/c 235. Mainly temperate regions on
the Northern Hemisphere. Usually herbs (rarely small trees). Nodes 1:1 or
≥3:≥3, unilacunar or trilacunar (rarely multilacunar) with one or more leaf
traces. Latex milky white, yellow, orange or red. Leaves with ptyxis of various
types. Colleters present. Flowers usually large. Outer tepals enclosing bud,
caducous. Inner (petaloid) tepals four or six, wrinkled, usually early caducous
(rarely absent). Nectary absent. Stamens (four to) numerous (possibly evolved
from a small number), in multiples of two or three. Style present or absent.
Stigmas often connate, Dry type. Placentation sometimes axile or almost axile.
Ovules numerous per carpel, anatropous or campylotropous. Hypostase present.
Outer integument (two to) four to ten cell layers thick. Inner integument two
to four cell layers thick. Parietal tissue two to four cell layers thick.
Nucellar cap approx. three cell layers thick. Antipodal cells uninucleate or
multinucleate. Embryogenesis irregularly solanad. Capsule often also
transversely dehiscing, sometimes siliquiform with replum (rarely a nut or
schizocarp). Exotegmen often with thickened outer cell walls, unlignified;
anticlinal cell walls sometimes sinuate. Endotegmen usually persistent. n =
5–10 (14, 19). Duplication of nuclear gene PAPACYL. – The
gametophytic self-incompatibility system in Papaveraceae is
non-RNase-based (differing from other Triaperturatae).
Eschscholzieae Baill.,
Hist. Plant. 3: 130, 142. 1871
3/13–15. Dendromecon
(2; D. rigida: California, Baja California; D. harfordii:
Channel Islands off California), Eschscholzia
(10–12; southwestern Canada, western United States), Hunnemannia
(1; H. fumariifolia; eastern Mexico). – Western North America,
northern and eastern Mexico. Stem with subepidermal collenchyma. Nodes usually
1:1. Hairs unicellular. Flowers in Eschscholtzia shortly perigynous.
Pollen grains polycolpate. Outer integument in Dendromecon
seven cell layers thick. Capsule explosively dehiscent from base. –
Eschscholzieae are sister-group to
[Papavereae+Chelidonieae].
Papavereae Dumort.,
Fl. Belg.: 130. 1827
11/c 170. Arctomecon (3; A.
californica, A. humilis, A. merriamii; Mojave Desert in
the United States), Argemone (23;
North America, the West Indies, South America, the Hawaiian Islands),
Cathcartia (4; C. cheidoniifolia, C. oliveriana,
C. smithiana, C. villosa; the Himalayas, Tibet, Burma,
China), Meconopsis
(c 50; the Himalayas to western China), ‘Papaver’ (c
80; Europe, the Cape Verde Islands, the Mediterranean, southern Africa,
temperate and subtropical Asia, western North America; paraphyletic),
Roemeria (3; R. hybrida, R. procumbens, R.
refracta; the Mediterranean to Afghanistan; in Papaver?), Romneya (1–2;
R. coulteri, R. trichocalyx; southern California, northern
Baja California), Canbya (2; C. aurea: western United States;
C. candida: western Mojave Desert), Hesperomecon (1; H.
linearis; southern Oregon, California), Meconella (3; M.
californica, M. denticulata, M. oregana; western United
States), Platystemon
(1; P. californicus; western United States, Baja California). –
Temperate regions on the Northern Hemisphere. Nodes often 1:1 or 3:3. Hairs
often multicellular, multiseriate. Hypogyny. Pistil composed of (three or) four
(diagonally arranged) to numerous (in Platystemon
up to c. 25) usually connate (in Platystemon
free) carpels. Style sometimes present. Epistase present. Megasporocytes
sometimes several. Capsule often poricidal, often with replum.
Benzylisoquinoline alkaloids in Papaver
synthesized in sieve tubes. – Papavereae are sister to
Chelidonieae.
Chelidonieae Dumort.,
Fl. Belg.: 130. 1827
9/53. Sanguinaria
(1; S. canadensis; southeastern Canada, eastern United States),
Eomecon (1; E. chionantha; eastern China), Bocconia
(10; Mexico, Central America, the West Indies, northern and central Andes), Dicranostigma
(8; the Himalayas, western China), Eomecon (1; E. chionantha;
eastern China), Glaucium (c
25; western Europe, the Mediterranean, southwestern to Central Asia), Chelidonium (1;
C. majus; Europe, temperate and subarctic Asia), Hylomecon (2;
H. hylomeconoides, H. vernalis; northeastern China, the
Korean Peninsula, Japan), Macleaya (2;
M. cordata: temperate China, Taiwan, Japan; M. microcarpa:
temperate China), Stylophorum
(3; S. sutchuenense: temperate China; S. lasiocarpum:
temperate China; S. diphyllum: eastern United States). – Europe,
Asia, eastern North America, Central and South America, the West Indies. Latex
yellow, orange, or red. Nodes 3–5(–9):3–5(–9). Hairs multicellular,
uniseriate at apex. Perianth in Sanguinaria
dimerous. Pollen grains also polyporate. Pistil sometimes composed of three
connate carpels. Gynophores present in Bocconia. Ovules sometimes one
per carpel (in Bocconia basal). Fruit elongate. Aril often present.
δ-acetylornithine present. – Chelidonieae are sister to
Papavereae.
Fumarioideae Eaton,
Bot. Dict., ed. 4: 46. Apr-Mai 1836 [‘Fumariaceae’]
20/c 520. Mainly temperate regions in
the Northern Hemisphere, southern Africa. Nodes usually 1:1, unilacunar with
one leaf trace (rarely multilacunar with up to five traces). Stem with
subepidermal collenchyma. Latex absent; watery juice present in often
non-articulated idioblasts (probably reduced laticifers). Flowers bisymmetric
or transversely zygomorphic. Tepals in multiples of two. Outer (sepaloid)
tepals minute, not enclosing inner tepals. Inner (petaloid) tepals four, often
spurred. Stamens four or six, free or connate in groups. Nectaries inserted at
staminal bases. Pollen grains sometimes tricolporoidate. Exine often spinulate.
Secondary pollen presentation frequent. Style present, often long. Stigma very
variable. Placentation sometimes axile. Ovules one to numerous per carpel,
campylotropous. Outer integument two to four cell layers thick. Inner
integument approx. two cell layers thick. Parietal tissue approx. four cell
layers thick. Nucellar cap usually absent. Suspensor haustorium present.
Embryogenesis caryophyllad. Fruit sometimes lomentum-like or a nutlet. Seed
curved. Exotesta often palisade. Endotesta without fibrillar reticulum.
Exotegmen not fibrous. Embryo sometimes elongate. n = (6–)8 (or more).
Coularine, δ-acetylornithine, and sometimes berberine present. –
Hypecoum is sister-group of Fumarieae.
Hypecoeae Dumort., Fl.
Belg.: 130. 1827
1/c 20. Hypecoum (c
20; the Mediterranean and eastwards to Central Asia, western and northern
China). – Outer tepals two, sepaloid, anterio-posterior, with open
aestivation. Inner tepals petaloid; outer ones not spurred; inner ones
trilobate. Stamens 2+2. Anthers latrorse, with monothecal median anthers fused
in pairs. Secondary pollen presentation: pollen deposited in bud within pockets
inside innermost tepals, which close prior to stigmatic maturation, and open at
touch of pollinators, these then becoming dusted with pollen. Pollen grains
dicolpate. Stylodia two, partially connate. Inner integument approx. three cell
layers thick. Fruit usually an elongate schizocarp detaching into one-seeded
units (resembling a lomentum), sometimes siliqua-like, acropetalous, and with
replum and persistent placentae. Seed coat with rectangular crystals. Suspensor
consisting of two massive cells. n = 9. – Pteridophyllum
was identified as sister to Hypecoum with
fairly high bootstrap support in Wang & al. (2009), using both
morphological and a number of molecular data. On the other hand, there are
arguments from many other analyses that Pteridophyllum
is instead sister to the entire Papaveraceae.
Fumarieae Dumort.,
Anal. Fam. Plant.: 51. 1829
19/c 500. Lamprocapnos
(1; L. spectabilis; Siberia, northern China, the Korean Peninsula,
Japan), Ehrendorferia (2; E. chrysantha, E.
ochroleuca; California, Baja California), Dicentra (10;
temperate Asia, North America), Ichtyoselmis (1; I.
macrantha; southern China, northern Burma), Adlumia (1; A.
fungosa; southeastern Canada, northeastern United States), Capnoides (1;
C. sempervirens; southeastern Canada, northeastern United States),
Dactylicapnos (10; the Himalayas to Southeast Asia), Corydalis (c
400; temperate regions on the Northern Hemisphere, tropical African mountains),
Cysticapnos
(2–3; C. cracca, C. vesicaria; southern Africa),
Discocapnos (1; D. mundtii; Western Cape),
Trigonocapnos (1; T. lichtensteinii; southern Africa), Fumaria (c 50;
Europe, the Mediterranean to Central Asia and the Himalayas, mountains in
tropical East Africa), Cryptocapnos (1; C. chasmophytica;
Afghanistan), Fumariola (1; F. turkestanica; Central Asia),
Rupicapnos (6; R. africana, R. calcarata, R.
muricata, R. numidica, R. ochracea, R.
sarcocapnoides; southern Spain, northwestern Africa), Pseudofumaria
(2; P. alba: northwestern Balkan Peninsula; P. lutea:
southern Alps), Platycapnos
(3; P. saxicola, P. spicata, P. tenuiloba; the
Iberian Peninsula, western Mediterranean, Macaronesia), Ceratocapnos
(3; C. claviculata, C. heterocarpa, C. turbinata;
western Europe, the Mediterranean), Sarcocapnos
(9; the Iberian Peninsula, western Mediterranean). – Mainly temperate regions
in the Northern Hemisphere (with their highest diversity in Himalaya and
southwestern China), southern and eastern Africa. Nodes sometimes with up to
five traces. Inflorescence often racemose. Flowers transversely zygomorphic or
bisymmetric. Outer (sepaloid) tepals minute. One or two outer petaloid tepals
spurred; inner petaloid tepals connate at apex, not lobate. Stamens in two
groups of three: two median dithecal and four lateral monothecal anthers (some
species of Dicentra with
six separate stamens). Secondary pollen presentation: pollen deposited on
stigma. Stigma Wet type, sometimes flattened, with marginal lobes. Ovule often
one per carpel. Fruit often a nut. Elaiosome often present. Exotesta usually
pigmented. Endotesta sometimes palisade, usually not crystalliferous. Suspensor
cells resembling a bunch of grapes. Embryo sometimes undifferentiated. –
Disymmetry of the flower is a synapomorphy of Fumarioideae and
zygomorphy has evolved secondarily from dissymmetry (Sauquet & al.
2015).
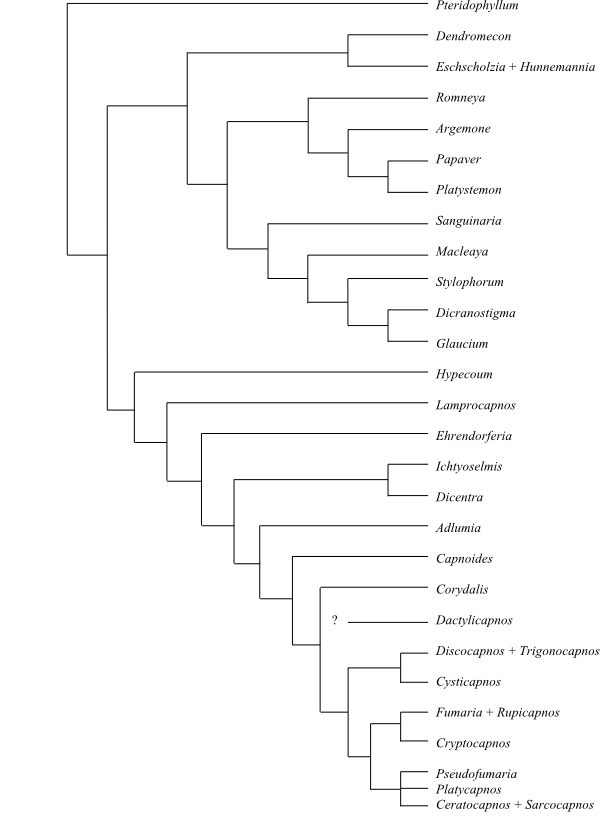 |
Phylogeny of Papaveraceae and Pteridophyllum
(Papaveroideae: strict consensus tree based on morphology and
DNA sequence data, Hoot & al. 1997; Fumarioideae: 50%
majority rule bootstrap tree based on DNA sequence data, Lidén &
al. 1997; Discocapnos to Sarcocapnos:
Bayesian consensus tree of ITS and cpDNA sequences, Pérez-Gutiérrez
& al. 2012, 2015). A similar topology was recovered by Sauquet
& al. (2015). Papaver is
sister to [Argemone+[Romneya+Platystemon]]
with high support, according to Hoot & al. (2015). Likewise,
Eomecon is sister to Sanguinaria.
In the same analysis, Dicentra
is sister to all other Fumarieae except Lamprocapnos,
and Ichtyoselmis successive sister to the rest. Moreover,
Dactylicapnos was found to be sister-group to the clade
[Corydalis+[Cysticapnos+Trigonocapnos]+[Sarcocapnos+[Pseudofumaria+[Rupicapnos+Fumaria]]]].
|
PTERIDOPHYLLACEAE
(Murb.) Nakai ex Reveal et Hoogland
|
( Back to Ranunculales )
|
Reveal et Hoogland in Bull. Mus. Natl. Hist. Nat.
(Paris), sér. 4, sect. B, Adansonia, 13: 91. 4 Oct 1991
Genera/species 1/1
Distribution Japan.
Fossils Unknown.
Habit Bisexual, perennial
herbs. Blackening when drying.
Vegetative anatomy Mycorrhiza
absent. Roots diarch. Phellogen? Secondary lateral growth absent. Vessel
elements with simple perforation plates; lateral pits? Imperforate tracheary
xylem elements libriform fibres with simple pits. Wood rays absent? Axial
parenchyma paratracheal. Sieve tube plastids Ss type. Nodes? Watery juice in
non-articulate idioblasts, probably representing reduced laticifers?
Crystals?
Trichomes Hairs multicellular,
pointed.
Leaves Alternate (spiral, in
basal rosette), pinnately compound (with ten to c. 20 pairs of leaflets) to
pinnetely lobed, with ? ptyxis. Leaf base surrounded by several large round
membranous cataphylls. Stipules and leaf sheath absent. Petiole vascular
bundles? Venation pinnate. Stomata anomocytic. Cuticular wax crystalloids? (as
platelets?). Mesophyll without sclerenchymatous idioblasts. Leaflet margins
serrate-dentate (glandular-serrate?).
Inflorescence Terminal, one-
to four-flowered, raceme-like (scapiflorous with separate one- to four-flowered
cyme).
Flowers Actinomorphic to
slightly bisymmetric. Pedicel thin. Hypogyny. Outer tepals two, with open
aestivation, median, petaloid, caducous, free. Inner tepals 2+2, petaloid,
outer ones with imbricate aestivation, caducous, free. Nectary? Disc absent.
Androecium Stamens four
(lateral two stamens absent; rarely six), in two alternipetalous diagonally
arranged groups. Filaments narrow, free from each other and from tepals.
Anthers basifixed, non-versatile, tetrasporangiate, extrorse, longicidal
(dehiscing by longitudinal slits). Tapetum secretory? Staminodia absent.
Pollen grains
Microsporogenesis simultaneous? Pollen grains usually tricolpate (rarely di- or
tetracolpate), shed as monads, bicellular at dispersal. Exine tectate, with
columellate? infratectum, perforate, echinulate.
Gynoecium Pistil composed of
two connate transverse carpels; carpel occluded by secretion. Ovary superior,
unilocular. Style single, simple, long. Stigmatic lobes commissural; stigmatic
area bifid, densely papillate, Dry? type. Pistillodium absent.
Ovules Placentation parietal.
Ovules usually one (rarely two) per carpel, anatropous (or amphitropous) to
almost campylotropous, bitegmic, crassinucellar. Micropyle endostomal. Outer
integument ? cell layers thick. Inner integument ? cell layers thick.
Megagametophyte monosporous, Polygonum type? Antipodal cells
persistent? Endosperm development nuclear. Endosperm haustoria?
Embryogenesis?
Fruit A many-seeded,
siliqua-like, septicidal (placenticidal) capsule with persistent placental
strands, acropetally dehiscing.
Seeds Aril absent. Seed coat
endotestal. Exotesta more or less collapsing. Endotesta well developed, with
coarse network of cellulose fibrils. Tegmen thin. Perisperm not developed.
Endosperm copious (starchy?). Embryo small, chlorophyll? Cotyledons two.
Germination?
Cytology n = 9
DNA
Phytochemistry Very
insufficiently known. Protopine present. Flavonols? Berberine?
Use Ornamental plant.
Systematics Pteridophyllum
(1; P. racemosum; Honshu in Japan).
Pteridophyllum
was identified as sister to Papaveroideae by Sauquet & al. (2015)
and as sister to Hypecoum with
fairly high bootstrap support in Wang & al. (2009), using both
morphological and a number of molecular data. On the other hand, there are
arguments from many other analyses that Pteridophyllum
is instead sister to the entire Papaveraceae.
de Jussieu, Gen. Plant.: 231. 4 Aug 1789, nom.
cons.
Thalictraceae Raf.,
Anal. Nat.: 176. Apr-Jul 1815 [‘Thalictria’];
Anemonaceae Vest, Anleit. Stud. Bot.: 264, 275. 1818
[’Anemonoideae’]; Helleboraceae Vest,
Anleit. Stud. Bot.: 264, 274. 1818 [‘Helleboroideae’];
Aconitaceae Bercht. et J. Presl, Přir. Rostlin: 216.
Jan-Apr 1820 [‘Aconiteae’]; Calthaceae
Martinov, Tekhno-Bot. Slovar: 92. 3 Aug 1820 [‘Calthoideae’];
Clematidaceae Martinov, Tekhno-Bot. Slovar: 134. 3
Aug 1820 [‘Clematites’]; Actaeaceae
Bercht. et J. Presl, Přir. Rostlin 1 (16*-53): 2, 140. 1823;
Xanthorhizaceae Bercht. et J. Presl, Přir. Rostlin
1(16*-53): 2, 145. 1823 [‘Xanthorhizeae’];
Cimicifugaceae (Arn.) Bromhead in Mag. Nat. Hist., n.
s., 4: 336, 338. Jul 1840 [’Cimicifugeae’];
Ranunculineae J. Presl in Nowočeská Bibl.
[Wšobecný Rostl.] 7:2, 10. 1846 [’Ranunculeae’];
Nigellaceae J. Agardh, Theoria Syst. Plant.: 76.
Apr-Sep 1858; Aquilegiaceae Lilja, Skånes Fl., ed.
2: 375, 861, 979. Apr-Dec 1870; Delphiniaceae
Brenner, Florist. Handb.: 52. 1886; Helleborales
Nakai in J. Jap. Bot. 24: 10. Dec 1949
Genera/species
43–44/1.730–1.880
Distribution Cosmopolitan,
with their largest diversity in temperate regions in the Northern and Southern
Hemispheres.
Fossil Numerous fossilized
fruits and seeds and a few records of fossil leaves are known from Cenozoic
sediments. Leefructus, a Lower Cretaceous fossil with leaves and
reproductive organs found in the Yixian Formation (125.8–123.0 Ma), may be
closely allied to Ranunculaceae (Wang & al.
2016).
Habit Usually bisexual (rarely
dioecious), usually perennial, biennial or annual herbs (in
Xanthorrhiza suffrutices; in Clematis lianas). Some species
of Ranunculus and Caltha are aquatic. Main root often early
withering and replaced by adventitious roots; some species with tuberous roots.
Roots and rhizomes in Xanthorhiza and Coptis and bark in
Xanthorhiza intensively yellow-coloured due to presence of berberine
(a major alkaloid).
Vegetative anatomyPhellogen ab
initio deeply seated. Medullary, primary, rays (in ligneous representatives)
wide, persistent. Primary vascular tissue consisting of one or several
cylinders of vascular bundles, or as scattered bundles, atactostele. Secondary
lateral growth usually absent (sometimes from normal cylindrical cambium).
Xylem usually V-shaped in cross-section, surrounding phloem. Vessel elements
with simple and/or scalariform (sometimes reticulate) perforation plates;
lateral pits alternate, bordered pits. Imperforate tracheary xylem elements
fibre tracheids or libriform fibres with simple pits, non-septate (vasicentric
tracheids usually absent, although present in many species of
Clematis, especially in temperate regions). Wood rays multiseriate,
heterocellular, or absent. Axial parenchyma apotracheal intervascular or scanty
vasicentric (Clematis) (paratracheal parenchyma absent or pervasive).
Wood elements sometimes (Clematis) partially storied. Phloem
surrounded by xylem. Sieve tube plastids Ss type. Nodes usually 3:3, trilacunar
with three leaf traces, or ≥5:≥5, multilacunar with at least five traces
(rarely 1:1, unilacunar with one trace, or 2:2, bilacunar with two traces).
Calciumoxalate crystals rhomboidal or absent.
Trichomes Hairs unicellular
och multicellular, uniseriate; glandular hairs often present.
Leaves Usually alternate
(spiral or sometimes distichous; in Clematis opposite), usually
compound (sometimes simple, sometimes two to several times pinnately compound),
usually lobed (sometimes entire); lamina and/or lobes with supervolute,
involute, curved-involute, or conduplicate ptyxis. Stipules intrapetiolar or
absent; petiole base often sheathing. Petioles in Clematis modified
into tactile tendrils. Petiole vascular bundle transection annular or arcuate.
Venation usually palmate (rarely parallelodromous; in some species of
Ranunculus flabellate, with open dichotomously branched veins).
Stomata usually anomocytic (sometimes paracytic), sometimes on adaxial side of
leaf only, or absent. Cuticular wax crystalloids usually as irregularly shaped
platelets (sometimes as clustered tubuli of Berberis type), chemically
dominated by nonacosan-10-ol, or absent. Hydathodes present in some
representatives. Idioblasts with ethereal oils absent. Leaf margin usually
glandular serrate, with chloranthoid teeth (sometimes crenate or entire).
Extrafloral nectaries present or absent.
Inflorescence Terminal or
axillary, usually cymose, simple or compound, of different types (rarely
racemes), or flowers solitary terminal. Bracts in Anemone often
sepaloid.
Flowers Usually actinomorphic
(in Aconitum and Delphinium zygomorphic). Receptacle usually
elongate (in Myosurus strongly prolonged). Hypogyny. Tepals spiral or
whorled. Outer tepals usually five (sometimes two to four, six or more), with
imbricate or valvate aestivation, sepaloid or petaloid, persistent or early
caducous, usually free. Inner tepals absent or one to 13, often interpreted as
staminodia, usually petaloid, with imbricate or valvate aestivation, free,
often (e.g. in Ranunculus) with one or several nectaries at base (in
Ranunculus giganteus up to c. 30) as nectar-producing pockets or spurs
(in Aquilegia five spurs; nectariferous spurs in Delphinium
pairwise). Nectariferous hairs present on carpels in Caltha. Disc
absent. Androgynophore present in some species of Ranunculus.
Androecium Stamens usually
(secondarily?) abundant (rarely few; in Laccopetalum up to more than
2.000), usually (secondarily?) spiral (rarely whorled). Filaments free from
each other and from tepals. Anthers basifixed to somewhat dorsifixed, usually
non-versatile, tetrasporangiate, extrorse, latrorse or introrse, usually
longicidal (dehiscing by longitudinal slits, rarely by longitudinal valves).
Tapetum usually secretory (in Actaea sometimes
amoeboid-periplasmodial), with binucleate to quadrinucleate cells. Staminodia
extrastaminal, petaloid and/or nectariferous, or absent.
Pollen grains
Microsporogenesis usually simultaneous (in Thalictrum successive).
Pollen grains tri-, hexa- or polycolpate or -porate (zono- or pantoaperturate
or syncolpate; rarely spiraperturate; in ’Souliea’ inaperturate),
shed as monads, usually bicellular (sometimes tricellular) at dispersal. Exine
usually tectate (rarely semitectate), with columellate infratectum, usually
perforate and scabrate or micro-echinate, sometimes spinulate (larger
columellae projecting through tectum) or punctate (in Helleborus
reticulate; in Trollius striate).
Gynoecium Carpels one to more
than 100 (in Ranunculus giganteus to more than 10.000), spiral or
whorled, usually free (sometimes, e.g. in Nigella, more or less
connate, compitum absent); carpel conduplicate-plicate, postgenitally usually
entirely fused, without canal (sometimes ascidiate). Closure by transverse slit
occasionally occurring together with longitudinal slit; closure of carpels
sometimes delayed. Ovary superior, unilocular (apocarpy or gynoecium
monomerous) or trilocular to quinquelocular. Stylodia short to long, or absent.
Stigmas of different types, papillate or non-papillate, Dry type. Pistillodium
absent.
Ovules Placentation marginal
or basal (when ovary unilocular) or axile (when ovary plurilocular). Ovules one
to numerous per carpel, usually anatropous (sometimes hemianatropous),
ascending, horizontal or pendulous, apotropous, usually bitegmic (sometimes
unitegmic, derived from integumentary shifting, especially when ovule single),
crassinucellar or pseudocrassinucellar (especially when unitegmic; in
Anemone-Clematis clade). Micropyle usually endostomal
(sometimes bistomal). Outer integument (two to) four to six (to ten) cell
layers thick. Inner integument (one or) two or three cell layers thick.
Parietal tissue one or two cell layers thick or absent. Nucellar cap present,
two to six cell layers thick (mainly in taxa with a single ovule per carpel),
or absent. Megagametophyte usually monosporous, Polygonum type
(sometimes disporous, Allium type; in Thalictrum etc.
sometimes tetrasporous, Adoxa type). Synergids sometimes with a
filiform apparatus. Antipodal cells usually persistent, often large,
proliferating (sometimes multinucleate). Endosperm development ab initio
nuclear. Endosperm haustoria? Embryogenesis usually onagrad (sometimes solanad,
rarely caryophyllad).
Fruit Usually an assemblage of
follicles or achenes (sometimes a capsule with more or less fused walls, or
single; in some species of Actaea a berry).
Seeds Aril absent. Seed coat
exotestal. Exotesta palisade, with often thickened non-lignified cell walls, or
seeds pachychalazal, with a thin testa. Mesotesta and endotesta unspecialized.
Tegmen unspecialized. Perisperm not developed. Endosperm scarce to copious
(rarely absent), usually starchy. Embryo very small to large, undifferentiated
to well differentiated at dispersal, without chlorophyll. Cotyledons usually
two (in some species of Anemone, Eranthis and
Ranunculus one), often connate (cotyledonary tube often present;
embryo without organs at seed dispersal, further growth intraseminal).
Germination phanerocotylar or cryptocotylar. Radicula often ephemeral.
Cytology x = 8, 9
(Coptoideae), x = 7 (Thalictroideae), x = 6–9
(Ranunculoideae) – Polyploidy and aneuploidy frequent; one group of
genera with small reniform T type (Thalictrum type) chromosomes and a
second group of genera with long two-armed and often curved R type
(Ranunculus type) chromosomes.
DNA Plastid gene infA
lost/defunct (Caltha with pseudogene, Clematis,
Ranunculus). Plastid inverted repeat in Anemone and
Clematis expanded by 4,1 kb into large single copy region. Two
inversions (39 and 24 kb, respectively) in plastid genome of Adonis
Subg. Adonis; one inversion of 42 kb in plastid genome of
Adonis Subg. Adonanthe. Four inversions in plastid genome of
Anemone and Clematis. Two (or four [two parallel]) inversions
in plastid genome of Clematis and at least three species of
Anemone. Plastid gene rps16 lost in Adonis Subg.
Adonis. One intron absent from plastid gene rps12 of
Anemone canadensis, A. dichotoma and A.
richardsonii. Mitochondrial intron coxII.i3 lost. Nuclear gene
AP3 triplicated.
Phytochemistry Flavonols,
(kaempferol, quercetin; frequently additional oxygenation at carbons 6 or 8 of
ring A in contrast to magnoliid clades), cyanidin, caffeic acid, cardioactive
bufadienolides (in Helleborus), Digitalis cardenolides (in
Adonis), diterpenoid alkaloids (e.g. strongly poisonous aconitine and
methyl lycaconitine, in Aconitum and Delphinium), damascenine
(a protoalkaloid), benzylisoquinoline alkaloids (berberine, quaternary
magnoflorine and its precursor corytuberine in Coptoideae and
Thalictroideae), quinolizidine alkaloids (in Actaea etc.),
pyrrolizidine alkaloids as macrocyclic diesters (in Caltha),
tyrosine-derived cyanogenic compounds (abundant in Thalictroideae),
ranunculines (in Ranunculeae, Anemoneae and
Helleborus in Ranunculoideae), triterpene saponins (in
Anemone, Ranunculus etc.), tetracyclic triterpene saponins
with lanostane sapogenins (in Actaea, Beesia and some species
of Thalictrum), and steroidal saponins (in Helleborus etc.),
chelidonic acid present. Ellagic acid and tannins not found. Mannitol
accumulation occurring in Aconitum and Delphinium. Oxalate
accumulation sometimes occurring.
Use Ornamental plants, spices
(seeds of Nigella sativa), medicinal plants, dyeing sources
(Xanthorhiza, Coptis).
Systematics Ranunculaceae may be sister
to the clade [Glaucidiaceae+Hydrastidaceae].
Coptoideae Tamura in
Sci. Rep. Coll. N. Coll. Osaka Univ. 17: 52. 1 Feb 1968
2/10–15. Coptis
(10–15; temperate regions on the Northern Hemisphere), Xanthorhiza
(1; X. simplicissima; southeastern Canada, eastern United States). –
Temperate regions on the Northern Hemisphere. Perennial herbs or suffrutices.
Nectaries five to ten, petaloid, thick, stipitate. Carpels stipitate. n = (8)
9. Chromosomes small and rod-like, T type (Thalictrum
type). Benzylisoquinoline alkaloids present in Coptis. –
Coptoideae may be sister to
[Thalictroideae+Ranunculoideae], although the support is
low.
Thalictroideae Raf.,
Anal. Nat.: 176. Apr-Jul 1815 [‘Thalictrinia’]
7–8/280–340. Thalictrum
(150–200; temperate regions on the Northern Hemisphere, tropical and southern
Africa, New Guinea, tropical America), Leptopyrum
(1; L. fumarioides; western Siberia to East Asia), Paraquilegia
(4; P. anemonoides, P. caespitosa, P. microphylla,
P. uniflora; western Iran to the Himalayas and western China),
Urophysa (2; U. henryi, U. rockii; China), Aquilegia
(100–110; temperate regions on the Northern Hemisphere), Dichocarpum
(18; the Himalayas, East Asia), Enemion
(6–7; E. biternatum, E. hallii, E. leveilleanum,
E. occidentale, E. raddeanum, E. savilei, E.
stipitatum; northeastern Asia, southwestern Canada, western United States;
in Isopyrum?), Isopyrum (3;
I. anemonoides, I. manshuricum, I. thalictroides;
Central Europe, temperate Asia; incl. Enemion?). – Temperate regions
on the Northern Hemisphere, tropical and southern Africa, New Guinea, tropical
America. Hairs capitate. Leaflets with curved-involute ptyxis, papillate.
Stipule-like processes present in Thalictrum.
Nectaries petaloid, stipitate. Intrastaminal staminodia sometimes present.
Integument single, seven or eight cell layers thick. x = 7. Chromosomes small
and reniform, T type (Thalictrum
type). Tyrosine-derived cyanogenic compounds often present. Benzylisoquinoline
alkaloids present in Isopyrum. –
Thalictroideae may be sister to Ranunculoideae.
Ranunculoideae Arn.,
Botany: 94. 9 Mar 1832 [’Ranunculineae’]
c 35/1.440–1.540. Calathodes
(4; C. oxycarpa, C. palmata, C. polycarpa, C.
unciformis; the Himalayas, China, Taiwan), Adonis (c 30;
Europe, temperate Asia), Trollius (c
30; temperate regions on the Northern Hemisphere), Callianthemum
(14–15; Central Europe to Central Asia), Caltha (12;
temperate regions on both hemispheres; section Psychrophila: the Andes
to Ecuador, Tierra del Fuego, Falkland Islands, southeastern Australia,
Tasmania, New Zealand), Helleborus
(c 20; West, Central and South Europe, the Mediterranean to the Caucasus and
northern Syria, one species, H. thibetanus, in Tibet and western
China); Delphinium
(300–320; temperate regions on the Northern Hemisphere),
Staphisagria (3; S. brevipes, S. hirtella, S.
moschata; the Mediterranean), Aconitum
(>250; temperate regions on the Northern Hemisphere), Gymnaconitum
(1; G. gymnandrum; Tibet, western China), Nigella (18;
Europe, the Mediterranean, temperate Asia); Actaea (29;
temperate regions on the Northern Hemisphere), Anemonopsis
(1; A. macrophylla; central Honshu in Japan), Beesia (2;
B. calthifolia, B. deltophylla; western and southwestern
China, northern Burma), Asteropyrum (2; A. cavaleriei, A.
peltatum; China), Eranthis
(8–9; Europe, temperate Asia). – Ranunculeae DC.,
Syst. Nat. 1: 130, 228. 1-15 Nov 1817. Kumlienia (1; K.
hystricula; Sierra Nevada in California), Callianthemoides (1;
C. semiverticillatus; the Andes in southern Chile and southern
Argentina), Hamadryas (7; H. argentea, H. delfini,
H. kingii, H. magellanica, H. paniculata, H.
sempervivoides, H. tomentosa; southern Chile, Tierra del Fuego),
Peltocalathos (1; P. baurii; southern Africa),
Beckwithia (3; B. andersonii, B. camissonis, B.
glacialis; temperate and polar regions on the Northern Hemisphere), Oxygraphis
(4–5; O. delavayi, O. endlicheri, O. polypetala,
O. shaftoanus, O. tenuifolia; temperate Asia), Halerpestes
(8; temperate Asia, Canada, United States), Arcteranthis (1; A.
cooleyae; Alaska, northwestern Canada), Trautvetteria (1; T.
caroliniensis; Japan, Sakhalin, southwestern Canada, western and eastern
United States), Coptidium
(2; C. lapponicum, C. pallasii; cold-temperate and arctic
regions on the Northern Hemisphere), Ficaria (4;
F. fascicularis, F. ficarioides, F. popovii, F.
verna; Europe, the Mediterranean to Central Asia), Myosurus
(5–6; M. apetalus, M. cupulatus, M. minimus,
M. nitidus, M. sessilis; temperate regions on both
hemisphere), Ceratocephala
(5; C. caulifolia, C. falcata, C. furfurascens,
C. pungens, C. testiculata; nearly cosmopolitan),
Krapfia (8–10; the Andes), Laccopetalum (1; L.
giganteum; the Andes in Peru), Ranunculus
(>400; polar, temperate and subtropical regions, tropical mountains),
Paroxygraphis (1; P. sikkimensis; eastern Himalayas). –
Anemoneae DC., Syst. Nat. 1: 129, 168. 1-15 Nov 1817.
Anemone
(160–170; temperate regions on the Northern Hemisphere, East African
mountains, southern Africa, Sumatra, Tasmania, New Zealand, North America to
the Andes in Chile), Clematis
(300–350; temperate regions on the Northern Hemisphere, tropical African
mountains, Madagascar, Pacific islands, South America). – Cosmopolitan. Hairs
clavate. Leaflets usually with involute (sometimes supervolute and/or curved)
ptyxis. Petiole vascular bundle transection sometimes arcuate; wing bundles and
medullary bundles sometimes present. Flowers sometimes zygomorphic. Ovule in
Ranunculeaeand Anemoneaeone per carpel, unitegmic,
pseudocrassinucellar, and integument six to twelve cell layers thick. Micropyle
sometimes bistomal. Parietal tissue one or two cell layers thick or absent.
Nucellar cap usually present. Endosperm development nuclear. Testa sometimes
vascularized. Exotesta sometimes short-palisade. Endotesta sometimes developed.
Embryo sometimes undifferentiated. x = (6–)8(–9). Chromosomes large,
elongated, two-armed and often curved, R type (Ranunculustype).
Lactone-forming glycosides (ranunculin, etc.). Cardenolides and bufadienolides
sometimes present. Berberine absent. Benzylisoquinoline alkaloids sparse or
absent. – Ranunculoideae are paraphyletic (Cossard & al.
2016).
 |
Cladogram (simplified) of Ranunculaceae based
on DNA sequence data (Ro & al. 1997; Wang & al. 2005; Wang
& Chen 2007, Wang & al. 2010, Cossard & al. 2016). The
positions of Actaea+Eranthis,
Adonis,
Asteropyrum, Callianthemum,
Caltha,
Helleborus,
and Nigella,
in particular, are very uncertain, and several other clades also have
low support clades have low support. Staphisagria is sister to
[Consolida+Delphinium]
(Cossard & al. 2016).
|
 |
Cladogram of Ranunculeae based on DNA
sequence data (Emadzade & al. 2010).
|
Literature
Adachi J, Kosuge K, Denda T, Watanabe K.
1995. Phylogenetic relationships of the Berberidaceae based on partial
sequences of the gapA gene. – In: Jensen U, Kadereit JW (eds),
Systematics and evolution of the Ranunculiflorae, Plant Syst. Evol. [Suppl.] 9:
351-353.
Adhikari B, Milne R, Pennington RT, Särkinen
T, Pendry CA. 2015. Systematics and biogeography of Berberis s.l.
inferred from nuclear ITS and chloroplast ndhF gene sequences. – Taxon 64:
39-48.
Ahmad SM, Hoot SB, Qazi PH, Verma V. 2009.
Phylogenetic patterns and genetic diversity of Indian Tinospora
species based on chloroplast sequence data and cytochrome P450 polymorphisms.
– Plant Syst. Evol. 281: 87-96.
Ahrendt LWA. 1961. Berberis and
Mahonia: a taxonomic revision. – Bot. J. Linn. Soc. 57: 1-410.
Aichele D, Schwegler HW. 1957. Die Taxonomie
der Gattung Pulsatilla. – Feddes Repert. 60: 1-230.
Aitzetmüller K. 1995. Fatty acid patterns of
Ranunculaceae seed oils:
phylogenetic relationships. – Plant Syst. Evol. [Suppl.] 9: 229-240.
Aitzetmüller K, Tsevegsüren N. 1994. Seed
fatty acids, ‘Front-end’ – desaturases and chemotaxonomy – a case study
in the Ranunculaceae. – J.
Plant Physiol. 143: 538-543.
Akkemik U, Akalin E, Ozhatay N. 2007.
Ranunculus anatolicus sp. nov. (Ranunculaceae) from northeast
Turkey. – Nord. J. Bot. 25: 311-314.
Al-Eisawi D. 1986. Pollen morphology of Ranunculaceae in Jordan. –
Pollen Spores 28: 311-328.
Aleykutty KM, Inamdar JA. 1980. Structure,
ontogeny and classification of trichomes in Ranales. – Feddes Repert. 91:
95-108.
Arber A. 1931. Studies in floral morphology
II. On the Fumarioideae, with special reference to the androecium. – New
Phytol. 30: 317-354.
Arber A. 1932. Studies in floral morphology
III. On the Hypecoideae with special reference to the androecium. – New
Phytol. 31: 145-173.
Arber A. 1936. Studies in flower structure
II. Vascular supply to the nectary in Ranunculus. – Ann. Bot. 50:
305-319.
Arber A. 1938. Studies in flower structure
IV. On the gynoeceum of Papaver and related genera. – Ann. Bot., N.
S., 2: 649-664.
Archangelsky DB. 1973. Palynological taxonomy
of Berberidaceae. – In:
Kuprianova LA (ed), Pollen and spore morphology of recent plants, Nauka,
Leningrad, pp. 18-21.
Aswal BS. 1985. Meconopsis bikramii
Aswal (Papaveraceae), a new
species from Lahul Valley, Himachal Pradesh, India. – Indian J. Forest 8:
84-85.
Avita S, Inamdar JA. 1980. Structure and
ontogeny of stomata in Ranunculaceae and Paeoniaceae. – Flora
171: 354-370.
Ba TL. 1974. Embryogénie comparée et
phylogénie des Helléborées. – Rev. Gen. Bot. 81: 151-191.
Balfour IB, Smith WW. 1914. Diagnoses
specierum novarum LI-CII (Species Chinenses). Kingdonia uniflora. –
Notes Roy. Bot. Gard. Edinb. 8: 191-192.
Balthazar M von, Pedersen KR, Friis EM. 2005.
Teixeiraea lusitanica, a new fossil flower from the Early Cretaceous
of Portugal with affinities to the Ranunculales. – Plant Syst. Evol.
255: 55-75.
Baltisberger M. 1980. Die Artengruppe des
Ranunculus polyanthemos L. in Europa. – Ber. Schweiz. Bot. Ges. 90:
143-188.
Baltisberger M. 1981.
Verwandtschaftsbeziehungen zwischen der gruppe des Ranunculus
polyanthemos L. und R. repens L. sowie Arten der Gruppen des
R. acris L. und R. bulbosus L. – Bot. Helvetica 91:
61-74.
Baltisberger M. 1994. Ranunculus
cacuminis and R. crenatus, representatives of the R.
alpestris-group (Ranunculaceae) on the Balkan
Peninsula. – Plant Syst. Evol. 190: 231-244.
Baltisberger M, Müller M. 1981.
Vergleichende cytotaxonomische Untersuchungen an Ranunculus seguieri
und der Artengruppe des R. alpestris (Ranunculaceae). – Plant Syst.
Evol. 138: 47-60.
Baltisberger M, Widmer A. 2005. Cytological
investigations on some Ranunculus-species from Crete. – Candollea
60: 335-344.
Barbosa-Filho JM, Leitão da-Cunha EV, Greay
AI. 2000. Alkaloids of the Menispermaceae. – The Alkaloids
54: 1-190.
Barbosa-Filho JM, Leitão da-Cunha EV, Greay
AI. 2000. Alkaloids of the Menispermaceae. – In: Cordell
GA (ed), The alkaloids: Chemistry and biology, vol. 54, Academic Press, San
Diego etc.
Barina Z, Caković D, Pifkó D, Schönswetter
P, Somogyi G, Frajiman B. 2017. Phylogenetic relationships, biogeography and
taxonomic revision of European taxa of Gymnospermium (Berberidaceae). – Bot. J. Linn.
Soc. 184: 298-311.
Barnáth J. 1998. Poppy: the genus
Papaver. – Harwood Academic, Australia.
Barneby RC. 1970. Revision of neotropical Menispermaceae tribe Tinosporeae.
– Mem. New York Bot. Gard. 20: 81-158.
Barneby RC. 1972. New and notable Menispermaceae tribe Tinosporeae.
– Mem. New York Bot. Gard. 22: 137-151.
Barneby RC. 1987. The fruit and tribal
affinity of genus Cionomene (Menispermaceae). – Brittonia
39: 258-259.
Barneby RC, Krukoff BA. 1971. Supplementary
notes on American Menispermaceae VIII. A generic
survey of the American Triclisieae and Anomospermeae. – Mem. New York Bot.
Gard. 22: 1-89.
Barthlott W, Theisen I. 1995. Epicuticular
wax structure and classification of Ranunculiflorae. – Plant Syst. Evol.
[Suppl.] 9: 39-45.
Baumberger H. 1970.
Chromosomenzahlbestimmungen und Karyotypanalysen bei den Gattungen
Anemone, Hepatica, und Pulsatilla. – Ber. Schweiz.
Bot. Ges. 80: 17-96.
Baytop A. 1983. A new Roemeria from
Turkey. – Notes Roy. Bot. Gard. Edinb. 41: 281.
Behnke H-D. 1971. Sieve-tube plastids of
Magnoliidae and Ranunculidae in relation to systematics. – Taxon 20:
723-730.
Behnke H-D. 1995. Sieve-element plastids,
phloem proteins, and the evolution of Ranunculanae. – Plant Syst. Evol.
[Suppl.] 9: 25-37.
Belyaeva NS. 1983. Ultrastructure of chalazal
end of ovule of Delphinium before fertilization. – In: Erdelska O
(ed), Fertilization and embryogenesis in ovulated plants, Veda, Bratislava, pp.
199-203.
Benson L. 1940. The North American
subdivisions of Ranunculus. – Amer. J. Bot. 27: 799-807.
Benson L. 1948. A treatise on the North
American Ranunculi. – Amer. Midl. Natur. 40: 1-264.
Benson L. 1954. Supplement to a treatise on
North American Ranunculi. – Amer. Midl. Natur. 52: 328-369.
Benzing DH. 1967a. Developmental patterns in
stem primary xylem of woody Ranales I. Species with unilacunar nodes. – Amer.
J. Bot. 54: 805-813.
Benzing DH. 1967b. Developmental patterns in
stem primary xylem of woody Ranales II. Species with trilacunar and
multilacunar nodes. – Amer. J. Bot. 54: 813-820.
Berg RY. 1966. Seed dispersal of
Dendromecon: its ecologic, evolutionary, and taxonomic significance.
– Amer. J. Bot. 53: 61-73.
Berg RY. 1967. Megagametogenesis and seed
development in Dendromecon rigida (Papaveraceae). – Phytomorphology
17: 223-232.
Berg RY. 1969. Adaptation and evolution in
Dicentra (Fumariaceae) with special reference to seed, fruit, and
dispersal mechanism. – Nytt Mag. Bot. 16: 49-75.
Berg RY. 1972. Dispersal ecology of
Vancouveria (Berberidaceae). – Amer. J. Bot.
59: 109-122.
Bersillon G. 1955. Rechèrches sur les
Papavéracées. Contribution à l’étude du développement des dicotylédones
herbacées. – Ann. Sci. Nat. Bot., sér. II, 16: 225-443.
Bhandari NN. 1965. Studies in the family Ranunculaceae VIII. Variations in
the development of the embryo sac of Anemone vitifolia. –
Phytomorphology 15: 285-291.
Bhandari NN. 1966. Studies in the family Ranunculaceae IX. Embryology of
Adonis. – Phytomorphology 16: 578-587.
Bhandari NN, Asnani S. 1966. Studies in the
family Ranunculaceae XI.
Morphology and embryology of Ceratocephalus falcatus Per. – Beitr.
Biol. Pflanzen 45: 271-290.
Bhandari NN, Vijayaraghavan MR. 1970. Studies
in the family Ranunculaceae
XII. Embryology of Aquilegia vulgaris. – Beitr. Biol. Pflanzen 46:
337-354.
Bhatnagar SP. 1965. Some observations on the
embryology of Holboellia latifolia Wall. – Curr. Sci. 54: 28-29.
Bhattarai S. 1989. Karyomorphological studies
of four species of Anemone. – Cytologia 54: 709-713.
Bird DA, Franceschi VR, Faccini PJ. 2003. A
tale of three cell types: alkaloid biosynthesis is localized to sieve elements
in opium poppy. – Plant Cell 15: 2626-2635.
Blackmore S, Stafford P, Persson V. 1995.
Palynology and systematics of Ranunculiflorae. – Plant Syst. Evol. [Suppl.]
9: 71-82.
Blanché C. 1990. Delphinium L.
Subgen. Delphinium: origin and evolutionary treds. – Collect. Bot.
(Barcelona) 19: 75-95.
Blanc-Louvel C. 1984. Le genre
“Ranunculus L.” dans le Berriasien (Crétace inf.) de la province
de Lérida (Espagne). – Inst. Est. Ilerdense Diputación Prov. Lleida 45:
83-92.
Blaque G, Maheu J. 1926. Les falsifications
actuelles de l’Hydrastis canadensis. – Bull. Sci. Pharmacol. 33:
375-384.
Blattner FR. 1996. Gattungsbeziehungen
innerhalb der Chelidonioideae (Papaveraceae): Analyse molekularer
und morphologischer Merkmale. – Ph.D. diss., Universität Mainz, Germany.
Blattner FR, Kadereit JW. 1995. Three
intercontinental disjunctions in Papaveraceae subfamily
Chelidonioideae: evidence from chloroplast DNA. – Plant Syst. Evol. [Suppl.]
9: 147-157.
Böcher TW. 1932. Beiträge zur Zytologie der
Gattung Anemone. – Bot. Tidsskr. 42: 183-206.
Böcher TW. 1938. Cytological studies in the
genus Ranunculus. – Dansk Bot. Ark. 9: 1-33.
Böhm H, Dolejs L, Prininger V, Santavy F,
Simanek V. 1975. Isolierung und Chemie die Alkaloide der Pflanzenfamilie Papaveraceae LXVIII. – Planta
Medica 28: 210-214.
Boivin B. 1957. Études thalictrologiques
III. Réduction du genre Anemonella Spach (Ranunculaceae). – Bull. Soc.
Roy. Bot. Belg. 89: 319-321.
Boivin JRB. 1944. American Thalictra
and their Old World allies. – Rhodora 46: 337-377, 391-445, 453-487.
Bonde SD. 1997. Fossil dicotyledonous liana
Anamirta pfeifferi sp. nov. (Menispermaceae) from the Deccan
Intertrappean beds of India. – The Palaeobotanist 47: 89-94.
Boraiah G. 1964. Phylogenetic studies in
Anemone stylosa. – Can. J. Bot. 43: 361-372.
Boraiah G, Heimburger M. 1964. Cytotaxonomic
studies on New World Anemone (section Eriocephalus) with
woody rootstocks. – Can. J. Bot. 42: 891-922.
Botha DJ. 1980a. The endocarp of the southern
African Menispermaceae. – J.
South African Bot. 46: 23-31.
Botha DJ. 1980b. The identity of Antizoma
harveyana Miers ex Harv. and A. capensis (L. f.) Diels. – J.
South Afr. Bot. 46: 1-5.
Bottini MCJ, Bustos A de, Sanso AM, Jouve N,
Poggio L. 2007. Relationships in Patagonian species of Berberis (Berberidaceae) based on the
characterization of rDNA internal transcribed spacer sequences. – Bot. J.
Linn. Soc. 153: 321-328.
Bowers H. 1891. A contribution to the
life-history of Hydrastis canadensis. – Bot. Gaz. (Crawfordsville)
16: 73-82.
Brett JF, Posluszny U. 1982. Floral
development in Caulophyllum thalictroides (Berberidaceae). – Can. J. Bot.
60: 2133-2141.
Briggs BG. 1960. Ranunculus
lappaceus and allied species of the Australian mainland. – Proc. Linn.
Soc. New South Wales 84: 295-324.
Briggs BG. 1994. A new species and new status
in Australian Ranunculus: R. diminutus and R.
acrophilus (Ranunculaceae). – Telopea 5:
583-587.
Britton NL. 1892. The American species of the
genus Anemone and the genera which have been referred to it. – Ann.
New York Acad. Sci. VI: 215-238.
Brouland M. 1935. Recherches sur l’anatomie
florale des Renonculacées. – Botaniste 27: 1-252.
Browicz K. 1976. Genus Bongardia C.
A. Mey. and its range. – Fragm. Flor. Geobot. 22: 435-444.
Brückner C. 1982. Zur Kenntnis der
Fruchtmorphologie der Papaveraceae Juss. s. str. und
Hypecoaceae (Prantl et Kündig) Nak. – Feddes Repert. 93: 153-212.
Brückner C. 1983a. Numerische Methoden in
der Karpomorphologie der Chelidonieae Reichenb. (Papaveraceae). – Gleditschia 10:
71-92.
Brückner C. 1983b. Zur Morphologie der
Samenschale in den Papaveraceae
Juss. s. str. und Hypecoaceae (Prantl et Kündig) Nak. – Feddes Repert. 94:
361-405.
Brückner C. 1984. Zur Narbenform und zur
karpelmorphologischen Stellung der Fumariaceae DC. in den Papaverales. –
Gleditschia 11: 5-16.
Brückner C. 1985a. Frucht- und Samenanatomie
von Pteridophyllum racemosum Sieb. et Zucc. und die Position der
monotypischen Gattung in den Papaverales. – Feddes Rep. 96: 199-213.
Brückner C. 1985b. Zur Samenmorphologie in
Corydalis Vent. (Fumariaceae DC.). – Gleditschia 13: 53-61.
Brückner C. 1992a. Gynoecium morphology and
fruit anatomy in Pseudofumaria Medik. (Fumariaceae), with discussion
of carpellary composition. – Bot. Jahrb. Syst. 114: 251-274.
Brückner C. 1992b. A tool for better
identification of Dicranostigma species (Papaveraceae). – Feddes Repert.
103: 399-409.
Brückner C. 1992c. Gynoecium ontogeny and
carpology in Corydalis DC. section Cheilanthifoliae Lidén
(Fumariaceae). – Flora 187: 299-316.
Brückner C. 1995. Comparative seed structure
in the Ranunculiflorae. – Plant Syst. Evol. [Suppl.] 9: 83-84.
Brückner C. 1996. Carpelloid stamens in Papaveraceae Juss. and Brassicaceae
Burnett (Cruciferae Juss.) and their bearing on theories of gynoecium
organization. – Feddes Repert. 107: 321-337.
Brückner C. 2000. Clarification of the
carpel number in Papaverales, Capparales, and Berberidaceae. – Bot. Rev. 66:
155-307.
Brummitt RK. 2000. Inclusion of
Clematopsis Hutch. in Clematis L. (Ranunculaceae). – Kew Bull. 55:
97-108.
Cai YF, Li SW, Liu Y, Quan S, Chen M, Xie YF,
Jiang HZ, Wei EZ, Yin NW, Wang L, Zhang R, Huang CL, He XH, Jiang MF. 2009.
Molecular phylogeny of Ranunculaceae based on internal
transcribed spacer sequences. – African J. Biotechn. 8: 5215-5224.
Candau P, Fernandez-Paniagua I. 1985. Polen
en Papaveraceae de Andalucia
occidental. – An. Asoc. Palinol. Leng. Esp. 2: 25-34.
Candau P, Soler A. 1981. Contribucion à la
palinologia de la familia Fumariaceae en la Peninsula Iberica. – Bot.
Macaronesica 8-9: 147-162.
Cao YN, Comes HP, Sakaguchi S, Chen LY, Qiu
YX. 2016. Evolution of East Asia’s Arcto-Tertiary relict Euptelea
(Eupteleaceae) shaped by Late
Neogene vicariance and Quaternary climate change. – BMC Evol. Biol. 16:
166.
Carlquist SJ. 1984. Wood and stem anatomy of
Lardizabalaceae, with
comments on the vining habit, ecology, and systematics. – Bot. J. Linn. Soc.
88: 257-277.
Carlquist SJ. 1995a. Wood and bark anatomy of
Ranunculaceae (including
Hydrastis) and Glaucidiaceae. – Aliso 14:
65-84.
Carlquist SJ. 1995b. Wood anatomy of Berberidaceae: ecological and
phylogenetic considerations. – Aliso 14: 85-103.
Carlquist SJ. 1995c. Wood anatomy of
Ranunculiflorae: a summary. – Plant Syst. Evol. [Suppl.] 9: 11-24.
Carlquist SJ. 1996. Wood and stem anatomy of
Menispermaceae. – Aliso 14:
155-170.
Carlquist SJ, Zona S. 1988. Wood anatomy of
Papaveraceae, with comments on
vessel restriction patterns. – IAWA Bull., N. S., 9: 253-267.
Carlquist SJ, Schneider EL, Miller RB. 1994.
Wood and bark anatomy of Argemone (Papaveraceae). – IAWA J. 15:
247-255.
Carolan JC, Hook ILI, Chase MW, Kadereit JW,
Hodkinson TR. 2006. Phylogenetics of Papaver and related genera based
on DNA sequences from ITS nuclear ribosomal DNA and plastid trnL
intron and trnL-F intergenic spacers. – Ann. Bot. (Oxford) 98:
141-155.
Chang H-L. 2005. Floral morphogenesis and
metamorphose in Ranunculaceae
and their systematic significance. – Ph.D. diss., Northwest University,
Xi’an, People’s Republic of China.
Chang K-T, Wang P-L. 1983. Study on the
pollen morphology of the family Berberidaceae. – Acta
Phytotaxon. Sin. 21: 130-142.
Chapman M. 1936. Carpel anatomy of the Berberidaceae. – Amer. J. Bot.
23: 340-348.
Chartier M, Dressler S, Schönenberger J,
Mora AR, Sarthou C, Wang W, Jabbour F. 2016. The evolution of afro-montane
Delphinium (Ranunculaceae): morphospecies,
phylogenetics and biogeography. – Taxon 65: 1313-1327.
Chaturvedi M, Datta K, Pal M. 1999 [2000].
Pollen anomaly – a clue to natural hybridity in Argemone (Papaveraceae). – Grana 38:
339-342.
Chen IS. 1975. The constituents of
Tinospora dentata Diels. – J. Chin. Chem. Soc.. 22: 271-274. [In
Chinese]
Cheng J, Xie L. 2014. Molecular phylogeny and
historical biogeography of Caltha (Ranunculaceae) based on analyses
of multiple nuclear and plastid sequences. – J. Syst. Evol. 52: 51-67.
Christenhusz MJM. 2012. An overview of Lardizabalaceae. – Curtis’s
Bot. Mag. 29: 235-276.
Chuang H. 1981. The systematic evolution and
the geographical distribution of Meconopsis Vig. – Acta Bot. Yunnan.
3: 139-146.
Clark C. 1978a. Pollen shed as tetrads by
plants of Eschscholzia californica (Papaveraceae). – Madroño 25:
59-60.
Clark C. 1978b. Systematic studies of
Eschscholzia (Papaveraceae) I. The origin and
affinities of E. mexicana. – Syst. Bot. 3: 374-385.
Clark C, Jernstedt JA. 1978. Systematic
studies of Eschscholzia (Papaveraceae) II. Seed coat
microsculpturing. – Syst. Bot. 3: 386-402.
Coles SM. 1971. The Ranunculus acris
complex in Europe. – Watsonia 8: 237-262.
Colombo ML, Tome F, Bugatti C. 1991. Lipid
content and fatty acid composition in hypogeous organs of Helleborus
species (Ranunculaceae). –
Plant Syst. Evol. 178: 55-63.
Compton JA, Culham A. 2002. Phylogeny and
circumscription of tribe Actaeeae (Ranunculaceae). – Syst. Bot. 27:
502-511.
Compton JA, Hedderson TAJ. 1997. A
morphometric analysis of the Cimicifuga foetida L. complex (Ranunculaceae). – Bot. J. Linn.
Soc. 123: 1-23.
Compton JA, Culham A, Jury SL. 1998.
Reclassification of Actaea to include Cimicifuga and
Souliea (Ranunculaceae): phylogeny inferred
from morphology, nrDNA ITS, and cpDNA trnL-F sequence variation. –
Taxon 47: 593-634.
Compton JA, Culham A, Gibbings JG, Jury SL.
1998. Phylogeny of Actaea including Cimicifuga (Ranunculaceae) inferred from nrDNA
ITS sequence variation. – Biochem. Syst. Ecol. 26: 185-197.
Cook CDK. 1962. Studies in
Ranunculus L. subgenus Batrachium (DC.) A. Gray I. Chromosome
numbers. – Watsonia 5: 123-126.
Cook CDK. 1963. Studies in
Ranunculus L. subgenus Batrachium (DC.) A. Gray II. General
morphological considerations in the taxonomy of the subgenus. – Watsonia 5:
294-303.
Cook CDK. 1966. A monographic study of
Ranunculus subgenus Batrachium (DC.) A. Gray. – Mitt. Bot.
Staatssamml. München 6: 47-237.
Cook CDK. 1970. Hybridization in the
evolution of Batrachium. – Taxon 19: 161-166.
Coonen LP. 1939. The chromosomes of
Ranunculus. – Amer. J. Bot. 26: 48-58.
Cossard G, Sannier J, Sauquet H, Damerval C,
Ronse de Craene L, Jabbour F, Nadot S. 2016. Subfamilial and tribal
relationships of Ranunculaceae:
evidence from eight molecular markers. – Plant Syst. Evol. 302: 419-431.
Cresson RA, Schneider EL. 1988. Ovule and
seed structure in Argemone aurantiaca (Papaveraceae). – Bull. Torrey
Bot. Club 115: 108-112.
Cumbie BG.1983. Developmental changes in the
wood of Bocconia vulcanica Donn. Smith. – IAWA Bull., N. S. 4:
131-140.
Dahl ÅE. 1989. Taxonomic and morphological
studies in Hypecoum sect. Hypecoum (Papaveraceae). – Plant Syst.
Evol. 163: 227-280.
Dahl ÅE. 1990. Infrageneric division of
Hypecoum (Papaveraceae). – Nord. J. Bot.
10: 129-140.
Dahl ÅE. 1992. Artificial crossing
experiments within Hypecoum sect. Hypecoum (Papaveraceae). – Nord. J. Bot.
12: 13-29.
Dahl ÅE, Wassgren A-B, Bergström G. 1990.
Floral scents in Hypecoum Sect. Hypecoum (Papaveraceae): chemical composition
an relevance to taxonomy and mating system. – Biochem. Syst. Ecol. 18:
157-168.
Dahlgren G. 1991. Karyological investigations
in Ranunculus subg. Batrachium on the Aegean islands. –
Plant Syst. Evol. 177: 193-211.
Dahlgren G. 1995. Differentiation patterns in
Ranunculus subgenus Batrachium (Ranunculaceae). – Plant Syst.
Evol. [Suppl.] 9: 305-317.
Damerval C, Nadot S. 2007. Evolution of
perianth and stamen characteristics with respect to floral symmetry in Ranunculales. – Ann. Bot. 100:
631-640.
Damerval C, Le Guilloux M, Jager M, Charon C.
2007. Diversity and evolution of CYCLOIDEA-like TCP genes in relation
to flower development in Papaveraceae. – Plant Physiol.
143: 759-772.
Damerval C, Citerne H, Le Guilloux M,
Domenichini S, Dutheil J, Ronse de Craene L, Nadot S. 2013. Asymmetric
morphogenetic cues along the transverse plane: shift from dissymmetry to
zygomorphy in the flower of Fumarioideae. – Amer. J. Bot. 100: 391-402.
Daumann E. 1969. Blütenmorphologie und
Bestäubungsökologie einiger Ranunculaceen (Cimicifuga L.,
Actaea L., Thalictrum L.). – Preslia 41: 213-219.
Davis KC. 1900. Native and cultivated
Ranunculi of North America and segregated genera. – Minn. Bot. Stud.
2: 459-507.
Davis PH. 1961. Materials for a flora of
Turkey IV. Ranunculaceae II.
Ranunculus L. – Notes Roy. Bot. Gard. Edinb. 23: 103-161.
Debnath HS, Nayar MP. 1989. A new species of
Meconopsis Vig. (Papaveraceae) from Nepal. – J.
Jap. Bot. 64: 157-159.
Décamps O. 1974. Types stomatiques chez les
Renonculacées. – Compt. Rend. Acad. Sci. Paris 279 D: 1527-1529.
Dekker AJFM. 1983. A revision of the genera
Penianthus Miers and Sphenocentrum Pierre (Menispermaceae) of West and
Central Africa. – Bull. Jard. Bot. Natl. Belg. 53: 17-66.
Delpino F. 1899. Rapporti tra la evoluzione e
la distribuzione geographica delle Ranunculaceae. – Mem. Reale
Accad. Sci. Inst. Bologna 8: 17-66.
De Maggio AE, Wilson CL. 1986. Floral
structure and organogenesis in Podophyllum peltatum L. (Berberidaceae). – Amer. J. Bot.
73: 21-32.
Dermen H. 1931. A study of chromosome number
in two genera of Berberidaceae:
Mahonia and Berberis. – J. Arnold Arbor. 12: 281-287.
Derviz-Sokolova TG, Elenevskij AG, Bulanyj
JI. 1986. Revizija ljutikov (Ranunculus L., Ranunculaceae) gruppy
Ranunculus dissectus Bieb. s.l. – Bjull. Moskovsk. Obšč. Isp.
Prir., Otd. Biol. 91: 101-108.
Despres L, Gielly L, Redoutet B, Taberlet P.
2003. Using AFLP to resolve phylogenetic relationships in a morphologically
diversified plant species complex when nuclear and chloroplast sequences fail
to reveal variability. – Mol. Phylogen. Evol. 27: 185-196.
Devar KV, Boraiah G, Khaleel TF. 1979.
Cytology of Clematis gouriana and Naravelia zeylanica. –
Bot. Not. 132: 310.
De Wet H, Wyk B-E van. 2008. An
ethnobotanical survey of southern African Menispermaceae. – South Afr. J.
Bot. 74: 2-9.
Dickson J. 1935. Studies in floral anatomy
II. The floral anatomy of Glaucium flavum with reference to other
members of the Papaveraceae. –
Bot. J. Linn. Soc. 50: 175-224.
Diels L. 1915. Neue Menispermaceae von Papuasien. –
Engl. Bot. Jahrb. Syst. 52: 187-190.
Diels L. 1921. Menispermaceae madagascarienses
novae. – Feddes Repert. 17: 312-313.
Diels L. 1931. Aufklärung der Gattung
Leichhardtia F. v. M. – Notizbl. Bot. Gart. Berlin-Dahlem 11:
308-310.
Diels L. 1932. Circaeaster: eine
hochgradig reduzierte Ranunculaceae. – Beih. Bot.
Centralbl. 49: 55-60.
Diosdado JC, Pastor JE. 1991. Estudios
cariosistemàticos del género Ranunculus sect.
Ranunculastrum DC. en la peninsula Ibérica. – Lgascalia 16:
269-290.
Diosdado JC, Pastor JE. 1991. Estudio
citotaxonómico del género Ranunculus L. sect. Flammula
(Webb ex Spach) Freyn en la Península Ibérica. – Candollea 46: 303.
Diosdado JC, Pastor JE. 1996. Consideraciones
citotaxonómicas del género Ranunculus L. (Ranunculaceae) en la Península
Ibérica. – An. Jard. Bot. Madrid 54: 166-178.
Donmez A, Mutlu B. 2004. A new species of
Nigella (Ranunculaceae) from Turkey. –
Bot. J. Linn. Soc. 146: 251-255.
Doria G, Jaramillo CA, Herrera F. 2008. Menispermaceae from the Cerrejón
Formation, middle to late Paleocene, Colombia. – Amer. J. Bot. 95:
954-973.
Doroszewska A. 1974. The genus
Trollius L.: a taxonomical study. – Monogr. Bot. 41: 1-167.
D’Ovidio R, Marchi P. 1990. DNA content,
karyotype structure analysis and karyotype symmetry in Ranunculus L.
(Ranunculaceae). Italian
species belonging to sections Flammula (Webb) Benson and
Micranthus (Ovcz.) Nyarady. – Caryologia 43: 99-115.
Drummond JR, Hutchinson J. 1920. A revision
of Isopyrum (Ranunculaceae) and its nearer
allies. – Kew Bull. 1920: 145-169.
Duncan T. 1980. A cladistic analysis of the
Ranunculus hispidus complex. – Taxon 29: 441-454.
Duncan T, Keener CS. 1991. A classification
of the Ranunculaceae with
special reference to the Western Hemisphere. – Phytologia 70: 24-27.
Duncan T, Perez T. 1979. Chromosomes of
Oreithales (Ranunculaceae). – Amer. J. Bot.
66: 989-990.
Echlin P, Godwin H. 1968a. The ultrastructure
and ontogeny of pollen in Helleborus foetidus L. II. Pollen grain
development through the callose special wall stage. – J. Cell Sci. 3:
175-186.
Echlin P, Godwin H. 1968b. The ultrastructure
and ontogeny of pollen in Helleborus foetidus L. III. The formation of
the pollen grain wall. – J. Cell Sci. 5: 459-477.
Ehdaie M, Russell SD. 1984. Megagametophyte
development of Nandina domestica and its taxonomic implications. –
Phytomorphology 34: 221-225.
Ehrendorfer F, Samuel R. 2001. Contributions
to a molecular phylogeny and systematics of Anemone and related genera
(Ranunculaceae – Anemoninae).
– Acta Phytotaxon. Sin. 39: 293-307.
Ehrendorfer F, Ziman SN, König C, Keener CS,
Dutton BE, Tsarenko ON, Bulakh EV, Boscaiu M, Médail F, Kästner A. 2009.
Taxonomic revision, phylogenetics and transcontinental distribution of
Anemone section Anemone (Ranunculaceae). – Bot. J. Linn.
Soc. 160: 312-354.
Eichler H. 1958. Revision der Ranunculaceen
Malesiens. – Bibl. Bot. 124: 1-110.
Elenevskij AG, Derviz-Sokolova TG. 1989.
Ljutiki (Ranunculus L., Ranunculaceae) Kavkaza. – Bjull.
Moskovsk. Obšč. Isp. Prir., Otd. Biol. 94: 112-123.
Emadzade K, Hörandl E. 2011. Northern
hemisphere origin, transoceanic dispersal, and diversification of Ranunculeae
DC. (Ranunculaceae) in the
Cenozoic. – J. Biogeogr. 38: 517-530.
Emadzade K, Lehnebach C, Lockhart P, Hörandl
E. 2010. A molecular phylogeny, morphology and classification of genera of
Ranunculeae (Ranunculaceae).
– Taxon 59: 809-828.
Emadzade K, Gehrke B, Linder HP, Hörandl E.
2011. The biogeographical history of the cosmopolitan genus Ranunculus
L. (Ranunculaceae) in the
temperate to meridional zones. – Mol. Phylogen. Evol. 58: 4-21.
Emura K. 1972. Cytotaxonomic studies on the
genus Thalictrum in Eurasia with special reference to Japanese
species. – J. Fac. Univ. Tokyo, Sect. III, Bot. 9: 93-135.
Endress PK. 1969. Gesichtspunkte zur
systematischen Stellung der Eupteleaceen (Magnoliales).
Untersuchungen über Bau und Entwicklung der generativen Region bei
Euptelea polyandra Sieb. & Zucc. – Ber. Schweiz. Bot. Ges. 79:
229-278.
Endress PK. 1989. Chaotic floral phyllotaxis
and reduced perianth in Achlys (Berberidaceae). – Bot. Acta 102:
159-163.
Endress PK. 1993. Eupteleaceae. – In: Kubitzki K,
Rohwer JG, Bittrich V (eds), The families and genera of vascular plants II.
Flowering plants. Dicotyledons. Magnoliid, hamamelid and caryophyllid families,
Springer, Berlin, Heidelberg, New York, pp. 299-301.
Endress PK. 1995. Floral structure and
evolution in Ranunculanae. – Plant Syst. Evol. [Suppl.] 9: 47-61.
Engell K. 1995. Embryo morphology of the Ranunculaceae. – Plant Syst.
Evol. [Suppl. 9]: 207-216.
Engler A. 1897a. Ranunculaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien, Nachträge zu III(2), W.
Engelmann, Leipzig, pp. 167-170.
Engler A. 1897b. Menispermaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien, Nachträge zu III(2), W.
Engelmann, Leipzig, pp. 170-172.
Erbar C. 1998. Coenocarpie ohne und mit
Compitum: ein Vergleich der Gynoeceen von Nigella (Ranunculaceae) und
Geranium (Geraniaceae). –
Beitr. Biol. Pflanzen 71: 13-39.
Erbar C, Kusma S, Leins P. 1999. Development
and interpretation of nectary organs in Ranunculaceae. – Flora 194:
317-332.
Erickson RO 1943. Taxonomy of
Clematis section Viorna. – Ann. Missouri Bot. Gard. 30:
1-62.
Ernst WR. 1962. A comparative morphology of
the Papaveraceae. – Ph.D.
diss., Stanford University, California.
Ernst WR. 1967. Floral morphology and
systematics of Platystemon and its allies Hesperomecon and
Meconella (Papaveraceae: Platystemonoideae).
– Univ. Kansas Sci. Bull. 47: 25-70.
Exell AW. 1960. 11. Papaveraceae; 12. Fumariaceae. –
In: Exell AW, Wild H (eds), Flora Zambesiaca 1 (Part 1), Crown Agents for
Oversea Governments and Administrations, London, pp. 180-181.
Exell AW, Milne-Redhead E. 1960. 4. Ranunculaceae. – In: Exell AW,
Wild H (eds), Flora Zambesiaca 1 (Part 1), Crown Agents for Oversea Governments
and Administrations, London, pp. 89-102.
Eymé J. 1963. Observations cytologiques sur
les nectaires de trios Renonculacées (Helleborus foetidus L., H.
niger L., Nigella damascena L.). – Le Botaniste 46: 137-179.
Eymé J, Le Blanc M. 1963. Contribution à
l’étude inframicroscopique d’inclusions cytoplasmiques présentes dans les
ovules de Ficaria et dans les nectaries d’Helleborus. –
Compt. Rend. Acad. Sci. Paris 256: 4958-4959.
Ezelarab GE, Dormer KJ. 1963. The
organization of the primary vascular system in Ranunculaceae. – Ann. Bot. N.
S., 27: 23-38.
Ezelarab GE, Dormer KJ. 1966. The
organization of the primary vascular system in the Rhoeadales. – Ann. Bot. N.
S., 30: 123-132.
Fabergé AC. 1942. Genetics of the
Scapiflora section of Papaver I. The garden Icelandic poppy.
– J. Genet. 44.
Fahselt D. 1972. The use of flavonoid
components in the characterization of the genus Corydalis
(Fumariaceae). – Can J. Bot. 50: 1605-1611.
Fairbairn JW, Williamson EM. 1978. Meconic
acid as a chemotaxonomic marker in the Papaveraceae. – Phytochemistry
17: 2087-2089.
Fay HAC. 1996. Evolutionary and taxonomic
relationships between fruit-piercing moths and the Menispermaceae. – Aust. Syst.
Bot. 9: 227-233.
Fedde F. 1905. Die geographische Verbreitung
der Papaveraceae. – Engl. Bot.
Jahrb. Syst. 36, Beiblatt 81: 28-43.
Fedde F. 1936. Papaveraceae. – In: Engler A
(†), Harms H (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 17b, W.
Engelmann, Leipzig, pp. 5-145.
Ferguson IK. 1975. Pollen morphology of the
tribe Triclisieae of the Menispermaceae in relation to its
taxonomy. – Kew Bull. 30: 49-75.
Ferguson IK. 1978. Pollen morphology of the
tribe Coscinieae of the Menispermaceae in relation to its
taxonomy. – Kew Bull. 32: 339-346.
Fernandez I. 1986. Contribucion al
conocimiento palinologico de la familia Ranunculaceae en Andalucia: 1.
Subf. Helleboroideae. – Lagascalia 14: 13-23.
Fisher FJF. 1965. The alpine
Ranunculi of New Zealand. – New Zealand Dept. Sci. Ind. Res. Bull.
165: 1-192.
Floden AJ, Schilling EE. 2018.
Trautvetteria fonticalcarea (Ranunculaceae: Ranunculeae), a new
tassel rue species endemic to calcareous seepage habitats in Tennessee, USA.
– Nord. J. Bot. 36: e01738
Flovik K. 1936. The somatic chromosomes of
certain arctic species of the genus Ranunculus. – Soc. Sci. Fenn.
Comm. Biol. 5: 1-17.
Folkestad K, Høiland K, Paulsen BS, Malterud
KE. 1988. Alkaloid chemotaxonomy of Nordic Papaver sect.
Scapiflora (Papaveraceae). – Nord. J. Bot. 8:
139-146.
Forgacs P, Buffard G, Jehanno A, Provost J,
Tiberghien R, Touche A. 1982. Composition chimique des Fumariacées.
Alcaloïdes de quatorce espèces de Fumaria. – Plant Med. Phytother.
16: 99-115.
Forman LL. 1956. The Menispermaceae of Malaysia I. –
Kew Bull. 1956: 41-69.
Forman LL. 1968. The Menispermaceae of Malesia V.
Tribe Cocculeae Hook. f. and Thomson. – Kew Bull. 22: 349-374.
Forman LL. 1974. The endocarps of
Cocculus (Menispermaceae). – Kew Bull.
29: 477-481.
Forman LL. 1975. The tribe Triclisieae Diels
in Asia, the Pacific and Australia. – Kew Bull. 30: 77-100.
Forman LL. 1981. The Menispermaceae of Malesia and
adjacent areas X. A revision of Tinospora (Menispermaceae) in Asia to
Australia and the Pacific. – Kew Bull. 36: 375-421.
Forman LL. 1982a. The correct names for the
tribes of Menispermaceae. –
Kew Bull. 37: 367-368.
Forman LL. 1982b. New Australian Menispermaceae. – Kew Bull. 37:
369-373.
Forman LL. 1984. The Menispermaceae of Malesia and
adjacent areas XII. A revision of tribe Tinosporeae (Menispermaceae) in Asia,
Australia and the Pacific. – Kew Bull. 39: 99-116.
Forman LL. 1985. The Menispermaceae of Malesia and
adjacent areas XIII. A revision of tribe Fibraureae (Menispermaceae) in Asia. – Kew
Bull. 40: 539-551.
Forman LL. 1986. Menispermaceae. – In: Steenis
CGGJ van, Wilde WJJO de (eds), Flora malesiana I, 10(2), Martinus Nijhoff, The
Hague, Boston, London, pp. 157-253.
Forman LL. 1988. A synopsis of Thai Menispermaceae. – Kew Bull. 43:
369-407.
Forman LL. 1997. A synopsis of
Hypserpa Miers (Menispermaceae). – Kew Bull.
52: 981-987.
Förster P. 1997. Die Keimpflanzen der Tribus
Ranunculeae DC. und der Tribus Adonideae Kunth (Ranunculaceae). – Flora 192:
133-142.
Foster AS. 1959. The morphological and
taxonomic significance of dichotomous venation in Kingdonia uniflora
Balfour f. et W. W. Smith. – Notes Roy. Bot. Gard. Edinb. 23: 1-12.
Foster AS. 1961. The floral morphology and
relationships of Kingdonia uniflora. – J. Arnold Arbor. 41:
397-410.
Foster AS. 1963. The morphology and
relationships of Circaeaster. – J. Arnold Arbor. 44: 299-327.
Foster AS. 1968. Further morphological
studies on anastomoses in the dichotomous venation of Circaeaster. –
J. Arnold Arbor. 49: 52-67.
Foster AS. 1970. Types of blind vein-endings
in the dichotomous venation of Circaeaster. – J. Arnold Arbor. 51:
70-80.
Foster AS. 1971. Additional studies on the
morphology of blind vein-endings in the leaf of Circaeaster agrestis.
– Amer. J. Bot. 58: 263-272.
Foster AS, Arnott HJ. 1960. Morphology and
dichotomous vasculature of the leaf of Kingdonia uniflora. – Amer.
J. Bot. 47: 684-698.
Francini E. 1931. Lo sviluppo del sistema
conduttore in plantule di Hydrastis canadensis L. – Nuovo Giorn.
Bot. Ital. 38: 336-357.
Friedel J. 1938a. Note sur la structure
anatomique du Pteridophyllum racemosum Sieb. et Zucc. – Bull. Soc.
Bot. France 85: 406-408.
Friedel J. 1938b. Anatomie comparée du
Pteridophyllum racemosum Sieb. et Zucc. et du Platystemon
californicum Benth. – Bull. Soc. Bot. France 85: 482-486.
Friis EM, Pedersen KR, Crane PR. 1995.
Appomattoxia ancistrophora gen. et sp. nov., a new Early Cretaceous
plant with similarities to Circaeaster and extant Magnoliidae. –
Amer. J. Bot. 82: 933-943.
Fu D-Z. 1988. A study on Dichocarpum
(Ranunculaceae). – Acta
Phytotaxon. Sin. 26: 249-264.
Fu D-Z. 1990. Phylogenetic considerations on
the subfamily Thalictroideae (Ranunculaceae). – Cathaya 2:
181-190.
Fukuhara T. 1992. Seed coat anatomy of
Japanese species of Corydalis and Dicentra (Papaveraceae: Fumarioideae). –
Bot. Mag. (Tokyo) 105: 303-321.
Fukuhara T. 1995. Vascular pattern in the
fruit of Trigonocapnos and Discocapnos (Papaveraceae; Fumarioideae). –
Intern. J. Plant Sci. 156: 547-554.
Fukuhara T. 1999. Seed and funicle morphology
of Fumariaceae-Fumarioideae: systematic implications and evolutionary patterns.
– Intern. J. Plant Sci. 160: 151-180.
Fukuhara T, Lidén M. 1995a. Pericarp anatomy
in Fumariaceae-Fumarioideae. – Bot. Jahrb. Syst. 117: 499-530.
Fukuhara T, Lidén M. 1995b. Seed-coat
anatomy in Fumariaceae-Fumarioideae. – Bot. J. Linn. Soc. 119: 323-365.
Furness CA. 2008. Successive
microsporogenesis in eudicots, with particular reference to Berberidaceae (Ranunculales). – Plant Syst.
Evol. 273: 211-223.
Gajewski W. 1946. Cytotaxonomic
investigations on Anemone L. I. Anemone janczewskii, a new
amphidiploid species of hybrid origin. – Acta Soc. Bot. Pol. 17: 129-194.
Garnock-Jones PJ. 1984. Ceratocephalus
pungens (Ranunculaceae): a
new species from New Zealand. – New Zealand J. Bot. 22: 135-137.
Gáyer G. 1909a. Vorarbeiten zu einer
Monographie der europäischen Aconitum-Arten. – Magyar Bot. Lapok 8:
114-206.
Gáyer G. 1909b. Die Aconitum-Arten
der Karpathen. – Allg. Bot. Zeit. 15: 109-112, 133-135.
Gáyer G. 1909c. Vorarbeiten zu einer
Monographie der europäischen Aconitum-Arten 5: Sectio
Lycoctonum. – Magyar Bot. Lapok 8: 310-327.
Gehrke B, Linder HP. 2009. The scramble for
Africa: pan-temperate elements on the African high mountains. – Proc. Roy.
Soc., Ser. B, 276: 2657-2665.
Ghimire B, Shin D-Y, Heo K. 2010. Embryology
of Gymnospermium microrrhynchum (Berberidaceae). – Korean J.
Plant Taxon. 40: 226-233.
Gilli A. 1954. Neue Ranunculaceae und Papaveraceae aus Afghanistan. –
Feddes Repert. 69: 155-175.
Gleissberg S, Kadereit JW. 1999. Evolution of
leaf morphogenesis: evidence from developmental and phylogenetic data in Papaveraceae. – Intern. J. Plant
Sci. 160: 787-794.
Goepfert D. 1974. Karyotypes and DNA content
in species of Ranunculus L. and related genera. – Bot. Not. 127:
464-489.
Goepfert D. 1976. Phylogenetic studies on
Ranunclus cortusifolius Willd. (Ranunculaceae), a Macaronesian
endemic species. – Bot. J. Linn. Soc. 72: 161-170.
Gomez B, Coiffard C, Sender LM, Luis M,
Martín-Closas C, Villanueva-Amado U, Ferrer J. 2009.
Klitzschophyllites, aquatic basal eudicots (Ranunculales?) from the Upper
Albian (Lower Cretaceous) of northeastern Spain. – Intern. J. Plant Sci. 170:
1075-1085.
Gonnermann C. 1980. Beiträge zur Kenntnis
der Gynoeceumsstruktur der Papaveraceae Juss. s. str. –
Feddes Repert. 91: 593-613.
Gonnermann C. 1982. Überblick über die
Testa-Morphologie der Papaveraceae Juss. s. str. –
Gleditschia 9: 17-25.
Gorysina TK. 1965. Anatomical structure of
leaves of prevernal nemoral ephemeroids. – Vestn. Leningr. Univ. 20, no. 3
(ser. Biol. no 1): 45-51. [In Russian with English summary]
Götz E. 1967. Die Aconitum
variegatum-Gruppe und ihre Bastarde in Europa. – Feddes Repert. 76:
1-62.
Grant V. 1976. Isolation between
Aquilegia formosa and A. pubescens: a reply and
reconsideration. – Evolution 30: 625-628.
Gray A. 1886. Contribution to American botany
1. A revision of the North American Ranunculi. – Proc. Amer. Acad. Sci. Arts
21: 363-378.
Gregory WC. 1941. Phylogenetic and
cytological studies in the Ranunculaceae Juss. – Trans.
Amer. Philos. Soc., N. S., 31: 443-521.
Gregson N. 1965. Chromosome morphology and
cytogenetics in the genus Ranunculus L. – Ph.D. diss., University of
Liverpool, England.
Grey-Wilson C. 1989. Clematis
orientalis (Ranunculaceae)
and its allies. – Kew Bull. 44: 33-60.
Grey-Wilson C. 2000. Poppies: a guide to the
poppy family in the wild and in cultivateion. – Timber Press, Portland,
Oregon.
Grey-Wilson C. 2012. Proposal to conserve the
name Meconopsis (Papaveraceae) with a conserved
type. – Taxon 61: 473-474.
Grey-Wilson C. 2014. The genus
Meconopsis blue poppies and their relatives. – Royal Botanical
Garden, Kew, United Kingdom.
Griffin SR, Mavraganis K, Eckert CG. 2000.
Experimental analysis of protogyny in Aquilegia canadensis (Ranunculaceae). – Amer. J. Bot.
87: 1246-1256.
Guédès M. 1977. Le gynécée de
Podophyllum (Berbéridacées): monomérie vraie et placentation
suturale de la portion congénitalement close du carpelle. – Compt. Rend.
Acad. Sci. Paris 285D: 755-758.
Guerrant EO. 1982. Neotenic evolution of
Delphinium nudicaule (Ranunculaceae): a
hummingbird-pollinated species. – Evolution 36: 699-712.
Guinochet M. 1935. Contribution à l’étude
génétique et cytologique du genre Anemone. – Rev. Cytol.
Cytophysiol. Vég. 1: 131-145.
Gunn CR. 1980. Seeds and fruits of Papaveraceae and Fumariaceae. –
Seed Sci. Technol. 8: 3-58.
Gunn CR, Seldin MJ. 1976. Seeds and fruits of
North American Papaveraceae. –
United States Department of Agriculture, Agricultural Research Service, Techn.
Bull. 1517: 1-96.
Günther K-F. 1975a. Beiträge zur
Morphologie und Verbreitung der Papaveraceae 1:
Infloreszenzmorphologie der Papaveraceae; Wuchsformen der
Chelidonieae. – Flora 164: 185-234.
Günther K-F. 1975b. Beiträge zur
Morphologie und Verbreitung der Papaveraceae 2: die Wuchsformen der
Papaveraceae, Eschscholzieae,
und Platystemonoideae. – Flora 164: 393-436.
Haccius B, Fischer E. 1959. Embryologische
und histogenetische Studien an ”monokotylen Dikotylen” III. Anemone
appenina L. – Österr. Bot. Zeitschr. 106: 373-389.
Hammond HD. 1955. Systematic serological
studies in Ranunculaceae. –
Bull. Serol. Mus. 14: 1-3.
Han M, Manchester SR, Fu Q-Y, Jin J-H, Quan
C. 2018. Paleogene fossil fruits of Stephania (Menispermaceae) from North
America and East Asia. – J. Syst. Evol. 56: 81-91.
Hance HF. 1884. Eomecon: genus
novum, e familia Papaveracearum. – J. Bot. 22: 346.
Hand R. 1998. On the identity of the
enigmatic Thalictrum morisonii (Ranunculaceae). – Taxon 47:
717-720.
Hand R. 2001. Revision der in Europa
vorkommenden Arten von Thalictrum subsection Thalictrum (Ranunculaceae). – Bot.
Naturschutz Hessen 9.
Hand R. 2005. Remarks on the taxonomy of
Thalictrum petaloideum L., Th. podolicum Lecoy. and Th.
uncinatum Rehmann (Ranunculaceae). – Candollea 60:
79-86.
Hanelt P. 1969. Revision der mongolischen
Taxa von Papaver L. sect. Scapiflora Rchb. sowie Studien zur
Systematik und Evolution dieser Sektion. – Habilitationsschrift,
Martin-Luther-Universität, Halle-Wittenberg, Germany.
Hang S, McLewin W, Fay MF. 2001. Molecular
phylogeny of Helleborus (Ranunculaceae), with an emphasis
on the East Asian-Mediterranean disjunction. – Taxon 50: 1001-1018.
Hannan LG. 1980. Heteromericarpy and dual
seed germination modes in Platystemon californicus (Papaveraceae). – Madroño 27:
163-170.
Hara H, Kurosawa S. 1956. Cytotaxonomic notes
on the Ranunculus acris group in Japan. – Bot. Mag. (Tokyo) 69:
345-352.
Harley MM. 1985. Pollen morphology and
taxonomy of the tribe Fibraureae (Menispermaceae). – Kew Bull.
40: 553-565.
Harley MM, Ferguson IK. 1982. Pollen
morphology and taxonomy of the tribe Menispermeae (Menispermaceae). – Kew Bull.
37: 353-366.
Harms H. 1897. Circaeaster Maxim.
– In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien. Nachtrag
und Register zu Teil II-IV, W. Engelmann, Leipzig, pp. 332-333.
Harvey-Gibson RJ, Horsman E. 1919. The
anatomy of the stem of the Berberidaceae. – Trans. Roy.
Soc. Edinb. 52: 501-515.
Hasegawa I. 1993. Molecular systematics of
the tribe Cimicifugeae and allied genera in the Ranunculaceae. – Ph.D. diss.,
City University of New York.
Hegnauer R. 1961. Die Gliederung der
Rhoeadales sensu Wettstein im Lichte der Inhaltsstoffe. – Planta Medica 9:
37-46.
Heimburger M. 1959. Cytotaxonomic studies of
the genus Anemone. – Can. J. Bot. 37: 587-612.
Heimburger M. 1961. A karyotype study of
Anemone drummondii and its hybrid with A. multifida. – Can.
J. Bot. 39: 497-502.
Heimburger M, Boraiah G. 1964. Genome
relationships of Anemone multifida. – Can. J. Gen. Cytol. 6:
529-539.
Heiss AG, Kropf M, Sontag S, Weber A. 2011.
Seed morphology of Nigella s.l. (Ranunculaceae): identification,
diagnostic traits, and their potential phylogenetic relevance. – Intern. J.
Plant Sci. 172: 267-284.
Henderson DM. 1965. The pollen morphology of
Meconopsis. – Grana Palynol. 6: 191-209.
Henderson EM. 1924. The stem structure of
Sargentodoxa cuneata Rehd. et Wils. – Trans. Proc. Bot. Soc. Edinb.
29: 57-62.
Herrera CM. 2005. Post-floral perianth
functionality: contribution of persistent sepals to seed development in
Helleborus foetidus (Ranunculaceae). – Amer. J. Bot.
92: 1486-1491.
Herrera F, Manchester SR, Hoot SB, Wefferling
KM, Carvalho MR, Jaramillo C. 2011. Phytogeographic implications of fossil
endocarps of Menispermaceae
from the Paleocene of Colombia. – Amer. J. Bot. 98: 2004-2017.
Hess H. 1954. Systematische und zytologische
Untersuchungen an einigen Ranunculus-Arten aus der
Nemorosus-Gruppe. – Ber. Schweiz. Bot. Ges. 65: 272-301.
Hidalgo O, Gleissberg S. 2010. Evolution of
reproductive morphology in the Papaveraceae s.l. (Papaveraceae and Fumariaceae, Ranunculales). – Intern. J. Plant
Devel. Biol. 4: 76-85.
Hidalgo O, Bartholmes C, Gleissberg S. 2012.
Virus-induced gene silencing (VIGS) in Cysticapnos vesicaria, a
zygomorphic-flowered Papaveraceae (Ranunculales, basal eudicots). –
Ann. Bot. 109: 911-920.
Hiepko P. 1965. Vergleichend-morphologische
und entwicklungsgeschichtliche Untersuchungen über das Perianth bei den
Polycarpicae I-II. – Bot. Jahrb. Syst. 84: 359-426, 427-508.
Hiepko P (ed). 1995. Die natürlichen
Pflanzenfamilien. 2. Aufl. Band 17a, IV, Angiospermae: Ordnung Ranunculales Fam. Ranunculaceae. – Duncker &
Humblot, Berlin.
Hiepko P, Tamura M. 1996. Proposal to
conserve the name Isopyrum L. (Ranunculaceae) with a conserved
type. – Taxon 45: 327-328.
Hill AW. 1918. The genus Caltha in
the southern hemisphere. – Ann. Bot. 32: 421-435.
Hill RS. 1989. Early Tertiary leaves of the
Menispermaceae from Nerriga,
New South Wales. – Alcheringa 13: 37-44.
Himmelbaur W. 1913. Die Berberidaceen und
ihre Stellung im System. – Denkschr. Kaiserl. Akad. Wiss. Wien,
Math.-Naturwiss. Kl. 89: 733-796.
Hind N. 2008. An introduction to the climbing
dicentras – the genus Dactylicapnos in cultivation. – Curtis’s
Bot. Mag. 25: 194-206.
Hodges SA. 1997. Rapid radiation due to a key
innovation in columbines (Ranunculaceae:
Aquilegia). – In: Givnish TJ, Sytsma KJ (eds), Molecular evolution
and adaptive radiation, Cambridge University Pres, New York, pp. 391-405.
Hodges SA, Arnold ML. 1994. Columbines: a
geographically wide-spread species flock. – Proc. Natl. Acad. Sci. U.S.A. 91:
5129-5132.
Hoe K, Suh Y. 2008. Taxonomic implications of
seed coat in the subtribe Calthinae (Ranunculaceae). – Korean J.
Plant Taxon. 38: 1-16.
Hoffmann MH, Hagen KB von, Hörandl E, Röser
M, Tkach NV. 2010. Sources of the arctic flora: origins of arctic species in
Ranunculus and related genera. – Intern. J. Plant Sci. 171:
90-106.
Holm T. 1899. Podophyllum peltatum,
a morphological study. – Bot. Gaz. (Crawfordsville) 27: 419-433.
Holm T. 1913. Medical plants of North America
75. Hydrastis canadensis. – Merck’s Rep. 22: 202-204.
Hong Y-P, Chen Z-D, Lu A-M. 2001. Phylogeny
of the tribe Menispermeae (Menispermaceae) reconstructed by
ITS sequence data. – Acta Phytotaxon. Sin. 32: 97-104. [In Chinese with
English summary]
Hong Y-P, Pan K-Y, Chen Z-D, Lu A-M. 2001.
Characters of leaf epidermis and their systematic significance in Menispermaceae. – Acta Bot.
Sin. 43: 615-623. [In Chinese with English summary]
Hoot SB. 1991. Phylogeny of the Ranunculaceae based on epidermal
microcharacters and macromorphology. – Syst. Bot. 16: 741-755.
Hoot SB. 1995. Phylogeny of the Ranunculaceae based on preliminary
atpB, rbcL and 18S nuclear ribosomal DNA sequence data. –
Plant Syst. Evol. [Suppl.] 9: 241-251.
Hoot SB, Crane PR. 1995. Interfamilial
relationships in the Ranunculidae based on molecular systematics. – Plant
Syst. Evol. [Suppl.] 9: 119-131.
Hoot SB, Palmer JD. 1994. Structural
rearrangements, including parallel inversions, within the chloroplast genome of
Anemone and related genera. – J. Mol. Evol. 38: 274-281.
Hoot SB, Reznicek AA, Palmer JD. 1994.
Phylogenetic relationships in Anemone (Ranunculaceae) based on morphology
and chloroplast DNA. – Syst. Bot. 19: 169-200.
Hoot SB, Culham A, Crane PR. 1995a. The
utility of atpB gene sequences in resolving phylogenetic
relationships: comparison with rbcL and 18S ribosomal DNA sequences in
the Lardizabalaceae. – Ann.
Missouri Bot. Gard. 82: 194-207.
Hoot SB, Culham A, Crane PR. 1995b.
Phylogenetic relationships of the Lardizabalaceae and
Sargentodoxaceae: chloroplast and nuclear DNA sequence evidence. – Plant
Syst. Evol. [Suppl.] 9: 195-199.
Hoot SB, Kadereit JW, Blattner FR, Jork KB,
Schwarzbach AE, Crane PR. 1997. Data congruence and phylogeny of the Papaveraceae s.l. based on four
data sets: atpB and rbcL sequences, trnK restriction
sites, and morphological characters. – Syst. Bot. 22: 575-590.
Hoot SB, Kramer J, Arroyo MTK. 2008.
Phylogenetic position of the South American dioecious genus Hamadryas
and related Ranunculeae (Ranunculaceae). – Intern. J.
Plant Sci. 169: 433-443.
Hoot SB, Zautke H, Harris DJ, Crane PR, Neves
SS. 2009. Phylogenetic patterns in Menispermaceae based on multiple
chloroplast sequence data. – Syst. Bot. 34: 44-56.
Hoot SB, Meyer KM, Manning JC. 2012.
Phylogeny and reclassification of Anemone (Ranunculaceae), with an emphasis
on Austral species. – Syst. Bot. 37: 139-152.
Hoot SB, Wefferling KM, Wulff JA. 2015.
Phylogeny and character evolution of Papaveraceae s. l. (Ranunculales). – Syst. Bot. 40:
474-488.
Hörandl E. 2002. Morphological
differentiation within the Ranunculus cassubicus group compared to
variation of isozymes, ploidy levels, and reproductive systems: implications
for taxonomy. – Plant Syst. Evol. 233: 65-78.
Hörandl E. 2004. Comparative analysis of
genetic divergence among sexual ancestors of apomictic complexes using isozyme
data. – Intern. J. Plant Sci. 165: 615-622.
Hörandl E. 2014. Nothing in taxonomy makes
sense except in the light of evolution: examples from the classification of
Ranunculus. – Ann. Missouri Bot. Gard. 100: 14-31.
Hörandl E, Emadzade K. 2011. The evolution
and biogeography of alpine species in Ranunculus (Ranunculaceae): a global
comparison. – Taxon 60: 415-426.
Hörandl E, Jakubowsky G, Dobes C. 2001.
Isozyme and morphological diversity within apomictic and sexual taxa of the
Ranunculus auricomus complex. – Plant Syst. Evol. 226: 165-185.
Hörandl E, Paun O, Johansson JT, Lehnebach
C, Armstrong T, Chen L, Lockhart P. 2005. Phylogenetic relationships and
evolutionary traits in Ranunculus s.l. (Ranunculaceae) inferred from ITS
sequence analysis. – Mol. Phylogen. Evol. 36: 305-327.
Hörandl E, Greilhuber J, Klímová K, Paun
O, Temsch E, Emadzade K, Hodálová I. 2009. Reticulate evolution and taxonomic
concepts in the Ranunculus auricomus complex (Ranunculaceae): insights from
analysis of morphological, karyological and molecular data. – Taxon 58:
1194-1215.
Horovitz A. 1976. Edaphic factors and
flower-colour distribution in the Anemoneae (Ranunculaceae). – Plant Syst.
Evol. 126: 239-242.
Hsiao P-K, Wag W-T. 1964. A new genus of Ranunculaceae –
Dichocarpum W. T. Wang et Hsiao. – Acta Phytotaxon. Sin. 9:
315-333. [In Chinese]
Hu A. 1987. Studies on the morphology of
Kingdonia uniflora Balf. f. et W. W. Sm. and Circaeaster
agrestis Maxim. – Intern. Bot. Cong., Berlin, 5-162b-3.
Hu J, Zhang J, Shan H, Chen Z. 2012.
Expression of floral MADS-box genes in Sinofranchetia chinensis (Lardizabalaceae): implications
for the nature of the nectar leaves. – Ann. Bot. 110: 57-69.
Hu Z-H, Yang J. 1987. Morphological studies
of Circaeaster agrestis Maxim. I. Process of embryological
development. – Acta Phytotaxon. Sin. 25: 350-356. [In Chinese]
Hu Z-H, Lang J, Jing R-Q, Dong Z-M. 1990.
Morphological studies on Circaeaster agrestis II. Morphology and
anatomy of flower, fruit, and seed. – Cathaya 2: 77-88.
Huang H-Y, Long H, Li L. 2010. Genesis of
microspore, megaspore and the development of male and female gametophyte in
Diphylleia sinensis. – Guihaia 30: 36-44. [In Chinese]
Huber W. 1989. Ranunculus kuepferi
Greuter & Burdet in Korsica (Gruppe R. pyrenaeus L.). –
Candollea 44: 630-637.
Hus H. 1907. The germination of Hydrastis
canadensis. – Ann. Rep. Missouri Bot. Gard. 18: 85-94.
Hutchinson J. 1920a. Bocconia and
Macleaya. – Kew Bull. 1920: 275-282.
Hutchinson J. 1920b. Clematopsis, a
primitive genus of Clematideae. – Kew Bull. 2: 12-22.
Hutchinson J. 1921. The genera of Fumariaceae
and their distribution. – Kew Bull. 3: 97-115.
Huth E. 1892. Revision der kleineren
Ranunculaceen-Gattungen Myosurus, Trautvetteria,
Hamadryas, Glaucidium, Hydrastis, Eranthis,
Coptis, Anemonopsis, Actaea, Cimicifuga und
Xanthorrhiza. – Engl. Bot. Jahrb. Syst. 16: 278-324.
Huynh KL. 1970a. Le pollen du genre
Anemone et du genre Hepatica (Ranunculaceae) et leur taxonomie.
– Pollen Spores 12: 329-364.
Huynh KL. 1970b. Le pollen et la
systématique du genre Pulsatilla. – Bot. Jahrb. Syst. 88:
204-268.
Huynh KL. 1970c. Pollen and the origin of the
Australasian anemones (Ranunculaceae). – Bot. J. Linn.
Soc. 63: 91-93.
Ichinoe Y, Tamura M. 1977. The characteristic
components and phylogenetic relationships of genus Aconitum and its
allies. – Bull. Dept. Gen. Educ., Coll. Sci. Tech., Nihon Univ. 22:71-8, 23:
27-36.
Il’Ina GM. 1968. K morfologii gineceja
ploskotycinocnika kalifornskojo (Platystemon californicus Benth.). –
Morfologiya vyssich rastenij 1968: 142-156.
Irie H, Uyeo S, Yamamoto K, Kinoshta K. 1967.
The structure of glaupalol, a novel furanocoumarin from Glaucidium
palmatum Sieb. et Zucc. – Chem. Commun. 1967. 547-548.
Iriki Y, Minamisawa H. 1983. D-galactose and
a ribitol-like substance in Hydrastis canadensis L. – Nippon
Nogeikagaku Kaishi 57: 319-321.
Iwashina T, Kitajima J. 2009. Flavonol
glycosides from the monotypic genus Ranzania endemic to Japan. –
Biochem. Syst. Ecol. 37: 122-123.
Iwashina T, Ootani S. 1990. Three flavonol
allosides from Glaucidium palmatum. – Phytochemistry 29:
3639-3641.
Iwatsuki K. 2006. Glaucidiaceae. – In: Iwatsuki K,
Boufford DE, Ohba H (eds), Flora of Japan IIa, Kodansha Ltd., Tokyo, p. 390.
Jabbour F, Renner SS. 2011a.
Consolida and Aconitella are an annual clade of
Delphinium (Ranunculaceae) that diversified in
the Mediterranean basin and the Irano-Turanian region. – Taxon 60:
1029-1040.
Jabbour F, Renner SS. 2011b. Resurrection of
the genus Staphisagria J. Hill, sister to all the other Delphinieae
(Ranunculaceae). – PhytoKeys
7: 21-26.
Jabbour F, Renner SS. 2012. A phylogeny of
Delphinieae (Ranunculaceae)
shows that Aconitum is nested within Delphinium and that Late
Miocene transitions to long life cycles in the Himalayas and Southwest China
coincide with bursts in diversification. – Mol. Phylogen. Evol. 62:
928-942.
Jabbour F, Ronse De Craene LP, Nadot S,
Damerval C. 2009. Establishment of zygomorphy on an ontogenetic spiral and
evolution of perianth in the tribe Delphinieae (Ranunculaceae). – Ann. Bot. 104:
809-822.
Jacques FMB. 2006. Histoire Evolutive des Menispermaceae. – Thèse,
Botanique Systématique, Museum National d’Histoire Naturelle, Paris.
Jacques FMB. 2009a. Survey of the Menispermaceae endocarps. –
Adansonia, sér. III, 31: 47-87.
Jacques FMB. 2009b. Fossil history of the Menispermaceae (Ranunculales). – Ann. Paléont.
95: 53-69.
Jacques FMB, Bertolino P. 2008. Molecular and
morphological phylogeny of Menispermaceae (Ranunculales). – Plant Syst.
Evol. 274: 83-97.
Jacques FMB, De Franceschi D. 2005. Endocarps
of Menispermaceae from Le
Quesnoy outcrop (Sparnacian facies, Lower Eocene, Paris Basin). – Rev.
Palaeobot. Palynol. 135: 61-70.
Jacques FMB, De Franceschi D. 2007. Menispermaceae wood anatomy and
cambial variants. – IAWA J. 28: 139-172.
Jacques FMB, Zhou Z. 2010. Geometric
morphometrics: a powerful tool for the study of shape evolution in Menispermaceae endocarps. –
Taxon 59: 881-895.
Jacques FMB, Gallut C, Vignes-Lebbe R,
Zaragüeta i Bagils R. 2007. Resolving phylogenetic reconstruction in Menispermaceae (Ranunculales) using fossils and a
novel statistical test. – Taxon 56: 379-392.
Jacques FMB, Wang W, Ortiz R del C, Li H-L,
Zhou Z-K, Chen Z-D. 2011. Integrating fossils in a molecular-based phylogeny
and testing them as calibration points for divergence time estimates in Menispermaceae. – J. Syst.
Evol. 49: 25-49.
Jalan S. 1963. Studies in the family Ranunculaceae IV. The embryology
of Actaea spicata Linn. – Phytomorphology 13: 338-347.
Jalan S. 1977. Morphogenesis of the stomata
of Holboellia latifolia Wall. with remarks on the taxonomy of the
aforementioned genus. – Geophytology 7: 61-64.
Janchen E. 1949. Die systematische Gliederung
der Ranunculaceen und Berberidaceen. – Österr. Akad. Wiss., Math.-Naturwiss.
Kl., Denkschr. 108, 4. Abh.: 1-82.
Janczewski ME de. 1892. Études
morphologiques sur le genre Anemone L. – Rev. Gén. Bot. 4:
241-258.
Janish JR. 1977. Nevada’s vanishing
bear-poppies. – Mentzelia 3: 2-10.
Jensen U. 1967 [1968]. Serologische Beiträge
zur Frage der Verwandtschaft zwischen Ranunculaceen und Papaveraceen. – Ber.
Deutsch. Bot. Ges. 80: 621-624.
Jensen U. 1968. Serologische Beiträge zur
Systematik der Ranunculaceae.
– Bot. Jahrb. Syst. 88: 204-268, 269-310.
Jensen U. 1971. Zur systematischen Stellung
der Helleborinae (Ranunculaceae). – Taxon 20:
747-758.
Jensen U. 1984. Legumin-like and vicilin-like
storage poteins in Nigella damascena (Ranunculaceae) and six other
dicotyledonous species. – J. Plant Physiol. 115: 161-170.
Jensen U. 1995a. Secondary compounds of the
Ranunculiflorae. – Plant Syst. Evol. [Suppl.] 9: 85-97.
Jensen U. 1995b. Serological legumin data and
the phylogeny of the Ranunculaceae. – Plant Syst.
Evol. [Suppl.] 9: 217-227.
Jensen U, Hoot SB, Johansson JT, Kosuge K.
1995. Systematics and phylogeny of the Ranunculaceae – a revised family
concept on the basis of molecular data. – Plant Syst. Evol. [Suppl.] 9:
273-280.
Jetter R, Riederer M, Seyer A, Mioskowski C.
1996. Homologous long-chain alkanediols from Papaver leaf cuticular
waxes. – Phytochemistry 42: 1617-1620.
Johansson JT. 1995. A revised chloroplast DNA
phylogeny of the Ranunculaceae.
– Plant Syst. Evol. [Suppl.] 9: 253-261.
Johansson JT. 1998. Chloroplast DNA
restriction site mapping and the phylogeny of Ranunculus (Ranunculaceae). – Plant Syst.
Evol. 213: 1-19.
Johansson JT, Jansen RK. 1993. Chloroplast
DNA variation and phylogeny of the Ranunculaceae. – Plant Syst.
Evol. 187: 29-49.
Johnston MC. 1976. A new species of prickly
poppy from Mexico. – Wrightia 5: 259-260.
Jork KB, Kadereit JW. 1995. Molecular
phylogeny of the Old World representatives of Papaveraceae subfamily
Papaveroideae with special emphasis on the genus Meconopsis. – Plant
Syst. Evol. [Suppl.] 9: 171-180.
Jork-Plessing K. 1989. Vergleichende
phytochemische Untersuchungen der Blüten ausgewählter Vertreter der Gattung
Papaver L. – Diplomarbeit, Universität Heidelberg, Germany.
Joseph C, Heimburger M. 1966. Cytotaxonomic
studies on New World species of Anemone (section
Eriocephalus) with tuberous rootstocks. – Can. J. Bot. 44:
899-928.
Joshi AC. 1937. Contribution to the
embryology of the Menispermaceae 1. Cocculus
villosus DC. – Proc. Indian Acad. Sci., Sect. B, 5: 57-63.
Joshi AC. 1939. Morphology of Tinospora
cordifolia, with some observations on the origin of the single integument,
nature of synergidae, and affinities of the Menispermaceae. – Amer. J. Bot.
26: 433-439.
Junell S. 1931. Die Entwicklungsgeschichte
von Circaeaster agrestis. – Svensk Bot. Tidskr. 25: 238-270.
Kaden NN. 1951. Fruits and seeds of Middle
Russian Nymphaeaceae
and Berberidaceae. – Bull.
Moscow Soc. Natur., Biol. Ser. 56: 81-90.
Kadereit JW. 1986a. A revision of
Papaver L. sect. Papaver (Papaveraceae). – Bot. Jahrb.
Syst. 108: 1-16.
Kadereit JW. 1986b. A revision of
Papaver Section Argemonidium. – Notes Roy. Bot. Gard.
Edinb. 44: 25-43.
Kadereit JW. 1987a. The taxonomy,
distribution and variability of the genus Roemeria Medic. (Papaveraceae). – Flora 179:
135-153.
Kadereit JW. 1987b. A revision of
Papaver sect. Carinatae (Papaveraceae). – Nord. J. Bot. 7:
501-504.
Kadereit JW. 1988a. Sectional affinities and
geographical distribution in the genus Papaver L. (Papaveraceae). – Beitr. Biol.
Pflanzen 63: 139-156.
Kadereit JW. 1988b. Papaver L. sect.
Californicum Kadereit, a new section of the genus. – Rhodora 90:
7-13.
Kadereit JW. 1989. A revision of
Papaver Section Rhoeadium Spach. – Notes Roy. Bot. Gard.
Edinb. 45: 225-286.
Kadereit JW. 1993. Papaveraceae. – In: Kubitzki K,
Rohwer JG, Bittrich V (eds), The families and genera of vascular plants II.
Flowering plants. Dicotyledons. Magnoliid, hamamelid and caryophyllid families,
Springer, Berlin, Heidelberg, New York, pp. 494-506.
Kadereit JW, Erbar C. 2011. Evolution of
gynoecium morphology in Old World Papaveroideae: a combined
phylogenetic/ontogenetic approach. – Amer. J. Bot. 98: 1243-1251.
Kadereit JW, Leins P. 1988. A wind tunnel
experiment on seed dispersal in Papaver L. sect. Argemonidium
Spach and Rhoeadium Spach (Papaveraceae). – Flora 181:
189-203.
Kadereit JW, Sytsma KJ. 1992. Disassembling
Papaver: a restriction site analysis of chloroplast DNA. – Nord. J.
Bot. 12: 205-217.
Kadereit JW, Blattner FR, Jork KB,
Schwarzbach A. 1994. Phylogenetic analysis of the Papaveraceae s.l. (incl.
Fumariaceae, Hypecoaceae, and Pteridophyllum) based on morphological
characters. – Bot. Jahrb. Syst. 116: 361-390.
Kadereit JW, Blattner FR, Jork KB,
Schwarzbach A. 1995. The phylogeny of the Papaveraceae sensu lato:
morphological, geographical and ecological implications. – Plant Syst. Evol.
[Suppl.] 9: 133-145.
Kadereit JW, Schwarzbach A, Jork KB. 1997.
The phylogeny of Papaver s.l. (Papaveraceae): polyphyly or
monophyly? – Plant Syst. Evol. 204: 75-98.
Kadereit JW, Preston CD, Valtueña FJ. 2011.
Is Welsh poppy, Meconopsis cambrica (L.) Vig. (Papaveraceae), truly a
Meconopsis? – New J. Bot. 1: 80-88.
Kadota Y. 1991. Taxonomic notes on some
alpine species of Ranunculus (Ranunculaceae) in the Himalaya.
– In: Ohba H, Malla SB (eds), The Himalayan plants 2, University of Tokyo
Press, Tokyo, pp. 95-115.
Kalis AJ. 1979. Papaveraceae. The Northwest
European pollen flora 20. – Rev. Palaeobot. Palynol. 28: 209-260.
Kang Y, Jabbour F, Cao S, Wang Y, Guo J,
Huang J. 2017. Leaf epidermal features of Chinese Stephania Lour. (Menispermaceae) and their
systematic significance. – Kew Bull. 72: 26 DOI 10.1007/S12225-017-9697-2
Kapil RN, Jalan S. 1962. Studies in the
family Ranunculaceae I. The
embryology of Caltha palustris Linn. – In: Plant embryology – a
symposium, Council of Scientific & Industrial Research, New Delhi, pp.
205-214.
Kaplan SM, Mulcahy DL. 1971. Mode of
pollination and floral sexuality in Thalictrum. – Evolution 25:
659-668.
Kapoor BM. 1966. Contribution to the cytology
of endosperm in some angiosperms X. Nigella damascena and N.
sativa. – Caryologia 19: 73-83.
Kapoor BM, Löve Á. 1970. Chromosomes of
Rocky Mountain Ranunculus. – Caryologia 23: 575-594.
Karrer AB. 1991. Blütenentwicklung und
systematische Stellung der Papaveraceae und Capparaceae. –
Ph.D. diss., Universität Zürich, Switzerland.
Kashyap M. 1979. Nodal organisation in some
species of Ranunculaceae. –
J. Indian Bot. Soc. 58: 148-153.
Kaul MLH. 1972. Studies on Argemone
mexicana Linn. VI. Pollen morphology, floral biology, and pollination
mechanism. – Proc. Indian Acad. Sci., Sect. B, 35: 86-93.
Kaute U. 1963. Beiträge zur Morphologie des
Gynoeceums der Berberidaceen mit einem Anhang über die Rhizomknospe von
Plagiorhegma dubium. – Ph.D. diss., Universität Berlin, Germany.
Kawano S, Ihara M. 1967 Chromosome morphology
of Caulophyllum robustum (Podophyllaceae) and its systematic
implications. – J. Jap. Bot. 42: 129-135.
Keener CS. 1967. A biosystematic study of
Clematis subsection Integrifoliae (Ranunculaceae). – J. Elisha
Mitchell Sci. Soc. 83: 1-41.
Keener CS. 1993. A review of the
classification of the genus Hydrastis (Ranunculaceae). – Aliso 13:
551-558.
Keener CS, Dennis WM. 1982. The subgeneric
classification of Clematis (Ranunculaceae) in temperate North
America north of Mexico. – Taxon 31: 37-44.
Keener CS, Reveal JL, Dutton BE, Ziman S.
1999. A list of suprageneric names in Ranunculaceae (Magnoliophyta). –
Taxon 48: 497-506.
Kessler PJA. 1993. Menispermaceae. – In: Kubitzki
K, Rohwer JG, Bittrich V (eds), The families and genera of vascular plants II.
Flowering plants. Dicotyledons. Magnoliid, hamamelid and caryophyllid families,
Springer, Berlin, Heidelberg, New York, pp. 402-418.
Khan HA. 1991 [1992]. Palynotaxonomy and
phylogeny of Ranunculaceae. –
Geophytology 21: 207-210.
Kidwai P. 1972. Development of stomata in
some Papaveraceae and
Fumaria. – Ann. Bot., N. S., 36: 1011-1018.
Killick DJB. 1977. The correct name for
Anemone capensis (Ranunculaceae). – Bothalia 12:
258.
Kim Y-D, Jansen RK. 1994. Characterization
and phylogenetic distribution of a chloroplast DNA rearrangement in the Berberidaceae. – Plant Syst.
Evol. 193: 107-114.
Kim Y-D, Jansen RK. 1995. Phylogenetic
implications of chloroplast DNA variation in the Berberidaceae. – Plant Syst.
Evol. [Suppl.] 9: 341-349.
Kim Y-D, Jansen RK. 1996. Phylogenetic
implications of rbcL and ITS sequence variation in the Berberidaceae. – Syst. Bot. 21:
381-396.
Kim Y-D, Jansen RK. 1998. Chloroplast DNA
restriction site variation and phylogeny of the Berberidaceae. – Amer. J. Bot.
85: 1766-1778.
Kim Y-D, Kim S-H, Landrum LR. 2004. Taxonomic
and phytogeographic implications from ITS phylogeny in Berberis (Berberidaceae). – J. Plant Res.
117: 175-182.
Kim Y-D, Kim S-H, Kim CH, Jansen RK. 2004.
Phylogeny of Berberidaceae
based on sequences of the chloroplast gene ndhF. – Biochem. Syst.
Ecol. 32: 291-301.
Kim YK, Park CW, Kim KJ. 2009. Complete
chloroplast DNA sequence from a Korean endemic genus, Megaleranthis
saniculifolia, and its evolutionary implications. – Molecules Cells 27:
365-381.
Kindler T. 1914. Gametophyt und Fruchtansatz
bei Ficaria ranunculoides. – Österr. Bot. Zeitschr. 64: 73-85.
Kingdon-Ward F. 1926. Notes on the genus
Meconopsis, with some additional species from Tibet. – Ann. Bot.
(Oxford) 40: 535-546.
Kita Y, Ito M. 2000. Nuclear ribosomal ITS
sequences and phylogeny in East Asian Aconitum subgenus
Aconitum (Ranunculaceae), with special
reference to extensive polymorphism in individual plants. – Plant Syst. Evol.
225: 1-13.
Kita Y, Ueda K, Kadota Y. 1995. Molecular
phylogeny and evaluation of the Asian Aconitum subgenus
Aconitum (Ranunculaceae). – J. Plant Res.
108: 429-442.
Kloimwieder R. 1929. Beiträge zur Kenntnis
der Schlauchzellen der Fumariaceae, spez. die Gattung Dicentra s.l.
SB. – Akad. Wiss. Wien, Math.-Nat. Kl. 138(1): 517-550.
Knaben G. 1959a. On the evolution of the
radicatum-group of the Scapiflora papavers as studied in 70
and 56 chromosome species A. Cytotaxonomical aspects. – Opera Bot. 2(3):
1-74.
Knaben G. 1959b. On the evolution of the
radicatum-group of the Scapiflora papavers as studied in 70
and 56 chromosome species B. Experimental studies. – Opera Bot. 3(3):
1-96.
Knaben G. 1979. Additional experimental
studies in the Papaver radicatum group. – Bot. Not. 132: 483-490.
Kölsch A, Gleissberg S. 2006.
Diversification of CYCLOIDEA-like TCP genes in the basal eudicot
families Fumariaceae and Papaveraceae. – Plant Biol. 8:
680-687.
Koontz JA, Soltis PS, Soltis DE. 2004. Using
phylogeny reconstruction to test hypotheses of hybrid origin in
Delphinium section Diedropetala (Ranunculaceae). – Syst. Bot. 29:
345-357.
Kootin-Sanwu M. 1964. Cytology and
distribution of Caltha. – Proc. Bot. Soc. Brit. Isles 5: 377-378.
Kordjum EL. 1959. Comparative embryological
investigation of the family Ranunculaceae DC. – Ukr. Bot.
Žurn. 16: 32-43. [In Russian]
Kosenko VN. 1980. Comparative
palynomorphological study of the family Berberidaceae: 2. Morphology of
the pollen grains of the genera Gymnospermium, Leontice,
Caulophyllum, Bongardia, Epimedium,
Vancouveria, Achlys, and Jeffersonia. – Bot.
Žurn. 65: 1412-1423. [In Russian]
Kosuge K. 1995. Petal evolution in Ranunculaceae. – Plant Syst.
Evol. [Suppl.] 9: 185-191.
Kosuge K, Okada H. 1989. Cytotaxonomical
studies on Dichocarpum (Ranunculaceae) in Japan. – J.
Jap. Bot. 64: 1-7.
Kosuge K, Tamura M. 1986. Isozymic variation
of glutamate dehydrogenase in the Ranunculaceae. – Acta
Phytotaxon. Geobot. 37: 167-179.
Kosuge K, Tamura M. 1988a. Morphology of the
petal in Aconitum. – Bot. Mag. (Tokyo) 101: 223-237.
Kosuge K, Tamura M. 1988b. Notes on
Dichocarpum Sect. Hutchinsonia. – Acta Phytotaxon. Geobot.
63: 37-46.
Kosuge K, Tamura M. 1989. Ontogenetic studies
on petals of the Ranunculaceae.
– J. Jap. Bot. 64: 65-74.
Kosuge K, Pu F-D, Tamura M. 1989. Floral
morphology and relationships of Kingdonia. – Acta Phytotaxon.
Geobot. 40: 61-67.
Kosuge K, Sawada K, Denda T, Adachi J,
Watanabe K. 1995. Phylogenetic relationships of some genera in the Ranunculaceae based on alcohol
dehydrogenase genes. – Plant Syst. Evol. [Suppl.] 9: 263-271.
Krahulcová A. 1982. Cytotaxonomic study of
Chelidonium majus L. s.l. – Folia Geobot. Phytotaxon. 17:
237-268.
Kramer EM, Hodges SA. 2010.
Aquilegia as a model system for the evolution and ecology of petals.
– Phil. Trans. Roy. Soc., Ser. B, 365: 477-490.
Kramer EM, Di Stilio VS, Schluter P. 2003.
Complex patterns of gene duplication in the APETALA3 and
PISTILLATA lineages of the Ranunculaceae. – Intern. J.
Plant Sci. 164: 1-11.
Krassilov VA, Volynets Y. 2008. Weedy Albian
angiosperms. – Acta Palaeobot. 48: 151-169.
Kratochwil A. 1988. Zur Bestäubungsstrategie
von Pulsatilla vulgaris. – Flora 181: 262-324.
Krukoff BA, Moldenke HN. 1938. Studies of
American Menispermaceae, with
special reference to species used in preparation of arrow-poisons. –
Brittonia 3: 1-74.
Krukoff BA, Smith AC. 1937. Notes on the
botanical components of curare. – Bull. Torrey Bot. Club 64: 401-409.
Krukoff BA, Smith AC. 1939. Notes on the
botanical components of curare-II. – Bull. Torrey Bot. Club 66: 305-314.
Kubitzki K. 1995. Ranunculiflorae –
delimitation, phylogeny, diversification. – Plant Syst. Evol. [Suppl.] 9:
1-10.
Kubitzki K, Reznik H. 1966. Flavonoid-Muster
der Polycarpicae als systematisches Merkmal I. Übersicht über die Familien.
– Beitr. Biol. Pflanzen 42: 445-470.
Kumar S, Jeelani SM, Rani S, Gupta RC, Kumari
S. 2013. Cytology of five species of subfamily Papaveroideae from the Western
Himalayas. – Protoplasma 250: 307-316.
Kumazawa M. 1930a. Morphology and biology of
Glaucidium palmatum Sieb. et Zucc. with notes of affinities to the
allied genera Hydrastis, Podophyllum, and
Diphylleia. – J. Fac. Sci. Univ. Tokyo, Sect. III, Bot. 2:
345-380.
Kumazawa M. 1930b. Structure and affinities
of Glaucidium and its allied genera. – Bot. Mag. (Tokyo) 44:
479-490. [In Japanese]
Kumazawa M. 1930c. Studies on the structure
of Japanese species of Ranunculus. – J. Fac. Sci. Univ. Tokyo, Sect.
III (Bot.), 2: 297-343.
Kumazawa M. 1932. The medullary bundle system
in the Ranunculaceae and allied
plants. – Bot. Mag. (Tokyo) 46: 327-332. [In Japanese with English
summary]
Kumazawa M. 1936a. Podophyllum
pleianthum Hance: a morphological study with supplementary notes on allied
plants. – Bot. Mag. (Tokyo) 50: 268-276. [In Japanese]
Kumazawa M. 1936b [1937]. Pollen grain
morphology in Ranunculaceae, Lardizabalaceae, and Berberidaceae. – Jap. J. Bot. 8:
19-46.
Kumazawa M. 1937a. Ranzania japonica
(Berberidac.). Its morphology, biology, and systematic affinities. – Jap. J.
Bot. 9: 55-70.
Kumazawa M. 1937b. Comparative studies on the
vernation in the Ranunculaceae
and Berberidaceae. – J. Jap.
Bot. 14: 573-586, 659-669, 713-726. [In Japanese with English summary]
Kumazawa M. 1938a. On the ovular structure in
the Ranunculaceae and Berberidaceae. – J. Jap. Bot.
14: 10-25.
Kumazawa M. 1938b. Systematic and
phylogenetic consideration of the Ranunculaceae and Berberidaceae. – Bot. Mag.
(Tokyo) 52: 9-15. [In Japanese]
Kundu BC, Guha S. 1976. New species of
Stephania and Rhaptonema (Menispermaceae). – Bot. Not.
129: 257-265.
Kundu BC, Guha S. 1977. The genus
Perichasma (Menispermaceae). – Adansonia,
n.s., 17: 221-234.
Kuntze O. 1885. Monographie der Gattung
Clematis. – Verh. Bot. Ver. Brandenburg 26: 83-202.
Kürbs S. 1973.
Vergleichend-entwicklungsgeschichtliche Studien an
Ranunculaceen-Fiederblättern. – Bot. Jahrb. Syst. 93: 130-167.
Kurita M. 1956a. Karyotype studies in Berberidaceae I. – Mem. Ehime
Univ., Sect. II (Sci.), Ser. B (Biol.), 2: 247-252.
Kurita M. 1956b. Cytological studies in Ranunculaceae XI. The karyotypes
of Nigella damascena and some other species. – Jap. J. Genet. 31:
330-333.
Kurita M. 1957a. Chromosome studies in Ranunculaceae III. Karyotypes of
the subtribe Ranunculinae. – Rep. Biol. Inst. Ehime Univ. 1: 1-18.
Kurita M. 1957b. Chromosome studies in Ranunculaceae VII. Karyotypes of
Eranthis and some other genera. – Mem. Ehime Univ., Sect. II (Sci.),
Ser. B (Biol.), 2: 235-334.
Kurita M. 1958a. Chromosome studies in Ranunculaceae VIII. Karyotype and
phylogeny. – Rep. Biol. Inst. Ehime Univ. 5: 1-14.
Kurita M. 1958b. Chromosome studies in Ranunculaceae IX. Comparison of
chromosome volume between a 14- and a 16-chromosome species in Anemone
and in Ranunculus. – Rep. Biol. Inst. Ehime Univ. 6: 1-7.
Kurita M. 1958c. Chromosome studies in Ranunculaceae X. Karyotypes and
chromosome numbers of some genera. – Rep. Biol. Inst. Ehime Univ. 6: 9-16.
Kurita M. 1959a. Chromosome studies in Ranunculaceae XII. Karyotype of
Nigella and kinetochore-structure of its metaphase chromosomes. –
Rep. Biol. Inst. Ehime Univ. 8: 1-5.
Kurita M. 1959b. Chromosome studies in Ranunculaceae XIV. Karyotypes of
several genera. – Mem. Ehime Univ., Sect. II (Sci.), Ser. B (Biol.), 3:
200-206.
Kurita M. 1960a. Chromosome studies in Ranunculaceae XVI. Comparison of
an aspect of nucleus and chromosome between several genera. – Mem. Ehime
Univ., Sect. II (Sci.), Ser. B (Biol.), 4: 53-58.
Kurita M. 1960b. Chromosome studies in Ranunculaceae XVII. Karyotypes of
some species. – Mem. Ehime Univ., Sect. II (Sci.), Ser. B (Biol.), 4:
59-66.
Kurita M. 1961a. Chromosome studies in Ranunculaceae XVIII. Karyotypes of
several species. – Mem. Ehime Univ., Sect. II (Sci.), Ser. B (Biol.), 4:
252-261.
Kurita M. 1961b. Chromosome studies in Ranunculaceae XIX. Chromosome size
in Ranunculus species in the eight chromosome series. – Mem. Ehime
Univ., Sect. II (Sci.), Ser. B (Biol.), 4: 263-268.
Kurita M. 1963. Chromosome studies in Ranunculaceae XXI. Karyotypes of
Myosurus and Adonis. – Mem. Ehime Univ., Sect. II (Sci.),
Ser. B (Biol.), 4: 487-492.
Kurita M. 1966. Chromosome studies in Ranunculaceae XXIV. – Mem. Ehime
Univ., Sect. II (Sci.), Ser. B (Biol.), 5: 31-36.
Kurita M. 1967. Chromosome studies in Ranunculaceae XXV. – Mem. Ehime
Univ., Sect. II (Sci.), Ser. B (Biol.), 5: 165-169.
Kuroki Y. 1965. Chromosome study in three
species of Berberidaceae. –
Mem. Ehime Univ., Sect. II (Sci.), Ser. B (Biol.), 5: 19-24.
Kuroki Y. 1967. Chromosome study in seven
species of Berberidaceae. –
Mem. Ehime Univ., Sect. II (Sci.), Ser. B (Biol.), 5: 175-181.
Kuroki Y. 1968. Chromosome study in five
species of Berberidaceae. –
Mem. Ehime Univ., Sect. II (Sci.), Ser. B (Biol.), 6: 11-16.
Kuroki Y. 1970a. Chromosome study in five
species of Berberidaceae. –
Mem. Ehime Univ., Sect. II (Sci.), Ser. B (Biol.), 6: 63-69.
Kuroki Y. 1970b. Chromosome study in four
species of Berberidaceae. –
Mem. Ehime Univ., Sect. II (Sci.), Ser. B (Biol.), 6: 215-221.
Lafferriere JE. 1997. Transfer of specific
and infraspecific taxa from Mahonia to Berberis (Berberidaceae). – Bot. Žurn.
82: 95-99.
Landolt E. 1954. Die Artengruppe des
Ranunculus montanus Willd. in den Alpen und im Jura
(zytologisch-systematische Untersuchungen). – Ber. Schweiz. Bot. Gesellsch.
64: 9-83.
Landolt E. 1956. Die Artengruppe des
Ranunculus montanus Willd. in den Pyrenäen und anderen europäischen
Gebirgen westlich der Alpen. – Ber. Schweiz. Bot. Gesellsch. 66: 92-117.
Lane AK, Augustin MM, Ayyampalayam S, Plant
A, Gleissberg S, Stilio VS di, Depamphilis CW, Wong GK-S, Kutchan TM,
Leebens-Mack JH. 2018. Phylogenomic analysis of Ranunculales resolves branching
events across the order. – Bot. J. Linn. Soc. 187: 157-166.
Langlet O. 1927. Beiträge zur Zytologie der
Ranunculaceen. – Svensk Bot. Tidskr. 21: 1-17.
Langlet O. 1928. Einige Beobachtungen über
die Zytologie der Berberidaceen. – Svensk Bot. Tidskr. 22: 169-184.
Langlet O. 1932. Über
Chromosomenverhältnisse und Systematik der Ranunculaceae. – Svensk Bot.
Tidskr. 26: 381-400.
Langlet O. 1936. Några bidrag till
kännedomen om kromosomtalen inom Nymphaeaceae, Ranunculaceae, Polemoniaceae, och
Compositae. – Svensk Bot. Tidskr. 30: 288-294.
Larter LNH. 1932. Chromosome variation and
behaviour in Ranunculus L. – J. Genet. 26: 255-283.
Lauener LA, Tamura M. 1979. A synopsis of
Aconitum subgenus Paraconitum I. – Notes Roy. Bot. Gard.
Edinb. 37: 113-124.
La Valva V, Sabato S, Gigliano GS. 1985.
Morphology and alkaloid chemistry of Papaver setigerum DC. (Papaveraceae). – Taxon 34:
191-196.
Lawrence MJ. 1975. The genetics of
self-incompatibility in Papaver rhoeas. – Proc. Roy. Soc. Lond.,
Sect. B, 188: 275-285.
Layka S. 1975. Les caractères d l’endexine
chez les Papavéracées. – Bull. Soc. Bot. Franç., Coll. Morphol. Pollin.
122: 103-107.
Layka S. 1976a. Le polymorphisme pollinique
dans le genre Argemone (Papaveraceae). – Pollen Spores
18:351-375.
Layka S. 1976b. Les méthodes modernes de la
palynologie appliqués à l’étude des Papaverales. – Ph.D. diss.,
l’Université de Montpellier, C.N.R.S.A.O. 12.535.
Layka S. 1977. Les caractères de
l’endexine chez les Papaveracées. – Bull. Soc. Bot. Franco 122:
103-107.
Lecoyer J-C. 1885. Monographie du genre
Thalictrum. – Bull. Soc. Roy. Bot. Belge 24: 78-325.
Lee C-H, Lee S-T, Suh Y-B, Yeau S-H, Lee N-S.
2004. A palynotaxonomic study of Korean Adonis (Ranunculaceae). – J. Plant Biol.
47: 383-390.
Lee H-W, Park C-W. 1998. A karyotypic study
on Korean taxa of Cimicifuga (Ranunculaceae). – Korean J.
Plant Taxon. 28: 385-398.
Lee N-S, Yeau S-H, Kim J-H, Kim M-J. 1998.
Taxonomic position and affinities of Isopyrum manshuricum within
Korean Isopyroideae (Ranunculaceae) based on molecular
data. – Korean J. Biol. Sci. 2: 133-141.
Lee S. 1989. Palynological evidence for the
relationships between Megaleranthis saniculifolia and
Trollius species. – Pollen Spores 31: 173-185.
Lee S. 1990. On the taxonomic position of
Megaleranthis saniculifolia Ohwi (Ranunculaceae). – Korean J.
Plant Taxon. 21: 1-8.
Lee S. 1992. Palynological relationships
among Calathodes and its related genera. – Korean J. Plant Taxon.
22: 23-31.
Lee S, Blackmore S. 1992. A palynotaxonomic
study of the genus Trollius (Ranunculaceae). – Grana 31:
81-100.
Lee Y-N. 1973. Taxonomic study on genus
Hylomecon. – J. Korean Res. Inst. Better Living 11: 127-136.
Lee Y-N, Yeau S-H. 1985. Taxonomic characters
of Megaleranthis saniculifolia Ohwi (Ranunculaceae). – Korean J.
Plant Taxon. 15: 127-131.
Léger LJ. 1894. Recherches sur l’appareil
végétatif des Papavéracées (Papavéracées et Fumariacées DC.). – Mém.
Soc. Linn. Normandie 18: 195-623.
Lehminger R. 1985. Entwicklungsgeschichtliche
Untersuchungen an Fumariaceen-Blüten. – Diplomarbeit Universität Bonn,
Germany.
Lehnebach CA. 2008. Phylogenetic affinities,
species delimitation and adaptive radiation of New Zealand Ranunculus.
– Ph.D. diss., Massey University, Palmerston North, New Zealand.
Lehnebach CA, Cano A, Monsalve C, McLenachan
P, Hörandl E, Lockhart P. 2007. Phylogenetic relationships of the monotypic
Peruvian genus Laccopetalum (Ranunculaceae). – Plant Syst.
Evol. 264: 109-116.
Lehtonen S, Christenhusz MJM, Falck D. 2016.
Sensitive phylogenetics of Clematis and its position in Ranunculaceae. – Bot. J. Linn.
Soc. 182: 825-867.
Leinfellner W. 1957. Zur Morphologie des
Gynözeums von Berberis. – Österr. Bot. Zeitschr. 103: 600-612.
Leinfellner W. 1958. Beiträge zur
Kronblattmorphologie VIII. Der peltate Bau der Nektarblätter von
Ranunculus, dargelegt an Hand jener von Ranunculus pallasii
Schlecht. – Österr. Bot. Zeitschr. 105: 184-192.
Leinfellner W. 1959. Über die
röhrenförmige Nectarschuppe an den Nectar-blättern verschiedener
Ranunculus und Batrachium-Arten. – Österr. Bot. Zeitschr.
106: 88.
Leinfellner W. 1969. Über die Karpelle
verschiedener Magnoliales VII.
Euptelea (Eupteleaceae). – Österr. Bot.
Zeitschr. 116: 159-166.
Lemèsle R. 1943. Les trachéides à
punctuations aréolées de Sargentodoxa cuneata Rehd. et Wils. et leur
importance dans la phylogénie des Sargentodoxacées. – Bull. Soc. Bot.
France 90: 104-107.
Lemèsle R. 1948. Position phylogénétique
de l’Hydrastis canadensis L. et du Crossosoma californicum
Nutt., d’après les particularités histologiques du xylème. – Compt.
Rend. Acad. Sci. Paris 227: 221-223.
Lemèsle R. 1950. L’Hydrastis
canadensis L. et ses principales falsifications. – Rev. Gén. Bot. 57:
5-23.
Lemèsle R. 1955. Contribution à l’étude
de quelques famille de dicotylédones considérées comme primitives. –
Phytomorphology 5: 11-45.
Leppik EE. 1964. Floral evolution in the Ranunculaceae. – Iowa State
Coll. J. Sci. 39: 1-101.
Lewitsky GA. 1931. The karyotype in
systematics, on the base of karyology of the subfamily Helleboreae. – Tr.
Prikl. Bot. 27: 187-240.
Li H-F, Ren Y. 2005. The variation of
perforation plates of vessels in the secondary xylem of Euptelea
pleiosperma (Eupteleaceae).
– Acta Phytotaxon. Sin. 43: 1-11. [In Chinese]
Li L-Q, Zhang W-X. 1989. Studies on pollen
morphology of Adonis L. – Bull. Bot. Res. Harbin 9: 123-137. [In
Chinese]
Li Y-L, Kvacek Z, Ferguson DK, Wang Y-F, Li
C-S, Yang J, Ying T-S, Ablaev AG, Liu H-M. 2010. The fossil record of
Berberis (Berberidaceae) from the Palaeocene
of NE China and interpretations of the evolution and phytogeography of the
genus. – Rev. Palaeot. Palynol. 160: 10-31.
Liao L, Xu L, Zhang, D, Fang L, Deng H, Shi
J, Li T. 2008. Multiple hybridization origin of Ranunculus
cantoniensis (4x): evidence from trnL-F and ITS sequences and
fluorescent in situ hybridization (FISH). – Plant Syst. Evol. 276: 31-37.
Lidén M. 1986. Synopsis of Fumarioideae
(Fumariaceae) with a monograph of the tribe Fumarieae. – Opera Bot. 88:
1-133.
Lidén M. 1988. Tuberous Corydalis
in the Mediterranean checklist area. – Notes Roy. Bot. Gard. Edinb. 45:
349-363.
Lidén M. 1989. Corydalis (Papaveraceae: Fumarioideae) in
Nepal. – Bull. Brit. Mus. Nat. Hist. (Bot.) 18: 479-538.
Lidén M. 1991. New tuberous species of
Corydalis (Papaveraceae). – Willdenowia 21:
175-179.
Lidén M. 1993a. Fumariaceae. – In:
Kubitzki K, Rohwer JG, Bittrich V (eds), The families and genera of vascular
plants II. Flowering plants. Dicotyledons. Magnoliid, hamamelid and
caryophyllid families, Springer, Berlin, Heidelberg, New York, pp. 310-318.
Lidén M. 1993b. Pteridophyllaceae. – In:
Kubitzki K, Rohwer JG, Bittrich V (eds), The families and genera of vascular
plants II. Flowering plants. Dicotyledons. Magnoliid, hamamelid and
caryophyllid families, Springer, Berlin, Heidelberg, New York, pp. 556-557.
Lidén M. 1995. 69. Papaveraceae. – In: Harling G,
Andersson L (eds), Flora of Ecuador 52, Nord. J. Bot., Copenhagen, pp. 1-13.
Lidén M. 1996. New taxa of tuberous
Corydalis (Fumariaceae). – Willdenowia 26: 23-35.
Lidén M. 2000. Notes on Dionysia,
Corydalis and Fumaria in Iran. – Iranian J. Bot. 8:
303-308.
Lidén M. 2007. New species, combinations and
records of Hypecoum, Dactylicapnos and Corydalis
(Fumariaceae) in China. – Nord. J. Bot. 25: 33-37.
Lidén M, Pathak MK. 2014. Studies in
Dactylicapnos (Papaveraceae-Fumarioideae) part II.
Revision of Dactylicapnos sect. Pogonosperma sect. nov., with
D. arunachalensis sp. nov. – Nord. J. Bot. 32: 176-184.
Lidén M, Fukuhara T, Axberg T. 1995.
Phylogeny of Corydalis, ITS and morphology. – Plant Syst. Evol.
[Suppl.] 9: 183-188.
Lidén M, Fukuhara T, Rylander J, Oxelman B.
1997. Phylogeny and classification of Fumariaceae, with emphasis on
Dicentra s.l., based on the plastid gene rps16 intron. –
Plant Syst. Evol. 206: 411-420.
Liu J-Q, Chen Z-D, Lu A-M. 2002. Molecular
evidence for the sister relationship of the eastern Asia-North American
intercontinental species pair in the Podophyllum group (Berberidaceae). – Bot. Bull.
Acad. Sin. 43: 147-154.
Liu S, Liu L, Huang X, Zhu Y, Xu Y. 2017. A
taxonomic revision of three Chinese spurless species of genus
Epimedium L. (Berberidaceae). – PhytoKeys 78:
23-36.
Liu Y, Liu Y, Yang F, Wang X. 2014. Molecular
phylogeny of Asian Meconopsis based on nuclear ribosomal and
chloroplast DNA sequence data. – PloS One 9: e104823.
Lockhart P, McLenachan PA, Havell D, Glenny
D, Huson D, Jensen U. 2001. Phylogeny, dispersal and radiation of New Zealand
alpine buttercups: molecular evidence under split decomposition. – Ann.
Missouri Bot. Gard. 88: 458-477.
Loconte H. 1993. Berberidaceae. – In: Kubitzki K,
Rohwer JG, Bittrich V (eds), The families and genera of vascular plants II.
Flowering plants. Dicotyledons. Magnoliid, hamamelid and caryophyllid families,
Springer, Berlin, Heidelberg, New York, pp. 147-152.
Loconte H, Blackwell WH. 1985. Intrageneric
taxonomy of Caulophyllum (Berberidaceae). – Rhodora 87:
463-469.
Loconte H, Estes JR. 1989a. Genetic
relationships within Leonticeae (Berberidaceae). – Can. J. Bot.
67: 2310-2316.
Loconte H, Estes JR. 1989b. Phylogenetic
systematics of Berberidaceae
and Ranunculales (Magnoliidae).
– Syst. Bot. 14: 565-579.
Loconte H, Campbell LM, Stevenson DW. 1995.
Ordinal and familial relationships of Ranunculid genera. – Plant Syst. Evol.
[Suppl.] 9: 99-118.
Lonay H. 1901. Contribution à l’anatomie
des renonculées. Structure des péricarpes et des spermodermes. – Arch.
Inst. Bot. Univ. Liège 3: 1-164.
Lourteig A. 1952. Ranunculáceas de
Sudamérica templada. – Darwiniana 9: 397-608.
Lourteig A. 1956. Ranunculáceas de
Sudamérica tropical. – Mem. Soc. Ci. Nat. ‘La Salle’ 16: 19-228.
Löve Á. 1955. Cytotaxonomical remarks on
the Icelandic Papaver. – Nytt Mag. Bot. 4: 5-18.
Lubbers AE. 1982. Spatial and temporal
variation in the reproductive characteristics of Thalictrum
thalictroides (L.) Eames and Bivin, a forest herbaceous perennial. –
Ph.D. diss., Duke University, North Carolina.
Lucas GL. 1962a. Fumariaceae. – In: Hubbard
CE, Milne-Redhead E (eds), Flora of tropical East Africa, Crown Agents for
Oversea Governments and Aministrations, London, pp. 1-6.
Lucas GL. 1962b. Papaveraceae. – In: Hubbard CE,
Milne-Redhead E (eds), Flora of tropical East Africa, Crown Agents for Oversea
Governments and Aministrations, London, pp. 1-3.
Luo Y, Zhang F-M, Yang Q-E. 2005. Phylogeny
of Aconitum subgenus Aconitum (Ranunculaceae) inferred from ITS
sequences. – Plant Syst. Evol. 252: 11-25.
Ma J, Yang BX, Zhu W, Sun LL, tian JK, Wang
X. 2013. The complete chloroplast genome sequence of Mahonia bealei
(Berberidaceae) reveals a
significant expansion of the inverted repeat and phylogenetic relationship with
other angiosperms. – Gene 528: 120-131.
McCain JW, Hennen JF. 1982. Is the taxonomy
of Berberis and Mahonia (Berberidaceae) supported by their
rust pathogens Cumminsiella santa sp. nov. and other
Cumminsiella species (Uredinales)? – Syst. Bot. 7: 48-59.
McDonald A. 1991. Plantae alpinae novae
mexicanae: Argemone subalpina (Papaveraceae). – Brittonia 43:
120-122.
McFadden SE. 1950. A series of related
cytological and biochemical studies of the Berberidaceae and its alliance
with Ranunculaceae. – Ph.D.
diss., University of Virginia, Charlottesville, Virginia.
McLewin W. 1999. Fundamental taxonomic
problems in and arising from the genus Helleborus. – In: Andrews S,
Leslie AC, Alexander C (eds), Taxonomy of cultivated plants, Kew, pp.
297-304.
McLewin W, Mathew B. 1995. Hellebores. –
New Plantsman 2: 112-122.
Madahar C. 1967. Mediterranean and Asian taxa
of Anemone (section Eriocephalus) with tuberous rootstocks.
– Can. J. Bot. 45: 725-735.
Maheu J. 1905. Sur l’existence de
lactifères à caoutchouc dans un genre de Ménispermacées: Tinomiscium. –
Compt. Rend. Scient. Séanc. Paris 141: 958.
Maheu J. 1906. Les organs sécréteures des
Ménispermacées. – Bull. Soc. Bot. France 53: 651.
Maia N, Venard P. 1976. Contribution à
l’étude cytotaxonomique d’espèces Mediterranéennes d’Anemone
et de leurs hybrides. – Can. J. Gen. Cytol. 18: 151-168.
Malyutin NI. 1987. The system of the genus
Delphinium L. based on the morphological features of seeds. – Bot.
Žurn. 72: 683-693.
Manning JC, Goldblatt P, Forest F. 2009. A
revision of Fumariaceae (Fumarioideae) in southern Africa, including
naturalized taxa. – Bothalia 39: 47-65.
Manning JC, Goldblatt P, Hoot SB. 2009. Ranunculaceae: the genus
Knowltonia subsumed within Anemone. – Bothalia 39:
217-240.
Marié P. 1885. Recherches sur la structure
des Renonculacées. – Ann. Sci. Nat. Bot. 20: 5-180.
Marks GE, Schweizer D. 1974. Giemsa banding:
karyotype differences in some species of Anemone and in Hepatica
nobilis. – Chromosoma 44: 405-416.
Martin PG, Dowd JM. 1984. The study of plant
phylogeny using amino acid sequences of ribulose-1,5-bisphosphate carboxylase
III. Addition of Malvaceae and Ranunculaceae to the phylogenetic
tree. – Aust. J. Bot. 32: 283-290.
Mathew B. 1989. Helleborus. –
Alpine Garden Society, Woking.
Mathias ME, Theobald WL. 1981. A revision of
the genus Hyperbaena (Menispermaceae). – Brittonia
33: 81-104.
Maue G. 1926. Zur Pharmakognosie der
Ranunculaceen und Berberidaceen. Anatomie des Laubblattes. – Ph.D. diss.,
Universität Basel, Switzerland.
Mauritzon J. 1936. Zur Embryologie der
Berberidaceen. – Acta Horti Gothob. 11: 1-18.
Meacham CA. 1980. Phylogeny of the Berberidaceae with an evaluation
of classification. – Syst. Bot. 5: 149-172.
Meacham CA. 1981. The estimation of
evolutionary history with reference to the Berberidaceae. – Ph.D. diss.,
University of Michigan, Ann Arbor, Michigan.
Melville R. 1983. The affinity of
Paeonia and a second genus of Paeoniaceae. – Kew
Bull. 38: 87-105.
Menadue Y, Crowden RK. 1985. Three new
species of Ranunculus (Ranunculaceae) from Tasmania. –
Brunonia 8: 373-380.
Mendes MM, Grimm GW, Pais J, Friis EM. 2014.
Fossil Kajanthus lusitanicus gen. et sp. nov. from Portugal: floral
evidence for Early Cretaceous Lardizabalaceae (Ranunculales, basal eudicot). –
Grana 53: 283-301.
Meng A, Zhang Z, Li J, De Craene LR, Wang H.
2012. Floral development of Stephania (Menispermaceae): impact of organ
reduction on symmetry. – Intern. J. Plant Sci. 173: 861-874.
Mennega AMW. 1982. Stem structure of the New
World Menispermaceae. – J.
Arnold Arbor. 63: 145-171.
Meyer KM, Hoot SB, Arroyo MTK. 2010.
Phylogenetic affinities of South American Anemone (Ranunculaceae), including the
endemic segregate genera, Barneoudia and Oreithales. –
Intern. J. Plant Sci. 171: 323-331.
Meyer NR, Iljina GM. 1986.
Palynomorphological data on the system of family Papaveraceae. – Vestnik Moscow
Univ. 16, Biol. 1: 16-21.
Miers J. 1871. A complete monograph of the Menispermaceae. – Contr. Bot.
3: 1-402, Williams & Norgate, London.
Miikeda O, Kita K, Handa T, Yukawa T. 2006.
Phylogenetic relationships of Clematis (Ranunculaceae) based on
chloroplast and nuclear DNA sequences. – Bot. J. Linn. Soc. 152: 153-168.
Milne-Redhead E, Turrill WB. 1952. Ranunculaceae. – In: Turrill WB,
Milne-Redhead E (eds), Flora of tropical East Africa, The Crown Agents for the
Colonies, London, pp. 1-23.
Min F, Lu A-M. 1998. Floral organogenesis and
its systematic significance of the genus Nandina (Berberidaceae). – Acta Bot. Sin.
40: 102-108.
Miyaji Y. 1927. Über die somatischen
Chromosome einiger Ranunculaceen. – Bot. Mag. (Tokyo) 41: 568-569. [In
Japanese]
Miyaji Y. 1930. Beiträge zur
Chromosomenphylogenie der Berberidaceen. – Planta 11: 650-659.
Moffet AA. 1932. Chromosome studies in
Anemone I. A new type of chiasma behaviour. – Cytologia 4: 26-37.
Mohana Rao PR. 1981. Seed and fruit anatomy
of Cocculus hirsutus (Menispermaceae). – Plant Syst.
Evol. 139: 95-102.
Moore RJ. 1963. Karyotype evolution in
Caulophyllum. – Can. J. Gen. Cytol. 5: 384-388.
Mory B. 1979a. Beiträge zur Kenntnis der
Sippenstruktur der Gattung Glaucium Miller. – Feddes Repert. 89:
499-594.
Mory B. 1979b. Ergebnisse einer Revision und
Bestimmungsschlüssel der Gattung Glaucium Miller (Papaveraceae). – Gleditschia 7:
117-126.
Mu X-J. 1984. Early development of the
endosperm of Kingdonia uniflora. – Acta Bot. Sin. 26: 668-671.
Mucher W. 1993. Systematics and chorology of
Aconitum ser. Toxicum (Ranunculaceae) in Europe. –
Phyton (Horn) 33: 51-76.
Müller M, Baltisberger M. 1984.
Cytotaxonomische Untersuchungen in der Artengruppe des Ranunculus
alpestris (Ranunculaceae).
– Plant Syst. Evol. 145: 269-289.
Murakami T, Mikami Y, Itokawa H. 1967. Die
Struktur des neu isolierten Glykosids aus den Rhizomen von Glaucidium
palmatum. – Chem. Pharm. Bull. 15: 1817-1818.
Murbeck S. 1912. Untersuchungen über den
Blütenbau der Papaveraceen. – Kungl. Sv. Vetensk.-Akad. Handl. 5(1):
1-168.
Nast CG, Bailey IW. 1946. Morphology of
Euptelea and comparison with Trochodendron. – J. Arnold
Arbor. 27: 186-192.
Nelson DR, Harper KT. 1991. Site
characteristics and habitat requirements of the endangered dwarf bear-claw
poppy (Arctomecon humilis Coville, Papaveraceae). – Great Basin
Natur. 51: 167-175.
Nelson DR, Welsh SL. 1993. Taxonomic revision
of Arctomecon Torr. & Frém. – Rhodora 95: 197-213.
Nesom GL. 1992. A second species of
Hunnemannia (Papaveraceae) and synopsis of the
genus. – Phytologia 73: 330-337.
Neves J de Barros. 1942. Sobre a cariología
de Ranunculus Ficaria L. – Bol. Soc. Brot. 16: 169-181.
Nickol MG. 1995. Phylogeny and inflorescence
of Berberidaceae – a
morphological survey. – Plant Syst. Evol. [Suppl.] 9: 327-340.
Nikolic T. 1995. Numerical taxonomy of the
family Ranunculaceae. – Acta
Bot. Hung. 39: 251-270.
Norris T. 1941. Torus anatomy and nectary
characteristics as phylogenetic criteria in the Rhoeadales. – Amer. J. Bot.
28: 101-113.
Novák J, Preininger V. 1980. Sect.
Glauca – nová sekce rodu Papaver. – Preslia 52:
97-101.
Nowicke JW, Skvarla JJ. 1981. Pollen
morphology and phylogenetic relationships of the Berberidaceae. – Smithsonian
Contr. Bot. 50: 1-83.
Nowicke JW, Skvarla JJ. 1982. Pollen
morphology and the relationships of Circaeaster, of
Kingdonia, and of Sargentodoxa to the Ranunculales. – Amer. J. Bot. 69:
990-998.
Nowicke JW, Skvarla JJ. 1983. A palynological
study of the genus Helleborus (Ranunculaceae). – Grana 22:
129-140.
Nowicke JW, Skvarla JJ. 1995. Angiospermae.
Ordnung Ranunculales. Fam. Ranunculaceae. I. General part.
Pollen morphology. – In: Hiepko P (ed), Natürliche Pflanzenfamilien, 2.
Aufl., 17aIV, Duncker & Humblot, Berlin, pp. 129-159.
Oganezowa GG. 1975. On the evolution of life
forms in the family Berberidaceae s.l. – Bot. Žurn.
60: 1665-1675. [In Russian]
Oh BU. 1988. The taxonomic characters of
Korean Corydalis (Fumariaceae) and their significance in phylogenetic
consideration. – Korean J. Plant Taxon. 18: 33-51.
Ohta T. 1949. Alkaloids of Nandina
domestica. – J. Pharm. Soc. Japan 69: 22-23.
Ohwi J. 1935. Megaleranthis, genus
novum ranunculacearum. – Acta Phytotaxon. Geobot. 4: 130-131.
Okada H, Tamura M. 1979. Karyomorphology and
relationship in the Ranunculaceae. – J. Jap. Bot.
54: 65-77.
Oliver D. 1895. Circaeaster agrestis
Maxim. – Hooker’s Icones Plantarum IV, 4: pl. 2366.
Ortiz-Gentry R del C. 2010. Phylogeny,
classification, and morphological diversification in Menispermaceae (moonseed family).
– Ph.D. diss., University of Missouri-St. Louis, St. Louis, Missouri.
Ortiz-Gentry R del C. 2011. The identity of
Synandropus and a new combination in Neotropical Menispermaceae. – Novon 21:
357-361.
Ortiz-Gentry R del C. 2012. Seed diversity in
Menispermaceae: developmental
anatomy and insights into the origin of the condyle. – Intern. J. Plant Sci.
173: 344-364.
Ortiz-Gentry R del C, Kellogg EA, Werff H van
der. 2007. Molecular phylogeny of the moonseed family (Menispermaceae): implications for
morphological diversification. – Amer. J. Bot. 94: 1425-1438.
Ortiz R del C, Wang W, Jacques FMB, Chen Z.
2016. Phylogeny and a revised tribal classification of Menispermaceae (moonseed family)
based on molecular and morphological data. – Taxon 65: 1288-1312.
Ott C. 1997. 54. Menispermaceae. – In: Harling
G, Andersson L (eds), Flora of Ecuador 58, Nord. J. Bot., Copenhagen, pp.
1-79.
Ownbey GB. 1958. Monograph of the genus
Argemone in North America and the West Indies. – Mem. Torrey Bot.
Club 21: 1-159.
Ownbey GB. 1961. The genus Argemone
in South America and Hawaii. – Brittonia 13: 91-109.
Oxelman B, Lidén M. 1995. The position of
Circaeaster – evidence from nuclear ribosomal DNA. – Plant Syst.
Evol. [Suppl.] 9: 189-193.
Pabón-Mora N, Hidalgo O, Gleissber G, Litt
A. 2013. Assessing duplication and loss of APETALA1/FRUITFULL homologs in Ranunculales. – Frontiers Plant
Sci. 4: 358.
Panov PP, Mollov MM, Panova LN. 1971.
Alkaloids from plants of the Berberidaceae family. – Comp.
Rend. Acad. Bulgare Sci. 24: 675-677.
Parkin J, Sledge WA. 1933. An
Anemone from New Zealand; a plant hitherto regarded as a species of
Ranunculus. – J. Linn. Soc., Bot. 49: 645-651.
Paun O, Lehnebach C, Johansson JT, Lockhart
P, Hörandl E. 2005. Phylogenetic relationships and biogeography of
Ranunculus and allied genera (Ranunculaceae) in the
Mediterranean region and the European alpine system. – Taxon 54: 911-930.
Pawłowski B. 1963. Dispositio systematica
specierum europaearum generis Delphinium L. – Fragm. Flor. Geobot.
9: 429-450.
Payne WW, Seago JL. 1968. The open
conduplicate carpel of Akebia quinata (Berberidales: Lardizabalaceae). – Amer. J.
Bot. 55: 575-581.
Pellmyr O. 1984. Yellow jackets disperse
Vancouveria seeds. – Madroño 32: 56.
Pellmyr O. 1992. The phylogeny of a
mutualism: evolution and coadaptation between Trollius and its
seed-parasitic pollinators. – Biol. J. Linn. Soc. 47: 337-365.
Pellmyr O, Bergström G, Groth I. 1987.
Floral fragrances in Actaea using differential chromatograms to
discern between floral and vegetative volatiles. – Phytochemistry 26:
1603-1606.
Peng Y, Chen S-B, Liu Y, Chen S-L, Xiao P-G.
2006. A pharmacophylogenetic study of the Berberidaceae (s.l.). – Acta
Phytotaxon. Sin. 44: 241-257.
Pereira A de L. 1942. Contribuïção ao
conhecimento cariológico do género Nigella L. – Bol. Soc. Brot.,
Ser. II, 16: 5-40.
Pérez-Gutiérrez MA. 2014. Systematics and
evolution of the subfamily Fumarioideae. – Ph.D. thesis, Universidad de
Granada, Spain.
Pérez-Gutiérrez MA, Romero-García AT,
Salinas MJ, Blanca G, Fernández MC, Suárez-Santiago VN. 2012. Phylogeny of
the tribe Fumarieae (Papaveraceae s.l.) based on
chloroplast and nuclear DNA sequences: evolutionary and biogeographic
implications. – Amer. J. Bot. 99: 517-528.
Pérez-Gutiérrez MA, Romero-García AT,
Fernández MC, Blanca G, Salinas-Bonillo MJ, Suárez-Santiago VN. 2015.
Evolutionary history of fumitories (subfamily Fumarioideae, Papaveraceae): an old story shaped
by the main geological and climatic events in the Northern Hemisphere. – Mol.
Phylogen. Evol. 88: 75-92.
Peterson RL, Scott MG, Miller SL. 1979. Some
aspects of carpel structure in Caltha palustris L. (Ranunculaceae). – Amer. J. Bot.
66: 334-342.
Pfeifer S, Banerjee SK. 1964. Über
Rotfärbungsalkaloide der Gattung Papaver. – Pharmazie 19:
286-289.
Pfenninger A, Moser DM. 2002. Eine neue
Aquilegia-Art aus den Judikarischen Alpen (Valvestino, Prov. di
Brescia, Italien): Aquilegia vestinae Pfenningen & D. M. Moser.
– Candollea 57: 317-327.
Picci V. 1969. Embryological research on the
genus Thalictrum II. – Giorn. Bot. Ital. 103: 475-483.
Pigg KB, DeVore ML. 2005.
Paleoactaea gen. nov. (Ranunculaceae) fruits from the
Paleogene of North Dakota and the London Clay. – Amer. J. Bot. 92:
1650-1659.
Pohl J. 1894. Botanische Mitteilung über
Hydrastis canadensis. – Bibl. Bot. 29: 1-12.
Polhill RM. 1966. Berberidaceae. – In:
Milne-Redhead E, Polhill RM (eds), Flora of tropical East Africa, Crown Agents
for Oversea Governments and Administrations, London, pp. 1-4.
Popova M. 1973. Cytotaxonomic investigation
in genus Ranunculus L. – Izv. Bot. Inst. (Sofia) 24: 233-239.
Powell AM. 1972. A new species of
Argemone (Papaveraceae)
from Mexico. – Southw. Natur. 17: 106-107.
Praglowski J. 1974. Pollen morphology of the
Trochodendraceae,
Tetracentraceae,
Cercidiphyllaceae,
and Eupteleaceae with reference
to taxonomy. – Pollen Spores 16: 449-467.
Prain D. 1895. Some additional Papaveraceae. – J. Asiat. Soc.
Bengal 2, Nat. Hist. 64: 303-327.
Prain D. 1906. A review of the genera
Meconopsis and Cathcartia. – Ann. Bot. (Oxford) 20:
323-370.
Prain D. 1915. Some additional species of
Meconopsis. – Bull. Misc. Inform. Roy. Gard. Kew 1915: 129-177.
Pramanik A, Thothathri K. 1987. Miscellaneous
notes on two Indian moonseeds (Menispermaceae). – Kew Bull.
42: 705-706.
Prantl K. 1888. Beiträge zur Morphologie und
Systematik der Ranunculaceen. – Engl. Bot. Jahrb. Syst. 9: 225-273.
Prantl K. 1891a. Trochodendraceae.
– In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(2), W.
Engelmann, Leipzig, pp. 21-23.
Prantl K. 1891b. Ranunculaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien III(2), W. Engelmann,
Leipzig, pp. 43-66.
Prantl K. 1891c. Lardizabalaceae. – In: Engler
A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(2), W. Engelmann,
Leipzig, pp. 67-70.
Prantl K. 1891d. Berberidaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien III(2), W. Engelmann,
Leipzig, pp. 70-77.
Prantl K. 1891e. Menispermaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien III(2), W. Engelmann,
Leipzig, pp. 78-91.
Prantl K, Kündig J. 1891. Papaveraceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien III(2), W. Engelmann,
Leipzig, pp. 130-145.
Preininger V. 1986. Chemotaxonomy of Papaveraceae and Fumariaceae. –
In: Manske RHF (ed), The alkaloids, Vol. 29, Academic Press, London, New York,
pp. 1-98.
Price M, Waser N. 1979. Pollen dispersal and
optimal outcrossing in Delphinium nelsoni. – Nature 277: 294-296.
Qin H-N. 1989. An investigation on carpels of
Lardizabalaceae in relation
to taxonomy and phylogeny. – Cathaya 1: 61-82.
Qin H-N. 1997. A taxonomic revision of the Lardizabalaceae. – Cathaya
8-9: 1-214.
Rachele LD. 1974. Pollen morphology of the Papaveraceae of the northeastern
United States and Canada. – Bull. Torrey Bot. Club 101: 152-159.
Ramirez JL, Cevallos-Ferriz SRS. 2000. Leaves
of Berberidaceae
(Berberis and Mahonia) from Oligocene sediments, near Tepexi
de Rodriguez, Puebla. – Rev. Palaeobot. Palynol. 110: 247-257.
Ramsey GW. 1965. A biosystematic study of the
genus Cimicifuga (Ranunculaceae). – Ph.D. diss.,
University of Tennessee, Nashville, Tennessee.
Ramstadt E. 1953. Über das Vorkommen und die
Verbreitung von Chelidonsäure in einigen Pflanzenfamilien. – Pharm. Acta
Helv. 28: 45-57.
Rändel U. 1974. Beiträge zur Kenntnis der
Sippenstruktur der Gattung Papaver L. sectio Scapiflora
(Reichenb. (Papaveraceae). –
Feddes Repert. 84: 655-732.
Rasmussen DA, Kramer EM, Zimmer EA. 2009. One
size fits all? Molecular evidence for a common inherited petal identity program
in Ranunculales. – Amer. J.
Bot. 96: 96-109.
Rasmussen H. 1979. The genus
Knowltonia (Ranunculaceae). – Opera Bot. 53:
3-44.
Rastipishe S, Pakravan M, Tavassoli A. 2011.
Phylogenetic relationships in Ranunculus species (Ranunculaceae) based on nrDNA ITS
and cpDNA trnL-F sequences. – Prog. Briol. Sci. 1: 41-47.
Ratter J. 1968. Cytological studies in
Meconopsis. – Not. Roy. Bot. Gard. Edinb. 28: 191-200.
Raubeson LA, Peery R, Chumley TW, Dziubek C,
Fourcade HM, Boore JL, Jansen RK. 2007. Comparative chloroplast genomics:
analyses including new sequences from the angiosperms Nuphar advena
and Ranunculus macranthus. – BMC Genomics 8: 174.
Réaubourg MG. 1906. Étude organographique
et anatomique de la famille des Lardizabalées. – L’Université de
Paris.
Ren Y, Hu Z-H. 1995. The morphology of the
vegetative organs of Circaeaster agrestis (Ranunculaceae) and its taxonomic
significance. – Cathaya 7: 177-188.
Ren Y, Hu Z-H. 1998. Anatomical studies on
root, node and leaf of Kingdonia uniflora. – Acta Bot. Bor.-Occid.
Sin. 18: 72-77. [In Chinese]
Ren Y, Wang ML, Hu Z-H. 1998.
Kingdonia, embryology and its systematic significance. – Acta
Phytotaxon. Sin. 36: 423-427.
Ren Y, Li Z-J,Chang H-L, Lei Y-L, Lu A-M.
2004. Floral development of Kingdonia (Ranunculaceae s.l., Ranunculales). – Plant Syst.
Evol. 247: 145-153.
Ren Y, Li H-F, Zhao L, Endress PK. 2007.
Floral morphogenesis in Euptelea (Eupteleaceae, Ranunculales). – Ann. Bot. 100:
185-193.
Ren Y, Chang H-L, Tian X-H, Song P, Endress
PK. 2009. Floral development in Adonideae (Ranunculaceae). – Flora 204:
506-517.
Ren Y, Chang H-L, Endress PK. 2010. Floral
development in Anemoneae (Ranunculaceae). – Bot. J. Linn.
Soc. 162: 77-100.
Ren Y, Gu T-Q, Chang H-L. 2011. Floral
development of Dichocarpum, Thalictrum, and
Aquilegia (Thalictroideae, Ranunculaceae). – Plant Syst.
Evol. 292: 203-213.
Rifot M. 1973. Evolution structurale du pole
chalazien du sac embryonnaire d’Aquilegia vulgaris en liaison avec
son sac activité trophique. – Compt. Rend. Acad. Sci. Paris 277:
1313-1316.
Ro K-E. 1996. Outgroup relationships of the
Ranunculaceae (buttercup
family) inferred from rbcL gene sequences. – Korean J. Plant Taxon.
26: 51-71.
Ro K-E, Mcpheron BA. 1997. Molecular
phylogeny of the Aquilegia group (Ranunculaceae) based on internal
transcribed spacers and 5.8S ribosomal DNA. – Biochem. Syst. Ecol. 25:
445-461.
Ro K-E, Keener CS, McPheron BA. 1997.
Molecular phylogenetic study of the Ranunculaceae: utility of the
nuclear 26S ribosomal DNA in inferring intrafamilial relationships. – Mol.
Phylogen. Evol. 8: 117-127. – Erratum: Mol. Phylogen. Evol. 9: 181.
Ro K-E, Han H-Y, Lee S-T. 1999. Phylogenetic
contributions of partial 26S rDNA sequences to the tribe Helleboreae (Ranunculaceae). – Korean J.
Biol. Sci. 3: 9-15.
Röder L. 1958. Anatomische und
fluoreszenz-optische Untersuchungen an Samen von Papaveraceae. – Österr. Bot.
Zeitschr. 104: 370-381.
Rohweder O. 1967. Karpellbau und Synkarpie
bei Ranunculaceen. – Ber. Schweiz. Bot. Ges. 77: 376-432.
Roland F. 1971. Characterization and
extraction of the polysaccharides of the intine and of the generative cell wall
in the pollen grains of some Ranunculaceae. – Grana 11:
101-106.
Roland-Heydacker F. 1974. Caractères
ultrastructuraux et cytochimiques particuliers du sporoderme des polen de
Berberis vulgaris L. et de Mahonia aquifolium Nutt. –
Compt. Rend. Acad. Sci. Paris 278: 1475-1477.
Romero AT, Fernández M del C. 2000.
Development of exine and apertures in Fumaria densiflora DC. from the
tetrad stage to maturity. – In: Harley MM, Morton CM, Blackmore S (eds),
Pollen and spores: morphology and biology, Royal Botanic Gardens, Kew, pp.
45-56.
Romero AT, Salinas MJ, Fernández MC. 2003.
Pollen wall development in Hypecoum imberbe Sm. (Fumariaceae). –
Grana 42: 91-101.
Ronse De Craene L-P, Smets E. 1990. The
systematic relationship between Begoniaceae and
Papaveraceae: a comparative
study of their floral development. – Bull. Jard. Bot. Nat. Belg. 60:
229-272.
Ronse De Craene L-P, Smets E. 1992. An
updated interpretation of the androecium of the Fumariaceae. – Can. J. Bot.
70: 1765-1776.
Ronse De Craene L-P, Smets E. 1995. Evolution
of the androecium in the Ranunculiflorae. – Plant Syst. Evol. [Suppl.] 9:
63-70.
Ross CA, Auge H, Durka W. 2008. Genetic
relationships among three native North-American Mahonia species,
invasive Mahonia populations from Europe, and commercial cultivars.
– Plant Syst. Evol. 275: 219-229.
Rothfels K, Sexsmith E, Heimburger M, Krause
M. 1966. Chromosome size and DNA content of species of Anemone L. and
related genera (Ranunculaceae).
– Chromosoma 20: 54-74.
Rousi A. 1956. Cytotaxonomy and reproduction
in the apomictic Ranunculus auricomus group. – Ann. Bot. Soc.
Zool.-Bot. Fennica, ‘Vanamo’ 29:2.
Rozefelds AC. 1991. Mid Tertiary
Sarcopetalum (Menispermaceae) from Glencoe,
mid-eastern Queensland. – Alcheringa 15: 145-149.
Rudolph K. 1909. Zur Kenntnis des
anatomischen Baues der Blattgelenke bei den Menispermaceen. – Ber. Deutsch.
Bot. Ges. 27: 411-421.
Ruijgrok HWL. 1966. The distribution of
ranunculin and cyanogenetic compounds in the Ranunculaceae. – In: Swain T
(ed), Comparative phytochemistry, Academic Press, London, New York, pp.
175-186.
Ruijgrok HWL. 1967. Over de verspreiding van
ranunculine en cyanogene verbindingen bij de Ranunculaceae. Een bijdrage tot de
chemotaxonomie van de familie. – Ph.D. diss., Universiteit Leiden, The
Netherlands.
Ruiz E, Crawford DJ, Stuessy TF, Conzález F,
Samuel R, Becerra J, Silva M. 2004. Phylogenetic relationships and genetic
divergence among endemic species of Berberis, Gunnera,
Myrceugenia and Sophora of the Juan Fernández Islands
(Chile) and their continental progenitors based on isozymes and nrITS
sequences. – Taxon 53: 321-332.
Ryberg M. 1955. A taxonomical survey of the
genus Corydalis Ventenat. – Acta Horti Berg. 17: 115-175.
Ryberg M. 1960. A morphological study of the
Fumariaceae and the taxonomic significance of the characters examined. – Acta
Horti Berg. 19: 122-248.
Sachar RC. 1955. The embryology of
Argemone mexicana: a reinvestigation. – Phytomorphology 5:
200-218.
Sachar RC, Mohan Ram HY. 1958. The embryology
of Eschscholzia californica Cham. – Phytomorphology 8: 114-124.
Saksena HB. 1954. Floral morphology and
embryology in Fumaria parviflora Lamk. – Phytomorphology 4:
409-417.
Salinas MJ, Romero AT, Blanca G, Herrán R de
la, Garrido-Ramos M, Ruiz-Rejón C, Morales C, Ruiz-Rejón M, Suárez V. 2003.
Contribution to the taxonomy and phylogeny of Sarcocapnos (DC.)
(Fumariaceae). – Plant Syst. Evol. 237: 153-164.
Salisbury EJ. 1919. Variation in Eranthis
hyemalis, Ficaria verna, and other members of the Ranunculaceae, with special
reference to trimery and the origin of the perianth. – Ann. Bot. 33:
47-79.
Salisbury EJ. 1931. On the morphology and
ecology of Ranunculus parviflorus, L. – Ann. Bot. 45: 539-578.
Sands MJS. 1973. New aspects on the floral
vascular anatomy in some members of the Rhoeadales sensu Hutchinson. – Kew
Bull. 28: 211-256.
Santavý F. 1970. Papaveraceae alkaloids. – In:
Manske RHF (ed), The alkaloids, vol. 12, Academic Press, New York, pp.
333-454.
Santavý F. 1979. Papaveraceae alkaloids II. – In:
Manske RHF (ed), The alkaloids, vol. 17, Academic Press, New York, pp.
385-544.
Santisuk T. 1979. A palynological study of
the tribe Ranunculeae. – Opera Bot. 48: 1-76.
Sastri RLN. 1954. Embryological studies in Menispermaceae I. Tiliacora
racemosa Coleb. – Proc. Natl. Inst. Sci. India, B, 20: 294-302.
Sastri RLN. 1964. Embryological studies in
the Menispermaceae II. Embryo
and seed development. – Bull. Torrey Bot. Club 91: 79-85.
Sastri RLN. 1969a. Floral morphology,
embryology, and relationships of the Berberidaceae. – Aust. J. Bot.
17: 69-79.
Sastri RLN. 1969b. Comparative morphology and
phylogeny of the Ranales. – Biol. Rev. Cambridge Philos. Soc. 44: 291-319.
Satake Y. 1949. A note on Coptis of
Japan. – J. Jap. Bot. 24: 69-74.
Sauquet H, Carrive L, Poullain N, Sannier J,
Damerval C, Nadot S. 2015. Zygomorphy evolved from dissymmetry in Fumarioideae
(Papaveraceae, Ranunculales): new evidence from an
expanded molecular phylogenetic framework. – Ann. Bot. 115: 895-914.
Savitsky VD. 1989. Morphology of pollen and
taxonomy of species of genus Helleborus L. – Ukr. Bot. Žurn. 46:
27-32. [In Ukrainian]
Schaeppi H. 1976. Über die männlichen
Blüten einiger Menispermaceen. – Beitr. Biol. Pflanzen 52: 207-215.
Schaeppi H, Frank K. 1962.
Vergleichend-morphologische Untersuchungen über die Karpellgestaltung,
insbesondere die Plazentation bei Anemoneen. – Bot. Jahrb. Syst. 81:
337-357.
Schiffner V. 1889. Die Gattung
Helleborus. – Engl. Bot. Jahrb. Syst. 11: 92-122.
Schiffner V. 1890. Monographia Hellebororum.
– Nova Acta Kön. Leop.-Carol. Deutsch. Akad. Naturf. 56: 1-198.
Schmidt E. 1928. Untersuchungen über
Berberidaceen. – Beih. Bot. Centralbl., Abt. 2, 45: 329-396.
Schneider EL, Nichols DM. 1984. Floral
biology of Argemone aurantiaca (Papaveraceae). – Bull. Torrey
Bot. Club 111: 1-7.
Schnyder N. 1982. Analyse divergenter
Fruchtdifferenzierung bei einheitlichem Bauplan am Beispiel von
Berberis, Vancouveria und Caulophyllum (Berberidaceae). – Unpubl.
diploma thesis, University of Zürich.
Schöffel K. 1932. Untersuchungen über den
Blütenbau der Ranunculaceen. – Planta 17: 315-371.
Schorn HE. 1966. Revision of the fossil
species of Mahonia from North America. – M.Sc. thesis, University of
California, Berkeley, California.
Schrödinger R. 1909. Der Blütenbau der
zygomorphen Ranunculaceen und seine Bedeutung für die Stammesgeschichte der
Helleboreen. – Abh. K. K. Zool.-Bot. Ges. Wien 4-5: 1-63.
Schrödinger R. 1914. Das Laubblatt der
Ranunculaceen. Eine organogeschichtliche Studie. – Abh. K. K. Zool.-Bot. Ges.
Wien 8-2: 1-71.
Schuettpelz E, Hoot SB. 2004. Phylogeny and
biogeography of Caltha (Ranunculaceae) based on
chloroplast and nuclear DNA sequences. – Amer. J. Bot. 91: 247-253.
Schuettpelz E, Hoot SB, Samuel R, Ehrendorfer
F. 2002. Multiple origins of southern hemisphere Anemone (Ranunculaceae) based on plastid
and nuclear sequence data. – Plant Syst. Evol. 231: 143-151.
Schwarzbach AE, Kadereit JW. 1995. Rapid
radiation of North American desert genera of the Papaveraceae: Evidence from
restriction site mapping of PCR-amplified chloroplast DNA fragments. – Plant
Syst. Evol. [Suppl.] 9: 159-170.
Scott RA. 1956. Evolution of some endocarpal
features in the tribe Tinosporeae (Menispermaceae). – Evolution
10: 74-81.
Seitz N. 1969. Die Taxonomie der Aconitum
napellus-Gruppe in Europa. – Feddes Repert. 80: 1-76.
Selin E. 2000. Morphometric differentiation
between populations of Papaver radicatum (Papaveraceae) in northern
Scandinavia. – Bot. J. Linn. Soc. 133: 263-284.
Shen Y-F. 1954. Phylogeny and wood anatomy of
Nandina. – Taiwania 5: 85-92.
Shneyer VS, Kutyavina NG, Morosova NS. 1995.
Serotaxonomical investigation in the Papaverales. – Plant Syst. Evol.
[Suppl.] 9: 181-182.
Sieber M, Kucera LJ. 1980. On the stem
anatomy of Clematis vitalba L. – IAWA Bull., N. S., 1: 49-54.
Siplivinsky V. 1972. Genus Trollius
in Asia boreali et orientali. – Novit. Syst. Plant. Vasc. Acad. Sci. USSR 9:
163-182. [In Russian]
Slavík J, Hanuš V, Slavíková L. 1991.
Alkaloids from Stylophorum lasiocarpum (Oliv.) Fedde. – Collect.
Czech. Chem. Commun. 56: 1116-1122.
Slavikovà Z. 1971. Zur Blütenmorphologie
von Adonis vernalis L. – Österr. Bot. Zeitschr. 119: 447-453.
Smit PG. 1973. A revision of Caltha
(Ranunculaceae). – Blumea 21:
119-130.
Smith AC. 1946. A taxonomic review of
Euptelea. – J. Arnold Arbor. 27: 175-185.
Smith GH. 1926. Vascular anatomy of ranalian
flowers I. Ranunculaceae. –
Bot. Gaz. (Chicago) 82: 1-29.
Smith GH. 1928. Vascular anatomy of ranalian
flowers II. Ranunculaceae
(continued), Menispermaceae,
Calycanthaceae, Annonaceae. – Bot. Gaz.
(Chicago) 85: 152-177.
Smith UR. 2001. Revision of the Cretaceous
fossil genus Palaeoaster (Papaveraceae) and clarification of
pertinent species of Eriocaulon, Palaeoaster, and
Sterculiocarpus. – Novon 11: 258-260.
Solstad H, Elven R, Nordal I. 2003. Isozyme
variation among and within North Atlantic species of Papaver sect.
Meconella (Papaveraceae) and taxonomic
implications. – Bot. J. Linn. Soc. 143: 255-269.
Sorarú SB. 1976. Nota sobre el género
Argemone (Papaveraceae)
en la República Argentina. – Darwiniana 20: 445-457.
Sorokin H. 1929. Idiograms, nucleoli, and
satellites of certain Ranunculaceae. – Amer. J. Bot.
16: 407-420.
Souèges R. 1941. Embryogénie des
Fumariacées. L’origine des corps de l’embryon chez le Fumaria
officinalis. – Compt. Rend. Acad. Sci. Paris 216: 354-356.
Souèges R. 1943a. Embryogénie des
Fumariacées. L’origine et les premières divisions de la cellule
embryonnaire proprement dite chez Hypecoum procumbens. – Compt.
Rend. Acad. Sci. Paris 216: 310-311.
Souèges R. 1943b. Embryogénie des
Fumariacées. La différentiation des regions fondamentales des corps chez
Hypecoum procumbens. – Compt. Rend. Acad. Sci. Paris 216:
354-356.
Souèges R. 1946a. Embryogénie des
Fumariacées. Les premiers ternes du développement de l’embryon chez le
Corydalis lutea. – Compt. Rend. Acad. Sci. Paris 222: 161-163.
Souèges R. 1946b. Embryogénie des
Fumariacées. La différentiation des regions fondamentales des corps chez le
Corydalis lutea. – Compt. Rend. Acad. Sci. Paris 222: 253-255.
Souèges R. 1949. L’embryon chez le
Corydalis cheilanthifolia et la classification embryogénique. –
Ann. Sci. Nat. Bot., ser. II, 7: 1-17.
Soza VL, Brunet J, Liston A, Smith PS, Di
Stilio VS. 2012. Phylogenetic insights into the correlates of dioecy in
meadowrues (Thalictrum, Ranunculaceae). – Mol. Phylogen.
Evol. 63: 180-192.
Sreenivasulu Y, Rana B, Chanda SK, Ahuja PS.
2010. Development of female gametophyte in Podophyllum hexandrum Royle
– an important medicinal herb. – J. Biol. Life Sci. 1: 16-21.
Stapf O. 1925. Sargentodoxa cuneata.
– Bot. Mag. 151: t. 9111-9112.
Starmühler W. 1998. Proposal to conserve the
name Aconitum (Ranunculaceae) with A.
variegatum as its type. – Taxon 47: 747-748.
Stearn WT. 1938. Epimedium and
Vancouveria (Berberidaceae), a monograph. –
Bot. J. Linn. Soc. 51: 409-535.
Stearn WT. 1990. Epimedium
dolichostemon (Berberidaceae) and other Chinese
species of Epimedium. – Kew Bull. 45: 685-692.
Stearn WT. 1993. The small-flowered Chinese
species of Epimedium (Berberidaceae). – Kew Bull. 48:
807-813.
Stearn WT. 1996. Epimedium
acuminatum and allied Chinese species (Berberidaceae). – Kew Bull. 51:
393-400.
Stearn WT. 1997. Four new Chinese species of
Epimedium (Berberidaceae). – Kew Bull. 52:
659-671.
Stearn WT. 1998. Four more Chinese species of
Epimedium (Berberidaceae). – Kew Bull. 53:
213-223.
Stearn WT. 2002. The genus Epimedium
and other herbaceous Berberidaceae (including the genus
Podophyllum, by Julian M. H. Shaw). – Timber Press, Portland,
Oregon.
Stermitz FR, Adamovics JA. 1977. Alkaloids of
Caltha leptosepala and Caltha biflora. – Phytochemistry 16:
500.
Stern KR. 1961. Revision of
Dicentra. – Brittonia 13: 1-52.
Stern KR. 1962. The use of pollen morphology
in the taxonomy of Dicentra. – Amer. J. Bot. 49: 362-368.
Stern KR. 1970. Pollen aperture variation and
phylogeny in Dicentra. – Madroño 20: 354-359.
Still SM, Potter D. 2013. California poppy
conuncrums: insights into relationships within tribe Eschscholtzieae (Papaveraceae). – Syst. Bot. 38:
1045-117.
Strid AK. 1965. Studies in the Aegean flora
VII. Chromosome morphology in the Nigella arvensis complex. – Bot.
Not. 118: 139-165.
Strid AK. 1969. Evolutionary trends in the
breeding system of Nigella (Ranunculaceae). – Bot. Not. 122:
380-397.
Strid AK. 1970. Studies in the Aegean flora
XVI. Biosystematics of the Nigella arvensis complex with special
reference to the problem of non-adaptive radiation. – Opera Bot. 28:
1-169.
Sugiura T. 1940. Chromosome studies on Papaveraceae with special reference
to the phylogeny. – Cytologia 10: 558-576.
Sugiyama M. 1981. Comparative studies of
vascular system of node-leaf continuum in the Ranalian complex 2.
Sargentodoxa cuneata Rehd. et Wils. – Jap. J. Bot. 58: 252-258.
Sugiyama M. 1984 [1985]. Comparative studies
of vascular system in node-leaf continuum in Ranalian complex 3. Lardizabalaceae. –
Phytomorphology 34: 99-109.
Sugiyama M, Hara N. 1988. Comparative study
of early ontogeny of compound leaves in Lardizabalaceae. –Amer. J.
Bot. 75: 1598-1605.
Sun G, Dilcher DL, Wang H, Chen Z. 2011. A
eudicot from the early Cretaceous of China. – Nature 471: 625-628.
Sun H, McLewin W, Fay MF. 2001. Molecular
phylogeny of Helleborus (Ranunculaceae), with an emphasis
on the East Asian-Mediterranean disjunction. – Taxon 50: 1001-1018.
Sun Y, Fung K-P, Leung P-C, Shaw PC. 2005. A
phylogenetic analysis of Epimedium (Berberidaceae) based on nuclear
ribosomal DNA sequences. – Mol. Phylogen. Evol. 35: 287-291.
Susplugas J, Massa V, Susplugas P, Taillade
R, Susplugas C, Salabert J. 1975. Fumeterres en Languedoc Roussillon. – Anal.
Inst. Bot. Cavanilles 32: 233-239.
Swamy BGL. 1953. Some observations on the
embryology of Decaisnea insignis Hook. f. et Thoms. – Proc. Natl.
Inst. Sci. India, Sect. B, 19: 307-310.
Tak MA, Wafai BA. 1996. Somatic chromosome
structure and nucleolar organization in Anemone coronaria L.,
Ranunculus asiaticus L. and Eranthis hyemalis Salisb. (Ranunculaceae). –
Phytomorphology 46: 377-385.
Takeda H. 1915. On the genus Achlys:
a morphological study. – Bot. Mag. (Tokyo) 29: 169-185.
Tamaio N, Cardoso-Vieira R, Angyalossy V.
2009. Origin of successive cambia on stem in three species of Menispermaceae. – Rev. Brasil.
Bot. 32: 839-848.
Tamaio N, Joffily A, Braga JMA, Rajput KS.
2010. Stem anatomy and pattern of secondary growth in some herbaceous vine
species of Menispermaceae. –
J. Torrey Bot. Club 137: 157-165.
Tamura A. 1972. Morphology and phyletic
relationship of the Glaucidiaceae. – Bot. Mag.
(Tokyo) 85: 29-41.
Tamura M. 1956. Notes on Clematis of
Eastern Asia III. – Acta Phytotaxon. Geobot. 16: 79-83.
Tamura M. 1962a. Petiolar anatomy in the Ranunculaceae I. Structure of the
proper part of petioles. – Sci. Rep. Osaka Univ. 11: 19-47.
Tamura M. 1962b. Morphology, ecology, and
phylogeny of the Ranunculaceae
I. – Sci. Rep. Osaka Univ. 11: 115-126.
Tamura M. 1962c. Taxonomical and
phylogenetical consideration of the Ranunculaceae. – Acta
Phytotaxon. Geobot. 20: 71-81. [In Japanese]
Tamura M. 1963. Morphology, ecology, and
phylogeny of the Ranunculaceae
II. – Sci. Rep. Osaka Univ. 12: 141-156.
Tamura M. 1964. Morphology, ecology, and
phylogeny of the Ranunculaceae
III. – Sci. Rep. Osaka Univ. 13: 25-35.
Tamura M. 1965. Morphology, ecology and
phylogeny of the Ranunculaceae
IV. Ranunculaceae of Eastern
Asia: general part IV. – Sci. Rep. Osaka Univ. 14: 53-71.
Tamura M. 1966. Morphology, ecology and
phylogeny of the Ranunculaceae
VI. – Sci. Rep. Osaka Univ. 15: 13-35.
Tamura M. 1967. Morphology, ecology and
phylogeny of the Ranunculaceae
VII. – Sci. Rep. Osaka Univ. 16: 21-43.
Tamura M. 1968a. Morphology, ecology and
phylogeny of the Ranunculaceae
VIII. – Sci. Rep. Osaka Univ. 17: 41-56.
Tamura M. 1968b. A revision of genus
Naravelia. – Acta Phytotaxon.Geobot. 37: 106-110.
Tamura M. 1972. Morphology and phyletic
relationships of the Glaucidiaceae. – Bot. Mag.
(Tokyo) 85: 29-41.
Tamura M. 1980. Change of phyllotaxis in
Clematis lasiandra Maxim. – J. Jap. Bot. 55: 257-265.
Tamura M. 1981. Morphology of Coptis
japonica and its meaning in phylogeny. – Bot. Mag. (Tokyo) 94:
165-174.
Tamura M. 1984. Phylogenetical consideration
in the Ranunculaceae. –
Korean J. Plant Taxon. 14: 33-42.
Tamura M. 1987. A classification of genus
Clematis. – Acta Phytotaxon. Geobot. 38: 33-44.
Tamura M. 1990-1992. A new classification of
the family Ranunculaceae 1-3.
– Acta Phytotaxon. Geobot. 41: 93-101; 42: 177-187; 43: 53-58.
Tamura M. 1993a. New species and combinations
in the Ranunculaceae. – Acta
Phytotaxon. Geobot. 44: 27-28.
Tamura M. 1993b. Ranunculaceae. – In: Kubitzki K,
Rohwer JG, Bittrich V (eds), The families and genera of vascular plants II.
Flowering plants. Dicotyledons. Magnoliid, hamamelid and caryophyllid families,
Springer, Berlin, Heidelberg, New York, pp. 563-583.
Tamura M. 1995a. Angiospermae. Ordnung Ranunculales. Fam. Ranunculaceae. II Systematic part.
– In: Hiepko P (ed), Natürliche Pflanzenfamilien, 2. Aufl., 17aIV, Duncker
& Humblot, Berlin, pp. 223-519.
Tamura M. 1995b. Phylogeny and classification
of the Ranunculaceae. – Plant
Syst. Evol. [Suppl.] 9: 201-206.
Tamura M, Kosuge K. 1989. Classification of
the Isopyroideae (Ranunculaceae). – Acta
Phytotaxon. Geobot. 40: 31-35.
Tamura M, Lauener AL. 1968a. A note on
Isopyrum anemonoides Kar. & Kir. – Notes Roy. Bot. Gard. Edinb.
28: 265-266.
Tamura M, Lauener AL. 1968b. A revision of
Isopyrum, Dichocarpum, and their allies. – Notes Roy. Bot.
Gard. Edinb. 28: 267-273.
Tamura M, Lauener AL. 1979. A synopsis of
Aconitum subgenus Lycoctonum 2. – Notes Roy. Bot. Gard.
Edinb. 37: 430-466.
Tamura M, Mizumoto Y. 1972. Stages of embryo
development in ripe seeds or achenes of the Ranunculaceae. – J. Jap. Bot.
47: 225-237.
Tamura M, Mizumoto Y. 1974. The cotyledon and
growing point in the monocotyledonous embryos of Shibateranthis
pinnatifida and Anemone flaccida. – J. Jap. Bot. 49:
123-128.
Tan K, Shuka L, Siljak-Yakovlev S, Malo S,
Pustahija F. 2011. The genus Gymnospermium (Berberidaceae) in the Balkans. –
Phytotaxa 25: 1-17.
Tanaka R, Takahashi C. 1981. Comparative
karyotype analysis in Epimedium species by C-banding 1. E.
sempervirens and E. perralderianum. – J. Jap. Bot. 56: 1-24.
Taylor BAS. 1967. Comparative morphology and
phylogeny of the Lardizabalaceae. – Ph.D.
diss., Indiana University, Bloomington, Indiana.
Taylor G. 1934. An account of the genus
Meconopsis. – New Flora and Silva Ltd., London.
Tepfer SS. 1953. Floral anatomy and ontogeny
in Aquilegia formosa v. truncata and Ranunculus
repens. – Univ. Calif. Publ. Bot. 25: 513-648.
Terabayashi S. 1977. Studies in the
morphology and systematics of Berberidaceae I. Floral anatomy of
Ranzania japonica. – Acta Phytotaxon. Geobot. 28: 45-57.
Terabayashi S. 1978. Studies in the
morphology and systematics of Berberidaceae II. Floral anatomy
of Mahonia japonica (Thunb.) DC. and Berberis thunbergii DC.
– Acta Phytotaxon. Geobot. 29: 106-118.
Terabayashi S. 1979. Studies in the
morphology and systematics of Berberidaceae III. Floral anatomy
of Epimedium grandiflorum Morr. et Decne. ssp. sempervirens
(Nakai) Kitam. and Vancouveria hexandra (Hook.) Morr. et Decne. –
Acta Phytotaxon. Geobot. 30: 153-168.
Terabayashi S. 1981. Studies in the
morphology and systematics of Berberidaceae IV. Floral anatomy
of Plagiorhegma dubia Maxim., Jeffersonia diphylla (L.)
Pers., and Achlys triphylla (Smith) DC. ssp. japonica
(Maxim.) Kitam. – Bot. Mag. (Tokyo) 94: 141-157.
Terabayashi S. 1983a. Studies in the
morphology and systematics of Berberidaceae V. Floral anatomy of
Caulophyllum Michx., Leontice L., Gymnospermium
Spach, and Bongardia Mey. – Mem. Fac. Sci. Kyoto Univ., Ser. Biol.,
8: 197-217.
Terabayashi S. 1983b. Studies on the
morphology and systematics of Berberidaceae VI. Floral anatomy
of Diphylleia Michx., Podophyllum L., and Dysosma
Woodson. – Acta Phyotaxon. Geobot. 34: 27-47.
Terabayashi S. 1983c. Studies in the
morphology and systematics of Berberidaceae VII. Floral anatomy
of Nandina domestica Thunb. – J. Phytogeogr. Taxon. 31: 16-21.
Terabayashi S.1985a. The comparative floral
anatomy and systematics of the Berberidaceae I. Morphology. –
Mem. Fac. Sci. Kyoto Univ., Ser. Biol. 10: 73-90.
Terabayashi S. 1985b. The comparative floral
anatomy of the Berberidaceae
II. Systematic considerations. – Acta Phytotaxon. Geobot. 36: 1-13.
Terabayashi S. 1987. Seedling morphology of
the Berberidaceae. – Acta
Phytotaxon. Geobot. 38: 63-74.
Thanikaimoni G. 1968. Morphologie des pollens
des Ménispermacées. – Inst. Franç. Pondichéry, Trav. Sect. Sci. Techn. 5:
1-57.
Thanikaimoni G. 1984. Ménispermacées:
palynologie et systématique. – Inst. Franç. Pondichéry, Trav. Sect. Sci.
Techn. 18: 1-135.
Thanikaimoni G. 1986. Evolution of Menispermaceae. – Can. J. Bot.
64: 3130-3133.
Thanikaimoni G, Roland F, Ferguson IK,
Cerceau MT, Derouet L. 1984. Ménispermacées: palynologie et systématique.
– Trav. Sect. Sci. Techn. Inst. Franç. Pondichéry 18: 1-135.
Tian X-H, Zhang L, Ren Y. 2005 [2006].
Development of flowers and inflorescences of Circaeaster (Circaeasteraceae, Ranunculales). – Plant Syst.
Evol. 256: 89-96.
Tian X-H, Zhao L, Ren Y, Zhang X-H. 2007.
Number of floral organs in Circaeaster agrestis (Circaeasteraceae) and possible
homeosis among floral organs. – Plant Syst. Evol. 265: 259-265.
Tiffney BH. 1993. Fruits and seeds of the
Tertiary Brandon Lignite VII. Sargentodoxa (Sargentodoxaceae). –
Amer. J. Bot. 80: 517-523.
Tilquin P. 1981. An unusual case of a complex
heterozygote presenting no taxonomical problem in Chelidonium majus L.
(Papaveraceae). – Experientia
37: 341-342.
Tischler G. 1902. Die Berberidaceen und
Podophyllaceen. Versuch einer morphologisch-biologischen Monographie. – Engl.
Bot. Jahrb. Syst. 31: 596-727.
Tobe H. 1980. Morphological studies on the
genus Clematis Linn. VI. Vascular anatomy of the androecial and
gynoecial regions of the floral receptacle. – Bot. Mag. (Tokyo) 93:
125-133.
Tobe H. 1981. Embryological studies in
Glaucidium palmatum Sieb. et Zucc. with a discussion on the taxonomy
of the genus. – Bot. Mag. (Tokyo) 94: 207-224.
Tobe H. 2003. Hydrastidaceae. – In: Kubitzki
K, Bayer C (eds), The families and genera of vascular plants V. Flowering
plants. Dicotyledons. Malvales, Capparales and
non-betalain Caryophyllales,
Springer, Berlin, Heidelberg, New York, pp. 405-409.
Tobe H, Keating RC. 1985. The morphology and
anatomy of Hydrastis (Ranunculaceae): systematic
reevaluation of the genus. – Bot. Mag. (Tokyo) 98: 291-316.
Tören J. 1950. Les caractères
morphologiques, anatomiques, et cytologiques des Bongardia chrysogonum
Boiss. – Rev. Fac. Sci. Univ. Istanbul, sér. B, 15: 239-263.
Tören J. 1954. Recherches sur des écotypes
de Bongardia chrysogonum. – Rev. Fac. Sci. Univ. Istanbul, sér. B,
19: 83-123.
Tören J. 1961. Recherches sur les Berberidaceae de la Turquie I.
Morphologie et anatomie du Leontice leontopetalum L. – Rev. Fac.
Sci. Univ. Istanbul, sér. B., 26: 125-162.
Tören J. 1962. Recherches sur les Berberidaceae de la Turquie II.
Caractères cytologiques du Leontice leontopetalum L. – Rev. Fac.
Sci. Univ. Istanbul, sér. B., 27: 229-250.
Tören J. 1971. Investigation of the Berberidaceae of Turkey VII.
Bongardia chrysogonum (L.) Boiss. – Rev. Fac. Sci. Univ. Istanbul,
sér. B., 36: 81-88.
Torres N, Saez L, Rossello JA, Blanche C.
2000. A new Delphinium subsp. from Formentera (Balearic Islands). –
Bot. J. Linn. Soc. 133: 371-377.
Toyokuni H, Toyokuni Y. 1964. Ein neuer
Anhalt für die Teilung der Podophyllaceae in zwei Unterfamilien. – Bot. Mag.
(Tokyo) 77: 197-198.
Troll W. 1933. Beiträge zur Morphologie des
Gynoeceums III. Über das Gynoeceum von Nigella und einiger anderer
Helleboreen. – Planta 21: 266-291.
Troupin G. 1949. Contribution à l’étude
des Menispermacées africaines I. – Bull. Jard. Bot. État Bruxelles 19:
409-435.
Troupin G. 1956. Menispermaceae. – In: Turrill
WB, Milne-Redhead E (eds), Flora of tropical East Africa, Crown Agents for
Oversea Governments and Administrations, London, pp. 1-32.
Troupin G. 1960. 7. Menispermaceae. – In: Exell AW,
Wild H (eds), Flora Zambesiaca 1 (Part 1), Crown Agents for Oversea Governments
and Administrations, London, pp. 150-171.
Troupin G. 1962. Monographie des Menispermaceae africaines. –
Mém. Acad. Roy. Sci. Outre-Mer, Cl. Sci. Nat. Méd. Coll., ser. II, 13:
1-313.
Trzaski L. 1999. Xylem distribution in the
achene of some European Ranunculus species as a taxonomical criterion
of Ranunculus genus. – Phytomorphology 49: 241-251.
Tschermak-Woess E. 1956. Notizen über die
Riesenkerne und “Riesenchromosomen” in den Antipoden von Aconitum.
– Chromosoma 8: 114-134.
Tucker SC. 1966. The gynoecial vascular
supply in Caltha. – Phytomorphology 16: 339-342.
Tucker SC, Hodges SA. 2005. Floral ontogeny
of Aquilegia, Semiaquilegia and Enemion (Ranunculaceae). – Intern. J.
Plant Sci. 166: 557-574.
Ulbrich E. 1905-1906. Über die systematische
Gliederung und geographische Verbreitung der Gattung Anemone L. –
Engl. Bot. Jahrb. Syst. 37: 172-334.
Ulbrich E. 1906. Ranunculaceae. – In: Urban I
(ed), Plantae novae andinae imprimis Weberbauerianae I, Engl. Bot. Jahrb. Syst.
37: 404.
Ulbrich E. 1922. Ranunculaceae novae vel criticae
V. – Notizbl. Bot. Gart. Berlin-Dahlem 8: 251-272.
Ulbrich E. 1925. Ranunculaceae novae vel criticae
VII. Ranunculaceae asiaticae.
– Notizbl. Bot. Gart. Berlin-Dahlem 9. 209-228.
Valen F van. 1978. Contribution to the
knowledge of cyanogenesis in angiosperms 9. Communication. Cyanogenesis in
Papaverales. – Proc. Kon. Ned. Akad. Wet., Ser. C, 81: 492-499.
Valpuesta M, Posadas N, Ruiz I, Silva MV,
Gomez AI, Suau R, Perez B, Pliego F, Cabezudo B. 1995. Alkaloids from
Ceratocapnos heterocarpa plants in vitro cultures. – Phytochemistry
38: 113-118.
Valtueña FJ, Preston CD, Kadereit JW. 2012.
Phylogeography of a Tertiary relict plant, Meconopsis cambrica (Papaveraceae), implies the
existence of northern refugia for a temperate herb. – Mol. Ecol. 21:
1426-1437.
Vent W. 1973. Beiträge zur Kenntnis der
Sippenstruktur der Gattungen Bocconia L. und Macleaya R. Br.
(Papaveraceae). – Acta Bot.
Acad. Sci. Hung. 19: 385-391.
Vent W, Mory B. 1973. Beiträge zur Kenntnis
der Sippenstruktur der Gattungen Glaucium Adans. und
Dicranostigma Hooker f. et Thomson (Papaveraceae). – Gleditschia 1:
33-41.
Vesprini JL, Pacini E. 2000. Breeding systems
in two species of the genus Helleborus (Ranunculaceae). – Plant Biosyst.
134: 193-197.
Vijayaraghavan MR. 1970. Ranunculaceae. – Bull. Indian
Nat. Sci. Acad. 41: 45-52.
Vollesen K. 1981. Anisocycla (Menispermaceae) new to East
Africa. – Kew Bull. 36: 217-218.
Waddington KD. 1981. Factors influencing
pollen flow in bumblebee-pollinated Delphinium virescens. – Oikos
37: 152-159.
Walker JW. 1976. Comparative pollen
morphology and phylogeny of the ranalean complex. – In: Beck CB (ed), Origin
and early evolution of angiosperms, Columbia University Press, New York, pp.
241-299.
Wang F-H, Chien N-F, Zhang Y-L. 1984. A study
on the pollen morphology in Trochodendron, Tetracentron,
Euptelea. – Acta Phytotaxon. Sin. 22: 456-460. [In Chinese]
Wang H, Meng A, Li J, Feng M, Chen Z, Wang W.
2006. Floral organogenesis of Cocculus orbiculatus and Stephania
diesliana (Menispermaceae). – Intern. J.
Plant Sci. 167: 951-960.
Wang H-C, Meng A-P, Li J-Q, He Z-C. 2004.
Chromosome numbers for eight species in five genera of Menispermaceae. – J. Jap. Bot.
79: 241-246.
Wang H-F, Friedman CR, Zhu Z-X, Qin H-N.
2009. Early reproductive developmental anatomy in Decaisnea (Lardizabalaceae) and its
systematic implications. – Ann. Bot. 104: 1243-1253.
Wang H-F, Kirchoff BK, Qin H-N, Zhu Z-X.
2009. Reproductive morphology of Sargentodoxa cuneata (Lardizabalaceae) and its
systematic implications. – Plant Syst. Evol. 280: 207-217.
Wang W, Chen Z-D. 2007. Generic level
phylogeny of Thalictroideae (Ranunculaceae) – implications
for the taxonomic status of Paropyrum and petal evolution. – Taxon
56: 811-821.
Wang W, Li R-Q, Chen Z-D. 2005. Systematic
position of Asteropyrum (Ranunculaceae) inferred from
chloroplast and nuclear sequences. – Plant Syst. Evol. 255: 41-54.
Wang W, Wang H-C, Chen Z-D. 2007. Phylogeny
and morphological evolution of tribe Menispermeae (Menispermaceae) inferred from
chloroplast and nuclear sequences. – Persp. Plant Ecol. Syst. 8: 141-154.
Wang W, Chen Z-D, Liu Y, Li R-Q, Li J-H.
2007. Phylogenetic and biogeographic diversification of Berberidaceae in the Northern
Hemisphere. – Syst. Bot. 32: 731-742.
Wang W, Lu A-M, Ren Y, Endress ME, Chen Z-D.
2009. Phylogeny and classification of Ranunculales: evidence from four
molecular loci and morphological data. – Persp. Plant Ecol. Evol. Syst. 11:
81-110.
Wang W, Hu H, Xiang X-G, Yu S-X, Chen Z-D.
2010. Phylogenetic placements of Calathodes and Megaleranthis
(Ranunculaceae): evidence from
molecular and morphological data. – Taxon 59: 1712-1720.
Wang W, Ortiz R del C, Jacques FMB, Xiang
X-G, Li H-L, Lin L, Li R-Q, Soltis PS, Soltis DE, Chen Z-D. 2012. Menispermaceae and the
diversification of tropical rainforests near the Cretaceous-Paleogene boundary.
– New Phytol. 195: 470-478.
Wang W, Liu Y, Yu S-X, Gao T-G, Chen Z-D.
2013. Gymnaconitum, a new genus of Ranunculaceae endemic to the
Qinghai-Tibetan Plateau. – Taxon 62: 713-722.
Wang W, Dilcher DL, Sun G, Wang H-S, Chen
Z-D. 2016. Accelerated evolution of early angiosperms: evidence from
ranunculalean phylogeny by integrating living and fossil data. – J. Syst.
Evol. 54: 336-341.
Wang W, Lin L, Xiang XG, Ortiz RDC, Liu Y,
Xiang KL, Yu SX, Xing YW, Chen ZD. 2016. The rise of angiosperm-dominated
herbaceous floras: insights from Ranunculaceae. – Sci. Rep. 6:
27259.
Wang W, Ortiz R Del C, Jacques FMB, Chung
S-W, Liu Y, Xiang X-G, Chen Z-D. 2017. New insights into the phylogeny of
Burasaieae (Menispermaceae)
with the recognition of a new genus and emphasis on the southern Taiwanese and
mainland Chinese disjunction. – Mol. Phylogen. Evol. 109: 11-20.
Wang W-Q, Deng Z-R, Hong D-Y. 1998. The
systematic position of Beesia: evidence from ITS (nrDNA) sequence
analysis. – Acta Phytotaxon. Sin. 36: 403-410.
Wang W-T. 1974. Notulae de Ranunculaceis
sinensibus III. – Acta Phytotaxon. Sin. 12: 155-180.
Wang W-T, Hsiao P-K. 1965. Notulae de
Ranunculaceis sinensibus II. – Acta Phytotaxon. Sin. Add. 1: 49-103.
Wang W-T, Hong L-Q, Wang Z. 1999. Notulae de
Ranunculaceis sinensibus XXIII. – Acta Phytotaxon. Sin. 37: 209-219.
Wang W-T, Wang H-C, Chen Z-D. 2007. Phylogeny
and morphological evolution of tribe Menispermeae (Menispermaceae) inferred from
chloroplast and nuclear sequences. – Persp. Plant Ecol. Evol. Syst. 8:
141-154.
Wang X, Gong J-Z, Zhao L, Che X-F, Li H-N,
Ren Y. 2016. Flower morphology and development of the monotypic Chinese genus
Anemoclema (Ranunculaceae). – Plant Syst.
Evol. 302: 683-690.
Wang X-M, Zhang P, Du Q-G, He H-X, Zhao L,
Ren Y, Endress PK. 2012. Heterodichogamy in Kingdonia (Circaeasteraceae, Ranunculales). – Ann. Bot. 109:
1125-1132.
Wang X-Q, Hong D-Y Li Z-Y. 1993. A study on
pollen and seed coat in the tribe Cimicifugeae and some allied genera (Ranunculaceae). – Cathaya 5:
131-149. [In Chinese]
Wang X-Q, Li Z-Y, Hong D-Y. 1994. A
karyomorphological study of nine species in four genera of Ranunculaceae. – Cathaya 6:
43-56. [In Chinese]
Wang Z-F, Ren Y. 2008. Ovule morphogenesis in
Ranunculaceae and its
systematic significance. – Ann. Bot. 101: 447-462.
Warncke K. 1964. Die europäischen Sippen der
Aconitum lycoctonum-Gruppe. – Ph.D. diss., Universität München,
Germany.
Warnock MJ. 1981. Biosystematics of the
Delphinium carolinianum complex (Ranunculaceae). – Syst. Bot. 6:
38-54.
Webster SD. 1991. A chromatographic
investigation of the flavonoids of Ranunculus L. subgenus
Batrachium (DC.) A. Gray (water buttercups) and selected species in
subgenus Ranunculus. – Aquatic Bot. 40: 11-26.
Wefferling KM, Hoot SB, Neves SS. 2013.
Phylogeny and fruit evolution in Menispermaceae. – Amer. J. Bot.
100: 883-905.
Werner K, Ebel F. 1994. Zur Lebensgeschichte
der Gattung Helleborus L. (Ranunculaceae). – Flora 189:
97-130.
Werth E. 1941. Die Blütennektarien der
Ranunculaceen und ihre phylogenetische Bedeutung. – Ber. Deutsch. Bot. Ges.
59: 246-256.
West WC. 1969. Ontogeny of oil cells in the
woody Ranales. – Bull. Torrey Bot. Club 96: 329-344.
Wheeler MJ, Graaf BHJ de, Hadjiosif N, Perry
RM, Poulter NS, Osman K, Vatovec S, Harper A, Franklin FCH, Franklin-Tong VE.
2009. Identification of the pollen self-incompatibility determinant in
Papaver rhoeas. – Nature 459: 992-995.
Wiegleb G. 1988. Notes on Japanese
Ranunculus subgenus Batrachium. – Acta Phytotaxon. Geobot.
34: 117-132.
Wild H. 1960. 8. Berberidaceae. – In: Exell AW,
Wild H (eds), Flora Zambesiaca 1 (Part 1), Crown Agents for Oversea Governments
and Administrations, London, pp. 171-173.
Wilkinson HP. 1978. Leaf anatomy of the tribe
Coscinieae Hook. f. & Thomson (Menispermaceae). – Kew Bull.
32: 347-360.
Wilkinson HP. 1986. Leaf anatomy of
Tinomiscium and Fibraurea (Menispermaceae tribe Fibraureeae)
with special reference to laticifers and astrosclereids. – Kew Bull. 41:
153-169.
Wilkinson HP. 1989. Leaf anatomy of the Menispermaceae tribe Tiliacoreae
Miers. – Bot. J. Linn. Soc. 99: 125-174.
Williams LHJ. 1972. Meconopsis
taylorii, a new species from Nepal. – Trans. Bot. Soc. Edinb. 41:
347-349.
Woo H-K, Kim J-H, Yeau S-H, Lee NS. 2002.
Morphological and isozyme divergence in Korean Hepatica sensu stricto
(Ranunculaceae). – Plant
Syst. Evol. 236: 33-44.
Woodcock CL, Bell PR. 1968. Features of
ultrastructure of female gametophyte of Myosurus minimus. – J.
Ultrastruct. Res. 22: 546-563.
Woodson RE. 1928. Dysosma: a new
genus of Berberidaceae. –
Ann. Missouri Bot. Gard. 15: 335-340.
Wu C-Y, Kubitzki K. 1993a. Circaeasteraceae. – In:
Kubitzki K, Rohwer JG, Bittrich V (eds), The families and genera of vascular
plants II. Flowering plants. Dicotyledons. Magnoliid, hamamelid and
caryophyllid families, Springer, Berlin, Heidelberg, New York, pp. 288-289.
Wu C-Y, Kubitzki K. 1993b. Lardizabalaceae. – In:
Kubitzki K, Rohwer JG, Bittrich V (eds), The families and genera of vascular
plants II. Flowering plants. Dicotyledons. Magnoliid, hamamelid and
caryophyllid families, Springer, Berlin, Heidelberg, New York, pp. 361-365.
Wu J-Y, Qin H-N, He S-Z. 2009. A new species
of Mahonia (Berberidaceae) from China. –
Bot. J. Linn. Soc. 159: 357-361.
Wu Z-Y, Chuang H. 1980. A study on the
taxonomic system of the genus Meconopsis. – Acta Bot. Yunnan. 2:
371-381.
Xi Y-Z, Ning J-C. 1993. Pollen morphology and
its taxonomic significance of tribe Cimicifugeae. – Yushania 10: 45-60. [In
Chinese]
Xi Y-Z, Ning J-C, Fu X-P. 1993. Pollen
morphology of the tribe Trollieae and its taxonomic significance. – Cathaya
5: 115-130. [In Chinese]
Xia Q, Kong J. 1990. A study on the leaf
morphology and anatomy of the Lardizabalaceae,
Sargentodoxaceae, and their significance in taxonomy. – Bull. Bot. Res.
North-East. For. Inst. (Harbin) 10: 113-128. [In Chinese]
Xia Q, Peng Z-X. 1989a. A study of the pollen
morphology of Lardizabalaceae,
Sargentodoxaceae, and its significance in taxonomy. – Bull. Bot. Res.
North-East. For. Inst. (Harbin) 9: 99-114. [In Chinese]
Xia Q, Peng Z-X. 1989b. A study on the seed
of Lardizabalaceae and
Sargentodoxaceae 1. A SEM examination of testa. – Acta Phytotaxon. Sin. 27:
273-276. [In Chinese]
Xiang K-L, Aytaç Z, Liu Y, Espinosa F,
Jabbour F, Byng JW, Zhang C-F, Erst AS, Wang W. 2017. Recircumscription of
Delphinium subg. Delphinium (Ranunculaceae) and implications
for its biogeography. – Taxon 66: 554-566.
Xiang K-L, Zhao L, Erst AS, Yu S-X, Jabbour
F, Wang W. 2017. A molecular phylogeny of Dichocarpum (Ranunculaceae): implications for
eastern Asian biogeography. – Mol. Phylogen. Evol. 107: 594-604.
Xiao P. 1980. A preliminary study of the
correlation between phylogeny, chemical constituents and pharmaceutical aspects
in the taxa of Chinese Ranunculaceae. – Acta
Phytotaxon. Sin. 18: 142-153. [In Chinese]
Xiao W. 2013. Molecular systematics of
Meconopsis Vig. (Papaveraceae): taxonomy, polyploidy
evolution, and historical biogeography from a phylogenetic insight. – Ph.D.
diss., the University of Texas at Austin, Texas.
Xiao W, Simpson BB. 2014. The role of the
triploid hybrid in the evolution of Meconopsis (Papaveraceae): a preliminary study
on ancient polyploid and hybrid speciation. – Lundellia 17: 5-17.
Xiao W, Simpson BB. 2015. Phylogenetic
analysis of Meconopsis (Papaveraceae) and evaluation of two
controversial taxonomic species. – Lundellia 18: 14-18.
Xiao W, Simpson BB. 2017. A new infrageneric
classification of Meconopsis (Papaveraceae) based on a
well-supported molecular phylogeny. – Syst. Bot. 42: 226-233.
Xie D, He J, Huang J, Xie H, Wang Y, Kang Y,
Jabbour F, Guo J. 2015. Molecular phylogeny of Chinese Stephania (Menispermaceae) and reassessment
of the subgeneric and sectional classifications. – Aust. Syst. Bot. 28:
246-255.
Xie L, Li L-Q. 2012. Variation of pollen
morphology, and its implications in the phylogeny of Clematis (Ranunculaceae). – Plant Syst.
Evol. 298: 1437-1453.
Xie L, Wen J, Li L-Q. 2011. Phylogenetic
analysis of Clematis (Ranunculaceae) based on sequences
of nuclear ribosomal ITS and three plastid regions. – Syst. Bot. 36:
907-921.
Yang Q-E. 1995. On the chromosomes of
Calathodes (Ranunculaceae) and its systematic
position. – Acta Phytotaxon. Sin. 33: 453-460. [In Chinese]
Yang Q-E. 1999. Correction of karyotype of
diploid Beesia and discovery of a tetraploid cytotype. – Acta
Phytotaxon. Sin. 37: 1-9.
Yang Q-E. 2002a. Cytology of the tribe
Trollieae and of the tribe Cimicifugeae in the Ranunculaceae: a comparative
study. – Acta Phytotaxon. Sin. 40: 52-65.
Yang Q-E. 2002b. Cytology of ten species in
Anemone, one in Anemoclema and six in Clematis
(Trib. Anemoneae, Ranunculaceae) from China. –
Acta Phytotaxon. Sin. 40: 396-405.
Yang Q-E, Gong X, Gu Z-J, Wu Q-A. 1993. A
karyomorphological study of five species in the Ranunculaceae from Yunnan, with a
special consideration on systematic positions of Asteropyrum and
Calathodes. – Acta Bot. Yunnan. 15: 179-190. [In Chinese]
Yang Q-E, Luo Y-B, Hong D-Y. 1994. A
karyotypic study of six species in the Ranunculaceae from Hunan in China.
– Guihaia 14: 27-36. [In Chinese]
Yang TYA, Moore DM. 1999. A revision of the
Viorna group of species (section Viorna sensu Prantl) in the
genus Clematis (Ranunculaceae). – Syst. Geogr.
Plants 68: 281-303.
Yang W-J, Li L-Q, Xie L. 2009. A revision of
Clematis sect. Atragene (Ranunculaceae). – J. Syst. Evol.
47: 552-580.
Ying M, Xie H, Nie Z, Gu Z, Yang Y. 2006. A
karyomorphological study on four species of Meconopsis Vig. (Papaveraceae) from the Hengduan
Mountains, SW China. – Caryologia 59: 1-6.
Ying T-S. 1975. On the Chinese species of
Epimedium. – Acta Phytotaxon. Sin. 13: 49-56. [In Chinese]
Ying T-S. 1979. On Dysosma Woodson
and Sinopodophyllum Ying, gen. nov. of the Berberidaceae. – Acta
Phytotaxon. Sin. 17: 15-23. [In Chinese]
Ying T-S, Terabayashi S, Boufford DE. 1984. A
monograph of Diphylleia (Berberidaceae). – J. Arnold
Arbor. 65: 57-94.
Yong W, Su Q. 1993. Researches of the anatomy
of vegetative organs in relation to the systematic position of the
Sinofranchetia Hemsl. – Act. Bot. Boreal.-Occident. Sin. 13: 57-59.
[In Chinese]
Yoshida O, Michikawa A. 1973. Embryological
studies of genus Akebia Decaisne. – J. Coll. Arts Chiba Univ., Sect.
B, 6: 25-37.
Yu C-C, Chung K-F. 2017. Why
Mahonia? Molecular recircumscription of Berberis s.l., with
the description of two new genera, Alloberberis and
Moranothamnus. – Taxon 66: 1371-1392.
Yuan C. 2002. The phylogeny, systematics and
biogeography of Meconopsis Vig. (Papaveraceae) and Craigia W. W. Sm.
& W. E. Evans (Tiliaceae). – Ph.D. diss., Zhong Shan University.
Guangzhou.
Yuan Q, Yang Q-E. 2006a. Tribal relationships
of Beesia, Eranthis and seven other genera of Ranunculaceae: evidence from
cytological characters. – Bot. J. Linn. Soc. 150: 267-289.
Yuan Q, Yang Q-E. 2006b. Polyploidy in
Aconitum subgenus Lycoctonum (Ranunculaceae). – Bot. J. Linn.
Soc. 150: 343-353.
Yuan Q, Yang Q-E. 2006c. Cytology,
palynology, and taxonomy of Asteropyrum and four other genera of Ranunculaceae. – Bot. J. Linn.
Soc. 152: 15-26.
Yuan Q, Yang Q-E. 2008. Low incidence of
polyploids and high uniformity of karyotypes displayed by Delphinium
(Ranunculaceae) in the Hengduan
Mountains region of South-West China. – Bot. J. Linn. Soc. 158: 172-188.
Yuan Y, Peng Z. 1987. The karyotype analysis
of Helleborus thibetanus Franch. – Acta Bot. Bor.-Occid. Sin. 7:
133-137. [In Chinese]
Zamora PM. 1966. Studies on the primary xylem
elements in the order Ranales (sensu lato): a systematic survey. – Philipp.
Agr. 50: 389-623.
Zhang L-B, Rao G-X. 2009. Aporphine,
protoberberine and morphine alkaloids from the tubers of Stephania
yunnanensis. – Biochem. Syst. Ecol. 37: 622-625.
Zhang M-L, Uhink CH, Kadereit JW. 2007.
Phylogeny and biogeography of Epimedium/Vancouveria (Berberidaceae): western North
America-East Asian disjunctions, the origin of European mountain plant taxa,
and East Asian species diversity. – Syst. Bot. 32: 81-92.
Zhang M-Y, Lu L, Li D-Z, Wang H. 2012.
Evolution of pollen in the family Berberidaceae. – Plant Div. Res.
34: 1-12. [in Chinese]
Zhang X-H, Ren Y. 2008. Floral morphology and
development in Sargentodoxa (Lardizabalaceae). – Intern. J.
Plant Sci. 169: 1148-1158.
Zhang X-H, Ren Y. 2011. Comparative floral
development in Lardizabalaceae (Ranunculales). – Bot. J. Linn.
Soc. 166: 171-184.
Zhang X-H, Tian X-H, Pan L-Z. 2005.
Anatomical studies on Sinofranchetia chinensis (Lardizabalaceae) and their
systematic significance. – Bot. J. Linn. Soc. 149: 271-281.
Zhang X-H, Ren Y, Tian X-H. 2009. Floral
morphogenesis in Sinofranchetia (Lardizabalaceae) and its
systematic significance. – Bot. J. Linn. Soc. 160: 89-92.
Zhang Y, Hon Y, Ren C, Tang M, Hoot S, Yang
Q-E. 2015. Palynology, cytology, and molecular systematics of Anemone
section Begoniifolia (Ranunculaceae). – Plant Syst.
Evol. 301: 411-424.
Zhang Y, Kong H-H, Yang Q-E. 2015.
Phylogenetic relationships and taxonomic status of the monotypic Chinese genus
Anemoclema (Ranunculaceae). – Plant Syst.
Evol. 301: 1335-1344.
Zhang Y-L. 1983. Pollen morphology of
Kingdonia uniflora and its taxonomic significance. – Acta
Phytotaxon. Sin. 21: 441-444. [In Chinese]
Zhang Z-Y. 1982. Chromosome observation of
three ranunculaceous genera in relation to their systematic position. – Acta
Phytotaxon. Sin. 20: 402-409. [In Chinese]
Zhao L, Liu P, Che X-F, Wang W, Ren Y. 2011.
Floral organogenesis of Helleborus thibetanus and Nigella
damascena (Ranunculaceae)
and its systematic significance. – Bot. J. Linn. Soc. 166: 431-443.
Zhao L, Bachelier JB, Chang, H-L, Tian X-H,
Ren Y. 2012. Inflorescence and floral development in Ranunculus and
three allied genera in Ranunculeae (Ranunculoideae, Ranunculaceae). – Plant Syst.
Evol. 198: 1057-1071.
Zhao L, Wang W, Ren Y, Bachelier JB. 2012.
Floral development in Asteropyrum (Ranunculaceae): implications for
its systematic position. – Ann. Bot. Fenn. 49: 31-42.
Zhou L-H. 1979. New taxa of
Meconopsis fron Qinghai-Tibet Plateau. – Acta Phytotaxon. Sin. 17:
112-114. [In Chinese]
Zhou L-H. 1980. Study on the
Meconopsis of Qinghai-Tibet Plateau. – Bull. Bot. Lab. N.E. Forest.
Inst. 8: 91-101. [In Chinese]
Zhuang X. 1993. The taxonomy, evolution and
distribution of Papaveraceae.
– Acta Bot. Yunnan. 15: 137-148. [In Chinese]
Ziman SN. 1983. Evolutionary tendencies and
phylogeny of the Helleborus L. genus. – Ukr. Bot. Žurn. 40: 43-46.
[In Ukrainian]
Ziman SN, Keener CS. 1989. A geographical
analysis of the family Ranunculaceae. – Ann. Missouri
Bot. Gard. 76: 1012-1049.
Ziman SN, Keener CS, Kadota Y, Bulakh EV,
Tsarenko ON. 2006. A revision of Anemone L. (Ranunculaceae) from the Southern
Hemisphere. – J. Jap. Bot. 81: 193-224.
Ziman SN, Bulakh EV, Kadota Y, Keener CS.
2008. Modern view on the taxonomy of the genus Anemone L. sensu
stricto (Ranunculaceae). – J.
Jap. Bot. 83: 127-155.
Zonneveld BJM. 2001. Nuclear DNA contents of
all species of Helleborus (Ranunculaceae) discriminate
between species and sectional divisions. – Plant Syst. Evol. 229: 125-130.
Zunnunzhanov A, Iskandarov S, Yunusov SY.
1971. Darvasamine – a new alkaloid from Leontice darvasica. –
Chem. Nat. Compd. 7: 838-839.