[Capparales+Malvales]
CAPPARALES Juss. ex Bercht. et
J. Presl
Berchtold et Presl, Přir. Rostlin: 218. Jan-Apr
1820 [‘Capparideae’]
Brassicales Bromhead
in Edinburgh New Philos. J. 24: 416. Apr 1838;
Capparanae Reveal in Phytologia 76: 3. 2 Mai 1994
Fossils Dressiantha
bicarpellata from the Turonian Raritan Formation in New Jersey (late
Cretaceous) comprises somewhat zygomorphic flowers with four sepals, five
petals, five antepetalous stamens with disporangiate monothecal anthers
containing tricolporate pollen grains, a ring of basally connate
alternipetalous staminodia, and two basally connate carpels inserted on top of
a well developed gynophore.
Habit Usually bisexual
(sometimes monoecious, andromonoecious, gynomonoecious, polygamomonoecious,
dioecious, or polygamodioecious), evergreen or deciduous trees, shrubs or
suffrutices, or perennial, biennial or annual herbs (rarely lianas).
Vegetative anatomy Phellogen
ab initio superficially or deeply seated (subepidermal or pericyclic), or
absent. Secondary lateral growth usually normal (sometimes anomalous, from
concentric cambia) or absent. Vessel elements with simple or scalariform
(rarely reticulate) perforation plates; lateral pits usually alternate (rarely
opposite or scattered), simple or bordered pits. Vestured pits often present.
Imperforate tracheary xylem elements usually fibre tracheids or libriform
fibres (sometimes tracheids) with simple or bordered pits, septate or
non-septate (also vasicentric tracheids). Wood rays uniseriate or multiseriate,
homocellular or heterocellular. Axial parenchyma apotracheal diffuse (sometimes
diffuse-in-aggregates), or paratracheal scanty vasicentric, confluent, or
banded (rarely aliform, lozenge-aliform or confluent). Wood elements often
storied. Intraxylary phloem rarely present. Sieve tube plastids usually
S0 or Ss type (rarely Pcs type). Nodes 1:1, 1:2, 2:2 or ≥3:≥3,
unilacunar to trilacunar (rarely multilacunar), with one or three leaf traces
(rarely two or more traces). Laticifers sometimes present. Calciumcarbonate,
calciumsulfate and/or calciumoxalate crystals (as prismatic crystals, sometimes
druses) often frequent (sometimes also silica).
Trichomes Hairs unicellular or
multicellular, uniseriate or multiseriate, simple, furcate or many-branched
(occasionally T-shaped malpighiaceous hairs), stalked or unstalked, medifixed
(with two branches parallel to epidermis), stellate (with several or many
branches, from common point, parallel to epidermis), candelabra-shaped,
dendritic, peltate or lepidote (rarely prickles), or absent; glands or
glandular hairs unicellular or multicellular, uniseriate, simple (occasionally
peltate, sometimes with calciumcarbonate), or absent.
Leaves Alternate (usually
spiral, rarely distichous; rarely opposite), simple or pinnately or palmately
compound (sometimes twice or three times compound), entire or pinnately or
palmately lobed, sometimes coriaceous or succulent (rarely almost absent), with
conduplicate, supervolute, involute, flat or curved ptyxis. Stipules
intrapetiolar or cauline (sometimes replaced by glands, extrafloral nectaries
or spines), or absent; leaf sheath absent. Petiole vascular bundle transection
arcuate or annular. Venation usually pinnate (sometimes palmate, rarely
flabellate). Stomata usually anomocytic or anisocytic (sometimes paracytic,
rarely helicocytic, cyclocytic, tetracytic, staurocytic, etc.). Cuticular wax
crystalloids sometimes as rosettes of platelets (Fabales type) or
tubuli, or absent. Stomatal (including guard cells) or idioblastic myrosin
cells (with myrosinase) usually frequent. Mesophyll often with crystalliferous
cells containing calciumoxalate crystals or druses, or with sclerenchymatous
idioblasts containing dendrosclereids or other types of sclereids. Epidermis
usually with mucilage cells; epidermal cells sometimes with crystals. Leaf
margin serrate, sinuate or entire.
Inflorescence Terminal or
axillary, corymb, panicle, thyrsoid, raceme, spike or umbel (rarely catkin), or
solitary axillary. Bracts and floral prophylls (bracteoles) usually absent.
Flowers Actinomorphic,
zygomorphic or bisymmetric. Hypanthium sometimes present. Usually hypogyny
(rarely half epigyny). Receptacle in zygomorphic flowers sometimes with
nectariferous appendage or gland inside adaxial sepal; often elongated into
androgynophore or gynophore. Sepals (two to) four or five (to ten), usually
with imbricate (sometimes valvate, contorted or open) aestivation, in one or
two whorls, usually caducous, usually free (rarely connate or absent). Petals
(two to) four to six (to nine), with imbricate, valvate or contorted (rarely
open) aestivation, alternisepalous, often clawed, usually free (rarely absent).
Nectariferous disc usually extrastaminal or intrastaminal, annular, or absent;
nectariferous glands of various shape, inserted on disc, perianth or stamen, or
absent. Disc present or absent.
Androecium Stamens usually
four, five or 2+4 (sometimes eight to ten, rarely one, two or up to c. 250), in
one or several whorls, usually haplostemonous or diplostemonous (rarely
triplostemonous). Filaments usually in one whorl, usually free (sometimes more
or less connate), usually free from tepals (sometimes adnate at base to petals,
epipetalous). Anthers basifixed or dorsifixed, versatile or non-versatile,
usually tetrasporangiate (rarely disporangiate), usually introrse (sometimes
latrorse or extrorse), longicidal (dehiscing by longitudinal slits). Tapetum
secretory. Staminodia usually absent (sometimes four to ten).
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually 3–4-colpate (sometimes
3–4-colporate or 3–4-colporoidate, rarely 2–11-colpate, 2–11-colporate,
hexacolporoidate or inaperturate), shed as monads, bicellular or tricellular at
dispersal. Exine tectate or semitectate, usually with columellate (rarely
acolumellate, sometimes granular) infratectum, perforate, finely punctate,
reticulate, microreticulate, striate, foveolate, scabrate, spinulate or
smooth.
Gynoecium Pistil composed of
two to six (to eight) connate and often paracarpous carpels. Ventral carpellary
vascular bundles fused and highly developed. Ovary usually superior (rarely
semi-inferior), trilocular or ab initio unilocular, and later usually bilocular
with membranous secondary septum (rarely quinquelocular to octalocular),
usually sessile (sometimes stipitate, with gynophore). Style single, simple,
bilobate or trilobate, or absent, or stylodia (four or) five free. Stigma one,
capitate or lobate, or stigmas (four or) five, truncate or punctate (sometimes
flabellate or almost petaloid), usually papillate, Dry type. Pistillodium
usually absent (male flowers sometimes with pistillodium). Strongly developed
fused ventral carpellary vascular bundles often present.
Ovules Placentation axile,
apical or parietal (sometimes intrusively parietal, rarely basal-parietal,
basal or laminar). Ovules one to more than 300 per carpel, in one or two rows,
usually anatropous or campylotropous (rarely amphitropous), ascending,
horizontal or pendulous, apotropous or epitropous, usually bitegmic (sometimes
unitegmic), usually crassinucellar (rarely weakly crassinucellar to
incompletely tenuinucellar). Micropyle usually bistomal (rarely endostomal),
sometimes Z-shaped (zig-zag). Nucellar cap sometimes present. Hypostase often
present. Archespore usually unicellar (sometimes bicellular or multicellular).
Megagametophyte usually monosporous, Polygonum type (rarely disporous,
Allium type, or modified Drusa type). Synergids sometimes
with a filiform apparatus. Endosperm development ab initio nuclear. Endosperm
haustorium chalazal, lateral or absent. Embryogenesis usually onagrad
(sometimes asterad, rarely solanad).
Fruit Usually a loculicidal
and/or septicidal capsule (often dehiscing from base upwards by usually two
valves, with membranous secondary septum and persistent replum consisting of
placental tissue (sometimes a nut; rarely berry, pumpkin fruit, drupe, samara,
schizocarp, drupaceous syncarp, or an assemblage of follicles).
Seeds Aril usually absent
(elaiosome rarely present). Seed coat usually endotestal (sometimes exotegmic,
rarely also mesotegmic). Testa sometimes vascularized and multiplicative (outer
testal epidermis sometimes modified into mucilaginous sarcotesta). Exotestal
cells sometimes palisade, thick-walled. Mesotestal cell walls sometimes
sclerified or tanniniferous. Endotestal cell walls sometimes sclerified and
crystalliferous. Tegmen often multiplicative. Exotegmen and mesotegmen usually
crushed. Exotegmen sometimes (rarely also mesotegmen) sclerified, fibrous or
non-fibrous. Endotegmic cell walls sometimes tanniniferous and lignified.
Perisperm rarely developed. Endosperm copious or sparse, oily, or absent.
Embryo curved, plicate or straight, well differentiated, oily, with or without
chlorophyll. Cotyledons two (or three). Germination usually phanerocotylar
(sometimes cryptocotylar).
Cytology x = (4–)8(–15)
Endoplasmic reticulum with protein-rich dilated organelle-like cisternae.
DNA Plastid gene infA
lost/defunct. Mitochondrial intron coxII.i3 lost.
Phytochemistry Flavonols
(kaempferol, quercetin), cyanidin, delphinidin, ellagic and gallic acids,
caffeic acid, oleanolic acid derivatives, proanthocyanidins (prodelphinidins),
protoalkaloids, pyrrolidine alkaloids, piperidine alkaloids, glucosinolates
(mustard oil glycosides with R-N=C=S group, i.e. 2-hydroxy-2-methylpropylic
benzylglucosinolate, 3,4-dihydroxybenzyl glucosinolate, phenethylglucosinolate,
etc.), derived from phenylalanine, tyrosine, methionine, valine, isoleucine
and/or leucine, saponins, cyanogenic compounds, hydroxyproline betaines,
mustard oils based on glucotropaeolin, glucocapparin, glucocleomin,
cholinesters (sinapin, cochlearin, hesperalin, isoferuloylcholin,
p-cumaroylcholin, etc.) etc., erucic acid, n-eicose-11-enoic
acid, docosadienoic acid and other unsaturated fatty acids, sinapic acid,
ferulic acid, benzylisothiocyanate, methoxybenzylisothiocyanates,
cucurbitacins, myo-inositol, sweet-tasting proteins (brazzein,
pentadin), and aromatic m-carboxycinnamic acids and proteolytic
enzymes (papain, carpain) present. Myricetin, tannins and other polyphenolic
compounds rare.
More than 130 different kinds of glucosinolates
are known. Digested into glucose and aglucones by thioglucoside glucohydrolase
(myrosinase, a β-thioglucohydrolase), when tissues are wounded. The aglucones
are transformed into thiocyanates (mustard oils with R-N=C=S), toxic
isothiocyanates and often toxic or non-toxic nitriles and other compounds.
Systematics Capparales are sister-group to
Malvales.
Akaniaceae and Tropaeolaceae have the
following potential synapomorphies in common (Stevens 2001 onwards): young stem
with separate vascular bundles; vessel elements with scalariform perforation
plates; axial parenchyma sparse vasicentric; absence of floral prophylls
(bracteoles); flowers obliquely zygomorphic, large; presence of perianth tube
or hypanthium; petals clawed; stamens eight, with short connective
prolongations; style elongated; placentation apical or apical-axile; ovules one
or two per carpel, epitropous; and testa vascularized.
The clade [[Moringaceae+Caricaceae]+[Setchellanthaceae+[Limnanthaceae+[[Koeberliniaceae+[Bataceae+Salvadoraceae]]+[Emblingiaceae+[Pentadiplandraceae+[Gyrostemonaceae+[Borthwickiaceae+Resedaceae]]+Tovariaceae+Brassicaceae]]]]] has the common
feature: more than six ovules per carpel.
Moringaceae and Caricaceae share the following
potential synapomorphies (Stevens 2001 onwards): woody habit, with stout stems
(often pachycaul); cambium storied; nodes also multilacunar; presence of
ER-dependent vacuoles; stipules modified into glands; colleters present on
petiole and/or lamina; leaf venation palmate; cuticular wax crystalloids as
rosettes of platelets; inflorescence thyrse; flowers pentamerous, whitish;
carpels antesepalous; ovary longitudinally sulcate; style hollow; placentation
parietal, with placental vascular strands opposite ventral bundles; ovules
numerous per carpel; micropyle bistomal; outer integument five or six cell
layers thick; testa multiplicative; and mesotesta lignified.
The clade [Setchellanthaceae+[Limnanthaceae+[[Koeberliniaceae+[Bataceae+Salvadoraceae]]+[Emblingiaceae+[Pentadiplandraceae+[[Gyrostemonaceae+[Borthwickiaceae+Resedaceae]+Tovariaceae+Brassicaceae]]]] have the
following synapomorphies, according to Stevens (2001 onwards): nodes 1:1; and
extension of 3’ terminus of plastid gene rbcL.
The clade [Limnanthaceae+[[Koeberliniaceae+[Bataceae+Salvadoraceae]]+[Emblingiaceae+ [Pentadiplandraceae+[[Gyrostemonaceae+[Borthwickiaceae+Resedaceae]]+Tovariaceae+Brassicaceae]]]] is
characterized by the potential synapomorphy (Stevens 2001 and onwards): root
hairs arranged in vertical rows. Indole glucosinolates sometimes present.
The clade [[Koeberliniaceae+[Bataceae+Salvadoraceae]]+[Emblingiaceae+[Pentadiplandraceae+[[Gyrostemonaceae+[Borthwickiaceae+Resedaceae]]+Tovariaceae+Brassicaceae]]]]: style/stylodia
short or absent; ovules campylotropous; seed coat exotegmic; exotegmen fibrous;
embryo strongly curved; and glucosinolates synthesized from chain-elongated
branched-chain amino acids.
The [Koeberliniaceae+[Bataceae+Salvadoraceae]] clade is
characterized by the following features: absence of idioblastic myrosin cells;
flowers tetramerous; pollen grains tricolporoidate; pistil composed of two
connate carpels; fruit indehiscent; exotestal cells well developed; and x = 11.
Adaptations to dry and/or salt environments are very common.
Bataceae and Salvadoraceae share the
characteristics: storied wood; non-bordered perforation plates; wide,
multiseriate wood rays; nodes 1:2, unilacunar with two traces; opposite leaves;
bracts with apical colleters; secondary veins palmate, ascending from at or
near leaf base; paracytic stomata; basal placentation; two ovules per carpel;
non-fibrous exotegmen; absence of endosperm; and straight to somewhat curved
embryo.
The clade [Emblingiaceae+[Pentadiplandraceae+[[Gyrostemonaceae+[Borthwickiaceae+Resedaceae]]+ Tovariaceae+Brassicaceae]] have the
following potential synapomorphies, according to Stevens (2001 onwards):
cisternae of endoplasmic reticulum dilated and vacuole-like; cuticular wax
crystalloids absent; inflorescence terminal, racemose; floral prophylls
(bracteoles) absent; floral development open; petals clawed; nectary
extrastaminal; ovules inserted in two rows; endotesta crystalliferous;
extension of 3’ terminus of plastid gene rbcL; glucosinolates
synthesized also from valine/isoleucine and/or leucine and indole
glucosinolates from tryptophane.
Gyrostemonaceae and Resedaceae share the
synapomorphies: absence of idioblastic myrosin cells; unicellular hairs;
presence of stylodia; calyx persistent in fruit; and presence of funicular
aril.
Tiganophytaceae (Swanepoel & al. 2020). Tiganophyton (1; T. karasense; the arid Karas Region in southeastern Namibia).

|
Cladogram of Capparales based on DNA
sequence data (Rodman & al. 1996; Rodman & al. 1998, Hall &
al. 2004; Su & al. 2012). Forchhammeria (Stixaceae) is often
identified as sister to Resedaceae
(Cardinal-McTeague & al. 2016). Forchhammeria,
Tirania and Resedaceae form an
unresolved trichotomy in some analyses. The sister-group relationship
between Tovariaceae and Brassicaceae is
disputable. – Tiganophytaceae are sister to Salvadoraceae (incl. Batis) and Koeberlinia spinosa successive sister to those two clades, according to Swanepoel & al. (2020). Tiganophyton karasense is a small evergreen shrub with aerial branches differentiated into long and short shoots. The dimorphic leaves are alternate and the singly borne flowers are bisexual and laterally flattened. The calyx, corolla and androecium are tetramerous. The sepals are connate and the petals free. The disc is staminal and nectariferous glands are absent. The style is gynobasic (provisional interpretation by Swanepoel & al. 2020). The bilobate bilocular ovary is supported by an S-shaped gynophore. Each locule is biovulate. The nut-like fruit is dry and one-seeded. The plant contains glucosinolates.
|
Stapf in Bull. Misc. Inform. 1912: 380. 13 Dec
1912, nom. cons.
Bretschneideraceae
Engl. et Gilg, Engler’s Syllabus, ed. 9-10: 218. 6 Nov 1924, nom. cons.;
Akaniales Doweld in Byull. Mosk. Obshch. Ispyt.
Prir., Biol. 105(5): 60. 9 Oct 2000
Genera/species 2/2
Distribution Southeastern
China, Vietnam, Taiwan, Thailand, eastern Australia.
Fossils Uncertain. Fossil
leaves attributed to Akania have been found in Paleocene layers in
Argentina.
Habit Bisexual, evergreen or
deciduous trees.
Vegetative anatomy Phellogen
ab initio subepidermal. Primary medullary strands usually wide. Young stem with
separate vascular bundles. Vessel elements with simple and/or scalariform
(sometimes reticulate) perforation plates; lateral pits alternate, simple
and/or bordered pits. Imperforate tracheary xylem elements libriform fibres
with simple (Bretschneidera) or bordered (Akania) pits,
septate or non-septate. Wood rays multiseriate, heterocellular. Axial
parenchyma paratracheal scanty vasicentric or banded. Wood elements not
storied. Sieve tube plastids ? type (Akania) or P type (?)
(Bretschneidera). Nodes 3:3, trilacunar with three leaf traces. Bark
and inflorescences with idioblastic and stomatal myrosin cells (in
Akania sometimes absent). Calciumoxalate in Akania as druses
and single crystals.
Trichomes Hairs simple
(Akania).
Leaves Alternate (spiral),
imparipinnate, in Akania large, coriaceous, with supervolute to curved
ptyxis. Stipules small or absent; leaf sheath absent. Petiole vascular bundle
transection? Petiolules swollen or articulated. Venation pinnate. Stomata in
Akania present in groups, in Bretschneidera evenly dispersed.
Cuticular waxes absent; cuticular cracks distinct. Leaflet margins
spinose-serrate (Akania) or entire (Bretschneidera).
Inflorescence Terminal, raceme
(Bretschneidera) or axillary panicle (Akania). Floral
prophylls (bracteoles) absent.
Flowers Actinomorphic
(Akania) or obliquely zygomorphic (Bretschneidera).
Hypanthium or perianth tube cupular. Hypogyny or perigyny. Sepals five, with
imbricate aestivation, free (Akania) or connate
(Bretschneidera). Petals five, with contorted or imbricate
aestivation, often clawed, inner petals smaller than outer, free. Nectariferous
disc in Bretschneidera annular, intrastaminal; nectary and disc absent
in Akania.
Androecium Stamens five outer
and three to five inner or three (rarely four), diplostemonous; abaxial stamen
in antepetalous whorl (Akania), in Bretschneidera bent down
and inserted below one petal. Filaments hairy at base, inserted at receptacular
base (in Bretschneidera at nectariferous disc), free from each other
and from tepals. Anthers subbasifixed (Akania) or dorsifixed
(Bretschneidera), versatile, tetrasporangiate, latrorse
(Akania) or introrse (Bretschneidera), longicidal (dehiscing
by longitudinal slits). Tapetum secretory. Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually tricolpate (rarely
dicolpate), shed as monads, bicellular at dispersal. Exinc semitectate, with
columellate infratectum, reticulate.
Gynoecium Pistil composed of
three (or five) connate carpels. Ovary superior or semi-inferior, trilocular
(or quinquelocular), stipitate (with gynophore). Style single, simple, narrow
(in Bretschneidera bent down). Stigma small, capitate
(Bretschneidera), trilobate (in Bretschneidera hollow), type?
Pistillodium absent.
Ovules Placentation
apical-axile. Ovules two (or three) per carpel, anatropous (Akania) or
campylotropous (Bretschneidera), pendulous, epitropous?, bitegmic,
crassinucellar. Micropyle bistomal. Outer integument approx. five cell layers
thick, multiplicative, vascularized. Inner integument two or three cell layers
thick. Hypostase present. Megagametophyte in Akania monosporous,
Polygonum type; in Bretschneidera disporous, 8-nucleate,
Allium type. Endosperm development ab initio nuclear. Endosperm
haustoria? Embryogenesis?
Fruit A septicidal coriaceous
capsule.
Seeds Aril absent. Testa
vascularized, multiplicative. Exotestal cells palisade, thick-walled. Mesotesta
thick, with thick sclerified cell walls. Endotesta thickened. Tegmen not
multiplicative, thin, more or less crushed. Exotegmen non-fibrous. Perisperm
not developed. Endosperm copious(Akania) or absent
(Bretschneidera). Embryo large, straight, well differentiated,
chlorophyll? Cotyledons two, large, thick. Germination phanerocotylar.
Cytology n = 9
(Bretschneidera)
DNA
Phytochemistry Flavonols
(kaempferol, quercetin), cyanidin, proanthocyanidins (prodelphinidins),
alkaloids, and glucosinolates (2-hydroxy-2-methylpropylic and
3,4-dihydroxybenzylic glucosinolate, synthesized from tyrosine, in
Bretschneidera also from valine/isoleucine and/or leucine) present.
Cyanogenic compounds? Ellagic acid and saponins not found.
Use Ornamental plants
(Bretschneidera)?
Systematics Akania
(1; A. bidwillii; coastal areas in southern Queensland and
northeastern New South Wales), Bretschneidera (1; B.
sinensis; Yunnan, Hunan, northern Vietnam, Taiwan, Thailand).
Akaniaceae are sister to Tropaeolaceae.
Perleb, Clav. Class.: 17. Jan-Mar 1838
[‘Batideae’], nom. cons.
Batales Engl.,
Syllabus, ed. 5: 111. Jul 1907 [‘Batidales’]
Genera/species 1/2
Distribution Coasts of
southern New Guinea and northern Australia, tropical and subtropical coasts in
America and the Galápagos Islands.
Fossils Unknown.
Habit Dioecious (B.
maritima) or monoecious (B. argillicola), evergreen suffrutices
or shrubs. Succulent halophytes or xerophytes. Young stems quadrangular in
cross-section.
Vegetative anatomy Phellogen
ab initio in pericyclic fibre bundles. Secondary lateral growth often absent.
Vessel elements with simple perforation plates (with rudimentary borders);
lateral pits usually alternate (rarely opposite), bordered pits. Vestured pits
present. Imperforate tracheary xylem elements fibre tracheids with bordered
pits, non-septate? Wood rays usually multiseriate, heterocellular. Axial
parenchyma apotracheal or paratracheal scanty vasicentric. Wood elements
(including parenchyma) sometimes storied. Sieve tube plastids S type. Nodes
2?:2, bilacunar? with two leaf traces. Myrosin cells stomatal; idioblastic
myrosin cells absent. Crystals?
Trichomes Hairs usually
absent.
Leaves Opposite, simple,
entire, fleshy, reduced, with ? ptyxis. Stipules very small, without vascular
bundles, intrapetiolar or cauline, often caducous; leaf sheath absent. Leaf
traces? disappearing adjacent to nodes; leaf supported by two vascular bundles
from stem margins. Venation consisting of two bundles. Stomata paracytic or
anomocytic. Cuticular wax crystalloids? Myrosin cells stomatal. Leaf margin
entire.
Inflorescence Axillary,
racemose, in Batis maritima cone-like dense catkin (strobilus); in
Batis argillicola loose spike. Bracts in male inflorescences with
cochleariform and imbricate aestivation; bracts in female inflorescence small
and partially incorporated in fleshy spike. Floral prophylls (bracteoles)
absent?
Flowers Zygomorphic (male
flowers) or actinomorphic? (female flowers), minute. Female flowers in
Batis maritima connate. Hypogyny? Sepals? (bracteoles?) in male
flowers two, unequal in size (adaxial flowers larger than and overlapping
abaxial flowers), median, enclosing flower (Batis argillicola), or
four, connate (Batis maritima), absent in female flowers. Petals? in
male flowers four, clawed, free or connate at base, absent in female flowers.
Nectary absent. Disc absent.
Androecium Stamens four,
haplostemonous, antesepalous?, alternipetalous. Filaments free from each other
and from tepals. Anthers dorsifixed, versatile, tetrasporangiate, introrse,
longicidal (dehiscing by longitudinal slits); connective slightly prolonged
apically. Tapetum secretory, with binucleate cells. Staminodia absent.
Pollen grains
Microsporogenesis simultaneous? Pollen grains 3(–4)-colpor(oid)ate, shed as
monads, bicellular at dispersal. Exine pertectate, with acolumellate
infratectum and undifferentiated ectexine, microverrucate, almost smooth.
Ectexine spongy, undifferentiated.
Gynoecium Pistil composed of
two connate carpels; carpel divided by secondary septum. Ovary largely
quadrilocular (unilocular at base); ovaries of adjacent flowers more or less
connate and adnate to bract bases. Style very short or absent. Stigma
capitate-penicillate to slightly bilobate, persistent, papillate, type? Male
flowers often with rudimentary pistillodium.
Ovules Placentation
basal-parietal. Ovules two per carpel, collateral, anatropous, ascending,
epitropous, bitegmic, crassinucellar. Micropyle bistomal, Z-shaped (zig-zag).
Outer integument ? cell layers thick. Inner integument ? cell layers thick.
Nucellar cap present. Megagametophyte monosporous, Polygonum type?
Endosperm development nuclear? Endosperm haustoria? Embryogenesis?
Fruit A drupaceous syncarp
composed of fused flowers (Batis maritima), or a drupe with four
one-seeded pyrenes (Batis argillicola). Endocarp lignified.
Seeds Aril absent. Seed coat
exotestal? Testa membranous, multiplicative? Tegmen multiplicative? Exotegmen
non-fibrous? Perisperm not developed. Endosperm absent. Embryo almost straight,
well differentiated, chlorophyll? Cotyledons two, carnose. Germination
phanerocotylar.
Cytology n = 11 (Batis
maritima)
DNA
Phytochemistry Hydroxyproline
betaines, benzylglucosinolate and myrosinase present. Ellagic acid,
proanthocyanidins, alkaloids, and cyanogenic compounds not found. Tannins?
Use Occasionally as
vegetables.
Systematics Batis (2;
B. argillicola: coasts of southern New Guinea and Queensland; B.
maritima: tropical and subtropical Atlantic and Pacific coasts of America
and the Galápagos Islands, introduced in the Hawaiian Islands.
Batis is
sister to Salvadoraceae.
BORTHWICKIACEAE Su,
Wang, Zhang et Chen
|
( Back to Capparales )
|
Su, Wang, Zhang et Chen in Taxon 61(3): 608. Jun
2012
Genera/species 1/1
Distribution Yunnan, Burma.
Fossils Unknown.
Habit Bisexual, evergreen
shrub or small tree.
Vegetative anatomy Phellogen?
Secondary lateral growth? Vessel elements with simple? perforation plates;
lateral pits alternate?, simple? or bordered? pits. Vestured pits? Imperforate
tracheary xylem elements ? with bordered? pits, non-septate? Wood rays? Axial
parenchyma? Sieve tube plastids S type? Nodes? Myrosin cells? Crystals?
Trichomes Hairs unicellular or
multicellular?, simple.
Leaves Opposite, palmately
ternate compound (trifoliolate), with ? ptyxis. Stipules and leaf sheath
absent. Petiole vascular bundle transection? Venation palmate. Stomata ?-cytic.
Cuticular wax crystalloids? Leaflet margins entire.
Inflorescence Terminal raceme.
Inflorescence bracts caducous.
Flowers Actinomorphic.
Hypogyny. Sepals five to eight, spiral, with imbricate? aestivation,
membranous, connate into tube. Petals five to eight, with proximally valvate
and distally imbricate aestivation. Androgynophore present. Nectary conical,
ascending from petal base to staminal base, surrounding androgynophore. Disc
absent.
Androecium Stamens c. 60 to c.
70. Filaments free from each other and from tepals. Anthers dorsifixed?,
versatile?, tetrasporangiate, latrorse?, longicidal (dehiscing by longitudinal
slits). Tapetum secretory? Staminodia absent.
Pollen grains
Microsporogenesis simultaneous? Pollen grains tricolporate, shed as monads?,
?-cellular at dispersal. Exine tectate, with columellate? infratectum,
perforate.
Gynoecium Pistil composed of
four to six connate carpels. Ovary superior, quadrilocular to sexalocular,
stipitate (androgynophore). Style single, simple. Stigma capitate?, type?
Pistillodium absent.
Ovules Placentation axile,
with ovules in two rows. Ovules several per carpel, bitegmic?, crassinucellar?
Micropyle ?-stomal. Outer integument ? cell layers thick. Inner integument ?
cell layers thick. Megagametophyte monosporous?, Polygonum type?
Endosperm development? Endosperm haustoria? Embryogenesis?
Fruit A many-seeded
loculicidal capsule, dehiscing along ventral suture from base to apex, with
persistent 4–6-ridged axis.
Seeds Aril? Testa? Tegmen?
Perisperm not developed? Endosperm sparse or absent? Embryo curved, poorly
differentiated, chlorophyll? Cotyledons two. Germination?
Cytology n = ?
DNA
Phytochemistry Unknown.
Systematics
Borthwickia (1; B. trifoliata; southern Yunnan, eastern and
northern Burma).
Borthwickia is sister, with high
bootstrap support, to a trichotomy comprising Resedaceae, Forchhammeria
and [Tirania+Stixis] (Su & al. 2012).
Burnett, Outl. Bot.: 854, 1093, 1123. Feb 1835,
nom. cons.
Capparaceae Juss.,
Gen. Plant.: 242. 4 Aug 1789 [‘Capparides’], nom. cons.;
Cruciferae Juss., Gen. Plant.: 237. 4 Aug 1789, nom.
cons. et nom. alt.; Drabaceae Martinov, Tekhno-Bot.
Slovar: 215. 3 Aug 1820; Erysimaceae Martinov,
Tekhno-Bot. Slovar: 238. 3 Aug 1820 [‘Erysimoides’];
Sisymbriaceae Martinov, Tekhno-Bot. Slovar: 583. 3
Aug 1820; Thlaspiaceae Martinov, Tekhno-Bot. Slovar:
633. 3 Aug 1820 [‘Thlaspiceae’];
Cleomaceae Bercht. et J. Presl., Přir. Rostlin
2(64): 253. 1825 [‘Cleomeae’];
Stanleyaceae Nutt. in J. Acad. Nat. Sci. Philadelphia
7: 85. 28 Oct 1834 [‘Stanleae’];
Arabidaceae Döll, Rhein. Fl.: 573. 24-27 Mai 1843
[‘Arabideae’]; Raphanaceae Horan., Char.
Ess. Fam.: 169. 30 Jun 1847 [‘Cruciferae s.
Raphanaceae’]; Schizopetalaceae A. Juss.
in V. V. D. d’Orbigny, Dict. Univ. Hist. Nat. 11: 419. 9 Sep 1848
[‘Schizopetaleae’]; Isatidaceae Döll,
Fl. Baden 3: 1310. 30-31 Dec 1861; Oxystylidaceae
Hutch., Evol. Phylog. Fl. Pl.: 516. 28 Aug 1969
Genera/species
340–342?/3.205–3.355
Distribution Cosmopolitan
except continental Antarctica.
Fossils Fossil fruits and
seeds of Brassicaceae
have been described from Oligocene onwards. Fossil pollen grains from the Late
Cretaceous of New Zealand have been assigned to Brassicaceae.
Habit Usually bisexual (rarely
monoecious, andromonoecious or dioecious), usually perennial, biennial or
annual herbs (sometimes evergreen or deciduous shrubs, suffrutices, rarely
lianas or small trees). Some species are succulents and some are aquatic. Many
species are xerophytes.
Vegetative anatomy Mycorrhiza
usually absent. Root hairs absent in Capparoideae and
Cleomoideae. Phellogen ab initio usually deeply (cortical etc.; rarely
superficially) seated. Cortical and medullary vascular bundles usually absent.
Secondary lateral growth usually normal (sometimes anomalous from concentric
cambia, e.g., in Boscia) or absent. Vessel elements with simple
perforation plates; lateral pits usually alternate (rarely opposite or
scattered), bordered pits. Vestured (also on lateral vessel walls) and
non-vestured pits present. Imperforate tracheary xylem elements usually fibre
tracheids (sometimes libriform fibres) with simple or bordered pits,
non-septate (also vasicentric tracheids). Wood rays uniseriate or multiseriate,
usually homocellular (sometimes heterocellular). Axial parenchyma apotracheal
diffuse, or paratracheal scanty vasicentric, confluent, or banded. Wood
elements often storied. Secondary phloem in Capparoideae stratified
into hard fibrous and soft parenchymatous layers. Intraxylary phloem present in
some genera. Sieve tube plastids usually S type (rarely Pcs type). Endodermis
sometimes prominent. Nodes 1:1–3 or 3:3, unilacunar or trilacunar (rarely
multilacunar), with one or three leaf traces. Myrosin cells idioblastic.
Calciumcarbonate, calciumsulphate and calciumoxalate crystals (prismatic
crystals, sometimes druses) present in many representatives (in
Capparis also silica).
Trichomes Hairs unicellular or
multicellular, uniseriate or multiseriate, simple, furcate or many-branched
(occasionally T-shaped malpighiacean hairs), stalked or sessile, medifixed
(with two branches parallel to epidermis), stellate (with several or many
branches, from common point, parallel to epidermis), candelabra-shaped,
dendritic, peltate or lepidote (rarely prickles), often with calciumcarbonate
(usually as calcite), or absent; glandular hairs unicellular or multicellular
(in Cleomoideae peltate) or absent.
Leaves Alternate (usually
spiral, rarely distichous; rarely opposite), usually simple (rarely pinnately
or palmately compound), entire or (usually pinnately) lobed, sometimes
coriaceous (rarely succulent), with conduplicate ptyxis. Stipules usually
absent (rarely present, sometimes replaced by glands or extrafloral nectaries;
in Capparis and Cleome sometimes as spines); leaf sheath
absent. Petiole vascular bundle transection annular or arcuate. Venation
usually pinnate (sometimes palmate, in Pringlea flabellate). Stomata
usually anomocytic or anisocytic (sometimes helicocytic, rarely cyclocytic,
tetracytic, staurocytic, etc.). Cuticular wax crystalloids as transversely
ridged rodlets, chemically dominated by ketones etc. Stomatal myrosin cells
absent. Mesophyll cells often with sclerenchymatous idioblasts (with sclereids
of different types, e.g., dendrosclereids in Boscia). Epidermis
usually with mucilaginous idioblasts; epidermal cells sometimes
crystalliferous. Leaf margin serrate, lobate or entire. Extrafloral nectaries
rarely present on lamina (e.g. in Crataeva).
Inflorescence Terminal or
axillary, raceme, corymb, often branched, spike or umbel-like, or solitary
axillary. Bracts and floral prophylls (bracteoles) usually suppressed.
Flowers Usually bisymmetric
(sometimes zygomorphic or actinomorphic), often small. Usually hypogyny (rarely
half epigyny). Receptacle flat, wide or elongate (sometimes laciniate at apex
or with corona), in zygomorphic flowers often with nectariferous appendage or
gland inside adaxial sepal; often prolonged into short or long androgynophore
or gynophore. Sepals usually 2+2, decussate (rarely three, five or six),
usually with imbricate (sometimes valvate or open) aestivation, in one or two
whorls, usually caducous, usually free (rarely connate at base; at anthesis
rarely forming caducous calyptra). Petals (two to) four (to six), usually with
imbricate (rarely contorted or open) aestivation, alternisepalous, of equal
size or one pair of larger petals and one pair of smaller petals, usually
clawed, free (rarely absent). Nectariferous disc usually extrastaminal
(sometimes intrastaminal), annular (sometimes with three or four small
episepalous appendages), or absent; nectariferous glands of various shape and
insertion (on disc, perianth or stamen).
Androecium Stamens usually 2+4
(rarely one, two, four or up to c. 250), usually haplostemonous or
diplostemonous, two outer stamens usually shorter than four inner stamens
(tetradynamous), centrifugally developing from four primordia. Filaments
filiform (sometimes with appendages), usually in one whorl, usually free (two
median ones rarely connate), articulated, free from tepals. Anthers basifixed
or dorsifixed, sometimes versatile, tetrasporangiate, introrse or extrorse,
longicidal (dehiscing by longitudinal slits); connective sometimes slightly
prolonged. Tapetum secretory, with binucleate (to quadrinucleate) cells.
Staminodia (four to approx. ten, median-dorsal) present in some zygomorphic
species.
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually tricolpate (sometimes
tricolporate, tricolporoidate, 2–11-colpate, 2–11-colporate or
inaperturate), shed as monads, usually tricellular at dispersal (in
Capparoideae often bicellular). Exine tectate or semitectate, with
columellate infratectum, perforate or reticulate (sometimes smooth, spinulate
or finely punctate).
Gynoecium Pistil composed of
two (to eight to twelve?) paracarpously connate carpels. Ovary usually superior
(rarely semi-inferior), first unilocular, later usually bilocular (in
Capparoideae and Cleomoideae sometimes multilocular) with
thin secondary septum, usually sessile (in Capparoideae and
Cleomoideae usually stipitate, with long gynophore). Style single,
usually simple (rarely bifid), often persistent, or absent. Stigma usually one,
punctate or capitate (occasionally somewhat lobate), usually papillate, Dry
type. Male flowers sometimes with pistillodium?
Ovules Placentation parietal
(sometimes intrusively). Ovules one to more than 300 per carpel, usually
anatropous or campylotropous (through intrusion of chalazal vascular bundle;
rarely amphitropous), usually pendulous or horizontal, bitegmic, usually
crassinucellar (rarely tenuinucellar). Micropyle usually bistomal (Z-shaped;
rarely endostomal). Outer integument two or three cell layers thick. Inner
integument one to four cell layers thick. Endothelium (integumental tapetum)
present. Hypostase often present. Megagametophyte monosporous,
Polygonum type. Synergids sometimes with a filiform apparatus.
Endosperm development ab initio nuclear. Endosperm haustorium chalazal.
Embryogenesis usually onagrad (rarely solanad). Suspensor of various shape,
filamentous. Polyembryony occurs.
Fruit A usually septicidal
(rarely also loculicidal) capsule, siliqua, dehiscing from base upwards by
usually two valves, usually with membranous secondary commissural septum and
persistent replum (absent in Capparoideae) consisting of placental
tissue (sometimes a nut; rarely a berry, pumpkin fruit, drupe, samara, or
schizocarp, lomentum).
Seeds Aril usually absent
(present in some Capparoideae). Oily elaiosome present in some
species. Seed coat usually endotestal (in Capparoideae and
Cleomoideae usually exotegmic, rarely also mesotegmic). Testa
non-multiplicative, often winged. Exotesta often palisade. Outer testal layers
often mucilaginous. Endotestal cell walls often U-shaped, inner walls
thickened, and unthickened wall facing periphery of seed. Tegmen
multiplicative. Exotegmic cell walls often (rarely also mesotegmic cells walls)
sclerified, fibrous (Capparoideae, Cleomoideae) or
non-fibrous (Brassicoideae); exo- and mesotegmen degenerating.
Endotegmic cell walls often lignified, often tanniniferous. Perisperm developed
in Cadaba, Capparis, Cleome, and Crateva.
Endosperm one to more than two cell layers thick or absent. Embryo usually
curved or plicate (rarely straight), well differentiated, oily, with
chlorophyll. Cotyledons two, flattened or plicate, oily. Radicula dorsal or
lateral, usually present in testal pouch. Germination usually phanerocotylar
(sometimes cryptocotylar).
Cytology n = (7–)10(–80)
(Capparoideae); n = (9–)10(–19)20(–35) (Cleomoideae); n
= 4–128; x = (4–)8(–13) (Brassicoideae+Atamisquea: n =
8) – Polyploidy and aneuploidy frequently occurring. Agamospermy present in,
e.g., Boechera. Endoplasmic reticulum with protein-rich, wide,
organelle-like cisternae.
DNA Plastid gene ndhF
with insertion of 6 bp in at least some Capparoideae. Plastid gene
infA transferred to nucleus (Arabidopsis). Ancestral KCS6/5
(β-ketoacyl coenzyme A synthases) gene duplicated.
Phytochemistry Flavonols
(kaempferol, quercetin; Capparoideae), anthocyanins, ellagic acid,
proanthocyanidins (Capparoideae), pyrrolidine alkaloids
(Capparoideae), cyanogenic compounds, glucosinolates (synthesized from
phenylalanine and/or tyrosine and methionin, chain-extended), methyl
glucosinolates (glucocapparine and glucocleomine; Capparoideae and
Cleomoideae), sinapic acid, sinapine, cochlearine etc. (choline esters
of sinapic acid; seeds of Brassicoideae), ferulic acid, erucic acid,
n-eicose-11-enoic acid (Brassicoideae), docosadienoic acid
(Brassicoideae), erucic acid, quaternary ammonium compounds (i.a.
betaines; Capparoideae and Cleomoideae), and phytoalexins
(Brassicoideae) present. Tannins almost absent. Heavy metals
accumulated in some species.
Use Ornamental plants,
vegetables (Brassica spp., Lepidium sativum,
Nasturtium spp.), spices (Brassica spp., Capparis
spinosa, Armoracia rusticana, Eutrema wasabi), seed oils
(Brassica, Raphanus, Sinapis), forage plants, dyeing
substances (Isatis tinctoria), genetic research (Arabidopsis
thaliana model plant).
Systematics Brassicaceae is part of a
polytomy also comprising Pentadiplandraceae to
Tovariaceae.
The taxonomy of Brassicoideae below
follows Al-Shehbaz & al. (2006), German & al. (2009) and Warwick &
al. (2010).
Neothorelia (1; N. laotica;
Thailand, northern Laos) has six sepals and six petals, androgynophore, and
very small pollen grains. Its position is unclear, although it may belong
somewhere within Brassicaceae or Resedaceae. Neothorelia
laotica has sometimes been assigned to Capparoideae
(Capparaceae; Williams & Chayamarit 2005).
Capparoideae Burnett,
Outlines Bot.: 867. Feb 1835 [‘Capparidae’]
12?/430–440. ‘Crateva’
(10; tropical and subtropical regions; paraphyletic); ‘Capparis’
(c 250; tropical and subtropical regions; diphyletic), Boscia (c 30;
tropical and southern Africa, Madagascar, the Arabian Peninsula),
Maerua (c 75; tropical regions in the Old World), Bachmannia
(1; B. woodii; Mozambique to KwaZulu-Natal and Eastern Cape),
Ritchiea (c 30; tropical Africa), Buchholzia (2; B.
coriacea, B. tholloniana; tropical West Africa),
Euadenia (3; E. brevipetala, E. eminens, E.
trifoliolata; tropical Africa), Cladostemon (1; C.
kirkii; Mozambique, Zimbabwe, Swaziland, KwaZulu-Natal), Dhofaria
(1; D. macleishii; Oman), Cadaba
(c 30; southern Africa, Madagascar, Indian Ocean islands, the Arabian
Peninsula, India, Australia), Apophyllum (1; A. anomalum;
eastern Australia; in Brassicoideae?). – Tropical and subtropical
regions. Usually trees or shrubs (sometimes herbs or lianas). Rhizoids (root
hairs) absent. Petiole vascular bundle transection annular or arcuate. Leaf
base in Crateva glanduliferous. Sclereids present. Inflorescence
usually raceme (sometimes fascicle). Flowers actinomorphic or zygomorphic.
Sepals usually free (sometimes connate; sepals and petals sometimes fused into
tube). Petals four to numerous (sometimes absent). Stamens (one to) four to
numerous, centrifugally developing. Filaments usually long. Tectum with various
sculpturing. Pistil composed of two to twelve connate carpels; when two
carpels, then transversely orientated (superposed-oblique). Gynophore long.
Locules sometimes divided by secondary septa. Style usually absent. Outer
integument approx. two cell layers thick. Inner integument three or four cell
layers thick. Fruit usually indehiscent, baccate (rarely a transversely
dehiscent schizocarp or a septicidal capsule). Endotesta sometimes
crystalliferous. Tegmen multiplicative, up to six cell layers thick. Exotegmen
radially enlarged, sclerified. Anticlinal endotegmic cell walls with lignified
bands. n = (7–)10(to more than 15). Insertion of 6 bp in plastid gene
ndhF. Methyl glucosinolates and pyrrolidine alkaloids present. –
‘Crateva’ is probably sister group to the remaining
Capparoideae. ‘Capparis’
seems to consist of one American clade and one Old World clade, not immediately
related to one another.
[Cleomoideae+Brassicoideae]
Usually herbaceous, often annuals.
Inflorescence corymb. Stamens six. Pistil composed of two connate carpels.
Septicidal capsule with persistent placental vascular strands. Placenta
lignified. Seeds 0,5–4 mm long.
Cleomoideae Burnett,
Outlines Bot.: 867. Feb 1835 [‘Cleomidae’]
6/260–270. Haptocarpum (1;
H. bahiense; eastern Brazil), Cleomella (c 10; southwestern
Canada, United States, Mexico), Isomeris (1; I. arborea;
California, Mexico), Wislizenia
(1–3; W. californica, W. palmeri, W. refracta; southwestern
United States, Baja California), Oxystylis (1; O. lutea;
Death Valley in southeastern California), Cleome (c 250; tropical
and subtropical regions on both hemispheres). – Tropical, subtropical and
warm-temperate, with their largest diversity in America. Usually bisexual (some
species of Cleome
monoecious, with basal male flowers) herbs (Isomeris shrub).
C4 photosynthesis rare (some species of Cleome).
Rhizoids (root hairs) absent. Petiole vascular bundle transection arcuate.
Leaves usually palmately compound (occasionally simple, palmately lobed).
Stipules usually absent. Inflorescence racemose (corymb?). Bracts usually
foliaceous. Flowers zygomorphic (sometimes initially bisymmetric). Sepals four,
with abaxial sepal often much larger than and in bud covering remaining floral
parts. Petals four, clawed. Stamens six. Filaments long. Anthers spirally
twisted at dehiscence. Tectum with various sculpturing, often spinulate. Pistil
usually composed of two (sometimes four, orthogonally orientated) connate
carpels. Gynophore usually present (androgynophore sometimes present). Placenta
usually thin, lignified, loop-shaped, persistent in fruit. Outer integument two
or three cell layers thick. Inner integument two to ten cell layers thick.
Parietal tissue three to five cell layers thick. Nucellar cap approx. two cell
layers thick. Endothelium usually absent. Fruit usually capsular (in the
Dipterygium group of Cleome a winged single-seeded nut).
Seeds usually without aril. Exotegmic cells radially enlarged, sclerified.
Endotegmic periclinal cell walls with lignified bands. Suspensor sometimes
massive, haustorial. Cotyledons usually incumbent. n = more than 9. Genome
duplication present (perhaps c. 20 million years old). Methyl glucosinolates
present.
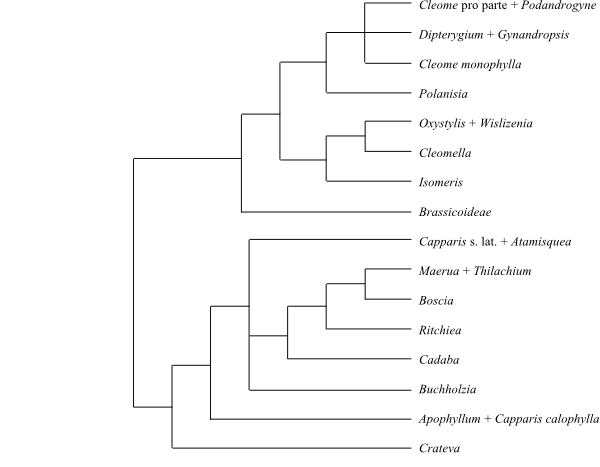 |
Cladogram of Cleomoideae and
Capparoideae based on DNA sequence data (Hall & al.
2002).
|
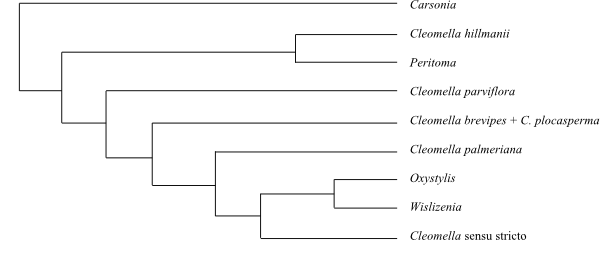 |
Cladogram (simplified) of Cleomoideae
pro parte based on DNA sequence data (Riser & al. 2013).
|
Brassicoideae Prantl,
Lehrbuch Bot.: 255. 1 Mar-15 Apr 1880 [‘Brassiceae’]
322–324/2.515–2.645. Cosmopolitan,
with their largest diversity in temperate regions in the Northern Hemisphere.
Usually herbs (sometimes shrubs, rarely trees). Mycorrhiza absent
(vesicular-arbuscular mycorrhiza possibly inhibited by glucosinolates;
arbuscular mycorrhiza reported from Thlaspi).
Phellogen at least usually deeply seated. Intraxylary phloem rare. Hairs
sometimes furcate, stellate or T-shaped. Stipules absent. Stomata anisocytic.
Inflorescence racemose (corymbose). Bracts usually absent (sometimes
foliaceous). Flowers usually bisymmetric (rarely zygomorphic), with closed
development. Petals rarely lobate or fimbriate. Stamens (two, four or) six (24
in Megacarpaea polyandra), two outer often shorter than four inner
(tetradynamous), approximately as long as petals. Lateral nectary lobes
positioned outside inner stamens. Pollen grains tricellular at dispersal.
Tectum often reticulate. Pistil composed of two connate carpels. Ovary usually
with commissural septum (absent in Pringlea). Style usually short
(rarely long). Gynophore usually absent. Stigma commissural (possibly
indicating a basically quadricarpellate gynoecium with axile placentation).
Outer integument two to four (or five) cell layers thick. Inner integument (two
or) three to eight (to 15) cell layers thick. Parietal tissue approx. one cell
layer thick. Hypostase present. Endothelium present. Archespore sometimes
multicellular. Fruit usually a siliqua (often explosively dehiscent) with
persistent lignified placental strands and membranous secondary septum, replum
(fruit occasionally latiseptate or angustiseptate). Seed plicate, but seed coat
not bulging inwards. Testa often mucilaginous, three-layered. Radial exotestal
cell walls reticulately thickened. Endotesta palisade, lignified, often with
U-shaped thickenings, without crystals. Tegmen often multiplicative,
non-persistent. Chalazal endosperm cyst present (possibly involved in transport
of metabolites into seed; transfer cells present around cyst). Endosperm
one-layered. Embryo plicate (sometimes spirally twisted). Cotyledons sometimes
conduplicate-incumbent. Radicula not present in testal pouch. x = 4 (before
Ata duplication), 7, 8 (after Ata duplication) (–13).
Sporophytic self-incompatibility frequent. Duplication of whole genome
(Ata palaeopolyploidization). Duplication of nuclear gene
PHYB leading to gene PHYD. Nortropane alkaloids sometimes
present. Methyl glucosinolates absent. Nickel or zinc accumulated in many
species. Selenium accumulated in, e.g., Stanleya pinnata.
–Aethionemateae (Aethionemodae) are sister-group to the
remaining Brassicoideae.
Aethionemateae
Al-Shehbaz, Beilstein et E. A. Kellogg in Plant Syst. Evol. 259: 110. 19 Jul
2006
1/c 55. Aethionema
(c 55; the Mediterranean, southeastern Europe and southwestern Asia to
Afghanistan). – Annual or perennial herbs or shrubs. Hairs absent. Petal claw
three-veined. Nectaries two, lateral. Gynoecium sessile or almost sessile.
Ovules (one or) two to four (to eight) per carpel. Fruit a flattened, winged
angustiseptate silicula, often nut-like. Testa sometimes mucilaginous. x = 7,
8, 11, 12, 14, 16, 18, 21, 22, 24, 30. Nortropane alkaloids present. – Aethionema
is sister to Brassicodae = the remaining Brassicoideae (Koch
& al. 2001, etc.).
Brassicodae V. E.
Avet. in Biol. Žurn. Armenii 43: 602. 12 Oct 1990
[‘Brassicidinae’]
321–323/2.460–2.590. Eglandular
hairs sometimes branched, stellate or T-shaped. Inflorescence rarely with
glandular stipules. Pollen grains covered by tryphine (not Pollenkitt). Genome
duplication present. – The phylogenetic relationships among the main clades
are in part very uncertain.
Scoliaxoneae
Al-Shehbaz & S. I. Warwick in Taxon 60: 1161. 4 Aug 2011
1/1. Scoliaxon (1; S.
mexicanus; northeastern Mexico). – Hairs simple or branched, minute.
Fruit a globose siliqua. – Scoliaxon is not included in any of the
studies by German & al. (2009) or Warwick & al. (2010). In the analyses
by Warwick & al. (2011) it was identified as a basal lineage.
[Erysimeae+[Cardamineae+[[[Descurainieae+Yinshanieae]+[Lepidieae+Smelowskieae]]+[Camelineae+[[Boechereae+[Halimolobeae+Oreophytoneae]]+[Turritideae+[[Crucihimalayeae+Physarieae]+Pachycladinae+Microlepidieae+[Dipoma+Hemilophia]+Alyssopsideae]]]]]]]
Erysimeae Dumort., Fl.
Belg.: 123. 1827
1/c 180. Erysimum
(c 180; Europe, Macaronesia, the Mediterranean, Africa, temperate Asia, North
America). – Hairs sometimes malpighiaceous. Cardenolides present.
[Cardamineae+[[[Descurainieae+Yinshanieae]+[Lepidieae+Smelowskieae]]+[Camelineae+[[Boechereae+[Halimolobeae+Oreophytoneae]]+[Turritideae+[[Crucihimalayeae+Physarieae]+Pachycladinae+Microlepidieae+[Dipoma+Hemilophia]+Alyssopsideae]]]]]]
Cardamineae Dumort.,
Fl. Belg.: 124. 1827
c 12/c 345. Aplanodes (2;
A. doidgeana: KwaZulu-Natal; A. sisymbrioides: Eastern Cape,
Lesotho), Armoracia
(3; A. macrocarpa, A. rusticana, A. sisymbrioides;
eastern Europe to Siberia, eastern North America), Barbarea
(22; Europe, northern Asia, North America), Cardamine
(c 200; cosmopolitan), Iodanthus (1; I. pinnatifidus; eastern
United States from Connecticut and Minnesota to Texas and Alabama), Leavenworthia
(8; southern and southeastern United States), Nasturtium
(c 15; temperate regions on the Northern Hemisphere), Ornithocarpa (2;
O. fimbriata, O. torulosa; Mexico), Planodes (2;
southern United States, northern Mexico), Rorippa
(c 85; nearly cosmopolitan, especially temperate regions), Selenia (5;
S. aurea, S. dissecta, S. geniculata, S.
grandis, S. jonesii; southern United States, Mexico),
Sisymbrella (1; S. aspera; western Mediterranean). – Hairs
simple or absent. Cotyledons accumbent. Fruit latiseptate or terete (in Armoracia
angustiseptate). x = 8. Plastid trnF pseudogene present.
[[[Descurainieae+Yinshanieae]+[Lepidieae+Smelowskieae]]+[Camelineae+[[Boechereae+[Halimolobeae+Oreophytoneae]]+[Turritideae+[[Crucihimalayeae+Physarieae]+Pachycladinae+Microlepidieae+[Dipoma+
Hemilophia]+Alyssopsideae]]]]]
[[Descurainieae+Yinshanieae]+[Lepidieae+Smelowskieae]]
[Descurainieae+Yinshanieae]
Descurainieae
Al-Shehbaz, Beilstein et E. A. Kellogg in Plant Syst. Evol. 259: 111. 19 Jul
2006
6/c 47. Descurainia
(c 35; Europe, Macaronesia, the Mediterranean, Asia, North and South America),
Robeschia (1; R. schimperi; the Middle East; in Descurainia?),
Trichotolinum (1; T. deserticola; Patagonia; in Descurainia?),
Hornungia
(5; H. alpina, H. aragonensis, H. pauciflora, H.
petraea, H. procumbens; Europe, the Mediterranean, one species,
H. procumbens, a widespread weed), Ianhedgea (1; I.
minutiflora; southwestern Asia to China), Tropidocarpum (4;
T. californicum, T. capparideum, T. gracile, T.
lanatum; California, Mexico, Chile). – Europe, Macaronesia, the
Mediterranean, Asia, North and South America. Hairs usually dendritic (rarely
dichotomous). Cotyledons incumbent. x = 6(Hornungia),
7.
Yinshanieae
Al-Shehbaz, Warwick, Mummenh. et M. A. Koch in Plant Syst. Evol. 285: 229. Mai
2010
1/7–13. Yinshania (7–13;
China, northern Vietnam). – Hairs simple, dichotomous or absent. Fruit a
latiseptate or angustiseptate silicula. Cotyledons incumbent. –
Yinshania is sister to Descurainieae, according to Warwick
& al. (2010).
[Lepidieae+Smelowskieae]
Lepidieae DC. in Mém.
Mus. Hist. Nat. 7(1): 240. 20 Apr 1821 [‘Lepidineae’]
3/175–220. Cyphocardamum (1;
C. aretioides; Afghanistan), Lepidium
(175–220; temperate and subtropical regions), Lithodraba (1; L.
mendociensis; Argentina). – Cosmopolitan. Hairs simple or absent. Ovule
one per locule. Fruit angustiseptate. Seeds often mucilaginous. Cotyledons
diplecolobal. x = 8.
Smelowskieae
Al-Shehbaz, Beilstein et E. A. Kellogg in Plant Syst. Evol. 259: 111. 19 Jul
2006
1/25. Smelowskia (25; Pakistan
to Central and northeastern Asia, northwestern North America). – Hairs
branched. Seeds not mucilaginous. Cotyledons incumbent. x = 6 (also n =
10–12). – Smelowskia is sister to Lepidieae.
[Camelineae+[[Boechereae+[Halimolobeae+Oreophytoneae]]+[Turritideae+[[Crucihimalayeae+Physarieae]+Pachycladinae+Microlepidieae+[Dipoma+Hemilophia]+Alyssopsideae]]]]
Camelineae DC. in
Mém. Mus. Hist. Nat. 7(1): 239. 20 Apr 1821
8/36. Arabidopsis
(11; Europe, tropical African mountains, temperate Asia, North America), Camelina
(8; central and southeastern Europe, eastern Mediterranean to central Asia), Capsella
(8; eastern Mediterranean, western Asia), Catolobus (1; C.
pendulus; eastern Europe, temperate Asia), Chrysochamela (4;
C. draboides, C. elliptica, C. noeana, C.
velutina; eastern Mediterranean to Russia), Neslia
(1; N. paniculata; southeastern Europe, the Mediterranean,
southwestern Asia), Noccidium (2; N. hastulatum, N.
tuberculatum; southwestern Asia), Pseudoarabidopsis (1; P.
toxophylla; Central Asia to western China). – Europe, temperate Asia,
northern North America. Hairs simple, stalked or sessile, or stellate. Fruit
usually a terete, latiseptate or quadrangular (in Capsella
angustiseptate) siliqua (in Camelina,
Capsella
and Neslia a
silicula). x = (6–)8(–11) (x = 4 in Stenopetalum; x = 5 in
Arabidopsis thaliana). – Camelineae are probably
non-monophyletic.
[[Boechereae+[Halimolobeae+Oreophytoneae]]+[Turritideae+[[Crucihimalayeae+Physarieae]+Pachycladinae+Microlepidieae+[Dipoma+Hemilophia]+Alyssopsideae]]]
[Boechereae+[Halimolobeae+Oreophytoneae]]
Boechereae
Al-Shehbaz, Beilstein et E. A. Kellogg in Plant Syst. Evol. 259: 111. 19 Jul
2006
8/c 118. Anelsonia (1; A.
eurycarpa; western United States), Boechera
(c 110; the Russian Far East, Canada, Greenland, the United States, northern
Mexico), Borodinia (1; B. macrophylla; eastern Siberia),
Cusickiella (2; C. douglasii, C. quadricostata;
western United States), Nevada (1; N. holmgrenii; Nevada),
Phoenicaulis (1; P. cheiranthoides; western North America),
Polyctenium (1; P. fremontii; western North America),
Sandbergia (1; S. whitedii; northwestern United States). –
The Russian Far East, North America. Hairs usually branched (in Nevada
simple; in Boechera
few or absent). x = 7.
[Halimolobeae+Oreophytoneae]
Halimolobeae
Al-Shehbaz, Beilstein et E. A. Kellogg in Plant Syst. Evol. 259: 111. 19 Jul
2006
5/45. Exhalimolobos (10;
Mexico to South America), Halimolobos (10; southwestern United States
to Central America), Mancoa (9; Central and South America),
Pennellia (10; southwestern United States, Mexico, Guatemala, Bolivia,
northern Argentina), Sphaerocardamum (6; S. compressum,
S. divaricatum, S. macropetalum, S. macrum, S.
nesliiforme, S. stellatum; Mexico). – Southwestern United
States to South America. Hairs branched. Seeds mucilaginous as wet. x = 8.
Plastid trnF pseudogene present.
Oreophytoneae
Al-Shehbaz, Warwick, Mummenh. et M. A. Koch in Plant Syst. Evol. 285: 229. Mai
2010
2/6. Murbeckiella
(5; M. boryi, M. huetii, M. pinnatifida, M.
sousae, M. zanonii; southwestern Europe, western Mediterranean,
the Caucasus), Oreophyton (1; O. falcatum; tropical mountains
in East and Northeast Africa). – Southwestern Europe, northwestern Africa,
tropical East Africa. Hairs simple or multifurcate, stipitate, or absent. Fruit
a latiseptate siliqua. Cotyledons incumbent.
[Turritideae+[[Crucihimalayeae+Physarieae]+Pachycladinae+Microlepidieae+[Dipoma+Hemilophia]+Alyssopsideae]]
Turritideae Buchenau,
Fl. Nordwestdeut. Tiefebene: 258. 28 Apr 1894 [‘Turritinae’]
1/4. Turritis
(4; T. brassica, T. chilensis, T. glabra, T.
laxa; Europe, Africa, western Asia; T. brassica: mountains in
central and southern Europe). – Fruit a siliqua, tetrangular in
cross-section. Hairs simple or stellate.
[[Crucihimalayeae+Physarieae]+Microlepidieae+[Dipoma+Hemilophia]+Alyssopsideae]
[Crucihimalayeae+Physarieae]
Crucihimalayeae D.
German et Al-Shehbaz in Nord. J. Bot. 28: 647. 15 Dec 2010
3/14. Crucihimalaya
(12; Central Asia, the Himalayas, Mongolia), Ladakiella (1; L.
klimesii; Ladak in northwestern India), Transberingia
(1; T. bursifolia; eastern Russia, western North America, southwestern
Greenland). – Northeastern Asia, one species also in North America and
southwestern Greenland.
Physarieae B. L.
Rob., Syn. Fl. N. Amer. 1: 100. 1895
7/133–138. Dimorphocarpa (4;
D. candicans, D. membranacea, D. pinnatifida, D.
wislizeni; southwestern Canada, western United States), Dithyrea
(2; D. californica, D. maritima; southwestern United States,
northwestern Mexico), Lyrocarpa (3; L. coulteri, L.
linearifolia, L. xantii; California, Mexico),
Nerisyrenia (9; southern United States, Mexico), Paysonia (8;
southeastern United States), Physaria
(105–110; southwestern Canada, western United States, northwestern Mexico),
Synthlipsis (2; S. densiflora, S. greggii; southern
United States, Mexico). – Southwestern Canada to northern Mexico, Bolivia,
Argentina. Hairs usually sessile, stellate (in Paysonia also simple,
dichotomous and stalked substellate). Pollen grains with at least four colpi.
Ovules two or more per locule. Fruit an angustiseptate or inflated silicula (in
Nerisyrenia sometimes a siliqua). x = 8 (n = 4–11).
Microlepidieae
Al-Shehbaz, Warwick, Mummenh. et M. A. Koch in Plant Syst. Evol. 285: 228. Mai
2010 (incl. Pachycladinae O. E. Schulz in Engler et Prantl, Nat.
Pflanzenfam. 86: 19, 181. 22 Jul 1924)
16/55. Pachycladon (10; the
Southern Alps in New Zealand, Tasmania), Menkea (6; M.
australis, M. crassa, M. draboides, M. lutea,
M. sphaerocarpa, M. villosula; southern Australia),
Cuphonotus (2; C. andraeanus, C. humistratus; arid
regions in Australia), Irenepharsus (3; I. magicus, I.
phasmatodes, I. trypherus; southeasternmost South Australia,
southeastern Victoria and New South Wales), ‘Arabidella’ (7;
A. chrysodema, A. eremigena, A. filifolia, A.
glaucescens, A. nasturtium, A. procumbens, A.
trisecta; drier regions in Australia; polyphyletic),
Phlegmatospermum (4; P. cochlearinum, P. drummondii,
P. eremaeum, P. richardsii; drier regions in southern
Australia), Harmsiodoxa (3; H. blennodioides, H.
brevipes, H. puberula; arid regions in Australia),
Stenopetalum (10; Australia), Drabastrum (1; D.
alpestre; eastern Victoria, southeastern New South Wales),
Pachymitus (1; P. cardaminoides; southeastern South
Australia, western Victoria, southern New South Wales), Ballantinia
(1; B. antipoda; south central Victoria, Tasmania), Geococcus
(1; G. pusillus; southern semi-arid regions in Australia),
Carinavalva (1; C. glauca; central Australia),
Microlepidium (2; M. alatum, M. pilosulum;
southwestern Western Australia, coastal South Australia), Scambopus
(1; S. curvipes; South Australia), Blennodia (2; B.
canescens, B. pterosperma; central Australia). – Australia, New
Zealand. Hairs simple or bi- to multifurcate, or sometimes stellate or absent.
Fruit an angustiseptate silicula or a terete siliqua. Cotyledons incumbent.
Dipoma clade
1 or 2/1 or 6. Dipoma (1;
D. iberideum; southwestern China); Hemilophia (5; H.
franchetii, H. pulchella, H. rockii, H.
serpens, H. sessilifolia; southwestern China). – Southwestern
China. Hairs simple or more or less malpighiaceous (sometimes slightly
dichotomous). Fruit a terete or slightly angustiseptate siliqua
(Dipoma) or a silicula (Hemilophia). – Dipoma fell
into a tetrachotomy also consisting of Physarieae,
Microlepidieae and Alyssopsideae, according to Warwick &
al. (2010). Hemilophia may possibly belong in this clade.
Alyssopsideae
Al-Shehbaz, Warwick, Mummenh. et M. A. Koch in Plant Syst. Evol. 285: 228. Mai
2010
4/9. Alyssopsis (2; A.
mollis, A. trinervis; Iran to Central Asia), Calymmatium
(2; C. draboides, C. notorrhizum;Central Asia),
Dielsiocharis (2; D. bactriana, D. kotschyi;
Iran, Tajikistan), Olimarabidopsis (3; O. cabulica, O.
pumila, O. umbrosa; eastern Mediterranean to western China). –
East Mediterranean to China. Hairs bi- or multifurcate, stalked. Cotyledons
accumbent or incumbent.
Alysseae DC.
in Mém. Mus. Hist. Nat. 7(1): 231. 20 Apr 1821 [‘Alyssineae’]
24/150–220. Alyssum
(100–170; Europe, the Mediterranean and North Africa to the Middle East,
western, Central and East Asia; non-monophyletic?), Hormathophylla (1;
H. halimifolia; western Mediterranean), Lutzia (1; L.
fruticosa; Crete, Karpathos, Astipalea, Kasos), Irania (5; I.
compacta, I. membranacea, I. multicaulis, I.
pendula, I. umbellata; Iraq, Iran, Afghanistan),
Clastopus (2; C. erubescens, C. vestitus; Turkey,
Iran, Iraq), Physoptychis (3; P. caspica, P.
haussknechtii, P. purpurascens; eastern Turkey to northwestern
Iran), Pterygostemon (1; P. spathulatus; northwestern China,
eastern Kazakhstan), Degenia (1; D. velebitica; Croatia),
Acuston (1; A. lunarioides; Crete and the Kikladhes in
Greece), Alyssoides (1; A. utriculata; southern and
southeastern Europe, Turkey), Resetnikia (1; R. triquetra;
coast of Croatia), Brachypus (1; B. asper; eastern Turkey and
Armenia to Iraq, Iran and Central Asia), Fibigia (6; F.
clypeata, F. eriocarpa, F. lunarioides, F.
macrocarpa, F. obovata, F. suffruticosa; eastern
Mediterranean and Ukraine to Iran and Afghanistan), Phyllolepidum (2;
P. cyclocarpum, P. rupestre; the Apennines, the Balkan
Peninsula, Turkey), Bornmuellera (9; the Balkan Peninsula, Turkey),
Takhtajaniella (1; T. globosa; Azerbaijan), Cuprella
(2; C. antiatlantica, C. homalocarpa; Morocco, Egypt to
Turkey, the Middle East, Iran, Pakistan and the Arabian Peninsula),
Aurinia (7; A. corymbosa, A. gionae, A.
leucadea, A. moreana, A. petraea, A. saxatilis,
A. sinuata; Europe, Turkey), Berteroa (5; B.
gintlii, B. incana, B. mutabilis, B. obliqua,
B. orbiculata; temperate regions in Eurasia), Lepidotrichum
(1; L. uechtritzianum; westernmost coast of the Black Sea in Bulgaria
and Turkey), Galitzkya (3; G. macrocarpa, G.
potaninii, G. spathulata; Central Asia and Russia to northwestern
China and Mongolia), Meniocus (4–7; M. aureus, M.
hirsutus, M. linifolius, M. serpyllifolius; Spain,
northwestern Africa, southeastern Europe, Ukraine and Turkey to Iran, the
Middle East, the Arabian Peninsula, Central Asia, Mongolia and China),
Clypeola (9; the Mediterranean and East Europe to the Middle East,
Iran, Central Asia and the Arabian Peninsula), Odontarrhena (87; South
and Southeast Europe, Turkey, the Caucasus to the Middle East, Central Asia and
East Asia, one species, O. obovata, in Alaska and western Canada). –
Mainly southern and southeastern Europe, northwestern Africa and southwestern
Asia. Hairs stellate. Filaments usually with appendages. Siliqua usually
latiseptate or terete (rarely angustiseptate). Seeds often winged. x = 8. –
Alysseae are plausible sister-group to the remaining
Brassicodae with moderate support (parsimony bootstrap value of
79%).
[Megacarpaeeae+[Heliophileae+Notothlaspideae]]
Megacarpaeeae Kamelin
ex D. German in Komarovia 6(2): 83. 19 May 2010
2/12. Megacarpaea (9; Europe,
western and Central Asia, the Himalayas, western China), Pugionium (3;
P. cornutum, P. dolabratum, P. pterocarpum; Russia,
Mongolia, northern China). – Eastern Europe to Central Asia. Hairs simple.
Fruit in Megacarpaea a deeply bilobate silicula with single-seeded
lobes.
[Heliophileae+Notothlaspi]
Heliophileae DC. in
Mém. Mus. Hist. Nat. 7(1): 256. 20 Apr 1821
1/c 90. Heliophila
(c 90; southern Africa, especially Northern, Western and Eastern Cape). –
Hairs simple or absent. Fruit a siliqua. Cotyledons diplecolobal. x = 10.
Notothlaspideae
Al-Shehbaz, Warwick, Mummenh. et M. A. Koch in Plant Syst. Evol. 285: 229. Mai
2010
1/2. Notothlaspi (2; N.
australe, N. rosulatum; the Southern Alps in New Zealand). –
Hairs simple or absent. Fruit a silicle, angustiseptate. Cotyledons incumbent.
– Notothlaspi is sister to Heliophila,
according to Warwick & al. (2010).
Iberideae Webb et
Berthel., Hist. Nat. Iles Canaries 3(2,1): 92. Nov 1837
2/29. Iberis
(27; Europe, the Mediterranean, northwestern Africa), Teesdalia
(2; T. coronopifolia, T. nudicaulis; Europe, the
Mediterranean, southwestern Asia). – Europe, the Mediterranean, northwestern
Africa, southwestern Asia. Hairs simple or absent. Flowers often zygomorphic.
Fruit usually strongly angustiseptate, with two seeds.
[Chamireae+[Conringieae+[Coluteocarpeae]]
Chamireae Sond. in
Abh. Naturwiss. Verein Hamburg 1: 267. 1846
1/1. Chamira (1; C.
circaeoides; Western Cape). – Annual herb. – Chamira may be
sister to the clade [Conringieae+Noccaeeae].
[Conringieae+Coluteocarpeae]
Conringieae D. A.
German et Al-Shehbaz in Harvard Pap. Bot. 13: 169. 30 Jun 2008
2/8. Conringia
(6; C. austriaca, C. clavata, C. grandiflora, C.
orientalis, C. persica, C. planisiliqua; southern
Europe, the Mediterranean to Central Asia), Zuvanda (2; Z.
exacoides, Z. meyeri; southwestern Asia). – Europe to Central
Asia. Hairs absent. Fruit a siliqua.
Coluteocarpeae V. I.
Dorof. In Turczinanowia 7(3): 51. 28 Sep 2004
3/c 125. Coluteocarpus (1;
C. reticulatus; mountains in southwestern Asia), Noccaea
(c 120; Europe, North Africa, temperate Asia, North America, Mexico,
Patagonia), Pseudosempervivum (4; P. amanum, P.
aucheri, P. gurulkanii, P. sempervivum; Turkey,
Armenia). – Europe, the Mediterranean, temperate Asia, North America,
Patagonia. Hairs absent. Fruit angustiseptate. x = 7.
[Thlaspideae+[Eutremeae+[Brassiceae+Bivonaeeae]+Thelypodieae+[Isatideae+[Sisymbrieae+Ochthodieae]]]]]
Thlaspideae DC. in
Mém. Mus. Hist. Nat. 7(1): 234. 20 Apr 1821 [‘Thlaspideae’]
12/36. Thlaspi (4; T.
arvense, T. ceratocarpum, T. huetii, T.
kochianum; temperate regions of the Northern Hemisphere),
Mummenhoffia (2; M. alliacea, M. olivieri; central
and southern Europe, East African high mountains), Didymophysa (2;
D. aucheri, D. fedtschenkoana; Iran to Central Asia and
western Himalayas), Peltaria (4; P. alliacea, P.
angustifolia, P. emarginata, P. turkmena; eastern
Mediterranean to Iran and Central Asia), Pseudocamelina (3; P.
aphragmodes, P. campylocarpa, P. glaucophylla; Iran,
northern Iraq, northern Pakistan), Peltariopsis (2; P. grossheimii, P.
planisiliqua; eastern Turkey, the Caucasus, northern Iran),
Graellsia (8; Morocco, Turkey to Pakistan), Parlatoria (1;
P. cakiloidea; Iran), Pachyphragma (1; P.
macrophyllum; the Caucasus), Pseudovesicaria (1; P.
digitata; the Caucasus), ‘Alliaria’ (3; A.
auriculata, A. brachycarpa, A. petiolata; Europe,
temperate Asia; diphyletic), Lysakia (1; L. rostrata;
northern Iran), Sobolewskia (4; S. caucasica, S.
clavata, S. sibirica, S. truncata; eastern Mediterranean
to the Caucasus). – Europe, the Mediterranean, southwestern and Central Asia,
the Himalayas. Hairs simple or absent. Seed coat striate or coarsely
reticulate. x = 7.
[Eutremeae+[Brassiceae+Bivonaeeae]+Thelypodieae+[Isatideae+[Sisymbrieae+Ochthodieae]]]]
Eutremeae Al-Shehbaz,
Beilstein et E. A. Kellogg in Plant Syst. Evol. 259: 112. 19 Jul 2006
3/34. Chalcanthus (1; C.
renifolius; mountains in Iran, Afghanistan and Central Asia),
Eutrema (26; Russia, Central Asia, the Himalayas, eastern Tibet, East
Asia, Colorado), Pegaeophyton (7; P. angustiseptatum, P.
minutum, P. nepalense,P. purii, P.
scapiflorum, P. sulphureum, P. watsonii; Central Asia,
the Himalayas to western China). – East Europe, temperate Asia, one species
also in North America. Hairs simple or absent. Fruit a siliqua. Cotyledons
incumbent. x = 7.
[[Brassiceae+Bivonaeeae]+Thelypodieae+[Isatideae+[Sisymbrieae+Ochthodieae]]]
[Brassiceae+Bivonaeeae]
Brassiceae DC. in
Mém. Mus. Hist. Nat. 7(1): 242. 20 Apr 1821
c 36/c 275. Nasturtiopsis (1;
N. arabica; North Africa to Israel); Ammosperma (2; A.
cinerea, A. variabile; North Africa), Brassica
(c 165; Europe, the Mediterranean, temperate Asia), Cakile
(c 6; C. arabica, C. constricta, C. edentula, C.
geniculata, C. lanceolata, C. maritima; coasts in
Europe, the Mediterranean, the Arabian Peninsula and North America; incl. Didesmus?,
Erucaria?),
Didesmus
(2; D. aegyptius, D. bipinnatus; eastern Mediterranean; in Cakile?),
Erucaria
(10; eastern Mediterranean, the Arabian Peninsula, Iran; in Cakile?),
Carrichtera
(1; C. annua; Macaronesia, the Mediterranean to Iran),
Cordylocarpus (1; C. muricatus; Morocco, Algeria),
Crambella (1; C. teretifolia; Morocco),
Enarthrocarpus (5; E. arcuatus, E. clavatus, E.
lyratus, E. pterocarpus, E. strangulatus; eastern
Mediterranean, North Africa), Crambe
(c 35; Europe, Macaronesia, the Mediterranean, mountains in northern tropical
Africa, western Asia), Douepea (2; D. arabica, D.
tortuosa; the Arabian Peninsula, Pakistan), Eremophyton (1;
E. chevallieri; Morocco, Algeria, Libya), Fezia (1; F.
pterocarpa; Morocco), Foleyola (1; F. billotii; western
Sahara), Fortuynia (1; F. garcinii; Iran, Afghanistan,
Baluchistan), Guiraoa (1; G. arvensis; Spain),
Hemicrambe (3; H. fruticosa, H. fruticulosa, H.
socotrana; Morocco, Socotra), Henophyton (1; H. deserti;
Morocco to Libya), Kremeriella (1; K. cordylocarpus;
northwestern Africa), Moricandia
(8; the Mediterranean to Pakistan), Morisia
(1; M. monanthos; Corsica, Sardinia), Muricaria (1; M.
prostrata; Morocco, Libya), Otocarpus (1; O. virgatus;
Algeria), Physorhynchus (2; P. brahuicus, P.
chamaerapistrum; southern Iran to northwestern India),
Pseuderucaria (2; P. clavata, P. teretifolia;
Morocco to Israel), Psychine
(1; P. stylosa; North Africa), Quezeliantha (1; Q.
tibestica; Sahara), Raffenaldia (2; R. platycarpa,
R. primuloides; Morocco, Algeria), Rytidocarpus
(1; R. moricandioides; Morocco), Savignya (1; S.
parviflora; arid regions in North Africa to the Middle East),
Schouwia (1; S. purpurea; North Africa, the Arabian
Peninsula), Succowia (1; S. balearica; the Canary Islands,
western Mediterranean), Vella
(>6; V. anremerica, V. bourgaeana, V. lucentina,
V. mairei, V. pseudocytisus, V. spinosa; western
Mediterranean), Zilla (2; Z. macroptera, Z. spinosa;
North Africa to the Arabian Peninsula), Orychophragmus (3; O.
limprichtianus, O. violaceus, O. ziguiensis; China). –
Europe, the Mediterranean, central and southern Africa, southwestern Asia, the
Atlantic coast of North America. Hairs simple or absent. Fruit usually
transversely segmented (heteroarthrocarpous; not in Nasturtiopsis).
Cotyledons usually conduplicate (not in Nasturtiopsis). – Brassica
in the present sense includes Brassica
s.str. (c 40; Europe, the Mediterranean, temperate Asia), Ceratocnemum
(1; C. rapistroides; Morocco), Coincya (6; C.
cheiranthus, C. cintrana, C. johnstonii, C.
monensis, C. richeri, C. wrightii; southern Europe, the
Mediterranean), Diplotaxis
(c 25; Europe, the Mediterranean to northwestern India), Eruca (1;
E. vesicaria; the Mediterranean, northeastern Africa), Erucastrum
(c 25; Europe, Macaronesia, the Mediterranean), Hirschfeldia (1;
H. incana; the Mediterranean), Raphanus
(4; R. caudatus, R. confusus, R. indicus, R.
raphanistrum; Europe, the Mediterranean to Central Asia), Rapistrum
(2; R. perenne, R. rugosum; Europe, the Mediterranean, West
Asia), Sinapidendron
(4; S. angustifolium, S. frutescens, S. rupestre,
S. sempervivifolium; Madeira), Sinapis
(6; S. alba, S. allionii, S. arvensis, S.
circinata, S. flexuosa, S. pubescens; Europe, the
Mediterranean), Trachystoma (3; T. aphanoneurum, T.
ballii, T. labasii; Morocco). Orychophragmus comprises
herbs with hairs simple or absent, filaments free from each other, fruit a
terete or slightly quadrangular silicula, and cotyledons conduplicate.
Bivonaeeae M. A. Koch
et Warwick in Taxon 61: 89. 21 Feb 2012
1/1. Bivonaea (1; B.
lutea; Sardinia, Sicily, northwestern Africa). – Fruit a silicula with
winged valves.
Thelypodieae Prantl in
Engler et Prantl, Nat. Pflanzenfam. III, 2: 154. Mar 1891
29/c 260. Chaunanthus (4;
C. acuminatus, C. gracielae, C. mexicanus, C.
petiolatus; Mexico), Chilocardamum (4; C. castellanosii,
C. longistylum, C. onuridifolium, C. patagonicum;
southern Argentina), Chlorocrambe (1; C. hastata; Oregon,
Utah), Dictyophragmus (3; D. englerianus, D.
lactucoides, D. punensis; Peru, Argentina), Dryopetalon
(8; southwestern North America), Englerocharis (2; E.
pauciflora, E. peruviana; the Andes), Eremodraba (2;
E. intricatissima, E. schulzii; Peru, northern Chile),
‘Hesperidanthus’ (5; H. argillaceus, H.
barnebyi, H. jaegeri, H. linearifolius, H.
suffrutescens; western North America; polyphyletic), Hollermayera
(1; H. silvatica; Chile), Ivania (1; I. juncalensis;
northern Chile), Mostacillastrum (c 30; Peru, Bolivia, Argentina),
Neuontobotrys (13; Chile, Argentina), Parodiodoxa (1; P.
chionophila; mountains in northern Argentina), Petroravenia (1;
P. eseptata; Argentina), Phlebolobium (1; P.
maclovianum; the Falkland Islands), Phravenia (1; P.
vireckii; northern Mexico), Polypsecadium (c 15; northern and
western South America), Pringlea (1; P. antiscorbutica;
Prince Edward Islands, Crozet Islands, Kerguélen Islands, Heard Island),
Romanschulzia (14; Central America), Sarcodraba (4; S.
andina, S. dusenii, S. karraikensis, S.
subterranea; the Andes), Sibara (13; western and southern North
America), Stanleya
(6–7; S. albescens, S. bipinnata, S.
confertiflora, S. lata, S. pinnata, S.
tomentosa, S. viridiflora; western North America), Streptanthus
(c 55; western and southern North America; paraphyletic?),
Thelypodiopsis (15; North America, Mexico, Guatemala),
Thelypodium (19; western North America, Central America), Thysanocarpus
(5; T. conchuliferus, T. curvipes, T. erectus,
T. laciniatus, T. radians; western United States),
Warea (4; W. amplexifolia, W. carteri, W.
cuneifolia, W. sessilifolia; southeastern United States),
Weberbauera (c 20; the Andes in Peru, Bolivia and Argentina),
Zuloagocardamum (1; Z. jujuyensis; northwesternmost
Argentina). – North, Central and South America, subantarctic islands.
[Isatideae+[Sisymbrieae+Ochthodieae]]
Isatideae DC. in Mém.
Mus. Hist. Nat. 7(1): 241. 20 Apr 1821
5/c 34. Chartoloma (1; C.
platycarpum; Central Asia), Glastaria (1; G.
glastifolia; southwestern Turkey, Syria, Iraq), Isatis
(c 30; Europe, the Mediterranean to Central Asia; paraphyletic), Myagrum
(1; M. perfoliatum; Central Europe, the Mediterranean to northwestern
India), Schimpera (1; S. arabica; eastern Mediterranean to
southern Iran). – Europe, Central and Southwest Asia. Hairs simple or absent.
Fruit nutlike, one- or two-seeded, often pendent. x = 7.
[Sisymbrieae+Ochthodieae]
Sisymbrieae DC. in
Mém. Mus. Hist. Nat. 7(1): 237. 20 Apr 1821 [‘Sisymbreae’]
1/44. Sisymbrium
(44; Europe, the Mediterranean, northern and southern Africa, Asia, one species
in North America). – Hairs simple or absent. Stigma bilobate. Fruit a terete
siliqua. x = 7.
Ochthodieae Bunge in
Arb. Naturf. Ver. Riga 1: 170. 1847
2/2. Ochthodium (1; O.
aegyptiacum; eastern Mediterranean); Pseudofortuynia (1; P.
esfandiarii; Iran). – Eastern Mediterranean, Iran. Fruit a silicula (in
Ochthodium lignified and indehiscent). – Ochthodium and
Pseudofortuynia form a clade with weak bootstrap support (less than
60%, according to Warwick & al. 2010), which may be sister to Sisymbrium.
[[Buniadeae+Kernereae]+[Asteae+[Schizopetaleae+[Cremolobeae+Eudemeae+Aphragmeae]]]]
[Buniadeae+Kernereae]
Buniadeae DC. in Mém.
Mus. Hist. Nat. 7(1): 245. 20 Apr 1821
1/3. Bunias
(3; B. cochlearioides, B. erucago, B. orientalis;
southern and eastern Europe, the Mediterranean, western Asia). – Hairs simple
or branched, glandular, or absent. Fruit an indehiscent silicula.
Kernereae Al-Shehbaz,
Warwick, Mummenh. et M. A. Koch in Plant Syst. Evol. 285: 228. Mai 2010
2/2. Kernera
(1; K. saxatilis; mountains in central and southern Europe),
Rhizobotrya (1; R. alpina; western Dolomites in central
Europe). – Central Europe. Hairs simple or dichotomous, stalked, or absent.
Fruit a siliqua, terete or latiseptate. Cotyledons accumbent.
[Asteae+[Schizopetaleae+[Cremolobeae+Eudemeae+Aphragmeae]]]
Asteae Al-Shehbaz,
Warwick, Mummenh. et M. A. Koch in Plant Syst. Evol. 285: 228. Mai 2010
1/1. Asta (1; A.
schaffneri; Mexico). – Hairs absent. Cotyledons incumbent. . –
Asta may be closely allied to Kernereae.
[Schizopetaleae+[Cremolobeae+Eudemeae+Aphragmeae]]
Schizopetaleae R. Br.
ex Barnéoud in Ann. Mag. Nat. Hist., ser. 1, 16: 68. Jul 1845
2/20. Atacama (1; A.
nivea; northern Chile); Mathewsia
(9; southern Peru, northern Chile), Schizopetalon (10; the Andes in
northern central Chile and northwestern Argentina). – Southern Peru, northern
Chile, northwestern Argentina, mainly in the Atacama desert. Hairs usually
simple (sometimes branched) or absent. Fruit usually a siliqua. x = (10–)14.
– Atacama was recovered as sister to Teesdalia
(Iberideae) in the analysis by Toro-Núñoz & al. (2015). –
Schizopetaleae may be sister-group to Cremolobeae.
[Cremolobeae+Eudemeae+Aphragmeae]
Cremolobeae R. Br. in
D. Denham et B. H. Clapperton, Narr. Travels Africa: 212. Mar-Apr 1826
3/32. Aimara (1; A.
rollinsii; northwestern Chile), Cremolobus (7; C.
bolivianus, C. chilensis, C. peruvianus, C.
rhomboideus, C. stenophyllus, C. subscandens, C.
suffruticosus; the Andes in Peru, Chile and Argentina),
Menonvillea (24; the Andes in Chile and Argentina). – Peru, Chile,
Argentina. Herbs or shrublets. Hairs simple. Fruit a silicula.
Eudemeae Al-Shehbaz,
Warwick, Mummenh. et M. A. Koch in Plant Syst. Evol. 285: 228. Mai 2010
9–10/42–43. Alshehbazia (1;
A. hauthalii; the Andes in southernmost Chile and southernmost
Argentina), Xerodraba (8; southern Argentina), Onuris (6;
O. alismatifolia, O. graminifolia, O. hatcheriana,
O. papillosa, O. reichei, O. spegazziniana; Chile,
Patagonia), Stenodraba (9; the Andes in Chile and Argentina;
diphyletic?), Aschersoniodoxa (4; A. cachensis, A.
mandoniana, A. pilosa, A. rusbyi; the Andes),
Brayopsis (6; B. alpaminae, B. calycina, B.
colombiana, B. diapensioides, B. gamosepala, B.
monimocalyx; the Andes), Dactylocardamum (1; D.
imbricatifolium; Peru), Delpinophytum (1; D.
patagonicum; Patagonia), Eudema (6; E. friesii, E.
hauthalii, E. incurva, E. nubigena, E.
rupestris, E. werdermannii; the Andes). – The Andes (especially
Chile), Patagonia. Hairs simple or dichotomous, stalked, or absent. Fruit a
siliqua or silicula, latiseptate or angustiseptate. Cotyledons incumbent. –
Horwoodia (1; H. dicksoniae; the Arabian Peninsula to Iraq)
was recovered with relatively low support as sister to Eutremeae in
the analysis by Toro-Núñez & al. (2015).
Aphragmeae D.
A. German et Al-Shehbaz in Harvard Pap. Bot. 13: 168. 30 Jun 2008
1/12. Aphragmus (12; the
Himalayas, Siberia, the Russian Far East, Alaska, Yukon). – Hairs simple or
dichotomous, small. Fruit a siliqua or silicula. x = 7. – Aphragmus
may be closely allied to Eudemeae.
[Calepineae+[Subularieae+[[[Arabideae+Stevenieae]+Biscutelleae]+[[Cochlearieae+Asperuginoides]+[Anchonieae+[Euclidieae+[Dontostemoneae+Chorisporeae]+[Hesperideae+Anastaticeae]]]]]]]
Calepineae Horan,
Char. Ess. Fam: 169. 17 Jun 1847
3/7. Calepina
(1; C. irregularis; southern and southwestern Europe, Macaronesia, the
Mediterranean), ‘Goldbachia’
(5; G. ikonnikovii, G. laevigata, G. pendula, G.
tetragona, G. verrucosa; southeastern Russia, Southwest to
Central Asia; paraphyletic), Leiocarpaea (1; L.
cochlearioides; southestern Russia, western Asia). – Southern Europe,
the Mediterranean, Southwest and Central Asia. Hairs simple (often papillary)
or absent. Fruit a silicula or siliqua (in ‘Goldbachia’
articulated with single-seeded segments; in Spirorhynchus
nut-like).
[Subularieae+[[[Arabideae+Stevenieae]+Biscutelleae]+[[Cochlearieae+Asperuginoides]+[Anchonieae+[Euclidieae+[Dontostemoneae+Chorisporeae]+[Hesperideae+Anastaticeae]]]]]]
Subularieae DC. in
Mém. Mus. Hist. Nat. 7(1): 257. 20 Apr 1821
3/4. Idahoa (1; I.
scapigera; western United States), Petrocallis
(1; P. pyrenaica; mountains in southwestern and central Europe),
Subularia (2; S. aquatica: temperate regions in Europe, Asia
and North America; S. monticola: mountains in tropical East and
Central Africa). – Temperate regions on the Northern Hemisphere. Hairs simple
or absent. Fruit a latiseptate silicula. – This clade has weak support
(parsimony bootstrap value less than 50%), according to Warwick & al.
(2010).
[[[Arabideae+Stevenieae]+Biscutelleae]+[[Cochlearieae+Asperuginoides]+[Anchonieae+[Euclidieae+[Dontostemoneae+Chorisporeae]+[Hesperideae+Anastaticeae]]]]]
[[Arabideae+Stevenieae]+Biscutelleae]
[Arabideae+Stevenieae]
Arabideae DC. in
Mém. Mus. Hist. Nat. 7(1): 229. 20 Apr 1821
17/490–495. Abdra (2; A.
aprica, A. brachycarpa; the United States), ’Arabis’
(c 60; temperate regions on the Northern Hemisphere, tropical African
mountains; paraphyletic), Athysanus
(1; A. pusillus; western United States), Aubrieta
(16; southern Europe to Iran), Draba (c
390; temperate regions on the Northern Hemisphere, the Andes),
Drabella (1; D. muralis; Europe to Turkey and the Caucasus,
Morocco), Pachyneurum (1; P. grandiflorum; Central Asia),
Baimashania (2; B. pulvinata, B. wangii; Yunnan,
Qinghai), Pseudodraba (1; P. hystrix; mountains in
Afghanistan and Pakistan), Sinoarabis (1; S. setosifolia;
Xizang), Arcyosperma (1; A. primulifolium; Nepal),
Borodiniopsis (1; B. alaschanica; western China),
Botschantzevia (1; B. karatavica; Kazakhstan),
Dendroarabis (1; D. fruticulosa; Altai), Parryodes
(1; P. axilliflora; Bhutan), Scapiarabis (4; S.
ariana, S. karategina, S. popovii, S. saxicola;
northern Pakistan, Central Asia, Tajikistan, Afghanistan, Xinjiang),
Tomostima (6; western North America, the Andes). – Temperate regions
on the Northern Hemisphere, tropical African mountains, the Andes. Hairs
branched. Cotyledons usually accumbent (in Berteroella incumbent).
Seeds not mucilaginous. x = 8 (polyploidy frequent in Draba).
Stevenieae
Al-Shehbaz, D. German et M. A. Koch in Plant Divers. Evol. 129: 72. 22 Mar
2011
3/8. Macropodium (2; M.
nivale, M. pterospermum; Central Asia, Sakhalin, Japan), Pseudoturritis
(1; P. turrita; southern and southeastern Europe), Stevenia
(5; S. alyssoides, S. cheiranthoides, S. incarnata,
S. maximowiczii, S. sergievskajae; China, the Korean
Peninsula, Japan, the Russian Far East). – Europe, Central and East Asia.
Biscutelleae Dumort.,
Fl. Belg.: 118. 1827
5/61. Heldreichia (1; H.
bupleurifolia; Turkey to Afghanistan), Biscutella
(46; central and southern Europe, the Mediterranean), Megadenia (1;
M. pygmaea; Siberia, China), Ricotia (9; eastern
Mediterranean), Lunaria (4; L. annua, L. elongata,
L. rediviva, L. telekiana; Europe). – Europe, the
Mediterranean, Asia. Fruit a compressed silicula (in Biscutella
didymous, indehiscent); Ricotia has a silique or a latiseptate
silicula; Lunaria has a latiseptate silicula and an unusually long
gynophore. – Biscutelleae are sister to
[Arabideae+Stevenieae].
[[Cochlearieae+Asperuginoides]+[Anchonieae+[Euclidieae+[Dontostemoneae+Chorisporeae]+[Hesperideae+Anastaticeae]]]]
[Cochlearieae+Asperuginoides]
Cochlearieae Buchenau,
Fl. Nordwestdeut. Tiefebene: 245. 28 Apr 1894 [‘Cochleariinae’]
2/c 35. Cochlearia
(c 30; Western Europe, the western Mediterranean, northwestern Africa, Arctic
regions in northeastern Asia, Alaska and northern Canada), Ionopsidium
(5; I. abulense, I. acaule, I. albiflorum, I.
prolongoi, I. savianum; western Mediterranean). – Europe, the
Mediterranean, North Africa, northeastern Asia, Alaska, northern Canada. Hairs
absent. Stigma entire. Fruit a terete or angustiseptate silicula. Seeds
biseriate. x = 6–7. – Cochlearieae may be sister-group to the
remaining Brassicodae. A sister-group relationship between
Cochlearieae and Asperuginoides is weakly supported in the
analyses by Warwick & al. (2010).
Asperuginoides
clade
1/1. Asperuginoides (1; A.
axillaris; Turkey and eastwards to Central Asia and Pakistan). – Hairs
stellate. Fruit a dorsally compressed silicula, covered with anchor-shaped
hairs (glochids). – Asperuginoides may be sister to
Cochlearieae, although its position in Brassicaceae is very
uncertain.
[Anchonieae+[Euclidieae+[Dontostemoneae+Chorisporeae]+[Hesperideae+Anastaticeae]]]
Anchonieae DC. in
Mém. Mus. Hist. Nat. 7(1): 242. 20 Apr 1821
9/66–68. Anchonium (2; A.
billardieri, A. elichrysifolium; West and Central Asia),
Eremoblastus (1; E. caspicus; western Kazakhstan to Central
Asia), Iskandera (2; I. alaica, I. hissarica;
Central Asia), ’Matthiola’
(48–50; western Europe, Macaronesia, the Mediterranean; polyphyletic),
Micrantha (1; M. multicaulis; Iran), Microstigma (3;
M. brachycarpum, M. deflexum, M. sajanense; Siberia,
Mongolia, China), Sterigmostemum (6; S. acanthocarpum, S.
caspicum, S. incanum, S. longistylum, S.
ramosissimum, S. sulphureum; southeastern Russia, western
Kazakhstan, Southwest and Central Asia), Synstemon (2; S.
lulianlianus, S. petrovii; China), Zerdana (1; Z.
anchonioides; mountains in Iran). – Europe, temperate Asia. Hairs
branched; glandular hairs multicellular with multiseriate stalk. Stigma often
strongly bilobate. x = 7 (x = 5–8 in Matthiola).
[Euclidieae+[Dontostemoneae+Chorisporeae]+[Hesperideae+Anastaticeae]]
Euclidieae DC. in
Mém. Mus. Hist. Nat. 7(1): 236. 20 Apr 1821
28/165. Anzhengxia (1; A.
yechengnica; Xinjiang Prov. in China), Atelanthera (1; A.
perpusilla; Central Asia, the Himalayas), Braya
(23; northern circumpolar, mountains in Central and North Europe, Central Asia,
the Himalayas), Catenulina (1; C. hedysaroides; Central
Asia), Christolea (5; C. borealis, C. crassifolia,
C. maidantalica, C. niyaensis, C. parryoides;
Central Asia, the Himalayas, East Asia), Cryptospora (3; C.
falcata, C. inconspicua, C. trichocarpa; Central Asia),
Cymatocarpus (3; C. grossheimii, C. heterophyllus,
C. pilosissimus; Transcaucasia to Central Asia),
Dichasianthus (1; D. subtilissimus; Central Asia),
Dilophia (2; D. ebracteata, D. salsa; Central Asia
to western China), Euclidium (1; E. syriacum; eastern Europe
to the Middle East), Lachnoloma (1; L. lehmannii; Iran to
China), Leiospora (8; Russia to East Asia), Lepidostemon (5;
L. everestianus, L. glaricola, L. gouldii, L.
pedunculosus, L. rosularis; the Himalayas), Leptaleum
(1; L. filifolium; eastern Mediterranean to Central Asia and
Baluchistan), Metashangrilaia (1; M. forrestii; Xizang,
Yunnan, Bhutan), Neotorularia (13; the Mediterranean to Central Asia
and Afghanistan), Octoceras (1; O. lehmannianum; Iran and
Afghanistan to Central Asia), Pycnoplinthopsis (1; P.
bhutanica; Bhutan), Pycnoplinthus (1; P. uniflora; the
Himalayas), Rhammatophyllum (9; Central Asia),
Rudolf-kamelinia (1; R. korolkowii; Central Asia, western
China, northern India), Shangrilaia (1; S. nana; Yunnan),
Sisymbriopsis (5; S. mollipila, S. pamirica, S.
schugnana, S. shuanghuica, S. yechengnica; Tajikistan,
China), Solms-laubachia
(33; China, one species also in Sikkim), Spryginia (7; S.
afghanica, S. crassifolia, S. falcata, S.
gracilis, S. pilosa, S. undulata, S. winkleri;
Central Asia), Streptoloma (2; S. desertorum, S.
sumbarense; Central Asia to Afghanistan), Strigosella (24; the
Mediterranean to western and Central Asia, North Africa), Tetracme
(10; eastern Mediterranean to Central Asia and Baluchistan). – Europe,
temperate and arctic Asia, arctic and alpine North America. Hairs usually
branched (rarely simple or absent). Multicellular glands absent. Stigma entire
or bilobate. Fruit often a terete to quadrangular siliqua or silicula.
Cotyledons incumbent. x = 7(–8).
[[Dontostemoneae+Chorisporeae]+[Hesperideae+Anastaticeae]]
[Dontostemoneae+Chorisporeae]
Dontostemoneae
Al-Shehbaz et Warwick in Harvard Pap. Bot. 12(2): 431. 21 Dec 2007
2/19. Clausia (6; C.
agideliensis, C. aprica, C. kasakhorum, C.
podlechii, C. robusta, C. trichosepala; southeastern
Europe to Central and East Asia), Dontostemon (13; Siberia, Mongolia,
China). – Southeastern Europe to Siberia and East Asia. Perennial herbs.
Eglandular hairs simple or dichotomous; glandular hairs when present
multicellular, multiseriate. Fruit a terete or latiseptate often tardily
dehiscent siliqua. Radicula accumbent.
Chorisporeae Ledeb.,
C. A. Mey. et Bunge, Fl. Altaic. 3: 104. Jul-Dec 1831
4/57. ’Chorispora’
(13; eastern Mediterranean to Central Asia; paraphyletic),
Diptychocarpus (1; D. strictus; eastern Europe to Central
Asia), Litwinowia (1; L. tenuisissima; southwestern Asia to
China), Parrya (42; northern temperate and arctic regions on the
Northern Hemisphere, circumpolar). – Europe, temperate Asia, northern North
America. Hairs simple; glandular hairs multicellular with multiseriate stalk.
Stigma connivent. Fruit a siliqua or a moniliform schizocarp (lomentum) with
one-seeded nutlike mericarps. x = 7 (in Chorispora
also x = 9).
[Hesperideae+Anastaticeae]
Hesperideae Prantl in
Engler et Prantl, Nat. Pflanzenfam. III, 2: 154. Mar 1891
2/c 30. Hesperis
(29–30; Europe, the Mediterranean to Iran, Central Asia and western China),
Tchihatchewia (1; T. isatidea; Turkey). – Europe, temperate
Asia. Hairs usually simple and/or dichotomous (rarely dendritic); glandular
hairs with uniseriate stalk and unicellular head (glands rarely absent).
Stigmatic lobes decurrent. Fruit a siliqua. x = 6–10.
Anastaticeae DC. in
Mém. Mus. Hist. Nat. 7(1): 236. 20 Apr 1821
13/96. Anastatica (1; A.
hierochuntica; Morocco to southern Iran), Cithareloma (2; C.
lehmannii, C. vernum; Iran, Central Asia), Diceratella
(10; northeastern tropical Africa, Iran), Eigia (1; E.
longistyla; Israel to northwestern Arabian Peninsula), Eremobium
(1; E. aegyptiacum; North Africa, the Middle East), Farsetia
(28; Morocco to northwestern India, mountains in Tanzania),
Lachnocapsa (1; L. spathulata; Socotra), Lobularia
(5; L. arabica, L. canariensis, L. libyca, L.
marginata, L. maritima; Macaronesia, the Mediterranean to the
Arabian Peninsula),‘Malcolmia’
(35; the Mediterranean to Afghanistan; polyphyletic), Maresia (3;
M. meyeri, M. nana, M. pulchella; the Mediterranean
to the Caspian area and southern Iran), Morettia (3; M.
canescens, M. parviflora, M. philaeana; North Africa to
the Arabian Peninsula), Notoceras (1; N. bicorne; the Canary
Islands, the Mediterranean to northwestern India), Parolinia
(5; P. filifolia, P. intermedia, P. ornata, P.
platypetala, P. schizogynoides; the Canary Islands). –
Macaronesia and the Mediterranean to Central Asia and northwestern India and
East African mountains. Hairs simple or branched, or absent. Fruit a siliqua or
a latiseptate silicula.
 |
Strict consensus tree (simplified) of
Brassicoideae based on DNA sequence data (Warwick & al.
2010).
|
 |
Bayesian inference tree (simplified) of
Brassicoideae based on DNA sequence data (German & al.
2009).
|
Unplaced
Brassicoideae
Andrzeiowskia (1; A.
cardamine; southeastern Europe, southwestern Asia),
Raphanorhyncha (1; R. crassa; Mexico), Veselskya (1;
V. griffithiana; Afghanistan).
Dumortier, Anal. Fam. Plant.: 37, 42. 1829, nom.
cons.
Papayaceae Blume,
Bijdr. 15: 940. Jul-Dec 1826, nom. illeg.; Papayales
Blume in C. F. P. von Martius, Consp. Regn. Veg.: 32. Sep-Oct 1835
[‘Papayaceae’], nom. illeg.; Caricales
L. D. Benson, Pl. Classif.: 648. 1957
Genera/species 6/c 34
Distribution Tropical Africa,
tropical America.
Fossils Unknown.
Habit Usually dioecious
(sometimes monoecious, andromonoecious, gynomonoecious, or polygamomonoecious;
rarely bisexual), evergreen trees or shrubs (rarely perennial herbs or climbing
shrubs; in Jarilla and Jacaratia corumbensis with a stout
tuberous stem). Stem usually unbranched, often spiny.
Vegetative anatomy Phellogen?
Xylem, except vessel elements, unlignified. Vessel elements with simple
perforation plates; lateral pits alternate or scalariform, bordered? pits.
Non-vestured pits present. Imperforate tracheary xylem elements? Wood rays
multiseriate?, heterocellular? Axial parenchyma paratracheal vasicentric,
usually non-lignified. Wood elements often storied. Secondary phloem stratified
into hard fibrous and soft parenchymatous layers. Phloem fibres as isolated
strands. Sieve tube plastids S type. Nodes ≥3:≥3?, trilacunar or
multilacunar with three or more? leaf traces. Parenchyma with cellular
(multinucleate as mature), articulated, anastomosing laticifers containing
latex-like exudate. Idioblastic myrosin cells absent. Calciumoxalate druses
present.
Trichomes Eglandular hairs
absent; stem and leaves (especially basal adaxial parts of lamina) in
Carica with multicellular, uniseriate, simple glandular hairs; leaves
in Carica and Jacaratia with adaxial glandular hairs crowned
by multicellular head. Stem apex and inflorescence in Carica with
colleters.
Leaves Alternate (spiral),
usually palmately compound (leaflets sometimes pinnately lobed) or palmately
lobed (leaves rarely entire or pinnately lobed), with flat-curved to involute
ptyxis. Stipules usually absent (in Vasconcellea stipulata modified
into spines); leaf sheath absent. Petiole vascular bundle transection? Venation
usually palmate (rarely pinnate). Stomata anomocytic. Cuticular wax
crystalloids as rosettes (Fabales type). Lamina with laticifers.
Myrosin cells stomatal. Mesophyll with cells containing calciumoxalate druses.
Leaf margin serrate or entire.
Inflorescence Axillary,
thyrsoid.
Flowers Actinomorphic.
Hypogyny. Sepals (four or) five, with open aestivation, free or entirely or
partially connate. Petals (four or) five, with contorted or valvate
aestivation, in male flowers connate into tube, in female flowers free or
connate in lower part. Nectaries in Carica and Jacaratia on
pistillodium in male flowers; female flowers without nectaries. Disc absent.
Androecium Stamens usually
(four or) five longer antesepalous and (four or) five shorter antepetalous,
diplostemonous (in Carica sometimes four or five, antesepalous).
Filaments sometimes hairy at base (in Carica and Jarilla
submoniliform hairs), free or connate at base, adnate to petals (epipetalous).
Anthers dorsifixed to subbasifixed, versatile?, usually tetrasporangiate
(anthers of inner stamens rarely disporangiate), introrse, longicidal
(dehiscing by longitudinal slits); connective often dorsally widened, often
with glandular apex. Tapetum secretory. Staminodia in male flowers sometimes
(four or) five (absent in female flowers).
Pollen grains
Microsporogenesis simultaneous. Pollen grains tricolporate, shed as monads,
bicellular at dispersal. Exine tectate or semitectate, with columellate
infratectum, perforate or reticulate.
Gynoecium Pistil composed by
(four or) five connate carpels. Ovary superior, unilocular (Carica,
Horovitzia, Jarilla) or divided by septa and secondarily
(quadrilocular or) quinquelocular (Cylicomorpha, Jacaratia,
Vasconcellea). Stylodia (four or) five, short, free, or style single,
(quadrilobate or) quinquelobate, or absent. Stigmas flabellate or almost
petaloid (rarely one capitate-truncate stigma), papillate, Dry type. Female
flowers in Jacaratia with white broad stigmas resembling stamens of
male flowers. Male flowers often with pistillodium.
Ovules Placentation
intrusively parietal (when ovary unilocular) or axile (when ovary
multilocular). Ovules usually numerous per carpel, anatropous, bitegmic,
crassinucellar. Micropyle bistomal. Outer integument ? cell layers thick. Inner
integument four to six cell layers thick. Megagametophyte usually monosporous,
Polygonum type (rarely tetrasporous). Synergids with a filiform
apparatus. Endosperm development ab initio nuclear. Endosperm haustoria?
Embryogenesis via irregular early divisions.
Fruit A many-seeded berry.
Seeds Aril usually absent
(sometimes present). Seed coat testal. Testa multiplicative, with outer
epidermis modified into mucilaginous sarcotesta. Mesotestal cell walls with
tannins and lignified ridges. Endotesta crystalliferous sclerotesta, sometimes
lignified. Tegmen not or only poorly multiplicative. Exotegmen fibrous or
non-fibrous, lignified. Endotegmen unspecialized. Perisperm not developed.
Endosperm copious, carnose, oily and proteinaceous. Embryo straight, well
differentiated, without chlorophyll. Cotyledons two, wide, flattened. Hypocotyl
swollen (often tuberous). Epicotyl swollen. Germination phanerocotylar.
Cytology n = 9
DNA
Phytochemistry Oleanolic acid
derivatives, gallic acid, alkaloids, saponins, benzylglucosinolates synthesized
from phenylalanine and/or tyrosine, benzylisothiocyanate (in wax layer of
fruit), myo-inositol, and proteolytic enzymes (carpain, papain in
latex) present. Cyclopentenoid cyanogenic glycosides? Flavonols, ellagic acid
and proanthocyanidins not found.
Use Ornamental plants, fruits,
papain for medical and technical purposes (softening of meat, tanning of
leather).
Systematics Jacaratia
(7; J. chocoensis, J. corumbensis, J. digitata,
J. dolichaula, J. heptaphylla, J. mexicana, J.
spinosa; southern Mexico, Central America, tropical South America),
Cylicomorpha (2; C. parviflora, C. solmsii; tropical
Africa), Carica (1;
C. papaya; southern Mexico, Central America, the West Indies, tropical
and subtropical South America), Jarilla (3; J. chocola,
J. heterophylla, J. nana; Mexico, Guatemala), Vasconcellea
(c 20; southern Mexico, Central America, the West Indies, tropical and
subtropical South America), Horovitzia (1; H. cnidoscoloides;
Oaxaca in Mexico).
Caricaceae are sister to
Moringa (Moringaceae).
Jacaratia
may be sister to the remaining Caricaceae, although
Horovitzia and Vasconcellea
are not analysed. Sometimes, Cylicomorpha is sister to the
remainder.
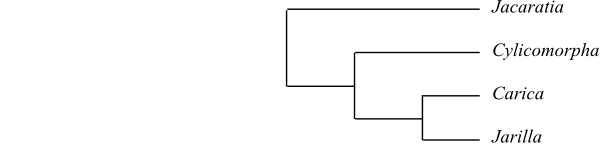 |
Cladogram of Caricaceae based on DNA
sequence data (Olson 2002).
|
EMBLINGIACEAE (Pax)
Airy Shaw
|
( Back to Capparales )
|
Airy Shaw in Kew Bull. 18: 257. 8 Dec 1965
Genera/species 1/1
Distribution Westernmost
Western Australia.
Fossils Unknown.
Habit Bisexual, more or less
woody procumbent suffrutex. Xerophyte.
Vegetative anatomy Phellogen
ab initio cortical. Cambium storied? Vessel elements with simple perforation
plates; lateral pits usually alternate (sometimes opposite). Imperforate
tracheary xylem elements fibre tracheids? with bordered pits. Wood rays
uniseriate or biseriate, homocellular? Axial parenchyma? Sieve tube plastids S
type? Nodes? Mesophyll with branched sclereids. Crystals?
Trichomes Hairs prickly;
glandular hairs absent?
Leaves Opposite, simple,
entire, with ? ptyxis. Stipules minute; leaf sheath absent. Petiole vascular
bundle transection? Venation pinnate? Stomata anomocytic. Cuticular wax
crystalloids? Leaf margin entire.
Inflorescence Flowers
axillary, solitary. Floral prophylls (bracteoles) absent.
Flowers Zygomorphic,
resupinate (twisted 180°). Hypogyny. Sepals five, with ? aestivation, connate
into tube in lower half; median sepal abaxial; calyx tube adaxially split to
base into two halves. Petals two, with valvate aestivation, latero-abaxial,
alternisepalous, sessile?, only with epidermis connate slipper-shaped. Nectary
extrastaminal, triangular, inserted on receptacle between petal bases and
staminal bases. Receptacle elongated into flattened adaxially curved
androgynophore, at apex more or less hidden behind petals.
Androecium Stamens four
abaxial fertile, and four or five adaxial staminodial, diplostemonous, forming
ring (torus) at apex of androphore; median stamens absent. Filaments connate at
base, free from tepals. Anthers basifixed?, non-versatile?, tetrasporangiate,
introrse, longicidal (dehiscing by longitudinal slits). Tapetum glandular?
Staminodia four or five.
Pollen grains
Microsporogenesis simultaneous? Pollen grains usually shortly tricolporate
(sometimes tetracolporate) with bulging apertures, shed as monads, tricellular?
at dispersal. Exine tectate, with columellate? infratectum, punctate,
smooth?
Gynoecium Pistil composed of
two or three connate carpels. Ovary superior, bilocular or trilocular
(unilocular?), shortly bialate, sessile, on androgynophore. Style absent.
Stigma somewhat bilobate or trilobate, type? Pistillodium absent.
Ovules Placentation
axile-basal. Ovule one per carpel, ?-tropous, bitegmic?, crassinucellar?
Funicle lobed. Micropyle ?-stomal. Outer integument ? cell layers thick. Inner
integument ? cell layers thick. Megagametophyte monosporous, Polygonum
type? Endosperm development? Endosperm haustoria? Embryogenesis?
Fruit A one-seeded nutlike
fruit. Pericarp thin, fused with seed.
Seeds Aril formed from apex of
outer integument. Testa thick (multiplicative?). Exotesta? Endotesta? Tegmen?
Perisperm not developed. Endosperm sparse. Embryo curved
(conduplicate-involute), well differentiated, chlorophyll? Cotyledons two.
Germination?
Cytology n = ?
DNA
Phytochemistry Virtually
unknown. Glucosinolates?
Use Unknown.
GYROSTEMONACEAE (Endl.)
A. Juss.
|
( Back to Capparales )
|
de Jussieu in V. V. D. d’Orbigny, Dict. Univ.
Hist. Nat. 6: 450. 1845 [‘Gyrostemoneae’], nom. cons.
Gyrostemonales
Takht., Divers. Classif. Fl. Pl.: 123. 24 Apr 1997;
Gyrostemonanae Takht., Divers. Classif. Fl. Pl.: 123.
24 Apr 1997
Genera/species 5/18
Distribution Australia,
Tasmania, with their highest diversity in southwestern Western Australia.
Fossils Unknown.
Habit Usually dioecious (in
Walteranthus monoecious), evergreen or deciduous shrubs or small trees
(Cypselocarpus and Tersonia annual or biennial herbs).
Xerophytes.
Vegetative anatomy Phellogen
ab initio subepidermal. Cambium more or less storied. Vessel elements with
simple perforation plates; lateral pits alternate or scalariform, simple pits.
Non-vestured pits present. Imperforate tracheary xylem elements tracheids with
bordered pits, non-septate. Wood rays uniseriate or multiseriate, homocellular.
Axial parenchyma, usually paratracheal scanty vasicentric or in tangential
bands (sometimes apotracheal diffuse or diffuse-in-aggregates). Wood elements
partially storied (shorter imperforate tracheary elements storied). Sieve tube
plastids Ss type. Nodes? Myrosin cells idioblastic. Secretory cavities absent.
Crystals absent.
Trichomes Hairs unicellular
(papilla-like) or absent.
Leaves Alternate (spiral),
simple, entire, often carnose or coriaceous, with flat ptyxis. Stipules minute
or absent; leaf sheath absent. Petiole vascular bundle transection arcuate.
Venation pinnate or leaves one-veined. Stomata anomocytic. Cuticular wax
crystalloids absent. Stomatal myrosin cells absent. Leaf margin entire.
Inflorescence Terminal or
axillary, simple or compound raceme or spike, or flowers solitary axillary.
Flowers Actinomorphic, small.
Receptacle flattened, discoid. Hypogyny. Tepals (sepals?) four or five (to
eight), in one whorl, with imbricate aestivation, persistent, connate into cup.
Petals probably absent. Nectary absent. Disc usually central.
Androecium Stamens seven to
more than 100, usually in one whorl inserted on receptacular margin (in some
species of Gyrostemon in several whorls covering receptacle),
centripetally developing. Filaments very short or absent, free from each other
and from tepals. Anthers basifixed, non-versatile, tetrasporangiate, introrse,
longicidal (dehiscing by longitudinal slits). Tapetum secretory, with
binucleate cells. Female flowers with or without staminodia?
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually tricolpate (sometimes
dicolpate or tetracolpate), shed as monads, bicellular at dispersal. Exine
tectate, with granular infratectum, psilate to scabrate. Ectexine spongy,
undifferentiated.
Gynoecium Pistil composed of
usually several to numerous (up to c. 60; in Gyrostemon few; in
Cypselocarpus one) free or connate carpels usually in one whorl
(sometimes two whorls); when two carpels, then transversely orientated. Ovary
superior, unilocular (apocarpy) or several- to multilocular (as many as
carpels). Stylodia as many as carpels (sometimes marginal), free or connate in
lower part (rarely cleft). Stigmas decurrent, large and often wide, type?;
stigmatic ring forming corona. Male flowers sometimes with pistillodium?
Ovules Placentation
apical-axile (when syncarpy) or marginal (when apocarpy). Ovule one per carpel,
campylotropous (anatropous?), apotropous, bitegmic, crassinucellar. Micropyle
bistomal. Outer integument two cell layers thick. Inner integument five cell
layers thick. Archespore unicellular (Gyrostemon) or bicellular to
quadricellular (Codonocarpus). Nucellar cap absent. Hypostase present.
Obturator absent. Megagametophyte monosporous, Polygonum type.
Porogamy. Endosperm development ab initio nuclear. Endosperm haustoria?
Embryogenesis?
Fruit A dry or carnose
schizocarp, with two to numerous ventrally (Gyrostemon) or marginally
(Codonocarpus) dehiscing samaroid to follicular mericarps, a syncarp
or nutlet (Cypselocarpus, Tersonia, Walteranthus),
often with persistent calyx.
Seeds Funicular exostomal aril
formed from apex of outer integument, surrounding hilum. Testa not
multiplicative. Exotestal cells compressed. Endotestal cells small, cuboid.
Tegmen not multiplicative. Exotegmen fibrous. Exotegmic cells elongate,
thick-walled. Remaining tegmic cells crushed. Perisperm not developed.
Endosperm copious, carnose, oily. Embryo curved, well differentiated,
chlorophyll? Cotyledons two. Germination?
Cytology n = 14
DNA
Phytochemistry Glucosinolates
(synthesized from?) and cyanogenic compounds present. Ellagic acid and
proanthocyanidins not found. Tannins?
Use Unknown.
Systematics
Codonocarpus (3; C. attenuatus, C. cotinifolius,
C. pyramidalis; South Australia, Victoria, New South WalesAustralia),
Gyrostemon
(12; Australia, Tasmania, with their highest diversity in southwestern Western
Australia), Tersonia
(1; T. cyathiflora; southwestern Western Australia),
Cypselocarpus (1; C. haloragoides; southwestern Western
Australia), Walteranthus (1; W. erectus; southwestern Western
Australia).
Gyrostemonaceae may be sister
to Resedaceae, although
Prijanto (1970) observed remarkable similarities in pollen morphology between
Batis
(Bataceae) and Gyrostemonaceae.
A phylogenetic analysis of Gyrostemonaceae is needed.
Engler in Engler et Prantl, Nat. Pflanzenfam.,
III, 6: 319. 14 Mai 1895, nom. cons.
Genera/species 1/2
Distribution Southern United
States, northern Mexico, tropical Bolivia.
Fossils Unknown.
Habit Bisexual, evergreen
shrubs or small trees. Xerophytes. Branches photosynthesizing, terminating in
an elongated spine. Prophylls basal on branches and spines.
Vegetative anatomy Phellogen
ab initio pericyclic. Cortex with bast bundles and brachysclereids and in older
parts secretory resinous ducts. Vessel elements with simple perforation plates;
lateral pits alternate, bordered pits. Vestured and non-vestured pits present.
Imperforate tracheary xylem elements tracheids and fibre tracheids with
bordered pits, non-septate (also vasicentric tracheids). Wood rays usually
multiseriate, homocellular. Axial parenchyma usually apotracheal diffuse or
diffuse-in-aggregates, or paratracheal scanty vasicentric. Wood elements not
storied. Sieve tube plastids S type? Nodes? Intercellular canals present.
Phloem with resinous ducts. Idioblastic myrosin cells present. Crystals? Druses
absent.
Trichomes Hairs unicellular;
glandular hairs absent.
Leaves Alternate (spiral),
simple, entire, very small, scale-like, early caducous, with ? ptyxis. Stipules
and leaf sheath absent. Petiole vascular bundle transection? Venation? Stomata
sunken. Cuticular wax crystalloids? Idioblastic myrosin cells present. Leaf
margin entire.
Inflorescence Axillary,
umbel-like raceme.
Flowers Actinomorphic, small
and indistinct. Hypogyny. Sepals usually 2+2, decussate (sometimes five), with
imbricate aestivation, free. Petals four (or five), diagonal, with imbricate
aestivation, shortly clawed, caducous, free. Nectaries inserted at staminal
bases. Disc absent.
Androecium Stamens 4+4 (or
5+5), diplostemonous. Filaments flattened, with basal appendages, free from
each other and from tepals. Anthers dorsifixed, versatile?, tetrasporangiate,
introrse (antesepalous stamens) or latrorse (antepetalous stamens), longicidal
(dehiscing by longitudinal slits). Tapetum secretory, with binucleate to
multinucleate cells. Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains (2–)3(–4)-colporate, shed as
monads, bicellular at dispersal. Exine semitectate, with columellate
infratectum, striate-reticulate.
Gynoecium Pistil composed of
two connate carpels, obliquely orientated. Ovary superior, bilocular (or
trilocular), shortly stipitate (gynophore present). Style single, simple,
subulate, persistent. Stigma slightly bifid, minutely expanded, type?
Pistillodium absent.
Ovules Placentation axile.
Ovules approx. ten per carpel, campylotropous, apotropous or epitropous,
bitegmic, tenuinucellar. Micropyle bistomal, Z-shaped (zig-zag). Outer
integument two cell layers thick, non-multiplicative. Inner integument three
cell layers thick. Obturator absent. Apical cells of megasporangium much
enlarged (functioning as “obturator”?). Hypostase absent. Parietal tissue
absent. Megasporangial epidermal cells radially enlarged. Megagametophyte
monosporous, Polygonum type. Antipodal cells ephemeral. Endosperm
development ab initio nuclear. Endosperm haustoria? Embryogenesis?
Fruit A one- or two-seeded
berry, with persistent style.
Seeds Arilloid small,
surrounding hilum (developed from apex of outer integument or exostome). Seed
coat exotestal-tegmic. Testa non-multiplicative. Exotesta with massive cuticle
and tannin-like substance; exotestal cells large and thick-walled. Endotestal
cells tiny. Tegmen non-multiplicative. Exotegmen fibrous, with elongate cells
and strongly thickened lignified cell walls. Mesotegmen crushed. Endotegmic
cells thick-walled, with tannin-like substance, almost collapsed. Perisperm not
developed. Endosperm sparse, thin. Embryo curved, well differentiated, with
chlorophyll. Cotyledons two, incumbent. Germination?
Cytology x = 11
DNA
Phytochemistry Virtually
unknown. Ellagic acid? Tannins? Glucosinolates not found.
Use Unknown.
Brown in London Edinburgh Philos. Mag. & J.
Sci. 3: 70. Jul 1833 [’Limnantheae’], nom. cons.
Limnanthales R. Br.
in C. F. P. von Martius, Consp. Regn. Veg.: 56. Sep-Oct 1835
[’Limnantheae’]; Limnanthineae Engl.,
Syllabus, ed. 2: 143. Mai 1898
Genera/species 1/7
Distribution Western United
States, northwestern Mexico.
Fossils Unknown.
Habit Bisexual, annual herbs.
Helophytes, sometimes aquatic. Main root early wilting and replaced by
adventitious roots.
Vegetative anatomy Phellogen
absent. Primary vascular tissue cylinder of vascular bundles. Secondary lateral
growth absent. Wood elements often only in root-stem junction. Vessel elements
with simple perforation plates; lateral pits alternate to pseudoscalariform,
simple pits. Non-vestured pits present. Xylem without imperforate tracheary
elements. Wood rays uniseriate or biseriate, homocellular. Axial parenchyma
paratracheal scanty. Wood non-storied. Sieve tube plastids S type. Nodes 1:1,
unilacunar with one leaf trace. Myrosin cells idioblastic. Wood without
crystals.
Trichomes Hairs unicellular
(sometimes verrucose) or absent; glandular hairs absent.
Leaves Alternate (spiral),
once or twice pinnately compound, or simple and pinnately lobed, with
conduplicate ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle
transection? Venation pinnate. Stomata anomocytic. Cuticular wax crystalloids?
Myrosin cells abundant; stomatal myrosin cells absent. Tanniniferous idioblasts
present. Leaf margin serrate. Apices of leaf and leaflets sometimes with
hydathodes.
Inflorescence Terminal, raceme
or flowers solitary axillary. Bracts and floral prophylls (bracteoles)
sometimes absent.
Flowers Actinomorphic.
Hypogyny or half epigyny. Sepals three or (four or) five, with valvate to
somewhat imbricate aestivation, persistent, free or slightly connate at base,
comparatively large. Petals three or (four or) five, with dextrorsely contorted
aestivation, caducous, free. Nectaries present on swollen abaxial bases of
antesepalous (alternipetalous, rarely also antepetalous) stamens, or absent.
Disc absent.
Androecium Stamens usually 3+3
or 5+5 (rarely three or 4+4), of two different lengths with longest stamens
antesepalous, usually diplostemonous. Filaments free from each other and from
tepals. Anthers dorsifixed, versatile, tetrasporangiate, ab initio introrse,
subsequently extrorse, longicidal (dehiscing by longitudinal slits). Tapetum
secretory. Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains zonocolporate (syncolpate?) of
unique type with two zonocolpi parallel to equator alternatively one colpus
parallel to and encircling equator, heteropolar with smaller distal and larger
proximal pole, shed as monads, bicellular at dispersal. Exine tectate, with
columellate infratectum, microperforate, striate, spinulate.
Gynoecium Pistil composed of
two or three or (four or) five carpels, bulging (seemingly almost free in lower
part); when three median carpels abaxial then carpels antesepalous; carpellary
walls without vascular bundles. Ovaries superior or partially semi-inferior,
two or three or (four or) five, unilocular, free. Style gynobasic, filiform,
hollow, bilobate to quinquelobate. Stigmas minutely capitate to punctate,
papillate, Dry type. Pistillodium absent.
Ovules Placentation
basal-parietal. Ovule one per carpel, anatropous, ascending, apotropous (or
epitropous?), unitegmic, tenuinucellar. Integument 14 to 16 cell layers thick.
Parietal tissue absent. Archespore multicellular. Megagametophyte tetrasporous,
pseudomonosporous (quadrinucleate or sexanucleate with one nucleus developing),
modified Drusa type (one or two antipodal cells absent). Synergids
sometimes with a filiform apparatus. Endosperm development ab initio nuclear.
Endosperm haustoria laterally micropylar (on funicular side) or absent.
Embryogenesis onagrad. Suspensor uniseriate.
Fruit A schizocarp, with
persistent and sometimes accrescent calyx and with (two or) three or five
muriculate (spinulate) one-seeded nutlike mericarps.
Seeds Aril absent. Testa
pachychalazal, thick, vascularized. Tegmen absent. Perisperm not developed.
Endosperm absent. Embryo straight, well differentiated, with chlorophyll.
Cotyledons two, thick, cordate, with backwardly directed lobes, with amyloid
(xyloglucans). Germination phanerocotylar. Radicula ephemeral.
Cytology n = 5
DNA
Phytochemistry Flavonols
(kaempferol, quercetin, myricetin), cyanidin, delphinidin, ellagic acid,
non-hydrolyzable tannins, caffeic acid and other polyphenols, glucosinolates
(m-methoxybenzyl isothiocyanate) synthesized from phenylalanine and/or
tyrosine, and erucic acid and other similar fatty acids (in seeds) present.
Saponins and cyanogenic compounds not found. Carbohydrate stored as
oligosaccharides with isokestose bonds.
Use Ornamental plants
(Limnanthes douglasii).
Martinov, Tekhno-Bot. Slovar: 404. 3 Aug 1820
[’Moringeae’], nom. cons.
Moringales R. Br. in
C. F. P. von Martius, Consp. Regn. Veg.: 51. Sep-Oct 1835
[‘Moringeae’]
Genera/species 1/12–13
Distribution Semiarid regions
in northeastern Africa, southern Angola, Namibia, southern Madagascar, southern
Arabian Peninsula, southern Iran, Pakistan, India.
Fossils Unknown.
Habit Bisexual, deciduous
trees or shrubs (sometimes pachycaul, sometimes stem succulents). Roots thick
and fleshy, sometimes with root tubers.
Vegetative anatomy Phellogen?
Primary medullary strands narrow. Medulla often with central lysigenous
mucilage canals. Vessel elements with simple perforation plates; lateral pits
alternate, simple or bordered pits. Vestured or non-vestured pits present.
Imperforate tracheary xylem elements libriform fibres with simple pits,
non-septate. Wood rays uniseriate to multiseriate, homocellular. Axial
parenchyma paratracheal aliform, lozenge-aliform, confluent, or vasicentric.
Wood elements partially storied. Sieve tube plastids S type. Nodes ?,
multilacunar. Stem, leaves and flowers with myrosine cells and cells containing
protein-rich cisternae from endoplasmic reticulum. Wood rays, phloem parenchyma
and cortex with schizogenous intercellular ducts containing gums (rubber).
Prismatic calciumoxalate crystals abundant. Cortex or xylem sometimes with
druses. Phloem rays sometimes with crystals or sclereids.
Trichomes Hairs unicellular,
simple, or absent.
Leaves Alternate (spiral),
twice or three times imparipinnate, usually with persistent rhachis, with ?
ptyxis. Stipules and stipulules as stalked glandular hairs or absent; leaf
sheath absent. Petiole vascular bundle transection? Leaf base and articulations
with distinct glands. Venation pinnate (to palmate). Stomata anomocytic.
Cuticular wax crystalloids as rosettes of platelets (Fabales type).
Epidermis often with mucilaginous idioblasts. Mesophyll with cells containing
calciumoxalate druses. Leaflet margins entire. Extrafloral nectaries sometimes
present between leaflets.
Inflorescence Axillary,
thyrsoid or paniculate.
Flowers Usually transversely
(obliquely) zygomorphic (sometimes actinomorphic). Hypanthium cupular or
shortly tubular, often oblique (partially formed by inwardly bent receptacle),
with basal whorl of nectaries. Hypogyny. Sepals five, with imbricate
aestivation, petaloid, often unequal in size, free. Petals five, with imbricate
aestivation; median petal adaxial and usually larger than recurved posterior
petal pair, or abaxial; two lateral petals erect (anterior petal largest),
free. Nectariferous disc annular, intrastaminal, at hypanthial base.
Androecium Stamens five,
antepetalous-antesepalous. Filaments unequal in length, hairy at base, inserted
at margin of nectariferous disc, free from each other and from tepals. Anthers
declinate, dorsifixed, versatile?, usually disporangiate (rarely
tetrasporangiate), introrse, longicidal (dehiscing by longitudinal slits;
forming tube, through which style grows). Tapetum secretory. Staminodia three
to five, extrastaminal, more or less antesepalous, alternating with fertile
stamens.
Pollen grains
Microsporogenesis simultaneous. Pollen grains (2–)3(–4)-colporate, shed as
monads, usually bicellular (rarely tricellular) at dispersal. Exine tectate,
with granular infratectum, sparsely perforate to punctate, psilate.
Gynoecium Pistil composed of
(two or) three (or four) connate carpels. Ovary superior, unilocular, curved,
stipitate (with gynophore). Style single, simple, narrow, curved, hollow (with
stylar canal). Stigma capitate to truncate-porate (punctate?), hollow, type?
Pistillodium absent.
Ovules Placentation parietal.
Ovules numerous per carpel, anatropous, pendulous, apotropous?, bitegmic,
crassinucellar. Micropyle bistomal to exostomal, Z-shaped (zig-zag). Outer
integument ? cell layers thick, vascularized. Inner integument approx. three
cell layers thick. Parietal tissue approx. three layers thick. Endothelium
present. Megagametophyte monosporous, Polygonum type. Synergids with a
filiform apparatus. Endosperm development ab initio nuclear. Endosperm
haustoria? Embryogenesis asterad.
Fruit Three- to twelve-angled
narrowly elongate explosion (explosively dehiscent) loculicidal capsule.
Seeds Seeds usually
three-angled to three-winged. Aril absent. Testa vascularized, multiplicative.
Mesotesta thick, multiplicative; outer and inner parts of cell walls with
spiral thickenings; middle part strongly thickened. Tegmen thin, usually not
multiplicative. Exotegmen not fibrous. Perisperm not developed. Endosperm
usually absent. Embryo large, straight, well differentiated, oily, with
chlorophyll. Cotyledons two (or three). Hypocotyl swollen (often tuberous).
Epicotyl sometimes swollen. Germination phanerocotylar or cryptocotylar. Leaves
in young plants palmately compound or simple, palmately veined.
Cytology n = 11, 14
DNA
Phytochemistry Flavonols
(kaempferol, quercetin) and glucosinolates (synthesized from phenylalanine
and/or tyrosine, and from valine/isoleucine and/or leucine) and alkaloids
present. Ellagic acid, tannins, proanthocyanidins, saponins, and cyanogenic
compounds not found.
Use Ornamental plants (e.g.
Moringa oleifera), vegetables, oil plants, medicinal plants, spices,
water purification (seeds).
Systematics Moringa
(12–13; northeastern Africa, southern Angola, Namibia, southern Madagascar,
southern Arabian Peninsula, southern Iran, Pakistan, India).
Moringa is sister to Caricaceae.
PENTADIPLANDRACEAE
Hutch. et Dalziel
|
( Back to Capparales )
|
Hutchinson et Dalziel, Fl. W. Trop. Afr. 1: 461.
Jul 1928
Genera/species 1/1
Distribution Tropical West and
Central Africa, southwestern tropical Africa.
Fossils Unknown.
Habit Polygamomonoecious,
evergreen shrub or liana.
Vegetative anatomy Phellogen?
Secondary lateral growth sometimes anomalous? Vessel elements with simple?
perforation plates; lateral pits?, simple pits. Non-vestured pits present.
Imperforate tracheary xylem elements ? with bordered pits. Wood rays? Axial
parenchyma? Wood elements storied? Sieve tube plastids S type? Nodes 1:1,
unilacunar with one leaf trace, or 3:3, trilacunar with three traces. Myrosin
cells present. Crystals?
Trichomes Hairs unicellular,
simple.
Leaves Alternate (spiral),
simple, entire, with ? ptyxis. Stipules minute; leaf sheath absent. Petiole
vascular bundle transection? Venation pinnate. Stomata anomocytic. Cuticular
wax crystalloids? Epidermis with mucilaginous idioblasts. Leaf margin
entire.
Inflorescence Axillary, short
corymbose raceme.
Flowers Actinomorphic to
bisymmetric. Hypogyny. Sepals five, with valvate aestivation, sometimes
persistent, free or somewhat connate at base. Petals five, with imbricate
aestivation, often clawed, arched below around nectaries/androgynophore and
connivent through lanate hairs at edges. Nectariferous disc extrastaminal,
annular, thick, fleshy, as short androgynophore.
Androecium Stamens (nine or)
ten (to 13), diplostemonous or triplostemonous. Filaments filiform, branched or
simple?, free or connate at base, free from tepals. Anthers basifixed,
non-versatile, tetrasporangiate, latrorse, longicidal (dehiscing by
longitudinal slits); connective slightly prolonged. Tapetum secretory? Female
flowers with ten staminodia.
Pollen grains
Microsporogenesis simultaneous? Pollen grains tricolporate, shed as monads,
tricellular? at dispersal. Exine semitectate, with columellate infratectum,
microreticulate.
Gynoecium Pistil composed of
(three to) five connate antesepalous carpels. Ovary superior, (trilocular to)
quinquelocular, shortly stipitate (gynophore). Style single, simple, long.
Stigma short, (trilobate to) quinquelobate, type? Male flowers with
pistillodium.
Ovules Placentation axile.
Ovules approx. ten per carpel (in two or three rows), anatropous?, bitegmic,
crassinucellar? Micropyle ?-stomal. Outer integument ? cell layers thick. Inner
integument ? cell layers thick. Megagametophyte monosporous, Polygonum
type. Endosperm development? Endosperm haustoria? Embryogenesis?
Fruit A many-seeded berry
(containing brazzein, a protein with extremely sweet taste).
Seeds Aril absent. Testa
lanate, with external layer of white, wooly, elongate cells. Tegmen? Perisperm
not developed. Endosperm absent. Embryo strongly curved, without chlorophyll.
Cotyledons two. Germination?
Cytology n = ?
DNA
Phytochemistry Insufficiently
known. Alkaloids, benzyl and 4-methoxybenzyl glucosinolates synthesized from
phenylalanine and/or tyrosine, saponins, sweet-tasting proteins (brazzein, c.
2.000 times sweeter than saccharose, pentadin) present. Ellagic acid?
Tannins?
Use Sweetening.
Martinov, Tekhno-Bot. Slovar: 541. 3 Aug 1820,
nom. cons.
Resedales Bercht. et
J. Presl, Přir. Rostlin: 218. Jan-Apr 1820 [‘Resedaceae’];
Astrocarpaceae A. Kern., Pflanzenleben 2: 688. 6-13
Jun 1891 [’Asterocarpaceae’]; Resedineae
Engl., Syllabus, ed. 2: 123. Mai 1898; Stixaceae
Doweld, New Syllabus Pl. Fam.: 746. Apr 2007
Genera/species 6/c 95
Distribution Mostly dry areas
in the southwestern United States, Mexico, Central America, the West Indies,
Macaronesia, northern and eastern Africa, South Africa, the Mediterranean,
Europe (except northern parts), the Arabic Peninsula, Socotra, western Asia to
northwestern India, western Central Asia, western Siberia, southeastern
Himalayas, Hainan, Southeast Asia, Malesia.
Fossils Unknown.
Habit Usually bisexusal (in
‘Ochradenus’ sometimes androdioecious, in Forchhammeria
dioecious), evergreen or deciduous shrubs or suffrutices, or perennial,
biennial or annual herbs, rarely climbing. Some species are xerophytes.
Vegetative anatomy Roots
rarely with mycorrhiza. Phellogen? Secondary lateral growth sometimes anomalous
from successive cambia. Vessel elements with simple perforation plates; lateral
pits alternate, bordered pits. Vestured and non-vestured pits present.
Imperforate tracheary xylem elements libriform fibres with usually simple
(sometimes bordered) pits, non-septate. Wood rays uniseriate or multiseriate,
homocellular or heterocellular. Axial parenchyma apotracheal diffuse, or
paratracheal scanty, confluent, vasicentric, or marginal-banded. Wood elements
not storied. Intraxylary phloem sometimes concentric. Sieve tube plastids S
type. Nodes 1:1, unilacunar with one leaf trace. Myrosin cells idioblastic.
Stem and foliar epidermis sometimes with mucilaginous idioblasts. Crystals?
Endoplasmic reticulum with ER-dependent cisternae.
Trichomes Hairs usually
unicellular (in Tirania multicellular, uniseriate), simple; glandular
hairs absent?
Leaves Alternate (spiral),
simple or pinnately compound (rarely trifoliolate), entire or pinnately lobed,
with ? ptyxis. Stipules small, intrapetiolar, often as glands, or absent; leaf
sheath absent (in Tirania as spines). Petiole vascular bundle
transection? Venation pinnate (rarely palmate) or leaves one-veined. Stomata
anomocytic. Cuticular wax crystalloids? Stomatal myrosin cells absent.
Epidermis with or without mucilaginous idioblasts. Mesophyll usually without
calciumoxalate crystals (in Forchhammeria with sclerenchymatous
cells). Leaf margin sinuate or entire. In Caylusea large water
absorbing hairs present along leaf margin.
Inflorescence Usually terminal
(sometimes axillary), simple or compound raceme, spike or panicle (in
Forchhammeria trifoliata sometimes cymose; flowers in Tirania
solitary axillary). Floral prophylls (bracteoles) absent.
Flowers Zygomorphic, usually
small. Hypogyny or half epigyny. Sepals (four to) six (to eight), with valvate
or somewhat imbricate aestivation, persistent, usually free (occasionally
somewhat connate at base); sepals in Tirania six, in
Forchhammeria numerous (bracts?) and in Stixis three to
numerous, with imbricate aestivation. Petals (two or four to) six (to eight),
with valvate or open aestivation, unequally sized (two adaxial petals largest,
lateral petals smaller and less split, abaxial petals smallest), clawed,
caducous or persistent, usually free (petals absent in
‘Ochradenus’), usually with adaxial ligule (absent in
‘Oligomeris’) inserted between claw and apically usually fringed
limb; petals in Tirania six, with imbricate aestivation, absent in
Forchhammeria and Stixis. Gynophore or androgynophore present
in some species; androgynophore present in Stixis. Nectariferous disc
extrastaminal (or sometimes absent), often widest adaxially, with nectariferous
gland on abaxial side; nectary sometimes petaloid (nectariferous disc absent in
‘Oligomeris’).
Androecium Stamens (three to)
16 to 22 (to more than 50; in ’Ochradenus’ numerous; in
’Oligomeris’ linifolia three), usually in one whorl
(sometimes in several whorls), centrifugally developing from annular
primordium. Filaments usually free (sometimes connate at base), free from
tepals; filaments in Forchhammeria inserted on annular outgrowth
surrounding base of androgynophore, free from each other and from tepals.
Anthers basifixed, non-versatile, tetrasporangiate, introrse, longicidal
(dehiscing by longitudinal slits); anthers in Forchhammeria with short
unicellular hairs provided with cuticular longitudinal folds, dorsifixed to
basifixed, often versatile, tetrasporangiate, latrorse, longicidal (dehiscing
by longitudinal slits). Tapetum secretory. Staminodia absent.
Pollen grains Microsporogenes
simultaneous. Pollen grains tricolpate, tricolporate or tricolporoidate, shed
as monads, bicellular at dispersal; pollen grains in Stixis
(2–)3(–4)-colporoidate and very small, in Forchhammeria
tricolporate. Exine tectate or semitectate, with columellate infratectum,
reticulate, microreticulate or microperforate, foveolate, striate or
rugulate.
Gynoecium Pistil composed of
(two or) three to six (to eight), connate or apically and adaxially often
incompletely paracarpously connate, antesepalous carpels (in
’Ochradenus’ finally closed); when carpels three, then median
carpel often adaxial (carpels in Caylusea connate only at base;
carpels in Sesamoides free, apocarpy). Ovary superior or
semi-inferior, usually unilocular (sometimes bilocular to multilocular),
shortly stipitate (gynophore and sometimes androgynophore); one locule and one
seed in Forchhammeria usually degenerating; ovary sessile in
Tirania. Style usually absent (sometimes single, simple or branched).
Stigmas two to five, usually small and narrow (in Forchhammeria
fleshy), commissural and/or dorsal, non-papillate, Dry type. Pistillodium
absent.
Ovules Placentation usually
parietal (in e.g. Tirania axile; in Caylusea basal; in
Sesamoides laminal). Ovules two to numerous (in Forchhammeria
and Sesamoides usually one, rarely two) per carpel, anatropous (to
hemianatropous?) to campylotropous, pendulous or ascending, bitegmic, usually
crassinucellar (rarely tenuinucellar). Micropyle usually endostomal (sometimes
bistomal). Outer integument approx. two cell layers thick. Inner integument
three or four cell layers thick. Hypostase present. Archespore multicellular.
Megagametophyte monosporous, Polygonum type. Endosperm development ab
initio nuclear. Endosperm haustoria? Embryogenesis onagrad.
Fruit Usually a soft,
thin-walled one- to many-seeded capsule apically open between stigmas (in
Sesamoides an assemblage of follicles, carpidia; in some species of
’Ochradenus’ baccate with fleshy pericarp).
Seeds Aril usually present,
formed from apex of outer integument. Elaiosome (carunculi?; lipid-rich tissue
adjacent to hilum) present in some species. Testa non-multiplicative. Exotesta?
Endotestal cells cuboid, with thickened unlignified walls and often
crystalliferous. Tegmen non-multiplicative. Exotegmic cells fibrous, with
lignified walls. Endotegmen crystalliferous. Perisperm not developed. Endosperm
sparse or absent. Embryo large, curved or plicate, well differentiated, oily,
with chlorophyll (at least in some species of Reseda). Cotyledons two,
sometimes unequal (to very unequal) in size. Germination phanerocotylar,
hypogeous or epigeous.
Cytology x = 5–15 –
Polyploidy and aneuploidy frequent.
DNA
Phytochemistry Flavonols
(kaempferol, quercetin), methylated flavonols, cyanogenic compounds,
glucosinolates (synthesized from phenylalanine and/or tyrosine), and aromatic
m-carboxycinnamic acids present. Ellagic acid, tannins, and
proanthocyanidins not found. BCAA glucosinolates absent. Sinapic acid not
accumulated. Methyl glucosinolates (synthesized from phenylalanine and/or
tyrosine and methionin?) present in Forchhammeria.
Use Ornamental plants,
vegetables (Caylusea abyssinica), dyeing sources (Reseda
luteola), perfumes (Reseda odorata).
Systematics Stixis
(7; S. hookeri, S. obtusifolia, S. ovata,
S. philippinensis, S. scandens, S. scortechinii,
S. suaveolens; northeastern India, Bhutan, Burma, southwestern China,
Hainan, northern Thailand, Vietnam, the Lesser Sunda Islands), Tirania
(1; T. purpurea; southern Vietnam); Forchhammeria (c 10;
California, Mexico, Central America, the West Indies); Caylusea (6;
C. abyssinica, C. canescens, C. hexagyna, C.
jaberi, C. latifolia, C. moquiniana; the Cape Verde
Islands, North and East Africa, Southwest Asia to northwestern India), Sesamoides
(6; S. interrupta, S. minor, S. prostrata, S.
purpurascens, S. spathulifolia, S. suffruticosa;
southwestern Europe, Madeira, western Mediterranean), Reseda (c
65; Europe, the Canary Islands, the Mediterranean to Central Asia).
Resedaceae may be sister to Gyrostemonaceae.
Caylusea is sister to [Reseda+Sesamoides]
(Martín-Bravo & al. 2007).
Borthwickiaceae and
Stixaceae were included in Resedaceae by APG IV (2016).
Tirania is sister to
Stixis (Su & al. 2012, Cardinal-McTeague & al. 2016). The
clade [Stixis+Tirania] is sister-group to the remaining
Resedaceae, and
Forchhammeria successive sister to the rest, according to
Cardinal-McTeague & al. (2016). All these genera, together with Borthwickiaceae, were
included in Resedaceae by
APG IV (2016).
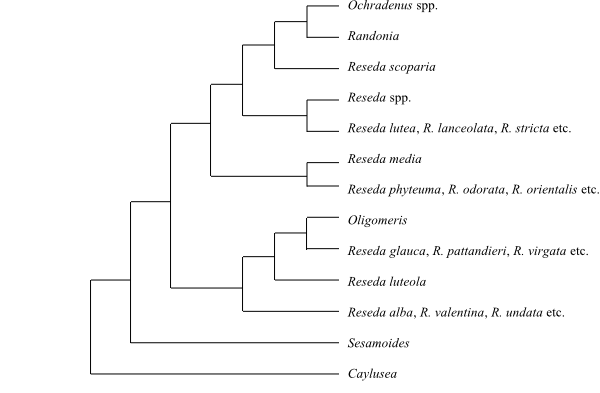 |
Bayesian inference tree (simplified) of Resedaceae based on DNA
sequence data (Martín-Bravo & al. 2007). Stixis,
Tirania and Forchhammeria were not included in the
analysis.
|
Lindley, Intr. Nat. Syst. Bot., ed. 2: 269. 13
Jun 1836, nom. cons.
Azimaceae Wight et
Gardner in Calcutta J. Nat. Hist. 6: 52. Apr 1845;
Salvadorineae Engl., Syllabus, ed. 2: 171. Mai 1898;
Salvadorales R. Dahlgren ex Reveal in Phytologia 74:
174. 25 Mar 1993
Genera/species 3/11
Distribution Africa,
Madagascar, the southern Arabian Peninsula, Pakistan, India, Sri Lanka,
southern China, Southeast Asia, West Malesia, the Philippines, often in drier
regions.
Fossils Unknown.
Habit Bisexual,
polygamomonoecious, dioecious, or polygamodioecious, evergreen shrubs
(sometimes climbing) or small trees. Xerophytes, sometimes spiny.
Vegetative anatomy Phellogen
ab initio superficial. Primary vascular tissue a cylinder of vascular bundles.
Secondary lateral growth anomalous, from simple cambial cylinder. Vessel
elements with simple perforation plates; lateral pits alternate, bordered pits.
Vestured pits present in Salvadora. Imperforate tracheary xylem
elements libriform fibres with simple pits, non-septate. Wood rays uniseriate
or multiseriate, homocellular (or slightly heterocellular). Axial parenchyma
paratracheal scanty vasicentric, or banded. Wood elements partially storied.
Intraxylary (concentric or diffuse) phloem present in Dobera and
Salvadora. Sieve tube plastids S type. Nodes 1:1, unilacunar with one
leaf trace (Salvadora), or 1:2, unilacunar with two traces
(Azima, Salvadora). Myrosin cells absent. Prismatic
calciumoxalate crystals abundant. Wood rays with rhomboidal calciumoxalate
crystals. Endoplasmic reticulum with proteinaceous vacuole-like dilated
cisternae.
Trichomes Hairs usually simple
(sometimes peltate to lepidote) or absent.
Leaves Opposite, simple,
entire, usually coriaceous, with flat to curved ptyxis (Salvadora).
Stipules very small; leaf sheath absent. Petiole vascular bundle transection?
Venation pinnate. Stomata usually paracytic (sometimes anomocytic or
anisocytic). Cuticular wax crystalloids as platelets. Mesophyll with cells
containing calciumoxalate druses. Leaf margin entire.
Inflorescence Terminal or
axillary, simple raceme or compound, fasciculate inflorescence. Floral
prophylls (bracteoles) usually present.
Flowers Usually actinomorphic
(occasionally somewhat zygomorphic), obliquely orientated, small. Short
hypanthium present in Salvadora. Hypogyny. Sepals two to four (or
five), with imbricate to contorted aestivation, connate at base into tube.
Petals two to four (or five), with imbricate to contorted aestivation, often
with glands (staminodia?) on adaxial side, free (Azima,
Dobera) or connate at base (Salvadora). Antepetalous
nectariferous glands (probably not staminal) alternating with stamens (in
Dobera extrastaminal, in Salvadora interstaminal) or absent
(Azima).
Androecium Stamens two to four
(or five), haplostemonous, antesepalous, alternipetalous. Filaments free
(Azima) or connate at base (Dobera), free from or adnate at
base to petals (Salvadora). Anthers dorsifixed, versatile?,
tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits).
Tapetum secretory. Staminodia absent (or possibly as glands?).
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually tricolporoidate
(sometimes hexacolporoidate), shed as monads, bicellular at dispersal. Exine
tectate, with columellate infratectum, punctate, perforate, reticulate or
striate.
Gynoecium Pistil composed of
two connate carpels, in Salvadora obliquely orientated. Ovary
superior, bilocular (Azima) or unilocular (pseudomonomerous;
Dobera, Salvadora); in Dobera and Azima
apically quadrilocular due to apical secondary septum; ovary shortly stipitate
(gynophore) in Salvadora. Style single, simple, short. Stigma capitate
(Salvadora) or slightly bilobate (Azima, Dobera),
type? Male flowers with pistillodium.
Ovules Placentation
basal-parietal (when ovary unilocular) or axile-basal (when ovary bilocular).
Ovules one or two per carpel, anatropous, ascending, apotropous, bitegmic,
crassinucellar. Micropyle usually endostomal (sometimes exostomal). Outer
integument approx. ten to 15 cell layers thick (in Azima
vascularized). Inner integument three to five cell layers thick. Obturator
dome-shaped, chalazal, present in Salvadora (absent in Azima
and Dobera). Megagametophyte monosporous, Polygonum type.
Endosperm development ab initio nuclear. Endosperm haustoria absent.
Embryogenesis asterad.
Fruit Usually a one-seeded
berry or one-seeded drupe.
Seeds Aril absent. Seed coat
exotestal. Testa multiplicative. Exotestal cells palisade, somewhat thickened,
crystalliferous, with inner walls mucilaginous. Endotesta present. Tegmen
non-multiplicative. Exotegmen fibrous or non-fibrous, finally crushed;
exotegmic cell walls unlignified. Endotegmen crushed. Perisperm not developed.
Endosperm absent. Embryo straight, well differentiated, chlorophyll? Cotyledons
two, cordate, thick, plano-convex, oily. Germination?
Cytology n = 11
(Azima), 12 (Salvadora)
DNA
Phytochemistry Flavonols
(quercetin), piperidine alkaloids, mustard oils based on glucotropaeolin, and
cyanogenic compounds present. Ellagic acid, tannins, and proanthocyanidins not
found.
Use Fruits, vegetables,
perfumes (Dobera), tooth-brushes.
Systematics Azima (4;
A. angustifolia, A. pubescens, A. sarmentosa, A.
tetracantha; eastern and southern Africa and Madagascar to Sri Lanka,
Southeast Asia, Hainan, the Philippines and the Lesser Sunda Islands),
Dobera (2; D. glabra, D. loranthifolia; tropical
East Africa, southern Arabian Peninsula to northwestern India),
Salvadora (5; S. alii, S. angustifolia, S.
australis, S. oleoides, S. persica; tropical and
subtropical Africa from South Africa to northeastern Africa and east to
tropical Asia).
Salvadoraceae are sister to Batis (Bataceae).
Judging from morphological data, Azima
may be sister to [Dobera+Salvadora].
Iltis in Taxon 48: 260. 17 Mai 1999
Genera/species 1/1
Distribution Northern and
south-central Mexico.
Fossils Unknown.
Habit Bisexual, evergreen
shrub with pungent smell and well differentiated long and short shoots.
Xerophyte.
Vegetative anatomy Phellogen
ab initio superficial. Primary medullary rays narrow. Primary vascular tissue
cylinder, without separate vascular bundles. Vessel elements with simple
perforation plates; lateral pits alternate. Non-vestured pits present.
Imperforate tracheary xylem elements fibre tracheids with bordered pits,
non-septate? (also vasicentric tracheids). Wood rays uniseriate. Axial
parenchyma usually paratracheal scanty (sometimes apotracheal diffuse or
diffuse-in-aggregates, or banded). Wood elements not storied. Sieve tube
plastids S type? Nodes 1:1, unilacunar with one leaf trace. Myrosin cells
absent. Crystals rare (rhomboidal) or absent; druses present in cortical
parenchyma cells.
Trichomes Unicellular T-shaped
malpighiacean hairs, situated on basal multicellular podium, with branches
parallel to longitudinal axis of organ (as in Brassicaceae).
Leaves Alternate (spiral),
simple, entire, isobifacial, often coriaceous, with ? ptyxis. Stipules and leaf
sheath absent. Petiole vascular bundle transection? Venation pinnate or
palmate; secondary veins subbasal (inserted near leaf base). Stomata
anomocytic. Cuticular wax crystalloids? Leaf margin entire.
Inflorescence Flowers
axillary, solitary.
Flowers Actinomorphic, large.
Receptacle elongated into short androphore and relatively short gynophore.
Hypogyny. Sepals (five or) six (or seven), with valvate? aestivation, swollen
at base, connate into calyptra and at anthesis irregularly dehiscing with one
or two valves, caducous or persistent. Petals (five or) six (or seven), with
imbricate aestivation, clawed, caducous, free. Nectary absent? Disc absent.
Androecium Stamens (c. 40 to)
c. 60 to 76 (from six staminal primordia), in five to seven vertical
alternipetalous rows of staminal pairs on receptacle, centrifugally developing.
Filaments free from each other (seemingly?), free from tepals. Anthers
basifixed, non-versatile, tetrasporangiate, introrse, longicidal (dehiscing by
longitudinal slits). Tapetum secretory? Staminodia absent.
Pollen grains
Microsporogenesis simultaneous? Pollen grains tricolpate, shed as monads,
?-cellular at dispersal. Exine semitectate, with columellate infratectum,
striato-rugulate.
Gynoecium Pistil composed of
three carpels, connate along their prolonged adaxial margins; commissural
fusion of marginal ventral carpellary vascular bundles. Ovary superior,
trilocular. Style single, simple, short, trifid. Stigmas subcapitate, type?
Pistillodium absent.
Ovules Placentation axile.
Ovules ten to 14 per carpel, arranged in two rows, anatropous, bitegmic,
crassinucellar. Micropyle ?-stomal. Outer integument ? cell layers thick. Inner
integument ? cell layers thick. Megagametophyte monosporous, Polygonum
type. Endosperm development? Endosperm haustoria? Embryogenesis?
Fruit A septifragal capsule,
dehiscing with three valves and with persistent thin central columella
corresponding to replum.
Seeds Seed at cotyledonary end
aleate, stellate in transverse section. Aril absent. Testa multiplicative?,
soft, thin, aleately expanded at chalazal end. Tegmen multiplicative? Exotegmen
non-fibrous. Endotegmen? Perisperm not developed. Endosperm sparse, one cell
layer thick. Embryo flat, straight, chlorophyll? Cotyledons two, cordate.
Germination phanerocotylar.
Cytology n = ?
DNA
Phytochemistry Uniknown.
Glucosinolates synthesized from phenylalanine and/or tyrosine?
Use Unknown.
Pax in Engler et Prantl, Nat. Pflanzenfam., III,
2: 207. Mar 1891, nom. cons.
Tovariales Nakai,
Chosakuronbun Mokuroku [Ord. Fam. Trib. Nov.]: 239. 20 Jul 1943
Genera/species 1/2
Distribution Tropical
America.
Fossils Unknown.
Habit Bisexual, annual or
perennial herbs, or evergreen shrubs or suffrutices (sometimes climbing or
arborescent). Leaves when dry with coumarin-like scent.
Vegetative anatomy Phellogen?
Vessel elements with simple perforation plates; lateral pits alternate or
scalariform, simple pits. Non-vestured pits present. Imperforate tracheary
xylem elements libriform fibres with simple pits, septate. Wood rays uniseriate
or multiseriate, heterocellular? Axial parenchyma scanty vasicentric. Wood
elements not storied. Sieve tube plastids S type. Nodes 1:1, unilacunar with
one leaf trace? Myrosin cells idioblastic or stomatal. Crystals? Cells with
organelle-like expanded ER-cisternae absent.
Trichomes Hairs unicellular,
simple, or absent.
Leaves Alternate (spiral),
trifoliolate, with ? ptyxis. Stipules cauline or present on leaf base, minute,
early caducous; leaf sheath absent. Petiole vascular bundle transection
arcuate. Venation palmate. Stomata anomocytic. Cuticular wax crystalloids?
Leaflet margins entire.
Inflorescence Terminal,
raceme.
Flowers Bisymmetric, small.
Hypogyny. Sepals (six to) eight (to ten), with imbricate or open aestivation,
in one whorl, caducous, free. Petals (six to) eight (or nine), with imbricate
aestivation, in one whorl, often shortly clawed, free. Nectariferous disc
largely extrastaminal, lobate, with nectaries between staminal bases.
Androecium Stamens (six to)
eight (to ten), haplostemonous, antesepalous, alternipetalous. Filaments
widened at base, sometimes hairy, free from each other and from tepals. Anthers
with short unicellular hairs provided with cuticular longitudinal folds,
basifixed, non-versatile, tetrasporangiate, introrse, longicidal (dehiscing by
longitudinal slits). Tapetum secretory. Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains (2–)3-colpor(oid)ate, shed as
monads, bicellular at dispersal. Exine semitectate, with columellate
infratectum, reticulate.
Gynoecium Pistil composed of
(five or) six (to eight) connate alternisepalous carpels. Ovary superior,
incompletely sexalocular (sometimes quinque-, septa- or octalocular), sometimes
stipitate (gynophore short or absent). Style single, simple, short, persistent,
or absent. Stigma peltate or sexalobate to octalobate, type? Pistillodium
absent.
Ovules Placentation axile in
lower part of ovary to intrusively parietal in upper part. Ovules numerous per
carpel, in several rows, ab initio anatropous, finally (after fertilization)
campylotropous, bitegmic, crassinucellar. Funicle long. Micropyle bistomal,
Z-shaped (zig-zag). Outer integument approx. two cell layers thick. Inner
integument approx. three cell layers thick. Megagametophyte monosporous,
Polygonum type. Synergids with a filiform apparatus. Endosperm
development ab initio nuclear. Endosperm haustoria? Embryogenesis?
Fruit A many-seeded berry. Two
of innermost pericarp layers lignified.
Seeds Aril absent. Seed coat
testal-tegmic? Testa non-multiplicative. Exotestal cells enlarged, with tannins
and thickened walls. Endotestal cells small. Tegmen non-multiplicative.
Exotegmic cells fibrous, with reticulately thickened walls. Endotegmen
tanniniferous. Perisperm not developed. Endosperm sparse, thin, oily. Embryo
curved along periphery of seed, well differentiated, oily, chlorophyll?
Cotyledons two, flattened. Germination?
Cytology n = 14
DNA
Phytochemistry Insufficiently
known. Glucosinolates (not synthesized from phenylalanine or tyrosine) and
cyanogenic compounds present. Tannins?
Use Unknown.
Systematics Tovaria
(2; T. diffusa, T. pendula; southern Mexico, Central America,
Jamaica, northwestern South America, especially in the Andes, to Peru and
Bolivia).
Tovaria is part of a polytomy
comprising Pentadiplandraceae to
Brassicaceae.
de Candolle, Prodr. 1: 683. med Jan 1824, nom.
cons.
Tropaeolales Bercht.
et J. Presl, Přir. Rostlin: 220. Jan-Apr 1820 [‘Tropaeoleae’]
Genera/species 1/80–100
Distribution Mountain areas
from southern Mexico to Tierra del Fuego, southern Brazil to southern
Argentina.
Fossils Unknown.
Habit Bisexual, perennial or
annual herbs, usually climbing with petioles or pedicel tendrils, more or less
fleshy (sometimes slightly succulent). Several species form root or stem tubers
from rhizome or from hypocotyl.
Vegetative anatomy Phellogen
ab initio superficial to deeply seated. Primary vascular tissue cylinder of
separate vascular bundles. Endodermis sometimes significant. Pericyclic fibres
absent. Vessels present in root and stem. Vessel elements with usually simple
(sometimes reticulate, scalariform or intermediate between scalariform and
multiperforate) perforation plates; lateral pits alternate, simple pits.
Non-vestured pits present. Imperforate tracheary xylem elements usually
tracheids (libriform fibres present in stem; also scarce vasicentric tracheids)
with simple or bordered pits, non-septate. Wood rays uniseriate (in root) or
multiseriate, heterocellular. Axial parenchyma paratracheal scanty vasicentric.
Wood elements not storied. Sieve tube plastids Ss type. Nodes 3:3, trilacunar
with three leaf traces. Myrosine cells only idioblastic. Crystals?
Trichomes Hairs multicellular
(few-celled), uniseriate, simple, or absent; glandular hairs sometimes
present.
Leaves Usually alternate
(sometimes opposite in lower parts), usually simple (sometimes palmately
compound), entire or palmately lobed, often peltate, with flat ptyxis. Stipules
small, fimbriate or more or less foliaceous or absent (often present only in
seedlings), usually caducous; leaf sheath absent. Petiole often climbing.
Petiole vascular bundle transection annular. Venation palmate. Stomata
anomocytic, without subsidiary cells. Cuticular wax crystalloids as clustered
tubuli (Berberis type), chemically dominated by nonacosan-10-ol.
Lamina sometimes gland-dotted. Epidermis sometimes with mucilaginous
idioblasts. Myrosine cells containing myrosinase abundant; stomatal myrosin
cells absent. Leaf margin serrate or entire.
Inflorescence Flowers usually
axillary solitary (rarely in axillary umbel-like inflorescence). Floral
prophylls (bracteoles) present or absent.
Flowers Zygomorphic, often
large. Hypogyny. Sepals five, with imbricate or valvate aestivation, petaloid;
adaxial, or adaxial and two lateral sepals expanded into nectariferous spur
(rarely absent), usually free (sometimes three connate), caducous. Petals
usually two upper adaxial and three lower abaxial (lower petals often absent),
with imbricate aestivation, often clawed, free. Nectary extrastaminal,
continuing into spur. Disc absent. Hairs on lower margin of abaxial (anterior)
petals forming barrier against small non-pollinating insects (nectar
thieves).
Androecium Stamens 4+4 (two
antepetalous – median – stamens suppressed). Filaments filiform, free from
each other and from tepals. Anthers basifixed, non-versatile, tetrasporangiate,
introrse-latrorse, longicidal (dehiscing by longitudinal slits). Tapetum
secretory. Staminodia absent. During anthesis in Tropaeolum majus,
stamens bend forth successively one after the other to nectariferous spur
liberating their pollen grains and subsequently returning to their original
position; style finally bending against spur.
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually tricolporate (sometimes
dicolporate), shed as monads, tricellular at dispersal. Exine semitectate, with
columellate infratectum, reticulate (sometimes partially striate).
Gynoecium Pistil composed of
three connate carpels; median carpel adaxial. Ovary superior, trilocular. Style
single, narrow, short. Stigma trilobate, non-papillate, Dry type. Pistillodium
absent.
Ovules Placentation
apical-axile. Ovules two per carpel (one degenerating), anatropous, pendulous,
epitropous, bitegmic, tenuinucellar, pachychalazal (massive chalaza becoming
meristematic and forming seed coat). Micropyle endostomal. Outer integument ?
cell layers thick. Inner integument ? cell layers thick. Megagametophyte
monosporous, Polygonum type. Endosperm development ab initio nuclear.
Two uppermost cells of proembryo several times dividing and forming basal
system of haustoria (at least in Tropaeolum majus). Peripheral cells
of basal cell mass (adjacent to suspensor and on opposite side of funicle)
forming rhizomorphic suspensor haustorium, penetrating integument below
micropyle, growing around ovule and into carpellary wall. Additional suspensor
haustorium developing from peripheral cells of basal cell mass adjacent to
funicle, growing through integument and funicle and reaching placental vascular
bundle, growing further along placenta. Embryogenesis solanad.
Fruit A schizocarp with
usually three (rarely one) drupaceous or nutlike mericarps (rarely a samara).
Carpophore rarely present.
Seeds Seeds pachychalazal
(seed coat formed by chalaza). Aril absent. Testa multiplicative, indistinct
(outer integument disappearing during maturation). Part of mesotesta suberized.
Endotesta (inner epidermis) lacking crystalliferous and sclerotic cells. Tegmen
non-multiplicative. Exotegmen not developing into sclerotic layer. Perisperm
not developed. Endosperm absent. Embryo large, straight, well differentiated,
with chlorophyll. Cotyledons two, thick, plano-convex, with amyloid
(xyloglucans). Germination cryptocotylar.
Cytology n = 12–15, 21
DNA
Phytochemistry Flavonols
(kaempferol, quercetin), benzyl and 4-methoxybenzyl glucosinolates (synthesized
from phenylalanine and/or tyrosine and valine/isoleucine and/or leucine),
erucic acid, eicosenoic acid, and cucurbitacins present. Ellagic acid,
proanthocyanidins and cyanogenic compounds not found.
Use Ornamental plants,
vegetables (Tropaeolum tuberosum etc.), pickled unripe fruits.
Systematics Tropaeolum
(80–100; mountain regions in southern Mexico to Tierra del Fuego, southern
Brazil to southern Argentina).
Tropaeolum
is sister to Akaniaceae.
Literature
Abdallah MS. 1967. The Resedaceae. A taxonomical revision of
the family. – Meded. Landbouwh. Wageningen 67-68: 1-98.
Abdallah MS, De Wit HCD. 1978. The Resedaceae. A taxonomical revision of
the family (final installment). – Meded. Landbouwh. Wageningen 78: 99-416.
Abraham M. 1885. Bau und
Entwicklungsgeschichte der Wandverdickungen in den Samenoberhautzellen einiger
Cruciferen. – Jahrb. Wiss. Bot. 16: 599-637.
Agerbirk N, Warwick SI, Hansen PR, Olsen CE.
2008. Sinapis phylogeny and evolution of glucosinolates and specific
nitrile degrading enzymes. – Phytochemistry 69: 2937-2949.
Aguinagalde I, Gómez-Campo C. 1984. The
phylogenetic significance of flavonoids in Crambe (Cruciferae). –
Bot. J. Linn. Soc. 89: 277-288.
Ahluwalia K. 1962. Morphological and
embryological studies in the families Zygophyllaceae
and Salvadoraceae I. Embryology
and systematic position of Peganum harmala Linn.; II. Some
embryological aspects of Salvadora persica Linn. – M.Sc. thesis,
University of Delhi, India.
Ahuja YR, Bhaduri PN. 1956. The embryology of
Brassica campestris L. var. toria Duth. & Full. –
Phytomorphology 6: 63-67.
Akhani H. 2003. Notes on the flora of Iran:
4. Two new records and synopsis of the new data on Iranian Cruciferae since
Flora Iranica. – Candollea 58: 369-385.
Alam Z. 1936. Cytological studies of some
Indian oleiferous Cruciferae III. – Ann. Bot. 50: 85-102.
Alexander I. 1952. Entwicklungsstudien an
Blüten von Cruciferen und Papaveraceen. – Planta 41: 125-144.
Alexander PJ, Windham MD, Govindarajulu R,
Al-Shehbaz IA, Bailey CD. 2010. Molecular phylogenetics and taxonomy of the
genus Thysanocarpus (Brassicaceae). – Syst. Bot. 35:
559-577.
Alexander PJ, Windham MD, Beck JB, Al-Shehbaz
IA, Allphin L, Bailey CD. 2013. Molecular phylogenetics and taxonomy of the
genus Boechera and related genera (Brassicaceae: Boechereae). – Syst.
Bot. 38: 192-209.
Aleykutty KM, Inamdar JA. 1978a. Studies in
the vessels of some Capparaceae. – Flora 167: 103-109.
Aleykutty KM, Inamdar JA. 1978b. Structure,
ontogeny, and taxonomic significance of trichomes and stomata in some
Capparidaceae. – Feddes Repert. 89: 19-30.
Allender CJ, Allainguillaume J, Lynn J, King
GJ. 2007. Simple sequence repeats reveal uneven distribution of genetic
diversity in chloroplast genomes of Brassica oleracea L. and (n = 9)
wild relatives. – Theor. Appl. Gen. 114: 609-618.
Al-Shehbaz IA. 1973. The biosystematics of
the genus Thelypodium (Cruciferae). – Contr. Gray Herb. 204:
3-148.
Al-Shehbaz IA. 1977. Protogyny in the
Cruciferae. – Syst. Bot. 2: 327-333.
Al-Shehbaz IA. 1982. Rollinsia, a
new genus of Cruciferae from Mexico. – Taxon 31: 421-422.
Al-Shehbaz IA. 1989a. New or noteworthy
Draba (Brassicaceae) from
South America. – J. Arnold Arbor. 70: 427-430.
Al-Shehbaz IA. 1989b.
Dactylocardamum (Brassicaceae): a remarkable new genus
from Peru. – J. Arnold Arbor. 70: 515-521.
Al-Shehbaz IA. 1989c. Systematics and
phylogeny of Schizopetalon (Brassicaceae). – Havard Pap. Bot.
1: 10-46.
Al-Shehbaz IA. 1989d. The South American
genera Brayopsis and Englerocharis (Brassicaceae). – Nord. J. Bot. 8:
619-625.
Al-Shehbaz IA. 1990a. The genus
Aschersoniodoxa (Brassicaceae). – Syst. Bot. 15:
387-393.
Al-Shehbaz IA. 1990b. The South American
Eremodraba (Brassicaceae). – Ann. Missouri Bot.
Gard. 77: 602-604.
Al-Shehbaz IA. 1990c. Generic limits and
taxonomy of Brayopsis and Eudema (Brassicaceae). – J. Arnold Arbor.
71: 93-109.
Al-Shehbaz IA. 1990d. A note on the Chilean
endemic Draba thlaspiformis (Brassicaceae). – J. Arnold Arbor.
71: 385-387.
Al-Shehbaz IA. 1991. Novelties in
Draba (Brassicaceae) from
Venezuela, Ecuador, and Peru. – Novon 1: 67-70.
Al-Shehbaz IA. 1992. Draba
barclayana (Brassicaceae), a
new species from Colombia. – Novon 2: 4-5.
Al-Shehbaz IA. 1994a. Petroravenia
(Brassicaceae), a new genus from
Argentina. – Novon 4: 191-196.
Al-Shehbaz IA. 1994b. Three new South
American species of Draba (Brassicaceae). – Novon 4:
197-202.
Al-Shehbaz IA. 1999. Generic placement of
species excluded from Arabidopsis (Brassicaceae). – Novon 9:
296-307.
Al-Shehbaz IA. 2000a. A revision of
Pegaeophyton (Brassicaceae). – Edinburgh J. Bot.
57: 157-170.
Al-Shehbaz IA. 2000b. Baimashania
(Brassicaceae), a new genus from
China. – Novon 10: 320-322.
Al-Shehbaz IA. 2000c. What is Nasturtium
tibeticum (Brassicaceae)? –
Novon 10: 334-336.
Al-Shehbaz IA. 2001. A review of gamosepaly
in the Brassicaceae and a revision
of Desideria, with a critical evaluation of related genera. – Ann.
Missouri Bot. Gard. 87: 549-563.
Al-Shehbaz IA. 2002a. Noccaea
nepalensis, a new species from Nepal, and four new combinations in
Noccaea (Brassicaceae).
– Adansonia, sér. III, 24: 89-92.
Al-Shehbaz IA. 2002b. New species of
Alyssum, Aphragmus, Arabis, and
Sinosophiopsis (Brassicaceae) from China and India.
– Novon 12: 309-313.
Al-Shehbaz IA. 2002c. Six new species of
Draba (Brassicaceae) from
the Himalayas. – Novon 12: 314-318.
Al-Shehbaz IA. 2003a. Proposal to conserve
the name Smelowskia against Redowskia (Brassicaceae). – Taxon 52:
360-361.
Al-Shehbaz IA. 2003b. Transfer of most North
American species of Arabis to Boechera (Brassicaceae). – Novon 13:
381-391.
Al-Shehbaz IA. 2003c. A synopsis of
Tropidocarpum (Brassicaceae). – Novon 13:
392-395.
Al-Shehbaz IA. 2003d. Aphragmus
bouffordii, a new species from Tibet and a synopsis of Aphragmus
(Brassicaceae). – Harvard Pap.
Bot. 8: 25-27.
Al-Shehbaz IA. 2004a. Novelties and notes on
miscellaneous Asian Brassicaceae.
– Novon 14: 153-157.
Al-Shehbaz IA. 2004b. Two new species of
Draba (Brassicaceae):
D. miehorum from Tibet and D. sagasteguii from Peru. –
Novon 14: 249-252.
Al-Shehbaz IA. 2004c. A synopsis of the South
American Neuontobotrys (Brassicaceae). – Novon 14:
253-257.
Al-Shehbaz IA. 2004d. A synopsis of the South
American Weberbauera (Brassicaceae). – Novon 14:
258-268.
Al-Shehbaz IA. 2005a. Proposal to conserve
the name Erucastrum against Kibera and Hirschfeldia
(Brassicaceae). – Taxon 54:
204-205.
Al-Shehbaz IA. 2005b. Nomenclatural notes on
Eurasian Arabis (Brassicaceae). – Novon 15:
519-524.
Al-Shehbaz IA. 2005c. Hesperidanthus
(Brassicaceae) revisited. –
Harvard Pap. Bot. 10: 47-51.
Al-Shehbaz IA. 2006a. The genus
Sisymbrium in South America, with synopses of the genera
Chilocardamum, Mostacillastrum, Neuontobotrys, and
Polypsecadium (Brassicaceae). – Darwiniana 44:
341-358.
Al-Shehbaz IA. 2006b. New or noteworthy taxa
of Argentinean and Chilean Brassicaceae (Cruciferae). –
Darwiniana 44: 359-362.
Al-Shehbaz IA. 2007a. Two new species of
Draba and Eutrema (Brassicaceae) from Sichuan, China.
– Harvard Pap. Bot. 11: 277-279.
Al-Shehbaz IA. 2007b. Generic limits of
Dryopetalon, Rollinsia, Sibara, and
Thelypodiopsis (Brassicaceae), and a synopsis of
Dryopetalon. – Novon 17: 397-402.
Al-Shehbaz IA. 2007c. Arabis
elgonensis (Brassicaceae), a
new species from Mount Elgon, Uganda. – Harvard Pap. Bot. 12: 387-388.
Al-Shehbaz IA. 2007d. The North American
genus Sandbergia (Boechereae, Brassicaceae). – Harvard Pap. Bot.
12: 425-427.
Al-Shehbaz IA. 2010a. The legacy of Asa
Gray’s South American botany: examples of amphitropical disjunctions from the
Brassicaceae. – Harvard Pap.
Bot. 15: 205-208.
Al-Shehbaz IA. 2010b. Ivania
juncalensis, a second species in the Chilean endemic Ivania (Brassicaceae). – Harvard Pap. Bot.
15: 343-345.
Al-Shehbaz IA. 2010c. A synopsis of the genus
Sibara (Brassicaceae).
– Harvard Pap. Bot. 15: 139-147.
Al-Shehbaz IA. 2010d. Menonvillea
zuloagaensis and Mostacillastrum hunzikeri (Brassicaceae), two new species from
Argentina. – Darwiniana 48: 59-63.
Al-Shehbaz IA. 2012a. A generic and tribal
synopsis of the Brassicaceae
(Cruciferae). – Taxon 61: 931-954.
Al-Shehbaz IA. 2012b. Notes on miscellaneous
species of the tribe Thelypodieae (Brassicaceae). – Harvard Pap. Bot.
17: 3-10.
Al-Shehbaz IA. 2013. Clypeola is
united with Alyssum (Brassicaceae). – Harvard Pap. Bot.
18: 125-128.
Al-Shehbaz IA. 2016. A revision of the
Mexican endemic Asta (Brassicaceae). – Novon 25: 8-11.
Al-Shehbaz IA. 2017. Five new species of
Lepidium (Brassicaceae):
L. pabotii (Iran), L. arequipa (Peru), and L.
lapazianum, L. linearilobum, and L. stephan-beckii
(Bolivia). – Novon 25: 403-413.
Al-Shehbaz IA, Al-Shammary KI. 1987.
Distribution and chemotaxonomic significance of glucosinolates in certain
Middle-Eastern Cruciferae. – Biochem. Syst. Ecol. 15: 559-569.
Al-Shehbaz IA, Appel O. 2002. A synopsis of
the Central Asian Rhammatophyllum (Brassicaceae). – Novon 12: 1-4.
Al-Shehbaz IA, Cano A. 2011.
Englerocharis dentata and Eudema peruviana (Brassicaceae), two new species from
Peru. – Harvard Pap. Bot. 16: 275-278.
Al-Shehbaz IA, Fuentes AF. 2008.
Polypsecadium apolobamba (Brassicaceae), a new species from
Bolivia. – Novon 18: 1-3.
Al-Shehbaz IA, German DA. 2013. A synopsis of
the genus Parrya (Brassicaceae). – Kew Bull. 68:
457-475.
Al-Shebaz IA, German DA. 2014. A synopsis of
the genus Braya (Brassicaceae). – Harvard Pap. Bot.
19: 161-174.
Al-Shebaz IA, German DA. 2016. Three new
genera in the tribe Euclidieae (Brassicaceae). – Novon 25:
12-17.
Al-Shehbaz IA, Guang Y. 2000a. A
reconsideration of the genus Eurycarpus (Brassicaceae). – Novon 10:
346-348.
Al-Shehbaz IA, Guang Y. 2000b. A revision of
the Chinese endemic Orychophragmus (Brassicaceae). – Novon 10:
349-353.
Al-Shehbaz IA, Iltis H. 1993.
Romanschulzia mexicana (Brassicaceae), a remarkable new
species from Guerrero Mexico. – Novon 3: 96-98.
Al-Shehbaz IA, Koch MA. 2003.
Drabopsis is united with Draba (Brassicaceae). – Novon 13:
172-173.
Al-Shehbaz IA, Marticorena C. 1990.
Menonvillea rollinsii (Brassicaceae), a new shrubby species
from Chile. – J. Arnold Arbor. 71: 135-138.
Al-Shehbaz IA, Mummenhoff K. 2005. Transfer
of the South African genera Brachycarpaea, Cycloptychis,
Schlechteria, Silicularia, and Thlaspeocarpa to
Heliophila (Brassiacaceae). – Novon 15: 385-397.
Al-Shehbaz IA, Mummenhoff K. 2011.
Stubendorffia and Winklera belong to the expanded
Lepidium (Brassicaceae).
– Edinburgh J. Bot. 68: 165-171.
Al-Shehbaz IA, O’Kane Jr SL. 1995.
Placement of Arabidopsis parvula in Thellungiella (Brassicaceae). – Novon 5:
309-310.
Al-Shehbaz IA, O’Kane SL Jr. 1999.
Ianhedgea (Brassicaceae),
a new generic name replacing the illegitimate Microsisymbrium. –
Edinburgh J. Bot. 56: 321-327.
Al-Shehbaz IA, O’Kane SL Jr. 2002a.
Taxonomy and phylogeny of Arabidopsis (Brassicaceae). – In: Somerville CR,
Meyerowitz EM (eds), The Arabidopsis Book, American Society of Plant
Biologists, Rockville, Maryland, pp.doi/10.1199/tab.0001,
http://www.aspb.org/publications/arabidopsis
Al-Shehbaz IA, O’Kane SL Jr. 2002b.
Lesquerella is united with Physaria (Brassicaceae). – Novon 12:
319-329.
Al-Shehbaz IA, Price RA. 1998. Delimitation
of the genus Nasturtium (Brassicaceae). – Novon 8:
124-126.
Al-Shehbaz IA, Price RA 2001. The Chilean
Agallis and Californian Tropidocarpum (Brassicaceae) are congeneric. –
Novon 11: 292-293.
Al-Shehbaz IA, Warwick SI. 1997. The generic
disposition of Quidproquo confusum and Sinapis aucheri (Brassicaceae). – Novon 7:
219-220.
Al-Shehbaz IA, Warwick SI. 2005. A synopsis
of Eutrema (Brassicaceae). – Harvard Pap. Bot.
10: 129-135.
Al-Shehbaz IA, Warwick SI. 2006. A synopsis
of Smelowskia (Brassicaceae). – Harvard Pap. Bot.
11: 91-99.
A.-Shebaz IA, Warwick SI. 2007. Two new
tribes (Dontostemoneae and Malcolmieae) in the Brassicaceae (Cruciferae). –
Harvard Pap. Bot. 12: 429-433.
Al-Shehbaz IA, Windham MD. 2007. New or
noteworthy North American Draba (Brassicaceae). – Harvard Pap. Bot.
12: 409-419.
Al-Shehbaz IA, Yang G. 1998. Notes on Chinese
Cardamine (Brassicaceae).
– Harvard Pap. Bot. 3: 73-77.
Al-Shehbaz IA, Yang G. 2006. A synopsis of
Eutrema (Brassicaceae).
– Harvard Pap. Bot. 10: 129-135.
Al-Shehbaz IA, Yang G, Lu L-L, Cheo T-Y.
1998. Delimitation of the Chinese genera Yinshania,
Hilliella, and Cochleariella (Brassicaceae). – Harvard Pap. Bot.
3: 79-94.
Al-Shehbaz IA, O’Kane SL Jr, Price RA.
1999. Generic placement of species excluded from Arabidopsis (Brassicaceae). – Novon 9:
296-307.
Al-Shehbaz IA, An ZY, Yang G. 1999. A
revision of Sisymbriopsis (Brassicaceae). – Novon 9:
308-312.
Al-Shehbaz IA, Arai K, Ohba H. 1999. A new
species of Hemilophia (Brassicaceae) from China. – Novon
9: 8-10.
Al-Shehbaz IA, Arai K, Ohba H. 2000. A
revision of the genus Lignariella (Brassicaceae). – Harvard Pap. Bot.
5: 113-121.
Al-Shehbaz IA, Taiyien C, Guang Y. 2000. The
status of Synstemon (Brassicaceae) and the discovery of a
second species. – Novon 10: 99-103.
Al-Shehbaz IA, Mummenhoff K, Appel O. 2002.
Cardaria, Coronopus, and Stroganowia are united with
Lepidium (Brassicaceae).
– Novon 12: 5-11.
Al-Shehbaz IA, Bartholomew B, Abbas A, Tumur
A. 2002. New or noteworthy species of Brassicaceae for the flora of China.
– Novon 12: 309-313.
Al-Shehbaz IA, Yue J, Sun H. 2004.
Shangrilaia (Brassicaceae), a new genus from
China. – Novon 14: 271-274.
Al-Shehbaz IA, Beilstein MA, Kellogg EA.
2006. Systematics and phylogeny of the Brassicaceae (Cruciferae). An
overview. – Plant Syst. Evol. 259: 89-120.
Al-Shehbaz IA, Mutlu B, Dönmez AA. 2007. The
Brassicaceae (Cruciferae) of
Turkey, updated. – Turk. J. Bot. 31: 327-336.
Al-Shehbaz IA, German DA, Karl R,
Jordon-Thaden I, Koch MA. 2011. Nomenclatural adjustments in the tribe
Arabideae. – Plant Divers. Evol. 129: 71-76.
Al-Shehbaz IA, Navarro E, Cano A. 2012.
Aschersoniodoxa peruviana (Brassicaceae), a remarkable new
species from Peru and a synopsis of the genus. – Kew Bull. 67: 483-486.
Al-Shehbaz IA, Cano A, Trinidad H, Navarro E.
2013. New species of Brayopsis, Descurainia, Draba,
Neuontobotrys and Weberbauera (Brassicaceae) from Peru. – Kew
Bull. 68: 219-231.
Amin A. 1978. Cytological studies on
Farsetia aegyptica Turra in Egypt. – United Arab. Rep. J. Bot. 21:
207-210.
An CH (ZX). 1981. New materials for Chinese
Cruciferae. – Bull. Bot. Res. North-East Forest Inst. 1: 97-107.
Ančev M. 2000. The trichomes of
Alyssum (Brassicaceae).
– Bot. Chron. 13: 151-168.
Ančev M, Deneva B. 1997. Pollen morphology
of seventeen species from family Brassicaceae (Cruciferae). –
Phytol. Balcanica 3: 75-82.
Ančev M, Goranova V. 2006. Trichome
morphology of eleven genera of the tribe Alysseae (Brassicaceae) occurring in Bulgaria.
– Willdenowia 36 (Spec. issue): 193-204.
Ancibor E. 1984. Estudio anatómico de la
vegetación de la Puna d Jujuy: V Anatomía de Aschersoniodoxa
mandoniana (Wedd.) Gilg et Muschler y Parodiodoxa chionophila
(Speg.) O. E. Schulz. – Parodiana 3: 103-111.
Anderson JK, Warwick SI. 1999. Chromosome
number evolution in the tribe Brassiceae (Brassicaceae): evidence from isozyme
number. – Plant Syst. Evol. 215: 255-285.
Andersson L. 1995. 70. Tovariaceae. – In: Harling G,
Andersson L (eds), Flora of Ecuador 45, Nord. J. Bot., Copenhagen, pp.
15-21.
Andersson L, Andersson S. 2000. A molecular
phylogeny of Tropaeolaceae and
its systematic implications. – Taxon 49: 721-736.
Appel O. 1998. The status of
Teesdaliopsis and Teesdalia (Brassicaceae). – Novon 8:
218-219.
Appel O. 1999. The so-called “beak”, a
character in the systematics of Brassicaceae? – Bot. Jahrb. Syst.
121: 85-98.
Appel O, Al-Shehbaz IA. 1997a. Re-evaluation
of the tribe Heliophileae (Brassicaceae). – Mitt. Inst. Allg.
Bot. Hamburg 27: 85-92.
Appel O, Al-Shehbaz IA. 1997b. Generic limits
and taxonomy of Hornungia, Pritzelago, and
Hymenolobus (Brassicaceae). – Novon 7:
338-340.
Appel O, Al-Shehbaz IA. 2001.
Dolichorhynchus is united with Douepea (Brassicaceae). – Novon 11:
296-297.
Appel O, Al-Shehbaz IA. 2002. Cruciferae. –
In: Kubitzki K, Bayer C (eds), The families and genera of vascular plants V.
Flowering plants. Dicotyledons. Malvales, Capparales and non-betalain Caryophyllales,
Springer, Berlin, Heidelberg, New York, pp. 75-174.
Appel O, Bayer C. 2002. Tovariaceae. – In: Kubitzki K, Bayer
C (eds), The families and genera of vascular plants V. Flowering plants.
Dicotyledons. Malvales, Capparales and non-betalain Caryophyllales,
Springer, Berlin, Heidelberg, New York, pp. 397-399.
Appelquist LA. 1976. Lipids in the
Cruciferae. – In: Vaughan JG, Macleod AJ, Jones BMG (eds), The biology and
chemistry of the Cruciferae, Academic Press, London, pp. 221-277.
Aradhya MK, Manshardt RM, Zee F, Morden CW.
1999. A phylogenetic analysis of the genus Carica L. (Caricaceae) based on fragment length
variation in a cpDNA intergenic spacer region. – Genet. Resourc. Crop Evol.
46: 579-586.
Aránega R. 1992. Estudio biosistemático de
Reseda L. sect. Leucoreseda DC. (Resedaceae) en el Mediterráneo
Occidental. – Ph.D. diss., Universidad Complutense de Madrid, Spain.
Aránega R. 1994. Notas sobre Reseda
sect. Leucoreseda DC. en la Península Ibérica. – An. Jard. Bot.
Madrid 52: 216-221.
Aránega R. 2005. Aclaraciones taxonómicas y
nomenclaturales sobre Reseda decursiva Forssk. y Reseda
gayana Boiss. en Andalucia. – Acta Bot. Malacit. 30: 189-197.
Arber A. 1931. Studies in floral morphology
I. On some structural features of the cruciferous flower. – New Phytol. 30:
11-41.
Arber A. 1942. Studies in flower structure
VII. On the gynoecium of Reseda with a consideration of paracarpy. –
Ann. Bot., N. S., 6: 43-48.
Arias T, Pires JC. 2012. A fully resolved
chloroplast phylogeny of the brassica crops and wild relatives (Brassicaceae: Brassiceae): novel
clades and potential taxonomic implications. – Taxon 61: 980-988.
Arias T, Beilstein MA, Tang M, McKain MR,
Pires JC. 2014. Diversification times among Brassica (Brassicaceae) crops suggest hybrid
formation after 20 million years of divergence. – Amer. J. Bot. 101:
86-91.
Arwidsson T. 1935. Capparidaceae. – In:
Norlindh T, Weimarck H (eds), Beiträge zur Kenntnis der Flora von
Süd-Rhodesia III, Bot. Not. 1935: 357-360.
Aryavand A. 1975. Contribution à l’étude
cytotaxinomique de quelques Crucifères de l’Iran et de la Turquie. – Bull.
Soc. Neuchâtel. Sci. Nat. 98: 43-58.
Aryavand A. 1978. Contribution à l’étude
cytotaxinomique de quelques Crucifères de l’Iran 2. – Bull. Soc.
Neuchâtel. Sci. Nat. 101: 95-106.
Aryavand A. 1983. Contribution à l’étude
cytotaxinomique de quelques Crucifères de l’Iran 3. – Bull. Soc.
Neuchâtel. Sci. Nat. 106: 123-130.
Aryavand A. 1996. Numerical taxonomic study
of the Iranian species of Alyssum L. based on morphological
characters. – J. Sci. Iran 7: 129-136.
Arnal C, Loiseau J. 1946. L’éperon de la
fleur du Tropaeolum majus. – Compt. Rend. Acad. Sci. Paris 223:
361-364.
Arunalakshmi V. 1985. Embryological studies
in Capparidaceae. Life history of Niebuhria apetala DC. – J. Indian
Bot. Soc. 64: 17-24.
Arunalakshmi V. 1989. Structure and
development of seed coat in Cleome. – J. Indian Bot. Soc. 68:
116-121.
Askerova RK. 1985. Zuvanda, a new
genus of the family Brassicaceae.
– Bot. Žurn. 70: 522-524.
Avetisian VE. 1963. Speciarum caucasicarum
generis Isatis synopsis critica. – Not. Syst. Geogr. Inst. Bot.
Thbilis. 23: 73-86.
Avetisian VE. 1976. Some modifications of the
system of the family Brassicaceae.
– Bot. Žurn. 61: 1198-1203. [In Russian]
Avetisian VE. 1983. The system of the family
Brassicaceae. – Bot. Žurn. 68:
1297-1305. [In Russian]
Avetisian VE. 1990. A review of the system of
Brassicaceae of flora of Caucasus.
– Bot. Žurn. 75: 1029-1032. [In Russian]
Avetisian VE. 2013. On the distribution of
tenus Takhtajaniella (Brassicaceae). – Takhtajania 2:
114.
Aytac Z, Aksoy A. 2000. A new species of
Bornmuellera Hausskn. (Brassicaceae) from South Anatolia,
Turkey. – Bot. J. Linn. Soc. 134: 485-490.
Aytac Z, Nordt B, Parolly G. 2006. A new
species of Noccaea (Brassicaceae) from South Anatolia,
Turkey. – Bot. J. Linn. Soc. 150: 409-416.
Backer CA. 1951. Salvadoraceae. – In: Steenis CGGJ
van (ed), Flora Malesiana I, 4(3), Noordhoff-Kolff N. V., Batavia, pp.
224-225.
Badillo VM. 1971. Monografía de la família
Caricaceae. – Publ. Asociación de
Profesores, Fac. Agron., Univ. Centr. Venezuela, Maracay.
Badillo VM. 1983. 131. Caricaceae. – In: Harling G, Sparre B
(eds), Flora of Ecuador 20, Swedish Natural Science Research Council,
Stockholm, pp. 27-48.
Badillo VM. 1993. Caricaceae. Segundo esquema. – Rev.
Fac. Agron. Univ. Centr. Venez. Alcance (Maracay) 43.
Baehni C, Macbride JF. 1937. Remarques sur
les Cruciferae – Sisymbrieae. – Candollea 7: 291-296.
Bailey CD. 2001. Systematics of
Sphaerocardamum (Brassicaceae). – Ph.D. diss.,
Cornell University, Ithaca, New York.
Bailey CD, Doyle JJ. 1999. Potential
phylogenetic utility of the low-copy nuclear gene pistillata in
dicotyledonous plants: comparison to nrDNA ITS and trnL intron in
Sphaerocardamum and other Brassicaceae. – Mol. Phylogen.
Evol. 13: 20-30.
Bailey CD, Price RA, Doyle JJ. 2002.
Systematics of the halimolobine Brassicaceae: evidence from three
loci and morphology. – Syst. Bot. 27: 318-332.
Bailey CD, Koch MA, Mayer M, Mummenhof K,
Okane SL Jr, Warwick SI, Windham MD, Al-Shehbaz IA. 2006. Toward a global
phylogeny of the Brassicaceae. –
Mol. Biol. Evol. 23: 2142-2160.
Bailey CD, Al-Shehbaz IA, Rajanikanth G.
2007. Generic limits in tribe Halimolobeae and description of the new genus
Exhalimolobos (Brassicaceae). – Syst. Bot. 32:
140-156.
Baillargeon G. 1985. Raphanus
boissieri Al-Shehbaz, an illegitimate synonym for Quidproquo
confusum Greuter & Burdet. – Cruciferae Newsl. 10: 8-9.
Baillargeon G. 1986. Eine taxonomische
Revision der Gattung Sinapis (Cruciferae: Brassiceae). – PhD. diss.,
Universität Berlin, Germany.
Baillon H. 1886. Quelques nouveaux types de
la flore du Congo. – Bull. Mens. Soc. Linn. Paris 1: 609-612.
Baker HG. 1976. “Mistake” pollination as
a reproductive system with special reference to the Caricaceae. – In: Burley J, Styles BT
(eds), Tropical trees – variation, breeding and conservation, Linn. Soc.
Symp. Ser. 2, Academic Press, London, pp. 161-169.
Ball PW. 1963. A review of Malcolmia
maritima and allied species. – Feddes Repert. 68: 179-186.
Ball PW. 1990. Notes on the genus
Erysimum L. in Europe. – Bot. J. Linn. Soc. 103: 200-213.
Banach-Pogan E. 1955. Dalsze badania
cytologiczne nad gatunkami rodzaju Cardamine L. – Acta Soc. Bot.
Polon. 24: 275-286.
Bannier JP. 1923. Untersuchungen über
apogame Fortpflanzung bei einigen elemataren Arten von Erophila verna.
– Rec. Trav. Bot. Néerl. 20: 1-106.
Barcley AS, Gentry HS, Jones Q. 1962. The
search for new industrial crops 2: Lesquerella as a source of new
oilseeds. – Econ. Bot. 16: 95-100.
Barker MS, Vogel H, Schranz ME. 2009.
Paleopolyploidy in Brassicales: analyses of the Cleome transcriptome
elucidate the history of genome duplications in Arabidopsis and other
Brassicales. – Genome Biol. Evol. 2009: 391-399.
Barnhart JH. 1910. Koeberliniaceae. – North Amer.
Flora 25: 101-102.
Barrett RL, Roalson EH, Ottewell K, Byrne M,
Govindwar SP, Yadav SR, Tamboli AS, Gholave AR. 2017. Resolving generic
boundaries in Indian-Australasian Cleomaceae: circumscription of
Areocleome, Arivela, and Corynandra as distinct
genera. – Syst. Bot. 42: 694-708.
Bartish IV, Aïnouche A, Jia D, Bergstrom D,
Chown SL, Winkworth RC, Hennion F. 2012. Phylogeny and colonization history of
Pringlea antiscorbutica (Brassicaceae), an emblematic endemic
from the south Indian Ocean Province. – Mol. Phylogen. Evol. 65: 748-756.
Bateman AJ. 1955a. Self-incompatibility
systems in angiosperms 3. Cruciferae. – Heredity 9: 53-68.
Bateman AJ. 1955b. Note on dioecy in the
Cruciferae. – Heredity 9: 415.
Baum DA, Yoon HS, Oldham RL. 2005. Molecular
evolution of the transcription factor LEAFY in Brassicaceae. – Mol. Phylogen.
Evol. 37: 1-14.
Bawa KS. 1980. Mimicry of male by female
flowers and intrasexual competition for pollinators in Jacaratia
dolichocaula (D. Smith) Woodson (Caricaceae). – Evolution 34:
467-474.
Bayer A. 1905. Beiträge zur systematischen
Gliederung der Cruciferen. – Bot. Centralbl. 18: 119-180.
Bayer C. 2000. On the identity of
Cotylonychia Stapf (Sterculiaceae). – Kew Bull. 55: 499-500.
Bayer C, Appel O. 2002a. Akaniaceae. – In: Kubitzki K, Bayer C
(eds), The families and genera of vascular plants V. Flowering plants.
Dicotyledons. Malvales, Capparales and non-betalain Caryophyllales,
Springer, Berlin, Heidelberg, New York, pp. 21-24.
Bayer C, Appel O. 2002b. Bataceae. – In: Kubitzki K, Bayer C
(eds), The families and genera of vascular plants V. Flowering plants.
Dicotyledons. Malvales, Capparales and non-betalain Caryophyllales,
Springer, Berlin, Heidelberg, New York, pp. 30-32.
Bayer C, Appel O. 2002c. Limnanthaceae. – In: Kubitzki K,
Bayer C (eds), The families and genera of vascular plants V. Flowering plants.
Dicotyledons. Malvales, Capparales and non-betalain Caryophyllales,
Springer, Berlin, Heidelberg, New York, pp. 220-224.
Bayer C, Appel O. 2002d. Pentadiplandraceae. – In:
Kubitzki K, Bayer C (eds), The families and genera of vascular plants V.
Flowering plants. Dicotyledons. Malvales, Capparales and non-betalain Caryophyllales,
Springer, Berlin, Heidelberg, New York, pp. 329-331.
Bayer C, Appel O. 2002. Tropaeolaceae. . – In: Kubitzki K,
Bayer C (eds), The families and genera of vascular plants V. Flowering plants.
Dicotyledons. Malvales, Capparales and non-betalain Caryophyllales,
Springer, Berlin, Heidelberg, New York, pp. 400-404.
Beck JB, Al-Shehbaz IA, Schaal BA. 2006.
Leavenworthia (Brassicaceae) revisited: testing
classic systematic and mating system hypotheses. – Syst. Bot. 31: 151-159.
Beck JB, Al-Shehbaz IA, O’Kane SL, Schaal
BA. 2007. Further insights into the phylogeny of Arabidopsis (Brassicaceae) from nuclear
Atmyb2 flanking sequence. – Mol. Phylogen. Evol. 42: 122-130.
Beentje HJ. 1989. Two new species of
Boscia and Maerua (Capparaceae) from Kenya. – Kew Bull. 44:
743-745.
Behnke H-D. 1977a. Phloem ultrastructure and
systematic position of Gyrostemonaceae. – Bot. Not.
130: 255-260.
Behnke H-D. 1977b. Dilatierte ER-Zisternen,
ein mikromorphologisches Merkmal der Capparales? – Ber. Deutsch. Bot. Ges.
90: 241-251.
Behnke H-D, Eschlbeck G. 1978. Dilated
cisternae in Capparales – an
attempt towards the characterization of a specific endoplasmatic reticulum. –
Protoplasma 97: 351-363.
Behnke H-D, Turner BL. 1971. On specific
sieve-tube plastids in Caryophyllales:
further investigations with special reference to the Bataceae. – Taxon 20: 731-737.
Beilstein MA, Windham MD. 2003. A
phylogenetic analysis of western North American Draba (Brassicaceae) based on nuclear
ribosomal DNA sequences from the ITS region. – Syst. Bot. 28: 584-592.
Beilstein MA, Al-Shehbaz IA, Kellogg EA.
2006. Brassicaceae phylogeny and
trichome evolution. – Amer. J. Bot. 93: 607-619.
Beilstein MA, Al-Shehbaz IA, Mathews Sarah,
Kellogg EA. 2008. Brassicaceae
phylogeny inferred from phytochrome A and ndhF sequence data: tribes
and trichomes revisited. – Amer. J. Bot. 95: 1307-1327.
Beilstein MA, Nagalingum NS, Clements MD,
Manchester SR, Mathews S. 2010. Dated molecular phylogenies indicate a Miocene
origin for Arabidopsis thaliana. – Proc. Natl. Acad. Sci. U.S.A.
107: 18724-18728.
Belyaeva LE, Fursa NS, Smichenko AA. 1977.
The embryology of Syrenia cana (Pill. et Mitt.) Neilr. (Brassicaceae) I. Development of the
embryo and endosperm. – Bot. Žurn. 62: 1453-1461. [In Russian]
Ben´tez de Rojas CE. 1974. Characteres
microscopicos de la epidermis foliar en Caricaceae. Genero Carica. –
Rev. Fac. Agron. Maracay VII: 195-274.
Bennett RN, Hick AJ, Dawson GW, Wallsgrove
RM. 1995. Glucosinolate biosynthesis. Further chracterization of the
aldoxime-forming microsomal monooxygenases in oilseed rape leaves. – Plant
Physiol. 109: 299-305.
Bergh E van den, Hofberger JA, Schranz ME.
2016. Flower power and the mustard bomb: comparative analysis of gene and
genome duplications in glucosinolate biosynthetic pathway evolution in
Cleomaceae and Brassicaceae. –
Amer. J. Bot. 103: 1212-1222.
Berkutenko AN. 1979. Notulae systematicae de
genere Draba L. in parte boreali-orientale URSS. – Novit. Syst.
Plant. Vasc. 16: 119-125. [In Russian]
Berkutenko AN. 2003. On the genus
Borodinia (Cruciferae). – Bot. Žurn. 88: 129-133. [In Russian]
Berkutenko AN. 2005. New combination in the
genus Arabis L. (Cruciferae), or once more on the genus Borodinia N.
busch. – Nov. Sist. Vysš. Rast. 37: 91-94. [In Russian]
Berry PE. 1992. A new lowland species of
Tropaeolum (Tropaeolaceae) from the Venezuelan
Guayana. – Novon 2: 182-184.
Bersier JD. 1960. L’ovule et la
placentation dans le genre Tropaeolum. – Arch. Sci. Genève 13:
566-567.
Bertoli IC. 1967. Ricerche sulla cariologia
di alcuni generi di Cruciferae della sezione Brassicinae. – Atti Accad. Naz.
Sci. Lett. Arti Modena, Ser. 6(9): 41-46.
Bhalla PL, Malik CP. 1982. Localization and
activity of some glycosidases during early embryogenesis in Tropaeolum
majus L. – J. Indian Bot. Soc. 61: 91-94.
Bhalla PL, Singh MB, Malik CP. 1979.
Physiology and sexual reproduction VI. Embryogenesis in Tropaeolum
majus L.: enzyme changes. – Acta Bot. Indica 7: 72-86.
Bhalla PL, Singh MB, Malik CP. 1982.
Post-fertilization developmental time table for Tropaeolum majus. –
Acta Bot. Indica 10: 201-205.
Bhide A, Schliesky S, Reich M, Weber AP,
Becker A. 2014. Analysis of the floral transcriptome of Tarenaya
hassleriana (Cleomaceae), a member of the sister group to the Brassicaceae: towards understanding
the base of morphological diversity in Brassicales. – BMC Genom. 15: 1-16.
Björkman R. 1976. Properties and function of
plant myrosinases. – In: Vaughan JG, MacLeod AJ, Jones BMG (eds), The biology
and chemistry of the Cruciferae, Academic Press, London, pp. 191-205.
Blanc G, Wolfe KH. 2004. Widespread
paleopolyploidy in model plant species inferred from age distributions of
duplicate genes. – Plant Cell 16: 1667-1678.
Blanc G, Hokamp K, Wolfe KH. 2003. A recent
polyploidy superimposed on older large-scale duplications in the
Arabidopsis genome. – Genome Res. 13: 137-144.
Bleeker W. 2007. Interspecific hybridization
in Rorippa (Brassicaceae): patterns and
processes. – Syst. Biodiv. 5: 311-319.
Bleeker W, Huthmann M, Hurka H. 1999.
Evolution of hybrid taxa in Nasturtium R. Br. (Brassicaceae). – Folia Geobot.
Phytotaxon. 34: 421-433.
Bleeker W, Weber-Sparenberg C, Hurka H. 2002.
Chlorolast DNA variation and biogeography in the genus Rorippa Scop.
(Brassicaceae). – Plant Biol. 4:
104-111.
Bleeker W, Franzke A, Pollmann K, Brown AHD,
Hurka H. 2002. Phylogeny and biogeography of southern hemisphere high-mountain
Cardamine species (Brassicaceae). – Aust. Syst. Bot.
15: 575-581.
Blua MJ, Hanscom Z. 1986. Isolation and
characterization of glucocapparin in Isomeris arborea Nutt. – J.
Chem. Ecol. 12: 1449-1458.
Böcher TW. 1951. Cytological and
embryological studies in the amphi-apomictic Arabis holboellii
complex. – Biol. Skr. 6: 1-59.
Böcher TW. 1966. Experimental and
cytological studies on plant species IX. Some Arctic and montane crucifers. –
Biol. Skr. Dan. Vid. Selsk. 14(7): 1-74.
Böcher TW. 1969. Further studies in
Arabis holboellii and allied species. – Bot. Tidsskr. 64:
141-161.
Boczantzeva VV. 1976. New genus
Asterotricha (Cruciferae) from Kazakhstan. – Bot. Žurn. 61:
930-931. [In Russian]
Boczantzeva VV. 1977. Chromosome numbers of
two species from the family Cruciferae. – Bot. Žurn. 62: 1504-1505. [In
Russian]
Boczantzeva VV. 1997. The genus
Arabis (Brassicaceae) in
middle Asia and Kazakhstan. – Bot. Žurn. 82: 115-119.
Boeke JH. 1971. Location of the postgenital
fusion in the gynoecium of Capsella bursa-pastoris. – Acta Bot.
Neerl. 20: 570-576.
Boelcke O. 1964. Notas sobre especies de
‘Lepidium’ de la Argentina. – Darwiniana 13: 506-528.
Boelcke O. 1984. El Género Onuris
(Cruciferae) endémico de la Patagonia. – Parodiana 3: 53-65.
Boesewinkel FD. 1990. Ovule and seed
development of Tovaria pendula Ruiz & Pavon. – Bot. Jahrb. Syst.
111: 389-401.
Bokhari MH, Hedge IC. 1975. Anatomical
characters in Capparis spinosa and its allies. – Notes Roy. Bot.
Gard. Edinb. 34: 231-240.
Bolenbaugh A. 1928. Microsporogenesis in
Tropaeolum majus with special reference to the cleavage process in
tetrad formation. – Bull. Torrey Bot. Club 55: 105-115.
Bolle F. 1936. Resedaceae. – In: Engler A (†),
Harms H (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 17b, W.
Engelmann, Leipzig, pp. 659-692.
Bolt Jørgensen L. 1981. Myrosin cells and
dilated cisternae of the endoplasmatic reticulum in the order Capparales. – Nord. J. Bot. 1:
433-445.
Bolt Jørgensen L. 1995. Stomatal myrosin
cells in Caricaceae. Taxonomic
implications for a glucosinolate-containing family. – Nord. J. Bot. 15:
523-540.
Bolt Jørgensen L. 1981a. Myrosin cells and
dilated cisternae of the endoplasmic reticulum in the order Capparales. – Nord. J. Bot. 1:
433-445.
Bolt Jørgensen L, Behnke H-D, Mabry TJ.
1977. Protein-accumulating cells and dilated cisternae of the endoplasmic
reticulum in three glucosinolate-containing genera: Armoracia,
Capparis, Drypetes. – Planta 137: 215-224.
Bones A, Iversen T-H. 1985. Myrosin cells and
myrosinase. – Israel J. Bot. 34:351-376.
Bones AM, Rossiter JT. 2006. The enzymic and
chemically induced decomposition of glucosinolates. – Phytochemistry 67:
1053-1067.
Bonnet ALM. 1963. Contribution à l’étude
caryologique du genre Alyssum L. (s. lat.). – Nat. Monspel., sér.
Bot., 15: 41-52.
Borgen L. 1987. Lobularia
(Cruciferae). A biosystematic study with special reference to the Macaronesian
region. – Opera Bot. 91: 1-96.
Borgen L. 1996. Genetic differentiation in
endemic Lobularia (Brassicaceae) in the Canary Islands.
– Nord. J. Bot. 16: 487-503.
Borsos O. 1967. Über einige
Rorippa- und Veronica-Arten. – Acta Bot. Acad. Sci. Hung.
13: 1-10.
Botschantzev VP. 1972a. On Parrya R.
Br., Neuroloma Andrz. and some other genera (Cruciferae). – Bot.
Žurn. 57: 667-673. [In Russian]
Botschantzev VP. 1972b. The genus
Strigosella Boiss. and its relation to the genus Malcolmia R.
Br. (Cruciferae). – Bot. Žurn. 57: 1033-1046. [In Russian]
Botschantzev VP. 1980. Two new genera of the
family Cruciferae. – Bot. Žurn. 65: 425-427.
Botschantzev VP. 1984. Rod
Stroganowia Kar. et Kir. (Cruciferae). – Nov. Sist. Vysš. Rast. 21:
72-81.
Botschantzev VP, Seifulin EM. 1988.
Alyssopsis Boiss. (Cruciferae): genus novum florae Asiae Mediae. –
Nov. Sist. Vysš. Rast. 25: 89-91. [In Russian]
Botschantzev VP, Vvedensky AI. 1948.
Cruciferae novae ex Asia Media. – Bot. Mater. Gerb. Inst. Bot. Zool. Akad.
Nauk Uzbeksk. S.S.R. 12: 3-12.
Bottomley W, White DE. 1950. The chemistry of
Western Australian plants II. The essential oil of Codonocarpus
cotinifolius (Desf.) F. Muell. – J. Proc. Roy. Aust. Chem. Inst. 17:
31-32.
Boufford DE, Kjær A, Øgaard Madsen J,
Skrydstrop T. 1989. Glucosinolates in Bretschneideraceae. – Biochem. Syst.
Ecol. 17: 375-379.
Bouman F. 1975. Integument initiation and
testa development in some Cruciferae. – Bot. J. Linn. Soc. 70: 213-229.
Bowman JL. 2006. Molecules and morphology:
comparative developmental genetics of the Brassicaceae. – Plant Syst. Evol.
259: 199-215.
Bowman JL, Smyth DR. 1998. Patterns of petal
and stamen reduction in Australian species of Lepidium L. (Brassicaceae). – Intern. J. Plant
Sci. 159: 65-74.
Bowman JL, Brüggemann H, Lee J-L, Mummenhoff
K. 1999. Evolutionary changes in floral structure within Lepidium L.
(Brassicaceae). – Intern. J.
Plant Sci. 160: 917-929.
Bramwell D. 1970. A revision of the genus
Parolinia Webb (Cruciferae) in the Canary Islands. – Bot. Not. 123:
394-400.
Brea M, Zucol AF, Bargo MS, Fernicola JC,
Vizcaíno SF. 2017. First Miocene record of Akaniaceae in Patagonia (Argentina): a
fossil wood from the early Miocene Santa Cruz formation and its
palaeobiogeographical implications. – Bot. J. Linn. Soc. 183: 334-347.
Brochmann C. 1992a. Polyploid evolution in
arctic-alpine Draba (Brassicaceae). – Sommerfeltia
Suppl. 4: 1-37.
Brochmann C. 1992b. Pollen and seed
morphology of Nordic Draba (Brassicaceae): phylogenetic and
ecological implications. – Nord. J. Bot. 12: 657-673.
Brochmann C, Soltis PS, Soltis DE. 1992a.
Recurrent formation and polyphyly of Nordic polyploids in Draba (Brassicaceae). – Amer. J. Bot. 79:
673-688.
Brochmann C, Soltis PS, Soltis DE. 1992b.
Multiple origins of the octoploid Scandinavian endemic Draba
cacuminum: electrophoretic and morphological evidence. – Nord. J. Bot.
12: 257-272.
Brochmann C, Stedje B, Borgen L. 1992. Gene
flow across ploidal levels in Draba (Brassicaceae). – Evol. Trends
Plants 6: 125-134.
Brochmann C, Soltis DE, Soltis PS. 1992.
Electrophoretic relationships and phylogeny of Nordic polyploids in
Draba (Brassicaceae). –
Plant Syst. Evol. 182: 35-70.
Brochmann C,Borgen L, Stedje CB. 1993.
Crossing relationships and chromosome numbers of Nordic populations of
Draba (Brassicaceae),
with emphasis on the D. alpina complex. – Nord. J. Bot. 13:
121-147.
Brock A, Herzfeld T, Paschke R, Koch M,
Dräger B. 2006. Brassicaceae
contain nortropane alkaloids. – Phytochemistry 67: 2050-2057.
Brooks RR, Morrison RS, Reeves RD, Dudley TR,
Akman Y. 1979. Hyperaccumulation of nickel by Alyssum L. (Cruciferae).
– Proc. Roy. Soc. London, Ser. B, Biol. Sci. 203: 387-403.
Brown RC, Lemmon BE, Nguyen H. 2004.
Comparative anatomy of the chalazal endosperm cyst in seeds of the Brassicaceae. – Bot. J. Linn. Soc.
144: 375-394.
Brückner C. 1996. Carpelloid stamens in Papaveraceae Juss.
and Brassicaceae Burnett
(Cruciferae Juss.) and their bearing on theories of gynoecium organization. –
Feddes Repert. 107: 321-337.
Brückner C. 2000. Clarification of the
carpel number in Papaverales, Capparales, and Berberidaceae.
– Bot. Rev. 66: 155-307.
Brunotte C. 1900. Recherches embryogéniques
et anatomiques sur quelques espèces des genres Impatiens (L.) et
Tropaeolum (L.). – Ph.D. diss., l’Université de Paris, France.
Buchenau F. 1896. Der Blütenbau von
Tropaeolum. – Abh. Nat. Ver. Bremen 13: 383-407.
Buchner R, Halbritter H, Pfunder G, Hesse M.
1990. Pollen of Limnanthes douglasii: a reinvestigation. – Grana 29:
207-211.
Burger W. 1991. 100. Tropaeolaceae. – Fieldiana Bot.
28: 21-23.
Burow M, Losansky A, Müller R, Plock A,
Kliebenstein DJ, Wittstock U. 2009. The genetic basis on constitutive and
herbivore-induced ESP-independent nitrile formation in Arabidopsis.
– Plant Physiology 149: 561-574.
Burtt BL. 1948. Farsetia socotrana
B. L. Burtt, nom. nov. – Kew. Bull. 1948: 162.
Burtt BL. 1949. On Farsetia
hamiltonii Royle. – Kew Bull. 1948: 495-498.
Burtt BL. 1951. The genus Ricotia.
– Kew Bull. 1: 123-132.
Busch A, Zachgo S. 2007. Control of corolla
monosymmetry in the Brassicaceae
Iberis amara. – Proc. Natl. Acad. Sci. U.S.A. 104: 16714-16719.
Busch A, Horn S, Mühlhausen A, Mummenhoff K,
Zachgo S. 2012. Corolla monosymmetry: evolution of a morphological novelty in
the Brassicaceae family. – Mol.
Evol. Ecol. 29: 1241-1254.
Buttler KP. 1967. Zytotaxonomische
Untersuchungen an Mittel- und Südeuropäischen Draba-Arten. – Mitt.
Bot. Staatssamml. München 6: 275-362.
Cacho NI, Burrell AM, Pepper AE, Strauss SY.
2014. Novel nuclear markers inform the systematics and the evolution of
serpentine use in Streptanthus and allies (Thelypodieae, Brassicaceae). – Mol. Phylogen.
Evol. 72: 71-81.
Cai L, Ma H. 2016. Using nuclear genes to
reconstruct angiosperm phylogeny at the species level: a case study with Brassicaceae species. – J. Syst.
Evol. 54: 438-452.
Cardinal-McTeague WM, Sytsma KJ, Hall JC.
2016. Biogeography and diversification of Brassicales: a 103 million year tale.
– Mol. Phylogen. Evol. 99: 204-224.
Carlquist SJ. 1971. Wood anatomy of
Macaronesian and other Brassicaceae. – Aliso 7:
365-384.
Carlquist SJ. 1978. Wood anatomy and
relationships of Bataceae, Gyrostemonaceae, and
Stylobasiaceae. – Allertonia 1: 297-330.
Carlquist SJ. 1985. Vegetative anatomy and
familial placement of Tovaria. – Aliso 11: 69-76.
Carlquist SJ. 1996. Wood anatomy of Akaniaceae and Bretschneideraceae: a
case of near-identity and its systematic implications. – Syst. Bot. 21:
607-616.
Carlquist SJ. 1998a. Wood and bark anatomy of
Caricaceae: correlations with
systematics and habit. – IAWA J. 19: 191-206.
Carlquist SJ. 1998b. Wood anatomy of Resedaceae. – Aliso 16: 127-135.
Carlquist SJ. 2002. Wood and bark anatomy of
Salvadoraceae: ecology,
relationships, histology of interxylary phloem. – J. Torrey Bot. Club 129:
10-20.
Carlquist SJ. 2016. Wood anatomy of
Brassicales: new information, new evolutionary concepts. – Bot. Rev. 82:
24-90.
Carlquist SJ, Donald CJ. 1996. Wood anatomy
of Limnanthaceae and Tropaeolaceae in relation to habit
and phylogeny. – Sida 17: 333-342.
Carlquist SJ, Miller RB. 1999. Vegetative
anatomy and relationships of Setchellanthus caeruleus (Setchellanthaceae). – Taxon
48: 289-302.
Carlquist SJ, Hansen BF, Iltis HH, Olson ME,
Geiger DL. 2013 [2014]. Forchhammeria and Stixis
(Brassicales): stem and wood anatomical diversity, ecological and phylogenetic
significance. – Aliso 31: 59-75.
Carlsen T, Bleeker W, Hurka H, Elven R,
Brochmann C. 2009. Biogeography and phylogeny of Cardamine (Brassicaceae). – Ann. Missouri Bot.
Gard. 96: 215-236.
Carlsen T, Elven R, Brochmann C. 2010. The
evolutionary history of Beringian Smelowskia (Brassicaceae) inferred from combined
microsatellite and DNA sequence data. – Taxon 59: 427-438.
Carlström A. 1984. A revision of
Cleome series Ornithopodioides Tzvelev (Capparaceae). –
Willdenowia 14: 119-130.
Carvalho FA, Renner SS. 2012. A dated
phylogeny of the papaya family (Caricaceae) reveals the crop’s
closest relatives and the family’s biogeographic history. – Mol. Phylogen.
Evol. 65: 46-53.
Carvalho FA, Filer D, Renner SS. 2015.
Taxonomy in the electronic age and an e-monograph of the papaya family (Caricaceae) as an example. –
Cladistics 31: 321-329.
Cecchi L. 2011. A reappraisal of
Phyllolepidum (Brassicaceae), a neglected genus of
the European flora, and its relationships in tribe Alysseae. – Plant Biosyst.
145: 818-831.
Cecchi L, Gabrielli R, Arnetoli M, Gonnelli
C, Hasko A, Selvi F. 2010. Evolutionary lineages of nickel hyperaccumulation
and systematics in European Alysseae (Brassicaceae): evidence from nrDNA
sequence data. – Ann. Bot. (Oxford) 106: 751-767.
Cecchi L, Colzi I, Coppi A, Gonnelli C, Selvi
F. 2013. Diversity and biogeography of Ni-hyperaccumulators of Alyssum
section Odontarrhena (Brassicaceae) in the central western
Mediterranean: evidence from karyology, morphology and DNA sequence data. –
Bot. J. Linn. Soc. 173: 269-289.
Çelik N, Akpulat HA, Donmez E. 2007. A new
species of Physoptychis (Brassicaceae) from Central Anatolia,
Turkey. – Bot. J. Linn. Soc. 154: 393-396.
Çetin Ö, Duran A, Martin E, Tuştaş S.
2012 A taxonomic study of the genus Fibigia Medik. (Brassicaceae). – Afr. J. Biotechn.
11: 109-119.
Chaban IA, Yakovev MS. 1974. The embryology
of Reseda lutea L. I. Megasporogenesis and development of embryo-sac.
– Bot. Žurn. 59: 24-37. [In Russian with English summary]
Chadefaud M. 1974. Sur la formule florale de
la capucine (Tropaeolum majus L.). – Bull. Bot. Soc. Fr. 121:
347-357.
Chandler GT, Bayer RJ. 2000. Phylogenetic
placement of the enigmatic Western Australian genus Emblingia based on
rbcL sequences. – Plant Species Biology 15: 67-72.
Charlesworth D, Liu FL, Zhang L. 1998. The
evolution of the alcohol dehydrogenase gene family by loss of introns of the
genus Leavenworthia (Brassicaceae). – Mol. Biol. Evol.
15: 552-559.
Chaturvedi M, Gupta S. 1983. Studies on the
pollen morphology of some Capparis L. (Capparaceae) species. – Proc.
Indian Acad. Sci., Sect. B, Plant Sci. 93: 29-34.
Chauhan E. 1979. Pollination by ants in
Coronopus didymus (L.) Sm. – Intern. Quart. J. Plant Sci. Res. 6:
39-40.
Chaw S-M, Peng C-I. 1987. Palynological notes
on Bretschneidera sinensis Hemsl. – Bot. Bull. Acad. Sin. (Taipei)
28: 55-60.
Chen H, Deng T, Yue J, Al-Shehbaz IA, Sun H.
2016. Molecular phylogeny reveals the non-monophyly of tribe Yinshanieae (Brassicaceae) and description of a
new tribe, Hillielleae. – Plant Div. 38: 171-182.
Cheng S, van den Bergh E, Zeng P, Zhong X, Xu
J, Liu X, Hofberger J, Bruijn S, Bhide AS, Bian C, Chen J, Fan G, Kaufmann K,
Hall JC, Becker A, Shi C, Zheng Z, Li W, Lu M, Tao Y, Xu X, Wang J, Zou H, Quan
Z, Hibberd J, Lim BL, Zhu X, Zhang G, Schranz ME. 2013. Insights into crucifer
evolution by sequencing the sister-lineage species Tarenaya
hassleriana. – Plant Cell 25: 2813-2830.
Chew FS. 1979. Cabbage butterflies as
indicators of chemical relationships among some Nearctic Cruciferae. – Symb.
Bot. Ups. 22(4): 100-106.
Choi Y-J, Shin H-D, Thines M. 2009. The host
range of Albugo candida extends from Brassicaceae through Cleomaceae to
Capparaceae. – Mycol. Progr. 8: 329-335.
Clauss MJ, Koch MA. 2006.
Arabidopsis and its poorly known relatives. – Trends Plant Sci. 11:
449-459.
Clemente Muñoz M, Hernandez Bermejo JE.
1978. El aparato nectarigeno en la tribu Brassiceae (Cruciferae). – Ann.
Inst. Bot. Cavanilles 35: 279-296.
Cole RA. 1976. Isothiocyanates, nitriles and
thiocyanates as products of autolysis of glucosinolate in Cruciferae. –
Phytochemistry 15: 759-762.
Contandriopoulos J. 1970. Contribution à
l’étude cytotaxinomique des Alysseae Adams de Grèce. – Ber. Schweiz. Bot.
Ges. 79: 313-334.
Cornejo X, Iltis HH. 2005. Studies in the
Capparaceae XXIII: Capparis coimbrana, a new species from Bolivia. –
Brittonia 57: 155-161.
Cornejo X, Iltis HH. 2006. New combinations
in Capparaceae sensu stricto for flora of Ecuador. – Harvard Pap. Bot. 11:
17-18.
Cornejo X, Iltis HH. 2008a. New combinations
in South American Capparaceae. – Harvard Pap. Bot. 13: 117-120.
Cornejo X, Iltis HH. 2008b. A revision of
Colicodendron Mart. (Capparaceae s.s.). – J. Bot. Res. Inst. Texas
2: 75-93.
Cornejo X, Iltis HH. 2008c. The reinstatement
of Capparidastrum (Capparaceae). – Harvard Pap. Bot. 13: 229-236.
Cornejo X, Iltis HH, Tomb AS. 2008.
Anisocapparis y Monilicarpa: dos nuevos géneros de
Capparaceae de América del Sur. – J. Bot. Res. Inst. Texas 2: 61-74.
Coulthart M, Denford KE. 1982. Isozyme
studies in Brassica I. Electrophoretic techniques for leaf enzymes and
comparison of B. napus, B. rapa, and B. oleracea
using phosphoglucoisomerase. – Can. J. Plant Sci. 62: 621-630.
Couvreur TLP, Franzke A, Al-Shehbaz IA,
Bakker FT, Koch MA, Mummenhoff K. 2010. Molecular phylogenetics, temporal
diversification and principles of evolution in the mustard family (Brassicaceae). – Mol. Biol. Evol.
27: 55-71.
Crespo MB. 1993. Reseda valentina
(Resedaceae), a legitimate name. –
Willdenowia 23: 103-106.
Crespo MB, Lledó MD, Fay MF, Chase MW. 2000.
Subtribe Vellinae (Brassiceae, Brassicaceae): a combined analysis of
ITS nrDNA sequences and morphological data. – Ann. Bot. 86: 53-62.
Crespo MB, Rios S, Vivero JL, Prados J,
Hernandez-Bermejo E, Lledo MD. 2005. A new spineless species of Vella
(Brassicaceae) from the high
mountains of south-eastern Spain. – Bot. J. Linn. Soc. 149: 121-128.
Crété P. 1936. Développement et structure
du tégument seminal chez le Reseda luteola L. – Bull. Soc. Bot.
France 83: 43-46.
Crété R. 1951. Embryogénie des
Capparidacées: développement de l’embryon chez le Cleome
graveolens Rafin. – Compt. Rend. Acad. Sci. 233: 562-564.
Crisp P. 1976. Trends in the breeding and
cultivation of cruciferous crops. – In: Vaughan JG, Macleod AJ, Jones BMG
(eds), The biology and chemistry of the Cruciferae, Academic Press, London, pp.
69-118.
Curtis PJ, Made PM. 1971. Cucurbitacins from
the Cruciferae. – Phytochemistry 10: 3081-3083.
Czapik R. 1974. Embryology of five species of
the Arabis hirsuta complex. – Acta Biol. Cracov. 17: 13-25.
Dammer U. 1893. Batidaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien, III(1a), W. Engelmann,
Leipzig, pp. 118-120.
D’Arbaumont J. 1890. Nouvelles observations
sur les cellules à mucilage des graines de Crucifères. – Ann. Sci. Nat.,
Bot. VII, 11: 125-181.
D’Arcy WG. 1979. Flora of Panama, part IV,
family 73A. Capparaceae-Tovarioideae. – Ann. Missouri Bot. Gard. 676:
117-121.
Das VSR, Rao KN. 1975. Phytochemical
phylogeny of the Brassicaceae
(Cruciferae) from the Capparidaceae. – Naturwissenschaften 62: 577-578.
Dass H, Nybom N. 1967. The relationships
between Brassica nigra, Brassica campestris, and Brassica
oleracea, and their amphidiploid hybrids studied by means of numerical
chemotaxonomy. – Can. J. Genet. Cytol. 9: 880-890.
Dassanayake M, Oh DH, Haas JS, Hernandez A,
Hong H, Ali S, Yun DJ, Bressan RA, Zhu JK, Bohnert HJ, Cheeseman JM. 2011. The
genome of the extremophile crucifer Thellungiella parvula. – Nat.
Genet. 43: 913-918.
Dathan ASR, Singh D. 1970. Female gametophyte
and seed of Carica candamarensis Hook. f. – Plant Sci. (Lucknow) 2:
52-60.
Datta PC. 1971. Chromosomal biotypes of
Carica papaya Linn. – Cytologia 36: 555-562.
Datta RM, Mitra IN. 1947. The systematic
position of the family Moringaceae
based on a study of Moringa pterigosperma Gaertn. (M.
oleifera Lam.). – J. Bombay Nat. Hist. Soc. 47: 355-358.
Dave YS, Patel ND, Desai SV. 1974. Pericarpal
studies in the developing fruit of Horse-radish (Moringa oleifera
Lamk.). – Flora 163: 398-404.
David E. 1938. Embryologische Untersuchungen
an Myoporaceen, Salvadoraceen, Sapindaceen und Hippocrateaceen. – Planta 28:
680-703.
Daxenbichler ME, Etten CH von, Brown FS.
1964. Oxazolidinethiones and volatile isothiocyanates in enzyme-treated seed
meals from 65 species of Cruciferae. – Agric. Food Chem. 12: 127-130.
Daxenbichler ME, Spencer GF, Carlson DG, Rose
GB, Brinker AM, Powell RG. 1991. Glucosinolate composition of seeds from 297
species of wild plants. – Phytochemistry 30: 2623-2638.
Delaveau P, Koudogbo B, Pousset J-L. 1973.
Alkaloides chez les Capparidaceae. – Phytochemistry 12: 2983-2985.
Denford KE. 1975. Isoenzyme studies in
members of the genus Brassica. – Bot. Not. 128: 455-462.
Deng Y, Hu Z. 1995. The comparative
morphology of the floral nectaries of Cruciferae. – Acta Phytotax. Sin. 33:
209-220.
Den Outer RW, Veenendaal WLH van. 1981. Wood
and bark anatomy of Azima tetracantha Lam. (Salvadoraceae). – Acta Bot. Neerl.
30: 199-207.
Detling LE. 1936. The genus Dentaria
in the Pacific states. – Amer. J. Bot. 23: 570-576.
Devi HM, Lakshmi VA. 1989. Reproductive
behaviour of Cleome aspera Koenig (Capparaceae). – J. Jap. Bot. 64:
10-17.
Devi S. 1952. Studies in the order Parietales
III. Vascular anatomy of the flower of Carica papaya L., with special
reference to the structure of the gynoecium. – Proc. Indian Acad. Sci., Sect.
B, 36: 59-69.
DeWolf GP. 1962. Notes on African
Capparidaceae III. – Kew Bull. 16: 75-83.
Díaz L CL, Lomelí S JA 1992. Revisión del
género Jarilla Rusby (Caricaceae). – Acta Bot. Mex. 20:
77-99.
Dierschke T, Mandáková T, Lysak MA,
Mummenhoff K. 2009. A bicontinental origin of polyploid Australian/New Zealand
Lepidium species (Brassicaceae)? Evidence from genomic
in situ hybridization. – Ann. Bot. 104: 681-688.
Dirmenci T, Satil F, Tumen G. 2006. A new
species of Matthiola R. Br. (Brassicaceae) from Turkey. – Bot.
J. Linn. Soc. 151: 431-435.
Dixon GR. 2007. Vegetable brassicas and
related crucifers. – CABI North America, Cambridge, Massachusetts.
Dobeš C, Mitchell-Olds T, Koch MA. 2004a.
Extensive chloroplast haplotype variation indicates Pleistocene hybridization
and radiation of North American Arabis drummondii, A. x
divaricarpa, and A. holboellii (Brassicaceae). – Mol. Ecol. 13:
349-370.
Dobeš C, Mitchell-Olds T, Koch MA. 2004b.
Intraspecific diversification in North American Boechera stricta (=
Arabis drummondii), Boechera x divaricarpa, and
Boechera holboellii (Brassicaceae) inferred from nuclear
and chloroplast molecular markers – an integrative approach. – Amer. J.
Bot. 91: 2087-2101.
Dobeš C, Koch M, Sharbel TF. 2006.
Embryology, karyology, and modes of reproduction in the North American genus
Boechera (Brassicaceae):
a compilation of seven decades of research. – Ann. Missiouri Bot. Gard. 93:
517-534.
Dobeš C, Sharbel TF, Koch M. 2007. Towards
understanding the dynamics of hybridization and apomixis in the evolution of
the genus Boechera (Brassicaceae). – Syst. Biodiv. 5:
321-331.
Dolan L, Costa S. 2001. Evolution and
genetics of root hair stripes in the root epidermis. – J. Experim. Bot. 52:
413-417.
Dole JA, Sun M. 1992. Field and genetic
survey of the endangered Butte county meadowfoam – Limnanthes
floccosa subsp. californica (Limnanthaceae). – Cons. Biol. 6:
549-558.
Dorn RD. 2003. A new species of
Boechera (Brassicaceae)
from Utah and Colorado. – Brittonia 55: 1-3.
Dorofeyev VI. 2004. System of family
Cruciferae B. Juss. (Brassicaceae
Burnett). – Turczaninowia 7: 43-52. [In Russian]
Doskotch RW, Ray AB, Beal JL. 1971.
Codonocarpine, a new lunaria-type alkaloid from Codonocarpus australis
A. Cunn. – J. Chem. Soc., Sect. D, 1971: 300-301.
Doweld AB. 1996a. The systematic relevance of
fruit and seed anatomy and morphology of Akania (Akaniaceae). – Bot. J. Linn. Soc.
120: 379-389.
Doweld AB. 1996b. The carpology and taxonomic
relationships of Bretschneidera (Bretschneideraceae). – Acta Bot.
Malacitania 21: 79-90.
Drury WH, Rollins RC. 1952. The North
American representatives of Smelowskia (Cruciferae). – Rhodora 54:
85-119.
Duarte JM, Wall PK, Edger PP, Landherr LL, Ma
H, Pires JC, Leebens-Mack J, dePamphilis CW. 2010. Identification of shared
single copy nuclear genes in Arabidopsis, Populus,
Vitis and Oryza and their phylogenetic utility across various
taxonomic levels. – BMC Evol. Biol. 10: 61; doi: 10.1186/1471-2148-10-61.
Dudley TR. 1964a. Studies in
Alyssum: Near Eastern representatives and their allies I. – J.
Arnold Arbor. 45: 57-100.
Dudley TR. 1964b. Synopsis of the genus
Alyssum. – J. Arnold Arbor. 45: 358-373.
Dudley TR. 1964c. Synopsis of the genus
Aurinia in Turkey. – J. Arnold Arbor. 45: 390-400.
Dudley TR. 1965a. Studies in
Alyssum: Near Eastern representatives and their allies II. Section
Meniocus and section Psilonema. – J. Arnold Arbor. 46:
181-217.
Dudley TR, Cullen J. 1965. Studies in the Old
World Alysseae Hayek. – Feddes Repert. 7: 218-228.
Dugand A. 1941. El género Capparis
en Colombia. – Caldasia 2: 29-54.
Dugand A. 1968. Acerca de unas
Capparis de la flora Colombiana. – Caldasia 10: 219-229.
Duman H. 2001. A new species of
Arabis L. (Brassicaceae)
from South Anatolia. – Bot. J. Linn. Soc. 137: 87-90.
Duman H, Duran A. 2001. A new species of
Arabis L. (Brassicaceae)
from south Anatolia. – Israel J. Plant Sci. 49: 237-240.
Duran A, Ocak A. 2005. Hesperis
turkmendaghensis (sect. Hesperis) (Cruciferae/Brassicaceae), a new species from the
Central Anatolia region, Turkey. – Bot. J. Linn. Soc. 147: 239-247.
Durkee AB, Harborne JB. 1973. Flavonol
glycosides in Brassica and Sinapis. – Phytochemistry 12:
1085-1089.
Dutt BSM. 1978a. Anther in Moringa
concanensis Nimmo. – Curr. Sci. 47: 589.
Dutt BSM. 1978b. Embryo development in
Moringa concanensis Nimmo. – Curr. Sci. 47: 693.
Dutt BSM. 1979. Ovule and seed of Moringa
concanensis Nimmo. – Curr. Sci. 48: 652-654.
Dutt BSM, Narayana LL, Parvathi A. 1978.
Floral anatomy of Moringa concanensis Nimmo. – Indian J. Bot. 1:
35-39.
Dutt BSM, Narayana LL, Radhakrishnaiah M,
Nageshwar G. 1984. Systematic position of Moringa. – J. Econ. Tax.
Bot. 5: 577-580.
Dvořák F. 1968a. A contribution to the
study of the variability of the nectaries. – Preslia 40: 13-17.
Dvořák F. 1968b. Study of the characters of
the genus Parrya R. Br. – Přirod Fak. Univ. Purk. Brno 497:
343-359.
Dvořák F. 1969. Study of the genus
Malcolmia R. Br. II. – Spisy Přírod. Univ. Purkyně Brně, L 36,
1969: 75-101.
Dvořák F. 1970. Study of the characters of
the genus Malcolmia R. Br. I. – Feddes Repert. 81: 387-416.
Dvořák F. 1971. On the evolutionary
relationship in the family Brassicaceae. – Feddes Repert. 82:
357-372.
Dvořák F. 1972. Study of the evolutional
relationship of the tribe Hesperideae. – Folia Fac. Sci. Nat. Univ.
Purkynianae Brun. Biol. 13: 1-82.
Dvořák F. 1973a. The importance of the
indumentum for the investigation of evolutional relationship in the family Brassicaceae. – Österr. Bot.
Zeitschr. 121: 155-164.
Dvořák F. 1973b. Infrageneric
classification of Hesperis L. – Feddes Repert. 84: 259-271.
Dvořák F, Konarikova V. 1970. Study of the
characters of the genus Malcolmia R. Br. 1. – Feddes Repert. 81:
387-416.
Eames AJ. 1930. Crucifer carpels. – Amer.
J. Bot. 17: 638-656.
Eames AJ, Wilson CL. 1928. Carpel morphology
in the Cruciferae. – Amer. J. Bot. 15: 251-270.
Eames AJ, Wilson CL. 1930. Crucifer carpels.
– Amer. J. Bot. 17: 638-656.
Easterly NW. 1963. Chromosome numbers of some
northwestern Ohio Cruciferae. – Castanea 28: 39-42.
Ebel AL. 1998. Notes on genus
Aphragmus Andrz. (Brassicaceae). – Turczaninowia 1:
20-27.
Eckardt T. 1959 [1960]. Das Blütendiagramm
von Batis P. Br. – Ber. Deutsch. Bot. Ges. 72: 411-418.
Eckardt T. 1971. Anlegung und Entwicklung der
Blüten von Gyrostemon ramulosus Desf. – Bot. Jahrb. Syst. 90:
434-446.
Edger PP, Tang M, Bird KA, Mayfield DR,
Conant G, Mummenhoff K, Koch MA, Pires JC. 2014. Secondary structure analyses
of the nuclear rRNA internal transcribed spacers and assessment of its
phylogenetic utility across the Brassicaceae (Mustards). – PLoS One
9: e101341.
Edger PP, Hall JC, Harkess A, Tang M, Coombs
J, Mohammadin S, Schranz ME, Xiong Z, Leebens-Mack J, Meyers BC, Sytsma KJ,
Koch MA, Al-Shehbaz IA, Pires C. 2018. Brassicales phylogeny inferred from 72
plastid genes: a reanalysis of the phylogenetic localization of two
paleopolyploid events and origin of novel chemical defenses. – Amer. J. Bot.
105: 463-469.
Eigsti OJ. 1936. Cytological studies in the
Resedaceae. – Bot. Gaz. 98:
363-369.
Eilert U, Wolters B, Nahrstedt A. 1981. The
antibiotic principle of seeds of Moringa oleifera and Moringa
stenopetala. – Planta Med. 42: 55-61.
Ekman E. 1926. Zur Kenntnis der nordischen
Hochgebirgs-Drabae II. – Kungl. Sv. Vetensk. Akad. Handl., ser. III,
2: 1-56.
Ekman E. 1931. Contribution to the
Draba flora of Greenland III. Some notes on the arctic, especially the
Greenland Drabas of the sections Aizopsis and Chrysodraba DC.
– Svensk Bot. Tidskr. 25: 465-494.
Ekman E. 1932. Contribution to the
Draba flora of Greenland IV. – Svensk Bot. Tidskr. 26: 133-142.
Ekman E. 1933. Contribution to the
Draba flora of Greenland V. – Svensk Bot. Tidskr. 27: 97-103.
Ekman E. 1941. Notes on the genus
Draba. A posthumous, unfinished fragment. – Svensk Bot. Tidskr. 35:
133-142.
Elffers J, Taylor P. 1958. Resedaceae. – In: Hubbard CE,
Milne-Redhead E (eds), Flora of tropical East Africa, Crown Agents for Oversea
Governments and Administrations, London, pp. 1-6.
Elffers J, Graham RA, Dewolf GP. 1964.
Capparidaceae. – In: Hubbard CE, Milne-Redhead E (eds), Flora of tropical
East Africa, Crown Agents for Oversea Governments and Administrations, London,
pp. 1-88.
El Hadidi MN, El Naggar SM, Hedge IC. 1988.
Taxonomic studies on Cruciferae in Egypt 1. Checklist and key to the genera.
– Taeckholmia 1: 73-86.
El-Menshawi B, Karawya, M, Wassel G. 1980.
Glucosinolates in the genus Zilla (Brassicaceae). – Lloydia 43:
534-536.
El Migirab S, Berger Y, Jadot J. 1977.
Isothiocyanates, thiourées et thiocarbamates isolés de Pentadiplandra
brazzeana. – Phytochemistry 16: 1719-1721.
El Naggar SM. 1987. Studies of the family
Cruciferae in Egypt. – Ph.D. diss., Assiut University, Egypt.
El Naggar SM. 1992. Systematic studies on the
tribe Brassiceae (Cruciferae) in Egypt. – Feddes Repert. 103: 515-522.
El Naggar SM. 1993. Numerical taxonomy of the
tribe Lepidieae and some other genera. – Feddes Repert. 104: 201-208.
El Naggar SM. 2002. Taxonomic significance of
pollen morphology in some taxa of Resedaceae. – Feddes Repert. 113:
518-527.
El Naggar SM, El Hadidi MN. 1998. The tribe
Alysseae Hayek (Brassicaceae) in
Egypt. – J. Union Arab Biol. Cairo 6(B): 501-520.
Elven R, Al-Shehbaz IA. 2008. Draba
simmonsii (Brassicaceae), a
new species of the D. micropetala complex from the Canadian Arctic
Archipelago. – Novon 18: 325-329.
Endress PK. 1992. Evolution and floral
diversity: the phylogenetic surroundings of Arabidopsis and
Antirrhinum. – Intern. J. Plant Sci. (Suppl.) 153: S106-S122.
Engel K, Schmidt B. 1972. – In: Löve Á
(ed), IOPB chromosome number reports 37, Taxon 21: 495.
Engler A. 1895. Koeberliniaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien, Bd. III(6), W. Engelmann,
Leipzig, pp. 319-321.
Engler A 1902. Cruciferae africanae. –
Engl. Bot. Jahrb. Syst. 32: 98-100.
Erbar C, Leins P. 1997a. Different patterns
of floral development in whorled flowers, exemplified by Apiaceae and Brassicaceae. – Intern. J. Plant
Sci. 158(Suppl.): S49-S64.
Erbar C, Leins P. 1997b. Studies on the early
floral development in Cleomoideae (Capparaceae) with emphasis on the androecial
development. – Plant Syst. Evol. 206: 119-132.
Erdtman G, Leins P, Melville R, Metcalfe CF.
1969. On the relationships of Emblingia. – Bot. J. Linn. Soc. 62:
169-186.
Erickson LR, Straus NA, Beversdorf WD. 1983.
Restriction patterns reveal origins of chloroplast genomes in Brassica
amphiploids. – Theor. Appl. Gen. 65: 201-206.
Esmailbegi S, Lysak MA, Rahiminejad MR,
Mirtadzadini M, Mummenhoff K, Al-Shehbaz IA. 2017. A taxonomic revision of the
genus Pseudocamelina (Brassicaceae, tribe Thlaspideae). –
Phytotaxa 313: 117-129.
Esmailbegi S, Al-Shehbaz IA, Pouch M,
Mandáková T, Mummenhoff K, Rahiminejad MR, Mirtadzadini M, Lysak MA. 2018.
Phylogeny and systematics of the tribe Thlaspideae (Brassicaceae) and the recognition of
two new genera. – Taxon 67: 324-340.
Ettlinger MG, Hodgkins JE. 1956. The mustard
oil of papya seed. – J. Org. Chem. 21: 204-205.
Ettlinger MG, Lundeen AJ. 1956. The mustard
oil of Limnanthes douglasii seed, m-methoxybenzyl isothiocyanate. –
J. Amer. Chem. Soc. 78: 1952-1954.
Evenari M, Gutterman Y. 1973. Some notes on
Salvadora persica L. in Sinai and its use as a toothbrush. – Flora
162: 118-125.
Exell AW. 1960. 13. Cruciferae. – In: Exell
AW, Wild H (eds), Flora Zambesiaca 1 (Part 1), Crown Agents for Oversea
Governments and Administrations, London, pp. 181-194.
Ezelarab GE, Dormer KJ. 1966. The
organization of the primary vascular system in the Rhoeadales. – Ann. Bot.,
N. S., 30: 123-132.
Fabbri LR, Valla JJ. 1998. Aspectos de la
biología reproductiva de Tropaeolum pentaphyllum (Tropaeolaceae). – Darwiniana 36:
51-58.
Farenholtz H. 1931. Tropaeolaceae. – In: Engler A
(†), Harms H, Pax F (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd.
19a, W. Engelmann, Leipzig, pp. 67-82.
Fathima T, Kusma Kumari P. 1970.
Embryological studies in Capparidaceae I. Sporogenesis and the development of
gametophytes in Cleome viscosa Linn. – Proc. Indian Acad. Sci.,
Sect. B, 72: 207-215.
Fawcett W, Rendle AB. 1914. Notes on Jamaican
species of Capparis. – J. Bot. 52: 142-144.
Fay MF, Christenhusz MJM. 2010. Brassicales
– an order of plants characterized by shared chemistry. – Curtis’s Bot.
Mag. 27: 165-196.
Feeny P. 1977. Defensive ecology of the
Cruciferae. – Ann. Missouri Bot. Gard. 64: 221-234.
Fenwick GR, Heaney RK, Mullin WJ. 1983.
Glucosinolates and their breakdown products in food and food plants. – CRC
Crit. Rev. Food Sci. Nutr. 18: 123-201.
Feodorova TA, Voznesenskaya EV, Edwards GE,
Roalson EH. 2010. Biogeographic patterns of diversification and the origins of
C4 in Cleome (Cleomaceae). – Syst. Bot. 35: 811-826.
Ferguson IK. 1985. The pollen morphology of
Moringaceae. – Kew Bull. 40:
25-34.
Fernald ML. 1934. Draba in temperate
northeastern America. – Rhodora 36: 241-261, 285-305, 314-344, 353-371,
392-404.
Fernández J. 1973. Sobre la dispersion
meridional de Tropaeolum tuberosum R. P. – Bol. Soc. Arg. Bot. 15:
106-112.
Fernández Peralta AM, González Aguilera JJ.
1982. Cytogenetic and evolutionary studies on the Spanish species of
Reseda L.: Section Luteola Dumort. (Resedaceae). – Taxon 31: 1-8.
Fici S. 2017. A taxonomic revision of the
genus Capparis (Capparaceae) in New Caledonia. – New Zealand J. Bot.
55: 407-423.
Finlayson AJ. 1976. The seed protein contents
of some Cruciferae. – In: Vaughan JG, Macleod AJ, Jones BMG (eds), The
biology and chemistry of the Cruciferae, Academic Press, London, pp.
279-306.
Fisch KJ, Weberling F. 1990. Untersuchungen
zur Morphologie und zur der Blüten von Tovaria pendula Ruiz &
Pavón und Tovaria diffusa Fawcett & Rendle (Tovariaceae). – Bot. Jahrb. Syst.
111: 365-387.
Fischer JB. 1980. The vegetative and
reproductive structure of papaya (Carica papaya). – Lloydia 1:
191-207.
Fisel KJ, Weberling F. 1990. Untersuchungen
zur Morphologie und Ontogenie der Blüten von Tovaria pendula Ruiz
& Pavon und Tovaria diffusa (Macfad.) Fawcett & Rendle (Tovariaceae). – Bot. Jahrb. Syst.
111: 365-387.
Fisher JB. 1980. The vegetative and
reproductive structure of papaya (Carica papaya). – Lyonia 1:
191-208.
Floyd AG. 1977. Family Akaniaceae. – Forest Commiss. New
South Wales 32: 80-83.
Foster LT. 1943. Morphological and
cytological studies in Carica papaya. – Bot. Gaz. 105: 116-126.
Franceschini MC, Tressens SG. 2004.
Morphology of fruits, seeds and embryos of Argentinian Capparis L.
(Capparaceae). – Bot. J. Linn. Soc. 145: 209-218.
Franchet MA. 1887. Sur les Cleome a
petals appendiculés. – J. Bot. (Morot) 1: 17-19, 37-41.
Francisco-Ortega J, Fuertes-Aguilar J,
Gomez-Campo C, Santos-Guerra A, Jansen RK. 1999. Internal transcribed spacer
sequence phylogeny of Crambe L. (Brassicaceae): molecular data reveal
two Old World disjunctions. – Mol. Phylogen. Evol. 11: 361-380.
Francisco-Ortega J, Fuertes-Aguilar J, Kim
SC, Santos-Guerra A, Crawford DJ, Jansen RK. 2002. Phylogeny of the
Macaronesian endemic Crambe section Dendrocrambe (Brassicaceae) based on internal
transcribed spacer sequences of nuclear ribosomal DNA. – Amer. J. Bot. 89:
1984-1990.
Franzke A, Hurka H. 2000. Molecular
systematics and biogeography of the Cardamine pratensis complex (Brassicaceae). – Plant Syst. Evol.
224: 213-234.
Franzke A, Mummenhoff K. 1999. Recent hybrid
speciation in Cardamine (Brassicaceae) – conversion of
nuclear ribosomal ITS sequences in statu nascendi. – Theor. Appl. Gen. 98:
831-834.
Franzke A, Pollmann K, Bleeker W, Kohrt R,
Hurka H. 1998. Molecular systematics of Cardamine and allied genera
(Brassicaceae): ITS and noncoding
chloroplast DNA. – Folia Geobot. Phytotaxon. 33: 225-240.
Franzke A, Hurka H, Janssen D, Neuffer B,
Friesen N, Markov M, Mummenhoff K. 2004. Molecular signals for Late
Tertiary/Early Quaternay range splits of an Eurasian steppe plant: Clausia
aprica (Brassicaceae). –
Mol. Ecol. 13: 2789-2795.
Franzke A, German D, Al-Shehbaz IA,
Mummenhoff K. 2009. Arabidopsis’s family ties: molecular phylogeny
and age estimates in the Brassicaceae. – Taxon 58:
425-437.
Franzke A, Lysák MA, Al-Shehbaz IA, Koch MA,
Mummenhoff K. 2011. Cabbage family affairs: the evolutionary history of Brassicaceae. – Trends Plant Sci.
16: 108-116.
Franzke A, Koch MA, Mummenhoff K. 2016.
Turnip time travels: age estimates in Brassicaceae. – Trends Plant Sci.
21: 554-561.
Franzke A, Samani B-RS, Neuffer B, Mummenhoff
K, Hurka H. 2017. Molecular evidence in Diplotaxis (Brassicaceae) suggests a Quaternary
origin of the Cape Verdean flora. – Plant Syst. Evol. 303: 467-479.
Freeling M, Lyons E, Pedersen M, Alam M, Ming
R, Lisch D. 2008. Many or most genes in Arabidopsis transposed after
the origin of the order Brassicales. – Genome Res. 18: 1924-1937.
Freeman JL, Quinn CF, Lindblom SD, Klamper
EM, Pilon-Smits EAH. 2009. Selenium protects the hyperaccumulator Stanleya
pinnata against black-tailed prairie dog herbivory in native seleniferous
habitats. – Amer. J. Bot. 96: 1075-1085.
Friedrich H-C. 1956. Studien über die
natürliche Verwandtschaft der Plumbaginales und Centrospermae. – Phyton 6:
221-263.
Fries M. 1936. Über die Chromosomenzahl bei
zwei Limnanthes-Arten. – Svensk Bot. Tidskr. 30: 440-442.
Friesen N, German DA, Hurka H, Herden T,
Oyuntsetseg B, Neuffer B. 2016. Dated phylogenies and historical biogeography
of Dontostemon and Clausia (Brassicaceae) mirror the
palaeogeographical history of the Eurasian steppe. – J. Biogeogr.
doi:10.1111/jbi.12658
Frohne D. 1962. Das Verhältnis von
vergleichender Serobotanik zur vergleichender Phytochemie, dargestellt an
serologischen Untersuchungen im Bereich der “Rhoeadales”. – Planta Medica
10: 283-297.
Fuchs C. 1975. Ontogenèse foliaire et
acquisition de la forme chez le Tropaeolum peregrinum L. I. Les
premiers stades de l’ontogenèse du lobe médian. – Ann. Sci. Nat., Bot.
XII Biol. Vég. 16: 321-389.
Fuchs C. 1976. Ontogenèse foliaire et
acquisition de la forme chez le Tropaeolum peregrinum L. II. Le
développement du lobe après la formation des lobules. – Ann. Sci. Nat.,
Bot. XII Biol. Vég. 17: 121-158.
Fuentes-Soriano S. 2004. A taxonomic revision
of Pennellia (Brassicaceae). – Harvard Pap. Bot.:
173-202.
Fuentes-Soriano S, Al-Shehbaz I. 2013.
Phylogenetic relationships of mustards with multiaperturate pollen (Physarieae,
Brassicaceae) based on the plastid
ndhF gene: implications for morphological diversification. – Syst.
Bot. 38: 178-191.
Fulcher WE. 1972. An anatomical and
morphological study of Batis maritima L. with systematic implications.
– Diss. Abstr. Int., Sect. B, 32: 6885.
Fursa NS, Belyaeva LE, Avetisian VE. 1986.
Natural compounds of the family Brassicaceae as possible
chemotaxonomic characters. – Report 4. Glucosinolates, alkaloids, sinapine.
– Rastitel’n. Resursy 22: 449-474.
Gadek PA, Quinn CJ, Rodman JE, Karol KG,
Conti E, Price RA, Fernando ES. 1992. Affinities of the Australian endemic Akaniaceae: new evidence from
rbcL sequences. – Aust. Syst. Bot. 5: 717-724.
Galloway GL, Malmberg RL, Price RA. 1998.
Phylogenetic utility of the nuclear gene arginine decarboxylase: an example
from Brassicaceae. – Mol. Biol.
Evol. 15: 1312-1320.
Gandolfo MA, Dibbern MC, Romero EJ. 1988.
Akania patagonica n. sp. and additional material on Akania
americana Romero & Hickey (Akaniaceae), from Paleocene sediments
of Patagonia. – Bull. Torrey Bot. Club 115: 83-88.
Gandolfo MA, Nixon KC, Crepet WL. 1998. A new
fossil flower from the Turonian of New Jersey: Dressiantha
bicarpellata gen. et sp. nov. (Capparales). – Amer. J. Bot. 85:
964-974.
Gandolfo MA, Hermsen EJ, Zamaloa MC, Nixon
KC, González CC, Wilf P, Cúneo NR, Johnson KR. 2011. Oldest known
Eucalyptus macrofossils are from South America. – PloS ONE 6(6):
e21084.
Garaventa A. 1940. El género
Mathewsia en Chile. – Rev. Univ. (Santiago) 25: 255-267.
Garnock-Jones PJ. 1978. Rorippa
(Cruciferae, Arabideae) in New Zealand. – New Zealand J. Bot. 16: 119-122.
Garnock-Jones PJ. 1991. Seed morphology and
anatomy of the New Zealand genera Cheesemania, Ischnocarpus,
Iti, Notothlaspi, and Pachycladon (Brassicaceae). – New Zealand J.
Bot. 29: 71-82.
Garnock-Jones PJ, Johnson PN. 1987. Iti
lacustris (Brassicaceae), a
new genus and species from southern New Zealand. – New Zealand J. Bot. 25:
603-610.
Garnock-Jones PJ, Jonsell B. 1988.
Rorippa divaricata (Brassicaceae): a new combination. –
New Zealand J. Bot. 26: 479-480.
Garnock-Jones PJ, Norton DA. 1995.
Lepidium naufragorum (Brassicaceae), a new species from
Westland, and notes on other coastal species of Lepidium. – New
Zealand J. Bot. 33: 43-51.
Gaur YD. 1968. Preliminary studies in
titrable acidity in xerophytic plants: Salvadora persica L. and
Prosopis juliflora D.C. – Experientia 24: 239-240.
Gauthier B, Rousseau C. 1973. L’écologie
du Floerkea proserpinacoides Willd. à l’îsle aux Grues, Montmagny
(Québec). – Nat. Can. 100. 371-383.
Gazet du Chatelier G. 1946. Le diagramme de
la fleur des Crucifères. – Rec. Trav. Inst. Bot. 2: 5-9.
Gazzani S, Li M, Maistri S, Scarponi E,
Graziola M, Barbaro E, Wunder J, Furini A, Saedler H, Varotto C. 2009.
Evolution of MIR168 paralogs in Brassicaceae BMC. – Evol. Biol. 9:
62.
Gentry HS, Miller RW. 1965. The search for
new industrial crops IV. Prospectus of Limnanthes. – Econ. Bot. 19:
25-32.
George AS. 1982. Gyrostemonaceae. – In: George AS
(ed), Flora of Australia 8, Australian Government Publ. Service, Canberra, pp.
362-379.
George AS. 2002. Gyrostemonaceae. – In: Kubitzki
K, Bayer C (eds), The families and genera of vascular plants V. Flowering
plants. Dicotyledons. Malvales, Capparales and non-betalain Caryophyllales,
Springer, Berlin, Heidelberg, New York, pp. 213-217.
German DA. 2003. Notes on the genus
Alyssum (Cruciferae) in Kazakhstan. – Turczaninowia 6: 45-57. [In
Russian]
German DA. 2004. New taxa of the genus
Erysimum L. (Cruciferae) from the Kazakhstanian Altai. –
Turczaninowia 7: 14-18. [In Russian]
German DA. 2005. Contribution to the taxonomy
of Arabidopsis s.l. The status of Transberingia and two new
combinations in Crucihimalaya (Cruciferae). – Turczaninowia 8:
5-15.
German DA. 2008. Six new synonyms in the
central Asian Cruciferae (Brassicaceae). – Nord. J. Bot. 26:
38-40.
German DA. 2009. New data on the species
composition and distribution of Mongolian mustards (Cruciferae). – Bot.
Žurn. 94: 1149-1158. [in Russian]
German DA. 2010. A check-list and the system
of the Cruciferae of Altai. – Komarovia 6: 83-92.
German DA. 2011. Taxonomical confusions in
the Cruciferae of North and Central Asia I. Alyssum fischerianum and
Alyssum canescens. – Turczaninowia 14: 18-28.
German DA. 2014a. New synonyms and
combinations in Eurasian Brassicaceae (Cruciferae). –
Phytotaxa 173: 31-40.
German DA. 2014b. Revised typifications and
nomenclatural notes in N Eurasian Cruciferae. – Willdenowia 44: 351-361.
German DA. 2014c. Some new and revised
typifications in North Eurasian Cruciferae. – Turczaninowia 17: 29-41.
German DA, Al-Shehbaz IA. 2008a. Five
additional tribes (Aphragmeae, Biscutelleae, Calepineae, Conringieae and
Erysimeae) in the Brassicaceae
(Cruciferae). – Harvard Pap. Bot. 13: 165-170.
German DA, Al-Shehbaz IA. 2008b.
Dendroarabis, a new Asian genus of Brassicaceae. – Harvard Pap. Bot.
13: 289-291.
German DA, Al-Shehbaz IA. 2010. Nomenclatural
novelties in miscellaneous Asian Brassicaceae (Cruciferae). – Nord.
J. Bot. 28: 646-651.
German DA, Ebel AL. 2005. Generic placement
of Arabidopsis rupicola (Cruciferae). – Turczaninowia 8: 5-12.
German DA, Friesen NW. 2014.
Shehbazia (Shehbazieae, Cruciferae), a new monotypic genus and tribe
of hybrid origin from Tibet. – Turczaninowia 17: 17-23.
German DA, Friesen N, Neuffer B, Al-Shehbaz
IA, Hurka H. 2009. Contribution to ITS phylogeny of the Brassicaceae, with special reference
to some Asian taxa. – Plant Syst. Evol. 283: 33-56.
German DA, Grant JR, Lysak MA, Al-Shehbaz IA.
2011. Molecular phylogeny and systematics of the tribe Chorisporeae (Brassicaceae). – Plant Syst. Evol.
294: 65-86.
Ghasi S, Nwodobo E, Ofili JO. 2000.
Hypocholesterolemic effects of crude extract of leaf of Moringa
oleifera Lam in highfat diet fed wistar rats. – J. Ethnopharm. 69:
21-25.
Gibbs PE, Marshall D, Brunton D. 1978.
Studies on the cytology of Oxalis tuberosa and Tropaeolum
tuberosum. – Notes Bot. Gard. Edinb. 37: 215-220.
Gibson AC. 1979. Anatomy of
Koeberlinia and Canotia revisited. – Madroño 26: 1-12.
Gilg E. 1897. Zwei neue
Capparidaceengattungen aus Afrika. – Engl. Bot. Jahrb. Syst. 24: 307-309.
Gilg E. 1904. Capparidaceae africanae. –
Engl. Bot. Jahrb. Syst. 33: 202-230.
Gilg E, Benedict C. 1915a. Monographische
Zusammenstellung sämtlicher Capparidaceae des tropischen und subtropischen
Afrika. – Engl. Bot. Jahrb. Syst. 53: 144-274.
Gilg E, Benedict C. 1915b.
Cercopetalum Gilg. – Engl. Bot. Jahrb. Syst. 53: 265-268.
Gilg E, Benedict C. 1915c. Nachträge und
Verbesserungen zu der “Monographischen Zusammenstellung sämtlicher
Capparidaceae des tropischen und subtropischen Africa”. – Engl. Bot. Jahrb.
Syst. 53: 452-454.
Gilg E, Muschler R. 1909. Aufzählung aller
zur Zeit bekannten südamerikansichen Cruciferen. – Engl. Bot. Jahrb. Syst.
42: 437-487.
Gill JJB. 1965. Diploids in the genus
Cochlearia. – Watsonia 6: 188-189.
Gill JJB. 1971. Cytogenetic studies in
Cochlearia L. – Ann. Bot., N. S., 35: 947-956.
Gill JJB. 1973. Cytogenetic studies in
Cochlearia L. (Cruciferae). the origins of C. officinalis L.
and C. micacea Marshall. – Genetica 44: 217-234.
Gill LS, Karatela YY, Lamina BL, Husaini SWH.
1985. Cytology and histomorphology of Moringa oleifera Lam. (Moringaceae). – Feddes Repert. 96:
299-305.
Glover JR, Chapple CCS, Rothwell S, Tober I,
Ellis BE. 1988. Allylglucosinolate biosynthesis in Brassica carinata.
– Phytochemistry 27: 1345-1348.
Gmelin R, Kjaer A. 1970a. Glucosinolates in
the Caricaceae. – Phytochemistry
9: 591-593.
Gmelin R, Kjaer A. 1970b. Glucosinolates in
Matthiola fruticulosa and related species: a reinvestigation. –
Phytochemistry 9: 569-573.
Goffman FD, Thies W, Velasco L. 1999.
Chemotaxonomic value of tocopherols in Brassicaceae. – Phytochemistry 50:
793-798.
Goldblatt P. 1976. Chromosome number and its
significance in Batis maritima (Bataceae). – J. Arnold Arbor. 57:
526-530.
Goldblatt P. 1978. Chromosome number in two
cytologically unknown New World families, Tovariaceae and Vivianiaceae. – Ann.
Missouri Bot. Gard. 65: 776-777.
Goldblatt P, Nowicke JW, Mabry TJ, Behnke
H-D. 1976. Gyrostemonaceae:
status and affinity. – Bot. Not. 129: 201-206.
Gómez SA. 1953. Capparidáceas Argentinas.
– Lilloa 26: 279-341.
Gómez-Campo C. 1978. Studies on Cruciferae
VI. Geographical distribution and conservation status of Boleum Desv.,
Guiraoa Coss. and Euzomodendron Coll. – An. Inst. Bot. A.
J. Cavanilles 35: 165-176.
Gómez-Campo C. 1980. Morphology and
morphotaxonomy of the tribe Brassiceae. – In: Tsunoda S, Hinata K,
Gómez-Campo C (eds), Brassica crops and wild allies, Japan Scientific
Societies Press, Tokyo, pp. 3-31.
Gómez-Campo C. 1981. Studies on Cruciferae
VIII. Nomenclatural adjustments in Diplotaxis DC. – An. Jard. Bot.
Madrid 38: 29-35.
Gómez-Campo C. 1982. Studies on Cruciferae
IX. Erucastrum rifanum (Emberger & Maire) Gómez-Campo, comb. nov.
– An. Jard. Bot. Madrid 38: 353-356.
Gómez-Campo C. 1983. Studies on Cruciferae
X. Concerning some West Mediterranean species of Erucastrum. – An.
Jard. Bot. Madrid 40: 63-72.
Gómez-Campo C. 1984. Studies on Cruciferae
XI. Erucastrum ifniense Gómez-Campo, sp. nov., and its allies. –
An. Jard. Bot. Madrid 41: 83-85.
Gómez-Campo C. 1999. Taxonomy. – In:
Gómez-Campo C (ed), Biology of Brassica coenospecies, Elsevier,
Amsterdam, pp. 3-32.
Gómez-Campo C, Hinata K. 1980. A check-list
of chromosome numbers in the tribe Brassiceae. – In: Tsunoda S, Hinata K,
Gómez-Campo C (eds), Brassica crops and wild allies, Japan Scientific
Societies Press, Tokyo, pp. 51-63.
Gómez-Campo C, Tortosa ME. 1974. The
taxonomic and evolutionary significance of some juvenile characters in the
Brassiceae. – Bot. J. Linn. Soc. 69: 105-124.
González Aguilera JJ, Fernández Peralta AM.
1981. Caryology and evolution in Sesamoides (Resedaceae). – Plant Syst. Evol. 139:
147-154.
González Aguilera JJ, Fernández Peralta AM.
1983. The nature of polyploidy in Reseda sect. Leucoreseda
(Resedaceae). – Plant Syst. Evol.
142: 223-237.
González Aguilera JJ, Fernández Peralta AM.
1984. Phylogenetic relationships in the family Resedaceae. – Genetica 64:
185-197.
González Aguilera JJ, Fernández Peralta AM,
Sañudo A. 1980a. Estudios citogenéticos y evolutivos en especies españolas
de la familia Resedaceae L. sección
Glaucoreseda DC. – An. Inst. Bot. Cavanilles 36: 311-320.
González Aguilera JJ, Fernández Peralta AM,
Sañudo A. 1980b. Cytogenetic and evolutive studies on the Spanish species of
the family Resedaceae L.: sections
Phyteuma L. and Resedastrum Duby. – Bol. Soc. Brot. 53:
519-536.
Goodson BE, Santos-Guerra A, Jansen RK. 2006.
Molecular systematics of Descurainia (Brassicaceae) in the Canary Islands:
biogeographic and taxonomic implications. – Taxon 55: 671-682.
Goodson BE, Rehman SK, Jansen RK. 2011.
Molecular systematics and biogeography of Descurainia (Brassicaceae) based on nuclear ITS
and non-coding chloroplast DNA. – Syst. Bot. 36: 957-980.
Gori C. 1957. Sull’embryologia e citologia
di alcune specie del genere Reseda. – Caryologia 10: 391-401.
Gowler ZR. 1998. A taxonomic revision of the
genus Matthiola R. Br. (Cruciferae) and related genera. – Ph.D.
diss., University of Edinburgh, Scotland.
Green ML. 1925. Standard species of the
Linnean genera of Tetradynamia. – Bull. Misc. Inform. Kew 1925: 49-58.
Greene CW. 1978. A nomarski interference
study of megasporogenesis and megagametogenesis in Smelowskia calycina
(Cruciferae). – Amer. J. Bot. 65: 353-358.
Greene EL. 1900. Studies in the Cruciferae
– III. – Pittonia 4: 187-207.
Gregory T, Chandler GT, Bayer RJ. 2000.
Phylogenetic placement of the enigmatic Western Australian genus
Emblingia based on rbcL sequences. – Plant Species Biology
15: 67-72.
Grennan AK. 2009. Identification of genes
involved in metal transport in plants. – Plant Physiology 149: 1623-1624.
Grillo O, Draper D, Venora G,
Martínez-Laborde JB. 2012. Seed image analysis and taxonomy of
Diplotaxis DC. (Brassicaceae, Brassiceea). – Syst.
Biodiv. 10: 57-70.
Grubb CD, Abel S. 2006. Glucosinolate
metabolism and its control. – Trends Plant Sci. 11: 89-100.
Grundt HH. 2003. The arctic-alpine polyploid
Draba lactea and its low-ploid relatives – evolution and taxonomy.
– Ph.D. diss., Faculty of Mathematics and Natural Sciences, University of
Oslo.
Grundt HH, Popp M, Brochmann C, Oxelman B.
2004. Polyploid origins in a circumpolar complex in Draba (Brassicaceae) inferred from cloned
nuclear DNA sequences and fingerprints. – Mol. Phylogen. Evol. 32:
695-710.
Grundt HH, Elven R, Brochmann C. 2005. A rare
case of self-incompatibility in arctic plants: Draba palanderiana (Brassicaceae). – Flora 200:
321-325.
Grundt HH, Obermayer R, Borgen L. 2005.
Ploidal levels in the arctic alpine polyploid Draba lactea (Brassicaceae) and its low-ploid
relatives. – Bot. J. Linn. Soc. 147: 333-347.
Guignard L. 1890. Recherches sur la
localization des principes actifs des Crucifères. – J. Bot. (Morot) 4:
385-394, 412-430, 435-455.
Guignard L. 1893. Recherches sur la
localisation des principés actifs chez les Capparidées, Tropéolées,
Limnanthées, Résédacées (suite). – J. Bot. (Morot) 7: 393-400.
Guinea E. 1963. El género
Biscutella. – An. Inst. Bot. Cavanilles 21: 387-405.
Günthart A. 1902. Beiträge zur
Blütenbiologie der Cruciferen, Crassulaceen und der Gattung
Saxifraga. – Bibl. Bot. 58: 1-97.
Gustafsson M, Bentzer B, Bothmer R von,
Snogerup S. 1976. Meiosis in Greek Brassica of the oleracea
group. – Bot. Not. 129: 73-84.
Hadač E, Chrtek J. 1973. A contribution to
the Brassicaceae of Iraq. – Acta
Univ. Carol., Biol. 1971: 231-265.
Hakki MI. 1974. Embryologische und
morphologische Beobachtungen an Succowia balearica (L.) Medik. (Brassicaceae). – Bot. Jahrb. Syst.
94: 360-382.
Halkier BA, Gershenzon J. 2006. Biology and
biochemistry of glucosinolates. – Ann. Rev. Plant Biol. 57: 303-333.
Hall JC. 2003. Systematics and floral
evolution of Capparaceae and other core Brassicales. – Ph.D. diss.,
University of Wisconsin, Madison, Wisconsin.
Hall JC. 2008. Systematics of Capparaceae and
Cleomaceae: an evaluation of the generic delimitations of Capparis and
Cleome using plastid DNA sequence data. – Botany 86: 682-696.
Hall JC, Sytsma KJ, Iltis HH. 2002. Phylogeny
of Capparaceae and Brassicaceae
based on chloroplast sequence data. – Amer. J. Bot. 89: 1826-1842.
Hall JC, Iltis HH, Sytsma KJ. 2004. Molecular
phylogenetics of core Brassicales, placement of orphan genera
Emblingia, Forchhammeria, Tirania, and character
evolution. – Syst. Bot. 29: 654-669.
Hall JC, Tisdale TE, Donoghue K, Wheeler A,
Al-Yahya MA, Kramer EM. 2011. Convergent evolution of a complex fruit structure
in the tribe Brassiceae (Brassicaceae). – Amer. J. Bot. 98:
1989-2003.
Hannig E. 1901. Untersuchungen über die
Scheidewände der Cruciferenfrüchte. – Bot. Zeitung Berlin, 2. Abt., 59:
207-245.
Hansen BF. 1977. A monograph of
Forchhammeria (Capparidaceae). – M.Sc. thesis, University of
Wisconsin, Madison, Wisconsin.
Hao G, Al-Shehbaz IA, Ahani H, Liang Q, Mao
K, Wang Q, Liu J. 2017. An integrative study of evolutionary diversification of
Eutrema (Eutremeae, Brassicaceae). – Bot. J. Linn. Soc.
184: 204-223.
Harberd DJ. 1972. A contribution to the
cytotaxonomy of Brassica (Cruciferae) and its allies. – Bot. J.
Linn. Soc. 65: 1-23.
Harberd DJ. 1976. Cytotaxonomic studies of
Brassica and related genera. – In: Vaughan JG, Macleod AJ, Jones BMG
(eds), The biology and chemistry of the Cruciferae, Academic Press, London, pp.
47-68.
Harberd DJ, McArthur ED. 1972a. The
chromosome constitution of Diplotaxis muralis (L.) DC. – Watsonia 9:
131-135.
Harberd DJ, McArthur ED. 1972b. Cytotaxonomy
of Rhynchosinapis and Hutera (Cruciferae-Brassiceae). –
Heredity 28: 254-257.
Harberd DJ, McArthur ED. 1980. Meiotic
analysis of some species and genus hybrids in the Brassiceae. – In: Tsunoda
S, Hinata K, Gómez-Campo C (eds), Brassica crops and wild allies,
Japan Scientific Societies Press, Tokyo, pp. 65-87.
Harms H. 1925. Caricaceae. – In: Engler A, Gilg E
(eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 21, W. Engelmann,
Leipzig, pp. 510-522.
Harms H. 1940. Akaniaceae. – In: Engler A, Prantl K
(eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 19b1, W. Engelmann,
Leipzig, pp. 173-175.
Harriman NA. 1965. The genus
Dentaria L. (Cruciferae) in eastern North America. – Ph.D. diss,
Vanderbilt University, Nashville, Tennessee.
Hasapis X, MacLeod AJ, Moreau M. 1981.
Glucosinolates of nine Cruciferae and two Capparaceae species. –
Phytochemistry 20: 2355-2358.
Hauptli H, Webster BD, Jain S. 1978.
Variation in nutlet morphology of Limnanthes. – Amer. J. Bot. 65:
615-624.
Hauser LA, Crovello TJ. 1982. Numerical
analysis of generic relationships in Thelypodieae (Brassicaceae). – Syst. Bot. 7:
249-268.
Hayek A. 1911. Entwurf eines
Cruciferen-Systems auf phylogenetischer Grundlage. – Beih. Bot. Centralbl.
27: 127-335.
Heap JW. 1997. Biology and control of
Reseda lutea L. 1. Seed biology and seedling growth. – Aust. J. Agr.
Res. 48: 511-515.
Hedge IC. 1969. Elbursia: a new
genus of Cruciferae from Iran. – Notes Roy. Bot. Gard. Edinb. 29: 181-184.
Hedge IC. 1976. A systematic and geographical
survey of the Old World Cruciferae. – In: Vaughan JG, MacLeod AJ, Jones BMG
(eds), The biology and chemistry of the Cruciferae, Academic Press, London, pp.
1-45.
Hedge IC, Kjaer A, Malver O. 1980.
Dipterygium – Cruciferae or Capparaceae? – Notes Roy. Bot. Gard.
Edinb. 38: 247-250.
Heel WA van. 1958. Additional investigations
on Batis argillicola van Royen. – Nova Guinea, N. S., 9: 1-7.
Heenan PB. 1999. Artificial intergeneric
hybrids between the New Zealand endemic Ischnocarpus and
Pachycladon (Brassicaceae). – New Zealand J.
Bot. 37: 595-601.
Heenan PB. 2009. A new species of
Pachycladon (Brassicaceae) from limestone in
eastern marlborough, New Zealand. – New Zealand J. Bot. 47: 155-161.
Heenan PB, Garnock-Jones PJ. 1999. A new
species combination in Cheesemania (Brassicaceae). – New Zealand J.
Bot. 37: 235-241.
Heenan PB; Mitchell AD. 2003. Phylogeny,
biogeography and adaptive radiation of Pachycladon (Brassicaceae) in the mountains of
South Island, New Zealand. – J. Biogeogr. 30: 1737-1749.
Heenan PB, Mitchell AD, Koch M. 2002.
Molecular systematics of the New Zealand Pachycladon (Brassicaceae) complex: generic
circumscription and relationships to Arabidopsis sens. lat. and
Arabis sens. lat. – New Zealand J. Bot. 40: 543-562.
Heenan PB, Goeke DG, Houliston GJ, Lysak MA.
2012. Phylogenetic analyses of ITS and rbcL DNA sequences for sixteen
genera of Australian and New Zealand Brassicaceae result in the expansion
of the tribe Microlepidieae. – Taxon 61: 970-979.
Heilborn O. 1927. Chromosome numbers in
Draba. – Hereditas 9: 59-68.
Heimerl A. 1934. Gyrostemonaceae. – In: Engler A
(†), Pax F, Harms H (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd.
16c, W. Engelmann, Leipzig, pp. 165-173.
Heinricher E. 1888. Die Eiweißschläuche der
Cruciferen und verwandte Elemente in der Rhoeadinenreihe. – Mitt. Bot. Inst.
Graz 1: 1-92.
Hellwig F. 1891. Resedaceae. – In: Engler A, Prantl K
(eds), Die natürlichen Pflanzenfamilien III(2), W. Engelmann, Leipzig, pp.
237-241.
Hempel FD, Feldman LJ. 1994. Bi-directional
inflorescence development in Arabidopsis thaliana: acropetal
initiation of flowers and basipetal initiation of paraclades. – Plant 192:
276-286.
Hemsley JH. 1958. Caricaceae. – In: Hubbard CE,
Milne-Redhead E (eds), Flora of tropical East Africa, Crown Agents for Oversea
Governments and Administrations, London, pp. 1-4.
Hennig L. 1929. Beiträge zur Kenntnis der
Resedaceen-Blüte und Frucht. – Planta 9: 507-563.
Hernández-Bermejo JE, Clemente-Muñoz M,
Pujadas Salvá A, Hidalgo B. 1986. Algunas consideraciones sobre
Biscutella L. sect. Laevigatae Malinow. en el sur de España.
– Lagascalia 14: 197-202.
Hernández-Hernández T, Colorado WB, Sosa V.
2013. Molecular evidence for the origin and evolutionary history of the rare
American desert monotypic family Setchellanthaceae. – Org.
Divers. Evol. 13: 485-496.
Hershkovitz MA, Hernández-Pellicer CC,
Arroyo MTK. 2006. Ribosomal DNA evidence for the diversification of
Tropaeolum sect. Chilensia (Tropaeolaceae). – Plant Syst.
Evol. 260: 1-24.
Hewson HJ. 1981. The genus Lepidium
L. (Brassicaceae) in Australia.
– Brunonia 4: 217-308.
Hewson HJ. 1982a. Capparaceae. – In: George
AS (ed), Flora of Australia 8, Australian Government Publ. Service, Canberra,
pp. 207-231.
Hewson HJ. 1982b. Brassicaceae (Cruciferae). – In:
George AS (ed), Flora of Australia 8, Australian Government Publ. Service,
Canberra, pp. 231-357.
Hewson HJ. 1982c. The genus Lepidium
L. (Brassicaceae) in New Guinea.
– Brunonia 5: 73-78.
Hewson HJ. 1982d. Irenepharsus, a
new genus in Brassicaceae in
Australia. – J. Adelaide Bot. Gard. 6: 1-4.
Hewson HJ. 1985. Akaniaceae. – In: George AS (ed),
Flora of Australia 25, Australian Government Publ. Service, Canberra, pp.
2-4.
Hildebrand F. 1879. Vergleichende
Untersuchungen über die Saftdrüsen der Cruciferen. – Jahrb. Wiss. Bot. 12:
10-40.
Hinata K, Nishio T. 1980.
Self-incompatibility in crucifers. – In: Tsunoda S, Hinata K, Gómez-Campo C
(eds), Brassica crops and wild allies, Japan Scientific Societies
Press, Tokyo, pp. 223-234.
Hirner A, Ladwig F, Stransky H, Okumoto S,
Keinath M, Harms A, Frommer WB, Koch W. 2006. Arabidopsis LHT1 is a
high-affinity transporter for cellular amino acid uptake in both root epidermis
and leaf mesophyll. – Plant Cell 18: 1931-1946.
Hitchcock CL. 1941. A revision of the Drabas
of Western North America. – Univ. Washington Publ. Biol. 11: 1-132.
Hitchcock CL. 1945. The South American
species of Lepidium. – Lilloa 11: 75-134.
Hofberger JA, Lyons E, Edger PP, Pires JC,
Schranz EM. 2013. Whole genome and tandem duplicate retention facilitated
glucosinolate pathway diversification in the mustard family. – Genome Biol.
Evol. 5: 2155-2173.
Hofmann U. 1987. Der Bau des Gynoeceums von
Tropaeolum und Pelargonium. – Bot. Jahrb. Syst. 108:
439-448.
Hofmann U, Ludewig J. 1985. Morphologie und
systematische Stellung von Limnanthes douglasii R. Brown, einem
repräsentativen Vertreter der Limnanthaceae. – Bot. Jahrb. Syst.
105: 401-431.
Höglund A-S, Lenman M, Rask L. 1992.
Myrosinase is localized to the interior of myrosin grains and is not associated
to the surrounding tonoplast membrane. – Plant Sci. 85: 165-170.
Hohmann N, Wolf EM, Lysak MA, Koch MA. 2015.
A time-calibrated road map of Brassicaceae species radiation and
evolutionary history. – Plant Cell 27: 2770-2784.
Holmes WC, Yip KL, Rushing AE. 2008. Taxonomy
of Koeberlinia (Koeberliniaceae). – Brittonia
60: 171-184.
Holmgren NH. 2004. Nevada: a new
genus for Smelowskia holmgrenii (Brassicaceae). – Brittonia 56:
238-244.
Holmgren PK. 2004. Lectotypifications and a
new combination in western North American Cleomaceae. – Brittonia 56:
103-106.
Hopkins RJ, Dam RJ van, Loon JJA van. 2009.
Role of glucosinolates in insect-plant relationships and multitrophic
interactions. – Ann. Rev. Entomology 54: 57-83.
Horovitz A, Galil J. 1972. Gynodioecism in
East Mediterranean Hirschfeldia incana, Cruciferae. – Bot. Gaz.
(London) 133: 127-131.
Horovitz S, Micheletti de Zerpa D, Arnal H.
1953. Frecuencias de equilibrio de las formas sexuales en poblaciones de
Carica papaya L. – Agron. Trop. 3: 149-174.
Hosaka K, Kianian SF, McGrath JM, Quiros CF.
1990. Development and chromosomal localization of genome-specific DNA markers
of Brassica and the evolution of amphi-diploids and n = 9 diploid
species. – Genome 33: 131-142.
Huang C-H, Sun R, Hu Y, Zeng LP, Zhang N, Cai
LM, Zhang Q, Koch MA, Al-Shehbaz IA, Edger PP, Pires JC, Tan D-Y, Zhong Y, Ma
H. 2016. Resolution of Brassicaceae phylogeny using nuclear
genes uncovers nested radiations and supports convergent morphological
evolution. – Mol. Biol. Evol. 33: 394-412.
Hufford L. 1996. Developmental morphology of
female flowers of Gyrostemon and Tersonia and floral
evolution among Gyrostemonaceae. – Amer. J. Bot.
83: 1471-1487.
Hunt GM, Holloway PJ, Baker EA. 1976.
Ultrastructure and chemistry of Clarkia elegans leaf wax: a
comparative study with Brassica leaf waxes. – Plant Sci. Letters 6:
353-360.
Hurka H, Paetsch M, Bleeker W, Neuffer B.
2005. Evolution within the Brassicaceae. – Nova Acta
Leopoldina, N. F., 92: 113-127.
Hutchinson J, Dalziel JM. 1928. Pentadiplandraceae. – In:
Dalziel JM (ed), Flora of West Tropical Africa 1, The Crown Agents for the
Colonies, London, p. 461.
Huynh K-L. 1968. Morphologie du pollen des
Tropaeolacées et des Balsaminacées. – Grana Palynol. 8: 88-184, 277-516.
Huynh K-L. 1970. Quelques caractères
cytologiques, anatomiques, et embryologiques distinctifs du genre
Tropaeolum et du genre Impatiens, et position taxinomique de
la famille des Balsaminacées. – Bull. Soc. Neuchatel. Sci. Nat. 93:
165-177.
Huynh K-L. 1971. The morphological
development of the pollen of Limnanthes douglasii (Limnanthaceae). – Grana 11:
58-61.
Huynh K-L. 1982. Le pollen du Limnanthes
douglasii (Limnanthaceae) en
microscopie électronique. – Pollen Spores 24: 211-234.
Hyam RD, Jury SL. 1990. A review of the genus
Graellsia Boiss., including Draba L. sect.
Helicodraba Schultz. – Bot. J. Linn. Soc. 102: 9-22.
Iljinska AP. 2005. Species of the genus
Alyssum L. (sect. Alyssum) in the flora of Ukraine. –
Ukrain. Bot. J. 62: 223-234.
Iltis HH. 1957. Studies in the Capparidaceae
III. Evolution and phylogeny of the western North American Cleomoideae. –
Ann. Missouri Bot. Gard. 44: 77-119.
Iltis HH. 1958. Studies in the Capparidaceae
IV. Polanisia Raf. – Brittonia 10: 33-58.
Iltis HH. 1959. Studies in the Capparidaceae
VI. Cleome sect. Physostemon: taxonomy, geography and
evolution. – Brittonia 11: 123-162.
Iltis HH. 1965. Studies in the Capparidaceae
IX. Capparis pachaca and C. oxysepala: taxonomy and
geography. – Southw. Natur. 10: 57-64.
Iltis HH. 1967. Studies in the Capparidaceae
XI. Cleome afrospina, a tropical African endemic with neotropical
affinities. – Amer. J. Bot. 54: 953-962.
Iltis HH. 1998. Cleome chapalaensis,
n. sp., a South American element on the Mexican plateau. – Bol. Inst. Bot.
(IBUG) 5: 413-443.
Iltis HH. 1999. Setchellanthaceae (Capparales), a new family for a
relictual, glucosinolate-producing endemic of the Mexican deserts. – Taxon
48: 257-275.
Iltis HH, Cochrane TS. 1939. Studies in the
Capparidaceae XVI. Podandrogyne. A new species and three new
combinations. – Rev. Acad. Colombiana Ci. Exact. Fisic. Nat. 17: 265-270.
Iltis HH, Cochrane TS. 2014. Studies in the
Cleomaceae VI: a new genus and sixteen new combinations for the Flora
Mesoamericana. – Novon 23: 51-58.
Iltis HH, Cornejo X. 2007. Studies in the
Capparaceae XXX: Capparicordis, a new genus from the neotropics. –
Brittonia 59: 245-254.
Iltis HH, Cornejo X. 2010. Studies in the
Capparaceae XXIX: synopsis of Quadrella, a Mesoamerican and West
Indies genus. – J. Bot. Res. Inst. Texas 4: 117-132.
Iltis HH, Cornejo X. 2011. Two new genera and
three new combinations in Neotropical Capparaceae. – Harvard Pap. Bot. 16:
65-75.
Iltis HH, Hall JC, Cochrane TS, Sytsma KJ.
2011. Studies in the Cleomaceae I. On the separate recognition of Capparaceae,
Cleomaceae, and Brassicaceae. –
Ann. Missouri Bot. Gard. 98: 28-36.
Inamdar JA. 1969. The stomatal structure and
ontogeny of Azima and Salvadora. – Flora 158: 519-525.
Inamdar JA, Rao NV. 1983. Light and scanning
electron microscopic studies on trichomes of some Brassicaceae. – Feddes Repert. 94:
183-190.
Inda LA, Torrecilla P, Catalán P,
Ruiz-Zapata T. 2008. Phylogeny of Cleome L. and its close relatives
Podandrogyne Ducke and Polanisia Raf. (Cleomoideae,
Cleomaceae) based on analysis of nuclear ITS sequences and morphology. –
Plant Syst. Evol. 274: 111-126.
Inocencio C, Rivera D, Concepción OM,
Alcaraz F, Barreña J-A. 2006. A systematic revision of Capparis
section Capparis (Capparaceae). – Ann. Missouri Bot. Gard. 93:
122-149.
Iversen T-H. 1970a. Cytochemical localization
of myrosinase (β-thioglucosidase) in root tips of Sinapis alba. –
Protoplasma 71: 451-466.
Iversen T-H. 1970b. The morphology,
occurrence, and distribution of dilated cisternae of the endoplasmatic
reticulum in tissues of plants of the Cruciferae. – Protoplasma 71:
467-477.
Jacobs M. 1960. Capparidaceae. – In:
Steenis CGGJ van (ed), Flora Malesiana I, 6, Wolters-Noordhoff, Groningen, pp.
61-105.
Jacobs M. 1963. The genus Stixis
(Capparaceae). A census. – Blumea 12: 5-12.
Jacobs M. 1964. The genus Crateva
(Capparaceae). – Blumea 12: 177-208.
Jacobs M. 1965. The genus Capparis
(Capparaceae) from the Indus to the Pacific. – Blumea 12: 385-541.
Jacquemoud F. 1984. Étude du genre
Anchonium DC. (Cruciferae). – Candollea 39: 715-769.
Jacquemoud F. 1985. Observations sur le genre
Zerdana Boiss. (Cruciferae). – Candollea 40: 347-376.
Jacquemoud F. 1988. Monographie du genre
Sterigmostemum M. Bieb. (Cruciferae-Hesperideae). – Boissiera 40:
4-161.
Jadin F. 1900. Localisation de la myrosine et
de la gomme chez les Moringa. – Compt. Rend. Acad. Sci. Paris 130:
733.
Jadin F, Boucher V. 1908. Sur la production
de la gomme chez Moringa. – Compt. Rend. Acad. Sci. Paris 146:
647.
Jaen-Molina R, Caujape-Castells J,
Reyes-Betancort JA, Akhani H, Fernandez-Palacios O, de Paz JP, Febles-Hernandez
R, Marrero-Rodríguez A. 2009. The molecular phylogeny of Matthiola R.
Br. (Brassicaceae) inferred from
ITS sequences, with special emphasis on the Macaronesian endemics. – Mol.
Phylogen. Evol. 53: 972-981.
Jafri SMH. 1957. The genus Farsetia
in Pakistan, India and Afghanistan. – Notes Roy. Bot. Gard. Edinb. 22:
208-216.
Jain SK. 1976. Meadowfoams – mermaids of
our vernal pools. – Fremontia 4: 19-21.
Jain SK. 1978. Local dispersal of
Limnanthes nutlets: an experiment with artificial vernal pools. –
Can. J. Bot. 56: 1995-1997.
Jain SK, Boussy IA, Hauptli H. 1978. Male
sterility in meadowfoam. – J. Heredity 69: 61-63.
Jaitly SC, Srivastava GN. 1970. Development
and structure of seed of Coronopus didymus (L.) Smith (Senebiera
pinnatifida DG.). – Agra Univ. J. Res. (Sci.) 19: 1-8.
Jallad W. 1975. Taxonomic and floristic
studies on the family Cruciferae in Jordan. – M.Sc. thesis, Faculty of
Science, University of Jordan, Jordania.
Janchen E. 1942. Das System der Cruciferen.
– Österr. Bot. Zeitschr. 91: 1-28.
Jaretzky R. 1928. Untersuchungen über
Chromosomen und Phylogenie bei einigen Cruciferen. – Jahrb. Wiss. Bot. 68:
1-45.
Jaretzky R. 1932. Beziehungen zwischen
Chromosomenzahl und Systematik bei den Cruciferen. – Jahrb. Wiss. Bot. 76:
485-527.
Jart A. 1978. The fatty acid composition of
various cruciferous seeds. – J. Amer. Oil Chem. Soc. 55: 873-875.
Javůrková-Kratochvilová V, Tomšovic P.
1972. Chromosome study of the genus Rorippa Scop. em. Reichenb. in
Czechoslovakia. – Preslia 44: 140-156.
Johns T, Towers GHN. 1980. Glucosinolates in
Tropaeolum tuberosum R. & P., an edible tuber producing plant from
the Andes. – Bot. Soc. Amer. Misc. Ser. Publ. 158: 56.
Johnson DS. 1935. The development of the
shoot, male flower, and seedling of Batis maritima L. – Bull. Torrey
Bot. Club 62: 19-31.
Johnson MAT. 1979. Chromosome numbers in
Akania and Cephalotus. – Kew Bull. 34: 37-38.
Johnston JS, Pepper AE, Hall AE, Chen ZJ,
Hodnett G, Drabek J, Lopez R, Price HJ. 2005. Evolution of gene size in Brassicaceae. – Ann. Bot. 95:
229-235.
Johri BM. 1970. Comparative embryology of
angiosperms: Limnanthaceae. –
Bull. Indian Natl. Sci. Acad. 41: 110-113.
Johri BM, Maheshwari P. 1951. The embryo sac
of Floerkea proserpinacoides Willd. – Curr. Sci. 20: 44-46.
Joly S, Heenan PB, Lockhart PJ. 2009. A
Pleistocene inter-tribal allopolyploidization event precedes the species
radiation of Pachycladon (Brassicaceae) in New Zealand. –
Mol. Phylogen. Evol. 51: 365-372.
Joly S, Heenan PB, Lockhart PJ. 2014. Species
radiation by niche shifts in New Zealand’s rockcresses (Pachycladon,
Brassicaceae). – Syst. Biol. 63:
192-202.
Jonsell B. 1968. Studies in the North-West
European species of Rorippa s. str. – Symb. Bot. Ups. 19(2):
1-222.
Jonsell B. 1971. The genus Rorippa
(Cruciferae) in eastern Siberia and the Soviet Far East. – Svensk Bot.
Tidskr. 65: 293-307.
Jonsell B. 1973. Taxonomy and distribution of
Rorippa (Cruciferae) in the southern U.S.S.R. – Svensk Bot. Tidskr.
67: 281-302.
Jonsell B. 1974. The genus Rorippa
(Cruciferae) in tropical Africa and Madagascar. – Svensk Bot. Tidskr. 68:
377-396.
Jonsell B. 1975. Lepidium L.
(Cruciferae) in tropical Africa. A morphological, taxonomical and
phytogeographical study. – Bot. Not. 128: 20-46.
Jonsell B. 1976. Some tropical African
Cruciferae. Chromosome numbers and taxonomic comments. – Bot. Not. 129:
123-130.
Jonsell B. 1978. New taxa of
Diceratella and Farsetia (Cruciferae) from E tropical Africa.
– Bot. Not. 131: 251-257.
Jonsell B. 1979. New taxa of Cruciferae from
East tropical Africa and Madagascar. – Bot. Not. 132: 521-535.
Jonsell B. 1982. Cruciferae. – In: Polhill
RM (ed), Flora of tropical East Africa, A. A. Balkema, Rotterdam, pp. 1-73.
Jonsell B. 1986a. A monograph of
Farsetia (Cruciferae). – Symb. Bot. Upsal. 25(3): 1-107.
Jonsell B. 1986b. Cruciferae. – In: Steenis
CGGJ van (†), Wilde WJJO de (eds), Flora Malesiana I, 10(3), Kluwer Academic
Publ., Dordrecht, Boston, London, pp. 541-560.
Jonsell B, Moggi G. 1983. Diceratella
alata (Cruciferae), a new species from the Horn of Africa. – Nord. J.
Bot. 3: 347-349.
Jordheim M, Andersen ØM, Nozzolillo C,
Amiguet VT. 2009. Acylated anthocyanins in inflorescence of spider flower
(Cleome hassleriana). – Phytochemistry 70: 740-745.
Jordon-Thaden IE. 2010. Species and genetic
diversity of Draba: phylogeny and phylogeography. – Ph.D. diss.,
Ruperto-Carola Universität Heidelberg, Germany.
Jordon-Thaden IE, Koch MA. 2008. Diversity
patterns in the genus Draba: a first global perspective. – Plant
Ecol. Divers. 1: 255-263.
Jordon-Thaden IE, Hase I, Al-Shehbaz I, Koch
MA. 2010. Molecular phylogeny and systematics of the genus Draba (Brassicaceae) and identification of
its most closely related genera. – Mol. Phylogen. Evol. 55: 524-540.
Jordon-Thaden IE, Al-Shehbaz IA, Koch MA.
2013. Species richness of the globally distributed, Arctic-alpine genus
Draba L. (Brassicaceae).
– Alpine Botany 123: 97-106.
Jørgensen LB. 1981. Myrosin cells and
dilated cisternae of the endoplasmic reticulum in the order Capparales. – Nord. J. Bot. 1:
433-445.
Jørgensen LB. 1995. Stomatal myrosin cells
in Caricaceae: implications for a
glucosinolate family. – Nord. J. Bot. 15: 523-540.
Jumelle MH. 1930. Les Moringa de
Madagascar. – Ann. Mus. Col. Marseille, sér. IV, 8: 1-20.
Kaercher W, Valdés Bermejo E. 1975.
Contribución al studio cariológico del género Reseda L. en España.
Nota I. Sección Leucoreseda DC. – An. Inst. Bot. Cavanilles 32:
165-174.
Kagale S, Robinson SJ, Nixon J, Xiao R,
Huebert T, Condie J, Kessler D, Clarke WE, Edger PP, Links MG, Sharpe AG,
Parkin IAP. 2014. Polyploid evolution of the Brassicaceae during the Cenozoic era.
– Plant Cell 26: 2777-2791.
Kalin Arroyo MT. 1973a. A taximetric study of
infraspecific variation in autogamous Limnanthes floccosa (Limnanthaceae). – Brittonia 25:
177-191.
Kalin Arroyo MT. 1973b. Chiasma frequency
evidence on the evolution of autogamy in Limnanthes floccosa (Limnanthaceae). – Evolution 27:
679-688.
Kamelin RV. 2002. The Cruciferae (brief
survey of the system). – Barnaul.
Kamelin RV, German DA. 2001. New species of
the genus Sterigmostemum Bieb. (Cruciferae) from East Kazakhstan. –
Turczaninowia 4: 5-9.
Kappert H. 1955. Einige für Evolutionsfragen
interessante Mutationen bei Matthiola. – Ber. Deutsch. Bot. Ges. 68:
413-422.
Karl R, Koch MA. 2013. A world-wide
perspective on crucifer speciation and evolution: phylogenetics, biogeography
and trait evolution in tribe Arabideae. – Ann. Bot. 112: 983-1001.
Karl R, Kiefer C, Ansell S, Koch MA. 2012.
Systematics and evolution of arctic-alpine Arabis alpina L. (Brassicaceae) and its closest
relatives in the eastern Mediterranean. – Amer. J. Bot. 99: 778-794.
Karol KG, Rodman JE, Conti E, Sytsma KJ.
1999. Nucleotide sequence of rbcL and phylogenetic relationships of
Setchellanthus caeruleus (Setchellanthaceae). – Taxon
48: 303-315.
Karrer AB. 1991. Blütenentwicklung und
systematische Stellung der Papaveraceae und
Capparaceae. – Ph.D. diss., Universität Zürich, Switzerland.
Kavousi K. 2001. Notes on the plant family
Cruciferae in Iran, new taxa and new records. – Iranian J. Bot. 9: 47-54.
Keighery GJ. 1975. Chromosome numbers in the
Gyrostemonaceae Endl. and the
Phytolaccaceae
Lindl.: a comparison. – Aust. J. Bot. 23: 335-338.
Keighery GJ. 1981. The breeding system of
Emblingia (Emblingiaceae). – Plant Syst.
Evol. 137: 63-65.
Keighery GJ. 1985. Walteranthus, a
new genus of Gyrostemonaceae
from Western Australia. – Bot. Jahrb. Syst. 106: 107-113.
Keighery GJ. 2002. Taxonomic notes on the
genus Stenopetalum (Brassicaceae). – Nuytsia 14:
393-403.
Keller S. 1979. A revision of the genus
Wislizenia (Capparidaceae) based on population studies. – Brittonia
31: 333-351.
Kerber E, Buchloh G. 1981. Sinapinfreie und
sinapinhaltige Brassicaceae (=
Cruciferen). – Beitr. Biol. Pflanzen 55: 377-384.
Kers LE. 1969a. Studies in Cleome
II. Cleome angustifolia Forssk. s. lat. and Cleome
semitetrandra Sond. – Svensk Bot. Tidskr. 63: 1-48.
Kers LE. 1969b. Studies in Cleome
III. Morphology and distribution of some African species. – Bot. Not. 122:
549-588.
Kers LE. 1972. Cleome oligandra sp.
nov. – a two-staminate species from Tanzania. – Bot. Not. 125: 157-160.
Kers LE. 1993. New taxa in Maerua
(Capparaceae) proposed for the Flora of Ethiopia. – Novon 3: 50-54.
Kers LE. 2002. Capparaceae. – In: Kubitzki
K, Bayer C (eds), The families and genera of vascular plants V. Flowering
plants. Dicotyledons. Malvales, Capparales and non-betalain Caryophyllales,
Springer, Berlin, Heidelberg, New York, pp. 36-56.
Kesseli RV, Jain SK. 1984. New variation and
biosystematic patterns detected by allozyme and morphological comparisons in
Limnanthes sect. Reflexae (Limnanthaceae). – Plant Syst.
Evol. 147: 133-165.
Khalik KA. 2005. Morphological studies on
trichomes of Brassicaceae in Egypt
and taxonomic significance. – Acta Bot. Croat. 64: 57-73.
Khalik KA, Maesen LJG van der, Koopman WJM,
Berg RG van den. 2002. Numerical taxonomic study of some tribes of Brassicaceae from Egypt. – Plant
Syst. Evol. 233: 207-221.
Khalik KA, Berg RG van den Maesen LJG van
der, El Hadidi MN. 2002. Pollen morphology of some tribes of Brassicaceae from Egypt and its
systematic implications. – Feddes Repert. 113: 211-223.
Khalilov II. 1991. Systematics of the genus
Crambe (Brassicaceae).
– Bot. Žurn. 76: 1612-1613.
Khanna KR, Rollins RC. 1965. A taxonomic
revision of Cremolobus (Cruciferae). – Contr. Gray Herb. 195:
135-157.
Khodashenas M. 2007. A taxonomic revision of
the genus Sisymbrium (Brassicaceae) in Iran. – Flora 13:
49-52.
Khosravi AR, Maassoumi AAR. 1998.
Contribution to the cytotaxonomy of some Cruciferae from Iran. – Iranian J.
Bot. 7: 193-206.
Khosravi AR, Mohsenzadeh S, Mummenhoff K.
2008a. Analysis of the phylogenetic position of Acanthocardamum
erinaceum (Boiss.) Thell. (Brassicaceae) based on ITS-sequences
shows that it should be transferred to Aethionema as A.
erinaceum. – Nord. J. Bot. 26: 25-30.
Khosravi AR, Mohsenzadeh S, Mummenhoff K.
2008b. Phylogenetic position of Brossardia papyracea (Brassicaceae) based on sequences of
nuclear ribosomal DNA. – Feddes Repert. 119: 13-23.
Khosravi AR, Jacquemoud F, Mohsenzadeh S,
Menke M, Mummenhoff K. 2009. Phylogenetic position and taxonomic classification
of Aethionema trinervium (Brassicaceae): a morphologically
variable subshrub from southwestern Asia. – Ann. Missouri Bot. Gard. 96:
564-574.
Khosravi AR, Mohsenzadeh S, Mummenhoff K.
2009. Phylogenetic relationships of Old World Brassicaceae from Iran based on
nuclear ribosomal DNA sequences. – Biochem. Syst. Ecol. 37: 106-115.
Kiefer C, Koch MA. 2012. A continental-wide
perspective: the gene pool of nuclear encoded ribosomal DNA and single-copy
gene sequences in North American Boechera (Brassicaceae). – PloS ONE 7(5):
e36491; doi: 10.1371/journal.pone.0036491.
Kiefer C, Dobeš C, Koch MA. 2009.
Boechera or not? Phylogeny and phylogeography of eastern North
American Boechera species (Brassicaceae). – Taxon 58:
1109-1121.
Kiefer C, Dobeš C, Sharbel TF, Koch MA.
2009. Phylogeographic structure of the chloroplast DNA gene pool in North
American Boechera – a genus and continental-wide perspective. –
Mol. Phylogen. Evol. 52: 303-311.
Kiefer M, Schmickl R, German DA, Mandáková
T, Lysak MA, Al-Shehbaz IA, Franzke A, Mummenhoff K, Stamatakis A, Koch MA.
2014. BrassiBase: introduction to a novel knowledge database on Brassicaceae evolution. – Plant
Cell Physiol. 55: e3(1-9). doi:10.1093/pcp/pct158
Kirti PB, Narasimhulu SB, Prakash S, Chopra
VL. 1992. Production and characterization of intergeneric somatic hybrids of
Trachystoma ballii and Brassica juncea. – Plant Cell Rep.
11: 90-92.
Kjær A. 1960. Naturally derived
isothiocyanates (mustard oils) and their parent glucosides. – Progr. Chem.
Org. Nat. Prod. 18: 122-176.
Kjær A. 1968. Glucosinolates in Tovariaceae. – Phytochemistry 7:
131-133.
Kjær A. 1973. The natural distribution of
glucosinolates: a uniform group of sulphur-containing glucosides. – In: Bendz
G, Santesson J (eds), Chemistry in botanical classification (eds), Academic
Press, New York, pp. 229-234.
Kjær A. 1976. Glucosinolates in the
Cruciferae. – In: Vaughan JG, MacLeod AJ, Jones BMG (eds), The biology and
chemistry of the Cruciferae, Academic Press, London, pp. 207-219.
Kjær A, Malver O. 1979. Glucosinolates in
Tersonia brevipes (Gyrostemonaceae). –
Phytochemistry 18: 1565.
Kjær A. Madsen JO, Maeda Y. 1978. Seed
volatiles within the family Tropaeolaceae. – Phytochemistry
17: 1285-1287.
Klimes L, German D. 2009. Draba
alshehbazii (Brassicaceae), a
new species from extreme altitudes of eastern Ladakh (Jammu and Kashmir,
India). – Bot. J. Linn. Soc. 158: 749-754.
Knaben G. 1966. Cytotaxonomical studies in
some Draba species. – Bot. Not. 119: 427-444.
Knaben G, Engelskjøn T. 1967. Chromosome
numbers of Scandinavian arctic-alpine plant species II. – Acta Borealia, ser.
A, Scientia 21: 1-57.
Knjasev M. 2011. Notes on some species of Brassicaceae in Urals and adjacent
territories. – Nov. Sist. Vyssh. Rast. 42: 136-146.
Knoblauch E. 1895. Salvadoraceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien IV(2), W. Engelmann, Leipzig,
pp. 17-19.
Koch MA. 2002. Genetic differentiation and
speciation in prealpine Cochlearia: allohexaploid Cochlearia
bavarica Vogt (Brassicaceae)
compared to its diploid ancestor Cochlearia pyrenaica DC. in Germany
and Austria. – Plant Syst. Evol. 232: 35-49.
Koch MA. 2003. Molecular phylogenetics,
evolution and population biology in Brassicaceae. – In: Sharma AK,
Sharma A (eds), Plant genome: biodiversity and evolution 1A: phanerogams,
Science Publ., Enfield, New Hampshire, pp. 1-35.
Koch MA. 2012. Mid-Miocene divergence of
Ionopsidium and Cochlearia and its impact on the systematics
and biogeography of the tribe Cochlearieae (Brassicaceae). – Taxon 61:
76-92.
Koch MA, Al-Shehbaz IA. 2000. Molecular
systematics of the Chinese Yinshania (Brassicaceae): evidence from plastid
and nuclear ITS DNA sequence data. – Ann.Missouri Bot. Gard. 87: 246-272.
Koch MA, Al-Shehbaz IA. 2002. Molecular data
indicate complex intra- and intercontinental differentiation of American
Draba (Brassicaceae). –
Ann. Missouri Bot. Gard. 89: 88-109.
Koch MA, Al-Shehbaz IA. 2004. Taxonomic and
phylogenetic evaluation of the American ‘Thlaspi’ species:
identity and relationship to the Eurasian genus Noccaea (Brassicaceae). – Syst. Bot. 29:
375-384.
Koch MA, Al-Shehbaz IA. 2009. Molecular
systematics and evolution of ’wild’ crucifers (Brassicaceae or Cruciferae). – In:
Gupta SK (ed), Biology and breeding of crucifers, Taylor and Francis Group,
Boca Raton, pp. 1-19.
Koch MA, Bernhardt KG. 2004. Comparative
biogeography of the cytotypes of annual Microthlaspi perfoliatum (Brassicaceae) in Europe using
isozymes and cpDNA data: refugia, diversity centers, and postglacial
colonization. – Amer. J. Bot. 91: 114-124.
Koch MA, Hurka H. 1999. Isozyme analysis in
the polyploid complex Microthlaspi perfoliatum (L.) F. K. Meyer:
morphology, biogeography and evolutionary history. – Flora 194: 33-48.
Koch MA, Kiefer C. 2006. Molecules and
migration: biogeographical studies in cruciferous plants. – Plant Syst. Evol.
259: 121-142.
Koch MA, Mummenhoff K. 2001. Thlaspi
s.str. (Brassicaceae) versus
Thlaspi s.l.: morphological and anatomical characters in the light of
ITS nrDNA sequence data. – Plant Syst. Evol. 227: 209-225.
Koch MA, Mummenhoff K. 2006. Evolution and
phylogeny of the Brassicaceae. –
Plant Syst. Evol. 259: 81-83.
Koch MA, Hurka H, Mummenhoff K. 1996.
Chloroplast DNA restriction site variation and RAPD-analyses in
Cochlearia (Brassicaceae): biosystematics and
speciation. – Nord. J. Bot. 16: 585-603.
Koch MA, Huthmann M, Hurka H. 1998. Molecular
biogeography and evolution of the Microthlaspi perfoliatum s.l.
polyploid complex (Brassicaceae):
chloroplast DNA and nuclear ribosomal DNA restriction site variation. – Can.
J. Bot. 76: 382-396.
Koch MA, Bishop J, Mitchell-Olds T. 1999.
Molecular systematics and evolution of Arabidopsis and
Arabis. – Plant Biol. 1: 529-537.
Koch MA, Mummenhoff K, Hurka H. 1999.
Molecular phylogenetics of Cochlearia L. (Brassicaceae) and allied genera based
on nuclear ribosomal ITS DNA sequence analysis contradict traditional concepts
of their evolutionary relationship. – Plant Syst. Evol. 216: 207-230.
Koch MA, Haubold B, Mitchell-Olds T. 2000.
Comparative evolutionary analysis of chalcone synthase and alcohol
dehydrogenase loci in Arabidopsis, Arabis, and related genera
(Brassicaceae). – Mol. Biol.
Evol. 17: 1483-1498.
Koch MA, Haubold B, Mitchell-Olds T. 2001.
Molecular systematics of the Brassicaceae: evidence from coding
plastidic matK and nuclear Chs sequences. – Amer. J. Bot.
88: 534-544.
Koch MA, Al-Shehbaz IA, Mummenhoff K. 2003.
Molecular systematics, evolution, and population biology in the mustard family
(Brassicaceae). – Ann. Missouri
Bot. Gard. 90: 151-171.
Koch MA, Bernhardt KG, Kochjarová J. 2003.
Cochlearia macrorrhiza (Brassicaceae): a bridging species
between Cochlearia taxa from the Eastern Alps and the Carpathians. –
Plant Syst. Evol. 242: 137-147.
Koch MA, Dobeš C, Matschinger M, Bleeker W,
Vogel J, Kiefer M, Mitchell-Olds T. 2005. Evolution of the trnF(GAA)
gene in Arabidopsis relatives and the Brassicaceae family: monophyletic
origin and subsequent diversification of a plastidic pseudogene. – Mol. Biol.
Evol. 22: 1032-1043.
Koch MA, Dobeš C, Kiefer C, Schmickl R,
Klimes L, Lysak MA. 2007. SuperNetwork identifies multiple events of plastid
trnF(GAA) pseudogene evolution in the Brassicaceae. – Mol. Biol. Evol.
24: 63-73.
Koch MA, Wernisch M, Schmickl R. 2008.
Arabidopsis thaliana wild relatives: an updated overview on
systematics, taxonomy, and evolution. – Taxon 57: 933-943.
Koch MA, Karl R, Kiefer M, Al-Shehbaz IA.
2010. Colonizing the American continent: systematics of the genus
Arabis in North America (Brassicaceae). – Amer. J. Bot. 97:
1040-1057.
Koch MA, Karl R, German DA, Al-Shehbaz IA.
2012. Systematics, taxonomy and biogeography of three new Asian genera of Brassicaceae tribe Arabideae: an
ancient distribution circle around the Asian high mountains. – Taxon 61:
955-969.
Koch MA, Kiefer M, German DA, Al-Shehbaz IA,
Franzke A, Mummenhoff K, Schmickl R. 2012. BrassiBase: tools and biological
resources to study characters and traits in the Brassicaceae – version 1.1. –
Taxon 61: 1001-1009.
Koch MA, Karl R, German DA. 2017.
Underexplored biodiversity of Eastern Mediterranean biota: systematics and
evolutionary history of the genus Aubrieta (Brassicaceae). – Ann. Bot. 119:
39-57.
Kolbe K-P. 1978. Serologische Beiträge zur
Systemaik der Capparales. – Bot.
Jahrb. Syst. 99: 468-489.
Korejo F, Ali SA, Tahir SS, Rajput MT, Akhter
MT. 2010. Comparative morphological and biochemical studies of
Salvadora species found in Sindh, Pakistan. – Pak. J. Bot.
1451-1463.
Koshy JK, Mathew PM. 1985. Cytology of the
genus Cleome Linn. – Cytologia 50: 283-287.
Koteyeva NK, Voznesenskaya EV, Roalson EH,
Edwards GE. 2011. Diversity in forms of C4 in the genus
Cleome (Cleomaceae). – Ann. Bot. 107: 269-283.
Koudogbo B, Delaveau P. 1974. Chimotaxonomie
des Capparidaceae. – Plant Méd. Phytothérapie 8: 96-103.
Koul KK, Nagpal R, Raina SN. 2000. Seed coat
microsculpturing in Brassica and allied genera (subtribes Brassicinae,
Raphaninae, Moricandiinae). – Ann. Bot. 86: 385-397.
Kowal E, Cutler DF. 1975. The wood anatomy of
Schouwia purpurea subsp. arabica and Fabrisinapis
fruticosus (Cruciferae). – Kew Bull. 30: 503-507.
Kruckeberg AR, Rodman JE, Worthington RD.
1982. Natural hybridization between Streptanthus arizonicus and S.
carinatus (Cruciferae). – Syst. Bot. 7: 291-299.
Kshetrapal S. 1970. A contribution to the
vascular anatomy of the flower of certain species of the Salvadoraceae. – J. Indian Bot.
Soc. 49: 92-99.
Kubitzki K. 2002a. Introduction to Capparales. – In: Kubitzki K, Bayer C
(eds), The families and genera of vascular plants V. Flowering plants.
Dicotyledons. Malvales, Capparales and non-betalain Caryophyllales,
Springer, Berlin, Heidelberg, New York, pp. 7-10.
Kubitzki K. 2002b. Conspectus of the families
of Capparales. – In: Kubitzki K,
Bayer C (eds), The families and genera of vascular plants V. Flowering plants.
Dicotyledons. Malvales, Capparales and non-betalain Caryophyllales,
Springer, Berlin, Heidelberg, New York, p. 11.
Kubitzki K. 2002c. Caricaceae. – In: Kubitzki K, Bayer C
(eds), The families and genera of vascular plants V. Flowering plants.
Dicotyledons. Malvales, Capparales and non-betalain Caryophyllales,
Springer, Berlin, Heidelberg, New York, pp. 57-61.
Kubitzki K. 2002d. Emblingiaceae. – In: Kubitzki K,
Bayer C (eds), The families and genera of vascular plants V. Flowering plants.
Dicotyledons. Malvales, Capparales and non-betalain Caryophyllales,
Springer, Berlin, Heidelberg, New York, pp. 206-208.
Kubitzki K. 2002e. Koeberliniaceae. – In: Kubitzki
K, Bayer C (eds), The families and genera of vascular plants V. Flowering
plants. Dicotyledons. Malvales, Capparales and non-betalain Caryophyllales,
Springer, Berlin, Heidelberg, New York, pp. 218-219.
Kubitzki K. 2002f. Moringaceae. – In: Kubitzki K, Bayer
C (eds), The families and genera of vascular plants V. Flowering plants.
Dicotyledons. Malvales, Capparales and non-betalain Caryophyllales,
Springer, Berlin, Heidelberg, New York, pp. 312-314.
Kubitzki K. 2002g. Resedaceae. – In: Kubitzki K, Bayer C
(eds), The families and genera of vascular plants V. Flowering plants.
Dicotyledons. Malvales, Capparales and non-betalain Caryophyllales,
Springer, Berlin, Heidelberg, New York, pp. 334-338.
Kubitzki K. 2002h. Salvadoraceae. – In: Kubitzki K,
Bayer C (eds), The families and genera of vascular plants V. Flowering plants.
Dicotyledons. Malvales, Capparales and non-betalain Caryophyllales,
Springer, Berlin, Heidelberg, New York, pp. 342-344.
Kubitzki K. 2002i. Setchellanthaceae. – In:
Kubitzki K, Bayer C (eds), The families and genera of vascular plants V.
Flowering plants. Dicotyledons. Malvales, Capparales and non-betalain Caryophyllales,
Springer, Berlin, Heidelberg, New York, pp. 353-354.
Kučera J, Valko I, Marhold K. 2005. On-line
database of the chromosome numbers of the genus Cardamine (Brassicacee). – Biologia 60:
473-476.
Kučera J, Lihova J, Marhold K. 2006.
Taxonomy and phylogeography of Cardamine impatiens and C.
pectinata (Brassicaceae). –
Bot. J. Linn. Soc. 152: 169-195.
Kučera J, Marhold K, Lihová J. 2010.
Cardamine maritima group (Brassicaceae) in the amphi-Adriatic
area: a hotspot of species diversity revealed by DNA sequences and
morphological variation. – Taxon 59: 148-164.
Kumar PR, Tsunoda S. 1980. Variation in oil
content and fatty acid composition among seeds from the Cruciferae. – In:
Tsunoda S, Hinata K, Gómez-Camp C (eds), Brassica crops and wild
allies, Japan Scientific Societies Press, Tokyo, pp. 235-252.
Kumar PV, Bahadur B. 1978. Seed morphology of
thirteen species of Cleome L. (Capparidaceae). – J. Indian Bot. Soc.
57: 39-46.
Kupicha FK. 1978. 85. Caricaceae. – In: Launert E (ed),
Flora Zambesiaca 4, Flora Zambesiaca Managing Committee, London, pp.
412-414.
Kyndt T, Droogenbroeck B van, Romeijn-Peeters
E, Romero-Motochi JP, Scheldeman X, Goetghebeur P, Damme P van, Gheysen G.
2005. Molecular phylogeny and evolution of Caricaceae based on rDNA internal
transcribed spacers and chloroplast sequence data. – Mol. Phylogen. Evol. 37:
442-459.
Kyndt T, Romeijn-Peeters E, Groogenbroek B
van, Romero-Motochi JP, Gheejsen G, Goetghebeur P. 2005. Species relationships
in the genus Vasconsellea (Caricaceae) based on molecular and
morphological evidence. – Amer. J. Bot. 92: 1033-1044.
Lagercrantz U. 1998. Comparative mapping
between Arabidopsis thaliana and Brassica nigra indicates the
Brassica genomes have evolved through extensive genome replication
accompanied by chromosome fusions and frequent rearrangements. – Genetics
150: 1217-1228.
Lagercrantz U, Lydiate D. 1996. Comparative
genome mapping in Brassica. – Genetics 144: 1903-1910.
Lagerheim G de 1892. Zur Kenntnis der
Tovariaceen. – Ber. Deutsch. Bot. Ges. 10: 163-169.
Lahham JN, Al-Eisawi D. 1987. Pollen
morphology of Jordanian Cruciferae. – Mitt. Bot. Staatssamml. München 23:
355-375.
Lamba LC. 1975. Structure and development of
seed in Brassica nigra Koch. – J. Indian Bot. Soc. 54: 225-233.
Lange PJ de, Heenan PB, Houliston GJ, Rolfe
JR, Mitchell AD. 2013. New Lepidium (Brassicaceae) from New Zealand. –
PhytoKeys 24: 1-147.
Lanning FC. 1961. Calcite in Lewquerella
ovalifolia trichomes. – Science 133: 380.
La-Serna Ramos I. 1996. Pollen characters of
Canary Resedaceae with special
reference to endemic taxa. – Grana 35: 16-23.
Leadlay EA, Heywood VH. 1990. The biology and
systematics of the genus Coincya Porta & Rigo ex Rouy
(Cruciferae). – Bot. J. Linn. Soc. 102: 313-398.
Lee J-Y, Mummenhoff K, Bowman JL. 2002.
Allopolyploidization and evolution of species with reduced floral structures in
Lepidium L. (Brassicaceae). – Proc. Natl. Acad.
Sci. U.S.A. 99: 16835-16840.
Leins P, Metzenauer G. 1979.
Entwicklungsgeschichtliche Untersuchungen an Capparis-Blüten. –
Bot. Jahrb. Syst. 100: 542-554.
Leins P, Sobick U. 1977. Die
Blütenentwicklung von Reseda lutea. – Bot. Jahrb. Syst. 98:
133-149.
Lenman M, Falk A, Xue J, Rask L. 1993.
Characterization of a Brassica napus myrosinase pseudogene:
myrosinases are members of the BGA family of β-glycosidases. – Plant Mol.
Biol. 21: 463-474.
Léonard J. 1977. Le genre irano-touranien
Drabopsis et son unique espèce D. nuda (Cruciferae). –
Publ. Cairo Univ. Herb. 7-8: 295-297.
Léonard J. 1980a. Contribution à la
connaissance de la flore de l’Iran 2. Petiniotia J. Léonard, genre
asiatique nouveau de Crucifères. – Bull. Jard. Bot. Belg. 50: 227-232.
Léonard J. 1980b. Contribution à la
connaissance de la flore de l’Iran 3. Les espèces iraniennes du genre
Tetracme Bunge (Crucifères). – Bull. Jard. Bot. Belg. 50:
233-235.
Léonard J. 1980c. Contribution à la
connaissance de la flore de l’Iran 4. Cithareloma Bunge, genre
irano-touranien de Crucifères nouveau pour l’Iran. – Bull. Jard. Bot.
Belg. 50: 371-374.
Léonard J. 1986. Neotorularia Hedge
& J. Léonard nom générique nouveau de Cruciferae. – Bull. Jard. Bot.
Belg. 56: 389-395.
Les DH. 1994. Molecular systematics and
taxonomy of lake cress (Neobeckia aquatica; Brassicaceae), an imperilled aquatic
mustard. – Aquatic Bot. 49: 149-165.
Li Y, Kong Y, Zhang Z, Yin YQ, Liu B, Lv GH,
Wang XY. 2014. Phylogeny and biogeography of Alyssum (Brassicaceae) based on nuclear
ribosomal ITS DNA sequences. – J. Genet. 93: 313-323.
Li Y, Feng Y, Lv G, Liu B, Qi A. 2015. The
phylogeny of Alyssum (Brassicaceae) inferred from molecular
data. – Nord. J. Bot. 33: 715-721.
Li Y, Lv G, He X, Zhang X, Yang X. 2017. The
complete chloroplast genome of the spring ephemeral plant Alyssum
desertorum and its implications for the phylogenetic position of the tribe
Alysseae within the Brassicaceae.
– Nord. J. Bot. 35: 644-652.
Lihová J, Marhold K. 2006. Phylogenetic and
diversity patterns in Cardamine (Brassicaceae) – a genus with
conspicuous polyploidy and reticulate evolution. – In: Sharma AK, Sharma A
(eds), Plant genome biodiversity and evolution, Vol. 1, Part C, Phanerogams
(Angiosperms-Dicotyledons), Science Publ., Enfield, New Hampshire, pp.
149-186.
Lihová J, Marhold K, Neuffer B. 2000.
Taxonomy of Cardamine amara (Cruciferae) in the Iberian Peninsula. –
Taxon 49: 747-763.
Lihová J, Aguilar JF, Marhold K, Feliner GN.
2003. Origin of the disjunct tetraploid Cardamine amporitana (Brassicaceae) assessed with nuclear
and chloroplast DNA sequence data. – Amer. J. Bot. 91: 1231-1242.
Lihová J, Tribsch A, Stuessy TF. 2004.
Cardamine apennina: a new endemic diploid species of the C.
pratensis group (Brassicaceae) from Italy. – Plant
Syst. Evol. 245: 69-92.
Link DA. 1992. The floral nectaries in the Limnanthaceae. – Plant Syst. Evol.
179: 235-243.
Liu C. 1986. Studies of pollen morphology in
the Bretschneideraceae and related families. – Acta Bot. Yunnan. 8: 441-450.
[In Chinese with English summary]
Liu L, Zhao B, Tan D, Wang J. 2011.
Phylogenetic relationships of Brassicaceae in China: insights from
a non-coding chloroplast, mitochondrial, and nuclear DNA set. – Biochem.
Syst. Ecol. 39: 600-608.
Lloyd DG. 1965. Evolution of
self-compatibility and racial differentiation in Leavenworthia
(Cruciferae). – Contr. Gray Herb. Harvard Univ. 195: 3-134.
Lobreau-Callen D. 1977. Les pollens des Celastrales:
illustrations, commentaires. – École Pratique des Hautes Études, Institut
de Montpellier.
Loiseau JE. 1947. Sur l’organisation du
gynécée chez les Tropaeolacées. – Ann. Fac. Sci. 26: 125-147.
López González G. 1990. Notas referentes al
género Sesamoides Gómez Ortega (Resedaceae). – An. Jard. Bot. Madrid
48: 97-100.
Lorence DH, Colin RT. 1988. Carica
cnidoscoloides (sp. nov.) and sect. Holostigma (sect. nov.) of Caricaceae from southern Mexico. –
Syst. Bot. 13: 107-110.
Louda SM, Rodman JE. 1983. Concentration of
glucosinolates in relation to habitat and insect herbivory for the native
crucifer Cardamine cordifolia. – Biochem. Syst. Ecol. 11:
199-207.
Lövkvist B. 1956. The Cardamine
pratensis complex. Outlines of its cytogenetics and taxonomy. – Symb.
Bot. Ups. 14(2): 1-131.
Lu S-Y, Shih K-H, Fan F-H. 1986.
Bretschneideraceae, a new family record for the flora of Taiwan. – Quart. J.
Chin. Forest. 19: 115-119.
Ludlow-Wiechers B. 1981. Catálogo
palinológico para la flora de Veracruz. No. 4. Familia Caricaceae. – Biotica 6: 33-42.
Ludlow-Wiechers B, Roldán-Ramos L. 1983.
Catálogo palinológico para la flora de Veracruz. No. 12. Fam. Bataceae. – Biotica 8: 31-36.
Lüning B, Kers LE, Seffers P. 1992. Methyl
glycosinolate confirmed in Puccionia and Dhofaria
(Capparidaceae). – Biochem. Syst. Ecol. 20: 394.
Lüthy B, Matile PH. 1984. The mustard oil
bomb: rectified analysis of the subcellular organisation of the myrosinase
system. – Bioch. Physiol. Pflanzen 179: 5-12.
Lysak MA. 2018. Brassicales: an update on
chromosomal evolution and ancient polyploidy. – Plant Syst. Evol. 304:
757-762.
Lysak MA, Koch MA. 2011. Phylogeny, genome,
and karyotype evolution of crucifers (Brassicaceae). – In: Schmidt R,
Bancroft I (eds), Genetics and genomics of the Brassicaceae. Plant genetics and
genomics: crops and models 9, Springer, pp. 1-31.
Lysak MA, Lexer C. 2006. Towards the era of
comparative evolutionary genomics in Brassicaceae. – Plant Syst. Evol.
259: 175-198.
Lysak MA, Koch MA, Pecinka A, Schubert I.
2005. Chromosome triplication found across the tribe Brassiceae. – Genome
Res. 15: 516-525.
Lysak MA, Berr A, Pecinka A, Schmidt R,
McBreen K, Schubert I. 2006. Mechanisms of chromosome number reduction in
Arabidopsis thaliana and related Brassicaceae species. – Proc. Natl.
Acad. Sci. U.S.A. 103: 5224-5229.
Lysak MA, Cheung K, Kitschke M, Bureš P.
2007. Ancestral chromosome blocks are triplicated in Brassiceae species with
varying chromosome number and genome size. – Plant Physiol. 145: 402-410.
Lysak MA, Koch MA, Beaulieu JM, Meister A,
Leitch IJ. 2009. The dynamic ups and downs in genome size evolution in Brassicaceae. – Mol. Biol. Evol.
21: 85-98.
Mabry TJ, Turner BL. 1964. Chemical
investigations of the Batidaceae. Betaxanthins and their systematic
implications. – Taxon 13: 197-200.
Macbride JF. 1938. Cruciferae. – In:
Macbride JF (ed), Flora of Peru, Publ. Field Mus. Nat. Hist. Bot. Ser. 13:
937-983.
McBreen K, Heenan PB. 2006. Phylogenetic
relationships of Pachycladon (Brassicaceae) species based on three
nuclear and two chloroplast DNA markers. – New Zealand J. Bot. 44:
377-386.
McGrath SP, Shen ZG, Zhao FJ. 1997. Heavy
metal uptake and chemical changes in the rhizosphere of Thlaspi
caerulescens and Thlaspi ochroleucum grown in contaminated soil.
– Plant and Soil 188: 153-159.
McLaughlin L. 1959. The woods and flora of
the Florida Keys: wood anatomy and phylogeny of Batidaceae. – Trop. Woods
110: 1-15.
McLean WFH, Blunden G, Jewers K. 1996.
Quaternary ammonium compounds in the Capparaceae. – Biochem. Syst. Ecol. 24:
427-434.
Macleod AJ. 1976. Volatile flavour compounds
in the Cruciferae. – In: Vaughan JG, Macleod AJ, Gómez-Campo C (eds), The
biology and chemistry of the Cruciferae, Academic press, London, pp.
307-330.
McNeill CL, Jain SK. 1983. Genetic
differentiation studies and phylogenetic inference in the plant genus
Limnanthes (section Inflexae). – Theor. Appl. Gen. 66:
257-269.
Machatschki-Laurich B. 1926. Die Arten der
Gattung Biscutella L. Sect. Thlaspidium (Med.) DC. – Bot.
Arch. 13: 1-115.
Magauer M, Schönswetter P, Jang T-S, Frajman
B. 2014. Disentangling relationships within disjunctly distributed Alyssum
ovirense/A. wulfenianum group (Brassicaceae), including description
of a novel species from the north-eastern Alps. – Bot. J. Linn. Soc. 176:
486-505.
Maheshwari Devi H. 1972. Salvadoraceae: a study of its
embryology and systematics. – J. Indian Bot. Soc. 51: 56-62.
Maheshwari P, Johri BM. 1956. The morphology
and embryology of Floerkea proserpinacoides Willd. with a discussion
on the systematic position of the family Limnanthaceae. – Bot. Mag. (Tokyo)
69: 410-423.
Maheshwari P, Khan R. 1953. Development of
the embryo sac, endosperm and embryo in Isomeris arborea – a
reinvestigation. – Phytomorphology 3: 446-459.
Malinowski E. 1910. Monographie du genre
Biscutella L. – Bull. Int. Acad. Sci. Cracovie, Cl. Sci. Math. 1910:
111-139.
Manchester SR, O’Leary EL. 2010.
Phylogenetic distribution and identification of fin-winged fruits. – Bot.
Rev. 76: 1-82.
Mandáková T, Lysak MA. 2008. Chromosomal
phylogeny and caryotype evolution in x=7 crucifer species (Brassicaceae). – Plant Cell 20:
2559-2570.
Mandáková T, Heenan PB, Lysak MA. 2010.
Island species radiation and karyotypic stasis in Pachycladon allopolyploids.
– BMC Evol. Biol. 10: 367. doi: 10.1186/1471-2148-10-367.
Mandáková T, Joly S, Krzywinski M,
Mummenhoff K, Lysak MA. 2010. Fast diploidization in close mesopolyploid
relatives of Arabidopsis. – Plant Cell 22: 2277-2290.
Mandáková T, Mummenhoff K, Al-Shehbaz IA,
Mucina L, Mühlhausen A, Lysak MA. 2012. Whole-genome triplication and species
radiation in the southern African tribe Heliophileae (Brassicaceae). – Taxon 61:
989-1000.
Manton I. 1932. Introduction to the general
cytology of the Cruciferae. – Ann. Bot. 46: 509-556.
Marais W. 1966. Notes on South African
Cruciferae. – Bothalia 9: 97-112.
Marhold K. 1995. Taxonomy of the genus
Cardamine L. (Cruciferae) in the Carpathians and Pannonia III. –
Folia Geobot. Phytotaxon. 30: 397-434.
Marhold K, Lihová J. 2006. Polyploidy,
hybridization and reticulate evolution: lessons from the Brassicaceae. – Plant Syst. Evol.
259: 143-174.
Marhold K, Huthmann M, Hurka H. 2002.
Evolutionary history of the polyploid complex of Cardamine amara (Brassicaceae): isozyme evidence. –
Plant Syst. Evol. 233: 15-28.
Marhold K, Lihová J, Perny M, Grupe R,
Neuffer B. 2002. Natural hybridization in Cardamine (Brassicaceae) in the Pyrenees:
evidence from morphological and molecular data. – Bot. J. Linn. Soc. 139:
275-294.
Marhold K, Lihová J, Perny M, Bleeker W.
2004. Comparative ITS and AFLP analysis of diploid Cardamine (Brassicaceae) taxa from closely
related polyploidy complexes. – Ann. Bot. 93: 507-520.
Martín-Bravo S, Meimberg H, Luceño M,
Märkl W, Valcárel V, Bräuchler C, Vargas P, Heubl G. 2007. Molecular
systematics and biogeography of Resedaceae based on ITS and
trnL-F sequences. – Mol. Phylogen. Evol. 44: 1105-1120.
Martín-Bravo S, Vargas P, Luceño M. 2009.
Is Oligomeris (Resedacae) indigenous to North America? Molecular
evidence for a natural colonization from the Old World. – Amer. J. Bot. 96:
507-518.
Martínez-Laborde JB. 1988a. Estudio
sistemático del género Diplotaxis DC. (Cruciferae, Brassiceae). –
Ph.D. diss., Universidad Politécnica de Madrid, Spain.
Martínez-Laborde JB. 1988b. El género
Diplotaxis (Cruciferae) en España. – Lagascalia 15 (Extra):
243-248.
Martínez-Laborde JB. 1990. On previous
misuses of the name Diplotaxis pitardiana Maire (Cruciferae:
Brassiceae). – Taxon 39: 117-118.
Martínez-Laborde JB. 1991a. Notes on the
taxonomy of Diplotaxis DC. (Brassiceae). – Bot. J. Linn. Soc. 106:
67-71.
Martínez-Laborde JB. 1991b. Diplotaxis
harra (Forskål) Boiss. in Europe. – Bot. J. Linn. Soc. 106: 97-119.
Martínez-Laborde JB. 1991c. Two additional
species of Diplotaxis DC. (Cruciferae, Brassiceae) with n = 8
chromosomes. – Willdenowia 21: 63-68.
Mason CT. 1951. Development of the embryo-sac
in the genus Limnanthes. – Amer. J. Bot. 38: 17-22.
Mason CT. 1952. A systematic study of the
genus Limnanthes R. Br. – Univ. Calif. Publ. Bot. 25: 455-511.
Mason CT. 1989. Infraspecific name changes in
Limnanthes (Limnanthaceae). – Madroño 36:
50-51.
Mateo G, Crespo MB. 2000. Three new Spanish
species of Biscutella L. (Brassicaceae) and remarks on B.
valentina (L.) Heywood. – Bot. J. Linn. Soc. 132: 1-17.
Mathews S, McBreen K. 2008. Phylogenetic
relationshps of B-related phytochromes in the Brassicaceae: redundancy and the
persistence of phytochrome D. – Mol. Phylogen. Evol. 49: 411-423.
Mathur N. 1956. The embryology of
Limnanthes. – Phytomorphology 6: 41-51.
Matile P. 1980. “Die Senfölbombe”: Zur
Kompartimentierung des Myrosinasesystems. – Biochem. Physiol. Pflanzen 175:
722-731.
Mauritzon J. 1934. Die Embryologie einiger
Capparidaceen sowie von Tovaria pendula. – Ark. f. Bot. 26A(15):
1-14.
Mayer MS, Soltis PS. 1994. The evolution of
serpentine endemics: a chloroplast DNA phylogeny of the Streptanthus
glandulosus complex (Cruciferae). – Syst. Bot. 19: 557-574.
Mayer MS, Soltis PS. 1999. Intraspecific
phylogeny analysis using ITS sequences: insights from studies of the
Streptanthus glandulosus complex (Cruciferae). – Syst. Bot. 24:
47-61.
Medve RJ. 1983. The mycorrhizal status of the
Cruciferae. – Amer. Midl. Natur. 109: 406-408.
Mehta IJ, Moseley MF Jr. 1981. The floral
anatomy of Koeberlinia Zucc.: systematic implications. – Amer. J.
Bot. 68: 482-497.
Meng J-L, Gan L. 1998. Studies on the
relationships between Moricandia and Brassica species. –
Acta Bot. Sin. 40: 508-514. [In Chinese with English summary]
Mengoni A, Baker AJM, Bazzicalupo M, Reeves
RD, Adigüzel N, Chianni E, Galardi F, Gabbrielli R, Gonelli C. 2003.
Evolutionary dynamics of nickel hyperaccumulation in Alyssum revealed
by its nrDNA analysis. – New Phytol. 159: 691-699.
Merxmüller H, Leins P. 1967. Die
Verwandschaftsbeziehungen der Kreuzblütler und Mohngewächse. – Bot. Jahrb.
Syst. 86: 113-129.
Meyer FK. 1973. Conspectus der
”Thaspi”-Arten Europas, Afrikas und Vorderasiens. – Feddes
Repert. 84: 449-470.
Meyer FK. 1979a. Kritische Revision der
”Thlaspi”-Arten Europas, Afrikas und Vorderasiens I. Geschichte,
Morphologie und Chorologie. – Feddes Repert. 90: 129-154.
Meyer FK. 1979b. Kritische Revision der
”Thlaspi”-Arten Europas, Afrikas und Vorderasiens. Spezieller
Teil. I. Thlaspi L. – Haussknechtia 8: 3-42.
Meyer FK. 1991. Seed-coat anatomy as a
character for a new classification Thlaspi. – Flora Veg. Mundi 9:
9-15.
Meyer FK. 2001. Kritische Revision der
”Thlaspi”-Arten Europas, Afrikas und Vorderasiens. Spezieller
Teil. I. Thlaspi L. – Haussknechtia 8: 3-42.
Meyer FK. 2003a. Kritische Revision der
“Thlaspi”-Arten Europas, Afrikas und Vorderasiens. Spezieller
Teil: V. Noccidium F. K. Mey. – Haussknechtia 9: 115-124.
Meyer FK. 2003b. Kritische Revision der
”Thlaspi”-Arten Europas, Afrikas und Vorderasiens. Spezieller
Teil: VI. Kotschyella F. K. Mey. – Haussknechtia 9: 125-134.
Meyer FK. 2006. Kritische Revision der
“Thlaspi”-Arten Europas, Afrikas und Vorderasiens. Spezieller
Teil: IX. Noccaea Moench. – Haussknechtia 12: 1-341.
Meyer FK. 2010. Anmerkungen zu einigen
Noccaea-Arten Nordasiens. – Haussknechtia 12: 5-18.
Miannay N. 1971. Embryologie des Crucifères.
Polyembryonie chez l’Arabis hisuta Scop. et l’Arabis
halleri L. – Bull. Soc. Bot. France 118: 341-344.
Mikolajczak KL, Miwa TK, Earle FR, Wolff IA,
Jones Q. 1961. Search for new industrial oils V. Oils of Cruciferae. – J.
Amer. Oil Chem. Soc. 38: 678-681.
Miller AG. 1978. A reassessment of the genus
Pseudocamelina. – Notes Roy. Bot. Gard. Edinburgh 36: 23-34.
Miller AG. 1984. A revision of
Ochradenus. – Notes Roy. Bot. Gard. Edinb. 41: 491-504.
Miller AG. 1988. Studies in the Flora of
Arabia XXII. Dhofaria, a new genus of Capparaceae from Oman. – Notes
Roy. Bot. Gard. Edinb. 45: 55-60.
Miller AG, Nyberg JA. 1994. Studies in the
flora of Arabia XXVII. Some new taxa from the Arabian Peninsula. – Edinburgh
J. Bot. 51: 33-47.
Miller AG, Short M, Sutton DA. 1982. A
revision of Schweinfurthia. – Notes Roy. Bot. Gard. Edinb. 40:
23-40.
Miller AG, Atkinson R, Al Khulaidi AW, Taleeb
N. 2002. Nesocrambe, a new genus of Cruciferae (Brassiceae) from
Soqotra, Yemen. – Willdenowia 32: 61-67.
Miller RW, Daxenbichler ME, Earle FR, Gentry
HS. 1964. Search for new industrial oils VIII. The genus Limnanthes.
– J. Amer. Oil Chem. Soc. 41: 167-169.
Ming D, Hellekant G. 1994. Brazzein, a new
high-potency sweet protein from Pentadiplandra brazzeana B. –
F.E.B.S. Lett. 335: 106-108.
Mirek Z. 1981. Genus Camelina in
Poland. Taxonomy, distribution and habitats. – Fragm. Flor. Geobot. 27:
445-507.
Misra RC. 1962. Contribution to the
embryology of Arabidopsis thalianum (Gay & Monn.). – Agra Univ.
J. Res. (Sci.) 11: 191-199.
Misra RC. 1966. Morphology studies on
Sisymbrium irio Linn. – J. Indian Bot. Soc. 45: 14-23.
Mitchell AD, Heenan PB. 2000. Systematic
relationships of New Zealand endemic Brassicaceae inferred from nrDNA ITS
sequence data. – Syst. Bot. 25: 98-105.
Mitchell-Olds T, Al-Shehbaz IA, Koch MA,
Sharbel TF. 2005. Crucifer evolution in the post-genomic era. – In: Henry RJ
(ed), Plant diversity and evolution: genotypic and phenotypic variation in
higher plants, CAB International, Wallingford, pp. 119-137.
Mithen R, Bennett R, Marquez J. 2010.
Glucosinolate biochemical diversity and innovation in the Brassicales. –
Phytochemistry 71: 2074-2086.
Mitra K. 1970. Pollen morphology of some
Indian Capparaceae. – J. Indian Bot. Soc. 49: 136-141.
Mitra K. 1975. Contribution to the pollen
morphology of the family Capparaceae. – Bull. Bot. Surv. India 17: 7-31.
Mitra K, Mitra SN. 1979. Pollen morphology in
relation to taxonomy and geography of Resedaceae. – Bot. Surv. India 18:
194-202.
Miyashita NT, Innan H, Terauchi R. 1996.
Intra- and interspecific variation of the alcohol dehydrogenase locus region in
wild plants Arabis gemmifera and Arabidopsis thaliana. –
Mol. Biol. Evol. 13: 433-436.
Miyashita NT, Kawabe A, Innan H, Terauchi R.
1998. Intra- and interspecific DNA variation and codon bias of the alcohol
dehydrogenase (Adh) locus in Arabis and Arabidopsis species.
– Mol. Biol. Evol. 15: 1420-1429.
Mizushima U. 1980. Genome analysis in
Brassica and allied genera. – In: Tsunoda S, Hinata K, Gómez-Campo
C (eds), Brassica crops and wild allies, Japan Scientific Societies
Press, Tokyo, pp. 89-105.
Mizushima U, Tsunoda S. 1967. A plant
exploration in Brassica and allied genera. – Tohoku J. Agric. Res.
17: 249-277.
Moazzeni H, Zarre S, Al-Shehbaz IA,
Mummenhoff K. 2007. Seed-coat microsculpturing and its systematic application
in Isatis (Brassicaceae)
and allied genera in Iran. – Flora 202: 447-454.
Moazzeni H, Zarre S, Al-Shehbaz IA,
Mummenhoff K. 2010. Phylogeny of Isatis (Brassicaceae) and allied genera based
on ITS sequences of nuclear ribosomal DNA and morphological characters. –
Flora 205: 337-343.
Moazzeni H, Zarre S, Pfeil BE, Bertrand YJK,
German DA, Al-Shehbaz IA, Mummenhof K, Oxelman B. 2014. Phylogenetic
perspectives on diversification and character evolution in the species-rich
genus Erysimum (Erysimeae; Brassicaceae) based on a densely
sampled ITS approach. – Bot. J. Linn. Soc. 175: 497-522.
Moggi G. 1965. Osservazioni tassonomiche e
corologiche sulle Hesperideae (Cruciferae). – Webbia 20: 241-273.
Moghe GD, Hufnagel DE, Tang HB, Xiao YL,
Dworkin I, Town CD, Conner JK, Shiu SH. 2014. Consequences of whole-genome
triplication as revealed by comparative genomic analyses of the wild radish
Raphanus raphanistrum and three other Brassicaceae species. – Plant Cell
26: 1925-1937.
Mohammadin S, Peterse K, van de Kerke SJ,
Chatrou LW, Dönmez AA, Mummenhoff K, Pires JC, Edger PP, Al-Shehbaz IA,
Schranz ME. 2107. Anatolian origins and diversification of Aethionema,
the sister lineage of the core Brassicaceae. – Amer. J. Bot. 104:
1042-1054.
Molloy BPJ, Edgar E, Heenan PB, De Lange PJ.
1999. New species of Poa (Gramineae) and Ischnocarpus (Brassicaceae) from limestone, North
Otago, South Island, New Zealand. – New Zealand J. Bot. 37: 41-50.
Morant AV, Bjarnholt N, Kragh ME, Kjærgaard
CH, Jørgensen K, Paquette SM, Piotrowski P, Imberty A, Olsen CE, Møller BL,
Back S. 2008. The β-glucosidases responsible for bioactivation of
hydroxynitrile glucosides in Lotus japonicus. – Plant Physiology
147: 1072-1091.
Mukherjee P. 1973. Chromosome study as an aid
in tracing the evolution of Cruciferae. – Cytologia 40: 727-734.
Müller A. 1961. Zur Charakterisierung der
Blüten und Infloreszenzen von Arabidopsis thaliana (L.) Heynh. –
Kulturpflanze 9: 364-393.
Mulligan GA. 1961. Chromosome numbers of the
family Cruciferae III. – Can. J. Bot. 39: 1057-1066.
Mulligan GA. 1964. Chromosome numbers of the
family Cruciferae I. – Can. J. Bot. 42: 1509-1519.
Mulligan GA. 1970a. Cytotaxonomic studies of
Draba glabella and its close allies in Canada and Alaska. – Can. J.
Bot. 48: 1431-1437.
Mulligan GA. 1970b. A new species of
Draba in the Kananaskis range of southwestern Alberta. – Can. J.
Bot. 48: 1897-1898.
Mulligan GA. 1971a. Cytotaxonomic studies of
the closely allied Draba cana, D. cinerea and D.
groenlandica in Canada and Alaska. – Can. J. Bot. 49: 89-93.
Mulligan GA. 1971b. Cytotaxonomic studies in
Draba species of Canada and Alaska: D. ventosa, D.
ruaxes and D. paysonii. – Can. J. Bot. 49: 1455-1460.
Mulligan GA. 1972. Cytotaxonomic studies of
Draba species in Canada and Alaska: D. oligosperma and D.
incerta. – Can. J. Bot. 50: 1763-1766.
Mulligan GA. 1974. Cytotaxonomic studies of
Draba nivalis and its close allies in Canada and Alaska. – Can. J.
Bot. 52: 1793-1801.
Mulligan GA. 1976. The genus Draba
in Canada and Alaska: key and summary. – Can. J. Bot. 54: 1386-1393.
Mulligan GA. 1996. Synopsis of the genus
Arabis (Brassicaceae) in
Canada, Alaska and Greenland. – Rhodora 97: 109-163.
Mulligan GA,. Calder JA. 1964. The genus
Subularia (Cruciferae). – Rhodora 66: 127-135.
Mulligan GA, Findlay JN. 1970. Sexual
reproduction and agamospermy in the genus Draba. – Can. J. Bot. 48:
269-270.
Mummenhoff K. 1989. Sippenstrukturen in der
Gattung Lepidium L. (Brassicaceae): isoelektrische
Fokussierungsmuster der Untereinheiten von Ribulose-1,5-Bisphosphate
Carboxylase/Oxygenase (RUBISCO) als systematischer und phylogenetischer Marker.
– PhD. diss., Universität Osnabrück, Germany.
Mummenhoff K. 1995. Should Cardaria
(L.) Desv. be classified within the genus Lepidium L. (Brassicaceae)? Evidence from subunit
polypeptide composition of RUBISCO. – Feddes Repert. 106: 25-28.
Mummenhoff K, Hurka H. 1991. Isoelectric
focusing analysis of Rubisco in Lepidium (Brassicaceae), sections
Lepia, Lepiocardamon and Cardamon. – Biochem.
Syst. Ecol. 19: 47-52.
Mummenhoff K, Hurka H. 1994. Subunit
polypeptide composition of rubisco and the origin of allopolyploid
Arabidopsis suecica (Brassicaceae). – Biochem. Syst.
Ecol. 22: 807-812.
Mummnhoff K, Hurka H. 1995. Allopolyploid
origin of Arabidopsis suecica (Fries) Norrlin – evidence from
chloroplast and nuclear genome markers. – Bot. Acta 108: 449-456.
Mummenhoff K, Koch M. 1994. Chloroplast DNA
restriction site variation and phylogenetic relationships in the genus
Thlaspi sensu lato (Brassicaceae). – Syst. Bot. 12:
73-88.
Mummenhoff K, Zunk K. 1991. Should
Thlaspi (Brassicaceae) be
split? Preliminary evidence from isoelectric focusing analysis of Rubisco. –
Taxon 40: 427-434.
Mummenhoff K, Kunth E, Koch M, Zunk K. 1995.
Systematic implications of chloroplast DNA variation in Lepidium
sections Cardamon, Lepiocardamon and Lepia (Brassicaceae). – Plant Syst. Evol.
196: 75-88.
Mummenhoff K, Franzke A, Koch M. 1997a.
Molecular data reveal convergence in fruit characters used in the
classification of Thlaspi s.l. (Brassicaceae). – Bot. J. Linn. Soc.
125: 183-199.
Mummenhoff K, Franzke A, Koch M. 1997b.
Molecular phylogenetics of Thlaspi s.l. (Brassicaceae) based on chloroplast
DNA restriction site variation and sequences of the internal transcribed
spacers of nuclear ribosomal DNA. – Can. J. Bot. 75: 469-482.
Mummenhoff K, Brüggemann H, Bowman JL. 2001.
Chloroplast DNA phylogeny and biogeography of Lepidium (Brassicaceae). – Amer. J. Bot. 88:
2051-2063.
Mummenhoff K. Coja U, Brüggemann H. 2001.
Pachyphragma and Gagria (Brassicaceae) revisited: molecular
data indicate close relationship to Thlaspi s. str. – Folia Geobot.
Phytotaxon. 36: 293-302.
Mummenhoff K, Linder P, Friesen N, Bowman JL,
Lee JY, Franzke A. 2004. Molecular evidence for bicontinental hybridogenous
genome constitution in Lepidium sensu stricto (Brassicaceae) species from Australia
and New Zealand. – New Zealand J. Bot. 91: 254-261.
Mummenhoff K, Al-Shehbaz IA, Bakker FT,
Linder HP, Mühlhausen A. 2005. Phylogeny, morphological evolution, and
speciation of endemic Brassicaceae
genera in the Cape flora of southern Africa. – Ann. Missouri Bot. Gard. 92:
400-424.
Mummenhoff K, Polster A, Muhlhausen A,
Theissen G. 2009. Lepidium as a model system for studying the
evolution of fruit development in Brassicaceae. – J. Exper. Bot. 60:
1503-1513.
Murley MR. 1951. Seeds of the Cruciferae of
northeastern North America. – Amer. Midl. Natur. 46: 1-81.
Murty YS. 1953. A contribution to the anatomy
and morphology of normal and some abnormal flowers of Gynandropsis
gynandra (L.) Briq. – J. Indian Bot. Soc. 32: 108-122.
Muschler R. 1908. Die Gattung
Coronopus (L.) Gaertn. – Engl. Bot. Jahrb. 41: 111-147.
Musil AF. 1948. Distinguishing the species of
Brassica by their seed. – Misc. Publ. U.S. Dept. Agric. 643.
Mutlu B. 2004. A new species of
Arabis (Brassicaceae)
from inner Anatolia. – Bot. J. Linn. Soc. 145: 251-256.
Nabiev MM. 1972. Botschantzevia
Nabiev – genus novum cruciferarum. – Nov. Sist. Vysš. Rast. 9: 186-187.
Nagaharu U. 1935. Genome analysis in
Brassica with special reference to the experimental formation of
B. napus and peculiar mode of fertilization. – Jap. J. Bot. 7:
389-452.
Nagpal R, Dar TH, Raina SN. 2008. Molecular
systematics of Brassica and allied genera in subtribes Brassicinae,
Raphaninae, Moricandiinae, and Cakilinae (Brassicaceae, tribe Brassiceae); the
organization and evolution of ribosomal gene families. – Bot. J. Linn. Soc.
157: 545-557.
Naqshi AR, Javied GN. 1984. Drabopsis
brevisiliqua (Cruciferae) – a new species from Kashmir. – J. Econ.
Taxon. Bot. 5: 963-966.
Narayana HS. 1962. Studies in the
Capparidaceae I. The embryology of Capparis decidua (Forssk.) Pax. –
Phytomorphology 12: 167-177.
Narayana HS. 1965. Studies in the
Capparidaceae II. Floral morphology and embryology of Cadaba indica
Lamk. and Crataeva nurvala Buch.-Ham. – Phytomorphology 15:
138-175.
Narayana HS. 1970. Comparative embryology of
angiosperms: Capparaceae, Moringaceae. – Bull. Natl. Sci.
Acad. India 41: 78-80, 81-83.
Narayana LL. 1962. Postfertilization study on
Moringa oleifera Lamk. – a reinvestigation. – Phytomorphology 12:
65-69.
Narayana LL. 1970a. Moringaceae. – Bull. Indian Natl.
Sci. Acad. 41: 81-83.
Narayana LL. 1970b. Tropaeolaceae. – Bull. Indian
Natl. Sci. Acad. 41: 121-122.
Narayana LL, Parvathi A. 1980. Chemotaxonomy
of Capparidaceae. – Phyta 2-3: 87-91.
Nasrallah JB. 2011. Self-incompatibility in
the Brassicaceae. – In: Schmidt
R, Bancroft I (eds), Genetics and genomics of the Brassicaceae, Springer, Berlin, pp.
389-411.
Neuffer B, Janche P. 1997. RAPD analysis of
hybridization events in Cardamine (Brassicaceae). – Folia Geobot.
Phytotaxon. 32: 57-67.
Neutofal F. 1927. Zytologische Studien über
die Kulturrassen von Brassica oleracea. – Österr. Bot. Zeitschr.
76: 105-115.
Nicola GR de, Nyegue M, Montaut S, Iori R,
Menut C, Tatibouët A, Rollin P, Ndoyé C, Zollo P-HA. 2012. Profile and
quantification of glucosinolates in Pentadiplandra brazzeana Baillon.
– Phytochemistry 73: 51-56.
Nordal I, Stabbetorp OE. 1990. Morphology and
taxonomy of the genus Cochlearia (Brassicaceae) in Northern
Scandinavia. – Nord. J. Bot. 10: 249-263.
Nordal I, Eriksen AB, Laane MM, Solberg Y.
1986. Biosystematic and biogeographic studies in the genus Cochlearia
in Northern Scandinavia. – Symb. Bot. Ups. XXVII(2): 149-159.
Nyárády EJ. 1928a. Vorstudium über einige
Arten der Section Odontarrhena generis Alyssum. – Bul. Grǎd. Bot. Univ. Cluj
7: 1-51, 65-160.
Nyárády EJ. 1928b. Vorstudium über einige
Arten der Section Odontarrhena generis Alyssum. – Bul. Grǎd. Bot. Univ. Cluj
8: 152-156.
Nyárády EJ. 1929. Vorstudium über einige
Arten der Section Odontarrhena generis Alyssum. – Bul. Grǎd. Bot. Univ. Cluj
9: 1-68.
O’Kane SL Jr, Al-Shehbaz IA. 1997. A
synopsis of Arabidopsis (Brassicaceae). – Novon 7:
323-327.
O’Kane SL Jr, Al-Shehbaz IA. 2002.
Paysonia, a new genus segregated from Lesquerella (Brassicaceae). – Novon 12:
379-381.
O’Kane SL Jr, Al-Shehbaz IA. 2003.
Phylogenetic position and generic limits of Arabidopsis (Brassicaceae) based on sequences of
nuclear ribosomal DNA. – Ann. Missouri Bot. Gard. 90: 603-612.
O’Kane SL Jr, Al-Shehbaz IA. 2004. The
genus Physaria (Brassicaceae) in South America. –
Novon 14: 198-207.
O’Kane SL Jr, Reveal JL. 2006. Physaria
pulvinata (Brassicaceae), a
new species from southwestern Colorado. – Brittonia 58: 74-77.
O’Kane SL Jr, Schaal BA, Al-Shehbaz IA.
1996. The origins of Arabidopsis suecica (Brassicaceae) as indicated by nuclear
rDNA sequences. – Syst. Bot. 21: 559-566.
Oliveira JTA, Silveira SB, Vasconcelos IM,
Cavada BS, Moreira RA. 1999. Compositional and nutritional attributes of seeds
from the multiple purpose tree Moringa oleifera Lamarck. – J. Sci.
Food Agric. 79: 815-820.
Olowokudejo JD. 1980. Systematic studies in
the genus Biscutella L. – Ph.D. diss., University of Reading,
England.
Olowokudejo JD. 1985. Scanning electron
microscopy of fruits in the genus Biscutella L. (Cruciferae). –
Phytomorphology 34: 273-286.
Olowokudejo JD. 1986. The taxonomic
importance of nectary variation in the genus Biscutella L. – Feddes
Repert. 97: 837-845.
Olowokudejo JD, Heywood VH. 1984.
Cytotaxonomy and breeding system of the genus Biscutella (Cruciferae).
– Plant Syst. Evol. 145: 291-309.
Olowokudejo JD, Heywood VH. 1995. Taxonomic
study of the Biscutella variegata complex (Cruciferae). –
Willdenowia 25: 25-38.
Olson ME. 2001a. Morphological and
ontogenetic diversity and phylogenetic relationships of Moringa (Moringaceae, Brassicales). – Ph.D.
diss., Graduate School of Arts and Sciences, Washington University, St. Louis,
Missouri.
Olson ME. 2001b. Stem and root anatomy in
Moringa (Moringaceae). –
Haseltonia 8: 56-96.
Olson ME 2002a. Intergeneric relationships
within the Caricaceae-Moringaceae clade (Brassicales), and
potential morphological synapomorphies of the clade and its families. –
Intern. J. Plant Sci. 163: 51-65.
Olson ME. 2002b. Combining data from DNA
sequences and morphology for a phylogeny of Moringaceae (Brassicales). – Syst.
Bot. 27: 55-73.
Olson ME. 2002c. Wood and bark anatomy in
Moringa (Moringaceae). –
Haseltonia 8: 85-121.
Olson ME. 2003. Ontogenetic origins of floral
bilateral symmetry in Moringaceae
(Brassicales). – Amer. J. Bot. 90: 49-71.
Olson ME. 2007. Wood ontogeny as a model for
studying heterochrony, with an example of paedomorphosis in Moringa
(Moringaceae). – Syst. Biodivers.
5: 145-158.
Olson ME, Carlquist SJ. 2001. Stem and root
anatomical correlations with life form diversity, ecology, and systematics in
Moringa (Moringaceae). –
Bot. J. Linn. Soc. 135: 315-348.
Olson ME, Razafimandimbison SG. 2000.
Moringa hildebrandtii: a tree extinct in the wild but preserved by
indigenous horticultural practices in Madagascar. – Adansonia, sér. III, 22:
217-221.
Olson ME, Rosell JA. 2006. Using heterochrony
to detect modularity in the evolution of stem diversity in the plant family Moringaceae. – Evolution 2006:
724-734.
Oltmann O. 1971.
Pollenmorphologisch-systematische Untersuchungen innerhalb der Geraniales. – Diss. Bot.
11: 127-129.
Ornduff R. 1971. Systematic studies of Limnanthaceae. – Madroño 21:
103-111.
Ornduff R, Crovello TJ. 1968. Numerical
taxonomy of Limnanthaceae. –
Amer. J. Bot. 55: 173-182.
Orr MY. 1919. The occurrence of tracheal
tissue enveloping the embryo in certain Capparidaceae. – Notes Roy. Bot.
Gard. Edinb. 11: 249-257.
Outer RW den, Veenendal WLH van. 1981. Wood
and bark anatomy of Azima tetracantha Lam. (Salvadoraceae) with description of
its included phloem. – Acta Bot. Neerl. 30: 199-207.
Özüdoğru B, Akaydın G, Erik S, Al-Shehbaz
IA, Mummenhoff K. 2015. Phylogeny, diversification and biogeographic
implications of the eastern Mediterranean endemic genus Ricotia (Brassicaceae). – Taxon 64:
727-740.
Özüdoğru B, Al-Shehbaz IA, Mummenhoff K.
2017. Tribal assignment of Heldreichia Boiss. (Brassicaceae): evbidence from nuclear
ITS and plastidic ndhF markers. – Plant Syst. Evol. 303: 329-335.
Paliwal GS. 1967. Ontogeny of stomata in some
Cruciferae. – Can. J. Bot. 45: 495-500.
Palmer JD, Shields CR, Cohen DB, Orton TJ.
1983. Chloroplast DNA evolution and the origin of amphiploid Brassica.
– Theor. Appl. Gen. 65: 181-189.
Pant DD, Kidwai PF. 1967. Development of
stomata in some Cruciferae. – Ann. Bot., N. S., 31: 513-521.
Parker WH. 1976. Comparison of numerical
taxonomic methods used to estimate flavonoid similarities in the Limnanthaceae. – Brittonia 28:
390-399.
Parker WH, Bohm BA. 1979. Flavonoids and
taxonomy of the Limnanthaceae.
– Amer. J. Bot. 66: 191-197.
Parolly G, Hein P. 2000. Arabis
lycia (Cruciferae), a new chasmophyte from the Taurus Mts, Turkey, and
notes on related species. – Willdenowia 30: 293-304.
Parolly G, Nordt B, Bleeker W, Mummenhoff K.
2010. Heldreichia Boiss. (Brassicaceae) revisited: a
morphological and molecular study. – Taxon 59: 187-202.
Patchell MJ, Bolton MC, Mankowski P, Hall JC.
2011. Comparative floral development in Cleomaceae reveals two distinct
pathways leading to monosymmetry. – Intern. J. Plant Sci. 172: 352-365.
Patchell MJ, Roalson EH, Hall JC. 2014.
Resolved phylogeny of Cleomaceae based on all three genomes. – Taxon 63:
315-328.
Pavlova D. 2007. A new species of
Aethionema (Brassicaceae)
from the Bulgarian flora. – Bot. J. Linn. Soc. 155: 533-540.
Pax F. 1887. Beiträge zur Kenntnis der
Capparidaceen. – Engl. Bot. Jahrb. Syst. 9: 39-77.
Pax F. 1891a. Tovariaceae. – In: Engler A, Prantl
K (eds), Die natürlichen Pflanzenfamilien III(2), W. Engelmann, Leipzig, pp.
207-208.
Pax F. 1891b. Capparidaceae. – In: Engler
A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(2), W. Engelmann,
Leipzig, pp. 209-236.
Pax F. 1891c. Moringaceae. – In: Engler A, Prantl
K (eds), Die natürlichen Pflanzenfamilien III(2), W. Engelmann, Leipzig, pp.
242-244.
Pax F. 1891d. Cleomodendron, eine
neue Gattung der Capparidaceen aus Somaliland. – Ber. Deutsch. Bot. Ges. 9:
32-34.
Pax F. 1936a. Moringaceae. – In: Engler A (†),
Harms H (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 17b, W.
Engelmann, Leipzig, pp. 693-698.
Pax F. 1936b. Bretschneideraceae. – In:
Engler A (†), Harms H (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd.
17b, W. Engelmann, Leipzig, pp. 699-700.
Pax F, Hoffmann K. 1936a. Capparidaceae. –
In: Engler A (†), Harms H (eds), Die natürlichen Pflanzenfamilien, 2. Aufl.,
Bd. 17b, W. Engelmann, Leipzig, pp. 146-223.
Pax F, Hoffmann K. 1936b. Tovariaceae. – In: Engler A (†),
Harms H (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 17b, W.
Engelmann, Leipzig, pp. 224-226.
Payne AM, Maun MA. 1981. Dispersal and
floating ability of dimorphic fruit segments of Cakile edentula var.
lacustris. – Can. J. Bot. 59: 2595-2602.
Payson EB. 1923. A monographic study of the
genus Thelypodium and its immediate allies. – Ann. Missouri Bot.
Gard. 9: 233-324.
Pepper AE, Norwood LE. 2001. Evolution of
Caulanthus amplexicaulis var. barbarae (Brassicaceae), a rare serpentine
endemic plant: a molecular phylogenetic perspective. – Amer. J. Bot. 88:
1479-1489.
Periasamy K, Indira C. 1986. The carpel of
Moringa. – Ann. Bot., N. S., 58: 897-901.
Perkins J. 1909. Resedaceae Africae tropicae. – In:
Engler A (ed), Beiträge zur Flora von Afrika XXXV, Bot. Jahrb. Syst. 43:
415-418.
Perný M, Tribsch A, Stuessy TF, Marhold K.
2005a. Allopolyploid origin of Cardamine silana (Brassicaceae) from Calabria (southern
Italy): karyological, morphological and molecular evidence. – Bot. J. Linn.
Soc. 148: 101-116.
Perný M, Tribsch A, Stuessy TF, Marhold K.
2005b. Taxonomy and cytogeography of Cardamine raphanifolia and C.
gallaecica (Brassicaceae) in
the Iberian Peninsula. – Plant Syst. Evol. 254: 69-91.
Persson J. 1971. Studies in the Aegean flora
XIX. Notes on Alyssum and some other genera of Cruciferae. – Bot.
Not. 124: 399-418.
Perveen A, Qaiser M. 1996. Pollen flora of
Pakistan VI. Salvadoraceae. –
Pak. J. Bot. 28: 151-154.
Perveen A, Qaiser M. 2001a. Pollen flora of
Pakistan XXVIII. Resedaceae. –
Turk. J. Bot. 25: 39-42.
Perveen A, Qaiser M. 2001b. Pollen flora of
Pakistan XXXI. Capparidaceae. – Turk. J. Bot. 25: 389-395.
Perveen A, Qaiser M. 2009. Pollen flora of
Pakistan LXIII. Moringaceae. –
Pak. J. Bot. 41: 987-989.
Pestalozzi A. 1898. Die Gattung
Boscia Lam. – Bull. Herb. Boissier 6: 1-152.
Phillips BE, Smith CR Jr, Tallent WH. 1971.
Glycerides of Limnanthes douglasii seed oil. – Lipids 6: 93-99.
Phitos D. 1970. Die Gattung Aubrieta
in Griechenland. – Candollea 25: 69-87.
Pillai A, Pillai SK. 1977. Some aspects of
the anatomy of Salvadora oleoides Dcne. – Flora 166: 211-218.
Platonova TF, Kusovkov AD. 1963. Ob
alkaloidach ložečnicy arktičeskoj Cochlearia arctica Schlechtd. –
Med. Ind. UdSSR 17: 19-20.
Ploch S, Thines M. 2011. Obligate biotrophic
pathogens of the genus Albugo are widespread as asymptotic endophytes
in natural populations of Brassicaceae. – Mol. Ecol. 20:
3692-3699.
Ploch S, Choi Y-J, Rost C, Shin H-D,
Schilling E, Thines M. 2010. Evolution of diversity in Albugo is
driven by high host specificity and multiple speciation events on closely
related Brassicaceae. – Mol.
Phylogen. Evol. 57: 812-820.
Pobedimova E. 1968. Species novae generis
Cochlearia. – Nov. Syst. Plant. Vasc. (Leningrad) 5: 130-135.
Pobedimova E. 1970. Revisio generis
Cochlearia L. 1. – Nov. Syst. Plant. Vasc. (Leningrad) 6: 67-106.
Pobedimova E. 1971. Revisio generis
Cochlearia L. 2. – Nov. Syst. Plant. Vasc. (Leningrad) 7:
167-195.
Pohle R. 1925. Drabae asiaticae.
Systematik und Geographie nord- und mittelasiatischer Draben. – Feddes
Repert. 32: 1-225.
Polatschek A. 1967. Cytotaxonomische
Beiträge zu den Gattungen Thlaspi und Hutchinsia. – Ann.
Naturhist. Mus. Wien 70: 29-35.
Polatschek A. 1976. Die Gattung
Erysimum auf den Kapverden, Kanaren und Madeira. – Ann. Naturhist.
Mus. Wien 80: 93-103.
Polatschek A. 1983. Chromosomenzahlen und
Hinweise auf Systematik und Verbreitung von Brassicaceae-Arten aus Europa,
Nordafrika, Asien und Australien. – Phyton 28: 127-139.
Polatschek A. 1994. Nomenklatorischer Beitrag
zur Gattung Erysimum (Brassicaceae). – Phyton 34:
189-202.
Pollock EG, Jensen W. 1967. Ontogeny and
cytochemistry of chalazal proliferating cells of Capsella
bursa-pastoris (L.) Medic. – New Phytol. 66: 413-417.
Polowick PL, Sawhney VK. 1986. A scanning
electron microscopic study on the initiation and development of floral organs
Brassica napus (cv. Westar). – Amer. J. Bot. 73: 254-263.
Polster A. 2005. The role of lignification
patterns in dehiscent and indehiscent fruits in Brassicaceae: a comparative
anatomical approach. – M.Sc. thesis, Universität Osnabrück, Germany.
Ponzi R, Pizzolongo P, Caputo G. 1978.
Ultrastructural particularities in ovular tissues of some Rhoeadales taxa and
their probable taxonomic value. – J. Submicr. Cytol. 10: 81-88.
Poulsen VA. 1878. Det ekstraflorale nektarium
hos Capparis cyanophallophora. – Vidensk. Medd. Nat. København 31:
35-44.
Pradhan AK, Prakash S, Mukhopadhyay A, Pental
D. 1992. Phylogeny of Brassica and allied genera based on variation
and mitochondrial DNA patterns: molecular and taxonomic classifications are
incongruous. – Theor. Appl. Gen. 85: 331-340.
Prakash S, Hinata K. 1980. Taxonomy,
cytogenetics, and origin of crop Brassica, a review. – Opera Bot.
55: 1-57.
Prantl K. 1891. Cruciferae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien III(2), W. Engelmann,
Leipzig, pp. 145-206.
Prasad K. 1974. Studies in the Cruciferae:
gametophytes, structure and development of seed in Eruca sativa Mill.
– J. Indian Bot. Soc. 53: 24-33.
Prasad K. 1975. Development and organization
of gametophytes in certain species of Cruciferae. – Acta Bot. Indica 3:
147-154.
Prasad K. 1976. Seed coat structure and
development in certain species of Cruciferae. – Intern. Quart. J. Plant Sci.
Res. 3: 95-103.
Prasad K. 1977a. Seed coat structure and
development in Lepidium sativum L. and Thlaspi perfoliatum L.
(Cruciferae). – Bot. Jahrb. Syst. 97: 508-514.
Prasad K. 1977b. The development and
structure of basal body in the ovule and seed of certain species of Cruciferae.
– Bot. Jahrb. Syst. 98: 266-272.
Prasad K. 1979. Morphology and histochemistry
of the nucellus and endosperm of Cruciferae. – Bot. Jahrb. Syst. 100:
536-541.
Preston RE. 1986. Pollen-ovule ratios in the
Cruciferae. – Amer. J. Bot. 73: 1732-1740.
Preston RE. 1991. The intrafloral phenology
of Streptanthus tortuosus (Brassicaceae). – Amer. J. Bot. 78:
1044-1053.
Price RA, Rollins RC. 1991. New taxa of
Draba (Cruciferae) from California, Nevada, and Colorado. – Harvard
Pap. Bot. 3: 71-77.
Price RA, Palmer JD, Al-Shehbaz IA. 1994.
Systematic relationships of Arabidopsis: a molecular and morphological
perspective. – In: Meyerowitz E, Somerville C (eds), Arabidopsis,
Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, pp. 7-19.
Price RA, Al-Shehbaz IA, O’Kane SL Jr.
2001. Beringia (Brassicaceae), a new genus of
Arabidopsoid affinities from Russia and North America. – Novon 11:
332-336.
Price RA, Bailey CD, Al-Shehbaz IA. 2001.
Transfer of the cupulate-flowered Arabis microsperma and A.
tricornuta (Brassicaceae) to
Pennellia. – Novon 11: 337-340.
Prijanto B. 1970. Batidaceae, Gyrostemonaceae. – In: Erdtman G
(ed), World Pollen and Spore Flora 3: 1-15.
Prina AO. 2000. A taxonomic revision of
Crambe, sect. Leptocrambe (Brassicaceae). – Bot. J. Linn. Soc.
133: 509-524.
Prina AO. 2001. Nuevas combinaciones en
Menonvillea (Brassicaceae). – Hickenia 3:
93-94.
Prina AO, Martinez-Laborde JB. 2008. A
taxonomic revision of Crambe section Dendrocrambe (Brassicaceae). – Bot. J. Linn. Soc.
156: 291-304.
Pritchard GG. 1957. Experimental taxonomic
studies on species of Cardamine Linn. in New Zealand. – Trans. Roy.
Soc. New Zealand 85: 75-89.
Propach H. 1934. Cytologische Untersuchungen
an Limnanthes douglasii R. Br. – Zeitschr. Zellforsch. 21:
357-375.
Puri V. 1934. A note on the embryo sac and
embryo of Moringa oleifera Lamk. – Proc. Indiana Acad. Sci., Ser. B,
1: 279-282.
Puri V. 1941a. Studies in floral anatomy I.
Gynoecium constitution in the Cruciferae. – Proc. Indian Acad. Sci., Sect. B,
14: 166-187.
Puri V. 1941b. The life-history of
Moringa oleifera Lam. – J. Indian Bot. Soc. 20: 263-284.
Puri V. 1942. Studies in floral anatomy II.
Floral anatomy of the Moringaceae
with special reference to gynoecium constitution. – Proc. Indian Natl. Inst.
Sci., Sect. B, 8: 71-88.
Puri V. 1950. Studies in floral anatomy VI.
Vascular anatomy of the flower of Crataeva religiosa Forst., with
special reference to the nature of the carpels in the Capparidaceae. – Amer.
J. Bot. 37: 363-370.
Quiros CF, Ochoa O, Douches DS. 1988.
Exploring the role of x = 7 species in Brassica evolution:
hybridization with B. nigra and B. oleracea. – J. Hered.
79: 351-358.
Rachmilevitz T, Fahn A. 1975. The floral
nectary of Tropaeolum majus L. The nature of the secretory cells and
the manner of nectar secretion. – Ann. Bot., N. S., 39: 721-728.
Raffaelli M. 1992. Biscutella sect.
Iondraba (Cruciferae) in the Mediterranean area. – Willdenowia 22:
19-36.
Raghavan TS. 1937. Studies on the
Capparidaceae I. The life-history of Cleome cholidonii Linn. fil. –
Bot. J. Linn. Soc. 51: 43-72.
Raghavan TS. 1938. Morphological and
cytological studies in the Capparidaceae II. Floral morphology and cytology of
Gynandropsis pentaphylla DC. – Ann. Bot., N. S., 2: 75-95.
Raghavan TS. 1939. Studies on the
Capparidaceae II. Floral anatomy and some structural features of the
capparidaceous flower. – Bot. J. Linn. Soc. 52: 239-257.
Raghavan TS, Venkatasubban KR. 1941a. Studies
in the Capparidaceae V. The floral morphology of Crataeva religiosa
Forst. – Beih. Bot. Centralbl. 60: 388-396.
Raghavan TS, Venkatasubban KR. 1941b. Studies
in the Capparidaceae VI. Floral ontogeny and anatomy of Crataeva
religiosa with special reference to the morphology of the carpel. –
Beih. Bot. Centralbl. 60: 397-416.
Raghavan TS, Venkatasubban KR. 1941c. Studies
in the Capparidaceae VII. The cytology of Capparis zeylanica Linn. and
related genera. – Cytologia 11: 319-331.
Rama Devi D. 1990a. Chemotaxonomy of Tropaeolaceae. – Indian J. Bot.
13: 136-141.
Rama Devi D. 1990b. Floral anatomy of Limnanthaceae. – J. Indian Bot.
Soc. 69: 271-274.
Rama Devi D, Narayana LL. 1994. Floral
anatomy of Tropaeolaceae. –
Feddes Repert. 105: 437-443.
Ranjbar M, Karami S, Rostami M. 2014. A
revision of Fibigia sect. Purpureae (Brassicaceae, Alysseae) in Iran, and
the description of three new species. – Biol. Div. Cons. 7: 32-43.
Rao AVN. 1967. Embryological studies in
Cleome monophylla Linn. – Proc. Indian Acad. Sci., Sect. B, 65:
249-256.
Rao NV, Avita S, Inambar JA. 1983. Studies on
the Moringaceae. – Feddes Repert.
94: 213-223.
Rao PSP, Rao BH. 1976. Embryo development in
Cleome aspera L. (Capparidaceae). – J. Jap. Bot. 51: 110-117.
Rao TA, Kelkar SS. 1951. Studies on foliar
sclereids in dicotyledons III. On sclereids in species of Loranthus
(Loranthaceae) and
Niebuhria apetala (Capparidaceae). – J. Univ. Bombay, N. S., B 20:
16-20.
Rao VS. 1938. Studies on Capparidaceae III.
Genus Capparis. – J. Indian Bot. Soc. 17: 69-80.
Rask L, Andréasson E, Ekbom E, Eriksson S,
Pontoppidan B, Meijer J. 2000. Myrosinase: gene family evolution and herbivore
defense in Brassicaceae. – Plant
Mol. Biol. 42: 93-113.
Rathore RKS. 1972. Structure and development
of seeds in Farsetia hamiltonii Royle. – Agra Univ. J. Res. (Sci.)
21: 13-17.
Rathore RKS, Singh RP. 1968. Embryological
studies in Brassica campestris L. var. Yellow Sarson Prain. – J.
Indian Bot. Soc. 47: 341-349.
Ravenna P. 1981. Taxonomical notes on the
Chilean Cruciferae. – Nord. J. Bot. 1: 140-142.
Rechinger KH. 1951. Cruciferae iranicae novae
vel minus cognitae. – Phyton 3: 44-68.
Record S. 1926. The wood of Koeberlinia
spinosa Zuccarini. – Trop. Woods 8: 15-17.
Reeves RD. 1988. Nickel and zinc accumulation
by species of Thlaspi L., Cochlearia L. and other genera of
the Brassicaceae. – Taxon 37:
309-318.
Reeves RD, Brooks RR, Macfarlane RM. 1981.
Nickel uptake by Californian Streptanthus and Caulanthus with
particular reference to the hyperaccumulator S. polygaloides Gray (Brassicaceae). – Amer. J. Bot. 68:
708-712.
Reeves RD, Brooks RR, Dudley TR. 1983. Uptake
of nickel by species of Alyssum, Bornmüllera and other
genera of Old World tribus Alysseae. – Taxon 32: 184-192.
Regvar M, Vogel K, Irgel N, Wraber T,
Hildbrandt U, Wilde P, Bothe H. 2003. Colonization of pennycresses
(Thlaspi spp.) of the Brassicaceae by arbuscular
mycorrhizal fungi. – J. Plant Physiol. 160: 615-626.
Reiche K. 1896a. Tropaeolaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien III(4), W. Engelmann,
Leipzig, pp. 23-27.
Reiche K. 1896b. Limnanthaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien III(5), W. Engelmann,
Leipzig, pp. 136-137.
Rešetnik I, Satovic Z, Schneeweiss GM, Liber
Z. 2013. Phylogenetic relationships in Brassicaceae tribe Alysseae inferred
from nuclear ribosomal and chloroplast DNA sequence data. – Mol. Phylogen.
Evol. 69: 772-786.
Rešetnik I, Schneeweiss GM, Liber Z. 2014.
Two new combinations in the genus Bornmuellera (Brassicaceae). – Phytotaxa 159:
298-300.
Reza Khosravi A, Mohsenzadeh S, Mummenhoff K.
2008a. Analysis of the phylogenetic position of Acanthocardamum
erinaceum (Brassicaceae)
based on ITS-sequences shows that it should be transferred to
Aethionema as A. erinaceum. – Nord. J. Bot. 26: 25-30.
Reza Khosravi A, Mohsenzadeh S, Mummenhoff K.
2008b. Phylogenetic position of Brassardia papyracea (Brassicaceae) based on sequences of
nuclear ribosomal DNA. – Feddes Repert. 119: 13-23.
Ricardi M, Marticorena C, Torres F. 1957.
Nota preliminar de los pólenes de Tropaeolaceae chilenas. – Bol.
Soc. Biol. Concepción 32: 17-19.
Riley R. 1956. The influence of the breeding
system on the genecology of Thlaspi alpestre L. – New Phytol. 55:
319-330.
Riser II JP, Cardinal-McTegue WM, Hall JC,
Hahn WJ, Sytsma KJ, Roalson EH. 2013. Phylogenetic relationships among the
North American cleomoids (Cleomaceae): a test of Iltis’s reduction series.
– Amer. J. Bot. 100: 2102-2111.
Risseeuw M. 1964. Primitiae Africanae V. A
revision of the genus Buchholzia Engler (Capp.). – Acta Bot. Neerl.
13: 161-174.
Ritland K, Jain S. 1984. The comparative life
histories of two annual Limnanthes species in a temporally variable
environment. – Amer. Natur. 124: 656-678.
Roalson EH, Hall JC. 2017. New generic
concepts for African Cleomaceae. – Syst. Bot. 42: 925-942.
Roalson EH, Hall JC, Riser II JP,
Cardinal-McTeague WM, Cochrane TS, Sytsma KJ. 2015. A revision of generic
boundaries and nomenclature in the North American cleomoid clade (Cleomaceae).
– Phytotaxa 205: 129-144.
Robinson BL. 1896. The fruit of
Tropidocarpum. – Erythea 4: 108-119.
Rodman JE. 1974. Systematics and evolution of
the genus Cakile (Cruciferae). – Contr. Gray Herb. 205: 3-146.
Rodman JE. 1976. Differentiation and
migration of Cakile (Cruciferae): seed glucosinolate evidence. –
Syst. Bot. 1: 137-148.
Rodman JE. 1978. Glucosinolates: methods of
analysis and some chemosystematic problems. – Phytochem. Bull. 11: 6-31.
Rodman JE. 1980. Population variation and
hybridization in sea-rockets (Cakile, Cruciferae): seed glucosinolate
characters. – Amer. J. Bot. 67: 1145-1159.
Rodman JE. 1981. Divergence, convergence, and
parallelism in phytochemical characters: the glucosinolate-myrosinase system.
– In: Young DA, Seigler DS (eds), Phytochemistry and angiosperm phylogeny,
Praeger, New York, pp. 43-79.
Rodman JE. 1986. Introduction establishment
and replacement of sea-rockets (Cakile, Cruciferae) in Australia. –
J. Biogeogr. 13: 159-171.
Rodman JE. 1991a. A taxonomic analysis of
glucosinolate-producing plants 1. Phenetics. – Syst. Bot. 16: 598-618.
Rodman JE. 1991b. A taxonomic analysis of
glucosinolate-producing plants 2. Cladistics. – Syst. Bot. 16: 619-629.
Rodman JE, Chew FS. 1980. Phytochemical
correlates of herbivory in a community of native and naturalized Cruciferae.
– Biochem. Syst. Ecol. 8: 43-50.
Rodman JE, Kruckeberg A, Al-Shehbaz IA. 1981.
Chemotaxonomic diversity and complexity in seed glucosinolates of
Caulanthus and Streptanthus (Cruciferae). – Syst. Bot. 6:
197-222.
Rodman JE, Brower L, Frey J. 1982.
Cardenolides in North American Erysimum (Cruciferae), a preliminary
chemotaxonomic report. – Taxon 31: 507-516.
Rodman J, Price RA, Karol K, Conti E, Sytsma
KJ, Palmer JD. 1993. Nucleotide sequences of the rbcL gene indicate
monophyly of mustard oil plants. – Ann. Missouri Bot. Gard. 80: 686-699.
Rodman JE, Karol KG, Price RA, Conti E,
Sytsma KJ. 1994. Nucleotide sequences of rbcL confirm the capparalean
affinity of the Australian endemic Gyrostemonaceae. – Aust. Syst.
Bot. 7: 57-69.
Rodman JE, Karol KG, Price RA, Sytsma KJ.
1996. Molecules, morphology, and Dahlgren’s expanded order Capparales. – Syst. Bot. 21:
289-307.
Rodman JE, Soltis PS, Soltis DE, Sytsma KJ,
Karol KG. 1998. Parallel evolution of glucosinolate biosynthesis inferred from
congruent nuclear and plastid gene phylogenies. – Amer. J. Bot. 85:
997-1006.
Rodríguez RR. 2003. Cleome sect.
Physostemon (Cleomaceae) in Cuba. – Willdenowia 33: 439-444.
Rodríguez RR, Greuter W. 2004. A study of
differentiation patterns in Capparis sect. Breyniastrum in
Cuba, with a nomenclatural and taxonomic survey of Cuban Capparis
(Capparaceae). – Willdenowia 34: 259-276.
Rohrbach P. 1869. Über den Blüthenbau von
Tropaeolum. – Bot. Zeitung 27: 833-839, 849-859.
Rollins RC. 1939a. The cruciferous genus
Physaria. – Rhodora 41: 391-414.
Rollins RC. 1939b. The cruciferous genus
Stanleya. – Lloydia 2: 109-127.
Rollins RC. 1941. A monographic study of
Arabis in western North America. – Rhodora 43: 289-325, 348-411,
425-485.
Rollins RC. 1947. Generic revisions in the
Cruciferae: Sibara. – Contr. Gray Herb. 165: 133-143.
Rollins RC. 1955. A revisionary study of the
genus Menonvillea (Cruciferae). – Contr. Gray Herb. 177: 3-57.
Rollins RC. 1956. Some new primitive Mexican
Cruciferae. – Rhodora 58: 148-157.
Rollins RC. 1957. Miscellaneous Cruciferae of
Mexico and western Texas. – Rhodora 59: 61-71.
Rollins RC. 1959. The genus
Synthlipsis (Cruciferae). – Rhodora 61: 253-264.
Rollins RC. 1963a. Protandry in two species
of Streptanthus (Cruciferae). – Rhodora 65: 45-49.
Rollins RC. 1963b. The evolution and
systematics of Leavenworthia (Cruciferae). – Contr. Gray Herb. 192:
3-98.
Rollins RC. 1966a. Chromosome numbers of
Cruciferae. – Contr. Gray Herb. Harv. Univ. 197: 43-65.
Rollins RC. 1966b. The genus
Mathewsia (Cruciferae). – Acta Bot. Neerl. 15: 102-116.
Rollins RC. 1971. Protogyny in the Cruciferae
and notes on Arabis and Caulanthus. – Contr. Gray Herb.
201: 1-10.
Rollins RC. 1979. Dithyrea and a
related genus (Cruciferae). – Publ. Bussey Inst. Harvard Univ. 1979: 3-20.
Rollins RC. 1981a. Studies in the genus
Physaria (Cruciferae). – Brittonia 33: 332-341.
Rollins RC. 1981b. Weeds of the Cruciferae
(Brassicaceae) in North America.
– J. Arnold Arbor. 62: 517-540.
Rollins RC. 1981c. Studies on Arabis
(Cruciferae) of western North America. – Syst. Bot. 6: 55-64.
Rollins RC. 1982. A new species of the
Asiatic genus Stroganowia (Cruciferae) from North America and its
biogeographic implications. – Syst. Bot. 7: 214-220.
Rollins RC. 1983. Interspecific hybridization
and taxon uniformity in Arabis (Cruciferae). – Amer. J. Bot. 7:
625-634.
Rollins RC. 1984a. Draba
(Cruciferae) in Mexico and Guatemala. – Contr. Gray Herb. 213: 1-10.
Rollins RC. 1984b. Sphaerocardamum
(Cruciferae). – Contr. Gray Herb. 213: 11-17.
Rollins RC. 1993. The Cruciferae of
continental North America: systematics of the mustard family from the Arctic to
Panama. – Stanford University Press, Stanford, California.
Rollins RC, Banerjee UC. 1975. Atlas of the
trichomes of Lesquerella (Cruciferae). – Publ. Bussey Inst. Harvard
Univ. 1975: 1-48.
Rollins RC, Banerjee UC. 1976. Trichomes in
studies of the Cruciferae. – In: Vaughan JG, Macleod AJ, Jones BMG (eds), The
biology and chemistry of the Cruciferae, Academic Press, London, pp.
145-166.
Rollins RC, Banerjee UC. 1979a. Pollen of the
Cruciferae. – Publ. Bussey Inst. Harvard Univ. 1979: 33-64.
Rollins RC, Banerjee UC. 1979b. Trichome
patterns in Physaria. – Harvard University Press, Cambridge,
Massachusetts.
Rollins RC, Price RA. 1988. High-elevation
Draba (Cruciferae) of the White Mountains of California and Nevada.
– Aliso 12: 17-27.
Rollins RC, Rüdenberg L. 1971. Chromosome
numbers of Cruciferae II. – Contr. Gray Herb. 201: 117-133.
Rollins RC, Rüdenberg L. 1977. Chromosome
numbers of Cruciferae III. – Contr. Gray Herb. 207: 101-116.
Rollins RC, Shaw EA. 1973. The genus
Lesquerella (Cruciferae) in North America. – Harvard University
Press, Cambridge, Massachusetts.
Romero EJ, Hickey LJ. 1976. A fossil leaf of
Akaniaceae from Paleocene beds in
Argentina. – Bull. Torrey Bot. Club 103: 126-131.
Ronse De Craene L-P. 2002. Floral development
and anatomy of Pentadiplandra (Pentadiplandraceae): a key
genus in the identification of floral morphological trends in the core
Brassicales. – Can. J. Bot. 80: 443-459.
Ronse De Craene L-P. 2005. Floral
developmental evidence for the systematic position of Batis (Bataceae). – Amer. J. Bot. 92:
752-760.
Ronse De Craene L-P, Haston E. 2006. The
systematic relationships of glucosinolate-producing plants and related
families: a cladistic investigation based on morphological and molecular
characters. – Bot. J. Linn. Soc. 151: 453-494.
Ronse De Craene L-P, Smets EF. 1997a. A
floral ontogenetic study of some species of Capparis and
Boscia, with special emphasis on the androecium. – Bot. Jahrb. Syst.
119: 231-255.
Ronse De Craene L-P, Smets EF. 1997b.
Evidence for carpel multiplications in the Capparaceae. – Belg. J. Bot. 130:
59-67.
Ronse De Craene L-P, Smets EF. 1999. The
floral development and anatomy of Carica papaya (Caricaceae). – Can. J. Bot. 77:
582-598.
Ronse De Craene L-P, Smets EF. 2001. Floral
developmental evidence for the systematic relationships of Tropaeolum
(Tropaeolaceae). – Ann. Bot.
88: 879-892.
Ronse De Craene L-P, Wanntorp L. 2009. Floral
development and anatomy of Salvadoraceae. – Ann. Bot. 104:
913-923.
Ronse De Craene L-P, Laet J de, Smets EF.
1998. Floral development and anatomy of Moringa oleifera (Moringaceae): what is the evidence for
a capparalean or sapindalean affinity? – Ann. Bot. 82: 273-284.
Ronse De Craene L-P, Yang TYA, Schols P,
Smets EF. 2002. Floral anatomy and systematics of Bretschneidera
(Bretschneideraceae). – Bot. J. Linn. Soc. 139: 29-45.
Ross JH. 1982. Bataceae. – In: George AS (ed), Flora
of Australia 8, Australian Government Publ. Service, Canberra, pp. 379-381.
Rossello JA, Torres N, Saez L. 1999. A new
Biscutella (Brassicaceae)
species from the western Balearic Islands. – Bot. J. Linn. Soc. 129:
155-164.
Rössler W. 1974. Myrosinzellen bei
Tovaria. – Phyton (Horn) 16: 231-238.
Roth I. 1972. Desarollo y anatomia del fruto
y de la semilla de Carica papaya L. (Lechosa). – Acta Bot. Venezuel.
7: 1-4.
Roy BA. 1995. The breeding systems of six
species of Arabis (Brassicaceae). – Amer. J. Bot 82:
869-877.
Roy BA. 2001. Patterns of association between
crucifers and their flower-mimic pathogens: host jumps are more common than
coevolution or cospeciation. – Evolution 55: 41-53.
Royen P van. 1956. A new Batidacea, Batis
argillicola. – Nova Guinea, N. S., 7: 187-195.
Royen P van. 1958. Batidaceae. – In:
Steenis CGGJ van (ed), Flora Malesiana I, 5(4), Noordhoff-Kolff N. V.,
Djakarta, pp. 414-415.
Rüger G. 1887. Beiträge zur Kenntniss der
Gattung Carica. – Ph.D. diss., Erlangen, Germany.
Ruíz Leal A, Perez-Moreau RL. 1964. Las
especies del género Magallana (Tropaeolaceae). – Darwiniana 13:
459-467.
Ruíz-Zapata T, Iltis HH. 1998. Capparaceae.
– In: Steyermark JA, Berry PE, Holst BK (eds), Flora of the Venezuelan
Guayana 4, Missouri botanical Garden Press, St. Louis, Missouri, pp.
132-156.
Russell AM. 1919. A comparative study of
Floerkea proserpinacoides and allies. – Contr. Bot. Lab. Univ.
Pennsylvania 4: 401-418.
Rustan ØH. 1980. Biosystematic studies in
Sinapidendron s. lat. (Brassicaceae) 1. The generic
delimitation. – Cand. scient. thesis, University of Oslo, Norway.
Rustan ØH. 1996. Revision of the genus
Diplotaxis (Brassicaceae)
in the Cape Verde Islands, W Africa. – Nord. J. Bot. 16: 19-50.
Rytz W. 1936. Systematische, ökologische und
geographische Probleme bei den Brassiceen. – Ber. Schweiz. Bot. Ges. 46:
517-544.
Sachar RC. 1956. The embryology of
Isomeris – a reinvestigation. – Phytomorphology 6: 346-363.
Salariato DL, Al-Shehbaz IA. 2014.
Zuloagocardamum (Brassicaceae: Thelypodieae) a new
genus from the Andes highlands of northern Argentina. – Syst. Bot. 39:
563-577.
Salariato DL, Zuloaga FO. 2015. Taxonomic
placement of Onuris hauthalii (Brassicaceae: Eudemeae), based on
morphology and multilocus species tree analyses, and the recognition of the new
genus Alshehbazia. – Kew Bull. 70: 49 DOI
10.1007/S12225-015-9602-9
Salariato DL, Zuloaga FO, Al-Shehbaz IA.
2013a. Revision and tribal placement of the Argentinean genus
Parodiodoxa (Brassicaceae). – Plant Syst. Evol.
299: 305-316.
Salariato DL, Zuloaga FO, Al-Shehbaz IA.
2013b. Molecular phylogeny of Menonvillea and recognition of the new
genus Aimara (Brassicaceae: Cremolobeae). – Taxon
62: 1220-1234.
Salariato DL, Zuloaga FO, Al-Shehbaz IA.
2014. A revision of the genus Menonvillea (Cremolobeae, Brassicaceae). – Phytotaxa 162:
241-298.
Salariato DL, Zuloaga FO, Al-Shehbaz IA.
2015. A taxonomic revision of the genus Xerodraba (Eudemeae, Brassicaceae). – Phytotaxa 20:
39-67.
Salariato DL, Zuloaga FO, Cano A, Al-Shehbaz
IA. 2015. Molecular phylogeny of the tribe Eudemeae (Brassicaceae) and implications for
its distribution and morphology. – Mol. Phylogen. Ecol. 82: 43-59.
Salariato DL, Zuloaga FO, Franzke A,
Mummenhoff K, Al-Shehbaz IA. 2016. Diversification patterns in the CES clade
(Brassicaceae tribes Cremolobeae,
Eudemeae, Schizopetaleae) in Andean South America. – Bot. J. Linn. Soc. 181:
543-566.
Salariato DL, Al-Shehbaz IA, Zuloaga FO.
2018. Reinstatement of the southern Andean genus Stenodraba (Brassicaceae) based on molecular data
and insights from its environmental and geographic distribution. – Syst. Bot.
43: 35-52.
Sánchez-Yélamo MD, Ortiz JM, Gogorcena Y.
1992. Comparative electrophoretic studies of seed proteins in some species of
the genera Diplotaxis, Erucastrum and Brassica
(Cruciferae: Brassiceae). – Taxon 41: 477-483.
Santisuk T. 1989. The monotypic family
Bretschneideraceae newly recorded for Thailand. – Nat. Hist. Bull. Siam Soc.
37: 173-176.
Santos Guerra M dos. 1989. The chromosome
number of Azima tetracantha (Salvadoraceae). – Plant Syst.
Evol. 168: 83-86.
Sato S, Nakamura Y, Kaneko T, Asamizu E,
Tabata S. 1999. Complete structure of the chloroplast genome of Arabidopsis
thaliana. – DNA Res. 6: 283-290.
Sauer H. 1933. Blüte und Frucht der
Oxalidaceen, Linaceen, Geraniaceen, Tropaeolaceen und Balsaminaceen.
Vergleichend-entwicklungsgeschichtliche Untersuchungen. – Planta 19:
417-481.
Saunders ER. 1923. The bractless
inflorescence of the Cruciferae. – New Phytol. 22: 150-156.
Saunders ER. 1929. On a new view of the
nature of the median carpels in the Cruciferae. – Amer. J. Bot. 16:
122-137.
Saunté LH. 1955. Cytogenetical studies in
the Cochlearia complex. – Hereditas 41: 499-515.
Saxena V, Gupta S. 2011. Wood anatomy of the
family Salvadoraceae from the
Indian subcontinent with special reference to the ultrastructure of the vessel
wall. – Aliso 29: 59-63.
Scheen A-C, Elven R, Brochmann C. 2002. A
molecular-morphological approach solves taxonomic controversy in arctic
Draba (Brassicaceae). –
Can. J. Bot. 80: 59-71.
Schmickl R, Kiefer C, Dobeš C, Koch MA.
2009. Evolution of trnF(GAA) pseudogenes in cruciferous plants. –
Plant Syst. Evol. 282: 229-240.
Schmidt R, Bancroft I (eds). 2011. Genetics
and genomics of the Brassicaceae.
Plant genetics and genomics: crops and models 9, Springer.
Schneider S. 1935. Untersuchungen über die
Samenschleuder-mechanismen verschiedener Rhoeadales. – Jahrb. Wiss. Bot. 81:
663-704.
Schönfelder P. 1968. Chromosomenzahlen
einiger Arten der Gattung Biscutella L. – Österr. Bot. Zeitschr.
115: 363-371.
Schranz ME, Mitchell-Olds T. 2006.
Independent ancient polyploidy events in the sister families Brassicaceae and Cleomaceae. –
Plant Cell 18: 1152-1165.
Schranz ME, Lysak MA, Mitchell-Olds T. 2006.
The ABC’s of comparative genomics in the Brassicaceae: building blocks of
crucifer genomes. – Trends Plant Sci. 11: 535-542.
Schranz ME, Song B-H, Windsor AJ,
Mitchell-Olds T. 2007. Comparative genomics in the Brassicaceae: a family-wide
perspective. – Curr. Opin. Plant Biol. 10: 168-175.
Schranz ME, Edger PP, Pires JC, van Dam NM,
Wheat CW. 2012. Comparative genomics in the Brassicales: ancient genome
duplications, glucosinolate diversification and Pierinae herbivore radiation.
– In: Edwards D, Batley J, Parkin I, Kole C (eds), Genetic, genomics and
breeding in crop plants: Brassica oilseeds, CRC Press, pp. 206-218.
Schraudolf H. 1969. Serotonin und
Indolglucosinolate in Tovaria pendula. – Naturwissenschaften 56:
462-463.
Schraudolf H, Schmidt B, Weberling F. 1971.
Das Vorkommen von “Myrosinase” als Hinweis auf die systematische Stellung
der Batidaceae. – Experientia 27: 1090-1091.
Schultz SR, Jensen WA. 1968a.
Capsella embryogenesis: the synergids before and after fertilization.
– Amer. J. Bot. 55: 541-552.
Schultz SR, Jensen WA. 1968b.
Capsella embryogenesis: the egg, zygote, and young embryo. – Amer.
J. Bot. 55: 807-819.
Schulz OE. 1903. Monographie der Gattung
Cardamine. – Engl. Bot. Jahrb. Syst. 32: 280-623.
Schulz OE. 1916. Neue Gattungen, Arten und
Kombinationen der Brassiceen. – Engl. Bot. Jahrb. Syst. 54, Beibl. 119:
52-56.
Schulz OE. 1928. Cruciferae. – In:
Werdermann E (ed), Beiträge zur Kenntnis der Flora von Chile, Notizblatt des
Königl. Botanischen Gartens und Museums zu Berlin 10: 460-475.
Schulz OE. 1929a. Cheesemania, eine
neue australische Cruciferengattung. – Notizbl. Bot. Gart. Mus. Berlin-Dahlem
10: 551-553.
Schulz OE. 1929b. Über Thlaspi
chionophilum Spegazzini. – Notizbl. Bot. Gar. Berlin-Dahlem 10:
781-783.
Schulz OE. 1931. Einige neue Cruciferen. –
Notizbl. Bot. Gart. Mus. Berlin-Dahlem 11: 224-236.
Schulz OE. 1932. Cruciferae variae. –
Notizbl. Bot. Gart. Berlin-Dahlem 11: 389-392.
Schulz OE. 1933. Kurze Notizen über neue
Gattungen, Sektionen und Arten der Cruciferen. – Bot. Jahrb. Syst. 66:
91-102.
Schulz OE. 1936. Cruciferae. – In: Engler A
(†), Harms H (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 17b, W.
Engelmann, Leipzig, pp. 227-658.
Schulz P, Jensen WA. 1971. Capsella
embryogenesis: the chalazal proliferating tissue. – Cell Sci. 8: 201-227.
Schweidler JH. 1905. Die systematische
Bedeutung der Eiweiß- und Myrosinzellen nebst Beiträgen zu ihrer
anatomisch-physiologischen Kenntnis. – Ber. Deutsch. Bot. Ges. 23:
274-285.
Schweidler JH. 1910. Die Eiweiss- oder
Myrosinzellen der Gattung Arabis L. – Beih. Bot. Centralbl. 26:
422-475.
Schweidler JH. 1911. Über den Grundtypus und
die systematische Bedeutung der Cruciferen-Nectarien I. – Beih. Bot.
Centralbl. 27: 337-390.
Schweingruber FH. 2006. Anatomical
characteristics and ecological trends in the xylem and phloem of Brassicaceae and Resedaceae. – IAWA J. 27: 419-442.
Selmeier A. 2005. Capparidoxylon
holleisii nov. spec., a silicified Capparis (Capparaceae) wood
with insect coprolites from the Neogene of southern Germany. – Zitteliana 45:
199-209.
Sen DN, Chawan DD, Sharma KD. 1972. Ecology
of Indian desert V. On the water relationships of Salvadora species.
– Flora 161: 463-471.
Sessions RA. 1997. Arabidopsis (Brassicaceae) flower development and
gynoecium patterning in wild type and ETTIN mutants. – Amer. J. Bot. 84:
1179-1191.
Shakirov EV, Salzberg SL, Alam M, Shippen DE.
2008. Analysis of Carica papaya telomeres and telomere-associated
proteins: insights into the evolution of telomere maintenance in Brassicales.
– Trop. Plant Biol. 1: 202-215.
Shamrov II. 2002. Ovule and seed study in
Capsella bursa-pastoris (Brassicaceae) with a peculiar
endothelium formation pattern. – Acta Biol. Cracov. 44: 79-90.
Sharbel TF, Mitchell-Olds T. 2001. Recurrent
polyploid origins and chloroplast phylogeography in the Arabis
holboellii complex (Brassicaceae). – Heredity 87:
59-68.
Sharma M. 1970. A study of brachysclereids in
two members of Capparidaceae. – Proc. Indian Acad. Sci., Sect. B, 72:
47-55.
Shaw EA. 1965. Taxonomic revision of some
Australian endemic genera of Cruciferae. – Trans. Roy. Soc. South Australia
89: 145-253.
Shaw EA. 1972. Revision of
Stenopetalum (Cruciferae). – J. Arnold Arbor. 53: 52-75.
Sikka SM. 1940. Cytogenetics of
Brassica hybrids and species. – J. Genet. 40: 441-509.
Silveira P, Paiva J, Samaniego NM. 2001. A
new endemic species of Arabis L. (Cruciferae) from central Portugal.
– Bot. J. Linn. Soc. 135: 299-303.
Singh B. 1944. A contribution to the anatomy
of Salvadora persica L. with special reference to the origin of the
included phloem. – J. Indian Bot. Soc. 23: 71-78.
Singh D. 1970. Caricaceae. – In: Proceedings of the
symposium on comparative embryology of angiosperms, Indian National Science
Academy, New Delhi, pp. 208-211.
Singh D, Gupta S. 1968. The seeds of the Violaceae and Resedaceae – a comparison. – J.
Indian Bot. Soc. 46: 248-256.
Singh S, Das S, Geeta R. 2018. A segmental
duplication in the common ancestor of Brassicaceae is responsible for the
origin of the paralogs KCS6-KCS5, which are not shared with other angiosperms.
– Mol. Phylogen. Evol. 126: 331-345.
Singh Raghuvanshi S, Pathak CS. 1974. Ploidy
barrier in Tropaeolum majus L. – Caryologia 27: 225-235.
Sjöstedt B. 1975. Revision of the genus
Cardamine L. (Cruciferae) in South and Central America. – Bot. Not.
128: 8-19.
Sleumer H. 1942. Salvadoraceae. – In: Engler A
(†), Harms H, Mattfeld J (eds), Die natürlichen Pflanzenfamilien, 2. Aufl.,
Bd. 20b, W. Engelmann, Leipzig, pp. 232-239.
Smith CG. 1974. The ultrastructural
development of spherosomes and oil bodies in the developing embryo of
Crambe abyssinica. – Plant 119: 125-142.
Snogerup S. 1967a. Studies in the Aegean
flora VIII. Erysimum sect. Cheiranthus A. Taxonomy. – Opera
Bot. 13: 1-70.
Snogerup S. 1967b. Studies in the Aegean
flora VIII. Erysimum sect. Cheiranthus B. Variation and
evolution in the small-population system. – Opera Bot. 14: 1-86.
Snogerup S, Gustafsson M, Bothmer R von.
1990. Brassica sect. Brassica (Brassicaceae) I. Taxonomy and
variation. – Willdenowia 19: 271-365.
Sobick U. 1983.
Blütenentwicklungsgeschichtliche Untersuchungen an Resedaceen unter besonderer
Berücksichtigung von Androeceum und Gynoeceum. – Bot. Jahrb. Syst. 104:
203-248.
Sobrino Vesperinas E. 1988. Obtainment of
some new intergeneric and interspecific hybrids between wild Brassiceae. –
Candollea 43: 499-504.
Solms-Laubach H zu. 1894. Caricaceae. – In: Engler A, Prantl K
(eds), Die natürlichen Pflanzenfamilien III(6a), W. Engelmann, Leipzig, pp.
94-99.
Sonboli A, Zehzad B, Assadi M, Azizian D.
2001. A taxonomic revision of the genera Sterigmostemum and
Petiniotia in Iran. – Rostaniha 2: 53-55.
Sønderby IE, Geu-Flores F, Halkier BA. 2010.
Biosynthesis of glucosinolates – gene discovery and beyond. – Trends Plant
Sci. 15: 283-290.
Song KM, Osborn TC. 1992. Polyphyletic
origins of Brassica napus: new evidence based on organelle and nuclear
RFLP analyses. – Genome 35: 992-1001.
Song KM, Osborn TC, Williams PH. 1988a.
Brassica taxonomy based on nuclear restriction fragment length
polymorphisms (RFLPs) 1. Genome evolution of diploid and amphidiploid species.
– Theor. Appl. Gen. 75: 784-794.
Song KM, Osborn TC, Williams PH. 1988b.
Brassica taxonomy based on nuclear restriction fragment length
polymorphisms (RFLPs) 2. Preliminary analysis of subspecies within B.
rapa (syn. campestris) and B. oleracea. – Theor. Appl.
Gen. 76: 593-600.
Song KM, Osborn TC, Williams PH. 1990.
Brassica taxonomy based on nuclear restriction fragment length
polymorphisms (RFLPs) 3. Genome relationships in Brassica and related
genera and the origin of B. oleracea and B. rapa (syn.
campestris). – Theor. Appl. Gen. 79: 497-506.
Song KM, Lu P, Tang K, Osborn TC. 1995. Rapid
genome change in synthetic polyploids of Brassica and its implications
for polyploid evolution. – Proc. Natl. Acad. Sci. U.S.A. 92: 7719-7723.
Sooda A, Song J, Mamieson PE, Clemens J.
2011. Phase change and flowering in Pachycladon exile and isolation of
LEAFY and TERMINAL FLOWER1 homologues. – New Zealand J. Bot. 49: 281-293.
Sørensen T. 1954. New species of
Hierochloë, Calamagrostis and Braya. – Meddel.
Grønland 136(8): 1-24.
Španiel S, Kempa M, Salmerón-Sánchez E,
Fuertes-Aguilar J, Mota JF, Al-Shehbaz IA, German DA, Olšavská K,
Šingliarová B, Zozomová-Lihová J, Marhold K. 2015. AlyBase: database of
names, chromosome numbers, and ploidy levels of Alysseae (Brassicaceae), with a new generic
concept of the tribe. – Plant Syst. Evol. 301: 2463-2491.
Sparre B. 1973. 89. Tropaeolaceae. – In: Harling G,
Sparre B (eds), Flora of Ecuador 2, Swedish Natural Science Research Council,
Stockholm, pp. 1-31.
Sparre B, Andersson L. 1991. A taxonomic
revision of the Tropaeolaceae.
– Opera Bot. 108: 1-139.
Spencer KC, Seigler DS. 1984. Cyanogenic
glycosides of Carica papaya and its phylogenetic position with respect
to the Violales and Capparales. –
Amer. J. Bot. 71: 1444-1447.
Spooner DM. 1984. Reproductive features of
Dentaria laciniata and D. diphylla (Cruciferae), and the
implications in the taxonomy of the eastern North American Dentaria
complex. – Amer. J. Bot. 71: 999-1005.
Spratt ER. 1932. The gynoecium of the family
Cruciferae. – J. Bot. 70: 308-314.
Sprecher A. 1943. Beitrag zur Morphologie von
Carica papaya L. – Ber. Schweiz. Bot. Ges. 53A: 517-549.
Stapf O. 1912. Akaniaceae: a new family of Sapindales. – Kew Bull.
9: 378-380.
Stenar H. 1925. Embryologische und
zytologische Studien über Limnanthes douglasii R. Br. – Svensk Bot.
Tidskr. 19: 133-152.
Stephens EL. 1910. The development of the
seed coat of Carica papaya. – Ann. Bot. 24: 607-610.
Stern WL, Brizicky GK, Tamolang FN. 1963. The
woods and flora of the Florida keys: Capparaceae. – Contr. U.S. Natl. Herb.
34, 2: 25-43.
Storey WB. 1976. Papaya. – In: Simmons NW
(ed), Evolution of crop plants, Longman, London, pp. 21-24.
Stork AL. 1971. Seed characters in European
taxa of Malcolmia R. Br. (Cruciferae). – Svensk Bot. Tidskr. 65:
283-292.
Stork AL. 1972a. Samen und Keimlinge von
Malcolmia sens. lat. (Cruciferae). – Svensk Bot. Tidskr. 66:
417-436.
Stork AL. 1972b. Studies in the Aegean flora
XX. Biosystematics of the Malcolmia maritima complex. – Opera Bot.
33: 1-118.
Stork AL, Wüest J. 1978. SEM studies of
seed-coats in Malcolmia (Cruciferae). – Arch. Sci. Genève 31:
229-237.
Stoudt HN. 1941. The floral morphology of
some of the Capparidaceae. – Amer. J. Bot. 28: 664-675.
Stout AB. 1923. Alternation of sexes and
intermittent production of fruit in the spider flower (Cleome
spinosa). – Amer. J. Bot. 10: 57-66.
Strid A. 1980. New species of
Aethionema and Peucedanum from the Greek mountains. – Bot.
Not. 133: 521-526.
Stuckey RL. 1972. Taxonomy and distribution
of the genus Rorippa (Cruciferae) in North America. – Sida 4:
279-430.
Su J-X, Wang W, Zhang L-B, Chen Z-D. 2012.
Phylogenetic placement of two enigmatic genera, Borthwickia and
Stixis, based on molecular and pollen data, and the description of a
new family of Brassicales, Borthwickiaceae. – Taxon 61:
601-611.
Subramanian D, Susheela G. 1988.
Cytotaxonomical studies of South Indian Capparidaceae. – Cytologia 53:
679-684.
Sulbha K. 1957. Embryology of Brassica
juncea Czern & Coss. – J. Indian Bot. Soc. 36: 292-301.
Swanepoel, W., Chase, M. W., Chistenhusz, M. J. M., Maurin, O.,
Forest F., van Wyk, A. E. 2020. From the frying pan: an unusual dwarf shrub from Namibia turns
out to be a new brassicalean family. – Phytotaxa 439(3): 171-185.
Sweeney PW, Price RA. 2000. Polyphyly of the
genus Dentaria (Brassicaceae): evidence from
trnL intron and ndhF sequence data. – Syst. Bot. 25:
468-478.
Sweeney PW, Price RA. 2001. A multivariate
morphological analysis of the Cardamine concatenata alliance (Brassicaceae). – Brittonia 53:
82-95.
Takahata Y, Hinata K. 1978. A description of
the genetic stocks in subtribe Brassicinae by chromosome numbers and numerical
characters. – Cruciferae Newsl. (EUCARPIA) 3: 47-51.
Takahata Y, Hinata K. 1983. Studies on
cytodemes in subtribe Brassicinae (Cruciferae). – Tôhoku J. Agric. Res. 33:
111-124.
Takahata Y, Hinata K. 1986. A consideration
of the species relationships in subtribe Brassicinae (Cruciferae) in view of
cluster analysis of morphological characters. – Plant Spec. Biol. 1:
79-88.
Takahata Y, Hinata K. 1992. Comparison of
species relationships in the subtribe Brassicinae based on morphology,
cytogenetics and isozymes. – J. Genet. & Breed. 46: 193-198.
Tamboli AS, Yadav PB, Gothe AA, Yadav SR,
Govindwar SP. 2018. Molecular phylogeny and genetic diversity of genus
Capparis (Capparaceae) based on plastid DNA sequences and ISSR
markers. – Plant Syst. Evol. 304: 205-217.
Tang C-S. 1971. Benzyl isothiocyanate of
papaya fruit. – Phytochemistry 10: 117-131.
Tang C-S. 1973. Localization of benzyl
glucosinolate and thioglucosidase in Carica papya fruit. –
Phytochemistry 12: 769-773.
Tang C-S, Hamilton RA. 1976. Benzyl
isothiocyanate in Cylicomorpha solmsii (Caricaceae). – Phytochemistry 15:
1767-1768.
Tang C-S, Syed MM, Hamilton RA. 1972. Benzyl
isothiocyanate in the Caricaceae.
– Phytochemistry 11: 2531-2533.
Tang Y. 1935. Notes on the systematic
position of Bretschneideraceae as shown by its timber anatomy. – Bull. Fan
Mem. Inst. Biol. 6: 153-157.
Tarutani Y, Shiba H, Iwano M, Kakizaki T,
Suzuki G, Watanabe M, Isogai A, Takayama S. 2010. Trans-acting small
RNA determines dominance relationships in Brassica
self-incompatibility. – Nature 466: 983-986.
Tatout C, Warwick S, Lenoir A, Deragon J-M.
1999. SINE insertions as clade markers for wild crucifer species. – Mol.
Biol. Evol. 16: 1614-1621.
Taylor P. 1960. 15. Resedaceae. – In: Exell AW, Wild H
(eds), Flora Zambesiaca 1 (Part 1), Crown Agents for Oversea Governments and
Administrations, London, pp. 245.
Thangstad OP, Winge P, Husebye H, Bones A.
1993. The myrosinase (thioglucoside glucohydrolase) gene family in Brassicaceae. – Plant Mol. Biol.
23: 511-524.
Thellung A. 1906a. Die afrikanischen
Lepidium-Arten. – Vierteljahrschr. Naturforsch. Ges. Zürich 51:
144-192.
Thellung A. 1906b. Die Gattung
Lepidium (L.) R. Br. – Mitt. Bot. Mus. Univ. Zürich 28: 1-340.
Thulin M. 1990. A new species of
Reseda from Somalia. – Kew Bull. 45: 667-669.
Thulin M. 1992. New species of
Maerua and Cleome (Capparaceae) from Somalia. – Nord. J.
Bot. 12: 423-426.
Thulin M. 1994. A new species of
Ochradenus (Resedaceae)
from southern Arabia. – Nord. J. Bot. 14: 383-384.
Thulin M, Roalson EH. 2017. Resurrection of
the genus Rorida (Cleomaceae), a distinctive old world segregate of
Cleome. – Syst. Bot. 42: 569-577.
Tieghem P van. 1900. Sur les Stachyuracées
et les Koeberliniacées. – J. Bot. (Morot) 14: 1-12.
Tieghem P van. 1903. Sur les Batidacées. –
J. Bot. (Morot) 17: 363-376.
Tiwari SC, Bouman F, Kapil RN. 1977. Ovule
ontogeny in Tropaeolum majus. – Phytomorphology 27: 350-358.
Tobe H, Peng CI. 1990. The embryology and
taxonomic relationships of Bretschneidera (Bretschneideraceae). –
Bot. J. Linn. Soc. 103: 139-152.
Tobe H, Raven PH. 1991. The embryology and
relationships of Gyrostemonaceae. – Aust. Syst.
Bot. 4: 407-420.
Tobe H, Raven PH. 1992. The embryology and
relationships of Bataceae. – Syst.
Bot. 19: 485-496.
Tobe H, Raven PH. 1995. Embryology and
relationships of Akania (Akaniaceae). – Bot. J. Linn. Soc.
118: 261-274.
Tobe H, Raven PH. 2008. Embryology of
Koeberlinia (Koeberliniaceae): evidence for
core-Brassicalean affinities. – Amer. J. Bot. 95: 1475-1486.
Tobe H, Takahashi M. 1995. Pollen morphology
of Gyrostemonaceae, Batidaceae,
and Koeberlinia. – J. Plant Res. 108: 283-288.
Tobe H, Carlquist S, Iltis HH. 1999.
Reproductive anatomy and relationships of Setchellantus caeruleus (Setchellanthaceae). – Taxon
48: 277-283.
Tolmachev AI. 1957. A contribution to the
history and geographical distribution of the genus Draba L. – Bot.
Žurn. 42: 1446-1456. [In Russian]
Tomb AS. 1999. Pollen morphology and
relationships of Setchellanthus caeruleus (Setchellanthaceae). – Taxon
48: 285-288.
Toro-Nuñez O, Mort ME, Ruiz-Ponce E,
Al-Shehbaz IA. 2013. Phylogenetic relationships of Mathewsia and
Schizopetalon (Brassicaceae) inferred from nrDNA and
cpDNA regions: taxonomic and evolutionary insights from an Atacama Desert
endemic lineage. – Taxon 62: 343-356.
Toro-Núñez O, Al-Shehbaz IA, Mort ME. 2015.
Phylogenetic study with nuclear and chloroplast data and ecological niche
reveals Atacama (Brassicaceae), a new monotypic genus
endemic from the Andes of the Atacama Desert, Chile. – Plant Syst. Evol. 301:
1377-1396.
Troll W. 1966. Caricaceae. – In: Bericht der
Kommission für biologische Forschung, Jahrb. Akad. Wiss. Lit. Mainz 1965, pp.
110-133.
Uphof JC. 1930. Biologische Beobachtungen an
Batis maritima L. – Österr. Bot. Zeitschr. 79: 355-367.
Urban L, Bailey CD. 2013. Phylogeny of
Levenworthia and Selenia (Brassicaceae). – Syst. Bot. 38:
723-736.
Urbanska KM, Hurka H, Landolt E, Neuffer B,
Mummenhoff K. 1997. Hybridization and evolution in Cardamine (Brassicaceae) at Urnerboden, central
Switzerland – biosystematic and molecular evidence. – Plant Syst. Evol.
204: 233-256.
Valdés Bermejo E, Kaercher W.1984. Dos
nuevos táxones ibéricos de género Reseda L., sect.
Leucoreseda DC. – An. Jard. Bot. Madrid 41: 198-201.
Vanderpool SS, Elisens WJ, Estes JR. 1991.
Pattern, tempo, and mode of evolutionary and biogeographic divergence in
Oxystylis and Wislizenia (Capparaceae). – Amer. J. Bot. 78:
925-937.
Vassilyeva AN. 1969. Critical notes on the
genus Parrya R. Br. – Notul. Syst. Herb. Inst. Bot. Acad. Sci.
KazSSR 6: 27-31.
Vaughan JG. 1956. The seed coat structure of
Brassica integrifolia (West) O. E. Schulz var. carinata (A.
Br.). – Phytomorphology 6: 363-367.
Vaughan JG. 1977. A multidisciplinary study
of the taxonomy and origin of Brassica crops. – BioScience 27:
35-40.
Vaughan JG, Denford KE. 1968. An acrylamide
gel electrophoretic study of the seed proteins of Brassica and
Sinapis species with special reference to their taxonomic value. –
J. Exp. Bot. 19: 724-732.
Vaughan JG, Waite A. 1967a. Comparative
electrophoretic studies of the seed proteins of certain species of
Brassica and Sinapis. – J. Exp. Bot. 18: 100-109.
Vaughan JG, Waite A. 1967b. Comparative
electrophoretic studies of the seed proteins of certain amphidiploid species of
Brassica. – J. Exp. Bot. 18: 269-276.
Vaughan JG, Whitehouse JM. 1971. Seed
structure and taxonomy of the Cruciferae. – Bot. J. Linn. Soc. 64:
383-409.
Vaughan JG, Gordon EI, Robinson D. 1968. The
identification of myrosinase after the electrophoresis of Brassica and
Sinapis seed proteins. – Phytochemistry 7: 1345-1348.
Vaughan JG, McLeod AJ, Jones BMG (eds). 1976.
The biology and chemistry of the Cruciferae. – Academic Press, London, New
York, San Francisco.
Vaughan JG, Phelan JR, Denford KE. 1976. Seed
studies in the Cruciferae. – In: Vaughan JG, McLeod AJ, Jones BMG (eds), The
biology and chemistry of the Cruciferae, Academic Press, London, New York, San
Francisco.
Verdcourt B. 1958. Moringaceae: a correction. – Kew
Bull. 13: 385.
Verdcourt B. 1968. Salvadoraceae. – In: Milne-Redhead
E, Polhill RM (eds), Flora of tropical East Africa, Crown Agents for Oversea
Governments and Administrations, London, pp. 1-9.
Verdcourt B. 1985. A synopsis of the Moringaceae. – Kew Bull. 48:
1-23.
Verdcourt B. 1986. Moringaceae. – In: Polhill RM (ed),
Flora of tropical East Africa, A. A. Balkema, Rotterdam, pp. 1-12.
Veselova PV. 2002. To the problem of the
taxonomic closeness of Erysimum L. and Syrenia Andrz. – In:
Problems of botany of South Siberia and Mongolia. Proceedings of the
1st International Scientific-Practical Conference (Barnaul, November
26-28, 2002), Barnaul, p. 34. [In Russian]
Vesperina ES. 1997. Interfertility in the
genus Moricandia DC. – Lagascalia 19: 839-844.
Vezey EL, Skvarla JJ, Vanderpool SS. 1991.
Characterizing pollen sculpture of three closely related Capparaceae species
using quantitative image analysis of scanning electron micrographs. – In:
Blackmore S, Barnes SH (eds), Pollen and spores, Syst. Assoc. Spec. Vol. 44,
pp. 291-300.
Vickery AR. 1983. 110. Salvadoraceae. – In: Launert E
(ed), Flora Zambesiaca 7 (Part 1), Flora Zambesiaca Managing Committee, London,
pp. 374-380.
Viegi L, Pagni AM, Corsi G, Renzoni GC. 1976.
Il sospensore embrionale nelle Cruciferae I. Morfologia e struttura. – Giorn.
Bot. Ital. 110: 347-357.
Villiers J-F. 1973. Pentadiplandraceae. – In:
Aubréville A, Leroy J-F (eds), Flore du Cameroun 15, Muséum National
d’Histoire Naturelle, Paris, pp. 163-167.
Vioque J, Pastor JE, Alaiz M, Vioque E. 1994.
Chemotaxonomic study of seed glucosinolate composition in Coincya Rouy
(Brassicaceae). – Bot. J. Linn.
Soc. 116: 343-350.
Vision TJ, Brown DG, Tanksley SD. 2000. The
origins of genomic duplications in Arabidopsis. – Science 290:
2114-2117.
Voelckel C, Mirzaei M, Reichelt M, Luo L,
Pascovici D, Heenan PB, Schmidt S, Janssen B, Haynes PA, Lockhart PJ. 2010.
Transcript and protein profiling identify candidate gene sets of adaptive
divergence in New Zealand Pachycladon. – BMC Evol. Biol. 10: 151;
doi: 10.1186/1471-2148-10-151.
Voytenko VF. 1968. The forms of heterocarpy
in the Brassicaceae Burn. family
and the evaluation of their evolutionary significance. – Bot. Žurn. 53:
1428-1439. [In Russian]
Voznesenskaya EV, Koteyeva NK, Chuong DX,
Ivanova AV, Barroca J, Craven LA, Edwards GE. 2007. Physiological, anatomical
and biochemical characterisation of photosynthetic types in the genus
Cleome (Cleomaceae). – Funct. Plant Biol. 34: 247-267.
Vuillemin P. 1915. Différences essentielles
entre la Capucine et les Géraniacées. – Compt. Rend. Acad. Sci. Paris 161:
297-301.
Walker RI. 1947. Megasporogenesis and embryo
sac development in Tropaeolum majus L. – Bull. Torrey Bot. Club 74:
240-249.
Warwick SI. 1993. Guide to the wild germplasm
of Brassica and allied crops IV. Wild species in the tribe Brassiceae
(Cruciferae) as sources of agronomic traits. – Agric. Can. Res. Branch Techn.
Bull. 1993-17E: 1-19.
Warwick SI, Al-Shehbaz IA. 1998. Generic
evaluation of Boleum, Euzomodendron, and Vella (Brassicaceae). – Novon 8:
321-325.
Warwick SI, Al-Shehbaz IA. 2003.
Nomenclatural notes on Sisymbrium (Brassicaceae). – Novon 13:
265-267.
Warwick SI, Al-Shehbaz IA. 2006. Brassicaceae: chromosome number index
and database on CD-Rom. – Plant Syst. Evol. 259: 237-248.
Warwick SI, Anderson KJ. 1993. Guide to the
wild germplasm of Brassica and allied crops II. Chromosome numbers in
the tribe Brassiceae (Cruciferae). – Agric. Can. Res. Branch Techn. Bull.
1993-15E: 1-22.
Warwick SI, Black LD. 1991. Molecular
systematics of Brassica and allied genera (Subtribe Brassicinae,
Brassiceae) – chloroplast genome and cytodeme congruence. – Theor. Appl.
Gen. 82: 81-92.
Warwick SI, Black LD. 1993a. Molecular
relationships in subtribe Brassicinae (Cruciferae, tribe Brassiceae). – Can.
J. Bot. 71: 906-918.
Warwick SI, Black LD. 1993b. Guide to the
wild germplasm of Brassica and allied crops III. Interspecific and
intergeneric hybridizations in the tribe Brassiceae (Cruciferae). – Agric.
Can. Res. Branch Techn. Bull. 1993-16E: 1-31.
Warwick Si, Black LD. 1994. Evaluation of the
subtribes Moricandiinae, Savignyinae, Vellinae, and Zillinae (Brassicaceae, tribe Brassiceae) using
chloroplast DNA restriction site variation. – Can. J. Bot. 72: 1692-1701.
Warwick SI, Black LD. 1997a. Phylogenetic
implications of chloroplast DNA restriction site variation in subtribes
Raphaninae and Cakilinae (Brassicaceae, tribe Brassiceae). –
Can. J. Bot. 75: 960-973.
Warwick SI, Black LD. 1997b. Molecular
phylogenies from theory to application in Brassica and allies (tribe
Brassiceae, Brassicaceae). –
Opera Bot. 132: 159-168.
Warwick SI, Francis A. 1994. Guide to the
wild germplasm of Brassica and allied crops V. Life history and
geographical data for wild species in the tribe Brassiceae (Cruciferae). –
Agric. Can. Res. Branch Techn. Bull. 1994-2E: 1-61.
Warwick SI, Hall JC. 2009. Phylogeny of
Brassica and wild relatives. – In: Gupta S (ed), Biology and
breeding of crucifers, CRC, Boca Raton, Florida, pp. 19-36.
Warwick SI, Sauder CA. 2005. Phylogeny of the
tribe Brassiceae (Brassicaceae)
based on chloroplast restriction site polymorphisms and nuclear ribosomal
internal transcribed spacer and chloroplast trnL intron sequences. –
Can. J. Bot. 83: 467-483.
Warwick SI, Black LD, Aguinagalde I. 1992.
Molecular systematics of Brassica and allied genera (subtribe
Brassicinae, Brassiceae) – chloroplast DNA variation in the genus
Diplotaxis. – Theor. Appl. Gen. 83: 839-850.
Warwick SI, Al-Shehbaz IA, Price RA, Sauder
CA. 2002. Phylogeny of Sisymbrium (Brassicaceae) based on ITS sequences
of nuclear ribosomal DNA. – Can. J. Bot. 80: 1002-1017.
Warwick SI, Al-Shehbaz IA, Sauder C, Harris
JG, Koch M. 2004. Phylogeny of Braya and Neotorularia (Brassicaceae) based on nucler
ribosomal internal transcribed spacer and chloroplast trnL intron
sequences. – Can. J. Bot. 82: 376-392.
Warwick SI, Al-Shehbaz IA, Sauder C, Murray
DF, Mummenhoff K. 2004. Phylogeny of Smelowskia and related genera (Brassicaceae) based on nuclear ITS
DNA and chloroplast trnL intron sequences. – Ann. Missouri Bot.
Gard. 91: 99-123.
Warwick SI, Al-Shehbaz IA, Sauder C. 2005.
Phylogeny and cytological diversity of Sisymbrium (Brassicaceae). – In: Sharma AK,
Sharma A (eds), Plant genome: biodiversity and evolution 1C: phanerogams
(angiosperms – dicotyledons), Science Publ., Enfield, New Hampshire.
Warwick SI, Al-Shehbaz IA, Sauder CA. 2006.
Phylogenetic position of Arabis arenicola and generic limits of
Eutrema and Aphragmus (Brassicaceae) based on sequences of
nuclear ribosomal DNA. – Can. J. Bot. 84: 269-281.
Warwick SI, Francis A, Al-Shehbaz IA. 2006.
Brassicaceae: species checklist
and database on CD-Rom. – Plant Syst. Evol. 259: 249-258.
Warwick SI, Sauder CA, Al-Shehbaz IA. 2006.
Molecular phylogeny, morphology and cytological diversity of
Sisymbrium (Brassicaceae). – In: Sharma AK,
Sharma A (eds), Plant genome biodiversity and evolution I, C: Phanerogams
(Angiosperms-Dicotyledons), Science Publ. Enfield, New Hampshire, pp.
218-250.
Warwick SI, Sauder CA, Al-Shehbaz IA,
Jacquemoud F. 2007. Phylogenetic relationships in the tribes Anchonieae,
Chorisporeae, Euclidieae, and Hesperideae (Brassicaceae) based on nuclear
ribosomal ITS DNA sequences. – Ann. Missouri Bot. Gard. 94: 56-78.
Warwick SI, Sauder CA, Al-Shehbaz IA. 2008.
Phylogenetic relationships in the tribe Alysseae (Brassicaceae) based on nuclear
ribosomal ITS DNA sequences. – Can. J. Bot. 86: 315-336.
Warwick SI, Sauder CA, Mayer MS, Al-Shehbaz
IA. 2009. Phylogenetic relationships in the tribes Schizopetaleae and
Thelypodieae (Brassicaceae) based
on nuclear ribosomal ITS region and chloroplast ndhF DNA sequences.
– Botany 87: 961-985.
Warwick SI, Francis A, Gugel RK. 2009. Guide
to the wild germplasm. Brassica and allied crops (tribe Brassiceae, Brassicaceae). –
http://www.brassica.info/.
Warwick SI, Mummenhoff K, Sauder CA, Koch MA,
Al-Shehbaz IA. 2010. Closing the gaps: phylogenetic relationships in the Brassicaceae based on DNA sequence
data of nuclear ribosomal ITS region. – Plant Syst. Evol. 285: 209-232.
Warwick SI, Sauder CA, Al-Shehbaz IA. 2011.
Systematic position of Ivania, Scoliaxon, and
Phravenia (Brassicaceae).
– Taxon 60: 1156-1164.
Watson JM, Flores AR. 2010a.
Tropaeolum section Chilensia: an overview. – Curtis’s
Bot. Mag. 27: 197-234.
Watson JM, Flores AR. 2010b. A synopsis of
perennial tuberous Tropaeolum L. section Chilensia Sparre (Tropaeolaceae), including validation
of three subsections and a new, reclassified natural hybrid. – Herbertia 64:
150-281.
Weber A. 1973. Stipularbildungen bei
Dentaria und ihr Wert für die morphologische Deutung der
Specherschuppen als Phyllodien. – Österr. Bot. Zeitschr. 121: 107-119.
Weberling F. 1968: Über die
Rudimentärstipeln der Resedaceae.
– Acta Bot. Neerl. 17: 360-376.
Weberling F. 1974. Weitere Untersuchungen zur
Morphologie des Unterblattes bei den Dikotylen VII. Polygalales; VIII.
Koeberlinia Zucc. – Beitr. Biol. Pflanzen 50: 277-289.
Weberling F, Müller L. 1980. Persistierende
Blütensporne bei Tropaeolum. – Flora 169: 295-298.
Weberling F, Uhlarz H. 1983. Zur Morphologie
und Morphogenese der Blüte von Cadaba juncea (Sparm.) Harv.
(Capparidaceae). – Beitr. Biol. Pflanzen 58: 267-281.
Wege JA, Lepschi BJ. 2007. A new species of
Arabidella (Brassicaceae)
from Western Australia. – Nuytsia 17: 453-458.
Weigend M, Förther H. 1999. Two new species
of Sisymbrium (Brassicaceae) from coastal Peru. –
Brittonia 51: 119-123.
Wel H van der; Larson G, Hladik A, Hellekant
G, Glaser D. 1989. Isolation and characterization of pentadin, the sweet
principles of Pentadiplandra brazzeana Baillon. – Chem. Senses 14:
75-79.
Werker E, Vaughan JG. 1974. Anatomical and
ultrastructural changes in aleurone and myrosin cells of Sinapis alba
during germination. – Planta 116: 243-255.
Widmer A, Baltisberger M. 1999a. Extensive
intrspecific chloroplast DNA (cpDNA) variation in the alpine Draba
aizoides L. (Brassicaceae):
haplotype relationships and population structure. – Mol. Ecol. 8:
1405-1415.
Widmer A, Baltisberger M. 1999b. Molecular
evidence for allopolyploid speciation and a single origin of the narrow endemic
Draba ladina (Brassicaceae). – Amer. J. Bot. 86:
1282-1289.
Wild H. 1960. 14. Capparidaceae. – In:
Exell AW, Wild H (eds), Flora Zambesiaca 1 (Part 1), Crown Agents for Oversea
Governments and Administrations, London, pp. 194-245.
Williams K, Chayamarit K. 2005. A revised
generic key to the Capparaceae of Thailand with a description of
Neothorelia laotica Gagnep. – Thai For. Bull. (Bot.) 33: 228-230.
Willis CG, Hall JC, Rubio de Casas R, Wang
TY, Donohue K. 2014. Diversification and the evolution of dispersal ability in
the tribe Brassiceae (Brassicaceae). – Ann. Bot. 114:
1675-1686.
Wills AB. 1966. Meiotic behaviour in the
Brassiceae. – Caryologia 19: 103-116.
Windham MD. 2000. Chromosome counts and
taxonomic notes on Draba (Brassicaceae) of the Intermountain
West 1: Utah and vicinity. – Madroño 47: 21-28.
Windham MD. 2004. Chromosome counts and
taxonomic notes on Draba (Brassicaceae) of the Intermountain
West 2: Idaho, Nevada and vicinity. – Madroño 50: 221-231.
Windham MD, Al-Shehbaz IA. 2006. New and
noteworthy species of Boechera (Brassicaceae) I: sexual diploids. –
Harvard Pap. Bot. 11: 61-88.
Windham MD, Al-Shehbaz IA. 2007a. New and
noteworthy species of Boechera (Brassicaceae) II: apomictic hybrids.
– Harvard Pap. Bot. 11: 257-274.
Windham MD, Al-Shehbaz IA. 2007b. New and
noteworthy species of Boechera (Brassicaceae) III: additional sexual
diploids and apomictic hybrids. – Harvard Pap. Bot. 12: 235-257.
Winge Ö. 1940. Taxonomic and evolutionary
studies in Erophila based on cytogenetic investigations. – Comp.
Rend. trav. Lab. Carlsberg, sér. Physiol. 23: 41-74.
Woodson RE. 1948. Gynandropsis,
Cleome, and Podandrogyne. – Ann. Missouri Bot. Gard. 35:
139-147.
Woycicki MZ. 1907. Über den Bau des
Embryosackes bei Tropaeolum majus. – Bull. Internat. Acad. Sci.
Cracovie 1907: 550-557.
Wroblewski T, Coulibaly S, Sadowski J, Quiros
CF. 2000. Variation and phylogenetic utility of the Arabidopsis
thaliana rps2 homolog in various species of the tribe Brassiceae.
– Mol. Phylogen. Evol. 16: 440-448.
Wunderlich R. 1991. Zur Frage nach der
systematischen Stellung der Limnanthaceae. – Stapfia 25:
3-59.
Xue J, Lenman M, Falk A, Rask L. 1992. The
glucosinolate-degrading enzyme myrosinase in Brassicaceae in encoded by a gene
family. – Plant Mol. Biol. 18: 387-398.
Xue J, Jørgensen M, Pihlgren U, Rask L.
1995. The myrosinase gene family in Arabidopsis thaliana: gene
organization expression and evolution. – Plant Mol. Biol. 27: 911-922.
Yanagino T, Takahata Y, Hinata K. 1987.
Chloroplast DNA variations among diploid species in Brassica and
allied genera. – Jap. J. Genet. 62: 119-125.
Yang D-Q, Hu C-M. 1985. The chromosomes of
Bretschneidera Hemsl. – Notes Roy. Bot. Gard. Edinb. 42: 347-349.
Yang Y-W, Lai K-N, Tai P-Y, Ma D-P, Li W-H.
1999. Molecular phylogenetic studies of Brassica, Rorippa,
Arabidopsis, and allied genera based on the internal transcribed
spacer region of 18S-25S rDNA. – Mol. Phylogen. Evol. 13: 455-462.
Yang Y-W, Tai P-Y, Chen Y, Li W-H. 2002. A
study of the phylogeny of Brassica rapa, B. nigra,
Raphanus sativus, and their related genera using noncoding regions of
chloroplast DNA. – Mol. Phylogen. Evol. 23: 268-275.
Yarnell SH. 1956. Cytogenetics of the
vegetable crops II. Crucifers. – Bot. Rev. 22: 81-166.
Yen C. 1959. On a new view of carpel
morphology in Brassica. – Acta Bot. Sin. 8: 271-288.
Yıldırımlı Ş. 2002. Two new species of
Draba L. (Brassicaceae)
from Turkey, D. terekemensis and D. narmanensis. – Ot 7:
1-6.
Yoffe MD. 1952. On the occurrence of
chlorophyll in the endosperm of Cruciferae. – Dokl. Akad. Nauk SSSR 84:
473-476. [In Russian]
Yue J-P, Gu Z-J, Al-Shehbaz IA, Sun H. 2004.
Cytological studies on the Sino-Himalayan endemic Solms-laubachia (Brassicaceae) and two related genera.
– Bot. J. Linn. Soc. 145: 77-86.
Yue J-P, Sun H, Al-Shehbaz IA, Li J-H. 2006.
Support for an expanded Solms-laubachia (Brassicaceae): evidence from
sequences of chloroplast and nuclear genes. – Ann. Missouri Bot. Gard. 93:
402-411.
Yue J-P, Sun H, Li J-H, Al-Shehbaz IA. 2008.
A synopsis of an expanded Solms-laubachia (Brassicaceae), and the description of
four new species from western China. – Ann. Missouri Bot. Gard. 95:
520-538.
Yue J-P, Sun H, Baum DA, Li J-H, Al-Shehbaz
IA, Ree R. 2009. Molecular phylogeny of Solms-laubachia (Brassicaceae) s.l., based on multiple
nuclear and plastid DNA sequences, and its biogeographic implications. – J.
Syst. Evol. 47: 402-415.
Zhao B, Liu L, Tan D, Wang J. 2010. Analysis
of phylogenetic relationships of Brassicaceae species based on
Chs sequences. – Biochem. Syst. Ecol. 38: 731-739.
Zhao Z, Zhang W, Stanley BA, Assmann SM.
2008. Functional proteomics of Arabidopsis thaliana guard cells
uncovers new stomatal signaling pathways. – Plant Cell 20: 3210-3226.
Zohary M. 1948a. Carpological studies in
Cruciferae. – Palestine J. Bot. (Jerusalem) 4: 158-165.
Zohary M. 1948b. Follicular dehiscence in
Cruciferae. – Lloydia 11: 226-228.
Zohary M. 1960. The species of
Capparis in the Mediterranean and the Near Eastern countries. –
Bull. Res. Council Israel 8D. 49-64.
Zozomová-Lihová J, Marhold K, Španiel S.
2014. Taxonomy and evolutionary history of Alyssum montanum (Brassicaceae) and related taxa in
southwestern Eruope and Morocco: diversification driven by polyploidy,
geographic and ecological isolation. – Taxon 63: 562-591.
Zuberi MI, Lewis D. 1988.
Gametophytic-sporophytic incompatibility in the Crucerae Brassica
campestris. – Heredity 61: 367-377.
Žukova PG, Petrovsky VV. 1984. A
cytotaxonomical study of some species of the family Brassicaceae in northern Asia. –
Bot. Žurn. 69: 236-240. [In Russian]
Zunk K, Mummenhoff K, Hurka H. 1993.
Chloroplast DNA restriction site variation in the Brassicaceae. – Plant Mol. Evol.
Newsl. 3: 40-44.
Zunk K, Mummenhoff K, Koch M, Hurka H. 1996.
Phylogenetic relationships of Thlaspi s.l. (subtribe Thlaspidinae,
Lepidieae) and allied genera based on chloroplast DNA restriction-site
variation. – Theor. Appl. Gen. 92: 375-381.
Zunk K, Mummenhoff K, Hurka H. 1999.
Phylogenetic relationships in tribe Lepidieae (Brassicaceae) based on chloroplast
DNA restriction site variation. – Can. J. Bot. 77: 1504-1512.






