ROSANAE
Takht.
Takhtajan, Sist. Filog. Cvetk. Rast. [Syst.
Phylog. Magnolioph.]: 264. 4 Feb 1967
Rosopsida Batsch,
Tab. Regni Veg.: 1. 1802 [’Rosaceae’];
Hamamelididae Takht, Sist. Filog. Cvetk. Rast. [Syst.
Phylog. Magnolioph.]: 461. 4 Feb 1967, pro parte;
Rosidae Takht., Sist. Filog. Cvetk. Rast. [Syst.
Phylog. Magnolioph.]: 264. 4 Feb 1967 pro parte;
Dilleniidae Takht. ex Reveal et Takht. in Phytologia
74: 171. 25 Mar 1993, pro parte
[Fabidae+Malvidae]
FABIDAE
W. S. Judd, D. E. Soltis et P. S. Soltis
Judd, Soltis et Soltis in Taxon 56: E29. Aug
2007
(eurosids I)
[Zygophyllales+COM
clade+Nitrogen Fixing clade]
ZYGOPHYLLALES Link
Link, Handbuch 2: 228. 4-11 Jul 1829
[’Zygophylleae’]
Zygophyllanae Doweld,
Tent. Syst. Plant. Vasc.: xxxviii. 23 Dec 2001
Habit Usually bisexual (rarely
dioecious), usually evergreen shrubs, suffrutices, or perennial or annual herbs
(rarely trees), sometimes with axillary, simple or branched, spines. Often
xerophytes or halophytes. C4-photosynthesis present.
Vegetative anatomy Mycorrhiza
probably absent. Phellogen ab initio superficially or deeply seated (pericyclic
to deep cortical). Vessel elements with simple perforation plates; lateral pits
alternate, bordered pits. Imperforate tracheary elements tracheids or fibre
tracheids with simple or bordered pits, non-septate (also vasicentric
tracheids). Wood rays uniseriate or multiseriate, homocellular or
heterocellular. Axial parenchyma usually apotracheal diffuse,
diffuse-in-aggregates or banded (rarely paratracheal scanty vasicentric). Sieve
tube plastids usually S type (rarely Pcs type, with two unequally sized protein
crystalloids). Nodes 1:1 or 3:3, unilacunar with one leaf trace or trilacunar
with three traces. Parenchyma often with sclerenchymatous idioblasts.
Tanniniferous secretory cells sometimes present. Dark-brown substances present
in heartwood in some species (e.g. Guaiacum officinale).
Calciumoxalate present as druses or styloids, prismatic or acicular crystals,
often in idioblasts.
Trichomes Hairs usually
unicellular (sometimes multicellular), simple or furcate (rarely peltate or
capitate); glandular hairs rarely present.
Leaves Usually alternate
(spiral) or opposite, usually pinnately compound (rarely unifoliolate,
bifoliolate or trifoliolate), leaflets entire, usually persistent (rarely
caducous), often coriaceous, sometimes succulent or modified into spines, with
? ptyxis. Stipules cauline or interpetiolar (sometimes modified into spines) or
absent; leaf sheath absent. Petiole vascular bundle transection arcuate or
annular. Venation usually pinnate (rarely palmate, leaves sometimes uninerved),
eucamptodromous or brochidodromous. Stomata usually anomocytic or paracytic
(rarely cyclocytic or actinocytic), sometimes transversely orientated.
Cuticular wax crystalloids at least sometimes as platelets. Epidermis and
mesophyll sometimes with mucilaginous idioblast. Tannins and crystals often
frequent. Lamina rarely gland-dotted. Leaves or leaflet margins entire.
Inflorescence Usually axillary
(sometimes terminal or leaf-opposite), usually few-flowered cymose or racemose
of different shape, or flowers solitary axillary. Floral prophylls (bracteoles)
small or absent.
Flowers Usually actinomorphic
or zygomorphic. Usually hypogyny (rarely half epigyny). Sepals (four or) five
(or six), with imbricate or valvate aestivation, sometimes caducous, usually
free (rarely connate at base). Petals (four or) five (or six), usually with
imbricate aestivation, often clawed, usually free (sometimes partially
connate). Disc annular or intrastaminal and lobate, or absent, sometimes also
with extrastaminal and/or intrastaminal nectariferous glands.
Androecium Stamens usually
four, eight or ten (sometimes three, five or twelve), in one or two whorls,
antesepalous and/or alternisepalous (antesepalous whorl sometimes consisting of
staminodia or absent). Filaments sometimes winged, sometimes with scales or
other appendages at base, free or connate at base, usually free from tepals.
Anthers dorsifixed or basifixed, versatile or non-versatile, tetrasporangiate,
introrse to latrorse, longicidal (dehiscing by longitudinal slits) or poricidal
(dehiscing by apical pores or short slits). Tapetum secretory. Staminodia
usually absent (extrastaminal antesepalous staminal whorl or median abaxial
stamen rarely staminodial).
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually tricolporate (sometimes
tricolpate, tricolporoidate, tetracolporate, triporate, synorate, or
polypantoporate, rarely hexarugorate), shed as monads, bicellular or
tricellular at dispersal. Exine tectate or semitectate, with columellate
infratectum, usually reticulate (sometimes striate, rarely rugulate,
columellate or retipilate).
Gynoecium Pistil composed of
two to five (or six) connate antepetalous carpels. Ovary usually superior
(rarely semi-inferior), bilocular or (quadrilocular or) quinquelocular (to
duodecemlocular); locules sometimes divided by secondary septa. Style single,
simple (sometimes gynobasic). Stigma punctate, capitate, clavate or as
commissural ridges down style, papillate, Dry or Wet type. Pistillodium
absent.
Ovules Placentation
axile(-marginal; sometimes apical). Ovules usually one to ten (sometimes more
than ten) per carpel, usually anatropous (rarely campylotropous, hemianatropous
or orthotropous), pendulous, epitropous, bitegmic, weakly crassinucellar.
Micropyle bistomal or endostomal. Megagametophyte monosporous,
Polygonum type. Endosperm development ab initio nuclear. Endosperm
haustoria? Embryogenesis usually solanad (rarely caryophyllad).
Fruit Usually a loculicidal
and/or septicidal capsule (sometimes a nutlike capsule or a schizocarp with two
or five nutlike mericarps; rarely a drupe).
Seeds Aril usually absent.
Seed coat usually testal or exotestal (rarely endotegmic). Exotesta often
palisade, sometimes with enlarged tanniniferous cells. Endotesta with crystals,
often lignified. Exotegmen? Endotegmic cells periclinally elongate, lignified.
Perisperm not developed. Endosperm usually absent (sometimes oily). Embryo
straight or somewhat curved, well differentiated, at least sometimes with
chlorophyll. Cotyledons two. Germination phanerocotylar or cryptocotylar.
Cytology x = 6, 8–13 (14)
DNA Plastid gene infA
present. Mitochondrial intron coxII.i3 lost.
Phytochemistry Flavonols
(kaempferol, quercetin), methylated flavonoids, ethereal oils (sesquiterpenes
etc., in wood), oleanolic acid derivatives, tannins, phlobaphene, indole and
quinazoline alkaloids (harman alkaloids, e.g. harmane, harmine, harmol),
steroidal and triterpene saponins, anthraquinones, lignans, neolignans,
nor-neolignans, pinitol, apiitol, and N-methyltyrosine present.
Ellagic acid, tannins, proanthocyanidins, and cyanogenic compounds not
found.
Systematics Zygophyllales may be
sister-group to [‘Nitrogen fixing clade’+’COM clade’] (Soltis & al.
2011). Zhao & al. (2016) recovered a sister-group relationship between
either Zygophyllales
and Myrtales, or
between Zygophyllales
and [the COM clade+malvids].
Dumortier, Anal. Fam. Plant.: 20, 23. 1829, nom.
cons.
Krameriales Kunth in
C. F. P. von Martius, Consp. Regn. Veg.: 44. Sep-Oct 1835 [’Krameriaceae’]
Genera/species 1/18
Distribution Southern United
States to Chile and Argentina.
Fossils Unknown.
Habit Bisexual, usually shrubs
or suffrutices (Krameria lanceolata is a perennial herb, usually with
woody rhizome). Root hemiparasites.
Vegetative anatomy Mycorrhiza
absent. Phellogen ab initio deeply seated (sometimes in pericycle). Vessel
elements with simple perforation plates; lateral pits alternate, bordered pits.
Non-vestured pits present? Imperforate tracheary xylem elements
(fibre?)tracheids with bordered pits, non-septate (also vasicentric tracheids).
Wood rays usually uniseriate (rarely biseriate), homocellular. Axial parenchyma
apotracheal diffuse or diffuse-in-aggregates, or paratracheal scanty, or
banded. Wood elements sometimes (axial parenchyma) partially storied. Wood
fluorescent? Phloem non-lignified. Sieve tube plastids S type. Nodes 1:1,
unilacunar with one leaf trace. Parenchyma with tanniniferous secretory cells.
Rhomboidal calciumoxalate crystals present especially in secondary phloem.
Trichomes Hairs unicellular,
simple.
Leaves Leaves alternate
(spiral), usually simple? (in reality unifoliolate?, rarely trifoliolate),
entire, small, with ? ptyxis. Stipules and leaf sheath absent. Petiole vascular
bundle transection arcuate (hippocrepomorphic, sometimes almost circular).
Venation pinnate? Stomata usually paracytic (sometimes anomocytic). Cuticular
wax crystalloids as narrow platelets. Mesophyll with sclerenchymatous
idioblasts with sclereids and calciumoxalate druses. Tannins and crystals
abundant. Leaf(let) margin entire.
Inflorescence Terminal or
axillary, raceme-like or botryoid-paniculate, or flowers solitary axillary.
Floral prophylls (bracteoles) foliaceous, small.
Flowers Zygomorphic, probably
inverted. Pedicel articulated. Hypogyny. Sepals (four or) five, with imbricate
aestivation, petaloid inside, free; median sepal abaxial, larger than
remainder; three outer sepals larger than two inner sepals, often almost
enclosing remaining parts of flower. Petals (four or) five, with imbricate
aestivation; median petal adaxial; (two or) three adaxial petals long-clawed,
usually connate at base into velum; two abaxial petals often modified into
sessile glands, excreting bee-attracting lipids (free β-acetoxy fatty acids)
from elaiophores in epidermis. Nectary absent. Disc absent.
Androecium Stamens (three or)
four, adaxial, antesepalous, alternipetalous. Filaments stout, usually connate
at base, usually free from petals (sometimes adnate to claws of adaxial
petals). Anthers basifixed, curved, non-versatile, tetrasporangiate, introrse,
poricidal (dehiscing by one or two apical pores or short slits). Tapetum?
Staminodia usually absent (median abaxial stamen rarely staminodial).
Pollen grains
Microsporogenesis simultaneous? Pollen grains tricolporate, tetracolporate,
triporate or synorate, shed as monads, ?-cellular at dispersal. Exine tectate,
with columellate infratectum, striate.
Gynoecium Pistil composed of
two connate carpels; adaxial carpel early degenerating. Ovary superior,
unilocular (pseudomonomerous, first bilocular). Style single, simple, stout,
curved. Stigma small, punctate, sunken, type? Pistillodium absent.
Ovules Placentation apical.
Ovules two per carpel, collateral, anatropous, pendulous, bitegmic, weakly
crassinucellar. Micropyle endostomal. Outer integument approx. six cell layers
thick. Inner integument four or five cell layers thick. Megagametophyte
monosporous, Polygonum type. Endosperm development? Endosperm
haustoria? Embryogenesis?
Fruit A one-seeded nutlike
capsule with hairs and barbed spines; thin pericarp irregularly dehiscing.
Seeds Aril absent. Seed coat
exotestal. Exotestal cells enlarged, tanniniferous. Endotesta? Tegmen up to
seven cell layers thick above, mostly degenerating. Perisperm not developed.
Endosperm absent. Embryo straight, well differentiated, chlorophyll? Haustoria
formed from young adventitious roots. Cotyledons two, large, ventrally
flattened, cordate. Germination? Seedling without root hairs.
Cytology n = 6
DNA Plastid gene
rps16 absent (lost). Plastid gene infA?
Phytochemistry Tannins of
catechin type, phlobaphene (red root pigment), neolignans, nor-neolignans,
apiitol, and N-methyltyrosine present. Saponins not found.
Harman-alkaloids?
Use Medicinal plants, dyeing
substances (roots), cosmetics.
Systematics Krameria
(18; southwestern and southern United States, Mexico, Central America, the West
Indies, northern and central South America to Chile and Argentina).
Krameria
is sister to Zygophyllaceae.
Brown in Flinders, Voy. Terra Austral. 2: 545. 19
Jul 1814 [’Zygophylleae’], nom. cons.
Balanitaceae M.
Roem., Fam. Nat. Syn. Monogr. 1:26. 14 Sept-15 Oct 1846
[‘Balaniteae’], nom. cons.;
Zygophyllineae J. Presl in Nowočeská Bibl.
[Wšobecný Rostl.] 7: 265, 266. 1846 [‘Zygophylleae’];
Tribulaceae Trautv., Estestv. Istorija Gub. Kievsk.
Uchebn. Okr. Bot. Sist.: 28. 1853; Balanitales C. Y.
Wu in Acta Phytotaxon. Sin. 40: 314. 2002
Genera/species 19/190–210
Distribution Mainly tropical
and subtropical regions on both hemispheres, with their largest diversity in
arid and subarid areas; some species in warm-temperate regions.
Fossils Unknown.
Habit Usually bisexual (rarely
dioecious), evergreen shrubs, suffrutices, or perennial or annual herbs (rarely
trees), sometimes with axillary, simple or branched, spines. Nodes often
swollen and/or articulated. Bark often bitter. Some species are succulent. Many
species are xerophytes or halophytes. C4 photosynthesis present
(almost all species in Kallstroemia have C4 photosynthesis;
nearly all in Zygophyllum are C3 photosynthetic).
Vegetative anatomy Mycorrhiza
usually absent (arbuscular mycorrhiza sometimes present in Larrea).
Phellogen ab initio usually superficially (sometimes deeply) seated. Primary
vascular tissue cylinder without separate vascular bundles. Primary medullary
rays usually narrow (in Balanites wide). Secondary lateral growth
normal or anomalous. Vessel elements with simple perforation plates; lateral
pits alternate, bordered pits. Vestured pits present. Imperforate tracheary
elements tracheids or fibre tracheids (or libriform fibres?) with simple and/or
bordered pits, non-septate (also vasicentric tracheids). Wood rays uniseriate
or multiseriate, one or two (to four) cells wide (in Balanites up to
c. 35 cells wide), homocellular or heterocellular. Axial parenchyma usually
apotracheal diffuse, diffuse-in-aggregates or in uniseriate bands (rarely
paratracheal scanty vasicentric). Wood elements usually storied. Wood often
fluorescent. Sieve tube plastids usually S type (rarely Pcs type, with two
unequally sized protein crystalloids). Nodes usually 1:1, unilacunar with one
leaf trace, often also with split laterals (trilacunar nodes present in
Viscainoa). Cortex and parenchyma often with fibres and
sclerenchymatous idioblasts. Secretory cavities absent. Dark-brown substances
present in heartwood in some species (e.g. Guaiacum officinale).
Calciumoxalate present as druses and styloid, prismatic and acicular crystals,
often in idioblasts.
Trichomes Hairs usually
unicellular (sometimes multicellular), simple or furcate (rarely peltate or
capitate); glandular hairs present in Fagonia.
Leaves Usually opposite (in
Morkillia and Viscainoa alternate, spiral), usually pinnately
compound (rarely bifoliolate or trifoliolate or probably seemingly simple:
unifoliolate?), leaflets entire, usually persistent (rarely caducous), often
coriaceous, sometimes succulent or modified into spines, with usually flat
ptyxis. Stipules cauline or one interpetiolar (sometimes modified into spines;
rarely absent); leaf sheath absent. Petiole vascular bundle transection
annular; petiole with wing bundles. Venation usually pinnate (rarely palmate,
leaves sometimes one-veined), eucamptodromous or brochidodromous. Stomata
usually anomocytic (rarely paracytic, cyclocytic or actinocytic). Cuticular wax
crystalloids? Epidermis sometimes with mucilaginous idioblasts. Mesophyll with
or without sclerenchymatous idioblasts. Lamina rarely gland-dotted. Leaflet
margins usually entire (sometimes serrate).
Inflorescence Usually axillary
(sometimes terminal or leaf-opposite), few-flowered cymose or racemose of
different appearance, or flowers solitary. Floral prophylls (bracteoles)
absent.
Flowers Usually actinomorphic
(rarely zygomorphic). Usually hypogyny (rarely partially perigynous). Sepals
(four or) five (or six), with imbricate or valvate aestivation, sometimes
caducous, usually free (rarely connate at base). Petals (four or) five (or
six), usually with imbricate (or contorted?) aestivation, often clawed, free
(absent in Seetzenia and one species of Zygophyllum). Disc
annular or as intrastaminal lobes or absent, sometimes also with extrastaminal
and/or intrastaminal nectariferous glands.
Androecium Stamens usually
eight or ten (sometimes five or twelve), in one or two whorls, antesepalous
and/or alternisepalous (antesepalous whorl staminodial or absent), often
obdiplostemonous. Filaments sometimes winged, sometimes with scales or other
appendages at base, free from each other and from tepals. Anthers dorsifixed,
versatile, tetrasporangiate, introrse or latrorse, longicidal (dehiscing by
longitudinal slits). Tapetum secretory. Staminodia usually absent
(extrastaminal antesepalous staminal whorl rarely staminodial).
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually tricolporate (sometimes
tricolpate, tricolporoidate or polypantoporate, rarely hexarugorate), shed as
monads, bicellular or tricellular at dispersal. Exine semitectate, with
columellate infratectum, usually reticulate (rarely rugulate, columellate or
retipilate).
Gynoecium Pistil composed of
(two to) five (or six) connate antepetalous carpels. Ovary usually superior
(rarely semi-inferior), (quadrilocular or) quinquelocular (to duodecemlocular),
often angular or winged (sometimes stipitate, with gynophore); locules
sometimes divided by secondary septa. Style single, simple, short to long,
narrow (in Zygophyllum gynobasic). Stigma capitate, punctate, clavate
or as commissural ridges down style, papillate, Dry or Wet type. Pistillodium
absent?
Ovules Placentation
axile(-marginal; rarely apical). Ovules usually one to ten (sometimes more than
ten) per carpel, usually anatropous (rarely campylotropous, hemianatropous or
orthotropous), pendulous, epitropous (sometimes apotropous?), bitegmic, weakly
crassinucellar. Micropyle endostomal or bistomal, usually not Z-shaped (in
Balanites zig-zag). Outer integument two to six (to eight) cell layers
thick. Inner integument two to four (to six) cell layers thick. Obturator
present. Hypostase present. Endothelium usually present (absent in
Seetzenia). Parietal tissue one or two (to four) cell layers thick.
Nucellar cap sometimes present. Archespore sometimes multicellular.
Megagametophyte monosporous, Polygonum type, elongated. Endosperm
development ab initio nuclear. Endosperm haustoria? Embryogenesis usually
solanad (rarely caryophyllad).
Fruit Usually a loculicidal
and/or septicidal capsule (sometimes a schizocarp with two or five nutlike
mericarps; in Balanites a one-seeded drupe with oily mesocarp).
Seeds Aril usually absent.
Seed coat usually testal (rarely endotegmic). Exotesta often palisade.
Endotesta usually crystalliferous, often lignified. Exotegmen? Endotegmic cells
periclinally elongate, lignified. Perisperm not developed. Endosperm usually
absent (sometimes present, oily). Embryo straight or somewhat curved, at least
sometimes with chlorophyll. Cotyledons two. Germination phanerocotylar or
cryptocotylar.
Cytology x = 6, 8–13 (14)
– Polyploidy occurring.
DNA The plastid gene
infA is present.
Phytochemistry Flavonoids
(kaempferol, quercetin, isorhamnetin-3-rutinoside in Zygophyllum),
methylated flavonoids, ethereal oils (sesquiterpenes etc., in wood), oleanolic
acid derivatives, quinazoline alkaloids (harmane, harmin, harmol and other
harmala alkaloids), indole alkaloids, steroidal and triterpene saponins,
anthraquinones, lignans, neolignans, and pinitol present. Ellagic acid,
tannins, proanthocyanidins, and cyanogenic compounds not found. Mustard
oils?
Use Ornamental plants, timber
and carpentries (extremely hard wood, Lignum vitae, from Guaiacum),
fruits (Balanites aegyptiaca), medicinal plants, edible flower
buds.
Systematics Zygophyllaceae are
sister-group to Krameria
(Krameriaceae).
[[Morkillioideae+Balanitoideae]+[Seetzenioideae+[Larreoideae+Zygophylloideae]]]
is a plausible topology (Sheahan & Case 2000).
[Morkillioideae+Balanitoideae]
Morkillioideae Thorne
et Reveal in Bot. Rev. (Lancaster) 73: 106. 29 Jun 2007
3/4. Morkillia (2; M.
acuminata, M. mexicana; Mexico), Viscainoa (1; V.
geniculata; Baja California in northwestern Mexico), Sericodes
(1; S. greggii; northern Mexico). – Mexico. Leaves usually alternate
(spiral).
Balanitoideae Engl. in
Engler et Prantl, Nat. Pflanzenfam. III, 4: 354, 355. Jul 1896
6/80–90. Tribulus
(40–45; tropical and subtropical regions on both hemispheres),
Kallstroemia (30–35; tropical and subtropical America),
Kelleronia (3; K. gillettiae, K. revoilii, K.
splendens; northeastern Africa, southern Arabian Peninsula),
Sisyndite (1; S. spartea; southern Namibia, Northern and
northwestern Western Cape), Neoluederitzia (1; N.
sericeocarpa; southern Namibia), Balanites (9; tropical Africa,
the Arabian Peninsula to India and Burma). – Tropical to warm-temperate
regions on both hemispheres. C4 photosynthesis frequent in
Kallstroemia. Fruit usually a schizocarp with four or five or up to
ten single-seeded nutlike mericarps. – Balanites are spiny shrubs or
trees with bitter bark. Medullary and wood rays very wide. Stipules tiny
(absent?). Petiole anatomy complicated. Stem stomata transversely orientated
relative to longitudinal axis. Leaves alternate (spiral) and pinnately compound
with one pair of leaflets. Corolla enclosing floral bud. Ovule one per carpel,
pendulous. Micropyle bistomal, Z-shaped (zig-zag). Outer integument four to
eight cell layers thick. Inner integument three to six cell layers thick. Fruit
a single-seeded drupe. Endosperm absent. Testa multiplicative, vascularized.
[Seetzenioideae+[Larreoideae+Zygophylloideae]]
Seetzenioideae M. C.
Sheahan et M. W. Chase in Bot. J. Linn. Soc. 122: 299. 18 Dec 1996
1/1. Seetzenia (1; S.
lanata; North Africa to Afghanistan and India, Northern and Eastern Cape).
– Prostrate perennial herb. Leaves opposite, trifoliolate. Petals absent.
Stamens five. Filaments without scale-like processes. Carpels five. Stylidia
five. Ovule one per carpel, epitropous. Micropyle bistomal. Outer integument
six or seven cell layers thick. Endothelium absent. Fruit a septicidal capsule,
dehiscing into five single-seeded cocci with fleshy exocarp and bony endocarp.
Endosperm present.
[Larreoideae+Zygophylloideae]
Larreoideae M. C.
Sheahan et M. W. Chase in Bot. J. Linn. Soc. 122: 299. 18 Dec 1996
8/32–36. Bulnesia
(8–10; South America), Guaiacum
(8–10; tropical and subtropical America), Porlieria (5; P.
angustifolia, P. arida, P. chilensis, P.
hygrometra, P. microphylla; Mexico, the Andes), Larrea
(6; L. ameghinoi, L. cuneifolia, L. divaricata,
L. nitida, L. simulans, L. tridentata; southwestern
United States, Mexico, South America), Pintoa (1; P.
chilensis; Chile), Plectrocarpa (2; P. rougesii, P.
tetracantha; temperate South America), Metharme (1; M.
lanata; northern Chile); unplaced: Izozogia (1; I.
nellii; Bolivia). – Southwestern United States, Mexico, Central and
South America to Chile and Argentina. Sieve tube plastids in Larrea
with protein and starch. Filaments often with scale-like processes. Ovary
stipitate or sessile. Capsule often winged. Seed one per locule. Endosperm
present.
Zygophylloideae Arn.,
Botany: 104. 9 Mar 1832 [‘Zygophylleae’]
1/80–85. Zygophyllum
(80–85; Macaronesia, the Mediterranean, southeastern Europe, northern and
northeastern Africa to Iran, Central Asia and northwestern India, China,
tropical and subtropical to southern Africa, Australia, southwestern United
States, Mexico). – Mainly drier regions of the Old World, southwestern United
States, Chile. Nectariferous disc octa- or decemangulate. Stamens eight or ten.
Filaments with basal scale-like processes. Style in Zygophyllum
simple, gynobasic. Stigma punctate. Outer and inner integuments in Zygophyllum
approx. two cell layers thick. Fruit an angular to winged capsule or
schizocarp.
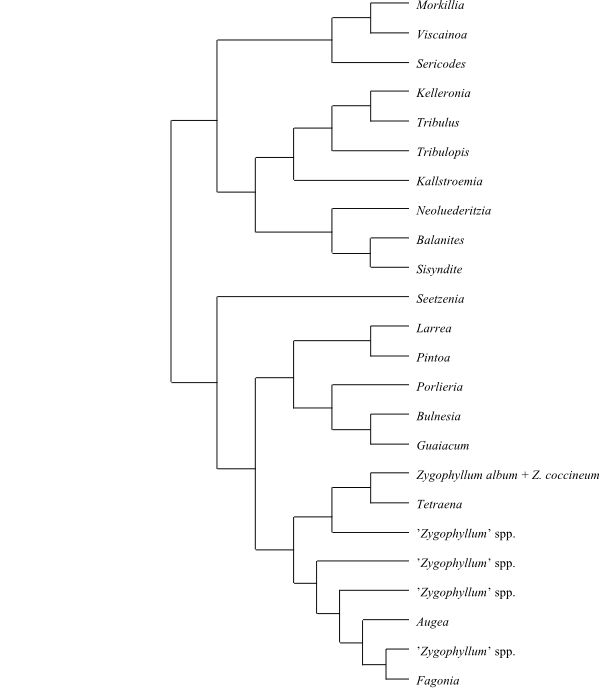 |
Cladogram (simplified) of Zygophyllaceae
based on DNA sequence data (Sheahan & Chase 2000).
|
Literature
Achenbach H, Gross J, Dominguez XA, Cano G,
Star JV, Brussolo L d C, Muñoz FSG, López L. 1987. Lignans, neolignans, and
nor-neolignans from Krameria cytisoides. – Phytochemistry 26:
1159-1166.
Achenbach H, Gross J, Dominguez XA, Star JV,
Salgado F. 1987. Ramosissin and other methoxylated nor-neolignans from
Krameria ramosissima. – Phytochemistry 26: 2041-2043.
Achenbach H, Gross J, Bauereiss P, Dominguez
XA, Vega HS, Star JV, Rombold C. 1989. Nor-lignans and
nor-neolignans from Krameria lanceolata. – Phytochemistry
28: 1959-1962.
Agababyan VS. 1964. Morphological types of
pollen and systematic classification of the Zygophyllaceae. – Izv. Akad.
Nauk Armyanskoi, SSR, Biol. Nauk 17: 39-45. [In Russian]
Axelrod FS. 2002. Proposal to conserve the
name Guaiacum (Zygophyllaceae) with that
spelling. – Taxon 51: 203-204.
Barker RJ. 1996. New taxa, new combinations,
keys and comments on generic concepts of Zygophyllum and a new species
of Tribulus (Zygophyllaceae) in the
manuscripts of the late H. J. Eichler. – J. Adelaide Bot. Gard. 17:
161-172.
Barker RJ. 1998a. Notes on the genus
Tribulopsis (Zygophyllaceae) in Australia.
– J. Adelaide Bot. Gard. 18: 77-93.
Barker RJ. 1998b. A trial key and notes on
Tribulus (Zygophyllaceae) in Australia,
including one new species and validation of Tribulus suberosus. –
Nuytsia 12: 9-35.
Behnke H-D. 1988. Sieve-element plastids and
systematic relationships of Rhizophoraceae, Anisophylleaceae
and allied groups. – Ann. Missouri Bot. Gard. 75: 1387-1409.
Beier B-A. 2005. A revision of the desert
shrub Fagonia (Zygophyllaceae). – Syst.
Biodivers. 3: 221-263.
Beier B-A, Thulin M. 2004. Proposal to
conserve the name Tetraena against Petrusia (Zygophyllaceae). – Taxon 53:
1078-1079.
Beier B-A, Chase MA, Thulin M. 2003.
Phylogenetic relationships and taxonomy of subfamily Zygophylloideae (Zygophyllaceae) based on
molecular and morphological data. – Plant Syst. Evol. 240: 11-39.
Beier B-A, Nylander JAA, Chase MA, Thulin M.
2004. Phylogenetic relationships and biogeography of the desert plant genus
Fagonia (Zygophyllaceae), inferred by
parsimony and Bayesian model averaging. – Mol. Phylogen. Evol. 33: 91-108.
Bellstedt DU, Zyl L van, Marais EM, Bytebier
B, Villiers CA de, Makwarela AM, Dreyer LL. 2008. Phylogenetic relationships,
character evolution and biogeography of southern African members of
Zygophyllum (Zygophyllaceae) based on three
plastid regions. – Mol. Phylogen. Evol. 47: 932-949.
Bellstedt DU, Zyl L van, Marais EM, Bytebier
B, Villiers CA de, Dreyer LL, Galley C, Pirie M, Linder HP. 2008. A molecular
phylogeny reveals evidence of rapid and recent radiation in Cape and Australian
members of the genus Zygophyllum. – South Afr. J. Bot. 74: 361.
Bellstedt DU, Galley C, Pirie MD, Linder HP.
2012. The migration of the Palaeotropical arid flora: Zygophylloideae as an
example. – Syst. Bot. 37: 951-959.
Boesewinkel FD. 1994. Ovules and seed
characters of Balanites aegyptiaca and the classification of the
Linales-Geraniales-Polygalales assembly.
– Acta Bot. Neerl. 43: 15-25.
Busse-Jung F. 1979. Phytoserologische
Untersuchungen zur Frage der systematischen Stellung von Krameria
triandra Ruiz et Pav. – Ph.D. diss., Christian-Albrechts-Universität,
Kiel, Germany.
Cannon WA. 1910. The root habits and
parasitism of Krameria canescens Gray. – In: MacDougall DT, Cannon
WA (eds), The conditions of parasitism in plants, Publ. Carnegie Inst.
Washington 129: 5-24.
Carlquist SJ. 2005. Wood anatomy of Krameriaceae with comparisons with
Zygophyllaceae: phylesis,
ecology and systematics. – Bot. J. Linn. Soc. 149: 257-270.
Carranco ME, Perez GF. 1989. A study on the
chemical composition and toxicological factors of Guayacan Viscainoa
geniculata. – Cuban J. Agricult. Sci. 23: 333-336.
Castro MA. 1981. Anatomia foliar del género
Plectrocarpa 35: 137-147.
Chodat M. 1890. Un travail monographique sur
la famille des Kramériacées. – Arch. Sci. Phys. Nat. 24: 495-499.
Crisci JV, Hunziker JH, Palacios RA, Naranjo
CA. 1979. A numerical-taxonomic study of the genus Bulnesia (Zygophyllaceae): cluster
analysis, ordination and simulation of evolutionary trees. – Amer. J. Bot.
66: 133-140.
Crookston RK, Moss DN. 1972. C4
and C3 carboxylation characteristics in the genus
Zygophyllum. – Ann. Missouri Bot. Gard. 59: 465-470.
Descole HR, O’Donnell CA, Lourteig A. 1940.
Revisión de las Zygofiláceas Argentinas. – Lilloa 5: 257-343.
Dhillon M. 1976. Vascular anatomy of the
flower of Krameria Parvifolia var. glandulosa Macbr. and its
bearing on its taxonomic status. – J. Res. Punjab Agric. Univ. 13:
197-201.
Domínguez XA, Espinoza BG, Rombold C, Utz W,
Achenbach H. 1992. Neolignans, norneolignans, and other compounds from
Krameria sonorae. – J. Med. Plant Res. 58: 382-383.
Eichler H. 1990. Four new species of
Zygophyllum (Zygophyllaceae) and one
lectotypification. – Telopea 4: 13-17.
El Hadidi MN. 1966. The genus
Fagonia L. in Egypt. – Candollea 21: 19-53.
El Hadidi MN. 1972a. Neue Beobachtungen an
der Gattung Fagonia L. – Candollea 27: 85-96.
El Hadidi MN. 1972b. The family Zygophyllaceae in Egypt I.
Fagonia L. and Seetzenia R. Br. – Bot. Not. 125:
523-535.
El Hadidi MN. 1973. Revision of
Fagonia species (Zygophyllaceae) with tri- to
unifoliolate and simple leaves. – Österr. Bot. Zeitschr. 121: 269-278.
El Hadidi MN. 1975. Zygophyllaceae in Africa. –
Boissiera 24: 317-323.
El Hadidi MN. 1977a. Tribulaceae as a
distinct family. – Publ. Cairo Univ. Herb. 7-8: 103-108.
El Hadidi MN. 1977b. Two new
Zygophyllum species from Arabia. – Publ. Cairo Univ. Herb. 7-8:
327-331.
El Hadidi MN. 1978a. The genus
Zygophyllum in Egypt. – Bot. Not. 131: 439-443.
El Hadidi MN. 1978b. Adumbratio florae
aethiopicae: Zygophyllaceae.
– Webbia 33: 45-101.
El Hadidi MN. 1985. Zygophyllaceae. – In: Polhill
RM (ed), Flora of tropical East Africa, A. A. Balkema, Rotterdam, pp. 1-15.
El Naggar SM, Abdel-Hafez SII. 2007. Pollen
morphology, leaf surfaces, mycobiota diversity and leaf spots of three species
of Zygophyllum growing along Cairo-Suez desert road, Eastern (Arabian)
desert in Egypt. – Feddes Repert. 118: 38-45.
Engler A. 1896a. Zygophyllaceae. – In: Engler
A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(4), W. Engelmann,
Leipzig, pp. 74-93.
Engler A. 1896b. Über die geographische
Verbreitung der Zygophyllaceen. – Königliche Akademie der Wissenschaften,
Berlin.
Engler A (†). 1931. Zygophyllaceae. – In: Engler A
(†), Harms H, Pax F (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd.
19a, W. Engelmann, Leipzig, pp. 144-184.
Fahn A, Shimony C. 1996. Glandular trichomes
of Fagonia L. (Zygophyllaceae) species:
structure, development and secreted materials. – Ann. Bot. 77: 25-34.
Gadek PA, Fernando ES, Quinn CJ, Hoot SB,
Terrazas T, Sheahan MC, Chase MW. 1996. Sapindales: molecular
delimitation and infraordinal groups. – Amer. J. Bot. 83: 802-811.
Hilu KW. 1970. Cytotaxonomy of some taxa of
Zygophyllaceae in Iraq. –
M.Sc. thesis, University of Baghdad, Iraq.
Hilu KW. 1981. Cytotaxonomical studies in
Tribulus terrestris and T. alatus (Zygophyllaceae). – Nord. J.
Bot. 1: 531-534.
Hosni HA. 1977. A new Tribulus
species with winged carpels. – Bot. Not. 130: 261-262.
Hosni HA. 1977. New Zygophyllum taxa
from Egypt. – Bot. Not. 130: 467-468.
Hunziker JH. 1975. On the geographical origin
of Larrea divaricata (Zygophyllaceae). – Ann.
Missouri Bot. Gard. 62: 497-500.
Hunziker JH, Palacios RA, Poggio L, Naranjo
CA, Yang T-W. 1977. Geographic distribution, morphology, hybridization,
cytogenetics, and evolution. – In: Mabry TJ, Hunziker JH, DiFeo DR Jr (eds),
Creosote bush: biology and chemistry of Larrea in New World deserts,
Dowden, Hutchinson & Ross, Stroudsburg, Pennsylvania, pp. 10-47.
Hussein SR, Kawashty SA, Tantawy ME, Saleh
NAM. 2009. Chemosystematic studies of Nitraria retusa and selected
taxa of Zygophyllaceae in
Egypt. – Plant Syst. Evol. 277: 251-264.
Huyssteen DC van. 1937.
Morphologisch-systematische Studien über die Gattung Zygophyllum. –
Ph.D. diss., Math.-Naturwiss. Fakultät, Friedrich-Wilhelms-Universität,
Berlin, Germany.
Inamdar JA. 1969. Epidermal structure,
stomatal ontogeny, and relationship of some Zygophyllaceae and Simaroubaceae. – Flora
158B: 360-368.
Inamdar JA, Patel RC. 1970. Epidermal
structure and development of stomata in vegetative and floral organs of
Fagonia cretica Linn. – Flora 159B: 63-70.
Keighery GJ. 1982. Geocarpy in
Tribulopsis R. Br. (Zygophyllaceae). – Flora 172:
329-333.
Kunz M. 1913. Die systematische Stellung der
Gattung Krameria unter besonderer Berücksichtigung der Anatomie. –
Beih. Bot. Centralbl. 30: 412-427.
Lahham JN, Al-Eisawi D. 1986. Pollen
morphology of Jordanian Zygophyllaceae. – Candollea
41: 325-328.
Launert E. 1963a. 36. Zygophyllaceae. – In: Exell
AW, Fernandes A, Wild H (eds), Flora Zambesiaca 2 (Part 1), Crown Agents for
Oversea Governments and Administrations, London, pp. 125-130.
Launert E. 1963b. 43. Balanitaceae. – In:
Exell AW, Fernandes A, Wild H (eds), Flora Zambesiaca 2 (Part 1), Crown Agents
for Oversea Governments and Administrations, London, pp. 221-224.
Lauterbach M, Wet van der Merwe P de, Keßler
L, Pirie MD, Bellstedt DU, Kadereit G. 2016. Evolution of leaf anatomy in arid
environments – a case study in southern African Tetraena and
Roepera (Zygophyllaceae). – Mol.
Phylogen. Evol. 97: 129-144.
Leinfellner W. 1971. Das Gynözeum von
Krameria und sie Vergleich mit jenem der Leguminosae und der Polygalaceae. –
Österr. Bot. Zeitschr. 119: 102-117.
Lia VV, Confalonieri VA, Comas CI, Hunziker
JH. 2001. Molecular phylogeny of Larrea and its allies (Zygophyllaceae): reticulate
evolution and the probable time of the creosote bush arrival to North America.
– Mol. Phylogen. Evol. 21: 309-320.
Ma Y-C, Zhang S. 1990. Study on the
systematic position of Tetraena Maxim. – Acta Phytotaxon. Sin. 28:
89-95.
Mabry TJ, Hunziker JH, DiFeo DR Jr (eds),
1977a. Creosote bush: biology and chemistry of Larrea in New World
deserts, Dowden, Hutchinson & Ross, Stroudsburg, Pennsylvania.
Mabry TJ, DiFeo DR Jr, Sakakibara M,
Bohnstedt CF Jr, Seigler D. 1977b. The natural products of Larrea. –
In: Mabry TJ, Hunziker JH, DiFeo DR Jr (eds), Creosote bush: biology and
chemistry of Larrea in New World deserts, Dowden, Hutchinson &
Ross, Stroudsburg, Pennsylvania, pp. 115-134.
Maksoud SA, El-Hadidi MN. 1988. The
flavonoids of Balanites aegyptiaca from Egypt. – Plant Syst. Evol.
160: 153-158.
Mathur A, Bhandari MM. 1983. Studies on
pollen grains of Fagonia and Seetzenia species. – J. Econ.
Taxon. Bot. 4: 331-334.
Mauritzon J. 1934. Etwas über die
Embryologie der Zygophyllaceen sowie einige Fragmente über die der
Humiriaceen. – Bot. Not. 87: 407-422.
Milby TH. 1971. Floral anatomy of
Krameria lanceolata. – Amer. J. Bot. 58: 569-576.
Mohamed AH. 2006. Taxonomic significance of
seed proteins and iso-enzymes in Tribulus (Zygophyllaceae). –Intern. J.
Agricult. Biol. 8: 573-575.
Musselman LJ. 1975. Parasitism and haustorial
structure in Krameria lanceolata (Krameriaceae). A preliminary
study. – Phytomorphology 25: 416-422.
Nag TN et al. 1986. Free endogenous ascorbic
acid from Zygophyllaceous plants growing in the arid zone of Rajasthan. – J.
Indian Bot. Soc. 9: 112-113.
Nair NC, Jain RK. 1956. Floral morphology and
embryology of Balanites roxburghii Planch. – Lloydia 19: 269-279.
Nair NC, Nathawat KS. 1958. Vascular anatomy
of the flower of some species of Zygophyllaceae 1. – J. Indian
Bot. Soc. 37: 172-180.
Narayana HS, Prakasa Rao CG. 1963. Floral
morphology and embryology of Seetzenia orientalis Decne. –
Phytomorphology 13: 197-205.
Narayana L, Satyauarayana P, Radhakrishnaiah
H. 1990. Systematic position of Balanitaceae. – In: Bilgrami K, Dogra J
(eds), Phytochemistry and plant taxonomy, CBS Publ., Delhi, pp. 157-164.
Navarro G. 1997. Izozogia nellii (Zygophyllaceae), Nuevo Género y
Especie del Gran Chaco de Santa Cruz (Bolivia). – Novon 7: 1-5.
O’Gara RW, Lee CW, Morton JF, Kapadia GJ,
Dunham LJ. 1974. Sarcoma induced in rats by extracts of plants and by
fractionated extracts of Krameria ixina. – J. Natl. Cancer Inst. 52:
445-448.
Ozenda P, Quézel P. 1956. Les
Zygophyllacées de l’Afrique du Nord et du Sahara. – Trav. Inst. Rech.
Sahariennes 14: 23-83.
Palacios RA, Hunziker JH. 1984. Revisión
taxonómica del género Bulnesia (Zygophyllaceae). – Darwiniana
25: 299-320.
Parameswaran N, Conrad H. 1982. Wood and bark
anatomy of Balanites aegyptiaca in relation to ecology and taxonomy.
– IAWA Bull., N. S., 3: 75-88.
Pederiva R, Kavka J, D’Archangelo A. 1975.
Chalcones and flavanones isolated from Larrea nitida. – Ann. Asoc.
Quim. Argent. 63: 85-90.
Perveen A, Qaiser M. 2006. Pollen flora of
Pakistan XLIX. Zygophyllaceae. – Pak. J. Bot.
38: 252-232.
Phatak VG. 1971. Embryology of
Zygophyllum coccineum L. and Z. fabago L. – Proc. Kon.
Nederl. Akad. Wetensch. 74C: 379-397.
Poggio L. 1978. Éstudios cromosómicos en
Bulnesia, Pintoa, Porlieria, Plectrocarpa y
Sericodes (Zygophyllaceae). – Darwiniana
21: 139-151.
Poggio L, Burghardt AD, Hunziker JH. 1989.
Nuclear DNA variation in diploid and polyploid taxa of Larrea (Zygophyllaceae). – Heredity
63: 321-328.
Poggio L, Hunziker JH, Wulff AF. 1992.
Cariotipo y contenido de ADN nuclear de Pintoa chilensis y
Sisyndite spartea (Zygophyllaceae). – Darwiniana
31: 11-15.
Popov G. 1925. Generis Zygophylli species
asiaticae. – Bull. Univ. l’Asie Centr. (Taschkent) 11: 105-122.
Popov G. 1926. Generis Zygophylli species
asiaticae. – Bull. Univ. l’Asie Centr. (Taschkent) 12: 109-125.
Porter DM. 1963. Taxonomy and distribution of
the Zygophyllaceae of Baja
California, Mexico. – Contr. Gray Herb. 192: 99-135.
Porter DM. 1969. The genus
Kallstroemia (Zygophyllaceae). – Contr. Gray
Herb. 198: 41-153.
Porter DM. 1971. Notes on the floral glands
in Tribulus (Zygophyllaceae). – Ann.
Missouri Bot. Gard. 58: 1-5.
Porter DM. 1974. Disjunct distributions in
the New World Zygophyllaceae.
– Taxon 23: 339-346.
Porter DM. 2005. 90. Zygophyllaceae. – In: Harling
G, Andersson L (eds), Flora of Ecuador 75, Botanical Institute, Göteborg
University, pp.17-29.
Praglowski J. 1987. Pollen morphology of
Tribulaceae. – Grana 26: 193-211.
Rose JN. 1909. Studies of Mexican and Central
American plants VI. – Contr. U.S. Natl. Herb. 12: 275.
Saleh NAM, El Hadidi MN. 1977. An approach to
the chemosystematics of the Zygophyllaceae. – Biochem.
Syst. Ecol. 5: 121-128.
Saleh N, El Hadidi MN, Ahmed A. 1982. The
chemosystematics of Tribulaceae. – Biochem. Syst. Ecol. 10: 313-317.
Sands MJS. 1983. Notes on Balanites
from the Somali Republic and Ethiopia. – Kew Bull. 38: 40.
Sands MJS. 1989. Balanitaceae. – In:
Hedberg I, Edwards S (eds), Flora of Ethiopia III, Addis Ababa University,
Ethiopia, and Uppsala University, Sweden, pp. 433-436.
Sands MJS. 2001. The desert date and its
relatives: a revision of the genus Balanites. – Kew Bull. 56:
1-128.
Sands MJS. 2003. Balanitaceae. – In:
Beentje HJ, Ghazanfar SA (eds), Flora of tropical East Africa, A. A. Balkema
Publ., Lisse, The Netherlands, pp. 1-14.
Sarma V, Rao SRS. 1991. Taxonomic importance
of epidermis in Simaroubaceae-Zygophyllaceae with special
reference to position of Balanites. – Feddes Repert. 102:
579-585.
Schweickerdt HG. 1937. An account of the
South African species of Tribulus Tour. ex Linn. – Bothalia 3:
159-178.
Seigler D, Simpson BB, Martin C, Neff JL.
1978. Free 3-acetoxy fatty acids in floral glands of Krameria species.
– Phytochemistry 17: 995-996.
Sheahan MC. 2006. Zygophyllaceae. – In: Kubitzki
K (ed), The families and genera of vascular plants IX. Flowering plants.
Eudicots. Berberidopsidales,
Buxales, Crossosomatales, Fabales p. p., Geraniales, Gunnerales, Myrtales p. p., Proteales, Saxifragales, Vitales, Zygophyllales, Clusiaceae Alliance, Passifloraceae
Alliance, Dilleniaceae, Huaceae, Picramniaceae, Sabiaceae, Springer, Berlin,
Heidelberg, New York, pp. 488-500.
Sheahan MC, Chase MW. 1996. A phylogenetic
analysis of Zygophyllaceae R.
Br. based on morphological, anatomical and rbcL DNA sequence data. –
Bot. J. Linn. Soc. 122: 279-300.
Sheahan MC, Chase MW. 2000. Phylogenetic
relationships within Zygophyllaceae based on DNA
sequences of three plastid regions, with special emphasis on Zygophylloideae.
– Syst. Bot. 25: 371-384.
Sheahan MC, Cutler DF. 1993. Contribution of
vegetative anatomy to the systematics of the Zygophyllaceae R. Br. – Bot.
J. Linn. Soc. 113: 227-262.
Simpson BB. 1982. Krameria (Krameriaceae) flowers: orientation
and elaiophore morphology. – Taxon 31: 517-528.
Simpson BB. 1989. Flora Neotropica. Monograph
49. Krameriaceae. – New York
Botanical Garden, Bronx, New York.
Simpson BB. 1991. The past and present uses
of rhatany (Krameria, Krameriaceae). – Econ. Bot. 45:
397-409.
Simpson BB. 2006. Krameriaceae. – In: Kubitzki K
(ed), The families and genera of vascular plants IX. Flowering plants.
Eudicots. Berberidopsidales,
Buxales, Crossosomatales, Fabales p. p., Geraniales, Gunnerales, Myrtales p. p., Proteales, Saxifragales, Vitales, Zygophyllales, Clusiaceae Alliance, Passifloraceae
Alliance, Dilleniaceae, Huaceae, Picramniaceae, Sabiaceae, Springer, Berlin,
Heidelberg, New York, pp. 208-212.
Simpson BB, Neff JL. 1978. Dynamics and
derivation of the pollination syndrome of Krameria (Krameriaceae). – Bot. Soc. Amer.
Misc. Publ. 156: 14.
Simpson BB, Skvarla JJ. 1981. Pollen
morphology and ultrastructure of Krameria (Krameriaceae): utility in
questions of intrafamilial and interfamilial classification. – Amer. J. Bot.
68: 277-294.
Simpson BB, Neff JL, Seigler DL. 1977.
Krameria, free fatty acids and oil-collecting bees. – Nature 267:
150-151.
Simpson BB, Neff JL, Moldenke AR. 1977.
Reproductive systems of Larrea. – In: Mabry TJ, Hunziker JH, DiFeo Dr Jr
(eds), Creosote bush: biology and chemistry of Larrea in New World
deserts, Dowden, Hutchinson & Ross, Stroudsberg, Pennsylvania, pp.
92-114.
Simpson BB, Seigler DS, Neff JL. 1978. Lipids
from the floral glands of Krameria. – Biochem. Syst. Ecol. 7:
193-194.
Simpson BB, Weeks A, Helfgott DM, Larkin LL.
2004. Species relationships in Krameria (Krameriaceae) based on ITS
sequences and morphology: implications for character utility and biogeography.
– Syst. Bot. 29: 97-108.
Stahl E, Ittel I. 1981. Neue lipophile
Benzofuranderivate aus Ratanhiawurzeln. – Planta Medica 42: 144-154.
Sterling CM. 1912. Krameria
canescens Gray. – Kansas Univ. Sci. Bull. 6: 363-372.
Taubert P. 1892. Leguminosae II. 6.
Caesalpinioideae-Kramerieae. – In: Engler A, Prantl K (eds), Die natürlichen
Pflanzenfamilien, III, 3, W. Engelmann, Leipzig, pp. 166-168.
Teppner H. 1984. Karyologie von Krameria
triandra (Krameriaceae).
– Mitteilungsbl. Kurzfass. Beiträge, Botaniker-Tagung, 1-14 September,
Wien.
Thulin M. 1993. Flora of Somalia 1. Zygophyllaceae (including
Tribulaceae). – Royal Botanic Gardens, Kew.
Turner BL. 1958. Chromosome numbers in the
genus Krameria: evidence for familial status. – Rhodora 60:
101-106.
Turner BL. 2016. Overview of
Kallstroemia (Zygophyllaceae) in the USA and
Mexico, and description of a new species: Kallstroemia porteri. –
Phytologia 98: 89-99.
Verkerke W. 1985. Ovule ontogeny and seed
coat development in Krameria Loefling (Krameriaceae). – Beitr. Biol.
Pflanzen 60: 341-351.
Weberling F. 1956. Weitere Untersuchungen zur
Morphologie des Unterblattes bei den Dikotylen III. Convolvulaceae;
IV. Zygophyllaceae. –
Beitr. Biol. Pflanzen 33: 149-161.
Wei N. 1991. A comparative anatomy on the
vegetative organs of Tetraena mongolica Maxim. and Zygophyllum
xanthoxylum (Bunge) Maxim. – Acta Scient. Natur. Univ. Intramongolicae
22: 528-533.
Wu S, Tu L. 1990. The embryology of
Tetraena mongolica Maxim. – Acta Scient. Natur. Univ.
Intramongolicae 21: 177-183.
Xi Y, Zhou S. 1989. Pollen morphology and its
exine ultrastructure of the Zygophyllaceae in China. –
Bot. Research (China) 4: 75-86.
Xi Y, Zhou S. 1992. A contribution to the
pollen morphology of Tetraena and Malpighiaceae, with
discussion of the affinity and taxonomic position of Tetraena. –
Chinese J. Bot. 4: 6-12.
Zohary M, Orshan G. 1954. Ecological studies
in the vegetation of the near eastern deserts V. The Zygophylletum dumosi and
its hydroecology in the Negev of Israel. – Vegetatio 5-6: 340-350.
Zyl L van. 2000. A systematic revision of
Zygophyllum (Zygophyllaceae) in the southern
African region. – Ph.D. diss., University of Stellenbosch, Republic of South
Africa.
Zyl L van, Marais EM. 1999. Three new species
of Zygophyllum (Zygophyllaceae) from Namibia and
the Northern Cape, South Africa. – Bothalia 29: 231-237.
