”The Nitrogen Fixing
clade”
[Polygalales+[Rosales+[Cucurbitales+Juglandales]]]
CUCURBITALES Juss. ex Bercht. et
J. Presl
Berchtold et Presl, Přir. Rostlin: 236. Jan-Apr
1820 [‘Cucurbitaceae’]
Cucurbitopsida
Brongn., Enum. Plant. Mus. Paris: xxx, 116. 12 Aug 1843
[’Cucurbitineae’]; Cucurbitanae Reveal
in Phytologia 76: 4. 2 Mai 1994
Habit Monoecious,
andromonoecious, gynomonoecious, polygamomonoecious, dioecious, androdioecious,
or gynodioecious (rarely bisexual), evergreen or deciduous trees, shrubs,
lianas, suffrutices, or perennial (rarely annual) herbs.
Vegetative anatomy Root
nodules containing nitrogen-fixing endosymbiotic actinobacteria
(Frankia) often present; Frankia infection sometimes via
intercellular penetration. Phellogen ab initio superficially or deeply seated.
Secondary lateral growth normal or anomalous (from concentric cambia or simple
cambial cylinder). Cambial initials storied, fusiform. Vessel elements with
usually simple (sometimes scalariform) perforation plates; lateral pits
alternate or scalariform, simple or reduced bordered pits. Imperforate
tracheary xylem elements libriform fibres with simple or bordered pits, septate
or non-septate (also vasicentric tracheids). Wood rays multiseriate,
homocellular or heterocellular, or absent. Axial parenchyma apotracheal,
diffuse or diffuse-in-aggregates, or paratracheal scanty, aliform,
lozenge-aliform, winged-aliform, confluent or vasicentric, or banded.
Intraxylary phloem usually present. Sieve tube plastids usually Ss type
(sometimes S0 type). Nodes 1:1 or 1:3, unilacunar with one or three
leaf traces, 3:1–3, trilacunar with one to three traces, or 5:5, pentalacunar
with five traces. Heartwood sometimes with resinous substances. Branched
idioblasts with bitter substances. Calciumoxalate as prismatic crystals, druses
and raphides.
Trichomes Hairs unicellular or
multicellular, uniseriate or multiseriate, simple or branched, sometimes
bristle-like, candelabra-shaped, stellate or prickles; glandular hairs often
present, sometimes lepidote glands and pearl glands; cell walls of hairs often
calcified; calcified cystoliths and similar structures often present at hair
bases or in adjacent cells.
Leaves Usually alternate
(spiral or distichous, sometimes opposite, rarely verticillate), simple or
usually palmately (rarely pinnately) compound, entire or palmately (rarely
pinnately) lobed, with conduplicate ptyxis. Stipules present (sometimes large,
cauline-extrapetiolar or intrapetiolar) or absent; leaf sheath absent. Petiole
vascular bundle transection arcuate or annular. Venation pinnate or palmate.
Stomata anomocytic, helicocytic, anisocytic, or paracytic. Cuticular wax
crystalloids usually absent (sometimes as rosettes of platelets,
Fabales type). Mesophyll often with sclerenchymatous idioblasts.
Cystoliths usually present. Leaf margin entire, crenate, dentate or serrate;
leaf teeth often begonioid or cucurbitoid.
Inflorescence Axillary,
panicle, thyrse, raceme, spike, bostrycoid, dichasial, or flowers solitary.
Floral prophylls (bracteoles) often absent.
Flowers Usually actinomorphic
(sometimes zygomorphic or bisymmetrical). Hypanthium often present. Usually
epigyny (sometimes hypogyny or half epigyny). Sepals two to five (to 16), with
imbricate, valvate or open aestivation, free from each other or more or less
connate (rarely absent). Petals two to five (to 16), with valvate,
induplicate-valvate or imbricate (sometimes open) aestivation, in lower part
often connate into campanulate corolla (rarely absent). Nectariferous disc
interstaminal and/or intrastaminal, or nectaries inserted on adaxial side of
hypanthium, on ovary, on hypanthial base, as nectariferous hairs, or absent.
Disc intrastaminal or absent.
Androecium Stamens usually
five, 4+4 or 5+5 (sometimes three, 3+3, 6+6 to more than 100, rarely two), in
one to five whorls. Filaments free from each other or more or less connate,
free from tepals or adnate to petals (epipetalous). Anthers basifixed or
dorsifixed, usually non-versatile, usually tetrasporangiate (sometimes
disporangiate, thecae rarely up to more than 50), introrse, latrorse or
extrorse, usually longicidal (dehiscing by longitudinal slits; rarely
poricidal, dehiscing by apical pore). Tapetum secretory. Staminodia absent or
present (female flowers often with staminodia).
Pollen grains
Microsporogenesis simultaneous. Pollen grains colpate, colporate or porate
(sometimes pororate or stephanoporate) with three to 16 apertures, usually shed
as monads (rarely tetrads), usually bicellular (sometimes tricellular) at
dispersal. Exine tectate or semitectate, usually with columellate (sometimes
granular or intermediate, acolumellate) infratectum, striate or reticulate,
scabrate, echinate, spinulate or psilate.
Gynoecium Pistil composed of
(one to) three or four (to six, rarely ten or twelve) usually more or less
connate (rarely free) carpels. Ovary usually inferior (sometimes superior or
semi-inferior), unilocular to quadrilocular (to sexalocular), sometimes with
roof-like structure. Style single, simple, or stylodia (two or) three or four
(to six), free or more or less connate; style often unifacial. Stigmas one or
(two or) three (to five), commissural, often bilobate (in association with
commissural lines), papillate or non-papillate, Dry or Wet type. Pistillodium
absent.
Ovules Placentation axile,
apical or (intrusively) parietal. Ovules one to more than 100 per carpel,
anatropous, pendulous, horizontal or ascending, apotropous, usually bitegmic
(rarely unitegmic), usually crassinucellar (sometimes tenuinucellar). Inner
integument, when present, often delayed in development. Micropyle usually
endostomal (sometimes bistomal, rarely absent). Nucellar cap or nucellar beak
present. Hypostase present or absent. Megagametophyte usually monosporous,
Polygonum type (rarely disporous, Allium type). Antipodal
cells sometimes degenerating prior to fertilization, sometimes persistent,
sometimes proliferating. Endosperm development ab initio nuclear. Endosperm
haustorium chalazal or absent. Embryogenesis onagrad (sometimes
caryophyllad?).
Fruit A loculicidal and/or
septicidal capsule, a drupe, a berry, a schizocarp or a berry-like gourd (pepo,
amphisarca?) with hard pericarp (rarely a samara or an assemblage of
achenes).
Seeds Aril sometimes present.
Operculum (formed by micropyle and hilum) often present. Seed coat testal,
exotestal or mesotestal. Testa multiplicative, often vascularized, with
lignified epidermis, unilayered or multilayered with sclerotic hypodermis and
lignified inner layer. Exotesta usually thick. Endotesta usually membranous.
Tegmen persistent, with tracheidal outer cells, or crushed or absent. Perisperm
usually not developed (sometimes well developed, enclosing embryo). Endosperm
rudimentary or absent. Embryo straight, usually well differentiated, without
chlorophyll. Cotyledons two. Germination phanerocotylar or cryptocotylar.
Cytology x = 7–12, 15, c.
23
DNA Plastid gene infA
lost/defunct. Mitochondrial intron coxII.i3 lost.
Phytochemistry Flavonols
(kaempferol, quercetin), cyanidin, bitter-tasting tetracyclic and pentacyclic
triterpenes (cucurbitacins etc.), toxic sesquiterpenoid compounds, methylated
and non-methylated ellagic acids, gallic acid, tannins, geraniins, karakin and
other poisonous alkaloids, very poisonous bitter-tasting glycosides, triterpene
saponins, cyclic polyvalent alcohols, punicic acid, eleostearic acid (isomere
of punicic acid), special long-chain fatty acids (present in seed oils),
myo-inisitol, and citrullin (α-amino-δ-ureidopentanoic acid, free
amino acid) present. Cyanogenic compounds not found.
Systematics A possible
topology of Cucurbitales is the following:
[Anisophylleaceae+[Cucur-bitaceae+[[Coriariaceae+Corynocarpaceae]+[Tetramelaceae+[Datiscaceae+Begoniaceae]]]]]. The
sister-group relationships of Apodanthaceae and Anisophylleaceae are still
unresolved, although the inclusion of the stem-holoparasitic Apodanthaceae in Cucurbitales is well supported
(Schaefer & Renner 2011).
The crown clade of Cucurbitales, [[Coriariaceae+Corynocarpaceae]+[Cucurbitaceae+ [Tetramelaceae+[Datiscaceae+Begoniaceae]]]] has the
potential synapomorphies (Stevens 2001 onwards): absence of uniseriate wood
rays; filaments shorter than anthers in bud; anthers basifixed; absence of
nectaries; and presence of free stylodia. Coriariaceae and Corynocarpaceae share the
following synapomorphies: wood rays wide; sieve tube plastids without starch
and protein inclusions; stomata paracytic; leaf margin entire; flowers small;
hypogyny; sepals with imbricate quincuncial aestivation; petals thick, with
broad base; carpels ascidiate; ovule one per carpel; vascular bundle extending
into outer integument; cotyledons very large; and presence of ellagic acid.
The clade [Cucurbitaceae+[Tetramelaceae+[Datiscaceae+Begoniaceae]]] is characterized
by the following features (Stevens 2001 onwards): flowers unisexual; perennial
herbaceous growth; young stem with separate vascular bundles; absence of
stipules; leaf margin serrate; medial vein ending in aggregation of translucent
cells, lateral vein also entering tooth; carpels antesepalous or median carpel
adaxial; roof-like structure present above ovary; stylodia marginal; stigmas
large, elongated, bilobate; placentation parietal; ovules numerous per carpel;
fruit many-seeded; presence of cucurbitacins; and absence of myricetin and
ellagic acid.
The clade [Tetramelaceae+[Datiscaceae+Begoniaceae]] has the potential
synapomorphies (Stevens 2001 onwards): pollen grains spheroidal; stigmas
elongate; fruit an apically dehiscent septicidal capsule; testa with operculum
formed by micropyle and hilum; exotestal cells honeycomb-shaped, inner walls
strongly thickened and lignified; and cotyledons medium-sized. Datisca
and Begoniaceae share
the synapomorphies: herbaceous growth; and outer and inner integuments two cell
layers thick. Tetramelaceae and Datiscaceae are both dioecious;
have completely isomerous but not regularly pentamerous flowers (not in male
plants of Datisca) with only small sepals and without petals (present
in male plants of Octomeles).
Cucurbitaceae have the
following features in common with [Tetramelaceae+[Datiscaceae+ Begoniaceae]] (Stevens 2001
onwards): herbaceous growth form; young stem with separate vascular bundles;
leaf margin serrate, with cucurbitoid teeth (i.e. medial vein ending in pad of
packed translucent cells, lateral veins also entering); absence of stipules;
flowers unisexual; epigyny; carpels antesepalous or median carpel adaxial;
extended roof-shaped structure on top of ovary (stylodia marginal), formed by
ventral carpellary segments; stigmas large, elongated, bilobate; placentation
parietal, often intrusive; ovules numerous per carpel; presence of
cucurbitacins (oxidized triterpenes); and absence of myricetin and ellagic
acid. The flowers are often androdioecious, the anthers basifixed and extrorse
or latrorse, the free carpellary parts bifurcate, and the gynoecium
trimerous.
Ridley, Fl. Malay Penins. 1: 700. 13-22 Jul 1922
[‘Anisophylleae’]
Polygonanthaceae
Croizat in Cact. Succ. J. (Los Angeles) 15: 64. Mai 1943;
Anisophylleales (Benth. et Hook.f.) Takht. ex Reveal
et Doweld in Novon 9: 550. 30 Dec 1999
Genera/species 4/35–40
Distribution Northern South
America (Amazonas), tropical Africa, southern India, Sri Lanka, West
Malesia.
Fossils Uncertain. Fossil
pollen grains of Anisophylleaceae type are
known from mid- and upper Miocene layers on Borneo. Fossil flowers of
Platydiscus, a possible Anisophylleaceae (or
Cunoniaceae?),
have been found in upper Cretaceous strata in southern Sweden.
Habit Usually monoecious
(sometimes polygamomonoecious or bisexual; in Combretocarpus
dioecious), evergreen trees or shrubs. Most species are anisophyllous
(Combretocarpus is isophyllous).
Vegetative anatomy Phellogen?
Cambial stratification? Vessel elements with simple perforation plates; lateral
pits alternate, simple or bordered pits. Imperforate tracheary xylem elements
fibres with bordered pits, non-septate (also vasicentric tracheids). Wood rays
multiseriate, heterocellular. Axial parenchyma apotracheal diffuse or
diffuse-in-aggregates, or paratracheal banded, aliform, lozenge-aliform or
winged-aliform. Sieve tube plastids S type. Nodes 1:1, unilacunar with one leaf
trace. Parenchyma in Poga with lysigenous secretory cavities.
Laticifers absent. Heartwood with resinous substances. Prismatic calciumoxalate
crystals frequent.
Trichomes Hairs usually
unicellular (sometimes multicellular, occasionally peltate), uniseriate,
simple; glandular hairs usually absent (present near leaf axils in
Anisophyllea disticha).
Leaves Alternate (spiral or
distichous; in Anisophyllea tetrastichous), simple, entire,
coriaceous, with ? ptyxis, usually dimorphic (in Combretocarpus
monomorphic). Stipules usually absent (rarely two to four, very small, arising
from petiole base, colleters?); leaf sheath absent. Petiole vascular bundle
transection simple. Leaf base in Anisophyllea asymmetrical. Venation
usually palmate (in Combretocarpus pinnate). Stomata usually
paracytic. Cuticular wax crystalloids as platelets (in Polygonanthus
cupular). Leaf margin entire.
Inflorescence Axillary,
usually panicle, raceme or spike (flowers rarely solitary).
Flowers Actinomorphic, usually
small. Epigyny. Sepals (three or) four (to 16), in one whorl, with valvate
aestivation, persistent, with mucilaginous inner epidermal walls, postgenitally
coherent. Petals (three or) four (to 16; in Combretocarpus often
absent), with valvate or open aestivation, clawed, usually trilobate,
quinquelobate or septalobate (in Poga entire), more or less enclosing
groups of free stamens. Nectariferous disc crenate, usually both interstaminal
and intrastaminal (nectariferous disc in Combretocarpus only
intrastaminal; in Anisophyllea formed by protrusions between and
behind each stamen).
Androecium Stamens 3+3, 4+4 or
5+5, obdiplostemonous. Filaments narrow, free from each other and from tepals,
inflexed in bud. Anthers dorsifixed, versatile?, tetrasporangiate, introrse,
longicidal (dehiscing by longitudinal slits). Tapetum secretory. Staminodia
present or absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains tricolporate to tricolporoidate
(in Anisophyllea usually dicolpate), shed as monads or tetrads (or
polyads?), bicellular at dispersal. Exine tectate or semitectate, with
columellate infratectum, usually reticulate or punctate (sometimes striate or
smooth).
Gynoecium Pistil composed of
(three or) four (or five) carpels connate in lower part. Ovary inferior,
(trilocular or) quadrilocular (or quinquelocular); sometimes with roof-like
structure above ovary. Stylodia (three or) four (or five), free, sometimes
hollow; compitum absent. Stigmas widened or punctate, type? Pistillodium often
present in male flowers.
Ovules Placentation
apical-axile or parietal. Ovule usually one per carpel, anatropous, pendulous,
epitropous, bitegmic or unitegmic (Anisophyllea,
Combretocarpus), crassinucellar. Micropyle bistomal (Poga,
Polygonanthum; in Anisophyllea slit-shaped). Outer integument
four or five cell layers thick. Inner integument approx. two cell layers thick.
Integument in Anisophyllea seven to nine cell layers thick (unitegmic
ovules). Parietal tissue approx. one cell layer thick. Nucellar cap present.
Megagametophyte usually monosporous, Polygonum type (in
Combretocarpus disporous, 8-nucleate, Allium type). Antipodal
cells degenerating prior to fertilization (mature megagametophyte
quinquecellular). Endosperm development ab initio nuclear. Endosperm haustoria?
Embryogenesis?
Fruit Usually a drupe (rarely
a capsule; in Combretocarpus a samara), sometimes with accrescent
sepals.
Seeds Aril present or absent.
Seed coat testal. Testa usually multiplicative, sometimes vascularized, approx.
ten to c. 30 cell layers thick, thin and one-layered (Combretocarpus),
or thick and multilayered (Anisophyllea, Poga). Outer
exotestal epidermis cells cuboid, with thick, lignified walls. Mesotestal cell
walls sometimes lignified. Tegmen crushed or absent. Exotegmen not sclerified.
Perisperm not developed. Endosperm absent. Embryo fusiform, with long
hypocotyl, chlorophyll? Cotyledons two, usually indistinct or absent (in
Combretocarpus small). Germination cryptocotylar.
Cytology x = 7, 8
DNA
Phytochemistry Very
insufficiently known. Tannins present. Alkaloids and saponins not found.
Aluminium accumulated.
Use Timber (Combretocarpus
rotundatus, Poga oleosa), seed oils (Poga).
Systematics
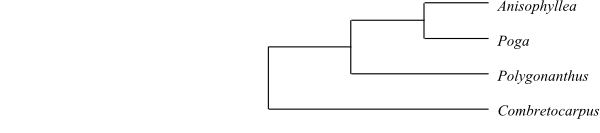
Anisophyllea (c 35; tropical regions on both hemispheres),
Poga (1; P. oleosa; Guinea to Congo), Polygonanthus
(2; P. amazonicus, P. punctulatus; Amazonia),
Combretocarpus (1; C. rotundatus; West Malesia).
According to Zhang & al. (2007), the
genetic distance between Anisophylleaceae and the
other Cucurbitales is
large, and no morphological synapomorphies are known. Anisophylleaceae appear to
be sister to the remaining Cucurbitales.
Combretocarpus is sister to the
remaining Anisophylleaceae.
APODANTHACEAE (R. Br.)
Tiegh. ex Takht.
|
( Back to Cucurbitales )
|
Takhtajan, Sist. Magnoliof. [Systema
Magnoliophytorum]: 42. 24 Jun 1987
Genera/species 2/13
Distribution Tropical East
Africa, southeastern Turkey to northern Iran, southwestern Australia, southern
California, Mexico, Central America, the West Indies, South America south to
central Argentina.
Fossils Unknown.
Habit Usually monoecious or
dioecious (rarely bisexual), achlorophyllous herbaceous endophytes without
rhizome or normal roots. Root or stem holoendoparasites (Apodanthes on
Salicaceae
[Casearia, Xylosma]; Pilostyles on
Papilionoideae in Fabaceae).
Vegetative anatomy Mycorrhiza
absent. Hypha-like cell-threads invading host plants, forming endophytic system
inside host roots. Phellogen absent. Secondary lateral growth absent. Vessels
absent. Imperforate tracheary elements? Wood rays absent. Axial parenchyma?
Stomata anomocytic. Sieve tube plastids S0 type, without starch or
protein inclusions. Cells with calciumoxalate crystals. Oils or mucilage
absent.
Trichomes Hairs (in flowers)
unicellular or multicellular, uniseriate; vesicular hairs present.
Leaves Absent.
Inflorescence Flowers
terminal, solitary.
Flowers Actinomorphic, small,
usually with evil smell. Epigyny or half epigyny. Floral (tepaloid) scales with
entire margin probably in three di-, tri-, tetra- or hexamerous whorls of eight
to 15 scales in total (2+4+4 or 3+6+6, etc.), at least inner scales probably
tepals and outer scales bracts, usually with open or imbricate (sometimes
contorted) aestivation, free or connate at base, usually persistent (outer
scales in Apodanthes caducous); inner whorl of scales with adaxial
hair tufts. Thin tissue, diaphragma, and special outgrowth, ramenta, inserted
on top of perigonal tube. Gynostemium present. Nectariferous disc somewhat
quadrangular or sexangular, extrastaminal (formed by base of innermost scales),
in Apodanthes and Pilostyles with stomata. Ring of vesicular
hairs present in male flowers on upper part of pistillodium, immediately above
synandrium.
Androecium Stamens
congenitally connate into synandrium, not differentiated into filaments and
anthers. Thecae five to more than 50, in (one or) two or three (or four)
whorls, extrorse, longicidal (in Pilostyles dehiscing by longitudinal
slits, sometimes between thecal whorls, and in Apodanthes by
longitudinal slits) or poricidal (dehiscing by apical pore). Endothecium
absent. Tapetum secretory. Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains monoporate, monocolpate,
triporate, tricolpate (Apodanthes, Pilostyles), or
inaperturate (Pilostyles), shed as monads, bicellular at dispersal.
Exine tectate, with columellate infratectum, psilate.
Gynoecium Pistil usually
composed of four (or five) distally connate antepetalous carpels. Ovary
inferior or semi-inferior, unilocular. Style single, simple, very short,
hollow, with H-shaped (in transverse view) stylar canal, compitum; nectary
present on stylar base. Stigma single, simple (Apodanthes,
Pilostyles) or somewhat quadrilobate (Pilostyles), annular,
hemispherical, relatively large, inserted below apex of central column,
papillate, Wet type. Male flowers with pistillodium with basal nectary and
vesicular hairs at least on margin.
Ovules Placentation parietal.
Ovules c. 15 to more than 90 per carpel, anatropous, bitegmic, tenuinucellar.
Micropyle bistomal or endostomal (Pilostyles), or absent
(Apodanthes, Pilostyles). Outer integument two cell layers
thick. Inner integument two cell layers thick. Megagametophyte monosporous,
Polygonum type. Endosperm development ab initio nuclear. Endosperm
haustoria? Embryogenesis?
Fruit A berry.
Seeds Aril absent. Elaiosome
present, formed from exotesta. Testa thin-walled, mucilaginous, exotestal.
Exotestal cells? Endotesta? Exo(?)tegmic cell walls strongly lignified.
Endotegmen? Perisperm not developed. Endosperm copious. Embryo rudimentary,
undifferentiated, without chlorophyll? Cotyledons two? Germination?
Cytology n = c. 12, 16?, 6,
30–31 + B (Pilostyles)
DNA
Phytochemistry Virtually
unknown. Tannins present in floral scales.
Use Unknown.
Systematics
Apodanthes (1; A. caseariae; Central America, tropical South
America), Pilostyles
(9; southeastern Turkey and Iran [P. haussknechtii], southwestern
Western Australia [P. coccoidea, P. collina, P.
hamiltonii], southeastern tropical Africa [P. aethiopica],
southwestern United States, Mexico, Central America, the West Indies, South
America).
Androecial and gynoecial features support the
insertion of Apodanthaceae into Cucurbitales. The sister-group
relationship of Apodanthaceae is
unresolved.
Agardh, Aphor. Bot.: 200. 13 Jun 1824, nom.
cons.
Begoniales Bercht. et
J. Presl, Přir. Rostlin: 270. Jan-Apr 1820 [‘Begoniae’];
Begoniineae Engl., Syllabus, ed. 2: 156. Mai 1898;
Begonianae Doweld, Tent. Syst. Plant. Vasc.: xxxix.
23 Dec 2001
Genera/species 2/>2.000
Distribution Tropical and
subtropical regions on both hemispheres, with their highest diversity in
tropical Asia and northern South America.
Fossils Unknown.
Habit Usually monoecious (male
flowers are produced ab initio, and subsequently female flowers; rarely
dioecious), usually more or less succulent perennial herbs with tuber (in
Hillebrandia round) or rhizome (in Begonia rhizome, rarely
tuber), sometimes climbing, sometimes somewhat woody below and frutescent.
Nodes swollen.
Vegetative anatomy Phellogen
ab initio subepidermal. Medulla sometimes storied by diaphragms. Cortical and
medullary vascular bundles usually present. Primary vascular tissue cylinder of
vascular bundles. Pericyclic envelope absent. Vessel elements with simple or
scalariform perforation plates; lateral pits scalariform, simple pits.
Imperforate tracheary xylem elements libriform fibres with simple or bordered
pits, non-septate. Wood rays multiseriate, heterocellular, or absent. Axial
parenchyma paratracheal scanty. Fibres sometimes storied. Tyloses often
abundant. Sieve tube plastids S type. Nodes 3:3, trilacunar with three leaf
traces, or 5:5, pentalacunar with five traces. Brachysclereids and
non-calcified cystoliths present. Calciumoxalate as prismatic crystals, druses
and raphides.
Trichomes Hairs unicellular or
multicellular, uniseriate or multiseriate, simple or branched, sometimes
bristle-like (setular), candelabra-shaped or stellate, often large and flat;
peltate-lepidote glands and sessile spherical pearl glands present.
Leaves Usually alternate
(usually distichous, sometimes spiral, rarely opposite), usually simple (rarely
palmately compound), entire or palmately lobed, with usually conduplicate
(vertically or laterally; sometimes supervolute-curved) ptyxis. Stipules
usually large, cauline-extrapetiolar or intrapetiolar, caducous or persistent;
leaf sheath absent. Petiole vascular bundle transection annular; petiole
sometimes with central vascular bundles. Leaf base usually strongly
asymmetrical. Venation palmate. Stomata helicocytic with three to six
subsidiary cells (often in two whorls) or anisocytic (rarely paracytic or
diacytic?), often only on adaxial side of lamina. Cuticular wax crystalloids?
Mesophyll with or without sclerenchymatous idioblasts. Cystoliths
(non-calcified) usually present. Hydathodes usually absent. Leaf margin usually
serrate or crenate (rarely entire); leaf teeth begonioid, supplied by several
veins; medial vein ending in aggregation of translucent cells, lateral vein
dominant, also entering tooth. Caducous pearl glands present.
Inflorescence Usually axillary
(in Begonia rarely epiphyllous), dichasial or bostrycoid. Female
flowers in Begonia usually developed in cymose inflorescence after
male flower anthesis (inflorescence in Symbegonia subclade racemose
and female flowers produced prior to male flowers).
Flowers More or less
zygomorphic or bisymmetrical. Usually epigyny (in Hillebrandia half
epigyny). Sepals usually two, with valvate or imbricate aestivation, petaloid
(rarely six to eight; in Hillebrandia five, larger to much larger than
petals), usually free (sometimes connate into tube). Petals usually two, with
valvate aestivation, or absent (in Hillebrandia five, smaller to much
smaller than sepals and possibly staminodia; in female flowers of
Begonia two to nine; tepals in male flowers of Begonia
usually two or four, sometimes three or five to eight), usually free (rarely
connate into campanulate corolla). Nectary usually absent (nectaries rarely
present at stylodium bases). Disc absent.
Androecium Stamens three to c.
50 to more than 100, in two to five whorls, centrifugally developing (in
Hillebrandia with branched vascular bundles). Filaments free or more
or less connate, free from tepals. Anthers basifixed, non-versatile,
tetrasporangiate, extrorse or latrorse, usually longicidal (dehiscing by
longitudinal slits; rarely poricidal, dehiscing by apical pores), yellow;
connective often exlpanded. Tapetum secretory. Female flowers often with
staminodia.
Pollen grains
Microsporogenesis simultaneous. Pollen grains tricolp(or)ate, shed as monads,
bicellular at dispersal. Exine tectate, with columellate infratectum, often
striate.
Gynoecium Pistil composed of
(two or) three (or six; in Hillebrandia five; in Begonia
sometimes one) carpels connate in lower part. Ovary usually inferior (in
Hillebrandia semi-inferior), unilocular (in Hillebrandia and
some species of Begonia) or secondarily usually trilocular (rarely
bilocular or sexalocular), usually three-winged; carpels strongly developed
ventrally forming roof-like structure above locule, this forming perianth tube
base; calyx tube with widely separated stylodia inserted on roof edge. Stylodia
(two or) three (or six), usually free (sometimes connate at base; in
Hillebrandia peripheral; in Begonia central), short, usually
deeply bifid. Nectaries inserted at stylodium bases in some ornithophilous
species. Stigmas often twisted (yellow, resembling anthers), papillate, Dry
type. Pistillodium absent.
Ovules Placentation usually
axile (in Hillebrandia axile at base and parietal at apex; in
Begonia axile to parietal), with large usually bilamellate placentae.
Ovules numerous per carpel, anatropous, bitegmic, crassinucellar. Micropyle
bistomal, Z-shaped (zig-zag). Outer integument ? cell layers thick. Inner
integument approx. two cell layers thick. Endothelium present. Parietal tissue
approx. two cell layers thick. Megagametophyte monosporous, Polygonum
type. Endosperm development ab initio nuclear. Endosperm haustoria?
Embryogenesis onagrad.
Fruit Usually a loculicidal
capsule (in Hillebrandia septicidal, dehiscing at apex between
stylodia; in Begonia rarely also septicidal), usually winged (often
asymmetrically winged; rarely a berry).
Seeds Aril absent. Funicle
surrounded by collar formed as extension of testa. Operculum formed by
micropyle and hilum, and surrounding annulus of collar cells. Seed coat
exotestal. Exotestal cells with honeycomb-like arrangement, with heavily
thickened and lignified inner walls? Endotesta absent or almost absent Tegmen
absent or almost absent. Perisperm not developed. Endosperm sparse or absent.
Embryo small, straight, little to well differentiated, chlorophyll? Cotyledons
two. Germination phanerocotylar.
Cytology n = 9–21 or more
(Begonia)
DNA
Phytochemistry Flavonols
(quercetin), cyanidin, cucurbitacins, tannins, calcium oxalate, oxalic acid,
proanthocyanidin, saponins and cyanidin glycosides present. Ellagic acid,
myricetin, alkaloids, and cyanogenic compounds not found.
Use Ornamental plants,
medicinal plants, occasionally as vegetables.
de Candolle, Prodr. 1: 739. med Jan 1824
[’Coriarieae’], nom. cons.
Coriariales Lindl.,
Nix. Plant.: 11. 17 Sep 1833 [‘Coriales’];
Coriariopsida Parl., Fl. Ital. 5: 486. 1872
[’Coriarineae’]; Coriariineae Engl.,
Syllabus, ed. 2: 142. Mai 1898
Genera/species 1/13
Distribution Western
Mediterranean, the Himalayas to Japan, Taiwan, the Philippines, New Guinea, New
Zealand, islands in southwestern Pacific, tropical America.
Fossils Macrofossils assigned
to Coriaria longaeva are known from Oligocene layers in France, and
seeds and pollen grains of Coriariaceae have been
described from the Miocene of several European sites.
Habit Usually dioecious
(sometimes monoecious, andromonoecious, gynomonoecious or polygamomonoecious,
rarely bisexual), evergreen or deciduous shrubs (sometimes suffrutices or small
trees). Some branches with limitied growth similar to pinnate leaves. Buds
usually perulate.
Vegetative anatomy Root
nodules containing nitrogen-fixing endosymbiotic actinobacteria
(Frankia) present in most species. Phellogen ab initio superficial.
Primary medullary strands wide. Vessel elements present in multiples. Vessel
elements with simple perforation plates; lateral pits alternate, bordered pits.
Imperforate tracheary xylem elements tracheids or libriform fibres with simple
or bordered pits, non-septate (also vasicentric tracheids). Wood rays
multiseriate, heterocellular. Axial parenchyma paratracheal scanty, confluent
or vasicentric. Wood elements partially storied. Sieve tube plastids S type.
Nodes 1:1, unilacunar with one leaf trace. Prismatic calciumoxalate crystals
abundant.
Trichomes Typical trichomoids
(half hair, half scale) present at nodes.
Leaves Usually opposite
(sometimes verticillate, rarely alternate spiral), simple, entire, coriaceous,
with flat ptyxis. Stipules minute, caducous; leaf sheath absent. Petiole
vascular bundle transection arcuate. Venation palmate. Stomata paracytic.
Cuticular waxes absent. Epidermis with secretory cells. Calciumoxalate crystals
abundant. Tannins very abundant. Leaf margin entire or weakly dentate.
Inflorescence Terminal,
raceme. Floral prophylls (bracteoles) absent.
Flowers Actinomorphic, small.
Hypogyny. Sepals five (or six), with imbricate quincuncial aestivation,
persistent, free. Petals five (or six), with valvate or open aestivation,
persistent, free, usually adaxially keeled. Nectary absent. Disc absent.
Androecium Stamens usually 5+5
(rarely 6+6), diplostemonous. Filaments thin, free from each other, free from
tepals or antepetalous filaments adnate to petal keel. Anthers basifixed or
slightly dorsifixed, versatile?, tetrasporangiate, introrse, longicidal
(dehiscing by longitudinal slits); connective narrow. Tapetum secretory, with
binucleate to quadrinucleate cells. Female flowers often with staminodia.
Pollen grains
Microsporogenesis simultaneous. Pollen grains (2–)3(–4)-colporate or
3(–4)-pororate, starchy, shed as monads, usually tricellular (sometimes
bicellular) at dispersal. Exine semitectate, with columellate infratectum,
reticulate.
Gynoecium Carpels usually
five, antesepalous (rarely ten or twelve), free from each other or connate at
base (although bulging and seemingly free); carpel ascidiate? Ovary superior,
unilocular (apocarpy). Stylodia usually five, filiform, subbasal; compitum
present. Stigmatic areas surrounding style, papillate, Dry type. Male flowers
often with pistillodium.
Ovules Placentation
apical-axile. Ovule one per carpel, anatropous, pendulous, apotropous,
bitegmic, crassinucellar. Micropyle endostomal. Outer integument three or four
cell layers thick. Inner integument two or three cell layers thick. Parietal
tissue approx. eight cell layers thick. Nucellar cap approx. four cell layers
thick. Megagametophyte monosporous, Polygonum type. Endosperm
development ab initio nuclear. Endosperm haustoria? Embryogenesis?
Fruit: A schizocarp with
nutlike mericarps or an assemblage of achenes with persistent subbasal stylodia
and enclosed by carnose accrescent petals, resulting in baccate or drupaceous
appearance.
Seeds Aril absent. Seed coat
exotestal. Exotesta consisting of cuboid, tanniniferous cells with thick
lignified? walls. Endotesta and tegmen indistinct. Perisperm not developed.
Endosperm sparse, oily, or absent. Embryo straight, oily, chlorophyll?
Cotyledons two, large. Germination phanerocotylar.
Cytology n = 10, 15
DNA
Phytochemistry Flavonols
(kaempferol, quercetin, myricetin), toxic sesquiterpenoid compounds, methylated
and non-methylated ellagic acids, gallic acid, tannins, alkaloids, geraniins,
cyclic polyvalent alcohols, coriolic fatty acid (in seeds), and
myo-inisitol present. Triterpene saponins? Proanthocyanidins and
cyanogenic compounds not found.
Use Ornamental plants,
tanning, insecticides, medicinal plants.
Systematics Coriaria
(c 17; western Mediterranean, the Himalayas to Japan, Taiwan, the Philippines
and New Guinea, Solomon Islands, Vanuatu, Fiji, New Zealand, Samoa, the Society
Islands and other islands in the South Pacific, Mexico, Central America, the
Andes from Colombia to central Chile).
Coriaria
is sister to Corynocarpus
(Corynocarpaceae).
The Eurasiatic clade of Coriaria
is sister to the remaining species. Species limits in Coriaria
are problematic.
Engler in Engler et Prantl, Nat. Pflanzenfam.,
Nachtr. 1: 215. Oct 1897, nom. cons.
Corynocarpales
Takht., Divers. Classif. Fl. Pl.: 340. 24 Apr 1997;
Corynocarpanae Takht., Divers. Classif. Fl. Pl.: 340.
24 Apr 1997
Genera/species 1/5
Distribution The Aru Islands,
New Guinea, New Britain, New Ireland, northeastern and eastern Australia,
Melanesia, New Zealand.
Fossils Unknown.
Habit Bisexual, evergreen
trees.
Vegetative anatomy Phellogen
ab initio subepidermal. Young stem with separate vascular bundles. Cambium
storied. Vessel elements with simple perforation plates; lateral pits
alternate, bordered pits. Imperforate tracheary xylem elements libriform fibres
with simple or bordered pits, non-septate. Wood rays multiseriate, homocellular
or heterocellular, very wide. Wood elements partially storied. Axial parenchyma
paratracheal scanty, confluent, vasicentric or wide-banded. Sieve tube plastids
S type. Nodes 3:3?, trilacunar with three? leaf traces. Prismatic
calciumoxalate crystals abundant.
Trichomes Typical trichomoids
(half hair, half scale) present at nodes.
Leaves Leaves alternate
(spiral), simple, entire, coriaceous, with conduplicate ptyxis. Stipule single,
intrapetiolar, caducous, or absent; leaf sheath absent. Petiole vascular bundle
transection forming line. Venation pinnate, brochidodromous. Stomata paracytic.
Cuticular wax crystalloids? Leaf margin entire.
Inflorescence Terminal,
branched panicle or raceme.
Flowers Actinomorphic, small.
Hypanthium short. Hypogyny. Sepals five, with imbricate aestivation, free.
Petals five, with imbricate aestivation, free. Nectaries five, intrastaminal,
staminodial. Disc consisting of five separate parts.
Androecium Fertile stamens
five, obhaplostemonous, alternisepalous, antepetalous. Filaments free, adnate
at base to petals, inflexed in bud. Anthers dorsifixed, non-versatile,
tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits).
Tapetum secretory? Staminodia five, petaloid, extrastaminal or interstaminal,
alternating with stamens, fimbriate, each with basal adaxial antesepalous
nectary.
Pollen grains
Microsporogenesis simultaneous? Pollen grains dicolp(or)ate, heteropolar (one
short colpus at each pole), shed as monads, ?-cellular at dispersal. Exine
tectate, with granular to intermediate infratectum, scabrate to psilate.
Gynoecium Pistil composed of
two connate carpels, one of which more or less reduced and sterile leading to
pseudomonomery. Ovary superior, unilocular or bilocular. Stylodium usually
single, simple, short, conduplicate (stylodia rarely two). Stigma capitate, Dry
type. Pistillodium absent.
Ovules Placentation apical.
Ovule one per fertile carpel, anatropous, pendulous, apotropous or epitropous?,
with erect micropyle, bitegmic, crassinucellar. Micropyle endostomal. Outer
integument c. 11 (to c. 30?) cell layers thick. Inner integument two or three
cell layers thick. Megagametophyte monosporous, Polygonum type.
Antipodal cells proliferating (with up to eight cells). Endosperm development
ab initio nuclear. Endosperm haustoria? Embryogenesis caryophyllad?
Fruit A drupe with persistent
eccentric styloid.
Seeds Aril absent. Testa
pachychalazal?, ab initio thick, vascularized, finally crushed. Tegmen?
Perisperm not developed. Endosperm sparse, starchy, or absent. Embryo straight,
well differentiated, oily and starchy, chlorophyll? Cotyledons two, large,
thick. Germination phanerocotylar.
Cytology n = (22?) 23 (46)
DNA
Phytochemistry Flavonols
(kaempferol), ellagic acid, very poisonous bitter glucosides (at least in bark
and seeds), and karakin (in fruits) present. Proanthocyanidins, saponin, and
cyanogenic compounds not found.
Use Timber (ship’s masts,
canoes etc.).
Systematics Corynocarpus
(5; C. cribbianus: the Aru Islands, New Guinea, New Britain, New
Ireland, Solomon Islands; C. rupestris: eastern Queensland, eastern
New South Wales; C. similis: Vanuatu; C. dissimilis: New
Caledonia; C. laevigatus: North Island in New Zealand).
Corynocarpus
is sister to Coriaria
(Coriariaceae).
de Jussieu, Gen. Plant.: 393. 4 Aug 1789, nom.
cons.
Nhandirobaceae T.
Lestib., Botanogr. Elém.: 515. Jun 1826 [’Nandhirobées’];
Zanoniaceae Dumort., Anal. Fam. Plant.: 28, 29. 1829;
Bryoniaceae G. Mey., Chloris Han.: 6, 112. Jul-Aug
1836; Cyclantheraceae Lilja, Skånes Fl., ed. 2: 716,
980. Apr-Dec 1870 [’Cyclanthereae’];
Cucurbitineae Engl., Syllabus, ed. 2: 190. Mai
1898
Genera/species 95/945–995
Distribution Tropical and
subtropical regions, with their largest diversity in South American rainforests
and arid regions in Africa, few species in Australasia and in temperate
regions.
Fossils Seeds have been
described from the Paleocene and the Eocene of England. Fossil hexacolpate or
stephanocolpate pollen grains, Hexacolpites echinatus, have been
described from the Oligocene of Cameroon.
Habit Monoecious,
andromonoecious, gynomonoecious, polygamomonoecious, dioecious, androdioecious,
and gynodioecious (in Actinostemma and Schizopepon sometimes
bisexual), usually perennial herbs, mostly climbing or winding (rarely lianas,
shrubs or tree, secondarily woody, or annual herbs; Dendrosicyos
extremely pachycaul and secondarily arborescent with soft juicy stem). Many
species are xerophytes. In leaf axils usually lateral and often branched
tendrils formed by modified lateral shoots with lower part corresponding to
stem branch and climbing upper part corresponding to strongly modified leaves
(tendrils sometimes modified into spines [i.a. in Acanthosicyos,
Momordica] or absent). Tendrils simple or branched; branched tendrils
with or without sensitive coiling base (zanonioid tendrils), sometimes with
terminal adhesive pads. Tendrils often part of axillary complex together with
axillary buds and inflorescences. Many species provided with epigeal tuberous
pachypodium (root stock) formed by swollen hypocotyl (cauduciform
succulents).
Vegetative anatomy Root
phellogen superficial. Stem phellogen ab initio superficially to deeply seated.
Primary vascular tissue with separate bundles or consisting of cylinder of
bundles. Young stem usually with bicollateral vascular bundles, often arranged
in two cylinders. Successive cambia present in at least Bryonia and
Ecballium. Cortical vascular bundles usually present. Cortex with
sclerenchymatous envelope. Pericyclic sheath absent. Secondary lateral growth
normal or anomalous (via concentric cambia or from simple cambial cylinder).
Vessel elements with simple perforation plates; lateral pits alternate,
bordered pits. Imperforate tracheary xylem elements libriform fibres with
simple or bordered pits, septate (also vasicentric tracheids). Wood rays
multiseriate, heterocellular. Axial parenchyma apotracheal or paratracheal
aliform or banded. Wood elements often storied. Tyloses often frequent.
Extrafascicular phloem usually present in cortex outside sclerenchymatous
envelope (in species with bicollateral vascular bundles). Sieve tube plastids S
type. Nodes 3:3, trilacunar with three leaf traces. Branched idioblasts with
bitter substances. Cystoliths usually present. Raphids present in at least
Cucurbita pepo and Ecballium; calcium oxalate crystals and
cystoliths, calcium carbonate bodies of varying shape (in some genera),
crystalline silica grains (in at least Cucurbita).
Trichomes Hairs unicellular,
simple, verrucate or often as prickles; glandular hairs often frequent; cell
walls often calcified; calcified cystoliths and similar structures often
present at hair bases or in adjacent cells.
Leaves Alternate (spiral),
palmately compound or simple and usually palmately lobed (sometimes entire),
with ? ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle
transection arcuate or as ring of arcuate bundles, unequal in size (larger
bundles bicollateral); leaf supplied by one vascular strand from outer ring of
bundles and by branches from two additional strands of outer bundle ring.
Pericyclic envelope usually absent. Extrafloral nectaries frequent on abaxial
surface of lamina and/or sometimes on petiole, node or leaf margin. Venation
usually palmate. Stomata usually anomocytic. Cuticular wax crystalloids usually
absent, sometimes as rosettes of platelets (Fabales type), or as
irregular platelets or terete rodlets, chemically dominated by triterpenoids,
etc. Hydathodes often present. Leaf margin usually serrate (rarely entire);
leaf teeth often cucurbitoid.
Inflorescence Axillary,
cymose, umbel, raceme, fascicle, pseudopanicle, or flowers solitary. Female
flowers often solitary in leaf axils on main branch, male inflorescence arising
from this first node. Floral prophylls (bracteoles) often absent. Floral bracts
sometimes with extrafloral nectaries.
Flowers Usually actinomorphic
(sometimes with zygomorphic androecium), often large. Hypanthium, formed by
lower parts of perianth and stamens, usually present. Usually epigyny (rarely
half epigyny). Sepals (three to) five (to seven), with imbricate or open
aestivation, usually more or less connate (rarely absent). Petals (three to)
five (to seven), with valvate, induplicate-valvate or imbricate quincuncial
aestivation, in lower parts usually connate into campanulate perigonal tube.
Nectary usually disc-shaped (sometimes covered by tissue lobe). Male flowers
usually with nectaries on adaxial side of hypanthium. Female flowers with
nectaries on ovary, on hypanthial base, or as nectariferous glandular hairs.
Oil-secreting hairs sometimes present. Disc intrastaminal or absent.
Androecium Stamens usually
five (sometimes three; in, e.g., Gurania, Helmontia and
Psiguria two), haplostemonous, antesepalous, alternipetalous.
Filaments usually entirely or almost entirely connate in fascicles 2+2+1 or all
five stamens into central columella (sometimes free or five free stamens or
three free filaments due to fusion of two adjacent stamens, although still five
anthers due to incomplete fusion of thecae; in Fevillea five free
stamens with bilocular anthers), often adnate to petals, usually inserted into
hypanthium. Anthers dorsifixed, non-versatile, straight or arcuate to
triplicate or (often strongly) sigmoid, often connate into tube (in
Thladiantha connate 4+1), when three anthers then usually two
tetrasporangiate and one disporangiate (monothecal), when five anthers then all
disporangiate (monothecal), extrorse, longicidal (dehiscing by longitudinal
slits); connective sometimes prolonged at apex. Tapetum secretory. Female
flowers with rudimentary staminodia (male flowers in Gerrardanthus
with one out of five stamens modified into staminodium).
Pollen grains
Microsporogenesis simultaneous. Pollen grains colpate, colporate or porate
(sometimes stephanoporate) with three to 16 apertures, starchy, sometimes
operculate, sometimes very large (40–70 µm or more), usually shed as monads
(in Gurania and Psiguria tetrads), bicellular at dispersal.
Exine tectate to semitectate, with columellate or acolumellate infratectum,
perforate or reticulate, echinate, spinulate, striate or microstriate. Pollen
grain germinating with several pollen tubes (polysiphony).
Gynoecium Pistil composed of
usually (two or) three (to five) connate carpels (in Sicyoeae often
one carpel); median carpel sometimes abaxial. Ovary usually inferior (rarely
semi-inferior), unilocular, although usually almost filled up by enlarged
placentae, sometimes fused in centre and seemingly multilocular; carpels
strongly developed ventrally forming roof-like structure above locule, this
forming perianth tube base; calyx tube with widely separated stylodia inserted
on roof edge. Stylodia (two or) three (to five), entirely (sometimes only
below) connate (style simple) or more or less separate. Stigmas one or (two or)
three (to five), commissural, often bilobate (in connection with commissural
lines), papillate or non-papillate, Dry or Wet type. Pistillodium absent (small
processes alternating with stamens perhaps representing reduced carpels).
Ovules Placentation
intrusively parietal (sometimes seemingly axile). Ovules usually numerous
(sometimes few; in Sicyoeae one) per carpel, anatropous, pendulous or
horizontal to ascending, bitegmic, crassinucellar. Micropyle usually endostomal
(rarely bistomal). Outer integument four to eight cell layers thick,
vascularized. Inner integument two or three (to at least six) cell layers
thick. Parietal tissue five to eleven cell layers thick. Nucellar cap and
nucellar beak present. Hypostase present or absent. Megagametophyte usually
monosporous, Polygonum type (rarely disporous, 8-nucleate,
Allium type), sometimes extremely long (cf Santalales).
Antipodal cells usually degenerating. Endosperm development ab initio nuclear.
Chalazal end of megagametophyte usually forming tubular endosperm haustorium.
Embryogenesis onagrad.
Fruit Usually a single- to
many-seeded berry or berry-like gourd (pepo, amphisarca?) with hard pericarp
(rarely a dry och fleshy capsule, a samara or a fleshy explosion fruit; fruit
in Actinostemma and Bolbostemma operculate).
Seeds Aril absent; arilloid
(derived from nearby carpellary tissue) often present. Seeds often flattened,
sometimes winged. Operculum? Seed coat mesotestal. Testa multiplicative,
vascularized, often complex, with lignified epidermis, unilayered or
multilayered sclerotic hypodermis (absent in Coniandreae) and finally
lignified inner layer, with single cell layer usually rich in asterosclereids
(sometimes osteosclereids), sclereid layers usually strongly separated from
other cells; testa often with aerenchyma inside epidermis, thick-walled and
lignified, but little differentiated from hypodermal layer; inner
sclerenchymatous layers often with anticlinal cell divisions, brachysclereid.
Exotesta usually thick, often palisade or cuboid (in some species with
multilayered epidermis). Endotesta usually membranous. Tegmen persistent, with
tracheidal outer cells. Perisperm usually not developed (sometimes well
developed, enclosing embryo). Endosperm rudimentary or absent. Embryo straight,
well differentiated (plumule often with distinct leaves), without chlorophyll;
often with crystalloid inclusions in protein bodies. Cotyledons two, large,
flat. Germination phanerocotylar or cryptocotylar. Seedlings often with
cortical outgrowth on lower side of axis in transition zone between root and
stem.
Cytology n = 7–24 (9–14,
16, 20, 22, 33, 44, 66, 88); x = 12 – Fixed polyploidy (n = 20) occurring in
Cucurbiteae.
DNA Plastid gene infA
lost/defunct (Luffa). Inversion of 35–40 bp present in plastid
trnL/trnF-intergenic spacer (absent in other Cucurbitales; absent in
Neoalsomitra and Cucurbita digitata). Cucurbitaceae usually with
labile genome organization: two (or three) cases (out of extremely few known
among angiosperms) of independent transfer of gene rbcL from plastid
to mitochondrial genome (Cucumis sativus, Cucurbita maxima
and Cucurbita pepo). Mitochondrial coxI intron present in at
least Citrullus, Cucumis and Melothria.
Cucumis with largest known mitochondrial genome among angiosperms
(1.500 kb in C. sativus, 2.400 kb in C. melo).
Phytochemistry Flavonols
(kaempferol, quercetin), extremely bitter-tasting tetra- and pentacyclic
triterpenoids (cucurbitacins etc.), alkaloids, triterpene saponins, punicic
acid (C18H30O2), eleostearic acid (isomere of
punicic acid, in Joliffieae), long-chain fatty acids (in seed oils)
and citrullin (free non-protein amino acid α-amino-δ-ureidopentanoic acid)
present. Ellagic acid, tannins, and proanthocyanidins not found.
Use Ornamental plants, fruits,
vegetables, water sources, medicinal plants, containers and musical instruments
(Lagenaria siceraria).
Systematics A probable
topology of Cucurbitaceae is (Schaefer
& Renner 2011): [Gomphogyneae+[Alsomitra
macrocarpa+[[Triceratieae+Zanonieae]+[Actinostemmateae+[Indofevilleeae+[Thladiantheae+[Siraitieae+[Momordiceae+[Joliffieae+[Bryonieae+[Sicyoeae+[Schizopeponeae+[Coniandreae+[Benincaseae+Cucurbiteae]]]]]]]]]]]]]].
Gomphogyneae Benth. et
Hook. f., Gen. Plant. 1: 820. Sep 1867
5/56. Bayabusua (1; B.
clarkei; the Malay Peninsula), Gomphogyne (6; G. bonii,
G. cirromitrata, G. cissiformis, G. heterosperma,
G. nepalensis, G. peekelii; eastern Himalayas to central
China and Southeast Asia), Gynostemma
(c 20; East and tropical Asia to New Guinea), Hemsleya (27; the
Himalayas, East Asia, Indochina, East Malesia), Neoalsomitra (2;
N. clavigera, N. sarcophylla; tropical Asia to eastern
Queensland and Fiji). – East and tropical Asia, eastern Queensland, Fiji,
with their largest diversity in Southeast Asia. Lianas. Tendrils usually
apically bifid, often with adhesive pads. Petals with multicellular
nectariferous hairs. Stamens three or five, largely connate. Filaments adnate
at base to petals. Anthers dithecal and/or monothecal. Fruit a capsule or
berry. n = 11 (Gynostemma),
14 (Hemsleya), 16 (Gomphogyne).
[Alsomitra
macrocarpa+[[Triceratieae+Zanonieae]+[Actinostemmateae+[Indofevilleeae+[Thladiantheae+[Siraitieae+[Momordiceae+[Joliffieae+[Bryonieae+[Sicyoeae+[Schizopeponeae+[Coniandreae+[Benincaseae+Cucurbiteae]]]]]]]]]]]]]
Alsomitra?
1/1. (1; A. macrocarpa;
Southeast Asia, Malesia to New Guinea). – Liana. n = ? – Alsomitra
has sometimes been identified as sister to the remaining Cucurbitaceae, yet this may
be the result of long-branch attraction.
[[Triceratieae+Zanonieae]+[Actinostemmateae+[Indofevilleeae+[Thladiantheae+[Siraitieae+[Momordiceae+[Joliffieae+[Bryonieae+[Sicyoeae+[Schizopeponeae+[Coniandreae+[Benincaseae+Cucurbiteae]]]]]]]]]]]]
[Triceratieae+Zanonieae]
Triceratieae A. Rich.
in R. de la Sagra, Hist. Fis. Cuba, Bot. 10: 298. 1845
[’Triceratiae’]
5/23. Fevillea (7; F.
anomalosperma, F. bahiensis, F. cordifolia, F.
narae, F. pedatifolia, F. pergamentacea, F.
trilobata; southern Mexico, Central America, tropical South America),
Anisosperma (1; A. passiflora; Brazil), Pteropepon
(4; P. argentinense, P. deltoideus, P. oleiferum,
P. parodii; Brazil, Peru, northern Argentina),
Cyclantheropsis (3; C. madagascariensis, C.
occidentalis, C. parviflora; tropical Africa, Madagascar),
Sicydium (8; tropical America). – Tropical Africa, Madagascar,
tropical America. Lianas. Tendrils simple or apically bifid. Petals with
multicellular nectariferous hairs? Stamens one to five. Filaments adnate at
base to petals. Anthers dithecal or monothecal. Female flowers with five
staminodia. Fruit a pepo, samara or achene. n = ?
Zanonieae Benth. et
Hook. f., Gen. Plant. 1: 820. Sep 1867
4/11. Gerrardanthus (5; G.
grandiflorus, G. lobatus, G. macrorhizus, G.
paniculatus, G. tomentosus; tropical and southern Africa),
Siolmatra (2; S. brasiliensis, S. pentaphylla;
Amazonas), Zanonia (1; Z. indica; tropical Asia), Xerosicyos
(3; X. danguyi, X. perrieri, X. pubescens;
Madagascar). – Tropical and southern Africa, Madagascar, tropical Asia to New
Guinea, the Amazon Basin. Lianas or twining herbs. Xerosicyos
comprises twining woody leaf succulents. Tendrils usually bifid. Inflorescence
with internode below leaf under first flower. Petals in Xerosicyos
free. Petals with multicellular nectariferous hairs. Stamens (four or) five.
Filaments adnate in lower parts to petals. Anthers monothecal. Fruit a capsule.
n = ?
[Actinostemmateae+[Indofevilleeae+[Thladiantheae+[Siraitieae+[Momordiceae+[Joliffieae+[Bryonieae+[Sicyoeae+[Schizopeponeae+[Coniandreae+[Benincaseae+Cucurbiteae]]]]]]]]]]]
Actinostemmateae H.
Schaef. et S. S. Renner in Taxon 60: 130. 1 Feb 2011
1/4. Actinostemma (4; A.
lobatum, A. paniculatum, A. parvifolium; two species in
China; A. tenerum; India, China and eastern Siberia to the Korean
Peninsula and Japan, Taiwan, Laos, Vietnam). – Twining herbs. Tendrils
usually bifid. Petals with multicellular nectariferous hairs. Stamens five or
2+2+1. Filaments adnate at base to petals. Anthers monothecal. Style single.
Ovule pendulous. Fruit a pyxidium. Testa often winged. n = 8.
[Indofevilleeae+[Thladiantheae+[Siraitieae+[Momordiceae+[Joliffieae+[Bryonieae+[Sicyoeae+[Schizopeponeae+[Coniandreae+[Benincaseae+Cucurbiteae]]]]]]]]]]
Usually only branches of tendrils
coiled. Young stem with bicollateral vascular bundles. Extrafascicular phloem
associated with adaxial phloem of vascular bundles. Axillary complex consisting
of tendril, bud, male inflorescence, and female flower (collateral). Nectary
usually disc-shaped, parenchymatous, with stomata. Male flowers with well
developed hypanthium. Style single. Secretion-filled stylar canal present.
Ovules horizontal to erect. Outer integument six to ten cell layers thick.
Inner integument (one or) two to five (or six) cell layers thick. Parietal
tissue five to eleven cell layers thick. Antipodal cells degenerating. Fruit
usually baccate. Testa not winged. Exotesta enlarged, palisade or cuboid,
mucilaginous, with distinctive sclereid layer and often strongly thickened cell
walls. Innermost layer chlorenchymatous. Non-protein amino acids present.
Indofevilleeae H.
Schaef. et S. S. Renner in Taxon 60: 130. 1 Feb 2011
1/1. Indofevillea (1; I.
khasiana; Assam, Bhutan, Tibet). – Liana. Tendrils zanonioid, apically
bifid. Stamens 2+2+1, adnate at base to corolla tube. Anthers monothecal.
Fruits dry, indehiscent, with thick lignified pericarp. n = ?
[Thladiantheae+[Siraitieae+[Momordiceae+[Joliffieae+[Bryonieae+[Sicyoeae+[Schizopeponeae+[Coniandreae+[Benincaseae+Cucurbiteae]]]]]]]]]
Thladiantheae H.
Schaef. et S. S. Renner in Taxon 60: 130. 1 Feb 2011
2/30–35. Baijiania (5; B.
borneensis, B. decipiens, B. smitinandii, B.
taiwaniana, B. yunnanensis; southern China, Thailand, Laos,
Borneo, Taiwan), Thladiantha
(25–30; southern Russia and western Asia, India, the Himalayas, Tibet, China,
the Korean Peninsula, Japan, Taiwan, Southeast Asia, Malesia to New Guinea).
– Temperate Asia, East and tropical Asia to Taiwan and Malesia. Lianas or
twining herbs. Tendrils simple or bifid. Stamens 2+2+1, adnate to corolla tube
(epipetalous). Anthers monothecal. Fruits baccate. n = 9 (Thladiantha),
16 (Baijiania).
[Siraitieae+[Momordiceae+[Joliffieae+[Bryonieae+[Sicyoeae+[Schizopeponeae+[Coniandreae+[Benincaseae+Cucurbiteae]]]]]]]]
Siraitieae H. Schaef.
et S. S. Renner in Taxon 60: 130. 1 Feb 2011
1/5. Siraitia (5; S.
grosvenorii, S. siamensis, S. sikkimensis, S.
silomaradjae: eastern Himalayas, southern China, Thailand, Vietnam,
Malesia; S. africana: in tropical Africa). – Twining herbs. Tendrils
zanonioid, apically bifid. Black to yellow glandular hairs frequent. Stamens
five or 2+2+1, adnate at base to corolla tube. Filaments separate. Anthers
monothecal. Fruit baccate. n = 14.
[Momordiceae+[Joliffieae+[Bryonieae+[Sicyoeae+[Schizopeponeae+[Coniandreae+[Benincaseae+Cucurbiteae]]]]]]]
Momordiceae H. Schaef.
et S. S. Renner in Taxon 60: 130. 1 Feb 2011
1/35–40. Momordica
(35–40; tropical and subtropical Africa, the Arabian Peninsula, tropical Asia
to eastern Queensland). – Usually lianas or twining herbs (rarely shrubs).
Tendrils simple or apically bifid. Stamens three or two, adnate to corolla tube
(epipetalous). Filaments separate. Two anthers dithecal and one anther
monothecal, or one anther trithecal and one dithecal. Fruit indehiscent or
capsular (dehiscing by three valves or irregularly). Aril often present. n =
11, 14.
[Joliffieae+[Bryonieae+[Sicyoeae+[Schizopeponeae+[Coniandreae+[Benincaseae+Cucurbiteae]]]]]]
Joliffieae Schrad. in
Linnaea 12: 402. Apr-Sep 1838
3/9. Cogniauxia (2; C.
podolaena, C. trilobata; Central Africa), Telfairia (3;
T. batesii, T. occidentalis, T. pedata; tropical
Africa), Ampelosycios (4; A. humblotii, A. major,
A. meridionalis, A. scandens; Madagascar). – Tropical
Africa, Madagascar. Lianas or twining herbs. Tendrils simple or bifid. Stamens
three (or five). Fruit fleshy. n = 12 (Telfairia). Eleostearic acid
(isomer of punicic acid) present.
[Bryonieae+[Sicyoeae+[Schizopeponeae+[Coniandreae+[Benincaseae+Cucurbiteae]]]]]
Bryonieae Dumort.,
Fl. Belg.: 54. 1827
3/18. Austrobryonia (4; A.
argillicola, A. centralis, A. micrantha, A.
pilbarensis; northwestern and central Australia), Bryonia
(13; Europe, the Canary Islands, the Mediterranean, North Africa, Southwest and
Central Asia), Ecballium
(1; E. elaterium; the Mediterranean). – Europe, the Mediterranean,
North Africa, the Canary Islands, Southwest and Central Asia, northwestern and
central Australia. Twining herbs. Tendrils simple or absent. Stamens three.
Fruit a berry (in Ecballium elaterium ejecting seeds by elastic
contraction). n = 9 (Ecballium),
10 (Bryonia).
[Sicyoeae+[Schizopeponeae+[Coniandreae+[Benincaseae+Cucurbiteae]]]]
Sicyoeae Schrad. in
Linnaea 12: 407. Apr-Sep 1838 [‘Secyoideae’]
12/244. Nothoalsomitra (1;
N. suberosa; southeastern Queensland); Luffa
(5; L. acutangula, L. aegyptiaca, L. cylindrica,
L. operculata, L. sepium; tropical regions on both
hemispheres); Trichosanthes
(83; tropical Asia to Queensland and islands in the Pacific),
Hodgsonia (2; H. heteroclita, H. macrocarpa; Assam,
Bhutan, southern China, Burma, Thailand, Indochina, Malesiatropical Asia),
Linnaeosicyos (1; L. amara; Hispaniola), Echinocystis
(1; E. lobata; eastern North America), Marah
(8; western and southeastern United States, Mexico), Frantzia (6;
F. panamensis, F. pittieri, F. tacaco, F.
talamancensis, F. venosa, F. villosa; Central America),
Sicyos
(67; eastern Australia, Tasmania, Norfolk Island, Lord Howe, New Zealand,
islands in the Pacific incl. the Hawaiian Islands, tropical and subtropical
America), Hanburia (7; H. caracasana, H.
grisebachii, H. mexicana, H. oerstedii, H.
parviflora, H. spectabilis, H. subcyclanthera; Mexico,
Central America to tropical South America), Cyclanthera
(42; southwestern United States, Mexico, tropical America), Echinopepon
(21; southern United States to northern Argentina, with their highest diversity
in western Mexico). – Pantropical. Lianas or twining herbs. Tendrils simple
or bifid to octafid. Multicellular nectariferous hairs present on hypanthium.
Stamens two to five. Anther thecae often curved, sigmoid, convolute or fused to
a flat or folded ring, dehiscing by continuous slit. Pistil sometimes
consisting of one carpel. Endosperm haustorium in Sicyos edulis up to
19 mm long. Fruit dry or fleshy, indehiscent, explosively dehiscent or a
pyxidium. n = 11–16(–44).
[Schizopeponeae+[Coniandreae+[Benincaseae+Cucurbiteae]]]
Schizopeponeae C.
Jeffrey in Kew Bull. 17: 475. 9 Apr 1964
2/9. Schizopepon (8; northern
India, the Himalayas, Tibet, China, eastern Siberia, Japan, Burma),
Herpetospermum (1; H. pedunculosum; eastern Himalayas to
Burma and southwestern China). – The Himalayas, Tibet, China, Japan. Twining
herbs. Tendrils bifid or trifid. Stamens three. Pollen grains sometimes
triporate. Fruit indehiscent or capsular. n = 10 (Schizopepon), 11
(Herpetospermum).
[Coniandreae+[Benincaseae+Cucurbiteae]]
Coniandreae Endl. ex
M. Roem., Fam. Nat. Syn. Monogr. 2: 6. Dec 1846
19/175–195. Bambekea (1;
B. racemosa; western and central tropical Africa), Eureiandra
(8; tropical and subtropical Africa, Madagascar, Socotra),
Dendrosicyos (1; D. socotranus; Yemen, Socotra),
Seyrigia (5; S. bosseri, S. gracilis, S.
humbertii, S. marnieri, S. multiflora; southern and
southwestern Madagascar), Trochomeriopsis (1; T.
diversifolia; Madagascar), Halosicyos (1; H. ragonesei;
central Argentina), Cucurbitella (1; C. asperata; Brazil,
Paraguay, Uruguay, Bolivia, Argentina), Corallocarpus (13–16;
tropical Africa, Madagascar, the Arabian Peninsula, Pakistan, India),
Kedrostis (c 20; tropical and subtropical Africa, Madagascar, the
Arabian Peninsula, India, Sri Lanka, West Malesia), Ceratosanthes (12;
Central America to northern Argentina), Doyerea (1; D.
emetocathartica; tropical America), Gurania (40–45; tropical
America), Psiguria (12–17; Central America, tropical South America;
incl. Helmontia?), Helmontia (4; H. cardiophylla,
H. leptantha, H. simplicifolia, H. trujilloi;
Venezuela, Guyana, Brazil; in Psiguria?), Melothrianthus (1;
M. smilacifolius; Brazil), Wilbrandia (c 15; Brazil,
Paraguay, Argentina), Apodanthera (30–35; tropical and subtropical
America), Tumamoca (2; T. macdougalii: near Tucson in
Arizona, Sonora in Mexico; T. mucronata: Zacatecas in central Mexico),
Ibervillea (10–11; Texas to Guatemala). – Tropical and subtropical
Africa, Madagascar, Socotra, the Arabian Peninsula, India, tropical Asia,
southern United States to northern Argentina. Usually lianas or twining herbs
(rarely trees). Tendrils simple or bifid or trifid (rarely absent). Stamens
two, three or five. Thecae often curved, convolute etc. Pollen grains sometimes
shed in tetrads. Fruit baccate. Testal hypodermis not sclerotic. n = 12 or 13
(Kedrostis), 13 (Seyrigia, Corallocarpus), 14
(Apodanthera).
[Benincaseae+Cucurbiteae]
Benincaseae Ser. in
Mém. Soc. Phys. Genève 3(1): 25. 1825 [‘Beninsaceae’]
24/230–235. Citrullus
(4; C. colocynthis, C. ecirrhosus, C. lanatus,
C. rehmii; eastern Mediterranean, North Africa, tropical and southern
Africa, western Asia), Peponium (c 20; tropical and subtropical
Africa, Madagascar, the Seychelles), Lagenaria
(6; L. abyssinica, L. breviflora, L. guineensis,
L. rufa, L. siceraria, L. sphaerica; tropical
Africa, Madagascar, one species, L. siceraria, pantropical),
Acanthosicyos (1; A. horridus; Angola, Namibia, Botswana,
South Africa), Raphidiocystis (4; R. brachypoda, R.
chrysocoma, R. jeffreyana, R. phyllocalyx; tropical
Africa, Madagascar), Cephalopentandra (1; C. ecirrhosa;
northeastern tropical Africa), Lemurosicyos (1; L. variegata;
Madagascar), Solena (3; S. amplexicaulis, S.
delavayi, S. heterophylla; tropical Asia), Borneosicyos
(1; B. simplex; Sarawak, Sabah), Benincasa
(2; B. hispida; New Caledonia, New Ireland, New Guinea, Queensland;
B. fistulosa: known only from cultivation), Ctenolepis (1;
C. lucorum; Madagascar), Dactyliandra (2; D.
welwitschii: the Namib desert in Angola and Namibia, the Thar desert in
Pakistan and India; D. nigrescens: Kenya), Khmeriosicyos (1;
K. harmandii; Cambodia; probably extinct), Papuasicyos (c 8;
New Guinea), Scopellaria (2; S. diversifolia, S.
marginata; Yunnan, Southeast Asia, West Malesia to the Philippines),
Trochomeria (8; subtropical and tropical Africa),
Indomelothria (2; I. blumei, I. chlorocarpa;
Southeast Asia, West Malesia), Melothria (16; southeastern United
States, Mexico, Central America, tropical South America; one species, M.
mannii, in western tropical Africa and in Central America and tropical
South America), Ruthalicia (2; R. eglandulosa, R.
longipes; tropical West Africa), Muellerargia (2; M.
jeffreyana: Madagascar; M. timorensis: the Lesser Sunda Islands,
Timor, tropical northern Australia), Cucumis
(c 55; subtropical and tropical regions in the Old World), Zehneria (c
60; tropical regions in the Old World), Diplocyclos (4–5; D.
decipiens, D. leiocarpus, D. palmatus, D.
schliebenii, D. tenuis; subtropical and tropical Africa, tropical
Asia, tropical Australia), Coccinia (c 25; tropical and southern
Africa, one species, C. cordifolia, also in tropical Asia). –
Eastern Mediterranean, Africa, Madagascar, the Seychelles, Aldabra, western and
tropical Asia to New Guinea, Australia and Melanesia, islands in the Pacific,
southeastern United States to South America, with their highest diversity in
tropical Africa and Madagascar. Usually lianas or twining herbs (rarely
shrubs). Tendrils simple, 2–5-fid or absent. Paired spines present at nodes
in Acanthosicyos. Stamens usually three (sometimes two, four or five).
Two anthers dithecal and one monothecal or all dithecal or all monothecal.
Thecae often curved or convolute, etc. Fruit usually baccate. n = 7, 11, 12,
24. Mitochondrial coxI intron present.
Cucurbiteae Dumort.,
Fl. Belg.: 54. 1827
11/95–110. Polyclathra (1 or
6; P. cucumerina; Mexico, Central America), Peponopsis (1;
P. adhaerens; Mexico), Cucurbita
(17; tropical and subtropical America), Calycophysum (5; C.
gracile, C. pedunculatum, C. spectabile, C.
villosum, C. weberbaueri; northwestern tropical South America),
Sicana (3; S. odorifera, S. sphaerica, S.
trinitensis; Central America, the West Indies), Penelopeia (2;
P. sphaerica, P. suburceolata; Hispaniola),
Tecunumania (1; T. quetzalteca; Mexico, Guatemala, Costa
Rica), Schizocarpum (11; Mexico, Guatemala), Cionosicyos (4;
C. excisus, C. guabubu, C. macranthus, C.
pomiformis; Central America, Cuba, Jamaica), Abobra (1; A.
tenuifolia; southern Brazil, Uruguay, Argentina), Cayaponia
(450–60; southern United States, Mexico, Central America, tropical South
America, one species, C. noronhae, endemic on Fernando de Noronha, one
species, C. africana, in western and central tropical Africa and
Madagascar). – Southern United States, Mexico, the Caribbean, Central and
South America, tropical Africa, Madagascar. Lianas or twining herbs. Tendrils
simple or 2–7-fid. Extrafascicular sieve tubes in Cucurbita
present inside cylinder of “pericyclic” fibres. Stamens usually three
(sometimes two or four). Thecae often reflexed, convolute, etc. Pollen grains
triporate to periporate (sometimes up to 200 μm). Fruit small dry and
indehiscent, or medium-sized or large pepos, or dry and capsular (dehiscing
with several valves). n = 20 (Cucurbita,
Sicana).
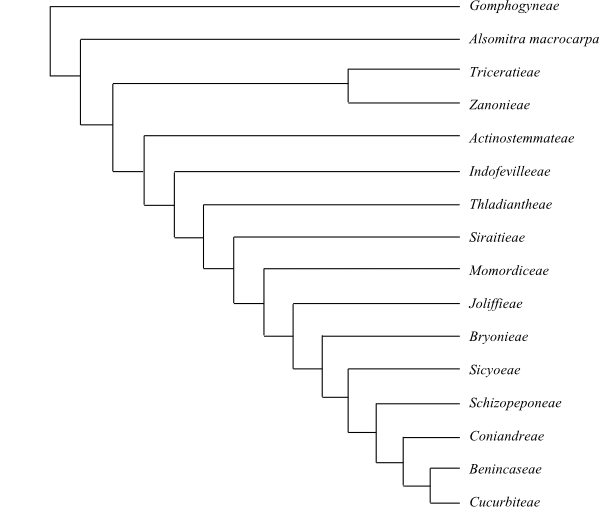 |
Phylogeny (simplified) of Cucurbitaceae based
on DNA data (Schaeffer & Renner 2011).
|
Berchtold et Presl in Přir. Rostlin: 217.
Jan-Apr 1820, nom. cons.
Datiscales Bercht. et
J. Presl, Přir. Rostlin: 217. Jan-Apr 1820 [‘Datisceae’];
Datiscineae Engl., Syllabus, ed. 2: 156. Mai 1898
Genera/species 1/2
Distribution Crete to Turkey
and the Caucasus, western Himalayas, northern California to northwestern
Mexico.
Fossils Unknown.
Habit Polygamomonoecious,
dioecious or androdioecious, perennial herbs. Superficially similar in habit to
Cannabis (Cannabaceae).
Vegetative anatomy Root
nodules containing nitrogen fixing endosymbiotic actinobacteria
(Frankia). Phellogen ab initio superficial. Medullary vascular bundles
present. Cambium and wood elements not storied. Vessel elements with simple
perforation plates; lateral pits alternate, simple pits. Imperforate tracheary
xylem elements libriform fibres with simple pits, non-septate? Wood rays
absent? Axial parenchyma paratracheal? Sieve tube plastids S type. Nodes 1:3,
unilacunar with three leaf traces. Tanniniferous sacs present. Crystals?
Trichomes Hairs unicellular or
multicellular, uniseriate, simple; glandular hairs?
Leaves Alternate (spiral),
deeply pinnately lobed to pinnately compound (imparipinnate), with conduplicate
ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle transection?
Venation pinnate. Stomata anomocytic. Cuticular wax crystalloids? Domatia as
pockets. Mesophyll without sclerenchymatous idioblasts. Leaf margin serrate.
Inflorescence Axillary,
compound, contracted thyrse.
Flowers Actinomorphic.
Epigyny. Sepals three to nine (to ten), with valvate aestivation (in male
flowers), persistent, free. Petals absent. Nectary absent. Disc absent.
Androecium Stamens in bisexual
flowers three to five, in male flowers eight to c. 25, outer stamens often
antesepalous. Filaments very short, free from each other and from tepals.
Anthers straight, basifixed, non-versatile, tetrasporangiate, extrorse,
longicidal (dehiscing by longitudinal slits). Tapetum secretory. Staminodia
usually absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains tricolporate, shed as monads,
bicellular at dispersal. Exine tectate, with columellate infratectum,
rugulate.
Gynoecium Pistil composed of
three to five (to eight) antesepalous carpels connate in lower part; carpels
strongly developed, ventrally forming roof-like structure above locule, this
forming perianth tube base; calyx tube with widely separated stylodia inserted
on roof edge. Ovary inferior, unilocular. Stylodia three to five (to eight),
subulate, deeply bifid; compitum possibly present. Stigmas papillate, Dry type.
Pistillodium absent.
Ovules Placentation parietal.
Ovules c. 30 to more than 100 per carpel, anatropous, pendulous to horizontal,
bitegmic, crassinucellar. Micropyle bistomal. Outer integument two cell layers
thick. Inner integument two cell layers thick. Parietal tissue three to five
cell layers thick. Nucellar cap two or three cell layers thick. Megagametophyte
disporous, 8-nucleate, Allium type. Antipodal cells persistent.
Endosperm development ab initio nuclear. Endosperm haustoria? Embryogenesis
onagrad.
Fruit A membranous septicidal?
capsule, apically dehiscing between persistent stylodia.
Seeds Aril absent. Funicle
surrounded by collar formed as extension of testa. Operculum formed by
micropyle and hilum, without surrounding annulus of collar cells. Seed coat
exotestal. Exotestal cells with honeycomb-like arrangement, with strongly
thickened and lignified inner walls. Endotesta absent. Exotegmic cells large,
cuboid. Endotegmen absent. Perisperm not developed. Endosperm sparse with oil
and aleurone, or absent. Embryo small, straight, well differentiated,
chlorophyll? Cotyledons two, oily. Germination phanerocotylar.
Cytology x = 11
DNA
Phytochemistry
B-ring-nonsubstituted and 2’-hydroxylated flavonols (kaempferol, quercetin)
and cucurbitacins present. Glucosides of unusual flavonols, galangin (5,
7-dihydroxyflavonol), 7-O-methylgalangin, datiscetin (3, 5, 7,
2’-tetrahydroxyflavonol) and 7-O-methyldatiscetin present. Ellagic acid,
tannins, proanthocyanidins, and cyanogenic compounds not found.
Use Ornamental plants.
Systematics Datisca
(2; D. cannabina: Crete, eastern Aegaean, Turkey, the Caucasus,
western Himalayas; D. glomerata: northern California to northern Baja
California in northwestern Mexico).
Datisca
is sister to Begoniaceae.
Airy Shaw in Kew Bull. 18: 267. 8 Dec 1965
Genera/species 2/2
Distribution Tropical Asia to
tropical Australia and Solomon Islands.
Fossils Fossil wood,
Tetrameleoxylon prenudiflora, has been described from the
Maastrichtian Deccan Intertrappean Beds in India.
Habit Dioecious, evergreen
trees. Often with large plank buttresses.
Vegetative anatomy Phellogen
ab initio superficial. Vessel elements with simple perforation plates; lateral
pits alternate, simple pits. Imperforate tracheary xylem elements libriform
fibres with simple or bordered pits, non-septate. Wood rays multiseriate,
heterocellular. Axial parenchyma paratracheal scanty, aliform, confluent,
vasicentric, or banded. Wood soft, sometimes fluorescent. Wood elements often
storied. Sieve tube plastids S type. Nodes usually 3:1–2, trilacunar with one
or two leaf traces from each lateral leaf gap. Branched sclereids and stone
cells present in cortex and medulla in Octomeles. Crystals?
Trichomes Hairs simple;
glandular, lepidote hairs often present.
Leaves Alternate (spiral),
simple, entire, asymmetrical, with ? ptyxis. Stipules and leaf sheath absent.
Petiole vascular bundle transection? Venation palmate. Stomata anomocytic.
Cuticular wax crystalloids absent? Mesophyll in at least Octomeles
with sclerenchymatous H-shaped idioblasts (reaching from one epidermis to
next). Leaf margin usually serrate (rarely entire).
Inflorescence Terminal or
axillary, compound or contracted spicate thyrse.
Flowers Actinomorphic.
Hypanthium present. Epigyny. Sepals four or six to eight, with valvate
aestivation, free or connate (postgenitally coherent). Petals usually absent
(in male flowers of Octomeles six to eight, small, with valvate
aestivation, free, inserted on sepals). Nectaries possibly present inside male
floral tube in Octomeles. Disc absent or present above ovary.
Androecium Stamens four or six
to eight, obhaplostemonous, alternisepalous, antepetalous. Filaments flattened
to narrowly elongate, free from each other and from tepals, inflexed in bud.
Anthers straight or curved, basifixed, non-versatile, tetrasporangiate,
extrorse, longicidal (dehiscing by longitudinal slits). Tapetum secretory.
Female flowers sometimes with staminodia.
Pollen grains
Microsporogenesis simultaneous. Pollen grains tricolporate, shed as monads,
bicellular at dispersal. Exine semitectate, with columellate infratectum,
reticulate.
Gynoecium Pistil composed of
(three or) four or six to eight carpels connate in lower part; carpels strongly
developed ventrally forming roof-like structure above locule, this forming
perianth tube base; calyx tube with widely separated stylodia inserted on roof
edge. Compitum possibly present in Octomeles. Ovary inferior,
unilocular, septate. Stylodia (three or) four or six to eight, short, straight,
free. Stigmas entire, decurrent to clavate or capitate, type? Male flowers
sometimes with pistillodium.
Ovules Placentation parietal,
protruding-diffuse; placentae in Octomeles bilobate. Ovules c. 20 to
more than 100 per carpel, anatropous, pendulous or horizontal, bitegmic,
crassinucellar. Micropyle bistomal. Outer integument approx. two cell layers
thick. Inner integument approx. two cell layers thick. Megagametophyte
monosporous, Polygonum type. Endosperm development ab initio nuclear.
Endosperm haustoria? Embryogenesis?
Fruit A septicidal capsule, in
Tetrameles apically dehiscing between persistent stylodia and along
sides, in Octomeles separating into two layers outer of which being
detached.
Seeds Aril absent? Funicle
surrounded by collar formed as extension of testa. Operculum formed by
micropyle and hilum, without surrounding annulus of cells. Seed coat exotestal.
Exotestal cells with honeycomb-like arrangement, with strongly thickened and
lignified inner walls? Endotesta? Tegmen? Perisperm not developed. Endosperm
very sparse or absent. Embryo straight, well differentiated, chlorophyll?
Cotyledons two. Germination?
Cytology n = c. 23
DNA
Phytochemistry Insufficiently
known. Flavonols (kaempferol, quercetin) and cucurbitacins present. Tannins and
cyanogenic compounds not found.
Use Timber, carpentries,
canoes.
Systematics Octomeles
(1; O. sumatrana; Sumatra, Borneo and the Philippines to New Guinea
and Solomon Islands), Tetrameles (1; T. nudiflora; Sri Lanka,
northeastern India, the Andaman Islands, southern Himalayas, southern Yunnan,
Southeast Asia, Malesia to New Guinea and New Britain, northeastern
Queensland).
Tetramelaceae are sister to
[Datiscaceae+Begoniaceae].
Literature
Akkermans ADL, Roelofsen W, Blom J,
Huss-Danell K, Harkink R. 1983. Utilization of carbon and nitrogen compounds by
Frankia in synthetic media and in root nodules of Alnus
glutinosa, Hippophae rhamnoides, and Datisca cannabina.
– Can. J. Bot. 61: 2793-2800.
Akkermans ADL, Hafeez F, Roelofsen W,
Chaudhary AH, Baas R. 1983. Ultrastructure and nitrogenase activity of
Frankia grown in pure culture and in actinorrhizae of Alnus,
Colletia and Datisca. – In: Veeger C, Newton WE (eds),
Advances in nitrogen fixation research, Nijhoff/Dr. W. Junk, The Hague, The
Netherlands, pp. 311-319.
Alvarado JL, Lira R, Caballero J. 1992.
Palynological evidence for the generic delimitation of Sechium sensu
lato (Cucurbitaceae) and its
allies. – Bull. Brit. Mus. (Nat. Hist.), Bot. 22: 109-121.
Alverson AJ, Wei X-X, Rice DW, Stern DB,
Barry K, Palmer JD. 2010. Insights into the evolution of mitochondrial genome
size from complete sequences of Citrullus lanatus and Cucurbita
pepo (Cucurbitaceae). –
Mol. Biol. Evol. 27: 1436-1448.
Amaral MM do, Ceccantini G. 2011. The
endoparasite Pilostyles ulei (Apodanthaceae-Cucurbitales) influences wood
structure in three host species of Mimosa. – IAWA J. 32: 1-13.
Anderson JAR, Muller J. 1975. Palynological
study of a Holocene peat and a Miocene coal deposit from NW Borneo. – Rev.
Paleobot. Palyn. 19: 291-351.
Arends JC. 1992. The biosystematics of B.
squamulosa Hook. f. and affiliated species in sect. Tetraphila A.
DC. – Wageningen Agric. Univ. Papers 91-6: 1-223.
Ashurmetov OA. 1995. On morphology and
taxonomy of the genera Cucumis L. and Melo Mill. – Feddes
Repert. 106: 155-159.
Ayala-Nieto ML, Lira Saade R, Alvarado JL.
1988. Morfología polínica de las Cucurbitaceae de la Península de
Yucatán, Mexico. – Pollen Spores 30: 5-28.
Baehni C, Dansereau P. 1939.
Polygonanthus, genre de Saxifragacées. – Bull. Soc. Bot. France 86:
183-186.
Barabé D. 1981. Vascularisation de la fleur
pistillée de Begonia handelii. – Can. J. Bot. 59: 819-825.
Barabé D, Chrétien L. 1983. Nouvelles
données sur la vascularisation de la fleur pistillée de Begonia (Begoniaceae). – Bull. Soc. Bot.
France, Lettr. Bot. 130: 307-316.
Barabé D, Brouillet L, Bertrand C. 1985. Les
Bégonias à placentation pariétale: Cas de Begonia masoniana
Irmscher. – Bull. Mus. Natl. Hist. Nat., sér. IV, Sect. B, Adansonia 7:
403-414.
Barabé D, Brouillet L, Bertrand C. 1990.
Analyse taxonumérique des caractères foliaires des Begoniaceae. – Bull. Mus. Natl.
Hist. Nat. Paris, sér. IV, sect. B, Adansonia 12: 337-353.
Barabé D, Brouillet L, Daigle S. 1992.
Organogénie de la feuille de Begonia radicans Vellozo et de B.
scabrida A. DC. (Begoniaceae). – Can. J. Bot. 70:
1107-1122.
Baranov A, Barkley FA. 1974. The sections of
the genus Begonia. – Northeastern University, Boston,
Massachusetts.
Barber KG. 1909. Comparative histology of
fruits and seeds of certain species of Cucurbitaceae. – Bot. Gaz. 47:
263-310.
Barkley FA. 1972. Begoniaceae. The genera, sections
and known species of each. – The Buxtonian I, suppl. 4: 1-20.
Barkley FA, Golding J. 1974. The species of
the Begoniaceae. – Northeastern
University, Boston, Massachusetts.
Barth OM, Pinto da Luz CF, Gomes-Klein VL.
2005. Pollen morphology of Brazilian species of Cayaponia Silva Manso
(Cucurbitaceae, Cucurbiteae).
– Grana 44: 129-13.
Bates DM, Robinson WR, Jeffrey C (eds). 1990.
Biology and utilisation of the Cucurbitaceae. – Cornell
University Press, Ithaca, New York.
Beevy SS, Kuriachan P. 1996. Chromosome
numbers of South Indian Cucurbitaceae and a note on the
cytological evolution in the family. – J. Cytol. Genet. 31: 65-71.
Behnke H-D. 1988. Sieve-element plastids and
systematic relationships of Rhizophoraceae, Anisophylleaceae, and allied
groups. – Ann. Missouri Bot. Gard. 75: 1387-1409.
Bell ME. 1974. Toxicology of karaka kernel,
karakin, and β-nitropropionic acid. – New Zealand J. Sci. 17: 327-334.
Bellot S, Renner SS. 2013. Pollination and
mating systems of Apodanthaceae
and the distribution of reproductive traits in parasitic angiosperms. – Amer.
J. Bot. 100: 1083-1094.
Bellot S, Renner SS. 2014a. Exploring new
dating approaches for parasites: the worldwide Apodanthaceae (Cucurbitales) as an example. –
Molec. Phylogen. Evol. 80: 1-10.
Bellot S, Renner SS. 2014b. The systematics
of the worldwide endoparasite family Apodanthaceae (Cucurbitales), with a key, a map,
and color photos of most species. – PhytoKeys 36: 41-57.
Benecke F. 1882. Beitrag zur Kenntniss der
Begoniaceen. – Engl. Bot. Jahrb. Syst. 3: 288-318.
Berg RG van den. 1983. Pollen characteristics
of the genera of the Begoniaceae.
– In: Wilde JJFE de (ed), Studies in Begoniaceae II, Meded. Landbouwh.
Wageningen 83: 55-66.
Berg RG van den 1984. Pollen morphology of
the genus Begonia in Africa. – In: Wilde JJFE de (ed), Studies in Begoniaceae II, Meded. Landbouwh.
Wageningen 84: 5-94.
Bhattachariya SS. 1954. Ein Beitrag zur
Morphologie des Andröceums von Benincasa hispida (Thunb.) Cogn. –
Ber. Deutsch. Bot. Ges. 67: 22-25.
Blarer A, Nickrent D, Endress PK. 2004.
Comparative floral structure and systematics in Apodanthaceae (Rafflesiales). –
Plant Syst. Evol. 245: 119-142.
Boer HJ de, Thulin M. 2012. Synopsis of
Trichosanthes (Cucurbitaceae) based on recent
molecular phylogenetic data. – PhytoKeys 12: 23-33.
Boesewinkel FD. 1984. Ovule and seed
structure in Datiscaceae. –
Acta Bot. Neerl. 33: 419-429.
Boesewinkel FD, De Lange A. 1983. Development
of ovule and seed in Begonia squamulosa Hook. f. – Acta Bot. Neerl.
32: 417-425.
Bohm BA. 1988. Flavonoid systematics of the
Datiscaceae. – Biochem. Syst.
Ecol. 16: 151-155.
Bohm BA, Ornduff R. 1981. Leaf flavonoids and
ordinal affinites of Coriariaceae. – Syst. Bot. 6:
15-26.
Bopp M. 1957. Untersuchungen über die
Verteilung und Vererbung von Anthocyanen in den Blättern von Begonien. –
Planta 48: 631-682.
Bouman F. 1985. Seed structure of B.
thomeana C. DC. – In: Wilde JJFE de (ed), Studies in Begoniaceae II, Agric. Univ.
Wageningen Papers 84-3: 122-124.
Bouman F, De Lange A. 1982. Micromorphology
of the seed coats in Begonia section Squamibegonia Warb. –
Acta Bot. Neerl. 31: 297-305.
Bouman F, Meijer W. 1994. Comparative
structure of ovules and seeds in Rafflesiaceae. –
Plant Syst. Evol. 193: 187-212.
Brouillet L, Bertrand A, Cuerrier A, Barabé
D. 1987. Les hydathodes des genres Begonia et Hillebrandia
(Begoniaceae). – Can. J. Bot.
65: 34-52.
Brown R. 1941. The growth of isolated
cotyledons of Cucurbita pepo. – Ann. Bot., N. S., 5: 175-192.
Bugnon P. 1956. Valeur morphologique de
l’ovaire infére chez les Begonia. – Bull. Soc. Linn. Normandie,
sér. VII, 9: 7-25.
Bugnon P. 1956. Valeur morphologique du
complexe axillaires chez les Cucurbitacées. – Ann. Sci. Nat. Bot., sér. IV,
11: 313-322.
Bugnon P, Bugnon F. 1953. La paroi de
l’ovaire infère des Begonia est-elle de nature axile ou
appendiculaire? – Bull. Sci. Bourgogne 14: 109-127.
Burt-Utley K. 1984. Studies on Middle
American Begonia (Begoniaceae) I. – Brittonia 36:
232-235.
Burt-Utley K. 1985. A revision of Central
American species of Begonia section Gireoudia (Begoniaceae). – Tulane Stud. Zool.
Bot. 25: 1-131.
Burt-Utley K. 1986. Studies on Middle
American Begonia (Begoniaceae) II. – Brittonia 38:
333-339.
Carlquist SJ. 1985a. Wood anatomy of Begoniaceae, with comments on
raylessness, paedomorphosis, relationships, vessel diameter, and ecology. –
Bull. Torrey Bot. Club 112: 59-69.
Carlquist SJ. 1985b. Wood anatomy of Coriariaceae: phylogenetic and
ecological implications. – Syst. Bot. 10: 174-183.
Carlquist SJ. 1992. Wood anatomy of selected
Cucurbitaceae and its
relationship to habit and systematics. – Nord. J. Bot. 12. 347-355.
Carlquist SJ, Miller RB. 2001. Wood anatomy
of Corynocarpaceae is
consistent with its cucurbitalean placement. – Syst. Bot. 26: 54-65.
Chakravarty HL. 1958. Morphology of the
staminate flowers in the Cucurbitaceae with special
reference to the evolution of the stamens. – Lloydia 21: 49-87.
Chakravorti AK. 1947. The development of the
female gametophyte and seed of Coccinia W. & A. – J. Indian Bot.
Soc. 26: 95-104.
Charpentier A, Brouillet L, Barabé D. 1989a.
Organogénèse de la fleur pistillée du Begonia dregei et de
l’Hillebrandia sandwicensis (Begoniaceae). – Can. J. Bot. 67:
3625-3639.
Charpentier A, Brouillet L, Barabé D. 1989b.
Organogénèse de la fleur pistillée du Begonia horticola (Begoniaceae). – Can. J. Bot. 67:
559-572.
Chaudhary AH. 1979. Nitrogen-fixing root
nodules in Datisca cannabina L. – Plant and Soil 51: 163-165.
Chen JCC, Chiu MH, Nie RL, Cordell GA, Qiu
SX. 2005. Cucurbitacins and cucurbitane glycosides: structures and biological
activities. – Nat. Prod. Rep. 22: 386-399.
Chen X, He H, Zhang L-B. 2013. A taxonomic
revision of Anisophyllea (Anisophylleaceae) from
Madagascar. – Phytotaxa 148: 32-46.
Chopra RN. 1955. Some observations on
endosperm development in the Cucurbitaceae. – Phytomorphology
5: 219-230.
Chopra RN, Basu B. 1965. Female gametophyte
and endosperm of some members of the Cucurbitaceae. – Phytomorphology
15: 217-223.
Chopra RN, Seth PN. 1977. Some aspects of
endosperm development in Cucurbitaceae. – Phytomorphology
27: 112-115.
Chung S-M, Decker-Walters DS, Staub JE. 2003.
Genetic relationships within the Cucurbitaceae as assessed by
consensus chloroplast simple sequence repeats (ccSSR) marker and sequence
analysis. – Can. J. Bot. 81: 814-832.
Clawson ML, Bourret A, Benson DR. 2004.
Assessing the phylogeny of Frankia-actinorhizal plant nitrogen-fixing
root nodule symbioses with Frankia 16SrRNA and glutamine synthetase
gene sequences. – Mol. Phylogen. Evol. 31: 131-138.
Clement WL, Tebbitt MC, Forrest LL, Blair JE,
Brouillet L, Eriksson T, Swensen SM. 2004. Phylogenetic position and
biogeography of Hillebrandia sandwicensis (Begoniaceae): a rare Hawaiian
relict. – Amer. J. Bot. 91: 905-917.
Condon MA, Gilbert LE. 1988. Sex expression
of Gurania and Psiguria (Cucurbitaceae): Neotropical vines
that change sex. – Amer. J. Bot. 75: 875-884.
Croizat L. 1943. New families
[Polygonanthaceae]. – Cact. Succ. J. 15: 64.
Cuerrier A, Brouillet L, Barabé D. 1990a.
Numerical taxonomic study of the Begoniaceae using the Mantel test on
leaf microcharacters. – Taxon 39: 549-560.
Cuerrier A, Brouillet L, Barabé D. 1990b.
Micromorphologie foliaire des Begoniaceae. – Bull. Mus. Natl.
Hist. Nat. Paris, sér. IV, sect. B, Adansonia 12: 297-335.
Cuerrier A, Brouillet L, Barabé D. 1991.
Analyse taxonumérique des characters foliaires des Begoniaceae. – Bull. Mus. Natl.
Hist. Nat. Paris IV, 12, sect. B, Adansonia: 337-353.
Dahlgren RMT. 1988. Rhizophoraceae and
Anisophylleaceae: summary
statement, relationships. – Ann. Missouri Bot. Gard. 75: 1259-1277.
Dane F. 1976. Evolutionary studies in the
genus Cucumis. – Ph.D. diss., C.S.U., Fort Collins Colorado.
Dane F, Tsuchiya T. 1979. Meiotic chromosome
and pollen morphological studies of polyploid Cucumis species. –
Euphytica 28: 53-567.
Dane F, Denna DW, Tsuchiya T. 1980.
Evolutionary studies of wild species in the genus Cucumis. –
Zeitschr. Pflanzenzücht. 85: 89-109.
Davidson C. 1972. An anatomical and
morphological study of Datiscaceae. – Ph.D. diss.,
Claremont Graduate School, Claremont, California.
Davidson C. 1973. An anatomical and
morphological study of Datiscaceae. – Aliso 8: 49-110.
Davidson C. 1976. Anatomy of the xylem and
phloem of Datiscaceae. – Nat.
Hist. County Museum Los Angeles Contr. Sci. 280: 1-28.
Dawson MI. 1997. Chromosome numbers in
Corynocarpus (Corynocarpaceae). – New
Zealand J. Bot. 35: 255-258.
De Lange A, Bouman F. 1986. Micromorphology
of the seeds in Begonia section Solananthera A. DC. – Acta
Bot. Neerl. 35: 489-495.
De Lima JE, Mamede MCH. 2004. Novelties in
Begonia (Begoniaceae)
from the coastal forests of Brazil. – Brittonia 56: 75-81.
Dell BD. 1984. Rafflesiaceae. –
In: George AS (ed), Flora of Australia 22, Australian Government Publ. Service,
Canberra, pp. 147-150.
Dell BD, Burbidge AH. 1981. Notes on the
biology of Pilostyles (Rafflesiaceae) in
Western Australia. – West. Aust. Herb. Res. Notes 5: 71-79.
Dell BD, Kuo J, Burbidge AH. 1982. Anatomy of
Pilostyles hamiltonii C. A. Gardner (Rafflesiaceae) in
stems of Daviesia. – Aust. J. Bot. 30: 1-9.
Deshpande BD, Kasat ML. 1966. Seedling
anatomy of certain members of the Cucurbitaceae. – Proc. Indian
Acad. Sci., Sect. B, 64: 62-67.
Deshpande PK, Bhuskute SM, Makde KH. 1986.
Microsporogenesis and male gametophyte in some Cucurbitaceae. – Phytomorphology
36: 145-150.
Devi HM, Naidu KC. 1979. Embryological
studies in the family Begoniaceae. – Indian J. Bot. 2:
1-7.
De Wilde JJFE. 1984. Begonia section
Cristasemen J. J. de Wilde, sect. nov. – In: Wilde JJFE de (ed),
Studies in Begoniaceae II, Meded.
Landbouwh. Wageningen 84: 113-129.
De Wilde JJFE. 1985a. Taxonomy of the
(African) Begoniaceae, and
introduction. – Acta Bot. Neerl. 34: 226-227.
De Wilde JJFE. 1985b. Studies in Begoniaceae II. Begonia
section Cristasemen J. J. de Wilde, sect. nov. – Wageningen Agric.
Univ. Pap. 84-3: 113-129.
De Wilde JJFE. 1988. Begonias als indicator
voor ijstijdinvloeden in tropisch woud. – Wageningen Bull. Bot. Tuinen 21:
10-11.
De Wilde JJFE. 2002. Studies in Begoniaceae VII. Begonia
section Tetraphila A. DC., a taxonomic revision. – Wageningen Agric.
Univ. Pap. 2001-2: 5-258.
De Wilde JJFE. 2011. Begoniaceae. – In: Kubitzki K
(ed), Families and genera of vascular plants X. Flowering plants. Eudicots. Sapindales, Cucurbitales, Myrtaceae. Springer, Heidelberg,
Dordrecht, London, New York, pp. 56-71.
De Wilde JJFE, Arends JC. 1980.
Begonia section Squamibegonia Warb.: a taxonomic revision.
– Misc. Pap. Landbouwhogeschool Wageningen 19: 377-421.
De Wilde JJFE, Arends JC. 1989. Begonia
salaziensis (Gaud.) Warb., taxonomy and placentation. – Acta Bot. Neerl.
38: 31-39.
De Wilde JJFE, Plana V. 2003. A new section
of Begonia (Begoniaceae)
from west central Africa. – Edinburgh J. Bot. 60: 121-130.
De Wilde WJJO, De Wilde-Duyfjes BEE. 1999.
Bayabusua, a new genus of Cucurbitaceae. – Sandakania 13:
1-16.
De Wilde WJJO, De Wilde-Duyfjes BEE. 2001.
Taxonomy of Hodgsonia (Cucurbitaceae), with a note on the
ovules and seeds. – Blumea 46: 169-179.
De Wilde WJJO, De Wilde-Duyfjes BEE. 2002.
Synopsis of Momordica (Cucurbitaceae) in SE Asia and
Malesia. – Bot. Žurn. 87: 132-148.
De Wilde WJJO, De Wilde-Duyfjes BEE. 2003a.
Revision of Neoalsomitra. – Blumea 48: 99-121.
De Wilde WJJO, De Wilde-Duyfjes BEE. 2003b.
The genus Baijiania (Cucurbitaceae). – Blumea 48:
279-284.
De Wilde WJJO, De Wilde-Duyfjes BEE. 2004.
Review of the genus Solena (Cucurbitaceae). – Blumea 49:
69-81.
De Wilde WJJO, De Wilde-Duyfjes BEE. 2004.
The genus Trichosanthes (Cucurbitaceae) in Sabah. –
Sandakania 14: 5-32.
De Wilde WJJO, De Wilde-Duyfjes BEE. 2006a.
Redefinition of Zehneria and four new related genera (Cucurbitaceae), with an
enumeration of the Australasian and Pacific species. – Blumea 51: 1-88.
De Wilde WJJO, De Wilde-Duyfjes BEE. 2006b.
Review of the genus Gymnopetalum (Cucurbitaceae). – Blumea 51:
281-296.
De Wilde WJJO, DeWilde-Duyfjes BEE. 2006c.
Scopellaria, a new genus name in Cucurbitaceae. – Blumea 51:
297-298.
De Wilde WJJO, De Wilde-Duyfjes BEE. 2006d.
The subtribe Thladianthinae (Cucurbitaceae) in Indochina and
Malesia. – Blumea 51: 493-518.
De Wilde WJJO, De Wilde-Duyfjes BEE. 2006e.
Mukia Arn. (Cucurbitaceae) in Asia, in
particular in Thailand. – Thai Forest Bull. (Botany) 34: 38-52.
De Wilde WJJO, De Wilde-Duyfjes BEE. 2007a.
Gynostemma (Cucurbitaceae) in Thailand and
Malesia. – Blumea 52: 263-280.
De Wilde WJJO, De Wilde-Duyfjes BEE. 2007b.
Mukia Arn. (Cucurbitaceae) in Asia, in
particular in Thailand. – Thai Forest Bull. (Bot.) 34: 38-52.
De Wilde WJJO, De Wilde-Duyfjes BEE. 2007c.
The wild species of Cucumis L. (Cucurbitaceae) in South-East Asia.
– Adansonia, sér. III, 29: 239-248.
De Wilde WJJO, De Wilde-Duyfjes BEE. 2009.
Miscellaneous Southeast Asian cucurbit news II. – Reinwardtia 12: 405-414.
De Wilde WJJO, De Wilde-Duyfjes BEE. 2012.
Keys to and checklist of species of the genus Trichosanthes L. (Cucurbitaceae) in Indochina. –
Adansonia 34: 265-278.
De Wilde WJJO, De Wilde-Duyfjes BEE, Ham RWJM
van der. 2003 [2007?]. Borneosicyos simplex (Cucurbitaceae) a veritable rare
plant peculiar of Kinabalu Park. – Flora Malesiana Bull. 14: 33-42.
De Wilde WJJO, De Wilde-Duyfjes BEE, Ham RWJM
van der. 2004. Khmeriosicyos, a new monotypic genus of Cucurbitaceae from Cambodia. –
Blumea 49: 441-446.
De Wilde WJJO, De Wilde-Duyfjes BEE, Ham RWJM
van der. 2007. Revision of the genus Gomphogyne (Cucurbitaceae). – Thai Forest
Bull. (Bot.) 35: 45-68.
De Wilde-Duyfjes BEE, De Wilde WJJO. 1998.
Revision of Alsomitra Spach. – In: Proceedings of the Fourth
International Flora Malesiana Symposium 1998, Forest Research Institute
Malaysia, Kuala Lumpur, pp. 101-105.
De Wilde-Duyfjes BEE, Ham RWJM van der, De
Wilde WJJO. 2003. Papuasicyos, a new genus of Cucurbitaeae. – Blumea 48:
123-128.
Dewitte A, Twyford AD, Thomas DC, Kidner CA,
Van Huylenbroeck J. 2011. The origin of diversity in Begonia: genome
dynamism, population processes and phylogenetic patterns. – In: Grillo O
(ed.), The dynamical processes of biodiversity – case studies of evolution
and spatial distribution, publ. by InTech, Rijeka & Shanghai, pp. 27-52.
Dewitte A, Twyford AD, Thomas DC, Kidner CA,
Van Huylenbroeck J. 2011. The origin of diversity in Begonia: genome
dynamism, population processes and phylogenetic patterns. – In: Grillo O
(ed.), The dynamical processes of biodiversity – case studies of evolution
and spatial distribution, publ. by InTech, Rijeka & Shanghai, pp. 27-52.
Dieterle JVA. 1974. A new geocarpic genus
from Mexico: Apatzingania (Cucurbitaceae). – Brittonia 26:
129-132.
Ding Hou. 1958. Rhizophoraceae. –
In: Steenis CGGJ van (ed), Flora Malesiana I, 5(4), Noordhoff-Kolff N. V.,
Djakarta, pp. 429-493.
Dittmer HJ, Roser ML. 1963. The periderm of
certain members of the Cucurbitaceae. – Southwest.
Naturalist 8: 1-9.
Doorenbos J. 1985. Domestication of
Begonia. – Acta Bot. Neerl. 34: 230-231.
Doorenbos J, Sosef MSM, de Wilde JJFE. 1998.
Studies in Begoniaceae VI. The
sections of Begonia including descriptions, keys and species lists.
– Wageningen Agric. Univ. Papers 98-2: 1-266.
Doskotch RW, Hufford CD. 1970.
Hexanor-cucurbitacin D, a degraded cucurbitacin from Begonia
tuberhybrida var. alba. – Can. J. Chem. 48: 1787-1788.
Doskotch RW, Malik MY, Beal JL. 1969.
Cucurbitacin B, the cytotoxic principle of Begonia tuberhybrida var.
alba. – Lloydia 32: 115-122.
Duchen P, Renner SS. 2010. The evolution of
Cayaponia (Cucurbitaceae): repeated shifts
from bat to bee pollination and long-distance dispersal to Africa 2–5 million
years ago. – Amer. J. Bot. 97: 1129-1141.
Dutt B, Roy RP. 1971. Cytogenetic
investigations in Cucurbitaceae
I. Interspecific hybridization in Luffa. – Genetica 42: 139-156.
Duyfjes BEE. 1993. Coriariaceae. – In: Kalkman C et
al. (eds), Flora Malesiana I, 11(2), Rijksherbarium/Hortus Botanicus, Leiden,
pp. 385-391.
Dwivedi MD, Barfield S, Pandey AK, Schaefer
H. 2018. Phylogeny of Zehneria (Cucurbitaceae) with special focus
on Asia. – Taxon 67: 55-65.
Eliasson UH. 1994. 59. Rafflesiaceae. –
In: Harling G, Andersson L (eds), Flora of Ecuador 51, Nord. J. Bot.,
Copenhagen, pp. 43-49.
Endriss W. 1902. Monographie von
Pilostyles ingae (Karst). – Flora 91: 209-236.
Engler A. 1912. Rafflesiaceae
Africanae. – Engl. Bot. Jahrb. Syst. 46: 293.
Engler A. 1896. Coriariaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien III(5), W. Engelmann,
Leipzig, pp. 128-129.
Engler A. 1897. Corynocarpaceae. – In: Engler
A, Prantl K (eds), Die natürlichen Pflanzenfamilien, Nachträge zu III(5), W.
Engelmann, Leipzig, pp. 215-217.
Fellerer C. 1892. Beiträge zur Anatomie und
Systematik der Begoniaceen. – Ph.D. diss., Universität München, Germany.
Fickel JF. 1876. Über die Anatomie und
Entwicklungsgeschichte der Samenschalen einiger Cucurbitaceen. – Bot. Zeit.
34: 738-778.
Filipowicz N, Renner SS. 2010. The worldwide
holoparasitic Apodanthaceae
confidently placed in the Cucurbitales by nuclear and
mitochondrial gene trees. – BMC Evol. Biol. 10: 219.
Floret J-J. 1979. À propos du contenu
seminal dans les genres Anisophyllea et Poga
(Rhizophoracées-Anisophylloidées). – Adansonia sér. II, 19: 109-115.
Foreman DB. 1978. Corynocarpaceae. – In:
Womersley JS (ed), Handbooks of the flora of Papua New Guinea 1, Melbourne
University Press, Carlton, Victoria, pp. 111-113.
Forrest LL. 2000. A phylogeny of Begoniaceae Bercht. & J. Presl.
– Ph.D. diss., University of Glasgow, Scotland.
Forrest LL, Hollingsworth PM. 2003. A
recircumscription of Begonia based on nuclear ribosomal sequences. –
Plant Syst. Evol. 241: 193-211.
Forrest LL, Hughes M, Hollingsworth PM. 2005.
A phylogeny of Begonia using nuclear ribosomal sequence data and
morphological characters. – Syst. Bot. 30: 671-682.
Fowden L. 1990. Amino acids as chemotaxonomic
indicators. – In: Bates DM, Robinson WR, Jeffrey C (eds), Biology and
utilisation of the Cucurbitaceae, Comstock Publ.,
Cornell University Press, Ithaca, New York, pp. 29-37.
Fukuhara T, Akimoto J. 1999. Floral
morphology and vasculature of Schizopepon bryoniaefolius (Cucurbitaceae). – Acta
Phytotaxon. Geobot. 50: 59-73.
Fursa TB. 1972a. On the taxonomy of the genus
Citrullus Schrad. – Bot. Žurn. 57: 31-41.
Fursa TB. 1972b. On the evolution of the
genus Citrullus Schrad. – Bot. Žurn. 57: 1365-1372.
Ganal M, Hemleben V. 1986. Comparison of the
ribosomal RNA genes in four closely related Cucurbitaceae. – Plant Syst.
Evol. 154: 63-77.
Gao X-F, Chen S-K, Gu Z-J, Zhao J-Z. 1995. A
chromosomal study on the genus Gynostemma (Cucurbitaceae). – Acta Bot.
Yunnan. 17: 312-316. [In Chinese]
Garcia-Mas J, Monforte AJ, Arús P. 2004.
Phylogenetic relationships among Cucumis species based on the
ribosomal internal transcribed spacer sequence and microsatellite markers. –
Plant Syst. Evol. 248: 191-203.
Garg M. 1981. Pollen morphology and
systematic position of Coriaria. – Phytomorphology 30: 5-10.
Garnock-Jones PJ, Brockie RE, FitzJohn RG.
2007. Gynodioecy, sexual dimorphism and erratic fruiting in Corynocarpus
laevigatus (Corynocarpaceae). – Aust. J.
Bot. 55: 803-808.
Gauthier R. 1950. The nature of the inferior
ovary in the genus Begonia. – Contr. Inst. Bot. Univ. Montréal 66:
5-91.
Gauthier R. 1959. L’anatomie vasculaire et
l’interprétation de la fleur pistillée de l’Hillebrandia
sandwicensis Oliv. – Phytomorphology 9: 72-87.
Gauthier R, Arros J. 1963. L’anatomie de la
fleur staminée de l’Hillebrandia sandwicensis Oliver et la
vascularisation de l’étamine. – Phytomorphology 13: 115-127.
Gentry AH. 1950. Taxonomy and evolution of
Vaseyanthus. – Madroño 10: 142-155.
Gentry AH. 1973. Flora of Panama. Rafflesiaceae. –
Ann. Missouri Bot. Gard. 60: 17-21.
Gentry AH, Wettach RH. 1986.
Fevillea – a new oil seed from Amazonian Peru. – Econ. Bot. 40:
177-185.
Gerrath JM, Guthrie TB Zitnak TA, Posluszny
U. 2008. Development of the axillary bud complex in Echinocystis
lobata (Cucurbitaceae):
interpreting the cucurbitaceous tendril. – Amer. J. Bot. 95: 773-781.
Getahoun A. 1973. Developmental anatomy and
germination of seeds of anchoté, Coccinia abyssinica (W. & A.)
Cogn. (Cucurbitaceae). – Bot.
Not. 126: 437-449.
Ghebretsinae AG, Thulin M, Barber JC. 2007.
Relationships of cucumbers and melons unravelled: molecular phylogenetics of
Cucumis and related genera (Benincaseae, Cucurbitaceae). – Amer. J. Bot.
94: 1256-1266.
Ghosh E. 1932. On the microstructure of the
stem of Bengal Cucurbitaceae
with reference to its value in taxonomy. – J. Indian Bot. Soc. 11:
259-270.
Gilg E. 1925a. Datiscaceae. – In: Engler A, Gilg
E (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 21, W. Engelmann,
Leipzig, pp. 543-547.
Golding J, Wasshausen DC. 2002. Begoniaceae. 2nd ed. Part
I: Annotated species lists; Part II: Illustrated key, abridgement and
supplement. – Contr. U.S. Natl. Herb. 43: 1-289.
Good RDO. 1930. The geography of the genus
Coriaria. – New Phytol. 29: 170-198.
Gomes-Klein VL, Pirani JR. 2005. Four new
species of Cayaponia (Cucurbitaceae) from Brazil and
Bolivia. – Brittonia 57: 108-117.
Gomez LD. 1983. Rafflesiaceae. –
In: Burger W (ed), Flora Costaricensis, Fieldiana Bot. II, 13: 89-93.
González F, Pabón-Mora N. 2017. Floral
development and morphoanatomy in the holoparasitic Pilostyles
boyacensis (Apodanthaceae,
Cucurbitales) reveal chimeric
half-staminate and half-carpellate flowers. – Intern. J. Plant Sci. 178:
522-536.
Goodall-Copestake WP, Harris DJ,
Hollingsworth PM. 2009. The origin of a megadiverse genus: dating
Begonia (Begoniaceae)
using alternative datasets, calibrations and relaxed clock models. – Bot. J.
Linn. Soc. 159: 363-380.
Goodall-Copestake WP, Pérez-Espona S, Harris
DJ, Hollingsworth PM. 2010. The early evolution of the mega-diverse genus
Begonia (Begoniaceae)
inferred from organelle DNA phylogenies. – Biol. J. Linn. Soc. 101:
243-250.
Goulet I, Barabé D, Brouillet L. 1994.
Analyse structurale et architecture de l’inflorescence des Begoniaceae. – Can. J. Bot. 72:
897-914.
Gregor H-J. 1980. Seeds of the genus
Coriaria Linné (Coriariaceae) in the European
Neogene. – Tertiary Res. 3: 61-69.
Grisebach H, Grambow HJ. 1968. Biosynthesis
of flavonoids XV. Occurrence and biosynthesis of flavonoids in Datisca
cannabina. – Phytochemistry 7: 51-56.
Guédès M. 1971. Carpel peltation and
syncarpy in Coriaria ruscifolia L. – New Phytol. 70: 213-227.
Gulyaiev VA. 1963. Comparative embryology of
Cucurbitaceae and its
significance for the systematics of the family. – Bot. Žurn. 48: 80-85.
Guymer GP. 1984. Corynocarpaceae. – In: George
AS (ed), Flora of Australia 22, Australian Government Publ. Service, Canberra,
pp. 214-215.
Hagerup O. 1930. Vergleichende morphologische
und systematische Studien über die Ranken und andere vegetative Organe der
Cucurbitaceen und Passifloraceen. – Dansk Bot. Ark. 6(8): 1-103.
Hagman FA, De Wilde JJFE. 1983.
Re-establishment of Begonia cavallyensis A. Chev. and the altitudinal
vicariad Begonia fusicarpa Irmsch. (sect. Tetraphila). – Studies in
Begoniaceae 1: 1-19.
Hall BA. 1949. The floral anatomy of
Drosera and Begonia and its bearing on the theory of carpel
polymorphism. – Amer. J. Bot. 36: 416-421.
Hallé N. 1972. Les Begonia
filicifoliés et quatre espèces nouvelles du Gabon (Begoniaceae). – Adansonia, sér.
II, 12: 359-374.
Ham RWJM van der. 1999. Pollen morphology of
Bayabusua (Cucurbitaceae) and its allies. –
Sandakania 13: 17-22.
Ham RWJM van der, Heuven BJ van. 2003. A new
type of Old World Cucurbitaceae
pollen. – Grana 42: 88-90.
Ham RWJM van der, Pruesapan K. 2006. Pollen
morphology of Zehneria s.l. (Cucurbitaceae). – Grana 45:
241-248.
Ham RWJM van der, Mennes C, Heuven BJ van.
2010. Fevilleoideae pollen (Cucurbitaceae): a study in striate
ornamentation. – Grana 49: 157-169.
Harms H. 1935. Rafflesiaceae. –
In: Engler A (†), Harms H (eds), Die natürlichen Pflanzenfamilien, 2. Aufl.,
Bd. 16b, W. Engelmann, Leipzig, pp. 243-281.
Havey MJ. 1997. Predominant paternal
transmission of the cucumber mitochondrial genome. – J. Hered. 88:
232-235.
Havey MJ, McCreight JD, Rhodes B, Taurik G.
1998. Differential transmission of the Cucumis organellar genomes. –
Theor. Appl. Gen. 97: 122-128.
Hebeler F. 2000. Structural and
ecophysiological shoot features on the leafless cucurbit Acanthosicyos
horridus, a keystone endemic of the Namib Desert. – Diplomarbeit,
Institut für Allgemeine Botanik und Pflanzenphysiologie,
Justus-Liebig-Universität, Giessen, Germany.
Heiser CB, Schilling EE. 1988. Phylogeny and
distribution of Luffa (Cucurbitaceae). – Biotropica 20:
185-191.
Heiser CB, Schilling EE. 1990. The genus
Luffa: a problem in phytogeography. – In: Bates DM, Robinson RW,
Jeffrey C (eds), Biology and utilization of the Cucurbitaceae, Comstock Publ.
Assoc., Cornell University Press, Ithaca, New York, pp. 120-133.
Heiser CB, Schilling EE, Dutt B. 1988. The
American species of Luffa (Cucurbitaceae). – Syst. Bot. 13:
138-145.
Helm MA, Hemleben V. 1997. Characterization
of a new prominent satellite DNA of Cucumis metuliferus and
differential distribution of satellite DNA in cultivated and wild species of
Cucumis and in related genera of Cucurbitaceae. – Euphytica 94:
219-226.
Hemleben V, Leweke B, Roth A, Stadler I.
1982. Organization of highly repetitive satellite DNA of two Cucurbitaceae species (Cucumis
melo and Cucumis sativus). – Nucleic Acids Res. 10: 631-644.
Hemsley WB. 1903. On the genus
Corynocarpus Forst., with descriptions of two new species. – Ann.
Bot. 17: 743-760.
Hildebrand FHG. 1859. Anatomische
Untersuchungen über die Stämme der Begoniaceae. – August Hirschwald,
Berlin.
Hill AW. 1916. Studies in seed germination.
The genus Marah (Megarrhiza), Cucurbitaceae. – Ann. Bot. 30:
215-222.
Himmelbaur W. 1909. Eine
blütenmorphologische und embryologische Studie über Datisca
cannabina L. – Sitzungsber. Kais. Akad. Wiss., Math.-Nat. Cl. II, Abt.
1, 118: 91-113.
Holroyd R. 1924. Morphology and physiology of
the axis in Cucurbitaceae. –
Bot. Gaz. 78: 1-44.
Holstein N. 2015. Monograph of
Coccinia (Cucurbitaceae). – PhytoKeys 54:
1-166.
Holstein N, Renner SS. 2010.
Coccinia (Cucurbitaceae) gains two new
species from East Africa, three new synonyms, and one new combination. – Kew
Bull. 65: 435-441.
Hoover WS. 1986. Stomata and stomatal
clusters in Begonia: ecological response in two Mexican species. –
Biotropica 18: 16-21.
Hoover WS, Karegeannes C, Wiriadinata H,
Hunter JM. 2004. Notes on the geography of South-East Asian Begonia
and species diversity in montane forests. – Telopea 10: 749-764.
Hopkins CY. 1990. Fatty acid of Cucurbitaceae seed oils in
relation to taxonomy. – In: Bates DM, Robinson WR, Jeffrey C (eds), Biology
and utilization of the Cucurbitaceae, Comstock Publ.,
Cornell University Press, Ithaca, New York, pp. 38-50.
Huang L, Yang B, Yue C. 1997. Pollen
morphology of Trichosanthes and its taxonomic significance. – Acta
Phytotaxon. Sin. 35: 125-135.
Huang S, Zhang Z, Gu X et al. (biology
analysis group). 2009. The genome of the cucumber, Cucumis sativus L.
– Nature Genetics 41: 1275-1281.
Hufford GN. 1938. Development and structure
of the watermelon seedling. – Bot. Gaz. 100: 100-122.
Hughes M, Hollingsworth PM. 2008. Population
genetic divergence corresponds with species-level biodiversity patterns in the
large genus Begonia. – Mol. Ecol. 17: 2643-2651.
Ilyas MHM. 1992. Studies on the extrafloral
nectaries in some members of Cucurbitaceae. – Acta Bot.
Indica 20: 116-119.
Imaichi R, Okamoto K. 1992. Comparative
androecium morphogenesis of Sicyos angulatus and Sechium
edule (Cucurbitaceae). –
Bot. Mag. (Tokyo) 105: 539-548.
Inamdar JA, Gangadhara M. 1976. Structure,
ontogeny, and taxonomic significance of stomata in some Cucurbitaceae. – Feddes Repert.
87: 293-310.
Ingle HD. 1956. A note on the wood anatomy of
the genus Corynocarpus. – Trop. Woods 105: 8-12.
Irmscher E. 1914. Die Verteilung der
Geschlechter in den Inflorescenzen der Begoniaceen unter Berücksichtigung der
morphologischen Verhältnisse. – Bot. Jahrb. Syst. Suppl. 50: 556-577.
Irmscher E. 1925. Begoniaceae. – In: Engler A, Gilg
E (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 21, W. Engelmann,
Leipzig, pp. 548-588.
Irmscher E. 1961. Monographische Revision der
Begoniaceen Afrikas I. Sekt. Augustia und Rostrobegonia sowie
einige neue Sippen aus anderen Sektionen. – Bot. Jahrb. Syst. 81: 106-188.
Isaev MI. 2000. Bryonia isoprenes II.
Cucurbitacin L and bryoamaride from Bryonia melanocarpa. – Chem.
Nat. Compounds 36: 292-294.
Jacques EL, Mamede MCH. 2004. Novelties in
Begonia (Begoniaceae)
from the coastal forests of Brazil. – Brittonia 56: 75-81.
Jeffrey C. 1962a. Notes on Cucurbitaceae, including a
proposed new classification of the family. – Kew Bull. 15: 337-371.
Jeffrey C. 1962b. The application of the
generic names Anguria and Elaterium (Cucurbitaceae). – Kew Bull. 16:
197-198.
Jeffrey C. 1962c. Notes on some species of
Fevillea L., Siolmatra Bail., and Pseudosicydium
Harms (Cucurbitaceae) in the
Amazon Basin. – Kew Bull. 16: 199-202.
Jeffrey C. 1964a. A note on pollen morphology
in Cucurbitaceae. – Kew Bull.
17: 473-476.
Jeffrey C. 1964b. Key to the Cucurbitaceae of West Tropical
Africa, with a guide to localities of rare and little-known species. – J.
West Afr. Sci. Assoc. 9: 79-97.
Jeffrey C. 1966. On the classification of the
Cucurbitaceae. – Kew Bull.
20: 417-426.
Jeffrey C. 1967. Cucurbitaceae. – In:
Milne-Redhead E, Polhill RM (eds), Flora of tropical East Africa, Crown Agents
for Oversea Governments, London, pp. 1-156.
Jeffrey C. 1969a. A review of the genus
Bryonia L. (Cucurbitaceae). – Kew Bull. 23:
441-461.
Jeffrey C. 1969b. The genus Mukia in
Asia, Malesia and Australia. – Hooker’s Icones Plant. 5th Ser.
7(3), Tab. 3661-3664: 1-12.
Jeffrey C. 1971. Further notes on Cucurbitaceae II. The tribe
Cucurbiteae. – Kew Bull. 25: 191-236.
Jeffrey C. 1978a. Further notes on Cucurbitaceae IV. Some New World
taxa. – Kew Bull. 33: 347-380.
Jeffrey C. 1978b. 86. Cucurbitaceae. – In: Launert E
(ed), Flora Zambesiaca 4, Flora Zambesiaca Managing Committee, London, pp.
414-499.
Jeffrey C. 1980. A review of the Cucurbitaceae. – Bot. J. Linn.
Soc. 81: 233-247.
Jeffrey C. 1982. Further notes on Cucurbitaceae VI. Cucurbitaceae of the Indian
Subcontinent: Corrigenda and Addenda. – Kew Bull. 36: 737-740.
Jeffrey C. 1985. Further notes on Cucurbitaceae VII. Preliminary to
the Flora of Ethiopia. – Kew Bull. 40: 209-211.
Jeffrey C. 1990a. Systematics of the Cucurbitaceae: an overview. –
In: Bates DM, Robinson RW, Jeffrey C (eds), Biology and utilization of the Cucurbitaceae, Cornell University
Press, Ithaca, New York, Appendix, pp. 3-9.
Jeffrey C. 1990b. Appendix: An outline
classification of the Cucurbitaceae. – In: Bates DM,
Robinson RW, Jeffrey C (eds), Biology and utilization of the Cucurbitaceae, Cornell University
Press, Ithaca, New York, Appendix, pp. 449-463.
Jeffrey C. 1992a. Notes on Cucurbitaceae including a proposed
new classification of the family. – Kew. Bull. 30: 475-493.
Jeffrey C. 1992b. The genus
Apodanthera (Cucurbitaceae) in Bahia State
(Brazil). – Kew Bull. 47: 517-528.
Jeffrey C. 2005. A new system of Cucurbitaceae. – Bot. Žurn. 90:
332-335.
Jeffrey C, De Wilde WJJO. 2006. A review of
the subtribe Thladianthinae (Cucurbitaceae). – Bot. Žurn.
91: 766-776.
Jin XB, Wang FH. 1994. Style and ovary
anatomy of Chinese Begonia and its taxonomic and evolutionary
implications. – Cathaya 6: 125-144.
Jobst J, King K, Hemleben V. 1998. Molecular
evolution of the internal transcribed spacers (ITS1 and ITS2) and phylogenetic
relationships among species of the family Cucurbitaceae. – Mol. Phylogen.
Evol. 9: 204-219.
Jones CS. 1992. Comparative ontogeny of a
wild cucurbit and its derived cultivar. – Evolution 46: 1827-1847.
Jones CS. 1993. Heterochrony and
heteroblastic leaf development in two subspecies of Cucurbita
argyrosperma (Cucurbitaceae). – Amer. J. Bot.
80: 787-795.
Juncosa AM, Tomlinson PB. 1988a. A historical
and taxonomic synopsis of Rhizophoraceae and
Anisophylleaceae. – Ann.
Missouri Bot. Gard. 75: 1278-1295.
Juncosa AM, Tomlinson PB. 1988b. Systematic
comparison and some biological characteristics of Rhizophoraceae and
Anisophylleaceae. – Ann.
Missouri Bot. Gard. 75: 1296-1318.
Kartusch B, Kartusch R. 2008. Stem anatomy of
Acanthosicyos horridus (Cucurbitaceae). – South Afr. J.
Bot. 74: 647-650.
Kater MM, Franken J, Carney KJ, Colombo L,
Angenent GC. 2001. Sex determination in the monoecious species cucumber is
confined to specific floral whorls. – Plant Cell 13: 481-493.
Kearns DM. 1992a. A revision of
Sechiopsis (Cucurbitaceae). – Syst. Bot. 17:
395-408.
Kearns DM. 1992b. A revision of
Polyclathra (Cucurbitaceae). – In:
Biosystematics of Mexican Cucurbitaceae, Ph.D. diss.,
University of Texas, Austin, Texas.
Kearns DM. 1994a. The genus
Ibervillea (Cucurbitaceae): an enumeration of
the species and two new combinations. – Madroño 41: 13-22.
Kearns DM. 1994b. A revision of
Tumamoca (Cucurbitaceae). – Madroño 1:
23-29.
Keating RC, Randrianasolo V. 1988. The
contribution of leaf architecture and wood anatomy to classification of the Rhizophoraceae and
Anisophylleaceae. – Ann.
Missouri Bot. Gard. 75: 1343-1368.
Keraudren M. 1968. Recherches sur les
cucurbitacées de Madagascar. – Mém. Mus. Natl. Hist. Nat., sér. B, Bot.
16: 122-330.
Keraudren- Aymonin M. 1971. La survivance des
Ampelosicyos (Cucurbitacées) à Madagascar. – Bull. Soc. Bot.
France 118: 281-286.
Khunwasi C. 1998. Palynology of the Cucurbitaceae. – Ph.D. diss.,
Leopold-Franzens-Universität, Innsbruck, Austria.
Kiew R. 2005. Begonias of Peninsular
Malaysia. – Natural History Publ., Kota Kinabalu, Sabah, Malaysia, and
Singapore Botanic Gardens, Singapore.
Kiew R. 2007. Notes on Vietnamese
Begonia (Begoniaceae),
including three new species. – Adansonia, sér. III, 29: 229-238.
King K, Torres RA, Zentgraf U, Hemleben V.
1993. Molecular evolution of the intergenic spacer in the nuclear ribosomal RNA
genes of Cucurbitaceae. – J.
Mol. Evol. 36: 144-152.
King K, Jobst J, Hemleben V. 1995.
Differential homogenization and amplification of two satellite DNAs in the
genus Cucurbita (Cucurbitaceae). – J. Mol. Evol.
41: 996-1005.
Kirkbride JH. 1993. Biosystematic monograph
of the genus Cucumis (Cucurbitaceae). – Parkway Publ.,
Boone, North Carolina.
Kirkwood JC. 1905. The comparative morphology
of the Cucurbitaceae. – Bull.
New York Bot. Gard. 3: 313-402.
Klazenga N, Wilde JJFE de, Quené RJ. 1994.
Begonia sect. Mezierea (Gaud.) Warb., a taxonomic revision.
– Bull. Jard. Bot. Natl. Belg. 3: 263-312.
Kocyan A, Zhang LB, Schaefer H, Renner SS.
2007. A multi-locus chloroplast phylogeny for the Cucurbitaceae and its implications
for character evolution and classification. – Mol. Phylogen. Evol. 44:
553-577.
Kolbe P-K, John J. 1979. Serologische
Untersuchungen zur Systematik der Violales. – Bot. Jahrb. Syst. 101: 3-15.
Kollmann LJC. 2003. Begonia ruschii
L. Kollmann (Begoniaceae), uma
nova espécie da Floresta Atlântica do Espírito Santo, Brasil. – Bol. Mus.
Biol. Prof. Mello-Leitão 15: 29-33.
Kollmann LJC. 2006. Begonia
novalombardiensis L. Kollmann (Begoniaceae), une nouvelle espèce
de la forêt atlantique de l’Etat de l’Espirito Santo, Brésil. –
Candollea 61: 89-92.
Kollmann LJC. 2007. Begonia callosa
L. Kollmann (Begoniaceae), a new
species of Espírito Santo, Brazil. – Candollea 62: 141-144.
Kollmann LJC. 2008. Duas novas espécies de
Begonia (Begoniaceae),
do Espírito Santo, Brasil. Rodriguesia 59: 259-363.
Kollmann LJC. 2009. Begonia
bullatifolia L. Kollmann and Begonia leopoldinensis L. Kollmann
(Begoniaceae), two new species
from the Atlantic forest in the State of Espírito Santo, Brazil. – Candollea
64: 117-122.
Konopa J, Matuszkiewicz A, Hrabowska M,
Onoszka K. 1974. Cucurbitacines, cytotoxic and antitumor substances from
Bryonia alba L. II: biological studies. – Arzneimettelforschung 24:
1741-1743.
Krause J. 1942. Corynocarpaceae. – In: Engler
A (†), Harms H, Mattfeld J (eds), Die natürlichen Pflanzenfamilien, 2.
Aufl., Bd. 20b, W. Engelmann, Leipzig, pp. 22-35.
Krauze-Baranowska M, Cisowski W. 1995.
Flavone C-glycosides from Bryonia alba and B. dioica. –
Phytochemistry 39: 727-729.
Kubitzki K. 2011a. Introduction to Cucurbitales. – In: Kubitzki K
(ed), Families and genera of vascular plants X. Flowering plants. Eudicots. Sapindales, Cucurbitales, Myrtaceae. Springer, Heidelberg,
Dordrecht, London, New York, pp. 4-6.
Kubitzki K. 2011b. Coriariaceae. – In: Kubitzki K
(ed), Families and genera of vascular plants X. Flowering plants. Eudicots. Sapindales, Cucurbitales, Myrtaceae. Springer, Heidelberg,
Dordrecht, London, New York, pp. 105-108.
Kubitzki K. 2011c. Corynocarpaceae. – In:
Kubitzki K (ed), Families and genera of vascular plants X. Flowering plants.
Eudicots. Sapindales, Cucurbitales, Myrtaceae. Springer, Heidelberg,
Dordrecht, London, New York, pp. 109-111.
Kuijt J, Bray D, Olson AR. 1985. Anatomy and
ultrastructure of the endophytic system of Pilostyles thurberi (Rafflesiaceae). –
Can. J. Bot. 63: 1231-1240.
Kumazawa M. 1964. Morphological
interpretation of axillary organs in the Cucurbitaceae. – Phytomorphology
14: 287-298.
Kummerow J. 1962. Pilostyles
berterii Guill., eine wenig bekannte Rafflesiaceae in
Mittelchile. – Zeitschr. Bot. 50: 321-337.
Kupchan SM, Sigel CW, Guttman LJ, Restivo RJ,
Bryan RF. 1972. Datiscoside, a novel antileukemic cucurbitacin glycoside from
Datisca glomerata. – J. Amer. Chem. Soc. 94: 1353-1354.
Kupchan SM, Tsou G, Sigel CW. 1973.
Datiscacin, a novel cytotoxic cucurbitacin 20-acetate from Datisca
glomerata. – J. Org. Chem. 38: 1420-1421.
Kupicha FK. 1978. 87. Begoniaceae. – In: Launert E (ed),
Flora Zambesiaca 4, Flora Zambesiaca Managing Committee, London, pp.
499-506.
Lakhanpal RN, Verma JK. 1965 [1966]. Fossil
wood of Tetrameles from the Deccan Intertrappean beds of Mohgaonkalan,
Madhya Pradesh. – Palaeobotanist 14: 209-213.
Lange A de, Bouman F. 1992. Seed
micromorphology of the genus Begonia in Africa: taxonomic and
ecological implications. – In: Wilde JJFE de (ed), Studies in Begoniaceae III, Wageningen Agric.
Univ. Pap. 91-4: 1-82.
Lange A de, Bouman F. 1999. Seed
micromorphology of neotropical begonias. – Smithsonian Contr. Bot. 90:
1-49.
Lassnig P. 1997. Verzweigungsmuster und
Rankenbau der Cucurbitaceae.
– Trop. Subtrop. Pflanzenwelt 98: 1-146.
Lee YS. 1974. A study of stem anatomy in
Begonia L. – Phytologia 27: 464-489.
Legro RAH, Doorenbos J. 1969, 1971, 1973.
Chromosome numbers in Begonia 1, 2 and 3. – Netherl. J. Agric. Sci.
17: 189-202, 19: 176-183, 21: 167-170.
Leins P, Bonnery-Brachtendorf R. 1977.
Entwicklungsgeschichtliche Untersuchungen an Blüten von Datisca
cannabina (Datiscaceae). –
Beitr. Biol. Pflanzen 53: 143-155.
Leins P, Galle P. 1971.
Entwiklungsgeschichtliche Untersuchungen an Cucurbitaceen-Blüten. – Österr.
Bot. Zeitschr. 119: 531-548.
Li H-Z, Ma H, Zhou Z-K, Guan K-Y. 2008. A new
species of Begonia (Begoniaceae) from Guangxi, China.
– Bot. J. Linn. Soc. 157: 83-90.
Li J-Q, Wu Z-Y, Lu A-M. 1993. Cytological
observation on the plants of Thladianthinae (Cucurbitaceae). – Acta Bot.
Yunnan. 15: 101-104.
Lindner K, Hu Y, Pandey AK, Schaefer H. 2017.
The Namib-Thar disjunction in Dactyliandra (Cucurbitaceae) is the result of a
recent introduction to India. – Syst. Bot. 42: 63-72.
Lira R. 1994. Especie nueva de
Microsechium (Cucurbitaceae, tribu Sicyeae,
subtribu Sicyinae) del estado de Oaxaca, México. – An. Inst. Biol. Univ.
Nac. Autón. México, Bot. 65: 73-81.
Lira R. 1995. Estudios taxonómicos en el
género Sechium P. Br. (Cucurbitaceae). – Ph.D. diss.,
Universidad Nacional Autónoma, México.
Lira R, Caballero J. 1997. A contribution to
the generic delimitation of Sechium (Cucurbitaceae, Sicyinae). –
Taxon 46: 269-282.
Lira R, Chiang F. 1992. Two new combinations
in Sechium (Cucurbitaceae) from Central
America and a new species from Oaxaca, Mexico. – Novon 2: 227-231.
Lira R, Alvarado JL, Castrejón J. 1994. Nota
sobre el polen de Sechium chinantlense Lira & Chiang y
Parasicyos dieterleae Lira & Torres (Cucurbitaceae). – Bol. Soc. Bot.
México 54: 275-280.
Lira R, Villasenor JL, Davila PD. 1997. A
cladistic analysis of the subtribe Sicyinae (Cucurbitaceae). – Syst. Bot. 22:
415-425.
Lira Saade R. 1991. Observaciones en el
género Sicana (Cucurbitaceae). – Brenesia 35:
19-59.
Lira Saade R. 2004a. El género
Sicydium (Cucurbitaceae, Zanonioideae,
Sicydiinae) en México. – Acta Bot. Mex. 68: 39-64.
Lira Saade R. 2004b. Etnoflora Yucatanense
22. Cucurbitaceae de la
Peninsula de Yucatán. Taxonomía, florística y etnobotánica. – Universidad
Autónoma de Yucatán/CONACyT, Mérida, Yucatán, México.
Lira Saade R, Alvarado JL, Ayala-Nieto ML.
1998. Pollen morphology in Sicydium (Cucurbitaceae, Zanonioideae). –
Grana 37: 215-221.
Liston A. Rieseberg LH, Elias TS. 1989.
Morphological stasis and molecular divergence in the intercontinental disjunct
genus Datisca (Datiscaceae). – Aliso 12:
525-542.
Liston A, Rieseberg LH, Elias TS. 1990.
Functional androdioecy in the flowering plant Datisca glomerata. –
Nature 343: 641-642.
Liston A, Rieseberg LH, Hanson MA. 1992.
Geographic partitioning of chloroplast DNA variation in the genus
Datisca (Datiscaceae).
– Plant Syst. Evol. 181: 121-132.
Lorence DH. 1987. The Hawaiian begonia. –
Bull. Pac. Trop. Bot. Gard. 17: 130-133.
Lu A-M. 1985. Studies on the genus
Schizopepon Max. (Cucurbitaceae). – Acta
Phytotaxon. Sin. 23: 106-120. [In Chinese]
MacCaughey V. 1918. An endemic begonia of
Hawaii. – Bot. Gaz. 66: 272-275.
McKay JW. 1931. Chromosome studies in the Cucurbitaceae. – Univ. Calif.
Publ. Bot. 16: 339-350.
McLellan T. 1990. Development of differences
in leaf shape in Begonia dregei (Begoniaceae). – Amer. J. Bot. 77:
323-337.
McLellan T. 1993. The roles of heterochrony
and heteroblasty in the diversification of leaf shape in Begonia
dregei (Begoniaceae). –
Amer. J. Bot. 80: 796-804.
McLellan T. 2000. Geographic variation and
plasticity of leaf shape and size in Begonia dregei and B.
homonyma (Begoniaceae). –
Bot. J. Linn. Soc. 132: 79-95.
Marticorena C. 1963. Material para una
monografía de la morfología del pollen de Cucurbitaceae. – Grana Palynol.
4: 78-91.
Martínez Crovetto R. 1952. El género
Pteropepon (Cucurbitaceae) en la República
Argentina. – Bol. Soc. Argent. Bot. 4: 177-182.
Martínez Crovetto R. 1956. Especies nuevas o
críticas del género Apodanthera (Cucurbitaceae) II. – Bol. Soc.
Argent. Bot. 6: 94-97.
Matienko BT. 1957. On the
anatomo-morphological nature of the flowers and fruits of Cucurbitaceae. – Trudy Bot.
Inst. Akad. Nauk SSSR, ser. 7, 4: 288-322.
Matolweni LO, Balkwill K, McLellan T. 2000.
Genetic diversity and gene flow in the morphologically variable, rare endemics
Begonia dregei and Begonia homonyma (Begoniaceae). – Amer. J. Bot. 87:
431-439.
Matthews ML, Endress PK. 2004. Comparative
floral structure and systematics in Cucurbitales (Corynocarpaceae, Coriariaceae, Tetramelaceae, Datiscaceae, Begoniaceae, Cucurbitaceae, Anisophylleaceae). – Bot. J.
Linn. Soc. 145: 129-185.
Matthews ML, Endress PK, Schönenberger J,
Friis EM. 2001. A comparison of floral structures of Anisophylleaceae and Cunoniaceae and the
problem of their systematic position. – Ann. Bot. 88: 439-455.
Mauritzon J. 1936. Zur Embryologie einiger
Parietales-Familien. – Svensk Bot. Tidskr. 30: 79-113.
Meeuse ADJ. 1965. The Cucurbitaceae of southern Africa.
– Bothalia 8: 59-82.
Meijer W. 1993. Rafflesiaceae. –
In: Kubitzki K, Rohwer JG, Bittrich V (eds), The families and genera of
vascular plants II. Flowering plants. Dicotyledons. Magnoliid, hamamelid and
caryophyllid families, Springer, Berlin, Heidelberg, New York, pp. 557-563.
Mercado P, Lira Saade R. 1994. Contribucion
al conocimiento de los numeros chromosomicos de los generos Sechium P.
Br. y Sicana Naudin (Cucurbitaceae). – Acta Bot. Mex.
27: 7-13.
Merrick LC. 1990. Systematics and evolution
of a domesticated squash, Cucurbita argyrosperma, and its wild and
weedy relatives. – In: Bates DA, Robinson RW, Jeffrey C (eds), Biology and
utilization of the Cucurbitaceae, Cornell University
Press, Ithaca, New York, pp. 77-95.
Merrick LC, Bates DM. 1989. Classification
and nomenclatural of Cucurbita argyrosperma Huber. – Baileya 23:
94-102.
Merxmüller H, Leins P. 1971. Zur
Entwicklungsgeschichte männlicher Begonienblüten. – Flora 160: 333-339.
Mirza MS, Hahn D, Dobritsa SV, Akkermans ADL.
1994. Phylogenetic studies on uncultered Frankia populations in
nodules of Datisca cannabina. – Can. J. Bot. 40: 313-318.
Molloy BPJ. 1990. The origin, relationships,
and use of karaka or kopi (Corynocarpus laevigatus). – In: Harris W,
Kapoor P (eds), Nga mahi maori o te wao nui a tane: contributions to an
international workshop on ethnobotany, te rehua marae, Botany Division,
Department of Scientific and Industrial Research, Christchurch, New Zealand,
pp. 48-53.
Monro AK, Stafford PJ. 1998. A synopsis of
the genus Echinopepon (Cucurbitaceae: Sicyoeae),
including three new taxa. – Ann. Missouri Bot. Gard. 85: 257-272.
Moonlight PW, Ardi WH, Padilla LA, Chung K-F,
Fuller D, Girmansyah D, Hollands R, Jara-Muñoz A, Kiew R, Leong W-C, Liu Y,
Mahardika A, Mareasinghe LDK, O’Connor M, Peng C-I, Pérez ÁJ, Phutthai T,
Pullan M, Rajbhandary S, Reynel C, Rubite RR, Sang J, Scherberich D, Shui Y-M,
Tebbitt MC, Thomas DC, Wilson HP, Zaini NH, Hughes M. 2018. Dividing and
conquering the fastest-growing genus: towards a natural sectional
classification of the mega-diverse genus Begonia (Begoniaceae). – Taxon 67:
267-323.
Müller EGO, Pax F. 1894. Cucurbitaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien IV(5), W. Engelmann, Leipzig,
pp. 1-39, 392-394.
Nakata M, Guan K, Li J, Lu Y, Li H. 2007.
Cytotaxonomy of Begonia rubropunctata and B. purpureofolia
(Begoniaceae). – Bot. J. Linn.
Soc. 155: 513-517.
Narayana LL, Narayana PS, Vatsavaya SR. 1986.
A contribution to the numerical chemotaxonomy of Corynocarpaceae. – J. Econ.
Taxon. Bot. 8: 249-254.
Narayana LL, Satyanarayana P, Raju VS,
Prakasa Rao PS. 1986. The floral anatomy of Corynocarpus laevigatus
(Corynocarpaceae). –
Phytomorphology 36: 325-329.
Nee M, Schaefer H, Renner SS. 2009. The
relationship between Anisosperma and Fevillea (Cucurbitaceae), and a new species
of Fevillea from Bolivia. – Syst. Bot. 34: 704-708.
Neubauer HF. 1967. Bemerkungen über den Bau
der Begoniaceen. – Ber. Deutsch. Bot. Ges. 80: 80-97.
Newcomb W, Pankhurst CE. 1982. Fine structure
of actinorhizal nodules of Coriaria arborea (Coriariaceae). – New Zealand J.
Bot. 20: 93-103.
Newstrom LE. 1986. Studies in the origin and
evolution of chayote, Sechium edule (Jacq.) Sw. (Cucurbitaceae). – Ph.D. diss.,
University of California, Berkeley, California.
Newstrom LE. 1990. Origin and evolution of
chayote, Sechium edule. – In: Bates DM, Robinson RW, Jeffrey C
(eds), Biology and utilization of the Cucurbitaceae, Ithaca, New York,
pp. 141-149.
Nickrent DL, Blarer A, Qiu YL, Vidal-Russell
R, Anderson FE. 2004. Phylogenetic inference in Rafflesiales: the influence of
rate heterogeneity and horizontal gene transfer. – BMC Evol. Biol. 4: 40.
Norton JD, Granberry DM. 1980.
Characteristics of progeny from an interspecific cross of Cucumis melo
with Cucumis metuliferus. – J. Amer. Soc. Hort. Sci. 105:
174-180.
Nowicke JW, Skvarla JJ. 1983. Pollen
morphology and the relationships of the Corynocarpaceae. – Taxon 32:
176-183.
Oginuma K, Nakata M, Suzuki M, Tobe H. 1991.
Karyomorphology of Coriaria (Coriariaceae): taxonomic
implications. – Bot. Mag. (Tokyo) 104: 297-308.
Okoli BE. 1984. Wild and cultivated cucurbits
in Nigeria. – Econ. Bot. 38: 350-357.
Okoli BE. 1987. Morphological and cytological
studies in Telfairia Hooker (Cucurbitaceae). – Feddes Repert.
98: 505-508.
Okoli BE, McEuen AR. 1986. Calcium-containing
crystals in Telfairia Hooker (Cucurbitaceae). – New Phytol.
102: 199-207.
Okoli BE, Onofeghara FA. 1984. Distribution
and morphology of extrafloral nectaries in some Cucurbitaceae. – Bot. J. Linn.
Soc. 89: 153-164.
Oliver FW. 1866. On Hillebrandia, a
new genus of Begoniaceae. –
Trans. Linn. Soc. London 25: 361-363.
Olson ME. 2003. Stem and leaf anatomy of the
arborescent Cucurbitaceae
Dendrosicyos socotrana with comments on the evolution of pachycauls
from lianas. – Plant Syst. Evol. 239: 199-214.
Oobayashi K, Yoshikawa K, Arihara S. 1992.
Structural revision of bryonoside and structure elucidation of minor saponins
from Bryonia dioica. – Phytochemistry 31: 943-946.
Page JS, Jeffrey C. 1975. A palyno-taxonomic
study of African Peponium (Cucurbitaceae). – Kew Bull. 30:
495-502.
Panda S, Wilde JJFE de. 1995. Diversity and
taxonomic value of stigmatic surfaces in Begoniaceae: SEM analysis. – Acta
Bot. Neerl. 44: 139-150.
Pant DD, Banerji R. 1965. Ontogeny of stomata
and hairs in some cucurbits and allied plants. – J. Indian Bot. Soc. 44:
193-197.
Patel RN. 1975. Wood anatomy of the
dicotyledons indigenous to New Zealand: 8. Corynocarpus. – New
Zealand J. Bot. 13: 19-29.
Patil VS, Marcati CR, Rajput KS. 2011.
Development of intra- and interxylary secondary phloem in Coccinia
indica (Cucurbitaceae).
– IAWA J. 32: 475-491.
Pax F, Engler A. 1897. Cucurbitaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien, Nachträge zu IV(5), W.
Engelmann, Leipzig, pp. 317-318.
Perl-Treves R. 1999. Male to female
conversion along the cucumber shoot: approaches to study sex genes and floral
development in Cucumis sativus. – In: Ainsworth CC (ed), Sex
determination in plants, BIOS Scientific Publ., Oxford, pp. 189-216.
Perl-Treves R, Zamir D, Navat N, Galun E.
1985. Phylogeny of Cucumis based on isoenzyme variability and its
comparison with plastome phylogeny. – Theor. Appl. Gen. 71: 430-436.
Philipson WR. 1987. Corynocarpus J.
R. and G. Forst. – an isolated genus. – Bot. J. Linn. Soc. 95: 9-18.
Pigott E. 1927. Observations on
Corynocarpus laevigata Forst., the karaka. – Trans. New Zealand
Inst. 58: 57-71.
Pires JM, Rodrigues WA. 1971. Notas sôbre os
gêneros Polygonanthus e Anisophyllea. – Acta Amazonica 1:
7-15.
Plana V. 2002. Systematics and biogeography
of the Afro-malagasy fleshy-fruited Begonia. – Ph.D. diss.,
University of Glasgow, United Kingdom.
Plana V. 2003. Phylogenetic relationships of
the Afro-Malagasy members of the large genus Begonia inferred from
trnL intron sequences. – Syst. Bot. 28: 693-704.
Plana V, Gascoigne A, Forrest LL, Harris D,
Pennington RT. 2003 [2004]. Pleistocene and pre-Pleistocene Begonia
speciation in Africa. – Mol. Phylogen. Evol. 31: 449-461.
Pozner R. 1993. Androsporangio,
androsporogénesis y androgametogénesis en Cayaponia citrullifolia,
Cayaponia bonariensis y Cucurbitella duriaei (Cucurbitaceae). – Darwiniana 32:
109-123.
Pozner R. 1994. Rudimento seminal y
ginosporogénesis en Cucurbitella duriaei y Cayaponia
bonariensis (Cucurbitaceae). – Kurtziana 23:
55-72.
Pozner R. 1998. Revisión del género
Cucurbitella (Cucurbitaceae). – Ann. Missouri
Bot. Gard. 85: 425-439.
Pozner R. 2004. A new species of
Echinopepon from Argentina and taxonomic notes on the subtribe
Cyclantherinae (Cucurbitaceae).
– Syst Bot. 29: 29: 599-608.
Praglowski J. 1970. Coriariaceae. – In: Erdtman G
(ed), World Pollen Flora, Scandinavian University Books, København, pp.
15-22.
Prance GT, da Silva MF, Albuquerque BW,
Araújo de J da SI, Carreira LMM, Braga MMN, Macedo M, da Conceição PN,
Lisbõa PLB, Braga PI, Lisbõa RCL, Vilhena RCQ. 1975. Revisão taxonômica das
espécies amazônicas de Rhizophoraceae. –
Acta Amazonica 5: 5-22.
Pruesapan K, Ham R van der. 2005. Pollen
morphology of Trichosanthes (Cucurbitaceae). – Grana 44:
75-90.
Puchalski JT, Robinson RW. 1990.
Electrophoretic analysis of isoenzymes in Cucurbita and
Cucumis and its application for phylogenetic studies. – In: Bates
DM, Robinson RW, Jeffrey C (eds), Biology and utilization of the Cucurbitaceae, Cornell University
Press, Ithaca, New York, pp. 60-76.
Puri V. 1954. Studies in floral anatomy VII.
On placentation in the Cucurbitaceae. – Phytomorphology
4: 278-299.
Raamsdonk LWD van, Visser DL. 1992.
Autotetraploidy in Cucumis zeyheri and a derived new species C.
diniae (Cucurbitaceae).
– Nord. J. Bot. 12: 327-334.
Raamsdonk LWD van, Nijs APM den, Jongerius
MC. 1989. Meiotic analyses of Cucumis hybrids and an evolutionary
evaluation of the genus Cucumis (Cucurbitaceae). – Plant Syst.
Evol. 163: 133-146.
Ramachadran C, Narayan RKJ. 1985. Chromosomal
DNA variation in Cucumis. – Theor. Appl. Gen. 69: 497-502.
Rauh W. 1996. Observations complementaires
sur Xerosicyos pubescens (Cucurbitaceae) de Madagascar. –
Bull. Mus. Natl. Hist. Nat., sér. B, Adansonia 18: 161-166.
Rehm S, Enslin PR, Meeuse ADJ, Wessels JH.
1957. Bitter principles of the Cucurbitaceae VII. The
distribution of bitter principles in this plant family. – J. Sci. Food Agric.
12: 679-680.
Reiche VK. 1921. Zur Kenntnis von Sechium
edule. – Flora 14: 232-248.
Reitsma T. 1983. Placentation in Begonias
from the African continent. – In: De Wilde JJFE (ed), Studies in Begoniaceae I, Meded. Landbouwh.
Wageningen 83: 21-53.
Renner SS, Schaefer H, Kocyan A. 2007.
Phylogenetics of Cucumis (Cucurbitaceae): C.
sativus (cucumber) belongs in an Australian/Asian clade far from African
C. melo (melon). – BMC Evol. Biol. 7: 58.
Rieseberg LH, Hanson MA, Philbrick CT. 1992.
Androdioecy is derived from dioecy in Datiscaceae: evidence from
restriction site mapping of PCR-amplified chloroplast DNA fragments. – Syst.
Bot. 17: 324-336.
Robinson BL. 1891. Two undescribed species of
Apodanthes. – Bot. Gaz. (Crawfordsville) 16: 82-84.
Robinson GL, Wunderlin RP. 2005a. Revision of
Siolmatra (Cucurbitaceae: Zanonieae). –
Sida 21: 1961-1969.
Robinson GL, Wunderlin RP. 2005b. Revision of
Fevillea (Cucurbitaceae: Zanonieae). –
Sida 21: 1971- 1996.
Rodriguez-Arévalo I. 2003. A new species of
Sicyos (Cucurbitaceae,
Sicyeae, Sicyinae) from Mexico and Guatemala. – Brittonia 55: 69-72.
Rodríguez-Arévalo I, Lira Saade R. 2001.
Nueva especie del género Sicyos L. (Cucurbitaceae) para la República
Mexicana. – Bol. Soc. Bot. México 68: 81-84.
Rodriguez-Arévalo I, Lira Saade R, Davila P.
2004. Two new species of Sicyos (Cucurbitaceae) from Guerrero and
Oaxaca, Mexico. – Bot. J. Linn. Soc. 145: 373-378.
Rodriguez-Arévalo I, Lira Saade R, Calzada
I. 2005. A new species of Sicyos L. (Cucurbitaceae) from Oaxaca,
Mexico. – Brittonia 57: 43-46.
Ronse De Craene L-P, Smets E. 1990. The
systematic relationship between Begoniaceae and Papaveraceae: a
comparative study of their floral development. – Bull. Jard. Bot. Natl. Belg.
60: 229-273.
Roy RP, Saran S. 1990. Sex expression in the
Cucurbitaceae. – In: Bates
DM, Robinson RW, Jeffrey C (eds), Biology and utilization of the Cucurbitaceae, Comstock Publ.
Ass., Cornell University Press, Ithaca, New York, pp. 251-268.
Roy RP, Saran S, Dutt B. 1991. Cytogenetics
of the Cucurbitaceae. – In:
Tschuchia T, Gupta PK (eds), Chromosome engineering in plants: genetics,
breeding, evolution, Elsevier, Amsterdam, pp. 181-200.
Rugayah EA, De Wilde WJJO. 1997.
Trichosanthes L. (Cucurbitaceae) in Java. – Blumea
42: 471-482.
Rugayah EA, De Wilde WJJO. 1999. Conspectus
of Trichosanthes (Cucurbitaceae) in Malesia. –
Reinwardtia 11: 227-280.
Rutherford RJ. 1970. The anatomy and cytology
of Pilostyles thurberi Gray (Rafflesiaceae). –
Aliso 7: 263-288.
Saade RL. 1996. Estudios taxonómicos y
ecogeográficos de las Cucurbitaceae Latinoamericas de
importancia económica. – Instituto de Biologia, UNAM.
Samuel R, Balasubramaniam S, Morawetz W.
1995. The karyology of some cultivated Cucurbitaceae of Sri Lanka. –
Ceylon J. Sci. (Biol. Sci.) 24: 17-22.
Sang J, Kiew R, Geri C. 2013. Revision of
Begonia ()Begoniaceae)
from the Melinau Limestone in Gunung Mulu National Park and Gunung Buda
National Park, Sarawak, Borneo, including thirteen new species. – Phytotaxa
99: 1-34.
Sanjur OI, Piperno DR, Andres TC,
Wessel-Beaver L. 2002. Phylogenetic relationships among domesticated and wild
species of Cucurbita (Cucurbitaceae) inferred from a
mitochondrial gene: implications for crop plant evolution and areas of origin.
– Proc. Natl. Acad. Sci. 99: 535-540.
Savolainen V, Manen J-F, Douzery E, Spichiger
R. 1994. Molecular phylogeny of families related to Celastrales based on
rbcL 5’ flanking sequences. – Mol. Phylogen. Evol. 3: 27-37.
Savolainen V, Spichiger R, Manen J-F. 1997.
Polyphyletism of Celastrales deduced from
a chloroplast noncoding DNA region. – Mol. Phylogen. Evol. 7: 145-157.
Schaefer H. 2007. Cucumis (Cucurbitaceae) must include
Cucumella, Dicoelospermum, Mukia,
Myrmecosicyos, and Oreosyce: a recircumscription based on
nuclear and plastid DNA data. – Blumea 52: 165-177.
Schaefer H, Renner SS. 2010a. A gift from the
New World? The West African crop Cucumeropsis mannii and the American
Posadaea sphaerocarpa (Cucurbitaceae) are the same
species. – Syst. Bot. 35: 534-540.
Schaefer H, Renner SS. 2010b. A three-genome
phylogeny of Momordica (Cucurbitaceae) suggests seven
returns from dioecy to monoecy and recent long-distance dispersal to Asia. –
Mol. Phylogen. Evol. 54: 553-560.
Schaefer H, Renner SS. 2011a. Cucurbitaceae. – In: Kubitzki K
(ed), Families and genera of vascular plants X. Flowering plants. Eudicots. Sapindales, Cucurbitales, Myrtaceae. Springer, Heidelberg,
Dordrecht, London, New York, pp. 112-174.
Schaefer H, Renner SS. 2011b. Phylogenetic
relationships in the order Cucurbitales and a new
classification of the gourd family (Cucurbitaceae). – Taxon 60:
122-138.
Schaefer H, Telford IRH, Renner SS. 2008.
Austrobryonia (Cucurbitaceae), a new Australian
endemic genus, is the closest living relative to the Eurasian and Mediterranean
Bryonia and Ecballium. – Syst. Bot. 33: 125-132.
Schaefer H, Kocyan A, Renner SS. 2008.
Linnaeosicyos (Cucurbitaceae): a new genus for
Trichosanthes amara, the Caribbean sister species of all Sicyeae. –
Syst. Bot. 33: 349-355.
Schaefer H, Heibl C, Renner SS. 2009. Gourds
afloat: a dated phylogeny reveals an Asian origin of the gourd family (Cucurbitaceae) and numerous
oversea dispersal events. – Proc. Roy. Soc., Ser. B, 276: 843-851.
Schemske DW, Ågren J, Le Corff J. 1996.
Deceit pollination in the monoecic, neotropical herb Begonia oaxacana
(Begoniaceae). – In: Lloyd DG,
Barrett SCH (eds), Floral biology: studies on floral evolution in
animal-pollinated plants, Chapman & Hall, New York, pp. 292-318.
Schimper AFW. 1893. Rhizophoraceae. –
In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(7), W.
Engelmann, Leipzig, pp. 42-56.
Schwarzbach AE, Ricklefs RE. 2000. Systematic
affinities of Rhizophoraceae and
Anisophylleaceae, and
intergeneric relationships within Rhizophoraceae,
based on chloroplast DNA, nuclear ribosomal DNA, and morphology. – Amer. J.
Bot. 87: 547-564.
Schwarzbach AE, Tomlinson PB. 2011. Anisophylleaceae. – In:
Kubitzki K (ed), Families and genera of vascular plants X. Flowering plants.
Eudicots. Sapindales, Cucurbitales, Myrtaceae. Springer, Heidelberg,
Dordrecht, London, New York, pp. 51-55.
Sebastian PM, Schaefer H, Renner SS. 2010.
Darwin’s Galapagos gourd: providing new insights 175 years after his visit.
– J. Biogeogr. 37: 975-980.
Sebastian P, Schaefer H, Telford IRH, Renner
SS. 2010. Cucumber (Cucumis sativus) and melon (C. melo) have
numerous wild relatives in Asia and Australia, and the sister species of melon
is from Australia. – Proc. Natl. Acad. Sci. U.S.A. 107: 14269-14273.
Seitner PG. 1972. Some observations on
Begonia seeds. – The Begonian 39: 47-55.
Sensarma P. 1955. Tendrils of the Cucurbitaceae: their morphological
nature on anatomical evidences. – Proc. Natl. Inst. Sci India 21: 162-169.
Setoguchi H, Kosuge K, Tobe H. 1999.
Molecular phylogeny of Rhizophoraceae
based on rbcL gene sequences. – J. Plant Res. 112: 443-455.
Sharma VK. 1968. Floral morphology, anatomy,
and embryology of Coriaria nepalensis Wall. with a discussion of the
interrelationships of the family Coriariaceae. – Phytomorphology
18: 143-153.
Shui YM, Peng CI, Wu CY. 2002. Synopsis of
the Chinese species of Begonia (Begoniaceae), with a reappraisal of
sectional delimitation. – Bot. Bull. Acad. Sin. 43: 313-327.
Shui YM, Peng CI, Wu CY. 2002. Synopsis of
the Chinese species of Begonia (Begoniaceae), with a reappraisal of
sectional delimitation. – Bot. Bull. Acad. Sin. 43: 313-327.
Silvester WB, Harris SL. 1989. Nodule
structure and nitrogenase activity of Coriaria arborea in response to
varying pO2. – Plant and Soil 118: 97-109.
Singh AK. 1979. Cucurbitaceae and polyploidy. –
Cytologia 44: 897-905.
Singh A, Dathan ASR. 1998. Morphology and
embryology. – In: Nayar NM, More TA (eds), Cucurbits, Science Publ., Enfield,
NH, pp. 67-84.
Singh A, Dathan ASR. 2001. Development and
structure of seed coat in the Cucurbitaceae and its implications
in systematics. – In: Chauhan SVS, Chaturvedi SN (eds), Botanical essays:
tribute to professor Bahadur Singh, Printwell, Jaipur, pp. 87-111.
Singh B. 1942. The anatomy of the stem, leaf
and petiole of Coccinia indica L. – J. Indian Bot. Soc.21:
319-326.
Singh B. 1952. Studies on the structure and
development of seeds of Cucurbitaceae I. Seeds of
Echinocystis Wrightii Cogn. – Phytomorphology 2: 201-209.
Singh B. 1953. Studies on the structure and
development of seeds of Cucurbitaceae. – Phytomorphology
3: 224-239.
Singh BP. 1991. Interspecific hybridization
in between New and Old-World species of Luffa and its phylogenetic
implication. – Cytologia 56: 359-365.
Singh D. 1961a. Studies on endosperm and
development of seed in the Cucurbitaceae and some related
families. – Agra Univ. J. Res. Sci. 10: 117-123.
Singh D. 1961b. Development of embryo in the
Cucurbitaceae. – J. Indian
Bot. Soc. 40: 620-623.
Singh D. 1964. A further contribution to the
endosperm of the Cucurbitaceae.
– Proc. Indian Acad. Sci., Sect. B, 60: 399-413.
Singh D. 1965a. Ovule and seed of
Dicoelospermum C. B. Clarke, together with a note on its systematic
position. – J. Indian Bot. Soc. 44: 181-190.
Singh D. 1965b. Ovule and seed of Sechium
edule Sw. – a reinvestigation. – Curr. Sci. 34: 696-697.
Singh D. 1967. Structure and development of
seedcoat in Cucurbitaceae I.
Seeds of Biswarea Cogn., Edgaria Clarke and
Herpetospermum Hook. f. – Proc. Indian Acad. Sci., Sect. B, 65:
267-274.
Singh D. 1970. Cucurbitaceae. – In: Proceedings
of the symposium on comparative embryology of angiosperms, Indian National
Science Academy, New Delhi, pp. 212-219.
Singh D, Bhandari MM. 1963. The identity of
an imperfectly known hermaphrodite Luffa, with a note on related
species. – Baileya 11: 132-141.
Singh D, Dathan ASR. 1974. Structure and
development of the seed coat of Cucurbitaceae IX. Seeds of
Corallocarpus, Kedrostis and Ibervillea. – Bull.
Torrey Bot. Club 101: 78-82.
Singh NP, Matta NK. 2008. Variation studies
on seed storage proteins and phylogenetics of the genus Cucumis. –
Plant Syst. Evol. 275: 209-218.
Skey W. 1871. Preliminary notes on the
isolation of bitter substances of the nut of the karaka tree (Corynocarpus
laevigata). – Trans. Proc. New Zealand Inst. 4: 316-321.
Skog LE. 1972. The genus Coriaria
(Coriariaceae) in the Western
Hemisphere. – Rhodora 74: 242-253.
Skog LE. 1987. 103. Coriariaceae. – In: Harling G,
Sparre B (eds), Flora of Ecuador 30, Swedish Natural Science Research Council,
Stockholm, pp. 3-7.
Smith LB, Schubert BG. 1958. Flora of Panama
VII. Begoniaceae. – Ann.
Missouri Bot. Gard. 45: 41-42.
Smith LB, Schubert BC. 1963. Begoniaceae Lindl. – Field Mass.
Nat. Hist. Bot. 13: 181-202.
Smith LB, Wasshausen DC. 1986. 133. Begoniaceae. – In: Harling G,
Sparre B (eds), Flora of Ecuador 25, Swedish Natural Science Research Council,
Stockholm, pp. 1-65.
Smith LB, Wasshausen DC, Golding J,
Karegeannes CE. 1986. Begoniaceae
I. Illustrated key II. Annotated species list. – Smithsonian Contr. Bot. 60:
1-584.
Solms-Laubach H. 1874. Über den Thallus von
Pilostyles haussknechtii. – Bot. Zeitung 32: 49-59.
Sosef MSM. 1994. Studies in Begoniaceae V. Refuge begonias:
taxonomy, phylogeny and historical biogeography of Begonia sect.
Loasibegonia and sect. Scutobegonia in relation to glacial
rain forest refuges in Africa. – Wageningen Agric. Univ. Pap. 94: 1-306.
Soyfer VN. 1964. Seed anatomy of the family
Cucurbitaceae Juss. as a
systematic character. – Bull. Moscow Soc. Nat., Section Biol., N. S., 69(1):
86-101.
Sridhar, Singh D. 1986. Development of anther
and male gametophyte in Cucurbitaceae. – J. Indian Bot.
Soc. 65: 487-493.
Stafford PJ, Sutton DA. 1994. Pollen
morphology of the Cyclantherinae C. Jeffr. (tribe Sicyeae Schrad., Cucurbitaceae) and its taxonomic
significance. – Acta Bot. Gall. 141: 171-182.
Stanley TD. 1982. Datiscaceae. – In: George AS (ed),
Flora of Australia 8, Australian Government Publ. Service, Canberra, pp.
199-200.
Steele PR. 2010. Taxonomic revision of the
neotropical genus Psiguria (Cucurbitaceae). – Syst. Bot. 35:
341-357.
Steele PR, Guisinger-Bellian M, Linder CR,
Jansen RK. 2008. Phylogenetic utility of 141 low-copy nuclear regions in taxa
at different taxonomic levels in two distantly related families of rosids. –
Mol. Phylogen. Evol. 48: 1013-1026.
Steele PR, Friar LM, Gilbert LE, jansen RK.
2010. Molecular systematics of the neotropical genus Psiguria (Cucurbitaceae): implications for
phylogeny and species identification. – Amer. J. Bot. 97: 156-173.
Steenis CGGJ van. 1951. Corynocarpaceae. – In: Steenis
CGGJ van (ed), Flora Malesiana I, 4(2), Noordhoff-Kolff N. V., Batavia, pp.
262-264.
Steenis CGGJ van. 1953. Datiscaceae. – In: Steenis CGGJ
van (ed), Flora Malesiana I, 4(4), Noordhoff-Kolff N. V., Batavia, pp.
382-387.
Stocking KM. 1955. Some considerations of the
genera Echinocystis and Echinopepon in the United States and
northern Mexico. – Madroño 13: 84-100.
Stults DZ, Axsmith BJ. 2011. First
macrofossil record of Begonia (Begoniaceae). – Amer. J. Bot. 98:
150-153.
Suzuki M, Yoda K. 1986. Comparative wood
anatomy of Coriaria in East Asia. – J. Jap. Bot. 61: 289-296,
333-342.
Swensen SM, Kubitzki K. 2011. Datiscaceae. – In: Kubitzki K
(ed), Families and genera of vascular plants X. Flowering plants. Eudicots. Sapindales, Cucurbitales, Myrtaceae. Springer, Heidelberg,
Dordrecht, London, New York, pp. 175-179.
Swensen SM, Mullin BC, Chase MW. 1994.
Phylogenetic affinities of Datiscaceae based on an analysis of
nucleotide sequences from the plastid rbcL gene. – Syst. Bot. 19:
157-168.
Swensen SM, Luthi JN, Rieseberg LH. 1998. Datiscaceae revisited: monophyly and
the sequence of breeding system evolution. – Syst. Bot. 23: 157-169.
Tebbitt MC. 2003. Taxonomy of Begonia
longifolia Blume (Begoniaceae) and related species.
– Brittonia 55: 19-29.
Tebbitt MC. 2005. Begonias: cultivation,
identification, and natural history. – Timber Press, Portland, Oregon.
Tebbitt MC, Dickson JH. 2000. Amended
edescriptions and revised sectional assignment of some Asian begonias (Begoniaceae). – Brittonia 52:
112-117.
Tebbitt MC, Maciver CM. 1999. The systematic
significance of the endothecium in Begoniaceae. – Bot. J. Linn. Soc.
131: 203-221.
Tebbitt MC, Lowe-Forrest L, Santoriella A,
Clement WL, Swensen S. 2006. Phylogenetic relationships of Asian
Begonia, with an emphasis on the evolution of rain-ballist and animal
dispersal mechanisms in sections Platycentrum, Sphenanthera,
and Leprosae. – Syst. Bot. 31: 327-336.
Telford IRH. 1982. Cucurbitaceae. – In: George AS
(ed), Flora of Australia 8, Australian Government Publ. Service, Canberra, pp.
158-198.
Telford IRH. 1989. Rediscovery of
Muellerargia timorensis (Cucurbitaceae). – Aust. Syst.
Bot. Soc. Newsletter 59: 4.
Telford IRH, Sebastian P, Lange PJ de, Bruhl
JJ, Renner SS. 2012. Morphological and molecular data reveal three rather than
one species of Sicyos (Cucurbitaceae) in Australia, New
Zealand and islands of the South West Pacific. – Aust. Syst. Bot. 25:
188-201.
Teppner H. 2004. Notes on Lagenaria
and Cucurbita (Cucurbitaceae) – review and new
contributions. – Phyton 44: 245-308.
Thakur GK, Sinha BMB. 1973. Cytological
investigation in some cucurbits. – J. Cytol. Genet. 7/8: 122-130.
Thiele KR, Wylie SJ, Maccarone L, Hollick P,
McComb JA. 2008. Pilostyles coccoidea (Apodanthaceae), a new species from
Western Australia described from morphological and molecular evidence. –
Nuytsia 18: 273-284.
Thomas DC. 2010. Phylogenetics and historical
biogeography of Southeast Asian Begonia L. (Begoniaceae). – PhD thesis.
University of Glasgow.
Thomas DC, Hughes M, Phutthai T, Rajbhandary
S, Rubite R, Ardi WH, Richardson JE. 2011. A non-coding plastid DNA phylogeny
of Asian Begonia (Begoniaceae): evidence for
morphological homoplasy and sectional polyphyly. – Mol. Phylogen. Evol. 60:
428-444.
Thomas DC, Hughes M, Phutthai T, Ardi WH,
Rajbhandary S, Rubite R, Twyford AD, Richardson JE. 2012. West to east
dispersal and subsequent rapid diversification of the mega-diverse genus
Begonia (Begoniaceae) in
the Malesian archipelago. – J. Biogeogr. 39: 98-113.
Thompson PN, Gornall RJ. 1995. Breeding
systems in Coriaria (Coriariaceae). – Bot. J. Linn.
Soc. 117: 293-304.
Thulin M. 1991. New species of
Cucumis (Cucurbitaceae) from northeast
tropical Africa. – Nord. J. Bot. 11: 535-542.
Thulin M. 1996. A new species of
Zehneria (Cucurbitaceae) from Somalia. –
Nord. J. Bot. 16: 297-299.
Thulin M. 2009. New species of
Coccinia and Momordica (Cucurbitaceae) from north-eastern
tropical Africa. – Kew Bull. 64: 485-489.
Thulin M, Al-Gifri AN. 1994. Cucumis
canoxyi (Cucurbitaceae)
– a new species from Yemen. – Nord. J. Bot. 14: 315-317.
Tobe H, Raven PH. 1987. Systematic embryology
of the Anisophylleaceae. –
Ann. Missouri Bot. Gard. 74: 1-26.
Tobe H, Raven PH. 1988a. Additional notes on
the embryology of Polygonanthus (Anisophylleaceae) and
relationships of the family. – Ann. Missouri Bot. Gard. 75: 1425-1428.
Tobe H, Raven PH. 1988b. Floral morphology
and evolution in Anisophylleaceae. – Bot. J.
Linn. Soc. 98: 1-25.
Tobe H, Suzuki M, Fukuhara T. 1992. Pericarp
anatomy and evolution in Coriaria (Coriariaceae). – Bot. Mag.
(Tokyo) 105: 289-302.
Tomlinson PB. 1988. Systematic comparison and
some biological characters of Rhizophoraceae and
Anisophylleaceae. – Ann.
Missouri Bot. Gard. 75: 1297-1318.
Turgeon R, Oparka K. 2010. The secret life of
pumpkins. – Proc. Natl. Acad. Sci. U.S.A. 107: 13201-13202.
Twyford AD, Kidner CA, Harrison N, Ennos RA.
2013. Population history and seed dispersal in widespread Central American
Begonia species (Begoniaceae) inferred from
plastome-derived microsatellite markers. – Bot. J. Linn. Soc. 171:
260-276.
Vasil IK. 1960. Studies on pollen germination
of certain Cucurbitaceae. –
Amer. J. Bot. 47: 239-247.
Vattimo I de. 1955. Notice sur la tribu
Apodantheae R. Br. (Rafflesiaceae). –
Taxon 4: 211-212.
Vattimo I de. 1956. Notes on Apodanthes
caseariae Poit. and Pilostyles calliandrae (Gardn.) R. Br. (Rafflesiaceae-Apodantheae).
– Not. Syst. 15: 225-229.
Vattimo I de. 1971 Contribuição ao
conhecimento da tribu Apodanthaceae R. Br. I. Conspecto
das espécies (Rafflesiaceae). –
Rodriguésia 26: 37-62.
Vattimo I de. 1978. Uma nova espécie de
Apodanthes Poit. (Rafflesiaceae). –
Rodriguésia 29: 269-306.
Verdcourt B. 1998. Rafflesiaceae. –
In: Beentje HJ, Whitehouse CM (eds), Flora of tropical East Africa, A. A.
Balkema, Rotterdam, The Netherlands, pp. 1-4.
Vezey EL, Shah VP, Skvarla JJ, Raven PH.
1988. Morphology and phenetics of Rhizophoraceae
pollen. – Ann. Missouri Bot. Gard. 75: 1369-1386.
Villiers JF. 1980. Corynocarpaceae. – In:
Aubréville A, Leroy JF (eds), Flore de la Nouvelle Calédonie et Dépendances
9, Muséum National d’Histoire Naturelle, Paris, pp. 175-178.
Vincent JR, Tomlinson PB. 1983. Architecture
and phyllotaxis of Anisophyllea disticha (Rhizophoraceae).
– Gard. Bull. (Singapore) 36: 3-18.
Vogel S. 1981a. Trichomatische
Blütennektaren bei Cucurbitaceen. – Beitr. Biol. Pflanzen 55: 325-353.
Vogel S. 1981b. Die Klebstoffhaare an den
Antheren von Cyclanthera pedata (Cucurbitaceae). – Plant Syst.
Evol. 137: 291-316.
Vogel S. 1990. Ölblumen und ölsammelnde
Biene 3. Momordica, Thladiantha und die Ctenoplectridae. –
Trop. Subtrop. Pflanzenwelt 73: 1-186.
Volz SM, Renner SS. 2008. Hybridization,
polyploidy, and evolutionary transitions between monoecy and dioecy in
Bryonia (Cucurbitaceae). – Amer. J. Bot.
95: 1297-1306.
Volz SM, Renner SS. 2009. Phylogeography of
the ancient Eurasian medicinal plant genus Bryonia (Cucurbitaceae) inferred from
nuclear and chloroplast sequences. – Taxon 58: 550-560.
Wagstaff SJ, Dawson MI. 2000. Classification,
origin, and patterns of diversification of Corynocarpus (Corynocarpaceae) inferred from
DNA sequences. – Syst. Bot. 25: 134-149.
Walters TW, Decker-Walters DS, Posluszny U,
Kevan PG. 1991. Determination and interpretation of comigrating allozymes among
genera of the Benincaseae (Cucurbitaceae). – Syst. Bot. 16:
30-40.
Warburg O. 1894a. Begoniaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien III(6a), W. Engelmann,
Leipzig, pp. 121-150.
Warburg O. 1894b. Datiscaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien III(6a), W. Engelmann,
Leipzig, pp. 150-155.
Ward BL, Anderson RS, Bendich AJ. 1981. the
mitochondrial genome is large and variable in a family of plants (Cucurbitaceae). – Cell 25:
793-803.
Weberling F. 1955. Die Stipularbildungen der
Coriariaceae. – Flora 142:
629-630.
Weiling F. 1959. Genomanalytische
Untersuchungen bei Kürbis (Cucurbita L.). – Züchter 29:
161-179.
Whitaker TW. 1933. Cytological and
phylogenetic studies in the Cucurbitaceae. – Bot. Gaz. 94:
780-790.
Whitaker TW, Bemis WP. 1964. Evolution in the
genus Cucurbita. – Evolution 18: 553-559.
Whitaker TW, Davis GN. 1962. Cucurbits:
botany, cultivation, and utilization. – Interscience, New York.
Whiting AG. 1938. Development and anatomy of
primary structures in the seedlings of Cucurbita maxima Duch. – Bot.
Gaz. 99: 497-528.
Widrlechner MP, Kirkbride JH, Ghebretinsae
AG, Reitsma KR. 2008. Cucumis zambianus (Cucurbitaceae), a new species from
northwestern Zambia. – Syst. Bot. 33: 732-738.
Wilde JJFE de. 2002. Studies in Begoniaceae VII. Begonia
section Tetraphila A. DC., a taxonomic revision. – Wageningen Agric.
Univ. Pap. 2001-2: 5-258.
Wilde JJFE de. 2011. Begoniaceae. – In: Kubitzki K
(ed), Families and genera of vascular plants X. Flowering plants. Eudicots. Sapindales, Cucurbitales, Myrtaceae. Springer, Heidelberg,
Dordrecht, London, New York, pp. 56-71.
Wilde WJJO de, Duyfjes BEE. 2006. Review of
the genus Gymnopetalum (Cucurbitaceae). – Blumea 51:
281-296.
Wilde WJJO de, Duyfjes BEE. 2007. The wild
species of Cucumis L. (Cucurbitaceae) in South-East Asia.
– Adansonia, sér. III, 29: 239-248.
Wilson HD. 1989. Discordant patterns of
allozyme and morphological variation in Mexican Cucurbita. – Syst.
Bot. 14: 612-623.
Wilson HD, Doebley J, Duvall M. 1992.
Chloroplast DNA diversity among wild and cultivated members of
Cucurbita (Cucurbitaceae). – Theor. Appl.
Gen. 84: 859-865.
Wilson PG. 1958. Contributions to the flora
of tropical America LXIII. – Kew Bull. 13: 161.
Wolf DE, Satkoski JA, White K, Rieseberg LH.
2001. Sex determination in the androdioecious plant Datisca glomerata
and its dioecious sister species D. cannabina. – Genetics 159:
1243-1257.
Worsdell WC. 1915. The origin and meaning of
medullary (intraxylary) phloem in the stem of dicotyledons 1. Cucurbitaceae. – Ann. Bot. 29:
567-590.
Wunderlin RP. 1976. Two new species and a new
combination in Frantzia (Cucurbitaceae). – Brittonia 28:
239-244.
Wunderlin RP. 1977. A new species of
Frantzia (Cucurbitaceae) from Panama. –
Bull. Torrey Bot. Club 104: 102-104.
Yasuda A. 1901. On the comparative anatomy of
the Cucurbitaceae, wild and
cultivated in Japan. – J. Col. Sci. Imp. Univ. Tokyo, Japan 18: 1-56.
Yoda K, Suzuki M. 1992. Comparative wood
anatomy of Coriaria. – Bot. Mag. (Tokyo) 105: 235-245.
Yokoyama J, Suzuki M, Iwatsuki K, Hasebe M.
2000. Molecular phylogeny of Coriaria, with special emphasis on the
disjunct distribution. – Mol. Phylogen. Evol. 14: 11-19.
Zentgraf U, Ganal M, Hemleben V. 1990. Length
heterogeneity of the rRNA precursor in cucumber (Cucumis sativus). –
Plant Mol. Biol. 15: 465-474.
Zentgraf U, King K, Hemleben V. 1992.
Repetitive sequences are valuable as molecular markers in studies of
phylogenetic relationships within the genus Cucumis. – Acta Bot.
Neerl. 41: 397-406.
Zhan Z-Y, Lu A-M. 1989. Pollen morphology of
the subtribe Thladiantinae (Cucurbitaceae) and its taxonomic
significance. – Cathaya 1: 23-36.
Zhang B, Tolstikov V, Turnbull C, Hicks LM,
Fiehn O. 2010. Divergent metabolome and proteome suggest functional
independence of dual phloem transport system in cucurbits. – Proc. Natl.
Acad. Sci. U.S.A. 107: 13532-13537.
Zhang C, Yu X, Ayre BG, Turgeon R. 2012. The
origin and composition of cucurbit “phloem” exudate. – Plant Physiol.
158: 1873-1882.
Zhang L-B, Simmons MP, Kocyan A, Renner SS.
2006. Phylogeny of the Cucurbitales based on DNA sequences
of nine loci from three genomes: implications for morphological and sexual
system evolution. – Mol. Phylogen. Evol. 39: 305-322.
Zhang L-B, Simmons MP, Renner SS. 2007. A
phylogeny of Anisophylleaceae based on six
nuclear and plastid loci: ancient disjunctions and recent dispersal between
South America, Africa, and Asia. – Mol. Phylogen. Evol. 44: 1057-1067.
Zheng Y-H, Alverson AJ, Wang Q-F, Palmer JD.
2013. Chloroplast phylogeny of Cucurbita: evolution of the
domesticated and wild species. – J. Syst. Evol. 51: 326-334.
Zimmermann A. 1922. Die Cucurbitaceen.
Beiträge zur Anatomie, Physiologie, Morphologie, Biologie, Pathologie und
Systematik. – Gustav Fisher, Jena.