SAPINDANAE Doweld
Doweld, Tent. Syst. Plant. Vasc.: xxxiv. 23 Dec
2001
Rutanae Takht., Sist.
Filog. Cvetk. Rast. [Syst. Phylog. Magnolioph.]: 311. 4 Feb 1967, nom.
illeg.; Rutidae Doweld, Tent. Syst. Plant.
Vasc.: xxxiii. 23 Dec 2001; Rutineae Doweld ex Reveal
in Kew Bull. 66: 48. Mar 2011
[Sapindales+[Huerteales+[Capparales+Malvales]]]
SAPINDALES Juss. ex Bercht. et
J. Presl
Berchtold et Presl, Přir. Rostlin: 224. Jan-Apr
1820 [‘Sapindeae’]
Fossils Chaneya, from
the Cenozoic of North America, Europe and Asia, is represented by flowers and
winged fruits. It may be assigned to some group of Sapindales.
Habit Bisexual, monoecious,
andromonoecious, polygamomonoecious, dioecious, androdioecious, gynodioecious,
or polygamodioecious (sometimes morphologically bisexual and functionally
monoecious), evergreen or deciduous trees, shrubs or lianas (sometimes
perennial or annual herbs).
Vegetative anatomy Phellogen
ab initio usually superficial (sometimes cortical or pericyclic; sometimes
absent). Secondary lateral growth normal or anomalous (from concentric cambia).
Vessels usually solitary and in radial multiples. Vessel elements usually with
simple (rarely scalariform or reticulate) perforation plates; lateral pits
usually alternate (rarely opposite or scalariform), simple or bordered pits.
Vestured pits sometimes present. Imperforate tracheary xylem elements usually
libriform fibres (sometimes fibre tracheids) with usually simple (sometimes
bordered) pits, septate or non-septate (also vasicentric tracheids). Wood rays
uniseriate or multiseriate, homocellular or heterocellular, or absent. Axial
parenchyma apotracheal diffuse or diffuse-in-aggregates, or paratracheal
scanty, aliform, lozenge-aliform, winged-aliform, confluent, vasicentric, or
banded, or absent. Secondary phloem sometimes stratified into hard fibrous and
soft parenchymatous layers. Sieve tube plastids S type. Nodes usually 3:3,
trilacunar with three leaf traces, or 5:5, pentalacunar with five traces
(sometimes 1:1, unilacunar with one trace, rarely heptalacunar). Laticifers
with latex or resinous substances sometimes present; resinous cells sometimes
frequent. Sclereids often present in cortex. Parenchyma sometimes with mucilage
cells, often having swollen and layered inner periclinal walls, or with
secretory cavities and ducts containing ethereal oils. Wood often silicified or
with silica bodies. Calciumoxalate as prismatic crystals or druses.
Trichomes Hairs unicellular or
multicellular, uniseriate or multiseriate, simple, furcate, stellate or
lepidote (sometimes complex capitate); glandular hairs multicellular (sometimes
peltate-lepidote; helical glands sometimes frequent).
Leaves Usually alternate
(spiral; sometimes opposite, rarely verticillate), usually pinnately or
palmately compound (sometimes bipinnate or unifoliolate, or simple and entire
or lobed), with conduplicate, supervolute or plicate (sometimes curved or flat)
ptyxis. Stipules usually absent (sometimes petiolar, rarely intrapetiolar or
cauline); stipules possibly being modified leaflets (described as
pseudostipules or metastipules, having typical stipule morphology, although
probably being modified pseudostipules); leaf sheath absent. Colleters often
abundant. Petiole vascular bundle transection arcuate or annular; petiole
sometimes with cortical or adaxial bundles (sometimes wing bundles). Venation
usually pinnate (sometimes palmate), brochidodromous, eucamptodromous, or
craspedodromous. Stomata usually anomocytic, paracytic (sometimes cyclocytic,
tetracytic, actinocytic or parallelocytic). Cuticular wax crystalloids usually
absent (rarely as tubuli). Domatia present as pits, pockets or hair tufts, or
absent. Lamina often gland dotted, usually without secretory cavities
(sometimes with lysigenous or schizogenous cavities and canals containing
resins or ethereal oils). Epidermis usually with mucilaginous idioblasts.
Mesophyll with or without spherical idioblasts containing ethereal oils, with
or without mucilaginous idioblasts, with or without sclerenchymatous
idioblasts. Mesophyll cells often with calciumoxalate druses or prismatic
crystals. Leaf margin and leaflet margins entire, crenate, or serrate. Leaf
teeth, when present, with transparent glandular tip, distally expanding and
with foramen and two accessory veins (or one vein, second vein running above
tooth).
Inflorescence Terminal or
axillary, panicle, thyrsoid, dichasial, raceme- or umbel-like, thyrse, raceme,
spike or head (rarely solitary axillary). Floral prophylls (bracteoles) rarely
absent.
Flowers Usually actinomorphic
(rarely zygomorphic). Usually hypogyny (occasionally epigyny). Sepals (two or)
four or five (to eight), usually with imbricate, valvate or induplicate-valvate
(rarely open) aestivation, free or connate in lower part (rarely absent).
Petals (two or) four or five (to 14), usually with imbricate or valvate
(sometimes induplicate-valvate or contorted) aestivation, often clawed and/or
with scale-like or other appendages or folds enclosing nectaries, usually free
(rarely connate at base; sometimes absent). Nectariferous disc extrastaminal or
intrastaminal, annular or unilateral (sometimes lobate or reduced to glandular
teeth), or nectariferous glands extrastaminal, alternipetalous, and disc
absent.
Androecium Stamens (three or)
five, 3+3, 4+4 or 5+5 (to 18) in one to three (to five) whorls, usually haplo-,
diplo- or obdiplostemonous. Filaments usually free from each other and from
tepals (sometimes more or less connate, sometimes connate into tube), sometimes
articulated. Anthers usually dorsifixed (sometimes basifixed), usually
versatile, tetrasporangiate, usually introrse (rarely extrorse or latrorse),
longicidal (dehiscing by longitudinal slits). Tapetum secretory. Staminodia one
to ten, extrastaminal or intrastaminal, or absent; female flowers often with
staminodia.
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually triporate, 2–4-colpate
or 2–5(–8)-colpor(oid)ate (sometimes acolpate, syncolporate or
parasyncolporate, rarely polyporate or rugate), usually shed as monads (rarely
tetrads), usually bicellular (sometimes tricellular) at dispersal. Exine
tectate or semitectate, with columellate infratectum, reticulate,
microreticulate, rugulate, scabrate, striate, gemmate, spinulate or psilate.
Gynoecium Pistil composed of
(two or) three to five (to 20) connate eusyncarpous antepetalous carpels, or
carpels secondarily free (apocarpy); odd carpel usually adaxial. Ovary usually
superior (rarely inferior), unilocular to quinquelocular (to 20-locular;
sometimes pseudomonomerous). Style single, simple or lobate (sometimes hollow),
or stylodia two to six, free or connate in lower part, or absent. Stigma one,
capitate, clavate, peltate or lobate, or stigmas two to five, punctate, usually
non-papillate, Dry or Wet type. Male flowers often with pistillodium.
Ovules Placentation axile,
apical, basal, or parietal. Ovules usually one or two (sometimes three to
numerous) per carpel, usually anatropous or campylotropous (sometimes
hemianatropous or amphitropous, rarely orthotropous), ascending, horizontal or
pendulous, apotropous or epitropous, usually bitegmic (sometimes unitegmic),
crassinucellar. Micropyle bistomal or endostomal. Placental obturator sometimes
present. Archespore sometimes multicellular. Nucellar cap sometimes present.
Megagametophyte usually monosporous, Polygonum type (rarely
tetrasporous, 16-nucleate, 13-celled, Penaea type). Synergids
sometimes with a filiform apparatus. Antipodal cells sometimes proliferating
(up to 14 cells), rarely persistent. Endosperm development ab initio nuclear.
Endosperm haustoria chalazal or absent. Embryogenesis asterad, solanad or
onagrad.
Fruit Usually a loculicidal
(sometimes septicidal, septifragal or irregularly dehiscent) capsule or a drupe
(sometimes a berry, samara, follicle, pyxidium, nut, hesperidium, syncarp, or
schizocarp with nutlike, samaroid, berry-like or drupaceous mericarps).
Seeds Aril usually absent.
Arillode, sarcotesta or elaiosome sometimes present. Testa often vascularized
(sometimes collapsed). Exotesta often palisade, lignified or non-lignified.
Mestotesta sometimes sclerotic or lignified. Endotesta sometimes with stellate
calciumoxalate crystals, sometimes lignified and tracheidal. Exotegmen often
lignified or fibrous, sometimes tracheidal, or crushed. Endotegmen sometimes
thickened and lignified (rarely tracheidal or fibrous), or crushed. Perisperm
not developed. Endosperm usually sparse or absent (rarely copious). Embryo
usually curved to spirally twisted (sometimes straight), usually well
differentiated, often large, oily, proteinaceous or often starchy, with or
without chlorophyll. Cotyledons two. Germination phanerocotylar or
cryptocotylar.
Cytology x = 5–13
DNA Plastid gene infA
lost/defunct. Mitochondrial intron coxII.i3 lost.
Phytochemistry Flavonols
(kaempferol, quercetin, myricetin), flavones, biflavones, flavone methyleters,
biflavonyls, 5-deoxyflavonoids, cyanidin, delphinidin, monoterpenes,
triterpenes, tetranor- and pentanortriterpenes, triterpenoid bitter substances
(meliacins, limonoids, simaroubalides-quassinoids), ethereal oils
(monoterpenes, sesquiterpenes, phenylpropans), ellagic acid (rare),
hydrolyzable and non-hydrolyzable tannins, phenols with unsaturated
side-chains, acridines, β-carbaline alkaloids, imidazole, quinoline alkaloids,
benzylisoquinoline alkaloids, anthranilic-derived alkaloids, toxic triterpene
saponins, pentacyclic terpene saponins, leucine- or phenylalanine-derived
cyanogenic compounds, polyacetate- or shikimic acid-derived arthroquinones,
pyranochromones, coumarins, furanocoumarins, quebrachitol (cyclitol), ethereal
oils with high content of aliphatic carbohydrates, polyacetylenes, cyclic
polyvalent alcohols, amides, type C18:3 fatty acids, cyclopropane
amino acids, and polygalitol present.
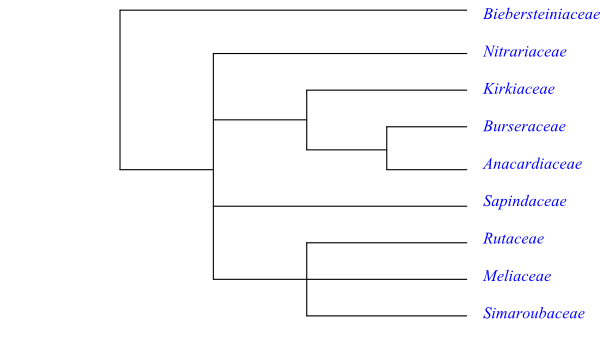
Systematics Sapindales may be sister-group to
the clade [Huerteales+[Capparales+Malvales]].
The sister-group relationships in Sapindales are only partly
resolved and several clades are only weakly supported.
According to Stevens (2001 onwards) Sapindales except
Biebersteinia (Biebersteiniaceae) are
supported by the synapomorphy: stigma papillate, stigmatic head arising from
postgenitally fused free carpellary apices. There is weak support from
molecular data for Nitrariaceae being sister to the
remaining Sapindales
(except Biebersteinia). The following potential synapomorphies are
suggested by Stevens (2001 onwards): remnants of floral apex persisting in
centre of gynoecium; ovules two per carpel, epitropous, superposed; micropyle
endostomal (inner integument elongated), S- or Z-shaped; and presence of
nucellar cap. The clade [Kirkiaceae+[Burseraceae+Anacar-diaceae]]
is usually well supported and has the following synapomorphies: inflorescence
thyrsoid (panicle consisting of cymes); carpels adnate to central receptacular
apex, synascidiate; stigma with uniseriate multicellular papillae, Wet type;
fruits containing one seed per carpel; and endocarp well developed. Finally,
Anacardiaceae and Burseraceae share the following
characters: phloem with vertical intercellular secretory resinous ducts, and
surrounded by light-coloured, sinuous sclerenchymatous band; glandular hairs
with uniseriate stalk; absence (usually) of cuticular waxes; flowers fairly
small; sepals often connate; petals little longer than sepals; central
receptacular apex more or less exposed in floral centre; ovule pachychalazal;
fruit an operculate drupe; endocarp cells lignified and not orientated; and
presence of biflavonoids.
[Sapindaceae+[Simaroubaceae+[Meliaceae+Rutaceae]]] is a clade with
relatively low support in molecular analyses. Muellner-Riehl & al. (2016)
present the following topology (Maximum Likelihood): [Sapindaceae+[Rutaceae+[Simaroubaceae+Meliaceae]]]. Stevens (2001
onwards) lists the following potential synapomorphies: anthers with pseudo-pit;
tapetal cells multinucleate, nuclei fusing to form polyploid mass; presence of
hypostase; and testa multiplicative and more than five cell layers thick. The
clade [Simaroubaceae+Rutaceae+Meliaceae] has the following
characters in common: absence of cuticular waxes; inflorescence branches
cymose; and presence of alkaloids and bitter-tasting pentanortriterpenes
(limonoids, protolimonoids, meliacins, cneorids, quassinoids, etc. – all
triterpenoids – are closely related biosynthetically). Moreover, Meliaceae and Rutaceae share the potential
synapomorphies: capitate stigma; and presence of flavones and
tetranortriterpenes (bitter-tasting).
Brown in J. H. Tuckey, Narr. Exped. Zaire: 431. 5
Mar 1818 [‘Cassuviae (or Anacardeae)’], nom. cons.
Terebinthaceae Juss.,
Gen. Plant.: 368. 4 Aug 1789 [’Terebintaceae’], nom. illeg.;
Cassuviaceae Juss. ex R. Br. in J. H. Tuckey, Narr.
Exped. Zaire: 431. 5 Mar 1818 [‘Cassuviae (or
Anacardeae)’], nom. illeg.; Comocladiaceae
Martinov, Tekhno-Bot. Slovar: 144. 3 Aug 1820 [‘Comocladieae’];
Pistaciaceae Martinov, Tekhno-Bot. Slovar: 485. 3 Aug
1820 [‘Pistaceae’]; Spondiadaceae
Martinov, Tekhno-Bot. Slovar: 594. 3 Aug 1820 [‘Spondiaceae’];
Terebinthales Juss. ex Bercht. et J. Presl, Přir.
Rostlin: 228. Jan-Apr 1820 [‘Terebinthaceae’], nom. illeg.;
Rhoaceae Spreng. ex J. Sadler, Fl. Comit. Pest. 2:
135. 30 Mai 1826 [‘Therebinthaceae, Rhoes Spr.,
Dumosae L.’]; Anacardiineae Link, Handbuch
2: 124. 4-11 Jul 1829 [‘Anacardiaceae’];
Spondiadineae Link, Handbuch 2: 126. 4-11 Jul 1829
[‘Spondiaceae’]; Terebinthopsida Bartl.,
Ord. Nat. Pl.: 229, 382. Sep 1830 [’Terebinthinae’], nom. illeg.;
Vernicaceae Schultz Sch., Nat. Syst. Pflanzenr.: 488.
30 Jan-10 Feb 1832 [’Verniceae’];
Cassuviales R. Br. in C. F. P. von Martius, Consp.
Regn. Veg.: 41. Sep-Oct 1835 [‘Cassuvieae’], nom. illeg.;
Spondiadales Kunth in C. F. P. von Martius, Consp.
Regn. Veg.: 56. Sep-Oct 1835 [‘Spondiaceae’];
Schinaceae Raf., Fl. Tellur. 3: 55. Nov-Dec 1837
[‘Schinidia’]; Sumachiaceae DC. ex
Perleb, Clav. Class.: 31. Jan-Mar 1838 [’Sumachineae’], nom.
illeg.; Lentiscaceae Horan., Tetractys: 25. Jun-Dec
1843 [‘Lentiscaceae (Pistaceae Link et Mart.)’];
Podoaceae Baill. ex Franch., Pl. Delav.: 145. Mai
1889 [‘Podoonaceae’]; Julianaceae Hemsl.
in J. Bot. 44: 379. Oct 1906 [‘Julianiaceae’], nom. cons.;
Julianales Engl., Syllabus, ed. 5: 111. Jul 1907
[‘Julianiales’]; Blepharocaryaceae Airy
Shaw in Kew Bull. 18: 254. 8 Dec 1965
Genera/species 77/830–875
Distribution Mainly tropical
and subtropical regions in the Northern and Southern Hemispheres; some species
in warm-temperate areas north to southern Canada, Central and East Europe, and
northeastern China.
Fossils Fruits are found in
the Early Eocene of North America and England, and Paleogene wood has been
attributed to Anacardiaceae.
Habit Monoecious,
andromonoecious, polygamomonoecious, dioecious, or gynodioecious (sometimes
bisexual), evergreen or deciduous trees or shrubs (sometimes with spines;
rarely lianas or perennial herbs to suffrutices).
Vegetative anatomy Phellogen
ab initio usually superficial (sometimes cortical). Medulla loose, shining.
Primary medullary rays narrow or wide. Vessel elements usually with simple
(rarely scalariform or reticulate) perforation plates; lateral pits alternate,
simple pits. Imperforate tracheary xylem elements libriform fibres with simple
or bordered pits, septate or non-septate (also vasicentric tracheids). Wood
rays uniseriate or multiseriate, homocellular or heterocellular. Axial
parenchyma usually paratracheal scanty, aliform, vasicentric, or banded
(sometimes absent). Wood often fluorescent. Tyloses often frequent. Sieve tube
plastids S type. Nodes usually 3:3, trilacunar with three leaf traces (rarely
unilacunar or 5:5, pentalacunar with five traces). Phloem with vertical
intercellular secretory ducts and surrounded by pale sinuous sclerenchymatous
band. Bark in many species with laticifers or vertical resinous ducts with
black, red to yellow, white or colour-less exudate. Wood often silicified or
with silica grains. Prismatic calciumoxalate crystals frequent.
Trichomes Hairs unicellular or
multicellular, uniseriate; stellate or peltate-lepidote glandular hairs often
present.
Leaves Usually alternate
(spiral; in, e.g., Bouea and Blepharocarya opposite, rarely
verticillate), trifoliolate or imparipinnate (sometimes unifoliolate, or simple
and entire or lobed; rarely palmate bipinnately compound), with conduplicate?
ptyxis. Stipules absent; leaf sheath absent. Petiole base often pulvinate.
Petiole vascular bundle transection?; petiole with cylinder of wing bundles.
Petiole base often swollen. Distal part of petiole sometimes with paired
extrafloral nectaries. Petiolules not articulated. Venation usually pinnate
(sometimes palmate). Stomata anomocytic, paracytic, cyclocytic, tetracytic or
parallelocytic. Cuticular wax crystalloids as clustered tubuli
(Berberis type), chemically dominated by nonacosan-10-ol. Domatia as
pits, pockets or hair tufts. Larger leaf veins and petiole phloem with usually
lysigenous (rarely schizogenous) secretory cavities and ducts containing resin,
balsam or latex. Mesophyll cells sometimes with calciumoxalate druses. Leaf
margin and leaflet margins serrate, crenate or entire. Extrafloral nectaries
sometimes present on stipules, petiole and lamina (e.g. in Anacardium
and Holigarna).
Inflorescence Terminal or
axillary, panicle, thyrsoid or raceme- or spike-like (flowers rarely solitary).
Extrafloral nectaries present on bracts in, e.g., Holigarna.
Flowers Usually actinomorphic
(rarely zygomorphic), small. Pedicel often articulated (in Anacardium
accrescent and swollen in fruit). Hypanthium sometimes present. Usually
hypogyny (in Drimycarpus and Holigarna epigyny). Sepals
(three or) four or five (to eight), with imbricate or valvate aestivation,
usually connate in lower part (sometimes absent), persistent or caducous,
sometimes accrescent in fruit. Petals (three or) four or five (to eight), with
imbricate or valvate (rarely open) aestivation, usually free (rarely connate at
base; sometimes absent), persistent (rarely accrescent) in fruit or caducous.
Nectariferous disc usually intrastaminal (in Mangifera extrastaminal),
usually annular (sometimes quinquelobate or modified into androphore or
gynophore, or absent). Floral tissues usually resiniferous.
Androecium Stamens usually
five, haplostemonous, antesepalous, alternipetalous, or 5+5, diplostemonous
(rarely one, in Anacardium, to four, 4+4, or more than ten to more
than 100). Filaments usually free (rarely connate at base), free from tepals,
sometimes inserted on nectariferous disc. Anthers usually dorsifixed (sometimes
basifixed), versatile, tetrasporangiate, usually introrse (rarely extrorse or
latrorse), longicidal (dehiscing by longitudinal slits). Tapetum secretory,
often with at least binucleate cells. Staminodia one to nine or absent; female
flowers often with staminodia.
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually (2–)3(–8)-colporate
(rarely colpate, rugate or polyporate), shed as monads, bicellular at
dispersal. Exine semitectate, with columellate infratectum, reticulate or
microreticulate to striate or striate-reticulate.
Gynoecium Carpels usually one
to five (rarely up to 13 [Pleiogynium]), usually connate (rarely free
in upper part; often only one carpel fertile leading to pseudomonomery). Ovary
usually superior (rarely inferior), usually bilocular to quinquelocular (rarely
up to 13-locular; often only a single locule fertile: pseudomonomerous), or
unilocular (monomerous). Style single, simple, or stylodia three to five
(rarely up to 13; sometimes gynobasic), connate in upper parts. Stigma(s) one
to five, usually capitate (sometimes discoid, spatulate or lobate, rarely
punctate), non-papillate, Dry type. Male flowers sometimes with
pistillodium.
Ovules Placentation apical to
axile or basal (when ovary multilocular), or parietal to basal (when ovary
unilocular). Ovule one per fertile carpel, usually anatropous (rarely
hemianatropous), ascending or pendulous, usually apotropous (sometimes
epitropous), unitegmic or bitegmic, crassinucellar. Funicle often long.
Micropyle usually bistomal, Z-shaped (zig-zag; sometimes endostomal). Outer
integument ? cell layers thick. Inner integument ? cell layers thick. Hypostase
present or absent. Often with a placental obturator, ponticulus, at funicular
base. Nucellar beak absent. Megagametophyte monosporous, Polygonum
type. Synergids hooked. Endosperm development ab initio nuclear. Endosperm
haustoria? Embryogenesis usually onagrad (sometimes asterad, Penaea or
Euphorbia type) Polyembryony occurring in several species. Chalazogamy
present in many species (pollen tube growing via ponticulus to chalaza).
Fruit Usually a drupe, often
asymmetrical, often operculate, flattened, sometimes with accrescent calyx
(sometimes a samara; rarely with accrescent corolla; rarely a berry- or nutlike
fruit, or a syncarp; fruits in Blepharocarya adnate to cupule-like
lignified inflorescence branches; fruit in Amphipterygium attached to
wing-like flat peduncle). Mesocarp sometimes with black resins. Endocarp often
consisting of unorientated sclerified and crystalliferous cells.
Seeds Aril absent. Seed often
pachychalazal. Seed coat usually reduced. Exotestal cells (and hypodermis)
sometimes thickened. Endotesta? Exotegmen? Endotegmen usually thickened, with
lignified cell walls. Perisperm not developed. Endosperm sparse, with oil and
sometimes starch, or absent. Embryo usually curved (sometimes straight, rarely
hippocrepomorphic), usually well differentiated, oily, with or without
chlorophyll. Cotyledons two, in e.g. Mangifera folded. Germination
phanerocotylar or cryptocotylar.
Cytology n = 7–12, 14–16,
21
DNA
Phytochemistry Flavonols
(kaempferol, quercetin, myricetin), biflavones, biflavonyls, 5-deoxyflavonoids,
cyanidin, delphinidin, balsams containing mono- and triterpenes, hydrolyzable
and condensed tannins, often extremely allergenic phenols with unsaturated
side-chains and cyclic polyvalent alcohols and their derivatives, often very
toxic and dermatitis-causing resins (e.g. campnospermonol, catechols,
resorcinols, anacardic acid, cardanol, cardol, and urushiol), triterpene
saponins and polyacetate-derived arthroquinones present. Ellagic acid and
cyanogenic compounds not found.
Use Ornamental plants, fruits
(Anacardium occidentale, Magnifera indica, Pistacia
vera, Schinus, Semecarpus, Spondias, etc.),
spices, terpentine, lacquer, resins, tannins, oils, varnish (Pistacia,
‘Rhus’ s.l., Toxicodendron), timber.
Systematics (under
construction) Anacardiaceae are sister to
Burseraceae.
Pegia and the doubtfully monophyletic
Spondias appear to form a sister-group (here Spondiadoideae
s.str.) to the remaining Anacardiaceae (Pell 2004).
Spondiadoideae Kunth
ex Arn., Botany: 106. 9 Mar 1832 [‘Spondiaceae’]
4/>20.
Haplospondias (1; H. brandisiana; Yunnan,
Burma),Pegia (2; P. nitida, P. sarmentosa; eastern
Himalayas, East Asia, West Malesia), Spondias (>16; Madagascar,
tropical Asia to tropical China and Indochina, islands in southern Pacific,
tropical America; monophyletic?), Poupartiopsis (1; P.
spondiocarpus; eastern Madagascar). – Madagascar, tropical Asia to West
Malesia, tropical America. Trees or lianas. Stamens eight or ten. Nectariferous
disc intrastaminal. Ovary quadrilocular or quinquelocular. Style one, simple,
or stylidia four or five, connate above. Fruit a drupe. Endocarp consisting of
unorientated sclerified cells and crystalliferous cells.
Anacardioideae Arn.,
Botany: 106. 9 Mar 1832 [’Anacardiaceae’]
Tapirireae Marchand,
Rév. Anacardiac.: 161. Jan–Jun 1869 [‘Tapirieae’]
10/85–90. Choerospondias (1;
C. axillaris; northeastern India to northern Thailand, Vietnam,
southeastern China, Taiwan and Japan), Cyrtocarpa (5; C.
caatingae, C. edulis, C. kruseana, C. procera,
C. velutinifolia; southern Baja California, western Mexico, northern
Colombia to Guyana, Venezuela and northern Brazil, the Caatinga in northeastern
Brazil), Pleiogynium (2; P. solandri, P. timoriense;
Indochina and Malesia to Queensland and islands in the Pacific),
Dracontomelon (8; Southeast Asia, Malesia to Fiji), Tapirira
(8–10; tropical America), Antrocaryon (4; A. klaineanum,
A. micraster, A. nannanii, A. schorkopfii;
tropical Africa, tropical South America), Poupartia (c 10; Madagascar,
the Mascarene Islands), Harpephyllum (1; H. caffrum; southern
Africa), Lannea (c 40; tropical Africa, Madagascar, Socotra, tropical
Asia), Operculicarya
(8; Madagascar, the Comoros, Aldabra). – Pantropical. Stamens
obdiplostemonous. Pistil composed of (one to) four or five (to twelve) connate
carpels. Ovary (1–)4–5(–12)-locular. When locule single, then ovary
possibly pseudomonomerous. Placentation apical. Ovules usually one (sometimes
two) per carpel, pendulous (when two then one ovule epitropous). Hypostase
present. Funicle massive. Exocarp thick, with or without operculum. Endocarp
consisting of unorientated sclerified cells and crystalliferous cells.
Exotestal cells thickened or not, persistent. Tegmen often absent. Hypostase
persistent, saddle-shaped. Biflavonoids present. Alkylcathechols and
alkylresorcinols absent.
Buchananieae Marchand,
Rév. Anacardiac.: 191. Jan-Jun 1869
3/43–50. Buchanania (25–30;
tropical Asia to islands in western Pacific), Campnosperma (>13;
Madagascar, the Seychelles, Sri Lanka, Southeast Asia, Malesia to New Guinea,
islands in western Pacific, tropical America), Pentaspadon (5; P.
annamensis, P. curtisii, P. motleyi, P.
poilanei, P. velutinus; Southeast Asia, Malesia to New Guinea,
Solomon Islands). – Madagascar, the Seychelles, tropical Asia to western
Pacific, tropical America. Pistil in Buchanania composed of six
partially connate carpels. – Buchanania is sister to the remaining
Anacardioideae (except the Lannea clade) in several analyses.
They differ from Anacardieae in the number of carpels (four to six),
the position of the fertile carpel, the endocarp anatomy (endocarp consisting
of unorientated sclerified cells and crystalliferous cells), and
phytochemistry. Campnosperma and Pentaspadon have similar
endocarp and Campnosperma may have bilocular fruit.
Anacardieae DC.,
Prodr. 2: 62. Nov (med.) 1825
39/555–585. Mainly tropical, but also
temperate regions. Leaves sometimes opposite, usually simple (sometimes
compound). Stamens usually five or ten (sometimes one+staminodia, or numerous).
Filaments sometimes connate at base. Pistil composed of usually one or three
connate carpels; only antesepalous carpel fertile. Styles sometimes connate.
Placentation apical to basal. Ponticulus sometimes present. Exocarp thin, with
lignified epidermis. Endocarp stratified, with up to three layers of lignified
palisade sclereids; inside these crystalliferous layer. Seed coat
undifferentiated.
Clade 1
7/185–190. Faguetia (1;
F. falcata; eastern Madagascar), Semecarpus (70–75;
tropical Asia to tropical Australia, New Caledonia and Fiji),
Fegimanra (3; F. acuminatissima, F. africana, F.
afzelii; tropical West and Central Africa), Anacardium (11;
southern Mexico, Central America, the West Indies, tropical South America),
Gluta (c 30; Madagascar, tropical Asia), Bouea (3; B.
macrophylla, B. oppositifolia, B. poilanei; Southeast
Asia, West Malesia), Mangifera
(c 70; tropical Asia to Solomon Islands). – Tropical Africa, Madagascar,
tropical Asia to New Caledonia and Fiji, tropical America.
Clade 2
22/280–300. Thyrsodium
(7–8; T. africanum, T. bolivianum, T. guianense,
T. herrerense, T. paraense, T. puberulum, T.
schomburgkianum, T. spruceanum; tropical South America),
Amphipterygium (4–5; A. adstringens, A.
amplifolium, A. glaucum, A. molle, A.
simplicifolium; western Mexico to northwestern Costa Rica),
Orthopterygium (1; O. huaucui; western Peru),
Loxopterygium (3; L. grisebachii, L. huasango,
L. sagotii; northern and southern tropical South America),
Astronium (11; southern Mexico, Central America, tropical South
America), Schinopsis (8; tropical South America), Bonetiella
(1; B. anomala; northern and central Mexico), Comocladia
(16–20; Mexico, Central America, the West Indies), Metopium (3;
M. brownei, M. toxiferum, M. venosa; Florida,
Mexico, the West Indies), Cotinus
(7; C. carranzae, C. chiangii, C. kanaka, C.
szechuanensis, C. coggygria: the Mediterranean to China; C.
nanus: northwestern Yunnan; C. obovatus: southeastern United
States), Mosquitoxylum (1; M. jamaicense; southern Mexico to
northwestern Ecuador, Jamaica; in Rhus?), Rhus
(35–40; temperate and subtropical regions on both hemispheres), Searsia
(110–115; the Mediterranean, tropical and subtropical Africa, Socotra, the
Middle East, the Arabian Peninsula, India, the Himalayas, Burma, southwestern
China), Baronia (1; B. taratana; Madagascar),
Malosma (1; M. laurina; southwestern California, Baja
California), Laurophyllus
(1; L. capensis; Western and Eastern Cape), Lithraea
(3; L. brasiliensis, L. caustica, L. molleoides;
South America), Schinus
(30–35; Mexico, Central America, the West Indies, tropical and subtropical
South America), Pachycormus (1; P. discolor; Baja California
in northwestern Mexico), Pistacia
(12; the Mediterranean, Asia to Malesia, southern United States to Central
America), Actinocheita (1; A. filicina; south central
Mexico), Toxicodendron
(22; tropical and East Asia to New Guinea, North America, Central America to
Bolivia). – Temperate to tropical regions on both hemispheres. – ‘Rhus’
is polyphyletic and needs extensive investigations.
Clade 3
10/90–95. Dobinea (2; D.
delavayi, D. vulgaris; eastern Himalayas to southern China);
Loxostylis (1; L. alata; KwaZulu-Natal, Western and Eastern
Cape), Smodingium (1; S. argutum; South Africa, Swaziland,
Lesotho), Sorindeia (9; tropical Africa, Madagascar, the Mascarene
Islands), Mauria (10–15; Central America, the Andes),
Protorhus (1; P. longifolia; Namibia, South Africa,
Swaziland, Madagascar), Abrahamia (19; Madagascar), Heeria
(1; H. argentea; Western Cape), Ozoroa (c 40; Africa,
Yemen)?, Micronychia (6; M. acuminata, M. danguyana,
M. humberti, M. macrophylla, M. madagascariensis,
M. tsiramiramy; Madagascar). – Tropical and southern Africa,
Madagascar, the Mascarene Islands, the Arabian Peninsula, eastern Himalayas to
southern China, the Andes.
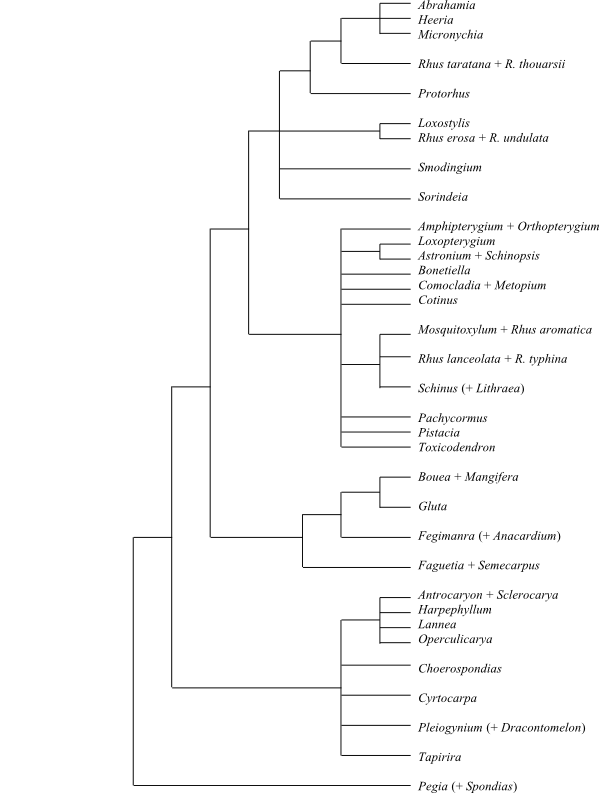 |
Bootstrap consensus tree of Anacardiaceae based on
DNA sequence data (Pell 2004).
|
Unplaced
Anacardioideae
Androtium (1; A.
astylum; West Malesia), Blepharocarya (2; B.
depauperata, B. involucrigera; Arnhem Land in Northern Territory,
northeastern Queensland), Campylopetalum (1; C. siamense;
Thailand), Cardenasiodendron (1; C. brachypterum; Bolivia),
Drimycarpus (3–4; D. anacardifolius, D. luridus,
D. maximus, D. racemosus; India to Borneo),
Euroschinus (>9; Malesia to New Guinea, eastern Queensland, eastern
New South Wales, New Caledonia), Haplorhus (1; H. peruviana;
the Andes in Peru and northern Chile), Melanochyla (c 30; southern
Thailand, Malesia), Nothopegia (>10; India, Sri Lanka),
Ochoterenaea (1; O. colombiana; Panamá, northern and central
Andes), Parishia (5; P. insignis, P. maingayi,
P. malabog, P. paucijuga, P. sericea; Burma,
Thailand, West Malesia), Pseudosmodingium (5; P. andrieuxii,
P. anomalum, P. barkleyi, P. multifolium, P.
perniciosum; Mexico), Rhodosphaera (1; R. rhodanthema;
southeastern Queensland, northeastern New South Wales), Swintonia (12;
the Andaman Islands, Southeast Asia, West and Central Malesia),
Trichoscypha (32; tropical and southern Africa).
Unplaced Anacardiaceae
Euleria (1; E.
tetramera; Cuba), Haematostaphis (1; H.
barteri; western tropical Africa to Nigeria), Hermogenodendron
(1; H. concinnum; Bahia and Espírito Santo in Brazil),
Holigarna (8; India, Southeast Asia), Koordersiodendron (1;
K. pinnatum; Borneo, the Philippines, Sulawesi, Maluku, New Guinea),
Pseudospondias (2; P. longifolia, P. microcarpa;
tropical West and Central Africa).
Schnizlein, Anal. Nat. Ordn. Gew.: 14. 1856
[‘Biebersteinieae’]
Biebersteiniales
Takht., Divers. Classif. Fl. Pl.: 327. 24 Apr 1997
Genera/species 1/5
Distribution Greece, Turkey,
the Caucasus and Iran to Central Asia and eastern Tibet.
Fossils Unknown.
Habit Bisexual, perennial
herbs with tuberous rhizome. Often evil-smelling.
Vegetative anatomy Phellogen
absent. Vessel elements with simple? perforation plates; lateral pits?
Imperforate tracheary xylem elements libriform fibres? Wood rays absent. Axial
parenchyma? Sieve tube plastids? Nodes? Crystals?
Trichomes Eglandular hairs
absent; glandular hairs with long multiseriate stalk and multicellular head.
Leaves Alternate (spiral), 2-
or 3-imparipinnate with lobed leaflets, or simple and pinnately lobed, with ?
ptyxis. Stipules petiolar, often lobed; leaf sheath absent. Petiole vascular
bundle transection? Venation pinnate. Stomata anomocytic. Cuticular wax
crystalloids? Leaflet margins serrate.
Inflorescence Terminal, erect
panicle or raceme.
Flowers Actinomorphic, large.
Hypogyny. Sepals five, with imbricate aestivation, persistent, free. Petals
five, with imbricate or contorted aestivation, often clawed, caducous, free.
Nectariferous glands extrastaminal, antesepalous, alternipetalous, fleshy
(staminodial?). Disc absent.
Androecium Stamens 5+5,
diplostemonous, antesepalous, alternipetalous, unequal in length. Filaments
connate at base, free from tepals. Anthers dorsifixed, versatile,
tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits).
Tapetum secretory, with up to duodecemnucleate cells. Staminodia absent
(alternatively as extrastaminal nectariferous glands?).
Pollen grains
Microsporogenesis simultaneous. Pollen grains dicolporate, shed as monads,
tricellular at dispersal. Exine tectate, with columellate infratectum,
striate.
Gynoecium Pistil composed of
five connate carpels. Ovary superior, deeply lobed, on short gynophore.
Stylodia five, compressed, gynobasic (inserted at base of ovary lobes), free in
lower part, connate at apex. Stigma capitate, type? Pistillodium absent.
Ovules Placentation apical.
Ovule one per carpel, anatropous, pendulous, epitropous, unitegmic,
crassinucellar. Micropyle endostomal. Integument four or five cell layers
thick. Funicle long, massive and irregularly bent. Obturator absent.
Megagametophyte tetrasporous, 16-nucleate, 13-celled, Penaea type.
Endosperm development ab initio nuclear. Endosperm haustoria? Embryogenesis?
Fruit A schizocarp with five
single-seeded nutlike mericarps, persistent central columella and accrescent
calyx.
Seeds Aril absent. Testa
thin-walled, more or less collapsed. Exotestal cells thick-walled,
non-lignified, non-fibrous, with sinuous anticlinal walls. Endotestal cells
polygonal, tanniniferous, with lignified walls. Perisperm not developed.
Endosperm sparse or absent. Suspensor absent. Embryo somewhat curved, well
differentiated, chlorophyll? Cotyledons two, foliaceous. Germination?
Cytology n = 5
DNA
Phytochemistry Flavone
methylethers, fatty acids of the type C18:3, and ethereal oils with
high content of aliphatic carbohydrates, alkaloids, bisabalone-type
sesquiterpene glycoside, myricetin, procyanidin and prodelphinidin present.
Ellagitannins and oxygenated sesquiterpenes not found.
Use Medicinal plants.
Systematics
Biebersteinia (5; B. emodii, B. heterostemon, B.
multifida, B. odora, B. orphanidis; Greece, Turkey, the
Caucasus and Iran to Central Asia, eastern Tibet and western Siberia).
Biebersteinia is sister to the
remaining Sapindales.
In Biebersteinia the pollen tube
reaches the apex of the megasporangium prior to micropyle formation
(pseudoporogamy; Yamamoto & al. 2014). The Penaea type of female
gametophyte is not known in other representatives of Sapindales (Kamelina & Konnova
1990).
Kunth in Ann. Sci. Nat. (Paris) 2: 346. Jul 1824,
nom. cons.
Balsameaceae Dumort.,
Anal. Fam. Plant.: 36, 41. 1829; Burserineae Link,
Handbuch 2: 127. 4-11 Jul 1829 [‘Burseraceae’];
Burserales Kunth in C. F. P. von Martius, Consp.
Regn. Veg.: 55. Sep-Oct 1835 [‘Burseraceae’];
Burseranae Doweld, Tent. Syst. Plant. Vasc.: xxxiv.
23 Dec 2001
Genera/species 19/>765
Distribution Tropical and
subtropical regions on both hemispheres north to California, the Himalayas and
eastern China, and south to Uruguay, South Africa and northern Australia.
Fossils Fruits and seeds have
been found in Eocene layers in North America and Eocene fruits in England.
Habit Usually dioecious or
polygamomonoecious (sometimes bisexual), evergreen or deciduous trees or shrubs
(Bursera standleyana epiphytic). Branches usually spinose. Sometimes
pachycaul. Stilt roots or plank buttresses often present. Resin often fragrant
(often like almond).
Vegetative anatomy Phellogen
usually superficial (in Santiria deeplier seated). Medulla often with
vascular strands. Vessel elements usually with simple (in Beiselia
scalariform) perforation plates; lateral pits alternate, bordered pits.
Imperforate tracheary xylem elements libriform fibres with simple or bordered
pits, usually septate. Wood rays uniseriate or multiseriate, homocellular or
heterocellular. Axial parenchyma usually paratracheal scanty vasicentric, or
absent. Tyloses often abundant. Secondary phloem sometimes stratified into hard
fibrous and soft parenchymatous layers. Sieve tube plastids S type. Nodes
usually 5:5, pentalacunar with five leaf traces (sometimes multilacunar).
Phloem with vertical intercellular secretory ducts and surrounded by light
sinuous sclerenchymatous band. Schizogenous secretory cavities and canals with
white or uncoloured resinous exudate, aromatic (often almond-scented).
Sclereids present in stem cortex. Parenchyma often with mucilage cells. Wood
often silicified or with silica grains. Prismatic calciumoxalate crystals
frequent.
Trichomes Hairs unicellular or
multicellular, uniseriate, usually simple (sometimes furcate or stellate);
helical glands (spirally twisted, uniseriate glandular hairs) abundant.
Leaves Alternate (spiral),
usually imparipinnate (sometimes unifoliolate, rarely bipinnate or seemingly
trifoliolate), lobed, with conduplicate? ptyxis. Stipules usually absent
(rarely petiolar or cauline, laciniate to entire, possibly in reality reduced
basal leaflets); leaf sheath absent. Colleters often abundant. Stipule-like
leaflets or colleters often present. Petiole and petiolules often pulvinate.
Petiole vascular bundle transection usually annular (in Commiphora
arcuate); petiole sometimes with medullary rays. Leaf base often swollen,
adaxially concave. Venation pinnate. Stomata anomocytic. Cuticular wax
crystalloids? Domatia as pits or hair tufts, or absent. Epidermis usually with
mucilage cells, sometimes with pellucid dots. Mesophyll with or without
mucilaginous idioblasts. Secretory cavities absent. Leaflet margins serrate or
entire.
Inflorescence Axillary,
thyrsoid, often raceme-, spike- or fascicle-like.
Flowers Actinomorphic, small.
Hypanthium present in, i.a., Garuga. Hypogyny. Sepals (three or) four
or five (to seven), with induplicate-valvate or imbricate (sometimes open)
aestivation, usually caducous (sometimes accrescent in fruit), usually connate
at base. Petals (three or) four or five (to seven), usually with
induplicate-valvate (in Boswellia and Canarium sometimes
imbricate) aestivation, usually free (sometimes connate at base; sometimes
absent). Nectariferous disc usually intrastaminal (in Aucoumea and
Triomma extrastaminal), usually annular or quinquepartite (sometimes
absent or as ovariodisc).
Androecium Stamens usually
3+3, 4+4 or 5+5, obdiplostemonous (sometimes three to five, haplostemonous,
alternisepalous, with antepetalous whorl absent; rarely antesepalous).
Filaments usually free from each other (in Canarium often more or less
connate), free from tepals. Anthers dorsifixed or basifixed, non-versatile or
somewhat versatile, tetrasporangiate, introrse or latrorse, longicidal
(dehiscing by longitudinal slits); connective sometimes slightly prolonged
apically. Tapetum secretory, with often binucleate cells. Female flowers often
with staminodia.
Pollen grains
Microsporogenesis simultaneous. Pollen grains 3(–6)-colporate, shed as
monads, bicellular at dispersal. Exine semitectate, with columellate
infratectum, reticulate, microreticulate, often spinulate, sometimes striate or
psilate.
Gynoecium Pistil composed of
(two or) three to five (nine to twelve in Beiselia) connate carpels
(odd carpel in Amyris abaxial); symplicate zone of carpel well
developed; occasionally only one carpel developing. Ovary usually superior
(when hypanthium present then semi-inferior), (bilocular or) trilocular to
quinquelocular (novem- to duodecemlocular in Beiselia); most locules
usually reduced and sterile. Style usually single, simple (stylodia sometimes
free at apex), usually short. Stigma capitate or (bilobate or) trilobate to
quinquelobate (novem- to duodecemlobate in Beiselia), with stigmatic
head formed by postgenital fusion, non-papillate?, type? Male flowers often
with pistillodium.
Ovules Placentation
apical-axile. Ovules (one or) two per carpel, usually anatropous or
hemianatropous to campylotropous (rarely orthotropous), collateral, pendulous,
epitropous (in Beiselia superposed), usually bitegmic (rarely
unitegmic), crassinucellar. Micropyle usually endostomal (sometimes bistomal,
Z-shaped, zig-zag). Outer integument approx. four cell layers thick. Inner
integument approx. four cell layers thick. Obturator absent. Megasporangium six
to twelve cell layers thick. Nucellar cap massive. Megagametophyte monosporous,
Polygonum type. Endosperm development ab initio nuclear. Endosperm
haustoria? Embryogenesis?
Fruit Usually a drupe with one
to five single-seeded pyrenes, or one multilocular pyrene (often with only one
locule developed); pyrene with valves (rarely winged); pseudo-aril sometimes
present (formed from pericarp in, e.g., Bursera and
Commiphora); mesocarp often dehiscing along loculicidal radius (rarely
a septifragal pseudocapsule with a columella; in Beiselia,
Boswellia and Triomma a dry schizocarp). Endocarp often
consisting of unorientated sclerified cells and crystalliferous cells.
Seeds Aril absent. Seed coat
testal. Exotesta sometimes with unthickened radially elongate cells. Endotesta
lignified, tracheidal. Tegmen? Perisperm not developed. Endosperm very sparse
or absent. Embryo small, usually straight (rarely curved), well differentiated,
with hemicellulose, oil and proteins, with chlorophyll. Cotyledons two, usually
entire (sometimes palmately lobed, sometimes folded, rarely transversely folded
twice). Germination phanerocotylar or cryptocotylar.
Cytology n = 11–13,
22–24
DNA Mitochondrial
coxI intron present in Bursera.
Phytochemistry Flavonols
(kaempferol, quercetin), biflavones, bitter-tasting triterpenoid substances
(triterpenes with ursane and oleanane components), oleoresins (with mono- and
bicyclic monoterpenes), ellagic acid, alkaloids, ethereal oils, cyanidin
pentacyclic terpene saponins, and polyacetylenes present.
Use Aromatic substances (myrrh
and frankincense from Commiphora, Boswellia etc.), medicinal
plants, timber, carpentry, varnish (Bursera).
Systematics Burseraceae are sister-group to
Anacardiaceae.
Beiselia is sister to the remaining
Burseraceae (Clarkson
2002; Thulin & al. 2008).
Beiselieae Thulin,
Beier et Razafim. in Nord. J. Bot. 26: 226. 22 Dec 2008
1/1. Beiselia (1; B.
mexicana; Michoacán in Mexico). – Vessel elements sometimes with
scalariform perforation plates. Leaf bases? persistent and provided with spine.
Pistil composed of nine to twelve connate carpels. Ovary deeply furrowed.
Ovules superposed. Fruit a capsule with pericarp dehiscing septifragally
separately from endocarp; mericarps winged at apex; columella provided with
deep flanges. Cotyledons simple. n = ?
Burseroideae Arn.,
Botany: 106. 9 Mar 1832 [‘Burseraceae’]
18/>765.
Protieae Marchand in Adansonia 8: 62. Oct 1867.
Crepidospermum (6; C. atlanticum, C. cuneifolium,
C. goudotianum, C. multijugum, C. prancei, C.
rhoifolium; northern South America), Tetragastris (9; Central
America, the West Indies, tropical South America), ’Protium’
(>180; Madagascar, Mauritius, tropical Asia from Pakistan to New Guinea,
tropical America; polyphyletic). – Bursereae DC.,
Prodr. 2: 75. Nov (med.) 1825 [’Burseraceae’].
Aucoumea (1; A. klaineana; Central Africa); Bursera
(c 100; southwestern United States, Mexico, Central America, the West Indies,
northern South America), Commiphora
(c 190; tropical and subtropical Africa, Madagascar, the Arabian Peninsula to
India and Sri Lanka, Vietnam, tropical South America). –
Garugeae Marchand in Adansonia 8: 66. Nov 1867.
Garuga (4; G. floribunda, G. forrestii, G.
pierrei, G. pinnata; the Himalayas, Southeast Asia, Malesia to
Melanesia and tropical Australia), ’Boswellia’ (c 20; semiarid
regions in tropical Africa, Madagascar, Socotra, drier parts of tropical and
subtropical Asia; polyphyletic). – Canarieae Engl.
in H. G. A. Engler et C. G. O. Drude, Veg. Erde 9(3, 1): 780. 22-29 Jul
1915.Triomma (1; T. malaccensis; Bihar in India, West
Malesia), Ambilobea (1; A. madagascariensis; northernmost
Madagascar), Canarium (c 120; tropical Africa, Madagascar, islands in
the Indian Ocean, tropical Asia to southern China and New Guinea, northern and
eastern Australia, Fiji, Micronesia, Tonga, Samoa), Dacryodes (c 77;
tropical regions on both hemispheres), Santiria (22–24; West Africa,
Malesia), Trattinnickia (13–14; Costa Rica to Brazil and Bolivia),
Rosselia (1; R. bracteata; Rossel Island in the Louisiade
Archipelago southeast of New Guinea). – Unplaced
Burseroideae Haplolobus (c 16; Borneo, Sulawesi, the
Moluccas, New Guinea to Fiji and Samoa, with their highest diversity on New
Guinea), Pseudodacryodes (1; P. leonardiana; Congo),
Scutinanthe (2; S. brunnea, S. engleri; Sri Lanka,
West Malesia to Sulawesi). – Pantropical, with their highest diversity in
tropical Asia and tropical America. Characters principally as for Burseraceae. – The phylogenetic
relationship among the four clades are not resolved.
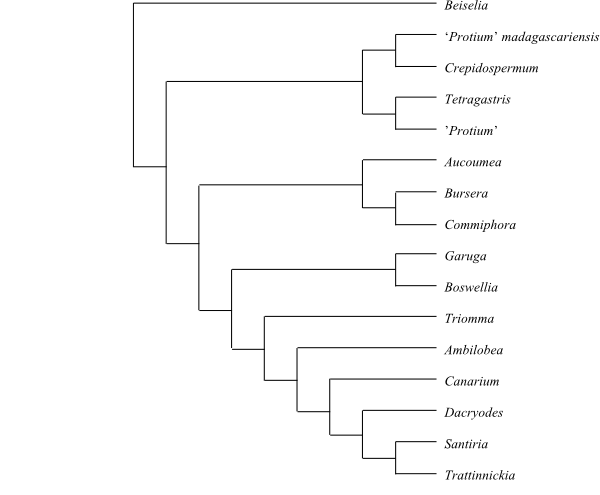 |
Bayesian majority rule consensus tree of
Burseraceae based
on DNA sequence data (Thulin & al. 2008).
|
Takhtajan, Sist. Filog. Cvetk. Rast. [Syst.
Phylog. Magnolioph.]: 321. 4 Feb 1967
Genera/species 1/6
Distribution Tropical and
subtropical East and Southeast Africa, Angola?, Madagascar.
Fossils Unknown.
Habit Morphologically
bisexual, functionally monoecious, polygamomonoecious or dioecious, usually
deciduous trees or shrubs. Heartwood often honey-scented.
Vegetative anatomy Phellogen?
Vessel elements with simple perforation plates; lateral pits alternate, simple
or bordered pits? Imperforate tracheary xylem elements libriform fibres with
simple or bordered pits, septate. Wood rays multiseriate, heterocellular. Axial
parenchyma paratracheal vasicentric. Tyloses frequent. Sieve tube plastids S
type. Nodes usually 3:3?, trilacunar with three? leaf traces. Parenchyma
without secretory cavities. Crystals?
Trichomes Hairs unicellular or
multicellular, uniseriate or multiseriate; glandular hairs with multiseriate
stalk and multicellular head.
Leaves Alternate (spiral),
imparipinnate, leaflets entire, with ? ptyxis. Stipules and leaf sheath absent.
Petiole vascular bundle transection annular; petiole with medullary bundles.
Venation pinnate. Stomata anomocytic or paracytic? Cuticular waxes absent.
Epidermis sometimes with mucilaginous idioblasts. Leaflet margins serrate.
Inflorescence Axillary,
compound thyrsoid consisting of dichasial and monochasial cymes, changing
between functionally female and functionally male flowers from one branching
order to next.
Flowers Actinomorphic, small.
Hypogyny. Sepals (three or) four (to six), decussate, usually with valvate
(sometimes imbricate quincuncial) and later open aestivation, free or connate
at base. Petals (three or) four (to six), with open aestivation at base and
imbricate aestivation in upper part, free. Nectariferous disc intrastaminal,
annular, angular, wide, fleshy, well developed in male flowers, reduced in
female flowers.
Androecium Stamens (three or)
four (to six), haplostemonous, antesepalous, alternipetalous. Filaments
conical, free from each other and from tepals. Anthers dorsobasifixed,
non-versatile, tetrasporangiate, introrse, longicidal (dehiscing by
longitudinal slits). Tapetum secretory. Female flowers with staminodia.
Pollen grains
Microsporogenesis simultaneous. Pollen grains trisyncolporate, shed as monads,
bicellular at dispersal. Exine semitectate, with columellate infratectum,
reticulate.
Gynoecium Pistil composed of
four (or eight) antepetalous connate carpels. Ovary superior, quadrilocular (or
octalocular), lobate. Gynophore short, glandular. Stylodia four (or eight),
separate, tightly connivent in lower part, connate in upper part, upright,
finally radiating. Stigmas four, short, connate, capitate, oblique, flattened,
papillate, Wet type. Male flowers with pistillodium.
Ovules Placentation apical to
axile. Ovules one (or two) per carpel, epitropous (longitudinal direction of
ovule opposite involution direction of carpel) and somewhat campylotropous,
pendulous, bitegmic, crassinucellar. Micropyle bistomal, Z-shaped (zig-zag),
elongate (outer integument longer than inner integument). Outer integument two
or three cell layers thick. Inner integument three or four cell layers thick.
Megagametophyte monosporous, Polygonum type. Endosperm development ab
initio nuclear. Endosperm haustoria? Embryogenesis?
Fruit A lignified schizocarp
with four (or seven or eight) nutlike mericarps, with ridges and persistent
columellar strands. Endocarp leathery, strongly lignified, with crystalliferous
cells and cells containing elongate sclereids. Mericarps single-seeded, pendant
from apex of central columella (carpophore) and dorsally with basal remnants of
stylodium recurved over apex.
Seeds Aril absent. Testa thin.
Exotesta? Endotesta? Tegmen? Perisperm not developed. Endosperm sparse or
absent. Embryo somewhat curved, well differentiated. chlorophyll? Cotyledons
two. Germination?
Cytology n = ?
DNA
Phytochemistry Ellagic acid,
lignans and nor-carotenoids ((+)-dihydrodehydrodiconiferyl alcohol;
(+)-lyoniresinol; (-)-ent-isolariciresinol;
(-)-4′,9,9′-trihydroxy-3′-methoxy-3.O.8′,4.O.7′-neolignan;
(+)-de-O-methyllasiodiplodin; (+)-(6S,7E,9R)-blumenol A;
(+)-(6S,7E)-dehydrovomifoliol;
(+)-4-ethanone-3,4-dihydro-6,8-dihydroxy-5-methylisocoumarin;
(+)-(2R,3R)-7-O-methylaromadendrin; etc.) present. Quassinoids and limonoids
not found.
Use Timber, carpentry,
medicinal plants.
Systematics Kirkia (6; K.
acuminata, K. burgeri, K. dewinteri, K.
leandrii, K. tenuifolia, K. wilmsii; tropical and
subtropical East and Southeast Africa from Ethiopia to Transvaal, Angola?,
Madagascar).
Kirkia is sister to [Anacardiaceae+Burseraceae].
de Jussieu, Gen. Plant.: 263. 4 Aug 1789
[’Meliae’], nom. cons.
Cedrelaceae R. Br. in
M. Flinders, Voy. Terra Austr. 2: 595, 596. 19 Jul 1814
[’Cedreleae’]; Meliales Juss. ex Bercht.
et J. Presl, Přir. Rostlin: 219. Jan-Apr 1820 [‘Meliaceae’];
Swieteniales Bercht. et J. Presl, Přir. Rostlin:
219. Jan-Apr 1820 [‘Swieteniae’];
Swieteniaceae E. D. M. Kirchn., Schul-Bot.: 415.
13-20 Oct 1831; Cedrelales R. Br. in C. F. P. von
Martius, Consp. Regn. Veg.: 61. Sep-Oct 1835 [‘Cedreleae’];
Aitoniaceae R. A. Dyer, Gen. S. Afr. Fl. Pl. 1: 298.
1975, nom. illeg.
Genera/species 48/650–665
Distribution Tropical and
subtropical lowland areas, mainly southern and southeastern Asia; few species
in warm-temperate regions; Xylocarpus consists of mangrove trees.
Fossils Eocene and Oligocene
fruits of Meliaceae are
found in North America and Eocene fruits in England.
Habit Bisexual, monoecious,
polygamomonoecious or dioecious, evergreen trees and shrubs (in
Munronia suffrutices, in Naregamia perennial herbs). Bark
often with a bitter taste, often with a strong garlic-like or sweet smell,
etc.
Vegetative anatomy Phellogen
ab initio superficial. Vessel elements with simple perforation plates; lateral
pits alternate, bordered pits. Imperforate tracheary xylem elements fibre
tracheids or libriform fibres with simple or bordered pits, septate or
non-septate. Wood rays uniseriate or multiseriate, homocellular or
heterocellular. Axial parenchyma apotracheal diffuse, or paratracheal scanty,
aliform, lozenge-aliform, winged-aliform, confluent, vasicentric, or banded.
Wood elements often storied. Secondary phloem sometimes stratified into hard
fibrous and soft parenchymatous layers. Sieve tube plastids S type (in, e.g.,
Azadirachta and Melia Ps type with protein crystalloids and
starch). Nodes usually 5:5, pentalacunar with five leaf traces (sometimes 3:3,
trilacunar with three traces). Secretory cells with resins and ethereal oils
present in leaves, cortex and medulla (rarely with secretory cavities).
Laticifers with white latex present in heartwood of some species. Prismatic
calciumoxalate crystals frequent.
Trichomes Hairs unicellular or
multicellular, simple, furcate or stellate, sometimes lepidote;
peltate-lepidote glandular hairs sometimes present.
Leaves Usually alternate
(spiral; in Turraea distichous; in Capuronianthus opposite),
usually paripinnate or imparipinnate (rarely trifoliolate or bipinnate; in
Nymania, Turraea and Vavaea simple [unifoliolate?]),
leaflets entire or lobed, with conduplicate? ptyxis. Stipules and leaf sheath
absent. Petiole vascular bundle transection annular? Leaf base swollen,
vertically elongate. Petiolules usually not articulated (in Walsura
often pulvinate). Venation pinnate. Stomata anomocytic. Cuticular wax
crystalloids? Domatia as pits, pockets or hair tufts. Epidermis with or without
mucilaginous idioblasts. Mesophyll with or without sclerenchymatous idioblasts.
Secretory cells with resins and ethereal oils. Leaflet margins (or margin)
usually entire (sometimes serrate). Extrafloral nectaries sometimes present on
petiole and abaxial side of lamina.
Inflorescence Usually axillary
(sometimes terminal), raceme, spike or panicle with thyrsoid partial
inflorescences (flowers sometimes solitary or pairwise, axillary; rarely
epiphylly).
Flowers Usually actinomorphic
(rarely slightly zygomorphic). Hypogyny. Sepals (two to) three to five (to
eight), usually with imbricate (rarely valvate or open) aestivation, usually
entirely or partially connate. Petals three to seven (to 14), usually with
imbricate or contorted (rarely valvate) aestivation, usually in one whorl
(rarely two whorls), usually free (sometimes connate below). Nectariferous disc
intrastaminal, annular, or absent.
Androecium Stamens usually
four to ten (sometimes three or up to 20 or more, rarely up to c. 30), twice as
many as petals, diplostemonous, or three to six, haplostemonous. Filaments
usually connate into tube around pistil (staminal tube sometimes very long
[rarely up to 14 cm!], sometimes corolla-like), often also adnate to petals
(filaments in Vavaea, Walsura, Cedrela and
Toona secondarily free or almost free). Anthers dorsifixed, versatile,
tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits).
Tapetum secretory, with binucleate to quadrinucleate (rarely up to
decemnucleate) cells. Staminodia few to numerous or absent; female flowers
often with staminodia. Secondary pollen display present in, e.g.,
Vavaea.
Pollen grains
Microsporogenesis simultaneous. Pollen grains (2–)3–5(–6)-colporate
(rarely porate), usually shed as monads (rarely tetrads), usually bicellular
(rarely tricellular) at dispersal. Exine tectate, with columellate infratectum,
rugulate, scabrate or psilate.
Gynoecium Pistil composed of
usually two to six (rarely one or in Turraea up to 20) antepetalous
connate carpels, postgenitally fused. Ovary superior, usually bilocular to
sexalocular (rarely unilocular or up to 20-locular). Style single, simple, or
absent. Stigma usually capitate, clavate or peltate (sometimes punctate or
lobate), usually large, papillate, Wet type. Male flowers often with
pistillodium.
Ovules Placentation usually
axile and ovary multilocular (rarely parietal and ovary unilocular). Ovules
usually two (sometimes one or more than two to numerous) per carpel,
anatropous, campylotropous or orthotropous (rarely amphitropous), usually
pendulous, epitropous, bitegmic, crassinucellar. Micropyle usually endostomal
(rarely bistomal or exostomal). Outer integument two to five cell layers thick.
Inner integument two to four cell layers thick. Placental obturator often
present. Parietal tissue three to nine (to 18) cell layers thick. Archespore
often multicellular. Nucellar cap present. Megagametophyte monosporous,
Polygonum type. Synergids sometimes with a filiform apparatus.
Endosperm development ab initio nuclear. Endosperm haustoria? Embryogenesis
onagrad.
Fruit A loculicidal or
septicidal capsule, a berry or drupe (rarely a nutlet).
Seeds Seeds often
pachychalazal, often with chalazal aril and/or sarcotesta (Melioideae)
or with suberinized/corky outer layer, or seeds winged and inserted at woody
central columella (Cedreloideae). Seed coat usually exotegmic
(sometimes reduced and undifferentiated). Testa vascularized, usually
multiplicative. Exotesta unspecialized. Endotesta with calciumoxalate crystals.
Tegmen usually multiplicative. Exotegmen usually fibrous. Endotegmen
unspecialized? Perisperm not developed. Endosperm usually absent (rarely
copious). Embryo straight or curved, elongate, well differentiated, with or
without chlorophyll. Cotyledons two. Germination phanerocotylar or
cryptocotylar.
Cytology n = 10–14
(sometimes more); x = 6 or 7
DNA Mitochondrial
coxI intron present.
Phytochemistry Flavonols
(kaempferol, quercetin), flavones, cyanidin, tetracyclic triterpenes,
tetranortriperpenes, pentanortriterpenes, limonoids and meliacins and other
bitter-tasting triterpenoid substances (turraeanthin, melianone, azadirone,
homoazadirone, azadiradione, gedunin, grandifolin, khivorin, khivol,
anthothecol, andirobin, methyl angolensate, nimbin, salannin, mexicanolide,
swietenine, astrotrichilin, etc.), tannins, proanthocyanidins, alkaloids,
triterpene saponins, polyacetylenes, coumarins, and ethereal oils present.
Ellagic acid and cyanogenic compounds not found.
Use Ornamental plants, fruits
(Lansium domesticum, Sandoricum koetjape), seed oils,
medicinal plants, cosmetics, timber (e.g. Swietenia, Khaya),
carpentries.
Systematics (under
construction) Meliaceae are
sister to Simaroubaceae.
The subdivision below follows Muellner &
al. 2003, and Muellner, Samuel & al. 2008. Two main clades can be
discerned, corresponding to Melioideae and Cedreloideae,
respectively.
Melioideae Arn.,
Botany: 103. 9 Mar 1832 [‘Melieae’]
34/580–585. Azadirachta (2;
A. excelsa, A. indica; tropical Asia), Melia (2;
M. volkensii: southern tropical Africa; M. azedarach:
tropical Asia to New Guinea), Astrotrichilia (c 12; Madagascar);
Quivisianthe (1–2; Q. papinae; Madagascar),
Walsura (16; India and Sri Lanka to New Guinea); Sandoricum
(4; S. bamboli, S. beccarianum, S. dubia, S.
koetjape; Malesia; three species endemic to Borneo); Munronia (3;
M. humilis, M. pinnata, M. unifoliolata; subtropical
China and tropical Asia to Timor), Lepidotrichilia (4; L.
ambrensis, L. convallariiodora, L. sambiranensis, L.
volkensii; tropical East Africa, Madagascar), Vavaea (4?; V.
amicorum, V. australiana, V. bantamensis, V.
pauciflora; Sumatra and the Philippines to northern Australia, Fiji, the
Caroline Islands and Tonga), Pseudoclausena (1; P.
chrysogyne; Indochina to New Guinea), Cipadessa (1; C.
baccifera; tropical and subtropical Asia from India, Sri Lanka and Nepal
to southern China, Indochina and Central Malesia), Ekebergia (4;
E. benguelensis, E. capensis, E. pterophylla, E.
pumila; tropical and southern Africa), Trichilia (c 95; tropical
Africa, Madagascar, tropical America), Owenia (5; O. acidula,
O. cepiodora, O. reliqua, O. reticulata, O.
vernicosa; northern, central and eastern Australia), Malleastrum
(23; Madagascar, the Comoros, Aldabra), Pterorhachis (2; P.
letestui, P. zenkeri; Central Africa), Nymania
(1; N. capensis; southern Namibia, Northern, Western and Eastern
Cape), Calodecaryia (2; C. crassifolia, C.
pauciflora; Madagascar), Humbertioturraea (10; Madagascar), Turraea (c
60; tropical and southern Africa, Madagascar, the Mascarene Islands, Socotra,
tropical Asia to northern and eastern Australia), Synoum (1; S.
glandulosum; eastern Queensland, eastern New South Wales),
Anthocarapa (1; A. nitidula; northeastern and southeastern
Queensland, northeastern New South Wales, New Guinea to New Caledonia and
Rotuma), Heckeldora (7; H. acuminata, H.
angustifolia, H. klainei, H. latifolia, H.
mangenotiana, H. staudtii, H. zenkeri; tropical West and
Central Africa), Turraeanthus (3; T. africana, T.
longipes, T. mannii; western and central tropical Africa),
Guarea (c 40; tropical Africa, Central America, tropical South
America), Ruagea (12; Guatemala to Peru), ’Dysoxylum’ (c
80; India and Sri Lanka to southern China, Indochina, Malesia to New Guinea,
Christmas Island, islands in southern and southwestern Pacific to northern and
eastern Australia, New Caledonia, Norfolk Island, Lord Howe, New Zealand, Tonga
to Niue; non-monophyletic?), Chisocheton (53–55; Assam, southern
China, Southeast Asia, Malesia to northeastern Queensland and Vanuatu),
Cabralea (1; C. canjerana; Central America, tropical South
America), Sphaerosacme (1; S. decandra; the Himalayas),
'Aglaia' (c 120; tropical Asia to islands in western Pacific;
paraphyletic; incl. Aphanamixis?), Aphanamixis (3; A.
borneensis, A. polystachya, A. sumatrana; tropical Asia
to Solomon Islands; in Aglaia?), Lansium (1; L.
parasiticum; Malesia; in Aglaia?), Reinwardtiodendron
(7; R. anamalaiense, R. celebicum, R. cinereum,
R. humile, R. kinabaluense, R. kostermansii, R.
merrillii; Southeast Asia, West and Central Malesia; in Aglaia?).
– Pantropical, with their largest diversity in tropical regions in the Old
World. Trees or shrubs. Buds naked. Stigma capitate. Ovules usually one to
three (rarely numerous) per carpel, epitropous. Fruit usually a loculicidal
capsule (rarely berry, drupe or nut). Seeds usually without wings (in
Quivisianthe winged), usually with arillode (in Naregamia
funicular aril) or sarcotesta. n = 8, 11, 12, 14, 15, 18 up to 140. –
Guarea in tropical America and Chisocheton in Malesia have
leaves with unlimited growth. The clade [Melia+Azadirachta]
is sister-group to the remaining Melioideae and has several unique
anatomical features, such as clusters of minute vessels with spiral wall
thickening (Muellner, Samuel & al. 2008). The position of
Ekebergia is doubtful. In the rbcL tree in Muellner, Samuel
& al. (2008), it is recovered as sister to Quivisianthe,
Sandoricum being sister to the other two genera. In the Bayesian ITS
nrDNA tree, Ekebergia is nested inside the
Turraeeae/Trichilieae clade and sister to Cipadessa,
whereas Quivisianthe and Walsura form a subbasal clade in
Melioideae.
Cedreloideae Arn.,
Botany: 103. 9 Mar 1832 [‘Cedreleae’]
14/70–80. Chukrasia (1;
C. tabularis; India, Sri Lanka and southern China to West Malesia),
Schmardaea (1; S. microphylla; the Andes from Venezuela to
Peru), Neobeguea (3; N. ankaranensis, N. leandriana,
N. mahafaliensis; Madagascar), Cedrela (c 17; southern
Mexico, Central America, the West Indies, tropical South America),
Toona (5; T. australis, T. ciliata, T.
fargesii, T. sinensis, T. sureni; eastern Pakistan to
southern China, Southeast Asia to eastern Queensland and eastern New South
Wales), Capuronianthus (2; C. mahafalensis, C.
vohemarensis; Madagascar; in Lovoa?), Lovoa (3; L.
swynnertonii, L. tomentosa, L. trichilioides; tropical
Africa; incl. Capuronianthus?), Carapa (10–20; tropical
Africa, Central America, the West Indies, tropical South America),
Xylocarpus (3; X. granatum, X. moluccensis, X.
rumphii; mangroves in East Africa to Tonga), Khaya (6; K.
anthotheca, K. grandifoliola, K. ivorensis, K.
madagascariensis, K. nyasica, K. senegalensis; tropical
Africa, Madagascar), Swietenia (5; S. aubrevilleana, S.
humilis, S. krukovii, S. macrophylla, S.
mahagoni; Florida, Mexico, Central America, the West Indies, tropical
South America), Entandrophragma (11; tropical Africa),
Pseudocedrela (1; P. kotschyi; tropical Africa),
Soymida (1; S. febrifuga; India, Sri Lanka). – Pantropical,
with their highest diversity in tropical regions in tropical Africa and
Madagascar. Buds usually perulate (in Capuronianthus naked). Stylar
apex usually discoid (rarely capitate). Ovules usually three to numerous (in
Capuronianthus two) per carpel, collateral. Fruit a septifragal
capsule with caducous valves, persisting central columella and winged seeds, or
rudimentary columella and seeds with massive woody or corky testa. n = 13, 18,
23, 25, 26, 28.
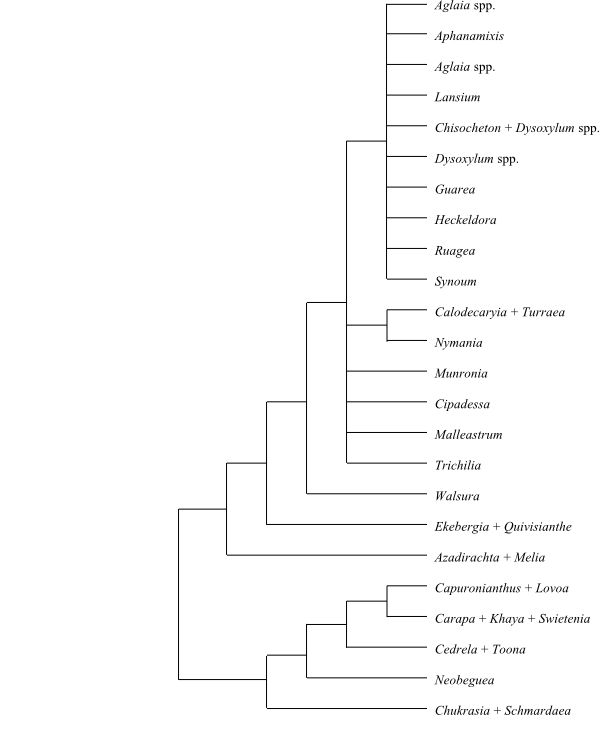 |
Cladogram of Meliaceae based on DNA
sequence data (Muellner & al. 2003).
|
Lindley, Intr. Nat. Syst. Bot.: 149. 27 Sep
1830
Nitrariales Bercht.
et J. Presl, Přir. Rostlin: 238. Jan-Apr 1820 [‘Nitrariae’];
Tetradiclidaceae (Engl.) Takht., Florist. Reg. World:
333. 27 Apr 1986; Peganaceae (Engl.) Tiegh. ex
Takht., Sist. Magnoliof. [Systema Magnoliophytorum]: 178. 24 Jun 1987
Genera/species 4/16–17
Distribution North Africa,
southern and southeastern Europe to Siberia and Mandshuria, Afghanistan,
Central Asia, the Arabian Peninsula, southern Australia, southeastern Texas,
northern Mexico.
Fossils Unknown.
Habit Bisexual, deciduous
shrubs (Malacocarpus, Nitraria) or perennial
(Peganum) or annual (Tetradiclis) herbs. Often xerophytic.
Often succulent. Sometimes spiny.
Vegetative anatomy Mycorrhiza
absent in at least Peganum. Phellogen ab initio inner-cortical.
Primary vascular tissue cylinder, without separate vascular bundles. Vessel
elements with simple perforation plates; lateral pits usually alternate,
bordered pits. Imperforate tracheary xylem elements libriform fibres usually
with bordered (sometimes simple) pits, non-septate (in Nitraria also
vasicentric tracheids). Wood rays uniseriate or multiseriate, homocellular or
heterocellular. Axial parenchyma usually apotracheal (at least in
Nitraria paratracheal aliform-confluent, vasicentric, or banded). Wood
elements and/or parenchyma storied. Sieve tube plastids S type. Nodes 3:3,
trilacunar with three leaf traces. Secretory cavities and mucilage cells
present in Nitraria. Heartwood in Nitraria with gum-like
substances. Prismatic calciumoxalate crystals frequent.
Trichomes Hairs unicellular or
multicellular, uniseriate and complex capitate hairs (not in
Nitraria); uniseriate glandular hairs present in Peganum.
Leaves Usually alternate
(spiral; in Nitraria often two or three per node; in Peganum
often two per node; two lowermost leaf pairs in leaf rosette of
Tetradiclis opposite), simple or pinnately or palmately compound,
entire or pinnately or palmately lobed, often carnose, sometimes coriaceous,
with ? ptyxis. Stipules small, bristle-like or foliaceous (in Peganum
often also divided), cauline or intrapetiolar, persistent or caducous (absent
in Tetradiclis); leaf sheath absent. Petiole vascular bundle
transection arcuate; petiole with wing bundles. Venation pinnate or palmate
(leaves sometimes one-veined). Stomata usually anomocytic (sometimes paracytic
or actinocytic). Cuticular waxes usually absent (wax crystalloids rarely as
platelets or rodlets). Epidermis with or without mucilaginous idioblasts.
Mesophyll in Nitraria sometimes with mucilaginous idioblasts and
occasionally with sclerenchymatous idioblasts. Calciumoxalate as raphides
(Peganum, Malacocarpus), druses and solitary prismatic
crystals. Leaflet margins usually entire (rarely serrate or lobate).
Inflorescence Terminal or
axillary, cymose (in Nitraria scorpioid), or flowers solitary
axillary. Floral prophyls (bracteoles) absent in Nitraria.
Flowers Actinomorphic.
Hypanthium present. Hypogyny. Sepals (three or) four or five (in
Tetradiclis usually four), with usually imbricate (in Peganum
valvate) aestivation, sometimes pinnately compound, often persistent, free or
more or less connate (Nitraria, Tetradiclis). Petals (three
or) four or five (in Tetradiclis usually four), with imbricate,
contorted or (induplicate-)valvate aestivation, free. Nectariferous disc
extrastaminal or intrastaminal, annular (in Nitraria and
Peganum as antepetalous nectaries; nectary in Tetradiclis
absent).
Androecium Stamens 5+5, 4+4+4,
or 15 in antesepalous fascicles of three, or in antepetalous pairs
(occasionally five; in Peganum sometimes paired, antepetalous; in
Tetradiclis usually four, antesepalous), obdiplostemonous (sometimes
haplostemonous). Filaments free from each other and from tepals, in
Nitraria inserted at hypanthium, often widened at base. Anthers
dorsifixed, versatile, tetrasporangiate, introrse, longicidal (dehiscing by
longitudinal slits). Tapetum secretory, with binucleate cells (in
Peganum finally often polyploid; in Tetradiclis finally
modified into false plasmodium). Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grans 3(–4)-colporate, shed as monads,
bicellular at dispersal. Exine tectate or semitectate, with columellate
infratectum, reticulate (sometimes reticulate-rugulate) or striate.
Gynoecium Pistil composed of
two to four (or six) connate carpels (carpels in Nitraria and
Tetradiclis alternisepalous, antepetalous; each of usually four
carpels in Tetradiclis divided into three partitions). Ovary superior,
bilocular to quadrilocular, in Tetradiclis on gynophore. Style single,
simple, usually terminal (in Nitraria and Tetradiclis basal),
sometimes hollow. Stigma usually two- to four-keeled (rarely six-keeled), as
commissural compital lines down upper part of style (stigma in
Tetradiclis clavate, tetrasulcate, with four short decurrent double
rows of papillae, stigmatic ridges running down widened apical part),
papillate, Dry type. Pistillodium absent.
Ovules Placentation axile to
apical (in Nitraria subapical; ovules in Tetradiclis
pendulous from free basal-central placenta). Ovules one (Nitraria) or
six (Tetradiclis: four ovules in central locellus and one ovule in
each of two lateral locelli) or numerous (Peganum,
Malacocarpus) per carpel, usually anatropous (in Tetradiclis
hemianatropous), pendulous, apotropous or epitropous, bitegmic, crassinucellar.
Micropyle bistomal, Z-shaped (zig-zag). Outer integument two to four cell
layers thick, in Tetradiclis with mucilage cells. Inner integument two
or three, or four to seven cell layers thick. Funicular-placental obturator
present in Malacocarpus. Parietal tissue approx. three cell layers
thick. Endothelium absent. Archespore in Peganum multicellular.
Megagametophyte monosporous, Polygonum type. Endosperm development ab
initio nuclear. Endosperm haustoria? Embryogenesis solanad
(Nitraria).
Fruit A loculicidal capsule
(Peganum, Tetradiclis), a single-seeded drupe with lignified
scleromesocarp (Nitraria), or a berry (Malacocarpus,
Peganum). In Tetradiclis only seeds of central locelli
liberated as capsule opens, seeds in lateral locelli liberated later.
Seeds Aril absent. Testa in
Peganum and Malacocarpus spongy, mucilaginous, in
Tetradiclis thin, in Peganum multiplicative. Exotesta and
endotesta (short) palisade; exotestal cells often enlarged (cells in
Tetradiclis inflated), sometimes mucilaginous; endotesta often
palisade. Exotegmen crushed. Endotegmic cells tangentially elongate; endotegmen
in Peganum almost fibriform, with lignified cells. Perisperm not
developed. Endosperm sparse to copious, oily, or absent. Embryo straight or
curved, well differentiated, sometimes with chlorophyll. Cotyledons two.
Germination phanerocotylar.
Cytology n = 7
(Tetradiclis), 12, 13 (Peganum, Malacocarpus,
Nitraria), 30 (Nitraria)
DNA
Phytochemistry Flavonols
(kaempferol, quercetin), flavonoids (apigenin, rutin, kaempferol,
isorhamnetin-3-rhamnogalactoside in Nitraria), β-carboline alkaloids (harmine,
harmaline, harmalol) and pyrroloquinazoline alkaloids (vasicine, vasicinone,
desoxyvasicinone, etc.) present. Nitrarin and carbolin present in Nitraria, in
Peganum also peganol and peganine; anabasin D in Malacocarpus. Mustard oils
reported from some species. Proanthocyanidins and cyanogenic compounds not
found. Ethereal oils?
Use Dyeing substances (turkish
red) from seeds used for dyeing hats (tarboosh), medicinal plants and narcotics
(Peganum harmala).
Systematics Nitraria
(9–10; salt deserts, salt marshes and coastal sand dunes in Southeast Europe,
North Africa, Southwest and Central Asia and China, one species, N.
billardieri, in southern Australia), Tetradiclis (1; T.
tenella; southeastern Russia and eastern Mediterranean to Central Asia),
Malacocarpus (1; M. crithmifolius; Central Asia), Peganum
(5; P. harmala: the Mediterranean, southwestern Asia to India; P.
mexicanum: Mexico; P. nigellastrum: Mongolia, China; P.
rothschildianum: Tunisia; P. texanum: southeastern Texas,
northern Mexico).
The sister-group relationship of Nitrariaceae is not resolved,
although there is weak support for Nitrariaceae being sister-group
to the remaining Sapindales
except Biebersteinia.
A possible topology, based on morphological and
molecular data, is the following: [Nitraria+[Tetradiclis+[Peganum+Malacocarpus]]]
(Sheahan & Chase 1996).
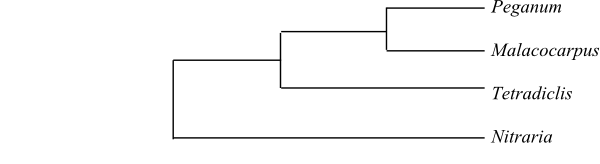 |
Phylogeny of Nitrariaceae based on
morphological and molecular data (Sheahan & Chase 1996,
Muellner-Riehl & al. 2016).
|
de Jussieu, Gen. Plant.: 296. 4 Aug 1789, nom.
cons.
Aurantiaceae Juss., Gen. Plant.: 259. 4 Aug 1789
[’Aurantia’]; Citraceae Roussel, Fl.
Calvados, ed. 2: 271. 1806; Cneoraceae Vest, Anleit.
Stud. Bot.: 267, 285. 1818 [’Cneoroideae’], nom. cons.;
Dictamnaceae Vest, Anleit. Stud. Bot.: 269, 287. 1818
[’Dictamnoideae’]; Rutales Juss. ex
Bercht. et J. Presl, Přir. Rostlin: 220. Jan-Apr 1820 [‘Rutaceae’];
Monieraceae Raf., Fl. Tellur. 4: 88. med 1838
[’Monierides’], nom. illeg.;
Jamboliferaceae Martinov, Tekhno-Bot. Slovar: 324. 3
Aug 1820 [’Jamboliferae’];
Zanthoxylaceae Martinov, Tekhno-Bot. Slovar: 682. 3
Aug 1820 [’Xanthoxyleae’]; Zanthoxylales
Bercht. et J. Presl, Přir. Rostlin: 227. Jan-Apr 1820
[‘Xanthoxyleae’]; Fraxinellaceae Nees et
Mart. in Nova Acta Phys.-Med. Acad. Caes. Leop.-Carol. Nat. Cur. 11: 147, 183.
3 Oct 1823 [’Fraxinellae’]; Amyridaceae
Kunth in Ann. Sci. Nat. (Paris) 2: 353. Jul 1824 [’Amyrideae’];
Pteleaceae Kunth in Ann. Sci. Nat. (Paris) 2: 354.
Jul 1824; Cuspariaceae (DC.) Tratt., Gen. Nov. Plant.
Fasc. 1: unpaged. 1825 [’Cuspariae’], nom. illeg.;
Aurantiales Link, Handbuch 2: 345. 4-11 Jul 1829
[‘Aurantia’]; Citrales Dumort., Anal.
Fam. Pl.: 43. 1829 [‘Citrarieae’];
Cneorales Link, Handbuch 2: 440. 4-11 Jul 1829
[‘Cneoreae’]; Pteleales Link, Handbuch
2: 118. 4-11 Jul 1829 [‘Pteleaceae’];
Amyridineae Link, Handbuch 2: 128. 4-11 Jul 1829
[‘Amyrideae’]; Diosmaceae R. Br. ex
Bartl., Ord. Nat. Pl.: 229, 386. Sep 1830;
Chamaeleaceae Bertol., Fl. Ital. 1: 196. 3 Feb 1834,
nom. illeg.; Rutopsida A. Juss. ex Meisn., Plant.
Vasc. Gen.: Tab. Diagn.: 59, Comm.: 43. 21-27 Mai 1837 [’Rutaceae’];
Aurantiineae Rchb., Deutsch. Bot. Herb.-Buch:
lxxxviii. Jul 1841 [‘Aurantiiflorae‘];
Amyridales J. Presl in Nowočeská Bibl. [Wšobecný
Rostl.] 7: 335. 1846 [‘Amyrideae’];
Diosmales J. Presl in Nowočeská Bibl. [Wšobecný
Rostl.] 7: 271. 1846 [‘Diosmeae’];
Dictamnineae J. Presl in Nowočeská Bibl.
[Wšobecný Rostl.] 7: 271, 272. 1846 [‘Dictamneae‘];
Diosmineae J. Presl in Nowočeská Bibl. [Wšobecný
Rostl.] 7: 271, 278. 1846; Pteleineae J. Presl in
Nowočeská Bibl. [Wšobecný Rostl.] 7: 280, 285. 1846
[‘Pteleaceae‘]; Zanthoxylineae J. Presl
in Nowočeská Bibl. [Wšobecný Rostl.] 7: 280, 282. 1846
[‘Zanthoxyleae‘]; Boroniaceae J. Agardh,
Theoria Syst. Plant.: 229. Apr-Sep 1858 [’Boronieae’];
Diplolaenaceae J. Agardh, Theoria Syst. Plant.: 229.
Apr-Sep 1858 [’Diplolaeneae’];
Pilocarpaceae J. Agardh, Theoria Syst. Plant.: 221.
Apr-Sep 1858 [’Pilocarpeae’];
Spatheliaceae J. Agardh, Theoria Syst. Plant.: 280.
Apr-Sep 1858 [’Spathelieae’];
Ptaeroxylaceae J.-F. Leroy in J. Agric. Trop. Bot.
Appl. 7: 456. 1960; Flindersiaceae (Luerss.) C. T.
White ex Airy Shaw in Kew Bull. 18: 257. 8 Dec 1965
Genera/species
142/1.840–1.890
Distribution Tropical,
subtropical and warm-temperate regions on both hemispheres, with their highest
diversity in the Cape Provinces and Australia.
Fossils Rutaspermum
biornatum comprises seeds of Rutaceae from the Maastrichtian of
Germany and the Eocene London Clay in England. Neogene seeds of Rutaceae have been found on many
places in North America, Europe and Asia. Wood and leaves are also known from
the mid-Eocene of North America and Europe.
Habit Usually bisexual (rarely
monoecious, andromonoecious, polygamomonoecious, or dioecious), evergreen or
deciduous trees, shrubs or lianas (rarely perennial herbs). Often strongly
aromatic. Many species are xerophytes.
Vegetative anatomy Phellogen
ab initio superficial. Vessel elements usually with simple (rarely scalariform)
perforation plates; lateral pits alternate, bordered pits. Imperforate
tracheary xylem elements libriform fibres with simple or bordered pits, usually
non-septate (also vasicentric tracheids). Wood rays usually multiseriate
(sometimes uniseriate), homocellular or heterocellular. Axial parenchyma
apotracheal diffuse, or paratracheal scanty, aliform, lozenge-aliform,
winged-aliform, confluent, vasicentric, or banded (rarely absent). Wood often
fluorescent. Wood elements often storied. Sieve tube plastids S type. Nodes
usually 3:3, trilacunar with three leaf traces (sometimes 1:1, unilacunar with
one trace). Parenchyma with secretory cavities containing ethereal oils.
Medulla, primary cortex and wood rays with resinous cells. Calciumoxalate as
prismatic crystals and druses abundant.
Trichomes Hairs unicellular or
multicellular, uniseriate or multiseriate, simple or branched, stellate,
sometimes furcate, peltate-lepidote or peltate and directed forwards; glands
(often with aromatic substances) frequent.
Leaves Usually alternate
(spiral; sometimes opposite, rarely verticillate), usually pinnately compound
(sometimes bipinnate), trifoliolate or unifoliolate (rarely simple, entire),
often ericoid or scale-like, often coriaceous, with conduplicate or flat
ptyxis. Stipules usually absent (in Metrodora intrapetiolar), or as
glands or spines; leaf sheath absent (petiole in Metrodorea
sheathing). Petiole vascular bundle transection arcuate or annular. Petiolules
usually articulated. Rhachis sometimes winged. Venation pinnate or leaves
one-veined. Stomata usually paracytic (rarely anomocytic or cyclocytic).
Cuticular wax crystalloids as platelets, rodlets or clustered tubuli
(Berberis type, chemically dominated by nonacosan-10-ol). Domatia
present as pockets or absent. Epidermis with or without mucilaginous
idioblasts. Mesophyll with or without sclerenchymatous idioblasts. Lamina
usually with pellucid (glandular) dots, and with schizogenous or lysigenous
secretory cavities containing ethereal oils (absent in Leptothyrsa and
Phellodendron). Leaf margin and leaflet margins usually entire or
crenate (sometimes serrate). Extrafloral nectaries sometimes present on abaxial
side of lamina.
Inflorescence Terminal or
axillary, usually panicle, corymb, etc. (sometimes racemose; flowers sometimes
solitary axillary; in one species of Erythrochiton seemingly
epiphyllous).
Flowers Usually actinomorphic
(rarely vertically or obliquely zygomorphic, e.g. Dictamnus and
Erythrochiton). Usually hypogyny (in Rutoideae usually
epigyny). Sepals (two to) four or five, usually with imbricate (sometimes
valvate) aestivation, often small, free or more or less connate (sepals in
Zanthoxylum opposite; calyx in, e.g., Pilocarpus reduced to
thin edge). Petals (two to) four or five, usually with imbricate (sometimes
valvate) aestivation, usually free (sometimes connate at base, rarely entirely
connate; absent in Empleurum). Nectariferous disc intrastaminal,
annular to cupular (sometimes unilateral or modified into androgynophore,
androphore or gynophore).
Androecium Stamens usually 4+4
or 5+5, usually obdiplostemonous (rarely diplostemonous; sometimes in one whorl
with two [Angostura alliance] to five antesepalous stamens,
haplostemonous, or three or four times as many as petals, or up to c. 60),
antepetalous staminal whorl often staminodial (rarely two or three stamens
fertile and remaining stamens staminodial). Filaments usually flattened,
usually free (sometimes connate at base all or in three to twelve fascicles;
sometimes connate into tube [Angostura alliance]), usually free from
tepals (sometimes adnate to petals). Anthers basifixed or dorsifixed,
versatile, tetrasporangiate, usually introrse (rarely latrorse), longicidal
(dehiscing by longitudinal slits); connective sometimes with glandular apex;
anthers sometimes with basal appendages. Tapetum secretory, with binucleate to
quadrinucleate cells. Staminodia (three or) four or five (to ten),
extrastaminal or intrastaminal, or absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains (2–)3–6(–8)-colporate, shed
as monads, usually bicellular (rarely tricellular) at dispersal. Exine tectate
or semitectate, with columellate infratectum, reticulate, gemmate, verrucate,
striate or striate-reticulate.
Gynoecium Pistil composed of
(one to) three to five (or six, or up to 20 [Aegle]), usually
laterally or only at base or at apex connate carpels (carpels sometimes free,
rarely entirely connate; in Zanthoxylum antesepalous); carpel fusion
possibly postgenital; compitum sometimes (e.g. in Boenninghausenia and
Ruta) restricted to stigmatic part. Ovary usually superior (sometimes
inferior), usually trilocular to quinquelocular (rarely unilocular, bilocular,
sexalocular or multilocular). Septal non-nectariferous cavities sometimes
present. Stylodia usually three to five, often free or connate only at apex,
impressed (style sometimes single, simple, marginal or, in some genera,
gynobasic). Stigma capitate or peltate, usually entire (sometimes lobate),
papillate or non-papillate, Dry or Wet type. Pistillodium often present in male
flowers.
Ovules Placentation usually
axile (rarely parietal or apical). Ovules one to numerous per carpel,
anatropous, hemianatropous, amphitropous or campylotropous, pendulous or
ascending, apotropous or epitropous, usually bitegmic (in, e.g.,
Glycosmis unitegmic), crassinucellar. Micropyle usually bistomal,
Z-shaped (zig-zag; rarely endostomal). Outer integument two to six cell layers
thick. Inner integument two to five (or six) cell layers thick. Hypostase
present or absent. Parietal tissue usually two to four (rarely five) cell
layers thick. Nucellar cap approx. two cell layers thick or absent.
Megagametophyte monosporous, Polygonum type. Synergids sometimes with
a filiform apparatus. Embryo sac haustoria chalazal. Endosperm development ab
initio nuclear. Endosperm haustorium absent. Embryogenesis usually onagrad or
solanad (rarely asterad). Polyembryony (nucellar) present in many species.
Agamospermy sometimes frequent.
Fruit A loculicidal and/or
entirely or partially septicidal capsule (often much lobed), a follicle, a
berry (in Aurantieae often a hesperidium), or a schizocarp with few to
numerous nutlike or drupaceous mericarps (sometimes a syncarp, rarely drupe or
samara). Exocarp often glandular and punctate. Mesocarp often with secretory
cavities. Mesocarp and endocarp often separating. Endocarp dividing
periclinally, capsule becoming layered.
Seeds Aril often present.
Elaiosome (endocarpial) present in some species (e.g. in Diosmeae).
Seed sometimes winged or hairy. Seed coat usually endotestal (sometimes reduced
and undifferentiated). Sarcotesta sometimes present (Aurantieae).
Exotesta often mucilaginous, irregularly palisade, lignified and/or fibrous.
Mesotesta sometimes sclerotic, rarely collenchyma-like. Endotesta sometimes
lignified. Endotestal cells sometimes with calciumoxalate crystals. Exotegmen
(sometimes also mesotegmen and endotegmen) usually tracheidal (in some species
fibrous). Mesotegmen sometimes tracheidal. Endotegmen absent or rudimentary.
Perisperm not developed. Endosperm copious, sparse or absent, oily. Embryo
usually straight (with micropyle close to hilum; sometimes, e.g. in
Dictyoloma, curved), well differentiated, with or without chlorophyll.
Cotyledons two, flat, folded or rolled. Germination phanerocotylar or
cryptocotylar.
Cytology n = 7–11, 18 or
more (in Erythrochiton n = 58)
DNA
Phytochemistry
Flavonols (kaempferol, quercetin, myricetin,
melisymplexin, ternatin, nobiletin, tangeretin, etc.), flavones, hydroxyflavones, polymethoxyflavones, flavonones
(naringin, hesperetin etc.), cyanidin, monoterpenes,
sesquiterpenes, bitter-tasting triterpenoid substances (flindissol,
bourjotinolone etc., cneorids, limonoids [limonin, nomilin, obacunone,
obacunoic acid, veprisone, etc.; in Cneoroideae also chromones]),
tetracyclic triterpenes, tetra- and pentanortriterpenes, tannins, proanthocyanidins (prodelphinidins), anthranilin-derived alkaloids (arborine, hortiacine, euxylophorine,
eduleine, cusparine, evocarpine, etc.), quinoline alkaloids (casimiroine,
oxirine, lunacridine, flindersine, isobalfouridine, lunacrine, skimmianine,
dictamnine, etc.), acridine alkaloids (acronycine, acridones, arborinine,
evoxanthidine, melicopicine, melicopidine, etc.), indolopyridoquinazoline,
furoquinoline and canthinone alkaloids, benzylisoquinoline alkaloids (produced
via at least nine different biosynthetic pathways; in some Rutoideae), polyacetate- or
shikimic acid-derived arthroquinone, ethereal oils (phenylpropans),
pyranochromones, furanocoumarins, and amides present. Saponins and
phenylalanine-derived cyanogenic compounds rare (often present in Amyridoideae). Ellagic acid not
found. Aluminium accumulated in some species.
Use Ornamental plants, fruits,
spices, flavouring, perfumes (Citrus, Aegle,
Casimiroa, Clausena, etc.), medicinal plants, herbicides,
insecticides, timber.
Systematics (under
construction) Rutaceae may be
sister to [Meliaceae+Simaroubaceae], but the support
is fairly weak.
Rutaceae are in urgent need of a
complete phylogenetic analysis.
Peltostigma has the floral formula
K3C3A9G[?5] and resembles Lauraceae.
Cneoroideae Webb in
London J. Bot. 1: 257. 1 Mai 1842 [‘Cneoreae’]
7/25. Dictyoloma (1; D.
vandellianum; Brazil, Peru, Bolivia), Spathelia (3; S.
excelsa, S. terminalioides, S. ulei; the West Indies,
northern South America); Harrisonia (2; H. abyssinica, H.
perforata; tropical Africa, tropical Asia, northern Australia), Cneorum (2;
C. pulverulentum: the Canary Islands; C. tricoccon: western
Mediterranean, introduced to Cuba), Ptaeroxylon (1; P.
obliquum; northeastern Tanzania to South Africa), Cedrelopsis (8;
C. ambanjensis, C. gracilis, C. grevei, C.
longibracteata, C. microfoliolata, C. procera, C.
rakotozafyi, C. trivalvis; Madagascar), Bottegoa (1;
B. insignis; southern Somalia, southeastern Ethiopia, northeastern
Kenya). – Tropical regions in the Old World, the Canary Islands, western
Mediterranean, the West Indies, tropical South America. Trees or shrubs. Oil
cavities usually absent (present in, e.g., Spathelia); oil cells
usually solitary. Medullary secretory ducts absent in Harrisonia.
Leaves in Cneorum
simple, non-punctate. Stipules usually absent (in Harrisonia modified
into spines adjacent to leaf bases). Petiole vascular bundle cylindrical,
consisting of two opposed plates; petiole bundle transection in
Bottegoa arcuate. Stomata in Ptaeroxylon anomocytic or
cyclocytic. Schizogenous cavities usually present. Extrafloral foliar nectaries
present in Harrisonia. Flower in Cneorum tricoccon and
Ptaeroxylon trimerous. Petals in Dictyoloma with valvate
aestivation. Nectaries present in Ptaeroxylon. Stamens usually four or
five, haplostemonous (sometimes eight or ten, obdiplostemonous). Filaments with
appendages (in Harrisonia scale-like). Exine in Ptaeroxylon
reticulate. Carpels in Harrisonia not connate; pistil in Cneorum
tricoccon composed of three connate carpels, with odd carpel adaxial.
Androgynophore (short) present in Ptaeroxylon. Ovules one or two (in
Harrisonia one; in Ptaeroxylon one to three; in
Dictyoloma four or five) per carpel, campylotropous, apotropous
(Harrisonia, Ptaeroxylon) or epitropous
(Dictyoloma). Micropyle endostomal (Cneorum).
Outer integument approx. two cell layers thick. Inner integument approx. three
cell layers thick. Hypostase present (Ptaeroxylon). Parietal tissue
approx. five cell layers thick. Fruit a loculicidal capsule (in
Ptaeroxylon with carpels dehiscing adaxially and separating laterally
and from columella), a follicle or a winged drupe (in Cneorum a
drupe or schizocarp). Testa multiplicative (Harrisonia,
Ptaeroxylon). Exotestal cells in Harrisonia large, with
thickened outer walls. Exotegmen tracheidal (Harrisonia).
Pyranochromones, diterpenoid cneorubin X, cedashnine (triterpenoid derivative),
quassinoids, etc. present (in Cneorum and
Harrisonia chromones [ptaeroxylins] and limonoids; in
Ptaeroxylon chromones but no limonoids). n = ? –
Cneoroideae are sister-group to the remaining Rutaceae.
[Amyridoideae+Rutoideae]
Amyridoideae Arn.,
Botany: 105. 9 Mar 1832 [‘Amyrideae’]
104/1.520–1.560. Casimiroa (c
10; mountain regions in southern Texas, Mexico to Costa Rica), Orixa (1;
O. japonica; China, the Korean Peninsula, Japan), Skimmia (6;
S. arborescens, S. japonica, S. laureola, S.
melanocarpa, S. multinervia, S. repens; Afghanistan, the
Himalayas, China, Japan, Southeast Asia, the Philippines), Dictamnus
(1; D. albus; Central and South Europe to northern China);
Flindersia (17; the Moluccas, New Guinea, Queensland, New South Wales,
New Caledonia); Pitavia (1; P. punctata; the coastal
Cordillera in southern Chile at c 35o–37oS); Ptelea (5;
P. baldwinii, P. crenulata, P. microcarpa, P.
serrata, P. trifoliata; North America, Mexico, Central America),
Pilocarpus (13–17; tropical South America); Acmadenia
(33; Western and Eastern Cape), Adenandra
(19; Western Cape), Agathosma
(>150; Western and Eastern Cape, KwaZulu-Natal, Lesotho, with their highest
diversity in Western Cape), Calodendrum (2; C. capense:
southern South Africa; C. eichii: Tanzania), Coleonema
(8; Western and southern Eastern Cape), Diosma (28;
Western, Northern and Eastern Cape), Empleurum (2; E.
fragrans, E. unicapsulare; Western and Eastern Cape), Euchaetis
(23; Western and Eastern Cape), Macrostylis (10; Western and Eastern
Cape), Phyllosma (2; P. barosmoides, P. capensis;
Western Cape), Sheilanthera (1; S. pubens; Cold Bokkeveld in
Western Cape); Balfourodendron (2; B. molle, B.
riedelianum; southern Brazil, Paraguay, northern Argentina),
Helietta (8; California, Texas, Mexico, Cuba, tropical South America),
Esenbeckia (30–35; southern Texas, Mexico, Central America, tropical
South America), Metrodorea (5; M. flavida, M.
maracasana, M. mollis, M. nigra, M. stipularis;
tropical South America), Raulinoa (1; R. echinata; Brazil);
Choisya (5; C. dumosa, C. katherinae, C.
neglecta, C. palmeri, C. ternata; Arizona, Mexico),
Decatropis (3; D. bicolor, D. coulteri, D.
paucijuga; Mexico, Guatemala, Honduras), Decacyx (2; D.
esparzae, D. macrophyllus; Mexico, Honduras), Megastigma
(1; M. skinneri; southern Mexico, Central America),
Peltostigma (2; P. guatemalense, P. pteleoides;
Central America, Jamaica, Ecuador, Peru), Plethadenia (2; P.
cubensis: eastern Cuba; P. granulata: Hispaniola),
Polyaster (1; P. boronoides; Mexico)?; Andreadoxa
(1; A. flava; Bahia in Brazil), Conchocarpus (c 45; Central
America, tropical South America), Ravenia (6; R. biramosa,
R. pseudalterna, R. rosea, R. spectabilis, R.
swartziana, R. urbanii; Central America, the West Indies,
northern South America), Raputia (10; tropical South America),
Neoraputia (5; N. alba, N. calliantha, N.
micrantha, N. paraensis, N. trifoliata; Peru, Brazil),
Almeidea (5; A. caerulea, A. lilacina, A.
limae, A. longifolia, A. rubra; coastal range from Bahia
to Paraná in Brazil), Angostura (c 20; Central America, Cuba,
tropical South America), Apocaulon (1; A. carnosum; the
Guayana Shield in southern Venezuela), Decagonocarpus (2; D.
cornutus, D. oppositifolius; the Guayana Shield in eastern
Colombia, southern Venezuela and northern Brazil), Desmotes (1; D.
incomparabilis; Coiba Island in Panamá), Erythrochiton (12–15;
Central America, tropical South America), Euxylophora (1; E.
paraensis; eastern Peru, Amazonian Brazil), Galipea (16; Central
America, tropical South America), Leptothyrsa (1; L. sprucei;
the Amazon), Lubaria (1; L. aroensis; Costa Rica, Venezuela),
Monniera (2–5; M. bahiensis, M. crenulata, M.
semiserrata, M. subserrata, M. trifoliata; southwestern
Mexico, Central America, tropical South America)?, Naudinia (1; N.
glabra; Colombia), Nycticalanthus (1; N.
speciosus; Amazonia), Raveniopsis (19; the Guayana Shield in
southern Venezuela and northern Brazil), Rutaneblina (1; R.
pusilla; the Guayana Shield in southern Venezuela), Spiranthera
(3; S. guianensis, S. odoratissima, S. parviflora;
northern South America), Ticorea (5; T. foetida, T.
froesii, T. longiflora, T. pedicellata, T.
tubiflora; Costa Rica to northern South America), Toxosiphon (4;
T. carinatus, T. lindenii, T. macropodus, T.
trifoliatus; southern Mexico, Central America, tropical South America),
Adiscanthus (1; A. fusciflorus; the Amazon), Hortia
(11; tropical South America); Zanthoxylum
(c 175; tropical and southern Africa, Madagascar, subtropical and temperate
East Asia, tropical Asia, northern and eastern Australia, Melanesia, eastern
North America, tropical and subtropical America), Fagaropsis (4;
F. angolensis, F. glabra, F. hildebrandtii, F.
velutina; tropical and northeastern Africa, Madagascar), Phellodendron
(5; P. amurense, P. chinense, P. japonicum, P.
sachalinense, P. sinii; China, the Korean Peninsula, Japan, the
Russian Far East, Taiwan, Southeast Asia), Tetradium (9;
the Himalayas, China, the Korean Peninsula, Japan, the Ryukyu Islands, Taiwan,
Southeast Asia, the Philippines, Sumatra, Java, Sumbawa), Euodia (5;
E. fraxinifolia, E. glabrifolia, E. robusta, E.
simplicifolia, E. tietaensis; New Guinea, eastern Queensland,
Solomon Islands to Samoa and Niue), Orixa (1; O. japonica;
China, the Korean Peninsula, Japan), Melicope (c
100; Madagascar, the Mascarene Islands, southern China, tropical Asia to New
Guinea, northern and eastern Australia, New Caledonia, Lord Howe, New Zealand,
Fiji to the Society Islands, the Marquesas Islands, Bonin Islands and the
Hawaiian Islands), Acronychia (c 50; southern China, tropical Asia to
New Guinea, eastern Queensland, eastern New South Wales, islands in western
Pacific, Taiwan; in Melicope?), Ivodea (11; Madagascar, the
Comoros), Vepris (80–85; tropical and southern Africa, Madagascar,
the Mascarene Islands, the Arabian Peninsula, southwestern India);
Acradenia (2; A. euodiiformis, A. frankliniae;
eastern Queensland, eastern New South Wales, Tasmania), Crossosperma
(2; C. cauliflora, C. velutina; New Caledonia),
Bosistoa (5; B. brassii, B. floydii, B.
medicinalis, B. pentacocca, B. transversa; eastern
Queensland, northeastern New South Wales), Bouchardatia (2; B.
cyanosperma, B. neurococca; New Guinea, southeastern Queensland,
northeastern New South Wales), Dinosperma (4; D.
erythrococca, D. longifolia, D. melanophloia, D.
stipitata; eastern Queensland, northeastern New South Wales),
Geijera (8; northern New Guinea, eastern and southern Australia, New
Caledonia; incl. Dendrosma?), Dendrosma (1; D.
deplanchei; New Caledonia; in Geijera?), Lunasia (1;
L. amara; the Philippines to Java, New Guinea, northeastern
Queensland)?, Pentaceras (1; P. australis; southeastern
Queensland, northeastern New South Wales); Boronia (c 150;
Australia, Tasmania, New Caledonia), Zieria (50–60; Z.
adenophora; eastern Queensland to southeastern South Australia, Tasmania,
one species, Z. chevalieri, in New Caledonia), Neobyrnesia
(1; N. suberosa; northern Arnhem Land in Northern Territory), Correa (12;
southeastern South Australia, Victoria, eastern New South Wales, Tasmania),
Perryodendron (1; P. parviflorum; the Moluccas, New Guinea,
New Britain), Brombya (2; B. platynema, B. smithii;
northeastern Queensland), Pitaviaster (1; P. haplophyllus;
northeastern Queensland), Medicosma (c 25; M. cunninghamii,
M. leratii; New Guinea, eastern Queensland, New Caledonia);
Asterolasia (18; southwestern Western Australia, southeastern South
Australia, Victoria, eastern New South Wales), Eriostemon (2; E.
australasius, E. banksia; eastern Queensland, eastern New South
Wales, Victoria, Tasmania), Leionema (21; southeastern Queensland,
eastern New South Wales, Victoria, southeastern South Australia, Tasmania, one
species, L. nudum, on North Island in New Zealand), Phebalium
(28; eastern and southern Australia, Tasmania), Philotheca
(c 45; southwestern Western Australia, southeastern South Australia to
southeastern Queensland, Tasmania), Diplolaena (15; western and
southwestern Western Australia), Geleznowia (1; G. verrucosa;
southwestern Western Australia), Drummondita (9; southwestern Western
Australia, Northern Territory, Queensland), Crowea (3;
C. angustifolia, C. exalata, C. saligna;
southwestern Western Australia, eastern New South Wales, Victoria),
Microcybe (3; M. albiflora, M. multiflora, M.
pauciflora; southern Western Australia, southern South Australia, western
Victoria)?, Muiriantha (1; M. hassellii; southwestern Western
Australia), Rhadinothamnus (3; R. anceps, R.
euphemiae, R. rudis; southwestern Western Australia), Chorilaena
(1; C. quercifolia; southwestern Western Australia),
Nematolepis (6; N. elliptica, N. frondosa, N.
ovatifolia, N. rhytidophylla, N. squamea, N.
wilsonii; southern Western Australia, eastern New South Wales, Victoria,
Tasmania), Tetractomia (7; T. acuminata, T.
holttumii, T. kostermansii, T. majus, T.
obovata, T. roxburghiana, T. tetrandra; Malesia to
Solomon Islands)?, Neoschmidia (2; N. calycina, N.
pallida; New Caledonia), Halfordia (3; H. kendack,
H. papuana, H. scleroxyla; New Guinea, eastern Queensland,
northeastern New South Wales, New Caledonia, Vanuatu), Myrtopsis
(8–9; New Caledonia), Amyris (c 60; tropical America),
Stauranthus (1–2; S. perforatus: southeastern Mexico to
Panamá; S. conzattii: Oaxaca in southern Mexico). – Tropical and subtropical
regions on both hemispheres, temperate East Asia, temperate Australia,
Tasmania, New Zealand, with their highest diversity in tropical Asia and
Australia. – Unplaced Zanthoxyloideae
Bouzetia (1; B. maritima; New Caledonia), Pseudiosma
(1; P. asiatica; Southeast Asia).
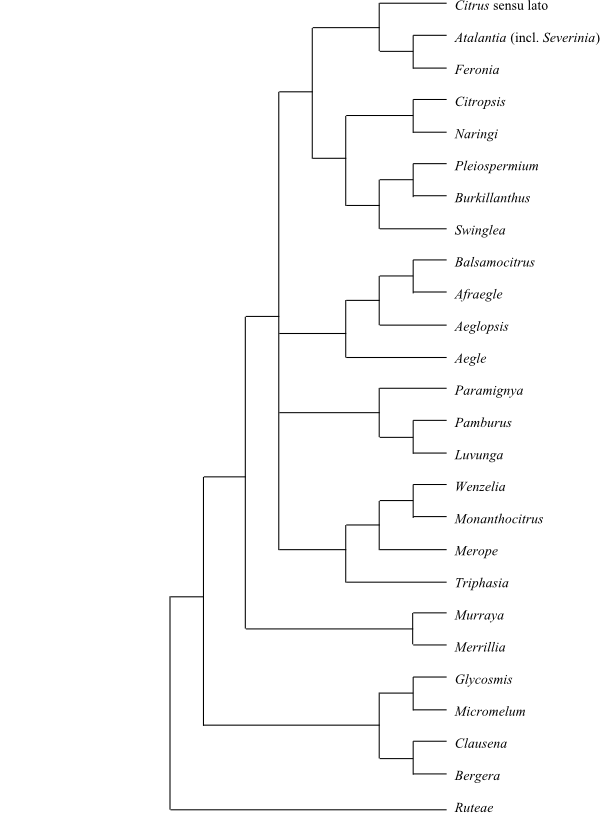 |
Cladogram (simplified) of Rutoideae
sensu stricto based on DNA sequence data (Bayer & al. 2009).
|
Rutoideae Arn.,
Encycl. Brit., ed. 7, 5: 104. 9 Mar 1832 [‘Ruteae’]
31/295–305. Exine sometimes striate.
Outer integument three to six cell layers thick. Inner integument two to five
(or six) cell layers thick. Parietal tissue five to twelve cell layers thick.
Nucellar cap several cell layers thick. Exotesta sometimes mucilaginous.
Ruteae Dumort., Anal.
Fam. Plant.: 45. 1829
5/25. Chloroxylon (3; C.
faho and C. falcatum: Madagascar; C. swietenia: southern
India, Sri Lanka), Psilopeganum (1; P. sinense; southern
China), Boenninghausenia
(2; B. albiflora, B. japonica; the Himalayas, Assam to
central Japan, Java and the Lesser Sunda Islands), Thamnosma (8; arid
regions of Namibia, South Africa, Botswana, Somalia, Yemen, Socotra,
southwestern United States, Mexico), Ruta (11;
Macaronesia, the Mediterranean, southwestern Asia). – Africa, Madagascar,
southwestern and southern Asia to eastern Siberia and Japan, southwestern
United States, Mexico. Usually trees or shrubs (rarely perennial herbs). Oil
cells often solitary. Leaves sometimes opposite. Flowers sometimes vertically
or obliquely zygomorphic. Petals in Ruta sometimes
with fimbriate margin. Quinolone and acridone alkaloids derived from
anthranilic acid or from pyranoquinolines or furopyranoquinolines.
Aurantieae Rchb., Fl.
Germ. Excurs. 2(2): 840. 1832
26/270–280. Cneoridium
(1; C. dumosum; southern California, Baja California),
Haplophyllum (65–70; the Mediterranean, northwestern Africa, the
Arabian Peninsula, southwestern Asia to eastern Siberia and temperate China);
Micromelum (9–10; India, Sri Lanka and Bangladesh to southern China
and through Indochina and Malesia to New Guinea, northern and eastern
Australia, New Caledonia, Tonga, Niue and Samoa); Glycosmis
(c 45;tropical Asia);Clausena (c 20; tropical
and southern Africa, tropical Asia to Malesia, New Guinea and tropical
Australia); Merrillia (1; M. caloxylon; Burma to West
Malesia), Murraya (8;
India, Sri Lanka to southern China and southern Ryukyu Islands, Southeast Asia,
Malesia to New Guinea, northern and eastern Australia, New Caledonia, Vanuatu,
the Mariana Islands); Paramignya (12–15; tropical Asia to
Queensland; incl. Luvunga?), Luvunga (10; L.
monophylla, L. scandens; tropical Asia to New Guinea and tropical
Australia; in. Paramignya?), Pamburus (1; P.
missionis; southern India, Sri Lanka), Merope (1; M.
spinosa; Burma, Indochina, Malesia to New Guinea), Triphasia (1;
T. trifolia; Southeast Asia, the Philippines, New Guinea),
Monanthocitrus (3; M. cornuta, M. oblanceolata,
M. paludosa; Borneo, New Guinea), Wenzelia (9; southern
Philippines, New Guinea, Bougainville, Solomon Islands, Fiji, the Hawaiian
Islands); Aegle (2; A. decandra, A. marmelos;
tropical Asia), Aeglopsis (5; A. alexandrae, A.
beguei, A. chevalieri, A. eggelingii, A.
mangenotii; western tropical Africa, Sudan, Uganda), Afraegle (4;
A. asso, A. gabonensis, A. mildbraedii, A.
paniculata; tropical West and Central Africa), Balsamocitrus (3;
B. camerunensis, B. dawei, B. gabonensis; tropical
East Africa); Swinglea (1; S. glutinosa; Luzon in the
Philippines), Burkillanthus (1; B. malaccensis; West
Malesia), Pleiospermium (3–6; P. alatum, P.
littorale, P. longisepalum; Southeast Asia, West Malesia),
Naringi (2; N. alata, N. crenulata; India to
Southeast Asia), Citropsis (10; tropical and southern Africa);
Atalantia (19; India, Southeast Asia, Malesia to New Guinea, Taiwan);
Citrus (c
30; India and southern China to Southeast Asia, Malesia to New Guinea, northern
and eastern Australia, New Caledonia and Southwest Pacific islands),
Limonia (1; L. acidissima; India, Sri Lanka, Java?). –
Tropical and southern Africa, East Asia to Taiwan, tropical Asia to New Guinea,
northern and eastern Australia, Melanesia, Micronesia, Polynesia. Trees or
shrubs (sometimes spiny). Foliar rhachis sometimes winged. Epigyny. Petals
often with imbricate aestivation. Stamens sometimes numerous. Carpels
postgenitally fused. Ovary with multicellular hairs inside locules. Pistil in
Triphasia composed of three connate carpels, with odd carpel adaxial.
Ovules in Glycosmis unitegmic. Nucellar polyembryony frequent. Fruit a
dry berry with mucilaginous pulp formed from endocarp and carnose hairs in
loculi. Exotesta mucilaginous, often fibrous, with lignified inner cell walls.
Exotesta and endotesta with crystalliferous cells. Exotegmen fibrous. Endosperm
absent. x = 9. Flavonoids formed by polymethoxylation, and 3-methyl carbazole
alkaloids (in the Clausena clade)present. – Appelhans & al.
(2016) recovered [Cneoridium+Haplophyllum] as sister-group to
Aurantieae.
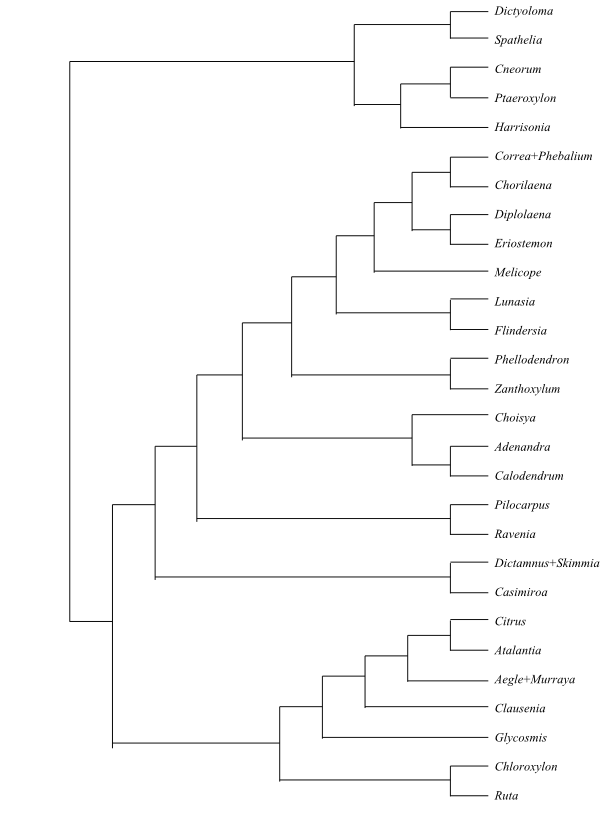 |
Phylogeny of Rutaceae based on Morton
& Telmer (2014).
|
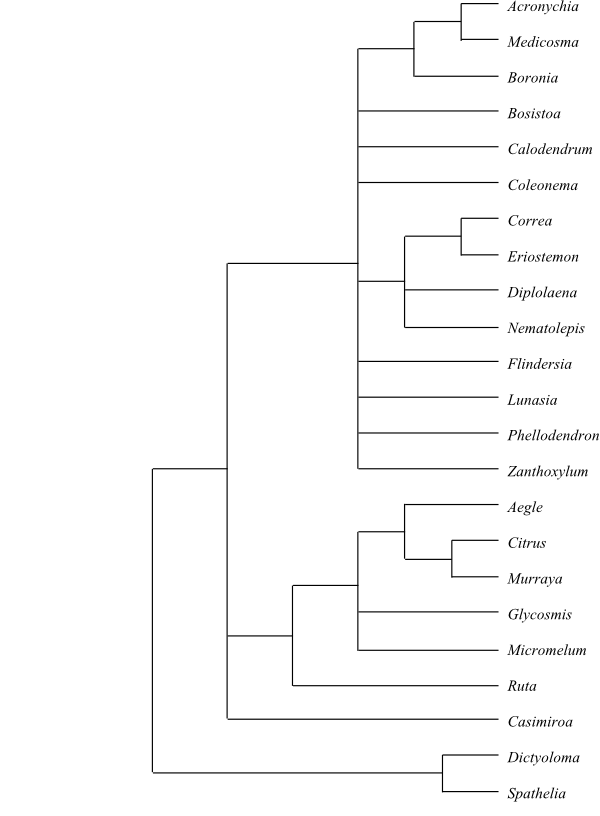 |
Bootstrap consensus tree of Rutaceae based on DNA
sequence data (Scott & al. 2000).
|
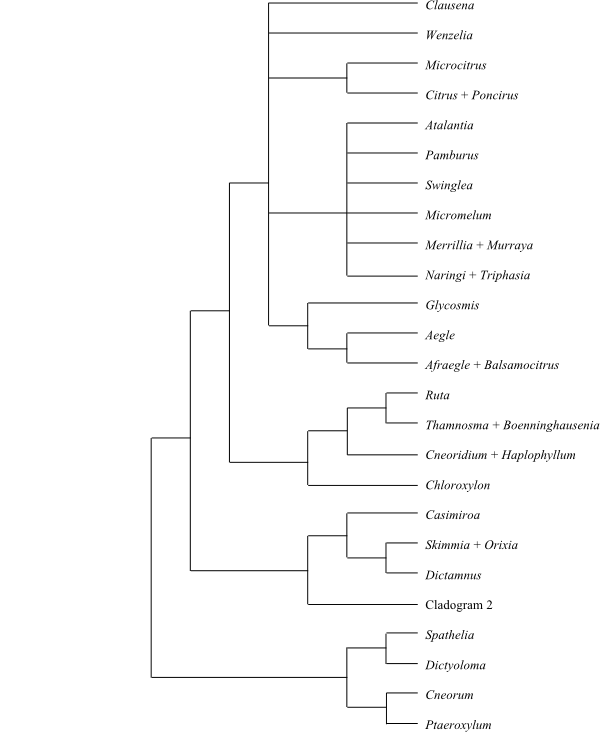 |
Cladogram 1 of Rutaceae based on DNA
sequence data (Groppo & al. 2008).
|
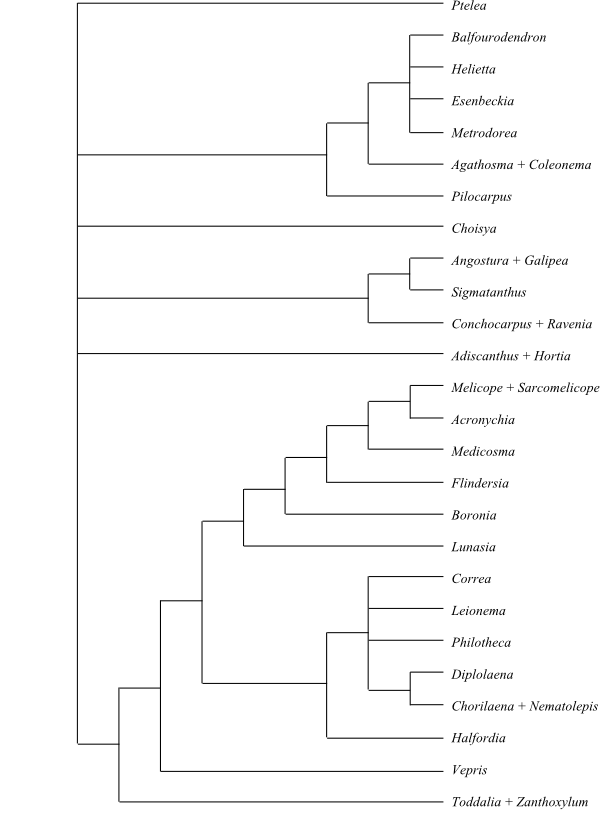 |
Cladogram 2 of Rutaceae based on DNA
sequence data (Groppo & al. 2008; Salvo & al. 2008).
|
de Jussieu, Gen. Plant.: 246. 4 Aug 1789
[’Sapindi’], nom. cons.
Aceraceae Juss., Gen.
Plant.: 250. 4 Aug 1789 [’Acera’], nom. cons.;
Acerales Bercht. et J. Presl, Přir. Rostlin: 225.
Jan-Apr 1820 [‘Acerinae’]; Aesculales
Bercht. et J. Presl, Přir. Rostlin: 224. Jan-Apr 1820
[‘Aesculeae’]; Allophylaceae Martinov,
Tekhno-Bot. Slovar: 19. 3 Aug 1820 [’Allophylleae’];
Ornithropaceae Martinov, Tekhno-Bot. Slovar: 443. 3
Aug 1820 [’Ornitropheae’];
Hippocastanaceae A. Rich., Bot. Méd.: 680. 1823
[’Hippocastaneae’], nom. cons.;
Hippocastanales Link, Handbuch 2: 335. 4-11 Jul 1829
[‘Hippocastaneae’]; Paviaceae Horan.,
Prim. Lin. Syst. Nat.: 100. 2 Nov 1834; Aesculaceae
Burnett, Outl. Bot.: 891, 1093, 1126. Feb 1835;
Aceropsida Endl., Gen. Plant.: 1055. Apr 1840
[’Acera’]; Aesculopsida Brongn., Enum.
Plant. Mus. Paris: xxiv, 86. 12 Aug 1843 [’Aesculineae’];
Sapindineae J. Presl in Nowočeská Bibl. [Wšobecný
Rostl.] 7: 222. 1846 [‘Sapindeae‘];
Koelreuteriaceae J. Agardh, Theoria Syst. Plant.:
227. Apr-Sep 1858 [’Koelreuterieae’];
Dodonaeaceae Kunth ex Small, Fl. S.E. U.S.: 724, 737.
22 Jul 1903, nom. cons.; Xanthoceraceae Buerki,
Callm. et Lowry in Plant Ecol. Evol. 143: 155. 2010
Genera/species c
138/1.930–1.965
Distribution Tropical,
subtropical and temperate regions on both hemispheres.
Fossils Schizocarpous winged
fruits similar to those in extant Acer have been found in the
Paleocene of North Dakota onwards. Vegetative organs of Sapindaceae are known from Eocene
and younger layers. Sapindoxylon type of wood is reported from the
Maastrichtian Deccan Intertrappean Beds in India and from Neogene strata of
Southeast Asia, Pakistan, Northeast Africa, Europe and Columbia. Eocene seeds
are represented by Palaealectryon, Palaeallophyllus and
Sapindospermum, the latter also known from the Late Turonian to the
Maastrichtian of Central Europe. Pollen grains of the
‘Cupaniopsis‘ type are reported from many areas including Africa,
a continent lacking extant Sapindaceae with this kind of
pollen.
Habit Monoecious,
andromonoecious, polygamomonoecious, dioecious, androdioecious, or
polygamodioecious (sometimes bisexual), evergreen or deciduous trees, shrubs or
lianas (Cardiospermum consists of perennial climbing herbs with
inflorescence branches modified into tendrils).
Vegetative anatomy Phellogen
ab initio usually superficial (sometimes outer-cortical; in Dodonaea
pericyclic). Primary medullary rays usually narrow. Secondary lateral growth in
lianas anomalous (sometimes via concentric cambia). Vessel elements usually
with simple (rarely scalariform) perforation plates; lateral pits usually
alternate (rarely opposite), bordered pits. Vestured pits present
(Aesculus). Imperforate tracheary xylem elements usually libriform
fibres (sometimes fibre tracheids) with simple pits, septate or non-septate
(also vasicentric tracheids). Wood rays uniseriate or multiseriate,
homocellular or heterocellular. Axial parenchyma apotracheal diffuse or
diffuse-in-aggregates, or paratracheal scanty, aliform, confluent, vasicentric,
or banded, or absent. Wood elements often storied. Secondary phloem sometimes
stratified into hard fibrous and soft parenchymatous layers. Sieve tube
plastids S type. Nodes usually 3:3, trilacunar with three leaf traces (rarely
multilacunar with more than three traces). Many representatives with laticifers
containing latex or resinous substances of unusual types. Silica bodies present
in parenchyma in some species. Prismatic calciumoxalate crystals and druses
frequent.
Trichomes Hairs unicellular,
simple or branched, or multicellular, uniseriate or often furcate, stellate or
lepidote; glandular hairs present (also peltate-lepidote).
Leaves Usually alternate
(spiral; in Hippocastanoideae opposite), usually pinnately or
palmately compound (sometimes bipinnate, or simple/unifoliolate and entire or
lobed), also with conduplicate-plicate ptyxis. Stipules usually absent
(sometimes petiolar); leaf sheath absent. Stipule-like leaflets or colleters
often present. Petiole base usually pulvinate. Petiole vascular bundle
transection ?; sometimes with cortical or adaxial bundles. Petiolules
articulated. Rhachis sometimes winged. Venation usually pinnate (sometimes
palmate), brochidodromous, eucamptodromous, craspedodromous, billioid, or
bohlenioid. Stomata usually anomocytic (sometimes paracytic). Cuticular waxes
usually absent (crystalloids rarely as platelets or rodlets). Domatia present
as pits, pockets or hair tufts, or absent. Lamina often gland-dotted, without
secretory cavities. Epidermis usually with mucilaginous idioblasts. Mesophyll
with or without spherical idioblasts with ethereal oils, with or without
mucilaginous idioblasts, with or without sclerenchymatous idioblasts. Mesophyll
cells often with calciumoxalate as druses or prismatic crystals. Leaf margin
and leaflet margins entire, crenate, or serrate. Extrafloral nectaries
sometimes present on stipules and/or lamina.
Inflorescence Terminal or
axillary, panicle, thyrsoid, raceme- or umbel-like (rarely solitary
axillary).
Flowers Actinomorphic or
zygomorphic (often obliquely, sometimes transversely), often small. Pedicel
articulated. Receptacle often prolonged to androphore or gynophore. Hypogyny.
Sepals (three or) four or five (to seven), usually with imbricate (rarely
valvate) aestivation, usually free (sometimes connate at base). Petals (three
or) four or five (or more than five), usually with imbricate (rarely valvate)
aestivation, often clawed and/or with scale-like or other appendages or folds
enclosing nectaries, free (sometimes absent). Nectariferous disc usually
extrastaminal, annular or unilateral (sometimes lobate or reduced to glandular
teeth, rarely absent; in Dodonaea intrastaminal).
Androecium Stamens usually 4+4
or 5+5 (rarely four to seven or numerous in three to five whorls), usually
diplostemonous. Filaments free from each other and usually from petals, often
pubescent or papillose. Anthers usually dorsifixed (sometimes basifixed to
somewhat ventrifixed), usually versatile, tetrasporangiate, introrse,
longicidal (dehiscing by longitudinal slits); connective usually somewhat
prolonged at apex. Tapetum secretory, with uni- to trinucleate cells. Female
flowers often with staminodia.
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually triporate, 2–4-colpate
or 2–4-colporate (sometimes acolpate, syncolporate or parasyncolporate), shed
as monads, bicellular at dispersal. Exine tectate or semitectate, with
columellate infratectum, reticulate, finely reticulate or scabrate, sometimes
striate or striate-reticulate.
Gynoecium Pistil composed of
(two or) three (to eight) connate carpels. Ovary superior, (bilocular or)
trilocular (to octalocular). Septal non-nectariferous cavities sometimes
present. Style single, simple or lobate (sometimes hollow), or stylodia two to
four, free or connate below. Stigma entire or lobate, usually non-papillate,
Dry type (in, e.g., Aesculus) or Wet type (in, e.g.,
Dodonaea). Male flowers often with pistillodium.
Ovules Placentation axile to
basal. Ovule usually one (ovules sometimes two; in Dodonaea two to
five; in Xanthoceras five to eight) per carpel, anatropous,
hemianatropous, amphitropous or campylotropous (rarely orthotropous),
ascending, horizontal or pendulous, apotropous or epitropous, bitegmic,
crassinucellar. Micropyle usually bistomal (rarely endostomal). Outer
integument three to twelve cell layers thick. Inner integument two to six cell
layers thick. Funicle absent. Placental obturator usually present, pressed to
ovules. Hypostase present. Parietal tissue four to 15 cell layers thick.
Megagametophyte monosporous, Polygonum type. Antipodal cells sometimes
proliferating (up to 14 cells), rarely long-lived. Endosperm development ab
initio nuclear. Endosperm haustoria? Embryogenesis asterad.
Fruit Usually a loculicidal
capsule (rarely a pyxidium or an irregularly dehiscent capsule) or a schizocarp
(often with carpophore) with two or three (to eight) one-seeded samaroid
mericarps (rarely a nut, samara, drupe or berry), usually with only one or two
fertile carpels. Capsule sometimes resembling a follicle, with only one carpel
developed, although dehiscing abaxially (in contrast to follicles).
Seeds Aril or arillode, formed
from integument or chalaza, and chalazal sarcotesta often present. Sarcotesta
sometimes present. Seed often pachychalazal. Seed coat sometimes with fold or
pocket. Seed coat usually exotestal (in Acer reduced). Testa
vascularized, multiplicative. Exotesta usually palisade, non-lignified.
Mesotestal cell walls sometimes thickened and lignified. Endotesta sometimes
with stellate calciumoxalate crystals. Exotegmen often lignified (in
Alectryon fibrous), sometimes multiplicative. Endotegmen? Perisperm
not developed. Endosperm usually absent (sometimes with starch). Embryo usually
curved to spirally twisted with micropyle adjacent to hilum (when straight then
micropyle opposite hilum), well differentiated, oily and starchy, with or
without amyloid, with or without chlorophyll. Radicula dorsal, often inserted
in testal pocket. Cotyledons two, usually folded or spirally twisted.
Germination phanerocotylar or cryptocotylar.
Cytology n = usually 10–12
(lianas) or 14–16 (non-lianas); x = 7
DNA
Phytochemistry Flavonols
(kaempferol, quercetin, myricetin), cyanidin, delphinidin, ellagic acid (in
some species of Acer), condensed tannins, alkaloids (e.g. gramine),
toxic triterpene saponins, leucine-derived cyanogenic compounds, quebrachitol
(cyclitol), cyclic polyvalent alcohols, type C18:3 fatty acids, and
non-protein cyclopropane amino acids (e.g. hypoglycine) present.
Use Ornamental plants, fruits
(Blighia, Dimocarpus, Litchi, Melicoccus,
Nephelium, Paullinia, Talisia), syrup
(Acer), oils (Schleichera), soap (Sapindus saponaria
etc.), medicinal plants, fish poison, timber.
Systematics The sister-group
relationship of Sapindaceae is unresolved. They
may be sister to the clade [Rutaceae+Meliaceae+Simaroubaceae], although the
support is weak.
Xanthoceras is sister to the remaining
Sapindaceae.
Xanthoceroideae thorne
et Reveal in Bot. Rev. (Lancaster) 73: 119. 29 Jun 2007
1/1. Xanthoceras (1; X.
sorbifolia; northern and northeastern China, the Korean Peninsula). –
Andromonoecious, deciduous tree or shrub. Secondary phloem sometimes
stratified. Leaves imparipinnate. Stomata anomocytic. Extrastaminal nectaries
complex golden-yellow. Flowers actinomorphic. Sepals five, with imbricate
aestivation. Petals five, shortly clawed. Nectariferous disc quinquelobate,
provided with apical-abaxial golden yellow, horn-shaped glands. Stamens eight.
Exine spinulate. Pistil composed of three connate carpels. Ovary trilocular.
Stigma papillate. Ovules six to eight per carpel, arranged in parallel,
campylotropous. Outer integument six to eight cell layers thick. Inner
integument three or four cell layers thick. Obturator absent. Fruit a
loculicidal schizocarp-like capsule. Pericarp coriaceous, thick, with fibre
bundles. Arillode absent. Mesotestal cell walls thickened. Tegmen
multiplicative. Inner tegmic cell layers with thickened walls. Cotyledons one
large and one small. n = 15.
[Hippocastanoideae+[Dodonaeoideae+Sapindoideae]]
Pericyclic envelope formed by phloem
fibres and sclerenchymatous cells. Flowers often strongly obliquely (in Aesculus
vertically) zygomorphic. Ovules two per carpel, apotropous. Funicular obturator
often present. Archespore sometimes multicellular. Fruit a loculicidal capsule
or a schizocarp with single-seeded samara-like mericarps.
Hippocastanoideae
Burnett, Outlines Bot.: 891, 1093, 1126. Feb 1835
[‘Hippocastanidae’]
5/180–185. Acer (c
165; temperate regions on the Northern Hemisphere, tropical mountains), Dipteronia
(2; D. dyeriana, D. sinensis; China; in Acer?);
Handeliodendron (1; H. bodinieri; southwestern China),
Billia (2; B. hippocastanum, B. rosea; southern
Mexico, Central America, tropical South America), Aesculus
(13; the Balkan Peninsula, the Himalayas to Japan and Indochina, North America,
northwestern Mexico). – Temperate regions in the Northern Hemispere, tropical
mountain regions south to Southeast Asia and tropical South America. Deciduous
trees or shrubs. Pericyclic envelope sometimes absent. Leaves alternate
(spiral) or opposite, usually palmately compound or lobed (rarely imparipinnate
or simple). Cuticular wax crystalloids often present in Acer.
Stomata usually actinocytic (sometimes anomocytic). Flowers actinomorphic or
zygomorphic. Petals usually clawed. Nectariferous disc in Acer often
intrastaminal. Stamens (five or) six to eight (to twelve). Stigma Dry type.
“Hippocastanoid” ovules (inserted back-to-back in each pair, half-way
twisted around one another, with elongate geniculate obturator) present in Aesculus,
Billia and Handeliodendron. Outer integument three to five
(in Acer) or
eight to ten cell layers thick. Inner integument three to six cell layers thick
(Handeliodendron). Nucellar cap present in Acer, eight
to ten cell layers thick. Hypostase usually present (absent in
Handeliodendron). Aril present in Handeliodendron. n = 13 (Acer), 20
(Aesculus).
Cyanogenic glucosides not found. – Hippocastanoideae are sister to
the remaining Sapindaceae
except Xanthoceras. Billia and Handeliodendron are
successive sister-groups to Aesculus.
[Dodonaeoideae+Sapindoideae]
Leaves usually paripinnate (sometimes
bicompound or simple). Seed often with chalazal or integumentary aril and
sarcotesta.
Dodonaeoideae Burnett,
Outlines Bot.: 889. Feb 1835 [‘Dodonidae’]
c 22/135–145. Tropical to
warm-temperate regions on both hemispheres, with their largest diversity in
tropical Asia and Australia. Phellogen pericyclic (Dodonaea).
Stomata cyclocytic (Dodonaea).
Petals sometimes absent. Stamens five to numerous. Placentation basal or
apical. Ovules usually two (sometimes one) per carpel, usually apotropous
(sometimes epitropous), sometimes pendulous. Outer integument eight to ten cell
layers thick. Inner integument three or four cell layers thick. Fruit usually a
longicidal (sometimes septicidal) capsule (sometimes a berry). Seed sometimes
with aril or sarcotesta. n = 10, 12, 14–16
Dodonaeeae Kunth ex
DC., Prodr. 1: 615. Jan (med.) 1824 [‘Dodonaeaceae’]
c 12/90–95. Averrhoidium
(4–5; A. dalyi, A. gardnerianum, A. paraguaiense,
A. spondioides; southern Mexico, southwestern Amazonas, southeastern
Brazil, Paraguay), Arfeuillea (1; A. arborescens; Southeast
Asia; in Majidea?), Majidea (4–5; M. cyanosperma,
M. forsteri, M. multijuga, M. zanguebarica; tropical
East Africa, Madagascar; incl. Arfeuillea?), Euphorianthus
(1; E. euneurus; the Philippines and Sulawesi to Vanuatu),
Eurycorymbus (1; E. cavaleriei; southern China, Taiwan),
Llagunoa (3–4; L. glandulosa, L. nitida, L.
venezuelana; the Andes), Cossinia (5; C. australiana,
C. pacifica, C. pinnata, C. triphylla; two species
on Mauritius, one species, C. trifoliata, in New Caledonia),
Diplopeltis (5; D. eriocarpa, D. huegelii, D.
intermedia, D. petiolaris, D. stuartii; Western
Australia, Northern Territory), Hirania (1; H. rosea;
southern central Somalia), Dodonaea
(65–70; tropical and subtropical regions on both hemispheres, with their
highest diversity in Australia), Loxodiscus
(1; L. coriaceus; New Caledonia), Diplokeleba (2; D.
floribunda, D. herzogii; southern South America). – East
Africa, Madagascar, tropical Asia to Taiwan, Australia, New Caledonia and Fiji,
Mexico to tropical South America (Dodonaea
pantropical). Perianth in Averrhoidium spirally arranged. Fruit a
capsule.
Harpullieae Radlk. in
Sitzungsber. Math.-Phys. Cl. Königl. Bayer. Akad. Wiss. München 20: 255. Jun
1890
2/25–30. Harpullia (25–30;
tropical Asia to New Guinea, northern Northern Territory, eastern Queensland,
northeastern New South Wales, New Caledonia, Tonga), Magonia (1;
M. pubescens; Brazil, Bolivia, Paraguay). – Tropical Africa,
Madagascar, tropical Asia to New Guinea, northeastern Australia, New Caledonia,
Tonga, Florida, the West Indies, southern tropical South America. Evergreen
trees. – Harpullia is sister to
[Cossinia+[Diplopeltis+Dodonaea]]
in the analyses by Buerki & al. (2012). Harpullia and
Magonia should possibly be transferred to Dodonaeeae.
DoratoxyleaeRadlk. in
Sitzungsber. Math.-Phys. Cl. Königl. Bayer. Akad. Wiss. München 20: 255. Jun
1890
8/19. Doratoxylon (5; D.
alatum, D. apetalum, D. chouxii, D. littorale,
D. stipulatum; Madagascar, the Comoros, Mauritius), Euchorium
(1; E. cubense; western Cuba), Exothea (3; E.
copalillo, E. diphylla, E. paniculata; Florida, Central
America, the West Indies, Colombia, Ecuador, Suriname), Filicium (3;
F. decipiens, F. longifolium, F. thouarsianum;
tropical East Africa, Madagascar, one species also in India and Sri Lanka),
Ganophyllum (2; G. falcatum, G. giganteum; Central
Africa, the Andaman Islands, the Nicobar Islands, Vietnam, the Malay Peninsula,
Sumatra, Java, the Philippines, New Guinea, northern Australia, Solomon
Islands), Hippobromus (1; H. pauciflorus; South Africa),
Zanha (3; Z. africana, Z. golungensis, Z.
suaveolens; southern Africa, Madagascar), Hypelate (1; H.
trifoliata; Florida, the West Indies). – Tropical and southern Africa,
Madagascar, the Comoros, Mauritius, tropical Asia to northern Australia,
tropical America. Fruit baccate.
Sapindoideae Burnett,
Outlines Bot.: 889. Feb 1835 [‘Sapindidae’]
c 110/1.615–1.640. Mainly tropical and
subtropical regions, few species in warm-temperate areas. Trees, shrubs
(sometimes lianas with anomalous secondary thickening; rarely herbaceous).
Petals often with complicated appendages. Stamens (four or) five (to numerous).
Pollen grains sometimes triporate. Ovule usually one per carpel, apotropous,
erect or ascending. Outer integument four to twelve cell layers thick. Inner
integument two to six cell layers thick. Antipodal cells in
Cardiospermum persistent. Fruit usually a loculicidal capsule
(sometimes septicidal or fruit indehiscent). Seed usually with aril, arillode
or sarcotesta. Amyloid (xyloglucans) present in seeds of Cardiospermum. n =
10–12, 14–16, etc.
Ungnadia clade
1/1 Ungnadia (1; U.
speciosa; Texas, northeastern Mexico). – Shrub or small tree. Flowers
zygomorphic. Ovules two per carpel.
Delavaya clade
1/1. Delavaya (1; D.
yunnanensis; southwestern China, northern Vietnam). – Dioecious shrub or
small tree. Leaves trifoliolate. Stamens eight. Pistil composed of two (or
three) connate carpels. Ovary bilocular (or trilocular). Ovules two per carpel.
Pericarp coriaceous, almost woody.
Koelreuterieae Radlk.
in Sitzungsber. Math.-Phys. Cl. Königl. Bayer. Akad. Wiss. München 20: 254.
Jun 1890
5/21–22. Koelreuteria
(3; K. bipinnata, K. elegans, K. paniculata;
southern China, Japan, Taiwan, Fiji?), Stadmania (6–7; S.
acuminata, S. excelsa, S. glauca, S. leandrii,
S. oppositifolia, S. serrulata; tropical East Africa,
Madagascar, Mauritius), Erythrophysa (9; Ethiopia, Northern Cape, Free
State, Northern Province, western Madagascar), Stocksia (1; S.
brahuica; eastern Iran, Afghanistan), Boniodendron (2; B.
minus, B. parviflorum; southern China, northern Vietnam)? –
Tropical East Africa, South Africa, Madagascar, Mauritius, southwestern Asia,
China, Taiwan, Fiji. Anthers hairy. Ovules two per carpel.
Schleichereae Radlk.
in Sitzungsber. Math.-Phys. Cl. Königl. Bayer. Akad. Wiss. München 20: 253,
287. Jun 1890
5/10. Amesiodendron (1; A.
chinense; southwestern China to West Malesia), Bizonula (1;
B. letestui; Gabon), Camptolepis (4; C. crassifolia,
C. grandiflora, C. hygrophila, C. ramiflora;
tropical eastern and northeastern Africa, Madagascar), Paranephelium
(3; P. hainanense, P. hystrix, P. spirei; Yunnan to
Sumatra, Borneo and the Philippines), Schleichera (1; S.
oleosa; tropical Asia). – Tropical Africa, Madagascar, southwestern
China, tropical Asia.
Pancovieae Baill.,
Hist. Plant. 5: 378, 414. Dec 1874
c 29/235–245. Deinbollia (c
40; tropical and subtropical Africa, Madagascar, the Mascarene Islands),
Atalaya (12; tropical Africa, tropical Asia to Australia),
Pseudima (2; P. frutescens, P. pallidum; tropical
continental America), Sapindus (c 10; tropical and subtropical regions
in the Old World, one species, S. oahuensis, on the Hawaiian Islands,
one species, S. saponaria, in southeastern United States to South
America), Hornea (1; H. mauritiana; Mauritius),
Porocystis (2; P. acuminata, P. toulicioides; French
Guiana, Amazonian Brazil), Thouinidium (3; T. decandrum,
T. insigne, T. oblongum; Mexico, Central America, the West
Indies), Toulicia (13; northern South America), Lepisanthes
(c 25; tropical regions in the Old World to northern Australia),
Pometia (2; P. eximia, P. pinnata; Sri Lanka, the
Andaman Islands, the Nicobar Islands, Indochina, Taiwan, Malesia, Fiji, Samoa,
Tonga), Nephelium (c 16; Assam and Yunnan to Hainan and Malesia),
Dimocarpus (8; India and Sri Lanka to northeastern Queensland; incl.
Otonephelium?), Otonephelium (1; O. stipulaceum;
western India; in Dimocarpus?), Litchi (1; L.
chinensis; southern China to West Malesia and the Philippines),
Lecaniodiscus (2; L. cupanioides, L. fraxinifolius;
tropical Africa), Eriocoelum (c 10; tropical Africa),
Lepidopetalum (6; L. fructoglabrum, L. micans,
L. montanum, L. perrottetii, L. subdichotomum,
L. xylocarpum; the Andaman Islands, the Nicobar Islands, Sumatra, the
Philippines to New Guinea, the Bismarck Archipelago, Bougainville, northernmost
Queensland), Blighia (6; B. kamerunensis, B.
laurentii, B. mildbraedii, B. sapida, B.
unijugata, B. welwitschii; tropical Africa), Cubilia (1;
C. cubili; eastern Borneo, the Philippines, Sulawesi, western
Moluccas), Haplocoelopsis (1; H. africana; Central and
tropical East Africa), Glenniea (8; tropical Africa, Madagascar, Sri
Lanka, Indochina, Malesia), Laccodiscus (4; L. ferrugineus,
L. klaineanus, L. pseudostipularis, L.
spinulosodentatus; tropical West and Central Africa), Xerospermum
(c 11; Bangladesh, Indochina, West Malesia), Chytranthus (25–30;
tropical West and Central Africa), Namataea (1; N.
simplicifolia; Cameroon), Pancovia (10–12; tropical West and
Central Africa), Placodiscus (c 10; tropical Africa),
Pseudopancovia (1; P. heterophylla; Central Africa),
Radlkofera (1; R. calodendron; tropical West Africa),
Zollingeria (4; Z. borneensis, Z. dongnaiensis,
Z. laotica, Z. macrocarpa; Southeast Asia, Borneo)? –
Pantropical. Ovules two per carpel. – The positions of Hornea,
Porocystis, Thouinidium, Toulicia and
Zollingeria are highly provisional.
'Cupanieae'
Blume, Rumphia 3: 157. Jun 1847
38/465–470. Diploglottis (12;
New Guinea, eastern Queensland, eastern New South Wales),
Podonephelium (4; New Caledonia), Alectryon
(c 25; East Malesia to New Guinea, Australia, Solomon Islands, New Caledonia,
Vanuatu, Fiji, New Zealand, Samoa, the Hawaiian Islands),
Elattostachys (c 20; Malesia to New Guinea, eastern Queensland,
northeastern New South Wales, Solomon Islands, Vanuatu, New Caledonia, Fiji,
Tonga, Niue, Samoa), Jagera (1; J. javanica; East Malesia to
New Guinea, eastern Queensland, northeastern New South Wales),
’Guioa’ (c 65; Thailand, Malesia to New Guinea, eastern
Queensland, eastern New South Wales, New Caledonia, Fiji, Samoa, Tonga;
non-monophyletic), Mischocarpus (c 15; Southeast Asia, Malesia to New
Guinea, eastern Queensland, northeastern New South Wales), Sarcopteryx
(12–13; the Moluccas, New Guinea, eastern Queensland, northeastern New South
Wales), Molinaea (9; Madagascar, the Mascarene Islands), Tina
(17; Madagascar), 'Matayba' (c 50; subtropical and tropical
America; polyphyletic), Vouarana (2; V. anomala, V.
guianensis; Costa Rica to northern Brazil), Cupania (c 50;
tropical and subtropical America), Mischarytera (3; M.
bullata, M. lautereriana, M. macrobotrys; New Guinea,
eastern Queensland), Gongrodiscus (3; G. bilocularis, G.
parvifolius, G. sufferrugineus; New Caledonia),
Podonephelium (9; New Caledonia), Storthocalyx
(5; S. chryseus, S. corymbosus, S. leioneurus,
S. pancheri, S. sordidus; New Caledonia),
Rhysotoechia (c 15; Borneo, the Philippines, Sulawesi, the Moluccas,
New Guinea, eastern Queensland, northeastern New South Wales),
Lepiderema (8; New Guinea, eastern Queensland),
’Sarcotoechia’ (10–11; the Moluccas, New Guinea, northeastern
Queensland; polyphyletic), Toechima (c 8; Flores, New Guinea, eastern
Queensland, northeastern New South Wales), Alatococcus (1; A.
siqueirae; Brazil), Arytera (28; northeastern India, Southeast
Asia, Malesia to New Guinea, Solomon Islands, northernmost Northern Territory,
eastern Queensland and northeastern New South Wales, Fiji, Samoa, Tonga),
Synima (1; S. cordieri; southeastern New Guinea, northeastern
Queensland), Aporrhiza (7; A. lastoursvillensis, A.
le-testui, A. multijuga, A. paniculata, A.
talbotii, A. tessmannii, A. urophylla; tropical Africa),
Blighiopsis (1; B. pseudostipularis; Congo),
Cnesmocarpon (4; C. dasyantha, C. dentata, C.
discoloroides, C. montana; New Guinea, eastern Queensland),
’Cupaniopsis’ (c 60; Sulawesi, New Guinea, Solomon Islands,
northern and eastern Australia, New Caledonia, Fiji, Samoa, the Caroline
Islands; polyphyletic), Dilodendron (3; D. bipinnatum, D.
costaricense, D. elegans; tropical continental America),
Gloeocarpus (1; G. patentivalvis; the Philippines),
Gongrospermum (1; G. philippinense; the Philippines),
Lychnodiscus (7; L. brevibracteatus, L. cerospermus,
L. dananensis, L. grandifolius, L. multinervis,
L. papillosus, L. reticulatus; tropical Africa),
Pavieasia (3; P. anamensis, P. kwangsiensis, P.
yunnanensis; southern China, northern Vietnam), Pentascyphus (1;
P. thyrsiflorus; French Guiana, Suriname, Amazonian Brazil),
Phyllotrichum (1; P. mekongense; Laos),
Scyphonychium (1; S. multiflora; French Guiana, northeastern
Brazil), Sisyrolepis (1; S. muricata; Thailand, Cambodia),
Trigonachras (9; Malesia), Tripterodendron (1; T.
filicifolium; eastern central Brazil). – Pantropical, with their highest
diversity in tropical Asia and tropical Australia. – ‘Cupanieae’
is a paraphyletic grade (Acevedo-Rodríguez & al. 2017).
Macphersonia clade
9/c 42. Pappea (1; P.
capensis; tropical East to southern Africa, Dhofar in southeastern Arabian
Peninsula), Tsingya (1; T. bemarana; Madagascar),
Plagioscyphus (c 10; Madagascar); Gereaua (1; G.
perrieri; Madagascar), Conchopetalum (2; C.
brachysepalum, C. madagascariense; Madagascar), Chouxia
(6; C. bijugata, C. borealis, C. macrophylla, C.
mollis, C. saboureaui, C. sorindeioides; northern
Madagascar), Macphersonia (c 8; tropical East Africa, Madagascar,
Aldabra), Pseudopteris (3; P. ankaranensis, P.
arborea, P. decipiens; Madagascar), Beguea (10; B.
apetala; Madagascar). – Africa, Madagascar, Dhofar, with their highest
diversity in Madagascar. Flowers actinomorphic. Ovule usually one per carpel
(in Conchopetalum two ovules).
Tristiropsis clade
2/>6. Dictyoneura (2; D.
acuminata, D. obtusa; eastern Borneo, the Philippines, Sulawesi,
the Moluccas, New Guinea, northeastern Queensland), Tristiropsis
(>4; T. acutangula, T. canarioides, T.
obtusangula, T. subangula; Borneo, the Philippines and Sulawesi
to New Guinea and the Bismarck Archipelago, northeastern Queensland, Solomon
Islands, Palau, Christmas Island, Guam). – Malesia to New Guinea, Queensland,
islands in western Pacific.
Blomia clade
3/10. Blomia (1; B.
cupanioides; Mexico, Belize, Guatemala), Haplocoelum (6; H.
acuminatum, H. congolanum, H. dekindtianum, H.
foliolosum, H. gallaense, H. intermedium; tropical
Africa, Madagascar), Guindilia (3; G. cristata, G.
dissecta, G. trinervis; Chile, Argentina). – Tropical Africa,
Madagascar, Mexico, Central America, southern South America.
Melicocceae Blume in
Rumphia 3: 142. Jun 1847
4/64. Castanospora (1; C.
alphandii; eastern Queensland, northeastern New South Wales),
Melicoccus (10; Hispaniola, tropical South America), Talisia
(52; southern Mexico, Central America, tropical South America),
Tristira (1; T. triptera; eastern Philippines, Sulawesi, the
Moluccas). – Central Malesia, eastern Australia, Central and tropical South
America.
Paulliniodae
Acev.-Rodr. in Syst. Bot. 42(1): 108. Jan–Mar 2017
Athyaneae Acev.-Rodr.
in Syst. Bot. 42(1): 108. Jan–Mar 2017
2/3. Athyana (1; A.
weinmanniifolia; Peru, Bolivia, Paraguay, northern Argentina),
Diatenopteryx (2; D. grazielae, D. sorbifolia;
Brazil, Bolivia, Paraguay, northern Argentina). – Western tropical South
America.
Bridgesieae
Acev.-Rodr. in Syst. Bot. 42(1): 108. Jan–Mar 2017
1/1. Bridgesia (1; B.
incisifolia; Andean Chile).
Thouinieae Blume in
Rumphia 3: 186. 1847
3/c 285? Thouinia (c 30; Mexico,
northern Central America, the Bahamas, the Greater Antilles),
Allophylus (c 255?; tropical and subtropical regions on both
hemispheres), Allophylastrum (1; A. frutescens; Guyana,
Roraima in northern Brazil). – Pantropical.
Paullinieae Kunth ex
DC., Prodr. 1: 601. Jan (med.) 1824 [‘Paullinieae’]
6/475–480. Thinouia (10–12;
Central America, tropical and subtropical South America), Lophostigma
(2; L. plumosum, L. schunkei; Ecuador, Peru, Bolivia),
Urvillea (c 21; Texas, Mexico, Central America, tropical South
America), Cardiospermum (c 14; tropical America, one species, C.
halicacabum, also in tropical Africa, two species pantropical),
Paullinia (c 200; tropical America, one species, P. pinnata,
also in tropical Africa), Serjania (c 230; Mexico, Central America,
the West Indies, South America). – Tropical and subtropical America.
Unplaced
Sapindoideae
Chonopetalum (1; C.
stenodictyum; Equatorial Guinea).
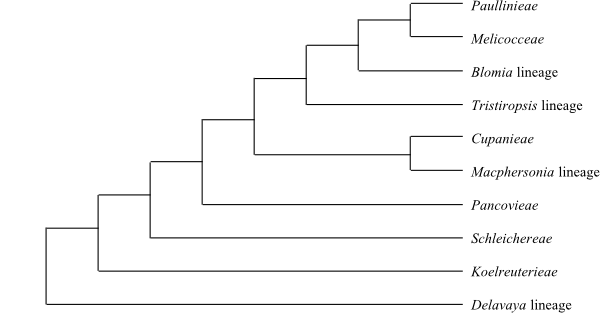 |
Cladogram (simplified) of Sapindaceae based on
morphology and DNA sequence data (Buerki & al. 2009).
|
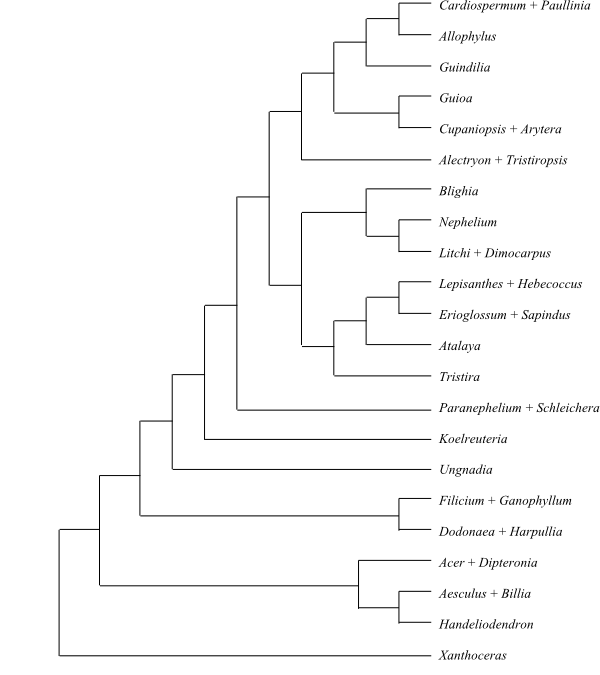 |
Cladogram of Sapindaceae based on DNA
sequence data (Harrington & al. 2005).
|
de Candolle in Nouv. Bull. Sci. Soc. Philom.
Paris 2: 209. Jan 1811 [‘Simarubeae’], nom. cons.
Quassiaceae Bertol.,
Prael. Rei Herb.: 262. Mar 1827 [’Quassiae’];
Simabaceae Horan., Char. Ess. Fam.: 179. 30 Jun 1847;
Ailanthaceae (Arn.) J. Agardh, Theoria Syst. Plant.:
223. Apr-Sep 1858 [‘Ailantheae’];
Castelaceae J. Agardh, Theoria Syst. Plant.: 181.
Apr-Sep 1858 [‘Casteleae’]; Soulameaceae
(Endl.) Pfeiffer, Nomencl. Bot. 2: 1202. 8 Mai 1874 [’Soulameae’];
Leitneriaceae Benth. et J. D. Hooker, Gen. Plant. 3:
vi, 396. 7 Feb 1880 [‘Leitnerieae’], nom. cons.;
Leitneriales Engl., Nat. Pflanzenfam. Nachtr. [1]:
345. Dec 1897
Genera/species 20/113–123
Distribution Tropical and
subtropical regions, few species (e.g. in Ailanthus) in temperate East
Asia.
Fossils Fruits and leaves
similar to those in Ailanthus are known from Eocene and younger strata
in the Northern Hemisphere. Fossils assigned to Leitneria have been
found in Oligocene layers in western Siberia and in Europe, East Asia and North
America. The fossil Cenozoic fruit of Chaneya from the Northern
Hemisphere may have been closely allied to Picrasma. Fossil wood under
the names of Ailanthoxylon and Simarouboxylon have been
described from the Deccan Intertrappean Beds from the Maastrichtian of
India.
Habit Monoecious,
polygamomonoecious, dioecious, or polygamodioecious (androdioecious) (sometimes
bisexual), evergreen or deciduous trees or shrubs. Bark, wood and seeds often
very bitter-tasting. Branch medulla characteristically light-coloured and
well-developed.
Vegetative anatomy Phellogen
ab initio superficial. Primary medullary rays at least in Leitneria
narrow and usually uniseriate. Vessel elements with simple perforation plates
(rarely also reticulate); lateral pits usually alternate (in Leitneria
scalariform to opposite), usually bordered (sometimes simple) pits. Imperforate
tracheary xylem elements fibre tracheids or libriform fibres with simple pits,
usually non-septate (also vasicentric tracheids). Wood rays uniseriate or
multiseriate, usually homocellular. Axial parenchyma apotracheal, or
paratracheal scanty, aliform, lozenge-aliform, winged-aliform, confluent,
vasicentric, or banded, or absent. Wood often fluorescent. Wood elements often
storied. Secondary phloem at least in Leitneria stratified into hard
fibrous and soft parenchymatous layers. Sieve tube plastids S type. Nodes
usually 3:3, trilacunar with three leaf traces (sometimes heptalacunar).
Secretory ducts with resinous substances sometimes present (especially in
medulla), often also vertical intercellular canals. Parenchyma often with
secretory cells containing oil, resin or mucilage. Sclereids frequent. Cortex
with or without cristarque cells. Silica bodies present in parenchyma in some
species. Prismatic calciumoxalate crystals or druses abundant.
Trichomes Hairs unicellular or
multicellular, usually simple; capitate glandular hairs sometimes present.
Leaves Usually alternate
(spiral; rarely opposite), usually imparipinnate (sometimes unifoliolate,
rarely trifoliolate, or simple and entire), with conduplicate or
supervolute-curved ptyxis (leaves in Holacantha scale-like or absent).
Stipules absent (in Picrasma and some species of Soulamea
petiolar or cauline pseudostipules); leaf sheath absent. Petiole vascular
bundles? Petiolules not articulated. Rhachis collapsing at nodes. Venation
pinnate, brochidodromous (sometimes reticulate). Stomata usually anomocytic
(sometimes paracytic). Cuticular wax crystalloids? Domatia as hair tufts or
absent. Adaxial or abaxial side of lamina often with flat to pitted glands and
glandular hairs. Epidermis with or without mucilaginous idioblasts. Mesophyll
with or without spherical idioblasts containing ethereal oils, usually with
sclerenchymatous idioblasts (containing sclereids of several types), usually
with calciumoxalate as druses or prismatic crystals. Leaf margin and leaflet
margins entire, serrate or basally sinuate. Extrafloral nectaries often
frequent on margin or adaxial side of lamina (rarely on petiole).
Inflorescence Terminal or
axillary, cymose thyrse, sometimes umbel- or raceme-like (in Leitneria
spike- or catkin-like), or simple or compound raceme, spike or panicle (flowers
rarely solitary). Flowers in Leitneria surrounded by large bracts.
Flowers Actinomorphic, small.
Receptacle sometimes prolonged into androphore or androgynophore. Hypogyny.
Sepals (three or) four or five (to eight), with imbricate or valvate
aestivation, usually connate at base (absent in Leitneria at least in
male flowers). Petals (three or) four or five (to eight), with imbricate,
valvate or contorted aestivation, free (absent in Leitneria).
Nectariferous disc extrastaminal (intrastaminal?) (sometimes modified into
gynophore or androgynophore) or absent.
Androecium Stamens usually
twice as many as petals (sometimes five, antesepalous, or ten to 18; in
Leitneria one to five), haplostemonous or diplostemonous. Filaments
free from each other and from tepals, often with lateral or basal hairy
scale-like appendages. Anthers usually dorsifixed (sometimes basifixed),
versatile or non-versatile, tetrasporangiate, usually introrse (sometimes
extrorse or latrorse), longicidal (dehiscing by longitudinal slits). Tapetum
secretory, with tri- to duodecemnucleate cells. Female flowers sometimes with
staminodia (male flowers of Eurycoma and Picrolemma with
outer whorl of staminodia).
Pollen grains
Microsporogenesis simultaneous. Pollen grains 3(–6)-colpor(oid)ate, shed as
monads, bicellular at dispersal. Exine usually tectate (rarely semitectate),
with columellate infratectum, perforate or striate (in Leitneria
microreticulate or reticulate).
Gynoecium Pistil composed of
(one or) two to five (to eight; in Leitneria one fertile carpel and
one carpel sterile, degenerating) usually more or less connate carpels (often
fused only in stylar parts; in Picrolemma and species of
Ailanthus free). Short gynophore often present. Ovary superior,
unilocular to quinquelocular (to octalocular). Style single, simple, often more
or less basal or lateral, or stylodia several or absent. Stigmas stellately
spreading, branched and more or less recurved (in Leitneria
decurrent), pointed, with prolonged receptive zone, or stigma single, capitate
to lobate, non-papillate, Dry type. Male flowers often with pistillodium.
Ovules Placentation usually
axile (in Leitneria parietal). Ovule one (or two) per carpel, usually
anatropous (rarely hemianatropous or amphitropous, in Leitneria
orthotropous), usually pendulous (in Leitneria ascending), epitropous,
unitegmic or bitegmic, crassinucellar. Micropyle usually endostomal (embryo
axis and micropyle forming acute angle; in Leitneria and
Samadera? Z-shaped [zig-zag]). Outer integument three to ten cell
layers thick. Inner integument two to eight cell layers thick (in
Leitneria long and folded). Hypostase present. Parietal tissue six to
20 cell layers thick. Nucellar cap approx. two cell layers thick.
Megagametophyte monosporous, Polygonum type. Synergids sometimes with
a filiform apparatus. Endosperm development ab initio nuclear. Endosperm
haustorium chalazal. Embryogenesis usually onagrad.
Fruit A one- or two-seeded
drupe with thin exocarp, a samara, or a schizocarp with (one or) two to five
berry-like, drupaceous or samaroid mericarps.
Seeds Aril absent. Seeds
sometimes pachychalazal. Seed coat usually testal (in Leitneria
endotegmic). Exotesta indistinct, membranous. Endotesta often somewhat
lignified. Testa in Leitneria multiplicative. Tegmen crushed.
Mesotegmen in Leitneria with reticulate thickenings and only
endotegmen persistent. Perisperm usually not developed (rarely developed,
thin). Endosperm sparse or absent (sometimes with hemicellulose as storage
polysaccharide, in Leitneria with starch). Embryo straight or curved,
well differentiated, oily, with chlorophyll. Cotyledons two, large. Germination
phanerocotylar or cryptocotylar.
Cytology n = 8–13; 16
(Leitneria)
DNA Plastid gene
rpl22 absent from Leitneria.
Phytochemistry Flavonols
(kaempferol, quercetin), tetracyclic triterpenes, pentanortriterpenes
(malabaricol etc.), bitter-tasting triterpenoid lactones (simaroubalides and
quassinoids, e.g. quassin, neoquassin, chaparrin, cedroniline, glaucarubol,
simorolide, melianodiol, samaderene B and C, brusatol and klaineanone;
especially in the bark), limonoids (limonin), ellagic acid, tannins, carboline
and canthinone alkaloids, chromones, polyacetylenes, and polyacetate-derived
arthroquinones present. Proanthocyanidins, saponins and cyanogenic compounds
not found.
Use Ornamental plants,
medicinal plants, insecticides, incense, timber, pulp.
SystematicsSimaroubaceae are probably
sister to Meliaceae,
although the support is fairly weak.
Picrasmateae are sister-group to the
remaining Simaroubaceae
and supported by a 6 bp deletion in the 5’-trnK intron. Leaves and
fruits are similar in Castela and Picrasma, whereas
Holacantha is a richly spiny shrub nested inside Castela.
The anemophilous Leitneria floridana
is sister to the [Brucea+Soulamea]
clade. Leitneria is characterized by a combination of features which
is unique to Simaroubaceae, i.e., dioecy,
silky young branches, bark not bitter, catkin-like cymose inflorescences
consisting of numerous reduced dichasia, reduced flowers without calyx (at
least in male flowers; in female flowers sepaloid bracts/bracteoles?), disc
absent, four stamens with basifixed anthers, pollen grains with reticulate
exine, one carpel (in reality two carpels, one of which reduced and rarely
developing), unilocular (pseudomonomerous) ovary, decurrent stigma, parietal
placentation, ascending ovules, bistomal micropyle, drupe, endotegmic
seed-coat, mesotegmen with reticulate thickenings, starchy endosperm, and n =
16. – Judging from fossils, the Leitneria clade was more diverse in
the past.
Picrasmateae Engl. in
Engler et Prantl, Nat. Pflanzenfam. III, 4: 207. Apr 1896
2/c 23. Picrasma (8; the
Himalayas to Japan and New Guinea, southern Mexico, Central America, the West
Indies, tropical South America), Castela (c 15; southern United
States, Mexico, Central America, the West Indies, tropical South America, the
Galápagos Islands). – Tropical Asia, tropical and subtropical America.
Simaroubeae Dumort.,
Anal. Fam. Plant.: 45. 1829
18/90–100. Ailanthus (6;
A. altissima, A. excelsa, A. fordii, A.
integrifolia, A. triphysa, A. vietnamensis; Turkestan,
India, Sri Lanka, China, Southeast Asia, Malesia to New Guinea, northern and
eastern Australia, Taiwan); Nothospondias (1; N. staudtii;
Central Africa); Leitneria (1; L. floridana; southeastern
United States), Brucea (8–10; tropical regions in the Old World), Soulamea
(14; one species, S. terminalioides, endemic to Mahé in the
Seychelles, eleven species endemic to New Caledonia, one species, S.
soulameoides, endemic to Fiji, one species, S. amara, widespread
in Southeast Asia and Malesia to Polynesia); Picrolemma (2; P.
huberi, P. sprucei; eastern Peru, Amazonian Brazil),
Samadera (5–6; Madagascar, Southeast Asia to eastern Queensland),
Quassia (2; Q. amara: tropical South America; Q.
africana: western tropical Africa), Gymnostemon (1; G.
zaizou; Côte d’Ivoire), Perriera (2; P.
madagascariensis, P. orientalis; Madagascar), Hannoa
(5–7; Central Africa), Odyendea (1; O. gabonensis; tropical
Africa), Eurycoma (3; E. apiculata, E. harmandiana,
E. longifolia; tropical Southeast Asia, West Malesia to the
Philippines), Iridosma (1; I. letestui; Central Africa),
Pierreodendron (2; P. africanum, P. kerstingii;
tropical West and Central Africa to Angola), Simaba (6; S.
arborea, S. guianensis, S. monophylla, S.
obovata, S. orinocensis, S. polyphylla; Florida, Central
America, the West Indies, tropical South America, with their highest diversity
in Amazonas), Homalolepis (25–30; Central America, tropical South
America), Simarouba (6; S. amara, S. berteroana,
S. glauca, S. laevis, S. tulae, S.
versicolor; Florida, Panamá, the West Indies, tropical South America).
– Pantropical, East Asia, southeastern United States.
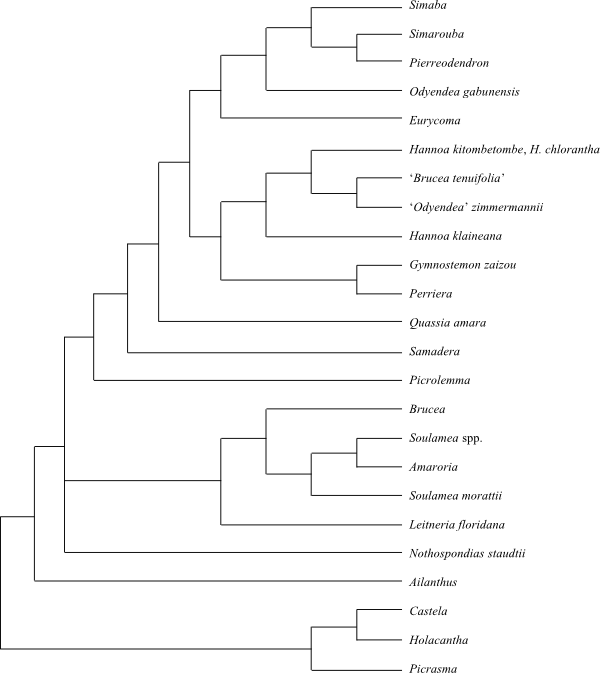 |
Strict consensus parsimony tree (simplified)
of Simaroubaceae
based on DNA sequence data (Clayton & al. 2007, 2009).
Nothospondias is sister to Picrolemma plus all the
remaining Simaroubaceae in the
majority rule consensus Bayesian tree. Picrolemma is sister to
Quassia and Pierreodendron sister to
Gymnostemon with high support in the analyses by Devecchi
& al. (2018).
|
Literature
Abbe EC. 1974. Flowers and inflorescences of
the ‘Amentiferae’. – Bot. Rev. 40: 159-261.
Abbe EC, Earle TT. 1940. Inflorescence,
floral anatomy, and morphology of Leitneria floridana. – Bull.
Torrey Bot. Club 67: 173-193.
Abdelgaleil S, Okamura H, Iwagawa T, Sato A,
Miyahara I, Doe M, Nakatani M. 2001. Khayanolides, rearranged phragmalin
limonoid antifeedants from Khaya senegalensis. – Tetrahedron 57:
119-126.
Abkenar AA, Isshiki S, Tashiro Y. 2004.
Phylogenetic relationships in the ‘true citrus fruit trees’ revealed by
PCR-RFLP analysis of cpDNA. – Sci. Horticult. 102: 233-242.
Aboutabl EA, El-Sakhawy FS, Fathy MM, Megid
RMA. 2000. Composition and antimicrobial activity of the leaf and fruit oils
from Amoora rohituka Wight et Arn. – J. Essential Oil Res. 12:
635-638.
Acevedo-Rodríguez P. 1990. Distributional
patterns in Brazilian Serjania (Sapindaceae). – Acta Bot. Bras. 4:
69-82.
Acevedo-Rodríguez P. 1993a. A revision of
Lophostigma (Sapindaceae).
– Syst. Bot. 18: 379-388.
Acevedo-Rodríguez P. 1993b. Systematics of
Serjania (Sapindaceae) I:
a revision of Serjania sect. Platycoccus. – Mem. New York
Bot. Gard. 67: 1-94.
Acevedo-Rodríguez P. 1997. Novelties in
Neotropical Sapindaceae. –
BioLlania 6: 143-151.
Acevedo-Rodríguez P. 1998a. Paullinia
lingulata (Sapindaceae), a new
species from French Guiana. – Brittonia 50: 514-516.
Acevedo-Rodríguez P. 1998b. Novelties in
Neotropical Sapindaceae II. Notes
on Averrhoidium, Serjania, and Porocystis. – Novon
8: 105-106.
Acevedo-Rodríguez P. 2003. Flora Neotropica.
Monograph 87. Melicocceae (Sapindaceae) – Melicoccus
and Talisia. – New York Botanical Garden, Bronx, New York, pp.
1-179.
Acevedo-Rodríguez P. 2011.
Allophylastrum: a new genus of Sapindaceae from northern South
America. – PhytoKeys 5: 39-43.
Acevedo-Rodríguez P. 2012.
Alatococcus, a new genus of Sapindaceae from Espirito Santo,
Brazil. – PhytoKeys 10: 1-5.
Acevedo-Rodríguez P, Ferrucci MS. 2002.
Averrhoidium dalyi (Sapindaceae): a new species from
Western Amazonia. – Brittonia 54: 112-115.
Acevedo-Rodríguez P, Somner GV. 2001. Two
new species of Serjania (Sapindaceae) from southeastern Brazil.
– Brittonia 53: 477-481.
Acevedo-Rodríguez P, Welzen PC van, Adema F,
Ham RWJM van der. 2011. Sapindaceae. – In: Kubitzki K (ed),
Families and genera of vascular plants X. Flowering plants. Eudicots. Sapindales, Cucurbitales, Myrtaceae. Springer, Heidelberg,
Dordrecht, London, New York, pp. 357-407.
Acevedo-Rodríguez P, Wurdack KJ, Ferrucci
MS, Johnson G, Dias P, Coelho RG, Somner GV, Steinmann VW, Zimmer EA, Strong
MT. 2017. Generic relationships and classification of tribe Paullinieae (Sapindaceae) with a new concept of
supertribe Paulliniodae. – Syst. Bot. 42: 96-114.
Ackerly DD, Donoghue MJ. 1998. Leaf size,
sapling allometry, and Corner’s rules: phylogeny and correlated evolution in
maples (Acer). – Amer. Natur. 152: 767-791.
Adams CD, Taylor DR, Warner JM. 1973.
N-methylflindersine from Spathelia sorbifolia. – Phytochemistry 12:
1359-1360.
Adawadkar PD, El Sohly MA. 1983. An urushiol
derivative from poison sumac. – Phytochemistry 22: 1280-1281.
Adema F. 1988. Notes on Cupaniopsis
(Sapindaceae) 1. New species from
New Caledonia. – Bull. Mus. Natl. Hist. Nat. Paris, sér. B, Adansonia 10:
263-270.
Adema F. 1991. Cupaniopsis Radlk.
(Sapindaceae), a monograph. –
Leiden Bot. Ser. 15: 1-190.
Adema F. 1993. Elattostachys (Blume)
Radlk. (Sapindaceae) in New
Caledonia. – Bull. Mus. Natl. Hist. Nat. B, Adansonia, sér. 4, 15:
141-151.
Adema F, Ham RWJM van der. 1993.
Cnesmocarpon (gen. nov.), Jagera, and Trigonachras
(Sapindaceae-Cupanieae): phylogeny
and systematics. – Blumea 38: 173-215.
Adema F, Leenhouts PW, Welzen PC van. 1994.
Sapindaceae. – In: Kalkman C et
al. (eds), Flora Malesiana I, 11(3), Flora Malesiana Foundation,
Rijksherbarium/Hortus Botanicus, Leiden, pp. 419-768.
Adsersen A, Smitt UW, Simonsen HT,
Christensen SB, Jaroszewski JW. 2007. Prenylated acetophenones from
Melicope obscura and Melicope obtusifolia ssp.
obtusifolia var. arborea and their distribution in Rutaceae. – Biochem. Syst. Ecol. 35:
447-453.
Agarwal M, Gupta S, Painuly V. 2005.
Xylotomic study of the family Sapindaceae: microstructure,
systematics and ecological trends. – Indian Forester 131: 1024-1040.
Agbedahunsi JM, Elujoba AA. 1998. Grandifolin
from Khaya grandifoliola stem bark. – Nig. J. Nat. Prod. Med. 2:
34-36.
Agostinho SMM, das GF da Silva MF, Fernandes
JB, Vieira PC, Pinheiro AL, Vilela EF. 1994. Limonoids from Toona
ciliata and speculations on their chemosystematic and ecological
significance. – Biochem. Syst. Ecol. 22: 323-328.
Aguilar-Ortigoza CJ, Sosa V. 2004a. The
evolution of toxic phenolic compounds in a group of Anacardiaceae genera. – Taxon 53:
357-364.
Aguilar-Ortigoza CJ, Sosa Y. 2004b. Taxonomic
revision of the genus Pseudosmodingium (Anacardiaceae). – Rhodora
106(928): 348-359.
Aguilar-Ortigoza CJ, Sosa V, Aguilar-Ortigoza
M. 2003. Toxic phenols in various Anacardiaceae species. – Econ.
Bot. 57: 354-364.
Aguilar-Ortigoza CJ, Sosa V, Angeles G. 2004.
Phylogenetic relationships of three genera in Anacardiaceae: Bonetiella,
Pseudosmodingium, and Smodingium. – Brittonia 56:
169-184.
Ahluwalia K. 1962. Morphological and
embryological studies in the families Zygophyllaceae
and Salvadoraceae I.
Embryology and systematic position of Peganum harmala Linn.; II. Some
embryological aspects of Salvadora persica Linn. – M.Sc. thesis,
University of Delhi, India.
Airy-Shaw HK, Forman LL. 1967. The genus
Spondias L. (Anacardiaceae) in tropical Asia. –
Kew Bull. 21: 1-19.
Akhani H. 2002. Notes on the flora of Iran:
1. Asparagus (Asparagaceae) and
Nitraria (Zygophyllaceae).
– Edinburgh J. Bot. 59: 295-302.
Al-Nowaihi AS, Khalifa SF. 1973. Studies on
some taxa of the Geraniales II. Floral
morphology of certain Linaceae, Rutaceae and Geraniaceae with a
reference to the consistency of some characters. – J. Indian Bot. Soc. 52:
198-206.
Álvarez R, Encina A, Pérez Hidalgo N. 2008.
Pistacia terebinthus L. leaflets: an anatomical study. – Plant Syst.
Evol. 272: 107-118.
Alves GGN, El Ottra JHL, Devecchi MF, Demarco
D, Pirani JR. 2017. Structure of the flower of Simaba (Simaroubaceae) and its anatomical
novelties. – Bot. J. Linn. Soc. 183: 162-176.
Andrés-Hernández AR, Organista DE. 2002.
Morfología de plántulas de Bursera Jacq. ex L. (Burseraceae) y sus implicaciones
filogenéticas. – Bol. Soc. Bot. México 70: 5-12.
Andrés-Hernández AR, Terrazas, T. 2009.
Leaf architecture of Rhus s. str. (Anacardiaceae). – Feddes Repert.
120: 293-306.
Andrés-Hernández AR, Espinosa D,
Fraile-Ortega M, Terrazas T. 2012. Venation patterns of Bursera
species Jacq. ex L. (Burseraceae)
and systematic significance. – Plant Syst. Evol. 298: 1723-1731.
Andrés-Hernández AR, Terrazas T, Salazar G,
Ochoterena H. 2014. Phylogenetic analysis based on structural and combined
analyses of Rhus s.s. (Anacardiaceae). – Bot. J. Linn.
Soc. 176: 452-468.
Anzótegui LM. 1971. El pollen de las Anacardiaceae del N.E. de la
Argentina. – Ameghiniana 8: 329-340.
Appanah S. 1982. Pollination of
androdioecious Xerospermum intermedium Radlk. (Sapindaceae) in a rain forest. –
Biol. J. Linn. Soc. 18: 11-34.
Appelhans MS, Smets E, Razafimandimbison SG,
Haevermans T, Marle EJ van, Couloux A, Rabarison H, Randrianarivelojosia M,
Kessler PJ. 2011. Phylogeny, evolutionary trends and classification of the
Spathelia-Paeroxylon clade: morphological and molecular
insights. – Ann. Bot. 107: 1259-1277.
Appelhans MS, Heuven BJ, Lens F, Baas P.
2012. Phylogenetic and ecological signals in the wood of Spathelioideae (Rutaceae). – IAWA J. 33: 337-353.
Appelhans MS, Keßler PJA, Smets E,
Razafimandimbison SG, Janssens SB. 2012. Age and historical biogeography of the
pantropically distributed Spathelioideae (Rutaceae, Sapindales). – J. Biogeogr. 39:
1235-1250.
Appelhans MS, Wen J, Wood KR, Allan GJ,
Zimmer EA, Wagner WL. 2014. Molecular phylogenetic analysis of Hawaiian Rutaceae (Melicope,
Platydesma and Zanthoxylum) and their different colonization
patterns. – Bot. J. Linn. Soc. 174: 425-448.
Appelhans MS, Wen J, Wagner WL. 2014. A
molecular phylogeny of Acronychia, Euodia, Melicope
and relatives (Rutaceae) reveals
polyphyletic genera and key innovations for species richness. – Molec.
Phylogen. Evol. 79: 54-68.
Appelhans MS, Krohm S, Manafzadeh S, Wen J.
2016. Phylogenetic placement of Psilopeganum, a rare monotypic genus
of Rutaceae (the citrus family)
endemic to China. – J. Syst. Evol 54: 535-544.
Appelhans MS, Wood KR, Wagner WL. 2017.
Reduction of the Hawaiian genus Platydesma into Melicope
section Pelea (Rutaceae) and
notes on the monophyly of the section. – PhytoKeys 91: 125-137.
Appelhans MS, Reichelt N, Groppo M, Paetzold
C, Wen J. 2018. Phylogeny and biogeography of the pantropical genus
Zanthoxylum and its closest relatives in the proto-Rutaceae group (Rutaceae). – Mol. Phylogen. Evol. 126:
31-44.
Araújo EF de, Queiroz LP de, Machado MA.
2003. What is Citrus? Taxonomic implications from a study of cp-DNA
evolution in tribe Citreae (Rutaceae
subfamily Aurantioideae). – Organisms Divers. Evol. 3: 55-62.
Aregullin M, Gompperb ME, Rodrigueza E. 2002.
Sesquiterpene lactone in Tratinnickia aspera. – Biochem. Syst. Ecol.
30: 187-188.
Arifkhodzhaev AO, Rakhimov DA. 1986.
Polysaccharides of saponin containing plants 3. Polysaccharides of the epigeal
organs of Biebersteinia multifida. – Khimiya Prirodnykh Soedinenii
6: 773-774.
Arifkhodzhaev AO, Rakhimov DA. 1993.
Polysaccharides of saponin containing plants 4. Structure of glucan-A,
glucane-B and glucan-C of Biebersteinia multifida. – Khimiya
Prirodnykh Soedinenii 2: 188-191.
Arifkhodzhaev AO, Rakhimov DA. 1994.
Polysaccharides of saponin-carrying plants 5. Structural study of glucane-A,
glucane-B, glucane-C and their oligosaccharides of Biebersteinia
multifida plants. – Khimiya Prirodnykh Soedinenii 6: 709-714.
Arifkhodzhaev AO, Arifkhodzhaev KA,
Kondratenko ES. 1985. Polysaccharides of saponin containing plants 2. Isolation
and characterization of polysaccharides from Biebersteinia multifida.
– Khimia Prirodnykh Soedinenii 6: 755-757.
Armstrong JA. 1991. Studies on pollination
and systematics in the Australian Rutaceae. – Ph.D. diss., University of
New South Wales, Sydney.
Armstrong JA. 2002. The genus Zieria
(Rutaceae): a systematic and
evolutionary study. – Aust. Syst. Bot. 15: 277-463.
Armstrong JA, Powell JM. 1980.
Neobyrnesia (Rutaceae): a new
genus endemic to northern Australia. – Telopea 1: 399-408.
Arrillaga-Maffei BR, Ziliani G, Ren J. 1973.
Anacardiáceas de Uruguay, Bol. 126. – Universidad de la Republica,
Montevideo.
Aryavand A. 1975. Contribution à l’étude
cytotaxonomique de Biebersteinia multifida DC. (Géraniacées). –
Compt. Rend. Acad. Sci. Paris, sér. D, 280: 1551-1554.
Aubréville A, Pellegrin F. 1936. Rutacée et
Méliacée nouvelles de la Côte d’Ivoire. – Bull. Soc. Bot. France 83:
488-489.
Aubréville A, Pellegrin F. 1937.
Gymnostemon A. et P., genre nouveau de la Côte d’Ivoire, voisin
d’un endémique de Madagascar. – Bull. Soc. Bot. France 84: 181-184.
Aubréville A, Pellegrin F. 1950. Rutacées.
– Notul. Syst. 14: 60.
Ayafor FJ, Sodengam BL, Ngo Bilon A, Tsamo E,
Kimbu SF. 1982. Furoquinoline alkaloids of Teclea oubanguiensis. –
J. Nat. Prod. 45: 714-717.
Ayafor JF, Sodengam BL, Ngo Bilon A. 1986.
Limonoids of Teclea oubanguiensis. – J. Nat. Prod. 49: 583-587.
Bacchetta G, Brullo S, Giusso del Galdo G.
2006. Ruta lamarmorae (Rutaceae), a new species from Sardinia.
– Edinburgh J. Bot. 63: 153-160.
Bachelier JB, Endress PK. 2007. Development
of inflorescences, cupules, and flowers in Amphipterygium and
comparison with Pistacia (Anacardiaceae). – Intern. J. Plant
Sci. 168: 1237-1253.
Bachelier JB, Endress PK. 2008. Floral
structure of Kirkia (Kirkiaceae) and its position in Sapindales. – Ann. Bot. 102:
539-550.
Bachelier JB, Endress PK. 2009. Comparative
floral morphology and anatomy of Anacardiaceae and Burseraceae (Sapindales), with a special focus on
gynoecium structure and evolution. – Bot. J. Linn. Soc. 159: 499-571.
Bachelier JB, Endress PK. Ronse De Craene LP.
2011. Comparative floral structure and development in Nitrariaceae (Sapindales) and systematic
implications. – In: Wanntorp L, Ronse De Craene LP (eds), Flowers on the Tree
of Life, Systematics Association, Spec. Vol. 80, Cambridge University Press,
Cambridge, pp. 181-217.
Backer HJ, Haack NH. 1938. Le principe
toxique des fruits de Renghas (Semecarpus heterophylla Bl.). – Rec.
Trav. Chim. Pays-Bas 57: 225-232.
Baehni C, Bonner EB. 1953. Les faisceaux
vasculaires dans l’ovaire de l’Aesculus parviflora. – Candollea
14: 85-91.
Baer H, Hooton M, Fales H, Wu A, Schaub F.
1980. Catecholic and other constituents of the leaves of Toxicodendron
radicans and variation of urushiol concentrations within one plant. –
Phytochemistry 19: 799-802.
Bailey VL. 1962. Revision of the genus
Ptelea (Rutaceae). –
Brittonia 14: 1-45.
Baillon H. 1876. Traité du développement de
la fleur et du fruit d’Anacardiées. – Bull. Mus. Natl. Hist. Nat., sér.,
III, Adansonia 11: 158-163.
Baily VL. 1962. Revision of the genus
Ptelea (Rutaceae). –
Brittonia 14: 1-45.
Bakker FT, Vassiliades DD, Morton C,
Savolainen V. 1998. Phylogenetic relationships of Biebersteinia
Stephan (Geraniaceae)
inferred from rbcL and atpB sequence comparisons. – Bot. J.
Linn. Soc. 127: 149-158.
Baksi SK. 1976. Pollen morphology of the
genera Gluta Linnaeus and Melanorrhoea Wallich (Anacardiaceae). – In: Ferguson IK,
Muller J (eds), The evolutionary significance of the exine, Academic Press,
London, pp. 379-395.
Bamba FA, Vanhaelen FR, Vanhaelen M, Lo
I,Toppet M, Ferster A, Fondu P. 1999. In vitro antisickling activity of a
rearranged limonoid isolated from Khaya senegalensis. – Planta Med.
65: 209-212.
Barber CA. 1892. On the nature and
development of the corky excrescences on stems of Zanthoxylum. –
Ann. Bot. 6: 155-166.
Bardot-Vaucoulon M. 2002. Une nouvelle
espèce de Commiphora (Burseraceae) du Nord de Madagascar.
– Adansonia 24: 43-47.
Barfod A. 1987. 104. Anacardiaceae. – In: Harling G,
Sparre B (eds), Flora of Ecuador 30, Swedish Natural Science Research Council,
Stockholm, pp. 11-49.
Barfod A. 1988. Inflorescence morphology of
some South American Anacardiaceae
and the possible phylogenetic trends. – Nord. J. Bot. 8: 3-11.
Barkley FA. 1937. A monographic study of
Rhus and its immediate allies in North and Central America, including
the West Indies. – Ann. Missouri Bot. Gard. 24: 265-498.
Barkley FA. 1940. Pseudosmodingium
and Mosquitoxylum. – Amer. Midl. Natur. 24: 666-680.
Barkley FA. 1942. A key to the genera of Anacardiaceae. – Amer. Midl. Nat.
28: 465-474.
Barkley FA. 1944. Schinus L. –
Brittonia 5: 160-198.
Barkley FA. 1957. Generic key to the Sumac
family (Anacardiaceae). –
Lloydia 20: 255-265.
Barkley FA. 1962. Anacardiaceae: Rhoideae:
Loxopterygium. – Lloydia 25: 109-122.
Barkley FA. 1963. A criticism of the
traditional concept of the genus Rhus. – Prosp. Iraq Biol. 3:
52-58.
Barkley FA, Reed MJ. 1939.
Actinocheita. – Amer. Midl. Natur. 21: 368-377.
Barrett HC, Rhodes AM. 1976. A numerical
taxonomic study of affinity relationships in cultivated Citrus and its
close relatives. – Syst. Bot. 1: 105-136.
Barrett RA, Bayly MJ, Duretto MF, Forster PI,
Ladiges PY, Cantrill DJ. 2015. A chloroplast phylogeny of Zieria (Rutaceae) in Australia and New Caledonia
shows widespread incongruence with species-level taxonomy. – Aust. Syst. Bot.
27: 427-449.
Barrett RA, Bayly MJ, Duretto MF, Forster PI,
Ladiges PY, Cantrill DJ. 2018. Phylogenetic analysis of Zieria (Rutaceae) in Australia and New Caledonia
based on nuclear ribosomal DNA shows species polyphyly, divergent paralogues
and incongruence with chloroplast DNA. – Aust. Syst. Bot. 31: 16-47.
Barros MA, Barth OM. 1994. Catálogo
sistemático do pólen das plantas arbóreas do Brasil meridional, vol. 28. Burseraceae e Clethraceae. –
Rev. Brasil. Biol. 54: 317-322.
Barth MO. 1982. Variações polínicas em
espécies brasileiras da familia Rutaceae. – Bol. Inst. Geoci. Univ.
São Paulo 13: 129-134.
Basak RK. 1963. Pollen morphology of Indian
Simaroubaceae. – Bull. Bot.
Surv. India 5: 381-397.
Basak RK. 1967. Studies on the pollen
morphology of Simaroubaceae. –
Bull. Bot. Surv. India 9: 63-67.
Baudouin G, Tillequin F, Koch M, Pusset J,
Sêvenet T. 1981. Plantes de Nouvelle-Calédonie. LXXIII. Alcaloïdes de
Dutaillyea oreophila et de Dutaillyea drupacea. – J. Nat.
Prod. 44: 546-550.
Baum H. 1950. Septalspalten im Gynözeum von
Koelreuteria paniculata. – Österr. Bot. Zeitschr. 97: 207-215.
Baumann TW, Schulthess BH, Hänni K. 1995.
Guaraná (Paullinia cupana) rewards seed dispersers without
intoxicating them by caffeine. – Phytochemistry 39: 1063-1070.
Bausher MG, Singh ND, Lee S-B, Jansen RK,
Daniell H. 2006. the complete chloroplast genome sequence of Citrus
sinensis (L.) Osbeck var. ‘Ridge Pineapple’: organization and
phylogenetic relationships to other angiosperms. – BMC Plant Biol. 6: 21.
Bawa KS. 1977. The reproductive biology of
Cupania guatemalensis Radlk. (Sapindaceae). –Evolution 31:
52-63.
Bayer RJ, Mabberley DJ, Morton C, Miller CH,
Sharma IK. Pfeil BE, Rich S, Hitchcock R, Sykes S. 2009. A molecular phylogeny
of the orange subfamily (Rutaceae:
Aurantioideae) using nine cpDNA sequences. – Amer. J. Bot. 96: 668-685.
Bayly MJ. 1994. Variation within
Eriostemon angustifolius (Rutaceae) and the recognition of a new
species, E. sporadicus. – Aust. Syst. Bot. 7: 275-280.
Bayly MJ. 1998. Notes on the Eriostemon
myoporoides (Rutaceae) species
complex, including new names and a new generic placement in
Philotheca. – Muelleria 11: 113-126.
Bayly MJ, Brophy JJ, Forster PI, Goldsack RJ,
Wilson PG. 1998. Reinstatement of Eriostemon banksii (Rutaceae), with a report on the
composition of leaf essential oils in E. banksii and E.
australasius s. str. – Aust. Syst. Bot. 11: 13-22.
Bayly MJ, Holmes GD, Forster PI, Cantrill DJ,
Ladiges PY. 2013. Major clades of Australasian Rutoideae (Rutaceae) based on rbcL and
atpB sequences. – PLoS ONE 8(8); e72493 doi:
10.1371/journal.pone.0072493
Bayly MJ, Duretto MF, Holmes GD, Forster PI,
Cantrill DJ, Ladiges PY. 2015. Transfer of the New Caledonian genus
Boronella to Boronia (Rutaceae) based on analyses of cpDNA and
nrDNA. – Aust. Syst. Bot. 28: 111-123.
Bayly MJ, Holmes GD, Forster PI, Munzinger J,
Cantrill DJ, Ladiges PY. 2016. Phylogeny, classification and biogeography of
Halfordia (Rutaceae) in
Australia and New Caledonia. – Plant Syst. Evol. 302: 1457-1470.
Beattie GAC, Holford P, Mabberley DJ, Haigh
AM, Bayer R, Broadbent P. 2008. On the origins of Citrus,
Huanglongbing, Diaphorina citri and Trioza erytreae. – In:
Proc. Intern. Res. Conf. on Huanglongbing, Ordlando, Florida, 2-5 Dec. 2008,
pp. 23-56.
Becerra JX. 2003. Evolution of Mexican
Bursera (Burseraceae)
inferred from ITS, ETS, and 5S nuclear ribosomal DNA sequences. – Mol.
Phylogen. Evol. 26: 300-309.
Becerra JX, Venable DL. 1999. Nuclear
ribosomal DNA phylogeny and its implication for evolutionary trends in Mexican
Bursera (Burseraceae). –
Amer. J. Bot. 86: 1047-1057.
Becerra JX, Venable DL, Evans PH, Bowers WS.
2001. Interactions between chemical and mechanical defences in the plant genus
Bursera and their implications for herbivores. – Amer. Zool. 41:
865-876.
Becerra JX, Noge K, Venable DL. 2009.
Macroevolutionary chemical escalation in an ancient plant-herbivore arms race.
– Proc. Natl. Acad. Sci. U.S.A. 106: 18062-18066.
Becerra JX, Noge K, Olivier S, Venable DL.
2012. The monophyly of Bursera and its impact for divergence times of
Burseraceae. – Taxon 61:
333-343.
Beck HT. 1991a. The taxonomy and economic
botany of the cultivated guaraná and its wild relatives and the generic limits
within the Paullinieae (Sapindaceae). – PhD diss., City
University of New York, New York.
Beck HT. 1991b. Typificiation of
Radlkofer’s infragenerica names in Paullinia L. (Sapindaceae). – Brittonia 43:
201-202.
Beck HT. 1992. Chimborazoa (Sapindaceae), a new genus from
Ecuador. – Brittonia 44: 306-311.
Benencia F, Courreges MC, Coulombie FC. 2000.
In vivo and in vitro immunomodulatory activities of Trichilia glabra
aqueous leaf extracts. – J. Ethnopharmac. 69: 199-205.
Bergen MA van, Ham RWJM van der, Turner H.
1995. Morphology and evolution of Arytera pollen (Sapindaceae-Cupanieae). – Blumea 40:
195-209.
Berry EW. 1924a. New Tertiary species of
Anacardium and Vantanea from Colombia. – Pan Amer. Geol.
42: 259-263.
Berry EW. 1924b. An Oligocene cashew nut from
South America. – Amer J. Sci. 8: 126-126.
Berry EW. 1929. An Anacardium from
the Eocene of Texas. – J. Washington Acad. Sci. 19: 37-39.
Beurton C. 1986. Phyllodienbildende
Zanthoxylum-Sippen in Cuba I. Zanthoxylum phyllopterum und
Z. rolandii (Fam. Rutaceae).
– Feddes Repert. 97: 29-41.
Beurton C. 1987. Phyllodienbildende
Zanthoxylum-Sippen in Cuba II. Z. dumosum, Z.
pseudodumosum, Z. ignoratum und Z. arnoldii (Fam. Rutaceae). – Feddes Repert. 98:
53-73.
Beurton C. 1994. Gynoecium and perianth in
Zanthoxylum s.l. (Rutaceae).
– Plant Syst. Evol. 189: 165-191.
Beurton C. 1996. Die Früchte und Samen der
kubanischen Zanthoxylum-Arten (Rutaceae). – Willdenowia 26:
283-299.
Beurton C. 2000a. The genus
Plethadenia (Rutaceae). –
Willdenowia 30: 115-123.
Beurton C. 2000b. Notes on
Zanthoxylum (Rutaceae) from
the Antilles. – Willdenowia 30: 125-130.
Bhatt JR, Ram HYM. 1992. Development and
ultrastructure of primary secretory ducts in the stem of Semecarpus
anacardium (Anacardiaceae).
– IAWA Bull., n.s., 13: 173-185.
Biesboer DD. 1975. Pollen morphology of the
Aceraceae. – Grana 15: 19-27.
Biondi E. 1981. Arganioxylon sardum
N. Gen., N. Sp. et Sclerocaryoxylon chiarugii N. Gen., N. Sp.: bois
fossils du Miocène de la Sardaigne (Italie). – Rev. Palaeobot. Palynol. 34:
301-320.
Blenk P. 1884. Ueber die durchsichtigen
Punkte in den Blättern. Simarubaceae. – Flora 67/1884: 291-299.
Boas F. 1913. Beiträge zur Anatomie und
Systematik der Simarubaceen. – Beitr. Bot. Centralbl. 29: 303-356.
Boesewinkel FD. 1977. Development of ovule
and testa in Rutaceae I.
Ruta, Zanthoxylum, and Skimmia. – Acta Bot. Neerl.
26: 193-211.
Boesewinkel FD. 1978. Development of ovule
and testa in Rutaceae III. Some
representatives of the Aurantioideae. – Acta Bot. Neerl. 27: 341-354.
Boesewinkel FD. 1980. Development of ovule
and seed-coat in the Rutales-Geraniales assembly. –
Academisch Proefschrift, Hugo de Vries Laboratory, University of Amsterdam, The
Netherlands.
Boesewinkel FD. 1984. Development of ovule
and seed coat in Cneorum tricoccum L. (Cneoraceae). – Acta Bot.
Neerl. 33: 61-70.
Boesewinkel FD. 1997. Seed structure and
phylogenetic relationships of the Geraniales. – Bot. Jahrb.
Syst. 119: 277-291.
Boesewinkel FD, Bouman F. 1978. Development
of ovule and testa in Rutaceae II. The
unitegmic and pachychalazal seed of Glycosmis cf. arborea
(Roxb.) DC. – Acta Bot. Neerl. 27: 69-78.
Bohning-Gaese K, Gaese BH, Rabemanantsoa SB.
1999. Importance of primary and secondary seed dispersal in the Malagasy tree
Commiphora guillaumini. – Ecology 8: 821-832.
Bombardelli E, Morazonni P, Griffini A. 1996.
Aesculus hippocastanum L. – Fitoterapia 67: 483-511.
Bonnefille R, Letouzey R. 1976. Fruits
fossiles de Antrocaryon dans la Vallée de l’Omo (Éthiopie). –
Adansonia, n.s., 16: 65-82.
Bortenschlager S. 1967. Vorläufige
Mitteilungen zur Pollenmorphologie in der Familie der Geraniaceen und ihre
systematische Bedeutung. – Grana Palynol. 7: 400-468.
Bosser J. 2002. Une nouvelle espèce de
Turraea (Meliaceae) des
Mascareignes. Localisation de T. thouarsiana et identité de T.
casimiriana. – Adansonia, sér. III, 24: 113-116.
Boulter M, Benfield J, Fisher H, Gee D,
Lhotak M. 1996. The evolution and global migration of Aceraceae. – Philos.
Trans., Ser. B, 351: 589-603.
Bourobou HB, Breteler FJ. 1999. Novitates
Gabonenses 35. Sorindeia oxyandra, another new Anacardiaceae from Gabon. – Syst.
Geogr. Plants 69: 115-117.
Braggins JE, Large MF, Mabberley DJ. 1999.
Sexual arrangements in kohekohe (Dysoxylum spectabile, Meliaceae). – Telopea 89: 315-324.
Breteler FJ. 2001. The genus
Trichoscypha (Anacardiaceae) in Upper Guinea: a
synoptic revision. – Adansonia, sér. III, 23: 247-264.
Breteler FJ. 2003. The African genus
Sorindeia (Anacardiaceae): a synoptic revision.
– Adansonia, sér. III, 25: 93-113.
Breteler FJ. 2004. The genus
Trichoscypha (Anacardiaceae) in Lower Guinea and
Congolia: a synoptic revision. – Adansonia, sér. III, 26: 97-127.
Brizicky GK. 1962. Taxonomic and
nomenclatural notes on Zanthoxylum and Glycosmis. – J.
Arnold Arbor. 43: 80-93.
Brizicky GK. 1963. Taxonomy and nomenclatural
notes on the genus Rhus (Anacardiaceae). – J. Arnold Arbor.
44: 60-80.
Broadhurst L. 2000. Morphometric analysis of
variation in Geleznowia verrucosa Turcz. (Rutaceae). – Aust. Syst Bot. 13:
479-490.
Brophy JJ, Forster PI, Goldsack RJ. 1994.
Diversity in Australian populations of Murraya paniculata (Rutaceae): new evidence from volatile
leaf oils. – Aust Syst. Bot. 7: 409-418.
Brophy JJ, Goldsack RJ, Forster PI. 2005. The
leaf oils of Coatesia and Geijera (Rutaceae) from Australia. – J. Ess. Oil
Res. 17: 169-174.
Brückner C. 1991. Fruchtanatomische Studien
an Dictamnus albus L., Zanthoxylum simulans Hance, Ptelea
trifoliata L. und Ruta graveolens L. (Rutaceae). – Feddes Repert. 102:
541-570.
Budantsev L. 1994. Fossil flowering plants of
Russia and adjacent states III. Leitneriaceae-Juglandaceae. – The
Komarov Botanical Institute, Russian Academy of Sciences, St. Petersburg.
Buerki S, Callmander MW, Lowry II PP,
Phillipson PB. 2009. A synoptic revision of the genus Lepisanthes
Blume (Sapindaceae) in Madagascar.
– Adansonia, sér. III, 31: 301-309.
Buerki S, Forest F, Acevedo-Rodriguez P,
Callmander MW, Nylander JAA, Harrington M, Sanmartin I, Kupfer P, Alvarez N.
2009. Plastid and nuclear DNA markers reveal intricate relationships at
subfamilial and tribal levels in the soapberry family (Sapindaceae). – Mol. Phylogen. Evol.
51: 238-258.
Buerki S, Lowry PP II, Phillipson PB,
Callmander MW. 2010. Molecular phylogenetic and morphological evidence supports
recognition of Gereaua, a new endemic genus of Sapindaceae from Madagascar. – Syst.
Bot. 35: 172-180.
Buerki S, Lowry PP II, Alvarez N,
Razafimandimbison SG, Küpfer P, Callmander MW. 2010. Phylogeny and
circumscription of Sapindaceae
revisited: molecular sequence data, morphology, and biogeography support
recognition of a new family, Xanthoceraceae. – Plant Ecol. Evol. 143:
148-159.
Buerki S, Forest F, Salamin N, Alvarez N.
2011. Comparative performance of supertree algorithms in large data sets using
the soapberry family (Sapindaceae)
as a case study. – Syst. Biol. 60: 32-44.
Buerki S, Forest F, Alvarez N, Nylander JAA,
Arrigo N, Sanmartín I. 2011. An evaluation of new parsimony-based versus
parametric inference methods in biogeography: a case study using the globally
distributed plant family Sapindaceae. – J. Biogeogr. 38:
531-550.
Buerki S, Lowry PP II, Andriambololonera S,
Phillipson PB, Vary L, Callmander MW. 2011. How to kill two genera with one
tree: clarifying generic circumscriptions in an endemic Malagasy clade of Sapindaceae. – Bot. J. Linn. Soc.
165: 223-234.
Buerki S, Callmander MW, Lowry PP II, Devey
DS, Munzinger J. 2012. Phylogenetic inference of New Caledonian lineages of Sapindaceae: molecular evidence
requires a reassessment of generic circumscriptions. – Taxon 61: 109-119.
Buerki S, Forest F, Stadler T, Alvarez N.
2013. The abrupt climate change at the Eocene-Oligocene boundary and the
emergence of South-East Asia triggered the spread of sapindaceous lineages. –
Ann. Bot. 112: 151-160.
Buerki S, Doherty R, Gautier L, Callmander
MW. 2014. Rediscovery of the genus Tsingya Capuron (Sapindaceae) and its phylogenetic
position. – Candollea 69: 195-200.
Buijsen JRM. 1995. Leaf anatomy of
Harpullia, Majidea and Conchopetalum (Sapindaceae). – Blumea 40:
345-361.
Buijsen JRM, Welzen PC van, Ham RWJM van der.
2003. A phylogenetic analysis of Harpullia (Sapindaceae) with notes on historical
biogeography. – Syst. Bot. 28: 106-117.
Burkill JH. 1931. An enumeration of the
species of Paramignya, Atalantia and Citrus, found
in Malaya. – Gard. Bull. 5: 212-223.
Burnham RJ, Carranco NL. 2004. Miocene winged
fruits of Loxopterygium (Anacardiaceae) from the Ecuadorian
Andes. – Amer. J. Bot. 91: 1767-1773.
But PP-H, Kong Y-C, Ng K, Chang H-T, Li Q, Yu
S, Waterman PG. 1986. A chemotaxonomic study of Murraya (Rutaceae) in China. – Acta Phytotaxon.
Sin. 24: 193-202.
But PP-H, Kong Y-C, Li Q, Chang H-T, Chang
K-L, Wong K-M, Gray AI, Waterman PG. 1988. Chemotaxonomic relationships between
Murraya and Merrillia (Rutaceae). – Acta Phytotaxon. Sin. 26:
205-210.
But PP-H, Poon AW-S, Shaw P-C, Simmons MP,
Greger H. 2009. Contribution of molecular cladistics to the taxonomy of Rutaceae in China. – J. Syst. Evol. 47:
144-150.
Buttrose MS, Lott JNA. 1978. Inclusions in
seed protein bodies in members of the Compositae and Anacardiaceae: comparison with other
dicotyledonous families. – Can. J. Bot. 56: 2062-2071.
Cambell WE, Majal T, Bean A. 1986. Coumarins
of the Rutoideae: tribe Diosmeae. – Phytochemistry 25: 655-657.
Campagna MN, Gattuso M, Martinez ML,
Rodriguez MV, Di Sapio O. 2017. Novel micromorphological features of wood and
bark of Argentinean Simaroubaceae. – New Zealand J.
Bot. 55: 134-150.
Cao L-M, Xia N-H. 2008. Structural characters
of leaf epidermis and their systematic significance in Sapindaceae from China. – Acta Bot.
Yunnan. 30: 405-421. [In Chinese]
Cao L-M, Xia N-H, Deng Y-F. 2008. Embryology
of Handeliodendron bodinieri (Sapindaceae) and its systematic value:
development of male and female gametophytes. – Plant Syst. Evol. 274:
17-23.
Capuron R. 1961. Contributions à l’étude
de la flore forestière de Madagascar III. Sur quelques plantes ayant
contribué au peuplement de Madagascar. – Adansonia, sér. II, 1: 65-92.
Capuron R. 1962. Contributions à l’étude
de la flore forestière de Madagascar VI. Note sur les Burseracées. –
Adansonia 2: 268-283.
Capuron R. 1967. Nouvelles observations sur
les Rutacées de Madagascar. – Adansonia, sér. II, 7: 479-500.
Capuron R. 1969. Révision des Sapindacées
de Madagascar et des Comores. – Mém. Mus. Natl. Hist. Nat. Paris, sér. B,
Bot. 19: 1-189.
Caris P, Smets E, Coster K de, Ronse De
Craene LP. 2006. Floral ontogeny of Cneorum tricoccon L. – Plant
Syst. Evol. 257: 223-232.
Carlquist SJ. 1988. Wood anatomy of
Cneoraceae: ecology, relationships, and generic definition. – Aliso 12:
7-16.
Carmello-Guerreiro SM, Paoli AAS. 1999.
Morfoligia e desenvolvimento pós-seminal de Schinus terebinthifolius
Raddi, Lithraea molleoides (Vell.) Engl., Myracrodruon
urundeuva Fr. Allem. y Astronium graveolens Jacq. (Anacardiaceae). – Naturalia (São
Paulo) 24: 127-138.
Carpenter RC, Sotheeswaran S, Sultanbawa MU,
Balasubramaniam S. 1980. (-)-5-methylmellein and catechol derivatives from four
Semecarpus species. – Phytochemistry 19: 445-447.
Case RJ, Tucker AO, Maciarello MJ, Wheeler
KA. 2003. Chemistry and ethnobotany of commercial incense Copals, Copal Blanco,
Copal Oro, and Copal Negro, of North America. – Econ. Bot. 57: 189-202.
Castañeda-Posadas C, Cevallos-Ferriz SRS.
2007. Swietenia (Meliaceae)
flower in Late Oligocene-Early Miocene amber from Simojovel de Allende,
Chiapas, Mexico. – Amer. J. Bot. 94: 1821-1827.
Cavalcante P. 1983. Revisão taxonômica do
gênero Simaba Aublet (Simaroubaceae) na América do Sul.
– Publ. Avulsas Mus. Goeldi 37, Belém, Pará, Brasil.
Champluvier D. 1999. Un Sorindeia
(Anacardiaceae) nouveau
d’Afrique Centrale. – Syst. Geogr. Plants 69: 39-44.
Chang C-S, Giannasi DE. 1991. Foliar
flavonoids af Acer sect. Palmata series Palmata. –
Syst. Bot. 16: 225-241.
Chang C-S, Kim H. 2003. Analysis of
morphological variation of the Acer tschonoskii complex in eastern
Asia: implications of inflorescence size and number of flowers within sect.
Macrantha. – Bot. J. Linn. Soc. 143: 29-42.
Chang KL, Wong KM, Gray AI, Waterman PG.
1988. Chemotaxonomic relationship between Murraya and
Merrillia (Rutaceae). –
Acta Phytotaxon. Sin. 26: 205-210. [In Chinese]
Chang YL. 1982. Sapindaceae. – In: Angiosperm pollen
flora of tropic and subtropic China, Institute of Botany and South China
Institute of Botany, Academia Sinica, pp. 343-344.
Chase MW, Morton CM, Kallunki JA. 1999.
Phylogenetic relationships of Rutaceae: a cladistic analysis of the
subfamilies using evidence from rbcL and atpB sequence
variation. – Amer. J. Bot. 86: 1191-1199.
Chaudhuri K. 1940. A note on the morphology
and chromosome number of Litchi chinensis Sonner. – Curr. Sci. 9:
416.
Chayamarit K. 1997. Molecular phylogenetic
analysis of Anacardiaceae in
Thailand. – Thai Forest Bull. 25: 1-13.
Cheek MR. 1989a. The systematic seed anatomy
of the Meliaceae. – Ph.D. diss.,
Bodleian Library, University of Oxford, England.
Cheek MR. 1989b. A new Trichilia (Meliaceae) from Tanzania and its
relationship with Pseudobersama. – Kew Bull. 44: 457-463.
Cheek MR. 1990a. A new species of
Turraea (Meliaceae) from
Madagascar and comments on the status of Naregamia. – Kew Bull. 45:
711-715.
Cheek MR. 1990b. Systematic seed anatomy of
the Turraeeae (Meliaceae); taxonomic
and ecological aspects. – Mitt. Inst. Allg. Bot. Hamburg 23b: 683-706.
Cheek MR. 1992. The wood anatomy of
Pseudobersama mossambicensis and Trichilia capitata (Meliaceae) compared. – Kew Bull. 47:
753-758.
Cheek MR. 1996. The identity of
Naregamia Wight & Arn. (Meliaceae). – Kew Bull. 51: 716.
Cheek MR, Haba P. 2016. Spiny African
Allophylus (Sapindaceae):
a synopsis. – Kew Bull. 71: 57 DOI 10.1007/S12225-016-9672-3
Cheek MR, Rakotozafy A. 1991. The identity of
Leroy’s fifth subfamily of the Meliaceae, and a new combination in
Commiphora (Burseraceae).
– Taxon 40: 231-237.
Cheek MR, Oben B, Heller T. 2009. The
identity of the West-Central African Oricia lecomteana Pierre with a
new combination in Vepris (Rutaceae). – Kew Bull. 64: 509-512.
Cheng YM, Ferguson DK, Li CS, Jiang XM, Wang
YF. 2006. Cedreloxylon cristalliferum, a new record of angiosperm wood
of Pliocene age from Yunnan, China. – IAWA J. 27: 145-152.
Chevalier A. 1949. Les Nitraria,
plantes utiles des déserts salés. – Rev. Bot. Appl. Agric. Trop. 29:
595-601.
Chiang F. 1989. Casimiroa gregii,
formerly in Sargentia (Rutaceae). – Taxon 38: 116-119.
Cho H-J, Kim S, Suh Y, Park C-W. 1996. ITS
sequences of some Acer species and phylogenetic implication. –
Korean J. Plant Taxon. 26: 271-291.
Choi B-K, Duretto MF, Hong S-P. 2012.
Comparative seed morphology of Boronia and related genera (Boroniinae:
Rutaceae) and its systematic
implications. – Nord. J. Bot. 30: 241-256.
Choux MP. 1925. Les Cupaniées malgaches. –
Compt. Rend. Acad. Sci. Paris 181: 71-72.
Choux MP. 1926. Quelques nouvelles
Sapindacées de Madagascar. – Compt. Rend. Acad. Sci. Paris 103: 712-714.
Choux MP. 1929. Nouvelles observations sur
les Sapindacées de Madagascar. – Ann. Mus. Hist. Nat. Marseille 22:
35-47.
Clark TP. 1994. The species of
Walsura and Pseudoclausena genus-novum (Meliaceae). – Blumea 38: 247-302.
Clarkson J, Chase MW, Harley MM. 2002.
Phylogenetic relationships in Burseraceae based on plastid
rps16 intron sequences. – Kew Bull. 57: 183-193.
Classen-Bockhoff R, Armstrong JA,
Ohligschläger M. 1991. The inflorescence of the Australian genera
Diplolaena R. Br. and Chorilaena Endl. (Rutaceae). – Aust. J. Bot. 39:
31-42.
Clayton JW. 2011. Simaroubaceae. – In: Kubitzki K
(ed), Families and genera of vascular plants X. Flowering plants. Eudicots. Sapindales, Cucurbitales, Myrtaceae. Springer, Heidelberg,
Dordrecht, London, New York, pp. 408-423.
Clayton JW, Fernando ES, Soltis PS, Soltis
DS. 2007. Molecular phylogeny of the tree-of-heaven family (Simaroubaceae) based on chloroplast
and nuclear markers. – Intern. J. Plant Sci. 168: 1325-1339.
Clayton JW, Soltis PS, Soltis DS. 2009.
Recent long-distance dispersal overshadows ancient biogeographical patterns in
a pantropical angiosperm family (Simaroubaceae, Sapindales). – Syst. Biol. 58:
395-410.
Coelho RLG. 2014. Estudos sistemáticos das
espécies neotropicais de Allophylus L. (Sapindaceae). – Ph.D. diss.,
Universidade Estadual de Campinas, São Paulo, Brazil.
Cojocaru M, Droby S, Glotter E, Goldman A,
Gottlieb HE, Jacoby B, Prusky D. 1986. 5-(12-heptadecenyl)-resorcinol, the
major component of the antifungal activity in the peel of mango fruit. –
Phytochemistry 25: 1093-1095.
Constantinidis TA. 1996. Biebersteinia
orphanidis Boiss. – Flora Mediterranea 6: 308-312.
Coombes PH, Naidoo D, Mulholland DA,
Randrianarivelojosia M. 2005. Quassinoids from the leaves of the Madagascan Simaroubaceae Samadera
madagascariensis. – Phytochemistry 66: 2734-2739.
Copeland HF. 1955. The reproductive
structures of Pistacia chinensis (Anacardiaceae). – Phytomorphology
5: 440-449.
Copeland HF. 1959. The reproductive
structures of Schinus molle (Anacardiaceae). – Madroño 15:
14-25.
Copeland HF. 1961. Observations on the
reproductive structures of Anacardium occidentale. – Phytomorphology
11: 315-325.
Copeland HF, Doyle BE. 1940. Some features of
the structure of Toxicodendron diversiloba. – Amer. J. Bot. 27:
932-939.
Corazza-Nunes MJ, Moreira Novelli V, Rosas
Moreira ALO, Carvalho Nunes WM de, Alves de Carvalho S, Macaho MA. 2006. The
phylogeny of Rutaceae: Contributions
from molecular systematics. – In: Sharma AK, Sharma A (eds), Plant genome
biodiversity and evolution, Vol. 1, Part C. Phanerogams
(Angiosperms-Dicotyledons), Science Publ., Enfield, New Hampshire, pp.
331-360.
Corbett SL, Manchester SR. 2004.
Phytogeography and fossil history of Ailanthus (Simaroubaceae). – Intern. J. Plant
Sci. 165: 671-690.
Corthout J, Janssens J, Pieters L, Vanden
Berghe D, Vlietninck AJ. 1989. The isolation of a long chain phenol from
Spondias mombin. – Planta Med. 55: 112-113.
Coulerie P, Maciuk A, Lebouvier N, Hnawia E,
Guillemot J-C, Canard B, Figadère B, Nour M. 2013. Phytochemical study of
Myrtopsis corymbosa, perspectives for anti-dengue natural compound
research. – Rec. Nat. Prod. 7(3): 250-253.
Coulleri J, Dematteis M, Ferrucci M. 2012. A
new insight into Serjania Mill. (Sapindaceae, Paullinieae) infrageneric
classification: a cytogenetic approach. – Plant Syst. Evol. 298:
1743-1753.
Coulleri JP, Urdampilleta JD, Ferrucci MS.
2014. Genome size evolution in Sapindaceae at subfamily level: a case
study of independence in relation to karyological and palynological traits. –
Bot. J. Linn. Soc. 174: 589-600.
Cowan RS, Brizicky GK. 1960. Taxonomic
relationships of Diomma Engler ex Harms. – Mem. New York Bot. Gard.
10: 38-64.
Craven LA, Dunlop CR. 2009. Quassia
arnhemensis, a new species of Simaroubaceae from Australia. –
Novon 19: 454-456.
Crayn DM, Fernando ES, Gadek PA, Quinn CJ.
1995. A reassessment of the familial affinities of the Mexican genus
Recchia Mocino & Sesse ex DC. – Brittonia 47: 397-402.
Croat TB. 1976. Flora of Panama. Sapindaceae. – Ann. Missouri Bot.
Gard. 63: 419-540.
Cronquist A. 1944a. Studies in the Simaroubaceae I. The genus
Castela. – J. Arnold Arbor. 25: 122-128.
Cronquist A. 1944b. Studies in the Simaroubaceae II. The genus
Simarouba. – Bull. Torrey Bot. Club 71: 226-234.
Cronquist A. 1944c. Studies in the Simaroubaceae III. The genus
Simaba. – Lloydia 7: 81-92.
Cronquist A. 1944d. Studies in the Simaroubaceae IV. Resumé of the
American genera. – Brittonia 5: 128-147.
Cruz R, Duarte M, Pirani JR, Melo-de-Pinna
GFA. 2017. Phylogenetic analysis and evolution of morphological characters in
Metrodorea and related species in Rutoideae (Rutaceae). – Plant Syst. Evol. 303:
927-943.
Dagne E, Yenesew A, Waterman PG, Gray AI.
1988. The chemical systematics of the Rutaceae, subfamily Toddalioideae, in
Africa. – Biochem. Syst. Ecol. 16: 179-188.
Daly DC. 1987. A taxonomic revision of
Protium Burm. f. in Eastern Amazonia and the Guianas. – Ph.D. diss.,
City University of New York, New York.
Daly DC. 1989. Studies in Neotropical Burseraceae II. Generic limits in New
World Protieae and Canarieae. – Brittonia 41: 17-27.
Daly DC. 1990. Studies in Neotropical Burseraceae III. The genus
Tetragastris and the forests of eastern Brazil. – Kew Bull. 45:
179-194.
Daly DC. 1992a. Studies in Neotropical Burseraceae V. Two new taxa of
Protium from eastern Brazil. – Kew Bull. 47: 713-719.
Daly DC. 1992b. Studies in Neotropical Burseraceae VI. New taxa and
combinations in Protium Burm. f. – Brittonia 44: 280-299.
Daly DC. 1997. Burseraceae. – In: Steyermark P,
Berry PE, Holst B (eds), Flora of the Venezuelan Guayana III, Timber Press,
Portland, Oregon, pp. 688-728.
Daly DC. 1999. Studies in Neotropical Burseraceae IX. Notes on
Trattinnickia, including a synopsis, in eastern Brazil’s Atlantic
forest complex. – Kew Bull. 54: 129-137.
Daly DC. 2002. Studies in Neotropical Burseraceae X. Crepidospermum
atlanticum sp. nov., a genus new to the Atlantic forest complex of Eastern
Brazil. – Kew Bull. 57: 471-477.
Daly DC. 2005. Studies in Neotropical Burseraceae XII. Dacryodes
edilsonii, a new species from southwestern Amazonia. – Brittonia 57:
118-122.
Daly DC. 2007. Studies in Neotropical Burseraceae XIII. A new section of
Protium from the Neotropics. – Brittonia 59: 1-24.
Daly DC, Martínez-Habibe MC. 2003. Studies
in Neotropical Burseraceae XI.
Notes on Dacryodes Vahl, including a new species from the Rio Negro
basin in Amazonia. – Brittonia 54: 266-274.
Daly DC, Martínez-Habibe MC. 2016. Seven new
species of Dacryodes from western Colombia. Studies in neotropical Burseraceae XXI. – Brittonia 68:
120-137.
Daly DC, Harley MM, Martínez-Habibe M-C,
Weeks A. 2011. Burseraceae. – In:
Kubitzki K (ed), Families and genera of vascular plants X. Flowering plants.
Eudicots. Sapindales, Cucurbitales, Myrtaceae. Springer, Heidelberg,
Dordrecht, London, New York, pp. 76-104.
DDaly DC, Raharimampionona J, Federman S.
2015. A revision of Canarium L. (Burseraceae) in Madagascar. –
Adansonia 37: 277-345.
Danguy P, Choux MP. 1926. Sapinda´cees
malgaches nouvelles ou peu connues. – Bull. Mus. Natl. Hist. Nat. Paris 32:
387-392.
Danguy P, Choux MP. 1927. Sapindacées
malgaches nouvelles ou peu connues. – Bull. Mus. Natl. Hist. Nat. Paris 33:
102-105.
Da Silva MF das GF, Gottlieb OR. 1987.
Evolution of quassinoids and limonoids in the Rutales. – Biochem. Syst. Ecol.
15: 85-103.
Da Silva MF das GF, Gottlieb OR, Dreyer DL.
1984. Evolution of limonoids in the Meliaceae. – Biochem. Syst. Ecol. 12:
299-310.
Da Silva MF das GF, Gottlieb OR, Ehrendorfer
F. 1988. Chemosystematics of Rutaceae:
suggestions for a more natural taxonomy and evolutionary interpretation of the
family. – Plant Syst. Evol. 161: 97-134.
Da Silva MF das GF, Agostinho SMM, Paula JR
de, Neto JO, Castro-Gamboa I, Filho ER, Fernandes JB, Vieira PV. 1999.
Chemistry of Toona ciliata and Cedrela odorata graft (Meliaceae): chemosystematic and
ecological significane. – Pure Appl. Chem. 71: 1083-1087.
Datta PC, Samanta P. 1977. Cytotaxonomy of Meliaceae. – Cytologia 42: 197-208.
Daumann E. 1974. Zur Frage nach dem Vorkommen
eines Septalnektariums bei Dicotyledonen, zugleich ein Beitrag zur
Blütenmorphologie und Bestäubungsökologie von Buxus L. und
Cneorum L. – Preslia 46: 97-109.
David E. 1938. Embryologische Untersuchungen
an Myoporaceen, Salvadoraceen, Sapindaceen und Hippocrateaceen. – Planta 28:
680-703.
Davies FG. 1997. A new genus
Haplocoelopsis (Sapindaceae) from East and Central
Africa. – Kew Bull. 52: 231-234.
Davies FG, Verdcourt B. 1998. Sapindaceae. – In: Beentje HJ,
Whitehouse CM (ed), Flora of tropical East Africa, A. A. Balkema, Rotterdam,
pp. 1-108.
Delendick TJ. 1990. A survey of foliar
flavonoids in the Aceraceae. – Mem. New York Bot. Gard. 54: 1-129.
Den Outer RW, Van Veenendaal W.L.H. 1986.
Distribution and development of secretory ducts in Trichoscypha (Anacardiaceae). – Acta Bot. Neerl.
35: 425-435.
De Nova JA, Medina R, Montero JC, Weeks A,
Rosell J, Olson ME, Eguiarte LE, Magallón S. 2012. Insights into the
historical construction of species-rich Mesoamerican seasonally dry tropical
forests: the diversification of Bursera (Burseraceae, Sapindales). – New Phytol. 193:
276-287.
dePamphilis C, Wyatt R. 1989. Hybridization
and introgression in buckeyes (Aesculus: Hippocastanaceae): a review
of the evidence and a hypothesis to explain long-distance gene flow. – Syst.
Bot. 14: 593-611.
Desai S. 1962. Cytology and embryology of the
Rutaceae. – Phytomorphology 12:
178-184.
Devecchi MF, Thomas WW, Plunkett GM, Pirani
JR. 2018. Testing the monophyly of Simaba (Simaroubaceae): evidence from five
molecular regions and morphology. – Mol. Phylogen. Evol. 120: 63-82.
Diakanamwa C, Diallo B, Vanhaelen-Fastre M.
1991. 3,3’-Di-O-methylellagic acid 4-O-â-D-xylopyronoside from Kirkia
acuminata roots. – Fitoterapia 62: 87-88.
Ding Hou. 1978. Anacardiaceae. – In: Steenis CGGJ
van (ed), Flora Malesiana I, 8(3), Sijthoff & Noordhoff International
Publ., Alphen aan den Rijn, The Netherlands, pp. 395-548.
Dreyer DL. 1969. Coumarins and alkaloids of
the genus Ptelea. – Phytochemistry 8: 1013-1020.
Dreyer DL. 1983. Limonoids of the Rutaceae. – In: Waterman PG, Grundon MF
(eds), Chemistry and chemical taxonomy of the Rutales, Academic Press, London,
pp. 215-245.
Du Y, Oshima R, Kumanotani J. 1984.
Reversed-phase liquid chromatographic separation and identification of
constituents of urushiol in the sap of the lac tree, Rhus vernicifera.
– J. Chromatogr. 284: 463-467.
Dugo G, Di Giacomo A (eds). 2002. Citrus. –
Taylor & Francis, London.
Duretto MF, Forster PI. 2007. A taxonomic
revision of the genus Zieria Sm. (Rutaceae) in Queensland. –
Austrobaileya 7: 473-544.
Duretto MF, Ladiges PY. 1997. Morphological
variation within the Boronia grandisepala group (Rutaceae) and the description of nine
taxa endemic to the Northern Territory, Australia. – Aust. Syst. Bot. 10:
249-302.
Duretto MF, Ladiges PY. 1999. A cladistic
analysis of Boronia section Valvatae (Rutaceae). – Aust. Syst. Bot. 11:
636-665.
Edman G. 1936. Zur Verkieselung und
Systematik der Simaroubaceae. –
Svensk Bot. Tidskr. 30: 491-514.
Edwards KJ, Gadek PA. 2001. Evolution and
biogeography of Alectryon (Sapindaceae). – Mol. Phylogen. Evol.
20: 14-26.
Eggers SH. 1974. Vesicant principles of
Smodingium argutum (Anacardiaceae). – J. South Afr.
Chem. Inst. 27: 99-104.
Eggli U. 1995. A synoptical revision of
Operculicarya (Anacardiaceae). – Adansonia, sér.
IV, 3-4: 149-158.
Elgin N. 1950. Les caractères morphologiques
et anatomiques du Peganum harmala L. (Zygophyllaceae).
– Rev. Fac. Sci. Univ. Istanbul, sér. B, 15: 333-361.
El Hadidi MM. 1975. Zygophyllaceae in
Africa. – Boissieria 24: 317-323.
El Ottra JHL, Pirani JR, Endress PK. 2013.
Fusion within and between whorls of floral organs in Galipeinae (Rutaceae): structural features and
evolutionary implications. – Ann. Bot. 111: 821-837.
Endress PK, Jenny M, Fallen ME. 1983.
Convergent elaboration of apocarpous gynoecia in higher advanced dicotyledons
(Sapindales, Malvales, Gentianales). – Nord.
J. Bot. 3: 293-300.
Engelmeier D, Hadacek F, Pacher T, Varodaya
S, Greger H. 2000. Cyclopenta[b]benzofurans from Aglaia species with
pronounced antifungal activity against rice blast fungus (Pyricularia
grisea). – J. Agric. Food Chem. 48: 1400-1404.
Engler A. 1883. Über die morphologischen
Verhältnisse und die geographische Verbreitung der Gattung Rhus, wie
der mit ihr verwandten, lebenden und ausgestorbenen Anacardiaceae. – Engl. Bot. Jahrb.
Syst. 1: 365-426.
Engler A. 1896a. Zygophyllaceae.
– In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(4), W.
Engelmann, Leipzig, pp. 74-93.
Engler A. 1896b. Cneoraceae. – In: Engler
A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(4), W. Engelmann,
Leipzig, pp. 93-94.
Engler A. 1896c. Rutaceae. – In: Engler A, Prantl K
(eds), Die natürlichen Pflanzenfamilien III(4), W. Engelmann, Leipzig, pp.
95-201.
Engler A. 1896d. Simarubaceae. – In: Engler
A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(4), W. Engelmann,
Leipzig, pp. 202-230.
Engler A. 1896e. Burseraceae. – In: Engler A, Prantl
K (eds), Die natürlichen Pflanzenfamilien III(4), W. Engelmann, Leipzig, pp.
231-257.
Engler A. 1896f. Anacardiaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien III(5), W. Engelmann,
Leipzig, pp. 138-178, 458-459.
Engler A. 1897a. V – Neue Arten aus
Transvaal. – Notizbl. Königl. Bot. Gart. Mus. Berlin 2: 25-26.
Engler A. 1897b. Rutaceae africanae. – Engl. Bot. Jahrb.
23: 150-154.
Engler A. 1902. Rutaceae africanae II. – Engl. Bot.
Jahrb. 32: 120.
Engler A. 1905. Anacardiaceae africanae III. –
Engl. Bot. Jahrb. 36: 216-217.
Engler A. 1911. Rutaceae africanae IV. – Engl. Bot.
Jahrb. 46: 410.
Engler A. 1913. Die Verbreitung der
afrikanischen Burseraceen im Verhältnis zu ihrer systematischen Gliederung und
die Einteilung der Gattung Commiphora. – Engl. Bot. Jahrb. Syst. 48:
443-490.
Engler A. 1917. Rutaceae africanae V. – Engl. Bot.
Jahrb. 54: 305-307.
Engler A (†). 1931a. Zygophyllaceae.
– In: Engler A (†), Harms H, Pax F (eds), Die natürlichen
Pflanzenfamilien, 2. Aufl., Bd. 19a, W. Engelmann, Leipzig, pp. 144-184.
Engler A (†). 1931b. Cneoraceae. – In:
Engler A (†), Harms H, Pax F (eds), Die natürlichen Pflanzenfamilien, 2.
Aufl., Bd. 19a, W. Engelmann, Leipzig, pp. 184-187.
Engler A (†). 1931c. Rutaceae. – In: Engler A (†), Harms
H, Pax F (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 19a, W.
Engelmann, Leipzig, pp. 187-359.
Engler A (†). 1931d. Simarubaceae. – In:
Engler A (†), Harms H, Pax F (eds), Die natürlichen Pflanzenfamilien, 2.
Aufl., Bd. 19a, W. Engelmann, Leipzig, pp. 359-405.
Engler A (†). 1931e. Burseraceae. – In: Engler A (†),
Harms H, Pax F (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 19a, W.
Engelmann, Leipzig, pp. 405-456.
Erwin DM, Stockey RA. 1990. Sapindaceous
flowers from the Middle Eocene (Allenby Fm.) of British Columbia, Canada. –
Can. J. Bot. 68: 2025-2034.
Etman B. 1994. A taxonomic and phylogenetic
analysis of Rhysotoechia (Sapindaceae). – Blumea 39: 41-71.
Exell AW. 1966. 56. Sapindaceae. – In: Exell AW,
Fernandes A, Wild H (eds), Flora Zambesiaca 2 (Part 2), Crown Agents for
Oversea Governments and Administrations, London, pp. 494-543.
Fahn A, Evert RF. 1974. Ultrastructure of the
secretory ducts of Rhus glabra L. – Amer. J. Bot. 61: 1-14.
Fang DQ, Krueger RR, Roose ML. 1998.
Phylogenetic relationships among selected Citrus germplasm accessions
revealed by inter-simple sequence repeat (ISSR) markers. – J. Amer. Soc.
Horticult. Sci. 123: 612-617.
Fang W-P. 1960. Additamenta ad floram
sinensem I. Hippocastanaceae. – Acta Sci. Nat. Univ. Szechuan 1960:
77-125.
Fang W-P. 1966. Revisio taxorum aceracearum
sinicarum. – Acta Phytotaxon. Sin. 11: 139-189.
Farsam H, Amanlou M, Reza Dehpour A,
Jahaniani F. 2000. Anti-inflammatory and analgesic activity of
Biebersteinia multifida DC. root extract. – J. Ethnopharmacol. 71:
443-447.
Federici CT, Fang DQ, Scora RW, Roose ML.
1998. Phylogenetic relationships within the genus Citrus (Rutaceae) and related genera as revealed
by RFLP and RAPD analysis. – Theor. Appl. Gen. 6: 812-822.
Fernandes RB 1966. Estudos nas Anacardiaceae africanas I –
Contribuição para o conhecimento do gén. Ozoroa Del. – Garcia de
Orta 14: 19-60.
Fernandes RB. 1975. Estudos nas Anacardiaceae africanas VIII – O
género Antrocaryon Pierre em Angola. – Garcia de Orta 2:
107-110.
Fernandes R, Fernandes A. 1966. 59. Anacardiaceae. – In: Exell AW,
Fernandes A, Wild H (eds), Flora Zambesiaca 2 (Part 2), Crown Agents for
Oversea Governments and Administrations, London, pp. 550-615.
Fernando ES, Quinn CJ. 1992. Pericarp anatomy
and systematics of the Simaroubaceae sensu lato. – Aust.
J. Bot. 40: 263-289.
Fernando ES, Quinn CJ. 1995. Picramniaceae, a new
family, and a recircumscription of Simaroubaceae. – Taxon 44:
177-182.
Fernando ES, Gadek PA, Quinn CJ. 1995. Simaroubaceae, an artificial
construct: evidence from rbcL sequence variation. – Amer. J. Bot.
82: 92-103.
Ferrucci MS. 1981. Recuentos cromosomicos en
Allophylus y Serjania (Sapindaceae). – Bol. Soc. Bot.
Argent. 24: 200-202.
Ferrucci MS. 1987. Houssayanthus
monogynus, nueva combinación en Sapindaceae. – Candollea 42:
805-807.
Ferrucci MS. 1989. Chromosomas en
Cardiospermum y Diplokeleba (Sapindaceae), significado taxonómico
y evolutivo. – Bonplandia 6: 151-164.
Ferrucci MS. 1991. Sapindaceae. – In: Spichiger RS,
Ramella L (eds), Flora del Paraguay 16: 1-144.
Ferrucci MS. 2000a. Cytotaxonomy of Sapindaceae with special reference to
the tribe Paullinieae. – Gen. Molec. Biol. 23: 941-946.
Ferrucci MS. 2000b. Revisión taxonómica de
los géneros Cardiospermum y Urvillea para el Neotrópico (Sapindaceae). – Ph.D. diss.,
Universidad Nacional de Córdoba, Córdoba, Argentina.
Ferrucci MS. 2006. A new species of
Urvillea (Sapindaceae)
from northwestern Venezuela. – Brittonia 58: 83-87.
Ferrucci MS, Acevedo-Rodríguez P. 1997. New
and noteworthy species in the Paullinieae tribe (Sapindaceae). – Brittonia 49:
441-448.
Ferrucci MS, Acevedo-Rodríguez P. 2005.
Three new species of Serjania (Sapindaceae) from South America. –
Syst. Bot. 30: 153-162.
Ferrucci MS, Anzótegui LM. 1993. El polen de
Paullinieae tribe (Sapindaceae).
– Bonplandia 6: 211-243.
Feuillet C. 1983. Le statut des genres
Quassia L., Samadera Gaertn., Simaba Aubl. et
Simarouba Aubl. (Simaroubaceae). – Bull. Jard. Bot.
Nat. Belg. 53: 510-511.
Fikenscher LH, Hegnauer R. 1977. Über die
cyanogenen Verbindungen bei einigen Compositae, bei den Oliniaceae und in der Rutaceae-Gattung Zieria. –
Pharmaceut. Weekbl. 112: 11-20.
Fine PV-A, Daly DC, Villa M FG, Mesones A I,
Cameron KM. 2005. The contribution of edaphic heterogeneity to the evolution
and diversity of Burseraceae trees
in the Western Amazon. – Evolution 29: 1464-1478.
Fine PV-A, Zapata F, Daly DC. 2014.
Investigating processes of Neotropical rain forest tree diversification by
examining the evolution and historical biogeography of the Protieae (Burseraceae). – Evolution 68:
1988-2004.
Fischer TC, Butzmann R. 1998. Citrus
meletensis (Rutaceae), a new
species from the Pliocene of Valdarno (Italy). – Plant Syst. Evol. 210:
51-55.
Fish F, Waterman PG. 1973. Chemosystematics
in the Rutaceae II. The
chemosystematics of the Zanthoxylum/Fagara complex. – Taxon
22: 177-203.
Fisher JB. 2002. Indeterminate leaves of
Chisocheton (Meliaceae):
survey of structure and development. – Bot. J. Linn. Soc. 139: 207-221.
Fisher JB, Rutishauser R. 1990. Leaves and
epiphyllous shoots in Chisocheton (Meliaceae): a continuum of woody leaf
and stem axes. – Can. J. Bot. 68: 2316-2328.
Forest F, Drouin JN, Charest R, Brouillet R,
Bruneau A. 2001. A morphological phylogenetic analysis of Aesculus L.
and Billia Peyr. (Sapindaceae). – Can. J. Bot. 79:
154-169.
Forman LL. 1954. A new genus from Thailand.
– Kew Bull. 4: 555-564.
Forman LL. 1958. The identity of
Feroniella pubescens Tanaka (Rutaceae). – Kew Bull. 1957:
503-504.
Forman LL. 1987. A new genus of Burseraceae from Mexico. – Kew Bull.
42: 262.
Forman LL, Brandham PE, Harley MM, Lawrence
TJ. 1989. Beiselia mexicana (Burseraceae) and its affinities. –
Kew Bull. 44: 1-31.
Forman LL, Ham RWJM van der, Harley MM,
Lawrence TJ. 1994. Rosselia, a new genus of Burseraceae from the Louisiade
Archipelago, Papua New Guinea. – Kew Bull. 49: 601-621.
Forster PI. 2005. New species of
Philotheca Rudge (Rutaceae)
from Queensland. – Austrobeileya 7: 175-181.
Forster PI, Brophy JJ, Goldsack RJ. 2004.
Variation in Australian populations of Halfordia kendack s.l. (Rutaceae): evidence from leaf essential
oils. – Aust. Syst. Bot. 17: 571-580.
Foster AS. 1955a. Comparative morphology of
foliar sclereids in Boronella Baill. – J. Arnold Arbor. 36:
189-198.
Foster AS. 1955b. Structure and ontogeny of
terminal sclereids in Boronia serrulata. – Amer. J. Bot. 42:
551-560.
Foster RC. 1933. Chromosome number in
Acer and Staphylea. – J. Arnold Arbor. 14: 386-393.
Franceschinelli EV, Thomas WW. 2000.
Simaba guianensis subsp. huberi, a new Venezuelan taxon of Simaroubaceae. – Brittonia 52:
311-314.
Franceschinelli EV, Yamamoto K, Shepherd GJ.
1999. Distinctions among three Simarouba species. – Syst. Bot. 23:
479-488.
Fraser L-A, Mulholland DA, Taylor DAH. 1995.
The chemotaxonomic significance of the limonoid, nymania-1, in Turraea
obtusifolia. – South Afr. J. Bot. 61: 281-282.
French PA, Brown GK, Bayly MJ. 2016.
Incongruent patterns of nuclear and chloroplast variation in Correa
(Rutaceae): introgression and
biogeography in south-eastern Australia. – Plant Syst. Evol. 302: 447-468.
Friis I. 1981. Notes on Somalian Sapindaceae. – Kew Bull. 36:
139-141.
Friis I. 1984. Additional notes on Somalian
Sapindaceae. – Kew Bull. 39:
779-783.
Friis I, Berdcourt B, Vollesen K. 1996. New
combinations in African Sapindaceae. – Kew Bull. 51: 802.
Fritsch FE. 1908. The anatomy of the
Julianiaceae considered from systematic point of view. –Trans. Linn. Soc.
London, ser. II, Bot. 7: 129-152.
Froelicher Y, Mouhaya W, Basserie J-B,
Costantino G, Kamiri M, Luro F, Morillon R, Ollitrault P. 2011. New universal
mitochondrial PCR markers reveal new information on maternal citrus phylogeny.
– Tree Gen. Genomes 7: 49-61.
Fukuhara T, Yokoyama J, Tsukaya H. 2003.
Phylogenetic relationships among species in the genera Chisocheton and
Guarea that have unique indeterminate leaves as inferred from
sequences of chloroplast data. – Intern. J. Plant Sci. 164: 13-24.
Furth DG, Young DA. 1988. Relationships of
herbivore feeding and plant flavonoids (Coleoptera: Chrysomelidae and Anacardiaceae: Rhus). –
Oecologia (Berlin) 74: 496-500.
Gabriel WJ. 1968. Dichogamy in Acer
saccharum. – Bot. Gaz. (Chicago) 129: 334-338.
Gadek PA, Fernando ES, Quinn CJ, Hoot SB,
Terrazas T, Sheahan MC, Chase MW. 1996. Sapindales: molecular delimitation and
infraordinal groups. – Amer. J. Bot. 83: 802-811.
Gallet F. 1913. Développement et structure
anatomique du tegument seminal des Rutacées. – PhD diss., Université de
Paris.
Gambaro V, Chamy MC, Brand E von, Gambarino
JA. 1986. 3-(pentadec-10-enyl)-catechol, a new allergenic compound from
Lithraea caustica (Anacardiaceae). – Planta Medica
44: 20-22.
Garudamma GK. 1957. Studies in the Meliaceae II. Gametogenesis in Melia
azadirachta Linn. – J. Indian Bot. Soc. 36: 227-231.
Gasson P, Cheek M. 1992. The wood anatomy of
Pseudobersama mossambicensis and Trichilia capitata (Meliaceae) compared. – Kew Bull. 47:
753-758.
Geisenheyner L. 1915. Der Schleuderapparat
von Dictamnus fraxinella Pers. – Ber. Deutsch. Bot. Ges. 33:
442-446.
Gelderen DM van, Jong PC de, Oterdoom HJ.
1994. Maples of the world. – Timber Press, Portland, Oregon.
Gentry AH, Steyermark J. 1987. A revision of
Dilodendron (Sapindaceae).
– Ann. Missouri Bot. Gard. 74: 533-538.
George AS, Erdtman G. 1969. A revision of the
genus Diplopeltis Endl. (Sapindaceae). – Grana Palynol. 9:
92-109.
Gereau R. 2001. New names in African Celastraceae and Rutaceae. – Novon 11: 43-44.
Ghosh PK, Roy SK. 1979. Chisochetonoxylon
bengalensis gen. et sp. nov., a new fossil wood of Meliaceae from the Tertiary beds of
Birbhum District, West Bengal, India. – Curr. Sci. 48: 737-739.
Giannasi DE. 1986. Phytochemical aspects of
phylogeny in Hamamelidae. – Ann. Missouri Bot. Gard. 73: 417-437.
Gibson AC. 1981. Vegetative anatomy of
Pachycormus (Anacardiaceae). – Bot. J. Linn.
Soc. 83: 273-284.
Gilbert MG. 1986. A reconsideration of the
Rhus glutinosa complex (Anacardiaceae). – Nord. J. Bot. 6:
571-572.
Gildenhuys E, Ellis AG, Carroll SP, Le Roux
JJ. 2015. Combining natal range distributions and phylogeny to resolve
biogeographic uncertainties in balloon vines (Cardiospermum, Sapindaceae). – Div. Distrib. 21:
163-174.
Gilg E, Pilger R. 1905. Rutaceae. – In: Pilger R (ed),
Beiträge zur Flora der Hylea nach den Sammlungen von E. Ule, Verh. Bot. Ver.
Prov. Brandenburg 47: 152-154.
Gillett JB. 1980. Commiphora (Burseraceae) in South America and its
relationship to Bursera. – Kew Bull. 34: 569-587.
Gillett JB. 1991. Burseraceae. – In: Polhill RM (ed),
Flora of tropical East Africa, A. A. Balkema, Rotterdam, The Netherlands, pp.
1-94.
Gillis WT. 1971. The systematics and ecology
of poison-ivy and the poison-oaks (Toxicodendron, Anacardiaceae). – Rhodora 73:
72-237, 370-443, 465-540.
Giménez AM, Moglia G. 1995. Estructura
cortical de Anacardiaceas Argentinas. – Invest. Agron, Sist. Recur. For. 4:
189-203.
Girard C, Muyard F, Bévalot F, Tillequin F,
Vaquette J, Sévenet T, Litaudon M. 1999. Polyoxygenated flavones from the
leaves of Comptonella microcarpa. – J. Nat. Prod. 62: 1188-1189.
Gleiser G, Verdú M. 2005. Repeated evolution
of dioecy from androdioey in Acer. – New Phytol. 165: 633-640.
Godfrey RK, Clewell AF. 1965.
Polygamodioecious Leitneria floridana (Leitneriaceae). – Sida 2:
172-173.
Golan-Goldhirsh A, Barazani O, Wang ZS,
Khadka DK, Saunders JA, Kostiukovsky V, Rowland LJ. 2004. Genetic relationships
among Mediterranean Pistacia species evaluated by RAPD and AFLP
markers. – Plant Syst. Evol. 246: 9-18.
Goldblatt P, Williams I. 1987. Notes on
chromosome cytology of Rutaceae-Diosmeae. – Ann. Missouri Bot.
Gard. 74: 443-444.
Goldblatt P, Tobe H, Carlquist S, Patel V.
1985. Familial position of the cape genus Empleuridium. – Ann.
Missouri Bot. Gard. 72: 167-183.
González AM, Vesprini JL. 2010. Anatomy and
fruit development in Schinopsis balansae (Anacardiaceae). – Anal. Jard. Bot.
Madrid 67: 103-112.
Goris MA. 1910. Contribution à l’étude
des Anacardiacées de la tribu Mangiférées. – Ann. Sci. Nat. (Bot.) IX, 11:
1-29.
Gostel MR, Phillipson PB, Weeks A. 2016.
Phylogenetic reconstruction of the myrrh genus, Commiphora (Burseraceae), reveals multiple
radiations in Madagascar and clarifies infrageneric relationships. – Syst.
Bot. 41: 67-81.
Gostel MR, Weeks A, Raharimampionona J,
Phillipson PB. 2016. A partial taxonomic revision of the Rhynchocarpa
clade of Commiphora (Burseraceae) endemic to Madagascar.
– Syst. Bot. 41: 1004-1019.
Gouvêa CF, Dornelas MC, Rodriguez APM. 2008.
Floral development in the tribe Cedreleae (Meliaceae, sub-family Swietenioideae):
Cedrela and Toona. – Ann. Bot. 101: 39-48.
Gouvêa CF, Dornelas MC, Martinelli AP. 2008.
Characterization of unisexual flower development in the endangered mahogany
tree Swietenia macrophylla King. (Meliaceae). – Bot. J. Linn. Soc. 156:
529-535.
Govindachari TR, Suresh G, Banumathy B,
Masilalami S, Gopalakrishnan G, Krishna KGN. 1999. Antifungal activity of some
B,D-seco limonoids from two meliaceous plants. – J. Chem. Ecol. 25:
923-933.
Grant M, Blackmore S, Morton C. 2000. Pollen
morphology of the subfamily Aurantioideae (Rutaceae). – Grana 39: 8-20.
Gray AI. 1983. Structural diversity and
distribution of coumarins and chromones in the Rutales. – In: Waterman PG,
Grundon MF (eds), Chemistry and chemical taxonomy of the Rutales, Academic
Press, London, pp. 97-146.
Gray AI, Waterman PG. 1978. Coumarins in the
Rutaceae. – Phytochemistry 17:
845-864.
Greenham J, Vassiliades DD, Harborne JB,
Williams CA, Eagles J, Grayer RJ, Veitch NC. 2001. A distinctive flavonoid
chemistry for the anomalous genus Biebersteinia. – Phytochemistry
56: 87-91.
Greger H, Zechner G, Hofer O, Vajrodaya S.
1996. Bioactive amides from Glycosmis species. – J. Nat. Prod. 59:
1163-1168.
Greger H, Pacher T, Brem B, Bacher M, Hofer
O. 2001. Insecticidal flavaglines and other compounds from Fijian
Aglaia species. – Phytochemistry 57: 57-64.
Gregor H-J. 1989. Aspects of the fossil
record and phylogeny of the family Rutaceae (Zanthoxyleae, Toddalioideae).
– Plant Syst. Evol. 162: 251-265.
Gregor V-J, Goth K. 1979. Erster Nachweis der
Gattung Canarium Stickman 1759 (Burseraceae) im europäischen
Alttertiär. – Stuttg. Beitr. Natkd. Ser. B (Geol. Palaeontol.) 47: 1-15.
Gregory M, Poole I, Wheeler EA. 2009. Meliaceae. – IAWA J., (Suppl.)6:
99-101.
Grieve CM, Scora RW. 1980. Flavonoid
distribution in the Aurantioideae (Rutaceae). – Syst. Bot. 5: 39-53.
Grimm GW, Denk T, Hemleben V. 2007.
Evolutionary history and systematics of Acer section Acer –
a case study of low-level phylogenetics. – Plant Syst. Evol. 267: 215-253.
Grison F. 1978. Note sur les fleurs de
l’okoumé (Aucoumea klaineana Pierre, Burseraceae). – Adansonia, sér. II,
17: 335-342.
Groppo M, Pirani JR. 2012. A revision of
Hortia (Rutaceae). – Syst.
Bot. 37: 197-212.
Groppo M, Kallunki JA, Pirani JR. 2005.
Synonymy of Hortia arborea with H. brasiliana (Rutaceae) and a new species from Brazil.
– Brittonia 57: 28-34.
Groppo M, Pirani JR, Salatino MLF, Blanco SR,
Kallunki JA. 2008. Phylogeny of Rutaceae based on two noncoding regions
from cpDNA. – Amer. J. Bot. 95: 985-1005.
Groppo M, Kallunki JA, Pirani JR, Antonelli
A. 2012. Chilean Pitavia more closely related to Oceania and Old World
Rutaceae than to Neotropical groups:
evidence from two cpDNA non-coding regions, with a new subfamilial
classification of the family. – PhytoKeys 19: 9-29.
Gross M, Baer H, Fales HM. 1975. Urushiols of
poisonous Anacardiaceae. –
Phytochemistry 14: 2263-2266.
Grudinski M, Pannell CM, Chase MW, Ahmad JA,
Muellner-Riehl AN. 2014. An evaluation of taxonomic concepts of the widespread
plant genus Aglaia and its allies across Wallace’s Line (tribe
Aglaieae, Meliaceae). – Molec.
Phylogen. Evol. 73: 65-76.
Grudinski M, Wanntorp L, Pannell CM,
Muellner-Riehl AN. 2014. West to east dispersal in a widespread
animal-dispersed woody angiosperm genus (Aglaia, Meliaceae) across the Indo-Australian
Archipelago. – J. Biogeogr. 41: 1149-1159.
Grundwag M. 1976. Embryology and fruit
development in four species of Pistacia L. (Anacardiaceae). – Bot. J. Linn.
Soc. 73: 355-370.
Grundwag M, Fahn A. 1969. The relation of
embryology to the low seed set in Pistacia vera (Anacardiaceae). – Phytomorphology
19: 225-235.
Guérin MP. 1901. Développement de la graine
et en particulier du tégument séminal de quelques Sapindacées. – J. Bot.
15: 336-362.
Guerra M dos S. 1984. New chromosome numbers
in Rutaceae. – Plant Syst. Evol.
146: 13-30.
Guerra M, Santos KGB, Silva AEB, Ehrendorfer
F. 2000. Heterochromatin banding pattern in Rutaceae-Aurantioideae – a case of
parallel chromosomal evolution. – Amer. J. Bot. 87: 735-747.
Guervin C. 1961. Contribution à l’étude
cyto-taxinomique des Sapindacées et caryologiques des Melianthacées et des
Didiereacées. – Rev. Cyt. Biol. Veg. 23: 4-87.
Guillaumin MA. 1907. Sur deux Burséracées
indo-chinoises. – Rev. Gen. Bot. 19: 161-166.
Guillaumin MA. 1909. Recherches sur la
structure et le développement der Burseracées. Applications à la
systématique. – Ann. Sci. Nat. IX, Bot. 10, 4: 201-301.
Gulati N, Mather S. 1977. Embryology and
taxonomy of Filicium decipiens Thw. – Phytomorphology 27:
261-266.
Gupta P, Shivanna KR, Mohan-Ram HY. 1996.
Apomixis and polyembryony in the guggul plant, Commiphora wightii. –
Ann. Bot. 78: 67-72.
Gupta S, Agarwal M. 2008. Wood anatomy of Anacardiaceae from India with
special reference to the systematic position of Rhus.- IAWA Bull. 29:
79-106.
Gut BJ. 1966. Beiträge zur Morphologie des
Gynoeceums und der Blütenachse einiger Rutaceen. – Bot. Jahrb. Syst. 85:
151-247.
Halim H, Locksley HD, Memon JJ. 1980.
Vesicant principles of poison ivy and related plants: synthesis of the
urushiols, 1,2-dihydroxy-3-((Z)-pentadec-8-enyl)benzene and
1,2-dihydroxy-3-pentadecylbenzene. – J. Chem. Soc., Perkin, Trans. 1:
2331-2337.
Hall BA. 1951. The floral anatomy of the
genus Acer. – Amer. J. Bot. 38: 793-799.
Hall BA. 1961. The floral anatomy of
Dipteronia. – Amer. J. Bot. 48: 918-924.
Hall JB, O’Brien EM, Sinclair FL. 2002.
Sclerocarya birrea: a monograph. – University of Wales, Bangor.
Hallier H. 1910. Über Juliania,
eine Terebinthaceen-Gattung mit Cupula und die wahren Stammeltern der
Kätzchenblütler. – Beih. Bot. Centralbl., II, 23: 81-265.
Ham RWJM van der. 1988. Types
harmomégathiques dans le pollen des Sapindaceae-Nephelieae. – Inst.
Franç. Pondichéry Trav. Sect. Sci. Techn. 25: 355-358.
Ham RWJM van der. 1990. Nephelieae pollen (Sapindaceae): form, function, and
evolution. – Natl. Herb. Nederl. Leiden, Bot. Ser. 13: 1-255.
Ham RWJM van der, Heuven BJ van. 1989.
Evolutionary trends in the morphology and harmomegathy of the pollen of the
genus Guioa (Sapindaceae-Cupanieae). – Blumea 34:
21-60.
Ham RWJM van der, Tomlik A. 1994.
Serjania pollen and the origin of the tribe Paullinieae (Sapindaceae). – Rev. Palaeobot.
Palynol. 83: 43-53.
Ham RWJM van der, Baas P, Bakker ME,
Boesewinkel FD, Bouman F, Heuven BJ van, Klaasen RKWM. 1995. Bottegoa
Chiov. transferred to Ptaeroxylaceae. – Kew Bull. 50: 243-265.
Harbaugh DT, Wagner WL, Allan G, Zimmer EA.
2009. The Hawaiian archipelago is a stepping-stone for dispersal in the
Pacific: an example from the plant genus Melicope (Rutaceae). – J. Biogeogr. 36:
230-241.
Harborne JB. 1983. The flavonoids of the
Rutales. – In: Waterman PG, Grundon MF (eds), Chemistry and chemical taxonomy
of the Rutales, Academic Press, London, pp. 147-174.
Hardin JW. 1957a. A revision of the American
Hippocastanaceae. – Brittonia 9: 145-195.
Hardin JW. 1957b. A revision of the American
Hippocastanaceae II. – Brittonia 9: 173-195.
Hardin JW. 1960. Studies in the
Hippocastanaceae V. Species of the Old World. – Brittonia 12: 26-38.
Hardin JW, Phillips LL. 1985. Atlas of foliar
surface features in woody plants VII. Rhus subg. Rhus (Anacardiaceae) of North America. –
Bull. Torrey Bot. Club 112: 1-10.
Harley MM, Clarkson JJ. 1999. Pollen
morphology of the African Burseraceae and related genera. –
In: Heine K (ed), Paleoecology of Africa and the surrounding islands, A. A.
Balkema Publ., Rotterdam, pp. 225-242.
Harley MM, Daly DC. 1995. Burseraceae Kunth, Protieae March. em.
Engl. – World Pollen and Spore Flora 20: 1-44.
Harley MM, Song U, Banks HI. 2005. Pollen
morphology and systematics of Burseraceae. – Grana 44: 282-299.
Harms H. 1896. Meliaceae. – In: Engler A, Prantl K
(eds), Die natürlichen Pflanzenfamilien III(4), W. Engelmann, Leipzig, pp.
258-308.
Harms H. 1917. Über eine Meliacee mit
blattbürtigen Blüten. – Ber. Deutsch. Bot. Gesellsch. 35: 338-348.
Harms H. 1940. Meliaceae. – In: Engler A (†), Harms
H, Mattfeld J (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 19bI, W.
Engelmann, Leipzig, pp. 1-172.
Harms H. 1940. Akaniaceae. – In: Engler
A (†), Harms H, Mattfeld J (eds), Die natürlichen Pflanzenfamilien, 2.
Aufl., Bd. 19bI, W. Engelmann, Leipzig, pp. 173-175.
Harrar ES. 1937. Notes on the genus
Flindersia R. Br. and the systematic anatomy of the important
flindersian timbers indigenous to Queensland. – J. Elisha Mitchell Sci. Soc.
53: 282-291.
Harrington MG, Gadek PA. 2009. A species well
travelled – the Dodonaea viscosa (Sapindaceae) complex based on
phylogenetic analyses of nuclear ribosomal ITS and ETS sequence. – J.
Biogeogr. 36: 2313-2323.
Harrington MG, Gadek PA. 2010. Phylogenetics
of hopbushes and pepperflowers (Dodonaea, Diplopeltis – Sapindaceae), based on nuclear
ribosomal ITS and partial ETS sequences incorporating secondary-structure
models. – Aust. Syst. Bot. 23: 431-442.
Harrington MG, Edwards KJ, Johnson SA, Chase
MW, Gadek PA. 2005. Phylogenetic inference in Sapindaceae sensu lato using plastid
matK and rbcL DNA sequences. – Syst. Bot. 30: 366-382.
Harrington MG, Biffin E, Gadek PA. 2009.
Comparative study of the evolution of nuclear ribosomal spacers incorporating
secondary structure analyzes within Dodonaeoideae, Hippocastanoideae and
Xanthoceroideae (Sapindaceae). –
Mol. Phylogen. Evol. 50: 364-375.
Harris AJ, Xiang Q-Y. 2009. Estimating
ancestral distributions of lineages with uncertain sister groups: a statistical
approach to dispersal-vicariance analysis and a case using Aesculus L.
(Sapindaceae) including fossils.
– J. Syst. Evol. 47: 349-368.
Harris AJ, Xiang Q-Y, Thomas DT. 2009.
Phylogeny, origin, and biogeographic history of Aesculus L. (Sapindales) – an update from combined
analysis of DNA sequences, morphology, and fossils. – Taxon 58: 108-126.
Harris AJ, Lutz S, Acevedo P, Wen J. 2015.
The utility of the morphological variation of pollen for resolving the
evolutionary history of Billia (subfam. Hippocastanoideae, Sapindaceae). – J. Syst. Evol. 53:
228-238.
Harris AJ, Fu C, Xiang Q-Y (J), Holland L,
Wen J. 2016. Testing the monophyly of Aesculus L. and Billia
Peyr., woody genera of tribe Hippocastaneae of the Sapindaceae. – Mol. Phylogen. Evol.
102: 145-151.
Hartl D. 1957a. Struktur und Herkunft des
Endokarps der Rutaceen. – Beitr. Biol. Pflanzen 34: 35-49.
Hartl D. 1957b. Die Pseudosympetalie von
Correa speciosa (Rutaceae)
und Oxalis tubiflora (Oxalidaceae). – Abh.
Akad. Wiss. Lit. Mainz, Abh. Math. Naturwiss. Kl. 2: 53-63.
Hartl D. 1958. Die Übereinstimmung des
Endokarps der Simaroubaceen, Rutaceen und Leguminosen. – Beitr. Biol.
Pflanzen 34: 452-455.
Hartley TG. 1969. A revision of the genus
Flindersia (Rutaceae). – J.
Arnold Arbor. 50: 481-526.
Hartley TG. 1974. A revision of the genus
Acronychia (Rutaceae). – J.
Arnold Arbor. 55: 469-523, 525-567.
Hartley TG. 1977a. A revision of the genus
Acradenia (Rutaceae). – J.
Arnold Arbor. 58: 171-181.
Hartley TG. 1977b. A revision of the genus
Bosistoa (Rutaceae). – J.
Arnold Arbor. 58: 416-436.
Hartley TG. 1979. A revision of the genus
Tetractomia (Rutaceae). –
J. Arnold Arbor. 60: 127-153.
Hartley TG. 1981. A revision of the genus
Tetradium (Rutaceae). –
Gard. Bull. (Singapore) 34: 91-131.
Hartley TG. 1982a. A revision of the genus
Sarcomelicope (Rutaceae). –
Aust. J. Bot. 30: 359-372.
Hartley TG. 1982b. Two new species of
Acronychia (Rutaceae) from
New Guinea. – Reinwardtia 10: 93-96.
Hartley TG. 1983. A revision of the genus
Comptonella (Rutaceae). –
Bull. Mus. Natn. Hist. Nat. Paris, Sect. B, Adansonia 5: 391-413.
Hartley TG. 1984. A revision of the genus
Dutaillyea (Rutaceae). –
Bull. Mus. Natn. Hist. Nat. Paris, Sect. B, Adansonia 6: 29-35.
Hartley TG. 1985. A revision of the genus
Medicosma (Rutaceae). –
Aust. J. Bot. 33: 27-64.
Hartley TG. 1986. Three new species of
Sarcomelicope (Rutaceae) from
New Caledonia (with a new key to the species of the genus). – Bull. Mus.
Natl. Hist. Nat. Paris, Sect. B, Adansonia 2: 183-189.
Hartley TG. 1990. A new species and new
combinations in Melicope (Rutaceae) in New South Wales. – Telopea
4: 33-35.
Hartley TG. 1991. A new combination in
Australian Acronychia (Rutaceae). – Aust. Syst. Bot. 4:
445-448.
Hartley TG. 1995. A new combination in
Boronella (Rutaceae) and a
view on relationships of the genus. – Bull. Mus. Natn. Hist. Paris, Sect. B,
Adansonia 17: 107-111.
Hartley TG. 1997. Five new rain forest genera
of Australasian Rutaceae. –
Adansonia, sér. III, 19: 189-212.
Hartley TG. 2000. On the taxonomy and
biogeography of Euodia and Melicope (Rutaceae). – Allertonia 8: 1-328.
Hartley TG. 2001a. Morphology and
biogeography in Australasian-Malesian Rutaceae. – Malaysian Nature J. 55:
197-219.
Hartley TG. 2001b. On the taxonomy and
biogeography of Euodia and Melicope (Rutaceae). – Allertonia 8: 1-319.
Hartley TG. 2003. Neoschmidia, a new
genus of Rutaceae from New Caledonia.
– Adansonia, sér. III, 25: 7-12.
Hartley TG, Hyland BMP. 1975. Additional
notes on the genus Flindersia (Rutaceae). – J. Arnold Arbor. 56:
243-247.
Hartley TG, Hyland BMP. 1982. A new species
of Acronychia (Rutaceae) from
Australia. – Austrobaileya 1: 451-454.
Hartley TG, Jessup LW. 1982. A name change in
the genus Flindersia (Rutaceae). – Brunonia 5: 109.
Hartley TG, Mabberley DJ. 2003. The identity
of Picrella Baill. (Rutaceae)
with a revision of the genus. – Adansonia, sér. III, 25: 251-259.
Hartley TG, Williams JB. 1983. A new species
of Acronychia (Rutaceae) from
Australia. – Brunonia 6: 251-255.
Hasebe M, Ando T, Iwatsuki K. 1998.
Intrageneric relationships of maple trees based on the chloroplast DNA
restriction fragment length polymorphisms. – J. Plant Res. 111: 441-451.
Hegnauer R. 1983. Chemical characters and the
classification of the Rutales. – In: Waterman PG, Grundon MF (eds), Chemistry
and chemical taxonomy of the Rutales, Academic Press, London, pp. 401-440.
Heimsch C. 1940. Wood anatomy and pollen
morphology of Rhus and allied genera. – J. Arnold Arbor. 21:
279-291.
Heimsch C. 1942. Comparative anatomy of the
secondary xylem in the ‘Gruinales’ and ‘Terebinthales’ of Wettstein
with reference to taxonomic grouping. – Lilloa 8: 83-198.
Heinrich G. 1969. Elektronenmikroskopische
Beobachtungen zur Entstehungsweise der Exkretbehälter von Ruta
graveolens, Citrus limon und Poncirus trifoliata. –
Österr. Bot. Zeitschr. 117: 397-403.
Heinrich G. 1970. Elektronenmikroskopische
Beobachtungen an den Drüsenzellen von Poncirus trifoliata; zugleich
ein Beitrag zur Wirkung aetherischer Öle auf Pflanzenzellen und eine Methode
zur Unterscheidung flüchtiger von nichtflüchtiger lipophilen Elementen. –
Protoplasma 69: 15-36.
Hemsley WB. 1908. On the Julianiaceae: a new
natural order of plants. – Philos. Trans, Ser. B, 199: 167-197.
Herrero R, Asins MJ, Pina JA, Carbonel EA,
Navarro L. 1996. Genetic diversity in the orange subfamily Aurantioideae II.
Genetic relationships among genera and species. – Theor. Appl. Gen. 93:
1327-1334.
Hess WR. 1949. Identification of New World
timbers II. Anacardiaceae. –
Trop. Woods 87: 11-34.
Hesse M. 1979. Ultrastruktur und Verteilung
des Pollenkitts in der Insekten- und windblütigen Gattung Acer (Aceraceae).
– Plant Syst. Evol. 131: 277-289.
Hewson HJ. 1985a. Burseraceae. – In: George AS (ed),
Flora of Australia 25, Australian Government Publ. Service, Canberra, pp.
165-170.
Hewson HJ. 1985b. Simaroubaceae. – In: George AS
(ed), Flora of Australia 25, Australian Government Publ. Service, Canberra, pp.
188-196.
Hill GA, Mattacotti V, Graham WD. 1934. The
toxic principle of the poison ivy. – J. Amer. Chem. Soc. 56: 2736-2738.
Hladik A, Hallé N. 1979. Note sur les
endocarps de quatre espèces de Spondias d’Amériques
(Anacardiacées). – Adansonia, n.s., 18: 487-492.
Hodgson RW. 1961. Taxonomy and nomenclature
in Citrus. – In: Price JR (ed), Proc. 2nd Conf. Intern. Org. Citrs
Virologists, Gainesville, Florida, pp. 1-7.
Hoehne FC. 1925. Sapindaceas Mattogrossenses.
– Arch. Bot. São Paulo 1: 131-142.
Hogbin PM, Crisp MD. 2003. Evolution of the
coastal neospecies Zieria prostrata (Rutaceae) and its relationships to the
Zieria smithii species complex. – Aust. Syst. Bot. 16: 515-525.
Horner HTJ, Lersten NR. 1971.
Microsporogenesis in Citrus limon (Rutaceae). – Amer. J. Bot. 58:
72-79.
Horton BM, Crayn DM, Clarke SW, Washington H.
2004. Leionema scopulinum (Rutaceae), a new species from Wollemi
National Park. – Telopea 10: 815-822.
Huang S-F, Ricklefs RE, Raven PH. 2002.
Phylogeny and historical biogeography of Acer I. Study history of the
infrageneric classification. – Taiwania 47: 203-218.
Hunziker AT. 1978. Notas críticas sobre
Sapindaceas argentinas III. Houssayanthus, genus novum Sapindacearum.
– Kurtziana 11: 7-24.
Hussein SR, Kawashty SA, Tantawy ME, Saleh
NAM. 2009. Chemosystematic studies of Nitraria retusa and selected
taxa of Zygophyllaceae in
Egypt. – Plant Syst. Evol. 277: 251-264.
Ibe RA, Leis RA. 1979. Pollen morphology of
Anacardiaceae of northeastern
North America. – Bull. Torrey Bot. Club 106: 140-144.
Ikuta A, Nakamura T, Urabe H. 1998.
Indolopyridoquinazoline, furoquinoline and canthinone type alkaloids from
Phellodendron amurense callus tissues. – Phytochemistry 48:
285-291.
Immelman KL. 1984. Flowering in Kirkia
wilmsii Engl. – Bothalia 15: 151-152.
Inamdar JA. 1969. Epidermal structure,
stomatal ontogeny, and relationship of some Zygophyllaceae
and Simaroubaceae. – Flora
158B: 360-368.
Inbar M. 2008. Systematics of
Pistacia: insights from specialist parasitic aphids. – Taxon 57:
238-242.
Iriarte J, Kindl F, Rosenkranz G, Sondheimer
F. 1956. The constituents of Casimiroa edulis Llave & Lex II. The
bark. – J. Chem. Soc. 1956: 4170-4173.
Jadin F. 1901. Contribution à l’étude des
Simarubacées. – Ann. Sci. Nat., sér. VIII, Bot. 13: 201-304.
Jaffré T, Fambart J. 2002. Quatre nouvelles
espèces de Soulamea (Simaroubaceae) de
Nouvelle-Calédonie. – Adansonia, sér. III, 24: 159-168.
Jarvis CE. 1989. A review of the order
Leitneriales. – In: Crane PR, Blackmore S (eds), Evolution, systematics, and
fossil history of Hamamelidae 2, ‘Higher’ Hamamelidae, Syst. Assoc. Special
Vol. 40B, Clarendon Press, Oxford, pp. 189-192.
Jessup LW. 1985. Anacardiaceae. – In: George AS
(ed), Flora of Australia 25, Australian Government Publ. Service, Canberra, pp.
170-187.
Jesus Freitas CM de, Lucchese AM, Silva FS.
2003. Coumarins, furoquinoline alkaloids and terpenes from Spiranthera
odoratissima (Rutaceae). – J.
Essential Oil Res. 31: 805-807.
Jiménez-Reyes N, Cuevas Figueroa XM. 2001.
Morfología del pollen de Amphipterygium Schiede ex Standley
(Julianiaceae). – Bol. IBUG 8(1/2): 65-73.
Joel DM. 1980. Resin ducts in the mango
fruit: a defense system. – J. Exp. Bot. 31: 1707-1718.
Joel DM, Fahn A. 1980a. Ultrastructure of the
resin ducts of Mangifera indica (Anacardiaceae) 1. –
Differentiation and senescence of shoot ducts. – Ann. Bot., N. S., 46:
225-233.
Joel DM, Fahn A. 1980b. Ultrastructure of the
resin ducts of Mangifera indica (Anacardiaceae) 2. – Resin
secretion on the primary shoot ducts. – Ann. Bot., N. S., 46: 779-783.
Johnson MAT, Taylor NP. 1989. Chromosome
counts in the genus Skimmia (Rutaceae). – Kew Bull. 44: 503-513.
Johri BM, Ahuja MR. 1957. A contribution to
the floral morphology and embryology of Aegle marmelos Correa. –
Phytomorphology 7: 10-24.
Jones TGH, Smith FB. 1928. Campnospermonol, a
ketonic phenol from Campnospermum brevipetiolatum. – J. Chem. Soc.
1928: 65-70.
Jong PC de. 1976. Flowering and sex
expression in Acer L. A biosystematic study. – Meded. Landbouwh.
Wageningen 76: 1-201.
Jong PC de. 1994. Taxonomy and reproductive
biology of maples. – In: Gelderen DM van, Jong PC de, Oterdoom HJ (eds),
Maples of the world, Timber Press, Portland, Oregon, pp. 69-103.
Juliano JB. 1937. Embryos of carabao mango,
Mangifera indica L. – Philipp. Agron. 25: 749-760.
Kaastra RC. 1982. Flora Neotropica Monograph
13. Pilocarpinae (Rutaceae). – New
York Botanical Garden, Bronx, New York.
Kadry AER. 1946. Embryology of
Cardiospermum halicacabum L. – Svensk Bot. Tidskr. 40: 111-126.
Kadry AER. 1951. Chromosome behavior in
Cardiospermum halicacabum L. – Svensk Bot. Tidskr. 45: 414-416.
Kadry AER. 1960. The seed of
Cardiospermum halicacabum L., a criticism. – Acta Bot. Neerl. 9:
330-332.
Kafkas S, Perl-Treves R. 2001. Morphological
and molecular phylogeny of Pistacia species in Turkey. – Theor.
Appl. Genet. 102: 908-915.
Kalkman C. 1953. Revision of the Burseraceae of the Malaysian area in a
wider sense. – Blumea 7: 470.
Kallunki JA. 1990. An emended description of
and new combinations in Raputia (Cuspariinae, Rutaceae). – Brittonia 42: 175-177.
Kallunki JA. 1992. A revision of
Erythrochiton sensu lato (Cuspariinae, Rutaceae). – Brittonia 44: 107-139.
Kallunki JA. 1994. Revision of
Raputia Aubl. (Cuspariinae, Rutaceae). – Brittonia 46: 279-295.
Kallunki JA. 1998. Andreadoxa flava
(Rutaceae, Cuspariinae): a new genus
and species from Bahia, Brazil. – Brittonia 50: 59-62.
Kallunki JA. 1998. Revision of
Ticorea Aubl. (Rutaceae,
Galipeinae). – Brittonia 50: 500-513.
Kallunki JA. 2009. Validation of
Neoraputia (Galipeae, Rutaceae) and description of two new
species from eastern Brazil. – Brittonia 61: 28-34.
Kallunki JA, Pirani JR. 1998. Synopses of
Angostura Roem. & Schult. and Conchocarpus J. C. Mikan.
– Kew Bull. 53: 257-334.
Kamelina OP. 1994. Embryology and systematic
position of Tetradiclis (Tetradiclidaceae). – Bot. Žurn. 79:
11-27.
Kamelina OP, Konnova VA. 1990. Embryological
characters of the genus Biebersteinia Steph. in relation to its
systematic position. – Doklady Akademii Nauk Tadzhikskoi SSR 33(3):
193-195.
Kamer P. 1939. The woods of Billia,
Cashalia, Henoonia, and Juliania. – Trop. Woods
58: 1-2.
Kamilya P, Paria N. 1995. Seedling morphology
in taxonomic study of some Indian members of the Anacardiaceae. – J. Ind. Bot. Soc.
74: 193-196.
Kaniewski K, Wazynska Z. 1970.
Sclerenchymatous endocarp with hairs in the fruit of Acer
pseudoplatanus L. – Bull. Acad. Polon. Sci. 18: 413-420.
Kannan K. 1994. Burning out the black dammar,
Canarium strictum Roxb. – J. Bombay Nat. Hist. Soc. 91: 159.
Kapil RN, Ahluwalia K. 1963. Embryology of
Peganum harmala Linn. – Phytomorphology 13: 127-140.
Karimi HR, Kafkas S, Zamani Z, Ebadi A,
Fatahi Moghadam MR. 2009. Genetic relationships among Pistacia species
using AFLP markers. – Plant Syst. Evol. 279: 21-28.
Keay RWJ. 1996. The future of the genus
Swietenia in its native forest. – Bot. J. Linn. Soc. 122: 3-7.
Kelkar SS. 1958a. Embryology of Rhus
mysurensis Heyne. – J. Ind. Bot. Soc. 37: 114-122.
Kelkar SS. 1958b. A contribution to the
embryology of Lannea coromandelica (Houtt.) Merr. – J. Univ. Bombay
26: 152-159.
Kenfack D, Tindo M, Gueye M. 2014.
Extranuptial nectaries in Carapa Aubl. (Meliaceae-Cedreloideae). – Adansonia,
sér. 3, 36: 335-349.
Khalid SA. 1983. Chemistry of the Burseraceae. – In: Waterman PG,
Grundon MF (eds), Chemistry and chemical taxonomy of the Rutales, Academic
Press, London, pp. 281-289.
Khosla PK, Styles BT. 1975. Karyological
studies and chromosome evolution in Meliaceae. – Silvae Genetica 24:
73-83.
Kimura H, Ogawa S, Jisaka M, Kimura Y,
Katsube T, Yokota K. 2006. Identification of novel saponins from edible seeds
of Japanese horse chestnut (Aesculus turbinata Blume) after treatment
with wooden ashes and their nutraceutical activity. – J. Pharmaceut. Biomed.
41: 1657-1665.
Klaassen RKWM. 1999. Wood anatomy of the Sapindaceae. – IAWA J. (Suppl.)2:
1-214.
Knuth R. 1931. Geraniaceae. – In:
Engler A (†), Harms H, Pax F (eds), Die natürlichen Pflanzenfamilien, 2.
Aufl., Bd. 19a, W. Engelmann, Leipzig, pp. 43-66.
Köcke AV, Muellner-Riehl AN, Pennington TD,
Schorr G, Schnitzler J. 2013. Niche evolution through time and across
continents: the story of Neotropical Cedrela (Meliaceae). – Amer. J. Bot. 100:
1800-1810.
Koenen EJM, Clarkson JJ, Pennington TD,
Chatrou LW. 2015. Recently evolved diversity and convergent radiations of
rainforest mahoganies (Meliaceae)
shed new light on the origins of rainforest hyperdiversity. – New Phytol.
207: 327-339.
Kokwaro JO. 1978. New taxa and combinations
in Rutaceae of E. and NE. Africa. –
Kew Bull. 32: 789-791.
Kokwaro JO. 1982. Rutaceae. – In: Polhill RM (ed), Flora
of tropical East Africa, A. A. Balkema, Rotterdam, The Netherlands, pp.
1-52.
Kokwaro JO. 1986. Anacardiaceae. – In: Polhill RM
(ed), Flora of tropical East Africa, A. A. Balkema, Rotterdam, The Netherlands,
pp. 1-59.
Kokwaro JO, Gillett J. 1980. Notes on the Anacardiaceae of Eastern Africa. –
Kew Bull. 34: 745-760.
Kong Y-C, Cheng K-F, Ng K-M, But PP-H, Li Q,
Yu S-X, Chang H-T, Cambie RC, Kinoshita T, Kan W-S, Waterman PG. 1986. A
chemotaxonomic division of Murraya based on the distribution of the
alkaloids yuehchukene and girinimbine. – Biochem. Syst. Ecol. 14: 491-497.
Kong Y-C, But PP-H, Ng K-M, Cheng K-F, Chang
K, Wong K, Gray A, Waterman PG. 1988. The biochemical systematics of
Merrillia; in relation to Murraya, the Clauseneae and the
Aurantioideae. – Biochem. Syst. Ecol. 16: 47-50.
Kong Y-C, But PP-H, Ng K-M, Li Q, Cheng K-F,
Waterman PG. 1988. Micromelum: a key genus in the chemosystematics of
the Clauseneae. – Biochem. Syst. Ecol. 16: 485-489.
Kostermans AJGH. 1981. Notes on
Spondias L. (Anacardiaceae). – Quart. J. Taiwan
Mus. 34: 105-111.
Kostermans AJGH. 1991. Kedondong,
Ambarella, Amra. The Spondioideae (Anacardiaceae) in Asia and the
Pacific area. – Publ. by the author, printed in Bogor, Indonesia by O.
Rachmat, Bina Karya 78 Printing Works, Jl. Semboja 13.
Kostermans AJGH. 1992. The identity of
Dracontomelum petelotii Tardieu-Blot (Anacardiaceae). – Reinwardtia 11:
55.
Kostermans AJGH, Bompard JM. 1993. The
mangoes: their botany, nomenclature, horticulture and utilization. – Academic
Press, London.
Kowalski R. 2008. Choerospondias
turovensis n. sp., a new Anacardiaceae species of the
European Neogene identified from the Turrow brown coal open-cast mine. –
Palaeontographica, Abt. B, Paläophytol. 284: 1-11.
Kribs DA. 1930. Comparative anatomy of the
woods of the Meliaceae. – Amer. J.
Bot. 17: 724-738.
Kryn JM. 1952. The anatomy of the wood of the
Anacardiaceae and its bearing on
the phylogeny and relationships of the family. – PhD diss., University of
Michigan, Ann Arbor, Michigan.
Kubitzki K. 2011. Introduction to Sapindales. – In: Kubitzki K (ed),
Families and genera of vascular plants X. Flowering plants. Eudicots. Sapindales, Cucurbitales, Myrtaceae. Springer, Heidelberg,
Dordrecht, London, New York, pp. 1-3.
Kubitzki K, Kallunki JA, Duretto M, Wilson
PG. 2011. Rutaceae. – In: Kubitzki K
(ed), Families and genera of vascular plants X. Flowering plants. Eudicots. Sapindales, Cucurbitales, Myrtaceae. Springer, Heidelberg,
Dordrecht, London, New York, pp. 276-356.
Kubo I, Tanis SP, Lee Y, Miura I, Nakanishi
K, Cahpya A. 1976. Harrisonin – a new limonoid from Harrisonia
abyssinica. – Heterocycles 5: 485.
Kurbanov D, Zharekeev BK. 1974. Alkaloids of
Biebersteinia multifida and Peganum harmala from
Karakalpakiya. – Khimia Prirodnykh Soedinenii 5: 685-686.
Labat J-N, Pignal M, Pascal O. 2005. Deux
espèces nouvelles et une combinaison nouvelle chez les Rutaceae de l’Archipel des Comores. –
Syst. Bot. Monogr. 104: 361-369.
Lahham JN, Al-Eisawi D. 1986. Pollen
morphology of Jordanian Zygophyllaceae.
– Candollea 41: 325-328.
Lal S. 1994. A contribution to the floral
anatomy of Cedreleae (Meliaceae). –
Feddes Repert. 105: 449-455.
Lal S, Narayana LL. 1994. Floral anatomy and
systematic position of Flindersia R. Br. – Feddes Repert. 105:
31-36.
Lam HJ. 1931. Beiträge zur Morphologie der
dreizähligen Burseraceae-Canarieae. – Ann. Bot.
Jard. Buitenz. 41: 23-56.
Lam HJ. 1932a. Beiträge zur Morphologie der
Burseraceae, insbesondere der
Canarieae. – Ann. Bot. Jard. Buitenz. 42: 97-226.
Lam HJ. 1932b. The Burseraceae of the Malay Archipelago
and Peninsula. – Bull. Jard. Bot. État, sér. III, 12: 281-561.
Lam HJ. 1938. Studies in phylogeny II. On the
phylogeny of the Malaysian Burseraceae, Canarieae in general, and
of Haplolobus in particular. – Blumea 3: 126-158.
Leenhouts PW. 1956. Burseraceae. – In: Steenis CGGJ van
(ed), Flora Malesiana I, 5(2), Noordhoff-Kolff N. V., Djakarta, pp. 209-296.
Leenhouts PW. 1959. Revision of the Burseraceae of the Malaysian area in a
wider sense Xa. Canarium Stickm. – Blumea 9: 275-475.
Leenhouts PW. 1967. A conspectus of the genus
Allophyllus (Sapindaceae).
The problem of a complex species. – Blumea 15: 301-358.
Leenhouts PW. 1972. A revision of
Haplolobus (Burseraceae).
– Blumea 20: 283-310.
Leenhouts PW. 1978a. The pollen morphology of
Burseraceae: a taxonomic comment.
– Grana 17: 175-177.
Leenhouts PW. 1978b. Systematic notes on the
Sapindaceae-Nephelieae. – Blumea
24: 395-403.
Leenhouts PW. 1985. An attempt towards a
natural system of Harpullia (Sapindaceae). – Blumea 31:
219-234.
Leenhouts PW. 1988a. Notes on some genera of
Sapindaceae-Cupanieae. – Blumea
33: 197-213.
Leenhouts PW. 1988b. A revision of
Alectryon (Sapindaceae) in
Malesia. – Blumea 33: 313-327.
Leenhouts PW, Vente M. 1982. A taxonomic
revision of Harpullia (Sapindaceae). – Blumea 28: 1-51.
Leinfellner W. 1958. Über die peltaten
Kronblätter der Sapindaceen. – Österr. Bot. Zeitschr. 105: 443-514.
Leroy J-F. 1959. Sur une petite famille de Sapindales proper à l’Afrique
australe et à Madagascar: Les Ptaeroxylaceae. – Compt. Rend. Acad. Sci.
Paris 248: 1001-1003.
Leroy J-F. 1976. Essais de taxonomie
syncrétique 1. Étude sur les Meliaceae de Madagascar. – Adansonia,
sér. II, 16: 167-203.
Leroy J-F, Lescot M. 1996. Taxons nouveaux de
Trichilieae (Meliaceae-Melioideae) de
Madagascar. – Bull. Mus. Natl. Hist. Nat. Paris, sect. B, Adansonia 18:
3-34.
Leroy J-F, Lobreau-Callen D, Lescot M. 1990.
Les Ptaeroxylaceae: espèces nouvelles du genre malgache Cedrelopsis
et palynologie de la famille. – Adansonia 12: 43-57.
Letouzey R. 1962. Deux Rutacées mal connues
d’Afrique centrale. – Adansonia, n.s. 2: 134.
Li B, Welle BJH ter, Klaassen R. 1995. Wood
anatomy of trees and shrubs from China VII. Sapindaceae. – IAWA J. 16:
191-215.
Li Q, Zhu LF, But PPH, Kong YC, Chang HT,
Waterman PG. 1988. Monoterpene and sesquiterpene rich oils from the leaves of
Murraya species: chemotaxonomic significance. – Biochem. Syst. Ecol.
16: 491-494.
Li S-W, Tu L-Z. 1991. The studies on the
embryology of Nitraria sibirica Pall. IV. The developmental anatomy of
the fruit and seed. – Acta Sci. Nat. Univ. Intramongolicae 22: 389-395.
Li S-W, Tu L-Z. 1994. The embryology and its
systematic significance of Nitraria. – Bull. Bot. Res. 3:
255-262.
Liang GY, Gray AI, Patalinghug WC, Skelton
BW, Waterman PG, White AH. 1989. Chemistry of the Burseraceae XI. X-ray-diffraction
studies on some tirucall-7-ene triterpenes from Aucoumea klaineana (Burseraceae). – Aust. J. Chem. 42:
1169-1175.
Lima MP, Braga PAC, Macedo ML, Silva MFGF,
Ferreira AG, Fernandes JB, Vieira PC. 2004. Phytochemistry of Trattinnickia
burserifolia, T. rhoifolia, and Dacryodes hopkinsii:
chemosystematic implications. – J. Braz. Chem. Soc. 15: 385-394.
Ling K-H, Wang Y, Poon W-S, Shaw P-C, But
PP-H. 2009. The relationship of Fagaropsis and Luvunga in Rutaceae. – Taiwania 54: 338-342.
Linney G. 1987. Nomenclature and taxonomic
changes in Hawaiian Alectryon (Sapindaceae). – Pacific Sci. 41:
68-73.
List AJ, Steward FC. 1965. The nucellus,
embryo sac, endosperm, and embryo of Aesculus and their
interdependence during growth. – Ann. Bot., N. S., 29: 1-15.
Liu C. 1986. Studies of pollen morphology in
the Bretschneideraceae and related families. – Acta Bot. Yunnan. 8: 441-450.
[In Chinese with English summary]
Liu JHQ, Ho TN, Chen SL, Lu AM. 2001.
Karyomorphology of Biebersteinia Stephan (Geraniaceae) and its
systematic and taxonomic significance. – Bot. Bull. Acad. Sin. (Taipei) 42:
61-66.
Lobreau-Callen D, Jérémie J. 1986.
L’espèce Cneorum tricoccon (Cneoraceae, Rutales) représentée à
Cuba. – Grana 25: 155-158.
Lobreau-Callen D, Nilsson S, Albers F, Straka
H. 1978. Les Cneoraceae (Rutales): étude taxonomique, palynologique, et
systématique. – Grana 17: 125-139.
Logacheva MD, Shipunov AB. 2017. Phylogenomic
analysis of Picramnia, Alvaradoa, and Leitneria
supports the independent Picramniales. – J.
Syst. Evol. 55: 171-176.
Lombello RA, Forni-Martins ER. 1998.
Chromosomal studies and evolution in Sapindaceae. – Caryologia 51:
81-93.
Love B. 1952. The active constituents of
poison ivy and related plants. Structure and synthesis. – PhD diss., Colombia
Unviersity, New York.
Ma JS, Cao W, Liu QR, Yu M, Han LJ. 2006. A
revision of the genus Phellodendron (Rutaceae). – Edinburgh J. Bot. 63:
131-151.
Mabberley DJ. 1979. The species of
Chisocheton (Meliaceae). –
Bull. Brit. Mus. (Nat. Hist.), Bot., ser. 6: 301-386.
Mabberley DJ. 1997. A classification for
edible Citrus (Rutaceae). –
Telopea 7: 167-172.
Mabberley DJ. 1998a. Australian Citreae with
notes on other Aurantioideae (Rutaceae). – Telopea 7: 333-344.
Mabberley DJ. 1998b. Notes on Australian Meliaceae. – Telopea 8: 47-48.
Mabberley DJ. 2001. Citrus reunited.
– Australian Plants 21: 52-54.
Mabberley DJ. 2004a. Citrus (Rutaceae): a review of recent advances in
etymology, systematics, and medical applications. – Blumea 49: 481-498.
Mabberley DJ. 2004b. A key to
Dysoxylum (Meliaceae) in
Australia, with a description of a new species from far north Queensland. –
Telopea 10: 725-730.
Mabberley DJ. 2010. The species of
Citrus (Rutaceae) with
pinnate leaves. – Blumea 55: 73-74.
Mabberley DJ. 2011. Meliaceae. – In: Kubitzki K (ed),
Families and genera of vascular plants X. Flowering plants. Eudicots. Sapindales, Cucurbitales, Myrtaceae. Springer, Heidelberg,
Dordrecht, London, New York, pp. 185-211.
Mabberley DJ, Pannell CM, Sing AM. 1995. Meliaceae. – In: Kalkman C et al.
(eds), Flora Malesiana I, 12(1), Flora Malesiana Foundation,
Rijksberbarium/Hortus Botanicus, Leiden, pp. 1-407.
Macbride JF. 1956. Flora of Peru. Sapindaceae. – Bot. Ser. 13:
291-391.
McClain AM, Manchester SR. 2001.
Dipteronia (Sapindaceae)
from the Tertiary of North America and implications for the phytogeographic
history of the Aceroideae. – Amer. J. Bot. 88: 1316-1325.
McGillivray DJ. 1975. Dodonaea (Sapindaceae): taxonomic notes. –
Telopea 1: 66-67.
MacGinitie HD. 1953. Fossil plants of the
Florissant Beds, Colorado. Sapindaceae. – Carnegie Institution
of Washington Publication no. 599, Washington, D.C., pp. 142-147.
McVaugh R, Rzedowski J. 1965. Synopsis of the
genus Bursera L. in western Mexico, with notes on the material of
Bursera collected by Sessé & Mociño. – Kew Bull. 18:
317-346.
Mai DH. 1984. Die Endokarpien bei der Gattung
Acer L. Eine biosystematische Studie. – Gleditschia 11: 17-46.
Manchester SR. 2001. Leaves and fruits of
Aesculus (Sapindales) from
the Paleocene of North America. – Intern. J. Plant Sci. 162: 985-998.
Manchester SR, Hermsen EJ. 2000. Flowers,
fruits, seeds, and pollen of Landeenia gen. nov., an extinct
sapindalean genus from the Eocene of Wyoming. – Amer. J. Bot. 87:
1909-1914.
Manchester SR, Wilde V, Collinson ME. 2007.
Fossil cashew nuts from the Eocene of Europe: biogeographic links between
Africa and South America. – Intern. J. Plant Sci. 168: 1199-1206.
Mandalari G, Bennett RN, Bisignano G,
Trombetta D, Saija A, Faulds CB, Gasson MJ, Narbad A. 2007. Antimicrobial
activity of flavonoids extracted from bergamot (Citrus bergamia Risso)
peel, a byproduct of the essential oil industry. – J Appl. Microbiol. 103:
2056-2064.
Marchand NL. 1867-1868. Recherches sur
l’organisation des Burseracées. – Adansonia 8: 17-71.
Marcone MR, Kakuda Y, Jahaniaval F, Yada RY,
Montevirgen LS. 2002. Characterization of the proteins of pili nut
(Canarium ovatum, Engl.). – Plant Foods Hum. Nutr. (Drodr.) 57:
107-120.
Marloth R. 1920. Notes on the function of the
staminal and staminodial gland in the flowers of Adenandra. – Ann.
Bol. Herb. 3: 38-39.
Marticorena C. 1968. Granas de pollen de
plantas chilenas – Anacardiaceae. – Gayana 17:
17-21.
Martínez E, Álvarez CHR. 2007. Un nuevo
género de Anacardiaceae de la
Peninsula de Yucatán. – Acta Bot. Hung. 49: 353-358.
Martínez-Pallé E, Herrero M. 1995. The
ponticulus – a structure bridging pollen tube access to the ovule in
Pistacia vera. – Sex. Plant Repr. 8: 217-222.
Matsuda H. 1997. Antiinflammatory effects of
escins Ia, Ib, IIa, and IIb from horse chestnut, the seeds of Aesculus
hippocastanum L. – Bioorg. Med. Chem. Lett. 7: 1611-1616.
Mauritzon J. 1935. Über die Embryologie der
Familie Rutaceae. – Svensk Bot.
Tidskr. 29: 319-347.
Mauritzon J. 1936. Zur Embryologie und
systematischen Abgrenzung der Reihen Terebinthales und Celastrales. – Bot.
Not. 1936: 161-212.
Mehra PN, Sareen TS, Khosla PK. 1972.
Cytological studies on Himalayan Meliaceae. – J. Arnold Arbor. 53:
558-568.
Meissner R, Markey A. 2007. Two new Western
Australian species of Drummondita (Rutaceae: Boronieae) from banded
ironstone ranges of the Yilgarn Craton. – Nuytsia 17: 273-280.
Mendonça FA. 1963. 40. Rutaceae. – In: Exell AW, Fernandes A,
Wild H (eds), Flora Zambesiaca 2 (Part 1), Crown Agents for Oversea Governments
and Administrations, London, pp. 180-210.
Mennega AM. 1972. Wood structure of the genus
Talisia (Sapindaceae). –
Acta Bot. Neerl. 21: 578-586.
Merrien A, Polonsky J. 1971. The natural
occurrence of melianodiol and its diacetate in Samadera
madagascariensis (Simaroubaceae): model experiments on
melianodiol directed towards simarolide. – J. Chem. Soc. D, 1971: 261-263.
Merxmüller H, Heine H. 1960. Simaroubaceae. – Mitt. Bot.
Staatssamml. München 3: 617-619.
Mester I. 1983. Structural diversity and
distribution of alkaloids in the Rutales. – In: Waterman PG, Grundon MF
(eds), Chemistry and chemical taxonomy of the Rutales, Academic Press, London,
pp. 31-96.
Mester I, Vicol EC. 1971. Contribution to the
chemotaxonomy of the Haplophyllum genus. – Rev. Roum. Biol. 16:
221-233.
Meyer FG. 1976. A revision of the genus
Koelreuteria (Sapindaceae). – J. Arnold Arbor. 57:
129-166.
Meyer FG. 1977. Sinoradlkofera: a
new genus of Sapindaceae. – J.
Arnold Arbor. 58: 182-188.
Miceli N, Taviano MF, Tzakou O, Yannitsaros
A, Vassiliades D, Giuffrida D, Galati EM. 2005. Biebersteinia
orphanidis Boiss. shows antioxidant and anti-inflammatory activity. –
Pharmacogn. Mag. 1: 54-58.
Mikesell J. 1990. Anatomy of terminal
haustoria in the ovule of plantain (Plantago major L.) with taxonomic
comparison to other angiosperm taxa. – Bot. Gaz. 151: 452-464.
Milanez FR. 1943. Anatomia das pricipais
madeiras brasileiras das Rutaceae. –
Rodriguésia 7: 5-22.
Miller AJ, Young DA, Wen J. 2001. Phylogeny
and biogeography of Rhus (Anacardiaceae) based on ITS sequence
data. – Intern. J. Plant Sci. 162: 1401-1407.
Milton G, Pirani JR, Salatino MLF, Blanco SR,
Kallunki JA. 2008. Phylogeny of Rutaceae based on two noncoding regions
from cpDNA. – Amer. J. Bot. 95: 985-1005.
Mitchell JD. 1990. The poisonous Anacardiaceae genera of the world.
– Adv. Econ. Bot. 8: 103-129.
Mitchell JD, Daly DC. 1991.
Cyrtocarpa (Anacardiaceae) in South America. –
Ann. Missouri Bot. Gard. 78: 1184-1189.
Mitchell JD, Daly DC. 1993. A revision of the
genus Thyrsodium (Anacardiaceae). – Brittonia 45:
115-129.
Mitchell JD, Daly DC. 1998. The
‘tortoise’s cajá’ – a new species of Spondias (Anacardiaceae) from southwestern
amazonia. – Brittonia 50: 447-451.
Mitchell JD, Daly DC. 2015. A revision of
Spondias L. (Anacardiaceae) in the Neotropics.
– PhytoKeys 55: 1-92.
Mitchell JD, Daly DC. 2017. Notes on
Astronium Jacq. (Anacardiaceae), including a dwarf
new species from the Brazilian Shield. – Brittonia 69: 457-464.
Mitchell JD, Mori SA. 1987. The cashew and
its relatives (Anacardium: Anacardiaceae). – Mem. N. Y. Bot.
Gard. 42: 1-76.
Mitchell JD, Daly DC, Pell SK, Randrianasolo
A. 2006. Poupartiopsis gen. nov. and its context in Anacardiaceae classification. –
Syst. Bot. 31: 337-348.
Mitchell JD, Daly DC, Randrianasolo A. 2012.
The first report of Spondias native to Madagascar: Spondias
tefyi, sp. nov. (Anacardiaceae). – Brittonia 64:
263-267.
Mitra K, Mondal M, Saha S. 1977. The pollen
morphology of Burseraceae. –
Grana 16: 75-79.
Modliszewski JL, Thomas DT, Fan C, Crawford
DJ, dePamphilis C, Xing Q-Y. 2006. Ancestral chloroplast polymorphism and
historical secondary contact in a broad hybrid zone of Aesculus (Sapindaceae). – Amer. J. Bot. 93:
377-388.
Moffett RO. 1999. A new species of
Rhus (Anacardiaceae),
endemic to serpentine near Barberton, Mpumalanga (Eastern Transvaal), South
Africa. – Bot. J. Linn. Soc. 130: 37-42.
Moffett RO. 2007. Name changes in the Old
World Rhus and recognition of Searsia (Anacardiaceae). – Bothalia 37:
165-175.
Mole BJ, Udovicic F, Ladiges PY, Duretto MF.
2004. Molecular phylogeny of Phebalium (Rutaceae: Boronieae) and related genera
based on the nrDNA regions ITS 1+2. – Plant Syst. Evol. 249: 197-212.
Molino J-F. 1991. Révision systématique du
genre Clausena Burm. f. (Rutaceae); application à la production
agro-industrielle d’anethole. – Thèse (Diplome de Doctorat), Université
de Montpellier II: 1-208.
Molino J-F. 1994a. Révision du genre
Clausena Burm. f. (Rutaceae).
– Adansonia 1: 105-153.
Mollemans F. 1993. Drummondita
wilsonii, Philotheca langei and P. basistyla (Rutaceae), new species from south-west
Western Australia. – Nuytsia 9: 95-109.
Momotani Y. 1961. Taxonomic study of the
genus Acer with special reference to the seed proteins I. Taxonomic
characters. – Mem. Coll. Sci. Kyoto Imp. Univer., Ser. B, Biology 28:
455-470.
Moncada M, Machado S. 1987. Los granos de
pollen de Simarubaceae. – Acta Bot. Cub. 45: 1-7.
Moncur MW, Wait AJ. 1986. Floral ontogeny of
the cashew, Anacardium occidentale L. (Anacardiaceae). – Sci. Horticult.
30: 203-211.
Mondon A, Epe B. 1983. Bitter principles of
Cneoraceae. – Progr. Chem. Org. Nat. Prod. 44: 101-187.
Monod T. 1979. Les arbres à encens
(Boswellia sacra Flückiger 1867) dans le Hadramaut (Yémen du Sud).
– Bull. Mus. Natl. Hist. Nat. Paris, sér. 4(1), sect. B: 131-169.
Moolla A, Vuuren SF van, Zyl RL van, Viljoen
AM. 2007. Biological activity and toxicity profile of 17 Agathosma (Rutaceae) species. – South Afr. J. Bot.
73: 588-592.
Moore GA. 2001. Oranges and lemons: clues to
the taxonomy of Citrus from molecular markers. – Trends Gen. 17:
536-540.
Moore J. 1936. Floral anatomy and phylogeny
in the Rutaceae. – New Phytol. 35:
318-322.
Moraes VR de S, Tomaleza DM, Ferracin RJ,
Garcia CF, Sannomiya M, Soriano M del PC, Silva MFGF da. 2003. Enzymatic
inhibition studies of selected flavonoids and chemosystematic significance of
polymethoxylated flavonoids and quinoline alkaloids in Neoraputia. –
J. Brazilian Chem. Soc. 14: 380-387.
Morales JF. 2003. A new species of
Paullinia (Sapindaceae)
from Costa Rica. – Brittonia 55: 173-175.
Morton CM. 2009. Phylogenetic relationships
of the Aurantioideae (Rutaceae) based
on the nuclear ribosomal DNA ITS region and three noncoding chloroplast DNA
regions, atpB-rbcL spacer, rps16, and
trnL-trnF. – Organisms Div. Evol. 9: 52-68.
Morton CM. 2015. Phylogenetic relationships
of Zieria (Rutaceae) inferred
from chloroplast, nuclear, and morphological data. – PhytoKeys 44: 15-38.
Morton CM, Kallunki JA. 1993. Pollen
morphology of the subtribe Cuspariinae (Rutaceae). – Brittonia 45: 286-314.
Morton CM, Telmer C. 2014. New subfamily
classification for the Rutaceae. –
Ann. Missouri Bot. Gard. 99: 620-641.
Morton CM, Chase MW, Kallunki JA. 1996.
Evaluation of the six subfamilies of Rutaceae using evidence from
rbcL sequence variation. – Amer. J. Bot. 83: 180-181.
Morton CM, Grant M, Blackmore S. 2003.
Phylogenetic relationships of the Aurantioideae inferred from chloroplast DNA
sequence data. – Amer. J. Bot. 90: 1463-1469.
Morton JF. 1978. Brazilian pepper – its
impact on people, animals and the environment. – Econ. Bot. 32: 353-359.
Mou F-J, Zhang D-X. 2009. Pollen morphology
supports the reinstatement of Bergera (Rutaceae). – Nord. J. Bot. 27:
298-304.
Mou F-J, Zhang D-X. 2012. Chromosome studies
in the tribe Clauseneae and the cytological homogeneity in the orange subfamily
(Aurantioideae, Rutaceae). – J.
Syst. Evol. 50: 460-466.
Mou F-J, Tu T-Y, Chen Y-Z, Zhang D-X. 2018.
Phylogenetic relationship of Clauseneae (Rutaceae) inferred from plastid and
nuclear DNA data and taxonomic implication for some major taxa. – Nord. J.
Bot. 36: e01552
Muellner AN. 2011a. Biebersteiniaceae. – In:
Kubitzki K (ed), Families and genera of vascular plants X. Flowering plants.
Eudicots. Sapindales, Cucurbitales, Myrtaceae. Springer, Heidelberg,
Dordrecht, London, New York, pp. 72-75.
Muellner AN. 2011b. Kirkiaceae. – In: Kubitzki K (ed),
Families and genera of vascular plants X. Flowering plants. Eudicots. Sapindales, Cucurbitales, Myrtaceae. Springer, Heidelberg,
Dordrecht, London, New York, pp. 180-184.
Muellner AN, Mabberley DJ. 2008. Phylogenetic
position and taxonomic disposition of Turraea breviflora (Meliaceae), a hitherto enigmatic
species. – Blumea 53: 607-616.
Muellner AN, Samuel R, Johnson SA, Cheek M,
Pennington TD, Chase MW. 2003. Molecular phylogenetics of Meliaceae (Sapindales) based on nuclear and
plastid DNA sequences. – Amer. J. Bot. 90: 471-480.
Muellner AN, Samuel R, Chase MW, Pannell CM,
Greger H. 2005. Aglaia (Meliaceae) – an evaluation of
taxonomic concepts based on DNA data and secondary metabolites. – Amer. J.
Bot. 92: 534-543.
Muellner AN, Savolainen V, Samuel R, Chase
MW. 2006. The mahogany family “out of Africa”: divergence time estimation,
global biogeographic patterns inferred from plastid rbcL DNA
sequences, extant, and fossil distribution of diversity. – Mol. Phylogen.
Evol. 40: 236-250.
Muellner AN, Vassiliades DD, Renner SS. 2007.
Placing Biebersteiniaceae, a
herbaceous clade of Sapindales, in a
temporal and geographic context. – Plant Syst. Evol. 266: 233-252.
Muellner AN, Pannell CM, Coleman A, Chase MW.
2008. The origin and evolution of Indomalesian, Australasian and Pacific island
biotas: insights from Aglaieae (Meliaceae, Sapindales). – J. Biogeogr. 35:
1769-1789.
Muellner AN, Samuel R, Chase MW, Coleman A,
Stuessy TF. 2008. An evaluation of tribes and generic relationships in
Melioideae (Meliaceae) based on
nuclear ITS ribosomal DNA. – Taxon 57: 98-108.
Muellner AN, Pennington TD, Chase MW. 2009.
Molecular phylogenetics of neotropical Cedreleae (mahogany family, Meliaceae) based on nuclear and plastid
DNA sequences reveal multiple origins of ‘Cedrela odorata’. –
Mol. Phylogen. Evol. 52: 461-469.
Muellner AN, Pennington TD, Köcke AV, Renner
SS. 2010. Biogeography of Cedrela (Meliaceae, Sapindales) in Central and South
America. – Amer. J. Bot. 97: 511-518.
Muellner AN, Schäfer H, Lahaye R. 2011.
Evaluation of DNA barcodes for economically important timber species of the
mahogany family (Meliaceae). – Mol.
Ecol. Res. 11: 450-460.
Muellner-Riehl AN, Weeks A, Clayton JW,
Buerki S, Nauheimer L, Chiang Y-C, Cody S, Pell SK. 2016. Molecular
phylogenetics and molecular clock dating of Sapindales based on plastid
rbcL, atpB and trnL-trnF DNA sequences. –
Taxon 65: 1019-1036.
Mulholland DA, Nair JJ, Taylor DAH. 1996.
Astrotrichilin, a limonoid from Astrotrichilia asterotricha. –
Phytochemistry 42: 1239-1241.
Mulholland DA, Kotsos M, Mahomed HA, Taylor
DAH. 1998. Triterpenoids from Owenia cepiodora. – Phytochemistry 49:
2457-2460.
Mulholland DA, Parel B, Coombes PH. 2000. The
chemistry of Meliaceae and
Ptaeroxylaceae of southern and eastern Africa and Madagascar. – Curr. Org.
Chem. 4: 1011-1054.
Mulholland DA, Cheplogoi P, Crouch NR. 2003.
Secondary metabolites from Kirkia acuminata and Kirkia
wilmsii (Kirkiacae). – Biochem. Syst. Ecol. 31: 793-797.
Mulholland DA, Naidoo D, Randrianarivelojosia
M, Cheplogoi PK, Coombes PH. 2003. Secondary metabolites from Cedrelopsis
grevei (Ptaeroxylaceae). – Phytochemistry 64: 631-635.
Muller J. 1985. Pollen morphology and
evolution of the genus Harpullia (Sapindaceae-Harpullieae). – Blumea
31: 161-218.
Muller J, Leenhouts PW. 1976. A general
survey of pollen types in Sapindaceae in relation to taxonomy.
– In: Ferguson IK, Muller J (eds), The evolutionary significance of the
exine, Linn. Soc. Symposium, No. 1, Academic Press, London, New York, pp.
407-445.
Muller J, Schuller M. 1989. Fam. 120: Sapindaceae. – Trop. Subtrop.
Pflanzenw. 67: 99-137.
Munzinger J, Lowry II PP, Callmander M,
Buerki S. 2013. A taxonomic revision of the endemic New Caledonian genus
Podonephelium Baillon (Sapindaceae). – Syst. Bot. 38:
1105-1124.
Munzinger J, Lowry II PP, Buerki S,
Callmander MW. 2016. A taxonomic revision of the endemic New Caledonian genus
Storthocalyx (Sapindaceae). – Syst. Bot. 41:
387-400.
Murray AE. 1970. A checklist of species of
Acer. – Kalmia 2: 22-45.
Murty YS, Gupta S. 1978a. Morphological
studies in Meliaceae II. A
reinvestigation of floral anatomy of members of Swietenieae and Tricilieae. –
Proc. Indian Acad. Sci., Ser. B, 87: 55-64.
Murty YS, Gupta S. 1978b. Morphological
studies in Meliaceae III. A
reinvestigation of floral anatomy of Azadirachta and Melia.
– J. Indian Bot. Soc. 57: 195-204.
Muscarella M, Kimber MC, Moody CJ. 2008.
Synthesis of ptaeroxylin (desoxykarenin): an unusual chromone from the
sneezewood tree Ptaeroxylon obliquum. – Synlett. 14: 2101.
Mziray W. 1992. Taxonomic studies in
Toddalieae Hook. f. (Rutaceae) in
Africa. – Symb. Bot. Ups. 30(1): 1-95.
Naidoo D,Coombes PH, Mulholland DA, Crouch
NR, Van Den Bergh AJJ. 2005. N-substituted acridone alkaloids from
Toddaliopsis bremekampii (Rutaceae: Toddalioideae) of south-central
Africa. – Phytochemistry 66: 1724-1728.
Nair GM, Venkaiah K, Shah J. 1983.
Ultrastructure of gumresin ducts in cashew (Anacardium occidentale).
– Ann. Bot., N. S., 51: 297-307.
Nair GV, Poti AN, Pillay PP. 1952a. The
constituents of lacquer-bearing trees of Travancore-Cochin I – chemical
examination of the constituents of Holigarna arnottiana Hook. f. –
J. Sci. Indust. Res. IIB: 294-297.
Nair GV, Poti AN, Pillay PP. 1952a. The
constituents of lacquer-bearing trees of Travancore-Cochin I – chemical
examination of the latex of Semecarpus travancorica Bedd. – J. Sci.
Indust. Res. IIB: 298-299.
Nair MNB. 1991. Wood anatomy of some members
of the Meliaceae. – Phytomorphology
41: 63-73.
Nair NC. 1959a. Studies in Meliaceae I. Floral morphology and
embryology of Naregamia alata W. et A. – J. Indian Bot. Soc. 38:
353-366.
Nair NC. 1959b. Studies in Meliaceae II. Floral morphology and
embryology of Melia azedarach Linn.: a reinvestigation. – J. Indian
Bot. Soc. 38: 366-378.
Nair NC. 1962. Studies in Meliaceae V. Morphology and anatomy of
the flower of the tribes Melieae, Trichileae, and Swietenieae. – J. Indian
Bot. Soc. 41: 226-242.
Nair NC. 1963. Studies in Meliaceae VI. Morphology and anatomy of
the flower of the tribe Cedrelieae and discussion of the floral anatomy of the
family. – J. Indian Bot. Soc. 42: 177-189.
Nair NC. 1970. Comparative embryology of
angiosperms: Meliaceae, Rhamnaceae, Vitaceae, Leeaceae. – Bull. Natl.
Sci. Acad. India 41: 151-155, 168-173, 174-179, 180-184.
Nair NC, Joseph TC. 1957. Floral morphology
and embryology of Samadera indica. – Bot. Gaz. 119: 104-115.
Nair NC, Joseph TC. 1960. Morphology and
embryology of Cardiospermum halicacabum L. – J. Indian Bot. Soc. 39:
176-194.
Nair NC, Joshi RK. 1958. Floral morphology of
some members of the Simaroubaceae. – Bot. Gaz. 120:
88-99.
Nair NC, Nathawat KS. 1958. Vascular anatomy
of the flower of some species of Zygophyllaceae 1.
– J. Indian Bot. Soc. 37: 172-180.
Nair NC, Sukumaran NP. 1960. Floral
morphology and embryology of Brucea amarissima. – Bot. Gaz. 121:
175-185.
Narayana LL. 1957. Embryology of two Simaroubaceae. – Curr. Sci. 26:
323-324.
Narayana LL. 1958a. Floral anatomy and
embryology of Cipadessa baccifera Miq. – J. Indian Bot. Soc. 37:
147-154.
Narayana LL. 1958b. Floral anatomy of Meliaceae I. – J. Indian Bot. Soc. 37:
365-374.
Narayana LL. 1959a. Floral anatomy of Meliaceae II. – J. Indian Bot. Soc.
38: 288-295.
Narayana LL. 1959b. Microsporogenesis and
female gametophyte in Boswellia serrata Roxb. – Curr. Sci. 28:
77-78.
Narayana LL. 1960a. Studies in Burseraceae I. – J. Indian Bot. Soc.
39: 204-209.
Narayana LL. 1960b. Studies in Burseraceae II. – J. Indian Bot.
Soc. 39: 402-409.
Narayana LL. 1963. A note on the embryology
of a few Rutaceae. – Curr. Sci. 32:
516-517.
Narayana LL, Sayeeduddin M. 1958. Floral
anatomy of Simaroubaceae I. –
J. Indian Bot. Soc. 37: 517-522.
Navarro C, Ward S, Hernandez M. 2002. The
tree Cedrela odorata (Meliaceae): a morphologically subdivided
species in Costa Rica. – Rev. Biol. Tropical 50: 21-29.
Navarro FB, Suarez-Santiago VN, Blanco G.
2004. A new species of Haplophyllum A. Juss. (Rutaceae) from the Iberian peninsula:
evidence from morphological, karyological and molecular analyses. – Ann. Bot.
94: 571-582.
Nene PM, Tilak VD. 1977. Placentation in the
Rutaceae. – Proc. Indian Acad. Sci.,
Sect. B, 85: 378-383.
Neto JO, das GF da Silva MF, Fo ER, Fernandes
JB, Vieira PC, Pinheiro AL. 1998. Norlimonoids from seeds of Toona
ciliata. – Phytochemistry 49: 1369-1373.
Ng K-M, But PP-H, Gray AI, Hartley TG, Kong
Y-C, Waterman PG. 1987. The biochemical systematics of Tetradium,
Euodia and Melicope, and their significance in the Rutaceae. – Biochem. Syst. Ecol. 15:
587-593.
Ng’ang’a MM, Hussain H, Chhabra S,
Langat-Thoruwa C, Krohn K. 2009. Chemical constituents from the root bark of
Ozoroa insignis. – Biochem. Syst. Ecol. 37: 116-119.
Nicolosi E, Deng Z-N, Gentile A, La Malfa S,
Continella G, Tribulato E. 2000. Citrus phylogeny and genetic origin
of important species as investigated by molecular markers. – Theor. Appl.
Gen. 100: 1155-1166.
Nie Z-L, Sun H, Meng Y, Wen J. 2009.
Phylogenetic analysis of Toxicodendron (Anacardiaceae) and its biogeographic
implications on the evolution of north temperate and tropical intercontinental
disjunctions. – J. Syst. Evol. 47: 416-430.
Noble JC, Whalley RDB. 1978. The biology and
autecology of Nitraria L. in Australia I. Distribution, morphology and
potential utilization. – Aust. J. Ecol. 3: 141-163.
Nooteboom HP. 1962a. Generic delimitation in
Simaroubaceae tribus Simaroubeae
and a conspectus of the genus Quassia L. – Blumea 11: 509-528.
Nooteboom HP. 1962b. Simaroubaceae. – In: Steenis CGGJ
van (ed), Flora Malesiana I, 6, Noordhoff, Leiden, pp. 193-226.
Nooteboom HP. 1966. Flavonols,
leuco-anthocyanins, cinnamic acids, and alkaloids in dried leaves of some
Asiatic and Malesian Simaroubaceae. – Blumea 14:
309-315.
Nooteboom HP. 1987. Laumoniera, a
new genus of Simaroubaceae from
Sumatra. – Blumea 32: 383-384.
Ogata K. 1967. A systematic study of the
Aceraceae. – Bull. Tokyo Imp. Univ. For. 63: 89-206.
Okorie DA. 1982. Chromones and limonoids from
Harrisonia abyssinica. – Phytochemistry 21: 2424-2426.
Oliver D. 1861. The natural order
Aurantiaceae with a synopsis of the Indian species. – J. Linn. Soc. 5 [Suppl.
2]: 1-55.
Oliver D. 1868. Kirkia acuminata.
– Hooker’s Icones Plantarum III, 11: 26-27, plate 1066.
Omoti U, Okiy DA. 1987. Characteristics and
composition of the pulp oil and cake of the African pear, Dacryodes
edulis (G. Don) H. J. Lam. – J. Sci. Food Agric. 38: 67-72.
Omurkamzinova VB, Maurel ND, Bikbulatova TN.
1991. Flavonoids from Biebersteinia multifida. – Khimiya Prirodnykh
Soedinenii 5: 720-721.
Onana JM. 2008. A synoptic revision of
Dacryodes (Burseraceae) in
Africa, with a new species from Central Africa. – Kew Bull. 63: 385-400.
Onana JM, Chevillotte H. 2015. Taxonomie des
Rutaceae-Toddalieae du Cameroun
revisitée: découverte de quatre espèces nouvelles, validation d’une
combinaison nouvelle et veritable identité de deux autres espèces de
Vepris Comm. ex A. Juss. – Adansonia 37: 103-129.
Oon BL, Choong CY, Mahani MC, Mat-Salleh K.
2000. Molecular phylogeny of Meliaceae based on chloroplast
trnL-trnF nucleotide sequences. – Malaysian Appl. Biol. 29:
127-132.
O’Sullivan J. 1983. Structural diversity
and distribution of lignans in the Rutales. – In: Waterman PG, Grundon MF
(eds), Chemistry and chemical taxonomy of the Rutales, Academic Press, London,
pp. 267-279.
Oterdom HJ. 1990. Paleobotany and evolution
of the maples. – Intern. Dendol. Soc. Yearbook: 12-13.
Othman RNA, Jordan GJ, Worth JRP, Steane DA,
Duretto MF. 2010. Phylogeny and infrageneric classification of Correa
Andrews (Rutaceae) on the basis of
nuclear and chloroplast DNA. – Plant Syst. Evol. 288: 127-138.
Paetow W. 1931. Embryologische Untersuchungen
an Taccaceen, Meliaceen und Dilleniaceen. – Planta 14: 441-470.
Palacios WA. 2007. 98. Meliaceae. – In: Harling G, Persson C
(eds), Flora of Ecuador 82, Department of Plant and Environmental Sciences,
Göteborg University, pp. 1-88.
Palamarev D, Uzunova K. 1970.
Morphologisch-anatomischer Nachweis der Gattung Skimmia in der
Tertiärflora Bulgariens. – Compt. Rend. Acad. Bulg. Sci. 23: 835-838.
Pan AD. 2010. Rutaceae leaf fossils from the Late
Oligocene (27.23 Ma) Guang River flora of northwestern Ethiopia. – Rev.
Palaeobot. Palynol. 159: 188-194.
Pan Y-L, Shen G-M, Chen P. 1999. A
preliminary research on taxonomy and systematics of genus Nitraria.
– Acta Bot. Yunnan. 21: 287-295. [In Chinese]
Pang X-M, Hu C-G, Deng X-X. 2007.
Phylogenetic relationships within Citrus and its related genera as
inferred from AFLP markers. – Gen. Res. Crop Evol. 54: 429-436.
Pannell CM. 1992. A taxonomic monograph of
the genus Aglaia Lour. (Meliaceae). – Kew Bull., Add. Ser. 16,
Her Majesty’s Stationery Office for the Royal Botanic Gardens, Kew,
London.
Pannell CM. 1993. A monograph of
Aglaia (Meliaceae): a
correction. – Kew Bull. 48: 244.
Pannell CM. 1995. A monograph of
Aglaia (Meliaceae): a second
correction. – Kew Bull. 50: 348.
Pannell CM. 2004. Three new species, two new
subspecies and five new combinations at the subspecific level in
Aglaia Lour. (Meliaceae).
– Kew Bull. 59: 87-94.
Panshin AJ. 1933. Comparative anatomy of the
woods of the Meliaceae, subfamily
Swietenioideae. – Amer. J. Bot. 20: 639-668.
Pantanelli E. 1900. Anatomia fisiologica
delle Zygophyllaceae.
– Atti società nat. mat. Modena IV, 2: 93-181.
Parfitt DE, Badenes ML. 1997. Phylogeny of
the genus Pistacia as determined from analysis of the chloroplast
genome. – Proc. Natl. Acad. Sci. U.S.A. 94: 7987-7992.
Parfitt DE, Badenes ML. 1998. Molecular
phylogenetic analysis of the genus Pistacia. – Acta Hort. 470:
143-151.
Park C-W, Oh S-H, Shin H. 1993. Reexamination
of vascular plants in Ullung Island, Korea II: taxonomic identity of Acer
takesimense Nakai (Aceraceae). – Kor. J. Plant Taxon. 23: 217-231.
Paula JED, Alves JLDH. 1973. Anatomia de
Anacardium spruceanum Benth. ex Engl. (Anacardiaceae da Amazonia). – Acta
Amaz. 3: 39-53.
Pax F. 1896a. Aceraceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien III(5), W. Engelmann,
Leipzig, pp. 263-272.
Pax F. 1896b. Hippocastanaceae. – In:
Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(5), W.
Engelmann, Leipzig, pp. 273-276.
Peck CJ, Lersten NR. 1991. Gynoecial ontogeny
and morphology, and pollen tube pathway in black maple, Acer saccharum
ssp. nigrum (Aceraceae). – Amer. J. Bot. 78: 247-259.
Pell SK. 2004. Molecular systematics of the
cashew family (Anacardiaceae).
– Ph.D. diss., Louisiana State University and Agricultural and Mechanical
College, Baton Rouge, Louisiana.
Pell SK, Mitchell JD, Lowry PP, Randrianasolo
A, Urbatsch LE. 2008. Phylogenetic split of Malagasy and African taxa of
Protorhus and Rhus (Anacardiaceae) based on cpDNA
trnL-trnF and nrDNA ETS and ITS sequence data. – Syst. Bot.
33: 375-383.
Pell SK, Mitchell JD, Miller AJ, Lobova TA.
2011. Anacardiaceae. – In:
Kubitzki K (ed), Families and genera of vascular plants X. Flowering plants.
Eudicots. Sapindales, Cucurbitales, Myrtaceae. Springer, Heidelberg,
Dordrecht, London, New York, pp. 7-50.
Penjor T, Anai T, Nagano Y, Matsumoto R,
Yamamoto M. 2010. Phylogenetic relationships of Citrus and its
relatives based on rbcL gene sequences. – Tree Gen. Genomes 6:
931-939.
Penjor T, Yamamoto M, Uehara M, Ide M,
Matsumoto N, Matsumoto R, Nagano Y. 2013. Phylogenetic relationships of
Citrus and its relatives based on matK gene sequences. –
PLoS ONE 8, e62574.
Pennington TD. 1981. Meliaceae. Flora Neotropica Monograph
28. – New York Botanical Garden, Bronx, New York.
Pennington TD, Styles BT. 1975. A generic
monograph of the Meliaceae. –
Blumea 22: 419-540.
Pennington TD, Styles BT, Taylor DAH. 1981.
Flora Neotropica. Monograph 28. Meliaceae. – The New York Botanical
Garden, Bronx, New York.
Pernet R. 1972. Phytochemie der Burseraceae. – Lloydia 35:
280-287.
Perrier de la Bâthie H. 1944. Révision des
Anacardiacées de Madagascar et des Comores. – Mém. Mus. Natl. Hist. Nat.
Paris 18:243-269.
Perrier de la Bâthie H. 1946.
114e Famille. Anacardiacées. – In: Humbert H (ed), Flore de
Madagascar et des Comores, Typ. Firmin-Didot & Co. Mesnil, France, pp.
1-85.
Perrier de la Bâthie H. 1948 [1949]. –
Révision des Rutacées de Madagascar et des Comores. – Mém. Acad. Sci.
Inst. France 67: 1-39.
Perrier de la Bâthie H. 1950a.
104e Famille. Rutacées. – In: Humbert H (ed), Flore de Madagascar
et des Comores, Typ. Firmin-Didot & Co. Mesnil, France, pp. 1-89.
Perrier de la Bâthie H. 1950b.
105e Famille. Simarubacées. – In: Humbert H (ed), Flore de
Madagascar et des Comores, Typ. Firmin-Didot & Co. Mesnil, France, pp.
1-7.
Perveen A, Qaiser M. 2006. Pollen flora of
Pakistan XLIX. Zygophyllaceae.
– Pak. J. Bot. 38: 252-232.
Petersen FP, Fairbrothers DE. 1983. A
serotaxonomic appraisal of Amphipterygium and Leitneria –
two amentiferous taxa of Rutiflorae (Rosidae). – Syst. Bot. 8: 134-148.
Petersen FP, Fairbrothers DE. 1985. A
serotaxonomic appraisal of the “Amentiferae”. – Bull. Torrey Bot. Club
112: 43-52.
Pfeiffer WM. 1912. The morphology of
Leitneria floridana. – Bot. Gaz. 53: 189-203.
Pfeil BE, Crisp MD. 2008. The age and
biogeography of Citrus and the orange subfamily (Rutaceae: Aurantioideae) in Australasia
and New Caledonia. – Amer. J. Bot. 95: 1621-1631.
Pfosser M, Guzy-Wrobelska J, Sun B, Stuessy
T, Sugawara T, Fujii N. 2002. The origin of species of Acer (Sapindaceae) endemic to Ullung Island,
Korea. – Syst. Bot. 27: 351-367.
Pierlot R. 1997. Pseudodacryodes
Pierlot, genre nouveau de Burseraceae de l’est de la Rep. dem.
du Congo. – Bull. Jard. Bot. Belg. 66: 175-186.
Pierre A-H, Le Moguédec G, Lowry II PP,
Munzinger J. 2014. Multivariate morphometric analysis and species delimitation
in the endemic New Caledonian genus Storthocalyx (Sapindaceae). – Bot. J. Linn. Soc.
176: 127-146.
Pierre L. 1898. Sur les genres
Oricia et Diphasia. – Bull. Mens. Soc. Linn. Paris, n.s. 8:
68-71.
Pijl L van der. 1957. On the arilloids of
Nephelium, Euphoria, Litchi and Aesculus,
and the seeds of Sapindaceae in
general. – Acta Bot. Neerl. 6: 618-641.
Pirani JR. 1998. A revision of
Helietta and Balfourodendron (Rutaceae, Pteleinae). – Brittonia 50:
348-380.
Pirani JR. 1999. Two new species of
Esenbeckia (Rutaceae,
Pilocarpinae) from Brazil and Bolivia. – Bot. J. Linn. Soc. 129: 305-313.
Pirani JR. 2004. Three new species of
Galipea (Rutaceae,
Galipeinae) from Brazil. – Bot. J. Linn. Soc. 144: 365-373.
Pirani JR, Kallunki JA. 2007. Two new species
of Galipea (Rutaceae,
Galipeeae) from Bolivia, Ecuador, and Peru. – Brittonia 59:343-349.
Pole M. 2010. Cuticle morphology of
Australasian Sapindaceae. – Bot.
J. Linn. Soc. 164: 264-292.
Polonsky J. 1983. Chemistry and biological
activity of the quassinoids. – In: Waterman PG, Grundon MF (eds), Chemistry
and chemical taxonomy of the Rutales, Academic Press, London, pp. 247-266.
Poon W-S, Shaw P-C, Simmons MP, But PP-H.
2007. Congruence of molecular, morphological and biochemical profiles in Rutaceae: a cladistic analysis of the
subfamilies Rutoideae and Toddalioideae. – Syst. Bot. 32: 837-846.
Porter DM. 1973. Flora of Panama, family 90,
Simaroubaceae. – Ann. Missouri
Bot. Gard. 60: 23-39.
Porter DM. 1974. Disjunct distributions in
the New World: Zygophyllaceae.
– Taxon 23: 339-346.
Potvin C, Bergeron Y, Simon J-P. 1983. A
numerical taxonomic study of selected Citrus species (Rutaceae) based on biochemical
characters. – Syst. Bot. 8: 127-133.
Powell JM, Armstrong JA. 1980. Seed surface
structure in the genus Zieria Sm. (Rutaceae). – Telopea 2: 85-112.
Pozhidaev AE. 1995. Pollen morphology of the
genus Aesculus (Hippocastanaceae). Patterns in the variety of
morphological characteristics. – Grana 34: 10-20.
Price JR. 1963. The distribution of alkaloids
in the Rutaceae. – In: Swain T (ed),
Chemical plant taxonomy, Academic Press, London, New York, pp. 429-452.
Provan GJ, Gray AI, Waterman PG. 1987.
Monoterpene-rich resins from some Kenyan Burseraceae. – Flavour Fragr. J. 2:
115-118.
Puri GS. 1945. Some fossil leaflets of
Aesculus indica Colebr. from the Karewa Beds at Laredura and Ningal
Nullah, Pir Panjal, Kashmir. – J. Indian Bot. Soc. 24: 147-151.
Quader A, Armstrong JA, Gray AI, Hartley TG,
Waterman PG. 1991. Chemosystematics of Acradenia and general
significance of acetophenones in the Rutaceae. – Biochem. Syst. Ecol. 19:
171-176.
Rabarimanarivo MN, Rakotonirina NH,
Phillipson PB, Lowry II PP, Labat J-N, Pignal M. 2015. Révision du genre
Ivodea Capuron (Rutaceae),
endémique de Madagascar et de l’archipel des Comores. – Adansonia 37:
63-102.
Radlkofer L. 1875. Monographie der
Sapindaceen-Gattung Serjania. – Verlag der Königl. Bayer. Akademie
Wissensch., München.
Radlkofer L. 1878a. Über Sapindus
und damit in Zusammenhang stehende Pflanzen. – Sitzungsber. Math.-Phys. Cl.
K. Bayer. Akad. Wiss. München 8: 334-338.
Radlkofer L. 1878b. Sopra un arillo speciale
di una Sapindacea. – Nuovo G. Bot. Italiano 10: 105-109.
Radlkofer L. 1879. Über Cupania und
damit verwandte Pflanzen. – Sitzungsber. Math.-Phys. Cl. K. Bayer. Akad.
Wiss. München 9: 457-678.
Radlkofer L. 1886. Ergänzungen zur
Monographie der Sapindaceen-Gattung Serjania. – Verlag der Königl.
Bayer. Akademie Wissensch., München.
Radlkofer L. 1890. Über die Gliederung der
Familie der Sapindaceen. – Sitzungsber. Königl. Bayer. Akademie Wissensch.
München, Math.-Physik. Cl., 20: 105-379.
Radlkofer L. 1895. Monographie der
Sapindaceen-Gattung Paullinia. – Verlag der Königl. Bayer. Akademie
Wissensch., München.
Radlkofer L. 1896. Sapindaceae. – In: Engler A, Prantl
K (eds), Die natürlichen Pflanzenfamilien III(5), W. Engelmann, Leipzig, pp.
277-366, 460-462.
Radlkofer L. 1908. Sapindaceae. – In: Engler A, Prantl
K (eds), Die natürlichen Pflanzenfamilien, Nachtr. 3-III.5, W. Engelmann,
Leipzig, pp. 202-209.
Radlkofer L. 1933. Sapindaceae 1. – W. Engelmann,
Leipzig.
Radlkofer L. 1934. Sapindaceae 2. – W. Engelmann,
Leipzig.
Ramírez DA. 1961. Cytology of Philippine
plants VII. Nephelium lappaceum Linn. – Philipp. Agric. 45:
340-342.
Ramírez JL, Cevallos-Ferriz SRS. 2002. A
diverse assemblage of Anacardiaceae from Oligocene
sediments, Tepexi de Rodriguez, Puebla, Mexico. – Amer. J. Bot. 89:
535-545.
Ramírez JJ, Flores KV, Durán RC. 2011.
Balsas (Sapindaceae),
género nuevo de la Cuenca del Río Balsas en el Estado de Guerrero, México.
– Novon 21: 196-200.
Ramos MFS, Guimarães AC, Siani AC. 2003.
Volatile monoterpenes from the oleoresin of Trattinnickia rhoifolia.
– Biochem. Syst. Ecol. 31: 309-311.
Ramp E. 1988. Struktur, Funktion und
systematische Bedeutung des Gynoeciums bei den Rutaceae und Simaroubaceae. – Ph.D. diss.,
Philos. Fakultät, Universität Zürich, Switzerland.
Randrianasolo A. 1998. Systematics and
evolution of three Malagasy genera of Anacardiaceae: Micronychia
Oliv., Protorhus Engl. and Rhus section Baronia
(Baker) H. Perrier. – Ph.D. diss., University of Missouri, St. Louis,
Missouri.
Randrianasolo A, Miller JS. 1999. A new
species of Poupartia (Anacardiaceae) from Madagascar. –
Novon 9: 546-548.
Randrianasolo A, Lowry II PP. 2006.
Operculicarya (Anacardiaceae) revisited: an updated
taxonomic treatment for Madagascar and the Comoro Islands, with descriptions of
two new species. – Adansonia, sér. III, 28: 359-371.
Randrianasolo A, Lowry II PP. 2009. Four new
species and one new combination in the Malagasy endemic genus
Micronychia Oliv. (Anacardiaceae). – Adansonia, sér.
II, 31: 157-168.
Rao BR, Rajput DK, Mallavarapu GR. 2011.
Chemical diversity in curry leaf (Murraya koenigii) essential oils.
– Food Chem. 126: 989-994.
Rao D. 1970. Comparative embryology of
Angiosperms: Simaroubaceae, Burseraceae. – Bull. Natl. Sci.
Acad. India 41: 142-147, 148-150.
Rao TA, Bhattacharya JB. 1981. Comparative
morphology of foliar sclereids in Boronia Sm. (Rutaceae). – Proc. Ind. Acad. Sci.
(Plant Sci.) 90: 9-29.
Razafimandimbison SG, Appenhans MS, Rabarison
H, Haevermans T, Rakotondrafara A, Rakotonandrasana SR, Ratsimbason M, Labat
JN, Kessler PJ, Smets E, Cruaud C, Couloux A, Randrianarivelojosia M. 2010.
Implications of a molecular phylogenetic study of the Malagasy genus
Cedrelopsis and its relatives (Ptaeroxylaceae). – Mol. Phylogen.
Evol. 57: 258-265.
Record SJ. 1939. American woods of the family
Anacardiaceae. – Trop. Woods
60: 11-45.
Record SJ. 1941. American timbers of the
Mahogany family. – Trop. Woods 66: 7-33.
Record SJ, Hess RW. 1940. American woods of
the family Rutaceae. – Trop. Woods
64: 1-28.
Reese G. 1958. Cyto-systematische Notizen zur
Gattung Nitraria (Zygophyllaceae).
– Flora 146: 478-488.
Rehder AA. 1935. Handeliodendron, a
new genus of Sapindaceae. – J.
Arnold Arbor. 16: 65-68.
Rehder AA. 1945. Moraceae, Hippocastanaceae et Vitaceae, nomina conservanda. –
J. Arnold Arbor. 26: 277-279.
Reiche K. 1896. Geraniaceae. – In:
Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(4), W.
Engelmann, Leipzig, pp. 1-14.
Renner SS, Beenken L, Grimm GW, Kocyan A,
Ricklefs RE. 2007. The evolution of dioecy, heterodichogamy, and labile sex
expression in Acer. – Evolution 61: 2701-2719.
Reynolds ST. 1981. Notes on Sapindaceae in Australia 1. –
Austrobeileya 1: 388-419.
Reynolds ST. 1987. Notes on Sapindaceae in Australia 5. –
Austrobaileya 2: 328-338.
Reynolds ST, West JG. 1985. Sapindaceae. – In: George AS (ed),
Flora of Australia 25, Australian Government Publ. Service, Canberra, pp.
4-164.
Rios MY, Delgado G. 1992. Polyprenols and
acylphloroglucinols from Esenbeckia nesiotica. – Phytochemistry 31:
3491-3494.
Rios MY, Rosas-Alonso E, Aguilar-Guadarrama
AB. 2002. Alkaloids, coumarins and sesquiterpenes from Esenbeckia
conspecta Kunt (Rutaceae). –
Biochem. Syst. Ecol. 30: 367-369.
Rios MY, Aguilar-Guadarrama AB, Delgado G.
2002. Furoquinoline alkaloids, furocoumarins and terpenes from Esenbeckia
litoralis (Rutaceae). –
Biochem. Syst. Ecol. 30: 977-979.
Ritchie E. 1964. Chemistry of
Flindersia species. – Rev. Pure Appl. Chemistry 14: 47-56.
Rivero-Cruz JF, Chávez D, Hernández B,
Anaya AL, Mata R. 1997. Separation and characterization of Metopium
brownei urushiol components. – Phytochemistry 45: 1003-1008.
Robbertse PJ, Teichman I von, Rensburg HJ
van. 1986. A re-evaluation of the structure of the mango ovule in comparison
with a few other Anacardiaceae
species. – South Afr. J. Bot. 52: 17-24.
Rodan BD, Campbell FT. 1996. CITES and the
sustainable management of Swietenia macrophylla King. – Bot. J.
Linn. Soc. 122: 83-87.
Ronse De Craene L-P, Smets EF. 1991.
Morphological studies in Zygophyllaceae I.
The floral development and vascular anatomy of Nitraria retusa. –
Amer. J. Bot. 78: 1438-1448.
Ronse De Craene L-P, De Laet J, Smets EF.
1996. Morphological studies in Zygophyllaceae
II. The floral development and vascular anatomy of Peganum harmala.
– Amer. J. Bot. 83: 201-215.
Ronse De Craene L-P, Smets E, Clinckemaillie
D. 2000. Floral anatomy and ontogeny in Koelreuteria with special
emphasis on monosymmetry and septal cavities. – Plant Syst. Evol. 223:
91-107.
Rossetto M. 2005. A simple molecular approach
for identifying a rare Acronychia (Rutaceae) provides new insights on its
multiple hybrid origins. – Biol. Cons. 121: 35-43.
Roth I. 1969. Estructura cortical de algunas
especies venezolanas de Anacardiaceae. – Acta Biol. Venez.
6: 146-160.
Rozefelds A. 2001a. Notes on the
Philotheca myoporoides complex (Rutaceae) in Victoria. – Muelleria 15:
15-18.
Rozefleds A. 2001b. The Tasmanian species of
Philotheca (Rutaceae). –
Muelleria 15: 19-26.
Rüdiger AL, Siani AC, Veiga VF. 2007. The
chemistry and pharmacology of the South American genus Protium Burm.
f. (Burseraceae). – Pharmacog.
Rev. 1: 93-104.
Rzedowski J, Kruse H. 1979. Algunas
tendencias evolutivas en Bursera (Burseraceae). – Taxon 28:
103-116.
Rzedowski J, Palacios-Chávez R. 1985. La
presencia de Commiphora (Burseraceae) en México. – Taxon 34:
207-210.
Sachar RC, Chopra RN. 1957. A study of the
endosperm and embryo in Mangifera L. – Ind. J. Agric. Sci. 27:
219-228.
Sachdev K, Kulshreshtha DK. 1983. Flavonoids
from Dodonaea viscosa. – Phytochemistry 22: 1253-1256.
Saleh NAM, El-Hadidi MH. 1977. An approach to
the chemosystematics of the Zygophyllaceae.
– Biochem. Syst. Ecol. 5: 121-128.
Salvo G, Bacchetta G, Ghahremaninejad F,
Conti E. 2008. Phylogenetic relationships of Ruteae (Rutaceae): new evidence from the chloroplast genome and
comparisons with non-molecular data. – Mol. Phylogen. Evol. 49: 736-748.
Salvo G, Ho SYW, Rosenbaum G, Ree R, Conti E.
2010. Tracing the temporal and spatial origins of island endemics in the
Mediterranean region: a case study from the citrus family (Ruta L., Rutaceae). – Syst. Biol. 59:
705-722.
Salvo G, Manafzadeh S, Ghahremaninejad F,
Tojibaev, K, Zeltner L, Conti E. 2011. Phylogeny, morphology, and biogeography
of Haplophyllum (Rutaceae), a
species-rich genus of the Irano-Turanian floristic region. – Taxon 60:
513-527.
Samuel R, Ehrendorfer F, Chase MW, Greger H.
2001. Phylogenetic analysis of Aurantioideae (Rutaceae) based on non-coding plastid DNA
sequences and phytochemical features. – Plant Biol. 3: 77-87.
San Miguel E. 2003. Rue (Ruta L., Rutaceae) in traditonal Spain: frequency
and distribution of its medicinal and symbolic applications. – Econ. Bot. 57:
231-244.
Santin D, Leitão-Filho H. 1991.
Restabelecimento e revisão taxonomica do género Myracrodruon Freie
Alemão (Anacardiaceae). – Rev.
Bras. Bot. 14: 133-145.
Santisuk T. 1992. Notes on the genus
Acer (Aceraceae) in Thailand. – Nord. J. Bot. 12: 695-698.
Sarma V, Rao SRS. 1991. Taxonomic importance
of epidermis in Simaroubaceae-Zygophyllaceae
with special reference to position of Balanites. – Feddes Repert.
102: 579-585.
Sato T. 2002. Phenology of sex expression and
gender variation in a heterodichogamous maple, Acer japonicum. –
Ecology 83: 1226-1238.
Schatz GE, Gereau RE, Lowry II PP. 1999. A
revision of the Malagasy endemic genus Chouxia Capuron (Sapindaceae). – Adansonia, sér.
III, 21: 51-62.
Schatz GE, Gereau RE, Lowry II PP. 2017. A
revision of the endemic Malagasy genus Beguea (Sapindaceae). – Candollea 72:
45-65.
Schneider C, Bohnenstengel FI, Nugroho BW,
Wray V, Witte L, Hung PD, Kiet LC, Proksch P. 2000. Insecticidal rocaglamide
derivatives from Aglaia spectabilis (Meliaceae). – Phytochemistry 54:
731-736.
Schneider H. 1968. The anatomy of
Citrus. – In: Reuther W, Batchelor LD, Webber HJ (eds), The Citrus
Industry 2, 2nd ed., University of California, Riverside, California, pp.
1-85.
Schönbeck-Temesy E. 1970. Geraniaceae:
Biebersteinia. – In: Rechinger KH (ed), Flora Iranica, Akademische
Druck- u. Verlagsanstalt, Graz, pp. 63-64.
Schwartz T. 2010. A phylogeny of the Rutaceae and a biogeographic study of its
subfamily Aurantioideae. – M.Sc. thesis, Dept. of Plant and Environmental
Science, University of Gothenburg, Sweden.
Schwartz T, Nylinder S, Ramadugu C, Antonelli
A, Pfeil BE. 2015. The origin of oranges: a multi-locus phylogeny of Rutaceae subfamily Aurantioideae. –
Syst. Bot. 40: 1053-1062.
Scora RW. 1975. IX. On the history and origin
of citrus. – Bull. Torrey Bot. Club 102: 369-375.
Scott KD, McIntyre CL, Playford J. 2000.
Molecular analyses suggest a need for a significant rearrangement of Rutaceae subfamilies and a minor
reassessment of species relationships within Flindersia. – Plant
Syst. Evol. 223: 15-27.
Segaar PJ, Ham RWJM van der. 1993. Pollen of
Scutinanthe brunnea compared with other burseraceous pollen types: a
remarkable case of divergence. – Rev. Palaeobot. Palyn. 79: 297-334.
Seigler DS, Kawahara W. 1976. New reports of
cyanolipids from sapindaceous plants. – Biochem. Syst. Ecol. 4: 263-265.
Setia RC, Parthasarathy MV, Shah JJ. 1977.
Development, histochemistry and ultrastructure of gum-resin ducts in
Commiphora mukul Engl. – Ann. Bot., N. S., 41: 999-1004.
Shan F, Yan G, Plummer JA. 2003. Karyotype
evolution in the genus Boronia (Rutaceae). – Bot. J. Linn. Soc. 142:
309-320.
Sharma MR. 1954. Studies in the family Anacardiaceae I. Vascular anatomy of
flowers of Mangifera indica. – Phytomorphology 4: 201-208.
Sheahan MC. 2011a. Nitrariaceae. – In: Kubitzki K
(ed), Families and genera of vascular plants X. Flowering plants. Eudicots. Sapindales, Cucurbitales, Myrtaceae. Springer, Heidelberg,
Dordrecht, London, New York, pp. 272-275.
Sheahan MC. 2011b. Tetradiclidaceae. – In:
Kubitzki K (ed), Families and genera of vascular plants X. Flowering plants.
Eudicots. Sapindales, Cucurbitales, Myrtaceae. Springer, Heidelberg,
Dordrecht, London, New York, pp. 424-429.
Sheahan MC, Chase MW. 1996. A phylogenetic
analysis of Zygophyllaceae R.
Br. based on morphological, anatomical and rbcL sequence data. –
Bot. J. Linn. Soc. 122: 279-300.
Sheahan MC, Cutler DF. 1993. Contribution of
vegetative anatomy to the systematics of the Zygophyllaceae R.
Br. – Bot. J. Linn. Soc. 113: 227-262.
Shen S, Huang R. 1997. Cytological and
morpho-anatomical studies of Biebersteinia heterostemon Maxim. –
Acta Biol. Plat. Sin. 13: 5-8.
Sherff EE. 1947. Further studies in the genus
Dodonaea L. – Publ. Field Mus. Bot. 23: 269-317.
Sherman-Broyles SL, Gibson JP, Hamrick JL,
Bucher MA, Gibson MJ. 1992. Comparisons of allozyme diversity among rare and
widespread Rhus species. – Syst. Bot. 17: 551-559.
Shivakumar VS, Appelhans MS, Johnson G,
Carlsen M, Zimmer EA. 2017. Analysis of whole chloroplast genomes from the
genera of the Clauseneae, the curry tribe (Rutaceae, Citrus family). – Mol.
Phylogen. Evol. 117: 135-140.
Shukla RD. 1955. On the morphology of the two
abnormal gynoecia of Peganum harmala. – J. Indian Bot. Soc. 34:
382-387.
Siddiqui BS, Ali ST, Ali SK. 2008. Chemical
wealth of Azadirachta indica (neem). – In: Singh KK, Phogat S, Tomar
A, Dhillon RS (eds), Neem – a treatise, I. K. International Publ., New Delhi,
pp. 171-207.
Silva JDE. 1973. Catalogo de nervação
foliar das Anacardiaceae da
caatinga I. – Arq. Jard. Bot. Rio de Janeiro 14: 249-256.
Simão SM, Barreiros EL, Silva MF das GF da,
Gottlieb OR. 1991. Chemogeographical evolution of quassinoids in Simaroubaceae. – Phytochemistry
30: 853-865.
Simmonds MSJ, Stevenson PC, Porter EA, Veitch
NC. 2001. Insect antifeedant activity of three new tetranortriterpenoids from
Trichilia pallida. – J. Nat. Prod. 64: 1117-1120.
Simpson DS, Jacobs H. 2005. Alkaloids and
coumarins from Esenbeckia pentaphylla (Rutaceae). – Biochem. Syst. Ecol. 33:
841-844.
Singh AK, Singh M, Singh AK. 1988. Antiviral
activity and physical properties of the extracts of Azadirachta indica
L. – Indian J. Virol. 4: 76-81.
Singh BP, Kaur I. 1998. Systematic position
of the genus Peganum. – J. Econ. Taxon. Bot. 22: 705-708.
Skepner AP, Krane DE. 1997. RAPD reveals
genetic similarity of Acer saccharum and Acer nigrum. –
Heredity 80: 422-428.
Skorupa LA, Pirani JR. 2004. A new species of
Pilocarpus (Rutaceae) from
northern Brazil. – Brittonia 56: 147-150.
Smith-White S. 1954. Chromosome numbers in
the Boronieae (Rutaceae) and their
bearing on the evolutionary development of the tribe in the Australian flora.
– Aust. J. Bot. 2: 287-303.
Snook LK. 1996. Catastrophic disturbance,
logging and the ecology of mahogany (Swietenia macrophylla King):
grounds for listing a major tropical timber species in CITES. – Bot. J. Linn.
Soc. 122: 35-46.
Solis SM, Ferrucci MS. 2009. Morpho-anatomy
and ontogeny of the floral nectaries of Cardiospermum grandiflorum and
Urvillea chacoensis (Sapindaceae). – Ann. Bot. Fenn. 46:
485-495.
Somner GV, Ferrucci MS. 1997. Paullinia
caerensis (Sapindaceae) nueva
especie de Brasil. – Bonplandia 9: 241-243.
Somner GV, Ferrucci MS. 2004. A new species
of Cupania sect. Trigonocarpus (Sapindaceae) from Brazil. – Bot. J.
Linn. Soc. 146: 217-221.
Song W-H, Li X-D, Li X-W, Huang H-W, Li J-Q.
2004. Genetic diversity and conservation strategy of Psilopeganum
sinense, a rare species in the three-gorges reservoir area. – Biodivers.
Sci. 12: 227-236.
Souèges R. 1953. Embryogénie des
Péganacées: développement de l’embryon chez le Peganum harmala L.
– Compt. Rend. Acad. Sci. 236: 2185-2188.
Souza LA, Mourão KSM, Moscheta IS, Rosa SM.
2003. Morfologia e anatomia da flor de Pilocarpus pennatifolius Lem.
(Rutceae). – Rev. Brasileira Bot. 26: 175-184.
Spiegel-Roy P, Goldschmidt EE. 1996. Biology
of Citrus. – Cambridge University Press, New York.
Spiekerkoetter H. 1924. Untersuchungen zur
Anatomie und Systematik ostafrikanischer Meliaceen, Burseraceen und
Simarubaceen. – Bot. Arch. 7: 274-320.
Sprague TA. 1910. XXVI. –
Entandrophragma, Leioptyx and Pseudocedrela. – Kew
Bull. 1910(6): 177-182.
Sprague TA. 1913. Diagnoses Africanae LIV.
Protorhus namaquensis. – Kew Bull. 4: 179.
Stace HM, Armstrong JA. 1992. New chromosome
numbers for Rutaceae. – Aust. Syst.
Bot. 5: 501-505.
Stace HM, Leach GJ. 1994. Cytological notes
in Rutaceae 2: Neobrynesia
suberosa. – Telopea 6: 167-168.
Stace HM, Armstrong JA, James SH. 1993.
Cytoevolutionary patterns in Rutaceae.
– Plant Syst. Evol. 187: 1-28.
Stannard BL. 1981. A revision of
Kirkia (Simaroubaceae).
– Kew Bull. 35: 829-839.
Stannard BL. 2000. Simaroubaceae. – In: Beentje,
Smith SAL, Whitehouse CM (eds), Flora of tropical East Africa, A. A. Balkema,
Rotterdam, The Netherlands, pp. 1-14.
Stannard BL. 2007. The inclusion of
Pleiokirkia in Kirkia (Kirkiaceae), and corresponding
combination. – Kew Bull. 62: 151-152.
Steingraeber DA, Fisher JB. 1986.
Indeterminate growth of leaves in Guarea (Meliaceae): a twig analogue. – Amer.
J. Bot. 73: 852-862.
Stern WL. 1952. The comparative anatomy of
the xylem and the phylogeny of the Julianiaceae. – Amer. J. Bot. 39:
220-229.
Stevens PF. 1975. Review of
Chisocheton (Meliaceae) in
Papuasia. – Contr. Herb. Australiense 11: 1-55.
Stone BC. 1962. A monograph of the genus
Platydesma (Rutaceae). – J.
Arnold Arbor. 43: 410-427.
Stone BC, Nair KN. 1994. A new species of
Clausena (Rutaceae) from
India. – Nord. J. Bot. 14: 491-493.
Stone BC, Lowry JB, Scora RW, Jong K. 1973.
Citrus halimii: a new species from Malaya and Peninsular Thailand. –
Biotropica 5: 102-110.
Straka H, Albers F, Mondon A. 1976. Die
Stellung und Gliederung der Familie Cneoraceae (Rutales). – Beitr. Biol.
Pflanzen 52: 267-310.
Strappaghetti G, Corsane S, Craveiro A,
Proieti G. 1982. Constituents of essential oil of Boswellia frereana.
– Phytochemistry 21: 2114-2115.
Strid AK. 1972. Revision of the genus
Adenandra (Rutaceae). –
Opera Bot. 32: 1-112.
Stuhlfauth T, Fock H, Huber H, Klug K. 1985.
The distribution of fatty acids including petroselinic and tariric acids in the
fruit and seed oils of the Pittosporaceae,
Araliaceae,
Umbelliferae, Simarubaceae and Rutaceae. – Phytochemistry 13:
447-453.
Styles BT. 1972. The flower biology of the Meliaceae and its bearing on tree
breeding. – Silvae Genet. 21: 175-182.
Styles BT, Bennett ST. 1992. Notes on the
morphology, chemistry, ecology, conservation status and cytology of
Schmardaea microphylla (Meliaceae). – Bot. J. Linn. Soc. 108:
359-373.
Styles BT, Khosla PK. 1976. Cytology and
reproductive biology of Meliaceae.
– In: Burley J, Styles BT (eds), Tropical trees: variation, breeding and
conservation, Academic Press, London, pp. 61-67.
Styles BT, Vosa CG. 1971. Chromosome numbers
in the Meliaceae. – Taxon 20:
485-499.
Suh Y, Cho H-J, Kim M, Park C-W. 1996.
Comparative analysis of ITS sequences from Acer species (Aceraceae) in
Korea. – J. Plant Biol. 39: 1-8.
Suh Y, Heo K, Park C-W. 2000. Phylogenetic
relationships of maples (Acer L.; Aceraceae) implied by nuclear
ribosomal ITS sequences. – J. Plant Res. 113: 193-202.
Sultana S, Ilyas M. 1986. A flavanone from
Lannea acida. – Phytochemistry 25: 963-964.
Sunnichan VG, Mohan Ram HY, Shivanna KR.
2005. Reproductive biology of Boswellia serrata, the source of salai
guggul, an important gum-resin. – Bot. J. Linn. Soc. 147: 73-82.
Swanepoel W. 2008. Commiphora
otjihipana (Burseraceae), a
new species from the Kaokoveld, Namibia. – South Afr. J. Bot. 74: 623-628.
Swart JJ. 1942. A monograph of the genus
Protium and some allied genera (Burseraceae). – Drukkerij Koch en
Knuttel, Gouda.
Swingle WT. 1914. Eremocitrus, a new
genus of hardy, drought-resistant citrous fruits from Australia. – J.
Agricult. Res. 2: 85-100.
Swingle WT. 1915a. A new genus,
Fortunella, comprising four species of kumquat oranges. – J.
Washington Acad. Sci. 5: 165-176.
Swingle WT. 1915b. Microcitrus,a new
genus of Australian citrous fruits. – J. Washington Acad. Sci. 5: 569-578.
Swingle WT. 1918. Merrillia, a new
rutaceous genus of the tribe Citreae from the Malay Peninsula. – Philipp. J.
Sci, Sect. C, Botany 13: 335-343.
Swingle WT. 1939. Clymenia and
Burkillanthus, new genera; also three new species of
Pleiospermium (Rutaceae-Aurantioideae). – J. Arnold
Arbor. 20: 250-263.
Swingle WT. 1943. The botany of
Citrus and its wild relatives of the orange subfamily. – In:
Batchelor LD, Webber HJ (eds), The citrus industry 1. History, world
distribution, botany, and varieties, University of California, Berkeley,
California, pp. 1129-474.
Swingle WT, Reece PC. 1967. The botany of
Citrus and its wild relatives. – In: Reuther W, Webber HJ, Bachelor
LD (eds), The citrus industry, rev. 2nd ed., 1. History, world
distribution, botany, and varieties, University of California, Berkeley,
California, pp. 190-430.
Takeda F, Crane JC, Lin J. 1979. Pistillate
flower bud development in pistachio. – J. Amer. Soc. Horticult. Sci. 104:
229-232.
Takeuchi W. 2001. A distinctive new
Rhysotoechia (Sapindaceae)
from Papua New Guinea. – Blumea 46: 569-573.
Tanaka T. 1929. Chalcas, a Linnean
genus, which includes many new types of Asian plants. – J. Soc. Trop.
Agricult. 1: 22-44.
Tanaka T. 1932. Philippine Rutaceae-Aurantioideae (Revisio
Aurantiacearum, VII.). – Trans. Nat. Hist. Soc. Formosa 22: 418-433.
Tanaka T. 1936. The taxonomy and nomenclature
of Rutaceae-Aurantioideae. – Blumea
2: 101-110.
Tanaka T. 1954. Species problem in
Citrus. – Jap. Soc. Promot. Sci., Ueno, Tokyo.
Tanaka T. 1961. Citrologia. Semi-centennial
commemoration papers on Citrus. – Citrologia Supporting Foundation,
Osaka.
Tang F, Ye Q, Yao X, Huang H. 2007. Isolation
and characterization of microsatellite loci in Psilopeganum sinense
Hemsl. (Rutaceae), an endangered herb
endemic to Yangtze River valley. – Mol. Ecol. Notes, publ. online, doi:
10.1111/j.1471-8286.2007.01933.x
Tardieu-Blot ML. 1961. Sur les
Dracontomelum d’Indochine. – Adansonia, n.s. 1: 55-58.
Tarus PK, Coombes PH, Crouch NR, Mulholland
DA, Moodley B. 2005. Furoquinoline alkaloids from the southern African Rutaceae Teclea natalensis. –
Phytochemistry 66: 703-706.
Tatsuhiro A. 2000. Dichogamy in fullmoon
maple (Acer japonicum Thunb.). – Bull. Hokkaido Forest Exper.
Station 37: 27-40.
Taylor DAH. 1982. Flora Neotropica Monograph
28. The occurrence of limonoids in the Meliaceae. – New York Botanical
Garden,Bronx, New York, pp. 450-459.
Taylor DAH. 1983. Biogenesis, distribution,
and systematic significance of limonoids in the Meliaceae, Cneoraceae, and allied taxa.
– In: Waterman PG, Grundon MF (eds), Chemistry and chemical taxonomy of the
Rutales, Academic Press, London, pp. 353-375.
Teichman I von. 1987. Development and
structure of the pericarp of Lannea discolor (Sonder) Engl. (Anacardiaceae). – Bot. J. Linn.
Soc. 95: 125-135.
Teichman I von. 1988. Notes on the ontogeny
and structure of the seed-coat of Sclerocarya birrea (Richard) Hochst.
subsp. caffra Kokwaro (Anacardiaceae). – Bot. J. Linn
Soc. 98: 153-158.
Teichman I von. 1990. Pericarp and seed coat
structure in Tapirira guianensis (Spondiadeae: Anacardiaceae). – South Afr. J.
Bot. 56: 435-439.
Teichman I von. 1991a. Ontogeny of the
seed-coat of Rhus lancea L. fil., and pachychalazy in the Anacardiaceae. – Bot. J. Linn.
Soc. 107: 35-47.
Teichman I von. 1991b. Pericarp structure in
Protorhus longifolia (Bernh.) Engl. (Anacardiaceae) and its taxonomic
significance. – Bot. Bull. Acad. Sin. 32: 121-128.
Teichman I von. 1994. Generic position of
Protorhus namaquensis Sprague (Anacardiaceae): evidence from seed
structure. – Bot. Bull. Acad. Sin. 35: 53-60.
Teichman I von. 1998. Micromorphological
structure of the fruit and seed of Smodingium argutum (Anacardiaceae), as an adaptation to
its natural habitat. – South Afr. J. Bot. 64: 121-127.
Teichman I von, Hardy DS. 1992. Flower and
fruit structure of Operculicarya decaryi H. Perrier (Anacardiaceae) from Madagascar. –
Bot. Bull. Acad. Sin., n.s., 33: 225-232.
Teichman I von, Robbertse L. 1986.
Development and structure of the drupe in Sclerocarya birrea (Richard)
Hochst. subsp. caffra Kokwaro (Anacardiaceae), with special
reference to the pericarp and the operculum. – Bot. J. Linn. Soc. 92:
303-322.
Teichman I von, Wyk AE van. 1988. The
ontogeny and structure of the pericarp and seed (coat) of Harpephyllum
caffrum Bernh. ex Krauss (Anacardiaceae). – Bot. J. Linn.
Soc. 98: 159-176.
Teichman I von, Wyk AE van. 1994. The generic
position of Protorhus namaquensis Sprague (Anacardiaceae): evidence from fruit
structure. – Ann. Bot., N. S., 73: 175-184.
Teichman I von, Wyk AE van. 1996. Taxonomic
significance of pericarp and seed structure in Heeria argentea
(Thunb.) Meisn. (Anacardiaceae),
including reference to pachychalazy and recalcitrance. – Bot. J. Linn. Soc.
122: 335-352.
Telford I, Copeland L. 2006. Philotheca
papillata (Rutaceae), a new
endangered species from north-eastern New South Wales. – Telopea 11:
105-109.
Temirbayeva K, Zhang M-L. 2015. Molecular
phylogenetic and biogeographical analysis of Nitraria based on nuclear
and chloroplast DNA sequences. – Plant Syst. Evol. 301: 1897-1906.
Teodoridis V, Kvaček Z. 2005. The extinct
genus Chaneya Wang et Manchester in the Tertiary of Europe: a revision
of Porana-like fruit remains from Öhningen and Bohemia. – Rev.
Palaeobot. Palynol. 134: 85-103.
Terrazas T. 1994. Wood anatomy of the Anacardiaceae: ecological and
phylogenetic interpretations. – Ph.D. diss., University of North Carolina,
Chapel Hill, North Carolina.
Terrazas T. 1995. Anatomia sistematica de la
familia Anacardiaceae en Mexico
I. La corteza de Tapirira Aublet. – Bot. Soc. Bot. Mexico 57:
103-112.
Thiv M, Van Dert Niet T, Rutschmann F, Thulin
M, Brtune T, Linder HP. 2011. Old-New World and trans-African disjunctions of
Thamnosma (Rutaceae):
intercontinental long-distance dispersal and local differentiation in the
succulent biome. – Amer. J. Bot. 98: 76-87.
Thomas DW, Harris DJ. 1999. New Sapindaceae from Cameroon and Nigeria.
– Kew Bull. 54: 951-957.
Thomas WW. 1985. The Simaba
guianensis complex in northern South America. – Acta Amazonica
15(suppl.): 71-79.
Thomas WW. 1990. The American genera of Simaroubaceae and their
distribution. – Acta Bot. Brasil. 4: 11-18.
Thulin M. 1999. Burseraceae. – In: Thulin M (ed),
Flora of Somalia, Royal Botanic Gardens, Kew, pp. 183-228.
Thulin M. 2001. Two new species of
frankincense trees (Boswellia, Burseraceae) from Socotra. – Kew
Bull. 56: 983-988.
Thulin M. 2004. A new genus of Sapindaceae from Somalia. – Nord. J.
Bot. 24: 509-511.
Thulin M, Warfa AM. 1987. The frankincense
trees (Boswellia spp., Burseraceae) of northern Somalia and
southern Arabia. – Kew Bull. 42: 487-500.
Thulin M, Beier B-A, Razafimandimbison SG,
Banks HI. 2008. Ambilobea, a new genus from Madagascar, the position
of Aucoumea, and comments on the tribal classification of the
frankincense and myrrh family (Burseraceae). – Nord. J. Bot. 26:
218-229.
Tieghem P van. 1898. Sur les Cnéoracées.
– Bull. Mus. Natl. Hist. Nat. Paris 4: 241-244.
Tieghem P van, Lecomte H. 1886. Structure et
affinité du Leitneria. – Bull. Soc. Bot. France 33: 181-184.
Tiffney BH. 1980. Fruits and seeds of the
Brandon Lignite V. Rutaceae. – J.
Arnold Arbor. 61: 1-40.
Tiffney BH. 1981. Euodia costata,
new combination (Rutaceae) from the
Eocene of southern England. – Paläontol. Zeitschr. 55: 185-190.
Tilak VD, Nene PM. 1978. Floral anatomy of
the Rutaceae. – Indian J. Bot. 1:
83-90.
Tilson AH, Bamford R. 1938. The floral
anatomy of the Aurantioideae. – Amer. J. Bot. 25: 780-793.
Tobe H. 2011. Embryological evidence supports
the transfer of Leitneria floribunda to the family Simaroubaceae. – Ann. Missouri
Bot. Gard. 98: 277-293.
Toledo CA. 1982. El género Bursera
(Burseraceae) en el estado de
Guerrero. – Ph.D. diss., Facultad de Ciencias, Universidad Nacional Autónoma
de México.
Tölke ED, Bachelier JB, Lima EA, Galetto L,
Demarco D, Carmello-Guerreiro SM. 2018. Diversity of floral nectary secretions
and structure, and implications for their evolution in Anacardiaceae. – Bot. J. Linn.
Soc. 187: 209-231.
Townsend CC. 1986. Taxonomic revision of the
genus Haplophyllum (Rutaceae). – Hooker’s Icones
Plantarum XL, I-III, Bentham-Moxon Trustees, Kew.
Traveset A. 1995. Reproductive ecology of
Cneorum tricoccon L. (Cneoraceae) in the Balearic Islands. – Bot. J.
Linn. Soc. 117: 221-232.
Trelease W. 1895. Leitneria
floridana. – Missouri Bot. Gard. Sixth Report, pp. 65-90.
Trinder-Smith TH, Linder HP, Niet T van der,
Verboom GA, Nowell TL. 2007. Plastid DNA sequences reveal generic paraphyly
within Diosmeae (Rutoideae, Rutaceae).
– Syst. Bot. 32: 847-855.
Trinder-Smith TH, Linder HP, Niet T van der,
Verboom GA, Nowell TL. 2008. The Cape’s orange genes: are they well stitched?
(Paraphyly of the Diosmeae, Rutiaceae). – South Afr. J. Bot. 74: 379.
Turland N, Xia N. 2005. A new combination in
Chinese Aesculus (Hippocastanaceae). – Novon 15: 488-489.
Turner GW, Berry AM, Gifford EM. 1998.
Schizogenous secretory cavities of Citrus limon (L.) Burm. f., and a
review of the lysigenous gland concept. – Intern. J. Plant Sci. 159:
75-88.
Turner H. 1995. Cladistic and biogeographic
analyses of Arytera Blume and Mischarytera gen. nov. (Sapindaceae), with notes on
methodology and a full taxonomic revision. – Blumea Suppl. 9: 1-230.
Turner H. 1996. Sapindaceae and the biogeography of
eastern Australia. – Aust. Syst. Bot. 9: 127-132.
Turner H, Ham RWJM van der. 1996. A taxonomic
and pollen morphological revision of the genus Gongrodiscus (Sapindaceae). – Bull. Mus. Natl.
Hist. Nat. Preis, sér. IV, 18: 339-349.
Turner SR, Cook A, Baskin JM, Baskin CC,
Tuckett RE, Steadman KJ, Dixon KW. 2009. Identification and characterization of
the water gap in the physically dormant seeds of Dodonaea petiolaris:
a first report for Sapindaceae. –
Ann. Bot. 104: 833-844.
Tutel B. 1984. Comparison on the taxonomy and
leaf anatomy of the genus Biebersteinia with the other genera of Geraniaceae in Turkey. –
Istanbul Univ. Fen Fak. Mecm., B, 47-48: 51-87.
Tyman JH, Morris LJ. 1967. The composition of
cashew nut-shell liquid (CNSL) and the detection of a novel phenolic
ingredient. – J. Chromatogr. 27: 287-288.
Tzakou O, Yannitsaros A, Vassiliades DD.
2001. Investigation of the C16:3/C18:3 fatty acid balance in leaf tissues of
Biebersteinia orphanidis Boiss. (Biebersteiniaceae). – Biochem.
Syst. Ecol. 29: 765-767.
Umadevi I, Daniel M. 1991. Chemosystematics
of the Sapindaceae. – Feddes
Repert. 102: 607-612.
Umadevi I, Daniel M, Sabnis SD. 1986.
Interrelationships among the families Aceraceae, Hippocastanaceae, Melianthaceae, Staphyleaceae. –
J. Plant Anat. Morph. 3: 169-172.
Umadevi I, Daniel M, Sabnis SD. 1990.
Chemotaxonomy of some Rutaceae. –
Indian J. Bot. 13: 23-28.
Urdampilleta JD, Ferrucci MS, Vanzela ALL.
2005. Karyotype differentiation between Koelreuteria bipinnata and
K. elegans ssp. formosana (Sapindaceae) based on chromosome
banding patterns. – Bot. J. Linn. Soc. 149: 451-455.
Urdampilleta JD, Ferrucci MS, Torezan JMD,
Vanzela ALL. 2006. Karyotype relationships among four South American species of
Urvillea (Sapindaceae:
Paullinieae). – Plant Syst. Evol. 258: 85-95.
Urdampilleta JD, Ferrucci MS, Vanzela ALL.
2007. Cytogenetic studies of four South American species of Paullinia
L. (Sapindaceae). – Bot. J. Linn.
Soc. 154: 313-320.
Urdampilleta JD, Ferrucci MS, Forni-Martins
ER. 2008. Chromosome studies of some Thinouia species (Sapindaceae) and the taxonomic
implications. – Ann. Bot. Fenn. 45: 68-73.
Urdampilleta JD, Coulleri JP, Ferrucci MS,
Forni-Martins ER. 2013. Karyotype evolution and phylogenetic analyses in the
genus Cardiospermum L. (Paullinieae, Sapindaceae). – Plant Biol. 15:
868-881.
Urdampilleta JD, Coulleri JP, Ferrucci MS.
2016. Insights into the Andean genera Bridgesia and Guindilia
(Sapindaceae): an integrated
approach. – Syst. Biodiv. 14: 583-598.
Valladares GR, Ferreyra D, Defago MT,
Carpinella MC, Palacios S. 1999. Effects of Melia azedarach on
Triatoma infestans. – Fitoterapia 70: 421-424.
Vassiliades D, Yannitsaros A. 2000.
Orphanides’s best discovery. – Bot. Chron. 13: 241-248.
Vassilyev AE. 2000. Quantitative
ultrastructure data of secretory duct epithelial cells in Rhus
toxicodendron. – Intern. J. Plant Sci. 161: 615-630.
Veken P van der. 1960. Nothospondias
Engl. Simaroubaceae africaine
meconnue. – Bull. Jard. Bot. État Bruxelles 30: 105-109.
Venkaiah K. 1992. Development, ultrastructure
and secretion of gum ducts in Lannea coromandelica (Houtt.) Merrill
(Anacardiaceae). – Ann. Bot.
69: 449-457.
Venning FD. 1948. The ontogeny of the
laticiferous canals in the Anacardiaceae. – Amer. J. Bot. 35:
637-644.
Verdcourt B. 1997. Proposal to conserve the
gender of Sapindus (Sapindaceae) as masculine. – Taxon
46: 360.
Verdcourt B, Davies FG. 1996. Ptaeroxylaceae.
– In: Polhill RM (ed), Flora of tropical East Africa, A. A. Balkema,
Rotterdam, The Netherlands, pp. 1-7.
Verdoorn IC. 1926. Revision of the African
Toddalieae. – Kew Bull. 1926: 389-416.
Verdu M, Climent J. 2007. Evolutionary
correlations of polycyclic shoot growth in Acer (Sapindaceae). – Amer. J. Bot. 94:
1316-1320.
Victor J, Wyk A van. 1999a. Pollen morphology
of Adenandra (Rutaceae:
Diosminae) and its taxonomic implications. – Grana 38: 1-11.
Victor J, Wyk A van. 1999b. Pollen morphology
of Diosma and Coleonema (Rutaceae: Diosminae) and its taxonomic
implications. – Grana 38: 12-19.
Victor J, Wyk A van. 2000. Pollen morphology
of Phyllosma and Sheilanthera (Diosminae: Rutaceae) and its taxonomic implications.
– Grana 39: 103-107.
Victor J, Wyk A van. 2001. Pollen morphology
of Euchaetis and Macrostylis (Diosminae-Rutaceae) and its taxonomic implications.
– Grana 40: 105-110.
Vieira PC, Lázaro AR, Fernandes JB, Da Silva
FGF. 1988. The chemosystematics of Dictyoloma. – Biochem. Syst.
Ecol. 16: 51-544.
Vieira PC, Lázaro AR, Fernandes JB, Da Silva
FGF. 1990. Limonoids, alkaloids and chromosomes from Dictyoloma
vandellianum, and their chemosystematic significance. – Quimica Nova 13:
287-233.
Vollesen K. 1984. Notes on Ethiopian Vitaceae and Burseraceae. – Nord. J. Bot. 4:
33-37.
Vollesen K. 1985. Studies in Burseraceae of northeastern Africa.
– Kew Bull. 40: 39-76.
Vollesen K. 1986. Commiphora, some
thoughts on the classification of an “impossible” genus. – In: Hedberg I
(ed), Research on the Ethiopian flora. Proceedings of the first Ethiopian flora
symposium held in Uppsala May 22-26, 1984, Almqvist & Wiksell
International, Uppsala, pp. 204-212.
Waffo AFK, Coombes PH, Crouch NR, Mulholland
DA, El Amin SMM, Smith PJ. 2007. Acridone and furoquinoline alkaloids from
Teclea gerrardii (Rutaceae:
Toddalioideae) of southern Africa. – Phytochemistry 68: 663-667.
Walsh N. 2004. A new species of
Leionema (Rutaceae) from
south-eastern New South Wales. – Telopea 10: 805-810.
Walt JJA van der. 1975. The fruit of
Commiphora. – Boissiera 24a: 325-330.
Walt JJA van der, Schijff HP van der. 1969.
The anatomy of the petiole as an aid to the identification of South African
Commiphora species. – Kirkia 9: 95-107.
Wang J, Zheng Y, Efferth T, Wang R, Shen Y,
Hao X. 2005. Indole and carbazole alkaloids from Glycosmis montana
with weak anti-HIV and cytotoxic activities. – Phytochemistry 66: 697-701.
Wang Q, Manchester SR, Gregor H-J, Shen S, Li
Z-Y. 2013. Fruits of Koelreuteria (Sapindaceae) from the Cenozoic
throughout the northern hemisphere: their ecological, evolutionary, and
biogeographic implications. – Amer. J. Bot. 100: 422-449.
Wang Y-F, Manchester SR. 2000.
Chaneya, a new genus of winged fruit from the Tertiary of North
America and eastern Asia. – Intern. J. Plant Sci. 161: 167-178.
Wannan BS. 1986. Systematics of the Anacardiaceae and its allies. –
Ph.D. diss., University of New South Wales, Sydney.
Wannan BS. 2006. Analysis of generic
relationships in Anacardiaceae.
– Blumea 51: 165-195.
Wannan BS, Quinn CJ. 1988. Biflavonoids in
the Julianiaceae. – Phytochemistry 27: 3161-3162.
Wannan BS, Quinn CJ. 1990. Pericarp structure
and generic affinities in the Anacardiaceae. – Bot. J. Linn.
Soc. 102: 225-252.
Wannan BS, Quinn CJ. 1991. Floral structure
and evolution in the Anacardiaceae. – Bot. J. Linn.
Soc. 107: 349-385.
Wannan BS, Quinn C. 1992. Inflorescence
structure and affinities of Laurophyllus (Anacardiaceae). – Bot. J. Linn.
Soc. 109: 235-245.
Wannan BS, Waterhouse JT, Gadek PA, Quinn CJ.
1985. Biflavonyls and the affinities of Blepharocarya. – Biochem.
Syst. Ecol. 13: 105-108.
Wannan BS, Waterhouse JT, Quinn CJ. 1987. A
taxonomic reassessment of Blepharocarya F. Muell. – Bot. J. Linn.
Soc. 95: 61-72.
Waterman PG. 1974. A review of the
chemosystematics of the genus Zanthoxylum (Rutaceae). – In: Stone BC (ed), The
role and goals of tropical botanic gardens, Kuala Lumpur, pp. 109-130.
Waterman PG. 1975. Alkaloids of the Rutaceae: their distribution and
systematic significance. – Biochem. Syst. Ecol. 3: 149-180.
Waterman PG. 1983. Phylogenetic implications
of the distribution of secondary metabolites within the Rutales. – In:
Waterman PG, Grundon MF (eds), Chemistry and chemical taxonomy of the Rutales,
Academic Press, London, pp. 377-400.
Waterman PG. 1990. Chemosystematics of the Rutaceae: comments on the interpretation
of Da Silva et al. – Plant Syst. Evol. 173: 39-48.
Waterman PG. 1993. Phytochemical diversity in
the order Rutales. – In: Downum KR, Romeo JT, Stafford HA (eds),
Phytochemical potential of tropical plants, Plenum Press, New York, pp.
203-233.
Waterman PG, Grundon MF (eds). 1983.
Chemistry and chemical taxonomy of the Rutales. – Academic Press, London, New
York.
Waterman PG, Khalid SA. 1981. The biochemical
systematics of Fagaropsis angolensis and its significance in the
Rutales. – Biochem. Syst. Ecol. 9: 45-51.
Webber IE. 1936. Systematic anatomy of the
woods of the Simarubaceae. – Amer. J. Bot. 23: 577-587.
Webber IE. 1941. Systematic anatomy of the
woods of the “Burseraceae”. –
Lilloa 6: 441-465.
Weberling F. 1976. Die Pseudostipeln der Sapindaceae. – Abhandl. Akad. Wiss.
Math.-Naturwiss. Kl. 2, 1976: 1-27.
Weberling F, Leenhouts PW. 1965.
Systematische-morphologische Studien an Terebinthales-Familien (Burseraceae, Simaroubaceae, Meliaceae, Anacardiaceae, Sapindaceae). – Abhandl. Akad. Wiss.
Lit. Mainz, Math.-Naturwiss. Kl., 10: 495-584.
Webster IE. 1936. Systematic anatomy of the
woods of the Simaroubaceae. –
Amer. J. Bot. 23: 577-587.
Weckerle CS, Reynel C. 2003. An overview of
the subspecies of Paullinia obovata (Sapindaceae-Paullinieae) in Peru. –
Novon 13: 145-152.
Weckerle CS, Rutishauser R. 2004. Comparative
morphology and systematic position of Averrhoidium within Sapindaceae. – Intern. J. Plant Sci.
164: 775-792.
Weckerle CS, Rutishauser R. 2005. Gynoecium,
fruit and seed structure of Paullinieae (Sapindaceae). – Bot. J. Linn.
Soc.147: 159-189.
Weckerle CS, Stutz MA, Baumann TW. 2003.
Purine alkaloids in Paullinia. – Phytochemistry 64: 735-742.
Weekley CW, Rutishauser R. 2005. Gynoecium,
fruit and seed structure of Paullinieae (Sapindaceae). – Bot. J. Linn. Soc.
147: 159-189.
Weeks A. 2003. The molecular systematics and
biogeography of the Burseraceae.
– Ph.D. diss., University of Texas, Austin, Texas.
Weeks A. 2009. Evolution of the pili nut
genus Canarium L. (Burseraceae) and its cultivated
species. – Gen. Res. Crop Evol. 56: 765-781.
Weeks A, Simpson BB. 2004. Molecular genetic
evidence for interspecific hybridization among endemic Hispaniolan
Bursera (Burseraceae). –
Amer. J. Bot. 91: 976-984.
Weeks A, Simpson BB. 2007. Molecular
phylogenetic analysis of Commiphora (Burseraceae) yields insight on the
evolution and historical biogeography of an ‘impossible’ genus. – Mol.
Phylogen. Evol. 42: 62-79.
Weeks A, Daly DC, Simpson BB. 2005. The
phylogenetic history and biogeography of the frankincense and myrrh family (Burseraceae) based on nuclear and
chloroplast sequence data. – Mol. Phylogen. Evol. 35: 85-101.
Weeks A, Zapata F, Pell SK, Daly DC, Mitchell
J, Fine PVA. 2014. To move or evolve: contrasting patterns of intercontinental
connectivity and climatic niche evolution in “Terebinthaceae” (Anacardiaceae and Burseraceae). – Frontiers Genet. 5:
409.
Wei F, Ma L-Y, Jin W-T, Ma S-C, Han G-Z, Khan
IA, Lin R-C. 2004. Antiinflammatoy triterpenoid saponins from the seeds of
Aesculus chinensis. – Chem. Pharm. Bull. 52: 12452: 1246-1248.
Wei L, Wang Y-Z, Li Z-Y. 2012. Floral
ontogeny of Ruteae (Rutaceae) and its
systematic implications. – Plant Biol. 14: 190-197.
Wei L, Xiang XG, Wang Y-Z, Li Z-Y. 2015.
Phylogenetic relationships and evolution of the androecia in Ruteae (Rutaceae). – PLoS ONE 10: e0137190.
doi:10.1371/journal.pone.0137190
Welzen PC van. 1990. Guioa Cav. (Sapindaceae): taxonomy, phylogeny, and
historical biogeography. – Leiden Bot. Ser. 12: 1-315.
Welzen PC van. 1991. Gloeocarpus
Radlk. (Sapindaceae) revised. –
Blumea 35: 389-392.
Welzen PC van. 1998. Indian Sapindaceae: interesting topic for
research? – In: Mathew P, Sivadasan M (eds), Diversity and taxonomy of
tropical flowering plants, Mentor Books, Calicut, pp. 135-165.
Welzen PC van, Turner H. 2001. Vicariance and
dispersal in Malesian Sapindaceae:
general patterns. – In: Saw LG, Chua LSL, Khoo KC (eds), Taxonomy, the
cornerstone of biodiversity, Forest Research Institute Malaysia, Kuala Lumpur,
pp. 233-251.
Welzen PC van, Piskaut P, Wandadri FI. 1992.
Lepidopetalum Blume (Sapindaceae): taxonomy, phylogeny, and
historical biogeography. – Blumea 36: 439-465.
Wendt T, Lott EJ. 1985. A new simple-leaved
species of Recchia (Simaroubaceae) from southeastern
Mexico. – Brittonia 37: 219-225.
West JG. 1980. A taxonomic revision of
Dodonaea (Sapindaceae) in
Australia. – Ph.D. diss, University of Adelaide, Australia.
West JG. 1984. A revision of
Dodonaea (Sapindaceae) in
Australia. – Brunonia 7: 1-194.
Weston PH. 1990. Notes on Boronia
(Rutaceae) in New South Wales,
including descriptions of three new species. – Telopea 4: 121-128.
Weston PH, Carolin R, Armstrong JA. 1984. A
cladistics analysis of Boronia Sm. and Boronella Baill. (Rutaceae). – Aust. J. Bot. 32:
187-203.
Whiffin T. 1982. Variation and evolution in
the genus Flindersia (Rutaceae) 1. Review of the genus. –
Aust. J. Bot. 30: 635-643.
White F. 1986. The taxonomy, chorology, and
reproductive biology of southern African Meliaceae and Ptaeroxylaceae. –
Bothalia 16: 143-168.
White F. 1990. Ptaeroxylon obliquum
(Ptaeroxylaceae), some other disjuncts, and the Quaternary history of African
vegetation. – Bull. Mus. Natl. Hist. Nat. Paris, sér. IV, 12: 139-185.
White F, Styles BT. 1963. 46. Meliaceae. – In: Exell AW, Fernandes
A, Wild H (eds), Flora Zambesiaca 2 (Part 1), Crown Agents for Oversea
Governments and Administrations, London, pp. 285-319.
White F, Styles BT. 1966. 58. Ptaeroxylaceae.
– In: Exell AW, Fernandes A, Wild H (eds), Flora Zambesiaca 2 (Part 2), Crown
Agents for Oversea Governments and Administrations, London, pp. 547-550.
White F, Styles BT. 1991. Meliaceae. – Flora of Tropical East
Africa, Balkema, Rotterdam.
Whitehouse C, Cheek M, Andrews S, Verdcourt
B. 2001. Tiliaceae & Muntingiaceae. – In:
Beentje HJ, Smith SAL (eds), Flora of tropical East Africa, A. A. Balkema,
Rotterdam, The Netherlands, pp. 1-120.
Wiens D, Barlow BA. 1971. The cytogeography
and relationships of the viscaceous and eremolepidaceous mistletoes. – Taxon
20: 313-332.
Wiger J. 1935. Embryological studies on the
families Buxaceae, Meliaceae, Simarubaceae, and Burseraceae. – Ph.D. diss.,
University of Lund, Sweden.
Wild H. 1959. A revised classification of the
genus Commiphora Jacq. – Bot. Soc. Broteriana 33: 67-95.
Wild H. 1963. 45. Burseraceae. – In: Exell AW,
Fernandes A, Wild H (eds), Flora Zambesiaca 2 (Part 1), Crown Agents for
Oversea Governments and Administrations, London, pp. 263-285.
Wild H, Phipps JB. 1963. 41. Simaroubaceae. – In: Exell AW,
Fernandes A, Wild H (eds), Flora Zambesiaca 2 (Part 1), Crown Agents for
Oversea Governments and Administrations, London, pp. 210-220.
Wilde JJFE de. 2007. Revision of the African
genus Heckeldora (Meliaceae). – Blumea 52: 179-199.
Wilkinson HP. 1971. Leaf anatomy of various
Anacardiaceae with special
reference to the epidermis. – Ph.D. diss., University of London, London.
Wilkinson HP. 1983. Leaf anatomy of
Gluta (L.) Ding Hou (Anacardiaceae). – Bot. J. Linn.
Soc. 86: 375-403.
Wilkinson HP. 1988. Sapindaceous pyritised
twigs from the Eocene of Sheppey, England. – Tertiary Res. 9: 81-86.
Williams I. 1984. Studies on the genera of
the Diosmeae (Rutaceae) 16. A key to
the genera of Diosmeae Benth. & Hook. (Rutaceae) and a description of a new
species of Agathosma (Rutaceae). – South Afr. J. Bot. 51:
149-151.
Wilson PG. 1970. A taxonomic revision of the
genera Crowea, Eriostemon and Phebalium (Rutaceae). – Nuytsia 1: 5-155.
Wilson PG. 1971. Taxonomic notes on the
family Rutaceae, principally of
Western Australia. – Nuytsia 1: 197-207.
Wilson PG. 1997. Brief notes on the genus
Crowea (Rutaceae). –
Nuytsia 11: 429-430.
Wilson PG. 1998a. Nomenclatural notes and new
taxa in the genera Asterolasia, Drummondita and
Microcybe (Rutaceae:
Boronieae). – Nuytsia 12: 83-88.
Wilson PG. 1998b. Notes on the genus
Correa (Rutaceaee). –
Nuytsia 12: 89-105.
Wilson PG. 1998c. New names and new taxa in
the genus Boronia (Rutaceae)
from Western Australia, with notes on seed characters. – Nuytsia 12:
119-154.
Wilson PG. 1998d. A taxonomic revision of the
genera Eriostemon and Philotheca (Rutaceae: Boronieae). – Nuytsia 12:
239-265.
Wilson PG. 1998e. New species and
nomenclatural changes in Phebalium and related genera (Rutaceae). – Nuytsia 12: 267-288.
Wilson PG, Armstrong JA, Griffin E. 1998.
Diplolaena (Rutaceae), new
taxa and nomenclatural notes. – Nuytsia 12: 107-118.
Wolfe JA, Tanai T. 1987. Systematics,
phylogeny, and distribution of Acer (maples) in the Cenozoic of
western North America. – J. Fac. Sci., Hokkaido Univ., Ser. IV, 22: 1-246.
Xi Y, Zhang J. 1991. Comparative studies of
pollen morphology between Nitraria and Meliaceae. – Bot. Res. (Inst. Bot.
Acad. Sinica) 5: 47-58. [In Chinese]
Xi Y, Zhou S. 1989. Pollen morphology and its
exine ultrastructure of the Zygophyllaceae in
China. – Bot. Res. (China) 4: 75-86. [In Chinese]
Xiang Q-Y, Crawford DJ, Wolfe AD, Tang Y-C,
dePamphilis CW. 1998. Origin and biogeography of Aesculus L.
(Hippocastanaceae): a molecular phylogenetic perspective. – Evolution 52:
988-997.
Xie L, Yang Z-Y, Wen J, Li D-Z, Yi T-S. 2014.
Biogeographic history of Pistacia (Anacardiaceae), emphasizing the
evolution of the Madrean-Tethyan and the eastern Asian-Tethyan disjunctions.
– Molec. Phylogen. Evol. 77: 136-146.
Yamamoto T, Vassiliades DD, Tobe H. 2014.
Embryology of Biebersteinia (Biebersteiniaceae, Sapindales): characteristics and
comparisons with related families. – J. Plant Res. 127: 599-615.
Yamamoto T, Fijridiyanto IA, Tobe H. 2016.
Embryology of Harrisonia (Cneoroideae, Rutaceae): a comparison with related
genera and families. – Bot. J. Linn. Soc. 180: 386-400.
Yan G, Shan F, Plummer JA. 2002. Genomic
relationships within Boronia (Rutaceae) as revealed by karyotype
analysis and RAPD molecular markers. – Plant Syst. Evol. 233: 147-161.
Yan H-X, Di Y-T, Fang X, Yang S-Y, He H-P, Li
S-L, Lu Y, Hao X-J. 2011. Chemical constituents from fruits of Harrisonia
perforata. – Phytochemistry 72: 508-513.
Yang DK, Qin YQ, Zhou JY, Li G, Zhu LH. 1996.
A study on chromosomes of Tribulus terrestris and Nitraria
sibirica. – Guihaia 16: 161-164. [In Chinese]
Yang J, Li S, Sun G, Yuan Y, Zhao G. 2008.
Population structure and genetic variation in the genus Dipteronia
Oliv. (Aceraceae) endemic to China as revealed by cpSSR analysis. – Plant
Syst. Evol. 272: 97-106.
Yang Y-Y, Meng Y, Wen J, Sun H, Nie Z-L.
2016. Phylogenetic analyses of Searsia (Anacardiaceae) from eastern Asia and
its biogeographic disjunction with its African relatives. – South Afr. J.
Bot. 106: 129-136.
Yanishevski DE. 1940. Tetradiclis
tenella (Ehrenb.) Litv. as the example of an ephemeral on the solonchaks
of the Mediterranean desert regions. – Trudy Bot. Inst. Akad. Nauk SSSR, ser.
IV, 4: 236-248. [In Russian with English summary]
Yannitsaros AG, Constantinidis TA,
Vassiliades DD. 1996. The rediscovery of Biebersteinia orphanidis
Boiss. (Geraniaceae) in
Greece. – Bot. J. Linn. Soc. 120: 239-242.
Ye X-L, Wang F-X, Qian N-F. 1992.
Embryological studies of Litchi chinensis. – Acta Bot. Yunnan. 14:
59-65. [In Chinese]
Yi T, Miller A, Wen J. 2004. Phylogenetic and
biogeographic diversification of Rhus (Anacardiaceae) in the Northern
Hemisphere. – Mol. Phylogen. Evol. 33: 861-879.
Yi T, Miller AJ, Wen J. 2007. Phylogeny of
Rhus (Anacardiaceae)
based on sequences of nuclear Nia-i3 intron and chloroplast
trnC-trnD. – Syst. Bot. 32: 379-391.
Yi T, Wen J, Golan-Goldhirsh A, Parfitt DE.
2008. Phylogenetics and reticulate evolution in Pistacia (Anacardiaceae). – Amer. J. Bot.
95: 241-251.
Yin B, Huo C, Shen L, Wang C, Zhao L, Wang Y,
Shi Q. 2009. Protolimonoids fro the seeds of Xylocarpus granatum. –
Biochem. Syst. Ecol. 37: 218-220.
Yonemori K, Honsho C, Kanzaki S, Eiadthong W,
Sugiura A. 2002. Phylogenetic relationships of Mangifera species
revealed by ITS sequences of nuclear ribosomal DNA and a possibility of their
hybrid origin. – Plant Syst. Evol. 231: 59-75.
Young DA. 1974. Comparative wood anatomy of
Malosma and related genera (Anacardiaceae). – Aliso 8:
133-146.
Young DA. 1976. Flavonoid chemistry and the
phylogenetic relationships of the Julianiaceae. – Syst. Bot. 1: 149-162.
Young DA. 1979. Heartwood flavonoids and the
infrageneric relationships of Rhus (Anacardiaceae). – Amer. J. Bot.
66: 502-510.
Yuh-Meei L, Fa-Ching C, Kuo-Hsiung L. 1989.
Hinokiflavone, a cytotoxic principle from Rhus succedanea and the
cytotoxicity of the related biflavonoids. – Planta Medica 55: 166-168.
Zakaria MB. 2001. The phytochemistry of Rutaceae species with special reference
to Melicope. – Malayan Nat. J. 55: 241-250.
Zavada MS, Dilcher DL. 1986. Comparative
pollen morphology and its relationship to phylogeny of pollen in the
Hamamelidae. – Ann. Missouri Bot. Gard. 73: 348-381.
Zavaleta-Mancera HA, Engleman EM. 1991.
Anatomía del fruto de Casimiroa edulis (Rutaceae), ’zapote blanco’, durante
su desarrollo. – Bol. Soc. Bot. México 51: 53-65.
Zhang X-F, Hu B-L, Zhou B-N. 1995. Studies on
the active constituents of Tibetan herb Biebersteinia heterostemon
Maxim. – Acta Pharmac. Sin. 30: 211-214.
Zhang Z, Li C, Li J. 2010. Conflicting
phylogenies of section Macrantha (Acer, Aceroideae, Sapindaceae) based on chloroplast and
nuclear DNA. – Syst. Bot. 35: 801-810.
Zhou Q, Wang Y, Jin X. 2002. Ontogeny of
floral organs and morphology of floral apex in Phellodendron amurense
(Rutaceae). – Aust. J. Bot. 50:
633-644.
Zhouy Q, Liu G. 2012. The embryology of
Xanthoceras and its phylogenetic implications. – Plant Syst. Evol.
298: 457-468.
Zini L, Solís S, Ferrucci M. 2014.
Anatomical and developmental studies on floral nectaries in
Cardiospermum species: an approach to the evolutionary trend in
Paullinieae. – Plant Syst. Evol. 300: 1515-1523.
Zohary M. 1952. A monographical study of the
genus Pistacia. – Palestine J. Bot. (Jerusalem Series) 5:
187-228.