CARYOPHYLLIDAE Takht.
Takhtajan, Sist Filog. Cvetk. Rast. [Syst.
Phylog. Magnolioph.]: 144. 4 Feb 1967
[Berberidopsidales+Caryophyllales]
CARYOPHYLLALES Juss. ex Bercht.
et J. Presl
Berchtold et Presl, Přir. Rostlin: 239. Jan-Apr
1820 [‘Caryophyllaceae’]
Caryophyllanae
Takht., Sist. Filog. Cvetk. Rast. [Syst. Phylog. Magnolioph.]: 144. 4 Feb
1967
Fossils There are relatively
few unambiguous fossils of Caryophyllales. Fossil
flowers (e.g. Caryophylloflora paleogenica) with pantoporate pollen
grains and campylotropous ovules, but with segmented ovary, have been found in
Late Cretaceous (Turonian) and Eocene layers.
Habit Usually bisexual
(sometimes monoecious, andromonoecious, gynomonoecious, polygamomonoecious,
dioecious, androdioecious, or gynodioecious), usually perennial, biennial or
annual herbs (sometimes evergreen or deciduous trees, shrubs, suffrutices or
lianas). Often leaf or stem succulents. Often halophytes. Often with spines.
C4 or CAM (crassulacean acid metabolism) physiologies often present
as well as Kranz’ anatomy. Often mucilaginous. Sometimes carnivorous.
Vegetative anatomy Mycorrhiza
absent in most groups (present in, i.a., Amaranthaceae and Nyctaginaceae). Root hair
cells arranged in vertical rows. Phellogen epidermal, subepidermal,
outer-cortical or pericyclic, or absent. Stem cortex often with two zones,
outer with thick-walled fibrous cells, inner with thin-walled cells. Secondary
lateral growth normal or anomalous (from concentric/successive cambia or from
inner whorl of vascular bundles), or absent. Vessel elements with simple
perforation plates; lateral pits usually alternate (sometimes opposite or
pseudoscalariform), usually simple (sometimes bordered) pits. Imperforate
tracheary xylem elements fibre tracheids or libriform fibres (sometimes
tracheids) with usually simple (sometimes bordered) pits, septate or
non-septate (sometimes also vasicentric tracheids). Vestured pits sometimes
present. Wood rays uniseriate or multiseriate, homocellular or heterocellular,
or absent. Axial parenchyma apotracheal, diffuse (sometimes
diffuse-in-aggregates), or paratracheal, scanty vasicentric, aliform,
winged-aliform, confluent, unilateral, or banded, or absent. Intraxylary phloem
rarely present. Sieve tube plastids S0, Ss, Pcs, P3c, P3cf, P3c’f,
P3f, P3c’’f, P3cfs, or P3c’’fs type. Nodes usually 1:1, 1:3 or 3:3,
unilacunar or with one or three leaf traces, or trilacunar with three traces
(sometimes 5–9:5–9, multilacunar with five to nine traces). Parenchyma
often with mucilaginous cells, often with sclereids. Wood with idioblasts
containing sphaerites. Tanniniferous cells sometimes present. Silica bodies
sometimes present in parenchyma cells. Calciumoxalate often present as druses,
sphaerites, styloids, raphides, prismatic or acicular crystals, or crystal
sand.
Trichomes Hairs unicellular or
multicellular, uniseriate or multiseriate, simple or branched (two-branched,
T-shaped, dendritic, candelabra-shaped, stellate, fasciculate, lepidote,
rosulate, barbed or vesicular), or absent; glandular hairs often frequent,
multicellular, stalked or sessile (occasionally peltate-lepidote; sometimes
secreting viscous mucilage).
Leaves Alternate (usually
spiral, rarely distichous) or opposite (rarely verticillate), simple, usually
entire (rarely lobed), often succulent, with conduplicate, supervolute, flat,
involute or circinate ptyxis (sometimes scale-like or absent). Stipules usually
absent (sometimes intrapetiolar); leaf sheath usually absent (leaf bases
sometimes sheathingly connate). Petiole vascular bundle transection arcuate or
annular, with peripheral ring of fibres. Venation pinnate or palmate,
brochidodromous or parallelodromous (sometimes indistinct; leaves sometimes
one-veined). Stomata usually paracytic, diacytic or anomocytic (sometimes
anisocytic, cyclocytic, tetracytic or actinocytic, rarely brachyparacytic).
Cuticular wax crystalloids as rodlets, threads or platelets, or absent. Leaf
margin usually entire (rarely dentate, sinuate, serrate or glandular-serrate).
Salt-secreting glands sometimes present.
Inflorescence Terminal or
axillary, cymose combinations of dichasia and cincinni, thyrsoid, fasciculate,
panicle, raceme-, spike- or head-like (flowers sometimes single). Floral
prophylls (bracteoles) sometimes numerous, sometimes absent.
Flowers Usually actinomorphic
(rarely zygomorphic). Hypanthium sometimes present. Usually hypogyny (rarely
half epigyny). Sepals (one to) five (to 23), usually with imbricate (sometimes
valvate, induplicate-valvate or open, rarely valvate-decussate, plicate or
descending-cochlear) aestivation, free or more or less connate. Petals alt.
petaloid staminodia (four or) five, with contorted or imbricate aestivation,
free, or absent. Nectaries/nectariferous disc present at staminal bases or in
tube formed by filament bases and petal bases or on inner side of receptacle,
or nectary and disc absent.
Androecium Stamens (one to)
five to more than 4.000 (staminal primordia usually five), in one or more
whorls or in several groups (outer stamens often initiated in pairs),
antetepalous when stamens isomerous relative to tepals; staminal development
usually centrifugal. Filaments free or more or less connate, sometimes adnate
to sepals or petals. Anthers basifixed or dorsifixed (sometimes latrorse),
versatile or non-versatile, usually tetrasporangiate (rarely disporangiate),
usually introrse (sometimes extrorse), usually longicidal (dehiscing by
longitudinal slits; rarely poricidal, dehiscing by apical pores or pore-like
slits); outer parietal cells developing directly into endothecium. Tapetum
usually secretory (rarely amoeboid-periplasmodial). Female flowers often with
staminodia (staminodia sometimes numerous in bisexual flowers). ‘Petaloids’
possibly in reality petaloid staminodia, developing simultaneously as or after
androecium (not prior to); petaloid staminodia (’petals’) and
’antepetalous’ stamens possibly forming a developmental unit.
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually tricolpate (rarely di- or
tricolporate, or di- or tricolporoidate) or tetra-, hexa- or polypantoporate
(sometimes tri- or hexarugate, rarely 4–12-colpate, spiraperturate,
triporate, etc.), shed as monads, usually tricellular rarely bicellular) at
dispersal. Exine tectate or semitectate (rarely intectate), with columellate
infratectum, perforate, microperforate, punctate, punctitegillate or
reticulate, scabrate, or spinulate.
Gynoecium Pistil composed of
(one or) two to five (to numerous) free or connate carpels; closure of carpels
sometimes delayed in at least Polygonaceae and in “the
betalain clade” (Caryophyllales s.str.).
Ovary superior, semi-inferior or inferior, unilocular to multilocular; ovary
sometimes with subepidermal cell layer containing large amounts of
calciumoxalate. Stylodia two to five, free or more or less connate, or style
single, simple; style sometimes unifacial. Stigmas two to five, or stigma one,
capitate or lobate, papillate, Dry type. Pistillodium usually absent (male
flowers often with pistillodium).
Ovules Placentation axile,
basal, basal-parietal, basal-lateral or free central (sometimes
parietal-laminar, rarely apical or basal-laminar). Ovules one to numerous per
carpel, campylotropous, hemianatropous or anacampylotropous (rarely anatropous,
circinotropous or amphitropous), ascending, erect, horizontal or pendulous,
apotropous or epitropous, usually bitegmic (rarely unitegmic), crassinucellar
(rarely tenuinucellar). Placental or funicular obturator sometimes present.
Micropyle usually endostomal (rarely bistomal). Funicle often with short hairs
directed against micropyle. Megasporangium usually thin. Archespore unicellular
to tricellular, only one developing further. Nucellar cap or nucellar beak
often present. Apical cells of megasporangium often radially elongate, forming
nucellar pad. Megagametophyte usually monosporous, Polygonum type
(rarely disporous or tetrasporous, Allium,
Adoxa,Endymion, Penaea, Drusa,
Fritillaria, Chrysanthemum, Plumbago, or
Plumbagella type). Synergids sometimes with a filiform apparatus.
Antipodal cells three, ephemeral, sometimes with early degenerating nuclei.
Chalazal caecum developed. Endosperm development ab initio usually nuclear
(sometimes cellular). Endosperm haustoria chalazal or absent. Embryogenesis
caryophyllad, chenopodiad or solanad (sometimes onagrad or asterad).
Fruit Usually a loculicidal
and/or septicidal (rarely denticidal, circumscissile or valvate) capsule
(sometimes a nut or an irregularly dehiscing capsule; rarely a berry, a
berry-like fruit, a drupe, a schizocarp or a syncarp), sometimes with
persistent calyx. Bracts and floral prophylls often partitioning in formation
of dispersal unit.
Seeds Aril usually absent.
Seed coat usually exotestal and/or endotegmic. Exotesta often tanniniferous;
outer exotestal wall sometimes with stalactite-like processes. Endotesta
sometimes thickened, often crystalliferous. Exotegmen? Endotegmen sometimes
with rod-shaped thickenings in cell walls, often tanniniferous. Perisperm
copious, starchy (with starch grains), surrounded by embryo, or not developed.
Endosperm copious, sparse or absent. Embryo usually lateral-peripheral, curved
around perisperm or straight (rarely cochleate or annular), with or without
chlorophyll. Cotyledons usually two (rarely one or three). Germination
phanerocotylar.
Cytology x = (4–)5–19
DNA Three large deletions
present in plastid ORF2280. Intron absent from plastid gene rpl2. The
plastid gene rpl23 is a pseudogene at least in the Caryophyllales analysed.
I copy of nuclear gene RPB2 lost. Mitochondrial intron
coxII.i3 sometimes lost.
Phytochemistry Flavonols
(kaempferol, quercetin, myricetin), flavonol sulphates,
flavone-C-glycosides (vitexin, isovitexin), flavones (e.g. luteolin),
isoflavones, glycoflavones, cyanidin, delphinidin, catechines, anthocyanins or
betalains (betacyanins, e.g. amaranthin, celosianin, betamin, phyllocactin, and
betaxanthins), oleanolic acid derivatives, sterols, methylated and
non-methylated ellagic acids, gallic acid, tannins, proanthocyanidins
(prodelphinidins), mesembrine, tyramine alkaloids, phenethylamines, peyote
alkaloids, indole alkaloids, tetrahydroisoquinoline alkaloids, acetogenic
benzylisoquinoline alkaloids, naphthyl-isoquinoline alkaloids (e.g.,
ancistrocline, dioncophylline, michellamines), cyanogenic compounds (e.g.
cyclopentenoid cyanogenic glycosides), betaine, triterpene saponins,
anthraquinones, acetophenones, naphthoquinones and naphthoquinone derivatives
(plumbagin, droserone, 5-O-methyl droserone, 7-methyljuglone,
hydroxyserone; toxic naphthoquinones and their derivatives act as antimicrobial
protectors and at least plumbagin as an insect ecdysis inhibitor e.g. in the
pitchers of Nepenthes), simmondsin, fagopyrine, protofagopyrine,
syringaresinol, oxalic acid, phytoferritin, phytoecdysones, cyclopeptides, and
pinitol present. Ferulic acid often present in non-lignified cell walls. Inulin
rarely present. Sulphated betalains present in the clade [Phytolaccaceae+ Petiveriaceae+Agdestis].
Benzylisoquinoline alkaloids and betalains both derived via shikimic acid
(especially phenylalanine) pathway.
Systematics Caryophyllales are
sister-group to Berberidopsidales.
Two strongly supported main clades can be
discerned. One large monophyletic group comprises “the carnivorous clade”
plus [[Tamaricaceae+Frankeniaceae]+[Polygonaceae+Plumbaginaceae]]. The
second main clade includes [Rhabdodendraceae+[Simmondsiaceae+[Asteropeiaceae+Physenaceae]]] plus the core
Caryophyllales in
the strict sense.
Potential synapomorphies of the first main
clade are according to Stevens (2001 onwards): presence of pit glands;
endosperm starchy; and presence of acetogenic naphthoquinones. The first of the
two subclades, “the carnivorous clade”, has the topology [[Droseraceae+Nepenthaceae]+[Drosophyllaceae+[Ancistrocladaceae+Dioncophyllaceae]]] and
is characterized by the following potential synapomorphies: presence of
vascularized multicellular glands; cymose inflorescence; corolla with contorted
aestivation; extrorse anthers; unilocular ovary; and presence of plumbagin
present. A clade identified in some analyses has the topology [Nepenthaceae+[Drosophyllaceae+[Ancistrocladaceae+Dioncophyllaceae]]] and
the synapomorphies: presence of fibriform vessel elements; wood rays one or two
cells wide; leaf vernation abaxially circinate; petiole vascular bundles
surrounded by massive sclerenchymatous cylinder with embedded bundles; presence
of wing bundles; and basifixed anthers. Ancistrocladaceae and
Dioncophyllaceae
share the following characters: climbing woody habit; phellogen deeply seated;
absence of cortical vascular bundles; petiole with inverted vascular bundles in
sclerenchyma cylinder; actinocyclocytic stomata; introrse anthers; and
acetogenic naphthyl isoquinoline alkaloids biosynthesized from polyketides (not
from aromatic amino acids).
The clade [[Frankeniaceae+Tamaricaceae]+[Plumbaginaceae+Polygonaceae]] is sometimes
discerned and has the potential synapomorphies (Stevens 2001 onwards): vessel
elements with minute lateral wall pits; outer and inner integuments two or
three cell layers thick; exotestal seed coat; and presence of sulphated
flavonols and ellagic acid. Frankeniaceae and Tamaricaceae share the
characters: wood storied; halophytic habit with small leaves covered by
salt-excreting glands; flowers small, tetra- to hexamerous; petals with basal
adaxial appendages; exine not spinulate; median carpel abaxial; placentation
usually parietal; loculicidal capsule; exotestal cells bulging or as hairs;
presence of endosperm; presence of bisulphated flavonols; and absence of
myricetin. Plumbaginaceae and Polygonaceae share the
synapomorphies: wood storied; absence of successive cambia; presence of
cortical and/or medullary vascular bundles; nodes 3:3; wide leaf bases; pollen
grains usually starchy; median carpel adaxial; ovary unilocular; placentation
basal; ovule one per carpel; fruit an anthocarp surrounded by accrescent calyx
forming part of dispersal unit; seed coat indistinguished except persistent
exotesta; loss of mitochondrial intron coxII.i3; and presence of
O-methylflavonols, myricetin and quinones.
The second main clade has the topology [Rhabdodendraceae+[Simmondsiaceae+[[Asteropeiaceae+Physenaceae]+[Macarthuriaceae+[Microteaceae+[[Caryophyllaceae+[Achatocarpaceae+Amaranthaceae]]+[Stegnospermataceae to
Cactaceae]]]]]]] and the
potential synapomorphies (Stevens 2001 onwards): filament much shorter than
anther; stylodia stigmatic (receptive) their entire length; ovules one or two
per carpel; fruit single-seeded; and endosperm sparse. This clade minus
Rhabdodendron is characterized by nodes 1:1 and absence of petals.
Asteropeia and Physena lack successive cambia; have a
vascular cylinder in the young stem; fibre tracheids; vasicentric tracheids;
wood rays one or two cells wide; aliform-confluent axial parenchyma; latrorse
anthers; and one-seeded fruit.
The core Caryophyllales – “the
betalain clade” – comprise [Macarthuriaceae+[Microteaceae+[[Caryophyllaceae+[Achatocarpaceae+Amaranthaceae]]+[Stegnospermataceae to
Cactaceae]]]]. Hence,
they include the caryophyllids in the traditional strict sense. It is a group
with very high bootstrap support and characterized by a large number of
potential synapomorphies (Stevens 2001 onwards): herbaceous habit; absence of
normal secondary lateral growth; CAM photosynthesis and C4
photosynthesis frequently present; sieve tube plastids P-type, with a central
angular protein crystal surrounded by a ring of protein filaments; absence of
pericyclic fibres; cymose inflorescence; presence of adaxial nectaries on
stamen bases; pollen grains tricellular at dispersal; exine with thin foot
layer; median carpel adaxial; ovary unilocular; stigmas papillate; placentation
free central or basal; ovules campylotropous; thickened exotestal and
endotegmic cells; endotegmic cells with bar-like thickenings; perisperm well
developed; absence of endosperm; starch grains clustered; embryo curved and
periferal; cotyledons incumbent; absence of mitochondrial gene rps10;
absence of plastid gene rpl2 intron; ferulic acid ester-linked to
unlignified primary cell walls; presence of flavonols and O-methylated
flavonols, quinones, usually betalains (chromoalkaloids) instead of
anthocyanins, triterpenoid saponins and phytoferritin; and absence of tannins
and myricetin. Usually phenylalanine-derived shikimic acid biosynthesis as
starting point for synthesis of benzylisoquinoline alkaloids and betalains. An
undifferentiated perianth evolved after the separation of the
Rhabdodendron lineage, and a differentiated perianth may have
originated at least nine times (Brockington & al. 2009):
Asteropeia, Caryophyllaceae,
Stegnosperma, species of Limeum, Corbichonia,
Mesembryanthemoideae and Ruschioideae in Aizoaceae, Mirabilis
in Nyctaginaceae,
Glinus in Molluginaceae,
Portulaca, Didiereaceae, Basellaceae, and Cactaceae.
The clade [Caryophyllaceae+[Achatocarpaceae+Amaranthaceae]] has the
potential synapomorphies: stamens as many as tepals, antetepalous; a single
ovule; parietal tissue approx. four cell layers thick; nucellar cap two to four
cell layers thick; especially outer exotestal cell walls thick, with
stalactite-like processes; mitochondrial genes rps1 and rps19
absent (lost); and often presence of phytoecdysteroids.
The lineages “above” Stegnosperma
are characterized by apotropous ovules. “The globular inclusion clade”
comprises Lophiocarpaceae to Cactaceae and posesses sieve
tube plastids with globular crystalloids. The clade comprising Sarcobataceae, Nyctaginaceae, Agdestidaceae, Phytolaccaceae and Petiveriaceae often forms
an unresolved polytomy (sometimes also including Gisekiaceae). It is
characterized by a single usually basal ovule per carpel; similarities in
ORF2280 sequence; and a 210 bp deletion in the plastid genome. They have
usually (Sarcobatus?) a subepidermal phellogen; also paracytic
stomata; and protein bodies in the nucleus. This clade minus Nyctaginaceae has racemose
inflorescence and a baccate fruit.
The second large clade of “the globular
inclusion lineage” comprises Molluginaceae to Cactaceae. The clade with the
topology (Brockington & al. 2013) [[[Montiaceae+Halophytaceae]+[Didieraceae+Basellaceae]]+[Talinaceae+[Portulacaceae+[Anacampserotaceae+Cactaceae]]]]
(Portulacineae, of Nyffeler & Eggli 2010; Cactineae of
Ocamp & Columbus 2010) has the following potential synapomorphies,
according to Stevens (2001 onwards): succulent leaves and/or stem; normal
secondary lateral growth; phloem parenchyma cells with phytoferritin; stem
epidermis with calciumoxalate crystals; presence of mucilage cells; leaves
amphistomatic; median inner pair of floral prophylls enclosing flower;
hypogyny; petaloid tepals; median tepal abaxial (opposite outer median floral
prophyll); pollen grains pantocolpate; absence of funicular obturator; and a
six bp deletion in plastid gene ndhF. The clade [Talinaceae+[Portulacaceae+[Anacampserotaceae+Cactaceae]]] is further
characterized by columellae narrowed towards middle or expanded towards base,
sometimes fused; pollen grains with granular internal surfaces; perforated foot
layer; and very thin non-apertural endexine (Nowicke 1996). Halophytaceae, Basellaceae and Didiereaceae sometimes form
a monophyletic group, with Halophytum sister to the remainder.
Potential synapomorphies are ovary with a single basal ovule; and a
single-seeded indehiscent fruit. Basellaceae may be
sister-group to Didiereaceae or even nested
within that clade (Didiereaceae and Basellaceae have often
paracytic stomata). In this case, Halophytum may be sister to Didiereaceae including
Basellaceae.
The monophyletic group [Talinaceae+[Portulacaceae+[Anacampserotaceae+Cactaceae]]] is supported by
the potential synapomorphies (Stevens 2001 onwards): presence of mucilaginous
cells; absence of pericyclic fibres; leaves with axillary uniseriate, biseriate
or multiseriate hairs, bristles or scales; stomata parallelocytic (stoma with a
lateral series of at least three alternating subsidiary cells increasing in
size away from guard cells; also present in Montiaceae); pericarp
two-layered; fruit covered by dry tepals; exocarp completely or almost
caducous. Portulacaceae and Cactaceae may be sister-groups,
according to Ocampo & Columbus (2010), using data mainly from non-coding
plastid DNA. Portulaca and at least Pereskia share, e.g., a
c. 500 bp deletion in rbcL, and non-lignified parenchyma cells are
sometimes present in the wood in Portulaca and Cactaceae. On the other hand,
Nyffeler & Eggli (2010), using sequence data from matK and
ndhF, identified Anacampserotaceae and
Cactaceae as
sister-groups (they share among morphological features the character of
numerous stamens). A special arrangement of testa cells along the dorsal
juncture, presence of a dry aril and a central field type of cuticular
ornamentation are characteristic features in several clades of Caryophyllales such as
Cactaceae and Portulacaceae (Barthlott
1984).
A characteristic feature of the “ACPT
clade” (Talinaceae,
Portulaca, Anacampserotaceae and
Cactaceae) are the
non-vascularized hair-, bristle- or scale-like trichomes present at the nodes
in the leaf axils (sometimes on internodes or on the lamina) (Ogburn &
Edwards 2009). They arise from the epidermis and are persistent. The hair-like
trichomes are either uniseriate or multiseriate (three or more cells in width).
The bristle-like trichomes are wide, flat and multiseriate (up to more than 20
cells in width). The species in Anacampseros sect. Avonia
possess wide scale-like trichomes which entirely surround the leaf distal to
the subtending leaf; these scales are apically lignified. Bristle-like
trichomes have been reported from species in Anacampserotaceae,
whereas hair-like trichomes are present in Portulaca, Anacampserotaceae and
Cactaceae. Membranous,
often paired, scale-like trichomes are present in Talinum (Talinaceae). They have often
been interpreted as homologous with the axillary trichomes, although they seem
to be apices of vascularized prophylls subsequently often developing into
leaves (Ogburn & Edwards 2009).
Problems concering homologies of the perianth
parts are notorious in Caryophyllales. A
uniseriate perianth is most probably plesiomorphic in Caryophyllales, and the
uniseriate floral organs may be homologous (Brockington & al. 2009). A
perianth may have originated by formation of homologous organs (perhaps in most
lineages of Caryophyllales), or by
differentiation of structures derived either from the androecium (in
Corbichonia in Lophiocarpaceae, in the
[Mesembryanthemoideae+Ruschioideae] clade of Aizoaceae, and in Molluginaceae) or from
bracts (in Nyctaginaceae and in the
“ACPT” clade), whereas ‘petaloid staminodia’ refer to floral parts that
are anambiguously derived from the androecium. The terms ‘sepaloid tepals’
and ‘petaloid tepals’ are applied to floral organs (with
quincuncial-imbricate aestivation) present in the core clade of Caryophyllales
(Brockington & al. 2009). ‘Petaloid tepals’ in Caryophyllaceae,
Stegnosperma, and Limeaceae have often been
interpreted as modified stamens (e.g. Ronse De Craene 2007, 2008, etc.).

|
Phylogeny of Caryophyllales
based on DNA sequence data (“core Caryophyllales”
– Caryophyllineae: Brockington & al. 2013; “basal
Caryophyllales”
– Polygonineae: Brockington & al. 2009).
‘Hypertelis’ on this tree (according to Brockington &
al. 2013) is now synonymous with Kewaceae, whereas the
monospecific Hypertelis s. str. (H. spergulacea) is
nested inside Molluginaceae>
(Christenhusz & al. 2014).
|
Heimerl in Engler et Prantl, Nat. Pflanzenfam.,
ed. 2, 16c: 174. Jan-Apr 1934, nom. cons.
Genera/species 2/c 11
Distribution California,
Texas, Mexico to Paraguay and Argentina.
Fossils Unknown.
Habit Dioecious, evergreen
trees or shrubs. Apices of short shoots often modified into spines.
Vegetative anatomy Phellogen
ab initio superficial. Phelloderm with thick-walled sclereid cells. Secondary
lateral growth normal (successive cambia absent). Vessel elements with simple
perforation plates; lateral pits opposite or alternate, simple pits.
Imperforate tracheary xylem elements libriform fibres with simple pits. Wood
rays uniseriate or multiseriate, homocellular or heterocellular. Axial
parenchyma (paratracheal) scanty vasicentric. Wood non-storied. Pericycle with
sclerenchyma and stone cells. Phloem fibres present (scattered in older
secondary phloem). Sieve tube plastids P3c’f type, with a single polygonal
central protein crystal and a subperipheral dense ring of protein fibrils.
Nodes? Tanniniferous cells often present. Calciumoxalate druses, sphaerites and
prismatic crystals present (raphides and styloids absent).
Trichomes Hairs absent on
older individuals; younger individuals with short hairs.
Leaves Alternate (spiral),
simple, entire, with ? ptyxis. Stipules and leaf sheath absent. Petiole
vascular bundles? Venation pinnate. Stomata anomocytic. Cuticular wax
crystalloids as lobed platelets (often arranged in groups). Leaf margin
entire.
Inflorescence Axillary,
raceme-like, fasciculate or panicle. Foliar prophylls (bracteoles) absent in
Achatocarpus.
Flowers Actinomorphic, small.
Hypogyny. Tepals five (Achatocarpus) or usually four (rarely five;
Phaulothamnus), with imbricate quincuncial (Achatocarpus) or
decussate (Phaulothamnus) aestivation, sepaloid, free. Nectary absent.
Disc absent.
Androecium Stamens ten to 20
(Achatocarpus) or twelve to 14 (Phaulothamnus). Filaments
thin, free or connate at base. Anthers basifixed, non-versatile,
tetrasporangiate, extrorse, longicidal (dehiscing by longitudinal slits).
Tapetum secretory? Staminodia absent.
Pollen grains
Microsporogenesis simultaneous? Pollen grains tetraporate to hexaporate or
apertures more or less irregular and often little delimited, shed as monads,
?-cellular at dispersal. Exine tectate, with columellate infratectum,
microperforate, scabrate (beset with microspinules) or coarsely granulate.
Gynoecium Pistil composed of
two usually free carpels (sometimes slightly connate at base), collateral or
superposed. Ovary superior, unilocular. Stylodia two, long, hairy and
papillate, free or connate at base. Stigmas two, acute, hairy and papillate,
type? Pistillodium absent.
Ovules Placentation basal.
Ovule usually one (rarely two) per ovary, campylotropous, ascending, bitegmic,
crassinucellar. Funicle present. Micropyle ?-stomal. Outer integument ? cell
layers thick. Inner integument ? cell layers thick. Parietal tissue?
Megagametophyte monosporous, Polygonum type? Endosperm development?
Endosperm haustoria? Embryogenesis?
Fruit A usually one-seeded
(occasionally two-seeded) berry.
Seeds Aril tiny (present at
hilum). Testa? Outer exotestal wall with stalactite-shaped outgrowths? Tegmen?
Perisperm copious, mealy. Endosperm rudimentary or absent. Embryo annular,
peripheral, enclosing perisperm, chlorophyll? Cotyledons two. Germination?
Cytology n = ?
DNA
Phytochemistry
Flavone-C-glycosides (vitexin, isovitexin) and tannins present.
Anthocyanin and betalains? Ferulic acid in non-lignified cell-walls? Ellagic
acid not found.
Use Medicinal plants.
Systematics
Achatocarpus (c 10; southern Mexico to Paraguay and Argentina),
Phaulothamnus (1; P. spinescens; California, Texas, northern
Mexico, Tres Marias Islands).
Nakai in J. Jap. Bot. 18: 104. 10 Mar 1942
Genera/species 1/1
Distribution Southern United
States to Nicaragua.
Fossils Unknown.
Habit Bisexual, evergreen,
more or less lignified liana. Roots often napiform.
Vegetative anatomy Phellogen
ab initio subepidermal. Secondary lateral growth anomalous (via
concentric/successive cambia). Vascular bundles present as concentric cylinders
in inner pericycle. Vessel elements with simple perforation plates; lateral
pits alternate, simple pits. Imperforate tracheary xylem elements libriform
fibres and tracheids with simple pits, septate or non-septate (also vasicentric
tracheids). Wood rays absent. Axial parenchyma apotracheal, diffuse, or
paratracheal. Wood non-storied. Tyloses sometimes present in vessels. Sieve
tube plastids P3cf type, with a central globular protein crystal surrounded by
a ring of protein filaments. Nodes? Parenchyma with idioblasts containing
calciumoxalate as coarse raphide-like crystals.
Trichomes Hairs?
Leaves Alternate (spiral),
simple, entire, with conduplicate ptyxis. Stipules and leaf sheath absent.
Petiole twisted at base. Petiole vascular bundles? Venation pinnate. Stomata
anomocytic. Cuticular wax crystalloids as rounded platelets. Idioblasts with
calciumoxalate as coarse raphide-like crystals. Leaf margin entire.
Inflorescence Axillary,
thyrsoid or panicle. Foliar prophylls (bracteoles) present.
Flowers Actinomorphic, small.
Half epigyny. Tepals usually four (in terminal flowers sometimes five), with
imbricate? aestivation, sepaloid, persistent, usually free (sometimes connate
at base). Nectariferous disc narrow.
Androecium Stamens (twelve to)
15 to c. 30, in alternitepalous fascicles. Filaments filiform, usually connate
at base (sometimes free), free from tepals, inserted at nectariferous disc.
Anthers dorsifixed, versatile?, tetrasporangiate, introrse, longicidal
(dehiscing by longitudinal slits). Tapetum secretory. Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains tricolpate, shed as monads,
tricellular at dispersal. Exine tectate, with columellate infratectum, punctate
or perforate, scabrate, spinulate or smooth.
Gynoecium Pistil composed of
(three or) four connate carpels. Ovary semi-inferior, ab initio (trilocular or)
quadrilocular, later usually unilocular by degeneration of remaining locules.
Style single, simple, short, cylindrical. Stigma (trilobate or) quadrilobate,
recurved, papillate on ventral side, type? Pistillodium absent.
Ovules Placentation basal to
axile. Ovule one per carpel, hemianatropous (or campylotropous?), bitegmic,
crassinucellar. Micropyle ?-stomal. Outer integument ? cell layers thick. Inner
integument ? cell layers thick. Parietal tissue approx. two cell layers thick.
Hypostase present. Nucellar cap massive. Megagametophyte monosporous,
Polygonum type. Endosperm development ab initio nuclear. Endosperm
haustoria? Embryogenesis?
Fruit A one-seeded unilocular
achene with wings formed by persistent and accrescent dry tepals. Pericarp
coriaceous, with reticulate pattern, adnate to seed coat.
Seeds Aril absent. Testa?
Tegmen? Perisperm probably copious and nutritious. Endosperm poorly developed
or absent. Embryo peripheral, curved around perisperm, well differentiated,
chlorophyll? Cotyledons two. Germination?
Cytology n = 9 – Cell nuclei
with protein bodies?
DNA 210 bp deletion present in
plastid DNA?
Phytochemistry Betacyanins and
betaxanthins present. Proanthocyanidins and alkaloids not found. Triterpenoid
saponins? Free oxalates accumulated?
Use Medicinal plant,
ornamental plant.
Systematics Agdestis
(1; A. clematidea; southern United States, Mexico, Central America
southwards to Nicaragua, probably introduced in the West Indies and Brazil).
Agdestis is sister to Sarcobatus,
according to Brockington & al. (2013).
Martinov, Tekhno-Bot. Slovar: 15. 3 Aug 1820
[’Aizoonides’], nom. cons.
Mesembryanthemaceae
Philib., Intr. Bot., ed. 2, 3: 268. 20 Dec 1801, [‘Mesembraceae’],
nom. rejic.; Galeniaceae Raf. in Amer. J. Sci. 1:
376. Mai-Dec 1819; Mesembryaceae Dumort., Anal. Fam.
Plant.: 37, 41. 1829 [‘Mesembryneae’];
Mesembryanthemales Link, Handbuch 2: 12. 4-11 Jul
1829 [‘Mesembrinae’]; Sesuviaceae
Horan., Prim. Lin. Syst. Nat.: 83. 2 Nov 1834 [‘Sesuviaceae
(Ficoideae)’]; Tetragoniaceae Lindl.,
Intr. Nat. Syst. Bot., ed. 2: 209. 13 Jun 1836, nom. cons.;
Aizoales Boerl., Handl. Fl. Nederl. Ind. 1: li. 2 Aug
1890; Aizoineae Doweld, Tent. Syst. Plant. Vasc.:
xli. 23 Dec 2001
Genera/species
124/1.675–1.695
Distribution Arid tropical and
subtropical regions including western and southern Australia, with their
highest diversity in southern and southwestern Africa.
Mesembryanthemoideae and, above all, Ruschioideae dominate
much of the succulent vegetation in the Karroo areas of South Africa, where
they constitute more than half the number of species and more than 90% of the
biomass.
Fossils Unknown.
Habit Usually bisexual (rarely
monoecious or dioecious), perennial or annual herbs (rarely climbing),
suffrutices or shrubs. Usually leaf succulents (sometimes stem succulents,
rarely root succulents; some species are almost entirely subterranean). Almost
all representatives are xerophytes; some species are halophytes. C4
or CAM (rarely C3) physiology present. Kranz’ anatomy present in
some species. Usually mucilaginous.
Vegetative anatomy Mycorrhiza
usually absent. Kranz’ anatomy present in some species. Phellogen ab initio
inner-cortical or endodermal, or absent. Primary medullary strands wide.
Medullary wide-band tracheid cells frequent, also present in foliar tissue
other than mid-vein; bands narrow yet very tall, cell lumen in places very
narrow. Endodermis significant. Secondary lateral growth normal or sometimes
anomalous (via concentric/successive cambia) or absent. Vessel elements usually
with simple perforation plates; lateral pits alternate? Imperforate tracheary
xylem elements libriform fibres with bordered pits? (in at least one species of
Ruschia also vasicentric tracheids). Wood rays usually absent (present
in Tetragonia). Axial parenchyma paratracheal? (in Tetragonia
also vasicentric). Wood elements often partially storied. Sieve element
plastids P3cf type, with a central globular protein crystal surrounded by
protein filaments. Nodes 1:1, unilacunar with one leaf trace, or 3:3,
trilacunar with three traces. Tanniniferous idioblasts often present in
water-storing tissue. Epidermis in many species with densely spaced
water-storing vesicular idioblasts with wide base. Calciumoxalate crystals
often present.
Trichomes Hairs usually
unicellular or multicellular and uniseriate (rarely stipitate, two-branched or
stellate), often vesicular, or absent.
Leaves Usually opposite (often
pairwise fused; sometimes alternate), simple, entire, often cuboidal, conical,
cylindrical or prismatic, with usually curved or flat ptyxis. Stipules usually
absent (petiole base in Sesuvioideae with stipule-like appendages);
leaf bases often membranous and sheathingly connate around stem (sometimes
entirely connate). Petiole vascular bundles forming cortical reticulum.
Venation pinnate or palmate, usually indistinct, with sunken main veins.
Stomata anomocytic, paracytic or anisocytic. Cuticular wax crystalloids as
rodlets (often terete), threads or platelets. Mesophyll sometimes with
idioblasts containing calciumoxalate raphides. Epidermis of upper side of
lamina often with numerous water-storing wide-based vesicular idioblasts. Outer
epidermal cell walls often with calciumoxalate crystals. Leaf margin entire,
dentate or serrate.
Inflorescence Terminal (often
seemingly axillary), cymose (sometimes capitate), or flowers usually solitary
terminal. Floral prophylls (bracteoles) usually large, often foliaceous.
Flowers Actinomorphic, often
large. Hypanthium usually present. Hypogyny, half epigyny or epigyny. Tepals
(three to) five (to eight), in one whorl, with usually imbricate quincuncial
(rarely valvate) aestivation, usually sepaloid (adaxial tepals sometimes
petaloid), persistent, connate at base, often with subapical abaxial appendage.
Nectary usually continuous, annular on adaxial side of perianth-stamen tube
(nectaries in Mesembryanthemoideae partially tubular). Disc present or
absent.
Androecium Stamens usually
numerous (to more than 2.000; sometimes four, five, eight, or ten), in one or
more whorls or in three to nine groups; staminal primordia five,
alternitepalous, or as annular meristem. Outer whorls usually consisting of
petaloid staminodia, inner whorls consisting of fertile stamens, median whorls
often intermediary. Filaments free or connate (all or in three to nine groups),
free from tepals. Anthers dorsifixed, often versatile, tetrasporangiate,
introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory, with
usually trinucleate (rarely septanucleate) cells. Staminodia petaloid,
extrastaminal, usually numerous (absent in some genera).
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually tricolpate (rarely
tetracolpate or tricolporoidate), shed as monads, tricellular at dispersal.
Exine tectate or semitectate, with columellate infratectum, perforate, punctate
or anulopunctate, spinulate (rarely reticulate or rugate).
Gynoecium Pistil composed of
(one or) two to five (to numerous) connate carpels. Ovary superior to inferior,
(unilocular or) bilocular to quinquelocular (to multilocular), usually entirely
septate (septa in Acrosanthes incomplete). Stylodia usually free
(rarely connate). Stigmatic areas adaxial, papillate, Dry or Wet type.
Pistillodium absent.
Ovules Placentation usually
axile (in early stage, primary; rarely basal or apical) to parietal (in later
stage, secondary). Ovules one to numerous per carpel (in Acrosanthes a
single basal ovule per carpel), campylotropous (hemianatropous?) to
anacampylotropous, bitegmic, crassinucellar. Micropyle endostomal. Outer
integument two or three cell layers thick. Inner integument two cell layers
thick. Obturator placental. Parietal tissue approx. three cell layer thick.
Nucellar cap micropylar. Apical cells of megasporangium often radially
elongate. Megagametophyte usually monosporous, Polygonum type (rarely
Endymion, Penaea, Drusa, or Adoxa type).
Endosperm development ab initio nuclear. Endosperm haustoria chalazal?
Embryogenesis caryophyllad or solanad.
Fruit Usually a loculicidal
capsule (sometimes a nut, rarely a pyxidium, a berry or a schizocarp; in
Gunniopsis a septicidal capsule), usually hydrochastic, dehiscing in
moist weather, when septa and other tissues swell (septal keels, reaching from
central axis to valve apices).
Seeds Aril sometimes present
(surrounding seed in Sesuvioideae). Exotesta palisade or tangentially
elongate. Endotesta and tegmen usually crushed. Perisperm copious, starchy.
Endosperm sparse or absent. Suspensor massive, usually biseriate to
multiseriate. Embryo peripheral, curved around perisperm, without chlorophyll.
Cotyledons two. Radicula dorsal. Germination phanerocotylar.
Cytology x = 8
(Sesuvioideae, Aizooideae); x = 9
(Mesembryanthemoideae, Ruschioideae) – Polyploidy
frequently occurring.
DNA Intron absent from plastid
gene rpoC1 (at least in Delospera and Faucaria).
Plastid gene infA transferred to nucleus (Mesembryanthemum;
pseudogene present in plastid genome).
Phytochemistry Flavonols
(kaempferol, quercetin), cyanidin, condensed tannins, betacyanins,
betaxanthins, mesembrine alkaloids (Phyllobolus etc.), and ferulic
acid (in non-lignified cell walls) present. C-glycosylflavonoids,
ellagic acid and saponins not found. Some species oxidize free oxalates.
Use Ornamental plants,
vegetables (Tetragonia tetragonioides), fruits (Carpobrotus
edulis), stabilization of soil (Carpobrotus etc.).
Systematics Aizoaceae are possibly sister
to a clade comprising Gisekia, Phytolaccaceae, Sarcobatus,
Agdestis, Nyctaginaceae, and Petiveriaceae.
There is strong bootstrap-support for the
following topology within Aizoaceae:
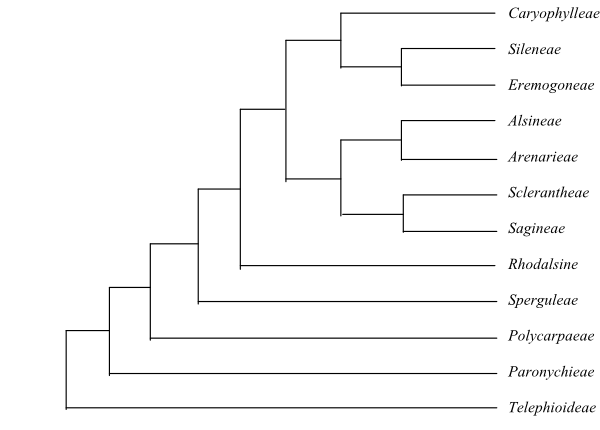
[Sesuvioideae+[Aizooideae+[Acrosanthoideae+[Mesembryanthemoideae+Ruschioideae]]]]
Sesuvioideae Lindl.,
Veg. Kingd.: 527. Jan-Mai 1846 [‘Sesuveae’]
6/63.
Anisostigmateae Klak in Taxon 66(5): 1167. 24 Oct
2017 Anisostigma (1; A. schenckii; Namibia),
Tribulocarpus (3; ‘T. dimorphanthus’ [non-monophyletic]:
Somalia, Namibia; T. retusus: Somalia);
Sesuvieae Fenzl in Ann. Wiener Mus. Naturgesch. 2:
289. 1839. Cypselea (3; C. humifusa, C. meziana,
C. rubriflora; southern Florida, the West Indies, Venezuela), Sesuvium (22;
tropical and subtropical coastal areas), Trianthema (27; Africa,
tropical and subtropical Asia, Australia, T. portulacastrum also in
northern South America), Zaleya (7; Z. camillei, Z.
decandra, Z. galericulata, Z. govindia, Z.
pentandra, Z. redimita, Z. sennii; northeastern and
eastern Africa, Madagascar, India, Sri Lanka, the Lesser Sunda Islands,
northern Australia). – Tropical and subtropical regions. C4
photosynthesis and Kranz’ anatomy present. Nodes 1:1 or 3:3. Stipules
petiolar. Inflorescence distinct from vegetative parts of plant. Prophylls
often prominent. Inflorescence bracteates, usually separated from vegetative
parts. Hypanthium often present. Nectary annular (holonectary). Stamens one to
five. Androecial primordia often antetepalous. Carpels (one or) two (to five),
alternitepalous. Ovules two to numerous per carpel. Fruit usually a pyxidium
(not a hygrochastic capsule). Seed glossy black, usually arillate.
Tribulocarpus, with a syncarpous fruit
fused with spiny bracts, and with non-arillate seeds, is sister to the
remaining Sesuvioideae.
[Aizooideae+[Acrosanthoideae+[Mesembryanthemoideae+Ruschioideae]]]
Inflorescence usually indistinct. Floral
prophylls foliaceous. Androecial primordia usually alternitepalous. Carpels
antetepalous. Fruit a hygrochastic capsule.
Aizooideae Spreng. ex
Arn., Botany: 112. 9 Mar 1832 [‘Aizoideae’]
5/c 100. Aizoanthemopsis (1;
A. hispanicum; the Mediterranean, northern Africa, the Middle East to
Iran), Gunniopsis (14; Australia except northern and eastern parts),
Tetragonia (c 50; southern Africa, Namibia, Morocco, Australia, New
Zealand, islands in the Pacific, temperate and subtropical South America, one
species, T. tetragonioides, worldwide), Aizoanthemum (4;
A. dinteri, A. galenioides, A. mossamedense, A.
rehmannii; southern Angola, northern Namibia), Aizoon (c 30;
southern Angola to South Africa, one species, A. canariense, in
Macaronesia, the Mediterranean, Zimbabwe, northern Kenya and Socotra to India).
– Drier parts of South Africa, South America, Australia
(Gunniopsis), a few species in North Africa, Southwest and East Asia.
Vesicular hairs with large vesicular terminal cell and multicellular stalk.
Accessory lateral branches often present. Inflorescence with leaves. Hypanthium
present. Hypogyny to epigyny. Nectary annular, continuous (holonectary),
present on apex of hypanthium. Stamens four to ten. Pistil composed of two to
ten connate carpels. Ovary superior to inferior. Ovule one per carpel, apical,
apotropous; or basal, several to numerous per carpel. Capsule usually a
loculicidal (in Gunniopsis a septicidal) capsule (in Tetragonia a
nutlet). Testal cell walls usually thickened (sometimes only little thickened).
Tetragonia
has a single pendulous ovule, megasporangium with druses, inner layer of the
inner integument well developed, and an indehiscent fruit. –
Aizoanthemopsis hispanicum is sister to the rest (Klak & al.
2017).
[Acrosanthoideae+[Mesembryanthemoideae+Ruschioideae]]
Leaves strongly succulent. Epigyny to
half epigyny. Nectary disrupted (meronectary). Stamens and petaloid staminodia
numerous. x = 9. – The androecial development is centrifugal. Basipetal
members become progressively more sterile and petaloid, and intermediates link
the outermost petals to the inner fertile stamens (Brockington & al. 2009).
Correspondingly, the outer pentamerous uniseriate perianth loses its petaloid
features and appears like a calyx.
Acrosanthoideae
Klak in Taxon 66(5): 1161. 24 Oct 2017
1/6. Ovules basal, shortly stipitate.
Capsule xerochastic, parchment-like. Acrosanthes (6; A.
anceps, A. angustifolia, A. humifusa, A.
microphylla, A. parviflora, A. teretifolia; Western
Cape). – Acrosanthes is sister-group to
[Mesembryanthemoideae+Ruschioideae], according to Klak &
al. (2017).
Mesembryanthemoideae
Burnett, Outlines Bot.: 736, 1092, 1131. Feb 1835
[‘Mesembryanthidae’]
6/c 95. Aspazoma (1; A.
amplectens; Namaqualand and Richtersveld in Northern and Western Cape),
Brownanthus (10; southern Angola, Namibia, Northern and Western Cape),
Caulipsolon (1; C. rapaceum; Namaqualand in Northern Cape),
Mesembryanthemum
(c 70; southern Angola, Namibia, western and central South Africa to Eastern
Cape), Psilocaulon
(13; southern Angola, western Namibia, western, southern and central South
Africa), Synaptophyllum (1; S. juttae; near Lüderitz in
southwestern Namibia). – Southern Africa. CAM photosynthesis present.
Cortical vascular bundles present. Stem sometimes with succulent persistent
green cortex. Wide-band tracheids absent. Stomata usually transversely
orientated. Inflorescence indistinct. Flowers tetra- or pentamerous. Half
epigyny. Tepals, petaloid staminodia and stamens often more or less united at
base into a tube. Nectaries usually hollow or koilomorphic (shell-shaped),
discontinuous (meronectary). Pistil composed of (three or) four or five (or
six) connate carpels. Placentation axile. Expanding fruit keels entirely
septal. Alkaloids usually present.
Ruschioideae Schwantes
in Ihlenfeldt, Schwantes et Straka in Taxon 11: 54. 28 Feb 1962
106/1.410–1.430.
Apatesieae Schwantes in H. D. Ihlenfeldt, G.
Schwantes et H. Straka in Taxon 11: 55. 28 Feb 1962. Apatesia (4;
A. helianthoides, A. mughani, A. pillansii, A.
sabulosa; Vanrhynsdorp to Cape Town in Western Cape), Carpanthea
(1; C. pomeridiana; southwesternmost Western Cape), Conicosia (3;
C. bijlii, C. elongata, C. pugioniformis; southern
Namibia, Northern and Western Cape), Hymenogyne (3; H.
conica, H. glabra, H. stephensiae; Cape Peninsula to
Clanwilliam in western Western Cape), Skiatophytum (1; S.
tripolium; southwestern Western Cape). –
Dorotheantheae Chesselet, G. F. Sm. et A. E. van Wyk
in Taxon 51: 306. 12 Jun 2002. Cleretum (14;
Northern and Western Cape). – Delospermeae
Chesselet, G. F. Sm. et A. E. van Wyk in Taxon 51: 306. 12 Jun 2002.
Dicrocaulon (7–9; Namaqualand in southern Northern Cape and northern
Western Cape), Diplosoma (2; D. luckhoffii, D.
retroversum; northwestern Western Cape), Jacobsenia (3; J.
halii, J. kolbei, J. vaginata; Vanrhynsdorp and
Vredendal in Northern and Western Cape), Meyerophytum
(1; M. meyeri; Richtersveld to southern Namaqualand in Northern Cape),
Mitrophyllum (7; M. abbreviatum, M. clivorum, M.
dissitum, M. grande, M. margaretae, M.
mitratum, M. roseum; Richtersveld in Northern Cape),
Monilaria (5; M. chrysoleuca, M. moniliformis,
M. obconica, M. pisiformis, M. scutata; Namaqualand
in Northern and Western Cape), Oophytum (2;
O. nanum, O. oviforme; Knersvlakte north of Vanrhynsdorp in
Western Cape), Disphyma (6;
D. australe, D. blackii, D. clavellatum, D.
crassifolium, D. dunsdonii, D. pupillatum; Western and
Eastern Cape, southern Australia, Tasmania, New Zealand), Glottiphyllum
(16; Western and Eastern Cape); Corpuscularia (2; C.
lehmannii, C. taylorii; Port Elizabeth to Grahamstown in Eastern
Cape), Delosperma (c
160; South Africa to eastern Africa and Arabian Peninsula, Madagascar,
Réunion), Drosanthemum
(c 110; southern Namibia, western, central and southern South Africa),
Knersia (1; K. diversifolia; western Western Cape), Malephora (c
17; southern Namibia, Northern, Western and Eastern Cape), Mestoklema
(7; M. albanicum, M. arboriforme, M. copiosum,
M. elatum, M. illepidum, M. macrorhizum, M.
tuberosum; Namibia, western parts of South Africa), Trichodiadema
(34; southern Namibia, western and southern parts of South Africa),’Lampranthus’
(c 95; southern Namibia, Northern, Western and Eastern Cape, KwaZulu-Natal;
polyphyletic), Oscularia (c
10; southern Northern Cape, Western Cape), Gibbaeum (c 18;
Little Karoo in Western Cape and southernmost Northern Cape), Muiria
(1; M. hortenseae; northern side of Langeberg in Western Cape);
Frithia (2; F. humilis, F. pulchra; northeastern
South Africa), Chasmatophyllum (6; C. braunsii, C.
maninum, C. musculinum, C. nelii, C. stanleyi,
C. willowmorense; Namibia, South Africa), Hammeria (3; H.
cedarbergensis, H. gracilis, H. meleagris; Tanqua Karoo
and Ceres Karoo in Northern and Western Cape), Rabiea (6; R.
albinota, R. albipuncta, R. comptonii, R.
difformis, R. jamesii, R. lesliei; eastern Northern
Cape, Eastern Cape, Free State), Rhinephyllum (c 10; Northern, Western
and Eastern Cape), Stomatium (40; western and southern parts of South
Africa), Mossia (1; M. intervallaris; Eastern Cape to eastern
Free State and northwestern Lesotho, Mpumalanga, Gauteng), Neohenricia
(2; N. sibbettii, N. spiculata; Northern Cape to Free State,
Eastern Cape), Faucaria (6–8; F. bosscheana, F.
britteniae, F. felina, F. gratiae, F. nemorosa,
F. subintegra, F. tigrina, F. tuberculosa; Eastern
Cape, eastern Western Cape), Orthopterum
(2; O. coeganum, O. waltoniae; Eastern Cape). –
Ruschieae Schwantes in H. D. Ihlenfeldt, G. Schwantes
et H. Straka in Taxon 11: 54. 28 Feb 1962. Aloinopsis (c 8; Western,
Eastern and Northern Cape), Deilanthe (3; D. hilmarii, D.
peersii, D. thudichumii; Western Cape and adjacent areas of
Northern and Eastern Cape, Free State), Ihlenfeldtia (2; I.
excavata, I. vanzyhlii; Northern Cape), Nananthus (5;
N. aloides, N. margaritiferus, N. pallens, N.
pole-evansii, N. vittatus; Namibia, Northern and Eastern Cape,
Free State, North-West, Gauteng), Titanopsis (3; T. calcarea,
T. hugo-schlechteri, T. schwantesii; southern Namibia,
central South Africa), Vanheerdia (2; V. primosii, V.
roodiae; Bushmanland in eastern Northern Cape), Didymaotus (1;
D. lapidiformis; Tanqua Karoo in Western Cape), Tanquana (3;
T. archeri, T. hilmarii, T. prismatica; Tanqua
Karoo, southwestern Great Karoo and Little Karoo to south of Laingsburg in
Western Cape), Dinteranthus (6; D. inexpectatus, D.
microspermus, D. pole-evansii, D. vallis-mariae, D.
vanzylii, D. wilmotianus; southeastern Namibia, northwestern
Northern Cape), Lapidaria (1; L. margaretae; southern
Namibia, northern Northern Cape), Lithops (36;
Namibia, South Africa, Botswana), Schwantesia (11; southern Namibia,
Northern Cape); Dracophilus (4; D. dealbatus, D.
delaetianus, D. montis-draconis, D. proximus; Lüderitz
in southwestern Namibia to northern Richtersveld in Northern Cape),
Hartmanthus (2; H. hallii, H. pergamentaceus;
Sperrgebiet in southern Namibia, northern Richtersveld in Northern Cape),
Jensenobotrya (1; J. lossowiana; Dolphin Head in Spencer Bay
in coastal Namibia), Juttadinteria (5; J. albata, J.
attenuata, J. ausensis, J. deserticola, J.
simpsonii; Lüderitz to Aus in Namibia, northern Richtersveld in Northern
Cape), Namibia (3; N. cinerea, N. pomonae, N.
ponderosa; around Lüderitz and east of Prince of Wales Bay in Namibia),
Nelia (4; N. meyeri, N. pillasii, N.
robusta, N. schlechteri; Richtersveld to Namaqualand in Northern
Cape), Psammophora (4; P. longifolia, P. modesta,
P. nissenii, P. saxicola; Lüderitz in western Namibia to
Richtersveld in Northern Cape), Ruschianthus (1; R. falcatus;
edge of the Namib desert in southern Namibia), Conophytum
(85–90; Northern and Western Cape to western Eastern Cape);
Bergeranthus (7–10; B. albomarginatus, B.
concavus, B. longisepalus, B. multiceps, B.
nanus, B. scapiger, B. vespertinus; Eastern Cape),
Machairophyllum (4; M. albidum, M. bijlii, M.
brevifolium, M. stayneri; Barrydale to Willowmore in Western
Cape, Zuurberg in Eastern Cape), Carruanthus
(2; C. peersii, C. ringens; around Willowmore in easternmost
Western Cape and westernmost Eastern Cape), Hereroa (c 30;
southern Namibia, South Africa), Bijlia (2; B. dilatata,
B. tugwelliae; near Prince Albert in Western Cape),
Cerochlamys (3; C. gemina, C. pachyphylla, C.
trigona; Little and Great Karoo in Western Cape); Antegibbaeum
(1; A. fissoides; Little Karoo in Western Cape), Braunsia (7;
B. apiculata, B. bina, B. edentula, B.
geminata, B. maximiliani, B. stayneri, B.
vanrensburgii; southwestern South Africa), Carpobrotus
(13; southern Africa, Australia, South America), Circandra (1; C.
serrata; at Ceres, Tulbagh and Villiersdorp in Western Cape),
Enarganthe (1; E. octonaria; Richtersveld in Northern Cape),
Erepsia (c 30; Western Cape, western Eastern Cape),
Esterhuysenia (5; E. alpina, E. drepanophylla,
E. inclaudens, E. mucronata, E. stokoei; at Caledon,
Ceres, Robertson and Worcester in Western Cape), Namaquanthus (1;
N. vanheerdii; northwest of Springbok in Northern Cape),
Scopelogena (2; S. bruynsii, S. verruculata; near
Cape Town and near Riversdale in Western Cape), Smicrostigma (1;
S. viride; north of Langeberg and Outeniqua Mountains in Western
Cape), Vlokia (2; V. ater, V. montana; near Montagu
in western Little Karoo in Western Cape), Wooleya (1; W.
farinosa; coastal parts of Namaqualand in Northern Cape),
Zeuktophyllum (2; Z. calycinum, Z. suppositum; at
Ladismith and Laingsburg in Western Cape), Octopoma (9–10; Little
Karoo in Western Cape); Acrodon (6; A. bellidiflorus, A.
deminutus, A. parvifolius, A. purpureostylus, A.
quarcicola, A. subulatus; coastal Little Karoo in Western Cape to
southwestern Eastern Cape), Arenifera (4; A. pillansii,
A. pungens, A. spinescens, A. stylosa;
Northern and Western Cape), Astridia (11; southern Namibia,
northern Northern Cape), Brianhuntleya (1; B. intrusa;
Worcester-Robertson Karroo in Western Cape), Ebracteola (6; E.
candida, E. derenbergiana, E. fulleri, E.
montis-moltkei, E. vallis-pacis, E. wilmaniae; central
Namibia, Northern Cape to central South Africa, North-West, Free State and
Gauteng), Khadia (6–8; K. acutipetala, K.
alticola, K. beswickii, K. borealis, K.
carolinensis, K. media; North-West, Gauteng, Mpumalanga and
KwaZulu-Natal in South Africa), Marlothistella (1; M.
stenophylla; Little Karoo in Western Cape), Polymita (2;
northern Namaqualand in Northern Cape), ‘Ruschia’
(220–225; Namibia, South Africa, Lesotho; non-monophyletic),
Stayneria (1; S. neilii; Breede River Valley in Western
Cape); Antimima (c 100; coastal parts of Namibia to Eastern Cape), Argyroderma
(11–12; Knersvlakte in southern Namaqualand in northwestern Western Cape),
Cephalophyllum (c 30; Namibia, Northern and Western Cape), Cheiridopsis
(23; Namibia, southwards to northern Western Cape), Cylindrophyllum
(5; C. calamiforme, C. comptonii, C. hallii, C.
obsubulatum, C. tugwelliae; Northern, Western and Eastern Cape),
Fenestraria (1; F. rhopalophylla; coastal parts of Namibia to
Richtersveld in Northern Cape), Hallianthus (1; H. planus;
Namaqualand in Northern Cape to Tanqua Karoo in Western Cape), Jordaaniella
(7; J. anemoniflora, J. clavifolia, J. cuprea,
J. dubia, J. maritima, J. spongiosa, J.
uniflora; southern Namibia, Northern and Western Cape),
Leipoldtia (8; Namibia, Northern, Western and Eastern Cape),
Octopoma (8; northern Namaqualand in Northern Cape, Little Karoo in
Western Cape), Odontophorus (4; O. angustifolius, O.
marlothii, O. nanus, O. pusillus; at Steinkopf in
Richtersveld in Northern Cape), Ottosonderia (1; O.
monticola; Namaqualand in southern Northern Cape and northern Western
Cape), Pleiospilos
(4–5; P. bolusii, P. compactus, P. leipoldtii,
P. nelii, P. simulans; Little Karoo in northern Western to
Great Karoo in western Eastern Cape), Schlechteranthus (15; northern
Namaqualand, especially Richtersveld, in Northern Cape), Vanzijlia (1;
V. annulata; coastal areas of Northern and Western Cape, Knersvlakte
in Namaqualand); Amphibolia (5; A. laevis, A.
obscura, A. rupis-arcuatae, A. saginata, A.
succulenta; coastal parts of Namibia south to southwestern Western Cape),
Eberlanzia (8; southern Namib Desert in southwestern Namibia, western
Namaqualand in Northern Cape), Ruschianthemum (1; R. gigas;
lower Orange River Valley in Namibia and Northern Cape), Stoeberia (5;
S. arborea, S. beetzii, S. carpii, S.
frutescens, S. utilis; Namaqualand in Namibia and Northern Cape),
Ectotropis (1; E. alpina; at Hogsback in the Amatola
Mountains and Katberg in Eastern Cape; in Delosperma?),
Rhombophyllum (5; R. albanense, R. dolabriforme,
R. dyeri, R. nelii, R. rhomboideum; around Uitenhage
and Port Elizabeth to Graaff-Reinet in Eastern Cape, southeastern Northern
Cape). – Southern Africa, with their largest diversity in the western coastal
part of the succulent karoo (Northern and Western Cape, Kalahari Desert etc.),
few species in eastern Africa, the Arabian Peninsula, Madagascar, Réunion and
Australia. CAM photosynthesis present. Wide-band tracheids usually frequent
(absent in basal taxa). Vesicular hairs usually absent. Leaves usually opposite
(sometimes spiral), succulent, of other form than flat (often terete or
trigonous). Inflorescence often distinct. Hypanthium usually present. Epigyny.
Tepal bases free. Nectary a koilomorphic meronectary, a wide and flat annular
holonectary, a lophomorphic (crested or lobed) holonectary, or nectary
inconspicuous or absent. Filaments usually free (sometimes connate at base),
papillate or hairy at base. Pistil composed of (three to) five to 15 (to 25)
connate carpels. Ovary inferior. Placentation basal or parietal. Fruit a
hygrochastic capsule, liberating a few seeds at a time. Expanding fruit keels
usually only on valves.
Apatesieae are sister-group to the
clade [Dorotheantheae+[Delospermeae+Ruschieae]] and
possess an annular holonectary. The capsule often lack hygrochastic properties
and has very reduced expanding keels. The clade
[Dorotheantheae+[Delospermeae+Ruschieae]] has a
covering membrane. Dorotheantheae are annual herbs with semi-succulent
leaves. The nectar is a wide and flat meronectary. The
[Delospermeae+Ruschieae] clade has a lophomorphic nectary and
a hygrochastic capsule. Moreover, the intron of the plastid gene rpoC1
is absent (lost). Delospermeae have a lophomorphic meronectary.
Ruschieae have a lophomorphic holonectary and the nuclear gene
ARP (a leaf developmental gene) is often duplicated (absent in some
species). Fruit characters are highly homoplasious. Apatesieae are
confined to the Cape Floristic Region.
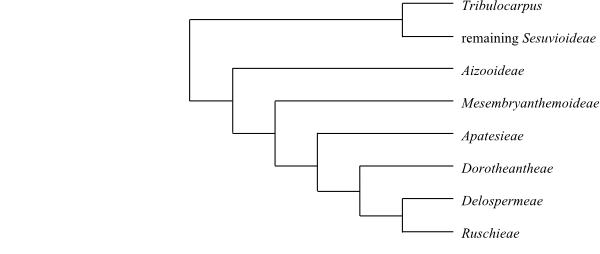 |
Cladogram (simplified) of Aizoaceae based on
morphology and DNA sequence data (Chesselet & al. 2002, etc.).
|
de Jussieu, Gen. Plant.: 87. 4 Aug 1789
[’Amaranthi’], nom. cons.
Atriplicaceae Juss.,
Gen. Plant.: 83. 4 Aug 1789 [’Atriplices’];
Chenopodiaceae Vent., Tabl. Règne Vég. 2: 253. 5
Mai 1799 [’Chenopodae’], nom. cons.;
Amaranthales R. Br. ex Bercht. et J. Presl, Přir.
Rostlin: 240. Jan-Apr 1820 [‘Amaranthaceae’];
Celosiaceae Martinov, Tekhno-Bot. Slovar: 117. 3 Aug
1820 [’Celosiae’]; Chenopodiales Juss.
ex Bercht. et J. Presl, Přir. Rostlin: 240. Jan-Apr 1820
[‘Chenopodeae’]; Salicorniaceae
Martinov, Tekhno-Bot. Slovar: 558. 3 Aug 1820 [’Salicorniae’];
Amaranthopsida Horan., Prim. Lin. Syst. Nat.: 58. 2
Nov 1834 [’Amaranthoideae’]; Betaceae
Burnett, Outl. Bot.: 591, 1091, 1142. Feb 1835;
Achyranthaceae Raf., Fl. Tellur. 3: 35. Nov-Dec 1837
[’Achyranthidia’]; Gomphrenaceae Raf.,
Fl. Tellur. 3: 38. Nov-Dec 1837 [’Gomphrenidia’];
Polycnemaceae Menge, Cat. Plat. Grudent. Gedan.: 161.
1839 [’Polycneminae’]; Salsolaceae
Menge, Cat. Plant. Grudent. Gedan.: 165. 1839 [’Salsolinae’];
Spinaciaceae Menge, Cat. Plant. Grudent. Gedan.: 166.
1839 [’Spinacinae’]; Chenopodiineae J.
Presl in Nowočeská Bibl. [Wšobecný Rostl.] 7: 1272, 1274. 1846;
Atriplicales Horan., Char. Ess. Fam.: 63. 30 Jun 1847
[‘Atriplicastra s. Curvembryae’];
Deeringiaceae J. Agardh, Theoria Syst. Plant.: 369.
Apr-Sep 1858 [’Deeringieae’]; Blitaceae
Post et Kuntze, Lex. Gen. Phan.: 637, 710. 20-30 Nov 1903;
Dysphaniaceae (Pax) Pax in Bot. Jahrb. Syst. 61: 230.
15 Jun 1927, nom. cons.
Genera/species
176/2.000–2.075
Distribution Cosmopolitan
except polar areas, with their highest diversity in saline, arid and semiarid
areas.
Fossils Fossil pollen (e.g.
Chenopodipollis) are known from Late Cretaceous (Maastrichtian),
Paleocene and Late Eocene layers in North America.
Habit Usually bisexual
(sometimes monoecious, andromonoecious, gynomonoecious, dioecious,
androdioecious, rarely polygamomonoecious), perennial, biennial or annual
herbs, evergreen or deciduous suffrutices or shrubs (rarely trees or lianas),
sometimes with spines. Often leaf or stem succulents. C4 plants with
c. 17 different types of foliar anatomy. Many species are halophytes or
xerophytes.
Vegetative anatomy Mycorrhiza
usually absent (sometimes with vesicular-arbuscular mycorrhiza). Kranz’
anatomy present in numerous species. Phellogen usually superficial or
pericyclic. Stem collenchyma well developed. Cortical and/or medullary bundles
usually present. Primary medullary strands usually wide. Endodermis usually
significant. Secondary lateral growth usually anomalous (polycyclic, anomalous
secondary vascular bundles from concentric cambia; not in
Polycnemoideae) or absent. Pericyclic fibres few or absent. Vessel
elements with simple perforation plates; lateral pits usually alternate
(sometimes opposite), simple or bordered pits. Imperforate tracheary xylem
elements libriform fibres (with cell nuclei) with simple or (reduced) bordered
pits, non-septate (also vasicentric tracheids). Wood rays usually absent
(sometimes uniseriate or multiseriate, homocellular or heterocellular). Axial
parenchyma paratracheal scanty, aliform, winged-aliform, confluent,
vasicentric, or banded. Vessel elements, fibres and/or parenchyma sometimes
partially or entirely storied. Intraxylary (concentric or diffuse) phloem
present. Sieve tube plastids P3cf type, without a central protein crystal, with
circular peripheral protein fibrils (sometimes with starch grains). Nodes 1:1
or 1:3, unilacunar with one or three leaf traces (sometimes 1:5, unilacunar
with five traces), often swollen. Heartwood sometimes with gum-like substances.
Calciumoxalate usually as crystal sand, druses or prismatic crystals (raphides
and styloids absent).
Trichomes Hair types
unicellular to multicellular, uniseriate, T-shaped or many-armed (also
malpighiaceous hairs), dendritic, stellate, candelabra-shaped, fasciculate,
lepidote, capitate and/or vesicular, often with salt-storing apical cell;
glandular hairs often present.
Leaves Alternate (spiral) or
opposite, simple, usually entire (sometimes lobed; in some stem succulents
reduced), with ? ptyxis. Stipules and leaf sheath absent. Petiole vascular
bundle transection arcuate or annular. Venation pinnate; leaves sometimes
one-veined. Stomata usually anomocytic (sometimes paracytic, diacytic or
anisocytic). Cuticular wax crystalloids as platelets (Chenopodioideae,
Salsoloideae etc.) or without platelets (Amaranthoideae etc.;
cuticular wax crystalloids absent in Dysphania). Domatia present in
many species. Hydathodes sometimes present. Leaf margin serrate, sinuate,
crenate or entire.
Inflorescence Terminal or
axillary, cymose, head-, raceme- or spike-like, thyrsoid, fasciculate or
panicle (flowers sometimes solitary, axillary). Lateral flowers of dichasial
partial inflorescences sometimes sterile and modified into scales, spines,
bristles or hairs. Bracts often membranous or coloured. Floral prophylls
(bracteoles) often petaloid (sometimes membranous). Extrafloral nectaries
rarely present (Iresine).
Flowers Actinomorphic, small.
Usually hypogyny (rarely half epigyny). Tepals (one or) three to five (to
eight), with usually imbricate (rarely valvate) aestivation, sepaloid, often
fleshy, often with tuberculate, spinulose or wing-like outgrowths, persistent,
free or connate at base, or rudimentary or absent. Nectary absent. Disc
(sometimes lobate) present in some species.
Androecium Stamens (one or)
three to five (to nine), usually as many as tepals, antetepalous, or absent.
Filaments free or connate in lower part (sometimes entirely) into a tube, often
adnate to tepals (epitepalous); tuberculate, scale-like or fringed
staminodium-like lobes, pseudostaminodia, often present between stamens and
usually fused with filaments. Anthers usually dorsifixed, often versatile,
usually tetrasporangiate (sometimes disporangiate), usually introrse (rarely
extrorse), longicidal (dehiscing by longitudinal slits); connective sometimes
with apical appendage (sometimes vesicular and coloured); anther wall
development monocotyledonous. Tapetum usually secretory, with binucleate or
multinucleate cells (sometimes amoeboid-periplasmodial). Staminodia one to
five, often petaloid, or absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains polypantoporate, shed as monads,
usually tricellular (rarely bicellular) at dispersal. Pollen grains often with
starch. Exine tectate or semitectate, with columellate infratectum,
microperforate, punctate or reticulate (in Gomphrenoideae
metareticulate), spinulate or tubuliferous.
Gynoecium Pistil composed of
(one or) two or three (to six) connate carpels (median carpel sometimes
abaxial); when two carpels then usually transverse. Ovary usually superior
(rarely semi-inferior), unilocular. Style single, simple, or stylodia two or
three, long or short, more or less connate. Stigma one, capitate (simple or
penicillate), or stigmas two or three (to six), narrowly elongate, papillate,
Dry type, often persistent. Pistillodium usually absent (male flowers sometimes
with pistillodium).
Ovules Placentation usually
basal (sometimes free central or apical). Ovule usually one (ovules sometimes
few or many) per ovary, campylotropous, anacampylotropous or circinotropous
(rarely amphitropous), erect to pendulous, bitegmic, crassinucellar. Micropyle
usually endostomal (rarely bistomal). Outer integument ? cell layers thick.
Inner integument ? cell layers thick. Parietal tissue? Nucellar beak present.
Apical cells of megasporangium often radially elongate. Megagametophyte usually
monosporous, Polygonum type (sometimes disporous, Allium
type). Synergids sometimes with a filiform apparatus. Antipodal cells usually
persistent. Chalazal caecum developed. Endosperm development ab initio nuclear.
Endosperm haustorium chalazal or absent. Embryogenesis chenopodiad or
solanad.
Fruit Usually a nut (often a
utriculus or an achene) or an irregularly dehiscent capsule (often a pyxidium,
rarely a berry, a baccaceous fruit or a drupe; adjacent ovaries sometimes
fusing and forming a syncarp), often surrounded by more or less fleshy perianth
forming an anthocarp; persistent and often accrescent bracts and floral
prophylls sometimes forming parts of dispersal unit.
Seeds Aril usually absent.
Seed coat usually exotestal-endotegmic. Outer exotestal cell walls with
stalactite-shaped appendages. Outer exotegmic cell walls often tanniniferous.
Endotegmen often thickened and lignified, tanniniferous. Perisperm usually
copious and starchy (rarely absent), usually surrounded by embryo. Endosperm
sparse or absent. Embryo curved or annular around perisperm (rarely straight;
in Salsoloideae spirally twisted), well differentiated, with or
without chlorophyll. Cotyledons usually two (rarely three). Radicula dorsal.
Germination phanerocotylar.
Cytology n = 6–36 –
Polyploidy frequently occurring.
DNA Intron absent from plastid
gene rpl2. Deletion of 300 bp in plastid IR in many clades. Plastid
genome in at least Chenopodium and Atriplex with 6 kb
inversion.
Phytochemistry Flavonols
(kaempferol, quercetin), 6-7-methylene-dioxyflavonols, isoflavones, flavonol
sulphates, betacyanins (e.g. amaranthin, celosianin and betamin or
phyllocactin), betaxanthins, isoquinoline alkaloids and other alkaloids
(particularly in Salsoleae), triterpene saponins, cyanogenic
compounds, betaine, anthraquinones, and sterols present. Ferulic acid present
in non-lignified cell walls. Ellagic acid and proanthocyanidins not found.
Nitrate or free oxalates accumulated in many species.
Use Ornamental plants,
vegetables (Beta vulgaris, Spinacia oleracea, Chenopodium
quinoa, Atriplex hortensis, etc.), sugar (Beta vulgaris
var. altissima), forage-plants (Beta vulgaris,
Atriplex, Chenopodium, Rhagodia,
Cornulaca), glass production (Salicornia, Salsola,
etc.), timber, wood carving, medicinal plants (Dysphania etc.).
Systematics Amaranthaceae are
sister-group to Achatocarpaceae or to the
clade [Caryophyllaceae+Achatocarpaceae].
A plausible topology is, according to Kadereit
& al. (2006) and Kadereit & Freitag (2011):
[Polycnemoideae+[[Amaranthoideae+[Betoideae+[Corispermoideae+Chenopodioideae]]]+[[Suaedoideae+Salicornioideae]+[‘Hammada’tamariscifolia+[Salsoloideae+Camphorosmoideae]]]]].
Polycnemoideae Raf.,
Fl. Tellur. 3: 44. Nov-Dec 1837 [‘Polycnemides’]
4/14. Hemichroa (1; H.
pentandra; Australia, Tasmania), Surreya (2; S.
diandra, S. mesembryanthema; Australia), Nitrophila (5;
N. atacamensis, N. australis, N. mexicana, N.
mohavensis, N. occidentalis; western United States, northwestern
Mexico, Chile, Argentina), Polycnemum (6; P. arvense, P.
fontanesii, P. heuffelii, P. majus, P. perenne,
P. verrucosum; central, southern and eastern Europe, the
Mediterranean, northewesternmost Africa, Central Asia). – Europe, the
Mediterranean, Central Asia, Australia, western United States, northwestern
Mexico. Normal secondary lateral growth present. Flowers unisexual. Filaments
connate at base. Anthers in Polycnemum monothecal (disporangiate).
Pollen grains smooth or spinulose. – Polycnemoideae were sister to
all other Amaranthaceae in one
matK/trnK analysis (Müller & Borsch 2005), but nested
deeply within Amaranthaceae in analyses
by Kadereit & Freitag (2011). Polycnemum is sister to the clade
[Nitrophila+[Hemichroa+Surreya]] (Masson &
Kadereit 2013).
[[Amaranthoideae+[Betoideae+[Corispermoideae+Chenopodioideae]]]+[[Suaedoideae+Salicornioideae]+[‘Hammada’tamariscifolia+[Salsoloideae+Camphorosmoideae]]]]
[Amaranthoideae+[Betoideae+[Corispermoideae+Chenopodioideae]]]
Amaranthoideae
Burnett, Outlines Bot.: 591, 593, 1091, 1142. Feb 1835
[‘Amarantidae’] (under construction)
76/800–830. Bosea (3;
B. yervamora: the Canary Islands; B. cypria: Cyprus; B.
amherstiana: western Himalayas); Charpentiera
(6; C. densiflora, C. elliptica, C. obovata, C.
ovata, C. tomentosa: the Hawaiian Islands; C. australis:
Tubuai in the Austral Islands). – Amarantheae
Rchb., Fl. Germ. Excurs. 2(2): 575, 583. 1832. Amaranthus
(c 50; cosmopolitan), Chamissoa (2; C. acuminata, C.
altissima; warmer regions in North to South America). –
Celosieae Fenzl in S. F. L. Endlicher, Gen. Plant.:
303. Oct 1837. Celosia (c
50; warmer regions in North to South America), Deeringia (12; tropical
and subtropical regions in the Old World), Henonia (1; H.
scoparia; Madagascar), Hermbstaedtia (c 15; tropical and southern
Africa except the Cape provinces), Pleuropetalum
(3; P. pleiogynum, P. sprucei: Mexico to Peru; P.
darwinii: the Galápagos Islands). – Pleuropetalum
has racemose inflorescence, five tepals, eight stamens developed in pairs,
filaments connate at base, pistil composed of five or six connate carpels,
basal placenta with several ovules, fruit ab initio fleshy, and n = 8 or 9. –
Aerveae Fenzl in S. F. L. Endlicher, Gen. Plant.:
302. Oct 1837. Aerva (c 20;
tropical and subtropical regions in the Old World), Nothosaerva (1;
N. brachiata; tropical Africa from Senegal to Ethiopia and northern
Somalia, south to southern Africa, Mauritius, Pakistan, India, Sri Lanka,
Burma, Mauritius), Ptilotus
(100–110; drier regions in Australia, one species, P. conicus, also
on Flores and Timor). – Gomphreneae Fenzl in S. F.
L. Endlicher, Gen. Plant.: 301. Oct 1837. Irenella (1; I.
chrysotricha; Ecuador), Iresine (35–40; tropical West Africa,
southern Japan, tropical and subtropical America), Woehleria (1;
W. serpyllifolia; Cuba); Alternanthera
(130–140; tropical and subtropical regions on both hemispheres, with their
largest diversity in tropical America), Pedersenia (9–10; tropical
America), Tidestromia (6; T. carnosa, T. lanuginosa,
T. rhizomatosa, T. suffruticosa, T. tenella, T.
valdesiana; southwestern United States, Mexico); Blutaparon (4;
B. portulacoides, B. rigidum [extinct], B.
vermiculare, B. wrightii; tropical West Africa, Ryukyu Islands,
tropical and subtropical America), Froelichia
(15; tropical and subtropical America, the Galápagos Islands),
Froelichiella (1; F. grisea; Brazil), ‘Gomphrena’
(130–135; tropical and subtropical regions in North to South America;
polyphyletic), Gossypianthus (2; G. lanuginosus, G.
tenuiflorus; United States, Mexico, Central America), Guilleminea
(8; Central America), Hebanthodes (1; H. peruviana; Peru),
Lecosia (2; L. formicarum, L. oppositifolia;
southeastern Brazil), Lithophila (2; L. radicata, L.
subscaposa; the Galápagos Islands), Pfaffia (c 30; tropical
South America), Pseudogomphrena (1; P. scandens; Brazil),
Pseudoplantago (2; P. bisteriliflora, P. friesii;
Venezuela, Argentina), Quaternella (3; Q. confusa, Q.
ephedroides, Q. glabratoides, Brazil), Xerosiphon (2;
X. angustiflorus, X. aphyllus; tropical South America). –
Pseudoplantago has cuboid or prismatic pollen grains. –
Achyrantheae Fenzl in S. F. L. Endlicher, Gen.
Plant.: 302. Oct 1837. Achyranthes
(12; tropical and subtropical regions in the Old World; incl. Nototrichum),
Nototrichium
(3; N. divaricatum, N. humile, N. sandwicense; the
Hawaiian Islands; in Achyranthes),
Calicorema (2; C. capitata, C. squarrosa; tropical
Africa and southwards to Namibia and Northern Cape), Cyathula (c 30;
tropical regions in Africa, Asia, America and the Pacific), Pandiaka
(13; tropical and southern Africa), Psilotrichum (16; tropical regions
in the Old World), Pupalia (4; P. grandiflora, P.
lappacea, P. micrantha, P. robbechii; tropical regions
in the Old World), Sericostachys (1; S. scandens; tropical
Africa). – Unplaced Amaranthoideae
Achyropsis (6; A. avicularis, A. filifolia, A.
fruticulosa, A. gracilis, A. laniceps, A.
leptostachya; tropical and southern Africa), Allmania (1; A.
nodiflora; tropical Asia), Allmaniopsis (1; A.
fruticulosa; eastern Kenya), Arthraerua (1; A.
leubnitziae; coast of Namibia), Centema (2; C.
angolensis, C. subfusca; southern tropical Africa to South
Africa), Centemopsis (13; tropical Africa), Centrostachys (1;
C. aquatica; northern Africa, India, Java), Chionothrix (2;
C. latifolia, C. somalensis; Somalia), Dasysphaera
(4; D. alternifolia, D. hyposericea, D. robecchii,
D. tomentosa; East Africa), Digera (1; D. muricata;
tropical regions in the Old World), Eriostylos (1; E.
stefaninii; Somalia), Herbstia (1; H. brasiliana;
Brazil), Indobanalia (1; I. thyrsiflora; southwestern India),
Kyphocarpa (3; K. angustifolia, K. cruciata, K.
trichinoides; southern tropical and southern Africa), Lagrezia
(9; Madagascar, Indian Ocean islands), Lecosia (2; L.
formicarum, L. oppositifolia; southeastern Brazil),
Leucosphaera (1; L. bainesii; Angola, Namibia, Botswana,
northwestern Northern Cape), Lopriorea (1; L. ruspolii; East
Africa), Marcelliopsis (3; M. denudata, M.
splendens, M. welwitschii; Namibia), Mechowia (1; M.
grandiflora; southern tropical Africa), Nelsia (3; N.
angolensis, N. quadrangula, N. tropidogyna; Angola,
southern Africa), Neocentema (2; N. alternifolia: Tanzania;
N. robecchii: Somalia), Nyssanthes (2; N. diffusa,
N. erecta; eastern Queensland, eastern New South Wales),
Omegandra (1; O. kanisii; northern Australia),
Pleuropterantha (3; P. revoilii, P. thulinii, P.
undulatifolia; northeastern tropical Africa), Polyrhabda (1;
P. atriplicifolia; Somalia), Pseudosericocoma (1; P.
pungens; southwestern and southern Africa), Psilotrichopsis (1;
P. curtisii; Thailand, the Malay Peninsula), Rosifax (1;
R. sabuletorum; Somalia), Saltia (1; S. papposa;
southern Arabian Peninsula), Sericocoma (3; S. avolans,
S. heterochiton, S. pungens; tropical Africa to Namibia,
Northern, Western and Eastern Cape), Sericocomopsis (2; S.
hildebrandtii, S. pallida; tropical East Africa),
Sericorema (2; S. remotiflora, S. sericea; southern
Africa), Stilbanthus (1; S. scandens; the Himalayas),
Trichuriella (1; T. monsoniae; southern and southeastern
Asia), Volkensinia (1; V. prostrata; East Africa). –
Subcosmopolitan with their largest diversity in tropical and subtropical
America and Africa. – Bosea and Charpentiera
are successive sisters to the remaining Amaranthoideae.
Gomphreneae, with bilocular anthers and metareticulate exine, are
sister-group to Achyrantheae.
[Betoideae+[Corispermoideae+Chenopodioideae]]
Betoideae Ulbr. in
Engler et Prantl, Nat. Pflanzenfam., ed. 2, 16c: 455. 28 Apr 1934
6/17. Acroglochin (2; A.
obtusifolia, A. persicarioides; Central and East Asia, the
Himalayas); Beta (9;
Europe, the Mediterranean, temperate Asia), Aphanisma (1; A.
blitoides; California, northwestern Mexico), Hablitzia
(1; H. tamnoides; the Caucasus), Oreobliton (1; O.
thesioides; Algeria, Tunisia), Patellifolia
(3; P. patellaris, P. procumbens, P. webbiana;
Macaronesia). – Europe, Macaronesia, the Mediterranean region, North Africa,
southwestern Asia, southwestern North America (Aphanisma). Annual to
perennial herbs (in Hablitzia
twining) or suffrutices. Tepals usually five (in Aphanisma three).
Fruit a pyxidium. – Betoideae are sister-group to the clade
[Corispermoideae+Chenopodioideae]. Acroglochin is
sister to the remaining Betoideae.
[Corispermoideae+Chenopodioideae]
Corispermoideae Raf.,
Fl. Tellur. 3: 45. Nov-Dec 1837 [‘Corispermides’]
3/c 80. Agriophyllum (6; A.
lateriflorum, A. latifolium, A. minus, A.
montasirii, A. paletzkianum, A. squarrosum; Europe,
western Asia to Central Asia), Anthochlamys (5; A. afghanica,
A. multinervis, A. polygaloides, A. tjanschanica,
A. turcomanica; southwestern and Central Asia), Corispermum
(c 70; temperate regions of Europe and Asia). – Eurasia. Annual herbs.
C3 metabolism and corispermoid non-Kranz’ leaf anatomy present.
Hairs on young shoots usually dendritic (not in Anthochlamys). Floral
prophylls (bracteoles) absent. Tepals one to five, membranous, caducous
(sometimes absent). Perisperm copious. Embryo erect. –
Corispermoideae are sister-group to Chenopodioideae.
Chenopodioideae
Burnett, Outlines Bot.: 591, 1091, 1142. Feb 1835
[‘Chenopodidae’]
23/460–470.
Dysphanieae Pax in H. G. A. Engler et K. A. E.
Prantl, Nat. Pflanzenfam. III, 1b: 69, 92. Mai 1889. Cycloloma (1;
C. atriplicifolium; western and central North America), Dysphania
(>40; warmer regions on both hemispheres), Suckleya (1; S.
suckleyana; Rocky Mountains), Teloxys (1;
T. vagans; Mongolia). – Axyrideae G.
Kadereit et Sukhor. in Amer. J. Bot. 97: 1682. Oct 2010. Axyris (6;
A. amaranthoides, A. caucasica, A. hybrida, A.
koreana, A. prostrata, A. sphaerosperma; Central Asia to
the Korean Peninsula), Ceratocarpus (1; C. arenarius; eastern
Europe, southwestern to Central Asia), Krascheninnikovia (3; K.
ceratoides, K. ewersmanniana, K. fruticulosa; the
Mediterranean, temperate Asia, western North America). –
Anserineae Dumort., Fl. Belg.: 20. 1827. Blitum (12;
Europe, northern and eastern Asia, Australia, Canada, United States, temperate
South America), Spinacia
(3; S. oleracea, S. tetrandra, S. turkestanica;
southwestern Asia, North Africa). – Atripliceae
Duby, Bot. Gall. 1: 394. 12-14 Apr 1828. Lipandra
(1; L. polysperma; Europe, the Mediterranean), Oxybasis
(5; O. chenopodioides, O. glauca, O. macrosperma,
O. rubra, O. urbica; temperate regions in Europe and Asia,
the Mediterranean), Chenopodiastrum
(5; C. badachschanicum, C. coronopus, C. hybridum,
C. murale, C. simplex; temperate regions on the Northern
Hemisphere), Atriplex
(250–260; cosmopolitan), Microgynoecium (1; M. tibeticum;
Tibet), Archiatriplex (1; A. nanpinensis; Sichuan),
Exomis (1; E. microphylla; Namibia, Northern, Western and
Eastern Cape, Free State), Extriplex (2; E. californica,
E. joaquinana; California), Grayia (3; G.
arizonica, G. plummeri, G. spinosa; western United
States), Holmbergia (1; H. tweedii; Uruguay, Paraguay,
Argentina), Manochlamys
(1; M. albicans; Namibia, Northern and Western Cape),
Proatriplex (1; P. pleiantha; the Navajo Basin in
southwestern United States), Stutzia (2; S. covillei, S.
dioica; western United States), Chenopodium
(c 115; cosmopolitan). – Chenopodioideae are sister-group to
Corispermoideae. The Atriplex
clade is sister-group to Chenopodium
with high support.
[[Suaedoideae+Salicornioideae]+[‘Hammada’tamariscifolia+[Salsoloideae+Camphorosmoideae]]]
[Suaedoideae+Salicornioideae]
Suaedioideae Ulbr. in
Engler et Prantl, Nat. Pflanzenfam., ed. 2, 16c: 445, 554. 28 Apr 1934
2/75–80. Bienertia (3; B.
cycloptera, B. kossinskyi, B. sinuspersici; southeastern
European Russia and Kazakhstan to western Central Asia), Suaeda
(70–75; cosmopolitan). – Subcosmopolitan. Annual or perennial herbs or
shrubs. Leaf-succulents, halophytes. C3 or C4 metabolism
with or without Kranz’ anatomy. Flowers in axillary cymes. Tepals five,
connate at base. Perisperm usually absent. Embryo spiral. –
Suaedioideae are sister-group to Salicornioideae.
Salicornioideae
Luerss., Hand. Syst. Bot. 2: 547. Nov 1880
12/105–110. Heterostachys (2;
H. olivascens: Argentina; H. ritteriana: Central America,
Hispaniola, South America), Allenrolfea (3; A. occidentalis:
southwestern United States, Mexico; A. patagonica, A.
vaginata: Argentina), Halopeplis (3; H. amplexicaulis:
the Mediterranean, North Africa; H. perfoliata: northeastern Africa,
the Arabian Peninsula to Pakistan; H. pygmaea: the Caucasus and Iraq
to Central Asia and Xinjiang), Halostachys (1; H.
belangeriana; southeastern Russia to Central Asia), Halocnemum
(2; H. strobilaceum, H. yurdakulolii; central Mediterranean
to Central Asia), Kalidium (6; K. capsicum, K.
cuspidatum, K. foliatum, K. gracile, K.
schrenkianum, K. sinicum; the Mediterranean to Central Asia),
Microcnemum (1; M. coralloides; the Mediterranean to the
Caucasus), Arthrocaulon (2; A. franzii, A.
macrostachyum; the Mediterranean, Macaronesia, northwestern and
northeastern Africa, southwestern Asia), Arthroceras (1; A.
subterminale; California, northern Mexico), Tecticornia (44;
Australia, one species, T. indica, along coasts of eastern Africa,
southern Asia and northern Australia), Mangleticornia (1; M.
ecuadorensis; coasts of southern Ecuador and northern Peru),
Salicornia (40–45; Europe, the Mediterranean, Africa, Asia,
Australia, America). – Subcosmopolitan. Annual or perennial herbs or shrubs.
Stem-succulents, halophytes. Usually with C3 metabolism (one species
of Tecticornia with C4 metabolism). Flowers in spike-like
thyrse. Embryo spiral. – Salicornioideae are sister to
Suaedioideae.
[‘Hammada’
tamariscifolia+[Salsoloideae+Camphorosmoideae]]
Salsoloideae Raf., Fl.
Tellur. 3: 45. Nov-Dec 1837 [‘Salsoloides’]
c 35–37/c 370.
Salsoleae Dumort., Fl. Belg.: 22. 1827.
Sympegma (1; S. regelii; Central Asia), Traganum
(2; T. moquinii, T. nudatum; the Canary Islands, eastern
Mediterranean, North Africa), Xylosalsola (4; X. arbuscula,
X. chiwensis, X. paletzkiana, X. richteri; Central
Asia), Turania (4; T. androssowii, T. aperta, T.
deserticola, T. sogdiana; Iran, Afghanistan, Central Asia,
northwestern China), Halothamnus (c 20; the Middle East to
Afghanistan, Somalia, Central Asia, northwestern China), Noaea (5;
N. cadmea, N. major, N. minuta, N.
mucronata, N. regelii; arid and semi-arid regions in eastern
Europe to Central Asia), Oreosalsola (9; Iran, Afghanistan, Central
Asia), ‘Salsola’
(60-65; Europe, Macaronesia, the Mediterranean, North Africa, southwestern and
Central Asia), Haloxylon (2; H. ammodendron, H. persicum; the
Mediterranean, North Africa, southwestern and Central Asia, Mongolia, western
and northwestern China), Cornulaca (7; C. alaschanica, C.
amblyacantha, C. aucheri, C. ehrenbergii, C.
korshinskyi, C. monacantha, C. setifera; Egypt to
Central Asia), Horaninovia (2; H. minor, H. ulicina;
southwestern and Central Asia), Hammada (3; H. griffithii,
H. leptoclada, H. wakhanica; southwestern and Central Asia),
Halogeton (4; H. arachnoideus, H. glomeratus, H.
sativus, H. tibeticus; the Mediterranean to Central Asia),
Girgensohnia (5; G. bungeana, G. diptera, G.
imbricata, G. minima, G. oppositiflora; southeastern
European Russia to Iran and Central Asia; incl. Cyathobasis and
Arthrophytum?), Cyathobasis (1; C. fruticulosa;
Turkey; in Girgensohnia?), Arthrophytum (8; western and
Central Asia; in Girgensohnia?), Anabasis (c 30; the
Mediterranean to Central Asia); unplaced Salsoleae: Iljinia
(1; I. regelii; Central Asia), Lagenantha (3; L.
cycloptera, L. gillettii, L. nogalensis; northeastern
Africa), Nucularia (1; N. perrinii; Algeria, Sahara),
Traganopsis (1; T. glomerata; Morocco). –
Caroxyloneae Akhani et Roalson in H. Akhani, G.
Edwards et E. H. Roalson in Intern. J. Plant Sci. 168: 947. Jul-Aug 2007. Caroxylon
(20–25; Macaronesia, the Mediterranean, northern, eastern and southern
Africa, Madagascar, the Arabian Peninsula, southwestern and Central Asia,
northwestern China, Xinjiang), Nanophyton (c 10; southeastern European
Russia to Central Asia), Halocharis (4–7; H. clavata,
H. hispida, H. sulphurea, H. violacea; southwestern
and Central Asia), Kaviria (10; western Asia to Iran and Central
Asia), Ofaiston (1; O. monandrum; southeastern European
Russia to western Asia), Petrosimonia (9; southeastern Europe to
Central Asia), Pyankovia (1; P. brachiata; southeastern
Europe, Crimea and the Caucasus to Central Asia and western Siberia),
Halimocnemis (6; H. karelinii, H. longifolia, H.
macrantha, H. occulta, H. sclerosperma, H.
villosa; southeastern European Russia to Central Asia),
Piptoptera (1; P. turkestana; Central Asia),
Climacoptera (40–45; southwestern Asia, Iran, Afghanistan, Central
Asia); unplaced Caroxyloneae: Halarchon (1; H.
vesiculosus; Afghanistan), Physandra (1; P.
halimocnemis; Central Asia). – Europe, Macaronesia, the Mediterranean,
Africa, southwestern and central Asia, with their highest diversity in central
and southwestern Asia. Annual or perennial herbs, shrubs or trees.
Leaf-succulents, halophytes. Usually C4 metabolism (in
Salsoleae usually NADP-malic enzyme subtype; in Caroxyloneae
NAD-malic enzyme subtype), although in a number of clades C3
metabolism (in S. arbusculiformis an intermediate between
C3 and C4 metabolism). Foliar prophylls (bracteoles)
present. Perianth usually with membranous wings in fruit. Perisperm poorly
developed or absent. Embryo spiral. – Hammada tamariscifolia (L.)
Iljin (= Salsola genistoides Juss. ex Poiret in Lam.; southern Spain)
is sister to the clade [Salsoloideae+Camphorosmoideae].
Camphorosmoideae
Luerss., Handb. Syst. Bot. 2: 546. Nov 1880 [‘Camphorosmeae’]
16/170–180. Chenolea (2;
C. convallis, C. diffusa; Namibia, Northern, Western and
Eastern Cape, KwaZulu-Natal), Spirobassia
(1; S. hirsuta; Atlantic coasts of Europe to central Mediterranean,
coasts of the Black Sea and the Caspian Sea), Neokochia (2; N.
americana, N. californica; southwestern North America),
Eokochia (1; E. saxicola; islands of Ischia, Capri and
Stromboli in Italy); Grubovia (7; G. brevidentata, G.
dasyphylla, G. eriophora, G. krylovii, G.
melanoptera, G. mucronata, G. sedoides; Central Asia,
Mongolia), ‘Maireana’ (57–60; continental and southern
Australia; non-monophyletic), Dissocarpus (4; D. biflorus,
D. fontinalis, D. latifolius, D. paradoxus;
continental Australia), Eriochiton (1; E. sclerolaenoides;
southern parts of Australia), Sclerolaena (60–65; Australia),
Didymanthus (1; D. roei; southwestern Western Australia),
Roycea (3; R. divaricata, R. pycnophylloides, R.
spinescens; southwestern Western Australia), Eremophea (2; E.
aggregata, E. spinosa; arid regions of Australia),
Malacocera (4; M. albolanata, M. biflora, M.
gracilis, M. tricornis; arid regions of Australia); Bassia (c 20;
western Mediterranean to eastern Asia), Sedobassia (1; S.
sedoides; eastern Europe to Siberia), Camphorosma (6; C.
annua, C. monandra, C. monspeliaca, C.
persepolitana, C. polygama, C. songorica; the
Mediterranean to Central Asia). – Temperate and subtropical Eurasia, northern
and southern Africa, Australia, with their highest diversity in Australia, few
species in South Africa or North America. C3 or C4
metabolism (one species intermediate between C3 and C4
metabolism). Camphorosmoideae are similar to Salsoloideae,
but differ through absence of floral prophylls (bracteoles), stigmas filiform
and with stigmatic papillae on their entire surface, annular or folded embryo
surrounding perisperm, wider pollen grains (>15 µm) with larger number of
pores (usually >70) with smaller diameter (usually <2000 nm), and exine
with smaller number of spinulae per operculum (>15). –
Camphorosmoideae are sister to Salsoloideae.
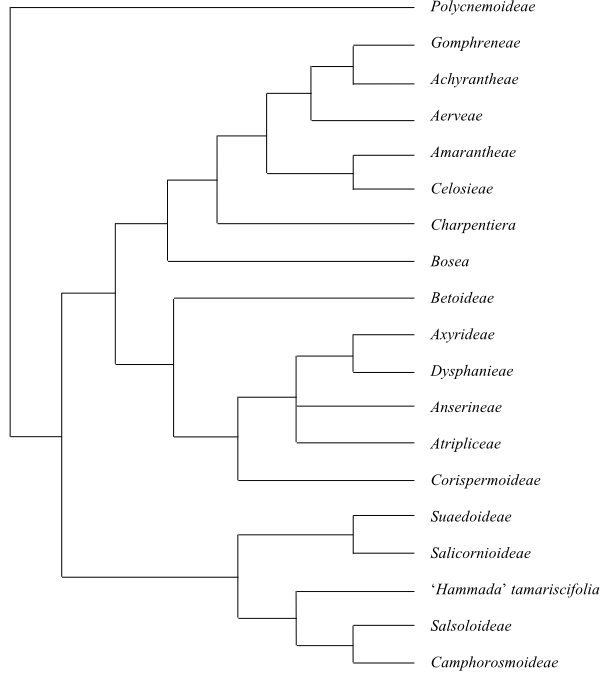 |
Simplified tree of Amaranthaceae based
on DNA sequence data (from numerous sources). The sister-group
relationship between Polycnemoideae and the remaining Amaranthaceae is
not very strong.
|
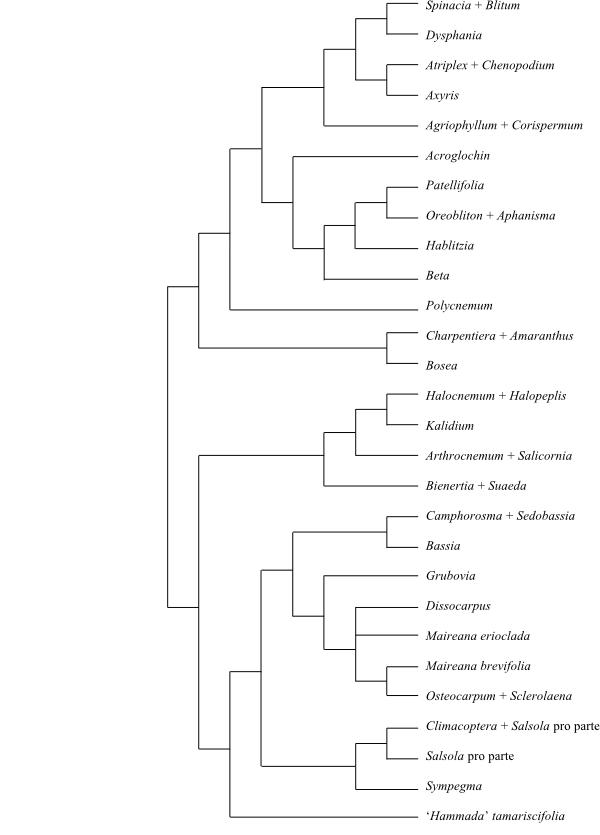 |
Maximum-likelihood tree (simplified) of Amaranthaceae based
on DNA sequence data (Kadereit & al. 2006; Kadereit & Freitag
2011). [Dysphania+Teloxys]
is sister-group to the Axyrideae clade in Fuentes-Bazan &
al. (2012).
|
Nyffeler et Eggli in Taxon 59: 232. Feb 2010
Genera/species 3/c 32
Distribution Southern and
southeastern Africa, Somalia, southwestern Arabian Peninsula, southwestern
United States, Mexico, Bolivia, Argentina, with their largest diversity in
South Africa.
Fossils Unknown.
Habit Bisexual, small shrubs
or suffrutices to thick-stemmed perennial herbs. Sometimes with a basal fleshy
caudex or tuberous main-root. Often stem succulents. Usually mucilaginous.
Vegetative anatomy Plants with
facultative CAM (or C4?) photosynthesis; leaves with Kranz’
anatomy. Stem epidermis usually without stomata (sometimes parallelocytic).
Phellogen ab initio usually epidermal (sometimes outer-cortical). Precocious
initiation of stem periderm. Phellem with lignified bands consisting of
thin-walled flattened cells. Medulla often with wide-band tracheids. Cortical
fibres absent. Fibre caps to stem vascular tissue absent. Secondary lateral
growth normal or absent. Vessel elements with simple perforation plates;
lateral pits with wide annular or helical secondary wall thickening.
Imperforate tracheary xylem elements usually absent (libriform fibres present
in stems of Grahamia bracteata and Talinopsis frutescens
lacking wide-band tracheids). Wood rays multiseriate or absent, often with
wide-band tracheids. Axial parenchyma paratracheal scanty vasicentric (in stems
of Talinopsis). Thick-walled pericyclic extraxylary phloem fibre caps
usually absent. Sieve tube plastids P3cf type? Nodes 1:1? Mucilaginous
tracheoidal idioblasts usually frequent in wood rays (not in
Grahamia). Sclereides (thick-walled) often present. Tanniniferous
cells absent. Phloem parenchyma cells with phytoferritin? Parenchyma and
epidermis often with numerous calciumoxalate crystals or druses.
Trichomes Hairs unicellular or
multicellular, uniseriate, or absent.
Leaves Usually alternate
(spiral; in Talinopsis opposite), simple, entire, often more or less
succulent (especially in Grahamia), usually globose to terete (rarely
flattened), with supervolute ptyxis? Stipules and leaf sheath absent. Leaves
with axillary biseriate or oligoseriate hairs, bristles or a pergamentaceous
scale enclosing diminutive leaves (in Anacampseros sect.
Avonia). Petiole vascular bundle transection arcuate? Venation
pinnate, ?-dromous. Stomata parallelocytic, usually transversely orientated.
Cuticular wax crystalloids usually absent (rarely as relatively irregular
platelets?). Mesophyll with mucilage idioblasts. Leaf margin entire.
Inflorescence Terminal or
axillary, few-flowered thyrsoid, sometimes compact (with short internodes) or
with scorpioid partial inflorescences. Involucre consisting of bracts often
present. Transverse floral prophylls (bracteoles) subtending axillary flowers;
median floral prophylls (in the same plane as bud-subtending prophylls) without
flowers; sepaloid floral prophylls two, persistent and dry in fruit.
Flowers Actinomorphic, small
to medium-sized. Hypanthium present. Hypogyny. Tepals five, with imbricate
aestivation, petaloid, free or slightly connate at base (rarely absent).
Nectary absent? Disc absent.
Androecium Stamens five to 15
(to c. 25). Annular androecial primordium often present in
Anacampseros. Filaments free from each other and from tepals. Anthers
dorsifixed, versatile, tetrasporangiate, introrse, longicidal (dehiscing by
longitudinal slits). Tapetum secretory, with multinucleate cells. Staminodia
absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually polypantocolpate,
polyrugate, shed as monads, tricellular at dispersal. Exine tectate, with
columellate infratectum, perforate, spinulate.
Gynoecium Pistil composed of
three connate carpels. Ovary superior, ab initio multilocular, later
unilocular. Stylodia (four or) five (or six). Stigmas receptive on both
surfaces, papillate, Dry type. Pistillodium absent.
Ovules Placentation usually
free central (rarely basal). Ovules numerous per ovary, anatropous?, bitegmic,
crassinucellar. Micropyle endostomal. Outer integument ? cell layers thick.
Inner integument ? cell layers thick. Parietal tissue? Apical cells of
megasporangium radially elongate. Megagametophyte monosporous,
Polygonum type. Synergids with a filiform apparatus? Antipodal cells
two?, ephemeral. Endosperm development ab initio nuclear. Endosperm haustoria?
Embryogenesis caryophyllad?
Fruit A loculicidal and/or
septicidal capsule dehiscing from apex into three or six valves, with caducous
exocarp usually separating from endocarp (not in Grahamia). Perianth
remnants and stamens persistent and forming a dry calyptra (Grahamia,
Talinopsis) or caducous (Anacampseros). Endocarp valves
forming small basket.
Seeds Aril absent. Testa
two-layered, sometimes winged. Exotesta in Talinaria and
Anacampseros thick, with dry cells with thin unlignified walls;
exotesta usually partially or entirely separating periclinally from endotesta.
Endotesta? Tegmen? Perisperm starchy, usually poorly developed. Endosperm
sparse or absent. Embryo usually only slightly curved, not surrounding
perisperm, parallel to perisperm, well differentiated, without chlorophyll.
Cotyledons two. Radicula dorsal. Germination phanerocotylar?
Cytology x = 9
DNA Intron absent from plastid
gene rpl2? 6 bp deletion in plastid gene ndhF? C. 500 bp
deletion in plastid gene rbcL?
Phytochemistry Betalains
present. Ellagic acid and cyanogenic compounds not found.
Use Ornamental plants.
Systematics
Talinopsis (1; T. frutescens; southern United States,
Mexico), Grahamia (1; G. bracteata; Bolivia, northern and
central Argentina), Anacampseros
(c 30; southeastern and southern Africa, Somalia, southwestern Arabian
Peninsula).
Anacampserotaceae are
sister-group to Cactaceae, according to
Brockington & al. (2013).
The axillary appendages may by remnants of
highly condensated axillary short shoots and thus homologous to the areoles in
the Cactaceae (Nyffeler
& Eggli 2010).
Six species of ‘Grahamia’ form a
basal pectination in the analysis by Nyffeler (1997). These are now included in
Anacampseros.
Grahamia bracteata is sister to Anacampseros
(Nyffeler & Eggli 2010).
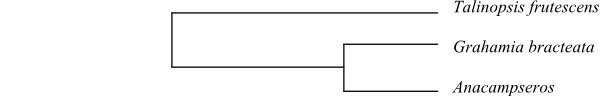 |
Cladogram (simplified) of Anacampserotaceae
based on DNA sequence data (Nyffeler & Eggli 2010).
|
Walpers in Ann. Bot. Syst. 2: 175. 15-16 Dec 1851
[‘Ancistrocladeae’], nom. cons.
Ancistrocladales
Takht. ex Reveal in Novon 2: 238. 13 Oct 1992
Genera/species 1/c 20
Distribution Tropical Africa,
India, Sri Lanka, eastern Himalayas to southeastern China, Southeast Asia and
West Malesia.
Fossils Unknown.
Habit Bisexual, evergreen
lianas with distinctly sympodial growth. Some lateral branches with circinately
branched, often leaf opposite, barbs (hooks, grapnels) in one plane.
Vegetative anatomy Phellogen
ab initio median-cortical. Primary cortex with thick-walled secretory cells.
Cortical vascular bundles absent? Vessel elements with simple perforation
plates; lateral pits alternate? Imperforate tracheary xylem elements fibre
tracheids with bordered pits, non-septate? (also vasicentric tracheids). Wood
rays uniseriate, homocellular. Axial parenchyma apotracheal, usually in
tangential bands, with fibriform vessels and spiral cells, without crystals.
Wood often fluorescent. Tyloses absent. Sieve tube plastids Ss type. Nodes 3:3,
trilacunar with three leaf traces. Cortical cells with pitted elongate
sclereids. Wood ray parenchyma cells with silica bodies (at least in African
species). Calciumoxalate crystals?
Trichomes Glandular hairs
multicellular, peltate-lepidote. Vascularized glands absent.
Leaves Alternate (spiral),
simple, entire, with supervolute ptyxis. Stipules very small, early caducous;
leaf sheath absent. Petiole articulated. Petiole vascular bundle transection
annular and with peripheral cylinder of small bundles. Venation pinnate.
Stomata actinocyclocytic (surrounded by delimited subsidiary cells). Cuticular
wax crystalloids as platelets. Abaxial side of lamina with small lepidote,
multicellular wax glands in small pocket-like domatia. Leaf margin entire.
Inflorescence Terminal or
axillary, panicle, raceme- or spike-like, racemes or spikes.
Flowers Actinomorphic to
zygomorphic, small. Pedicel articulated. Half epigyny. Sepals five, with
imbricate quincuncial aestivation, unequal, persistent, connate, sometimes with
abaxial glands. Petals five, with contorted or imbricate aestivation, fleshy,
free or connate at base. Nectaries? Disc absent.
Androecium Stamens usually ten
(rarely five or 15), in one whorl (staminal primordia five?); antepetalous
stamens larger. Filaments short, fleshy, connate at base, adnate to petal bases
(epipetalous). Anthers basifixed, non-versatile, tetrasporangiate, introrse or
latrorse, longicidal (dehiscing by longitudinal slits). Tapetum secretory?
Staminodia absent.
Pollen grains
Microsporogenesis simultaneous? Pollen grains 3(–4)-colp(or)ate, shed as
monads, ?-cellular at dispersal. Exine tectate, with columellate infratectum,
spinulate.
Gynoecium Pistil composed of
usually four (sometimes three) connate carpels. Ovary semi-inferior,
unilocular. Stylodia usually four (sometimes three), free or connate at base,
articulated, slightly widened in upper part. Stigmas (three or) four,
crescent-shaped or pinnatifid, Dry? type. Pistillodium absent.
Ovules Placentation
basal-lateral. Ovule one per ovary, hemianatropous, bitegmic, crassinucellar.
Micropyle ?-stomal Outer integument ? cell layers thick. Inner integument ?
cell layers thick. Parietal tissue? Megagametophyte monosporous,
Polygonum type? Endosperm development cellular. Endosperm haustoria?
Embryogenesis?
Fruit A nut surrounded by
hypanthium and with persistent strongly enlarged wing-like calyx lobes.
Seeds Aril absent. Seed coat
exotestal? Testa membranous. Exotesta? Endotesta? Tegmen? Perisperm not
developed. Endosperm hard, starchy, ruminate. Embryo small, straight,
chlorophyll? Cotyledons two, strongly plicate. Germination phanerocotylar.
Cytology n = ?
DNA Intron present in plastid
gene rpl2.
Phytochemistry Flavonols
(kaempferol, quercetin, myricetin), cyanidin, delphinidin, betulinic acid,
polyketide-derived naphthyl isoquinoline alkaloids (e.g., ancistrocline,
dioncophylline, michellamines), and naphthoquinones (plumbagin, droserone)
present. Ellagic acid?
Use Michellamines (dimerous
naphthyl isoquinoline alkaloids) in Ancistrocladus korupensis are
anti-HIV active substances.
Systematics
Ancistrocladus (c 20; tropical Africa, India, Sri Lanka, eastern
Himalayas to southeastern China, Burma, Southeast Asia, the Andaman Islands and
West Malesia to Borneo).
Ancistrocladus is sister to Dioncophyllaceae.
The non-carnivorous habit in
Ancistrocladus is obviously secondary.
ASTEROPEIACEAE
(Szyszył.) Takht. ex Reveal et Hoogl.
|
( Back to Caryophyllales
)
|
Reveal et Hoogland in Bull. Mus. Natl. Hist. Nat.
Paris, sér. 4, sect. B, Adansonia, 12: 205. 24 Nov 1990
Genera/species 1/8
Distribution Madagascar.
Fossils Unknown.
Habit Bisexual, evergreen
trees or climbing to creeping shrubs.
Vegetative anatomy
Ectomycorrhiza present. Phellogen ab initio subepidermal. Pericyclic fibres
present. Primary medullary rays narrow. Continuous sclerenchyma cylinder
surrounding vascular cylinder in young stems. Vessel elements with simple
perforation plates; lateral pits alternate, bordered pits. Imperforate
tracheary xylem elements fibre tracheids or libriform fibres (tracheids absent)
with small bordered pits, non-septate. Wood rays usually uniseriate (rarely
biseriate), homocellular (consisting of exclusively procumbent cells). Axial
parenchyma apotracheal diffuse, or paratracheal aliform, winged-aliform,
confluent or unilateral. Wood diffuse to diffuse porose, non-storied. Sieve
tube plastids Ss type. Nodes 1:1, unilacunar with one leaf trace. Medullary
parenchyma with sclereids. Secretory cavities? Calciumoxalate crystals and
silica bodies absent.
Trichomes Hairs absent?
Leaves Alternate (spiral),
simple, entire, with ? ptyxis. Stipules and leaf sheath absent. Petiole
articulated. Petiole vascular bundle transection annular. Venation pinnate,
brochidodromous, indistinct. Stomata paracytic? Cuticular waxes absent.
Mesophyll with sclerenchymatous idioblasts containing brachysclereids. Leaf
margin entire.
Inflorescence Terminal or
axillary, thyrso-paniculate. Floral prophylls (bracteoles) numerous.
Flowers Actinomorphic, small.
Hypogyny. Sepals five, with imbricate aestivation, connate at base, persistent
and accrescent. Petals five, with imbricate aestivation, early caducous, free,
adnate to sepals. Nectary absent. Disc absent.
Androecium Stamens usually
5+5, diplostemonous (sometimes 3+3+3 or 5+5+5, triplostemonous). Filaments
widened at base and connate into a ring, free from tepals. Anthers dorsifixed,
versatile, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal
slits). Tapetum secretory? Staminodia absent.
Pollen grains
Microsporogenesis simultaneous? Pollen grains usually tri- or
hexacolp(oroid)ate (sometimes hexarugate, rarely dicolp[oroid]ate), shed as
monads, ?-cellular at dispersal. Exine tectate, with columellate infratectum,
echinate to spinulate.
Gynoecium Pistil composed of
(two or) three connate carpels. Ovary superior, usually trilocular (sometimes
bilocular), with usually incomplete septa. Stylodia (two or) three, free or
more or less connate. Stigmas (two or) three, punctuate, type? Pistillodium
absent.
Ovules Placentation
axile(-apical). Ovules usually two (sometimes four or five, rarely more than
five) per carpel, anatropous, pendulous, epitropous, bitegmic, tenuinucellar?
Micropyle ?-stomal. Outer integument ? cell layers thick. Inner integument ?
cell layers thick. Parietal tissue? Nucellar cap absent. Megagametophyte
monosporous, Polygonum type? Endosperm development nuclear? Endosperm
haustoria? Embryogenesis?
Fruit A usually one-seeded
(sometimes many-seeded) thick-walled nut, with persistent staminal filaments
and strongly accrescent winged and leathery to membranous calyx, or an
irregularly dehiscing capsule with weak septa.
Seeds Aril absent. Testa?
Tegmen? Perisperm not developed. Endosperm almost absent. Embryo curved, well
differentiated?, chlorophyll? Cotyledons two, large, spirally twisted.
Germination?
Cytology n = ?
DNA
Phytochemistry Virtually
unknown. Ellagic acid? Alkaloids?
Use Locally used for
timber.
Systematics
Asteropeia (8; A. amblyocarpa, A. densiflora, A.
labatii, A. matrambody, A. mcphersonii, A.
micraster, A. multiflora, A. rhopaloides;
Madagascar).
The sister-group of Asteropeia is
Physena (Physenaceae).
Nakai in J. Jap. Bot. 18: 105. 10 Mar 1942
Genera/species 1/1
Distribution Madagascar.
Fossils Unknown.
Habit Bisexual, evergreen
liana. Blackening when drying.
Vegetative anatomy Phellogen
superficial. Cortical fibres present. Secondary lateral growth anomalous (as
concentric cylinders of vascular bundles in inner pericycle from successive
cambia). Vessel elements with simple perforation plates; lateral pits
alternate. Imperforate tracheary xylem elements tracheids (and libriform
fibres?) with simple pits, non-septate? Wood rays multiseriate, homocellular.
Axial parenchyma paratracheal vasicentric, scanty (sometimes apotracheal,
diffuse). Intraxylary phloem present. Sieve tube plastids P3cf type, with a
polygonal protein crystal surrounded by a ring of protein filaments. Nodes 1:1,
unilacunar with one leaf trace. Sclereids present in primary cortex.
Calciumoxalate sphaeroits, rhomboidal crystals or druses present.
Trichomes Hairs absent?
Leaves Alternate (spiral),
simple, entire, with ? ptyxis. Stipules and leaf sheath absent. Petiole
articulated at base. Petiole vascular bundles? Venation pinnate. Stomata
anomocytic. Cuticular waxes? Leaf margin entire.
Inflorescence Axillary,
racemes.
Flowers Actinomorphic.
Hypogyny. Tepals five, with imbricate quincuncial aestivation, sepaloid,
persistent, free. Nectariferous disc annular.
Androecium Stamens c. 20–25,
in several (four or five?) whorls. Filaments short, free from each other and
from tepals, inserted at annular disc. Anthers subbasifixed, non-versatile?,
tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits).
Tapetum secretory. Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains 3(–6)-colporoidate or
3(–6)-rugate, shed as monads, tricellular at dispersal. Exine tectate, with
columellate infratectum, undulate to verrucate.
Gynoecium Pistil composed of
two connate carpels. Ovary superior, bilocular. Stylodia two, linear, slightly
flattened, directed outwards to upright, somewhat connate at base. Stigmas two,
flattened, papillate adaxially and along margins, type? Pistillodium absent.
Ovules Placentation basal.
Ovule one per carpel, campylotropous, ascending, bitegmic, crassinucellar.
Micropyle ?-stomal. Outer integument ? cell layers thick. Inner integument ?
cell layers thick. Parietal tissue? Megagametophyte monosporous,
Polygonum type. Endosperm development ab initio nuclear. Endosperm
haustoria? Embryogenesis?
Fruit A one- or two-seeded
lignified loculicidal capsule.
Seeds Aril partially enclosing
seed. Seed coat testal? Testal cells elongate, with sinuate anticlinal walls.
Exotesta? Endotesta? Tegmen? Perisperm copious and nutritious. Endosperm
sparse. Embryo peripheral, curved around perisperm, well differentiated.
Cotyledons two. Germination?
Cytology n = ?
DNA
Phytochemistry Virtually
unknown. Betalains? Triterpenoid saponins?
Use Unknown.
Systematics Barbeuia
(1; B. madagascariensis; Madagascar).
Barbeuia is probably sister to the
“globular inclusion clade” minus Lophiocarpaceae and
Hypertelis.
Rafinesque, Fl. Tellur. 3: 44. Nov-Dec 1837
[‘Basellides’], nom. cons.
Anrederaceae J.
Agardh, Theoria Syst. Plant.: 357. Apr-Sep 1858 [’Anredereae’];
Basellineae Nakai in J. Jap. Bot. 18: 108. 10 Mar
1942 [‘Baselliineae’]; Ullucaceae Nakai
in J. Jap. Bot. 18: 109. 10 Mar 1942
Genera/species 4/19
Distribution Tropical East and
southeastern Africa, Madagascar, Indian Ocean islands, tropical Asia to New
Guinea and islands in the Pacific, southeastern United States to tropical South
America.
Fossils Unknown.
Habit Usually bisexual (rarely
monoecious; in Anredera vesicaria functionally unisexual), winding or
climbing perennial herbs or lianas, slightly to distinctly fleshy. Stem bases
and rhizome usually swollen and tuberous; roots sometimes tuberous.
Vegetative anatomy CAM
photosynthesis? Phellogen ab initio outer-cortical. Primary vascular tissue a
single cylinder of vascular strands with intraxylary phloem in larger bundles,
bicollateral (at least in larger bundles); vessels only present in central
portions of fascicular areas. Anomalous secondary lateral growth from
concentric/successive cambia sometimes present. Vessel elements with simple
perforation plates; lateral pits alternate? Imperforate tracheary xylem
elements? Wood rays? Axial parenchyma often ray-adjacent. Intraxylary phloem
present. Sieve tube plastids P3cf type, with a central globular protein crystal
surrounded by a ring of protein filaments. Nodes? Pericycle sclerenchymatous.
Parenchyma with mucilaginous cells. Phloem parenchyma cells with phytoferritin?
Stem epidermis with numerous calciumoxalate druses (sometimes also prismatic
and octaedric crystals).
Trichomes Hairs absent.
Leaves Alternate (spiral) or
nearly opposite (at stem base), simple, usually entire (rarely lobed), often
somewhat fleshy, in Anredera with conduplicate ptyxis. Stipules and
leaf sheath absent. Petiole vascular bundles? Venation pinnate or palmate.
Stomata paracytic or anomocytic. Cuticular wax crystalloids absent. Mesophyll
with mucilaginous idioblasts. Leaf margin usually entire (in Tournonia
glandular-serrate).
Inflorescence Terminal or
axillary, spike, raceme, panicle or dichasium (in Tournonia). Floral
prophylls (bracteoles) 2+2, inner pair median. Sepaloid floral prophylls two,
opposite, persistent, free or partially connate at base.
Flowers Actinomorphic, small.
Hypanthium present. Usually hypogyny (rarely half epigyny). Tepals (four or)
five (to 13), with imbricate quincuncial aestivation, petaloid, persistent,
connate at base to more than half-way up. Nectariferous disc annular,
extrastaminal or intrastaminal.
Androecium Stamens (four or)
five (to nine), often as many as tepals, antetepalous. Filaments widened and
connate at base, in lower part adnate to tepals (epitepalous). Anthers
basifixed to dorsifixed, versatile, tetrasporangiate, introrse, longicidal
(dehiscing by longitudinal slits) or poricidal (dehiscing by apical pores or
pore-like slits). Tapetum secretory, with multinucleate cells. Female flowers
with staminodia.
Pollen grains
Microsporogenesis simultaneous. Pollen grains polypantocolpate or
polypantoporate (often hexacolpate), sometimes cuboid, shed as monads,
tricellular at dispersal. Exine tectate or semitectate, with columellate
infratectum, punctate or reticulate, spinulate.
Gynoecium Pistil composed of
(two or) three connate carpels. Ovary usually superior (rarely semi-inferior),
unilocular (sometimes trilocular at base; in young stages trilocular). Stylodia
usually three, free or connate in lower part (style sometimes single, shortly
lobate). Stigmas usually three, capitate or clavate (sometimes one, trilobate),
type? Male flowers with pistillodium.
Ovules Placentation basal.
Ovule one per ovary or carpel, campylotropous to orthoamphitropous
(anatropous?), ascending, bitegmic, crassinucellar. Micropyle endostomal. Outer
integument ? cell layers thick. Inner integument ? cell layers thick. Parietal
tissue? Nucellar cap/beak present. Megagametophyte monosporous,
Polygonum type. Synergids without filiform apparatus. Antipodal cells
three or more. Endosperm development ab initio nuclear. Endosperm haustoria?
Embryogenesis chenopodiad.
Fruit A thin-walled achene
(utriculus), often sunken into pergamentaceous or carnose persistent perianth
or surrounded by persistent winged remnants of floral prophylls.
Seeds Aril absent. Seed coat
testal-tegmic? Testa membranous. Exotesta and endotesta more or less thickened.
Exotegmen? Endotegmen thickened. Perisperm scarce, surrounded by embryo.
Endosperm usually copious (absent?), with clusters of starch grains. Embryo
annular to cochlear, well differentiated, with chlorophyll. Cotyledons two.
Germination phanerocotylar.
Cytology n = 12, 18, 22, 24
DNA 6 bp deletion in plastid
gene ndhF.
Phytochemistry Flavonol
(quercetin), flavone-C-glycosides, betacyanins, and triterpene
saponins present. Ellagic acid, proanthocyanidins and cyanogenic compounds not
found.
Use Ornamental plants,
vegetables, dyeing of food.
Systematics Anredera (12;
Texas, southern Florida, Central America, the West Indies, tropical South
America to Argentina), Basella (5;
B. paniculata: tropical East and southeastern Africa; B.
excavata, B. leandriana, B. madagascariensis:
Madagascar; B. alba: tropical Asia from India to New Guinea, possibly
introduced in Africa, tropical America and on oceanic islands),
Tournonia (1; T. hookeriana; Colombia), Ullucus (1;
U. tuberosus; the Andes).
Basellaceae may be
sister-group to Didiereaceae (Ocampo &
Columbus 2010; Brockington & al. 2013) or even to the Portulacaria-Ceraria
clade in Didiereaceae
(Arakaki & al. 2011).
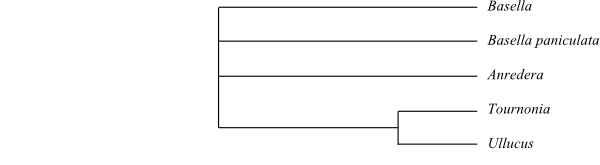 |
Cladogram (simplified) of Basellaceae based on
morphology (Eriksson 2007).
|
de Jussieu, Gen. Plant.: 310. 4 Aug 1789
[’Cacti’], nom. cons.
Opuntiaceae Desv. in
S. Gérardin de Mirecourt et N. A. Desvaux, Dict. Rais. Bot.: 52, 385. 12-19
Apr 1817; Cactales Juss. ex Bercht. et J. Presl,
Přir. Rostlin: 238. Jan-Apr 1820 [‘Cacteae’];
Cereaceae Spreng. ex DC. et Spreng., Elem. Philos.
Bot.: 142. Jul 1821 [‘Cereae’];
Opuntiopsida Endl., Gen. Plant.: 942. Nov 1839
[’Opuntiae’]; Cactopsida Brongn., Enum.
Plant Mus. Paris: xxviii, 105. 12 Aug 1843 [’Cactoideae’];
Opuntiales Endl. ex Willk., Anleit. Stud. Bot. 2:
295. 19-20 Jan 1854 [‘Opuntieae’];
Nopaleaceae Schmid et Curtman, Pflanzenr. 2: 135.
10-11 Jun 1856 [‘Nopaleae’]; Cactineae
Bessey in C. K. Adams, Johnson’s Universal Cyclop. 8: 462. 15 Nov 1895
[‘Cactales’]
Genera/species
131/1.720–1.815
Distribution Mainly arid and
semiarid regions of North and South America (British Columbia and Alberta south
to Patagonia); also epiphytes and lianas in rain forests and other moist
forests (Rhipsalis also in tropical Africa, Madagascar, the
Seychelles, the Mascarenes and Sri Lanka, possibly introduced?).
Fossils Proposed fossils of
Cactaceae are
ambiguous.
Habit Usually bisexual (at
least in Mammillaria dioica and Selenicereus innesii
functionally dioecious), usually perennial herbs (sometimes climbing or
epiphytic; rarely deciduous shrubs or small trees [Rhodocactus,
Pereskia]). Usually xerophytic. Almost all species are stem succulents
with elongate and branched or unbranched, or almost globular pachycaul
photosynthesizing stem; some genera with flattened almost foliaceous stem
segments, phylloclades. Stem and branch surfaces usually with areolae –
modified axillary short shoots, brachyblasts – with numerous spines –
modified leaves or foliar lobes.
Vegetative anatomy CAM
physiology dominating (also C4 physiology present). Roots usually
fibrous or tuberous (Peniocereus and Pterocactus with
napiform nutritious roots). Stem epidermis with thick cuticle and thick cell
walls, usually with parallelocytic stomata. Apical meristem very wide (often c.
0,5–1,5 mm). Phellogen ab initio usually in epidermis (rarely
outer-cortical). Precocious or delayed initiation of stem periderm. Hypodermis
present. Cortex and medulla with or without vascular bundles and usually
modified into water-storing tissue with vacuolized parenchyma cells. Apical
meristem 400–1.500 μm wide. Wide-band tracheid cells usually present in
xylem strands and medulla in particular. Primary vascular tissue a cylinder of
vascular bundles. Secondary lateral growth normal (inner phloem absent;
secondary lateral growth absent in some groups). Vessel elements usually with
simple (rarely reticulate) perforation plates; lateral pits alternate or
pseudoscalariform. Imperforate tracheary xylem elements libriform fibres with
simple pits (also vasicentric tracheids). Wood rays multiseriate?, wide and
tall, starchy. Axial parenchyma usually paratracheal scanty. Thick-walled
pericyclic extraxylary phloem fibre caps present. Sieve tube plastids P3cf
type, with a central globular protein crystalloid and a subspherical ring of
protein filaments, without starch. Nodes 1–?:1–>5, at least unilacunar
with one to many leaf traces (trace often with two or several branches). Cortex
(sometimes also medulla) with mucilaginous idioblasts and often sclereids.
Laticifers in Mammillaria with white latex containing mucilaginous
polysaccharides. Tanniniferous cells absent. Phloem parenchyma cells with
phytoferritin? Idioblasts with crystals. Calciumoxalate (often very frequent)
usually as prismatic crystals or druses (sometimes crystal sand, rarely
raphides). Calciumoxalate as whewellite
(CaC2O4.H2O) or weddellite
(CaC2O4.2H2O). Cuticular wax
crystalloids as platelets or rodlets.
Trichomes Hairs multicellular,
uniseriate, or absent.
Leaves Usually almost absent
(present as microscopical vestiges); in Rhodocactus and
Pereskia usually alternate (spiral); leaves when present simple,
entire and flat (in Rhodocactus and Pereskia) or terete (in
Maihuenia and young plantlets of Opuntioideae); in
Maihuenia and Opuntioideae succulent; in Pereskia
with supervolute ptyxis. Stipules and leaf sheath absent. Leaves with axillary
hairs (in Pereskia, Pereskiopsis and Quiabentia
usually uniseriate) and sometimes multiseriate bristles or scales (hairs in
Cactaceae s. str.
sometimes in areolae). Petiole vascular bundles? Venation pinnate (rarely
palmate) or indistinct. Stomata in Cactaceae parallelocytic, often
opuntioid (subsidiary cells overlapping ends of guard cells). Cuticular wax
crystalloids usually as platelets or rodlets (in Cereus clade as
spiral rodlets) or as continuous crust. Mesophyll with mucilaginous idioblasts.
Leaf margin entire. Extrafloral nectaries frequent at or near areolae in
several genera.
Inflorescence Flowers usually
solitary (rarely two or more) on lateral or subapical areolae (rarely terminal;
in Pereskia cymose or racemose, panicle). Median floral prophylls
absent.
Flowers Usually actinomorphic
(rarely zygomorphic, e.g. Schlumbergera, Zygocactus), often
large. Usually epigyny (rarely half epigyny or hypogyny). Hypanthium present or
absent. Tepals few to many, spiral, with imbricate aestivation
(Rhodocactus, Pereskia), usually petaloid, free or connate
into perianth tube, downwards grading via sepaloid tepals and tepaloid bracts
into scale-like bracts. Nectariferous disc (in, e.g., Pereskia and
Rhipsalis) or nectaries at base of hypanthium and androecium (in some
species of Opuntia on annular or cupular outgrowth at stylar base).
Androecium Stamens c. 20 to
more than 4.000, usually at least initially spiral, later often in one or two
whorls, or more than perianth tube length (staminal primordia initiated in
fascicles and often continuing tepal spiral phyllotaxis). Androecial ring
primordium sometimes present. Filaments free, usually adnate to perianth tube.
Anthers basifixed or dorsifixed, sometimes versatile, tetrasporangiate,
introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory.
Staminodia present in some genera.
Pollen grains
Microsporogenesis simultaneous. Pollen grains tricolpate, hexacolpate or
polypantocolpate (in most Opuntioideae porate), usually shed as monads
(rarely tetrads), tricellular at dispersal. Exine tectate or semitectate, with
columellate infratectum, perforate or reticulate, spinulate (rarely verrucate
or psilate).
Gynoecium: Pistil composed of
three to more than 20 connate carpels. Ovary usually inferior (in
Pereskia superior or semi-inferior), usually unilocular (sometimes
partially multilocular due to secondary septa). Style usually single, simple,
long. Stigma trilobate to more than 20-lobate, non-papillate, Wet type.
Pistillodium usually absent.
Ovules Placentation usually
parietal-laminar (in Rhodocactus and Pereskia basal-laminar).
Ovules numerous per ovary, usually campylotropous or circinotropous (rarely
anatropous), bitegmic, crassinucellar. Micropyle endostomal. Outer integument
usually approx. two (in Cereus three or four) cell layers thick. Inner
integument two (or three) cell layers thick. Parietal tissue approx. one cell
layer thick. Apical cells of megasporangium often radially elongate. Lateral
epidermal cells anticlinally divided. Nucellar cap present. Megagametophyte
monosporous, Polygonum type. Synergids sometimes with a filiform
apparatus. Endosperm development ab initio nuclear. Endosperm haustoria?
Embryogenesis? Polyembryony known from several genera.
Fruit Usually a berry, often
dehiscent (sometimes a nut; in Copiapoa a capsule; sometimes with
persistent areola, hypanthium and/or remnants of tepals). Pericarp not
two-layered.
Seeds Aril (funicular) present
or absent, in Opuntioideae bony. Funicle fleshy, with short apical
hairs. Hilum and micropyle with various shapes (in many Cactoideae
with spongy hilum/micropyle region). Testa sometimes winged. Operculum present
in some representatives. Endotegmic cell walls often thickened. Perisperm
copious and nutritious or almost absent. Endosperm sparse or absent. Embryo
peripheral, usually strongly curved over perisperm (sometimes nearly straight
or spirally twisted), well differentiated to rudimentary, without chlorophyll.
Cotyledons two, usually more or less reduced. Radicula sometimes aborted.
Germination phanerocotylar.
Cytology x = (9) 11 (12) –
Polyploidy frequently occurring in Opuntioideae and
Cactoideae. Adventitious embryony present at least in
Opuntioideae.
DNA Plastid genome in at least
Pereskia with inversion of 6 kb in LSC region, and c. 500 bp deletion
in rbcL (cf. Portulaca!). Plastid gene infA
lost/defunct (Notocactus). Intron absent from Cactoideae
plastid gene rpoC1. 6 bp deletion in plastid gene ndhF.
Phytochemistry Flavonols
(kaempferol, quercetin, isorhamnetin, apigenin, etc.), flavones, flavanones,
dihydroflavonol glycosides, cyanidin, delphinidin, sterols, betacyanins,
betaxanthins, tyramine alkaloids, phenylethylamines, pilocereine, peyote
alkaloids (e.g. mescaline), and tetrahydroisoquinoline alkaloids (e.g.
anhalamine, anhalidine, pellotine and anhalonidine), saponins, sapogenins and
other triterpenes, and phytoferritin (in phloem parenchyma cells) present.
Ellagic acid and cyanogenic compounds not found.
Use Ornamental plants, fruits
(Opuntia, Selenicereus megalanthus, Hylocereus,
Stenocereus, etc.), narcotics (Lophophora williamsii), fences
and hedges (e.g. Opuntia), carpentries.
Systematics Cactaceae are sister-group to
Anacampserotaceae,
according to Brockingty & al. (2013).
The generic and specific delimitations are
notorious in Cactaceae.
Hence, the species numbers within the genera are often highly provisional.
Cactaceae have undergone a
tremendous increase in number of perianth parts as compared to their closest
relatives (Anacampserotaceae,
Portulacaceae,
Talinaceae, Basellaceae, Didiereaceae and Halophytaceae). The
spirally arranged perianth parts in Cactaceae may be bracteal
rather than staminodial in their homology (Ronse De Craene 2007, 2008, etc.).
The perianth may have evolved through differentiation and inclusion of
supernumerary bracts (Ronse De Craene 2008) or through development of
additional bracts. Brockington & al. (2009) suggest that this increase in
perianth parts may be partially the result of a corresponding increase in
meristem size and merosity of reproductive organs.
The subdivision of Opuntioideae and
Cactoideae are according to Nyffeler & Eggli (2010c).
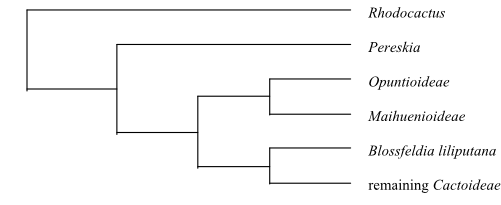 |
Cladogram (simplified) of Cactaceae based on DNA
sequence data (Nyffeler 2007; Ogburn & Edwards 2009; Nyffeler &
Eggli 2010). The clade [Opuntioideae+Maihuenioideae]
collapsed in the Nyffeler & Eggli analysis.
|
Rhodocactus
1/5. Rhodocactus (5; R.
bahiensis, R. grandifolia, R. nemorosa, R.
sacharosa, R. stenantha; Mexico, the Antilles, northwestern South
America, southeastern Brazil). – Phellogen outer-cortical. Precocious
initiation of stem periderm. Stem epidermis without stomata or with
parallel-orientated parallelocytic stomata, opuntioid except innermost cell
pair, with subsidiary cells randomly arranged; leaf stomata randomly
orientated; stomata opening only during rains and at night. Concentric
(stratified) layers of sclereids (often thick-walled) usually present in
phellem of periderm. Wide-band tracheids absent. Hypogyny or half epigyny.
Pollen grains colpate. Placentation basal-laminar.
[Pereskia+[Opuntioideae+[Maihuenia+[Blossfeldia+Cactoideae]]]
Stem cuticle often thick. Epidermis
persistent. Phellogen usually epidermal. Usually delayed initiation of stem
periderm (not in Pereskia aculeata). Stomata usually present in stem
epidermis (absent in P. nemorosa). Stem mucilage cells present.
Cortical sclereids absent.
Pereskioideae Engelm.
in W. H. Brewer et al., Bot. California 1: 243. 1876
[‘Peirescieae’]
1/4. Pereskia (4;
P. aculeata, P. diaz-romeroana, P. horrida, P.
weberiana; the Andes, Central America, northern and central South
America). – Roots sometimes tuberous. Stem stomata parallel-orientated,
opuntioid. Concentric (stratified) layers of sclereids (often thick-walled)
usually present in phellem of periderm. Wide-band tracheids absent. Sclereids
(often thick-walled) present in phloem. Lamina with supervolute ptyxis. Stomata
randomly orientated, opening only during rains and at night. Hypogyny, half
epigyny or epigyny. Stamens with centrifugal development from five primordia.
Pollen grains polycolpate. Placentation basal-laminar.
[Opuntioideae+[Maihuenia+[Blossfeldia+Cactoideae]]]
Stem succulents with very short
internodes. CAM or facultative CAM photosynthesis present. Hypodermis
collenchymatous. Cortical chlorenchyma forming mesophyllar tissue with
intercellular spaces. Wide-band tracheids in secondary xylem at least of
seedlings with annular thickenings. Flowers solitary, axillary. Hypanthium
present. Epigyny. Stamens developing from annular primordium. Placentation
parietal-laminar.
Opuntioideae Burnett,
Outlines Bot.: 742, 1130. Feb 1835 [’Opuntidae’]
18/170–200.
Cylindropuntieae Doweld in Sukkulenty 1(2): 25. 25
Jul 1999. Austrocylindropuntia (8–10; tropical northern South
America), Cumulopuntia (19–20; the Andes), Punotia (1;
P. lagopus; Peru, Bolivia?), Maihueniopsis (1; M.
glomerata; Peru, Bolivia, Chile, Argentina), Quiabentia (2;
Q. verticillata, Q. zehntneri; eastern Brazil, Paraguay,
Bolivia, Argentina), Pereskiopsis (2; P. aquosa, P.
porteri; Mexico, Central America), Micropuntia (1; M.
pulchella; the Mojave Desert in southwestern United States),
'Corynopuntia' (16; southwestern United States, Mexico; polyphyletic),
‘Cylindropuntia’
(20–25; southwestern United States, Mexico, Central America;
non-monophyletic), Grusonia (1;
G. bradtiana; southwestern United States, northwestern Mexico); Tephrocactus
(13; Argentina). – Opuntieae DC., Prodr. 3: 458.
Mar (med.) 1828. Miqueliopuntia (1; M. miquelii; coast of
Chile), Tunilla (12; Peru, Bolivia, Chile, Argentina),
Brasiliopuntia (2; B. brasiliensis, B.
schickendantzii; Peru, Brazil, Paraguay, eastern Bolivia, northern
Argentina), Tacinga (9; Venezuela, northeastern Brazil), ‘Opuntia’
(45–65; southwestern Canada to South America, the Galápagos Islands;
non-monophyletic), Consolea (10; C. corallicola, C.
moniliformis, C. spinosissima; Florida Keys, the West Indies).
– Unplaced Opuntioideae
Pterocactus (9; Patagonia in southern and western Argentina). –
Southwestern Canada to southern Patagonia. Roots often tuberous. Stem terete,
usually articulated, often flattened. Stem stomata parallel-orientated,
opuntioid. Calciumoxalate as whewellite
(CaC2O4.H2O). Leaves usually small,
terete, succulent, caducous, present only on young stems (in
Pereskiopsis large, persistent, bifacial, with lamina and petiole; in
Quiabentia terete, persistent, unifacial). Areolae with glochidia
(minute acicular spines with retrorse barbs). Leaf stomata parallel-oriented.
Subsidiary cells of stomata not or only slightly overlapping ends of guard
cells. Short hypanthium sometimes present. Pollen grains usually polyporate.
Seeds covered by bony funicular aril. Outer exotestal cells with little
thickened periclinal walls. Cotyledons sometimes nutrient storing organs.
Deletion of plastid gene accD. – Cumulopuntia is nested in
Maihueniopsis and Puna sister to this clade in the analyses
by Griffith & Porter (2009).
[Maihuenia+[Blossfeldia+Cactoideae]]
Testa with interstitial craters or pits.
Outer exotestal cells with strongly thickened periclinal walls.
Maihuenioideae P.
Fearn, Rev. Orig. Cactus Fam.: unpaged. [53]. 10 Mar 1996
1/2. Maihuenia (2;
M. patagonica, M. poeppigii; the Patagonian Andes Patagonia
in southern Chile and southern Argentina). Caespitose shrubs. Phellogen
epidermal. Phellogen and cork formation not delayed. epidermis persistent. Stem
usually without stomata. Photosynthetic parenchyma present at base of areolar
crypts. Stomata sometimes present in areolar crypts, parallelocytic. Medulla
and cortex with large mucilage reservoirs. Leaves terete, succulent,
persistent. Leaf with cylindrical reticulum of vascular bundles; external xylem
surrounding central mucilage reservoir. Leaf stomata transversely orientated.
Pollen grains tricolpate. Funicles in fruit long, mucilaginous.
[Blossfeldia+Cactoideae]
Stem stomata parallelocytic, usually
unoriented (in epiphytes transverse). Leaves minute or absent. Pollen grains
tricolpate to polycolpate (sometimes porate). Seeds with conspicuous spongy
hilum-miropyle region. Intron absent from plastid gene rpoC1.
Blossfeldioideae
Crozier in Phytologia 86: 55. 24 Sep 2004
1/1. Blossfeldia (1; B.
liliputana; the eastern Andes in Bolivia and Argentina). Spines absent.
Stem cuticle and epidermal wall thin, early replaced by phellogen.
Photosynthetic parenchyma present at base of areolar crypts. Stem stomata few,
present at base of areolar crypts. Hypodermis absent. Vascular bundles without
cap of phloem fibres. Leaf stomata absent. Seeds round, with strophiolate
funicular aril. Testa with a single short narrow hair per cell. –
Blossfeldia is sister to Cactoideae.
Cactoideae Eaton, Bot.
Dict., ed. 4: 43. Apr-Mai 1836 [‘Cacteae’]
109/1.560–1.625. Southern Canada to
the southwestern United States and southwards (a few species of Rhipsalis
possibly introduced by man into Africa and Madagascar). Some species are
epiphytic. Primary root at least in some Cactoideae with limited
growth. Stem usually ribbed (sometimes tuberculate). Stomata usually
unoriented. Wide-band tracheids sometimes absent. Cortex wide, succulent.
Cortical vascular bundles usually present. Cuticular wax crystalloids in
Cereae as spiral-shaped rodlets. Calciumoxalate often as weddellite
(CaC2O4.2H2O). Leaves absent.
Flowers usually actinomorphic (sometimes zygomorphic). Hypanthium usually
present. Pollen grains tricolpate to polycolpate (sometimes porate). Seeds
usually non-arillate. Testa with interstitial craters or pits. Outer exotestal
cells with strongly thickened periclinal walls. Hypocotyl sometimes nutrient
storing organ. Intron absent from plastid gene rpoC1. Alkaloids
sometimes present.
Nyffeler & Eggli (2009) found the following
topology in Cactoideae:
[Cacteae+[Phyllocacteae+[Rhipsalideae+[Notocacteae+Cereeae]]]].
Cacteae Rchb., Fl.
Germ. Excurs. 2(2): 561. 1832
26/430–440. Geohintonia (1;
G. mexicana; Nuevo Léon in northeastern Mexico; in
Aztekium?), Aztekium (3; A. hintonii, A.
ritteri, A. valdesii; Nuevo Léon in northeastern Mexico, incl.
Geohintonia?), Echinocactus
(15; southwestern United States, Mexico), Astrophytum
(6; A. asterias, A. capricorne, A. caput-medusae,
A. coahuilense, A. myristigma, A. ornatum; the
Chihuahuan desert in southern Texas and northern Mexico); Sclerocactus
(15–19; southwestern United States), Pediocactus (9; western United
States), Echinomastus (6–9; E. erectocentrus, E.
intertextus, E. johnsonii, E. mariosensis, E.
unguispinus, E. warnockii; southwestern United States, northern
Mexico); ‘Ferocactus’
(23; southwestern United States, Mexico; paraphyletic), Leuchtenbergia
(1; L. principis; the Chihuahuan desert in northern Mexico; in Ferocactus?),
Stenocactus
(10; the Chihuahuan desert in Mexico; in Ferocactus?),
Thelocactus
(11; Texas, northern and central Mexico; in Ferocactus?);
Epithelantha (2; E. bokei, E. micromeris; the
Chihuahuan desert in southern Texas, southern Arizona and northern Mexico);
Strombocactus (1; S. disciformis; eastern central Mexico),
Ariocarpus (6; A. agavoides, A. bravoanus, A.
fissuratus, A. kotschoubeyanus, A. retusus, A.
scaphirostris; the Chihuahuan desert in southern Texas and northern
Mexico), ‘Turbinicarpus’ (c 17; northern central Mexico;
paraphyletic); Obregonia (1; O. denegrii; northestern
Mexico), Lophophora (8; southern Texas, northern and eastern Mexico),
Acharagma (3; A. aguirreanum, A. huasteca, A.
roseanum; Coahuila and Nuevo Léon in northern Mexico),
Rapicactus (6; Mexico); Neolloydia (5; N. conoidea,
N. mandragora, N. matehualensis, N. subterranea,
N. texensis; the Chihuahuan desert in Texas and northern Mexico),
Cumarinia (1; C. odorata; Mexico), ‘Escobaria’
(23; southwestern Canada to Mexico, Cuba; diphyletic), Pelecyphora (2;
P. aselliformis, P. strobiliformis; northern central to
northeastern Mexico), Ortegocactus (1; O. macdougallii;
Oaxaca province in southeastern Mexico), ‘Coryphantha’
(c 55; southwestern and southern United States, Mexico, with their highest
diversity in southern United States and northern Mexico; polyphyletic), Mammillaria
(>200; southwestern United States and Mexico to Colombia and Venezuela, the
West Indies, with their highest diversity in Mexico). – North America incl.
Mexico, with their highest diversity in the Chihuahuan desert in southern Texas
and northern Mexico.
[Lymanbensonieae+[Frailea+[Phyllocacteae+[Rhipsalideae+[Notocacteae+Cereeae]]]]]
Lymanbensonieae N.
Korotkova et Barthlott in Willdenowia 40: 166. 9 Dec 2010
3/c 30. Copiapoa (c 25; coastal
deserts in northern Chile), Calymmanthium (1; C. substerile;
Cajamarca in northern Peru), Lymanbensonia (3; L. brevispina,
L. incachacana, L. micrantha; southern Ecuador, Peru,
Bolivia). – Southern Ecuador to Bolivia and northern Chile. –
Lymanbensonieae are sister-group to the clade
[Frailea+[Phyllocacteae+[Rhipsalideae+[Notocacteae+Cereeae]]]],
according to Korotkova & al. (2010).
[Frailea+[Phyllocacteae+[Rhipsalideae+[Notocacteae+Cereeae]]]]
Frailea (26; central South America).
[Phyllocacteae+[Rhipsalideae+[Notocacteae+Cereeae]]]
Phyllocacteae
(Salm-Dyck) Rchb., Deutsch. Bot. Herb.-Buch: 161. Jul 1841
32/335–355. ‘Eulychnia’
(5; E. acida, E. breviflora, E. castanea, E.
iquiquensis, E. procumbens; coastal deserts in Peru and northern
Chile; paraphyletic), Austrocactus (5; A. bertinii, A.
coxii, A. patagonicus, A. philippii, A.
spiniflorus; Chile, southern Argentina), Corryocactus (c 35;
southern Peru, Bolivia, northern Chile), Castellanosia (1; C.
caineana; northeastern Paraguay, Cochabamba and Santa Cruz in Bolivia),
Armatocereus (6; A. cartwrightianus, A. godingianus,
A. laetus, A. matucanensis, A. procerus, A.
rauhii; Colombia, Ecuador, Peru), Leptocereus (15; Cuba,
Hispaniola, Puerto Rico), Neoraimondia (4; N. arequipensis,
N. herzogiana, N. gigantea, N. macrostibas; Peru,
Bolivia, northern Chile), Acanthocereus (6; A. basaniensis,
A. colombianus, A. horridus, A. occidentalis, A.
subinermis, A. tetragonus; Florida, southern Mexico, Central
America to Colombia and Venezuela, the West Indies), Lemaireocereus
(35–40; Mexico to northern South America), Bergerocactus
(1; B. emoryi; coastal areas of San Diego in California and Baja
California), Myrtillocactus (4; M. cochai, M.
eichlamii, M. geometrizans, M. schenckii; Mexico,
Guatemala), Carnegiea (1; C. gigantea; Sonoran desert in
southern Arizona, southeasternmost California and northern Mexico),
Pachycereus (9–13; southwestern United States, Mexico, northern
Central America), Cephalocereus (14; eastern and southern Mexico),
Peniocereus (19; southwestern North America, Mexico, Central America),
Morangaya (1; M. pensilis; southern Baja California Peninsula
in northwestern Mexico), Escontria (1; E. chiotilla; southern
Mexico), Stenocereus
(24; southern United States, Mexico, Central America, the West Indies, northern
South America), Polaskia (2; P. chende, P. chichipe;
southern Mexico), Echinocereus
(55–60; South Dakota to Mexico). – Unplaced
Phyllocacteae Brachycereus (1; B.
nesioticus; the Galápagos Islands), Carnegiea (1;
C. gigantea; Sonoran desert in southern Arizona, southeasternmost
California and northern Mexico), Dendrocereus (2; Cuba: D.
nudiflorus; Hispaniola: D. undulosus), Isolatocereus (1;
I. dumortieri; central to southwestern Mexico), Jasminocereus
(1; J. thouarsii; the Galápagos Islands),
Pseudoacanthocereus (2; P. brasiliensis: Minas Gerais in
Brazil; P. sicariguensis: Lara in Venezuela).–
Hylocereeae Buxb. in Madroño 14: 179. 2 Mai 1958
[‘Hylocereae’]. Epiphyllum (15–20; Mexico, Central
America, the West Indies, northern South America), Pseudorhipsalis (8;
Central America, northern South America), Disocactus (13; southern
Mexico, Central America, the West Indies, Colombia, Venezuela),
Hylocereus (11–13; southern Mexico, Central America, northern
tropical South America), Selenicereus (28; southern United States and
Mexico, Central America, the West Indies, tropical South America),
Strophocactus (3; S. chontalensis, S. testudo,
S. wittii; tropical America; in Selenicereus?),
Weberocereus (9; southern Mexico, Central America to Ecuador). –
Southern United States to southern South America, the West Indies, the
Galápagos Islands.
[Rhipsalideae+[Notocacteae+Cereeae]]
Rhipsalideae DC.,
Prodr. 3: 475. Mar (med.) 1828
4/c 70. Schlumbergera
(9; mountains in southeastern Brazil), Lepismium (16–17; L.
cruciforme, L. houlletianum, L. lorentzianum, L.
lumbricoides, L. warmingianum; eastern Bolivia, southwestern
Brazil, northwestern Argentina), Hatiora (7;
H. cylindrica, H. epiphylloides, H. gaertneri,
H. herminiae, H. pentaptera, H. rosea, H.
salicornioides; eastern and southeastern Brazil), Rhipsalis (c
40; Central America, the West Indies, tropical and subtropical South America,
especially Brazil, one species also in Africa, Madagascar and Sri Lanka). –
Central and South America, Africa to Sri Lanka.
[Notocacteae+Cereeae]
Notocacteae Buxb. in
Madroño 14: 191. 2 Mai 1958
4/125–130. Eriosyce (42;
Peru, Chile), Neowerdermannia (2; N. chilensis, N.
vorwerkii; Peru, Bolivia, Chile, Argentina), Parodia
(80–85; eastern South America), Yavia (1; Y. cryptocarpa;
Jujuy in Argentina). – South America.
Cereeae Salm-Dyck in
Allg. Gartenzeitung 8: 58. 22 Feb 1840 [‘Cereastrae’]
39/545–575.
Coleocephalocereus (9; eastern and southeastern Brazil),
Browningia (13; the Andes in Peru, Bolivia and northern Chile),
Stetsonia (1; S. coryne; Paraguay, Bolivia, Argentina),
Uebelmannia (5; U. buiningi, U. gummifera, U.
horrida, U. meninensis, U. pectinifera; mountains in
eastern Brazil), Cereus (21; the
West Indies, eastern South America), Micranthocereus (11; central and
eastern Brazil), Cleistocactus
(c 40; central Peru to Bolivia and northern Argentina, Paraguay, Uruguay),
Yungasocereus (1; Y. inquisivensis; La Paz in Bolivia),
Samaipaticereus (1; S. corroanus; Santa Cruz in Bolivia),
Matucana (15; Peru), Haageocereus (c 30; deserts in Peru and
northern Chile), ‘Echinopsis’
(c 130; South America; polyphyletic), Acanthocalycium
(5; A. ferrarii, A. glaucum, A. klimpelianum, A.
spiniflorum, A. thionanthum; Argentina; in Echinopsis?),
Gymnocalycium
(c 60; southeastern South America), Harrisia (18; Florida, the West
Indies, northern and eastern South America to Argentina), Oreocereus
(6; O. celsianus, O. doelzianus, O. hempelianus,
O. pseudofossulatus, O. leucotrichus, O. trollii;
Peru, Bolivia, Chile, Argentina), Rauhocereus (1; R.
riosaniensis; northern Peru). – Unplaced
Cereeae Arrojadoa (5; A. dinae,
A. heimenii, A. marylaniae, A. penicillata, A.
rhodantha; Bahia, Minas Gerais and Piaui in eastern Brazil),
Arthrocereus (4; A. glaziovii, A. melanurus, A.
rondonianus, A. spinosissimus; Minas Gerais and Mato Grosso in
Brazil), Brasilicereus (2; B. markgrafii, B.
phaeacanthus; Bahia and Minas Gerais in eastern Brazil),
Cipocereus (6; C. bradei, C. crassisepalus, C.
laniflorus, C. minensis, C. pleurocarpus, C.
pusilliflorus; Minas Gerais in Brazil), Denmoza (1; D.
rhodacantha; western and northwestern Argentina), Discocactus
(11; Brazil, Bolivia), Espostoa (12; Ecuador, Peru, Bolivia),
Espostoopsis (1; E. dybowskii; northern Bahia in eastern
Brazil), Facheiroa (4; F. cephaliomelana, F.
pubiflora, F. squamosa, F. ulei; Bahia in northeastern
Brazil), Lasiocereus (2; L. fulvus, L. rupicola;
Peru)?, Leocereus (1; L. bahiensis; Bahia and Minas Gerais in
Brazil), Melocactus (c
35; western Mexico, Central America, the West Indies, tropical South America to
southern Peru and eastern Brazil), Mila (3; M. caespitosa,
M. colorea, M. nealeana; central Peru), Oroya (7;
O. baumannii, O. borchersii, O. gibbosa, O.
laxiareolata, O. neoperuviana, O. peruviana, O.
subocculta; Peru), Pierrebraunia (2; P. bahiensis,
P. brauniorum; Minas Gerais in eastern Brazil; intergeneric hybrid
incl. Pilosocereus?)?,
Pilosocereus
(c 55; southern United States and Mexico, Central America, the West Indies,
tropical South America), Praecereus (2; P. euchlorus, P.
saxicola; the West Indies, South America), Pygmaeocereus (3;
P. bieblii, P. bylesianus, P. familiaris; Peru), Rebutia
(12–41; southern Bolivia, northwestern Argentina), Cintia (1; C.
knizei; Bolivia; in Rebutia?),
Stephanocereus (2; S. leucostele, S. luetzelburgii;
Bahia in eastern Brazil), Weberbauerocereus
(9; Peru, northern Chile). – Florida and Mexico to South America.
 |
Phylogeny of Cactaceae based on DNA
sequence data (Nyffeler 2002).
|
de Jussieu, Gen. Plant.: 299. 4 Aug 1789
[’Caryophylleae’], nom. cons.
Illecebraceae R. Br.,
Prodr. Fl. Nov.-Holl.: 413. 27 Mar 1810 [’Illecebrearum’
(Illecebreae)], nom. cons.; Paronychiaceae
Juss. in Mém. Mus. Natl. Hist. Nat. 2: 386. 1815
[’Paronychieae’]; Cerastiaceae Vest,
Anleit. Stud. Bot.: 271, 292. 1818 [’Cerastoideae’];
Dianthaceae Vest, Anleit. Stud. Bot.: 271, 293. 1818
[’Dianthoideae’]; Herniariaceae
Martinov, Tekhno-Bot. Slovar: 307. 3 Aug 1820 [’Herniariae’];
Illecebrales R. Br. ex Bercht. et J. Presl, Přir.
Rostlin: 239. Jan-Apr 1820 [‘Illecebreae’];
Ortegaceae Martinov, Tekhno-Bot. Slovar: 443. 3 Aug
1820 [’Ortegiae’]; Saginaceae Bercht. et
J. Presl, Přir. Rostlin: 239. Jan-Apr 1820 [’Sagineae’];
Scleranthales Bercht. et J. Presl, Přir. Rostlin:
240. Jan-Apr 1820 [‘Sclerantheae’];
Stellariaceae Bercht. et J. Presl, Přir. Rostlin:
239. Jan-Apr 1820 [’Stellariae’];
Stellariales Bercht. et J. Presl, Přir. Rostlin:
239. Jan-Apr 1820 [‘Stellariae’];
Telephiaceae Martinov, Tekhno-Bot. Slovar: 633. 3 Aug
1820 [’Thelephides ili Telephides’];
Scleranthaceae J. Presl et C. Presl, Delic. Prag.:
66. Jul 1822 [’Sclerantheae’];
Alsinaceae Bartl. in F. G. Bartling et H. L.
Wendland, Beitr. Bot. 2: 159. Dec 1825 [’Alsineae’], nom. cons.;
Caryophyllopsida Bartl. in Bartl. et Wendl. in Beitr.
Bot.: 137. Dec 1825 [’Caryophyllinae’];
Silenaceae (DC.) Bartl. in F. G. Bartling et H. L.
Wendland, Beitr. Bot. 2: 160. Dec 1825 [’Sileneae’];
Spergulaceae Bartl. in F. G. Bartling et H. L.
Wendland, Beitr. Bot. 2: 158. Dec 1825 [’Sperguleae’];
Corrigiolaceae (Dumort.) Dumort., Anal. Fam. Plant.:
44, 49. 1829; Paronychiales Link, Handbuch 2: 420.
4-11 Jul 1829 [‘Paronychiaceae’];
Telephiales Link, Handbuch 2: 45. 4-11 Jul 1829
[‘Telephiaceae’]; Silenales Lindl., Nix.
Plant.: 14. 17 Sep 1833; Dianthales Burnett, Outl.
Bot.: 1117. Jun 1835 [‘Dianthinae’], nom. illeg.;
Minuartiaceae Mart., Consp. Regn. Veg.: 49. Sep-Oct
1835 [’Minuartieae’]; Polycarpaeaceae
Mart., Consp. Regn. Veg.: 49. Sep-Oct 1835 [’Polycarpaeae’];
Lychnidaceae Döll, Rhein. Fl.: 638. 24-27 Mai 1843
[’Lychnideae’]; Sabulinaceae Döll,
Rhein. Fl.: 623. 24-27 Mai 1843 [’Sabulineae’];
Caryophyllineae Bessey in C. K. Adams, Johnson’s
Universal Cyclop. 8: 461. 15 Nov 1895 [‘Caryophyllales‘]
Genera/species
82–84/2.515–2.660
Images Caryophyllaceae
Distribution Cosmopolitan
although mainly temperate regions in the Northern Hemisphere, the Arctic,
temperate parts of the Southern Hemisphere (including the Antarctic continent),
tropical mountains, with their largest diversity in the Mediterranean and West
and Central Asia.
Fossils The fossilized
inflorescence of Caryophylloflora paleogenica from Tasmania has been
assigned to Caryophyllaceae. Fossil
pollen grains, Caryophyllidites polyoratus, are described from the
Oligocene of New Zealand, and similar pollen fossils have been found in the
Miocene onwards of Europe.
Habit Usually bisexual (rarely
monoecious, andromonoecious, dioecious or gynodioecious), usually perennial,
biennial or annual herbs (sometimes suffrutices; rarely shrubs, small trees or
lianas).
Vegetative anatomy Mycorrhiza
usually absent. Phellogen usually pericyclic (rarely subepidermal or in outer
cortex). Pericyclic fibres present. Secondary lateral growth usually anomalous
(also in roots; sometimes from concentric/successive cambia or from an inner
cylidner of vascular bundles) or absent (sometimes from normal cambium). Vessel
elements with simple perforation plates; lateral pits alternate or
pseudoscalariform. Imperforate tracheary xylem elements usually fibre tracheids
or libriform fibres (in Gymnocarpos tracheids) with usually simple (in
Gymnocarpos bordered) pits. Wood rays usually absent (sometimes
uniseriate or multiseriate, homocellular). Axial parenchyma usually absent
(sometimes as marginal bands). Wood occasionally storied. Wood with idioblasts
containing sphaerites. Intraxylary phloem present in some species. Sieve tube
plastids P3c’f type, with a single polygonal central protein crystal and a
subperipheral dense ring of protein filaments. Endodermis often significant.
Nodes usually 1:1, unilacunar with one leaf trace, often swollen (with
geotropic adjustment). Calciumoxalate as druses or crystal sand.
Trichomes Hairs unicellular or
multicellular, usually uniseriate (rarely branched, dendritic); glandular hairs
present or absent.
Leaves Usually opposite
(rarely verticillate or alternate), simple, entire, sometimes succulent, with
conduplicate or flat ptyxis. Stipules usually absent (sometimes small,
membranous); leaf sheath absent. Leaf bases often connate around stem. Petiole
vascular bundle transection annular, arcuate etc. Venation pinnate. Stomata
usually diacytic or anomocytic (rarely anisocytic). Cuticular wax crystalloids
as rodlets or platelets or as tubuli dominated by β-diketones. Leaf margin
entire. Extrafloral nectaries present or absent.
Inflorescence Usually terminal
or axillary, cymose combinations of dichasia and cincinni, thyrsoid (sometimes
paniculate or capitate; flowers sometimes single).
Flowers Actinomorphic.
Epicalyx consisting of one or more pairs of floral prophylls (bracteoles)
sometimes present. Anthophore (prolonged internode between sepal and ovary, and
between petals and stamens) sometimes present. Usually hypogyny (rarely
perigyny). Sepals (three to) five (to 23), usually with imbricate (rarely
valvate) aestivation, free or more or less connate. Petals (petaloid
staminodia?) usually five (sometimes four), with usually contorted (rarely
imbricate) aestivation, free, often bifid and/or clawed (sometimes fimbriate),
often with a scale-like appendage, ‘corona’, in transition zone between
claw and limb (‘petals’ sometimes absent); ‘corona’(in, e.g.,
Silene) arising from two bulges on adaxial side of ’petals’
(possibly former staminal thecae). Nectariferous disc at staminal bases or in
tube formed by filament bases and petal bases or on inner side of a cupular
receptacle (and sometimes nectar glands on abaxial side of base of episepalous
stamens).
Androecium Stamens usually
five or 5+5 (rarely one to five or more than ten), in one or two whorls,
usually haplostemonous or diplostemonous (rarely 15, obdiplostemonous), usually
antesepalous (rarely alternisepalous). Filaments connate at base or free from
each other, often adnate to tepals (epitepalous). Anthers usually dorsifixed,
versatile, tetrasporangiate, introrse, longicidal (dehiscing by longitudinal
slits). Outer secondary parietal cell dividing. Tapetum secretory, with
binucleate or multinucleate cells. Female flowers often with staminodia.
Pollen grains
Microsporogenesis simultaneous. Pollen grains tricolpate (rarely tricolporate)
or hexa- to polypantoporate (rarely 4–12-colpate, spiraperturate or
triporate), shed as monads, tricellular at dispersal. Exine tectate, with
columellate infratectum, usually punctitegillate (sometimes anulopunctate,
rarely reticulate), spinulate.
Gynoecium Pistil usually
composed of two to five (to ten) connate antepetalous or antesepalous carpels,
sometimes with gynophores; when three carpels then odd carpel adaxial. Ovary
usually superior (rarely semi-inferior), unilocular (below and/or as young
sometimes bi- to quinquelocular). Stylodia two to five, usually filiform,
usually free (sometimes connate in lower part). Stigmatic area along entire or
part of adaxial side of style or only at apex, papillate, Dry type. Male
flowers often with pistillodium.
Ovules Placentation usually
free central (as young often basally axile; rarely basal or on free septa in
upper part; Uebelinia has a basal placenta with nearly circinotropous
ovule). Ovules usually numerous (sometimes one or few) per carpel,
hemianatropous to campylotropous, ascending, bitegmic, tenuinucellar or
crassinucellar. Micropyle endostomal. Outer integument two (to five) cell
layers thick. Inner integument usually two cell layers thick. Archespore
unicellular to tricellular, only one cell developing. Parietal tissue sometimes
three or four cell layers thick. Nucellar cap/beak present. Megagametophyte
usually monosporous, Polygonum type (rarely Allium or
Adoxa type). Synergids sometimes with a filiform apparatus. Antipodal
cells three, ephemeral; antipodal nuclei sometimes early degenerating. Chalazal
caecum developed. Endosperm development ab initio nuclear. Endosperm haustoria
chalazal or absent. Embryogenesis caryophyllad or solanad.
Fruit Usually a septicidal
and/or loculicidal (denticidal) capsule or opening by valves (rarely a
pyxidium, irregularly dehiscing capsule, a nut or a berry), sometimes with
persistent calyx.
Seeds Aril usually absent
(funicular aril present in Moehringia and Petrocoptis).
Operculum absent. Seed coat exotestal. Exotesta usually hard and thick, often
tanniniferous; outer exotestal cell walls with stalactite-shaped processes.
Endotesta sometimes thickened. Exotegmen? Endotegmen usually without rod-shaped
cell wall thickenings, often tanniniferous. Perisperm copious and with starch
grains, surrounded by embryo. Endosperm sparse or absent. Embryo
lateral-peripheral, curved around perisperm (sometimes straight, rarely
spirally twisted), well differentiated, without chlorophyll. Cotyledons two.
Radicula usually dorsal. Germination phanerocotylar.
Cytology x = 5–19 –
Polyploidy often occurring. Protein bodies present in nucleus. Some species of
Silene have a sex determination system with X and Y chromosomes.
DNA Plastid gene infA
lost/defunct (Dianthus, Stellaria). Mitochondrial intron
coxII.i3 lost.
Phytochemistry Flavonols
(kaempferol, quercetin), flavone-C-glycosides, anthocyanins,
triterpene saponins, alkaloids, pinitol, anthraquinones, phytoferritin,
sterols, phytoecdysones, and cyclopeptides present. Ferulic acid present in
non-lignified cell walls. Cyanidin rare. Ellagic acid, betalains and cyanogenic
compounds not found.
Use Ornamental plants,
detergents for washing clothes (Acanthophyllum,
Saponaria).
Systematics Caryophyllaceae are
sister-group to Achatocarpaceae or to the
clade [Achatocarpaceae+ Amaranthaceae].
The petals have possibly androecial origin
(see, e.g., Ronse De Craene & al. 1998, Ronse De Craene 2007 and 2008).
However, it is ambiguous whether the absence of petals is ancestral or whether
loss of petals have occurred several times in Caryophyllaceae.
The subdivision below follows Harbaugh &
al. (2010) and Greenberg & Donoghue (2011), although it is still under
construction (many generic names unplaced). The descriptions of the clades are
mainly according to Stephens and Harbaugh & al. (2010).
Telephioideae
Beilschm. in Flora 16(Beibl. 7): 92, 111. 14 Jun 1833
[‘Telephieae’]
2/16. Corrigiola
(12; nearly cosmopolitan, especially Europe, Africa and Chile), Telephium
(4; T. eriglaucum, T. imperati, T. oligospermum,
T. sphaerospermum; the Mediterranean, northern Africa). –
Subcosmopolitan. Leaves alternate (spiral). Stipules auriculate. Sepal margins
membranous. Ovary with incomplete septa. Embryogenesis solanad. Corrigiola
with thick-walled triangular nutlets. Telephium
with irregularly three-valved and fairly thin-walled capsules. Endotegmic cells
without bar-shaped thickenings. – Telephioideae are sister-group to
the remaining Caryophyllaceae.
Caryophylloideae Arn.,
Botany: 99. 9 Mar 1832 [‘Caryophylleae’]
Leaves opposite.
Paronychieae Dumort.,
Fl. Belg.: 86. 1827
6/110–160. Herniaria
(30–40; Europe, the Canary Islands, the Mediterranean, North Africa, Somalia,
southern Africa, southwestern Asia to India, Bolivia, northern Argentina),
‘Paronychia’
(70–>110; nearly cosmopolitan, especially the Mediterranean, Turkey,
southeastern United States and the central Andes in Peru to Bolivia;
paraphyletic), Chaetonychia
(1; C. cymosa; western Mediterranean; in Paronychia?),
‘Polycarpaea’
corymbosa (tropical and subtropical weed), Gymnocarpos
(10; the Canary Islands to East Asia, northeastern Africa), Cometes
(2; C. abyssinica, C. surattensis; northeastern Africa and
Ethiopia to northwestern India)? – Subcosmopolitan. Stipules paired,
subadaxial to petiole; paired, connate, adaxial; single, concave, adaxial or
interpetiolar. Tepals hooded, with subapical abaxial awn, sometimes with
membranous margins. Petals usually absent (sometimes filiform). Staminodia
usually absent. Embryogenesis solanad. Fruit a nutlet. –
Paronychieae are sister-group to the remaining
Caryophylloideae.
[Polycarpaeae+[Sperguleae+[Rhodalsine+[[Sclerantheae+Sagineae]+[[Arenarieae+Alsineae]+[Caryophylleae+[Sileneae+Eremogoneae]]]]]]]
Corolla present.
Polycarpaeae DC.,
Prodr. 3: 373. Mar (med.) 1828
20/155–165. Cardionema
(6; C. andina, C. burkartii, C. camphorosmoides,
C. congesta, C. kurtzii, C. ramosissimum;
southwestern Canada and western United States to Chile), Dicheranthus
(1; D. plocamoides; the Canary Islands), Pteranthus (1;
P. dichotomus; North Africa and Cyprus to Iran), Illecebrum
(1; I. verticillatum; Europe, the Canary Islands, the Mediterranean),
Loeflingia (4; L. baetica: southern Iberian Peninsula; L.
hispanica: western Mediterranean; L. tavaresiana: southern
Portugal; L. squarrosa: United States, Mexico), ’Polycarpon’
(9; warmer regions of both hemispheres; polyphyletic), ‘Polycarpaea’
(40–50; warmer regions of both hemispheres;
polyphyletic),‘Scopulophila’ (2; S. parryi: Mexico;
S. rixfordii: California, Nevada; diphyletic), Sphaerocoma
(1; S. hookeri; northeastern Sudan, southern Arabian Peninsula, Iran),
Pollichia (1; P. campestris; northern Arabian Peninsula,
Ethiopia, tropical East to southern Africa), Achyronychia (1; A.
cooperi; southwestern United States, Mexico), ‘Drymaria’ (c
55; western United States to Patagonia; the Galapagos Islands, one species,
D. cordata, pantropical; polyphyletic; incl. Cerdia,
Ortegia and Pycnophyllum?), Cerdia (1; C.
virescens; Mexico; in Drymaria?), Ortegia (1; O.
hispanica; the Iberian Peninsula; in Drymaria?),
Pycnophyllum (c 25; the Andes; in Drymaria?),
Krauseola (2; K. gillettii: northern Kenya, southern
Ethiopia; K. mosambicina: Mozambique)?, Microphyes (2; M.
litoralis, M. minima; Chile)?, Pirinia (1; P.
koenigii; southwestern Bulgaria)?, Polytepalum (1; P.
angolense; Angola)?, Stipulicida (1; S. setacea;
southeastern United States, Cuba)? – Subcosmopolitan, with their highest
diversity in drier subtropical regions. Stipules interpetiolar, fimbriate, from
common primordium. Sepals usually hooded, awned, sometimes with membranous
margins. Corolla usually absent (sometimes present, deeply lobate to entire).
Stylodia connate at base. Embryogenesis solanad. Fruit usually a nutlet
(sometimes a capsule). – Polycarpaeae are sister to
Caryophylloideae minus Paronychieae.
[Sperguleae+[Rhodalsine+[[Sclerantheae+Sagineae]+[[Arenarieae+Alsineae]+[Caryophylleae+[Sileneae+Eremogoneae]]]]]]
Wood rays absent. Hypanthium absent.
Stamens ten or more. Nectary position. Placentation sometimes axile at least
basally when ovary young. Fruit usually a septicidal and loculicidal capsule
(sometimes a nutlet). Fruit with two or more seeds.
Sperguleae Dumort.,
Anal. Fam. Plant.: 49. 1829
3/c 65. Spergula
(13; temperate regions in Europe Asia, the Mediterranean, northern Patagonia),
Thylacospermum (1; T. caespitosum; Central Asia, the
Himalayas, western China), Spergularia
(c 50; cosmopolitan). – Cosmopolitan. Stipules single, interpetiolar, connate
and encircling stem below leaves. Sepals with membranous margin. Embryogenesis
solanad. – Thylacospermum caespitosum is sister to Spergula
(Greenberg & Donoghue 2011; Dillenberger & Kadereit 2014).
Spergularia manicata (San Ambrosio Island off Chile) is a small
tree.
[Rhodalsine+[[Sclerantheae+Sagineae]+[[Arenarieae+Alsineae]+[Caryophylleae+[Sileneae+Eremogoneae]]]]]
Wood rays absent. Stipules absent.
Hypanthium absent. Stamens ten or more. Nectary position. Placentation
sometimes axile at least basally when ovary young. Fruit usually a septicidal
and loculicidal capsule (sometimes a nutlet). Fruit with two or more seeds.
Rhodalsine clade
1/1. Rhodalsine (1; R.
geniculata; the Mediterranean, the Canary Islands, Morocco to northern
Egypt, northern Somalia). – Rhodalsine, lacking stipules, is sister
to the remaining Caryophyllaceae
(Dillenberger & Kadereit 2014). Rhodalsine is sister-group to
[Spergula+Spergularia], according to Kool & Thulin
(2017). Thylacospermum caespitosum was not included in their
analysis.
[[[Sclerantheae+Sagineae]+[[Arenarieae+Alsineae]+[Caryophylleae+[Sileneae+Eremogoneae]]]]
Sclerantheae Link ex
DC., Prodr. 3: 377. Mar (med.) 1828
9–10/85–100.
Pseudocherleria (12; the Caucasus and adjacent regions, arctic Asia to
Japan, western North America), Triplateia (1; T.
moehringioides; central Mexico), ‘Stellaria’
pro parte, Wilhelmsia (1; W. physodes; arctic regions in
northeastern Asia and northwestern North America), Honckenya
(1; H. peploides; coasts of temperate regions on the Northern
Hemisphere, southern Patagonia), Schiedea
(26; the Hawaiian Islands), Mononeuria (10; eastern North America,
southwestern Greenland), Scleranthus
(13; temperate regions in Europe and Asia, the Mediterranean, Ethiopia, New
Guinea, Australia), Cherleria
(18; central and southeastern Europe, the Mediterranean, Arctic Eurasia, the
Caucasus, Central Asia, western and Arctic North America),
Pentastemonodiscus (1; P. monochlamydeus; Afghanistan)? –
The Northern Hemisphere, Ethiopia, New Guinea, Australia, Patagonia. Hypanthium
present or absent. Sepals usually without membranous margins. Petals entire or
absent. Stamens one to ten. Staminodia sometimes five. Embryogenesis solanad.
Fruit a capsule dehiscing by as many valves as stylodia, or a single-seeded
nutlet. – Sclerantheae are sister to Sagineae.
Sagineae J. Presl in
Nowočeská Bibl. [Wšobecný Rostl.] 7: 1609, 1621. 1846
[‘Sagusariae’]
10/210–215. Drypis (1;
D. spinosa; the Mediterranean to Lebanon); Bufonia (34; the
Canary Islands, the Mediterranean, the Middle East), Mcneillia
(5; M. graminifolia, M. moraldoi, M.
pseudosaxifraga, M. saxifraga, M. stellata; southern and
southeastern Europe, northwestern Anatolia in Turkey), Minuartia
(c 55; Europe, North Africa, southwestern Asia, the Caucasus, northern India),
Habrosia (1; H. spinuliflora; Syria, Iraq, western Iran),
Minuartiella (4; M. acuminata, M. dianthifolia,
M. elmalia, M. pestalozzae; mountain regions from Anatolia in
Turkey to Nakhichivan in Azerbaijan and northern Iran), Colobanthus (c
25; mountains of southeastern Australia and Tasmania, New Zealand and adjacent
islands, temperate South America and the southern Andes, the Falkland Islands,
the Antarctic Peninsula and adjacent islands, South Georgia, New Amsterdam,
Kerguélen, one species, C. quitensis, also in tropical South America
to Mexico), Sagina (21;
arctic, temperate and alpine regions on the Northern Hemisphere, mountains in
East Africa and New Guinea, the Himalayas, the Andes), Facchinia (7;
F. cerastiifolia, F. cherlerioides, F. grignensis,
F. herniarioides, F. lanceolata, F. rupestris,
F. valentina; alpine areas in Central Europe), Sabulina
(c 60; southern and Central Europe, the Mediterranean, Anatolia, the Caucasus,
southwestern and Central Asia, North America, Chile, Argentina). – Temperate,
arctic and alpine regions on both hemispheres. Hypanthium present or absent.
Sepals sometimes awned, sometimes with membranous margins (in Drypis
hooded). Petals usually entire (in Drypis
bifid; petals rarely absent). Stamens sometimes as many as petals.
Embryogenesis solanad. Fruit usually a capsule dehiscing by as many valves as
stylodia (in Drypis a
single-seeded nutlet). – Sagineae are sister to
Sclerantheae. Drypis may
be sister to the remaining Sagineae, although it is morphologically
aberrant.
[[Arenarieae+Alsineae]+[Caryophylleae+[Sileneae+Eremogoneae]]]
Hypanthium absent. Sepals connate.
Petals clawed, with closed venation and adaxial ligulae. Capsule often
denticidal, with twice as many teeth as stylodia. Embryogenesis usually
caryophyllad.
Arenarieae Kitt.,
Taschenb. Fl. Deutschl., ed. 2, 2: 981. 1844
2/200–>210. Moehringia
(c 30; temperate Europe, Asia and North America), Arenaria
(170–>180; temperate regions in Europe and Asia, mountains in East Africa,
North America, Mexico, Central America). – The Northern Hemisphere, Central
and western South America. Petals entire (rarely absent). Fruit a valvicidal
capsule, dehiscing by twice as many valves as stylodia. Oily funicular aril
present in Moehringia.
Cotyledons incumbent. – Arenarieae are sister-group to
Alsineae.
Alsineae Lam. et DC.,
Syn. Plant. Fl. Gall.: 392. 30 Jun 1806
12/400–410? Lepyrodiclis (3;
L. holosteoides, L. stellarioides, L. tenera; Turkey
to the Himalayas), Pseudostellaria
(17; Europe, Afghanistan, Central Asia and western China to the Korean
Peninsula and Japan, Canada, United States), Odontostemma (c 65;
China, Tibet, Sikkim), Shivparvatia (7; S. ciliolata, S.
forrestii, S. glanduligera, S. ludlowii, S.
ramellata, S. rhodantha, S. stracheyi; northern India,
the Himalayas, southwestern China), Holosteum
(5; H. glutinosum, H. kobresietorum, H. marginatum,
H. polygamum, H. umbellatum; temperate regions in Europe and
Asia, Ethiopia), Moenchia
(4; M. coerulea, M. erecta, M. graeca, M.
mantica; West and Central Europe, the Mediterranean), Cerastium
(180–190?; nearly cosmopolitan), Stellaria
(c 120; Europe, the Mediterranean, mountains in Africa, Asia, few species
cosmopolitan), Brachystemma (1; B. calycinum; the Himalayas,
China, Indochina)?, Pseudocerastium (1; P. stellarioides;
Anhui in eastern China)?, Pycnophyllopsis (4; P. cryptantha,
P. keraiopetala, P. muscosa, P. tetrasticha; the
Andes in Bolivia and Patagonia)?, Thurya (1; T. capitata;
southwestern Asia)? – Subcosmopolitan, with their largest diversity in Europe
to East Asia. Hypanthium usually absent. Sepals sometimes with membranous
margins. Petals usually retuse to deeply bifid (rarely absent). Fruit usually a
denticidal capsule (rarely a single-seeded nutlet). – Alsineae are
sister to Arenarieae.
[Caryophylleae+[Sileneae+Eremogoneae]]
Veins at leaf apex intramarginal. Sepals
connate. Anthophore (prolongation between sepals and remaining floral parts)
sometimes present. Petals with contorted aestivation, clawed, often with retuse
apex, with closed venation. Coronal scale present or absent. Nectary usually
adaxial on stamens. – The sister-group relationships among
Caryophylleae, Eremogoneae and Sileneae are not
clarified.
Caryophylleae Lam. et
DC., Syn. Plant. Fl. Gall.: 386. 30 Jun 1806
13/665–685. Psammosilene (1;
P. tunicoides; Yunnan); Gypsophila (c 150; temperate regions
of Eurasia, eastern Mediterranean, the Middle East, Egypt, Somalia, the Arabian
Peninsula, one species, G. australis, in Australia and New Zealand;
polyphyletic), Saponaria (c 30; temperate regions of Eurasia, with the
highest diversity in the Mediterranean and the Middle East),
Acanthophyllum (90–100; southwestern, southern and Central Asia,
Siberia), Heterochroa (6; H. antoninae, H.
desertorum, H. microphylla, H. petraea, H.
turkestanica, H. violacea; Kazakhstan, western Siberia, Altai,
the Russian Far East, the Kamchatka Peninsula, Mongolia, northern China),
Cyathophylla (2; C. chlorifolia: mountains in Greece and
Turkey; C. viscosa: Azerbaijan), Petroana (2; P.
montserratii: the Iberian Peninsula; P. montana: Somalia, Yemen,
Socotra, Oman), Bolanthus (c 10; southern Bulgaria, Greece, southern
and central Turkey, Syria, Lebanon, Palestine), Psammophiliella (5;
P. filipes, P. floribunda, P. kermanensis, P.
muralis, P. picta; Europe to Central Asia), Balkana (1;
B. spergulifolia; western and central Balkan Peninsula),
Graecobolanthus (8; G. chelmicus, G. creutzburgii,
G. fruticulosus, G. graecus, G. intermedius, G.
laconicus, G. thessalus, G. thymifolius; Greece, Crete),
Petrorhagia (c 30; Europe, the Canary Islands, the Mediterranean,
southwestern and Central Asia to Kashmir), Dianthus (330–340;
temperate regions in Europe and Asia, the Mediterranean, mountains in East
Africa, one species, D. repens, also in northwestern North America).
– Eurasia, Africa, one species in North America and one species in Australia
and New Zealand. “Epicalyx” (consisting of floral prophylls) sometimes
present. Calyx tubular. Sepals without commissural veins. Petals with usually
dextrorse-contorted (sometimes imbricate) aestivation. Pistil composed of two
(or three) connate carpels. n = 12–15, 16. – Psammosilene is
sister to the remaining Caryophylleae (Madhani & al. 2018).
Caryophylleae are possibly sister-group to
[Sileneae+Eremogoneae]. On the other hand, Sadeghian &
al. (2015) recovered Caryophylleae as sister to either
Eremogoneae or Sileneae, although with low
support.
[Sileneae+Eremogoneae]
Sileneae DC., Prodr.
1: 351. Jan (med.) 1824
2/520–530. Agrostemma
(3; A. brachylobum, A. githago, A. gracile;
temperate regions of Europe and Asia, the Mediterranean), Silene
(520–530; Europe, Macaronesia, the Mediterranean, Africa, Asia, North
America, the Hawaiian Islands). – North temperate to subtropical regions,
montane regions in Africa. Calyx tubular. Sepals with commissural veins. Petals
with usually contorted (sometimes imbricate) aestivation. Placentation
sometimes axile at base. – Sileneae are sister-group to
Eremogoneae. – Evolution of protease gene clpP often
accelerated.
Eremogoneae Rabeler et
W. L. Wagner in D. T. Harbaugh et al., Intern. J. Plant Sci. 171: 196. 14 Jan
2010
1–3/90–100. Eremogone
(90–95; temperate regions on the Northern Hemisphere). – Western North
America, Eurasia, the Middle East. Usually perennial herbs. Leaves filiform to
subulate. Hypanthium usually poorly developed. Sepals free (calyx not tubular),
with membranous margins. Ovary usually poorly semi-inferior. Cotyledons
accumbent. – Eremogoneae are sister-group to Sileneae.
Phlebanthia (here included in Eremogone) is sister to
Eremogone, according to Harbaugh & al. (2010). –
Dolophragma (5–7; D. globiflorum, D. juniperinum,
D. oreophilum, D. polytrichoides, D. przewalskii,
D. smithianum; the Himalayas, western China) may be sister to
Eremogone, according to Sadeghian & al. (2015).
Unplaced Caryophyllaceae
Kabulia (1; K.
akhtarii; Afghanistan).
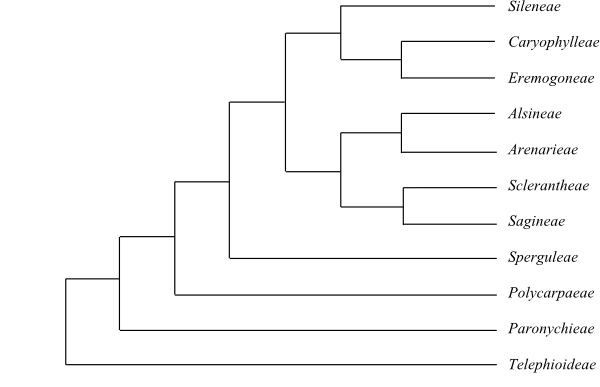 |
Phylogeny of Caryophyllaceae
based on DNA sequence data (Harbaugh & al. 2010; Greenberg &
Donoghue 2011).
|
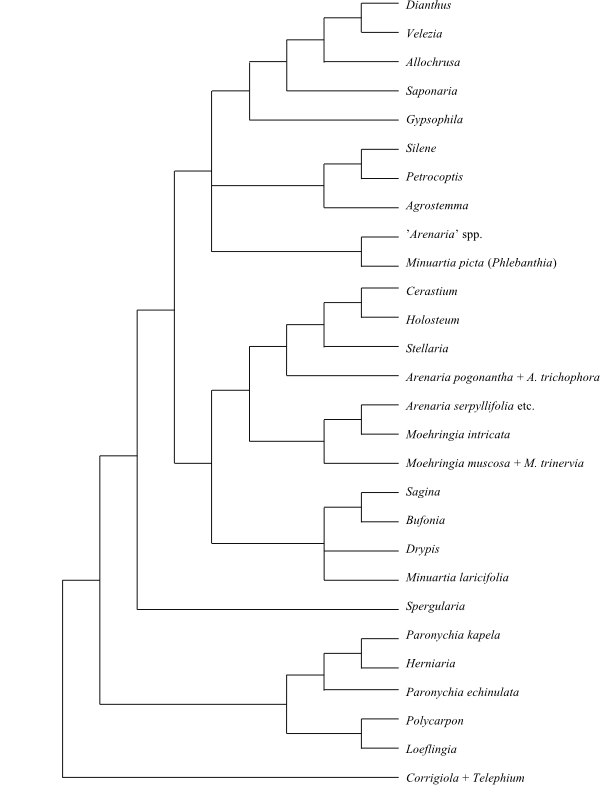 |
Cladogram (simplified) of Caryophyllaceae
based on DNA sequence data (Fior & al. 2006).
|
Radlkofer in Engler et Prantl, Nat. Pflanzenfam.,
III, 5: 462. 23 Apr 1896 [‘Didiereae’], nom. cons.
Portulacariaceae
(Fenzl) Doweld, Tent. Syst. Plant. Vasc.: xlii. 23 Dec 2001
Genera/species 6/20
Distribution Southwestern
Madagascar, southern and eastern Africa.
Fossils Unknown.
Habit Bisexual
(Calyptrotheca, Portulacaria) or dioecious (in
Ceraria and Decaryia rarely gynodioecious), evergreen or
deciduous trees or shrubs (rarely climbing). Many species are cactus-like
xerophytic stem succulents, often with short shoots (brachyblasts) modified
into a small and definite number of spines.
Vegetative anatomy CAM or
facultative CAM photosynthesis present. Stem epidermis with or without
parallelocytic stomata. Phellogen ab initio superficial? Precocious initiation
of periderm. Phellem in at least Ceraria and Portulacaria
with lignified bands consisting of thin-walled flattened cells. Primary
medullary strands wide. Secondary lateral growth normal. Vessel elements with
simple perforation plates; lateral pits alternate to pseudoscalariform or
scalariform, simple pits. Imperforate tracheary xylem elements libriform fibres
with simple pits, non-septate (also vasicentric tracheids). Wide-band tracheids
sometimes present. Wood rays multiseriate, heterocellular. Axial parenchyma
apotracheal diffuse, or paratracheal vasicentric or banded. Thick-walled
pericyclic extraxylary phloem fibre caps present. Sieve tube plastids P3cf
type, with a central globular protein crystal surrounded by a ring of protein
filaments. Intraxylary phloem absent. Nodes 1:1? Cortex and medulla often with
tanniniferous cells (not in Calyptrotheca), mucilage cells and
secretory mucilage ducts. Sclereids (often thin-walled, elongated with
lignified walls) often present. Mucilaginous idioblasts and tanniniferous cells
abundant. Phloem parenchyma cells with phytoferritin? Parenchyma and epidermis
often with numerous prismatic crystals or druses of calciumoxalate.
Trichomes Stem epidermis often
with papillae or multicellular, uniseriate hairs. Spines without trichomes.
Leaves Alternate (spiral) or
opposite, simple, entire (caducous in some species; in Calyptrotheca
succulent), with ? ptyxis. Stipules and leaf sheath absent. Petiole vascular
bundles? Venation usually pinnate (in Ceraria and
Portulacaria palmate). Stomata parallelocytic (transversely
orientated). Cuticular wax crystalloids as cross-bars or rodlets. Mesophyll
with mucilaginous idioblasts. Leaf margin entire.
Inflorescence Terminal or
axillary, dichasium, capitate, fasciculate, panicle etc., or flowers solitary
axillary. Floral prophylls (bracteoles) 2+2, with inner pair (subtending
flower) median. Some genera with two free, sepaloid (sometimes coloured,
petaloid), often persistent floral prophylls subtending flower. Transverse
floral prophylls absent. Sepaloid floral prophylls with imbricate
aestivation.
Flowers Actinomorphic. Usually
hypogyny (rarely half epigyny). Tepals four or five, with imbricate or
decussate aestivation, petaloid, usually free (in Portulacaria connate
into a tube), or absent. Nectariferous disc intrastaminal, annular.
Androecium Stamens four or
five, in one whorl, alternitepalous, or seven to numerous (in
Calyptrotheca up to c. 60), in several whorls; developed from annular
primordium. Filaments free or connate at base into ring of adaxial nectaries,
usually free from tepals (in Portulacaria adnate to sepals,
episepalous). Anthers dorsifixed, often versatile, tetrasporangiate, introrse,
longicidal (dehiscing by longitudinal slits). Tapetum secretory? Female flowers
sometimes with staminodia.
Pollen grains
Microsporogenesis simultaneous? Pollen grains usually polypantoporate or tetra-
to heptazonocolpate (in Ceraria and Portulacaria tricolpate;
in Calyptrotheca polypantoporate), shed as monads, tricellular at
dispersal. Exine tectate, with columellate infratectum (due to fusions?),
microperforate, spinulate and globulate.
Gynoecium Pistil composed of
(two or) three (or four) connate carpels (in Ceraria and
Portulacaria a single carpel). Ovary usually superior (rarely
semi-inferior), usually ab initio trilocular (sometimes bilocular or
quadrilocular), later secondarily unilocular (median/adaxial locule) due to
degeneration of remaining locules. Style single, usually simple (in
Portulacaria trifid). Stigmas more or less peltate, fimbriate (bifid
or) trifid (or quadrifid), type? Male flowers sometimes with pistillodium.
Ovules Placentation basal.
Ovules one to six (Calyptrotheca) per ovary (when carpel adaxial) or
one per carpel, campylotropous to hemicampylotropous, ascending, apotropous,
bitegmic, crassinucellar. Micropyle endostomal?, directed downwardly. Outer
integument ? cell layers thick. Inner integument ? cell layers thick. Parietal
tissue? Megagametophyte monosporous, Polygonum type. Endosperm
development ab initio nuclear. Endosperm haustoria? Embryogenesis
chenopodiad.
Fruit Usually a one-seeded
achene (in some groups enclosed inside two dry sepaloid bracteoles; in
Ceraria a samara; dehiscence in Calyptrotheca circumscissile
at base and splitting upwards into valves in upper part, with strongly
accrescent calyx).
Seeds Seeds with funicular
strophiole or (in, e.g., Calyptrotheca) aril. Testa? Tegmen? Perisperm
sparse or absent. Endosperm entirely or almost entirely absent. Embryo large,
curved around perisperm, well differentiated, chlorophyll? Cotyledons two,
often fleshy. Germination phanerocotylar.
Cytology n = 8–12(–17),
22, 24, 86, 120 – Polyploidy frequently occurring.
DNA 6 bp deletion in plastid
gene ndhF.
Phytochemistry
C-methylated flavonoids, cyanidin, delphinidin, betacyanins,
betaxanthins, and phytoferritin present. Triterpene saponins? Cyanogenic
compounds not found.
Use Ornamental plants.
Systematics Portulacaria
(7; P. afra, P. armiana, P. carrissoana, P.
fruticulosa, P. longipedunculata, P. namaquensis, P.
pygmaea; tropical and southern Africa); Calyptrotheca (2; C.
somalensis, C. taitensis; northeastern tropical Africa);
Alluaudiopsis (2; A. fiherensis, A. marnieriana;
southern and southwestern Madagascar), Alluaudia
(6; A. ascendens, A. comosa, A. dumosa, A.
humbertii, A. montagnacii, A. procera; southern and
southwestern Madagascar), Decarya (1; D. madagascariensis;
southwestern Madagascar), Didierea (2; D. madagascariensis,
D. trollii; southern and southwestern Madagascar).
Didiereaceae may be
sister-group to Basellaceae (Ocampo &
Columbus 2010; Brockington & al. 2013). However, some analyses (e.g.
Arakaki & al. 2011) include Basellaceae in Didiereaceae.
DNA analyses give the topology [Portulacaria+[Calyptrotheca+[Alluaudia+Alluaudiopsis+Decarya+Didierea]]].
Lignified bands consisting of thin-walled
flattened cells are present in the phellem in Ceraria fruticulosa and
Portulacaria afra (Ogburn & Edwards 2009).
The axillary appendages may be remnants of
highly condensed axillary short shoots and homologous to the areolae in Cactaceae (Nyffeler & Eggli
2010).
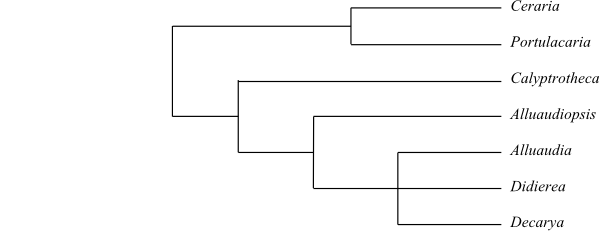 |
Cladogram of Didiereaceae based
on DNA sequence data (Applequist & Wallace 2000; Nyffeler &
Eggli 2010).
|
Airy Shaw in Kew Bull. 6(1951): 333. 26 Jan 1952,
nom. cons.
Dioncophyllales
Takht. ex Reveal in Phytologia 74: 174. 25 Mar 1993
Genera/species 3/3
Distribution Tropical West and
Central Africa.
Fossils Unknown.
Habit Bisexual, evergreen
lianas or climbing and scrambling shrubs (climbing up to c. 70 m high).
Carnivorous (at least Triphyophyllum).
Vegetative anatomy Phellogen?
Cortex with vascular bundles and massive fibrous bands. Secondary lateral
growth in Dioncophyllum anomalous (from concentric/successive cambia).
Vessel elements with simple perforation plates; lateral pits alternate.
Imperforate tracheary xylem elements tracheids and fibre tracheids with
bordered pits (also vasicentric tracheids). Wood rays usually uniseriate,
homocellular. Axial parenchyma apotracheal, diffuse, or paratracheal, scanty
vasicentric. Intraxylary phloem present. Sieve tube plastids S type. Nodes?
Crystals?
Trichomes Glandular hairs
multicellular, insensitive, stalked or sessile, secreting a viscous mucilage
with and without proteolytic enzymes, respectively. Glandular heads in
Triphyophyllum consisting of two layers of secretory cells and inside
these layers an endodermis; glandular stalk usually vascularized (xylem and
phloem).
Leaves Alternate (spiral),
simple, entire, often coriaceous, ’midrib’ leaves in
Triphyophyllum with circinate ptyxis. Stipules and leaf sheath absent.
Rosette leaves long, densely parallelodromous; higher leaves with short lamina
and, in Triphyophyllum, stalked and sessile multicellular vascularized
glandular hairs (with proteolytic enzymes in secretory drips) on prolonged
mid-vein on abaxial side of lamina; terminal leaves (on climbing parts of
shoots) with paired recurved apical barbs (hooks, grapnels).
Dioncophyllum and Habropetalum with simple leaves and barbed
leaves. Triphyophyllum with barbed leaves on older shoots, simple
leaves on short shoots, and abaxially circinate filiform densely glandular
leaves on young short shoots. Petiole vascular bundle transection
crescent-arcuate. Venation usually pinnate (on some leaves of
Triphyophyllum parallelodromous); mid-vein usually prolonged and
dichotomizing in two recurved barb- or hook-shaped tendrils. Stomata
actinocyclocytic or cyclocytic. Cuticular wax crystalloids as platelets. Leaf
margin entire or crenulate.
Inflorescence Axillary or
supra-axillary, cymose, of various shape.
Flowers Actinomorphic, small.
Hypogyny. Sepals five, with open or valvate aestivation, persistent, free or
connate at base into a tube. Petals five, with contorted aestivation, caducous,
free. Nectary absent. Disc absent.
Androecium Stamens ten or 25
to 30 (in Dioncophyllum sometimes five antepetalous). Filaments free
or slightly connate at base, free from tepals. Anthers basifixed,
non-versatile, tetrasporangiate, introrse, longicidal (dehiscing by
longitudinal slits); connective somewhat prolonged. Tapetum secretory.
Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains 3- or 4-colp(or)ate, shed as
monads, ?-cellular at dispersal. Exine tectate, with columellate infratectum,
spinulate or scabrate.
Gynoecium Pistil composed of
two (Triphyophyllum) or five connate carpels; carpels sometimes
opening as seed develops. Ovary superior, unilocular. Stylodia two
(Triphyophyllum) or five, filiform, short, free or slightly connate at
base, or absent. Stigmas two capitate (Triphyophyllum), or five
punctate, capitate or plumose (Triphyophyllum), non-papillate, Dry
type. Pistillodium absent.
Ovules Placentation parietal.
Ovules c. 30 to more than 100 per ovary, anatropous, bitegmic, crassinucellar.
Micropyle exostomal? Outer integument ? cell layers thick, forming an envelope.
Inner integument ? cell layers thick. Parietal tissue? Megagametophyte
monosporous, Polygonum type. Endosperm development nuclear?
Megagametophyte and endosperm transversely elongate. Endosperm haustoria?
Embryogenesis?
Fruit A loculicidal capsule,
dehiscing before maturation with two (Triphyophyllum) or five valves
exposing unripe seeds.
Seeds Seeds flattened, on
elongated stiff funicles, often broadly winged. Aril absent? Seed coat thick.
Testa? Tegmen? Perisperm not developed. Endosperm copious, starchy, surrounding
larger part of embryo. Embryo large, straight, well differentiated,
chlorophyll? Cotyledons two, semicircular. Germination cryptocotylar.
Cytology n = 12, 18?
(Triphyophyllum peltatum)
DNA Intron present in plastid
gene rpl2.
Phytochemistry Betulinic acid,
polyketide-derived naphthyl isoquinoline alkaloids (e.g. ancistrocladine,
dioncophylline, habropetaline and habropetaloic acid), cyclopentenoid
cyanogenic glycosides, naphthoquinones (plumbagin), cis- and
trans-isoshinanolone, and habropetalal present. Ellagic acid?
Use Medicinal plants.
Systematics
Dioncophyllum (1; D. thollonii; Congo, Gabon),
Triphyophyllum (1; T. peltatum; Sierra Leone, Liberia, Ivory
Coast), Habropetalum (1; H. dawei; Sierra Leone).
Dioncophyllaceae are
sister-group to Ancistrocladus (Ancistrocladaceae).
The young leaves in Triphyophyllum
resemble those in Drosophyllum:
circinate and insectivorous. The carnivorous habit is lost in
Dioncophyllum and Habropetalum, or in their common ancestor.
A cladistic treatment of Dioncophyllaceae is
needed in order to answer this question.
Salisbury, Parad. Lond. 2: ad t. 95. 1 Feb 1808
[‘Drosereae’], nom. cons.
Droserales Bercht. et
J. Presl, Přir. Rostlin: 217. Jan-Apr 1820 [‘Drosereae’];
Dionaeaceae Raf., Fl. Tellur. 3: 35. Nov-Dec 1837
[‘Dionidia’]; Aldrovandaceae Nakai in J.
Jap. Bot. 24: 10. Dec 1949
Genera/species 3/probably
>200
Distribution Cosmopolitan
except polar and arid regions, with their largest species diversity in
Australia and New Zealand.
Fossils Seeds assigned to
Aldrovanda (c. fossil 20 species described) have been found in the
Oligocene and the Miocene of Siberia and the Eocene of Europe. Some cenozoic
pollen grains assigned to Droseraceae have been
described under the name of Saxonipollis. Fossil pollen of
Drosera have been reported from the Miocene of New Zealand and
Europe.
Habit Bisexual, perennial or
annual herbs (in Drosera sometimes climbing). Corm or tuberous rhizome
present in some species of Drosera. Most species are hygrophytes;
Aldrovanda is aquatic with submersed leaves. Carnivorous;
Aldrovanda: ‘snaptraps’ with c. 20 trigger hairs per foliar lobe;
Dionaea: ‘snaptraps’ with three trigger hairs per foliar lobe;
Drosera: ‘flypaper traps’.
Vegetative anatomy Mycorrhiza
absent. Main root usually ephemeral and replaced by adventitious roots (roots
absent in mature plants of Aldrovanda). Phellogen? Young stem with
separate vascular bundles in one or two cylinders. Medullary vascular bundles
present in some species of Drosera; medullary rays wide. Cambium and
secondary lateral growth absent. Vessel elements with simple perforation
plates; lateral pits? Imperforate tracheary xylem elements? Wood rays absent.
Axial parenchyma? Sieve tube elements S type. Nodes 1:1, unilacunar with one
leaf trace. Crystals?
Trichomes Eglandular hairs
present as multicellular uniseriate trigger hairs in Aldrovanda,
Dionaea and Drosera glanduligera. Characteristic
multicellular glandular hairs and sessile glands. Glands in Aldrovanda
sessile, non-vascular, quadrifid, adaxial; in Dionaea sessile,
non-vascular, adaxial and stellate, abaxial; and in Drosera stalked,
vascular (with xylem, without phloem), adaxial. Tip of ‘catching glands’ in
Drosera consisting of two layers with endodermis inside.
Leaves Usually alternate
(usually in a basal rosette; in Aldrovanda verticillate), simple,
usually entire (in Drosera sometimes once or several times
dichotomously lobed), often incurved and with adaxially circinate ptyxis;
leaves in Aldrovanda and some species of Drosera bifid,
articulated along mid-vein. Stipules intrapetiolar or absent; leaf sheath
absent. Petiole in Dionaea and some species of Drosera
horizontally widened. Petiole vascular bundle transection annular, arcuate etc.
Venation? Stomata usually anomocytic (sometimes tetracytic or actinocytic) or
absent. Cuticular wax crystalloids as threads (round in cross-section) forming
felt-like covering. Leaf margin usually entire (in Dionaea coarsely
serrate). Lamina in Aldrovanda and Dionaea modified into a
’snaptrap’ with foldable laminal halves provided with sensitive trigger
hairs, or margins and adaxial side of lamina provided with sensitive viscid
long-stalked glandular hairs and sessile glands, secreting proteolytic enzymes;
leaf margins in Drosera bending inwards when touched.
Inflorescence Usually
terminal, thyrsopaniculate, scorpioid or cincinnus (flowers in
Aldrovanda solitary). Bracts and floral prophylls (bracteoles)
sometimes absent.
Flowers Actinomorphic.
Hypogyny. Sepals (four or) five (to eight), with imbricate aestivation,
marcescent, usually connate at base. Petals (four or) five (to eight), with
convolute (imbricate or contorted?) aestivation, short-stalked, marcescent,
free. Nectaries absent? Disc absent.
Androecium Stamens as many as
sepals, antesepalous, (four or) five (Drosera, Aldrovanda) or
ten to 15 (to 20) (Dionaea). Filaments usually free (in
Dionaea connate at base), free from tepals. Anthers basifixed,
non-versatile, tetrasporangiate, usually extrorse (rarely introrse), longicidal
(dehiscing by longitudinal slits); connective sometimes expanded. Tapetum
usually secretory (sometimes amoeboid-periplasmodial). Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains triporate (Aldrovanda)
or 10–30-porate (Dionaea, Drosera), shed as tetrads (in
Drosera usually with radial discs), usually bicellular (sometimes
tricellular) at dispersal. Exine usually tectate (sometimes intectate), with
columellate infratectum, usually spinulate (not in Dionaea), in
Drosera often operculate.
Gynoecium Pistil composed of
three (to five) paracarpous and connate carpels; median carpel abaxial. Ovary
superior, unilocular. Stylodia three (to five), free, in Aldrovanda
and Drosera simple or more or less bilobate (style in Dionaea
single, usually simple). Stigmas three (to five), expanded, capitate,
papillate, Dry type. Pistillodium absent.
Ovules Placentation usually
parietal (in Dionaea basal). Ovules one to numerous per carpel,
anatropous, ascending, bitegmic, usually crassinucellar (in some species of
Drosera tenuinucellar). Micropyle endostomal. Outer integument ? cell
layers thick. Inner integument ? cell layers thick. Megasporangial epidermal
cells enlarged. Parietal tissue often absent. Megagametophyte monosporous,
Polygonum type. Synergids with a filiform apparatus. Megagametophyte
haustorium present. Endosperm development nuclear. Endosperm haustoria?
Embryogenesis caryophyllad to solanad or onagrad to asterad.
Fruit Usually a loculicidal
capsule (in Aldrovanda a nutlet).
Seeds Aril absent. Seed coat
testal (Aldrovanda), exotestal (Dionaea) or endotestal
(Drosera). Exotesta thick (Aldrovanda, Dionaea) or
thin (Drosera), often palisade. Endotestal cell walls occasionally
with U-shaped thickenings. Exotegmen crushed. Endotegmic cells small,
tanniniferous, sclerotized or mucilaginous. Megasporangium in Drosera
strongly prolonged during seed development. Perisperm not developed. Endosperm
copious, oily (and starchy?). Embryo straight, well differentiated, sometimes
short, chlorophyll? Cotyledons two; their tips with haustorial function.
Germination phanerocotylar or cryptocotylar. Radicula usually ephemeral.
Cytology n = 5 or more
(Drosera), n = 16 (Dionaea), n = 24 (Aldrovanda) –
Polyploid and aneuploid series frequent in Drosera (n = 5–8,
10–12, 14–19); chromosomes less than 1,5 µm long (in Dionaea up
to 6 µm long). Drosera and Aldrovanda (not Dionaea)
have diffuse centromeres.
DNA Plastid gene infA
lost/defunct (Dionaea). Intron in plastid gene rpl2 rarely
lost. Mitochondrial coxI intron present (Dionaea).
Phytochemistry Flavonols
(kaempferol, quercetin, myricetin), hyperoside (quercetin
3-O-galactoside), cyanidin, ellagic acid, gallic acid,
proanthocyanidins (prodelphinidins), cyanogenic compounds, and naphthoquinones
(plumbagin, droserone and 7-methyljuglone) present. Alkaloids and saponins not
found.
Use Ornamental plants,
medicinal plants.
Systematics Drosera
(probably >200; cosmopolitan, especially Australia), Dionaea (1;
D. muscipula; North and South Carolina), Aldrovanda
(1; A. vesiculosa; central and eastern Europe, Africa, Asia to
Queensland).
Droseraceae may be sister to
Nepenthes
(Nepenthaceae) or,
possibly, to the clade [Nepenthaceae+[Drosophyllaceae+[Ancistrocladaceae+Dioncophyllaceae]]].
Dionaea is
sister to Aldrovanda.
The two clades share the synapomorphies leaves with ’snaptraps’, sensitive
multicellular trigger hairs, sessile non-vascularized glands, and large smooth
seeds with a thick exotesta. Dionaea
differs from Aldrovanda
by presence of stellate glands, 15 stamens, multiaperturate non-spinulate
pollen grains with alternate pores at tetrad insertion, connate stylodia and
basal placentation.
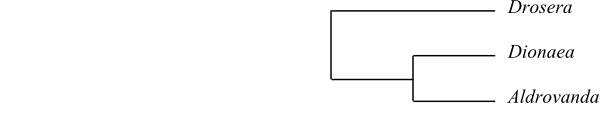 |
Cladogram of Droseraceae based on
DNA sequence data (Cameron & al. 2002).
|
DROSOPHYLLACEAE Chrtek,
Slavíková et Studnička
|
( Back to Caryophyllales
)
|
Chrtek, Slavíková et Studnička in Preslia 61:
122. 10 Apr 1989
Genera/species 1/1
Distribution Portugal,
southern Spain, northern Morocco.
Fossils Unknown.
Habit Bisexual, suffrutex with
long-lived taproot (sometimes a biennial herb). Carnivorous. Leaves with sweet
scent.
Vegetative anatomy Mycorrhiza
probably absent. Phellogen ab initio superficial. Cortical vascular bundles
inverted. Secondary lateral growth normal. Vessel elements with simple
perforation plates; lateral pits alternate, bordered pits. Imperforate
tracheary xylem elements tracheids with bordered pits. Wood rays uniseriate or
biseriate (consisting of mostly upright elements), usually homocellular. Axial
parenchyma apotracheal diffuse (sometimes diffuse-in-aggregates), or
paratracheal scanty vasicentric. Sieve tube plastids S type? Nodes?
Crystals?
Trichomes Eglandular hairs
absent. Stem and leaves covered with rows of stalked mucilage tentacles
(mucilage hygroscopic) and irregularly distributed sessile digesting glands,
otherwise glabrous. Glandular head composed of two layers of secretory cells
and inside these endodermis; glandular stalk vascularized (with xylem and
phloem).
Leaves Alternate (spiral),
simple, entire, linear, with abaxially circinate ptyxis. Stipules and leaf
sheath absent. Petiole vascular bundle transection arcuate. Venation? Stomata?
Cuticular wax crystalloids? Lamina immobile, on margins and abaxial side with
sessile glands, and longitudinal rows of stalked viscid non-sensitive glandular
hairs secreting proteolytic enzymes, and immobile sensitive hairs for capturing
insects. Leaf margin entire?
Inflorescence Terminal,
few-flowered, thyrso-paniculate.
Flowers Actinomorphic, often
large. Hypogyny. Sepals five, with imbricate aestivation, glandular, connate at
base. Petals five, with contorted aestivation, marcescent, free. Nectaries?
Disc absent.
Androecium Stamens 5+5,
antesepalous longer than antepetalous. Filaments free from each other and from
tepals. Anthers basifixed, non-versatile, tetrasporangiate, extrorse,
longicidal (dehiscing by longitudinal slits). Tapetum secretory? Staminodia
absent.
Pollen grains
Microsporogenesis simultaneous? Pollen grains polypantoporate with c. 40 pores,
shed as monads, tricellular at dispersal. Exine tectate, with columellate
infratectum, microperforate, spinulate.
Gynoecium Pistil composed of
five paracarp and connate antesepalous carpels. Ovary superior, unilocular.
Stylodia five, free. Stigmas capitate, papillate, Dry type? Pistillodium
absent.
Ovules Placentation basal.
Ovules numerous per ovary, anatropous, bitegmic, crassinucellar, with long
funicle. Micropyle endostomal. Outer integument ? cell layers thick. Inner
integument ? cell layers thick. Parietal tissue? Megagametophyte monosporous,
Polygonum type? Endosperm development ab initio nuclear. Endosperm
haustoria? Embryogenesis?
Fruit Septicidal and in upper
part loculicidal capsule, with antesepalous valves.
Seeds Aril absent. Seeds
operculate. Seed coat testal-tegmic. Testa thick. Exotesta non-palisade.
Endotestal cells with crystals and U-shaped wall thickenings. Exotegmen
thick-walled. Endotegmen partially sclerotized. Perisperm not developed.
Endosperm copious, fleshy, starchy. Embryo small, cordate, well differentiated,
chlorophyll? Cotyledons two; cotyledon tips with haustorial function.
Germination cryptocotylar.
Cytology n = 6 – Chromosomes
more than 15 µm long (much larger than in Droseraceae). Centromere
localized.
DNA Intron present in plastid
gene rpl2.
Phytochemistry Flavones (e.g.
luteolin), proanthocyanidins, naphthoquinones (plumbagin etc.), and inulin
present.
Use Ornamental plant.
Desvaux in S. Gérardin de Mirecourt et N. A.
Desvaux, Dict. Rais. Bot.: 188. 12-19 Apr 1817 [’Frankeniae’],
nom. cons.
Frankeniales Link,
Handbuch 2: 229. 4-11 Jul 1829 [‘Frankeniaceae’]
Genera/species 1/70–80
Distribution Subtropical and
warm-temperate dry regions.
Fossils Unknown.
Habit Usually bisexual
(sometimes polygamomonoecious), evergreen shrubs, suffrutices or perennial
(sometimes annual) herbs. Usually halophytic or xerophytic, sometimes
gypsophilous or calciphilous.
Vegetative anatomy Mycorrhiza
usually absent. Phellogen subepidermal or pericyclic (intraxylary cork-tissue
present in some species). Secondary lateral growth in some species of
Frankenia anomalous (from concentric/successive cambia). Fibriform
vessel elements present. Vessel elements with simple perforation plates;
lateral pits alternate, simple pits? Imperforate tracheary xylem elements
libriform fibres usually with simple pits (fibres in some species replaced by
axial parenchyma; also vasicentric tracheids). Wood rays absent. Axial
parenchyma? Wood elements partly storied. Sieve tube plastids S type. Nodes?
Crystals?
Trichomes Hairs usually
unicellular, uniseriate, sometimes stellate as tufts; salt glands present.
Leaves Opposite, simple,
entire, linear, often ericoid, with ? ptyxis. Stipules absent; leaves in each
pair fused by a common sheath. Petiole vascular bundles? Leaf single-veined.
Stomata usually anomocytic (sometimes paracytic). Cuticular wax crystalloids
absent. Mesophyll often with sclerenchymatous idioblasts in association with
vascular strands. Epidermis often with salt-secreting glands (consisting of six
crescent cells in two layers of three). Leaf margin entire, usually
reflexed.
Inflorescence Terminal or
axillary, cymose of various shapes, or flowers solitary axillary.
Flowers Actinomorphic, small.
Hypogyny. Sepals usually five (sometimes four, six or seven), with
induplicate-valvate aestivation, persistent, usually connate into a tube (calyx
in Frankenia triandra campanulate or urceolate). Petals usually five
(sometimes four, six or seven), with imbricate aestivation, clawed, usually
with adaxial scale-like appendage, ligule, with nectary on stipe, free. Disc
absent.
Androecium Stamens (three to)
3+3 (to 24). Filaments free or slightly connate at base, free from tepals.
Anthers basifixed, versatile, tetrasporangiate, extrorse, longicidal (dehiscing
by longitudinal slits). Tapetum secretory, with binucleate cells. Inner
staminal whorl rarely staminodial.
Pollen grains
Microsporogenesis simultaneous. Pollen grains (2–)3(–4)-colpate, shed as
monads, tricellular at dispersal. Exine semitectate, with columellate
infratectum, finely reticulate.
Gynoecium Pistil composed of
(one to) three (or four) connate paracarpous carpels; median/odd carpel adaxial
(abaxial?). Ovary superior, unilocular. Style single, elongate, narrow, usually
branched. Stigmas (one to) three (or four), papillate, Dry type. Pistillodium
absent.
Ovules Placentation parietal
or basal-parietal (rarely basal). Ovules (one or) two to six (to numerous) per
ovary, anatropous, ascending, bitegmic, tenuinucellar (pseudocrassinucellar).
Micropyle endostomal. Outer integument two or three cell layers thick. Inner
integument two or three cell layers thick. Parietal tissue absent. Nucellar cap
present. Megagametophyte monosporous, Polygonum type. Synergids with a
filiform apparatus. Endosperm development ab initio nuclear. Endosperm
haustorium bicellular or quadricellular, coenocytic, micropylar. Embryogenesis
solanad. Polyembryony sometimes present.
Fruit A loculicidal apicidal
capsule with persistent calyx.
Seeds Aril usually present.
Seed coat exotestal? Exotestal cells large; cell wall papillae with terminal
nail-like thickenings. Endotestal cells thin-walled. Exotegmen? Endotegmen with
thick cuticle, tanniniferous. Perisperm not developed? Endosperm copious,
starchy. Embryo large, straight, chlorophyll? Suspensor absent. Cotyledons two.
Germination phanerocotylar.
Cytology n = 5, 10, 15
DNA Intron present in plastid
gene rpl2.
Phytochemistry Flavonol
bisulphates (kaempferol, quercetin), cyanidin, tannins, proanthocyanidins
(prodelphinidins), and pinitol present. Cyanogenic compounds, alkaloids,
saponins and myricetin not found. Sulphated compounds frequently present.
Ellagic acid?
Use Ornamental plants, fish
poison.
Systematics Frankenia
(70–80; subtropical and warm-temperate dry regions, especially on sea-shores,
in southern Europe, northernmost and southernmost Africa, Macaronesia, the
Mediterranean, southwestern Asia and Australia, St. Helena, southwestern United
States, southwestern South America, with their largest diversity in
Australia).
Frankenia
is sister to Tamaricaceae.
Frankenia margaritae has a single
carpel with one ovule.
Nakai in J. Jap. Bot. 18: 102. 10 Mar 1942
Genera/species 1/7
Distribution Tropical and
subtropical regions in the Old World.
Fossils Unknown.
Habit Usually bisexual (rarely
unisexual), usually annual (sometimes perennial) herbs.
Vegetative anatomy Phellogen
absent? C4 photosynthesis present. Kranz anatomy atriplicoid.
Secondary lateral growth absent. Vessel elements? Imperforate tracheary xylem
elements? Wood rays absent. Axial parenchyma? Sieve tube plastids P3cf type?
Nodes unilacunar? with ? leaf traces. Calciumoxalate raphides present.
Trichomes Hairs?
Leaves Usually opposite
(rarely almost verticillate), simple, entire, with ? ptyxis. Stipules and leaf
sheath absent. Petiole vascular bundles? Venation pinnate. Stomata? Cuticular
waxes? Leaf margin entire. Epidermis with calciumoxalate raphides.
Inflorescence Axillary or
terminal, leaf-opposed panicle (sometimes umbel-like cyme) with dichasial
partial inflorescences.
Flowers Actinomorphic, small.
Hypogyny. Tepals five, with imbricate-quincuncial aestivation, sepaloid, free.
Nectariferous disc flattened.
Androecium Stamens usually
five or ten to 15 (rarely eight, sometimes 20 or more, in fascicles of three),
alternitepalous. Filaments widened at base, free. Anthers basifixed,
non-versatile, tetrasporangiate, introrse?, longicidal (dehiscing by
longitudinal slits). Tapetum secretory. Female flowers with staminodia.
Pollen grains
Microsporogenesis simultaneous. Pollen grains tricolpate to polypantoporate,
shed as monads, tricellular at dispersal. Exine tectate, with columellate
infratectum, punctate or perforate, scabrate, spinulate or smooth.
Gynoecium Pistil composed of
(three to) five (to ten) carpels, whorled, antetepalous, seemingly free
(pseudapocarpy). Partial ovaries superior, unilocular. Stylodia short, ventral.
Stigmas? Male flowers with pistillodium.
Ovules Placentation basal.
Ovule one per carpel, anatropous (campylotropous?), bitegmic, crassinucellar.
Micropyle ?-stomal. Outer integument three cell layers thick. Inner integument
? cell layers thick. Parietal tissue approx. two cell layers thick. Nucellar
cap approx. three cell layers thick. Megasporangial cells radially expanded.
Megagametophyte monosporous, Polygonum type. Synergids with a filiform
apparatus. Endosperm development ab initio nuclear. Endosperm haustoria?
Embryogenesis?
Fruit An achene with
membranous epicarp covered by warts or prickles (sometimes with wing-like
lateral processes).
Seeds Aril absent. Exotestal
cells tangentially elongate. Endotesta? Exotegmic cells thickened. Endotegmen?
Perisperm copious and nutritious. Endosperm poorly developed or absent. Embryo
peripheral, curved around perisperm, well differentiated?, chlorophyll?
Suspensor apically curved. Cotyledons two. Germination phanerocotylar?
Cytology x = 9
DNA
Phytochemistry Very
insufficiently known. Betacyanins and betaxanthins present. Anthocyanins?
Triterpene saponins?
Use Unknown.
Systematics Gisekia
(7; G. africana, G. diffusa, G. haudica, G.
paniculata, G. pharnaceoides, G. polylopha, G.
scabridula; tropical and subtropical regions in Africa, Madagascar, the
Mascarene Islands, southern Asia to southeastern China and Indochina, with
their highest diversity in East Africa).
Gisekia may be closely allied to
Petiveriaceae, but
its position is uncertain. It may be part of a Gisekia-Sarcobatus-Phytolaccaceae-Petiveriaceae-Agdestis-Nyctaginaceae clade.
Soriano in Bol. Soc. Argent. Bot. 23: 161. 29 Aug
1984
Genera/species 1/1
Distribution Argentina.
Fossils Unknown.
Habit Monoecious, annual herb.
Leaf succulent.
Vegetative anatomy Phellogen?
Cortex succulent. Secondary lateral growth anomalous (from
concentric/successive cambia). Vessel elements? Imperforate tracheary xylem
element libriform fibres with bordered pits? Extraxylary fibres present. Wood
rays absent from secondary xylem. Axial parenchyma? Sieve tube plastids P3cf
type, with a globular central protein crystal surrounded by a subperipheral
dense ring of protein filaments. Nodes 1:1? Phloem parenchyma cells with
phytoferritin? Calciumoxalate druses present.
Trichomes Hairs absent.
Leaves Alternate (spiral),
simple, usually entire (sometimes absent), succulent, subterete, flattened on
adaxial side, convex on abaxial side, with ? ptyxis. Stipules and leaf sheath
absent. Petiole vascular bundles? Leaf single-veined. Stomata paracytic or
anomocytic? Cuticular waxes? Leaf margin entire. Young buds surrounded by two
opposite scale-like leaves (prophylls?).
Inflorescence Male flowers
numerous in a terminal, dense, spike-like, bracteate inflorescence. Female
flowers four or five together in axillary fascicles (or female flowers in
reality solitary axillary?) in uppermost four or five leaf axils. Male flowers
with two bracts or floral prophylls (bracteoles; transverse floral prophylls
absent). Each female flower sunken into cortex and enclosed by one (or two?)
small bracts and two very unequally sized median? floral prophylls.
Flowers Actinomorphic, small.
Hypogyny? Tepals four in male flowers, with valvate-decussate aestivation,
somewhat petaloid, membranous, whorled, free or connate at base. Female flowers
without tepals.
Androecium Stamens four,
alternitepalous. Filaments filiform, free from each other and from tepals.
Anthers dorsifixed to subbasifixed, protruding, versatile, tetrasporangiate,
extrorse, poricidal (dehiscing by pores due to contraction of connective).
Endothecial anticlinal cell walls with frame-like thickening. Tapetum
secretory? Staminodia absent.
Pollen grains
Microsporogenesis simultaneous? Pollen grains hexaporate, cuboid (with one pore
on each side), shed as monads, ?-cellular at dispersal. Exine tectate, with
columellate infratectum (fusions?), punctate, microspinulate.
Gynoecium Pistil composed of
three connate carpels; median adaxial carpel fertile. Ovary superior,
unilocular. Style single, simple. Stigmas three, finally protruding, papillate,
type? Pistillodium absent.
Ovules Placentation basal.
Ovule one per ovary, anatropous (campylotropous?), bitegmic, crassinucellar.
Micropyle ?-stomal. Outer integument ? cell layers thick. Inner integument ?
cell layers thick. Parietal tissue? Megagametophyte monosporous,
Polygonum type. Endosperm development ab initio nuclear. Endosperm
haustoria? Embryogenesis?
Fruit Thin-walled and nutlike.
Hardening inflorescence axis enclosing several nutlets aggregated into a dry
syncarp.
Seeds Aril absent. Testa?
Tegmen? Perisperm well developed and nutritious (with polygonal and unusually
large starch grains when compared to other Caryophyllales),
surrounded by embryo. Endosperm almost absent. Embryo annular, chlorophyll?
Cotyledons two. Germination phanerocotylar?
Cytology n = 12
DNA
Phytochemistry Very
insufficiently known. Betanin (a tyrosine-derived glucose glycoside of
betanidin) present. Proanthocyanidins not found.
Use Unknown.
Christenhusz in Phytotaxa 181: 240. 8 Oct 2014
Genera/species 1/8
Distribution Eastern and
southern Africa, Madagascar, St. Helena.
Fossils Unknown.
Habit Bisexual herbs or
suffrutices.
Vegetative anatomy Phellogen?
Secondary lateral growth? Vessel elements? Imperforate tracheary xylem
elements? Wood rays? Axial parenchyma? Sieve tube plastids P3cf type, with
central globular protein crystal surrounded by ring of protein filaments.
Nodes? Calciumoxalate crystals as clusters and crystal sand.
Trichomes Hairs absent.
Leaves Alternate (spiral,
often pseudoverticillate), simple, entire, linear, terete, with ? ptyxis.
Stipules membranous, adnate to dilated leaf base forming a sheath; leaf sheath
absent. Petiole vascular bundles? Leaf one-veined. Stomata? Cuticular wax
crystalloids as rodlets. Leaf margin entire.
Inflorescence Terminal (often
seemingly axillary), umbel-like thyrse.
Flowers Actinomorphic.
Hypogyny. Tepals five, with imbricate aestivation, often petaloid, persistent,
free. Nectaries present on adaxial side of inner staminal filaments. Disc
absent.
Androecium Stamens (three to)
five to 15 (to c. 30), diplostemonous. Filaments connate at base, free from
tepals. Anthers basifixed, non-versatile, tetrasporangiate, introrse,
longicidal (dehiscing by longitudinal slits). Tapetum secretory? Staminodia
absent.
Pollen grains
Microsporogenesis simultaneous? Pollen grains tricolpate?, shed as monads,
?-cellular at dispersal. Exine tectate?, with columellate? infratectum,
sculpturing?
Gynoecium Pistil composed of
three to five connate antesepalous carpels. Ovary superior, trilocular to
quinquelocular. Style absent. Stigmas three to five, papillate?, type?
Pistillodium absent.
Ovules Placentation axile.
Ovules numerous per carpel, ?-tropous, bitegmic?, crassinucellar? Micropyle
?-stomal. Outer integument ? cell layers thick. Inner integument ? cell layers
thick. Obturator? Parietal tissue? Megagametophyte monosporous,
Polygonum type? Endosperm development ab initio nuclear, finally
cellular. Endosperm haustoria? Embryogenesis solanad.
Fruit A loculicidal membranous
capsule or a schizocarp with nutlike mericarps.
Seeds Aril absent. Operculum
present. Seed coat? Exotesta? Endotesta? Exotegmen? Endotegmic cell walls with
rod-shaped thickenings? Perisperm copious, hard, starchy. Endosperm sparse or
absent? Embryo peripheral?, curved around perisperm?, well differentiated?,
without chlorophyll? Cotyledons two. Radicula dorsal? Germination?
Cytology n = 8
DNA
Phytochemistry Virtually
unknown. Anthocyanins present. Betalains absent?
Use Unknown.
Systematics Kewa (8; K.
angrae-pequenae, K. arenicola, K. bowkeriana, K.
caespitosa, K. salsoloides: eastern and southern Africa; K.
suffruticosa: Madagascar; K. acida: St. Helena).
According to Brockington & al. (2013), Kewa
(‘Hypertelis’ in their paper) belongs in a clade also comprising
Lophiocarpaceae,
Barbeuiaceae, Aizoaceae, Gisekia, Sarcobatus,
Agdestis, Phytolaccaceae, Nyctaginaceae, and Petiveriaceae. Kewa is here
successive sister to the remaining “globular inclusion clade” minus Lophiocarpaceae.
The genus comprises all the former species of
Hypertelis except the type Hypertelis spergulacea
and some recently recombined speciespreviously included
inMollugo;
(Christin & al. 2011, Christenhusz & al. 2014, Thulin & al.
2016).
Shipunov ex Reveal in Bot. Rev. (Lancaster) 71:
128. 20 Mai 2005
Genera/species 1/c 25
Distribution Sub-Saharan
Africa, the Arabian Peninsula, southern Asia east to India.
Fossils Unknown.
Habit Bisexual, perennial
herbs or suffrutices with lignified basal parts and often green
photosynthesizing branches.
Vegetative anatomy Phellogen?
Secondary lateral growth usually anomalous (from meristematic rings of
concentric cambia in phloem or pericycle; rarely normal). Vessel elements with
simple? perforation plates; lateral pits alternate? Imperforate tracheary xylem
elements? Wood rays absent? Axial parenchyma? Sieve tube plastids P3c’’f
type, with a central cuboidal protein crystal surrounded by a ring of protein
filaments. Nodes? Calciumoxalate usually as druses.
Trichomes Hairs usually
absent; glandular hairs present in some species.
Leaves Alternate (spiral),
simple, entire, with ? ptyxis. Stipules and leaf sheath absent. Petiole
vascular bundles? Venation? Stomata? Cuticular waxes? Leaf margin entire.
Inflorescence Terminal,
raceme-like to capitate, cymose.
Flowers Actinomorphic.
Hypogyny. Sepals five, free. Petals usually five (absent in some species),
free. Nectary absent? Disc absent.
Androecium Stamens (five to)
seven (to ten). Filaments connate at base, adnate to tepals (epitepalous).
Anthers dorsifixed, versatile?, tetrasporangiate, extrorse, longicidal
(dehiscing by longitudinal slits). Tapetum secretory. Staminodia absent.
Pollen grains
Microsporogenesis simultaneous? Pollen grains 3(–4)-colpate, shed as monads,
?-cellular at dispersal. Exine tectate, with columellate infratectum, punctate,
smooth or spinulate.
Gynoecium Pistil composed of
two to seven connate antesepalous carpels. Ovary superior, pseudomonomerous,
two carpels being initiated: adaxial carpel sterile and much smaller than
abaxial carpel, in which two ovules are developed and separated by secondary
septum. Stylodia two or three or absent. Stigmas two or three, filiform, type?
Pistillodium absent.
Ovules Placentation axile.
Ovules one to three per carpel, anatropous, bitegmic, crassinucellar. Micropyle
endostomal. Outer integument ? cell layers thick. Inner integument ? cell
layers thick. Obturator placental. Parietal tissue? Megagametophyte
monosporous, Polygonum type. Antipodal cells often persistent.
Endosperm development ab initio nuclear. Endosperm haustoria? Embryogenesis
caryophyllad.
Fruit A membranous capsule or
a dimerous schizocarp with nutlike (sometimes winged) mericarps.
Seeds Dry aril/elaiosome
(funicular) present or absent. Seed coat? Testa with cells in rows along dorsal
suture. Tegmen? Perisperm copious, starchy. Endosperm almost absent. Embryo
peripheral, curved around perisperm?, chlorophyll? Cotyledons two.
Germination?
Cytology n = 9 (Limeum
indicum)
DNA
Phytochemistry Virtually
unknown. Anthocyanin present.
Use Unknown.
Doweld et Reveal, New Syllabus Pl. Fam.: 795. Jan
2007
Corbichoniaceae Thulin
in Taxon 65(4): 790. Aug 2016
Genera/species 2/6
Distribution Sub-Saharan
Africa, southern Asia.
Fossils Unknown.
Habit Bisexual, suffrutices or
perennial herbs (in Corbichonia often with procumbent stems).
Vegetative anatomy Phellogen?
Secondary lateral growth usually normal (sometimes anomalous from concentric
meristematic rings in phloem or pericycle). Vessel elements with simple?
perforation plates; lateral pits alternate? Imperforate tracheary xylem
elements? Wood rays absent? Axial parenchyma? Sieve tube plastids P3cf type,
with a central globular protein crystal surrounded by a ring of protein
filaments. Nodes 1:1, unilacunar with one leaf trace. Calciumoxalates as druses
or sphaeroites.
Trichomes Hairs?
Leaves Alternate (spiral),
simple, entire, somewhat fleshy, with ? ptyxis. Stipules and leaf sheath
absent. Petiole vascular bundles? Venation pinnate? Stomata? Cuticular wax
crystalloids as platelets. Leaf margin entire.
Inflorescence Terminal or
seemingly leaf-opposite, cymose (Corbichonia), or raceme or spike
arranged in groups of usually three (sometimes two) partial inflorescences
(Lophiocarpus).
Flowers Actinomorphic.
Hypogyny. Sepals (four or) five, with usually quincuncial aestivation, back and
front sepals covering remaining ones (Corbichonia), free. Petals 15 to
numerous, with valvate aestivation, free (Corbichonia), or absent
(Lophiocarpus). Nectariferous disc present.
Androecium Stamens four
(Lophiocarpus; occasionally seemingly three) or ten to c. 30
(Corbichonia). Filaments free from each other and from tepals. Anthers
basifixed, elongate (Lophiocarpus), non-versatile, tetrasporangiate,
introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory
(bicellular or tricellular). Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains tricolpate, shed as monads,
tricellular at dispersal. Exine tectate, with columellate infratectum,
perforate to finely punctate, spinulate.
Gynoecium Pistil composed of
two (Lophiocarpus) or five (Corbichonia) connate antesepalous
carpels. Ovary superior, unilocular (Lophiocarpus) or quinquelocular
(Corbichonia). Style single, simple, very short
(Corbichonia), or absent (Lophiocarpus). Stigmas four
(Lophiocarpus), type? Pistillodium absent.
Ovules Placentation axile
(Corbichonia) or basal (Lophiocarpus). Ovule one per ovary
(Lophiocarpus) or numerous per carpel (Corbichonia),
campylotropous (Lophiocarpus), bitegmic, thinly crassinucellar.
Micropyle ?-stomal. Outer integument ? cell layers thick. Inner integument ?
cell layers thick. Obturator placental. Parietal tissue two or three cell
layers thick. Megagametophyte monosporous, Polygonum type. Endosperm
development ab initio nuclear. Endosperm haustoria? Embryogenesis solanad
(Corbichonia) or caryophyllad (Lophiocarpus).
Fruit An achene or one-seeded
drupe (Lophiocarpus) or a loculicidal capsule
(Corbichonia).
Seeds Funicular aril present
(Corbichonia) or absent. Seeds with small strophiole
(Corbichonia). Seed coat? Exotestal cells radially elongate.
Endotesta? Tegmen? Perisperm copious and nutritious. Endosperm almost absent.
Embryo peripheral, curved around perisperm, chlorophyll? Cotyledons two.
Germination?
Cytology n = ?
DNA
Phytochemistry Very
insufficiently known. Anthocyanins and saponins present in
Corbichonia. Betalains absent.
Use Unknown.
Systematics
Corbichonia (2; C. decumbens, C. rubriviolacea;
tropical and southwestern Africa, southwestern and southern Asia),
Lophiocarpus (4; L. dinteri, L. latifolius, L.
polystachyus, L. tenuissimus; Namibia, South Africa,
Botswana).
Lophiocarpaceae are
sister to the remaining members of the “globular inclusion clade” also
comprising Hypertelis, Barbeuiaceae, Aizoaceae, Gisekia, Sarcobatus,
Agdestis, Phytolaccaceae, Nyctaginaceae, and Petiveriaceae.
The sieve tube plastids in
Lophiocarpus are similar to those of Phytolaccaceae. The ovary
organization resembles that in Amaranthaceae.
Christenhusz in Phytotaxa 181: 240. 8 Oct 2014
Genera/species 1/9
Distribution Australia.
Fossils Unknown.
Habit Bisexual, shrubs or
suffrutices with lignified basal parts and often green photosynthesizing
branches.
Vegetative anatomyPhellogen?
Secondary lateral growth anomalous (from meristematic cylinders of concentric
cambia in phloem or pericycle). Vessel elements with simple? perforation
plates; lateral pits alternate? Imperforate tracheary xylem elements? Wood rays
absent? Axial parenchyma? Sieve tube plastids P3c’’fs type. Nodes 1:1?
Calciumoxalate usually as druses.
Trichomes Hairs usually
absent.
Leaves Alternate (spiral),
simple, entire, often reduced and scale-like, with ? ptyxis. Stipules and leaf
sheath absent. Petiole vascular bundles? Venation? Stomata? Cuticular waxes?
Leaf margin entire.
Inflorescence Terminal,
raceme-like to capitate, cymose.
Flowers Actinomorphic, often
small. Hypogyny. Sepals five, free. Petals five, free, or absent. Nectary
absent? Disc absent.
Androecium Stamens 4+4.
Filaments connate at base, adnate to tepals (epitepalous). Anthers dorsifixed,
versatile?, tetrasporangiate, extrorse, longicidal (dehiscing by longitudinal
slits). Tapetum secretory. Staminodia absent.
Pollen grains
Microsporogenesis simultaneous? Pollen grains 3(–4)-colpate, shed as monads,
?-cellular at dispersal. Exine tectate, with columellate infratectum, punctate,
smooth or spinulate.
Gynoecium Pistil composed of
three to seven connate antesepalous carpels. Ovary superior, with two carpels
initiated: adaxial carpel sterile and much smaller than abaxial carpel, in
which two ovules develop and are separated by secondary septum. Stylodia two or
three or absent. Stigmas two or three, filiform, type? Pistillodium absent.
Ovules Placentation
basal-axile. Ovules one to three per carpel, anatropous, bitegmic,
crassinucellar. Micropyle endostomal. Outer integument ? cell layers thick.
Inner integument ? cell layers thick. Obturator placental. Parietal tissue?
Megagametophyte monosporous, Polygonum type. Antipodal cells usually
ephemeral. Endosperm development ab initio nuclear. Endosperm haustoria?
Embryogenesis?
Fruit A membranous loculicidal
capsule.
Seeds Dry aril/elaiosome
(funicular) present. Testa with cells in rows along dorsal suture. Tegmen?
Perisperm copious, starchy. Endosperm almost absent. Embryo peripheral, curved
around perisperm?, chlorophyll? Cotyledons two. Germination?
Cytologyn = ?
DNA
Phytochemistry Very
insufficiently known. O-glycosylflavonoids and anthocyanins present.
Betalains not found.
Use Unknown.
Systematics Macarthuria
(9; M. apetala, M. australis, M. complanata, M.
ephedroides, M. georgeana, M. intricata, M.
keigheryi, M. neocambrica, M. vertex; southwestern,
northern and eastern Australia).
Macarthuria
is sister to the core Caryophyllales
(“beyond” the [Asteropeia+Physena] clade) (Brockington
& al. 2013).
Schäferhoff et Borsch in Willdenowia 39: 223.
Dec 2009
Genera/species 1/9
Distribution Mexico, Central
America, the West Indies.
Fossils Unknown.
Habit Bisexual, usually annual
herbs (occasionally perennial and somewhat lignified at base).
Vegetative anatomy Phellogen?
Secondary lateral growth usually normal (sometimes anomalous from concentric
meristematic cylinders in phloem or pericycle). Vessel elements with simple?
perforation plates; lateral pits alternate? Imperforate tracheary xylem
elements? Wood rays absent? Axial parenchyma? Sieve tube plastids P3f type
without central protein crystal and with central starch grain. Nodes 1:1,
unilacunar with one leaf trace. Calciumoxalates as druses or sphaeroites or
absent?
Trichomes Hairs absent.
Leaves Alternate (spiral),
simple, entire, somewhat fleshy, with ? ptyxis. Stipules and leaf sheath
absent. Petiole vascular bundles? Venation pinnate? Stomata? Cuticular wax
crystalloids as platelets. Leaf margin entire.
Inflorescence Terminal or
seemingly leaf-opposite, thyrsoid, with racemes or spikes arranged in groups of
usually three (sometimes two) partial inflorescences. Floral prophylls
(bracteoles two or absent).
Flowers Actinomorphic.
Hypogyny. Tepals (four or) five, with quincuncial aestivation, free.
Nectariferous disc present.
Androecium Stamens (two to)
five to eight (or nine), alternitepalous. Filaments free from each other and
from tepals. Anthers basifixed to dorsifixed, non-versatile, tetrasporangiate,
introrse, longicidal (dehiscing by apical slits). Tapetum secretory. Staminodia
absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains 9–29-polypantoporate, shed as
monads, tricellular? at dispersal. Exine tectate, with columellate infratectum,
perforate to finely punctate, microspinulate.
Gynoecium Pistil composed of
two to five connate carpels (with various orientation). Ovary superior,
unilocular (without traces of a suture). Stylodia two to five. Stigmas two to
five, papillate, type? Pistillodium absent.
Ovules Placentation free
central. Ovule one per ovary, basal, campylotropous, bitegmic, crassinucellar.
Micropyle ?-stomal. Outer integument ? cell layers thick. Inner integument ?
cell layers thick. Obturator placental. Parietal tissue? Megagametophyte
monosporous, Polygonum type. Endosperm development ab initio nuclear.
Endosperm haustoria? Embryogenesis?
Fruit A muricate to spiny
achene.
Seeds Funicular aril present.
Testa crustaceous. Tegmen? Perisperm copious and nutritious. Endosperm almost
absent. Embryo peripheral, curved around perisperm, chlorophyll? Cotyledons
two. Germination?
Cytology n = ?
DNA
Phytochemistry Virtually
unknown. Anthocyanins? Betalains?
Use Medicinal plants.
Systematics Microtea
(9; M. debilis, M. glochidiata, M. longebracteata,
M. maypurensis, M. paniculata, M. portoricensis,
M. scabrida, M. sulcicaulis, M. tenuifolia; Baja
California to Central America, the West Indies)
Microtea is sister to the remaining
core Caryophyllales
“above” Macarthuriaceae,
according to Brockington & al. (2013).
The sieve tube plastids resemble those in
Amaranthaceae.
Bartling in F. G. Bartling et H. L. Wendland,
Beitr. Bot. 2: 158. Dec 1825 [’Mollugineae’], nom. cons.
Pharnaceaceae
Martinov, Tekhno-Bot. Slovar: 477. 3 Aug 1820 [’Pharnaceae’];
Glinaceae Mart., Consp. Regn. Veg.: 64. Sep-Oct 1835
[’Glinoideae’]; Adenogrammaceae (Fenzl)
Nakai in J. Jap. Bot. 18: 101. 10 Mar 1942;
Polpodaceae (Fenzl) Nakai in J. Jap. Bot. 18: 109. 10
Mar 1942
Genera/species 11/c 90
Distribution Mainly tropical
and subtropical regions, with their highest diversity i southern Africa, some
species in warm-temperate areas.
Fossils Unknown.
Habit Usually bisexual (in
Mollugo ulei dioecious), usually annual or perennial herbs (sometimes
suffrutices). Often somewhat succulent.
Vegetative anatomy
C4 photosynthesis (‘Hypertelis’) or intermediates
between C3 and C4 photosynthesis sometimes present.
Phellogen? Secondary lateral growth usually anomalous (from concentric cambia
as successive meristematic cylinders in phloem or pericycle) or absent.
Pericyclic fibres present. Vessel elements with simple perforation plates;
lateral pits alternate?, bordered pits. Imperforate tracheary xylem elements?
Wood rays usually absent. Axial parenchyma? Sieve tube plastids P3cf type, with
a central globoid protein crystal surrounded by a ring of protein filaments (in
Glinus P3cfs type, with starch grains). Nodes 1:1, unilacunar with one
leaf trace. Calciumoxalate usually as single crystals, lumps or druses (rarely
as crystal sand, rhomboidal crystals or raphides).
Trichomes Hairs usually absent
(in Glinus stellate hairs; in Mollugo ulei glandular
hairs).
Leaves Usually alternate
(spiral), often as a basal rosette or pseudoverticillate (sometimes opposite),
simple, entire, often somewhat succulent or ericoid, with ? ptyxis. Stipules
large, membranous and sometimes lobed, small or absent; leaf sheath absent.
Petiole vascular bundles? Venation pinnate. Stomata usually anomocytic
(sometimes paracytic, diacytic or anisocytic?). Cuticular wax crystalloids in
as platelets or rodlets. Leaf margin entire.
Inflorescence Usually terminal
or seemingly axillary, cymose of various shape (in Polpoda axillary,
few-flowered, cymose, or flowers solitary). Prophylls often prominent.
Flowers Actinomorphic, small.
Usually hypogyny (in Coelanthum half epigyny). Tepals usually five (in
Polpoda four), with imbricate aestivation, usually sepaloid (sometimes
petaloid), persistent, usually free (in Coelanthum connate at base).
Petaloid staminodia five to c. 20 or absent. Nectaries usually on adaxial side
of inner staminal filaments (absent in Polpoda). Nectariferous disc
annular or absent.
Androecium Stamens (two to)
four to ten (to c. 30, in fascicles), haplostemonous or diplostemonous, usually
antetepalous (in Polpoda alternitepalous) or – when more than five
– in alternitepalous and epitepalous whorls. Filaments widened at base and
usually connate into a tube, free from tepals. Anthers basifixed or dorsifixed,
often versatile, tetrasporangiate, introrse, longicidal (dehiscing by
longitudinal slits). Tapetum secretory. Staminodia petaloid or absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually tricolpate (sometimes
tetracolpate; in Mollugo sometimes polypantocolpate or
polypantoporate), shed as monads, tricellular at dispersal. Exine tectate, with
columellate infratectum, tubuliferopunctate, scabrate-punctate or spinulate.
Gynoecium Pistil composed of
usually two (in, e.g., Polpoda) to five (rarely more than five)
connate carpels (in Adenogramma a single carpel); carpels antetepalous
or median carpel adaxial. Ovary usually superior (rarely semi-inferior),
usually bilocular to quinquelocular (in Adenogramma unilocular,
monomerous). Style single, simple, or stylodia two to five, short, usually free
(in Polpoda connate at base), or absent. Stigma single, bilobate to
quinquelobate, or stigmas two to five, adaxially papillate, type? Pistillodium
absent?
Ovules Placentation axile.
Ovule one seemingly basal (in Adenogramma, Polpoda and
Psammotropha) to numerous per carpel or ovary, (hemi)anatropous to
(ana)campylotropous, bitegmic, crassinucellar. Micropyle endostomal. Outer
integument two cell layers thick. Inner integument two cell layers thick.
Obturator funicular. Parietal tissue? Megagametophyte monosporous,
Polygonum type. Endosperm development ab initio nuclear, finally
cellular. Endosperm haustoria? Embryogenesis solanad.
Fruit A loculicidal capsule or
dehiscing by transverse slits (in Adenogramma a nutlet).
Seeds Aril present or absent.
Funicle short. Operculum sometimes present. Seed coat exotestal-endotegmic.
Exotestal cells indistinguished in shape. Endotesta and exotegmen crushed.
Endotegmic cell walls with rod-shaped thickenings. Perisperm copious, hard,
starchy. Endosperm sparse or absent. Embryo peripheral, curved around
perisperm, well differentiated, without chlorophyll. Cotyledons two. Radicula
dorsal. Germination?
Cytology n = 9, 18
(Glinus) – Polyploidy occurring in Mollugo s.lat. (n = 18,
27, 32, 36).
DNA
Phytochemistry Insufficiently
known. Flavonols (kaempferol?, quercetin?), C-glycosyl-flavonoids,
proanthocyanidins, anthocyanins, hopane saponins, and cyanogenic compounds
present. Betalains not found in investigated species.
Use Medicinal plants,
vegetables.
Systematics
Trigastrotheca (3; T. molluginea, T. pentaphylla,
T. stricta; tropical and subtropical Asia and Australia),
Mollugo (c 15; tropical to warmtemperate regions in North to South
America, one species, M. disticha, in India and Sri Lanka),
Glinus (c 10; tropical to warmtemperate regions on both hemispheres),
Paramollugo (6; P. nudicaulis: tropical Africa, Madagascar,
Yemen, Socotra, tropical Asia; P. angustifolia: Somalia; P.
decandra, P. elliotii, P. simulans: Madagascar; P.
digyna: New Caledonia), Hypertelis (5; H. cerviana,
H. fragilis, H. spergulacea, H. umbellata, H.
walteri; southwestern and southeastern Europe, tropical and subtropical
regions, South Africa), Polpoda (2; P. capensis, P.
stipulacea; Western Cape), Psammotropha (11; tropical and
southern Africa), Adenogramma
(10–11; Northern and Western Cape), Suessenguthiella (1; S.
scleranthoides; Namibia, Northern and Western Cape), Coelanthum
(3; C. grandiflorum, C. semiquinquefidum, C.
verticillatum; southern Namibia, Northern and Western Cape), Pharnaceum
(c 28; southern Africa).
Molluginaceae are
sister-group to the clade [[[Montiaceae+Halophytaceae]+[Didiereaceae+Basellaceae]]+[Talinaceae+[Portulacaceae+[Anacampserotaceae+Cactaceae]]]] (Brockington
& al. 2013).
‘Mollugo’
is highly polyphyletic, as shown by Christin & al. (2011). It should
perhaps be split into at least eight different taxa.
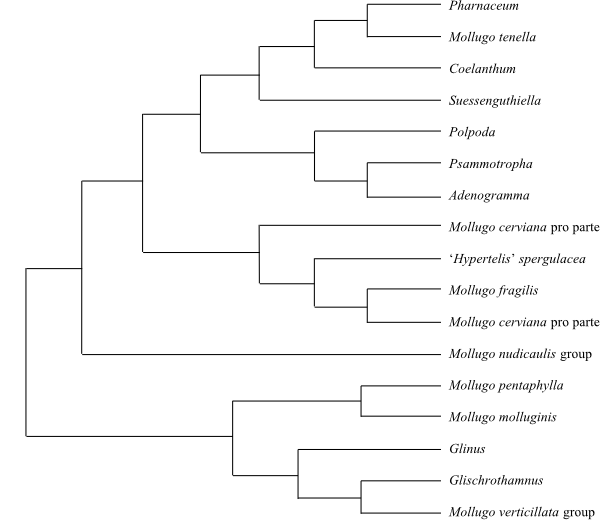 |
Phylogeny (simplified) of Molluginaceae based
on DNA sequence data (Christin & al. 2010).
|
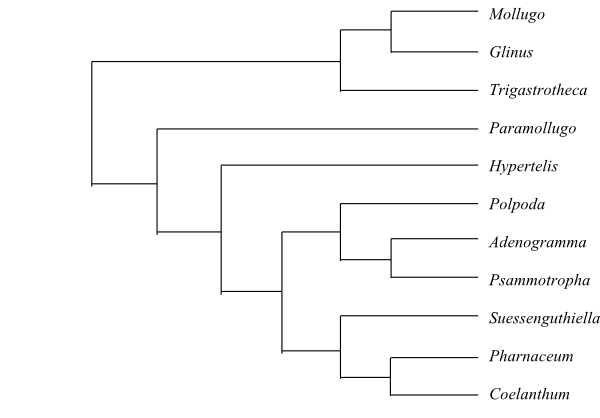 |
Phylogeny (simplified) of Molluginaceae based
on DNA sequence data (Thulin & al. 2016).
|
Rafinesque in Ann. Gén. Sci. Phys. Bruxelles 5:
349. Jul-Sep 1820 [’Montidia’]
Hectorellaceae
Philipson et Skipworth in Trans. Roy. Soc. New Zealand Bot. 1: 31. 20 Sep
1961
Genera/species c
10/285–290
Distribution Cosmopolitan
except polar areas (mainly temperate regions on the Northern Hemisphere),
Australia, Tasmania, New Zealand, Kerguélen, North and South America, with
their largest diversity in western North America, western South America and
southern Australia.
Fossils Unknown.
Habit Usually bisexual (rarely
unisexual), perennial or annual herbs (rarely suffrutices), often succulent.
Roots sometimes tuberous. Certain species? of Lewisia
(’Erocallis’) with corm. Stem frequently absent. Some species are
xerophytic.
Vegetative anatomy Mycorrhiza
absent. At least some genera with CAM photosynthesis. At least some species
with stomata present in stem epidermis. Phellogen ab initio superficial,
present near stem apex. Precocious or delayed initiation of stem periderm;
periderm usually absent. Endodermis sometimes prominent, with casparian dots
(Montia). Primary vascular tissue usually a cylinder of bundles.
Cortical and medullary parenchyma early degenerating. Secondary lateral growth
normal, poor (without concentric cambia or inner phloem), or absent. Vessel
elements with simple perforation plates; lateral pits alternate,
pseudoscalariform, or intermediate between pseudoscalariform and helical
thickenings. Imperforate tracheary xylem elements libriform fibres;
non-septate. Wood rays multiseriate or absent. Axial parenchyma? Thick-walled
pericyclic extraxylary phloem fibre caps absent. Sieve tube plastids P3cf type,
with a central globular protein crystal surrounded by a ring of protein
filaments. Nodes 1:1, unilacunar with one leaf trace. Sclereids absent. Stem
usually without mucilaginous idioblasts. Tanniniferous cells absent. Phloem
parenchyma cells with phytoferritin? Crystal sand or druses of calciumoxalate
often present; stem epidermis often with calciumoxalate crystals. At least some
genera with CAM photosynthesis.
Trichomes Hairs unicellular or
multicellular, uniseriate (sometimes glandular), or absent.
Leaves Alternate (spiral) or
opposite, simple, entire, often perfoliate, often fleshy, with ? ptyxis.
Stipules and leaf sheath absent. Leaves without paired axillary hairs, bristles
or scales. Petiole vascular bundles? Venation pinnate? Stomata brachyparacytic
(longitudinally orientated, with two subsidiary cells surrounding but not
completely enclosing guard cells; sometimes parallelocytic or anomocytic).
Cuticular wax crystalloids as procumbent platelets. Mesophyll usually with
mucilaginous idioblasts (absent in Claytonia and Parakeelya).
Sclereids absent. Crystals abundant. Leaf margin entire.
Inflorescence Terminal or
axillary, dichasia and/or monochasia (sometimes head-, spike or raceme-like),
often scorpioid, or flowers solitary axillary. Floral prophylls (bracteoles)
usually two or 2+2 (inner pair median; rarely three, up to nine
[Lewisia] or absent), with imbricate aestivation, lateral, apical or
basal, at equal or different heights, usually persistent and dry in fruit, free
or connate at base (transverse floral prophylls sometimes absent).
Montiopsis sometimes with trilobate involucral bracts.
Flowers Usually actinomorphic
(rarely zygomorphic). Hypogyny. Tepals (two or) three to five (to seven; in
Lewisia up to 19), in one or two whorls, with imbricate aestivation,
petaloid, persistent or caducous, usually free (rarely connate at base), or
absent. Nectariferous disc present.
Androecium Stamens usually
three to five (rarely six, seven or up to c. 100; in Lewisia up to 19;
in Calandrinia/Monocosmia and Calyptridium one), in
one whorl, usually antetepalous (in Lyallia alternitepalous).
Filaments free or connate (all together or in fascicles), free from tepals or
adnate at base. Anthers dorsifixed, versatile, tetrasporangiate, introrse,
longicidal (dehiscing by longitudinal slits). Tapetum secretory, with
multinucleate cells. Staminodia usually absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains tri- to polyzonacolpate, tri- to
polyzonacolporate, tri- to polyzonaporate, polypantoporate, -rugate or
-foraminate (in ’Montiastrum’ tholate, polypantocolpate; centres
of mesocolpi vaulted forming spiniferous upraised tholi), shed as monads,
tricellular at dispersal. Exine tectate, with columellate infratectum,
punctate, spinulate.
Gynoecium Pistil composed of
two to four (to nine) connate carpels. Ovary superior, unilocular. Style
single, simple, or stylodia two to four (to nine), free or connate. Stigmas
capitate or lobate, papillate, Dry type. Pistillodium usually absent.
Ovules Placentation basal to
free central. Ovules two to more than 100 per ovary, anatropous to
amphitropous, bitegmic, crassinucellar. Micropyle endostomal. Outer integument
? cell layers thick. Inner integument ? cell layers thick. Parietal tissue?
Megagametophyte monosporous, Polygonum type. Synergids sometimes with
a filiform apparatus. Antipodal cells three, ephemeral. Endosperm development
ab initio nuclear. Endosperm haustoria? Embryogenesis caryophyllad or
solanad.
Fruit Usually a two- or
three-valved capsule and/or circumscissile at base (Lewisia,
Lewisiopsis; elastically or passively dehiscent; rarely poricidal;
sometimes irregularly dehiscent; sometimes a one-seeded capsular utriculus, in
Lenzia indehiscent or tardily dehiscent, in Philippiamra
irregularly dehiscent or indehiscent; in Lyallia one- or two-seeded
successively disintegrating indehiscent capsules). Fruit sometimes with
deciduous calyptra formed by dry perianth and staminal remnants.
Seeds Aril usually absent (in
Phemeranthus carnose or chartaceous). Seed coat testal. Strophiole or
elaiosome present or absent. Outer exotestal cell walls thickened, with
stalactite-shaped processes. Endotesta? Tegmen? Perisperm copious, starchy.
Endosperm sparse or absent. Embryo peripheral, curved and surrounding
perisperm, well differentiated, without chlorophyll. Cotyledons usually two
(rarely three or four). Radicula dorsal. Germination phanerocotylar.
Cytology n = 6–13 or more
– Claytonia virginiana shows extreme variation in chromosome number
with n = 6 to c. 95; Lyallia: n = 48.
DNA 6 bp deletion in plastid
gene ndhF.
Phytochemistry Flavonols
(kaempferol), cyanidins, betalains (betacyanins, betaxanthins), alkaloids, and
triterpene saponins present. Ellagic acid and cyanogenic compounds not found.
Free oxalates often accumulated.
Use Ornamental plants,
vegetables (Claytonia perfoliata).
Systematics
Phemeranthus (18–20; southern Canada, United States, Mexico; incl.
’Talinum’ pro
parte); Cistanthe (c
40; America; incl. Lenzia? and Montiopsis?),
Lenzia (1; L. chamaepitys; Chile; in Cistanthe?),
Montiopsis (18; western South America, with the highest diversity in
Chile; in Cistanthe?), ’Calandrinia’
(>150; Australia, western North America, western South America, especially
Chile; non-monophyletic), Schreiteria (1; S. macrocarpa;
Argentina; in Calandrinia?),
Parakeelya (2; P. nana, P. spergularina; arid
regions in Australia; in Calandrinia?), Lyallia (3; L.
andicola: Atacama in northern Chile, L. kerguelensis: Kerguélen
Islands; L. caespitosa: South Island of New Zealand), Lewisia (20;
western North America), Claytonia
(24; East Asia, North America), Montia (11–12;
temperate regions on both hemispheres).
Montiaceae may be sister-group
to Halophytaceae.
Phemeranthus is sister (with high
support) to the remaining genera in the ndhF analysis by Applequist
& Wallace (2001). Claytonia has
tricolpate pollen grains, whereas its sister-group Montia has
pantocolpate pollen.
 |
Phylogeny (simplified) of Montiaceae based on
DNA sequence data (Applequist & Wallace 2001). Baitaria,
Lyallia, Parakeelya, and Philippiamra were
not included in the study. Lyallia is sister to the
[Lewisia+[Montia+Claytonia]] clade in
analyses by Ogburn & Edwards (2009).
|
Dumortier, Anal. Fam. Plant.: 14, 16. 1829
[‘Nepenthideae’], nom. cons.
Nepenthales Bercht.
et J. Presl, Přir. Rostlin: 267. Jan-Apr 1820 [‘Nepenthaceae’];
Nepenthineae Link, Handbuch 1: 369. 4-11 Jul 1829;
Nepenthanae Takht. ex Reveal in Phytologia 79: 71. 29
Apr 1996
Genera/species 1/170
Distribution Madagascar, the
Seychelles, Sri Lanka, Assam, Southeast Asia, southeastern China, Malesia to
northeastern Australia, New Caledonia.
Fossils Fossil pollen grains,
which may be assigned to Nepenthes, have been found in Late Cretaceous
layers in South America and Africa, and in the Eocene and the Miocene of
Europe.
Habit Dioecious, usually
scrambling and climbing (sometimes epiphytic) perennial herbs (rarely
lignified). Carnivorous (largely utilizing excrements and other detrimental
waste products from animals living in the water solution of the pitcher and
feeding on prey falling into the pitcher). Often hygrophytes.
Vegetative anatomy Mycorrhiza
absent. Phellogen ab initio pericyclic. Primary vascular tissue a cylinder of
bundles. Medullary vascular bundles may be present; young stems with cortical
bundles. Medulla, pericycle and bark tissues with large idioblasts having
spiral thickenings and cap-like apices. Pith and cortex with idioblastic
helical-banded fibre-sclereids. A certain amount of lateral growth may occur.
Vessel elements dimorphic, with simple (sometimes vestigial scalariform)
perforation plates; lateral pits alternate, bordered pits. Imperforate
tracheary xylem elements tracheids with bordered pits, usually non-septate
(occasionally septate). Wood rays usually uniseriate or biseriate (rarely
multiseriate), homocellular or heterocellular. Axial parenchyma apotracheal
diffuse or diffuse-in-aggregates, or paratracheal banded. Sieve tube plastids S
type. Nodes 5–9:5–9, multilacunar with five to nine leaf traces. Older
parts of secondary xylem with gum-like deposits. Silica bodies present.
Crystals?
Trichomes Hairs usually
multicellular (rarely unicellular), filiform, fasciculate, rosulate, dendroid,
or absent. Nectariferous glands and peltate hydathodes abundant on stems,
leaves and other organs. Vascularized glands absent.
Leaves Alternate (spiral),
simple, entire, with abaxially circinate, involute ptyxis. Stipules absent;
leaf base sheathing, wide, gradually expanding into an even broader
photosynthesizing discoid part, phyllodium, distally narrowing into a
partially spirally twisted climbing organ, cirrhus, probably
corresponding to petiole. This part grading into a pendant pitcher- or
urn-shaped, often many-coloured organ, ascidium, catching insects.
Ascidium probably corresponding to lamina and provided with lid,
operculum, above mouth. Below lid, outside pitcher a spur, which may
correspond to leaf apex. Pitcher differentiated into a recurved border zone,
peristome, with numerous nectar-secreting glands (extrafloral nectaries) and,
below these on inner side, a wax-secreting slippery zone, and at base a
digesting glandular zone with proteolytic enzymes and absorbing cells partially
or entirely covered by epidermis. Petiole vascular bundle transection arcuate.
Chlorophyllous cells at pitcher margin oxygenating solution. On ventral side of
pitcher two elongate narrowly dentate wings, corresponding to leaf margins.
Venation parallelodromous to palmate. Stomata anomocytic. Cuticular waxes
absent? Adaxial hypodermis present. Leaf margin entire. Water-absorbing peltate
glandular hairs, hydathodes, sunken into depressions on foliar surface.
Inflorescence Terminal,
raceme-like or paniculate thyrse or botryoid. Bracts and floralp prophylls
(bracteoles) absent.
Flowers Actinomorphic, small.
Hypogyny. Tepals (sepals?) (three or) four, with imbricate decussate
aestivation, with large flat adaxial nectariferous glands, usually free
(sometimes connate at base). Petals? absent. Tepal nectaries present. Disc
absent.
Androecium Stamens (four to)
eight to 24. Filaments connate into a central column, free from tepals. Anthers
basifixed to subbasifixed?, connivent into a tube, non-versatile,
tetrasporangiate, extrorse, longicidal (dehiscing by longitudinal slits).
Tapetum secretory. Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains inaperturate or with indistinct
apertures, shed as tetrads, tricellular at dispersal. Exine tectate, with
columellate infratectum, spinulate or psilate.
Gynoecium Pistil composed of
(three or) four (to six) connate antesepalous carpels. Ovary superior, usually
quadrilocular (rarely trilocular, quinquelocular or sexalocular). Style single,
simple, very short or absent. Stigma single, capitate to discoid, papillate,
Dry type. Pistillodium absent.
Ovules Placentation axile (to
laminar). Ovules c. 10 to more than 50 per carpel, anatropous, bitegmic,
crassinucellar. Micropyle bistomal. Outer integument ? cell layers thick. Inner
integument ? cell layers thick. Parietal tissue one cell layer thick.
Megagametophyte monosporous, Polygonum type. Chalazal projection
present. Endosperm development ab initio nuclear. Endosperm haustoria?
Embryogenesis?
Fruit A coriaceous loculicidal
capsule.
Seeds Seeds very small,
numerous, fusiform, usually winged. Aril absent. Seed coat exotestal. Exotesta
with strongly thickened inner cell walls. Chalaza with hairpin-shaped vascular
bundle. Outer integument strongly elongating after fertilization and forming
exostome. Parietal cell of ovule not further dividing. Tegmen? Perisperm not
developed. Endosperm abundant, starchy, oily and proteinaceous. Embryo small,
straight, well differentiated, chlorophyll? Cotyledons two; apices with
haustorial function? Germination phanerocotylar.
Cytology n = 40 –
Paleopolyploidy probable.
DNA Intron present in plastid
gene rpl2.
Phytochemistry Flavonols
(kaempferol, quercetin), cyanidin, tannins, and naphthoquinones (plumbagin,
droserone, hydroxyserone) present. Ellagic acid and cyanogenic compounds not
found.
Use Ornamental plants.
Systematics Nepenthes
(c 170; Madagascar, the Seychelles, Sri Lanka, Assam, southeastern China,
Southeast Asia, Malesia to New Guinea, Queensland, New Caledonia, with their
largest diversity in West Malesia and the Philippines).
Nepenthes
is sister to Droseraceae.
The pollen grains in Nepenthes
are very similar to those in Droseraceae.
de Jussieu, Gen. Plant.: 90. 4 Aug 1789
[’Nyctagines’], nom. cons.
Jalapaceae Batsch,
Tab. Affin. Regn. Veg.: 224. 2 Mai 1802 [’Jalapinae’], nom.
illeg.; Nyctaginales Juss. ex Bercht. et J. Presl,
Přir. Rostlin: 241. Jan-Apr 1820 [’Nyctagineae’];
Allioniaceae Horan., Prim. Lin. Syst. Nat.: 68. 2 Nov
1834 [’Allioniaceae [Nyctagineae]’];
Bougainvilleaceae J. Agardh, Theoria Syst. Plant.:
364. Apr-Sep 1858 [’Bugainvilleae’];
Pisoniaceae J. Agardh, Theoria Syst. Plant.: 363.
Apr-Sep 1858 [’Pisonieae’];
Mirabilidaceae W. R. B. Oliver in Trans. Roy. Soc.
New Zealand 66: 294. 1936; Nyctaginineae Nakai in J.
Jap. Bot. 18: 98. 10 Mar 1942
Genera/species 31/430–470
Distribution Tropical,
subtropical and warm-temperate regions in the Northern and Southern
Hemispheres, with their largest diversity in North and South America;
Phaeoptilum in southwestern Africa.
Fossils Pollen grains assigned
to Nyctaginaceae
have been found in Eocene layers in Argentina, and the Late Campanian
Retitricolpites multibaculates from the island of Sakhalin in Russia
also resemble nyctaginaceous pollen.
Habit Usually bisexual (rarely
monoecious, andromonoecious, gynomonoecious or dioecious), evergreen or
deciduous trees (Leucastereae), shrubs or lianas, perennial or annual
herbs. Roots sometimes fleshy or tuberous. Band-shaped sticky secretions
present on internodes in Anulocaulis, Cyphomeris, and some
species of Boerhavia.
Vegetative anatomy Mycorrhiza
usually absent (Pisonieae may form ectomycorrhiza together with a
diverse array of basidiomycetes). Many species with C4 physiology.
Phellogen usually superficial (sometimes cortical). Lateral meristem in
parenchyma producing secondary cortex outwards and wood rays inwards,
conjunctive tissue and a series of cambia from which isolated areas of vascular
tissue (not rays) develop. Secondary lateral growth usually anomalous (usually
from concentric/successive cambia). Vessel elements usually with simple
(sometimes reticulate) perforation plates; lateral pits alternate, bordered
pits. Imperforate tracheary xylem elements libriform fibres with simple or
reduced bordered pits, septate or non-septate (also vasicentric tracheids).
Wood rays uniseriate or multiseriate, usually heterocellular (sometimes
homocellular), or absent. Axial parenchyma apotracheal diffuse, or paratracheal
vasicentric or banded (usually very scarce). Wood elements often partially
storied. Phloem often intraxylary (as islands or concentric cylinders,
sometimes diffuse). Sieve tube plastids P3cf type, with a central globular
protein crystal surrounded by ring of protein filaments. Endodermis in
Boerhaavia with thick cell walls. Nodes 1:1?, unilacunar with one?
leaf trace, often swollen. Epidermis sometimes with tanniniferous idioblasts.
Heartwood often with resins etc. Calciumoxalate raphides abundant (absent in
Leucastereae); styloids and/or prismatic or elongate crystals present;
crystal sand present or absent.
Trichomes Hairs usually
multicellular, uniseriate, or absent (sometimes dendritic or stellate; in
Boldoa barbed hairs; Leucastereae with stellate or lepidote
hairs); usually uniseriate glandular hairs (rarely branched) present in some
species.
Leaves Usually opposite
(sometimes alternate, spiral, rarely verticillate), simple, entire or lobed,
with ? ptyxis. Stipules and leaf sheath absent. Petiole vascular bundles?
Venation indistinct. Stomata usually anomocytic or paracytic (rarely
actinocytic). Cuticular wax crystalloids usually absent (in some species as
platelets or granules). Mesophyll usually with calciumoxalate raphides. Leaf
margin usually entire (rarely serrate).
Inflorescence Terminal or
axillary, panicle, spike- or umbel-like etc., or flowers solitary axillary.
Partial inflorescences or single flowers often in pseudanthia, surrounded by
free or connate sepaloid or petaloid bracts forming an involucre.
Flowers Usually actinomorphic
(in species of Colignonia and Allionia zygomorphic).
Hypogyny. Tepals (three or) four or five (to seven), in a single whorl, with
induplicate-valvate or contorted (plicate?) aestivation, usually petaloid, with
lower part usually carnose or coriaceous, connate into a tube or
infundibuliform to campanulate, with upper part usually early caducous and
lower part usually persistent around fruit. Nectary usually absent (sometimes
present on receptacle). Disc present (often annular and enclosing ovary) or
absent.
Androecium Stamens one to ten
(to c. 40), in one or sometimes two whorls, usually alternisepalous. Filaments
usually connate at base (in Leucastereae free), usually free from
tepals. Anthers basifixed to dorsifixed, often versatile, tetrasporangiate,
latrorse or partially introrse, longicidal (dehiscing by longitudinal slits).
Tapetum secretory, with binucleate cells. Female flowers in Pisonieae
with staminodia.
Pollen grains
Microsporogenesis simultaneous. Pollen grains 3(–4)-colpate
(Leucastereae), 6–18-pantocolpate (Belemia,
Phaeoptilum) or polypantoporate (some species with twelve to numerous
pores), shed as monads, tricellular at dispersal. Some species (e.g. in
Mirabilis) with pollen grains having a diameter of at least 200 μm
(some of the largest among angiosperms). Exine tectate or semitectate, with
columellate infratectum, reticulate or punctate to anulopunctate, spinulate,
echinate or tegillate.
Gynoecium Pistil composed of a
single carpel. Ovary superior, unilocular (monomerous), sometimes stipitate,
often with subepidermal cell layer containing numerous calciumoxalate raphides.
Style usually single, simple, apical or lateral (rarely absent). Stigma linear,
capitate, lobate, fimbriate, penicillate etc., papillate, Dry type.
Pistillodium?
Ovules Placentation basal.
Ovule one per ovary, hemianatropous or (following fertilization)
anacampylotropous, ascending, usually bitegmic (in Abronia and
Boerhavia unitegmic), crassinucellar. Micropyle endostomal. Outer
integument ? cell layers thick. Inner integument ? cell layers thick. Parietal
tissue? Megagametophyte usually monosporous, Polygonum type (in
species of Mirabilis Tridax type: nuclei arranged according
to the principle 1:2:1). Antipodal cells usually ephemeral (often persistent,
long-lived and somewhat proliferating). Endosperm development ab initio
nuclear. Endosperm haustoria? Embryogenesis asterad. Polyembryony present in
Boerhavia.
Fruit A thin-coated nutlet or
achene (often drupaceous), usually surrounded by accrescent basal part of calyx
forming carnose, coriaceous or lignified diclesium ’anthocarp’ (sometimes
covered with viscid secretion from glandular hairs; sometimes with prickles,
warts, ridges, wings, or other supraepidermal processes, in Allionia
boat-shaped with two rows of inwardly directed teeth; perianth in
Leucastereae and Boldoeae persistent but not accrescent and
’anthocarp’ usually absent; fruits rarely free). Pericarp weakly developed.
’Okenia’ (nested in Boerhavia) with geocarpy.
Seeds Aril absent. Seed coat
testal. Testa in Pisonia multiplicative (on raphe side), unstructured.
Exotesta up to seven cell layers thick. Endotesta rarely (in
Mirabilis) thickened. Tegmen? Perisperm usually abundant, usually
mealy (rarely gelatinous). Endosperm sparse, dome-shaped above radicula, or
absent. Embryo straight or curved around perisperm, well differentiated, with
chlorophyll. Cotyledons usually two (in Abronia one), sometimes (e.g.
Pisonia) unequally sized. Germination phanerocotylar.
Cytology n = 8, 10, 11,
13–17, 20, 21, 26, 27, 29, 33, 40, 42, 44, 46, 47, 56, 58, 68 – Polyploidy
and aneuploidy frequently occurring. Nuclei with protein bodies?
DNA Intron absent (lost) from
plastid gene rpl2. 210 bp deletion in plastid genome.
Phytochemistry Flavonols
(kaempferol, quercetin), flavone-C-glycosides, cyanidin, alkaloids,
triterpene saponins, and betalains (betacyanins, betaxanthins) present. Ellagic
acid and cyanogenic compounds not found. Free oxalates often accumulated.
Use Ornamental plants,
vegetables (Pisonia), medicinal plants, bird lime
(Pisonia).
Systematics Nyctaginaceae may be
sister-group to Petiveriaceae (Brockington
& al. 2013). In some other analyses they are part of an unresolved clade
also comprising Sarcobatus
(Sarcobataceae),
Phytolaccaceae,
Petiveriaceae,
Agdestis (Agdestidaceae) and
Gisekia (Gisekiaceae).
A plausible topology of Nyctaginaceae is
[Leucasteroideae+[Boldooideae+Nyctaginoideae]]. The
following subdivision principally follows Douglas & Spellenberg (2010).
Leucasteroideae
Heimerl, Beitr. Syst. Nyctag.: 15. Mai-Jul 1897
[‘Leucastereae’]
4/5. Reichenbachia (2; R.
colombiana, R. hirsuta; tropical southeastern South America),
Andradea (1; A. floribunda; southeastern Brazil),
Leucaster (1; L. caniflorus; southeastern Brazil),
Ramisia (1; R. brasiliensis; southeastern Brazil). –
Southeastern South America, with their highest diversity in southeastern
Brazil. Raphides absent. Leaves alternate (spiral). Hairs stellate or lepidote.
Perianth contracted in middle ot entirely tubular, accrescent. Stamens usually
two or three (sometimes twelve to 20). Filaments connate at base. Pollen grains
tricolpate (or tetracolpate). Style linare, thick or absent. Stigma lateral,
crested, or sulcate. Anthocarp usually absent (sometimes twelve-ribbed). Tepals
in Ramisia accrescent in fruit. Embryo hooked. –
Leucastereae are sister-group to the remaining Nyctaginaceae.
[Boldooideae+Nyctaginoideae]
Style long and filiform.
Boldooideae Heimerl,
Beitr. Syst. Nyctag.: 16. Mai-Jul 1897 [‘Boldoeae’]
3/3. Boldoa (1; B.
purpurascens; Central America), Cryptocarpus (1; C.
pyriformis; western South America, the Galápagos Islands),
Salpianthus (1; S. arenarius; Mexico, Central America). –
Mexico, Central America to Bolivia and northwestern and northern South America,
the West Indies. Leaves alternate (spiral). Hairs straight or hooked. Bracts
and floral prophylls (bracteoles) absent. Perianth tri- to quinquelobate,
tubular to campanulate, not contracted above ovary. Stamens three to five.
Filaments free. Pollen grains tricolpate. Style short, linear to filiform
(sometimes absent). Stigma inconspicuous (sometimes fimbriate). Anthocarp
coriaceous. Embryo curved. – Boldooideae are sister-group to
Nyctaginoideae.
Nyctaginoideae Eaton,
Bot. Dict., ed. 4: 31. Apr-Mai 1836 [‘Nyctagineae’]
23/420–460. Distribution as for Nyctaginaceae. Leaves
usually opposite (in Bougainvilleeae alternate, spiral). Tepals one to
numerous. Sepals bifid. Perianth tube stout; perianth rim thin. Pollen grains
usually pantoporate (sometimes tricolpate, etc.). Stigma capitate or crested.
Fruit with accrescent base of perianth tube. Cotyledons sometimes unevenly
long. – Nyctaginoideae may have the topology
[Colignonieae+Nyctagineae+[Bougainvilleae+Pisonieae]].
Colignonieae Heimerl,
Beitr. Syst. Nyctag.: 15. Mai-Jul 1897 [‘Coligoniinae’].
1/6. Colignonia (6; C.
glomerata, C. ovalifolia, C. parviflora, C.
pentoptera, C. rufopilosa, C. scandens; the Andes in
Colombia to Argentina). – Lianas or scandent shrubs. Leaves opposite or
verticillate. Hairs tricellular or quadricellular. Bracts usually showy, often
foliaceous. Tepals trilobite to quinquelobate, connate only at base. Stamens
five, epitepalous. Filaments connate at base. Filaments flat, nectariferous.
Pollen grains 12-pantoporate. Ovary stipitate. Style clavate. Stigma
penicillate. Anthocarp winged or angular. Embryo curved.
Nyctagineae Horan.,
Char. Ess. Fam.: 106. 17 Jun 1847.
12/240–260.Acleisanthes (16;
Sonoran and Chihuahuan deserts in southwestern United States and northern
Mexico, one species, A. somalensis, in Somalia); Abronia
(20–25; southwestern United States, northern Mexico), Tripterocalyx
(4; T. carneus, T. crux-maltae, T. micranthus,
T. wootonii; southwestern Canada, western United States; in Abronia?);
Mirabilis
(c 60; tropical and subtropical regions in America, with their highest
diversity in southwestern United States and Mexico, one species in the
Himalayas), Commicarpus (30–35; tropical and subtropical regions on
both hemispheres), Allionia
(2; A. choisyi, A. incarnata; central and western United
States, Mexico, Central America, the West Indies, South America to Chile and
Argentina), Cyphomeris (2; C. crassifolia, C.
gypsophiloides; New Mexico, Texas, northern Mexico), Anulocaulis
(5; A. annulatus, A. eriosolenus, A. gypsogenus,
A. leiosolenus, A. reflexus; southwestern United States,
northern Mexico; incl. Nyctaginia?), Nyctaginia (1; N.
capitata; New Mexico, Texas, northern Mexico; in Anulocaulis?),
Boerhavia
(100–110; tropical and subtropical regions on both hemispheres),
Okenia (1–2; O. hypogaea; southeastern Florida, Mexico,
Nicaragua; in Boerhavia?),
Cuscatlania (1; C. vulcanicola; El Salvador)? –
Distribution as for Nyctaginaceae. Suffrutices
or annual or perennial herbs, sometimes scandent, sometimes with with viscid
exudates on internodes. C4 photosynthesis sometimes present. Leaves
opposite (often anisophyllous), often glandular. Involucres of three to 20
connate or free bracts, or one or two caducous or persistent foliaceous or
membranous bracts subtending each flower or terminal cyme. Flowers
actinomorphic or zygomorphic. Perianth campanulate, tubular or
hypocraterimorphic, (quadrilobate or) quinquelobate, constricted above ovary.
Stamens (one or) two to five (to 18). Filaments connate at base, sometimes
adnate to perianth tube. Pollen grains tricolpate or pantoporate. Style
filiform. Stigma linear, capitates or peltate. Outer integument in Mirabilis
five to seven cell layers thick. Anthocarp globose, turbinate, clavate,
obpyramidal, fusiform etc., usually coriaceous, often glandular or with
membranous wings. Endotesta in Mirabilis
thickened. Embryo hooked.
[Bougainvilleeae+Pisonieae]
Bougainvilleeae Choisy
in A. P. de Candolle et A. L. P. P. de Candolle, Prodr. 13(2): 427, 436. 5 Mai
1849 [‘Bougainvilleae’].
3/12–20. Bougainvillea
(10–18; Central America, the West Indies, tropical South America),
Belemia (1; B. fucsioides; Brazil), Phaeoptilum (1;
P. spinosum; Namibia, northern South Africa, Botswana). – Central
and northern South America, southwestern Africa. Trees or shrubs, sometimes
scandent. Leaves alternate (spiral) or opposite. Bracts three (often showy) or
absent. Flowers often inserted on bracts. Perianth often tubular or
hypocraterimorphic. Stamens five to twelve. Filaments often connate at base.
Pollen grains tricolpate or pantocolpate. Style short, filiform or stout.
Stigma linear to penicillate or multifid. Outer integument four to six cell
layers thick (when a single integument then three to six cell layers thick).
Parietal tissue approx. four cell layers thick. Anthocarp fusiform and
five-ribbed or with four membranous wings. Embryo curved.
Pisonieae Meisn.,
Plant. Vasc. Gen.: Tab. Diagn. 318, Comm. 230. 18-24 Jul 1841.
7/160–170. Pisoniella (1;
P. arborescens; tropical and subtropical regions in America), Pisonia (22;
tropical and subtropical regions on both hemispheres, especially America),
Neea (70–80; southern Mexico, Central America, the West Indies,
tropical South America; in Guapira?), Guapira (c 70; southern
Mexico, Central America, the West Indies, tropical South America; incl.
Neea?), Cephalotomandra (2; C. fragrans, C.
panamensis; Central America)?, Grajalesia (1; G.
fasciculata; Mexico)?, Neeopsis (1; N. flavifolia;
Guatemala)? – Distribution as for Nyctaginaceae. Trees or
(often scandent) shrubs. Leaves alternate (spiral), opposite or verticillate
(sometimes anisophyllous), often glandular. Bracts two or three subtending each
flower, caducous or persistent. Flowers sometimes zygomorphic. Perianth
campanulate, urceolate, hypocraterimorphic or tubular, quinquelobate. Stamens
(two to) five to ten (to numerous). Filaments connate at base, often adnate to
pistil base. Pollen grains usually tricolpate. Stigma penicillate or papillate.
Anthocarp oblong, clavate or ellipsoid, five-ribbed, coriaceous and
glandular-viscid, or globose, carnose, glabrous. Testa in Pisonia
multiplicative, unstructured. Embryo straight.
Unplaced Nyctaginaceae
Caribea (1; C.
litoralis; Cuba).
Caribea was assigned to
Caribeeae Douglas et Spellenb. in Taxon 59: 909. Jun 2010 by Douglas
& Spellenberg (2010). Tufted perennial herb. Leaves opposite. Leaf base
sheathing, stipule-like. Flower subtended by an involucres consisting of three
to five free bracts. Perianth quinquelobate, constricted above ovary. Stamens
two. Filaments adnate to perianth base. Style filiform. Stigma capitates.
Anthocarp subglobose, smooth. Embryo unknown.
 |
Cladogram of Nyctaginaceae based
on DNA sequence data (Douglas & Manos 2007; Douglas &
Spellenberg 2010).
|
Agardh, Aphor. Bot.: 221. 13 Jun 1824
[’Petivereae’]
Rivinaceae C. Agardh,
Aphor. Bot.: 218. 13 Jun 1824 [’Rivineae’];
Petiveriales Link, Handbuch 1: 392. 4-11 Jul 1829
[‘Petiveriaceae’];
Riviniales C. Agardh in C. F. P. von Martius, Consp.
Regn. Veg.: 16. Sep-Oct 1835 [‘Riviaceae’];
Hilleriaceae Nakai in J. Jap. Bot. 18: 99. 10 Mar
1942; Rivinineae Nakai in J. Jap. Bot. 18: 99. 10 Mar
1942; Seguieriaceae Nakai in J. Jap. Bot. 18: 99. 10
Mar 1942
Genera/species 9/24
Distribution Southern United
States to tropical South America, the West Indies, easternmost Australia,
Melanesia.
Fossils Unknown.
Habit Usually bisexual (in
Ledenbergia and Monococcus dioecious), evergreen trees (in
Seguieria with spines), shrubs or lianas, perennial herbs (sometimes
with lignified base).
Vegetative anatomy Phellogen
ab initio subepidermal. Medulla with or without diaphragms. Secondary lateral
growth at least sometimes anomalous (from concentric/successive cambia). Vessel
elements with simple perforation plates; lateral pits alternate, simple and/or
bordered pits. Imperforate tracheary xylem elements fibre tracheids or
libriform fibres with simple pits, usually non-septate (in Rivina?)
(also vasicentric fibres). Wood rays usually multiseriate, heterocellular.
Axial parenchyma apotracheal diffuse, or paratracheal vasicentric scanty or
banded. Intraxylary (concentric) phloem present. Sieve tube plastids P3cf type,
with a central globular protein crystal surrounded by a ring of protein
filaments. Nodes 1:1, unilacunar with one leaf trace. Calciumoxalate as
prismatic crystals, styloids and elongate crystals (in Gallesia and
Seguieria hexagonal crystals).
Trichomes Hairs usually
unicellular or multicellular, uniseriate.
Leaves Alternate (spiral),
simple, entire, with conduplicate ptyxis. Stipules present (rarely as tubercles
or prickles) or absent; leaf sheath absent. Petiole vascular bundles? Venation
pinnate. Stomata usually anomocytic (sometimes paracytic). Cuticular wax
crystalloids as platelets. Abaxial domatia as hair tufts (in
Gallesia). Epidermis often with mucilaginous idioblasts. Leaf margin
entire. Extrafloral nectaries sometimes (Petiveria) present in leaf
axils.
Inflorescence Terminal or
axillary, raceme och spike, or cymose (in Seguieria panicle or
raceme-like; in Gallesia spicate raceme or compound spike). Floral
prophylls (bracteoles) usually lateral (sometimes slightly adaxial or abaxial).
Extrafloral nectaries sometimes (Petiveria) present.
Flowers Usually actinomorphic
(in Hilleria somewhat zygomorphic), small. Usually hypogyny (rarely
epigyny or half epigyny). Tepals in various numbers, usually four (in
Monococcus and Petiveria four diagonal, in Seguieria
five orthogonal), with imbricate aestivation, sepaloid or petaloid, whorled,
usually persistent, usually free (in Hilleria three tepals somewhat
connate). Nectary absent. Disc absent.
Androecium Stamens four to c.
65 (in Rivina four, in Hilleria four to 13, in
Seguieria up to c. 65), in one or two whorls. Filaments free or
somewhat connate at base, free from tepals. Anthers dorsifixed, sometimes
versatile, tetrasporangiate, introrse or extrorse, longicidal (dehiscing by
longitudinal slits). Tapetum secretory. Female flowers in Ledenbergia
with four to six staminodia.
Pollen grains
Microsporogenesis simultaneous. Pollen grains tricolpate (Gallesia,
Seguieria) to 7–17-polypantoporate (Monococcus,
Petiveria, Schindleria), shed as monads, tricellular at
dispersal. Exine tectate, with columellate infratectum, punctate or perforate,
scabrate, spinulate or smooth.
Gynoecium Carpel usually one
(rarely several, secondarily free or connate). Ovary usually superior (rarely
inferior or semi-inferior), usually unilocular (apocarpy, monomerous). Style
single, simple, in Seguieria and Galleria flattened and
wing-like, in Rivina and Monococcus short, in
Trichostigma, Schindleria, Ledenbergia and
Petiveria short or absent. Stigmas one to four, plumose, penicillate
(Trichostigma) or capitate (Rivina), in Seguieria
and Gallesia laterally decurrent, type? Pistillodium?
Ovules Placentation basal.
Ovule one per carpel, campylotropous, bitegmic, crassinucellar. Micropyle
endostomal. Outer integument ? cell layers thick. Inner integument ? cell
layers thick. Parietal tissue two to 18 cell layers thick. Hypostase present.
Nucellar cap approx. two cell layers thick. Nucellar beak present in
Petiveria. Apical cells of megasporangium often radially elongate.
Megagametophyte monosporous, Polygonum type. Endosperm development ab
initio nuclear. Endosperm haustoria? Embryogenesis?
Fruit A one-seeded berry
(Rivina, Trichostigma), a nutlet (in Petiveria with
bristle-like processes) or a samara (in Seguieria and
Gallesia with enlarged persistent lignified sepals; in
Schindleria, Ledenbergia and Hilleria a utriculus;
in Ledenbergia with wing-like sepals). Pericarp usually adnate to
seed.
Seeds Aril absent. Seed coat
exotestal, sometimes thin. Endotesta and tegmen usually collapsed. Perisperm
copious and nutritious (nutrient tissue absent in mature seed in, e.g.,
Gallesia and Seguieria). Endosperm almost absent. Embryo
peripheral, curved around perisperm, well differentiated, without chlorophyll.
Cotyledons two. Germination phanerocotylar.
Cytology n = 18
(Hilleria, Petiveria), 36 (Petiveria,
Trichostigma), 54 (Rivina) – Nuclei with protein bodies?
DNA 210 bp deletion present in
plastid genome.
Phytochemistry Very
insufficiently known. Flavone-C-glycosides (Trichostigma),
betalains (betacyanin, betaxanthin) and sterols present. Flavonols? Alkaloids?
Triterpene saponins? Ellagic acid, proanthocyanidins and cyanogenic compounds
not found. Gallesia with a garlic-like smell. Free oxalates
accumulated?
Use Ornamental plants,
medicinal plants.
Systematics Gallesia
(1; G. integrifolia; Peru, Brazil), Hilleria
(4; H. latifolia, H. longifolia, H. secunda, H.
subcordata; tropical South America, one species, H. latifolia,
also in tropical Africa, Madagascar and the Mascarene Islands),
Ledenbergia (3; L. macrantha, L. peruviana, L.
seguierioides; Mexico, Central America to Colombia and Venezuela),
Monococcus (1; M. echinophorus, southeastern Queensland,
northeastern New South Wales, New Caledonia, Vanuatu), Petiveria (1;
P. alliacea; Florida, southern Texas, Mexico, Central America, the
West Indies, tropical South America), Rivina (1;
R. humilis; southern United States, Mexico, Central America, the West
Indies, tropical South America), Schindleria (3; S.
densiflora, S. racemosa, S. rosea-aenia; Peru, Bolivia),
Seguieria (6; S. americana, S. brevithyrsa, S.
langsdorfii, S. macrophylla, S. paraguayensis, S.
parvifolia; tropical South America), Trichostigma
(4; T. octandrum, T. peruvianum, T. polyandrum,
T. rivinoides; Central America, the West Indies, tropical South
America).
Petiveriaceae are sister to
Nyctaginaceae,
according to Brockington & al. (2013).
Petiveriaceae have
generally been included in Phytolaccaceae, but differ
from the latter clade by usually having a single carpel and by the shape of its
calciumoxalate crystals, and possibly by a number of chemical features,
although the phytochemistry of this group is poorly known.
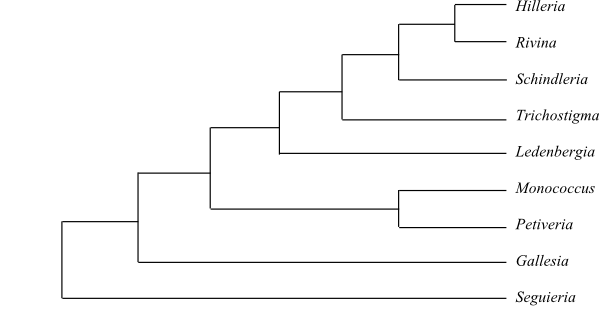 |
Cladogram of Petiveriaceae based
on DNA sequence data (Brockington & al. 2013).
|
Takhtajan in Bot. Žurn. 70: 1692. 13 Dec 1985
Physenales Takht.,
Divers. Classif. Fl. Pl.: 168. 24 Apr 1997
Genera/species 1/2
Distribution Madagascar.
Fossils Unknown.
Habit Dioecious, evergreen
shrubs or small to medium-sized trees.
Vegetative anatomy Phellogen
ab initio subepidermal. Primary vascular tissue a cylinder, without separate
vascular bundles. Continuous sclerenchyma cylinder surrounding vascular
cylinder in young stems. Vessel elements with simple perforation plates;
lateral pits alternate, bordered pits. Imperforate tracheary xylem elements
fibre tracheids or libriform fibres with small simple pits or reduced bordered
pits, non-septate (also vasicentric tracheids). Wood rays uniseriate,
homocellular or heterocellular (consisting of erect or square cells). Axial
parenchyma apotracheal diffuse, paratracheal aliform, confluent, or unilateral.
Sieve tube plastids? Nodes 1:1, unilacunar with one leaf trace. Pericyclic
sclereids present. Secondary phloem with brachysclereids. Medullary parenchyma
with sclereids. Crystals usually absent (rarely a single crystal per cell);
silica grains absent. Prismatic crystals sometimes present in wood ray
cells.
Trichomes Hairs absent.
Leaves Alternate (distichous),
simple, entire, coriaceous, with ? ptyxis. Stipules and leaf sheath absent.
Petiole articulated? Petiole vascular bundles arcuate. Venation pinnate,
brochidodromous. Stomata anomocytic. Cuticular waxes? Mesophyll cells with
calciumoxalate druses. Leaf margin entire.
Inflorescence Axillary,
few-flowered raceme, or flowers solitary axillary.
Flowers Actinomorphic, small.
Hypogyny. Tepals five to nine, with somewhat imbricate aestivation, sepaloid,
persistent, with multicellular simple hairs inside, free or somewhat connate at
base. Nectary absent. Disc absent.
Androecium Stamens (eight to)
ten to 14 (to 25), in one whorl. Filaments filiform, free or partially connate
at base, free from tepals. Anthers basifixed, non-versatile, tetrasporangiate,
latrorse, longicidal (dehiscing by longitudinal slits). Tapetum? Staminodia
absent.
Pollen grains
Microsporogenesis? Pollen grains 3(–5)-colpate, shed as monads, ?-cellular at
dispersal. Exine tectate, with columellate infratectum, echinate or spinulate,
traversed by numerous microchannels.
Gynoecium Pistil composed of
two connate carpels. Ovary superior, bilocular (with incomplete septum) at base
and apex, unilocular in middle. Stylodia two, long, filiform, usually connate
at base. Stigmatic surface papillate, decurrent almost the entire length of
stylodia, type? Male flowers with rudimentary pistillodium.
Ovules Placentation (sub)basal
to axile; placental vascular bundles inverted. Ovules two per carpel,
campylotropous, ascending, bitegmic, weakly crassinucellar. Micropyle
endostomal. Outer integument? cell layers thick. Inner integument ? cell layers
thick. Parietal tissue? Megagametophyte monosporous, Polygonum-type?
Endosperm development? Endosperm haustoria? Embryogenesis?
Fruit A dry one-seeded,
somewhat inflated, capsular nutlike (almost drupaceous) fruit with persistent
calyx.
Seeds Seed large. Aril absent.
Seed coat vascularized, 16 to 20 cell layers thick, with insignificantly
thickened cell walls. Exotesta? Endotesta? Tegmen? Perisperm not developed.
Endosperm absent. Embryo straight, chlorophyll? Cotyledons two, unequally
sized. Germination?
Cytology n = ?
DNA
Phytochemistry Virtually
unknown. Cytotoxic 16-β-[(D-xylopyranosyl)oxy]oxohexadecanyl triterpene
glycosides and oxohexadecanoic acid present. Ellagic acid? Alkaloids?
Use Unknown.
Systematics Physena
(2; P. madagascariensis: Andohahela PN to Montagne d’Ambre PN;
P. sessiliflora: Ampanihy to Antsiranana).
Physena is sister to
Asteropeia (Asteropeiaceae).
Brown in J. H. Tuckey, Narr. Exped. Zaire: 454. 5
Mar 1818 [’Phytolaceae’], nom. cons.
Phytolaccales Link,
Handbuch 1: 390. 4-11 Jul 1829 [‘Phytolacceae’];
Sarcocaceae Raf., Fl. Tellur. 3: 55. Nov-Dec 1837
[’Sarcocidia’]; Phytolaccineae Engl.,
Syllabus, ed. 2: 112. Mai 1898
Genera/species
3–4/30–35
Distribution Tropical and
subtropical regions, with their largest diversity in South America.
Fossils Unknown.
Habit Usually bisexual (rarely
dioecious), evergreen trees, shrubs or lianas (Ercilla), perennial or
annual herbs (Anisomeria is a succulent). Anisomeria and some
species of Phytolacca with napiform roots.
Vegetative anatomy Mycorrhiza
usually absent. Phellogen ab initio subepidermal. Medulla in
Phytolacca sometimes septated by diaphragms. Secondary lateral growth
normal or anomalous (from concentric/successive inner-cortical cambia). Vessel
elements with simple perforation plates; lateral pits alternate, non-bordered
pits. Vestured pits present. Imperforate tracheary xylem elements fibre
tracheids or libriform fibres with simple or reduced bordered pits, septate
(also vasicentric fibres). Wood rays uniseriate or multiseriate,
heterocellular. Axial parenchyma apotracheal diffuse. Intraxylary phloem
present in Phytolacca dioica. Sieve tube plastids P3cf type, with a
central globular protein crystal surrounded by a ring of protein filaments.
Nodes 1:1, unilacunar with one leaf trace. Calciumoxalate styloids and raphides
present.
Trichomes Hairs usually
unicellular or multicellular, uniseriate.
Leaves Alternate (spiral),
simple, entire, with conduplicate ptyxis. Stipules and leaf sheath absent.
Petiole vascular bundles? Venation pinnate; finer veins indistinct. Stomata
anomocytic or paracytic. Cuticular wax crystalloids as platelets. Epidermis
often with mucilaginous idioblasts. Leaf margin entire.
Inflorescence Terminal or
axillary, or leaf-opposite, raceme or spike.
Flowers Usually actinomorphic
(in some species of Anisomeria zygomorphic), small. Hypogyny. Tepals
(four or) five, in, e.g., Phytolacca spiral, usually with quincuncial
(in Anisomeria descending-cochlear) aestivation, sepaloid, fleshy,
usually persistent, free. Nectariferous disc absent in Anisomeria.
Androecium Stamens five to c.
30. Filaments usually connate at base (sometimes free), free from tepals.
Anthers dorsifixed, versatile?, tetrasporangiate, introrse, longicidal
(dehiscing by longitudinal slits). Tapetum secretory. Female flowers sometimes
with staminodia?
Pollen grains
Microsporogenesis simultaneous. Pollen grains tricolpate, shed as monads,
tricellular at dispersal. Exine tectate, with columellate infratectum, punctate
or perforate, scabrate, spinulate or smooth.
Gynoecium Carpels three to 16,
seemingly or secondarily free or connate below and with free stylodia (carpels
in Nowickea on gynophore), initiated in a ring around receptacular
apex, alternitepalous or antetepalous. Ovary single, superior, unilocular
(pseudapocarpy). Stylodia three to 16, free or connate, more or less gynobasic.
Stigmas free, type? Male flowers sometimes with pistillodium.
Ovules Placentation basal or
subbasal. Ovule one per carpel, campylotropous (amphitropous?), apotropous,
bitegmic, crassinucellar. Micropyle endostomal. Outer integument ? cell layers
thick. Inner integument ? cell layers thick. Obturator present. Hypostase
present. Parietal tissue approx. two cell layers thick. Nucellar cap massive.
Apical cells of megasporangium radially elongate. Megagametophyte monosporous,
Polygonum type. Antipodal cells sometimes slightly proliferating.
Endosperm development ab initio nuclear. Endosperm haustoria? Embryogenesis
onagrad, caryophyllad or chenopodiad.
Fruit An assemblage of
one-seeded berries or a berry-like syncarpous fruit.
Seeds Aril absent. Seed coat
exotestal. Exotestal cells thick-walled? Endotesta and tegmen largely
collapsed. Perisperm copious and nutritious. Endosperm poorly developed or
absent. Embryo peripheral, curved around perisperm, well differentiated,
without chlorophyll. Cotyledons two. Germination phanerocotylar.
Cytology n = 9, 18, 36
(Phytolacca) – Nuclei with protein bodies?
DNA 210 bp deletion present in
plastid genome.
Phytochemistry Flavonols
(kaempferol), betalains (betacyanins, betaxanthins), alkaloids, triterpene
saponins, sterols, and pinitol present. Ellagic acid, proanthocyanidins and
cyanogenic compounds not found. Free oxalates accumulated.
Use Ornamental plants, dyeing
sources (Phytolacca), medicinal plants.
Systematics Anisomeria
(3; A. bistrata, A. coriacea, A. littoralis; central
and southern Chile, Tierra del Fuego), Ercilla (2; E.
spicata, E. syncarpellata; Chile), Nowickea (2;
Nowickea glabra, N. xolocotzii; central Mexico; ‘monstruous
forms of Phytolacca?’;
probably in Phytolacca),
Phytolacca
(25–30; tropical and subtropical regions on both hemispheres; probably incl.
Nowickea).
Phytolaccaceae are
possibly sister-group to [Agdestidaceae+Sarcobataceae].
de Jussieu, Gen. Plant.: 92. 4 Aug 1789
[’Plumbagines’], nom. cons.
Staticaceae Cassel,
Lehrb. Nat. Pflanzenordn.: 215. Apr-Mai 1817 [’Staticeae’];
Plumbaginales Juss. ex Bercht. et J. Presl, Přir.
Rostlin: 241. Jan-Apr 1820 [‘Plumbagineae’];
Staticales Link, Handbuch 2: 262. 4-11 Jul 1829
[‘Staticinae’]; Armeriaceae Horan.,
Prim. Lin. Syst. Nat.: 68. 2 Nov 1834;
Plumbaginopsida Endl., Gen. Plant.: 346. Dec 1837
[’Plumbagines’]; Plumbaginineae J. Presl
in Nowočeská Bibl. [Wšobecdný Rostl.] 7: 1238.
1846[‘Plumbagines‘]; Limoniaceae Ser.,
Fl. Pharm.: 456. 1851, nom. cons.; Aegialitidaceae
Lincz. in Novosti Sist. Vyssik Rast. [Nov. Syst. Plant. Vasc.] 1968: 173. 18
Dec 1968; Plumbaginanae Takht. ex Reveal in Novon 2:
236. 13 Oct 1992; Plumbaginidae C. Y. Wu in Acta
Phytotaxon. Sin. 40: 291. 2002
Genera/species 28/625–835
Distribution Cosmopolitan
except Antarctica, with their largest diversity in arid and saline environments
in the Mediterranean and Southwest to Central Asia.
Fossils Uncertain.
Habit Bisexual, usually
perennial herbs or shrubs (sometimes annual herbs, rarely lianas). Many species
are xerophytes or halophytes.
Vegetative anatomy Phellogen
ab initio subepidermal, later cortical. Cortical and medullary bundles often
present. Secondary lateral growth in Acantholimon, Aegialitis
and Limoniastrum anomalous (often from successive/concentric cambia).
Vessel elements with simple perforation plates; lateral pits alternate, simple
pits? Imperforate tracheary xylem elements libriform fibres with simple pits,
non-septate? (also vasicentric tracheids). Wood rays multiseriate, homocellular
or absent. Axial parenchyma paratracheal scanty. Wood elements sometimes
storied. Intraxylary phloem present in some species. Sieve tube plastids S
type. Nodes 3:3, trilacunar with three leaf traces. Silica bodies often
abundant. Crystals?
Trichomes Hairs unicellular or
multicellular, uniseriate; glands stalked or unstalked (sometimes shaggy).
Vascularized mucilage glands present.
Leaves Alternate (spiral),
simple, entire or pinnately lobed, usually herbaceous (rarely scale-like; in
Aegialitis coriaceous), with convolute, involute or flat ptyxis.
Petiole vascular bundle transection arcuate. Stipules usually absent (rarely
well developed); leaf sheath usually absent (present in Aegialitis?).
Venation usually pinnate (sometimes palmate; in Aegialitis
parallelodromous). Stomata anomocytic, anisocytic or paracytic (Ranunculaceae
or Rubiaceae type).
Cuticular wax crystalloids usually absent (sometimes as irregular platelets).
Epidermis often with chalk glands secreting water and calcium salts; epidermal
salt-excreting glands present in halophytes. Extrafloral nectaries sometimes
present on abaxial side of midvein in Plumbaginoideae. Mesophyll often
with secretory glands; abaxial vein axils often with upraised vascularized
mucilage glands; in Staticoideae with sclerenchymatous idioblasts
(with branched sclereids; sclerenchyma in Limonium with
asterosclereids, osteosclereids, filiform sclereids, etc.). Leaf margin
sinuate, crenate or entire.
Inflorescence Terminal,
racemose (Plumbaginoideae) or cymose (Staticoideae), simple
or compound; capitate inflorescence in Armeria composed of
glomera/drepania consisting of cincinni; floral bracts aborting; glomeral
bracts forming involucrum around capitulum; lower parts growing downwards
forming sheath around uppermost part of peduncle. Bracts often sheathing, dry
and membranous. Floral prophylls (bracteoles) in Plumbaginoideae often
with extrafloral nectaries.
Flowers Actinomorphic.
Hypogyny. Sepals five, with valvate or plicate aestivation, persistent, often
membranous or petaloid, with five or ten ridges, usually connate into a tube
(rarely free). Petals five, with usually contorted (sometimes imbricate)
aestivation, often persistent, connate usually only at base (sometimes
entirely), in Aegialitis coriaceous; petal and staminal primordia
common. Nectaries often present (sometimes as five glands alternating with
stamens). Disc absent.
Androecium Stamens five,
alternisepalous, antepetalous. Filaments free or connate, free from petals
(Plumbaginoideae) or adnate at base to petals (Staticoideae);
adaxial side often with basal nectary. Anthers usually dorsifixed (in
Aegialitis basifixed), sometimes versatile, tetrasporangiate,
introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory or
amoeboid-periplasmodial. Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually 3(–5)-colpate (rarely
pantocolpate), tetra- or hexarugate or irregular, shed as monads, usually
tricellular (sometimes bicellular) at dispersal. Exine tectate or semitectate,
with columellate infratectum, in Plumbaginoideae tectate and
verrucate, in Staticoideae reticulate. Pollen grains in
Plumbaginoideae Plumbago type (columellae very irregular,
tectum continuous), in Staticoideae Armeria type (columellae
straight and irregular, tectum discontinuous, reticulate). Pollen grains in
Staticoideae often dimorphic.
Gynoecium Pistil composed of
five connate eusyncarpous carpels. Ovary superior, unilocular. Style single,
lobate at apex (Plumbaginoideae), or stylodia five, free or connate
below (Staticoideae). Stigmas five, capitate to cylindrical, papillate
(sometimes with multicellular papillae), Dry type. Pistillodium absent.
Heterostyly frequent in Plumbaginoideae.
Ovules Placentation basal.
Ovule one per ovary, anatropous or circinotropous, pendulous, bitegmic,
crassinucellar. Micropyle endostomal. Outer integument ? cell layers thick.
Inner integument ? cell layers thick. Funicle long and curled. Obturator
usually present between ovary apex and micropyle. Parietal tissue two or three
cell layers thick. Nucellar cap in Plumbagella approx. two cell layers
thick. Megagametophyte tetrasporous, 4- or 8?-nucleate, Plumbago type
(Ceratostigma, Plumbago, Dyerophytum),
Plumbagella type (Plumbagella), Fritillaria type
(Limonium, Armeria), Adoxa type?, or Penaea
type? Proendosperm nucleus triploid or tetraploid. Endosperm development ab
initio nuclear. Endosperm haustorium chalazal. Embryogenesis solanad.
Fruit Usually a membranous
nutlet (sometimes a capsule [denticidal?] or a pyxidium), entirely or partially
enclosed by persistent calyx.
Seeds Aril absent. Seed coat
exotestal-endotegmic, winged. Exotesta? Endotesta? Exotegmen? Endotegmen
persistent. Perisperm not developed. Endosperm usually copious (sometimes
sparse or absent), with simple starch grains and proteins, tetraploid or
pentaploid. Embryo large, straight, well differentiated, with chlorophyll.
Cotyledons two, flat. Germination phanerocotylar.
Cytology n = 6, 7
(Plumbaginoideae) or x = 8, 9 (Staticoideae) – Polyploidy
and aneuploidy frequent (especially in Staticoideae). Agamospermy
present in Limonium etc.
DNA Intron present in plastid
gene rpl2. Intron in plastid gene rpl16 absent (lost) in
Staticeae (e.g. Limonium gmelinii). Mitochondrial intron
coxII.i3 lost.
Phytochemistry Flavonols
(kaempferol, quercetin, myricetin), flavonol sulphates
(cholin-O-sulphate), and gallic acid present. Hydrolyzable tannins
based on ellagic acid and condensed tannins based on leucodelphinidin present
in Staticoideae. Naphthoquinone (plumbagin) present in all
investigated Plumbaginoideae (not found in Staticoideae).
Cyanidin and prodelphinidins present in Plumbaginoideae. Alkaloids and
cyanogenic compounds present in Plumbago. Ellagic acid present in
Aegialitis. Glycine betaines (quaternary ammonium compounds) present
in some species of Plumbago and Limonium. Saponins not found.
Oxalate sometimes accumulated.
Use Ornamental plants,
medicinal plants.
Systematics Plumbaginaceae are
sister-group to Polygonaceae.
Plumbaginoideae
Burnett, Outlines Bot.: 1028, 1095, 1101. Feb 1835
[‘Plumbaginidae’]
4/34–36. Ceratostigma
(8; northeastern tropical Africa, Tibet, China, Southeast Asia), Dyerophytum
(3; D. africanum, D. pendulum, D. socotranum;
Namibia, Northern and Western Cape, Socotra, the Arabian Peninsula to India),
Plumbagella (1; P. micrantha; Central Asia), Plumbago
(22–24; tropical to warm-temperate regions on both hemispheres, the
Mediterranean). – Tropical to warm-temperate regions in the Northern and
Southern Hemispheres, the Mediterranean. Shrubs or perennial herbs. Vegetative
and reproductive shoots similar. Stem angular, striated. Continuous cylinder of
sclerenchyma present outside phloem. Leaves usually entire (rarely deeply
lobed). Cauline stipules present in Plumbago.
Inflorescence racemose. Calyx herbaceous, glandular. Corolla lobes
truncate-emarginate, later apiculate. Pollen receptive surfaces in fascicles
along branch. Style single, with stigmatic areas as groups along branches.
Fruit a pyxidium, dehiscing at base, with persistent herbaceous calyx. n = 6,
7. Plumbagin, 5-O-methylated flavonols present; glycine betaines
present in some species of Plumbago.
Staticoideae Burnett,
Outlines Bot.: 1028, 1095, 1101. Feb 1835 [’Staticidae’]
Mainly coastal areas and arid regions in
Northern and Southern Hemispheres. Vegetative and reproductive shoots usually
dissimilar. Separate vascular bundles (fascicles) present. Leaves usually
cartilaginous or coriaceous, with five to ten rows of whitish cells along
margins. Petals connate. Filaments adnate to corolla. Stylodia usually separate
or connate below. Stigmas usually capitate (sometimes filiform). Fruit an
achene or a pyxidium. x = 8, 9. Deletion present in intron of plastid gene
rpl16. β-alanine betaines (quaternary ammonium compounds included in
salt excretion) present; glycine betaines present in some species of Limonium.
Plumbagin not found.
Aegialitideae (Lincz.)
T. H. Peng, Fl. Reipubl. Popularis Sion. 60(1): 1. 1987
1/2. Aegialitis (2; A.
rotundifolia: coasts along eastern India to Burma, the Andaman Islands;
A. annulata: coasts along northern Australia and southern New Guinea).
– Mangrove shrubs. Successive cambia present. Cortical vascular bundles
present. Branched sclereids present. Leaves with involute ptyxis and with
sheathing base. n = ? Ellagic acid present. Glycine betaine not found.
Staticeae Bartl., Ord.
Nat. Plant.: 127. Sep 1830 [‘Staticea’]
23/590–800. Acantholimon
(290–300; eastern Mediterranean to Central Asia), Armeria (c
95; temperate regions on the Northern Hemisphere, the Andes south to Tierra del
Fuego), Bakerolimon (2; B. peruvianum, B. plumosum;
Peru, northern Chile), Bamiania (1; B. pachycormum;
Afghanistan), Bukiniczia
(1; B. cabulica; Afghanistan, Pakistan), Cephalorhizum (6;
C. coelicolor, C. micranthum, C. oopodum, C.
pachycormum, C. popovii, C. turcomanicum; Central Asia),
Ceratolimon
(3; C. feei, C. migiurtinum, C. weygandiorum; the
Mediterranean), Chaetolimon (3; C. limbatum, C.
setiferum, C. sogdianum; Central Asia), Dictyolimon (2;
D. griffithii, D. macrorrhabdos; Afghanistan to India),
Ghaznianthus (1; G. rechingeri; Afghanistan),
Gladiolimon (1; G. speciosissimum; Afghanistan), Goniolimon
(c 20; Russia and the Balkan Peninsula to Mongolia), Ikonnikovia (1;
I. kaufmanniana; Central Asia, northwestern China), Limoniastrum
(1; L. monopetalum; the Mediterranean), Limoniopsis (2;
L. davisii, L. owerinii; Turkey to the Caucasus), Limonium
(150–350; cosmopolitan, with their highest diversity in maritime and arid
habitats on the Northern Hemisphere), Muellerolimon (1; M.
salicorniaceum; Western Australia), Myriolimon (2; M.
diffusum, M. ferulaceum; western and central Mediterranean),
Neogontscharovia (2; N. mira, N. miranda;
Afghanistan, Central Asia), Popoviolimon (1; P. turcomanicum;
Central Asia), Psylliostachys (c 7; P. afghanicus, P.
anceps, P. beludshistanica, P. leptostachya, P.
spicata, P. suworowii, P. volkii; eastern Mediterranean
to the Caucasus and Central Asia), Saharanthus (1; S.
ifniensis; southern Morocco, northern Sahara), Vassilczenkoa (1;
V. sogdiana; Afghanistan, Central Asia). – Mainly irano-turanian and
mediterranean, also in southern Africa, southern South America and Western
Australia. Shrubs or perennial herbs. Leaves in basal rosette. Leaf margin
usually entire. Inflorescence cymose (capitate or branched), with channeled
axis. Inflorescence leaves reduced or absent. Calyx membranous (sometimes
petaloid). Pollen grains often dimorphic. Exine with regular columellae and
incomplete tectum, reticulate. Heterostyly frequent. Fruit an achene or a
pyxidium, often with calyx as part of dispersal unit. Deletion of intron in
plastid gene rpl16. β-alanine betaines present; glycine betaines
present in some species of Limonium.
– The generic delimitations are very uncertain and a comprehensive
phylogenetic analysis of Staticeae is needed.
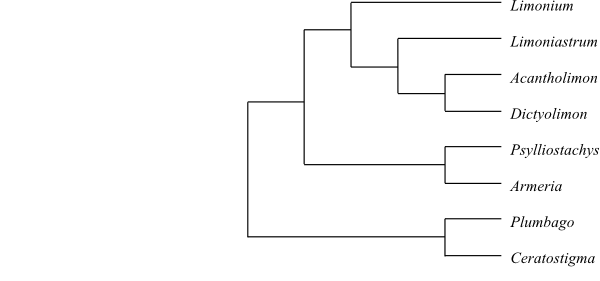 |
One of three most-parsimonious cladograms from
successive weighting of Plumbaginaceae
based on DNA sequence data (Lledó & al. 1998).
|
de Jussieu, Gen. Plant.: 82. 4 Aug 1789
[’Polygoneae’], nom. cons.
Persicariaceae
Martinov, Tekhno-Bot. Slovar: 473. 3 Aug 1820 [’Persicariae’];
Polygonales Juss. ex Bercht. et J. Presl, Přir.
Rostlin: 240. Jan-Apr 1820 [‘Polygoneae’];
Rumicaceae Martinov, Tekhno-Bot. Slovar: 554. 3 Aug
1820 [’Rumoides’]; Rumicales Burnett,
Outl. Bot.: 1141. Jun 1835 [‘Rumicinae’], nom. illeg.;
Eriogonaceae (Dumort.) G. Don in R. Sweet, Hort.
Brit., ed. 3: 580. 23 Oct 1839 [’Eriogoneae’];
Polygonopsida Brongn., Enum. Plant. Mus. Paris:
xxvii, 100. 12 Aug 1843 [’Polygonoideae’];
Atraphaxidineae H. Gross in Bot. Jahrb. Syst. 49:
250. 1913; Calligonaceae Khalk. in Dokl. Akad. Nauk.
Uzbeksk. SSR 1985(11): 45. Nov 1985; Polygonanae
Takht. ex Reveal in Novon 2: 236. 13 Oct 1992;
Polygonidae C. Y. Wu in Acta Phytotaxon. Sin. 40:
294. 2002
Genera/species
42/1.480–1.530
Distribution Cosmopolitan
except Antarctica, with their largest diversity in tempererate regions on the
Northern Hemisphere.
Fossils Numerous fossilized
fruits have been found in Late Cretaceous (Maastrichtian) and Paleocene later
layers in North America, but also from Cenozoic strata in Asia and Europe.
Lower Paleocene leaf fossils, Paranymphaea crassfolia, from North
America were assigned to Polygonaceae (McIver &
Basinger 1993).
Habit Usually bisexual
(sometimes monoecious, polygamomonoecious or dioecious), usually perennial or
annual herbs (often climbing or twining) or shrubs (rarely trees or lianas).
Stem and branches often sulcate, geniculate, striate and/or hollow, often with
swollen nodes (branches in Muehlenbeckia and Calligonum
photosynthesizing phyllocladia).
Vegetative anatomy Mycorrhiza
usually absent (endomycorrhiza present in Eriogonum; ectomycorrhiza
present in Coccoloba). Phellogen ab initio usually subepidermal
(sometimes pericyclic). Subepidermal collenchyma or sclerenchyma strands
frequently present in stem. Endodermis often significant. Lignified species
with normal or anomalous secondary lateral growth (in Antigonon from
successive/concentric cambia). Vessel elements with simple perforation plates;
lateral pits alternate, bordered pits. Vestured pits sometimes present
(Muehlenbeckia). Imperforate tracheary xylem elements tracheids or ?
with simple or bordered pits, septate or non-septate (also vasicentric
tracheids). Wood rays usually uniseriate (sometimes multiseriate), homocellular
or heterocellular (with dark-stained inclusions). Axial parenchyma apotracheal
diffuse, or paratracheal scanty, vasicentric, confluent, or banded. Wood
elements sometimes partially storied. Intraxylary phloem present or absent.
Sieve tube plastids usually S type (sieve tube plastids with protein fibrils
present in Triplarieae). Nodes 3:3, trilacunar with three leaf traces,
or ≥5:≥5, multilacunar with five or more traces. Mucilage cells abundant.
Calciumoxalate as prismatic crystals, crystal sand and druses often present.
Trichomes Hairs unicellular or
multicellular, usually uniseriate (sometimes stellate or glandular, also
peltate-lepidote).
Leaves Usually alternate
(spiral; rarely opposite or verticillate), usually simple (rarely pinnately
compound), entire or lobate, sometimes fleshy or coriaceous (in
Muehlenbeckia and Calligonum rudimentary), usually with
revolute (in Muehlenbeckia convolute) ptyxis. Stipules usually
enclosing stem as a caducous or persistent, membranous, often lobate or
fimbriate tubular ocrea (usually absent in the Eriogonum clade;
rudimentary in some species of Chorizanthe). Colleters present in
association with leaves. Foliar tendrils present in Antigonon. Petiole
vascular bundle transection usually annular (sometimes D-shaped; bundles
scattered in some species of Coccoloba). Extrafloral nectaries present
in some species on abaxial side of petiolar pulvinus. Venation pinnate or
palmate. Stomata usually anomocytic (rarely diacytic, anisocytic, helicocytic,
or paracytic). Cuticular wax crystalloids as platelets or rodlets. Lamina
sometimes gland-dotted. Epidermis often with mucilage cells. Leaf margin
usually entire (sometimes crenate or lobed).
Inflorescence Usually terminal
or axillary, simple or compound panicle, thyrsoid, or spike-, head- or
raceme-like (flowers rarely single axillary). Each flower usually subtended by
a persistent membranous, tubular ocreola consisting of two connate floral
prophylls (bracteoles). Partial inflorescence in the Eriogonum clade
surrounded by and partially enclosed by involucrum.
Flowers Actinomorphic, small.
Pedicel articulated. Hypanthium present or absent. Hypogyny. Tepals usually
five or 3+3 (rarely 2+2 or 3+6), with imbricate quincuncial aestivation,
sepaloid or petaloid, spiral, usually persistent, connate at base.
Nectariferous disc annular, inserted around ovary base, or single nectaries
present between staminal bases (sometimes absent).
Androecium Stamens usually 3+3
(sometimes two, three, 2+2, five, 4+4, or 3+6), alternisepalous or
antesepalous. Filaments filiform, free or connate at base, often adnate to
tepal bases forming a ring. Anthers basifixed or dorsifixed, often versatile,
tetrasporangiate, usually introrse (sometimes latrorse or extrorse), longicidal
(dehiscing by longitudinal slits). Tapetum secretory. Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains tricolpate, tricolporate or
pantoporate, shed as monads, usually tricellular (rarely bicellular) at
dispersal. Exine tectate or semitectate, with columellate infratectum,
reticulate, punctate eller striate, often spinulate or echinulate.
Gynoecium Pistil composed of
(two or) three (or four) connate eusyncarpous carpels; median carpel adaxial;
when tepals 3+3, then carpels antesepalous. Ovary superior, unilocular
(sometimes seemingly multilocular by secondary septa). Style single, simple, or
stylodia (two or) three (or four), usually long (rarely short), separate or
connate below. Stigma one or stigmas several, entire or lobate, filiform,
penicillate, peltate, or capitate, papillate or non-papillate, Dry type.
Pistillodium? Heterstyly occur in some genera.
Ovules Placentation basal and
free central. Ovule one per ovary, usually orthotropous (occasionally
campylotropous to anatropous), ascending, usually bitegmic (rarely unitegmic),
crassinucellar. Funicle present (representing a reduced free central
placenta?), or absent. Micropyle endostomal. Outer integument ? cell layers
thick. Inner integument ? cell layers thick. Nucellar beak present. Hypostase
present or absent. Archespore usually unicellular (rarely multicellular).
Megagametophyte monosporous, Polygonum type. Synergids sometimes with
a filiform apparatus. Antipodal cells usually uninucleate (in Rumex
multinucleate). Endosperm development ab initio nuclear. Endosperm haustoria?
Embryogenesis asterad.
Fruit A usually triangular
(rarely lenticulate) achene, sometimes winged or with bristles (rarely a
berry), often surrounded by persistent and sometimes accrescent tepals (in
Emex and Oxygonum surrounded by receptacle/hypanthium).
Seeds Aril absent. Seed coat
testal. Exotesta? Endotesta? Tegmen? Perisperm entirely or almost absent.
Endosperm copious, mealy or horny (in Coccolobeae and
Triplareae ruminate), with simple starch grains, oil and proteins.
Embryo usually straight to curved (rarely plicate), usually lateral to
peripheral (rarely central), without chlorophyll. Cotyledons two. Germination
phanerocotylar.
Cytology x =
(4–)7–13(–17) – Polyploidy and aneuploidy frequently occurring.
Dioecious species of Rumex with a sex-determining X-autosome balance
system.
DNA Intron present in the
plastid gene rpl2. Mitochondrial intron coxII.i3 lost.
Plastid IR expanded.
Phytochemistry Flavonols
(kaempferol, quercetin, myricetin), hyperoside (quercetin
3-O-galactoside), flavonol sulphates, flavone-C-glycosides,
catechins, O-methylated flavonoids, hexaoxygenated flavonoids,
condensed and hydrolyzable tannins, proanthocyanidins (prodelphinidins),
methylated and non-methylated ellagic acids, gallic acid, caffeic acid, indole
alkaloids and other alkaloids, saponins, sesquiterpene lactones?, free soluble
oxalic acid, oleanolic acid derivatives, pinitol, fagopyrine and
protofagopyrine, polyacetate derived anthraquinones (oxymethyl anthraquinone
etc.), and naphthoquinones and acetophenones present. Cyanogenic compounds not
found.
Use Ornamental plants,
vegetables (Rheum, Rumex etc.), fruits (Coccoloba
uvifera), starch sources (Fagopyrum).
Systematics Polygonaceae are
sister-group to Plumbaginaceae.
Symmeria and Afrobrunnichia
are successive sister-groups to the remaining analysed genera of Polygonaceae, which are
subdivided into a mostly ligneous clade (with, e.g., the Eriogonum
group) and a mainly herbaceous clade.
Symmerioideae Meisn.
in DC., Prodr. 14: 4, 185. mid Oct 1856
1/1. Symmeria (1; S.
paniculata; tropical West and Central Africa, northern South America). –
Dioecious. Petiole bases expanded and winged, enclosing developing shoot
meristem, not forming closed tube, hence ocrea (sheathing stipule) absent.
Tepals 3+3. Stamens 40–50. Ovary trilocular, with basal partitions. Achene
pyramidal, with three tepals adnate to pericarp. n = ?
[Afrobrunnichia+[Polygonoideae+Eriogonoideae]]
Closed tubular stipule (ocrea) usually
present, ensheathing stem, or reduced to circular scar. Stamens 3–15. Achene
lenticular, triquetrous or trigonous.
Afrobrunnichia
Afrobrunnichia (2; A.
africana, A. erecta; tropical West Africa). – Lianas. Tendrils
axillary, bifid. Pedicel winged on both sides. Tepals five, connate at base.
Fruit drupaceous. Seed deeply longitudinally trisulcate, irregularly ruminate.
n = ?
[Polygonoideae+Eriogonoideae]
Polygonoideae Eaton,
Bot. Dict., ed. 4: 30. Apr-Mai 1836 [‘Polygoneae’]
17/880–900. Oxygoneae
T. M. Schust. et Reveal in Taxon 64: 1199. 2015.
Oxygonum (c 30; tropical and southern Africa, Madagascar). –
Persicarieae Dumort., Fl. Belg.: 17. 1827. Koenigia
(24–26; arctic and temperate regions in Asia and North America, South and
East Asia to Pakistan, the Himalayas and Japan, one species, K.
islandica, almost cosmopolitan), Rubrivena (1; R.
polystachya; Afghanistan?, southern Himalayas, Xizang, northern Burma,
western China), Bistorta
(40–45; temperate and arctic regions in Europe, Asia and North America), Persicaria
(120–125; almost cosmopolitan). – Fagopyreae
Yonek. in I. Kunio, D. E. Boufford et H. Ohba, Fl. Jap. 2a: 132. 24 Feb 2006.
Fagopyrum
(15–16; East Africa, Asia; incl. Eskemukerjea?),
Eskemukerjea (1; E. megacarpum; the Himalayas; in Fagopyrum?).
– Rumiceae Dumort., Fl. Belg.: 17. 1827. Rumex (c 200;
temperate regions on both hemispheres, especially the Northern Hemisphere), Oxyria (2–3;
O. caucasica, O. digyna, O. sinensis; arctic and
alpine regions, circumboreal south to California), Rheum (55–60;
Europe, temperate and subtropical regions in Asia). –
Calligoneae C. A. Mey. in Mém. Acad. Imp. Sci.
Saint-Pétersbourg, sér. 6, Sci. Math., Sec. Pt. Sci. Nat. 6(2): 142. 25 Oct
1840. Calligonum (80–85; the Mediterranean to India; incl.
Pteroxygonum?). – Polygoneae Rchb., Fl.
Germ. Excurs. 2(2): 563, 568. 1832. Oxygonum (22; tropical and
southern Africa, Madagascar); Knorringia (1; K. sibirica;
Siberia, Afghanistan, Pakistan, Central Asia, the Himalayas, western China); Muehlenbeckia
(c 30; New Guinea, Solomon Islands, Australia, Tasmania, New Zealand, the
Chatham Islands, Honduras, Chile), Fallopia (c
20; temperate regions on the Northern Hemisphere); Fallopia
denticulata (southern China), F. cilinodis (North America); Atraphaxis
(40–45; southeastern Europe, northern Africa, western Asia to the Himalayas
and eastern Siberia), Duma (3; D. coccoloboides, D.
florulenta, D. horrida; Australia), Polygonum
(210–220; temperate regions on the Northern Hemisphere, one species, P.
maritimum, also in southern South America). – Cosmopolitan, with their
largest diversity in temperate regions. Shrubs, lianas or perennial or annual
herbs, often climbing and twining (with axillary tendrils). Stipule membranous.
Stamens sometimes nine. n = 7 or more. – Oxygonum, consisting of
polygamous heterostylous annual or perennial herbs or shrubs, is sister to the
remaining Polygoneae in the combined molecular analysis by Schuster
& al. (2011) and Knorringia is successive sister to the rest. The
inclusion of Pteroxygonum in Calligonum is provisional
(Sanchez & al. 2011).
Eriogonoideae Arn.,
Botany: 126. 9 Mar 1832 [’Eriogoneae’]
23/600–630.
Brunnichieae C. A. Mey. in Mém. Acad. Imp. Sci.
Saint-Petersbourg, sér. 6, Sci. Math., Sec. Pt. Sci. Nat. 6(2): 150. 25 Oct
1840 [‘Brunnichiaceae’]. Antigonon (3; A.
flavescens, A. guatemalense, A. leptopus; Mexico,
Central America), Brunnichia (5; B. africana, B.
chirrhosa, B. congoensis, B. erecta, B. ovata;
southeastern United States). – Coccolobeae Dumort.,
Anal. Fam. Plant.: 18. 1829. Podopterus (3; P. cordifolius,
P. mexicanus, P. paniculatus; Mexico, Guatemala), Coccoloba
(120–130; tropical and subtropical regions from Mexico to tropical South
America), Neomillspaughia (2; N. emarginata, N.
paniculata; Central America). – Leptogoneae
Jan. M. Burke et Adr. Sanchez in Brittonia 63(4): 517. 1 Dec 2011.
Leptogonum (1; L. domingense; Hispaniola). –
Triplarideae C. A. Mey. In Mém. Acad. Imp. Sci.
Saint-Pétersbourg, sér. 6, Sci. Math., Seconde Pt. Sci. Nat. 6(2): 147. 1840.
Salta (1; S. triflora; Bolivia, Paraguay, northern
Argentina); 'Ruprechtia' (c 35; tropical America; non-monophyletic),
Magoniella (1; M. obidensis; Venezuela, Brazil, Bolivia),
Triplaris (15–20; southern Mexico, Central America, the West Indies,
tropical South America). – Gymnopodieae Jan. M.
Burke et Adr. Sanchez in Brittonia 63(4): 518. 1 Dec 2011. Gymnopodium
(2; G. floribundum, G. ovatifolium; southern Mexico,
Guatemala). – Eriogoneae Dumort., Anal. Fam.
Plant.: 17. 1829. Harfordia (1; H. macroptera; Baja
California, Mexico), Pterostegia
(1; P. drymarioides; western and southwestern United States),
Gilmania (1; G. luteola; Death Valley in southeastern
California), Dedeckera (1; D. eurekensis; California),
Stenogonum (2; S. flexum, S. salsuginosum; western
United States), Sidotheca (3; S. caryophylloides, S.
emarginata, S. trilobata; California, northwestern Mexico),
Lastarriaea (2; L. chilensis: northern and central Chile;
L. coriacea: California), Oxytheca (7; O.
dendroidea, O. emarginata, O. insignis, O.
parishii, O. perfoliata, O. trilobata, O.
watsonii; western United States, northwestern Mexico, Chile, Argentina),
Goodmania (1; G. luteola; California, Nevada), Eriogonum
(340–350; western North America, Mexico, western South America), Chorizanthe
(60–65; southwestern Canada, western Unites States, northwestern Mexico,
southern Peru, Chile, western Argentina), Hollisteria (1; H.
lanata; central California). – Mainly tropical and subtropical
(especially western North America to South America; Eriogoneae
especially in western North America). Usually bisexual (sometimes dioecious)
shrubs or lianas, usually with branch tendrils (in Antigonon foliar
tendrils) (rarely trees or herbs). Sheathing stipule (ocrea) absent in
Eriogoneae. Inflorescence usually racemose (in Eriogoneae
cymose with involucrum). n = 9, 11–12, 14, 16–22, 40–44. –
Harfordia and Pterostegia
form a monophyletic clade (Pterostegieae Torr. et A. Gray in Proc.
Amer. Acad. Arts 8: 146. 26 Jan 1870) sister to Eriogoneae in the
analyses by Kempton (2012). On the other hand, they are nested inside
Eriogoneae in the study by Sanchez & al. (2009). – Several
species of Chorizanthe
(C. interposita, C. rigida, C. watsonii of the
subgenus Amphietes) do not fit into the Eriogonoideae sensu
stricto clade in the analyses by Kempton (2012); possibly, they form a new
genus.
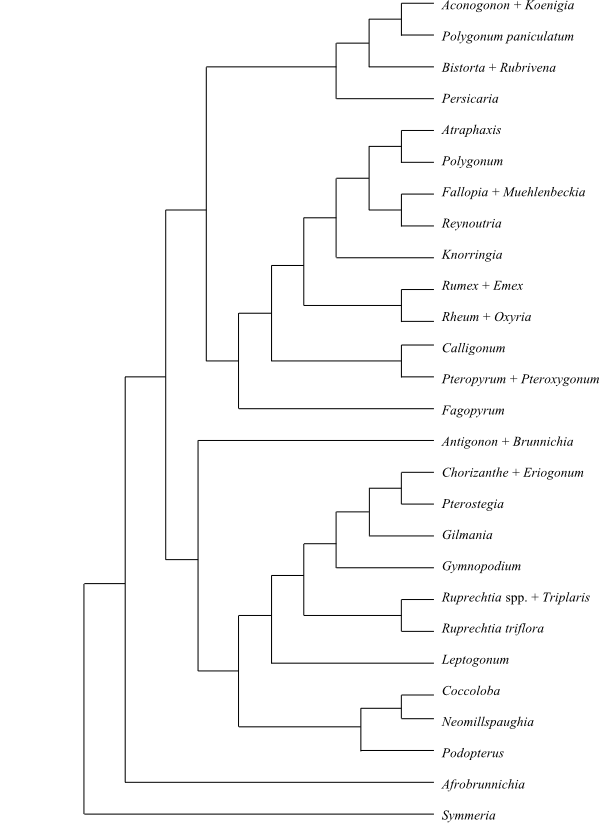 |
Cladogram of Polygonaceae based
on DNA sequence data (ITS; Sanchez & al. 2009; Burke & al.
2010; Sanchez & al. 2011; Schuster & al. 2011).
|
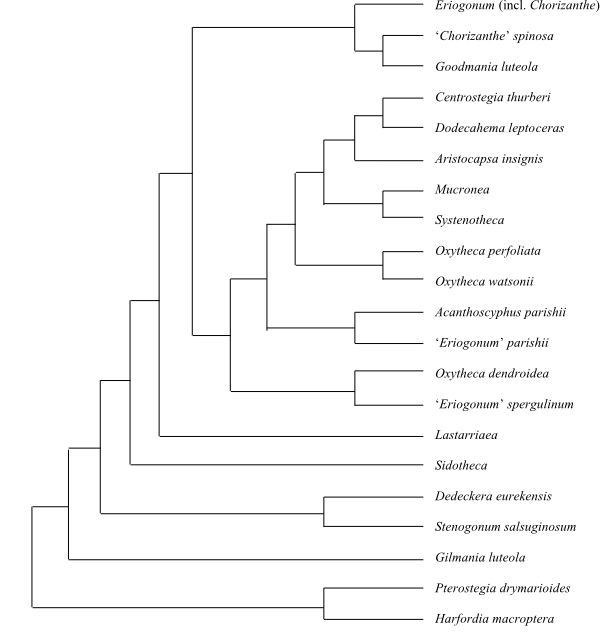 |
Cladogram (Bayesian inference) of
Eriogoneae based on DNA sequence data (Kempton 2012).
|
de Jussieu, Gen. Plant.: 312. 4 Aug 1789
[’Portulaceae’], nom. cons.
Portulacales Juss. ex
Bercht. et J. Presl, Přir. Rostlin: 238. Jan-Apr 1820
[‘Portulaceae’]; Portulacineae Engl.,
Syllabus, ed. 2: 113. Mai 1898
Genera/species 1/100–115
Distribution Cosmopolitan
except polar areas, with their largest diversity in tropical and subtropical
parts of North and South America.
Fossils Unknown.
Habit Bisexual, perennial or
annual herbs (in Portulaca suffrutescens somewhat lignified at stem
base). Roots often tuberous. Usually leaf succulents.
Vegetative anatomy Mycorrhiza
usually absent. C4 photosynthesis alternating with CAM
photosynthesis; leaves with Kranz’ anatomy. Root cortex often with
subelliptic thin-walled brachysclereids. Stem epidermis with or without
parallelocytic stomata. Phellogen ab initio epidermal. Delayed initiation of
stem periderm. Medulla sometimes with wide-band tracheids. Cortical fibres
absent. Secondary lateral growth normal or absent. Vessel elements with simple
perforation plates; lateral pits alternate. Imperforate tracheary xylem
elements libriform fibres with simple pits; non-septate. Wood rays multiseriate
or absent. Axial parenchyma? Thick-walled pericyclic extraxylary phloem fibre
caps absent. Sieve tube plastids P3cf type? Nodes 1:1, unilacunar with one leaf
trace? Sclereids often present. Mucilaginous idioblasts abundant. Tanniniferous
cells absent. Phloem parenchyma cells with phytoferritin? Parenchyma and
epidermis often with numerous calciumoxalate crystals or druses.
Trichomes Hairs unicellular or
multicellular, uniseriate; stem epidermis sometimes with papillae or
multicellular hairs.
Leaves Usually alternate
(rarely opposite), simple, entire, usually succulent, flat to terete, with
various ptyxis? Stipules and leaf sheath absent. Leaves with or without few to
numerous axillary biseriate or oligoseriate hairs or bristles (in Portulaca
somalica and P. wightiana multiseriate, scale-like). Petiole
vascular bundle transection arcuate? Venation pinnate. Stomata parallelocytic,
usually transversely orientated. Cuticular wax crystalloids usually absent
(rarely as relatively irregular platelets?). Mesophyll with mucilaginous
idioblasts. Leaf margin entire.
Inflorescence Terminal,
few-flowered, capitate, cymose, often surrounded by involucral bracts. Floral
prophylls (bracteoles) 2+2, inner pair median. Two large floral prophylls
(bracteoles) sometimes subtending flower and four smaller prophylls in a whorl
separated from first pair by short internode. Transverse floral prophylls with
axillary flowers, or absent; median prophylls without flowers. Sepaloid floral
prophylls two.
Flowers Actinomorphic, small.
Hypanthium present. Epigyny to half epigyny. Tepals (three to) five (to eight),
with imbricate aestivation, petaloid, free or slightly connate at base (rarely
absent). Nectary absent? Disc absent.
Androecium Stamens four to
numerous. Androecial ring primordium present. Filaments free from each other
and from tepals. Anthers dorsifixed, versatile, tetrasporangiate, introrse,
longicidal (dehiscing by longitudinal slits). Tapetum secretory, with
multinucleate cells. Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually polypantocolpate
(polyrugate), shed as monads, tricellular? at dispersal. Exine tectate, with
columellate infratectum, spinulate or echinate.
Gynoecium Pistil composed of
(four or) five (to eight) connate carpels. Ovary inferior or semi-inferior, ab
initio multilocular, later unilocular. Stylodia (four or) five (or six).
Stigmas papillate, Dry type. Pistillodium absent.
Ovules Placentation usually
free central (rarely basal). Ovules numerous per ovary, anatropous?, bitegmic,
crassinucellar. Micropyle endostomal. Outer integument ? cell layers thick.
Inner integument ? cell layers thick. Parietal tissue? Apical cells of
megasporangium often radially elongate. Megagametophyte monosporous,
Polygonum type. Synergids with a filiform apparatus? Antipodal cells
two?, ephemeral. Endosperm development ab initio nuclear. Endosperm haustoria?
Embryogenesis caryophyllad.
Fruit A pyxidium with apical
operculum, dehiscing together with perianth remnants, stamens and style as a
dry calyptra. Pericarp undifferentiated.
Seeds Aril hilar, spongy. Seed
coat? Anticlinal testal cell walls sinuous. Tegmen? Perisperm copious, starchy.
Endosperm sparse or absent. Embryo curved, well differentiated, without
chlorophyll. Cotyledons two. Radicula dorsal. Germination phanerocotylar?
Cytology n = (8–)10
DNA Intron absent from plastid
gene rpl2. 6 bp deletion in plastid gene ndhF. C. 500 bp
deletion in plastid gene rbcL.
Phytochemistry Insufficiently
known. Betalains (betacyanins, betaxanthins) and sterols present. Ellagic acid
and cyanogenic compounds not found.
Use Ornamental plants,
vegetables, medicinal plants.
Systematics Portulaca
(100–115; cosmopolitan except polar areas, with their largest diversity in
tropical and subtropical parts of North and South America; rare as indigenous
in temperate regions).
Portulaca
is sister to Cactaceae
in some analyses (e.g. Ocampo & Columbus 2010) or to the clade [Anacampserotaceae+Cactaceae] (e.g. Brockington
& al. 2013).
The axillary appendages may by remnants of
highly condensated axillary short shoots and hence homologous to the areoles in
Cactaceae (Nyffeler
& Eggli 2010).
Prance in Bull. Jard. Bot. État 38: 141. 30 Jun
1968
Rhabdodendrales
Doweld, Tent. Syst. Plant. Vasc.: xli. 23 Dec 2001;
Rhabdodendranae Doweld, Tent. Syst. Plant. Vasc.:
xli. 23 Dec 2001
Genera/species 1/3
Distribution Tropical South
America.
Fossils Unknown.
Habit Usually bisexual (rarely
androdioecious), evergreen trees or shrubs.
Vegetative anatomy Phellogen?
Cortical vascular bundles sometimes present. Anomalous secondary lateral growth
from successive cambia present in two species (not in Rhabdodendron
macrophyllum). Vessel elements with simple perforation plates; lateral
pits scalariform or alternate, bordered pits. Imperforate tracheary xylem
elements tracheids (libriform fibres absent) with simple or bordered pits,
non-septate. Vestured pits present. Wood rays uniseriate or multiseriate,
usually heterocellular (sometimes homocellular). Axial parenchyma abaxial,
apotracheal sparsely diffuse, or paratracheal scanty, vasicentric, or banded.
Intraxylary (concentric) phloem present. Sieve tube plastids Pcs type, with one
peripheral polygonal protein crystalloid and multiple starch grains. Nodes ?:?,
multilacunar with ? leaf traces. Parenchyma with resinous secretory cavities.
Dark-staining substances present especially in wood rays. Sclereids present.
Silica bodies sometimes present in wood. Acicular or elongate crystals,
styloids, crystal sand and other types of crystals and crystal complexes (e.g.
sphaerocrystals) abundant.
Trichomes Hairs multicellular,
fimbriate, short-stalked, peltate.
Leaves Alternate (spiral),
simple, entire, coriaceous, with revolute ptyxis. Stipules small, caducous or
absent; leaf sheath absent. Petiole vascular bundles transection annular, with
often separate bundles; wing bundles present; medullary bundles sometimes
present. Leaf base usually widened. Venation pinnate. Stomata anomocytic,
without subsidiary cells. Cuticular waxes? Lamina revolute, gland-dotted,
lysigenic secretory cavities with resins, scattered lipid bodies and on abaxial
side small short-stalked peltate hairs with silica inclusions. Mesophyll with
sclerenchymatous idioblasts, traversed by fibre-like simple or branched
sclereids representing elongations of vein endings; many mesophyll cells with
silica bodies and silicified walls. Leaf margin entire.
Inflorescence Axillary or
supra-axillary, simple or compound, raceme-like or racemose.
Flowers Actinomorphic.
Hypanthium present. Hypogyny. Sepals five, with imbricate quincuncial
aestivation, partially or entirely connate. Petals (four or) five, with
cochleate, quincuncial or valvate (imbricate below) aestivation, sepaloid,
gland-dotted, caducous, free. Nectary absent. Disc absent.
Androecium Stamens 27 to 53,
in three whorls. Filaments flattened, very short, persistent, free from each
other and from petals. Anthers basifixed, non-versatile, tetrasporangiate,
introrse?, longicidal (dehiscing by longitudinal slits), with monocotyledonous
wall development. Tapetum secretory. Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually tricolp(or)ate (rarely
tetracolp[or]ate), shed as monads, bicellular (or tricellular?) at dispersal.
Exine tectate or semitectate, with columellate infratectum, punctate to finely
reticulate.
Gynoecium Pistil composed of a
single carpel. Ovary superior, unilocular. Stylodium gynobasic, thick.
Stigmatic surfaces usually reaching from base or centre of abaxial side to apex
(unilateral, dorsal stigma), type? Pistillodium absent?
Ovules Placentation basal.
Ovules ab initio usually two, later one (one ovule degenerating) per ovary,
(hemi)campylotropous, epitropous, bitegmic in micropylar end (unitegmic
elsewhere), crassinucellar. Micropyle ?-stomal. Outer integument four or five
cell layers thick. Inner integument two to five cell layers thick. Parietal
tissue ten or more cell layers thick. Nucellar cap absent. Archespore
multicellular. Megagametophyte monosporous, aberrant Polygonum type
(unique variation). Endothelium not formed. Endosperm development ab initio
nuclear. Endosperm haustoria? Embryogenesis?
Fruit A small short-stalked
dry one-seeded drupe, surrounded at base by persistent calyx/hypanthium and
with swollen lignified distal part of pedicel.
Seeds Aril absent. Seed coat
testal. Testa thin. Exotestal cells tangentially elongate. Endotestal cells
shortly tracheidal. Tegmen? Perisperm poorly developed. Endosperm poorly
developed. Embryo curved, well differentiated, with chlorophyll. Radicula
curved inwards against hilum. Cotyledons two, large, fleshy. Germination
cryptocotylar.
Cytology n = 10
DNA
Phytochemistry Triterpenoids,
methylated and non-methylated ellagic acid, O-alkylated ellagic acid
derivatives, prodelphinidin, oleanolic acid derivatives, and cyanogenic
compounds present. Alkaloids not found.
Use Timber.
Systematics
Rhabdodendron (3; R. amazonicum, R. gardnerianum,
R. macrophyllum; tropical South America, especially the Amazon
Basin).
Rhabdodendron is sister to all other
core Caryophyllales
“above” the tamaricoid, polygonoid and droseroid lineages.
Behnke in Taxon 46: 503. 15 Aug 1997
Genera/species 1/1–2
Distribution Arid regions in
southwestern North America.
Fossils Unknown.
Habit Monoecious (sometimes
dioecious?), evergreen? shrubs with spines. Xeromorphic halophytes.
Vegetative anatomy Phellogen?
Secondary lateral growth anomalous (from concentric/successive cambia). Vessel
elements with simple perforation plates; lateral pits alternate, bordered pits.
Non-vestured pits present. Imperforate tracheary xylem elements libriform
fibres with simple or (reduced) bordered pits, non-septate (also vasicentric
tracheids). Wood rays absent. Axial parenchyma paratracheal vasicentric.
Intraxylary (concentric or diffuse) phloem present. Vessel and phloem elements
and/or parenchyma storied. Sieve tube plastids P3cf type, with a globular
central protein crystal and a subperipheral dense ring of protein filaments.
Nodes? Heartwood with gums etc. Parenchyma often with idioblasts containing
elongate and rhomboidal crystals or solitary styloids.
Trichomes Hairs ’sub-bladder
trichomes’ (Carolin 1983).
Leaves Alternate (spiral),
simple, entire, fleshy, linear, terete, with ? ptyxis. Stipules and leaf sheath
absent. Petiole vascular bundles? Venation? Stomata? Cuticular waxes? Raphides?
Leaf margin entire.
Inflorescence Male
inflorescence terminal, spike- or catkin-like. Female flowers axillary,
solitary or pairwise. Floral prophylls (bracteoles) absent at least in male
inflorescences (in female flowers sometimes present, bilobate, pairwise connate
into a tube).
Flowers Actinomorphic, small.
Probably half epigyny. Tepaloid structures (bracts or sepaloid tepals?) in
female flowers two, scale-like, fleshy, persistent, connivent into a tube,
tepals adnate to ovary. Male flowers inserted inside stalked peltate scales,
without tepals. Nectary absent. Disc absent.
Androecium Stamens usually one
to three (to four), inserted below a stalked peltate scale. Filaments short,
free. Anthers basifixed, non-versatile, tetrasporangiate, latrorse, longicidal
(dehiscing by longitudinal slits). Tapetum secretory? Staminodia absent.
Pollen grains
Microsporogenesis simultaneous? Pollen grains pantoporate, foraminate, with
raised pore margins, shed as monads, tricellular? at dispersal. Exine tectate,
with columellate infratectum, sculpturing?
Gynoecium Pistil composed of
two connate carpels. Ovary inferior?, unilocular. Style single, simple, short,
or absent. Stigmas two, horizontally expanded, papillate, type? Pistillodium
absent.
Ovules Placentation basal.
Ovule one per ovary, campylotropous?, bitegmic, crassinucellar. Micropyle
?-stomal. Outer integument ? cell layers thick. Inner integument ? cell layers
thick. Parietal tissue? Megagametophyte monosporous, Polygonum type?
Endosperm development? Endosperm haustoria? Embryogenesis?
Fruit A nutlet surrounded by
persistent and accrescent fleshy tepals, horizontally winged at apex.
Seeds Seed vertical. Aril
absent. Testa membranous. Exotesta? Endotesta? Tegmen? Perisperm copious?,
nutritious. Endosperm absent. Embryo peripheral, spirally twisted around
perisperm, with chlorophyll. Cotyledons two. Germination?
Cytology n = 9
DNA Deletion of 210 bp in
plastid genome?
Phytochemistry Insufficiently
known. Betalains (betacyanins and betaxanthins) present. Oxalic acid often
accumulated. Triterpene saponins? Anthocyanins not found.
Use Tools.
Systematics Sarcobatus
(2; S. baileyi: Nevada; S. vermiculatus: southwestern Canada,
western United States, northern Mexico).
Sarcobatus
is sister to Agdestis (e.g. Brockington 2013) or to Nyctaginaceae (with weak
support in matK analyses).
van Tieghem in Bot. Jahresber. (Just) 25(2): 422.
19 Jan 1900 [emend. Tiegh. ex Reveal et Hoogland, Bull. Mus. Natl. Hist. Nat.
(Paris), ser. 4, B Adansonia 12: 206. 24 Nov 1990]
Simmondsiales Reveal
in Novon 2: 239. 13 Oct 1992; Simmondsianae Doweld,
Tent. Syst. Plant. Vasc.: xliii. 23 Dec 2001;
Simmondsiineae Reveal in Kew Bull. 66: 48. Mar
2011
Genera/species 1/1
Distribution Southwestern
United States, northwestern Mexico.
Fossils Unknown.
Habit Dioecious, evergreen
shrubs. Xerophytes.
Vegetative anatomy Phellogen
ab initio usually pericyclic. Secondary lateral growth anomalous (from
concentric/successive cambia). Vessel elements with simple perforation plates;
lateral pits alternate or opposite, bordered pits. Imperforate tracheary xylem
elements tracheids with large bordered pits, non-septate. Wood rays rare,
uniseriate or multiseriate, heterocellular. Axial parenchyma rare, apotracheal
diffuse, or paratracheal scanty, or absent. Intraxylary phloem present. Sieve
tube plastids Ss type, with few small starch grains. Nodes 1:1, unilocular with
one leaf trace. Calciumoxalate as druses and prismatic crystals.
Trichomes Hairs short,
unicellular or multicellular, uniseriate.
Leaves Opposite, simple,
entire, coriaceous, articulated near leaf insertion, with flat ptyxis. Stipules
and leaf sheath absent. Petiole vascular bundle transection arcuate? Venation
pinnate, brochidodromous. Stomata cyclocytic or laterocytic (or anomocytic?),
sunken. Cuticular waxes? (organ specific). Mesophyll with calciumoxalate as
druses and single prismatic crystals. Leaf margin entire.
Inflorescence Male
inflorescence terminal or axillary, cymose, head-like. Female flowers usually
solitary axillary (rarely fascicular in axillary racemes).
Flowers Actinomorphic, small.
Hypogyny. Tepals (four or) five (or six), with imbricate aestivation, sepaloid,
in male flowers fimbriate, in female flowers foliaceous, persistent. Petals
absent. Nectary absent. Disc absent.
Androecium Stamens (4+4 or)
5+5 (to 8+8), diplostemonous. Filaments short, stout, free from each other and
from tepals. Anthers longer than connectives, basifixed or subbasifixed,
non-versatile, tetrasporangiate, extrorse (or latrorse?), longicidal (dehiscing
by longitudinal slits). Tapetum secretory, in later stages with a tendency to
disintegration of membranes. Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains tricolpate to indistinctly
triporate (aperture with operculoid central part), shed as monads, bicellular
at dispersal. Exine tectate, with columellate infratectum, punctate-scabrate,
beset with small dots of tiny spinules.
Gynoecium Pistil composed of
three (or four) connate carpels. Ovary superior, usually trilocular (sometimes
quadrilocular). Stylodia three (or four), free, long protruding, subulate,
recurved, papillate and hairy, caducous, with stylar canal. Stigmas little
differentiated, papillate, type? Pistillodium absent.
Ovules Placentation apical to
subapical-axile. Ovule one (or two) per carpel, anatropous, pendulous,
apotropous, bitegmic, tenuinucellar (crassinucellar?). Micropyle endostomal.
Outer integument six to ten cell layers thick. Inner integument three to five
cell layers thick. Obturator absent. Parietal tissue (two to) five cell layers
thick. Nucellar cap present (sometimes poorly developed). Megagametophyte
monosporous, Polygonum type. Synergids with a filiform apparatus.
Endosperm development ab initio nuclear (heavily reduced). Endosperm haustoria?
Embryogenesis?
Fruit A usually one-seeded
(rarely two- och three-seeded) loculicidal capsule, with strongly accrescent
calyx and persistent columella.
Seeds Aril absent. Seed coat
testal. Testa multiplicative, vascularized (prominent postchalazal vascular
bundles reaching to micropyle). Exotestal cells palisade, with thickened walls.
Mesotesta aerenchymatous. Endotesta mainly collapsed. Tegmen collapsed.
Perisperm not developed. Endosperm sparse or absent. Embryo large, straight,
well differentiated, chlorophyll? Cotyledons two, thick, fleshy, with
simmondsin (a nitrile glycoside), aleurone grains and a liquid wax. Germination
phanerocotylar?
Cytology n = 13
DNA Intron present in the
plastid gene rpl2.
Phytochemistry Foliar
flavonoids, condensed tannins, proanthocyanidins, anthocyanin, pinitol and
simmondsinoid compounds present (simmondsin in jojoba-meal from ground seeds).
Waxes consisting of high-molecular esters (C36 to C46 long straight-chain wax
esters) of monoethylenic acids. Ellagic acid and alkaloids not found.
Use Oils and waxes (jojoba),
medicinal plant.
Nakai in J. Jap. Bot. 18: 108. 10 Mar 1942
Stegnospermatineae
Nakai in J. Jap. Bot. 18: 108. 10 Mar 1942
Genera/species 1/4
Distribution Coastal areas in
northwestern and western Mexico and Central America, the West Indies.
Fossils Unknown.
Habit Bisexual, evergreen
shrubs or small trees, more or less climbing.
Vegetative anatomy Phellogen
in periderm. Secondary lateral growth anomalous (from concentric/successive
cambia). Vessel elements with simple perforation plates; lateral pits
alternate. Imperforate tracheary xylem elements tracheids with bordered pits.
Wood rays usually multiseriate (sometimes uniseriate), homocellular or
heterocellular. Axial parenchyma apotracheal diffuse, or paratracheal scanty,
or absent. Tyloses sometimes abundant. Phloem fibres absent. Sieve tube
plastids P3c’f type, with a central polygonal protein crystal and a
subperipheral dense ring of protein filaments. Nodes? Parenchyma, cortex and
medulla with idioblasts containing sphaerite crystals.
Trichomes Hairs absent.
Leaves Alternate (spiral),
simple, entire, fleshy, with ? ptyxis. Stipules and leaf sheath absent. Petiole
vascular bundles? Venation pinnate, brochidodromous. Stomata anomocytic.
Cuticular waxes? Lamina with idioblasts containing sphaerite crystals. Leaf
margin entire, transparent.
Inflorescence Terminal or
axillary, thyrsoid, raceme- or umbel-like.
Flowers Actinomorphic.
Hypogyny. Tepals five, with quincuncial aestivation, sepaloid, persistent,
free. ‘Petals’ (two to) five, with imbricate aestivation, probably
staminodial in origin, free. Nectaries present in depressions at carpel bases.
Disc absent.
Androecium Stamens five or
eight to ten, in one whorl. Filaments connate at base, antepetalous stamens
adnate to ‘petals’ (epipetalous). Anthers basifixed to subbasifixed,
non-versatile, tetrasporangiate, introrse, longicidal (dehiscing by
longitudinal slits). Tapetum secretory. Staminodia probably present,
petaloid.
Pollen grains
Microsporogenesis simultaneous? Pollen grains tricolpate, shed as monads,
tricellular at dispersal. Exine tectate, with columellate infratectum,
punctate, spinulate. Endexine massive.
Gynoecium Pistil composed of
two to five connate alternisepalous (alternitepalous) carpels. Ovary superior,
ab initio bilocular to quinquelocular, later unilocular. Style single, simple,
short, or absent. Stigmas two to five, subulate, recurved, ventrally papillate,
type? Pistillodium absent.
Ovules Placentation basal,
gradually free central. Ovule one per carpel, amphitropous, erect, epitropous,
bitegmic, crassinucellar. Micropyle ?-stomal. Outer integument ? cell layers
thick. Inner integument ? cell layers thick. Obturator placental. Parietal
tissue. Nucellar cap absent. Megagametophyte monosporous, Polygonum
type. Endosperm development ab initio nuclear. Endosperm haustoria?
Embryogenesis?
Fruit A coriaceous capsule,
dehiscing by three to five valves.
Seeds Aril present, covering
larger part of seed. Seed coat exotestal-endotegmic? Exotesta palisade, with
non-lignified cell walls. Endotesta? Exotegmen? Endotegmen enlarged,
persistent. Perisperm copious, nutritious. Endosperm almost absent. Embryo
curved, chlorophyll? Cotyledons two. Germination?
Cytology n = 36
DNA
Phytochemistry Very
insufficiently known. Betalains (betacyanins, betaxanthins) present. Triterpene
saponins probably abundant. Alkaloids? Pinitol? Proanthocyanidins not found.
Use Soap.
Systematics
Stegnosperma (4; S. cubense, S. halimifolium, S.
sanchezii, S. watsonii; coastal areas in Baja California, the
Sonoran Desert in Mexico, Central America, the Greater Antilles).
Stegnosperma is sister to the
remaining “betalain clade” above the Achatocarpaceae-Amaranthaceae-Caryophyllaceae clade.
The sieve tube plastids resemble those in
Caryophyllaceae
and Achatocarpaceae.
Doweld, Tent. Syst. Plant. Vasc.: xlii. 23 Dec
2001
Genera/species 2/c 15
Distribution Africa,
Madagascar, western North America to South America, the West Indies, with their
largest diversity in Mexico.
Fossils Unknown.
Habit Bisexual, usually small
shrubs (sometimes perennial herbs, suffrutices or small trees?;
‘Talinella’ lianoid shrubs) with somewhat succulent leaves.
Subterraneous organs often tuberous. Aerial parts often ephemeral.
Vegetative anatomy
C3 photosynthesis alternating with CAM photosynthesis. Stem
epidermis usually with parallelocytic stomata, parallel to stem axis. Phellogen
ab initio usually epidermal (rarely outer-cortical). Precocious or delayed
initiation of stem periderm. Vessel elements with simple perforation plates;
lateral pits alternate to pseudoscalariform or scalariform. Imperforate
tracheary xylem elements usually libriform fibres (occasionally
fibre-tracheids) with simple pits, septate. Wood rays multiseriate or absent.
Axial parenchyma scanty vasicentric. Tyloses sometimes present. Thick-walled
pericyclic extraxylary phloem fibre caps present. Sieve tube plastids P3cf
type? Nodes 1:1, unilacunar with one leaf trace? Sclereids (thin-walled,
elongated with lignified walls) often present. Mucilage idioblasts and
tanniniferous cells abundant. Phloem parenchyma cells with phytoferritin?
Epidermis and parenchyma often with numerous crystals or druses of
calciumoxalate.
Trichomes Hairs multicellular,
uniseriate, or absent; stem epidermis often with papillae or multicellular
hairs.
Leaves Alternate (spiral),
simple, entire, succulent, with ? ptyxis. Stipules and leaf sheath absent.
Leaves usually with single or paired axillary scale-like vascularized prophylls
representing rudimentary axillary short shoots (sometimes with tannins).
Petiole vascular bundle transection arcuate; wing bundles present. Venation
pinnate, brochidodromous? Stomata parallelocytic, transversely orientated.
Cuticular wax crystalloids? Mesophyll with mucilaginous idioblasts. Leaf margin
entire.
Inflorescence Terminal,
thyrsoid paniculate cymose (sometimes raceme-like; in ‘Talinella’
congested heads), or flowers solitary axillary. Sepaloid floral prophylls
(bracteoles) two, deciduous or persistent, often scale-like.
Flowers Actinomorphic.
Hypogyny. Tepals (two to) five, with quincuncial imbricate aestivation,
petaloid, caducous, free or connate at base. Nectaries extrastaminal, inserted
at tepal bases. Disc absent.
Androecium Stamens c. 15 to c.
35. Filaments free from each other, free or adnate at base to tepals. Anthers
dorsifixed, versatile, tetrasporangiate, extrorse, longicidal (dehiscing by
longitudinal slits). Tapetum secretory? Staminodia absent.
Pollen grains
Microsporogenesis simultaneous? Pollen grains polyforate or polyrugate, shed as
monads, tricellular? at dispersal. Exine tectate, with columellate infratectum,
spinulate.
Gynoecium Pistil composed of
three (to five) connate carpels. Ovary superior, usually unilocular (in
‘Talinella’ multilocular). Style single, thin. Stigma bilobate or
trilobate, type? Pistillodium absent.
Ovules Placentation free
central. Ovaries numerous per ovary, anatropous?, bitegmic, crassinucellar.
Micropyle ?-stomal. Outer integument ? cell layers thick. Inner integument ?
cell layers thick. Parietal tissue? Apical cells of megasporangium radially
elongate? Megagametophyte monosporous, Polygonum type? Endosperm
development nuclear? Endosperm haustoria? Embryogenesis?
Fruit Usually a loculicidal
valvicidal capsule (dehiscent from apex and/or base), often with caducous
exocarp separating from fibrous persistent endocarp (capsule in
‘Talinella’ berry-like, juicy and mucilaginous; capsule in
Amphipetalum a pyxidium, opening by a circumscissile operculum).
Pericarp epidermis papillate. Capsule covered by dry remnants of perianth,
stamens and style, these shed together as a calyptra.
Seeds Testa with strophiole
near base. Seed coat? Exotesta? Endotesta? Tegmen? Perisperm copious, starchy?
Endosperm absent? Embryo curved and sourrounding perisperm. Cotyledons two.
Germination phanerocotylar?
Cytology n = 8 eller 12?
DNA 6 bp deletion in plastid
gene ndhF.
Phytochemistry Virtually
unknown. Betalains present.
Use Ornamental plants.
Systematics Talinum (c 14;
southern Africa, Madagascar, Mexico, Central and South America, the West
Indies), Amphipetalum (1; A. paraguayense; Bolivia,
Paraguay).
Talinaceae may be sister-group
to a clade comprising Portulacaceae, Anacampserotaceae and
Cactaceae (e.g.
Brockington & al. 2013).
The axillary scale-like prophylls are probably
remnants of highly condensed axillary short shoots and homologous to the
areoles in the Cactaceae
(Nyffeler & Eggli 2010).
Link, Enum. Hort. Berol. Alt. 1: 291. 16 Mar-30
Jun 1821, nom. cons.
Tamaricales Bercht.
et J. Presl, Přir. Rostlin: 233. Jan-Apr 1820 [‘Tamariscinae’];
Reaumuriaceae Ehrenb. ex Lindl., Intr. Nat. Syst.
Bot.: 48. 27 Sep 1830 [‘Reaumurieae’];
Reaumuriales Lindl. in C. F. P. von Martius, Consp.
Regn. Veg.: 61. Sep-Oct 1835 [‘Reaumurieae’];
Tamaricineae Engl., Syllabus, ed. 2: 151. Mai 1898
Genera/species
3–4/75–90
Distribution Mostly drier
regions of northern, northeastern and southwestern Africa, and Eurasia
eastwards to East Asia, with their largest diversity in the Mediterranean and
the irano-turanian areas; naturalized in North America.
Fossils Fossil wood of
Tamarix has been found in Pleistocene (possibly also Pliocene) layers
in North Africa.
Habit Usually bisexual (in
Tamarix rarely dioecious), evergreen trees, shrubs or suffrutices.
Xerophytes, rheophytes or halophytes.
Vegetative anatomy Phellogen
ab initio usually superficially (rarely deeply) sited. Vessel elements with
simple perforation plates; lateral pits alternate, bordered pits. Imperforate
tracheary xylem elements usually libriform fibres (sometimes tracheids) with
simple or (reduced) bordered pits, non-septate. Wood rays uniseriate or
multiseriate, heterocellular. Axial parenchyma paratracheal scanty, confluent,
vasicentric, or banded. Vessel elements and/or parenchyma partially storied.
Sieve tube plastids S type. Nodes? Heartwood often with resins etc.
Calciumsulphate crystals sometimes present. Prismatic calciumoxalate crystals
often abundant.
Trichomes Hairs unicellular or
multicellular, uniseriate; salt glands common.
Leaves Alternate (spiral),
simple, entire, usually small and scale-like or subulate (in Reaumuria
coriaceous or fleshy, not scale-like), with ? ptyxis. Stipules and leaf sheath
absent. Venation? (often single-veined). Stomata usually anomocytic (sometimes
paracytic), often on adaxial side only, often deeply sunken. Cuticular wax
crystalloids as tubuli or curled rodlets. Epidermis often with sunken,
multicellular, salt-excreting glands. Leaf margin entire.
Inflorescence Usually
terminal, simple or branched, catkin-, spike- or raceme-like, botryoid to
paniculate (flowers in Reaumuria solitary terminal). Floral prophylls
(bracteoles) absent.
Flowers Actinomorphic, usually
small. Hypogyny. Sepals four or five (or six), with imbricate aestivation,
persistent, usually free (in Reaumuria connate below). Petals four or
five (or six), with usually imbricate (in Reaumuria contorted)
aestivation, persistent or caducous, usually without scale-like appendage (in
Reaumuria two adaxial scale-like appendages at petal base, without
nectary), free or slightly connate at base, usually inserted at fleshy
nectariferous disc. Nectariferous disc usually present below petals and
stamens, often annular (rarely intrastaminal and/or extrastaminal, in
Reaumuria absent).
Androecium Stamens in
Myricaria 5+5 (antesepalous longer than antepetalous), in
Tamarix and Myrtama four or five (to 14), in
Reaumuria five to more than twelve (from ten primordia). Filaments
usually connate at base, usually in a single group (in Reaumuria often
five antepetalous groups), sometimes free, usually inserted on fleshy
nectariferous disc, free from tepals. Anthers dorsifixed, versatile?,
tetrasporangiate, introrse, latrorse or extrorse, longicidal (dehiscing by
longitudinal slits). Tapetum secretory. Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains tri- or tetracolpate, shed as
monads or tetrads, bicellular (Tamarix) or tricellular (Myricaria
germanica) at dispersal. Exine semitectate, with columellate infratectum,
reticulate or microreticulate.
Gynoecium Pistil composed of
(two or) three or four (or five) antepetalous connate paracarpous carpels;
median carpel abaxial? Ovary superior, unilocular (sometimes seemingly
bilocular to quinquelocular due to intrusive placentae). Stylodia (two or)
three or four (or five), free or partially connate (absent in
Myricaria). Stigmas with usually widened lobes, non-papillate, Wet
type. Pistillodium absent?
Ovules Placentation parietal,
basal or basal-parietal. Ovules two to numerous per carpel, anatropous,
ascending, bitegmic, weakly crassinucellar. Micropyle endostomal. Outer
integument two or three cell layers thick. Inner integument two or three cell
layers thick. Megasporangium very thin. Parietal tissue approx. one cell layer
thick (parietal cell often not dividing). Megagametophyte tetrasporous,
Fritillaria, Adoxa, Drusa or Chrysanthemum
type (also within the same species of Tamarix), sometimes 16-nucleate,
bipolar. Synergids occasionally with a filiform apparatus. Endosperm
development ab initio nuclear or cellular. Endosperm haustoria absent.
Embryogenesis solanad. Polyembryony frequent.
Fruit A loculicidal
capsule.
Seeds Seeds with long
unicellular hairs (Reaumuria) or a chalazal coma of long hairs
(Myricaria, Tamarix, Myrtama). Aril absent. Seed
coat exotestal. Exotestal cells periclinally elongate, thick-walled. Endotestal
cells thin-walled, with crystals. Tegmen entirely or largely absent. Perisperm
often copious, thin. Endosperm usually absent (occasionally sparse then usually
oily and proteinaceous; in Reaumuria sparse, starchy). Embryo large,
straight, well differentiated, chlorophyll? Suspensor massive. Cotyledons two.
Germination phanerocotylar.
Cytology n = (10) 12 (18, 24,
36) (Tamarix); n = 12 (Myricaria); n = 11
(Reaumuria)
DNA Intron present in plastid
gene rpl2?
Phytochemistry Flavonol
sulphates and bisulphates (kaempferol, quercetin or tamarixin), cyanidin,
methylated and non-methylated ellagic acids, gallic acid, gallic and ellagic
tannins, alkaloids, pinitol, and syringaresinol present. Myricetin, saponins
and cyanogenic compounds not found.
Use Ornamental plants,
medicinal plants, dyeing substances, tanning.
Systematics Reaumuria
(c 12; eastern Mediterranean to Pakistan and Central Asia); Tamarix
(50–60; southern Europe, the Mediterranean to eastern and southern Asia,
Africa), Myricaria
(10–15; temperate regions of Europe to Central Asia; incl.
Myrtama?), Myrtama (1; M. elegans; Pakistan,
Kashmir, Tibet; in Myricaria?).
Tamaricaceae are
sister-group to Frankenia
(Frankeniaceae).
Myrtama is morphologically
intermediate between Myricaria
and Tamarix. The
clade [Hololachna+Reaumuria] is sister to the remaining
Tamaricaceae (Gaskin
& al. 2004).
 |
Cladogram of Tamaricaceae based
on DNA sequence data (Gaskin & al. 2004).
|
Literature
Abdel Bari E. 1973. Cytological studies in
the genus Silene L. – New Phytol. 72: 833-838.
Acosta JM, Perreta M, Amsler A, Vegetti AC.
2009. The flowering unit in the synflorescences of Amaranthaceae. – Bot. Rev. 75:
363-376.
Adamec L. 1995. Ecological requirements and
recent European distribution of the aquatic carnivorous plant Aldrovanda
vesiculosa L.: a review. – Folia Geobot. Phytotaxon. 30: 53-61.
Adamec L. 2009. Ecophysiological
investigation on Drosophyllum lusitanicum: why doesn’t the plant dry
out? – Carniv. Plant Newsl. 38: 71-74.
Adamec L, Kohout P, Benes K. 2006. Root
anatomy of three carnivorous plant species. – Carniv. Plant Newsl. 35:
19-22.
Adams LG, West JG, Cowley KJ. 2008. Revision
of Spergularia (Caryophyllaceae) in Australia.
– Aust. Syst. Bot. 21: 251-270.
Adamson RS. 1955a. The South African species
of Aizoaceae I.
Adenogramma and Polpoda. – J. South Afr. Bot. 21: 83-95.
Adamson RS. 1955b. The South African species
of Aizoaceae II.
Tetragonia. – J. South Afr. Bot. 21: 109-154.
Adamson RS. 1956. The South African species
of Aizoaceae III.
Galenia. – J. South Afr. Bot. 22:87-127.
Adamson RS. 1958. The South African species
of Aizoaceae IV.
Mollugo, Pharnaceum, Coelanthum, and
Hypertelis. – J. South Afr. Bot. 24: 11-66.
Adamson RS. 1959a. The South African species
of Aizoaceae VI.
Acrosanthes. – J. South Afr. Bot. 25: 23-28.
Adamson RS. 1959b. The South African species
of Aizoaceae VII.
Aizoon. – J. South Afr. Bot. 25: 29-51.
Adamson RS. 1959c. The South African species
of Aizoaceae VIII.
Psammotropha. – J. South Afr. Bot. 25: 51-68.
Adamson RS. 1960. The phytogeography of Molluginaceae with reference to
southern Africa. – J. South Afr. Bot. 26: 17-35.
Adamson RS. 1961a. The South African species
of Aizoaceae X. Gisekia.
– J. South Afr. Bot. 27: 131-137.
Adamson RS. 1961b. The South African species
of Aizoaceae XI.
Plinthus. – J. South Afr. Bot. 27: 147-151.
Adlassnig W, Peroutka M, Lendl T. 2011. Traps
of carnivorous pitcher plants as a habitat: composition of the fluid,
biodiversity and mutualistic activities. – Ann. Bot. 107: 181-194.
Aellen P. 1929. Beitrag zur Systematik der
Chenopodium-Arten Amerikas, vorwiegend auf Grund der Sammlung des
United States National Museum in Washington, D.C. I. – Feddes Repert. 26:
31-64.
Aellen P. 1930. Die Wolladventiven
Chenopodien Europas. – Verh. Naturf. Ges. Basel 41: 77-104.
Aellen P. 1937-1938. Revision der
australischen und neuseeländischen Chenopodiaceae I: Theleophyton,
Atriplex, Morrisiella, Blackiella,
Senniella, Pachypharynx. – Bot. Jahrb. Syst. 68:
345-434.
Aellen P. 1938a. Halimione Aellen,
eine rehabilitierte Chenopodiaceen-Gattung. – Verh. Naturforsch. Ges. Basel
49: 118-130.
Aellen P. 1938b. Die orientalischen
Obione-Arten. – Verh. Naturforsch. Ges. Basel 49: 131-137.
Aellen P. 1939a. Die Atriplex-Arten
des Orients. – Bot. Jahrb. Syst. 70: 1-66.
Aellen P. 1939b. Exomis und
Manochlamys in Südafrika. – Bot. Jahrb. Syst. 70: 373-381.
Aellen P, Just T. 1943. Key and synopsis of
the American species of the genus Chenopodium L. – Amer. Midl.
Natur. 30: 47-76.
Aellen P. 1967. New Chenopodiaceae from
Turkey. – Notes Roy. Bot. Gard. Edinb. 28: 29-34.
Aellen P, Just T. 1934. Key and synopsis of
the American species of the genus Chenopodium L. – Amer. Midl.
Natur. 30: 47-76.
Aellen P, Townsend C. 1972. Fadenia
– a new genus of Chenopodiaceae from tropical Africa. – Kew Bull. 27:
501.
Ahmed M, Khaleduzzaman M, Rashid MA. 1988.
Chalcone derivatives from Polygonum lapathifolium. – Phytochemistry
27: 2359-2360.
Ahmed M, Khaleduzzaman M, Islam MS. 1990.
Isoflavan-4-ol, dihydrochalcone and chalcone derivatives from Polygonum
lapathifolium. – Phytochemistry 29: 2009-2011.
Airy Shaw HK. 1952. On the Dioncophyllaceae, a
remarkable new family of flowering plants. – Kew Bull. 1951: 327-347.
Akhani H. 2008. Taxonomic revision of the
genus Salicornia L. (Chenopodiaceae) in central and southern Iran. –
Pakistan J. Bot. 40: 1635-1655.
Akhani H, Trimborn P, Ziegler H. 1997.
Photosynthetic pathways in Chenopodiaceae from Africa, Asia and Europe with
their ecological, phytogeographical and taxonomical importance. – Plant Syst.
Evol. 206: 187-221.
Akhani H, Ghobadnejhad M, Hashemi SMH. 2003.
Ecology, biogeography and pollen morphology of Bienertia cycloptera
Bunge ex Boiss. (Chenopodiaceae), an enigmatic C4 plant without Kranz anatomy.
– Plant Biol. 5: 167-178.
Akhani H, Barroca J, Koteeva N, Voznesenskaya
E, Franceschi V, Edwards G, Ghaffari SM, Ziegler H. 2005. Bienertia
sinuspersici (Chenopodiaceae): a new species from Southwest Asia and
discovery of a third terrestrial C4 plant without Kranz anatomy. – Syst. Bot.
30: 290-301.
Akhani H, Edwards G, Roalson EH. 2007.
Diversification of the Old World Salsoleae s.l. (Chenopodiaceae): molecular
phylogenetic analysis of nuclear and chloroplast data sets and a revised
classification. – Intern. J. Plant Sci. 168: 931-956.
Akhani H, Chatrenoor T, Dehghani M,
Khoshravesh R, Mahdavi P, Matinzadeh Z. 2012. A new species of
Bienertia (Chenopodiaceae) from Iranian salt deserts: a third species
of the genus and discovery of a fourth terrestrial C4 plant without Kranz
anatomy. – Plant Biosyst. 16: 550-559.
Akhani H, Malekmohammadi M, Hahdavi P,
Gharibiyan A, Chase MW. 2013. Phylogenetics of the Irano-Turanian taxa of
Limonium (Plumbaginaceae) based on ITS
nrDNA sequences and leaf anatomy provides evidence for species delimitation and
relationships of lineages. – Bot. J. Linn. Soc. 171: 519-550.
Akhani H, Greuter W, Roalson EH. 2014. Notes
on the typification and nomenclature of Salsola and Kali
(Chenopodiaceae). – Taxon 63: 647-650.
Akhani H, Khoshravesh R, Malekmohammadi M.
2016. Taxonomic novelties from Irano-Turanian region and NE Iran:
Oreosalsola, a new segregate from Salsola s.l., two new
species in Anabasis and Salvia, and two new combinations in
Caroxylon and Seseli. – Phytotaxa 249(1). DOI:
http://dx.doi.org/10.11646/phytotaxa.249.1.7
Aksoy A, Hamzaoglu E, Kilic S. 2009. A new
species of Silene L. (Caryophyllaceae) from Turkey.
– Bot. J. Linn. Soc. 158: 730-733.
Alain H. 1960. Novedades en la flora cubana
XIII. – Candollea 17: 113-121.
Albert VA, Williams SE, Chase MW. 1992.
Carnivorous plants: phylogeny and structural evolution. – Science 257:
1491-1495.
Al-Eisawi D. 1989. Pollen morphology of Caryophyllaceae in Jordan. –
Mitt. Bot. Staatssamml. München 28: 599-614.
Alioshina LA. 1963. Morphology of pollen
grains in the genus Claytonia Gronov. and allied genera. – Bot.
Žurn. 48: 1191-1196.
Almaraz-Abarca N, Campos MG, Delgado-Alvarado
EA, Ávila-Reyes JA, Herrera-Corral J, González-Valdez LS, Naranjo-Jiménez N,
Frigerio C, Tomatas AF, Almeida AJ, Vieira A, Uribe-Soto JN. 2008. Pollen
flavonoid/phenolic acid composition of four species of Cactaceae and its taxonomic
significance. – Amer. J. Agric. Biol. Sci. 3: 534-543.
Altesor A, Silva C, Ezcurra E. 1994.
Allometric neoteny and the evolution of succulence in cacti. – Bot. J. Linn.
Soc. 114: 282-292.
Altesor A, Silva C, Ezcurra E. 1994.
Allometric neoteny and the evolution of succulence in cacti. – Bot. J. Linn.
Soc. 114: 282-292.
Al-Turki TA. 1992. Systematic and ecological
studies of Suaeda and Salicornia from Saudi Arabia and
Britain. – Ph.D. diss., University of East Anglia, Norwich, England.
Al-Turki TA, Omer S, Ghafoor A. 2000. A
synopsis of the genus Atriplex L. (Chenopodiaceae) in Saudi Arabia.
– Feddes Repert. 111: 261-293.
Al-Turki TA, Swarupanadan K, Wilson PG. 2003.
Primary vasculature in Chenopodiaceae: a re-interpretation and implications for
systematics and evolution. – Bot. J. Linn. Soc. 143: 337-374.
Amini E, Zarre SH, Asadi M. 2011. Seed
micromorphology and its systematic significance in Gypsophila (Caryophyllaceae) and allied
genera. – Nord. J. Bot. 29: 660-669.
Amiri N, Sharifnia F. 2007. Taxonomic
revision of Polygonum sections in Iran by palynological characters.
– Rostaniha 8: 85-93. [In Iranian]
Anderson EF. 1960. A revision of
Ariocarpus (Cactaceae)
I. The status of the proposed genus Roseocactus. – Amer. J. Bot. 47:
582-589.
Anderson EF. 1962. A revision of
Ariocarpus (Cactaceae)
II. The status of the proposed genus Neogomesia. – Amer. J. Bot. 49:
615-622.
Anderson EF. 1967. A study of the proposed
genus Obregonia (Cactaceae). – Amer. J. Bot. 54:
897-903.
Anderson EF. 1979. Peyote, the divine cactus.
– University of Arizona Press, Tucson, Arizona.
Anderson EF. 1986. A revision of the genus
Neolloydia B. & R. (Cactaceae). – Bradleya 4: 1-28.
Anderson EF. 1987. A revision of the genus
Thelocactus B. & R. (Cactaceae). – Bradleya 5:
49-76.
Anderson EF. 1996. The genus Opuntia
in the Galápagos Islands. – Cact. Succ. J. (US) 68: 298-305.
Anderson EF. 1999. Some nomenclatural changes
in the Cactaceae, subfamily
Opuntioideae. – Cact. Succ. J. (Los Angeles) 71: 324-325.
Anderson EF. 2001. The cactus family. –
Timber Press, Portland, Oregon.
Anderson EF. 2005. Das grosse
Kakteen-Lexikon. – Ulmer, Stuttgart.
Anderson EF, Boke NH. 1969. The genus
Pelecyphora (Cactaceae):
resolution of a controversy. – Amer. J. bot. 56: 314-326.
Anderson EF, Fitz Maurice WA. 1998.
Ariocarpus revisited. – Haseltonia 5: 1-20.
Anderson EF, Ralston ME. 1978. A study of
Thelocactus (Cactaceae)
I. The status of the proposed genus Gymnocactus. – Cact. Succ. J.
(U.S.) 50: 216-224.
Anderson EF, Skillman SM. 1984. A comparison
of Aztekium and Strombocactus (Cactaceae). – Syst. Bot. 9:
42-49.
Anton MA, Hernández-Hernández T, De-Nova A,
Sosa V. 2014. Evaluating the phylogenetic position of the monotypic family Halophytaceae (Portulacineae, Caryophyllales) based on
plastid and nuclear molecular data sets. – Bot. Sci. 92: 351-361.
Applequist WL. 2005. A revision of the
Malagasy endemic Talinella (Portulacaceae). – Adansonia,
sér. III, 27: 47-80.
Applequist WL, Pratt DB. 2005. The Malagasy
endemic Dendroportulaca (Portulacaceae) is referable to
Deeringia (Amaranthaceae): molecular and
morphological evidence. – Taxon 54: 681-687.
Applequist WL, Wallace RS. 2000. Phylogeny of
the Madagascan endemic family Didiereaceae. – Plant Syst.
Evol. 221: 157-166.
Applequist WL, Wallace RS. 2001. Phylogeny of
the portulacaceous cohort based on ndhF sequence data. – Syst. Bot.
26: 406-419.
Applequist WL, Wallace RS. 2002. Deletions in
the plastid trnT-trnL intergenic spacer define clades within
Cactaceae subfamily Cactoideae.
– Plant Syst. Evol. 231: 153-162.
Applequist WL, Wallace RS. 2003. Expanded
circumscription of Didiereaceae and its division
into three subfamilies. – Adansonia, sér. III, 25: 13-16.
Applequist WL, Wagner WL, Zimmer EA,
Nepokroeff M. 2006. Molecular evidence resolving the systematic position of
Hectorella (Portulacaceae). – Syst. Bot.
31: 310-319.
Arakaki M. 2003. Relaciones taxonómicas en
el género Weberbauerocereus Backeberg. – Quepo 17: 62-72.
Arakaki M, Soltis DE, Speranza P. 2007. New
chromosome counts and evidence of polyploidy in Haageocereus and
related genera in tribe Trichocereeae and other tribes of Cactaceae. – Brittonia 59:
290-297.
Arakaki M, Christin P-A, Nyffeler R, Lendel
A, Eggli U, Ogburn RM, Spriggs E, Moore MJ, Edwards EJ. 2011. Contemporaneous
and recent radiations of the world’s major succulent lineages. – Proc.
Natl. Acad. Sci. U.S.A. 108: 8379-8384.
Arber A. 1910. The Cactaceae and the study of
seedlings. – New Phytol. 9: 333-337.
Arber A. 1939. Studies in flower structure V.
On the interpretation of the petal and ‘corona’ in Lychnis. –
Ann. Bot., N. S., 3: 337-346.
Arber A. 1941. On the morphology of the
pitcher-leaves in Heliamphora, Sarracenia,
Darlingtonia, Cephalotus, and Nepenthes. – Ann.
Bot., N. S., 5: 563-578.
Archibald EEA. 1939. The development of the
ovule and seed of jointed cactus (Opuntia aurantiaca Lindley). –
South Afr. J. Sci. 36: 195-211.
Areces-Mallea AE. 2001. A new opuntioid
cactus from the Cayman Islands, B.W.I., with a discussion and key to the genus
Consolea Lemaire. – Brittonia 53: 96-107.
Arias S, Sánchez E. 2010. Una especie nueva
de Strombocactus (Cactaceae) del Rio Moctezuma,
Querétaro, México. – Rev. Mexicana Biodivers. 81: 619-624.
Arias S, Terrazas T. 2006. Análisis
cladístico del género Pachycereus (Cactaceae) con caracteres
morfológicos. – Brittonia 58: 197-216.
Arias S, Terrazas T. 2009. Taxonomic revision
of Pachycereus (Cactaceae). – Syst. Bot. 34:
68-83.
Arias S, Terrazas T, Cameron K. 2003.
Phylogenetic analysis of Pachycereus (Cactaceae, Pachycereeae) based on
chloroplast and nuclear DNA sequences. – Syst. Bot. 28: 547-557.
Arias S, Terrazas T, Arreola-Nava HJ,
Vázquez-Sánchez M, Cameron KM. 2005. Phylogenetic relationships in
Peniocereus (Cactaceae)
inferred from plastid DNA sequence data. – J. Plant Res. 118: 317-328.
Arreola-Nava HJ, Terrazas T. 2003. Especies
de Stenocereus con aréolas morenas: clave y descripciones. – Acta
Bot. Mex. 64: 1-18.
Arreola-Nava HJ, Terrazas T. 2004.
Stenocereus zopilotensis Arreola-Nava and Terrazas (Cactaceae), a new species from
Mexico. – Brittonia 56: 96-100.
Arrigoni PV, Diana S. 1993. Contribution à
la connaissance du genre Limonium en Corse. – Candollea 48:
631-677.
Arrigoni PV, Diana S. 1999. Karyology,
chorology and bioecology of the genus Limonium (Plumbaginaceae) in Sardinia.
– Plant Biosyst. 133: 63-71.
Arroyo-Cosultchi G, Terrazas T, Arias S,
Arreola-Nava HJ. 2006. The systematic significance of seed morphology in
Stenocereus (Cactaceae).
– Taxon 55: 983-992.
Arruda E, Melo-de-Pinna GF. 2010. Wide-band
tracheids (WBTs) of photosynthetic and non-photosynthetic stems in species of
Cactaceae. – J. Torrey Bot.
Club 137: 16-29.
Artelari R. 1989a. Limonium creticum
(Plumbaginaceae), a new
species from Kriti island (Aegean Sea) Greece. – Candollea 44: 415-421.
Artelari R. 1989b. Biosystematic study of the
genus Limonium (Plumbaginaceae) in the Aegean
area (Greece) I. Some Limonium species from the Kikladhes islands. –
Willdenowia 18: 399-408.
Artelari R. 1989c. Biosystematic study of the
genus Limonium (Plumbaginaceae) in the Aegean
area (Greece) II. Limonium hierapetrae Rech. fil. from Kriti island.
– Webbia 43: 33-40.
Artelari R, Erben M. 1986. Limonium
brevipetiolatum – eine neue hexaploide Sippe aus Süd-Griechenland. –
Mitt. Bot. Stattssamml. München 22: 507-511.
Artelari R, Georgiou O. 1999. Two new species
of Limonium (Plumbaginaceae) from the island
of Kithira (Greece). – Bot. J. Linn. Soc. 131: 399-415.
Artelari R, Georgiou O. 2002. Biosystematic
study of the genus Limonium (Plumbaginaceae) in the Aegean
area (Greece) III. Limonium on the islands Kithira and Antikithira and
the surrounding islets. – Nord. J. Bot. 22: 483-501.
Artelari R, Kamari G. 1986. A karyological
study of ten Limonium species (Plumbaginaceae) endemic in the
Ionian area. – Willdenowia 15: 497-513.
Artelari R, Kamari G. 1995. Limonium
kardamylii (Plumbaginaceae), a new species
from S Peloponnisos (Greece). – Phyton 35: 131-137.
Artelari R, Kamari G. 2000. Limonium
messeniacum (Plumbgainaceae), a new species from S Peloponnisos (Greece).
– Bot. Chron. 13: 45-49.
Aryavand A, Favarger C. 1980. Contribution à
l’étude cytotaxonomique des Caryophyllacées de l’Iran. – Biol. &
Écol. Médit. 7: 15-26.
Augustin K, Hentzschel G. 2002. Die Gattung
Weingartia Werdermann. Teil 1: Besprechung und Neuordnung. –
Gymnocalycium 15: 453-472.
Augustin K, Gertel W, Hentzschel G. 2000.
Sulcorebutia. Kakteenzwerge der bolivianischen Anden. – E. Ulmer,
Stuttgart.
Aydin Z, Ertekin AS, Långström E, Oxelman
B. 2014. A new section of Silene (Caryophyllaceae) including a
new species from south Anatolia, Turkey. – Phytotaxa 178: 98-112.
Ayodele AE, Olowokudejo JD. 2006. The family
Polygonaceae in West Africa:
taxonomic significance of leaf epidermal characters. – South Afr. J. Bot. 72:
442-459.
Aytaç Z, Duman H. 2004. Six new taxa (Caryophyllaceae) from Turkey.
– Ann. Bot. Fenn. 41: 213-221.
Backeberg C. 1936. Über die Abgrenzung der
Gattungen Trichocereus, Echinopsis, u.s.w. – Blätt.
Kakteenf. 1936(12), Nachtrag 13.
Backeberg C. 1937. Neolloydia Br. & R.
– Bl. Kakteenforsch. 3.
Backeberg C. 1938. Cactaceae Lindley, Neubearbeitung
der systematischen Übersicht. New systematic synopsis. – Blätt.
Kakt.-forsch. 6: 1-24.
Backeberg C. 1942. Zur Geschichte der Kakteen
im Verlauf der Entwicklung des amerikanischen Kontinentalbildes. – Cactaceae. Jahrb. Deutsch.
Kakteen-Ges. 1942: 4-72.
Backeberg C. 1958-1962. Die Cactaceae 1-6. – Gustav Fischer,
Jena.
Backeberg C. 1966. Das Kakteenlexikon. –
Gustav Fischer, Jena.
Backeberg C. 1977. Cactus lexicon. English
ed., transl. L. Glass. – Blandford Press, Poole.
Backeberg C, Knuth FM. 1936. Kaktus-ABC. En
haandbog for fagfolk og amatører. – Nordisk Forlag, København.
Backer CA. 1949a. Amaranthaceae. – In: Steenis
CGGJ van (ed), Flora Malesiana I, 4(2), Noordhoff-Kolff N. V., Batavia, pp.
69-98.
Backer CA. 1949b. Chenopodiaceae. – In:
Steenis CGGJ van (ed), Flora Malesiana I, 4(2), Noordhoff-Kolff N. V., Batavia,
pp. 99-106.
Bagci Y, Uysal T, Ertugrul K, Demirelma H.
2007. Silene kucukodukii sp. nov. (Caryophyllaceae) from south
Anatolia, Turkey. – Nord. J. Bot. 25: 306-310.
Bailey DC. 1980. Anomalous growth and
vegetative anatomy of Simmondsia chinensis. – Amer. J. Bot. 67:
147-161.
Bailey IW. 1964. Comparative anatomy of the
leaf-bearing Cactaceae XI: the
xylem of Pereskiopsis and Quiabentia. – J. Arnold Arbor.
45: 140-157.
Bailey IW. 1966. The significance of
reduction of vessels in the Cactaceae. – J. Arnold Arbor. 47:
288-292.
Bailey JP, Stace CA. 1992. Chromosome number,
morphology, pairing, and DNA values of species and hybrids in the genus
Fallopia (Polygonaceae). – Plant Syst.
Evol. 180: 29-52.
Baird WV, Blackwell WH. 1980. Secondary
growth in the axis of Halogeton glomeratus (Bieb.) Meyer
(Chenopodiaceae). – Bot. Gaz. 141: 269-276.
Baker HG. 1948a. Relationships in the Plumbaginaceae. – Nature 161:
400.
Baker HG. 1948b. Dimorphism and monomorphism
in the Plumbaginaceae I. A
survey of the family. – Ann. Bot., N. S., 12: 207-219.
Baker HG. 1953a. Dimorphism and monomorphism
in the Plumbaginaceae II.
Pollen and stigmata in the genus Limonium. – Ann. Bot., N. S., 17:
433-445.
Baker HG. 1953b. Dimorphism and monomorphism
in the Plumbaginaceae III.
Correlation of geographical distribution patterns with dimorphism and
monomorphism in Limonium. – Ann. Bot., N. S., 17: 615-627.
Baker HG. 1964. Variation in style length in
relation to outbreeding in Mirabilis. – Evolution 18: 507-509.
Baker HG. 1966. The evolution, functioning
and breakdown of heteromorphic incompatibility systems I. The Plumbaginaceae. – Evolution
20: 349-368.
Baker M. 2006. A new florally dimorphic
hexaploid, Echinocereus yavapaiensis sp. nov. (section
Triglochidiatus, Cactaceae) from central Arizona. –
Plant Syst. Evol. 258: 63-83.
Balfour E. 1965. Anomalous secondary
thickening in Chenopodiaceae, Nyctaginaceae, and Amaranthaceae. –
Phytomorphology 15: 111-122.
Ball PW. 1964. A taxonomic review of
Salicornia in Europe. – Feddes Repert. Spec. Nov. Regni Veg. 69:
1-8.
Ball PW, Heywood VH. 1964. A revision of the
genus Petrorhagia. – Bull. Brit. Mus. (Nat. Hist.) Bot. 3:
119-172.
Ball PW, Cornejo X, Kadereit G. 2017.
Mangleticornia (Amaranthaceae:
Salicornioideae) – a new sister for Salicornia from the
Pacific coast of South America. – Willdenowia 47: 145-153.
Bao BJ, Li AR. 1993. A study of the genus
Atraphaxis in China and the system of Atraphaxideae (Polygonaceae). – Acta
Phytotaxon. Sin. 31: 127-139.
Baquar SR, Olusi OO. 1988. Cytomorphological
and phylogenetic studies of the genus Amaranthus from Nigeria. –
Kromosomo 51-52: 1665-1674.
Baranova MA. 1980. Comparative stomatographic
studies in the families Buxaceae and Simmondsiaceae. – In: Žilin
SG (ed), Systematics and evolution of higher plants, Nauka, Leningrad, pp.
68-75. [In Russian]
Bárcenas RT. 2016. A molecular phylogenetic
approach to the systematics of Cylindropuntieae (Opuntioideae, Cactaceae). – Cladistics 32:
351-359.
Bárcenas RT, Yesson C, Hawkins JA. 2011.
Molecular systematics of the Cactaceae. – Cladistics 27:
470-489.
Barkoudah YI. 1962. A revision of
Gypsophila, Bolanthus, Ankyropetalum and
Phryna. – Wentia 9: 1-203.
Barnley B. 1982. Frankeniaceae. – In: George AS
(ed), Flora of Australia 8, Australian Government Publ. Service, Canberra, pp.
112-146.
Barthlott W. 1977. Cacti. Botanical aspects.
Descriptions and cultivation. – Stanley Thornes Ltd., Cheltenham, England.
Barthlott W. 1983. Biogeography and evolution
in neo- and palaeotropical Rhipsalinae. – In: Kubitzki K (ed), Dispersal and
distribution, Sonderbd. Naturbd. Naturwiss. Ver. Hamburg 7, P. Parey, Hamburg,
pp. 241-248.
Barthlott W. 1987. New names in Rhipsalidinae
(Cactaceae). – Bradleya 5:
97-100.
Barthlott W. 1988. Über die systematischen
Gliederungen der Cactaceae. –
Beitr. Biol. Pflanzen 63: 17-40.
Barthlott W. 1994. Epicuticular wax
ultrastructure and systematics. – In: Behnke H-D, Mabry TJ (eds) Caryophyllales: evolution and
systematics, Springer, Berlin, Heidelberg, pp. 75-86.
Barthlott W, Hunt DR. 1993. Cactaceae. – In: Kubitzki K,
Rohwer JG, Bittrich V (eds), The families and genera of vascular plants II.
Flowering plants. Dicotyledons. Magnoliid, hamamelid and caryophyllid families,
Springer, Berlin, Heidelberg, New York, pp. 161-197.
Barthlott W, Hunt DR. 2000. Seed-diversity in
the Cactaceae, subfamily
Cactoideae. – Succ. Plant Res. 5: 1-176.
Barthlott W, Porembski S. 1996. Ecology and
morphology of Blossfeldia liliputana (Cactaceae): a poikilohydric and
almost astomate succulent. – Bot. Acta 109: 161-166.
Barthlott W, Taylor NP. 1995. Notes towards a
monograph of Rhipsalideae (Cactaceae). – Bradleya 13:
43-79.
Barthlott W, Voit G. 1979. Mikromorphologie
der Samenschalen und Taxonomie der Cactaceae: ein
raster-elektronenmikroskopischer Überblick. – Plant Syst. Evol. 132:
205-229.
Barthlott W, Porembski S, Kluge M, Hopke J,
Schmidt L. 1997. Selenicereus wittii (Cactaceae): an epiphyte adapted to
Amazonian Igapó inundation forests. – Plant Syst. Evol. 206: 175-185.
Basak RK, Subramanyan K. 1966. Pollen grains
of some species of Nepenthes. – Phytomorphology 16: 334-338.
Bassett IJ, Crompton CW. 1982. The genus
Chenopodium in Canada. – Can. J. Bot. 60: 586-610.
Batalin A. 1877. Mechanik der Bewegungen der
insektenfressenden Pflanzen. – Flora 35: 54-58; 60: 33-39, 54-58, 65-73,
105-111, 129-154.
Bateman AJ. 1968. The role of heterostyly in
Narcissus and Mirabilis. – Evolution 22: 645-646.
Batenburg LH, Moeliono BM. 1982. Oligomery
and vasculature in the androecium of Mollugo nudicaulis Lam. (Molluginaceae). – Acta Bot.
Neerl. 31: 215-220.
Batenburg LH, Geluk PCW, Moeliono BM. 1984.
Morphology and vascular system of the inflorescences of Mollugo
nudicaulis Lam. and Hypertelis bowkeriana Sond. (Molluginaceae). – Acta Bot.
Neerl. 33: 101-110.
Báthori M, Méthé I Jr, Solymosi P,
Szendrei K. 1987. Phytoecdysteroids in some species of Caryophyllaceae and
Chenopodiaceae. – Acta Bot. Hung. 33: 377-385.
Bauer R. 1922. Entwickelungsgeschichtliche
Untersuchungen an Polygonaceenblüthen. – Flora 115: 273-292.
Bauer R. 2003a. A synopsis of the tribe
Hylocereeae F. Buxb. – Cact. Syst. Init. No. 17: 1-63.
Bauer R. 2003b. The genus
Pseudorhipsalis Britton & Rose. – Haseltonia 9: 94-120.
Bauer U, Bohn HF, Federle W. 2008. Harmless
nectar source or deadly trap: Nepenthes pitchers are activated by
rain, condensation and nectar. – Proc. Roy. Soc. London, Sect. B, 275:
259-265.
Baum BR. 1964. On the vernales-aestivales
character in Tamarix and its diagnostic value. – Israel J. Bot. 13:
30-35.
Baum BR. 1966. Monographic revision of the
genus Tamarix. – Final Res. Rep. U. S. Dept. Agric., pp. 141-142.
Baum BR. 1978. The genus Tamarix.
– Israel Academy of Sciences and Humanities, Jerusalem.
Baum BR, Bassett IJ, Crompton CW. 1971.
Pollen morphology of Tamarix species and its relationship to the
taxonomy of the genus. – Pollen Spores 13: 495-521.
Bayón ND. 2015. Revisión taxonómica de las
especies monoicas de Amaranthus (Amaranthaceae):
Amaranthus subg. Amaranthus y Amaranthus subg.
Albersia. – Ann. Missouri Bot. Gard. 101: 261-383.
Bean AR. 2008. A synopsis of
Ptilotus (Amaranthaceae) in eastern
Australia. – Telopea 12: 227-250.
Beard EC. 1937. Some chromosome complements
in the Cactaceae and a study of
meiosis in Echinocereus papillosus. – Bot. Gaz. 99: 1-21.
Beck E, Merxmüller H, Wagner H. 1962.
Anthocyane bei Plumbaginaceen, Alsinoideen, und Molluginaceen. – Planta 58:
220-224.
Becker H. 1913. Über die Keimung
verschiedenartiger Früchte und Samen bei derselben Spezies III.
Chenopodiaceae. – Beih. Bot. Centralbl. 29: 122-132.
Beckstrom-Sternberg S. M. 1989.
Two-dimensional gel electrophoresis as a taxonomic tool: evidence from
Centrospermae. – Biochem. Syst. Ecol. 17: 573-582.
Bedell HG. 1980. A taxonomic and
morphological re-evaluation of Stegnospermaceae (Caryophyllales). – Syst. Bot.
5: 419-431.
Beer SS, Beer AS, Sokoloff DD. 2010. Flower
and inflorescence development in Salicornia (Chenopodiaceae). –
Feddes Repert. 121: 229-247.
Behnke H-D. 1969. Über Siebröhren-Plastiden
und Plastidenfilamente der Caryophyllales. Untersuchungen
zum Feinbau und zur Verbreitung eines weiteren spezifischen Plastidentyps. –
Planta 89: 275-283.
Behnke H-D. 1974. Elektronenmikroskopische
Untersuchungen an Siebröhren-Plastiden und ihre Aussage über die
systematische Stellung von Lophiocarpus. – Bot. Jahrb. Syst. 94:
114-119.
Behnke H-D. 1975. Hectorella
caespitosa: ultrastructural evidence against its inclusion into Caryophyllaceae. – Plant
Syst. Evol. 124: 31-34.
Behnke H-D. 1976a. A tabulated survey of some
characters of systematic importance in centrospermous families. – Plant Syst.
Evol. 125: 95-98.
Behnke H-D. 1976b. Delimitation and
classification of the order Caryophyllales (Centrospermae)
according to ultrastructure data from sieve-element plastids: a survey based on
146 species. – Plant Syst. Evol. 126: 31-54.
Behnke H-D. 1976c. Die Siebelement-Plastiden
der Caryophyllaceae, eine
weitere spezifische Form der P-Typ-Plastiden bei Centrospermen. – Bot. Jahrb.
Syst. 95: 327-333.
Behnke H-D. 1976d. Sieve-element plastids of
Fouquieria, Frankenia (Tamaricales), and
Rhabdodendron (Rutaceae), taxa
sometimes allied with Centrospermae (Caryophyllales). – Taxon 25:
265-268.
Behnke H-D. 1977a. Regular occurring massive
deposits of phytoferritin in the phloem of succulent Centrospermae. –
Zeitschr. Pflanzenphysiol. 85: 89-92.
Behnke H-D. 1977b. Zur Skulptur der
Pollen-Exine bei drei Centrospermen (Gisekia, Limeum,
Hectorella) bei Gyrostemaceen und Rhabdodendraceen. – Plant Syst.
Evol. 128: 227-235.
Behnke H-D. 1977c. Transmission electron
microscopy and systematics of flowering plants. – Plant Syst. Evol. [Suppl.]
1: 155-178.
Behnke H-D. 1978. Elektronenoptische
Untersuchungen am Phloem sukkulenter Centrospermen (incl. Didiereaceen). –
Bot. Jahrb. Syst. 99: 341-352.
Behnke H-D. 1981. Sieve-element characters.
– Nord. J. Bot. 1: 381-400.
Behnke H-D. 1982a. Geocarpon
minimum: sieve-element plastids as additional evidence for its inclusion
in the Caryophyllaceae. –
Taxon 31: 45-47.
Behnke H-D. 1982b. Sieve-element plastids,
exine sculpturing, and the systematic affinities of the Buxaceae. – Plant Syst.
Evol. 139: 257-266.
Behnke H-D. 1993. Further studies of the
sieve-element plastids of the Caryophyllales including
Barbeuia, Corrigiola, Lyallia, Microtea,
Sarcobatus, and Telephium. – Plant Syst. Evol. 186:
231-243.
Behnke H-D. 1994. Sieve-element plastids:
their significance for the evolution and systematics of the order. – In:
Behnke H-D, Mabry TJ (eds), Caryophyllales: evolution and
systematics, Springer, Berlin, Heidelberg, New York, pp. 87-121.
Behnke H-D. 1997. Sarcobataceae – a new family
of Caryophyllales. – Taxon
46: 495-507.
Behnke H-D. 1999. P-type sieve-element
plastids present in members of the tribes Triplareae and Coccolobeae (Polygonaceae) renew the links
between the Polygonales and the Caryophyllales. – Plant Syst.
Evol. 214: 15-27.
Behnke H-D, Barthlott W. 1983. New evidence
from ultrastructural and micromorphological fields in angiosperm
classification. – Nord. J. Bot. 3: 43-66.
Behnke H-D, Mabry TJ (eds). 1994. Caryophyllales: systematics and
evolution. – Springer, Berlin, Heidelberg, New York.
Behnke H-D, Turner BL. 1971. On specific
sieve-tube plastids in Caryophyllales. Further
investigations with special reference to the Bataceae. – Taxon
20: 731-737.
Behnke H-D, Chang C, Eifert IJ, Mabry TJ.
1974. Betalains and P-type sieve-tube plastids in Petiveria and
Agdestis (Phytolaccaceae). – Taxon 23:
541-542.
Behnke H-D, Mabry TJ, Eifert IJ, Pop L. 1975.
P-type sieve-element plastids and betalains in Portulacaceae (including
Ceraria, Portulacaria, Talinella). – Can. J. Bot.
53: 2103-2109.
Behnke H-D, Mabry TJ, Neumann P, Barthlott W.
1983. Ultrastructural, micromorphological and phytochemical evidence for a
”central position” of Macarthuria (Molluginaceae) within the Caryophyllales. – Plant Syst.
Evol. 143: 151-161.
Behnke H-D, Pop L, Sivarajan VV. 1983.
Sieve-element plastids of Caryophyllales: additional
investigations with special reference to the Caryophyllaceae and Molluginaceae. – Plant Syst.
Evol. 142: 109-115.
Behre K. 1929. Physiologische und
zytologische Untersuchungen über Drosera. – Planta 7: 208-306.
Bennett ST, Cheek M. 1990. The cytology and
morphology of Drosera slackii and its relatives in South Africa. –
Kew Bull. 45: 375-381.
Benson L. 1982. The cacti of the United
States and Canada. – Stanford University Press, Stanford, California.
Berger A. 1926. Die Entwicklungslinien der
Kakteen. – Gustav Fischer, Jena.
Berger A. 1929. Kakteen. –
Verlagsbuchhandlung von Eugen Ulmer, Stuttgart.
Bernasconi G, Antonovics J, Biere A,
Charlesworth D, Delph LF, Filatov D, Giraud T, Hood ME, Marais GA, McCauley D,
Pannell JR, Shykoff JA, Vyskot B, Wolfe LM, Widmer A. 2009. Silene as
a model system in ecology and evolution. – Heredity 103: 5-14.
Bhambie S, Joshi MC, Gupta ML. 1977.
Anatomical studies on certain members of Aizoaceae. – Proc. Indian Acad.
Sci. 85: 399-406.
Bidwell GL, Wooton EO. 1925. Saltbushes and
their allies in the United States. – U.S.D.A. Dept. Bull. 1345: 1-39.
Bisalputra T. 1960. Anatomical and
morphological studies in the Chenopodiaceae I. Inflorescence of
Atriplex and Bassia. – Aust. J. Bot. 8: 226-242.
Bisalputra T. 1961. Anatomical and
morphological studies in the Chenopodiaceae II. Vascularization of the
seedlings. – Aust. J. Bot. 9: 1-19.
Bisalputra T. 1962. Anatomical and
morphological studies in the Chenopodiaceae III. The primary vascular system
and nodal anatomy. – Aust. J. Bot. 10: 13-24.
Bissinger K, Khoshravesh R, Kotrade JP,
Oakley J, Sage TL, Sage RF, Hartmann HEK, Kadereit G. 2014. Gisekia
(Gisekiaceae): phylogenetic relationships, biogeography, and ecophysiology of a
poorly known C4 lineage in the Caryophyllales. – Amer. J. Bot. 101:
499-509.
Bittrich V. 1986 [1987]. Untersuchungen zu
Merkmalbestand, Gliederung und Abgrenzung der Unterfamilie Mesembryanthemoideae
(Mesembryanthemaceae Fenzl). – Mitt. Inst. Allg. Bot. Hamburg 21: 5-116.
Bittrich V 1990. Systematic studies in Aizoaceae. – Mitt. Inst. Allg.
Bot. Hamburg 23b: 491-507.
Bittrich V. 1993a. Introduction to
Centrospermae. – In: Kubitzki K, Rohwer JG, Bittrich V (eds), The families
and genera of vascular plants II. Flowering plants. Dicotyledons. Magnoliid,
hamamelid and caryophyllid families, Springer, Berlin etc, pp. 13-19.
Bittrich V. 1993b. Achatocarpaceae. – In:
Kubitzki K, Rohwer JG, Bittrich V (eds), The families and genera of vascular
plants II. Flowering plants. Dicotyledons. Magnoliid, hamamelid and
caryophyllid families, Springer, Berlin, Heidelberg, New York, pp. 35-36.
Bittrich V. 1993c. Caryophyllaceae. – In:
Kubitzki K, Rohwer JG, Bittrich V (eds), The families and genera of vascular
plants II. Flowering plants. Dicotyledons. Magnoliid, hamamelid and
caryophyllid families, Springer, Berlin, Heidelberg, New York, pp. 206-236.
Bittrich V. 1993d. Halophytaceae. – In: Kubitzki
K, Rohwer JG, Bittrich V (eds), The families and genera of vascular plants II.
Flowering plants. Dicotyledons. Magnoliid, hamamelid and caryophyllid families,
Springer, Berlin, Heidelberg, New York, pp. 320-321.
Bittrich V, Amaral MC. 1991.
Proanthocyanidins in the testa of centrospermous seeds. – Biochem. Syst.
Evol. 19: 319-321.
Bittrich V, Hartmann HEK. 1988. The Aizoaceae – a new approach. –
Bot. J. Linn. Soc. 97: 239-254.
Bittrich V, Ihlenfeldt H-D. 1984. Morphologie
früher Keimungstadien bei Mesembryanthemaceae: eine Anpassung an aride
Umweltbedingungen. – Mitt. Inst. Allg. Bot. Hamburg 19: 123-139.
Bittrich V, Kühn U. 1993. Nyctaginaceae. – In: Kubitzki
K, Rohwer JG, Bittrich V (eds), The families and genera of vascular plants II.
Flowering plants. Dicotyledons. Magnoliid, hamamelid and caryophyllid families,
Springer, Berlin, Heidelberg, New York, pp. 473-486.
Bittrich V, Struck M. 1989. What is primitive
in the Mesembryanthemaceae? An analysis of evolutionary polarity of character
states – South Afr. J. Bot. 55: 321-331
Black RF. 1954. The leaf anatomy of
Australian members of the genus Atriplex. – Aust. J. Bot. 2:
269-286.
Blackburn KB, Morton JK. 1957. The incidence
of polyploidy in the Caryophyllaceae of Britain and
of Portugal. – New Phytol. 56: 344-351.
Blackwell WH Jr. 1977. The subfamilies of the
Chenopodiaceae. – Taxon 26: 395-397.
Blackwell WH Jr, Powell MJ. 1981. A
preliminary note on pollination in the Chenopodiaceae. – Ann. Missouri Bot.
Gard. 68: 524-526.
Blackwell WH Jr, Baechle MD, Williamson G.
1978. Synopsis of Kochia (Chenopodiaceae) in North America. – Sida
7: 248-254.
Blake SF. 1921. Neomillspaughia, a
new genus of Polygonaceae,
with remarks on related genera. – Bull. Torrey Bot. Club 48: 77-89.
Blanche C, Molero J. 1987. The genus
Halopeplis Ung.-Sternb. (Salicorniaceae) in the Iberian peninsula. –
Coll. Bot. (Barcelona) 17: 67-77.
Blum W, Lange M, Rischer W, Rutow J. 1998.
Echinocereus. Monographie. – Bietigheim & al. (publ. by the
authers).
Blum W, Felix D, Waldeis D. 2008.
Echinocereus. Die Sektion Wilcoxia. – Arbeitsgruppe
Echinocereus der Deutschen Kakteen-Gesellschaft e. V., Rhauderfehn.
Blunden G, Yang M-H, Janicsák G, Málthé L,
Carabot-Cuervo A. 1999. Betaine distribution in the Amaranthaceae. – Biochem.
Syst. Ecol. 27: 87-92.
Bobrov EG. 1966. A review of the genus
Reaumuria L. in connection with the problem of the origin of the
Afro-Asiatic desert flora. – Bot. Žurn. 51: 1057-1072.
Bocquet G. 1959 [1960]. The structure of the
placental column in the genus Melandrium (Caryophyllaceae). –
Phytomorphology 9: 217-221.
Boedeker F. 1930. Eine neue, eigenartige
Coryphantha. – Monatsschr. Deutsch. Kakteen-Ges. 2: 168-170.
Boesewinkel FD. 1989. Ovule and seed
development in Droseraceae. –
Acta Bot. Neerl. 38: 295-311.
Bogle AL, Swain T, Thomas RD, Kohn ED. 1971.
Geocarpon: Aizoaceae or
Caryophyllaceae? – Taxon
20: 473-477.
Bohley K, Joos O, Hartmann H, Sage R,
Liede-Schumann S, Kadereit G. 2015. Phylogeny of Sesuvioideae (Aizoaceae) – biogeography, leaf
anatomy and the evolution of C4 photosynthesis. – Persp. Plant Ecol. Evol.
Syst. 17: 116-130.
Bohley K, Winter PJD, Kadereit G. 2017. A
revision of Sesuvium (Aizoaceae, Sesuvioideae). – Syst.
Bot. 42: 124-147.
Bohlin J-E. 1988. A monograph of the genus
Colignonia (Nyctaginaceae). – Nord. J.
Bot. 8: 231-252.
Bohn HF, Federle W. 2004. Insect aquaplaning:
Nepenthes pitcher plants capture prey with the peristome, a fully
wettable water-lubricated anisotropic surface. – Proc. Natl. Acad. Sci.
U.S.A. 101: 14138-14143.
Bohrer VL. 1975. Recognition and
interpretation of prehistoric remains of Mirabilis multiflora in the
Sacramento Mountains of New Mexico. – Bull. Torrey Bot. Club 102: 21-25.
Boke NH. 1941. Zonation in the shoot apices
of Trichocereus spachianus and Opuntia cylindrica. – Amer.
J. Bot. 28: 656-664.
Boke NH. 1944. Histogenesis of the leaf and
areole in Opuntia cylindrica. – Amer. J. Bot. 31: 299-316.
Boke NH. 1963. Anatomy and development of the
flower and fruit of Pereskia pititache. – Amer. J. Bot. 50:
843-858.
Boke NH. 1964. The cactus gynoecium: a new
interpretation. – Amer. J. Bot. 51: 598-610.
Boke NH. 1966. Ontogeny and structure of the
flower and fruit of Pereskia aculeata. – Amer. J. Bot. 53:
534-542.
Boke NH. 1980. Developmental morphology and
anatomy in Cactaceae. –
Bioscience 30: 605-610.
Bokhari MH. 1970. Morphology and taxonomic
significance of foliar sclereids in Limonium. – Notes Roy. Bot.
Gard. Edinb. 30: 43-53.
Bokhari MH. 1972a. A brief synopsis of stigma
and pollen types in Acantholimon and Limonium. – Notes Roy.
Bot. Gard. Edinb. 32: 79-84.
Bokhari MH. 1972b. Anatomical characters and
their taxonomic importance in Acantholimon. – Notes Roy. Bot.Gard.
Edinb. 32: 85-92.
Bokhari MH, Wendelbo P. 1978. On anatomy,
adaptation to xerophytism and taxonomy of Anabasis inclusive
Esfandiaria. – Bot. Not. 131: 279-292.
Bolus HML. 1928. Notes on
Mesembryanthemum and some allied genera 1. – University of Cape
Town, Cape Town.
Bolus HML. 1928-1935. Notes on
Mesembryanthemum and allied genera 2. – University of Cape Town,
Cape Town.
Bolus HML. 1936-1958. Notes on
Mesembryanthemum and allied genera 3. – University of Cape Town,
Cape Town.
Borkowski B, Drost K. 1965. Alkaloide aus
Salicornia herbacea L. – Pharmazie 20: 390-393.
Borsch T. 1998. Pollen types in the Amaranthaceae. Morphology and
evolutionary significance. – Grana 37: 129-142.
Borsch T, Pedersen TM. 1997. Restoring the
generic rank of Hebanthe Martius (Amaranthaceae). – Sendtnera 4:
13-31.
Borsch T, Ortuño T, Nee MH. 2011.
Phylogenetics of the neotropical liana genus Pedersenia (Amaranthaceae: Gomphrenoideae)
and discovery of a new species from Bolivia based on molecules and morphology.
– Willdenowia 41: 5-14.
Borsch T, Hernández-Ledesma P, Berendsohn
WG, Flores-Olvera H, Ochoterena H, Zuloaga FO, von Mering S, Kilian N. 2015. An
integrative and dynamic approach for monographing species-rich plant groups –
building the global synthesis of the angiosperm order Caryophyllales. – Persp.
Plant Ecol. Evol. Syst. 17: 284-300.
Borsch T, Flores-Olvera H, Zumaya S, Müller
K. 2018. Pollen characters and DNA sequence data converge on a monophyletic
genus Iresine (Amaranthaceae, Caryophyllales) and help to
elucidate its species diversity. – Taxon 67: 944-976.
Bortenschlager S. 1973. Morphologie
pollinique des Phytolaccaceae. – Pollen
Spores 15: 227-253.
Bortenschlager S, Auimger A, Blaha J,
Simonsburger P. 1972. Pollen morphology of Achatocarpaceae
(Centrospermae). – Bot. Naturwiss.-Med. Vereins Innsbruck 59: 7-13.
Borzova LM. 1968. The pollen of some species
of the genus Polygonum L. 2 species of the section Knorringia
Czuk. – Vestnik. Moskov. Univ. ser. 6, Biol. 5: 108-110. [In Russian]
Borzova LM. 1973. Pollen morphology of the
genus Polygonum L. s. lat. In Tadzhikistan. – In: Pollen and spore
morphology of the recent plants, Nauka, Leningrad, pp. 78-82. [In Russian]
Borzova LM, Sladkov AN. 1969. Pollen
morphology within the representatives of Polygonum L. s. lat. in
Tadzhikistan, SSR. – Vestn. Mosk. Univ. 4: 47-54. [In Russian]
Botschantzev VP. 1967. Sevadinae Botsch., a
new subtribe of the family Chenopodiaceae. – Bot. Žurn. 52: 800-810. [In
Russian]
Botschantzev VP. 1975. Species of the
subtribe Sevadinae (Chenopodiaceae). – Kew Bull. 30: 367-370.
Botschantzev VP. 1976. New genus
Chenoleoides (Ulbrich) Botsch. – Bot. Žurn. 61: 1408-1409. [In
Russian]
Botschantzev VP. 1977. The genus
Agathophora (Fenzl) Bunge. – Bot. Žurn. 62: 1447-1452. [In
Russian]
Boulos L. 1991a. Studies in the
Chenopodiaceae of Arabia 2. Notes on Suaeda Forssk. ex Scop. – Kew
Bull. 46: 291-296.
Boulos L. 1991b. Studies in the
Chenopodiaceae of Arabia 4. A synopsis of Chenopodium L. – Kew Bull.
46: 301-305.
Boulos L. 1992. Studies in the Chenopodiaceae
of Arabia 5. Notes on Agathophora (Fenzl) Bunge and Cornulaca
Del. – Kew Bull. 47: 283-287.
Boulos L, Friis I, Gilbert MG. 1991. Notes on
the Chenopodiaceae of Ethiopia, Somalia and southern Arabia. – Nord. J. Bot.
11: 309-316.
Boyd MR, Hallock YF, Cardellina JH, Manfredi
KP, Blunt JW, McMahon JB, Buckheit RW Jr, Bringmann G, Schäffer M, Cragg GM,
Thomas DW, Jato JG. 1994. Anti-HIV michellamines from Ancistrocladus
korpuensis. – J. Med. Chem. 37: 1740-1745.
Boyes JW, Bataglia E. 1951. Embryo sac
development in the Plumbaginaceae. – Caryologia
3: 305-310.
Brandbyge J. 1984. Three new species of the
genus Triplaris (Polygonaceae). – Nord. J. Bot.
4: 761-764.
Brandbyge J. 1986. A revision of the genus
Triplaris (Polygonaceae). – Nord. J. Bot.
6: 545-570.
Brandbyge J. 1989a. 34. Polygonaceae. – In: Harling G,
Andersson L (eds), Flora of Ecuador 38, Nord. J. Bot., Copenhagen, pp. 1-61.
Brandbyge J. 1989b. Two new species of the
genus Coccoloba (Polygonaceae). – Nord. J. Bot.
9: 205-208.
Brandbyge J. 1990a. The diversity of
micromorphological features in the genus Coccoloba (Polygonaceae). – Nord. J. Bot.
10: 25-44.
Brandbyge J. 1990b. Woody Polygonaceae from Brazil: new
species and a new interpretation. – Nord. J. Bot. 10: 155-160.
Brandbyge J. 1990c. The genus
Leptogonum (Polygonaceae). – Nord. J. Bot.
10: 487-492.
Brandbyge J. 1992. The genus
Muehlenbeckia (Polygonaceae) in South and
Central America. – Bot. Jahrb. Syst. 114: 349-416.
Brandbyge J. 1993. Polygonaceae. – In: Kubitzki K,
Rohwer JG, Bittrich V (eds), The families and genera of vascular plants II.
Flowering plants. Dicotyledons. Magnoliid, hamamelid and caryophyllid families,
Springer, Berlin, Heidelberg, New York, pp. 531-544.
Brandbyge J, Øllgaard B. 1984. Inflorescence
structure and generic delimitation of Triplaris and
Ruprechtia (Polygonaceae). – Nord. J. Bot.
4: 765-769.
Brandegee K. 1894. Studies in Portulacaceae. – Proc. Calif.
Acad. Sci., Ser. II, 4: 86-91.
Brandt R, Lomonosova M, Weising K, Wagner N,
Freitag H. 2015. Phylogeny and biogeography of Suaeda subg.
Brazia (Chenopodiaceae/Amaranthaceae) in the Americas.
– Plant Syst. Evol. 301: 2351-2375.
Brantjes NBM, Leemans JAAM. 1976. Silene
otites (Caryophyllaceae) pollinated by
nocturnal Lepidoptera and mosquitoes. – Acta Bot. Neerl. 25: 281-295.
Braun PJ, Esteves Pereira E. 2007. Beautiful
and bizarre Arrojadoa. The taxonomy of subgenus
Albertbuiningia. – Cact. Succ. J. (US) 79: 254-263.
Braun PJ, Esteves Pereira E. 2008.
Siccobaccatus insigniflorus. A new status for a marvellous columnar
cactus from Brazil. – Cact. Succ. J. (US) 80: 36-41.
Bravo-Hollis H. 1953. Un nuevo género de la
familia de las cactáceas, Backebergia. – An. Inst. Biol. Univ. Nac.
México 24: 215-232.
Bravo-Hollis H. 1978. Las Cactáceas de
México, 2nd ed. – Universidad Nactional Autónoma de México, México.
Bravo-Hollis H, Sánchez-Mejorada H.
1978-1991. Las Cactáceas de Mexico. 2nd ed. I-III. – Universidad
Nacional Autonoma de Mexico, Mexico City.
Breckenridge III FG, Miller JM. 1982.
Pollination biology, distribution, and chemotaxonomy of the Echinocereus
enneacanthus complex (Cactaceae). – Syst. Bot. 7:
365-378.
Bregman R. 1992. Seed studies in the subtribe
Borziacactinae Buxbaum (Cactaceae); morphology, taxonomy,
phylogeny and biogeography. – Bot. Jahrb. Syst. 114: 201-250.
Bregman R. 1996. The genus Matucana.
Biology and systematics of fascinating Peruvian cacti. – A. A. Balkema,
Rotterdam & Brookfield.
Bregman R, Bouman F. 1983. Seed germination
in Cactaceae. – Bot. J. Linn.
Soc. 86: 357-374.
Brenan JPM. 1953. Tropical African plants
XXIV. Chenopodiaceae. – Kew Bull. 8: 432-434.
Brenan JPM. 1954. Chenopodiaceae. – Turrill
WB, Milne-Redhead E. (2010), Flora of Tropical east Africa, Crown Agents for
Oversea Governments and Administrations, London, pp. 1-26.
Brett OE. 1952. Basic chromosome numbers in
the genus Cerastium. – Nature 170: 251-252.
Brett OE. 1954. Cyto-taxonomy of the genus
Cerastium I. Cytology. – New Phytol. 54: 138-148.
Brightmore D. 1979. Biological flora of the
British Isles 146. Frankenia laevis L. – J. Ecol. 67: 1097-1107.
Brignone NF, Denham SS, Pozner R. 2016.
Synopsis of the genus Atriplex (Amaranthaceae, Chenopodioideae)
for South America. – Aust. Syst. Bot. 29: 324-357.
Bringmann G, Pokorny F, Reuscher H, Lisch D,
Aké Assi L. 1990. Novel Ancistrocladaceae and Dioncophyllaceae type
naphthylisoquinoline alkaloids from Ancistrocladus abbreviatus: a
phylogenetic link between the two families? – Planta Med. 56: 496-497.
Bringmann G, Lisch D, Reuscher H, Assi LA,
Günther K. 1991. Atrop-diastereomer separation by racemate resolution
techniques: N-methyl-dioncophylline A and its 7-epimer from Ancistrocladus
abbreviatus. – Phytochemistry 30: 1307-1310.
Bringmann G, Rübenacker M, Vogt P, Busse H,
Assi LA, Peters K, Shering HG von. 1991. Dioncopeltine A and dioncolactone A:
alkaloids from Triphyophyllum peltatum. – Phytochemistry 30:
1691-1696.
Bringmann G, François G, Ake Assi L,
Schlauer J. 1998. The alkaloids of Triphyophyllum peltatum (Dioncophyllaceae). – Chimia
52: 18-28.
Bringmann G, Saeb W, God R, Schäffer M,
François G, Peters K, Peters E, Proksch P, Hostettmann K, Assi LA. 1998.
5’-O-Demethyldioncophylline A, a new antimalarial alkaloid from
Triphyophyllum peltatum. – Phytochemistry 49: 1667-1673.
Bringmann G, Wenzel M, Bringmann HP, Schlauer
J. 2001. Uptake of the amino acid alanine by digestive leaves: proof of
carnivory in the tropical liana Triphyophyllum peltatum (Dioncophyllaceae). –
Carniv. Plants Newsl. 30: 15-21.
Bringmann G, Rüdenauer S, Irmer A, Bruhn T,
Brun R, Heimberger T, Stühmer T, Bargou R, Chatterjee M. 2008. Antitumoral and
antileishmanial dioncoquinones and ancistroquinones from cell cultures of
Triphyophyllum peltatum (Dioncophyllaceae) and
Ancistrocladus abbreviatus (Ancistrocladaceae). –
Phytochemistry 69: 2501-2509.
Britton NL, Rose JN. 1909. The genus
Cereus and its allies in North America. – Contr. U.S. Natl. Herb.
12: 413-437.
Britton NL, Rose JN. 1919-1923. The Cactaceae. Descriptions and
illustrations of plants of the cactus family, 1-4. – The Carnegie
Institution, Publ. 248, Washington, D.C.
Brochmann C, Lobin W, Sunding P, Stabbetorp
O. 1995. Parallel ecoclinal evolution and taxonomy of Frankenia (Frankeniaceae) in the Cape Verde
Islands, W Africa. – Nord. J. Bot. 15: 603-623.
Brockington SF, Alexandre R, Ramdial J, Moore
MJ, Crawley S, Dhingra A, Hilu K, Soltis DE, Soltis PS. 2009. Phylogeny of the
Caryophyllales sensu lato:
revisiting hypotheses on pollination biology and perianth differentiation in
the core Caryophyllales. –
Intern. J. Plant Sci. 170: 627-643.
Brockington SF, Walker RH, Glover BJ, Soltis
PS, Soltis DE. 2011. Complex pigment evolution in the Caryophyllales. – New Phytol.
190: 854-864.
Brockington SF, Rudall PJ, Frohlich MW,
Oppenheimer DG, Soltis PS, Soltis DE. 2011. ‘Living stones’ reveal
alternative petal identity programs within the core eudicots. – The Plant J.
69: 193-203.
Brockington SF, Dos Santos P, Glover B, Ronse
De Craene L. 2013. Androecial evolution in Caryophyllales in light of a
paraphyletic Molluginaceae.
– Amer. J. Bot. 100: 1757-1778.
Brockington SF, Yang Y, Gandia-Herrero F,
Covshoff S, Hibberd JM, Sage RF, Wong GKS, Moore MJ, Smith SA. 2015.
Lineage-specific gene radiations underlie the evolution of novel betalain
pigmentation in Caryophyllales. – New Phytol.
207: 1170-1180.
Brown GK, Varadarajan GS. 1985. Studies in Caryophyllales I: re-evaluation
of classification of Phytolaccaceae s.l. – Syst.
Bot. 10: 49-63.
Brown NE. 1925. Mesembryanthemum and
some new genera separated from it. – Gard. Chron. 78: 272-273, 412.
Brullo S. 1980. Taxonomic and nomenclatural
notes on the genus Limonium in Sicily. – Bot. Not. 133: 281-293.
Brullo S. 1982. Notes on the genus
Salsola (Chenopodiaceae) I. The Salsola oppositifolia and
S. longifolia groups. – Willdenowia 12: 241-247.
Brullo S. 1984. Taxonomic consideration on
the genus Darniella (Chenopodiaceae). – Webbia 38: 301-328.
Brullo S, Pavone P. 1981. Chromosome numbers
in the Sicilian species of Limonium Miller (Plumbaginaceae). – An. Jard.
Bot. Madrid 37: 535-555.
Brullo S, Pavone P. 1987. Cremnophyton
lanfrancoi: a new genus and species of Chenopodiaceae from Malta. –
Candollea 42: 621-625.
Brummer-Dinger CH. 1955. Notes on Guiana Droseraceae. – Acta Bot. Neerl.
4: 136-138.
Bruyns PV, Oliveira-Neto M, Melo-de-Pinna GF,
Klak C. 2014. Phylogenetic relationships in the Didiereaceae with special
reference to subfamily Portulacarioideae. – Taxon 63: 1053-1064.
Brysting AK, Elven R. 2000. The Cerastium
alpinum-C. arcticum complex (Caryophyllaceae): numerical
analyses of morphological variation and a taxonomic revision of C.
arcticum Lange s.l. – Taxon 49: 189-216.
Buchinger M. 1957. Notas sobre la
subdivisión de la familia de las Polygonáceas. – Bol. Soc. Argent. Bot. 7:
42-43.
Buchmann SL. 1987. Floral biology of jojoba
(Simmondsia chinensis), an anemophilous plant. – Desert Plants 8:
111-124.
Buining AFH. 1980. Die Gattung
Discocactus Pfeiffer. Eine Revision bekannter und Diagnosen neuer
Arten. – Succulenta, Venlo.
Burke JM, Sanchez A. 2011. Revised subfamily
classification for Polygonaceae, with a tribal
classification for Eriogonoideae. – Brittonia 63: 510-520.
Burke JM, Sanchez A, Kron K, Luckow M. 2010.
Placing the woody tropical genera of Polygonaceae: a hypothesis of
character evolution and phylogeny. – Amer. J. Bot. 97: 1377-1390.
Burret F, Rabesa Z, Zandonella P, Voirin B.
1981. Contribution biochimique à la systématique de l’ordre des
Centrospermales. – Biochem. Syst. Ecol. 9: 257-262.
Burret F, Lebreton P, Voirin B. 1982. Les
aglycones flavoniques de Cactées: distribution, signification. – J. Nat.
Prod. 45: 687-693.
Buschi CA, Pomilio AB, Gros EG. 1979. A new
flavone from Gomphrena martiana. – Phytochemistry 18: 1249-1250.
Buschi CA, Pomilio AB, Gros EG. 1980. New
methylated flavones from Gomphrena martiana. – Phytochemistry 19:
903-904.
Buschi CA, Pomilio AB, Gros EG. 1981.
5,6,7-Trisubstituted flavones from Gomphrena martiana. –
Phytochemistry 20: 1178-1179.
Buschmann A. 1938. Über einige ausdauernde
Cerastium-Arten aus der Verwandtschaft des C. tomentosum
Linn. – Feddes Repert. 43: 118-143.
Butnik AA. 1981. The carpological
characteristics of the Chenopodiaceae. – Bot. Žurn. 66: 1433-1443. [in
Russian]
Butterworth CA. 2006a. Resolving
“Nyffeler’s Puzzle” – the intriguing taxonomic position of
Blossfeldia. – Haseltonia 12: 3-10.
Butterworth CA. 2006b. Molecular
phylogenetics of Cactaceae
Jussieu – a review. – In: Sharma AK, Sharma A (eds), Plant genome
biodiversity and evolution 1 C. Phanerogams (Angiosperm-Dicotyledons), Science
Publ., Enfield, New Hampshire, pp. 489-524.
Butterworth CA, Edwards EJ. 2008.
Investigating Pereskia and the earliest divergences in Cactaceae. – Haseltonia 14:
46-53.
Butterworth CA, Wallace RS. 2004.
Phylogenetic studies of Mammillaria (Cactaceae) – insights from
chloroplast sequence variation and hypothesis testing using the parametric
bootstrap. – Amer. J. Bot. 91: 1086-1098.
Butterworth CA, Wallace RS. 2005. Molecular
phylogenetics of the leafy cactus genus Pereskia (Cactaceae). – Syst. Bot. 30:
800-808.
Butterworth CA, Cota-Sanchez JH, Wallace RS.
2002. Molecular systematics of tribe Cacteae (Cactaceae: Cactoideae): a phylogeny
based on rpl16 intron sequence variation. – Syst. Bot. 27:
257-270.
Butterworth CA, Butterworth KM, Fitz Maurice
WA, Fitz Maurice B. 2007. A localized loss of the chloroplast rpl16
intron in Mammillaria series Stylothelae (Cactaceae) delineates members of the
M. crinita group. – Bradleya 25: 187-192.
Buttler KP. 1977. Revision von Beta
section Corollinae (Chenopodiaceae) I. Selbststerile Basisarten. –
Mitt. Bot. Staatssamml. Münchn 13: 255-336.
Buxbaum F. 1942. Rapicactus Buxb. et
Oehme, Gen. nov. – Cactaceae.
Jahrb. Deutsch. Kakteen-Ges. 1: 1-24.
Buxbaum F. 1944. Untersuchungen zur
Morphologie der Kakteenblüte I. Das Gynoeceum. – Bot. Arch. 45: 190-247.
Buxbaum F. 1948. Zur Klärung der
phylogenetischen Stellung der Aizoaceae und Cactaceae im Pflanzenreich. –
Jahrb. Schweiz. Kakt. Ges. 2: 3-16.
Buxbaum F. 1949. Vorläufer des
Kakteen-Habitus bei den Phytolaccaceen. – Österr. Bot. Zeitschr. 96:
5-14.
Buxbaum F. 1950-1954. The morphology of cacti
I-III. – Abbey Garden Press, Pasadena, California.
Buxbaum F. 1951a. Stages and lines of
evolution of the tribe Euechinocactineae. – Cact. Succ. J. (US) 23:
193-197.
Buxbaum F. 1951b. Die phylogenie der
nordamerikanischen Echinocacteen. Trib. Euechinocactineae F. Buxb. – Österr.
Bot. Zeitschr. 98: 44-104.
Buxbaum F. 1953. Vorarbeiten zu einem
phylogenetischen System der Cactaceae. – Kakt. Sukk. 4:
2-7.
Buxbaum F. 1956. Das Gesetz der Verkürzung
der vegetativen Phase in der Familie der Cactaceae. – Österr. Bot.
Zeitschr. 103: 354-362.
Buxbaum F. 1957-1960. Morphologie der
Kakteen. – In: Krainz H (ed), Die Kakteen, Franckh, Stuttgart.
Buxbaum F. 1958. The phylogenetic division of
the subfamily Cereoideae, Cactaceae. – Madroño 14:
177-216.
Buxbaum F. 1961a. Vorläufige Untersuchungen
über Umfang, systematische Stellung und Gliederung der Caryophyllales (Centrospermae).
– Beitr. Biol. Pflanzen 36: 3-56.
Buxbaum F. 1961b. Die Entwicklungslinien der
Tribus Pachycereae F. Buxb. – Bot. Studien 12: 1-107.
Buxbaum F. 1962. Stellung der Kakteen im
Pflanzenreich. – Kakt. Sukk. 13: 194-197.
Buxbaum F. 1967. Der gegenwärtige Stand der
stammesgeschichtlichen Erforschung der Kakteen. – Kakt. Sukk. 18: 3-9,
22-27.
Buxbaum F. 1969. Die Entwicklungswege der
Kakteen in Südamerika. – In: Fittkau EJ, Illies J, Klinge H, Schwabe GH,
Sioli H (eds), Biogeography and ecology in South America, W. Junk, The Hague,
pp. 583-623.
Buxbaum F. 1980. Kakteenleben. Eine
biologische Plauderei für jeden Naturfreund. – Albert Philler Verlag,
Minden, Germany.
Cabrera J. 2007. Phylogeny and historical
biogeography of the Australian Camphorosmeae (Chenopodiaceae). – Ph.D. diss.,
Universität Mainz, Germany.
Cabrera JF, Jacobs SWL, Kadereit G. 2009.
Phylogeny of the Australian Camphorosmeae (Chenopodiaceae) and the taxonomic
significance of the fruiting perianth. – Intern. J. Plant Sci. 170:
505-521.
Cabrera JF, Jacobs SWL, Kadereit G. 2011.
Biogeography of Camphorosmeae (Chenopodiaceae): tracking the Tertiary history
of Australian aridification. – Telopea 13: 313-326.
Caccavari de Filice MA. 1979. Granos de polen
de Nyctagináceas Argentinas. – Rev. Mus. Argent. Ci. Nat. Bernardino
Rivadavia 5: 211-227.
Calderón N, Zappi DC, Taylor NP, Ceroni A.
2007. Taxonomy and conservation of Haageocereus Backeb. (Cactaceae) in Peru. – Bradleya 25:
45-124.
Calvente A. 2012. A new subgeneric
classification of Rhipsalis (Cactoideae, Cactaceae). – Syst. Bot. 37:
983-988.
Calvente A, Andreata RHP, Vieira RC. 2008.
Stem anatomy of Rhipsalis (Cactaceae) and its relevance for
taxonomy. – Plant Syst. Evol. 276: 1-7.
Calvente A, Zappi DC, Forest F, Lohmann LG.
2011a. Molecular phylogeny, evolution, and biogeography of South American
epiphytic cacti. – Intern. J. Plant Sci. 172: 902-914.
Calvente A, Zappi DC, Forest F, Lohmann LG.
2011b. Molecular phylogeny of the tribe Rhipsalideae (Cactaceae) and taxonomic
implications for Schlumbergera and Hatiora. – Mol.
Phylogen. Evol. 58: 456-468.
Calvente A, Moraes EM, Lavor P, Bonatelli
IAS, Nacaguma P, Versieux LM, Taylor NP, Zappi DC. 2017. Phylogenetic analyses
of Pilosocereus (Cactaceae) inferred from plastid and
nuclear sequences. – Bot. J. Linn. Soc. 183: 25-38.
Cameron KM, Wurdack KJ, Jobson RW. 2002.
Molecular evidence for the common origin of snaptraps among carnivorous plants.
– Amer. J. Bot. 89: 1503-1509.
Campagna ML, Downie SR. 1998. The intron in
chloroplast gene rpl16 is missing from the flowering plant families Geraniaceae, Goodeniaceae
and Plumbaginaceae. –
Trans. Illinois State Acad. Sci. 9: 1-11.
Campbell N, Thomson WW. 1976. The
ultrastructure of Frankenia salt glands. – Ann. Bot., N. S., 40:
681-686.
Candau P. 1978a. Palinologia de Caryophyllaceae del sur de
España I. Subfamilia Paronychioideae. – Lagascalia 7: 143-157.
Candau P. 1978b. Palinologia en Caryophyllaceae del sur de
Espagna II. Subfamilia Alsinoideae. – Lagascalia 8: 39-51.
Capuron R. 1968. Sur le genre
Physena Noronh. ex Thouars. – Adansonia, sér. II, 8: 355-357.
Capuron R. 1974. Une variété nouvelle
d’Asteropeia amblyocarpa Tul., Théacée de Madagascar. –
Adansonia, sér. II, 14: 291-292.
Carlquist SJ. 1981. Wood anatomy of Nepenthaceae. – Bull. Torrey
Bot. Club 108: 324-330.
Carlquist SJ. 1982. Wood anatomy of Buxaceae: correlations with
ecology and phylogeny. – Flora 172: 463-491.
Carlquist SJ. 1995. Wood anatomy of Caryophyllaceae: ecological,
habital, systematic, and phylogenetic implications. – Aliso 14: 1-17.
Carlquist SJ. 1998a. Wood anatomy of Portulacaceae and
Hectorellaceae: ecological, habital, and systematic implications. – Aliso 16:
137-153.
Carlquist SJ. 1998b. Wood and stem anatomy of
Petiveria and Rivina (Caryophyllales): systematic
implications. – IAWA J. 19: 383-391.
Carlquist SJ. 1999a. Wood, stem and root
anatomy of Basellaceae with
relation to habit, systematics, and cambial variants. – Flora 194: 1-12.
Carlquist SJ. 1999b. Wood anatomy of
Agdestis (Caryophyllales): systematic
position and nature of the successive cambia. – Aliso 18: 35-43.
Carlquist SJ. 1999c. Wood and stem anatomy of
Stegnosperma (Caryophyllales); phylogenetic
relationships; nature of lateral meristems and successive cambial activity. –
IAWA J. 20: 149-163.
Carlquist SJ. 1999d. Wood anatomy, stem
anatomy, and cambial activity of Barbeuia (Caryophyllales). – IAWA J.
20: 431-440.
Carlquist SJ. 2000a. Wood and stem anatomy of
phytolaccoid and rivinoid Phytolaccaceae (Caryophyllales): ecology,
systematics, nature of successive cambia. – Aliso 19: 13-29.
Carlquist SJ. 2000b. Wood and stem anatomy of
Sarcobatus (Caryophyllales): systematic and
ecological implications. – Taxon 49: 27-34.
Carlquist SJ. 2000c. Wood and bark anatomy of
Achatocarpaceae. – Sida
19: 71-78.
Carlquist SJ. 2001. Wood and stem anatomy of
Rhabdodendraceae is
consistent with placement in Caryophyllales sensu lato. –
IAWA J. 22: 171-181.
Carlquist SJ. 2002. Wood anatomy and
successive cambia in Simmondsia (Simmondsiaceae): evidence for
inclusion in Caryophyllales
s.l. – Madroño 49: 158-164.
Carlquist SJ. 2003a. Wood anatomy of Polygonaceae: analysis of a
family with exceptional wood diversity. – Bot. J. Linn. Soc. 141: 25-51.
Carlquist SJ. 2003b. Wood and stem anatomy of
woody Amaranthaceae s.s.:
ecology, systematics, and the problem of defining rays in dicotyledons. –
Bot. J. Linn. Soc. 143: 1-19.
Carlquist SJ. 2004. Lateral meristems,
successive cambia and their products: a reinterpretation based on roots and
stems of Nyctaginaceae. –
Bot. J. Linn. Soc. 146: 129-143.
Carlquist SJ. 2006. Asteropeia and
Physena (Caryophyllales): a case study
in comparative wood anatomy. – Brittonia 58: 301-313.
Carlquist SJ. 2007. Successive cambia in Aizoaceae: products and process. –
Bot. J. Linn. Soc. 153: 141-155.
Carlquist S. 2010. Caryophyllales: a key group for
understanding wood anatomy character states and their evolution. – Bot. J.
Linn. Soc. 164: 342-393.
Carlquist SJ, Boggs CJ. 1996. Wood anatomy of
Plumbaginaceae. – Bull.
Torrey Bot. Club 123: 135-147.
Carlquist SJ, Lowrie A. 1990. A new species
of tuberous Drosera from Western Australia. – Phytologia 69:
160-162.
Carlquist SJ, Lowrie A. 1992. Eight new taxa
of Drosera from Australia. – Phytologia 73: 98-116.
Carlquist SJ, Wilson EJ. 1995. Wood anatomy
of Drosophyllum (Droseraceae): ecological and
phylogenetic considerations. – Bull. Torrey Bot. Club 122: 185-189.
Carolin RC. 1983. The trichomes of the
Chenopodiaceae and Amaranthaceae. – Bot. Jahrb.
Syst. 103: 451-466.
Carolin RC. 1987. A review of the family Portulacaceae. – Aust. J. Bot.
35: 383-412.
Carolin RC. 1993. Portulacaceae. – In: Kubitzki
K, Rohwer JG, Bittrich V (eds), The families and genera of vascular plants II.
Flowering plants. Dicotyledons. Magnoliid, hamamelid and caryophyllid families,
Springer, Berlin, Heidelberg, New York, pp. 544-555.
Carolin RC, Jacobs SWL, Vesk M. 1975. Leaf
structure in Chenopodiaceae. – Bot. Jahrb. Syst. 95: 226-255.
Carolin RC, Jacobs SWL, Vesk M. 1978. Kranz
cells and mesophyll in the Chenopodiales. – Aust. J. Bot. 26: 683-698.
Castro M, Rossello JA. 2007. Karyology of
Limonium (Plumbaginaceae) species from
the Balearic Islands and the western Iberian Peninsula. – Bot. J. Linn. Soc.
155: 257-272.
Castroviejo S, Lago E. 1992a. Nuevos datos
cariológicos de Chenopodiaceae Ibéricas. – Nova Acta Ci. Comp. Biol. 3:
201-203.
Castroviejo S, Lago E. 1992b. Datos acerca de
la hibridación en el género Sarcocornia (Chenopodiaceae). – An.
Jard. Bot. Madrid 50: 163-170.
Cavaco A. 1962. Les Amaranthaceae de l’Afrique au
sud du Tropique du Cancer et de Madagascar. – Mém. Mus. Natl. Hist. Nat.
Paris, sér. B, 13: 1-254.
Cave RL, Birch CJ, Hammer GL, Erwin JE,
Johnston ME. 2010. Floral ontogeny in Brunonia australis (Goodeniaceae)
and Calandrinia speciosa (Portulacaceae). – Aust. J.
Bot. 58: 61-69.
Çelebioğlu T, Favarger C, Huynh KL. 1983.
Contribution à la micromorphologie de la testa des graines du genre
Minuartia (Caryophyllaceae I. sect.
Minuartia. – Adansonia 4: 415-435.
Cevallos-Ferriz SRS, Estrada-Ruiz E,
Pérez-Hernández BR. 2008. Phytolaccaceae infructescences
from Cerro del Pueblo formation, upper Cretaceous (late Campanian), Coahuila,
Mexico. – Amer. J. Bot. 95: 77-83.
Chamberland M. 1997. Systematics of the
Echinocactus polycephalus complex (Cactaceae). – Syst. Bot. 22:
303-313.
Chan KF, Sun M. 1997. Genetic diversity and
relationships detected by isozyme and RAPD analysis of crop and wild species of
Amaranthus. – Theor. Appl. Gen. 95: 865-873.
Chanda S. 1965. The pollen morphology of Droseraceae with special reference
to taxonomy. – Pollen Spores 7: 509-528.
Chaney RW. 1944. A fossil cactus from the
Eocene of Utah. – Amer. J. Bot. 31: 507-528.
Chang C, Mabry TJ. 1974. The constitution of
the order Centrospermae: RNA-DNA hybridization studies among betalain- and
anthocyanin-producing families. – Biochem. Syst. 1: 185-190.
Charles G. 1998. Copiapoa. – Cirio
Publ. Services Ltd., Holbury.
Charles G. 1999. The genus Espostoa
Br. & R. – Brit. Cact. Succ. J. 17: 68-79.
Charles G. 2008. Notes on
Maihueniopsis Spegazzini (Cactaceae). – Bradleya 26:
63-74.
Chaturvedi SK. 1989. A new device of
self-pollination in Boerhaavia diffusa L. (Nyctaginaceae). – Beitr. Biol.
Pflanzen 64: 55-58.
Chaudhri MN. 1968. A revision of the
Paronychiinae. – Meded. Bot. Mus. Herb. Rijksuniv. Utrecht 258: 1-440.
Chavez RP, Nava RF, Grafström E, Pfaler M
von, Nilsson S. 1998. On the pollen of Salpianthus (Nyctaginaceae) – a
morphological and image analysis approach. – Grana 37: 352-357.
Cheek MR. 2000. A synoptic revision of
Ancistrocladus (Ancistrocladaceae) in
Africa, with a new species from Western Cameroon. – Kew Bull. 55: 871-882.
Cheek MR, Jebb M. 1998. Two new Philippine
Nepenthes. – Kew Bull. 53: 966.
Cheek MR, Jebb M. 1999. Nepenthes
(Nepenthaceae) in Palawan,
Philippines. – Kew Bull. 54: 887-895.
Cheek MR, Jebb M. 2001. Nepenthaceae. – In: Nooteboom
HP (ed), Flora Malesiana 1, 15, Foundation Flora Malesiana, Nationaal Herbarium
Nederland, Leiden; pp. 1-161.
Cheek MR, Jebb M, Murphy B, Mambor F. 2018.
Nepenthes section Insignes in Indonesia, with two new
species. – Blumea 62: 174-178.
Chen D, Wang Q-F. 2004. Pentamerous flowers
in the genus Phytolacca have been derived from trimerous flowers –
new evidence from the floral organogenesis of Phytolacca dodecandra.
– Acta Phytotaxon. Sin. 42: 345-351.
Chen L, James SH, Stace HM. 1997. Self
incompatibility, seed abortion and clonality in the breeding system of several
western Australian Drosera species (Droseraceae). – Aust. J. Bot.
45: 191-201.
Cheng Z-M, Pan H-X, Yin L-K. 2000. Study on
the phytochemistry taxonomy of Tamarix L. and Myricaria Desv.
– Acta Bot. Boreal.-Occid. Sin. 20: 275-282.
Chesselet P, Van Wyk AE. 2002. Mesembs with
nutlike schizocarpic fruit and Ruschianthemum Friedrich sunk under
Stoeberia Dinter & Schwantes. – Bothalia 32: 187-190.
Chesselet P, Mössmer M, Smith GF. 1995.
Research priorities in the succulent plant family Mesembryanthemaceae Fenzl.
– South Afr. J. Sci. 91: 197-209.
Chesselet P, Smith GF, Wyk AE van. 2000.
Systematic and evolutionary significance of morphology in the
Mesembryanthemaceae: interactive database and illustrated atlas for
identification. – Aloe 37: 46-51.
Chesselet P, Smith GF, Wyk AE van. 2001. A
new tribal classification for the Mesembryanthemaceae Fenzl based on characters
of the floral nectary. – Aloe 38: 25-28.
Chesselet P, Smith GF, Wyk AE van. 2002. A
new tribal classification of Mesembryanthemaceae: evidence from floral
nectaries. – Taxon 51: 295-308.
Chesselet P, Hammer S, Oliver I. 2003.
Brianhuntleya, a new genus endemic to the Worcester-Robertson Karoo,
South Africa. – Bothalia 30: 160-164.
Chesselet P, Wyk AE van, Smith GF. 2004.
Mesembryanthemaceae: a new tribe and adjustments to infrafamilial
classification. – Bothalia 34: 47-51.
Chin L, Moran JA, Clarke C. 2010. Trap
geometry in three giant montane pitcher plant species from Borneo is a function
of tree shrew body size. – New Phytol. 186: 461-470.
Chinnappa CC, Morton JK. 1984. Studies on the
Stellaria longipes complex (Caryophyllaceae) –
biosystematics. – Syst. Bot. 9: 60-73.
Chinnock RJ. 1983. The Australian genus
Gunniopsis Pax (Aizoaceae). – J. Adelaide Bot.
Gard. 6: 133-179.
Chinnock RJ. 2010. Some observations on
Salsola L. (Chenopodiaceae) in Australia. – J. Adelaide Bot. Gard.
24: 75-79.
Chopin J, Besson E, Dellamonica G, Nair AGR.
1982. Structure of a 6,8-di-C-pentosylapigenin from Mollugo
pentaphylla. – Phytochemistry 21: 2367-2369.
Chopra RN. 1957. The mode of embryo sac
development in Opuntia aurantiaca Lindl. – a reinvestigation. –
Phytomorphology 7: 403-406.
Chorinsky F. 1931. Vergleichend-anatomische
Untersuchung der Haar-gebilde bei Portulacaceen und Cactaceen. – Österr.
Bot. Zeitschr. 80: 308-327.
Choudhuri HC. 1942. Chromosome studies in
some British species of Limonium. – Ann. Bot., N. S., 6: 183-217.
Choux P. 1934. Les Didiéréacées:
xérophytes de Madagascar. – Mém. Acad. Malgache 18: 1-69.
Chowdhuri PK. 1957. Studies in the genus
Silene. – Notes Roy. Bot. Gard. Edinb. 22: 221-278.
Christenhusz MJM, Brockington SF, Christin
PA, Sage RF. 2014. On the disintegration of Molluginaceae: a new genus and
family – Kewa, Kewaceae
– segregated from Hypertelis, and placement of Macarthuria
in Macarthuriaceae. –
Phytotaxa 181: 238-242.
Christin P-A, Sage TL, Edwards EJ, Ogburn RM,
Khoshravesh R, Sage RF. 2011. Complex evolutionary transitions and the
significance of C3-C4 intermediate forms of
photosynthesis in Molluginaceae. – Evolution 65:
643-660.
Chrtek J, Křisa B. 1999. A revision of Asian
species of the genus Bufonia L. – Acta Univ. Carol., Biol. 43:
77-118.
Chrtek J, Slavíková Z. 1987.
Leitbündelanordnung in den Kronblättern von ausgewählten Arten der Familie
Stellariaceae. – Preslia 60: 11-21.
Chrtek J, Slaviková Z. 1996. Comments on the
families Drosophyllaceae
and Droseraceae. – Casopis
Národního Rada Prirovedna 165: 139-141.
Chrtek J, Slaviková Z. 1999. Genera and
families of the Droserales order. – Novit. Bot. Univ. Carol. Praha 13:
39-46.
Chrtek J, Slaviková Z, Studnička M. 1989.
Beitrag zur Leitbündelanordnung in den Kronblättern ausgewählter Arten der
fleischfressenden Pflanzen. – Preslia 61: 107-124.
Chu G-L. 1987. Archiatriplex, a new
Chenopodiaceous genus from China. – J. Arnold Arbor. 68: 461-469.
Chu G-L, Sanderson SC. 2008. The genus
Kochia (Chenopodiaceae) in North America. – Madroño 55. 251-256.
Chu GL, Stutz HC, Sanderson SC. 1991.
Morphology and taxonomic position of Suckleya suckleyana
(Chenopodiaceae). – Amer. J. Bot. 78: 63-68.
Chuong SDX, Franceschi VR, Edwards GE. 2006.
The cytoskeleton maintains organelle partitioning required for single cell
C4 photosynthesis in Chenopodiaceae species. – Plant Cell 18:
2207-2223.
Cires E, Prieto JA. 2015. Phylogenetic
relationships of Petrocoptis A. Braun ex Endl. (Caryophyllaceae), a discussed
genus from the Iberian Peninsula. – J. Plant Res. 128: 223-238.
Cisneros A, Garcia RB, Tel-Zur N. 2011. Ovule
morphology, embryogenesis and seed development in three Hylocereus
species (Cactaceae). – Flora
206: 1076-1084.
Clement J-S, Mabry TJ. 1996. Pigment
evolution in the Caryophyllales: a systematic
overview. – Bot. Acta 109: 360-367.
Clement J-S, Mabry TJ, Wyler H, Dreiding AS.
1994. Chemical review and evolutionary significance of betalains. – In:
Behnke H-D, Mabry TJ (eds), Caryophyllales: evolution and
systematics, Springer, Berlin, Heidelberg, New York, pp. 247-261.
Clinckemaillie D, Smets EF. 1992. Floral
similarities between Plumbaginaceae and Primulaceae:
systematic significance. – Belg. J. Bot. 125: 151-153.
Cocucci AE. 1957. El género
Ruprechtia (Polygonaceae) en Argentina,
Paraguay y Uruguay. – Rev. Fac. Ci. Exact. Fis. Nat. Cordoba 19: 559-618.
Cocucci AE. 1961a. Embriología de
Trianthema argentina (Aizoaceae). – Kurtziana 1:
105-122.
Cocucci AE. 1961b. Revisión del género
Ruprechtia (Polygonaceae). – Kurtziana 1:
217-269.
Cohn FM. 1913. Beiträge zur Kenntnis der
Chenopodiaceen. – Flora 106: 51-89.
Cole DT, Cole NA. 2005. Lithops:
Flowering stones. 2nd ed. – Cactus & Co.
Conn BJ. 1980. A review of Drosera
in Papuasia. – Brunonia 3: 209-216.
Connor HE. 1984. Gynodioecism in
Sarcocornia quinqueflora (Salicornieae) in New Zealand. – New
Zealand J. Bot. 22: 433-439.
Conran JG, Jaudzems VG, Hallam ND. 1997. Droseraceae germination patterns
and their taxonomic significance. – Bot. J. Linn. Soc. 123: 211-223.
Conran JG, Jaudzems G, Hallam ND. 2007. Droseraceae gland and germination
patterns revisited: support for recent molecular phylogenetic studies. –
Carniv. Plants Newsl. 36: 14-20.
Consaul LL, Warwick SI, McNeill J. 1991.
Allozyme variation in the Polygonum lapathifolium complex. – Can. J.
Bot. 69: 2261-2270.
Contandriopoulos J, Favarger C. 1983. Sur
quelques espèces de Turquie du genre Arenaria L. (étude
cytotaxonomique). – Candollea 38: 733-743.
Conti F. 2003. Minuartia
graminifolia (Caryophyllaceae), a south-east
European species. – Bot. J. Linn. Soc. 143: 419-432.
Coons MP. 1975. The genus Amaranthus
in Ecuador. – Ph.D. diss., University of Michigan, Ann Arbor, Michigan.
Coons MP. 1977. The status of Amaranthus
hybridus L. in South America. – Ci. Naturaleza Ci. Nat. 18: 80-87.
Coons MP. 1978. The status of Amaranthus
hybridus L. in South America 2: the taxonomic problem. – Ci. Naturaleza
Ci. Nat. 19: 66-71.
Coons MP. 1982. Relationships of
Amaranthus caudatus. – Econ. Bot. 36: 129-146.
Cornejo DO, Simpson BB. 1997. Analysis of
form and function in North American columnar cacti (tribe Pachycereeae). –
Amer. J. Bot. 84: 1482-1501.
Correa AMD, Silva TR dos S. 2005. Flora
Neotropica Monograph 96. Drosera (Droseraceae). – New York
Botanical Garden, Bronx, New York.
Correa MN. 1966. Las Frankeniaceae Argentinas. –
Darwiniana 14: 68-94.
Correa MD, Dos Santos Silva TR, Stefano RD
de. 2005. 68. Droseraceae. –
In: Harling G, Andersson L (eds), Flora of Ecuador 75, Botanical Institute,
Göteborg University, pp. 11-16.
Correia E, Freitas H. 2002. Drosophyllum
lusitanicum, an endangered west Mediterranean endemic carnivorous plant:
threats and its ability to control available resources. – Bot. J. Linn. Soc.
140: 383-390.
Costea M, DeMason DA. 2001. Stem in
Amaranthus L. – Taxonomic significance. – Bull. Torrey Bot. Club
128: 254-281.
Costea M, Tardif FJ. 2003. The bracteoles in
Amaranthus (Amaranthaceae): their
morphology, structure, function, and taxonomic significance. – Sida 20:
969-985.
Costea M, Tardif FJ. 2005. Taxonomy of the
Polygonum douglasii (Polygonaceae) complex with a new
species from Oregon. – Brittonia 57: 1-27.
Costea M, Sanders A, Waines G. 2001.
Preliminary results toward a revision of the Amaranthus hybridus
species complex (Amaranthaceae). – Sida 19:
931-974.
Costea M, Waines G, Sanders A. 2001 [2003].
Structure of the pericarp in some Amaranthus L. (Amaranthaceae) species and its
taxonomic significance. – Aliso 20: 51-60.
Costea M, Weaver E, Tardif FJ. 2004. The
biology of Canadian weeds 130. Amaranthus retroflexus L., A.
powellii S. Watson and A. hybridus L. – Can. J. Plant Sci. 84:
631-668.
Cota JH. 1997. A phylogenetic study of
Ferocactus Britton and Rose (Cactaceae: Cactoideae). – Ph.D.
diss., Iowa State University, Ames, Iowa.
Cota JH, Wallace RS. 1995. Karyotypic studies
in the genus Echinocereus (Cactaceae) and their taxonomic
significance. – Caryologia 48: 105-122.
Cota JH, Wallace RS. 1997. Chloroplast DNA
evidence for divergence in Ferocactus and its relationships to North
American columnar cacti (Cactaceae: Cactoideae). – Syst.
Bot. 22: 529-542.
Cota JH, Rebman JP, Wallace RS. 1996.
Chromosome numbers in Ferocactus (Cactaceae: Cactoideae). –
Cytologia 61: 431-437.
Cota-Sánchez JH, Reyes-Olivas Á,
Sánchez-Soto B. 2007. Vivipary in coastal cacti: a potential reproductive
strategy in halophytic environments. – Amer. J. Bot. 94: 1577-1581.
Coulter JM. 1896. Preliminary revision of the
North American species of Echinocactus, Cereus, and
Opuntia. – Contr. U.S. Natl. Herb. 3: 406.
Covas G. 1939. Los géneros de Amarantáceas
Argentinas. – Rev. Argent. Agron. 6: 282-303.
Covas G. 1941. Las Amarantáceas Bonarienses.
– Darwiniana 5: 329-368.
Cranwell LM. 1961. Subantarctic pollen and
spores I. Lyallia of Kerguelen. – Pollen Spores 3: 11-20.
Cranwell LM. 1963. The Hectorellaceae: pollen
type and taxonomic speculation. – Grana Palynol. 4: 195-202.
Crawford DJ, Evans K. 1978. The affinities of
Chenopodium flabellifolium: evidence from seed coat surface and
flavonoid chemistry. – Brittonia 30: 313-318.
Crawley SS, Hilu KW. 2012a. Impact of missing
data, gene choice, and taxon sampling on phylogenetic reconstruction: the Caryophyllales (angiosperms).
– Plant Syst. Evol. 298: 297-312.
Crawley SS, Hilu KW. 2012b. Caryophyllales: evaluating
phylogenetic signal in trnK intron versus matK. – J. Syst.
Evol. 50: 387-410.
Crespo MB, Lledó MD. 2000. Two new North
African genera related to Limoniastrum (Plumbaginaceae). – Bot. J.
Linn. Soc. 132: 165-174.
Cronquist A, Thorne RF. 1994. Nomenclatural
and taxonomic history. – In: Behnke H-D, Mabry TJ (eds) Caryophyllales: evolution and
systematics, Springer, Berlin, Heidelberg, New York, pp. 87-121.
Crow GE. 1978. A taxonomic revision of
Sagina (Caryophyllaceae) in North
America. – Rhodora 80: 1-91.
Crow GE. 1979. The systematic significance of
seed morphology in Sagina (Caryophyllaceae) under
scanning electron microscopy. – Brittonia 31: 52-63.
Crozier BS. 2004. Subfamilies of Cactaceae Juss., including
Blossfeldioideae subfam. nov. – Phytologia 86: 52-64.
Crozier BS. 2005. Systematics of Cactaceae Juss.: phylogeny, cpDNA
evolution, and classification, with emphasis on the genus Mammillaria
Haw. – Ph.D. diss., University of Texas, Austin, Texas.
Cruden RW. 1970. Hawkmoth pollination of
Mirabilis. – Bull. Torrey Bot. Club 97: 89-91.
Cruden RW. 1973. Reproductive biology of
weedy and cultivated Mirabilis. – Amer. J. Bot. 60: 802-809.
Cruz MÁ, Arias S, Terrazas T. 2016.
Molecular phylogeny and taxonomy of the genus Disocactus (Cactaceae), based on the DNA
sequences of six chloroplast markers. – Willdenowia 46: 145-164.
Cuadrado GA, Garralla SS. 2009. Palinología
de los géneros de Cactaceae
Maihuenia (Maihuenioideae) y Pereskia (Pereskioideae) de
Argentina. – Bonplandia 18: 5-12.
Cuénoud P. 2006. Phylogeny, evolution and
diversification of Caryophyllales. – In: Sharma
AK, Sharma A (eds), Plant genome biodiversity and evolution 1 C, Phanerogams
(Angiosperms-Dicotyledons), Science Publ., Enfield, New Hampshire, pp.
187-218.
Cuénoud P, Savolainen V, Chatrou LW, Powell
M, Grayer RJ, Chase MW. 2002. Molecular phylogenetics of Caryophyllales based on nuclear
18S rDNA and plastid rbcL, atpB, and matK DNA
sequences. – Amer. J. Bot. 89: 132-144.
Cufodontis G. 1953. Caryophyllaceae. Enumeratio
plantarum Aethiopiae. – Bull. Jard. Bot. Bruxelles 23: 97-98.
Cui D-F, Liao W-B, Zhang B. 2000.
Determination of flavonoid compounds of Reaumuria L. (Tamaricaceae) and their
taxonomical significance. – Acta Bot. Boreal.-Occid. Sin. 20: 283-287.
Culham A, Gornall RJ. 1994. The taxonomic
significance of naphthoquinones in the Droseraceae. – Biochem. Syst.
Ecol. 22: 507-515.
Cullen DC, Suárez A de. 1953. Las espécies
argentinas del género Calandrinia (Portulacaceae). – Bol. Soc.
Argent. Bot. 5: 1-112.
Curran MK. 1885. Classification of the
Eriogoneae as affected by some connecting forms. – Bull. Calif. Acad. Sci. 1:
272-275.
Curtis GJ. 1968. Observations on fruit shape
and other characters in the species of the section Patellares, genus
Beta. – Euphytica 17: 485-491.
Czukavina A. 1966. Knorringia, a new
section in Polygonum L. – Novit. Syst. Plant. Vasc. 1966: 92-93. [In
Russian]
Dahlgren KVO. 1916. Zytologische und
embryologische Studien über die reihen Primulales und Plumbaginales. –
Kungl. Sv. Vetensk.-Akad. Handl. 56(4): 1-80.
Dahlgren RMT, Van Wyk AE. 1988. Structures
and relationships of families endemic to or centered in southern Africa. –
In: Goldblatt P, Lowry PP II (eds), Modern systematic studies in African
botany, Monographs in Systematic Botany 25, Missouri Botanical Garden, St.
Louis, Missouri, pp. 1-95.
Dajoz I, Till-Bottraud I, Gouyon P-H. 1991.
Evolution of pollen morphology. – Science 253: 66-68.
Dalby DH. 1962. Chromosome number, morphology
and breeding behaviour in the British Salicornieae. – Watsonia 5: 150-162.
Dalby DH. 1963. Seed dispersal in
Salicornia pusilla. – Nature 199: 197-198.
Damboldt J, Phitos D. 1966. Ein Beitrag zur
Zytotaxonomie der Gattung Silene L. in Griechenland. – Österr. Bot.
Zeitschr. 113: 169-175.
Dammer U. 1893a. Polygonaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien III(1a), W. Engelmann,
Leipzig, pp. 1-36.
Dammer U. 1893b. Die
Verbreitungsausrüstungen der Polygonaceen. – Engl. Bot. Jahrb. Syst. 15:
260-285.
Danin A. 2001. A new species of
Bufonia (Caryophyllaceae) from Israel:
B. ramonensis. – Willdenowia 31: 95-100.
Danin A, Baker I, Baker HG. 1978.
Cytogeography and taxonomy of the Portulaca oleracea L. polyploid
complex. – Israel J. Bot. 27: 177-211.
Danser BH. 1927. Die Polygonaceen
Niederländisch-Ostindiens. – Bull. Jard. Bot. Buitenzorg, sér. III, 8:
117-261.
Danser BH. 1928. The Nepenthaceae of the Netherlands
Indies. – Bull. Jard. Bot. Buitenzorg, sér. III, 9: 249-438.
Daston JS. 1946. Three noteworthy cacti of
southwestern Utah. – Amer. Midl. Natur. 36: 661-662.
Datson B. 2002. Samphires in Western
Australia: a field guide to Chenopodiaceae tribe Salicornieae. – Department
of Conservation and Land Management, Perth, Western Australia.
Daumann E. 1930. Das Blütennektarium von
Nepenthes. – Beih. Bot. Centralbl. 47: 1-14.
Davidson BL. 2000. Lewisias. – Timber
Press, Portland, Oregon.
Davis RJ. 1966. The North American perennial
species of Claytonia. – Brittonia 18: 285-303.
Daxenbichler M, Spencer GF, Carlson DG, Rose
GB, Brinker AM, Powell RG. 1991. Glucosinolate composition of seeds from 297
species of wild plants. – Phytochemistry 30: 2623-2638.
DeBuhr LE. 1977. Sectional reclassification
of Drosera subgenus Ergaleium (Droseraceae). – Aust. J. Bot.
25: 209-218.
DeFraine E. 1912. The anatomy of the genus
Salicornia. – Bot. J. Linn. Soc. 41: 317-348.
DeFraine E. 1916. The morphology and anatomy
of the genus Statice as represented at Blakeney Point. – Ann. Bot.
30: 240-280.
Degraeve N. 1980. Étude de diverses
particularités caryotypiques des genres Silene, Lychnis et
Melandrium. – Bol. Soc. Brot., ser. II, 53: 595-643.
Dehghani M, Akhani H. 2009. Pollen
morphological studies in subfamily Suaedoideae (Chenopodiaceae). – Grana 48:
79-101.
Dehmer KJ. 2003. Molecular diversity in the
genus Amaranthus. – In: Knüpffer H, Ochsmann J (eds), Schriften zu
Genetischen Resourcen 22, Rudolf Mansfeld and Plant Genetic Resources, ZADI,
Bonn, pp. 208-215.
Dehn M. 1992. Untersuchungen zum
Verwandtschaftskreis der Ruschiinae (Mesembryanthemaceae). – Mitt. Inst.
Allg. Bot. Hamburg 24: 91-198.
De Laet J, Clinckemaille J, Jansen S, Smets
E. 1995. Floral ontogeny of the Plumbaginaceae. – J. Plant
Res. 108: 289-304.
de la Fuente V, Oggerin M, Rufo L, Rodríguez
N, Ortuñez E, Sánchez-Mata D, Amils R. 2013. A micromorphological and
phylogenetic study of Sarcocornia A. J. Scott (Chenopodiaceae) on the
Iberian Peninsula. – Plant Biosyst. 147: 158-173.
Demaio PH, Barfuss MHJ, Till W, Chiapella J.
2010. Entwicklungsgeschichte und infragenerische Klassifikation der Gattung
Gymnocalycium: Erkenntnisse aus molekularen Daten. Phylogenetic
relationships and infrageneric classification of the genus
Gymnocalycium: insights from molecular data. –
Gymnocalycium 23(Sonderausgabe): 925-946.
Demaio PH, Barfuss MHJ, Kiesling R, Till W,
Chiapella JO. 2011. Molecular phylogeny of Gymnocalycium (Cactaceae): assessment of
alternative infrageneric systems, a new subgenus, and trends in the evolution
of the genus. – Amer. J. Bot. 98: 1841-1854.
Desfeux C, Lejeune B. 1996. Systematics of
Euromediterranean Silene (Caryophyllaceae): evidence
from a phylogenetic analysis using ITS sequences. – Compt. Rend. Acad. Sci.
Paris, sciences de la vie 319: 351-358.
Desfeux C, Maurice S, Henry J-P, Lejeune B,
Gouyon P-H. 1996. Evolution of reproductive systems in the genus
Silene. – Proc. Biol. Sci., Roy. Soc. 263: 409-414.
Devi HM, Rao MSR. 1976. Embryology of
Suaeda maritima (L.) Dumort. – Curr. Sci. 45: 383-385.
Dicht RF, Lüthy AD. 2003.
Coryphantha. Kakteen aus Nordamerika. – E. Ulmer, Stuttgart.
Dickie SL. 1996. Phylogeny and evolution in
the subfamily Opuntioideae (Cactaceae): insights from
rpl16 intron sequence evolution. – M.Sc. thesis, Iowa State
University, Ames, Iowa.
Dickison WC. 2002. Physenaceae. – In: Kubitzki K,
Bayer C (eds), The families and genera of vascular plants V. Flowering plants.
Dicotyledons. Malvales,
Capparales and
non-betalain Caryophyllales,
Springer, Berlin, Heidelberg, New York, pp. 332-333.
Dickison WC, Miller RB. 1993. Morphology and
anatomy of the Malagasy genus Physena (Physenaceae), with a discussion on
the relationships of the genus. – Bull. Mus. Natl. Hist. Nat. (Paris), sér.
IV, sect. B, Adansonia 15: 85-106.
Diels L. 1936. Droseraceae. – In: Engler A
(†), Harms H (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 17b, W.
Engelmann, Leipzig, pp. 766-784.
Di Fulvio TE. 1975. Estomatogenesis en
Halophytum ameghinoi (Halophytaceae). – Kurtziana 8:
17-29.
Dillenberger MS, Kadereit JW. 2014. Maximum
polyphyly: multiple origins and delimitation with plesiomorphic characters
require a new circumscription of Minuartia (Caryophyllaceae). – Taxon
63: 64-88.
Dillenberger MS, Kadereit JW. 2015. A
revision of Facchinia (Minuartia s.l., Caryophyllaceae). –
Edinburgh J. Bot. 72: 353-389.
Dinan L, Whiting P, Scott AJ. 1998. Taxonomic
distribution of phytoecdysteroids in seeds of members of Chenopodiaceae. –
Biochem. Syst. Ecol. 26: 553-576.
Di Tomaso JM. 1998. Impact, biology, and
ecology of saltcedar (Tamarix spp.) in the southwestern United States.
– Weed Technol. 12: 326-336.
Di Vincenzo V, Gruenstaeudl M, Nauheimer L,
Wondafrash M, Kamau P, Demissew S, Borsch T. 2018. Evolutionary diversification
of the African achyranthoid clade (Amaranthaceae) in the context of
sterile flower evolution and epizoochory. – Ann. Bot. 122: 69-85.
Dixon KW, Pate SJ. 1978. Phenology,
morphology and reproductive biology of the tuberous sundew, Drosera
erythrorhiza Lindl. – Aust. J. Bot. 26: 441-454.
Dnyansagar VR, Malkhede SR. 1963. Development
of the seed of Trianthema portulacastrum Linn. – Proc. Indian Acad.
Sci., Sect. B, 57: 343-355.
Doğan M, Akaydin GP. 2002a. A new species of
Acantholimon Boiss. (Plumbaginaceae) from Turkey.
– Bot. J. Linn. Soc. 138: 365-368. – Erratum: 139: 223.
Doğan M, Akaydin GP. 2002b. A new species of
Acantholimon Boiss. (Plumbaginaceae) from Ankara,
Turkey. – Bot. J. Linn. Soc. 140: 443-448.
Doğan M, Akaydin G. 2007. Synopsis of
Turkish Acantholimon Boiss. (Plumbaginaceae). – Bot. J.
Linn. Soc. 154: 397-419.
Doida Y. 1960. Cytological studies in
Polygonum and related genera I. – Bot. Mag. (Tokyo) 37: 337-340.
Dolcher T, Pignatti S. 1971. Un’ ipotesi
sull evoluzione dei Limonium del bacino del Mediterraneo. – Giorn.
Bot. Ital. 105: 95-107.
Donald J, Rowley GD. 1966. Reunion of the
genus Neoporteria. – Cact. Succ. J. Gr. Brit. 28: 54-58.
Donati D, Zanovello C. 2011.
Epithelantha. – Associazione Cactus Trentino Südtirol,
Villazzano.
Douglas NA, Manos PS. 2007. Molecular
phylogeny of Nyctaginaceae:
taxonomy, biogeography, and characters associated with radiation of xerophytic
genera in North America. – Amer. J. Bot. 94: 856-872.
Douglas N, Spellenberg R. 2010. A new tribal
classification of Nyctaginaceae. – Taxon 59:
905-910.
Doweld AB. 1999. Tribal taxonomy of
Pereskioideae and Opuntioideae (Cactaceae). – Sukkulenty I:
25-26.
Doweld AB. 2000a. Proposal to conserve the
name Brasilicactus against Acanthocephala (Cactaceae). – Taxon 49:
564-565.
Doweld AB. 2000b. Proposal to conserve the
name Eriocactus against Eriocephala (Cactaceae). – Taxon 49:
566-568.
Downie SR, Palmer JD. 1994a. A chloroplast
DNA phylogeny of the Caryophyllales based on
structural and inverted repeat restriction site variation. – Syst. Bot. 19:
236-252. – Erratum: Syst. Bot. 19: 487.
Downie SR, Palmer JD. 1994b. Phylogenetic
relationships using restriction site variation of the chloroplast DNA inverted
repeat. – In: Behnke H-D, Mabry TJ (eds), Caryophyllales: evolution and
systematics, Springer, Berlin, Heidelberg, New York, pp. 223-233.
Downie SR, Katz-Downie DS, Cho K-J. 1997.
Relationships in the Caryophyllales as suggested by
phylogenetic analyses of partial chloroplast DNA ORF2280 homolog sequences. –
Amer. J. Bot. 84: 253-273.
Doyle JJ. 1983. Flavonoid races of
Claytonia virginica (Portulacaceae). – Amer. J.
Bot. 70: 1085-1092.
Drude O. 1891. Droseraceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien III(2), W. Engelmann,
Leipzig, pp. 261-272.
Ducousso M, Ramanankierana H, Duponnois R,
Rabévohitra R, Randrihasipara L, Vincelett M, Dreyfus B, Prin Y. 2008.
Mycorrhizal status of native trees and shrubs from eastern Madagascar littoral
forests with special emphasis on one new ectomycorrhizal endemic family, the Asteropeiaceae. – New Phytol.
178: 233-238.
Dugand A. 1952. Notas sobre algunas
Triplaris (Polygonaceae) de Venezuela y la
costa caribe de Colombia. – Mutisia 10: 1-6.
Duke JA. 1960. Polygonaceae in Flora of Panama.
– Ann. Missouri Bot. Gard. 47: 323-359.
Duke JA. 1961. Preliminary revision of the
genus Drymaria. – Ann. Missouri Bot. Gard. 48: 173-268.
Dulberger R. 1975. Intermorph structural
differences between stigmatic papillae and pollen grains in relation to
incompatibility in Plumbaginaceae. – Proc. Roy.
Soc. London, Biol. 188: 2578-274.
Dunbar A. 1967. Wachs im Sporoderm von
Plumbago capensis Thunb. – Grana Palynol. 7: 10-15.
Dupont S. 1968. Épidermes et plantules des
mesembryanthemacées. Systématique, évolution. – Bull. Soc. Hist. Nat.
Toulouse 104: 7-64.
Dupont S. 1977. Notes on the pollen of the
Mesembryanthemaceae. Principal types, variation and problems requiring study.
– Cact. Succ. J. Great Britain 39: 57-63.
Duran A, Menemen Y. 2003. A new species of
Silene (Caryophyllaceae) from South
Anatolia, Turkey. – Bot. J. Linn. Soc. 143: 109-113.
Durand R, Zenk MH. 1974. The homogenisate
ring-cleavage pathway in the biosynthesis of acetate-derived naphthoquinones of
the Droseraceae. –
Phytochemistry 13: 1483-1492.
Durović S, Schönswetter P, Niketić M,
Tomović G. Frajman B. 2017. Disentangling relationships among the members of
the Silene saxifraga alliance (Caryophyllaceae): phylogenetic
structure is geographically rather than taxonomically segregated. – Taxon 66:
343-364.
Ebrahimzadeh H, Ataei-Azimi A, Akhani H,
Noori-Daloii MR. 1994. Studies on the caryology of some species of the genus
Suaeda (Chenopodiaceae) in Iran. – J. Sci. Islamic Rep. Iran 5:
81-88.
Eckardt T. 1954. Morphologische und
systematische Auswertung der Placentation von Phytolaccaceen. – Ber. Deutsch.
Bot. Ges. 67: 113-129.
Eckardt T. 1955. Nachweis der
Blattbürtigkeit (‘Phyllosporie’) grundständiger Samenanlagen bei
Centrospermen. – Ber. Deutsch. Bot. Ges. 68: 167-182.
Eckardt T. 1967a. Vergleich von
Dysphania mit Chenopodium und mit Illecebraceae. – Bauhinia
3: 327-344.
Eckardt T. 1967b. Blütenbau und
Blütenentwicklung von Dysphania myriocephala Benth. – Bot. Jahrb.
Syst. 86: 20-37.
Eckardt T. 1967c. Blütenmorphologie von
Dysphania plantaginella F. v. M. – Phytomorphology 17: 165-172.
Eckardt T. 1974. Vom Blütenbau der
Centrospermen-Gattung Lophiocarpus Turcz. – Phyton (Horn) 16:
13-27.
Eckardt T. 1976. Classical morphological
features of centrospermous families. – Plant Syst. Evol. 126: 5-25.
Edman G. 1929. Zur Entwicklungsgeschichte der
Gattung Oxyria Hill, nebst zytologischen, embryologischen, und
systematischen Bemerkungen über einige andere Polygonaceen. – Acta Horti
Berg. 9: 165-291.
Edwards EJ. 2006. Correlated evolution of
stem and leaf hydraulic traits in Pereskia (Cactaceae). – New Phytol. 172:
479-789.
Edwards EJ, Diaz M. 2006. Ecological
physiology of Pereskia guamacho, a cactus with leaves. – Plant Cell
Envir. 29: 247-256.
Edwards EJ, Donoghue MJ. 2006.
Pereskia and the origin of the cactus life form. – Amer. Natur. 167:
777-793.
Edwards EJ, Nyffeler R, Donoghue MJ. 2005.
Basal cactus phylogeny: implications of Pereskia paraphyly for the
transition to the cactus life form. – Amer. J. Bot. 92: 1177-1188.
Eggens F, Popp M, Nepokroeff M, Wagner WL,
Oxelman B. 2007. The origin and number of introductions of the Hawaiian endemic
Silene species (Caryophyllaceae). – Amer. J.
Bot. 94: 210-218.
Eggli U. 1984. Stomatal types of Cactaceae. – Plant Syst. Evol.
146: 197-214.
Eggli U. 1997. A synopsis of woody Portulacaceae in Madagascar. –
Adansonia, sér. III, 19: 45-59.
Eggli U (ed). 2002. Illustrated handbook of
succulent plants. Dicotyledons. – Springer, Berlin, Heidelberg, New York.
Eggli U, Nyffeler R. 1998. Proposal to
conserve Parodia against Frailea. – Taxon 47: 475-476.
Eggli U, Taylor NP. 1991. Index of names of
Cactaceae published 1950-1990.
– Royal Botanic Gardens, Kew.
Eggli U, Muñoz Schick M, Leuenberger BE.
1995 [1996]. Cactaceae of South
America: the Ritter collections. – Englera 16.
Ehler N, Barthlott W. 1978. Die epicuticulare
Skulptur der Testa-Zellwände einiger Mesembryanthemaceae. – Bot. Jahrb.
Syst. 99: 329-340.
Ehrendorfer F. 1976a. Chromosome numbers and
differentiation of centrospermous families. – Plant Syst. Evol. 126:
27-30.
Ehrendorfer F. 1976b. Closing remarks:
systematics and evolution of centrospermous families. – Plant Syst. Evol.
126: 99-105.
Elghamry MI, Grunert E, Aehnelt E. 1971. An
active principle responsible for estrogenicity in the leaves of Beta
vulgaris. – Plant Med. 19: 208-214.
Eliasson UH. 1987. 44. Amaranthaceae. – In: Harling
G, Sparre B (eds), Flora of Ecuador 28, Swedish Natural Science Research
Council, Stockholm, pp. 1-137.
Eliasson UH. 1988. Floral morphology and
taxonomic relations among the genera of Amaranthaceae in the New World
and the Hawaiian Islands. – Bot. J. Linn. Soc. 96: 235-283.
Eliasson UH. 1993. 35A. Phytolaccaceae, 35B. Achatocarpaceae. – In:
Harling G, Andersson L (eds), Flora of Ecuador 46, Nord. J. Bot., Copenhagen,
pp. 1-51.
Eliassson UH. 1996. 37. Molluginaceae, 38. Aizoaceae, 39. Portulacaceae. – In: Harling
G, Andersson L (eds), Flora of Ecuador 55, Nord. J. Bot., Copenhagen, pp.
1-53.
Ellis AG, Weis AE. 2006. Coexistence and
differentiation of ‘flowering stones’: the role of local adaptation to soil
microenvironment. – J. Ecol. 94: 322-335.
Ellis AG, Weis AE, Gaut BS. 2006.
Evolutionary radiation of “stone plants” in the genus Argyroderma
(Aizoaceae): unravelling the
effects of landscape, habitat, and flowering time. – Evolution 60: 39-55.
Ellis JR, Janick J. 1960. The chromosomes of
Spinacia oleracea. – Amer. J. Bot. 47: 210-214.
Emberger L. 1939. La structure de la fleur
des Polygonacées. – Compt. Rend. Acad. Sci. Paris 208: 370-372.
Endler J, Buxbaum F. 1973. Die
Pflanzenfamilie der Kakteen. Ein systematischer Wegweiser für Liebhaber und
Erwerbszüchter mit einer kompletten Liste der Gattungssynonyme. Dritte
Auflage. – Verlag A. Philler, Minden, Germany.
Endress ME, Bittrich V. 1993. Molluginaceae. – In: Kubitzki
K, Rohwer JG, Bittrich V (eds), The families and genera of vascular plants II.
Flowering plants. Dicotyledons. Magnoliid, hamamelid and caryophyllid families,
Springer, Berlin, Heidelberg, New York, pp. 419-426.
Engel T, Barthlott W. 1988. Micromorphology
of epicuticular waxes in centrosperms. – Plant Syst. Evol. 161: 71-85.
Engleman EM. 1960. Ovule and seed development
in certain cacti. – Amer. J. Bot. 47: 460-467.
Engler A. 1912. Caryophyllaceae africanae. –
Engl. Bot. Jahrb. Syst. 48: 380-384.
Engler A. 1925. Reihe Opuntiales. Historische
Entwicklung der Ansichten über die systematische Stellung der Reihe. – In:
Engler A (ed), Die Natürlichen Pflanzenfamilien. 2. Aufl. 21: 592-594.
Engler A (†). 1931. Rutaceae. – In:
Engler A (†), Harms H, Pax F (eds), Die natürlichen Pflanzenfamilien, 2.
Aufl., Bd. 19a, W. Engelmann, Leipzig, pp. 187-359.
Erbar C, Leins P. 2006. Floral ontogeny and
systematic position of the Didiereaceae. – Plant Syst.
Evol. 261: 165-185.
Erben M. 1978. Die Gattung Limonium
im südwestmediterranen Raum. – Mitt. Bot. Staatssamml. München 14:
361-631.
Erben M. 1979. Karyotype differentiation and
its consequences in Mediterranean Limonium. – Webbia 34: 409-417.
Erben M. 1986. Bemerkungen zur Taxonomie der
Gattung Limonium III. – Mitt. Bot. Staatssamml. München 22:
203-220.
Erben M. 1988. Bemerkungen zur Taxonomie der
Gattung Limonium IV. – Mitt. Bot. Staatssamml. München 27:
281-406.
Erben M. 1991. Bemerkungen zur Taxonomie der
Gattung Limonium VI. – Mitt. Bot. Staatssamml. München 30:
459-478.
Erben M, Crespo MB. 2003.
Myriolepis, a new genus segregated from Limonium (Plumbaginaceae). – Taxon 52:
67-73.
Erdtman G. 1948. Pollen morphology and plant
taxonomy VIII. Didiereaceae.
– Bull. Mus. Natl. Hist. Nat. Paris, sér. II, 20: 387-394.
Erdtman G. 1958. A note on the pollen
morphology in the Ancistrocladaceae and Dioncophyllaceae. –
Veröff. Geobot. Inst. Rübel Zürich 33: 47-49.
Erdtman G. 1968. On the exine in
Stellaria crassipes Hult. – Grana Palynol. 8: 271-276.
Erdtman G, Dunbar A. 1966. Notes on electron
micrographs illustrating the pollen morphology in Armeria maritima and
A. sibirica. – Grana Palynol. 6: 435-475.
Eriksson R. 1996. 40. Basellaceae. – In: Harling G,
Andersson L (eds), Flora of Ecuador 55, Nord. J. Bot., Copenhagen, pp.
53-86.
Eriksson R. 2007. A synopsis of Basellaceae. – Kew Bull. 62:
297-320.
Erixon P, Oxelman B. 2008. Reticulate or
tree-like chloroplast DNA evolution in Sileneae (Caryophyllaceae)? – Mol.
Phylogen. Evol. 48: 313-325.
Eröz Poyraz İ, Ataşlar E. 2010. Pollen and
seed morphology of Velezia L. (Caryophyllaceae) genus in
Turkey. – Turkish J. Bot. 34: 179-190.
Ertter B. 1980. A revision of the genus
Oxytheca (Polygonaceae). – Brittonia 32:
70-102.
Ertter B. 1981. Notes on Goodmania
and Oxytheca (Polygonaceae: Eriogonoideae). –
Brittonia 33: 37-38.
Esau K. 1934. Ontogeny of phloem in the sugar
beet (Beta vulgaris L.). – Amer. J. Bot. 21: 632-644.
Esau K, Cheadle VJ. 1969. Secondary growth in
Bougainvillea. – Ann. Bot., N. S., 33: 807-819.
Eyma PJ. 1934. Polygonaceae. – In: Pulle A
(ed), Flora of Suriname, Vereeniging Kolonial Institute te Amsterdam, Meded.
no. xxx. Afd. Handelsmuseum no. 11, pp. 49-71.
Fagerlind F. 1937. Der Embryosack von
Plumbagella und Plumbago. – Ark. f. Bot. 29B(1): 1-8.
Fahn A. 1958. Xylem structure and annual
rhythm of development in trees and shrubs of the desert 1. Tamarix
aphylla, T. jordanis var. negevensis, T.
gallica var. marismortui. – Trop. Woods 109: 81-94.
Fahn A. 1963. The fleshy cortex of
articulated Chenopodiaceae. – J. Indian Bot. Soc. 42A: 39-45.
Fahn A, Arzee T. 1959. Vascularization of
articulated Chenopodiaceae and the nature of their fleshy cortex. – Amer. J.
Bot. 46: 330-338.
Fahn A, Dembo N. 1964. Structure and
development of the epidermis in articulated Chenopodiaceae. – Israel J. Bot.
13: 177-192.
Fahn A, Shchori Y. 1967. The organization of
the secondary conducting tissues in some species of the Chenopodiaceae. –
Phytomorphology 17: 147-154.
Falatoury AN, Assadi M, Ghahremaninejad F.
2016. New species and new synonymy in the genus Gypsophila L. subgenus
Pseudosaponaria Williams (Caryophyllaceae). –
Adansonia 38: 257-265.
Falkovich MI, Kovalev OV. 2007. An overview
of classification of the higher taxa of the Chenopodiaceae and the family
origin. – In: Kovalev OV, Zhilin SG (eds), Phase transitions in biological
systems and the evolution of biodiversity, PIYaF RAN, St. Petersburg, Russia,
pp. 80-115. [in Russian with English summary]
Favarger C. 1962. Contribution à l’étude
cytologique des genres Minuartia et Arenaria. – Bull. Soc.
Neuchâtel. Sci. Nat. 85: 53-81.
Favarger C. 1965. A striking polyploid
complex in the alpine flora: Arenaria ciliata L. – Bot. Not. 118:
273-280.
Favarger C. 1967. Nombres chromosomiques de
quelques taxa principalement balkaniques du genre Minuartia (L.)
Hiern. – Bot. Jahrb. Syst. 86: 282.
Favarger C. 1972. Sur quelques
Arenaria d’Europe et d’Asie occidentale. – Bot. Not. 125:
465-476.
Fawzi NM, Fawzi AM, Mohamed AA. 2010. Seed
morphological studies on some species of Silene L. (Caryophyllaceae). – Intern.
J. Bot. 6: 287-292.
Fay MF, Cameron KM, Prance GT, Lledó MD,
Chase MW. 1997. Familial relationships of Rhabdodendron (Rhabdodendraceae): plastid
rbcL sequences indicate a caryophyllid placement. – Kew Bull. 52:
923-932.
Fearn PJ. 1996. A review of the origins of
the cactus family and the search for a system of classification. – Wimborne,
United Kingdom. [Publ. by the author]
Fellows CE. 1976. Chromosome counts and new
combinations in Claytonia sect. Limnia (Portulacaceae). – Madroño 23:
296-297.
Feodorova TA. 2011. Phylogenetic relations of
the south African species of Caroxylon sect. Caroxylon and
Tetragonae (Chenopodiaceae) based on the morphology and nrITS
sequences. – Turczaninowia 14: 69-76.
Ferguson DJ. 1991. In defense of the genus
Glandulicactus Backeb. – Cactus Succ. J. (U.S.) 63: 87-91.
Ferguson DJ. 1992. The genus
Echinocactus Link & Otto, subgenus Homalocephala (Br.
& R.) stat. nov. – Cactus Succ. J. (U.S.) 64: 169-172.
Ferguson DJ. 2001. Phemeranthus and
Talinum (Portulacaceae) in New Mexico.
– New Mexico Botanist 20: 1-7.
Ferguson DJ, Kiesling R. 1997. Puna
bonnieae (Cactaceae), a new
species from Argentina. – Cactus Succ. J. 69: 287-293.
Fernald ML. 1919. The unity of the genus
Arenaria. – Rhodora 21: 1-22.
Fernandes A, Leitao MT. 1971. Contribution à
la connaissance cytotaxonomique des Spermatophyta du Portugal III. Caryophyllaceae. – Bol. Soc.
Broteriana, sér. II, 45: 143-176.
Fiedler H. 1910. Beiträge zur Kenntnis der
Nyctaginaceae. – Engl. Bot.
Jahrb. Syst. 44: 572-605.
Fior S, Karis PO. 2007. Phylogeny, evolution
and systematics of Moehringia (Caryophyllaceae) as inferred
from molecular and morphological data: a case for homology reassessment. –
Cladistics 23: 362-372.
Fior S, Karis PO, Casazza G, Minuto L, Sala
F. 2006. Molecular phylogeny of the Caryophyllaceae (Caryophyllales) inferred from
chloroplast matK and nuclear rDNA ITS sequences. – Amer. J. Bot. 93:
399-411.
Flores-Olvera H, Davis JI. 2001. A cladistics
analysis of Atripliceae (Chenopodiaceae) based on morphological data. – J.
Torrey Bot. Soc. 128: 297-319.
Flores-Olvera H, Vrijdaghs A, Ochoterena H,
Smets E. 2011. The need to re-investigate the nature of homoplastic characters:
an ontogenetic case study of the ‘bracteoles’ in Atripliceae
(Chenopodiaceae). – Ann. Bot. 108: 847-865.
Flowers TJ, Galal HK, Bromham L. 2010.
Evolution of halophytes: multiple origins of salt tolerance in land plants. –
Funct. Plant Biol. 37: 604-612.
Ford DI. 1992. Systematics and evolution of
Montiopsis subgenus Montiopsis. – Ph.D. diss., Washington
University, St. Louis, Missouri.
Ford DI. 1993. New combinations in
Montiopsis Kuntze (Portulacaceae). – Phytologia
74: 273-278.
Ford-Lloyd BV, Williams JT. 1975. A revision
of Beta section Vulgares (Chenopodiaceae), with new light on
the origin of cultivated beets. – Bot. J. Linn. Soc. 71: 89-102.
Forterre Y, Skotheim JM, Dumais J, Mahadevan
L. 2005. How the Venus flytrap snaps. – Nature 433: 421-425.
Fosberg FR. 1978. Studies in the genus
Boerhavia L. (Nyctaginaceae) 1-5. –
Smithsonian Contr. Bot. 39: 1-20.
Foster PF, Sork VL. 1997. Population and
genetic structure of the West African rain forest liana Ancistrocladus
korupensis (Ancistrocladaceae). –
Amer. J. Bot. 84: 1078-1091.
Fowler BA, Turner BL. 1977. Taxonomy of
Selinocarpus and Ammocodon (Nyctaginaceae). – Phytologia
37: 177-208.
Fraine E de. 2013. The anatomy of the genus
Salicornia. – Bot. J. Linn. Soc. 41: 317-348.
Fraine E de, Salisbury ES. 1916. The
morphology and anatomy of the genus Statice as represented at Blakeney
Point I. Statice binervosa G. E. Sm. and S. bellidifolia D.
C. – Ann. Bot. 30: 239-282.
Frajman B, Oxelman B. 2007. Reticulate
phylogenetics and phytogeographical structure of Heliosperma
(Sileneae, Caryophyllaceae)
inferred from chloroplast and nuclear DNA sequences. – Mol. Phylogen. Evol.
43: 140-155.
Frajman B, Eggfens F, Oxelman B. 2009. Hybrid
origins and homoploid reticulate evolution within Heliosperma
(Sileneae, Caryophyllaceae): a multigene
phylogenetic approach with relative dating. – Syst. Biol. 58: 328-345.
Frajman B, Heidari N, Oxelman B. 2009.
Phylogenetic relationships of Atocion and Viscaria (Sileneae,
Caryophyllaceae) inferred
from chloroplast, nuclear ribosomal, and low-copy gene DNA sequences. – Taxon
58: 811-824.
Frajman B, Thollesson M, Oxelman B. 2013.
Taxonomic revision of Atocion and Viscaria (Sileneae, Caryophyllaceae). – Bot. J.
Linn. Soc. 173: 194-210.
Franchet AR. 1895. Sur quelques
Rheum nouveaux du Thibet oriental et du Yunnan. – Bull Mus. Natl.
Hist. Nat. Paris 1: 211-213.
Franck AR. 2012. Synopsis of
Harrisia including a newly described species, several typifications,
new synonyms, and a key to species. – Haseltonia 18: 95-104.
Franck AR, Cochrane BJ, Garey JR. 2013a.
Relationships and dispersal of the Caribbean species of Harrisia
(sect. Harrisia; Cactaceae) using AFLPs and seven DNA
regions. – Taxon 62: 486-497.
Franck AR, Cochrane BJ, Garey JR. 2013b.
Phylogeny, biogeography, and infrageneric classification of Harrisia
(Cactaceae). – Syst. Bot. 38:
210-223.
Franco FF, Rodrigues Silva GA, Moraes EM,
Taylor N, Zappi DC, Jojima CL, Machado MC. 2017. Plio-Pleistocene
diversification of Cereus (Cactaceae, Cereeae) and closely
allied genera. – Bot. J. Linn. Soc. 183: 199-210.
François G, Bringmann G, Phillipson JD, Aké
Assi L, Dochez C, Rübenacker M, Schneider C, Warhurst DC, Kirby GC. 1994.
Activity of extracts and naphthylisoquinoline alkaloids from Triphyophyllum
peltatum, Ancistrocladus abbreviatus and A. barteri
against Plasmodium falciparum in vitro. – Phytochemistry 35:
1461-1464.
Franz E. 1908. Beiträge zur Kenntnis der
Portulacaceen und Basellaceen. – Engl. Bot. Jahrb. Syst. (Beibl. 97) 42:
1-46.
Freitag H, Kadereit G. 2014. C3 and C4 leaf
anatomy types in Camphorosmeae (Camphorosmoideae, Chenopodiaceae). – Plant
Syst. Evol. 300: 665-687.
Freitag H, Stichler W. 2002. Bienertia
cycloptera Bunge ex Boiss., Chenopodiaceae, another C4 plant without Kranz
tissues. – Plant Biol. 4: 121-132.
Freitag H, Vural M, Adigüzel N. 1999. A
remarkable new Salsola and some new records of Chenopodiaceae from
Central Anatolia, Turkey. – Willdenowia 29: 123-139.
Freson R. 1967. Note sur la distribution
africaine du genre Ancistrocladus Wall. (Ancistrocladaceae). –
Bull. Jard. Bot. Nat. Belg. 37: 73-76.
Friedrich H-C. 1955. Beiträge zur Kenntnis
einiger Familien der Centrospermae. – Mitt. Bot. Staatssamml. München 2:
56-66.
Friedrich H-C. 1956a. Studien über die
natürliche Verwandtschaft der Plumbaginales und Centrospermae. – Phyton 6:
220-263.
Friedrich H-C. 1956b. Beiträge zur Kenntnis
der Molluginaceen. Revision der Gattung Limeum L. – Mitt. Bot.
Staatssamml. München 2: 133-258.
Friedrich H-C. 1957. Aizoanthemum
Dinter ex Friedr., eine wenig beachtete Gattung der Ficoideae aus
Südwestafrika. – Mitt. Bot. Staatssamml. München 2: 339-347.
Friedrich H-C. 1959. Beiträge zur Kenntnis
einiger Familien der Centrospermae. – Mitt. Bot. Staatssamm. München 12:
56-66.
Friedrich H-C. 1970. Aizoaceae. – In: Merxmüller H
(ed), Prodromus einer Flora von Südwestafrika 27, J. Cramer, München, pp.
58-74.
Frye ASL, Kron KA. 2003. rbcL
phylogeny and character evolution in Polygonaceae. – Syst. Bot. 28:
326-332.
Fuente V de la, Oggerin M, Rufo L, Rodrígues
N, Ortuñez E, Sánchez-Mata D, Amils R. 2013. A micromorphological and
phylogenetic study of Sarcocornia A. J. Scott (Chenopodiaceae) on the
Iberian Peninsula. – Plant Biosyst. 147: 158-173.
Fuente V de la, Rufo L, Rodrígues N,
Sánchez-Mata D, Franco A, Amils R. 2016. A study of Sarcocornia A. J.
Scott (Chenopodiaceae) from Western Mediterranean Europe. – Plant Biosyst.
150: 343-356.
Fuentes-Bazán S, Mansion G, Borsch T. 2012.
Towards a species level tree of the globally diverse genus Chenopodium
(Chenopodiaceae). – Mol. Phylogen. Evol. 62: 359-374.
Fuentes-Bazán S, Uotila P, Borsch T. 2012. A
novel phylogeny-based generic classification for Chenopodium sensu
lato, and a tribal rearrangement of Chenopodioideae (Chenopodiaceae). –
Willdenowia 42: 5-24.
Fuertes-Aguilar J, Nieto Feliner G. 2003.
Additive polymorphisms and reticulation in an ITS phylogeny of thrifts
(Armeria, Plumbaginaceae). – Mol.
Phylogen. Evol. 28: 430-447.
Fuertes-Aguilar J, Rossello JA, Nieto Feliner
G. 1999. Nuclear ribosomal DNA (nrDNA) concerted evolution in natural and
artificial hybrids of Armeria (Plumbaginaceae). – Mol. Ecol.
8: 1341-1346.
Gadella TWJ, Kliphuis E, Naber J. 1979.
Chromosome numbers in the tribe Rhipsalinae (Cactaceae). – Bot. Not. 132:
294.
Gage AT. 1903. A census of the Indian
Polygonums. – Rec. Bot. Surv. India 2: 371-452.
Gagnepain F. 1918. Place de quelques genre
soi-disant de la famille des Ficoîdes. – Bull. Soc. Bot. France 65: 7-10.
Gail PA. 1964. Simmondsia chinensis
(Link) Schneider: anatomy and morphology of flowers. – M.A. thesis,
Claremont.
Galasso G, Banfi E, Mattia F di, Grassi F,
Sgorbati S, Labra M. 2009. Molecular phylogeny of Polygonum L. s.l.
(Polygonoideae, Polygonaceae),
focusing on European taxa: preliminary results and systematic considerations
based on rbcL plastidial sequence data. – Atti Soc. Ital. Sci. Nat.
Mus. Civ. Storia Nat. Milano 150: 113-148.
Galle P. 1977. Untersuchungen zur
Blütenentwicklung der Polygonaceen. – Bot. Jahrb. Syst. 98: 449-489.
Galloway LA. 1975. Systematics of the North
American desert species of Abronia and Tripterocalyx (Nyctaginaceae). – Brittonia
27: 328-347.
Gama-López S, Arlas S. 1998 Una nueva
especie de Pachycereus (Cactaceae) del occidente de México.
– Novon 8: 359-363.
Ganong WF. 1898. Contributions to a knowledge
of the morphology and ecology of the Cactaceae II. The comparative
morphology of the embryos and seedlings. – Ann. bot. 12: 423-474.
Gao Y, Su Y, Yan S, Wu Z, Zhang X, Wang T,
Gao X. 2010. Hexaoxygenated flavonoids from Pteroxygonum giraldii. –
Nat. Prod. Commun. 5: 223-226.
García C, Pastor J, Luque T. 1989.
Contribucion al estudio cariologico del género Rumex (Polygonaceae). – Acta Bot.
Malacit. 14: 129-140.
Garg SP, Bhushan R, Kapoor RC. 1979. Chrysin
7-galactoside: a new flavonoid from Aerva persica Burm. f. – Indian
J. Chem. 17B: 416-417.
Garralla S, Cuadrado GA. 2007. Pollen
morphology of Austrocylindropuntia Backeb., Maihuenopsis
Speg., Opuntia Mill. and Tephrocactus Lem. (Cactaceae, Opuntioideae) of
Argentina. – Rev. Palaeobot. Palynol. 146: 1-17.
Gaskin JF. 2002. Tamaricaceae. – In: Kubitzki K,
Bayer C (eds), The families and genera of vascular plants V. Flowering plants.
Dicotyledons. Malvales,
Capparales and
non-betalain Caryophyllales,
Springer, Berlin, Heidelberg, New York, pp. 363-368.
Gaskin JF, Ghahremaninejad F, Zhang D-Y,
Londo JP. 2004. A systematic overview of Frankeniaceae and Tamaricaceae from nuclear rDNA
and plastid sequence data. – Ann. Missouri Bot. Gard. 91: 401-409.
Geesink R. 1969. An account of the genus
Portulaca in Indo-Australia and the Pacific (Portulacaceae). – Blumea 17:
275-307.
Geitler L. 1932. Zur Morphologie der Blüten
von Polygonum. – Österr. Bot. Zeitschr. 78: 229-241.
Genc GE, Kandemir A, Genc I. 2007. A new
species of Silene (Caryophyllaceae) from east
Anatolia, Turkey. – Nord. J. Bot. 25: 58-63.
Gentry HS. 1958. The natural history of
jojoba (Simmondsia chinensis) and its cultural aspects. – Econ. Bot.
12: 261-295.
Gerbaulet M. 1992a. Die Gattung
Anacampseros L. (Portulacaceae) I. Untersuchungen
zur Systematik. – Bot. Jahrb. Syst. 113: 477-564.
Gerbaulet M. 1992b. Die Gattung
Anacampseros L. (Portulacaceae) II.
Untersuchungen zur Biogeographie. – Bot. Jahrb. Syst. 113: 565-576.
Gerbaulet M. 1993. Die Gattung
Anacampseros L. (Portulacaceae) III.
Untersuchungen zur Standort-Ökologie der afrikanischen Arten. – Bot. Jahrb.
Syst. 114: 1-28.
Gerbaulet M. 1995. Phyllobolus N. E.
Br. emend. Bittrich (Aizoaceae):
a reassessment of generic boundaries. – Bot. Jahrb. Syst. 117: 385-399.
Gerbaulet M. 1996a. Revision of the genus
Sceletium N. E. Br. (Aizoaceae). – Bot. Jahrb. Syst.
118: 9-24.
Gerbaulet M. 1996b. Revision of the genus
Prenia N. E. Br. (Aizoaceae). – Bot. Jahrb. Syst.
118: 25-40.
Gerbaulet M. 1996c. Revision of the genus
Aridaria N. E. Br. (Aizoaceae). – Bot. Jahrb. Syst.
118: 41-58.
Gerbaulet M. 1997. Revision of the genus
Phyllobolus N. E. Br. (Aizoaceae). – Bot. Jahrb. Syst.
119: 145-211.
Gerbaulet M. 2012. One or many genera in
Mesembryanthemoideae (Aizoaceae)?
Discussion of a conflict in genus perception. – Bradleya 30: 187-198.
Gerloff N, Neduchal J. 2004. Taxonomische
Neubearbeitung der Gattung Notocactus Fric. – Internoto 25:
35-128.
Gerloff N, Neduchal J, Stuchlik M. 1995.
Notokakteen. Gesamtdarstellung aller Notokakteen. – Kveten, Brno.
Germishuizen G. 1987. Notes on African
plants: Polygonaceae: a new
variety of Oxygonum alatum. – Bothalia 17: 185-187.
Germishuizen G. 1989. Polygonaceae: Oxygonum
altissimum, a new species from central Somalia. – Bothalia 19:
210-211.
Ghaffari S. M. 2004. Cytotaxonomy of some
species of Acanthophyllum (Caryophyllaceae) from Iran.
– Biologia (Bratislava) 59: 53-60.
Giannasi DE, Zurawski G, Learn G, Clegg MT.
1992. Evolutionary relationships of the Caryophyllidae based on comparative
rbcL sequences. – Syst. Bot. 17: 1-15.
Gibson AC. 1973. Comparative anatomy of
secondary xylem in Cactoideae (Cactaceae). – Biotropica 5:
29-65.
Gibson AC. 1977. Vegetative anatomy of
Maihuenia (Cactaceae)
with some theoretical discussions of ontogenetic changes in xylem cell types.
– Bull. Torrey Bot. Club 104: 35-48.
Gibson AC. 1978. Rayless secondary xylem of
Halophytum. – Bull. Torrey Bot. Club 105: 39-44.
Gibson AC. 1982. Phylogenetic relationships
of Pachycereeae. – In: Barker F, Starmer WT (eds), Ecological, genetics and
evolution. The cactus-yeast-Drosophila model system, Academy Press,
Sydney, pp. 3-16.
Gibson AC. 1988a. The systematics and
evolution of subtribe Stenocereinae 2. Polaskia. – Cact. Succ. J.
(US) 60: 55-62.
Gibson AC. 1988b. The systematics and
evolution of subtribe Stenocereinae 3. Myrtillocactus. – Cact. Succ.
J. (US) 60: 109-116.
Gibson AC. 1988c. The systematics and
evolution of subtribe Stenocereinae 4. Escontria. – Cact. Succ. J.
(US) 60: 161-167.
Gibson AC. 1991. The systematics and
evolution of subtribe Stenocereinae 11. Stenocereus dumortieri versus
Isolatocereus dumortieri. – Cact. Succ. J. (US) 63: 184-190.
Gibson AC. 1994. Vascular tissues. – In:
Behnke H-D, Mabry TJ (eds), Caryophyllales: evolution and
systematics, Springer, Berlin, pp. 45-74.
Gibson AC, Horak KE. 1978. Systematic anatomy
and phylogeny of Mexican columnar cacti. – Ann. Missouri Bot. Gard. 65:
999-1057.
Gibson AC, Nobel PS. 1986. The cactus primer.
– Harvard University Press, Cambridge, Massachusetts.
Gibson AC, Spencer KC, Bajaj R, McLaughlin
JL. 1986. The ever-changing landscape of cactus systematics. – Ann. Missouri
Bot. Gard. 73: 532-555.
Gibson R, Conn BJ, Bruhl JJ. 2012.
Morphological evaluation of the Drosera peltata complex (Droseraceae). – Aust. Syst. Bot.
25: 49-80.
Gibson TC, Waller DM. 2009. Evolving
Darwin’s ‘most wonderful’ plant: ecological steps to a snap-trap. – New
Phytol. 183: 575-587.
Gilbert MG. 1987a. The taxonomic position of
the genera Telephium and Corrigiola. – Taxon 36: 47-49.
Gilbert MG. 1987b. Two new species of
Polycarpaea (Caryophyllaceae) from Somalia.
– Kew Bull. 42: 701-704.
Gilbert MG. 1991. Notes on the Caryophyllaceae of Ethiopia
and Somalia. – Nord. J. Bot. 11: 451-457.
Gilbert MG. 1993. A review of
Gisekia (Gisekiaceae).
– Kew Bull. 48: 343-356.
Gilbert MG. 1994. Three new species of
Portulaca sect. Neossia from east and northeast Africa. –
Nord. J. Bot. 14: 307-309.
Gilbert MG. 2000. A review of the
opposite-leaved species of Portulaca in Africa and Arabia. – Kew
Bull. 55: 769-802.
Gilbert MG, Thulin M. 1993. A new species of
Mollugo (Molluginaceae) from Somalia. –
Nord. J. Bot. 13: 169-170.
Gilg E. 1895. Ancistrocladaceae. – In:
Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(6), W.
Engelmann, Leipzig, pp. 274-276.
Gilg E. 1897. Amarantaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien, Nachträge zu III(1a), pp.
151-154.
Gilg E. 1925. Ancistrocladaceae. – In:
Engler A, Gilg E (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 21, W.
Engelmann, Leipzig, pp. 589-592.
Gilmer K, Thomas H-P. 1998. Die Gattung
Tephrocactus Lemaire s. str. – Taxonomie, Ökologie und Kultur. –
Schumannia 2: 85-141.
Glass C (ed). 1998. Guide to the threatened
cacti of Mexico. – Cante AC., San Miguel de Allende, Guanajuato, Mexico.
Glass C, Foster R. 1972. Gymnocactus
aguirreanus. A new species from southern Coahuila, Mexico. – Cactus
Succ. J. (U.S.) 44: 80-81.
Glass C, Foster R. 1975. The genus
Echinomastus in the Chihuahuan Desert. – Cact. Succ. J. (US) 47:
218-223.
Glass C, Foster R. 1977. A revision of the
genus Turbinicarpus. – Cactus Succ. J. (U.S.) 49: 161-176.
Glass C, Foster R. 1978. A revision of the
genus Epithelantha. – Cact. Succ. J. (US) 50: 184-187.
Godofredo VR, Melo-de-Pinna GF. 2008.
Occurrence of wide-band tracheids in Cactaceae: wood variation during
Pilosocereus aurisetus development. – J. Torrey Bot. Soc. 135:
94-102.
Gómez-Hinostrosa C, Hernández HM, Terrazas
T, Correa-Cano ME. 2014. Studies on Mexican Cactaceae V. Taxonomic notes on
Selenicereus tricae. – Brittonia 66: 51-59.
Gonçalves ML. 1978a. 88. Cactaceae. – In: Launert E (ed),
Flora Zambesiaca 4, Flora Zambesiaca Managing Committee, London, pp.
506-508.
Gonçalves ML. 1978b. 89a. Aizoaceae. – In: Launert E (ed),
Flora Zambesiaca 4, Flora Zambesiaca Managing Committee, London, pp.
508-521.
Gonçalves ML. 1978c. 89b. Molluginaceae. – In: Launert E
(ed), Flora Zambesiaca 4, Flora Zambesiaca Managing Committee, London, pp.
522-548.
Gonçalves ML. 1978d. 89c.
Mesembryanthemaceae. – In: Launert E (ed), Flora Zambesiaca 4, Flora
Zambesiaca Managing Committee, London, pp. 548-553.
Gonçalves ML. 1978e. 89d. Tetragoniaceae.
– In: Launert E (ed), Flora Zambesiaca 4, Flora Zambesiaca Managing
Committee, London, pp. 553-555.
Gonella PM, Fleischmann A, Rivadavia F, Neill
DA, Sano PT. 2016. A revision of Drosera (Droseraceae) from the central and
northern Andes, including a new species from the Cordillera del Cóndor (Peru
and Ecuador). – Plant Syst. Evol. 302: 1419-1432.
Goodman GJ. 1934. A revision of the North
American species of the genus Chorizanthe. – Ann. Missouri Bot.
Gard. 21: 1-102.
Gorelick R. 2002. DNA sequences and cactus
classification: a short review. – Bradleya 20: 1-4.
Gorelick R. 2004. Resolving the phylogenetic
placement of Blossfeldia liliputana (Cactaceae): reticulate evolution,
chloroplast inheritance, and graft-chimeras. – Bradleya 22: 9-14.
Gottwald H, Parameswaran N. 1968. Das
sekundäre Xylem und die systematische Stellung der Ancistrocladaceae und Dioncophyllaceae. – Bot.
Jahrb. Syst. 88: 49-69.
Goyder DJ. 1988. A revision of
Arenaria section Plinthine (Caryophyllaceae). – Bot. J.
Linn. Soc. 97: 9-32.
Grady BR, Reveal JL. 2011. New combinations
and a new species of Eriogonum (Polygonaceae: Eriogonoideae) from
the Great Basin Desert, United States. – Phytotaxa 24: 33-38.
Graham RA. 1957. A revision of
Oxygonum. – Kew Bull. 12: 145-172.
Graham RA. 1958. Polygonaceae. – In: Turrill WB,
Milne-Redhead E (eds), Flora of tropical East Africa, Crown Agents for Oversea
Governments and Administrations, London, pp. 1-40.
Grant V, Grant KA. 1983. Hawkmoth pollination
of Mirabilis longiflora (Nyctaginaceae). – Proc. Natl.
Acad. Sci. U.S.A. 80: 1298-1299.
Grant WF. 1959. Cytogenetic studies in
Amaranthus II. Natural interspecific hybridization between
Amaranthus dubius and A. spinosus. – Can. J. Bot. 37:
1063-1070.
Green S, Green TL, Heslop-Harrison Y. 1979.
Seasonal heterophylly and leaf gland features in Triphyophyllum (Dioncophyllaceae), a new
carnivorous plant genus. – Bot. J. Linn. Soc. 78: 99-116.
Greenberg AK, Donoghue MJ. 2011. Molecular
systematics and character evolution in Caryophyllaceae. – Taxon 60:
1637-1652.
Greene EL. 1904. Certain polygonaceous
genera. – Leafl. Bot. Observ. Crit. 1: 17-50.
Grego-Valencia D, Terrazas T, Lara-Martínez
R, Jiménez-García LF. 2015. La membrane de la punteadura en dos especies de
Cacteae, Cactaceae. – Bot. Sci.
93: 209-219.
Greuter W. 1995a. Silene (Caryophyllaceae) in Greece: a
subgeneric and sectional classification. – Taxon 44: 543-581.
Greuter W. 1995b. Studies in Greek
Caryophylloideae: Agrostemma, Silene and Vaccaria.
– Willdenowia 25: 105-142.
Greuter W, Böhling N, Jahn R. 2002. The
Cerastium scaposum group (Caryophyllaceae): three annual
taxa endemic to Crete (Greece), two of them new. – Willdenowia 32: 45-54.
Grevenstuk T, Gonçalves S, Nogueira JMF,
Romano A. 2008. Plumbagin recovery from field specimens of Drosophyllum
lusitanicum (L.) Link. – Phytochem. Analysis 19: 229-235.
Grey-Wilson C, Wadhwa BM. 1987. Two new
species of Chenopodiaceae from the Western Himalaya. – Kew Bull. 42:
471-475.
Griffith MP. 2002. Grusonia
pulchella reclassification and its impact on the genus Grusonia:
morphological and molecular evidence. – Haseltonia 9: 86-93.
Griffith MP. 2003. Using molecular data to
elucidate reticulate evolution in Opuntia (Cactaceae). – Madroño 50:
162-169.
Griffith MP. 2004a. What did the first cactus
look like? An attempt to reconcile the morphological and molecular evidence.
– Taxon 53: 493-499.
Griffith MP. 2004b. Early cactus evolution: a
postmodern view. – Haseltonia 10: 3-11.
Griffith MP. 2004c. The origins of an
important cactus crop, Opuntia ficus-indica (Cactaceae): new molecular evidence.
– Amer. J. Bot. 91: 1915-1921.
Griffith MP. 2005. Cactus evolution and
systematics: studies in the Opuntioideae. – Ph.D. diss., Claremont Graduate
University, Claremont, California.
Griffith MP. 2008. Pereskia, Portulacaceae, photosynthesis
and phylogenies: implications for early Cactaceae. – Haseltonia 14:
37-45.
Griffith MP. 2009. Evolution of leaf and
habit characters in Opuntioideae (Cactaceae): reconstruction of
ancestral form. – Bradleya 27: 49-58.
Griffith MP, Porter JM. 2009. Phylogeny of
Opuntioideae (Cactaceae). –
Intern. J. Plant Sci. 170: 107-116.
Grigore M-N, Ivanescu L, Toma T. 2014.
Halophytes: an integrative anatomical study. – Springer-Verlag, Cham etc.
Grigorjev GS. 1933. Neue Arten der Gattung
Polygonum aus der Flora Mittel-Asiens. – Acta Inst. Bot. Acad. Sci.
URSS, ser. 1: 101-104.
Griss A. 1959. Neue Primulaceen und
Chenopodiaceen aus Afghanistan. – Feddes Repert. 62: 22-26.
Gross MH. 1913a. Beiträge zur Kenntnis der
Polygonaceen. – Engl. Bot. Jahrb. Syst. 49: 234-239.
Gross MH. 1913b. Remarques sur les
Polygonées de l’Asie Orientale. – Bull. Acad. Intern. Géogr. Bot. 23:
7-32.
Guaralnick LJ, Jackson MD. 2001. The
occurrence and phylogenetics of crassulacean acid metabolism in the Portulacaceae. – Intern. J.
Plant Sci. 162: 257-262.
Gubellini L, Lakušić D, Conti F, Santangelo
A. 2001. Alcune note su Silene sect. Saxifragoides (Caryophyllaceae) nell’Italia
peninsulare. – Inform. Bot. Ital. 33: 520-523.
Güerere I, Tezara W, Herrera C, Fernández
MD, Herrera A. 1996. Recycling of CO2 during induction of CAM by
drought in Talinum paniculatum (Portulacaceae). – Physiol.
Plant. 98: 471-476.
Guerrero PC, Arroyo MTK, Bustamante RO,
Duarte M, Hagemann TK, Walter HE. 2011. Phylogenetics and predictive
distribution modelling provide insights in the geographic divergence of
Eriosyce subgen. Neoporteria (Cactaceae). – Plant Syst. Evol.
297: 113-128.
Guilliams CM. 2009. Phylogenetic
reconstruction, character evolution, and conservation in the genus
Calyptridium (Montiaceae). – M.Sc. thesis, San
Diego State University, California.
Guilló A, Alonso M, Juan A. 2013. New
insights into seminal and stomatal morphology and their contribution to the
taxonomy of the Old World succulent perennial Salicornioideae. – Plant Syst.
Evol. 299: 1185-1203.
Guilló A, Alonso MA, Lendínez ML, Salazar
C, Juan A. 2014. Taxonomical identity of Sarcocornia fruticosa and
S. hispanica in the Iberian Peninsula. – An. Jard. Bot. Madrid
71(2): e011.
Gundersen A. 1927. The Frankeniaceae as a link in the
classification of the dicotyledons. – Torreya 27: 65-71.
Gupta AK, Murty YS. 1987. Floral anatomy in
Tamaricaceae. – J. Indian
Bot. Soc. 66: 275-282.
Guralnick LJ, Jackson MD. 2001. The
occurrence and phylogenetics of Crassulacean acid metabolism in the Portulacaceae. – Intern. J.
Plant Sci. 162: 257-262.
Guralnick LJ, Marsh C, Asp R, Karjala A.
2001. Physiological and anatomical aspects of CAM-cycling in Lewisia
cotyledon var. cotyledon (Portulacaceae). – Madroño 48:
131-137.
Guralnick LJ, Edwards GE, Ku MSB, Hockema B,
Franceschi V. 2002. Photosynthetic and anatomical characteristics in the
C4-crassulacean acid metabolism-cycling plant Portulaca
grandiflora. – Funct. Plant Biol. 29: 763-773.
Guralnick LJ, Cline A, Smith M, Sage RF.
2008. Evolutionary physiology: the extent of C4 and CAM
photosynthesis in the genera Anacampseros and Grahamia of the
Portulacaceae. – J.
Experim. Bot. 59: 1735-1742.
Gustafsson M. 1976. Evolutionary trends in
the Atriplex prostrata group of Scandinavia. Taxonomy and
morphological variation. – Opera Bot. 39: 1-64.
Gustafsson M. 1986. Taxonomic position and
distribution of Atriplex lapponica (Chenopodiaceae). – Nord. J. Bot.
6: 11-13.
Gustavsson LÅ. 1976. New species of
Anchusa and Arenaria from Sterea Ellas, Greece. – Bot. Not.
129: 273-278.
Gutermann W. 2011. Notulae nomenclaturales
41-45. Neue Namen bei Cruciata und Kali sowie einige kleinere
Korrekturen. – Phyton 51: 95-102.
Guzmán U, Arias S, Dávila P. 2003.
Catálogo de Cactáceas Mexicanas. – Universidad Nacional Autónoma de
México y Comisión Nactional para el Conocimiento y Uso de la Biodiversidad,
México D.F.
Haas R. 1976. Morphologische, anatomische und
entwicklungsgeschichtliche Untersuchungen an Blüten und Früchten
hochsukkulenter Mesembryanthemaceen-Gattungen. – Diss. Bot. 33: 1-256.
Haccius B. 1954. Embryologische und
histogenetische Studien an ”monokotylen Dikotylen” I. Claytonia
virginica L. – Österr. Bot. Zeitschr. 101: 285-303.
Haccius B, Troll W. 1961. Über die
sogenannten Wurzelhaare an den keimpflanzen von Drosera- und
Cuscuta-Arten. – Beitr. Biol. Pflanzen 36: 139-157.
Hakki MI. 2013. On flower anatomy and
embryology of Lophiocarpus polystachyus (Lophiocarpaceae). –
Willdenowia 43: 185-194.
Hakki MJ. 1972. Blütenmorphologische und
embryologische Untersuchungen an Chenopodium capitatum und C.
foliosum sowie weiteren Chenopodiaceae. – Bot. Jahrb. Syst. 92:
178-330.
Halbritter H, Hesse M, Weber M. 2012. The
unique design of pollen tetrads in Dionaea and Drosera. –
Grana 51: 148-157.
Halket AC. 1928. The morphology of
Salicornia – an abnormal plant. – Ann. Bot. (Oxford) 42:
525-530.
Hall BA. 1949. The floral anatomy of
Drosera and Begonia and its bearing on the theory of carpel
polymorphism. – Amer. J. Bot. 36: 416-421.
Hall HM, Clements FC. 1923. The phylogenetic
method in taxonomy. The North American species of Artemisia,
Chrysothamnus, and Atriplex. – Publ. Carnegie Inst.
Washington 326: 1-355.
Hallier H. 1923. Beiträge zur Kenntnis der
Linaceae (DC.
1819) Dumort. – Beih. Bot. Centralbl. 39: 1-178.
Hallock YF, Manfredi KP, Dai J-R, Cardellina
JH II, Gulkowski JR, McMahon JB, Schäffer M, Stahl M, Gulden K-P, Bringmann G,
François G, Boyd MR. 1997. Michellamines D-F, new HIV-ionhibitory dimeric
napththylisoquinoline alkaloids, and korupensamine E, a new antimalarial
monomer, from Ancistrocladus korupensis. – J. Nat. Prod. 60:
677-683.
Hammer SA. 1991. The genus
Conophytum: a monograph. – Succulent Plant Publ., Pretoria.
Hammer T, Davis R, Thiele K. 2015. A
molecular framework phylogeny for Ptilotus (Amaranthaceae): evidence for
the rapid diversification of an arid Australian genus. – Taxon 64:
272-285.
Hanelt P. 1970. Vorkommen und
Vergesellschaftung von Nanophyton erinaceum (Pall.) Bunge in der
Mongolischen Volksrepublik. – Arch. Naturschutz Landschaftsforsch. 10:
19-40.
Hannon DP. 1993. When to split and when to
lump. – Cactus Succ. J. 65: 205.
Hänsel R, Hörhammer L. 1954.
Phytochemisch-systematische Untersuchung über die Flavonglykoside einiger
Polygonaceen. – Arch. Pharm. 287: 189-198.
Hanson CA. 1962. Perennial Atriplex
of Utah and the northern desert. – Thesis, Brigham Young University, Provo,
Utah.
Hanson AD, Rathinassabapathi B, Rivoal J,
Burnet M, Dillon MO, Gage DA. 1994. Osmoprotective compounds in the Plumbaginaceae: a natural
experiment in metabolic engineering of stree tolerance. – Proc. Natl. Acad.
Sci. U.S.A. 91: 306-310.
Hara H. 1962. Sunania Rafin. versus
Tovara Adanson. – J. Jap. Bot. 37: 326-332.
Haraldson K. 1978. Anatomy and taxonomy in Polygonaceae subfam.
Polygonoideae Meissn. emend. Jaretzky. – Symb. Bot. Upsal. 22(2): 1-95.
Harbaugh DT, Nepokroeff M, Rabeler RK,
McNeill J, Zimmer EA, Wagner WL. 2010. A new lineage-based tribal
classification of the family Caryophyllaceae. – Intern.
J. Plant Sci. 171: 185-198.
Harborne JB. 1967. Comparative biochemistry
of the flavonoids IV. Correlations between chemistry, pollen morphology, and
systematics in the family Plumbaginaceae. –
Phytochemistry 6: 1415-1428.
Harborne JB. 1975. Flavonoid bisulphates and
their co-occurrences with ellagic acid in the Bixaceae, Frankeniaceae, and related
families. – Phytochemistry 14: 1331-1337.
Hardham CB. 1989. Chromosome numbers of some
annual species of Chorizanthe and related genera (Polygonaceae: Eriogonoideae). –
Phytologia 66: 89-94.
Harms H. 1936. Nepenthaceae. – In: Engler A
(†), Harms H (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 17b, W.
Engelmann, Leipzig, pp. 728-765.
Harpke D, Peterson A. 2006. Non-concerted ITS
evolution in Mammillaria (Cactaceae). – Mol. Phylogen. Evol.
41: 579-593.
Harpke D, Peterson A, Hoffmann MH, Röser M.
2006. Phylogenetic evaluation of chloroplast trnL-trnF DNA
sequence variation in the genus Mammillaria. – Schlechtendalia 14:
7-16.
Harriman NA. 1999. Synopsis of New World
Commicarpus (Nyctaginaceae). – Sida 18:
679-684.
Harris EM, Horn JW, Wagner WL. 2012. Floral
development of the divergent endemic Hawaiian genus Schiedea (Caryophyllaceae), with special
emphasis on the floral nectaries. – Taxon 61: 576-591.
Harris FS, Martin CE. 1991. Correlation
between CAM-cycling and photosynthetic gas exchange in five species of
Talinum (Portulacaceae). – Plant
Physiol. 96: 1118-1124.
Hartl D, Klapper H, Barbier B, Ensikat HJ,
Dronskowski R, Müller P, Ostendorp G, Tye A, Bauer R, Barthlott W. 2007.
Diversity of calcium oxalate crystals in Cactaceae. – Can. J. Bot. 85:
501-517.
Hartl WP, Barbier B, Klapper H, Müller P,
Barthlott W. 2003. Dimorphism of calcium oxalate crystals in stem tissues of
Rhipsalideae (Cactaceae) – a
contribution to the systematics and taxonomy of the tribe. – Bot. Jahrb.
Syst. 124: 287-302.
Hartman RL, Rabeler RK. 2004. New
combinations in North American Eremogone (Caryophyllaceae). – Sida 21:
237-247.
Hartmann HEK. 1976. Monographie der Gattung
Odontophorus N. E. Br. (Mesembryanthemaceae Fenzl). – Bot. Jahrb.
Syst. 97: 161-225.
Hartmann HEK. 1978. Monographie der Gattung
Argyroderma N. E. Br. (Mesembryanthemaceae Fenzl). – Mitt. Inst.
Allg. Bot. Hamburg 15: 121-235.
Hartmann HEK. 1979. Surface structure of
leaves: their ecological and taxonomical significance in members of the
subfamily Ruschioideae Schw. (Mesembryanthemaceae Fenzl). – In: Cutler DF,
Hartmann HEK (eds), Scanning electron microscope studies of the leaf epidermis
in some succulents, Trop. Subtrop. Pflanzenwelt 28: 31-55.
Hartmann HEK. 1983. Untersuchungen zum
Merkmalsbestand und zur Taxonomie der Subtribus Leipoldtiinae
(Mesembryanthemaceae). – Bibl. Bot. 136: 1-67.
Hartmann HEK. 1986. Chromosome numbers in the
genus Cephalophyllum N. E. Br. (Mesembryanthemaceae). – Cact. Succ.
J. (U.S.) 58: 263-266.
Hartmann HEK. 1987. Phytogeography of the
subtribe Leipoldtiinae (Mesembryanthemaceae). – Bothalia 17: 205-212.
Hartmann HEK. 1988. Fruit types in
Mesembryanthema. – Beitr. Biol. Pflanzen 63: 313-349.
Hartmann HEK. 1989. Monographien der
Subtribus Leipoldtiinae VIII. Monographie der Gattung Cephalophyllum
(Mesembryanthemaceae). – Mitt. Inst. Allg. Bot. Hamburg 22: 93-187.
Hartmann HEK. 1991. Mesembryanthema.
– Contr. Bolus Herb. 13: 75-157.
Hartmann HEK. 1992. Ihlenfeldtia, a
new genus in Mesembryanthema (Aizoaceae). – Bot. Jahrb. Syst.
114: 29-50.
Hartmann HEK. 1993. Aizoaceae. – In: Kubitzki K,
Rohwer JG, Bittrich V (eds), The families and genera of vascular plants II.
Flowering plants. Dicotyledons. Magnoliid, hamamelid and caryophyllid families,
Springer, Berlin, Heidelberg, New York, pp. 37-69.
Hartmann HEK. 1994. On the phytogeography and
evolution of Mesembryanthema (Aizoaceae). – In: Seyani JH,
Chikuni AC (eds), Proceedings of the 13th Plenary Meeting AETFAT,
Malawi, vol. 2, pp. 1165-1180.
Hartmann HEK. 1998. New combinations in
Ruschioideae, based on studies in Ruschia (Aizoaceae). – Bradleya 16:
44-91.
Hartmann HEK (ed). 2001a. Illustrated
handbook of succulent plants. Aizoaceae A-E. – Springer,
Berlin.
Hartmann HEK (ed). 2001b. Illustrated
handbook of succulent plants. Aizoaceae F-Z. – Springer,
Berlin.
Hartmann HEK. 2004. Adaptations and
phytogeography in the ice-plant family Aizoaceae – the interaction of the
genetic equipment and ecological parameters I. One leaf-pair is the plant. –
Bradleya 22: 21-36.
Hartmann HEK. 2006. Adaptations and
phytogeography in the ice-plant family Aizoaceae – the interaction of the
genetic equipment and ecological parameters II. Hide-and-seek: plants sunk in
the ground. – Bradleya 24: 1-38.
Hartmann HEK. 2007. Studies in Aizoaceae: eight new subgenera in
Drosanthemum Schwantes. – Bradleya 25: 145-176.
Hartmann HEK. 2008. A carnival of flowers in
Drosanthemum subgenus Speciosa (Aizoaceae). – Bradleya 26:
99-120.
Hartmann HEK, Bittrich V. 1990. Nomenclature
in Mesembryanthema (Aizoaceae): the generic names by
Rappa & Camarrone. – Bothalia 20: 153-157.
Hartmann HEK, Bittrich V. 1991. Typification
of suprageneric names – some nomenclatural changes in Aizoaceae. – South Afr. J. Bot.
57: 73-75.
Hartmann HEK, Bruckmann C. 2000. The capsules
of Drosanthemum Schwantes (Ruschioideae, Aizoaceae). – Bradleya 18:
75-112.
Hartmann HEK, Dehn M. 1987. Monographien der
Leipoldtiinae VII. Monographie der Gattung Cheiridopsis
(Mesembryanthemaceae). – Bot. Jahrb. Syst. 108: 567-663.
Hartmann HEK, Liede S. 1986. Die Gattung
Pleiospilos s. lat. (Mesembryanthemaceae). – Bot. Jahrb. Syst. 106:
433-485.
Hartmann HEK, Liede-Schumann S. 2013.
Knersia gen. nov., an emigrant from Drosanthemum (Ruschieae,
Ruschioideae, Aizoaceae). –
Bradleya 31: 116-127.
Hartmann HEK, Niesler IM. 1991. On the
identity of Trichodiadema schimperi (Engl.) Herre (Mesembryanthema).
– Cactus Succ. J. (U.S.) 63: 143-149.
Hartmann HEK, Niesler IM. 2009. On the
evolution of nectaries in Aizoaceae. – Bradleya 27:
69-120.
Hartmann HEK, Rust S. 1994. Monographs of
Leipoldtiinae IX. Monograph of the genus Leipoldtia L. Bolus s. lat.
– Verh. Naturwiss. Vereins Hamburg 34: 275-351.
Hartmann HEK, Stüber D. 1993. On spiny
Mesembryanthema and the genus Eberlanzia (Aizoaceae). – Contr. Bol. Herb.
15: 1-75.
Hartmann HEK, Meve U, Liede-Schumann S. 2011.
Towards a revision of Trianthema, the Cinderella of Aizoaceae. – Plant Ecol. Evol.
144: 177-213.
Hartmann S, Nason JD, Bhattacharya D. 2001.
Extensive ribosomal DNA genic variation in the columnar cactus
Lophocereus. – J. Mol. Evol. 53: 124-134.
Hartmann S, Nason JD, Bhattacharya D. 2002.
Phylogenetic origins of Lophocereus (Cactaceae) and the senita
cactus-senita moth pollination mutualism. – Amer. J. Bot. 89: 1085-1092.
Hartmeyer I, Hartmeyer SRH. 2010.
Snap-tentacles and runway lights: summary of comparative examination of
Drosera tentacles. – Carniv. Plants Newsl. 39: 101-113.
Hartvig P. 1979. Cerastium
smolikanum Hartvig, sp. nov. and C. vourinense from N Greece. –
Bot. Not. 132: 359-361.
Hassan NS, Hartmann HEK, Liede-Schumann S.
2005. Conspectus of Aizoaceae, Gisekiaceae and Molluginaceae of Egypt and
Sudan. – Feddes Repert. 116: 1-42. – Nachtrag: Feddes Repert. 116 (2005):
406.
Hassan NMS, Meve U, Liede-Schumann S. 2005.
Seed coat morphology of Aizoaceae-Sesuvioideae, Gisekiaceae and Molluginaceae and its systematic
significance. – Bot. J. Linn. Soc. 148: 189-206.
Hassan NMS, Thiede J, Liede-Schumann S. 2005.
Phylogenetic analysis of Sesuvioideae (Aizoaceae) inferred from nrDNA
internal transcribed spacer (ITS) sequences and morphological data. – Plant
Syst. Evol. 255: 121-143.
Hatschbach G, Guimarães O. 1973.
Fitolacáceas do estado do Paraná. – Bol. Mus. Bot. Munic. Curitiba 8:
1-24.
Haug I, Weiß M, Homeier J, Oberwinkler F,
Kottke I. 2005. Russulaceae and Thelephoraceae form ectomycorrhizas with
members of Nyctaginaceae (Caryophyllales) in the tropical
mountain rain forest of southern Ecuador. – New Phytol. 165: 923-936.
Heath PV. 1992. The restoration of
Rathbunia Britton & Rose. – Calix (Brighton, Enland) 2:
102-115.
Hedberg O. 1946. Pollen morphology in the
genus Polygonum L. s. lat. and its taxonomical significance. –
Svensk Bot. Tidskr. 40: 371-404.
Hedberg O. 1954. Taxonomic studies in
afroalpine Caryophyllaceae.
– Svensk Bot. Tidskr. 48: 199-210.
Hedberg O. 1988. Koenigia delicatula
(Meisn.) Hara subsp. relicta O. Hedb. n. subsp. (Polygonaceae) – a new taxon
linking the genera Koenigia L. and Persicaria Mill. – J.
Jap. Bot. 63: 71-77.
Hedberg O. 1995. Studies in the genus
Polycarpaea (Caryophyllaceae) in Ethiopia.
– Nord. J. Bot. 15: 513-517.
Hedberg O. 1997. The genus Koenigia
L. emend. Hedberg (Polygonaceae). – Bot. J. Linn.
Soc. 124: 295-330.
Hedlund RW, Engelhardt DW. 1970.
Rugaepollis fragilis sp. nov. from the Tertiary of Kachemak Bay,
Alaska. – Pollen Spores 12: 173-176.
Heek W van, Strecker W. 2007. Die Gattung
Arrojadoa. – Kakt. Sukk. 58: 85-92.
Heenan PB. 1999. A taxonomic revision of
Neopaxia Ö. Nilss. (Portulacaceae). – New Zealand
J. Bot. 37: 213-234.
Heil KD, Porter JM. 1994.
Sclerocactus (Cactaceae): a revision. –
Haseltonia 2: 20-46.
Heil KD, Armstrong B, Schleser D. 1981. A
review of the genus Pediocactus. – Cact. Succ. J. (US) 53: 17-39.
Heimerl A. 1886. Über die Einlagerung von
Calciumoxalat in die Zellwand bei Nyctagineen. – Sitzungsber. Kais. Akad.
Wiss. Wien, Math.-Nat. Kl. 93(I): 231-246.
Heimerl A. 1889a. Beiträge zur Anatomie der
Nyctaginaceen-Früchte. – Sitzungsber. Kais. Akad. Wiss. Wien, Math.-Nat. Cl.
97(I): 692-703.
Heimerl A. 1889b. Phytolaccaceae. – In: Engler
A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(1b), W. Engelmann,
Leipzig, pp. 1-14.
Heimerl A. 1889c. Nyctaginaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien III(1), W. Engelmann,
Leipzig, pp. 14-32; Heimerl A. 1897. Nachträge zu III(1b), pp. 154-156.
Heimerl A. 1901. Monographie der
Nyctaginaceen. – Denkschr. Akad. Wissensch. 70: 132-137.
Heimerl A. 1908. Nyctaginaceae andinae. – In:
Urban I (ed), Plantae novae andinae imprimis weberbauerianae IV, Engl. Bot.
Jahrb. Syst. 42: 78-79.
Heimerl A. 1916. Nyctaginaceae andinae. – Bot.
Jahrb. Syst. LIV. Beibl. 117: 2-4.
Heimerl A. 1934a. Nyctaginaceae. – In: Engler A
(†), Pax F, Harms H (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd.
16c, W. Engelmann, Leipzig, pp. 86-134.
Heimerl A. 1934b. Phytolaccaceae. – In: Engler
A (†), Pax F, Harms H (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd.
16c, W. Engelmann, Leipzig, pp. 135-164.
Heimerl A. 1934c. Achatocarpaceae. – In:
Engler A (†), Pax F, Harms H (eds), Die natürlichen Pflanzenfamilien, 2.
Aufl., Bd. 16c, W. Engelmann, Leipzig, pp. 174-178.
Heklau H. 2006. Proposal to conserve the name
Krascheninnikovia against Ceratoides (Chenopodiaceae). –
Taxon 55: 1044-1045.
Heklau H, Röser M. 2008. Delineation,
taxonomy and phylogenetic relationships of the genus Krascheninnikovia
(Amaranthaceae subtribe
Axyridinae). – Taxon 57: 563-576.
Helsen P, Browne RA, Anderson DJ, Verdyck P,
Dongen S. 2009. Galapagos’ Opuntia (prickly pear) cacti: extensive
morphological diversity, low genetic variability. – Biol. J. Linn. Soc. 96:
451-461.
Henrickson J. 1987. A taxonomic reevaluation
of Gossypianthus and Guilleminea (Amaranthaceae). – Sida 12:
307-337.
Henrickson J, Sundberg S. 1986. On the
submersion of Dicraurus into Iresine (Amaranthaceae). – Aliso 11:
355-364.
Hentzschel G, Augustin K. 2008. Die Gattung
Weingartia Werdermann. Teil 2. Weingartia, Sulcorebutia und
Cintria – eine untrennbare Einheit – Merkmalsvergleiche und
Neukombinationen. – Gymnocalycium 21: 767-782.
Hernándes-Lopes J, Oliveira-Neto MA,
Melo-de-Pinna GFA. 2016. Different ways to build succulent leaves in
Portulacineae (Caryophyllales). – Intern. J.
Plant Sci. 177: 198-208.
Hernández HM. 1990. Autopolinización en
Mirabilis longiflora L. (Nyctaginaceae). – Acta Bot.
Mex. 12: 25-30.
Hernández HM, Bárcenas RT. 1995. Endangered
cacti in the Chihuahuan desert I. Distribution patterns. – Cons. Biol. 9:
1176-1188.
Hernández-Hernández T, Hernández HM,
De-Nova JA, Puente R, Eguiarte LE, Magallón S. 2011. Phylogenetic
relationships and evolution of growth form in Cactaceae (Caryophyllales,
Eudicotyledoneae). – Amer. J. Bot. 98: 44-61.
Hernández-Ledesma, P, Olvera HF, Ochoterena
H. 2010. Cladistic analysis and taxonomic synopsis of Anulocaulis (Nyctaginaceae) based on
morphological data. – Syst. Bot. 35: 858-876.
Hernández-Ledesma P, Bárcenas RT. 2017.
Phylogenetic utility of the trnH-psbA IGR and stem-loop diversity of the 3’
UTR in Cactaceae (Caryophyllales). – Plant
Syst. Evol. 303: 299-315.
Hernández-Ledesma P, Berendsohn WG, Borsch
T, Von Mering S, Akhani H, Arias S, Castañeda-Noa I, Eggli U, Eriksson R,
Flores-Olvera H, Fuentes-Bazán S, Kadereit G, Klak C, Korotkova N, Nyffeler R,
Ocampo G, Ochoterena H, Oxelman B, Rabeler RK, Sanchez A, Schlumpberger BO,
Uotila P. 2015. A taxonomic backbone for the global synthesis of species
diversity in the angiosperm order Caryophyllales. – Willdenowia
45: 281-383.
Herre H. 1971. The genera of the
Mesembryanthemaceae. – Tafelberg, Cape Town.
Herre H, Volk OH. 1948. Mesembryanthemaceae
Herre et Volk, familia nova. – Sukkulentenkunde 2: 38.
Herrera A, Delgado J, Paraguatey J. 1991.
Occurrence of inducible crassulacean acid metabolism in leaves of Talinum
triangulare (Portulacaceae). – J. Experim.
Bot. 42: 493-499.
Hershkovitz MA. 1989. Phylogenetic studies in
Centrospermae: a brief appraisal. – Taxon 38: 602-608.
Hershkovitz MA. 1990a. Phylogenetic and
morphological studies in Portulacaceae. – Ph.D. diss.,
University of California, Davis, California.
Hershkovitz MA. 1990b. Nomenclatural changes
in Portulacaceae. –
Phytologia 68: 267-270.
Hershkovitz MA. 1991a. Phylogenetic
assessment and revised circumscription of Cistanthe Spach (Portulacaceae). – Ann.
Missouri Bot. Gard. 78: 1009-1021.
Hershkovitz MA. 1991b. Taxonomic notes on
Cistanthe, Calandrinia, and Talinum (Portulacaceae). – Phytologia
70: 209-225.
Hershkovitz MA. 1992. Leaf morphology and
taxonomic analysis of Cistanthe tweedyi (nee Lewisia tweedyi;
Portulacaceae). – Syst.
Bot. 17: 220-238.
Hershkovitz MA. 1993a. Revised
circumscriptions and subgeneric taxonomies of Calandrinia and
Montiopsis (Portulacaceae) with notes on
phylogeny of the portulacaceous alliance. – Ann. Missouri Bot. Gard. 80:
333-365.
Hershkovitz MA. 1993b. Leaf morphology of
Calandrinia and Montiopsis (Portulacaceae). – Ann.
Missouri Bot. Gard. 80: 366-396.
Hershkovitz MA. 1998. Parakeelya: a
new genus segregated from Calandrinia (Portulacaceae). – Phytologia
84: 98-106.
Hershkovitz MA. 2006. Ribosomal and
chloroplast DNA evidence for diversification of Western American Portulacaceae in the Andean
region. – Gayana Bot. 63: 13-74.
Hershkovitz MA, Zimmer EA. 1997. On the
evolutionary origin of the cacti. – Taxon 46: 217-232.
Hershkovitz MA, Zimmer EA. 2000. Ribosomal
DNA evidence and disjunctions of western American Portulacaceae. – Mol.
Phylogen. Evol. 15: 419-439.
Heslop-Harrison J. 1963. An ultrastructural
study of pollen wall ontogeny in Silene pendula. – Grana Palynol. 4:
7-24.
Hesse M. 1979. Entwicklungsgeschichte und
Ultrastruktur von Pollenkitt und Exine bei nahe verwandten entomo- und
anemophilen Angiospermen: Polygonaceae. – Flora 168:
558-577.
Heubl GR, Wistuba A. 1995. A cytological
study of the genus Nepenthes L. (Nepenthaceae). – Sendtnera 4:
169-174.
Heubl GR, Bringmann G, Meimberg H. 2006.
Molecular phylogeny and character evolution of carnivorous plant families in Caryophyllales – revisited.
– Plant Biol. 8: 821-830.
Heubl GR, Turini F, Mudogo V, Kajahn I,
Bringmann G. 2010. Ancistrocladus iliboensis (D. R. Congo), a new
liana with unique alkaloids. – Plant Ecol. Evol. 143: 63-69.
Hewson HJ. 1984. Phytolaccaceae. – In: George
AS (ed), Flora of Australia 4, Australian Government Publ. Service, Canberra,
pp. 1-5.
Hickman JC. 1971. Arenaria, section
Eremogone (Caryophyllaceae) in the
Pacific Northwest: a key and discussion. – Madroño 21: 201-207.
Higashi S, Nagashima A, Ozaki H, Abe M,
Uchiumi T. 1993. Analysis of feeding mechanisms in a pitcher of Nepenthes
hybrida. – J. Plant Res. 106: 47-54.
Hind DJN. 1988. The biology and systematics
of Moehringia L. (Caryophyllaceae). – Ph.D.
diss., University of Reading, England.
Hindmarsh GJ. 1966. An embryological study of
five species of Bassia. – Proc. Linn. Soc. New South Wales 90:
274-289.
Hinton WF. 1975. Systematics of the
Calyptridium umbellatum complex (Portulacaceae). – Brittonia
27: 197-208.
Hirrel MC, Mehravaran H, Gerdemann JW. 1978.
Vesicular-arbuscular mycorrhizae in Chenopodiaceae and Cruciferae: do they
occur? – Can. J. Bot. 56: 2813-2817.
Hochstätter F. 2005. The genus
Sclerocactus, tribe Cacteae, family Cactaceae. – Mannheim & Hamlyn
Valley (publ. by the author).
Hochstätter F. 2007. The genera
Pediocactus, Navajoa, Toumeya (Cactaceae), family Cactaceae, subfamily Cactoideae,
tribe Cacteae. – Sine loco (publ. by the author).
Hoffmann AE. 1989. Cactáceas en la flora
silvestre de Chile. – Santiago de Chile.
Hoffmann AE, Walter H. 2005. Cactáceas en la
flora Silvestre de Chile. Ed. 2. – Ediciones Fundación Claudio Gay, Santiago
de Chile.
Hofmann U. 1973. Centrospermen-Studien 6.
Morphologische Untersuchungen zur Umgrenzung und Gliederung der Aizoaceen. –
Bot. Jahrb. Syst. 92: 247-324.
Hofmann U. 1977. Centrospermen-Studien 9. Die
Stellung von Stegnosperma innerhalb der Centrospermen. – Ber.
Deutsch. Bot. Ges. 90: 39-52.
Hofmann U. 1994. Flower morphology and
ontogeny. – In: Behnke H-D, Mabry TJ (eds), Caryophyllales: Evolution and
systematics, Springer, Berlin, Heidelberg, New York, pp. 123-166.
Hohmann S, Kadereit JW, Kadereit G. 2006.
Understanding Mediterranean-Californian disjunctions: molecular evidence from
Chenopodiaceae-Betoideae. – Taxon 55: 67-78.
Hollingsworth ML, Bailey JP, Hollingsworth
PM, Ferris C. 1999. Chloroplast DNA variation and hybridization between
invasive populations of Japanese knotweed and giant knotweed
(Fallopia, Polygonaceae). – Bot. J.
Linn.Soc. 129: 139-154.
Holm T. 1905. Claytonia Gronov. A
morphological and anatomical study. – Mem. Natl. Acad. Sci., Washington 10:
27-37.
Holub J. 1970. Fallopia Adans. 1763
instead of Bilderdykia Dum. 1827. – Folia Geobot. Phytotaxon. 6:
171-177.
Holub J. 1998. Trommsdorffia Bernh.
1800 is a validly published generic name. – Preslia 70: 179-182.
Hong S-P. 1988. A pollenmorphological
re-evaluation of Harpagocarpus and Eskemukerjea (Polygonaceae). – Grana 27:
291-295.
Hong S-P. 1989. Knorringia (=
Aconogonon sect. Knorringia), a new genus in the Polygonaceae. – Nord. J. Bot.
9: 343-357.
Hong S-P. 1990. A new species of
Aconogonon from Upper Burma and Yunnan. – Notes Roy. Bot. Gard.
Edinb. 46: 361-363.
Hong S-P. 1991a. A revision of
Aconogonon (= Polygonum sect. Aconogonon, Polygonaceae) in North America.
– Rhodora 93: 322-346.
Hong S-P. 1991b. The dimorphic heterostyly in
Aconogonon campanulatum (Polygonaceae). – Plant Syst.
Evol. 176: 125-131.
Hong S-P. 1992. Taxonomy of the genus
Aconogonon (Polygonaceae) in Himalaya and
adjacent regions. – Symb. Bot. Ups. 30(2): 1-118.
Hong S-P. 1993. Reconsideration of the
generic status of Rubrivena (Polygonaceae, Persicarieae). –
Plant Syst. Evol. 186: 95-122.
Hong S-P. 1995. Pollen morphology of
Parapteropyrum and some putatively related genera (Polygonaceae-Atraphaxideae). –
Grana 134: 153-159.
Hong S-P. 1999. Pollen dimorphism in
heterostylous species of Oxygonum (Polygonaceae, Polygoneae). –
Syst. Geogr. Plants 68: 245-252.
Hong S-P. 2008. Knorringia
(=Aconogonon sect. Knorringia), a new genus in the Polygonaceae. – Nord. J. Bot.
9: 343-357.
Hong S-P, Hedberg O. 1990. Parallel evolution
of aperture numbers and arrangement in the genera Koenigia,
Persicaria and Aconogonon (Polygonaceae). – Grana 29:
177-184.
Hong S-P, Lee S-T. 1983. A palynotaxonomic
study of the Korean Polygonaceae. – Korean J. Plant
Taxon. 13: 63-76.
Hong S-P, Ronse De Craene LP, Smets E. 1998.
Systematic significance of tepal surface morphology in tribes Persicarieae and
Polygoneae (Polygonaceae). –
Bot. J. Linn. Soc. 127: 91-116.
Hong S-P, Oh I-C, Son S-H. 1998. Pollen
morphology of the tribe Pterostegieae (Polygonaceae: Eriogonoideae). –
Grana 37: 15-21.
Hong S-P, Oh I-C, Ronse De Craene LP. 2005.
Pollen morphology of the genera Polygonum s. str. and
Polygonella (Polygoneae: Polygonaceae). – Plant Syst.
Evol. 254: 13-30.
Hood ME, Mena-Ali JI, Gibson AK, Oxelman B,
Giraud T, Yockteng R, Arroyo MTK, Conti F, Petersen AB, Gladieux P, Antonovics
J. 2010. Distribution of the anther-smut pathogen Microbotryum on
species of the Caryophyllaceae. – New
Phytol. 187: 217-229.
Hooker JD. 1859. On the origin and
development of the pitcher of Nepenthes, with an account of some new
Bornean plants of the genus. – Trans. Linn. Soc. London 22: 415-424.
Horak KE. 1981a. The three-dimensional
structure of vascular tissues in Stegnosperma (Phytolaccaceae). – Bot. Gaz.
142: 545-549.
Horak KE. 1981b. Anomalous secondary
thickening in Stegnosperma (Phytolaccaceae). – Bull.
Torrey Bot. Club 108: 189-197.
Hörhammer L, Hänsel R, Kriesmair G, Endes
W. 1955. Zur Kenntnis der Polygonaceenflavone 1. – Arch. Pharm. (Weinheim)
288: 419-425.
Horton JS. 1957. Inflorescence development in
Tamarix pentandra Pallas (Tamaricaceae). – Southw. Natur.
2: 135-139.
Horton J. 1963. A taxonomic revision of
Polygonella (Polygonaceae). – Brittonia 15:
177-203.
Hoshi Y, Hotta K. 1998. A chromosome
phylogeny of the Droseraceae by
using CMA-DAPI fluorescent banding. – Cytologia 63: 329-339.
Hosseus C. 1939. Notas sobre Cactaceas
Argentinas I. – Arch. Esc. Farm. Fac. Ci. Med. Córdoba 9: 106.
Howard RA. 1949. The genus Coccoloba
in Cuba. – J. Arnold Arbor. 30: 388-424.
Howard RA. 1957a. Studies in the genus
Coccoloba III. The Jamaican species. – J. Arnold Arbor. 38:
81-106.
Howard RA. 1957b. Studies in the genus
Coccoloba IV. The species from Puerto Rico and the Virgin Islands and
from the Bahama Islands. – J. Arnold Arbor. 38: 211-242.
Howard RA. 1958. Studies in the genus
Coccoloba V. The genus in Haiti and the Dominican Republic. – J.
Arnold Arbor. 39: 1-48.
Howard RA. 1959a. Studies in the genus
Coccoloba VI. The species from the Lesser Antilles, Trinidad and
Tobago. – J. Arnold Arbor. 40: 68-93.
Howard RA. 1959b. Studies in the genus
Coccoloba VII. A synopsis and key to the species in Mexico and Central
America. – J. Arnold Arbor. 40: 176-220.
Howard RA. 1960. Studies in the genus
Coccoloba IX. A critique of the South American species. – J. Arnold
Arbor. 41: 213-229, 231-258, 357-390.
Howard RA. 1961. Studies in the genus
Coccoloba X. New species and a summary of distribution in South
America. – J. Arnold Arbor. 42: 87-95.
Howell T. 1893. A rearrangement of American
Portulacaceae. – Erythea 1:
29-41.
Huber JA. 1924. Zur Morphologie von
Mesembryanthemum. – Bot. Arch. 5: 7-25.
Hunt DR. 1966. Tamaricaceae. – In:
Milne-Redhead E, Polhill RM (eds), Flora of tropical East Africa, Crown Agents
for Oversea Governments and Administrations, London, pp. 1-4.
Hunt DR. 1968. Cactaceae. – In: Hubbard CE,
Milne-Redhead E (eds), Flora of tropical East Africa, Crown Agents for Oversea
Governments and Administrations, London, pp. 1-7.
Hunt DR. 1971. Schumann and Buxbaum
reconciled. – Cactus Succ. J. (Great Britain) 33: 53-72.
Hunt DR. 1977. Schumann and Buxbaum
recompiled 2-3. – Cactus Succ. J. (Great Britain) 39: 37-40, 71-74.
Hunt DR. 1981. Revised classified list of the
genus Mammillaria. – Cactus Succ. J. (Great Britain) 43: 41-48.
Hunt DR. 1986. The genera of the Cactaceae: towards a new consensus.
– Bradleya 4: 65-78.
Hunt DR. 1987. A new review of
Mammillaria names. – British Cactus & Succulent Society,
Oxford.
Hunt DR. 1992. CITES Cactaceae Checklist. – Royal
Botanic Gardens and International Organization for Succulent Plant Study (IOS),
Kew.
Hunt DR. 1999. CITES Cactaceae Checklist. 2nd
ed. – Royal Botanic Gardens and International Organization for Succulent
Plant Study (IOS), Kew.
Hunt DR. 2000. Notes on miscellaneous genera
of Cactaceae subfam. Cactoideae.
– Cact. Syst. Init. 9: 13-18.
Hunt DR. 2003. Astrophytum. – Cactaceae Systematics Initiatives
15: 5-6.
Hunt DR. 2006a. Further comments on
Mammillaria gene-sequence data. – Cact. Syst. Init. 21: 21-24.
Hunt DR (ed). 2006b. The new cactus lexicon
I-II. – DH Books, Milborne Port, United Kingdom.
Hunt DR. 2012a. Taxonomic implications of DNA
studies relating to Cactaceae
subfam. Cactoideae. – Cactaceae Syst. Init. 26: 5-11.
Hunt DR. 2012b. New cactus lexicon updates
etc. – Cactaceae syst. Init.
26: 12-20.
Hunt DR. 2013. Leuenbergeria. – Cactaceae Syst. Init. 31: 16-17.
Hunt DR, Taylor N. 1986. The genera of the Cactaceae: towards a new consensus.
– Bradleya 4: 65-78.
Hunt DR, Taylor N. 1990. The genera of Cactaceae: progress towards
consensus. – Bradleya 8: 85-107.
Hunt DR, Taylor N. 1991. Notes on
miscellaneous genera of Cactaceae. – Bradleya 9: 81-92.
Hunt DR, Taylor N. 1992. Notes on
miscellaneous genera of Cactaceae
2. – Bradleya 10: 17-32.
Hunt DR, Taylor NP, Glass H. 2006. The new
cactus lexicon. – Remous Ltd., Milborne Port, United Kingdom.
Hunziker JH, Behnke H-D, Eifert IJ, Mabry TJ.
1974. Halophytum ameghinoi: a betalain-containing and P-type
sieve-tube plastid species. – Taxon 23: 537-539.
Hunziker JH, Pozner R, Escobar A. 2000.
Chromosome number in Halophytum ameghinoi (Halophytaceae). – Plant Syst.
Evol. 221: 125-127.
Husson P. 1966. Stomates et cellules annexes:
types stomatiques chez les Polygonacées. – Bull. Soc. Hist. Nat. Toulouse
102: 308-318.
Iamonico D. 2016. Nomenclatural notes on four
Linnaean names in Arenaria (Caryophyllaceae). – Taxon
65: 610-616.
Ihlenfeldt H-D. 1960.
Entwicklungsgeschichtliche, morphologische und systematische Untersuchungen an
Mesembryanthemen. – Feddes Repert. 63: 1-104.
Ihlenfeldt H-D. 1971. Zur Morphologie und
Taxonomie der Mitrophyllinae Schwantes. – Ber. Deutsch. Bot. Ges. 84:
655-660.
Ihlenfeldt H-D. 1975. Some trends in the
evolution of the Mesembryanthemaceae. – Boissiera 24: 249-254.
Ihlenfeldt H-D. 1978. Monographie der
Mitrophyllinae Schwantes III. Morphologie und Taxonomie der Gattung
Oophytum N. E. Br. (Mesembryanthemaceae). – Bot. Jahrb. Syst. 99:
303-328.
Ihlenfeldt H-D. 1980. Der Haarapparat
(‘Diadem’) der Gattung Trichodiadema Schwant.
(Mesembryanthemaceae). – Mitt. Inst. Allg. Bot. Hamburg 17: 145-163.
Ihlenfeldt H-D. 1983. Dispersal of
Mesembryanthemaceae in arid habitats. – Sonderbd. Naturwiss. Ver. Hamburg 7:
381-390.
Ihlenfeldt H-D. 1994. Diversification in an
arid world: the Mesembryanthemaceae. – Ann. Rev. Ecol. Syst. 25: 521-546.
Ihlenfeldt H-D, Gerbaulet M. 1990.
Untersuchungen zum Merkmalsbestand und zur Taxonomie der Gattungen
Apatesia N. E. Br., Carpanthea N. E. Br., Conicosia
N. E. Br., Herrea Schwantes und Hymenogyne Haw.
(Mesembryanthemaceae Fenzl). – Bot. Jahrb. Syst. 111: 457-498.
Ihlenfeldt H-D, Hartmann HEK. 1982. Leaf
surfaces in Mesembryanthemaceae. – In: Cutler DF, Alvin KL, Price CE (eds),
The plant cuticle, Academic Press, London, pp. 397-423.
Ihlenfeldt H-D, Straka H. 1962. Über die
systematische Stellung und Gliederung der Mesembryanthemen. – Ber. Deutsch.
Bot. Ges. 74: 485-492.
Ihlenfeldt H-D, Struck M. 1987. Morphologie
und Taxonomie der Dorotheanthinae Schwantes (Mesembryanthemaceae). – Beitr.
Biol. Pflanzen 61: 411-453.
Ihlenfeldt H-D, Schwantes G, Straka H. 1962.
Die höheren Taxa der Mesembryanthemaceae. – Taxon 11: 52-56.
Ikonnikov SS. 1973. Notes on Caryophyllaceae 1. On the
genus Dichodon (Bartl.) Reichb. – Nov. Sist. Vyssh. Rast. 10:
140-142. [In Russian]
Ikonnikov SS. 1976. Notes on Caryophyllaceae 3. On the
genus Psammophiliella Ikonn. – Nov. Sist. Vyssh. Rast. 13: 116-117.
[In Russian]
Iijima T, Sibaoka T. 1985. Membrane
potentials in excitable cells of Aldrovanda vesiculosa trap-lobes. –
Plant Cell Physiol. 26: 1-14.
Iliff J. 2002. The Andean opuntias: an
annotated checklist of the indigenous non-platyopuntioid opuntias (Cactaceae-Opuntioideae) of South
America. – Succ. plant Res. 6: 133-244.
Iljin MM. 1929. Novye vidy roda
Corispermum L. – Izv. Glavn. Bot. Sada SSSR 28: 637-654.
Iljin MM. 1936. On the systematics of the
genus Suaeda Forssk. and the tribe Suaedeae Rchb. – Sovetsk. Bot. 5:
39-49. [In Russian]
Iljin MM. 1937. Novye vidy sem.
Chenopodiaceae iz Nahičevanskoj ASSR. – Bot. Mater. Gerb. Bot. Inst.
Komarova Akad. Nauk SSSR 7: 203-218.
Iljin MM. 1946. Entomofilija u sem
Chenopodiaceae, eje rasprostranenije i znacenije. – Sov. Bot. (Leningrad) 14:
247-254.
Illing N, Klak C, Johnson C, Negrao N, Baine
F, Kets V van, Ramchurn KR, Seoighe C, Roden L. 2009. Duplication of the
Asymmetric Leaves 1/Rough Sheath 2/Phantastica (ARP) gene
precedes the explosive radiation of the Ruschioideae. – Evol. Genes Devel.
219: 331-338.
Inamdar JA. 1968. Epidermal structure and
ontogeny of stomata in some Nyctaginaceae. – Flora 158:
159-166.
Inamdar JA, Gangadhara M, Morge PG, Patel RM.
1977. Epidermal structure and ontogeny of stomata in some Centrospermae. –
Feddes Repert. 88: 465-475.
Inamdar JA, Gangadhara M, Avita S, Rao NV.
1980. Epidermal studies in some Indian cultivars of bougainvilleas. – Feddes
Repert. 91: 259-266.
Ingrouille MJ. 1984. A taxonomic analysis of
Limonium (Plumbaginaceae) in Western
Europe. – Plant Syst. Evol. 147: 103-118.
Ingrouille MJ, Stace CA. 1985. Pattern of
variation of agamospermous Limonium (Plumbaginaceae) in the British
Isles. – Nord. J. Bot. 5: 113-125.
Ingvarsson PK, Ribstein S, Taylor DR. 2003.
Molecular evolution of insertions and deletions in the chloroplast genome of
Silene. – Mol. Biol. Evol. 20: 1737-1740.
Inoue M, Ohtani K, Kasai R, Okukubo M,
Andriansiferana M, Yamasaki K, Koike T. 2009. Cytotoxic
16-β-[(D-xylopyranosyl)oxy]oxohexadecanyl triterpene glycosides from a
Malagasy plant, Physena sessiliflora. – Phytochemistry 70:
1195-1202.
Iones K. 1964. Pollen structure and
development in Drosera. – J. Linn. Soc. Bot. 59: 81-87.
Isomiddinova D, Sokolov PD. 1977. Comparative
anatomic study of leaves of some Polygonum L. species. – Rast.
Resur. 13: 11-24. [In Russian]
Iwarsson M. 1977. Pollen morphology of East
African Caryophyllaceae.
– Grana 16: 15-22.
Jacobs SWL. 1988. Notes on Aizoaceae and Chenopodiaceae. –
Telopea 3: 139-143.
Jacobs SWL. 2001. Review of leaf anatomy and
ultrastructure in the Chenopodiaceae (Caryophyllales). – J. Torrey
Bot. Soc. 128: 236-253.
Jaeger EJ. 1992. Die Verbreitung von
Frankenia in der Mongolei, in Westeurasien und im Weltmaßstab. –
Flora 186: 177-186.
James LE, Kyhos DW. 1961. The nature of the
fleshy shoot of Allenrolfea and allied genera. – Amer. J. Bot. 48:
101-108.
Jansen S, Ronse Decraene LP, Smets E. 2000.
On the wood and stem anatomy of Monococcus echinophorus (Phytolaccaceae s.l.). – Syst.
Geogr. Plants 70: 171-179.
Jaretzky R. 1925. Beiträge zur Systematik
der Polygonaceen unter Berücksichtigung des Oxymethyl-Anthrachinon-Vorkommens.
– Feddes Repert. 22: 49-83.
Jaretzky R. 1928. Histologische und
karyologische Studien an Polygonaceen. – Jahrb. Wiss. Bot. 69: 357-490.
Jassem B. 1976. Embryology and genetics of
apomixes in the section Corollinae of the genus Beta. –
Acta Biol. Cracov. Bot. 19: 149-172.
Jassem B. 1980. Origin and reproduction of
higher polyploids within the Corollinae section of the genus
Beta. – Genet. Polon. 21: 18-27.
Jay M, Lebreton P. 1973. Recherches
chimiotaxinomiques sur les plantes vasculaires XXVI. Les flavanoides des
Sarracéniacées, Nepenthacées, Droséracées, et Céphalotacées; étude
critique de l’ordre des Sarracéniales. – Nat. Canadien 91: 607-613.
Jeanmonod D. 1984a. Révision de la section
Siphonomorpha Otth du genre Silene L. (Caryophyllaceae) en
Méditerranée occidentale II: le groupe du S. mollissima. –
Candollea 39: 195-259.
Jeanmonod D. 1984b. Révision de la section
Siphonomorpha Otth du genre Silene L. (Caryophyllaceae) en
Méditerranée occidentale III: aggrégat italica et espèces
affinés. – Candollea 39: 549-639.
Jeanmonod D. 1985a. Révision de la section
Siphonomorpha Otth du genre Silene L. (Caryophyllaceae) en
Méditerranée occidentale IV: species caeterae. – Candollea 40: 5-34.
Jeanmonod D. 1985b. Révision de la section
Siphonomorpha Otth du genre Silene L. (Caryophyllaceae) en
Méditerranée occidentale V: synthèse. – Candollea 40: 35-56.
Jebb M, Cheek M. 1997. A skeletal revision of
Nepenthes (Nepenthaceae). – Blumea 42:
1-106.
Jeffrey C. 1961. Aïzoaceae. – In: Hubbard
CE, Milne-Redhead E (eds), Flora of tropical East Africa, Crown Agents for
Oversea Governments and Administrations, London, pp. 1-37.
Jensen U. 1965. Serologische Untersuchungen
zur Frage der systematischen Einordnung der Didiereaceae. – Bot. Jahrb.
Syst. 84: 233-253.
Jia A-Q, Tan N-H, Yang Y-P, Wu S-G,Wang L-Q,
Zhou J. 2004. Cyclopeptides from three arctic Caryophyllaceae plants,
chemotaxonomy and distribution significance of Caryophyllaceae cyclopeptides.
– Acta Bot. Sin. 46: 625-630.
Joesting F. 1902. Beiträge zur Anatomie der
Sperguleen, Polycarpeen, Paronychieen, Sclerantheen und Pterantheen. – Beih.
Bot. Centralbl. 12: 139-181.
Johri BM, Kak D. 1954. The embryology of
Tamarix Linn. – Phytomorphology 4: 230-247.
Jordan GJ, Macphail MK. 2003. A Middle-Late
Eocene inflorescence of Caryophyllaceae from Tasmania,
Australia. – Amer. J. Bot. 90: 761-768.
Joshi AC. 1937. Some salient points in the
evolution of the secondary vascular cylinder of Amaranthaceae and
Chenopodiaceae. – Amer. J. Bot. 24: 3-9.
Joshi AC. 1938. The nature of the ovular
stalk in Polygonaceae and some
related families. – Ann. Bot., N. S., 2: 957-959.
Joshi AC, Rao VS. 1933. Floral anatomy of
Rivina humilis and the theory of carpel polymorphism. – New Phytol.
32: 359-363.
Joshi AC, Rao VSR. 1934. Vascular anatomy of
flowers of four Nyctaginaceae. – J. Indian
Bot. Soc. 13: 169-186.
Joshi AC, Rao VSR. 1936. The embryology of
Gisekia pharnaceoides L. – Proc. Indian Acad. Sci., Sect. B, 3:
71-92.
Juan R, Pastor J, Alaiz M, Vioque J. 2007.
Electrophoretic characterization of Amaranthus L. seed proteins and
its systematic implications. – Bot. J. Linn. Soc. 155: 57-63.
Jürgens N. 1986. Untersuchungen zur
Ökologie sukkulenter Pflanzen des südlichen Afrika. – Mitt. Inst. Allg.
Bot. Hamburg 21: 139-365.
Kadereit G, Freitag H. 2011. Molecular
phylogeny of Camphorosmeae (Camphorosmoideae, Chenopodiaceae): implications for
biogeography, evolution of C4 photosynthesis and taxonomy. – Taxon
60: 51-78.
Kadereit G, Yaprak AE. 2008. Microcnemum
coralloides (Salicornioideae, Chenopodiaceae): an example of intraspecific
east-west disjunction in the Mediterranean region. – An. Jard. Bot. Madrid
65: 415-426.
Kadereit G, Borsch T, Weising K, Freitag H.
2003. Phylogeny of Amaranthaceae and Chenopodiaceae
and the evolution of C4 photosynthesis. – Intern. J. Plant Sci.
164: 959-986.
Kadereit G, Gotzek D, Jacobs S, Freitag H.
2005. Origin and age of Australian Chenopodiaceae. – Organisms Divers. Evol.
5: 59-80.
Kadereit G, Mucina L, Freitag H. 2006.
Phylogeny of Salicornioideae (Chenopodiaceae): diversification, biogeography,
and evolutionary trends in floral morphology. – Taxon 55: 617-642.
Kadereit G, Hohmann S, Kadereit JW. 2006. A
synopsis of Chenopodiaceae subfam. Betoideae and notes on the taxonomy of
Beta. – Willdenowia 36 (Spec. issue): 9-19.
Kadereit G, Ball P, Beer S, Mucina L,
Sokoloff D, Teege P, Yaprack AE, Freitag H. 2007. A taxonomic nightmare comes
true: phylogeny and biogeography of glassworts (Salicornia L.,
Chenopodiaceae). –Taxon 56: 1143-1170.
Kadereit G, Mavrodiev EV, Zacharias EH,
Sukhorukov AP. 2010. Molecular phylogeny of Atripliceae (Chenopodioideae,
Chenopodiaceae): implications for systematics, biogeography, flower and fruit
evolution, and the origin of C4 photosynthesis. – Amer. J. Bot.
97: 1664-1687.
Kadereit G, Piirainen M, Lambinon J,
Vanderpoorten A. 2012. Cryptic taxa should have names: reflections in the
glasswort genus Salicornia (Amaranthaceae). – Taxon 61:
1227-1239.
Kadereit G, Ackerly D, Pirie MD. 2012. A
broader model for C4 photosynthesis evolution in plants inferred from the
goosefoot family (Chenopodiaceae s.s.). – Proc. Roy. Soc. Biol. Sci. Ser. B
279: 3304-3311.
Kadereit G, Lauterbach M, Pirie MD, Arafeh R,
Freitag H. 2014. When do different C4 leaf anatomies indicate independent C4
origins? – Parallel evolution of C4 leaf types in Camphorosmeae
(Chenopodiaceae). – J. Exp. Bot 65: 3499-3511.
Kajale LB. 1940a. Structure and development
of the male and female gametophytes of Sesuvium portulacastrum Linn.
– Proc. Indian Acad. Sci., Sect. B, 10: 82-89.
Kajale LB. 1940b. A contribution to the
embryology of the Amaranthaceae. – Proc. Natl.
Inst. Sci. India 6: 597-625.
Kajale LB. 1954. Contribution to the
embryology of the Phytolaccaceae II.
Fertilization and development of embryo, seed, and fruit in Rivina
humilis Linn. and Phytolacca dioica Linn. – J. Indian Bot. Soc.
33: 206-225.
Kamari G, Constantinidis T. 1994. The
Minuartia verna complex in Greece. – Bot. Chron. 11: 41-54.
Kamelina OP, Proskurina OB. 1985. On the
embryology of Stegnosperma halimifolium (Stenospermataceae).
Distribution of embryological features in Caryophyllales. – Bot. Žurn.
70: 721-730. [In Russian with English summary]
Kapil RN, Prakash N. 1966. Coexistence of
mono-, bi- and tetrasporic embryo sacs in Delosperma cooperi (Hook.
f.) L. Bol. (Aizoaceae). –
Beitr. Biol. Pflanzen 42: 381-392.
Kapil RN, Prakash N. 1969. Embryology of
Cereus jamacaru and Ferocactus wislizeni and comments on the
systematic position of the Cactaceae. – Bot. Not. 122:
409-426.
Kaplan A, Çölgeçen H, Büyükkartal HN.
2009. Seed morphology and histology of some Paronychia taxa. –
Bangladesh J. Bot. 38: 171-176.
Kapralov MV, Akhani H, Voznesenskaya EV,
Edwards G, Franceschi V, Roalson EH. 2006. Phylogenetic relationships in the
Salicornioideae/Suaedoideae/Salsoloideae s.l. (Chenopodiaceae) clade and a
clarification of the phylogenetic position of Bienertia and
Alexandra using multiple DNA sequence datasets. – Syst. Bot. 31:
571-585.
Karis PO. 2004. Taxonomy, phylogeny and
biogeography of Limonium sect. Pteroclados (Plumbaginaceae), based on
morphological data. – Bot. J. Linn. Soc. 144: 461-482.
Karschon R. 1973. Seedling morphology and
schizocotyly in Hammada salicornia (Moq.) Iljin. – Leafl. For. Div.
Agric. Res. Org. Ilanot 46.
Kato M. 1993. Floral biology of Nepenthes
gracilis (Nepenthaceae)
in Sumatra. – Amer. J. Bot. 80: 924-927.
Kattermann F. 1994. Eriosyce (Cactaceae). The genus revised and
amplified. – Succ. Plant Res. 1: 1-176.
Kaul RB. 1933. Vergleichende
entwicklungsgeschichtliche Untersuchungen an der Insectivore
Nepenthes. – Beih. Bot. Centralbl. 51: 311-334.
Kaul RB. 1982. Floral and fruit morphology of
Nepenthes lowii and N. villosa, montane carnivores of Borneo.
– Amer. J. Bot. 69: 793-803.
Keeler KH, Fredericks MS. 1979. Nocturnal
pollination of Abronia fragrans. – Southw. Natur. 24: 692-693.
Keighery GJ. 1975. Chromosome numbers in the
Gyrostemonaceae
Endl. and the Phytolaccaeae Lindl.: a comparison. – Aust. J. Bot. 23:
335-338.
Keighery GJ. 1982. Macarthuria
intricata sp. nov. (Aizoaceae), a new species from South
Western Australia. – Nord. J. Bot. 2: 5-6.
Kellner A, Ritz CM, Schlittenhardt P, Hellwig
FH. 2011. Genetic differentiation of the genus Lithops L.
(Ruschioideae, Aizoaceae) reveals
a high level of convergent evolution and reflects geographic distribution. –
Plant Biol. 13: 368-380.
Kelly WA. 1973. Pollen morphology and
relationships in the genus Calandrinia H.B.K. – Ph.D. diss.,
California State University, Northridge, California.
Kemp MS, Burden RS, Brown C. 1979. A new
naturally occurring flavanone from Tetragonia expansa. –
Phytochemistry 18: 1765-1766.
Kempton EA. 2010. Systematics, character
evolution and ecological diversification within Eriogonoideae s.s. – Ph.D.
diss., Claremont Graduate University, Claremont, California.
Kempton EA. 2012. Systematics of
Eriogonoideae s. s. (Polygonaceae). – Syst. Bot. 37:
723-737.
Kendrick RE, Hillman WS. 1971. Absence of
phytochrome dark reversion in seedlings of the Centrospermae. – Amer. J. Bot.
58: 424-428.
Keng H. 1967. Observations on
Ancistrocladus. – Gard. Bull. (Singapore) 22: 113-121.
Keng H. 1970. Further observations on
Ancistrocladus tectorius (Ancistrocladaceae). –
Gard. Bull. (Singapore) 25: 235-237.
Kennedy RA, Eastburn JL, Jensen KG. 1980.
C3-C4 photosynthesis in the genus Mollugo:
structure, physiology and evolution of intermediate characteristics. – Amer.
J. Bot. 67: 1207-1217.
Keskin M. 2009. Polygonum
istanbulicum Keskin sp. nov. (Polygonaceae) from Turkey. –
Nord. J. Bot. 27: 11-15.
Kharadze AL. 1955. Ad cognitionem generum
monotypicum Caryophyllacearum. – Not. Syst. Geogr. Inst. Bot. Thbilissiensis
18: 72-83.
Khatib A. 1959. Contribution à l’étude
systematique, anatomique, phylogénetique et écologique des Chènopodiacées
de la Syrie. – Ph.D. diss. l’Université de Montpellier, France.
Khoshoo TN, Bhatia SK. 1965. Biosystematics
of Indian plants 1. Saponaria vaccaria Linn. – Bull. Nat. Bot. Gard.
(Lucknow) 116: 1-54.
Khullar SP, Dutta M. 1973. Cytotaxonomic
studies on the genus Portulaca from Chandigarh. – Bangladesh J. Bot.
2: 95-100.
Kiesling R. 1978. El género
Trichocereus (Cactaceae)
I. Las species de la Rep. Argentina. – Darwiniana 21: 263-330.
Kiesling R. 1982a. The genus
Pterocactus. – Cactus Succ. J. Gr. Brit. 44: 51-56.
Kiesling R. 1982b. Problemas nomenclaturales
en el género Cereus (Cactaceae). – Darwiniana 24:
443-453.
Kiesling R. 1984. Estudios en Cactaceae de Argentina:
Maihueniopsis, Tephrocactus y géneros afines (Opuntioideae).
– Darwiniana 25: 171-215.
Kiesling R, Piltz J. 2001. Yavia
cryptocarpa R. Kiesling & Piltz, gen. & spec. nov. – Kakt. Sukk.
52: 57-63.
Kiger RW. 2001. New combinations in
Phemeranthus Rafinesque (Portulacaceae). – Novon 11:
319-321.
Kihara H, Ono T. 1926. Chromosomenzahlen und
systematische Gruppierung der Rumex-Arten. – Zeitschr. Zellforschung
Mikroskop. Anatomie 4: 475-481.
Kilian N, Leyens T. 1994. Limonium
lobinii (Plumbaginaceae), a new species
from the Cape Verde Islands, W Africa. – Willdenowia 24: 59-63.
Kim I, Carr GD. 1990. Cytogenetics and
hybridization of Portulaca in Hawaii. – Syst. Bot. 15: 370-377.
Kim JY, Park C-W. 2000. Morphological and
chromosomal variation in Fallopia section Reynoutria (Polygonaceae) in Korea. –
Brittonia 52: 34-48.
Kim S-T, Donoghue MJ. 2008a. Molecular
phylogeny of Persicaria (Persicarieae, Polygonaceae). – Syst. Bot. 33:
77-86.
Kim S-T, Donoghue MJ. 2008b. Incongruence
between cpDNA and nrITS trees indicates extensive hybridization within
Eupersicaria (Polygonaceae). – Amer. J. Bot.
95: 1122-1135.
Kim S-T, Sultan SE, Donoghue MJ. 2008.
Allopolyploid speciation in Persicaria (Polygonaceae): insights from a
low-copy nuclear region. – Proc. Natl. Acad. Sci. U.S.A. 105: 12370-12375.
Kimnach M. 1960. A revision of
Borzicactus. – Cact. Succ. J. (Los Angeles) 32: 8-13, 57-60.
Kimnach M. 1983. A revision of
Acanthorhipsalis. – Cact. Succ. J. (US) 55: 177-182.
Kimnach M. 1984. Lymanbensonia (Cactaceae), a new genus for
Acanthorhipsalis micrantha. – Cact. Succ. J. (Los Angeles) 56:
100-101.
Kimnach M. 1993. The genus
Disocactus. – Haseltonia 1: 95-139.
Kircheimer F von. 1941. Über ein Vorkommen
der Gattung Aldrovanda Linné im Alttertiär Thüringens. –
Braunkohle 40: 308-311.
Kirchoff BK, Fahn A. 1984a. The primary
vascular system and medullary bundle structure of Phytolacca dioica
(Phytolaccaceae). – Can.
J. Bot. 62: 2432-2440.
Kirchoff BK, Fahn A. 1984b. Initiation and
structure of the secondary vascular system in Phytolacca dioica (Phytolaccaceae). – Can. J.
Bot. 62: 2580-2586.
Kishima Y, Ogura K, Mizukami K, Mikami T,
Adachi T. 1995. Chloroplast DNA analysis in buckwheat species: phylogenetic
relationships, origin of the reproductive systems and extended inverted
repeats. – Plant Sci. 108: 173-179.
Klak C. 2003. New combinations, a new genus
and five new species in the Aizoaceae. – Bradleya 21:
107-120.
Klak C. 2005. Ruschiella, a new
genus of Aizoaceae and new
combinations in Phiambolia and Phyllobolus. – Bradleya 23:
97-104.
Klak C. 2010. Phylogeny and diversification
of Aizoaceae: progress and
prospects. – Schumannia 6: 87-102.
Klak C, Bruyns PV. 2012. Phylogeny of the
Dorotheantheae (Aizoaceae), a
tribe of succulent annuals. – Taxon 61: 293-307.
Klak C, Bruyns PV. 2013. A new infrageneric
classification for Mesembryanthemum L. (Aizoaceae: Mesembryanthemoideae).
– Bothalia 43: 197-206.
Klak C, Bruyns PV. 2016. Expansion of
Schlechteranthus (Ruschioideae, Aizoaceae) to include
Polymita, with a new species from Namaqualand, South Africa. – South
Afr. J. Bot. 103: 70-77.
Klak C, Linder HP. 1998. Systematics of
Psilocaulon N. E. Br. and Caulipsolon Klak gen. nov.
(Mesembryanthemoideae, Aizoaceae). – Bot. Jahrb. Syst.
120: 301-375.
Klak C, Hedderson TA, Linder HP. 2003. A
molecular systematic study of the Lampranthus group (Aizoaceae) based on the chloroplast
trnL-trnF and nuclear ITS and 5S NTS sequence data. – Syst.
Bot. 28: 70-85.
Klak C, Khunou A, Reeves G, Hedderson T.
2003. A phylogenetic hypothesis for the Aizoaceae (Caryophyllales) based on four
plastid DNA regions. – Amer. J. Bot. 90: 1433-1445.
Klak C, Reeves G, Hedderson T. 2004.
Unmatched tempo of evolution in southern African semidesert ice plants. –
Nature 427: 63-65.
Klak C, Nowell TL, Hedderson TAJ. 2006.
Phylogeny and revision of Brownanthus and its close allies
Aspazoma and Dactylopsis (Aizoaceae) based on morphology and
four DNA regions. – Kew Bull. 61: 353-400.
Klak C, Bruyns PV, Hedderson TAJ. 2007. A
phylogeny and new classification for Mesembryanthemoideae (Aizoaceae). – Taxon 56:
737-756.
Klak C, Bruyns PV, Hanáček P. 2013. A
phylogenetic hypothesis for the recently diversified Ruschieae (Aizoaceae) in southern Africa. –
Mol. Phylogen. Evol. 69: 1005-1020.
Klak C, Hanáček P, Bruyns PV. 2014.
Phylogeny and taxonomy for Mesembryanthemum subg.
Volkeranthus (Aizoaceae-Mesembryanthemoideae). –
South Afr. J. Bot. 95: 112-122.
Klak C, Hanáček P, Bruyns PV. 2015. A
phylogeny and revised classification for the Apatesieae (Aizoaceae: Ruschioideae) with a
comparison of centres of diversity. – Taxon 64: 507-522.
Klak C, Hanáček P, Bruyns PV. 2017a. Out of
southern Africa: origin, biogeography and age of the Aizooideae (Aizoaceae). – Mol. Phylogen. Evol.
109: 203-216.
Klak C, Hanaček P, Bruyns PV. 2017b.
Disentangling the Aizooideae: new generic concepts and a new subfamily in Aizoaceae. – Taxon 66:
1147-1170.
Kluge M, Ting IP. 1978. Crassulacean acid
metabolism. Analysis of an ecological adaptation. – Springer-Verlag,
Berlin.
Kobayashi N, Schmidt J, Mimtz M, Wray V,
Schliemann W. 2000. Betalains from Christmas cactus. – Phytochemistry 54:
419-426.
Koch KE, Kennedy RA. 1980. Characteristics of
crassulacean acid metabolism in the succulent C4 dicot,
Portulaca oleracea L. – Plant Physiol. 65: 193-197.
Koch KE, Kennedy RA. 1982. Crassulacean acid
metabolism in the succulent C4 dicot, Portulaca oleracea L.
under natural environmental conditions. – Plant Physiol. 69: 757-761.
Köhler E. 1993. Blattnervatur-Muster der Buxaceae Dumortier und Simmondsiaceae van Tieghem. –
Feddes Repert. 104: 145-167.
Köhler E. 2002. Simmondsiaceae. – In:
Kubitzki K, Bayer C (eds), The families and genera of vascular plants V.
Flowering plants. Dicotyledons. Malvales, Capparales and
non-betalain Caryophyllales,
Springer, Berlin, Heidelberg, New York, pp. 355-358.
Köhler E, Brückner P. 1983. Zur
Pollenmorphologie und systematischen Stellung der Gattung Simmondsia
Nutt. – Wiss. Zeitschr. Friedrich-Schiller-Univ. Jena, Math.-Naturwiss.
Reihe, 32: 945-955.
Kondo K. 1973. The chromosome numbers of
Striga asiatica and Triphyophyllum peltatum. – Phyton 31:
1-2.
Kondo K. 1976. A cytotaxonomic study in some
species of Drosera. – Rhodora 78: 532-541.
Kondo K, Lavarack PS. 1984. A cytotaxonomic
study of some Australian species of Drosera. – Bot. J. Linn. Soc.
88: 317-333.
Kondo K, Olivier MC. 1979. Chromosome numbers
of four species of Drosera (Droseraceae). – Ann. Missouri
Bot. Gard. 66: 584-587.
Kondo K, Segawa M. 1988. A cytotaxonomic
study in artificial hybrids between Drosera anglica Huds. and its
certain closely related species in series Drosera, section
Drosera, subgenus Drosera, Drosera. – La Kromosoma
II-51-52: 1697-1709.
Kondo K, Segawa M, Nehira K. 1976. A
cytotaxonomic study in four species of Drosera. – Mem. Fac. Integr.
Arts Sci. Hiroshima Univ., Ser. IV, 2: 27-36.
Kondorskaya VR. 1984. Features of
inflorescence structure in the tribe Atripliceae C. A. Mey. (Chenopodiaceae).
– Bull. Mosk. Obsch. Ispyt. Prir. (Bul. MOIP), sér. Biology 89: 104-114. [In
Russian]
Kool A, Thulin M. 2013. Proposal to reject
the name Psammanthe (Caryophyllaceae). – Taxon
62: 833.
Kool A, Thulin M. 2017a. A giant spurrey on a
tiny island: on the phylogenetic position of Sanctambrosia manicata
(Caryophyllaceae) and the
generic circumscriptions of Spergula, Spergularia and
Rhodalsine. – Taxon 66: 615-622.
Kool A, Thulin M. 2017b. A plant that
Linnaeus forgot: taxonomic revision of Rhodalsine (Caryophyllaceae). –
Willdenowia 47: 317-323.
Kool A, Bengtson A, Thulin M. 2007. Polyphyly
of Polycarpon (Caryophyllaceae) inferred from
DNA sequence data. – Taxon 56: 775-782.
Kool A, Perrigo A, Thulin M. 2012. Bristly
versus juicy: phylogenetic position and taxonomy of Sphaerocoma (Caryophyllaceae). – Taxon
61: 67-75.
Korn RW. 2011. Window patterns in
Lithops. – Intern. J. Plant Sci. 172: 1101-1109.
Korotkova N, Zabel L, Quandt D, Barthlott W.
2010. A phylogenetic analysis of Pfeiffera and the reinstatement of
Lymanbensonia as an independently evolved lineage of epiphytic Cactaceae with a new tribe
Lymanbensonieae. – Willdenowia 40: 151-172.
Korotkova N, Borsch T, Quandt D, Taylor NP,
Müller KF, Barthlott W. 2011. What does it take to resolve relationships and
to identify species with molecular markers? An example from the epiphytic
Rhipsalideae (Cactaceae). –
Amer. J. Bot. 98: 1549-1572.
Korzshinsky S. 1886. Über die Samen der
Aldrovanda vesiculosa L. – Bot. Centralbl. 27: 302-304, 334-335.
Kosová V, Chldek M, Petrik F. 1958. Ein
Beitrag zur Pharmakognosie und Wertbestimmung einiger Arten der Gattung
Chenopodium. – Pharmazie 13: 631-647.
Kothe-Heinrich G. 1993. Revision der Gattung
Halothamnus (Chenopodiaceae). – Bibl. Bot. 143.
Kovácik J, Repcák M. 2006. Naphtoquinone
content of some sundews (Drosera L.). – Carniv. Plant Newsl. 35:
49-51.
Kowal K. 1961. Studia nad morfologia i
anatomia nasion Portulacaceae
Reichb. – Monogr. Bot. 12: 1-48.
Kozhanchikov VI. 1967. Morphological
characters of the seeds of the family Caryophyllaceae and probable
ways of their evolution. – Bot. Žurn. 52: 1277-1280.
Kraft E. 1917. Experimentelle und
entwicklungsgeschichtliche Untersuchungen an Caryophyllaceen-Blüten. – Flora
109: 283-362.
Krainz H (ed). 1956-1976. Die Kakteen. –
Franckh., Stuttgart.
Král M. 1969. Aconogonon
polystachyum comb. nov. – Preslia (Praha) 41: 258-260.
Král M. 1984. Pirinia, a new genus
of the Caryophyllaceae. –
Preslia (Praha) 56: 161-163.
Král M. 1985. Rubrivena, a new
genus of Polygonaceae. –
Preslia (Praha) 57: 65-67.
Kraybill AA, Martin CE. 1996. Crassulacean
acid metabolism in three species of the C4 genus Portulaca.
– Intern. J. Plant Sci. 157: 103-109.
Kress A. 1970. Zytotaxonomische
Untersuchungen an einigen Insektenfängern (Droseraceae, Byblidaceae,
Cephalotaceae,
Roridulaceae, Sarraceniaceae).
– Ber. Deutsch. Bot. Ges. 83: 55-62.
Kristen U. 1976. Die Morphologie der
Schleimsekretion im Fruchtknoten von Aptenia cordifolia. –
Protoplasma 89: 221-233.
Ku MSB, Shieh YJ, Reger BJ, Black CC. 1981.
Photosynthetic characteristics of Portulaca grandiflora, a succulent
C4 dicot. – Plant Physiol. 768: 1073-1080.
Krutzsch W. 1985. Über
Nepenthes-Pollen im europäischen Tertiär. – Gleditschia 13:
89-93.
Kubitzki K. 1993a. Didiereaceae. – In: Kubitzki K,
Rohwer JG, Bittrich V (eds), The families and genera of vascular plants II.
Flowering plants. Dicotyledons. Magnoliid, hamamelid and caryophyllid families,
Springer, Berlin, Heidelberg, New York, pp. 292-295.
Kubitzki K. 1993b. Plumbaginaceae. – In:
Kubitzki K, Rohwer JG, Bittrich V (eds), The families and genera of vascular
plants II. Flowering plants. Dicotyledons. Magnoliid, hamamelid and
caryophyllid families, Springer, Berlin, Heidelberg, New York, pp. 523-530.
Kubitzki K. 1994. A note on the relationship
of the order within the angiosperms. – In: Behnke H-D, Mabry TJ (eds), Caryophyllales: evolution and
systematics, Springer, Berlin, Heidelberg, New York, pp. 317-320.
Kubitzki K. 2002a. Asteropeiaceae. – In:
Kubitzki K, Bayer C (eds), The families and genera of vascular plants V.
Flowering plants. Dicotyledons. Malvales, Capparales and
non-betalain Caryophyllales,
Springer, Berlin, Heidelberg, New York, pp. 28-29.
Kubitzki K. 2002b. Droseraceae. – In: Kubitzki K,
Bayer C (eds), The families and genera of vascular plants V. Flowering plants.
Dicotyledons. Malvales,
Capparales and
non-betalain Caryophyllales,
Springer, Berlin, Heidelberg, New York, pp. 198-202.
Kubitzki K. 2002c. Drosophyllaceae. – In:
Kubitzki K, Bayer C (eds), The families and genera of vascular plants V.
Flowering plants. Dicotyledons. Malvales, Capparales and
non-betalain Caryophyllales,
Springer, Berlin, Heidelberg, New York, pp. 203-205.
Kubitzki K. 2002d. Frankeniaceae. – In: Kubitzki
K, Bayer C (eds), The families and genera of vascular plants V. Flowering
plants. Dicotyledons. Malvales, Capparales and
non-betalain Caryophyllales,
Springer, Berlin, Heidelberg, New York, pp. 209-212.
Kubitzki K. 2002e. Nepenthaceae. – In: Kubitzki K,
Bayer C (eds), The families and genera of vascular plants V. Flowering plants.
Dicotyledons. Malvales,
Capparales and
non-betalain Caryophyllales,
Springer, Berlin, Heidelberg, New York, pp. 320-324.
Kühl R. 1933. Vergleichende
entwicklungsgeschichtliche Untersuchungen an der Insektivore
Nepenthes. – Beih. Bot. Centralbl. I, 51: 311-334.
Kühn U, Bittrich V, Carolin R, Freitag H,
Hedge IC, Uotila P, Wilson PG. 1993. Chenopodiaceae. – In: Kubitzki K, Rohwer
JG, Bittrich V (eds), The families and genera of vascular plants II. Flowering
plants. Dicotyledons. Magnoliid, hamamelid and caryophyllid families, Springer,
Berlin, Heidelberg, New York, pp. 253-281.
Kuprianova LA. 1973. Pollen morphology within
the genus Drosera. – Grana 13: 103-107.
Kurata K, Jaffré T, Setoguchi H. 2008.
Genetic diversity and geographical structure of the pitcher plant Nepenthes
vieillardii in New Caledonia: a chloroplast DNA haplotype analysis. –
Amer. J. Bot. 95: 1632-1644.
Kurata S. 1976. Nepenthes of Mount
Kinabalu. – Sabah Nat. Parks Publ. 2: 1-80.
Kuroyanagi M, Yamamoto Y, Fukushima S, Ueno
A, Noro T, Miyase T. 1982. Chemical studies on the constituents of
Polygonum nodosum. – Chem. Pharm. Bull. 30: 1602-1608.
Kurtz EG. 1962. Pollen morphology of the Cactaceae. – Pollen Spores 4:
359-360.
Kurzweil H. 2005. Observations on the
development of the placentas and closing bodies in the fruit capsules of some
Mesembryanthema (Aizoaceae). – Bot. Jahrb. Syst.
126: 385-401.
Kuz’min VI. 1971. On the sex dimorphism of
Polygonum coriarium Grig. – Rast. Resur. 7: 288-291. [In Russian]
Kuz’min VI. 1989. Morphology of
inflorescence and sexual dimorphism in Polygonum spp. L. from sect.
Aconogonon Meisn. in connection with fruiting. – Rast. Resur. 25:
137-145. [In Russian]
Labbe A. 1962. Les Plumbaginacées:
structure, développement, repartition, conséquences en systématique. –
Thesis, l’Université de Grenoble, France.
LaCroix C, Sattler R. 1988. Phyllotactix
theories and tepal-stamen superposition in Basella rubra. – Amer. J.
Bot. 75: 906-917.
Lahondère C. 2004. Les salicornes s.l.
(Salicornia L., Sarcocornia A. J. Scott et
Arthrocnemum Moq.) sur le côtes françaises. – Bull. Soc. Bot.
Centre-Ouest, sér. II, num. spec. 24.
Lakomski K. 2003. Haftowanie taksonomii
kaktusów. – Swiat Kakt. 37: 59-73.
Lambert JG. 1985. Het argentijnse geslacht
Tephrocactus. – Succulenta 64: 164-168.
Lamb-Frye AS, Kron KA. 2003. Phylogeny and
character evolution in Polygonaceae. – Syst. Bot. 28:
326-332.
Landrum JV. 2001. Wide-band tracheids in
leaves of genera of Aizoaceae:
the systematic occurrence of a novel cell type and its implications for the
monophyly of the subfamily Ruschioideae. – Plant Syst. Evol. 227: 49-61.
Landrum JV. 2002. Four succulent families and
40 million years of evolution and adaptation to xeric environments: what can
stem and leaf anatomical characters tell us about their phylogeny? – Taxon
51: 463-473.
Landrum JV. 2006. Wide band tracheids in
genera of Portulacaceae:
novel, non-xylary tracheids possibly evolved as an adaptation to water stress.
– J. Plant Res. 119: 497-504.
Lange W, Brandenburg WA, de Bock TSM. 1999.
Taxonomy and cultonomy of beet (Beta vulgaris L.). – Bot. J. Linn.
Soc. 130: 81-96.
Larridon I, Walter HE, Guerrero PC, Duarte M,
Cisternas MA, Hernández CP, Bauters K, Asselman P, Goetghebeur P, Samain M-S.
2015. An integrative approach to understanding the evolution and diversity of
Copiapoa (Cactaceae), a
threatened endemic Chilean genus from the Atacama Desert. – Amer. J. Bot.
102: 1506-1520.
Larsen K. 2002. Caryophyllaceae. – In:
Nooteboom HP (ed), Flora Malesiana 1, 16, Foundation Flora Malesiana, Nationaal
Herbarium Nederland, Leiden; pp. 1-51.
Larsen K, Larsen SS, Pedersen TM. 1987.
Siamosia thailandica, gen. et sp. nov. from Thailand (Amaranthaceae). – Nord. J.
Bot. 7: 271-276.
Las Peñas ML, Bernardello G, Kiesling R.
2008. Karyotypes and fluorescent chromosome banding in Pyrrhocactus
(Cactaceae). – Plant Syst.
Evol. 272: 211-222.
Laubengayer RA. 1937. Studies in the anatomy
and morphology of the polygonaceous flower. – Amer. J. Bot. 24: 329-343.
Laundon JR. 1959. Droseraceae. – In: Hubbard CE,
Milne-Redhead E (eds), Flora of tropical East Africa, Crown Agents for Oversea
Governments and Administrations, London, pp. 1-6.
Laundon JR. 1978. 68. Droseraceae. – In: Launert E
(ed), Flora Zambesiaca 4, Flora Zambesiaca Managing Committee, London, pp.
62-68.
Lawrence GHM. 1940. Armerias, native and
cultivated. – Gentes Herb. 4: 391-418.
Leach GJ, Townsend CC, Harley MM. 1993.
Omegandra, a new genus of Amaranthaceae from Australia.
– Kew Bull. 48: 787-793.
Lebègue A. 1955. Embryogénie des
Mésembryanthemacées. Développement de l’embryon chez le
Mesembryanthemum canaliculatum. – Compt. Rend. Acad. Sci. Paris 240:
450-452.
Le Duc A. 1995. A revision of
Mirabilis section Mirabilis (Nyctaginaceae). – Sida 16:
613-648.
Lee CW, Sherman RA. 1985. Meiosis in jojoba,
Simmondsia chinensis. – Israel J. Bot. 34: 1-6.
Lee J, Kim SY, Park SH, Ali MA. 2013.
Molecular phylogenetic relationships among members of the family Phytolaccacee sensu lato
inferred from internal transcribed spacer sequences of nuclear ribosomal DNA.
– Genet. Mol. Res. 12: 4515-4525. DOI: 10.4238/2013.February.28.15.
Lee KK, Harrison DK, Johnston ME, Williams
RR. 2007. Molecular taxonomic clarification of Ptilotus exaltatus and
Ptilotus nobilis (Amaranthaceae). – Aust. Syst.
Bot. 20: 72-81.
Lee MAB, Brothers T. 1981. Seed dispersal in
hybrid Salsola. – Great Basin Natur. 41: 367-370.
Lee ST, Kim SK, Hong S-P. 1985. A
palynotaxonomic study of the Korean Rumex (Polygonaceae). – J. Sung Kyun
Kwan Univ. (Nat. Sci.) 36: 39-48. [In Korean with English summary]
Lee ST, Kim JG, Hong S-P. 1985. Pollen
surface pattern and its taxonomic significance of the Korean Polygonaceae pollen. – Korean
J. Plant Taxon. 15: 37-48.
Legrand CD. 1949. Las especies del género
Portulaca en la Argentina. – Lilloa 17: 311-376.
Legrand CD. 1953. Demarcación del género
Portulaca. – Comun. Bot. Mus. Hist. Nat. Montevideo 3: 1-16.
Legrand CD. 1958. Desmembración del género
Portulaca II. – Comun. Bot. Mus. Hist. Nat. Montevideo 3: 1-17.
Legrand CD. 1962. Las espécies americanas de
Portulaca. – An. Mus. Hist. Nat. Montevideo 2a, 7, 3: 1-147.
Leighton Boyce G, Iliff J. 1973. The subgenus
Tephrocactus. A historical survey with notes on cultivation. –
Morden.
Leinfellner W. 1959. Die falschen
Rollblätter der Frankeniaceen, in Vergleich gesetzt mit jenen der Ericaceen.
– Österr. Bot. Zeitschr. 106: 325-351.
Leinfellner W. 1965. Über die Kronblätter
der Frankeniaceen. – Österr. Bot. Zeitschr. 112: 44-55.
Leins P, Erbar C. 1994. Putative origin and
relationships of the order from the viewpoint of developmental flower
morphology. – In: Behnke H-D, Mabry TJ (eds), Caryophyllales: evolution and
systematics, Springer, Berlin, pp. 303-316.
Leins P, Schwitalla S. 1985. Studien an
Cactaceen Blüten I. – Beitr. Biol. Pflanzen 60: 313-323.
Leins P, Schwitalla S. 1988. Placentation in
Cactaceae. – In: Leins P,
Tucker SC, Endress PK (eds), Aspects of floral development, J. Cramer,
Bornträger, Berlin, pp. 57-68.
Leins P, Walter A, Erbar C. 2001. Eine
morphogenetische Interpretation der Caryophyllaceen-Kronblätter. – Bot.
Jahrb. Syst. 123: 355-367.
Léonard J. 1982. Ancistrocladaceae. – In:
Bamps P (ed), Flore d’Afrique Centrale. – Jardin Botanique National de
Belgique, Meise.
Léonard J. 1986. Ancistrocladaceae. – In:
Polhill RM (ed), Flora of tropical East Africa, A. A. Balkema, Rotterdam, The
Netherlands, pp. 1-4.
Lepschi BJ. 1996. A taxonomic revision of
Macarthuria (Molluginaceae) in Western
Australia. – Nuytsia 11: 37-54.
Letschert JPW, Lange W, Frese L, Berg RG van
den. 1994. Taxonomy of Beta Section Beta. – J. Sugar Beet
Res. 31: 69-85.
Leuenberger BE. 1976a. Die Pollenmorphologie
der Cactaceae und ihre Bedeutung
für die Systematik. – Diss. Bot. 31, J. Cramer, A. R. Gantner, Vaduz,
Liechtenstein.
Leuenberger BE. 1976b. Pollen morphology of
the Cactaceae – an SEM survey
of exine sculpturing and its tentative implications for taxonomy and phylogeny.
– Cact. Succ. J. (Great Britain) 38: 79-94.
Leuenberger BE. 1986. Pereskia (Cactaceae). – Mem. New York Bot.
Gard. 41: 1-141.
Leuenberger BE. 1993. The genus
Denmoza Britton & Rose (Cactaceae): taxonomic history and
typification. – Haseltonia 1: 86-94.
Leuenberger BE. 1997. Maihuenia –
monograph of a Patagonian genus of Cactaceae. – Bot. Jahrb. Syst.
119: 1-92.
Leuenberger BE. 2002. The South American
Opuntia ser. Armatae (= O. ser. Elatae) (Cactaceae). – Bot. Jahrb. Syst.
123: 413-439.
Leuenberger BE. 2008. Pereskia,
Maihuenia, and Blossfeldia – taxonomic history, updates,
and notes. – Haseltonia 14: 54-93.
Leuenberger BE, Eggli U. 1999. Notes on the
genus Blossfeldia (Cactaceae) in Argentina. –
Haseltonia 6: 2-13.
Leuenberger BE, Eggli U. 2000. The genus
Eulychnia (Cactaceae) in
Chile: notes on the taxonomy, types, and other old specimens. – Haseltonia 7:
63-76.
Levin RA. 2000. Phylogenetic relationships
within Nyctaginaceae tribe
Nyctagineae: evidence from nuclear and chloroplast genomes. – Syst. Bot. 25:
738-750.
Levin RA. 2002. Taxonomic status of
Acleisanthes, Selinocarpus, and Ammocodon (Nyctaginaceae). – Novon 12:
58-63.
Lewis PO. 1991. Allozyme variation and
evolution in Polygonella (Polygonaceae). – Ph.D. diss.,
Ohio State University, Columbus, Ohio.
Lewis PO, Crawford DJ. 1995. Pleistocene
refugium endemics exhibit greater allozymic diversity than widespread congeners
in the genus Polygonella (Polygonaceae). – Amer. J. Bot.
82: 141-149.
Lewis WH, Suda Y. 1968. Karyotypes in
relation to classification and phylogeny in Claytonia. – Ann.
Missouri Bot. Gard. 55: 64-67.
Li A-J. 1981. Parapteropyrum A. J.
Li – unum genus novum Polygonacearum sinicum. – Acta Phytotaxon. Sin. 19:
330-332.
Li B, Fan D, Lei S, Zhang Z. 2013. New
combinations in Persicaria (Polygonaceae: Persicarieae) for
the Flora of China. – Phytotaxa 91: 24-26.
Li F-L, Zeller F, Huang K-F, Shi T-X, Chen
Q-F. 2013. Improvement of fluorescent chromosome in situ PCR and its
application in the phylogeny of the genus Fagopyrum Mill. using
nuclear genes of chloroplast origin (cpDNA). – Plant Syst. Evol. 299:
1679-1691.
Li H-L. 1952. The genus Tovara (Polygonaceae). – Rhodora 54:
19-25.
Liede S. 1989. Untersuchungen zum
Merkmalsbestand und zur taxonomie der ‘Erepsiinae’ (Mesembryanthemaceae).
– Beitr. Biol. Pflanzen 64: 391-479.
Liede-Schumann S, Hartmann HEK. 2009.
Mesembryanthemum – back to the roots? – Taxon 58: 345-346.
Linczevsky I. 1968. Tentamentum systematis
ordinis Plumbaginalium Lindl. – Novit. Syst. Plant. Vasc. (Leningrad) 1968:
171-177.
Linczevsky I. 1971. Notes on Limoniaceae 3.
– Bot. Žurn. 56: 1633-1635.
Lindau G. 1890. Monographia generis
Coccolobae. – Engl. Bot. Jahrb. Syst. 13: 106-229.
Lindsay G. 1963. The genus
Lophocereus. – Cact. Succ. J. (Los Angeles) 35: 176-192.
Liu MT. 1995. General study on
Tamarix L. and its large scale generalizing and application. –
Lanzhou Univ. Publ. House, Lanzhou.
Livingstone DA, Tomlinson M, Friedman G,
Broome R. 1973. Stellate pore ornamentation in pollen grains of the Amaranthaceae. – Pollen Spores
15: 345-351.
Lledó MD, Crespo MB. 2000. Polyphyly of
Limoniastrum (Plumbaginaceae): evidence from
DNA sequences of plastid rbcL, trnL intron and trnL-F intergene spacer. –
Bot. J. Linn. Soc. 132: 175-191.
Lledó MD, Crespo MB, Cameron KM, Fay MF,
Chase MW. 1998. Systematics of Plumbaginaceae based upon
cladistic analysis of rbcL sequence data. – Syst. Bot. 23: 21-29.
Lledó MD, Crespo MB, Cox AV, Fay MF, Chase
MW. 2000. Polyphyly of Limoniastrum (Plumbaginaceae): evidence from
DNA sequences of plastid rbcL, trnL intron and
trnL-F intergene spacer. – Bot. J. Linn. Soc. 132: 175-191.
Lledó MD, Karis PO, Crespo MB, Fay MF, Chase
MW. 2001. Phylogenetic position and taxonomic status of the genus
Aegialitis and subfamilies Staticoideae and Plumbaginoideae (Plumbaginaceae): evidence from
plastid DNA sequences and morphology. – Plant Syst. Evol. 229: 107-124.
Lledó MD, Erben M, Crespo MB. 2005.
Myriolimon, a new name for the recently published Myriolepis
(Plumbaginaceae). – Taxon
54: 811-812.
Lledó MD Crespo MB, Fay MF, Chase MW. 2005.
Molecular phylogenetics of Limonium and related genera (Plumbaginaceae):
biogeographical and systematic implications. – Amer. J. Bot. 92:
1189-1198.
Llorens L. 1985. Revisión
sistemático-taximétrica del género Limonium Miller en la isla de
Mallorca I. – Lazaroa 8: 11-68.
Lloyd FE. 1942. The carnivorous plants. –
Chronica Botanica, Waltham, Massachusetts.
Lobin W, Leyens T, Kilian N, Erben M,
Lewejohann K. 1995. The genus Limonium (Plumbaginaceae) on the Cape
Verde Islands, W Africa. – Willdenowia 25: 197-214.
Logacheva MD, Samigullin TH, Dhingra A, Penin
AA. 2008. Comparative chloroplast genomics and phylogenetics of Fagopyrum
esculentum ssp. ancestrale – a wild ancestor of cultivated
buckwheat. – BMC Plant Biol. 8: 59.
Lomonosova MN, Freitag H. 2003. A new species
of Suaeda (Chenopodiaceae) from the Altai, Central Asia. –
Willdenowia 33: 139-147.
Lomonosova MN, Krasnikov AA. 1993. Chromosome
numbers in some members of the Chenopodiaceae. – Bot. Žurn. 78: 158-159.
Lomonosova MN, Krasnikov AA, Krasnikova SA.
2001. Chromosome numbers of the Chenopodiaceae species from Siberia. – Bot.
Žurn. 86: 145-146.
Lomonosova MN, Krasnikov AA, Krasnikova SA.
2003. Chromosome numbers of the Chenopodiaceae family members of the Kazakhstan
flora. – Bot. Žurn. 88: 134-135.
Lomonosova MN, Brandt R, Freitag H. 2008.
Suaeda corniculata (Chenopodiaceae) and related new taxa from Eurasia.
– Willdenowia 38: 81-109.
Lonsing A. 1939. Über einjährige
europäische Cerastium-Arten aus der Verwandtschaft der Gruppen
‘Ciliatopetala’ Fenzl und ’Cryptodon’ Pax. – Feddes
Repert. 46: 139-165.
López HA, Vegetti AC, Anton AM. 1998.
Estructura de la inflorescencia en especies de Boerhavia L. subgénero
Boerhavia (Nyctaginaceae). – Kurtziana
26: 117-128.
López González G. 2010. Sobre el género
Spergula L. (Caryophyllaceae) y sus
especies en la Península Ibérica e Islas Baleares. – Lagascalia 30:
7-18.
Lopriore GG. 1904. Staminodi delle
Amaranthacee dal punto di vista morfologico, biologico e sistematico. – In:
Urban I, Graebner P (eds), Ascherson Festschrift, Berlin, pp. 413-430.
Lorz A. 1937. Cytological investigations on
five chenopodiaceous genera with special emphasis on chromosome morphology and
somatic doubling in Spinacia. – Cytologia 8: 241-276.
Lourteig A. 1991. El género Montia
(Portulacaceae) en el
Hemispherio Austral. – Rev. Acad. Colombiana Ci. Ex. Fis. Nat. Corr.
Española 18: 41-48.
Lourteig A. 1994. Lyallia
kerguelensis Hook. f. and its artificial propagation. – In: Behnke H-D,
Mabry TJ (eds), Caryophyllales: evolution and
systematics, Springer, Berlin, pp. 321-327.
Löve Á. 1943. Cytogenetic studies on
Rumex subgenus Acetosella. – Hereditas 30: 1-136.
Löve Á, Löve D. 1956. Chromosome number
and taxonomy of eastern North American Polygonum. – Can. J. Bot. 34:
501-521.
Löve Á, Sarkar P. 1957. Heat tolerance of
Koenigia islandica. – Bot. Not. 110: 478-481.
Lowrie A. 2005. A taxonomic revision of
Drosera section Stolonifera (Droseraceae) from south-west
Western Australia. – Nuytsia 15: 355-394.
Lowrie A, Conran JG. 2007. A revision of the
Drosera omissa/D. nitidula complex (Droseraceae) from south-west
Western Australia. – Taxon 56: 533-544.
Lowrie A, Conran JG. 2008. A review of
Drosera whittakeri s. lat. (Droseraceae) and a description of
a new species from Kangaroo Island, South Australia. – Telopea 12:
147-165.
Loza-Cornejo S, Terrazas T. 1996. Anatomía
del tallo y de la raíz de dos especies de Wilcoxia Britton y rose (Cactaceae) del noreste de México.
– Bol. Soc. Bot. México 59: 13-23.
Loza-Cornejo S, Terrazas T. 2011.
Morfo-anatomía de plántulas en especies de Pachycereeae: Hasta cuándo son
plántulas? – Bol. Soc. Bot. México 88: 1-13.
Lu DQ. 1988. Commicarpus (Nyctaginaceae) in China. –
Acta Bot. Bor. Occid. Sin. 8: 125-128.
Lüders H. 1907. Systematische Untersuchungen
über die Caryophyllaceen mit einfachem Diagramm. – Bot. Jahrb. Syst. 40,
Beibl. 91: 1-38.
Luo Y, Bian F-H, Luo Y-B. 2012. Different
patterns of floral ontogeny in dimorphic flowers of Pseudostellaria
heterophylla (Caryophyllaceae). – Intern.
J. Plant Sci. 173: 150-160.
Luteyn J. 1990. 151. Plumbaginaceae. – In: Harling
G, Andersson L (eds), Flora of Ecuador 39, Nord. J. Bot., Copenhagen, pp.
39-47.
Lüthy JM. 1995. Taxonomische Untersuchung
der Gattung Mammilaria Haw. (Cactaceae). – Arbeitskreis für
Mammillarienfreunde, Frankenthal.
Lüthy JM. 2001. A revised classification of
the ‘primitive’ mammillarias. – J. Mammillaria Soc. 41: 6-7.
Lüthy JM. 2002. Further comments on
Turbinicarpus and a key to species. – Cact. Syst. Init. 14:
21-25.
Lüthy JM. 2003a. Rapicactus Buxbaum
& Oehme. Revisione del genere – revision of the genus. – Cactus &
Co. 7: 4-43.
Lüthy JM. 2003b. Revision of the genus
Rapicactus Buxbaum & Oehme. – Turbi-Now 14: 3-13.
Lüthy JM. 2007. Comments on
Sclerocactus. – Cact. Syst. Init. 22: 19-24.
Lüthy JM, Moser U. 2002. The cacti of CITES
Appendix I. – Bundesamt für Veterinärwesen, Bern.
Lütolf GA. 1969. Beziehungen zwischen Portulacaceae und Cactaceae. – Ph.D. diss.,
Universität Zürich, Switzerland.
Mabry TJ. 1974. Is the order Centrospermae
monophyletic? – In: Bendz G, Santesson J (eds), Chemistry in botanical
classification, Proceedings of the 25th Nobel Symposium, Academic
Press, New York, pp. 275-280.
Mabry TJ. 1976. Pigment dichotomy and DNA-RNA
hybridization data for centrospermous families. – Plant Syst. Evol. 126:
79-94.
Mabry TJ. 1977. The order Centrospermae. –
Ann. Missouri Bot. Gard. 64: 210-220.
Mabry TJ, Behnke H-D (eds). 1976. Evolution
of the centrospermous families. – Plant Syst. Evol. 126: 1-106.
Mabry TJ, Taylor A, Turner BL. 1963. The
betacyanins and their distribution. – Phytochemistry 2: 61-64.
Mabry TJ, Kimler L, Chang C. 1972. The
betalains: structure, function, and biogenesis and the plant order
Centrospermae. – In: Runeckles VC, Tso TC (eds), Recent advances in
phytochemistry 4, New York, pp. 105-134.
Mabry TJ, Behnke H-D, Eifert IJ. 1976.
Betalains and P-type sieve-element plastids in Gisekia L.
(Centrospermae). – Taxon 25: 112-114.
Mabry TJ, Neumann P, Philipson WP. 1978.
Hectorella: a member of the betalain-suborder Chenopodiineae of the
order Centrospermae. – Plant Syst. Evol. 130: 163-165.
McCann C. 1953. Notes on the genus
Salicornia L. – Bombay Nat. Hist. Soc. 50: 872-877.
McCauley RA. 2004. New taxa and a new
combination in the North American species of Froelichia (Amaranthaceae). – Syst. Bot.
29: 64-76.
McCauley RA, Ballard HE. 2007a. Systematics
of North American Froelichia (Amaranthaceae subfam.
Gomphrenoideae) I: identification of consistent morphological variation and
segregation of species complexes. – Brittonia 59: 255-274.
McCauley RA, Ballard HE. 2007b. Systematics
of North American Froelichia (Amaranthaceae subfam.
Gomphrenoideae) II: phylogeny and biogeographic speciation patterns inferred
from nrITS sequence data. – Brittonia 59: 275-289.
MacDaniels LH. 1981. A study of cultivars in
Bougainvillea. – Baileya 21: 77-100.
McDonald CB. 1980. A biosystematic study of
the Polygonum hydropiperoides (Polygonaceae) complex. – Amer.
J. Bot. 67: 664-670.
Mackenzie KK. 1914. A new genus from
Missouri. – Torreya 14: 67-68.
McMillan AJS, Horobin JF. 1995. Christmas
cacti. The genus Schlumbergera and its hybrids. – Succ. Plant Res.
4: 1-160.
McNeill J. 1962. Taxonomic studies on the
Alsinoideae I. Generic and infra-generic groups. – Notes Roy. Bot. Gard.
Edinb. 24: 79-155.
McNeill J. 1963. Taxonomic studies in the
Alsinoideae II. A revision of the species in the Orient. – Notes Roy. Bot.
Gard. Edinb. 24: 241-404.
McNeill J. 1972. New taxa of
Claytonia section Claytonia (Portulacaceae). – Can. J. Bot.
50: 1895-1898.
McNeill J. 1973. Gypsophila and
Stellaria: an unexpected problem in generic delimitation. – Notes
Roy. Bot. Gard. Edinb. 32: 389-395.
McNeill J. 1974. Synopsis of a revised
classification of the Portulacaceae. – Taxon 23:
725-728.
McNeill J. 1975. A generic revision of Portulacaceae tribe Montieae
using techniques of numerical taxonomy. – Can. J. Bot. 53: 789-809.
McNeill J. 1978. Silene alba and
Silene dioica in North America and the generic delimitation of
Lychnis, Melandrium, and Silene (Caryophyllaceae). – Can. J.
Bot. 56: 297-308.
McNeill J. 1980. The delimitation of
Arenaria (Caryophyllaceae) and related
genera in North America, with 11 combinations in Minuartia. –
Rhodora 82: 495-501.
McNeill J, Bassett IJ. 1974. Pollen
morphology and the infrageneric classification of Minuartia (Caryophyllaceae). – Can. J.
Bot. 52: 1225-1231.
McNeill J, Crompton W. 1978. Pollen
dimorphism in Silene alba (Caryophyllaceae). – Can. J.
Bot. 56: 1280-1286.
McNeill J, Findlay JN. 1971. The systematic
position of Claytonia bostockii. – Can. J. Bot. 49: 713-715.
McNeill J, Majumdar NC. 1980. A new species
of Arenaria subgenus Odontostemma from Tibet, with a review
of the status of the genus Gooringia (Caryophyllaceae). – Bot. J.
Linn Soc. 80: 371-378.
McNeill J, Bassett IJ, Crompton CW,
Taschereau PM. 1983. Taxonomic and nomenclatural notes on Atriplex L.
(Chenopodiaceae). – Taxon 32: 549-556.
McPherson S. 2008. Pitcher plants of the
Americas. – McDonald & Woodward, Granville, Ohio.
McPherson S. 2009. Pitcher plants of the Old
World I-II. – Redfern Natural History Prod., Poole.
McPherson S. 2011. New Nepenthes 1.
– Redfern Natural History Prod., Poole.
Machado MC. 2007. Fascinating
Frailea 2. Review of the species from Rio Grande do Sul. – Cact.
World 25: 65-76.
Madhani H, Rabeler R, Pirani A, Oxelman B,
Heubl G, Zarre S. 2018. Untangling phylogenetic patterns and taxonomic
confusion in tribe Caryophylleae (Caryophyllaceae) with special
focus on generic boundaries. – Taxon 67: 83-112.
Madsen JE. 1989. 45. Cactaceae. – In: Harling G,
Andersson L (eds), Flora of Ecuador 35, Nord. J. Bot., Copenhagen, pp. 1-78.
Maekawa F. 1964. On the phylogeny in the Polygonaceae. – J. Jap. Bot.
39: 14-18.
Magin N. 1984. Die “Frucht” von
Pollichia campestris Aiton (Caryophyllaceae). – Bot.
Jahrb. Syst. 104: 455-467.
Maguire B. 1947. Studies in the Caryophyllaceae III: a
synopsis of the North American species of Arenaria, section
Eremogone Fenzl. – Bull. Torrey Bot. Club 74: 38-56.
Maguire B. 1951. Studies in the Caryophyllaceae V.
Arenaria in America north of Mexico: a conspectus. – Amer. Midl.
Natur. 46: 493-511.
Maguire B. 1958. Arenaria rossii and
some of its relatives in America. – Rhodora 60: 44-53.
Maheshwari P, Chopra RN. 1954. Polyembryony
in Opuntia dillenii L. – Curr. Sci. 23: 130-131.
Maheshwari P, Chopra RN. 1955. The structure
and development of the ovule and seed of Opuntia dillenii Haw. –
Phytomorphology 5: 112-122.
Maheshwari Devi H, Pulliah T. 1975. Life
history of Basella rubra Linn. and taxonomic status of the family Basellaceae. – J. Indian Bot.
Soc. 54: 154-166.
Mahrt M, Spellenberg R. 1995. Taxonomy of
Cyphomeris (Nyctaginaceae) based on
multivariate analyses of geographic variation. – Sida 16: 679-697.
Maihle NJ, Blackwell WH. 1978. A synopsis of
N American Corispermum (Chenopodiaceae). – Sida 7: 382-391.
Maiti GG, Dutta RM, Babu CR. 1981.
Aconogonon kuttiense (Polygonaceae) – a new species
from N. W. Himalaya. – J. Bombay Nat. Hist. Soc. 77: 303-305.
Maiti GG, Sikdar JL. 1985. A census to Polygonaceae of West Bengal. –
Indian J. For. 8: 187-198.
Majure LC, Puente R. 2014. Phylogenetic
relationships and morphological evolution in Opuntia s. str. and
closely related members of tribe Opuntieae. – Succ. Plant Res. 8: 9-30.
Majure LC, Puente R, Griffith MP, Judd WS,
Soltis PS, Soltis DE. 2012. Phylogeny of Opuntia s.s. (Cactaceae): clade delineation,
geographic origins, and reticulate evolution. – Amer. J. Bot. 99: 847-864.
Majure LC, Puente R, Griffith MP, Soltis DE,
Judd WS. 2013. Opuntia lilae, another Tacinga hidden in
Opuntia s.l. (Cactaceae). – Syst. Bot. 38:
444-450.
Malekmohammadi M, Akhani H, Borsch T. 2017.
Phylogenetic relationships of Limonium (Plumbaginaceae) inferred from
multiple chloroplast and nuclear loci. – Taxon 66: 1128-1146.
Mallingson F. 1922. Serodiagnostische
Untersuchungen über die Verwandtschaften innerhalb des Centrospermen-Astes des
Pflanzenreiches. – Bot. Arch. 1: 2-20.
Mandák B, Bímová K, Pyšek P, Štěpánek
J, Plačková I. 2005. Isoenzyme diversity in Reynoutria (Polygonaceae) taxa: escape from
sterility by hybridization. – Plant Syst. Evol. 253: 219-230.
Manfredi KP, Blunt JW, Cardellina JH, McMahon
JB, Pannell LL, Cragg GM, Boyd MR. 1991. Novel alkaloids from the tropical
plant Ancistrocladus abbreviatus inhibit cell killing by HIV-1 and
HIV-2. – J. Med. Chem. 34: 3402-3405.
Manhart JR, Rettig JH. 1994. Gene sequence
data. – In: Behnke H-D, Mabry TJ (eds), Caryophyllales: evolution and
systematics, Springer, Berlin, Heidelberg, New York, pp. 235-246.
Manning JC, Goldblatt P, Forest F. 2011.
Adenogramma natans, a remarkable new aquatic species from Western
Cape, South Africa. – Bothalia 41: 189-193.
Maradufu A, Ouma JH. 1978. A new chalcone as
a natural molluscicide from Polygonum senegalense. – Phytochemistry
17: 823-824.
Marburger JE. 1979. Glandular leaf structure
of Triphyophyllum peltatum (Dioncophyllaceae): a
“fly-paper” insect trapper. – Amer. J. Bot. 66: 404-411.
Marchant NG, Lowrie A. 1992. New names and
new combinations in 34 taxa of Western Australian tuberous and pygmy
Drosera. – Kew Bull. 47: 315-328.
Marchant NG, Aston HI, George AS. 1982. Droseraceae. – In: George AS
(ed), Flora of Australia 8, Australian Government Publ. Service, Canberra, pp.
9-66.
Marek S. 1954. Morphological and anatomical
features of the fruits of genera Polygonum L. and Rumex L.
and keys for their determination. – Monogr. Bot. 2: 77-161. [In Polish with
English summary]
Marek S. 1958. European genera of Polygonaceae in the light of
anatomical and morphological investigations on their fruits and seeds. –
Monogr. Bot. 6: 57-79. [In Polish with English summary]
Martin CE, Wallace RS. 2000. Photosynthetic
pathway variation in leafy members of two subfamilies of the Cactaceae. – Intern. J. Plant Sci.
161: 639-650.
Martin CE, Higley M, Wang W. 1988.
Ecophysiological significance of CO2-recycling via crassulacean acid
metabolism in Talinum calycinum Engelm. (Portulacaceae). – Plant
Physiol. 86: 562-568.
Martin PG, Dowd JM. 1984. The study of plant
phylogeny using amino acid sequences of ribulose-1,5-biphosphate carboxylase V.
Magnoliaceae, Polygonaceae and the concept of
primitiveness. – Aust. J. Bot. 32: 301-309.
Martínez-Crovetto R. 1967. Catalogo
preliminar de las Caryophyllaceae de la
Argentina y del Uruguay. – Bonplandia 2: 187-264.
Martínez Del Río C, Búrquez A. 1986.
Nectar production and temperature dependent pollination in Mirabilis
jalapa L. – Biotropica 18: 28-31.
Martínez-García J, McDonald JA. 1989.
Nowickea (Phytolaccaceae), a new genus
with two new species from Mexico. – Brittonia 41: 399-403.
Martis B de, Loi MC, Polo MB. 1986.
Contribution to a better understanding of the genus Tamarix in
Portugal. – Candollea 41: 293-298.
Masson R, Kadereit G. 2013. Phylogeny of
Polycnemoideae (Amaranthaceae): implications for
biogeography, character evolution and taxonomy. – Taxon 62: 100-111.
Mata R, Navarrete A, Alvarez L,
Pereda-Miranda R, Delgado G, Romo de Vivar A. 1987. Flavonoids and terpenoids
of Chenopodium graveolens. – Phytochemistry 26: 191-193.
Mathew B. 1989. The genus Lewisia.
– Royal Botanic Gardens, Kew.
Matta R, McLaughlin JL. 1982. Cactus
alkaloids. A comprehensive tabular summary. – Rev. Latinoameric. Quím. 12:
95-117.
Mattfeld J. 1921. Enumeratio specierum
generis Minuartia (L.) emend. Hier. – Engl. Bot. Jahrb. Syst. 57,
Beibl. 126: 27-33.
Mattfeld J. 1922a. Beitrag zur Kenntnis der
systematischen Gliederung und geographischen Verbreitung der Gattung
Minuartia. – Engl. Bot. Jahrb. Syst. 57, Beibl. 127: 13-63.
Mattfeld J. 1922b. Geographisch-genetische
Untersuchungen über die Gattung Minuartia (L.) Hiern. – Feddes
Repert. Beih. 15: 1-228.
Mattfeld J. 1934. Nachtrag zu den Caryophyllaceae. – In:
Engler A (†), Pax F, Harms H (eds), Die natürlichen Pflanzenfamilien, 2.
Aufl., Bd. 16c, W. Engelmann, Leipzig, pp. 365-367.
Mattfeld J. 1938. Über eine angebliche
Drymaria Australiens nebst Bemerkungen über die Staminaldrüsen und
die Petalen der Caryophyllaceae. – Feddes
Repert. Beih. 100: 147-164.
Matthew OR, Edwards EJ. 2009. Anatomical
variation in Cactaceae and
relatives: trait lability and evolutionary innovation. – Amer. J. Bot. 96:
391-408.
Matthews JF, Levins PA. 1986. The systematic
significance of seed morphology in Portulaca (Portulacaceae) under scanning
electron microscopy. – Syst. Bot. 11: 302-308.
Matthews JF, Faircloth WR, Allison JR. 1991.
Portulaca biloba Urban (Portulaceae), a species new to the United
States. – Syst. Bot. 16: 736-740.
Mauritzon J. 1933. Über die Embryologie der
Turneraceae
und Frankeniaceae. – Bot.
Not. 86: 543-554.
Mauritzon J. 1934. Ein Beitrag zur
Embryologie der Phytolaccaceen und Cactaceen. – Bot. Not. 1934: 111-135.
Mauseth JD. 1982-1984. Introduction to cactus
anatomy 1-11. – Cact. Succ. J. (Los Angeles) 54: 263-266; 55: 18-21, 42,
84-89, 113-118, 171-175, 272-276; 56: 33-37, 131-135, 181-184, 212-216,
250-255.
Mauseth JD. 1990. Continental drift, climate,
and the evolution of cacti. – Cact. Succ. J. (Los Angeles) 62: 301-308.
Mauseth JD. 1999. Anatomical adaptations to
xeric conditions in Maihuenia (Cactaceae), a relictual,
leaf-bearing cactus. – J. Plant Res. 112: 307-315.
Mauseth JD. 2004. Wide-band tracheids are
present in almost all species of Cactaceae. – J. Plant Res. 117:
69-76.
Mauseth JD. 2005. Anatomical characters,
other than wood, in subfamily Opuntioideae (Cactaceae). – Haseltonia 11:
113-125.
Mauseth JD. 2006a. Structure-function
relationships in highly modified shoots of Cactaceae. – Ann. Bot. 98:
901-926.
Mauseth JD. 2006b. Blossfeldia lacks
cortical bundles and persistent epidermis; is it basal within Cactoideae? –
Bradleya 24: 73-82.
Mauseth JD. 2006c. Wood in the cactus
subfamily Opuntioideae has extremely diverse structure. – Bradleya 24:
93-106.
Mauseth JD. 2007. Tiny but complex foliage
leaves occur in many “leafless” cacti (Cactaceae). – Intern. J. Plant
Sci. 168: 845-853.
Mauseth JD, Landrum JV. 1997. Relictual
vegetative anatomical characters in Cactaceae: the genus
Pereskia. – J. Plant Res. 110: 55-64.
Mauseth JD, Plemons BJ. 1995. Developmentally
variable, polymorphic woods in cacti. – Amer. J. Bot. 82: 1199-1205.
Mauseth JD, Plemons-Rodriguez BJ. 1998.
Evolution of extreme xeromorphic characters in wood: a study of nine
evolutionary lines in Cactaceae.
– Amer. J. Bot. 85: 209-218.
Mauseth JD, Sajeva M. 1992. Cortical bundles
in the persistent, photosynthetic stems of cacti. – Ann. Bot. (Oxford), n.s.
70: 317-324.
Mauseth JD, Uozumi Y, Plemons BJ, Landrum JK.
1995. Structural and systematic study of an unusual tracheid type in cacti. –
J. Plant Res. 108: 517-526.
Mauseth JD, Terrazas T, Loza-Cornejo S. 1998.
Anatomy of relictual members of subfamily Cactoideae, IOS Group 1a (Cactaceae). – Bradleya 16:
31-43.
Mayer MS, Williams LM, Rebman JP. 2000.
Molecular evidence for the hybrid origin of Opuntia prolifera (Cactaceae). – Madroño 47:
109-115.
Mayol M. 1999. A synopsis of Silene
subgenus Petrocoptis (Caryophyllaceae). – Taxon
48: 471-482.
Mayonde SG, Cron GV, Gaskin JF, Byrne MJ.
2015. Evidence of Tamarix hybrids in South Africa, as inferred by
nuclear ITS and plastid trnS-trnG DNA seuqnces. – South Afr. J. Bot. 96:
122-131.
Mears JA. 1967. Revision of
Guilleminea (Bryulinea) including Gossypianthus. –
Sida 3: 137-152.
Mears JA. 1982. A summary of
Blutaparon Rafinesque including species earlier known as
Philoxerus R. Brown (Amaranthaceae). – Taxon 31:
111-117.
Mehra PN, Malik CP. 1963. Cytology of some
Indian Chenopodiaceae. – Caryologia 16: 67-84.
Meikle RD. 1978. A key to
Commicarpus. – Notes Roy. Bot. Gard. Edinb. 36: 235-249.
Meikle RD. 1979. Supplementary notes on
Commicarpus (Nyctaginaceae). – Kew Bull.
34: 341-343.
Meikle RD. 1983. Additional notes on
Commicarpus (Nyctaginaceae). – Kew Bull.
38: 481-484.
Meikle RD, Hewson HJ. 1984. Nyctaginaceae. – In: George AS
(ed), Flora of Australia 4, Australian Government Publ. Service, Canberra, pp.
5-18.
Meimberg H. 2002. Molekular-Systematische
Untersuchungen an den Familien Nepenthaceae und Ancistrocladaceae sowie
verwandter Taxa aus der Unterklasse Caryophyllidae s.l. – Ph.D. diss.,
Universität München, Germany.
Meimberg H, Heubl G. 2006. Introduction of a
nuclear marker for phylogenetic analysis of Nepenthaceae. – Plant Biol. 8:
831-840.
Meimberg H, Dittrich P, Bringmann G, Schlauer
J, Heubl G. 2000. Molecular phylogeny of Caryophyllales s.l. based on
matK sequences with special emphasis on carnivorous taxa. – Plant
Biol. 2: 218-228.
Meimberg H, Wistuba A, Dittrich P, Heubl G.
2001. Molecular phylogeny of Nepenthaceae based on cladistic
analysis of plastid trnK intron sequence data. – Plant Biol. 3:
164-175.
Melikian AP. 1968. The systematic position of
the families Buxaceae
and Simmondsiaceae. – Bot.
Žurn. 53: 1043-1047. [In Russian with English summary]
Melo-de-Pinna GF. 2009. Non-lignified
parenchyma in Cactaceae and Portulacaceae. – Bot. J. Linn.
Soc. 159: 322-329.
Melo-de-Pinna GFA, Ogura AS, Arruda ECP, Klak
C. 2014. Repeated evolution of endoscopic peripheral vascular bundles in
succulent leaves of Aizoaceae (Caryophyllales). – Taxon 63:
1037-1052.
Melville R. 1952. Tianthema
pentandra L. and some related species. – Kew Bull. 1952: 261-269.
Melzer H. 1971. Oxybaphus
nyctagineus (Michx.) Sweet, eine neue Adventivpflanze in der Flora
Österreichs. – Österr. Bot. Zeitschr. 119: 564-566.
Melzheimer V. 1974. Bemerkungen zur Cytologie
einiger Arten der Gattung Silene L. von der Balkan-Halbinsel. –
Candollea 29: 337-343.
Melzheimer V. 1975. Pollensystematische
Untersuchungen in der Gattung Silene L. (Caryophyllaceae). – Bot.
Jahrb. Syst. 95: 215-225.
Melzheimer V. 1977. Biosystematische Revision
einiger Silene-Arten (Caryophyllaceae) der
Balkanhalbinsel (Griechenland). – Bot. Jahrb. Syst. 98: 1-92.
Melzheimer V. 1980. Revision einiger
balkanischer Arten von Silene Sect. Inflatae (Caryophyllaceae). – Bot.
Jahrb. Syst. 101: 153-190.
Melzheimer V, Damboldt J. 1973. Zur
Morphologie und cytology tetraploider Sippen von Silene vulgaris (Caryophyllaceae). –
Willdenowia 7: 83-100.
Mendoza F JM, Wood JRI. 2013. Taxonomic
revision of Talinum (Talinaceae) in Bolivia with a note
on the occurrence of Phemeranthus (Montiaceae). – Kew Bull. 68:
233-247.
Meregalli M. 1985. Il genere
Gymnocalycium Pfeiffer. – Piante Grasse 5: 5-63.
Meregalli M, Metzing D, Kiesling R, Tosatto
S, Caramiello R. 2002. Systematics of the Gymnocalycium
paraguayense-fleischerianum group (Cactaceae): morphological and
molecular data. – Candollea 57: 299-315.
Meregalli M, Ercole E, Rodda M. 2010.
Molecular phylogeny vs morphology: shedding light on the infrageneric
classification of Gymnocalycium (Cactaceae). – Schumannia 6:
257-275.
Merxmüller H. 1950. Untersuchungen über
eine alpine Cerastien-gruppe. – Ber. Bayrisch. Bot. Ges. 28: 219-238.
Merxmüller H, Grau J. 1967.
Moehringia-Studien. – Mitt. Bot. Staatssamml. München 6:
257-273.
Metcalfe CR. 1951. The anatomical structure
of the Dioncophyllaceae in
relation to the taxonomic affinities of the family. – Kew Bull. 1951:
351-368.
Metzing D. 1992. Zur Benennung einiger
Gymnocalycium-Untergattungen und -Sektionen. – Gymnos 9: 3-6.
Metzing D, Kiesling R. 2006. Notes on the
diversity, biology, and taxonomy of Frailea (Cactaceae). – Bradleya 24:
115-128.
Metzing D, Kiesling R. 2007.
Winterocereus (Cactaceae) is the correct name for
Hildewintera. – Taxon 56: 226-228.
Metzing D, Kiesling R. 2008. The study of
cactus evolution: the pre-DNA era. – Haseltonia 14: 6-25.
Metzing D, Thiede J. 2001. Testa sculpture in
the genus Frailea (Cactaceae). – Bot. J. Linn. Soc.
137: 65-70.
Metzing D, Meregalli M, Kiesling R. 1995. An
annotated checklist of the genus Gymnocalycium Pfeiffer ex Mittler (Cactaceae). – Allionia 33:
181-228.
Meulen-Bruijns C van der. 1976. The vascular
pattern in the flower of some Mesembryanthemaceae: Aptenia cordifolia
and Dorotheanthus bellidiformis. – Blumea 23: 189-201.
Meunier A. 1890. Les téguments séminaux des
cyclospermées. – Cellule 6: 299-392.
Meyberg M, Kristen U. 1981. The nectaries of
Aptenia cordifolia. Ultrastructure, translocation of
14C-labelled sugars, and a possible pathway of secretion. –
Zeitschr. Pflanzenphys. 104: 139-147.
Mikesell JE. 1979. Anomalous secondary
thickening in Phytolacca americana L. (Phytolaccaceae). – Amer. J.
Bot. 66: 997-1005.
Milby TH. 1980. Studies in the floral anatomy
of Claytonia. – Amer. J. Bot. 67: 1046-1050.
Miller JM. 1988. Floral pigments and
phylogeny in Echinocereus (Cactaceae). – Syst. Bot. 13:
173-183.
Miller JM, Chambers KL. 1977. Chromosome
numbers and relationships of Claytonia saxosa and C.
arenicola (Portulacaceae). – Madroño 43:
62-63.
Miller JM, Chambers KL. 1993. Nomenclatural
changes and new taxa in Claytonia (Portulacaceae). – Novon 3:
268-272.
Miller JM, Chambers KL. 2006. Systematics of
Claytonia (Portulacaceae). – Syst. Bot.
Monogr. 78: 1-236.
Miller JM, Chambers KL, Fellows CE. 1984.
Cytogeographic patterns and relationships in the Claytonia sibirica
complex (Portulacaceae). –
Syst. Bot. 9: 266-271.
Miller RM. 1979. Some occurrences of
vesicular-arbuscular mycorrhiza in natural and disturbed ecosystems of the Red
Desert. – Can. J. Bot. 57: 619-623.
Miller SL. 1994. Phylogenetic analyses of
selected species of the genus Silene (Caryophyllaceae) using
morphological characters and nucleotide sequences of the ITS region of nuclear
ribosomal DNA. – Masters thesis, Duke University, Durham, North Carolina.
Minuto L, Fior S, Roccotiello E, Casazza G.
2006. Seed morphology in Moehringia L. and its taxonomic significance
in comparative studies within the Caryophyllaceae. – Plant
Syst. Evol. 262: 189-208.
Minuto L, Roccotiello E, Casazza G. 2011. New
seed morphological features in Moehringia L. (Caryophyllaceae) and their
taxonomic and ecological significance. – Plant Biosystems 145: 60-67.
Misra AN, Tiwari HP. 1971. Constituents of
roots of Boerhavia diffusa. – Phytochemistry 10: 3318-3319.
Mihöfer A. 2011. Carnivorous pitcher plants:
insights in an old topic. – Phytochemistry 72: 1678-1682.
Mitra JN. 1956. On the systematic position of
the family Cactaceae as based
from the studies of the morphological characters of the flowers of
Solenicereus grandiflorus Brit. et Rose (Cereus grandiflora)
and Opuntia dillenii Haw. – Sci. Cult. 21: 460-461.
Mitroiu N. 1971. Contribution à la
connaissance de la morphologie du pollen des Molluginacées. – Rev. Roum.
Biol. Bot. 16: 91-96.
Moharrek F, Kasempour Osaloo S, Assadi M.
2014. Molecular phylogeny of Plumbaginaceae with emphasis on
Acanthlimon Boiss. based on nuclear and plastid DNA sequences in Iran.
– Biochem. Syst. Ecol. 57: 117-127.
Moharrek F, Kazempour-Osaloo S, Assadi M,
Feliner GN. 2017. Molecular phylogenetic evidence for a wide circumscription of
a characteristic Irano-Turanian element: Acantholimon (Plumbaginaceae: Limonioideae).
– Bot. J. Linn. Soc. 184: 366-386.
Mondal MS. 1997. Pollen morphology and
systematic relationship of the family Polygonaceae. – Botanical
Survey of India, Calcutta, India.
Monje PV, Baran EJ. 2002. Characterization of
calcium oxalates generated as biominerals in cacti. – Plant Physiol. 128:
707-713.
Monnier P. 1968. Synopsis du genre
Spergularia (Pers.) Presl au Maroc. – Nat. Monspel. Ser. Bot. 19:
87-113.
Monoszon MK. 1964. Pollen of halophytes and
xerophytes of the Chenopodiaceae family in the periglacial zone of the Russian
plain. – Pollen Spores 6: 147-155.
Moore AJ, Dillenberger MS. 2017. A conspectus
of the genus Cherleria (Minuartia s.l., Caryophyllaceae). –
Willdenowia 47: 5-14.
Moore AJ, Kadereit JW. 2013. The evolution of
substate differentiation in Minuartia series Laricifoliae (Caryophyllaceae) in the
European Alps: in situ origin or repeated colonization? – Amer. J. Bot. 100:
2412-2425.
Moore DM, Yates B. 1974. Armeria L.
in South America. – Bot. Not. 127: 183-192.
Moran J, Booth WE, Charles JK. 1999. Aspects
of pitcher morphology and spectral characteristics of six Bornean
Nepenthes pitcher plant species: implications for prey capture. –
Amer. J. Bot. 83: 521-528.
Moran R. 1965. Revisión de
Bergerocactus. – Cact. Suc. Mex. 10: 51-59.
Moreno NC, Amarilla LD, Las Peñas ML,
Bernardello G. 2015. Molecular cytogenetic insights into the evolution of the
epiphytic genus Lepismium (Cactaceae) and related genera. –
Bot. J. Linn. Soc. 177: 263-277.
Morton CM, Karol KG, Chase MW. 1997.
Taxonomic affinities of Physena (Physenaceae) and
Asteropeia (Theaceae). – Bot. Rev.
63: 231-239.
Mosaferi S, Keshavarzi M. 2011.
Micromorphological study of Polygonaceae tribes in Iran. –
Phytol. Balcan. 17: 89-100.
Mosco A, Zanovello C. 2002.
Thelocactus. An introduction to the genus/Un’ introduzione al
genere. – Cactus & Co. 6: 144-171.
Moss CE. 1954. The species of
Arthrocnemum and Salicornia in southern Africa. – J. South
Afr. Bot. 20: 1-22.
Mostafavi G, Assadi M, Nejadsattari T,
Sharifnia F, Mehregan I. 2013. Seed micromorphological survey of the
Minuartia species (Caryophyllaceae) in Iran. –
Turk. J. Bot. 37: 446-454.
Mosti S, Lewke Bandara N, Papini A. 2011.
Further insights and new combinations in Aylostera (Cactaceae) based on molecular and
morphological data. – Pakistan J. Bot. 43: 2769-2785.
Mosyakin SL. 1988. Krytychnyi pereglyad vydiv
rodu Corispermum flory Ukayiny. – Ukrayns’k Bot. Žurn. 45:
19-23.
Mosyakin SL. 1994. New infrageneric taxa of
Corispermum L. (Cenopodiaceae). – Novon 4: 153-154.
Mosyakin SL. 1995. New taxa of
Corispermum L. (Chenopodiaceae), with preliminary comments on the
taxonomy of the genus in N America. – Novon 5: 340-354.
Mosyakin SL. 1997. New subsections in
Corispermum L. (Chenopodiaceae). – Thaiszia 7: 9-15.
Mosyakin SL. 2002. The system and
phytogeography of Chenopodium subg. Blitum I. Hiitonen
(Chenopodiaceae). – Ukrayins’kyi Bot. Žurn. 59: 696-701.
Mosyakin SL. 2005. On the origin of dioecious
amaranths (Amaranthus L., Amaranthaceae Juss.). –
Ukrayins’kyi Bot. Žurn. 62: 3-9.
Mosyakin SL. 2013. New nomenclatural
combinations in Blitum, Oxybasis, Chenopodiastrum,
and Lipandra (Chenopodiaceae). – Phytoneuron 2013-56: 1-8.
Mosyakin SL, Clemants SE. 1996. New
infrageneric taxa and combinations in Chenopodium L. (Chenopodiaceae).
– Novon 6: 398-403.
Mosyakin SL, Clemants SE. 2002. New
nomenclatural combinations in Dysphania R. Br. (Chenopodiaceae): taxa
occurring in North America. – Ukrayins’kyi Bot. Žurn. 59: 380-385.
Mosyakin SL, Clemants SE. 2008. Further
transfers of glandular-pubescent species from Chenopodium subg.
Ambrosia to Dysphania (Chenopodiaceae). – J. Bot. Res.
Inst. Texas 2: 425-431.
Mosyakin SL, Robertson KR. 1996. New
infrageneric taxa and combinations in Amaranthus (Amaranthaceae). – Ann. Bot.
Fenn. 33: 275-281.
Mottram R. 1990. A contribution to a new
classification of the cactus family and index of suprageneric and supraspecific
taxa. – Whitestone Gardens, Thirsk, England.
Mottram R. 2001. Rimacactus, a new
genus of Cactaceae. – Bradleya
19: 75-82.
Mottram R. 2006. Osservazioni
sulle/Observations in Borzicactinae. – Cactus & Co. 10: 183-190.
Mozaffarian V. 2012. A revision of
Polygonum L. sensu lato (Polygonaceae) in Iran. – Iran.
J. Bot. 18: 159-174.
Müller K. 1908. Beiträge zur Systematik der
Aizoaceen. – Engl. Bot. Jahrb. 42, Beiblatt 97: 54-97.
Müller K, Borsch T. 2005a. Phylogenetics of
Amaranthaceae based on
matK/trnK sequence data – evidence from parsimony,
likelihood, and Bayesian analysis. – Ann. Missouri Bot. Gard. 92: 66-102.
Müller K, Borsch T. 2005b. Multiple origins
of a unique pollen feature: stellate pore ornamentation in Amaranthaceae. – Grana 44:
266-281.
Munshi AH, Javeid GN. 1986. Systematic
studies in Polygonaceae of
Kashmir Himalaya. – J. Ecol. Taxon. Bot., Add. Ser. 2(IV), Syringer.
Murakeözy EP, Aïnouche A, Meudec A,
Deslandes E, Poupart N. 2007. Phylogenetic relationships and genetic diversity
of the Salicornieae (Chenopodiaceae) native to the Atlantic coasts of France.
– Plant Syst. Evol. 264: 217-237.
Musa DA, Akaydin GP. 2004. Three new species
with two-flowered spikelets in Acantholimon (Plumbaginaceae) from East
Anatolia, Turkey. – Bot. J. Linn. Soc. 144: 497-505.
Musa DA, Galíp A. 2005. A new species of
Acantholimon Boiss. sect. Glumaria Boiss. (Plumbaginaceae) from Elaziğ,
Turkey. – Bot. J. Linn. Soc. 149: 351-356.
Naciri Y, Pasquier P-ED, Lundberg M,
Jeanmonod D, Oxelman B. 2017. A phylogenetic circumscription of Silene
sect. Siphonomorpha (Caryophyllaceae) in the
Mediterranean Basin. – Taxon 66: 91-108.
Nageshwar G, Radhakrishnaiah M. 1993.
Affinities of Basella L. – Feddes Repert. 104: 241-244.
Nair NC, Nair VJ. 1961. Studies on the
morphology of some members of the Nyctaginaceae I. Nodal anatomy
of Boerhavia. – Proc. Indian Acad. Sci., Sect. B, 54: 281-294.
Nair PKK, Khan HA. 1965. Pollen grains of
Indian plants 7. Nyctaginaceae. – Bull. Lucknow
Natl. Bot. Gard. 111: 1-13.
Nair PKK, Rehman K, Saxena AK. 1976.
Contribution to the pollen morphology of Indian Polygonaceae. – J. Palynol. 12:
1-18.
Nakai T. 1926. A new classification of
Linnaean Polygonum. – Rigakkai [Sci. World] 24: 289-301. [In
Japanese]
Narayana HS. 1962. Seed structure in Aizoaceae. – In: Maheshwari P,
Johri BM, Vasil IK (eds), Proceedings of the summer school of botany held June
2-15, 1960, at Darjeeling, Government of India, New Delhi, pp. 220-230.
Narayana HS, Lodha BC. 1963. Embryology of
Orygia decumbens Forssk. (Aizoaceae). – Phytomorphology 13:
54-59.
Narayana HS, Lodha BC. 1972. Embryology of
Glinus lotoides Linn. – Proc. Indian Acad. Sci., Ser. B, 75:
77-85.
Narayana PS, Narayana LL. 1986. The
embryology of Stegnospermataceae, with a
discussion on its status, affinities, and systematic position. – Plant Syst.
Evol. 154: 137-146.
Narayana PS, Narayana LL. 1988. Systematic
position of Gisekia L.: a numerical assessment. – Feddes Repert. 99:
189-193.
Navajas-Perez R, Herrán R de la, López
González G, Jamilena M, Lozano R, Ruiz Rejón C, Ruiz Rejón M, Garrido-Ramos
MA. 2005. The evolution of reproductive systems and sex-determining mechanisms
within Rumex (Polygonaceae) inferred from
nuclear and chloroplastidial sequence data. – Mol. Biol. Evol. 22:
1929-1939.
Negron-Ortiz V. 2007. Chromosome numbers,
nuclear DNA content, and polyploidy in Consolea (Cactaceae), an endemic cactus of the
Caribbean Islands. – Amer. J. Bot. 94: 1360-1370.
Nelson B, Prance GT. 1984. Observations on
the pollination of Rhabdodendron macrophyllum (Spr. ex Benth.) Huber.
– Acta Amazonica 14: 411-426.
Nelson EA, Sage TL, Sage RF. 2005. Functional
leaf anatomy of plants with crassulacean acid metabolism. – Funct. Plant
Biol. 32: 409-419.
Nepokroeff M, Wagner WL, Zimmer EA, Weller
SG, Sakai AK, Rabeler RK. 2001. Origin of the Hawaiian subfam. Alsinoideae and
preliminary relationships in Caryophyllaceae inferred from
matK and trnL C-F sequence data. – Botany 2001: The annual
meeting of the Botanical Society of America, in Albuquerque, New Mexico,
website https://urlproxy.sunet.se/canit/urlproxy.php?_q=aHR0cDovL3d3dy5ib3RhbnkyMDAxLm9yZy9zZWN0aW9uMTIvYWJzdHJhY3RzLzE5Ni5zaHRtbA%3D%3D&_s=ZGVmYXVsdA%3D%3D&_c=61d52405&_r=c3Utc2U%3D
Nepokroeff M, Wagner WL, Rabeler RK, Zimmer
EA, Weller SG, Sakai AK. 2002. Relationships within Caryophyllaceae inferred from
molecular sequence data. – Botany 2002: The annual meeting of the Botanical
Society of America, in Madison, Wisconsin, website
https://urlproxy.sunet.se/canit/urlproxy.php?_q=aHR0cDovL3d3dy5ib3RhbnkyMDAyLm9yZy9zeW1wb3MxMi9hYnN0cmFjdHMvNi5zaHRtbA%3D%3D&_s=ZGVmYXVsdA%3D%3D&_c=139adc2d&_r=c3Utc2U%3D
Neumann M. 1935. Die Entwicklung des Pollens,
der Samenanlage und des Embryosackes von Pereskia amapola var.
argentina. – Österr. Bot. Zeitschr. 84: 1-30.
Neumayer H. 1923. Die Frage der
Gattungsabgrenzung innerhalb der Silenoideen. – Verh. Zool.-Bot. Ges. Wien
72: (53)-(59).
Ng SY, Philipson WR, Walker JRL. 1975.
Hectorellaceae – member of the Centrospermae. – New Zealand J. Bot. 13:
567-570.
Nicola MV, Pozner R. 2013. A new species of
Minuartia (Caryophyllaceae) restricted to
the high Andes of South America. – Phytotaxa 111: 53-56.
Niedenzu F. 1895a. Frankeniaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien III(6), W. Engelmann,
Leipzig, pp. 283-289.
Niedenzu F. 1895b. Tamaricaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien III(6), W. Engelmann,
Leipzig, pp. 289-298.
Niedenzu F. 1925a. Frankeniaceae. – In: Engler A,
Gilg E (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 21, W.
Engelmann, Leipzig, pp. 276-281.
Niedenzu F. 1925b. Tamaricaceae. – In: Engler A,
Gilg E (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 21, W.
Engelmann, Leipzig, pp. 282-289.
Niesler IM, Hartmann HEK. 2007. Nectaries in
Aizoaceae: the case of
Glottiphyllum. – Bradleya 25: 177-186.
Nieto Feliner G. 1994. Growth-form and
taxonomy in Arenaria sect. Plinthine (Caryophyllaceae). – Taxon
43: 45-50.
Nieto Feliner G, Aguilar JF, Rosselloa JA.
2001. A new species of Armeria (Plumbaginaceae) from southern
Spain with molecular and morphometric evidence on its origin. – Bot. J. Linn.
Soc. 135: 71-84.
Nieto Feliner G, Aguilar JF, Rosselló JA.
2002. Reticulation or divergence: the origin of a rare serpentine endemic
assessed with chloroplast, nuclear and RAPD markers. – Plant Syst. Evol. 231:
19-38.
Nieto Feliner G, Larena BG, Aguilar JF. 2004.
Finer-scale geographical structure, intra-individual polymorphism and
recombination in nuclear ribosomal internal transcribed spacers in
Armeria (Plumbaginaceae). – Ann. Bot.,
93: 189-200.
Nijs CJM den 1984. Biosystematic studies of
the Rumex acetosella complex (Polygonaceae) VIII. A taxonomic
revision. – Feddes Repert. 95: 43-66.
Niketić M, Stevanovic V. 2007. A new species
of Heliosperma (Caryophyllaceae) from Serbia
and Montenegro. – Bot. J. Linn. Soc. 154: 55-63.
Niketić M, Siljak-Yakovlev S, Frajman B,
Lazarević M, Stevanović B, Tomović G, Stevanović V. 2013. Towards resolving
the systematics of Cerastium subsection Cerastium (Caryophyllaceae): a
cytogenetic approach. – Bot. J. Linn. Soc. 172: 205-224.
Nilsson Ö. 1966a. Studies in Montia
L., Claytonia L. and allied genera I. Two new genera, Mona
and Paxia. – Bot. Not. 119: 265-285. – Corrections and additions:
Bot. Not. 119: 469.
Nilsson Ö. 1966b. Studies in Montia
L., Claytonia L. and allied genera II. Some chromosome numbers. –
Bot. Not. 119: 464-468.
Nilsson Ö. 1967. Studies in Montia
L., Claytonia L. and allied genera III. Pollen morphology. – Grana
Palynol. 7: 277-363.
Nilsson Ö. 1970. Studies in Montia
L., Claytonia L. and allied genera IV. The genus Crunocallis
Rydb. – Bot. Not. 123: 119-148.
Nilsson Ö. 1971a. Studies in Montia
L., Claytonia L. and allied genera V. The genus Montiastrum
(Gray) Rydb. – Bot. Not. 124: 87-121.
Nilsson Ö. 1971b. Studies in Montia
L., Claytonia L. and allied genera VI. The genera Limnalsine
Rydb. and Maxia Ö. Nilss. – Bot. Not. 124: 187-207.
Nilsson Ö. 1977. Studies in Montia
L., Claytonia L. and allied genera. – Ph.D. diss., University of
Uppsala, Sweden.
Nishimoto Y, Ohnishi O, Hasegawa M. 2003.
Topological incongruence between nuclear and chloroplast DNA trees suggesting
hybridization in the urophyllum group of the genus Fagopyrum
(Polygonaceae). – Genes
Genet. Syst. 78: 139-153.
Nobel PS. 1982. Low-temperature tolerance and
cold hardening of cacti. – Ecology 63: 1650-1656.
Nobel PS. 1988. Environmental biology of
agaves and cacti. – Cambridge University Press, Cambridge.
Nobel PS (ed). 2002. Cacti: biology and uses.
– University of California Press, Berkeley.
Nobel PS, Hartsock TL. 1986. Leaf and stem
CO2 uptake in the three subfamilies of the Cactaceae. – Plant Physiol. 80:
913-917.
Nobs MA. 1981. Evidence for apomixis in
Atriplex (Chenopodiaceae). – Carnegie Inst. Washington Year Book 81:
92.
Nowicke JW. 1968. Palynotaxonomic study of
the Phytolaccaceae. – Ann.
Missouri Bot. Gard. 55: 294-364.
Nowicke JW. 1970. Pollen morphology in the Nyctaginaceae I. Nyctagineae
(Mirabileae). – Grana 10: 79-88.
Nowicke JW. 1975. Pollen morphology in the
order Centrospermae. – Grana 15: 51-77.
Nowicke JW. 1994. Pollen morphology and exine
ultrastructure. – In: Behnke H-D, Mabry TJ (eds), Caryophyllales: evolution and
systematics, Springer, Berlin, Heidelberg, New York, pp. 167-221.
Nowicke JW. 1996. Pollen morphology, exine
structure and the relationships of Basellaceae and Didiereaceae to Portulacaceae. – Syst. Bot.
21: 187-208.
Nowicke JW, Luikart TJ. 1971. Pollen
morphology of the Nyctaginaceae II. Colignonieae,
Boldoeae, and Leucastereae. – Grana 11: 145-150.
Nowicke JW, Skvarla JJ. 1977. Pollen
morphology and the relationship of the Plumbaginaceae, Polygonaceae, and Primulaceae to the
order Centrospermae. – Smithsonian Contr. Bot. 37: 1-64.
Nowicke JW, Skvarla JJ. 1984. Pollen
morphology and relationships of Simmondsia chinensis to the order
Euphorbiales. – Amer. J. Bot. 71: 210-215.
Nñez Mariel C, Engleman EM, Márquez Guzmán
J. 2001. Embriología de Pachycereus militaris (Audot) Hunt (Cactaceae). – Bol. Soc. Bot.
México 68: 5-13.
Nyananyo BL. 1986a. Taxonomic significance of
stomatal complex in the Portulacaceae. – Feddes
Repert. 97: 763-766.
Nyananyo BL. 1986b. The systematic position
of the genus Calyptrotheca Gilg (Portulacaceae). – Feddes
Repert. 97: 767-770.
Nyananyo BL. 1986c. The taxonomic position of
Talinella Baillon (Portulacaceae). – Feddes
Repert. 97: 771-773.
Nyananyo BL. 1986d. Tribal and generic
relationship and classification in the Portulacaceae (Centrospermae).
– Ph.D. diss. University of Reading, England.
Nyananyo BL. 1987. Seed coat morphology in
Calandrinia (Portulacaceae) and its taxonomic
significance. – J. Plant Sci. Res. 3: 93-97.
Nyananyo BL. 1988a. The systematic
significance of seed morphology and anatomy in the Portulacaceae (Centrospermae).
– Folia Geobot. Phytotaxon. 23: 275-279.
Nyananyo BL. 1988b. Leaf anatomical studies
in the Portulacaceae
(Centrospermae), with regards to photosynthetic pathways. – Folia Geobot.
Phytotaxon. 23: 99-101
Nyananyo BL. 1990. Tribal and generic
relationship in the Portulacaceae (Centrospermae).
– Feddes Repert. 101: 237-241.
Nyananyo BL. 1992. Pollen morphology in the
Portulacaceae
(Centrospermae). – Folia Geobot. Phytotaxon. 27: 387-400.
Nyananyo BL, Heywood VH. 1987. A new
combination in Lyallia (Portulacaceae). – Taxon 36:
640-641.
Nyananyo BL, Okoli BE. 1987. Cytological and
morphological studies on Nigerian species of Portulaca (Portulacaceae) in relation to
their taxonomy. – Feddes Repert. 98: 583-587.
Nyffeler R. 1998. The genus
Uebelmannia Buining (Cactaceae: Cactoideae). – Bot.
Jahrb. Syst. 120: 145-163.
Nyffeler R. 2000. Should Pfeiffera
be resurrected? – Cactaceae Syst. Init. 10: 10-11.
Nyffeler R. 2001. What about the tribe
Notocacteae? – Cact. Syst. Init. 12: 25-27.
Nyffeler R. 2002. Phylogenetic relationships
in the cactus family (Cactaceae)
based on evidence from trnK/matK and
trnL-trnF sequences. – Amer. J. Bot. 89: 312-326.
Nyffeler R. 2007. The closest relatives of
cacti: insights from phylogenetic analyses of chloroplast and mitochondrial
sequences with special emphasis on relationships in the tribe Anacampseroteae.
– Amer. J. Bot. 94: 89-101.
Nyffeler R, Eggli U. 1997. Comparative stem
anatomy and systematics of Eriosyce sensu lato (Cactaceae). – Ann. Bot. (Oxford),
n.s. 80: 767-786.
Nyffeler R, Eggli U. 2010a. Disintegrating Portulacaceae: a new familial
classification of the suborder Portulacineae (Caryophyllales) based on
molecular and morphological data. – Taxon 59: 227-240.
Nyffeler R, Eggli U. 2010b. An up-to-date
familial and suprafamilial classification of succulent plants. – Bradleya 28:
125-144.
Nyffeler R, Eggli U. 2010c. A farewell to
dated ideas and concepts – molecular phylogenetics and a revised suprageneric
classification of the family Cactaceae. – Schumannia 6:
109-149.
Nyffeler R, Eggli U, Ogburn M, Edwards E.
2008. Variations on a theme: repeated evolution of succulent life forms in the
Portulacineae (Caryophyllales). – Haseltonia
14: 26-36.
O’Callaghan M. 1992. The ecology and
identification of the southern African Salicorniae (Chenopodiaceae). – South
Afr. J. Bot. 58: 430-439.
Ocampo G. 2002. Transferencia de tres
especies mexicanas de Talinum Adans. a Phemeranthus Raf. (Portulacaceae). – Acta Bot.
Mex. 59: 75-80.
Ocampo G. 2003. Una combinación nueva en
Phemeranthus (Portulacaceae). – Acta Bot.
Mex. 63: 55-57.
Ocampo G. 2013. Morphological
characterization of seeds in Portulacaceae. – Phytotaxa
141: 1-24.
Ocampo G. 2015. Systematic implications of
seed morphological diversity in Portulacaceae (Caryophyllales). – Plant
Syst. Evol. 301: 1215-1226.
Ocampo G, Columbus JT. 2010. Molecular
phylogenetics of suborder Cactineae (Caryophyllales), including
insights into photosynthetic diversification and historical biogeography. –
Amer. J. Bot. 97: 1827-1847.
Ocampo G, Columbus JT. 2012. Molecular
phylogenetics, historical biogeography, and chromosome evolution of
Portulaca (Portulacaceae). – Mol.
Phylogen. Evol. 63: 97-112.
Ocampo G, Koteyeva NK, Voznesenskaya EV,
Edwards GE, Sage TL, Sage RF, Columbus JT. 2013. Evolution of leaf anatomy and
photosynthetic pathways in Portulacaceae. – Amer. J. Bot.
100: 2388-2402.
Ochiauri D. 1965. Charesia E. Busch
genus novum pro florae Georgia. – Not. Syst. Geogr. Inst. Bot. Thbilissiensis
24: 87-88. [In Russian]
Ogburn RM. 2007. Anatomical variation in Cactaceae sensu lato. – Master
thesis, University of Missouri, St. Louis, Missouri.
Ogburn RM, Edwards EJ. 2009. Anatomical
variation in Cactaceae and
relatives: trait lability and evolutionary innovation. – Amer. J. Bot. 96:
391-408.
Ogburn RM, Edwards EJ. 2010. The ecological
water use strategies of succulent plants. – Adv. Bot. Res. 55: 179-225.
Ogburn RM, Edwards EJ. 2012. Quantifying
succulence: a rapid, physiologically meaningful metric of plant water storage.
– Plant Cell Environm. 35: 1533-1542.
Ogburn RM, Edwards EJ. 2013. Repeated origin
of three-dimensional venation releases constraints on the evolution of
succulence in plants. – Curr. Biol. 23: 722-726.
Ogburn RM, Edwards EJ. 2015. Life history
lability underlies rapid climate niche evolution in the angiosperm clade Montiaceae. – Mol. Phylogen.
Evol. 92: 181-192.
Ogundipe OT, Chase M. 2009. Phylogenetic
analyses of Amaranthaceae
based on matK DNA sequence data with emphasis on West African species.
– Turkish J. Bot. 33: 153-161.
Ohsako T, Ohnishi O. 2000. Intra- and
interspecific phylogeny of wild Fagopyrum (Polygonaceae) species based on
nucleotide sequences of noncoding regions in chloroplast DNA. – Amer. J. Bot.
87: 573-582.
Ohsako T, Fukuoka S, Bimb HP, Baniya BK,
Yasui Y, Ohnishi O. 2001. Phylogenetic analysis of the genus Fagopyrum
(Polygonaceae), including the
Nepali species F. megacarpum, based on nucleotide sequence of the
rbcL-accD region in chloroplast DNA. – Fagopyrum 18:
9-14.
Okabe T, Iwakiri Y, Mori H, Ogawa T, Ohyama
T. 2005. An S-like ribonuclease gene is used to generate a trap-leaf enzyme in
the carnivorous plant Drosera adelae. – FEBS Letters 579:
5729-5733.
Okamoto M. 1984. Centrifugal ovule inception
I. Sequence of ovule inception in Silene cucubalus. – Bot. Mag.
(Tokyo) 97: 345-353.
Olson ME, Gaskin JF, Ghahremani-nejad F.
2003. Stem anatomy is congruent with molecular phylogenies placing
Hypericopsis persica in Frankenia (Frankeniaceae): comments on
vasicentric tracheids. – Taxon 52: 525-532.
Olvera HF. 2003. Classification of the North
American species of Atriplex section Obione (Chenopodiaceae)
based on numerical taxonomic analysis. – Taxon 52: 247-260.
Olvera HF, Davis JI. 2001. A cladistic
analysis of Atripliceae (Chenopodiaceae) based on morphological data. – J.
Torrey Bot. Soc. 128: 297-319.
Olvera HF, Fuentes-Soriano S, Hernández EM.
2006. Pollen morphology and systematics of Atripliceae (Chenopodiaceae). –
Grana 45: 175-194.
Olvera HF, Smets E, Vrijdaghs A. 2008. Floral
and inflorescence morphology and ontogeny in Beta vulgaris, with
special emphasis on the ovary position. – Ann. Bot. 102: 643-651.
Olvera HF, Vrijdaghs A, Ochoterena H, Smets
E. 2011. The need to re-investigate the nature of homoplastic characters: an
ontogenetic case study of the ‘bracteoles’ in Atripliceae (Chenopodiaceae).
– Ann. Bot. 108: 847-865.
Opel MR. 2005. Leaf anatomy of
Conophytum N. E. Br. (Aizoaceae). – Haseltonia 11:
27-52.
Opel MR. 2005. A morphological phylogeny of
the genus Conophytum N. E. Br. (Aizoaceae). – Haseltonia 11:
53-77.
O’Quinn R, Hufford L. 2005. Molecular
systematics of Montieae (Portulacaceae): implications for
taxonomy, biogeography and ecology. – Syst. Bot. 30: 314-331.
O’Quinn R, Hufford L. 2006. Shoot
morphology in the Claytonia sibirica complex (Portulacaceae). – Madroño 53:
1-10.
Ormond WT, Cortella C de, Bezerra Pinheiro
AR, Rodrigues Correia MC. 1978. Contribuição ao estudo anatômico das
populações diplóide e tetraplóide de Petiveria alliacea L. –
Bol. Mus. Nac. Rio de Janeiro, Bot. II, 51: 1-13.
Ortega Olivencia A, Carrasco Claver JP,
Devesa Alcarez JA. 1995. Floral and reproductive biology of Drosophyllum
lusitanicum (L.) Link (Droseraceae). – Bot. J. Linn.
Soc. 118: 331-351.
Ortiz S. 2003. Two new species of the
Oxygonum stuhlmannii-atriplicifolium complex (Polygonaceae) from Somalia. –
Bot. J. Linn. Soc. 142: 341-345.
Ortiz S, Paiva JAR. 1999. Taxonomic notes on
Polygonaceae from southern
tropical Africa. – Bot. J. Linn. Soc. 131: 167-176.
Ortiz S, Carbajal R, Serrano M. 2008. A new
species of Polygonum L. (Polygonaceae) from South Africa.
– Bot. J. Linn. Soc. 157: 111-114.
Osman AK, El-Garf IA. 2006. Pollen morphology
of the Egyptian species of genus Limonium Mill. (Plumbaginaceae). – Feddes
Repert. 117: 476-485.
Osmond CB, Björkman O, Anderson DJ. 1980.
Physiological processes in plant ecology. Towards a synthesis with
Atriplex. – Springer, Berlin, Heidelberg, New York.
Ostolaza C. 2006. El género
Armatocereus Backeberg. – Zonas Aridas (Lima) 10: 144-154.
Ousted S. 1985. A taxonomic revision of the
genus Uebelinia Hochst. (Caryophyllaceae). – Bull.
Jard. Bot. Nat. Belg. 55: 421-459.
Ovczinnikov PN, Kinzikaeva GK. 1977.
Myrtama Ovcz. et Kinz. gen. nov.: the new genus from the family Tamaricaceae Link. – Dokl.
Akad. Nauk. Tadzhiksk. SSR 20, 7: 54-57. [In Russian]
Oxelman B. 1995. A revision of the Silene
sedoides-group (Caryophyllaceae). –
Willdenowia 25: 143-169.
Oxelman B, Jonsell B. 2001. Proposal to
conserve the name Viscaria against Steris (Caryophyllaceae, Sileneae).
– Taxon 50: 281-282.
Oxelman B, Lidén M. 1995. Generic boundaries
in the tribe Sileneae (Caryophyllaceae) as inferred
from nuclear rDNA sequences. – Taxon 44: 525-542.
Oxelman B, Jonsell B. 2001. Proposal to
conserve the name Viscaria against Steris (Caryophyllaceae, Sileneae).
– Taxon 50: 281-282.
Oxelman B, Lidén M, Berglund D. 1997.
Chloroplast rps16 intron phylogeny of the tribe Sileneae (Caryophyllaceae). – Plant
Syst. Evol. 206: 392-410.
Oxelman B, Lidén M, Rabeler RK, Popp M.
2001. A revised generic classification of the tribe Sileneae (Caryophyllaceae). – Nord. J.
Bot. 20: 743-748.
Oxelman B, Ahlgren B, Thulin M. 2002.
Circumscription and phylogenetic relationships of Gymnocarpos (Caryophyllaceae-Paronychioideae).
– Edinburgh J. Bot. 59: 221-237.
Pal GD, Maiti GG. 1985. A new species of
Aconogonum (Polygonaceae) from Eastern
Himalaya. – Bull. Bot. Surv. India 26: 95-96.
Pal M, Khoshoo TN. 1965. Origin of
Amaranthus dubius. – Curr. Sci. 34: 370-341.
Pal S, Murty YS. 1974. Studies on the nodal
and floral anatomy of some species of Stellaria L. – J. Indian Bot.
Soc. 53: 100-110.
Palacios C, Rossello JA, Gonzalez-Candelas F.
2000. Study of the evolutionary relationships among Limonium species
(Plumbaginaceae) using
nuclear and cytoplasmic molecular markers. – Mol. Phylogen. Evol. 14:
232-249.
Paliwal CS, Gupta BP, Malasi CB. 1930.
Structure and development of stomata in Centrospermae: I. Amaranthaceae and Nyctaginaceae. – Ind. Forest
J. 3: 135-139.
Paliwal GS. 1965. The development of stomata
in Basella rubra Linn. – Phytomorphology 15: 50-53.
Palmer EJ, Steyermark J. 1950. Notes on
Geocarpon minimum Mackenzie. – Bull. Torrey Bot. Club 77:
268-273.
Palmer J. 1998. A taxonomic revision of
Gomphrena (Amaranthaceae) in Australia. –
Aust. Syst. Bot. 11: 73-151.
Palomino G, Segura MD, Bye R, Mercado PR.
1990. Cytogenetic distinction between Teloxys and Chenopodium
(Chenopodiaceae). – Southw. Natur. 35: 351-353.
Pancho JV, Capinpin JM. 1961-1962. Haploidy
in Bougainvillea. – Philipp. Agric. 45: 88-94.
Pant DD, Bhatnagar S. 1977. Morphological
studies in Nepenthes (Nepenthaceae). –
Phytomorphology 27: 13-34.
Pant DD, Kidwai PF. 1968. Structure and
ontogeny of stomata in some Caryophyllaceae. – Bot. J.
Linn. Soc. 60: 309-314.
Pant RP. 2000. Phylogenetic relationships and
character evolution in Eriogonoideae (Polygonaceae): inferred from
molecular and morphological data. – Ph.D. diss., Claremont Graduate School,
Claremont, California.
Papanicolaou K, Kokkini S. 1982.
Armeria (Plumbaginaceae) in Greek
montains. – Willdenowia 12: 213-219.
Park C-W. 1987. Flavonoid chemistry of
Polygonum Sect. Echinocaulon: a systematic survey. – Syst.
Bot. 12: 167-179.
Park C-W. 1988. Taxonomy of
Polygonum section Echinocaulon (Polygonaceae). – Mem. New York
Bot. Gard. 47: 1-82.
Parkhurst RM, Thomas DW, Skinner WA, Cary LW.
1973. Molluscicidal saponins of Phytolacca dodecandra:
oleanoglycotoxin-A. – Phytochemistry 12: 1437-1442.
Parolin P. 2001. Seed expulsion in fruits of
Mesembryanthema (Aizoaceae), a
mechanistic approach to study the effect of fruit morphological structures on
seed dispersal. – Flora 196: 313-322.
Parolin P. 2006. Ombrohydrochory:
rain-operated seed dispersal in plants – with special regard to jet-action
dispersal in Aizoaceae. – Flora
201: 511-518.
Patankar TBV. 1956. Further contribution to
the embryology of Drosera burmannii Vahl. – Proc. Indian Acad. Sci.
43B: 161-171.
Patterson GW, Xu S. 1990. Sterol composition
in five families of the order Caryophyllales. –
Phytochemistry 29: 3539-3541.
Pavlovic A, Masarovicová E, Hudák J. 2007.
Carnivorous syndrome in Asian pitcher plants of the genus Nepenthes.
– Ann. Bot., N. S., 100: 527-536.
Pax F. 1889a. Aizoaceae (Ficoideae,
Mesembrianthemaceae). – In: Engler A, Prantl K (eds), Die natürlichen
Pflanzenfamilien III(1b), W. Engelmann, Leipzig, pp. 33-51.
Pax F. 1889b. Portulacaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien III(1), W. Engelmann,
Leipzig, pp. 51-60.
Pax F. 1889c. Caryophyllaceae. – In:
Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(1), W.
Engelmann, Leipzig, pp. 61-94.
Pax F. 1891. Plumbaginaceae. – In: Engler
A, Prantl K (eds), Die natürlichen Pflanzenfamilien IV(1), W. Engelmann,
Leipzig, pp. 116-125.
Pax F. 1896. Buxaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien III(5), W. Engelmann,
Leipzig, pp. 130-135.
Pax F. 1927. Zur Phylogenie der Caryophyllaceae. – Engl.
Bot. Jahrb. Syst. 61: 223-241.
Pax F, Hoffmann K. 1934a. Aizoaceae. – In: Engler A (†),
Pax F, Harms H (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 16c, W.
Engelmann, Leipzig, pp. 179-233.
Pax F, Hoffmann K. 1934b. Portulacaceae. – In: Engler A
(†), Pax F, Harms H (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd.
16c, W. Engelmann, Leipzig, pp. pp. 234-262.
Pax F, Hoffmann K. 1934c. Dysphaniaceae. –
In: Engler A (†), Pax F, Harms H (eds), Die natürlichen Pflanzenfamilien, 2.
Aufl., Bd. 16c, W. Engelmann, Leipzig, pp. 272-274.
Pax F, Hoffmann K. 1934d. Caryophyllaceae. – In:
Engler A (†), Pax F, Harms H (eds), Die natürlichen Pflanzenfamilien, 2.
Aufl., Bd. 16c, W. Engelmann, Leipzig, pp. 275-364.
Payne MA. 1933. The morphology and anatomy of
Mollugo verticillata L. – Univ. Kansas Sci. Bull. 21: 399-419.
Pedersen TM. 1983. Two new species of
Stellaria from South America, with a description of Stellaria
arvalis F. Phil. – Bonplandia 5: 203-210.
Pedersen TM. 1990. Studies in South American
Amaranthaceae III. – Bull.
Mus. Natl. Hist. Nat., B, Adansonia 12: 69-97.
Pedersen TM. 1997. Studies in South American
Amaranthaceae IV. –
Adansonia, sér. 3, 19: 217-251.
Pedersen TM. 2010. Studies in South American
Amaranthaceae V. –
Bonplandia 10: 83-112.
Pendry CA. 2004. Monograph of
Ruprechtia (Polygonaceae). – Syst. Bot.
Monogr. 67: 1-113.
Penzig O. 1877. Untersuchungen über
Drosophyllum lusitanicum Link. – Inaugural Diss., Phil. Fak.,
Breslau Universität.
Perdrigeat M C-A. 1900. Anatomie comparée
des polygonées et ses rapports avec la morphologie et la classification. –
Actes Soc. Linn. Bordeaux 55: 1-91.
Perrier de la Bâthie H. 1950. Caryophyllaceae. – In:
Humbert H (ed), Flore de Madagascar et des Comores, Muséum National
d’Histoire Naturelle, Paris.
Perveen A, Qaiser M. 2001. Pollen flora of
Pakistan XXVII. Nyctaginaceae. – Turk. J. Bot.
25: 385-388.
Petri A, Oxelman B. 2011. Phylogenetic
relationships within Silene (Caryophyllaceae) section
Physolychnis. – Taxon 60: 953-968.
Petrusson L, Thulin M. 1996. Taxonomy and
biogeography of Gymnocarpos (Caryophyllaceae,
Paronychioideae). – Edinburgh J. Bot. 53: 1-26.
Philbrick RN. 1963. Zygotic and agamospermous
reproduction in Opuntia littoralis. – Amer. J. Bot. 50: 637.
Philipp M. 1974. Flower biology of
Stellaria longipes. – Bot. Tidsskr. 69: 239-244.
Philips SM. 2000. Notes on Portulaca
L. (Portulacaceae) in
tropical East Africa. – Kew Bull. 55: 687-698.
Philipson WR. 1993. Hectorellaceae. – In:
Kubitzki K, Rohwer JG, Bittrich V (eds), The families and genera of vascular
plants II. Flowering plants. Dicotyledons. Magnoliid, hamamelid and
caryophyllid families, Springer, Berlin, Heidelberg, New York, pp. 331-334.
Philipson WR, Skipworth JP. 1961.
Hectorellaceae: a new family of dicotyledons. – Trans. Roy. Soc. New Zealand
Bot. 1: 31.
Phillips SM. 2002. Portulacaceae. – In: Beentje
HJ, Smith SAL (eds), Flora of tropical East Africa, A. A. Balkema, Rotterdam,
pp. 1-40.
Phitos D. 1981. To genos Bolanthus
(Caryophyllaceae) stin
Ellada. – Bot. Hron. 1: 35-45.
Piatelli M, Imperato F. 1970. Betacyanins
from Bougainvillea. – Phytochemistry 9: 455-458.
Piatelli M, Imperato F. 1971. Betacyanins of
some Chenopodiaceae. – Phytochemistry 10: 3133-3134.
Piirainen M, Liebisch O, Kadereit G. 2017.
Phylogeny, biogeography, systematics and taxonomy of Salicornioideae (Amaranthaceae/Chenopodiaceae)
– a cosmopolitan, highly specialized hygrohalophyte lineage dating back to
the Oligocene. – Taxon 66: 109-132.
Pilbeam J. 1985. Sulcorebutia and
Weingartia. A collector’s guide. – B. T. Batsford Ltd., London.
Pilbeam J. 1995. Gymnocalycium. A
collector’s guide. – A. A. Balkema, Rotterdam & Brookfield.
Pilbeam J. 1996. Thelocactus. The
cactus file handbook 1. – Cirio Publ. Services, Holbury.
Pilbeam J. 1997. Rebutia. – Cirio
Publ. Services, Southampton.
Pilbeam J. 1999. Mammillaria. –
Cirio Publ. Services, Southampton.
Pilbeam J, Bowdery D. 2005.
Ferocactus. – British Cactus & Succulent Society, Hornchurch.
Pilbeam J, Hunt DR. 2004. A Sulco gallery.
Sulcorebutias in pictures. – David Hunt, Milborne Port.
Pilbeam J, Weightman B. 2006.
Ariocarpus et cetera. The special, smaller genera of Mexican cacti.
– British Cactus & Succulent Society, Hornchurch.
Pilz GE. 1978. Systematics of
Mirabilis subgenus Quamoclidion (Nyctaginaceae). – Madroño 25:
113-132.
Pinkava DJ. 2002. On the evolution of
continental North American Opuntioideae. – Succ. Plant Res. 6: 59-98.
Pinkava DJ, Baker MA, Parfitt BD, Mohlenbrock
MW, Worthington RD. 1985. Chromosome numbers in some cacti of western North
America V. – Syst. Bot. 10: 471-483.
Pinkava D, Parfitt B, Baker M, Worthington R.
1992. Chromosome numbers in some cacti of western North America VI, with
nomenclatural changes. – Madroño 39: 98-113.
Pinkava DJ, Rebman J, Baker M. 1998.
Chromosome numbers in some cacti of western North America VII. – Haseltonia
6: 32-41.
del-Pino IS, Borsch T, Motley TJ. 2009.
trnL-F and rpl16 sequence data and dense taxon sampling
reveal monophyly of unilocular anthered Gomphrenoideae (Amaranthaceae) and an improved
picture of their internal relationships. – Syst. Bot. 34: 57-67.
Pirani A, Zarre S, Pfeil BE, Bertrand YJK,
Assadi M, Oxelman B. 2014. Molecular phylogeny of Acanthophyllum (Caryophyllaceae:
Caryophylleae), with emphasis on infrageneric classification. – Taxon 63:
592-607.
Pires JM. 1981. Notas de Herbário I.
Belemia Pires n. gen. – Bol. Mus. Para. Emilio Goeldi 52: 1-4.
Planchuelo AM. 1974 [1975]. Estudio de los
frutos y semillas del genero Chenopodium en la Argentina. –
Darwiniana 19: 528-565.
Poellnitz K von. 1932. Claytonia
Gronov. and Montia Mich. – Feddes Repert. 30: 279-325.
Poellnitz K von. 1933. Anacampseros
L. Versuch einer Monographie. – Bot. Jahrb. Syst. 65: 382-448.
Poellnitz K von. 1934a. Monographie der
Gattung Talinum Adans. – Feddes Repert. 35: 1-34.
Poellnitz K von. 1934b. Die
Calandrinia-Arten Australiens. – Feddes Repert. 35: 161-173.
Poellnitz K von. 1934c. Versuch zu einer
Monographie der Gattung Portulaca L. – Feddes Repert. 37:
240-320.
Poggio L, Guaglianone ER, Greizerstein EJ.
1986. Estudios cromosómicos en Phytolacca dioica, P.
tetramera y P. bogotensis (Phytolaccaceae). – Darwiniana
27: 19-27.
Poindexter DB, Bennett KE, Weakley AS. 2014.
A morphologically based taxonomic reevaluation of the genus
Stipulicida (Caryophyllaceae), with
comments on rank. – J. Bot. Res. Inst. Texas 8: 419-430.
Pole M. 1993. Early Miocene flora of the
Manuherikia Group, New Zealand 5. Smilacaceae, Polygonaceae, Elaeocarpaceae.
– J. Roy. Soc. New Zealand 23: 289-302.
Polhill RM. 1971. Phytolaccaceae. – In:
Milne-Redhead E, Polhill RM (eds), Flora of tropical East Africa, Crown Agents
for Oversea Governments and Aministrations, London, pp. 1-8.
Pope CL. 1976. A phylogenetic study of the
suffrutescent shrubs in the genus Atriplex. – Ph.D. diss., Brigham
Young University, Provo, Utah.
Popp M, Oxelman B. 2001. Inferring the
history of the polyploid Silene aegaea (Caryophyllaceae) using plastid
and homoeologous nuclear DNA seqences. – Mol. Phylogen. Evol. 20: 474-481.
Popp M, Oxelman B. 2004. Evolution of a RNA
polymerase gene family in Silene (Caryophyllaceae): incomplete
concerted evolution and topological congruence among paralogues. – Syst.
Biol. 53: 914-932.
Popp M, Oxelman B. 2007. Origin and evolution
of North American polyploid Silene (Caryophyllaceae). – Amer. J.
Bot. 94: 330-349.
Popp M, Erixon P, Eggens F, Oxelman B. 2005.
Origin and evolution of a circumpolar polyploid species complex in
Silene (Caryophyllaceae) inferred from
low copy nuclear RNA polymerase introns, rDNA, and chloroplast DNA. – Syst.
Bot. 30: 302-313.
Popp M, Gizaw A, Nemomissa S, Suda J,
Brochmann C. 2008. Colonization and diversification in the African ‘sky
islands’ by Eurasian Lychnis L. (Caryophyllaceae). – J.
Biogeogr. 35: 1016-1029.
Poppendieck H-H, Ihlenfeldt H-D. 1978.
Delosperma harazianum (Deflers) Poppendieck & Ihlenfeldt, eine
wenig bekannte Mesembryanthemaceae aus dem Südjemen. – Mitt. Inst. Allg.
Bot. Hamburg 16: 183-187.
Poppinga S, Hartmeyer SRH, Masselter T,
Hartmeyer I, Speck T. 2013. Trap diversity and evolution in the family Droseraceae. – Plant Signal.
& Behav. 8: 7, e24685.
Porembski S. 2002. Ancistrocladaceae. – In:
Kubitzki K, Bayer C (eds), The families and genera of vascular plants V.
Flowering plants. Dicotyledons. Malvales, Capparales and
non-betalain Caryophyllales,
Springer, Berlin, Heidelberg, New York, pp. 25-27.
Porembski S, Barthlott W. 2002. Dioncophyllaceae. – In:
Kubitzki K, Bayer C (eds), The families and genera of vascular plants V.
Flowering plants. Dicotyledons. Malvales, Capparales and
non-betalain Caryophyllales,
Springer, Berlin, Heidelberg, New York, pp. 178-181.
Porsch O. 1938-1939. Das Bestäubungsleben
der Kakteenblüte. – Jahrb. Deutsch. Kakteenges. 1938/1, 1939/1. Neumann,
Neudamm.
Porter JM, Kinney MS, Heil KD. 2000.
Relationships between Sclerocactus and Toumeya (Cactaceae) based on chloroplast
trnL-trnF sequences. – Haseltonia 7: 8-23.
Powell AM, Weedin JF. 2004. Cacti of the
trans-Pecos and adjacent areas. – Texas Tech University Press, Lubbock.
Powell JW, Whalley WB. 1969. Triterpenoid
saponins from Phytolacca dodecandra. – Phytochemistry 8:
2105-2107.
Powell RF, Boatwright JS, Klak C, Mgee AR.
2016. Phylogenetic placement and generic re-circumscriptions of the
multilocular genera Arenifera, Octopoma and
Schlechteranthus (Aizoaceae: Ruschieae): evidence from
anatomical, morphological and plastid DNA data. – Taxon 65: 249-261.
Pozner R, Cocucci A. 2006. Floral structure,
anther development, and pollen dispersal of Halophytum ameghinoi (Halophytaceae). – Intern. J.
Plant Sci. 167: 1091-1098.
Prakash N. 1966. Aizoaceae and Cactaceae. A study of their
embryology and relationships. – Ph.D. diss., University of Delhi, India.
Prakash N. 1967a. Life history of
Tetragonia tetragonioides (Pall.) O. Kuntze. – Aust. J. Bot. 15:
413-424.
Prakash N. 1967b. Gametogenesis and seed
development in Hereroa hesperantha (Dtr.) Dtr. et Schwant. (Aizoaceae). – Aust. J. Bot. 15:
425-435.
Prakash N. 1967c. Aizoaceae – a study of its
embryology and systematics. – Bot. Not. 120: 305-323.
Prance GT. 1968. The systematic position of
Rhabdodendron Gilg and Pilg. – Bull. Jard. Bot. Natl. État,
Bruxelles, 38: 127-146.
Prance GT. 1972. Flora Neotropica Monograph
11. Rhabdodendraceae. –
Hafner, New York.
Prance GT. 2002. Rhabdodendraceae. – In:
Kubitzki K, Bayer C (eds), The families and genera of vascular plants V.
Flowering plants. Dicotyledons. Malvales, Capparales and
non-betalain Caryophyllales,
Springer, Berlin, Heidelberg, New York, pp. 339-341.
Pratov U. 1986. Rod Climacoptera
Botsch. – Tashkent.
Pratov U. 1985. Overview of the genus
Nanophyton Less. (Chenopodiaceae). – Nov. Sist. Vyssh. Rast. 22:
81-88. [in Russian]
Pratov U. 1986. The genus
Climacoptera Botsch. (Systematics, geography, phylogeny and
conservation questions). – Izdatel’stvo Fan Uzbekskoi AN, Tashkent. [in
Russian]
Pratt DB. 2003. Phylogeny and morphological
evolution of the Chenopodiaceae-Amaranthaceae alliance. –
Ph.D. diss., Iowa State University, Ames, Iowa.
Prentice HC, Mastenbroek O, Berendsen W,
Hogeweg P. 1984. Geographic variation in the pollen of Silene
latifolia (S. alba, S. pratensis): a quantitative
morphological analysis of population data. – Can. J. Bot. 62: 1259-1267.
Prescott A, Venning J. 1984. Aizoaceae. – In: George AS (ed),
Flora of Australia 4, Australian Government Publ. Service, Canberra, pp.
19-62.
Presting D, Straka H, Friedrich B. 1983.
Palynologia Madagassica et Mascarenica, Familien 128-146 (Fam. 134: Theaceae). – Akad.
Wiss. Lit. Mainz, Trop. Subtrop. Pflanzenwelt 44.
Price TM. 2012. Phylogeny and evolution of
Phemeranthus (Montiaceae) in North American xeric
habitats. – Electronic Theses and Dissertations. Paper 724.
http://openscholarship.wustl.edu/etd/724.
Price TM, Ferguson DJ. 2012. A new
combination in Phemeranthus (Montiaceae) and notes on the
circumscription of Phemeranthus and Talinum (Talinaceae) from the southwestern
United States and northern Mexico. – Novon 22: 67-69.
Puff CA, Weber A. 1976. Contributions to the
morphology, anatomy, and caryology of Rhabdodendron, and a
reconsideration of the systematic position of the Rhabdodendronaceae. – Plant
Syst. Evol. 125: 195-222.
Pusalkar PK, Sing DK. 2015. Taxonomic
rearrangement of Arenaria (Caryophyllaceae) in Indian
Western Himalaya. – J. Jap. Bot. 90: 77-91.
P’yankov VI, Artyusheva EG, Edwards GE,
Black CC, Soltis PS. 2001. Phylogenetic analysis of tribe Salsoleae
(Chenopodiaceae) based on ribosomal ITS sequences: implications for the
evolution of photosynthesis types. – Amer. J. Bot. 88: 1189-1198.
P’yankov VI, Ziegler H, Kuz’min A,
Edwards G. 2001. Origin and evolution of C4 photosynthesis in the
tribe Salsoleae (Chenopodiaceae) based on anatomical and biochemical types in
leaves and cotyledons. – Plant Syst. Evol. 230: 43-74.
Qaiser M. 1983. The genus Tamarix
(Tamaricaceae) in Pakistan.
– Iran. J. Bot. 2, 1: 21-68.
Qaiser M. 1987. Studies in the seed
morphology of the family Tamaricaceae from Pakistan. –
Bot. J. Linn. Soc. 94: 469-484.
Qaiser M, Ali SZ. 1978. Tamaricaria,
a new genus of Tamaricaceae.
– Blumea 24: 151-155.
Quézel P, Sintès S. 1960. Les
nyctaginacées d’Afrique du Nord et du Sahara. – Bull. Soc. Hist. Nat. Afr.
Nord 50: 222-256.
Rabeler RK. 2017. New combinations and
typification in Shivparvatia (Alsineae, Caryophyllaceae). –
Phytotaxa 303: 293-296.
Rabeler RK, Bittrich V. 1993. Suprageneric
nomenclature in the Caryophyllaceae. – Taxon 42:
857-863.
Rabeler RK, Wagner WL. 2015.
Eremogone (Caryophyllaceae): new
combinations for Old World taxa. – PhytoKeys 50: 35-42.
Rabeler RK, Wagner WL. 2016. New combinations
in Odontostemma (Caryophyllales). – PhytoKeys
63: 77-97.
Rabesa ZA. 1982a. Definition de deux sections
du genre Alluaudia (Didiereaceae). – Taxon 31:
736-737.
Rabesa ZA. 1982b. Recherches
chimiosystématiques sur les flavonoides des Didiéréacées. – Trop.
Subtrop. Pflanzenw. 37: 339-358.
Radlkofer L. 1896. Didierea Baill.
– In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(5), W.
Engelmann, Leipzig, pp. 461-462.
Raghavan TS, Srinivasan VK. 1940. Studies in
the Indian Aizoaceae. – Ann.
Bot., N. S., 4: 651-661.
Ragleti HWJ, Weintraub M, Lo E. 1972.
Characteristics of Drosera tentacles I. Anatomical and cytological
detail. – Can. J. Bot. 50: 159-168.
Ragonese AM. 1966. Anatomía de las
Frankeniáceas Argentinas. – Darwiniana 14: 95-129.
Rahiminejad MR, Gornall RJ. 2004. Flavonoid
evidence for allopolyploidy in the Chenopodium album aggregate (Amaranthaceae). – Plant Syst.
Evol. 246: 77-87.
Raj G, Kurup R, Hussain AA, Baby S. 2011.
Distribution of naphthoquinones, plumbagin, droserone, and 5-O-methyl
droserone in chitin-induced and uninduced Nepenthes khasiana:
molecular events in prey capture. – J. Exp. Bot. 62: 5429-5436.
Rajendru G, Prasad JSR, Rama Das VS. 1986.
C3–C4 intermediate species in Alternanthera
(Amaranthaceae). – Plant
Physiol. 80: 409-414.
Rajput KS. 2002. Stem anatomy of Amaranthaceae: rayless nature of
xylem. – Flora 197: 224-232.
Ramanna MS. 1976. Are there heteromorphic sex
chromosomes in spinach (Spinacia oleracea L.). – Euphytica 25:
277-284.
Rao AR, Awasthi P, Khare P. 1965.
Palynological studies in Indian Aizoaceae (Ficoideae, Molluginaceae). – Palynol.
Bull. 1: 50-51.
Rao BSS. 1975. Embryo development in five
species of Mollugo. – Curr. Sci. 44: 712-713.
Rao TA, Das GC. 1968. Foliar sclereids in
some species of Limonium. – Curr. Sci. 37: 252-254.
Rao TA, Cheluviah MC, Chakraborti S. 1984. On
foliar sclereids in Asteropeia Thou. – Curr. Sci. 53: 45-48.
Rao VS. 1936. A contribution to the
morphology of Antigonon leptopus Hook. & Arn. – J. Indian Bot.
Soc. 15: 105-114.
Rao VS. 1969. The floral anatomy of
Ancistrocladus. – Proc. Indian Acad. Sci. 70B: 215-222.
Rappa F. 1912. Per una classificazione
naturale dei Mesembriantemi. – Boll. Reale Orto. Bot. Giard. Colon. Palermo
11: 21-36.
Rappa F, Camarrone V. 1953. Mesembrianthemum
e Mesembryanthemum. Una rivendicazione. – Lav. Ist. Bot. Giard. Colon.
Palermo 14: 1-39.
Rappa F, Camarrone V. 1962. La
classificazione naturale delle Mesembriantemacee. – Lav. Ist. Bot. Giard.
Colon. Palermo 18: 11-32.
Ratter JA. 1964. Cytogenetic studies in
Spergularia I. Cytology of some old world species. – Notes Roy. Bot.
Gard. Edinb. 25: 293-302.
Ratter JA. 1976. Cytogenetic studies in
Spergularia IX. Summary and conclusions. – Notes Roy. Bot. Gard.
Edinb. 34: 411-428.
Rauh W. 1956. Morphologische,
entwicklungsgeschichtliche, histogenetische, und anatomische Untersuchungen an
den Sprossen der Didiereaceen. – Abh. Akad. Wiss. Lit., Math.-Nat. Kl., 6:
343-444.
Rauh W. 1961. Weitere untersuchungen an
Didiereaceen I. Beitrag zur Kenntnis der Wuchsformen der Didiereaceen, unter
besonderer Berücksichtigung neuer Arten – Sitzungsber. Heidelberger Akad.
Wiss., Math.-Nat. Kl., 1960-1961(7): 185-300.
Rauh W. 1963. 121e Fam. Didiereaceae. – In: Humbert H
(ed), Flore de Madagascar et des Comores, Muséum National d’Histoire
Naturelle, Paris.
Rauh W. 1979. Kakteen an ihren Standorten
unter besonderer Berücksichtigung ihrer Morphologie und Systematik. – P.
Parey, Berlin & Hamburg.
Rauh W. 1983. The morphology and systematic
position of the Didiereaceae
of Madagascar. – Bothalia 14: 839-843.
Rauh W, Dittmar K. 1970. Weitere
Untersuchungen an Didiereaceen 3. Vergleichend-anatomische Untersuchungen an
den Sprossachsen und den Dornen der Didiereaceen. – Sitzungsber. Heidelberger
Akad. Wiss., Math.-Nat. Kl., 1969/70(4): 1-88.
Rauh W, Reznik H. 1961. Zur Frage der
systematischen Stellung der Didiereaceen. – Bot. Jahrb. Syst. 81: 94-105.
Rauh W, Schölch H-F. 1965. Weitere
Untersuchungen an Didiereaceen 2. Infloreszenz-, blütenmorphologische und
embryologische Untersuchungen mit Ausblick auf die systematische Stellung der
Didiereaceen. – Sitzungsber. Heidelberger Akad. Wiss., Math.-Nat. Kl.,
1965(3): 1-218, 221-434.
Rausch W. 1975-1976. Lobivia. Die
tagblütige Echinopsidinae aus arealgeographischer Sicht. I-III. – R. Herzig,
Wien.
Rausch W. 1987. Lobivia 85. – R.
Herzig, Wien.
Rautenberg A, Filatov D, Svennblad B, Heidari
N, Oxelman B. 2008. Conflicting phylogenetic signals in the SIX1/Y1
gene in Silene. – BMC Evol. Biol. 8: 299. DOI:
10.1186/1471-2148-8-299.
Rautenberg A, Sloan DB, Aldén V, Oxelman B.
2012. Phylogenetic relationships of Silene multinervia and
Silene Section Conoimorpha (Caryophyllaceae). – Syst.
Bot. 37: 226-237.
Ravn RL. 1987. Montiapollis n.gen.,
possible Portulacaceae pollen
from the Cenomanian of Iowa. – Grana 26: 243-247.
Rayder L, Ting IP. 1981. Carbon metabolism in
two species of Pereskia (Cactaceae). – Plant Physiol. 68:
139-142.
Rea J. 1969. Biología floral de la quinua
(Ch. quinoa). – Turrialba 19: 91-96.
Realini MF, González GE, Font F, Picca PI,
Poggio L, Gottlieb AM. 2015. Phylogenetic relationships in Opuntia (Cactaceae, Opuntioideae) from
southern South America. – Plant Syst. Evol. 301: 1123-1134.
Rebman JP. 1995. Biosystematic study of
Opuntia subgenus Cylindropuntia (Cactaceae), the chollas of Lower
California, México. – Ph.D. diss., Arizona State University, Tempe,
Arizona.
Rebman JP. 2002. Nomenclatural changes in
Cylindropuntia, Grusonia, and Nopalea (Cactaceae). – J. Ariz. Nev. Acad.
Sci. 34: 45.
Rebman JP. 2015. Seven new cacti (Cactacee: Opuntioideae) from the
Baja California Region, México. – Madroño 62: 46-67.
Rechinger KH. 1933a. Vorarbeiten zu einer
Monographie der Gattung Rumex II. Die Arten der Subsektion
Patientiae. – Feddes Repert. 31: 226-283.
Rechinger KH. 1933b. Vorarbeiten zu einer
Monographie der Gattung Rumex III. Die süd- und zentralamerikanischen
Arten der Gattung Rumex. – Ark. f. Bot. 26A(3): 1-58.
Rechinger KH. 1937. Vorarbeiten zu einer
Monographie der Gattung Rumex V. The North American species of
Rumex. – Publ. Field Mus. Nat. Hist., Bot. Ser. 17: 1-150.
Rechinger KH. 1943. Die Rumex-Arten
der Balkanhalbinsel. – Mitt. Thüring. Bot. Ver., Ser. II, 50: 193-217.
Rechinger KH. 1949. Vorarbeiten zu einer
Monographie der Gattung Rumex VII. Rumices asiatici. – Candollea 12:
9-152.
Rechinger KH. 1954. Vorarbeiten zu einer
Monographie der Gattung Rumex VIII. Monograph of the genus
Rumex in Africa. – Bot. Not. Suppl. 3(3): 1-114.
Rechinger KH. 1984. Rumex (Polygonaceae) in Australia: a
reconsideration. – Nuytsia 5: 75-122.
Record SJ. 1934. The woods of
Rhabdodendron and Duckeodendron. – Trop. Woods 33: 6-10.
Remski MF. 1954. Cytological investigations
in Mammillaria and some associated genera. – Bot. Gaz. 116:
163-171.
Renner T, Specht CD. 2011. A sticky
situation: assessing adaptations for plant carnivory in the Caryophyllales by means of
stochastic character mapping. – Intern. J. Plant Sci. 172: 889-901.
Reppenhagen W. 1991. Die Gattung
Mammillaria. Monographie 1. – Steinhart, Titisee-Neustadt.
Reppenhagen W. 1992. Die Gattung
Mammillaria. Monographie 2. – Steinhart, Titisee-Neustadt.
Rettig JH, Wilson HD, Manhart JR. 1992.
Phylogeny of the Caryophyllales – gene
sequence data. – Taxon 41: 201-209.
Reveal JL. 1968. Notes on Eriogonum
IV. A revision of the Eriogonum deflexum complex. – Brittonia 20:
13-33.
Reveal JL. 1969a. A revision of the genus
Eriogonum. – Ph.D. diss., Brigham Young University, Provo, Utah.
Reveal JL. 1969b. The subgeneric concept in
Eriogonum (Polygonaceae). – In: Gunckel J
(ed), Current topics in plant science, Academic Press, New York, pp.
229-249.
Reveal JL. 1978. Distribution and phylogeny
of Eriogonoideae (Polygonaceae). – In: Harper KT,
Reveal JL (eds), Intermountain biogeography: a symposium, Great Basin Natur.
Mem. 2, Brigham Young University Press, Provo, Utah, pp. 169-190.
Reveal JL. 1983. The Demoulin rule and newly
mandated combinations in Eriogonum (Polygonaceae). – Taxon 32:
292-295.
Reveal JL. 1989a. Remarks on the genus
Pterostegia (Polygonaceae:
Eriogonoideae). – Phytologia 66: 228-235.
Reveal JL. 1989b. Notes on selected genera
related to Eriogonum (Polygonaceae: Eriogonoideae). –
Phytologia 66: 236-245.
Reveal JL. 1989c. A checklist of the
Eriogonoideae (Polygonaceae).
– Phytologia 66: 266-294.
Reveal JL. 1989d. The eriogonoid flora of
California (Polygonaceae:
Eriogonoideae). – Phytologia 66: 295-414.
Reveal JL. 2004a. Johanneshowellia
(Polygonaceae: Eriogonoideae),
a new genus from the Intermountain West. – Brittonia 56: 299-306.
Reveal JL. 2004b. Nomenclatural summary of Polygonaceae subfamily
Eriogonoideae. – Harvard Pap. Bot. 9: 143-230.
Reveal JL. 2004c. Proposal to conserve the
name Gilmania Coville against Phyllogonum Coville (Polygonaceae: Eriogonoideae) –
a case of mistaken homonymy. – Taxon 53: 573.
Reveal JL, Bjoerk CR. 2004. Eriogonum
soliceps (Polygonaceae:
Eriogonoideae), a new species from east-central Idaho and southwestern Montana.
– Brittonia 56: 295-298.
Reveal JL, Doweld A. 2008. Proposal to
conserve the name Aizoaceae
against Mesembryanthemaceae, a “superconservation” proposal. – Taxon 57:
302.
Reveal JL, Ertter BJ. 1977.
Goodmania (Polygonaceae), a new genus from
California. – Brittonia 28: 427-429.
Reveal JL, Hardham CB. 1989a. Three new
monospecific genera of Polygonaceae subfamily
Eriogonoideae from California. – Phytologia 66: 83-88.
Reveal JL, Hardham CB. 1989b. A revision of
the annual species of Chorizanthe (Polygonaceae: Eriogonoideae). –
Phytologia 66: 98-198.
Reveal JL, Howell JT. 1976.
Dedeckera (Polygonaceae), a new genus from
California. – Brittonia 28: 245-251.
Reyes-Rivera J, Canché-Escamilla G,
Soto-Hernández M, Terrazas T. 2015. Wood chemical composition in species of Cactaceae: the relationship between
lignification and stem morphology. – PLoS ONE 10:e0123919.
Reyes-Salas M, Martínez-Hernández E. 1982.
Palynological catalog for the flora of Veracruz, Mexico 8. Nyctaginaceae family. –
Biotica (Mexico) 7: 423-456.
Reznicek T, Britton DM. 1971. Chromosome
studies on the Spring Beauties, Claytonia, in Ontario. – Mich. Bot.
10: 51-62.
Reznik H. 1955. Die Pigmente der
Centrospermen als systematisches Element. – Zeitschr. Bot. 43: 499-530.
Reznik H. 1957. Die Pigmente der
Centrospermen als systematisches Element II. Untersuchung über das
ionophoretische Verhalten. – Planta 49: 406-434.
Reznik H. 1975. Betalaine. – Ber. Deutsch.
Bot. Ges. 88: 179-190.
Richardson PM. 1978. Flavonols and
C-glycosylflavonoids of the Caryophyllales. – Biochem.
Syst. Ecol. 6: 283-286.
Richardson PM. 1981. Flavonoids of some
controversial members of the Caryophyllales (Centrospermae).
– Plant Syst. Evol. 138: 227-233.
Riha J, Riha JJ. 1975. The genus
Mammilloydia Buxbaum. – Cactus Succ. J. (U.S.) 47: 195-197.
Rilke S. 1999a. Species diversity and
polymorphism in Salsola sect. Salsola sensu lato
(Chenopodiaceae). – Syst. Geogr. Plants 68: 305-314.
Rilke S. 1999b. Revision der Sektion
Salsola s.l. der Gattung Salsola (Chenopodiaceae). – Bibl.
Bot. 149: 1-189.
Ritter F. 1962. Calymmanthium –
eine neue Cereengattung aus Peru. – Kakt. Sukk. 13: 24-28.
Ritter F. 1979-1981. Kakteen in Südamerika.
Ergebnisse meiner 20-jährigen Feldforschungen 1-4. – Selbstverlag,
Spangenberg, Germany.
Ritz CM, Mecklenburg R. 2008. Die Phylogenie
von Rebutia und ihren Verwandten spiegelt die geologische Geschichte
Südamerikas wider. – Kakt. Sukk. 59: 157-170.
Ritz CM, Martins L, Mecklenburg R, Goremykin
V, Hellwig FK. 2007. The molecular phylogeny of Rebutia (Cactaceae) and its allies
demonstrates the influence of paleogeography on the evolution of South American
mountain cacti. – Amer. J. Bot. 94: 1321-1332.
Ritz CM, Reiker J, Charles G, Hoxey P, Hunt
D, Lowry M, Stuppy W, taylor N. 2012. Molecular phylogeny and character
evolution in terete-stemmed Andean opuntias (Cactaceae-Opuntioideae). – Mol.
Phylogen. Evol. 65: 668-681.
Ritz CM, Fickenscher K, Föller J, Herrmann
K, Mecklenburg R, Wahl R. 2016. Molecular phylogenetic relationships of the
Andean genus Aylostera Speg. (Cactaceae, Trichocereeae), a new
classification and a morphological identification key. – Plant Syst. Evol.
302: 763-780.
Rivadavia F, Kondo K, Kato M, Hasebe M. 2003.
Phylogeny of the sundews, Drosera (Droseraceae), based on chloroplast
rbcL and nuclear 18S ribosomal DNA sequences. – Amer. J. Bot. 90:
123-130.
Rivadavia F, Miranda VFO de, Hoogenstrijd G,
Pinheiro F, Heubl G, Fleischmann A. 2012. Is Drosera meristocaulis a
pygmy sundew? Evidence of a long-distance dispersal between Western Australia
and northern South America. – Ann. Bot. 110: 11-21.
Rivera ER, Smith BN. 1979. Crystal morphology
and 13carbon/12carbon composition of solid oxalate in
cacti. – Plant Physiol. 64: 966-970.
Roberts PR, Oosting HJ. 1958. Responses of
Venus’ flytrap to factors involved in its endemism. – Ecol. Monogr. 28:
193-218.
Robertson JH. 1983. Greasewood
(Sarcobatus vermiculatus (Hook.) Torr.). – Phytologia 54:
309-324.
Roberty G, Vautier S. 1964. Les genres des
Polygonacées. – Boissiera 10: 7-128.
Robins RJ, Juniper BE. 1980a. The secretory
cycle of Dionaea muscipula Ellis I. The fine structure and the effect
of stimulation on the fine structure of the digestive gland cells. – New
Phytol. 86: 279-296.
Robins RJ, Juniper BE. 1980b. The secretory
cycle of Dionaea muscipula Ellis III. The mechanism of release of
digestive secretion. – New Phytol. 85: 313-327.
Robinson AS, Fleischmann AS, McPherson SR,
Heinrich VB, Gironella EP, Pena CQ. 2009. A spectacular new species of
Nepenthes L. (Nepenthaceae) pitcher plant from
central Palawan, Philippines. – Bot. J. Linn. Soc. 159: 195-202.
Robinson H. 1973. New combinations in the Cactaceae subfamily Opuntioideae.
– Phytologia 26: 175-176.
Robinson H. 1974. Scanning electron
microscope studies of the spines and glochids of the Opuntioideae (Cactaceae). – Amer. J. Bot. 61:
278-283.
Rocén T. 1927. Zur Embryologie der
Centrospermen. – Ph.D. diss., University of Uppsala, Sweden.
Rodkiewicz B, Bedmara J, Kudlicka K. 1983.
Egg apparatus walls and vacuoles in Stellaria media. – In: Erdelska
O (ed), Fertilization and embryogenesis in ovulated plants, Veda, Bratislava,
pp. 211-214.
Rodman JE. 1990. The Centrospermae revisited
1. – Taxon 39: 383-393.
Rodman JE. 1994. Cladistic and phenetic
studies. – In: Behnke H-D, Mabry TJ (eds), Caryophyllales: evolution and
systematics, Springer, Berlin, pp. 279-301.
Rodman JE, Oliver MK, Nakamura RR, McClammer
Jr JU, Bledsoe AH. 1984. A taxonomic analysis and revised classification of
Centrospermae. – Syst. Bot. 9: 297-323.
Rodríguez-Rodríguez JF, Shiskova S,
Napsucialy-Mendivil S, Dubrovsky JG. 2003. Apical meristem organization and
lack of establishment of the quiescent center in Cactaceae roots with determinate
growth. – Planta 217: 849-857.
Rohrbach P. 1868. Monographie der Gattung
Silene. – Lipsiae.
Rohweder O. 1965a. Centrospermen-Studien 1.
Der Blütenbau bei Uebelinia kiwuensis T. C. E. Fries (Caryophyllaceae). – Bot.
Jahrb. Syst. 83: 406-418.
Rohweder O. 1965b. Centrospermen-Studien 2.
Entwicklung und morphologische Deutung der Gynöciums bei Phytolacca.
– Bot. Jahrb. Syst. 84: 509-526.
Rohweder O. 1967. Centrospermen-Studien 3.
Blütenentwicklung und Blütenbau bei Silenoideen (Caryophyllaceae). – Bot.
Jahrb. Syst. 86: 130-185.
Rohweder O. 1970. Centrospermen-Studien 4.
Morphologie und Anatomie der Blüten, Früchte, und Samen bei Alsinoideen und
Paronychioideen s. lat. (Caryophyllaceae). – Bot.
Jahrb. Syst. 90: 201-271.
Rohweder O, Huber K. 1974.
Centrospermen-Studien 7. Beobachtungen und Anmerkungen zur Morphologie und
Entwicklungsgeschichte einiger Nyctaginaceen. – Bot. Jahrb. Syst. 94.
327-359.
Rohweder O, König K. 1971.
Centrospermen-Studien 5. Bau der Blüten, Früchte, und Samen von
Pteranthus dichotomus Forsk. (Caryophyllaceae). – Bot.
Jahrb. Syst. 90: 447-468.
Rohweder O, Urmi E. 1978.
Centrospermen-Studien 10. Untersuchungen über den Bau der Blüten und Früchte
von Cucubalus baccifer L. und Drypis spinosa L. (Caryophyllaceae-Silenoideae).
– Bot. Jahrb. Syst. 100: 1-25.
Rohweder O, Urmi-König K. 1975.
Centrospermen-Studien 8. Beiträge zur Morphologie, Anatomie, und
systematischen Stellung von Gymnocarpos Forsk. und Paronychia
argentea Lam. (Caryophyllaceae). – Bot.
Jahrb. Syst. 96: 375-409.
Rohwer JG. 1982. A taxonomic revision of the
genera Seguieria Loefl. and Gallesia Casar. (Phytolaccaceae). – Mitt. Bot.
Staatssamml. München 18: 231-288.
Rohwer JG. 1993a. Phytolaccaceae. – In:
Kubitzki K, Rohwer JG, Bittrich V (eds), The families and genera of vascular
plants II. Flowering plants. Dicotyledons. Magnoliid, hamamelid and
caryophyllid families, Springer, Berlin, Heidelberg, New York, pp. 506-515.
Rohwer JG. 1993b. Stegnospermaceae. – In:
Kubitzki K, Rohwer JG, Bittrich V (eds), The families and genera of vascular
plants II. Flowering plants. Dicotyledons. Magnoliid, hamamelid and
caryophyllid families, Springer, Berlin, Heidelberg, New York, pp. 592-594.
Roland F. 1967. Mise en évidence d’une
membrane aperturale particulière dans le pollen de quelques Aizoacées. –
Compt. Rend. Acad. Sci. Paris 264: 2986-2988.
Ronse De Craene L-P. 1990. Morphological
studies of Tamaricales I. Floral ontogeny and anatomy of Reaumuria
vermiculata L. – Beitr. Biol. Pflanzen 65: 181-203.
Ronse De Craene L-P. 2013. Reevaluation of
the perianth and androecium in Caryophyllales: implications
for flower evolution. – Plant Syst. Evol. 299: 1599-1636.
Ronse De Craene L-P, Akeroyd JR. 1988.
Generic limits in Polygonum and related genera (Polygonaceae) on the basis of
floral characters. – Bot. J. Linn. Soc. 98: 321-371.
Ronse De Craene L-P, Smets EF. 1991a. The
floral nectaries of Polygonum s.l. and related genera (Persicarieae
and Polygoneae): position, morphological nature and semophylesis. – Flora
185: 165-185.
Ronse De Craene L-P, Smets EF. 1991b. The
floral ontogeny of some members of the Phytolaccaceae (subfamily
Rivinoideae) with a discussion of the evolution of the androecium in the
Rivinoideae. – Biol. Jaarb. Dodonaea 59: 77-99.
Ronse De Craene L-P, Vanvinckenroye P, Smets
E. 1997. A study of the floral morphological diversity in Phytolacca
(Phytollacaceae) based on early floral ontogeny. – Intern. J. Plant Sci. 158:
56-72.
Ronse De Craene L-P, Smets EF, Vanvinckenroye
P. 1998. Pseudodiplostemony, and its implications for the evolution of the
androecium in the Caryophyllaceae. – J. Plant
Res. 111: 25-43.
Ronse De Craene L-P, Volgin SA, Smets EF.
1999. The floral development of Pleuropetalum darwinii, an anomalous
member of Amaranthaceae. –
Flora 194: 189-199.
Ronse De Craene L-P, Hong S-P, Smets E. 2000.
Systematic significance of fruit morphology and anatomy in tribes Persicarieae
and Polygoneae (Polygonaceae).
– Bot. J. Linn. Soc. 134: 301-337.
Ronse De Craene L-P, Hong S-P, Smets E. 2004.
What is the taxonomic status of Polygonella? Evidence of floral
morphology. – Ann. Missouri Bot. Gard. 91: 320-345.
Rose JN, Standley PC. 1911. The genus
Talinum in Mexico. – Contr. U. S. Natl. Herb. 13: 281-288.
Ross R. 1981. Chromosome counts, cytology,
and reproduction in the Cactaceae. – Amer. J. Bot. 68:
463-470.
Rossbach RP. 1943. El género
Spergularia (Caryophyllaceae) en Chile. –
Darwiniana 6: 211-256.
Rost TL, Samper AD, Schell P, Allen S. 1977.
Anatomy of jojoba (Simmondsia chinensis) seed and the utilization of
liquid wax during germination. – Econ. Bot. 31: 140-147.
Roth I. 1953. Zur Entwicklungsgeschichte und
Histogenese der Schlauchblätter von Nepenthes. – Planta 42:
177-208.
Roth I. 1954. Entwicklung und
histogenetischer Vergleich der Nektar- und Verdauungsdrüsen von
Nepenthes. – Planta 43: 361-378.
Roth I. 1962a. Histogenese und morphologische
Deutung der basilären Plazenta von Armeria. – Österr. Bot.
Zeitschr. 109: 19-40.
Roth I. 1962b. Histogenese und morphologische
Deutung der Kronblätter von Armeria. – Port. Acta Biol., ser. A, 6:
211-230.
Roth I. 1963. Histogenese und morphologische
Deutung der Zentralplacenta von Cerastium. – Bot. Jahrb. Syst. 82:
100-118.
Rousi A, Yli-Rekola M, Jokela P, Kalliola R,
Pietilä L, Salo J. 1988. The fruit of Ullucus (Basellaceae), an old enigma. –
Taxon 37: 71-75.
Rowley GD. 1958. Reunion of the genus
Opuntia Mill. – Natl. Cact. Succ. J. 13: 3-6.
Rowley GD. 1974. The unhappy medium:
Morangaya a new genus of Cactaceae. – Ashingtonia 1:
43-45.
Rowley GD. 1978. Reunion of some genera of
Mesembryanthemaceae. – Cact. Succ. J. (Great Britain) 33: 6-9.
Rowley GD. 1992. Didiereaceae. ‘Cacti of the Old
World’. – British Cactus and Succulent Society, Kew.
Rowley GD. 1994. Anacampseros and
allied genera – a reassessment. – Bradleya 12: 105-112.
Rowley GD. 1995. Anacampseros,
Avonia, Grahamia: A grower’s guide. – British Cactus and
Succulent Society, Hull Road.
Rowley GD. 1997. A history of succulent
plants. – Strawberry Press, Mill Valley, California.
Rowley GD. 2004a. Spontaneous bigeneric
hybrids in Cactaceae. –
Bradleya 12: 2-7.
Rowley GD. 2004b. Intergeneric hybrids in Cactaceae – 2004 update. – Brit.
Cact. Succ. J. 22: 64-65.
Runemark H. H. 1974. Studies in the Aegean
flora XXII. Goniolimon Boiss (Plumbaginaceae). – Bot. Not.
127: 540-545.
Runemark H. 1980. Studies in the Aegean flora
XXIII. The Dianthus fruticosus complex (Caryophyllaceae). – Bot.
Not. 133: 475-490.
Russell AD. 2003. Phylogenetic analysis and
morphological study of the subfamily Eriogonoideae (Polygonaceae) with an emphasis on
the genus Chorizanthe. – M.Sc. thesis, San Diego State University,
San Diego, California.
Rutishauser R. 1981. Basale Blattauswüchse
bei Centrospermen. – In: Cotthem W van (ed), Morphologie, Anatomie und
Systematik der Pflanzen, 5. Symposium Gent 1979, Waegeman, Ninove (Belgium),
pp. 21-27.
Ruxton GD, Schaefer HM. 2011. Alternative
explanations for apparent mimicry. – J. Ecol. 99: 899-904.
Sadeghian S, Zarre S, Heubl G. 2014.
Systematic implication of seed micromorphology in Arenaria (Caryophyllaceae) and allied
genera. – Flora 209: 513-529.
Sadeghian S, Zarre S, Rabeler RK, Heubl G.
2015. Molecular phylogenetic analysis of Arenaria (Caryophyllaceae: tribe
Arenarieae) and its allies inferred from nuclear DNA internal transcribed
spacer and plastid DNA rps16 sequences. – Bot. J. Linn. Soc. 178: 648-669.
Sage RF, Sage TL, Pearcy RW, Borsch T. 2007.
The taxonomic distribution of C4 photosynthesis in Amaranthaceae sensu stricto. –
Amer. J. Bot. 94: 1992-2003.
Sage RF, Christin PA, Edwards EJ. 2011. The
C4 plant lineages of planet Earth. – J. Exp. Bot. 62: 3155-3169.
Sahashi N, Ikuse M. 1973. Pollen morphology
of Aldrovanda vesiculosa L. – J. Jap. Bot. 48: 374-379.
Sakai AK, Weller SG, Karoly K. 1989.
Inbreeding depression in Schiedea globosa and S. salicaria
(Caryophyllaceae),
subdioecious and gynodioecious Hawaiian species. – Amer. J. Bot. 76:
437-444.
Sakai AK, Weller SG, Wagner WL, Solis PS,
Soltis DE. 1997. Phylogenetic perspectives on the evolution of dioecy: adaptive
radiation in the endemic Hawaiian genera Schiedea and
Alsinidendron (Caryophyllaceae: Alsinoideae).
– In: Givnish TJ, Sytsma KJ (eds), Molecular evolution an adaptive radiation,
Cambridge University Press, New York, pp. 455-473.
Sakai AK, Weller SG, Wagner WL, Nepokroeff M,
Culley TM. 2006. Adaptive radiation and evolution of breeding systems in
Schiedea (Caryophyllaceae), an endemic
Hawaiian genus. – Ann. Missouri Bot. Gard. 93: 49-63.
Salgues R. 1961. Le genre Polygonum
(Polygonacées). Études chimiques et toxicologiques. – Qual. Pl. Mater. Veg.
8: 367-395.
Samuelsson G. 1930. Polygonaceae. – Acta Horti
Gothob. 5: 1-11.
Sanchez A, Kron KA. 2008. Phylogenetics of Polygonaceae with an emphasis on
the evolution of Eriogonoideae. – Syst. Bot. 33: 87-96.
Sanchez A, Kron KA. 2009. Phylogenetic
relationships of Afrobrunnichia Hutch. & Dalziel (Polygonaceae) based on three
chloroplast genes and ITS. – Taxon 58: 781-792.
Sanchez A, Kron KA. 2011. Phylogenetic
relationships of Triplaris and Ruprechtia: re-delimitation of
the recognized genera and two new genera for tribe Triplarideae (Polygonaceae). – Syst. Bot. 36:
702-710.
Sanchez A, Schuster TM, Kron KA. 2009. A
large-scale phylogeny of Polygonaceae based on molecular
data. – Intern. J. Plant Sci. 170: 1044-1055.
Sanchez A, Schuster TM, Burke JM, Kron KA.
2011. Taxonomy of Polygonoideae (Polygonaceae): a new tribal
classification. – Taxon 60: 151-160.
Sánchez D, Arias S, Terrazas T. 2014.
Phylogenetic relationships in Echinocereus (Cactaceae, Cactoideae). – Syst.
Bot. 39: 1183-1196.
Sánchez-del Pino I. 2007. Phylogeny and
floral evolution of the subfamily Gomphrenoideae (Amaranthaceae). – Ph.D. diss.,
City University of New York.
Sánchez-del Pino I, Flores Olvera H. 2002.
New taxa and a new combination in Tidestromia (Amaranthaceae) from North
America. – Novon 12: 399-407.
Sánchez-del Pino I, Flores Olvera H. 2006.
Phylogeny of Tidestromia (Amaranthaceae, Gomphrenoideae)
based on morphology. – Syst. Bot. 31: 689-701.
Sánchez-del Pino I, Borsch T, Motley TJ.
2009. trnL-F and rpl16 sequence data and dense taxon sampling
reveal monophyly of unilocular anthered Gomphrenoideae (Amaranthaceae) and an improved
picture of their internal relationships. – Syst. Bot. 34: 57-67.
Sánchez-del Pino I, Motley TJ, Borsch T.
2012. Molecular phylogenetics of Alternanthera (Gomphrenoideae, Amaranthaceae): resolving a
complex taxonomi chistory caused by different interpretations of morphological
characters in a lineage with C4 and C3-C4
intermediate species. – Bot. J. Linn. Soc. 169: 493-517.
Sánchez-del Pino I, Espadas C, Pool R. 2013.
Taxonomy and richness of nine genera of Amaranthaceae s.s. (Caryophyllales) in the Yucatan
Peninsula Biotic Province. – Phytotaxa 107: 1-74.
Sánchez-Mejorda H. 1974. Revisión del
género Peniocereus (Las Cactáceas). – Gobierno del Estado de
México, Dirección de Agricultura y Ganaderia, Toluca, México.
Sanderson SC, Ge-Ling C, McArthur ED, Stutz
HC. 1988. Evolutionary loss of flavonoids an other chemical characters in the
Chenopodiaceae. – Biochem. Syst. Ecol. 16: 143-149.
Santa Anna del Conde-Juárez H,
Contreras-Medina R, Luna-Vega I. 2009. Biogeographic analysis of endemic cacti
of the Sierra Madre Oriental, Mexico. – Biol. J. Linn. Soc. 97: 373-389.
Santoni S, Bervillé A. 1992.
Characterization of the nuclear ribosomal DNA units and phylogeny of
Beta L. wild forms and cultivated beets. – Theor. Appl. Gen. 83:
533-542.
Sattler R, La Croix C. 1988. Development and
evolution of basal cauline placentation: Basella rubra. – Amer. J.
Bot. 75: 913-927.
Sattler R, Perlin L. 1982. Floral development
of Bougainvillea spectabilis Willd., Boerhavia diffusa L. and
Mirabilis jalapa L. (Nyctaginaceae). – Bot. J.
Linn. Soc. 84: 161-182.
Sauer W. 1965. Die Moehringia
bavarica-Gruppe. – Bot. Jahrb. Syst. 84: 257-273.
Schaeppi H. 1936. Zur Morphologie des
Gynoeceums der Phytolaccaceen. – Flora 131: 41-59.
Schäferhoff B, Müller KF, Borsch T. 2009.
Caryophyllales
phylogenetics: disentangling Phytolaccaceae and Molluginaceae and description of
Microteaceae as a new isolated
family. – Willdenowia 39: 209-228.
Schatz GE, Lowry PP II, Wolf A-E. 1999.
Endemic families of Madagascar IV. A synoptic revision of Asteropeia
(Asteropeiaceae). –
Adansonia, sér. III, 21: 255-268.
Scheen AC, Brochmann C, Brysting AK, Elven R,
Morris A, Soltis DE, Soltis PS, Albert VA. 2004. Northern Hemisphere
biogeography of Cerastium (Caryophyllaceae): insights
from phylogenetic analysis of noncoding plastidnucleotide sequences. – Amer.
J. Bot. 91: 943-952.
Schenk HJ, Ferren WR. 2001. On the sectional
nomenclature of Suaeda (Chenopodiaceae). – Taxon 50: 857-873.
Schill R, Rauh W, Wieland H-P. 1974. Weitere
Untersuchungen an Didiereaceen 4. Die Chromosomenzahlen der einzelnen Arten.
– Akad. Wiss. Lit. Math.-Nat. Kl., Trop. Subtrop. Pflanzenwelt 11: 1-14.
Schiman-Czeika H. 1987. Notes on the capsule
dehiscence in Acanthophyllum (Caryophyllaceae) and allied
genera. – Plant Syst. Evol. 155: 67-69.
Schinz H. 1893. Amaranthaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien III(1a), W. Engelmann,
Leipzig, pp. 91-118.
Schinz H. 1894. Ficoideae. – Bull. Herb.
Boiss. 2: 204-205.
Schinz H. 1934. Amaranthaceae. – In: Engler A
(†), Pax F, Harms H (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd.
16c, W. Engelmann, Leipzig, pp. 7-85.
Schinz H, Autran E. 1893. Des Genres
Achatocarpus Triana et Bosia Linné et de leur place dans le
système naturel. – Bull. Herb. Boiss. 1: 1-14.
Schlauer J. 1996. A dichotomous key to the
genus Drosera L. (Droseraceae). – Carniv. Plant
Newsl. 25: 67-88.
Schlegel U. 2009. The composite structure of
cactus spines. – Bradleya 27: 129-138.
Schlumpberger BO. 2009. More on the phylogeny
and evolution of Echinopsis s.l. – IOS-Bull. 15: 48.
Schlumpberger BO. 2012. New combinations in
the Echinopsis alliance. – Cactaceae Syst. Init. 28: 30-32.
Schlumpberger BO, Renner SS. 2012. Molecular
phylogenetics of Echinopsis (Cactaceae): polyphyly at all levels
and convergent evolution of pollination modes and growth forms. – Amer. J.
Bot. 99: 1335-1349.
Schmalzel RJ, Nixon RT, Best AL, Tress JA.
2004. Morphometric variation in Coryphantha robustispina (Cactaceae). – Syst. Bot. 29:
553-568.
Schmid R. 1964. Die systematische Stellung
der Dioncophyllaceen. – Bot. Jahrb. Syst. 83: 1-56.
Schmid R. 1978. Floral and fruit anatomy of
jojoba (Simmondsia chinensis). – Mem. 2nd Intern. Conf.
Jojoba y su Aprov. Ensenada, México, 1976, pp. 143-148.
Schmid W. 1925. Morphologische, anatomische
und entwicklungsgeschichtliche Untersuchungen an Mesembryanthemum
pseudotruncatellum Berger. – Beibl. Vierteljahrsschr. Naturforsch. Ges.
Zürich 8: 1-80.
Schmid-Hollinger R. 1970.
Nepenthes-Studien I. Homologien von Deckel (operculum, lid) und
Spitzchen (calcar, spur). – Bot. Jahrb. Syst. 90: 275-296.
Schmid-Hollinger R. 1971.
Nepenthes-Studien II. Die Haare der Nepenthaceen und ihre
phylogenetische Bedeutung. – Bot. Jahrb. Syst. 91: 61-90.
Schmid-Hollinger R. 1977.
Nepenthes-Studien IV. Eine neue Nepenthes-Art aus Madagascar.
Nepenthes masoalensis sp. nov. – Bot. Jahrb. Syst. 97: 575-585.
Schmid-Hollinger R. 1979. Die Kannenformen
der westlichen Nepenthes-Arten. – Bot. Jahrb. Syst. 100: 379-405.
Schmidt R. 1964. Die systematische Stellung
der Dioncophyllaceen. – Bot. Jahrb. Syst. 83: 1-56.
Schmitz-Linneweber C, Maier RM, Alcaraz JP,
Cottet A, Herrmann RG, Mache R. 2001. The plastid chromosome of spinach
(Spinacia oleracea): complete nucleotide sequence and gene
organization. – Plant Mol. Biol. 45: 307-315.
Schölch H-F. 1963. Die systematische
Stellung der Didiereaceen im Lichte neuer Untersuchungen über ihren
Blütenbereich. – Ber. Deutsch. Bot. Ges. 76: 49-55.
Schönland S. 1903. Morphological and
biological observations on the genus Anacampseros L.
(Rulingia Ehrh.). – South Afr. Rep. Sci. 1: 295-301.
Schoute JC. 1935. Observations on the
inflorescence in the family of the Plumbaginaceae. – Rec. Trav.
Bot. Néerl. 32: 406-424.
Schulz R. 2006. Copiapoa in their
environment. – Schulz Publ., Teesdale.
Schulz R, Machado M. 2000.
Uebelmannia and their environment. – Schulz Publ., Teesdale.
Schulze W, Schulze ED, Pate JS, Gillison AN.
1997. The nitrogen supply from soils and insects during growth of the pitcher
plants Nepenthes mirabilis, Cephalotus follicularis and
Darlingtonia californica. – Oecologia 112: 464-471.
Schumann K. 1894. Cactaceae. – In: Engler A, Prantl
K (eds), Die natürlichen Pflanzenfamilien III(6a), W. Engelmann, Leipzig, pp.
156-205; Schumann K. 1897. Nachträge zu III(6a), pp. 258-260.
Schumann K. 1897. Neue Kakteen aus dem
Andengebiet. – Monatsschr. Kakteenkunde 7: 6-9.
Schumann K. 1897-1899. Gesamtbeschreibung der
Kakteen (Monographia Cactacearum). – J. Neumann, Neudamm, Germany.
Schumann K. 1899. Die Verbreitung der Cactaceae im Verhältnis zu ihrer
systematischen Gliederung. – Abhandl. Königl. Akad. Wissensch. Berlin 2:
1-114.
Schumann K. 1903. Gesamtbeschreibung der
Kakteen (Monographia Cactacearum). Nachträge 1898-1902. – J. Neumann,
Neudamm.
Schuster TM, Reveal JL, Kron KA. 2011.
Phylogeny of Polygoneae (Polygonaceae: Polygonoideae). –
Taxon 60: 1653-1666.
Schuster TM, Wilson KL, Kron KA. 2011.
Phylogenetic relationships of Muehlenbeckia, Fallopia, and
Reynoutria (Polygonaceae) investigated with
chloroplast and nuclear sequence data. – Intern. J. Plant Sci. 172:
1053-1058.
Schuster TM, Setaro SD, Kron KA. 2013. Age
estimates for the buckwheat family Polygonaceae based on sequence
data calibrated by fossils and with a focus on the Amphi-Pacific
Muehlenbeckia. – PloS ONE 8:e61261
Schuster TM, Reveal JL, Bayly MJ, Kron KA.
2015. An updated molecular phylogeny of Polygonoideae (Polygonaceae): relationships of
Oxygonum, Pteroxygonum, and Rumex, and a new
circumscription of Koenigia. – Taxon 64: 1188-1208.
Schütte KH, Steyn R, Westhuizen M van der.
1967. Crassulacean acid metabolism in South African succulents: a preliminary
investigation in its occurrence in various families. – J. South Afr. Bot. 33:
107-110.
Schütz B. 1962. K systematice rodu
Gymnocalycium. – Friciana 1: 1-8.
Schütz B. 1969. Rodu Gymnocalycium.
– Friciana 7: 3-23.
Schütz B. 1986. Monografie rodu
Gymnocalycium. – Vydal Klub Kaktusaru Astrophytum, Brno.
Schütze PW. 2008. Molekulare Systematik der
Gattung Suaeda (Chenopodiaceae) und Evolution des
C4-Photosynthesesyndroms. – Ph.D. thesis, Fachbereich Naturwissenschaften der
Universität Kassel.
Schütze P, Freitag H, Weising K. 2003. An
integrated molecular and morphological study of the subfamily Suaedoideae Ulbr.
(Chenopodiaceae). – Plant Syst. Evol. 239: 257-286.
Schwallier R, Gravendeel B, Boer H de,
Nylinder S, Heuven BJ van, Sieder A, Sumail S, Vugt R van, Lens F. 2017.
Evolution of wood anatomical characters in Nepenthes and close
relatives of Caryophyllales.
– Ann. Bot. 119: 1179-1193.
Schwantes G. 1927. Zur Systematik der
Mesembryanthemen. – Sukkulentenkunde 3: 14-30.
Schwantes G. 1947. System der
Mesembryanthemaceen. – Sukkulentenkunde 1: 34-40.
Schwantes G. 1952. Die Früchte der
Mesembryanthemaceen. – Vierteljahrsschr. Naturforsch. Ges. Zürich 97, Beih.
2: 1-38.
Schwantes G. 1957a. Ficoidaceae (Juss.) em.
Hutchinson. – Kakteen Sukk. 8: 167-169.
Schwantes G. 1957b. Flowering stones and
mid-day flowers. – Ernest Benn Ltd., London.
Schwarz O. 2003. Atriplex micrantha
C. A. Mey. in Ledeb. und andere Meldearten. Nomenklatur, Morphologie,
Verbreitung, Ökologie und Taxonomie. – Jahresh. Ges. Naturk. Württemberg
159: 113-195.
Schwarzová T. 1986. Chromosome numbers of
some species of the genus Chenopodium L. from localities in
Czechoslovakia. – Acta Fac. Rerum Nat. Univ. Comen. 33: 37-40.
Schweingruber FH. 2007. Stem anatomy of Caryophyllaceae. – Flora
202: 281-292.
Scogin R. 1980. Serotaxonomy of
Simmondsia chinensis (Simmondsiaceae). – Aliso 9:
555-559.
Scogin R, Brown S. 1979. Leaf flavonoids of
Simmondsia chinensis (Simmondsiaceae). – Aliso 9:
475-477.
Scott AJ. 1977. Reinstatement and revision of
Salicorniaceae J. Agardh (Caryophyllales). – Bot. J.
Linn. Soc. 75: 357-374.
Scott AJ. 1978a. Rhagodiinae: a new subtribe
in the Chenopodiaceae. – Feddes Repert. 89: 1-12.
Scott AJ. 1978b. A revision of the
Camphorosmoideae (Chenopodiaceae). – Feddes Repert. 89: 101-119.
Scott AJ. 1978c. A review of the
classification of Chenopodium L. and related genera. – Bot. Jahrb.
Syst. 100: 205-220.
Scott AJ, Ford-Lloyd BV, Williams JT. 1977.
Patellifolia, nomen novum (Chenopodiaceae). – Taxon 26: 284.
Seine R, Barthlott W. 1992. Ontogeny and
morphological quality of the marginal bristles of Dionaea muscipula
Ellis (Droseraceae). – Beitr.
Biol. Pflanzen 67: 289-294.
Seine R, Barthlott W. 1993. On the morphology
of trichomes and tentacles of Droseraceae Salisb. – Beitr.
Biol. Pflanzen 67: 345-366.
Seine R, Barthlott W. 1994. Some proposals on
the infrageneric classification of Drosera L. – Taxon 43:
583-589.
Sekar KC, Srivastava SK. 2007. Stellaria
pinvalliaca (Caryophyllaceae), a new
species from India. – Feddes Repert. 118: 20-23.
Sell PD, Whitehead FH. 1964. Notes on the
annual species of Cerastium in Europe. – Feddes Repert. 69:
14-24.
Selvi F. 2009. Armeria saviana sp.
nov. (Plumbaginaceae) from
central Italy. – Nord. J. Bot. 27: 125-133.
Senda M, Mikami T, Kinoshita T, Mikami T.
1995. Mitochondrial gene variation and phylogenetic relationships in the genus
Beta. – Theor. Appl. Gen. 90: 914-919.
Sharma A, Ghosh S. 1968. Cytotaxonomy of
Ficoideae. – Cytologia 33: 439-452.
Sharma AK, Banik M. 1965. Cytological
investigation of different genera of Amaranthaceae with a view to
trace their interrelationships. – Bull. Bot. Soc. Bengal 19: 40-50.
Sharma HP. 1954. Studies in the order
Centrospermales I. Vascular anatomy of the flower of certain species of the Portulacaceae. – J. Indian
Bot. Soc. 47: 98-111.
Sharma HP. 1961. Contribution to the
morphology and anatomy of Basella rubra Linn. – Bull. Bot. Soc.
Bengal 15: 43-48.
Sharma HP. 1962a. Contributions to the
morphology of the Nyctaginaceae I. Anatomy of the
node and inflorescence of some species. – Proc. Indian Acad. Sci., Sect. B,
56: 35-50.
Sharma HP. 1962b. Studies in the order
Centrospermales III. Vascular anatomy of the flower of some species of the
family Ficoidaceae. – Proc. Indian Acad. Sci., Sect. B, 56: 269-285.
Sharma HP. 1963a. Contributions to the
morphology of the Nyctaginaceae II. Floral anatomy
of some species. – Proc. Indian Acad. Sci., Sect. B, 57: 149-163.
Sharma HP. 1963b. Studies in the order
Centrospermales II. Vascular anatomy of the flower of certain species of the Molluginaceae, Nyctaginaceae, and Portulacaceae. – J. Indian
Bot. Soc. 42: 19-32, 637-645.
Sharsmith CW. 1938. On the identity of
Claytonia nevadensis Watson. – Madroño 4: 171-176.
Sheikh SA, Kondo K. 1995. Differential
staining with orcein, Giemsa, CMA, and DAPI for comparative chromosome study of
12 species of Australian Drosera. – Amer. J. Bot. 82: 1278-1286.
Sheikh SA, Kondo K, Hoshi Y. 1995. Study on
diffused centromeric nature of Drosera chromosomes. – Cytologia 60:
43-47.
Shen Y, Ford-Lloyd BV, Newbury HJ. 1998.
Genetic relationships within the genus Beta determined using both
PCR-based marker and DNA sequencing techniques. – Heredity 80: 624-632.
Shepherd KA. 2007. Three new species of
Tecticornia (formerly Halosarcia) (Chenopodiaceae:
Salicornioideae) from the Ememaean Botanical Province, Western Australia. –
Nuytsia 17: 353-366.
Shepherd KA, Lyons MN. 2009. Three new
species of Tecticornia (Chenopodiaceae, subfamily Salicornioideae)
identified through Salinity Action Plan surveys of the wheatbelt region,
Western Australia. – Nuytsia 19: 167-180.
Shepherd KA, Wilson PG. 2007. Incorporation
of the Australian genera Halosarcia, Pachycornia,
Sclerostegia and Tegicornia into Tecticornia
(Salicornioideae, Chenopodiaceae). – Aust. Syst. Bot. 20: 319-331.
Shepherd KA, Yan G. 2003. Chromosome number
and size variations in the Australian Salicornioideae (Chenopodiaceae) –
evidence of polyploidisation. – Aust. J. Bot. 51: 441-452.
Shepherd KA, Waycott M, Calladine A. 2004.
Radiation of the Australian Salicornioideae (Chenopodiaceae) – based on
evidence from nuclear and chloroplast DNA sequences. – Amer. J. Bot. 91:
1387-1397.
Shepherd KA, Macfarlane TD, Waycott M. 2005.
Phylogenetic analysis of the Australian Salicornioideae (Chenopodiaceae) based
on morphology and nuclear DNA. – Aust. Syst. Bot. 18: 89-115.
Shepherd KA, Macfarlane TD, Colmer TD. 2005.
Morphology, anatomy, and histochemistry of fruits and seeds of the
Salicornioideae (Chenopodiaceae). – Ann. Bot. 95: 917-933.
Sherbrooke WC, Haase EF. 1974. Jojoba: a
wax-producing shrub of the Sonoran Desert. – Arid Lands Resource Information
Paper 5, University of Sonora, Office of Arid Lands Studies, Tucson,
Arizona.
Sherry RA, Eckard KJ, Lord EM. 1993. Flower
development in dioecious Spinacia oleracea (Chenopodiaceae). – Amer.
J. Bot. 80: 283-291.
Shmida A. 1991.Tamarisks in Israel. –
Israel Land Nature 16: 119-125.
Shults VA. 1989. Rod myl’nyanka
(Saponaria L. s.l.) vo flore SSSR. – Zinatne, Riga
Shykoff JA. 1988. Maintenance of gynodioecy
in Silene acaulis (Caryophyllaceae):
stage-specific fecundity and viability selection. – Amer. J. Bot. 75:
844-850.
Silva AR Pinto da. 1956. As espécies
portuguesas de Silene sect. Botryosilene Rohrb. – Agron.
Lusit. 18: 24-29.
Simmler G. 1910. Monographie der Gattung
Saponaria. – Denkschr. Kaiserl. Akad. Wiss., Wien. Math.-Naturwiss.
Kl. 85: 433-509.
Simonds NW. 1965. The grain chenopods of the
tropical American highland. – Econ. Bot. 19: 223-235.
Simonet M. 1928. Le nombre des chromosomes
dans le genre Iris. – Compt. Rend. Soc. Biol. Paris 99:
1314-1316.
Simonet M. 1930. Nouvelles recherches sur le
nombre des chromosomes chez les Iris et sur l’existence de mitoses
didiploïdes dans ce genre. – Compt. Rend. Soc. Biol. Paris 103:
1197-1200.
Simonet M. 1932. Recherches cytologiques et
génétiques chez les Iris. – Bull. Biol. France Belg. 105:
255-444.
Simonet M. 1934. Nouvelles recherches
cytologiques et génétiques chez les Iris. – Ann. Sci. Nat. Bot.,
sér. X, 16: 229-383.
Simonet M. 1952. Nouveaux dénombrements
chromosomiques chez les Iris. – Compt. Rend. Acad. Sci. Paris 235:
1244-1246.
Simpson RB. 1995. Nepenthes and
conservation. – Curtis’s Bot. Mag. 12: 111-119.
Singh K, Buys MH, Liedt T. 2009. Assessing
relationships between Oscularia (Aizoaceae) and possible sister taxa
using pollen. – South Afr. J. Bot. 75: 440.
Sirrine E. 1895. Structure of the seed coats
of Polygonaceae. – Proc.
Iowa Acad. Sci. 2: 128-135.
Sivarajan VV. 1988. A preliminary taxonomic
revision of Indian Molluginaceae. – J. Taiwan
Mus. 41: 79-93.
Sivarajan VV, Gopinathan MC. 1985. Seedcoat
micromorphology of Caryophyllales: observations on
some Molluginaceae. – Proc.
Indian Acad. Sci. (Plant Sci.) 94: 51-47.
Skipwort JP. 1961. The taxonomic position of
Hectorella caespitose Hook. f. – Trans. Roy. Soc. New Zealand Bot.
1: 17-30.
Skipwort JP. 1962. The primary vascular
system and phyllotaxis in Hectorella caespitosa Hook. f. – New
Zealand J. Sci. 5: 253-258.
Skvarla JJ, Nowicke JW. 1976. Ultrastructure
of pollen exine in the centrospermous families. – Plant Syst. Evol. 126:
55-78.
Skvarla JJ, Nowicke JW. 1982. Pollen fine
structure and relationships of Achatocarpus Triana and
Phaulothamnus A. Gray. – Taxon 31: 244-249.
Slenzka A, Mucina W, Kadereit G. 2013.
Salicornia L. (Amaranthaceae) in South Africa
and Namibia: rapid spread and ecological diversification of cryptic species.
– Bot. J. Linn. Soc. 172: 175-186.
Slepyan EI. 1962. Galls and bud teratisms and
their pathogens in Tamarix L. – Byull. Mosk. Obshch. Ispyt. Prir.
67: 61-65. [In Russian]
Sloan DB, Alverson AJ, Wu M, Palmer JD,
Taylor DR. 2012. Recent acceleration of plastid sequence and structural
evolution coincides with extreme mitochondrial divergence in the angiosperm
genus Silene. – Genom Biol. Evol. 4: 294-306.
Sloan DB, Triant DA, Forrester NJ, Bergner
LM, Wu M, Taylor DR. 2014. A recurring syndrome of accelereated plastid genome
evolution in the angiosperm tribe Sileneae (Caryophyllaceae). – Mol.
Phylogen. Evol. 72: 82-89.
Small JK. 1895. A monograph of the North
American species of the genus Polygonum. – Mem. Dept. Bot. Columbia
Coll. 1: 1-183.
Smissen RD. 1999. Systematics of
Scleranthus. – Ph.D. diss., Victoria University, Wellington, New
Zealand.
Smissen RD, Garnock-Jones PJ. 2002.
Relationships, classification and evolution of Scleranthus (Caryophyllaceae) as inferred
from analysis of morphological characters. – Bot. J. Linn. Soc. 140:
15-29.
Smissen RD, Clement JC, Garnock-Jones PJ,
Chambers GK. 2002. Subfamilial relationships within Caryophyllaceae as inferred
from 5’ndhF sequences. – Amer. J. Bot. 89: 1336-1341.
Smissen RD, Garnock-Jones PJ, Chambers GK.
2003. Phylogenetic analysis of ITS sequences suggests a Pliocene origin for the
bipolar distribution of Scleranthus (Caryophyllaceae). – Aust.
Syst. Bot. 16: 301-315.
Smith CM. 1929. Development of Dionaea
muscipula I. Flowers and seed. – Bot. Gaz. 87: 507-530.
Smith GF, Chesselet P, Jaarsveld EJ van,
Hartmann H, Hammer S, Wyk B-E van, Burgoyne P, Klak C, Kurzweil H. 1998.
Mesembs of the world. – Briza Publ., Pretoria.
Smith JM. 1976. A taxonomic study of
Acleisanthes (Nyctaginaceae). – Wrightia 5:
261-276.
Sivarajan VV, Usha T. 1983. On reinstating
Mollugo stricta L. (Molluginaceae). – Taxon 32:
123-126.
Sobolevskaya KA, Vysochina GI. 1972.
Ecological and geographical aspects and some problems of the chemosystematics
of section Aconogonon Meisn. of genus Polygonum. – Izv.
Sib. Otd. Akad. Nauk. SSSR, ser. Biol. Med. Nauk. 3: 29-39. [In Russian with
English summary]
Sohmer H. 1972. Revision of the genus
Charpentiera (Amaranthaceae). – Brittonia
24:283-312.
Sohmer H. 1976a. Herbstia, a new
genus in the Amaranthaceae.
– Brittonia 28: 448-452.
Sohmer H. 1976b. The genus
Indobanalia. – Phytologia 34: 235-239.
Sohmer H. 1977. A revision of
Chamissoa (Amaranthaceae). – Bull. Torr.
Bot. Club 104: 111-126.
Soják J. 1974. Bemerkungen zur Gattung
Truellum Houtt. (Polygonaceae). – Preslia
(Praha) 46: 139-156.
Sokolov PD, Kondratenkova TD. 1983.
Chromosome numbers of some species of the genus Polygonum (Polygonaceae) of the section
Aconogonon. – Bot. Žurn. 68: 638-640. [In Russian]
Söllner R. 1952. Nouvelle contribution à la
cytotaxinomie du genre Cerastium. – Experientia 8: 104-105.
Söllner R. 1953. Sur l’emploi des
critères cytologiques dans la taxinomie du genre Cerastium. – Bull.
Soc. Neuchât. Sci. Nat. 76: 121-132.
Söllner R. 1954. Recherches cytotaxinomiques
sur le genre Cerastium. – Ber. Schweiz. Bot. Ges. 64: 221-354.
Soltis PS, Soltis DE, Weller SG, Sakai AK,
Wagner WL. 1996. Molecular phylogenetic analysis of the Hawaiian endemics
Schiedea and Alsinidendron (Caryophyllaceae). – Syst.
Bot. 21: 365-379.
Solymosi P, Pusztai T. 1984. Cytological
study of stable viable morphological changes appearing in Amaranthus
weed populations of maize monocultures. – Acta Bot. Hung. 30: 47-52.
Soriano A. 1946. Halophytaceae, nueva familia del
orden Centrospermae. – Notas Mus. La Plata 11: 161-175.
Soriano A. 1948. Los generous de
Quenopodiáceas de la flora Argentina. – Rev. Argent. Agron. 15: 1-19.
Soriano A. 1984. Halophytaceae. – Bol. Soc.
Argent. Bot. 23: 161-162.
Sosa V, Ochoterena H, Escamilla M. 2006. A
revision of Cerdia (Caryophyllaceae). – Bot. J.
Linn. Soc. 152: 1-13.
Souková M. 1978. Caryophyllaceae subfam.
Dianthoideae – Begrenzung, Charakteristik und Gliederung. – Preslia 50:
139-152.
Spegazzini C. 1905. Cactearum Platensium
Tentamen. – An. Mus. Nac. Buenos Aires 11: 477-521.
Spellenberg R. 1993. Taxonomy of
Anulocaulis (Nyctaginaceae). – Sida 15:
373-389.
Spellenberg R, Delson RK. 1977. Aspects of
reproduction in Chihuahuan Desert Nyctaginaceae. – In: Wauer RH,
Riskind DH (eds), Transactions of the symposium on the biological resources of
the Chihuahuan Desert region, United States and Mexico, U.S. Department of the
Interior, pp. 273-287.
Spellenberg R, Poole JM. 2003. Nomenclatural
adjustments and comments in Abronia and Acleisanthes (Nyctaginaceae). – Sida 20:
885-889.
Spencer JL. 1955. A cytological study of the
Cactaceae of Puerto Rico. –
Bot. Gaz. 117: 33-37.
Sperling CR. 1987. Systematics of the Basellaceae. – Ph.D. diss.,
Dept. of Organismic and Evolutionary Botany, Harvard University, Cambridge,
Massachusetts.
Sperling CR, Bittrich V. 1993. Basellaceae. – In: Kubitzki K,
Rohwer JG, Bittrich V (eds), The families and genera of vascular plants II.
Flowering plants. Dicotyledons. Magnoliid, hamamelid and caryophyllid families,
Springer, Berlin, Heidelberg, New York, pp. 143-147.
Sprague TA. 1920. Stellaria and
Alsine. – Kew Bull. 6: 308-318.
Srivastava AK, Misra KC. 1966. Chromosome
numbers in Boerhavia diffusa L. – Sci. Cult. 32: 315.
Stafford HA. 1994. Anthocyanins and
betalains: evolution of the mutually exclusive pathways. – Plant Sci. 101:
91-98.
Standley PC. 1909. The Allioniaceae of the
United States with notes on Mexican species. – Contr. U.S. Natl. Herb. 12:
303-389.
Standley PC. 1911. The Allioniaceae of Mexico
and Central America. – Contr. U.S. Natl. Herb. 13: 377-430.
Standley PC. 1916a. Ammocodon, a new
genus of Allioniaceae from the southwestern United States. – J. Washington
Acad. Sci. 6: 629-631.
Standley PC. 1916b. New or notable
Allioniaceae. – Contr. U. S. Natl. Herb. 18: 98-101.
Standley PC. 1931a. Studies of American
plants: Nyctaginaceae. –
Field Mus. Bot. Ser. 8: 304-311.
Standley PC. 1931b. The Nyctaginaceae and Chenopodiaceae
of northwestern South America. – Field Mus. Bot. Ser. 11: 71-126.
Standley PC, Steyermark JA. 1946. Polygonaceae. – Fieldiana, Bot.
24: 104-137.
Stanford EE. 1925a. The inflorescence and
flower-form in Polygonum, subgenus Persicaria. – Rhodora
27: 41-47.
Stanford EE. 1925b. Possibilities of
hybridism as a cause of variation in Polygonum. – Rhodora 27:
81-89.
Stanley TD. 1982. Nepenthaceae. – In: George AS
(ed), Flora of Australia 8, Australian Government Publ. Service, Canberra, pp.
7-8.
Steenis CGGJ van. 1949a. Ancistrocladaceae– In:
Steenis CGGJ van (ed), Flora Malesiana I, 4(2), Noordhoff-Kolff N. V., Batavia,
pp. 8-10.
Steenis CGGJ van. 1949b. Plumbaginaceae. – In: Steenis
CGGJ van (ed), Flora Malesiana I, 4(2), Noordhoff-Kolff N. V., Batavia, pp.
107-112.
Steenis CGGJ van. 1953. Droseraceae. – In: Steenis CGGJ
van (ed), Flora Malesiana I, 4(4), Noordhoff-Kolff N. V., Batavia, pp.
377-381.
Steffen S, Mucina L, Kadereit G. 2007.
Phylogeny and ecological diversification of South African Sarcocornia
(Chenopodiaceae). – South Afr. J. Bot. 73: 337.
Steffen S, Mucina L, Kadereit G. 2009. Three
new species of Sarcocornia (Chenopodiaceae) from South Africa. – Kew
Bull. 64: 447-459.
Steffen S, Mucina L, Kadereit G. 2010. A
revision of Sarcocornia in South Africa, Namibia and Mozambique. –
Syst. Bot. 35: 390-408.
Steffen S, Ball P, Mucina L, Kadereit G.
2015. Phylogeny, biogeography and ecological diversification of
Sarcocornia (Salicornioideae, Amaranthaceae). – Ann. Bot.
(Oxford) 115: 353-368.
Steiner E. 1944. Cytogenetic studies on
Talinum and Portulaca. – Bot. Gaz. 105: 374-379.
Stemmerick JF. 1964a. Florae Malesianae
Precursores 28. Notes on Pisonia L. in the Old World. – Blumea 12:
275-284.
Stemmerick JF. 1964b. Nyctaginaceae. – In: Steenis
CGGJ van (ed), Flora Malesiana, I, 6, Wolters-Noordhoff, Groningen, pp.
450-468.
Stephens EL. 1926. A new sundew, Drosera
regia Stephens, from the Cape Province. – Trans. Roy. Soc. South Africa
8: 309-312.
Sterk AA, Dijkhuizen L. 1972. The relation
between the genetic determination and the ecological significance of the seed
wing in Spergularia media and S. marina. – Acta Bot. Neerl.
21: 481-490.
Stern K. 1917. Beiträge zur Kenntnis der Nepenthaceae. – Flora 109:
213-282.
Stetter MG, Schmid KJ. 2017. Analysis of
phylogenetic relationships and genome size evolution of the Amaranthus
genus using GBS indicates the ancestors of an ancient crop. – Mol. Phylogen.
Evol. 109: 80-92.
Steward AN. 1930. The Polygonaceae of eastern Asia. –
Contr. Gray Herb. Harvard Univ. 5: 1-129.
Stewart D, Wiens D. 1971. Chromosome races in
Claytonia lanceolata (Portulacaceae). – Amer. J.
Bot. 58: 41-47.
Stohr G. 1982. Entfaltungszentren der Gattung
Coccoloba L. (Polygonaceae) in der Neotropis.
– Rev. Jard. Bot. Nac. Univ. Habana 3: 129-144.
Stokes SG. 1936. The genus
Eriogonum, a preliminary study based on geographical distribution. –
J. H. Neblett Press, San Francisco.
Stokes SG, Stebbins GL. 1955. Chromosome
numbers in the genus Eriogonum. – Leafl. Western Bot. 7: 228-233.
Stone-Palmquist ME, Mauseth JD. 2002. The
structure of enlarged storage roots in cacti. – Intern. J. Plant Sci. 163:
89-98.
Stoughton TR, Kriebel R, Jolles DD, O’Quinn
RL. 2018. Next-generation lineage discovery: a case study of tuberous
Claytonia L. – Amer. J. Bot. 105: 536-548.
Straka H. 1955. Anatomische und
entwicklungsgeschichtliche Untersuchungen an Früchten paraspermer
Mesembryanthemen. – Nova Acta Leopold. 17(118): 127-190.
Straka H. 1965. Die Pollenmorphologie der
Didiereaceen. – Sitzungsber. Heidelb. Akad. Wiss., Math.-Nat. Kl., 3:
219-227.
Straka H. 1975. Palinologie et
différentiation systématique d’une famille endémique de Madagascar: les
Didieréacées. – Boissiera 24: 245-248.
Straka H, Friedrich B. 1983. Palynologica
Madagassica et Mascarenica. Fam. 121-127. Microscopie électronique à balayage
et addenda. – Pollen Spores 25: 49-73.
Straka H, Friedrich B. 1988. Fam. 73. Caryophyllaceae. – In:
Palynologica Madagassica et Mascarenica, Trop. Subtrop. Pflanzenwelt 61:
29-33.
Strandmark PW. 1887. Förgreningen och
bladställningen hos Montia särskildt med afseende på frågan om
blommans orientering. – Bot. Not. 1887: 164-174.
Studholme WP, Philipson WR. 1966. A
comparison of the cambium in two woods with included phloem:
Heimerliodendron brunonianum and Avicennia resinifera. –
New Zealand J. Bot. 4: 355-365.
Stuppy W. 2001. A new combination in
Tephrocactus Lem. (Cactaceae). – Kew bull. 56:
1003-1005.
Stuppy W. 2002. Seed characters and the
generic classification of the Opuntioideae (Cactaceae). – Succ. Plant Res. 6:
25-58.
Stutz HC, Chu G-L, Sanderson SC. 1990.
Evolutionary studies of Atriplex: phylogenetic relationships of
Atriplex pleiantha. – Amer. J. Bot. 77: 364-369.
Styles BT. 1962. The taxonomy of
Polygonum aviculare and its allies in Britain. – Watsonia 5:
177-214.
Suárez-García C, Paz JP, Febles R,
Caujapé-Castells J. 2009. Genetic diversity and floral dimorphism in
Limonium dendroides (Plumbaginaceae), a woody
Canarian species on the way of extinction. – Plant Syst. Evol. 280:
105-117.
Suh Y, Kim S, Park C-W. 1997. A phylogenetic
study of Polygonum sect. Tovara (Polygonaceae) based on ITS
sequences of nuclear ribosomal DNA. – J. Plant Biol. 40: 47-52.
Sukhorukov AP. 1999a. Eine neue asiatische
Chenopodium-Art aus der Sektion Pseudoblitum Hook. f.
(Chenopodiaceae). – Feddes Repert. 110: 493-497.
Sukhorukov AP. 1999b. Some general
evolutionary trends in the genus Atriplex L. s.l. (Chenopodiaceae).
– In: Melikyan AP, Lotova LI (eds), Tenth Moscow Meeting in Plant Phylogeny
(Materials), Moscow, pp. 166-168. [in Russia]
Sukhorukov AP. 2000. Atriplex
altaica Sukhor. – eine neue Art aus der Flora des Altai-Gebirges. –
Feddes Repert. 111: 175-179.
Sukhorukov AP. 2005. Karpologische
Untersuchung der Axyris-Arten (Chenopodiaceae) im Zusammenhang mit
ihrer Diagnostik und Taxonomie. – Feddes Repert. 116: 168-176.
Sukhorukov AP. 2006. Zur Systematik und
Chorologie der in Rußland und benachbarten Staaten (in den Grenzen der
ehemaligen UdSSR) vorkommenden Atriplex-Arten (Chenopodiaceae). –
Ann. Naturhist. Mus. Wien 108B: 307-420.
Sukhorukov AP. 2007a. Einige neue und wenig
bekannte Taxa aus der Familie Chenopodiaceae in Europa und im östlichen
Mittelmeergebiet. – Feddes Repert. 118: 73-83.
Sukhorukov AP. 2007b. Fruit anatomy and its
taxonomic significance in Corispermum (Corispermoideae,
Chenopodiaceae). – Willdenowia 37: 63-87.
Sukhorukov AP. 2007c. Notes on the taxonomy
of Girgensohnia (Chenopodiaceae/Amaranthaceae). – Edinburgh J.
Bot. 64: 317-330.
Sukhorukov AP. 2008. Fruit anatomy of the
genus Anabasis (Salsoloideae, Chenopodiaceae). – Aust. Syst. Bot.
21: 431-442.
Sukhorukov AP. 2011. Axyris
(Chenopodiaceae s.str. or Amaranthaceae s.l.) in the
Himalayas and Tibet. – Willdenowia 41: 75-82.
Sukhorukov AP. 2012. Taxonomic notes on
Dysphania and Atriplex (Chenopodiaceae). – Willdenowia 42:
169-180.
Sukhorukov AP, Danin A. 2009. Taxonomic notes
on Atriplex sect. Teutliopsis and sect. Atriplex in
Israel and Syria. – Flora Mediterranea 19: 15-23.
Sukhorukov AP, Kushunina M. 2014. Taxonomic
revision of Chenopodiaceae in Nepal. – Phytotaxa 191: 10-44.
Sukhorukov AP, Kushunina M. 2015. Taxonomy
and chorology of Corbichonia (Lophiocarpaceae s.l.) with
further description of a new species from southern Africa. – Phytotaxa 218:
227-240.
Sukhorukov AP, Kushunina M. 2016. Taxonomic
revision and distribution of herbaceous Paramollugo (Molluginaceae) in the Eastern
Hemisphere. – PhytoKeys 73: 93-116.
Sukhorukov AP, Kushunina M. 2017. Taxonomic
significance of seed morphology in the genus Mollugo s.l. (Molluginaceae). – Israel J.
Plant Sci. 64: 31-47.
Sukhorukov AP, Nilova MV. 2016. A new species
of Arthrocnemum (Salicornioideae: Chenopodiaceae-Amaranthaceae) from West Africa,
with a revised characterization of the genus – Bot. Lett. 163: 237-250.
Sukhorukov AP, Uotila P, Zhang M, Zhang H-X,
Speranskaya AS, Krinitsyna AA. 2013. New combinations in Asiatic
Oxybasis (Amaranthaceae s.l.): evidence
from morphological, carpological and molecular data. – Phytotaxa 144:
1-12.
Sukhorukov AP, Mavrodiev EV, Struwig M,
Nilova MV, Dzhalilova KK, Balandin SA, Erst A, Krinitsyna AA. 2015. One-seeded
fruits in the core Caryophyllales: their origin
and structural diversity. – PloS ONE 10(2): e0117974.
http://dx.doi.org/10.1371/journal.pone.1007974
Sukhorukov AP, Zhang M-L, Kushunina M, Nilova
MV, Krinitsina A, Zaika MA, Mazei Y. 2018. Seed characters in Molluginaceae (Caryophyllales): implications
for taxonomy and evolution. – Bot. J. Linn. Soc. 187: 167-208.
Sultan SE, Wilczek AM, Hann SD, Brosi BJ.
1998. Contrasting ecological breadth of co-occurring annual Polygonum
species. – J. Ecol. 86: 363-383.
Summerhayes VS. 1930. A revision of the
Australian species of Frankenia. – Bot. J. Linn. Soc. 48:
337-387.
Sun W, Zhou Z-Z, Liu M-Z, Wan H-W, Dong X.
2008. Reappraisal of the generic status of Pteroxygonum (Polygonaceae) on the basis of
morphology, anatomy and nrDNA ITS sequence analysis. – J. Syst. Evol. 46:
73-79.
Sun Y-X, Zhang M-L. 2012. Molecular phylogeny
of tribe Atraphaxideae (Polygonaceae) evidenced from five
cpDNA genes. – J. Arid Land 4: 180-190.
Sun Y-X, Wang A, Wan D, Wang Q, Liu J. 2012.
Rapid radiation of Rheum (Polygonaceae) and parallel
evolution of morphological traits. – Mol. Phylogen. Evol. 63: 150-158.
Surgis E. 1921. Étude sur les
Frankéniacées. – André Lesot, Nemours.
Swanepoel W. 2007. Ceraria
kaokoensis, a new species from Namibia, with notes on gynodioecy in the
genus. – Bothalia 37: 202-206.
Swanson JR. 1966. A synopsis of relationships
in Montioideae (Portulacaceae). – Brittonia
18: 229-241.
Syeda ST. 1980. Three new species of
Calandrinia (Portulacaceae) from inland
Australia. – Telopea 2: 59-61.
Sykes WR. 1987. The parapara, Pisonia
brunoniana (Nyctaginaceae). – New Zealand
J. Bot. 25: 459-466.
Szyszyłowicz I von. 1895b. Theaceae (Ternstroemiaceae).
– In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(6), W.
Engelmann, Leipzig, pp. 175-192.
Takahashi H. 1988. Ontogenetic development of
pollen tetrads of Drosera capensis L. – Bot. Gaz. 149: 275-282.
Takahashi H, Sohma K. 1982. Pollen morphology
of the genus Drosera and its related taxa. – Sci. Rep. Tohoku Imp.
Univ., Ser. IV, Biology 38: 81-156.
Talavera S, Muñoz Garmendia F. 1989.
Sinopsis del género Silene L. (Caryophyllaceae) en la
Península Ibérica y Baleares. – An. Jard. Bot. Madrid 45: 407-460.
Tanaka R, Tanaka A. 1980. Karyomorphological
studies on halophytic plants I. Some taxa of Chenopodium. –
Cytologia 45: 257-269.
Tapia HJ, Bárcenas-Argüello ML, Terrazas T,
Arias S. 2017. Phylogeny and circumscription of Cephalocereus (Cactaceae) based on molecular and
morphological evidence. – Syst. Bot. 42: 709-723.
Tavakkoli S, Osaloo SK, Maassoumi AA. 2010.
The phylogeny of Calligonum and Pteropyrum (Polygonaceae) based on nuclear
ribosomal DNA ITS and chloroplast trnL-F sequences. – Iran. J.
Biotechn. 8: 7-15.
Tavakkoli S, Osaloo SK, Mozaffarian V,
Maassoumi AA. 2015. Molecular phylogeny of Atraphaxis and the woody
Polygonum species (Polygonaceae): taxonomic
implications based on molecular and morphological evidence. – Plant Syst.
Evol. 301: 1157-1170.
Taylor CM. 1994. Revision of
Tetragonia (Aizoaceae)
in South America. – Syst. Bot. 19: 575-589.
Taylor CM, Gereau RE, Walters GM. 2005.
Revision of Ancistrocladus Wall. (Ancistrocladaceae). – Ann.
Missouri Bot. Gard. 92: 360-399.
Taylor NP. 1978. Review of the genus
Escobaria B. & R. – Cact. Succ. J. (Great Britain) 40: 31-37.
Taylor NP. 1979a. A commentary on the genus
Echinofossulocactus Lawr. – Cact. Succ. J. (Great Britain) 41:
35-42.
Taylor NP. 1979b. Notes in
Ferocactus B. & R. – Cact. Succ. J. (Great Britain) 41:
88-94.
Taylor NP. 1980. Ferocactus and
Stenocactus united. – Cact. Succ. J. (Great Britain) 42: 108.
Taylor NP. 1983. Comments on proposal 673 to
conserve 5408 Stenocactus (Schumann) Berger (1929) over various
generic names (Cactaceae). –
Taxon 32: 641-643.
Taylor NP. 1984. A review of
Ferocactus Britton & Rose. – Bradleya 2: 19-38.
Taylor NP. 1985. The genus
Echinocereus. – Collingridge & The Royal Botanic Gardens, Kew,
London.
Taylor NP. 1986. The identification of
Escobarias (Cactaceae).
– Cactus Succ. J. (Great Britain) 4: 36-44.
Taylor NP. 1991. The genus
Melocactus (Cactaceae)
in Central and South America. – Bradleya 9: 1-80.
Taylor NP. 2005. 526. Maihuenia
poeppigii. Cactaceae. –
Curtis’s Bot. Mag. 22: 105-108.
Taylor NP, Zappi DC. 1989. An alternative
view on generic delimitation and relationships in tribe Cereeae (Cactaceae). – Bradleya 7:
13-40.
Taylor NP, Zappi DC. 2004. Cacti of Eastern
Brazil. – Royal Botanic Gardens, Kew, Richmond.
Taylor NP, Stuppy W, Barthlott W. 2002.
Realignment and revision of the Opuntioideae of Eastern Brazil. – Succ. Plant
Res. 6: 99-132.
Teege P, Kadereit JW, Kadereit G. 2011.
Tetraploid European Salicornia species are best interpreted as
ecotypes of multiple origin. – Flora 206: 910-920.
Teeri JA, Stowe LG, Murawski DA. 1978. The
climatology of two succulent plant families: Cactaceae and Crassulaceae. –
Can. J. Bot. 56: 1750-1758.
Teillier S, Macaya-Berti J. 2018. Cinco
nuevas especies de Chorizanthe (Polygonaceae-Eriogonoideae) del
Norte de Chile. – Novon 26: 37-52.
Teillier S, Taylor C. 1997. Maireana
Moq. a new genus for the flora of Chile (Desventuradas Islands). – Gayana,
Bot. 54: 15-17.
Telford IRH. 1984. Cactaceae. – In: George AS (ed),
Flora of Australia 4, Australian Government Publ. Service, Canberra, pp.
62-80.
Terrazas T, Arlas S. 1999. Las ramas
dimórficas de la tribu Pachycereeae (Cactaceae). – In: Vásquez MA
(ed), Resúmenes del II congreso mexicano y I congreso Latinoamericano y del
Caribe de cactáceas y otras plantas suculentas, Sociedad Mexicana de
Cactología, Oaxaca, p. 71.
Terrazas T, Arias S. 2003. Comparative stem
anatomy in the subfamily Cactoideae. – Bot. Rev. 68: 444-473.
Terrazas T, Loza-Cornejo S. 2002.
Phylogenetic relationships of Pachycereeae: a cladistic analysis based on
anatomical-morphological data. – In: Fleming TH, Vallente-Banuet A (eds),
Evolution, ecology, and conservation of columnar cacti and their mutualists,
Arizona University Press, Tucson, pp. 66-86.
Terrazas T, Escamilla-Molina R,
Vázquez-Sánchez M. 2016. Variation in the tracheary elements in species of
Coryphantha (Cacteae-Cactoideae) with contrasting morphology: the
bottleneck model. – Brazil. J. Bot. 39: 669-678.
Thiede J. 2004. Phylogenetics, systematics
and classification of the Aizoaceae: a reconsideration based
on molecular data. – Schumannia 4: 51-58.
Thiede J, Schmidt SA, Rudolph B. 2007.
Phylogenetic implication of the chloroplast rpoC1 intron loss in the
Aizoaceae (Caryophyllales). – Biochem.
Syst. Ecol. 35: 372-380.
Thiv M, Thulin M, Kilian N, Linder HP. 2006.
Eritreo-Arabian affinities of the Socotran flora as revealed from the molecular
phylogeny of Aerva (Amaranthaceae). – Syst. Bot.
31: 560-570.
Thomas SM, Murray BG. 1983. Chromosome
studies in species and hybrids of Petrorhagia sect.
Kohlrauschia (Caryophyllaceae). – Plant
Syst. Evol. 141: 243-255.
Thomson BF. 1942. The floral morphology of
the Caryophyllaceae. –
Amer. J. Bot. 29: 333-349.
Thorne RF. 1985. Phylogenetic relationships
of the monotypic family Simmondsiaceae. – Jojoba
Happen. 13: 8.
Throughton JH, Card KA. 1974. Leaf anatomy of
Atriplex buchananii. – New Zealand J. Bot. 12: 167-177.
Thulin M. 1990. Four new species of
Commicarpus (Nyctaginaceae) from NE tropical
Africa. – Nord. J. Bot. 10: 403-409.
Thulin M. 1993a. Notes on Tetragonia
(Aizoaceae-Tetragonioideae) in
Somalia. – Nord. J. Bot. 13: 165-167.
Thulin M. 1993b. Nyctaginaceae. – In: Thulin M
(ed), Flora of Somalia, Royal Botanic Gardens, Kew, pp. 168-175.
Thulin M. 1996. A new species of
Polycarpaea (Caryophyllaceae) from Somalia.
– Nord. J. Bot. 16: 291-293.
Thulin M. 2002. Anacampseros (Portulacaceae) in the Horn of
Africa region. – Kew Bull. 57: 741-745.
Thulin M, Rydberg A, Thiede J. 2010. Identity
of Tetragonia pentandra and taxonomy and distribution of
Patellifolia (Chenopodiaceae). – Willdenowia 40: 5-11.
Thulin M, Thiede J, Liede-Schumann S. 2012.
Phylogeny and taxonomy of Tribulocarpus (Aizoaceae): a paraphyletic species
and an adaptive shift from zoochorous trample burrs to anemochorous nuts. –
Taxon 61: 55-66.
Thulin M, Moore AJ, El-Seedi H, Larsson A,
Christin P-A, Edwards EJ. 2016. Phylogeny and generic delimitation in Molluginaceae, new pigment data
in Caryophyllales, and the
new family Corbichoniaceae.
– Taxon 65: 775-793.
Tiaghi YD. 1954. Studies in the floral
morphology of Opuntia dillenii Haworth I. Development of the ovules
and the gametophytes. – Bot. Not. 107: 343-356.
Tiaghi YD. 1955. Studies in floral morphology
II. Vascular anatomy of the flower of certain species of the Cactaceae. – J. Indian Bot. Soc.
34: 408-428.
Tiaghi YD. 1956. Polyembryony in
Mammillaria tenuis DC. – Bull. Bot. Soc. Univ. Saugar 8: 25-27.
Tiaghi YD. 1963. Studies in floral morphology
VII. A further study of the vascular anatomy of the flower of certain species
of the Cactaceae. – J. Indian
Bot. Soc. 42: 545-558.
Tiaghi YD. 1967. Contribution to the
embryology of the genus Pereskia. – Proc. 54th Indian
Sci. Congr. 3: 324-325.
Tiaghi YD. 1970. Comparative embryology of
angiosperms: Cactaceae. – Bull.
Natl. Sci. Acad. India 41: 25-29.
Tian X, Liu R, Tian B, Liu J. 2009.
Karyological studies of Parapteropyrum and Atraphaxis (Polygonaceae). – Caryologia 62:
261-266.
Tian X, Luo J, Wang A, Mao K, Liu J. 2011. On
the origin of the woody buckwheat Fagopyrum tibeticum
(=Parapteropyrum tibeticum) in the Qinghai-Tibetan plateau. – Mol.
Phylogen. Evol. 61: 515-520.
Tieghem P van. 1898. Sur le genre
Simmondsia, considéré comme type d’une famille distincte, les
Simmondsiacées. – J. Bot. (Paris) 12: 103-112.
Tieghem P van. 1903. Sur les
Ancistrocladacées. – J. Bot. (Paris) 17: 151-168.
Till H, Amerhauser H, Till W. 2008.
Neuordnung der Gattung Gymnocalycium II. – Gymnocalycium
21(Sonderausgabe): 815-838.
Tillett SS. 1967. The maritime species of
Abronia (Nyctaginaceae). – Brittonia
19: 299-327.
Timson J. 1964. A study of hybridization in
Polygonum section Persicaria. – Bot. J. Linn. Soc. 59:
155-160.
Tobe H, Raven PH. 1989. The embryology and
systematic position of Rhabdodendron (Rhabdodendraceae). – In:
Tan K, Mill RR, Elias TS (eds), The Davis and Hedge Festschrift: Plant
taxonomy, phytogeography, and related subjects, Edinburgh University Press,
Edinburgh, pp. 233-248.
Tobe H, Yasuda S, Oginuma K. 1992. Seed coat
anatomy, karyomorphology, and relationships of Simmondsia (Simmondsiaceae). – Bot. Mag.
(Tokyo) 105: 529-538.
Toekes ZA, Woon WC, Chambers SM. 1974.
Digestive enzymes secreted by the carnivorous plant Nepenthes
macfarlanei L. – Planta 119: 39-46.
Tölken HR. 1967. The species of
Arthrocnemum and Salicornia (Chenopodiaceae) in southern
Africa. – Bothalia 9: 255-307.
Tölken HR. 1969. The genus Talinum
(Portulacaceae) in southern
Africa. – Bothalia 10: 19-28.
Torrey J, Gray A. 1870. A revision of the
Eriogoneae. – Proc. Amer. Acad. Arts Sci. 8: 145-200.
Townsend CC. 1974. Notes on Amaranthaceae II. – Kew Bull.
29: 461-475.
Townsend CC. 1982. Notes on Amaranthaceae XIV. A new species
of African Celosia and a new conspectus of Hermbstaedtia. –
Kew Bull. 37: 81-90.
Townsend CC. 1983. Notes on Amaranthaceae XV. The generic
position of Chionothrix hyposericea. – Kew Bull. 38: 345-346.
Townsend CC. 1984a. Notes on Amaranthaceae XV. Two new
species of Gomphrena (Amaranthaceae) from Bahia,
Brazil. – Kew Bull. 39: 117-120.
Townsend CC. 1984b. Notes on Amaranthaceae XVI. A new genus
and species of Amaranthaceae
from Somalia. – Kew Bull. 39: 775-777.
Townsend CC. 1985. Amaranthaceae. – In: Polhill
RM (ed), Flora of tropical East Africa, A. A. Balkema, Rotterdam, The
Netherlands, pp. 1-136.
Townsend CC. 1988. Notes on Amaranthaceae XVIII. Two new
species of Amaranthaceae from
South America. – Kew Bull. 43: 103-106.
Townsend CC. 1990. Notes on Amaranthaceae XX. Three new
species of Psilotrichum from Somalia. – Kew Bull. 45: 661-665.
Townsend CC. 1991. Notes on Amaranthaceae XXII. A new genus
of Amaranthaceae from
Somalia. – Kew Bull. 46: 101-103.
Townsend CC. 1993. Amaranthaceae. – In: Kubitzki
K, Rohwer JG, Bittrich V (eds), The families and genera of vascular plants II.
Flowering plants. Dicotyledons. Magnoliid, hamamelid and caryophyllid families,
Springer, Berlin, Heidelberg, New York, pp. 70-91.
Troll W, Weberling F. 1981.
Infloreszenzstudien an Aizoaceen, Mesembryanthemaceen und Tetragoniaceen. –
Trop. Subtrop. Pflanzenwelt 35: 1-99 (195)-(289).
Tsukada M. 1964. Pollen morphology and
identification II. Cactaceae. –
Pollen Spores 6: 45-84.
Tsymbalyuk ZM. 2008. Palynomorphological
peculiarities of members of the family Chenopodiaceae. – Bot. Žurn. 93:
430-438. [In Russian]
Tugay O, Ertugrul K. 2008. A new species of
Silene (Caryophyllaceae) from East
Anatolia, Turkey. – Bot. J. Linn. Soc. 156: 463-466.
Turesson G. 1925. Studies in the genus
Atriplex. – Lunds Univ. Årsskr., N. F., Avd. II, 21: 1-15.
Turki Z, El-Shayeb F, Shehata F. 2006.
Taxonomic studies in the Camphorosmeae (Chenopodiaceae) in Egypt 1. Subtribe
Kochiinae (Bassia, Kochia and Chenolea). – Flora
Mediterr. 16: 275-294.
Turner BL. 1991. A new gypsophilic species of
Mirabilis (Nyctaginaceae) from Nuevo León,
Mexico. – Phytologia 70: 44-46.
Turner BL. 1993. A new species of
Anulocaulis (Nyctaginaceae) from southern
Coahuila, Mexico. – Sida 15: 613-615.
Turner BL. 1994a. Chromosome numbers and
their phyletic interpretations. – In: Behnke H-D, Mabry TJ (eds), Caryophyllales: evolution and
systematics, Springer, Berlin, Heidelberg, New York, pp. 27-43.
Turner BL. 1994b. Revisionary study of the
genus Allionia (Nyctaginaceae). – Phytologia
77: 45-55.
Turrill WB. 1956. Caryophyllaceae. – In:
Turrill WB, Milne-Redhead E (eds), Flora of tropical East Africa, Crown Agents
for Oversea Governments and Administrations, London, pp. 1-38.
Tzvelev NN. 1987. Notulae de Polygonaceis in
flora Orientis extremi. – Nov. Syst. Plant. Vasc. 24: 72-79.
Tzvelev NN. 1993. Notes on Chenopodiaceae of
eastern Europe. – Ukrains’k Bot. Žurn. 50: 78-85. [in Russian]
Tzvelev NN. 2001. De generibus tribus
Sileneae DC. (Caryophyllaceae) in Europa
orientali. – Nov. Syst. Plant. Vasc. 33: 90-113.
Ulbrich E. 1934a. Basellaceae. – In: Engler A
(†), Pax F, Harms H (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd.
16c, W. Engelmann, Leipzig, pp. 263-271.
Ulbrich E. 1934b. Chenopodiaceae. – In:
Engler A (†), Pax F, Harms H (eds), Die natürlichen Pflanzenfamilien, 2.
Aufl., Bd. 16c, W. Engelmann, Leipzig, pp. 379-584.
Ungar IA. 1979. Seed dimorphism in
Salicornia europaea L. – Bot. Gaz. 140: 102-108.
Unger G. 1992. Die grossen Kugelkakteen
Nordamerikas. Handbuch. Vollständige Gesamtbearbeitung aller bisher bekannten
Taxa und Synonyme der Gattungen Echinocactus Link et Otto und
Ferocactus Britton et Rose. – Selbstverlag, Graz.
Uotila P. 1973. Chromosome counts in the
genus Chenopodium L. from SE Europe and SW Asia. – Ann. Bot. Fenn.
10: 337-340.
Uotila P. 1974. Pollen morphology in European
species of Chenopodium sect. Chenopodium, with special
reference to C. album and C. suecicum. – Ann. Bot. Fenn.
11: 44-58.
Uotila P. 1993. Taxonomic and nomenclatural
notes on Chenopodium in the Flora iranica area. – Ann. Bot. Fenn.
30: 189-194.
Uotila P. 2001. Taxonomic and nomenclatural
notes on Chenopodium (Chenopodiaceae) for Flora Nordica. – Ann. Bot.
Fenn. 38: 95-97.
Uotila P. 2013. Dysphania sect.
Botryoides (Amaranthaceae s. lat.) in Asia.
– Willdenowia 43: 65-80.
Urban I. 1885. Über den Blüthenbau der
Phytolaccaceen-Gattung Microtea. – Ber. Deutsch. Bot. Ges. 3:
324-332.
Urmi-König K. 1981. Blütentragende
Sproß-Systeme einiger Chenopodiaceae. – Diss. Bot. 63: 1-150.
Valcárcel V, Vargas P, Feliner GN. 2006.
Phylogenetic and phylogeographic analysis of the western Mediterranean
Arenaria section Plinthine (Caryophyllaceae) based on
nuclear, plastid, and morphological markers. – Taxon 55: 297-312.
Valente LM, Savolainen V, Vargas P. 2010.
Unparalleled rates of species diversification in Europe. – Proc. Roy. Soc.,
Sect. B, 277: 1489-1496.
Valente LM, Britton AW, Powell MP,
Papadopulos AST, Burgoyne PM, Savolainen V. 2014. Correlates of hyperdiversity
in southern African ice plants (Aizoaceae). – Bot. J. Linn. Soc.
174: 110-129.
Vanvinckenroye P, Smets E. 1996. Floral
ontogeny of five species of Talinum and of related taxa (Portulacaceae). – J. Plant
Res. 109: 387-402.
Vanvinckenroye P, Smets E. 1999. Floral
ontogeny of Anacampseros subgen. Anacampseros sect.
Anacampseros (Portulacaceae). – Syst. Geogr.
Plants 68: 173-194.
Vanvinckenroye P, Cresens E, Ronse De Craene
LP, Smets E. 1993. A comparative floral developmental study in
Pisonia, Bougainvillea and Mirabilis (Nyctaginaceae) with special
emphasis on the gynoecium and floral nectaries. – Bull. Jard. Bot. Natl.
Belg. 62: 69-96.
Vaupel F. 1925. Cactaceae. – In: Engler A, Gilg E
(eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 21, W. Engelmann,
Leipzig, pp. 594-651.
Vaupel F. 1925-1926. Die Kakteen. Monographie
der Cactaceae. – Selbstverlag
des Verfassers, Berlin.
Vautier S. 1949. La vascularisation florale
chez les Polygonacées. – Candollea 12: 219-343.
Vázquez-Lobo A, Morales GA, Arias S, Golubov
J, Hernández-Hernández T, Mandujano MC. 2015. Phylogeny and biogeographic
history of Astrophytum (Cactaceae). – Syst. Bot. 40:
1022-1030.
Vázquez-Sánchez M, Terrazas T. 2011. Stem
and wood allometric relationships in Cacteae (Cactaceae). – Trees Struct. Func.
25: 755-767.
Vázquez-Sánchez M, Terrazas T, Arias S.
2012. El hábito y la forma de crecimiento en Cacteae (Cactaceae). – Bot. Sci. 90:
97-108.
Vázquez-Sánchez M, Terrazas T, Arias S,
Ochoterena H. 2013. Molecular phylogeny, origin and taxonomic implications of
the tribe Cacteae (Cactaceae).
– Syst. Biodiv. 11: 103-116.
Vázquez-Sánchez M, Terrazas T,
Grego-Valencia D, Arias S. 2017. Growth form and wood evolution in the tribe
Cacteae (Cactaceae). – Willdenowia 47:
49-67.
Vekemans X, Lefèbvre C, Belalia L, Meerts P.
1990. The evolution and breakdown of the heteromorphic incompatibility system
of Armeria maritima revisited. – Evol. Trends Plants 4: 15-23.
Velazco MCG, Nevárez RM. 2002. Nuevo género
de la familia Cactaceae en el
estado de Nuevo León, México: Digitostigma caput-medusae Velazco et
Nevárez sp. nov. – Cactaceas y Suculentas Mexicanas 47: 76-86.
Venkatasuban KR. 1950a. Studies in the Droseraceae I. The cytology of
D. indica L., D. burmanni Vahl, and D. peltata Sm.
with special reference to pollen mitosis. – Proc. Indian Acad. Sci., Sect. B,
31: 308-330.
Venkatasuban KR. 1950b. Studies in the Droseraceae II. A contribution to
the embryology of three species of Drosera. – Proc. Indian Acad.
Sci., Sect. B, 32: 23-38.
Venugopal N, Rashi Devi N. 2003. Development
of the anther in Nepenthes khasiana Hook. f. (Nepenthaceae), an endemic and
endangered insectivorous plant of North East India. – Feddes Repert. 115:
69-73.
Verdcourt B. 1968. Basellaceae. – In: Milne-Redhead
E, Polhill RM (eds), Flora of tropical East Africa, Crown Agents for Oversea
Governments and Administrations, London, pp. 1-4.
Vickery AR. 1983. 103. Plumbaginaceae. – In: Launert
E (ed), Flora Zambesiaca 7 (Part 1), Flora Zambesiaca Managing Committee,
London, pp. 181-184.
Vishnu-Mittre P. 1963. Pollen morphology of
Indian Amaranthaceae. – J.
Indian Bot. Soc. 42: 86-101.
Vishnu-Mittre P, Gupta HP. 1964. Studies of
Indian pollen grains III. Caryophyllaceae. – Pollen
Spores 6: 99-111.
Voit G. 1979. Untersuchungen zur
Mikromorphologie der Cactaceen-Samen unter Berücksichtigung taxonomischer
Aspekte. – Ph.D. diss., Universität Heidelberg, Germany.
Volgin SA. 1988. Vergleichende Morphologie
und Gefässbündelanatomie der Blüte bei den Rivinoideae (Phytolaccaceae). – Flora 181:
325-337.
Volkens G. 1893a. Chenopodiaceae. – In:
Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien, III(1a), W.
Engelmann, Leipzig, pp. 36-91.
Volkens G. 1893b. Basellaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien III(1), W. Engelmann,
Leipzig, pp. 124-128.
Volkov AG, Adesina T, Markin VS, Jovanov E.
2008. Kinetics and mechanism of Dionaea muscipula trap closing. –
Plant Physiol. 146: 694-702.
Volkova EV. 1964. A review of the species of
Claytonia in USSR. – Bot. Žurn. 49: 1760-1768. [In Russian]
Volponi CR. 1983. Sinopsis de las especies
argentinas de Stellaria (Caryophyllaceae). – Lilloa
36: 69-75.
Volponi CR. 1986a. Sobre Stellaria
cuspidata (Caryophyllaceae) y especies
afines en Argentina. – Kurtziana 18: 93-107.
Volponi CR. 1986b. Contribución a la
espermatología de especies argentinas de Stellaria (Caryophyllaceae). – Bol.
Soc. Argent. Bot. 24: 283-294.
Volponi CR. 1993. Stellaria
cuspidata (Caryophyllaceae) and some
related species in the Andes. – Willdenowia 23: 193-209.
Voznesenskaya EV, Artyusheva EG, Franceschi
VR, Pyankov VI, Kiirats O, Ku MSB, Edwards GE. 2001. Salsola
arbusculiformis, a C3-C4intermediate in Salsoleae
(Chenopodiaceae). – Ann. Bot. 88: 337-348.
Voznesenskaya EV, Edwards GE, Kiirats O,
Artyusheva EG, Franceschi VR. 2003. Development of biochemical specialization
and organelle partitioning in the single-cell C4 system in leaves of
Borszczowia aralocaspica (Chenopodiaceae). – Amer. J. Bot. 90:
1669-1680.
Voznesenskaya EV, Koteyeva NK, Edwards GE,
Ocampo G. 2010. Revealing diversity in structural and biochemical forms of
C4 photosynthesis and a C3–C4 intermediate
in genus Portulaca L. (Portulacaceae). – J. Experim.
Bot. 61: 3647-3662.
Voznesenskaya EV, Koteyeva NK, Akhani H,
Roalson EH, Edwards GE. 2013. Structural and physiological analyses in
Salsoleae (Chenopodiaceae) indicate multiple transitions among C3,
intermediate, and C4 photosynthesis. – J. Experim. Bot. 64: 3583-3604.
Vrijdaghs A, Flores-Olvera H, Smets E. 2014.
Enigmatic floral structures in Alternanthera, Iresine, and
Tidestromia (Gomphrenoideae, Amaranthaceae). A developmental
homology assessment. – Plant Ecol. Evol. 147: 49-66.
Vural C. 2008. A new species of
Dianthus (Caryophyllaceae) from Mount
Erciyes, Central Anatolia, Turkey. – Bot. J. Linn. Soc. 158: 55-61.
Wagenitz G. 1959. Neue und bemerkenswerte
Chenopodiaceen Inneranatoliens. – Ber. Deutsch. Bot. Ges. 72: 151-158.
Wagner WL, Harris EM. 2000. A unique Hawaiian
Schiedea (Caryophyllaceae: Alsinoideae)
with only five fertile stamens. – Amer. J. Bot. 87: 153-160.
Wagner WL, Weller SG, Sakai AK. 1995.
Phylogeny and biogeography in Schiedea and Alsinidendron (Caryophyllaceae). – In:
Wagner WL, Funk VA (eds), Hawaiian biogeography: evolution on a hot spot
archipelago, Smithsonian Institution Press, Washington, D.C., pp. 221-258.
Wagner WL, Weller SG, Sakai AK. 2005.
Monograph of Schiedea (Caryophyllaceae-Alsinoideae).
– Syst. Bot. Monogr. 72: 1-169.
Wagstaff SJ, Hennion F. 2007. Evolution and
biogeography of Lyallia and Hectorella (Portulacaceae), geographically
isolated sisters from the Southern Hemisphere. – Antarctic Sci. 19:
417-426.
Walia K, Kapil RN. 1965. Embryology of
Frankenia Linn. with some comments on the systematic position of the
Frankeniaceae. – Bot. Not.
118: 412-429.
Walker JF, Yang Y, Feng T, Timoneda A,
Mikenas J, Hutchison V, Edwards C, Wang N, Ahluwalia S, Olivieri J, Walker-Hale
N, Majure LC, Puente R, Kadereit G, Lauterbach M, Eggli U, Flores-Olvera H,
Ochoterena H, Brockington SF, Moore MJ, Smith SA. 2018. From cacti to
carnivores: improved phylotranscriptomic sampling and hierarchical homology
inferenceprovide further insight into the evolution of Caryophyllales. – Amer. J.
Bot. 105: 446-462.
Wallace RS. 1987. Seed characters,
biogeography, and systematics of the genus Mollugo (Molluginaceae). – Amer. J.
Bot. 74: 763.
Wallace RS. 1995. Molecular systematic study
of the Cactaceae: using
chloroplast DNA variation to elucidate cactus phylogeny. – Bradleya 13:
1-12.
Wallace RS. 2002a. The phylogeny and
systematics of columnar cacti: an overview. – In Fleming TH, Valiente-Banuet
A (eds), Columnar cacti and their mutualists, University of Arizona Press,
Tucson, pp. 42-65.
Wallace RS. 2002b. An overview of columnar
cactus evolution and systematics. – In: Fleming TH, Valiente Banuet A (eds),
Columnar cacti and their mutualists: evolution, ecology and conservation,
University of Arizona Press, Tucson, pp. 66-86.
Wallace RS, Cota JH. 1996. An intron loss in
the chloroplast gene rpoC1 supports a monophyletic origin for the
subfamily Cactoideae of the Cactaceae. – Curr. Gen. 29:
275-281.
Wallace RS, Dickie SL. 2002. Systematic
implication of chloroplast DNA sequence variation in subfam. Opuntioideae (Cactaceae). – In: Hunt D, Taylor N
(eds), Studies in the Opuntioideae, David Hunt, Sherborne, United Kingdom, pp.
9-24.
Wallace RS, Gibson AC. 2002. Evolution and
systematics. – In: Nobel PS (ed), Cacti: biology and uses, University of
California Press, Berkeley, pp. 1-21.
Walter H. 1906. Die Diagramme der
Phytolaccaceen. – Engl. Bot. Jahrb. Syst. 37, Beibl. 85: 1-57.
Waly NM. 1999. Wood anatomical characters of
the Egyptian Tamarix L. species and its taxonomic significance. –
Taeckholmia 19: 115-125.
Wang A, Yang M, Liu J. 2005. Molecular
phylogeny, recent radiation and evolution of gross morphology of the rhubarb
genus Rheum (Polygonaceae) inferred from
chloroplast DNA trnL-F sequences. – Ann. Bot. 96: 489-498.
Wang Y, Chen C, Ding H. 1992. Development of
ovule and female gametophyte in Rheum palmatum L. – Phytomorphology
42: 71-79.
Wang Y, Liu Y, Liu S, Huang H. 2009.
Molecular phylogeny of Myricaria (Tamaricaceae): implications for
taxonomy and conservation in China. – Bot. Stud. 50: 343-352.
Warburg O. 1894. Flacourtiaceae. – In:
Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(6a), W.
Engelmann, Leipzig, pp. 1-56.
Warming E. 1920. Structure and biology of
arctic flowering plants II. Caryophyllaceae. – Medd.
Grønland 37: 231-342.
Waselkov KE, Boleda AS, Olsen KM. 2018. A
phylogeny of the genus Amaranthus (Amaranthaceae) based on several
low-copy nuclear loci and chloroplast regions. – Syst. Bot. 43: 439-458.
Watson SL. 1874. Revision of the North
American Chenopodiaceae. – Proc. Amer. Acad. Arts Sci. 9: 82-126.
Watson SL. 1877. Descriptions of new species
of plants, with revisions of Lychnis, Eriogonum and
Chorizanthe. – Proc. Amer. Acad. Arts Sci. 12: 246-278.
Weber WA. 1950. A new species and subgenus of
Atriplex from southwestern Colorado. – Madroño 10: 187-191.
Weberling F. 1956. Weitere Untersuchungen zur
Morphologie des Unterblattes bei den Dikotylen I. Balsaminaceae: II.
Plumbaginaceae. – Beitr.
Biol. Pflanzen 33: 17-32.
Weberling F. 1970. Weitere Untersuchungen zur
Morphologie des Unterblattes bei den Dikotylen VI. Polygonaceae. – Beitr. Biol.
Pflanzen 47: 127-140.
Weller SG, Sakai AK, Wagner WL, Herbst DR.
1990. Evolution of dioecy in Schiedea (Caryophyllaceae: Alsinoideae)
in the Hawaiian Islands: biogeographical and ecological factors. – Syst. Bot.
15: 266-276.
Weller SG, Wagner WL, Sakai AK. 1995. A
phylogenetic analysis of Schiedea and Alsinidendron
(Caryophllaceae: Alsinoideae): implications for the evolution of breeding
systems. – Syst. Bot. 20: 315-337.
Weller SG, Sakai AK, Straub C. 1996. Allozyme
diversity and genetic identity in Schiedea and Alsinidendron
(Caryophyllaceae:
Alsinoideae) in the Hawaiian Islands. – Evolution 50: 23-34.
Weller SG, Sakai AK, Rankin AE, Golonka A,
Kutcher B, Ashby KE. 1998. Dioecy and the evolution of pollination systems in
Schiedea and Alsinidendron (Caryophyllaceae: Alsinoideae)
in the Hawaiian Islands. – Amer. J. Bot. 85: 1377-1388.
Weller SG, Sakai AK, Wagner WL. 2001.
Artificial and natural hybridization in Schiedea and
Alsinidendron (Caryophyllaceae: Alsinoideae):
the importance of phylogeny, genetic divergence, breeding system, and
population size. – Syst. Bot. 26: 571-584.
Wen Z-B, Zhang M-L, Zhu G-L, Sanderson SC.
2010. Phylogeny of Salsoleae s.l. (Chenopodiaceae) based on DNA sequence data
from ITS psbB-psbH, and rbcL, with emphasis on taxa
of northwestern China. – Plant Syst. Evol. 288: 25-42.
Werker E, Many T. 1974. Heterocarpy and its
ontogeny in Aellenia autrani (Post.) Zoh. Light- and
electron-microscope study. – Israel J. Bot. 23: 132-144.
Wetterwald X. 1889. Blatt- und Sprossbildung
bei Euphorbien und Cacteen. – Nova Acta Acad. Caes. Leop.-Carol. German. Nat.
Cur. 53: 379-440.
Whalen MA. 1987. Wood anatomy of the American
frankenias (Frankeniaceae):
systematic and evolutionary implications. – Amer. J. Bot. 74: 1211-1223.
Whalen MA. 1989. Systematics of
Frankenia (Frankeniaceae) in North and
South America. – Syst. Bot. Monogr. 17: 1-93.
Wheat D. 1977. Successive cambia in the stem
of Phytolacca dioica. – Amer. J. Bot. 64: 1209-1217.
Whitehouse C. 1996. Nyctaginaceae. – In: Polhill
RM (ed), Flora of tropical East Africa, A. A. Balkema, Rotterdam, pp. 1-20.
Wiger J. 1935. Embryological studies on the
families Buxaceae, Meliaceae,
Simarubaceae and Burseraceae. –
Ph.D. diss., Lund University, Sweden.
Wild H. 1961a. 21. Caryophyllaceae; 22.
Illecebraceae. – In: Exell AW, Wild H (eds), Flora Zambesiaca 1 (Part 2),
Crown Agents for Oversea Governments and Administrations, London, pp.
337-362.
Wild H. 1961b. 23. Portulacaceae. – In: Exell AW,
Wild H (eds), Flora Zambesiaca 1 (Part 2), Crown Agents for Oversea Governments
and Administrations, London, pp. 362-372.
Willert DJ von. 1979. Vorkommen und
Regulation des CAM bei Mittagsblumengewächsen (Mesembryanthemaceae). – Ber.
Deutsch. Bot. Ges. 92: 133-144.
Willert DJ von, Curdts E, Willert K von.
1977. Veränderung der PEP-Carboxylase während einer durch NaCl geförderten
Ausbildung eines CAM bei Mesembryanthemum crystallinum. – Biochem.
Physiol. Pflanzen 171: 101-107.
Williams FN. 1895. On the genus
Arenaria L. – Bull. Herb. Boissier 3: 593-603.
Williams FN. 1896. A revision of the genus
Silene L. – Bot. J. Linn. Soc. 32: 1-196.
Williams FN. 1898. A revision of the genus
Arenaria Linn. – J. Linn. Soc. 38: 395-407.
Williams FN. 1909. The Caryophyllaceae of Tibet. –
Bot. J. Linn. Soc. 38: 395-407.
Williams JT, Ford-Lloyd BV. 1974. The
systematics of the Chenopodiaceae. – Taxon 23: 353-354.
Williams SE. 1976. Comparative sensory
physiology of the Droseraceae
– the evolution of a plant sensory system. – Proc. Amer. Philos. Soc. 120:
187-204.
Williams SE, Albert VA, Chase MW. 1994.
Relationships of Droseraceae: a
cladistic analysis of rbcL sequence and morphological data. – Amer.
J. Bot. 81: 1027-1037.
Willis JC. 1892. The distribution of the seed
in Claytonia. – Ann. Bot. 6: 382-383.
Willson J, Spellenberg R. 1977. Observations
on anthocarp anatomy in the subtribe Mirabilinae (Nyctaginaceae). – Madroño 24:
104-111.
Willyard A, Wallace LE, Wagner WL, Weller SG,
Sakai Ak, Nepokroeff M. 2011. Estimating the species tree for Hawaiian
Schiedea (Caryophyllaceae) from multiple
loci in the presence of reticulate evolution. – Mol. Phylogen. Evol. 60:
29-48.
Wilmot-Dear CM. 1976. Plumbaginaceae. – In: Polhill
RM (ed), Flora of tropical East Africa, Crown Agents for Oversea Governments
and Administrations, London, pp. 1-12.
Wilms HJ. 1981. Ultrastructure of the egg
apparatus of Spinacia. – Acta Soc. Bot. Polon. 50: 165-168.
Wilson HD. 1988. Allozyme variation and
morphological relationships of Chenopodium hircinum (s.l.). – Syst.
Bot. 13: 215-228.
Wilson J. 1890. The mucilage – and other
glands of the Plumbaginaceae. – Ann. Bot.
4: 231-258.
Wilson KL. 1988. Polygonum sensu
lato (Polygonaceae) in
Australia. – Telopea 3: 177-182.
Wilson KL. 1990. Some widespread species of
Persicaria (Polygonaceae) and their allies.
– Kew Bull. 45: 621-636.
Wilson KL. 1996. Nomenclatural notes on Polygonaceae in Australia. –
Telopea 7: 83-94.
Wilson PG. 1972. A taxonomic revision of the
genus Tecticornia. – Nuytsia 1: 277-288.
Wilson PG. 1975. A taxonomic revision of the
genus Maireana. – Nuytsia 2: 2-83.
Wilson PG. 1980. A revision of the Australian
species of Salicornieae (Chenopodiaceae). – Nuytsia 3: 3-154.
Wilson PG. 1983. A taxonomic revision of the
tribe Chenopodieae (Chenopodiaceae) in Australia. – Nuytsia 4: 135-262.
Wilson PG. 1984. Chenopodiaceae. – In:
George AS (ed), Flora of Australia 4, Australian Government Publ. Service,
Canberra, pp. 81-317, 322-330.
Wilson RC. 1972. Distribution, ecology and
habit of nine species of Abronia found in California. – Aliso 7:
421-437.
Wilson RC. 1974. Abronia 1.
Anthocarp polymorphism and anatomy for 9 Abronia species found in
California. – Aliso 8: 113-128.
Wilson RC. 1975. Abronia 2. Pericarp
and seed coat anatomy and its ecological implications for nine species of
Abronia. – Aliso 8: 289-299.
Wodehouse RP. 1931. Pollen grains in the
identification of plants VI. Polygonaceae. – Amer. J. Bot.
18: 749-764.
Wofford BE. 1981. External seed morphology of
Arenaria (Caryophyllaceae) of the
southeastern United States. – Syst. Bot. 6: 126-135.
Wohlpart A, Mabry TJ. 1968. The distribution
and phylogenetic significance of the betalains with respect to the
Centrospermae. – Taxon 17: 148-152.
Wolfe GR, Xu S, Patterson GW, Salt TA. 1989.
Polygonales and Plumbaginales: sterol composition in relation to the
Caryophyllidae. – Phytochemistry 28: 143-149.
Wolter-Filho W, Da Rocha AI, Yoshida M,
Gottlieb OR. 1985. Ellagic acid derivatives from Rhabdodendron
macrophyllum. – Phytochemistry 24: 1991-1993.
Wolter-Filho W, Da Rocha AI, Yoshida M,
Gottlieb OR. 1989. Chemosystematics of Rhabdodendron. –
Phytochemistry 28: 2355-2357.
Woo W-S. 1975. The structure of esculentic
acid: a new triterpene from Phytolacca esculenta. – Phytochemistry
14: 1885-1888.
Woo W-S, Kang S-S. 1976. Phytolaccoside B:
triterpene glucoside from Phytolacca americana. – Phytochemistry 15:
1315-1317.
Wood CE. 1995. Evidence for the hybrid origin
of Drosera anglica. – Rhodora 57: 105-130.
Woroschilov WN. 1942. Revision des espèces
de Chenopodium de la section Ambrina (Spach) Hook. fil. –
Bot. Žurn. 27: 33-47. [In Russian with French summary]
Wulff HD. 1936. Die Polysomatie der
Chenopodiaceen. – Planta 26: 275-290.
Wunschmann E. 1891. Nepenthaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien III(2), W. Engelmann,
Leipzig, pp. 253-260.
Wyatt R. 1984. The evolution of
self-pollination in granite outcrop species of Arenaria I.
Morphological correlates. – Evolution 38: 804-816.
Xi Y-Z. 1988. Study on pollen morphology of
the Tamaricaceae from China.
– Bull. Bot. Res. Harbin 8: 23-42.
Xu F, Sun M. 2001. Comparative analysis of
phylogenetic relationships of grain amaranths and their wild relatives
(Amaranthus; Amaranthaceae) using internal
transcribed spacer, amplified fragment length polymorphism, and double-primer
fluorescent intersimple sequence repeat markers. – Mol. Phylogen. Evol. 21:
372-387.
Xue JJ, Zhang ML. 2011. Monophyly and
infrageneric variation of Corispermum L. (Chenopodiaceae), evidence
from sequence data psbB-psbH, rbcL and ITS. – J.
Arid Land 3: 240-253.
Yakubovskaya TV. 1991. The genus
Aldrovanda (Droseraceae) in the Pleistocene of
the Belorussian SSR. – Bot. Žurn. 76: 109-118. [In Russian]
Yamazaki T. 1987. The floral anatomy of the
genus Phytolacca with reference to the flower of the Caryophyllaceae. – Acta
Phytotaxon. Geobot. 38: 21-32.
Yang Y, Moore MJ, Brockington SF, Soltis DE,
Wong GK-S, Carpenter EJ, Zhang Y, Chen L, Yan Z, Xie Y et al. 2015. Dissecting
molecular evolution in the highly diverse plant clade Caryophyllales using
transcriptome sequencing. – Mol. Biol. Evol. 32: 2001-2014.
Yao G. 2016. A contribution to the Flora of
China: three new combinations in Solitaria (Caryophyllaceae). –
Phytotaxa 298: 2. http://dx.doi.org/10.11646/phytotaxa.298.2.11
Yaprak AE, Kadereit G. 2009. A new species of
Halocnemum M. Bieb. (Amaranthaceae) from southern
Turkey. – Bot. J. Linn. Soc. 158: 716-721.
Yasmin G, Khan MA, Shaheen N, Hayat MQ. 2009.
Micromorphological investigation of foliar anatomy of genera
Aconogonon and Bistorta of family Polygonaceae. – Intern. J.
Agricult. Biol. 11: 285-289.
Yasmin G, Khan MA, Shaheen N. 2010. Pollen
morphology of selected Polygonum L. species (Polygonaceae) from Pakistan and
its taxonomic significance. – Pakistan J. Bot. 42: 3693-3703.
Yasui Y, Ohnishi O. 1996. Comparative study
of rbcL gene sequences in Fagopyrum and related taxa. –
Genes Genet. Syst. 71: 219-224.
Yasui Y, Ohnishi O. 1998. Interspecific
relationships in Fagopyrum (Polygonaceae) revealed by the
nucleotide sequences of the rbcL and accD genes and their
intergenic region. – Amer. J. Bot. 85: 1134-1142.
Yıldız K. 2002. Seed morphology of Caryophyllaceae species from
Turkey (North Anatolia). – Pakistan J. Bot. 34: 161-171.
Yıldız K, Çırpıcı A. 1998. Seed
morphological studies of Silene L. from Turkey. – Pakistan J. Bot.
30: 173-188.
Yıldız K, Minareci E, Çırpıcı A,
Dadandi MY. 2009. A karyotypic study on Silene, section
Siphonomorpha species of Turkey. – Nord. J. Bot. 26: 368-374.
Yonekura K, Ohashi H. 1997. New combinations
of East Asian species of Polygonum s.l. – J. Jap. Bot. 72:
154-161.
Yu W, Fan S, Xu C, Zhu L, Hou Y, Lin F, Li F.
2008. Systematic position of Reynoutria and Polygonum
sibiricum inferred from sequences of chloroplast trnL-F and
matK. – J. Syst. Evol. 46: 676-681.
Yuasa H, Shimizu H, Kashiwai S, Kondo N.
1974. Chromosome numbers and their bearing on the geographic distribution in
the subfamily Opuntioideae (Cactaceae). – Rep. Inst. Breed.
Res., Tokyo Univ. Agric. 4: 1-10.
Yurtseva OV, Troitsky AV, Bobrova VK,
Voylokova VN. 2010. A taxonomical revision of Polygonum s. str. (Polygonaceae): phylogenetic and
morphological data. – Bot. Žurn. 95: 226-247. [In Russian]
Yurtseva OV, Severova EE, Bovina I. 2014.
Pollen morphology and taxonomy of Atraphaxis (Polygoneae, Polygonaceae). – Plant Syst.
Evol. 300: 749-766.
Zacharias EH. 2007. Evolutionary studies in
American Atripliceae (Chenopodiaceae). – Ph.D. diss., University of
California, Berkeley, California.
Zacharias EH, Baldwin BG. 2010. A molecular
phylogeny of North American Atripliceae (Chenopodiaceae), with implications for
floral and photosynthetic pathway evolution. – Syst. Bot. 35: 839-857.
Zamyatnin BN. 1951. On the inferior ovary of
the axial origin. – Bot. Žurn. 36: 89-92. [In Russian]
Zandonella P. 1967. Les nectaires des
Alsinoideae: Stellaria et Cerastium sensu lato. – Compt.
Rend. Acad. Sci. Paris, sér. D, 264: 2466-2469.
Zandonella P. 1972. Le nectaire floral des
Centrospermales. Localisation, morphologie, anatomie, histology, cytology. –
Ph.D. diss., l’Université Claude-Bernard, Lyon, France.
Zandonella P. 1977. Apports de l’étude
comparée des nectaires floraux à la conception phylogénétique de l’ordre
des Centrospermales. – Ber. Deutsch. Bot. Ges. 90: 105-125.
Zandonella P, Lecocq M. 1977. Morphologie
pollinique et mode de pollinisation chez les Amaranthaceae. – Pollen Spores
19: 119-141.
Zappetini G. 1953. The taxonomy of
Halogeton glomeratus. – Amer. Midl. Natur. 50: 238-247.
Zappi DC. 1994. Pilosocereus, Cactaceae. The genus in Brazil. –
Succ. Plant Res. 3: 1-160.
Zenk MH, Fürbringer M, Steglich W. 1969.
Occurrence and distribution of 7-methyljuglone and plumbagin in the Droseraceae. – Phytochemistry 8:
2199-2200.
Zhang D-Y, Chen Z-D, Sun H-Y, Yin L-K, Pan
B-R. 2000. Systematic studies on some questions of Tamaricaceae based on ITS
sequence. – Acta Bot. Boreal.-Occid. Sin. 20: 421-431.
Zhang D-Y, Pan B-R, Yin L-K. 2003. The
photogeographical studies of Tamarix (Tamaricaceae). – Acta Bot.
Yunnan. 25: 415-427.
Zhang P-Y, Zhang Y-J. 1984. A study on the
taxonomy of the genus Myricaria Desv. in China. – Acta Bot.
Boreal.-Occid. Sin. 4: 67-80.
Zhang Q, Huang L-J, Wang X-F. 2018. The
unusual gynoecium structure and extragynoecial pollen-tube pathway in
Phytolacca (Phytolaccaceae). – Plant
Syst. Evol. 304: 885-894.
Zhang Y-M. 2001. Pollen morphology of the Tamaricaceae from China and its
taxonomic significance. – Acta Bot. Boreal.-Occid. Sin. 21: 857-864.
Zhang Y-M, Pan B-R, Yin L-K. 1998. Seed
morphology of Tamaricaceae in
China arid areas and its systematic evolution. – J. Plant Resourc. Environm.
7: 22-27.
Zheng H-C, Lu A-M, Hu Z-H. 2004. Floral
organogenesis in Phytolacca (Phytolaccaceae). – Acta
Phytotaxon. Sin. 42: 352-364. [In Chinese]
Zheng H-C, Ma S-W, Chai T-Y. 2010. Ovular
development and perisperm formation in Phytolacca americana (Phytolaccaceae) and their
systematic significance in Caryophyllales. – J. Syst.
Evol. 48: 318-325.
Zhou LH. 1996. On the geographical
distribution of Arenaria L. – Acta Phytotaxon. Sin. 3: 229-241.
Zhou Z-Z, Zhang X-P, Xu R-X. 2004. Pollen
morphology of Koenigia from China. – Acta Phytotaxon. Sin. 42:
513-523.
Zibareva L, Volodin V, Saatov Z, Savchenko T,
Whiting P, Lafont R, Dinan L. 2003. Distribution of phytoecdyosteroids in the
Caryophyllaceae. –
Phytochemistry 64: 499-517.
Zimmerman AD. 1985. Systematics of the genus
Coryphantha (Cactaceae).
– Ph.D. diss., University of Texas, Austin, Texas.
Zimmerman CA. 1977. A comparison of breeding
systems and seed physiologies in three species of Portulaca L. –
Ecology 58: 860-868.
Zmarzty Sue. 1993. A reconsideration of
Gypsophila Series Deserticolae Barikoudah (Caryophyllaceae) including
G. capillaris (Forssk.) C. Chr. – Kew Bull. 48: 683-697.
Zohary M, Baum B. 1965. On the androecium of
Tamarix flower and its evolutionary trends. – Israel J. Bot. 14:
101-111.
Zohary M, Danin A. 1970. The genus
Reaumuria in the Near East. – Israel J. Bot. 19: 305-313.