
Cladogram of Liliales based on DNA sequence data (Kim & al. 2013; Corsiaceae added). Corsiaceae are sister to Campynemataceae in the analyses by Givnish & al. (2016).
[Liliales+[Iridales+Commelinidae]]
Liriopsida Brongn., Enum. Plant. Mus. Paris: xv, 17. 12 Aug 1843 [’Lirioideae’]
Fossils Monocotylostrobus bracteatus, an inflorescence from the Deccan Intertrappean Beds, has been assigned to Liliaceae, although this assessment may be questioned.
Habit Usually bisexual (sometimes andromonoecious, polygamous, dioecious, androdioecious, or gynodioecious), perennial herbs (sometimes with more or less lignified stem). Usually with a bulb (sometimes a corm, rarely a tuberous rhizome). Sometimes twining or climbing.
Vegetative anatomy Usually Paris type arbuscular mycorrhiza. Bulb with or without tunica. Velamen sometimes present. Phellogen absent. Secondary lateral growth absent. Vessels present in roots (sometimes also in stem). Vessel elements usually with scalariform (sometimes simple) perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial parenchyma? Sieve tube plastids P2c type, with cuneate protein crystals, without starch or protein filaments. Nodes multilacunar with several leaf traces. Silica bodies absent. Calciumoxalate as raphides or druses (sometimes crystal sand or solitary crystals) present or absent.
Trichomes Hairs usually absent (sometimes unicellular, bicellular or multicellular, uniseriate, simple).
Leaves Alternate (spiral or distichous; sometimes seemingly opposite or verticillate), simple, entire, usually linear (sometimes subulate; sometimes resupinate; rarely differentiated into pseudopetiole and pseudolamina), with convolute, supervolute, conduplicate, curved or flat ptyxis. Stipules absent; leaf sheath usually absent (sometimes well developed, open or closed). Venation usually parallelodromous (sometimes acrodromous or palmatireticulate; rarely reticulate or pinnate-campylodromous). Stomata anomocytic. Cuticular wax crystalloids as parallel platelets (Convallaria type, resembling ‘electromagnetic field lines’; sometimes as stalk-like structures). Mesophyll with or without idioblasts containing calciumoxalate raphides (sometimes single prismatic crystals). Tanniniferous cells sometimes present. Leaf margin usually entire (rarely serrate).
Inflorescence Usually terminal (sometimes axillary), paniculate, thyrsoid, botryoid, stachyoid, cincinnus, umbellate, capitate, spicate or raceme-like, or racemose, simple or branched, or flowers solitary terminal. Floral prophylls (bracteoles) lateral or absent.
Flowers Usually actinomorphic (sometimes zygomorphic). Usually hypogyny (sometimes epigyny, rarely half epigyny). Tepals 3(–6)+3(–6) (rarely three, four, seven, twelve or more than twelve), with imbricate or contorted aestivation, usually petaloid (sometimes sepaloid), often spotted or striate, usually free (rarely connate at base). Tepal nectaries (often as nectariferous glands) present at tepal bases (sometimes androecial nectaries; nectary sometimes absent). Septal nectaries absent. Disc absent.
Androecium Stamens usually as many as tepals, in two whorls (rarely only three outer stamens, or up to 24 in several whorls). Filaments free from each other, usually free from tepals (sometimes epitepalous in lower part). Anthers pseudobasifixed (filament apex surrounded by tubular connective), basifixed or dorsifixed, versatile or non-versatile, tetrasporangiate, extrorse, introrse or latrorse, usually longicidal (dehiscing by longitudinal slits, rarely poricidal, dehiscing by apical pores). Tapetum usually secretory (sometimes amoeboid-periplasmodial). Staminodia absent.
Pollen grains Microsporogenesis successive. Pollen grains usually monosulcate (rarely 2–4-sulcate, 2–4-foraminate, tetraporate, or inaperturate), shed as monads, bicellular or tricellular at dispersal. Exine tectate or semitectate (rarely intectate), usually with columellate (rarely granular) infratectum, reticulate, striate or striato-reticulate, foveolate, verrucate-areolate, verrucate, rugulate, clavate, corrugate, echinate, spinulate, gemmate, or psilate.
Gynoecium Pistil composed of (one to) three (to ten) connate carpels. Ovary usually superior (sometimes inferior, rarely semi-inferior), trilocular (rarely unilocular, bilocular or quadrilocular to decemlocular). Style single, simple or lobate, or stylodia free. Stigma single, capitate or slightly lobate, or stigmas several, punctate, papillate (with unicellular papillae), usually Dry (sometimes Wet) type. Pistillodium absent.
Ovules Placentation usually axile (rarely parietal or subparietal). Ovules one to more than 100 per carpel, usually anatropous (sometimes campylotropous, rarely hemianatropous, orthotropous or amphitropous), ascending to pendulous, apotropous?, epitropous, plagiotropous, basitropous, pleurotropous, or hypotropous, usually bitegmic (rarely unitegmic), usually tenuinucellar (sometimes crassinucellar). Micropyle usually endostomal (sometimes bistomal). Funicular obturator often present. Parietal cell not formed, or formed from archesporial cell. Periclinal cell divisions sometimes occurring at single-layered megasporangial apex. Nucellar cap sometimes present. Megagametophyte monosporous, Polygonum type, or tetrasporous, Fritillaria type (rarely Clintonia type, 8-nucleate, Adoxa type, or 16-nucleate, Drusa type, or disporous, Allium type, Veratrum lobelianum type or Scilla type). Synergids sometimes with a filiform apparatus. Antipodal cells persistent, sometimes proliferating. Endosperm development usually nuclear (sometimes helobial). Endosperm haustoria? Embryogenesis onagrad, asterad or chenopodiad.
Fruit Usually a loculicidal capsule (sometimes septicidal, ventricidal or irregularly dehiscent; occasionally a berry or berry-like).
Seeds Aril absent (strophiole or caruncle sometimes present). Elaiosome (from raphe and chalaza, or from raphe and hilum) sometimes present. Testa thin (often with two cell layers), with cells often flat or collapsed (sometimes with lignified walls; exotestal epidermis sometimes with phlobaphene). Sarcotesta rarely present. Exotesta sometimes palisade, sometimes collapsing. Tegmen strongly compressed, thin, often collapsing. Endotegmic cells sometimes with phlobaphene. Perisperm not developed. Endosperm copious, with aleurone and/or oils (starch absent), with thick and pitted cell walls with hemicellulose. Embryo straight, little differentiated to well differentiated, without chlorophyll. Cotyledon one, usually photosynthesizing, bifacial. Cotyledon hyperphyll usually elongate (sometimes compact), usually assimilating. Hypocotyl internode short or absent. Coleoptile absent. Radicula persistent. Germination phanerocotylar.
Cytology x = 5–14(–19)
DNA Mitochondrial gene sdh3 lost.
Phytochemistry Flavonols (kaempferol, quercetin), flavones, flavanones, luteolin, apigenin, etc., chalcones, tannins, alkaloids without 7-C-tropolone ring (androcymbine, floramultine, kreysigine, etc.) or with 7-C-tropolone ring (e.g. colchicine), steroidal alkaloids (ceveratrum and jerveratrum alkaloids, veratramine, zigadenine, jervine, etc.), steroidal saponins (e.g. lilagenin, yuccagenin, diosgenin), anthraquinones, tulipalines (butyrolactone), chelidonic acid fructans, glucose esters (tuliposides), and saccharose esters of diferulic or triferulic acid present. Ellagic acid, proanthocyanidins, and cyanogenic compounds not found.
Systematics Liliales are sister-group to the [Iridales+Commelinidae] clade in most molecular analyses.
Corsiaceae, Campynemataceae and the remaining Liliales form a basal trichotomy (Fay & al. 2006). This third clade has relatively high bootstrap support, but no unambiguous morphological synapomorphies can be postulated so far (the fruit is often a berry). A subbasal trichotomy comprises the clades Melanthiaceae, [[Philesiaceae+Rhipogonaceae]+[Smilacaceae+Liliaceae]] and [Petermanniaceae+[Colchicaceae+[Alstroemeriaceae+Luzuriagaceae]]]. Arachnitis (Corsiaceae) and Campynemataceae formed a sister-group to the remaining Liliales and the Melanthiaceae were placed as sister to the PR+SL clade in the analysis by Givnish & al. (2016). In Petersen & al. (2013) Arachnitis was placed as sister-group to the remaining Liliales, whereas Mennes & al. (2015) found the Corsiaceae (Corsia+Arachnitis) to be sister-group to the Campynemataceae. The Philesiaceae-Liliaceae clade is supported by the potential synapomorphies (Stevens 2001 onwards): broad leaves with reticulate venation; relatively small flowers; and a baccate fruit. Philesiaceae and Rhipogonaceae share the synapomorphies: leaves with pseudopetiole, pseudolamina and a midrib; cuticular wax crystalloids as parallel platelets; tepals not spotted; ovules crassinucellar; testa disintegrating; and absence of stem fructans. Smilacaceae and Liliaceae may share the basic chromosome number x=8.
The clade [Petermanniaceae+[Colchicaceae+[Luzuriagaceae+Alstroemeriaceae]]] is characterized by a well developed seedling primary root (Stevens 2001 onwards). Alstroemeriaceae and Luzuriagaceae have the following features in common: resupinate leaves; placentation sometimes parietal; testa and tegmen thin-walled; and a bimodal karyotype.
Colchicaceae, Corsiaceae, Liliaceae, and Melanthiaceae, have tepals with three traces, whereas in Petermannia and Smilacaceae each tepal has a single trace.

|
Cladogram of Liliales based on DNA sequence data (Kim & al. 2013; Corsiaceae added). Corsiaceae are sister to Campynemataceae in the analyses by Givnish & al. (2016). |
ALSTROEMERIACEAE Dumort. |
( Back to Liliales ) |
Alstroemeriales Hutch., Fam. Fl. Pl., Monocot. 2: 12, 111. 20 Jul 1934
Genera/species 2–3/210–240
Distribution Central Mexico and the West Indies southwards to Chile and Argentina, with the largest diversity in the Andes.
Fossils Unknown.
Habit Bisexual, usually perennial (in Alstroemeria rarely annual) herbs. Sometimes climbing. Roots often swollen, fusiform or tuberous, storing water and nutrients.
Vegetative anatomy Roots usually fibrous, without velamen. Phellogen absent. Stem vascular bundles usually present inside a sclerenchymatous cylinder. Secondary lateral growth absent. Vessels present in roots and stem. Vessel elements usually with scalariform (in roots often also simple) perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial parenchyma? Sieve tube plastids P2c type, with cuneate protein crystals. Nodes? Calciumoxalate raphides present in Alstroemeria.
Trichomes Hairs usually absent (sometimes unicellular, bicellular or multicellular, uniseriate, with a basal cell).
Leaves Alternate (spiral), simple, entire, more or less resupinate (twisted 180° at base), often linear, with conduplicate or supervolute? ptyxis. Stipules and leaf sheath absent. Venation parallelodromous. Stomata anomocytic. Cuticular wax crystalloids as parallel platelets (Convallaria type). Mesophyll with calciumoxalate raphides. Leaf margin entire.
Inflorescence Usually terminal (sometimes axillary) umbel-like, thyrse, simple or branched, consisting of helicoid cymes (flowers rarely solitary terminal), often subtended by large green foliaceous bracts. Foliar prophylls (bracteoles) lateral.
Flowers Actinomorphic or zygomorphic, often large. Epigyny. Tepals 3+3, petaloid, often dotted to striated, usually caducous, free; in Alstroemeria median tepal of outer whorl adaxial. Inner tepals usually with canaliculate base with tepal nectaries (in Leontochir with nectariferous pocket). Septal nectaries absent. Disc absent.
Androecium Stamens 3+3. Filaments filiform, free from each other and from tepals. Anthers pseudobasifixed, non-versatile, tetrasporangiate, introrse to latrorse, longicidal (dehiscing by longitudinal slits). Tapetum secretory, with binucleate cells. Staminodia absent.
Pollen grains Microsporogenesis successive. Pollen grains monosulcate, shed as monads, bicellular at dispersal. Exine tectate or semitectate, with columellate infratectum, striate to striato-reticulate (Alstroemeria) or reticulate (Bomarea, Leontochir).
Gynoecium Pistil composed of three connate carpels. Ovary inferior, unilocular (Leontochir, the ’Schickendantzia clade’ of Alstroemeria) or trilocular (Alstroemeria, Bomarea). Style single, simple, filiform. Stigma trilobate, Wet type (at least in Alstroemeria). Pistillodium absent.
Ovules Placentation axile (Alstroemeria, Bomarea) or parietal (Leontochir, the ’Schickendantzia clade’ of Alstroemeria). Ovules c. 20 to more than 100 per carpel, anatropous, bitegmic, tenuinucellar. Micropyle endostomal. Outer integument ? cell layers thick. Inner integument ? cell layers thick. Parietal cell not formed (parietal tissue absent). Nucellar cap absent. Megagametophyte monosporous, Polygonum type. Endosperm development ab initio nuclear. Endosperm haustoria? Embryogenesis?
Fruit Usually a loculicidal capsule (dry to fleshy, rarely indehiscent; in Alstroemeria usually explosively dehiscent; fruit in some species of Bomarea berry-like).
Seeds Aril absent. Seed coat testal. Testa undifferentiated or more or less thick-walled, usually dry. Sarcotesta present in Bomarea and Leontochir. Exotesta? Endotesta? Tegmen collapsing and membranous. Perisperm not developed. Endosperm copious, with oils and aleurone (starch absent), with thick and pitted cell walls. Embryo straight, often short, well differentiated, chlorophyll? Cotyledon one, usually not photosynthesizing (in Alstroemeria graminea, an annual species, photosynthesizing). Cotyledon hyperphyll elongate and assimilating, or compact and not assimilating. Hypocotyl internode short. Coleoptile absent. Radicula persistent. Germination?
Cytology n = 8, 9 – Karyotype bimodal. Chromosomes 6–19 µm long.
DNA Very large genomes (with a C value of c. 350 pg or more) are found in some species.
Phytochemistry Flavonols (kaempferol, quercetin), steroidal saponins, tannins, anthraquinones, chelidonic acid, and glucose esters (tuliposides) present. Flavones, ellagic acid, proanthocyanidins, alkaloids, and cyanogenic compounds not found.
Use Ornamental plants, starch sources (Alstroemeria ligtu, Bomarea edulis).
Systematics Leontochir (1; L. ovallei; coast of northern Chile; in Bomarea?), Bomarea (110–120; Mexico, Central America, tropical South America; incl. Leontochir?), Alstroemeria (100–120; western and eastern South America, especially the Andes).
Alstroemeriaceae are sister to Luzuriagaceae.
Based on morphology, Bomarea appears to be paraphyletic, when Leontochir is excluded.
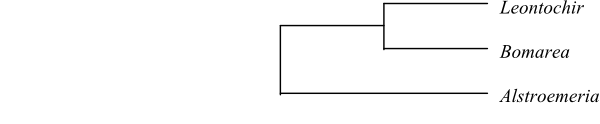 |
Cladogram (simplified) of Alstroemeriaceae based on DNA sequence data (Aagesen & Sanso 2003). |
CAMPYNEMATACEAE Dumort. |
( Back to Liliales ) |
Campynematales Doweld, Tent. Syst. Plant. Vasc.: lvi. 23 Dec 2001; Campynematineae Reveal in Kew Bull. 66: 47. Mar 2011
Genera/species 2/4
Distribution New Caledonia,Tasmania.
Fossils Unknown.
Habit Bisexual, perennial herbs.
Vegetative anatomy Phellogen absent. Secondary lateral growth absent. Vessels present in roots (Campynema) or absent (Campynemanthe). Vessel elements with scalariform perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial parenchyma? Sieve tube plastids probably P2c type. Nodes? Calciumoxalate raphides present in idioblasts.
Trichomes Hairs absent.
Leaves Alternate (spiral), simple, entire, linear, with ? ptyxis. Stipules absent; leaf sheath well developed. Leaf base fibrous, persistent. Venation parallelodromous; reticulate fine venation absent. Stomata anomocytic. Cuticular wax crystalloids? Mesophyll with idioblasts containing calciumoxalate raphides. Leaf margin entire (leaf apex in Campynemanthe tridentate).
Inflorescence Terminal, umbel-like (pseudo-umbel) or raceme-like, cymose.
Flowers Actinomorphic. Epigyny (Campynema) or half epigyny (Campynemanthe). Tepals 3+3, sepaloid to petaloid, persistent, free. Tepal nectaries present in Campynemanthe. Septal nectaries absent. Disc absent.
Androecium Stamens 3+3. Filaments free from each other, adnate to tepal bases. Anthers dorsifixed (Campynema) or basifixed (Campynemanthe), usually non-versatile, tetrasporangiate, extrorse (Campynema) or introrse to almost latrorse (Campynemanthe), longicidal (dehiscing by longitudinal slits). Tapetum secretory, with binucleate (Campynemanthe) or multinucleate (Campynema) cells. Staminodia absent.
Pollen grains Microsporogenesis successive. Pollen grains monosulcate (Campynema) or inaperturate to indistinctly sulcate (Campynemanthe), shed as monads, bicellular at dispersal. Exine tectate, with columellate? infratectum, rugulate (Campynemanthe).
Gynoecium Pistil composed of three connate carpels. Ovary inferior (Campynema) or semi-inferior (Campynemanthe), unilocular (Campynema) or trilocular (Campynemanthe). Style single, simple (Campynemante), or stylodia three free (Campynema). Stigma single, capitate, or stigmas three, punctate, type? Pistillodium absent.
Ovules Placentation axile (when ovary trilocular) or parietal (when ovary unilocular). Ovules three to more than 50 per carpel, anatropous, bitegmic, weakly crassinucellar. Micropyle endostomal. Outer integument two cell layers thick. Inner integument two cell layers thick. Parietal cell (producing parietal tissue) formed from archesporial cell. Megagametophyte monosporous, Polygonum type. Endosperm development ab initio nuclear. Endosperm haustoria? Embryogenesis?
Fruit A capsule, dehiscing by degradation of lateral or dorsal walls, or indehiscent, with persistent and accrescent perianth.
Seeds Aril absent. Exotestal epidermis with large non-compressed cells. Endotesta? Exotegmen? Endotegmic epidermis with large non-compressed cells; endotegmic cells with (reddish-brown) phlobaphene. Perisperm not developed. Endosperm copious, oily, with hemicellulose in cell walls. Embryo very small, chlorophyll? Cotyledon one. Cotyledon hyperphyll? Hypocotyl internode? Coleoptile? Germination?
Cytology n = 11 – Chromosomes up to 3 µm long.
DNA
Phytochemistry Virtually unknown. Steroidal saponins and sapogenins present.
Use Unknown.
Systematics Campynemanthe (3; C. neocaledonica, C. parva, C. viridiflora; New Caledonia), Campynema (1; C. lineare; Tasmania).
Campynemataceae are sister to the remaining Liliales in the analyses by Kim & al. (2013), although Corsiaceae were not included in their study. Givnish & al. (2016) recovered Corsiaceae as sister-group to Campynemataceae in their phylogenomic study of Liliales.
COLCHICACEAE DC. |
( Back to Liliales ) |
Merenderaceae Mirb., Hist. Nat. Plant. 8: 211. 1804 [‘Merenderae’]; Colchicales Dumort., Anal. Fam. Plant.: 59. 1829 [‘Colchicarieae’]; Methonicaceae E. Mey., Preuss. Pfl.-Gatt.: 43. 1839 [‘Menthoniceae’], nom. illeg.; Uvulariaceae A. Gray ex Kunth, Enum. Plant. 4: 199. 17-19 Jul 1843 [‘Uvularieae’], nom. cons.; Bulbocodiaceae Salisb., Gen. Plant.: 52. 15-31 Mai 1866 [‘Bulbocodeae’]; Burchardiaceae Takht. in Bot. Žurn. 81(2): 85. Mai-Jun 1996
Genera/species 15/275–280
Distribution Europe, Africa, Southwest, East and Southeast Asia to New Guinea, Australia and New Zealand, North America, with their largest diversity in South Africa and Australia.
Fossils Unknown.
Habit Usually bisexual (in Wurmbea rarely dioecious), perennial herbs. Usually with a starch-rich corm (with basal innovation; in Gloriosa a hypopodial tuber; corm in Gloriosa and Sandersonia stoloniferous). Stem usually herbaceous (in Kuntheria more or less lignified), in Gloriosa twining with apical foliar tendrils, in Gloriosa, Uvularia etc. branched.
Vegetative anatomy Roots usually fibrous (in Burchardia tuberous). Phellogen absent. Secondary lateral growth absent. Vessels present in fibrous roots (in some genera also in stem). Vessel elements with scalariform perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial parenchyma? Sieve tube plastids P2c type, with cuneate protein crystals. Nodes? Laticifers absent. Silica bodies absent. Calciumoxalate raphides usually absent. Crystal sand present in some species.
Trichomes Hairs unicellular or multicellular, uniseriate, or absent.
Leaves Alternate (distichous) or (pseudo?)verticillate, simple, entire, often linear (sometimes subulate), sometimes differentiated into pseudopetiole and pseudolamina, with conduplicate ptyxis. Stipules absent; leaf sheath often well developed. Venation parallelodromous, often with distinct mid-vein (sometimes with reticulate fine venation). Stomata anomocytic. Cuticular wax crystalloids as parallel platelets (Convallaria type). Mesophyll with calciumoxalate as crystal sand. Leaf margin entire. Leaf apex in Gloriosa often transformed into a tendril.
Inflorescence Axillary, usually umbel-, head-, spike- or raceme-like (flowers sometimes solitary).
Flowers Actinomorphic or somewhat zygomorphic, often large. Hypogyny. Tepals usually 3+3 (rarely seven to twelve), at base U-shaped in bud and folded around each stamen, petaloid, sometimes clawed (rarely with basal spur), persistent or caducous, free or more or less connate at base (in, e.g., Colchicum and Wurmbea connivent into a tube). Nectaries present at tepal bases (tepal nectaries) and/or stamens (androecial nectaries in Colchicum), or absent. Septal nectaries absent. Disc absent.
Androecium Stamens 3+3. Filaments subulate or flattened in transverse section (sometimes with basal appendages), usually free (sometimes connate), free from or adnate to tepals. Anthers dorsifixed or basifixed, sometimes versatile, tetrasporangiate, usually extrorse (sometimes latrorse, rarely introrse), longicidal (dehiscing by longitudinal slits). Tapetum secretory. Staminodia absent.
Pollen grains Microsporogenesis successive. Pollen grains usually monosulcate (sometimes 2–4-sulcate or 2–4-foraminate), shed as monads, bicellular at dispersal. Exine tectate or semitectate, with columellate infratectum, usually reticulate or microreticulate (rarely foveolate-spinulate). Pollen grains sometimes operculate.
Gynoecium Pistil composed of (two or) three (or four) connate carpels. Ovary superior, usually trilocular (rarely bilocular or quadrilocular). Style single, simple (Camptorrhiza) or trilobate, or stylodia three, free. Stigma capitate or trilobate, with reflexed lobes, Dry or Wet type. Pistillodium absent.
Ovules Placentation axile. Ovules two to more than 50 per carpel, anatropous to campylotropous, usually ascending, epitropous, plagiotropous or basitropous, usually bitegmic (rarely unitegmic), (pseudo)crassinucellar. Micropyle bistomal. Outer integument ? cell layers thick. Inner integument ? cell layers thick. Nucellar cap sometimes present. Parietal cell not formed (parietal tissue absent). Periclinal cell divisions often taking place in megasporangial epidermis (e.g. Colchicum, Gloriosa, Iphigenia; not in Uvularia). Megagametophyte monosporous, Polygonum type. Antipodal cells multinucleate, sometimes proliferating (at least in Iphigenia). Endosperm development ab initio nuclear. Endosperm haustoria? Embryogenesis onagrad or asterad. Nucellar (megasporangial) embryony occurring in Colchicum.
Fruit Usually a septicidal and/or loculicidal capsule (in Disporum a berry).
Seeds Strophiole, caruncle or aril present (in, e.g., Uvularia) or absent. Seed coat testal. Unripe testa starchy. Exotestal epidermis often with phlobaphene, a reddish-brown pigment; exotestal cell walls usually thick (in Colchicum narrower). Tegmen more or less collapsing, often pigmented. Perisperm not developed. Endosperm copious, with oils and aleurone (rarely starch), usually with thick and pitted cell walls. Embryo usually small, straight, chlorophyll? Cotyledon one, usually bifacial (sometimes ligulate), coleoptile-like, often photosynthesizing. Cotyledon hyperphyll compact, not assimilating. Hypocotyl internode and mesocotyl absent. Radicula ephemeral or persistent. Germination?
Cytology x = 5–12(–19) – Polyploidy and aneuploidy occurring. Chromosomes 1–16 µm long.
DNA
Phytochemistry Flavonols, tannins, alkaloids without a 7-C-tropolone ring (androcymbine, floramultine, kreysigine, etc.) or with a 7-C-tropolone ring (colchicines etc.), anthraquinones, and chelidonic acid present. Flavones and tricine (flavone-3’,5’-dimethyl ether of tricetin) rare. Ellagic acid, proanthocyanidins, steroidal saponins, and cyanogenic compounds not found.
Use Ornamental plants, medicinal plants (Colchicum, Gloriosa etc.), plant breeding (Colchicum autumnale).
Systematics Colchicaceae are sister to the clade [Luzuriagaceae+Alstroemeriaceae].
[Uvularieae+[Burchardieae+[Tripladenieae+[Colchiceae+[Iphigenieae+Anguillarieae]]]]]
Uvularieae are sister-group to remaining Colchicaceae (Kim & al. 2013; Thi Nguyen & al. 2013). Uvularieae and Tripladenieae are rhizomatous herbs possessing enlarged cells in the megasporangial epidermis. They have flavonols, but seem to lack alkaloids.
Uvularieae A. Gray ex Meisn., Plant. Vasc. Gen.: Tab. Diagn. 397, 402, Comm. 306. 17-20 Aug 1842
2/c 25. Uvularia (5; U. floridana, U. grandiflora, U. perfoliata, U. puberula, U. sessilifolia; southeastern Canada, eastern United States), Disporum (c 20; northern India, East and Southeast Asia to Japan and West Malesia). – Northern India, East and Southeast Asia, West Malesia, eastern North America. Uvularieae are rhizomatous herbs with distichous leaves, often with disulcate pollen grains. Nucellar cap is not formed. n = 7 (Uvularia) or 8, 15, 16 (Disporum). – Chacón & al. (2014) recovered Uvularia as sister to the remaining Colchicaceae except Burchardia.
[Burchardieae+[Tripladenieae+[Colchiceae+[Iphigenieae+Anguillarieae]]]]
Burchardieae (Benth.) J. C. Manning et Vinn. in Taxon 56: 177. 12 Mar 2007
1/6. Burchardia (6; B. bairdiae, B. congesta, B. monantha, B. multiflora, B. rosea: southwestern Western Australia; B. umbellata: southeasternmost Queensland, eastern New South Wales, South Australia, Victoria, Tasmania; paraphyletic). – The vertical rhizome in Burchardia is short and wears papery scales, the leaf base is sheathing, styluli are present, and the capsule is septicidal (Stevens 2001 onwards). n = 24, 48. – Burchardia is possibly sister-group to the remaining Colchicaceae with axillary flowers (Thi Nguyen & al. 2013). On the other hand, Burchardia formed a basal grade in the analysis by Vinnersten & Manning (2007) and was sister to all other Colchicaceae in Chacón & al. (2014).
[Tripladenieae+[Colchiceae+[Iphigenieae+Anguillarieae]]]
Tripladenieae Vinn. et J. C. Manning in Taxon 56: 177. 12 Mar 2007
3/4. Tripladenia (1; T. cunninghamii; southeasternmost Queensland, northeastern New South Wales), Schelhammera (2; S. multiflora: East Malesia, New Guinea, northeastern Queensland; S. undulata: eastern New South Wales, Victoria), Kuntheria (1; K. pedunculata; northeastern Queensland). – East Malesia, New Guinea, southwestern and eastern Australia. Rhizomatous herbs with distichous leaves. n = 7, 18.
[Colchiceae+[Iphigenieae+Anguillarieae]]
Stevens (2001 onwards) lists the following synapomorphies of this clade: corm with tunica; sheathing leaf base; nectaries usually present on filament bases; and presence of alkaloids with a 7-C-tropolone ring.
Colchiceae T. Nees et C. H. Eberm. ex Endl., Gen. Plant.: 137. Dec 1836
5/c 180. Colchicum (c 160; the Canary Islands, the Mediterranean to Ethiopia, Somalia and southern Africa, Central Asia, northern India), Hexacyrtis (1; H. dickiana; Namibia), Ornithoglossum (8; tropical and southern Africa), Sandersonia (1; S. aurantiaca; South Africa, Swaziland), Gloriosa (c 12; tropical and southern Africa, the Arabian Peninsula, tropical Asia to Malesia). – Europe, Africa, Central Asia to Malesia. Usually with nectaries median on tepals or staminal bases. The antipodal cells are often proliferating. n = 9–11, 18–21, 27 (Colchicum), 11 (Hexacyrtis), 11, 22, 33 (Gloriosa), or 12 (Ornithoglossum, Sandersonia). – Sandersonia has vessels in the stem.
[Iphigenieae+Anguillarieae]
Iphigenieae Hutch., Fam. Fl. Plants 2: 84, 101. 20 Jul 1934
2/12–13. Iphigenia (11–12; tropical and southern Africa, Madagascar, Socotra, India, Australia, New Zealand), Camptorrhiza (1; C. strumosa; southern Africa to Zimbabwe and Mozambique). – Old World tropical regions, South Africa. Nectaries absent. n = 11. – The antipodal cells in Iphigenia are persistent. Iphigenia novae-zelandiae in New Zealand should perhaps be transferred to Wurmbea (Anguillarieae).
Anguillarieae (D. Don) Pfeiff., Nomencl. Bot. 1(1): 192. ante 10 Mai 1872
2/35–40. Baeometra (1; B.
uniflora; Western Cape), Wurmbea
(c 40; South Africa, Swaziland, Australia). n = 7, 10, 20 (Wurmbea)
or 11 (Baeometra). – Southern Africa, Australia (possibly New
Zealand). Inflorescence is an ebracteate raceme or spike.
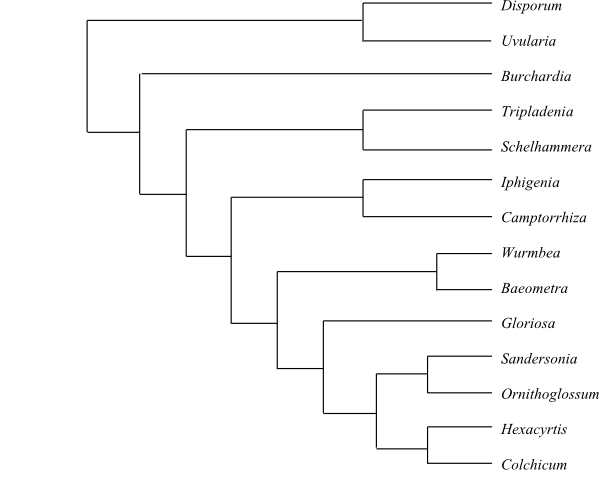 |
Cladogram (simplified) of Colchicaceae based on DNA sequence data (Kim & al. 2013; Thi Nguyen 2013). |
CORSIACEAE Becc. |
( Back to Liliales ) |
Genera/species 1/c 25
Distribution Southern China, New Guinea, Solomon Islands, northern Queensland (Australia), Bolivia, central and southern Chile, the Falkland Islands.
Fossils Unknown.
Habit Bisexual (extremely protandrous), perennial herbs. Achlorophyllous mycoheterotrophic holoparasites.
Vegetative anatomy Arbuscular mycorrhiza (Glomeraceae). Roots long, widely branched. Phellogen absent. Collateral or concentric stem vascular bundles forming a cylinder, each bundle often surrounded by sclerenchymatous layers. Secondary lateral growth absent. Vessels absent. Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial parenchyma? Sieve tube plastids probably P2c type. Silica bodies absent. Raphides absent.
Trichomes Hairs absent.
Leaves Alternate (spiral or distichous), simple, entire, scale-like. Stipules absent; leaf sheath closed. Venation parallelodromous. Stomata absent? Cuticular wax crystalloids in Corsia as parallel platelets (Convallaria type), in Arachnitis tubuli, chemically dominated by nonacosan-10-ol. Leaf margin entire.
Inflorescence Flowers solitary terminal, without bracts.
Flowers Zygomorphic, usually resupinate. Epigyny. Tepals 3+3, petaloid, persistent?, free; median outer tepal, ’labellum’, strongly enlarged, with a ’callus’ at base consisting of thicker tissue and with outgrowths of various shape (nectariferous in some species). Tepal nectaries sometimes present on median outer tepal. Septal nectaries absent. Disc absent.
Androecium Stamens in Corsia 3+3, in Arachnitis 3 antepetalous. Filaments free from each other and from tepals, in Corsia adnate at base to style into a gynostemium. Anthers dorsifixed, non-versatile, tetrasporangiate, extrorse, longicidal (dehiscing by longitudinal slits). Tapetum? Antesepalous staminodium present adjacent to ‘labellum’.
Pollen grains Microsporogenesis successive. Pollen grains monoporate, shed as monads, bicellular at dispersal. Exine semitectate, with columellate? infratectum, reticulate.
Gynoecium Pistil composed of three connate carpels. Ovary inferior, unilocular. Style in Corsia single; in Arachnitis stylodia three, free. Stigma trilobate, papillate, type? Pistillodium absent.
Ovules Placentation intrusively parietal. Ovules c. 25 to more than 100 per ovary, anatropous, pendulous, bitegmic, crassinucellar (tenuinucellar?). Micropyle ?-stomal. Outer integument two cell layers thick. Inner integument two cell layers thick. Funicle long. Parietal tissue one cell layer thick. Nucellar cap? Sometimes two cell layers present above megasporocyte. Megagametophyte monosporous, Polygonum type. Endosperm development helobial. Endosperm haustoria? Embryogenesis?
Fruit A loculicidal? capsule, in Arachnitis circumscissilely dehiscent.
Seeds Aril absent. Seeds extremely small, winged. Seed coat testal. Exotestal epidermis with prosenchymatous, elongate cells with convex periclinal walls (in Arachnitis with collapsing membranous outer periclinal cell walls). Endotesta? Tegmen collapsed? Perisperm not developed. Endosperm small, multicellular, proteinaceous and often oily, with hemicellulose in cell walls. Embryo c. 50-cellular (in Arachnitis tricellular), undifferentiated, chlorophyll? Suspensor short. Cotyledon one. Germination phanerocotylar?
Cytology n = 9
DNA
Phytochemistry Unknown.
Use Unknown.
Systematics Corsia (c 25), Arachnitis (1; A. uniflora; Bolivia, central and southern Chile, the Falkland Islands).
Davis & al. (2004) suggested that Corsiaceae should be included in Liliales. This position has relatively weak support, yet largely corresponds with morphological features and their opinion is followed here (see Mennes & al. 2015, Givnish & al. 2016).
Arachnitis is sister to Thismia, according to some 26SrDNA sequence analyses (Neyland & Hennigan 2003). Like several Thismiaceae Arachnitis has a tuber with a single shoot, whereas Corsia has a rhizome with several shoots. In a study of familial relationships in the Liliales, Kim & al. (2013) added Arachnitis to the analyses and did not find any close affinity between A. uniflora and the investigated taxa of Liliales. Unfortunately, they did not analyse any member of Corsia and used only one locus for the phylogenetic analysis. According to Mennes & al. (2015), the placement of Arachnitis outside of Liliales was probably the result of DNA contamination. However, Givnish & al. (2016) using 75 plastid genes found Arachnitis to be sister to Corsia, although having an extremely long branch-length. Like Corsia but unlike Thismiaceae, Arachnitis has hemicellulose in the cell walls of the endosperm. On the other hand, Arachnitis differs from Corsia in, e.g., having tubular cuticular wax crystalloids, three antepetalous stamens, three free stylodia, capsule with a terminal opening instead of three lateral valves, seeds similar to those in Orchidaceae, and embryo tricellular instead of multicellular.
LILIACEAE Juss. |
( Back to Liliales ) |
Liriaceae Batsch ex Borkh., Bot. Wörterb. 1: 374. 1797 [‘Liria’]; Tulipaceae Batsch ex Borkh., Bot. Wörterb. 2: 391. 1797; Erythroniaceae Martinov, Tekhno-Bot. Slovar: 238. 3 Aug 1820 [‘Erythronides’]; Calochortaceae Dumort., Anal. Fam. Plant.: 53. 1829 [‘Calocorthineae’]; Compsoaceae Horan., Prim. Lin. Syst. Nat.: 51. 2 Nov 1834 [‘Hypoxideae s. Compsoaceae’]; Liriales K. Koch, Nat. Syst. Pflanzenr.: 151. 3-9 Nov 1839 [‘Liranthae’]; Fritillariaceae Salisb., Gen. Plant.: 56. 15-31 Mai 1866 [‘Fritillareae’]; Medeolaceae (S. Watson) Takht., Sist. Magnoliof. [Systema Magnoliophytorum]: 291. 24 Jun 1987; Scoliopaceae Takht. in Bot. Žurn. 81(2): 86. Mai-Jun 1996; Tricyrtidaceae Takht., Divers. Classif. Fl. Pl.: 482. 24 Apr 1997, nom. cons.
Genera/species 16/575–>600
Distribution Temperate and subtropical regions on the Northern Hemisphere, Central America, with their highest diversity in West and East Asia and the Himalayas.
Fossils Uncertain.
Habit Bisexual, perennial herbs. Usually with a bulb (in Medeola a tuberous rhizome). Axillary bulbils sometimes present (in, e.g., some species of Lilium).
Vegetative anatomy Roots often contractile, with or without velamen. Root hypodermis dimorphic. Bulb with or without tunica. Phellogen absent. Secondary lateral growth absent. Vessels present at least in roots (in Tricyrtis also in stem). Vessel elements with scalariform perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial parenchyma? Sieve tube plastids P2c type, with cuneate protein crystals. Nodes? Calciumoxalate raphides absent.
Trichomes Hairs usually absent (sometimes unicellular or multicellular, uniseriate).
Leaves Alternate (usually spiral; sometimes seemingly opposite or verticillate), simple, entire, usually linear (sometimes subulate), with convolute to curved or flat ptyxis. Stipules absent; leaf sheath usually absent. Venation usually parallelodromous (rarely reticulate). Stomata anomocytic. Cuticular wax crystalloids as non-orientated tubuli or granules, chemically dominated by nonacosan-10-ol (in Tricyrtis absent). Mesophyll with or without calciumoxalate crystals. Leaf margin entire. Extrafloral nectaries sometimes present.
Inflorescence Usually terminal (in Streptopus axillary?), thyrsoid, umbel-like or racemose, or flowers solitary. Floral prophylls (bracteoles) lateral or absent.
Flowers Usually actinomorphic (in some species of Fritillaria slightly zygomorphic), often large. Hypogyny. Tepals 3+3, usually all petaloid (inner tepals rarely, e.g. in Nomocharis, fimbriate or pubescent), often spotted or striated, free. Tepal nectaries (often as nectariferous glands) present at tepal bases. Septal nectaries absent. Disc absent.
Androecium Stamens usually 3+3 (in Scoliopus only three outer stamens). Filaments often filiform, sometimes flattened (rarely swollen with needle-like apex), free, in Calochortus and Streptopeae adnate in lower part to tepals. Anthers pseudobasifixed (filament apex surrounded by tubular connective), basifixed or dorsifixed, often versatile, tetrasporangiate, extrorse, latrorse or introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory, with binucleate or multinucleate cells. Staminodia absent.
Pollen grains Microsporogenesis successive. Pollen grains usually monosulcate (rarely disulcate, trisulcate or inaperturate; in Tulipa sometimes operculate), shed as monads, bicellular or tricellular at dispersal. Exine tectate or semitectate, with columellate infratectum, reticulate, verrucate-areolate, gemmate or rugulate.
Gynoecium Pistil composed of three connate carpels. Ovary superior, usually trilocular (rarely unilocular or bilocular). Style single, long, short or rudimentary, or trifid at apex. Stigma capitate or trilobate, papillate (with unicellular papillae), usually Dry (sometimes Wet) type. Pistillodium absent.
Ovules Placentation usually axile (in Medeola, Calochortus, and Scoliopus parietal). Ovules two to more than 50 per carpel, anatropous, pendulous, pleurotropous, hypotropous or epitropous, bitegmic, tenuinucellar. Micropyle endostomal. Outer integument with two to more than three (to six) cell layers thick. Inner integument two? cell layers thick. Funicular obturator sometimes present. Megasporangial apex one-layered, usually without periclinal cell divisions (occurring in Calochortus and Streptopeae). Nucellar cap usually one to three cell layers thick (sometimes absent?). Parietal cell not formed (parietal tissue absent). Megagametophyte usually tetrasporous, Fritillaria type (in Calochortus and Streptopeae monosporous, Polygonum type; in Streptopus disporous, 8-nucleate, Allium type; in Clintonia and some Tulipa tetrasporous, Clintonia type; in some Erythronium and Tulipa tetrasporous, 8-nucleate, Adoxa type; in some Tulipa tetrasporous, 16-nucleate, Drusa type). Synergids sometimes with a filiform apparatus. Antipodal cells usually uninucleate. Endosperm development at least usually nuclear. Endosperm haustoria? Embryogenesis onagrad, asterad or chenopodiad. Polyembryony sometimes present.
Fruit Usually a loculicidal capsule (sometimes septicidal or irregularly dehiscing; in Medeola, Prosartes, and Streptopus a berry).
Seeds Seed sometimes winged. Aril absent. Elaiosome sometimes present (developing from raphe and chalaza in Gagea, from raphe and hilum in Scoliopus). Seed coat testal. Testa thin (often with two cell layers), with thickened cells often flat or collapsed (sometimes with lignified walls). Exotesta in Medeola palisade. Tegmen usually strongly compressed (in Calochortus and Streptopeae two cell layers thick). Perisperm not developed. Endosperm copious, with aleurone and oils (starch absent), with thickened and pitted cell walls. Embryo usually small, little differentiated to well differentiated (embryo often growing after seed dispersal but prior to germination, e.g. in Cardiocrinum), without chlorophyll (Fritillaria, Tulipa). Cotyledon one, usually photosynthesizing, bifacial. Cotyledon hyperphyll usually elongate (sometimes compact), usually assimilating (sometimes not assimilating). Hypocotyl internode short or absent. Coleoptile absent. Germination phanerocotylar.
Cytology x = 6–14 (27) – Polyploidy and aneuploidy occurring. Chromosomes 1,5–19 µm long. The high level of variation in megagametophyte formation in Tulipa may depend on the use of apomictic and otherwise non-fertile cultivated material for most of the investigations.
DNA Plastid gene infA lost (Tricyrtis; pseudogene present). Very large genomes (with C value of c. 350 pg or more) found in some species.
Phytochemistry Flavonols (quercetin, kaempferol), flavanones, chalcones, tannins, steroidal alkaloids (in, e.g., Fritillaria and Notholirion), steroidal saponins (e.g. the sapogenins lilagenin and yuccagenin), tulipalines (α-methylene-γ-butyrolactone, a fungicide), glucose esters (tuliposides), saccharose esters of diferulic or triferulic acid, and fructans present. Ellagic acid, proanthocyanidins, cyanogenic compounds, and chelidonic acid not found.
Use Ornamental plants, medicinal plants, vegetables.
Systematics Liliaceae are sister-group to the clade [Smilacaceae+[Philesiaceae+Rhipogonaceae]].
The tree below mainly follows Vinnersten & al. (2001) and Kim & al. (2013). Alternative phylogenies are found by, e.g., Patterson & Givnish (2002), Fay & al. (2006) and Peruzzi & al. (2009).
1/c 70? Calochortus (c 70?; temperate regions on the Northern Hemisphere, with their highest diversity in western and eastern North America and East Asia. – Rhizomatous herbs. Bulb sometimes present. Vessels sometimes present in stem. Foliar venation parallelodromous, without reticulation. Flowers large. Tepals pubescent. Tepal nectaries saccate. Style developed, trifid. Placental epidermis papillate. Polar nuclei fusing prior to fertilization. Capsule sometimes septicidal. Seeds of various shape. Exotegmic and endotegmic cuticles developed. Hypocotyl short? n = 6–13; chromosomes 1,1–6,5(–15) μm long. γ-methylene glutamic acid sometimes present. – Calochortus s.lat. may not be a monophyletic group. – Both Calochortus and Streptopeae have an apically divided style. However, the support for the sister-group relationship [Calochortus+Streptopeae] is fairly low.
Streptopeae Baker in Gard. Chron., n.s., 2: 660. 21 Nov 1874
4/36. Tricyrtis (18; the Himalayas, China, the Korean Peninsula, Japan, Taiwan); Streptopus (10; central and southern Europe to the Himalayas, Tibet, East Asia, Siberia, Greenland, Canada, United States), Scoliopus (2; S. bigelovii, S. hallii; western United States), Prosartes (6; P. hookeri, P. lanuginosa, P. maculata, P. parvifolia, P. smithii, P. trachycarpa; southern and western Canada, United States). – Cold-temperate regions on the Northern Hemisphere, with their largest diversity in eastern North America and East Asia. Rhizomatous herbs. Style developed, trifid. Megagametophyte development in Streptopus disporous, 8-nucleate, Allium type. Seeds striate. n = 8. – Tricyrtis appears to be sister to the clade [Streptopus+[Scoliopus+Prosartes]], although it has sometimes been recovered as sister to all other Liliaceae (with fairly low support).
Lilioideae Eaton, Bot. Dict., ed. 4: 27. Apr-Mai 1836 [‘Liliaceae’]
Medeoleae Benth. et Hook. f., Gen. Plant. 3: 750, 762. 14 Apr 1883
2/6. Medeola (1; M. virginiana; southeastern Canada, eastern United States), Clintonia (5; C. andrewsiana, C. borealis, C. udensis, C. umbellulata, C. uniflora; the Himalayas and Burma to China, the Korean Peninsula, Japan and the Russian Far East, Canada, western and eastern United States). – East Asia, North America. Fruit a berry. – Clintonia has a diploid endosperm. The megagametophyte has a tetrasporous development of special type: Clintonia type (also found in, e.g., some species of Tulipa).
Lilieae Lam. et DC., Syn. Plant. Fl. Gall.: 159. 30 Jun 1806 [’Liliaceae’]
9/460–490. Gagea (90–>>100; Europe, temperate Asia), Lloydia (10–12; Europe, temperate Asia, southwestern Canada, western United States), Tulipa (80–90; Europe, the Mediterranean, North Africa, West and Central Asia, China, with their highest diversity in southwestern Asia), Erythronium (27; Europe, temperate Asia, temperate North America, with their largest diversity in western North America), Fritillaria (130–140; Europe, the Mediterranean, temperate Asia to Japan); Notholirion (4–5; N. bulbiferum, N. koeiei, N. macrophyllum, N. thomsonianum; Afghanistan to western China), Cardiocrinum (3; C. cathayanum, C. cordatum, C. giganteum; the Himalayas, Tibet, northern Burma, East Asia), Lilium (c 110; temperate and subtropical regions on the Northern Hemisphere south to the Philippines; incl. Nomocharis?), Nomocharis (7–8; northern Assam, southestern Tibet, western China, northeastern Burma; in Lilium?). – Temperate regions on the Northern Hemisphere, with their largest diversity in North America and East Asia. Roots often contractile. Bulb often present. Stem simple. Venation usually parallelodromous, without reticulation. Flowers often large. Median outer tepal adaxial? Style entire or absent. Stigma crested. Megagametophyte tetrasporous (Fritillaria type, with three chalazal megaspores fusing and two subsequent cell divisions; Adoxa and Drusa types also observed). Capsule septicidal. Seeds often flattened. Elaiosome sometimes present. Funicle long. Seed coat testal-tegmic. Exotesta palisade or lignified. Tegmen also persistent. Endosperm thick-walled, often pentaploid, in Erythronium without pits. Cotyledon sometimes unifacial. n = 9, 11–14; chromosomes (1,1–)5–27 μm long. Steroidal alkaloids (sometimes present), tuliposides, di- and triferulic acid saccharose esters, and γ-methylene glutamic acid present.
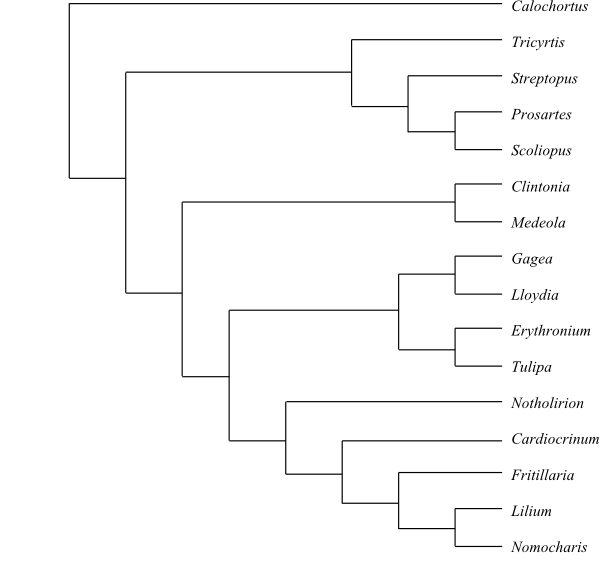 |
Cladogram of Liliaceae based on DNA sequence data (Vinnersten & Bremer 2001, etc.; Kim & al. 2013). Nomocharis is nested inside Lilium, according to Gao & al. (2012). Streptopeae are sister-group to the remaining Liliaceae, according to Kim & al. (2013), and Calochortus sister to the rest, although with weak support. |
LUZURIAGACEAE Lotsy |
( Back to Liliales ) |
Genera/species 2/5
Distribution Easternmost and southeasternmost Australia, Tasmania, New Zealand, Stewart Island, Peru to Tierra del Fuego, the Falkland Islands.
Fossils The fossil flowers Luzuriaga peterbannisteri and Liliacidites contortus were reported from Early Miocene layers in New Zealand. The fossil Acaciaephyllum spatulatum from the Potomac Formation resembles extant Luzuriagaceae in its foliar anatomy.
Habit Bisexual, perennial herbs, often with more or less lignified stem. Twining, climbing or epiphytic.
Vegetative anatomy Roots fibrous, with uniseriate exodermis. Phellogen? Cortex with a ring of fibre bundles and small collateral vascular bundles; centre of stem with scattered vascular bundles. Secondary lateral growth absent. Vessels present in roots and stem. Vessel elements with scalariform or simple perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays? Axial parenchyma? Sieve tube plastids P2c type, with cuneate protein crystals. Nodes? Laticifers and mucilage cells absent. Tanniniferous cells present in Drymophila. Silica bodies absent. Calciumoxalate raphides and druses present in Drymophila.
Trichomes Hairs absent.
Leaves Alternate (distichous), simple, entire, resupinate (twisted 180º at base), with conduplicate or supervolute ptyxis. Stipules absent; leaf sheath open or absent. Venation parallelodromous, acrodromous, with three to five longitudinal primary veins (mid-vein distinct) and transversal and reticulate secondary and tertiary veins (in Drymophila with free ends). Stomata anomocytic. Cuticular wax crystalloids as stalk-like structures (Convallaria type?). Mesophyll with mucilaginous idioblasts containing calciumoxalate raphides. Leaf margin entire.
Inflorescence Axillary, few-flowered cincinnus, or flowers solitary.
Flowers Actinomorphic. Pedicel articulated. Hypogyny. Tepals 3(–4)+3(–4), petaloid, persistent (Luzuriaga) or caducous (Drymophila), free. Tepal nectaries present at tepal bases. Septal nectaries absent. Disc absent.
Androecium Stamens 3+3. Filaments filiform, free from each other and from tepals. Anthers basifixed, non-versatile, tetrasporangiate, introrse or extrorse, usually longicidal (dehiscing by longitudinal slits); in some species of Luzuriaga poricidal (dehiscing by apical pores). Tapetum amoeboid-periplasmodial, binucleate (Drymophila). Staminodia absent.
Pollen grains Microsporogenesis successive (Drymophila). Pollen grains monosulcate, shed as monads, bicellular at dispersal. Exine tectate or semitectate, with columellate infratectum, reticulate or finely foveolate.
Gynoecium Pistil composed of usually three (rarely four) connate carpels. Ovary superior, usually trilocular (rarely unilocular or quadrilocular). Style single, in Luzuriaga simple, in Drymophila deeply trilobate. Stigma single, capitate, Dry type (Luzuriaga), or stigmas three, Wet type (Drymophila). Pistillodium absent.
Ovules Placentation axile or subparietal. Ovules three to nine per carpel, hemianatropous, bitegmic, crassinucellar. Micropyle endostomal. Outer integument ? cell layers thick. Inner integument ? cell layers thick. Megagametophyte monosporous, Polygonum type. Endosperm development nuclear? Endosperm haustoria? Embryogenesis?
Fruit A berry, in Luzuriaga with persistent tepals.
Seeds Aril absent. Testa and tegmen thin-walled. Exotesta collapsing, sometimes caducous. Endotesta? Tegmen? Perisperm not developed. Endosperm copious, with thick-walled cells, with aleurone and oil (starch absent). Embryo straight, chlorophyll? Cotyledon one, little differentiated, not photosynthesizing? Cotyledon hyperphyll compact, not assimilating. Hypocotyl internode short. Coleoptile absent. Radicula well developed, persistent. Epicotyl elongate. Ligule absent. Germination?
Cytology x = 10 – Karyotype bimodal.
DNA
Phytochemistry Virtually unknown. Chelidonic acid present. Alkaloids? Steroidal saponins? Flavonols, ellagic acid, proanthocyanidins, and cyanogenic compounds not found.
Use Ornamental plants, ropes (Luzuriaga radicans).
Systematics Luzuriaga (4; L. marginata, L. polyphylla, L. radicans: southern Chile, Patagonia, Tierra del Fuego, Falkland Islands; L. parviflora: New Zealand incl. Stewart Island), Drymophila (2; D. cyanocarpa: southern New South Wales, Victoria, Tasmania; D. moorei: southeastern Queensland, northeastern New South Wales).
Luzuriagaceae are sister to Alstroemeriaceae.
Drymophila has resupinate leaves, twisted 180° at base. Its taxonomic position has been uncertain, although it was identified as sister to Luzuriaga in the analyses by Kim & al. (2013).
MELANTHIACEAE Borkh. |
( Back to Liliales ) |
Veratraceae Salisb. in Trans. Linn. Soc. London 8: 10. 1807 [’Veratreae’]; Paridaceae Dumort., Fl. Belg.: 131. 1827 [’Parideae’]; Trilliaceae Chevall., Fl. Gén. Env. Paris 2: 297. 1827, nom. cons.; Melanthiales Link, Handbuch 1: 145. 4-11 Jul 1829 [‘Melanthaceae’]; Paridales Link, Handbuch 1: 277. 4-11 Jul 1829 [‘Parideae’]; Veratrales Dumort., Anal. Fam. Plant.: 59. 1829 [’Veratrarieae’]; Heloniadaceae J. Agardh, Theoria Syst. Plant.: 4. Apr-Sep 1858 [’Helonieae’]; Chionographidaceae (Nakai) Takht. in Bot. Žurn. 81(2): 85. Mai-Jun 1996; Xerophyllaceae Takht. in Bot. Žurn. 81(2): 86. Mai-Jun 1996; Trilliales Takht., Divers. Classif. Fl. Pl.: 485. 24 Apr 1997; Melanthianae Doweld, Tent. Syst. Plant. Vasc.: lv. 23 Dec 2001
Genera/species 17/175–180
Distribution Europe, the Mediterranean, temperate Asia, the Himalayas, northeastern India, East Asia, Burma, Indochina, North America to the northern Andes.
Fossils Unknown.
Habit Bisexual, andromonoecious, polygamous, dioecious, androdioecious, or gynodioecious, evergreen or deciduous perennial herbs (rarely somewhat lignified at base). Sometimes with a bulb without nutrient-storing scales (in some species of Schoenocaulon a corm).
Vegetative anatomy Roots fibrous. Phellogen absent. Secondary lateral growth absent. Vessels present in roots. Vessel elements with scalariform perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial parenchyma? Sieve tube plastids P2c type, with cuneate protein crystals. Nodes? Idioblasts with calciumoxalate raphides, druses or single crystals.
Trichomes Hairs unicellular or absent.
Leaves Alternate (in Parideae seemingly verticillate), simple, entire, with usually ? (sometimes curved-plicate) ptyxis, sometimes differentiated into pseudopetiole and pseudolamina. Stipules absent; leaf sheath sometimes well developed. Venation parallelodromous or palmatireticulate, acrodromous, with primary veins connected by reticulate veins (Parideae). Stomata anomocytic (in Parideae with four cells). Cuticular wax crystalloids as parallel platelets with indistinct orientation or absent. Mesophyll with idioblasts containing calciumoxalate as raphides and single prismatic crystals. Leaf margin usually entire (sometimes serrate).
Inflorescence Terminal, panicle, umbel-like, botryoid and raceme-like, or stachyoid and spike-like, simple or branched, or flowers solitary. Floral prophylls (bracteoles) absent.
Flowers Usually actinomorphic (in Chionographis zygomorphic). Usually hypogyny (in Stenanthium and sometimes Zigadenus half epigyny; in some species of Veratrum epigyny). Tepals usually 3+3, 4+4, 5+5 or 6+6 (rarely three, four or more than twelve), with imbricate or contorted (inner tepals in Parideae) aestivation, sepaloid or petaloid (in Parideae differentiated into outer sepaloid and inner petaloid tepals), persistent or caducous (in Melanthium clawed), usually free (sometimes connate at base). Tepal nectaries often (e.g. in Trillium, Veratrum and Zigadenus) present at inner tepal bases. Septal nectaries usually absent. Disc absent.
Androecium Stamens usually as many as tepals (rarely up to 24 in six whorls), in Parideae usually persistent. Filaments filiform to subulate, free from each other and usually free from tepals (sometimes adnate to tepal bases). Anthers basifixed or dorsifixed, non-versatile, tetrasporangiate, usually extrorse (rarely latrorse or introrse), longicidal (dehiscing by longitudinal slits or flaps); connective in, e.g., Parideae with long apical appendage. Tapetum secretory, with binucleate cells. Staminodia absent.
Pollen grains Microsporogenesis successive. Pollen grains usually monosulcate or inaperturate (in Chamaelirium and Chionographis tetraporate; in Trillium omniaperturate), shed as monads, bicellular at dispersal. Exine tectate or semitectate (rarely intectate), with columellate infratectum, reticulate, foveolate, clavate, gemmate, corrugate, verrucate, echinate, spinulate, or psilate.
Gynoecium Pistil composed of three (to ten) more or less connate carpels (in Amianthium and Stenanthium in lower part only, otherwise half-way or largely connate; rarely one carpel). Ovary usually superior (sometimes semi-inferior, rarely inferior), usually trilocular (to decemlocular) at least in lower part (rarely unilocular). Style single, simple, or stylodia free. Stigma single, capitate, or stigmas three adaxial, more or less papillate, usually Dry (rarely Wet) type. Pistillodium absent?
Ovules Placentation usually axile (rarely parietal, when ovary unilocular). Ovules two to more than 50 per carpel, anatropous or campylotropous, apotropous? or epitropous, bitegmic, crassinucellar. Micropyle endostomal. Outer integument ? cell layers thick. Inner integument ? cell layers thick. Nucellar cap, formed by periclinal divisions of apical megasporangial epidermal cells, present in at least Amianthium, Chionographis and Trillium. Parietal cell formed from archesporial cell (at least in some genera; parietal tissue one or two cell layers thick). Megagametophyte usually monosporous, Polygonum type (in Veratrum monosporous, modified Polygonum type, or disporous, Allium type or Veratrum lobelianum type: an ephemeral triad formed during second meiotic division, since micropylar cells divides earlier than chalazal cell; in Parideae usually disporous, Allium or Scilla type). Synergids in Amianthium with a filiform apparatus. Antipodal cells inAmianthium, Heloniopsis and Veratrum multinucleate, proliferating (in Veratrum). Endosperm development usually helobial (in Paris nuclear). Endosperm haustoria? Embryogenesis asterad (Heloniopsis).
Fruit A berry (many species in Parideae) or a loculicidal and/or septicidal or ventricidal (in Veratreae) capsule (in some species of Trillium irregularly dehiscing) with persistent tepals.
Seeds Aril or sarcotesta present in some species of Parideae. Testa sometimes winged or with terminal appendages, caudate. Phlobaphene present in at least Amianthium. Exotesta? Endotesta? Tegmen collapsed. Perisperm not developed. Endosperm copious, with aleurone and oils (sometimes also with starch grains). Embryo usually small (rarely large), well or little differentiated, without chlorophyll. Cotyledon one, unifacial or bifacial. Cotyledon hyperphyll elongate, assimilating. Hypercotyl internode short or absent. Coleoptile absent. Radicula little differentiated. Germination?
Cytology n = 5, 8, 10–12, 15–17, 21, 22, 27 – Polyploidy occurring in, e.g., Veratrum and Parideae. Chromosomes usually 1–6 µm long (in Parideae 6–>40 μm long).
DNA Very large genomes (with a C value of c. 350 pg or more) are found in some species.
Phytochemistry Flavonols (kaempferol, quercetin), flavones, luteolin, apigenin, etc., tannins, steroidal alkaloids (ceveratrum and jerveratrum alkaloids, veratramine, zigadenine, jervine, etc.), steroidal saponins, anthraquinones, and chelidonic acid present. Ellagic acid and cyanogenic compounds not found.
Use Ornamental plants, medicinal plants (Veratrum etc.), insecticides (Schoenocaulon).
Systematics Melanthiaceae are sister-group to Petermannia (Petermanniaceae), according to Kim & al. (2013).
Melanthieae Griseb., Spic. Fl. Rumel. 2: 377. Jan 1846
8/80–85. Zigadenus (1; Z. glaberrimus; southeastern United States), Toxicoscordion (9; southern Canada, western and central United States), Schoenocaulon (c 25; Florida, Mexico, Central America, northern South America to Peru), Anticlea (11; Mongolia, China, the Korean Peninsula, Japan, the Russian Far East, Canada, United States, Mexico, Central America to Guatemala), Stenanthium (5; S. densum, S. diffusum, S. gramineum, S. leimanthoides, S. occidentale; southeastern and eastern United States), Melanthium (4; M. latifolium, M. parviflorum, M. virginicum, M. woodii; central and eastern United States; in Veratrum?), Veratrum (25–30; temperate regions on the Northern Hemisphere), Amianthium (1; A. muscitoxicum; eastern United States). – Temperate regions on the Northern Hemisphere and southwards to the Andes in Peru. Bulb present (in Amianthium with very toxic substances). Leaves sometimes with curved-plicate ptyxis. Leaf sheath in Veratrum closed. Anthers reniform, dehiscing by valves. Capsule septicidal-ventricidal. n = 8 (10, 11, 16). Strongly oxygenated esterified C-nor-D-homosteroidal alkaloids. Alkaloids in Veratrum and its closest relatives are very complex and typical.
[Heloniadeae+Chionographideae]
Stevens (2001 onwards) lists the following characters: cuboid calciumoxalate crystals; inflorescence without bracts; confluent thecae; and intectate exine.
Helonias
3/13. Helonias (1; H. bullata; eastern United States), Heloniopsis (6; H. kawanoi, H. koreana, H. leucantha, H. orientalis, H. tubiflora, H. umbellata; the Korean Peninsula, Japan, Sakhalin, Taiwan), Ypsilandra (6; Y. alpina, Y. cavaleriei, Y. jinpingensis, Y. kansuensis, Y. thibetica, Y. yunnanensis; the Himalayas, Tibet, western China, Burma, northern Vietnam, Taiwan). – Eastern United States, the Himalayas, East Asia. Raphides absent. Pollen grains with spinulate exine. Seeds linear, at each end with a long caudate appendage. n = 17.
Chionographideae Nakai, Chosakuronbun Mokuroku [Ord. Fam. Trib. Nov.]: 229. 20 Jul 1943
2/7. Chamaelirium (1; C. luteum; eastern United States), Chionographis (6; C. chinensis, C. hisauchiana, C. japonica, C. koidzumiana, C. merrilliana, C. shiwandashanensis; southern China to Japan). – Eastern United States, East Asia. Flowers often unisexual. Tepal one-veined. Pollen grains tetraporate. Exine with clavate processes. Capsule septicidal. Seeds winged (sometimes at one end only). n = 12 (21, 22).
[Xerophylleae+Parideae]
Characterized by distinct thecae (Stevens 2001 onwards).
Xerophylleae S. Watson in Proc. Amer. Acad. Arts 14: 225. 2 Aug 1879
1/2. Xerophyllum (2; X. asphodeloides: eastern United States; X. tenax: western North America). – Bulb present. Pericycle two or three cell layers thick. Leaves long and linear, xeromorphic. Tepal nectaries absent. Styloids present. Ovules two to four per carpel. n = 15. – Xerophyllum is anatomically very distinct and adapted to arid conditions.
Parideae Bartl., Ord. Nat. Plant.: 53. Sep 1830 [‘Paridea’]
3/c 73. Pseudotrillium (1; P. rivale; Siskiyou Mountains in southern Oregon and northern California, usually on ultrabasic soils), Trillium (c 45; temperate regions on the Northern Hemisphere), Paris (27; Eurasia to Southeast Asia, with the highest diversity in China). – Temperate and mountainous regions on the Northern Hemisphere. Rhizome monopodial. Root hypodermis dimorphic. Raphides absent. Cuboidal crystals present. Stomata tetracytic. Leaves verticillate, with mid-vein, pseudolamina and sometimes pseudopetiole. Flowers solitary, terminal, up to 12-merous. Hypogyny. Outer tepals three to ten, sepaloid, free (sometimes absent). Inner petals three to six (to eight), petaloid, free (sometimes absent). Septal nectaries sometimes present. Stamens six to 24. Anthers introrse, latrorse or extrorse. Pistil composed of three (to ten) connate carpels. Ovary superior. Style branched or simple. Stigma Dry type. Placentation axile to parietal. Ovules usually numerous per carpel, with various positions, crassinucellar. Nucellar cap two to four cell layers thick. Parietal tissue one or two cell layers thick. Megagametophyte usually disporous, 8-nucleate, Allium or Scilla type. Endosperm development nuclear or helobial. Berry or usually loculicidal (sometimes also septicidal) capsule, often with persistent tepals and stamens. Aril or sarcotesta sometimes present. Embryo very small, undifferentiated (prior to germination growing to approx. the length of the seed). Cotyledon unifacial, sometimes differentiated into pseudopetiole and pseudolamina. n = 5; chromosomes heteromorphic, 6–>40 μm long. Flavonols present.
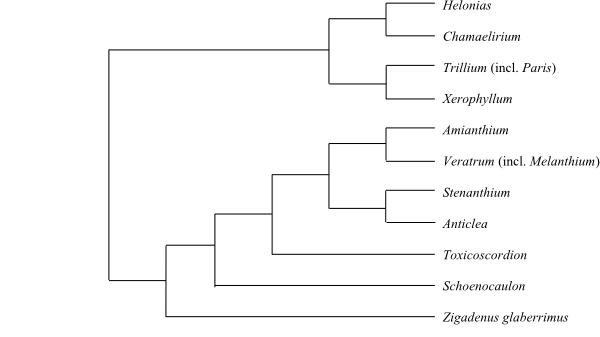 |
Cladogram of Melanthiaceae based on DNA sequence data (Zomlefer & al. 2001). |
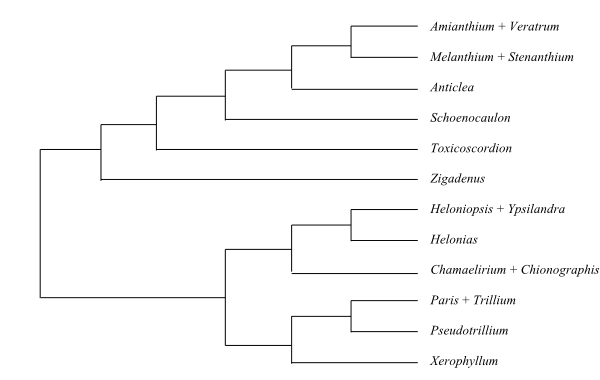 |
Cladogram of Melanthiaceae based on DNA sequence data (Kim & al. 2016). |
PETERMANNIACEAE Hutch. |
( Back to Liliales ) |
Genera/species 1/1
Distribution Easternmost Australia.
Fossils The fossil species Petermanniopsis angleseaensis (with paracytic stomata) from the Eocene of Victoria resembles the extant Petermannia cirrosa.
Habit Bisexual, perennial herb with lignified rhizome. Aerial stem climbing, with spines and branched leaf-opposite tendrils.
Vegetative anatomy Roots fibrous, with uniseriate velamen. Phellogen? Cortex with a cylinder of fibre bundles and small collateral vascular bundles; centre of stem with scattered bundles. Secondary lateral growth absent. Vessels present in roots, stem and sometimes leaves. Vessel elements with scalariform perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays absent? Axial parenchyma? Sieve tube plastids P2c type, with cuneate protein crystals. Nodes? Calciumoxalate raphides abundant.
Trichomes Hairs absent; prickles abundant on stem.
Leaves Alternate (spiral), simple, entire, linear, differentiated in pseudopetiole and pseudolamina, with conduplicate ptyxis. Stipules and leaf sheath absent. Venation pinnate-campylodromous, with three to five longitudinal primary veins connected by reticulate secondary and tertiary veins. Stomata anomocytic. Cuticular wax crystalloids? Mesophyll with cells containing calciumoxalate raphides. Tanniniferous cells present. Leaf margin entire.
Inflorescence Terminal (gradually leaf-opposed due to growth of axillary sympodial shoot), panicle or raceme-like. Inflorescence partially transformed into tendrils.
Flowers Actinomorphic. Pedicel not articulated. Epigyny. Tepals 3+3, petaloid to sepaloid, caducous, free or connate at base into a tube. Tepal nectaries present at tepal bases. Septal nectaries absent. Disc absent.
Androecium Stamens 3+3. Filaments filiform, free from each other and from tepals. Anthers basifixed, non-versatile?, tetrasporangiate, extrorse, longicidal (dehiscing by longitudinal slits). Tapetum amoeboid-periplasmodial, with binucleate cells. Staminodia absent.
Pollen grains Microsporogenesis successive. Pollen grains monosulcate, shed as monads, bicellular at dispersal. Exine tectate, with columellate? infratectum, spinulate. Pollen grains containing starch.
Gynoecium Pistil composed of three connate carpels. Ovary inferior, unilocular (sometimes incompletely trilocular due to placental fusion). Style single, simple, long, narrow. Stigma capitate, slightly trilobate, Wet type. Pistillodium absent.
Ovules Placentation parietal. Ovules c. 15 to more than 50 per ovary, anatropous, bitegmic, crassinucellar. Micropyle ?-stomal. Outer integument ? cell layers thick. Inner integument ? cell layers thick. Parietal cell formed from archesporial cell. Nucellar cap formed by periclinal divisions in megasporangial epidermis. Megagametophyte monosporous, Polygonum type. Endosperm development ab initio nuclear. Endosperm haustoria? Embryogenesis?
Fruit A many-seeded berry.
Seeds Aril absent. Seed coat testal. Exotesta and endotesta thickened. Mesotesta several cell layers thick. Cuticle present between testa and tegmen. Tegmic cells collapsed (crushed), thin-walled. Perisperm not developed. Endosperm copious, without starch. Embryo straight, chlorophyll? Cotyledon one, compact. Cotyledon hyperphyll? Hypocotyl internode? Coleoptile? Radicula well developed. Primary leaves cataphylls. Germination?
Cytology n = 5
DNA
Phytochemistry Virtually unknown. Saponins not found.
Use Unknown.
Systematics Petermannia (1; P. cirrosa; southeastern Queensland, northeastern New South Wales).
Petermannia is sister to Melanthiaceae, according to Kim & al. (2013).
PHILESIACEAE Dumort. |
( Back to Liliales ) |
Lapageriaceae Kunth, Enum. Plant. 5: 283. 10-11 Jun 1850 [’Lapagerieae’]
Genera/species 2/2
Distribution Southern Chile.
Fossils Unknown.
Habit Bisexual, suffrutex (Philesia) or liana (Lapageria). Aerial roots absent. Rhizome, when present, short, woody, sometimes branched.
Vegetative anatomy Roots fibrous. Velamen present. Phellogen? Cortex with a cylinder of fibre bundles and small collateral vascular bundles; centre of stem with scattered bundles. Secondary lateral growth anomalous? Vessels present or absent in roots and/or stem. Vessel elements with scalariform perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays? Axial parenchyma? Sieve tube plastids P2c type, with cuneate protein crystals. Nodes? Tanniniferous cells, mucilage cells and laticifers absent. Silica bodies absent. Calciumoxalate present as raphides.
Trichomes Hairs absent.
Leaves Alternate (spiral or distichous), simple, entire, coriaceous or scleromorphic, differentiated into pseudopetiole and pseudolamina or almost ericoid, with conduplicate-flat or curved ptyxis; pseudolamina resupinated (twisted 180° at base). Stipules absent; leaf sheath more or less developed. Venation parallelodromous (seemingly palmate), acrodromous; secondary veins reticulate or transversal. Stomata anomocytic (in Philesia transversely oriented relative to foliar long axis). Cuticular wax crystalloids as parallel or non-orientated platelets. Leaf margin entire.
Inflorescence Terminal or axillary, few-flowered, or flowers solitary and pendant. Pedicel usually with many scale-like bracts and floral prophylls (bracteoles).
Flowers Actinomorphic, often large. Pedicel not articulated. Hypogyny. Tepals 3+3, all petaloid (Lapageria) or outer tepals sepaloid and inner tepals petaloid and spotted on adaxial side (Philesia), caducous, usually free (sometimes connate at base), forming an infundibuliform or campanulate perianth; perianth tube absent. Nectar secreted from pouch-shaped nectaries on tepal bases (tepal nectaries). Septal nectaries absent. Disc absent.
Androecium Stamens 3+3. Filaments free (Lapageria) or connate in lower half into a tube (Philesia), free from tepals. Anthers subbasifix, non-versatile?, tetrasporangiate, extrorse (Philesia) or introrse (Lapageria), poricidal (dehiscing by pores). Tapetum probably secretory. Staminodia absent.
Pollen grains Microsporogenesis successive. Pollen grains inaperturate, shed as monads, bicellular at dispersal. Exine tectate, with granular infratectum, spinulate to echinulate.
Gynoecium Pistil composed of three connate carpels. Ovary superior, unilocular. Style single, simple, filiform, with stylar canal. Stigma capitate or slightly trilobate, papillate, Dry (Philesia) or Wet (Lapageria) type. Pistillodium absent.
Ovules Placentation intrusively parietal. Ovules ten to more than 100 per ovary, anatropous or amphitropous, bitegmic, crassinucellar. Micropyle endostomal. Outer integument ? cell layers thick. Inner integument ? cell layers thick. Megasporangial epidermis with periclinal and anticlinal cell divisions. Parietal cell formed from archesporial cell. Megagametophyte monosporous, Polygonum type. Synergids with a filiform apparatus. Endosperm development nuclear? Endosperm haustoria? Embryogenesis?
Fruit A few- to many-seeded berry.
Seeds Aril absent. Seed exotegmic-endotegmic. Testa thin, collapsed. Exotegmen? Endotegmen? Perisperm not developed. Endosperm copious, with oils and aleurone, with thin cell walls. Embryo well differentiated, straight, chlorophyll? Cotyledon one, not photosynthesizing. Cotyledon hyperphyll compact, not assimilating. Hypocotyl internode short. Coleoptile absent. Radicula well developed, persistent. Primary leaf a dorsiventral cataphyll, alternating with cotyledon. Germination?
Cytology n = 15 (Lapageria); n = 19 (Philesia) – Chromosomes 2,5–12 µm long.
DNA
Phytochemistry Very insufficiently known. Steroidal saponins (diosgenin) present in Philesia. Chelidonic acid? Stem fructans not found.
Use Ornamental plants.
Systematics Philesia (1; P. magellanica; southern Chile), Lapageria (1; L. rosea; southern Chile, adjacent parts of Argentina).
Philesiaceae are sister to Rhipogonum (Rhipogonaceae).
RHIPOGONACEAE Conran et Clifford |
( Back to Liliales ) |
Genera/species 1/6
Distribution New Guinea, eastern Australia, New Zealand.
Fossils Fossil Rhipogonum is known from the Early Eocene in Tasmania and South America, and from the Early Eocene to the Miocene in New Zealand.
Habit Bisexual, evergreen shrubs or lianas with short rhizome and without tendrils. Lateral ligules and/or spines present. Aerial roots absent.
Vegetative anatomy Roots fibrous. Root exodermis with thickened cell walls. Phellogen ab initio superficial? Cortex with a cylinder of fibre bundles and small collateral vascular bundles. Rhizome with specialized layer of spirally arranged vascular strands. Stem bundles arranged in a cylinder; two leaf traces arising from each node and connected with one another, stem vascular tissue originating from leaf traces. Secondary lateral growth absent. Vessels present in roots and stem. Vessel elements with scalariform perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays? Axial parenchyma? Sieve tube plastids P2c type, with cuneate protein crystals. Nodes ?:2, ?-lacunar with two leaf traces. Laticifers absent. Tanniniferous cells present. Secretory cavities with mucilage often present. Calciumoxalate as raphides.
Trichomes Hairs absent.
Leaves Usually alternate (distichous) or opposite (sometimes verticillate), simple, entire, usually coriaceous, differentiated into pseudopetiole and pseudolamina, with conduplicate or curved ptyxis. Stipules and leaf sheath absent. Pseudopetiole without tendrils or spines. Venation parallelodromous, acrodromous or campylodromous (or brochidodromous?), with five longitudinal primary veins and reticulate fine venation with sometimes free vein endings. Stomata anomocytic (mesoperigenous). Cuticular wax crystalloids as parallel platelets? Leaf margin entire.
Inflorescence Terminal or axillary, simple or compound paniculate, often raceme- or spike-like.
Flowers Actinomorphic, small. Hypogyny. Pedicel not articulated. Tepals 3+3, petaloid, caducous, free. Tepal nectaries present at tepal bases. Septal nectaries absent. Disc absent.
Androecium Stamens 3+3. Filaments free from each other and from tepals. Anthers basifixed, non-versatile, tetrasporangiate, latrorse or introrse, longicidal (dehiscing by longitudinal slits). Tapetum amoeboid-periplasmodial, with binucleate cells. Staminodia absent.
Pollen grains Microsporogenesis successive. Pollen grains monosulcate, shed as monads, bicellular at dispersal. Exine semitectate, thin, with columellate infratectum, reticulate and somewhat foveolate.
Gynoecium Pistil composed of three connate carpels. Ovary superior, trilocular. Style single, simple, short, or absent. Stigma trilobate, papillate, Wet type. Pistillodium absent.
Ovules Placentation basal or axile. Ovules two per carpel, anatropous, pendulous, bitegmic, crassinucellar. Micropyle ?-stomal. Outer integument ? cell layers thick. Inner integument ? cell layers thick. Megasporangial epidermis with periclinal and anticlinal cell divisions. Parietal cell formed from archesporial cell. Megagametophyte monosporous, Polygonum-type. Antipodal cells two to six. Endosperm development ab initio nuclear. Endosperm haustoria? Embryogenesis?
Fruit A many-seeded berry.
Seeds Aril absent. Seed coat tegmic. Testa degenerating? Thick cuticle present between testa and tegmen (and on inner tegmic epidermis?). Tegmen well developed? Perisperm not developed. Endosperm copious, with thickened cell walls?, with starch, aleurone and oils. Embryo straight, well differentiated, small, chlorophyll? Cotyledon one, not photosynthesizing. Cotyledon hyperphyll compact, not assimilating. Hypocotyl internode not elongate. Epicotyl long. Coleoptile absent. Ligule absent. Radicula well developed, persistent. Germination?
Cytology n = 15 – Karyotype bimodal.
DNA
Phytochemistry Flavonols (kaempferol, quercetin), tannins, steroidal saponins, and chelidonic acid present. Ellagic acid, proanthocyanidins, and stem fructans not found.
Use Basketry.
Systematics Rhipogonum (6; R. album, R. brevifolium, R. discolor, R. elseyanum, R. fawcettianum: eastern Queensland, eastern New South Wales, R. album also in eastern Victoria and on New Guinea; R. scandens: New Zealand on North Island, South Island, Stewart Island and Chatham Islands).
Rhipogonum is sister to Philesiaceae.
SMILACACEAE Vent. |
( Back to Liliales ) |
Genera/species: 1–2/310–360
Distribution Tropical, subtropical and temperate regions on both hemispheres.
Fossils Fossil leaves referred to Smilax are known from the Maastrichtian, the Eocene and the Miocene.
Habit Usually dioecious, twining evergreen shrubs, suffrutices or perennial herbs. Tuberous stem present in some species. Lateral ligules and/or spines or pairwise petiolar tendrils present. Aerial roots absent. Rhizome woody.
Vegetative anatomy Roots fibrous. Root exodermis with unthickened cell walls. Phellogen ab initio superficial? Cortex with a cylinder of fibre bundles and small collateral vascular bundles. Stem vascular bundles cylindrically arranged. Secondary lateral growth absent. Vessels usually present at least in roots and stem (in some species of Heterosmilax also in leaves). Vessel elements with scalariform or simple perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays? Axial parenchyma? Sieve tube plastids P2c type, with cuneate protein crystals. Nodes?, with three main bundles often entering leaf base. Laticifers absent. Tanniniferous cells present. Secretory cavities with mucilage often present. Silica bodies absent. Mucilage cells with calciumoxalate raphides abundant in rhizome and tuberous stem.
Trichomes Hairs usually absent; prickles (often large and spine-like) present in numerous species.
Leaves Usually alternate (spiral or distichous; sometimes opposite), simple, entire, usually coriaceous, differentiated into pseudopetiole and pseudolamina, with conduplicate or supervolute-involute ptyxis. Stipules and leaf sheath absent. Pseudopetiole often with pair of tendrils or spines. One pair of pseudostipules (stipule-like outgrowths) often present at pseudopetiole base. Venation parallelodromous, acrodromous or campylodromous (brochidodromous at margins), with five to seven stout longitudinal primary veins and reticulate fine venation with sometimes free vein endings. Stomata anomocytic or irregular. Cuticular wax crystalloids as usually parallel (sometimes non-orientated) platelets. Leaf margin entire. Extrafloral nectaries sometimes present on abaxial side of pseudolamina.
Inflorescence Terminal or axillary, simple or compound, often umbel-, raceme- or spike-like.
Flowers Actinomorphic, small. Hypogyny. Pedicel not articulated. Tepals 3+3, petaloid to sepaloid, caduous, usually free (in Heterosmilax connate into a tube). Tepal nectaries often present on inner tepal bases in male flowers. Septal nectaries absent. Disc absent.
Androecium Stamens usually 3+3 (in Heterosmilax three in one whorl or nine, twelve, 15 or 18 in several whorls). Filaments usually free from each other (sometimes entirely or partially connate into a tube), free from tepals. Anthers basifixed, non-versatile, disporangiate (dithecal or sometimes confluent and seemingly monothecal), latrorse or introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory, with binucleate cells. Female flowers often with staminodia. Nectariferous hairs often present on stamens.
Pollen grains Microsporogenesis successive. Pollen grains inaperturate, shed as monads, bicellular at dispersal. Exine tectate, thin, with granular infratectum, granulate to microspinulate to spinulate.
Gynoecium Pistil composed of usually three connate carpels (rarely one carpel). Ovary superior, usually trilocular (rarely unilocular, monomerous). Stylodia usually three, short (rarely rudimentary, rarely connate at base). Stigmas usually three, papillate, Dry type. Male flowers sometimes with pistillodium.
Ovules Placentation basal, apical or axile. Ovules one or two per carpel, usually orthotropous (sometimes hemianatropous or campylotropous), pendulous, bitegmic, crassinucellar. Micropyle ?-stomal. Outer integument ? cell layers thick. Inner integument ? cell layers thick. Megasporangial epidermis with periclinal and anticlinal cell divisions. Parietal cell formed from archesporial cell. Megagametophyte monosporous, Polygonum type. Antipodal cells? Endosperm development ab initio nuclear. Endosperm haustoria? Embryogenesis?
Fruit A one- or three-seeded berry.
Seeds Aril absent. Seed coat tegmic. Testa degenerating. Outer testal epidermis obliterated. Thick cuticle present between testa and tegmen and on inner tegmic epidermis. Tegmen well developed. Perisperm not developed. Endosperm copious, with thick scalariform-pitted cell walls, with aleurone and oil (starch absent). Embryo straight, linear, well differentiated, small, chlorophyll? Cotyledon one, compact, not photosynthesizing. Cotyledon hyperphyll compact, non assimilating. Hypocotyl internode not elongate. Epicotyl long. Coleoptile absent. Ligule present. Radicula well developed, persistent. Germination?
Cytology n = 14–16, 30–32 – Chromosomes 1,25–9,7 µm long.
DNA Plastid gene infA lost/defunct (Smilax).
Phytochemistry Flavonols (kaempferol, quercetin), tannins, steroidal saponins, and fructans present. Anthocyanin glycosides sometimes present. Ellagic acid and proanthocyanidins not found.
Use Ornamental plants, medicinal plants, flavouring of sweets and beverages, vegetables.
Systematics Smilax (300–350; temperate to tropical regions on both hemispheres; incl. Heterosmilax?), Heterosmilax (12; China, Southeast Asia, West Malesia to Borneo, Taiwan; in Smilax?).
Smilacaceae are sister to the clade [Philesiaceae+Rhipogonaceae].
The leaves in Smilax are developed from the upper part of the foliar primordium.
Literature
Aagesen L, Sanso AM. 2003. The phylogeny of the Alstroemeriaceae, based on morphology, rps16 intron, and rbcL sequence data. – Syst. Bot. 28: 47-69.
Aizen MA, Basilio A. 1995. Within and among flower sexphase distribution in Alstroemeria aurea (Alstroemeriaceae). – Can. J. Bot. 73: 1986-1994.
Aker S, Healy W. 1990. The phytogeography of the genus Alstroemeria. – Herbertia 46: 76-87.
Alden I. 1912. A contribution to the life history of Uvularia sessilifolia. – Bull. Torrey Bot. Club 39: 439-446.
Allen GA. 2001. Hybrid speciation in Erythronium (Liliaceae): a new allotetraploid species from Washington State. – Syst. Bot. 26: 263-272.
Allen GA, Soltis DE, Soltis PS. 2003. Phylogeny and biogeography of Erythronium (Liliaceae) inferred from chloroplast matK and nuclear rDNA ITS sequences. – Syst. Bot. 28: 512-523.
Alzate F, Mort ME, Ramirez M. 2008. Phylogenetic analyses of Bomarea (Alstroemeriaceae) based on combined analyses of nrDNA ITS, psbA-trnH, rpoB-trnC and matK sequences. – Taxon 57: 853-862.
Ambrose JD. 1975. Comparative anatomy and morphology of the Melanthioideae (Liliaceae). – Ph.D. diss., Cornell University, Ithaca, New York.
Ambrose JD. 1980. A re-evaluation of the Melanthioideae (Liliaceae) using numerical analyses. – In: Brickell CD, Cutler DF, Gregory M (eds), Petaloid monocotyledons, Linn. Soc. Symposium Ser. 8, Academic Press, London, pp. 65-81.
Anderson CE. 1940. Some studies on the floral anatomy of the Liliales. – Ph.D. diss., Cornell University, Ithaca, New York.
Andreata RHP. 2009. A new species of Smilax and a key to all species from Minas Gerais, Brazil. – Syst. Bot. 34: 28-31.
Arber A. 1920. Tendrils of Smilax. – Bot. Gaz. 60: 438-442.
Arroyo SC, Leuenberger BE. 1988a. Leaf morphology and taxonomic history of Luzuriaga (Philesiaceae). – Willdenowia 17: 159-172.
Arroyo SC, Leuenberger BE. 1988b. A note on Luzuriaga marginata (Philesiaceae) from Patagonia. – Herbertia 44: 17-21.
Assis MC de. 2002. Novas espécies de Alstroemeria L. (Alstroemeriaceae) de Minas Gerais, Brasil. – Rev. Brasileira Bot. 25: 177-182.
Assis MC de. 2006. A new species of Alstroemeria (Alstroemeriaceae) from Pará, Brazil. – Brittonia 58: 267-269.
Athanasiou K. 1988. Some cytogeographical notes on the genus Tulipa L. (Liliaceae) in Greece. – Bot. Hron. 8: 51-59.
Badawi AA, Elwan Z. 1986. A taxonomic study of Liliaceae sensu lato 1-2. – Phytologia 60: 201-221.
Baker JG. 1871. A new synopsis of all the known lilies. – Gard. Chron. 28: 104.
Baker JG. 1879. A synopsis of Colchicaceae and the aberrant tribes of Liliaceae. – Bot. J. Linn. Soc. 17: 405-510.
Baker JG. 1888. Handbook of the Amaryllideae including the Alstroemerieae and Agaveae. – George Bell and Sons, London.
Baker JG. 1895. Iphigenia somaliensis. – Kew Bull. 1895: 228.
Bakhshi Khaniki G. 1998. Taxonomy and karyology of the genus Fritillaria s. lat. (Liliaceae) in South West Asia with special reference to the species in Iran. – Ph.D. diss., Göteborg University, Sweden.
Bakhshi Khaniki G, Persson K. 1997. Nectary morphology in South West Asian Fritillaria (Liliaceae). – Nord. J. Bot. 17: 579-611.
Bambacioni-Mezzetti V. 1931. Sullo sviluppo dell’embrione in Tulipa gesneriana. – Ann. Bot. (Roma) 19: 145-155.
Baranova MV. 1988. A synopsis of the system of the genus Lilium (Liliaceae). – Bot. Žurn. 73: 1319-1329. [In Russian]
Barker AJD. 1940. A contribution to the life history of Ripogonum scandens J. R. et G. Forster. – M.Sc. thesis, Victoria University, Wellington, New Zealand.
Barret SCH. 1992. Gender variation and the evolution of dioecy in Wurmbea dioica (Liliaceae). – J. Evol. Biol. 5: 423-444.
Bartha L, Sramkó G, Volkova PA, Surina B, Ivanov AL, Banciu HL. 2015. Patterns of plastid DNA differentiation in Erythronium (Liliaceae) are consistent with allopatric lineage divergence in Europe across longitude and latitude. – Plant Syst. Evol. 301: 1747-1758.
Bayer E. 1987. Die Gattung Alstroemeria in Chile. – Mitt. Bot. Staatssamml. München 24: 1-362.
Bayer E. 1988. Beitrag zur Cytologie der Alstroemeriaceae. – Mitt. Bot. Staatssamml. München 27: 1-6.
Bayer E. 1998a. Taltalia – eine neue Gattung in der Familie der Alstroemericaeae. – Sendtnera 5: 5-14.
Bayer E. 1998b. Alstroemeriaceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 79-83.
Beal JM, Ownbey M. 1943. Cytological studies in relation to the classification of the genus Calochortus III. – Bot. Gaz. 104: 553-562.
Behnke H-D. 2003. Sieve-element plastids and evolution of monocotyledons with emphasis on Melanthiaceae sensu lato and Aristolochiaceae-Asaroideae, a putative dicotyledon sister group. – Bot. Rev. 68: 524-544.
Berg RY. 1958. Seed dispersal, morphology, and phylogeny of Trillium. – Skr. Norske Vidensk.-Akad. Oslo I, Mat.-Naturvidensk. Kl., N. S., 1: 1-36.
Berg RY. 1959. Seed dispersal, morphology, and taxonomic position of Scoliopus, Liliaceae. – Skr. Norske Vidensk.-Akad. Oslo I, Mat.-Naturvidensk. Kl., N. S., 4: 1-56.
Berg RY. 1960. Ovary, ovule, and endosperm of Calochortus amabilis with remarks on the taxonomic position of Calochortus. – Nytt Mag. Bot. 8: 189-206.
Berg RY. 1962a. Morphology and taxonomic position of Medeola, Liliaceae. – Skr. Norske Vidensk.-Akad. Oslo, I, Mat.-Naturvidensk. Kl., N. S., 3: 1-55.
Berg RY. 1962b. Contribution to the comparative embryology of the Liliaceae: Scoliopus, Trillium, Paris, and Medeola. – Skr. Norske Vidensk.-Akad. Oslo, I, Mat.-Naturvidensk. Kl., N. S., 4: 1-64.
Bodin SS, Kim JS, Kim JH. 2013. Complete chloroplast genome of Chionographis japonica (Willd.) Maxim. (Melanthiaceae): comparative genomics and evolution of universal primers for Liliales. – Plant Mol. Biol. Rep. 31: 1407-1421.
Bodkin NL. 1978. A revision of North American Melanthium L. (Liliaceae). – Ph.D. diss., University of Maryland, College Park, Maryland.
Bonde SD, Kumaran KPN. 1993. A liliaceous inflorescence from the Deccan Intertrappean beds of India. – Curr. Sci. 65: 776-778.
Brinker RR. 1942. Monograph of Schoenocaulon. – Ann. Missouri Bot. Gard. 29: 287-315.
Buitendijk JH, Ramanna MS. 1996. Giemsa C-banded karyotypes of eight species of Alstroemeria L. and some of their hybrids. – Ann. Bot., N. S., 78: 449-457.
Buxbaum F. 1925. Vergleichende Anatomie der Melanthioideae. – Feddes Repert. Beih. 29: 1-80.
Buxbaum F. 1927. Nachträge zur vergleichenden Anatomie der Melanthioideae I. – Beih. Bot. Centralbl. 44: 255-263.
Buxbaum F. 1936. Die Entwicklungslinien der Lilioideae I. Die systematische Stellung der Gattung Gagea. – Bot. Arch. 38: 213-293.
Buxbaum F. 1937. Die Entwicklungslinien der Lilioideae II. Wurmbeoideae. – Bot. Arch. 38: 305-398.
Buxbaum F. 1951. Die Grundachse von Alstroemeria und Einheit ihres morphologischen Typus mit den echten Liliaceae. – Phytomorphology 1: 170-184.
Buxbaum F. 1954. Morphologie der Blüte und Frucht von Alstroemeria und der Anschluß der Alstroemerioideae bei den echten Liliaceen. – Österr. Bot. Zeitschr. 101: 337-352.
Buxbaum F. 1958. Der morphologische Typus und die systematische Stellung der Gattung Calochortus. – Beitr. Biol. Pflanzen 34: 405-452.
Buxbaum F. 1959. Beiträge zur Morphologie der Gattung Tricyrtis. – Beitr. Biol. Pflanzen 35: 55-75.
Cameron KM, Fu C-X. 2006. A nuclear rDNA phylogeny of Smilax (Smilacaceae). – In: Columbus JT, Friar EA, Porter JM, Prince LM, Simpson MG (eds), Monocots: comparative Biology and Evolution. Excluding Poales, Rancho Santa Ana Botanical Garden, Claremont, California, pp. 598-605.
Campbell MA, Brown KS, Hassell JR, Horigan EA, Keeler RF. 1985. Inhibition of limb chondrogenesis by a Veratrum alkaloid; temporal specificity in vivo and in vitro. – Dev. Biol. 111: 464-470.
Carpenter RJ, Wilf P, Conran JG, Cúneo NR. 2014. A Paleogene trans-Antarctic distribution for Ripogonum (Ripogonaceae: Liliales)? – Palaeontol. Electr. 17: 39A: 1-9. palaeo-electronica.org/content/2014/921-early-eocene-ripogonum.
Case AL. 2000. The evolution of combined versus separate sexes in Wurmbea (Colchicaceae). – Ph.D. diss., University of Toronto, Ontario.
Case AL, Graham SW, Macfarlane TD, Barrett SCH. 2008. A phylogenetic study of evolutionary transitions in sexual systems in Australasian Wurmbea (Colchicaceae). – Intern. J. Plant Sci. 169: 141-156.
Caujapé-Castells J, Jansen RK, Pedrola-Monfort J, Membrives N. 2000. Chloroplast DNA restriction site phylogeny of the genus Androcymbium (Colchicaceae). – Syst. Bot. 24: 581-597.
Caujapé-Castells J, Jansen RK, Membrives N, Pedrola-Monfort J, Montserrat JM, Ardanuy A. 2001. Historical biogeography of Androcymbium Willd. (Colchicaceae) in Africa: evidence from cpDNA RFLPs. – Bot. J. Linn. Soc. 136: 379-392.
Caujapé-Castells J, Jansen RK, Pedrola-Monfort J, Membrives N. 2002. Space-time diversification of Androcymbium Willd. (Colchicaceae) in western South Africa. – Plant Syst. Evol. 232: 73-88.
Cauneau-Pigot A. 1988. Biopalynological study of Lapageria rosea and Iris unguicularis. – Grana 27: 297-312.
Cave MS. 1941. Megasporogenesis and embryo sac development in Calochortus. – Amer. J. Bot. 28: 390-394.
Cave MS. 1966. The female gametophytes of Lapageria rosea and Philesia magellanica. – Gayana Bot. 15: 25-31.
Cave MS. 1970. Chromosomes of the California Liliaceae. – Univ. Calif. Publ. Bot. 57: 1-58.
Chacón J, Sousa A, Baeza CM, Renner SS. 2012. Ribosomal DNA distribution and a genus-wide phylogeny reveal patterns of chromosomal evolution in Alstroemeria (Alstroemeriaceae). – Amer. J. Bot. 99: 1501-1512.
Chacón J, Camargo M, Meerow AW, Renner SS.
2012. From East Gondwana to Central America: historical biogeography of the Alstroemeriaceae. – J. Biogeogr.
39: 1806-1818.
Chacón J, Cusimano N, Renner SS. 2014. The
evolution of Colchicaceae, with a
focus on chromosome numbers. – Syst. Bot. 39: 415-427.
Chang H-J, Hsu C-C. 1974. A cytotaxonomical study on some Formosan Liliaceae. – Taiwania 19: 58-71.
Chauhan KPS, Brandham PE. 1985. The significance of Robertsonian fusion and monosomy in Cardiocrinum (Liliaceae). – Kew Bull. 40: 567-571.
Cheadle VI. 1970. Vessels in Pontederiaceae, Ruscaceae, Smilacaceae, and Trilliaceae. – In: Robson NKB, Cutler DF, Gregory M (eds), New research in plant anatomy, Bot. J. Linn. Soc. 63 [Suppl. 1]: 45-50.
Cheadle VI, Kosakai H. 1971. Vessels in Liliaceae. – Phytomorphology 21: 320-333.
Cheadle VI, Kosakai H. 1975. Vessels in Alstroemeriales. – In: Mohan Ram HY et al. (eds), Form, structure and function in plants, Meerut, pp. 292-299.
Chen H-N, Zhao C-H, Liu X-R, Liu J-X. 2012. Pollen development of Cardiocrinum giganteum (Wall.) Makina in China. – Plant Syst. Evol. 298: 1557-1565.
Chen L, Su Y-F, Yin Z-Y, Luo X-H, Wu Z-H, Gao X-M, Zhang B-L. 2009. Flavonoids from Disporum cantoniense (Liliaceae). – Biochem. Syst. Ecol. 37: 609-612.
Chen S-C, Hsia K-C. 1977. A taxonomic study of the Chinese drug Bei-mu (Fritillaria). – Acta Phytotaxon. Sin. 15: 31-46. [In Chinese with English summary]
Chen S-C, Qiu Y-X, Wang A-L, Cameron KM, Fu C-X. 2006. A phylogenetic analysis of Smilacaceae based on morphological data. – Acta Phytotaxon. Sin. 44: 113-125.
Chen S-C, Zhang X-P, Ni S-F, Fu C-X, Cameron KM. 2006. The systematic value of pollen morphology in Smilacaceae. – Plant Syst. Evol. 259: 19-37.
Chen S-C, Yang H, Li S, Zhu J, Fu C-X. 2007. Bayesian inference and its application in the molecular phylogeny of Liliales. – Acta Bot. Yunnan. 29: 161-166.
Christenhusz MJM, Govaerts R, David JC, Hall T, Borland K, Roberts PS, Tuomisto A, Buerki S, Chase MW, Fay MF. 2013. Tiptoe through the tulips – cultural history, molecular phylogenetics and classification of Tulipa (Liliaceae). – Bot. J. Linn. Soc. 172: 280-328.
Chupov VS. 1984a. The position of Liliaceae s. str. (subfamily Lilioideae of the family Liliaceae s.l.) in the system. Serological study. – Bot. Žurn. 69: 762-771. [In Russian]
Chupov VS. 1984b. The position of the family Liliaceae s. str. (subfamily Lilioideae of the family Liliaceae s.l.) in the system. An analysis of characters. – Bot. Žurn. 69: 1451-1461. [In Russian]
Chupov VS. 1994. Phylogeny and systematics of the Liliales and Asparagales. – Bot. Žurn. 79: 1-12. [In Russian with English summary]
Chupov VS, Kutiavina NG. 1978. The comparative immunoelectrophoretic investigation of seed proteins of Liliaceae. – Bot. Žurn. 63: 473-493.
Chupov VS, Kutiavina NG. 1981a. Serological studies in the order Liliales. – Bot. Žurn. 66: 75-81.
Chupov VS, Kutiavina NG. 1981b. Serological studies in the order Liliales II. – Bot. Žurn. 66: 408-416.
Clément C, Audran J-C. 1993a. Electron microscope evidence for a membrane around the core of the Ubisch body in Lilium (Liliaceae). – Grana 32: 311-314.
Clément C, Audran J-C. 1993b. Orbicule wall surface characteristics in Lilium (Liliaceae). – Grana 32: 348-353.
Clennett JGB, Chase NMW, Forest F, Maurin O, Wilkin P. 2012. Phylogenetic systematics of Erythronium (Liliaceae): morphological and molecular analyses. – Bot. J. Linn. Soc. 170: 504-528.
Clifford HT, Conran JG, Henderson RJF, Hewson HJ, Keighery GJ, Williams JB et al. 1987. Liliaceae. – In: George AS (ed), Flora of Australia 45, Australian Government Publ. Service, Canberra, pp. 148-419, 466-496.
Coetzee J, Schijff HP van der. 1969. Secondary thickening in geophytic Liliaceae. – J. South Afr. Bot. 35: 99-108.
Comber HF. 1949. A new classification of the genus Lilium. – Lily Year-Book 13: 86-105.
Conant GH, Haquist CW. 1944. Gametophyte development in Lilium michiganensis. – Cardina Biol. Suppl. Co., Elon College, North Carolina.
Conover MH. 1983. The vegetative anatomy of the reticulate-veined Liliiflorae. – Telopea 2: 401-412.
Conover MH. 1991. Epidermal patterns of the reticulate-veined Liliiflorae and their parallel-veined allies. – Bot. J. Linn. Soc. 107: 295-312.
Conran JG. 1985a. The taxonomic affinities of the genus Drymophila (Liliaceae s.l.). – Ph.D. diss., University of Queensland, St. Lucia, Australia.
Conran JG. 1985b. IOPB Chromosome number reports 87 (ed. Á. Löve): chromosome number reports on Colchicaceae, Petermanniaceae and Philesiaceae. – Taxon 34: 346-347.
Conran JG. 1987. A phenetic study of the relationships of the genus Drymophila R. Br. within the reticulate-veined Liliiflorae. – Aust. J. Bot. 35: 283-300.
Conran JG. 1988a. Embryology and possible relationships of Petermannia cirrhosa (Petermanniaceae). – Nord. J. Bot. 8: 13-17.
Conran JG. 1988b. Observations on the pollination ecology of Drymophila moorei Baker (Luzuriagaceae) in Southeast Queensland. – Victorian Natur. 105: 43-47.
Conran JG. 1989. Cladistic analyses of some net-veined Liliiflorae. – Plant Syst. Evol. 168: 123-141.
Conran JG. 1995. Family distributions in the Liliiflorae and their biogeographical implications. – J. Biogeogr. 22: 1023-1034.
Conran JG. 1998. Smilacaceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 417-422.
Conran JG, Clifford HT. 1986a. The taxonomic affinities of the genus Ripogonum. – Nord. J. Bot. 5: 215-219.
Conran JG, Clifford HT. 1986b. Smilacaceae. – In: George AS (ed), Flora of Australia 46, Australian Government Publ. Service, Canberra, pp. 180-196.
Conran JG, Clifford HT. 1998a. Luzuriagaceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 365-369.
Conran JG, Clifford HT. 1998b. Petermanniaceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 406-408.
Conran JG, Clifford HT. 1998c. Philesiaceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 409-411.
Conran JG, Christophel DC, Scriben LJ. 1994. Petermanniopsis angleseaënsis: an Australian fossil net-veined monocotyledon from Eocene Victoria. – Intern. J. Plant Sci. 155: 816-827.
Conran JG, Carpenter RJ, Jordan GJ. 2009. Early Eocene Ripogonum (Liliales: Ripogonaceae) leaf macrofossils from southern Australia. – Aust. Syst. Bot. 22: 219-228.
Conran JG, Bannister JM, Mildenhall DC, Lee
DE, Chacón J, Renner SS. 2014. Leaf fossils of Luzuriaga and a
monocot flower with in situ pollen of Liliacidites contortus Mildenh.
& Bannister sp. nov. (Alstroemeriaceae) from the Early
Miocene. – Amer. J. Bot. 101: 141-155.
Conran JG, Kennedy EM, Bannister JM. 2018. Early Eocene Ripogonaceae leaf macrofossils from New Zealand. – Aust. Syst. Bot. 31: 8-15.
Cooper DC. 1934. Development of the embryo sac of Lilium henryi. – Proc. Natl. Acad. Sci. (Washington) 20: 163-166.
Cooper DC. 1939. Development of megagametophyte in Erythronium albidum. – Bot. Gaz. 100: 862-867.
Cooper DC. 1943. Haploid-diploid twin embryos in Lilium and Nicotiana. – Amer. J. Bot. 30: 408-413.
Cowley EJ. 1989. Smilacaceae. – In: Polhill RM (ed), Flora of tropical East Africa, A. A. Balkema, Rotterdam, The Netherlands, pp. 1-4.
Cribb PJ. 1985. The saprophytic genus Corsia in the Solomon Islands. – Kew Mag. 3: 320-323.
Cribb PJ, Wilken P, Clements M. 1995. Corsiaceae: a new family for the Falkland Islands. – Kew Bull. 50: 171-172.
Dahlgren RMT, Lu A-M. 1985. Campynemanthe (Campynemaceae): morphology, microsporogenesis, early ovule ontogeny, and relationships. – Nord. J. Bot. 5: 321-330.
Davis PH, Henderson DM. 1970. A revision of Turkish lilies. – Lily Year-Book 33: 212-223.
Day PD, Berger M, Hill L, Fay MF, Leitch AR,
Leitch IJ, Kelly LJ. 2014. Evolutionary relationships in the medicinally
important genus Fritillaria L. (Liliaceae). – Molec. Phylogen. Evol. 80:
11-19.
Denk T, Velitzelos D, Güner HT, Ferrufino-Acosta L. 2015. Smilax (Smilacaceae) from the Miocene of western Eurasia with Caribbean biogeographic affinities. – Amer. J. Bot. 102: 423-438.
Díaz Lifante Z, Díez MJ, Fernández I. 1990. Morfología polínica de las subfamilies Melanthioideae y Asphodeloideae (Liliaceae) en la Península Ibérica y su importantcía taxonómica. – Lagascalia 16: 211-225.
Dimitri JM. 1972. Una nueva especie del género Arachnitis Phil. (Corsiaceae). – Rev. Fac. Agron. Univ. Nac. La Plata 48: 37-45.
Do HDK, Kim JS, Kim JH. 2013. Comparative genomics of four Liliales families inferred from the complete chloroplast genome sequence of Veratrum patulum O. Loes. (Melanthiaceae). – Gene 530: 229-235.
Do HDK, Kim JS, Kim JH. 2014. A trnL-CAU triplication event in the complete chloroplast genome of Paris verticillata M. Bieb. (Melanthiaceae, Liliales). – Genome Biol. Evol. 6: 1699-1706.
Dominguez LS, Sersic A. 2006. “Prepackaged symbioses”: propagules on roots of the mycoheterotrophic plant Arachnitis uniflora. – New Phytol. 169: 191-198.
Dubouzet JG, Shinoda K. 1999. Phylogenetic analysis of the Internal Transcribed Spacer region of Japanese Lilium species. – Theor. Appl. Gen. 98: 954-960.
El-Hamidi A. 1952. Vergleichend-morphologische Untersuchungen am Gynoeceum der Unterfamilien Melanthioideae und Asphodelioideae der Liliaceae. – Arb. Inst. Allg. Bot., Univ. Zürich, Ser. A, 4: 1-50.
Engler A. 1888. Liliaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien II(5), W. Engelmann, Leipzig, pp. 10-91; Engler A. 1897. Nachträge zu II(5), pp. 71-77.
Engler A. 1893. Iphigenia oliveri. – Engl. Bot. Jahrb. Syst. 15: 467.
Eunus AM. 1951a. Contribution to the embryology of the Liliaceae V. Life history of Amianthium muscaetoxicum Walt. – Phytomorphology 1: 73-79.
Eunus AM. 1951b. Development of the embryo sac and fertilization in Fritillaria pudica Spring. – Pakistan J. Sci. Res. 3: 106-113.
Farmer SB. 2006. Phylogenetic analyses and biogeography of Trilliaceae. – In: Columbus JT, Friar EA, Porter JM, Prince LM, Simpson MG (eds), Monocots: comparative biology and evolution. Excluding Poales, Rancho Santa Ana Botanical Garden, Claremont, California, pp. 579-592.
Farmer SB, Schilling EE. 2002. Phylogenetic analyses of Trilliaceae based on morphological and molecular data. – Syst. Bot. 27: 674-692.
Fassett NC. 1935. Notes from the herbarium of the University of Wisconsin XII. A study of Streptopus. – Rhodora 37: 88-113.
Fay MF, Chase MW. 2000. Modern concepts of Liliaceae with a focus on the relationships of Fritillaria. – Curtis’s Bot. Mag. 17: 146-149.
Fay MF, Chase MW, Rønsted N, Devey DS, Pillon Y, Pires JC, Petersen G, Seberg O, Davis JI. 2006. Phylogenetics of Liliales: summarized evidence from combined analyses of five plastid and one mitochondrial loci. – In: Columbus JT, Friar EA, Porter JM, Prince LM, Simpson MG (eds), Monocots: comparative biology and evolution. Excluding Poales, Rancho Santa Ana Botanical Garden, Claremont, California. – Aliso 22: 559-565.
Feinbrun N. 1958. Chromosome numbers and evolution in the genus Colchicum. – Evolution 12: 173-188.
Field DV. 1972. The identity of Gloriosa simplex. – Kew Bull. 25: 243-244.
Finnie JF, Staden J van. 1991. Isolation of colchicine from Sandersonia aurantiaca and Gloriosa superba. Variation in the alkaloid levels of plants grown in vivo. – J. Plant Physiol. 138: 691-695.
Fowden L. 1959. Nitrogenous compounds and nitrogen metabolism in the Liliaceae 6. Changes in the nitrogenous composition during the growth of Convallaria and Polygonatum. – Biochem. J. 71: 643-648.
Fowden L, Steward FC. 1957. Nitrogenous compounds and nitrogen metabolism in the Liliaceae 1. The occurrence of soluble nitrogen compounds. – Ann. Bot., N. S., 21: 53-67.
Frame DM. 1990. A revision of Schoenocaulon (Liliaceae-Melanthieae). – Ph.D. diss., Dept. of Biology, City University of New York, New York.
Frame DM. 2001. Chromosome studies in Schoenocaulon (Liliaceae-Melanthieae) a relict genus. – An. Inst. Biol. Univ. Nac. Aut. México, Ser. Bot. 72: 123-129.
Frame DM, Espejo A, López-Ferrari AR. 1999. A conspectus of Mexican Melanthiaceae including a description of new taxa of Schoenocaulon and Zigadenus. – Acta Bot. Mexicana 48: 27-50.
Fredericks NA. 1989. Morphological comparison of Calochortus howellii and a new species from southwestern Oregon, C. umpquaensis (Liliaceae). – Syst. Bot. 14: 7-15.
Freeman JD. 1975. Revision of Trillium subgenus Phyllantherum (Liliaceae). – Brittonia 27: 1-62.
Fu C, Kong H, Qui Y, Cameron KM. 2005. Molecular phylogeny of the East Asian-North American disjunct Smilax sect. Nemexia (Smilacaceae). – Intern. J. Plant Sci. 166: 301-309.
Fuchsig H. 1911. Vergleichende Anatomie der Vegetationsorgane der Lilioideen. – Sitzungsber. K. Akad. Wiss. Wien, Math.-Naturw. Kl., 120: 1-43.
Fukuda I. 1990. Breeding systems in American and Asian Trillium species by means of chromosome analyses. – Plant Spec. Biol. 5: 65-72.
Fukuda I, Channell RB. 1975. Distribution and evolutionary significance of chromosome variation in Trillium ovatum. – Evolution 29: 257-266.
Fukuhara T, Shinwari ZK. 1994. Seed-coat anatomy in Uvulariaceae (Liliales) of the Northern Hemisphere: systematic implications. – Acta Phytotaxon. Geobot. 45: 1-14.
Furlani J. 1904. Zur Embryologie von Colchicum autumnale. – Österr. Bot. Zeitschr. 54: 318-324, 373-379.
Furness CA, Gregory T, Rudall PJ. 2015. Pollen structure and diversity in Liliales. – Intern. J. Plant Sci. 176: 697-723.
Fuse S, Tamura MN. 2000. A phylogenetic analysis of the plastid matK gene with emphasis on Melanthiaceae sensu lato. – Plant Biol. 2: 415-427.
Fuse S, Lee NS, Tamura MN. 2004. Biosystematic studies on the genus Heloniopsis (Melanthiaceae) II. Two new species from Korea based on morphological and molecular evidence. – Taxon 53: 949-958.
Gao Y-D, Hohenegger m, harris AJ, Zhou S-D, He X-J, Wan J. 2012. A new species in the genus Nomocharis Franchet (Liliaceae): evidence that brings the genus Nomocharis into Lilium. – Plant Syst. Evol. 298: 69-85.
Gao Y-D, Harris AJ, Zhou S-D, He X-J. 2013. Evolutionary events in Lilium (including Nomocharis, Liliaceae) are temporally correlated with orogenies of the Q-T plateau and the Hengduan Mountains. – Mol. Phylogen. Evol. 68: 443-460.
Gates RR. 1918. A systematic study of the North American Melanth[i]aceae from a genetic standpoint. – Bot. J. Linn. Soc. 44: 131-172.
Geitler L. 1938. Weitere cytogenetische Untersuchungen an natürlichen Populationen von Paris quadrifolia. – Zeitschr. Indukt. Abst. Vererbsl. 75: 161-190.
Givnish TJ, Zuluaga A, Marques I, Lam VKY, Gomez MS, Iles WJD, Ames M, Spalink D, Moeller JR, Briggs BG, Lyon SP, Stevenson DW, Zomlefer W, Graham SW. 2016. Phylogenomics and historical biogeography of the monocot order Liliales: out of Australia and through Antarctica. – Cladistics 32: 581-605.
Goldblatt P. 1986. Systematics and relationships of the bigeneric Pacific family Campynemataceae (Liliales). – Bull. Mus. Natl. Hist. Nat., sér. B, Adansonia IV, 8: 117-132.
Goldblatt P. 1995. The status of R. Dahlgren’s orders Liliales and Melanthiales. – In: Rudall, P. J., Cribb, P. J., Cutler, D. F., Humphries, C. J. (eds.), Monocotyledons: systematics and evolution, Vol. 1, Royal Botanic Gardens, Kew, pp. 181-200.
Goldblatt P, Henrich JE, Rudall P. 1984. Occurrence of crystals in Iridaceae and allied families and their phylogenetic significance. – Ann. Missouri Bot. Gard. 71: 1013-1020.
Goodspeed TH. 1940. Amaryllidaceae from the University of California botanical expedition to the Andes. – Herbertia 7: 17-31.
Grove A. 1933. Lilium michiganense. – Gard. Chron. 1933, 2: 120-121.
Guédès M. 1967. Stipules médianes et stipules ligulaires chez quelques Liliacées, Joncacées et Cypéracées. – Beitr. Biol. Pflanzen 43: 59-103.
Haccius B, Hausner G. 1972. Frühembryogenese und Polyembryonie-Problem in der Gattung Tulipa L. – Beitr. Biol. Pflanzen 48: 207-228.
Haga T. 1934. The comparative morphology of the chromosome complement in the tribe Parideae. – J. Fac. Sci. Hokkaido Univ., Ser. V, Bot. 3: 1-32.
Haga T, Channell RB. 1982. Three species groups in American trilliums as revealed by compatibility with an Asian species. – Bot. Mag. (Tokyo) 95: 77-80.
Han T, De Jeu M, Eck H van, Jacobsen E. 2000. Genetic diversity of Chilean and Brazilian Alstroemeria species assessed by AFLP analysis. – Heredity 84: 564-569.
Handa K, Tsuji S, Tamura MN. 2001. Pollen morphology of Japanese Asparagales and Liliales (Lilianae). – Jap. J. Hist. Bot. 9: 85-125.
Haque A. 1951. The embryo sac of Erithronium americanum. – Bot. Gaz. 112: 495-500.
Hara H. 1968. A revision of the genus Chionographis (Liliaceae). – J. Jap. Bot. 43: 257-267.
Hara H. 1969. Variations in Paris polyphylla Smith, with reference to other Asiatic species. – J. Fac. Sci. Univ. Tokyo III, 10: 141-180.
Hara H. 1988. A revision of the Asiatic species of the genus Disporum (Liliaceae). – Univ. Mus., Univ. Tokyo, Bull. 31: 163-209.
Harling G, Neuendorf M. 2003. 200. Alstroemeriaceae. – In: Harling G, Andersson L (eds), Flora of Ecuador 71, Botanical Institute, Göteborg University, pp. 3-108.
Hayashi K, Kawano S. 2000. Molecular systematics of Lilium and allied genera (Liliaceae): Phylogenetic relationships among Lilium and related genera based on the rbcL and matK gene sequence data. – Plant Species Biol. 15: 73-93.
Hayashi K, Yoshida S, Kato H, Utech FH, Wigham D, Kawano S. 1998. Molecular systematics of the genus Uvularia and selected Liliales based on matK and rbcL gene sequence data. – Plant Species Biol. 13: 129-146.
Heatley M. 1916. A study of the life history of Trillium cernuum L. – Bot. Gaz. 1: 425-429.
Hess WJ, Sivinski RC. 1995. A new species of Zigadenus (Liliaceae) from New Mexico, with additional comments on section Anticlea. – Sida 16: 389-400.
Heyn CC, Dafni A. 1971. Studies in the genus Gagea (Liliaceae) I. The platyspermous species in Israel and neighbouring areas. – Israel J. Bot. 20: 214-233.
Heyn CC, Dafni A. 1977. Studies in the genus Gagea (Liliaceae) II. The non-platyspermous species from the Galilee, the Golan heights and Mt. Hermon. – Israel J. Bot. 26: 11-22.
Higashi S, Tsuyuzaki S, Ohara M, Ito F. 1989. Adaptive advantages of ant-dispersed seeds in the myrmecochorous plant Trillium tschonoskii (Liliaceae). – Oikos 54: 389-394.
Hofreiter A. 2004. A new species of Bomarea Mirbel, subgenus Wichuraea (M. Roem.) Baker (Alstroemeriaceae). – Feddes Repert. 115: 438-440.
Hofreiter A. 2006. Bomarea edulis (Tussac) Herb., a nearly forgotten pre-Columbian cultivated plant and its closest relatives (Alstroemeriaceae). – Feddes Repert. 117: 85-95.
Hofreiter A. 2007. Biogeography and ecology of the Alstroemeriaceae-Luzuriagaceae clade in the high-mountain regions of Central and South America. – Harvard Papers Bot. 12: 259-284.
Hofreiter A. 2008a. Revision of Bomarea Mirbel subgenus Baccata Hofreiter (Alstroemeriaceae). – Feddes Repert. 119: 1-12.
Hofreiter A. 2008b. A revision of Bomarea subgenus Bomarea s. str. Section Multiflorae (Alstroemeriaceae). – Syst. Bot. 33: 661-684.
Hofreiter A, Lyshede OB. 2006. Functional leaf anatomy of Bomarea Mirb. (Alstroemeriaceae). – Bot. J. Linn. Soc. 152: 73-90.
Hofreiter A, Tillich H-J. 2002. The delimitation, infrageneric subdivision, ecology and distribution of Bomarea Mirbel (Alstroemeriaceae). – Feddes Repert. 113: 528-544.
Hofreiter A, Tillich H-J. 2003. Revision of the subgenus Wichuraea (M. Roemer) Baker of Bomarea Mirbel (Alstroemeriaceae). – Feddes Repert. 115: 208-239.
Hong D, Zhu X. 1987. Cytotaxonomical studies on Liliaceae (s.l.) I. Report on karyotypes of 10 species of 6 genera. – Acta Phytotaxon. Sin. 25: 245-253.
Hong H-H, Wang A-L, Lee J, Fu CX. 2007. Studies of systematic evolution and karyotypic variation in Smilax and Heterosmilax (Smilacaceae). – Acta Phytotaxon. Sin. 45: 257-273. [In Chinese]
Hong W-P, Greenham J, Jury SL, Williams CA. 1999. Leaf flavonoid patterns in the genus Tricyrtis (Tricyrtidaceae sensu stricto, Liliaceae sensu lato). – Bot. J. Linn. Soc. 130: 261-266.
Hoyo A del, Pedrola-Monfort J. 2008. Phylogeny of Androcymbium (Colchicaceae) based on morphology and DNA sequences. – Plant Syst. Evol. 273: 151-167.
Hoyo A del, Pedrola-Monfort J. 2006. Missing links between disjunct populations of Androcymbium (Colchicaceae) in Africa using chloroplast DNA noncoding sequences. – In: Columbus JT, Friar EA, Porter JM, Prince LM, Simpson MG (eds), Monocots: comparative biology and evolution. Excluding Poales, Rancho Santa Ana Botanical Garden, Claremont, California. – Aliso 22: 606-618.
Hoyo A del, Pedrola-Monfort J. 2008. Phylogeny of Androcymbium (Colchicaceae) based on morphology and DNA sequences. – Plant Syst. Evol. 273: 151-167.
Hoyo A del, Garcia-Marin JL, Pedrola-Monfort J. 2009. Temporal and spatial diversification of the African disjunct genus Androcymbium (Colchicaceae). – Mol. Phylogen. Evol. 53: 848-861.
Hruby C. 1938. Embryo sac development in Erithronium dens canis. – Chron. Bot. 4: 20-21.
Humphrey LE. 1914. A cytological study of the stamens of Smilax herbacea. – Ohio Nat. 15: 357-369.
Hunziker AT. 1973. Notas sobre Alstroemeriaceae. – Kurtziana 7: 133-135.
Hunziker JH. 1991. Protandry in Alstroemeria psittacina (Alstroemeriaceae). – Polish Bot. Stud. 2: 195-198.
Hunziker JH, Xifreda CC. 1990. Chromosome studies in Bomarea and Alstroemeria (Alstroemeriaceae). – Darwiniana 30: 179-183.
Ibisch P:, Neinhuis C, Rojas NP. 1996. On the biology, biogeography, and taxonomy of Arachnitis Phil. nom. cons. (Corsiaceae) in respect to a new record from Bolivia. – Willdenowia 26: 321-332.
İkıncı N, Oberprieler C, Güner A. 2006. On the origin of European lilies: phylogenetic analysis of Lilium section Liriotypus (Liliaceae) using sequences of the nuclear ribosomal transcribed spacers. – Willdenowia 36: 647-656.
Jeffrey EC. 1895. Polyembryony in Erythronium americanum. – Ann. Bot. 9: 537-541.
Jeffrey EC. 1939. The production of unfertilized seeds in Trillium. – Science 90: 81-82.
Jessop JP. 1979. Liliaceae I. – In: Steenis CGGJ van (ed), Flora Malesiana I, 9(1), Sijthoff & Noordhoff International Publ., Alphen aan den Rijn, The Netherlands, pp. 189-235.
Ji Y, Fritsch PW, Li H, Xiao T, Zhou Z. 2006. Phylogeny and classification of Paris (Melanthiaceae) inferred from DNA sequence data. – Ann. Bot. 98: 245-256.
Johansen DA. 1932. The chromosomes of the Californian Liliaceae I. – Amer. J. Bot. 19: 779-783.
Jones DL, Gray B. 2008. Corsia dispar D. L. Jones & B. Gray (Corsiaceae), a new species from Australia, and a new combination of Corsia for a New Guinea taxon. – Austrobaileya 7: 717-722.
Joshi AC. 1940. Development of the embryo sac of Gagea fascicularis Salisb. – Bull. Torrey Bot. Club 67: 155-158.
Judd WS, Zomlefer WB. 2002. Resurrection of segregates of the polyphyletic genus Zigadenus s.l. (Liliales: Melanthiaceae) and resulting new combinations. – Novon 12: 299-308.
Kamari G. 1984. Karyosystematic studies on Fritillaria L. (Liliaceae) in Greece I. – Webbia 38: 723-731.
Kamari G. 1991. The genus Fritillaria L. in Greece: taxonomy and karyology. – Bot. Chron. 10: 253-270.
Kamari G. 1996. Fritillaria species (Liliaceae) with yellow or yellowish-green flowers in Greece. – Bocconea 5: 221-238.
Kamari G, Phitos D. 2006. Karyosystematic study of Fritillaria messanensis s.l. (Liliaceae). – Willdenowia 36: 217-233.
Kato H, Kawano S, Terauchi R, Ohara M, Utech FH. 1995. Evolutionary biology of Trillium and related genera (Trilliaceae) I. Restriction site mapping and variation of chloroplast DNA and its systematic implications. – Plant Spec. Biol. 10: 17-29.
Kato H, Terauchi R, Utech FH, Kawano S. 1995. Molecular systematics of the Trilliaceae sensu lato as inferred from rbcL sequence data. – Mol. Phylogen. Evol. 4: 184-193.
Kauntze R, Trounce J. 1951. The hypotensive action of veriloid (Veratrum viride). A clinical investigation. – Lancet 260: 549-555.
Kawano S, Kato H. 1995. Evolutionary biology of Trillium and related genera (Trilliaceae) II. Cladistic analyses on gross morphological characters, and phylogeny and evolution of the genus Trillium. – Plant Spec. Biol. 10: 169-183.
Kawano S, Hiratsuka A, Hayashi K. 1982. Life history characteristics and survivorship of Erythronium japonicum. – Oikos 38: 129-149.
Kawano S, Ohara M, Utech FH. 1992. Life history studies on the genus Trillium (Liliaceae) VI. Life history characteristics of three western North American species and their evolutionary-ecological implications. – Plant Spec. Biol. 7: 21-36.
Kazempour Osaloo S, Kawano S. 1999. Molecular systematics of Trilliaceae II. Phylogenetic analysis of Trillium and its allies using sequences of rbcL and matL genes of cpDNA and internal transcribed spacers of 18S-26S nrDNA. – Plant Species Biol. 14: 75-94.
Kazempour Osaloo S, Utech FH, Ohara M, Kawano S. 1999. Molecular systematics of Trilliaceae I. Phylogenetic analyses of Trillium using matK gene sequences. – J. Plant Res. 112: 35-49.
Keighery GJ. 1984. Chromosome numbers of Australian Liliaceae. – Feddes Repert. 95: 523-532.
Killip EP. 1936. Bomarea, a genus of showy Andean plants. – Nat. Hort. Mag. 15: 115-129.
Kim JS, Kim JH. 2013. Comparative genome analysis and phylogenetic relationship of order Liliales: insight from the complete plastid genome sequences of two lilies (Lilium longiflorum and Alstroemeria aurea). – PLoS One 8: e68180.
Kim JS, Hong J-K, Chase MW, Fay MF, Kim J-H. 2013. Familial relationships of the monocot order Liliales based on a molecular phylogenetic analysis using four plastid loci: matK, rbcL, atpB and atpF-H. – Bot. J. Linn. Soc. 172: 5-21.
Kim S-C, Kim JS, Chase MW, Fay MF, Kim J-H. 2016. Molecular phylogenetic relationships of Melanthiaceae (Liliales) based on plastid DNA sequences. – Bot. J. Linn. Soc. 181: 567-584.
Knuth R. 1930. Dioscoreaceae. – In: Engler A (ed), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd 15a, W. Engelmann, Leipzig, pp. 438-462.
Komar GA. 1978. Arils and aril-like formations in some Liliales. – Bot. Žurn. 63: 937-955.
Kondo T, Sato C, Baskin JM, Baskin CC. 2006. Post-dispersal embryo development, germination phenology, and seed dormancy in Cardiocrinum cordatum var. glehnii (Liliaceae s. str.), a perennial herb of the broadleaved deciduous forest in Japan. – Amer. J. Bot. 93: 849-859.
Kong H-H, Wang A-L, Lee J, Fu C-X. 2007. Studies of systematic evolution and karyotypic variation in Smilax and Heterosmilax (Smilacaceae). – Acta Phytotaxon. Sin. 45: 257-273. [in Chinese]
Kores P, White DA, Thien LB. 1978. Chromosomes of Corsia (Corsiaceae). – Amer. J. Bot. 65: 584-585.
Kosenko VN. 1987. Pollen morphology of Tofieldieae, Narthecieae, Xerophylleae, and Melanthieae (Melanthiaceae). – Bot. Žurn. 72: 1318-1330. [In Russian]
Kosenko VN. 1988. Pollen morphology in Chionographideae, Uvularieae, Tricyrtideae, Scoliopeae, Anguillarieae, Iphigenieae, Glorioseae, and Colchiceae (Melanthiaceae). – Bot. Žurn. 73: 172-185. [In Russian]
Kosenko VN. 1991. Palynomorphology of the family Liliaceae s. str. – Bot. Žurn. 76: 1696-1710. [In Russian with English summary]
Kosenko VN. 1992. Pollen morphology and systematic problems of the Liliaceae family. – Bot. Žurn. 77: 1-15. [In Russian with English summary]
Kosenko VN. 1994. Pollen morphology of the family Alstroemeriaceae. – Bot. Žurn. 79: 1-8. [In Russian with English summary]
Kosenko VN. 1999. Contributions to the pollen morphology and taxonomy of the Liliaceae. – Grana 38: 20-30.
Koul AK, Wafai BA. 1980. Chromosome polymorphism and nucleolar organization in some species of Fritillaria. – Cytologia 45: 675-682.
Koul AK, Wafai BA. 1981. Analysis of breeding system in the genus Tulipa I. Sporogenesis, gametogenesis and embryogeny in diploid T. aitchisonii. – Phytomorphology 31: 11-17.
Koul AK, Wakhlu AK. 1985. Studies on the genus Gagea 5. Embryology of Gagea reticulata Schult. – J. Jap. Bot. 60: 361-369.
Koyama T. 1978. Smilacaceae. – In: Li H, Lui T, Huang T, Koyama T, De Vol CE (eds), Flora of Taiwan 5, Epoch Publ., Taipei, pp. 110-137.
Koyama T. 1980. Materials toward a monograph of the genus Smilax. – Quart. J. Taiwan Mus. 8: 1-62.
Koyama T. 1984. A taxonomic revision of the genus Heterosmilax (Smilacaceae). – Brittonia 36: 184-205.
Krause K. 1921. Revision der Gattung Androcymbium Willd. – Notizbl. Bot. Gart. Berlin-Dahlem 70: 512-526.
Krause K. 1930. Liliaceae. – In: Engler A (ed), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 15a, W. Engelmann, Leipzig, pp. 227-386.
Kubitzki K. 1998. Campynemataceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 173-175.
Kupchan SM, Gruenfeld N. 1959. The hypotensive principles of cryptenamine, a Veratrum viride alkaloid preparation. – J. Amer. Pharm. Assoc. Sci. Ed. 48: 727-730.
Kupchan SM, Zimmerman JH, Afonso A. 1961. The alkaloids and taxonomy of Veratrum and related genera. – Lloydia 24: 1-26.
Kurabayashi M. 1958. Evolution of variation in Japanese species of Trillium. – Evolution 12: 286-310.
La Cour LF. 1978. Two types of constitutive heterochromatin in the chromosomes of some Fritillaria species. – Chromosoma 67: 67-76.
Lavaud C, Massiot G, Le Men Olivier L. 1993. Steroidal saponins of Smilax krausiana. – Plant Med. Phytother. 26: 64-73.
Lee C-S, Kim S-C, Yeau S-Y, Lee N-S. 2011. Major lineages of the genus Lilium (Liliaceae) based on nrDNA ITS sequences, with special emphasis on the Korean species. – J. Plant Biol. 54: 159-171.
Lee N-S. 1979. A taxonomic study of the genus Disporum in Korea. – Korean J. Plant Taxon. 9: 67-87.
Lee N-S, Yeau S-H. 1990. A palynological study on Streptopus ovalis (Ohwi) Wang et Y. C. Tang and the relative species (tribe Polygonateae, Liliaceae). – Korean J. Plant Taxon. 20: 81-94. [In Korean with English summary]
Leinfellner W. 1963. Das Perigon der Liliaceen ist staminaler Herkunft. – Österr. Bot. Zeitschr. 110: 448-467.
Leitch IJ, Beaulieu JM, Cheung K, Lysak MA, Fay MF. 2007. Punctuated genome size evolution in Liliaceae. – J. Evol. Biol. 20: 2296-2308.
Levichev IG. 1990. The synopsis of the genus Gagea (Liliaceae) from the western Tien-Shan. – Bot. Žurn. 75: 225-234.
Li H. 1986. A study on the taxonomy of the genus Paris L. – Bull. Bot. Res. Kunming 6: 109-144. [In Chinese]
Li H. 1998. The phylogeny of the genus Paris L. – In: Li H (ed), The genus Paris (Trilliaceae), Science Press, Beijing, pp. 8-65.
Li H, Gu Z, Na H. 1988. Cytogeographic study of the genus Paris. – Acta Phytotaxon. Sin. 26: 1-10.
Li P, Qi Z, Chen S, Cameron KM, Fu C. 2011. Smilax ligneoriparia sp. nov.: a link between herbaceous and woody Smilax (Smilacaceae) based on morphology, karyotype and molecular phylogenetic data. – Taxon 60: 1104-1112.
Li P, Li M, Shi Y, Zhao Y, Wan Y, Fu C, Cameron KM. 2013. Phylogeography of North American herbaceous Smilax (Smilacaceae): combined AFLP and cpDNA data support a northern refugium in the driftless area. – Amer. J. Bot. 100: 801-814.
Liao W-J, Yuan Y-M, Zhang D-Y. 2007. Biogeography and evolution of flower color in Veratrum (Melanthiaceae) through inference of a phylogeny based on multiple DNA markers. – Plant Syst. Evol. 267: 177-190.
Lighty RW. 1968. Evolutionary trends in lilies. – Lily Year-Book 31: 40-44.
Liu J, Qi Z-C, Zhao Y-P, Fu C-X, Xiang Q-Y. 2012. Complete cpDNA genome sequence of Smilax china and phylogenetic placement of Liliales – influences of gene partitions and taxon sampling. – Mol. Phylogen. Evol. 64: 545-562.
Loesener O. 1926. Studien über die Gattung Veratrum und ihre Verbreitung. – Verh. Bot. Ver. Prov. Brandenburg 68: 106-166.
Loesener O. 1927. Übersicht über Arten der Gattung Veratrum 1. – Feddes Repert. 24: 61-72.
Loesener O. 1928. Übersicht über Arten der Gattung Veratrum 2. – Feddes Repert. 25: 1-10.
Lord JM. 2009. Comment: Clintonia’s unique embryology not apomixis. – Intern. J. Plant Sci. 170: 699.
Lovka M. 1975. A contribution to the cytotaxonomy of Yugoslav Spermatophyta I. Liliaceae s. lat. – Biol. Vestn. (Ljubljana) 23: 25-40.
Lowry II PP, Goldblatt P, Tobe H. 1987. Notes on the floral biology, cytology, and embryology of Campynemanthe (Liliales: Campynemataceae). – Ann. Missouri Bot. Gard. 74: 573-576.
Lyshede OB. 2002. Comparative and functional leaf anatomy of selected Alstroemeriaceae of mainly Chilean origin. – Bot. J. Linn. Soc. 140: 261-272.
Macfarlane TD. 1980. A revision of Wurmbea (Liliaceae) in Australia. – Brunonia 3: 145-208.
Macfarlane TD. 1987a. Wurmbea (Liliaceae). – In: George AS, Purdie RW (eds), Flora of Australia 45, Griffin Press Ltd., Canberra, Australia, pp. 387-405.
Macfarlane TD. 1987b. Burchardia. – In: George AS, Purdie RW (eds), Flora of Australia 45, Griffin Press Ltd., Canberra, Australia, pp. 405-410.
Macmillan BW. 1972. The biological flora of New Zealand 7. Ripogonum scandens J. R. et G. Forst. (Smilacaceae), Supplejack, Kareao. – New Zealand J. Bot. 10: 641-672.
McRae EA. 1998. Lilies: a guide for growers and collectors. – Portland, Oregon.
Maheshwari P. 1934. Contributions to the morphology of some Indian Liliaceae I. – Proc. Indian Acad. Sci., Sect. B, 1: 197-204.
Maheshwari P. 1946. The Fritillaria type of embryo sac. A critical review. – J. Indian Bot. Soc., M. D. P. Iyengar Comm. Vol.: 101-119.
Maki M. 1992a. Allozyme variation in Japanese species of Chionographis (Liliaceae). – Bot. Mag. (Tokyo) 105: 149-160.
Maki M. 1992b. Fixation indices and genetic diversity in hermaphroditic and gynodioiecious populations of Japanese Chionographis (Liliaceae). – Heredity 68: 329-336.
Maki M. 1993a. Outcrossing and fecundity advantage of females in gynodioecious Chionographis japonica var. kurohimensis (Liliaceae). – Amer. J. Bot. 80: 629-634.
Maki M. 1993b. Floral sex ratio variation in hermaphrodites of gynodioecious Chionographis japonica var. kurohimensis Ajima et Satomi (Liliaceae). – J. Plant Res. 106: 181-186.
Maki M, Masuda M. 1993. Spatial autocorrelation of genotypes in a gynodioecious population of Chionographis japonica var. kurohimensis (Liliaceae). – Intern. J. Plant Sci. 154: 467-472.
Maki M, Masuda M. 1994. Spatial genetic structure within two populations of a self-incompatible perennial, Chionogrphis japonica var. japonica (Liliaceae). – J. Plant Res.107: 283-287.
Mandenova I. 1940. Lilii Kavkaza. – Trudy Tbilissk. Bot. Inst. 8: 149-208.
Manning JC, Forest F, Vinnersten A. 2007. The genus Colchicum L. redefined to include Androcymbium Willd. based on molecular evidence. – Taxon 56: 872-882.
Marsh CD, Clawson AB, Marsh H. 1915. Zygadenus, or death camas. – Bull. U.S. Dept. Agric. 125: 1-46.
Marsh CD, Clawson AB, Roe GC. 1926. Nuttall’s death camas (Zygadenus Nuttallii) as a poisonous plant. – Bull. U.S. Dept. Agric. 137: 1-13.
Martin BF, Tucker SC. 1985. Developmental studies in Smilax (Liliaceae) I. Organography and the shoot apex. – Amer. J. Bot. 72: 66-74.
Mathew B. 1989. A review of Veratrum. – Plantsman 11: 35-61.
Meerow AW, Tombolato AFC, Meyer F. 1999. Two new species of Alstroemeria (Alstroemeriaceae) from Brazil. – Brittonia 51: 439-444.
Mehra PN, Sacchdeva SK. 1976. Cytological observations on some W. Himalayan monocots II. Smilacaceae, Liliaceae and Trilliaceae. – Cytologia 41: 5-22.
Membrives N, Pedrola-Monfort J, Caujapé-Castells J. 2001. Relative influence of biological versus historical factors on isozyme variation of the genus Androcymbium (Colchicaceae) in Africa. – Plant Syst. Evol. 229: 237-260.
Mennes CB, Lam VKY, Rudall PJ, Lyon SP, Graham SW, Smets EF, Merckx VS. 2015. Ancient Gondwana break-up explains the distribution of the mycoheterotrophic family Corsiaceae (Liliales). – J. Biogeogr. 42: 1123-1136.
Měsiček J, Hrouda L. 1974. Chromosome numbers in Czechoslovak species of Gagea (Liliaceae). – Folia Geobot. Phytotaxon. 9: 359-368.
Meurer B, Strack D, Wiermann R. 1984. The systematic distribution of ferulic acid-sucrose esters in anthers of the Liliaceae. – Planta Medica 50: 376-380.
Meyer NR, Yaroshevskaya AS. 1976. The phylogenetic significance of the development of pollen grain walls in Liliaceae, Juncaceae and Cyperaceae. – In: Ferguson IK, Muller J (eds), The evolutionary significance of the exine, Linn. Soc. Symposium Series No. 1, pp. 91-100.
Minoletti ML. 1986. Arachnitis uniflora Phil. una curiosa monocotiledónea de la flora chilena. – Bol. Soc. Biol. Concepción (Chile) 57: 7-20.
Miyamoto J, Kurita S, Gu Z, Li H. 1992. C-banding patterns in 18 taxa of the genus Paris sensu Li, Liliaceae. – Cytologia 57: 181-194.
Morita H. 1931. On new species of the genera Cinnamomum and Smilax from the Miocene deposits of Oguni-Machi, Uzen Province, Japan. – Jap. J. Geol. Geogr. 9: 1-8.
Mulay BN, Deshpande BD. 1959. Velamen in terrestrial monocots – I. Ontogeny and morphology in the Liliaceae. – J. Indian Bot. Soc. 38: 383-390.
Müller-Doblies U, Müller-Doblies D. 2002. De Liliifloris Notulae 7. De decuria altera specierum novarum generis Androcymbium (Colchicaceae) in Africa Australi s.l. – Feddes Repert. 113: 545-599.
Mulligan GA, Munro DB. 1987. The biology of Canadian weeds. 77. Veratrum viride Ait. – Can. J. Plant Sci. 67: 777-786.
Narita M, Takahashi H. 2008. A comparative study of shoot and floral development in Paris tetraphylla and P. verticillata (Trilliaceae). – Plant Syst. Evol. 272: 67-78.
Nawa N. 1928. Some cytological observations in Tricyrtis, Sagittaria and Lilium. – Bot. Mag. (Tokyo) 42: 33-36.
Neinhuis C, Ibisch PL. 1998. Corsiaceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 198-201.
Nesom GL, La Duke JC. 1985. Biology of Trillium nivale (Liliaceae). – Can. J. Bot. 63: 7-14.
Ness BD. 1989. Seed morphology and taxonomic relationships in Calochortus (Liliaceae). – Syst. Bot. 14: 495-505.
Neuendorf M. 1977. Pardinae, a new section of Bomarea (Alstroemeriaceae). – Bot. Not. 130: 55-60.
Neuss N. 1953. A new alkaloid from Amianthium muscaetoxicum Gray. – J. Amer. Chem. Soc. 75: 2772-2773.
Neves J de B. 1973. Contribution à la connaissance cytotaxonomique des Spermatophyta du Portugal VIII. Liliaceae. – Bol. Soc. Brot., ser.IIa, 47: 157-212.
Neyland R. 2002. A phylogeny inferred from large-subunit (26S) ribosomal DNA sequences suggests that Burmanniales are polyphyletic. – Aust. Syst. Bot. 15: 19-28.
Neyland R, Hennigan M. 2003. A phylogeny inferred from large-subunit (26S) ribosome DNA sequences suggests that the Corsiaceae are polyphyletic. – New Zealand J. Bot. 41: 1-11.
Nguyen TPA, Kim JS, Kim J-H. 2013. Molecular phylogenetic relationships and implications for the circumscription of Colchicaceae (Liliales). – Bot. J. Linn. Soc. 172: 255-269.
Nishikawa T, Okazaki K, Uchino T, Arakawa K, Nagamine T. 1999. A molecular phylogeny of Lilium in the Internal Transcribed Spacer region of nuclear ribosomal DNA. – J. Mol. Evol. 49: 238-249.
Noda S. 1991. Chromosomal variation and evolution in the genus Lilium. – In: Tsuchiya T, Gupta PK (eds), Chromosome engineering in plants: genetics, breeding, evolution, part B, Elsevier, Amsterdam, pp. 507-524.
Nordal I, Bingham MG. 1998. Description of a new species, Gloriosa sessiliflora (Colchicaceae), with notes on the relationship between Gloriosa and Littonia. – Kew Bull. 53: 479-482.
Nordenstam B. 1964. Studies in South African Liliaceae I. New species of Wurmbea. – Bot. Not. 117: 173-182.
Nordenstam B. 1982. A monograph of the genus Ornithoglossum (Liliaceae). – Opera Bot. 64: 1-51.
Nordenstam B. 1986. The genus Wurmbea (Colchicaceae) in the Cape Region. – Opera Bot. 87: 1-41.
Nordenstam B. 1998. Colchicaceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 175-185.
Obermeyer AA. 1971. Iphigenia and Camptorrhiza in southern Africa. – Kirkia 1: 84-87.
Obermeyer AA. 1992. Luzuriagaceae. – In: Leistner OA (ed), Flora of Southern Africa, Vol. 5(3), National Botanical Institute, Pretoria, pp. 83-84.
Oganezova GG. 1984a. Morphological and anatomical specific features of seed and fruit in some representatives of the subfamily Melanthioideae (Liliaceae) in relation with their systematics and phylogeny. – Bot. Žurn. 69: 772-781. [In Russian with English summary]
Oganezova GG. 1984b. Morphologo-anatomical peculiarities of the fruit and seed in some representatives of the subfamily Wurmbaeoideae (Liliaceae) in connection with systematic and phylogeny. – Bot. Žurn. 69: 1317-1327. [In Russian]
Oganezova GH. 2000. Systematic position of the Trilliaceae, Smilacaceae, Herreriaceae, Tecophilaeaceae, Dioscoreaceae families and the volume and phylogeny of the Asparagales (based on seed structure). – Bot. Žurn. 85: 9-25. [In Russian]
Ogihara R. 1971. Interspecific differentiation of basic chromosome number from x = 13 to x = 12 in natural populations of Tricyrtis hirta. – Rep. Fac. Technol. Kanagawa Univ. 9: 26-30. [In Japanese with English summary]
Ogura H. 1964. On the embryo sac of two species of Tricyrtis. – Sci. Rep. Tohoku Univ. Ser. IV (Biology) 30: 219-222.
Ohara M. 1989. Life history evolution in the genus Trillium. – Plant Species Biol. 4: 1-28.
Ohara M, Higashi S. 1987. Interference by ground beetles with the dispersal by ants of seeds of Trillium species (Liliaceae). – J. Ecol. 75: 1091-1098.
Ohara M, Kawano S. 1986. Life history studies on the genus Trillium (Liliaceae) IV. Stage class structures and spatial distribution of four Japanese species. – Plant Species Biol. 1: 147-161.
Ohara M, Utech FH. 1986. Life history studies on the genus Trillium (Liliaceae) III. Reproductive biology of six sessile-flowered species occurring in the southeastern United States with special reference to vegetative reproduction. – Plant Species Biol. 1: 135-145.
Ohara M, Utech FH. 1988. Life history studies on the genus Trillium (Liliaceae) V. Reproductive biology and survivorship of three declinate-flowered species. – Plant Species Biol. 3: 35-41.
Oikawa K. 1937. A note on the development of the embryo sac in Cardiocrinum cordatum. – Sci. Rep. Tohoku Imp. Univ., 4th ser., 11: 303-306.
Oikawa K. 1940. The embryo sac of Erythronium japonicum. – Bot. Mag. (Tokyo) 54: 366-369. [In Japanese]
Oikawa K. 1961. The embryo sac of Chionographis japonica Maxim. – Sci. Rep. Tohoku Imp. Univ., 4th ser. (Biol.), 2: 155-158.
Ono T. 1926. Embryologische Studien an Heloniopsis breviscapa. – Sci. Rep. Tohoku Imp. Univ., 4th ser. (Biol.), 2: 93-104.
Ono T. 1928. Endosperm formation in the Liliaceae. – Bot. Mag. (Tokyo) 42: 445-449.
Osaloo SK, Kawano S. 1999. Molecular systematics of Trilliaceae II. Phylogenetic analyses of Trillium and its allies using sequences of rbcL and matK genes of cpDNA and internal transcribed spacers of 18S-26S nrDNA. – Plant Species Biol. 14: 75-94.
Osaloo SK, Utech FH, Ohara M, Kawano S. 1999. Molecular systematics of Trilliaceae I. Phylogenetic analyses of Trillium using matK gene sequences. – J. Plant Res. 112: 35-49.
Ownbey M. 1940. A monograph of the genus Calochortus. – Ann. Missouri Bot. Gard. 27: 371-560.
Ownbey M. 1969. Calochortus. – Univ. Washington Publ. 17: 765-779.
Özdemir C. 2003. Morphological, anatomical and cytological characteristics of endemic Lilium ciliatum P. H. Davis (Liliaceae) in Turkey. – Pakistan J. Bot. 35: 99-110.
Pascher A. 1904. Übersicht über die Arten der Gattung Gagea. – Sitzungsber. Deutsch. Naturwiss.-Med. Ver. Böhmen “Lotos” (Prag) 24: 109-131.
Patterson TB, Givnish TJ. 2002. Phylogeny, concerted convergence, and phylogenetic niche conservatism in the core Liliales: insights from rbcL and ndhF sequence data. – Evolution 56: 233-252.
Pax F, Hoffmann K. 1930. Amaryllidaceae. – In: Engler A (ed), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd 15a, W. Engelmann, Leipzig, pp. 391-430.
Pechenitsyn UP. 1972. Double fertilization in species of Tulipa with Fritillaria-type embryo sac. – Bot. Žurn. 57: 465-469. [In Russian]
Pedrola-Monfort J, Caujape-Castells J. 1994. Allozymic and morphological relationships among Androcymbium gramineum, A. europaeum, and A. psammophilum (Colchicaceae). – Plant Syst. Evol. 191: 111-126.
Pellicer J, Kelly LJ, Leitch IJ, Zomlefer WB, Fay MF. 2014. A universe of dwarfs and giants: genome size and chromosome evolution in the monocot family Melanthiaceae. – New Phytologist 201: 1484-1497.
Persson K. 1988. New species of Colchicum (Colchicaceae) from the Greek mountains. – Willdenowia 18: 29-46.
Persson K. 1993. Reproductive strategies and evolution in Colchicum. – Proceedings of the 5th OPTIMA meeting, Istanbul University, Istanbul, pp. 394-414.
Persson K. 1999. New and revised species of Colchicum (Colchicaceae) from the Balkan Peninsula. – Plant Syst. Evol. 217: 55-80.
Persson K. 2001. A new soboliferous species of Colchicum in Turkey. – Bot. J. Linn. Soc. 135: 85-88.
Persson K. 2007. Nomenclatural synopsis of the genus Colchicum (Colchicaceae), with some new species and combinations. – Bot. Jahrb. Syst. 127: 165-242.
Persson K. 2008. A new species of Colchicum (Colchicaceae) from southern Italy. – Bot. Jahrb. Syst. 127: 283-288.
Persson K. 2009. A new species of Colchicum (Colchicaceae) from southern Italy. – Bot. Jahrb. Syst. 127: 489.
Persson K, Petersen G, Hoyo A del, Seberg O, Jørgensen T. 2011. A phylogenetic analysis of the genus Colchicum L. (Colchicaceae) based on sequences from six plastid regions. – Taxon 60: 1349-1365.
Peruzzi L. 2003. Contribution to the cytotaxonomical knowledge of Gagea Salisb. (Liliaceae) sect. Foliatae A. Terrac. and synthesis of karyological data. – Caryologia 56: 115-128.
Peruzzi L, Aquaro G. 2005. Contribution to the cytotaxonomical knowledge of Gagea Salisb. (Liliaceae) II. Further karyological studies on Italian populations. – Candollea 60: 237-253.
Peruzzi L, Bartolucci F, Frignani F, Minutillo F. 2007. Gagea tisoniana, a new species of Gagea Salisb. sect. Gagea (Liliaceae) from Central Italy. – Bot. J. Linn. Soc. 155: 337-347.
Peruzzi L, Peterson A, Tison J-M, Peterson J. 2008. Phylogenetic relationships of Gagea Salisb. (Liliaceae) in Italy, inferred from molecular and morphological data matrices. – Plant Syst. Evol. 276: 219-234.
Peruzzi L, Tison J-M, Peterson A, Peterson J. 2008. On the phylogenetic position and taxonomic value of Gagea trinervia (Viv.) Greuter and Gagea sect. Anthericoides A. Terracc. (Liliaceae). – Taxon 57: 1201-1214.
Peruzzi L, Leitch IJ, Caparelli KF. 2009. Chromosome diversity and evolution in Liliaceae. – Ann. Bot. 103: 459-475.
Petersen G, Seberg O, Davis JI. 2013. Phylogeny of the Liliales (monocotyledons) with special emphasis on data partition congruence and RNA editing. – Cladistics 29: 274-295.
Peterson A, John H, Koch E, Peterson J. 2004. A molecular phylogeny of the genus Gagea (Liliaceae) in Germany inferred from non-coding chloroplast and nuclear DNA sequences. – Plant Syst. Evol. 245: 145-162.
Peterson A, Levichev IG, Peterson J. 2008. Systematics of Gagea and Lloydia (Liliaceae) and infrageneric classification of Gagea based on molecular and morphological data. – Mol. Phylogen. Evol. 46: 446-465.
Peterson A, Harpke D, Peruzzi L, Levichev IG, Tison JM, Peterson J. 2009. Hybridization drives speciation in Gagea (Liliaceae). – Plant Syst. Evol. 278: 133-148.
Petrova TF. 1977. Cytoembryology of the Liliaceae subfamily Lilioideae. – Moscow. [In Russian]
Pijewska L, Kaul JL, Joshi RK, Šantavý F. 1967. Substances from the plants of the subfamily Wurmbaeoideae and their derivatives LXVI. The presence of alkaloids in some tribes of the subfamily Wurmbaeoideae. – Collect. Czech. Chem. Commun. 32: 158-170.
Pole M. 1993. Early Miocene flora of the Manuherikia Group, New Zealand 5. Smilacaceae, Polygonaceae, Elaeocarpaceae. – J. Roy. Soc. New Zealand 23: 289-302.
Popova M. 1966. Some remarks on the lilies of the Balkans. – The Lily Year-Book 29: 66-70.
Potĕšilová H, Alcaraz C, Šantavý F. 1969. Substances from the plants of the subfamily Wurmbeoideae and their derivates LXXI. Isolation of alkaloids from the plants Colchicum byzanthinum Park., C. cupani Guss., C. libanoticum Ehrenb., Merendera filifolia Camb., C. luteum Baker, and Kreysigia multiflora Reichb. – Collect. Czech. Chem. Commun. 34: 2128-2133.
Potĕšilová H, Macfarlane TD, Guénard D, Šimánek V. 1987. Alkaloids and phenolics of Wurmbea and Burchardia species. – Phytochemistry 26: 1031-1032.
Preece SJ. 1956. A cytotaxonomic study of the genus Zigadenus. – Ph.D. diss., State College of Washington, Pullman, Washington.
Qi Z, Cameron KM, Li P, Zhao Y, Chen S, Chen G, Fu C. 2013. Phylogenetics, character evolution, and distribution patterns of the greenbriers, Smilacaceae (Liliales), a near-cosmopolitan family of monocots. – Bot. J. Linn. Soc. 173: 535-548.
Raamsdonk LWD, Vries T. 1992. Biosystematic studies in Tulipa sect. Eriostemones (Liliaceae). – Plant Syst. Evol. 179: 27-41.
Raamsdonk LWD, Vries T. 1995. Species relationships and taxonomy in Tulipa subg. Tulipa (Liliaceae). – Plant Syst. Evol. 195: 13-44.
Radulescu D. 1973. Recherches morpho-palynologiques sur la famille Liliaceae. – Acta Bot. Horti Bucur. 1972-1973: 133-248.
Ramsey M, Vaughton G. 2002. Maintenance of gynodioecy in Wurmbea biglandulosa (Colchicaceae): gender differences in seed production and progeny success. – Plant Syst. Evol. 232: 189-200.
Rasmussen FN, Frederiksen S, Johansen B, Jørgensen LB, Petersen G, Seberg O. 2006. Fleshy fruits in liliiflorous monocots. – In: Columbus JT, Friar EA, Porter JM, Prince LM, Simpson MG (eds), Monocots: comparative biology and evolution. Excluding Poales, Rancho Santa Ana botanical Garden, Claremont, California. – Aliso 22: 135-147.
Rasmussen H. 1983. Stomatal development in families of Liliales. – Bot. Jahrb. Syst. 104: 261-287.
Ravenna P. 2000. New or interesting Alstroemeriaceae I. – Onira 4: 33-46.
Renny M, Acosta MC, Cofré N, Domínguez LS, Bidartondo MI, Sérsic AN. 2017. Genetic diversity patterns of arbuscular mycorrhizal fungi associated with the mycoheterotroph Arachnitis uniflora Phil. (Corsiaceae). – Ann. Bot. 119: 1279-1294.
Rešetnik I, Liber Z, Satovic Z, Cigić P, Nikolić T. 2007. Molecular phylogeny and systematics of the Lilium carniolicum group (Liliaceae) based on nuclear ITS sequences. – Plant Syst. Evol. 265: 45-58.
Reveal JL. 1998. Proposals to conserve the names Tricyrtidaceae and Walleriaceae (Magnoliophyta). – Taxon 47: 173-174.
Reynaud C, Tison J-M. 1997. Étude phytochimique de quelques échantillons de Gagea (Liliaceae). – Bull. Mens. Soc. Linn. Lyon 66: 141-144.
Rix EM. 1971. The taxonomy of the genus Fritillaria in the Eastern Mediterranean region. – Ph.D. diss., University of Cambridge, England.
Rix EM. 1975. Notes on Fritillaria in the eastern Mediterranean region III. – Kew Bull. 30: 153-162.
Rix EM. 2001. Fritillaria. A revised classification. – The Fritillaria Group of the Alpine Garden Society.
Rix EM, Rast D. 1975. Nectar sugars and subgeneric classification in Fritillaria. – Biochem. Syst. Ecol. 2: 207-209.
Rix EM, Woods RG. 1981. Gagea bohemica (Zauschner) J. A. & J. H. Schultes in the British Isles, and a general review of the G. bohemica species complex. – Watsonia 13: 265-270.
Rodríguez R, Marticorena C. 1987. Las especies del género Luzuriaga R. et P. – Gayana Bot. 44: 3-15.
Romanov ID. 1936. Die Embryosackentwicklung in der Gattung Gagea Salisb. – Planta 25: 438-459.
Romanov ID. 1938. Eine neue Form des Embryosackes von Adoxa-Typus bei Tulipa tetraphylla und T. ostrovskiana. – Dokl. Akad. Nauk SSSR (Bot.) 19: 113-115.
Romanov ID. 1939. Two new forms of embryo sac in the genus Tulipa. – Dokl. Akad. Nauk SSSR (Bot.) 22: 139-141.
Romanov ID. 1961. The origin of a particular structure of endosperm nuclei in Gagea. – Dokl. Akad. Nauk SSSR 141: 984-986.
Rønsted N, Law S, Thornton H, Fay MF, Chase MW. 2005. Molecular phylogenetic evidence for the monophyly of Fritillaria and Lilium (Liliaceae; Liliales) and the infrageneric classification of Fritillaria. – Mol. Phylogen. Evol. 35: 509-527.
Rosen WG, Thomas HR. 1970. Secretory cells of lily pistils I. Fine structure and function. – Amer. J. Bot. 57: 1108-1114.
Roux LG le, Robbertse PJ. 1994. Tuber ontogeny, morphology and vegetative reproduction of Gloriosa superba L. – South Afr. J. Bot. 60: 321-324.
Royen P van. 1972. Sertulum Papuanum 17. Corsiaceae of New Guinea and surrounding areas. – Webbia 27: 223-255.
Rübsamen T. 1986. Morphologische, embryologische und systematische Untersuchungen an Burmanniaceae und Corsiaceae (mit Ausblick auf die Orchidaceae-Apostasioideae). – Diss. Bot. 92, J. Cramer, Berlin, Stuttgart.
Rudall PJ, Eastman A. 2002. The questionable affinities of Corsia (Corsiaceae): evidence from floral anatomy and pollen morphology. – Bot. J. Linn. Soc. 138: 315-324.
Rudall PJ, Stobart KL, Hong W-P, Conran JG, Furness CA, Kite GC, Chase MW. 2000. Consider the lilies: Systematics of Liliales. – In: Wilson KL, Morrison DA (eds), Monocots: systematics and evolution, CSIRO Publ., Melbourne, pp. 347-359.
Ruiz Réjon M. 1978. Estudios cariologicos en especies espñolas del orden Liliales III. Familia Liliaceae. – An. Inst. Bot. Antonio José Cavanilles 34: 733-759.
Rydberg PA. 1903. Some generic segregations. – Bull. Torrey Bot. Club 30: 271-281.
Samejima J, Samejima K. 1962. Studies on the eastern Asiatic Trillium (Liliaceae). – Acta Horti Gothob. 25: 157-257.
Samejima K, Samejima J. 1987. Trillium genus. – Hokkaido University Press, Sapporo.
Sanso AM. 1996. El género Alstroemeria (Alstroemeriaceae) en Argentina. – Darwiniana 34: 349-382.
Sanso AM. 2002. Chromosome studies in Andean taxa of Alstroemeria (Alstroemeriaceae). – Bot. J. Linn. Soc. 138: 451-459.
Sanso AM, Hunziker JH. 1998. Karyological studies in Alstroemeria and Bomarea (Alstroemeriaceae). – Hereditas 129: 67-74.
Sanso AM, Xifreda CC. 1995. El género Bomarea (Alstroemeriaceae) en Argentina. – Darwiniana 33: 315-336.
Sanso AM, Xifreda CC. 1999. The synonymy of Schickendantzia with Alstroemeria (Alstroemeriaceae). – Syst. Geogr. Plants 68: 315-323.
Sanso AM, Xifreda CC. 2001. Generic delimitation between Alstroemeria and Bomarea (Alstroemeriaceae). – Ann. Bot. 88: 1057-1069.
Šantavý F. 1956. Substanzen der Herbstzeitlose und ihre Derivate XLV. Verbreitung der Colchicinalkaloide im Pflanzenreich. – Österr. Bot. Zeitschr. 103: 300-311.
Šantavý F. 1967. The presence of colchicine alkaloids in Kreysigia multiflora Reichb. – Experientia 23: 273.
Sargant E. 1896. The formation of the sexual nuclei in Lilium martagon I. Oögenesis. – Ann. Bot. 10: 445-477.
Sarwar AKMG, Hoshino Y, Araki H. 2010. Pollen morphology and infrageneric classification of Alstroemeria (Alstroemeriaceae). – Grana 49: 227-242.
Satô D. 1942. Karyotype alteration and phylogeny in Liliaceae and allied families. – Jap. J. Bot. 12: 58-161.
Schaffner JH. 1901. A contribution to the life history and cytology of Erythronium. – Bot. Gaz. 31: 369-387.
Schlittler J. 1949. Die systematische Stellung der Gattung Petermannia F. v. Muell. und ihre phylogenetischen Beziehungen zu den Luzuriagoideae Engl. und den Dioscoreaceae Lindl. – Vierteljahresschr. Naturf. Ges. Zürich 94, Beih. 1: 1-28.
Schlittler J. 1953. Die Blütenartikulation und die Phyllokladien der Liliaceen organphylogenetisch betrachtet. – Feddes Repert. 55: 154-258.
Schlittler J. 1965 [1966]. Sind die Luzuriagoideen wirkliche Liliaceen oder haben die Ericales und Ternstroemiales organo-phylogenetisch und stammesgeschichtlich Beziehungen zu primitiven Liliifloren? – Ber. Schweiz. Bot. Ges. 75: 96-109.
Schmid M. 1938. Vergleichende Untersuchungen der leitenden Elemente im Gynoeceum einiger Liliaceae. – Ph.D. diss., Universität Wien, Austria.
Schmidt C. 1891. Über die Blätter einiger xerophiler Liliifloren. – Beih. Bot. Centralbl. 47: 1-6, 33-42, 97-107, 164-170.
Schnarf K. 1929. Die Embryologie der Liliaceae und ihre systematische Bedeutung. – S. B. Akad. Wiss. Wien I, 138: 69-92.
Schnarf K. 1949. Der Umfang der Lilioideae im natürlichen System. – Österr. Bot. Zeitschr. 95: 257-269.
Schulze R. 1893. Beiträge zur vergleichenden Anatomie der Liliaceen, Haemodoraceen, Hypoxidaceen und Velloziaceen. – Engl. Bot. Jahrb. Syst. 17: 295-394.
Schulze W. 1975. Beiträge zur Taxonomie der Liliifloren II. Colchicaceae. – Wiss. Zeitschr. Friedrich-Schiller-Univ. Jena, Math.-Naturwiss. Reihe, 24: 417-428.
Schulze W. 1978a. Beiträge zur Taxonomie der Liliifloren III. Alstroemeriaceae. – Beitr. Phytotaxonomie, 5. Folge, Wiss. Zeitschr. Friedrich-Schiller-Univ. Jena, Math.-Naturwiss. Reihe, 27: 79-85.
Schulze W. 1978b. Beiträge zur Taxonomie der Liliifloren IV. Melanthiaceae. – Wiss. Zeitschr. Friedrich-Schiller-Univ. Jena, Math.-Naturwiss. Reihe, 27: 87-95.
Schulze W. 1982a. Beiträge zur Taxonomie der Liliifloren VII. Philesiaceae. – Wiss. Zeitschr. Friedrich-Schiller-Univ. Jena, Math.-Naturwiss. Reihe, 31: 277-283.
Schulze W. 1982b. Beiträge zur Taxonomie der Liliifloren VIII. Smilacaceae. – Wiss. Zeitschr. Friedrich-Schiller-Univ. Jena, Math.-Naturwiss. Reihe, 31: 285-289.
Schwartz FC. 1994. Molecular systematics of Zigadenus section Chitonia (Liliaceae). – Ph.D. diss., University of Washington, Seattle, Washington.
Sen S. 1973. Polysomaty and its significance in Liliales. – Cytologia 38: 737-751.
Sen S. 1975. Cytotaxonomy of Liliales. – Feddes Repert. 86: 255-305.
Shamrov II. 1999. The ovule and seed development in some representatives of the orders Liliales and Amaryllidales. – Bot. Žurn. 84: 13-33. [In Russian]
Sheldon JM, Dickinson HG. 1983. Determination of patterning in the pollen wall of Lilium henryi. – J. Cell Sci. 63: 191-208.
Shinwari ZK, Terauchi R, Utech FH, Kawano S. 1994. Recognition of the New World Disporum section Prosartes as Prosartes (Liliaceae) based on the sequence data of the rbcL gene. – Taxon 43: 353-366.
Shneyer VS. 1983. The relationships between the Iridaceae and Liliaceae s.l. as revealed by the serological analysis of seed proteins. – Bot. Žurn. 68: 49-54. [In Russian]
Shuzukhina EA. 1994. Anatomical structure and ultrastructure of the seeds of Campynemataceae. – Bot. Žurn. 79: 58-62. [In Russian]
Siljak-Yakovlev S, Peccenini S, Muratović E, Zoldos V, Robin O, Vallés J. 2003. Chromosomal differentiation and genome size in thre three European mountain Lilium species. – Plant Syst. Evol. 236: 151-163.
Simpson PG, Philipson WR. 1969. Vascular anatomy in vegetative shoots of Rhipogonum scandens Forst. (Smilacaceae). – New Zealand J. Bot. 7: 3-29.
Slob A. 1973. Tulip allergens in Alstroemeria and some other Liliiflorae. – Phytochemistry 12: 811-815.
Smith J. 1909. Burmanniaceae, Corsiaceae. – Nova Guinea 8: 193-197.
Smyth DR, Kongsuwan K, Wisudharomn S. 1989. A survey of C-band patterns in chromosomes of Lilium (Liliaceae). – Plant Syst. Evol. 163: 53-69.
Sokolowska-Kulchycka A. 1973. Development of bisporic embryo sacs in Veratrum lobelianum Bernh. – Acta Biol. Cracov., Ser. Bot. 16: 85-98.
Sokolowska Kulczycka A. 1976. Development of bisporic embryo sacs in Veratrum nigrum L. – Acta Biol. Cracov. Ser. Bot. 20: 11-24.
Sopova M, Starova U, Sekovski Z. 1984. Study in the genus Gagea II. Cytology and distribution of seven Gagea species from Sr. Macedonia. – Acta Mus. Maced. Sci. Nat. 17: 103-131.
Spangler RC. 1925. Female gametophyte of Trillium sessile. – Bot. Gaz. 79: 217-221.
Stapf O. 1934. Lilium, Notholirion and Fritillaria. – Kew Bull. 1934: 94-96.
Stefanoff B. 1926. Monografiya na roda Colchicum L. – Sbornik na Bulg. Akad. Nauk (Sofiya) 22: 1-100.
Stenar H. 1927. Entwicklung des siebenkernigen Embryosacks bei Gagea lutea Ker-Gawler. – Svensk Bot. Tidskr. 21: 344-360.
Stenar H. 1928. Zur Embryologie der Veratrum- und Anthericum-Gruppe. – Bot. Not. 1928: 357-378.
Stenar H. 1952. Notes on the embryology and anatomy of Luzuriaga latifolia Poir. – Acta Horti Berg. 16: 219-232.
Stergios B, Dorr L. 2003. Bomarea amilcariana (Amaryllidaceae). – Acta Bot. Venezuel. 26: 31-40.
Sterling C. 1972. Comparative morphology of the carpel in the Liliaceae: Neodraegeae. – Bot. J. Linn. Soc. 65: 163-171.
Sterling C. 1973a. Comparative morphology of the carpel in the Liliaceae: Wurmbeeae. – Bot. J. Linn. Soc. 66: 75-82.
Sterling C. 1973b. Comparative morphology of the carpel in the Liliaceae: Colchiceae (Colchicum). – Bot. J. Linn. Soc. 66: 213-221.
Sterling C. 1973c. Comparative morphology of the carpel in the Liliaceae: Colchiceae (Androcymbium). – Bot. J. Linn. Soc. 67: 149-156.
Sterling C. 1974a. Comparative morphology of the carpel in the Liliaceae: Baeometra, Burchardia, and Walleria. – Bot. J. Linn. Soc. 68: 115-125.
Sterling C. 1974b. Comparative morphology of the carpel in the Liliaceae: Iphigenieae. – Bot. J. Linn. Soc. 68: 283-290.
Sterling C. 1975. Comparative morphology of the carpel in the Liliaceae: Glorioseae. – Bot. J. Linn. Soc. 70: 341-349.
Sterling C. 1977. Comparative morphology of the carpel in the Liliaceae: Uvularieae. – Bot. J. Linn. Soc. 74: 345-354.
Sterling C. 1978. Comparative morphology of the carpel of the Liliaceae: Hewardieae, Petrosavieae, and Tricyrteae. – Bot. J. Linn. Soc. 77: 95-106.
Sterling C. 1980. Comparative morphology of the carpel in the Liliaceae: Helonieae. – Bot. J. Linn. Soc. 80: 341-356.
Sterling C. 1982. Comparative morphology of the carpel in the Liliaceae: Veratreae. – Bot. J. Linn. Soc. 84: 57-77.
Stewart RN. 1947. The morphology of somatic chromosomes in Lilium. – Amer. J. Bot. 34: 9-26.
Stewart RN, Bamford R. 1942. The chromosomes and nucleoli of Medeola virginiana. – Amer. J. Bot. 29: 301-303.
Stoker F. 1938. The carniolicum group of lilies. – Lily Year-Book 7: 86-107.
Stoker F. 1939. European lilies of the Isolirion section. – Lily Year-Book 8: 15-29.
Stroh G. 1937. Die Gattung Gagea Salisb. – Beih. Bot. Centralbl. 57B: 485-520.
Subramanyam K, Govindu HC. 1949. The development of the male and female gametophyte in Lilium neilgherrense Wight. – J. Indian Bot. Soc. 28: 36-41.
Sulbha 1954.The embryology of Iphigenia indica Kunth. – Phytomorphology 4: 180-191.
Sun Z, Dilcher DL. 1988. Fossil Smilax from Eocene sediments in western Tennessee. – Amer. J. Bot. 75: 118.
Swamy BGL. 1948-1949. On the post-fertilization development of Trillium undulatum. – La Cellule 52: 5-14.
Synge PM. 1980. Lilies. A revision of Elwes’ Monograph of the genus Lilium and its supplements. – London.
Takahashi H. 1980. A taxonomic study on the genus Tricyrtis. – Sci. Rep. Fac. Educ., Gifu Univ. (Nat. Sci.) 6: 583-635.
Takahashi H. 1982a. Pollen morphology in North American species of Trillium. – Amer. J. Bot. 69: 1185-1195.
Takahashi H. 1982b. Pollen morphology in the genus Heloniopsis (Liliaceae). – Grana 21: 175-177.
Takahashi H. 1984a. The floral biology of Tricyrtis latifolia Maxim. (Liliaceae). – Bot. Mag. (Tokyo) 97: 207-217.
Takahashi H. 1984b. Pollen morphology in Paris and its related genera. – Bot. Mag. (Tokyo) 97: 233-245.
Takahashi H. 1987. A comparative floral and pollination biology of Tricyrtis flava Maxim., T. nana Yatabe and T. ohsumiensis Masamune (Liliaceae). – Bot. Mag. (Tokyo) 100: 185-203.
Takahashi H. 1988. The pollination biology of Heloniopsis orientalis (Thunb.) C. Tanaka (Liliaceae). – Plant Spec. Biol. 3: 117-123.
Takahashi H. 1991. Karyotype variations of Tricyrtis hirta Hook. (Liliaceae). – Acta Phytotaxon. Geobot. 42: 113-124.
Takahashi H. 1993. Floral biology of Tricyrtis macranthopsis Masamune and T. ishiiana (Kitagawa et T. Koyama) Ohwi et Okuyama var. surugensis Yamazaki (Liliaceae). – Acta Phytotaxon. Geobot. 44: 141-150.
Takahashi H. 1994. Floral biology of Tricyrtis macropoda Miq. (Liliaceae). – Acta Phytotaxon. Geobot. 45: 33-40.
Takahashi M. 1982. Pollen morphology in North American species of Trillium. – Amer. J. Bot. 69: 1185-1195.
Takahashi M. 1983. Pollen morphology in Asiatic species of Trillium. – Bot. Mag. (Tokyo) 96: 377-384.
Takahashi M. 1984. Pollen morphology in Paris and its related genera. – Bot. Mag. (Tokyo) 97: 233-245.
Takahashi M. 1987. Pollen morphology in the genus Erythronium (Liliaceae) and its systematic implications. – Amer. J. Bot. 74: 1254-1261.
Takahashi M, Kawano S. 1989. Pollen morphology of the Melanthiaceae and its systematic implications. – Ann. Missouri Bot. Gard. 76: 863-876.
Takahashi M, Sohma K. 1982. Pollen morphology of the genus Clintonia (Liliaceae). – Sci. Rep. Tohoku Imp. Univ., Ser. IV, Biol. 38: 157-164.
Takhtajan A. 1983. A revision of Daiswa (Trilliaceae). – Brittonia 35: 255-270.
Tamura MN. 1995. A karyological review of the orders Asparagales and Liliales (Monocotyledonae). – Feddes Repert. 1: 83-111.
Tamura MN. 1998a. Calochortaceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 164-172.
Tamura MN. 1998b. Liliaceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 343-353.
Tamura MN. 1998c. Melanthiaceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 369-380.
Tamura MN. 1998d. Trilliaceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 444-452.
Tamura MN, Utech FH, Kawano S. 1992. Biosystematic studies in Disporum (Liliaceae-Polygonateae) IV. Karyotype analysis of some Asiatic and North American taxa with special reference to their systematic status. – Plant Spec. Biol. 7: 103-120.
Tamura MN, Yamada M, Fuse S, Hotta M. 2013. Molecular phylogeny and taxonomy of the genus Disporum (Colchicaceae). – Acta Phytotaxon. Geobot. 64: 137-147.
Tan D-Y, Li X-R, Hong D-Y. 2007. Amana kuocangshanica (Liliaceae), a new species from South-East China. – Bot. J. Linn. Soc. 154: 435-442.
Tanaka NY. 1985. Variation of sexual system in Chionographis species. – Shuseibutsugaku-kenkyu 8: 11-19. [In Japanese]
Tanaka NY. 1997. Taxonomic significance of some floral characters in Helonias and Ypsilandra. – J. Jap. Bot. 72: 110-116.
Tanaka NY, Tanaka N. 1977. Chromosome studies in Chionographis (Liliaceae) I. On the holokinetic nature of chromosomes in Chionographis japonica Maxim. – Cytologia 42: 753-763.
Tanaka NY, Tanaka N. 1979. Chromosome studies in Chionographis (Liliaceae) II. Morphological characteristics of the somatic chromosomes of four Japanese members. – Cytologia 44: 935-949.
Tanaka NY, Tanaka N. 1980. Chromosome studies in Chionographis (Liliaceae) III. The mode of meiosis. – Cytologia 45: 809-817.
Tatewaki M, Suto T. 1935. On the new genus Kinugasa. – Trans. Sapporo Nat. Hist. Soc. 14: 34-37.
Taylor CA. 1956. The culture of false hellebore. – Econ. Bot. 10: 155-165.
Terracciano A. 1904. Gagearum novarum diagnoses. – Boll. Soc. Ortic. Palermo 2: 3-10.
Terracciano A. 1905a. Gagearum species florae orientalis. – Bull. Herb. Boissier, sér. II, 5: 1113-1129.
Terracciano A. 1905b. Les espèces du genre Gagea dans la flore de l’Afrique boréale. – Bull. Soc. Bot. France 52: 1-26.
Terracciano A. 1906. Gagearum species florae orientalis. Pars secunda. – Bull. Herb. Boissier, sér. II, 6: 105-120.
Therman E, Denniston C. 1984. Random arrangement of chromosomes in Uvularia (Liliaceae). – Plant Syst. Evol. 147: 289-297.
Thulin M. 1995. Iphigenia socotrana sp. nov. (Colchicaceae), with a note on I. oliveri. – Nord. J. Bot. 15: 403-405.
Tison J-M. 1996. Revision des Gagea du groupe bohemica en France. – Monde Plant. 455: 11-17.
Tison J-M. 1997. Les Gagea du groupe lutea en France. – Monde Plant. 460: 15-16.
Tison J-M. 1998. Notes complementaires sur quelques Gagea française. – Monde Plant 462: 7-8.
Tison J-M. 2004a. Contribution à la connaissance du genre Gagea Salisb. (Liliaceae) en Afrique du Nord. – Lagascalia 24: 67-87.
Tison J-M. 2004b. Identité et situation taxonomique de Gagea polymorpha Boiss. – Candollea 59: 109-117.
Tomko J, Votický Z. 1973. Steroid alkaloids: the Veratrum and Buxus groups. – In: Manske RHF (ed), The alkaloids XIV, Academic Press, New York, pp. 5-82.
Tomlinson PB, Ayensu ES. 1969. Notes on the vegetative morphology and anatomy of the Petermanniaceae (Monocotyledones). – Bot. J. Linn. Soc. 62: 17-26.
Tsuchiya T, Hang A. 1989. Cytogenetics in the genus Alstroemeria. – Herbertia 45: 163-170.
Tsuchiya T, Hang A, Healey WE Jr, Hughes H. 1987. Chromosome studies in the genus Alstroemeria I. Chromosome numbers in 10 cultivars. – Bot. Gaz. 148: 519-524.
Turktas M, Metin ÖK, Baştuğ B, Ertuğrul F, Saraç YI, Kaya E. 2013. Molecular phylogenetic analysis of Tulipa (Liliaceae) based on noncoding plastid and nuclear DNA sequences with an emphasis on Turkey. – Bot. J. Linn. Soc. 172: 270-279.
Turner BL. 1992. A new species of Zigadenus (Liliaceae) from eastern Mexico. – Phytologia 72: 378-382.
Turrill WB. 1937. The genus Fritillaria L. in the Balkan Peninsula and Asia Minor. – J. Roy. Hort. Soc. 62: 329-335.
Turrill WB. 1953. The lilies of the Balkan Peninsula. – Lily Year-Book 17: 30-39.
Tzanoudakis D, Iatrou G, Kypriotakis Z, Christodoulakis D. 1991. Cytogeographical studies in some Aegean Liliaceae. – Bot. Chron. 10: 761-775.
Ugarte E, Arriagada J. 1983. Presencia de Arachnitis uniflora Phil. (Corsiaceae) en la peninsula de Hualpén, Concepción, Chile. – Bol. Soc. Biol. Concepción 54: 167-170.
Uphof JCT. 1952. A review of the genus Alstroemeria. – Plant Life 8: 36-53.
Utech FH. 1978a. Floral vascular anatomy of Medeola virginiana L. (Liliaceae-Parideae = Trilliaceae) and tribal note. – Ann. Carnegie Mus. 47: 13-28.
Utech FH. 1978b. Floral vascular anatomy of Scoliopus bigelovii Torrey (Liliaceae-Parideae = Trilliaceae) and tribal note. – Ann. Carnegie Mus. 47: 43-71.
Utech FH. 1978c. Comparison of the vascular anatomy of Xerophyllum asphodeloides (L.) Nutt. and X. tenax (Pursh) Nutt. (Liliaceae-Melanthioideae). – Ann. Carnegie Mus. 47: 147-167.
Utech FH. 1978d. Vascular floral anatomy of Helonias bullata L. (Liliaceae-Holonieae) with a comparison to the Asian Heloniopsis orientalis. – Ann. Carnegie Mus. 47: 169-191.
Utech FH. 1978e. Somatic karyotype analysis of Uvularia floridana Chapman (Liliaceae). – Cytologia 43: 671-678.
Utech FH. 1980. Somatic karyotype analysis of Helonias bullata L. (Liliaceae), with a comparison to the Asian Heloniopsis orientalis (Thunb.) C. Tanaka. – Ann. Carnegie Mus. 49: 153-160.
Utech FH. 1986. Floral morphology and vascular anatomy of Amianthium muscaetoxicum (Walter) A. Gray (Liliaceae-Veratreae) with notes on distribution and taxonomy. – Ann. Carnegie Mus. 55: 481-504.
Utech FH. 1987a. Biosystematic studies in Stenanthium (Liliaceae: Veratreae) I. Floral morphology, floral vascular anatomy, geography and taxonomy of S. occidentale A. Gray. – Ann. Carnegie Mus. 56: 113-135.
Utech FH. 1987b. Biosystematic studies in Stenanthium (Liliaceae: Veratreae) II. Floral morphology, floral vascular anatomy, geography and taxonomy of the Mexican S. frigidum (Schlecht. & Cham.) Kunth. – Ann. Carnegie Mus. 56: 197-212.
Utech FH. 1992. Biology of Scoliopus (Liliaceae) I. Phytogeography and systematics. – Ann. Missouri Bot. Gard. 79: 126-142.
Utech FH, Kawano S. 1975a. Biosystematic studies in Erythronium (Liliaceae-Tulipeae) I. Floral biology of E. japonicum Decne. – Bot. Mag. (Tokyo) 88: 163-176.
Utech FH, Kawano S. 1975b. Biosystematic studies in Erythronium (Liliaceae-Tulipeae) II. Floral anatomy of E. japonicum Decne. – Bot. Mag. (Tokyo) 88: 177-185.
Utech FH, Kawano S. 1980. Vascular floral anatomy of the Japanese Paris tetraphylla A. Gray (Liliaceae-Parideae). – J. Phytogeogr. Taxon. 28: 17-23.
Utech FH, Kawano S. 1981. Vascular floral anatomy of the East Asian Heloniopsis orientalis (Thunb.) C. Tanaka (Liliaceae-Helonieae). – Bot. Mag. (Tokyo) 94: 295-311.
Vaughton G, Ramsey M. 1998. Floral display, pollinator visitation and reproductive success in the dioecious perennial herb Wurmbea dioica (Liliaceae). – Oecologia 115: 93-101.
Vaughton G, Ramsey M. 2002. Evidence of gynodioecy and sex ratio variation in Wurmbea biglandulosa (Colchicaceae). – Plant Syst. Evol. 232: 167-179.
Veldkamp J, Zonneveld B. 2012. The infrageneric nomenclature of Tulipa (Liliaceae). – Plant Syst. Evol. 298: 87-92.
Vijayavalli B, Mathew PM. 1990. Cytotaxonomy of the Liliaceae and allied families. – Kerala.
Vinnersten A, Bremer K. 2001. Age and biogeography of major clades in Liliales. – Amer. J. Bot. 88: 1695-1703.
Vinnersten A, Bremer K. 2003. Historical biogeography of Colchicaceae inferred from dispersal-vicariance analysis. – In: Vinnersten A (ed), Tracing history. Phylogenetic, taxonomic and biogeographic research in the Colchicum family, Ph.D. diss., University of Uppsala, Sweden.
Vinnersten A, Larsson S. 2003. Colchicine distribution in Colchicaceae investigated by mass spectrometry. – In: Vinnersten A (ed), Tracing history. Phylogenetic, taxonomic and biogeographic research in the Colchicum family, Ph.D. diss., University of Uppsala, Sweden.
Vinnersten A, Larsson S. 2010. Colchicine is still a chemical marker for the expanded Colchicaceae. – Biochem. Syst. Ecol. 38: 1193-1198.
Vinnersten A, Manning J. 2007. A new classification of Colchicaceae. – Taxon 56: 171-178.
Vinnersten A, Reeves G. 2003. Phylogenetic relationships within Colchicaceae. – Amer. J. Bot. 90: 1455-1462.
Vinnersten A, Macfarlane TD, Case AL. 2003. A note on Iphigenia novae-zelandiae (Colchicaceae). – In: Vinnersten A (ed), Tracing history. Phylogenetic, taxonomic and biogeographic research in the Colchicum family, Ph.D. diss., University of Uppsala, Sweden.
Wafai BA, Koul AK. 1982. Analysis of breeding system in Tulipa II. Sporogenesis, gametogenesis and embryogeny in tetraploid T. clusiana. – Phytomorphology 32: 289-301.
Walsh OS. 1940. A systematic study of the genus Zigadenus Michx. – Ph.D. diss., University of California, Berkeley, California.
Warmke HE. 1937. Cytology of the Pacific Coast Trilliums. – Amer. J. Bot. 24: 376-383.
Watson S. 1879. Contributions to American botany I. Revision of the North American Liliaceae. – Proc. Amer. Acad. Arts Sci. 14: 213-288, 301.
Wendelbo P. 1967. New species of Bellevalia, Ornithogalum and Tulipa from Iran. – Nytt Mag. Bot. 14: 96-100.
Whyte RO. 1929. Chromosome studies I. Relationships of the genera Alstroemeria and Bomarea. – New Phytol. 28: 319-344.
Wietsma WA, Deinum D, Teunissen HAS, Berg RG van den. 2015. Phylogenetic relationships within Fritillaria section Petilium based on AFLP fingerprints. – Plant Syst. Evol. 301: 1043-1054.
Wilbur RL. 1963. A revision of North American genus Uvularia (Liliaceae). – Rhodora 65: 158-188.
Wilbur RL. 2004. The subgeneric nomenclature for the herbaceous-stemmed Smilax species (Smilacaceae) of North America. – Brittonia 56: 166-168.
Wilbur RL. 2006. Addendum to “The subgeneric nomenclature for the herbaceous-stemmed Smilax species (Smilacaceae) of North America” and comments on the problems of infrageneric nomenclature. – Brittonia 58: 88-89.
Wilford R. 2006. Tulips – species and hybrids for the gardener. – Timber Press.
Wilkin P. 1997. Leontochir ovallei. – Kew Mag. 14: 7-12.
Williams CA. 1975. Biosystematics of the Monocotyledoneae – flavonoid patterns in leaves of the Liliaceae. – Biochem. Syst. Ecol. 3: 229-244.
Wilson EH. 1925. The lilies of Eastern Asia, a monograph. – London.
Woodard TM Jr. 1948. Difference in form and reaction to cold in root-tip and apical bud chromosomes of Medeola. – Bull. Torr. Bot. Club 75: 250-255.
Wright CH. 1909. Diagnoses africanae: xxx. Neodregea. – Kew Bull. 1909: 308-309.
Wunderlich R. 1936. Vergleichende Untersuchungen von Pollenkörnern einiger Liliaceen und Amaryllidaceen. – Österr. Bot. Zeitschr. 85: 30-55.
Xie X-Y, Gu Z-J, Wu QA. 1992. Cytological studies of the genus Nomocharis and its related genera. – Acta Phytotaxon. Sin. 30: 487-497. [In Chinese]
Yamagishi M. 1995. Detection of section-specific random amplified polymorphic DNA (RAPD) markers in Lilium. – Theor. Appl. Gen. 91: 830-835.
Yang L-Q, Hu H-Y, Xie C, Lai S-P, Yang M, He X-J, Zhou S-D. 2017. Molecular phylogeny, biogeography and ecological niche modelling of Cardiocrinum (Liliaceae): insights into the evolutionary history of endemic genera distributed across the Sino-Japanese floristic region. – Ann. Bot. 119: 59-72.
Zaharof E. 1987. Biometrical and cytological studies of the genus Fritillaria L. in Greece. – Ph.D. diss., University of Thessaloniki, Greece.
Zaharof E. 1988. A phenetic study of Fritillaria (Lilaceae) in Greece. – Plant syst. Evol. 161: 23-34.
Zaharof E. 1989. Karyological studies of twelve Fritillaria species from Greece. – Caryologia 43: 91-102.
Zakhariyeva OI, Makushenko LM. 1969. Chromosome numbers of monocotyledons belonging to the families Liliaceae, Iridaceae, Amaryllidaceae and Araceae. – Bot. Žurn. 54: 1213-1227. [In Russian]
Zarrei M, Zarre S, Wilkin P, Rix M. 2007. Systematic revision of the genus Gagea Salisb. (Liliaceae) in Iran. – Bot. J. Linn. Soc. 154: 559-588.
Zarrei M, Wilkin P, Fay MF, Ingouille MJ, Chase MW. 2009. Molecular systematics of Gagea and Lloydia (Liliaceae; Liliales): implications of analyses of nuclear ribosomal and plastid DNA sequences for infrageneric classification. – Ann. Bot. 104: 125-142.
Zarrei M, Wilkin P, In-Grouille MJ, Chase MW. 2011. A revised infrageneric classification for Gagea Salisb. (Tulipeae; Liliaceae): insights from DNA sequence and morphological data. – Phytotaxa 15: 44-56.
Zemskova EA, Levichev IG. 1988. Chromosome numbers of the species of the genus Gagea (Liliaceae). – Bot. Žurn. 83: 136-138. [In Russian]
Zhang D, Saunders RMK, Hu C-M. 1999. Corsiopsis chinensis gen. et sp. nov. (Corsiaceae): first record of the family in Asia. – Syst. Bot. 24: 311-314.
Zimmerman JH. 1958. A monograph of Veratrum. – Ph.D. diss., University of Wisconsin, Madison, Wisconsin.
Zomlefer WB. 1999. Advances in angiosperm systematics: examples from the Liliales and Asparagales. – J. Torrey Bot. Soc. 125: 58-62.
Zomlefer WB. 2003. Documented chromosome numbers 2003: 1. Chromosome number of Toxicoscordion nuttallii (Liliales: Melanthiaceae) and clarification of the genus. – Sida 20: 1085-1092.
Zomlefer WB, Judd WS. 2002. Resurrection of segregates of the polyphyletic genus Zigadenus s.l. (Liliales: Melanthiaceae) and resulting new combinations. – Novon 12: 299-308.
Zomlefer WB, Judd WS. 2008. Two new species of Schoenocaulon (Liliales: Melanthiaceae) from Mexico supported by ITS sequence data. – Syst. Bot. 33: 117-124.
Zomlefer WB, Perkins KD. 1999. Phylogeny of the Melanthiaceae. – Available at http://www.flmnh.ufl.edu/natsci/herbarium/molecular/melan/ and http://ajbsupp.botany.org/v88/zomlefer/
Zomlefer WB, Smith GL. 2002. Documented chromosome numbers 2002: 1. Chromosome number of Stenanthium (Liliales: Melanthiaceae) and its significance in the taxonomy of tribe Melanthieae. – Sida 20: 221-226.
Zomlefer WB, Williams NH, Whitten WM, Judd WS. 2001. Generic circumscription and relationships in the tribe Melanthieae (Liliales, Melanthiaceae), with emphasis on Zigadenus: evidence from ITS and trnL-F sequence data. – Amer. J. Bot. 88: 1657-1669.
Zomlefer WB, Whitten WM, Williams NH, Judd WS. 2003. An overview of Veratrum s.l. (Liliales: Melanthiaceae) and an infrageneric phylogeny based on ITS sequence data. – Syst. Bot. 28: 250-269.
Zomlefer WB, Whitten WM, Williams NH, Judd WS. 2006. Infrageneric phylogeny of Schoenocaulon (Liliales: Melanthiaceae) with clarification of cryptic species based on ITS sequence data and geographical distribution. – Amer. J. Bot. 93: 1178-1192.
Zomlefer WB, Judd WS, Whitten WM, Williams NH. 2006. A synopsis of Melanthiaceae (Liliales) with focus on character evolution in tribe Melanthieae. – In: Columbus JT, Friar EA, Porter JM, Prince LM, Simpson MG (eds), Monocots: comparative biology and evolution (excluding Poales), Rancho Santa Ana Botanic Garden, Claremont, California. – Aliso 22: 566-578.
Zomlefer WB, McKain M, Rentsch J. 2014.
Documentation of the chromosome number for Zigadenus glaberrimus (Liliales: Melanthiaceae) and its significance in
the taxonomy of tribe Melanthieae). – Syst. Bot. 39: 411-414.
Zonneveld BJM. 2009. The systematic value of nuclear genome size for “all” species of Tulipa L. (Liliaceae). – Plant Syst. Evol. 281: 217-245.
Zweigelt F. 1913. Vergleichende Anatomie der Asparagoideae, Ophiopogonoideae, Aletroideae, Luzuriagoideae und Smilacoideae nebst Bemerkungen über die Beziehungen Ophiopogonoideae und Dracaenoideae. – Denkschr. Akad. Wiss. Wien 88: 397-476.