
Cladogram of Alismatales based on DNA sequence data.
[Alismatales+[Petrosaviaceae+[Taccales+Pandanales]+[Liliales+[Iridales+Commelinidae]]]]
Alismatineae Engl., Syllabus, ed. 2: 73. Mai 1898; Alismatanae Takht., Sist. Filog. Cvetk. Rast. [Syst. Phylog. Magnolioph.]: 461. 4 Feb 1967; Alismatidae Takht., Sist. Filog. Cvetk. Rast. [Syst. Phylog. Magnolioph.]: 461. 4 Feb 1967
Fossils Pennistemon portugallicus is represented by a flower with spirally arranged stamens and pollen with reticulate exine and acolumellate granular infratectum. These and similar pollen grains (Pennipollis type) from the Late Aptian to the Cenomanian are known from Europe, Africa and North America. Pennicarpus tenuis consists of fossilized unilocular one-seeded fruits from the Early Cretaceous of Portugal, which may emanate from the same plants as Pennistemon. Thalassotaenia debeyi and other Maastrichtian and Danian fossils from western Europe comprise leaves similar to those of extant Cymodoceaceae or Posidonia. Numerous fruits from the Late Paleocene onwards of Asia and Europe resemble Ruppia and Potamogetonaceae and have been described under the name of Limnocarpus.
Habit Bisexual, monoecious, andromonoecious, gynomonoecious, polygamomonoecious, dioecious, or gynodioecious, usually perennial rhizomatous (rarely annual) herbs. Usually aquatic or hygrophilous (sometimes marine).
Vegetative anatomy Mycorrhiza absent. Root stele often triarch to pentarch. Phellogen absent. Secondary lateral growth absent. Vessels often present in roots and sometimes in stem (sometimes entirely absent). Vessel elements with usually scalariform (sometimes simple, rarely reticulate) perforation plates; lateral pits scalariform, simple pits. Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial parenchyma? Sieve tube plastids P2cs or P2cfs type (rarely Ss type?). Nodes multilacunar with several leaf traces. Laticifers sometimes present. Schizogenous ducts and cavities with resins and oils sometimes present. Trichosclereids sometimes present. Silica bodies absent. Calciumoxalate as raphides (H-shaped in cross-section), solitary prismatic crystals, or druses, or absent.
Trichomes Hairs usually absent (sometimes unicellular or multicellular, simple, lepidote, stellate, or prickly).
Leaves Alternate (spiral or distichous), compound (rarely pinnately compound) or simple, entire or lobed, often differentiated into pseudopetiole and pseudolamina, with supervolute, involute or convolute ptyxis (rarely absent). Pseudolamina usually developing from leaf base zone (sometimes from upper part of foliar primordium). Stipules absent or as axillary scales inside leaf sheath (rarely lateral); leaf sheath usually well developed, often with axillary intravaginal (sometimes hair-like) scales/colleters, squamulae intravaginales. Pseudopetiole vascular bundle transection arcuate; inverted bundles frequent. Venation usually pinnate or palmate, pedate, curvipalmate, parallelodromous, acrodromous, campylodromous, sometimes reticulodromous (then usually with tertiary veins). Stomata often absent (sometimes paracytic, tetracytic, anomocytic, or cyclocytic; adjacent cells with or without oblique divisions). Cuticular waxes absent. Mesophyll often with mucilaginous idioblasts containing calciumoxalate raphides, prismatic crystals or druses. Schizogenous laticiferous cavities sometimes present. Tanniniferous cells sometimes abundant. Leaf margin usually entire (sometimes serrate to dentate). Pseudolamina sometimes with apical pore.
Inflorescence Terminal or axillary, simple or branched, panicle, spike or raceme, spike-, raceme- or umbel-like, or fleshy cymose spadix, or flowers solitary. Peduncle sometimes laticiferous. Inflorescence bract sometimes large and showy. Floral bracts and prophylls (bracteoles) often absent (lateral bracteole rarely present).
Flowers Usually actinomorphic (sometimes zygomorphic or asymmetrical). Hypanthium rarely present. Usually hypogyny (sometimes epigyny). Tepals (two or 2+2 or) 3+3 (rarely 4+4 or 6+6), all sepaloid or petaloid, or outer tepals sepaloid with valvate aestivation and inner tepals petaloid, crumpled in bud, free or entirely or partially connate, or absent. Septal nectaries present or absent. Disc absent.
Androecium Stamens one to three, 2+2 or 3+3 (sometimes 18 to c. 100, rarely 4+4, 6+3, 6+4 or 6+6), whorled. Filaments short, free or sometimes connate into synandrium, usually free from tepals (rarely adnate to tepals, epitepalous). Anthers basifixed or subbasifixed, non-versatile, sometimes connate into synandrium, usually tetrasporangiate (sometimes disporangiate or 1–12-sporangiate), usually extrorse (rarely latrorse or introrse), longicidal (dehiscing by longitudinal slits) or poricidal (dehiscing by apical or subapical pores, or short transverse slits); connective often expanded (laminar connective outgrowths sometimes perianth-like). Tapetum amoeboid-periplasmodial, with uninucleate cells. Staminodia usually absent (sometimes extrastaminal or intrastaminal; female flowers sometimes with staminodia).
Pollen grains Microsporogenesis usually successive (sometimes simultaneous). Pollen grains sulcate, meridionosulcate (zonate), disulcate, trichotomosulcate, forate (periporate), di-, tri- or polypantoporate, or inaperturate, usually shed as monads (rarely tetrads), bicellular or tricellular at dispersal. Exine tectate or semitectate, usually with columellate (sometimes granular) infratectum, or intectate, reticulate, microreticulate, foveolate, foraminate, scabrate, spinulate, echinate, verrucate, fossulate, psilate, or smooth (rarely gemmate, areolate, rugulate, or striate), or absent.
Gynoecium Carpels (one to) three or six (to c. 100), in one whorl or seemingly spiral, secondarily free or connate at base, carpel plicate or conduplicate; or pistil composed of (one to) three (to 15) usually congenitally fused (rarely free) carpels; carpel ascidiate to intermediate, with completely non-fused canals, seemingly occluded by secretion. Closure by transverse slit occurring together with longitudinal slit. Ovary usually superior (sometimes inferior), usually unilocular to trilocular (to 47-locular), usually with mucilaginous hair on inner side. Style single, simple, usually very small, or absent, or stylodia terminal, lateral, gynobasic (sometimes connate at base) or absent. Stigma capitate, lobate or peltate, or stigmas usually linear, papillate or non-papillate, Dry or Wet type. Pistillodium usually absent (male flowers sometimes with pistillodia).
Ovules Placentation axile, parietal, basal, apical, laminar, or (sub)marginal. Ovules one to more than 100 per carpel, anatropous, hemianatropous, orthotropous, amphitropous, anacampylotropous, campylotropous or intermediate, ascending, horizontal or pendulous, apotropous, usually bitegmic (rarely unitegmic), usually tenuinucellar (sometimes crassinucellar or pseudocrassinucellar). Micropyle bistomal, endostomal or exostomal. Parietal cell formed from archesporial cell or absent. Nucellar cap often present. Megagametophyte usually monosporous, Polygonum type (rarely 10- to 12-nucleate; rarely disporous, Allium, Veratrum lobelianum, Endymion or Scilla type). Synergids often with a filiform apparatus. Antipodal cells usually persistent, often proliferating (sometimes absent). Endosperm development helobial or nuclear. Endosperm appendage (’endosperm haustorium’, ’basal apparatus’) chalazal. Embryogenesis onagrad, asterad, caryophyllad, or solanad.
Fruit Usually a berry, a drupe, a nut, a nutlike or drupaceous fruit, a loculicidal, septicidal or ventricidal capsule, or an assemblage of achenes or follicles (multifolliculus; rarely an indehiscent or dehiscent syncarp with baccate or nutlike mericarps).
Seeds Aril absent. Operculum sometimes present. Testa thick or thin, multiplicative, often parenchymatous (sometimes absent). Exotesta often fleshy and viscid, often with thick epidermal cell walls. Mesotesta sometimes parenchymatous. Endotesta sometimes absent. Mesotestal and endotestal cells usually thick-walled. Exotegmen often reduced or collapsing. Endotegmen tanniniferous or collapsing. Perisperm rarely developed. Endosperm copious, sparse or absent, starchy (starch grains usually Pteridophyte type, amylophilic) or with aleurone and lipids. Embryo large to small (often macropodous), straight or curved, sometimes with starch, well or poorly differentiated, sometimes with chlorophyll. Cotyledon one, not photosynthesizing. Cotyledon hyperphyll assimilating or modified into haustorium or nutrient-storing organ. Hypocotyl internode usually present (sometimes as swollen nutrient-storing organ). Coleoptile absent. Collar rhizoids or collar roots sometimes present. Radicula absent in some genera. Germination phanerocotylar. Seedling with hypocotyl and root well developed.
Cytology x = 5–15
DNA Deletion of 3 bp in atpA in many Alismatales (and in Acorus), but not in Tofieldiaceae or Cymodoceaceae; mitochondrial intron processing in most Alismatales according to cis-splicing mechanism
Phytochemistry Flavonols (kaempferol, quercetin), flavones, flavone-C-glycosides, flavone-O-glycosides, flavone aglucones, flavonoid sulfates, protocatechinic aldehyde, cyanidin, tannins, cinnamic acid, caffeic acid derivatives (including caffeic acid sulfate), chlorogenic acid, polyphenolic glycosides with caffeic acid, phenolic sulfates (sulfonated phenolic acids), alkaloids, tyrosine-derived cyanogenic glycosides (e.g. triglochinin), steroidal saponins, chelidonic acid, 5-alkylic- and 5-alkenylic resorcinols homogentisic acid and their glycosides, amines, and bitter substances present. Proanthocyanidins rare or absent. Ellagic acid not found. Sulphated compounds frequent in Juncaginaceae and some allied groups.
Systematics Alismatales are sister-group to all Liliidae except Acorus.
Squamulae intravaginales are a synapomorphy of a main clade in Alismatales (cf. Acoraceae). These structures are intravaginal or axillary non-vascularized scale-, gland- or finger-like trichomes occurring in pairs or multiples in the leaf axils. They are usually multicellular and two-layered and secrete a protective mucilage. Tanniniferous cells are often present at their base, in the middle parts, at the distal end or at the tooth-like border. Alismatales are one of the very few monocot clades in which chlorophyllous embryos have been found. Furthermore, Alismatales comprise most entirely marine angiosperms and the only known marine flowering plants, in which sulphated phenolic compounds are frequent. In some Alismatales intermediates between floral bracts and abaxial tepals seem to be present. It has been suggested that the flowers in at least many taxa are actually pseudanthia.
In most Alismatales, the leaves appear to have developed from the upper part of the leaf primordium (cf. Acorus). However, in many Araceae the leaves probably develop according to the normal monocotyledon principle.
Araceae are usually placed as sister-group to the remaining Alismatales, yet an unresolved basal trichotomy consisting of Araceae, Tofieldiaceae and the remainder has been identified in some analyses, and sometimes Tofieldiaceae are recovered as the sister-group either of all other Alismatales or to Araceae.
Apocarpous gynoecium is a synapomorphy of Alismatales except Araceae, according to Stevens (2001 onwards). Alismatales, except Araceae and Tofieldiaceae, are further characterized by the following potential synapomorphies: aquatic habit; stem with lacunae; absence of druses and raphides; absence of indumentum (trichomes) and bulliform cells; squamulae intravaginales often present; pseudolamina often with an apical pore; pollen grains tricellular at dispersal; absence of endosperm in mature seed; and presence of seedling collar and collar rhizoids.
The clade [Alismataceae+[Butomus+Hydrocharitaceae]] is characterized by, e.g.: bifurcating apical meristems of vegetative axes; inflorescence scapose, cymose and bracteate; outer tepals sepaloid and inner tepals petaloid; both perianth whorls with numerous leaf traces; stamens paired; carpels plicate; seed coat exotestal; and presence of flavone-C-glycosides. Butomus and Hydrocharitaceae have the character of ovary loculi with secretion in common.
The clade [Scheuchzeria+[Aponogeton+[Juncaginaceae+[[Posidoniaceae+[Ruppia+Cymodoceaceae]]+[Maundia+[Zosteraceae+Potamogetonaceae]]]]]] has a poorly developed radicula, and tepals (when present) with a single leaf trace.
The clade [Juncaginaceae+[[Posidonia+[Ruppia+Cymodoceaceae]]+[Maundia+[Zosteraceae+ Potamogetonaceae]]]] is characterized by the following potential synapomorphies (Stevens 2001 onwards): linear ligulate leaves with auriculate base; tepals absent or reduced to a retinaculum (‘abaxial outgrowths’ from the stamens); absence of nectary and filaments; pollen grains inaperturate; carpels with complete postgenital fusion; fruit indehiscent; endosperm development nuclear; presence of sulphated phenolic acids; and absence of flavones and proanthocyanidins.
The clade [[Posidonia+[Ruppia+Cymodoceaceae]]+[Maundia+[Zosteraceae+Potamo-getonaceae]]] has, e.g., the following characteristic features: rhizome with endodermis; epidermis with chlorophyll; one apical orthotropous ovule per carpel; fruit drupaceous; embryo with a massive elongate hypocotyls; and collar or base of hypocotyls much enlarged. [Posidonia+[Ruppia+Cymodoceaceae]] lack vessels, have distichous leaves without stomata, and produce filiform pollen grains. Ruppia and Cymodoceaceae have serrulate leaves and two stamens.
The [Zosteraceae+Potamogetonaceae] clade has the following potential synapomorphies: flowers unisexual; leaf sheath closed; leaves with an apical pore (also present in Scheuchzeria); and staminal pair with a single vascular trace. So far, no obvious morphological synapomorphy uniting Maundia with Zosteraceae and Potamogetonaceae has been found. On the other hand, Maundia is still very poorly investigated.
Petersen & al. (2016) found Acorus as sister to all Alismatales except Tofieldiaceae and Araceae. However, Acorus appeared outside of the Alismatales clade in the analysis by Ross & al. (2016).
According to Ross & al. (2016) and Petersen & al. (2016), Aponogeton (Aponogetonaceae) was sister-group to the remaining Alismatales (with low to moderate support), whereas Scheuchzeria (Scheuchzeriaceae) was successive sister to the rest. Both studies recovered the alternative topology [Maundiaceae+[[Potamogetonaceae+Zosteraceae]+[Posidoniaceae+[Ruppiaceae+Cymodoceaceae]]]].

|
Cladogram of Alismatales based on DNA sequence data. |
ALISMATACEAE Vent. |
( Back to Alismatales ) |
Damasoniaceae Nakai, Chosakuronbun Mokuroku [Ord. Fam. Trib. Nov.]: 213. 20 Jul 1943; Elismataceae Nakai, Chosakuronbun Mokuroku [Ord. Fam. Trib. Nov.]: 213. 20 Jul 1943, nom. illeg.; Limnocharitaceae Takht. ex Cronq., Integr. Syst. Class. Fl. Pl.: 1048. 10 Aug 1981
Genera/species 18/100–105
Distribution Allmost cosmopolitan, with their highest diversity in North and South America.
Fossils Cardstonia tolmanii comprises fossilized leaves from the Campanian to the Maastrichtian of Alberta. Moreover, fruits and seeds of Alismataceae are frequent in Cenozoic layers from the Eocene onwards.
Habit Usually bisexual (sometimes unisexual: in Sagittaria monoecious or polygamomonoecious, in Limnophyton polygamomonoecious, in Burnatia dioecious), usually perennial (rarely annual) herbs. Aquatic or helophytic. Tuberous stem, corm or stolons sometimes present.
Vegetative anatomy Roots fibrous, often septate. Turions sometimes formed (e.g. in Sagittaria). Phellogen absent. Secondary lateral growth absent. Rhizome in Limnochariteae and allied genera with endodermis (similar to root endodermis). Vessels in roots only, as a single vascular strand (sometimes absent). Vessel elements usually with simple (sometimes scalariform) perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids. Sieve tube plastids P2c type, with cuneate protein crystalloid bodies, without starch or protein filaments. Nodes? Schizogenous ducts with milky latex. Calciumoxalate as druses and single prismatic crystals.
Trichomes Hairs unicellular or multicellular, uniseriate or stellate, or absent.
Leaves Alternate (spiral or distichous), simple, entire, often differentiated into pseudopetiole and pseudolamina, with involute or supervolute ptyxis. Stipules as axillary scales inside leaf sheath (primary leaves in some genera with lateral stipules); leaf sheath well developed; intravaginal scales absent? Venation parallelodromous; mid-vein prominent; usually with transveral veins between primary veins; secondary and tertiary venation reticulate; inverted vascular bundles sometimes present. Stomata usually paracytic, with parallel cell divisions of subsidiary cells (rarely tetracytic). Cuticular wax absent. Mesophyll with prismatic calciumoxalate crystals or druses. Schizogenous secretory cavities with latex. Leaf margin entire (sometimes undulating). Heterophylly frequently occurring. Abaxial surface with an apical subepidermal pore. Extrafloral nectaries present or absent.
Inflorescence Usually terminal or axillary, paniculate, whorled, spike- or umbel-like, with one verticil (flowers sometimes solitary axillary). Sometimes with involucre-like bracts.
Flowers Actinomorphic. Hypogyny. Tepals 3+3, free; outer tepals sepaloid, with valvate aestivation, sometimes persistent; inner tepals petaloid, thin, crumpled in bud (in Burnatia reduced or absent), caducous. Gynoecial-septal nectaries present on lateral walls of carpel bases (in Echinodorus also at tepal bases or stamens; in Sagittaria also on staminodia and filaments of fertile stamens; nectar in Limnocharis and allied genera secreted on entire surface of ovaries). Disc absent.
Androecium Stamens three, six (paired, antesepalous, alternipetalous), 6+3 or numerous (18 to c. 100), whorled (often seemingly spiral). Filaments simple, free from each other and from tepals. Anthers basifixed or subbasifixed, non-versatile, tetrasporangiate, usually extrorse (in Limnochariteae and allied genera usually latrorse), longicidal (dehiscing by longitudinal slits). Tapetum amoeboid-periplasmodial, with uninucleate cells. Outer staminal whorl in, e.g., some species of Hydrocleys and Limnocharis transformed into extrastaminal staminodia.
Pollen grains Microsporogenesis successive. Pollen grains 9–30-pantoporate (rarely di- or triporate or inaperturate), shed as monads, tricellular at dispersal. Exine tectate, with columellate infratectum, foraminate, usually spinulate (in, e.g., Limnocharis and Alisma granulate). Pollen grains often starchy. Excess pollen in one carpel grow out of this carpel through flower base and into another carpel (Endress 2011).
Gynoecium Carpels six, nine (to 20, rarely 20 to c. 100), in a single whorl or seemingly spiral, ab initio antesepalous (sometimes on gynoecial bulges), free (secondary apocarpy) or connate at base; carpel plicate and ascidiate. Ovaries superior, unilocular. Styluli terminal, lateral, gynobasic or absent. Stigmas usually linear (in Limnocharis capitate, in Butomopsis and Hydrocleys hippocrepomorphic), papillate, Dry type. Pistillodia usually absent (male flowers sometimes with pistillodia).
Ovules Placentation in Limnocharis, Butomopsis and Hydrocleys laminar-diffuse (lateral, ovules scattered over carpellary surface), in Alismateae usually basal (in Damasonium marginal). Ovules usually one (sometimes two; in Damasonium two to ten), in Limnocharis, Butomopsis and Hydrocleys numerous per carpel, usually anatropous (rarely campylotropous or amphitropous), ascending, apotropous, bitegmic, tenuinucellar or pseudocrassinucellar. Micropyle usually endostomal. Outer integument two or three cell layers thick. Inner integument approx. two cell layers thick. Parietal tissue often not formed. Nucellar cap formed by periclinal cell divisions of megasporangial epidermis. Megagametophyte usually tetrasporic, quadrinucleate, Allium type (sometimes monosporic, quadrinucleate). Synergids usually with a filiform apparatus. Endosperm development ab initio helobial (Echinodorus, Limnophyton, Sagittaria, Limnocharis, Butomopsis and Hydrocleys) or nuclear (Alisma, Damasonium, Luronium). Endosperm haustoria? Embryogenesis caryophyllad.
Fruit Usually an assemblage of achenes (in Limnocharis, Butomopsis, Hydrocleys and Damasonium polyspermum follicles), often with persistent style and sometimes persistent outer tepals.
Seeds Aril absent. Seeds U-shaped. Seed coat testal(-tegmic). Exotesta with thickened epidermal cell walls. Testal cells in Limnocharis thin-walled, with upright ends (in Butomopsis and Hydrocleys with glandular hairs). Tegmen more or less reduced or with thickened cell walls. Perisperm? starchy. Endosperm absent. Basal suspensor cell enlarged. Embryo hippocrepomorphic, without chlorophyll. Cotyledon one. Cotyledon hyperphyll elongate, assimilating. Hypocotyl internode present (in Baldellia long). Coleoptile absent. Germination phanerocotylar.
Cytology x = 5–13 (x = 11) – Chromosomes 2,4–14,4 μm in length (in Limnocharis, Butomopsis and Hydrocleys 2,6–13,6 μm).
DNA
Phytochemistry Flavonols (kaempferol, quercetin), flavone-C-glycosides (Sagittaria), flavone sulphates, cyanidin, tannins (Echinodorus, Luronium, Wiesneria), phenolic sulphates, and alkaloids present. Proanthocyanidins usually absent. Ellagic acid and saponins not found. Rhizome containing starch and sugar.
Use Ornamental plants, aquarium plants, vegetables (corms and roots of Sagittaria, leaves of Limnocharis), forage plants.
Systematics Alismataceae are sister-group to [Butomaceae+Hydrocharitaceae]. A comprehensive molecular phylogenetical investigation of Alismataceae is needed.
Alismateae Dumort., Fl. Belg.: 135. 1827 [‘Alismeae’]
4/19. Luronium (1; L. natans; western Europe to Scandinavia), Damasonium (6; D. alisma, D. bourgaei, D. californicum, D. constrictum, D. minus, D. polyspermum; West and Central Europe, the Mediterranean, Russia, western and Central Asia, India, North Africa, southeastern Australia, Tasmania, western United States), Baldellia (3; B. alpestris: northern Iberian Peninsula; B. ranunculoides: the Azores, Europe, the Mediterranean to Turkey, Morocco; B. repens: the Canary Islands, Europe, Algeria), Alisma (9; temperate regions on the Northern Hemisphere). – Temperate regions on the Northern Hemisphere. – Baldellia is sister to [Alisma+Luronium] in a study of Baldellia phylogeny by Arrigo & al. (2011).
Limnochariteae Pichon in Notul. Syst. 12: 183. Feb 1946
14/c 85. Burnatia (1; B. enneandra; tropical to southern Africa), Limnocharis (2; L. flava, L. laforestii; Mexico, Central America, the West Indies, tropical South America), Butomopsis (1; B. latifolia; tropical Africa, South Asia to northern Australia), Hydrocleys (5; H. martii, H. mattogrossensis, H. modesta, H. nymphoides, H. parviflora; Central America, tropical South America); Albidella (1; A. nymphaeifolia; tropical Africa, coastal areas along the Indian Ocean to tropical Australia, Yucatán and Cuba), Ranalisma (2; R. humile: tropical Africa; R. rostrata: India, Southeast Asia, southern China), Helanthium (3; H. bolivianum, H. tenellum, H. zombiense; eastern United States, southern Mexico, Central America, the West Indies, tropical South America), Caldesia (3; C. grandis, C. oligococca, C. parnassifolia; central, southern and eastern Europe, Africa, Asia to tropical Australia), Echinodorus (28; southern United States to tropical South America), Albidella (1; A. nymphaeifolia; Mexico, Honduras, Cuba), Sagittaria (c 30; America, few species in Europe, Africa and Asia), Wiesneria (3; W. schweinfurthii: tropical Africa; W. filifolia: Madagascar; W. triandra: southern India), Limnophyton (3; L. angolense, L. fluitans, L. obtusifolium; tropical Africa, Madagascar, South and Southeast Asia, Malesia), Astonia (1; A. australiensis; northeastern Queensland). – Pantropical, Eurasia, eastern and southern North America.
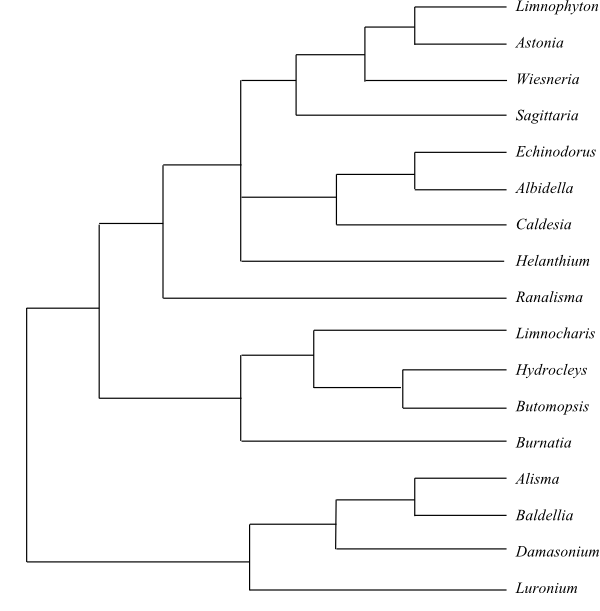 |
Phylogeny (Bayesian tree, simplified) of Alismataceae based on DNA data (Chen & al. 2012; Butomopis added according to Chen & al. 2013). Burnatia is sister-group to [Luronium+[Damasonium+[Baldellia+Alisma]]] and Albidella sister to the majority of Limnochariteae, according to Lehtonen (2017). |
APONOGETONACEAE Planch. |
( Back to Alismatales ) |
Aponogetonales Hutch., Fam. Fl. Pl., Monocot.: 10, 43. 20 Jul 1934
Genera/species 1/50–55
Distribution Tropical and subtropical regions of the Old World, with their largest diversity in southern Africa and Madagascar.
Fossils Fossil leaves similar to Aponogeton have been found in Late Oligocene layers in Central Asia.
Habit Usually monoecious (rarely dioecious or bisexual?), perennial herbs with corm or rhizome. Aquatic.
Vegetative anatomy Phellogen absent. Apical stem meristem usually bipartite. Secondary lateral growth absent. Vessels usually absent (rarely present in roots, with scalariform perforation plates and ? lateral pits). Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial parenchyma? Sieve tube plastids P2c type, with cuneate protein crystalloid bodies, without starch or protein filaments. Nodes? Articulated laticifers containing tannins or oils. Calciumoxalate crystals present in rhomboedrical aggregations.
Trichomes Hairs absent.
Leaves Alternate (spiral), simple, often differentiated into pseudopetiole and pseudolamina, entire, with involute or supervolute ptyxis. Stipules absent; leaf sheath well developed, with axillary intravaginal scales, squamulae intravaginales. Venation parallelodromous, with transversal veins connecting primary veins; tertiary veins absent (leaves in Aponogeton madagascariensis reticulate, fenestrate, due to absence of mesophyll between veins). Stomata paracytic. Cuticular wax absent. Mesophyll cells in floating leaves with rhomboedrical accumulations of calciumoxalate crystals. Leaf margin entire. Old pseudolamina with apical pore. Heterophylly often present.
Inflorescence Terminal, with flowers usually spirally arranged in one to 15 spike-like, simple or branched, inflorescences above water level (in Aponogeton ranunculiflorus reduced into few-flowered pseudanthia); each inflorescence as young surrounded by a usually caducous spathe-like bract. Peduncle (scape) with articulated laticifers. Floral bracts absent.
Flowers More or less zygomorphic, small. Hypogyny. Tepals usually two (abaxial lateral, corresponding to inner? tepals; rarely one tepal [one tepal possibly adnate to bract]; in Aponogeton hexatepalus six tepals), petaloid, usually persistent (rarely caducous), free (absent in female flowers of dioecious species). Gynoecial-septal nectaries usually present on lateral walls of carpel bases. Disc absent.
Androecium Stamens usually 3+3 (in Aponogeton distachyos eight to 16 in several whorls, sometimes paired). Filaments free from each other and from tepals. Anthers basifixed, non-versatile, tetrasporangiate, usually extrorse (sometimes introrse), longicidal (dehiscing by longitudinal slits). Tapetum amoeboid-periplasmodial, with ab initio uninucleate cells. Female flowers in monoecious species with staminodia.
Pollen grains Microsporogenesis successive or simultaneous. Pollen grains monosulcate, shed as monads, usually tricellular (sometimes bicellular) at dispersal. Exine semitectate, with columellate infratectum, perreticulate, beset with supratectal spinules.
Gynoecium Carpels (two or) three (to nine), alternitepalous, free or connate at base; odd carpel anterior (carpella anterioris). Ovary superior, unilocular (apocarpy). Stylulus short. Stigma adaxially decurrent, papillate, Dry type. Male flowers with pistillodia.
Ovules Placentation basal or marginal. Ovules (one or) two to twelve per carpel, anatropous to hemianatropous, ascending, usually bitegmic (in Aponogeton distachyos unitegmic), crassinucellar. Micropyle ?-stomal. Outer integument usually three cell layers thick. Inner integument two cell layers thick. Parietal cell formed from archesporial cell. Nucellar cap formed by periclinal divisions of megasporangial epidermal cells. Megagametophyte monosporous, Polygonum type. Antipodal cells not proliferating. Endosperm development helobial. Endosperm haustoria? Embryogenesis caryophyllad (sometimes Sagittaria type).
Fruit A multifolliculus with almost free (basally connate) individual follicles.
Seeds Aril absent. Seed coat testal, mucilaginous. Exotesta thick or thin. Mesotesta often parenchymatous. Endotesta present or absent. Exotegmen collapsing. Endotegmen tanniniferous, or thin and undifferentiated. Perisperm not developed. Starch present. Endosperm absent. Suspensor absent. Embryo straight, with or without chlorophyll. Cotyledon one, large, bifacial. Cotyledon hyperphyll elongate, assimilating. Hypocotyl internode short. Coleoptile absent. Germination phanerocotylar.
Cytology n = 8, 12, 16, 20, 28 – Polyploidy and aneuploidy frequently occurring. Agamospermy occurring. Chromosomes 0,7–2,3 μm long.
DNA
Phytochemistry Flavonols (kaempferol, quercetin), cyanidin, tannins (in isolated leaf cells), polyphenolic glycosides with caffeic acid, proanthocyanidins, and chlorogenic acid present. Flavone-C-glycosides, ellagic acid, alkaloids, saponins, cyanogenic compounds, and cell wall bound ferulate not found.
Use Ornamental plants, aquarium plants, vegetables.
Systematics Aponogeton (50–55; Africa south to the Cape Provinces, Madagascar, India to Malesia and northern Australia).
Aponogetonaceae are usually sister-group to the clade [Juncaginaceae+[[Posidoniaceae+ [Ruppia+Cymodoceaceae]]+[Maundia+[Zosteraceae+Potamogetonaceae]]]], although Apono-geton has sometimes been recovered as sister to Scheuchzeria (Kato & al. 2003).
ARACEAE Juss. |
( Back to Alismatales ) |
Arales Juss. ex Bercht. et J. Presl, Přir. Rostlin: 263. Jan-Apr 1820 [‘Aroideae’]; Lemnaceae Gray, Nat. Arr. Brit. Pl. 2: 729. 10 Jan 1822 [‘Lemnadeae’], nom. cons.; Pistiaceae Rich. ex C. Agardh, Aphor. Bot. (9): 130. 19 Jun 1822; Lemnales Link, Handbuch 1: 289. 4-11 Jul 1829 [‘Lemnaceae’]; Aropsida Bartl., Ord. Nat. Pl.: 25, 65. Sep 1830 [’Aroideae’]; Callaceae Reichb. ex Bartl., Ord. Nat. Plant.: 25, 66. Sep 1830; Orontiaceae Bartl., Ord. Nat. Plant.: 24, 68. Sep 1830; Pistiales Rich. in C. F. P. von Martius, Consp. Regn. Veg.: 5. Sep-Oct 1835 [’Pistiaceae’]; Arisaraceae Raf., Fl. Tellur. 4: 16. med 1838 [’Arisaria’]; Pothaceae Raf., Fl. Tellur. 4: 16. med 1838 [‘Pothidia’]; Orontiales J. Presl in Nowočeská Bibl. [Wšobecný Rostl.] 7: 1653 ['1553']. 1846 [‘Orontiaceae’]; Cryptocorynaceae J. Agardh, Theoria Syst. Plant.: 32. Apr-Sep 1858 [‘Cryptocoryneae’]; Caladiaceae Salisb., Gen. Plant.: 5. 15-31 Mai 1866 [‘Caladeae’]; Dracontiaceae Salisb., Gen. Plant.: 7. 15-31 Mai 1866 [‘Draconteae’]; Colocasiaceae Vines, Stud. Text-book Bot. 2: 541. Mar 1895 [’Colocasioideae’]; Lasiaceae Vines, Stud. Text-book Bot. 2: 540. Mar 1895 [‘Lasioideae’]; Monsteraceae Vines, Stud. Text-book Bot. 2: 540. Mar 1895 [‘Monsteroides’]; Philodendraceae Vines, Stud. Text-book Bot. 2: 540. Mar 1895 [‘Philodendroideae’]; Wolffiaceae (Gray) Bubani, Fl. Pyren. 4: 22. 15-28 Feb 1902; Aranae Thorne ex Reveal in Novon 2: 235. 13 Oct 1992; Aridae Takht., Divers. Classif. Fl. Pl.: 579. 24 Apr 1997
Genera/species 117/3.440–3.475
Distribution Mainly tropical but also subtropical regions, and approx. ten genera in temperate regions in the Northern Hemisphere.
Fossils The fossil record of Cretaceous and Cenozoic Araceae is very rich, with a peak during the Paleocene and Eocene (e.g. with the Proxapertites pollen type). Fossils of Orontium have been found in Late Cretaceous and Eocene layers in North America. Fossil stems, leaves, stamens and pollen of Limnobiophyllum have been found in Late Cretaceous to Miocene layers of Europe, Israel, North America and East Asia. Limnobiophyllum had stolons and rosettes of floating leaves or foliaceous branches. This genus has often been interpreted as intermediary between Lemnoideae and other Araceae and is placed in cladistic analyses as sister to Lemnoideae. Fossil pollen grains of Pothooideae-Monstereae are known from the Late Barremian to the Early Aptian of Portugal. These include the Early Cretaceous Mayoa portugallica, if correctly interpreted as a fossil pollen, inaperturate, with striate exine and granular infratectum, similar to Holochlamys in Monsteroideae. Pennipollis may possibly belong here. The Proxapertites pollen grains have a tectate exine, an acolumellate infratectum and an aperture encircling the equator. The infructescence Albertarum pueri from the Campanian of Alberta has been assigned to Orontioideae, and leaves resembling Orontioideae were described from the Early Campanian to Maastrichtian of Europe and North America. Leaves of Cobbania have been found in the Campanian to the Maastrichtian of Asia and North America. Fossil flowers, fruits and seeds occur in Cretaceous and Cenozoic layers of North America and Europe, and Neogene seeds of Araceae (e.g. Pistia) are frequent in European and Siberian lignites. Lasioideaecidites, from the latest Campanian to the earliest Maastrichtian Vilui Basin sediments in Siberia, was assigned to Lasioideae.
Habit Bisexual, monoecious (often with female flowers in lower part of inflorescence and male flowers in middle and upper parts), andromonoecious, gynomonoecious, polygamomonoecious, dioecious, or gynodioecious, usually perennial (rarely annual) herbs (some are giant herbs, whereas others are extremely small [e.g. Wolffia]), often epiphytic or climbing with stem or roots (rarely suffrutices). In the aquatic Lemnoideae, leaves are absent and the stem is reduced to a usually flat thalloid floating structure with or without roots on the lower side. Some genera have a starchy stem tuber (e.g. in Lasioideae and Aroideae). Many representatives are aquatic (e.g. Pistia) or hygrophilous. Turions are formed in, e.g., Spirodela, Wolffia and some species of Lemna.
Vegetative anatomy Roots with mycorrhiza. Water-absorbing velamen, formed from epidermis, present in some representatives (e.g. Anthurium). Root hypodermis dimorphic. Phellogen absent. Secondary lateral growth absent? Vessels present in roots in many species (in some species also in stems). Vessel elements with scalariform perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial parenchyma? Sieve tube plastids usually P2c, P2cs or P2cfs type (in Pistia Ss type). Nodes multilacunar with several leaf traces. Laticifers (simple or branched, sometimes anastomosing) usually present; latex white or colour-less, often tanniniferous. Schizogenous canals and cavities with resins and oils present in some genera. Trichosclereids abundant in Spathiphylleae and Monstereae. Calciumoxalate as ‘twinned’ raphides (H-shaped in cross-section), druses, prismatic crystals, etc. Silica bodies absent.
Trichomes Hairs usually absent (rarely unicellular or multicellular, lepidote, or as prickles; in Pistia ‘jointed’ uniseriate hairs).
Leaves Alternate (spiral), compound (rarely pinnately compound) or simple, entire or lobed (sometimes fenestrate or perforate; rarely peltate), usually differentiated into pseudopetiole and pseudolamina, or absent (in Lemnoideae), with supervolute ptyxis. Stipules absent; leaf sheath usually well developed (some genera with axillary intravaginal hair-like scales/colleters, squamulae intravaginales). Pseudopetiole often with distal pulvinus, geniculum. Vascular bundles of pseudopetiole scattered. Venation usually pinnate or palmate (sometimes pedate, arcuate from leaf base, curvipalmate, parallelodromous, etc.; only in Gymnostachys distinctly parallel), acrodromous, campylodromous etc.; mid-vein compound; fine venation reticulate to parallel-pinnate (parallel to primary lateral veins). Stomata usually paracytic (sometimes anomocytic, cyclocytic, or tetracytic); adjacent cells usually dividing. Cuticular wax crystalloids as non-orientated platelets, threads or tubuli, or absent. Mesophyll often with mucilage cells containing calciumoxalate raphides. Leaf margin usually entire. Extrafloral nectaries present in some species.
Inflorescence Usually a terminal, thick fleshy cymose spadix (in Lemnoideae reduced to one or two flowers) usually with spiral (rarely whorled) flowers subtended by a usually showy bract, spathe, often with odorous appendage (in Gymnostachys, Orontium and Lemnoideae very small or strongly reduced). Floral prophylls (bracteoles) and bracts absent.
Flowers Actinomorphic to asymmetrical, small (in Lemnoideae extremely small and strongly reduced). Hypogyny. Tepals 2+2 or 3+3 (when dimerous then outer tepals lateral; when trimerous then median tepal in outer whorl adaxial; rarely 4+4 or 6+6), sepaloid, scale-like, free or entirely or partially connate, or absent. Septal nectaries absent (perigonal nectaries present in Anthurium; staminodial nectaries in Aglaonema). Disc absent.
Androecium Stamens one, 2+2 or 3+3 (rarely 4+4 or 6+6), antetepalous. Filaments short, free or connate into a synandrium, free from tepals. Anthers usually basifixed (sometimes ventrifixed), non-versatile, sometimes connate into a synandrium, usually tetrasporangiate (sometimes disporangiate), usually extrorse (rarely latrorse or introrse), longicidal (dehiscing by longitudinal slits) or poricidal (dehiscing by apical or subapical pores, or short transversal slits); connective often expanded. Tapetum amoeboid-periplasmodial, with usually uninucleate (some species with binucleate) cells. Female flowers sometimes with staminodia.
Pollen grains Microsporogenesis usually successive (rarely simultaneous). Pollen grains sulcate, meridionosulcate (zonate), disulcate (diaperturate), forate (periporate), or inaperturate (omniaperturate), usually shed as monads (rarely tetrads), bicellular or tricellular at dispersal. Exine tectate or semitectate, with columellate or granular infratectum, or intectate, pertectate, perforate, reticulate or foveolate, smooth, psilate, scabrate, spinulate, or fossulate (rarely gemmate, verrucate, areolate, regulate, or striate); endexine spongy. Pollen grains usually starchy.
Gynoecium Pistil composed of (one to) three (to 15) usually congenitally fused (rarely free) carpels; carpel ascidiate to intermediate, eusyncarpous, seemingly occluded by secretion. Ovary superior, usually unilocular to trilocular (in Spathicarpeae unilocular to octolocular; in Philodendron bilocular to 47-locular), usually with a mucilaginous hair on inner side. Style single, usually very small, or absent. Stigma capitate or lobate, non-papillate, Dry or Wet type. Male flowers sometimes with pistillodium.
Ovules Placentation axile to parietal, basal to apical. Ovules one or few to numerous per carpel, anatropous, hemianatropous, orthotropous, amphitropous, anacampylotropous or intermediate, pendulous, horizontal or ascending, usually bitegmic (in Gymnostachys unitegmic), usually tenuinucellar or pseudocrassinucellar (in, e.g., Calla and Symplocarpus crassinucellar). Micropyle bistomal or exostomal. Outer integument (two to) eight to 26 cell layers thick (at base). Inner integument two to ten cell layers thick. Parietal cell often formed from archesporial cell. Nucellar cap, usually two or three cell layers thick, formed by periclinal cell divisions of megasporangial epidermis. Megagametophyte usually monosporous, Polygonum type (in Nephthytis 10- to 12-nucleate; in Lemna, Wolffia and Wolffiella disporous, Allium or Scilla type). Antipodal cells sometimes proliferating. Hypostase present or absent. Endosperm development usually ab initio helobial or nuclear; first cell division usually very asymmetrical; subsequent initial divisions often entirely in micropylar chamber. Endosperm appendage (’endosperm haustorium’, ’basal apparatus’) chalazal. Embryogenesis onagrad, asterad, caryophyllad, or solanad.
Fruit Usually a juicy berry (sometimes dry or leathery; in Lagenandra dehiscing at base; in some genera fused into an indehiscent or dehiscent syncarp), often with mucilaginous envelope and sometimes partially mucilaginous pericarp; in Lemnoideae a nutlike fruit.
Seeds Aril absent. Testa thick or thin, multiplicative, often parenchymatous (absent in Gymnostachys, Nephthytis and Orontium). Operculum present. Exotesta often fleshy and viscid. Mesotestal and endotestal cells usually thick-walled. Tegmen unspecialized, often collapsing. Perisperm rarely present (in, e.g., Pistia). Endosperm copious, sparse or absent, starchy or with aleurone and lipids. Embryo large to small (often macropodous), straight or curved, with or without starch, usually little differentiated, usually with chlorophyll. Cotyledon one, not photosynthesizing. Cotyledon hyperphyll assimilating or transformed into haustorium or nutrient-storing organ. Hypocotyl internode usually present (sometimes as a swollen nutrient-storing organ). Coleoptile absent. Collar rhizoids or collar roots sometimes present. Radicula absent in some genera. Germination phanerocotylar.
Cytology n = 7–84 – Polyploidy and aneuploidy frequent in some genera.
DNA Mitochondrial coxI intron present in numerous genera.
Phytochemistry Flavonols (kaempferol, quercetin), O-flavones, flavone-C-glycosides, cyanidin, ethereal oils (in, e.g., Homalomena), cinnamic acid, alkaloids (including benzylisoquinoline alkaloids), cyanogenic glycosides (triglochinin etc.), steroidal saponins, tyrosine-dervided cyanogenic substances, amines, and bitter substances present as well as several irritating or allergenic substances (e.g. protocatechinic aldehyde, homogentisic acid and their glycosides, and 5-alkylic- and 5-alkenylic resorcinols). Ellagic acid and chelidonic acid not found.
Use Ornamental plants, aquarium plants (Cryptocoryne, Pistia etc.), starch sources and vegetables (Colocasia esculenta, Xanthosoma sagittifolium, Amorphophallus paeoniifolius), fruits (Monstera deliciosa), gels in food-stuffs (A. konjac), medicinal plants, arrow poisons, fibres for basketry, animal forage (Lemnoideae).
Systematics Araceae are sister-group to the remaining Alismatales. The clade [Gymnostachys+Orontioideae] is sister to all other Araceae and characterized by, e.g., continuous tectum, orthotropous ovule, an inner integument three or four cell layers thick and multiplicative, and a seedling possessing cataphylls.
[Gymnostachys+Orontioideae]
Gymnostachydoideae Bogner et Nicolson in Willdenowia 21: 37. 11 Dec 1991
1/1. Gymnostachys (1; G. anceps; eastern Queensland, eastern New South Wales). – Vascular bundles with fibre envelopes and girders. Leaves distichous, linear, without pseudopetiole. Venation parallelodromous. Stomata parallel to foliar axis. Leaf margin finely serrate. Inflorescence complex, branched. Spatha absent. Flowers dimerous. Tepals four. Stamens four. Anther thecae running out in a tip above the slit. Pistil composed of a single ascidiate carpel. Ovary unilocular, with non-secreting? locule. Stigma Dry type. Placentation apical. Ovule one per carpel, orthotropous, unitegmic. Micropyle absent. Testa absent. Endosperm copious, starchy, with chlorophyll. Embryo with chlorophyll. Collar rhizoids absent. n = 12. – Gymnostachys is sister-group to the remaining Araceae, according to some molecular analyses. On the other hand, Cabrera & al. (2008) identified Gymnostachys as sister to Orontioideae.
Orontioideae R. Br. ex Müll. Berol. in Ann. Bot. Syst. 5: 898. 1860 [‘Orontiaceae’]
3/8. Orontium (1; O. aquaticum; southeastern Canada, eastern United States), Lysichiton (2; L. camtschatcensis: Kamchatka, Sakhalin, the Kuriles; L. americanus: western North America), Symplocarpus (5; S. egorovii, S. nabekuraensis, S. nipponicus, S. renifolius: northeastern Asia; S. foetidus: southeastern Canada, northeastern United States). – Temperate East Asia, western and eastern North America. Collenchyma sometimes arranged in cortical bands. Vascular bundles often with fibre strands. Simple laticifers present in Orontium. Sieve tube plastids without starch. Leaves spiral, elliptic. Spatha absent in Orontium. Tepals usually 2+2 (in Orontium usually 3+3). Usually epigyny (in Orontium hypogyny). Ovary unilocular or bilocular. Placentation basal or subbasal. Ovule one or two per carpel, usually orthotropous (in Orontium hemianatropous), crassinucellar. Outer integument 22 or more cell layers thick (integument in Symplocarpus lobate). Inner integument five to ten cell layers thick. Parietal tissue usually present. Endosperm absent or rudimentary. n = 13–15. Flavonols present. Flavone glycosides absent. – Orontium is sister to [Lysichiton+Symplocarpus].
[Lemnoideae+[[Pothoideae+Monsteroideae]+[Lasioideae+[Zamioculcadoideae s.lat.+Aroideae]]]]
Megasporangial tissue disappearing during maturation of the ovule. Endothelium present. (Steven 2001 onward)
Lemnoideae Bab., Man. Brit. Bot.: 320. Mai-Jul 1843 [‘Lemneae’]
5/37. Spirodela (3; S. oligorrhiza, S. polyrrhiza, S. sichuanensis; cosmopolitan), Landoltia (1; L. punctata; southern Asia, Australia), Lemna (12; cosmopolitan), Wolffia (11; nearly cosmopolitan; paraphyletic; incl. Wolffiella?), Wolffiella (10; tropical and southern Africa, southern Asia, southeastern United States to tropical South America). – Mainly temperate and subtropical regions on the Northern Hemisphere, the Andes, Argentina, eastern and southern Africa, Madagascar, South Asia, Australia, Tasmania, New Zealand. Usually monoecious (rarely dioecious). Annual herbs. Floating, aquatic herbs with usually thalloid stems. Roots single, several or absent. Collenchyma and bundle fibres absent. Vessels absent. Venation only as primary veins. Mucilage cells present. Leaves absent. Prophyll present or absent. Spathe extremely reduced, present at side of leaf sheath, or absent. Spadix extremely reduced. Tepals absent. Stamen single, disporangiate (Wolffia and Wolffiella) or tetrasporangiate. Anther dehiscing by transversal or longitudinal slits or pores (sometimes apically). Pollen grains tricellular at dispersal. Exine ulcerate, spinulate. Pistil composed of a single carpel. Stigma infundibuliform. Placentation basal. Ovules one to seven per carpel, orthotropous, anatropous or hemianatropous, ascending, bitegmic, crassinucellar. Micropyle bistomal or endostomal. Inner integument two or three cell layers thick. Parietal tissue one (or two) cell layer thick. Nucellar cap sometimes two cell layers thick. Megagametophyte Polygonum or Allium type. Endosperm development probably cellular. Embryogenesis onagrad or irregular. Fruit an achene. Seed operculate. Endosperm starchy. Embryo undifferentiated. Hypocotyl and radicula absent. Cotyledon sheath wide, photosynthesizing. x = 10. Polyploidy and dysploidy frequently occurring. Flavonols, proanthocyanidins, alkaloids, and cyanogenic substances absent. – Lemnoideae are sister-group to the remaining Araceae (except Gymnostachys and Orontioideae). The thallus is usually interpreted as a combination of a reduced stem and a reduced leaf. The reproductive organ is interpreted as a small bisexual flower or a much reduced inflorescence with a single female flower and one or two male flowers. Spirodela is sister to the clade [Lemna+[Landoltia+[Wolffia+Wolffiella]]]. Wolffia and Wolffiella lack roots and veins on the thallus.
[[Pothoideae+Monsteroideae]+[Lasioideae+[Zamioculcadoideae s.lat.+Aroideae]]]
Araceae sensu stricto
108/3.395–3.430. According to Stevens (2001 onwards) characterized by, e.g., shoot consisting of a reiterated sympodial unit (continuation shoot); branching from the axil of a penultimate foliar organ outside the spathe; pseudopetiole distinctly differentiated from the sheathing base; spathe large and often showy; presence of peduncle; and non-multiplicative inner integument. The collenchyma is of the Type B. Seedling cataphylls may be present or absent. – The Bisexual Climbers clade is sister-group to the remainder.
The Bisexual Climbers clade (sensu Cabrera & al. 2008)
16/>1.420. Stems usually hemiepiphytic, aerial, climbing (plants sometimes terrestrial, rheophytic or helophytic). Vascular bundles often surrounded by fibres. Styloids present. H- or T-shaped trichosclereids usually present. Leaves spiral or distichous. Pseudopetiole distally geniculate. Embryo often surrounded by crystals. Separate cortical vascular bundles may occur, as well as vessels in the stem (Steven 2001 onwards). – Pothoideae are sister to Monsteroideae.
[Pothoideae+Monsteroideae]
Pothoideae Engl. in Nova Acta Acad. Caes. Leop.-Carol. German. Nat. Cur. 39: 140. 1876
4/>1.060. Anthuriumclade Anthurium (>1.000; Mexico, Central America, the West Indies, tropical South America). – Potheae Bartl., Ord. Nat. Plant.: 67. Sep 1830 [‘Pothoina’]. Pothos (c 55; Madagascar, tropical Asia to eastern Queensland, northeastern New South Wales and Melanesia), Pedicellarum (1; P. paiei; Borneo), Pothoidium (1; P. lobbianum; the Philippines, Sulawesi, the Moluccas, Orchid Island in Taiwan). – Tropical America, Madagascar, tropical Asia, northeastern Australia, Melanesia. Spadix not enclosed by the spatha. Flowers dimerous or trimerous. Anther thecae often forming a tip above anther slit. Pollen grains in Anthurium porate. Placentation basal-parietal. Ovules one or two per carpel, anatropous?, apotropous. Outer integument six to eight cell layers thick, multiplicative. Inner integument at least sometimes five to seven cell layers thick. Spatha persistent in fruit. Endosperm sparse, starchy, or absent (in Anthurium copious). Embryo sometimes with chlorophyll. n = (10) 12 (14) (15). Seedling internode sometimes long. Unifacial part of cotyledon sometimes very short. Cataphyll absent in Anthurium. – Anthurium is sister to the remaining Pothoideae, Potheae, which are characterized by shoot monopodial, endosperm sparse or absent, and x = 12.
Monsteroideae Engl. in Nova Acta Acad. Caes. Leop.-Carol. German. Nat. Cur. 39: 142. 1876
12/c 360. Heteropsideae Engl., Nat. Pflanzenr. 21: 20. 13 Jun 1905. Stenospermation (c 50; southern Mexico, Central America, tropical South America), Heteropsis (17; tropical South America), Rhodospatha (c 30; Costa Rica, Panamá, tropical South America), Alloschemone (2; A. inopinata, A. occidentalis; Brazil, Bolivia). – Spathiphylleae Engl. in Engler et Prantl, Nat. Pflanzenfam. II, 3: 112, 121. Aug 1884. Spathiphyllum (c 40; Central Malesia to Solomon Islands, Florida, southern Mexico, Central America, tropical South America), Holochlamys (1; H. beccarii; New Guinea, New Britain). – Rhaphidophoreae Engl. in Nova Acta Acad. Caes. Leop.-Carol. German. Nat. Cur. 39: 143. 1876 [‘Raphidophoreae’]. ‘Rhaphidophora’ (c 100; tropical Africa, Madagascar, tropical Asia to islands in the Pacific; paraphyletic), Anadendrum (12; southern China, Southeast Asia, Malesia to Sulawesi and the Philippines), Scindapsus (c 35; tropical Asia from India to Hainan and Solomon Islands), Monstera (c 50; southern Mexico, Central America, the West Indies, tropical South America), Epipremnum (15–17; the Himalayas, southern China, Southeast Asia, Malesia to tropical Australia and islands in western Pacific), Amydrium (5; A. hainanense, A. humile, A. medium, A. sinense, A. zippelianum; southern China, Southeast Asia, Malesia to New Guinea). – Pantropical (few species in Africa). Separate stem cortical vascular tissue sometimes present. Vessels sometimes present in stem. Laticifers sometimes present. Spatha early caducous. Flowers usually dimerous (in Spathiphylleae trimerous). Tepals often absent. Pollen grains inaperturate, expanded-monosulcate or zonate. Style often with abundant trichosclereids. Placentation often basal. Ovules one to four (to numerous) per carpel, sometimes hemianatropous. Seed often surrounded by mucilage. Endosperm usually absent (present in Spathiphylleae). Embryo often surrounded by crystals. n = 12, 14, 15, 21. Polyploidy frequently occurring. –Heteropsideae are sister-group to the remaining Monsteroideae and have x = 14. Spathiphylleae are characterized by smaller trichosclereids in bundles, and pollen grains polyplicate-multiaperturate.
[Lasioideae+[Zamioculcadoideae s.lat.+Aroideae]]
The Podolasia clade
92/1.975–2.010. Leaves usually spirally arranged. – Lasioideae are sister to the remaining Podolasia clade.
Lasioideae Engl. in Nova Acta Acad. Caes. Leop.-Carol. German. Nat. Cur. 39: 144. 1876
10/49. Dracontium (24; Central America, the West Indies, tropical South America), Dracontioides (2; D. desciscens, D. salvianii; eastern Brazil), Anaphyllopsis (3; A. americanum, A. cururuana, A. pinnata; tropical South America), Pycnospatha (2; P. arietina, P. palmata; Southeast Asia), Anaphyllum (2; A. beddomei, A. wightii; Laccadive Islands), Cyrtosperma (12; New Guinea to islands in the Pacific), Lasiomorpha (1; L. senegalensis; tropical West and Central Africa), Podolasia (1; P. stipitata; West Malesia), Lasia (2; L. concinna, L. spinosa; India, Tibet, China, Taiwan, Southeast Asia, Malesia to New Guinea), Urospatha (11; Central America, tropical South America). – Tropical West and Central Africa, tropical Asia, Pacific islands, tropical America. Terrestrial or rooted aquatic (helophytic) plants with tuber or rhizome. Vascular bundles surrounded by fibres. Sieve tube plastids with sparse starch. Laticifers present. Pseudopetiole, abaxial surface of primary leaf veins, and peduncle very often prickly and warty. Pseudopetiole geniculate distally, with Type 4 ground tissue. Type 4 foliar spongy aerenchyma. Order of anthesis basipetal. Anther with oblique pore-like slits. Pollen grain without starch; sulcus unique among angiosperms, with ectexine lamella and thick two-layered endexine: outer layer flaky or lamellate and inner layer spongy. Pistil composed of one to three (to 16) connate carpels. Placentation of various types. Ovules one or two (to numerous) per locule, anacampylotropous, apotropous. Mesotestal and endotestal cell walls lignified. Endosperm thin or absent. Embryo curved, with chlorophyll. x = 13. – The phylogeny of Lasioideae is largely unresolved.
[Zamioculcadoideae s.lat.+Aroideae]
The Unisexual Flowers clade (sensu Cabrera & al. 2008)
82/1.925–1.960. Flowers usually unisexual (in Calla bisexual). Spatha differentiated into ‘tube’ and ‘lamina’. Spadix differentiated into zones with female flowers and male flowers, respectively. Pollen grains usually inaperturate (in Zamioculcas and Gonatopus aperturate). – This clade has weak support in molecular analyses, although the Stylochaeton clade [Stylochaeton+Zamioculcadoideae] may be sister to Aroideae.
The Stylochaeton clade (Zamioculcadoideae s.lat.)
3/26. Tropical, with the highest diversity in tropical Africa. Rhizomatous geophytes. Sterile flowers often present between female and male floral zones in inflorescence, developing in various ways. Tepals present. Stamens usually free (sometimes connate). Anthers introrse to extrorse (in Zamioculcas introrse). Pollen grains large, often with encircling sulcus. Columellae twisted, forming an ‘inner tectum’ together with outer tectum. Endexine lamellate. Intine thin. Pistil composed of two connate carpels. Placentation axile. Ovule one per carpel, ascending. Endosperm almost absent. Pistillodia present in male flowers. Ovules tenuinucellar. Micropyle bistomal. Nucellar cap and integumentary endothelium present. – Stylochaeton is sister to a clade consisting of Zamioculcas and Gonatopus.
Stylochaeton
1/20. Stylochaeton (20; central and eastern tropical Africa to Angola). – Biforines present. Leaves simple. Tepals connate. Pollen grains inaperturate. Sporopollenin ectexine very thin, undifferentiated. x = 14. – Stylochaeton resembles Zamioculcadoideae in its floral morphology. However, pollen and leaf morphology correspond well with main stream Araceae.
Zamioculcadoideae Bogner et Hesse in Aroideana 28: 13. Jul 2005
2/6. Zamioculcas (1; Z. zamiifolia; southeastern tropical Africa), Gonatopus (5; G. angustus, G. boivinii, G. clavatus, G. marattioides, G. petiolulatus; tropical East and southeastern Africa). – Kenya to northeastern South Africa. Stem condensed, thickened. Biforines absent. Leaves pinnately compound. Pseudopetiole geniculate. Pollen grains zonasulcate (extended-monosulcate). x = 17. –The pollen grains in Lasioideae, with a lamellate endexine, are relatively similar to those in Zamioculcadoideae.
Aroideae Arn., Botany: 136. 9 Mar 1832 [‘Arineae’], sensu lato
79/1.900–1.935. Tropical and subtropical regions on both hemispheres, temperate regions in Europe and eastern North America. Collenchyma usually in cortical bands or in bundle-associated strands, or absent. Laticifers usually present (simple and articulated or anastomosing; sometimes absent). Biforines often present; biforine walls thick, lignified, papillate at ends, with mucilaginous cell cytoplasm. Leaves spiral. Spatha differentiated into tube and lamina. Tepals absent. Filaments usually connate (rarely free). Anthers usually extrorse (sometimes introrse), longicidal (sometimes poricidal), with thick connectives. Pollen grains usually bicellular (sometimes tricellular) at dispersal. Exine with various sculpturing. Ectexine thin, usually without sporopollenin (consisting of polysaccharides). Endexine thick, spongy. Intine massive. Female flowers usually with staminodia. Stylodia usually free (sometimes connate). Male flowers usually with pistillodium. Placentation apical, basal or parietal. Ovules one to numerous per carpel, usually orthotropous, usually tenuinucellar (in Calla crassinucellar). Outer integument four to ten cell layers thick. Endosperm present, often starchy, or absent. Embryo sometimes chlorophyllous. Collar rhizoids sometimes present.
Callopsideae Engl. in Engler et Prantl, Nat. Pflanzenfam. Nachtr. 2: 29, 34. Apr 1906
1/1. Callopsis (1; C. volkensii; Tanzania). – Callopsis may be sister to the remaining Aroideae.
Anubiadeae Engl. in Nova Acta Acad. Caes. Leop.-Carol. German. Nat. Cur. 39: 147. 1876
1/8. Anubias (8; tropical West and Central Africa). – Aquatic or semi-aquatic herbs. Exine smooth. – Anubias may be successive sister to the remaining Aroideae except Callopsis.
Montrichardieae Engl. in Nova Acta Acad. Caes. Leop.-Carol. German. Nat. Cur. 39: 144. 1876
1/2. Montrichardia (2; M. arborescens, M. linifera; southern Mexico, Central America, the West Indies, tropical South America to Peru and Brazil). – Helophytic herbs. Exine smooth. – Montrichardia may be sister to the remaining Aroideae s.lat. except Callopsideae and Anubiadeae. A fossil species, M. aquatica, has been described from Paleocene layers in Colombia.
Aroideae sensu stricto
76/1.890–1.925. Tropical and warm-temperate. Usually monoecious (sometimes dioecious; in Calla bisexual). Leaves very variable. Pollen grains inaperturate. Cotyledons sometimes nutrient storing. – Zantedeschieae are sister-group to the remainder (Philonotieae).
Zantedeschieae Engl. in Engler et Prantl, Nat. Pflanzenfam. II, 3: 113, 136. Aug 1887
24/770–775. Exine often smooth. – The basal branching of Zantedeschieae is unresolved.
The Anchomanes clade
5/36. Aglaonemateae Engl. in Nova Acta Acad. Caes. Leop.-Carol. German. Nat. Cur. 39: 148. 1876. [‘Aglaonemeae’] Aglaonema (21; tropical Asia), Aglaodorum (1; A. griffithii; West Malesia). – Nephthytideae Engl. in Engler et Prantl, Nat. Pflanzenfam. II, 3: 112, 128. Aug 1887. Nephthytis (6; N. afzelii, N. bintuluensis, N. hallaei, N. mayombensis, N. poissonii, N. swalnei; tropical West and Central Africa, one species, N. bintuluensis, on Borneo), Pseudohydrosme (2; P. buettneri, P. gabunensis; Gabon), Anchomanes (6; A. abbreviatus, A. boehmii, A. dalzielii, A. difformis, A. giganteus, A. nigritianus; tropical Africa). – Tropical Africa, tropical Asia. Foliar collenchyma usually Type Sb. Spatha boat-shaped, early caducous or marcescent. Nephthytideae are characterized by very strongly developed basal foliar ribs, having dracontioid lobing.
Homalomeneae (Schott) M. Hotta in Mem. Fac. Sci. Kyoto Univ., ser. Biol., 4: 89. 1970
5/645–650. Culcasieae Engl. in Engler et Prantl, Nat. Pflanzenfam. II, 3: 112, 116. Aug 1887. Culcasia (27; tropical Africa), Cercestis (10; tropical West and Central Africa). – Philodendreae Schott, Syn. Aroid.: 71. Mar 1856. Philodendron (c 490; Florida, southern Mexico, Central America, the West Indies, tropical South America), Adelonema (16; tropical America), Homalomena (100–105; tropical Asia, tropical America). – Pantropical. Roots with sclerotic hypodermis and resin canals. Anthers usually without endothecial thickenings (present in Homalomena). – Culcasieae are hemiepiphytic climbing plants. Philodendreae are characterized by female-sterile-male spadix floral zonation. Thaumatophyllum (tropical South America; in Philodendron s.l.) is sometimes recovered as sister-group to Philodendron s.str. (Vasconcelos & al. 2018, Sakuragui & al. 2018).
The Zantedeschia clade sensu stricto
14/88–90. Zantedeschiaclade Zantedeschia (8; eastern tropical and southern Africa). – Spathicarpeae Schott, Syn. Aroid.: 214. Mar 1856. Lorenzia (1; L. umbrosa; Amapá in northern Brazil), Bognera (1; B. recondita; Brazil), Gearum (1; G. brasiliense; central Brazil), Dieffenbachia (c 55; southern Mexico, Central America, the West Indies, tropical South America), Mangonia (2; M. tweedieana, M. uruguaya; Brazil, Uruguay), Incarum (1; I. pavonii; Ecuador, Peru, Bolivia), Gorgonidium (8; Peru, Bolivia, northern Argentina), Spathantheum (2; S. fallax, S. orbignyanum; the Andes in Peru, Bolivia and northern Argentina), Synandrospadix (1; S. vermitoxicus; Bolivia, northern Argentina), Croatiella (1; C. integrifolium; Ecuador), Spathicarpa (3; S. gardneri, S. hastifolia, S. lanceolata; southern tropical South America), Taccarum (6; T. caudatum, T. crassispathum, T peregrinum, T. ulei, T. warmingii, T. weddellianum; tropical South America), Asterostigma (8; Brazil). – Tropical and southern Africa, tropical America. Spathicarpeae are characterized by connate stamens and x = 17.
Philonotieae S. Y. Wong et P. C. Boyce in S. Y. Wong et al., Taxon 59: 121. 5 Feb 2010
52/1.120–1.150. Calla is sometimes recovered as sister to the Dracunculus clade, although with weak support (Cusimano & al. 2011), and sometimes as sister to the clade [Rheophytes clade+Dracunculeae] (Henriquez & al. 2014).
Calleae Bartl., Ord. Nat. Plant.: 67. Sep 1830 [‘Callea’]
1/1. Calla
(C. palustris; temperate regions on the Northern Hemisphere). –
Rooted helophytic herb. Bisexual. Vascular bundles amphivasal. Simple
articulated laticifers present. Biforines absent. Leaves distichous. Leaf
sheath elongate, ligulate. Tepals absent. Pollen grains diporate. Pistil
composed of a single carpel. Placentation basal. Ovules six to nine per carpel,
crassinucellar. Endosperm present. Embryo with chlorophyll. Seedling roots with
chlorophyll. n = 18, 27. – The bisexual flowers are remarkable, yet may be
due to a reversal.
The Rheophytes clade
(sensu Cabrera & al. 2008)
15/255–265. Philonotion clade Philonotion (1; P. spruceanum; northernmost South America). – Cryptocoryneae Blume, Rumphia 1: 83. Jul-Nov 1836. Lagenandra (15; southern India, Sri Lanka, Assam, Bangladesh), Cryptocoryne (65; India and Sri Lanka to southern China and Southeast Asia, Malesia to New Guinea). – Schismatoglottideae Nakai, Chosakuronbun Mokuroku [Ord. Fam. Trib. Nov.]: 218. 20 Jul 1943. Piptospatha (12; peninsular Thailand, the Malay Peninsula, Borneo), Ooia (2; O. grabowskii, O. kinabaluensis; Borneo), Pichinia (1; P. disticha; Sarawak), Schottarum (2; S. josefii, S. sarikeensis; Kanowit-Song-Ai area in Sarawak), Phymatarum (1; P. borneense; Borneo), Schismatoglottis (100–110; southern China, Southeast Asia, Malesia to Solomon Islands and Vanuatu, Taiwan, with their highest diversity on Borneo), Aridarum (10; Borneo), Schottariella (1; S. mirifica; Borneo), Bucephalandra (30; Borneo), Bakoa (1; B. lucens; Sarawak), Apoballis (12; Thailand, West Malesia, Timor), Hestia (1; H. longifolia; Sarawak). – Northernmost South America, tropical Asia, with their highest diversity on Borneo. Mainly aquatics and rheophytes. Anthers with horned thecae. Exine often smooth. – The Rheophytes clade corresponds to Schismatoglottideae sensu lato, with the addition of Philonotion. Cryptocoryneae are characterized by absence of laticifers, foliar Type Sv collenchyma, presence of squamulae intravaginales, spatha and spadix forming two chambers by partial fusion, spatha with internal flap and connate margins, and n = 18. Schismatoglottideae have erect aerial stems, early caducous or marcescent upper pseudolamina, leaf sheath with long ligules at apex, and spatha with persistent basal half.
Dracunculeae Schott in Schott et Endlicher, Melet. Bot.: 16. 1832 [‘Dracunculinae’]
36/865–885. Foliar collenchyma Type Sv. Sympodial inframarginal vein usually present on each side of pseudolamina. Exine usually spinulate. Stamens often more or less connate into a synandrium. – Amorphophalleae are sister-group to the remaining Dracunculeae.
Amorphophalleae Engl. in Nova Acta Acad. Caes. Leop.-Carol. German. Nat. Cur. 39: 144. 1876
12/c 410. Thomsonieae Blume in Rumphia 1: 138. Apr-Jun 1837. Amorphophallus (c 200; tropical regions in the Old World). – Caladieae Schott in Schott et Endlicher, Melet. Bot.: 18. 1832. Jasarum (1; J. steyermarkii; Venezuela, Guyana), Hapaline (8; Burma to Borneo), Caladium (14; Costa Rica, Panamá, tropical South America), Syngonium (c 35; Florida, Mexico, Central America, the West Indies, tropical ), Filarum (1; F. manserichense; Peru), Ulearum (2; U. donburnsii, U. sagittatum; Amazonia in Brazil), Xanthosoma (c 75; Mexico, Central America, the West Indies, tropical South America), Chlorospatha (c 70; Costa Rica, Panamá, tropical South America, with the largest diversity in the northwestern Andes), Scaphispatha (1; S. gracilis; Bolivia), Zomicarpella (2; Z. amazonica: northwestern Brazil; Z. maculata: Colombia, Peru), Zomicarpa (2; Z. pythonium, Z. steigeriana; northeastern Brazil). – Pantropical. Thomsonieae have tripartite primary leaf development with decompound subdivision, involute ptyxis, often no sterile zone between the male and female zones, and presence of smooth or staminodial spadix appendix. Caladieae are characterized by anastomosing laticifers, persistent spathe tube, and early caducous or marcescent spathe lamina.
Ambrosineae Schott in Schott et Endlicher, Melet. Bot.: 16. 1832 [‘Ambrosinieae’]
24/455–475. The Colletogyne clade is sister to Pistieae.
The Colletogyne clade
7/19. Arisareae Dumort., Fl. Belg.: 162. 1827. Arisarum (3; A. proboscideum, A. simorrhinum, A. vulgare; Macaronesia except Cape Verde Islands, the Mediterranean to the Caucasus), Ambrosina (1; A. bassii; central Mediterranean). – Typhonodoreae Engl. in Nova Acta Acad. Caes. Leop.-Carol. German. Nat. Cur. 39: 146. 1876. Peltandrinae Schott, Syn. Aroid.: 50. Mar 1856. Peltandra (2; P. sagittifolia: southeastern United States; P. virginica: southeastern Canada, eastern and southeastern United States, Cuba), Typhonodorum (1; T. lindleyanum; coastal regions in East Africa, Madagascar, the Mascarene Islands). – Arophyteae A. Lemee ex Bogner in Bot. Jahrb. Syst. 92: 9. 1972. Arophyton (7; A. buchetii, A. crassifolium, A. humbertii, A. pedatum, A. rhizomatosum, A. simplex, A. tripartitum; Madagascar), Carlephyton (3; C. diegonse, C. glaucophyllum, C. madagascariense; northern Madagascar), Colletogyne (1; C. perrieri; Madagascar). – The Mediterranean to the Caucasus, Macaronesia, East Africa, Madagascar, the Mascarene Islands, North America. Arisareae are characterized by striate exine, connate spathe margin, and adnation of the spadix female zone to spathe. Typhonodoreae have staminodia in female zone, endosperm sparse to absent, embryo large, and cotyledon nutrient-storing. Peltandrinae are helophytes with parallel-pinnate fine venation, spathae with a persistent tube and caducous or marcescent lamina, female-sterile-male-sterile zonation of spadix, staminodial spadix appendices, and entirely connate stamens. Arophyteae, in Madagascar, have female-male zonation of spadix, connate stamens, globose pollen grains, and spinulate exine.
Pistieae Lecoq et Juill., Dict. Rais. Term. Bot.: 56, 493. 1831 [‘Pistiaceae’]
17/435–440. Protarumclade Protarum (1; P. sechellarum; the Seychelles). – Pistia clade Pistia (1; P. stratiotes; tropical East Africa?). – Colocasieae Brongn., Enum. Plant. Mus. Paris: 13. 12 Aug 1843. Ariopsis (2; A. peltata: Western Ghats, Sikkim, Assam; A. protanthera: Bhutan, Burma), Steudnera (8; the Himalayas, Southeast Asia to the Malay Peninsula), Remusatia (4; R. hookerana, R. pumila, R. vivipara, R. yunnanensis; tropical Africa, Madagascar, India, Sri Lanka, the Himalayas, Tibet, Burma, southern China, Southeast Asia, Malesia to tropical Australia, Taiwan and islands in the Pacific), Colocasia (10; the Himalayas, Tibet, Burma, southern China, Southeast Asia, the Nicobar Islands, West Malesia). – Englerarum (1; E. hypnosum; southwestern Yunnan, Laos, Thailand). – Alocasiinae Schott, Syn. Aroid.: 43. Mar 1856 [‘Alocasinae’]. Alocasia (c 80; the Hmalayas and southeastern China to Southeast Asia, Malesia to New Guinea and tropical Australia); Arisaema (180–185; eastern Africa, southern Arabian Peninsula, India, the Himalayas, Tibet, East and tropical Asia to New Guinea, southeastern Canada, eastern and southeastern United States, Mexico); Pinellia (9; China, the Korean Peninsula, Japan, the Ryukyu Islands). – Areae Theriophonum (7; T. dalzellii, T. danielii, T. fischeri, T. infaustum, T. manickamii, T. minutum, T. sivaganganum; central and southern India, Sri Lanka), Typhonium (c 70; Mongolia, China, tropical Asia to New Guinea and tropical Australia, Taiwan), Eminium (9; eastern Mediterranean to Iran, Afghanistan and Central Asia), Biarum (21; the Mediterranean from Morocco to Syria and Egypt), Arum (c 30; Europe, the Mediterranean to Iran and the Himalayas), Dracunculus (3; D. canariensis: the Canary Islands, Madeira; D. muscivorus: Corsica, Sardinia, the Balearic Islands; D. vulgaris: central and eastern Mediterranean). – Europe, Macaronesia, the Mediterranean, tropical Africa, Madagascar, the Arabian Peninsula, western and Central Asia, East and tropical Asia to tropical Asia and islands in the Pacific, eastern North America. Pistia has exostomal ovules. Colocasieae have peltate leaves, usually anastomosing laticifers, colocasioid fine leaf venation, and entirely connate stamens. Areae are a subclade within the clade Alocasiinae and are characterized by reticulate fine leaf venation, usually smooth spadix appendices, spinulate exine, bristle- or hair-like staminodia (together with Arisaema), and orthotropous ovules.
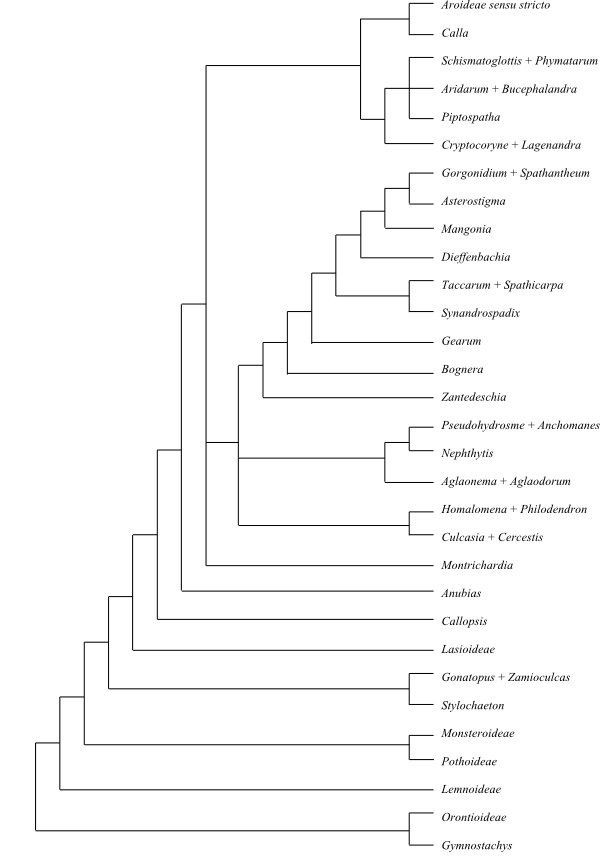 |
Bayesian summary tree of Araceae based on DNA sequence data (Cabrera & al. 2008). Gymnostachys is identified in some other analyses as sister to the remaining Araceae. In many analyses Lasioideae are sister-group to the Zamioculcadoideae clade plus the rest; this alternative is followed in the text above. |
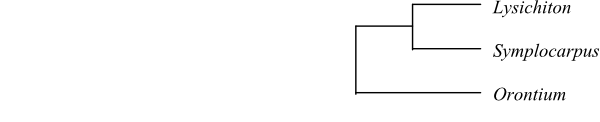 |
Bayesian summary tree of Orontioideae based on DNA sequence data (Cabrera & al. 2008). |
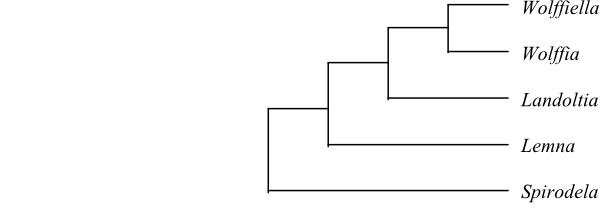 |
Bayesian summary tree of Lemnoideae based on DNA sequence data (Rothwell & al. 2004; Cabrera & al. 2008). |
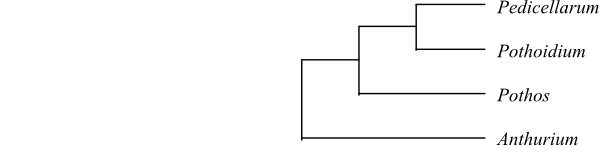 |
Bayesian summary tree of Pothoideae based on DNA sequence data (Cabrera & al. 2008). |
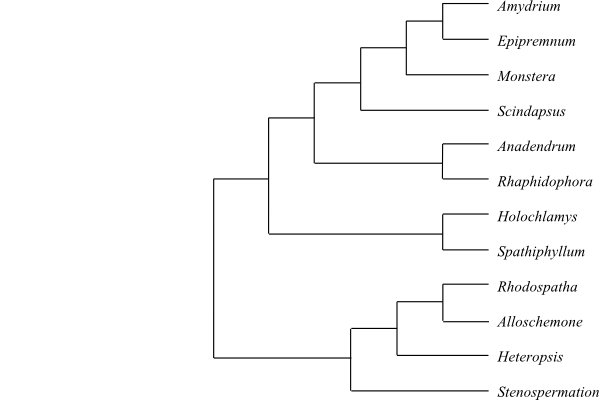 |
Bayesian summary tree of Monsteroideae based on DNA sequence data (Cabrera & al. 2008). |
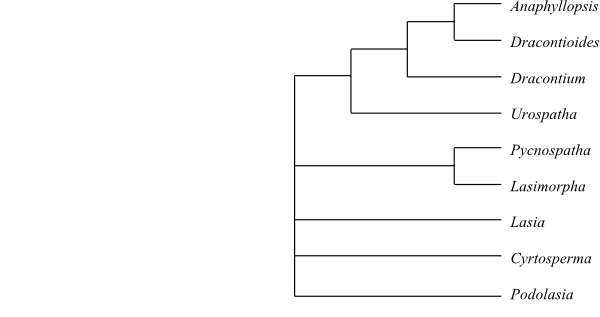 |
Bayesian summary tree of Lasioideae based on DNA sequence data (Cabrera & al. 2008). |
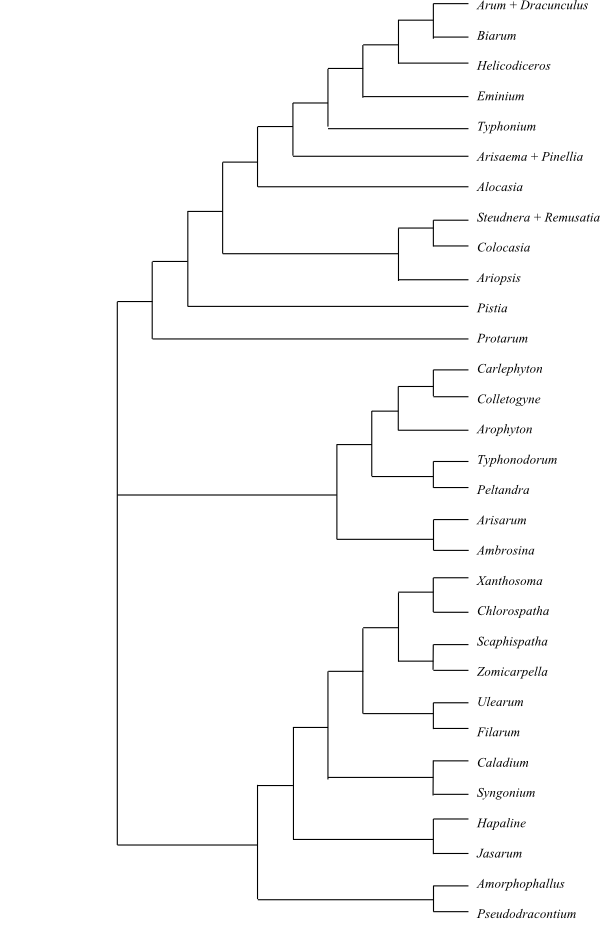 |
Bayesian summary tree of Aroideae sensu stricto based on DNA sequence data (Cabrera & al. 2008). |
BUTOMACEAE Mirb. |
( Back to Alismatales ) |
Butomineae Engl., Syllabus, ed. 2: 73. Mai 1898; Butomales Rich. in C. F. P. von Martius, Consp. Regn. Veg.: 8. Sep-Oct 1835 [‘Butomeae’]; Butomanae Takht. ex Reveal in Novon 2: 235. 13 Oct 1992
Genera/species 1/1
Distribution Temperate regions in Eurasia and North Africa.
Fossils Seeds that may be attributed to Butomaceae have been described from the Oligocene onwards in Europe.
Habit Bisexual, perennial herb. Aquatic or helophytic. Axillary buds sometimes developing into short-stalked bulbils. Rhizome starchy and with monopodial branching.
Vegetative anatomy Roots sometimes contractile. Phellogen absent. Secondary lateral growth absent. Endodermis absent. Vessels present in roots. Vessel elements with usually simple (sometimes scalariform) perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial parenchyma? Sieve tube plastids P2c type, with cuneate protein crystalloid bodies, without starch or protein filaments. Nodes multilacunar with several leaf traces. Schizogenous secretory canals absent; laticifers absent. Outer part of rhizome cortex with crystals as irregular polygonal groups or single styloids.
Trichomes Hairs absent.
Leaves Alternate (distichous), simple, entire, linear, triangular in cross-section, not differentiated into pseudopetiole and pseudolamina, with ? ptyxis. Stipules absent; leaf sheath well developed. Leaf base with a row of intravaginal hairlike scales, squamulae intravaginales. Venation parallelodromous. Stomata paracytic. Cuticular wax crystalloids non-orientated. Schizogenous secretory canals and cavities. Mesophyll cells often with calciumoxalate crystals. Leaf margin entire.
Inflorescence Terminal, umbel-like, cymose, on a long peduncle (scape) from rhizome, subtended by usually three (sometimes two or four) spathe-like bracts. Floral bracts sometimes absent.
Flowers Actinomorphic. Hypogyny. Tepals 3+3, free; outer tepals petaloid-sepaloid, with imbricate aestivation; inner tepals petaloid, crumpled in bud, caducous. Gynoecial-septal nectaries present on lateral walls of carpel bases. Disc absent.
Androecium Stamens 6+3 (outer stamens paired, bifid?, alternipetalous, alternating with inner stamens). Filaments flat, free from each other and from tepals. Anthers basifixed, non-versatile, tetrasporangiate, latrorse, longicidal (dehiscing by longitudinal slits). Corolla/androecium-primordia with one pair of stamens probably first differentiating and subsequently one adaxial stamen. Tapetum amoeboid-periplasmodial, with uninucleate cells. Staminodia absent.
Pollen grains Microsporogenesis successive. Pollen grains monosulcate, shed as monads, tricellular at dispersal. Exine semitectate, with columellate infratectum, reticulate, smooth.
Gynoecium Carpels six, in one whorl, usually at best postgenitally fused; carpel plicate or conduplicate, incompletely closed at apex (carpellary margins held together distally by interwoven hairs). Stylodia very short. Stigmatic surfaces ventral, more or less decurrent, bilobate, papillate, Dry type. Pistillodia absent.
Ovules Placentation laminar-diffuse (lateral, ovules scattered over carpellary surface); placentae covered with secretions. Ovules c. 20 to more than 100 per carpel, anatropous, bitegmic, crassinucellar. Micropyle ?-stomal. Outer integument ? cell layers thick. Inner integument ? cell layers thick. Parietal cell formed from archesporial cell. Parietal tissue one cell layer thick. Megagametophyte monosporous, Polygonum type. Synergids with a filiform apparatus. Antipodal cells not proliferating. Endosperm development helobial. Endosperm haustoria? Embryogenesis caryophyllad.
Fruit An assemblage of follicles.
Seeds Aril absent. Seed coat testal-tegmic. Exotesta with thickened epidermal cell walls and with incrustations. Endotesta? Tegmen persistent. Perisperm not developed. Endosperm absent. Embryo large, straight, well differentiated, starchy, without chlorophyll. Cotyledon one. Cotyledon hyperphyll elongate, assimilating. Hypocotyl internode long. Coleoptile absent. Germination phanerocotylar.
Cytology n = (7) 8, 10–15, 20, 21 – Triploid individuals with 2n = 39 are frequent in Europe. Chromosomes 3,7–8,3 μm long.
DNA
Phytochemistry Flavone-C-glycosides present. Flavonols, ellagic acid, proanthocyanidins, alkaloids, saponins, and cyanogenic compounds not found.
Use Ornamental plants, starch source (locally).
Systematics Butomus (1; B. umbellatus; temperate regions in Europe, northern Africa and Asia east to Manchuria and eastern China).
Butomus is sister to Hydrocharitaceae.
CYMODOCEACEAE Vines |
( Back to Alismatales ) |
Cymodoceales Nakai, Chosakuronbun Mokuroku [Ord. Fam. Trib. Nov.]: 211. 20 Jul 1943
Genera/species 5/16
Distribution Mainly tropical and subtropical coastal marine areas; some species in warm-temperate seas along coasts of Australia and the Mediterranean.
Fossils Thalassocharis was described from the late Cretaceous in the Netherlands, and Thalassodendron auricula-leporis from the Mid-Eocene of Florida, USA. These may be assigned to Cymodoceaceae. Furthermore, Cymodocea is known from several Cenozoic fossils.
Habit Monoecious or dioecious, perennial herbs. Marine. Submersed. Rhizome in Cymodocea, Halodule and Syringodium herbaceous, monopodially branched, in Amphibolis and Thalassodendron lignified, sympodially branched.
Vegetative anatomy Mycorrhiza absent. Roots often with bacterial colonies (nitrogen-fixing?). Epidermal cells of roots usually lignified. Phellogen absent. Endodermal and some outer-cortical and epidermal cell walls of rhizome lignified. Secondary lateral growth absent. Vessels absent (also in roots). Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial parenchyma? Sieve elements in Halodule with thick nacreous cell walls (absent from roots). Sieve tube plastids P2c type, with cuneate protein crystalloid bodies, without starch or protein filaments. Nodes? Tanniniferous cells abundant. Calciumoxalate crystals absent.
Trichomes Hairs absent.
Leaves Alternate (distichous), simple, entire, linear to terete, with ? ptyxis. Stipules absent; leaf sheath well developed, with basal membranous sheath-like ligule in transition zone to lamina; axillary intravaginal scales, squamulae intravaginales, two or more. Venation parallelodromous; primary veins connected by transversal veins. Stomata absent. Cuticular wax absent. Mesophyll without calciumoxalate crystals. Epidermis with tanniniferous cells. Leaf margin subentire, at apex finely serrate.
Inflorescence Flowers axillary, usually solitary (in Syringodium in few-flowered cymose inflorescences), surrounded by a spatha (bract).
Flowers Zygomorphic?, small. Tepals absent? (in Amphibolis transformed into an attachment organ). Nectary absent. Disc absent.
Androecium Stamens two. Filaments connate. Anthers somewhat twisted, connate into a synandrium, basifixed, non-versatile, tetrasporangiate, longicidal (dehiscing by longitudinal slits); connective in Amphibolis and Thalassodendron with two or three apical appendages on each lobe. Tapetum amoeboid-periplasmodial, with uninucleate cells. Staminodia absent.
Pollen grains Microsporogenesis usually successive (in Thalassodendron simultaneous). Pollen grains inaperturate, filiform (in Amphibolis up to 5 mm long), in anthers spirally twisted, shed as monads, tricellular at dispersal. Exine absent; pollen surface usually with pitted longitudinal ridges.
Gynoecium Carpels two, free (apocarpy); usually only one carpel developing into fruit. Ovary unilocular. Stylodia long, simple (in Halodule) or bilobate (bibrachiate) or trilobate (tribrachiate; in Amphibolis). Stigmas long, narrow, Dry type. Pistillodium absent?
Ovules Placentation apical (Syringodium) or basal (Thalassodendron). Ovule one per carpel, anatropous (Syringodium, Thalassodendron) or orthotropous (Syringodium), pendulous, bitegmic, crassinucellar. Micropyle ?-stomal. Outer integument ? cell layers thick. Inner integument ? cell layers thick. Parietal cell? Megagametophyte monosporous, Polygonum type? Endosperm development ab initio nuclear. Endosperm haustoria? Embryogenesis? In Amphibolis an attachment structure with four hardening fleshy pericarp lobes with bristle hairs formed from ovary wall.
Fruit Nutlike or drupaceous (Cymodocea, Halodule, Syringodium; in Thalassodendron a pseudofruit with innermost bracts enclosing carpels during fruit stage).
Seeds Aril absent. Testa present in Cymodocea, Halodule, and Syringodium, absent in Amphibolis and Thalassodendron. Tegmen? Perisperm not developed? Endosperm absent. Embryo large, straight? (macropodous), well differentiated, chlorophyll? Cotyledon usually one. Hypocotyl in Cymodocea, Halodule and Syringodium large, rich in starch. Amphibolis, Thalassodendron and Cymodocea antarctica viviparous, often with small or no cotyledon, but with transfer cells between embryo and mother tissue.
Cytology n = 7, 8, 10, 14, 15, 22
DNA Deletion of 3 bp in atpA absent.
Phytochemistry Insufficiently known. Polyphenols, phenolic sulphates (sulphonated phenolic acids), proanthocyanidins and cyclitols (cyclic polyols) present.
Use Unknown.
Systematics Syringodium (2; S. filiforme: shores of the Gulf of Mexico and the West Indies; S. isoetifolium: coastal marine waters of the Indian Ocean and western Pacific from Mozambique and Madagascar to southern China, New Guinea, northern Australia and Micronesia), Halodule (6; H. bermudensis, H. ciliata, H. emarginata, H. pinifolia, H. uninervis, H. wrightii; tropical coastal marine waters), 'Cymodocea' (4; C. angustata, C. nodosa, C. rotundata, C. serrulata; coastal marine waters along the Canary Islands, the Mediterranean, West Africa, tropical Asia to the Pacific; non-monophyletic); Amphibolis (2; A. antarctica, A. griffithii; coastal marine waters along western and southern Australia), Thalassodendron (3; T. ciliatum, T. leptocaule, T. pachyrhizum; coastal marine waters along eastern Africa including the Red Sea, western Indian Ocean, East Malesia and western and northeastern Australia).
Cymodoceaceae are sister-group to Ruppia (Ruppiaceae).
One clade consists of [Amphibolis+Thalassodendron] and this is sister to Syringodium, Halodule and Cymodocea.
HYDROCHARITACEAE Juss. |
( Back to Alismatales ) |
Najadaceae Juss., Gen. Plant.: 18. 4 Aug 1789 [’Naiades’], nom. cons.; Najadopsida Hoffmanns. et Link, Fl. Portug. 1: 59. 1 Sep 1809 [’Naïdes’]; Hydrocharitales Juss. ex Bercht. et J. Presl, Přir. Rostlin: 271. Jan-Apr 1820 [‘Hydrocharideae’]; Najadales Bercht. et J. Presl, Přir. Rostlin: 271. Jan-Apr 1820 [‘Najadeae’]; Elodeaceae Dumort., Anal. Fam. Plant.: 54. 1829; Vallisneriaceae (Dumort.) Dumort., Anal. Fam. Plant.: 54, 55. 1829; Hydrocharitopsida Bartl., Ord. Nat. Plant.: 25, 74. Sep 1830 [’Hydrocharideae’]; Stratiotaceae Schultz Sch., Nat. Syst. Pflanzenr.: 274. 30 Jan-10 Feb 1832 [’Stratioteae’]; Halophilaceae J. Agardh, Theoria Syst. Plant.: 50. Apr-Sep 1858 [’Halophileae’]; Otteliaceae Chatin in Casp., Jahrb. Wiss. Bot. 1: 482. 1858, nom. illeg.; Hydrillaceae Prantl, Lehrb. Bot., ed. 3: 188. 24 Jan 1879 [’Hydrilleae’]; Enhalaceae Nakai, Chosakuronbun Mokuroku [Ord. Fam. Trib. Nov.]: 210, 211. 20 Jul 1943; Thalassiaceae Nakai, Chosakuronbun Mokuroku [Ord. Fam. Trib. Nov.]: 210. 20 Jul 1943; Blyxaceae (Aschers. et Gürcke) Nakai in J. Jap. Bot. 24: 14. Dec 1949; Vallisneriales Nakai in J. Jap. Bot. 24: 14. Dec 1949; Elodeales Nakai in J. Jap. Bot. 25: 5. Feb 1950; Najadanae Takht. ex Reveal in Novon 2: 236. 13 Oct 1992
Genera/species 18/c 120
Distribution Mainly in tropical and subtropical but also temperate regions: Eurasia, Australia, North America, coastal marine areas in the West Indies, and the Indian Ocean to the Pacific.
Fossils Stratiotes is known from a large number of fossil seeds from the Late Paleocene and onwards. Fossil Hydrocharis has been found in Neogene layers of Germany och Switzerland. Fossil seeds of Najas and Najadites are known from the Oligocene of England and Russia. The extinct genus Ceratostratiotes from the early Miocene of northern Austria resembles Blyxa, but should perhaps be assigned to Ceratophyllaceae.
Habit Usually monoecious, polygamomonoecious, or dioecious (sometimes bisexual), usually perennial (sometimes annual) herbs. Aquatic, often submersed. Some genera are growing in salt or brackish water. Roots usually unbranched (in Najas fibrous). Stems monomorphic, dimorphic or polymorphic.
Vegetative anatomy Mycorrhiza absent in marine species. Root epidermis in Hydrocharis arising from inner epidermis. Phellogen absent. Secondary lateral growth absent. Endodermis indistinct or thick-walled. Vessels usually absent (present in roots of some species). Vessel elements with scalariform perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial parenchyma? Sieve tube plastids P2c type, with cuneate protein crystalloid bodies, without starch or protein filaments. Nodes? Laticifers and schizogenous secretory canals absent. Calciumoxalate crystals and silica bodies absent. Hydrilla, Egeria, etc. have C4 photosynthesis in specialized cells.
Trichomes Hairs absent or unicellular and confined to leaf margins.
Leaves Usually alternate (sometimes distichous; rarely verticillate; in Najas seemingly opposite, although in reality alternate and distichous), simple, entire, often differentiated into pseudopetiole and pseudolamina, often linear, sometimes scale-like (consisting of a mere leaf sheath), with supervolute (involute) or convolute ptyxis. Stipules membranous, single median or paired lateral, or absent; leaf sheath well developed or absent. Pseudopetiole sometimes with wings or spines. Intravaginal scales, squamulae intravaginales, (one or) two to more than ten, mucilaginous? Venation parallelodromous or acrodromous; often with transversal veins. Some species with inverted vascular bundles. Stomata usually absent (paracytic when present). Cuticular wax absent. Leaf margin serrate (sometimes serrate-dentate), dentate or entire. Leaf apex in Najas with one to three unicellular or multicellular teeth on each side.
Inflorescence Terminal or axillary, cymose of various shape (often umbel-like panicles), or flowers solitary (female flowers often solitary), with one or two free or connate spathe-like bracts (flowers in Najas solitary or few together, axillary; each female flower surrounded by one and each male flower by two membranous scale-like bracts). Floral prophyll (bracteole) varying in number and position (sometimes lateral or pairwise).
Flowers Usually actinomorphic (in some genera zygomorphic as young), often small; male flowers in some genera detached from plant immediately prior to anthesis; bisexual flowers often cleistogamous. Hypanthium present in female flowers in some genera. Epigyny. Outer tepals three, sepaloid, with valvate aestivation, caducous or persistent, free (absent in Najas); inner tepals usually three (rarely fewer than three or absent), petaloid, thin, crumpled in bud, caducous, free. Nectaries at stylodial bases usually three, probably staminodial in origin (rarely more than three; sometimes absent). Disc absent.
Androecium Stamens (one to) three or 3+3 (sometimes up to six [to ten] whorls, each one with three stamens, antesepalous stamens sometimes paired; in Najas a single stamen). Filaments (branched? or) simple, free from each other and from tepals. Anthers basifixed or dorsifixed, non-versatile?, usually tetrasporangiate (in Enhalus and Halophila disporangiate; in some species of Najas monosporangiate), introrse, latrorse or extrorse, longicidal (usually dehiscing by longitudinal slits; in Najas with a short apical slit), sometimes connate. Tapetum amoeboid-periplasmodial, with uninucleate cells. Staminodia very varying in number, shape and function, extrastaminal or intrastaminal (absent in Najas).
Pollen grains Microsporogenesis successive. Pollen grains inaperturate, usually shed as monads (in Elodea often as tetrads; in Halophila and Thalassia filiform, cohering in moniliform chain-like bands covered by mucilage), usually tricellular (sometimes bicellular) at dispersal. Exine semitectate to intectate, often very thin, with columellate infratectum, psilate, verrucate, echinate or spinulate, sometimes confined to scattered columellae and spinules (absent in some species). Pollen grains at least in Najas with starch grains.
Gynoecium Pistil composed of (one to) three to six (to more than 20) usually partially connate (sometimes free?) carpels (in Elodea antepetalous); carpel plicate (sometimes ascidiate at base), occluded by secretion only. Ovary inferior, unilocular (often seemingly septate by intrusion of free carpellary margins; ovary wall in Najas two cell layers thick), sometimes filled with mucilage (from mucilaginous hairs?). Style single, or stylodia three to more than 20, sometimes connate at base. Stigmas filiform, branched, papillate (with multicellular papillae), Dry type. Male flowers often with usually trilobate pistillodia (lobes often split).
Ovules Placentation usually parietal to laminal (in, e.g., Najas basal), often strongly intrusive. Ovules one or few to more than 100 per carpel, usually anatropous (sometimes orthotropous, rarely amphitropous), ascending to pendulous, bitegmic, crassinucellar or pseudocrassinucellar. Micropyle usually bistomal (in, e.g., Najas endostomal). Outer integument often three or more cell layers thick. Inner integument ? cell layers thick. Parietal cell formed from archesporial cell. Parietal tissue one or two cell layers thick. Nucellar cap usually three cell layers thick. Megagametophyte monosporous, Polygonum type. Synergids sometimes with a filiform apparatus. Antipodal cells not proliferating. Endosperm development usually helobial (in Najas nuclear). Endosperm haustorium chalazal. Embryogenesis caryophyllad.
Fruit A berry or a capsule (sometimes fleshy; in Najas a nutlike fruit, with gradually decaying wall).
Seeds Aril absent. Seed coat testal. Exotesta present. Mesotesta and endotesta often with stone cells. Testal cell walls often thickened. Endotegmic cell walls with unique small tubercles, with only tuberculate inner wall persistent. Perisperm not developed. Starch present. Endosperm usually absent (in Ottelia sparse). Suspensor haustorium unicellular. Embryo large (often macropodous), usually straight, little or well differentiated, usually without chlorophyll (in Najas with chlorophyll). Cotyledon one, bifacial; embryo at germination often with several leaves; hypocotyl massive often with unicellular attachment hairs. Cotyledon hyperphyll elongate, assimilating. Hypocotyl internode well developed. Coleoptile absent. Radicula absent in, e.g., Stratiotes. Germination phanerocotylar. Synergid polyembryony known from several species of Najas.
Cytology n = 6–66 – Polyploidy and aneuploidy frequently occurring. Chromosomes (0,8–)1,6–11,7 μm long.
DNA Significant losses of mitochondrial genes; introne 2 in the mitochondrial gene nad1 is absent in at least some species.
Phytochemistry Flavones, flavone-C-glycosides, flavone sulphates, cyanidin, 5-methylcyanidin (in Egeria) caffeic acid derivatives, cyanine and polyphenolic glycosides, chlorogenic acids, phenolic sulphates, and hemicellulose-bound apiose present. Cell wall bound ferulate absent in unlignified cell walls. Flavonols, ellagic acid, alkaloids, and saponins not found.
Use Ornamental plants, aquarium plants, vegetables (e.g. Ottelia cordata).
Systematics Hydrocharitaceae are sister-group to Butomus (Butomaceae). The following classification is in accordance with Les & al. (2006). A possible topology is: [Hydrocharitoideae+[Stratiotoideae+[Anacharidoideae+[Najadeae+[Halophileae+Vallisnerieae]]]]].
Hydrocharitoideae Eaton, Bot. Dict., ed. 4: 29, 44. Apr-Mai 1836 [‘Hydrocharides’, ‘Hydrocharideae’]
2/4. Hydrocharis (3; H. morsus-ranae: Europe, North Africa, the Caucasus, Central Asia, Siberia; H. chevalieri: tropical West and Central Africa; H. dubia: East and tropical Asia to the Russian Far East and New Guinea), Limnobium (1; L. spongia; southeastern United States). – Temperate and subtropical regions. Monoecious. Leaves wide, with involute or convolute ptyxis. Foliar vascular bundles inverted. Pseudopetiole present. Ligulae basal, adaxial or paired lateral, entirely enclosing young leaves. Leaf margin entire. Male flowers with three sepaloid tepals and three petaloid tepals. Female flowers with three sepaloid tepals and three or no petaloid tepals. Stamens (one or) three or 3+3. Pistil composed of three to nine connate carpels. Placentae strongly intrusive. Exotestal cells much enlarged. Exotegmic tuberculae present. n = 7, 8, 10, 11, 13–15. –Hydrocharitoideae are sister to the remaining Hydrocharitaceae.
[Stratiotoideae+[Anacharidoideae+[Najadeae+[Halophileae+Vallisnerieae]]]]
Stratiotoideae Luerss., Handb. Syst. Bot. 2: 309. Dec 1879 [‘Stratiotideae’]
1/1. Stratiotes (1; S. aloides; Europe, temperate Asia). – Dioecious. Roots unbranched. Leaves tristichous, spiny, in rosettes. Stamens numerous, inner five to 17 of which fertile. Female flowers with staminodia. Carpels six, free, in two whorld (outer carpels antesepalous). n = >10. – Stratiotes is sister to [Anacharidoideae+Hydrilloideae].
[Anacharidoideae+[Najadeae+[Halophileae+Vallisnerieae]]]
Anacharidoideae Thomé, Fl. Deutschl. 1: 84. 1886 [‘Anacharideae’]
7/51–52. Apalanthe (1; A. granatensis; tropical South America), Appertiella (1; A. hexandra; Madagascar), Lagarosiphon (9; tropical and southern Africa, Madagascar), Egeria (3; E. densa, E. heterostemon, E. najas; subtropical South America), Elodea (5–6; E. bifoliata, E. callitrichoides, E. canadensis, E. granatensis, E. nuttallii, E. potamogeton; Canada, the United States, South America), Blyxa (12; tropical Africa, Madagascar, tropical and subtropical Asia to tropical Australia and islands in western Pacific), Ottelia (c 20; tropical Africa, East and tropical Asia to Australia and Solomon Islands, one species, O. brasiliensis, in tropical South America). – Tropical to temperate regions on both hemispheres, with their largest diversity in North and South America. Usually dioecious (in Apalanthe and Ottelia bisexual) herbs with submerged leaves. Roots usually unbranched. Leaves distichous or pseudoverticillate. Pseudopetiole usually absent. Leaf margin finely serrate to spinose-serrate. Inflorescence axillary. Male flowers usually liberated as buds, usually with 3+3 (sometimes three) tepals. Fertile stamens three (then sometimes also three staminodia) or 3+3. Female flowers with usually long hypanthium, 3+3 tepals and staminodia. Pistil composed of three connate carpels. Placentae usually little intruded. Ovules usually few per carpel. Fruit not or irregularly dehiscent. Seeds usually fewer than 30. n = 6?, 8, 11, 12, 14, etc. – Anacharidoideae are sister-group to Hydrilloideae with low bootstrap support. In Apalanthe and species of Ottelia the flowers are bisexual. Root trichoblasts are absent in Blyxa. Blyxa has secondary pollen presentation and small chromosomes. The leaves of Ottelia have pseudopetiole and a wide lamina and the stamens are three to 15. The female flowers in Blyxa possess hypanthium, staminodia and a pistil composed of three to 20 or more connate carpels.
Hydrilloideae Luerss., Handb. Syst. Bot. 2: 308. Dec 1879 [’Hydrilleae’]
8/c 62. Tropical and subtropical regions, especially in the Old World. Roots usually unbranched. Usually dioecious herbs with submerged leaves. Pollen grains with discontinuous exine and little or no sculpturing. Pollination epi- or hypohydrophilous. Placentae usually little intruded. Ovules usually few per carpel.
[Najadeae+[Halophileae+Vallisnerieae]]
Najadeae Dumort., Fl. Belg.: 164. 1827 [‘Nayeae’]
2/c 40. Hydrilla (1; H. verticillata; temperate regions in Europe, North and East Africa, Asia and northern and eastern Australia), Najas (c 38; nearly cosmopolitan). – Subcosmopolitan. Dioecious. Root system well developed, with simple roots and numerous root hairs. Leaves more or less alternate (often seemlingly opposite), narrow. Pseudopetiole absent. Leaf base with auricle-like structure. Leaf margin serrate. Male flower with two spathae. Stamen single. Hydrophilous. Female flower with one spatha. Carpel one?, ascidiate. Style simple or bifid. Placentation basal. Ovule one. Fruit an achene? Exotegmen sometimes present. Endosperm nuclear. Embryo with chlorophyll. Cotyledon unifacial. n = 6–8. – Najas is sister to [Halophileae+Vallisnerieae] with low bootstrap support.
[Halophileae+Vallisnerieae]
Halophileae Aschers. in Flora 35: 172. Jul 1867
3/22. Enhalus (1; E. acoroides; tropical Asia to western Pacific), Thalassia (2; T. hemprichtii: the Indian Ocean, the Pacific; T. testudinum: the West Indies), Halophila (19; tropical and subtropical coastal marine waters in the Indian Ocean and the Pacific, the West Indies). – Tropical and subtropical coastal marine waters in the Indian and the Pacific Oceans, the West Indies. With rhizome and leaf-bearing short shoots. Tanniniferous cells present or absent. Leaves distichous, narrow. Leaf margin usually entire (sometimes serrate). Male flowers with tepals in two whorls, undifferentiated or three. Stamens three (to 13). Pollen grains deliberated in strands. Usually hydrophilous (not in Enhalus). Female flowers with tepals in two whorls, uniseriate or reduced. Pistil composed of three to nine connate carpels. Fruit fleshy, a capsule or irregularly dehiscent. n = ? – Halophileae are sister-group to Vallisnerieae, although with low bootstrap support. The ovary in Thalassia is elongated into a hypanthium. The lamina in Halophila is wide and has a pseudopetiole.
Vallisnerieae Dumort., Fl. Belg.: 135. 1827
3/16. Nechamandra (1; N. alternifolia; India to Southeast Asia), Vallisneria (14; tropical and subtropical regions on both hemispheres), Maidenia (1; M. rubra; northwestern Australia). – Tropical and subtropical regions on both hemispheres, with their largest diversity in the Old World. Leaves distichous or pseudoverticillate, narrow. Pseudopetiole absent. Leaf margin serrate. Inflorescence axillary. Male flowers liberated as buds, with three sepaloid tepals and (one or) no petaloid tepals. Stamens one to three; staminodium sometimes present. Female flowers with hypanthium, (two or) three sepaloid tepals and two or three petaloid tepals (sometimes absent). Female flowers with (two or) three staminodia. Pistil composed of two or three connate carpels. Fruit irregularly dehiscing. Exotegmic tuberculae sometimes present. n = 8, 10, 12, 15. – Root trichoblasts are absent in Vallisneria. In Hydrilla etc. the hypanthium is strongly elongating, while the anthers reach the water surface in small gas bubbles formed by the male flowers.
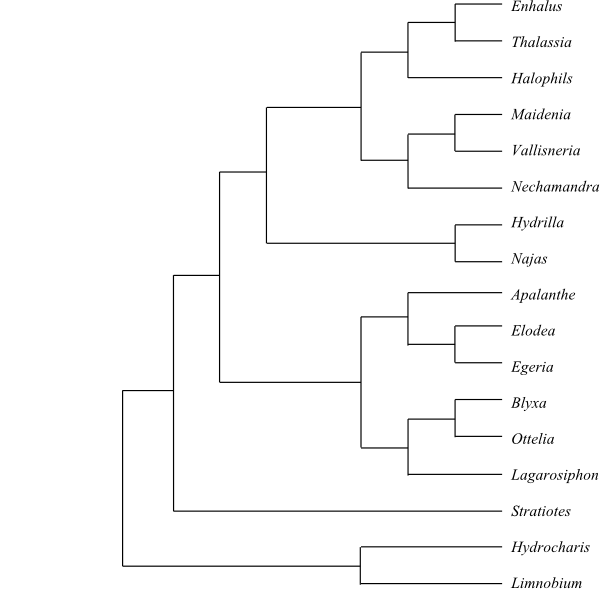 |
Single most parsimonious tree recovered from maximum parsimony analysis of Hydrocharitaceae based on morphological and DNA sequence data (Les & al. 2006). |
JUNCAGINACEAE A. Rich. |
( Back to Alismatales ) |
Triglochinaceae Bercht. et J. Presl, Přir. Rostlin 1(7*-13): 1, 2. 1823 [’Triglochineae’]; Lilaeaceae Dumort., Anal. Fam. Plant.: 62, 65. 1829 [’Lilaearieae’], nom. cons.; Juncaginales Rich. in C. F. P. von Martius, Consp. Regn. Veg.: 6. Sep-Oct 1835 [‘Juncagineae’]; Heterostylaceae Hutch., Fam. Fl. Pl. 2: 41. 20 Jul 1934
Genera/species 3/c 35?
Distribution Cold-temperate regions on both hemispheres.
Fossils Uncertain.
Habit Bisexual, monoecious or polygamomonoecious (in Tetroncium dioecious), usually perennial (rarely annual) herbs. Aquatic or helophytic, in fresh or brackish water.
Vegetative anatomy Mycorrhiza absent. Roots usually fibrous (in Triglochin sometimes tuberous). Apical stem meristem usually bipartite. Phellogen absent. Secondary lateral growth absent. Endodermis present or absent. Vessel elements with scalariform perforation plates; lateral pits scalariform, simple pits. Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial parenchyma? Sieve tube plastids P2c type, with cuneate protein crystalloid bodies, without starch or protein filaments. Nodes? Laticifers present in Triglochin scilloides (‘Lilaea’). Calciumoxalate as single (prismatic?) crystals.
Trichomes Hairs absent.
Leaves Alternate (usually distichous, sometimes spiral), simple, entire, linear, with ? ptyxis. Stipules absent; leaf sheath open, with two auricles (biauriculate) and numerous filiform intravaginal scales, squamulae intravaginales (in Triglochin a ligule). Venation parallelodromous. Primary vascular bundles usually with sclerenchymatous envelope, outermost layer of which often with U-shaped cell wall thickenings. Stomata paracytic or tetracytic (subsidiary cells with parallel cell divisions). Cuticular wax absent. Hypodermis in Triglochin scilloides laticiferous. Mesophyll in Triglochin sometimes with cells containing calciumoxalate as druses or single prismatic crystals. Condensed tannins sometimes present (e.g., in mesophyll cells in Triglochin scilloides). Leaf margin entire.
Inflorescence Terminal, usually spike (rarely raceme). Floral bracts usually absent (present in Triglochin scilloides).
Flowers Actinomorphic (or possibly somewhat zygomorphic), small. Hypogyny. Tepals probably absent. Nectary absent. Disc absent.
Androecium Stamens one (Triglochin scilloides), three, 2+2 or 3+3 (rarely 4+4). Filaments short, free, or absent. Anthers basifixed, non-versatile, usually tetrasporangiate (anthers in bisexual, lower, flowers of Triglochin scilloides disporangiate), extrorse, longicidal (dehiscing by longitudinal slits); connective with a tepaloid appendage, retinaculum. Tapetum amoeboid-periplasmodial, with ab initio uninucleate cells. Staminodia three or four or absent; female flowers usually with staminodia with tepaloid connective appendages.
Pollen grains Microsporogenesis successive. Pollen grains inaperturate, shed as monads, bicellular or tricellular at dispersal. Exine semitectate, with columellate infratectum, reticulate.
Gynoecium Carpels one (in bisexual flowers of Triglochin scilloides), 2+2 or 3+3 (sometimes up to ten; three carpels in Triglochin often sterile, alternipetalous), antepetalous, plicate, usually somewhat connate (rarely entirely free), separating during fruit maturation. Ovary superior, usually trilocular, quadrilocular or sexalocular (rarely unilocular, apocarpy). Stylodia usually short or absent (female flowers in some species of Triglochin with narrow style up to 3 dm). Stigmas penicillate or plumose, papillate, Dry type. Pistillodia (as sterile carpels) present in Triglochin.
Ovules Placentation usually basal. Ovules one or few per carpel, usually anatropous, usually ascending, bitegmic, crassinucellar. Micropyle ?-stomal. Outer integument three or more cell layers thick. Inner integument ? cell layers thick. Parietal cell formed from archesporial cell. Parietal tissue four to six cell layers thick. Megagametophyte monosporous, Polygonum type. Antipodal cells in Triglochin proliferating. Endosperm development ab initio nuclear. Endosperm haustoria? Embryogenesis caryophyllad.
Fruit Usually an assemblage of nutlets (in Triglochin scilloides three-winged or hooked) or follicles (in Triglochin a schizocarp with four or six nutlike mericarps).
Seeds Aril absent. Seed coat exotestal-endotegmic. Exotesta and endotegmen with cuticle; remaining layers crushed. Perisperm not developed? Starch present. Endosperm absent. Suspensor absent. Embryo straight, well differentiated, with short thick ephemeral hypocotyl, chlorophyll? Cotyledon one, large. Cotyledon hyperphyll elongate, assimilating. Hypocotyl internode absent. Coleoptile absent. Germination phanerocotylar. Seedling in Triglochin with distichous leaves.
Cytology n = 6, 9, 12, 15, 16, 18, 24, 30, 48, 60 – Polyploidy occurring. Chromosomes 0,6–1,1 μm long.
DNA
Phytochemistry Flavone-C-glycosides, flavone-O-glycosides and tyrosine-derived cyanogenic glucoside (triglochinin) present. Flavonols, ellagic acid, proanthocyanidins, alkaloids, and saponins not found.
Use Vegetables (Triglochin).
Systematics Tetroncium (1; T. magellanicum; western Patagonia northward to 40° south latitude, Andean Patagonia, Tierra del Fuego, Falkland Islands), Triglochin (c 25?; cosmopolitan), Cycnogeton (9; northern and southwestern Western Australia, Victoria).
Juncaginaceae are sister-group to the clade [[Posidonia+[Ruppia+Cymodoceaceae]]+ [Maundia+[Zosteraceae+Potamogetonaceae]]].
Tetroncium is sister to [Triglochin+Cycnogeton] with weak support (van Mering & Kadereit 2010). ‘Lilaea’ is sister to Triglochin bulbosa and hence nested inside Triglochin s.str. Cycnogeton is excluded from and sister-group to Triglochin.
The inner ’tepal whorl’ is situated inside the outer stamens. The abaxial tepal resembles a bract.
Maundia triglochinoides, formerly included in Juncaginaceae, is placed in Maundiaceae and sister to [Potamogetonaceae+Zosteraceae], according to van Mering & Kadereit (2010).
MAUNDIACEAE Nakai |
( Back to Alismatales ) |
Genera/species 1/1
Distribution Eastern Australia.
Fossils Unknown.
Habit Bisexual, perennial rhizomatous herb. Aquatic or hygrophilous, in fresh water.
Vegetative anatomy Roots fibrous. Apical stem meristem bipartite. Phellogen absent. Secondary lateral growth absent. Endodermis present? Vessel elements with scalariform perforation plates; lateral pits scalariform, simple pits. Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial parenchyma? Sieve tube plastids probably P2c type. Nodes? Laticifers absent. Calciumoxalate as single crystals.
Trichomes Hairs absent.
Leaves Alternate (distichous), simple, entire, triangular in transverse section, with ? ptyxis. Stipules absent; leaf sheath absent or almost so, with two auricles (biauriculate) and numerous filiform intravaginal scales, squamulae intravaginales. Venation parallelodromous. Primary vascular bundles with sclerenchymatous envelope. Stomata paracytic. Cuticular wax probably absent. Leaf margin entire.
Inflorescence Terminal, spike. Floral bracts two (or four).
Flowers Actinomorphic, small. Hypogyny. Tepals probably absent (or possibly two, transversal-abaxial). Nectary absent. Disc absent.
Androecium Stamens (2+2 or) 3+3. Filaments very short, free. Anthers basifixed, non-versatile, usually tetrasporangiate, extrorse, longicidal (dehiscing by longitudinal slits); connective with a tepaloid appendage, retinaculum. Tapetum amoeboid-periplasmodial, with uninucleate cells? Staminodia absent.
Pollen grains Microsporogenesis successive? Pollen grains inaperturate, shed as monads, ?-cellular at dispersal. Exine semitectate?, with columellate infratectum?, reticulate?
Gynoecium Carpels (two or three) or four, plicate, adaxially connate, with free recurved apices, fused at fruit maturation. Ovary superior, usually quadrilocular. Styluli marginal, recurved. Stigmas linear, papillate, Dry type. Pistillodia absent.
Ovules Placentation apical. Ovules one per carpel, orthotropous, pendulous, bitegmic, crassinucellar. Micropyle ?-stomal. Outer integument ? cell layers thick. Inner integument ? cell layers thick. Parietal cell formed from archesporial cell? Chalazal part of megasporangium coenocytic. Megagametophyte monosporous, Polygonum type. Endosperm development ab initio nuclear? Endosperm haustoria? Embryogenesis?
Fruit A schizocarp with nutlike mericarps.
Seeds Aril absent. Exotesta and endotegmen with cuticle; remaining layers crushed. Perisperm not developed? Starch present. Endosperm absent. Embryo straight, well differentiated, with short thick hypocotyl, chlorophyll? Suspensor absent. Cotyledon one, large. Cotyledon hyperphyll elongate, assimilating. Hypocotyl internode absent. Coleoptile absent. Germination phanerocotylar?
Cytology n = ?
DNA
Phytochemistry Unknown.
Use Unknown.
Systematics Maundia (1; M. triglochinoides; southeasternmost Queensland, northeastern New South Wales).
Maundia is sister to [Potamogetonaceae+Zosteraceae], according to Mering & Kadereit (2010). Five sheath-like tubular cataphylls split longitudinally. The leaves and the peduncle have inverted peripheral vascular bundles, a feature which is almost unique among monocots. The outer ‘tepals’ (Sokoloff & al. 2013) may be interpreted as floral bracts. A nucellar coenocyte is formed in the fertilized ovules.
POSIDONIACEAE Vines |
( Back to Alismatales ) |
Cauliniaceae J. Presl in Nowočeská Bibl. [Wšobecný Rostl.] 7: 1687. 1846, nom. illeg.; Posidoniales Nakai, Chosakuronbun Mokuroku [Ord. Fam. Trib. Nov.]: 211. 20 Jul 1943
Genera/species 1/9
Distribution Southwest European Atlantic coasts, Mediterranean coasts, and coastal waters off South Australia and Tasmania, with their highest diversity along Australian coasts.
Fossils Single ambiguous fossils which may possibly be attributed to Posidoniaceae are known from Cretaceous and Eocene layers.
Habit Usually bisexual, perennial herbs. Marine (sometimes in brackish water). Submersed. Rhizome with one scale at each node and covered by remnants of old leaf sheaths. Old foliar fibres detached from rhizomes, rolled by sea-waves into balls and washed onto beaches along Mediterranean and southern Australian coasts.
Vegetative anatomy Mycorrhiza absent. Phellogen absent. Rhizome stele with four to ten cortical vascular bundles. Secondary lateral growth absent. Vessels absent (also in roots). Imperforate tracheary xylem elements tracheids with little lignified cell walls and with simple? pits. Wood rays absent. Axial parenchyma? Cortical parenchyma with numerous tanniniferous cells and small starch grains. Sieve tube plastids P2c type, with cuneate protein crystalloid bodies, without starch or protein filaments. Nodes? Crystals absent.
Trichomes Hairs absent.
Leaves Alternate (distichous), simple, entire, linear, with ? ptyxis. Stipules absent; leaf sheath open, biauriculate, without chloroplasts, with basal membranous sheath-like ligule in transition zone to lamina, and strongly lignified fibres, long persistent after leaf has fallen; axillary intravaginal scales, squamulae intravaginales, numerous. Venation parallelodromous; transversal veins connecting? primary veins, each of these enclosed by one cell layer with lignified cell walls. Stomata absent. Cuticular wax absent. Mesophyll without calciumoxalate crystals. Tanniniferous cells frequent. Leaf margin entire or finely serrate.
Inflorescence Axillary, botryoid or panicle, with flowers in two rows in flattened and long-stalked, three- to five-flowered, branched spike-like partial inflorescences, surrounded by fibrous leaf sheaths forming spathae (bracts). Floral bracts present.
Flowers Actinomorphic, small. Hypogyny. Tepals absent. Nectary absent. Disc absent.
Androecium Stamens three. Filaments absent or nearly so. Anthers basifixed or subbasifixed, non-versatile, tetrasporangiate, extrorse, longicidal (dehiscing by longitudinal basal slits); connective wide, discoid, tepaloid and with a prolonged midvein. Tapetum amoeboid-periplasmodial? Staminodia absent.
Pollen grains Microsporogenesis successive? Pollen grains inaperturate, filiform, shed as monads, tricellular at dispersal. Exine absent. Pollen surface smooth.
Gynoecium Pistil composed of one carpel; ventral side of carpel abaxial. Ovary superior, unilocular (monomerous, monocarpellate). Style single, simple, very short, or absent. Stigma irregularly lobate, almost discoid, with cuticle, covered with secretions, type? Pistillodium absent.
Ovules Placentation apical. Ovule one per carpel, campylotropous, pendulous, bitegmic, crassinucellar, with integumentary outgrowth. Micropyle ?-stomal. Outer integument ? cell layers thick. Inner integument ? cell layers thick. Parietal cell? Megagametophyte monosporous, Polygonum type. Endosperm development helobial. Endosperm haustoria? Embryogenesis?
Fruit A one-seeded, follicle-like fruit with fleshy pericarp.
Seeds Aril absent. Seed coat absent. Testa and tegmen membranous, without phytomelan. Perisperm not developed. Endosperm absent. Embryo large, straight (macropodous), well differentiated, chlorophyll? Cotyledon one. Cotyledon hyperphyll? Hypocotyl internode? Coleoptile absent? Hypocotyl large, starchy. Germination?
Cytology x = 10 – Karyotype bimodal, with five pairs of larger and five pairs of smaller chromosomes.
DNA
Phytochemistry Proanthocyanidins and phenolic sulphates (sulphonated phenolic acids) present. Flavone sulphates, alkaloids and saponins not found.
Use Fibres for textiles etc.
Systematics Posidonia (9; marine areas along the Mediterranean and Southwest European Atlantic coasts, coastal waters off South Australia and Tasmania).
Posidonia is usually identified as sister to [Ruppiaceae+Cymodoceaceae].
POTAMOGETONACEAE Bercht. et J. Presl |
( Back to Alismatales ) |
Genera/species 6/115–120
Distribution Cosmopolitan except polar areas.
Fossils Fossil leaves and fruits of Potamogetonaceae are described from Cenozoic layers of Europe and Asia.
Habit Bisexual (Groenlandia, Potamogeton, and Stuckenia), or usually monoecious (Zannichellia, Pseudalthenia and Althenia; rarely dioecious), usually perennial (rarely annual) herbs, usually without tuber. Shoot apices in Potamogeton often transformed into turions. Aquatic (in fresh water, some species in brackish water). Many species are entirely submersed.
Vegetative anatomy Mycorrhiza absent? Roots fibrous, non-septate. Outer root cortex often with somewhat lignified cells. Stem apical meristem in Zannichellia bifid. Phellogen absent. Vascular tissue usually reduced. Secondary lateral growth absent. Vessels present only in roots in some species of Potamogeton. Vessel elements with scalariform perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial parenchyma? Sieve tube plastids P2c type, with cuneate protein crystalloid bodies, without starch or protein filaments. Nodes?, with three main bundles often entering leaf base. Tanniniferous cells frequent. Calciumoxalate crystals and silica bodies absent.
Trichomes Hairs absent.
Leaves Alternate (usually distichous, sometimes spiral; in Groenlandia pseudo-opposite), simple, entire, often differentiated into pseudopetiole and pseudolamina, with involute ptyxis, often linear, sometimes scale-like. Stipules absent; leaf sheath well developed, open, sometimes auriculate, with basal membranous sheath-like ligule in transition zone to lamina, sometimes densely pressed to (adnate to?) leaf base (absent in Zannichellia); axillary intravaginal scales, squamulae intravaginales, (one or) two to more than ten. Venation parallelodromous (leaves often one-veined); main veins usually connected to transversal veins. Stomata paracytic (often with aberrant development) or absent. Cuticular wax absent. Leaf margin usually entire (rarely crenulate or finely serrulate). Leaf in Potamogeton with apical pore. Heterophylly often present (submersed leaves different from floating leaves).
Inflorescence Terminal or axillary, scapose, usually two- to many-flowered spike- or head-like (flowers rarely solitary axillary), each one surrounded by a spatha. Floral bracts usually absent. Inflorescence in Zannichellia developing sympodially.
Flowers Actinomorphic, small. Hypogyny. Tepals absent. Nectary absent. Disc absent.
Androecium Stamens one or (two to) four (Groenlandia, Potamogeton, and Stuckenia). Filaments free. Anthers basifixed, non-versatile, 1–8(–12)-sporangiate (anthers in Althenia, Pseudalthenia, and Zannichellia with one or several disporangiate units), extrorse, longicidal (dehiscing by longitudinal slits); connective usually with abaxial tepaloid appendage (absent in male flowers of Pseudalthenia and Zannichellia); stamen and tepaloid connective appendage supported by a common trace (vascular strand branch). Tapetum amoeboid-periplasmodial, usually with ab initio uninucleate (rarely binucleate) and later multinucleate cells. Female flowers with staminodia provided with tepaloid connective appendages (in Pseudalthenia and Zannichellia as a tubular structure surrounding carpels; in Althenia as three separate parts each enveloping one carpel).
Pollen grains Microsporogenesis successive. Pollen grains usually inaperturate (rarely monosulcate), shed as monads, tricellular at dispersal. Exine usually semitectate (absent in some species), with columellate infratectum, reticulate, sometimes spinulate (sometimes absent). Pollen grains of some Althenia and Zannichellia dispersed within a common gelatinous envelope.
Gynoecium Carpels (one to) four (to eight; in Groenlandia, Potamogeton, and Stuckenia usually four), usually ‘alternitepalous’, sometimes shortly stipitate (in Zannichellia with often long stipe), free; carpel plicate and ascidiate. Ovary superior, unilocular (apocarpy). Stylodia short to long or absent. Stigmas capitate (Groenlandia, Potamogeton, and Stuckenia), peltate or infundibuliform (in Althenia bilocularis with fimbriate margin), non-papillate, Dry type. Pistillodia absent.
Ovules Placentation ventral-marginal, lateral or apical. Ovule one per carpel, orthotropous (Groenlandia, Potamogeton, and Stuckenia) or campylotropous to anatropous, pendulous, apotropous, bitegmic, crassinucellar. Micropyle endostomal. Outer integument ? cell layers thick. Inner integument ? cell layers thick. Hypostase present or absent. Parietal cell formed from archesporial cell. Megagametophyte monosporous, Polygonum type (in Groenlandia, Potamogeton, and Stuckenia) or disporous, Allium or Endymion type (in Zannichellia, Pseudalthenia and Althenia). Antipodal cells sometimes persistent and proliferating up to four or five cells. Suspensor prominent, vesicular. Endosperm development ab initio helobial. Endosperm haustoria? Embryogenesis caryophyllad.
Fruit Usually a drupe (in Groenlandia densa a berry, dehiscing by degradation of pericarp wall; in Althenia a follicle). Photosynthesizing.
Seeds Aril absent. Seed coat usually exotestal. Testa membranous, without phytomelan. Exotesta usually well developed. Tegmen unspecialized. Testa crushed in some species of Potamogeton and Zannichellia. Perisperm not developed. Endosperm absent. Embryo large, curved, spirally twisted (usually macropodous), well differentiated, without chlorophyll. Cotyledon one. Cotyledon hyperphyll elongate, assimilating. Hypocotyl large, rich in starch, with long internode. Coleoptile absent. Germination phanerocotylar.
Cytology n = 7, 12, 14–18, 26, 52, etc. – Polyploidy and aneuploidy frequently occurring. Chromosomes 0,5–2,3 μm long.
DNA
Phytochemistry Flavone aglucones, flavone-C-glycosides (in Zannichellia flavone-O-glycosides), and tannins (in Potamogeton) present. Flavonols and flavone sulphates present in Zannichellia. Anthocyanins, ellagic acid, proanthocyanidins, alkaloids, saponins, and cyanogenic compounds not found. Rhodoxanthin gives some species a red colour.
Use Aquarium plants (some species of Potamogeton).
Systematics Potamogeton (c 100; nearly cosmopolitan), Groenlandia (1; G. densa; western Europe, northern Africa, southwestern Asia), Stuckenia (7; S. amblyophylla, S. filiformis, S. macrocarpa, S. pamirica, S. pectinata, S. striata, S. vaginata; nearly cosmopolitan), Althenia (8; A. australis, A. bilocularis, A. cylindrocarpa, A. filiformis, A. marina, A. orientalis, A. patentifolia, A. preissii; Atlantic coasts of Morocco and southwestern Europe north to France, Mediterranean coasts, Turkey, central Siberia, coastal waters in Namibia and southwestern South Africa to Eastern Cape, southern Australia, Tasmania, New Zealand), Pseudalthenia (1; P. aschersoniana; coastal areas in western Western Cape), Zannichellia (1–5; Z. palustris; nearly cosmopolitan).
Potamogetonaceae are sister-group to Zosteraceae.
According to some DNA analyses, Potamogeton, Groenlandia, Stuckenia, Lepilaena, and Zannichellia form a clade, sister to [Althenia+Pseudalthenia]. On the other hand, Ito et al. (2016) found Lepilaena to be paraphyletic relative to Althenia. Hence, they included Lepilaena in Althenia.
 |
Frequency difference jacknife consensus tree of four genera in Potamogetonaceae based on DNA sequence data (Lindqvist & al. 2006). |
RUPPIACEAE Horan. |
( Back to Alismatales ) |
Ruppiales Nakai in J. Jap. Bot. 25: 5. Feb 1950
Genera/species 1/3–11
Distribution Temperate and subtropical regions on both hemispheres. Mainly in coastal marine environments.
Fossils Fossil fruits described as Limnocarpus from the Paleocene of western Europe have sometimes been assigned to Ruppiaceae.
Habit Bisexual, usually annual (rarely perennial) herbs. Aquatic in salt or brackish (rarely fresh) water. Submersed. Shoot apices usually not transformed into turions (present in Ruppia tuberosa).
Vegetative anatomy Mycorrhiza absent. Roots fibrous, non-septate. Phellogen absent. Secondary lateral growth absent. Vessels absent. Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial parenchyma? Sieve tube plastids P2c type, with cuneate protein crystalloid bodies, without starch or protein filaments. Nodes? Tanniniferous cells frequent. Calciumoxalate crystals and silica bodies absent.
Trichomes Hairs absent.
Leaves Alternate (distichous), simple, entire, linear, often filiform, with ? ptyxis. Stipules absent; leaf sheath well developed, open, more or less auriculate, with basal membranous sheath-like ligule in transition zone to lamina; axillary intravaginal scales, squamulae intravaginales, numerous. Venation parallelodromous (leaf one-veined). Foliar vascular tissue reduced to a central strand and two indistinct strands along leaf margin. Stomata absent. Cuticular wax absent. Leaf margin subentire, at apex finely serrate.
Inflorescence Terminal, scapose one- or few-flowered head- or umbel-like spike, each one at an early stage surrounded by two bracts. Peduncle (scape) during fruit maturation much prolonged and approaching water surface or spiralling and forcing fruits downwards below water surface. Floral bracts absent.
Flowers Actinomorphic, small. Hypogyny. Tepals absent. Nectary absent. Disc absent.
Androecium Stamens two, median, free. Filaments absent. Anthers basifixed, non-versatile, tetrasporangiate, extrorse, longicidal (dehiscing by longitudinal slits)?; connective wide, with abaxial tepaloid appendage. Tapetum amoeboid-periplasmodial, with ab initio uninucleate cells. Staminodia absent.
Pollen grains Microsporogenesis successive. Pollen grains inaperturate, heteropolar, bilateral, elongate, often curved, floating on water surface, shed as monads, tricellular at dispersal. Exine semitectate (intectate at ends), with columellate infratectum, reticulate.
Gynoecium Carpels (two to) four (to 16), free. Ovary superior, unilocular (apocarpy), stipitate. Style absent. Stigmas peltate, lobate, non-papillate, Dry type. Pistillodia absent.
Ovules Placentation apical or lateral. Ovule one per carpel, campylotropous, pendulous, bitegmic, crassinucellar. Micropyle bistomal. Outer integument ? cell layers thick. Inner integument ? cell layers thick. Megagametophyte monosporous, Polygonum type. Endosperm development helobial. Endosperm haustoria? Embryogenesis caryophyllad.
Fruit A drupe, dehiscing by degradation of fleshy outer pericarp layers. Endocarp strongly sclerified, with two perforations in placental part, through which embryo germinates.
Seeds Aril absent. Testa two-layered. Exotesta? Endotesta? Exotegmic cells large with branched processes from walls, all crushed. Endotegmen? Perisperm not developed? Starch present. Endosperm absent. Embryo straight, curved or spirally twisted (macropodous), well differentiated, chlorophyll? Cotyledon one. Cotyledon hyperphyll elongate, assimilating. Hypocotyl large, with long internode. Coleoptile absent. Germination phanerocotylar?
Cytology n = 8, 10, 12, 20 – Karyotype bimodal, with nine small and one large chromosome. Chromosomes 0,7–4,4 μm long.
DNA
Phytochemistry Flavonols, flavone-O-glycosides, ellagic acid, and proanthocyanidins present. Sulphates? Alkaloids and saponins not found.
Use Unknown.
Systematics Ruppia (3–11; temperate and subtropical regions on both hemispheres). The number of species in Ruppia is uncertain due to difficulties in species delimitation.
Ruppia is usually sister to Cymodoceaceae, although it was recovered as sister to [Posidonia+ Cymodoceaceae] in a study by Kato & al. (2003).
SCHEUCHZERIACEAE F. Rudolphi |
( Back to Alismatales ) |
Scheuchzeriales B. Boivin in Bull. Soc. Bot. France 103: 494. 20 Jan 1957
Genera/species 1/1
Distribution Cold-temperate regions on the Northern Hemisphere.
Fossils Uncertain.
Habit Bisexual, perennial herb. Helophytes.
Vegetative anatomy Phellogen absent. Outer cycle of vascular bundles connected by sclerenchyma and forming a continuous cylinder. Secondary lateral growth absent. Endodermis present in stem. Vessels present in roots. Vessel elements with scalariform perforation plates; lateral pits scalariform, simple pits. Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial parenchyma? Sieve tube plastids P2c type, with cuneate protein crystalloid bodies, without starch or protein filaments. Nodes? Tanniniferous cells present. Silica bodies absent. Calciumoxalate as druses or single prismatic crystals.
Trichomes Hairs absent.
Leaves Alternate (distichous), simple, entire, linear, with ? ptyxis. Stipules absent; leaf sheath open, with two small auricles (biauriculate) and numerous filiform intravaginal scales, squamulae intravaginales. Venation parallelodromous. Stomata tetracytic. Cuticular wax absent. Air lacunae present. Mesophyll with cells containing calciumoxalate as druses or single prismatic crystals. Hypodermal rows of crystals surrounding vascular bundles. Tanniniferous cells abundant. Leaf margin entire. Pseudolamina with adaxial apical pore.
Inflorescence Terminal, raceme-like panicle.
Flowers Actinomorphic, small. Hypogyny. Tepals 3+3, sepaloid to petaloid, free. Nectary absent. Disc absent.
Androecium Stamens 3+3. Filaments free from each other and from tepals. Anthers basifixed, non-versatile, tetrasporangiate, extrorse, longicidal (dehiscing by longitudinal slits). Tapetum amoeboid-periplasmodial, with ab initio uninucleate cells with degenerating walls. Staminodia absent.
Pollen grains Microsporogenesis successive? Pollen grains inaperturate, shed as dyads, tricellular at dispersal. Exine semitectate, with columellate infratectum, reticulate.
Gynoecium Pistil composed of usually three (rarely six) basally connate plicate antesepalous carpels, usually congenitally fused (carpel edges not entirely coherent). Ovary superior, usually trilocular (rarely sexalocular). Style absent. Stigma adaxially decurrent, papillate, Dry type. Pistillodium absent.
Ovules Placentation subbasal-axile. Ovules usually two (sometimes one) per carpel, anatropous, ascending, bitegmic, crassinucellar. Micropyle endostomal. Outer integument five or six cell layers thick. Inner integument approx. four cell layers thick. Parietal cell formed from archesporial cell. Megagametophyte monosporous, Polygonum type. Antipodal cells not formed; antipodal nuclei early degenerating. Endosperm development ab initio helobial. Endosperm haustorium micropylar? (absent?). Embryogenesis probably caryophyllad.
Fruit An assemblage of one- or two-seeded follicles (a schizocarp with three or rarely six mericarps).
Seeds Aril absent. Testa smooth. Tegmen? Perisperm not developed? Starch present. Endosperm absent. Embryo well differentiated, with chlorophyll. Cotyledon one, large, rounded, non-photosynthesizing. Cotyledon hyperphyll elongate to short (nutrient storing, with starch?). Hypocotyl internode absent. Coleoptile absent. Germination phanerocotylar.
Cytology x = 11 – Chromosomes 0,8–2 μm long.
DNA
Phytochemistry Tyrosine-derived cyanogenic glucosides (triglochinin) and cyanidin? present. Flavonoids, alkaloids and saponins not found. Unlignified cell walls lacking ferulate.
Use Unknown.
Systematics Scheuchzeria (1; S. palustris; cold-temperate regions on the Northern Hemisphere).
Scheuchzeriaceae are usually sister-group to the clade [Aponogeton+[Juncaginaceae+ [[Posidoniaceae+[Ruppia+Cymodoceaceae]]+[Maundia+[Zosteraceae+Potamogetonaceae]]]]], although Scheuchzeria has sometimes been recovered as sister to Aponogeton (Kato & al. 2003).
TOFIELDIACEAE Takht. |
( Back to Alismatales ) |
Tofieldiales Reveal et Zomlefer in Novon 8: 176. 15 Jul 1998
Genera/species 4/23
Distribution Temperate Eurasia, with their largest diversity in East Asia, southeastern United States, northwestern South America.
Fossils Unknown.
Habit Bisexual, perennial rhizomatous herbs.
Vegetative anatomy Roots fibrous. Phellogen absent. Vessel elements with scalariform (to reticulate) perforation plates; lateral pits? Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial parenchyma? Phloem with alternating sieve cells and fibres. Sieve tube plastids P2ccp type, with starch, many cuneate protein crystals and a single polygonal protein crystal. Nodes? Parenchyma with a unique type of calciumoxalate druses. Envelopes of vascular bundles with prismatic crystals. Calciumoxalate raphides absent at least in Tofieldia.
Trichomes Hairs absent?
Leaves Alternate (distichous), simple, entire, usually equitant (ensiform), isobifacial, with ? ptyxis. Stipules absent; leaf sheath well developed; ligule present in Pleea. Venation parallelodromous. Stomata anomocytic. Cuticular wax absent. Leaf margin entire.
Inflorescence Terminal, usually spike or raceme (flowers in Harperocallis rarely solitary). Floral bracts in Pleea spathe-like. A calyculus – consisting of one to three floral prophylls (bracteoles) – subtending each flower (absent in some species of Tofieldia).
Flowers Actinomorphic, small. Hypogyny. Tepals 3+3, petaloid, usually free (sometimes connate at base); median outer tepal in Tofieldia adaxial. Triradiate septal? nectaries usually present, on inner bases of connate carpellary stipes (perigonal nectaries sometimes present as well). Disc absent.
Androecium Stamens usually 3+3 (in Pleea 6+3, or rarely 6+4 or 6+6), usually free (sometimes connate at base), sometimes adnate to tepal bases. Anthers basifixed or subbasifixed, usually non-versatile, tetrasporangiate, introrse or latrorse, longicidal (dehiscing by longitudinal slits). Tapetum secretory, with binucleate cells. Staminodia absent.
Pollen grains Microsporogenesis simultaneous (Tofieldia). Pollen grains in Harperocallis at least sometimes monosulcate, in Pleea and Tofieldia usually disulcate (sometimes trichotomosulcate), shed as monads, bicellular at dispersal. Exine tectate or semitectate, with columellate infratectum, reticulate, microreticulate, rugulate, verrucate or gemmate (in species of Tofieldia).
Gynoecium Pistil composed of three at base (Pleea), half-way up (Tofieldia, Triantha?) or for most of their length (Harperocallis) connate carpels; carpel usually plicate, postgenitally fused with secretory canal; compitum formed by main parts of carpels. Ovary superior, usually trilocular (sometimes unilocular). Stylodia three, usually free (sometimes connate in lower part or only at base, in Harperocallis connate into a single simple style). Stigma capitate, type? Pistillodium absent.
Ovules Placentation usually axile (sometimes parietal, then ovary unilocular). Ovules five to numerous per carpel, anatropous to campylotropous, usually bitegmic? (in Tofieldia iridacea and Harperocallis duidae unitegmic), faintly crassinucellar. Micropyle ?-stomal. Outer integument ? cell layers thick. Inner integument ? cell layers thick. Obturator integumentary. Parietal tissue weakly developed. Nucellar cap sometimes present. Hypostase present. Megagametophyte in Tofieldia monosporous, modified Polygonum type (seven-celled and 11-nucleate), or disporous, Allium type or Veratrum lobelianum type (micropylar cell dividing prior to chalazal cell during second meiotic division of megagametophyte mother cell, leading to formation of ephemeral triad). Antipodal cells in Tofieldia binucleate, persistent, often proliferating. Long chalazal (funicular?) hooked extension present in Pleea and species of Tofieldia. Endosperm development helobial. Endosperm haustoria? Embryogenesis?
Fruit Usually a septicidal (Pleea, Harperocallis) or ventricidal (Tofieldia, Triantha) capsule or an assemblage of follicles (sometimes, e.g., in Isidrogalvia with persistent tepals).
Seeds Aril absent. Seeds in Pleea and Triantha with filiform terminal appendages at both ends, in Harperocallis with filiform chalazal appendage, decurrent along seed. Phlobaphene present at least in Tofieldia. Tegmen thin. Perisperm not developed. Endosperm copious. Embryo large, well differentiated, chlorophyll? Cotyledon one. Cotyledon hyperphyll? Hypocotyl internode? Coleoptile? Rhizoids in Harperocallis flava collar type. Radicula absent? Germination phanerocotylar.
Cytology x = (14) 15 (16) – Polyploidy rare. Chromosomes 0,9–2,5 µm long.
DNA Deletion of 3 bp in atpA absent.
Phytochemistry Flavonols (kaempferol, quercetin), chelidonic acid, glycosides, and steroidal saponins present. Flavones not found.
Use Ornamental plants.
Systematics Pleea (1; P. tenuifolia; Alabama, Florida, North and South Carolina), Tofieldia (7–10; temperate regions on the Northern Hemisphere), Triantha (4; T. japonica: Japan; T. glutinosa, T. occidentalis, T. racemosa: North America), Harperocallis (11; Florida, the Andes from Colombia and Venezuela to Bolivia, the Guayana Highlands in southern Venezuela, Guyana and northern Brazil).
Tofieldiaceae are probably sister to all other Alismatales except Araceae. They are, however, sometimes recovered as sister to Araceae or to the remaining Alismatales or part of a basal trichotomy.
Using matK and rbcL sequences, Tamura & al. (2004) recovered Isidrogalvia (excl. Harperocallis flava) as nested inside Nartheciaceae (Taccales).
 |
Phylogeny of Tofieldiaceae based on DNA sequence data (Remizowa & al. 2011). Harperocallis includes Isidrogalvia (Campbell & Dorr 2013). |
ZOSTERACEAE Dumort. |
( Back to Alismatales ) |
Zosterales J. Presl in Nowočeská Bibl. [Wšobecný Rostl.] 7: 1667. 1846 [‘Zostereae’]; Zosteranae Doweld, Tent. Syst. Plant. Vasc.: lviii. 23 Dec 2001
Genera/species 4/21
Distribution Temperate marine coastal waters on both hemispheres; some species in tropical and subtropical marine coastal waters.
Fossils The Maastrichtian Archaeozostera from Japan may be assigned to Zosteraceae.
Habit Monoecious (Zostera, Nanozostera, Heterozostera) or dioecious (Phyllospadix), usually perennial (rarely annual) herbs. Marine (sometimes in brackish water). Submersed. Main stem with monopodial growth. Branches leaf-opposite.
Vegetative anatomy Mycorrhiza absent. Phellogen absent. Rhizome cortex usually with fibre strands (absent in Phyllospadix) and vascular bundles. Secondary lateral growth absent. Vessels absent. Imperforate tracheary xylem elements tracheids. Wood rays absent. Axial parenchyma? Sieve elements with thick nacreous cell walls (absent from roots). Sieve tube plastids P2c type, with cuneate protein crystalloid bodies, without starch or protein filaments. Nodes? Tanniniferous cells frequent. Crystals absent.
Trichomes Hairs absent.
Leaves Alternate (distichous), simple, entire, linear, with convolute (supervolute) ptyxis? Stipules absent; leaf sheath well developed, open or closed, auriculate, with ligule in transition zone to lamina; axillary intravaginal filiform scales, squamulae intravaginales, two to four. Venation parallelodromous; primary veins connected? to transversal veins. Stomata absent. Cuticular wax absent. Mesophyll without calciumoxalate crystals. Leaf margin entire. Leaf with apical pore.
Inflorescence Axillary, flattened spike-like spadix with incurved margins and with flowers arranged into two dorsal-abaxial rows and enclosed by a sheathing spatha; inflorescence interpreted as a panicle consisting of one or more rhipidia, each of which with (one or) two to five spathae. In Zostera two male flowers alternating with one female flower along two inflorescence rows. Male or female flowers in Phyllospadix zig-zag arranged in spadix. Floral bracts and prophylls (bracteoles) absent. Inflorescence axis usually with marginal appendages, retinacula (absent from Zostera), each one surrounding and almost enclosing a single stamen.
Flowers Actinomorphic? (zygomorphic by reduction), small. Hypogyny? Tepals absent. Nectary absent. Disc absent.
Androecium Stamen one. Filament absent. Anther dorsifixed, non-versatile?, tetrasporangiate, extrorse, longicidal (dehiscing by longitudinal slits); connective with enlarged tepaloid appendage; stamen and tepaloid connective appendage supported by a common trace (vascular strand branch). Tapetum amoeboid-periplasmodial. Staminodia absent.
Pollen grains Microsporogenesis successive. Pollen grains inaperturate, filiform (up to 2 mm long), shed as monads, bicellular (Zostera, Nanozostera, Heterozostera) or tricellular (Phyllospadix) at dispersal. Exine absent. Pollen surface smooth.
Gynoecium Pistil composed of seemingly one carpel (in reality two carpels one of which reduced and sterile). Ovary superior?, unilocular (pseudomonomerous). Stylodium single, bilobate, short. Stigmas two, type? Pistillodium absent?
Ovules Placentation apical. Ovule one per ovary, orthotropous, pendulous, bitegmic, pseudocrassinucellar or tenuinucellar. Micropyle endostomal. Outer integument up to seven cell layers thick. Inner integument ? cell layers thick. Parietal cell not formed. Nucellar cap formed by periclinal cell divisions of megasporangial epidermis. Megagametophyte monosporous, Polygonum type. Antipodal cells not proliferating. Hypostase present. Endosperm development ab initio nuclear? Endosperm haustoria? Embryogenesis caryophyllad.
Fruit A usually nutlike fruit (in Phyllospadix with soft exocarp and hard fibrous endocarp).
Seeds Aril absent. Seed coat exotestal. Exotestal cells more or less anticlinally and periclinally elongate; remaining cells persistent, often more or less thickened. Tegmen degenerating. Perisperm not developed? Endosperm absent. Embryo somewhat curved (usually macropodous), well differentiated, chlorophyll? Cotyledon one. Starch? Cotyledon hyperphyll elongate, assimilating. Hypocotyl large, with long internode, basally as a large disc with rhizoids. Coleoptile absent. Radicula minute or absent. Germination phanerocotylar.
Cytology x = 6, 9, 10, 12 (Zostera), 18 (Heterozostera) – Chromosomes 0,9–1,6 μm long.
DNA
Phytochemistry Flavones, flavone sulphates, and sulphonated phenolic acids present. Flavonols, ellagic acid, and proanthocyanidins not found.
Use Fences, packing material.
Systematics Phyllospadix (6; P. iwatensis, P. japonicus, P. juzepczukii, P. scouleri, P. serrulatus, P. torreyi; marine coastal waters from China, the Korean Peninsula and Japan to western North America and northwestern Mexico); Zostera (4; Z. asiatica, Z. caespitosa, Z. caulescens, Z. marina; marine coastal waters along northern Atlantic and northern Pacific Oceans), Nanozostera (7; N. capensis, N. capricorni, N. japonica, N. mucronata, N. muelleri, N. noltii, N. novazelandica; marine coastal waters on both hemispheres), Heterozostera (4; H. nigricaulis, H. polychlamys, H. tasmanica: marine coastal waters along southern Australia, Tasmania; H. chilensis: Chile).
Zosteraceae are sister to Potamogetonaceae.
 |
Cladogram of Zosteraceae based on DNA data (Coyer & al. 2013). |
Literature
Aalto M. 1970. Potamogetonaceae fruits I. Recent and subfossil endocarps of the Fennoscandian species. – Acta Bot. Fenn. 88: 1-85.
Aalto M. 1974. Potamogetonaceae fruits II. Potamogeton robbinsii, a seldom fruiting North American species. – Ann. Bot. Fennici 11: 29-33.
Ackerman JD. 1993. Pollen germination and pollen tube growth in the marine angiosperm, Zostera marina L. – Aquatic Bot. 46: 189-202.
Agrawal JS. 1952. The embryology of Lilaea subulata H. B. K. with a discussion on its systematic position. – Phytomorphology 2: 15-29.
Aioi K, Komatsu T, Morita K. 1998. The world’s longest seagrass, Zostera caulescens from northeastern Japan. – Aquatic Bot. 61: 87-93.
Albergoni FG, Basso B, Tedesco G. 1978. Considerations sur l’anatomie de Posidonia oceanica (Zosteraceae). – Plant Syst. Evol. 130: 191-210.
Alberte RS, Suba GK, Procaccini G, Zimmerman RC, Fain SR. 1994. Assessment of genetic diversity of seagrass populations using DNA fingerprinting: implications for population stability and management. – Proc. Natl. Acad. Sci. U.S.A. 91: 1049-1053.
Ancibor E. 1979. Systematic anatomy of vegetative organs of the Hydrocharitaceae. – Bot. J. Linn. Soc. 78: 237-266.
Anderson LWJ. 1978. Abscisic acid induces formation of floating leaves in the heterophyllous aquatic angiosperm Potamogeton nodosus. – Science 201: 1135-1138.
Andrade IM, Mayo SJ. 2010. Molecular and morphometric patterns in Araceae from fragmented Northeast Brazilian forests. – In: Seberg O, Petersen G, Barfod AS, Davis JI (eds), Diversity, phylogeny, and evolution in the monocotyledons, Aarhus University Press, Århus, pp. 115-128.
Andrejev A. 1989. Aponogeton czokusianus A. Andrejev sp. nov. – In: Zhilin SG (ed), History of the development of the temperate forest flora in Kazakhstan, USSR, from the Oligocene to Early Miocene, Bot. Rev. 55: 292.
Appert O. 1996. Zur Verbreitung und Biologie der Gattung Appertiella (Hydrocharitaceae). – Bot. Helvetica 106: 57-71.
Arber A. 1919. On the vegetative morphology of Pistia and the Lemnaceae. – Proc. Roy. Soc. London, Ser. B, Biol. Sci. 91: 96-103.
Arber A. 1920. The vegetative morphology of Pistia and the Lemnaceae. – Proc. Roy. Soc. London, Ser. B, 91: 96-103.
Arber A. 1921. Leaves of the Helobiae. – Bot. Gaz. 72: 31-38.
Arber A. 1923. On the “squamulae intravaginales” of the Helobiae. – Ann. Bot. 37: 31-41.
Arber A. 1924. Leaves of Triglochin. – Bot. Gaz. 77: 50-62.
Arber A. 1925. On the “squamulae intravaginales” of the Alismataceae and Butomaceae. – Ann. Bot. 39: 169-173.
Arber A. 1940. Studies in flower structure VI. On the residual vascular tissue in the apices of reproductive shoots, with special reference to Lilaea and Amherstia. – Ann. Bot., N. S., 2: 617-627.
Arends JC, Laan FM van der. 1978. Somatic chromosome numbers in Lagenandra Dalzell. – Meded. Landbouwhog. Wageningen 78: 46-48.
Arends JC, Laan FM van der. 1982. Somatic chromosome numbers in Anubias Schott. – Arideana 5: 3-7.
Arends JC, Bastmeijer JD, Jacobsen N. 1982. Chromosome numbers and taxonomy in Cryptocoryne (Araceae) II. – Nord. J. Bot. 2: 453-463.
Argue CL. 1971. Pollen of the Butomaceae and Alismataceae I. Development of the pollen wall in Butomus umbellatus L. – Grana 11: 131-144.
Argue CL. 1973. The pollen of Limnocharis flava Buch., Hydrocleis nymphoides (Willd.) Buch., and Tenagocharis latifolia (Don) Buch. (Limnocharitaceae). – Grana 13: 108-112.
Argue CL. 1974. Pollen studies in the Alismataceae (Alismaceae). – Bot. Gaz. 135: 338-344.
Argue CL. 1976. Pollen studies in the Alismataceae with special reference to taxonomy. – Pollen Spores 18: 157-201.
Arrigo N, Buerki S, Sarr A, Guadagnuolo R, Kozlowski G. 2011. Phylogenetics and phylogeography of the monocot genus Baldellia (Alismataceae): mediterranean refugia, suture zones and implications for conservation. – Mol. Phylogen. Evol. 58: 33-42.
Asana JJ, Sutaria RN. 1935. On the number of chromosomes of some Indian Araceae I. Chromosomes of Arisaema murrayi (Graham) Hook. – J. Univ. Bombay 3: 24-31.
Asana JJ, Sutaria RN. 1937. On the number of chromosomes of some Indian Araceae II. – J. Univ. Bombay 7: 58-62.
Ascherson P. 1889. Potamogetonaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien II(1), W. Engelmann, Leipzig, pp. 194-214.
Ascherson P, Gürke M. 1889. Hydrocharitaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien II(1), W. Engelmann, Leipzig, pp. 238-258.
Aston HI. 1987. Limnophyton australiense sp. nov. (Alismataceae): a new generic record for Australia. – Muelleria 6: 311-316.
Azuma H, Tobe H. 2011. Molecular phylogenetic analysis of Tofieldiaceae (Alismatales): family circumscription and infrafamilial relationships. – J. Plant Res. 124: 349-357.
Backman TWH. 1991. Genotypic and phenotypic variability of Zostera marina on the west coast of North America. – Can. J. Bot. 69: 1361-1371.
Balfour IB. 1870. On the genus Halophila. – Trans. Proc. Bot. Soc. Edinb. 13: 290-343.
Banerjee M. 1971. Chromosome studies in Lemnaceae. – Rev. Roum. Embryol. Ser. Cytol. 8: 21-27.
Barabé D. 2013. Aroid floral morphogenesis in relation to phylogeny. – In: Wilkin P, Mayo SJ (eds.), Early events in monocot evolution, Systematics Association Spec. Vol. Series, Cambridge University Press, Cambridge. doi:10.1017/CBO9781139002950.012
Barabé D, Forget S. 1988. Anatomie des fleurs fertiles et sterile de Zamioculcas (Araceae). – Bull. Mus. Natl. Hist. Nat., sect. B, Adansonia 10: 411-419.
Barabé D, Lacroix C. 2001. The developmental floral morphology of Montrichardia arborescens (Araceae) revisited. – Bot. J. Linn. Soc. 135: 413-420.
Barabé D, Lacroix C. 2008a. Developmental morphology of the flower of Anthurium jenmanii: a new element in our understanding of basal Araceae. – Botany 86: 45-52.
Barabé D, Lacroix C. 2008b. Developmental morphology of the flower of Anaphyllopsis americana and its relevance to our understanding of basal Araceae. – Botany 86: 1467-1473.
Barabé D, Lacroix C. 2008c. Hierarchical developmental morphology: the case of the inflorescence of Philodendron ornatum (Araceae). – Intern. J. Plant Sci. 169: 1013-1022.
Barabé D, Chretien L, Forget S. 1987. On the pseudomonomerous gynoecia of the Araceae. – Phytomorphology 37: 139-143.
Barabé D, Bruneau A, Forest F, Lacroix C. 2002. The correlation between development of atypical bisexual flowers and phylogeny in the Aroideae (Araceae). – Plant Syst. Evol. 232: 1-19.
Barabé D, Lacroix C, Jeune B. 2002. Study of homeosis in the flower of Philodendron (Araceae): a qualitative and quantitative approach. – Ann. Bot. 90: 579-592.
Barabé D, Lacroix C, Bruneau A, Archambault, A, Gilbernau M. 2004. Floral development and phylogenetic position of Schismatoglottis (Araceae). – Intern. J. Plant Sci. 165: 173-189.
Barabé D, Lacroix C, Chouteau M, Gibernau M. 2004. On the presence of extracellular calcium oxalate crystals on the inflorescences of Araceae. – Bot. J. Linn. Soc. 146: 181-190.
Barabé D, Lacroix C, Gibernau M. 2012. Developmental floral morphology of Syngonium in the context of the tribe Caladieae (Araceae). – Willdenowia 42: 297-305.
Barnabas AD. 1982. Fine structure of the leaf epidermis of Thalassodendron ciliatum (Forsk.) den Hartog. – Aquatic Bot. 12: 41-55.
Barnabas AD. 1983. Composition and fine structural features of longitudinal veins in leaves of Thalassodendron ciliatum. – South Afr. J. Bot. 2: 317-325.
Barnabas AD. 1988. Apoplastic tracers studies in leaves of a seagrass I. Pathway through epidermal and mesophyll tissue. – Aquatic Bot. 32: 63-77.
Barnabas AD. 1989. Apoplastic tracers studies in leaves of a seagrass II. Pathway into leaf veins. – Aquatic Bot. 35: 375-386.
Barnabas AD. 1994a. Apoplastic and symplastic pathways in leaves and roots of the seagrass Halodule uninervis (Forsk.) Aschers. – Aquatic Bot. 47: 155-174.
Barnabas AD. 1994b. Anatomical, histochemical and ultrastructural features of the seagrass Phyllospadix scouleri Hook. – Aquatic Bot. 49: 167-182.
Barnabas AD, Arnott HJ. 1987. Zostera capensis Setchell: root structure in relation to function. – Aquatic Bot. 27: 309-322.
Barnabas AD, Kasavan S. 1983. Structural features of the leaf epidermis of Halodule uninervis. – South Afr. J. Bot. 2: 311-316.
Barnes E. 1934. Some observations on the genus Arisaema on the Nilgiri Hills, South India. – J. Bombay Nat. Hist. Soc. 37: 630-639.
Barroso GM. 1957. Araceae novae. – Arch. Jard. Bot. Rio de Janeiro 15: 89-98.
Baude E. 1956. Die Embryoentwicklung von Stratiotes aloides L. – Planta 46: 649-671.
Baytop A. 1982. Deux nouvelles aracées pur la Turquie. – Istanbul Univ. Ecz. Fak. Mecm. 18: 60-64.
Beal EO, Wooten JW, Kaul RB. 1982. Review of the Sagittaria engelmanniana complex (Alismataceae) with environmental correlations. – Syst. Bot. 7: 417-432.
Bedalov M. 1972. Novi broj kromosoma za vrstu Dracunculus vulgaris Schott. – Acta Bot. Croat. 31: 87-89.
Bedalov M. 1975. Cytotaxonomical and phytogeographical investigation of the species Arum italicum Mill. in Jugoslavia. – Acta Bot. Croat. 34: 143-150.
Bedalov M. 1976. Cytotaxonomical investigation in the genus Dracunculus Adans. – Biol. Écol. Médit. 3: 41-43.
Bedalov M. 1978. Sur quelques espèces diploïdes du genre Arum L. – Bull. Soc. Neuchâtel. Sci. Nat. 101: 85-93.
Bedalov M. 1981. Cytotaxonomy of the genus Arum (Araceae) in the Balkans and the Aegean area. – Bot. Jahrb. Syst. 101: 183-200.
Bedalov M. 1982. Die Gattung Arum in den Ostalpenländern. – Stapfia 10: 95-97.
Bedalov M. 1984. A new pentaploid of the genus Arum (Araceae). – Bot. Helvetica 94: 385-390.
Beentje HJ. 1999. Zosteraceae. – In: Beentje HJ (ed), Flora of tropical East Africa, A. A. Balkema, Rotterdam, The Netherlands, pp. 1-4.
Beentje HJ. 2000. Zannichelliaceae. – In: Beentje HJ, Smith SAL (eds), Flora of tropical East Africa, A. A. Balkema, Rotterdam, The Netherlands, pp. 1-4.
Beentje HJ. 2002. Cymodoceaceae. – In: Beentje HJ, Smith SAL (eds), Flora of tropical East Africa, A. A. Balkema, Rotterdam, The Netherlands, pp. 1-10.
Behnke H-D. 1995. P-type sieve element plastids and the systematics of the Arales (sensu Cronquist 1988) – with S-type plastids in Pistia. – Plant Syst. Evol. 195: 87-119.
Benzecry A, Brack-Hanes SD. 2008. A new hydrocharitacean seagrass from the Eocene of Florida. – Bot. J. Linn. Soc. 157: 19-30.
Benzing L. 1969. Beitrag zur Klärung der Verwandtschaftsverhältnisse der Tribus Areae (Aroideae-Araceae) auf vergleichend-blütenmorphologischer Grundlage. – Ph.D. diss., Universität Mainz, Germany.
Beppu T, Takimoto A. 1981. Geographical distribution and cytological variation of Lemna paucicostata Hegelm. – Bot. Mag. (Tokyo) 94: 11-20.
Beuret E. 1971. Répartition de quelque Arum des groupes Maculatum L. et Italicum Mill. – Bull. Soc. Neuchâtel. Sci. Nat. 94: 29-36.
Bhanthumnavin K, McGarry GM. 1971. Wolffia arrhiza as a possible source of inexpensive protein. – Nature 232: 495.
Bhattacharya GN. 1972. Investigations on the study of structure and behaviour of chromosomes as an aid in the interpretation of phylogeny, affinities and evolution with special reference to the family Araceae. – Ph.D. diss., University of Calcutta, India.
Bhattacharya GN. 1974. Cytological studies in the genus Alocasia G. Don. – In: Kachroo P (ed), Advancing frontiers in cytogenetis, pp. 118-122.
Bhattacharya GN. 1976 [1978]. A cytological study on the tribe Anthurieae (Araceae). – Bull. Bot. Soc. Bengal 30: 51-56.
Bhattacharyya UC. 1957. Karyotypic variation in Scindapsus officinalis Schott (Aroideae). – Caryologia 9: 286-292.
Bierzychudek P. 1982. The demography of jack-in-the-pulpit, a forest perennial that changes sex. – Ecol. Monogr. 52: 335-351.
Bigley RE,Barreca JL. 1982. Evidence for synonymizing Zostera americana den Hartog with Zostera japonica Aschers. et Graebn. – Aquatic Bot. 14: 349-356.
Björkqvist I. 1967. Studies in Alisma L. I. Distribution, variation and germination. – Opera Bot. 17: 1-128.
Björkqvist I. 1968. Studies in Alisma L. II. Chromosome studies, crossing experiments and taxonomy. – Opera Bot. 19: 1-138.
Black JM. 1913. The flowering and fruiting of Pectinella antarctica (Cymodocea antarctica). – Trans. Proc. Roy. Soc. South Aust. 37: 1-5.
Blackburn KB. 1933. Notes on the chromosomes of duckweeds (Lemnaceae) introducing the question of chromosome size. – Proc. Univ. Durham Phil. Soc. 9: 84-90.
Blanc P. 1977. Contribution à l’étude des aracées II. Remarques sur la croissance sympodiale chez l’Anthurium scandens Engl., le Philodendron fenzlii Engl. et le Philodendron speciosum Schott. – Rev.Gén. Bot. 84: 319-331.
Blanc P. 1978. Aspects de la ramification chez des aracées tropicales. – Ph.D. diss., Université Pierre et Marie Curie, Paris, France.
Blanc P. 1980. Observations sur les flagelles des Araceae. – Adansonia, sér. II, 20: 325-338.
Blodgett FH. 1923. The embryo of Lemna. – Amer. J. Bot. 10: 336-342.
Bloedel CA, Hirsch AM. 1979. Developmental studies of the leaves of Sagittaria latifolia and their relationship to the leaf base theory of monocotyledonous leaf morphology. – Can. J. Bot. 57: 420-434.
Bogin C. 1955. Revision of the genus Sagittaria. – Mem. New York Bot. Gard. 9: 179-233.
Bogner J. 1972. Revision der Arophyteae (Araceae). – Bot. Jahrb. Syst. 92: 1-63.
Bogner J. 1973 [1974]. Die Gattung Pycnospatha Thorel ex Gagnep. (Araceae). – Österr. bot. Zeitschr. 122: 199-216.
Bogner J. 1976. Die systematische Stellung von Acoropsis Conwentz, einer fossilen Aracee aus dem Bernstein. – Mitt. Bayer. Staatssamml. Paläontol. Hist. Geol. 16: 95-98.
Bogner J. 1979a. A critical list of the aroid genera. – Aroideana 1: 63-73.
Bogner J. 1979b. Two new Aridarum species and one new variety from Sarawak. – Aroideana 2: 110-121.
Bogner J. 1980. The genus Scaphispatha Brongn. ex Schott. – Aroideana 3: 4-12.
Bogner J. 1987. Morphological variations in aroids. – Aroideana 10: 4-16.
Bogner J. 1997a. New taxa of Araceae. – Sendtnera 4: 5-12.
Bogner J. 1997b. The pollen of Chlorospatha longipoda (K. Krause) Madison. – Aroideana 20: 6-10.
Bogner J. 2003. Aronstabgewächse (Araceen), anmutige und vielgestaltige Exoten. – Sukkulentenwelt (Zürich) 8: 26-29.
Bogner J. 2007. Zomicarpella maculata (Araceae) rediscovered, with notes on the tribe Zomicarpeae. – Willdenowia 37: 523-534.
Bogner J. 2008. Gorgonidium beckianum (Araceae), a new species from Bolivia. – Willdenowia 38: 195-200.
Bogner J. 2009. The free-floating aroids (Araceae) – living and fossil. – Zitteliana A48: 113-128.
Bogner J, Boyce P. 2008. Eminium jaegeri (Araceae), a new species from northwestern Iran. – Willdenowia 38: 149-153.
Bogner J, Gonçalves EG. 1999. The genus Gearum N. E. Brown (Araceae: tribe Spathicarpeae). – Aroideana 22: 20-29.
Bogner J, Gonçalves EG. 2002. Two new aroids from South America. – Willdenowia 32: 323-329.
Bogner J, Gonçalves EG. 2005. Two new species of Xanthosoma (Araceae) from South America and notes on the tribe Caladieae. – Willdenowia 35: 333-344.
Bogner J, Hannon L (†). 2007. New species of Xanthosoma and Chlorospatha (Araceae) from Colombia and a new combination in Chlorospatha. – Willdenowia 37: 331-337.
Bogner J, Hay A. 2000. Schismatoglottideae (Araceae) in Malesia II. Aridarum, Bucephalandra, Phymatarum and Piptospatha. – Telopea 9: 179-222.
Bogner J, Hesse M. 2005. Zamioculcadoideae, a new subfamily of Araceae. – Aroideana 28: 3-20.
Bogner J, Kvacek Z. 2009. A fossil Vallisneria plant (Hydrocharitaceae) from the Early Miocene freshwater deposits of the Most Basin (North Bohemia). – Aquatic Bot. 90: 119-123.
Bogner J, Nicolson DH. 1988. Revision of the South American genus Gorgonidium Schott (Araceae: Spathicarpeae). – Bot. Jahrb. Syst. 109: 529-554.
Bogner J, Nicolson DH. 1991. A revised classification of the Araceae with dichotomous keys. – Willdenowia 21: 35-50.
Bogner J, Nusbaumer L. 2012. A new species of Carlephyton (Araceae) from northern Madagascar with notes on the species of this genus. – Willdenowia 42: 209-217.
Bogner J, Petersen G. 2007. The chromosome numbers of the aroid genera. – Aroideana 30: 82-90.
Bogner J, Van Du Nguyen. 2008. A new Homalomena species (Araceae) from Vietnam. – Willdenowia 38: 524-531.
Bogner J, Mayo SJ, Sivadasan M. 1985. New species and changing concepts in Amorphophallus. – Aroideana 8: 15-25.
Bogner J, Hoffman GL, Aulenback KR. 2005. A fossilized aroid infructescence, Albertarum pueri gen. nov. et sp. nov. of Late Cretaceous (Late Campanian) age from the Horseshoe Canyon Formation of southern Alberta, Canada. – Can. J. Bot. 83: 591-598.
Bogner J, Johnson KR, Kvaček Z, Upchurch GR. 2007. New fossil leaves of Araceae from the Late Cretaceous and Paleogene of western North America. – Zitteliana A47: 133-147.
Bolkhovskikh ZV. 1983. On the morphology of pollen grains of Najas major (Najadaceae). – Bot. Žurn. 68: 448-452. [In Russian]
Bonde SD. 2000. Rhodospathodendron tomlinsonii gen. et sp. nov., an araceous viny axis from the Nawargaon intertrappean beds of India. – The Palaeobotanist 49: 85-92.
Bornet E. 1864. Recherches sur le Phucagrostis major Cavol. – Ann. Sci. Nat. Bot. V, 1: 5-51.
Bornkamm R. 1965. Die Rolle des Oxalats im Stoffwechsel höherer grüner Pflanzen. Untersuchungen an Lemna minor L. – Flora 156: 139-171.
Bouman F. 1985. Embryology. – In: Bruggen HWE van (ed), Monograph of the genus Aponogeton (Aponogetonaceae), Bibl. Bot. 137: 4-9.
Bown D. 1988. Aroids. Plants of the Arum family. – Century Press, London.
Bown D. 2000. Aroids. Plants of the Arum family. 2nd ed. – Timber Press, Portland, Oregon.
Boyce PC. 1989. A new classification of Arum with keys to the infrageneric taxa. – Kew Bull. 44: 383-395.
Boyce PC. 1993. The genus Arum. – Royal Botanic Gardens, Kew, and HMSO.
Boyce PC. 1994a. The genera Dracunculus and Helicodiceros (Araceae: Aroideae). – Thaiszia 4: 175-182.
Boyce PC. 1994b. New species of Araceae from Brunei. – Kew Bull. 49: 793-801.
Boyce PC. 1995. Introduction to the family Araceae. – Curtis’s Bot. Mag. 12: 122-125.
Boyce PC. 1994 [1995]. The genus Arum (Araceae) in Greece and Cyprus. – Ann. Mus. Goulandris 9: 27-38.
Boyce PC. 1996. The genus Hapaline (Araceae: Aroideae: Caladieae). – Kew Bull. 51: 63-82.
Boyce PC. 2006. Arum – a decade of change. – Aroideana 29: 132-137.
Boyce PC, Hay AD. 2001. A taxonomic revision of Araceae tribe Potheae (Pothos, Pothoidium and Pedicellarum) for Malesia, Australia and the tropical Western Pacific. – Telopea 9: 449-571.
Boyce PC, Nguyen Van Dzu. 1995. Pothos grandis (Araceae: Pothoideae) described and validated and architectural notes on Pothos subgen. Pothos. – Kew Bull. 50: 753-759.
Boyce PC, Poulsen AD. 1994. Notes on Pothos insignis (Araceae: Pothoideae). – Kew Bull. 49:523-529.
Boyce PC, Wong SY. 2008. Studies on Schismatoglottideae (Araceae) of Borneo VII: Schottarum and Bakoa, two new genera from Sarawak, Malaysian Borneo. – Bot. Stud. (Taipei, Taiwan) 49: 393-404.
Boyce PC, Wong SY. 2009. Schottariella
mirifica P. C. Boyce & S. Y. Wong: a new name for Schottarum
sarikeense (Araceae:
Schismatoglottideae). – Bot. Stud. 50: 269-271.
Brasier MD. 1975. An outline history of seagrass communities. – Palaeontlogy 18: 681-702.
Bröderbauer D, Diaz A, Weber A. 2012. Reconstructing the origin and elaboration of insect-trapping inflorescences in the Araceae. – Amer. J. Bot. 99: 1666-1679.
Brooks JS. 1940. The cytology and morphology of the Lemnaceae. – Ph.D. diss., Cornell University, Ithaca, New York.
Bruggen HWE van. 1968. Revision of the genus Aponogeton (Aponogetonaceae) I. – Blumea 16:243-265.
Bruggen HWE van. 1969. Revision of the genus Aponogeton (Aponogetonaceae) II. – Blumea 17: 121-137.
Bruggen HWE van. 1970. Revision of the genus Aponogeton (Aponogetonaceae) III. – Blumea 18: 457-486.
Bruggen HWE van. 1973. Revision of the genus Aponogeton (Aponogetonaceae) IV. – Bull. Jard. Bot. Natl. Belg. 43: 193-233.
Bruggen HWE van. 1985. Monograph of the genus Aponogeton (Aponogetonaceae). – Bibl. Bot. 137: 1-76.
Bruggen HWE van. 1987. Aponogetonaceae. – In: Dassanayake MD (ed), Revised Handbook Flora Ceylon 6, Amerind, New Delhi, pp. 3-16.
Bruggen HWE van. 1990. Die Gattung Aponogeton (Aponogetonaceae). – Aqua-Planta Sonderheft 2.
Bruggen HWE van. 1991. Neue Erkenntnisse über die Aponogetonaceae. – Aqua-Planta 16: 46-54.
Bruggen HWE van. 1998. Aponogetonaceae. – In: Kubitzki K (ed), The families and genera of vascular plants IV. Flowering plants. Monocotyledons. Alismatanae and Commelinanae (except Gramineae), Springer, Berlin, Heidelberg, New York, pp. 21-25.
Brunaud A. 1974. Organisation de la pousse végétative d’une lemnacée: Spirodela polyrrhiza (L.) Schleid. – Compt. Rend. Acad. Sci. Paris, sect. D, 278: 1183-1186.
Brunaud A. 1976. Ramification chez les Hydrocharitaceae I. Ontogénie du système des pousses. – Rev. Gén. Bot. 83: 397-413.
Brunaud A. 1977. Ramification chez les Hydrocharitaceae II. Organisation des rameaux latéraux. . – Rev. Gén. Bot. 84: 137-157.
Buchenau F. 1882. Beiträge zur Kenntnis der Butomaceen, Alismaceen, und Juncaginaceen. – Engl. Bot. Jahrb. Syst. 2: 465-510.
Buchenau F. 1889a. Alismaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien II(1), W. Engelmann, Leipzig, pp. 227-232.
Buchenau F. 1889b. Butomaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien II(1), W. Engelmann, Leipzig, pp. 232-234.
Buchenau F, Hieronymus G. 1889. Juncaginaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien II(1), W. Engelmann, Leipzig, pp. 222-227.
Bunting GS. 1960. The genus Schismatoglottis (Section Philonotion) in America. – Ann. Missouri Bot. Gard. 47: 69-71.
Bunting GS. 1964. Studies in Araceae. – Ann. Missouri Bot. Gard. 50: 28.
Bunting GS. 1977. Nuevas especies para la revisión de las Araceas Venezolanos. – Acta Bot. Venez. 10: 263-335.
Bunting GS. 1988. Urospathella, new genus of Venezuelan Araceae. – Phytologia 65: 391-392.
Bunting GS. 1989. A reconsideration of Urospathella (Araceae). – Phytologia 67: 139-141.
Buscalioni L, Lanza D. 1935. Le basi morfologiche, anatomiche, teratologiche della nuova famiglia delle Pistiaceae (Buscalioni e Lanza) rappresentate dai due generi Pistia ed Ambrosinia. – Malpighia 34: 103-180.
Buzgó M. 1994. Inflorescence development of Pistia stratiotes (Araceae). – Bot. Jahrb. Syst. 115: 557-570.
Buzgó M. 2001. Flower structure and development of Araceae compared with alismatids and Acoraceae. – Bot. J. Linn. Soc. 136: 393-425.
Buzgó M, Soltis DE, Soltis PS, Kim S, Ma H, Hauser BA, Leebens-Mack J, Johansen B. 2006. Perianth development in the basal monocot Triglochin maritima (Juncaginaceae). – In: Columbus JT, Friar EA, Porter JM, Prince LM, Simpson MG (eds), Monocots: comparative biology and evolution. Excluding Poales, Rancho Santa Ana Botanical Garden, Claremont, California. – Aliso 22: 107-125.
Cabrera LI, Salazar GA, Chase MW, Mayo SJ, Bogner J, Dávila P. 2008. Phylogenetic relationships of aroids and duckweeds (Araceae) inferred from coding and noncoding plastid DNA. – Amer. J. Bot. 95: 1153-1165.
Cambridge ML, Kuo J. 1979. Posidonia sinuosa and P. angustifolia (Posidoniaceae); two new taxa of seagrasses from Australia. – Aquatic Bot. 6: 307-328.
Cambridge ML, Kuo J. 1982. Morphology, anatomy and histochemistry of the Australian seagrasses genus Posidonia König (Posidoniaceae) III. Posidonia sinuosa Cambridge & Kuo. – Aquatic Bot. 14: 1-14.
Cambridge ML, Carstairs SA, Kuo J. 1983. An unusual method of vegetative propagation in Australian Zosteraceae. – Aquatic Bot. 15: 201-203.
Camenisch M, Cook CDK. 1996. Wiesneria triandra (Dalzell) Micheli (Alismataceae): a rare and unusual south Indian endemic. – Aquatic Bot. 55: 115-131.
Campbell DH. 1897. A morphological study of Najas and Zannichellia. – Proc. Calif. Acad. Sci., 3rd ser., 1: 1-61.
Campbell DH. 1898. Development of the flower and embryo of Lilaea subulata H. B. K. – Ann. Bot. 12: 1-12.
Campbell DH. 1899. Notes on the structure of the embryo-sac in Sparganium and Lysichiton. – Bot. Gaz. 27: 153-166.
Campbell DH. 1900. Studies on the Araceae. – Ann. Bot. 14: 1-24.
Campbell DH. 1905. Studies on the Araceae III. – Ann. Bot. 19: 329-349.
Campbell GKG. 1936. The anatomy of Potamogeton pectinatus. – Trans. Proc. Bot. Soc. Edinb. 32: 179-186.
Campbell LM. 2010. Four new species of Isidrogalvia (Tofieldiaceae) from the Guayana Highlands. – Harvard Pap. Bot. 15: 51-62.
Campbell LM, Dorr LJ. 2013. A synopsis of Harperocallis (Tofieldiaceae, Alismatales) with ten new combinations. – PhytoKeys 21: 37-52.
Cannon JFM. 1979. An experimental investigation of Posidonia balls. – Aquatic Bot. 6: 407-410.
Carlquist SJ, Schneider EL. 1998. Origins and nature of vessels in monocotyledons 5. Araceae subfamily Colocasioideae. – Bot. J. Linn. Soc. 128: 71-96.
Carlsen MM, Croat TB. 2013. A molecular phylogeny of the species-rich Neotropical genus Anthurium (Araceae) based on combined chloroplast and nuclear DNA. – Syst. Bot. 38: 576-588.
Carter S. 1960a. Alismataceae. – In: Hubbard CE, Milne-Redhead E (eds), Flora of tropical East Africa, Crown Agents for Oversea Governments and Administrations, London, pp. 1-16.
Carter S. 1960b. Butomaceae. – In: Hubbard CE, Milne-Redhead E (eds), Flora of tropical East Africa, Crown Agents for Oversea Governments and Administrations, London, pp. 1-3.
Caye G, Meinzies A. 1985. Observations on the vegetative development, flowering and seedling of Cymodocea nodosa (Ucria) Ascherson on the Mediterranean coasts of France. – Aquatic Bot. 22: 277-289.
Cevallos-Ferriz SRS, Stockey RA. 1988. Permineralized fruits and seeds from the Princeton chert (Middle Eocene) of British Columbia: Araceae. – Amer. J. Bot. 75: 1099-1113.
Chanda S, Nilsson S, Blackmore S. 1988. Phylogenetic trends in the Alismatales with reference to pollen grains. – Grana 27: 257-272.
Chandler C. 1943. The number of chromosomes in two species of Amorphophallus. – Bull. Torrey Bot. Club 70: 612-614.
Chandra S (ed). 1984. Edible aroids. – Clarendon Press, Oxford.
Charlton WA. 1968. Studies in the Alismataceae I. Developmental morphology of Echinodorus tenellus. – Can. J. Bot. 46: 1345-1360.
Charlton WA. 1973. Studies in the Alismataceae II. Inflorescences of Alismataceae. – Can. J. Bot. 51: 775-789.
Charlton WA. 1981. Features of the inflorescence of Triglochin maritimum. – Can. J. Bot. 59: 2108-2115.
Charlton WA. 1991. Studies in the Alismataceae IX. Development of the flower of Ranalisma humile. – Can. J. Bot. 69: 2790-2796.
Charlton WA. 1999. Studies in the Alismataceae X. Floral organogenesis in Luronium natans (L.) Raf. – Can. J. Bot. 77: 1560-1568.
Charlton WA. 2004. Studies in the Alismataceae XII. Floral organogenesis in Damasonium alisma and Baldellia ranunculoides, and comparisons with Butomus umbellatus. – Can. J. Bot. 82: 528-539.
Charlton WA, Ahmed A. 1973a. Studies in the Alismataceae III. Floral anatomy of Ranalisma humile. – Can. J. Bot. 51: 891-897.
Charlton WA, Ahmed A. 1973b. Studies in the Alismataceae IV. Developmental morphology of Ranalisma humile and comparison with two members of the Butomaceae, Hydrocleis nymphoides and Butomus umbellatus. – Can. J. Bot. 51: 899-910.
Charlton WA, Posluszny U. 1991. Meristic variation in Potamogeton flowers. – Bot. J. Linn. Soc. 106: 265-293.
Chatterjee D. 1954. Indian and Burmese species of Arisaema. – Bull. Bot. Soc. Beng. 8: 118-139.
Chaudhuri JB, Sharma A. 1979. Chromosome studies in certain members of Araceae. – Genet. Iber. 30-31: 161-187.
Chauhan KPS, Brandham PE. 1985. Chromosome and DNA variation in Amorphophallus (Araceae). – Kew Bull. 40: 745-758.
Chen J-K. 1989. Systematic and evolutionary botanical studies on Chinese Sagittaria. – Wuhan University Press, Wuhan.
Chen J-M, Chen D, Robert GW, Wang Q-F, Guo Y-H. 2004. Evolution of apocarpy in Alismatidae using phylogenetic evidence from chloroplast rbcL sequence data. – Bot. Bull. Acad. Sin. 45: 33-40.
Chen J-M, Robert GW, Wang Q-F. 2004. Evolution of aquatic life forms in Alismatidae: phylogenetic estimation from chloroplast rbcL sequence data. – Israel J. Plant Sci. 52: 323-329.
Chen L-Y, Chen J-M, Gituru RW, Temam TD, Wang Q-F. 2012. Generic phylogeny and historical biogeography of Alismataceae, inferred from multiple DNA sequences. – Mol. Phylogen. Evol. 63: 407-416.
Chen L-Y, Chen J-M, Gituru RW, Wang Q-F. 2012. Generic phylogeny, historical biogeography and character evolution of the cosmopolitan aquatic plant family Hydrocharitaceae. – BMC Evol. Biol. 12: 30.
Chen L-Y, Chen J-M, Gituru RW, Wang Q-F. 2013. Eurasian origin of Alismatidae inferred from statistical dispersal-vicariance analysis. – Mol. Phylogen. Evol. 67: 38-42.
Cho Y, Palmer JD. 1999. Multiple acquisitions via horizontal transfer of a group I intron in the mitochondrial coxI gene during evolution of the Araceae family. – Mol. Biol. Evol. 16: 1155-1165.
Chouteau M, Gibernau M, Barabé D. 2008. Relationships between floral characters, pollination mechanisms, life forms, and habitats in Araceae. – Bot. J. Linn. Soc. 156: 29-42.
Chrysler MA. 1907. The structure and relationships in Potamogetonaceae and allied families. – Bot. Gaz. 44: 161-188.
Chum S. 1985. La variation intra-individuelle du nombre chromosomique dans la famille des Aracées. – Bull. Soc. Bot. France, Acta Bot. 132: 73-79.
Clark HL, Thieret JW. 1968. The duckweeds of Minnesota. – Michigan Bot. 7: 67-76.
Claudel C, Buerki S, Chatrou LW, Antonelli A, Alvarez N, Hetterscheid W. 2017. Large-scale phylogenetic analysis of Amorphophallus (Araceae) derived from nuclear and plastid sequences reveals new subgeneric delineation. – Bot. J. Linn. Soc. 184: 32-45.
Coccuci A. 1966. Embriología de Synandrospadix vermitoxicus. – Kurtziana 3: 157-181.
Coiffard C, Mohr BAR. 2015. Lejalia sagenopteroides gen. nov. et comb. nov.: a new tropical member of Araceae from Late Cretaceous strata of northern Gondwana (Jebel Abyad, Sudan). – Taxon 64: 987-997.
Coiffard C, Mohr BAR, Bernardes-de-Oliveira MEC. 2013. The Early Cretaceous aroid, Spixiarum kipea gen. et sp. nov., and implications on early dispersal and ecology of basal monocots. – Taxon 62: 997-1008.
Collinson ME. 1982. A reassessment of fossil Potamogetoneae fruits with description of new material from Saudi Arabia. – Tertiary Res. 4: 83-104.
Collinson ME, Kvaček Z, Zastawniak E. 2002. The aquatic plants Salvinia (Salviniales) and Limnobiophyllum (Arales) from the Late Miocene flora of Sosnica (Poland). – Acta Palaeobotanica 41: 253-282.
Colombo PM, Rascio N, Cinelli F. 1983. Posidonia oceanica (L.) Delile: a structural study of the photosynthetic apparatus. – Mar. Ecol. 4: 133-145.
Conacher CA, Pointer IR, Donohue MO. 1994. Morphology, flowering and seed production of Zostera capricorni Aschers. in subtropical Australia. – Aquatic Bot. 49: 33-46.
Cook CDK. 1982. Pollination mechanisms in the Hydrocharitaceae. – In: Symoens JJ, Hooper SS, Compère P (eds), Studies on aquatic vascular plants. Proceedings of The International Colloquium on Aquatic Vascular Plants (Brussels, 23-25 January, 1981), Royal Botanic Society of Belgium, Brussels, pp. 1-15.
Cook CDK. 1985. A revision of the genus Apalanthe (Hydrocharitaceae). – Aquatic Bot. 21: 157-164.
Cook CDK. 1998a. Butomaceae. – In: Kubitzki K (ed), The families and genera of vascular plants IV. Flowering plants. Monocotyledons. Alismatanae and Commelinanae (except Gramineae), Springer, Berlin, Heidelberg, New York, pp. 100-102.
Cook CDK. 1998b. Hydrocharitaceae. – In: Kubitzki K (ed), The families and genera of vascular plants IV. Flowering plants. Monocotyledons. Alismatanae and Commelinanae (except Gramineae), Springer, Berlin, Heidelberg, New York, pp. 234-248.
Cook CDK, Lüönd R. 1982a. A revision of the genus Hydrilla (Hydrocharitaceae). – Aquatic Bot. 13: 485-504.
Cook CDK, Lüönd R. 1982b. A revision of the genus Nechamandra (Hydrocharitaceae). – Aquatic Bot. 13: 505-513.
Cook CDK, Lüönd R. 1982c. A revision of the genus Hydrocharis (Hydrocharitaceae). – Aquatic Bot. 14: 177-204.
Cook CDK, Lüönd R. 1983. A revision of the genus Blyxa (Hydrocharitaceae). – Aquatic Bot. 15: 1-52.
Cook CDK, Urmi-König K. 1983a. A revision of the genus Stratiotes (Hydrocharitaceae). – Aquatic Bot. 16: 213-249.
Cook CDK, Urmi-König K. 1983b. A revision of the genus Limnobium including Hydromystria (Hydrocharitaceae). – Aquatic Bot. 17: 1-27.
Cook CDK, Urmi-König K. 1983c. A revision of the genus Ottelia (Hydrocharitaceae) 2. The species of Eurasia, Australia and America. – Aquatic Bot. 20: 131-177.
Cook CDK, Urmi-König K. 1984. A revision of the genus Egeria (Hydrocharitaceae). – Aquatic Bot. 19: 73-96.
Cook CDK, Urmi-König K. 1985. A revision of the genus Elodea (Hydrocharitaceae). – Aquatic Bot. 21: 111-156.
Cook CDK, Lüönd R, Nair B. 1981. Floral biology of Blyxa octandra (Roxb.) Planchon ex Thwaites (Hydrocharitaceae). – Aquatic Bot. 10: 61-68.
Cook CDK, Symoens J-J, Urmi-König K. 1983. A revision of the genus Ottelia (Hydrocharitaceae) 1. Generic considerations. – Aquatic Bot. 18: 263-274.
Cook MT. 1908. The development of the embryo sac and embryo of Potamogeton lucens. – Bull. Torrey Bot. Club 35: 209-218.
Cooper LW, McRoy CP. 1988. Anatomical adaptation to rocky substrates and surf exposure by the seagrass genus Phyllospadix. – Aquatic Bot. 32: 365-381.
Costa JY, Forni-Martins ER. 2003. Karyology of some Brazilian species of Alismataceae. – Bot. J. Linn. Soc. 143: 159-164.
Costa JY, Forni-Martins ER, Vanzela ALL. 2006. Karyotype characterization of five Brazilian species of Echinodorus (Alismataceae) with chromosomal banding and 45S rDNA FISH. – Plant Syst. Evol. 257: 119-127.
Coté GG. 2009. Diversity and distribution of idioblasts producing calcium oxalate crystals in Dieffenbachia seguine (Araceae). – Amer. J. Bot. 96: 1245-1254.
Cox PA, Humphries CJ. 1993. Hydrophilous pollination and breeding system evolution in seagrasses: a phylogenetic approach to the evolutionary ecology of the Cymodoceaceae. – Bot. J. Linn. Soc. 113: 217-226.
Cox PA, Knox RB. 1989. Two-dimensional pollination in hydrophilous plants: convergent evolution in the genera Halodule (Cymodoceaceae), Halophila (Hydrocharitaceae), Ruppia (Ruppiaceae), and Lepilaena (Zannichelliaceae). – Amer. J. Bot. 76: 164-175.
Cox PA, Tomlinson PB. 1988. Pollination ecology of a seagrass, Thalassia testudinum (Hydrocharitaceae), in St. Croix. – Amer. J. Bot. 75: 958-965.
Coyer JA, Hoarau G, Kuo J, Tronholm A, Veldsink J, Olsen JL. 2013. Phylogeny and temporal divergence of the seagrass family Zosteraceae using one nuclear and three chloroplast loci. – Syst. Biodiv. 11: 271-284.
Crawford DJ, Landolt E. 1993. Allozyme studies in Spirodela (Lemnaceae): variation among conspecific clones and divergence among the species. – Syst. Bot. 18: 389-394.
Crawford DJ, Landolt E. 1995. Allozyme divergence among species of Wolffia (Lemnaceae). – Plant Syst. Evol. 197: 59-70.
Crawford DJ, Landolt E, Les DH, Tepe E. 1995. Allozyme divergence among species of Wolffiella (Lemnaceae). – Amer. J. Bot. 82: 122-123.
Crawford DJ, Landolt E, Les DH. 1996. An allozyme study of two sibling species of Lemna (Lemnaceae) with comments on their morphology, ecology, and distribution. – Bull Torrey Bot. Club 123: 1-6.
Crawford DJ, Landolt E, Les DH, Tepe E. 1997. Allozyme variation and the taxonomy of Wolffiella (Lemnaceae). – Aquatic Bot. 58: 43-54.
Crawford DJ, Landolt E, Les DH, Kimball RT. 2001. Allozyme studies in Lemnaceae: variation and relationships in Lemna sections Alatae and Biformes. – Taxon 50: 987-999.
Crawford DJ, Landolt E, Les DH, Kimball RT. 2006. Speciation in duckweeds (Lemnaceae): phylogenetic and ecological inferences. – In: Columbus JT, Friar EA, Porter JM, Prince LM, Simpson MG (eds), Monocots: comparative biology and evolution. Excluding Poales, Rancho Santa Ana Botanical Garden, Claremont, California. – Aliso 22: 231-242.
Crepet WL. 1978. Investigations of angiosperms from the Eocene of North America: an aroid inflorescence. – Rev. Palaeobot. Palyn. 25: 241-252.
Croat TB. 1979. The distribution of Araceae. – In: Larsen K, Holm-Nielsen L (eds), Tropical botany, Academic Press, London, pp. 291-308.
Croat TB. 1980. Flowering behaviour of the neotropical genus Anthurium (Araceae). – Amer. J. Bot. 67: 888-904.
Croat TB. 1981. A revision of Syngonium (Araceae). – Ann. Missouri Bot. Gard. 68: 565-651.
Croat TB. 1983. A revision of Anthurium (Araceae of Mexico and Central Am. Part I. Mexico and Middle Am.). – Ann. Missouri Bot. Gard. 70: 211-420.
Croat TB. 1988. Ecology and life-forms of Araceae. – Aroideana 11: 4-56.
Croat TB. 1990 [1992]. A comparison of aroid classification systems. – Aroideana 13: 44-63.
Croat TB. 1997. A revision of Philodendron subgenus Philodendron (Araceae) for Mexico and Central America. – Ann. Missouri Bot. Gard. 84: 311-704.
Croat TB. 1998a. History and current status on systematic research with Araceae. – Aroideana 21: 26-145.
Croat TB, Yu G. 2006. Four new species of Philodendron (Araceae) from South America. – Willdenowia 36: 885-894.
Croat TB. 1998b. Tropical aroids: taxonomy, diversity and ecology. – In: Mathew P, Sivadasan M (eds), Diversity and Taxonomy of Tropical Flowering Plants, Mento Books, Calicut, pp. 235-286.
Croat TB, Bunting GS, 1979. Standardization of Anthurium descriptions. – Aroideana 2: 15-25.
Croat TB, Carlsen MM. 2013. A reassessment of Anthurium species with palmately divided leaves, and a reinterpretation of Anthurium section Dactylophyllium (Araceae). – PhytoKeys 23: 41-54.
Croat TB, Hannon LP. 2004. Chlorospatha of Antioquia (Colombia). – Aroideana 27: 2-37.
Croat TB, Hannon LP. 2015. A revision of the genus Chlorospatha (Araceae). – Ann. Missouri Bot. Gard. 101: 1-259.
Croat TB, Yates ED, Hayworth DA. 2005. New taxa of Anthurium and Philodendron (Araceae) from western Amazonia. – Willdenowia 35: 345-358.
Croat TB, Yu G. 2006. Four new species of Philodendron (Araceae) from South America. – Willdenowia 36: 885-894.
Cruden RW. 1991. A revision of Isidrogalvia (Liliaceae): recognition for Ruiz and Pavon’s genus. – Syst. Bot. 16: 270-282.
Cruden RW, Dorr LJ. 1992. A previously unrecognized Isidrogalvia (Liliaceae) from Bolivia. – Brittonia 44: 368-369.
Cuenca A. Petersen G, Seberg O. 2013. the complete sequence of the mitochondrial genome of Butomus umbellatus – a member of an early branching lineage of Monocotyledons. – PloS ONE 8, e61552.
Culley DD, Rejmankova E, Kvet J, Frye JB. 1981. Production, chemical quality and use of duckweeds (Lemnaceae) in aquaculture, waste management, and animal feeds. – J. World Maric. Soc. 12: 27-49.
Cusimano N, Zhang L-B, Renner SS. 2008. Reevaluation of the cox1 group I intron in Araceae and angiosperms indicates a history dominated by loss rather than horizontal transfer. – Mol. Biol. Evol. 25: 265-276.
Cusimano N, Barrett MD, Hetterscheid WLA, Renner SS. 2010. A phylogeny of the Areae (Araceae) implies that Typhonium, Sauromatum, and the Australian species of Typhonium are distinct clades. – Taxon 59: 439-447.
Cusimano N, Bogner J, Mayo SJ, Boyce PC, Wong SY, Hesse M, Hetterscheid WLA, Keating RC, French JC. 2011. Relationships within the Araceae: comparison of morphological patterns with molecular phylogenies. – Amer. J. Bot. 98: 654-668.
Cusimano N, Sousa A, Renner SS. 2012. Maximum likelihood inference implies a high, not a low, ancestral haploid chromosome number in Araceae, with a critique of the bias introduced by ‘x’. – Ann. Bot. 109: 681-692.
Dahlgren KVO. 1939. Endosperm- und Embryobildung bei Zostera marina. – Bot. Not. 1939: 607-615.
Damerval C. 1980. Contribution à l’étude caryosystématique des Aracées. – Rev. Cytol. Biol. Vég. Bot. 3: 291-300.
Dandy JE. 1937. The genus Potamogeton L. in tropical Africa. – Bot. J. Linn. Soc. 50: 507-540.
Dandy JE. 1970. Potamogeton and Ruppia in the Azores. – Bol. Soc. Brot. 44: 1-7.
Dash S, Kanungo SK, Dinda SC. 2013. Detailed anatomical study of Aponogeton natans (Linn.) Engl. & Krause. An important folklore medicine. – Res. J. Pharmacy Technol. 6: 398-402.
Daubs EN. 1965. A monograph of Lemnaceae. – Illinois Biological Monographs 34, The University of Illinois Press, Urbana, Illinois.
Daumann E. 1931. Nektarabscheidung in der Blütenregion einiger Araceen. Zugleich ein Hinweis auf die Bargersche Methode. – Planta 12: 34-48.
Daumann E. 1963. Zur Frage nach dem Ursprung der Hydrogamie. Zugleich ein Beitrag zur Blütenökologie von Potamogeton. – Preslia 35: 23-30.
Daumann E. 1964. Zur Morphologie der Blüte von Alisma plantago-aquatica L. – Preslia 36: 226-239.
Daumann E. 1965. Insekten- und Windbestäubung bei Alisma plantago-aquatica L. Ein Beitrag zur experimentellen Blütenökologie. – Österr. Bot. Zeitschr. 112: 295-310.
De Cock AWAM. 1978. Germination of the thread like pollen grains of the seagrass Zostera marina L. – Bull. Soc. Bot. France Act. Bot. 1-2: 145-148.
De Cock AWAM. 1980. Flowering pollination and fruiting in Zostera marina L. – Aquatic Bot. 9: 201-220.
Dilcher DL, Daghlian CP. 1977. Investigations of angiosperms from the Eocene of south-eastern North America: Philodendron leaf remains. – Amer. J. Bot. 64: 526-534.
Doohan ME, Newcomb EH. 1975. Leaf ultrastructure and 13C values of three seagrasses from the Great Barrier Reef. – Aust. J. Plant Physiol. 3: 9-23.
Dorofeev PI. 1978. On the taxonomy of the Neogene genus Caulinia Willd. – Bot. Žurn. 63: 1089-1101.
Drew EA. 1983. Sugars, cyclitols and seagrass phylogeny. – Aquatic Bot. 15: 387-408.
Dring JV, Kite GC, Nash RJ, Reynolds T. 1995. Chemicals in aroids: a survey, including new results for polyhydroxy alkaloids and alkylresorcinols. – Bot. J. Linn. Soc. 117: 1-12.
Ducker SC, Knox RB. 1976. Submarine pollination in seagrasses. – Nature 263: 705-706.
Ducker SC, Foord NJ, Knox RB. 1977. Biology of Australian sea grasses: the genus Amphibolis C. Agardh (Cymodoceaceae). – Aust. J. Bot. 25: 67-95.
Ducker SC, Pettitt JM, Knox RB. 1978. Biology of Australian sea grasses: pollen development and submarine pollination in Amphibolis antarctica and Thalassodendron ciliatum (Cymodoceaceae). – Aust. J. Bot. 26: 265-285.
Dudley MG. 1937. Morphological and cytological studies of Calla palustris. – Bot. Gaz. 98: 556-571.
Duggar BM. 1900. Studies in the development of the pollengrain of Symplocarpus foetidus and Peltandra undulata. – Bot. Gaz. 29: 81-98.
Earl G. 1955. Chromosome numbers in the Araceae. – Virginia J. Sci., N. S., 6: 249-250.
Earl G. 1956. The chromosome morphology and speciation of some species in the genus Aglaonema. – Virginia J. Sci., N. S., 7: 287.
Earl G. 1957. Chromosome numbers and speciation in the genus Zantedeschia. – Plant Life 13: 140-146.
Eber E. 1934. Karpellbau und Plazentationsverhältnisse in der Reihe der Helobiae. – Flora 127: 273-330.
Eie S. 1972. Floral anatomy in Tofieldia pusilla (Michx.) Pers. with special reference to the gynoecium. – Norsk Bot. Tidskr. 19: 31-36.
Engler A. 1877a. Vergleichende Untersuchungen über die morphologischen Verhältnisse der Araceae I. – Nova Acta Leopoldina. Abhandl. Kaiserl. Leopoldinisch-Carolinischen Deutsch. Akad. Naturf. 39: 135-158.
Engler A. 1877b. Vergleichende Untersuchungen über die morphologischen Verhältnisse der Araceae II. Über Blattstellung und Sproßverhältnisse der Araceae. – Nova Acta Leopoldina. Abhandl. Kaiserl. Leopoldinisch-Carolinischen Deutsch. Akad. Naturf. 39: 159-232.
Engler A. 1889a. Aponogetonaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien II(1), W. Engelmann, Leipzig, pp. 218-222.
Engler A. 1889b. Araceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien II(3), W. Engelmann, Leipzig, pp. 102-153.
Engler A. 1889c. Lemnaceae (Wasserlinsen). – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien II(3), W. Engelmann, Leipzig, pp. 154-164.
Engler A. 1990. Comparative studies on the morphology of the Araceae II. On leaf placement and shoot organization of Araceae. Transl. by TS Ray and SS Renner. – Englera 12: 1-140.
Ernst-Schwarzenbach M. 1945. Zur Blütenbiologie einiger Hydrocharitaceen. – Ber. Schweiz. Bot. Ges. 55: 33-69.
Ertl PO. 1932. Vergleichende Untersuchungen über die Entwicklung der Blattnervatur der Araceen. – Flora 126: 115-248.
Erwin DM, Stockey RA. 1989. Permineralized monocotyledons from the Middle Eocene Princeton chert (Allenby Formation) of British Columbia: Alismataceae. – Can. J. Bot. 67: 2636-2645.
Espíndola A, Buerki S, Bedalov M, Küpfer P, Alvarez N. 2010. New insights into the phylogenetics and biogeography of Arum (Araceae): unravelling its evolutionary history. – Bot. J. Linn. Soc. 163: 14-32.
Ewin DM, Stockey RA. 1989. Permineralized monocotyledons from the Middle Eocene Princeton chert (Allenby Formation) of British Columbia: Alismataceae. – Can. J. Bot. 67: 2636-2645.
Eyde RH, Nicolson DH, Sherwin P. 1967. A survey of floral anatomy in Araceae. – Amer. J. Bot. 54: 478-497.
Fassett NC. 1955. Echinodorus of the American tropics. – Rhodora 57: 133-156, 174-188, 202-212.
Feitoza LL, Felix LP, Castro AAJF, Carvalho R. 2009. Cytogenetics of Alismatales s.l.: chromosomal evolution and C-banding. – Plant Syst. Evol. 280: 119-131.
Feitoza LL, Martins MIG, Castro AAJF, Félix LP, Carvalho R. 2010. Cytogenetics of Alismataceae and Limnocharitaceae: CMA/DAPI banding and 45S rDNA sites. – Plant Syst. Evol. 286: 199-208.
Fernald ML. 1918. The diagnostic character of Vallisneria americana. – Rhodora 20: 108-110.
Fernald ML. 1932. The linear-leaved North American species of Potamogeton, section Axillares. – Mem. Amer. Acad. Arts Sci., N. S., 17: 1-183.
Fernando DD, Cass DD. 1997. Developmental assessment of sexual reproduction in Butomus umbellatus (Butomaceae): female reproductive component. – Ann. Bot. 80: 457-467.
Fox MG, French JC. 1988. Systematic occurrence of sterols in latex of Araceae: Colocasioideae. – Amer. J. Bot. 75: 132-137.
French JC. 1985a. Patterns of endothecial wall thickenings in Araceae: subfamilies Calloideae, Lasioideae and Philodendroideae. – Bot. Gaz. 146: 521-533.
French JC. 1985b. Patterns of endothecial wall thickenings in Araceae: subfamilies Pothoideae and Monsteroideae. – Amer. J. Bot. 72: 472-486.
French JC. 1986a. Ovular vasculature in Araceae. – Bot. Gaz. 147: 478-495.
French JC. 1986b. Patterns of endothecial wall thickenings in Araceae: subfamilies Colocasioideae, Aroideae, and Pistioideae. – Bot. Gaz. 147: 166-179.
French JC. 1986c. Patterns of stamen vasculature in the Araceae. – Amer. J. Bot. 73: 434-449.
French JC. 1987a. Systematic occurrence of a sclerotic hypodermis in roots of Araceae. – Amer. J. Bot. 74: 891-903.
French JC. 1987b. Structure of ovular and placental trichomes of Araceae. – Bot. Gaz. 148: 198-208.
French JC. 1987c. Systematic survey of resin canals in roots of Araceae. – Bot. Gaz. 148: 360-371.
French JC. 1988. Systematic occurrence of anastomosing laticifers in Araceae. – Bot. Gaz. 149: 71-81.
French JC. 1997. Vegetative anatomy. – In: Mayo SJ, Bogner J, Boyce PC (eds), The genera of Araceae, Royal Botanic Gardens, Kew, pp. 9-24.
French JC, Fox JC. 1988. Systematic occurrence of sterols in latex of Araceae: subfamily Colocasioideae. – Amer. J. Bot. 75: 132-137.
French JC, Tomlinson PB. 1981a. Vascular patterns in stems of Araceae: subfamily Pothoideae. – Amer. J. Bot. 68: 713-729.
French JC, Tomlinson PB. 1981b. Vascular patterns in stems of Araceae: subfamily Monsteroideae. – Amer. J. Bot. 68: 1115-1129.
French JC, Tomlinson PB. 1981c. Vascular patterns in stems of Araceae: subfamilies Calloideae and Lasioideae. – Bot. Gaz. 142: 366-381.
French JC, Tomlinson PB. 1981d. Vascular patterns in stems of Araceae: subfamily Philodendroideae. – Bot. Gaz. 142: 550-563.
French JC, Tomlinson PB. 1983. Vascular patterns in stems of Araceae: subfamilies Colocasioideae, Aroideae and Pistioideae. – Amer. J. Bot. 70: 756-771.
French JC, Tomlinson PB. 1984. Patterns of stem vasculature in Philodendron. – Amer. J. Bot. 71: 1432-1443.
French JC, Chung MG, Hur YK. 1995. Chloroplast DNA phylogeny of the Ariflorae. – In: Rudall PJ, Cribb PJ, Cutler DF, Humphries CJ (eds), Monocotyledons: systematics and evolution, Royal Botanic Gardens, Kew, pp. 255-275.
Friis EM. 1985. Angiosperm fruits and seeds from the Middle Miocene of Jutland (Denmark). Alismataceae. – Kong. Danske Vidensk. Selsk., Biol. Skr. 24(3): 71-73.
Friis EM, Crane PR, Pedersen KR. 2004. Araceae from the early Cretaceous of Portugal: evidence on the emergence of monocotyledons. – Proc. Natl. Acad. Sci. U.S.A. 101: 16565-16570.
Fukai S. 2004. Floral initiation and development of the sex-changing plant Arisaema sikokianum (Araceae). – Intern. J. Plant Sci. 165: 739-744.
Furness CA, Banks H. 2010. Pollen evolution in the early-divergent monocot order Alismatales. – Intern. J. Plant Sci. 171: 713-739.
Furtado CX. 1939. Notes on some Indo-Malaysian Homalomena species. – Gard. Bull. Straits Settlement 10: 183-238.
Gaiser L. 1927. Chromosome numbers and species characters in Anthurium. – Proc. Trans. Roy. Soc. Canada 21: 1-137.
Gamerro JC. 1968. Observaciones sobre la biologia floral y morfología de la potamogetonácea Ruppia cirrhosa (Petag.) Grande (=’R. spiralis’ L. ex Dum.). – Darwiniana 14: 575-608.
Gandolfo MA, Zamaloa MC, Cúneo RN, Archangelsky A. 2009. Potamogetonaceae fossil fruits from the Tertiary of Patagonia, Argentina. – Intern. J. Plant Sci. 170: 419-428.
Gardner RO. 1976. Binucleate pollen in Triglochin L. – New Zealand J. Bot. 14: 115-116.
Gatin C-L. 1921. Première contribution a l’étude de l’embryon et de la germination des Aracées. – Ann. Sci. Natur. Bot., sér. 10, 3: 145-180.
Gauthier M-P L, Barabé D, Bruneau A. 2008. Molecular phylogeny of the genus Philodendron (Araceae): delimitation and infrageneric classification. – Bot J. Linn. Soc. 156: 13-27.
Gibernau M, Chartier M, Barabé D. 2010. Recent advances towards an evolutionary comprehension of Araceae pollination. – In: Seberg O, Petersen G, Barfod AS, Davis JI (eds), Diversity, phylogeny, and evolution in the monocotyledons, Aarhus University Press, Århus, pp. 101-114.
Gibson RJH. 1905. The axillary scales of aquatic monocotyledons. – Bot. J. Linn. Soc. 37: 228-236.
Gill LS, Chiannappa CC. 1971 [1972]. Another tetraploid species in Anthurium (Araceae). – Baileya 18: 93-95.
Godziemba-Czyk J. 1970. Vegetative and resting forms of Wolffia arrhiza 2. Anatomy, physical and physiological properties. – Acta Soc. Bot. Pol. 39: 421-443.
Goldberg B. 1941. Life history of Peltandra virginica. – Bot. Gaz. 102: 641-662.
Gonçalves EG. 1999a. A new pedate-leaved species of Xanthosoma Schott (Araceae: tribe Caladieae) with linear leaflets, from the Brazilian Pantanal. – Aroideana 22: 3-6.
Gonçalves EG. 1999b. A revised key for the genus Asterostigma Fisch. & C. A. Mey. (Araceae: tribe Spathicarpeae) and a new species from southeastern Brazil. – Aroideana 22: 30-33.
Gonçalves EG. 2000. Two new species of Philodendron (Araceae) from Central Brazil. – Kew Bull. 55: 175-180.
Gonçalves EG. 2002. Sistemática e evolução da tribo Spathicarpeae (Araceae). – Ph.D. diss., Universidade de São Paulo, Brazil.
Gonçalves EG. 2002 [2003]. New aroid taxa from Brazil. – Aroideana 25: 16-35.
Gonçalves EG. 2005a. A new species of Anthurium (Araceae) from Espírito Santo State, eastern Brazil. – Feddes Repert. 116: 92-95.
Gonçalves EG. 2005b. Two new Andean genera for the tribe Spathicarpeae (Araceae). – Willdenowia 35: 319-326.
Gonçalves EG. 2012a. Lorenzia (Araceae – Spathicarpeae): a new genus from northern Brazil supported by matK sequence data. – Syst. Bot. 37: 48-52.
Gonçalves EG. 2012b. A revision of the small genus Zomicarpa Schott. – Kew Bull. 67: 443-449.
Gonçalves EG, Temponi LG. 2004. A new Monstera (Araceae: Monsteroideae) from Brazil. – Brittonia 56: 72-74.
Gonçalves EG, Pavia EAS, Nadruz Coelho M-A. 2004. A preliminary survey of petiolar collenchyma in the Araceae. – Ann. Missouri Bot. Gard. 91: 473-484.
Gonçalves EG, Diener PSA, Sousa C, Alarcao G de, Pina GO. 2004. A preliminary survey of gynoecium morphology in Xanthosoma (Araceae). – Aroideana 27: 182-189.
Gonçalves EG, Mayo SJ, Sluys M-A van, Salatino A. 2007. Combined genotypic-phenotypic phylogeny of the tribe Spathicarpeae (Araceae) with reference to independent events of invasion to Andean regions. – Mol. Phylogen. Evol. 43: 1023-1039.
Gonçalves-Souza P, Gonçalves EG, Paiva EAS. 2016. Extrafloral nectaries in Philodendron (Araceae): distribution and structure. – Bot. J. Linn. Soc. 180: 229-240.
Goor ACJ van. 1919. Het zeegrass (Zostera marina L.) en zijn beteekenis voor het leven der visschen. – Rapp. Verh. Rijksinst. Visserijonderz. 1: 415-498.
Gottsberger G, Amaral A Jr. 1984. Pollination strategies in Brazilian Philodendron species. – Ber. Deutsch. Bot. Ges. 97: 391-410.
Gottsberger G, Silberbauer-Gottsberger I. 1991. Olfactory and visual attraction of Erioscelis emarginata (Cyclo-cephalini, Dynastidae) to the inflorescences of Philodendron selloum (Araceae). – Biotropica 23: 23-28.
Govaerts R, Frodin DG, Bogner J, Boos J, Boyce P, Cosgriff B, Croat TB et al. 2002. World checklist and bibliography of Araceae and Acoraceae. – Royal Botanic Gardens, Kew, United Kingdom.
Govindappa DA, Najdu TRB. 1956. The embryo sac and endosperm Blyxa oryzetorum Hook. f. – J. Indian Bot. Soc. 35: 417-422.
Gow JE. 1907. Morphology of Spathyema foetida. – Bot. Gaz. 43: 131-136.
Gow JE. 1908. Studies in the Araceae. – Bot. Gaz. 46: 35-42.
Gow JE. 1913a. Observations on the morphology of the aroids. – Bot. Gaz. 56: 127-142.
Gow JE. 1913b. Phylogeny of the Araceae. – Proc. Iowa Acad. Sci. 20: 161-168.
Graaf A de, Arends JC. 1986. The occurrence of Cryptocoryne and Lagenandra (Araceae) on Sri Lanka. – Nord. J. Bot. 6: 757-764.
Grafl I. 1940. Cytologische Untersuchungen an Sauromatum guttatum. – Österr. Bot. Zeitschr. 89: 81-118.
Graves AH. 1908. The morphology of Ruppia maritima. – Conn. Acad. Arts Sci. 14: 59-170.
Grayum MH. 1984. Palynology and phylogeny of the Araceae. – Ph.D. diss., University of Massachusetts, Amherst, Massachusetts.
Grayum MH. 1985. Evolutionary and ecological significance of starch storage in pollen of the Araceae. – Amer. J. Bot. 72: 1565-1577.
Grayum MH. 1986a. Correlations between pollination biology and pollen morphology in the Araceae, with some implications for angiosperm evolution. – In: Ferguson IK, Blackmore S (eds), Pollen and spores: form and function, Elsevier, London, pp. 313-327.
Grayum MH. 1986b. Phylogenetic implications of pollen nuclear number in the Araceae. – Plant Syst. Evol. 151: 145-161.
Grayum MH. 1986c. New taxa of Caladium, Chlorospatha, and Xanthosoma (Araceae: Colocasioideae) from southern Central America and northwestern Colombia. – Ann. Missouri Bot. Gard. 73: 462-474.
Grayum MH. 1990. Evolution and phylogeny of the Araceae. – Ann. Missouri Bot. Gard. 77: 628-697.
Grayum MH. 1991. Systematic embryology of the Araceae. – Bot. Rev. 57: 167-203.
Grayum MH. 1992. Comparative external pollen ultrastructure of the Araceae and putatively related taxa. – Monogr. Syst. Bot. Missouri Bot. Gard. 43: 1-167.
Grayum MH. 1996. Revision of Philodendron subgen. Pteromischum for Pacific and Caribbean tropical America. – Monogr. Syst. Bot. Missouri Bot. Gard. 47: 1-233.
Grear JW. 1966. Cytogeography of Orontium aquaticum. – Rhodora 68: 25-34.
Green EP, Short FT (eds). 2003. World atlas of seagrasses. – University of California Press, Berkeley.
Gregor H-J. 1980. Trapa zapfei Berger aus dem Untermiozän von Langau bei Geras (Niederösterreich) – eine Hydrocharacee. – Ann. Naturhist. Mus. Wien 83: 105-118.
Gregor H-J. 1991. Ein neues fossils Seegras – Posidocea frickhinger nov. gen. et spec. im Paläogen Oberitaliens (Verona). – Zeitschr. Docum. Nat. 65: 1-11.
Gregor H-J, Bogner J. 1984. Fossile Araceen Mitteleuropas und ihre rezenten Vergleichsformen. – Doc. Nat. 19: 1-12.
Gregor H-J, Bogner J. 1989. Neue Untersuchungen an tertiären Araceen II. – Doc. Nat. 49: 12-22.
Greuter W. 1984. Les Arum de la Crète. – Bot. Helvetica 94: 15-22.
Grob GBJ, Gravendeel BB, Eurlings MCM, Hetterscheid WLA. 2002. Phylogeny of the tribe Thomsonieae (Araceae) based on chloroplast matK and trnL intron sequences. – Syst. Bot. 27: 453-467.
Grob GBJ, Gravendeel BB, Eurlings MCM. 2004. Potential phylogenetic utility of the nuclear FLORICAULA/LEAFY second intron: comparison with three chloroplast DNA regions in Amorphophallus (Araceae). – Mol. Phylogen. Evol. 30: 13-23.
Guha R, Mondal MS. 1999. Taxonomy and pollen morphology of the genus Ruppia L. (Ruppiaceae) in India with special reference to systematic position. – Geophytologist 29: 17-23.
Guignard L. 1901. La double fécondation dans le Najas major. – J. Bot. 15: 205-213.
Guo Y-H, Cook CDK. 1989. Pollination efficiency of Potamogeton pectinatus L. – Aquatic Bot. 34: 381-384.
Guo Y-H, Cook CDK. 1990. The floral biology of Groenlandia densa (L.) Fourreau (Potamogetonaceae). – Aquatic Bot. 38: 283-288.
Gupta BL. 1935. Studies on the development of the pollen grain and embryo sac of Wolffia arrhiza. – Curr. Sci. 4: 104-105.
Gusman G. 2001. Arisaema omkoiense (Araceae), a new species described from North Thailand. – Syst. Geogr. Plants 71: 3-7.
Gusman G. 2003. The intraspecific variation of Arisaema speciosum (Araceae). – Syst. Geogr. Plants 73: 63-70.
Guttenberg H von, Tober A, Dretke W, Ruickholdt M. 1960. Embryologische Studien an Monokotyledonen III. Die Embryogenese von Triglochin maritimum L., Arum maculatum L. und Typha latifolia L. – Flora 149: 243-281.
Haccius B, Lakshmanan KK. 1966. Vergleichende Untersuchung der Entwicklung von Kotyledon und Sproßscheitel bei Pistia stratiotes und Lemna gibba: ein Beitrag zum Problem der sogenannten terminalen Blattorgane. – Beitr. Biol. Pflanzen 42: 425-443.
Haggard C, Tiffney BH. 1997. The flora of the early Miocene Brandon Lignite, Vermont, USA, VIII. Caldesia (Alismataceae). – Amer. J. Bot. 84: 239-252.
Hagström JO. 1911. Three species of Ruppia. – Bot. Not. 1911: 137-144.
Hagström JO. 1916. Critical researches on the Potamogeton. – Kungl. Sv. Vetensk.-Akad. Handl. 55(5): 1-281.
Hall JG. 1902. An embryological study of Limnocharis emarginata. – Bot. Gaz. (Chicago) 33: 214-219.
Ham RWJM van der, Hetterscheid WLA, Heuven BJ van. 1998. Notes on the genus Amorphophallus (Arceae) – 8. Pollen morphology of Amorphophallus and Pseudodracontium. – Rev. Palaeobot. Palynol. 103: 95-142.
Ham RWJM van der, Konijnenburg-van Cittert JHA van, Indeherberge L. 2007. Seagrass foliage from the Maastrichtian type area (Maastrichtian, Danian, NE Belgium, SE Netherlands). – Rev. Palaeobot. Palynol. 144: 301-321.
Harada I. 1956. Cytological studies in Helobiae I. Chromosome idiograms and a list of chromosome numbers in seven families. – Cytologia 21: 306-328.
Harborne JB, Williams CA. 1976. Occurrence of sulphated flavones and caffeic acid esters in members of the Fluviales. – Biochem. Syst. Ecol. 4: 37-41.
Harley MM. 1982. Palynological evidence of a close association between Butomopsis Kunth and Hydrocleys L. C. Rich. (Limnocharitaceae). – In: Symoens JJ, Hooper SS, Compère P (eds), Studies on aquatic vascular plants, Royal Bot. Soc. Belgium (Brussels), pp. 61-65.
Harrison PG, Bigley RE. 1982. The recent introduction of the seagrass Zostera japonica to the Pacific coast of North America. – Can. J. Fish. Aquatic Sci. 39: 1642-1648.
Hartog C den. 1957a. Alismataceae. – In: Steenis CGGJ van (ed), Flora Malesiana I, 5(3), Noordhoff-Kolff N. V., Djakarta, pp. 317-334.
Hartog C den. 1957b. Hydrocharitaceae. – In: Steenis CGGJ van (ed), Flora Malesiana I, 5(4), Noordhoff-Kolff N. V., Djakarta, pp. 381-413.
Hartog C den. 1970. The seagrasses of the world. – Verh. Kon. Nederl. Akad. Wetensch., Afd. Natuurk., Sect. II, 59(1): 1-275.
Hartog C den. 1975. Thoughts about the taxonomical relationships within the Lemnaceae. – Aquatic Bot. 1: 407-416.
Hartog C den. 2002. Potamogetonaceae, Zosteraceae, Cymodoceaceae. – In: Nooteboom HP (ed), Flora Malesiana, I, 16, Flora Malesiana Foundation, Nationaal Herbarium Nederland, Leiden, pp. 167-216.
Hartog C den, Kuo J. 2006. Taxonomy and biogeography of seagrasses. – In: Larkum AWD, Orth RJ, Duarte CM (eds), Seagrasses: Biology, Ecology and Conservation, Springer, Dordrecht, pp. 1-23.
Hartog C den, Plas F van der. 1970. A synopsis of the Lemnaceae. – Blumea 18: 355-368.
Hartog C den, Loenhoud PJ van, Roelofs JGM, Sande JCPM van de. 1979. Chromosome numbers of three seagrasses from The Netherlands Antilles. – Aquatic Bot. 7: 267-271.
Hartog C den, Hennen J, Norten TMPA, Wijk RJ van. 1987. Chromosome number of the European seagrasses. – Plant Syst. Evol. 156: 55-59.
Hauman L. 1915. Note sur Hydromystria stolonifera Mey. – An. Mus. Nac. Hist. Nat. Buenos Aires 27: 325-331.
Hay A. 1986. Cyrtosperma Griff. and the origin of the aroids. – Ph.D. diss., Oxford University, England.
Hay A. 1988. Cyrtosperma (Araceae) and its Old World allies. – Blumea 33: 427-469.
Hay A. 1989. Anaphyllopsis: a new neotropical genus of Araceae-Lasieae. – Aroideana 11: 25-31.
Hay A. 1992a. Tribal and subtribal delimitation and circumscription of the genera of Araceae tribe Lasieae. – Ann. Missouri Bot. Gard. 79: 184-205.
Hay A. 1992b. A new Australian genus of Araceae, with notes on generic limits and biogeography of the Areae. – Bot. J. Linn. Soc. 109: 427-434.
Hay A. 1993. The genus Typhonium (Araceae-Areae) in Australasia. – Blumea 37: 345-376.
Hay A, Mabberley DJ. 1991. ’Transference of Function’ and the origin of aroids: their significance in early angiosperm evolution. – Bot. Jahrb. Syst. 113: 339-428.
Hay A, Wise R. 1991. The genus Alocasia (Araceae) in Australasia. – Blumea 35: 499-545.
Hay A, Yuzammi. 2000. Schismatoglottideae (Araceae) in Malesia I. Schismatoglottis. – Telopea 9: 1-177.
Haynes RR. 1974. A revision of North American Potamogeton subsection Pusilli (Potamogetonaceae). – Rhodora 76: 564-649.
Haynes RR. 1985. A revision of the clasping-leaved Potamogeton (Potamogetonaceae). – SIDA 11: 173-188.
Haynes RR, Holm-Nielsen LB. 1982. A new species of Potamogeton (Potamogetonaceae) from the northern Andes. – Syst. Bot. 7: 498-500.
Haynes RR, Holm-Nielsen LB. 1986. Notes on Echinodorus (Alismataceae). – Brittonia 38: 325-332.
Haynes RR, Holm-Nielsen LB. 1987. A generic treatment of Alismatidae in the neotropics with special reference to Brazil. – Acta Amazonica [Suppl.] 15: 153-193.
Haynes RR, Holm-Nielsen LB. 1989. Speciation of Alismatidae in the Neotropics. – In: Holm-Nielsen LB, Nielsen IC, Balslev H (eds), Tropical forests. Botanical dynamics, speciation and diversity, Academic press, London, pp. 211-219.
Haynes RR, Holm-Nielsen LB. 1992. Flora Neotropica Monograph 56. Limnocharitaceae. – New York Botanical Garden, Bronx, New York.
Haynes RR, Holm-Nielsen LB, 1994. Flora Neotropica Monograph 64. Alismataceae. – New York Botanical Garden, Bronx, New York.
Haynes RR, Holm-Nielsen LB. 2003. Flora Neotropica Monograph 85. Potamogetonaceae. – New York Botanical Garden, Bronx, New York.
Haynes RR, Holm-Nielsen LB, Les DH. 1998a. Najadaceae. – In: Kubitzki K (ed), The families and genera of vascular plants IV. Flowering plants. Monocotyledons. Alismatanae and Commelinanae (except Gramineae), Springer, Berlin, Heidelberg, New York, pp. 301-306.
Haynes RR, Holm-Nielsen LB, Les DH. 1998b. Ruppiaceae. – In: Kubitzki K (ed), The families and genera of vascular plants IV. Flowering plants. Monocotyledons. Alismatanae and Commelinanae (except Gramineae), Springer, Berlin, Heidelberg, New York, pp. 445-448.
Haynes RR, Les DH, Holm-Nielsen LB. 1998a. Alismataceae. – In: Kubitzki K (ed), The families and genera of vascular plants IV. Flowering plants. Monocotyledons. Alismatanae and Commelinanae (except Gramineae), Springer, Berlin, Heidelberg, New York, pp. 11-18.
Haynes RR, Les DH, Holm-Nielsen LB. 1998b. Limnocharitaceae. – In: Kubitzki K (ed), The families and genera of vascular plants IV. Flowering plants. Monocotyledons. Alismatanae and Commelinanae (except Gramineae), Springer, Berlin, Heidelberg, New York, pp. 271-275.
Haynes RR, Les DH, Holm-Nielsen LB. 1998c. Juncaginaceae. – In: Kubitzki K (ed), The families and genera of vascular plants IV. Flowering plants. Monocotyledons. Alismatanae and Commelinanae (except Gramineae), Springer, Berlin, Heidelberg, New York, pp. 260-263.
Haynes RR, Les DH, Holm-Nielsen LB. 1998d. Potamogetonaceae. – In: Kubitzki K (ed), The families and genera of vascular plants IV. Flowering plants. Monocotyledons. Alismatanae and Commelinanae (except Gramineae), Springer, Berlin, Heidelberg, New York, pp. 408-415.
Haynes RR, Les DH, Holm-Nielsen LB. 1998e. Scheuchzeriaceae. – In: Kubitzki K (ed), The families and genera of vascular plants IV. Flowering plants. Monocotyledons. Alismatanae and Commelinanae (except Gramineae), Springer, Berlin, Heidelberg, New York, pp. 449-451.
Haynes RR, Les DH, Holm-Nielsen LB. 1998f. Zannichelliaceae. – In: Kubitzki K (ed), The families and genera of vascular plants IV. Flowering plants. Monocotyledons. Alismatanae and Commelinanae (except Gramineae), Springer, Berlin, Heidelberg, New York, pp. 470-474.
Haynes RR, Les DH, Král M. 1998. Two new combinations in Stuckenia, the correct name for Coleogeton (Potamogetonaceae). – Novon 8: 241.
Hegelmaier F. 1868. Die Lemnaceen. Eine monographische Untersuchung. – W. Engelmann, Leipzig.
Hegelmaier F. 1870. Über die Entwicklung der Blüthenteile von Potamogeton. – Bot. Zeitschr. 18: 283-320.
Hegelmaier F. 1871. Über die Fruktifikationstheile von Spirodela. – Bot. Zeitung 29: 645-666.
Hegelmaier F. 1895. Systematische Übersicht der Lemnaceen. – Engl. Bot. Jahrb. Syst. 21: 268-305.
Hegnauer R. 1997. Phytochemistry and chemotaxonomy. – In: Mayo SJ, Bogner J, Boyce PC (eds), The genera of Araceae, Royal Botanic Gardens, Kew, pp. 36-43.
Hellquist CB. 1980. Correlation of alkalinity and the distribution of Potamogeton in New England. – Rhodora 82: 331-344.
Hellquist CB, Hilton RL. 1983. A new species of Potamogeton (Potamogetonaceae) from northeastern United States. – Syst. Bot. 8: 86-92.
Hellquist CB, Jacobs SWL. 1998. Aponogetonaceae of Australia, with descriptions of six new taxa. – Telopea 8: 7-19.
Hendricks AJ. 1957. A revision of the genus Alisma (Dill.) L. – Amer. Midl. Nat. 58: 470-493.
Henriquez CL, Arias T, Pires JC, Croat TB,
Schaal BA. 2014. Phylogenomics of the plant family Araceae. – Molec. Phylogen. Evol. 75:
91-102.
Hepper FN. 1973. Lemnaceae. – In: Polhill RM (ed), Flora of tropical East Africa, Crown Agents of Oversea Governments and Administrations, London, pp. 1-10.
Herrera FA, Jaramillo CA, Dilcher DL, Wing SL, Gómez-N. C. 2008. Fossil Araceae from a Paleocene neotropical rainforest in Colombia. – Amer. J. Bot. 95: 1569-1583.
Hesse M. 2002. The uniquely designed pollen aperture in Lasioideae (Araceae). – Aroideana 25: 51-59.
Hesse M. 2006a. Pollen wall ultrastructure in Araceae and Lemnaceae in relation to molecular classifications. – In: Columbus JT, Friar EA, Porter JM, Prince LM, Simpson MG (eds), Monocots: comparative biology and evolution. Excluding Poales, Rancho Santa Ana Botanical Garden, Claremont, California. – Aliso 22: 204-208.
Hesse M. 2006b. Reasons and consequences of the lack of sporopollenin ektexine in Aroideae (Araceae). – Flora 201: 421-428.
Hesse M. 2006c. Conventional and novel modes of exine patterning in members of the Araceae – the consequences of ecological paradigm shifts? – Protoplasma 228: 145-149.
Hesse M, Zetter R. 2007. The fossil pollen record of Araceae. – Plant Syst. Evol. 263: 93-115.
Hesse M, Weber M, Halbritter H-M. 1999. Pollen walls of Araceae, with special reference to their fossilization potential. – Grana 38: 203-209.
Hesse M, Bogner J, Halbritter H, Weber M. 2001. Palynology of the perigoniate Aroideae: Zamioculcas, Gonatopus and Stylochaeton (Araceae). – Grana 40: 26-34.
Hetterscheid WLA, Boyce PC. 2000. A reclassification of Sauromatum Schott and new species of Typhonium Schott (Araceae). – Aroideana 23: 48-55.
Hetterscheid WLA, Ham RWJM van der. 2001. Notes on the genus Amorphophallus (Araceae) 11: new and obsolete species from East Malaysia and continental Southeast Asia. – Blumea 46: 253-282.
Hetterscheid WLA, Ittenbach S. 1996. Everything you always wanted to know about Amorphophallus, but were afraid to stick your nose into!!!! – Aroideana 19: 7-131.
Hetterscheid WLA, Yadav SR, Patil KS. 1994. Notes on the genus Amorphophallus (Araceae) 5. Amorphophallus konkanensis, a new species from India, and taxonomic reflections on Amorphophallus section Rhaphiophallus. – Blumea 39: 289-294.
Hetterscheid WLA, Ittenbach S, Bogner J. 1999. Notes on the genus Amorphophallus (Araceae) 10. Revision of the endemic Amorphophallus species of Madagascar. – Bot. Jahrb. Syst. 121: 1-17.
Hetterscheid WLA, Ibisch PL, Gonçalves EG. 2003. Two new species of Araceae tribe Spathicarpeae from Bolivia. – Brittonia 55:37-41.
Hettiarachchi P, Triest L. 1991. Isozyme polymorphism in the genus Potamogeton (Potamogetonaceae). – In: Triest L (ed), Isozymes in water plants, Op. Bot. Belg. 4, National Botanic Garden of Belgium, Meise, pp. 87-114.
Hill TG. 1900. The structure and development of Triglochin maritimum L. – Ann. Bot. 14: 83-107.
Hillman WS. 1959. Experimental control of flowering in Lemna I. General methods. Photoperiodism in Lemna pe[r]pusilla 6746. – Amer. J. Bot. 46: 466-473.
Hillman WS. 1961a. Test-tube studies on flowering: experiments with the Lemnaceae, or duckweeds. – Bull. Torrey Bot. Club 88: 327-336.
Hillman WS. 1961b. The Lemnaceae, or duckweeds. A review of the descriptive and experimental literature. – Bot. Rev. 27: 221-287.
Hillman WS. 1961c. Photoperiodism, chelating agents, and flowering of Lemna perpusilla and L. gibba in aseptic culture. – In: McElroy WD, Glass B (eds), Light and life, John Hopkins Press, Baltimore, pp. 673-686.
Hillman WS, Culley DD. 1978. The uses of duckweeds. – Amer. Sci. 66: 442-451.
Hirahaya M, Kadono Y. 1995. Biosystematic study of Lemna minor L. sensu lato (Lemnaceae) in Japan with special reference to allozyme variation. – Acta Phytotaxon. Geobot. 46: 117-129.
Hocking PJ, Cambridge ML, McComb AJ. 1980. Nutrient accumulation in the fruits of two species of seagrass, Posidonia australis and Posidonia sinuosa. – Ann. Bot., N. S., 45: 149-161.
Hocking PJ, Cambridge ML, McComb AJ. 1981. Nitrogen and phosphorus nutrition of developing plants of two seagrasses, Posidonia australis and Posidonia sinuosa. – Aquatic Bot. 11: 245-261.
Hofmann C-C, Zetter R. 2010. Upper Cretaceous sulcate pollen from the Timerdyakh formation, Vilui Basin (Siberia). – Grana 49: 170-193.
Holferty GM. 1901. Ovule and embryo of Potamogeton natans. – Bot. Gaz. 31: 339-346.
Hollingsworth PM, Preston CD, Gornall RJ. 1998. Euploid and aneuploid evolution in Potamogeton (Potamogetonaceae): a factual basis for interpretation. – Aquat. Bot. 60: 337-358.
Holloway SJ, Friedman WE. 2008. Embryological features of Tofieldia glutinosa and their bearing on the early diversification of monocotyledonous plants. – Ann. Bot. 102: 167-182.
Holm-Nielsen LB, Haynes RR. 1986. 191. Alismataceae. – In: Harling G, Sparre B (eds), Flora of Ecuador 26, Swedish Natural Science Research Council, Stockholm, pp. 3-24.
Holm-Nielsen LB, Haynes RR. 1986. 192-197. Limnocharitaceae, Hydrocharitaceae, Juncaginaceae, Potamogetonaceae, Zannichelliaceae, Najadaceae. – In: Harling G, Sparre B (eds), Flora of Ecuador 26, Swedish Natural Science Research Council, Stockholm, pp. 37-82.
Holub J. 1997. Stuckenia Börner 1912: the correct name for Coleogeton (Potamogetonaceae). – Preslia 69: 361-366.
Hooper SS, Symoens JJ. 1982. Observations on the family Limnocharitaceae Takhtajan ex Hooper et Symoens. – In: Symoens JJ, Hooper SS, Compère P (eds), Studies on aquatic vascular plants, Royal Botanic Society of Belgium (Brussels), pp. 55-60.
Horen F van. 1869. Observations sur la physiologie des Lemnacées. – Bull. Soc. Roy. Bot. Belge 8: 15-88.
Hotta M. 1962. On Colocasia gigantea Hook. f. – Acta Phytotaxon. Geobot. 18: 157.
Hotta M. 1967. Notes on Bornean plants II. – Acta Phytotaxon. Geobot. 22: 153-162.
Hotta M. 1970. A system of the family Araceae in Japan and adjacent areas I. – Mem. Fac. Sci. Kyoto Imp. Univ., Ser. Biol. 4: 72-96.
Hotta M. 1971. Study of the family Araceae. General remarks. – Jap. J. Bot. 40: 269-310.
Hotta M. 1976. Notes on Bornean plants III. Pedicellarum and Heteroaridarum, two new genera of the aroids. – Acta Phytotaxon. Geobot. 27: 61-65.
Hotta M. 1981. A new genus of the family Araceae from West Sumatra. – Acta Phytotaxon. Geobot. 32: 142-146.
Hotta M. 1985. New species of the genus Homalomena (Araceae) from Sumatra with a short note on the genus Furtadoa. – Gard. Bull. (Singapore) 38: 43-54.
Hotta M, Okada H, Ito M, Bebasari L. 1984. Variation in local populations of Schismatoglottis in G. Gadut area. – In: Hotta M et al. (eds), Ecology and speciation in tropical rain forest of Malesia (Sumatra): forest ecology and flora of G. Gadut, West Sumatra, Sumatra Nature Study (Botany), Kyoto, pp. 49-88.
Hroudová Z, Zákravský P. 1993a. Ecology of two cytotypes of Butomus umbellatus II. Reproduction, growth and biomass production. – Folia Geobot. Phytotaxon. 28: 413-424.
Hroudová Z, Zákravský P. 1993b. Ecology of two cytotypes of Butomus umbellatus III. Distribution and habitat differentiation in the Czech and Slovak Republics. – Folia Geobot. Phytotaxon. 28: 425-435.
Huang L-J, Wang X-W, Wang X-F. 2014. The structure and development of incompletely closed carpels in an apocarpous species, Sagittaria trifolia (Alismataceae). – Amer. J. Bot. 101: 1229-1234.
Huggett B, Tomlionson PB. 2010. Aspects of vessel dimensions in the aerial roots of epiphytic Araceae. – Intern. J. Plant Sci. 171: 362-369.
Hunziker AT. 1982. Observaciones biologicas y taxonomicas sobre Hydromystria laevigata (Hydrocharitaceae). – Taxon 31: 472-477.
Huotari T, Korpelainen H. 2012. Complete chloroplast genome sequence of Elodea canadensis and comparative analyses with other monocot plastid genomes. – Gene 15: 96-105.
Huttleston DG. 1953. A taxonomic study of the temperate North American Araceae. – Ph.D. diss., Cornell University, Ithaca, New York.
Iida S, Kosuge K, Kadono Y. 2004. Molecular phylogeny of Japanese Potamogeton species in light of noncoding chloroplast sequences. – Aquat. Bot. 80: 115-127.
Iles WJD, Smith SY, Graham SW. 2013. A well-supported phylogenetic framework for the monocot order Alismatales reveals multiple losses of the plastid NADH dehydrogenase complex and a strong long-branch effect. – In: Wilkin P, Mayo SJ (eds.), Early events in monocot evolution, Systematics Association Spec. Vol. 83, Cambridge University Press, Cambridge, United Kingdom, pp. 1-28.
Isaac FM. 1969. Floral structure and germination in Cymodocea ciliata. – Phytomorphology 19: 44-51.
Islam AS. 1950. A contribution to the life history of Ottelia alismoides Pers. – J. Indian Bot. Soc. 29: 79-91.
Ito T. 1942. Chromosomen und Sexualität der Araceae I. Somatische Chromosomenzahlen einiger Arten. – Cytologia 12: 313-325.
Ito Y, Ohi-Toma T, Murata J, Tanaka N. 2010. Hybridization and polyploidy of an aquatic plant, Ruppia (Ruppiaceae), inferred from plastid and nuclear DNA phylogenies. – Amer. J. Bot. 97: 1156-1167.
Ito Y, Ohi-Toma T, Murata J, Tanaka N. 2013. Comprehensive phylogenetic analyses of the Ruppia maritima complex focusing on taxa from the Mediterranean. – J. Plant Res. 126: 753-762.
Ito Y, Ohi-Toma T, Tanaka N, Murata J, Muasya AM. 2015. Phylogeny of Ruppia (Ruppiaceae) revisited: molecular and morphological evidence for a new species from Western Cape, South Africa. – Syst. Bot. 40: 942-949.
Ito Y, Tanaka N, García-Murillo P, Muasya AM. 2016. A new delimitation of the Afro-Eurasian plant genus Althenia to include its Australasian relative, Lepilaena (Potamogetonaceae) – evidence from DNA and morphological data. – Mol. Phylogen. Evol. 98: 261-270.
Ito Y, Tanaka N, Gale SW, Yano O, Li J. 2017. Phylogeny of Najas (Hydrocharitaceae) revisited: implications for systematics and evolution. – Taxon 66: 309-323.
Ittenbach S. 1997. Revision der afrikanischen Arten der Gattung Amorphophallus (Araceae). – Ph.D. diss., Universität Bonn, Germany.
Ittenbach S, Lobin W. 1997. Notes on the genus Amorphophallus (Araceae) 6. Six new species and two new subspecies from Africa. – Willdenowia 27: 147-160.
Ivanova IE. 1973. On the systematics of the family Lemnaceae. – Bot. Žurn. 58: 1413-1428. [In Russian]
Iwashina T, Konishi T, Takayama A, Fukada M, Ootani S. 1999. Isolation and identification of the flavonoids in the leaves of taro. – Ann. Tsukuba Bot. Gard. 18: 71-74.
Jacobs SWL. 1997. Astonia (Alismataceae), a new genus for Australia. – Telopea 7: 139-141.
Jacobs SWL, Brock MA. 1982. A revision of the genus Ruppia (Potamogetonaceae) in Australia. – Aquatic Bot. 14: 325-337.
Jacobs SWL, Brock MA. 2011. Ruppiaceae. – In: Wilson A (ed), Flora of Australia, Vol. 39, Alismatales to Arales, ABRS/CSIRO, Melbourne, Victoria, pp. 95-98.
Jacobs SWL, Frank KA. 1997. Notes on Vallisneria (Hydrocharitaceae) in Australia, with descriptions of two new species. – Telopea 7: 111-118.
Jacobs SWL, Les DH. 2009. New combinations in Zostera (Zosteraceae). – Telopea 12: 419-423.
Jacobs SWL, Williams A. 1980. Notes on the genus Zostera s. lat. in New South Wales. – Telopea 1: 451-455.
Jacobs SWL, Les DH, Moody ML. 2006. New combinations in Australasian Zostera (Zosteraceae). – Telopea 11: 127-128.
Jacobs SWL, Les DH, Moody ML, Hellquist CB. 2006. Two new species of Aponogeton (Aponogetonaceae), and a new key to species from Australia. – Telopea 11: 129-134.
Jacobsen N. 1976. Notes on the Cryptocoryne of Sri Lanka (Ceylon). – Bot. Not. 129: 179-190.
Jacobsen N. 1977. Chromosome numbers and taxonomy in Cryptocoryne (Araceae). – Bot. Not. 130: 71-87.
Jacobsen N. 1979a. Cryptocoryne gasseri N. Jacobsen, sp. nov. (Araceae). – Bot. Not. 132: 114.
Jacobsen N. 1979b. Cryptocoryne. – Clausen Bøger, Copenhagen.
Jacobsen N. 1980a. The Cryptocoryne albida group of mainland Asia (Araceae). – Meded. Landbouwhog. Wageningen 19: 183-204.
Jacobsen N. 1980b. Cryptocoryne villosa N. Jacobsen, sp. nov. – Aroideana 3: 109, 118-119.
Jacobsen N. 1981. Cryptocoryne undulata Wendt und Bemerkungen zu anderen Arten. – Aqua Planta 81: 29, 31-37, Nachschrift, 92-94.
Jacobsen N. 1982. Cryptocorynen. – A. Kernen, Stuttgart.
Jacobsen N. 1985. The Cryptocoryne (Araceae) of Borneo. – Nord. J. Bot. 5: 31-50.
Jacobsen N, Sivadasan M, Bogner J. 1989. Ungewöhnliche vegetative Vermehrung bei der Gattung Cryptocoryne. – Aqua Planta 14: 83-88, 127-132.
Jacobson A, Hedrén M. 2007. Phylogenetic relationships in Alisma (Alismataceae) based on RAPDs, and sequence data from ITS and trnL. – Plant Syst. Evol. 265: 27-44.
Jayalakshmi SK. 2004. Pollen apertural diversity and its evolution in the Araceae. – J. Palyn. 40: 51-65.
Jérémie J, Lobreau-Callen D, Couderc H, Jossang A. 2001. Une nouvelle espèce d’Echinodorus (Alismataceae) de Guadeloupe (Petites Antilles). Observations palynologiques, cytogénétiques et chimiques. – Adansonia, sér. III, 23: 191-203.
Johri BM. 1936. The life-history of Butomopsis lanceolata Kunth. – Proc. Indian Acad. Sci., Sect. B, 4: 139-162.
Johri BM. 1938a. The embryo sac of Hydrocleis nymphoides Buchen. – Beih. Bot. Centralbl. 58A: 165-172.
Johri BM. 1938b. The embryo sac of Limnocharis emarginata L. – New Phytol. 37: 279-285.
Johri BM. 1970. Comparative embryology of angiosperms: Limnanthaceae, Alismataceae and Butomaceae. – Bull. Indian Nat. Sci. Acad. 41: 110-113, 334-335.
Jones GE. 1957. Chromosome numbers and phylogenetic relationships in the Araceae. – Ph.D. diss., University of Virginia, Charlotteville, Virginia.
Jordan WC, Courtney MW, Neigel JE. 1996. Low levels of intraspecific genetic variation at a rapidly evolving chloroplast DNA locus in North American duckweeds (Lemnaceae). – Amer. J. Bot. 83: 430-439.
Jos JS, Magoon ML. 1970. Pollen mitotic studies in some aroids. – Chrom. Inf. Serv. 11: 4-7.
Jos JS, Vijaya Bai K, Hrishi N. 1977. A high polyploidy in Aglaonema. – Chrom. Inf. Serv. 22: 19-20.
Jüssen FJ. 1929. Die Haploidgeneration der Araceen und ihre Verwendung für das System. – Engl. Bot. Jahrb. Syst. 62: 155-283.
Kandeler R, Hügel B. 1974. Wiederentdeckung der echten Lemna perpusilla Torr. und Vergleich mit L. paucicostata Hegelmeier. – Plant Syst. Evol. 123: 83-96.
Kaplan Z. 2002. Phenotypic plasticity in Potamogeton (Potamogetonaceae). – Folia Geobot. Phytotaxon. 37: 141-170.
Kato Y, Aioi K, Omori Y, Takahata N, Satta Y. 2003. Phylogenetic analyses of Zostera species based on rbcL and matK sequences: implications for the origin and diversification of seagrasses in Japanese waters. – Genes Genet. Syst. 78: 329-342.
Kaul RB. 1967a. Development and vasculature of the flowers of Lophotocarpus calycinus and Sagittaria latifolia (Alismataceae). – Amer. J. Bot. 54: 914-920.
Kaul RB. 1967b. Ontogeny and anatomy of the flower of Limnocharis flava (Butomaceae). – Amer. J. Bot. 54: 1223-1230.
Kaul RB. 1968a. Floral development and vasculature in Hydrocleis nymphoides (Butomaceae). – Amer. J. Bot. 55: 236-242.
Kaul RB. 1968b. Floral morphology and phylogeny in the Hydrocharitaceae. – Phytomorphology 18: 13-35.
Kaul RB. 1969. Morphology and development of the flowers of Boottia cordata, Ottelia alismoides, and their synthetic hybrid (Hydrocharitaceae). – Amer. J. Bot. 58: 951-959.
Kaul RB. 1970. Evolution and adaptation of inflorescences in the Hydrocharitaceae. – Amer. J. Bot. 57: 708-715.
Kaul RB. 1976. Conduplicate and specialized carpels in the Alismatales. – Amer. J. Bot. 63: 175-182.
Kaul RB. 1992. Distribution, habitats, and taxonomy of Ruppia maritima L. and R. occidentalis S. Watson in Nebraska. – Trans. Nebraska Acad. Sci. 16: 67-74.
Kaul RB. 1993. Meristic and organogenetic variation in Ruppia occidentalis and R. maritima. – Intern. J. Plant Sci. 154: 416-424.
Kausik SB. 1939. Pollination and its influences on the behavior of the pistillate flower in Vallisneria spiralis. – Amer. J. Bot. 26: 207-211.
Kausik SB. 1941. Structure and development of the staminate flower and male gametophyte of Enalus acoroides (L. fil.), Steud. – Proc. Indian Acad. Sci., Sect. B, 14: 1-15.
Kausik SB, Rao PKV. 1942. The male gametophyte of Halophila ovata Gaudich. – Half-Yearly J. Mysore Univ., N. S., 3: 41-49.
Kay QON. 1971. Floral structure in the marine angiosperms Cymodocea serrulata and Thalassodendron ciliatum (Cymodocea ciliata). – Bot. J. Linn. Soc. 64: 423-429.
Keating RC. 2000. Collenchyma in Araceae: trends and relation to classification. – Bot. J. Linn. Soc. 134: 203-214.
Keating RC. 2002. 9. Acoraceae and Araceae. – In: Gregory M, Cutler DF (eds), Anatomy of the Monocotyledons, Oxford University Press, Oxford, pp. 1-327.
Keating RC. 2003. Leaf anatomical characters and their value in understanding morphoclines in the Araceae. – Bot. Rev. 68: 510-523.
Keating RC. 2004a. Vegetative antomical data and its relationship to a revised classification of the genera of Araceae. – Ann. Missouri Bot. Gard. 91: 485-494.
Keating RC. 2004b. Systematic occurrence of raphide crystals in Araceae. – Ann. Missouri Bot. Gard. 91: 495-504.
Keeley JE. 1998. CAM photosynthesis in submerged aquatic plants. – Bot. Rev. 64: 121-175.
Keener BR. 2005. Molecular systematics and revision of the aquatic monocot genus Sagittaria (Alismataceae). – PhD. diss., The University of Alabama, Tuscaloosa, Alabama.
Kenton A. 1981. A Robertsonian relationship in the chromosomes of two species of Hydrocleys (Butomaceae sens. lat.). – Kew Bull. 36:487-492.
Kimball RT, Crawford DJ, Les DH, Landolt E. 2003. Out of Africa: molecular phylogenetics and biogeography of Wolffiella (Lemnaceae). – Biol. J. Linn. Soc. 2003: 565-576.
Kirkman H. 1975. Male floral structure in the marine angiosperm Cymodocea serrulata (R. Br.) Ascherson & Magnus (Zannichelliaceae). – Bot. J. Linn. Soc. 70: 267-268.
Kirkman H, Kuo J. 1996. Seagrasses of the southern coast of Western Australia. – In: Kuo J, Phillips RC, Walker DI, Kirkman H (eds), Seagrass biology. Proceedings of an International Workshop, Rottnest Island, Western Australia, 25-29 January 1996, Science, UWA, Perth, pp. 51-56.
Kishimoto E. 1941. Chromosomenzahlen einiger Arten von Amorphophallus und Arisaema. – Bot. & Zool. 9: 433-434.
Knecht M. 1983. Contribution à l’étude biosystématique des représentants d’Aracées de la Côte d’Ivoire. – Cramer, Vaduz.
Knoll F. 1926. Die Arum-Blütenstände und ihre Besucher (Insekten och Blumen IV). – Abh. Zool. Bot. Ges. Wien 12: 379-481.
Ko SC. 1983. A taxonomic study on genus Arisaema in Korea. – Ph.D. diss. Korea University, Seoul, Republic of Korea.
Köcke AV, Mering S von, Mucina L, Kadereit JW. 2010. Revision of the Mediterranean and southern African Triglochin bulbosa complex (Juncaginaceae). – Edinburgh J. Bot. 67: 353-398.
Koriba K, Miki S. 1931. Observation on Archaeozostera (new named) from Upper Cretaceous Izumi Sandstones. – Chikyu 15: 165-201. [In Japanese]
Koriba K, Miki S. 1958. Archaeozostera, a new genus from the Upper Cretaceous in Japan. – Paleobotanist 7: 107-110.
Kosenko VN. 1987. Pollen morphology of Tofieldieae, Narthecieae, Xerophylleae, and Melanthieae (Melanthiaceae). – Bot. Žurn. 72: 1318-1330. [In Russian]
Kovar-Eder J. 1992. A remarkable preservation state of fossil leaves recognized in Potamogeton. – Courier Forschungsinst. Senckenberg 147: 393-397.
Krahulcová A, Jarolímová V. 1993. Ecology of two cytotypes of Butomus umbellatus I. Karyology and breeding behaviour. – Folia Geobot. Phytotaxon. 28: 385-411.
Krause K. 1930. Liliaceae. – In: Engler A (ed), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 15a, W. Engelmann, Leipzig, pp. 227-386.
Krishnan R, Magoon ML, Vijaya Bai K. 1970a. Cytological studies in Xanthosoma sagittifolium (L.) Schott. – The Nucleus 13: 142-148.
Krishnan R, Magoon ML, Vijaya Bai K. 1970b. Karyological studies in Amorphophallus campanulatus. – Can. J. Genet. Cytol. 12: 187-196.
Kudryashov LV, Savich EI. 1963. Some data on the embryology of Alisma plantago-aquatica L. – Bull. Moscow Soc. Natur. Div. Biol. 68(4): 50-63. [In Russian]
Kulkarni AR, Dosi D, Manoj VM. 1990. Fruit and seed structure in Araceae. – Proc. Indian Acad. Sci., Sect. B, 100: 61-70.
Kuo J. 1978. Morphology, anatomy and histochemistry of Australian seagrasses of the genus Posidonia König (Posidoniaceae) I. Leaf blade and leaf sheath of Posidonia australis Hook. f. – Aquatic Bot. 5: 171-190.
Kuo J. 1983a. Notes on the biology of Australian seagrasses. – Proc. Linn. Soc. New South Wales 106: 225-245.
Kuo J. 1983b. The nacreous walls of sieve elements in seagrasses. – Amer. J. Bot. 70: 159-164.
Kuo J. 1993a. Functional leaf anatomy and ultrastructure in a marine angiosperm, Syringodium isoetifolium (Aschers.) Dandy (Cymodoceaceae). – Aust. J. Mar. Freshwater Res. 44: 59-73.
Kuo J. 1993b. Root anatomy and rhizosphere ultrastructure in tropical seagrasses. – Aust. J. Mar. Freshwater Res. 44: 75-84.
Kuo J. 2001. Chromosome numbers of the Australian Zosteraceae. – Plant Syst. Evol. 226: 155-163.
Kuo J. 2005. A revision of the genus Heterozostera (Zosteraceae). – Aquatic Bot. 81: 97-140.
Kuo J. 2013. Chromosome numbers of the Australian Cymodoceaceae. – Plant Syst. Evol. 299: 1443-1448.
Kuo J, Cambridge ML. 1978. Morphology, anatomy, and histochemistry of Australian sea grasses of the genus Posidonia König (Posidoniaceae) II. Rhizome and root of Posidonia australis Hook. f. – Aquatic Bot. 5: 191-206.
Kuo J, Cambridge ML. 1984. A taxonomic study of the Posidonia ostenfeldii complex (Posidoniaceae) with description of four new Australian seagrasses. – Aquatic Bot. 20: 267-295.
Kuo J, Hartog C den. 2001. Seagrass taxonomy and identification key. – In: Short FT, Coles RG (eds), Global seagrass research methods, Elsevier, Amsterdam, pp. 123-140.
Kuo J, Kirkman H. 1987. Floral and seedling morphology and anatomy of Thalassodendron pachyrhizum den Hartog (Cymodoceaceae). – Aquatic Bot. 29: 1-17.
Kuo J, Kirkman H. 1990. Anatomy of viviparous seagrasses seedlings of Amphibolis and Thalassodendron and their nutrient supply. – Bot. Mar. 33: 117-126.
Kuo J, Kirkman H. 1996. Seedling development of selected Posidonia species from southwest Australia. – In: Kuo J, Phillips RC, Walker DI, Kirkman H (eds), Seagrass biology. Proceedings of an International Workshop, Rottnest Island, Western Australia. 25-29 January 1996, Science, UWA, Perth, pp. 57-64.
Kuo J, McComb AJ. 1989. Seagrass taxonomy, structure and development. – In: Larkum AWD, McComb AJ, Shepherd SA (eds), Biology of seagrasses. A treatise on the biology of seagrasses with special reference to the Australian region, Elsevier, Amsterdam, pp. 6-73.
Kuo J, McComb AJ. 1998a. Cymodoceaceae. – In: Kubitzki K (ed), The families and genera of vascular plants IV. Flowering plants. Monocotyledons. Alismatanae and Commelinanae (except Gramineae), Springer, Berlin, Heidelberg, New York, pp. 133-140.
Kuo J, McComb AJ. 1998b. Posidoniaceae. – In: Kubitzki K (ed), The families and genera of vascular plants IV. Flowering plants. Monocotyledons. Alismatanae and Commelinanae (except Gramineae), Springer, Berlin, Heidelberg, New York, pp. 404-408.
Kuo J, McComb AJ. 1998c. Zosteraceae. – In: Kubitzki K (ed), The families and genera of vascular plants IV. Flowering plants. Monocotyledons. Alismatanae and Commelinanae (except Gramineae), Springer, Berlin, Heidelberg, New York, pp. 496-502.
Kuo J, McComb AJ, Cambridge ML. 1981. Ultrastructure of the seagrass rhizosphere. – New Phytol. 89: 139-143.
Kuo J, Cook IH, Kirkman H. 1987. Observations of propagation shoots in the seagrass genus Amphibolis C. Agardh (Cymodoceaceae). – Aquatic Bot. 27: 291-293.
Kuo J, Aioi K, Lizumi H. 1988. Comparative leaf structure and its functional significance in Phyllospadix iwatensis Makino and Phyllospadix japonicus Makino (Zosteraceae). – Aquatic Bot. 30: 169-187.
Kuo J, Seto K, Nasu T, Iizumi H, Aioi K. 1989. Notes on Archaeozostera in relation to the Zosteraceae. – Aquatic Bot. 34: 317-328.
Kuo J, James SH, Kirkman H, Hartog C den. 1990. Chromosome numbers and their systematic implications in Australian marine angiosperms: the Posidoniaceae. – Plant Syst. Evol. 171: 199-204.
Kuo J, Ridge RW, Lewis S. 1990. Leaf internal morphology and ultrastructure of Zostera muelleri Irmisch ex Aschers.: a comparative study of intertidal and subtidal forms. – Aquatic Bot. 36: 217-236.
Kuo J, Iizumi H, Nilsen BE, Aioi K. 1992. Fruit anatomy, seed germination and seedling development in the Japanese seagrass Phyllospadix (Zosteraceae). – Aquatic Bot. 37: 229-245.
Kuprianova LA, Tarasevich VF. 1984. The ultrastructure of the surface of pollen grain wall in some genera of the family Lemnaceae and the related genera of the family Araceae. – Bot. Žurn. 69: 1656-1661. [In Russian]
Kurakubo Y. 1940. Über die Chromosomenzahlen von Araceae-Arten. – Bot. & Zool. 8: 1492.
Kvaček Z. 1995. Limnobiophyllum Krassilov – a fossil link between the Araceae and the Lemnaceae. – Aquatic Bot. 50: 49-61.
Kvaček Z, Smith SY. 2015. Orontiophyllum, a new genus for foliage of fossil Orontioideae (Araceae) from the Cretaceous of central Europe. – Bot. J. Linn. Soc. 178: 489-500.
Lacroix CR, Kemp JR. 1997. Developmental morphology of the androecium and gynoecium in Ruppia maritima L.: considerations for pollination. – Aquatic Bot. 59: 253-263.
Lakshmanan KK. 1961. Embryological studies in the Hydrocharitaceae I. Blyxa octandra Planch. – J. Madras Univ. 31B: 133-142.
Lakshmanan KK. 1963. Embryological studies in the Hydrocharitaceae II. Halophila ovata Gaudich. – J. Indian Bot. Soc. 42: 15-18.
Lakshmanan KK. 1965a. Embryological studies in the Hydrocharitaceae III. Nechamandra alternifolia. – Phyton (Buenos Aires) 20: 49-58.
Lakshmanan KK. 1965b. Embryological studies in the Hydrocharitaceae IV. Post-fertilization development in the Hydrilla verticillata Royle. – Phyton (Buenos Aires) 22: 13-14.
Lakshmanan KK. 1965c. Note on the endosperm formation in Zannichellia palustris L. (Potamogetonaceae). – Phyton Rev. Intern. Bot. Exp. 22: 13-14.
Lakshmanan KK. 1970. Hydrocharitaceae, Scheuchzeriaceae, Juncaginaceae, Potamogetonaceae, Zannichelliaceae, Naiadaceae. – In: Comparative Embryology of Angiosperms (Symposium), Bull. Indian Natl. Sci. Acad. 41: 336-357.
Landolt E. 1980a. Key to the determination of taxa within the family of Lemnaceae. – Veröff. Geobot. Inst. E.T.H. Stiftung Rübel, Zürich, 70: 13-21.
Landolt E. 1980b. Description of six new species of Lemnaceae. – Veröff. Geobot. Inst. E.T.H. Stiftung Rübel, Zürich, 70: 22-29.
Landolt E. 1980c. Bibliographie der Familie der Lemnaceae. – Veröff. Geobot. Inst. E.T.H. Stiftung Rübel, Zürich, 70: 142-204.
Landolt E. 1986. Biosystematic investigations in the family of duckweeds (Lemnaceae). The family of Lemnaceae – a monographic study 1. Morphology; karyology; ecology; geographic distribution; systematic position; nomenclature; descriptions. – Veröff. Geobot. Inst. E.T.H. Stiftung Rübel, Zürich, 71: 1-566.
Landolt E. 1992. Wolffiella caudata, a new Lemnaceae species from the Bolivian Amazon region. – Ber. Geobot. Inst. E.T.H. Stiftung Rübel, Zürich, 58: 121-123.
Landolt E. 1994. Taxonomy and ecology of the section Wolffia of the genus Wolffia (Lemnaceae). – Veröff. Geobot. Inst. E.T.H. Stiftung Rübel, Zürich, 60: 137-151.
Landolt E. 1998a. Lemna yungensis, a new duckweed species from rocks of the Andean Yungas in Bolivia. – Ber. Geobot. Inst. E.T.H. Stiftung Rübel, Zürich, 64: 15-21.
Landolt E. 1998b. Lemnaceae. – In: Kubitzki K (ed), The families and genera of vascular plants IV. Flowering plants. Monocotyledons. Alismatanae and Commelinanae (except Gramineae), Springer, Berlin, Heidelberg, New York, pp. 264-270.
Landolt E, Kandeler R. 1987. Biosystematic investigations in the family of duckweeds (Lemnaceae). The family of Lemnaceae 2. Phytochemistry; physiology; application; bibliography. – Veröff. Geobot. Inst. E.T.H. Stiftung Rübel, Zürich, 95: 1-638.
Landolt E, Urbanska-Worytkiewicz K. 1980. List of the studied Lemnaceae samples: origin and chromosome numbers. – In: Landolt E (ed), Biosystematische Untersuchungen in der Familie der Wasserlinsen (Lemnaceae) 1, Veröff. Geobot. Inst. E.T.H. Stiftung Rübel, Zürich, 70: 205-247.
Langdon KR. 1960. Limnobium, an additional genus to the flora of Oklahoma. – Proc. Oklahoma Acad. Sci. 40: 9.
Langland KA. 1990. Hydrilla: a continuing problem in Florida waters. – Circular No. 884, Institute of food and agricultural sciences, University of Florida, Gainesville, Florida, pp. 1-21.
Larkum AWD, Hartog C den. 1989. Evolution and biogeography of seagrasses. – In: Larkum AWD, McComb AJ, Shepherd SA (eds), Biology of seagrasses. A treatise on the biology of seagrasses with special reference to the Australian region, Elsevier, Amsterdam, pp. 112-156.
Larkum AWD, McComb AJ, Shepherd SA (eds). 1989. Biology of seagrasses. A treatise on the biology of seagrasses with special reference to the Australian region. – Elsevier, Amsterdam.
Larkum AWD, Roberts G, Kuo J, Strother S. 1989. Gaseous movement in seagrasses. – In: Larkum AWD, McComb AJ, Shepherd SA (eds), Biology of seagrasses. A treatise on the biology of seagrasses with special reference to the Australian region, Elsevier, Amsterdam, pp. 686-722.
Larsen K. 1966. Cytotaxonomical note on Lilaea. – Bot. Not. 119: 496-497.
Larsen K. 1969. Cytology of vascular plants III. A study of Thai aroids. – Dansk Bot. Ark. 27: 39-59.
Lawalrée A. 1945. La position systématique des Lemnaceae et leur classification. – Bull. Soc. Roy. Bot. Belg. 77: 27-38.
Lawalreé A. 1952. L’embryolgie des Lemnaceae. Observations sur Lemna minor L. – Cellule 54: 305-326.
Lawalrée A. 1961. La pollinisation de Lemna minor L. – Nat. Belg. 42: 164-165.
Lehtonen S. 2006. Phylogenetics of Echinodorus (Alismataceae) based on morphological data. – Bot. J. Linn. Soc. 150: 291-305.
Lehtonen S. 2009a. An integrative approach to species delimitation in Echinodorus (Alismataceae) and the description of two new species. – Kew Bull. 63: 525-563.
Lehtonen S. 2009b. Systematics of the Alismataceae – a morphological evaluation. – Aquatic Bot. 91: 279-290.
Lehtonen S. 2017. Splitting Caldesia in favour of Albidella (Alismataceae). – Aust. Syst. Bot. 30: 64-69.
Lehtonen S, Myllys L. 2008. Cladistic analysis of Echinodorus (Alismataceae): simultaneous analysis of molecular and morphological data. – Cladistics 24: 218-239.
Leins P, Stadtler P. 1973. Entwicklungsgeschichtliche Untersuchungen am Androeceum der Alismatales. – Österr. Bot. Zeitschr. 121: 51-63.
Lemon GD, Posluszny U. 2000a. Shoot development in Pistia stratiotes (Araceae). – Intern. J. Plant Sci. 161: 721-732.
Lemon GD, Posluszny U. 2000b. Comparative shoot development and evolution in the Lemnaceae. – Intern. J. Plant Sci. 161: 733-748.
Lersten NR, Curtis JD. 1977. Anatomy and distribution of secretory glands and other emergences in Tofieldia (Liliaceae). – Ann. Bot. 41: 879-882.
Les DH. 1983. Taxonomic implications of aneuploidy and polyploidy in Potamogeton (Potamogetonaceae). – Rhodora 85: 301-323.
Les DH, Crawford, DJ. 1999. Landoltia (Lemnaceae), a new genus of duckweeds. – Novon 9: 530-533.
Les DH, Haynes RR. 1995. Systematics of subclass Alismatidae: a synthesis of approaches. – In: Rudall PJ, Cribb PJ, Cutler DF, Humphries CJ (eds), Monocotyledons: systematics and evolution 2, Royal Botanic Gardens, Kew, pp. 353-377.
Les DH, Haynes RR. 1996. Coleogeton (Potamogetonaceae), a new genus of pondweeds. – Novon 6: 389-391.
Les DH, Sheridan DJ. 1990a. Hagström’s concept of phylogenetic relationships in Potamogeton L. (Potamogetonaceae). – Taxon 39: 41-58.
Les DH, Sheridan DJ. 1990b. Biochemical heterophylly and flavonoid evolution in North American Potamogeton (Potamogetonaceae). – Amer. J. Bot. 77: 453-465.
Les DH, Tippery NP. 2013. In time and with water … the systematics of alismatid monocotyledons. – In: Wilkin P, Mayo SJ (eds.), Early events in monocot evolution, Systematics Association Spec. Vol. Series, Cambridge University Press, Cambridge, pp. 118-164.
Les DH, Garvin DK, Wimpee CF. 1993. Phylogenetic studies in the monocot subclass Alismatidae: evidence for a reappraisal of the aquatic order Najadales. – Mol. Phylogen. Evol. 2: 304-314.
Les DH, Landolt E, Crawford DJ. 1994. Molecular systematics of the Lemnaceae. – Amer. J. Bot. 81: 168-169.
Les DH, Cleland MA, Waycott M. 1997. Phylogenetic studies in Alismatidae II: evolution of marine angiosperms (‘seagrasses’) and hydrophily. – Syst. Bot. 22: 443-463.
Les DH, Landolt E, Crawford DJ. 1997. Systematics of the Lemnaceae (duckweeds): inferences from micromolecular and morphological data. – Plant Syst. Evol. 204: 161-177.
Les DH, Crawford DJ, Landolt E, Gabel JD, Kimball RT. 2002. Phylogeny and systematics of Lemnaceae, the duckweed family. – Syst. Bot. 27: 221-240.
Les DH, Moody ML, Jacobs SWL, Bayer RJ. 2002. Systematics of seagrasses (Zosteraceae) in Australia and New Zealand. – Syst. Bot. 27: 468-484.
Les DH, Crawford DJ, Kimball RT, Moody ML, Landolt E. 2003. Biogeography of discontinuously distributed hydrophytes: a molecular appraisal of intercontinental disjunctions. – Intern. J. Plant Sci. 164: 917-932.
Les DH, Moody ML, Jacobs SWL. 2005. Phylogeny and systematics of Aponogeton (Aponogetonaceae): the Australian species. – Syst. Bot. 30: 503-519.
Les DH, Moody ML, Soros CL. 2006. A reappraisal of phylogenetic relationships in the monocotyledon family Hydrocharitaceae (Alismatidae). – In: Columbus JT, Friar EA, Porter JM, Prince LM, Simpson MG (eds), Monocots: comparative biology and evolution. Excluding Poales, Rancho Santa Ana Botanical Garden, Claremont, California. – Aliso 22: 211-230.
Les DH, Jacobs SWL, Tippery NP, Chen L, Moody ML, Wilstermann-Hildebrand M. 2008. Systematics of Vallisneria (Hydrocharitaceae). – Syst. Bot. 33: 49-65.
Les DH, Murray Nm, Tippery NP. 2009. Systematics of two imperilled pondweeds (Potamogeton vaseyi, P. gemmiparus) and taxonomic ramifications for Subsection Pusilli. – Syst. Bot. 34: 643-651.
Li H. 2000. New species of Arisaema from China (Araceae: Arisaemateae). – Kew Bull. 55: 417-426.
Li H, Hay A. 1992. Notes on the classification of genera Remusatia and Gonantanthus in Araceae. – Acta Bot. Yunnan. [Suppl.] 5: 27-33.
Li X-X, Zhou Z-K. 2009. Phylogenetic studies of the core Alismatales inferred from morphology and rbcL sequences. – Progr. Nat. Sci. 19: 931-945.
Lieu SM. 1979a. Organogenesis in Triglochin striata. – Can. J. Bot. 57: 1418-1438.
Lieu SM. 1979b. Growth forms in the Alismatales II. Two rhizomatous species: Sagittaria lancifolia and Butomus umbellatus. – Can. J. Bot. 57: 2353-2373.
Lindqvist C, De Laet J, Haynes RR, Aagesen L, Keener BR, Albert VA. 2006. Molecular phylogenetics of an aquatic plant lineage, Potamogetonaceae. – Cladistics 22: 568-588.
Linz J, Stökl J, Urru I, Krügel T, Stensmyr MC, Hansson BS. 2010. Molecular phylogeny of the genus Arum (Araceae) inferred from multi-locus sequence data and AFLPs. – Taxon 59: 405-415.
Liu K-M, Lei L-G, Hu G-W. 2002.Developmental study on the inflorescence and flower of Caldesia grandis Samuel (Alismataceae). – Bot. J. Linn. Soc. 140: 39-47.
Liu P-Y, Zhang D-P, Zhao L. 1985. The karyotype analysis and protein study of two species of Amorphophallus. – J. Southw. Agric. Univ. 4: 39-43. [In Chinese]
Lobin W, Boyce P. 1991. Eminium koenenianum (Araceae), a new species from NE Turkey and a key to the genus Eminium. – Willdenowia 20: 43-51.
Lock IE, Sokoloff DD, Remizowa MV. 2011. Morphogenetic lability of the Ruppia maritima (Ruppiaceae, Alismatales) reproductive organs: from two lateral flowers to a terminal flower. – Russ. J. Devel. Biol. 42: 247-260.
Loh JP, Kiew R, Hay A, Kee A, Gan LH, Gan Y-Y. 2000. Intergeneric and interspecific relationships in Araceae tribe Caladieae and development of molecular markers using amplified fragment length polymorphism (AFLP). – Ann. Bot. 85: 371-378.
Lohammar G. 1954a. Bulbils in the inflorescence of Butomus umbellatus. – Svensk Bot. Tidskr. 48: 485-488.
Lohammar G. 1954b. Matsmältningens inverkan på Potamogeton-frönas groning. – Fauna Flora 49: 17-32.
Long CI, Li H. 2000. Colocasia gongii (Araceae), a new species from Yunnan, China. – Feddes Repert. 111: 559-560.
Low SL, Wong SY, Boyce PC. 2014.
Schottarum (Schismatoglottideae: Araceae) substantiated based on combined
nuclear and plastid DNA sequences. – Plant Syst. Evol. 300: 607-617.
Lowden RM. 1982. An approach to the taxonomy of Vallisneria L. (Hydrocharitaceae). – Aquatic Bot. 13: 269-298.
Lozano C G. 1984. Consideraciones sobre el género Dugandiodendron (Magnoliaceae). – Taxon 33: 691-696.
Lumbert SH, Hartog C den, Phillips RC, Olsen FS. 1984. The occurrence of fossil seagrasses in the Avon Park formation (Late Middle Eocene), Levy County, Florida (USA). – Aquatic Bot. 20: 121-129.
Lye KA. 1986. The geographical variation in Aponogeton abyssinicus A. Rich. – Lydia 1: 67-80.
Lye KA. 1989. Aponogetonaceae. – In: Polhill RM (ed), Flora of tropical East Africa, A. A. Balkema, Rotterdam, pp. 1-10.
McClure JW. 1964. Taxonomic significance of the flavonoid chemistry and the morphology of the Lemnaceae in axenic culture. – Ph.D. diss., University of Texas, Austin, Texas.
McClure JW, Alston RE. 1964. Patterns of selected chemical components of Spirodela polyrhiza formed under various conditions of axenic culture. – Nature 201: 311-313.
McClure JW, Alston RE. 1966. A chemotaxonomic study of Lemnaceae. – Amer. J. Bot. 53: 849-860.
McConchie CA. 1983. Floral development of Maidenia rubra Rendle (Hydrocharitaceae). – Aust. J. Bot. 31: 585-603.
McConchie CA, Kadereit JW. 1987. Floral structure of Vallisneria caulescens Bailey & F. Mueller. – Aquatic Bot. 29: 101-110.
McConchie CA, Knox RB. 1989a. Pollination and reproductive biology of seagrasses. – In: Larkum AWD, McComb AJ, Shepherd SA (eds), Biology of seagrasses. A treatise on the biology of seagrasses with special reference to the Australian region, Elsevier, Amsterdam, pp. 74-111.
McConchie CA, Knox RB. 1989b. Pollen-stigma interaction in the seagrass Posidonia australis. – Ann. Bot., N. S., 63: 235-248.
McConchie CA, Ducker SC, Knox RB. 1982. Biology of Australian seagrasses: floral development and morphology in Amphibolis (Cymodoceaceae). – Aust. J. Bot. 30: 251-264.
McConchie CA, Knox RB, Ducker SC. 1982. Pollen wall structure and cytochemistry in the seagrass Amphibolis griffithii (Cymodoceaceae). – Ann. Bot., N. S., 50: 729-732.
McDaniel S. 1968. Harperocallis. A new genus of the Liliaceae from Florida. – J. Arnold Arbor. 49: 35-40.
McMillan C. 1980a. Isozymes of tropical seagrasses from the Indo-Pacific and the Gulf of Mexico-Caribbean. – Aquatic Bot. 8: 163-172.
McMillan C. 1980b. Flowering under controlled conditions by Cymodocea serrulata, Halophila stipulacea, Syringodium isoetifolium, Zostera capensis and Thalassia hemprichii from Kenya. – Aquatic Bot. 8: 323-336.
McMillan C. 1981. Morphological variation and isozymes under laboratory conditions in Cymodocea serrulata. – Aquatic Bot. 10: 365-370.
McMillan C. 1982. Isozymes in seagrasses. – Aquatic Bot. 14: 231-243.
McMillan C. 1983a. Comparison of secondary compounds in Heterozostera tasmanica from Australia and Chile. – Aquatic Bot. 15: 205-207.
McMillan C. 1983b. Seed germination in Halodule wrightii and Syringodium filiforme from Texas and the US Virgin Islands. – Aquatic Bot. 15: 217-220.
McMillan C, Bragg LH. 1987. Comparison of fruits of Syringodium (Cymodoceaceae) from Texas, the US Virgin Islands and the Philippines. – Aquatic Bot. 28: 97-100.
McMillan C, Parker PL, Fry B. 1980. C13/C12 ratios in seagrasses. – Aquatic Bot. 9: 237-249.
McMillan C, Zapata O, Escobar L. 1980. Sulphated phenolic compounds in seagrasses. – Aquatic Bot. 8: 267-278.
McMillan C, Williams SC, Escobar L, Zapata O. 1981. Isozymes, secondary compounds and experimental cultures of Australian seagrasses in Halophila, Halodule, Zostera, Amphibolis and Posidonia. – Aust. J. Bot. 29: 247-260.
McRoy CP, Helfferich C (eds). 1977. Seagrass ecosystems. A scientific perspective. – Marcel Dekker, New York.
Mader E, Vierssen W van, Schwenk K. 1998. Clonal diversity in the submerged macrophyte Potamogeton pectinatus L. inferred from nuclear and cytoplasmic variation. – Aquat. Bot. 62: 147-160.
Madison M. 1976. Luctatio Aroideis I. Caladium and Xanthosoma. – Phytologia 35: 105.
Madison M. 1977. A revision of Monstera (Araceae). – Contr. Gray Herb. Harvard Univ. 207: 3-100.
Madison M. 1978. A synopsis of Caladiopsis. – Contr. Gray Herb. 208: 95-98.
Madison M. 1981. Notes on Caladium (Araceae) and its allies. – Selbyana 5: 342-377.
Magnus P. 1889. Najadaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien II(1), W. Engelmann, Leipzig, pp. 214-218.
Magnus P. 1894. Über die Gattung Najas. – Ber. Deutsch. Bot. Ges. 12: 214-224.
Maheshwari SC. 1954. The embryology of Wolffia. – Phytomorphology 4: 355-365.
Maheshwari SC. 1956a. The endosperm and embryo of Lemna and systematic position of Lemnaceae. – Phytomorphology 6: 51-55.
Maheshwari SC. 1956b. Endosperm and seed of Wolffia. – Nature 178: 925-926.
Maheshwari SC. 1958a. Spirodela polyrrhiza: the link between the aroids and the duckweeds. – Nature 181: 1745-1746.
Maheshwari SC. 1958b. The Lemnaceae. A contribution to their biology, morphology and systematics. – Ph.D. diss., University of Delhi, India.
Maheshwari SC, Kapil RN. 1963a. Morphological and embryological studies on the Lemnaceae I. The floral structure and gametophytes of Lemna paucicostata. – Amer. J. Bot. 50: 677-686.
Maheshwari SC, Kapil RN. 1963b. Morphological and embryological studies on the Lemnaceae II. The endosperm and embryo of Lemna paucicostata. – Amer. J. Bot. 50: 907-914.
Maheshwari SC, Khanna PP. 1956. The embryology of Arisaema wallichianum Hook. f. and the systematic position of the Araceae. – Phytomorphology 6: 379-388.
Maheshwari SC, Maheshwari N. 1963. The female gametophyte, endosperm and embryo of Spirodela polyrrhiza. – Beitr. Biol. Pflanzen 39: 179-188.
Malik CP. 1961. Chromosome morphology of some species of Arisaema. – Phyton 16: 69-76.
Malvesin-Fabre G. 1945. Contribution à la caryologie des Aracées. – Thèse, Sciences, l’Université de Bordeaux, France.
Manafi M. 1975. Caryologie du genre Dieffenbachia Schott. – Compt. Rend. Acad. Sci. Paris 280: 2745-2748.
Mannino AM, Menéndez M, Obrador B, Sfriso A, Triest L. 2015. The genus Ruppia L. (Ruppiaceae) in the Mediterranean region: an overview. – Aquatic Bot. 124: 1-9.
Mansion G, Rosenbaum G, Schoenenberger N, Bacchetta G, Rosselló J, Conti E. 2008. Phylogenetic analysis informed by geological history supports multiple, sequential invasions of the Mediterranean basin by the angiosperm family Araceae. – Syst. Biol. 57: 269-285.
Marchant CJ. 1970. Chromosome variation in Araceae I. Pothoeae to Stylochitoneae. – Kew Bull. 24: 315-322.
Marchant CJ. 1971a. Chromosome variation in Araceae II. Richardieae to Colocasieae. – Kew Bull. 25: 47-56.
Marchant CJ. 1971b. Chromosome variation in Araceae III. Philodendreae to Pythonieae. – Kew Bull. 25: 323-329.
Marchant CJ. 1972. Chromosome variation in Araceae IV. Areae. – Kew Bull. 26: 395-404.
Marchant CJ. 1973 [1974].Chromosome variation in Araceae V. Acoreae to Lasieae. – Kew Bull. 28: 199-210.
Mardanov AV, Ravin NV, Kuznetsov BB, Samigullin TH, Antonov AS, Kolganova TV, Skyabin KG. 2008. Complete sequence of the duckweed (Lemna minor) chloroplast genome: structural organization and phylogenetic relationships to other angiosperms. – J. Mol. Evol. 66: 555-564.
Marie-Victorin F. 1943. Les Vallisnéries américaines. – Contr. Inst. Bot. Univ. Montréal, Canada, 46.
Markgraf F. 1936. Blütenbau und Verwandtschaft bei den einfachsten Helobiae. – Ber. Deutsch. Bot. Ges. 54: 191-229.
Martin AC, Uhler FM. 1939. Food of game ducks in the United States and Canada. – U.S.D.A. Tech. Bull. No. 634.
Mason R. 1967. The species of Ruppia in New Zealand. – New Zealand J. Bot. 5: 519-531.
Mayeda Y. 1932. Cytological studies in Colocasia. (Preliminary report). – Proc. Crop Sci. (Tokyo) 43: 524-540.
Mayo SJ. 1980. Aroid symposium at Selby Gardens. – Aroideana 3: 69-71.
Mayo SJ. 1982. Anthurium acaule (Jacq.) Schott (Araceae) and West Indian ‘Bird’s nest’ Anthuriums. – Kew Bull. 36: 691-719.
Mayo SJ. 1985. Araceae. – In: Polhill RM (ed), Flora of tropical East Africa, A. A. Balkema, Rotterdam, pp. 1-71.
Mayo SJ. 1986. Systematics of Philodendron Schott (Araceae) with special reference to inflorescence characters. – Ph.D. diss, University of Reading, England.
Mayo SJ. 1989. Observations of gynoecial structure in Philodendron (Araceae). – Bot. J. Linn. Soc. 100: 139-172.
Mayo SJ. 1990. History and infrageneric nomenclature of Philodendron (Araceae). – Kew Bull. 45: 37-71.
Mayo SJ. 1991. A revision of Philodendron subgenus Meconostigma (Araceae). – Kew Bull. 46: 601-681.
Mayo SJ. 1993. Aspects of aroid geography. – In: George W, Lavocat R (eds), The Africa-South America connection, Clarendon Press, Oxford, pp. 44-58.
Mayo SJ, Bogner J. 1988. A new species of Caladium (Araceae) with notes on generic delimitation in the Colocasioideae-Caladieae. – Willdenowia 18: 231-242.
Mayo SJ, Bogner J. 2013. The first evolutionary classification of Araceae: A. Engler’s natural system. – In: Wilkin P, Mayo SJ (eds.), Early events in monocot evolution, Systematics Association Spec. Vol. Series, Cambridge University Press, Cambridge. doi:10.1017/CBO9781139002950.011
Mayo SJ, Gilbert MG. 1986. A preliminary revision of Arisaema (Araceae) in tropical Africa and Arabia. – Kew Bull. 41: 261-278.
Mayo SJ, Bogner J, Boyce PC. 1994. Gearum (Araceae) rediscovered. – Kew Bull. 49: 785-788.
Mayo SJ, Bogner J, Boyce PC. 1995a. The Arales. – In: Rudall PJ, Cribb PJ, Cutler DF, Humphries CJ (eds), Monocotyledons: systematics and evolution, Royal Botanic Gardens, Kew, pp. 277-286.
Mayo SJ, Bogner J, Boyce PC. 1995b. The acolytes of the Araceae. – Curtis’s Bot. Mag. 12: 153-168.
Mayo SJ, Bogner J, Boyce PC. 1997. The genera of Araceae. – The Trustees, Royal Botanic Gardens, Kew, United Kingdom.
Mayo SJ, Bogner J, Boyce PC. 1998. Araceae. – In: Kubitzki K (ed), The families and genera of vascular plants IV. Flowering plants. Monocotyledons. Alismatanae and Commelinanae (except Gramineae), Springer, Berlin, Heidelberg, New York, pp. 26-74.
Mayo SJ, Bogner J, Cusimano N. 2013. Recent progress in the phylogenetics and classification of Araceae. – In: Wilkin P, Mayo SJ (eds.), Early events in monocot evolution, Systematics Association Spec. Vol. Series, Cambridge University Press, Cambridge. doi:10.1017/CBO9781139002950.010
Mayr F. 1943. Beiträge zur Anatomie der Alismataceen: die Blattanatomie von Caldesia parnassifolia (Bassi) Parl. – Beih. Bot. Centralbl. 62: 61-77.
Meeuse BJD. 1975. Thermogenic respiration in aroids. – Ann. Rev. Plant Physiol. 26: 117-125.
Meeuse BDJ, Raskin I. 1988. Review. Sexual reproduction in the arum lily family, with emphasis on thermogenicity. – Sex. Plant Reprod. 1: 3-15.
Mercado-Noriel LR, Mercado BT. 1978. Floral anatomy and seed morphology of water lettuce (Pistia stratiotes). – Philipp. Agric. 61: 281-290.
Mering S von. 2013. Tetroncium and its only species, T. magellanicum (Juncaginaceae): distribution, ecology and lectotypification. – Willdenowia 43: 13-24.
Mering S von, Kadereit JW. 2010. Phylogeny, systematics, and recircumscription of Juncaginaceae – a cosmopolitan wetland family. – In: Seberg O, Petersen G, Barfod AS, Davis JI (eds), Diversity, phylogeny, and evolution in the monocotyledons, Aarhus University Press, Århus, pp. 55-79.
Mering S von, Kadereit JW. 2015. Phylogeny,
biogeography and evolution of Triglochin L. (Juncaginaceae) – morphological
diversification is linked to habitat shifts rather than to genetic
diversification. – Mol. Phylogen. Evol. 83: 200-212.
Meyer FJ. 1932-1936. Beiträge zur Anatomie der Alismataceen. – Beih. Bot. Centralbl. 49(1): 54-63, 272-291, 309-368 (1932); 50(1): 54-63 (1933); 52B: 96-111 (1935); 54A: 156-169 (1936).
Michell MR. 1916. The embryosac of the Richardia africana Kth. – Bot. Gaz. 61: 325-336.
Miki S. 1934. On the seagrasses in Japan II. Cymodoceaceae and marine Hydrocharitaceae. – Bot. Mag. (Tokyo) 48: 131-142.
Miki S. 1937. The origin of Najas and Potamogeton. – Bot. Mag. (Tokyo) 51: 472-480.
Monod T. 1949. Sur une Lemnacée africaine: Wolffiella Welwitschii (Hegelmaier, 1865) comb. nov. – Mém. Soc. Hist. Nat. Afrique Nord, Hors Sér. 2: 229-242.
Montalvo AM, Ackerman JD. 1986. Relative pollinator effectiveness and evolution of floral traits to Spathiphyllum friedrichsthalii (Araceae). – Amer. J. Bot. 73: 1665-1676.
Montesantos M. 1913. Morphologische und biologische Untersuchungen über einige Hydrocharideen. – Flora, N. F., 5: 1-32.
Monti C, Garbari F. 1974. Appunti citotassonomici sul genera Biarum Schott (Araceae) in Italia. – Giorn. Bot. Ital. 108: 19-26.
Moodie GEE. 1976. Heat production and pollination in Araceae. – Can. J. Bot. 54: 545-546.
Mookerjea A. 1955. Cytology of different species of aroids with a view to trace the basis of their evolution. – Caryologia 7: 221-291.
Mora M, Bernal R, Croat T, Jácome J. 2006. A phytogeographic analysis of Araceae of Cabo Corrientes (Chocó, Colombia) and comparable lowland tropical American floras. – Ann. Missouri Bot. Gard. 93: 359-366.
Mosyakin AS, Bezusko AG, Tsymbalyuk ZM. 2009. Palynomorphological peculiarities of representatives of the family Tofieldiaceae (Liliopsida) and Isidrogalvia: evolutionary aspects. – Naukovy Zapiski, Biologiya ta Ecologiya 93: 16-22.
Mtchedlishvili ND, Shakhmoundes VA. 1973. Occurrence of Araceae pollen in the Lower Cretaceous sediments. – In: Chlonova AF (ed), Palynology of mesophytes, Nauka, Moscow, pp. 137-142. [In Russian with English summary]
Muenscher WC. 1936. The germination of seeds of Potamogeton. – Ann. Bot., N. S., 50: 805-821.
Mues R. 1983. Species specific flavone glucuronides in Elodea species. – Biochem. Syst. Ecol. 11: 261-265.
Mujawar II, Gaikwad SP, Yadav SR. 2003. Cytological studies in Wiesneria triandra (Dalz.) Micheli (Alismataceae). – Cytology 68: 375-378.
Mukai H. 1993. Biogeography of the tropical seagrasses in the Western Pacific. – Mar. Freshw. Res. 44: 1-17.
Münchberg P. 1956. Zur bindung der Libelle Aeschna viridis Eversm. an die Pflanze Stratiotes aloides L. – Nachrichtenbl. Bayer. Entomol. 5: 113-118.
Murata J. 1986. A revision of the Arisaema amurense group (Araceae). – J. Fac. Sci. Univ. Tokyo 3: 49-68.
Murata J. 1990. Present status of Arisaema systematics. – Bot. Mag. (Tokyo) 103: 371-382.
Murata J. 1991. The systematic position of Arisaema nepenthoides and A. wattii (Araceae). – Kew Bull. 46: 119-128.
Murata J, Iijima M. 1983. New and noteworthy chromosome records in Arisaema (Araceae). – J. Jap. Bot. 58: 270-280.
Murbeck S. 1902. Über die Embryologie von Ruppia rostellata Koch. – Kungl. Sv. Vetensk.-Akad. Handl. 36(5): 1-21.
Nahrstedt A. 1975. Triglochinin in Araceen. – Phytochemistry 14: 2627-2628.
Nahrstedt A, Kant JD, Hösel W. 1984. Aspects on the biosynthesis of the cyanogenic glucoside triglochinin in Triglochin maritima. – Planta Med. 50: 394-397.
Nakamura T, Kadono Y. 1993. Chromosome number and geographical distribution of monoecious and dioecious Hydrilla verticillata (L. f.) Royle (Hydrocharitaceae) in Japan. – Acta Phytotaxon. Geobot. 44: 123-140.
Naidoo Y, Lawton JR, Barnabas AD, Goetzee J. 1990. Ultrastructure and cytochemistry of squamulae intravaginales of the marine angiosperm Halophila ovalis. – South Afr. J. Bot. 56: 546-553.
Napper DM. 1971. Juncaginaceae. – In: Milne-Redhead E, Polhill RM (eds), Flora of tropical East Africa, Crown Agents for Oversea Governments and Administrations, London, pp. 1-3.
Nauheimer L, Boyce PC. 2014.
Englerarum (Araceae,
Aroideae): a new genus supported by plastid and nuclear phylogenies. – Plant
Syst. Evol. 300: 709-715.
Nauheimer L, Boyce PC, Renner SS. 2012. Giant taro and its relatives: a phylogeny of the large genus Alocasia (Araceae) sheds light on Miocene floristic exchange in the Malesian region. – Mol. Phylogen. Evol. 63: 43-51.
Nauheimer L, Metzler D, Renner SS. 2012. Global history of the ancient monocot family Araceae inferred with models accounting for past continental positions and previous ranges based on fossils. – New Phytol. 195: 938-950.
Nawa N. 1928. Some cytological observations in Tricyrtis, Sagittaria and Lilium. – Bot. Mag. (Tokyo) 42: 33-36.
Nguyen Van Dzu, Boyce PC. 1999. The genus Amydrium (Araceae: Monsteroideae: Monstereae) with particular reference to Thailand and Indochina. – Kew Bull. 54: 379-393.
Nicolson DH. 1959. The occurrence of trichosclereids and crystalline deposits in the Monsteroideae (Araceae). – M.Sc. thesis, Cornell University, Ithaca, New York.
Nicolson DH. 1960. A brief review of classifications in the Araceae. – Baileya 8: 62-67.
Nicolson DH. 1983. Translation of Engler’s classification of Araceae with updating. – Aroideana 5: 67-88.
Nicolson DH. 1984. Suprageneric names attributable to Araceae. – Taxon 33: 680-690.
Nicolson DH. 1987. History of aroid systematics. – Aroideana 10: 23-30.
Nicolson DH. 1994. Spathiphyllum sect. nov. Chlaenophyllum (Araceae). – Aroideana 15: 19-21.
Nicolson DH. 2006. Proposal to conserve the name Caladium (Araceae) with a conserved type. – Taxon 55: 529-530.
Nie Z-L, Sun H, Li H, Wen J. 2006. Intercontinental biogeography of subfamily Orontioideae (Symplocarpus, Lysichiton, and Orontium) of Araceae in eastern Asia and North America. – Mol. Phylogen. Evol. 40: 155-165.
Nikiticheva ZI, Proskurina OB. 1992. Embryology of Scheuchzeria palustris (Scheuchzeriaceae). – Bot. Žurn. 77: 3-18. [In Russian with English summary]
Novelo RA. 1991. Ruppia didymia (Potamogetonaceae) en México y las Antillas. – An. Inst. Biol. Univ. Nac. Autón. Méx. Ser. Bot. 62: 173-180.
Nunes ELP, Lima MC de, Moço MCC. 2012. Floral development in Potamogeton (Potamogetonaceae, Alismatales) with emphasis on gynoecial features. – Aquatic bot. 100: 56-61.
Ogden EC. 1943. The broad-leaved species of Potamogeton of North America north of Mexico. – Rhodora 45: 57-241.
Ohi-Toma T, Wu S, Yadav SR, Murata H, Murata J. 2010. Molecular phylogeny of Typhonium sensu lato and its allied genera in the tribe Areae of the subfamily Aroideae (Araceae) based on sequences of six chloroplast regions. – Syst. Bot. 35: 244-251.
Ohi-Toma T, Wu S, Murata H, Murata J. 2016. An updated genus-wide phylogenetic analysis of Arisaema (Araceae) with reference to sections. – Bot. J. Linn. Soc. 182: 100-114.
Okada H, Tsukaya H, Mori Y. 1999. A new species of Schismatoglottis (Schismatoglottidinae, Araceae) from West Kalimantan and observations of its peculiar bulbil development. – Syst Bot. 24: 62-68.
Olah LV. 1956. Cytological investigation in the genus Amorphophallus Bl. – Ann. Bogor. 2: 125-136.
Ostenfeld CH. 1908. On the ecology and distribution of the Grass-Wrack (Zostera marina) in Danish waters. – Rep. Danish Biol. Station 16: 3-62.
Packer JG. 1993. Two new combinations in Triantha (Liliaceae). – Novon 3: 278-279.
Petch T. 1928. Notes on
Cryptocoryne. – Ann. Roy. Bot. Gard. Peradeniya 11: 11-26.
Peredo EL, King UM, Les DH. 2013. The plastid genome of Najas flexilis: adaptation to submersed environments is accompanied by the complete loss of the NDH complex in an aquatic angiosperm. – PLoS ONE 8, e68591.
Petersen G. 1989. Cytology and systematics of Araceae. – Nord. J. Bot. 9: 119-166.
Petersen G. 1993. New chromosome numbers in Araceae. – Willdenowia 23: 239-244.
Petersen G, Seberg O, Davis JI, Stevenson DW. 2006. RNA editing and phylogenetic reconstruction in two monocot mitochondrial genes. – Taxon 55: 871-886.
Petersen G, Seberg O, Short FT, Fortes MD. 2014. Complete genomic congruence but non-monophyly of Cymodocea (Cymodoceaceae), a small group of seagrasses. – Taxon 63: 3-8.
Petersen G, Seberg O, Cuenca A, Stevenson DW, Thadeo M, Davis JI, Graham S, Ross TG. 2016. Phylogeny of the Alismatales (Monocotyledons) and the relationship of Acorus (Acorales?). – Cladistics 32: 141-159.
Pettitt JM. 1976. Pollen and stigma surface in the marine angiosperms Thalassia and Thalassodendron. – Micron 7: 21-32.
Pettitt JM. 1980. Reproduction in sea grasses: nature of the pollen and receptive surface of the stigma in the Hydrocharitaceae. – Ann. Bot., N. S., 45: 257-271.
Pettitt JM. 1981. Reproduction in sea grasses: pollen development in Thalassia hemprichii, Halophila stipulacea, and Thalassodendron ciliatum. – Ann. Bot., N. S., 48: 609-622.
Pettitt JM. 1984. Aspects of flowering and pollination in marine angiosperms. – Oceanogr. Mar. Biol. Ann. Rev. 22: 315-342.
Pettitt JM, Jermy AC. 1975. Pollen in hydrophilous angiosperms. – Micron 5: 377-405.
Pettitt JM, Ducker SC, Knox RB. 1978. Amphibolis pollen has no exine. – Helobiae Newsl. 2: 19-22.
Pettitt JM, McConchie CA, Ducker SC, Knox RB. 1980. Unique adaptations for submarine pollination in seagrasses. – Nature 286: 487-489.
Pettitt JM, Ducker SC, Knox RB. 1981. Submarine pollination. – Sci. Amer. 244: 92-101.
Pettitt JM, McConchie CA, Ducker SC, Knox RB. 1983. Reproduction in seagrasses: pollination in Amphibolis antarctica. – Proc. Roy. Soc. London, Sect. B, 219: 119-135.
Pettitt JM, McConchie CA, Ducker SC, Knox RB. 1984. Reproduction in seagrasses: pollen wall morphogenesis in Amphibolis antarctica and wall structure in filiform grains. – Nord. J. Bot. 4: 199-216.
Pfitzer P. 1957a. Chromosomenzahlen von Araceen. – Chromosoma 8: 436-446.
Pfitzer P. 1957b. Constancy and variability of the chromosome numbers of several species of Anthurium with special regard to the B-chromosomes. – Chromosoma 8: 547-572.
Philbrick CT. 1983. Aspects of floral biology in three species of Potamogeton (pondweeds). – Mich. Bot. 23: 35-38.
Philbrick CT, Anderson GJ. 1987. Implications of pollen/ovule ratios and pollen size for the reproductive biology of Potamogeton and autogamy in aquatic angiosperms. – Syst. Bot. 12: 98-105.
Phillips RC. 1972. The ecological life history of Zostera marina L. (eelgrass) in Puget Sound, Washington. – Ph.D. diss., University of Washington, Seattle, Washington.
Phillips RC. 1980. Phenology and taxonomy of seagrasses. – In: Phillips RC, McRoy CP (eds), Handbook of seagrass biology. An ecosystem perspective, Garland STPM Press, New York, pp. 29-40.
Phillips RC, McRoy CP (eds). 1980. Handbook of seagrass biology. – STPM Press, Garland, New York.
Phillips RC, Meñez EG. 1988. Seagrasses. – Smithsonian Contr. Mar. Sci. 34: 1-104.
Phillips RC, Shaw RF. 1976. Zostera noltii Hornem. in Washington, U.S.A. – Syesis 9: 355-358.
Phillips RC, McMillan C, Bridges KW. 1983. Phenology of eelgrass, Zostera marina L., along latitudinal gradients in North America. – Aquatic Bot. 15: 145-156.
Pichon M. 1946. Sur les Alismatacées et les Butomacées. – Notulae Syst. (Paris) 12: 170-183.
Plas F van der. 1971. Lemnaceae. – In: Steenis CGGJ van (ed), Flora Malesiana I, 7, Noordhoff, Leiden, pp. 219-237.
Platonova AG, Remizowa MV, Briggs BG, Mering S von, Lock IE, Sokoloff DD. 2016. Vegetative morphology and anatomy of Maundia (Maundiaceae: Alismatales) and patterns of peripheral bundle orientation in angiosperm leaves with three-dimensional venation. – Bot. J. Linn. Soc. 182: 757-790.
Plowman T. 1969. Folk uses of New World aroids. – Econ. Bot. 23: 97-122.
Pneva GP. 1988. Eine neue tertiäre Art aus der Gattung Aponogeton (Aponogetonaceae) aus Kazakhstan und Karakalpakiya. – Bot. Žurn. 73: 1597. [In Russian]
Posluszny U. 1981. Unicarpellate floral development in Potamogeton zosteriformis. – Can. J. Bot. 59:495-504.
Posluszny U. 1983. Re-evaluation of certain key relationships in the Alismatidae: floral organogenesis of Scheuchzeria palustris. – Amer. J. Bot. 70: 925-933.
Posluszny U, Charlton WA. 1993. Evolution of the helobial flower. – Aquatic Bot. 44: 303-324.
Posluszny U, Sattler R. 1973. Floral development of Potamogeton densus. – Can. J. Bot. 51: 647-656.
Posluszny U, Sattler R. 1974a. Floral development of Potamogeton richardsonii. – Amer. J. Bot. 61: 209-216.
Posluszny U, Sattler R. 1974b. Floral development of Ruppia maritima var. maritima. – Can. J. Bot. 52: 1607-1612.
Posluszny U, Sattler R. 1976a. Floral development of Zannichellia palustris. – Can. J. Bot. 54: 651-662.
Posluszny U, Sattler R. 1976b. Floral development of Najas flexilis. – Can. J. Bot. 54: 1140-1151.
Posluszny U, Tomlinson PB. 1977. Morphology and development of floral shoots and organs in certain Zannichelliaceae. – Bot. J. Linn. Soc. 75: 21-46.
Posluszny U, Charlton WA, Jain DK. 1986. Morphology and development of the reproductive shoots of Lilaea scilloides (Poir.) Hauman (Alismatidae). – Bot. J. Linn. Soc. 92: 323-342.
Posluszny U, Charlton WA, Les DH. 2000. Modularity in helobial flowers. – In: Wilson KL, Morrison DA (eds), Monocots: systematics and evolution, CSIRO, Collingwood, pp. 63-74.
Preston CD. 1995. Pondweeds of Great Britain and Ireland. BSBI Handbook No. 8. – Botanical Society of the British Islaes, London.
Prime CT. 1955. Problems of speciation in the British species of Arum. – In: Lousley JE (ed), Species studies in the British flora, London, pp. 136-139.
Prime CT. 1960. Lords and ladies. – Collins New Naturalist Spec. Vol., Collins, London.
Prime CT. 1961. Taxonomy and nomenclature in some species of the genus Arum L. – Watsonia 5: 106-109.
Procaccini G, Mazzella L. 1996. Genetic variability and reproduction in two Mediterranean seagrasses. – In: Kuo J, Phillips RC, Walker DI, Kirkman H (eds), Segrass biology. Proceedings of an International Workshop, Rottnest Island, Western Australia, 25-29 January 1996, UWA, Science, Perty, pp. 85-92.
Prychid CJ, Jabaily RS, Rudall PJ. 2008. Cellular ultrastructure and crystal development in Amorphophallus (Araceae). – Ann. Bot. 101: 983-995.
Ramachandran K. 1977. Karyological studies on four South Indian species of Amorphophallus. – Cytologia (Japan) 42: 645-652.
Ramachandran K. 1978. Cytological studies on South Indian Araceae. – Cytologia (Japan) 43: 289-303.
Ramírez BW, Gómez PLD. 1978. Production of nectar and gums by flowers of Monstera deliciosa (Araceae) and of some species of Clusia (Guttiferae) collected by New World Trigona bees. – Brenesia 14-15: 407-412.
Rangasamy K. 1941. A morphological study of the flower of Blyxa echinosperma Hook. f. – J. Indian Bot. Soc. 20: 123-133.
Rao YS. 1953. Karyo-systematic studies of Helobiales I. Butomaceae. – Proc. Natl. Inst. Sci. India, Sect. B, 19: 563-581.
Rataj K. 1975a. Revizion of the genus Echinodorus Rich. – Studie ČSAV (Praha), č. 2: 1-160.
Rataj K. 1975b. Revision of the genus Cryptocoryne Fischer. – Studie ČSAV (Praha), č. 3: 1-74.
Rataj K. 2004. A new revision of the swordplant genus Echinodorus Richard 1848 (Alismataceae). – J. Ichthyol. Aquat. Biol. (Aqua) Spec. Publ. 1.
Raunkiær C. 1903. Anatomical Potamogeton-studies and Potamogeton fluitans. – Bot. Tidsskr. 25: 253-380.
Ray TS. 1987a. Leaf types in the Araceae. – Amer. J. Bot. 74: 1359-1372.
Ray TS. 1987b. Diversity of shoot organization in Araceae. – Amer. J. Bot. 74: 1373-1387.
Ray TS. 1988. Survey of shoot organization in the Araceae. – Amer. J. Bot. 75: 56-84.
Ray TS, Renner SS. 1990. Comparative studies on the morphology of the Araceae. Transl. from A. Engler, 1876, with an introduction, updated nomenclature, and a glossary. – Englera 12: 1-140.
Reese G. 1962. Zur intragenerischen Gliederung der Gattung Ruppia L. – Zeitschr. Bot. 50: 237-264.
Reinecke P. 1964. A contribution to the morphology of Zannichellia aschersoniana Graebn. – J. South Afr. Bot. 30: 93-101.
Remizowa M, Sokoloff DD. 2003. Inflorescence and floral morphology in Tofieldia (Tofieldiaceae) compared with Araceae, Acoraceae and Alismatales s.str. – Bot. Jahrb. Syst. 124: 255-271.
Remizowa M, Sokoloff DD, Moskvicheva LA. 2005. Morphology and development of flower and shoot system in Tofieldia pusilla (Tofieldiaceae). – Bot. Žurn. 90: 840-853. [in Russian]
Remizowa M, Sokoloff DD, Rudall PJ. 2006a. Evolution of the monocot gynoecium: evidence from comparative morphology and development in Tofieldia, Japonolirion, Petrosavia and Narthecium. – Plant Syst. Evol. 258: 183-209.
Remizowa M, Sokoloff DD, Rudall PJ. 2006b. Comparative patterns of floral orientation, bracts and bracteoles in Tofieldia, Japonolirion, and Narthecium. – Aliso 24: 157-169.
Remizowa M, Sokoloff DD, Rudall PJ. 2006c. Patterns of floral structure and orientation in Japonolirion, Narthecium and Tofieldia. – In: Columbus JT, Friar EA, Porter JM, Prince LM, Simpson MG (eds), Monocots: comparative biology and evolution. Excluding Poales, Rancho Santa Ana Botanical Garden, Claremont, California. – Aliso 22: 159-171.
Remizowa MV, Sokoloff DD, Timonin AC, Rudall PJ. 2010. Floral vasculature in Tofieldia (Tofieldiaceae) is correlated with floral morphology and development. – In: Seberg O, Petersen G, Barfod AS, Davis JI (eds), Diversity, phylogeny, and evolution in the monocotyledons, Aarhus University Press, Århus, pp. 81-99.
Remizowa MV, Sokoloff DD, Campbell LM, Stevenson DW, Rudall PJ. 2011. Harperocallis is congeneric with Isidrogalvia (Tofieldiaceae, Alismatales): evidence from comparative floral morphology. – Taxon 60: 1076-1094.
Remizowa MV, Sokoloff DD, Calvo S, Tomasello A, Rudall PJ. 2012. Flowers and inflorescences of the seagrass Posidonia (Posidoniaceae, Alismatales). – Amer. J. Bot. 99: 1592-1608.
Rendle AE. 1899. A systematic revision of the genus Najas. – Trans. Linn. Soc. London, Bot., Ser. II, 5: 379-444.
Rendle AE. 1916. Maidenia: a new genus of Hydrocharitaceae. – J. Bot. 54: 313-316.
Renner SS, Zhang L-B. 2004. Biogeography of the Pistia clade (Araceae): based on chloroplast and mitochondrial DNA sequences and Bayesian divergence time inference. – Syst. Biol. 53: 422-432.
Renner SS, Zhang L-B, Murata J. 2004. A chloroplast phylogeny of Arisaema (Araceae) illustrates Tertiary floristic links between Asia, North America, and East Africa. – Amer. J. Bot. 91: 881-888.
Reumer JWF. 1984. Cytotaxonomy and evolution in Cryptocoryne (Araceae). – Genetica 65: 149-158.
Reuter M, Piller WE, Harzhauser M, Kroh A, Rögl F, Ćorić S. 2011. The Quilon Limestone, Kerala Basin, India: an archive for Miocene Indo-Pacific seagrass beds. – Lethaia 44: 76-86.
Reznik H, Menschick R. 1969. Flavonoide in Sommergliedern und Winterknospen von Spirodela polyrhiza (L.) Schleid. – Zeitschr. Pflanzenphysiol. 61: 348-349.
Rich TCG, Nicholls-Vuille FL. 2001. Taxonomy and distribution of European Damasonium (Alismataceae). – Edinb. J. Bot. 58: 45-55.
Riedl H. 1969. Kritische Untersuchungen über die Gattung Eminium (Blume) Schott nebst Bemerkungen zu einigen anderen Aroideen der südwestasiatischen Flora. – Ann. Naturhist. Mus. Wien 73: 103-121.
Riedl H. 1980a. Tentative keys for the identification of species in Biarum and Emnium, with notes on some taxa included in Biarum. – Aroideana 3: 24-31.
Riedl H. 1980b. The importance of ecology for generic and specific differentiation in Araceae-Aroideae. – Aroideana 3: 49-54.
Riley MG, Stockey RA. 2004. Cardstonia tolmanii gen. et sp. nov. (Limnocharitaceae) from the Upper Cretaceous of Alberta, Canada. – Intern. J. Plant Sci. 165: 897-916.
Roberts ML, Haynes RR. 1986. Flavonoid systematics of Potamogeton subsections Perfoliati and Praelongi (Potamogetonaceae). – Nord. J. Bot. 6: 291-294.
Robertson EL. 1984. Seagrasses. – In: Womersley HBS (ed), The marine benthic flora of Southern Australia I, Government Printer, South Australia, pp. 57-122.
Roper RB. 1952. The embryo sac of Butomus umbellatus L. – Phytomorphology 2: 61-74.
Rosenberg O. 1901. Über die Pollenbildung von Zostera. – Medd. Stockholms Högskola 4: 3-21.
Rosendahl CO. 1909. Embryo-sac development and embryology of Symplocarpus foetidus. – Minnesota Bot. Stud., IV, Bot. Ser. 7(4): 1-9.
Ross TG, Barrett CF, Gomez MS, Lam VKY, Henriquez CL, Les DH, Davis JI, Cuenca A, Petersen G, Seberg O, Thadeo M, Givnish TJ, Conran J, Stevenson DW, Graham SW. 2016. Plastid phylogenomics and molecular evolution of Alismatales. – Cladistics 32: 160-178.
Roth I. 1961. Histogenese der Laubblätter von Zostera nana. – Bot. Jahrb. Syst. 80: 500-507.
Rothwell GW, Van Atta MR, Ballard HE Jr, Stockey RA. 2004. Molecular phylogenetic relationships among Lemnaceae and Araceae using the chloroplast trnL-trnF intergenic spacer. – Mol. Phylogen. Evol. 30: 378-385.
Ruggiero MV, Procaccini G. 2004. The rDNA ITS region in the Lessepsian marine angiosperm Halophila stipulacea (Forssk.) Aschers. (Hydrocharitaceae): intragenomic variability and putative pseudogenic sequences. – J. Mol. Evol. 58: 115-121.
Saeger A. 1929. The flowering of Lemnaceae. – Bull. Torrey Bot. Club 56: 351-358.
Sakuragui CM, Mayo SJ. 1997. Three new species of Philodendron (Araceae) from South-Eastern Brazil. – Kew Bull. 52: 673-681.
Sakuragui CM, Mayo SJ, Zappi DC. 2006. Taxonomic revision of Brazilian species of Philodendron Section Macrobelium. – Kew Bull. 60: 465-513.
Sakuragui CM, Calazans LSB, Oliveira LL de, Morais EB de, Benko-Iseppon AM, Vasconcelos S, Schrago CEG, Mayo SJ. 2018. Recognition of the genus Thaumatophyllum Schott – formerly Philodendron subg. Meconostigma (Araceae) – based on molecular and morphological evidence. – PhytoKeys 98: 51-71.
Salisbury EJ. 1926. Floral construction in Helobiales. – Ann. Bot. 40: 419-455.
Sane YK. 1939. A contribution to the embryology of the Aponogetonaceae. – J. Indian Bot. Soc. 18: 79-91.
Sargent JA, Wangermann E. 1959. The effect of some growth regulators on the vascular system of Lemna minor. – New Phytol. 58: 345-363.
Sarkar AK, Dutta N, Chatterjee UC. 1979. Chromosome studies in Cryptocoryne (Araceae). – Caryologia 32: 1-4.
Sastrapradja S, Rijanti AM. 1972. On the cytology of Javanese Colocasia. – Ann. Bogor. 5: 117-122.
Satô D. 1942. Karyotype alteration and phylogeny in Liliaceae and allied families. – Jap. J. Bot. 12: 57-161.
Sattler R. 1965. Perianth development of Potamogeton richardsonii. – Amer. J. Bot. 52: 35-41.
Sattler R, Singh V. 1973. Floral development of Hydrocleis nymphoides. – Can. J. Bot. 51: 2455-2458.
Sattler R, Singh V. 1977. Floral organogenesis of Limnocharis flava. – Can. J. Bot. 55: 1076-1086.
Sattler R, Singh V. 1978. Floral organogenesis of Echinodorus amazonicus Rataj and floral construction of the Alismatales. – Bot. J. Linn. Soc. 77: 141-156.
Sauvageau C. 1891. Sur les feuilles de quelques monocotyledons aquatiques. – Ann. Sci. Nat. Bot., Sér. 7, 13: 103-296.
Schaffner JH. 1904. Some morphological peculiarities of the Nymphaeaceae and Helobiae. – Ohio Naturalist 4: 83-92.
Schiestl FP, Dötterl S. 2012. The evolution of floral scent and olfactory preferences in pollinators: coevolution or pre-existing bias? – Evolution 66: 2042-2055.
Schneider EL, Carlquist SJ. 1997. Origin and nature of vessels in monocotyledons 2. Juncaginaceae and Scheuchzeriaceae. – Nord. J. Bot. 17: 397-401.
Schneider EL, Carlquist SJ. 1998. Origins and nature of vessels in monocotyledons 4. Araceae subfamily Philodendroideae. – J. Torrey Bot. Soc. 125: 253-260.
Schott HW. 1865. Beiträge zur Aroideenkunde. – Oesterreich. Bot. Zeitschr. 15: 33-35.
Schwanitz G. 1967. Untersuchungen zur postmeiotischen Mikrosporogenese I. Morphogenese des Ruppia-Pollens. – Pollen Spores 9: 9-48.
Schulz B. 1962. Wasserlinsen. – Neue Brehmbücherei, Witternberg.
Scribailo RW, Posluszny U. 1983. The reproductive biology of Hydrocharis morsus-ranae 1. Floral biology. – Can. J. Bot. 62: 2779-2787.
Scribailo RW, Posluszny U. 1985. Floral development of Hydrocharis morsus-ranae (Hydrocharitaceae). – Amer. J. Bot. 72: 1578-1589.
Sculthorpe CD. 1967. The biology of aquatic vascular plants. – Edward Arnold, London.
Sedayu A, Eurlings MCM, Gravendeel B, Hetterscheid WLA. 2010. Morphological character evolution in Amorphophallus (Araceae) based on a combined phylogenetic analysis of trnL, rbcL, and LEAFY second intron sequences. – Bot. Stud. 51: 473-490.
Selling OH. 1947. Aponogetonaceae in the Cretaceous of South America. – Svensk Bot. Tidskr. 41: 182.
Semroud R, Verlaque R, Crouzet A, Boudouresque CF. 1992. On a broad-leaved form of the seagrass Posidonia oceanica (Posidoniaceae) from Algiers (Algeria). – Aquatic Bot. 43: 181-198.
Serebryanyi MM. 1995. A taxonomic revision of Pseudodracontium (Araceae-Aroideae-Thomsonieae). – Blumea 40: 217-235.
Serguéeff M. 1907. Contribution à la morphologie et la biologie des Aponogétonacées. – W. Kündig & fils, Geneva.
Serizawa S. 1980. Studies in the genus Arisaema in Japan 1. Group of Arisaema undulatifolium. – J. Jap. Bot. 55: 148-156.
Serizawa S. 1981. Studies on the genus Arisaema in Japan 4. Arisaema amurense group and A. longipedunculatum group. – Acta Phytotaxon. Geobot. 32: 22-30.
Setchell WA. 1927. Zostera marina latifolia: ecad or ecotype? – Bull. Torrey Bot. Club 54: 1-6.
Setchell WA. 1933. A preliminary survey of the species of Zostera. – Proc. Natl. Acad. Sci. U.S.A. 19: 810-817.
Setchell WA. 1935. Geographic elements of the marine flora of the North Pacific Ocean. – Amer. Natur. 69: 560-577.
Setchell WA. 1946. The genus Ruppia L. – Proc. Calif. Acad. Sci. 25: 469-478.
Seubert E. 1993. Die Samen der Araceen: die Samenmerkmale der Araceen und ihre Bedeutung für die Gliederung der Familie. – Ph.D. diss., Universität Kaiserlautern, Germany. Koeltz Scientific Books, Königstein, Germany.
Shadowsky AF. 1931. Einige Angaben über die Embryogenie von Pistia stratiotes L. – Ber. Deutsch. Bot. Ges. 49: 350-356.
Shaffer-Fehre M. 1991a. The endotegmen tuberculae: an account of little known structures from the seed coat of the Hydrocharitoideae (Hydrocharitaceae) and Najas (Najadaceae). – Bot. J. Linn. Soc. 107: 169-188.
Shaffer-Fehre M. 1991b. The position of Najas within the subclass Alismatidae (Monocotyledones) in the light of new evidence from seed coat structures in the Hydrocharitoideae (Hydrocharitales). – Bot. J. Linn. Soc. 107: 189-209.
Sharma AK, Bhattacharya GN. 1966. A taxonomic study on some species of Araceae. – Genet. Iber. 18: 237-262.
Sharma AK, Bhattacharyya UC. 1961. Structure and behaviour of chromosomes in species of Anthurium with special reference to the accessory chromosomes. – Proc. Nat. Inst. Sci. India, Sect. B, Biol. Sci. 27: 317-328.
Sharma AK, Das NK. 1954. Study of karyotypes and their alternations in aroids. – Agron. Lusit. 16: 23-48.
Sharma AK, Datta KB. 1961. A cytological study to work out the trends of evolution in Aglaonema and Richardia. – Caryologia 14: 439-453.
Sharma AK, Mukhopadhyay S. 1965a. Cytological study on two genera of Araceae and correct assessment of their taxonomic status. – Genet. Agrar. 18: 603-616.
Sharma AK, Mukhopadhyay S. 1965b. Chromosome studies in Typhonium and Arisaema with a view to find out the mode of origin and affinity of the two. – Caryologia 30: 58-66.
Sharma AS, Chatterjee T. 1967. Cytotaxonomy of Helobiae with special reference to the mode of evolution. – Cytologia 32: 286-307.
Shaw DE, Cantrell BK. 1983. A study of the pollination of Alocasia macrorrhiza (L.) G. Don (Araceae) in Southeast Queensland. – Proc. Linn. Soc. New South Wales 106: 323-335.
Sheffer RD, Croat TB. 1983. Chromosome numbers in the genus Anthurium (Araceae) II. – Amer. J. Bot. 70: 858-871.
Sheffer RD, Kamemoto H. 1976. Chromosome numbers in the genus Anthurium (Araceae). – Amer. J. Bot. 63: 74-81.
Shepherd SA, Womersley HBS. 1981. The algal and seagrass ecology of Waterloo Bay, South Australia. – Aquatic Bot. 11: 305-391.
Sille NP, Collinson ME, Kucera M, Hooker JJ. 2006. Morphological evolution of Stratiotes through the Paleogene in Europe. – Palaios 21: 272-288.
Silva CJ da. 1981. Observações sobre a biologia reprodutiva de Pistia stratiotes L. (Araceae). – Acta Amazônica 11: 487-504.
Simmonds NW. 1950. Notes on the biology of the Araceae of Trinidad. – J. Ecol. 38: 277-291.
Simpson D. 1989. Hydrocharitaceae. – In: Polhill RM (ed), Flora of tropical East Africa, A. A. Balkema, Rotterdam, pp. 1-29.
Singh V. 1964. Morphological and anatomical studies in Helobiae I. Vegetative anatomy of some members of Potamogetonaceae. – Proc. Indian Acad. Sci., Sect. B, 60: 214-231.
Singh V. 1965a. Morphological and anatomical studies in Helobiae II. Vascular anatomy of the flower of Potamogetonaceae. – Bot. Gaz. (Crawfordsville) 126: 137-144.
Singh V. 1965b. Morphological and anatomical studies in Helobiae III. Vascular anatomy of the node and flower of Najadaceae. – Proc. Indian Acad. Sci., Sect. B, 61: 98-108.
Singh V. 1965c. Morphological and anatomical studies in Helobiae IV. Vegetative and floral anatomy of Aponogetonaceae. – Proc. Indian Acad. Sci., Sect. B, 61: 147-159.
Singh V. 1966a. Morphological and anatomical studies in Helobiae VI. Vascular anatomy of the flower of Alismaceae. – Proc. Indian Acad. Sci., Sect. B, 36: 329-344.
Singh V. 1966b. Morphological and anatomical studies in Helobiae VII. Vascular anatomy of the flower of Butomus umbellatus Linn. – Proc. Indian Acad. Sci., Sect. B, 63: 313-320.
Singh V. 1966c. Morphological and anatomical studies in Helobiae VIII. Vascular anatomy of the flower of Hydrocharitaceae-Stratioideae and Thalassioideae. – Agra Univ. J. Res. Sci. 15: 43-59.
Singh V. 1966d. Morphological and anatomical studies in Helobiae IX. Vascular anatomy of the flower of Hydrocharitaceae-Vallisnerioideae and Halophiloideae. – Agra Univ. J. Res. Sci. 15: 84-106.
Singh V. 1973. Development of gynoecium in Triglochin in three dimensions. – Curr. Sci. 42: 813-815.
Singh V, Sattler R. 1972. Floral development of Alisma triviale. – Can. J. Bot. 50: 619-627.
Singh V, Sattler R. 1973. Nonspiral androecium and gynoecium of Sagittaria latifolia. – Can. J. Bot. 51: 1093-1095.
Singh V, Sattler R. 1974. Floral development of Butomus umbellatus. – Can. J. Bot. 52: 223-230.
Singh V, Sattler R. 1977a. Development of the inflorescence and flower of Sagittaria cuneata. – Can. J. Bot. 55: 1087-1105.
Singh V, Sattler R. 1977b. Floral development of Aponogeton natans and A. undulatus. – Can. J. Bot. 55: 1106-1120.
Sivadasan M, Nicolson DH. 1982. A revision of Theriophonum (Araceae). – Kew Bull. 37: 277-290.
Skillicorn P, Spira W, Journey W. 1993. Duckweed aquaculture: a new aquatic farming system for developing countries. – The World Bank, Washington, D.C.
Sloover J-L de. 1961. Note sur le pollen de Lemna minor L. – Pollen Spores 3: 5-10.
Smith SY, Stockey RA. 2003. Aroid seeds from the Middle Eocene Princeton Chert (Keratosperma allenbyense, Araceae): comparisons with extant Lasioideae. – Intern. J. Plant Sci. 164: 239-250.
Soares ML, Mayo SJ. 2001. Three new species of Philodendron (Araceae) from the Ducke Forest Reserve, central Amazonas, Brazil. – Feddes Repert. 112: 37-45.
Soares ML, Mayo SJ, Croat TB, Gribel R. 2009. Two new species and a new combination in Amazonian Heteropsis (Araceae). – Kew Bull. 64: 263-270.
Sokoloff DD, Mering S von, Jacobs SWL, Remizowa MV. 2013. Morphology of Maundia supports its isolated phylogenetic position in the early-divergent monocot order Alismatales. – Bot. J. Linn. Soc. 173: 13-48.
Sokoloff DD, Mering S von, Remizowa MV. 2015. Female flower and fruit anatomy of Tetroncium magellanicum: implications for gynoecium evolution in the early divergent monocot order Alismatales. – Bot. J. Linn. Soc. 179: 712-724.
Sokolowska-Kulczycka A. 1980. Embryological studies of Tofieldia calyculata (L.) Whlb. – Acta Biol. Cracov., Ser. Bot. 22: 113-128.
Solereder H. 1913. Systematisch-anatomische Untersuchung des Blattes der Hydrocharitaceen. – Beih. Bot. Centralbl. 30, Abt. I, 1: 24-104.
Somogyi J. 2006. Taxonomic, nomenclatural and chorological notes on several taxa of the genus Echinodorus (Alismataceae). – Biologia 61: 381-385.
Soros-Pottruff C, Posluzny U. 1994. Developmental morphology of reproductive structures of Phyllospadix (Zosteraceae). – Intern. J. Plant Sci. 155: 405-420.
Soros-Pottruff CL, Posluszny U. 1995. Developmental morphology of reproductive structures of Zostera and a reconsideration of Heterozostera (Zosteraceae). – Intern. J. Plant Sci. 156: 143-158.
Sorsa P. 1988. Pollen morphology of Potamogeton and Groenlandia (Potamogetonaceae) and its taxonomic significance. – Ann. Bot. Fenn. 25: 179-199.
Souèges R. 1943. Embryogénie des Scheuchzériacées: Développement de l’embryon chez le Triglochin maritimum L. – Compt. Rend. Acad. Sci. 216: 746-748.
Spence DHN, Milburn TR, Ndawula-Senyimba M, Roberts E. 1971. Fruit biology and germination of two tropical Potamogeton species. – New Phytol. 70: 197-212.
Stant MY. 1964. Anatomy of the Alismataceae. – Bot. J. Linn. Soc. 59: 1-42.
Stant MY. 1967. Anatomy of the Butomaceae. – Bot. J. Linn. Soc. 60: 31-60.
Steenis CGGJ van. 1948. The genus Arisaema in Java (Araceae). – Bull. Jard. Bot. Buitenzorg, sér. III, 17: 447-456.
Steenis CGGJ van. 1955. Butomaceae. – In: Steenis CGGJ van (ed), Flora Malesiana I, 5(1), Noordhoff-Kolff N. V., Djakarta, pp. 118-120.
Stenar H. 1935. Embryologische Beobachtungen über Scheuchzeria palustris L. – Bot. Not. 1935: 78-86.
Sterling C. 1979. Comparative morphology of the carpel in the Liliaceae: Tofieldieae. – Bot. J. Linn. Soc. 79: 321-332.
Stewart JG, Lüdenberg L. 1980. Microsporocyte growth and meiosis in Phyllospadix torreyi, a marine monocotyledon. – Amer. J. Bot. 67: 949-954.
Stockey RA, Hoffman GL, Rothwell GW. 1997. The fossil monocot Limnobiophyllum scutatum: resolving the phylogeny of Lemnaceae. – Amer. J. Bot. 84: 355-368.
Stockey RA, Rothwell GW, Johnson KR. 2007. Cobbania corrugata gen. et comb. nov. (Araceae): a floating aquatic monocot from the Upper Cretaceous of western North America. – Amer. J. Bot. 94: 609-624.
Stockey RA, Gemmell JL, Beard G. 2010. An Eocene aroid inflorescence from the Appian Way locality on Vancouver Island. – In: Seberg O, Petersen G, Barfod AS, Davis JI (eds), Diversity, phylogeny, and evolution in the monocotyledons, Aarhus University Press, Århus, pp. 129-144.
Storey WS. 1954. Cytology of taro and other edible aroids. – Proc. Hawaii Acad. Sci. 29: 14-15.
Strong DJ, Ray TS. 1975. Host tree location behavior of a tropical vine (Monstera gigantea) by skototropism. – Science 190: 804-806.
Subramanian D, Munian M. 1988. Cytotaxonomical studies in South Indian Araceae. – Caryologia 53: 59-66.
Svedelius N. 1932. On the different types of pollination in Vallisneria spiralis L. and Vallisneria americana Michx. – Svensk Bot. Tidskr. 26: 1-12.
Swamy BGL, Lakshmanan KK. 1962. Contributions to the embryology of the Naiadaceae. – J. Indian Bot. Soc. 41: 247-267.
Symoens JJ, Triest L. 1983. Monograph of the African genus Lagarosiphon Harvey (Hydrocharitaceae). – Bull. Jard. Bot. Natl. Belg. 53: 441-488.
Takahashi M. 1994. Pollen development in a submerged plant Ottelia alismoides (L.) Pers. (Hydrocharitaceae). – J. Plant Res. 107: 161-164.
Takaso T, Bouman F. 1984. Ovule ontogeny and seed development in Potamogeton natans L. (Potamogetonaceae), with a note on the campylotropous ovule. – Acta Bot. Neerl. 33: 519-533.
Talavera S. 1976. Revisión de las especies españolas del genero Biarum Schott. – Lagascalia 6: 275-296.
Tam S-M, Boyce PC, Upson TM, Barabé D, Bruneau A, Forest F, Parker JS. 2004. Intergeneric and infrafamilial phylogeny of subfamily Monsteroideae (Araceae) revealed by chloroplast trnL-F sequences. – Amer. J. Bot. 91: 490-498.
Tamura MN. 1998. Nartheciaceae. – In: Kubitzki K (ed), The families and genera of vascular plants III. Flowering plants. Monocotyledons. Lilianae (except Orchidaceae), Springer, Berlin, Heidelberg, New York, pp. 381-392.
Tamura MN, Fus S, Azuma H, Hasebe M. 2004. Biosystematic studies on the family Tofieldiaceae I. Phylogeny and circumscription of the family inferred from DNA sequences of matK and rbcL. – Plant Biol. 6: 562-567.
Tamura MN, Azuma H, Yamashita J, Fuse S, Ishii T. 2010. Biosystematic studies on the family Tofieldiaceae II. Phylogeny of species of Tofieldia and Triantha inferred from plastid and nuclear DNA sequences. – Acta Phytotaxon. Geobot. 60: 131-140.
Tamura MN, Fuse S, Lee NS, Kim JO, Yamashita J, Ishii T. 2011. Biosystematic studies on the family Tofieldiaceae III. Classification of Tofieldia nuda into three species and three varieties. – Taxon 60: 1339-1348.
Tanaka N, Setoguchi H, Murata J. 1997. Phylogeny of the family Hydrocharitaceae inferred from rbcL and matK gene sequence data. – J. Plant Res. 110: 329-337.
Tanaka N, Kuo J, Omori Y, Nakaoka M, Aioi K. 2003. Phylogenetic relationships in the genera Zostera and Heterozostera (Zosteraceae) based on matK sequence data. – J. Plant Res. 116: 273-279.
Tanaka N, Uehara K, Murata J. 2004. Correlation between pollen morphology and pollination mechanisms in the Hydrocharitaceae. – J. Plant Res. 117: 265-276.
Tanaka N, Uehara K, Murata J. 2013. Evolution of floral traits in relation to pollination mechanisms in Hydrocharitaceae. – In: Wilkin P, Mayo SJ (eds.), Early events in monocot evolution, Systematics Association Spec. Vol. Series, Cambridge University Press, Cambridge. doi:10.1017/CBO9781139002950.008
Tarasevich VF. 1990. Palynological evidence on the position of the Lemnaceae family in the system of flowering plants. – Bot. Žurn. 75: 959-965. [In Russian]
Taylor ARA. 1957a. Studies of the development of Zostera marina L. 1. The embryo and seed. – Can. J. Bot. 35: 477-499.
Taylor ARA. 1957b. Studies of the development of Zostera marina L. 2. Germination and seedling development. – Can. J. Bot.35: 681-695.
Taylor ML, Altrichter KM, Aeilts LB. 2018. Pollen ontogeny in Ruppia (Alismatidae). – Intern. J. Plant Sci. 179: 217-230.
Tenorio V, Sakuragui CS, Vieira RC. 2012. Stem anatomy of Philodendron Schott (Araceae) and its contribution to the systematics of the genus. – Plant Syst. Evol. 298: 1337-1347.
Tenorio V, Sakuragui CS, Viera RC. 2014. Structures and functions of adventitious roots in species of the genus Philodendron Schott (Araceae). – Flora 209: 547-555.
Teodoridis V. 2007. Revision of Potamogeton fossils from the Most Basin and their palaeoecological significance (Early Miocene, Czech Republic). – Bull. Geosci. 82: 409-418.
Tepper JGO. 1882. Further observations on the propagation of Cymodocea antarctica. – Proc. Roy. Soc. South Aust. 4: 47-49.
Terechin ES, Chubarov SI. 1991. The embryological and carpological investigation of Althenia filiformis (Zannichelliaceae). – Bot. Žurn. 76: 226-236. [In Russian]
Terpó A. 1973. Kritische Revision der Arum-Arten des Karpatenbeckens. – Acta Bot. Acad. Sci. Hung. 18: 215-255.
Thanikaimoni G. 1969. Esquisse palynologique des aracées. – Trav. Sect. Sci. Tech. Inst. Franç. Pondichéry 5: 1-31.
Thompson CH. 1896. The ligulate Wolffias of the United States. – Rep. Missouri Bot. Gard. 7: 101-111.
Thompson CH. 1898. A revision of the American Lemnaceae occurring North of Mexico. – Rep. Missouri Bot. Gard. 9: 21-42.
Thompson S. 1982. Cyrtosperma chamissonis (Araceae): ecology, distribution and economic importance in the South Pacific. – J. Agric. Tradit. Bot. Appl. 29: 185-203.
Tillich H-J. 1985. Keimlingsbau und verwandtschaftliche Beziehungen der Araceae. – Gleditschia 13: 63-73.
Tillich H-J. 2003. Seedling diversity in Araceae and its systematic implications. – Feddes Repert. 114: 454-487.
Tippery NP, Les DH, Crawford DJ. 2014.
Evaluation of phylogenetic relationships in Lemnaceae using nuclear ribosomal
data. – Plant Biol. 17, Spec. Issue: 50-58.
Tobe H, Kadokawa T. 2008. Embryology of the Araceae: variation and character evolution. – Makinoa, N.S. 7: 29-53.
Tobe H, Kadokawa T. 2010. Endosperm development in the Araceae (Alismatales) and evolution of developmental modes in monocots. – J. Plant Res. 123: 731-739.
Toh CA. 1975. Cytotaxonomic studies on the Arisaema. – J. Korean Res. Better Living 14: 165-174.
Tomlinson PB. 1969a. On the morphology and anatomy of turtle grass, Thalassia testudinum (Hydrocharitaceae) II. Anatomy and development of the root in relation to function. – Bull. Marine Sci. 19: 57-71.
Tomlinson PB. 1969b. On the morphology and anatomy of turtle grass, Thalassia testudinum (Hydrocharitaceae) III. Floral morphology and anatomy. – Bull. Mar. Sci. 19: 286-305.
Tomlinson PB. 1972. On the morphology and anatomy of turtle grass, Thalassia testudinum (Hydrocharitaceae) IV. Leaf anatomy and development. – Bull. Marine Sci. 22: 75-93.
Tomlinson PB. 1974. Vegetative morphology and meristem dependence. The foundation of productivity in seagrasses. – Aquaculture 4: 107-130.
Tomlinson PB. 1982. Helobiae (Alismatidae), including the seagrasses. – In: Metcalfe CR (ed), Anatomy of Monocotyledons 7, Clarendon Press, Oxford, pp. 126-225.
Tomlinson PB, Posluszny U. 1976. Generic limits in the Zannichelliaceae (sensu Dumortier). – Taxon 25: 273-279.
Tomlinson PB, Posluszny U. 1978. Aspects of floral morphology and development in the sea grass Syringodium filiforme (Cymodoceaceae). – Bot. Gaz. 139: 333-345.
Tomlinson PB, Posluszny U. 2001. Generic limits in the seagrass family Zosteraceae. – Taxon 50: 429-437.
Triest L. 1988. A revision of the genus Najas L. (Najaceae) in the Old World. – Mem. Acad. Roy. Sci. Outre-Mer 22(1).
Triest L. 1989. Najadaceae. – In: Polhill RM (ed), Flora of tropical East Africa, A. A. Balkema, Rotterdam, The Netherlands, pp. 1-9.
Troll W. 1931. Beiträge zur Morphologie dees Gynaeceums I. Über das Gynaeceum der Hydrocharitaceen. – Planta 14: 1-18.
Troll W. 1932. Beiträge zur Morphologie des Gynaeceums II. Über das Gynaeceum von Limnocharis Humbl. et Bonpl. – Planta 17: 453-460.
Tsuchiya T, Takada M. 1962. Chromosome studies in five species of Araceae. – Chrom. Inf. Serv. 3: 36-38.
Tur N. 1982. Revisión del género Potamogeton L. en la Argentina. – Darwiniana 24: 214-265.
Tutin TG. 1938. The autecology of Zostera marina in relation to its wasting disease. – New Phytol. 37: 50-71.
Uchiyama H. 1989. Karyomorphological studies on some taxa of the Helobiae (Alismatidae). – J. Sci. Hiroshima Univ., ser. B, div. 2 (Botany) 22: 271-352.
Uchiyama H. 1993. Karyomorphology of seagrasses and their allied genera. – Proceedings of an International Workshop on Seagrass Biology, Kominato 1993, ORI, University of Tokyo, Japan, pp. 51-56.
Uchiyama H. 1996. An easy method for investigating molecular systematic relationships in the genus Zostera, Zosteraceae. – In: Kuo J, Phillips RC, Walker DI, Kirkman H (eds), Seagrass biology. Proceedings of an International Workshop on Seagrass Biology, Rottnest Island, 25-29 January 1996, Science, University of Western Australia, Perth, pp. 79-84.
Uhlarz H. 1985. Ist Pinellia tripartita (Blume) Schott (Araceae-Aroideae) habituell anemophil geitonogam? – Beitr. Biol. Pflanzen 60: 277-291.
Ulrich S, Hesse M, Bröderbauer D, Wong SY, Boyce PC. 2012. Schismatoglottis and Apoballis (Araceae: Schismatoglottideae): a new example for the significance of pollen morphology in Araceae systematics. – Taxon 61: 281-292.
Ulrich S, Hesse M, Bröderbauer D, Bogner J, Weber M, Halbritter H. 2013. Calla palustris (Araceae): new palynological insights with special regard to its controversial systematic position and to closely related genera. – Taxon 62: 701-712.
Urbanska-Worytkiewicz K. 1980. Cytological variation within the family of Lemnaceae. – Veröff. Geobot. Inst. E.T.H. Stiftung Rübel, Zürich, 70: 30-101.
Utech FH. 1978. Floral vascular anatomy of Pleea tenuifolia Michx. (Liliaceae-Tofieldieae) and its reassignment to Tofieldia. – Ann. Carnegie Mus. 47: 423-453.
Utech FH. 1979. Karyotype analysis, palynology, and external seed morphology of Tofieldia tenuifolia (Michx.) Utech (Liliaceae-Fofieldieae). – Ann. Carnegie Mus. 48: 171-184.
Valerio CE. 1984. Insect visitors to the inflorescence of the aroid Dieffenbachia oerstedii (Araceae) in Costa Rica. – Brenesia 22: 139-146.
Vasconcelos S, Lourdes Soares M de, Sakuragui CM, Croat TB, Oliveira G, Benko-Iseppon AM. 2018. New insights on the phylogenetic relationships among the traditional Philodendron subgenera and the other groups of the Homalomena clade (Araceae). – Mol. Phylogen. Evol. 127: 168-178.
Vasseur L, Aarssen LW, Bennett T. 1993. Allozymic variation in local apomictic populations of Lemna minor (Lemnaceae). – Amer. J. Bot. 80: 974-979.
Veen J. 1975. Preliminary studies of the flavonoid pattern of Lemna gibba L. and Lemna minor L. – Aquatic Bot. 1: 417-421.
Venkatesh CS. 1956. Structure and dehiscence of the anther in Najas. – Bot. Not. 109: 75-82.
Vierssen W van. 1982. The ecology of communities dominated by Zannichellia-taxa in western Europe I. Characterization and autecology of the Zannichellia taxa. – Aquatic Bot. 12: 103-155.
Vierssen W van, Zee jr van der. 1982. On the pollination mechanism of some eurysaline Potamogetonaceae. – Aquatic Bot. 14: 339-347.
Vierssen W van, Wijk RJ van, Zee JR van der. 1981. Some additional notes on the cytotaxonomy of Ruppia taxa in Western Europe. – Aquatic Bot. 11: 297-301.
Viinikka Y. 1973. The occurrence of B chromosomes and their effect on meiosis in Najas marina. – Hereditas 75: 207-212.
Vijayaraghavan MR, Kapoor T. 1985. Embryogenesis in Najas marina L.: structural and histochemical approach. – Aquatic Bot. 22: 45-60.
Vijayaraghavan MR, Vidya Kumari A. 1974. Embryology and systematic position of Zannichellia palustris L. – J. Indian Bot. Soc. 53: 292-302.
Vogel S, Martens J. 2000. A survey of the function of the lethal kettle traps of Arisaema (Araceae), with records of pollinating fungus gnats from Nepal. – Bot. J. Linn. Soc. 133: 61-100.
Voigt E, Domke W. 1955. Thalassocharis bosquetii Debey ex Miquel, ein strukturell erhaltenes Seegras aus der holländischen Kreide. – Mitt. Geol. Staatsinst. Hamburg 24: 87-102.
Vuille FL. 1988. The reproductive biology of the genus Baldellia (Alismataceae). – Plant Syst. Evol. 159: 173-183.
Wagner R. 1918. Über den Aufbau der Limnocharis laforestii Duchass. – Sitzungsber. Akad. Wiss. Wien, Math.-Nat. Kl. I, 127: 317-327.
Walker DI. 1985. Correlation between salinity and growth of the seagrass Amphibolis antarctica (Labill.) Sonder & Aschers. in Shark Bay, Western Australia, using a new method for measuring production rate. – Aquatic Bot. 23: 13-26.
Wang J-K (ed). 1984. Taro: A review of Colocasia esculenta and its potentials. – University of Hawaii Press, Honolulu.
Wang Q-D, Zhang T, Wang J-B. 2007. Phylogenetic relationships and hybrid origin of Potamogeton species (Potamogetonaceae) distributed in China: insights from the nuclear ribosomal internal transcribed spacer sequence (ITS). – Plant Syst. Evol. 267: 65-78.
Wang Q-F, Chen J-K. 1997. Floral organogenesis of Caldesia parnassifolia (Bassi ex L.) Parl. (Alismataceae). – Acta Phytotaxon. Sin. 35: 289-292. [In Chinese]
Wang Q-F, Zhang Z-Y, Chen J-K. 1997. Pollen morphology of the Alismataceae. – Acta Phytotaxon. Sin. 35: 225-235.
Wang X-F, Tan Y-Y, Chen J-H, Lu Y-T. 2006. Pollen tube reallocation in two preanthesis cleistogamous species, Ranalisma rostratum and Sagittaria guyanensis ssp. lappula (Alismataceae). – Aquatic Bot. 85: 233-240.
Wang X-F, Tao Y-B, Lu Y-T. 2002. Pollen tubes enter neighboring ovules by way of receptacle tissue, resulting in increased fruit-set in Sagittaria potamogetifolia Merr. – Ann. Bot. 89: 791-796.
Wangerman E, Ashby E. 1951. Studies in the morphogenesis of leaves VII, 1. Effects of light intensity and temperature on the cycle of ageing and rejuvenation in the vegetative history of Lemna minor. – New Phytol. 50: 186-199.
Wangerman E, Lacy HJ. 1952. Some effects of ultra-violet radiation on Lemna minor. – Nature 170: 126-127.
Waycott M, Les DH. 1996. An integrated approach to the evolutionary study of seagrasses. – In: Kuo J, Phillips RC, Walker DI, Kirkman H (eds), Seagrass biology. Proceedings of an International Workshop, Rottnest Island, Western Australia, 25-29 January 1996, Science, University of West Australia, Perth, pp. 71-78.
Waycott M, Walker DI, James SH. 1996. Genetic uniformity in a dioecious seagrass, Amphibolis antarctica. – Heredity 76: 578-585.
Waycott M, Procaccini GA, Les DH, Reusch TBH. 2006. Seagrass evolution, ecology and conservation: a genetic perspective. – In: Larkum AWD, Orth RJ, Duarte CM (eds), Seagrasses: biology, ecology and conservation, Springer, Dordrecht, pp. 25-50.
Wcislo H. 1970. Karyological studies in Polish representatives of Spadiciflorae. – Acta Biol. Cracov., Ser. Bot. 13: 79-88.
Weber H. 1950. Über das Wachstum des Rhizoms von Butomus umbellatus L. – Planta 38: 196-204.
Weber M, Halbritter H. 2007. Exploding pollen in Montrichardia arborescens (Araceae). – Plant Syst. Evol. 263: 51-57.
Weber M, Halbritter H, Hesse M. 1999. The basic pollen wall types in Araceae. – Intern. J. Plant Sci. 160: 415-423.
Wen J, Jansen RK, Kilgore K. 1996. Evolution of the eastern Asia and eastern North American disjunct genus Symplocarpus (Araceae): insights from chloroplast DNA restriction site data. – Biochem. Syst. Ecol. 24: 735-747.
Wiegleb G. 1988. Notes on pondweeds, outline for a monographical treatment of the genus Potamogeton L. – Feddes Repert. 99: 249-266.
Wiegleb G, Kaplan Z. 1998. An account of the species of Potamogeton L. (Potamogetonaceae). – Folia Geobot. Phytotaxon. 33: 241-316.
Wilde V, Kvaček Z, Bogner J. 2005. Fossil leaves of the Araceae from the European Eocene and notes on other aroid fossils. – Intern. J. Plant Sci. 166: 157-183.
Wilde WJJO de. 1962. Najadaceae. – In: Steenis CGGJ van (ed), Flora Malesiana I, 6, Noordhoff-Kolff N.V., pp. 157-171.
Wilder GJ. 1974a. Symmetry and development of Butomus umbellatus (Butomaceae) and Limnocharis flava (Limnocharitaceae). – Amer. J. Bot. 61: 379-394.
Wilder GJ. 1974b. Symmetry and development of Limnobium spongia (Hydrocharitaceae). – Amer. J. Bot. 61: 624-642.
Wilder GJ. 1974c. Symmetry and development of pistillate Vallisneria americana (Hydrocharitaceae). – Amer. J. Bot. 61: 846-866.
Wilder GJ. 1975. Phylogenetic trends in the Alismatidae (Monocotyledoneae). – Bot. Gaz. 136: 159-170.
Willby NJ, Eaton JW. 1993. The distribution, ecology and conservation of Luronium natans (L.) Raf. in Britain. – J. Aquatic Plant Manage 31: 70-76.
Williams CA, Harborne JB, Mayo SJ. 1981. Anthocyanin pigments and leaf flavonoids in the family Araceae. – Phytochemistry 20: 217-234.
Williams NH, Dressler RL. 1976. Euglossine pollination of Spathiphyllum (Araceae). – Selbyana 1: 349-356.
Winterbottom DC. 1917. Marine fibre. – Dept. Chem. South Aust. Bull. 4: 1-36.
Wit HCD de. 1970. A key to the species of Cryptocoryne Fish. ex Wydl. (Araceae). – Belmontia 4: 257-280.
Witmer SW. 1937. Morphology and cytology of Vallisneria spiralis L. – Amer. Midl. Natur. 18: 309-333.
Wodehouse RP. 1936. Pollen grains in the identification and classification of plants VIII. The Alismataceae. – Amer. J. Bot. 23: 535-539.
Wolek J. 1981. Assessment of the possibility of exoornithochory of duckweeds (Lemnaceae) in the light of researches into the resistance of these plants to desiccation. – Ekol. Pol. 29: 405-419.
Wong S-Y. 2009. Phylogenetic and systematic studies of the Schismatoglottideae (Araceae: Aroideae). – Ph.D. diss., Universiti Malaysia Sarawak, Kuching, Malaysia.
Wong S-Y. 2013. Rheophytism in Bornean Schismatoglottideae (Araceae). – Syst. Bot. 38: 32-45.
Wong S-Y, Boyce PC. 2010a. Studies on Schismatoglottideae (Araceae) of Borneo IX: a new genus, Hestia, and resurrection of Apoballis. – Bot. Stud. 51: 249-255.
Wong S-Y, Boyce PC. 2010b. Studies on Schismatoglottideae (Araceae) of Borneo X: Pichinia, a remarkable novel genus from Sarawak, Malaysian Borneo. – Gard. Bull. Singapore 61: 297-304.
Wong S-Y, Boyce PC. 2010c. Studies on Schismatoglottideae (Araceae) of Borneo XI: Ooia, a new genus, and a new generic delimitation for Piptospatha. – Bot. Stud. 51: 543-552.
Wong S-Y, Boyce PC. 2014a. Studies on Schismatoglottideae (Araceae) of Borneo XXX – new species and combinations for Bucephalandra. – Willdenowia 44: 149-199.
Wong S-Y, Boyce PC. 2014b. Studies on
Schismatoglottideae (Araceae) of
Borneo XXXI: additional new species of Bucephalandra. – Willdenowia
44: 415-421.
Wong S-Y, Boyce PC, Sofiman Othman A, Leaw CP. 2010. Molecular phylogeny of tribe Schismatoglottideae (Araceae) based on two plastid markers and recognition of a new tribe, Philonotieae, from the Neotropics. – Taxon 59: 117-124.
Wong S-Y, Tan PJ, Ng KK, Sofiman OA, Lee HB, Fasihuddin Badruddin A, Boyce PC. 2013. Phylogeny of Asian Homalomena (Araceae) based on ITS region combined with morphological and chemical data. – Syst. Bot. 38: 589-599.
Wongso S, Bastmeijer JD, Budianto H, Ipor IB, Munk KR, Ørgaard M, Jacobsen N. 2017. Six new Cryptocoryne taxa (Araceae) from Kalimantan, Borneo. – Willdenowia 47: 325-339.
Woodhead TW, Kirchner O. 1908. Butomaceae. – In: Kirchner O, Loew E, Schröter C (eds), Lebensgeschichte der Blütenpflanzen Mitteleuropas 1 (1a), E. Ulmer, Stuttgart.
Woolard GR, Jones JK. 1978. Polysaccharides of the sea grass Phyllospadix torreyi. – Carbohydr. Res. 63: 327-332.
Wright H, Doorn WG van, Gunawardena AHLAN. 2009. In vivo study of developmental programmed cell death using the lace plant (Aponogeton madagascariensis; Aponogetonaceae) leaf model system. – Amer. J. Bot. 96: 865-876.
Wylie RB. 1917. The pollination of Vallisneria spiralis. – Bot. Gaz. (Chicago) 63: 135-145.
Wyllie-Echeverria S, Cox PA, Churchill AC, Brotherson JD, Wyllie-Echeverria T. 2003. Seed size variation within Zostera marina L. (Zosteraceae). – Bot. J. Linn. Soc. 142: 281-288.
Yabe H, Endo S. 1935. Potamogeton remains from the Lower Cretaceous? Lycoptera bed of Jehol. – Proc. Imp. Acad. Tokyo 11: 274-276.
Yadav SR. 1993. Mechanism of apomixis in Aponogeton decaryi Jumelle. – Phytomorphology 43: 201-207.
Yadav SR. 1995. Die Hybride zwischen Aponogeton decaryi Jumelle und Aponogeton satarensis Raghavan, Kulkarni & Yadav. – Aqua-Planta 20: 71-80.
Yamashita T. 1970. Eigenartige Wurzelanlage des Embryos bei Lilaea subulata Humb. & Bonpl. und Triglochin maritimum L. – J. Fac. Sci. Univ. Tokyo, Sect. III, 10: 181-205.
Yamashita T. 1972. Eigenartige Wurzelanlage der Embryos bei Ruppia maritima L. – Beitr. Biol. Pflanzen 48: 157-170.
Yamashita T. 1973. Über die Embryo- und Wurzelentwicklung bei Zostera japonicus Aschers. et Graebn. – J. Fac. Sci. Univ. Tokyo, Ser. III, Bot. 11: 175-193.
Yamashita T. 1976a. Über die Pollenbildung bei Halodule pinifolia und H. uninervis. – Beitr. Biol. Pflanzen 52: 217-226.
Yamashita T. 1976b. Über die Embryo- und Wurzelentwicklung bei Aponogeton madagascariensis (Mirbel) van Bruggen. – J. Fac. Sci. Univ. Tokyo, Sect. III, 12: 37-64.
Yeng WS. 2013. Rheophytism in Bornean Schismatoglottideae (Araceae). – Syst. Bot. 38: 32-45.
Yeng WS, Jean TP, Kiaw NK, Othman AS, Boon LH, Ahmad FB, Boyce PC. 2013. Phylogeny of Asian Homalomena (Araceae) based on the ITS region combined with morphological and chemical data. – Syst. Bot. 38: 589-599.
Yeng WS, Meerow AW, Croat TB. 2016. Resurrection and new species of the Neotropical genus Adelonema (Araceae: Philodendron Clade). – Syst. Bot. 41: 32-48.
Yeo RR, Falk RH, Thurston JR. 1984. The morphology of hydrilla (Hydrilla verticillata (L. f.) Royle). – J. Aquatic Plant Manag. 22: 1-17.
Yi T-S, Li H, Li D-Z. 2005. Chromosome variation in the genus Pinellia (Araceae) in China and Japan. – Bot. J. Linn. Soc. 147: 449-455.
Yip M. 1988. The anatomy and morphology of the vegetative and floral parts of Zostera L. – M.Sc. thesis, Guelph University, Guelph, Ontario.
Young HJ. 1986. Beetle pollination of Dieffenbachia longispatha (Araceae). – Amer. J. Bot. 73: 931-944.
Zakhariyeva OI, Makushenko LM. 1969. Chromosome numbers of monocotyledons belonging to the families Liliaceae, Iridaceae, Amaryllidaceae and Araceae. – Bot. Žurn. 54: 1213-1227. [In Russian]
Zapata O, McMillan C. 1979. Phenolic acids in seagrasses. – Aquatic Bot. 7: 307-317.
Zennie TM, McClure JW. 1977. The flavonoid chemistry of Pistia stratiotes L. and the origin of the Lemnaceae. – Aquatic Bot. 3: 49-54.
Zhang XI, Gituru RW, Yang C, Guo Y. 2009. Variations of floral traits among different life forms illustrate the evolution of pollination systems in Potamogeton species from China. – Aquatic Bot. 90: 124-128.
Zhao L-C, Wu Z-Y. 2008. A review on the taxonomy and evolution of Ruppia. – J. Syst. Evol. 46: 467-478.
Zhao L-C, Collinson ME, Li C-S. 2004. Fruits and seeds of Ruppia (Potamogetonaceae) from the Pliocene of Yushe Basin, Shanxi, northern China, and their ecological implications. – Bot. J. Linn. Soc. 145: 317-329.
Zhilin SG. 1974. Die erste tertiäre Art aus der Gattung Aponogeton (Aponogetonaceae). – Bot. Žurn. 59: 1203-1206. [In Russian]
Zhu, G, Li H, Li R. 2007. A synopsis and a new species of the E Asian genus Pinellia (Araceae). – Willdenowia 37: 503-522.